User login
Carvedilol fails to reduce variceal bleeds in acute-on-chronic liver failure
WASHINGTON – Treatment with carvedilol reduced the incidence of sepsis and acute kidney injury and improved survival at 28 days but did not significantly reduce the progression of esophageal varices in patients with acute-on-chronic liver failure.
A total of 136 patients with acute-on-chronic liver failure with small or no esophageal varices and a hepatic venous pressure gradient (HVPG) of 12 mm Hg or greater were enrolled in a single center, prospective, open-label, randomized controlled trial: 66 were randomized to carvedilol and 70 to placebo, according to Sumeet Kainth, MD, of the Institute of Liver and Biliary Sciences in New Delhi.
More than 90% of patients were men with a mean age of 44 years, and composition of the treatment and placebo groups was similar. About 70% in each group had alcoholic hepatitis (the reason for acute liver failure in most). Mean Model for End-Stage Liver Disease (MELD) scores were about 25. Hemodynamic parameters also were comparable, with a mean HVPG of about 19, Dr. Kainth said at the annual meeting of the American Association for the Study of Liver Diseases.
Patients in the treatment group received a median maximum tolerated dose of carvedilol of 12.5 mg, with a range of 3.13 mg to 25 mg.
Morbidity and mortality were high, as is expected with acute-on-chronic liver failure, he noted. A total of 36 patients died before the end of the 90-day study period. Another 23 experienced adverse events and 2 progressed to liver transplant.
HVPG at 90 days decreased significantly in both groups. In the carvedilol group, 90-day HVPG was 16 mm Hg, compared with 19.7 mm Hg at baseline (P less than .01). For placebo patients, 90-day HVPG spontaneously improved to 14.8 mm Hg, compared with a baseline of 17.2 mm Hg (P less than .01).
Carvedilol did not significantly slow the development or growth of varices, however, Dr. Kainth said. At 90 days, varices had progressed in 9 of 40 patients (22.5%) of patients on carvedilol and 8 of 31 (25.8%) of placebo patients.
Significantly fewer patients in the carvedilol group developed acute kidney injury at 28 days (14% vs. 38% on placebo) and sepsis (5% vs. 20%). Mortality also was reduced significantly at 28 days (11% vs. 24%), he reported.
Treatment with carvedilol did not achieve significant reductions in variceal bleeding, “possibly due to the low number of bleeds seen in the study [because of] the exclusion of patients with large varices,” Dr. Kainth said.
The study was sponsored by Institute of Liver and Biliary Sciences. Dr. Kainth reported no relevant conflicts of interest.
[email protected]
On Twitter @denisefulton
WASHINGTON – Treatment with carvedilol reduced the incidence of sepsis and acute kidney injury and improved survival at 28 days but did not significantly reduce the progression of esophageal varices in patients with acute-on-chronic liver failure.
A total of 136 patients with acute-on-chronic liver failure with small or no esophageal varices and a hepatic venous pressure gradient (HVPG) of 12 mm Hg or greater were enrolled in a single center, prospective, open-label, randomized controlled trial: 66 were randomized to carvedilol and 70 to placebo, according to Sumeet Kainth, MD, of the Institute of Liver and Biliary Sciences in New Delhi.
More than 90% of patients were men with a mean age of 44 years, and composition of the treatment and placebo groups was similar. About 70% in each group had alcoholic hepatitis (the reason for acute liver failure in most). Mean Model for End-Stage Liver Disease (MELD) scores were about 25. Hemodynamic parameters also were comparable, with a mean HVPG of about 19, Dr. Kainth said at the annual meeting of the American Association for the Study of Liver Diseases.
Patients in the treatment group received a median maximum tolerated dose of carvedilol of 12.5 mg, with a range of 3.13 mg to 25 mg.
Morbidity and mortality were high, as is expected with acute-on-chronic liver failure, he noted. A total of 36 patients died before the end of the 90-day study period. Another 23 experienced adverse events and 2 progressed to liver transplant.
HVPG at 90 days decreased significantly in both groups. In the carvedilol group, 90-day HVPG was 16 mm Hg, compared with 19.7 mm Hg at baseline (P less than .01). For placebo patients, 90-day HVPG spontaneously improved to 14.8 mm Hg, compared with a baseline of 17.2 mm Hg (P less than .01).
Carvedilol did not significantly slow the development or growth of varices, however, Dr. Kainth said. At 90 days, varices had progressed in 9 of 40 patients (22.5%) of patients on carvedilol and 8 of 31 (25.8%) of placebo patients.
Significantly fewer patients in the carvedilol group developed acute kidney injury at 28 days (14% vs. 38% on placebo) and sepsis (5% vs. 20%). Mortality also was reduced significantly at 28 days (11% vs. 24%), he reported.
Treatment with carvedilol did not achieve significant reductions in variceal bleeding, “possibly due to the low number of bleeds seen in the study [because of] the exclusion of patients with large varices,” Dr. Kainth said.
The study was sponsored by Institute of Liver and Biliary Sciences. Dr. Kainth reported no relevant conflicts of interest.
[email protected]
On Twitter @denisefulton
WASHINGTON – Treatment with carvedilol reduced the incidence of sepsis and acute kidney injury and improved survival at 28 days but did not significantly reduce the progression of esophageal varices in patients with acute-on-chronic liver failure.
A total of 136 patients with acute-on-chronic liver failure with small or no esophageal varices and a hepatic venous pressure gradient (HVPG) of 12 mm Hg or greater were enrolled in a single center, prospective, open-label, randomized controlled trial: 66 were randomized to carvedilol and 70 to placebo, according to Sumeet Kainth, MD, of the Institute of Liver and Biliary Sciences in New Delhi.
More than 90% of patients were men with a mean age of 44 years, and composition of the treatment and placebo groups was similar. About 70% in each group had alcoholic hepatitis (the reason for acute liver failure in most). Mean Model for End-Stage Liver Disease (MELD) scores were about 25. Hemodynamic parameters also were comparable, with a mean HVPG of about 19, Dr. Kainth said at the annual meeting of the American Association for the Study of Liver Diseases.
Patients in the treatment group received a median maximum tolerated dose of carvedilol of 12.5 mg, with a range of 3.13 mg to 25 mg.
Morbidity and mortality were high, as is expected with acute-on-chronic liver failure, he noted. A total of 36 patients died before the end of the 90-day study period. Another 23 experienced adverse events and 2 progressed to liver transplant.
HVPG at 90 days decreased significantly in both groups. In the carvedilol group, 90-day HVPG was 16 mm Hg, compared with 19.7 mm Hg at baseline (P less than .01). For placebo patients, 90-day HVPG spontaneously improved to 14.8 mm Hg, compared with a baseline of 17.2 mm Hg (P less than .01).
Carvedilol did not significantly slow the development or growth of varices, however, Dr. Kainth said. At 90 days, varices had progressed in 9 of 40 patients (22.5%) of patients on carvedilol and 8 of 31 (25.8%) of placebo patients.
Significantly fewer patients in the carvedilol group developed acute kidney injury at 28 days (14% vs. 38% on placebo) and sepsis (5% vs. 20%). Mortality also was reduced significantly at 28 days (11% vs. 24%), he reported.
Treatment with carvedilol did not achieve significant reductions in variceal bleeding, “possibly due to the low number of bleeds seen in the study [because of] the exclusion of patients with large varices,” Dr. Kainth said.
The study was sponsored by Institute of Liver and Biliary Sciences. Dr. Kainth reported no relevant conflicts of interest.
[email protected]
On Twitter @denisefulton
AT THE LIVER MEETING 2017
Key clinical point:
Major finding: At 90 days, varices had progressed in 9 of 40 (22.5%) patients on carvedilol vs. 8 of 31 (25.8%) of placebo patients.
Data source: A single-center, prospective, open-label, randomized controlled trial of 136 patients with acute-on-chronic liver failure.
Disclosures: The study was sponsored by the Institute of Liver and Biliary Sciences. Dr. Kainth reported no relevant conflicts of interest.
How to Interpret Positive Troponin Tests in CKD
Q) Recently, when I have sent my patients with chronic kidney disease (CKD) to the emergency department (ED) for complaints of chest pain or shortness of breath, their troponin levels are high. I know CKD increases risk for cardiovascular disease, but I find it hard to believe that every CKD patient is having an MI. What gives?
Cardiovascular disease remains the most common cause of death in patients with CKD, accounting for 45% to 50% of all deaths. Therefore, accurate diagnosis of acute myocardial infarction (AMI) in this patient population is vital to assure prompt identification and treatment.1,2
Cardiac troponins are the gold standard for detecting myocardial injury in patients presenting to the ED with suggestive symptoms.1 But the chronic baseline elevation in serum troponin levels among patients with CKD often results in a false-positive reading, making the detection of AMI difficult.1
With the recent introduction of high-sensitivity troponin assays, as many as 97% of patients on hemodialysis exhibit elevated troponin levels; this is also true for patients with CKD, on a sliding scale (lower kidney function = higher baseline troponins).2 The use of high-sensitivity testing has increased substantially in the past 15 years, and it is expected to become the benchmark for troponin evaluation. While older troponin tests had a false-positive rate of 30% to 85% in patients with stage 5 CKD, the newer troponin tests display elevated troponins in almost 100% of these patients.1,2
Numerous studies have been conducted to determine the best way to interpret positive troponin tests in patients with CKD to ensure an accurate diagnosis of AMI.2 One study determined that a 20% increase in troponin levels was a more accurate determinant of AMI in patients with CKD than one isolated positive level.3 Another study demonstrated that serial troponin measurements conducted over time yielded higher diagnostic accuracy than one measurement above the 99th percentile.4
The American College of Cardiology Foundation task force found that monitoring changes in troponin concentration over time (3-6 h) is more accurate than a single elevated troponin when diagnosing AMI in symptomatic patients.3 Correlation between elevated troponin levels and clinical suspicion proved helpful in determining the significance of troponin results and the probability of AMI in patients with CKD.2
The significance and interpretation of elevated troponin levels in patients with CKD remains an important topic for further study, as cardiovascular disease continues to be the leading cause of mortality in patients with kidney dysfunction.1,2 More definitive studies need to be conducted on patients with CKD as high-sensitivity troponin assay testing becomes standard for diagnosing AMI.
So, the reason you see more positive troponin results in your CKD population is due to both the increased accuracy of the newer tests and the fact that CKD often causes a false-positive result. Monitoring your patients with serial troponins for at least three hours is essential to confirm or rule out an AMI. —MS-G
Marlene Shaw-Gallagher, MS, PA-C
University of Detroit Mercy, Michigan
Division of Nephrology, University of Michigan, Ann Arbor
1. Robitaille R, Lafrance JP, Leblanc M. Altered laboratory findings associated with end-stage renal disease. Semin Dial. 2006;19(5):373.
2. Howard CE, McCullough PA. Decoding acute myocardial infarction among patients on dialysis. J Am Soc Nephrol. 2017;28(5):1337-1339.
3. Newby LK, Jesse RL, Babb JD, et al. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2012; 60(23):2427-2463.
4. Mahajan VS, Petr Jarolim P. How to interpret elevated cardiac troponin levels. Circulation. 2011;124:2350-2354.
Q) Recently, when I have sent my patients with chronic kidney disease (CKD) to the emergency department (ED) for complaints of chest pain or shortness of breath, their troponin levels are high. I know CKD increases risk for cardiovascular disease, but I find it hard to believe that every CKD patient is having an MI. What gives?
Cardiovascular disease remains the most common cause of death in patients with CKD, accounting for 45% to 50% of all deaths. Therefore, accurate diagnosis of acute myocardial infarction (AMI) in this patient population is vital to assure prompt identification and treatment.1,2
Cardiac troponins are the gold standard for detecting myocardial injury in patients presenting to the ED with suggestive symptoms.1 But the chronic baseline elevation in serum troponin levels among patients with CKD often results in a false-positive reading, making the detection of AMI difficult.1
With the recent introduction of high-sensitivity troponin assays, as many as 97% of patients on hemodialysis exhibit elevated troponin levels; this is also true for patients with CKD, on a sliding scale (lower kidney function = higher baseline troponins).2 The use of high-sensitivity testing has increased substantially in the past 15 years, and it is expected to become the benchmark for troponin evaluation. While older troponin tests had a false-positive rate of 30% to 85% in patients with stage 5 CKD, the newer troponin tests display elevated troponins in almost 100% of these patients.1,2
Numerous studies have been conducted to determine the best way to interpret positive troponin tests in patients with CKD to ensure an accurate diagnosis of AMI.2 One study determined that a 20% increase in troponin levels was a more accurate determinant of AMI in patients with CKD than one isolated positive level.3 Another study demonstrated that serial troponin measurements conducted over time yielded higher diagnostic accuracy than one measurement above the 99th percentile.4
The American College of Cardiology Foundation task force found that monitoring changes in troponin concentration over time (3-6 h) is more accurate than a single elevated troponin when diagnosing AMI in symptomatic patients.3 Correlation between elevated troponin levels and clinical suspicion proved helpful in determining the significance of troponin results and the probability of AMI in patients with CKD.2
The significance and interpretation of elevated troponin levels in patients with CKD remains an important topic for further study, as cardiovascular disease continues to be the leading cause of mortality in patients with kidney dysfunction.1,2 More definitive studies need to be conducted on patients with CKD as high-sensitivity troponin assay testing becomes standard for diagnosing AMI.
So, the reason you see more positive troponin results in your CKD population is due to both the increased accuracy of the newer tests and the fact that CKD often causes a false-positive result. Monitoring your patients with serial troponins for at least three hours is essential to confirm or rule out an AMI. —MS-G
Marlene Shaw-Gallagher, MS, PA-C
University of Detroit Mercy, Michigan
Division of Nephrology, University of Michigan, Ann Arbor
Q) Recently, when I have sent my patients with chronic kidney disease (CKD) to the emergency department (ED) for complaints of chest pain or shortness of breath, their troponin levels are high. I know CKD increases risk for cardiovascular disease, but I find it hard to believe that every CKD patient is having an MI. What gives?
Cardiovascular disease remains the most common cause of death in patients with CKD, accounting for 45% to 50% of all deaths. Therefore, accurate diagnosis of acute myocardial infarction (AMI) in this patient population is vital to assure prompt identification and treatment.1,2
Cardiac troponins are the gold standard for detecting myocardial injury in patients presenting to the ED with suggestive symptoms.1 But the chronic baseline elevation in serum troponin levels among patients with CKD often results in a false-positive reading, making the detection of AMI difficult.1
With the recent introduction of high-sensitivity troponin assays, as many as 97% of patients on hemodialysis exhibit elevated troponin levels; this is also true for patients with CKD, on a sliding scale (lower kidney function = higher baseline troponins).2 The use of high-sensitivity testing has increased substantially in the past 15 years, and it is expected to become the benchmark for troponin evaluation. While older troponin tests had a false-positive rate of 30% to 85% in patients with stage 5 CKD, the newer troponin tests display elevated troponins in almost 100% of these patients.1,2
Numerous studies have been conducted to determine the best way to interpret positive troponin tests in patients with CKD to ensure an accurate diagnosis of AMI.2 One study determined that a 20% increase in troponin levels was a more accurate determinant of AMI in patients with CKD than one isolated positive level.3 Another study demonstrated that serial troponin measurements conducted over time yielded higher diagnostic accuracy than one measurement above the 99th percentile.4
The American College of Cardiology Foundation task force found that monitoring changes in troponin concentration over time (3-6 h) is more accurate than a single elevated troponin when diagnosing AMI in symptomatic patients.3 Correlation between elevated troponin levels and clinical suspicion proved helpful in determining the significance of troponin results and the probability of AMI in patients with CKD.2
The significance and interpretation of elevated troponin levels in patients with CKD remains an important topic for further study, as cardiovascular disease continues to be the leading cause of mortality in patients with kidney dysfunction.1,2 More definitive studies need to be conducted on patients with CKD as high-sensitivity troponin assay testing becomes standard for diagnosing AMI.
So, the reason you see more positive troponin results in your CKD population is due to both the increased accuracy of the newer tests and the fact that CKD often causes a false-positive result. Monitoring your patients with serial troponins for at least three hours is essential to confirm or rule out an AMI. —MS-G
Marlene Shaw-Gallagher, MS, PA-C
University of Detroit Mercy, Michigan
Division of Nephrology, University of Michigan, Ann Arbor
1. Robitaille R, Lafrance JP, Leblanc M. Altered laboratory findings associated with end-stage renal disease. Semin Dial. 2006;19(5):373.
2. Howard CE, McCullough PA. Decoding acute myocardial infarction among patients on dialysis. J Am Soc Nephrol. 2017;28(5):1337-1339.
3. Newby LK, Jesse RL, Babb JD, et al. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2012; 60(23):2427-2463.
4. Mahajan VS, Petr Jarolim P. How to interpret elevated cardiac troponin levels. Circulation. 2011;124:2350-2354.
1. Robitaille R, Lafrance JP, Leblanc M. Altered laboratory findings associated with end-stage renal disease. Semin Dial. 2006;19(5):373.
2. Howard CE, McCullough PA. Decoding acute myocardial infarction among patients on dialysis. J Am Soc Nephrol. 2017;28(5):1337-1339.
3. Newby LK, Jesse RL, Babb JD, et al. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2012; 60(23):2427-2463.
4. Mahajan VS, Petr Jarolim P. How to interpret elevated cardiac troponin levels. Circulation. 2011;124:2350-2354.
Oral anticoagulation ‘reasonable’ in advanced kidney disease with A-fib
BARCELONA – Oral anticoagulation had a net overall benefit for patients with atrial fibrillation and advanced chronic kidney disease, based on results of a large observational study reported at the annual congress of the European Society of Cardiology.
The novel direct-acting oral anticoagulants (NOACs) and warfarin were all similarly effective in this study of 39,241 patients who had stage 4 or 5 chronic kidney disease (CKD), atrial fibrillation, and were not on dialysis. Compared with no oral anticoagulation, the drugs cut in half the risk of stroke or systemic embolism, with no increased risk of major bleeding.
“In patients with advanced CKD, it appears that OACs [oral anticoagulants] are reasonable,” concluded Peter A. Noseworthy, MD, of the Mayo Clinic in Rochester, Minn.
This is a potentially practice-changing finding given the “striking underutilization” of OACs in advanced CKD, he noted. Indeed, only one-third of the patients in this study were prescribed an OAC and picked up their prescriptions. And while the study has the limitations inherent to an observational study reliant upon data from a large U.S. administrative database – chiefly, the potential for residual confounding because of factors that couldn’t be adjusted for statistically – these real-world data may be as good as it gets, since patients with advanced CKD were excluded from the pivotal trials of the NOACs.
Apixaban (Eliquis) was the winner in this study: It separated itself from the pack by reducing the major bleeding risk by 57%, compared with warfarin, although it wasn’t significantly more effective than the other drugs in terms of stroke prevention. In contrast, the major bleeding rates for dabigatran (Pradaxa) and rivaroxaban (Xarelto) weren’t significantly different from warfarin in this challenging patient population.
In a related analysis of 10,712 patients with atrial fibrillation and advanced CKD who were on dialysis, use of an OAC was once again a winning strategy: It resulted not only in an impressive 58% reduction in the risk of stroke or systemic embolism, but also a 26% reduction in the risk of major bleeding, compared with no OAC.
Here again, apixaban was arguably the drug of choice. None of the 125 dialysis patients on apixaban experienced a stroke or systemic embolism. In contrast, dabigatran and rivaroxaban were associated with greater than threefold higher stroke rates than in patients on warfarin, although these differences didn’t achieve statistical significance because of small numbers, just 36 patients on dabigatran and 56 on rivaroxaban, the cardiologist continued.
For these analyses of the relationship between OAC exposure and stroke and bleeding outcomes, Dr. Noseworthy and his coinvestigators used propensity scores based upon 59 clinical and sociodemographic characteristics.
Asked why rates of utilization of OACs are so low in patients with advanced CKD, Dr. Noseworthy replied that he didn’t find that particularly surprising.
“Even if you look only at patients without renal dysfunction, there is incredible undertreatment of atrial fibrillation with OACs. And adherence is very poor,” he observed.
Moreover, in talking with nephrologists, he finds many of them have legitimate reservations about prescribing OACs for patients with end-stage renal disease on hemodialysis.
“They’re undergoing a lot of procedures. They’re having a ton of lines placed; they’re having fistulas revised; and they have very high rates of GI bleeding. In some studies the annual risk of bleeding is 20%-40% in this population. And they’re a frail population with frequent falls,” Dr. Noseworthy said.
He reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
BARCELONA – Oral anticoagulation had a net overall benefit for patients with atrial fibrillation and advanced chronic kidney disease, based on results of a large observational study reported at the annual congress of the European Society of Cardiology.
The novel direct-acting oral anticoagulants (NOACs) and warfarin were all similarly effective in this study of 39,241 patients who had stage 4 or 5 chronic kidney disease (CKD), atrial fibrillation, and were not on dialysis. Compared with no oral anticoagulation, the drugs cut in half the risk of stroke or systemic embolism, with no increased risk of major bleeding.
“In patients with advanced CKD, it appears that OACs [oral anticoagulants] are reasonable,” concluded Peter A. Noseworthy, MD, of the Mayo Clinic in Rochester, Minn.
This is a potentially practice-changing finding given the “striking underutilization” of OACs in advanced CKD, he noted. Indeed, only one-third of the patients in this study were prescribed an OAC and picked up their prescriptions. And while the study has the limitations inherent to an observational study reliant upon data from a large U.S. administrative database – chiefly, the potential for residual confounding because of factors that couldn’t be adjusted for statistically – these real-world data may be as good as it gets, since patients with advanced CKD were excluded from the pivotal trials of the NOACs.
Apixaban (Eliquis) was the winner in this study: It separated itself from the pack by reducing the major bleeding risk by 57%, compared with warfarin, although it wasn’t significantly more effective than the other drugs in terms of stroke prevention. In contrast, the major bleeding rates for dabigatran (Pradaxa) and rivaroxaban (Xarelto) weren’t significantly different from warfarin in this challenging patient population.
In a related analysis of 10,712 patients with atrial fibrillation and advanced CKD who were on dialysis, use of an OAC was once again a winning strategy: It resulted not only in an impressive 58% reduction in the risk of stroke or systemic embolism, but also a 26% reduction in the risk of major bleeding, compared with no OAC.
Here again, apixaban was arguably the drug of choice. None of the 125 dialysis patients on apixaban experienced a stroke or systemic embolism. In contrast, dabigatran and rivaroxaban were associated with greater than threefold higher stroke rates than in patients on warfarin, although these differences didn’t achieve statistical significance because of small numbers, just 36 patients on dabigatran and 56 on rivaroxaban, the cardiologist continued.
For these analyses of the relationship between OAC exposure and stroke and bleeding outcomes, Dr. Noseworthy and his coinvestigators used propensity scores based upon 59 clinical and sociodemographic characteristics.
Asked why rates of utilization of OACs are so low in patients with advanced CKD, Dr. Noseworthy replied that he didn’t find that particularly surprising.
“Even if you look only at patients without renal dysfunction, there is incredible undertreatment of atrial fibrillation with OACs. And adherence is very poor,” he observed.
Moreover, in talking with nephrologists, he finds many of them have legitimate reservations about prescribing OACs for patients with end-stage renal disease on hemodialysis.
“They’re undergoing a lot of procedures. They’re having a ton of lines placed; they’re having fistulas revised; and they have very high rates of GI bleeding. In some studies the annual risk of bleeding is 20%-40% in this population. And they’re a frail population with frequent falls,” Dr. Noseworthy said.
He reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
BARCELONA – Oral anticoagulation had a net overall benefit for patients with atrial fibrillation and advanced chronic kidney disease, based on results of a large observational study reported at the annual congress of the European Society of Cardiology.
The novel direct-acting oral anticoagulants (NOACs) and warfarin were all similarly effective in this study of 39,241 patients who had stage 4 or 5 chronic kidney disease (CKD), atrial fibrillation, and were not on dialysis. Compared with no oral anticoagulation, the drugs cut in half the risk of stroke or systemic embolism, with no increased risk of major bleeding.
“In patients with advanced CKD, it appears that OACs [oral anticoagulants] are reasonable,” concluded Peter A. Noseworthy, MD, of the Mayo Clinic in Rochester, Minn.
This is a potentially practice-changing finding given the “striking underutilization” of OACs in advanced CKD, he noted. Indeed, only one-third of the patients in this study were prescribed an OAC and picked up their prescriptions. And while the study has the limitations inherent to an observational study reliant upon data from a large U.S. administrative database – chiefly, the potential for residual confounding because of factors that couldn’t be adjusted for statistically – these real-world data may be as good as it gets, since patients with advanced CKD were excluded from the pivotal trials of the NOACs.
Apixaban (Eliquis) was the winner in this study: It separated itself from the pack by reducing the major bleeding risk by 57%, compared with warfarin, although it wasn’t significantly more effective than the other drugs in terms of stroke prevention. In contrast, the major bleeding rates for dabigatran (Pradaxa) and rivaroxaban (Xarelto) weren’t significantly different from warfarin in this challenging patient population.
In a related analysis of 10,712 patients with atrial fibrillation and advanced CKD who were on dialysis, use of an OAC was once again a winning strategy: It resulted not only in an impressive 58% reduction in the risk of stroke or systemic embolism, but also a 26% reduction in the risk of major bleeding, compared with no OAC.
Here again, apixaban was arguably the drug of choice. None of the 125 dialysis patients on apixaban experienced a stroke or systemic embolism. In contrast, dabigatran and rivaroxaban were associated with greater than threefold higher stroke rates than in patients on warfarin, although these differences didn’t achieve statistical significance because of small numbers, just 36 patients on dabigatran and 56 on rivaroxaban, the cardiologist continued.
For these analyses of the relationship between OAC exposure and stroke and bleeding outcomes, Dr. Noseworthy and his coinvestigators used propensity scores based upon 59 clinical and sociodemographic characteristics.
Asked why rates of utilization of OACs are so low in patients with advanced CKD, Dr. Noseworthy replied that he didn’t find that particularly surprising.
“Even if you look only at patients without renal dysfunction, there is incredible undertreatment of atrial fibrillation with OACs. And adherence is very poor,” he observed.
Moreover, in talking with nephrologists, he finds many of them have legitimate reservations about prescribing OACs for patients with end-stage renal disease on hemodialysis.
“They’re undergoing a lot of procedures. They’re having a ton of lines placed; they’re having fistulas revised; and they have very high rates of GI bleeding. In some studies the annual risk of bleeding is 20%-40% in this population. And they’re a frail population with frequent falls,” Dr. Noseworthy said.
He reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: The risk of stroke/systemic embolism in patients with advanced chronic kidney disease who were on oral anticoagulation was reduced by 49% among those not on hemodialysis and by 58% in those who were, compared with similar patients not on oral anticoagulation.
Data source: This was an observational study of nearly 50,000 patients with atrial fibrillation and stage 4 or 5 chronic kidney disease in a large U.S. administrative database.
Disclosures: The presenter reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
Do PPIs Pose a Danger to Kidneys?
Q) Is it true that PPI use can cause kidney disease?
Proton pump inhibitors (PPIs) have been available in the United States since 1990, with OTC options available since 2009. While these medications play a vital role in the treatment of gastrointestinal (GI) conditions, observational studies have linked PPI use to serious adverse events, including dementia, community-acquired pneumonia, hip fracture, and Clostridium difficile infection.1-4
Studies have also found an association between PPI use and kidney problems such as acute kidney injury (AKI), acute interstitial nephritis, and incident chronic kidney disease (CKD).5-7 One observational study used the Department of Veterans Affairs (VA) national databases to track the renal outcomes of 173,321 new PPI users and 20,270 new histamine H2 receptor antagonist (H2RA) users over the course of five years. Those who used PPIs demonstrated a significant risk for decreased renal function, lower estimated glomerular filtration rate (eGFR), doubled serum creatinine levels, and progression to end-stage renal disease (ESRD).8
Another study of 10,482 patients (322 PPI; 956 H2RA; 9,204 nonusers) and a replicate study of 248,751 patients (16,900 PPI; 6,640 H2RA; 225,211 nonusers) with an initial eGFR ≥ 60 mL/min/1.73m2 also found an association between PPI use and incident CKD, which persisted when compared to the other groups. Additionally, twice-daily PPI use was associated with a higher CKD risk than once-daily use.9
The pathophysiology of PPI use and kidney deterioration is poorly understood at this point. It is known that AKI can increase the risk for CKD, and AKI has been an assumed precursor to PPI-associated CKD. However, a study by Xie and colleagues reported an association between PPI use and increased risk for CKD, progression of CKD, and ESRD in the absence of preceding AKI. Using the VA databases, the researchers identified 144,032 new users of acid-suppressing medications (125,596 PPI; 18,436 H2RA) who had no history of kidney disease and followed them for five years. PPI users were found to be at increased risk for CKD, and a graded association was discovered between length of PPI use and risk for CKD.10
While these studies are observational and therefore do not prove causation, they do suggest a need for attentive monitoring of kidney function in patients using PPIs. Evaluating the need for PPIs and inquiring about OTC use of these medications is highly recommended, as research has found 25% to 70% of PPI prescriptions are not prescribed for an appropriate indication.11 Considerations regarding PPI use should include dosage, length of use, and whether alternate use of an H2RA is appropriate. —CAS
Cynthia A. Smith, DNP, CNN-NP, FNP-BC, APRN
Renal Consultants, PLLC, South Charleston, West Virginia
1. Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
2. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PloS One. 2015;10(6):e0128004.
3. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006; 296(24):2947-2953.
4. Dial S, Alrasadi K, Manoukian C, et al. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171(1):33-38.
5. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150.
6. Blank ML, Parkin L, Paul C, et al. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86:837-844.
7. Antoniou T, Macdonald EM, Hollands S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3(2):E166-171.
8. Xie Y, Bowe B, Li T, et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2016;27(10):3153-3163.
9. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238-246.
10. Xie Y, Bowe B, Li T, et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 2017;91(6):1482-1494.
11. Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2-3.
Q) Is it true that PPI use can cause kidney disease?
Proton pump inhibitors (PPIs) have been available in the United States since 1990, with OTC options available since 2009. While these medications play a vital role in the treatment of gastrointestinal (GI) conditions, observational studies have linked PPI use to serious adverse events, including dementia, community-acquired pneumonia, hip fracture, and Clostridium difficile infection.1-4
Studies have also found an association between PPI use and kidney problems such as acute kidney injury (AKI), acute interstitial nephritis, and incident chronic kidney disease (CKD).5-7 One observational study used the Department of Veterans Affairs (VA) national databases to track the renal outcomes of 173,321 new PPI users and 20,270 new histamine H2 receptor antagonist (H2RA) users over the course of five years. Those who used PPIs demonstrated a significant risk for decreased renal function, lower estimated glomerular filtration rate (eGFR), doubled serum creatinine levels, and progression to end-stage renal disease (ESRD).8
Another study of 10,482 patients (322 PPI; 956 H2RA; 9,204 nonusers) and a replicate study of 248,751 patients (16,900 PPI; 6,640 H2RA; 225,211 nonusers) with an initial eGFR ≥ 60 mL/min/1.73m2 also found an association between PPI use and incident CKD, which persisted when compared to the other groups. Additionally, twice-daily PPI use was associated with a higher CKD risk than once-daily use.9
The pathophysiology of PPI use and kidney deterioration is poorly understood at this point. It is known that AKI can increase the risk for CKD, and AKI has been an assumed precursor to PPI-associated CKD. However, a study by Xie and colleagues reported an association between PPI use and increased risk for CKD, progression of CKD, and ESRD in the absence of preceding AKI. Using the VA databases, the researchers identified 144,032 new users of acid-suppressing medications (125,596 PPI; 18,436 H2RA) who had no history of kidney disease and followed them for five years. PPI users were found to be at increased risk for CKD, and a graded association was discovered between length of PPI use and risk for CKD.10
While these studies are observational and therefore do not prove causation, they do suggest a need for attentive monitoring of kidney function in patients using PPIs. Evaluating the need for PPIs and inquiring about OTC use of these medications is highly recommended, as research has found 25% to 70% of PPI prescriptions are not prescribed for an appropriate indication.11 Considerations regarding PPI use should include dosage, length of use, and whether alternate use of an H2RA is appropriate. —CAS
Cynthia A. Smith, DNP, CNN-NP, FNP-BC, APRN
Renal Consultants, PLLC, South Charleston, West Virginia
Q) Is it true that PPI use can cause kidney disease?
Proton pump inhibitors (PPIs) have been available in the United States since 1990, with OTC options available since 2009. While these medications play a vital role in the treatment of gastrointestinal (GI) conditions, observational studies have linked PPI use to serious adverse events, including dementia, community-acquired pneumonia, hip fracture, and Clostridium difficile infection.1-4
Studies have also found an association between PPI use and kidney problems such as acute kidney injury (AKI), acute interstitial nephritis, and incident chronic kidney disease (CKD).5-7 One observational study used the Department of Veterans Affairs (VA) national databases to track the renal outcomes of 173,321 new PPI users and 20,270 new histamine H2 receptor antagonist (H2RA) users over the course of five years. Those who used PPIs demonstrated a significant risk for decreased renal function, lower estimated glomerular filtration rate (eGFR), doubled serum creatinine levels, and progression to end-stage renal disease (ESRD).8
Another study of 10,482 patients (322 PPI; 956 H2RA; 9,204 nonusers) and a replicate study of 248,751 patients (16,900 PPI; 6,640 H2RA; 225,211 nonusers) with an initial eGFR ≥ 60 mL/min/1.73m2 also found an association between PPI use and incident CKD, which persisted when compared to the other groups. Additionally, twice-daily PPI use was associated with a higher CKD risk than once-daily use.9
The pathophysiology of PPI use and kidney deterioration is poorly understood at this point. It is known that AKI can increase the risk for CKD, and AKI has been an assumed precursor to PPI-associated CKD. However, a study by Xie and colleagues reported an association between PPI use and increased risk for CKD, progression of CKD, and ESRD in the absence of preceding AKI. Using the VA databases, the researchers identified 144,032 new users of acid-suppressing medications (125,596 PPI; 18,436 H2RA) who had no history of kidney disease and followed them for five years. PPI users were found to be at increased risk for CKD, and a graded association was discovered between length of PPI use and risk for CKD.10
While these studies are observational and therefore do not prove causation, they do suggest a need for attentive monitoring of kidney function in patients using PPIs. Evaluating the need for PPIs and inquiring about OTC use of these medications is highly recommended, as research has found 25% to 70% of PPI prescriptions are not prescribed for an appropriate indication.11 Considerations regarding PPI use should include dosage, length of use, and whether alternate use of an H2RA is appropriate. —CAS
Cynthia A. Smith, DNP, CNN-NP, FNP-BC, APRN
Renal Consultants, PLLC, South Charleston, West Virginia
1. Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
2. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PloS One. 2015;10(6):e0128004.
3. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006; 296(24):2947-2953.
4. Dial S, Alrasadi K, Manoukian C, et al. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171(1):33-38.
5. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150.
6. Blank ML, Parkin L, Paul C, et al. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86:837-844.
7. Antoniou T, Macdonald EM, Hollands S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3(2):E166-171.
8. Xie Y, Bowe B, Li T, et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2016;27(10):3153-3163.
9. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238-246.
10. Xie Y, Bowe B, Li T, et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 2017;91(6):1482-1494.
11. Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2-3.
1. Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416.
2. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PloS One. 2015;10(6):e0128004.
3. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006; 296(24):2947-2953.
4. Dial S, Alrasadi K, Manoukian C, et al. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171(1):33-38.
5. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150.
6. Blank ML, Parkin L, Paul C, et al. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86:837-844.
7. Antoniou T, Macdonald EM, Hollands S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3(2):E166-171.
8. Xie Y, Bowe B, Li T, et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2016;27(10):3153-3163.
9. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238-246.
10. Xie Y, Bowe B, Li T, et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 2017;91(6):1482-1494.
11. Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2-3.
Is pregnancy safe after kidney transplant?
Since the first successful pregnancy in a kidney transplant recipient in 1958,1 hundreds of kidney recipients have had successful pregnancies. Chronic kidney disease disrupts the hypothalamic-pituitary-gonadal axis, leading to anovulation and infertility. However, within 6 months of kidney transplant, the hypothalamic-pituitary-gonadal axis and sex hormone levels return to normal,2 and the renal allograft is able to adapt to the various physiologic changes of pregnancy.3
Successful pregnancy after kidney transplant requires a team approach to care that includes the primary care physician, a transplant nephrologist, and an obstetrician with expertise in high-risk pregnancies. But equally important is educating and counseling the patient about the risks and challenges. This should begin at the first pretransplant visit.4
Below are answers to questions often asked by renal transplant recipients who wish to become pregnant.
WHAT IS THE IDEAL TIME TO BECOME PREGNANT AFTER KIDNEY TRANSPLANT?
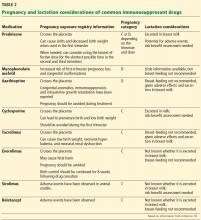
Mycophenolate mofetil and sirolimus are contraindicated in pregnancy and should be stopped at least 6 weeks before conception. Mycophenolate mofetil increases the risk of congenital malformations and spontaneous abortion. Data on sirolimus from clinical studies are limited, but in animal studies it is associated with delay in ossification of skeletal structure and with an increase in fetal mortality.7
WHAT INCREASES THE RISK OF A POOR PREGNANCY OUTCOME AFTER RENAL TRANSPLANT?
Risk factors for poor maternal and fetal outcomes include an elevated prepregnancy serum creatinine level (≥ 1.4 mg/dL), hypertension, and proteinuria (≥ 500 mg/24 hours). Younger age at transplant and at conception is associated with better pregnancy outcome.5,8
WHAT ARE THE POSSIBLE MATERNAL COMPLICATIONS?
Kidney transplant recipients who become pregnant have a risk of developing preeclampsia 6 times higher than normal, and the incidence rate ranges between 24% and 38%.9,10 The risk of cesarean delivery is 5 times higher than in the general population, and the incidence rate is 43% to 64%.10,11
Low-dose aspirin reduces the risk of preeclampsia and should be prescribed to all pregnant women who are kidney transplant recipients. Angiotensin-converting enzyme inhibitors are contraindicated due to the risk of teratogenic effects, ie, pulmonary hypoplasia and oligohydramnios.4
WHAT ARE THE POSSIBLE FETAL COMPLICATIONS?
Women who become pregnant after kidney transplant are at greater risk of preterm delivery (40% to 60% higher risk), having a baby with low birth weight (42% to 46% higher risk), and intrauterine growth restriction (30% to 50% higher risk). But the risk of perinatal mortality is not increased in the absence of the above-mentioned risk factors.10,11
DOES PREGNANCY INCREASE THE RISK OF GRAFT FAILURE?
Pregnancy does not increase the risk of allograft loss as long as the patient has a prepregnancy serum creatinine below 1.4 mg/dL, no hypertension, and urine protein excretion less than 500 mg/24 hours.12
WHAT CHANGES TO IMMUNE SUPPRESSION ARE REQUIRED BEFORE AND DURING PREGNANCY?
Careful management of immunosuppression is critical in renal transplant recipients before and during pregnancy because of the risks of teratogenicity and other adverse effects.
As stated above, mycophenolate mofetil and sirolimus are teratogenic and should be stopped 6 weeks before conception. The recommended maintenance immunosuppression during pregnancy includes calcineurin inhibitors (tacrolimus and cyclosporine), azathioprine, and low-dose prednisone.
A 20% to 25% increase in the dose of calcineurin inhibitor is required during pregnancy due to an increase in metabolic activity of cytochrome P450 and an increase in the volume of distribution.5,6,13 However, this dosing increase requires more frequent monitoring throughout the pregnancy to ensure the safest possible therapeutic levels.
DOES PREGNANCY INCREASE THE RISK OF INFECTION?
Because of their immunosuppressed state, renal transplant recipients are prone to infection; the incidence rate of urinary tract infection is as high as 40% due to mild reflux and pregnancy-related dilation of ureters and collecting ducts.6 Women should be screened for urinary tract infection at every visit with urine dipstick testing and with urine culture every 4 weeks. Antibiotics such as nitrofurantoin, amoxicillin, and cephalexin are safe to treat urinary tract infection during pregnancy.6
IS BREAST-FEEDING SAFE IN RENAL TRANSPLANT RECIPIENTS?
Breast-feeding is considered safe for women with renal transplant who are on prednisone, azathioprine, cyclosporine, and tacrolimus. Women should avoid breast-feeding if they are taking mycophenolate mofetil, sirolimus, everolimus, or belatacept, as clinical data on safety are not adequate.14
- Murray JE, Reid DE, Harrison JH, Merrill JP. Successful pregnancies after human renal transplantation. N Engl J Med 1963; 269:341–343.
- Saha MT, Saha HH, Niskanen LK, Salmela KT, Pasternack AI. Time course of serum prolactin and sex hormones following successful renal transplantation. Nephron 2002; 92:735–737.
- Davison JM. The effect of pregnancy on kidney function in renal allograft recipients. Kidney Int 1985; 27:74–79.
- Shah S, Verma P. Overview of pregnancy in renal transplant patients. Int J Nephrol 2016; 2016:4539342.
- McKay DB, Josephson MA, Armenti VT, et al; Women’s Health Committee of the American Society of Transplantation. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant 2005; 5:1592–1599.
- EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: long-term management of the transplant recipient. IV.10. Pregnancy in renal transplant recipients. Nephrol Dial Transplant 2002; 17(suppl 4):50–55.
- Armenti VT, Moitz MJ, Cardonick EH, Davison JM. Immunosuppression in pregnancy: choices for infant and maternal health. Drugs 2002; 62:2361–2375.
- Bramham K, Chusney G, Lee J, Lightstone L, Nelson-Piercy C. Breastfeeding and tacrolimus: serial monitoring in breast-fed and bottle-fed infants. Clin J Am Soc Nephrol 2013; 8:563–567.
- Deshpande NA, James NT, Kucirka LM, et al. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant 2011; 11:2388–2404.
- Bramham K, Nelson-Piercy C, Gao H, et al. Pregnancy in renal transplant recipients: a UK national cohort study. Clin J Am Soc Nephrol 2013; 8:290–298.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 65–85.
- Sibanda N, Briggs JD, Davison JM, Johnson RJ, Rudge CJ. Pregnancy after organ transplantation: a report from the UK transplant pregnancy registry. Transplantation 2007; 83:1301–1307.
- Kim H, Jeong JC, Yang J, et al. The optimal therapy of calcineurin inhibitors for pregnancy in kidney transplantation. Clin Transplant 2015; 29:142–148.
- Constantinescu S, Pai A, Coscia LA, Davison JM, Moritz MJ, Armenti VT. Breast-feeding after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1163–1173.
Since the first successful pregnancy in a kidney transplant recipient in 1958,1 hundreds of kidney recipients have had successful pregnancies. Chronic kidney disease disrupts the hypothalamic-pituitary-gonadal axis, leading to anovulation and infertility. However, within 6 months of kidney transplant, the hypothalamic-pituitary-gonadal axis and sex hormone levels return to normal,2 and the renal allograft is able to adapt to the various physiologic changes of pregnancy.3
Successful pregnancy after kidney transplant requires a team approach to care that includes the primary care physician, a transplant nephrologist, and an obstetrician with expertise in high-risk pregnancies. But equally important is educating and counseling the patient about the risks and challenges. This should begin at the first pretransplant visit.4
Below are answers to questions often asked by renal transplant recipients who wish to become pregnant.
WHAT IS THE IDEAL TIME TO BECOME PREGNANT AFTER KIDNEY TRANSPLANT?
Mycophenolate mofetil and sirolimus are contraindicated in pregnancy and should be stopped at least 6 weeks before conception. Mycophenolate mofetil increases the risk of congenital malformations and spontaneous abortion. Data on sirolimus from clinical studies are limited, but in animal studies it is associated with delay in ossification of skeletal structure and with an increase in fetal mortality.7
WHAT INCREASES THE RISK OF A POOR PREGNANCY OUTCOME AFTER RENAL TRANSPLANT?
Risk factors for poor maternal and fetal outcomes include an elevated prepregnancy serum creatinine level (≥ 1.4 mg/dL), hypertension, and proteinuria (≥ 500 mg/24 hours). Younger age at transplant and at conception is associated with better pregnancy outcome.5,8
WHAT ARE THE POSSIBLE MATERNAL COMPLICATIONS?
Kidney transplant recipients who become pregnant have a risk of developing preeclampsia 6 times higher than normal, and the incidence rate ranges between 24% and 38%.9,10 The risk of cesarean delivery is 5 times higher than in the general population, and the incidence rate is 43% to 64%.10,11
Low-dose aspirin reduces the risk of preeclampsia and should be prescribed to all pregnant women who are kidney transplant recipients. Angiotensin-converting enzyme inhibitors are contraindicated due to the risk of teratogenic effects, ie, pulmonary hypoplasia and oligohydramnios.4
WHAT ARE THE POSSIBLE FETAL COMPLICATIONS?
Women who become pregnant after kidney transplant are at greater risk of preterm delivery (40% to 60% higher risk), having a baby with low birth weight (42% to 46% higher risk), and intrauterine growth restriction (30% to 50% higher risk). But the risk of perinatal mortality is not increased in the absence of the above-mentioned risk factors.10,11
DOES PREGNANCY INCREASE THE RISK OF GRAFT FAILURE?
Pregnancy does not increase the risk of allograft loss as long as the patient has a prepregnancy serum creatinine below 1.4 mg/dL, no hypertension, and urine protein excretion less than 500 mg/24 hours.12
WHAT CHANGES TO IMMUNE SUPPRESSION ARE REQUIRED BEFORE AND DURING PREGNANCY?
Careful management of immunosuppression is critical in renal transplant recipients before and during pregnancy because of the risks of teratogenicity and other adverse effects.
As stated above, mycophenolate mofetil and sirolimus are teratogenic and should be stopped 6 weeks before conception. The recommended maintenance immunosuppression during pregnancy includes calcineurin inhibitors (tacrolimus and cyclosporine), azathioprine, and low-dose prednisone.
A 20% to 25% increase in the dose of calcineurin inhibitor is required during pregnancy due to an increase in metabolic activity of cytochrome P450 and an increase in the volume of distribution.5,6,13 However, this dosing increase requires more frequent monitoring throughout the pregnancy to ensure the safest possible therapeutic levels.
DOES PREGNANCY INCREASE THE RISK OF INFECTION?
Because of their immunosuppressed state, renal transplant recipients are prone to infection; the incidence rate of urinary tract infection is as high as 40% due to mild reflux and pregnancy-related dilation of ureters and collecting ducts.6 Women should be screened for urinary tract infection at every visit with urine dipstick testing and with urine culture every 4 weeks. Antibiotics such as nitrofurantoin, amoxicillin, and cephalexin are safe to treat urinary tract infection during pregnancy.6
IS BREAST-FEEDING SAFE IN RENAL TRANSPLANT RECIPIENTS?
Breast-feeding is considered safe for women with renal transplant who are on prednisone, azathioprine, cyclosporine, and tacrolimus. Women should avoid breast-feeding if they are taking mycophenolate mofetil, sirolimus, everolimus, or belatacept, as clinical data on safety are not adequate.14
Since the first successful pregnancy in a kidney transplant recipient in 1958,1 hundreds of kidney recipients have had successful pregnancies. Chronic kidney disease disrupts the hypothalamic-pituitary-gonadal axis, leading to anovulation and infertility. However, within 6 months of kidney transplant, the hypothalamic-pituitary-gonadal axis and sex hormone levels return to normal,2 and the renal allograft is able to adapt to the various physiologic changes of pregnancy.3
Successful pregnancy after kidney transplant requires a team approach to care that includes the primary care physician, a transplant nephrologist, and an obstetrician with expertise in high-risk pregnancies. But equally important is educating and counseling the patient about the risks and challenges. This should begin at the first pretransplant visit.4
Below are answers to questions often asked by renal transplant recipients who wish to become pregnant.
WHAT IS THE IDEAL TIME TO BECOME PREGNANT AFTER KIDNEY TRANSPLANT?
Mycophenolate mofetil and sirolimus are contraindicated in pregnancy and should be stopped at least 6 weeks before conception. Mycophenolate mofetil increases the risk of congenital malformations and spontaneous abortion. Data on sirolimus from clinical studies are limited, but in animal studies it is associated with delay in ossification of skeletal structure and with an increase in fetal mortality.7
WHAT INCREASES THE RISK OF A POOR PREGNANCY OUTCOME AFTER RENAL TRANSPLANT?
Risk factors for poor maternal and fetal outcomes include an elevated prepregnancy serum creatinine level (≥ 1.4 mg/dL), hypertension, and proteinuria (≥ 500 mg/24 hours). Younger age at transplant and at conception is associated with better pregnancy outcome.5,8
WHAT ARE THE POSSIBLE MATERNAL COMPLICATIONS?
Kidney transplant recipients who become pregnant have a risk of developing preeclampsia 6 times higher than normal, and the incidence rate ranges between 24% and 38%.9,10 The risk of cesarean delivery is 5 times higher than in the general population, and the incidence rate is 43% to 64%.10,11
Low-dose aspirin reduces the risk of preeclampsia and should be prescribed to all pregnant women who are kidney transplant recipients. Angiotensin-converting enzyme inhibitors are contraindicated due to the risk of teratogenic effects, ie, pulmonary hypoplasia and oligohydramnios.4
WHAT ARE THE POSSIBLE FETAL COMPLICATIONS?
Women who become pregnant after kidney transplant are at greater risk of preterm delivery (40% to 60% higher risk), having a baby with low birth weight (42% to 46% higher risk), and intrauterine growth restriction (30% to 50% higher risk). But the risk of perinatal mortality is not increased in the absence of the above-mentioned risk factors.10,11
DOES PREGNANCY INCREASE THE RISK OF GRAFT FAILURE?
Pregnancy does not increase the risk of allograft loss as long as the patient has a prepregnancy serum creatinine below 1.4 mg/dL, no hypertension, and urine protein excretion less than 500 mg/24 hours.12
WHAT CHANGES TO IMMUNE SUPPRESSION ARE REQUIRED BEFORE AND DURING PREGNANCY?
Careful management of immunosuppression is critical in renal transplant recipients before and during pregnancy because of the risks of teratogenicity and other adverse effects.
As stated above, mycophenolate mofetil and sirolimus are teratogenic and should be stopped 6 weeks before conception. The recommended maintenance immunosuppression during pregnancy includes calcineurin inhibitors (tacrolimus and cyclosporine), azathioprine, and low-dose prednisone.
A 20% to 25% increase in the dose of calcineurin inhibitor is required during pregnancy due to an increase in metabolic activity of cytochrome P450 and an increase in the volume of distribution.5,6,13 However, this dosing increase requires more frequent monitoring throughout the pregnancy to ensure the safest possible therapeutic levels.
DOES PREGNANCY INCREASE THE RISK OF INFECTION?
Because of their immunosuppressed state, renal transplant recipients are prone to infection; the incidence rate of urinary tract infection is as high as 40% due to mild reflux and pregnancy-related dilation of ureters and collecting ducts.6 Women should be screened for urinary tract infection at every visit with urine dipstick testing and with urine culture every 4 weeks. Antibiotics such as nitrofurantoin, amoxicillin, and cephalexin are safe to treat urinary tract infection during pregnancy.6
IS BREAST-FEEDING SAFE IN RENAL TRANSPLANT RECIPIENTS?
Breast-feeding is considered safe for women with renal transplant who are on prednisone, azathioprine, cyclosporine, and tacrolimus. Women should avoid breast-feeding if they are taking mycophenolate mofetil, sirolimus, everolimus, or belatacept, as clinical data on safety are not adequate.14
- Murray JE, Reid DE, Harrison JH, Merrill JP. Successful pregnancies after human renal transplantation. N Engl J Med 1963; 269:341–343.
- Saha MT, Saha HH, Niskanen LK, Salmela KT, Pasternack AI. Time course of serum prolactin and sex hormones following successful renal transplantation. Nephron 2002; 92:735–737.
- Davison JM. The effect of pregnancy on kidney function in renal allograft recipients. Kidney Int 1985; 27:74–79.
- Shah S, Verma P. Overview of pregnancy in renal transplant patients. Int J Nephrol 2016; 2016:4539342.
- McKay DB, Josephson MA, Armenti VT, et al; Women’s Health Committee of the American Society of Transplantation. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant 2005; 5:1592–1599.
- EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: long-term management of the transplant recipient. IV.10. Pregnancy in renal transplant recipients. Nephrol Dial Transplant 2002; 17(suppl 4):50–55.
- Armenti VT, Moitz MJ, Cardonick EH, Davison JM. Immunosuppression in pregnancy: choices for infant and maternal health. Drugs 2002; 62:2361–2375.
- Bramham K, Chusney G, Lee J, Lightstone L, Nelson-Piercy C. Breastfeeding and tacrolimus: serial monitoring in breast-fed and bottle-fed infants. Clin J Am Soc Nephrol 2013; 8:563–567.
- Deshpande NA, James NT, Kucirka LM, et al. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant 2011; 11:2388–2404.
- Bramham K, Nelson-Piercy C, Gao H, et al. Pregnancy in renal transplant recipients: a UK national cohort study. Clin J Am Soc Nephrol 2013; 8:290–298.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 65–85.
- Sibanda N, Briggs JD, Davison JM, Johnson RJ, Rudge CJ. Pregnancy after organ transplantation: a report from the UK transplant pregnancy registry. Transplantation 2007; 83:1301–1307.
- Kim H, Jeong JC, Yang J, et al. The optimal therapy of calcineurin inhibitors for pregnancy in kidney transplantation. Clin Transplant 2015; 29:142–148.
- Constantinescu S, Pai A, Coscia LA, Davison JM, Moritz MJ, Armenti VT. Breast-feeding after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1163–1173.
- Murray JE, Reid DE, Harrison JH, Merrill JP. Successful pregnancies after human renal transplantation. N Engl J Med 1963; 269:341–343.
- Saha MT, Saha HH, Niskanen LK, Salmela KT, Pasternack AI. Time course of serum prolactin and sex hormones following successful renal transplantation. Nephron 2002; 92:735–737.
- Davison JM. The effect of pregnancy on kidney function in renal allograft recipients. Kidney Int 1985; 27:74–79.
- Shah S, Verma P. Overview of pregnancy in renal transplant patients. Int J Nephrol 2016; 2016:4539342.
- McKay DB, Josephson MA, Armenti VT, et al; Women’s Health Committee of the American Society of Transplantation. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant 2005; 5:1592–1599.
- EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: long-term management of the transplant recipient. IV.10. Pregnancy in renal transplant recipients. Nephrol Dial Transplant 2002; 17(suppl 4):50–55.
- Armenti VT, Moitz MJ, Cardonick EH, Davison JM. Immunosuppression in pregnancy: choices for infant and maternal health. Drugs 2002; 62:2361–2375.
- Bramham K, Chusney G, Lee J, Lightstone L, Nelson-Piercy C. Breastfeeding and tacrolimus: serial monitoring in breast-fed and bottle-fed infants. Clin J Am Soc Nephrol 2013; 8:563–567.
- Deshpande NA, James NT, Kucirka LM, et al. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant 2011; 11:2388–2404.
- Bramham K, Nelson-Piercy C, Gao H, et al. Pregnancy in renal transplant recipients: a UK national cohort study. Clin J Am Soc Nephrol 2013; 8:290–298.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 65–85.
- Sibanda N, Briggs JD, Davison JM, Johnson RJ, Rudge CJ. Pregnancy after organ transplantation: a report from the UK transplant pregnancy registry. Transplantation 2007; 83:1301–1307.
- Kim H, Jeong JC, Yang J, et al. The optimal therapy of calcineurin inhibitors for pregnancy in kidney transplantation. Clin Transplant 2015; 29:142–148.
- Constantinescu S, Pai A, Coscia LA, Davison JM, Moritz MJ, Armenti VT. Breast-feeding after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1163–1173.
FDA approves biosimilar to bevacizumab
The Food and Drug Administration has approved a biosimilar to bevacizumab (Avastin) for the treatment of certain colorectal, lung, brain, kidney, and cervical cancers.
Bevacizumab-awwb is the first biosimilar approved in the United States for the treatment of cancer, the FDA said in a press release.
Approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrate bevacizumab-awwb is biosimilar to bevacizumab, the FDA said.
• Metastatic colorectal cancer, in combination with intravenous 5-fluorouracil-based chemotherapy for first- or second-line treatment.
• Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan–based or fluoropyrimidine-oxaliplatin–based chemotherapy for the second-line treatment of patients who have progressed on a first-line bevacizumab product–containing regimen.
• Non-squamous non–small cell lung cancer, in combination with carboplatin and paclitaxel for first line treatment of unresectable, locally advanced, recurrent, or metastatic disease.
• Glioblastoma with progressive disease following prior therapy, based on improvement in objective response rate.
• Metastatic renal cell carcinoma, in combination with interferon alfa.
• Cervical cancer that is persistent, recurrent, or metastatic, in combination with paclitaxel and cisplatin or paclitaxel and topotecan.
Common expected side effects of the biosimilar include epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Serious expected side effects include perforation or fistula, arterial and venous thromboembolic events, hypertension, posterior reversible encephalopathy syndrome, proteinuria, infusion-related reactions, and ovarian failure. Women who are pregnant should not take bevacizumab-awwb.
The biosimilar to bevacizumab carries a similar boxed warning regarding the increased risk of gastrointestinal perforations; surgery and wound healing complications; and severe or fatal pulmonary, gastrointestinal, central nervous system, and vaginal hemorrhage.
The biosimilar approval was granted to Amgen, which will market the drug under the trade name Mvasi.
The Food and Drug Administration has approved a biosimilar to bevacizumab (Avastin) for the treatment of certain colorectal, lung, brain, kidney, and cervical cancers.
Bevacizumab-awwb is the first biosimilar approved in the United States for the treatment of cancer, the FDA said in a press release.
Approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrate bevacizumab-awwb is biosimilar to bevacizumab, the FDA said.
• Metastatic colorectal cancer, in combination with intravenous 5-fluorouracil-based chemotherapy for first- or second-line treatment.
• Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan–based or fluoropyrimidine-oxaliplatin–based chemotherapy for the second-line treatment of patients who have progressed on a first-line bevacizumab product–containing regimen.
• Non-squamous non–small cell lung cancer, in combination with carboplatin and paclitaxel for first line treatment of unresectable, locally advanced, recurrent, or metastatic disease.
• Glioblastoma with progressive disease following prior therapy, based on improvement in objective response rate.
• Metastatic renal cell carcinoma, in combination with interferon alfa.
• Cervical cancer that is persistent, recurrent, or metastatic, in combination with paclitaxel and cisplatin or paclitaxel and topotecan.
Common expected side effects of the biosimilar include epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Serious expected side effects include perforation or fistula, arterial and venous thromboembolic events, hypertension, posterior reversible encephalopathy syndrome, proteinuria, infusion-related reactions, and ovarian failure. Women who are pregnant should not take bevacizumab-awwb.
The biosimilar to bevacizumab carries a similar boxed warning regarding the increased risk of gastrointestinal perforations; surgery and wound healing complications; and severe or fatal pulmonary, gastrointestinal, central nervous system, and vaginal hemorrhage.
The biosimilar approval was granted to Amgen, which will market the drug under the trade name Mvasi.
The Food and Drug Administration has approved a biosimilar to bevacizumab (Avastin) for the treatment of certain colorectal, lung, brain, kidney, and cervical cancers.
Bevacizumab-awwb is the first biosimilar approved in the United States for the treatment of cancer, the FDA said in a press release.
Approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrate bevacizumab-awwb is biosimilar to bevacizumab, the FDA said.
• Metastatic colorectal cancer, in combination with intravenous 5-fluorouracil-based chemotherapy for first- or second-line treatment.
• Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan–based or fluoropyrimidine-oxaliplatin–based chemotherapy for the second-line treatment of patients who have progressed on a first-line bevacizumab product–containing regimen.
• Non-squamous non–small cell lung cancer, in combination with carboplatin and paclitaxel for first line treatment of unresectable, locally advanced, recurrent, or metastatic disease.
• Glioblastoma with progressive disease following prior therapy, based on improvement in objective response rate.
• Metastatic renal cell carcinoma, in combination with interferon alfa.
• Cervical cancer that is persistent, recurrent, or metastatic, in combination with paclitaxel and cisplatin or paclitaxel and topotecan.
Common expected side effects of the biosimilar include epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Serious expected side effects include perforation or fistula, arterial and venous thromboembolic events, hypertension, posterior reversible encephalopathy syndrome, proteinuria, infusion-related reactions, and ovarian failure. Women who are pregnant should not take bevacizumab-awwb.
The biosimilar to bevacizumab carries a similar boxed warning regarding the increased risk of gastrointestinal perforations; surgery and wound healing complications; and severe or fatal pulmonary, gastrointestinal, central nervous system, and vaginal hemorrhage.
The biosimilar approval was granted to Amgen, which will market the drug under the trade name Mvasi.
Obtaining cystatin-C levels useful in chronic kidney disease
SAN FRANCISCO – Routine lab tests for estimating the average glomerular filtration rate (GFR) are too imprecise, according to Michael G. Shlipak, MD.
“If you study 100 patients, the average GFR based on creatinine is going to be pretty close to estimated GFR,” Dr. Shlipak said at the UCSF Annual Advances in Internal Medicine meeting. “But with individual patients, the average GFR is going to be plus or minus 30%, which is a lot. If the estimated GFR is 70 mL/min per 1.73 m2, the real GFR could be between 50 and 90 mL/min per 1.73 m2; so that’s a wide range.”
“The main appeal of the Cockcroft-Gault equation is that you can almost do it in your head, so that’s a real positive,” said Dr. Shlipak, who is also scientific director of the Kidney Health Research Collaborative at the University of California, San Francisco, and professor of internal medicine, epidemiology, and biostatistics at UCSF Medical Center. “The main disadvantage is that it’s really a terrible equation. The Cockcroft-Gault equation is clearly inadequate, as it is standardized to creatinine clearance and very inaccurate, so it should no longer be used. The MDRD and the CKD-EPI are newer equations that are at least validated to real GFR and not creatinine clearance. In our system, the pharmacy uses the Cockcroft-Gault equation, and the lab gives us the MDRD GFR equation, so it’s quite confusing.”
Dr. Shlipak described using estimated GFR in clinical decision making as “better than using just creatinine, because it integrates demographic characteristics, which are determinants in part of the creatinine production, which is what determines how much creatinine is in the blood before it gets filtered. The equations make us think of GFR and kidney function instead of just the lab value.”
The downsides of using GFRs, he added, include the fact that they are mostly validated in younger patients with kidney disease, they rely on the assumption that demographic characteristics alone can define muscle mass, they were only developed in whites and blacks, and estimated GFR can be interpreted only as “suggested GFR.”
A blood test of kidney function known as cystatin C has been shown to be an alternative, better marker of creatinine, compared with GFR, and is supported by the Kidney Disease: Improving Global Outcomes CKD work group’s 2012 clinical practice guidelines for the evaluation and management of CKD. “Because cystatin C is not related to muscle mass, age, sex, and race, it has major advantages over creatinine,” Dr. Shlipak said. “It is a reliable, standardized, and automated measure that is available for clinical use.”
He and his associates conducted a meta-analysis comparing cystatin C and creatinine in determining prognosis for patients with baseline kidney disease. They included 16 studies involving 90,750 patients and compared associations of estimated GFR (eGFR) as measured with creatinine, cystatin C, and both creatinine and cystatin C with mortality risk; they also determined proportions reclassified by cystatin C in each subgroup of eGFR using creatinine and by the effect on risk associations (N Engl J Med. 2013 Sep 5; 369[10]:932-43).
In the general-population cohorts, the prevalence of an eGFR of less than 60 mL/min per 1.73 m2 of body surface area was higher with the cystatin C–based eGFR than with the creatinine-based eGFR (13.7% vs. 9.7%). Across all eGFR categories, the reclassification of the eGFR to a higher value with the measurement of cystatin C, compared with creatinine, was associated with a reduced risk of all three study outcomes. Reclassification to a lower eGFR was associated with an increased risk.
“When we looked at the threshold for where risk begins, traditionally we’ve said it starts when the GFR declines below 60 mL/min per 1.73 m2,” Dr. Shlipak explained. “That’s exactly what we found with creatinine. But with cystatin C, the threshold of risk was at 88 mL/min per 1.73 m2.
“So, from 88 mL/min per 1.73 m2 downward, every incremental reduction in GFR is associated with higher mortality and cardiovascular risk,” he added. “So cystatin C opens this new window of between 60 and 90 mL/min per 1.73 m2 to start measuring relative declines in kidney function. If you combine creatinine and cystatin C together in an equation, you get a similar estimate. Many advocate that the combined CKD-EPI creatinine-cystatin C equation is the best way to measure GFR.”
Dr. Shlipak reported that he is on the scientific advisory boards of TAI Diagnostics and Cricket Health.
SAN FRANCISCO – Routine lab tests for estimating the average glomerular filtration rate (GFR) are too imprecise, according to Michael G. Shlipak, MD.
“If you study 100 patients, the average GFR based on creatinine is going to be pretty close to estimated GFR,” Dr. Shlipak said at the UCSF Annual Advances in Internal Medicine meeting. “But with individual patients, the average GFR is going to be plus or minus 30%, which is a lot. If the estimated GFR is 70 mL/min per 1.73 m2, the real GFR could be between 50 and 90 mL/min per 1.73 m2; so that’s a wide range.”
“The main appeal of the Cockcroft-Gault equation is that you can almost do it in your head, so that’s a real positive,” said Dr. Shlipak, who is also scientific director of the Kidney Health Research Collaborative at the University of California, San Francisco, and professor of internal medicine, epidemiology, and biostatistics at UCSF Medical Center. “The main disadvantage is that it’s really a terrible equation. The Cockcroft-Gault equation is clearly inadequate, as it is standardized to creatinine clearance and very inaccurate, so it should no longer be used. The MDRD and the CKD-EPI are newer equations that are at least validated to real GFR and not creatinine clearance. In our system, the pharmacy uses the Cockcroft-Gault equation, and the lab gives us the MDRD GFR equation, so it’s quite confusing.”
Dr. Shlipak described using estimated GFR in clinical decision making as “better than using just creatinine, because it integrates demographic characteristics, which are determinants in part of the creatinine production, which is what determines how much creatinine is in the blood before it gets filtered. The equations make us think of GFR and kidney function instead of just the lab value.”
The downsides of using GFRs, he added, include the fact that they are mostly validated in younger patients with kidney disease, they rely on the assumption that demographic characteristics alone can define muscle mass, they were only developed in whites and blacks, and estimated GFR can be interpreted only as “suggested GFR.”
A blood test of kidney function known as cystatin C has been shown to be an alternative, better marker of creatinine, compared with GFR, and is supported by the Kidney Disease: Improving Global Outcomes CKD work group’s 2012 clinical practice guidelines for the evaluation and management of CKD. “Because cystatin C is not related to muscle mass, age, sex, and race, it has major advantages over creatinine,” Dr. Shlipak said. “It is a reliable, standardized, and automated measure that is available for clinical use.”
He and his associates conducted a meta-analysis comparing cystatin C and creatinine in determining prognosis for patients with baseline kidney disease. They included 16 studies involving 90,750 patients and compared associations of estimated GFR (eGFR) as measured with creatinine, cystatin C, and both creatinine and cystatin C with mortality risk; they also determined proportions reclassified by cystatin C in each subgroup of eGFR using creatinine and by the effect on risk associations (N Engl J Med. 2013 Sep 5; 369[10]:932-43).
In the general-population cohorts, the prevalence of an eGFR of less than 60 mL/min per 1.73 m2 of body surface area was higher with the cystatin C–based eGFR than with the creatinine-based eGFR (13.7% vs. 9.7%). Across all eGFR categories, the reclassification of the eGFR to a higher value with the measurement of cystatin C, compared with creatinine, was associated with a reduced risk of all three study outcomes. Reclassification to a lower eGFR was associated with an increased risk.
“When we looked at the threshold for where risk begins, traditionally we’ve said it starts when the GFR declines below 60 mL/min per 1.73 m2,” Dr. Shlipak explained. “That’s exactly what we found with creatinine. But with cystatin C, the threshold of risk was at 88 mL/min per 1.73 m2.
“So, from 88 mL/min per 1.73 m2 downward, every incremental reduction in GFR is associated with higher mortality and cardiovascular risk,” he added. “So cystatin C opens this new window of between 60 and 90 mL/min per 1.73 m2 to start measuring relative declines in kidney function. If you combine creatinine and cystatin C together in an equation, you get a similar estimate. Many advocate that the combined CKD-EPI creatinine-cystatin C equation is the best way to measure GFR.”
Dr. Shlipak reported that he is on the scientific advisory boards of TAI Diagnostics and Cricket Health.
SAN FRANCISCO – Routine lab tests for estimating the average glomerular filtration rate (GFR) are too imprecise, according to Michael G. Shlipak, MD.
“If you study 100 patients, the average GFR based on creatinine is going to be pretty close to estimated GFR,” Dr. Shlipak said at the UCSF Annual Advances in Internal Medicine meeting. “But with individual patients, the average GFR is going to be plus or minus 30%, which is a lot. If the estimated GFR is 70 mL/min per 1.73 m2, the real GFR could be between 50 and 90 mL/min per 1.73 m2; so that’s a wide range.”
“The main appeal of the Cockcroft-Gault equation is that you can almost do it in your head, so that’s a real positive,” said Dr. Shlipak, who is also scientific director of the Kidney Health Research Collaborative at the University of California, San Francisco, and professor of internal medicine, epidemiology, and biostatistics at UCSF Medical Center. “The main disadvantage is that it’s really a terrible equation. The Cockcroft-Gault equation is clearly inadequate, as it is standardized to creatinine clearance and very inaccurate, so it should no longer be used. The MDRD and the CKD-EPI are newer equations that are at least validated to real GFR and not creatinine clearance. In our system, the pharmacy uses the Cockcroft-Gault equation, and the lab gives us the MDRD GFR equation, so it’s quite confusing.”
Dr. Shlipak described using estimated GFR in clinical decision making as “better than using just creatinine, because it integrates demographic characteristics, which are determinants in part of the creatinine production, which is what determines how much creatinine is in the blood before it gets filtered. The equations make us think of GFR and kidney function instead of just the lab value.”
The downsides of using GFRs, he added, include the fact that they are mostly validated in younger patients with kidney disease, they rely on the assumption that demographic characteristics alone can define muscle mass, they were only developed in whites and blacks, and estimated GFR can be interpreted only as “suggested GFR.”
A blood test of kidney function known as cystatin C has been shown to be an alternative, better marker of creatinine, compared with GFR, and is supported by the Kidney Disease: Improving Global Outcomes CKD work group’s 2012 clinical practice guidelines for the evaluation and management of CKD. “Because cystatin C is not related to muscle mass, age, sex, and race, it has major advantages over creatinine,” Dr. Shlipak said. “It is a reliable, standardized, and automated measure that is available for clinical use.”
He and his associates conducted a meta-analysis comparing cystatin C and creatinine in determining prognosis for patients with baseline kidney disease. They included 16 studies involving 90,750 patients and compared associations of estimated GFR (eGFR) as measured with creatinine, cystatin C, and both creatinine and cystatin C with mortality risk; they also determined proportions reclassified by cystatin C in each subgroup of eGFR using creatinine and by the effect on risk associations (N Engl J Med. 2013 Sep 5; 369[10]:932-43).
In the general-population cohorts, the prevalence of an eGFR of less than 60 mL/min per 1.73 m2 of body surface area was higher with the cystatin C–based eGFR than with the creatinine-based eGFR (13.7% vs. 9.7%). Across all eGFR categories, the reclassification of the eGFR to a higher value with the measurement of cystatin C, compared with creatinine, was associated with a reduced risk of all three study outcomes. Reclassification to a lower eGFR was associated with an increased risk.
“When we looked at the threshold for where risk begins, traditionally we’ve said it starts when the GFR declines below 60 mL/min per 1.73 m2,” Dr. Shlipak explained. “That’s exactly what we found with creatinine. But with cystatin C, the threshold of risk was at 88 mL/min per 1.73 m2.
“So, from 88 mL/min per 1.73 m2 downward, every incremental reduction in GFR is associated with higher mortality and cardiovascular risk,” he added. “So cystatin C opens this new window of between 60 and 90 mL/min per 1.73 m2 to start measuring relative declines in kidney function. If you combine creatinine and cystatin C together in an equation, you get a similar estimate. Many advocate that the combined CKD-EPI creatinine-cystatin C equation is the best way to measure GFR.”
Dr. Shlipak reported that he is on the scientific advisory boards of TAI Diagnostics and Cricket Health.
AT THE ANNUAL ADVANCES IN INTERNAL MEDICINE
AWARD-7: Dulaglutide benefits patients with diabetic renal disease
LISBON – Patients with type 2 diabetes mellitus who also have moderate-to-severe chronic kidney disease (CKD) may be as effectively treated with the glucagonlike peptide–1 receptor agonist dulaglutide (Trulicity) as insulin glargine, the results of an international study showed.
Although comparable effects on glycemic control were seen, patients who received dulaglutide rather than insulin glargine in conjunction with prandial insulin lispro (Humalog) exhibited greater weight loss, had fewer episodes of hypoglycemia, and experienced beneficial effects on two important renal parameters.
The adverse event profile of dulaglutide was typical of that seen with GLP-1 receptor antagonism, noted Dr. Tuttle, who is the executive director of Providence Medical Research Center, Providence Sacred Heart Medical Center in Spokane, Washington, and clinical professor of medicine in the nephrology division at the University of Washington in Seattle.
In Europe, the use of dulaglutide in patients with severely reduced kidney function is not currently recommended, and monitoring is required in the United States in those with gastrointestinal side effects.
AWARD-7 was a randomized, open-label parallel-arm study comparing once-weekly dulaglutide (0.75 mg or 1.5 mg) with insulin glargine plus prandial insulin lispro in 576 subjects who had T2DM and stage 3-4 chronic kidney disease. The aim of the trial was to show noninferiority of the dulaglutide regimen to the insulin glargine regimen.
The trial was open label because the dose of insulin glargine had to be regulated, Dr. Tuttle explained, but the dose of dulaglutide given was blinded. The dose of insulin glargine was targeted to fasting plasma glucose to achieve a value between 5.6 mmol/L and 8.3 mmol/L. The dose of insulin lispro was also adjusted to target preprandial plasma glucose between 6.7 mmol/L and 10.0 mmol/L.
Patients were included if they had a glycated hemoglobin (HbA1c) level of at least 7.5% but less than or equal to 10.5% (at least 57 but less than or equal to 91 mmol/mol) and an eGFR less than 60 but greater than or equal to 15 mL/min per 1.73 m2 at screening.
Approximately 45% of dulaglutide- and 52% of glargine-treated subjects were women; the average age of participants was 65 years, with an average duration of diabetes of around 18 years. The mean HbA1c level at study entry was 8.6% (70.5 mmol/mol), with around half of participants having a HbA1c above 8.5% (69.4 mmol/mol) at entry.
The majority of patients included had an eGFR of less than 45 mL/min per 1.73 m2, and about 30% of patients had stage 4 CKD (eGFR at least 15 but less than 30 mL/min per 1.73 m2), about 45% had macroalbuminuria, and a third or more had microalbuminuria.
The primary endpoint was change in HbA1c from baseline to week 26, and this was comparable for both dulaglutide (at around –1.1% to –1.2%) and insulin glargine (around –1.1%). The effects were maintained at 52 weeks, Dr. Tuttle said, adding that similar percentages (approximately 70% or more) of patients in the groups achieved a target HbA1c of less than 8.0 (64 mmol/mol) at 26 and at 52 weeks. Similar results were seen when the more conventional target of less than 7% (less than 52 mmol/mol) was used, with around 30% of patients achieving this target at 26 and at 52 weeks.
As for weight change, the insulin-treated patients gained about 1 kg in weight over the full course of the study while a dose-dependent decrease in weight of about 2-3 kg was seen with dulaglutide treatment.
“Rates of hypoglycemia were consistently lower in the dulaglutide groups [than in the glargine group],” Dr. Tuttle said, noting “the lowest rates of hypoglycemia were actually seen with the highest dose of dulaglutide.”
The rate of total hypoglycemia (less than or equal to 3.9 mmol/mol) in the 1.5-mg and 0.75-mg dulaglutide groups was 50% and 59.8% of patients, respectively, versus almost 75% of the glargine-treated patients. Rates for documented symptomatic (40.5%, 48.1%, 63.4%), nocturnal (20.5%, 23.8%, 47.9%), and severe (0%, 2.6%, 6.7%) hypoglycemia followed a similar pattern.
“Albuminuria was reduced in all study groups, but there were greater reductions in the patients receiving dulaglutide at 26 weeks,” Dr. Tuttle said. The mean change in UACR from baseline to week 26 was –27.7 for the 1.5-mg dose of dulaglutide, –26.7 for the 0.75-mg dose, and –16.4 for insulin glargine.
The expected rate of eGFR decline at week 26 was also lower with dulaglutide 1.5 mg and 0.75 mg than with glargine, at a respective –0.8%, –3.3%, and –7.7% or –0.1, –0.4, and –1.9 mL/min per 1.73 m2.
“In patients at this stage of CKD, we expect about a 4- to 5-mL per minute loss, so they are right on target or as expected in the insulin group, but this was essentially extinguished in the dulaglutide groups, where there was no significant loss in eGFR during the 26-week time period,” said Dr. Tuttle.
The only difference in side effect profiles between the dulaglutide groups and the glargine group was a higher rate of gastrointestinal side effects. Nausea was seen in 19.8%, 14.2%, and 4.6% of patients given the dulaglutide 1.5 mg, dulaglutide 0.75 mg, and glargine, respectively, with vomiting reported by 13.5%, 8.4%, and 4.6%.
Eli Lilly funded the study. Dr. Tuttle disclosed acting as a consultant on therapies for diabetic kidney disease for Eli Lilly, Boehringer Ingelheim, Gilead, and AstraZeneca.
This article was updated September 28, 2017.
LISBON – Patients with type 2 diabetes mellitus who also have moderate-to-severe chronic kidney disease (CKD) may be as effectively treated with the glucagonlike peptide–1 receptor agonist dulaglutide (Trulicity) as insulin glargine, the results of an international study showed.
Although comparable effects on glycemic control were seen, patients who received dulaglutide rather than insulin glargine in conjunction with prandial insulin lispro (Humalog) exhibited greater weight loss, had fewer episodes of hypoglycemia, and experienced beneficial effects on two important renal parameters.
The adverse event profile of dulaglutide was typical of that seen with GLP-1 receptor antagonism, noted Dr. Tuttle, who is the executive director of Providence Medical Research Center, Providence Sacred Heart Medical Center in Spokane, Washington, and clinical professor of medicine in the nephrology division at the University of Washington in Seattle.
In Europe, the use of dulaglutide in patients with severely reduced kidney function is not currently recommended, and monitoring is required in the United States in those with gastrointestinal side effects.
AWARD-7 was a randomized, open-label parallel-arm study comparing once-weekly dulaglutide (0.75 mg or 1.5 mg) with insulin glargine plus prandial insulin lispro in 576 subjects who had T2DM and stage 3-4 chronic kidney disease. The aim of the trial was to show noninferiority of the dulaglutide regimen to the insulin glargine regimen.
The trial was open label because the dose of insulin glargine had to be regulated, Dr. Tuttle explained, but the dose of dulaglutide given was blinded. The dose of insulin glargine was targeted to fasting plasma glucose to achieve a value between 5.6 mmol/L and 8.3 mmol/L. The dose of insulin lispro was also adjusted to target preprandial plasma glucose between 6.7 mmol/L and 10.0 mmol/L.
Patients were included if they had a glycated hemoglobin (HbA1c) level of at least 7.5% but less than or equal to 10.5% (at least 57 but less than or equal to 91 mmol/mol) and an eGFR less than 60 but greater than or equal to 15 mL/min per 1.73 m2 at screening.
Approximately 45% of dulaglutide- and 52% of glargine-treated subjects were women; the average age of participants was 65 years, with an average duration of diabetes of around 18 years. The mean HbA1c level at study entry was 8.6% (70.5 mmol/mol), with around half of participants having a HbA1c above 8.5% (69.4 mmol/mol) at entry.
The majority of patients included had an eGFR of less than 45 mL/min per 1.73 m2, and about 30% of patients had stage 4 CKD (eGFR at least 15 but less than 30 mL/min per 1.73 m2), about 45% had macroalbuminuria, and a third or more had microalbuminuria.
The primary endpoint was change in HbA1c from baseline to week 26, and this was comparable for both dulaglutide (at around –1.1% to –1.2%) and insulin glargine (around –1.1%). The effects were maintained at 52 weeks, Dr. Tuttle said, adding that similar percentages (approximately 70% or more) of patients in the groups achieved a target HbA1c of less than 8.0 (64 mmol/mol) at 26 and at 52 weeks. Similar results were seen when the more conventional target of less than 7% (less than 52 mmol/mol) was used, with around 30% of patients achieving this target at 26 and at 52 weeks.
As for weight change, the insulin-treated patients gained about 1 kg in weight over the full course of the study while a dose-dependent decrease in weight of about 2-3 kg was seen with dulaglutide treatment.
“Rates of hypoglycemia were consistently lower in the dulaglutide groups [than in the glargine group],” Dr. Tuttle said, noting “the lowest rates of hypoglycemia were actually seen with the highest dose of dulaglutide.”
The rate of total hypoglycemia (less than or equal to 3.9 mmol/mol) in the 1.5-mg and 0.75-mg dulaglutide groups was 50% and 59.8% of patients, respectively, versus almost 75% of the glargine-treated patients. Rates for documented symptomatic (40.5%, 48.1%, 63.4%), nocturnal (20.5%, 23.8%, 47.9%), and severe (0%, 2.6%, 6.7%) hypoglycemia followed a similar pattern.
“Albuminuria was reduced in all study groups, but there were greater reductions in the patients receiving dulaglutide at 26 weeks,” Dr. Tuttle said. The mean change in UACR from baseline to week 26 was –27.7 for the 1.5-mg dose of dulaglutide, –26.7 for the 0.75-mg dose, and –16.4 for insulin glargine.
The expected rate of eGFR decline at week 26 was also lower with dulaglutide 1.5 mg and 0.75 mg than with glargine, at a respective –0.8%, –3.3%, and –7.7% or –0.1, –0.4, and –1.9 mL/min per 1.73 m2.
“In patients at this stage of CKD, we expect about a 4- to 5-mL per minute loss, so they are right on target or as expected in the insulin group, but this was essentially extinguished in the dulaglutide groups, where there was no significant loss in eGFR during the 26-week time period,” said Dr. Tuttle.
The only difference in side effect profiles between the dulaglutide groups and the glargine group was a higher rate of gastrointestinal side effects. Nausea was seen in 19.8%, 14.2%, and 4.6% of patients given the dulaglutide 1.5 mg, dulaglutide 0.75 mg, and glargine, respectively, with vomiting reported by 13.5%, 8.4%, and 4.6%.
Eli Lilly funded the study. Dr. Tuttle disclosed acting as a consultant on therapies for diabetic kidney disease for Eli Lilly, Boehringer Ingelheim, Gilead, and AstraZeneca.
This article was updated September 28, 2017.
LISBON – Patients with type 2 diabetes mellitus who also have moderate-to-severe chronic kidney disease (CKD) may be as effectively treated with the glucagonlike peptide–1 receptor agonist dulaglutide (Trulicity) as insulin glargine, the results of an international study showed.
Although comparable effects on glycemic control were seen, patients who received dulaglutide rather than insulin glargine in conjunction with prandial insulin lispro (Humalog) exhibited greater weight loss, had fewer episodes of hypoglycemia, and experienced beneficial effects on two important renal parameters.
The adverse event profile of dulaglutide was typical of that seen with GLP-1 receptor antagonism, noted Dr. Tuttle, who is the executive director of Providence Medical Research Center, Providence Sacred Heart Medical Center in Spokane, Washington, and clinical professor of medicine in the nephrology division at the University of Washington in Seattle.
In Europe, the use of dulaglutide in patients with severely reduced kidney function is not currently recommended, and monitoring is required in the United States in those with gastrointestinal side effects.
AWARD-7 was a randomized, open-label parallel-arm study comparing once-weekly dulaglutide (0.75 mg or 1.5 mg) with insulin glargine plus prandial insulin lispro in 576 subjects who had T2DM and stage 3-4 chronic kidney disease. The aim of the trial was to show noninferiority of the dulaglutide regimen to the insulin glargine regimen.
The trial was open label because the dose of insulin glargine had to be regulated, Dr. Tuttle explained, but the dose of dulaglutide given was blinded. The dose of insulin glargine was targeted to fasting plasma glucose to achieve a value between 5.6 mmol/L and 8.3 mmol/L. The dose of insulin lispro was also adjusted to target preprandial plasma glucose between 6.7 mmol/L and 10.0 mmol/L.
Patients were included if they had a glycated hemoglobin (HbA1c) level of at least 7.5% but less than or equal to 10.5% (at least 57 but less than or equal to 91 mmol/mol) and an eGFR less than 60 but greater than or equal to 15 mL/min per 1.73 m2 at screening.
Approximately 45% of dulaglutide- and 52% of glargine-treated subjects were women; the average age of participants was 65 years, with an average duration of diabetes of around 18 years. The mean HbA1c level at study entry was 8.6% (70.5 mmol/mol), with around half of participants having a HbA1c above 8.5% (69.4 mmol/mol) at entry.
The majority of patients included had an eGFR of less than 45 mL/min per 1.73 m2, and about 30% of patients had stage 4 CKD (eGFR at least 15 but less than 30 mL/min per 1.73 m2), about 45% had macroalbuminuria, and a third or more had microalbuminuria.
The primary endpoint was change in HbA1c from baseline to week 26, and this was comparable for both dulaglutide (at around –1.1% to –1.2%) and insulin glargine (around –1.1%). The effects were maintained at 52 weeks, Dr. Tuttle said, adding that similar percentages (approximately 70% or more) of patients in the groups achieved a target HbA1c of less than 8.0 (64 mmol/mol) at 26 and at 52 weeks. Similar results were seen when the more conventional target of less than 7% (less than 52 mmol/mol) was used, with around 30% of patients achieving this target at 26 and at 52 weeks.
As for weight change, the insulin-treated patients gained about 1 kg in weight over the full course of the study while a dose-dependent decrease in weight of about 2-3 kg was seen with dulaglutide treatment.
“Rates of hypoglycemia were consistently lower in the dulaglutide groups [than in the glargine group],” Dr. Tuttle said, noting “the lowest rates of hypoglycemia were actually seen with the highest dose of dulaglutide.”
The rate of total hypoglycemia (less than or equal to 3.9 mmol/mol) in the 1.5-mg and 0.75-mg dulaglutide groups was 50% and 59.8% of patients, respectively, versus almost 75% of the glargine-treated patients. Rates for documented symptomatic (40.5%, 48.1%, 63.4%), nocturnal (20.5%, 23.8%, 47.9%), and severe (0%, 2.6%, 6.7%) hypoglycemia followed a similar pattern.
“Albuminuria was reduced in all study groups, but there were greater reductions in the patients receiving dulaglutide at 26 weeks,” Dr. Tuttle said. The mean change in UACR from baseline to week 26 was –27.7 for the 1.5-mg dose of dulaglutide, –26.7 for the 0.75-mg dose, and –16.4 for insulin glargine.
The expected rate of eGFR decline at week 26 was also lower with dulaglutide 1.5 mg and 0.75 mg than with glargine, at a respective –0.8%, –3.3%, and –7.7% or –0.1, –0.4, and –1.9 mL/min per 1.73 m2.
“In patients at this stage of CKD, we expect about a 4- to 5-mL per minute loss, so they are right on target or as expected in the insulin group, but this was essentially extinguished in the dulaglutide groups, where there was no significant loss in eGFR during the 26-week time period,” said Dr. Tuttle.
The only difference in side effect profiles between the dulaglutide groups and the glargine group was a higher rate of gastrointestinal side effects. Nausea was seen in 19.8%, 14.2%, and 4.6% of patients given the dulaglutide 1.5 mg, dulaglutide 0.75 mg, and glargine, respectively, with vomiting reported by 13.5%, 8.4%, and 4.6%.
Eli Lilly funded the study. Dr. Tuttle disclosed acting as a consultant on therapies for diabetic kidney disease for Eli Lilly, Boehringer Ingelheim, Gilead, and AstraZeneca.
This article was updated September 28, 2017.
AT EASD 2017
Key clinical point: Dulaglutide had more beneficial effects on renal parameters than insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease.
Major finding: The primary endpoint of change in HbA1c level from baseline to week 26 was comparable for both dulaglutide and insulin glargine.
Data source: A randomized, open-label parallel-arm study comparing once-weekly dulaglutide (0.75 or 1.5 mg) or insulin glargine plus prandial insulin lispro in 576 subjects with type 2 diabetes mellitus and stage 3–4 CKD.
Disclosures: Eli Lilly funded the study. The presenting author has been a consultant on therapies for diabetic kidney disease for Eli Lilly, Boehringer Ingelheim, Gilead, and AstraZeneca.
For interstitial cystitis, restrictive diet pays off
ESTES PARK, CO. – When patients with interstitial cystitis (IC) learn that first-line therapy is a rigorous diet designed to eliminate common bladder irritants, they tend to react in one of two ways, according to Julie A. Chacko, MD, a urologist in private practice in Santa Barbara, Calif.
Some “are just so grateful that they’re not crazy, which is what they’ve been told after 15 negative urine cultures. (Others) “look at the diet and think I’m sentencing them to death,” she said.
The sole medication approved by the Food and Drug Administration for IC is pentosan polysulfate sodium (Elmiron), and it should be reserved for the minority of patients who don’t experience significant improvement after giving the diet a reasonable shot, Dr. Chako advised. “When Elmiron works it’s great, but it’s not usually my go-to agent because it’s very expensive, you have to take it for 3-6 months to know for sure if it’s efficacious, and it has to be taken on an empty stomach. It’s a difficult medication.”
She advises patients to work with the diet. “Over time, they’re going to be able to find what I call their island – a point where they know very well their limitations and become quite comfortable with them,” she said at a conference on internal medicine sponsored by the University of Colorado.
A poorly understood yet common disorder, IC has a prevalence estimated at 0.5%-4% in women, less in men. Although typically diagnosed in the fourth decade or later, IC occurs at all ages. In some studies, the delay from first appearance of symptoms to arrival at a diagnosis is up to 8 years.
Interstitial cystitis is increasingly being called bladder pain syndrome in the literature, said Dr. Chako, who added, “I personally don’t love bladder pain syndrome as a description for this process. This syndrome has variable symptoms, and patients can have no pain at all.”
The mechanisms that result in IC are a mystery. The leading theory is that a bladder permeability problem allows urinary irritants to reach the interstitium. Nearly 80% of patients with IC can, with coaxing, identify dietary triggers for their symptoms, thereby basically establishing the diagnosis.
Other proposed mechanisms include an infectious agent that’s yet to be identified, allergic reaction, and neuromodulatory dysfunction. Common triggers other than foods include menses, copulation, emotional distress, and bladder trauma, including transvaginal ultrasound.
Conditions commonly associated with IC include fibromyalgia, irritable bowel syndrome, chronic fatigue, vulvodynia, migraines, depression, and anxiety.
The most common symptoms of IC are urinary urgency and frequency. Many affected patients have dysuria. Some have pain, which is typically suprapubic. However, pain can be present anywhere in a band circumscribing the whole central section of the torso, including the lower back, lower abdomen, urethra, vagina, and vulva. Patients describe a range of pain – burning, aching, stabbing, itching, buzzing, or a feeling of pressure.
“Most women who come in with IC are married to the idea that they’re having recurrent UTIs. They’re going to get antibiotics any way they can for their UTIs: over the phone, at urgent care. You need to get them to buy into the idea that even though UTIs are common, maybe not all of their flares are infections. They ask, ‘Then why do I feel better when I’m on antibiotics for recurrent UTI even though the cultures are negative?’ I say, ‘You feel less stress and anxiety because you think you’re on effective treatment,” Dr. Chacko said.
The diagnosis of IC is one of exclusions. Diagnoses to rule out before arriving at IC include recurrent UTI; overactive bladder, which should present with pure urge frequency and respond to medications for that condition; kidney stone disease present at the end of the ureter where it enters the bladder; gastrointestinal pathology; bladder cancer; and ovarian or uterine pathology.
Referral to a urologist for cystoscopy and cytology is appropriate in patients with microscopic hematuria, a significant smoking history predisposing to bladder cancer, or severe pain with severe frequency, which raises the possibility of Hunner’s ulcers, considered pathognomic for IC, respond “beautifully” to fulguration, she said.
Otherwise, IC can readily be managed by interested primary care physicians. The IC diet initially calls for 2 weeks of strict avoidance of all high-risk foods, most of which are acidic foods. These include fruits and fruit juices, especially citrus and cranberry juices; tomatoes and tomato products, including ketchup; yogurt; chocolate; coffee and tea, including decaf; vinegar; spicy foods; and carbonated beverages, water included.
These foods can later be added back one at a time to the diet while watching for IC flares, which typically occur within hours to several days of re-introducing the food. The return to coffee consumption, if that’s something important to the patient, should be with low-acid coffee. If that triggers an IC flare, try decaf. In time, many patients find they can consume some trigger foods in modest amounts.
“I tell patients it will take 12-18 months to get a good handle on their IC,” Dr. Chacko noted.
The use of OTC alkalizing agents such as Prelief may diffuse dietary triggers. A teaspoon of baking soda in water is also effective.
Second-line treatments include oral hydroxyzine 10-20 mg at bedtime; amitriptyline 10-20 mg at bedtime, mainly for patients with predominant pain symptoms; cimeditine; and pentosan polysulfate at 100 mg TID.
For IC patients with pelvic muscle tightness on pelvic examination, referral to a physical therapist adept at pelvic floor trigger point release can work wonders, she added.
One second-line option is bladder instillations of dimethyl sulfoxide weekly for 6 weeks, cutting back to once monthly maintenance therapy if the more intensive regimen is effective. Instillation of “heparin with lidocaine is a rescue solution. If it’s going to work, it kicks in within a few hours and usually lasts for 24-72 hours. It gets patients through a weekend, a wedding, or a funeral. A response can help make the IC diagnosis, too,” Dr. Chacko said.
She reported having no financial conflicts of interest regarding her presentation.
ESTES PARK, CO. – When patients with interstitial cystitis (IC) learn that first-line therapy is a rigorous diet designed to eliminate common bladder irritants, they tend to react in one of two ways, according to Julie A. Chacko, MD, a urologist in private practice in Santa Barbara, Calif.
Some “are just so grateful that they’re not crazy, which is what they’ve been told after 15 negative urine cultures. (Others) “look at the diet and think I’m sentencing them to death,” she said.
The sole medication approved by the Food and Drug Administration for IC is pentosan polysulfate sodium (Elmiron), and it should be reserved for the minority of patients who don’t experience significant improvement after giving the diet a reasonable shot, Dr. Chako advised. “When Elmiron works it’s great, but it’s not usually my go-to agent because it’s very expensive, you have to take it for 3-6 months to know for sure if it’s efficacious, and it has to be taken on an empty stomach. It’s a difficult medication.”
She advises patients to work with the diet. “Over time, they’re going to be able to find what I call their island – a point where they know very well their limitations and become quite comfortable with them,” she said at a conference on internal medicine sponsored by the University of Colorado.
A poorly understood yet common disorder, IC has a prevalence estimated at 0.5%-4% in women, less in men. Although typically diagnosed in the fourth decade or later, IC occurs at all ages. In some studies, the delay from first appearance of symptoms to arrival at a diagnosis is up to 8 years.
Interstitial cystitis is increasingly being called bladder pain syndrome in the literature, said Dr. Chako, who added, “I personally don’t love bladder pain syndrome as a description for this process. This syndrome has variable symptoms, and patients can have no pain at all.”
The mechanisms that result in IC are a mystery. The leading theory is that a bladder permeability problem allows urinary irritants to reach the interstitium. Nearly 80% of patients with IC can, with coaxing, identify dietary triggers for their symptoms, thereby basically establishing the diagnosis.
Other proposed mechanisms include an infectious agent that’s yet to be identified, allergic reaction, and neuromodulatory dysfunction. Common triggers other than foods include menses, copulation, emotional distress, and bladder trauma, including transvaginal ultrasound.
Conditions commonly associated with IC include fibromyalgia, irritable bowel syndrome, chronic fatigue, vulvodynia, migraines, depression, and anxiety.
The most common symptoms of IC are urinary urgency and frequency. Many affected patients have dysuria. Some have pain, which is typically suprapubic. However, pain can be present anywhere in a band circumscribing the whole central section of the torso, including the lower back, lower abdomen, urethra, vagina, and vulva. Patients describe a range of pain – burning, aching, stabbing, itching, buzzing, or a feeling of pressure.
“Most women who come in with IC are married to the idea that they’re having recurrent UTIs. They’re going to get antibiotics any way they can for their UTIs: over the phone, at urgent care. You need to get them to buy into the idea that even though UTIs are common, maybe not all of their flares are infections. They ask, ‘Then why do I feel better when I’m on antibiotics for recurrent UTI even though the cultures are negative?’ I say, ‘You feel less stress and anxiety because you think you’re on effective treatment,” Dr. Chacko said.
The diagnosis of IC is one of exclusions. Diagnoses to rule out before arriving at IC include recurrent UTI; overactive bladder, which should present with pure urge frequency and respond to medications for that condition; kidney stone disease present at the end of the ureter where it enters the bladder; gastrointestinal pathology; bladder cancer; and ovarian or uterine pathology.
Referral to a urologist for cystoscopy and cytology is appropriate in patients with microscopic hematuria, a significant smoking history predisposing to bladder cancer, or severe pain with severe frequency, which raises the possibility of Hunner’s ulcers, considered pathognomic for IC, respond “beautifully” to fulguration, she said.
Otherwise, IC can readily be managed by interested primary care physicians. The IC diet initially calls for 2 weeks of strict avoidance of all high-risk foods, most of which are acidic foods. These include fruits and fruit juices, especially citrus and cranberry juices; tomatoes and tomato products, including ketchup; yogurt; chocolate; coffee and tea, including decaf; vinegar; spicy foods; and carbonated beverages, water included.
These foods can later be added back one at a time to the diet while watching for IC flares, which typically occur within hours to several days of re-introducing the food. The return to coffee consumption, if that’s something important to the patient, should be with low-acid coffee. If that triggers an IC flare, try decaf. In time, many patients find they can consume some trigger foods in modest amounts.
“I tell patients it will take 12-18 months to get a good handle on their IC,” Dr. Chacko noted.
The use of OTC alkalizing agents such as Prelief may diffuse dietary triggers. A teaspoon of baking soda in water is also effective.
Second-line treatments include oral hydroxyzine 10-20 mg at bedtime; amitriptyline 10-20 mg at bedtime, mainly for patients with predominant pain symptoms; cimeditine; and pentosan polysulfate at 100 mg TID.
For IC patients with pelvic muscle tightness on pelvic examination, referral to a physical therapist adept at pelvic floor trigger point release can work wonders, she added.
One second-line option is bladder instillations of dimethyl sulfoxide weekly for 6 weeks, cutting back to once monthly maintenance therapy if the more intensive regimen is effective. Instillation of “heparin with lidocaine is a rescue solution. If it’s going to work, it kicks in within a few hours and usually lasts for 24-72 hours. It gets patients through a weekend, a wedding, or a funeral. A response can help make the IC diagnosis, too,” Dr. Chacko said.
She reported having no financial conflicts of interest regarding her presentation.
ESTES PARK, CO. – When patients with interstitial cystitis (IC) learn that first-line therapy is a rigorous diet designed to eliminate common bladder irritants, they tend to react in one of two ways, according to Julie A. Chacko, MD, a urologist in private practice in Santa Barbara, Calif.
Some “are just so grateful that they’re not crazy, which is what they’ve been told after 15 negative urine cultures. (Others) “look at the diet and think I’m sentencing them to death,” she said.
The sole medication approved by the Food and Drug Administration for IC is pentosan polysulfate sodium (Elmiron), and it should be reserved for the minority of patients who don’t experience significant improvement after giving the diet a reasonable shot, Dr. Chako advised. “When Elmiron works it’s great, but it’s not usually my go-to agent because it’s very expensive, you have to take it for 3-6 months to know for sure if it’s efficacious, and it has to be taken on an empty stomach. It’s a difficult medication.”
She advises patients to work with the diet. “Over time, they’re going to be able to find what I call their island – a point where they know very well their limitations and become quite comfortable with them,” she said at a conference on internal medicine sponsored by the University of Colorado.
A poorly understood yet common disorder, IC has a prevalence estimated at 0.5%-4% in women, less in men. Although typically diagnosed in the fourth decade or later, IC occurs at all ages. In some studies, the delay from first appearance of symptoms to arrival at a diagnosis is up to 8 years.
Interstitial cystitis is increasingly being called bladder pain syndrome in the literature, said Dr. Chako, who added, “I personally don’t love bladder pain syndrome as a description for this process. This syndrome has variable symptoms, and patients can have no pain at all.”
The mechanisms that result in IC are a mystery. The leading theory is that a bladder permeability problem allows urinary irritants to reach the interstitium. Nearly 80% of patients with IC can, with coaxing, identify dietary triggers for their symptoms, thereby basically establishing the diagnosis.
Other proposed mechanisms include an infectious agent that’s yet to be identified, allergic reaction, and neuromodulatory dysfunction. Common triggers other than foods include menses, copulation, emotional distress, and bladder trauma, including transvaginal ultrasound.
Conditions commonly associated with IC include fibromyalgia, irritable bowel syndrome, chronic fatigue, vulvodynia, migraines, depression, and anxiety.
The most common symptoms of IC are urinary urgency and frequency. Many affected patients have dysuria. Some have pain, which is typically suprapubic. However, pain can be present anywhere in a band circumscribing the whole central section of the torso, including the lower back, lower abdomen, urethra, vagina, and vulva. Patients describe a range of pain – burning, aching, stabbing, itching, buzzing, or a feeling of pressure.
“Most women who come in with IC are married to the idea that they’re having recurrent UTIs. They’re going to get antibiotics any way they can for their UTIs: over the phone, at urgent care. You need to get them to buy into the idea that even though UTIs are common, maybe not all of their flares are infections. They ask, ‘Then why do I feel better when I’m on antibiotics for recurrent UTI even though the cultures are negative?’ I say, ‘You feel less stress and anxiety because you think you’re on effective treatment,” Dr. Chacko said.
The diagnosis of IC is one of exclusions. Diagnoses to rule out before arriving at IC include recurrent UTI; overactive bladder, which should present with pure urge frequency and respond to medications for that condition; kidney stone disease present at the end of the ureter where it enters the bladder; gastrointestinal pathology; bladder cancer; and ovarian or uterine pathology.
Referral to a urologist for cystoscopy and cytology is appropriate in patients with microscopic hematuria, a significant smoking history predisposing to bladder cancer, or severe pain with severe frequency, which raises the possibility of Hunner’s ulcers, considered pathognomic for IC, respond “beautifully” to fulguration, she said.
Otherwise, IC can readily be managed by interested primary care physicians. The IC diet initially calls for 2 weeks of strict avoidance of all high-risk foods, most of which are acidic foods. These include fruits and fruit juices, especially citrus and cranberry juices; tomatoes and tomato products, including ketchup; yogurt; chocolate; coffee and tea, including decaf; vinegar; spicy foods; and carbonated beverages, water included.
These foods can later be added back one at a time to the diet while watching for IC flares, which typically occur within hours to several days of re-introducing the food. The return to coffee consumption, if that’s something important to the patient, should be with low-acid coffee. If that triggers an IC flare, try decaf. In time, many patients find they can consume some trigger foods in modest amounts.
“I tell patients it will take 12-18 months to get a good handle on their IC,” Dr. Chacko noted.
The use of OTC alkalizing agents such as Prelief may diffuse dietary triggers. A teaspoon of baking soda in water is also effective.
Second-line treatments include oral hydroxyzine 10-20 mg at bedtime; amitriptyline 10-20 mg at bedtime, mainly for patients with predominant pain symptoms; cimeditine; and pentosan polysulfate at 100 mg TID.
For IC patients with pelvic muscle tightness on pelvic examination, referral to a physical therapist adept at pelvic floor trigger point release can work wonders, she added.
One second-line option is bladder instillations of dimethyl sulfoxide weekly for 6 weeks, cutting back to once monthly maintenance therapy if the more intensive regimen is effective. Instillation of “heparin with lidocaine is a rescue solution. If it’s going to work, it kicks in within a few hours and usually lasts for 24-72 hours. It gets patients through a weekend, a wedding, or a funeral. A response can help make the IC diagnosis, too,” Dr. Chacko said.
She reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM THE ANNUAL INTERNAL MEDICINE PROGRAM
Reproductive planning for women after solid-organ transplant
Increasing numbers of women of childbearing age are receiving solid-organ transplants. All need counseling on how to prevent pregnancy while they are taking immunosuppressive agents. Some want to become pregnant after their transplant and thus require counseling and follow-up to maintain good health during pregnancy (Table 1).1
Primary care physicians can assist with basic contraception counseling and pregnancy planning for their patients who have had solid-organ transplants. In this review, we describe contraceptive options and pregnancy planning for these women.
TRANSPLANTS IN WOMEN ARE INCREASING
Over the past 20 years, the number of solid-organ transplants in US women has increased steadily. Since 1988, 38% of the 634,000 transplants performed were in women, and 47% of these women were of childbearing age (ages 18 to 49).2 Kidneys accounted for 60% of solid-organ transplants,2 and kidney transplant is now commonly performed in women of childbearing age. In 2012, of 176,000 patients with a functioning renal graft, 40.5% were women, and recipients between ages 20 and 44 composed the second-largest age group.3
FERTILITY IN WOMEN WITH END-STAGE RENAL DISEASE
Women in their reproductive years who have end-stage renal disease have lower fertility rates than women in the general population. In women undergoing peritoneal dialysis or hemodialysis, conception rates decrease to around 0.5% per year.4 This lower rate is most likely related to hypothalamic-pituitary-gonadal dysfunction, leading to reduced or total impairment of ovulation, menstrual irregularities, and infertility.5
Fertility often returns within a few months after transplant,1,6 and reported posttransplant pregnancy rates range from 3.3% to 18%,7–9 with up to one-third of pregnancies being unintended.6,10 These numbers are likely an underestimate because they do not reflect all pregnancies that are terminated, as many women do not voluntarily report having had an abortion.
Fertility is also severely diminished in women with end-stage liver disease. After liver transplant, sex hormone levels return to normal for many women, and menses soon resume.11
In 2005, the National Transplantation Pregnancy Registry reported 1,418 pregnancies in 919 female recipients of solid-organ transplants. In 2010, this number had increased to 1,940 pregnancies in 1,185 recipients, of whom 75% were kidney transplant recipients.12
A successful pregnancy outcome is most likely when a minimum of 1 year intervenes between transplant and conception.12,13
TERATOGENICITY OF IMMUNOSUPPRESSANTS
Immunosuppressant drugs commonly used for maintenance therapy after solid-organ transplant include the following:
- Calcineurin inhibitors (eg, cyclosporine, tacrolimus)
- Antiproliferative and antimetabolite agents (eg, mycophenolate mofetil, azathioprine)
- Corticosteroids
- Mammalian target of rapamycin inhibitors (eg, sirolimus, everolimus)
- T-cell costimulation blockers (eg, belatacept).14
The US Food and Drug Administration (FDA) previously classified mycophenolate mofetil and azathioprine in pregnancy risk category D (positive evidence of human fetal risk). The teratogenic risk of mycophenolate mofetil is well established in studies documenting specific congenital malformations and fetal loss in the first trimester.13,15 The teratogenic risk of azathioprine, on the other hand, is estimated to be minimal to small.16 Many of the associated fetal abnormalities may be related to the complexity of the underlying medical condition of the mother rather than to the medication.16
In June 2015, the FDA’s new Pregnancy and Lactation Labeling Rule went into effect, which removes the pregnancy letter categories A, B, C, D, and X from labeling.17 This rule was designed to help providers counsel their patients regarding the specific risks and benefits of a drug when used by pregnant or nursing women. However, the ABCDX categories are still commonly used. Table 2 shows information about the risks during pregnancy and lactation posed by the immunosuppressive drugs commonly used by posttransplant patients.18
CRITERIA FOR A SUCCESSFUL PREGNANCY
To ensure a safe and successful pregnancy with the fewest fetal and maternal complications, women are generally advised to avoid pregnancy for at least 1 year after transplant.19,20
In addition, women should meet certain clinical prerequisites after transplant before they conceive, as outlined by the American Society of Transplantation.19,20 These include:
- No rejection within the previous year
- Adequate and stable graft function (eg, serum creatinine < 1.5 mg/dL and urinary protein excretion < 500 mg/24 hours)
- No acute infection that might affect the fetus
- Maintenance immunosuppression at stable dosages.
Other circumstances to consider include episodes of rejection in the first year after transplant (as evidenced by biopsy results or glomerular filtration rate), the woman’s age (advanced maternal age is unfavorable), or any history of noncompliance.
Every pregnancy in a transplant recipient must be carefully planned. Primary care providers should encourage patients to meet with their transplant team and obstetricians early and often to allow time for the care team to adjust the type and dosing of immunosuppressant drugs, to ensure stable graft function, and to optimize any current chronic medical conditions such as diabetes mellitus or hypertension before conception.
CONTRACEPTIVE COUNSELING AFTER TRANSPLANT
Pregnancy should be avoided while transplant patients are taking FDA category D immunosuppressant drugs and, as already mentioned, during the first year after transplant. Unintended pregnancy can have serious health consequences for the mother and the fetus, as well as poor pregnancy outcomes. The US Centers for Disease Control and Prevention (CDC) lists solid-organ transplant within the past 2 years as a condition that can lead to adverse events as a result of pregnancy.21 After a transplant, a woman’s risks from an unintended pregnancy are always greater than the risks from any contraceptive, and this is important to reinforce in counseling.
Two forms of reliable contraception should be used at all times, and consistent condom use should be encouraged as one of the methods. Condoms are not reliable when used as the sole contraceptive method because they have an 18% typical-use failure rate. However, they are an excellent adjunct to other contraceptive methods because they have the additional benefit of protecting against sexually transmitted disease.
Choosing the appropriate contraceptive method for recipients of solid-organ transplants can be challenging because of several factors, including the recipient’s preexisting medical problems and drug interactions of immunosuppressant medications.
CDC criteria and categories for contraceptive use
In 2010, the CDC released the US version of the Medical Eligibility Criteria (US MEC) for contraceptive use, which was based on the 2009 World Health Organization Medical Eligibility Criteria (WHO MEC); these criteria were revised in August 2016.21
- Category 1: A condition for which there is no restriction for the use of the contraceptive method
- Category 2: A condition for which the advantages of using the method generally outweigh the theoretical or proven risks
- Category 3: A condition for which the theoretical or proven risks usually outweigh the advantages of using the method
- Category 4: A condition that represents an unacceptable health risk if the contraceptive method is used.
These recommendations aimed to improve family planning options by clarifying the possible safe and effective contraceptive options available while considering the patient’s medical condition. The CDC added solid-organ transplant recipients to this document because of the prevalence of this group in the United States.
The CDC categorizes a patient’s medical condition after transplant as either complicated or uncomplicated. Complicated conditions include acute or chronic graft failure, graft rejection, and cardiac allograft vasculopathy.21
Effectiveness of contraceptive methods
Contraceptive methods can be divided into 4 categories based on estimated effectiveness, ie, the pregnancy rate with “typical use” of that particular method in 1 year21–23:
- Very effective (0%–0.9%)
- Effective (1%–9%)
- Moderately effective (10%–25%)
- Less effective (26%–32%).
Typical use refers to failure rates for women and men whose use is not consistent nor always correct. Correct use, also described in the sections that follow, refers to failure rates for those whose use is consistent and always correct.
Women should be counseled regarding all available contraceptive options that are medically suitable for them, so they can choose the method that best fits their needs and lifestyle. They should receive counseling on emergency contraception, barrier protection against sexually transmitted disease, and the correct use of the contraceptive method they choose. They should be advised that if their chosen contraceptive method is unsatisfactory for any reason, they can switch to another method. Most importantly, providers need to impress on their patients that the risks associated with unintended pregnancy are far greater than the risks from any of the contraceptive methods.
VERY EFFECTIVE CONTRACEPTIVES (UNINTENDED PREGNANCY RATE 0%–0.9%)
This tier of contraception is the most effective regardless of the patient’s adherence; it includes long-acting, reversible contraceptives and permanent sterilization (both male and female) (Table 3).21–23
Long-acting reversible contraceptives include intrauterine devices (IUDs) and the subdermal etonogestrel implant. Given their efficacy and favorable safety profile, long-acting reversible contraceptives are being promoted for use in women who have chronic medical conditions, such as transplants.24
Intrauterine devices
IUDs are long-acting and reversible. They can be used by women who are nulliparous and those of all ages, including adolescents.22
Two types of IUDs are available in the United States: nonhormonal (copper) and hormonal (levonorgestrel). The copper IUD is effective for at least 10 years, whereas the levonorgestrel IUDs last for 3 to 5 years.22
Four levonorgestrel IUDs are currently available in the United States. Their sizes and doses vary: Mirena (52 µg), Skyla (13.5 µg), Liletta (52 µg), and Kyleena (19.5 µg).
Fewer than 1% of women become pregnant in the first year of IUD use.22,23 IUDs are an ideal option for women with solid-organ transplants because they are so effective and because the patient does not have to do anything once the IUD is in.22–24 The levonorgestrel IUD Mirena has the additional advantage of reducing heavy menstrual bleeding and is currently the only hormonal IUD with FDA approval for the management of menorrhagia.
About 12% of women in the general population use IUDs as their contraceptive method of choice,25 whereas after solid-organ transplantation about 15% to 20% of women do.26
Two historic concerns regarding IUDs may explain their low rate of use in transplant recipients.
First, IUDs were believed to be less effective in women on immunosuppressive drugs because IUDs act by inducing a local inflammatory reaction. However, IUDs involve macrophage activation, which is independent of the immune processes modified by immunosuppressants (primarily T-cell function).27 A recent pilot study showed a strong inflammatory reaction in the endometrium of transplant recipients after levonorgestrel IUD insertion.28
Second, there was concern about the increased risk of pelvic inflammatory disease with IUDs, but studies have shown levonorgestrel IUDs to be safe in transplant patients.29,30
The CDC21 lists copper and levonorgestrel IUDs in MEC category 3 (the risks generally outweigh the advantages) for initiation in patients with complicated transplants and in category 2 (advantages generally outweigh the risks) in patients with uncomplicated organ transplants. The devices are in category 2 for both complicated and uncomplicated cases if the IUD is already in place.
Subdermal implant
A subdermal implant consisting of a single rod containing 68 mg of etonogestrel is commercially available in the United States. It is one of the most effective contraceptive methods, with the lowest rates of pregnancy—less than 1% per year, with protection lasting at least 3 years.22,23 This low risk makes the subdermal implant a suitable method of contraception after transplant. Daily compliance is not required, and there are no hepatic first-pass effects, which results in higher bioavailability and less chance of drug interactions.
The main disadvantage of the subdermal implant and IUDs is unscheduled bleeding. An important benefit is prolonged amenorrhea, not only for patient convenience, but for reduction of endometrial cancer risk. Insertion and removal of the implant are considered minor office procedures. The implants are classified as US MEC category 2 in uncomplicated cases; initiation in complicated cases is considered category 3 but continuation is considered category 2.21
Permanent sterilization
Permanent sterilization is another option for women and men. In women, the fallopian tubes can be occluded with a coil system implanted vaginally through a hysteroscope, or they can be severed, tied, or clamped in a laparoscopic procedure or during cesarean delivery. Pregnancy rates after tubal ligation are less than 1%,23,31 although concern exists for high failure rates with the hysteroscopic method.
Because younger patients are more likely than older patients to subsequently regret having the procedure done, all available contraceptive options should be discussed with them.31
For men, permanent sterilization is done by vasectomy, which has less associated risk and cost compared with sterilization for women.
EFFECTIVE CONTRACEPTIVE METHODS (UNINTENDED PREGNANCY RATE 1%–9%)
Effective contraceptive methods, the next tier down from very effective methods, include injectable contraceptives, combined hormonal contraceptives, and progestin-only contraceptives (Table 4).
Injectable contraceptives
Depot medroxyprogesterone acetate is an injectable progestin-only contraceptive that carries a pregnancy risk of 6% with typical use and less than 1% with correct use.23 Thus, some failures are due to patients not returning for follow-up, but in some patients this method is not effective. Injections are given intramuscularly once every 3 months, avoiding the need for daily use.
A valid concern for transplant patients is that medroxyprogesterone acetate reduces bone mineral density. Although the bone effects are reversible in healthy adult women, caution is needed when prescribing this option to transplant patients who are already at increased risk of bone disease attributable to renal osteodystrophy and chronic corticosteroid use. 32,33
Recently, a subcutaneous formulation of depot medroxyprogesterone acetate (104 mg)was added to the WHO MEC for contraceptive use.34,35 The recommendations for the subcutaneous form are similar to those for the intramuscular form. In healthy women, the subcutaneous formulation is as safe and effective as the intramuscular form,36 but its efficacy after solid-organ transplant has not been determined. Both forms of depot medroxyprogesterone acetate are category 2 in the US MEC for both complicated and uncomplicated transplant cases.21
Combined hormonal contraceptives
Combined hormonal contraceptives contain both estrogen and progesterone and are available as pills, patches, or rings. Each product has an unintended pregnancy risk of 9% with typical use and less than 1% with correct use.23 They require strict patient adherence to regular daily use, which likely explains their high failure rate with typical use.
Combined hormonal contraceptives reduce mortality risk in women in the general population,37 but their effect on mortality risk after transplant is unknown and needs further study. In women who received liver transplants, low-dose combined hormonal contraceptives have been found to be effective and well tolerated, but initiation should be delayed at least 6 months until postoperative organ stability is demonstrated.11
Combined oral contraceptives are the most widely prescribed because they are convenient and familiar and have an acceptable safety profile in transplant patients,11,33,37 despite their high failure rate with typical use. They regulate the menstrual cycle and reduce anemia associated with menstruation.
The transdermal contraceptive patch has a mechanism of action similar to that of the combined oral contraceptives, but it delivers estrogen and progesterone transdermally through the abdominal wall, thus avoiding first-pass metabolism in the liver and enzymatic degradation in the gut. It delivers 35 µg of ethinyl estradiol and 150 µg of norelgestromin (an active metabolite of norgestimate) daily.38 It may cause higher circulating levels of estrogen than a combined oral contraceptive and may be associated with a higher risk of venous thromboembolism, but the evidence is conflicting.39–42
The vaginal ring, made of Silastic, delivers ethinyl estradiol in a low dose (15 µg/day) and etonorgestrel 0.12 mg/day. Like the patch, it has the advantage of bypassing first-pass metabolism in the liver, making it a good option for transplant patients who are taking antirejection drugs, thus avoiding drug interactions.41
Both the transdermal patch and vaginal ring were studied in transplant patients and had favorable results.24,43 The combined hormonal oral contraceptive pills, patch, and ring are in category 4 (unacceptable health risk) in the US MEC in patients with complicated cases, but they are in category 2 in uncomplicated cases.21
Combined hormonal contraceptives should not be considered first-line options by themselves for transplant patients because of their high failure rate with typical use.24
Progestin-only pills
Although progestin-only pills have not been studied specifically in transplant patients, they can be considered for women who have contraindications to estrogen use. Estrogen use is contraindicated in women with a history of venous thromboembolism, thrombogenic mutations, estrogen-dependent neoplasia, hepatocellular adenoma, severe hypertension, vascular disease, and Budd-Chiari syndrome.
Progestin-only pills inhibit ovulation in only about half of a woman’s cycles, but they prevent conception by other mechanisms as well, such as causing thickening of the cervical mucus. They also alter the endometrium to make it unfavorable for implantation and reduce the ciliary activity of the fallopian tube.
Strict adherence is important for effectiveness because progestin-only pills have a shorter half-life than combined hormonal contraceptives and also suppress ovulation less effectively.22 Failure rates are similar or somewhat higher than with combined hormonal contraceptives; with typical use, about 9 in 100 women can become pregnant in the first year.23 According to the US MEC,21 progestin-only pills are classified as category 2 for patients after both complicated and uncomplicated transplants.
MODERATELY EFFECTIVE METHODS (PREGNANCY RATE 10%–25%)
This tier of contraceptives includes all barrier methods, ie, male and female condoms, vaginal diaphragms, cervical caps, and sponges (Table 5).
Condoms (male and female)
When male condoms are used as the only birth control method, pregnancy occurs less often (18% with typical use and 2% with correct use) than with female condoms (21% with typical use and 5% with correct use).23 Male and female condoms are the only contraceptive methods that also prevent transmission of sexually transmitted disease.24
Caps, sponges, diaphragms
Cervical caps, vaginal sponges, and vaginal diaphragms are other forms of barrier contraceptives. All barrier methods should be combined with another contraceptive method to provide reliable protection against pregnancy. These methods are considered category 1 according to the US MEC.
LESS-EFFECTIVE METHODS
Fertility awareness-based methods such as the rhythm method have an associated pregnancy rate of about 25% with typical use and 3% to 5% with correct use23 and cannot be relied on for use by transplant recipients.24
Withdrawal and spermicides are considered least effective and unreliable for pregnancy prevention.
KNOW YOUR OPTIONS
With the growing number of women in their reproductive years receiving solid-organ transplants in the United States, it is increasingly important for healthcare providers to be aware of contraceptive options and reproductive life planning for this high-risk population.
Safe and effective forms of contraception are available, and additional information to guide the choice can be found in the Summary Chart of US MEC for Contraceptive Use, which is also available in a free smart phone app through the CDC.44
Pregnancy after transplant carries high risks, requiring these patients to have special counseling and monitoring. Fortunately, planned pregnancy at least 1 year after transplant can lead to successful outcomes in these women.
- McKay DB, Josephson MA. Pregnancy in recipients of solid organs: effects on mother and child. N Engl J Med 2006; 354:1281–1293.
- US Department of Health and Human Services. Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/. Accessed July 17, 2017.
- United States Renal Data System. 2014 annual data report. https://www.usrds.org/2014/view/Default.aspx. Accessed July 17, 2017.
- Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 1999; 33:235–252.
- Josephson MA, McKay DB. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis 2013; 20:433–440.
- Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 2009; 9:1541–1549.
- Mohapatra A, Basu G. Pregnancy in kidney disease. Health Sciences 2012; 1(2). http://healthsciences.ac.in/july-sep-12/downloads/pregnancy_in_kidney_disease.pdf. Accessed July 25, 2017.
- Potluri K, Moldenhauer J, Karlman R, Hou S. Beta HCG levels in a pregnant dialysis patient: a cautionary tale. NDT Plus 2011; 4:42–43.
- Kennedy C, Hussein W, Spencer S, et al. Reproductive health in Irish female renal transplant recipients. Ir J Med Sci 2012; 181:59–63.
- Ghazizadeh S, Lessan-Pezeshki M, Khatami M, et al. Unwanted pregnancy among kidney transplant recipients in Iran. Transplant Proc 2005; 37:3085–3086.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 24:65–85.
- Mohamed-Ahmed O, Nelson-Piercy C, Bramham K, et al. Pregnancy outcomes in liver and cardiothoracic transplant recipients: a UK national cohort study. PLoS One 2014; 9:e89151.
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care 2015; 21(suppl 1):s12–s23.
- Hoeltzenbein M, Elefant E, Vial T, et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A 2012; 158A:588–596.
- Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 2002; 65:240–261.
- Dinatale M. The pregnancy and lactation labeling rule (PLLR). US Food and Drug Administration, 2016. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM520454.pdf. Accessed July 25, 2017.
- Lexicomp. http://online.lexi.com/lco/action/api/find/globalid/6612?utd=1. Accessed July 27, 2017.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(suppl 3):S1–S155.
- Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol 2013; 6:116–125.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2011; 118:184–196.
- Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404.
- Krajewski CM, Geetha D, Gomez-Lobo V. Contraceptive options for women with a history of solid-organ transplantation. Transplantation 2013; 95:1183–1186.
- Stern LF, Simons HR, Kohn JE, Debevec EJ, Morfesis JM, Patel AA. Differences in contraceptive use between family planning providers and the U.S. population: results of a nationwide survey. Contraception 2015; 91:464–469.
- Rafie S, Lai S, Garcia JE, Mody SK. Contraceptive use in female recipients of a solid-organ transplant. Prog Transplant 2014; 24:344–348.
- Labied S, Galant C, Nisolle M, et al. Differential elevation of matrix metalloproteinase expression in women exposed to levonorgestrel-releasing intrauterine system for a short or prolonged period of time. Hum Reprod 2009; 24:113–121.
- Kim CR, Martinez-Maza O, Magpantay L, et al. Immunologic evaluation of the endometrium with a levonorgestrel intrauterine device in solid organ transplant women and healthy controls. Contraception 2016; 94:534–540.
- Ramhendar T, Byrne P. Use of the levonorgestrel-releasing intrauterine system in renal transplant recipients: a retrospective case review. Contraception 2012; 86:288–289.
- Huguelet PS, Sheehan C, Spitzer RF, Scott S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: a case series. Contraception 2017; 95:378–381.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the US Collaborative Review of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007; 18:1319–1328.
- Krajewski C, Sucato G. Reproductive health care after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1222–1234.
- World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. http://apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf. Accessed July 27, 2017.
- Pietrzak B, Bobrowska K, Jabiry-Zieniewicz Z, et al. Oral and transdermal hormonal contraception in women after kidney transplantation. Transplant Proc 2007; 39:2759–2762.
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004; 70:269–275.
- Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet 2003; 362:185–191.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2006; 73:223–228.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- Estes CM, Westhoff C. Contraception for the transplant patient. Semin Perinatol 2007; 31:372–377.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Paternoster DM, Riboni F, Bertolino M, et al. The contraceptive vaginal ring in women with renal and liver transplantation: analysis of preliminary results. Transplant Proc 2010; 42:1162–1165.
- Centers for Disease Control and Prevention (CDC). Summary chart of US medical eligibility criteria for contraceptive use. https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf. Accessed July 17, 2017.
Increasing numbers of women of childbearing age are receiving solid-organ transplants. All need counseling on how to prevent pregnancy while they are taking immunosuppressive agents. Some want to become pregnant after their transplant and thus require counseling and follow-up to maintain good health during pregnancy (Table 1).1
Primary care physicians can assist with basic contraception counseling and pregnancy planning for their patients who have had solid-organ transplants. In this review, we describe contraceptive options and pregnancy planning for these women.
TRANSPLANTS IN WOMEN ARE INCREASING
Over the past 20 years, the number of solid-organ transplants in US women has increased steadily. Since 1988, 38% of the 634,000 transplants performed were in women, and 47% of these women were of childbearing age (ages 18 to 49).2 Kidneys accounted for 60% of solid-organ transplants,2 and kidney transplant is now commonly performed in women of childbearing age. In 2012, of 176,000 patients with a functioning renal graft, 40.5% were women, and recipients between ages 20 and 44 composed the second-largest age group.3
FERTILITY IN WOMEN WITH END-STAGE RENAL DISEASE
Women in their reproductive years who have end-stage renal disease have lower fertility rates than women in the general population. In women undergoing peritoneal dialysis or hemodialysis, conception rates decrease to around 0.5% per year.4 This lower rate is most likely related to hypothalamic-pituitary-gonadal dysfunction, leading to reduced or total impairment of ovulation, menstrual irregularities, and infertility.5
Fertility often returns within a few months after transplant,1,6 and reported posttransplant pregnancy rates range from 3.3% to 18%,7–9 with up to one-third of pregnancies being unintended.6,10 These numbers are likely an underestimate because they do not reflect all pregnancies that are terminated, as many women do not voluntarily report having had an abortion.
Fertility is also severely diminished in women with end-stage liver disease. After liver transplant, sex hormone levels return to normal for many women, and menses soon resume.11
In 2005, the National Transplantation Pregnancy Registry reported 1,418 pregnancies in 919 female recipients of solid-organ transplants. In 2010, this number had increased to 1,940 pregnancies in 1,185 recipients, of whom 75% were kidney transplant recipients.12
A successful pregnancy outcome is most likely when a minimum of 1 year intervenes between transplant and conception.12,13
TERATOGENICITY OF IMMUNOSUPPRESSANTS
Immunosuppressant drugs commonly used for maintenance therapy after solid-organ transplant include the following:
- Calcineurin inhibitors (eg, cyclosporine, tacrolimus)
- Antiproliferative and antimetabolite agents (eg, mycophenolate mofetil, azathioprine)
- Corticosteroids
- Mammalian target of rapamycin inhibitors (eg, sirolimus, everolimus)
- T-cell costimulation blockers (eg, belatacept).14
The US Food and Drug Administration (FDA) previously classified mycophenolate mofetil and azathioprine in pregnancy risk category D (positive evidence of human fetal risk). The teratogenic risk of mycophenolate mofetil is well established in studies documenting specific congenital malformations and fetal loss in the first trimester.13,15 The teratogenic risk of azathioprine, on the other hand, is estimated to be minimal to small.16 Many of the associated fetal abnormalities may be related to the complexity of the underlying medical condition of the mother rather than to the medication.16
In June 2015, the FDA’s new Pregnancy and Lactation Labeling Rule went into effect, which removes the pregnancy letter categories A, B, C, D, and X from labeling.17 This rule was designed to help providers counsel their patients regarding the specific risks and benefits of a drug when used by pregnant or nursing women. However, the ABCDX categories are still commonly used. Table 2 shows information about the risks during pregnancy and lactation posed by the immunosuppressive drugs commonly used by posttransplant patients.18
CRITERIA FOR A SUCCESSFUL PREGNANCY
To ensure a safe and successful pregnancy with the fewest fetal and maternal complications, women are generally advised to avoid pregnancy for at least 1 year after transplant.19,20
In addition, women should meet certain clinical prerequisites after transplant before they conceive, as outlined by the American Society of Transplantation.19,20 These include:
- No rejection within the previous year
- Adequate and stable graft function (eg, serum creatinine < 1.5 mg/dL and urinary protein excretion < 500 mg/24 hours)
- No acute infection that might affect the fetus
- Maintenance immunosuppression at stable dosages.
Other circumstances to consider include episodes of rejection in the first year after transplant (as evidenced by biopsy results or glomerular filtration rate), the woman’s age (advanced maternal age is unfavorable), or any history of noncompliance.
Every pregnancy in a transplant recipient must be carefully planned. Primary care providers should encourage patients to meet with their transplant team and obstetricians early and often to allow time for the care team to adjust the type and dosing of immunosuppressant drugs, to ensure stable graft function, and to optimize any current chronic medical conditions such as diabetes mellitus or hypertension before conception.
CONTRACEPTIVE COUNSELING AFTER TRANSPLANT
Pregnancy should be avoided while transplant patients are taking FDA category D immunosuppressant drugs and, as already mentioned, during the first year after transplant. Unintended pregnancy can have serious health consequences for the mother and the fetus, as well as poor pregnancy outcomes. The US Centers for Disease Control and Prevention (CDC) lists solid-organ transplant within the past 2 years as a condition that can lead to adverse events as a result of pregnancy.21 After a transplant, a woman’s risks from an unintended pregnancy are always greater than the risks from any contraceptive, and this is important to reinforce in counseling.
Two forms of reliable contraception should be used at all times, and consistent condom use should be encouraged as one of the methods. Condoms are not reliable when used as the sole contraceptive method because they have an 18% typical-use failure rate. However, they are an excellent adjunct to other contraceptive methods because they have the additional benefit of protecting against sexually transmitted disease.
Choosing the appropriate contraceptive method for recipients of solid-organ transplants can be challenging because of several factors, including the recipient’s preexisting medical problems and drug interactions of immunosuppressant medications.
CDC criteria and categories for contraceptive use
In 2010, the CDC released the US version of the Medical Eligibility Criteria (US MEC) for contraceptive use, which was based on the 2009 World Health Organization Medical Eligibility Criteria (WHO MEC); these criteria were revised in August 2016.21
- Category 1: A condition for which there is no restriction for the use of the contraceptive method
- Category 2: A condition for which the advantages of using the method generally outweigh the theoretical or proven risks
- Category 3: A condition for which the theoretical or proven risks usually outweigh the advantages of using the method
- Category 4: A condition that represents an unacceptable health risk if the contraceptive method is used.
These recommendations aimed to improve family planning options by clarifying the possible safe and effective contraceptive options available while considering the patient’s medical condition. The CDC added solid-organ transplant recipients to this document because of the prevalence of this group in the United States.
The CDC categorizes a patient’s medical condition after transplant as either complicated or uncomplicated. Complicated conditions include acute or chronic graft failure, graft rejection, and cardiac allograft vasculopathy.21
Effectiveness of contraceptive methods
Contraceptive methods can be divided into 4 categories based on estimated effectiveness, ie, the pregnancy rate with “typical use” of that particular method in 1 year21–23:
- Very effective (0%–0.9%)
- Effective (1%–9%)
- Moderately effective (10%–25%)
- Less effective (26%–32%).
Typical use refers to failure rates for women and men whose use is not consistent nor always correct. Correct use, also described in the sections that follow, refers to failure rates for those whose use is consistent and always correct.
Women should be counseled regarding all available contraceptive options that are medically suitable for them, so they can choose the method that best fits their needs and lifestyle. They should receive counseling on emergency contraception, barrier protection against sexually transmitted disease, and the correct use of the contraceptive method they choose. They should be advised that if their chosen contraceptive method is unsatisfactory for any reason, they can switch to another method. Most importantly, providers need to impress on their patients that the risks associated with unintended pregnancy are far greater than the risks from any of the contraceptive methods.
VERY EFFECTIVE CONTRACEPTIVES (UNINTENDED PREGNANCY RATE 0%–0.9%)
This tier of contraception is the most effective regardless of the patient’s adherence; it includes long-acting, reversible contraceptives and permanent sterilization (both male and female) (Table 3).21–23
Long-acting reversible contraceptives include intrauterine devices (IUDs) and the subdermal etonogestrel implant. Given their efficacy and favorable safety profile, long-acting reversible contraceptives are being promoted for use in women who have chronic medical conditions, such as transplants.24
Intrauterine devices
IUDs are long-acting and reversible. They can be used by women who are nulliparous and those of all ages, including adolescents.22
Two types of IUDs are available in the United States: nonhormonal (copper) and hormonal (levonorgestrel). The copper IUD is effective for at least 10 years, whereas the levonorgestrel IUDs last for 3 to 5 years.22
Four levonorgestrel IUDs are currently available in the United States. Their sizes and doses vary: Mirena (52 µg), Skyla (13.5 µg), Liletta (52 µg), and Kyleena (19.5 µg).
Fewer than 1% of women become pregnant in the first year of IUD use.22,23 IUDs are an ideal option for women with solid-organ transplants because they are so effective and because the patient does not have to do anything once the IUD is in.22–24 The levonorgestrel IUD Mirena has the additional advantage of reducing heavy menstrual bleeding and is currently the only hormonal IUD with FDA approval for the management of menorrhagia.
About 12% of women in the general population use IUDs as their contraceptive method of choice,25 whereas after solid-organ transplantation about 15% to 20% of women do.26
Two historic concerns regarding IUDs may explain their low rate of use in transplant recipients.
First, IUDs were believed to be less effective in women on immunosuppressive drugs because IUDs act by inducing a local inflammatory reaction. However, IUDs involve macrophage activation, which is independent of the immune processes modified by immunosuppressants (primarily T-cell function).27 A recent pilot study showed a strong inflammatory reaction in the endometrium of transplant recipients after levonorgestrel IUD insertion.28
Second, there was concern about the increased risk of pelvic inflammatory disease with IUDs, but studies have shown levonorgestrel IUDs to be safe in transplant patients.29,30
The CDC21 lists copper and levonorgestrel IUDs in MEC category 3 (the risks generally outweigh the advantages) for initiation in patients with complicated transplants and in category 2 (advantages generally outweigh the risks) in patients with uncomplicated organ transplants. The devices are in category 2 for both complicated and uncomplicated cases if the IUD is already in place.
Subdermal implant
A subdermal implant consisting of a single rod containing 68 mg of etonogestrel is commercially available in the United States. It is one of the most effective contraceptive methods, with the lowest rates of pregnancy—less than 1% per year, with protection lasting at least 3 years.22,23 This low risk makes the subdermal implant a suitable method of contraception after transplant. Daily compliance is not required, and there are no hepatic first-pass effects, which results in higher bioavailability and less chance of drug interactions.
The main disadvantage of the subdermal implant and IUDs is unscheduled bleeding. An important benefit is prolonged amenorrhea, not only for patient convenience, but for reduction of endometrial cancer risk. Insertion and removal of the implant are considered minor office procedures. The implants are classified as US MEC category 2 in uncomplicated cases; initiation in complicated cases is considered category 3 but continuation is considered category 2.21
Permanent sterilization
Permanent sterilization is another option for women and men. In women, the fallopian tubes can be occluded with a coil system implanted vaginally through a hysteroscope, or they can be severed, tied, or clamped in a laparoscopic procedure or during cesarean delivery. Pregnancy rates after tubal ligation are less than 1%,23,31 although concern exists for high failure rates with the hysteroscopic method.
Because younger patients are more likely than older patients to subsequently regret having the procedure done, all available contraceptive options should be discussed with them.31
For men, permanent sterilization is done by vasectomy, which has less associated risk and cost compared with sterilization for women.
EFFECTIVE CONTRACEPTIVE METHODS (UNINTENDED PREGNANCY RATE 1%–9%)
Effective contraceptive methods, the next tier down from very effective methods, include injectable contraceptives, combined hormonal contraceptives, and progestin-only contraceptives (Table 4).
Injectable contraceptives
Depot medroxyprogesterone acetate is an injectable progestin-only contraceptive that carries a pregnancy risk of 6% with typical use and less than 1% with correct use.23 Thus, some failures are due to patients not returning for follow-up, but in some patients this method is not effective. Injections are given intramuscularly once every 3 months, avoiding the need for daily use.
A valid concern for transplant patients is that medroxyprogesterone acetate reduces bone mineral density. Although the bone effects are reversible in healthy adult women, caution is needed when prescribing this option to transplant patients who are already at increased risk of bone disease attributable to renal osteodystrophy and chronic corticosteroid use. 32,33
Recently, a subcutaneous formulation of depot medroxyprogesterone acetate (104 mg)was added to the WHO MEC for contraceptive use.34,35 The recommendations for the subcutaneous form are similar to those for the intramuscular form. In healthy women, the subcutaneous formulation is as safe and effective as the intramuscular form,36 but its efficacy after solid-organ transplant has not been determined. Both forms of depot medroxyprogesterone acetate are category 2 in the US MEC for both complicated and uncomplicated transplant cases.21
Combined hormonal contraceptives
Combined hormonal contraceptives contain both estrogen and progesterone and are available as pills, patches, or rings. Each product has an unintended pregnancy risk of 9% with typical use and less than 1% with correct use.23 They require strict patient adherence to regular daily use, which likely explains their high failure rate with typical use.
Combined hormonal contraceptives reduce mortality risk in women in the general population,37 but their effect on mortality risk after transplant is unknown and needs further study. In women who received liver transplants, low-dose combined hormonal contraceptives have been found to be effective and well tolerated, but initiation should be delayed at least 6 months until postoperative organ stability is demonstrated.11
Combined oral contraceptives are the most widely prescribed because they are convenient and familiar and have an acceptable safety profile in transplant patients,11,33,37 despite their high failure rate with typical use. They regulate the menstrual cycle and reduce anemia associated with menstruation.
The transdermal contraceptive patch has a mechanism of action similar to that of the combined oral contraceptives, but it delivers estrogen and progesterone transdermally through the abdominal wall, thus avoiding first-pass metabolism in the liver and enzymatic degradation in the gut. It delivers 35 µg of ethinyl estradiol and 150 µg of norelgestromin (an active metabolite of norgestimate) daily.38 It may cause higher circulating levels of estrogen than a combined oral contraceptive and may be associated with a higher risk of venous thromboembolism, but the evidence is conflicting.39–42
The vaginal ring, made of Silastic, delivers ethinyl estradiol in a low dose (15 µg/day) and etonorgestrel 0.12 mg/day. Like the patch, it has the advantage of bypassing first-pass metabolism in the liver, making it a good option for transplant patients who are taking antirejection drugs, thus avoiding drug interactions.41
Both the transdermal patch and vaginal ring were studied in transplant patients and had favorable results.24,43 The combined hormonal oral contraceptive pills, patch, and ring are in category 4 (unacceptable health risk) in the US MEC in patients with complicated cases, but they are in category 2 in uncomplicated cases.21
Combined hormonal contraceptives should not be considered first-line options by themselves for transplant patients because of their high failure rate with typical use.24
Progestin-only pills
Although progestin-only pills have not been studied specifically in transplant patients, they can be considered for women who have contraindications to estrogen use. Estrogen use is contraindicated in women with a history of venous thromboembolism, thrombogenic mutations, estrogen-dependent neoplasia, hepatocellular adenoma, severe hypertension, vascular disease, and Budd-Chiari syndrome.
Progestin-only pills inhibit ovulation in only about half of a woman’s cycles, but they prevent conception by other mechanisms as well, such as causing thickening of the cervical mucus. They also alter the endometrium to make it unfavorable for implantation and reduce the ciliary activity of the fallopian tube.
Strict adherence is important for effectiveness because progestin-only pills have a shorter half-life than combined hormonal contraceptives and also suppress ovulation less effectively.22 Failure rates are similar or somewhat higher than with combined hormonal contraceptives; with typical use, about 9 in 100 women can become pregnant in the first year.23 According to the US MEC,21 progestin-only pills are classified as category 2 for patients after both complicated and uncomplicated transplants.
MODERATELY EFFECTIVE METHODS (PREGNANCY RATE 10%–25%)
This tier of contraceptives includes all barrier methods, ie, male and female condoms, vaginal diaphragms, cervical caps, and sponges (Table 5).
Condoms (male and female)
When male condoms are used as the only birth control method, pregnancy occurs less often (18% with typical use and 2% with correct use) than with female condoms (21% with typical use and 5% with correct use).23 Male and female condoms are the only contraceptive methods that also prevent transmission of sexually transmitted disease.24
Caps, sponges, diaphragms
Cervical caps, vaginal sponges, and vaginal diaphragms are other forms of barrier contraceptives. All barrier methods should be combined with another contraceptive method to provide reliable protection against pregnancy. These methods are considered category 1 according to the US MEC.
LESS-EFFECTIVE METHODS
Fertility awareness-based methods such as the rhythm method have an associated pregnancy rate of about 25% with typical use and 3% to 5% with correct use23 and cannot be relied on for use by transplant recipients.24
Withdrawal and spermicides are considered least effective and unreliable for pregnancy prevention.
KNOW YOUR OPTIONS
With the growing number of women in their reproductive years receiving solid-organ transplants in the United States, it is increasingly important for healthcare providers to be aware of contraceptive options and reproductive life planning for this high-risk population.
Safe and effective forms of contraception are available, and additional information to guide the choice can be found in the Summary Chart of US MEC for Contraceptive Use, which is also available in a free smart phone app through the CDC.44
Pregnancy after transplant carries high risks, requiring these patients to have special counseling and monitoring. Fortunately, planned pregnancy at least 1 year after transplant can lead to successful outcomes in these women.
Increasing numbers of women of childbearing age are receiving solid-organ transplants. All need counseling on how to prevent pregnancy while they are taking immunosuppressive agents. Some want to become pregnant after their transplant and thus require counseling and follow-up to maintain good health during pregnancy (Table 1).1
Primary care physicians can assist with basic contraception counseling and pregnancy planning for their patients who have had solid-organ transplants. In this review, we describe contraceptive options and pregnancy planning for these women.
TRANSPLANTS IN WOMEN ARE INCREASING
Over the past 20 years, the number of solid-organ transplants in US women has increased steadily. Since 1988, 38% of the 634,000 transplants performed were in women, and 47% of these women were of childbearing age (ages 18 to 49).2 Kidneys accounted for 60% of solid-organ transplants,2 and kidney transplant is now commonly performed in women of childbearing age. In 2012, of 176,000 patients with a functioning renal graft, 40.5% were women, and recipients between ages 20 and 44 composed the second-largest age group.3
FERTILITY IN WOMEN WITH END-STAGE RENAL DISEASE
Women in their reproductive years who have end-stage renal disease have lower fertility rates than women in the general population. In women undergoing peritoneal dialysis or hemodialysis, conception rates decrease to around 0.5% per year.4 This lower rate is most likely related to hypothalamic-pituitary-gonadal dysfunction, leading to reduced or total impairment of ovulation, menstrual irregularities, and infertility.5
Fertility often returns within a few months after transplant,1,6 and reported posttransplant pregnancy rates range from 3.3% to 18%,7–9 with up to one-third of pregnancies being unintended.6,10 These numbers are likely an underestimate because they do not reflect all pregnancies that are terminated, as many women do not voluntarily report having had an abortion.
Fertility is also severely diminished in women with end-stage liver disease. After liver transplant, sex hormone levels return to normal for many women, and menses soon resume.11
In 2005, the National Transplantation Pregnancy Registry reported 1,418 pregnancies in 919 female recipients of solid-organ transplants. In 2010, this number had increased to 1,940 pregnancies in 1,185 recipients, of whom 75% were kidney transplant recipients.12
A successful pregnancy outcome is most likely when a minimum of 1 year intervenes between transplant and conception.12,13
TERATOGENICITY OF IMMUNOSUPPRESSANTS
Immunosuppressant drugs commonly used for maintenance therapy after solid-organ transplant include the following:
- Calcineurin inhibitors (eg, cyclosporine, tacrolimus)
- Antiproliferative and antimetabolite agents (eg, mycophenolate mofetil, azathioprine)
- Corticosteroids
- Mammalian target of rapamycin inhibitors (eg, sirolimus, everolimus)
- T-cell costimulation blockers (eg, belatacept).14
The US Food and Drug Administration (FDA) previously classified mycophenolate mofetil and azathioprine in pregnancy risk category D (positive evidence of human fetal risk). The teratogenic risk of mycophenolate mofetil is well established in studies documenting specific congenital malformations and fetal loss in the first trimester.13,15 The teratogenic risk of azathioprine, on the other hand, is estimated to be minimal to small.16 Many of the associated fetal abnormalities may be related to the complexity of the underlying medical condition of the mother rather than to the medication.16
In June 2015, the FDA’s new Pregnancy and Lactation Labeling Rule went into effect, which removes the pregnancy letter categories A, B, C, D, and X from labeling.17 This rule was designed to help providers counsel their patients regarding the specific risks and benefits of a drug when used by pregnant or nursing women. However, the ABCDX categories are still commonly used. Table 2 shows information about the risks during pregnancy and lactation posed by the immunosuppressive drugs commonly used by posttransplant patients.18
CRITERIA FOR A SUCCESSFUL PREGNANCY
To ensure a safe and successful pregnancy with the fewest fetal and maternal complications, women are generally advised to avoid pregnancy for at least 1 year after transplant.19,20
In addition, women should meet certain clinical prerequisites after transplant before they conceive, as outlined by the American Society of Transplantation.19,20 These include:
- No rejection within the previous year
- Adequate and stable graft function (eg, serum creatinine < 1.5 mg/dL and urinary protein excretion < 500 mg/24 hours)
- No acute infection that might affect the fetus
- Maintenance immunosuppression at stable dosages.
Other circumstances to consider include episodes of rejection in the first year after transplant (as evidenced by biopsy results or glomerular filtration rate), the woman’s age (advanced maternal age is unfavorable), or any history of noncompliance.
Every pregnancy in a transplant recipient must be carefully planned. Primary care providers should encourage patients to meet with their transplant team and obstetricians early and often to allow time for the care team to adjust the type and dosing of immunosuppressant drugs, to ensure stable graft function, and to optimize any current chronic medical conditions such as diabetes mellitus or hypertension before conception.
CONTRACEPTIVE COUNSELING AFTER TRANSPLANT
Pregnancy should be avoided while transplant patients are taking FDA category D immunosuppressant drugs and, as already mentioned, during the first year after transplant. Unintended pregnancy can have serious health consequences for the mother and the fetus, as well as poor pregnancy outcomes. The US Centers for Disease Control and Prevention (CDC) lists solid-organ transplant within the past 2 years as a condition that can lead to adverse events as a result of pregnancy.21 After a transplant, a woman’s risks from an unintended pregnancy are always greater than the risks from any contraceptive, and this is important to reinforce in counseling.
Two forms of reliable contraception should be used at all times, and consistent condom use should be encouraged as one of the methods. Condoms are not reliable when used as the sole contraceptive method because they have an 18% typical-use failure rate. However, they are an excellent adjunct to other contraceptive methods because they have the additional benefit of protecting against sexually transmitted disease.
Choosing the appropriate contraceptive method for recipients of solid-organ transplants can be challenging because of several factors, including the recipient’s preexisting medical problems and drug interactions of immunosuppressant medications.
CDC criteria and categories for contraceptive use
In 2010, the CDC released the US version of the Medical Eligibility Criteria (US MEC) for contraceptive use, which was based on the 2009 World Health Organization Medical Eligibility Criteria (WHO MEC); these criteria were revised in August 2016.21
- Category 1: A condition for which there is no restriction for the use of the contraceptive method
- Category 2: A condition for which the advantages of using the method generally outweigh the theoretical or proven risks
- Category 3: A condition for which the theoretical or proven risks usually outweigh the advantages of using the method
- Category 4: A condition that represents an unacceptable health risk if the contraceptive method is used.
These recommendations aimed to improve family planning options by clarifying the possible safe and effective contraceptive options available while considering the patient’s medical condition. The CDC added solid-organ transplant recipients to this document because of the prevalence of this group in the United States.
The CDC categorizes a patient’s medical condition after transplant as either complicated or uncomplicated. Complicated conditions include acute or chronic graft failure, graft rejection, and cardiac allograft vasculopathy.21
Effectiveness of contraceptive methods
Contraceptive methods can be divided into 4 categories based on estimated effectiveness, ie, the pregnancy rate with “typical use” of that particular method in 1 year21–23:
- Very effective (0%–0.9%)
- Effective (1%–9%)
- Moderately effective (10%–25%)
- Less effective (26%–32%).
Typical use refers to failure rates for women and men whose use is not consistent nor always correct. Correct use, also described in the sections that follow, refers to failure rates for those whose use is consistent and always correct.
Women should be counseled regarding all available contraceptive options that are medically suitable for them, so they can choose the method that best fits their needs and lifestyle. They should receive counseling on emergency contraception, barrier protection against sexually transmitted disease, and the correct use of the contraceptive method they choose. They should be advised that if their chosen contraceptive method is unsatisfactory for any reason, they can switch to another method. Most importantly, providers need to impress on their patients that the risks associated with unintended pregnancy are far greater than the risks from any of the contraceptive methods.
VERY EFFECTIVE CONTRACEPTIVES (UNINTENDED PREGNANCY RATE 0%–0.9%)
This tier of contraception is the most effective regardless of the patient’s adherence; it includes long-acting, reversible contraceptives and permanent sterilization (both male and female) (Table 3).21–23
Long-acting reversible contraceptives include intrauterine devices (IUDs) and the subdermal etonogestrel implant. Given their efficacy and favorable safety profile, long-acting reversible contraceptives are being promoted for use in women who have chronic medical conditions, such as transplants.24
Intrauterine devices
IUDs are long-acting and reversible. They can be used by women who are nulliparous and those of all ages, including adolescents.22
Two types of IUDs are available in the United States: nonhormonal (copper) and hormonal (levonorgestrel). The copper IUD is effective for at least 10 years, whereas the levonorgestrel IUDs last for 3 to 5 years.22
Four levonorgestrel IUDs are currently available in the United States. Their sizes and doses vary: Mirena (52 µg), Skyla (13.5 µg), Liletta (52 µg), and Kyleena (19.5 µg).
Fewer than 1% of women become pregnant in the first year of IUD use.22,23 IUDs are an ideal option for women with solid-organ transplants because they are so effective and because the patient does not have to do anything once the IUD is in.22–24 The levonorgestrel IUD Mirena has the additional advantage of reducing heavy menstrual bleeding and is currently the only hormonal IUD with FDA approval for the management of menorrhagia.
About 12% of women in the general population use IUDs as their contraceptive method of choice,25 whereas after solid-organ transplantation about 15% to 20% of women do.26
Two historic concerns regarding IUDs may explain their low rate of use in transplant recipients.
First, IUDs were believed to be less effective in women on immunosuppressive drugs because IUDs act by inducing a local inflammatory reaction. However, IUDs involve macrophage activation, which is independent of the immune processes modified by immunosuppressants (primarily T-cell function).27 A recent pilot study showed a strong inflammatory reaction in the endometrium of transplant recipients after levonorgestrel IUD insertion.28
Second, there was concern about the increased risk of pelvic inflammatory disease with IUDs, but studies have shown levonorgestrel IUDs to be safe in transplant patients.29,30
The CDC21 lists copper and levonorgestrel IUDs in MEC category 3 (the risks generally outweigh the advantages) for initiation in patients with complicated transplants and in category 2 (advantages generally outweigh the risks) in patients with uncomplicated organ transplants. The devices are in category 2 for both complicated and uncomplicated cases if the IUD is already in place.
Subdermal implant
A subdermal implant consisting of a single rod containing 68 mg of etonogestrel is commercially available in the United States. It is one of the most effective contraceptive methods, with the lowest rates of pregnancy—less than 1% per year, with protection lasting at least 3 years.22,23 This low risk makes the subdermal implant a suitable method of contraception after transplant. Daily compliance is not required, and there are no hepatic first-pass effects, which results in higher bioavailability and less chance of drug interactions.
The main disadvantage of the subdermal implant and IUDs is unscheduled bleeding. An important benefit is prolonged amenorrhea, not only for patient convenience, but for reduction of endometrial cancer risk. Insertion and removal of the implant are considered minor office procedures. The implants are classified as US MEC category 2 in uncomplicated cases; initiation in complicated cases is considered category 3 but continuation is considered category 2.21
Permanent sterilization
Permanent sterilization is another option for women and men. In women, the fallopian tubes can be occluded with a coil system implanted vaginally through a hysteroscope, or they can be severed, tied, or clamped in a laparoscopic procedure or during cesarean delivery. Pregnancy rates after tubal ligation are less than 1%,23,31 although concern exists for high failure rates with the hysteroscopic method.
Because younger patients are more likely than older patients to subsequently regret having the procedure done, all available contraceptive options should be discussed with them.31
For men, permanent sterilization is done by vasectomy, which has less associated risk and cost compared with sterilization for women.
EFFECTIVE CONTRACEPTIVE METHODS (UNINTENDED PREGNANCY RATE 1%–9%)
Effective contraceptive methods, the next tier down from very effective methods, include injectable contraceptives, combined hormonal contraceptives, and progestin-only contraceptives (Table 4).
Injectable contraceptives
Depot medroxyprogesterone acetate is an injectable progestin-only contraceptive that carries a pregnancy risk of 6% with typical use and less than 1% with correct use.23 Thus, some failures are due to patients not returning for follow-up, but in some patients this method is not effective. Injections are given intramuscularly once every 3 months, avoiding the need for daily use.
A valid concern for transplant patients is that medroxyprogesterone acetate reduces bone mineral density. Although the bone effects are reversible in healthy adult women, caution is needed when prescribing this option to transplant patients who are already at increased risk of bone disease attributable to renal osteodystrophy and chronic corticosteroid use. 32,33
Recently, a subcutaneous formulation of depot medroxyprogesterone acetate (104 mg)was added to the WHO MEC for contraceptive use.34,35 The recommendations for the subcutaneous form are similar to those for the intramuscular form. In healthy women, the subcutaneous formulation is as safe and effective as the intramuscular form,36 but its efficacy after solid-organ transplant has not been determined. Both forms of depot medroxyprogesterone acetate are category 2 in the US MEC for both complicated and uncomplicated transplant cases.21
Combined hormonal contraceptives
Combined hormonal contraceptives contain both estrogen and progesterone and are available as pills, patches, or rings. Each product has an unintended pregnancy risk of 9% with typical use and less than 1% with correct use.23 They require strict patient adherence to regular daily use, which likely explains their high failure rate with typical use.
Combined hormonal contraceptives reduce mortality risk in women in the general population,37 but their effect on mortality risk after transplant is unknown and needs further study. In women who received liver transplants, low-dose combined hormonal contraceptives have been found to be effective and well tolerated, but initiation should be delayed at least 6 months until postoperative organ stability is demonstrated.11
Combined oral contraceptives are the most widely prescribed because they are convenient and familiar and have an acceptable safety profile in transplant patients,11,33,37 despite their high failure rate with typical use. They regulate the menstrual cycle and reduce anemia associated with menstruation.
The transdermal contraceptive patch has a mechanism of action similar to that of the combined oral contraceptives, but it delivers estrogen and progesterone transdermally through the abdominal wall, thus avoiding first-pass metabolism in the liver and enzymatic degradation in the gut. It delivers 35 µg of ethinyl estradiol and 150 µg of norelgestromin (an active metabolite of norgestimate) daily.38 It may cause higher circulating levels of estrogen than a combined oral contraceptive and may be associated with a higher risk of venous thromboembolism, but the evidence is conflicting.39–42
The vaginal ring, made of Silastic, delivers ethinyl estradiol in a low dose (15 µg/day) and etonorgestrel 0.12 mg/day. Like the patch, it has the advantage of bypassing first-pass metabolism in the liver, making it a good option for transplant patients who are taking antirejection drugs, thus avoiding drug interactions.41
Both the transdermal patch and vaginal ring were studied in transplant patients and had favorable results.24,43 The combined hormonal oral contraceptive pills, patch, and ring are in category 4 (unacceptable health risk) in the US MEC in patients with complicated cases, but they are in category 2 in uncomplicated cases.21
Combined hormonal contraceptives should not be considered first-line options by themselves for transplant patients because of their high failure rate with typical use.24
Progestin-only pills
Although progestin-only pills have not been studied specifically in transplant patients, they can be considered for women who have contraindications to estrogen use. Estrogen use is contraindicated in women with a history of venous thromboembolism, thrombogenic mutations, estrogen-dependent neoplasia, hepatocellular adenoma, severe hypertension, vascular disease, and Budd-Chiari syndrome.
Progestin-only pills inhibit ovulation in only about half of a woman’s cycles, but they prevent conception by other mechanisms as well, such as causing thickening of the cervical mucus. They also alter the endometrium to make it unfavorable for implantation and reduce the ciliary activity of the fallopian tube.
Strict adherence is important for effectiveness because progestin-only pills have a shorter half-life than combined hormonal contraceptives and also suppress ovulation less effectively.22 Failure rates are similar or somewhat higher than with combined hormonal contraceptives; with typical use, about 9 in 100 women can become pregnant in the first year.23 According to the US MEC,21 progestin-only pills are classified as category 2 for patients after both complicated and uncomplicated transplants.
MODERATELY EFFECTIVE METHODS (PREGNANCY RATE 10%–25%)
This tier of contraceptives includes all barrier methods, ie, male and female condoms, vaginal diaphragms, cervical caps, and sponges (Table 5).
Condoms (male and female)
When male condoms are used as the only birth control method, pregnancy occurs less often (18% with typical use and 2% with correct use) than with female condoms (21% with typical use and 5% with correct use).23 Male and female condoms are the only contraceptive methods that also prevent transmission of sexually transmitted disease.24
Caps, sponges, diaphragms
Cervical caps, vaginal sponges, and vaginal diaphragms are other forms of barrier contraceptives. All barrier methods should be combined with another contraceptive method to provide reliable protection against pregnancy. These methods are considered category 1 according to the US MEC.
LESS-EFFECTIVE METHODS
Fertility awareness-based methods such as the rhythm method have an associated pregnancy rate of about 25% with typical use and 3% to 5% with correct use23 and cannot be relied on for use by transplant recipients.24
Withdrawal and spermicides are considered least effective and unreliable for pregnancy prevention.
KNOW YOUR OPTIONS
With the growing number of women in their reproductive years receiving solid-organ transplants in the United States, it is increasingly important for healthcare providers to be aware of contraceptive options and reproductive life planning for this high-risk population.
Safe and effective forms of contraception are available, and additional information to guide the choice can be found in the Summary Chart of US MEC for Contraceptive Use, which is also available in a free smart phone app through the CDC.44
Pregnancy after transplant carries high risks, requiring these patients to have special counseling and monitoring. Fortunately, planned pregnancy at least 1 year after transplant can lead to successful outcomes in these women.
- McKay DB, Josephson MA. Pregnancy in recipients of solid organs: effects on mother and child. N Engl J Med 2006; 354:1281–1293.
- US Department of Health and Human Services. Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/. Accessed July 17, 2017.
- United States Renal Data System. 2014 annual data report. https://www.usrds.org/2014/view/Default.aspx. Accessed July 17, 2017.
- Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 1999; 33:235–252.
- Josephson MA, McKay DB. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis 2013; 20:433–440.
- Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 2009; 9:1541–1549.
- Mohapatra A, Basu G. Pregnancy in kidney disease. Health Sciences 2012; 1(2). http://healthsciences.ac.in/july-sep-12/downloads/pregnancy_in_kidney_disease.pdf. Accessed July 25, 2017.
- Potluri K, Moldenhauer J, Karlman R, Hou S. Beta HCG levels in a pregnant dialysis patient: a cautionary tale. NDT Plus 2011; 4:42–43.
- Kennedy C, Hussein W, Spencer S, et al. Reproductive health in Irish female renal transplant recipients. Ir J Med Sci 2012; 181:59–63.
- Ghazizadeh S, Lessan-Pezeshki M, Khatami M, et al. Unwanted pregnancy among kidney transplant recipients in Iran. Transplant Proc 2005; 37:3085–3086.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 24:65–85.
- Mohamed-Ahmed O, Nelson-Piercy C, Bramham K, et al. Pregnancy outcomes in liver and cardiothoracic transplant recipients: a UK national cohort study. PLoS One 2014; 9:e89151.
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care 2015; 21(suppl 1):s12–s23.
- Hoeltzenbein M, Elefant E, Vial T, et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A 2012; 158A:588–596.
- Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 2002; 65:240–261.
- Dinatale M. The pregnancy and lactation labeling rule (PLLR). US Food and Drug Administration, 2016. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM520454.pdf. Accessed July 25, 2017.
- Lexicomp. http://online.lexi.com/lco/action/api/find/globalid/6612?utd=1. Accessed July 27, 2017.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(suppl 3):S1–S155.
- Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol 2013; 6:116–125.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2011; 118:184–196.
- Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404.
- Krajewski CM, Geetha D, Gomez-Lobo V. Contraceptive options for women with a history of solid-organ transplantation. Transplantation 2013; 95:1183–1186.
- Stern LF, Simons HR, Kohn JE, Debevec EJ, Morfesis JM, Patel AA. Differences in contraceptive use between family planning providers and the U.S. population: results of a nationwide survey. Contraception 2015; 91:464–469.
- Rafie S, Lai S, Garcia JE, Mody SK. Contraceptive use in female recipients of a solid-organ transplant. Prog Transplant 2014; 24:344–348.
- Labied S, Galant C, Nisolle M, et al. Differential elevation of matrix metalloproteinase expression in women exposed to levonorgestrel-releasing intrauterine system for a short or prolonged period of time. Hum Reprod 2009; 24:113–121.
- Kim CR, Martinez-Maza O, Magpantay L, et al. Immunologic evaluation of the endometrium with a levonorgestrel intrauterine device in solid organ transplant women and healthy controls. Contraception 2016; 94:534–540.
- Ramhendar T, Byrne P. Use of the levonorgestrel-releasing intrauterine system in renal transplant recipients: a retrospective case review. Contraception 2012; 86:288–289.
- Huguelet PS, Sheehan C, Spitzer RF, Scott S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: a case series. Contraception 2017; 95:378–381.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the US Collaborative Review of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007; 18:1319–1328.
- Krajewski C, Sucato G. Reproductive health care after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1222–1234.
- World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. http://apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf. Accessed July 27, 2017.
- Pietrzak B, Bobrowska K, Jabiry-Zieniewicz Z, et al. Oral and transdermal hormonal contraception in women after kidney transplantation. Transplant Proc 2007; 39:2759–2762.
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004; 70:269–275.
- Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet 2003; 362:185–191.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2006; 73:223–228.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- Estes CM, Westhoff C. Contraception for the transplant patient. Semin Perinatol 2007; 31:372–377.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Paternoster DM, Riboni F, Bertolino M, et al. The contraceptive vaginal ring in women with renal and liver transplantation: analysis of preliminary results. Transplant Proc 2010; 42:1162–1165.
- Centers for Disease Control and Prevention (CDC). Summary chart of US medical eligibility criteria for contraceptive use. https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf. Accessed July 17, 2017.
- McKay DB, Josephson MA. Pregnancy in recipients of solid organs: effects on mother and child. N Engl J Med 2006; 354:1281–1293.
- US Department of Health and Human Services. Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/. Accessed July 17, 2017.
- United States Renal Data System. 2014 annual data report. https://www.usrds.org/2014/view/Default.aspx. Accessed July 17, 2017.
- Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 1999; 33:235–252.
- Josephson MA, McKay DB. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis 2013; 20:433–440.
- Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 2009; 9:1541–1549.
- Mohapatra A, Basu G. Pregnancy in kidney disease. Health Sciences 2012; 1(2). http://healthsciences.ac.in/july-sep-12/downloads/pregnancy_in_kidney_disease.pdf. Accessed July 25, 2017.
- Potluri K, Moldenhauer J, Karlman R, Hou S. Beta HCG levels in a pregnant dialysis patient: a cautionary tale. NDT Plus 2011; 4:42–43.
- Kennedy C, Hussein W, Spencer S, et al. Reproductive health in Irish female renal transplant recipients. Ir J Med Sci 2012; 181:59–63.
- Ghazizadeh S, Lessan-Pezeshki M, Khatami M, et al. Unwanted pregnancy among kidney transplant recipients in Iran. Transplant Proc 2005; 37:3085–3086.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 24:65–85.
- Mohamed-Ahmed O, Nelson-Piercy C, Bramham K, et al. Pregnancy outcomes in liver and cardiothoracic transplant recipients: a UK national cohort study. PLoS One 2014; 9:e89151.
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care 2015; 21(suppl 1):s12–s23.
- Hoeltzenbein M, Elefant E, Vial T, et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A 2012; 158A:588–596.
- Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 2002; 65:240–261.
- Dinatale M. The pregnancy and lactation labeling rule (PLLR). US Food and Drug Administration, 2016. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM520454.pdf. Accessed July 25, 2017.
- Lexicomp. http://online.lexi.com/lco/action/api/find/globalid/6612?utd=1. Accessed July 27, 2017.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(suppl 3):S1–S155.
- Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol 2013; 6:116–125.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2011; 118:184–196.
- Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404.
- Krajewski CM, Geetha D, Gomez-Lobo V. Contraceptive options for women with a history of solid-organ transplantation. Transplantation 2013; 95:1183–1186.
- Stern LF, Simons HR, Kohn JE, Debevec EJ, Morfesis JM, Patel AA. Differences in contraceptive use between family planning providers and the U.S. population: results of a nationwide survey. Contraception 2015; 91:464–469.
- Rafie S, Lai S, Garcia JE, Mody SK. Contraceptive use in female recipients of a solid-organ transplant. Prog Transplant 2014; 24:344–348.
- Labied S, Galant C, Nisolle M, et al. Differential elevation of matrix metalloproteinase expression in women exposed to levonorgestrel-releasing intrauterine system for a short or prolonged period of time. Hum Reprod 2009; 24:113–121.
- Kim CR, Martinez-Maza O, Magpantay L, et al. Immunologic evaluation of the endometrium with a levonorgestrel intrauterine device in solid organ transplant women and healthy controls. Contraception 2016; 94:534–540.
- Ramhendar T, Byrne P. Use of the levonorgestrel-releasing intrauterine system in renal transplant recipients: a retrospective case review. Contraception 2012; 86:288–289.
- Huguelet PS, Sheehan C, Spitzer RF, Scott S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: a case series. Contraception 2017; 95:378–381.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the US Collaborative Review of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007; 18:1319–1328.
- Krajewski C, Sucato G. Reproductive health care after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1222–1234.
- World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. http://apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf. Accessed July 27, 2017.
- Pietrzak B, Bobrowska K, Jabiry-Zieniewicz Z, et al. Oral and transdermal hormonal contraception in women after kidney transplantation. Transplant Proc 2007; 39:2759–2762.
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004; 70:269–275.
- Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet 2003; 362:185–191.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2006; 73:223–228.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- Estes CM, Westhoff C. Contraception for the transplant patient. Semin Perinatol 2007; 31:372–377.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Paternoster DM, Riboni F, Bertolino M, et al. The contraceptive vaginal ring in women with renal and liver transplantation: analysis of preliminary results. Transplant Proc 2010; 42:1162–1165.
- Centers for Disease Control and Prevention (CDC). Summary chart of US medical eligibility criteria for contraceptive use. https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf. Accessed July 17, 2017.
KEY POINTS
- The number of solid-organ transplants in US women of childbearing age has increased over the past 20 years.
- Women should wait at least 1 year after receiving a solid-organ transplant before attempting to become pregnant, and then should do so only when cleared by the transplant team and obstetrician, with close monitoring.
- The various types of contraception can be grouped by their effectiveness and by the medical eligibility criteria set by the US Centers for Disease Control and Prevention.
- Transplant recipients of childbearing age should use 2 contraceptive methods concurrently, one of which should be condoms.