User login
Acute Kidney Injury in the ICU: Medication Dosing
Q: As a hospitalist, I often see patients in our ICU develop AKI. Our pharmacist helps us with medication dosing, but sometimes I feel as if we're pulling a dose out of the air. Are there any studies or guidelines we can refer to?
Standard medication dosing adjustments for patients with impaired renal function are generally based on estimated glomerular filtration rate (eGFR). Because SCr is a lagging indicator of AKI, all methods of deriving eGFR from SCr are valid only when the patient is in a steady state.10
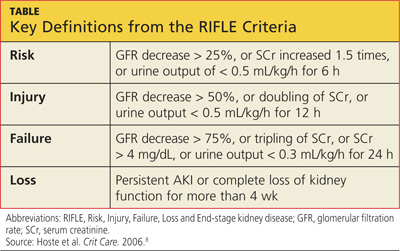
SCr has yet to be replaced by a real-time biomarker for AKI; this has left clinicians in the ICU setting with no simple or concise method for real-time assessment of renal function. In response to this common clinical conundrum, the RIFLE criteria,8 mentioned above, incorporates urinary output and relative increase in SCr as assessment criteria (see table for definitions).
This revised classification system may help the clinician define the severity of AKI in the acute setting. However, no medication dosing guidelines currently correspond with RIFLE staging. To further complicate the picture, there is evidence to suggest that AKI may affect drug metabolism through nonrenal pathways, such as hepatic clearance and transport functions.11 Add to this the potential for impaired drug absorption, distribution, and/or clearance due to variance in intravascular volume status, hepatic hypoperfusion, hypoxia, decreased protein synthesis, and competitive inhibition from concomitant medications—in short, the variables become too complex for calculating therapeutic drug dosing to be possible.
In the absence of definitive guidelines, the clinician plays a critical role in medication dosing adjustment for the ICU patient with AKI. The clinician must use astute clinical judgment to assess and prioritize the unique constellation of factors in any given case. Some of the factors that should be carefully considered when estimating medication dose adjustments in this context include RIFLE staging, trend in SCr, baseline SCr, nephrotoxicity of the medication to be administered, the drug's volume of distribution, the metabolic pathways of drug excretion, and the patient's weight.
A serum drug level, when available, is generally the best guide for dosing adjustment.10 The RIFLE staging does offer some clinical pearls that may be helpful. Though not evidence-based recommendations, these guides are commonly used in the clinical environment.
When patients are in the Failure stage, for example (see specifics in the table), they are generally considered to have an eGFR of less than 15 mL/min for purposes of drug dose adjustment (personal communication, Gideon Kayanan, PharmD, February 2013). However, patients in this category are much more likely than others to be undergoing dialysis, in which case the pharmacokinetics and pharmacodynamics are further complicated. In some cases, it may be appropriate to order creatinine clearance studies with a 6- or 12-hour urine collection and extrapolate a 24-hour creatinine clearance from this value.
The dearth of literature addressing this topic (despite the prevalence of AKI in the acute care setting) is a clear indication of the complexity of creating guidelines to address such a dynamic, multivariate pharmacokinetic process. Review of the literature clearly demonstrates that medical science in this area is not yet sufficiently developed to produce a standardized, data-driven guideline for dose adjustment calculation in patients with AKI.10 Until biomarkers are detected that offer real-time assessment of renal function and that can be used in the clinical setting, there will continue to be a component of estimation, analysis of trends, and reliance on clinical judgment in adjusting medication doses for inpatients with AKI. —AC
References
1. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2010 Annual Data Report. www.usrds.org/2010/view/default.asp. Accessed March 5, 2013.
2. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2011 Annual Data Report. www.usrds.org/2011/view/v2_00_appx.asp. Accessed March 5, 2013.
3. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844-861.
4. Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370.
5. Hsu RK, McCulloch CE, Dudley RA, et al. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2012;24:37-42.
6. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35:349-355.
7. Nisula S, Kaukonen KM, Vaara ST, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420-428.
8. Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73.
9. Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961-973.
10. Matzke GR, Aronoff GR, Atkinson AJ Jr, et al. Drug dosing consideration in patients with acute and chronic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:1122-1137.
11. Vilay AM, Churchwell MD, Mueller BA. Clinical review: drug metabolism and nonrenal clearance in acute kidney injury. Crit Care. 2008;12:235.
Q: As a hospitalist, I often see patients in our ICU develop AKI. Our pharmacist helps us with medication dosing, but sometimes I feel as if we're pulling a dose out of the air. Are there any studies or guidelines we can refer to?
Standard medication dosing adjustments for patients with impaired renal function are generally based on estimated glomerular filtration rate (eGFR). Because SCr is a lagging indicator of AKI, all methods of deriving eGFR from SCr are valid only when the patient is in a steady state.10
SCr has yet to be replaced by a real-time biomarker for AKI; this has left clinicians in the ICU setting with no simple or concise method for real-time assessment of renal function. In response to this common clinical conundrum, the RIFLE criteria,8 mentioned above, incorporates urinary output and relative increase in SCr as assessment criteria (see table for definitions).
This revised classification system may help the clinician define the severity of AKI in the acute setting. However, no medication dosing guidelines currently correspond with RIFLE staging. To further complicate the picture, there is evidence to suggest that AKI may affect drug metabolism through nonrenal pathways, such as hepatic clearance and transport functions.11 Add to this the potential for impaired drug absorption, distribution, and/or clearance due to variance in intravascular volume status, hepatic hypoperfusion, hypoxia, decreased protein synthesis, and competitive inhibition from concomitant medications—in short, the variables become too complex for calculating therapeutic drug dosing to be possible.
In the absence of definitive guidelines, the clinician plays a critical role in medication dosing adjustment for the ICU patient with AKI. The clinician must use astute clinical judgment to assess and prioritize the unique constellation of factors in any given case. Some of the factors that should be carefully considered when estimating medication dose adjustments in this context include RIFLE staging, trend in SCr, baseline SCr, nephrotoxicity of the medication to be administered, the drug's volume of distribution, the metabolic pathways of drug excretion, and the patient's weight.
A serum drug level, when available, is generally the best guide for dosing adjustment.10 The RIFLE staging does offer some clinical pearls that may be helpful. Though not evidence-based recommendations, these guides are commonly used in the clinical environment.
When patients are in the Failure stage, for example (see specifics in the table), they are generally considered to have an eGFR of less than 15 mL/min for purposes of drug dose adjustment (personal communication, Gideon Kayanan, PharmD, February 2013). However, patients in this category are much more likely than others to be undergoing dialysis, in which case the pharmacokinetics and pharmacodynamics are further complicated. In some cases, it may be appropriate to order creatinine clearance studies with a 6- or 12-hour urine collection and extrapolate a 24-hour creatinine clearance from this value.

The dearth of literature addressing this topic (despite the prevalence of AKI in the acute care setting) is a clear indication of the complexity of creating guidelines to address such a dynamic, multivariate pharmacokinetic process. Review of the literature clearly demonstrates that medical science in this area is not yet sufficiently developed to produce a standardized, data-driven guideline for dose adjustment calculation in patients with AKI.10 Until biomarkers are detected that offer real-time assessment of renal function and that can be used in the clinical setting, there will continue to be a component of estimation, analysis of trends, and reliance on clinical judgment in adjusting medication doses for inpatients with AKI. —AC
References
1. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2010 Annual Data Report. www.usrds.org/2010/view/default.asp. Accessed March 5, 2013.
2. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2011 Annual Data Report. www.usrds.org/2011/view/v2_00_appx.asp. Accessed March 5, 2013.
3. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844-861.
4. Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370.
5. Hsu RK, McCulloch CE, Dudley RA, et al. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2012;24:37-42.
6. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35:349-355.
7. Nisula S, Kaukonen KM, Vaara ST, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420-428.
8. Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73.
9. Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961-973.
10. Matzke GR, Aronoff GR, Atkinson AJ Jr, et al. Drug dosing consideration in patients with acute and chronic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:1122-1137.
11. Vilay AM, Churchwell MD, Mueller BA. Clinical review: drug metabolism and nonrenal clearance in acute kidney injury. Crit Care. 2008;12:235.
Q: As a hospitalist, I often see patients in our ICU develop AKI. Our pharmacist helps us with medication dosing, but sometimes I feel as if we're pulling a dose out of the air. Are there any studies or guidelines we can refer to?
Standard medication dosing adjustments for patients with impaired renal function are generally based on estimated glomerular filtration rate (eGFR). Because SCr is a lagging indicator of AKI, all methods of deriving eGFR from SCr are valid only when the patient is in a steady state.10
SCr has yet to be replaced by a real-time biomarker for AKI; this has left clinicians in the ICU setting with no simple or concise method for real-time assessment of renal function. In response to this common clinical conundrum, the RIFLE criteria,8 mentioned above, incorporates urinary output and relative increase in SCr as assessment criteria (see table for definitions).
This revised classification system may help the clinician define the severity of AKI in the acute setting. However, no medication dosing guidelines currently correspond with RIFLE staging. To further complicate the picture, there is evidence to suggest that AKI may affect drug metabolism through nonrenal pathways, such as hepatic clearance and transport functions.11 Add to this the potential for impaired drug absorption, distribution, and/or clearance due to variance in intravascular volume status, hepatic hypoperfusion, hypoxia, decreased protein synthesis, and competitive inhibition from concomitant medications—in short, the variables become too complex for calculating therapeutic drug dosing to be possible.
In the absence of definitive guidelines, the clinician plays a critical role in medication dosing adjustment for the ICU patient with AKI. The clinician must use astute clinical judgment to assess and prioritize the unique constellation of factors in any given case. Some of the factors that should be carefully considered when estimating medication dose adjustments in this context include RIFLE staging, trend in SCr, baseline SCr, nephrotoxicity of the medication to be administered, the drug's volume of distribution, the metabolic pathways of drug excretion, and the patient's weight.
A serum drug level, when available, is generally the best guide for dosing adjustment.10 The RIFLE staging does offer some clinical pearls that may be helpful. Though not evidence-based recommendations, these guides are commonly used in the clinical environment.
When patients are in the Failure stage, for example (see specifics in the table), they are generally considered to have an eGFR of less than 15 mL/min for purposes of drug dose adjustment (personal communication, Gideon Kayanan, PharmD, February 2013). However, patients in this category are much more likely than others to be undergoing dialysis, in which case the pharmacokinetics and pharmacodynamics are further complicated. In some cases, it may be appropriate to order creatinine clearance studies with a 6- or 12-hour urine collection and extrapolate a 24-hour creatinine clearance from this value.

The dearth of literature addressing this topic (despite the prevalence of AKI in the acute care setting) is a clear indication of the complexity of creating guidelines to address such a dynamic, multivariate pharmacokinetic process. Review of the literature clearly demonstrates that medical science in this area is not yet sufficiently developed to produce a standardized, data-driven guideline for dose adjustment calculation in patients with AKI.10 Until biomarkers are detected that offer real-time assessment of renal function and that can be used in the clinical setting, there will continue to be a component of estimation, analysis of trends, and reliance on clinical judgment in adjusting medication doses for inpatients with AKI. —AC
References
1. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2010 Annual Data Report. www.usrds.org/2010/view/default.asp. Accessed March 5, 2013.
2. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2011 Annual Data Report. www.usrds.org/2011/view/v2_00_appx.asp. Accessed March 5, 2013.
3. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844-861.
4. Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370.
5. Hsu RK, McCulloch CE, Dudley RA, et al. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2012;24:37-42.
6. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35:349-355.
7. Nisula S, Kaukonen KM, Vaara ST, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420-428.
8. Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73.
9. Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961-973.
10. Matzke GR, Aronoff GR, Atkinson AJ Jr, et al. Drug dosing consideration in patients with acute and chronic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:1122-1137.
11. Vilay AM, Churchwell MD, Mueller BA. Clinical review: drug metabolism and nonrenal clearance in acute kidney injury. Crit Care. 2008;12:235.
Acute Kidney Injury in the ICU: Increasing Prevalence
Q: In 10 years as a hospitalist advanced practitioner, I've been seeing more and more AKI in our ICU. Is this true everywhere, or are we doing something wrong?
AKI is on the rise nationwide (see hospitalization data in figure), and it carries grim implications for patient outcomes.1-3 AKI with a rise in serum creatinine (SCr) as modest as 0.3 mg/dL is associated with a 70% increase in mortality risk. A rise in SCr exceeding 0.5 mg/dL has been associated with a 6.5-fold rise in the risk for death, even when adjusted for age and gender.4 This is higher than the mortality rate for inpatients admitted with cardiovascular disease or cancer, and just slightly more favorable than the mortality risk associated with sepsis (odds ratios, 6.6 and 7.5, respectively). AKI management in the non-ICU setting incurs the third highest median direct hospital cost, after acute MI and stroke.3
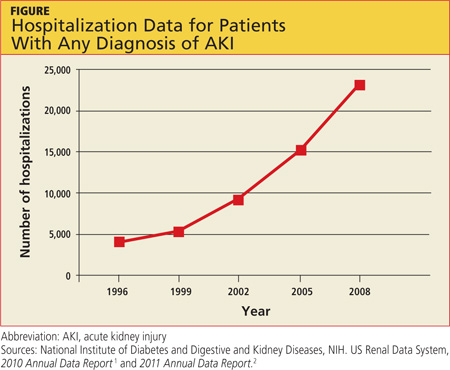
A recent retrospective analysis of hospital admissions nationwide from 2000 to 2009 shows a 10% annual increase in the incidence of AKI requiring dialysis, with at least doubling of the incidence and the number of deaths during that 10-year time period.5 Analyzing the incidence of AKI not requiring dialysis over time is more challenging because the criteria to define AKI have not been static; however, the rise in AKI requiring dialysis has mirrored the rise in AKI not requiring dialysis—suggesting that there is in fact an increased incidence of AKI, independent of variability in the defining criteria.3
Researchers reported in 2012 that during the previous year, the incidence of AKI among all hospitalized patients was 1 in 5.6 In the ICU, incidence of AKI has been reported at 39%, with a mortality rate of 25%.7 Based on the RIFLE criteria (a recently revised classification system whose name refers to Risk, Injury, Failure; Loss and End-stage kidney disease),8 as many as two-thirds of patients admitted to the ICU meet criteria for a diagnosis of AKI.

Predictors for AKI include advancing age, baseline SCr below 1.2 mg/dL, the presence of diabetes, use of IV contrast, acute coronary syndromes, sepsis, liver or heart failure, and use of nephrotoxic medications.3
It is important for clinicians to recognize the implications of AKI, even when it manifests as a relatively minor rise in SCr. In addition to its association with poor outcomes in hospitalized patients, AKI increases the risk for chronic kidney disease and for readmissions within six months after hospital discharge.9 Unfortunately, our increased awareness of the implications of AKI in the inpatient setting has yet to translate into significant improvement in outcomes.
The evolution and availability of epidemiologic and outcome data, we can only hope, will serve to direct more resources and further study toward this issue. Clinicians' efforts to prevent and treat AKI can have profound implications for many of our nation's most chronically and critically ill patients. —AC
REFERENCES
1. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2010 Annual Data Report. www.usrds.org/2010/view/default.asp. Accessed March 5, 2013.
2. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2011 Annual Data Report. www.usrds.org/2011/view/v2_00_appx.asp. Accessed March 5, 2013.
3. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844-861.
4. Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370.
5. Hsu RK, McCulloch CE, Dudley RA, et al. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2012;24:37-42.
6. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35:349-355.
7. Nisula S, Kaukonen KM, Vaara ST, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420-428.
8. Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73.
9. Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961-973.
10. Matzke GR, Aronoff GR, Atkinson AJ Jr, et al. Drug dosing consideration in patients with acute and chronic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:1122-1137.
11. Vilay AM, Churchwell MD, Mueller BA. Clinical review: drug metabolism and nonrenal clearance in acute kidney injury. Crit Care. 2008;12:235.
Q: In 10 years as a hospitalist advanced practitioner, I've been seeing more and more AKI in our ICU. Is this true everywhere, or are we doing something wrong?
AKI is on the rise nationwide (see hospitalization data in figure), and it carries grim implications for patient outcomes.1-3 AKI with a rise in serum creatinine (SCr) as modest as 0.3 mg/dL is associated with a 70% increase in mortality risk. A rise in SCr exceeding 0.5 mg/dL has been associated with a 6.5-fold rise in the risk for death, even when adjusted for age and gender.4 This is higher than the mortality rate for inpatients admitted with cardiovascular disease or cancer, and just slightly more favorable than the mortality risk associated with sepsis (odds ratios, 6.6 and 7.5, respectively). AKI management in the non-ICU setting incurs the third highest median direct hospital cost, after acute MI and stroke.3

A recent retrospective analysis of hospital admissions nationwide from 2000 to 2009 shows a 10% annual increase in the incidence of AKI requiring dialysis, with at least doubling of the incidence and the number of deaths during that 10-year time period.5 Analyzing the incidence of AKI not requiring dialysis over time is more challenging because the criteria to define AKI have not been static; however, the rise in AKI requiring dialysis has mirrored the rise in AKI not requiring dialysis—suggesting that there is in fact an increased incidence of AKI, independent of variability in the defining criteria.3
Researchers reported in 2012 that during the previous year, the incidence of AKI among all hospitalized patients was 1 in 5.6 In the ICU, incidence of AKI has been reported at 39%, with a mortality rate of 25%.7 Based on the RIFLE criteria (a recently revised classification system whose name refers to Risk, Injury, Failure; Loss and End-stage kidney disease),8 as many as two-thirds of patients admitted to the ICU meet criteria for a diagnosis of AKI.

Predictors for AKI include advancing age, baseline SCr below 1.2 mg/dL, the presence of diabetes, use of IV contrast, acute coronary syndromes, sepsis, liver or heart failure, and use of nephrotoxic medications.3
It is important for clinicians to recognize the implications of AKI, even when it manifests as a relatively minor rise in SCr. In addition to its association with poor outcomes in hospitalized patients, AKI increases the risk for chronic kidney disease and for readmissions within six months after hospital discharge.9 Unfortunately, our increased awareness of the implications of AKI in the inpatient setting has yet to translate into significant improvement in outcomes.
The evolution and availability of epidemiologic and outcome data, we can only hope, will serve to direct more resources and further study toward this issue. Clinicians' efforts to prevent and treat AKI can have profound implications for many of our nation's most chronically and critically ill patients. —AC
REFERENCES
1. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2010 Annual Data Report. www.usrds.org/2010/view/default.asp. Accessed March 5, 2013.
2. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2011 Annual Data Report. www.usrds.org/2011/view/v2_00_appx.asp. Accessed March 5, 2013.
3. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844-861.
4. Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370.
5. Hsu RK, McCulloch CE, Dudley RA, et al. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2012;24:37-42.
6. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35:349-355.
7. Nisula S, Kaukonen KM, Vaara ST, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420-428.
8. Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73.
9. Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961-973.
10. Matzke GR, Aronoff GR, Atkinson AJ Jr, et al. Drug dosing consideration in patients with acute and chronic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:1122-1137.
11. Vilay AM, Churchwell MD, Mueller BA. Clinical review: drug metabolism and nonrenal clearance in acute kidney injury. Crit Care. 2008;12:235.
Q: In 10 years as a hospitalist advanced practitioner, I've been seeing more and more AKI in our ICU. Is this true everywhere, or are we doing something wrong?
AKI is on the rise nationwide (see hospitalization data in figure), and it carries grim implications for patient outcomes.1-3 AKI with a rise in serum creatinine (SCr) as modest as 0.3 mg/dL is associated with a 70% increase in mortality risk. A rise in SCr exceeding 0.5 mg/dL has been associated with a 6.5-fold rise in the risk for death, even when adjusted for age and gender.4 This is higher than the mortality rate for inpatients admitted with cardiovascular disease or cancer, and just slightly more favorable than the mortality risk associated with sepsis (odds ratios, 6.6 and 7.5, respectively). AKI management in the non-ICU setting incurs the third highest median direct hospital cost, after acute MI and stroke.3

A recent retrospective analysis of hospital admissions nationwide from 2000 to 2009 shows a 10% annual increase in the incidence of AKI requiring dialysis, with at least doubling of the incidence and the number of deaths during that 10-year time period.5 Analyzing the incidence of AKI not requiring dialysis over time is more challenging because the criteria to define AKI have not been static; however, the rise in AKI requiring dialysis has mirrored the rise in AKI not requiring dialysis—suggesting that there is in fact an increased incidence of AKI, independent of variability in the defining criteria.3
Researchers reported in 2012 that during the previous year, the incidence of AKI among all hospitalized patients was 1 in 5.6 In the ICU, incidence of AKI has been reported at 39%, with a mortality rate of 25%.7 Based on the RIFLE criteria (a recently revised classification system whose name refers to Risk, Injury, Failure; Loss and End-stage kidney disease),8 as many as two-thirds of patients admitted to the ICU meet criteria for a diagnosis of AKI.

Predictors for AKI include advancing age, baseline SCr below 1.2 mg/dL, the presence of diabetes, use of IV contrast, acute coronary syndromes, sepsis, liver or heart failure, and use of nephrotoxic medications.3
It is important for clinicians to recognize the implications of AKI, even when it manifests as a relatively minor rise in SCr. In addition to its association with poor outcomes in hospitalized patients, AKI increases the risk for chronic kidney disease and for readmissions within six months after hospital discharge.9 Unfortunately, our increased awareness of the implications of AKI in the inpatient setting has yet to translate into significant improvement in outcomes.
The evolution and availability of epidemiologic and outcome data, we can only hope, will serve to direct more resources and further study toward this issue. Clinicians' efforts to prevent and treat AKI can have profound implications for many of our nation's most chronically and critically ill patients. —AC
REFERENCES
1. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2010 Annual Data Report. www.usrds.org/2010/view/default.asp. Accessed March 5, 2013.
2. National Institute of Diabetes and Digestive and Kidney Diseases, NIH. US Renal Data System, 2011 Annual Data Report. www.usrds.org/2011/view/v2_00_appx.asp. Accessed March 5, 2013.
3. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844-861.
4. Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370.
5. Hsu RK, McCulloch CE, Dudley RA, et al. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2012;24:37-42.
6. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35:349-355.
7. Nisula S, Kaukonen KM, Vaara ST, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420-428.
8. Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73.
9. Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961-973.
10. Matzke GR, Aronoff GR, Atkinson AJ Jr, et al. Drug dosing consideration in patients with acute and chronic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:1122-1137.
11. Vilay AM, Churchwell MD, Mueller BA. Clinical review: drug metabolism and nonrenal clearance in acute kidney injury. Crit Care. 2008;12:235.
Acute Kidney Injury in an Unexpected Patient Population
Q: When I was doing sports physicals at the high school this week, several students asked if it was true that marijuana causes kidney failure. I had not heard this. Is it true? What should I tell teens who ask about this?
Synthetic marijuana, which goes by the street names of Spice, K2, Black Mamba, Fake Weed, Genie, and Zohai, is a mixture of herbs and spices that is sprayed with a synthetic THC-type compound.1 These can be sold over the Internet as "incense" or "bath salts." However, as is often the case with drugs purchased online or from a neighborhood dealer, other compounds toxic to humans can be cut and mixed in with these substances. While hypertension, nausea, cognitive dysfunction, and dizziness have all been associated with Spice, there has been a recent flurry of reports of severe and lasting cardiac and renal damage following use of these drugs.
In 2011, three cases of Spice-associated acute coronary syndrome were reported in the pediatric literature.2 In late 2012, four residents of the same Alabama community developed AKI after using Spice. While all four eventually recovered kidney function, they now have some permanent chronic kidney damage, and all four patients required kidney biopsies.3 Similarly, the CDC recently reported 14 cases of AKI in Wyoming that developed in patients who had smoked Spice.4 Six cases were reported from Oregon, two each from New York and Oklahoma, and one each from Rhode Island and Kansas. Half of the case patients required hemodialysis and kidney biopsy. All had residual chronic kidney disease after recovery.4
The patients' presentations were similar: they were all young and healthy with no history of kidney problems—then, wham! After they had smoked Spice, severe nausea and vomiting with flank pain took them to the ER. On admission, serum creatinine (SCr) was mildly abnormal, but it rose to an average of 8 mg/dL, with one patient's SCr peaking at 21 mg/dL.4
While there have been no Spice-associated deaths reported, the critical care needed for these young people included hemodialysis. Perhaps a graphic description of the standard 15-gauge needles we use for dialysis would be helpful during a discussion of drug use with teens.
Kim Zuber, PA-C, MSPS, DFAAPA, Metropolitan Nephrology, Alexandria, VA, and Clinton, MD
REFERENCES
1. US Drug Enforcement Administration. Drug Fact Sheet: K2 or Spice. www.justice.gov/dea/druginfo/drug_data_sheets/K2_Spice.pdf. Accessed March 15, 2013.
2. Mir A, Obafemi A, Young A, Kane C. Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics. 2011;128:e1622-e1627.
3. Bhanushali GK, Jain G, Fatima H, et al. AKI associated with synthetic cannabinoids: a case series. Clin J Am Soc Nephrol. 2012 Dec 14. [Epub ahead of print]
4. CDC. Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:93-98.
Q: When I was doing sports physicals at the high school this week, several students asked if it was true that marijuana causes kidney failure. I had not heard this. Is it true? What should I tell teens who ask about this?
Synthetic marijuana, which goes by the street names of Spice, K2, Black Mamba, Fake Weed, Genie, and Zohai, is a mixture of herbs and spices that is sprayed with a synthetic THC-type compound.1 These can be sold over the Internet as "incense" or "bath salts." However, as is often the case with drugs purchased online or from a neighborhood dealer, other compounds toxic to humans can be cut and mixed in with these substances. While hypertension, nausea, cognitive dysfunction, and dizziness have all been associated with Spice, there has been a recent flurry of reports of severe and lasting cardiac and renal damage following use of these drugs.
In 2011, three cases of Spice-associated acute coronary syndrome were reported in the pediatric literature.2 In late 2012, four residents of the same Alabama community developed AKI after using Spice. While all four eventually recovered kidney function, they now have some permanent chronic kidney damage, and all four patients required kidney biopsies.3 Similarly, the CDC recently reported 14 cases of AKI in Wyoming that developed in patients who had smoked Spice.4 Six cases were reported from Oregon, two each from New York and Oklahoma, and one each from Rhode Island and Kansas. Half of the case patients required hemodialysis and kidney biopsy. All had residual chronic kidney disease after recovery.4
The patients' presentations were similar: they were all young and healthy with no history of kidney problems—then, wham! After they had smoked Spice, severe nausea and vomiting with flank pain took them to the ER. On admission, serum creatinine (SCr) was mildly abnormal, but it rose to an average of 8 mg/dL, with one patient's SCr peaking at 21 mg/dL.4
While there have been no Spice-associated deaths reported, the critical care needed for these young people included hemodialysis. Perhaps a graphic description of the standard 15-gauge needles we use for dialysis would be helpful during a discussion of drug use with teens.
Kim Zuber, PA-C, MSPS, DFAAPA, Metropolitan Nephrology, Alexandria, VA, and Clinton, MD
REFERENCES
1. US Drug Enforcement Administration. Drug Fact Sheet: K2 or Spice. www.justice.gov/dea/druginfo/drug_data_sheets/K2_Spice.pdf. Accessed March 15, 2013.
2. Mir A, Obafemi A, Young A, Kane C. Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics. 2011;128:e1622-e1627.
3. Bhanushali GK, Jain G, Fatima H, et al. AKI associated with synthetic cannabinoids: a case series. Clin J Am Soc Nephrol. 2012 Dec 14. [Epub ahead of print]
4. CDC. Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:93-98.
Q: When I was doing sports physicals at the high school this week, several students asked if it was true that marijuana causes kidney failure. I had not heard this. Is it true? What should I tell teens who ask about this?
Synthetic marijuana, which goes by the street names of Spice, K2, Black Mamba, Fake Weed, Genie, and Zohai, is a mixture of herbs and spices that is sprayed with a synthetic THC-type compound.1 These can be sold over the Internet as "incense" or "bath salts." However, as is often the case with drugs purchased online or from a neighborhood dealer, other compounds toxic to humans can be cut and mixed in with these substances. While hypertension, nausea, cognitive dysfunction, and dizziness have all been associated with Spice, there has been a recent flurry of reports of severe and lasting cardiac and renal damage following use of these drugs.
In 2011, three cases of Spice-associated acute coronary syndrome were reported in the pediatric literature.2 In late 2012, four residents of the same Alabama community developed AKI after using Spice. While all four eventually recovered kidney function, they now have some permanent chronic kidney damage, and all four patients required kidney biopsies.3 Similarly, the CDC recently reported 14 cases of AKI in Wyoming that developed in patients who had smoked Spice.4 Six cases were reported from Oregon, two each from New York and Oklahoma, and one each from Rhode Island and Kansas. Half of the case patients required hemodialysis and kidney biopsy. All had residual chronic kidney disease after recovery.4
The patients' presentations were similar: they were all young and healthy with no history of kidney problems—then, wham! After they had smoked Spice, severe nausea and vomiting with flank pain took them to the ER. On admission, serum creatinine (SCr) was mildly abnormal, but it rose to an average of 8 mg/dL, with one patient's SCr peaking at 21 mg/dL.4
While there have been no Spice-associated deaths reported, the critical care needed for these young people included hemodialysis. Perhaps a graphic description of the standard 15-gauge needles we use for dialysis would be helpful during a discussion of drug use with teens.
Kim Zuber, PA-C, MSPS, DFAAPA, Metropolitan Nephrology, Alexandria, VA, and Clinton, MD
REFERENCES
1. US Drug Enforcement Administration. Drug Fact Sheet: K2 or Spice. www.justice.gov/dea/druginfo/drug_data_sheets/K2_Spice.pdf. Accessed March 15, 2013.
2. Mir A, Obafemi A, Young A, Kane C. Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics. 2011;128:e1622-e1627.
3. Bhanushali GK, Jain G, Fatima H, et al. AKI associated with synthetic cannabinoids: a case series. Clin J Am Soc Nephrol. 2012 Dec 14. [Epub ahead of print]
4. CDC. Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:93-98.
NSAIDs for BPH
Benign prostatic hyperplasia is an uncommon cause of mortality but a common cause of morbidity.
More than 80% of men aged older than 80 years have histologic evidence of benign prostatic hyperplasia (BPH). As most of us know, the most common symptoms of BPH are urinary frequency, nocturia, hesitancy, and weak urine stream. But it seems like nocturia is the source of most complaints in my panel.
Interestingly, many men will experience stabilization or improvement over time without therapy. We likely do not remember this, because men who present to us are not typically enamored with the idea of "watchful waiting" when they are up all night standing at the latrine. But in fact, 38% of men will have symptom improvement over 2.6 to 5 years of follow-up without intervention.
Treatment is symptom driven. Treatment modality selections are cost and convenience driven. Many of us will reach for an alpha-adrenergic antagonist or a 5-alpha-reductase inhibitor as first-line therapy for patients with mild but annoying symptoms.
But how many of us consider NSAIDs in this setting?
Dr. Arman Kahokehr and colleagues conducted a systematic review of the literature examining the effects of NSAIDs in the treatment of men with BPH. Trials were included if they were randomized and included objective outcomes such as urologic symptom scales or urodynamics (BJU Int. 2013;111:304-11).
Three randomized trials enrolling 183 men and lasting 4-24 weeks were included in the meta-analysis. The trials used rofecoxib plus finasteride, celecoxib, and tenoxicam plus doxazosin. NSAIDs improved scores on the International Prostate Symptom Score (IPSS; P less than .001) and increased urine flow (0.89 mL/s; P = .01). No increased side effects were observed.
The authors highlight that inflammatory infiltration is seen in 43%-98% of BPH tissue, and men with acute or chronic inflammation have larger prostate volumes.
COX-2 inhibitors were used in the included studies, but nonselective NSAIDs may have as powerful an effect on the inflammatory process associated with BPH – and may be associated with less concern for adverse cardiovascular outcomes. The long-term effects of NSAIDs on kidney function and the gastrointestinal mucosa, especially in older patients, need to be considered.
But the use of NSAIDs in combination with other BPH pharmacologic agents before changing doses, medications, or intervention approach may be an attractive short-term clinical option.
Dr. Ebbert is professor of medicine and a primary care clinician at the Mayo Clinic in Rochester, Minn. He reports having no conflicts of interest. The opinions expressed are those of the author.
Benign prostatic hyperplasia is an uncommon cause of mortality but a common cause of morbidity.
More than 80% of men aged older than 80 years have histologic evidence of benign prostatic hyperplasia (BPH). As most of us know, the most common symptoms of BPH are urinary frequency, nocturia, hesitancy, and weak urine stream. But it seems like nocturia is the source of most complaints in my panel.
Interestingly, many men will experience stabilization or improvement over time without therapy. We likely do not remember this, because men who present to us are not typically enamored with the idea of "watchful waiting" when they are up all night standing at the latrine. But in fact, 38% of men will have symptom improvement over 2.6 to 5 years of follow-up without intervention.
Treatment is symptom driven. Treatment modality selections are cost and convenience driven. Many of us will reach for an alpha-adrenergic antagonist or a 5-alpha-reductase inhibitor as first-line therapy for patients with mild but annoying symptoms.
But how many of us consider NSAIDs in this setting?
Dr. Arman Kahokehr and colleagues conducted a systematic review of the literature examining the effects of NSAIDs in the treatment of men with BPH. Trials were included if they were randomized and included objective outcomes such as urologic symptom scales or urodynamics (BJU Int. 2013;111:304-11).
Three randomized trials enrolling 183 men and lasting 4-24 weeks were included in the meta-analysis. The trials used rofecoxib plus finasteride, celecoxib, and tenoxicam plus doxazosin. NSAIDs improved scores on the International Prostate Symptom Score (IPSS; P less than .001) and increased urine flow (0.89 mL/s; P = .01). No increased side effects were observed.
The authors highlight that inflammatory infiltration is seen in 43%-98% of BPH tissue, and men with acute or chronic inflammation have larger prostate volumes.
COX-2 inhibitors were used in the included studies, but nonselective NSAIDs may have as powerful an effect on the inflammatory process associated with BPH – and may be associated with less concern for adverse cardiovascular outcomes. The long-term effects of NSAIDs on kidney function and the gastrointestinal mucosa, especially in older patients, need to be considered.
But the use of NSAIDs in combination with other BPH pharmacologic agents before changing doses, medications, or intervention approach may be an attractive short-term clinical option.
Dr. Ebbert is professor of medicine and a primary care clinician at the Mayo Clinic in Rochester, Minn. He reports having no conflicts of interest. The opinions expressed are those of the author.
Benign prostatic hyperplasia is an uncommon cause of mortality but a common cause of morbidity.
More than 80% of men aged older than 80 years have histologic evidence of benign prostatic hyperplasia (BPH). As most of us know, the most common symptoms of BPH are urinary frequency, nocturia, hesitancy, and weak urine stream. But it seems like nocturia is the source of most complaints in my panel.
Interestingly, many men will experience stabilization or improvement over time without therapy. We likely do not remember this, because men who present to us are not typically enamored with the idea of "watchful waiting" when they are up all night standing at the latrine. But in fact, 38% of men will have symptom improvement over 2.6 to 5 years of follow-up without intervention.
Treatment is symptom driven. Treatment modality selections are cost and convenience driven. Many of us will reach for an alpha-adrenergic antagonist or a 5-alpha-reductase inhibitor as first-line therapy for patients with mild but annoying symptoms.
But how many of us consider NSAIDs in this setting?
Dr. Arman Kahokehr and colleagues conducted a systematic review of the literature examining the effects of NSAIDs in the treatment of men with BPH. Trials were included if they were randomized and included objective outcomes such as urologic symptom scales or urodynamics (BJU Int. 2013;111:304-11).
Three randomized trials enrolling 183 men and lasting 4-24 weeks were included in the meta-analysis. The trials used rofecoxib plus finasteride, celecoxib, and tenoxicam plus doxazosin. NSAIDs improved scores on the International Prostate Symptom Score (IPSS; P less than .001) and increased urine flow (0.89 mL/s; P = .01). No increased side effects were observed.
The authors highlight that inflammatory infiltration is seen in 43%-98% of BPH tissue, and men with acute or chronic inflammation have larger prostate volumes.
COX-2 inhibitors were used in the included studies, but nonselective NSAIDs may have as powerful an effect on the inflammatory process associated with BPH – and may be associated with less concern for adverse cardiovascular outcomes. The long-term effects of NSAIDs on kidney function and the gastrointestinal mucosa, especially in older patients, need to be considered.
But the use of NSAIDs in combination with other BPH pharmacologic agents before changing doses, medications, or intervention approach may be an attractive short-term clinical option.
Dr. Ebbert is professor of medicine and a primary care clinician at the Mayo Clinic in Rochester, Minn. He reports having no conflicts of interest. The opinions expressed are those of the author.
Bilateral adrenal masses
To the Editor: In their article “The clinical picture: bilateral adrenal masses” in the December 2012 issue,1 Drs. Saberi and Esfandiari provide excellent points about adrenal hemorrhage as a differential diagnosis for adrenal masses. However, there are two points worth emphasizing when mentioning this diagnosis, especially in the case they presented.
Drs. Saberi and Esfandiari cryptically mention this patient’s coagulopathy (with thrombocytopenia and a rise in creatinine) and anticoagulation as the probable causes of adrenal hemorrhage. We wonder if a diagnosis of antiphospholipid syndrome (APS) was overlooked. Even though overt Addison disease is reported in only 0.4% of patients with APS2 and APS is diagnosed in fewer than 0.5% of all patients with Addison disease,3 we think that in this case, since the patient initially presented with an arterial thrombus in the abdominal aorta, screening for APS would have been warranted.
Second, though it is rare, bilateral adrenal hemorrhage with normal imaging on initial presentation has been described,2,4 which raises this additional question: Should screening for adrenal insufficiency in a patient with possible APS or other coagulopathy be done early while waiting for repeat computed tomography to reveal hemorrhage? Occasionally, intraparenchymal microhemorrhages may not be recognized by sectional imaging but can nonetheless compromise adrenal function.4
- Saberi S, Esfandiari NH. The clinical picture: bilateral adrenal masses. Cleve Clin J Med 2012; 79:841–842.
- Espinosa G, Santos E, Cervera R, et al. Adrenal involvement in the antiphospholipid syndrome: clinical and immunologic characteristics of 86 patients. Medicine (Baltimore) 2003; 82:106–118.
- Presotto F, Fornasini F, Betterle C, Federspil G, Rossato M. Acute adrenal failure as the heralding symptom of primary antiphospholipid syndrome: report of a case and review of the literature. Eur J Endocrinol 2005; 153:507–514.
- Satta MA, Corsello SM, Della Casa S, et al. Adrenal insufficiency as the first clinical manifestation of the primary antiphospholipid antibody syndrome. Clin Endocrinol (Oxf) 2000; 52:123–126.
To the Editor: In their article “The clinical picture: bilateral adrenal masses” in the December 2012 issue,1 Drs. Saberi and Esfandiari provide excellent points about adrenal hemorrhage as a differential diagnosis for adrenal masses. However, there are two points worth emphasizing when mentioning this diagnosis, especially in the case they presented.
Drs. Saberi and Esfandiari cryptically mention this patient’s coagulopathy (with thrombocytopenia and a rise in creatinine) and anticoagulation as the probable causes of adrenal hemorrhage. We wonder if a diagnosis of antiphospholipid syndrome (APS) was overlooked. Even though overt Addison disease is reported in only 0.4% of patients with APS2 and APS is diagnosed in fewer than 0.5% of all patients with Addison disease,3 we think that in this case, since the patient initially presented with an arterial thrombus in the abdominal aorta, screening for APS would have been warranted.
Second, though it is rare, bilateral adrenal hemorrhage with normal imaging on initial presentation has been described,2,4 which raises this additional question: Should screening for adrenal insufficiency in a patient with possible APS or other coagulopathy be done early while waiting for repeat computed tomography to reveal hemorrhage? Occasionally, intraparenchymal microhemorrhages may not be recognized by sectional imaging but can nonetheless compromise adrenal function.4
To the Editor: In their article “The clinical picture: bilateral adrenal masses” in the December 2012 issue,1 Drs. Saberi and Esfandiari provide excellent points about adrenal hemorrhage as a differential diagnosis for adrenal masses. However, there are two points worth emphasizing when mentioning this diagnosis, especially in the case they presented.
Drs. Saberi and Esfandiari cryptically mention this patient’s coagulopathy (with thrombocytopenia and a rise in creatinine) and anticoagulation as the probable causes of adrenal hemorrhage. We wonder if a diagnosis of antiphospholipid syndrome (APS) was overlooked. Even though overt Addison disease is reported in only 0.4% of patients with APS2 and APS is diagnosed in fewer than 0.5% of all patients with Addison disease,3 we think that in this case, since the patient initially presented with an arterial thrombus in the abdominal aorta, screening for APS would have been warranted.
Second, though it is rare, bilateral adrenal hemorrhage with normal imaging on initial presentation has been described,2,4 which raises this additional question: Should screening for adrenal insufficiency in a patient with possible APS or other coagulopathy be done early while waiting for repeat computed tomography to reveal hemorrhage? Occasionally, intraparenchymal microhemorrhages may not be recognized by sectional imaging but can nonetheless compromise adrenal function.4
- Saberi S, Esfandiari NH. The clinical picture: bilateral adrenal masses. Cleve Clin J Med 2012; 79:841–842.
- Espinosa G, Santos E, Cervera R, et al. Adrenal involvement in the antiphospholipid syndrome: clinical and immunologic characteristics of 86 patients. Medicine (Baltimore) 2003; 82:106–118.
- Presotto F, Fornasini F, Betterle C, Federspil G, Rossato M. Acute adrenal failure as the heralding symptom of primary antiphospholipid syndrome: report of a case and review of the literature. Eur J Endocrinol 2005; 153:507–514.
- Satta MA, Corsello SM, Della Casa S, et al. Adrenal insufficiency as the first clinical manifestation of the primary antiphospholipid antibody syndrome. Clin Endocrinol (Oxf) 2000; 52:123–126.
- Saberi S, Esfandiari NH. The clinical picture: bilateral adrenal masses. Cleve Clin J Med 2012; 79:841–842.
- Espinosa G, Santos E, Cervera R, et al. Adrenal involvement in the antiphospholipid syndrome: clinical and immunologic characteristics of 86 patients. Medicine (Baltimore) 2003; 82:106–118.
- Presotto F, Fornasini F, Betterle C, Federspil G, Rossato M. Acute adrenal failure as the heralding symptom of primary antiphospholipid syndrome: report of a case and review of the literature. Eur J Endocrinol 2005; 153:507–514.
- Satta MA, Corsello SM, Della Casa S, et al. Adrenal insufficiency as the first clinical manifestation of the primary antiphospholipid antibody syndrome. Clin Endocrinol (Oxf) 2000; 52:123–126.
Even mild kidney dysfunction raises recurrent stroke risk
Diminished renal function is an independent risk factor for recurrent stroke within the first 6 months following hospitalization for an acute ischemic stroke, Dr. Abraham Thomas reported at the International Stroke Conference.
The short-term risk of recurrent stroke climbs in stepwise fashion with decreasing renal function. Even patients categorized as having stage 2 renal function by National Kidney Foundation criteria – those with an estimated glomerular filtration rate of 60-89 mL/min/1.73 m2 – have a 60% increased risk compared with those who have an estimated GFR of 90 or greater, according to Dr. Thomas of the University of California, San Francisco.
He presented an observational study involving 2,882 patients admitted with acute ischemic stroke to 12 Northern California Kaiser Permanente hospitals during 2004-2007. Twenty-four percent had stage 1 renal function upon admission, with an eGFR of at least 90 mL/min/1.73 m2. Forty-seven percent were stage 2, 25% were stage 3 as defined by an eGFR of 30-59, and the rest had stage 4 chronic kidney disease.
In a multivariate analysis, stage 2 renal function was independently associated with a 60% greater risk of recurrent stroke within 6 months compared with those who were stage 1. Patients with stage 3 renal function were at 70% greater risk than were those who were stage 1, while stage 4 patients were at 80% increased risk.
Renal dysfunction is an established risk factor for first-time cardiovascular events, including stroke. But the relationship between renal function and short-term risk of recurrent stroke has not previously been scrutinized.
One possible mechanism by which impaired renal function might predict an increased short-term risk of recurrent stroke involves poor blood pressure control, Dr. Thomas observed. The prevalence of hypertension was 19% with stage 1 renal function, 24% in those who were stage 2, and 26% in patients with stage 3 or 4 renal function. And at 6 months’ follow-up, blood pressure control was less than half as common among stage 4 patients than in those who were stages 1-3.
The conference was sponsored by the American Heart Association. Dr. Thomas reported having no financial conflicts.
National Kidney Foundation, glomerular, he University of California
Diminished renal function is an independent risk factor for recurrent stroke within the first 6 months following hospitalization for an acute ischemic stroke, Dr. Abraham Thomas reported at the International Stroke Conference.
The short-term risk of recurrent stroke climbs in stepwise fashion with decreasing renal function. Even patients categorized as having stage 2 renal function by National Kidney Foundation criteria – those with an estimated glomerular filtration rate of 60-89 mL/min/1.73 m2 – have a 60% increased risk compared with those who have an estimated GFR of 90 or greater, according to Dr. Thomas of the University of California, San Francisco.
He presented an observational study involving 2,882 patients admitted with acute ischemic stroke to 12 Northern California Kaiser Permanente hospitals during 2004-2007. Twenty-four percent had stage 1 renal function upon admission, with an eGFR of at least 90 mL/min/1.73 m2. Forty-seven percent were stage 2, 25% were stage 3 as defined by an eGFR of 30-59, and the rest had stage 4 chronic kidney disease.
In a multivariate analysis, stage 2 renal function was independently associated with a 60% greater risk of recurrent stroke within 6 months compared with those who were stage 1. Patients with stage 3 renal function were at 70% greater risk than were those who were stage 1, while stage 4 patients were at 80% increased risk.
Renal dysfunction is an established risk factor for first-time cardiovascular events, including stroke. But the relationship between renal function and short-term risk of recurrent stroke has not previously been scrutinized.
One possible mechanism by which impaired renal function might predict an increased short-term risk of recurrent stroke involves poor blood pressure control, Dr. Thomas observed. The prevalence of hypertension was 19% with stage 1 renal function, 24% in those who were stage 2, and 26% in patients with stage 3 or 4 renal function. And at 6 months’ follow-up, blood pressure control was less than half as common among stage 4 patients than in those who were stages 1-3.
The conference was sponsored by the American Heart Association. Dr. Thomas reported having no financial conflicts.
Diminished renal function is an independent risk factor for recurrent stroke within the first 6 months following hospitalization for an acute ischemic stroke, Dr. Abraham Thomas reported at the International Stroke Conference.
The short-term risk of recurrent stroke climbs in stepwise fashion with decreasing renal function. Even patients categorized as having stage 2 renal function by National Kidney Foundation criteria – those with an estimated glomerular filtration rate of 60-89 mL/min/1.73 m2 – have a 60% increased risk compared with those who have an estimated GFR of 90 or greater, according to Dr. Thomas of the University of California, San Francisco.
He presented an observational study involving 2,882 patients admitted with acute ischemic stroke to 12 Northern California Kaiser Permanente hospitals during 2004-2007. Twenty-four percent had stage 1 renal function upon admission, with an eGFR of at least 90 mL/min/1.73 m2. Forty-seven percent were stage 2, 25% were stage 3 as defined by an eGFR of 30-59, and the rest had stage 4 chronic kidney disease.
In a multivariate analysis, stage 2 renal function was independently associated with a 60% greater risk of recurrent stroke within 6 months compared with those who were stage 1. Patients with stage 3 renal function were at 70% greater risk than were those who were stage 1, while stage 4 patients were at 80% increased risk.
Renal dysfunction is an established risk factor for first-time cardiovascular events, including stroke. But the relationship between renal function and short-term risk of recurrent stroke has not previously been scrutinized.
One possible mechanism by which impaired renal function might predict an increased short-term risk of recurrent stroke involves poor blood pressure control, Dr. Thomas observed. The prevalence of hypertension was 19% with stage 1 renal function, 24% in those who were stage 2, and 26% in patients with stage 3 or 4 renal function. And at 6 months’ follow-up, blood pressure control was less than half as common among stage 4 patients than in those who were stages 1-3.
The conference was sponsored by the American Heart Association. Dr. Thomas reported having no financial conflicts.
National Kidney Foundation, glomerular, he University of California
National Kidney Foundation, glomerular, he University of California
AT THE INTERNATIONAL STROKE CONFERENCE
Survival higher with surveillance of small kidney tumors
Older patients with small kidney tumors are up to 70% less likely to die from any cause when managed by watchful waiting rather than by surgery, based on findings from a large retrospective study.
Surveillance appears to be safe for these small lesions, with a kidney cancer mortality of just 3% over a 5-year period. In addition, watchful waiting seems to confer a cardiovascular benefit; these patients had a 49% lower cardiac mortality risk compared with that for patients who had kidney surgery, said lead author Dr. William C. Huang of New York University Medical Center.
The link between kidney surgery and heart problems is probably mediated by compromised kidney function, Dr. Huang said. "It’s believed that when surgery takes excess normal kidney tissue, it hastens the acceleration of kidney failure," leading to cardiovascular problems, he said.
The study findings are going to be increasingly valuable as imaging turns up more and more incidental asymptomatic kidney tumors. Last year alone, about 65,000 of these lesions were diagnosed, most of them during a work-up for other abdominal complaints.
While the trend toward surveillance of small asymptomatic lesions is growing, Dr. Huang said surgery is still the treatment mode for more than half of cases. Most procedures in the retrospective study were radical nephrectomies, with kidney removal in about half of those. "The majority of these small lesions could be removed laparoscopically, but even then you’re taking out normal kidney tissue that you might really want back someday," he said at a press briefing at the Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
"Physicians can comfortably tell an elderly patient, especially a patient who is not healthy enough to tolerate general anesthesia and surgery, that the likelihood of dying of kidney cancer is low and that kidney surgery is unlikely to extend their lives," he said. "However, since it is difficult to identify which tumors will become lethal, elderly patients who are completely healthy and have an extended life expectancy, may opt for surgery."
The study examined mortality data in the Surveillance, Epidemiology, and End Results database for 8,317 patients, aged 66 years or older, who were diagnosed from 2002 to 2007 with kidney tumors smaller than 1.5 cm. The patients were followed for a median of 59 months; 78% were managed with surgery and 22%, with surveillance. The use of surveillance increased over the course of the study, from about 25% in 2002 to almost 40% in 2007.
Over the study period, 2,078 (25%) of the patients died, including 277 (3%) who died of kidney cancer. At least one cardiovascular event occurred in 24% of the patients.
Kidney cancer mortality rates did not vary between the treatment groups. However, surgical patients had a significantly increased risk of death from any cause. At 7-36 months, those who had surveillance were 30% less likely to have died than those managed by surgery (hazard ratio, 0.70). After 36 months, patients were 63% less likely to have died if their tumors were managed by surveillance instead of surgery (HR, 0.37).
Dr. Huang also demonstrated that those in the surveillance group experienced a significant cardiovascular benefit as well. By the end of the study, 25% of the deaths were due to a cardiovascular event. Patients in the surveillance group had a 49% reduction in the chance of an event.
Dr. Huang said he had no relevant financial disclosures.
Older patients with small kidney tumors are up to 70% less likely to die from any cause when managed by watchful waiting rather than by surgery, based on findings from a large retrospective study.
Surveillance appears to be safe for these small lesions, with a kidney cancer mortality of just 3% over a 5-year period. In addition, watchful waiting seems to confer a cardiovascular benefit; these patients had a 49% lower cardiac mortality risk compared with that for patients who had kidney surgery, said lead author Dr. William C. Huang of New York University Medical Center.
The link between kidney surgery and heart problems is probably mediated by compromised kidney function, Dr. Huang said. "It’s believed that when surgery takes excess normal kidney tissue, it hastens the acceleration of kidney failure," leading to cardiovascular problems, he said.
The study findings are going to be increasingly valuable as imaging turns up more and more incidental asymptomatic kidney tumors. Last year alone, about 65,000 of these lesions were diagnosed, most of them during a work-up for other abdominal complaints.
While the trend toward surveillance of small asymptomatic lesions is growing, Dr. Huang said surgery is still the treatment mode for more than half of cases. Most procedures in the retrospective study were radical nephrectomies, with kidney removal in about half of those. "The majority of these small lesions could be removed laparoscopically, but even then you’re taking out normal kidney tissue that you might really want back someday," he said at a press briefing at the Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
"Physicians can comfortably tell an elderly patient, especially a patient who is not healthy enough to tolerate general anesthesia and surgery, that the likelihood of dying of kidney cancer is low and that kidney surgery is unlikely to extend their lives," he said. "However, since it is difficult to identify which tumors will become lethal, elderly patients who are completely healthy and have an extended life expectancy, may opt for surgery."
The study examined mortality data in the Surveillance, Epidemiology, and End Results database for 8,317 patients, aged 66 years or older, who were diagnosed from 2002 to 2007 with kidney tumors smaller than 1.5 cm. The patients were followed for a median of 59 months; 78% were managed with surgery and 22%, with surveillance. The use of surveillance increased over the course of the study, from about 25% in 2002 to almost 40% in 2007.
Over the study period, 2,078 (25%) of the patients died, including 277 (3%) who died of kidney cancer. At least one cardiovascular event occurred in 24% of the patients.
Kidney cancer mortality rates did not vary between the treatment groups. However, surgical patients had a significantly increased risk of death from any cause. At 7-36 months, those who had surveillance were 30% less likely to have died than those managed by surgery (hazard ratio, 0.70). After 36 months, patients were 63% less likely to have died if their tumors were managed by surveillance instead of surgery (HR, 0.37).
Dr. Huang also demonstrated that those in the surveillance group experienced a significant cardiovascular benefit as well. By the end of the study, 25% of the deaths were due to a cardiovascular event. Patients in the surveillance group had a 49% reduction in the chance of an event.
Dr. Huang said he had no relevant financial disclosures.
Older patients with small kidney tumors are up to 70% less likely to die from any cause when managed by watchful waiting rather than by surgery, based on findings from a large retrospective study.
Surveillance appears to be safe for these small lesions, with a kidney cancer mortality of just 3% over a 5-year period. In addition, watchful waiting seems to confer a cardiovascular benefit; these patients had a 49% lower cardiac mortality risk compared with that for patients who had kidney surgery, said lead author Dr. William C. Huang of New York University Medical Center.
The link between kidney surgery and heart problems is probably mediated by compromised kidney function, Dr. Huang said. "It’s believed that when surgery takes excess normal kidney tissue, it hastens the acceleration of kidney failure," leading to cardiovascular problems, he said.
The study findings are going to be increasingly valuable as imaging turns up more and more incidental asymptomatic kidney tumors. Last year alone, about 65,000 of these lesions were diagnosed, most of them during a work-up for other abdominal complaints.
While the trend toward surveillance of small asymptomatic lesions is growing, Dr. Huang said surgery is still the treatment mode for more than half of cases. Most procedures in the retrospective study were radical nephrectomies, with kidney removal in about half of those. "The majority of these small lesions could be removed laparoscopically, but even then you’re taking out normal kidney tissue that you might really want back someday," he said at a press briefing at the Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
"Physicians can comfortably tell an elderly patient, especially a patient who is not healthy enough to tolerate general anesthesia and surgery, that the likelihood of dying of kidney cancer is low and that kidney surgery is unlikely to extend their lives," he said. "However, since it is difficult to identify which tumors will become lethal, elderly patients who are completely healthy and have an extended life expectancy, may opt for surgery."
The study examined mortality data in the Surveillance, Epidemiology, and End Results database for 8,317 patients, aged 66 years or older, who were diagnosed from 2002 to 2007 with kidney tumors smaller than 1.5 cm. The patients were followed for a median of 59 months; 78% were managed with surgery and 22%, with surveillance. The use of surveillance increased over the course of the study, from about 25% in 2002 to almost 40% in 2007.
Over the study period, 2,078 (25%) of the patients died, including 277 (3%) who died of kidney cancer. At least one cardiovascular event occurred in 24% of the patients.
Kidney cancer mortality rates did not vary between the treatment groups. However, surgical patients had a significantly increased risk of death from any cause. At 7-36 months, those who had surveillance were 30% less likely to have died than those managed by surgery (hazard ratio, 0.70). After 36 months, patients were 63% less likely to have died if their tumors were managed by surveillance instead of surgery (HR, 0.37).
Dr. Huang also demonstrated that those in the surveillance group experienced a significant cardiovascular benefit as well. By the end of the study, 25% of the deaths were due to a cardiovascular event. Patients in the surveillance group had a 49% reduction in the chance of an event.
Dr. Huang said he had no relevant financial disclosures.
FROM THE GENITOURINARY CANCERS SYMPOSIUM
Major Finding: After 36 months, patients were 63% less likely to have died if their tumors were managed by surveillance instead of surgery (HR, 0.37).
Data Source: Mortality data from the Surveillance, Epidemiology, and End Results database for 8,317 patients, aged 66 years or older, who were diagnosed from 2002 to 2007 with kidney tumors smaller than 1.5 cm.
Disclosures: Dr. Huang said he had no relevant financial disclosures.
CDC: Multiple cases link synthetic cannabinoid, kidney injury
Sixteen cases of acute kidney injury following the use of synthetic cannabinoids were identified in the United States in 2012, according to a report from the Centers for Disease Control and Prevention.
The cases, which occurred in six states and were unrelated in all but two incidents, underscore the importance of awareness on the part of health care providers about renal and other unexpected toxicities from the use of synthetic cannabinoid (SC) compounds, the CDC said in the Feb. 15 issue of the Morbidity and Mortality Weekly Report.
This is particularly true given the increasing use of SCs, also known as synthetic marijuana, "spice," or "K2, and in light of prior reports of toxicities associated with SC.
The initial four cases of acute kidney injury (AKI) following recent SC use were reported in Wyoming in March 2012, and an additional 12 cases were subsequently identified, including 6 in Oregon, 2 in New York, 2 in Oklahoma, and 1 each in Rhode Island and Kansas (MMWR 2013;62:93-8).
The patients – 15 adolescent or adult males aged 15-33 years, and one 15-year-old female – all visited emergency departments complaining of nausea and vomiting within days or hours of SC use; 12 also reported abdominal, flank, and/or back pain, and none had preexisting renal dysfunction or used medications associated with renal problems. All were hospitalized.
"The highest serum creatinine concentrations (creatinine peak) among the 16 patients ranged from 3.3 to 21.0 mg/dL (median: 6.7 mg/dL; normal 0.6-1.3 mg/dL) and occurred 1-6 days after symptom onset (median: 3 days)," according to the report.
Urinalysis results were variable, demonstrating proteinuria in eight patients, casts in five patients, white blood cells in nine patients, and red blood cells in eight patients. Renal ultrasonography in 12 patients showed that 9 had a nonspecific increase in renal cortical echogenicity. None had hydronephrosis.
Renal biopsy in eight patients demonstrated acute tubular injury in six cases, and features of acute interstitial nephritis in three.
Most patients experienced kidney function recovery within 3 days of creatinine peak, but five required hemodialysis, and four received corticosteroids.
Toxicologic analysis of the implicated SC product and clinical specimens were possible in seven cases, and two products were linked with the first three cases. These products contained 3-(1-naphthoyl) indole, a precursor to several aminoalkylindole synthetic cannabinoids, and one also contained AM 2201, a potent SC previously linked to human disease and death, but not to AKI.
With one exception, other product samples and/or blood or urine specimens from patients contained XLR-11 (a previously undescribed fluorinated-derivative of the known SC compound UR-144) either alone or in combination with an N-pentanoic acid metabolite of XLR-11 or UR-144.
These reports of AKI following SC use are concerning given the worldwide distribution of SC products, which are "packaged in colorful wrappers designed to appeal to teens, young adults, and first-time drug users," according to an editorial note in the report, which also states that SCs often are packaged with disingenuous labels claiming the products are not for human consumption, although it is widely known that they are smoked like marijuana.
Despite federal and state regulations prohibiting SC sale and distribution, illicit use continues, and reports of illness are increasing.
"The increasing use of synthetic cannabinoids in adolescents is particularly concerning because these substances can contain multiple active and inactive substances with a variety of short-term and potentially unknown long-term effects," Dr. Joanna S. Cohen said in an interview.
In a case study published last year, Dr. Cohen, of the departments of pediatrics and emergency medicine at George Washington University, Washington, noted the increasing use of SCs among adolescents, the potential dangers of SCs with respect to the developing brain, and the need for providers to become familiar with the presenting signs and symptoms of SC-related intoxication (Pediatrics 2012;129:e1064-7).
According to the MMWR report, SCs are related to the active ingredient in marijuana (delta-9-tetrahydrocannabinol), but are up to three times more likely to be associated with sympathomimetic effects such as tachycardia and hypertension, and about five times more likely to be associated with hallucinations.
An increase in seizure occurrence also has been reported with SC use.
"Given the rapidity with which new SC compounds enter the marketplace and their increasing use in the past 3 years, outbreaks of unexpected toxicity associated with their use are likely to increase," the CDC said.
Furthermore, no antidote currently exists; management of SC toxicity is symptomatic and supportive. All patients in this report of 16 cases of AKI recovered creatinine clearance during their hospital stay, but a risk for long-term kidney sequelae is possible. Findings from recent studies suggest the risk for chronic and end-stage renal disease is increased among patients who experience AKI, regardless of etiology and initial recovery.
The CDC recommends that physicians caring for adolescents and young adults with unexplained AKI inquire about SC use. Cases of suspected SC poisoning should be reported to the appropriate state health department, and to a regional poison center by calling 800-222-1222.
The authors reported on disclosures.
Sixteen cases of acute kidney injury following the use of synthetic cannabinoids were identified in the United States in 2012, according to a report from the Centers for Disease Control and Prevention.
The cases, which occurred in six states and were unrelated in all but two incidents, underscore the importance of awareness on the part of health care providers about renal and other unexpected toxicities from the use of synthetic cannabinoid (SC) compounds, the CDC said in the Feb. 15 issue of the Morbidity and Mortality Weekly Report.
This is particularly true given the increasing use of SCs, also known as synthetic marijuana, "spice," or "K2, and in light of prior reports of toxicities associated with SC.
The initial four cases of acute kidney injury (AKI) following recent SC use were reported in Wyoming in March 2012, and an additional 12 cases were subsequently identified, including 6 in Oregon, 2 in New York, 2 in Oklahoma, and 1 each in Rhode Island and Kansas (MMWR 2013;62:93-8).
The patients – 15 adolescent or adult males aged 15-33 years, and one 15-year-old female – all visited emergency departments complaining of nausea and vomiting within days or hours of SC use; 12 also reported abdominal, flank, and/or back pain, and none had preexisting renal dysfunction or used medications associated with renal problems. All were hospitalized.
"The highest serum creatinine concentrations (creatinine peak) among the 16 patients ranged from 3.3 to 21.0 mg/dL (median: 6.7 mg/dL; normal 0.6-1.3 mg/dL) and occurred 1-6 days after symptom onset (median: 3 days)," according to the report.
Urinalysis results were variable, demonstrating proteinuria in eight patients, casts in five patients, white blood cells in nine patients, and red blood cells in eight patients. Renal ultrasonography in 12 patients showed that 9 had a nonspecific increase in renal cortical echogenicity. None had hydronephrosis.
Renal biopsy in eight patients demonstrated acute tubular injury in six cases, and features of acute interstitial nephritis in three.
Most patients experienced kidney function recovery within 3 days of creatinine peak, but five required hemodialysis, and four received corticosteroids.
Toxicologic analysis of the implicated SC product and clinical specimens were possible in seven cases, and two products were linked with the first three cases. These products contained 3-(1-naphthoyl) indole, a precursor to several aminoalkylindole synthetic cannabinoids, and one also contained AM 2201, a potent SC previously linked to human disease and death, but not to AKI.
With one exception, other product samples and/or blood or urine specimens from patients contained XLR-11 (a previously undescribed fluorinated-derivative of the known SC compound UR-144) either alone or in combination with an N-pentanoic acid metabolite of XLR-11 or UR-144.
These reports of AKI following SC use are concerning given the worldwide distribution of SC products, which are "packaged in colorful wrappers designed to appeal to teens, young adults, and first-time drug users," according to an editorial note in the report, which also states that SCs often are packaged with disingenuous labels claiming the products are not for human consumption, although it is widely known that they are smoked like marijuana.
Despite federal and state regulations prohibiting SC sale and distribution, illicit use continues, and reports of illness are increasing.
"The increasing use of synthetic cannabinoids in adolescents is particularly concerning because these substances can contain multiple active and inactive substances with a variety of short-term and potentially unknown long-term effects," Dr. Joanna S. Cohen said in an interview.
In a case study published last year, Dr. Cohen, of the departments of pediatrics and emergency medicine at George Washington University, Washington, noted the increasing use of SCs among adolescents, the potential dangers of SCs with respect to the developing brain, and the need for providers to become familiar with the presenting signs and symptoms of SC-related intoxication (Pediatrics 2012;129:e1064-7).
According to the MMWR report, SCs are related to the active ingredient in marijuana (delta-9-tetrahydrocannabinol), but are up to three times more likely to be associated with sympathomimetic effects such as tachycardia and hypertension, and about five times more likely to be associated with hallucinations.
An increase in seizure occurrence also has been reported with SC use.
"Given the rapidity with which new SC compounds enter the marketplace and their increasing use in the past 3 years, outbreaks of unexpected toxicity associated with their use are likely to increase," the CDC said.
Furthermore, no antidote currently exists; management of SC toxicity is symptomatic and supportive. All patients in this report of 16 cases of AKI recovered creatinine clearance during their hospital stay, but a risk for long-term kidney sequelae is possible. Findings from recent studies suggest the risk for chronic and end-stage renal disease is increased among patients who experience AKI, regardless of etiology and initial recovery.
The CDC recommends that physicians caring for adolescents and young adults with unexplained AKI inquire about SC use. Cases of suspected SC poisoning should be reported to the appropriate state health department, and to a regional poison center by calling 800-222-1222.
The authors reported on disclosures.
Sixteen cases of acute kidney injury following the use of synthetic cannabinoids were identified in the United States in 2012, according to a report from the Centers for Disease Control and Prevention.
The cases, which occurred in six states and were unrelated in all but two incidents, underscore the importance of awareness on the part of health care providers about renal and other unexpected toxicities from the use of synthetic cannabinoid (SC) compounds, the CDC said in the Feb. 15 issue of the Morbidity and Mortality Weekly Report.
This is particularly true given the increasing use of SCs, also known as synthetic marijuana, "spice," or "K2, and in light of prior reports of toxicities associated with SC.
The initial four cases of acute kidney injury (AKI) following recent SC use were reported in Wyoming in March 2012, and an additional 12 cases were subsequently identified, including 6 in Oregon, 2 in New York, 2 in Oklahoma, and 1 each in Rhode Island and Kansas (MMWR 2013;62:93-8).
The patients – 15 adolescent or adult males aged 15-33 years, and one 15-year-old female – all visited emergency departments complaining of nausea and vomiting within days or hours of SC use; 12 also reported abdominal, flank, and/or back pain, and none had preexisting renal dysfunction or used medications associated with renal problems. All were hospitalized.
"The highest serum creatinine concentrations (creatinine peak) among the 16 patients ranged from 3.3 to 21.0 mg/dL (median: 6.7 mg/dL; normal 0.6-1.3 mg/dL) and occurred 1-6 days after symptom onset (median: 3 days)," according to the report.
Urinalysis results were variable, demonstrating proteinuria in eight patients, casts in five patients, white blood cells in nine patients, and red blood cells in eight patients. Renal ultrasonography in 12 patients showed that 9 had a nonspecific increase in renal cortical echogenicity. None had hydronephrosis.
Renal biopsy in eight patients demonstrated acute tubular injury in six cases, and features of acute interstitial nephritis in three.
Most patients experienced kidney function recovery within 3 days of creatinine peak, but five required hemodialysis, and four received corticosteroids.
Toxicologic analysis of the implicated SC product and clinical specimens were possible in seven cases, and two products were linked with the first three cases. These products contained 3-(1-naphthoyl) indole, a precursor to several aminoalkylindole synthetic cannabinoids, and one also contained AM 2201, a potent SC previously linked to human disease and death, but not to AKI.
With one exception, other product samples and/or blood or urine specimens from patients contained XLR-11 (a previously undescribed fluorinated-derivative of the known SC compound UR-144) either alone or in combination with an N-pentanoic acid metabolite of XLR-11 or UR-144.
These reports of AKI following SC use are concerning given the worldwide distribution of SC products, which are "packaged in colorful wrappers designed to appeal to teens, young adults, and first-time drug users," according to an editorial note in the report, which also states that SCs often are packaged with disingenuous labels claiming the products are not for human consumption, although it is widely known that they are smoked like marijuana.
Despite federal and state regulations prohibiting SC sale and distribution, illicit use continues, and reports of illness are increasing.
"The increasing use of synthetic cannabinoids in adolescents is particularly concerning because these substances can contain multiple active and inactive substances with a variety of short-term and potentially unknown long-term effects," Dr. Joanna S. Cohen said in an interview.
In a case study published last year, Dr. Cohen, of the departments of pediatrics and emergency medicine at George Washington University, Washington, noted the increasing use of SCs among adolescents, the potential dangers of SCs with respect to the developing brain, and the need for providers to become familiar with the presenting signs and symptoms of SC-related intoxication (Pediatrics 2012;129:e1064-7).
According to the MMWR report, SCs are related to the active ingredient in marijuana (delta-9-tetrahydrocannabinol), but are up to three times more likely to be associated with sympathomimetic effects such as tachycardia and hypertension, and about five times more likely to be associated with hallucinations.
An increase in seizure occurrence also has been reported with SC use.
"Given the rapidity with which new SC compounds enter the marketplace and their increasing use in the past 3 years, outbreaks of unexpected toxicity associated with their use are likely to increase," the CDC said.
Furthermore, no antidote currently exists; management of SC toxicity is symptomatic and supportive. All patients in this report of 16 cases of AKI recovered creatinine clearance during their hospital stay, but a risk for long-term kidney sequelae is possible. Findings from recent studies suggest the risk for chronic and end-stage renal disease is increased among patients who experience AKI, regardless of etiology and initial recovery.
The CDC recommends that physicians caring for adolescents and young adults with unexplained AKI inquire about SC use. Cases of suspected SC poisoning should be reported to the appropriate state health department, and to a regional poison center by calling 800-222-1222.
The authors reported on disclosures.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Major finding: Sixteen cases of acute kidney injury occurred after synthetic cannabinoid use in six states in 2012.
Data source: The cases are based on state reports and surveillance by the Centers for Disease Control and Prevention.
Disclosures: The authors reported no disclosures.
Age, race impact prostate cancer risk
Prostate cancers detected during screening are much more likely to be high risk when they affect black men and men aged 75 years or older.
Men over age 74 years were nine times more likely to have high-risk disease after a positive prostate-specific antigen test, and black men of all ages were twice as likely to have high-risk disease as were white men, based on a study of 4 years of data extracted from the Surveillance, Epidemiology and End Results (SEER) database.
The findings make the case for a more personalized approach to screening, Dr. Hong Zhang said at a press briefing during the 2013 Genitourinary Cancers Symposium.
Without prostate-specific antigen (PSA) screening, "we have no other way to detect prostate cancer sufficiently early to have the best chance of helping this group of high-risk patients," said Dr. Zhang of the University of Rochester (N.Y.).
The study brings a bit of context to current PSA screening guidelines, which are "all over the map," according to session moderator Dr. Bruce Roth of Washington University, St. Louis. In 2011, the United States Preventive Services Task Force determined that routine screening harms more men that it helps.
"The American Cancer Society recommends just screening older men and the USPTF recommends that nobody get screened," Dr. Roth said. Based on these results, the presumption that older men will die first of something other than their prostate cancer is not necessarily true. "In fact, a significant number of these men present with high-risk disease. Age is not the greatest determinant of who should and should not be screened."
During 2004-2008, 70,345 men were diagnosed with T1cN0M0 prostate cancer in SEER. Of these, 48% had low-risk disease (PSA less than 10 mg/L or Gleason score of 6 or less), 36% intermediate-risk disease (PSA between 10 mg/L and 20 mg/L or Gleason score 7), and 16% high-risk disease (PSA at least 20 mg/L, or Gleason score of 8 or higher).
The median age of patients with low-risk disease was 67 years; for those with intermediate-risk disease, median age was 70 years; and for high-risk disease, it was 72 years. Men 75 years or older accounted for 12% of the population, but for 24% of intermediate-risk and 26% of high-risk disease.
In a multivariate analysis, Dr. Zhang determined that, compared with younger men, those aged 75 years and older were almost five times more likely to have intermediate-risk disease and nine times more likely to have high-risk disease.
Blacks made up 13% of the low-risk category, 16% of the intermediate-risk category, and 18% of the high-risk category. Compared with whites, blacks were 1.5 times more likely to have intermediate-risk disease and twice as likely to have high-risk disease.
Dr. Zhang and Dr. Roth had no financial disclosures.
Prostate cancers detected during screening are much more likely to be high risk when they affect black men and men aged 75 years or older.
Men over age 74 years were nine times more likely to have high-risk disease after a positive prostate-specific antigen test, and black men of all ages were twice as likely to have high-risk disease as were white men, based on a study of 4 years of data extracted from the Surveillance, Epidemiology and End Results (SEER) database.
The findings make the case for a more personalized approach to screening, Dr. Hong Zhang said at a press briefing during the 2013 Genitourinary Cancers Symposium.
Without prostate-specific antigen (PSA) screening, "we have no other way to detect prostate cancer sufficiently early to have the best chance of helping this group of high-risk patients," said Dr. Zhang of the University of Rochester (N.Y.).
The study brings a bit of context to current PSA screening guidelines, which are "all over the map," according to session moderator Dr. Bruce Roth of Washington University, St. Louis. In 2011, the United States Preventive Services Task Force determined that routine screening harms more men that it helps.
"The American Cancer Society recommends just screening older men and the USPTF recommends that nobody get screened," Dr. Roth said. Based on these results, the presumption that older men will die first of something other than their prostate cancer is not necessarily true. "In fact, a significant number of these men present with high-risk disease. Age is not the greatest determinant of who should and should not be screened."
During 2004-2008, 70,345 men were diagnosed with T1cN0M0 prostate cancer in SEER. Of these, 48% had low-risk disease (PSA less than 10 mg/L or Gleason score of 6 or less), 36% intermediate-risk disease (PSA between 10 mg/L and 20 mg/L or Gleason score 7), and 16% high-risk disease (PSA at least 20 mg/L, or Gleason score of 8 or higher).
The median age of patients with low-risk disease was 67 years; for those with intermediate-risk disease, median age was 70 years; and for high-risk disease, it was 72 years. Men 75 years or older accounted for 12% of the population, but for 24% of intermediate-risk and 26% of high-risk disease.
In a multivariate analysis, Dr. Zhang determined that, compared with younger men, those aged 75 years and older were almost five times more likely to have intermediate-risk disease and nine times more likely to have high-risk disease.
Blacks made up 13% of the low-risk category, 16% of the intermediate-risk category, and 18% of the high-risk category. Compared with whites, blacks were 1.5 times more likely to have intermediate-risk disease and twice as likely to have high-risk disease.
Dr. Zhang and Dr. Roth had no financial disclosures.
Prostate cancers detected during screening are much more likely to be high risk when they affect black men and men aged 75 years or older.
Men over age 74 years were nine times more likely to have high-risk disease after a positive prostate-specific antigen test, and black men of all ages were twice as likely to have high-risk disease as were white men, based on a study of 4 years of data extracted from the Surveillance, Epidemiology and End Results (SEER) database.
The findings make the case for a more personalized approach to screening, Dr. Hong Zhang said at a press briefing during the 2013 Genitourinary Cancers Symposium.
Without prostate-specific antigen (PSA) screening, "we have no other way to detect prostate cancer sufficiently early to have the best chance of helping this group of high-risk patients," said Dr. Zhang of the University of Rochester (N.Y.).
The study brings a bit of context to current PSA screening guidelines, which are "all over the map," according to session moderator Dr. Bruce Roth of Washington University, St. Louis. In 2011, the United States Preventive Services Task Force determined that routine screening harms more men that it helps.
"The American Cancer Society recommends just screening older men and the USPTF recommends that nobody get screened," Dr. Roth said. Based on these results, the presumption that older men will die first of something other than their prostate cancer is not necessarily true. "In fact, a significant number of these men present with high-risk disease. Age is not the greatest determinant of who should and should not be screened."
During 2004-2008, 70,345 men were diagnosed with T1cN0M0 prostate cancer in SEER. Of these, 48% had low-risk disease (PSA less than 10 mg/L or Gleason score of 6 or less), 36% intermediate-risk disease (PSA between 10 mg/L and 20 mg/L or Gleason score 7), and 16% high-risk disease (PSA at least 20 mg/L, or Gleason score of 8 or higher).
The median age of patients with low-risk disease was 67 years; for those with intermediate-risk disease, median age was 70 years; and for high-risk disease, it was 72 years. Men 75 years or older accounted for 12% of the population, but for 24% of intermediate-risk and 26% of high-risk disease.
In a multivariate analysis, Dr. Zhang determined that, compared with younger men, those aged 75 years and older were almost five times more likely to have intermediate-risk disease and nine times more likely to have high-risk disease.
Blacks made up 13% of the low-risk category, 16% of the intermediate-risk category, and 18% of the high-risk category. Compared with whites, blacks were 1.5 times more likely to have intermediate-risk disease and twice as likely to have high-risk disease.
Dr. Zhang and Dr. Roth had no financial disclosures.
FROM THE 2013 GENITOURINARY CANCERS SYMPOSIUM
Major Finding: Men aged 75 years or older accounted for 12% of the population, but for 24% of intermediate-risk and 26% of high-risk disease.
Data Source: The study looked at more than 70,000 men diagnosed with prostate cancer from 2004-2008.
Disclosures: Dr. Zhang had no financial disclosures.
Resistant hypertension: Diagnostic strategies and management
Poor control of blood pressure is one of the most common risk factors for death worldwide, responsible for 62% of cases of cerebral vascular disease and 49% of cases of ischemic heart disease as well as 7.1 million deaths annually. As our population ages and the prevalence of obesity, diabetes, and chronic kidney disease increases, resistant hypertension will be seen more often in general practice.
Using a case study, this article will provide a strategy for diagnosing and treating resistant hypertension.
CASE: A WOMAN WITH LONG-STANDING HIGH BLOOD PRESSURE
A 37-year-old woman was referred for help with managing difficult-to-control hypertension. She had been diagnosed with hypertension at age 32, and it was well controlled until about 2 years ago. Various combinations of antihypertensive drugs had been tried, and a search for a cause of secondary hypertension revealed no clues.
On examination, her blood pressure averaged 212/124 mm Hg, and her heart rate was 109 beats per minute. Her medications were:
- Amlodipine (Norvasc), a calcium channel blocker, 10 mg once daily
- Valsartan (Diovan), an angiotensin II receptor antagonist, 160 mg once daily
- Carvedilol (Coreg), a beta-blocker, 25 mg twice daily
- Labetalol (Normodyne), a beta-blocker, 400 mg three times daily
- Clonidine (Catapres), a sympatholytic agent, 0.05 mg three times daily
- Doxazosin (Cardura), a peripheral alpha-blocker, 16 mg once daily
- Xylometazoline (Xylomet), an alpha agonist nasal spray for nasal congestion.
She had previously been taking spironolactone (Aldactone), hydralazine (Apresoline), and hydrochlorothiazide, but they were discontinued because of adverse effects.
Does this patient have resistant hypertension? How should her condition be managed?
RESISTANT HYPERTENSION DEFINED
The seventh Joint National Committee and the American Heart Association define resistant hypertension as an office blood pressure above the appropriate goal of therapy (< 140/90 mm Hg for most patients, and < 130/80 mm Hg for those with ischemic heart disease, diabetes, or renal insufficiency) despite the use of three or more antihypertensive drugs from different classes at full dosages, one of which is a diuretic.1,2
In this definition, the number of antihypertensive drugs required is arbitrary. More importantly, the concept of resistant hypertension is focused on identifying patients who may have a reversible cause of hypertension, as well as those who could benefit from special diagnostic or therapeutic intervention because of persistently high blood pressure.
This definition does not apply to patients who have recently been diagnosed with hypertension.
Resistant hypertension is not synonymous with uncontrolled hypertension, which includes all cases of hypertension that is not optimally controlled despite treatment, including apparent resistance (ie, pseudoresistance) and true resistance (defined below).
COMMON, BUT ITS PREVALENCE IS HARD TO PINPOINT
The prevalence of resistant hypertension is unknown because of inadequate sample sizes in published studies. However, it is common and is likely to become more common with the aging of the population and with the increasing prevalence of obesity, diabetes mellitus, and chronic kidney disease.
In small studies, the prevalence of resistance in hypertensive patients ranged from 5% in general medical practice to more than 50% in nephrology clinics. In the National Health and Nutrition Examination Survey in 2003 to 2004, only 58% of people being treated for hypertension had achieved blood pressure levels lower than 140/90 mm Hg,3 and the control rate in those with diabetes mellitus or chronic kidney disease was less than 40%.4
Isolated systolic hypertension—elevated systolic pressure with normal diastolic pressure—increases in prevalence with age in those with treated, uncontrolled hypertension. It accounted for 29.1% of cases of treated, uncontrolled hypertension in patients ages 25 to 44, 66.1% of cases in patients ages 45 to 64, and 87.6% of cases in patients age 65 and older.5
Even in clinical trials, in which one would expect excellent control of hypertension, rates of control ranged from 45% to 82%.6–10
APPARENT RESISTANCE VS TRUE RESISTANCE
Resistant hypertension can be divided arbitrarily into two broad categories: apparent resistance and true resistance, with the prevalence of apparent resistance being considerably higher. Each broad category has a long list of possible causes; most are readily identifiable in the course of a thorough history and physical examination and routine laboratory testing. If resistance to therapy persists, referral to a hypertension specialist is a logical next step.
Detecting pseudoresistance
Causes of apparent resistance include improper technique in measuring blood pressure, such as not having the patient rest before measurement, allowing the patient to have coffee or to smoke just before measurement, or not positioning the patient’s arm at the level of the heart during measurement.
Many elderly patients have calcified arteries that are hard to compress, leading to erroneously high systolic blood pressure measurements, a situation called pseudohypertension and a cause of pseudoresistance. The only way to measure blood pressure accurately in such cases is intra-arterially. These patients often do not have target-organ disease, which would be expected with high systolic pressure.
The white-coat phenomenon is another common cause of apparent resistance. It is defined as persistently elevated clinic or office blood pressure (> 140/90 mm Hg), together with normal daytime ambulatory blood pressure (the “white-coat effect” is the difference between those blood pressures).
Finally, poor patient adherence to treatment is estimated to account for 40% of cases of resistant hypertension.4,5,11 Poor adherence is difficult to prove because patients often claim they are compliant, but certain clues are indicative. For example, patients taking a diuretic should have increased uric acid levels, so normal uric acid levels in a patient on a diuretic could be a clue that he or she is not taking the medication. If poor adherence is suspected, patients should be admitted to the hospital to take the medications under close observation.
Many factors can contribute to true resistance
Many cases of resistant hypertension are drug-induced, particularly in patients taking a nonsteroidal anti-inflammatory drug or a cyclooxygenase II inhibitor. Use of ginseng, ma huang, and bitter lemon should also be suspected. Drugs or herbal preparations contributing to high blood pressure should be discontinued or minimized.
Alcohol intake in excess of two drinks (1 oz of alcohol) per day for men and half that amount for women can also contribute to hypertension.
Volume overload is common and has many causes, including a compensatory response to vasodilators, excessive salt intake, or an undetected reduction in the glomerular filtration rate causing retention of salt and water.
Drug considerations
A common cause of apparent resistant hypertension is physicians not following blood pressure treatment guidelines by not increasing the dosage when needed or by prescribing inappropriate drug combinations.
We commonly see furosemide (Lasix) being misused, ie, being prescribed once daily for hypertension. (It has a shorter duration of action than thiazide diuretics, the usual class of diuretics used for hypertension.)
For a patient who is already on many medications but whose hypertension is not responding, the first step should be to give a diuretic of an appropriate class in an appropriate dosage.
Diuretics are often inappropriately stopped if a patient develops hypokalemia. Potassium supplementation should always be an adjunct to diuretic therapy. Potassium itself is a potent vasodilator and, given as a supplement, has been shown to reduce stroke risk in rats.
The combination of an angiotensin receptor blocker and an angiotensin-converting enzyme inhibitor should not be used for patients with true resistant hypertension. The direct renin inhibitor aliskiren (Tekturna) should not be used in combination with these drugs, and the combination of aliskiren and valsartan (Valturna) has now been taken off the market.
Spironolactone (Aldactone) is sometimes used for resistant hypertension in the belief that in some cases primary aldosteronism is the underlying cause. A study in 1,400 participants confirms that it lowers blood pressure,9 but the reason is unclear: the blood pressure response was unrelated to levels of renin, angiotensin, or the plasma aldosterone-to-renin ratio.
Identify secondary causes of hypertension
Patients should be evaluated for kidney disease, which is the most common secondary medical reason for resistant hypertension. For patients with poor renal function (estimated glomerular filtration rate < 50 mL/minute), hydrochlorothiazide is not effective against hypertension, but chlorthalidone is. In addition, patients with poor renal function should be given loop diuretics such as furosemide two or three times daily, or the long-acting drug torsemide (Demadex) should be used instead.
Genetic variation can cause different rates of metabolism of drugs, contributing to resistant hypertension. Certain people metabolize hydralazine very fast, making it less effective. The same is true for some beta-blockers.
Obesity and diabetes can also contribute to resistant hypertension.
Ancillary neurohumoral studies are occasionally indicated to rule out identifiable causes of secondary hypertension that may be correctable. There are many identifiable causes of hypertension, but detailing each is beyond the scope of this article.
Patients should be tested for thyroid disease. Hypothyroidism can cause high blood pressure, although usually diastolic rather than systolic hypertension. Hyperthyroidism can cause marked systolic hypertension.
Table 1 provides a step-by-step guide for evaluating and managing patients with resistant hypertension.
EXPERIMENTAL DRUG THERAPY
Endothelin receptor antagonists are currently under investigation for the treatment of resistant hypertension. The protein endothelin-1 (ET-1) is a potent vasoconstrictor (30–50 times more potent than angiotensin II and norepinephrine) and has a long duration of action. ET-1 binds to two receptors with opposing effects: ET-A promotes vasoconstriction, and ET-B promotes vasodilation and clears ET-1.
Darusentan, a selective blocker of ET-A, was tested in the phase III DORADO trial, which was discontinued because the initial results did not meet primary outcome measures. Initial findings had indicated that it might not be as useful as hoped. Side effects included headache, flushing, and edema.
EXPERIMENTAL NONPHARMACOLOGIC THERAPIES
Electrical stimulation of carotid sinus baroreceptors is being tried under the assumption that a high sympathoexcitatory state contributes to resistant hypertension. Devices are placed around the carotid artery bifurcation, and stimulation is believed to increase the depressor influences that modulate blood pressure. Large-scale trials are under way, but it is too early to tell if the approach will be useful. Patients complain of neck pain from the device.
Renal denervation is another experimental approach.12 The kidney has a central role in blood pressure regulation: efferent nerves regulate renal vascular resistance, renal blood flow, and renin release from the juxtaglomerular apparatus; afferent nerves modulate sympathetic output from the central nervous system. The results of the Renal Denervation in Patients With Uncontrolled Hypertension (Symplicity HTN) trials 1 and 2 have been encouraging. The Symplicity HTN-3 trial will begin soon in the United States.
OUR PATIENT UNDERGOES ADDITIONAL STUDIES
To rule out the white-coat effect in our patient, we measured her blood pressure with an automated device that takes several readings without the clinician in the room. (This topic has been reviewed by Vidt et al in this journal13). The average of the automated readings was 183/113 mm Hg, and her average pulse was 109 beats per minute, arguing against a white-coat effect.
Her blood pressure was also markedly elevated (average 198/129) during 24-hour ambulatory blood pressure monitoring.
Findings on physical examination were unremarkable except for grade III hypertensive retinopathy. She had no carotid or abdominal bruits. Her peripheral pulses were strong and synchronous bilaterally.
Laboratory testing found the patient had normal serum electrolyte levels and good renal function but relatively low urinary sodium, 90 mmol/day (normal 40–220), and very low renin activity, 0.7 μg/L/h (normal up-right 0.8–5.8 μg/L/h, supine 0.5–1.8 μg/L/h), calling into question the wisdom of treatment with an angiotensin receptor blocker.
Hemodynamic studies were performed using impedance cardiography and found very high systemic vascular resistance with normal cardiac output, indicating that the patient had a high preload, which could be from hypervolemia or intense venous constriction. It is especially interesting that her vascular resistance was high despite her treatment regimen that included an angiotensin receptor blocker and a vasodilator, perhaps an indication of nonadherence with her medications.
Diuresis reduces her blood pressure
The patient was admitted to the hospital, and because her laboratory results indicated that plasma renin activity was suppressed, the angiotensin receptor blocker valsartan was discontinued.
On day 1, her weight was 162 lb and average blood pressure was 194/128 mm Hg. After 4 days of diuresis with escalating doses of furosemide, her weight was 153 lb and blood pressures ranged from 140 to 158 over 82 to 98 mm Hg. Her heart rate was 90 beats per minute. The hospital stay showed that volume overload was one of the factors maintaining her hypertension. She was discharged on metoprolol succinate (Toprol-XL) 100 mg twice daily and furosemide 80 mg twice daily.
Her blood pressure fluctuates widely after discharge
Over the next 5 days after discharge, the patient’s blood pressure rose steadily to 180/122 mm Hg, her heart rate was in excess of 100 beats per minute, and her weight increased to 158 lb. Blood screening found that the level of metoprolol was undetectable, and a diuretic screen showed no furosemide in the urine. Both the patient and her husband were adamant that she was taking her medications.
Hydrochlorothiazide 25 mg daily was added, and nadolol (Corgard) 80 mg once daily was started in place of metoprolol. On a return visit, her blood pressure and heart rate were finally good at 138/86 mm Hg and 60 beats per minute (sitting) and 134/92 and 63 (standing).
On 24-hour monitoring, some fluctuations of elevated blood pressure were still evident, with an average of 142/91 mm Hg, so nifedipine (Procardia) 60 mg daily was added.
Her final list of medications is hydrochlorothiazide 25 mg, nadolol 80 mg, and nifedipine XL 60 mg, all taken once daily.
Volume overload complicated by nonadherence
In summary, the main pathogenetic mechanism that sustained this patient’s hypertension was volume overload. Her urinary sodium level indicated that she was not taking excessive amounts of sodium. The volume overload may have been a compensatory response to the concomitant use of peripheral vasodilators plus sympatholytic agents.
In addition, she was not adherent to her antihypertensive regimen. The fact that her heart rate was 109 beats per minute despite having a drug regimen that included five sympathetic blocking agents was a strong clue. She eventually admitted that she did not like taking diuretics because they made her skin wrinkle.
In general, in a case like this, I try to minimize the number of drugs and give a diuretic as well as different classes of appropriate drugs.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2007; 51:1403–1419.
- Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment and control of hypertension among United States adults 1999–2004. Hypertension 2007; 49:69–75.
- Sarafidis PA, Li S, Chen SC, et al. Hypertension awareness, treatment, and control in chronic kidney disease. Am J Med 2008; 121:332–340.
- Sarafidis PA, Bakris GL. State of hypertension management in the United States: confluence of risk factors and the prevalence of resistant hypertension. J Clin Hypertens (Greenwich) 2008; 10:130–139.
- Jamerson K, Bakris GL, Dahlöf B, et al; for the ACCOMPLISH Investigators. Exceptional early blood pressure control rates: the ACCOMPLISH trial. Blood Pressure 2007; 16:80–86.
- Dahlöf B, Devereux RB, Kjeldsen S, et al; for the LIFE study group. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–003.
- Cushman WC, Ford CE, Cutler JA, et al; for the ALLHAT Collaborative Research Group. Success and predictors of blood pressure control in diverse North American settings: the Antihypertensive and Lipid-Lowering and Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens (Greenwich) 2002; 4:393–404.
- Chapman N, Dobson J, Wilson S, et al; on behalf of the Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007; 49:839–845.
- Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al; INVEST Investigators. A calcium antagonist vs a noncalcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003; 290:2805–2816.
- Calhoun DA, Jones D, Textor S, et al. AHA Scientific Statement. Resistant hypertension: diagnosis, evaluation, and treatment. Circulation 2008; 17:e510–e526.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension: guarded optimism. Cleve Clin J Med 2012; 79:501–510.
- Vidt DG, Lang RS, Seballos RJ, Misra-Hebert A, Campbell J, Bena JF. Taking blood pressure: too important to trust to humans? Cleve Clin J Med 2010; 77:683–688.
Poor control of blood pressure is one of the most common risk factors for death worldwide, responsible for 62% of cases of cerebral vascular disease and 49% of cases of ischemic heart disease as well as 7.1 million deaths annually. As our population ages and the prevalence of obesity, diabetes, and chronic kidney disease increases, resistant hypertension will be seen more often in general practice.
Using a case study, this article will provide a strategy for diagnosing and treating resistant hypertension.
CASE: A WOMAN WITH LONG-STANDING HIGH BLOOD PRESSURE
A 37-year-old woman was referred for help with managing difficult-to-control hypertension. She had been diagnosed with hypertension at age 32, and it was well controlled until about 2 years ago. Various combinations of antihypertensive drugs had been tried, and a search for a cause of secondary hypertension revealed no clues.
On examination, her blood pressure averaged 212/124 mm Hg, and her heart rate was 109 beats per minute. Her medications were:
- Amlodipine (Norvasc), a calcium channel blocker, 10 mg once daily
- Valsartan (Diovan), an angiotensin II receptor antagonist, 160 mg once daily
- Carvedilol (Coreg), a beta-blocker, 25 mg twice daily
- Labetalol (Normodyne), a beta-blocker, 400 mg three times daily
- Clonidine (Catapres), a sympatholytic agent, 0.05 mg three times daily
- Doxazosin (Cardura), a peripheral alpha-blocker, 16 mg once daily
- Xylometazoline (Xylomet), an alpha agonist nasal spray for nasal congestion.
She had previously been taking spironolactone (Aldactone), hydralazine (Apresoline), and hydrochlorothiazide, but they were discontinued because of adverse effects.
Does this patient have resistant hypertension? How should her condition be managed?
RESISTANT HYPERTENSION DEFINED
The seventh Joint National Committee and the American Heart Association define resistant hypertension as an office blood pressure above the appropriate goal of therapy (< 140/90 mm Hg for most patients, and < 130/80 mm Hg for those with ischemic heart disease, diabetes, or renal insufficiency) despite the use of three or more antihypertensive drugs from different classes at full dosages, one of which is a diuretic.1,2
In this definition, the number of antihypertensive drugs required is arbitrary. More importantly, the concept of resistant hypertension is focused on identifying patients who may have a reversible cause of hypertension, as well as those who could benefit from special diagnostic or therapeutic intervention because of persistently high blood pressure.
This definition does not apply to patients who have recently been diagnosed with hypertension.
Resistant hypertension is not synonymous with uncontrolled hypertension, which includes all cases of hypertension that is not optimally controlled despite treatment, including apparent resistance (ie, pseudoresistance) and true resistance (defined below).
COMMON, BUT ITS PREVALENCE IS HARD TO PINPOINT
The prevalence of resistant hypertension is unknown because of inadequate sample sizes in published studies. However, it is common and is likely to become more common with the aging of the population and with the increasing prevalence of obesity, diabetes mellitus, and chronic kidney disease.
In small studies, the prevalence of resistance in hypertensive patients ranged from 5% in general medical practice to more than 50% in nephrology clinics. In the National Health and Nutrition Examination Survey in 2003 to 2004, only 58% of people being treated for hypertension had achieved blood pressure levels lower than 140/90 mm Hg,3 and the control rate in those with diabetes mellitus or chronic kidney disease was less than 40%.4
Isolated systolic hypertension—elevated systolic pressure with normal diastolic pressure—increases in prevalence with age in those with treated, uncontrolled hypertension. It accounted for 29.1% of cases of treated, uncontrolled hypertension in patients ages 25 to 44, 66.1% of cases in patients ages 45 to 64, and 87.6% of cases in patients age 65 and older.5
Even in clinical trials, in which one would expect excellent control of hypertension, rates of control ranged from 45% to 82%.6–10
APPARENT RESISTANCE VS TRUE RESISTANCE
Resistant hypertension can be divided arbitrarily into two broad categories: apparent resistance and true resistance, with the prevalence of apparent resistance being considerably higher. Each broad category has a long list of possible causes; most are readily identifiable in the course of a thorough history and physical examination and routine laboratory testing. If resistance to therapy persists, referral to a hypertension specialist is a logical next step.
Detecting pseudoresistance
Causes of apparent resistance include improper technique in measuring blood pressure, such as not having the patient rest before measurement, allowing the patient to have coffee or to smoke just before measurement, or not positioning the patient’s arm at the level of the heart during measurement.
Many elderly patients have calcified arteries that are hard to compress, leading to erroneously high systolic blood pressure measurements, a situation called pseudohypertension and a cause of pseudoresistance. The only way to measure blood pressure accurately in such cases is intra-arterially. These patients often do not have target-organ disease, which would be expected with high systolic pressure.
The white-coat phenomenon is another common cause of apparent resistance. It is defined as persistently elevated clinic or office blood pressure (> 140/90 mm Hg), together with normal daytime ambulatory blood pressure (the “white-coat effect” is the difference between those blood pressures).
Finally, poor patient adherence to treatment is estimated to account for 40% of cases of resistant hypertension.4,5,11 Poor adherence is difficult to prove because patients often claim they are compliant, but certain clues are indicative. For example, patients taking a diuretic should have increased uric acid levels, so normal uric acid levels in a patient on a diuretic could be a clue that he or she is not taking the medication. If poor adherence is suspected, patients should be admitted to the hospital to take the medications under close observation.
Many factors can contribute to true resistance
Many cases of resistant hypertension are drug-induced, particularly in patients taking a nonsteroidal anti-inflammatory drug or a cyclooxygenase II inhibitor. Use of ginseng, ma huang, and bitter lemon should also be suspected. Drugs or herbal preparations contributing to high blood pressure should be discontinued or minimized.
Alcohol intake in excess of two drinks (1 oz of alcohol) per day for men and half that amount for women can also contribute to hypertension.
Volume overload is common and has many causes, including a compensatory response to vasodilators, excessive salt intake, or an undetected reduction in the glomerular filtration rate causing retention of salt and water.
Drug considerations
A common cause of apparent resistant hypertension is physicians not following blood pressure treatment guidelines by not increasing the dosage when needed or by prescribing inappropriate drug combinations.
We commonly see furosemide (Lasix) being misused, ie, being prescribed once daily for hypertension. (It has a shorter duration of action than thiazide diuretics, the usual class of diuretics used for hypertension.)
For a patient who is already on many medications but whose hypertension is not responding, the first step should be to give a diuretic of an appropriate class in an appropriate dosage.
Diuretics are often inappropriately stopped if a patient develops hypokalemia. Potassium supplementation should always be an adjunct to diuretic therapy. Potassium itself is a potent vasodilator and, given as a supplement, has been shown to reduce stroke risk in rats.
The combination of an angiotensin receptor blocker and an angiotensin-converting enzyme inhibitor should not be used for patients with true resistant hypertension. The direct renin inhibitor aliskiren (Tekturna) should not be used in combination with these drugs, and the combination of aliskiren and valsartan (Valturna) has now been taken off the market.
Spironolactone (Aldactone) is sometimes used for resistant hypertension in the belief that in some cases primary aldosteronism is the underlying cause. A study in 1,400 participants confirms that it lowers blood pressure,9 but the reason is unclear: the blood pressure response was unrelated to levels of renin, angiotensin, or the plasma aldosterone-to-renin ratio.
Identify secondary causes of hypertension
Patients should be evaluated for kidney disease, which is the most common secondary medical reason for resistant hypertension. For patients with poor renal function (estimated glomerular filtration rate < 50 mL/minute), hydrochlorothiazide is not effective against hypertension, but chlorthalidone is. In addition, patients with poor renal function should be given loop diuretics such as furosemide two or three times daily, or the long-acting drug torsemide (Demadex) should be used instead.
Genetic variation can cause different rates of metabolism of drugs, contributing to resistant hypertension. Certain people metabolize hydralazine very fast, making it less effective. The same is true for some beta-blockers.
Obesity and diabetes can also contribute to resistant hypertension.
Ancillary neurohumoral studies are occasionally indicated to rule out identifiable causes of secondary hypertension that may be correctable. There are many identifiable causes of hypertension, but detailing each is beyond the scope of this article.
Patients should be tested for thyroid disease. Hypothyroidism can cause high blood pressure, although usually diastolic rather than systolic hypertension. Hyperthyroidism can cause marked systolic hypertension.
Table 1 provides a step-by-step guide for evaluating and managing patients with resistant hypertension.
EXPERIMENTAL DRUG THERAPY
Endothelin receptor antagonists are currently under investigation for the treatment of resistant hypertension. The protein endothelin-1 (ET-1) is a potent vasoconstrictor (30–50 times more potent than angiotensin II and norepinephrine) and has a long duration of action. ET-1 binds to two receptors with opposing effects: ET-A promotes vasoconstriction, and ET-B promotes vasodilation and clears ET-1.
Darusentan, a selective blocker of ET-A, was tested in the phase III DORADO trial, which was discontinued because the initial results did not meet primary outcome measures. Initial findings had indicated that it might not be as useful as hoped. Side effects included headache, flushing, and edema.
EXPERIMENTAL NONPHARMACOLOGIC THERAPIES
Electrical stimulation of carotid sinus baroreceptors is being tried under the assumption that a high sympathoexcitatory state contributes to resistant hypertension. Devices are placed around the carotid artery bifurcation, and stimulation is believed to increase the depressor influences that modulate blood pressure. Large-scale trials are under way, but it is too early to tell if the approach will be useful. Patients complain of neck pain from the device.
Renal denervation is another experimental approach.12 The kidney has a central role in blood pressure regulation: efferent nerves regulate renal vascular resistance, renal blood flow, and renin release from the juxtaglomerular apparatus; afferent nerves modulate sympathetic output from the central nervous system. The results of the Renal Denervation in Patients With Uncontrolled Hypertension (Symplicity HTN) trials 1 and 2 have been encouraging. The Symplicity HTN-3 trial will begin soon in the United States.
OUR PATIENT UNDERGOES ADDITIONAL STUDIES
To rule out the white-coat effect in our patient, we measured her blood pressure with an automated device that takes several readings without the clinician in the room. (This topic has been reviewed by Vidt et al in this journal13). The average of the automated readings was 183/113 mm Hg, and her average pulse was 109 beats per minute, arguing against a white-coat effect.
Her blood pressure was also markedly elevated (average 198/129) during 24-hour ambulatory blood pressure monitoring.
Findings on physical examination were unremarkable except for grade III hypertensive retinopathy. She had no carotid or abdominal bruits. Her peripheral pulses were strong and synchronous bilaterally.
Laboratory testing found the patient had normal serum electrolyte levels and good renal function but relatively low urinary sodium, 90 mmol/day (normal 40–220), and very low renin activity, 0.7 μg/L/h (normal up-right 0.8–5.8 μg/L/h, supine 0.5–1.8 μg/L/h), calling into question the wisdom of treatment with an angiotensin receptor blocker.
Hemodynamic studies were performed using impedance cardiography and found very high systemic vascular resistance with normal cardiac output, indicating that the patient had a high preload, which could be from hypervolemia or intense venous constriction. It is especially interesting that her vascular resistance was high despite her treatment regimen that included an angiotensin receptor blocker and a vasodilator, perhaps an indication of nonadherence with her medications.
Diuresis reduces her blood pressure
The patient was admitted to the hospital, and because her laboratory results indicated that plasma renin activity was suppressed, the angiotensin receptor blocker valsartan was discontinued.
On day 1, her weight was 162 lb and average blood pressure was 194/128 mm Hg. After 4 days of diuresis with escalating doses of furosemide, her weight was 153 lb and blood pressures ranged from 140 to 158 over 82 to 98 mm Hg. Her heart rate was 90 beats per minute. The hospital stay showed that volume overload was one of the factors maintaining her hypertension. She was discharged on metoprolol succinate (Toprol-XL) 100 mg twice daily and furosemide 80 mg twice daily.
Her blood pressure fluctuates widely after discharge
Over the next 5 days after discharge, the patient’s blood pressure rose steadily to 180/122 mm Hg, her heart rate was in excess of 100 beats per minute, and her weight increased to 158 lb. Blood screening found that the level of metoprolol was undetectable, and a diuretic screen showed no furosemide in the urine. Both the patient and her husband were adamant that she was taking her medications.
Hydrochlorothiazide 25 mg daily was added, and nadolol (Corgard) 80 mg once daily was started in place of metoprolol. On a return visit, her blood pressure and heart rate were finally good at 138/86 mm Hg and 60 beats per minute (sitting) and 134/92 and 63 (standing).
On 24-hour monitoring, some fluctuations of elevated blood pressure were still evident, with an average of 142/91 mm Hg, so nifedipine (Procardia) 60 mg daily was added.
Her final list of medications is hydrochlorothiazide 25 mg, nadolol 80 mg, and nifedipine XL 60 mg, all taken once daily.
Volume overload complicated by nonadherence
In summary, the main pathogenetic mechanism that sustained this patient’s hypertension was volume overload. Her urinary sodium level indicated that she was not taking excessive amounts of sodium. The volume overload may have been a compensatory response to the concomitant use of peripheral vasodilators plus sympatholytic agents.
In addition, she was not adherent to her antihypertensive regimen. The fact that her heart rate was 109 beats per minute despite having a drug regimen that included five sympathetic blocking agents was a strong clue. She eventually admitted that she did not like taking diuretics because they made her skin wrinkle.
In general, in a case like this, I try to minimize the number of drugs and give a diuretic as well as different classes of appropriate drugs.
Poor control of blood pressure is one of the most common risk factors for death worldwide, responsible for 62% of cases of cerebral vascular disease and 49% of cases of ischemic heart disease as well as 7.1 million deaths annually. As our population ages and the prevalence of obesity, diabetes, and chronic kidney disease increases, resistant hypertension will be seen more often in general practice.
Using a case study, this article will provide a strategy for diagnosing and treating resistant hypertension.
CASE: A WOMAN WITH LONG-STANDING HIGH BLOOD PRESSURE
A 37-year-old woman was referred for help with managing difficult-to-control hypertension. She had been diagnosed with hypertension at age 32, and it was well controlled until about 2 years ago. Various combinations of antihypertensive drugs had been tried, and a search for a cause of secondary hypertension revealed no clues.
On examination, her blood pressure averaged 212/124 mm Hg, and her heart rate was 109 beats per minute. Her medications were:
- Amlodipine (Norvasc), a calcium channel blocker, 10 mg once daily
- Valsartan (Diovan), an angiotensin II receptor antagonist, 160 mg once daily
- Carvedilol (Coreg), a beta-blocker, 25 mg twice daily
- Labetalol (Normodyne), a beta-blocker, 400 mg three times daily
- Clonidine (Catapres), a sympatholytic agent, 0.05 mg three times daily
- Doxazosin (Cardura), a peripheral alpha-blocker, 16 mg once daily
- Xylometazoline (Xylomet), an alpha agonist nasal spray for nasal congestion.
She had previously been taking spironolactone (Aldactone), hydralazine (Apresoline), and hydrochlorothiazide, but they were discontinued because of adverse effects.
Does this patient have resistant hypertension? How should her condition be managed?
RESISTANT HYPERTENSION DEFINED
The seventh Joint National Committee and the American Heart Association define resistant hypertension as an office blood pressure above the appropriate goal of therapy (< 140/90 mm Hg for most patients, and < 130/80 mm Hg for those with ischemic heart disease, diabetes, or renal insufficiency) despite the use of three or more antihypertensive drugs from different classes at full dosages, one of which is a diuretic.1,2
In this definition, the number of antihypertensive drugs required is arbitrary. More importantly, the concept of resistant hypertension is focused on identifying patients who may have a reversible cause of hypertension, as well as those who could benefit from special diagnostic or therapeutic intervention because of persistently high blood pressure.
This definition does not apply to patients who have recently been diagnosed with hypertension.
Resistant hypertension is not synonymous with uncontrolled hypertension, which includes all cases of hypertension that is not optimally controlled despite treatment, including apparent resistance (ie, pseudoresistance) and true resistance (defined below).
COMMON, BUT ITS PREVALENCE IS HARD TO PINPOINT
The prevalence of resistant hypertension is unknown because of inadequate sample sizes in published studies. However, it is common and is likely to become more common with the aging of the population and with the increasing prevalence of obesity, diabetes mellitus, and chronic kidney disease.
In small studies, the prevalence of resistance in hypertensive patients ranged from 5% in general medical practice to more than 50% in nephrology clinics. In the National Health and Nutrition Examination Survey in 2003 to 2004, only 58% of people being treated for hypertension had achieved blood pressure levels lower than 140/90 mm Hg,3 and the control rate in those with diabetes mellitus or chronic kidney disease was less than 40%.4
Isolated systolic hypertension—elevated systolic pressure with normal diastolic pressure—increases in prevalence with age in those with treated, uncontrolled hypertension. It accounted for 29.1% of cases of treated, uncontrolled hypertension in patients ages 25 to 44, 66.1% of cases in patients ages 45 to 64, and 87.6% of cases in patients age 65 and older.5
Even in clinical trials, in which one would expect excellent control of hypertension, rates of control ranged from 45% to 82%.6–10
APPARENT RESISTANCE VS TRUE RESISTANCE
Resistant hypertension can be divided arbitrarily into two broad categories: apparent resistance and true resistance, with the prevalence of apparent resistance being considerably higher. Each broad category has a long list of possible causes; most are readily identifiable in the course of a thorough history and physical examination and routine laboratory testing. If resistance to therapy persists, referral to a hypertension specialist is a logical next step.
Detecting pseudoresistance
Causes of apparent resistance include improper technique in measuring blood pressure, such as not having the patient rest before measurement, allowing the patient to have coffee or to smoke just before measurement, or not positioning the patient’s arm at the level of the heart during measurement.
Many elderly patients have calcified arteries that are hard to compress, leading to erroneously high systolic blood pressure measurements, a situation called pseudohypertension and a cause of pseudoresistance. The only way to measure blood pressure accurately in such cases is intra-arterially. These patients often do not have target-organ disease, which would be expected with high systolic pressure.
The white-coat phenomenon is another common cause of apparent resistance. It is defined as persistently elevated clinic or office blood pressure (> 140/90 mm Hg), together with normal daytime ambulatory blood pressure (the “white-coat effect” is the difference between those blood pressures).
Finally, poor patient adherence to treatment is estimated to account for 40% of cases of resistant hypertension.4,5,11 Poor adherence is difficult to prove because patients often claim they are compliant, but certain clues are indicative. For example, patients taking a diuretic should have increased uric acid levels, so normal uric acid levels in a patient on a diuretic could be a clue that he or she is not taking the medication. If poor adherence is suspected, patients should be admitted to the hospital to take the medications under close observation.
Many factors can contribute to true resistance
Many cases of resistant hypertension are drug-induced, particularly in patients taking a nonsteroidal anti-inflammatory drug or a cyclooxygenase II inhibitor. Use of ginseng, ma huang, and bitter lemon should also be suspected. Drugs or herbal preparations contributing to high blood pressure should be discontinued or minimized.
Alcohol intake in excess of two drinks (1 oz of alcohol) per day for men and half that amount for women can also contribute to hypertension.
Volume overload is common and has many causes, including a compensatory response to vasodilators, excessive salt intake, or an undetected reduction in the glomerular filtration rate causing retention of salt and water.
Drug considerations
A common cause of apparent resistant hypertension is physicians not following blood pressure treatment guidelines by not increasing the dosage when needed or by prescribing inappropriate drug combinations.
We commonly see furosemide (Lasix) being misused, ie, being prescribed once daily for hypertension. (It has a shorter duration of action than thiazide diuretics, the usual class of diuretics used for hypertension.)
For a patient who is already on many medications but whose hypertension is not responding, the first step should be to give a diuretic of an appropriate class in an appropriate dosage.
Diuretics are often inappropriately stopped if a patient develops hypokalemia. Potassium supplementation should always be an adjunct to diuretic therapy. Potassium itself is a potent vasodilator and, given as a supplement, has been shown to reduce stroke risk in rats.
The combination of an angiotensin receptor blocker and an angiotensin-converting enzyme inhibitor should not be used for patients with true resistant hypertension. The direct renin inhibitor aliskiren (Tekturna) should not be used in combination with these drugs, and the combination of aliskiren and valsartan (Valturna) has now been taken off the market.
Spironolactone (Aldactone) is sometimes used for resistant hypertension in the belief that in some cases primary aldosteronism is the underlying cause. A study in 1,400 participants confirms that it lowers blood pressure,9 but the reason is unclear: the blood pressure response was unrelated to levels of renin, angiotensin, or the plasma aldosterone-to-renin ratio.
Identify secondary causes of hypertension
Patients should be evaluated for kidney disease, which is the most common secondary medical reason for resistant hypertension. For patients with poor renal function (estimated glomerular filtration rate < 50 mL/minute), hydrochlorothiazide is not effective against hypertension, but chlorthalidone is. In addition, patients with poor renal function should be given loop diuretics such as furosemide two or three times daily, or the long-acting drug torsemide (Demadex) should be used instead.
Genetic variation can cause different rates of metabolism of drugs, contributing to resistant hypertension. Certain people metabolize hydralazine very fast, making it less effective. The same is true for some beta-blockers.
Obesity and diabetes can also contribute to resistant hypertension.
Ancillary neurohumoral studies are occasionally indicated to rule out identifiable causes of secondary hypertension that may be correctable. There are many identifiable causes of hypertension, but detailing each is beyond the scope of this article.
Patients should be tested for thyroid disease. Hypothyroidism can cause high blood pressure, although usually diastolic rather than systolic hypertension. Hyperthyroidism can cause marked systolic hypertension.
Table 1 provides a step-by-step guide for evaluating and managing patients with resistant hypertension.
EXPERIMENTAL DRUG THERAPY
Endothelin receptor antagonists are currently under investigation for the treatment of resistant hypertension. The protein endothelin-1 (ET-1) is a potent vasoconstrictor (30–50 times more potent than angiotensin II and norepinephrine) and has a long duration of action. ET-1 binds to two receptors with opposing effects: ET-A promotes vasoconstriction, and ET-B promotes vasodilation and clears ET-1.
Darusentan, a selective blocker of ET-A, was tested in the phase III DORADO trial, which was discontinued because the initial results did not meet primary outcome measures. Initial findings had indicated that it might not be as useful as hoped. Side effects included headache, flushing, and edema.
EXPERIMENTAL NONPHARMACOLOGIC THERAPIES
Electrical stimulation of carotid sinus baroreceptors is being tried under the assumption that a high sympathoexcitatory state contributes to resistant hypertension. Devices are placed around the carotid artery bifurcation, and stimulation is believed to increase the depressor influences that modulate blood pressure. Large-scale trials are under way, but it is too early to tell if the approach will be useful. Patients complain of neck pain from the device.
Renal denervation is another experimental approach.12 The kidney has a central role in blood pressure regulation: efferent nerves regulate renal vascular resistance, renal blood flow, and renin release from the juxtaglomerular apparatus; afferent nerves modulate sympathetic output from the central nervous system. The results of the Renal Denervation in Patients With Uncontrolled Hypertension (Symplicity HTN) trials 1 and 2 have been encouraging. The Symplicity HTN-3 trial will begin soon in the United States.
OUR PATIENT UNDERGOES ADDITIONAL STUDIES
To rule out the white-coat effect in our patient, we measured her blood pressure with an automated device that takes several readings without the clinician in the room. (This topic has been reviewed by Vidt et al in this journal13). The average of the automated readings was 183/113 mm Hg, and her average pulse was 109 beats per minute, arguing against a white-coat effect.
Her blood pressure was also markedly elevated (average 198/129) during 24-hour ambulatory blood pressure monitoring.
Findings on physical examination were unremarkable except for grade III hypertensive retinopathy. She had no carotid or abdominal bruits. Her peripheral pulses were strong and synchronous bilaterally.
Laboratory testing found the patient had normal serum electrolyte levels and good renal function but relatively low urinary sodium, 90 mmol/day (normal 40–220), and very low renin activity, 0.7 μg/L/h (normal up-right 0.8–5.8 μg/L/h, supine 0.5–1.8 μg/L/h), calling into question the wisdom of treatment with an angiotensin receptor blocker.
Hemodynamic studies were performed using impedance cardiography and found very high systemic vascular resistance with normal cardiac output, indicating that the patient had a high preload, which could be from hypervolemia or intense venous constriction. It is especially interesting that her vascular resistance was high despite her treatment regimen that included an angiotensin receptor blocker and a vasodilator, perhaps an indication of nonadherence with her medications.
Diuresis reduces her blood pressure
The patient was admitted to the hospital, and because her laboratory results indicated that plasma renin activity was suppressed, the angiotensin receptor blocker valsartan was discontinued.
On day 1, her weight was 162 lb and average blood pressure was 194/128 mm Hg. After 4 days of diuresis with escalating doses of furosemide, her weight was 153 lb and blood pressures ranged from 140 to 158 over 82 to 98 mm Hg. Her heart rate was 90 beats per minute. The hospital stay showed that volume overload was one of the factors maintaining her hypertension. She was discharged on metoprolol succinate (Toprol-XL) 100 mg twice daily and furosemide 80 mg twice daily.
Her blood pressure fluctuates widely after discharge
Over the next 5 days after discharge, the patient’s blood pressure rose steadily to 180/122 mm Hg, her heart rate was in excess of 100 beats per minute, and her weight increased to 158 lb. Blood screening found that the level of metoprolol was undetectable, and a diuretic screen showed no furosemide in the urine. Both the patient and her husband were adamant that she was taking her medications.
Hydrochlorothiazide 25 mg daily was added, and nadolol (Corgard) 80 mg once daily was started in place of metoprolol. On a return visit, her blood pressure and heart rate were finally good at 138/86 mm Hg and 60 beats per minute (sitting) and 134/92 and 63 (standing).
On 24-hour monitoring, some fluctuations of elevated blood pressure were still evident, with an average of 142/91 mm Hg, so nifedipine (Procardia) 60 mg daily was added.
Her final list of medications is hydrochlorothiazide 25 mg, nadolol 80 mg, and nifedipine XL 60 mg, all taken once daily.
Volume overload complicated by nonadherence
In summary, the main pathogenetic mechanism that sustained this patient’s hypertension was volume overload. Her urinary sodium level indicated that she was not taking excessive amounts of sodium. The volume overload may have been a compensatory response to the concomitant use of peripheral vasodilators plus sympatholytic agents.
In addition, she was not adherent to her antihypertensive regimen. The fact that her heart rate was 109 beats per minute despite having a drug regimen that included five sympathetic blocking agents was a strong clue. She eventually admitted that she did not like taking diuretics because they made her skin wrinkle.
In general, in a case like this, I try to minimize the number of drugs and give a diuretic as well as different classes of appropriate drugs.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2007; 51:1403–1419.
- Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment and control of hypertension among United States adults 1999–2004. Hypertension 2007; 49:69–75.
- Sarafidis PA, Li S, Chen SC, et al. Hypertension awareness, treatment, and control in chronic kidney disease. Am J Med 2008; 121:332–340.
- Sarafidis PA, Bakris GL. State of hypertension management in the United States: confluence of risk factors and the prevalence of resistant hypertension. J Clin Hypertens (Greenwich) 2008; 10:130–139.
- Jamerson K, Bakris GL, Dahlöf B, et al; for the ACCOMPLISH Investigators. Exceptional early blood pressure control rates: the ACCOMPLISH trial. Blood Pressure 2007; 16:80–86.
- Dahlöf B, Devereux RB, Kjeldsen S, et al; for the LIFE study group. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–003.
- Cushman WC, Ford CE, Cutler JA, et al; for the ALLHAT Collaborative Research Group. Success and predictors of blood pressure control in diverse North American settings: the Antihypertensive and Lipid-Lowering and Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens (Greenwich) 2002; 4:393–404.
- Chapman N, Dobson J, Wilson S, et al; on behalf of the Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007; 49:839–845.
- Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al; INVEST Investigators. A calcium antagonist vs a noncalcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003; 290:2805–2816.
- Calhoun DA, Jones D, Textor S, et al. AHA Scientific Statement. Resistant hypertension: diagnosis, evaluation, and treatment. Circulation 2008; 17:e510–e526.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension: guarded optimism. Cleve Clin J Med 2012; 79:501–510.
- Vidt DG, Lang RS, Seballos RJ, Misra-Hebert A, Campbell J, Bena JF. Taking blood pressure: too important to trust to humans? Cleve Clin J Med 2010; 77:683–688.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2007; 51:1403–1419.
- Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment and control of hypertension among United States adults 1999–2004. Hypertension 2007; 49:69–75.
- Sarafidis PA, Li S, Chen SC, et al. Hypertension awareness, treatment, and control in chronic kidney disease. Am J Med 2008; 121:332–340.
- Sarafidis PA, Bakris GL. State of hypertension management in the United States: confluence of risk factors and the prevalence of resistant hypertension. J Clin Hypertens (Greenwich) 2008; 10:130–139.
- Jamerson K, Bakris GL, Dahlöf B, et al; for the ACCOMPLISH Investigators. Exceptional early blood pressure control rates: the ACCOMPLISH trial. Blood Pressure 2007; 16:80–86.
- Dahlöf B, Devereux RB, Kjeldsen S, et al; for the LIFE study group. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–003.
- Cushman WC, Ford CE, Cutler JA, et al; for the ALLHAT Collaborative Research Group. Success and predictors of blood pressure control in diverse North American settings: the Antihypertensive and Lipid-Lowering and Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens (Greenwich) 2002; 4:393–404.
- Chapman N, Dobson J, Wilson S, et al; on behalf of the Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007; 49:839–845.
- Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al; INVEST Investigators. A calcium antagonist vs a noncalcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003; 290:2805–2816.
- Calhoun DA, Jones D, Textor S, et al. AHA Scientific Statement. Resistant hypertension: diagnosis, evaluation, and treatment. Circulation 2008; 17:e510–e526.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension: guarded optimism. Cleve Clin J Med 2012; 79:501–510.
- Vidt DG, Lang RS, Seballos RJ, Misra-Hebert A, Campbell J, Bena JF. Taking blood pressure: too important to trust to humans? Cleve Clin J Med 2010; 77:683–688.
KEY POINTS
- Resistant hypertension is arbitrarily divided into two categories: apparent resistance (pseudoresistant hypertension) and true resistance. Apparent resistance is much more common.
- Common causes of true resistant hypertension are volume overload, excessive alcohol use, some drugs (eg, nonsteroidal anti-inflammatory drugs), and some over-the-counter supplements.
- Volume overload commonly results from excess sodium intake, kidney disease, or a counterregulatory response to arterial vasodilation.
- To address volume overload, an appropriate diuretic at an adequate dosage is a cornerstone of therapy, along with potassium supplementation.
- Hospitalization may be needed to monitor drug intake if poor compliance is suspected.






