User login
Missed visits during pandemic cause ‘detrimental ripple effects’
according to a new report from the Urban Institute.
Among the adults who postponed or missed care, 32.6% said the gap worsened one or more health conditions or limited their ability to work or perform daily activities. The findings highlight “the detrimental ripple effects of delaying or forgoing care on overall health, functioning, and well-being,” researchers write.
The survey, conducted among 4,007 U.S. adults aged 18-64 in September 2020, found that adults with one or more chronic conditions were more likely than adults without chronic conditions to have delayed or missed care (40.7% vs. 26.4%). Adults with a mental health condition were particularly likely to have delayed or gone without care, write Dulce Gonzalez, MPP, a research associate in the Health Policy Center at the Urban Institute, and colleagues.
Doctors are already seeing the consequences of the missed visits, says Jacqueline W. Fincher, MD, president of the American College of Physicians.
Two of her patients with chronic conditions missed appointments last year. By the time they resumed care in 2021, their previsit lab tests showed significant kidney deterioration.
“Lo and behold, their kidneys were in failure. … One was in the hospital for 3 days and the other one was in for 5 days,” said Dr. Fincher, who practices general internal medicine in Georgia.
Dr. Fincher’s office has been proactive about calling patients with chronic diseases who missed follow-up visits or laboratory testing or who may have run out of medication, she said.
In her experience, delays mainly have been because of patients postponing visits. “We have stayed open the whole time now,” Dr. Fincher said. Her office offers telemedicine visits and in-person visits with safety precautions.
Still, some patients have decided to postpone care during the pandemic instead of asking their primary care doctor what they should do.
“We do know that chronic problems left without appropriate follow-up can create worse problems for them in terms of stroke, heart attack, and end organ damage,” Dr. Fincher said.
Lost lives
Future studies may help researchers understand the effects of delayed and missed care during the pandemic, said Russell S. Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston.
“Although it is still early, and more data on patient outcomes will need to be collected, I anticipate that the ... delays in diagnosis, in cancer screening, and in management of chronic illness will result in lost lives and will emphasize the important role that primary care plays in saving lives,” Dr. Phillips said.
During the first several months of the pandemic, there were fewer diagnoses of hypertension, diabetes, and depression, Dr. Phillips said.
“In addition, and most importantly, the mortality rate for non-COVID conditions increased, suggesting that patients were not seeking care for symptoms of stroke or heart attack, which can be fatal if untreated,” he said. “We have also seen substantial decreases in cancer screening tests such as colonoscopy, and modeling studies suggest this will cost more lives based on delayed diagnoses of cancer.”
Vaccinating patients against COVID-19 may help primary care practices and patients get back on track, Dr. Phillips suggested.
In the meantime, some patients remain reluctant to come in. “Volumes are still lower than prepandemic, so it is challenging to overcome what is likely to be pent-up demand,” he told this news organization in an email. “Additionally, the continued burden of evaluating, testing, and monitoring patients with COVID or COVID-like symptoms makes it difficult to focus on chronic illness.”
Care most often skipped
The Urban Institute survey asked respondents about delays in prescription drugs, general doctor and specialist visits, going to a hospital, preventive health screenings or medical tests, treatment or follow-up care, dental care, mental health care or counseling, treatment or counseling for alcohol or drug use, and other types of medical care.
Dental care was the most common type of care that adults delayed or did not receive because of the pandemic (25.3%), followed by general doctor or specialist visits (20.6%) and preventive health screenings or medical tests (15.5%).
Black adults were more likely than White or Hispanic/Latinx adults to have delayed or forgone care (39.7% vs. 34.3% and 35.5%), the researchers found. Compared with adults with higher incomes, adults with lower incomes were more likely to have missed multiple types of care (26.6% vs. 20.3%).
The report by the Urban Institute researchers was supported by the Robert Wood Johnson Foundation. Dr. Phillips is an adviser to two telemedicine companies, Bicycle Health and Grow Health. Dr. Fincher has disclosed no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
according to a new report from the Urban Institute.
Among the adults who postponed or missed care, 32.6% said the gap worsened one or more health conditions or limited their ability to work or perform daily activities. The findings highlight “the detrimental ripple effects of delaying or forgoing care on overall health, functioning, and well-being,” researchers write.
The survey, conducted among 4,007 U.S. adults aged 18-64 in September 2020, found that adults with one or more chronic conditions were more likely than adults without chronic conditions to have delayed or missed care (40.7% vs. 26.4%). Adults with a mental health condition were particularly likely to have delayed or gone without care, write Dulce Gonzalez, MPP, a research associate in the Health Policy Center at the Urban Institute, and colleagues.
Doctors are already seeing the consequences of the missed visits, says Jacqueline W. Fincher, MD, president of the American College of Physicians.
Two of her patients with chronic conditions missed appointments last year. By the time they resumed care in 2021, their previsit lab tests showed significant kidney deterioration.
“Lo and behold, their kidneys were in failure. … One was in the hospital for 3 days and the other one was in for 5 days,” said Dr. Fincher, who practices general internal medicine in Georgia.
Dr. Fincher’s office has been proactive about calling patients with chronic diseases who missed follow-up visits or laboratory testing or who may have run out of medication, she said.
In her experience, delays mainly have been because of patients postponing visits. “We have stayed open the whole time now,” Dr. Fincher said. Her office offers telemedicine visits and in-person visits with safety precautions.
Still, some patients have decided to postpone care during the pandemic instead of asking their primary care doctor what they should do.
“We do know that chronic problems left without appropriate follow-up can create worse problems for them in terms of stroke, heart attack, and end organ damage,” Dr. Fincher said.
Lost lives
Future studies may help researchers understand the effects of delayed and missed care during the pandemic, said Russell S. Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston.
“Although it is still early, and more data on patient outcomes will need to be collected, I anticipate that the ... delays in diagnosis, in cancer screening, and in management of chronic illness will result in lost lives and will emphasize the important role that primary care plays in saving lives,” Dr. Phillips said.
During the first several months of the pandemic, there were fewer diagnoses of hypertension, diabetes, and depression, Dr. Phillips said.
“In addition, and most importantly, the mortality rate for non-COVID conditions increased, suggesting that patients were not seeking care for symptoms of stroke or heart attack, which can be fatal if untreated,” he said. “We have also seen substantial decreases in cancer screening tests such as colonoscopy, and modeling studies suggest this will cost more lives based on delayed diagnoses of cancer.”
Vaccinating patients against COVID-19 may help primary care practices and patients get back on track, Dr. Phillips suggested.
In the meantime, some patients remain reluctant to come in. “Volumes are still lower than prepandemic, so it is challenging to overcome what is likely to be pent-up demand,” he told this news organization in an email. “Additionally, the continued burden of evaluating, testing, and monitoring patients with COVID or COVID-like symptoms makes it difficult to focus on chronic illness.”
Care most often skipped
The Urban Institute survey asked respondents about delays in prescription drugs, general doctor and specialist visits, going to a hospital, preventive health screenings or medical tests, treatment or follow-up care, dental care, mental health care or counseling, treatment or counseling for alcohol or drug use, and other types of medical care.
Dental care was the most common type of care that adults delayed or did not receive because of the pandemic (25.3%), followed by general doctor or specialist visits (20.6%) and preventive health screenings or medical tests (15.5%).
Black adults were more likely than White or Hispanic/Latinx adults to have delayed or forgone care (39.7% vs. 34.3% and 35.5%), the researchers found. Compared with adults with higher incomes, adults with lower incomes were more likely to have missed multiple types of care (26.6% vs. 20.3%).
The report by the Urban Institute researchers was supported by the Robert Wood Johnson Foundation. Dr. Phillips is an adviser to two telemedicine companies, Bicycle Health and Grow Health. Dr. Fincher has disclosed no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
according to a new report from the Urban Institute.
Among the adults who postponed or missed care, 32.6% said the gap worsened one or more health conditions or limited their ability to work or perform daily activities. The findings highlight “the detrimental ripple effects of delaying or forgoing care on overall health, functioning, and well-being,” researchers write.
The survey, conducted among 4,007 U.S. adults aged 18-64 in September 2020, found that adults with one or more chronic conditions were more likely than adults without chronic conditions to have delayed or missed care (40.7% vs. 26.4%). Adults with a mental health condition were particularly likely to have delayed or gone without care, write Dulce Gonzalez, MPP, a research associate in the Health Policy Center at the Urban Institute, and colleagues.
Doctors are already seeing the consequences of the missed visits, says Jacqueline W. Fincher, MD, president of the American College of Physicians.
Two of her patients with chronic conditions missed appointments last year. By the time they resumed care in 2021, their previsit lab tests showed significant kidney deterioration.
“Lo and behold, their kidneys were in failure. … One was in the hospital for 3 days and the other one was in for 5 days,” said Dr. Fincher, who practices general internal medicine in Georgia.
Dr. Fincher’s office has been proactive about calling patients with chronic diseases who missed follow-up visits or laboratory testing or who may have run out of medication, she said.
In her experience, delays mainly have been because of patients postponing visits. “We have stayed open the whole time now,” Dr. Fincher said. Her office offers telemedicine visits and in-person visits with safety precautions.
Still, some patients have decided to postpone care during the pandemic instead of asking their primary care doctor what they should do.
“We do know that chronic problems left without appropriate follow-up can create worse problems for them in terms of stroke, heart attack, and end organ damage,” Dr. Fincher said.
Lost lives
Future studies may help researchers understand the effects of delayed and missed care during the pandemic, said Russell S. Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston.
“Although it is still early, and more data on patient outcomes will need to be collected, I anticipate that the ... delays in diagnosis, in cancer screening, and in management of chronic illness will result in lost lives and will emphasize the important role that primary care plays in saving lives,” Dr. Phillips said.
During the first several months of the pandemic, there were fewer diagnoses of hypertension, diabetes, and depression, Dr. Phillips said.
“In addition, and most importantly, the mortality rate for non-COVID conditions increased, suggesting that patients were not seeking care for symptoms of stroke or heart attack, which can be fatal if untreated,” he said. “We have also seen substantial decreases in cancer screening tests such as colonoscopy, and modeling studies suggest this will cost more lives based on delayed diagnoses of cancer.”
Vaccinating patients against COVID-19 may help primary care practices and patients get back on track, Dr. Phillips suggested.
In the meantime, some patients remain reluctant to come in. “Volumes are still lower than prepandemic, so it is challenging to overcome what is likely to be pent-up demand,” he told this news organization in an email. “Additionally, the continued burden of evaluating, testing, and monitoring patients with COVID or COVID-like symptoms makes it difficult to focus on chronic illness.”
Care most often skipped
The Urban Institute survey asked respondents about delays in prescription drugs, general doctor and specialist visits, going to a hospital, preventive health screenings or medical tests, treatment or follow-up care, dental care, mental health care or counseling, treatment or counseling for alcohol or drug use, and other types of medical care.
Dental care was the most common type of care that adults delayed or did not receive because of the pandemic (25.3%), followed by general doctor or specialist visits (20.6%) and preventive health screenings or medical tests (15.5%).
Black adults were more likely than White or Hispanic/Latinx adults to have delayed or forgone care (39.7% vs. 34.3% and 35.5%), the researchers found. Compared with adults with higher incomes, adults with lower incomes were more likely to have missed multiple types of care (26.6% vs. 20.3%).
The report by the Urban Institute researchers was supported by the Robert Wood Johnson Foundation. Dr. Phillips is an adviser to two telemedicine companies, Bicycle Health and Grow Health. Dr. Fincher has disclosed no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Postoperative Neurologic Deficits in a Veteran With Recent COVID-19
Anesthesia providers should be aware of COVID-19 sensitive stroke code practices and maintain heightened vigilance for the need to implement perioperative stroke mitigation strategies.
The risk of perioperative stroke in noncardiac, nonneurologic, nonvascular surgery ranges from 0.1 to 1.9% and is associated with increased mortality.1,2 Stroke mechanisms include both ischemia (large and small vessel occlusion, cardioembolism, anemic-tissue hypoxia, cerebral hypoperfusion) and hemorrhage.1 Risk factors for perioperative stroke include prior cerebral vascular accident (CVA), hypertension, aged > 62 years, acute renal insufficiency, dialysis, and recent myocardial infarction (MI).2
Introduction
COVID-19 was declared a pandemic by the World Health Organization in March 2020.3 COVID-19 has certainly affected the veteran population; between February and May 2020, more than 60,000 veterans were tested for COVID-19 with a positive rate of about 9%.4 While primarily affecting the respiratory system, there are increasing reports of COVID-19 neurologic manifestations: headache, hypogeusia, hyposomia, seizure, encephalitis, and acute stroke.5 In an early case series from Wuhan, China, 36% of 214 patients with COVID-19 reported neurologic complications, and acute CVAs were more common in patients with severe (compared to milder) viral disease presentations (5.7% vs 0.8%).6 Large vessel stroke was a presenting feature in another report of 5 patients aged < 50 years.7
The mechanism of ischemic stroke in the setting of COVID-19 is unclear.8 Indeed, stroke and COVID-19 share similar risk factors (eg, hypertension, diabetes mellitus [DM], older age), and immobile critically ill patients may already be prone to developing stroke.5,9 However, COVID-19 is associated with arterial and venous thromboembolism, elevated D-dimer and fibrinogen levels, and antiphospholipid antibody production. This prothrombotic state may be linked to cytokine-induced endothelial damage, mononuclear cell activation, tissue factor expression, and ultimately thrombin propagation and platelet activation.8
The rates of perioperative stroke may change as more patients with COVID-19 present for surgery, and the anesthesiology care team must prioritize mitigation efforts in high-risk patients, including veterans. Reducing the elevated stroke burden within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) is a public health priority.10 We present the case of a veteran with prior CVA and recent positive COVID-19 testing who experienced transient weakness and dysarthria following plastic surgery. The patient discussed provided written Health Insurance Portability and Accountability Act consent for publication of this report.
Case Presentation
A 75-year-old male veteran presented to the Minneapolis VA Medical Center in Minnesota with chronic left foot ulceration necessitating debridement and flap coverage. His medical history was significant for hypertension, type 2 DM, anemia of chronic disease, and coronary artery disease (left ventricular ejection fraction, 50%). Additionally, he had prior ischemic strokes in the oculomotor nucleus (in 2004 with internuclear ophthalmoplegia) and left ventral medulla (in 2019 with right hemiparesis). During his 2019 poststroke rehabilitation, he was diagnosed with mild neurocognitive deficit not attributable to his strokes. The patient’s medications included amlodipine, lisinopril, atorvastatin, clopidogrel (lifelong for secondary stroke prevention), metformin, and glipizide. The debridement procedure was initially delayed 3 weeks due to positive routine preoperative COVID-19 nasopharyngeal testing, though he reported no respiratory symptoms or fever. During the delay, the primary team prescribed daily oral rivaroxaban for thrombosis prophylaxis in addition to clopidogrel. One week prior to surgery, his repeat COVID-19 test was negative and prophylactic anticoagulation stopped.
On the day of surgery, the patient was hemodynamically stable: heart rate 86 beats/min, blood pressure 167/93 mm Hg (baseline 120-150 mm Hg systolic pressure), respiratory rate 16 breaths/min, oxygen saturation 99% without supplemental oxygen, temperature 97.1 °F. He received amlodipine and clopidogrel, but not lisinopril, that morning. No focal neurologic deficits were appreciated on preoperative examination, and resolution of symptoms related to the 2 prior MIs was confirmed. Preoperative glucose was 163 mg/dL. Femoral and sciatic peripheral nerve blocks were done for postoperative analgesia. A preinduction arterial line was placed and 2 mg of midazolam was administered for anxiolysis. Induction of general anesthesia with oral endotracheal intubation proceeded uneventfully; he was positioned prone.
Given his stroke risk factors, mean arterial pressure was maintained > 70 mm Hg for the duration of surgery. No vasoactive infusions were necessary and no β-blocking agents were administered. Insulin infusion was required; the maximum-recorded glucose was 219 mg/dL. Arterial blood gas samples were routinely drawn; acid-base balance was well maintained, PaO2 was > 185 mm Hg, and PaCO2 ranged from 29.4 to 38.5 mm Hg. The patient received 2 units of packed red blood cells for nadir hemoglobin of 7.5 mg/dL. At surgery end, we fully reversed neuromuscular blockade with suggamadex. The patient was returned to a supine position and extubated uneventfully after demonstrating the ability to follow commands.
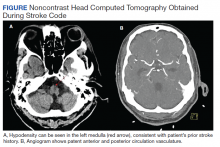
During postanesthesia care unit (PACU) handoff, the patient exhibited acute speech impairment. He was able to state his name on repetition but seemed confused and sedated. Prompt formal neurology evaluation (stroke code) was sought. Initial National Institutes of Health (NIH) stroke scale score was 8 (1 for level of consciousness, 1 for minor right facial droop, 1 for right arm drift, 3 for right leg with no effort against gravity, 1 for right partial sensory loss, and 1 for mild dysarthria). The patient was oriented only to self. Other findings included mild right facial droop and dysarthria. On a 5-point strength scale, he scored 4 for the right deltoid, biceps, triceps, wrist extensors, right knee flexion, right dorsiflexion, and plantarflexion, 2 for right hip flexion, and ≥ 4 for right knee extension. Positive sensory findings were notable for decreased pin prick sensation on the right limbs.
We obtained emergent head computed tomography (CT) that was negative for acute abnormalities; CT angiography was negative for large vessel occlusion or clinically significant stenosis (Figure). On returning to the PACU from the CT scanner, the patient regained symmetric strength in both arms, right leg was antigravity, and his speech had normalized. Prior to PACU discharge 2 hours later, the patient was back to his prehospitalization neurologic function and NIH stroke scale was 0. Given this rapid clinical resolution, no acute stroke interventions were done, though permissive hypertension was recommended by the neurologist during PACU recovery.
The neurology team concluded that the patient’s symptoms were likely secondary to recrudescence of previous stroke symptoms in the setting of brief postoperative delirium (POD). However, we could not exclude transient ischemic attack or new cardioembolism, therefore patient was started on dual antiplatelet therapy for 3 weeks. Unfortunately, elective confirmatory magnetic resonance imaging (MRI) was not sought to confirm new ischemic changes due hospital COVID-19 restrictions on nonessential scanning. Neurology did not recommend carotid duplex ultrasound given patent vasculature on the head and neck CT angiography. Finally, the patient had undergone surface echocardiography 3 weeks prior to surgery that showed a left ventricular ejection fraction of 50% without significant valvular abnormalities, thrombus, or interatrial shunting, so repeated study was deferred.
Formal neurology consultation did not extend beyond postoperative day 1. One month after surgery, the anesthesiology team visited the patient during inpatient rehabilitation; he had not developed further focal neurologic symptoms or delirium. His strength was equal bilaterally and no speech deficits were noted. Unfortunately, the patient was readmitted to the hospital for continued foot wound drainage 2 months postoperatively, though no focal neurologic deficits were documented on his medical admission history and physical. No long term sequalae of his COVID-19 infection have been suspected.
Discussion
We report a veteran with prior stroke and COVID-19 who experienced postoperative speech and motor deficit despite deliberate risk factor mitigation. This case calls for increased vigilance by anesthesia providers to employ proper perioperative stroke management and anticoagulation strategies, and to be prepared for prompt intervention with COVID-19-sensitive practices should the need for advanced airway management or thrombectomy arises.
The exact etiology of the postoperative neurologic deficit in our patient is unknown. The most likely possibility is that this represents poststroke recrudescence (PSR), knowing he had a previous left medullary infarct that presented similarly.11 PSR is a phenomenon in which prior stroke symptoms recur acutely and transiently in the setting of physiologic stressors—also known as locus minoris resistantiae.12 Triggers include γ aminobutyric acid (GABA) mediating anesthetic agents such as midazolam, opioids (eg, fentanyl or hydromorphone), infection, or relative cerebral hypoperfusion.11,13,14 The focality of our patient’s presentation favors PSR in the context of brief POD; of note, these entities share similar risk factors.15 Our patient did indeed receive low-dose preoperative midazolam in the context of mild preoperative neurocognitive deficit, which may have predisposed him to POD.
Though less likely, our patient’s presentation could have been explained by a new cerebrovascular event—transient ischemic attack vs new MI. Speech and right-sided motor/sensory deficits can localize to the left middle cerebral artery or small penetrating arteries of the left brainstem or deep white matter. MRI was not performed to exclude this possibility due to hospital-wide COVID-19 precautions minimizing nonessential MRIs unlikely to change clinical management. We speculate, however, that due to recent SARS-CoV-2 infection, our patient may have been at higher risk for cerebrovascular events due to subclinical endothelial damage and/or microclot in predisposed neurovasculature. Though our patient had interval COVID-19 negative tests, the timeframe of coronavirus procoagulant effects is unknown.16
There are well-established guidelines for perioperative stroke management published by the Society for Neuroscience in Anesthesiology and Critical Care (SNACC).17 This case exemplifies many recommendations including tight hemodynamic and glucose control, optimized oxygen delivery, avoidance of intraoperative β blockade, and prompt neurologic consultation. Additionally, special precaution was taken to ensure continuation of antiplatelet therapy on the day of surgery; in light of COVID-19 prothrombosis risk we considered this essential. Low-dose enoxaparin was also instituted on postoperative day 1. Prophylactic anticoagulation with low molecular weight heparin (LMWH) is recommended for hospitalized COVID-19–positive patients, though perioperatively, this must be weighed against hemorrhagic stroke transformation and surgical bleeding.8,16 Interestingly, the benefit of LMWH may partly relate to its anti-inflammatory effects, of which higher levels are observed in COVID-19.16,18
Though substantial health care provider energy and hospital resource utilization is presently focused on controlling the COVID-19 pandemic, the importance of appropriate stroke code processes must not be neglected. Recently, SNACC released anesthetic guidelines for endovascular ischemic stroke management that reflect COVID-19 precautions; highlights include personal protective equipment (PPE) utilization, risk-benefit analysis of general anesthesia (with early decision to intubate) vs sedation techniques for thrombectomy, and airway management strategies to minimize aerosolization exposure.19 Finally, negative pressure rooms relative to PACU and operating room locations need to be known and marked, as well as the necessary airway equipment and PPE to transfer patients safely to and from angiography suites.
Conclusions
We discuss a surgical patient with prior SARS-CoV-2 infection at elevated stroke risk that experienced recurrence of neurologic deficits postoperatively. This case informs anesthesia providers of the broad differential diagnosis for focal neurological deficits to include PSR and the possible contribution of COVID-19 to elevated acute stroke risk. Perioperative physicians, including VHA practitioners, with knowledge of current COVID-19 practices are primed to coordinate multidisciplinary efforts during stroke codes and ensuring appropriate anticoagulation.
Acknowledgments
The authors would like to thank perioperative care teams across the world caring for COVID-19 patients safely.
1. Vlisides P, Mashour GA. Perioperative stroke. Can J Anaesth. 2016;63(2):193-204. doi:10.1007/s12630-015-0494-9
2. Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114(6):1289-1296. doi:10.1097/ALN.0b013e318216e7f4
3. Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157-160. Published 2020 Mar 19. doi:10.23750/abm.v91i1.9397
4. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Covid-19 by Race and Ethnicity: A National Cohort Study of 6 Million United States Veterans. Preprint. medRxiv. 2020;2020.05.12.20099135. Published 2020 May 18. doi:10.1101/2020.05.12.20099135
5. Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin Neurol Neurosurg. 2020;194:105921. doi:10.1016/j.clineuro.2020.105921
6. Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. doi:10.1001/jamaneurol.2020.1127
7. Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020;382(20):e60. doi:10.1056/NEJMc2009787
8. Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(8):889-891. doi:10.1136/jnnp-2020-323586
9. Needham EJ, Chou SH, Coles AJ, Menon DK. Neurological Implications of COVID-19 Infections. Neurocrit Care. 2020;32(3):667-671. doi:10.1007/s12028-020-00978-4
10. Lich KH, Tian Y, Beadles CA, et al. Strategic planning to reduce the burden of stroke among veterans: using simulation modeling to inform decision making. Stroke. 2014;45(7):2078-2084. doi:10.1161/STROKEAHA.114.004694
11. Topcuoglu MA, Saka E, Silverman SB, Schwamm LH, Singhal AB. Recrudescence of Deficits After Stroke: Clinical and Imaging Phenotype, Triggers, and Risk Factors. JAMA Neurol. 2017;74(9):1048-1055. doi:10.1001/jamaneurol.2017.1668
12. Jun-O’connell AH, Henninger N, Moonis M, Silver B, Ionete C, Goddeau RP. Recrudescence of old stroke deficits among transient neurological attacks. Neurohospitalist. 2019;9(4):183-189. doi:10.1177/194187441982928813. Karnik HS, Jain RA. Anesthesia for patients with prior stroke. J Neuroanaesthesiology Crit Care. 2018;5(3):150-157. doi:10.1055/s-0038-1673549
14. Minhas JS, Rook W, Panerai RB, et al. Pathophysiological and clinical considerations in the perioperative care of patients with a previous ischaemic stroke: a multidisciplinary narrative review. Br J Anaesth. 2020;124(2):183-196. doi:10.1016/j.bja.2019.10.021
15. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium [published correction appears in Eur J Anaesthesiol. 2018 Sep;35(9):718-719]. Eur J Anaesthesiol. 2017;34(4):192-214. doi:10.1097/EJA.0000000000000594
16. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
17. Mashour GA, Moore LE, Lele AV, Robicsek SA, Gelb AW. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurologic surgery: consensus statement from the Society for Neuroscience in Anesthesiology and Critical Care*. J Neurosurg Anesthesiol. 2014;26(4):273-285. doi:10.1097/ana.0000000000000087
18. Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267(11):3135-3153. doi:10.1007/s00415-020-09990-2
19. Sharma D, Rasmussen M, Han R, et al. Anesthetic Management of Endovascular Treatment of Acute Ischemic Stroke During COVID-19 Pandemic: Consensus Statement From Society for Neuroscience in Anesthesiology & Critical Care (SNACC): Endorsed by Society of Vascular & Interventional Neurology (SVIN), Society of NeuroInterventional Surgery (SNIS), Neurocritical Care Society (NCS), European Society of Minimally Invasive Neurological Therapy (ESMINT) and American Association of Neurological Surgeons (AANS) and Congress of Neurological Surgeons (CNS) Cerebrovascular Section. J Neurosurg Anesthesiol. 2020;32(3):193-201. doi:10.1097/ANA.0000000000000688
Anesthesia providers should be aware of COVID-19 sensitive stroke code practices and maintain heightened vigilance for the need to implement perioperative stroke mitigation strategies.
Anesthesia providers should be aware of COVID-19 sensitive stroke code practices and maintain heightened vigilance for the need to implement perioperative stroke mitigation strategies.
The risk of perioperative stroke in noncardiac, nonneurologic, nonvascular surgery ranges from 0.1 to 1.9% and is associated with increased mortality.1,2 Stroke mechanisms include both ischemia (large and small vessel occlusion, cardioembolism, anemic-tissue hypoxia, cerebral hypoperfusion) and hemorrhage.1 Risk factors for perioperative stroke include prior cerebral vascular accident (CVA), hypertension, aged > 62 years, acute renal insufficiency, dialysis, and recent myocardial infarction (MI).2
Introduction
COVID-19 was declared a pandemic by the World Health Organization in March 2020.3 COVID-19 has certainly affected the veteran population; between February and May 2020, more than 60,000 veterans were tested for COVID-19 with a positive rate of about 9%.4 While primarily affecting the respiratory system, there are increasing reports of COVID-19 neurologic manifestations: headache, hypogeusia, hyposomia, seizure, encephalitis, and acute stroke.5 In an early case series from Wuhan, China, 36% of 214 patients with COVID-19 reported neurologic complications, and acute CVAs were more common in patients with severe (compared to milder) viral disease presentations (5.7% vs 0.8%).6 Large vessel stroke was a presenting feature in another report of 5 patients aged < 50 years.7
The mechanism of ischemic stroke in the setting of COVID-19 is unclear.8 Indeed, stroke and COVID-19 share similar risk factors (eg, hypertension, diabetes mellitus [DM], older age), and immobile critically ill patients may already be prone to developing stroke.5,9 However, COVID-19 is associated with arterial and venous thromboembolism, elevated D-dimer and fibrinogen levels, and antiphospholipid antibody production. This prothrombotic state may be linked to cytokine-induced endothelial damage, mononuclear cell activation, tissue factor expression, and ultimately thrombin propagation and platelet activation.8
The rates of perioperative stroke may change as more patients with COVID-19 present for surgery, and the anesthesiology care team must prioritize mitigation efforts in high-risk patients, including veterans. Reducing the elevated stroke burden within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) is a public health priority.10 We present the case of a veteran with prior CVA and recent positive COVID-19 testing who experienced transient weakness and dysarthria following plastic surgery. The patient discussed provided written Health Insurance Portability and Accountability Act consent for publication of this report.
Case Presentation
A 75-year-old male veteran presented to the Minneapolis VA Medical Center in Minnesota with chronic left foot ulceration necessitating debridement and flap coverage. His medical history was significant for hypertension, type 2 DM, anemia of chronic disease, and coronary artery disease (left ventricular ejection fraction, 50%). Additionally, he had prior ischemic strokes in the oculomotor nucleus (in 2004 with internuclear ophthalmoplegia) and left ventral medulla (in 2019 with right hemiparesis). During his 2019 poststroke rehabilitation, he was diagnosed with mild neurocognitive deficit not attributable to his strokes. The patient’s medications included amlodipine, lisinopril, atorvastatin, clopidogrel (lifelong for secondary stroke prevention), metformin, and glipizide. The debridement procedure was initially delayed 3 weeks due to positive routine preoperative COVID-19 nasopharyngeal testing, though he reported no respiratory symptoms or fever. During the delay, the primary team prescribed daily oral rivaroxaban for thrombosis prophylaxis in addition to clopidogrel. One week prior to surgery, his repeat COVID-19 test was negative and prophylactic anticoagulation stopped.
On the day of surgery, the patient was hemodynamically stable: heart rate 86 beats/min, blood pressure 167/93 mm Hg (baseline 120-150 mm Hg systolic pressure), respiratory rate 16 breaths/min, oxygen saturation 99% without supplemental oxygen, temperature 97.1 °F. He received amlodipine and clopidogrel, but not lisinopril, that morning. No focal neurologic deficits were appreciated on preoperative examination, and resolution of symptoms related to the 2 prior MIs was confirmed. Preoperative glucose was 163 mg/dL. Femoral and sciatic peripheral nerve blocks were done for postoperative analgesia. A preinduction arterial line was placed and 2 mg of midazolam was administered for anxiolysis. Induction of general anesthesia with oral endotracheal intubation proceeded uneventfully; he was positioned prone.
Given his stroke risk factors, mean arterial pressure was maintained > 70 mm Hg for the duration of surgery. No vasoactive infusions were necessary and no β-blocking agents were administered. Insulin infusion was required; the maximum-recorded glucose was 219 mg/dL. Arterial blood gas samples were routinely drawn; acid-base balance was well maintained, PaO2 was > 185 mm Hg, and PaCO2 ranged from 29.4 to 38.5 mm Hg. The patient received 2 units of packed red blood cells for nadir hemoglobin of 7.5 mg/dL. At surgery end, we fully reversed neuromuscular blockade with suggamadex. The patient was returned to a supine position and extubated uneventfully after demonstrating the ability to follow commands.
During postanesthesia care unit (PACU) handoff, the patient exhibited acute speech impairment. He was able to state his name on repetition but seemed confused and sedated. Prompt formal neurology evaluation (stroke code) was sought. Initial National Institutes of Health (NIH) stroke scale score was 8 (1 for level of consciousness, 1 for minor right facial droop, 1 for right arm drift, 3 for right leg with no effort against gravity, 1 for right partial sensory loss, and 1 for mild dysarthria). The patient was oriented only to self. Other findings included mild right facial droop and dysarthria. On a 5-point strength scale, he scored 4 for the right deltoid, biceps, triceps, wrist extensors, right knee flexion, right dorsiflexion, and plantarflexion, 2 for right hip flexion, and ≥ 4 for right knee extension. Positive sensory findings were notable for decreased pin prick sensation on the right limbs.
We obtained emergent head computed tomography (CT) that was negative for acute abnormalities; CT angiography was negative for large vessel occlusion or clinically significant stenosis (Figure). On returning to the PACU from the CT scanner, the patient regained symmetric strength in both arms, right leg was antigravity, and his speech had normalized. Prior to PACU discharge 2 hours later, the patient was back to his prehospitalization neurologic function and NIH stroke scale was 0. Given this rapid clinical resolution, no acute stroke interventions were done, though permissive hypertension was recommended by the neurologist during PACU recovery.
The neurology team concluded that the patient’s symptoms were likely secondary to recrudescence of previous stroke symptoms in the setting of brief postoperative delirium (POD). However, we could not exclude transient ischemic attack or new cardioembolism, therefore patient was started on dual antiplatelet therapy for 3 weeks. Unfortunately, elective confirmatory magnetic resonance imaging (MRI) was not sought to confirm new ischemic changes due hospital COVID-19 restrictions on nonessential scanning. Neurology did not recommend carotid duplex ultrasound given patent vasculature on the head and neck CT angiography. Finally, the patient had undergone surface echocardiography 3 weeks prior to surgery that showed a left ventricular ejection fraction of 50% without significant valvular abnormalities, thrombus, or interatrial shunting, so repeated study was deferred.
Formal neurology consultation did not extend beyond postoperative day 1. One month after surgery, the anesthesiology team visited the patient during inpatient rehabilitation; he had not developed further focal neurologic symptoms or delirium. His strength was equal bilaterally and no speech deficits were noted. Unfortunately, the patient was readmitted to the hospital for continued foot wound drainage 2 months postoperatively, though no focal neurologic deficits were documented on his medical admission history and physical. No long term sequalae of his COVID-19 infection have been suspected.
Discussion
We report a veteran with prior stroke and COVID-19 who experienced postoperative speech and motor deficit despite deliberate risk factor mitigation. This case calls for increased vigilance by anesthesia providers to employ proper perioperative stroke management and anticoagulation strategies, and to be prepared for prompt intervention with COVID-19-sensitive practices should the need for advanced airway management or thrombectomy arises.
The exact etiology of the postoperative neurologic deficit in our patient is unknown. The most likely possibility is that this represents poststroke recrudescence (PSR), knowing he had a previous left medullary infarct that presented similarly.11 PSR is a phenomenon in which prior stroke symptoms recur acutely and transiently in the setting of physiologic stressors—also known as locus minoris resistantiae.12 Triggers include γ aminobutyric acid (GABA) mediating anesthetic agents such as midazolam, opioids (eg, fentanyl or hydromorphone), infection, or relative cerebral hypoperfusion.11,13,14 The focality of our patient’s presentation favors PSR in the context of brief POD; of note, these entities share similar risk factors.15 Our patient did indeed receive low-dose preoperative midazolam in the context of mild preoperative neurocognitive deficit, which may have predisposed him to POD.
Though less likely, our patient’s presentation could have been explained by a new cerebrovascular event—transient ischemic attack vs new MI. Speech and right-sided motor/sensory deficits can localize to the left middle cerebral artery or small penetrating arteries of the left brainstem or deep white matter. MRI was not performed to exclude this possibility due to hospital-wide COVID-19 precautions minimizing nonessential MRIs unlikely to change clinical management. We speculate, however, that due to recent SARS-CoV-2 infection, our patient may have been at higher risk for cerebrovascular events due to subclinical endothelial damage and/or microclot in predisposed neurovasculature. Though our patient had interval COVID-19 negative tests, the timeframe of coronavirus procoagulant effects is unknown.16
There are well-established guidelines for perioperative stroke management published by the Society for Neuroscience in Anesthesiology and Critical Care (SNACC).17 This case exemplifies many recommendations including tight hemodynamic and glucose control, optimized oxygen delivery, avoidance of intraoperative β blockade, and prompt neurologic consultation. Additionally, special precaution was taken to ensure continuation of antiplatelet therapy on the day of surgery; in light of COVID-19 prothrombosis risk we considered this essential. Low-dose enoxaparin was also instituted on postoperative day 1. Prophylactic anticoagulation with low molecular weight heparin (LMWH) is recommended for hospitalized COVID-19–positive patients, though perioperatively, this must be weighed against hemorrhagic stroke transformation and surgical bleeding.8,16 Interestingly, the benefit of LMWH may partly relate to its anti-inflammatory effects, of which higher levels are observed in COVID-19.16,18
Though substantial health care provider energy and hospital resource utilization is presently focused on controlling the COVID-19 pandemic, the importance of appropriate stroke code processes must not be neglected. Recently, SNACC released anesthetic guidelines for endovascular ischemic stroke management that reflect COVID-19 precautions; highlights include personal protective equipment (PPE) utilization, risk-benefit analysis of general anesthesia (with early decision to intubate) vs sedation techniques for thrombectomy, and airway management strategies to minimize aerosolization exposure.19 Finally, negative pressure rooms relative to PACU and operating room locations need to be known and marked, as well as the necessary airway equipment and PPE to transfer patients safely to and from angiography suites.
Conclusions
We discuss a surgical patient with prior SARS-CoV-2 infection at elevated stroke risk that experienced recurrence of neurologic deficits postoperatively. This case informs anesthesia providers of the broad differential diagnosis for focal neurological deficits to include PSR and the possible contribution of COVID-19 to elevated acute stroke risk. Perioperative physicians, including VHA practitioners, with knowledge of current COVID-19 practices are primed to coordinate multidisciplinary efforts during stroke codes and ensuring appropriate anticoagulation.
Acknowledgments
The authors would like to thank perioperative care teams across the world caring for COVID-19 patients safely.
The risk of perioperative stroke in noncardiac, nonneurologic, nonvascular surgery ranges from 0.1 to 1.9% and is associated with increased mortality.1,2 Stroke mechanisms include both ischemia (large and small vessel occlusion, cardioembolism, anemic-tissue hypoxia, cerebral hypoperfusion) and hemorrhage.1 Risk factors for perioperative stroke include prior cerebral vascular accident (CVA), hypertension, aged > 62 years, acute renal insufficiency, dialysis, and recent myocardial infarction (MI).2
Introduction
COVID-19 was declared a pandemic by the World Health Organization in March 2020.3 COVID-19 has certainly affected the veteran population; between February and May 2020, more than 60,000 veterans were tested for COVID-19 with a positive rate of about 9%.4 While primarily affecting the respiratory system, there are increasing reports of COVID-19 neurologic manifestations: headache, hypogeusia, hyposomia, seizure, encephalitis, and acute stroke.5 In an early case series from Wuhan, China, 36% of 214 patients with COVID-19 reported neurologic complications, and acute CVAs were more common in patients with severe (compared to milder) viral disease presentations (5.7% vs 0.8%).6 Large vessel stroke was a presenting feature in another report of 5 patients aged < 50 years.7
The mechanism of ischemic stroke in the setting of COVID-19 is unclear.8 Indeed, stroke and COVID-19 share similar risk factors (eg, hypertension, diabetes mellitus [DM], older age), and immobile critically ill patients may already be prone to developing stroke.5,9 However, COVID-19 is associated with arterial and venous thromboembolism, elevated D-dimer and fibrinogen levels, and antiphospholipid antibody production. This prothrombotic state may be linked to cytokine-induced endothelial damage, mononuclear cell activation, tissue factor expression, and ultimately thrombin propagation and platelet activation.8
The rates of perioperative stroke may change as more patients with COVID-19 present for surgery, and the anesthesiology care team must prioritize mitigation efforts in high-risk patients, including veterans. Reducing the elevated stroke burden within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) is a public health priority.10 We present the case of a veteran with prior CVA and recent positive COVID-19 testing who experienced transient weakness and dysarthria following plastic surgery. The patient discussed provided written Health Insurance Portability and Accountability Act consent for publication of this report.
Case Presentation
A 75-year-old male veteran presented to the Minneapolis VA Medical Center in Minnesota with chronic left foot ulceration necessitating debridement and flap coverage. His medical history was significant for hypertension, type 2 DM, anemia of chronic disease, and coronary artery disease (left ventricular ejection fraction, 50%). Additionally, he had prior ischemic strokes in the oculomotor nucleus (in 2004 with internuclear ophthalmoplegia) and left ventral medulla (in 2019 with right hemiparesis). During his 2019 poststroke rehabilitation, he was diagnosed with mild neurocognitive deficit not attributable to his strokes. The patient’s medications included amlodipine, lisinopril, atorvastatin, clopidogrel (lifelong for secondary stroke prevention), metformin, and glipizide. The debridement procedure was initially delayed 3 weeks due to positive routine preoperative COVID-19 nasopharyngeal testing, though he reported no respiratory symptoms or fever. During the delay, the primary team prescribed daily oral rivaroxaban for thrombosis prophylaxis in addition to clopidogrel. One week prior to surgery, his repeat COVID-19 test was negative and prophylactic anticoagulation stopped.
On the day of surgery, the patient was hemodynamically stable: heart rate 86 beats/min, blood pressure 167/93 mm Hg (baseline 120-150 mm Hg systolic pressure), respiratory rate 16 breaths/min, oxygen saturation 99% without supplemental oxygen, temperature 97.1 °F. He received amlodipine and clopidogrel, but not lisinopril, that morning. No focal neurologic deficits were appreciated on preoperative examination, and resolution of symptoms related to the 2 prior MIs was confirmed. Preoperative glucose was 163 mg/dL. Femoral and sciatic peripheral nerve blocks were done for postoperative analgesia. A preinduction arterial line was placed and 2 mg of midazolam was administered for anxiolysis. Induction of general anesthesia with oral endotracheal intubation proceeded uneventfully; he was positioned prone.
Given his stroke risk factors, mean arterial pressure was maintained > 70 mm Hg for the duration of surgery. No vasoactive infusions were necessary and no β-blocking agents were administered. Insulin infusion was required; the maximum-recorded glucose was 219 mg/dL. Arterial blood gas samples were routinely drawn; acid-base balance was well maintained, PaO2 was > 185 mm Hg, and PaCO2 ranged from 29.4 to 38.5 mm Hg. The patient received 2 units of packed red blood cells for nadir hemoglobin of 7.5 mg/dL. At surgery end, we fully reversed neuromuscular blockade with suggamadex. The patient was returned to a supine position and extubated uneventfully after demonstrating the ability to follow commands.
During postanesthesia care unit (PACU) handoff, the patient exhibited acute speech impairment. He was able to state his name on repetition but seemed confused and sedated. Prompt formal neurology evaluation (stroke code) was sought. Initial National Institutes of Health (NIH) stroke scale score was 8 (1 for level of consciousness, 1 for minor right facial droop, 1 for right arm drift, 3 for right leg with no effort against gravity, 1 for right partial sensory loss, and 1 for mild dysarthria). The patient was oriented only to self. Other findings included mild right facial droop and dysarthria. On a 5-point strength scale, he scored 4 for the right deltoid, biceps, triceps, wrist extensors, right knee flexion, right dorsiflexion, and plantarflexion, 2 for right hip flexion, and ≥ 4 for right knee extension. Positive sensory findings were notable for decreased pin prick sensation on the right limbs.
We obtained emergent head computed tomography (CT) that was negative for acute abnormalities; CT angiography was negative for large vessel occlusion or clinically significant stenosis (Figure). On returning to the PACU from the CT scanner, the patient regained symmetric strength in both arms, right leg was antigravity, and his speech had normalized. Prior to PACU discharge 2 hours later, the patient was back to his prehospitalization neurologic function and NIH stroke scale was 0. Given this rapid clinical resolution, no acute stroke interventions were done, though permissive hypertension was recommended by the neurologist during PACU recovery.
The neurology team concluded that the patient’s symptoms were likely secondary to recrudescence of previous stroke symptoms in the setting of brief postoperative delirium (POD). However, we could not exclude transient ischemic attack or new cardioembolism, therefore patient was started on dual antiplatelet therapy for 3 weeks. Unfortunately, elective confirmatory magnetic resonance imaging (MRI) was not sought to confirm new ischemic changes due hospital COVID-19 restrictions on nonessential scanning. Neurology did not recommend carotid duplex ultrasound given patent vasculature on the head and neck CT angiography. Finally, the patient had undergone surface echocardiography 3 weeks prior to surgery that showed a left ventricular ejection fraction of 50% without significant valvular abnormalities, thrombus, or interatrial shunting, so repeated study was deferred.
Formal neurology consultation did not extend beyond postoperative day 1. One month after surgery, the anesthesiology team visited the patient during inpatient rehabilitation; he had not developed further focal neurologic symptoms or delirium. His strength was equal bilaterally and no speech deficits were noted. Unfortunately, the patient was readmitted to the hospital for continued foot wound drainage 2 months postoperatively, though no focal neurologic deficits were documented on his medical admission history and physical. No long term sequalae of his COVID-19 infection have been suspected.
Discussion
We report a veteran with prior stroke and COVID-19 who experienced postoperative speech and motor deficit despite deliberate risk factor mitigation. This case calls for increased vigilance by anesthesia providers to employ proper perioperative stroke management and anticoagulation strategies, and to be prepared for prompt intervention with COVID-19-sensitive practices should the need for advanced airway management or thrombectomy arises.
The exact etiology of the postoperative neurologic deficit in our patient is unknown. The most likely possibility is that this represents poststroke recrudescence (PSR), knowing he had a previous left medullary infarct that presented similarly.11 PSR is a phenomenon in which prior stroke symptoms recur acutely and transiently in the setting of physiologic stressors—also known as locus minoris resistantiae.12 Triggers include γ aminobutyric acid (GABA) mediating anesthetic agents such as midazolam, opioids (eg, fentanyl or hydromorphone), infection, or relative cerebral hypoperfusion.11,13,14 The focality of our patient’s presentation favors PSR in the context of brief POD; of note, these entities share similar risk factors.15 Our patient did indeed receive low-dose preoperative midazolam in the context of mild preoperative neurocognitive deficit, which may have predisposed him to POD.
Though less likely, our patient’s presentation could have been explained by a new cerebrovascular event—transient ischemic attack vs new MI. Speech and right-sided motor/sensory deficits can localize to the left middle cerebral artery or small penetrating arteries of the left brainstem or deep white matter. MRI was not performed to exclude this possibility due to hospital-wide COVID-19 precautions minimizing nonessential MRIs unlikely to change clinical management. We speculate, however, that due to recent SARS-CoV-2 infection, our patient may have been at higher risk for cerebrovascular events due to subclinical endothelial damage and/or microclot in predisposed neurovasculature. Though our patient had interval COVID-19 negative tests, the timeframe of coronavirus procoagulant effects is unknown.16
There are well-established guidelines for perioperative stroke management published by the Society for Neuroscience in Anesthesiology and Critical Care (SNACC).17 This case exemplifies many recommendations including tight hemodynamic and glucose control, optimized oxygen delivery, avoidance of intraoperative β blockade, and prompt neurologic consultation. Additionally, special precaution was taken to ensure continuation of antiplatelet therapy on the day of surgery; in light of COVID-19 prothrombosis risk we considered this essential. Low-dose enoxaparin was also instituted on postoperative day 1. Prophylactic anticoagulation with low molecular weight heparin (LMWH) is recommended for hospitalized COVID-19–positive patients, though perioperatively, this must be weighed against hemorrhagic stroke transformation and surgical bleeding.8,16 Interestingly, the benefit of LMWH may partly relate to its anti-inflammatory effects, of which higher levels are observed in COVID-19.16,18
Though substantial health care provider energy and hospital resource utilization is presently focused on controlling the COVID-19 pandemic, the importance of appropriate stroke code processes must not be neglected. Recently, SNACC released anesthetic guidelines for endovascular ischemic stroke management that reflect COVID-19 precautions; highlights include personal protective equipment (PPE) utilization, risk-benefit analysis of general anesthesia (with early decision to intubate) vs sedation techniques for thrombectomy, and airway management strategies to minimize aerosolization exposure.19 Finally, negative pressure rooms relative to PACU and operating room locations need to be known and marked, as well as the necessary airway equipment and PPE to transfer patients safely to and from angiography suites.
Conclusions
We discuss a surgical patient with prior SARS-CoV-2 infection at elevated stroke risk that experienced recurrence of neurologic deficits postoperatively. This case informs anesthesia providers of the broad differential diagnosis for focal neurological deficits to include PSR and the possible contribution of COVID-19 to elevated acute stroke risk. Perioperative physicians, including VHA practitioners, with knowledge of current COVID-19 practices are primed to coordinate multidisciplinary efforts during stroke codes and ensuring appropriate anticoagulation.
Acknowledgments
The authors would like to thank perioperative care teams across the world caring for COVID-19 patients safely.
1. Vlisides P, Mashour GA. Perioperative stroke. Can J Anaesth. 2016;63(2):193-204. doi:10.1007/s12630-015-0494-9
2. Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114(6):1289-1296. doi:10.1097/ALN.0b013e318216e7f4
3. Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157-160. Published 2020 Mar 19. doi:10.23750/abm.v91i1.9397
4. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Covid-19 by Race and Ethnicity: A National Cohort Study of 6 Million United States Veterans. Preprint. medRxiv. 2020;2020.05.12.20099135. Published 2020 May 18. doi:10.1101/2020.05.12.20099135
5. Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin Neurol Neurosurg. 2020;194:105921. doi:10.1016/j.clineuro.2020.105921
6. Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. doi:10.1001/jamaneurol.2020.1127
7. Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020;382(20):e60. doi:10.1056/NEJMc2009787
8. Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(8):889-891. doi:10.1136/jnnp-2020-323586
9. Needham EJ, Chou SH, Coles AJ, Menon DK. Neurological Implications of COVID-19 Infections. Neurocrit Care. 2020;32(3):667-671. doi:10.1007/s12028-020-00978-4
10. Lich KH, Tian Y, Beadles CA, et al. Strategic planning to reduce the burden of stroke among veterans: using simulation modeling to inform decision making. Stroke. 2014;45(7):2078-2084. doi:10.1161/STROKEAHA.114.004694
11. Topcuoglu MA, Saka E, Silverman SB, Schwamm LH, Singhal AB. Recrudescence of Deficits After Stroke: Clinical and Imaging Phenotype, Triggers, and Risk Factors. JAMA Neurol. 2017;74(9):1048-1055. doi:10.1001/jamaneurol.2017.1668
12. Jun-O’connell AH, Henninger N, Moonis M, Silver B, Ionete C, Goddeau RP. Recrudescence of old stroke deficits among transient neurological attacks. Neurohospitalist. 2019;9(4):183-189. doi:10.1177/194187441982928813. Karnik HS, Jain RA. Anesthesia for patients with prior stroke. J Neuroanaesthesiology Crit Care. 2018;5(3):150-157. doi:10.1055/s-0038-1673549
14. Minhas JS, Rook W, Panerai RB, et al. Pathophysiological and clinical considerations in the perioperative care of patients with a previous ischaemic stroke: a multidisciplinary narrative review. Br J Anaesth. 2020;124(2):183-196. doi:10.1016/j.bja.2019.10.021
15. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium [published correction appears in Eur J Anaesthesiol. 2018 Sep;35(9):718-719]. Eur J Anaesthesiol. 2017;34(4):192-214. doi:10.1097/EJA.0000000000000594
16. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
17. Mashour GA, Moore LE, Lele AV, Robicsek SA, Gelb AW. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurologic surgery: consensus statement from the Society for Neuroscience in Anesthesiology and Critical Care*. J Neurosurg Anesthesiol. 2014;26(4):273-285. doi:10.1097/ana.0000000000000087
18. Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267(11):3135-3153. doi:10.1007/s00415-020-09990-2
19. Sharma D, Rasmussen M, Han R, et al. Anesthetic Management of Endovascular Treatment of Acute Ischemic Stroke During COVID-19 Pandemic: Consensus Statement From Society for Neuroscience in Anesthesiology & Critical Care (SNACC): Endorsed by Society of Vascular & Interventional Neurology (SVIN), Society of NeuroInterventional Surgery (SNIS), Neurocritical Care Society (NCS), European Society of Minimally Invasive Neurological Therapy (ESMINT) and American Association of Neurological Surgeons (AANS) and Congress of Neurological Surgeons (CNS) Cerebrovascular Section. J Neurosurg Anesthesiol. 2020;32(3):193-201. doi:10.1097/ANA.0000000000000688
1. Vlisides P, Mashour GA. Perioperative stroke. Can J Anaesth. 2016;63(2):193-204. doi:10.1007/s12630-015-0494-9
2. Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114(6):1289-1296. doi:10.1097/ALN.0b013e318216e7f4
3. Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157-160. Published 2020 Mar 19. doi:10.23750/abm.v91i1.9397
4. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Covid-19 by Race and Ethnicity: A National Cohort Study of 6 Million United States Veterans. Preprint. medRxiv. 2020;2020.05.12.20099135. Published 2020 May 18. doi:10.1101/2020.05.12.20099135
5. Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin Neurol Neurosurg. 2020;194:105921. doi:10.1016/j.clineuro.2020.105921
6. Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. doi:10.1001/jamaneurol.2020.1127
7. Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020;382(20):e60. doi:10.1056/NEJMc2009787
8. Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(8):889-891. doi:10.1136/jnnp-2020-323586
9. Needham EJ, Chou SH, Coles AJ, Menon DK. Neurological Implications of COVID-19 Infections. Neurocrit Care. 2020;32(3):667-671. doi:10.1007/s12028-020-00978-4
10. Lich KH, Tian Y, Beadles CA, et al. Strategic planning to reduce the burden of stroke among veterans: using simulation modeling to inform decision making. Stroke. 2014;45(7):2078-2084. doi:10.1161/STROKEAHA.114.004694
11. Topcuoglu MA, Saka E, Silverman SB, Schwamm LH, Singhal AB. Recrudescence of Deficits After Stroke: Clinical and Imaging Phenotype, Triggers, and Risk Factors. JAMA Neurol. 2017;74(9):1048-1055. doi:10.1001/jamaneurol.2017.1668
12. Jun-O’connell AH, Henninger N, Moonis M, Silver B, Ionete C, Goddeau RP. Recrudescence of old stroke deficits among transient neurological attacks. Neurohospitalist. 2019;9(4):183-189. doi:10.1177/194187441982928813. Karnik HS, Jain RA. Anesthesia for patients with prior stroke. J Neuroanaesthesiology Crit Care. 2018;5(3):150-157. doi:10.1055/s-0038-1673549
14. Minhas JS, Rook W, Panerai RB, et al. Pathophysiological and clinical considerations in the perioperative care of patients with a previous ischaemic stroke: a multidisciplinary narrative review. Br J Anaesth. 2020;124(2):183-196. doi:10.1016/j.bja.2019.10.021
15. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium [published correction appears in Eur J Anaesthesiol. 2018 Sep;35(9):718-719]. Eur J Anaesthesiol. 2017;34(4):192-214. doi:10.1097/EJA.0000000000000594
16. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
17. Mashour GA, Moore LE, Lele AV, Robicsek SA, Gelb AW. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurologic surgery: consensus statement from the Society for Neuroscience in Anesthesiology and Critical Care*. J Neurosurg Anesthesiol. 2014;26(4):273-285. doi:10.1097/ana.0000000000000087
18. Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267(11):3135-3153. doi:10.1007/s00415-020-09990-2
19. Sharma D, Rasmussen M, Han R, et al. Anesthetic Management of Endovascular Treatment of Acute Ischemic Stroke During COVID-19 Pandemic: Consensus Statement From Society for Neuroscience in Anesthesiology & Critical Care (SNACC): Endorsed by Society of Vascular & Interventional Neurology (SVIN), Society of NeuroInterventional Surgery (SNIS), Neurocritical Care Society (NCS), European Society of Minimally Invasive Neurological Therapy (ESMINT) and American Association of Neurological Surgeons (AANS) and Congress of Neurological Surgeons (CNS) Cerebrovascular Section. J Neurosurg Anesthesiol. 2020;32(3):193-201. doi:10.1097/ANA.0000000000000688
Risdiplam study shows promise for spinal muscular atrophy
, according to results of part 1 of the FIREFISH study.
A boost in SMN expression has been linked to improvements in survival and motor function, which was also observed in exploratory efficacy outcomes in the 2-part, phase 2-3, open-label study.
“No surviving infant was receiving permanent ventilation at month 12, and 7 of the 21 infants were able to sit without support, which is not expected in patients with type 1 spinal muscular atrophy, according to historical experience,” reported the FIREFISH Working Group led by Giovanni Baranello, MD, PhD, from the Dubowitz Neuromuscular Centre, National Institute for Health Research Great Ormond Street Hospital Biomedical Research Centre, Great Ormond Street Institute of Child Health University College London, and Great Ormond Street Hospital Trust, London.
However, “it cannot be stated with confidence that there was clinical benefit of the agent because the exploratory clinical endpoints were analyzed post hoc and can only be qualitatively compared with historical cohorts,” they added.
The findings were published online Feb. 24 in the New England Journal of Medicine.
A phase 2-3 open-label study
The study enrolled 21 infants with type 1 SMA, between the ages of 1 and 7 months. The majority (n = 17) were treated for 1 year with high-dose risdiplam, reaching 0.2 mg/kg of body weight per day by the twelfth month. Four infants in a low-dose cohort were treated with 0.08 mg/kg by the twelfth month. The medication was administered once daily orally in infants who were able to swallow, or by feeding tube for those who could not.
The primary outcomes of this first part of the study were safety, pharmacokinetics, pharmacodynamics (including the blood SMN protein concentration), and selection of the risdiplam dose for part 2 of the study. Exploratory outcomes included event-free survival, defined as being alive without tracheostomy or the use of permanent ventilation for 16 or more hours per day, and the ability to sit without support for at least 5 seconds.
In terms of safety, the study recorded 24 serious adverse events. “The most common serious adverse events were infections of the respiratory tract, and four infants died of respiratory complications; these findings are consistent with the neuromuscular respiratory failure that characterizes spinal muscular atrophy,” the authors reported. “The risdiplam-associated retinal toxic effects that had been previously observed in monkeys were not observed in the current study,” they added.
Regarding SMN protein levels, a median level of 2.1 times the baseline level was observed within 4 weeks after the initiation of treatment in the high-dose cohort, they reported. By 12 months, these median values had increased to 3.0 times and 1.9 times the baseline values in the low-dose and high-dose cohorts, respectively.
Looking at exploratory efficacy outcomes, 90% of infants survived without ventilatory support, and seven infants in the high-dose cohort were able to sit without support for at least 5 seconds. The higher dose of risdiplam (0.2 mg/kg per day) was selected for part 2 of the study.
The first oral treatment option
Risdiplam is the third SMA treatment approved by the Food and Drug Administration, “and has the potential to expand access to treatment for people with SMA,” commented Mary Schroth, MD, chief medical officer of Cure SMA, who was not involved in the research. She added that the exploratory outcomes of the FIREFISH study represent “a significant milestone for symptomatic infants with SMA type 1.”
While the other two approved SMA therapies – nusinersen and onasemnogene abeparvovec – have led to improvements in survival and motor function, they are administered either intrathecally or intravenously respectively, while risdiplam is an oral therapy.
Dr. Schroth says there are currently no studies comparing the different SMA treatments. “Cure SMA is actively collecting real-world experience with risdiplam and other SMA treatments through multiple pathways,” she said. “Every individual and family, in collaboration with their health care provider, should discuss SMA treatments and make the decision that is best for them.”
Writing in Neuroscience Insights, a few months after risdiplam’s FDA approval last summer, Ravindra N. Singh MD, from the department of biomedical sciences, Iowa State University, Ames, wrote that, as an orally deliverable small molecule, risdiplam “is a major advancement for the treatment of SMA.”
Now, the FIREFISH study is “welcome news,” he said in an interview. “The results look promising so far,” he added. “I am cautiously optimistic that risdiplam would prove to be a viable alternative to the currently available invasive approaches. However, long-term studies (with appropriate age and sex-matched cohorts) would be needed to fully rule out the potential side effects of the repeated administrations.”
The therapy “is particularly great news for a group of SMA patients that might have tolerability and/or immune response concerns when it comes to nusinersen and gene therapy,” he noted in his article, adding that the ability to store and ship the drug at ambient temperatures, as well as its comparatively low cost are added benefits.
The study was supported by F. Hoffmann–La Roche. Dr. Baranello disclosed that he serves as a consultant for AveXis, F. Hoffmann-La Roche, and Sarepta Therapeutics, as well as PTC Therapeutics, from whom he also receives speaker honoraria. Dr. Schroth disclosed no personal conflicts and is an employee of Cure SMA. Cure SMA works to develop strategic relationships with corporate partners with the goal of working together to lead the way to a world without SMA. In advancement of that mission, Cure SMA has received funding from multiple corporate sources including Aetna, Biogen, Blue Cross Blue Shield, Genentech, Kaiser Permanente, Novartis Gene Therapies, Scholar Rock, and United HealthCare. Cure SMA has no financial stake in any treatment and does not advocate for one treatment over another. Dr. Singh disclosed that Spinraza (Nusinersen), the first FDA-approved SMA drug, is based on the target (US patent # 7,838,657) that was discovered in his former laboratory at UMASS Medical School, Worcester, Mass.
, according to results of part 1 of the FIREFISH study.
A boost in SMN expression has been linked to improvements in survival and motor function, which was also observed in exploratory efficacy outcomes in the 2-part, phase 2-3, open-label study.
“No surviving infant was receiving permanent ventilation at month 12, and 7 of the 21 infants were able to sit without support, which is not expected in patients with type 1 spinal muscular atrophy, according to historical experience,” reported the FIREFISH Working Group led by Giovanni Baranello, MD, PhD, from the Dubowitz Neuromuscular Centre, National Institute for Health Research Great Ormond Street Hospital Biomedical Research Centre, Great Ormond Street Institute of Child Health University College London, and Great Ormond Street Hospital Trust, London.
However, “it cannot be stated with confidence that there was clinical benefit of the agent because the exploratory clinical endpoints were analyzed post hoc and can only be qualitatively compared with historical cohorts,” they added.
The findings were published online Feb. 24 in the New England Journal of Medicine.
A phase 2-3 open-label study
The study enrolled 21 infants with type 1 SMA, between the ages of 1 and 7 months. The majority (n = 17) were treated for 1 year with high-dose risdiplam, reaching 0.2 mg/kg of body weight per day by the twelfth month. Four infants in a low-dose cohort were treated with 0.08 mg/kg by the twelfth month. The medication was administered once daily orally in infants who were able to swallow, or by feeding tube for those who could not.
The primary outcomes of this first part of the study were safety, pharmacokinetics, pharmacodynamics (including the blood SMN protein concentration), and selection of the risdiplam dose for part 2 of the study. Exploratory outcomes included event-free survival, defined as being alive without tracheostomy or the use of permanent ventilation for 16 or more hours per day, and the ability to sit without support for at least 5 seconds.
In terms of safety, the study recorded 24 serious adverse events. “The most common serious adverse events were infections of the respiratory tract, and four infants died of respiratory complications; these findings are consistent with the neuromuscular respiratory failure that characterizes spinal muscular atrophy,” the authors reported. “The risdiplam-associated retinal toxic effects that had been previously observed in monkeys were not observed in the current study,” they added.
Regarding SMN protein levels, a median level of 2.1 times the baseline level was observed within 4 weeks after the initiation of treatment in the high-dose cohort, they reported. By 12 months, these median values had increased to 3.0 times and 1.9 times the baseline values in the low-dose and high-dose cohorts, respectively.
Looking at exploratory efficacy outcomes, 90% of infants survived without ventilatory support, and seven infants in the high-dose cohort were able to sit without support for at least 5 seconds. The higher dose of risdiplam (0.2 mg/kg per day) was selected for part 2 of the study.
The first oral treatment option
Risdiplam is the third SMA treatment approved by the Food and Drug Administration, “and has the potential to expand access to treatment for people with SMA,” commented Mary Schroth, MD, chief medical officer of Cure SMA, who was not involved in the research. She added that the exploratory outcomes of the FIREFISH study represent “a significant milestone for symptomatic infants with SMA type 1.”
While the other two approved SMA therapies – nusinersen and onasemnogene abeparvovec – have led to improvements in survival and motor function, they are administered either intrathecally or intravenously respectively, while risdiplam is an oral therapy.
Dr. Schroth says there are currently no studies comparing the different SMA treatments. “Cure SMA is actively collecting real-world experience with risdiplam and other SMA treatments through multiple pathways,” she said. “Every individual and family, in collaboration with their health care provider, should discuss SMA treatments and make the decision that is best for them.”
Writing in Neuroscience Insights, a few months after risdiplam’s FDA approval last summer, Ravindra N. Singh MD, from the department of biomedical sciences, Iowa State University, Ames, wrote that, as an orally deliverable small molecule, risdiplam “is a major advancement for the treatment of SMA.”
Now, the FIREFISH study is “welcome news,” he said in an interview. “The results look promising so far,” he added. “I am cautiously optimistic that risdiplam would prove to be a viable alternative to the currently available invasive approaches. However, long-term studies (with appropriate age and sex-matched cohorts) would be needed to fully rule out the potential side effects of the repeated administrations.”
The therapy “is particularly great news for a group of SMA patients that might have tolerability and/or immune response concerns when it comes to nusinersen and gene therapy,” he noted in his article, adding that the ability to store and ship the drug at ambient temperatures, as well as its comparatively low cost are added benefits.
The study was supported by F. Hoffmann–La Roche. Dr. Baranello disclosed that he serves as a consultant for AveXis, F. Hoffmann-La Roche, and Sarepta Therapeutics, as well as PTC Therapeutics, from whom he also receives speaker honoraria. Dr. Schroth disclosed no personal conflicts and is an employee of Cure SMA. Cure SMA works to develop strategic relationships with corporate partners with the goal of working together to lead the way to a world without SMA. In advancement of that mission, Cure SMA has received funding from multiple corporate sources including Aetna, Biogen, Blue Cross Blue Shield, Genentech, Kaiser Permanente, Novartis Gene Therapies, Scholar Rock, and United HealthCare. Cure SMA has no financial stake in any treatment and does not advocate for one treatment over another. Dr. Singh disclosed that Spinraza (Nusinersen), the first FDA-approved SMA drug, is based on the target (US patent # 7,838,657) that was discovered in his former laboratory at UMASS Medical School, Worcester, Mass.
, according to results of part 1 of the FIREFISH study.
A boost in SMN expression has been linked to improvements in survival and motor function, which was also observed in exploratory efficacy outcomes in the 2-part, phase 2-3, open-label study.
“No surviving infant was receiving permanent ventilation at month 12, and 7 of the 21 infants were able to sit without support, which is not expected in patients with type 1 spinal muscular atrophy, according to historical experience,” reported the FIREFISH Working Group led by Giovanni Baranello, MD, PhD, from the Dubowitz Neuromuscular Centre, National Institute for Health Research Great Ormond Street Hospital Biomedical Research Centre, Great Ormond Street Institute of Child Health University College London, and Great Ormond Street Hospital Trust, London.
However, “it cannot be stated with confidence that there was clinical benefit of the agent because the exploratory clinical endpoints were analyzed post hoc and can only be qualitatively compared with historical cohorts,” they added.
The findings were published online Feb. 24 in the New England Journal of Medicine.
A phase 2-3 open-label study
The study enrolled 21 infants with type 1 SMA, between the ages of 1 and 7 months. The majority (n = 17) were treated for 1 year with high-dose risdiplam, reaching 0.2 mg/kg of body weight per day by the twelfth month. Four infants in a low-dose cohort were treated with 0.08 mg/kg by the twelfth month. The medication was administered once daily orally in infants who were able to swallow, or by feeding tube for those who could not.
The primary outcomes of this first part of the study were safety, pharmacokinetics, pharmacodynamics (including the blood SMN protein concentration), and selection of the risdiplam dose for part 2 of the study. Exploratory outcomes included event-free survival, defined as being alive without tracheostomy or the use of permanent ventilation for 16 or more hours per day, and the ability to sit without support for at least 5 seconds.
In terms of safety, the study recorded 24 serious adverse events. “The most common serious adverse events were infections of the respiratory tract, and four infants died of respiratory complications; these findings are consistent with the neuromuscular respiratory failure that characterizes spinal muscular atrophy,” the authors reported. “The risdiplam-associated retinal toxic effects that had been previously observed in monkeys were not observed in the current study,” they added.
Regarding SMN protein levels, a median level of 2.1 times the baseline level was observed within 4 weeks after the initiation of treatment in the high-dose cohort, they reported. By 12 months, these median values had increased to 3.0 times and 1.9 times the baseline values in the low-dose and high-dose cohorts, respectively.
Looking at exploratory efficacy outcomes, 90% of infants survived without ventilatory support, and seven infants in the high-dose cohort were able to sit without support for at least 5 seconds. The higher dose of risdiplam (0.2 mg/kg per day) was selected for part 2 of the study.
The first oral treatment option
Risdiplam is the third SMA treatment approved by the Food and Drug Administration, “and has the potential to expand access to treatment for people with SMA,” commented Mary Schroth, MD, chief medical officer of Cure SMA, who was not involved in the research. She added that the exploratory outcomes of the FIREFISH study represent “a significant milestone for symptomatic infants with SMA type 1.”
While the other two approved SMA therapies – nusinersen and onasemnogene abeparvovec – have led to improvements in survival and motor function, they are administered either intrathecally or intravenously respectively, while risdiplam is an oral therapy.
Dr. Schroth says there are currently no studies comparing the different SMA treatments. “Cure SMA is actively collecting real-world experience with risdiplam and other SMA treatments through multiple pathways,” she said. “Every individual and family, in collaboration with their health care provider, should discuss SMA treatments and make the decision that is best for them.”
Writing in Neuroscience Insights, a few months after risdiplam’s FDA approval last summer, Ravindra N. Singh MD, from the department of biomedical sciences, Iowa State University, Ames, wrote that, as an orally deliverable small molecule, risdiplam “is a major advancement for the treatment of SMA.”
Now, the FIREFISH study is “welcome news,” he said in an interview. “The results look promising so far,” he added. “I am cautiously optimistic that risdiplam would prove to be a viable alternative to the currently available invasive approaches. However, long-term studies (with appropriate age and sex-matched cohorts) would be needed to fully rule out the potential side effects of the repeated administrations.”
The therapy “is particularly great news for a group of SMA patients that might have tolerability and/or immune response concerns when it comes to nusinersen and gene therapy,” he noted in his article, adding that the ability to store and ship the drug at ambient temperatures, as well as its comparatively low cost are added benefits.
The study was supported by F. Hoffmann–La Roche. Dr. Baranello disclosed that he serves as a consultant for AveXis, F. Hoffmann-La Roche, and Sarepta Therapeutics, as well as PTC Therapeutics, from whom he also receives speaker honoraria. Dr. Schroth disclosed no personal conflicts and is an employee of Cure SMA. Cure SMA works to develop strategic relationships with corporate partners with the goal of working together to lead the way to a world without SMA. In advancement of that mission, Cure SMA has received funding from multiple corporate sources including Aetna, Biogen, Blue Cross Blue Shield, Genentech, Kaiser Permanente, Novartis Gene Therapies, Scholar Rock, and United HealthCare. Cure SMA has no financial stake in any treatment and does not advocate for one treatment over another. Dr. Singh disclosed that Spinraza (Nusinersen), the first FDA-approved SMA drug, is based on the target (US patent # 7,838,657) that was discovered in his former laboratory at UMASS Medical School, Worcester, Mass.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Certain DMTs in MS may attenuate COVID-19 vaccines
“There’s no reason to think any of the three authorized vaccines are in any way more dangerous in people with MS, or in the context of MS DMTs. It’s only a question of whether certain DMTs will influence the degree of benefit you get from the vaccine,” said Amit Bar-Or, MD, director of the Center for Neuroinflammation and Neurotherapeutics, chief of the multiple sclerosis division, and Melissa and Paul Anderson President’s Distinguished Professor at the University of Pennsylvania, Philadelphia. He spoke at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, and he also answered questions in a follow-up interview.
“The merits of being protected by the COVID-19 vaccines far outweigh any risks that one would consider associated with vaccines and individuals with MS,” said Dr. Bar-Or. “And there’s reason to think that the RNA vaccines may even be safer than prior, more traditional vaccines. They are nonlive, noninactivated vaccines, and there is no risk in terms of interacting with MS.”
Where do DMTs fit in? In an interview, Hesham Abboud, MD, PhD, of University Hospitals of Cleveland and Case Western Reserve University, also in Cleveland, said there’s reason for caution regarding DMTs that deplete immune cells or entrap them in the lymph nodes. “What is not clearly known is the effect of the fumarates, which do not act through cell depletion but can occasionally deplete immune cells as a side effect. These likely have no negative effect on vaccine efficacy in patients with normal immune cell count but may have a negative effect in those with significant immune cell reduction. Luckily, significant immune cell reduction is rare in patients taking fumarates.”
In addition, he said, “interferons and natalizumab are generally thought to have no impact on vaccine efficacy while glatiramer acetate and teriflunomide are thought to have no or only little impact on vaccines. Most of these concepts are derived from studies of non–COVID-19 vaccines.”
Dr. Bar-Or highlighted specific DMTs. Teriflunomide (Aubagio) “has a relatively mild effect on the immune system and is not thought to be particularly immune suppressive or deplete immune cells,” Dr. Bar-Or said, as shown in a 2015 study he led (Neurol Neuroimmunol Neuroinflamm. 2015 Feb 12;2[2]:e70). In contrast, a 2020 study, also led by Dr. Bar-Or, showed that nonlive vaccinations given after treatment with ocrelizumab (Ocrevus) – an anti-CD20 monoclonal antibody – are “attenuated, compared with untreated or interferon-beta–treated patients, but they can still be expected to be protective.”
Dr. Bar-Or pointed to National MS Society guidelines about the timing of the Pfizer and Moderna mRNA vaccines for patients with MS who are on DMT. In patients with stable MS, the society recommends no adjustments in timing for patients starting or remaining on several DMTs. The list includes teriflunomide, glatiramer acetate (Copaxone), and dimethyl fumarate, among others.
Patients shouldn’t start fingolimod (Gilenya), siponimod (Mayzent), or ozanimod (Zeposia) until 4 weeks or more after their second vaccine dose, the guidelines suggest. Vaccine doses are recommended 3-5 days after the final dose of high-dose steroids. And there are more complicated recommendations regarding a number of other DMTs – ocrelizumab, ofatumumab (Kesimpta), alemtuzumab (Lemtrada), cladribine (Mavenclad), and rituximab (Rituxan).
Dr. Bar-Or cautioned that the guidelines are an imperfect “first pass” and are being updated.
He added that the guidelines are not set in stone: “Scheduling is not always possible in terms of adjusting the vaccine timing. Patients in general are recommended to take the vaccine when it becomes available, as it may be more important for them to get the vaccine than to try to time the vaccine relative to the DMT.”
Guidance regarding the newly authorized Johnson & Johnson vaccine is expected soon, said neurologist Barbara Giesser, MD, of Pacific Neuroscience Institute in Santa Monica, Calif., in an interview. As for her advice to patients, she said that, “in general, I am recommending that patients get [vaccinated] as soon as it is available to them with adjustment of timing of some DMTs as may be appropriate.”
Dr. Bar-Or has received consulting fees and/or grant support from – or participated as a speaker in events sponsored by – Accure, Atara Biotherapeutics, Biogen, Bristol-Myer Squibb/Celgene/Receptos, GlaxoSmithKline, Gossamer, Janssen/Actelion, Medimmune, Merck/EMD Serono, Novartis, Roche/Genentech, and Sanofi-Genzyme. He also receives research funding from various organizations and agencies. Dr. Abboud reported receiving consulting fees from Biogen, Genentech, Bristol-Myer Squibb, Alexion, and Viela Bio. He receives research support from Novartis, Bristol-Myer Squibb, Genentech, and Sanofi-Genzyme. Dr. Giesser reports no disclosures.
“There’s no reason to think any of the three authorized vaccines are in any way more dangerous in people with MS, or in the context of MS DMTs. It’s only a question of whether certain DMTs will influence the degree of benefit you get from the vaccine,” said Amit Bar-Or, MD, director of the Center for Neuroinflammation and Neurotherapeutics, chief of the multiple sclerosis division, and Melissa and Paul Anderson President’s Distinguished Professor at the University of Pennsylvania, Philadelphia. He spoke at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, and he also answered questions in a follow-up interview.
“The merits of being protected by the COVID-19 vaccines far outweigh any risks that one would consider associated with vaccines and individuals with MS,” said Dr. Bar-Or. “And there’s reason to think that the RNA vaccines may even be safer than prior, more traditional vaccines. They are nonlive, noninactivated vaccines, and there is no risk in terms of interacting with MS.”
Where do DMTs fit in? In an interview, Hesham Abboud, MD, PhD, of University Hospitals of Cleveland and Case Western Reserve University, also in Cleveland, said there’s reason for caution regarding DMTs that deplete immune cells or entrap them in the lymph nodes. “What is not clearly known is the effect of the fumarates, which do not act through cell depletion but can occasionally deplete immune cells as a side effect. These likely have no negative effect on vaccine efficacy in patients with normal immune cell count but may have a negative effect in those with significant immune cell reduction. Luckily, significant immune cell reduction is rare in patients taking fumarates.”
In addition, he said, “interferons and natalizumab are generally thought to have no impact on vaccine efficacy while glatiramer acetate and teriflunomide are thought to have no or only little impact on vaccines. Most of these concepts are derived from studies of non–COVID-19 vaccines.”
Dr. Bar-Or highlighted specific DMTs. Teriflunomide (Aubagio) “has a relatively mild effect on the immune system and is not thought to be particularly immune suppressive or deplete immune cells,” Dr. Bar-Or said, as shown in a 2015 study he led (Neurol Neuroimmunol Neuroinflamm. 2015 Feb 12;2[2]:e70). In contrast, a 2020 study, also led by Dr. Bar-Or, showed that nonlive vaccinations given after treatment with ocrelizumab (Ocrevus) – an anti-CD20 monoclonal antibody – are “attenuated, compared with untreated or interferon-beta–treated patients, but they can still be expected to be protective.”
Dr. Bar-Or pointed to National MS Society guidelines about the timing of the Pfizer and Moderna mRNA vaccines for patients with MS who are on DMT. In patients with stable MS, the society recommends no adjustments in timing for patients starting or remaining on several DMTs. The list includes teriflunomide, glatiramer acetate (Copaxone), and dimethyl fumarate, among others.
Patients shouldn’t start fingolimod (Gilenya), siponimod (Mayzent), or ozanimod (Zeposia) until 4 weeks or more after their second vaccine dose, the guidelines suggest. Vaccine doses are recommended 3-5 days after the final dose of high-dose steroids. And there are more complicated recommendations regarding a number of other DMTs – ocrelizumab, ofatumumab (Kesimpta), alemtuzumab (Lemtrada), cladribine (Mavenclad), and rituximab (Rituxan).
Dr. Bar-Or cautioned that the guidelines are an imperfect “first pass” and are being updated.
He added that the guidelines are not set in stone: “Scheduling is not always possible in terms of adjusting the vaccine timing. Patients in general are recommended to take the vaccine when it becomes available, as it may be more important for them to get the vaccine than to try to time the vaccine relative to the DMT.”
Guidance regarding the newly authorized Johnson & Johnson vaccine is expected soon, said neurologist Barbara Giesser, MD, of Pacific Neuroscience Institute in Santa Monica, Calif., in an interview. As for her advice to patients, she said that, “in general, I am recommending that patients get [vaccinated] as soon as it is available to them with adjustment of timing of some DMTs as may be appropriate.”
Dr. Bar-Or has received consulting fees and/or grant support from – or participated as a speaker in events sponsored by – Accure, Atara Biotherapeutics, Biogen, Bristol-Myer Squibb/Celgene/Receptos, GlaxoSmithKline, Gossamer, Janssen/Actelion, Medimmune, Merck/EMD Serono, Novartis, Roche/Genentech, and Sanofi-Genzyme. He also receives research funding from various organizations and agencies. Dr. Abboud reported receiving consulting fees from Biogen, Genentech, Bristol-Myer Squibb, Alexion, and Viela Bio. He receives research support from Novartis, Bristol-Myer Squibb, Genentech, and Sanofi-Genzyme. Dr. Giesser reports no disclosures.
“There’s no reason to think any of the three authorized vaccines are in any way more dangerous in people with MS, or in the context of MS DMTs. It’s only a question of whether certain DMTs will influence the degree of benefit you get from the vaccine,” said Amit Bar-Or, MD, director of the Center for Neuroinflammation and Neurotherapeutics, chief of the multiple sclerosis division, and Melissa and Paul Anderson President’s Distinguished Professor at the University of Pennsylvania, Philadelphia. He spoke at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, and he also answered questions in a follow-up interview.
“The merits of being protected by the COVID-19 vaccines far outweigh any risks that one would consider associated with vaccines and individuals with MS,” said Dr. Bar-Or. “And there’s reason to think that the RNA vaccines may even be safer than prior, more traditional vaccines. They are nonlive, noninactivated vaccines, and there is no risk in terms of interacting with MS.”
Where do DMTs fit in? In an interview, Hesham Abboud, MD, PhD, of University Hospitals of Cleveland and Case Western Reserve University, also in Cleveland, said there’s reason for caution regarding DMTs that deplete immune cells or entrap them in the lymph nodes. “What is not clearly known is the effect of the fumarates, which do not act through cell depletion but can occasionally deplete immune cells as a side effect. These likely have no negative effect on vaccine efficacy in patients with normal immune cell count but may have a negative effect in those with significant immune cell reduction. Luckily, significant immune cell reduction is rare in patients taking fumarates.”
In addition, he said, “interferons and natalizumab are generally thought to have no impact on vaccine efficacy while glatiramer acetate and teriflunomide are thought to have no or only little impact on vaccines. Most of these concepts are derived from studies of non–COVID-19 vaccines.”
Dr. Bar-Or highlighted specific DMTs. Teriflunomide (Aubagio) “has a relatively mild effect on the immune system and is not thought to be particularly immune suppressive or deplete immune cells,” Dr. Bar-Or said, as shown in a 2015 study he led (Neurol Neuroimmunol Neuroinflamm. 2015 Feb 12;2[2]:e70). In contrast, a 2020 study, also led by Dr. Bar-Or, showed that nonlive vaccinations given after treatment with ocrelizumab (Ocrevus) – an anti-CD20 monoclonal antibody – are “attenuated, compared with untreated or interferon-beta–treated patients, but they can still be expected to be protective.”
Dr. Bar-Or pointed to National MS Society guidelines about the timing of the Pfizer and Moderna mRNA vaccines for patients with MS who are on DMT. In patients with stable MS, the society recommends no adjustments in timing for patients starting or remaining on several DMTs. The list includes teriflunomide, glatiramer acetate (Copaxone), and dimethyl fumarate, among others.
Patients shouldn’t start fingolimod (Gilenya), siponimod (Mayzent), or ozanimod (Zeposia) until 4 weeks or more after their second vaccine dose, the guidelines suggest. Vaccine doses are recommended 3-5 days after the final dose of high-dose steroids. And there are more complicated recommendations regarding a number of other DMTs – ocrelizumab, ofatumumab (Kesimpta), alemtuzumab (Lemtrada), cladribine (Mavenclad), and rituximab (Rituxan).
Dr. Bar-Or cautioned that the guidelines are an imperfect “first pass” and are being updated.
He added that the guidelines are not set in stone: “Scheduling is not always possible in terms of adjusting the vaccine timing. Patients in general are recommended to take the vaccine when it becomes available, as it may be more important for them to get the vaccine than to try to time the vaccine relative to the DMT.”
Guidance regarding the newly authorized Johnson & Johnson vaccine is expected soon, said neurologist Barbara Giesser, MD, of Pacific Neuroscience Institute in Santa Monica, Calif., in an interview. As for her advice to patients, she said that, “in general, I am recommending that patients get [vaccinated] as soon as it is available to them with adjustment of timing of some DMTs as may be appropriate.”
Dr. Bar-Or has received consulting fees and/or grant support from – or participated as a speaker in events sponsored by – Accure, Atara Biotherapeutics, Biogen, Bristol-Myer Squibb/Celgene/Receptos, GlaxoSmithKline, Gossamer, Janssen/Actelion, Medimmune, Merck/EMD Serono, Novartis, Roche/Genentech, and Sanofi-Genzyme. He also receives research funding from various organizations and agencies. Dr. Abboud reported receiving consulting fees from Biogen, Genentech, Bristol-Myer Squibb, Alexion, and Viela Bio. He receives research support from Novartis, Bristol-Myer Squibb, Genentech, and Sanofi-Genzyme. Dr. Giesser reports no disclosures.
FROM ACTRIMS FORUM 2021
Sleep apnea and cognitive impairment are common bedfellows
“The study shows obstructive sleep apnea is common in patients with cognitive impairment. The results suggest that people with cognitive impairment should be assessed for sleep apnea if they have difficulty with sleep or if they demonstrate sleep-related symptoms,” said study investigator David Colelli, MSc, research coordinator at Sunnybrook Health Sciences Centre in Toronto.
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology..
Linked to cognitive impairment
OSA is a common sleep disorder and is associated with an increased risk of developing cognitive impairment. It is also prevalent in the general population, but even more common among patients with dementia.
However, the investigators noted, the frequency and predictors of OSA have not been well established in Alzheimer’s disease and other related conditions such as vascular dementia.
The investigators had conducted a previous feasibility study investigating a home sleep monitor as an OSA screening tool. The current research examined potential correlations between OSA detected by this monitor and cognitive impairment.
The study included 67 patients with cognitive impairment due to neurodegenerative or vascular disease. The range of disorders included Alzheimer’s disease, mild cognitive impairment caused by Alzheimer’s disease, dementia caused by Parkinson’s or Lewy body disease, and vascular conditions.
Participants had a mean age of 72.8 years and 44.8% were male. The mean body mass index (BMI) was 25.6 kg/m2.
These participants completed a home sleep apnea test, which is an alternative to polysomnography for the detection of OSA.
Researchers identified OSA in 52.2% of the study population. This, Mr. Colelli said, “is in the range” of other research investigating sleep and cognitive impairment.
“In the general population, however, this number is a lot lower – in the 10%-20% range depending on the population or country you’re looking at,” Mr. Colelli said.
He emphasized that, without an objective sleep test, some patients may be unaware of their sleep issues. Those with cognitive impairment may “misjudge how they’re sleeping,” especially if they sleep without a partner, so it’s possible that sleep disorder symptoms often go undetected.
Bidirectional relationship?
Participants answered questionnaires on sleep, cognition, and mood. They also completed the 30-point Montreal Cognitive Assessment (MoCA) to assess language, visuospatial abilities, memory and recall, and abstract thinking.
Scores on this test range from 0 to 30, with a score of 26 or higher signifying normal, 18-25 indicating mild cognitive impairment, and 17 or lower indicating moderate to severe cognitive impairment. The average score for study participants with OSA was 20.5, compared with 23.6 for those without the sleep disorder.
Results showed OSA was significantly associated with a lower score on the MoCA scale (odds ratio, 0.40; P = .048). “This demonstrated an association of OSA with lower cognitive scores,” Mr. Colelli said.
The analysis also showed that OSA severity was correlated with actigraphy-derived sleep variables, including lower total sleep time, greater sleep onset latency, lower sleep efficiency, and more awakenings.
The study was too small to determine whether a specific diagnosis of cognitive impairment affected the link to OSA, Mr. Colelli said. “But definitely future research should be directed towards looking at this.”
Obesity is a risk factor for OSA, but the mean BMI in the study was not in the obese range of 30 and over. This, Mr. Colelli said, suggests that sleep apnea may present differently in those with cognitive impairment.
“Sleep apnea in this population might not present with the typical risk factors of obesity or snoring or feeling tired.”
While the new study “adds to the understanding that there’s a link between sleep and cognitive impairment, the direction of that link isn’t entirely clear,” Mr. Colelli said.
“It’s slowly becoming appreciated that the relationship might be bidirectionality, where sleep apnea might be contributing to the cognitive impairment and cognitive impairment could be contributing to the sleep issues.”
The study highlights how essential sleep is to mental health, Mr. Colelli said. “I feel, and I’m sure you do too, that if you don’t get good sleep, you feel tired during the day and you may not have the best concentration or memory.”
Identifying sleep issues in patients with cognitive impairment is important, as treatment and management of these issues could affect outcomes including cognition and quality of life, he added.
“Future research should be directed to see if treatment of sleep disorders with continuous positive airway pressure (CPAP), which is the gold standard, and various other treatments, can improve outcomes.” Future research should also examine OSA prevalence in larger cohorts.
Common, undertreated
Commenting on the resaerch, Lei Gao, MD, assistant professor of anesthesia at Harvard Medical School, Boston, whose areas of expertise include disorders of cognition, sleep, and circadian rhythm, believes the findings are important. “It highlights how common and potentially undertreated OSA is in this age group, and in particular, its link to cognitive impairment.”
OSA is often associated with significant comorbidities, as well as sleep disruption, Dr. Gao noted. One of the study’s strengths was including objective assessment of sleep using actigraphy. “It will be interesting to see to what extent the OSA link to cognitive impairment is via poor sleep or disrupted circadian rest/activity cycles.”
It would also be interesting “to tease out whether OSA is more linked to dementia of vascular etiologies due to common risk factors, or whether it is pervasive to all forms of dementia,” he added.
A version of this article first appeared on Medscape.com.
“The study shows obstructive sleep apnea is common in patients with cognitive impairment. The results suggest that people with cognitive impairment should be assessed for sleep apnea if they have difficulty with sleep or if they demonstrate sleep-related symptoms,” said study investigator David Colelli, MSc, research coordinator at Sunnybrook Health Sciences Centre in Toronto.
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology..
Linked to cognitive impairment
OSA is a common sleep disorder and is associated with an increased risk of developing cognitive impairment. It is also prevalent in the general population, but even more common among patients with dementia.
However, the investigators noted, the frequency and predictors of OSA have not been well established in Alzheimer’s disease and other related conditions such as vascular dementia.
The investigators had conducted a previous feasibility study investigating a home sleep monitor as an OSA screening tool. The current research examined potential correlations between OSA detected by this monitor and cognitive impairment.
The study included 67 patients with cognitive impairment due to neurodegenerative or vascular disease. The range of disorders included Alzheimer’s disease, mild cognitive impairment caused by Alzheimer’s disease, dementia caused by Parkinson’s or Lewy body disease, and vascular conditions.
Participants had a mean age of 72.8 years and 44.8% were male. The mean body mass index (BMI) was 25.6 kg/m2.
These participants completed a home sleep apnea test, which is an alternative to polysomnography for the detection of OSA.
Researchers identified OSA in 52.2% of the study population. This, Mr. Colelli said, “is in the range” of other research investigating sleep and cognitive impairment.
“In the general population, however, this number is a lot lower – in the 10%-20% range depending on the population or country you’re looking at,” Mr. Colelli said.
He emphasized that, without an objective sleep test, some patients may be unaware of their sleep issues. Those with cognitive impairment may “misjudge how they’re sleeping,” especially if they sleep without a partner, so it’s possible that sleep disorder symptoms often go undetected.
Bidirectional relationship?
Participants answered questionnaires on sleep, cognition, and mood. They also completed the 30-point Montreal Cognitive Assessment (MoCA) to assess language, visuospatial abilities, memory and recall, and abstract thinking.
Scores on this test range from 0 to 30, with a score of 26 or higher signifying normal, 18-25 indicating mild cognitive impairment, and 17 or lower indicating moderate to severe cognitive impairment. The average score for study participants with OSA was 20.5, compared with 23.6 for those without the sleep disorder.
Results showed OSA was significantly associated with a lower score on the MoCA scale (odds ratio, 0.40; P = .048). “This demonstrated an association of OSA with lower cognitive scores,” Mr. Colelli said.
The analysis also showed that OSA severity was correlated with actigraphy-derived sleep variables, including lower total sleep time, greater sleep onset latency, lower sleep efficiency, and more awakenings.
The study was too small to determine whether a specific diagnosis of cognitive impairment affected the link to OSA, Mr. Colelli said. “But definitely future research should be directed towards looking at this.”
Obesity is a risk factor for OSA, but the mean BMI in the study was not in the obese range of 30 and over. This, Mr. Colelli said, suggests that sleep apnea may present differently in those with cognitive impairment.
“Sleep apnea in this population might not present with the typical risk factors of obesity or snoring or feeling tired.”
While the new study “adds to the understanding that there’s a link between sleep and cognitive impairment, the direction of that link isn’t entirely clear,” Mr. Colelli said.
“It’s slowly becoming appreciated that the relationship might be bidirectionality, where sleep apnea might be contributing to the cognitive impairment and cognitive impairment could be contributing to the sleep issues.”
The study highlights how essential sleep is to mental health, Mr. Colelli said. “I feel, and I’m sure you do too, that if you don’t get good sleep, you feel tired during the day and you may not have the best concentration or memory.”
Identifying sleep issues in patients with cognitive impairment is important, as treatment and management of these issues could affect outcomes including cognition and quality of life, he added.
“Future research should be directed to see if treatment of sleep disorders with continuous positive airway pressure (CPAP), which is the gold standard, and various other treatments, can improve outcomes.” Future research should also examine OSA prevalence in larger cohorts.
Common, undertreated
Commenting on the resaerch, Lei Gao, MD, assistant professor of anesthesia at Harvard Medical School, Boston, whose areas of expertise include disorders of cognition, sleep, and circadian rhythm, believes the findings are important. “It highlights how common and potentially undertreated OSA is in this age group, and in particular, its link to cognitive impairment.”
OSA is often associated with significant comorbidities, as well as sleep disruption, Dr. Gao noted. One of the study’s strengths was including objective assessment of sleep using actigraphy. “It will be interesting to see to what extent the OSA link to cognitive impairment is via poor sleep or disrupted circadian rest/activity cycles.”
It would also be interesting “to tease out whether OSA is more linked to dementia of vascular etiologies due to common risk factors, or whether it is pervasive to all forms of dementia,” he added.
A version of this article first appeared on Medscape.com.
“The study shows obstructive sleep apnea is common in patients with cognitive impairment. The results suggest that people with cognitive impairment should be assessed for sleep apnea if they have difficulty with sleep or if they demonstrate sleep-related symptoms,” said study investigator David Colelli, MSc, research coordinator at Sunnybrook Health Sciences Centre in Toronto.
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology..
Linked to cognitive impairment
OSA is a common sleep disorder and is associated with an increased risk of developing cognitive impairment. It is also prevalent in the general population, but even more common among patients with dementia.
However, the investigators noted, the frequency and predictors of OSA have not been well established in Alzheimer’s disease and other related conditions such as vascular dementia.
The investigators had conducted a previous feasibility study investigating a home sleep monitor as an OSA screening tool. The current research examined potential correlations between OSA detected by this monitor and cognitive impairment.
The study included 67 patients with cognitive impairment due to neurodegenerative or vascular disease. The range of disorders included Alzheimer’s disease, mild cognitive impairment caused by Alzheimer’s disease, dementia caused by Parkinson’s or Lewy body disease, and vascular conditions.
Participants had a mean age of 72.8 years and 44.8% were male. The mean body mass index (BMI) was 25.6 kg/m2.
These participants completed a home sleep apnea test, which is an alternative to polysomnography for the detection of OSA.
Researchers identified OSA in 52.2% of the study population. This, Mr. Colelli said, “is in the range” of other research investigating sleep and cognitive impairment.
“In the general population, however, this number is a lot lower – in the 10%-20% range depending on the population or country you’re looking at,” Mr. Colelli said.
He emphasized that, without an objective sleep test, some patients may be unaware of their sleep issues. Those with cognitive impairment may “misjudge how they’re sleeping,” especially if they sleep without a partner, so it’s possible that sleep disorder symptoms often go undetected.
Bidirectional relationship?
Participants answered questionnaires on sleep, cognition, and mood. They also completed the 30-point Montreal Cognitive Assessment (MoCA) to assess language, visuospatial abilities, memory and recall, and abstract thinking.
Scores on this test range from 0 to 30, with a score of 26 or higher signifying normal, 18-25 indicating mild cognitive impairment, and 17 or lower indicating moderate to severe cognitive impairment. The average score for study participants with OSA was 20.5, compared with 23.6 for those without the sleep disorder.
Results showed OSA was significantly associated with a lower score on the MoCA scale (odds ratio, 0.40; P = .048). “This demonstrated an association of OSA with lower cognitive scores,” Mr. Colelli said.
The analysis also showed that OSA severity was correlated with actigraphy-derived sleep variables, including lower total sleep time, greater sleep onset latency, lower sleep efficiency, and more awakenings.
The study was too small to determine whether a specific diagnosis of cognitive impairment affected the link to OSA, Mr. Colelli said. “But definitely future research should be directed towards looking at this.”
Obesity is a risk factor for OSA, but the mean BMI in the study was not in the obese range of 30 and over. This, Mr. Colelli said, suggests that sleep apnea may present differently in those with cognitive impairment.
“Sleep apnea in this population might not present with the typical risk factors of obesity or snoring or feeling tired.”
While the new study “adds to the understanding that there’s a link between sleep and cognitive impairment, the direction of that link isn’t entirely clear,” Mr. Colelli said.
“It’s slowly becoming appreciated that the relationship might be bidirectionality, where sleep apnea might be contributing to the cognitive impairment and cognitive impairment could be contributing to the sleep issues.”
The study highlights how essential sleep is to mental health, Mr. Colelli said. “I feel, and I’m sure you do too, that if you don’t get good sleep, you feel tired during the day and you may not have the best concentration or memory.”
Identifying sleep issues in patients with cognitive impairment is important, as treatment and management of these issues could affect outcomes including cognition and quality of life, he added.
“Future research should be directed to see if treatment of sleep disorders with continuous positive airway pressure (CPAP), which is the gold standard, and various other treatments, can improve outcomes.” Future research should also examine OSA prevalence in larger cohorts.
Common, undertreated
Commenting on the resaerch, Lei Gao, MD, assistant professor of anesthesia at Harvard Medical School, Boston, whose areas of expertise include disorders of cognition, sleep, and circadian rhythm, believes the findings are important. “It highlights how common and potentially undertreated OSA is in this age group, and in particular, its link to cognitive impairment.”
OSA is often associated with significant comorbidities, as well as sleep disruption, Dr. Gao noted. One of the study’s strengths was including objective assessment of sleep using actigraphy. “It will be interesting to see to what extent the OSA link to cognitive impairment is via poor sleep or disrupted circadian rest/activity cycles.”
It would also be interesting “to tease out whether OSA is more linked to dementia of vascular etiologies due to common risk factors, or whether it is pervasive to all forms of dementia,” he added.
A version of this article first appeared on Medscape.com.
FROM AAN 2021
Do antidepressants increase the risk of brain bleeds?
Contrary to previous findings, results of a large observational study show. However, at least one expert urged caution in interpreting the finding.
“These findings are important, especially since depression is common after stroke and SSRIs are some of the first drugs considered for people,” Mithilesh Siddu, MD, of the University of Miami/Jackson Memorial Hospital, also in Miami, said in a statement.
However, Dr. Siddu said “more research is needed to confirm our findings and to also examine if SSRIs prescribed after a stroke may be linked to risk of a second stroke.”
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Widely prescribed
SSRIs, the most widely prescribed antidepressant in the United States, have previously been linked to an increased risk of ICH, possibly as a result of impaired platelet function.
To investigate further, the researchers analyzed data from the Florida Stroke Registry (FSR). They identified 127,915 patients who suffered ICH from January 2010 to December 2019 and for whom information on antidepressant use was available.
They analyzed the proportion of cases presenting with ICH among antidepressant users and the rate of SSRI prescription among stroke patients discharged on antidepressant therapy.
The researchers found that 11% of those who had been prescribed antidepressants had an ICH, compared with 14% of those who had not.
Antidepressant users were more likely to be female; non-Hispanic White; have hypertension; have diabetes; and use oral anticoagulants, antiplatelets, and statins prior to hospital presentation for ICH.
In multivariable analyses adjusting for age, race, prior history of hypertension, diabetes and prior oral anticoagulant, antiplatelet and statin use, antidepressant users were just as likely to present with spontaneous ICH as nonantidepressant users (odds ratio, 0.92; 95% confidence interval, 0.85-1.01).
A total of 3.4% of all ICH patients and 9% of those in whom specific antidepressant information was available were discharged home on an antidepressant, most commonly an SSRI (74%).
The authors noted a key limitation of the study: Some details regarding the length, dosage, and type of antidepressants were not available.
Interpret with caution
In a comment, Shaheen Lakhan, MD, PhD, a neurologist in Newton, Mass., and executive director of the Global Neuroscience Initiative Foundation, urged caution in making any firm conclusions based on this study.
“We have two questions here: One, is SSRI use a risk factor for first-time intracerebral hemorrhage, and two, is SSRI use after an ICH a risk factor for additional hemorrhages,” said Dr. Lakhan, who was not involved with the study.
“This study incompletely addresses the first because it is known that SSRIs have a variety of potencies. For instance, paroxetine is a strong inhibitor of serotonin reuptake, whereas bupropion is weak. Hypothetically, the former has a greater risk of ICH. Because this study did not stratify by type of antidepressant, it is not possible to tease these out,” Dr. Lakhan said.
“The second question is completely unaddressed by this study and is the real concern in clinical practice, because the chance of rebleed is much higher than the risk of first-time ICH in the general population,” he added.
The study had no specific funding. Dr. Siddu and Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Contrary to previous findings, results of a large observational study show. However, at least one expert urged caution in interpreting the finding.
“These findings are important, especially since depression is common after stroke and SSRIs are some of the first drugs considered for people,” Mithilesh Siddu, MD, of the University of Miami/Jackson Memorial Hospital, also in Miami, said in a statement.
However, Dr. Siddu said “more research is needed to confirm our findings and to also examine if SSRIs prescribed after a stroke may be linked to risk of a second stroke.”
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Widely prescribed
SSRIs, the most widely prescribed antidepressant in the United States, have previously been linked to an increased risk of ICH, possibly as a result of impaired platelet function.
To investigate further, the researchers analyzed data from the Florida Stroke Registry (FSR). They identified 127,915 patients who suffered ICH from January 2010 to December 2019 and for whom information on antidepressant use was available.
They analyzed the proportion of cases presenting with ICH among antidepressant users and the rate of SSRI prescription among stroke patients discharged on antidepressant therapy.
The researchers found that 11% of those who had been prescribed antidepressants had an ICH, compared with 14% of those who had not.
Antidepressant users were more likely to be female; non-Hispanic White; have hypertension; have diabetes; and use oral anticoagulants, antiplatelets, and statins prior to hospital presentation for ICH.
In multivariable analyses adjusting for age, race, prior history of hypertension, diabetes and prior oral anticoagulant, antiplatelet and statin use, antidepressant users were just as likely to present with spontaneous ICH as nonantidepressant users (odds ratio, 0.92; 95% confidence interval, 0.85-1.01).
A total of 3.4% of all ICH patients and 9% of those in whom specific antidepressant information was available were discharged home on an antidepressant, most commonly an SSRI (74%).
The authors noted a key limitation of the study: Some details regarding the length, dosage, and type of antidepressants were not available.
Interpret with caution
In a comment, Shaheen Lakhan, MD, PhD, a neurologist in Newton, Mass., and executive director of the Global Neuroscience Initiative Foundation, urged caution in making any firm conclusions based on this study.
“We have two questions here: One, is SSRI use a risk factor for first-time intracerebral hemorrhage, and two, is SSRI use after an ICH a risk factor for additional hemorrhages,” said Dr. Lakhan, who was not involved with the study.
“This study incompletely addresses the first because it is known that SSRIs have a variety of potencies. For instance, paroxetine is a strong inhibitor of serotonin reuptake, whereas bupropion is weak. Hypothetically, the former has a greater risk of ICH. Because this study did not stratify by type of antidepressant, it is not possible to tease these out,” Dr. Lakhan said.
“The second question is completely unaddressed by this study and is the real concern in clinical practice, because the chance of rebleed is much higher than the risk of first-time ICH in the general population,” he added.
The study had no specific funding. Dr. Siddu and Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Contrary to previous findings, results of a large observational study show. However, at least one expert urged caution in interpreting the finding.
“These findings are important, especially since depression is common after stroke and SSRIs are some of the first drugs considered for people,” Mithilesh Siddu, MD, of the University of Miami/Jackson Memorial Hospital, also in Miami, said in a statement.
However, Dr. Siddu said “more research is needed to confirm our findings and to also examine if SSRIs prescribed after a stroke may be linked to risk of a second stroke.”
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Widely prescribed
SSRIs, the most widely prescribed antidepressant in the United States, have previously been linked to an increased risk of ICH, possibly as a result of impaired platelet function.
To investigate further, the researchers analyzed data from the Florida Stroke Registry (FSR). They identified 127,915 patients who suffered ICH from January 2010 to December 2019 and for whom information on antidepressant use was available.
They analyzed the proportion of cases presenting with ICH among antidepressant users and the rate of SSRI prescription among stroke patients discharged on antidepressant therapy.
The researchers found that 11% of those who had been prescribed antidepressants had an ICH, compared with 14% of those who had not.
Antidepressant users were more likely to be female; non-Hispanic White; have hypertension; have diabetes; and use oral anticoagulants, antiplatelets, and statins prior to hospital presentation for ICH.
In multivariable analyses adjusting for age, race, prior history of hypertension, diabetes and prior oral anticoagulant, antiplatelet and statin use, antidepressant users were just as likely to present with spontaneous ICH as nonantidepressant users (odds ratio, 0.92; 95% confidence interval, 0.85-1.01).
A total of 3.4% of all ICH patients and 9% of those in whom specific antidepressant information was available were discharged home on an antidepressant, most commonly an SSRI (74%).
The authors noted a key limitation of the study: Some details regarding the length, dosage, and type of antidepressants were not available.
Interpret with caution
In a comment, Shaheen Lakhan, MD, PhD, a neurologist in Newton, Mass., and executive director of the Global Neuroscience Initiative Foundation, urged caution in making any firm conclusions based on this study.
“We have two questions here: One, is SSRI use a risk factor for first-time intracerebral hemorrhage, and two, is SSRI use after an ICH a risk factor for additional hemorrhages,” said Dr. Lakhan, who was not involved with the study.
“This study incompletely addresses the first because it is known that SSRIs have a variety of potencies. For instance, paroxetine is a strong inhibitor of serotonin reuptake, whereas bupropion is weak. Hypothetically, the former has a greater risk of ICH. Because this study did not stratify by type of antidepressant, it is not possible to tease these out,” Dr. Lakhan said.
“The second question is completely unaddressed by this study and is the real concern in clinical practice, because the chance of rebleed is much higher than the risk of first-time ICH in the general population,” he added.
The study had no specific funding. Dr. Siddu and Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2021
Functional neurological disorder: A practical guide to an elusive Dx
CASE
John D,* a 25-year-old patient with an otherwise unremarkable medical history, describes 2 months of daily headache, lower-extremity weakness, and unsteady gait that began fairly suddenly during his first deployment in the US Army. He explains that these symptoms affected his ability to perform his duties and necessitated an early return stateside for evaluation and treatment.
Mr. D denies precipitating trauma or unusual environmental exposures. He reports that, stateside now, symptoms continue to affect his ability to work and attend to personal and family responsibilities.
Asked about stressors, Mr. D notes the birth of his first child approximately 3 months ago, while he was deployed, and marital stressors. He denies suicidal or homicidal ideation.
* The patient’s name has been changed to protect his identity.
The challenge of identifying and managing FND
A functional neurological disorder (FND) is a constellation of psychological, physiological, and neurological symptoms, without an identifiable organic etiology, a conscious decision, or secondary gain for the patient,1 that adversely impacts functioning in 1 or more significant life domains.
Given the high throughput of patients in primary care practices, family physicians can expect to encounter suspected cases of FND in their practices. Regrettably, however, a lack of familiarity with the disorder and its related problems (eg, nonorganic paralysis, sensory loss, nonepileptic seizures, and abnormal movements) can add as much as $20,000 in excess direct and indirect costs of care for every such patient.1 In this article, we synthesize the recent literature on FND so that family physicians can expand their acumen in understanding, identifying, and evaluating patients whose presentation suggests FND.
An underrecognized entity
A precise estimate of the prevalence of FND is difficult to determine because the disorder is underrecognized and misdiagnosed and because it is often accompanied by the confounding of psychological and physiological comorbidities. A 2012 study estimated the annual incidence of FND to be 4 to 12 cases for every 100,000 people2; in primary care and outpatient neurology settings, prevalence is 6% to 22% of all patients.3,4 Stone and colleagues identified functional neurological symptoms as the second most common reason for outpatient neurology consultation,5 with 1 nonepileptic seizure patient seen for every 6 epileptic patients, and functional weakness presenting at the same rate as multiple sclerosis.6
Continue to: Demographics of patients with FND...
Demographics of patients with FND vary, depending on presenting neurological symptoms and disorder subtype. Existing data indicate a correlation between FND and younger age, female sex, physical disability,7 and a history of abuse or trauma.3,8 A challenge in concretely ascertaining the prevalence of FND is that conditions such as fibromyalgia, chronic pelvic pain, globus hystericus, and nonepileptic seizures can also be characterized as medically unexplained functional disorders, even within the network of neurology care.4
Misdiagnosis and bias are not uncommon
Ambiguity in classifying and evaluating FND can affect physicians’ perceptions, assessment, and care of patients with suggestive presenting symptoms. A major early challenge in diagnosing FND is the inconsistency of characterizing terminology (pseudoneurological, somatic, dissociative, conversion, psychogenic, hysterical, factitious, functional, medically unexplained9,10) and definitions in the literature. Neurological symptoms of unidentifiable organic cause can greatly diminish quality of life4; FND is a scientifically and clinically useful diagnosis for many combinations of nonrandomly co-occurring symptoms and clinical signs.
The pitfall of misdiagnosis. Remain cautious about making a diagnosis of FND by exclusion, which might yield an incorrect or false-negative finding because of an atypical presentation. It is important to avoid misdiagnosis by prematurely closing the differential diagnosis; instead, keep in mind that a medically unexplained diagnosis might be better explained by conducting a robust social and medical history and obtaining additional or collateral data, or both, along with appropriate consultation.4,9
Misdiagnosis can lead to a circuitous and costly work-up, with the potential to increase the patient’s distress. You can reduce this burden with early recognition of FND and centralized management of multidisciplinary care, which are more likely to lead to an accurate and timely diagnosis—paramount to empowering patients with access to the correct information and meaningful support needed to enhance treatment and self-care.9
Bias, haste, and dismissal are unproductive. Even with a clear definition of FND, it is not uncommon for a physician to rapidly assess a patient’s clinical signs, make a diagnosis of “unknown etiology,” or openly question the veracity of complaints. Furthermore, be aware of inadvertently characterizing FND using the prefix “pseudo” or the term “hysterical,” which can be psychologically discomforting for many patients, who legitimately experience inexplicable symptoms. Such pejoratives can lead to stigmatizing and misleading assessments and treatment paths4—courses of action that can cause early and, possibly, irreparable harm to the patient–physician relationship and increase the patient’s inclination to go “doctor-shopping,” with associated loss of continuity of care.
Why is it difficult to diagnose FND?
The latest (5th) edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) describes conversion, somatoform disorder, and FND synonymously.DSM-5 diagnostic criteria for conversion disorderare11:
- a specified type of symptom or deficit of altered voluntary motor or sensory function (eg, weakness, difficulty swallowing, slurred speech, seizures)
- clinical evidence of the incompatibility of the symptom or deficit and any recognized neurological or medical disorder
- incapability of better explaining the symptom or deficit as another medical or mental disorder.
- The symptom or deficit causes distress or impairment that (1) is clinically significant in occupational, social, or other important areas of function or (2) warrants medical evaluation.
The overarching feature of these criteria is the inconsistency of symptoms with recognized neurological, physiological, or psychiatric conditions. Although identification of psychological factors can help clarify and provide a treatment direction, such identification is not essential for making a diagnosis of FND. Malingering does not need to be refuted as part of establishing the diagnosis.12
Continue to: In contrast...
In contrast, the World Health Organization’s ICD-10 Classification of Mental and Behavioural Disorders groups diagnostic criteria for FND among the dissociative disorders13:
- Clinical features are specified for the individual dissociative disorder (motor, sensory, convulsions, mixed).
- Evidence is absent of a physical disorder that might explain symptoms.
- Evidence of psychological causation is present in clear temporal association with stressful events and problems or disturbed relationships, even if the patient denies such association.
Note the emphasis on psychological causation and exclusion of purposeful simulation of symptoms, as opposed to a primarily unconscious disconnection from the patient’s body or environment.
ICD-10 guidelines acknowledge the difficulty of finding definitive evidence of a psychological cause and recommend provisional diagnosis of FND if psychological factors are not readily apparent.14 Of note, many patients with FND are affected psychologically by their condition, with an impact on mood, behaviors, and interpersonal interactions, although not necessarily to a clinically diagnostic degree. Therefore, a psychiatric diagnosis alone is not a necessary precursor for the diagnosis of an FND.
CASE
History. Mr. D’s history is positive for light alcohol consumption (“2 or 3 cans of beer on weekends”) and chewing tobacco (he reports stopping 6 months earlier) and negative for substance abuse. The family history is positive for maternal hypertension and paternal suicide when the patient was 10 years old (no other known paternal history).
Physical findings. The review of systems is positive for intermittent palpitations, lower-extremity weakness causing unsteady gait, and generalized headache.
Vital signs are within normal limits, including blood pressure (120/82 mm Hg) and heart rate (110 beats/min). The patient is not in acute distress; he is awake, alert, and oriented × 3. No murmurs are heard; lungs are clear bilaterally to auscultation. There is no tenderness on abdominal palpation, and no hepatomegaly or splenomegaly; bowel sounds are normal. No significant bruising or lacerations are noted.
Neurology exam. Cranial nerves II-XII are intact. Pupils are equal and reactive to light. Reflexes are 2+ bilaterally. Muscle strength and tone are normal; no tremors are noted. Babinski signs are normal. A Romberg test is positive (swaying).
Continue to: Mr. D has an antalgic gait...
Mr. D has an antalgic gait with significant swaying (without falling); bent posture; and unsteadiness that requires a cane. However, he is able to get up and off the exam table without assistance, and to propel himself, by rolling a chair forward and backward, without difficulty.
Conducting a diagnostic examination
Taking the history. Certain clues can aid in the diagnosis of FND (TABLE 1).15 For example, the patient might have been seen in multiple specialty practices for a multitude of vague symptoms indicative of potentially related conditions (eg, chronic fatigue, allergies and sensitivities, fibromyalgia, and other chronic pain). The history might include repeated surgeries to investigate those symptoms (eg, laparoscopy, or hysterectomy at an early age). Taking time and care to explore all clinical clues, patient reports, and collateral data are therefore key to making an accurate diagnosis.
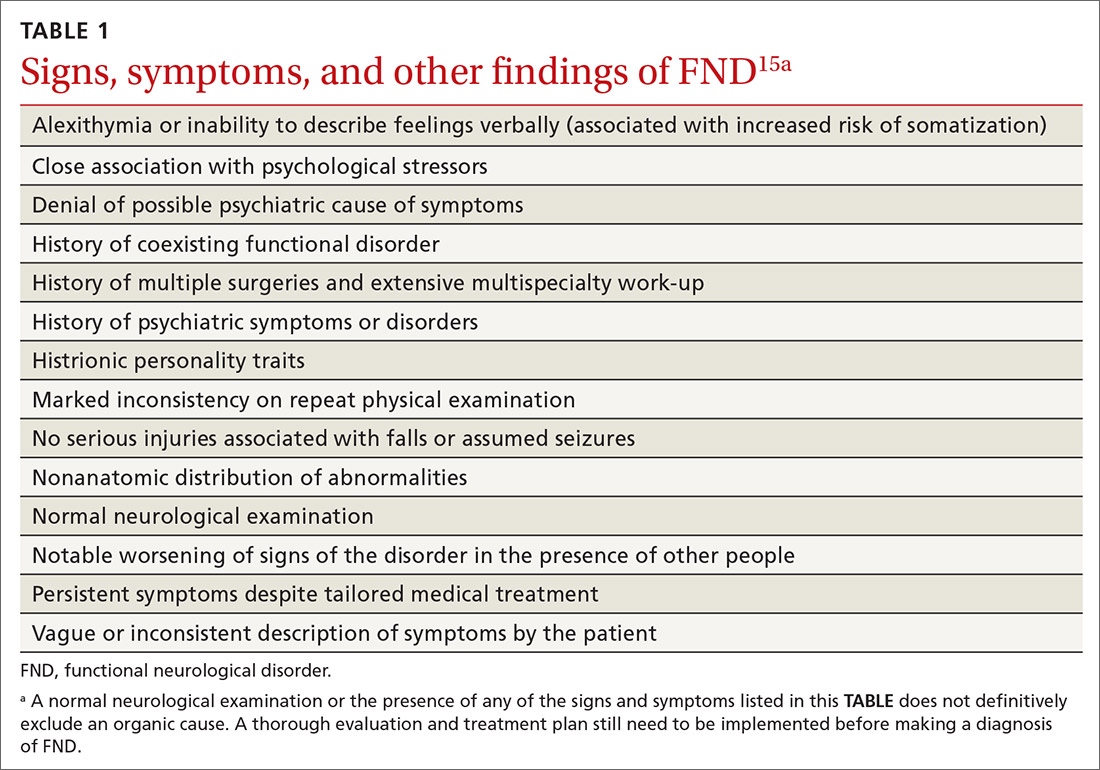
Note any discrepancies between the severity of reported symptoms and functional ability. A technique that can help elucidate a complex or ambiguous medical presentation is to ask the patient to list all their symptoms at the beginning of the interview. This has threefold benefit: You get a broad picture of the problem; the patient is unburdened of their concerns and experiences your validation; and a long list of symptoms can be an early clue to a diagnosis of FND.
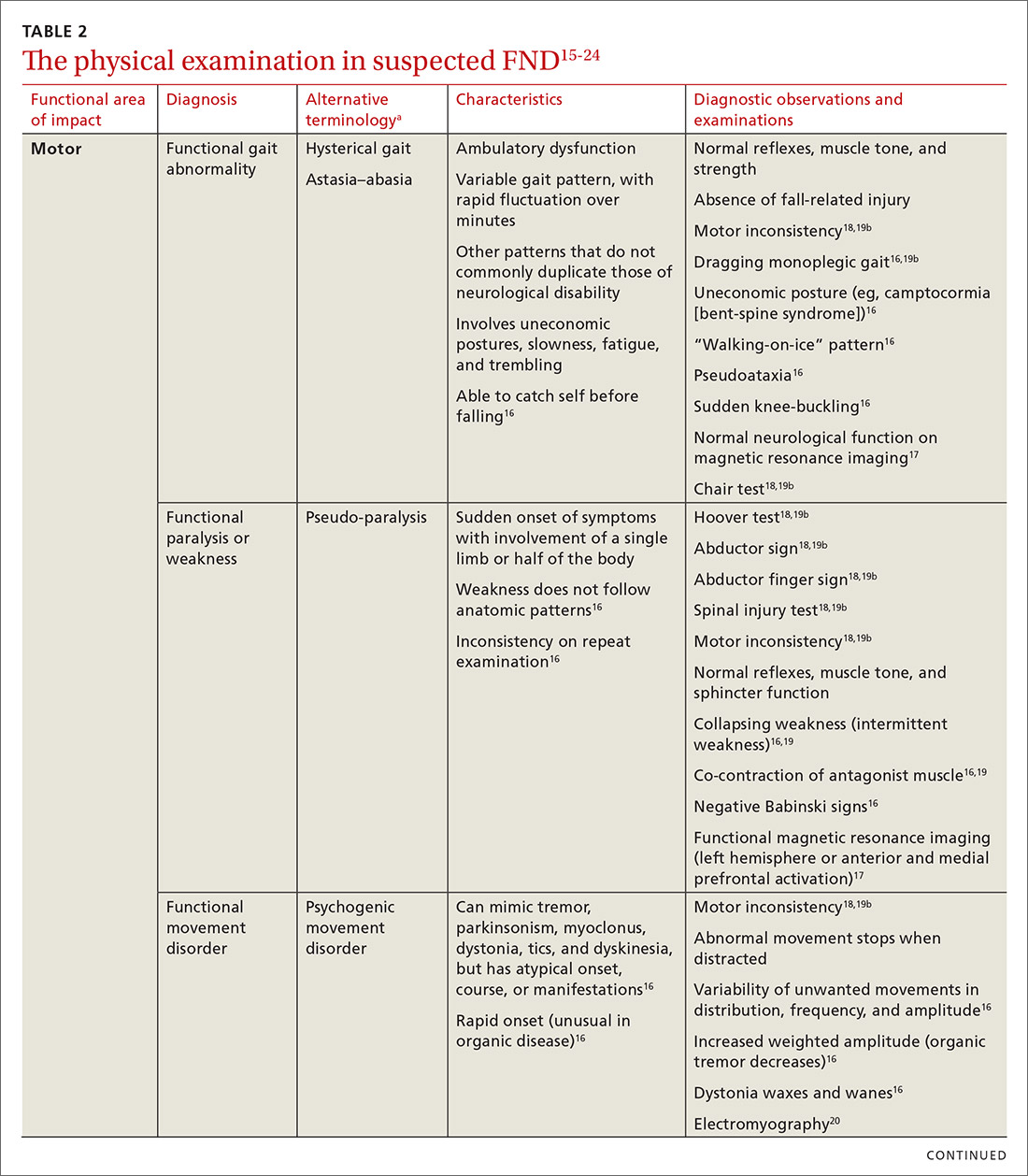
Other helpful questions to determine the impact of symptoms on the patient’s well-being include inquiries about16:
- functional impairment
- onset and course of symptoms
- potential causal or correlating events
- dissociative episodes
- previous diagnoses and treatments
- the patient’s perceptions of, and emotional response to, their illness
- a history of abuse.
The physical examination to determine the presence of FND varies, depending on the functional area of impact (eg, motor, neurological, sensory, speech and swallowing). Pay particular attention to presenting signs and clues, and balance them with the patient’s report (or lack of report). Endeavor to demonstrate positive functional signs, such as a positive Hoover test, which relies on the principle of synergistic muscle contraction. You might see evidence of inconsistency, such as weakness or a change in gait, under observation, that seemingly resolves when the patient is getting on and off the exam table.16Table 215-24 describes areas affected by FND, characteristics of the disorder, and related diagnostic examinations.
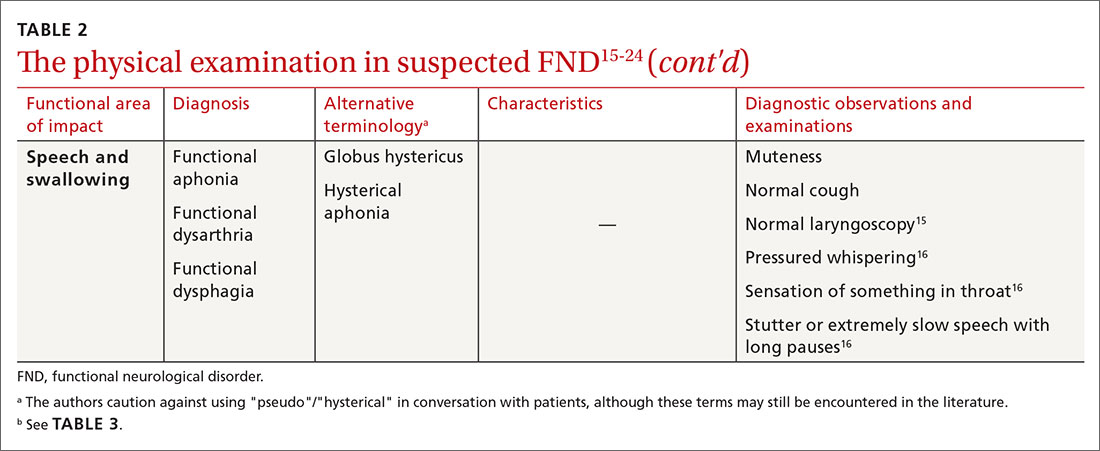
Table 315,18,19 reviews validated special exams that can aid in making the diagnosis. Additional special tests are discussed in the literature.15-24 These tests can be helpful in narrowing the differential diagnosis but have not been validated and should be used with caution.

Some clinical signs associated with FND might be affected by other factors, including socioeconomic status, limited access to health care, low health literacy, poor communication skills, and physician bias. Keep these factors in mind during the visit, to avoid contributing further to health disparities among groups of patients affected by these problems.
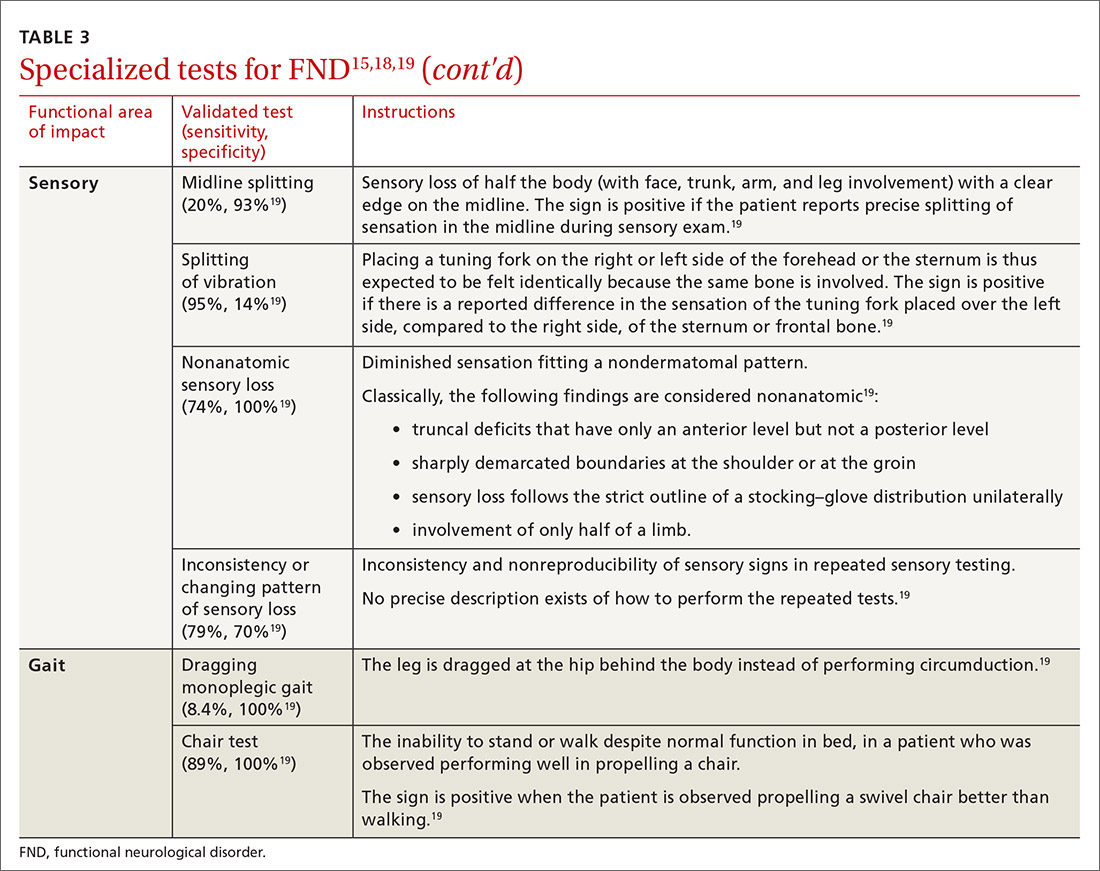
Continue to: CASE
CASE
The work-up over the next month for Mr. D includes numerous studies, all yielding results that are negative or within normal limits: visual acuity; electrocardiography and an event monitor; laboratory testing (including a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, folate, and vitamin D); magnetic resonance imaging of the brain and lumbar spine; lumbar puncture; and electromyography.
The score on the 9-item Patient Health Questionnaire for depression is 4 (severity: “none or minimal”); on the 7-item Generalized Anxiety Disorder scale, 0 (“no anxiety disorder”).
Referral. A neurology work-up of headache, lower extremity weakness, and unsteady gait to address several diagnostic possibilities, including migraine and multiple sclerosis, is within normal limits. A cardiology work-up of palpitations is negative for arrhythmias and other concerning findings.
Mr. D declines psychiatric and psychological evaluations.
Building a differential diagnosisis a formidable task
The differential diagnosis of FND is vast. It includes neurological, physiological, and psychiatric symptoms and disorders; somatization; and malingering (Table 4).6 Any disorder or condition in these areas that is in the differential diagnosis can be precipitated or exacerbated by stress; most, however, do not involve loss of physical function.12 In addition, the diagnosis of an FND does not necessarily exclude an organic disorder.
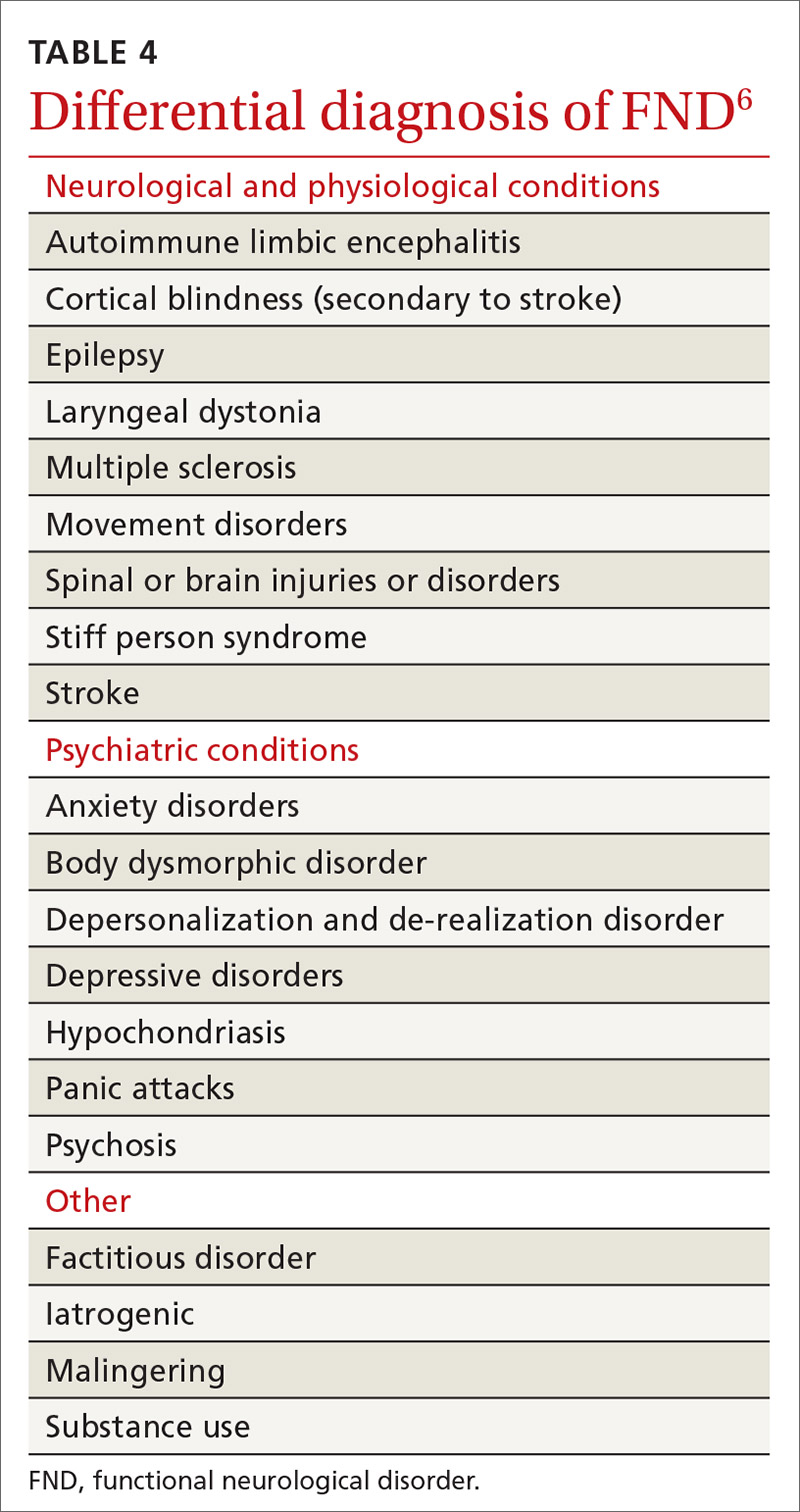
A patient’s presentation becomes complicated—and more difficult to treat—when functional symptoms and an unrelated underlying or early-stage neurological condition coexist. For example, a patient with epilepsy might also have dissociative seizures atop their organic disorder. Neurological disease is considered a risk factor for an overlying FND—just as the risk of depression or anxiety runs concurrently with other chronic diseases.14
Focus on clinical signs to narrow the differential. A thorough social and medical history and physical examination, as discussed earlier, help narrow the differential diagnosis of organic and medically unexplained disorders. Well-defined imaging or laboratory protocols do not exist to guide physicians to a definitive diagnosis, however.
Continue to: Psychiatric conditions
Psychiatric conditions can coexist with the diagnosis of FND, but might be unrelated. A systematic review of the literature showed that 17% to 42% of patients with FND had a concurrent anxiety disorder. Depression disorders were co-diagnosed in 19% to 71% of patients with FND; dissociative and personality disorders were noted, as well.25 However, coexisting psychiatric diagnosis might more likely be associated with distress from the presenting functional neurological symptoms, not linked to the FND diagnosis itself.12 This shift in understanding is reflected in the description of FND in the DSM-5.11
CASE
Mr. D reports debilitating headaches at return office visits. Trials of abortive triptans provide no relief; neither do control medications (beta-blockers, coenzyme Q10, magnesium, onabotulinumtoxinA [Botox], topiramate, and valproate). Lower-extremity weakness and unsteadiness are managed with supportive devices, including a cane, and physical therapy.
Importance of establishing a multidisciplinary approach
The complexity of FND lends itself to a multidisciplinary approach during evaluation and, eventually, for treatment. The assessment and diagnostic intervention that you provide, along with the contributions of consulted specialists (including neurology, physical and occupational therapy, psychiatry, psychology, and other mental health professionals) establishes a team-based approach that can increase the patient’s sense of support and reduce excessive testing and unnecessary medications, surgeries, and other treatments.26
Family physicians are in the ideal position to recognize the patient’s functional capacity and the quality of symptoms and to provide timely referral (eg, to Neurology and Psychiatry) for confirmation of the diagnosis and then treatment.
Evidence-based treatment options include:
- psychotherapy, with an emphasis on cognitive behavioral therapy
- physical therapy
- psychopharmacology
- promising combinations of physical and psychological treatment to improve long-term functionality.27
A promising diagnostic tool
The most significant update in the FND literature is on functional neuroimaging for assessing the disorder. Early findings suggest an intricate relationship between mind and body regarding the pathological distortion in FND. And, there is clear evidence that neuroimaging—specifically, functional magnetic resonance imaging—shows changes in brain activity that correspond to the patient’s symptom report. That said, imaging is not the recommended standard of care in the initial work-up of FND because of its cost and the fact that the diagnosis is principally a clinical undertaking.17,28
Call to action
Offer a generous ear. Begin the diagnostic pursuit by listening carefully and fully to the patient’s complaints, without arriving at a diagnosis with unwarranted bias or haste. This endeavor might require support from other clinical staff (eg, nurses, social workers, case managers) because the diagnostic process can be arduous and lengthy.
Continue to: Convey the diagnosis with sensitivity
Convey the diagnosis with sensitivity. Inquire about the patient’s perceptions and impairments to best personalize your diagnostic explanations. Delivery of the diagnosis might affect the patient’s acceptance and compliance with further testing and treatment of what is generally a persistent and treatment-resistant disorder; poor delivery of diagnostic information can impair the patient–physician relationship and increase the risk of disjointed care. Many patients find that improved patient–physician communication is therapeutic.29
Let the patient know that you’re taking her seriously. Validate patient concerns with a nonstigmatizing diagnostic label; discuss the diagnostic parameters and cause of symptoms in layman’s terms; and emphasize the potential for reversibility.30 Some patients are not satisfied with having a diagnosis of FND until they are reassured with normal results of testing and provided with referral; even then, some seek further reassurance.
Key tenets of managing care for patients who have been given a diagnosis of FND include:
- nonjudgmental, positive regard
- meaningful expression of empathy
- multidisciplinary coordination
- avoidance of unnecessary testing and harmful treatments
- descriptive and contextual explanations of the diagnosis.
Last, keep in mind that the course of treatment for FND is potentially prolonged and multilayered.
CASE
After many visits with his family physician and the neurology and cardiology specialists, as well as an extensive work-up, the physician approaches Mr. D with the possibility of a diagnosis of FND and proposes a multidisciplinary plan that includes:
- a course of physical and occupational therapy
- development of individualized cognitive behavioral tools
- weekly personal and marital counseling
- initiation of a selective serotonin reuptake inhibitor for anxiety
- monthly visits with his family physician.
Months after his return from deployment for evaluation and treatment, Mr. D is able to return to military duty. He reports that his quality of life has improved.
CORRESPONDENCE
Roselyn W. Clemente Fuentes, MD, FAAFP, Eglin Family Medicine Residency, 307 Boatner Road, Eglin AFB, FL 32547; [email protected].
1. Konnopka A, Schaefert R, Heinrich S, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265-275.
2. Carson AJ, Brown R, David AS, et al; on behalf of UK-FNS. Functional (conversion) neurological symptoms: research since the millennium. J Neurol Neurosurg Psychiatry. 2012;83:842-850.
3. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
4. Evens A, Vendetta L, Krebs K, et al. Medically unexplained neurologic symptoms: a primer for physicians who make the initial encounter. Am J Med. 2015;128:1059-1064.
5. Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol. 2013;13:104-113.
6. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537-1551.
7. Carson A, Stone J, Hibberd C, et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J Neurol Neurosurg Psychiatry. 2011;82:810-813.
8. Fink P, Hansen MS, Oxhøj M-L. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res. 2004;56:413-418.
9. Thomas LE. Are your patient’s medically unexplained symptoms really “all in her head”? Med Hypotheses. 2012;78:542-547.
10. Ding JM, Kanaan RAA. What should we say to patients with unexplained neurological symptoms? How explanation affects offence. J Psychosom Res. 2016;91:55-60.
11. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association; 2013.
12. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM-5: Conversion disorder. Am J Psychiatry. 2010;167:626-627.
13. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1994. Accessed January 21, 2021. www.who.int/classifications/icd/en/bluebook.pdf
14. Stone J, Carson A, Duncan R, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease.” J Neurol. 2012;259:33-38.
15. Shaibani A, Sabbagh M. Pseudoneurologic syndromes: recognition and diagnosis. Am Fam Physician. 1998;57:2485-2494.
16. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
17. Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323-337.
18. McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics. 2018;59:358-368.
19. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190.
20. Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001;16:595-599.
21. Ludwig L, McWhirter L, Williams S, et al. Functional coma. In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology: Volume 139: Functional Neurologic Disorders. 1st ed. Academic Press; 2016:313.
22. Miller NR, Subramanian PS, Patel VR. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 3rd ed. Wolters Kluwer; 2016:512-513.
23. Takazaki K, Stransky AD, Miller G. Psychogenic nonepileptic seizures: diagnosis, management, and bioethics. Pediatr Neurol. 2016;62:3-8.
24. Sahaya K, Dholakia SA, Sahota PK. Psychogenic non-epileptic seizures: a challenging entity. J Clin Neurosci. 2011;18:1602-1607.
25. Gelauff J, Stone J, Edwards M, et al. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220-226.
26. Kranick SM, Gorrindo T, Hallett M. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics. 2011;52:109-116.
27. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250-260.
28. Burgmer M, Kugel H, Pfleiderer B, et al. The mirror neuron system under hypnosis—brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex. 2013;49:437-445.
29. van Bokhoven MA, Koch H, van der Weijden T, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints, Ann Fam Med. 2009;7:112-120.
30. Stone J, Carson A, Hallet M. Explanation as treatment for functional neurologic disorders. Handb Clin Neurol. 2016;139:543-553.
CASE
John D,* a 25-year-old patient with an otherwise unremarkable medical history, describes 2 months of daily headache, lower-extremity weakness, and unsteady gait that began fairly suddenly during his first deployment in the US Army. He explains that these symptoms affected his ability to perform his duties and necessitated an early return stateside for evaluation and treatment.
Mr. D denies precipitating trauma or unusual environmental exposures. He reports that, stateside now, symptoms continue to affect his ability to work and attend to personal and family responsibilities.
Asked about stressors, Mr. D notes the birth of his first child approximately 3 months ago, while he was deployed, and marital stressors. He denies suicidal or homicidal ideation.
* The patient’s name has been changed to protect his identity.
The challenge of identifying and managing FND
A functional neurological disorder (FND) is a constellation of psychological, physiological, and neurological symptoms, without an identifiable organic etiology, a conscious decision, or secondary gain for the patient,1 that adversely impacts functioning in 1 or more significant life domains.
Given the high throughput of patients in primary care practices, family physicians can expect to encounter suspected cases of FND in their practices. Regrettably, however, a lack of familiarity with the disorder and its related problems (eg, nonorganic paralysis, sensory loss, nonepileptic seizures, and abnormal movements) can add as much as $20,000 in excess direct and indirect costs of care for every such patient.1 In this article, we synthesize the recent literature on FND so that family physicians can expand their acumen in understanding, identifying, and evaluating patients whose presentation suggests FND.
An underrecognized entity
A precise estimate of the prevalence of FND is difficult to determine because the disorder is underrecognized and misdiagnosed and because it is often accompanied by the confounding of psychological and physiological comorbidities. A 2012 study estimated the annual incidence of FND to be 4 to 12 cases for every 100,000 people2; in primary care and outpatient neurology settings, prevalence is 6% to 22% of all patients.3,4 Stone and colleagues identified functional neurological symptoms as the second most common reason for outpatient neurology consultation,5 with 1 nonepileptic seizure patient seen for every 6 epileptic patients, and functional weakness presenting at the same rate as multiple sclerosis.6
Continue to: Demographics of patients with FND...
Demographics of patients with FND vary, depending on presenting neurological symptoms and disorder subtype. Existing data indicate a correlation between FND and younger age, female sex, physical disability,7 and a history of abuse or trauma.3,8 A challenge in concretely ascertaining the prevalence of FND is that conditions such as fibromyalgia, chronic pelvic pain, globus hystericus, and nonepileptic seizures can also be characterized as medically unexplained functional disorders, even within the network of neurology care.4
Misdiagnosis and bias are not uncommon
Ambiguity in classifying and evaluating FND can affect physicians’ perceptions, assessment, and care of patients with suggestive presenting symptoms. A major early challenge in diagnosing FND is the inconsistency of characterizing terminology (pseudoneurological, somatic, dissociative, conversion, psychogenic, hysterical, factitious, functional, medically unexplained9,10) and definitions in the literature. Neurological symptoms of unidentifiable organic cause can greatly diminish quality of life4; FND is a scientifically and clinically useful diagnosis for many combinations of nonrandomly co-occurring symptoms and clinical signs.
The pitfall of misdiagnosis. Remain cautious about making a diagnosis of FND by exclusion, which might yield an incorrect or false-negative finding because of an atypical presentation. It is important to avoid misdiagnosis by prematurely closing the differential diagnosis; instead, keep in mind that a medically unexplained diagnosis might be better explained by conducting a robust social and medical history and obtaining additional or collateral data, or both, along with appropriate consultation.4,9
Misdiagnosis can lead to a circuitous and costly work-up, with the potential to increase the patient’s distress. You can reduce this burden with early recognition of FND and centralized management of multidisciplinary care, which are more likely to lead to an accurate and timely diagnosis—paramount to empowering patients with access to the correct information and meaningful support needed to enhance treatment and self-care.9
Bias, haste, and dismissal are unproductive. Even with a clear definition of FND, it is not uncommon for a physician to rapidly assess a patient’s clinical signs, make a diagnosis of “unknown etiology,” or openly question the veracity of complaints. Furthermore, be aware of inadvertently characterizing FND using the prefix “pseudo” or the term “hysterical,” which can be psychologically discomforting for many patients, who legitimately experience inexplicable symptoms. Such pejoratives can lead to stigmatizing and misleading assessments and treatment paths4—courses of action that can cause early and, possibly, irreparable harm to the patient–physician relationship and increase the patient’s inclination to go “doctor-shopping,” with associated loss of continuity of care.
Why is it difficult to diagnose FND?
The latest (5th) edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) describes conversion, somatoform disorder, and FND synonymously.DSM-5 diagnostic criteria for conversion disorderare11:
- a specified type of symptom or deficit of altered voluntary motor or sensory function (eg, weakness, difficulty swallowing, slurred speech, seizures)
- clinical evidence of the incompatibility of the symptom or deficit and any recognized neurological or medical disorder
- incapability of better explaining the symptom or deficit as another medical or mental disorder.
- The symptom or deficit causes distress or impairment that (1) is clinically significant in occupational, social, or other important areas of function or (2) warrants medical evaluation.
The overarching feature of these criteria is the inconsistency of symptoms with recognized neurological, physiological, or psychiatric conditions. Although identification of psychological factors can help clarify and provide a treatment direction, such identification is not essential for making a diagnosis of FND. Malingering does not need to be refuted as part of establishing the diagnosis.12
Continue to: In contrast...
In contrast, the World Health Organization’s ICD-10 Classification of Mental and Behavioural Disorders groups diagnostic criteria for FND among the dissociative disorders13:
- Clinical features are specified for the individual dissociative disorder (motor, sensory, convulsions, mixed).
- Evidence is absent of a physical disorder that might explain symptoms.
- Evidence of psychological causation is present in clear temporal association with stressful events and problems or disturbed relationships, even if the patient denies such association.
Note the emphasis on psychological causation and exclusion of purposeful simulation of symptoms, as opposed to a primarily unconscious disconnection from the patient’s body or environment.
ICD-10 guidelines acknowledge the difficulty of finding definitive evidence of a psychological cause and recommend provisional diagnosis of FND if psychological factors are not readily apparent.14 Of note, many patients with FND are affected psychologically by their condition, with an impact on mood, behaviors, and interpersonal interactions, although not necessarily to a clinically diagnostic degree. Therefore, a psychiatric diagnosis alone is not a necessary precursor for the diagnosis of an FND.
CASE
History. Mr. D’s history is positive for light alcohol consumption (“2 or 3 cans of beer on weekends”) and chewing tobacco (he reports stopping 6 months earlier) and negative for substance abuse. The family history is positive for maternal hypertension and paternal suicide when the patient was 10 years old (no other known paternal history).
Physical findings. The review of systems is positive for intermittent palpitations, lower-extremity weakness causing unsteady gait, and generalized headache.
Vital signs are within normal limits, including blood pressure (120/82 mm Hg) and heart rate (110 beats/min). The patient is not in acute distress; he is awake, alert, and oriented × 3. No murmurs are heard; lungs are clear bilaterally to auscultation. There is no tenderness on abdominal palpation, and no hepatomegaly or splenomegaly; bowel sounds are normal. No significant bruising or lacerations are noted.
Neurology exam. Cranial nerves II-XII are intact. Pupils are equal and reactive to light. Reflexes are 2+ bilaterally. Muscle strength and tone are normal; no tremors are noted. Babinski signs are normal. A Romberg test is positive (swaying).
Continue to: Mr. D has an antalgic gait...
Mr. D has an antalgic gait with significant swaying (without falling); bent posture; and unsteadiness that requires a cane. However, he is able to get up and off the exam table without assistance, and to propel himself, by rolling a chair forward and backward, without difficulty.
Conducting a diagnostic examination
Taking the history. Certain clues can aid in the diagnosis of FND (TABLE 1).15 For example, the patient might have been seen in multiple specialty practices for a multitude of vague symptoms indicative of potentially related conditions (eg, chronic fatigue, allergies and sensitivities, fibromyalgia, and other chronic pain). The history might include repeated surgeries to investigate those symptoms (eg, laparoscopy, or hysterectomy at an early age). Taking time and care to explore all clinical clues, patient reports, and collateral data are therefore key to making an accurate diagnosis.

Note any discrepancies between the severity of reported symptoms and functional ability. A technique that can help elucidate a complex or ambiguous medical presentation is to ask the patient to list all their symptoms at the beginning of the interview. This has threefold benefit: You get a broad picture of the problem; the patient is unburdened of their concerns and experiences your validation; and a long list of symptoms can be an early clue to a diagnosis of FND.

Other helpful questions to determine the impact of symptoms on the patient’s well-being include inquiries about16:
- functional impairment
- onset and course of symptoms
- potential causal or correlating events
- dissociative episodes
- previous diagnoses and treatments
- the patient’s perceptions of, and emotional response to, their illness
- a history of abuse.

The physical examination to determine the presence of FND varies, depending on the functional area of impact (eg, motor, neurological, sensory, speech and swallowing). Pay particular attention to presenting signs and clues, and balance them with the patient’s report (or lack of report). Endeavor to demonstrate positive functional signs, such as a positive Hoover test, which relies on the principle of synergistic muscle contraction. You might see evidence of inconsistency, such as weakness or a change in gait, under observation, that seemingly resolves when the patient is getting on and off the exam table.16Table 215-24 describes areas affected by FND, characteristics of the disorder, and related diagnostic examinations.

Table 315,18,19 reviews validated special exams that can aid in making the diagnosis. Additional special tests are discussed in the literature.15-24 These tests can be helpful in narrowing the differential diagnosis but have not been validated and should be used with caution.

Some clinical signs associated with FND might be affected by other factors, including socioeconomic status, limited access to health care, low health literacy, poor communication skills, and physician bias. Keep these factors in mind during the visit, to avoid contributing further to health disparities among groups of patients affected by these problems.

Continue to: CASE
CASE
The work-up over the next month for Mr. D includes numerous studies, all yielding results that are negative or within normal limits: visual acuity; electrocardiography and an event monitor; laboratory testing (including a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, folate, and vitamin D); magnetic resonance imaging of the brain and lumbar spine; lumbar puncture; and electromyography.
The score on the 9-item Patient Health Questionnaire for depression is 4 (severity: “none or minimal”); on the 7-item Generalized Anxiety Disorder scale, 0 (“no anxiety disorder”).
Referral. A neurology work-up of headache, lower extremity weakness, and unsteady gait to address several diagnostic possibilities, including migraine and multiple sclerosis, is within normal limits. A cardiology work-up of palpitations is negative for arrhythmias and other concerning findings.
Mr. D declines psychiatric and psychological evaluations.
Building a differential diagnosisis a formidable task
The differential diagnosis of FND is vast. It includes neurological, physiological, and psychiatric symptoms and disorders; somatization; and malingering (Table 4).6 Any disorder or condition in these areas that is in the differential diagnosis can be precipitated or exacerbated by stress; most, however, do not involve loss of physical function.12 In addition, the diagnosis of an FND does not necessarily exclude an organic disorder.

A patient’s presentation becomes complicated—and more difficult to treat—when functional symptoms and an unrelated underlying or early-stage neurological condition coexist. For example, a patient with epilepsy might also have dissociative seizures atop their organic disorder. Neurological disease is considered a risk factor for an overlying FND—just as the risk of depression or anxiety runs concurrently with other chronic diseases.14
Focus on clinical signs to narrow the differential. A thorough social and medical history and physical examination, as discussed earlier, help narrow the differential diagnosis of organic and medically unexplained disorders. Well-defined imaging or laboratory protocols do not exist to guide physicians to a definitive diagnosis, however.
Continue to: Psychiatric conditions
Psychiatric conditions can coexist with the diagnosis of FND, but might be unrelated. A systematic review of the literature showed that 17% to 42% of patients with FND had a concurrent anxiety disorder. Depression disorders were co-diagnosed in 19% to 71% of patients with FND; dissociative and personality disorders were noted, as well.25 However, coexisting psychiatric diagnosis might more likely be associated with distress from the presenting functional neurological symptoms, not linked to the FND diagnosis itself.12 This shift in understanding is reflected in the description of FND in the DSM-5.11
CASE
Mr. D reports debilitating headaches at return office visits. Trials of abortive triptans provide no relief; neither do control medications (beta-blockers, coenzyme Q10, magnesium, onabotulinumtoxinA [Botox], topiramate, and valproate). Lower-extremity weakness and unsteadiness are managed with supportive devices, including a cane, and physical therapy.
Importance of establishing a multidisciplinary approach
The complexity of FND lends itself to a multidisciplinary approach during evaluation and, eventually, for treatment. The assessment and diagnostic intervention that you provide, along with the contributions of consulted specialists (including neurology, physical and occupational therapy, psychiatry, psychology, and other mental health professionals) establishes a team-based approach that can increase the patient’s sense of support and reduce excessive testing and unnecessary medications, surgeries, and other treatments.26
Family physicians are in the ideal position to recognize the patient’s functional capacity and the quality of symptoms and to provide timely referral (eg, to Neurology and Psychiatry) for confirmation of the diagnosis and then treatment.
Evidence-based treatment options include:
- psychotherapy, with an emphasis on cognitive behavioral therapy
- physical therapy
- psychopharmacology
- promising combinations of physical and psychological treatment to improve long-term functionality.27
A promising diagnostic tool
The most significant update in the FND literature is on functional neuroimaging for assessing the disorder. Early findings suggest an intricate relationship between mind and body regarding the pathological distortion in FND. And, there is clear evidence that neuroimaging—specifically, functional magnetic resonance imaging—shows changes in brain activity that correspond to the patient’s symptom report. That said, imaging is not the recommended standard of care in the initial work-up of FND because of its cost and the fact that the diagnosis is principally a clinical undertaking.17,28
Call to action
Offer a generous ear. Begin the diagnostic pursuit by listening carefully and fully to the patient’s complaints, without arriving at a diagnosis with unwarranted bias or haste. This endeavor might require support from other clinical staff (eg, nurses, social workers, case managers) because the diagnostic process can be arduous and lengthy.
Continue to: Convey the diagnosis with sensitivity
Convey the diagnosis with sensitivity. Inquire about the patient’s perceptions and impairments to best personalize your diagnostic explanations. Delivery of the diagnosis might affect the patient’s acceptance and compliance with further testing and treatment of what is generally a persistent and treatment-resistant disorder; poor delivery of diagnostic information can impair the patient–physician relationship and increase the risk of disjointed care. Many patients find that improved patient–physician communication is therapeutic.29
Let the patient know that you’re taking her seriously. Validate patient concerns with a nonstigmatizing diagnostic label; discuss the diagnostic parameters and cause of symptoms in layman’s terms; and emphasize the potential for reversibility.30 Some patients are not satisfied with having a diagnosis of FND until they are reassured with normal results of testing and provided with referral; even then, some seek further reassurance.
Key tenets of managing care for patients who have been given a diagnosis of FND include:
- nonjudgmental, positive regard
- meaningful expression of empathy
- multidisciplinary coordination
- avoidance of unnecessary testing and harmful treatments
- descriptive and contextual explanations of the diagnosis.
Last, keep in mind that the course of treatment for FND is potentially prolonged and multilayered.
CASE
After many visits with his family physician and the neurology and cardiology specialists, as well as an extensive work-up, the physician approaches Mr. D with the possibility of a diagnosis of FND and proposes a multidisciplinary plan that includes:
- a course of physical and occupational therapy
- development of individualized cognitive behavioral tools
- weekly personal and marital counseling
- initiation of a selective serotonin reuptake inhibitor for anxiety
- monthly visits with his family physician.
Months after his return from deployment for evaluation and treatment, Mr. D is able to return to military duty. He reports that his quality of life has improved.
CORRESPONDENCE
Roselyn W. Clemente Fuentes, MD, FAAFP, Eglin Family Medicine Residency, 307 Boatner Road, Eglin AFB, FL 32547; [email protected].
CASE
John D,* a 25-year-old patient with an otherwise unremarkable medical history, describes 2 months of daily headache, lower-extremity weakness, and unsteady gait that began fairly suddenly during his first deployment in the US Army. He explains that these symptoms affected his ability to perform his duties and necessitated an early return stateside for evaluation and treatment.
Mr. D denies precipitating trauma or unusual environmental exposures. He reports that, stateside now, symptoms continue to affect his ability to work and attend to personal and family responsibilities.
Asked about stressors, Mr. D notes the birth of his first child approximately 3 months ago, while he was deployed, and marital stressors. He denies suicidal or homicidal ideation.
* The patient’s name has been changed to protect his identity.
The challenge of identifying and managing FND
A functional neurological disorder (FND) is a constellation of psychological, physiological, and neurological symptoms, without an identifiable organic etiology, a conscious decision, or secondary gain for the patient,1 that adversely impacts functioning in 1 or more significant life domains.
Given the high throughput of patients in primary care practices, family physicians can expect to encounter suspected cases of FND in their practices. Regrettably, however, a lack of familiarity with the disorder and its related problems (eg, nonorganic paralysis, sensory loss, nonepileptic seizures, and abnormal movements) can add as much as $20,000 in excess direct and indirect costs of care for every such patient.1 In this article, we synthesize the recent literature on FND so that family physicians can expand their acumen in understanding, identifying, and evaluating patients whose presentation suggests FND.
An underrecognized entity
A precise estimate of the prevalence of FND is difficult to determine because the disorder is underrecognized and misdiagnosed and because it is often accompanied by the confounding of psychological and physiological comorbidities. A 2012 study estimated the annual incidence of FND to be 4 to 12 cases for every 100,000 people2; in primary care and outpatient neurology settings, prevalence is 6% to 22% of all patients.3,4 Stone and colleagues identified functional neurological symptoms as the second most common reason for outpatient neurology consultation,5 with 1 nonepileptic seizure patient seen for every 6 epileptic patients, and functional weakness presenting at the same rate as multiple sclerosis.6
Continue to: Demographics of patients with FND...
Demographics of patients with FND vary, depending on presenting neurological symptoms and disorder subtype. Existing data indicate a correlation between FND and younger age, female sex, physical disability,7 and a history of abuse or trauma.3,8 A challenge in concretely ascertaining the prevalence of FND is that conditions such as fibromyalgia, chronic pelvic pain, globus hystericus, and nonepileptic seizures can also be characterized as medically unexplained functional disorders, even within the network of neurology care.4
Misdiagnosis and bias are not uncommon
Ambiguity in classifying and evaluating FND can affect physicians’ perceptions, assessment, and care of patients with suggestive presenting symptoms. A major early challenge in diagnosing FND is the inconsistency of characterizing terminology (pseudoneurological, somatic, dissociative, conversion, psychogenic, hysterical, factitious, functional, medically unexplained9,10) and definitions in the literature. Neurological symptoms of unidentifiable organic cause can greatly diminish quality of life4; FND is a scientifically and clinically useful diagnosis for many combinations of nonrandomly co-occurring symptoms and clinical signs.
The pitfall of misdiagnosis. Remain cautious about making a diagnosis of FND by exclusion, which might yield an incorrect or false-negative finding because of an atypical presentation. It is important to avoid misdiagnosis by prematurely closing the differential diagnosis; instead, keep in mind that a medically unexplained diagnosis might be better explained by conducting a robust social and medical history and obtaining additional or collateral data, or both, along with appropriate consultation.4,9
Misdiagnosis can lead to a circuitous and costly work-up, with the potential to increase the patient’s distress. You can reduce this burden with early recognition of FND and centralized management of multidisciplinary care, which are more likely to lead to an accurate and timely diagnosis—paramount to empowering patients with access to the correct information and meaningful support needed to enhance treatment and self-care.9
Bias, haste, and dismissal are unproductive. Even with a clear definition of FND, it is not uncommon for a physician to rapidly assess a patient’s clinical signs, make a diagnosis of “unknown etiology,” or openly question the veracity of complaints. Furthermore, be aware of inadvertently characterizing FND using the prefix “pseudo” or the term “hysterical,” which can be psychologically discomforting for many patients, who legitimately experience inexplicable symptoms. Such pejoratives can lead to stigmatizing and misleading assessments and treatment paths4—courses of action that can cause early and, possibly, irreparable harm to the patient–physician relationship and increase the patient’s inclination to go “doctor-shopping,” with associated loss of continuity of care.
Why is it difficult to diagnose FND?
The latest (5th) edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) describes conversion, somatoform disorder, and FND synonymously.DSM-5 diagnostic criteria for conversion disorderare11:
- a specified type of symptom or deficit of altered voluntary motor or sensory function (eg, weakness, difficulty swallowing, slurred speech, seizures)
- clinical evidence of the incompatibility of the symptom or deficit and any recognized neurological or medical disorder
- incapability of better explaining the symptom or deficit as another medical or mental disorder.
- The symptom or deficit causes distress or impairment that (1) is clinically significant in occupational, social, or other important areas of function or (2) warrants medical evaluation.
The overarching feature of these criteria is the inconsistency of symptoms with recognized neurological, physiological, or psychiatric conditions. Although identification of psychological factors can help clarify and provide a treatment direction, such identification is not essential for making a diagnosis of FND. Malingering does not need to be refuted as part of establishing the diagnosis.12
Continue to: In contrast...
In contrast, the World Health Organization’s ICD-10 Classification of Mental and Behavioural Disorders groups diagnostic criteria for FND among the dissociative disorders13:
- Clinical features are specified for the individual dissociative disorder (motor, sensory, convulsions, mixed).
- Evidence is absent of a physical disorder that might explain symptoms.
- Evidence of psychological causation is present in clear temporal association with stressful events and problems or disturbed relationships, even if the patient denies such association.
Note the emphasis on psychological causation and exclusion of purposeful simulation of symptoms, as opposed to a primarily unconscious disconnection from the patient’s body or environment.
ICD-10 guidelines acknowledge the difficulty of finding definitive evidence of a psychological cause and recommend provisional diagnosis of FND if psychological factors are not readily apparent.14 Of note, many patients with FND are affected psychologically by their condition, with an impact on mood, behaviors, and interpersonal interactions, although not necessarily to a clinically diagnostic degree. Therefore, a psychiatric diagnosis alone is not a necessary precursor for the diagnosis of an FND.
CASE
History. Mr. D’s history is positive for light alcohol consumption (“2 or 3 cans of beer on weekends”) and chewing tobacco (he reports stopping 6 months earlier) and negative for substance abuse. The family history is positive for maternal hypertension and paternal suicide when the patient was 10 years old (no other known paternal history).
Physical findings. The review of systems is positive for intermittent palpitations, lower-extremity weakness causing unsteady gait, and generalized headache.
Vital signs are within normal limits, including blood pressure (120/82 mm Hg) and heart rate (110 beats/min). The patient is not in acute distress; he is awake, alert, and oriented × 3. No murmurs are heard; lungs are clear bilaterally to auscultation. There is no tenderness on abdominal palpation, and no hepatomegaly or splenomegaly; bowel sounds are normal. No significant bruising or lacerations are noted.
Neurology exam. Cranial nerves II-XII are intact. Pupils are equal and reactive to light. Reflexes are 2+ bilaterally. Muscle strength and tone are normal; no tremors are noted. Babinski signs are normal. A Romberg test is positive (swaying).
Continue to: Mr. D has an antalgic gait...
Mr. D has an antalgic gait with significant swaying (without falling); bent posture; and unsteadiness that requires a cane. However, he is able to get up and off the exam table without assistance, and to propel himself, by rolling a chair forward and backward, without difficulty.
Conducting a diagnostic examination
Taking the history. Certain clues can aid in the diagnosis of FND (TABLE 1).15 For example, the patient might have been seen in multiple specialty practices for a multitude of vague symptoms indicative of potentially related conditions (eg, chronic fatigue, allergies and sensitivities, fibromyalgia, and other chronic pain). The history might include repeated surgeries to investigate those symptoms (eg, laparoscopy, or hysterectomy at an early age). Taking time and care to explore all clinical clues, patient reports, and collateral data are therefore key to making an accurate diagnosis.

Note any discrepancies between the severity of reported symptoms and functional ability. A technique that can help elucidate a complex or ambiguous medical presentation is to ask the patient to list all their symptoms at the beginning of the interview. This has threefold benefit: You get a broad picture of the problem; the patient is unburdened of their concerns and experiences your validation; and a long list of symptoms can be an early clue to a diagnosis of FND.

Other helpful questions to determine the impact of symptoms on the patient’s well-being include inquiries about16:
- functional impairment
- onset and course of symptoms
- potential causal or correlating events
- dissociative episodes
- previous diagnoses and treatments
- the patient’s perceptions of, and emotional response to, their illness
- a history of abuse.

The physical examination to determine the presence of FND varies, depending on the functional area of impact (eg, motor, neurological, sensory, speech and swallowing). Pay particular attention to presenting signs and clues, and balance them with the patient’s report (or lack of report). Endeavor to demonstrate positive functional signs, such as a positive Hoover test, which relies on the principle of synergistic muscle contraction. You might see evidence of inconsistency, such as weakness or a change in gait, under observation, that seemingly resolves when the patient is getting on and off the exam table.16Table 215-24 describes areas affected by FND, characteristics of the disorder, and related diagnostic examinations.

Table 315,18,19 reviews validated special exams that can aid in making the diagnosis. Additional special tests are discussed in the literature.15-24 These tests can be helpful in narrowing the differential diagnosis but have not been validated and should be used with caution.

Some clinical signs associated with FND might be affected by other factors, including socioeconomic status, limited access to health care, low health literacy, poor communication skills, and physician bias. Keep these factors in mind during the visit, to avoid contributing further to health disparities among groups of patients affected by these problems.

Continue to: CASE
CASE
The work-up over the next month for Mr. D includes numerous studies, all yielding results that are negative or within normal limits: visual acuity; electrocardiography and an event monitor; laboratory testing (including a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, folate, and vitamin D); magnetic resonance imaging of the brain and lumbar spine; lumbar puncture; and electromyography.
The score on the 9-item Patient Health Questionnaire for depression is 4 (severity: “none or minimal”); on the 7-item Generalized Anxiety Disorder scale, 0 (“no anxiety disorder”).
Referral. A neurology work-up of headache, lower extremity weakness, and unsteady gait to address several diagnostic possibilities, including migraine and multiple sclerosis, is within normal limits. A cardiology work-up of palpitations is negative for arrhythmias and other concerning findings.
Mr. D declines psychiatric and psychological evaluations.
Building a differential diagnosisis a formidable task
The differential diagnosis of FND is vast. It includes neurological, physiological, and psychiatric symptoms and disorders; somatization; and malingering (Table 4).6 Any disorder or condition in these areas that is in the differential diagnosis can be precipitated or exacerbated by stress; most, however, do not involve loss of physical function.12 In addition, the diagnosis of an FND does not necessarily exclude an organic disorder.

A patient’s presentation becomes complicated—and more difficult to treat—when functional symptoms and an unrelated underlying or early-stage neurological condition coexist. For example, a patient with epilepsy might also have dissociative seizures atop their organic disorder. Neurological disease is considered a risk factor for an overlying FND—just as the risk of depression or anxiety runs concurrently with other chronic diseases.14
Focus on clinical signs to narrow the differential. A thorough social and medical history and physical examination, as discussed earlier, help narrow the differential diagnosis of organic and medically unexplained disorders. Well-defined imaging or laboratory protocols do not exist to guide physicians to a definitive diagnosis, however.
Continue to: Psychiatric conditions
Psychiatric conditions can coexist with the diagnosis of FND, but might be unrelated. A systematic review of the literature showed that 17% to 42% of patients with FND had a concurrent anxiety disorder. Depression disorders were co-diagnosed in 19% to 71% of patients with FND; dissociative and personality disorders were noted, as well.25 However, coexisting psychiatric diagnosis might more likely be associated with distress from the presenting functional neurological symptoms, not linked to the FND diagnosis itself.12 This shift in understanding is reflected in the description of FND in the DSM-5.11
CASE
Mr. D reports debilitating headaches at return office visits. Trials of abortive triptans provide no relief; neither do control medications (beta-blockers, coenzyme Q10, magnesium, onabotulinumtoxinA [Botox], topiramate, and valproate). Lower-extremity weakness and unsteadiness are managed with supportive devices, including a cane, and physical therapy.
Importance of establishing a multidisciplinary approach
The complexity of FND lends itself to a multidisciplinary approach during evaluation and, eventually, for treatment. The assessment and diagnostic intervention that you provide, along with the contributions of consulted specialists (including neurology, physical and occupational therapy, psychiatry, psychology, and other mental health professionals) establishes a team-based approach that can increase the patient’s sense of support and reduce excessive testing and unnecessary medications, surgeries, and other treatments.26
Family physicians are in the ideal position to recognize the patient’s functional capacity and the quality of symptoms and to provide timely referral (eg, to Neurology and Psychiatry) for confirmation of the diagnosis and then treatment.
Evidence-based treatment options include:
- psychotherapy, with an emphasis on cognitive behavioral therapy
- physical therapy
- psychopharmacology
- promising combinations of physical and psychological treatment to improve long-term functionality.27
A promising diagnostic tool
The most significant update in the FND literature is on functional neuroimaging for assessing the disorder. Early findings suggest an intricate relationship between mind and body regarding the pathological distortion in FND. And, there is clear evidence that neuroimaging—specifically, functional magnetic resonance imaging—shows changes in brain activity that correspond to the patient’s symptom report. That said, imaging is not the recommended standard of care in the initial work-up of FND because of its cost and the fact that the diagnosis is principally a clinical undertaking.17,28
Call to action
Offer a generous ear. Begin the diagnostic pursuit by listening carefully and fully to the patient’s complaints, without arriving at a diagnosis with unwarranted bias or haste. This endeavor might require support from other clinical staff (eg, nurses, social workers, case managers) because the diagnostic process can be arduous and lengthy.
Continue to: Convey the diagnosis with sensitivity
Convey the diagnosis with sensitivity. Inquire about the patient’s perceptions and impairments to best personalize your diagnostic explanations. Delivery of the diagnosis might affect the patient’s acceptance and compliance with further testing and treatment of what is generally a persistent and treatment-resistant disorder; poor delivery of diagnostic information can impair the patient–physician relationship and increase the risk of disjointed care. Many patients find that improved patient–physician communication is therapeutic.29
Let the patient know that you’re taking her seriously. Validate patient concerns with a nonstigmatizing diagnostic label; discuss the diagnostic parameters and cause of symptoms in layman’s terms; and emphasize the potential for reversibility.30 Some patients are not satisfied with having a diagnosis of FND until they are reassured with normal results of testing and provided with referral; even then, some seek further reassurance.
Key tenets of managing care for patients who have been given a diagnosis of FND include:
- nonjudgmental, positive regard
- meaningful expression of empathy
- multidisciplinary coordination
- avoidance of unnecessary testing and harmful treatments
- descriptive and contextual explanations of the diagnosis.
Last, keep in mind that the course of treatment for FND is potentially prolonged and multilayered.
CASE
After many visits with his family physician and the neurology and cardiology specialists, as well as an extensive work-up, the physician approaches Mr. D with the possibility of a diagnosis of FND and proposes a multidisciplinary plan that includes:
- a course of physical and occupational therapy
- development of individualized cognitive behavioral tools
- weekly personal and marital counseling
- initiation of a selective serotonin reuptake inhibitor for anxiety
- monthly visits with his family physician.
Months after his return from deployment for evaluation and treatment, Mr. D is able to return to military duty. He reports that his quality of life has improved.
CORRESPONDENCE
Roselyn W. Clemente Fuentes, MD, FAAFP, Eglin Family Medicine Residency, 307 Boatner Road, Eglin AFB, FL 32547; [email protected].
1. Konnopka A, Schaefert R, Heinrich S, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265-275.
2. Carson AJ, Brown R, David AS, et al; on behalf of UK-FNS. Functional (conversion) neurological symptoms: research since the millennium. J Neurol Neurosurg Psychiatry. 2012;83:842-850.
3. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
4. Evens A, Vendetta L, Krebs K, et al. Medically unexplained neurologic symptoms: a primer for physicians who make the initial encounter. Am J Med. 2015;128:1059-1064.
5. Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol. 2013;13:104-113.
6. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537-1551.
7. Carson A, Stone J, Hibberd C, et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J Neurol Neurosurg Psychiatry. 2011;82:810-813.
8. Fink P, Hansen MS, Oxhøj M-L. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res. 2004;56:413-418.
9. Thomas LE. Are your patient’s medically unexplained symptoms really “all in her head”? Med Hypotheses. 2012;78:542-547.
10. Ding JM, Kanaan RAA. What should we say to patients with unexplained neurological symptoms? How explanation affects offence. J Psychosom Res. 2016;91:55-60.
11. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association; 2013.
12. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM-5: Conversion disorder. Am J Psychiatry. 2010;167:626-627.
13. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1994. Accessed January 21, 2021. www.who.int/classifications/icd/en/bluebook.pdf
14. Stone J, Carson A, Duncan R, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease.” J Neurol. 2012;259:33-38.
15. Shaibani A, Sabbagh M. Pseudoneurologic syndromes: recognition and diagnosis. Am Fam Physician. 1998;57:2485-2494.
16. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
17. Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323-337.
18. McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics. 2018;59:358-368.
19. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190.
20. Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001;16:595-599.
21. Ludwig L, McWhirter L, Williams S, et al. Functional coma. In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology: Volume 139: Functional Neurologic Disorders. 1st ed. Academic Press; 2016:313.
22. Miller NR, Subramanian PS, Patel VR. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 3rd ed. Wolters Kluwer; 2016:512-513.
23. Takazaki K, Stransky AD, Miller G. Psychogenic nonepileptic seizures: diagnosis, management, and bioethics. Pediatr Neurol. 2016;62:3-8.
24. Sahaya K, Dholakia SA, Sahota PK. Psychogenic non-epileptic seizures: a challenging entity. J Clin Neurosci. 2011;18:1602-1607.
25. Gelauff J, Stone J, Edwards M, et al. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220-226.
26. Kranick SM, Gorrindo T, Hallett M. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics. 2011;52:109-116.
27. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250-260.
28. Burgmer M, Kugel H, Pfleiderer B, et al. The mirror neuron system under hypnosis—brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex. 2013;49:437-445.
29. van Bokhoven MA, Koch H, van der Weijden T, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints, Ann Fam Med. 2009;7:112-120.
30. Stone J, Carson A, Hallet M. Explanation as treatment for functional neurologic disorders. Handb Clin Neurol. 2016;139:543-553.
1. Konnopka A, Schaefert R, Heinrich S, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265-275.
2. Carson AJ, Brown R, David AS, et al; on behalf of UK-FNS. Functional (conversion) neurological symptoms: research since the millennium. J Neurol Neurosurg Psychiatry. 2012;83:842-850.
3. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
4. Evens A, Vendetta L, Krebs K, et al. Medically unexplained neurologic symptoms: a primer for physicians who make the initial encounter. Am J Med. 2015;128:1059-1064.
5. Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol. 2013;13:104-113.
6. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537-1551.
7. Carson A, Stone J, Hibberd C, et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J Neurol Neurosurg Psychiatry. 2011;82:810-813.
8. Fink P, Hansen MS, Oxhøj M-L. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res. 2004;56:413-418.
9. Thomas LE. Are your patient’s medically unexplained symptoms really “all in her head”? Med Hypotheses. 2012;78:542-547.
10. Ding JM, Kanaan RAA. What should we say to patients with unexplained neurological symptoms? How explanation affects offence. J Psychosom Res. 2016;91:55-60.
11. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association; 2013.
12. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM-5: Conversion disorder. Am J Psychiatry. 2010;167:626-627.
13. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1994. Accessed January 21, 2021. www.who.int/classifications/icd/en/bluebook.pdf
14. Stone J, Carson A, Duncan R, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease.” J Neurol. 2012;259:33-38.
15. Shaibani A, Sabbagh M. Pseudoneurologic syndromes: recognition and diagnosis. Am Fam Physician. 1998;57:2485-2494.
16. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
17. Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323-337.
18. McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics. 2018;59:358-368.
19. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190.
20. Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001;16:595-599.
21. Ludwig L, McWhirter L, Williams S, et al. Functional coma. In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology: Volume 139: Functional Neurologic Disorders. 1st ed. Academic Press; 2016:313.
22. Miller NR, Subramanian PS, Patel VR. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 3rd ed. Wolters Kluwer; 2016:512-513.
23. Takazaki K, Stransky AD, Miller G. Psychogenic nonepileptic seizures: diagnosis, management, and bioethics. Pediatr Neurol. 2016;62:3-8.
24. Sahaya K, Dholakia SA, Sahota PK. Psychogenic non-epileptic seizures: a challenging entity. J Clin Neurosci. 2011;18:1602-1607.
25. Gelauff J, Stone J, Edwards M, et al. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220-226.
26. Kranick SM, Gorrindo T, Hallett M. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics. 2011;52:109-116.
27. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250-260.
28. Burgmer M, Kugel H, Pfleiderer B, et al. The mirror neuron system under hypnosis—brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex. 2013;49:437-445.
29. van Bokhoven MA, Koch H, van der Weijden T, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints, Ann Fam Med. 2009;7:112-120.
30. Stone J, Carson A, Hallet M. Explanation as treatment for functional neurologic disorders. Handb Clin Neurol. 2016;139:543-553.
PRACTICE RECOMMENDATIONS
› Avoid using stigmatizing terminology (eg, adding the prefix “pseudo” or the adjective “hysterical”) to characterize a suspected functional neurological disorder (FND) or a medically unexplained disorder. C
› Refrain from ordering functional magnetic resonance imaging as part of the routine evaluation of suspected FND. C
› Validate the patient‘s concerns with an appropriate diagnostic label; use layman’s terms to discuss the diagnostic parameters of FND and the cause of symptoms; and emphasize treatment possibilities and plans. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Late-window stroke thrombolysis not linked to clot migration
In patients with acute ischemic stroke, the use of thrombolysis in the late window of 4.5-9 hours after symptom onset was not associated with an increase in clot migration that would cause reduced clot accessibility to endovascular therapy, a new analysis from the EXTEND trial shows.
“There was no significant difference in the incidence of clot migration leading to clot inaccessibility in patients who received placebo or (intravenous) thrombolysis,” the authors report.
“Our results found no convincing evidence against the use of bridging thrombolysis before endovascular therapy in patients with acute ischemic stroke who present outside the 4.5-hour window,” they conclude.
“This information is important because it provides some comfort for neurointerventionists that IV thrombolysis does not unduly increase the risk of clot migration,” senior author, Bernard Yan, DMedSci, FRACP, told this news organization.
The study was published online in Stroke on Feb. 16.
The Australian researchers explain that endovascular thrombectomy is the standard of care in patients presenting with acute ischemic stroke caused by large-vessel occlusion, and current treatment guidelines recommend bridging thrombolysis for all patients receiving thrombectomy within the 4.5-hour time window.
While thrombectomy is also recommended in selected patients up to 24 hours after onset of symptoms, it remains unclear whether thrombolysis pretreatment should be administered in this setting.
One of the issues that might affect use of thrombolysis is distal clot migration. As proximal clot location is a crucial factor determining suitability for endovascular clot retrieval, distal migration may prevent successful thrombectomy, they note.
“Clot migration can happen any time and makes life more difficult for the neurointerventionist who performs the endovascular clot retrieval,” added Dr. Yan, who is a neurologist and neurointerventionist at the Royal Melbourne Hospital, Australia.
In the current paper, the researchers report a retrospective analysis of data from the EXTEND trial of late thrombolysis, defined as 4.5-9 hours after symptom onset, to investigate the association between thrombolysis and clot migration leading to clot irretrievability.
The analysis included a total of 220 patients (109 patients in the placebo group and 111 in the thrombolysis group).
Results showed that retrievable clot was seen on baseline imaging in 69% of patients in the placebo group and 61% in the thrombolysis group. Clot resolution occurred in 28% of patients in the placebo group and 50% in the thrombolysis group.
No significant difference was observed in the incidence of clot migration leading to inaccessibility between groups. Clot migration from a retrievable to nonretrievable location occurred in 19% of the placebo group and 14% of the thrombolysis group, with an odds ratio for clot migration in the thrombolysis group of 0.70 (95% confidence interval, 0.35-1.44). This outcome was consistent across subgroups.
The researchers note that, to their knowledge, this is the first randomized controlled study to assess the effect of thrombolysis on clot migration and accessibility in an extended time window.
They acknowledge that a limitation of this study is that they only assessed clot migration from a retrievable to a nonretrievable location; therefore, the true frequency of any clot migration occurring was likely to be higher, and this could explain why other reports have found higher odds ratios of clot migration.
But they point out that they chose to limit their analysis in this way specifically to guide decision-making regarding bridging thrombolysis incorporating endovascular therapy in the extended time window.
“The findings of this study are highly relevant in the current clinical environment, where there are multiple ongoing trials looking at removing thrombolysis pretreatment within the 4.5-hour time window in thrombectomy patients,” the authors write.
“We have demonstrated that thrombolysis in the 4.5- to 9-hour window is not associated with reduced clot accessibility, and this information will be useful in future trial designs incorporating this extended time window,” they add.
Commenting on the study for this news organization, Michael Hill, MD, University of Calgary (Alta.), said: “Thrombus migration does happen and is likely part of the natural history of ischemic stroke, which may be influenced by therapeutics such as thrombolysis. This paper’s top-line result is that thrombus migration occurs in both treated and untreated groups – and therefore that this is really an observation of natural history.”
Dr. Hill says that, at present, patients should be treated with thrombolysis before endovascular therapy if they are eligible, and these results do not change that recommendation.
“The results of the ongoing trials comparing direct thrombectomy with thrombolysis plus thrombectomy will help to understand the potential clinical outcome relevance of this phenomenon,” he added.
The EXTEND trial was supported by grants from the Australian National Health and Medical Research Council of Australia and the Commonwealth Scientific and Industrial Research Organization Flagship Program. Dr. Yan reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with acute ischemic stroke, the use of thrombolysis in the late window of 4.5-9 hours after symptom onset was not associated with an increase in clot migration that would cause reduced clot accessibility to endovascular therapy, a new analysis from the EXTEND trial shows.
“There was no significant difference in the incidence of clot migration leading to clot inaccessibility in patients who received placebo or (intravenous) thrombolysis,” the authors report.
“Our results found no convincing evidence against the use of bridging thrombolysis before endovascular therapy in patients with acute ischemic stroke who present outside the 4.5-hour window,” they conclude.
“This information is important because it provides some comfort for neurointerventionists that IV thrombolysis does not unduly increase the risk of clot migration,” senior author, Bernard Yan, DMedSci, FRACP, told this news organization.
The study was published online in Stroke on Feb. 16.
The Australian researchers explain that endovascular thrombectomy is the standard of care in patients presenting with acute ischemic stroke caused by large-vessel occlusion, and current treatment guidelines recommend bridging thrombolysis for all patients receiving thrombectomy within the 4.5-hour time window.
While thrombectomy is also recommended in selected patients up to 24 hours after onset of symptoms, it remains unclear whether thrombolysis pretreatment should be administered in this setting.
One of the issues that might affect use of thrombolysis is distal clot migration. As proximal clot location is a crucial factor determining suitability for endovascular clot retrieval, distal migration may prevent successful thrombectomy, they note.
“Clot migration can happen any time and makes life more difficult for the neurointerventionist who performs the endovascular clot retrieval,” added Dr. Yan, who is a neurologist and neurointerventionist at the Royal Melbourne Hospital, Australia.
In the current paper, the researchers report a retrospective analysis of data from the EXTEND trial of late thrombolysis, defined as 4.5-9 hours after symptom onset, to investigate the association between thrombolysis and clot migration leading to clot irretrievability.
The analysis included a total of 220 patients (109 patients in the placebo group and 111 in the thrombolysis group).
Results showed that retrievable clot was seen on baseline imaging in 69% of patients in the placebo group and 61% in the thrombolysis group. Clot resolution occurred in 28% of patients in the placebo group and 50% in the thrombolysis group.
No significant difference was observed in the incidence of clot migration leading to inaccessibility between groups. Clot migration from a retrievable to nonretrievable location occurred in 19% of the placebo group and 14% of the thrombolysis group, with an odds ratio for clot migration in the thrombolysis group of 0.70 (95% confidence interval, 0.35-1.44). This outcome was consistent across subgroups.
The researchers note that, to their knowledge, this is the first randomized controlled study to assess the effect of thrombolysis on clot migration and accessibility in an extended time window.
They acknowledge that a limitation of this study is that they only assessed clot migration from a retrievable to a nonretrievable location; therefore, the true frequency of any clot migration occurring was likely to be higher, and this could explain why other reports have found higher odds ratios of clot migration.
But they point out that they chose to limit their analysis in this way specifically to guide decision-making regarding bridging thrombolysis incorporating endovascular therapy in the extended time window.
“The findings of this study are highly relevant in the current clinical environment, where there are multiple ongoing trials looking at removing thrombolysis pretreatment within the 4.5-hour time window in thrombectomy patients,” the authors write.
“We have demonstrated that thrombolysis in the 4.5- to 9-hour window is not associated with reduced clot accessibility, and this information will be useful in future trial designs incorporating this extended time window,” they add.
Commenting on the study for this news organization, Michael Hill, MD, University of Calgary (Alta.), said: “Thrombus migration does happen and is likely part of the natural history of ischemic stroke, which may be influenced by therapeutics such as thrombolysis. This paper’s top-line result is that thrombus migration occurs in both treated and untreated groups – and therefore that this is really an observation of natural history.”
Dr. Hill says that, at present, patients should be treated with thrombolysis before endovascular therapy if they are eligible, and these results do not change that recommendation.
“The results of the ongoing trials comparing direct thrombectomy with thrombolysis plus thrombectomy will help to understand the potential clinical outcome relevance of this phenomenon,” he added.
The EXTEND trial was supported by grants from the Australian National Health and Medical Research Council of Australia and the Commonwealth Scientific and Industrial Research Organization Flagship Program. Dr. Yan reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with acute ischemic stroke, the use of thrombolysis in the late window of 4.5-9 hours after symptom onset was not associated with an increase in clot migration that would cause reduced clot accessibility to endovascular therapy, a new analysis from the EXTEND trial shows.
“There was no significant difference in the incidence of clot migration leading to clot inaccessibility in patients who received placebo or (intravenous) thrombolysis,” the authors report.
“Our results found no convincing evidence against the use of bridging thrombolysis before endovascular therapy in patients with acute ischemic stroke who present outside the 4.5-hour window,” they conclude.
“This information is important because it provides some comfort for neurointerventionists that IV thrombolysis does not unduly increase the risk of clot migration,” senior author, Bernard Yan, DMedSci, FRACP, told this news organization.
The study was published online in Stroke on Feb. 16.
The Australian researchers explain that endovascular thrombectomy is the standard of care in patients presenting with acute ischemic stroke caused by large-vessel occlusion, and current treatment guidelines recommend bridging thrombolysis for all patients receiving thrombectomy within the 4.5-hour time window.
While thrombectomy is also recommended in selected patients up to 24 hours after onset of symptoms, it remains unclear whether thrombolysis pretreatment should be administered in this setting.
One of the issues that might affect use of thrombolysis is distal clot migration. As proximal clot location is a crucial factor determining suitability for endovascular clot retrieval, distal migration may prevent successful thrombectomy, they note.
“Clot migration can happen any time and makes life more difficult for the neurointerventionist who performs the endovascular clot retrieval,” added Dr. Yan, who is a neurologist and neurointerventionist at the Royal Melbourne Hospital, Australia.
In the current paper, the researchers report a retrospective analysis of data from the EXTEND trial of late thrombolysis, defined as 4.5-9 hours after symptom onset, to investigate the association between thrombolysis and clot migration leading to clot irretrievability.
The analysis included a total of 220 patients (109 patients in the placebo group and 111 in the thrombolysis group).
Results showed that retrievable clot was seen on baseline imaging in 69% of patients in the placebo group and 61% in the thrombolysis group. Clot resolution occurred in 28% of patients in the placebo group and 50% in the thrombolysis group.
No significant difference was observed in the incidence of clot migration leading to inaccessibility between groups. Clot migration from a retrievable to nonretrievable location occurred in 19% of the placebo group and 14% of the thrombolysis group, with an odds ratio for clot migration in the thrombolysis group of 0.70 (95% confidence interval, 0.35-1.44). This outcome was consistent across subgroups.
The researchers note that, to their knowledge, this is the first randomized controlled study to assess the effect of thrombolysis on clot migration and accessibility in an extended time window.
They acknowledge that a limitation of this study is that they only assessed clot migration from a retrievable to a nonretrievable location; therefore, the true frequency of any clot migration occurring was likely to be higher, and this could explain why other reports have found higher odds ratios of clot migration.
But they point out that they chose to limit their analysis in this way specifically to guide decision-making regarding bridging thrombolysis incorporating endovascular therapy in the extended time window.
“The findings of this study are highly relevant in the current clinical environment, where there are multiple ongoing trials looking at removing thrombolysis pretreatment within the 4.5-hour time window in thrombectomy patients,” the authors write.
“We have demonstrated that thrombolysis in the 4.5- to 9-hour window is not associated with reduced clot accessibility, and this information will be useful in future trial designs incorporating this extended time window,” they add.
Commenting on the study for this news organization, Michael Hill, MD, University of Calgary (Alta.), said: “Thrombus migration does happen and is likely part of the natural history of ischemic stroke, which may be influenced by therapeutics such as thrombolysis. This paper’s top-line result is that thrombus migration occurs in both treated and untreated groups – and therefore that this is really an observation of natural history.”
Dr. Hill says that, at present, patients should be treated with thrombolysis before endovascular therapy if they are eligible, and these results do not change that recommendation.
“The results of the ongoing trials comparing direct thrombectomy with thrombolysis plus thrombectomy will help to understand the potential clinical outcome relevance of this phenomenon,” he added.
The EXTEND trial was supported by grants from the Australian National Health and Medical Research Council of Australia and the Commonwealth Scientific and Industrial Research Organization Flagship Program. Dr. Yan reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Maternal chronic conditions predict cerebral palsy in offspring
Several maternal chronic conditions increase the risk of giving birth to a child with cerebral palsy, based on data from more than 1.3 million Norwegian children.
Mothers with autoimmune disorders, such as diabetes and lupus, had the greatest risks, reported lead author Marianne S. Strøm, MD, of the University of Bergen (Norway) and colleagues.
“The etiologies of cerebral palsy are complex, and only a few prenatal risk factors have been identified,” the investigators wrote in Pediatrics. “Among these possible risk factors are maternal chronic conditions, although studies are typically underpowered and limited to one or two conditions.”
According to Dr. Strøm and colleagues, several components of maternal chronic conditions have been linked with cerebral palsy, including placental abnormalities, altered thrombotic state, and inflammation. Furthermore, mothers with chronic conditions are more likely to give birth prematurely and have children with congenital malformations, both of which have also been associated with cerebral palsy.
To date, however, “there has been no systematic description of maternal chronic conditions and risk of cerebral palsy in offspring,” the investigators noted.
The present, prospective cohort study aimed to meet this need with a population of 1,360,149 children born in Norway from 1990 to 2012, among whom 3,575 had cerebral palsy. Case data were extracted from the Norwegian Patient Registry and the National Insurance Scheme. Information about maternal chronic conditions was extracted from the Medical Birth Registry of Norway and the Norwegian Patient Registry, with the latter also providing information about paternal chronic conditions.
Using log binomial regression models, the investigators determined relative risks of having children with cerebral palsy among parents with chronic conditions versus parents from the general population. This revealed that chronic conditions in fathers had no correlation with cerebral palsy. In contrast, mothers with chronic conditions had a 30% increased risk (relative risk, 1.3; 95% confidence interval, 1.2-1.5), which could be further stratified by number of chronic conditions; mothers with one chronic condition, for instance, had a 20% increased risk (RR, 1.2; 95% CI, 1.1-1.4), while those with two chronic conditions had a 60% increased risk (RR, 1.6; 95% CI, 1.1-2.2), and those with more than two chronic conditions had triple the risk (RR, 3.1; 95% CI, 1.4-6.8)
“The lack of associations between the father’s chronic illness and cerebral palsy risk supports the interpretation that cerebral palsy risk in offspring is the direct result of the mother’s condition and not genetic predisposition or unmeasured situational factors,” the investigators wrote.
Maternal autoimmune conditions were particularly relevant, as they were associated with a 40% increased risk of cerebral palsy (RR, 1.4; 95% CI, 1.1-1.7), a rate that climbed dramatically, to 270%, among mothers with more than one autoimmune condition (RR, 2.7; 95% CI, 1.1-6.6).
“The role of autoimmune diseases in cerebral palsy risk (and maternal inflammation specifically) deserves closer attention,” the investigators wrote. “Using studies with larger sample sizes and a more clinical focus, including measures of placental structure and perinatal blood assays, researchers may be able to explore these possible connections between maternal autoimmune diseases and fetal neurodevelopment.”
Specifically, cerebral palsy in offspring was most strongly associated with maternal Crohn’s disease (RR, 2.1; 95% CI, 1.0-4.1), type 1 diabetes (RR, 2.2; 95% CI, 1.4-3.4), lupus erythematosus (RR, 2.7; 95% CI, 0.9-8.3), and type 2 diabetes (RR, 3.2; 95% CI, 1.8-5.4). Associations were also found for migraine (RR, 1.6; 95% CI, 1.2-2.2), multiple sclerosis (RR, 1.8; 95% CI, 0.8-4.4), and rheumatoid arthritis (RR, 2.0; 95% CI, 1.3-2.9). Several “weaker and less convincing associations” were detected for ulcerative colitis, thyroid disorder, epilepsy, asthma, anemia, and hypertension. Adjusting for parental education level, age, smoking status, and single-mother status did not significantly alter findings. Poisson and logistic regression models generated similar results.
In an accompanying editorial, Sandra Julsen Hollung, PhD, of the Cerebral Palsy Registry of Norway, Vestfold Hospital Trust, Tønsberg, and colleagues, advised that clinicians maintain perspective when discussing these findings with the general public.
“As the authors state, the absolute risk of cerebral palsy associated with at least one chronic maternal condition is low,” wrote Dr. Hollung and colleagues. “Among 1,000 pregnant women with any chronic and/or autoimmune disorder, more than 990 will deliver an infant who will not be diagnosed with cerebral palsy.”
They went on to emphasize that the study findings should not be viewed as firm evidence of causal relationships.
“Thus, the study cannot give clues to any specific preventive treatment,” wrote Dr. Hollung and colleagues. “However, if these disorders are part of a causal pathway, optimal treatment might reduce the risk of cerebral palsy.”
Although Dr. Hollung and colleagues advised that such efforts “would hardly affect the birth prevalence of cerebral palsy,” they also cited the Royal College of Obstetricians and Gynaecologists in the United Kingdom, noting that “each baby counts.”
Emeritus Professor Alastair MacLennan, AO, MB ChB, FRCOG, FRANZCOG, head of the Australian Collaborative Cerebral Palsy Research Group at the University of Adelaide (Australia) suggested that the findings may guide future research.
“An increasing proportion of cerebral palsy cases are being diagnosed by genome sequencing and other genetic techniques to have causative genetic variations,” Dr. MacLennan said. “The possibility of epigenetic interactions are also likely and are still to be investigated. Maternal disorders such as diabetes, lupus, or Crohn’s disease are possible epigenetic factors and this study helps to target these in future genetic and environmental studies of cerebral palsy causation. The days of attributing cerebral palsy to ‘birth asphyxia’ are over.”
The study was supported by the National Institutes of Health and the Western Norwegian Regional Health Authorities. The investigators reported no conflicts of interest.
Several maternal chronic conditions increase the risk of giving birth to a child with cerebral palsy, based on data from more than 1.3 million Norwegian children.
Mothers with autoimmune disorders, such as diabetes and lupus, had the greatest risks, reported lead author Marianne S. Strøm, MD, of the University of Bergen (Norway) and colleagues.
“The etiologies of cerebral palsy are complex, and only a few prenatal risk factors have been identified,” the investigators wrote in Pediatrics. “Among these possible risk factors are maternal chronic conditions, although studies are typically underpowered and limited to one or two conditions.”
According to Dr. Strøm and colleagues, several components of maternal chronic conditions have been linked with cerebral palsy, including placental abnormalities, altered thrombotic state, and inflammation. Furthermore, mothers with chronic conditions are more likely to give birth prematurely and have children with congenital malformations, both of which have also been associated with cerebral palsy.
To date, however, “there has been no systematic description of maternal chronic conditions and risk of cerebral palsy in offspring,” the investigators noted.
The present, prospective cohort study aimed to meet this need with a population of 1,360,149 children born in Norway from 1990 to 2012, among whom 3,575 had cerebral palsy. Case data were extracted from the Norwegian Patient Registry and the National Insurance Scheme. Information about maternal chronic conditions was extracted from the Medical Birth Registry of Norway and the Norwegian Patient Registry, with the latter also providing information about paternal chronic conditions.
Using log binomial regression models, the investigators determined relative risks of having children with cerebral palsy among parents with chronic conditions versus parents from the general population. This revealed that chronic conditions in fathers had no correlation with cerebral palsy. In contrast, mothers with chronic conditions had a 30% increased risk (relative risk, 1.3; 95% confidence interval, 1.2-1.5), which could be further stratified by number of chronic conditions; mothers with one chronic condition, for instance, had a 20% increased risk (RR, 1.2; 95% CI, 1.1-1.4), while those with two chronic conditions had a 60% increased risk (RR, 1.6; 95% CI, 1.1-2.2), and those with more than two chronic conditions had triple the risk (RR, 3.1; 95% CI, 1.4-6.8)
“The lack of associations between the father’s chronic illness and cerebral palsy risk supports the interpretation that cerebral palsy risk in offspring is the direct result of the mother’s condition and not genetic predisposition or unmeasured situational factors,” the investigators wrote.
Maternal autoimmune conditions were particularly relevant, as they were associated with a 40% increased risk of cerebral palsy (RR, 1.4; 95% CI, 1.1-1.7), a rate that climbed dramatically, to 270%, among mothers with more than one autoimmune condition (RR, 2.7; 95% CI, 1.1-6.6).
“The role of autoimmune diseases in cerebral palsy risk (and maternal inflammation specifically) deserves closer attention,” the investigators wrote. “Using studies with larger sample sizes and a more clinical focus, including measures of placental structure and perinatal blood assays, researchers may be able to explore these possible connections between maternal autoimmune diseases and fetal neurodevelopment.”
Specifically, cerebral palsy in offspring was most strongly associated with maternal Crohn’s disease (RR, 2.1; 95% CI, 1.0-4.1), type 1 diabetes (RR, 2.2; 95% CI, 1.4-3.4), lupus erythematosus (RR, 2.7; 95% CI, 0.9-8.3), and type 2 diabetes (RR, 3.2; 95% CI, 1.8-5.4). Associations were also found for migraine (RR, 1.6; 95% CI, 1.2-2.2), multiple sclerosis (RR, 1.8; 95% CI, 0.8-4.4), and rheumatoid arthritis (RR, 2.0; 95% CI, 1.3-2.9). Several “weaker and less convincing associations” were detected for ulcerative colitis, thyroid disorder, epilepsy, asthma, anemia, and hypertension. Adjusting for parental education level, age, smoking status, and single-mother status did not significantly alter findings. Poisson and logistic regression models generated similar results.
In an accompanying editorial, Sandra Julsen Hollung, PhD, of the Cerebral Palsy Registry of Norway, Vestfold Hospital Trust, Tønsberg, and colleagues, advised that clinicians maintain perspective when discussing these findings with the general public.
“As the authors state, the absolute risk of cerebral palsy associated with at least one chronic maternal condition is low,” wrote Dr. Hollung and colleagues. “Among 1,000 pregnant women with any chronic and/or autoimmune disorder, more than 990 will deliver an infant who will not be diagnosed with cerebral palsy.”
They went on to emphasize that the study findings should not be viewed as firm evidence of causal relationships.
“Thus, the study cannot give clues to any specific preventive treatment,” wrote Dr. Hollung and colleagues. “However, if these disorders are part of a causal pathway, optimal treatment might reduce the risk of cerebral palsy.”
Although Dr. Hollung and colleagues advised that such efforts “would hardly affect the birth prevalence of cerebral palsy,” they also cited the Royal College of Obstetricians and Gynaecologists in the United Kingdom, noting that “each baby counts.”
Emeritus Professor Alastair MacLennan, AO, MB ChB, FRCOG, FRANZCOG, head of the Australian Collaborative Cerebral Palsy Research Group at the University of Adelaide (Australia) suggested that the findings may guide future research.
“An increasing proportion of cerebral palsy cases are being diagnosed by genome sequencing and other genetic techniques to have causative genetic variations,” Dr. MacLennan said. “The possibility of epigenetic interactions are also likely and are still to be investigated. Maternal disorders such as diabetes, lupus, or Crohn’s disease are possible epigenetic factors and this study helps to target these in future genetic and environmental studies of cerebral palsy causation. The days of attributing cerebral palsy to ‘birth asphyxia’ are over.”
The study was supported by the National Institutes of Health and the Western Norwegian Regional Health Authorities. The investigators reported no conflicts of interest.
Several maternal chronic conditions increase the risk of giving birth to a child with cerebral palsy, based on data from more than 1.3 million Norwegian children.
Mothers with autoimmune disorders, such as diabetes and lupus, had the greatest risks, reported lead author Marianne S. Strøm, MD, of the University of Bergen (Norway) and colleagues.
“The etiologies of cerebral palsy are complex, and only a few prenatal risk factors have been identified,” the investigators wrote in Pediatrics. “Among these possible risk factors are maternal chronic conditions, although studies are typically underpowered and limited to one or two conditions.”
According to Dr. Strøm and colleagues, several components of maternal chronic conditions have been linked with cerebral palsy, including placental abnormalities, altered thrombotic state, and inflammation. Furthermore, mothers with chronic conditions are more likely to give birth prematurely and have children with congenital malformations, both of which have also been associated with cerebral palsy.
To date, however, “there has been no systematic description of maternal chronic conditions and risk of cerebral palsy in offspring,” the investigators noted.
The present, prospective cohort study aimed to meet this need with a population of 1,360,149 children born in Norway from 1990 to 2012, among whom 3,575 had cerebral palsy. Case data were extracted from the Norwegian Patient Registry and the National Insurance Scheme. Information about maternal chronic conditions was extracted from the Medical Birth Registry of Norway and the Norwegian Patient Registry, with the latter also providing information about paternal chronic conditions.
Using log binomial regression models, the investigators determined relative risks of having children with cerebral palsy among parents with chronic conditions versus parents from the general population. This revealed that chronic conditions in fathers had no correlation with cerebral palsy. In contrast, mothers with chronic conditions had a 30% increased risk (relative risk, 1.3; 95% confidence interval, 1.2-1.5), which could be further stratified by number of chronic conditions; mothers with one chronic condition, for instance, had a 20% increased risk (RR, 1.2; 95% CI, 1.1-1.4), while those with two chronic conditions had a 60% increased risk (RR, 1.6; 95% CI, 1.1-2.2), and those with more than two chronic conditions had triple the risk (RR, 3.1; 95% CI, 1.4-6.8)
“The lack of associations between the father’s chronic illness and cerebral palsy risk supports the interpretation that cerebral palsy risk in offspring is the direct result of the mother’s condition and not genetic predisposition or unmeasured situational factors,” the investigators wrote.
Maternal autoimmune conditions were particularly relevant, as they were associated with a 40% increased risk of cerebral palsy (RR, 1.4; 95% CI, 1.1-1.7), a rate that climbed dramatically, to 270%, among mothers with more than one autoimmune condition (RR, 2.7; 95% CI, 1.1-6.6).
“The role of autoimmune diseases in cerebral palsy risk (and maternal inflammation specifically) deserves closer attention,” the investigators wrote. “Using studies with larger sample sizes and a more clinical focus, including measures of placental structure and perinatal blood assays, researchers may be able to explore these possible connections between maternal autoimmune diseases and fetal neurodevelopment.”
Specifically, cerebral palsy in offspring was most strongly associated with maternal Crohn’s disease (RR, 2.1; 95% CI, 1.0-4.1), type 1 diabetes (RR, 2.2; 95% CI, 1.4-3.4), lupus erythematosus (RR, 2.7; 95% CI, 0.9-8.3), and type 2 diabetes (RR, 3.2; 95% CI, 1.8-5.4). Associations were also found for migraine (RR, 1.6; 95% CI, 1.2-2.2), multiple sclerosis (RR, 1.8; 95% CI, 0.8-4.4), and rheumatoid arthritis (RR, 2.0; 95% CI, 1.3-2.9). Several “weaker and less convincing associations” were detected for ulcerative colitis, thyroid disorder, epilepsy, asthma, anemia, and hypertension. Adjusting for parental education level, age, smoking status, and single-mother status did not significantly alter findings. Poisson and logistic regression models generated similar results.
In an accompanying editorial, Sandra Julsen Hollung, PhD, of the Cerebral Palsy Registry of Norway, Vestfold Hospital Trust, Tønsberg, and colleagues, advised that clinicians maintain perspective when discussing these findings with the general public.
“As the authors state, the absolute risk of cerebral palsy associated with at least one chronic maternal condition is low,” wrote Dr. Hollung and colleagues. “Among 1,000 pregnant women with any chronic and/or autoimmune disorder, more than 990 will deliver an infant who will not be diagnosed with cerebral palsy.”
They went on to emphasize that the study findings should not be viewed as firm evidence of causal relationships.
“Thus, the study cannot give clues to any specific preventive treatment,” wrote Dr. Hollung and colleagues. “However, if these disorders are part of a causal pathway, optimal treatment might reduce the risk of cerebral palsy.”
Although Dr. Hollung and colleagues advised that such efforts “would hardly affect the birth prevalence of cerebral palsy,” they also cited the Royal College of Obstetricians and Gynaecologists in the United Kingdom, noting that “each baby counts.”
Emeritus Professor Alastair MacLennan, AO, MB ChB, FRCOG, FRANZCOG, head of the Australian Collaborative Cerebral Palsy Research Group at the University of Adelaide (Australia) suggested that the findings may guide future research.
“An increasing proportion of cerebral palsy cases are being diagnosed by genome sequencing and other genetic techniques to have causative genetic variations,” Dr. MacLennan said. “The possibility of epigenetic interactions are also likely and are still to be investigated. Maternal disorders such as diabetes, lupus, or Crohn’s disease are possible epigenetic factors and this study helps to target these in future genetic and environmental studies of cerebral palsy causation. The days of attributing cerebral palsy to ‘birth asphyxia’ are over.”
The study was supported by the National Institutes of Health and the Western Norwegian Regional Health Authorities. The investigators reported no conflicts of interest.
FROM PEDIATRICS
NfL levels linked to worse disability in real-world MS
according to new findings from a large, diverse population of patients with MS. “This is one of the largest studies to evaluate serum neurofilament light chain levels in people with MS,” said lead author Elias S. Sotirchos, MD, an assistant professor of neurology at Johns Hopkins University, Baltimore.
“An important strength of this cohort is that it is a real-world cohort of patients followed in U.S. and European MS centers,” he said. “The study captures the diversity of the MS population, including demographics, comorbidities, lifestyle factors, and clinical characteristics that may otherwise not be captured in a clinical trial population.”
The research was presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Scrutinizing serum neurofilament light chain levels in a real-world cohort
Neurofilaments – neuron-specific proteins that release in response to neuroaxonal injury – have been observed to be elevated in a variety of neurologic disorders, and with a need for biomarkers in MS, there is high interest of their role in the disease. But studies involving real-world, heterogeneous MS populations are lacking, the researchers noted.
To take a broader look at the issue, Dr. Sotirchos and colleagues conducted a cross-sectional evaluation of 6,968 people with MS in the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS), a large network of MS centers in the United States and Europe.
Participants’ baseline serum neurofilament light chain levels were compared with those of 201 healthy controls in the cohort using a novel, high-throughput immunoassay (Siemens Healthineers).
Of those with MS, 1,202 (17.2%) showed elevated serum neurofilament light chain levels, above the age-specific 97.5th percentile derived from the healthy controls.
A look at key factors associated with elevations showed significant links to having progressive MS (odds ratio, 1.63), non-White race (OR, 1.43), type 2 diabetes (OR, 1.89), and smoking (current vs. never smoker; OR, 1.49).
Associations with age and symptom duration were somewhat complex, but overall, younger patients and those with shorter disease duration had the highest frequency of elevated serum neurofilament light chain levels.
Interestingly, those with a higher body mass index (BMI) showed a reduced odds of having elevated serum neurofilament light chain levels (OR, 0.83 per 5 kg/m2 increase in BMI).
Evaluation of neuroperformance measures – including walking speed, manual dexterity and processing speed, and MRI data – showed that those with elevated serum neurofilament light chain levels had worse neurologic function, lower brain parenchymal fraction, lower thalamic volume, and higher T2 lesion volume (P < .001 for all).
Dr. Sotirchos noted that the higher rates of elevations in younger people, also observed in previous clinical trials, may reflect higher early-stage disease activity. “Generally, people who are younger and earlier in the course of disease tend to have more inflammatory disease activity in MS, and that could be what we’re capturing here, but we need to better understand the pathologic correlates of elevated serum neurofilament light chain levels.”
The lower levels of neurofilament light chain with higher BMI, also recently reported in another study, likewise need further investigation, including in healthy controls, Dr. Sotirchos added. “Having lower serum neurofilament light chain levels with increasing BMI could have to do with effects of blood volume and how the serum neurofilament light chain levels is distributed in the body,” he explained.
The findings suggest that interpretation of serum neurofilament light chain levels without accounting for BMI could result in false-negative or false-positive results, Dr. Sotirchos noted. “It will be important to further evaluate this observation in control populations and account for BMI in neurofilament light chain reference ranges.”
Dr. Sotirchos added that the 17% rate of elevated serum neurofilament light chain levels seen in people with MS in the study is likely an underestimate.
“This is a cross-sectional study and represents one sample per patient, so it is a snapshot in time,” he said. “With the nature of MS, we know that people’s levels fluctuate over time.” In addition, most patients were on disease-modifying therapy for MS, so serum neurofilament light chain elevations could have been suppressed.
Applying the findings to individual patients
Commenting on the findings, Jennifer Graves, MD, PhD, director of the neuroimmunology research program at the University of California, San Diego, said the study is an important addition to the ongoing evidence on serum neurofilament light chain in MS.
“The current presented research importantly addresses the gaps we have in understanding how best to apply serum filament light chain levels to individual patients and not just using them to assess group level means of outcome measures,” she said.
“The MS PATHS collaborative is looking at multiple factors (in addition to MS activity) that drive serum neurofilament light chain levels so meaningful and practical cutoffs for what’s abnormal can be created,” said Dr. Graves, who also directs the Rady Children’s Pediatric MS Clinic in San Diego.
Dr. Graves noted that the findings on BMI were unexpected. “Elevated BMI has been shown to be associated with greater brain atrophy and greater relapses and disability in MS participants, so to have an opposite effect with serum neurofilament light chain is interesting.
“My thoughts would be that obesity is somehow affecting measurable blood levels of this marker. I think it less likely BMI has a protective effect against neurodegeneration given the observations with other MS outcome measures,” she added.
Future research
In terms of future directions, Dr. Sotirchos noted that the researchers are following the group longitudinally to further assess changes in neurofilament light chain over time, and will be looking at associations with longitudinal, clinical, and radiologic outcomes.
The current research, meanwhile, offers important insights in terms of developing precision reference ranges, he noted.
“It appears that reference ranges may need to account for sex, race, BMI, and comorbid/lifestyle factors,” Dr. Sotirchos said, “in order to potentially improve the performance of serum neurofilament light chain as a biomarker in MS and other neurological diseases.”
The study received funding from Biogen and the MS PATHS network receives funding from Biogen. Dr. Sotirchos has served on scientific advisory boards for Alexion, Viela Bio, and Genentech, and has received speaker honoraria from Viela Bio and Biogen. Dr. Graves has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new findings from a large, diverse population of patients with MS. “This is one of the largest studies to evaluate serum neurofilament light chain levels in people with MS,” said lead author Elias S. Sotirchos, MD, an assistant professor of neurology at Johns Hopkins University, Baltimore.
“An important strength of this cohort is that it is a real-world cohort of patients followed in U.S. and European MS centers,” he said. “The study captures the diversity of the MS population, including demographics, comorbidities, lifestyle factors, and clinical characteristics that may otherwise not be captured in a clinical trial population.”
The research was presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Scrutinizing serum neurofilament light chain levels in a real-world cohort
Neurofilaments – neuron-specific proteins that release in response to neuroaxonal injury – have been observed to be elevated in a variety of neurologic disorders, and with a need for biomarkers in MS, there is high interest of their role in the disease. But studies involving real-world, heterogeneous MS populations are lacking, the researchers noted.
To take a broader look at the issue, Dr. Sotirchos and colleagues conducted a cross-sectional evaluation of 6,968 people with MS in the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS), a large network of MS centers in the United States and Europe.
Participants’ baseline serum neurofilament light chain levels were compared with those of 201 healthy controls in the cohort using a novel, high-throughput immunoassay (Siemens Healthineers).
Of those with MS, 1,202 (17.2%) showed elevated serum neurofilament light chain levels, above the age-specific 97.5th percentile derived from the healthy controls.
A look at key factors associated with elevations showed significant links to having progressive MS (odds ratio, 1.63), non-White race (OR, 1.43), type 2 diabetes (OR, 1.89), and smoking (current vs. never smoker; OR, 1.49).
Associations with age and symptom duration were somewhat complex, but overall, younger patients and those with shorter disease duration had the highest frequency of elevated serum neurofilament light chain levels.
Interestingly, those with a higher body mass index (BMI) showed a reduced odds of having elevated serum neurofilament light chain levels (OR, 0.83 per 5 kg/m2 increase in BMI).
Evaluation of neuroperformance measures – including walking speed, manual dexterity and processing speed, and MRI data – showed that those with elevated serum neurofilament light chain levels had worse neurologic function, lower brain parenchymal fraction, lower thalamic volume, and higher T2 lesion volume (P < .001 for all).
Dr. Sotirchos noted that the higher rates of elevations in younger people, also observed in previous clinical trials, may reflect higher early-stage disease activity. “Generally, people who are younger and earlier in the course of disease tend to have more inflammatory disease activity in MS, and that could be what we’re capturing here, but we need to better understand the pathologic correlates of elevated serum neurofilament light chain levels.”
The lower levels of neurofilament light chain with higher BMI, also recently reported in another study, likewise need further investigation, including in healthy controls, Dr. Sotirchos added. “Having lower serum neurofilament light chain levels with increasing BMI could have to do with effects of blood volume and how the serum neurofilament light chain levels is distributed in the body,” he explained.
The findings suggest that interpretation of serum neurofilament light chain levels without accounting for BMI could result in false-negative or false-positive results, Dr. Sotirchos noted. “It will be important to further evaluate this observation in control populations and account for BMI in neurofilament light chain reference ranges.”
Dr. Sotirchos added that the 17% rate of elevated serum neurofilament light chain levels seen in people with MS in the study is likely an underestimate.
“This is a cross-sectional study and represents one sample per patient, so it is a snapshot in time,” he said. “With the nature of MS, we know that people’s levels fluctuate over time.” In addition, most patients were on disease-modifying therapy for MS, so serum neurofilament light chain elevations could have been suppressed.
Applying the findings to individual patients
Commenting on the findings, Jennifer Graves, MD, PhD, director of the neuroimmunology research program at the University of California, San Diego, said the study is an important addition to the ongoing evidence on serum neurofilament light chain in MS.
“The current presented research importantly addresses the gaps we have in understanding how best to apply serum filament light chain levels to individual patients and not just using them to assess group level means of outcome measures,” she said.
“The MS PATHS collaborative is looking at multiple factors (in addition to MS activity) that drive serum neurofilament light chain levels so meaningful and practical cutoffs for what’s abnormal can be created,” said Dr. Graves, who also directs the Rady Children’s Pediatric MS Clinic in San Diego.
Dr. Graves noted that the findings on BMI were unexpected. “Elevated BMI has been shown to be associated with greater brain atrophy and greater relapses and disability in MS participants, so to have an opposite effect with serum neurofilament light chain is interesting.
“My thoughts would be that obesity is somehow affecting measurable blood levels of this marker. I think it less likely BMI has a protective effect against neurodegeneration given the observations with other MS outcome measures,” she added.
Future research
In terms of future directions, Dr. Sotirchos noted that the researchers are following the group longitudinally to further assess changes in neurofilament light chain over time, and will be looking at associations with longitudinal, clinical, and radiologic outcomes.
The current research, meanwhile, offers important insights in terms of developing precision reference ranges, he noted.
“It appears that reference ranges may need to account for sex, race, BMI, and comorbid/lifestyle factors,” Dr. Sotirchos said, “in order to potentially improve the performance of serum neurofilament light chain as a biomarker in MS and other neurological diseases.”
The study received funding from Biogen and the MS PATHS network receives funding from Biogen. Dr. Sotirchos has served on scientific advisory boards for Alexion, Viela Bio, and Genentech, and has received speaker honoraria from Viela Bio and Biogen. Dr. Graves has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new findings from a large, diverse population of patients with MS. “This is one of the largest studies to evaluate serum neurofilament light chain levels in people with MS,” said lead author Elias S. Sotirchos, MD, an assistant professor of neurology at Johns Hopkins University, Baltimore.
“An important strength of this cohort is that it is a real-world cohort of patients followed in U.S. and European MS centers,” he said. “The study captures the diversity of the MS population, including demographics, comorbidities, lifestyle factors, and clinical characteristics that may otherwise not be captured in a clinical trial population.”
The research was presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Scrutinizing serum neurofilament light chain levels in a real-world cohort
Neurofilaments – neuron-specific proteins that release in response to neuroaxonal injury – have been observed to be elevated in a variety of neurologic disorders, and with a need for biomarkers in MS, there is high interest of their role in the disease. But studies involving real-world, heterogeneous MS populations are lacking, the researchers noted.
To take a broader look at the issue, Dr. Sotirchos and colleagues conducted a cross-sectional evaluation of 6,968 people with MS in the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS), a large network of MS centers in the United States and Europe.
Participants’ baseline serum neurofilament light chain levels were compared with those of 201 healthy controls in the cohort using a novel, high-throughput immunoassay (Siemens Healthineers).
Of those with MS, 1,202 (17.2%) showed elevated serum neurofilament light chain levels, above the age-specific 97.5th percentile derived from the healthy controls.
A look at key factors associated with elevations showed significant links to having progressive MS (odds ratio, 1.63), non-White race (OR, 1.43), type 2 diabetes (OR, 1.89), and smoking (current vs. never smoker; OR, 1.49).
Associations with age and symptom duration were somewhat complex, but overall, younger patients and those with shorter disease duration had the highest frequency of elevated serum neurofilament light chain levels.
Interestingly, those with a higher body mass index (BMI) showed a reduced odds of having elevated serum neurofilament light chain levels (OR, 0.83 per 5 kg/m2 increase in BMI).
Evaluation of neuroperformance measures – including walking speed, manual dexterity and processing speed, and MRI data – showed that those with elevated serum neurofilament light chain levels had worse neurologic function, lower brain parenchymal fraction, lower thalamic volume, and higher T2 lesion volume (P < .001 for all).
Dr. Sotirchos noted that the higher rates of elevations in younger people, also observed in previous clinical trials, may reflect higher early-stage disease activity. “Generally, people who are younger and earlier in the course of disease tend to have more inflammatory disease activity in MS, and that could be what we’re capturing here, but we need to better understand the pathologic correlates of elevated serum neurofilament light chain levels.”
The lower levels of neurofilament light chain with higher BMI, also recently reported in another study, likewise need further investigation, including in healthy controls, Dr. Sotirchos added. “Having lower serum neurofilament light chain levels with increasing BMI could have to do with effects of blood volume and how the serum neurofilament light chain levels is distributed in the body,” he explained.
The findings suggest that interpretation of serum neurofilament light chain levels without accounting for BMI could result in false-negative or false-positive results, Dr. Sotirchos noted. “It will be important to further evaluate this observation in control populations and account for BMI in neurofilament light chain reference ranges.”
Dr. Sotirchos added that the 17% rate of elevated serum neurofilament light chain levels seen in people with MS in the study is likely an underestimate.
“This is a cross-sectional study and represents one sample per patient, so it is a snapshot in time,” he said. “With the nature of MS, we know that people’s levels fluctuate over time.” In addition, most patients were on disease-modifying therapy for MS, so serum neurofilament light chain elevations could have been suppressed.
Applying the findings to individual patients
Commenting on the findings, Jennifer Graves, MD, PhD, director of the neuroimmunology research program at the University of California, San Diego, said the study is an important addition to the ongoing evidence on serum neurofilament light chain in MS.
“The current presented research importantly addresses the gaps we have in understanding how best to apply serum filament light chain levels to individual patients and not just using them to assess group level means of outcome measures,” she said.
“The MS PATHS collaborative is looking at multiple factors (in addition to MS activity) that drive serum neurofilament light chain levels so meaningful and practical cutoffs for what’s abnormal can be created,” said Dr. Graves, who also directs the Rady Children’s Pediatric MS Clinic in San Diego.
Dr. Graves noted that the findings on BMI were unexpected. “Elevated BMI has been shown to be associated with greater brain atrophy and greater relapses and disability in MS participants, so to have an opposite effect with serum neurofilament light chain is interesting.
“My thoughts would be that obesity is somehow affecting measurable blood levels of this marker. I think it less likely BMI has a protective effect against neurodegeneration given the observations with other MS outcome measures,” she added.
Future research
In terms of future directions, Dr. Sotirchos noted that the researchers are following the group longitudinally to further assess changes in neurofilament light chain over time, and will be looking at associations with longitudinal, clinical, and radiologic outcomes.
The current research, meanwhile, offers important insights in terms of developing precision reference ranges, he noted.
“It appears that reference ranges may need to account for sex, race, BMI, and comorbid/lifestyle factors,” Dr. Sotirchos said, “in order to potentially improve the performance of serum neurofilament light chain as a biomarker in MS and other neurological diseases.”
The study received funding from Biogen and the MS PATHS network receives funding from Biogen. Dr. Sotirchos has served on scientific advisory boards for Alexion, Viela Bio, and Genentech, and has received speaker honoraria from Viela Bio and Biogen. Dr. Graves has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACTRIMS 2021