User login
Statins beneficial in elderly, guidelines should be strengthened
Contrary to historical evidence, two new studies show.
“By contrast with previous historical studies, our data show that LDL cholesterol is an important risk factor for myocardial infarction and atherosclerotic cardiovascular disease in a contemporary primary prevention cohort of individuals aged 70 to 100 years,” Borge Nordestgaard, MD, of the University of Copenhagen, and colleagues noted in the first of the two studies, published this week in the Lancet.
“By lowering LDL cholesterol in healthy individuals aged 70-100 years, the potential for preventing myocardial infarctions and atherosclerotic cardiovascular disease is huge, and at a substantially lower number needed to treat when compared with those aged 20-69 years,” they added.
“These findings support the concept of the cumulative burden of LDL cholesterol over one’s lifetime and the progressive increase in risk for atherosclerotic cardiovascular disease, including myocardial infarction, with age,” added Frederick J. Raal, PhD, and Farzahna Mohamed, MB BCh, of the University of the Witwatersrand, Johannesburg, South Africa, in an editorial published with both new studies in the Lancet (2020 Nov 10. doi: 10.1016/S0140-6736[20]32333-3).
The studies underscore the need for clinicians to consider continued risks associated with elevated LDL cholesterol in older age, they stressed, adding that statins are also beneficial for younger persons at risk to prevent conditions from worsening.
“The average age of patients in all the trials analyzed was older than 60 years, an age when atherosclerotic cardiovascular disease is already well established,” the editorialists wrote.
“Lipid-lowering therapy should be initiated at a younger age, preferably before age 40 years, in those at risk to delay the onset of atherosclerosis, rather than try to manage the condition once fully established or advanced,” they stressed.
No RCTs have included patients older than 70
For persons aged 40-75 years, elevated LDL cholesterol levels are a known risk factor for MI and atherosclerotic cardiovascular disease, and there is consensus in guidelines regarding treatment with statins.
However, the risk for people older than 70 is controversial. Some studies show little or no association between elevated LDL cholesterol levels and an increased risk for MI.
Contributing to the uncertainty is that few of the randomized, controlled trials that have investigated the question have included patients aged older than 70 years.
As a consequence, many practice guidelines have noted that the level of evidence in older patients is low, and some organizations have lowered the strength of recommendations regarding the treatment for older patients in comparison with younger patients.
Primary prevention: CV events increase with elevated LDL cholesterol in older age
Dr. Nordestgaard and colleagues studied data on 91,131 people living in Copenhagen who did not have atherosclerotic cardiovascular disease or diabetes at baseline and were not taking statins.
Of the participants, 10,592 were aged 70-79 years, and 3,188 participants were aged 80-100 years.
Over an average follow-up period of 7.7 years, 1,515 participants had a first MI, and 3,389 developed atherosclerotic cardiovascular disease.
In the primary-prevention cohort, after multivariate adjustment, the risk of having a heart attack per 1.0 mmol/L increase in LDL cholesterol was increased in the group overall (hazard ratio, 1.34). The increased risk was observed for all age groups, including those aged 80-100 years (HR, 1.28), 70-79 (HR, 1.25), 60-69 (HR, 1.29), 50-59 (HR, 1.28), and 20-49 (HR, 1.68).
Risk for atherosclerotic cardiovascular disease was also raised per 1.0 mmol/L increase in LDL cholesterol overall (HR, 1.16) and in all age groups, particularly those aged 70-100 years.
Greater elevations in LDL cholesterol (5.0 mmol/L or higher, indicative of possible familial hypercholesterolemia) were associated with a notably higher risk for heart attack after multivariate adjustment in people aged 80-100 (HR, 2.99). Risk was also higher among those aged 70-79 (HR, 1.82).
The highest incidence was in those older than 70. The rate was 8.5 heart attacks per 1,000 people per year among those aged 80-100 and 5.2 heart attacks per 1,000 in those aged 70-79. The rates were 2.5 per 1,000 among those 60-69, 1.8 for those aged 50-59, and 0.8 for those aged 20-49.
“The absolute risk [of cardiovascular events] is of course much higher in the elderly than those under the age of 75, but what was a surprise was how clear our results were on a relative risk scale, that the risk associated with elevated LDL [cholesterol] was as high in people aged 80-100 as the younger patients,” Dr. Nordestgaard said in an interview.
With regard to the benefits of cholesterol-lowering drugs, the study showed that the number needed to prevent one heart attack over 5 years was 80 among those aged 80-100; the number was 439 for people aged 50-59.
With regard to stronger statins, when moderate-intensity statins were used, the number needed to treat to prevent one cardiovascular disease event of any type dropped to 42 for patients aged 80-100. It was 88 for those aged 70-79, 164 for those aged 60-69, 345 for those aged 50-59, and 769 for those aged 20-49.
“The clinical significance of this is that it appears those in older age groups indeed benefit from cholesterol-lowering therapy,” Dr. Nordestgaard said. “I think many people have this idea that LDL [cholesterol] is not important over the age of about 70-75, but that’s not the case.”
“These robust findings are novel,” he and his colleagues stressed.
Despite these observational findings, the South African editorialists noted that “whether lipid-lowering therapy should be initiated for primary prevention in people aged 75 years or older is unclear,” owing to the host of risks and benefits that need to be balanced.
The findings of an ongoing randomized, placebo-controlled trial (STAREE) may answer this question, they wrote. It is investigating primary prevention in 18,000 older patients (≥70 years) who are being randomly assigned to receive atorvastatin 40 mg/d or placebo. The study is seeking to determine whether statin treatment extends the length of a disability-free life, which will be assessed on the basis of survival outside permanent residential care. Results are expected in 2022-2023.
Unequivocal reductions in events in elderly, comparable with younger patients
In the second study (Lancet. 2020 Nov 10. doi: 10.1016/S0140-6736[20]32332-1), Baris Gencer, MD, of Brigham and Women’s Hospital, Boston, =and colleagues evaluated the effects of statins and other cholesterol-lowering drugs, including ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors, in older versus younger patients.
The systematic review and meta-analysis of 29 randomized controlled trials, also published in the Lancet, were presented virtually as a poster as part of the 2020 American Heart Association scientific session. It included data on 244,090 patients, including 21,492 aged 75 years and older.
The meta-analysis included studies of cardiovascular outcomes of a guideline-recommended LDL cholesterol–lowering drug, with a median follow-up of at least 2 years and inclusion of data on patients aged 75 years and older.
The results showed that over a median follow-up of 2.2 to 6 years, statin use by older patients was associated with a relative risk reduction of major vascular events of 26% per 1 mmol/L reduction in LDL cholesterol (P = .0019), which was comparable with a risk reduction of 15% per 1 mmol/L reduction in LDL cholesterol for patients younger than 75 years (P = .37, compared with older patients).
Treatment of older patients with LDL cholesterol–lowering drugs was also associated with significantly improved outcomes in cardiovascular death (risk ratio, 0.85), MI (RR, 0.80), stroke (RR, 0.73), and coronary revascularization (RR, 0.80).
“We found an unequivocal reduction in the risk of major vascular events with both statin and nonstatin LDL cholesterol-lowering treatments, which was similar to that seen in younger patients,” the authors wrote.
“Cholesterol-lowering medications are affordable drugs that have reduced risk of heart disease for millions of people worldwide, but until now, their benefits for older people have remained less certain,” said lead author Marc Sabatine, MD, also of Brigham and Women’s Hospital, in a Lancet press release.
“Our analysis indicates that these therapies are as effective in reducing cardiovascular events and deaths in people aged 75 years and over as they are in younger people. We found no offsetting safety concerns, and together, these results should strengthen guideline recommendations for the use of cholesterol-lowering medications, including statin and nonstatin therapy, in elderly people.”
The editorialists agreed: “More than 80% of fatal cardiovascular events occur in individuals older than 65 years, and the incidence of cardiovascular events is increasing in those older than 80 years; therefore, the findings of Gencer and colleagues’ study should encourage the use of lipid-lowering therapy in older patients.”
The authors of the two studies have disclosed no relevant financial relationships. Dr. Raal has received research grants, honoraria, or consulting fees for advisory board membership, professional input, and lectures on lipid-lowering drug therapy from Amgen, Regeneron, Sanofi, Novartis, and the Medicines Company.
A version of this article originally appeared on Medscape.com.
Contrary to historical evidence, two new studies show.
“By contrast with previous historical studies, our data show that LDL cholesterol is an important risk factor for myocardial infarction and atherosclerotic cardiovascular disease in a contemporary primary prevention cohort of individuals aged 70 to 100 years,” Borge Nordestgaard, MD, of the University of Copenhagen, and colleagues noted in the first of the two studies, published this week in the Lancet.
“By lowering LDL cholesterol in healthy individuals aged 70-100 years, the potential for preventing myocardial infarctions and atherosclerotic cardiovascular disease is huge, and at a substantially lower number needed to treat when compared with those aged 20-69 years,” they added.
“These findings support the concept of the cumulative burden of LDL cholesterol over one’s lifetime and the progressive increase in risk for atherosclerotic cardiovascular disease, including myocardial infarction, with age,” added Frederick J. Raal, PhD, and Farzahna Mohamed, MB BCh, of the University of the Witwatersrand, Johannesburg, South Africa, in an editorial published with both new studies in the Lancet (2020 Nov 10. doi: 10.1016/S0140-6736[20]32333-3).
The studies underscore the need for clinicians to consider continued risks associated with elevated LDL cholesterol in older age, they stressed, adding that statins are also beneficial for younger persons at risk to prevent conditions from worsening.
“The average age of patients in all the trials analyzed was older than 60 years, an age when atherosclerotic cardiovascular disease is already well established,” the editorialists wrote.
“Lipid-lowering therapy should be initiated at a younger age, preferably before age 40 years, in those at risk to delay the onset of atherosclerosis, rather than try to manage the condition once fully established or advanced,” they stressed.
No RCTs have included patients older than 70
For persons aged 40-75 years, elevated LDL cholesterol levels are a known risk factor for MI and atherosclerotic cardiovascular disease, and there is consensus in guidelines regarding treatment with statins.
However, the risk for people older than 70 is controversial. Some studies show little or no association between elevated LDL cholesterol levels and an increased risk for MI.
Contributing to the uncertainty is that few of the randomized, controlled trials that have investigated the question have included patients aged older than 70 years.
As a consequence, many practice guidelines have noted that the level of evidence in older patients is low, and some organizations have lowered the strength of recommendations regarding the treatment for older patients in comparison with younger patients.
Primary prevention: CV events increase with elevated LDL cholesterol in older age
Dr. Nordestgaard and colleagues studied data on 91,131 people living in Copenhagen who did not have atherosclerotic cardiovascular disease or diabetes at baseline and were not taking statins.
Of the participants, 10,592 were aged 70-79 years, and 3,188 participants were aged 80-100 years.
Over an average follow-up period of 7.7 years, 1,515 participants had a first MI, and 3,389 developed atherosclerotic cardiovascular disease.
In the primary-prevention cohort, after multivariate adjustment, the risk of having a heart attack per 1.0 mmol/L increase in LDL cholesterol was increased in the group overall (hazard ratio, 1.34). The increased risk was observed for all age groups, including those aged 80-100 years (HR, 1.28), 70-79 (HR, 1.25), 60-69 (HR, 1.29), 50-59 (HR, 1.28), and 20-49 (HR, 1.68).
Risk for atherosclerotic cardiovascular disease was also raised per 1.0 mmol/L increase in LDL cholesterol overall (HR, 1.16) and in all age groups, particularly those aged 70-100 years.
Greater elevations in LDL cholesterol (5.0 mmol/L or higher, indicative of possible familial hypercholesterolemia) were associated with a notably higher risk for heart attack after multivariate adjustment in people aged 80-100 (HR, 2.99). Risk was also higher among those aged 70-79 (HR, 1.82).
The highest incidence was in those older than 70. The rate was 8.5 heart attacks per 1,000 people per year among those aged 80-100 and 5.2 heart attacks per 1,000 in those aged 70-79. The rates were 2.5 per 1,000 among those 60-69, 1.8 for those aged 50-59, and 0.8 for those aged 20-49.
“The absolute risk [of cardiovascular events] is of course much higher in the elderly than those under the age of 75, but what was a surprise was how clear our results were on a relative risk scale, that the risk associated with elevated LDL [cholesterol] was as high in people aged 80-100 as the younger patients,” Dr. Nordestgaard said in an interview.
With regard to the benefits of cholesterol-lowering drugs, the study showed that the number needed to prevent one heart attack over 5 years was 80 among those aged 80-100; the number was 439 for people aged 50-59.
With regard to stronger statins, when moderate-intensity statins were used, the number needed to treat to prevent one cardiovascular disease event of any type dropped to 42 for patients aged 80-100. It was 88 for those aged 70-79, 164 for those aged 60-69, 345 for those aged 50-59, and 769 for those aged 20-49.
“The clinical significance of this is that it appears those in older age groups indeed benefit from cholesterol-lowering therapy,” Dr. Nordestgaard said. “I think many people have this idea that LDL [cholesterol] is not important over the age of about 70-75, but that’s not the case.”
“These robust findings are novel,” he and his colleagues stressed.
Despite these observational findings, the South African editorialists noted that “whether lipid-lowering therapy should be initiated for primary prevention in people aged 75 years or older is unclear,” owing to the host of risks and benefits that need to be balanced.
The findings of an ongoing randomized, placebo-controlled trial (STAREE) may answer this question, they wrote. It is investigating primary prevention in 18,000 older patients (≥70 years) who are being randomly assigned to receive atorvastatin 40 mg/d or placebo. The study is seeking to determine whether statin treatment extends the length of a disability-free life, which will be assessed on the basis of survival outside permanent residential care. Results are expected in 2022-2023.
Unequivocal reductions in events in elderly, comparable with younger patients
In the second study (Lancet. 2020 Nov 10. doi: 10.1016/S0140-6736[20]32332-1), Baris Gencer, MD, of Brigham and Women’s Hospital, Boston, =and colleagues evaluated the effects of statins and other cholesterol-lowering drugs, including ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors, in older versus younger patients.
The systematic review and meta-analysis of 29 randomized controlled trials, also published in the Lancet, were presented virtually as a poster as part of the 2020 American Heart Association scientific session. It included data on 244,090 patients, including 21,492 aged 75 years and older.
The meta-analysis included studies of cardiovascular outcomes of a guideline-recommended LDL cholesterol–lowering drug, with a median follow-up of at least 2 years and inclusion of data on patients aged 75 years and older.
The results showed that over a median follow-up of 2.2 to 6 years, statin use by older patients was associated with a relative risk reduction of major vascular events of 26% per 1 mmol/L reduction in LDL cholesterol (P = .0019), which was comparable with a risk reduction of 15% per 1 mmol/L reduction in LDL cholesterol for patients younger than 75 years (P = .37, compared with older patients).
Treatment of older patients with LDL cholesterol–lowering drugs was also associated with significantly improved outcomes in cardiovascular death (risk ratio, 0.85), MI (RR, 0.80), stroke (RR, 0.73), and coronary revascularization (RR, 0.80).
“We found an unequivocal reduction in the risk of major vascular events with both statin and nonstatin LDL cholesterol-lowering treatments, which was similar to that seen in younger patients,” the authors wrote.
“Cholesterol-lowering medications are affordable drugs that have reduced risk of heart disease for millions of people worldwide, but until now, their benefits for older people have remained less certain,” said lead author Marc Sabatine, MD, also of Brigham and Women’s Hospital, in a Lancet press release.
“Our analysis indicates that these therapies are as effective in reducing cardiovascular events and deaths in people aged 75 years and over as they are in younger people. We found no offsetting safety concerns, and together, these results should strengthen guideline recommendations for the use of cholesterol-lowering medications, including statin and nonstatin therapy, in elderly people.”
The editorialists agreed: “More than 80% of fatal cardiovascular events occur in individuals older than 65 years, and the incidence of cardiovascular events is increasing in those older than 80 years; therefore, the findings of Gencer and colleagues’ study should encourage the use of lipid-lowering therapy in older patients.”
The authors of the two studies have disclosed no relevant financial relationships. Dr. Raal has received research grants, honoraria, or consulting fees for advisory board membership, professional input, and lectures on lipid-lowering drug therapy from Amgen, Regeneron, Sanofi, Novartis, and the Medicines Company.
A version of this article originally appeared on Medscape.com.
Contrary to historical evidence, two new studies show.
“By contrast with previous historical studies, our data show that LDL cholesterol is an important risk factor for myocardial infarction and atherosclerotic cardiovascular disease in a contemporary primary prevention cohort of individuals aged 70 to 100 years,” Borge Nordestgaard, MD, of the University of Copenhagen, and colleagues noted in the first of the two studies, published this week in the Lancet.
“By lowering LDL cholesterol in healthy individuals aged 70-100 years, the potential for preventing myocardial infarctions and atherosclerotic cardiovascular disease is huge, and at a substantially lower number needed to treat when compared with those aged 20-69 years,” they added.
“These findings support the concept of the cumulative burden of LDL cholesterol over one’s lifetime and the progressive increase in risk for atherosclerotic cardiovascular disease, including myocardial infarction, with age,” added Frederick J. Raal, PhD, and Farzahna Mohamed, MB BCh, of the University of the Witwatersrand, Johannesburg, South Africa, in an editorial published with both new studies in the Lancet (2020 Nov 10. doi: 10.1016/S0140-6736[20]32333-3).
The studies underscore the need for clinicians to consider continued risks associated with elevated LDL cholesterol in older age, they stressed, adding that statins are also beneficial for younger persons at risk to prevent conditions from worsening.
“The average age of patients in all the trials analyzed was older than 60 years, an age when atherosclerotic cardiovascular disease is already well established,” the editorialists wrote.
“Lipid-lowering therapy should be initiated at a younger age, preferably before age 40 years, in those at risk to delay the onset of atherosclerosis, rather than try to manage the condition once fully established or advanced,” they stressed.
No RCTs have included patients older than 70
For persons aged 40-75 years, elevated LDL cholesterol levels are a known risk factor for MI and atherosclerotic cardiovascular disease, and there is consensus in guidelines regarding treatment with statins.
However, the risk for people older than 70 is controversial. Some studies show little or no association between elevated LDL cholesterol levels and an increased risk for MI.
Contributing to the uncertainty is that few of the randomized, controlled trials that have investigated the question have included patients aged older than 70 years.
As a consequence, many practice guidelines have noted that the level of evidence in older patients is low, and some organizations have lowered the strength of recommendations regarding the treatment for older patients in comparison with younger patients.
Primary prevention: CV events increase with elevated LDL cholesterol in older age
Dr. Nordestgaard and colleagues studied data on 91,131 people living in Copenhagen who did not have atherosclerotic cardiovascular disease or diabetes at baseline and were not taking statins.
Of the participants, 10,592 were aged 70-79 years, and 3,188 participants were aged 80-100 years.
Over an average follow-up period of 7.7 years, 1,515 participants had a first MI, and 3,389 developed atherosclerotic cardiovascular disease.
In the primary-prevention cohort, after multivariate adjustment, the risk of having a heart attack per 1.0 mmol/L increase in LDL cholesterol was increased in the group overall (hazard ratio, 1.34). The increased risk was observed for all age groups, including those aged 80-100 years (HR, 1.28), 70-79 (HR, 1.25), 60-69 (HR, 1.29), 50-59 (HR, 1.28), and 20-49 (HR, 1.68).
Risk for atherosclerotic cardiovascular disease was also raised per 1.0 mmol/L increase in LDL cholesterol overall (HR, 1.16) and in all age groups, particularly those aged 70-100 years.
Greater elevations in LDL cholesterol (5.0 mmol/L or higher, indicative of possible familial hypercholesterolemia) were associated with a notably higher risk for heart attack after multivariate adjustment in people aged 80-100 (HR, 2.99). Risk was also higher among those aged 70-79 (HR, 1.82).
The highest incidence was in those older than 70. The rate was 8.5 heart attacks per 1,000 people per year among those aged 80-100 and 5.2 heart attacks per 1,000 in those aged 70-79. The rates were 2.5 per 1,000 among those 60-69, 1.8 for those aged 50-59, and 0.8 for those aged 20-49.
“The absolute risk [of cardiovascular events] is of course much higher in the elderly than those under the age of 75, but what was a surprise was how clear our results were on a relative risk scale, that the risk associated with elevated LDL [cholesterol] was as high in people aged 80-100 as the younger patients,” Dr. Nordestgaard said in an interview.
With regard to the benefits of cholesterol-lowering drugs, the study showed that the number needed to prevent one heart attack over 5 years was 80 among those aged 80-100; the number was 439 for people aged 50-59.
With regard to stronger statins, when moderate-intensity statins were used, the number needed to treat to prevent one cardiovascular disease event of any type dropped to 42 for patients aged 80-100. It was 88 for those aged 70-79, 164 for those aged 60-69, 345 for those aged 50-59, and 769 for those aged 20-49.
“The clinical significance of this is that it appears those in older age groups indeed benefit from cholesterol-lowering therapy,” Dr. Nordestgaard said. “I think many people have this idea that LDL [cholesterol] is not important over the age of about 70-75, but that’s not the case.”
“These robust findings are novel,” he and his colleagues stressed.
Despite these observational findings, the South African editorialists noted that “whether lipid-lowering therapy should be initiated for primary prevention in people aged 75 years or older is unclear,” owing to the host of risks and benefits that need to be balanced.
The findings of an ongoing randomized, placebo-controlled trial (STAREE) may answer this question, they wrote. It is investigating primary prevention in 18,000 older patients (≥70 years) who are being randomly assigned to receive atorvastatin 40 mg/d or placebo. The study is seeking to determine whether statin treatment extends the length of a disability-free life, which will be assessed on the basis of survival outside permanent residential care. Results are expected in 2022-2023.
Unequivocal reductions in events in elderly, comparable with younger patients
In the second study (Lancet. 2020 Nov 10. doi: 10.1016/S0140-6736[20]32332-1), Baris Gencer, MD, of Brigham and Women’s Hospital, Boston, =and colleagues evaluated the effects of statins and other cholesterol-lowering drugs, including ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors, in older versus younger patients.
The systematic review and meta-analysis of 29 randomized controlled trials, also published in the Lancet, were presented virtually as a poster as part of the 2020 American Heart Association scientific session. It included data on 244,090 patients, including 21,492 aged 75 years and older.
The meta-analysis included studies of cardiovascular outcomes of a guideline-recommended LDL cholesterol–lowering drug, with a median follow-up of at least 2 years and inclusion of data on patients aged 75 years and older.
The results showed that over a median follow-up of 2.2 to 6 years, statin use by older patients was associated with a relative risk reduction of major vascular events of 26% per 1 mmol/L reduction in LDL cholesterol (P = .0019), which was comparable with a risk reduction of 15% per 1 mmol/L reduction in LDL cholesterol for patients younger than 75 years (P = .37, compared with older patients).
Treatment of older patients with LDL cholesterol–lowering drugs was also associated with significantly improved outcomes in cardiovascular death (risk ratio, 0.85), MI (RR, 0.80), stroke (RR, 0.73), and coronary revascularization (RR, 0.80).
“We found an unequivocal reduction in the risk of major vascular events with both statin and nonstatin LDL cholesterol-lowering treatments, which was similar to that seen in younger patients,” the authors wrote.
“Cholesterol-lowering medications are affordable drugs that have reduced risk of heart disease for millions of people worldwide, but until now, their benefits for older people have remained less certain,” said lead author Marc Sabatine, MD, also of Brigham and Women’s Hospital, in a Lancet press release.
“Our analysis indicates that these therapies are as effective in reducing cardiovascular events and deaths in people aged 75 years and over as they are in younger people. We found no offsetting safety concerns, and together, these results should strengthen guideline recommendations for the use of cholesterol-lowering medications, including statin and nonstatin therapy, in elderly people.”
The editorialists agreed: “More than 80% of fatal cardiovascular events occur in individuals older than 65 years, and the incidence of cardiovascular events is increasing in those older than 80 years; therefore, the findings of Gencer and colleagues’ study should encourage the use of lipid-lowering therapy in older patients.”
The authors of the two studies have disclosed no relevant financial relationships. Dr. Raal has received research grants, honoraria, or consulting fees for advisory board membership, professional input, and lectures on lipid-lowering drug therapy from Amgen, Regeneron, Sanofi, Novartis, and the Medicines Company.
A version of this article originally appeared on Medscape.com.
Concussion linked to risk for dementia, Parkinson’s disease, and ADHD
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From Family Medicine and Community Health
Nearly one in five develop mental illness following COVID-19
One in five COVID-19 patients are diagnosed with a psychiatric disorder such as anxiety or depression within 3 months of testing positive for the virus, new research suggests.
“People have been worried that COVID-19 survivors will be at greater risk of psychiatric disorders, and our findings in a large and detailed study show this to be true,” principal investigator Paul Harrison, BM, DM, professor of psychiatry, University of Oxford, Oxford, United Kingdom, said in a statement.
Health services “need to be ready to provide care, especially since our results are likely to be underestimates of the actual number of cases,” said Harrison.
The study also showed that having a psychiatric disorder independently increases the risk of getting COVID-19 – a finding that’s in line with research published earlier this month.
“Having a psychiatric illness should be added to the list of risk factors for COVID-19,” study coauthor Maxime Taquet, PhD, University of Oxford, said in the release.
The study was published online Nov. 9 in The Lancet Psychiatry.
Double the risk
The investigators took advantage of the TriNetX analytics network, which captured deidentified data from electronic health records of a total of 69.8 million patients from 54 healthcare organizations in the United States.
Of those patients, 62,354 adults were diagnosed with COVID-19 between Jan. 20 and Aug. 1, 2020.
To assess the psychiatric sequelae of COVID-19, the investigators created propensity score–matched cohorts of patients who had received a diagnosis of other conditions that represented a range of common acute presentations.
In 14 to 90 days after being diagnosed with COVID-19, 5.8% of patients received a first recorded diagnosis of psychiatric illness. Among patients with health problems other than COVID, 2.5% to 3.4% of patients received a psychiatric diagnosis, the authors report. The risk was greatest for anxiety disorders, depression, and insomnia.
Older COVID-19 patients had a two- to threefold increased risk for a first dementia diagnosis, a finding that supports an earlier UK study.
Some of this excess risk could reflect misdiagnosed cases of delirium or transient cognitive impairment due to reversible cerebral events, the authors noted.
The study also revealed a bidirectional relationship between mental illness and COVID-19. Individuals with a psychiatric diagnosis were about 65% more likely to be diagnosed with COVID-19 in comparison with their counterparts who did not have mental illness, independently of known physical health risk factors for COVID-19.
“We did not anticipate that psychiatric history would be an independent risk factor for COVID-19. This finding appears robust, being observed in all age strata and in both sexes, and was substantial,” the authors write.
At present, “we don’t understand what the explanation is for the associations between COVID and mental illness. We are looking into this in more detail to try and understand better what subgroups are particularly vulnerable in this regard,” Harrison told Medscape Medical News.
“Ambitious” research
Commenting on the findings for Medscape Medical News, Roy H. Perlis, MD, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, said this is “an ambitious effort to understand the short-term consequences of COVID in terms of brain diseases.”
Perlis said he’s not particularly surprised by the increase in psychiatric diagnoses among COVID-19 patients.
“After COVID infection, people are more likely to get close medical follow-up than usual. They’re more likely to be accessing the healthcare system; after all, they’ve already had COVID, so they’re probably less fearful of seeing their doctor. But, that probably also means they’re more likely to get a new diagnosis of something like depression,” he said.
Dementia may be the clearest illustration of this, Perlis said. “It seems less likely that dementia develops a month after COVID; more likely, something that happens during the illness leads someone to be more likely to diagnose dementia later on,” he noted.
Perlis cautioned against being “unnecessarily alarmed” by the findings in this study.
“We know that rates of depression in the UK and the US, as in much of the world, are substantially elevated right now. Much of this is likely a consequence of the stress and disruption that accompanies the pandemic,” said Perlis.
The study was funded by the National Institute for Health Research. Harrison has disclosed no relevant financial relationships. One author is an employee of TriNetX. Perlis has received consulting fees for service on scientific advisory boards of Belle Artificial Intelligence, Burrage Capital, Genomind, Psy Therapeutics, Outermost Therapeutics, RID Ventures, and Takeda. He holds equity in Psy Therapeutics and Outermost Therapeutics.
This article first appeared on Medscape.com.
One in five COVID-19 patients are diagnosed with a psychiatric disorder such as anxiety or depression within 3 months of testing positive for the virus, new research suggests.
“People have been worried that COVID-19 survivors will be at greater risk of psychiatric disorders, and our findings in a large and detailed study show this to be true,” principal investigator Paul Harrison, BM, DM, professor of psychiatry, University of Oxford, Oxford, United Kingdom, said in a statement.
Health services “need to be ready to provide care, especially since our results are likely to be underestimates of the actual number of cases,” said Harrison.
The study also showed that having a psychiatric disorder independently increases the risk of getting COVID-19 – a finding that’s in line with research published earlier this month.
“Having a psychiatric illness should be added to the list of risk factors for COVID-19,” study coauthor Maxime Taquet, PhD, University of Oxford, said in the release.
The study was published online Nov. 9 in The Lancet Psychiatry.
Double the risk
The investigators took advantage of the TriNetX analytics network, which captured deidentified data from electronic health records of a total of 69.8 million patients from 54 healthcare organizations in the United States.
Of those patients, 62,354 adults were diagnosed with COVID-19 between Jan. 20 and Aug. 1, 2020.
To assess the psychiatric sequelae of COVID-19, the investigators created propensity score–matched cohorts of patients who had received a diagnosis of other conditions that represented a range of common acute presentations.
In 14 to 90 days after being diagnosed with COVID-19, 5.8% of patients received a first recorded diagnosis of psychiatric illness. Among patients with health problems other than COVID, 2.5% to 3.4% of patients received a psychiatric diagnosis, the authors report. The risk was greatest for anxiety disorders, depression, and insomnia.
Older COVID-19 patients had a two- to threefold increased risk for a first dementia diagnosis, a finding that supports an earlier UK study.
Some of this excess risk could reflect misdiagnosed cases of delirium or transient cognitive impairment due to reversible cerebral events, the authors noted.
The study also revealed a bidirectional relationship between mental illness and COVID-19. Individuals with a psychiatric diagnosis were about 65% more likely to be diagnosed with COVID-19 in comparison with their counterparts who did not have mental illness, independently of known physical health risk factors for COVID-19.
“We did not anticipate that psychiatric history would be an independent risk factor for COVID-19. This finding appears robust, being observed in all age strata and in both sexes, and was substantial,” the authors write.
At present, “we don’t understand what the explanation is for the associations between COVID and mental illness. We are looking into this in more detail to try and understand better what subgroups are particularly vulnerable in this regard,” Harrison told Medscape Medical News.
“Ambitious” research
Commenting on the findings for Medscape Medical News, Roy H. Perlis, MD, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, said this is “an ambitious effort to understand the short-term consequences of COVID in terms of brain diseases.”
Perlis said he’s not particularly surprised by the increase in psychiatric diagnoses among COVID-19 patients.
“After COVID infection, people are more likely to get close medical follow-up than usual. They’re more likely to be accessing the healthcare system; after all, they’ve already had COVID, so they’re probably less fearful of seeing their doctor. But, that probably also means they’re more likely to get a new diagnosis of something like depression,” he said.
Dementia may be the clearest illustration of this, Perlis said. “It seems less likely that dementia develops a month after COVID; more likely, something that happens during the illness leads someone to be more likely to diagnose dementia later on,” he noted.
Perlis cautioned against being “unnecessarily alarmed” by the findings in this study.
“We know that rates of depression in the UK and the US, as in much of the world, are substantially elevated right now. Much of this is likely a consequence of the stress and disruption that accompanies the pandemic,” said Perlis.
The study was funded by the National Institute for Health Research. Harrison has disclosed no relevant financial relationships. One author is an employee of TriNetX. Perlis has received consulting fees for service on scientific advisory boards of Belle Artificial Intelligence, Burrage Capital, Genomind, Psy Therapeutics, Outermost Therapeutics, RID Ventures, and Takeda. He holds equity in Psy Therapeutics and Outermost Therapeutics.
This article first appeared on Medscape.com.
One in five COVID-19 patients are diagnosed with a psychiatric disorder such as anxiety or depression within 3 months of testing positive for the virus, new research suggests.
“People have been worried that COVID-19 survivors will be at greater risk of psychiatric disorders, and our findings in a large and detailed study show this to be true,” principal investigator Paul Harrison, BM, DM, professor of psychiatry, University of Oxford, Oxford, United Kingdom, said in a statement.
Health services “need to be ready to provide care, especially since our results are likely to be underestimates of the actual number of cases,” said Harrison.
The study also showed that having a psychiatric disorder independently increases the risk of getting COVID-19 – a finding that’s in line with research published earlier this month.
“Having a psychiatric illness should be added to the list of risk factors for COVID-19,” study coauthor Maxime Taquet, PhD, University of Oxford, said in the release.
The study was published online Nov. 9 in The Lancet Psychiatry.
Double the risk
The investigators took advantage of the TriNetX analytics network, which captured deidentified data from electronic health records of a total of 69.8 million patients from 54 healthcare organizations in the United States.
Of those patients, 62,354 adults were diagnosed with COVID-19 between Jan. 20 and Aug. 1, 2020.
To assess the psychiatric sequelae of COVID-19, the investigators created propensity score–matched cohorts of patients who had received a diagnosis of other conditions that represented a range of common acute presentations.
In 14 to 90 days after being diagnosed with COVID-19, 5.8% of patients received a first recorded diagnosis of psychiatric illness. Among patients with health problems other than COVID, 2.5% to 3.4% of patients received a psychiatric diagnosis, the authors report. The risk was greatest for anxiety disorders, depression, and insomnia.
Older COVID-19 patients had a two- to threefold increased risk for a first dementia diagnosis, a finding that supports an earlier UK study.
Some of this excess risk could reflect misdiagnosed cases of delirium or transient cognitive impairment due to reversible cerebral events, the authors noted.
The study also revealed a bidirectional relationship between mental illness and COVID-19. Individuals with a psychiatric diagnosis were about 65% more likely to be diagnosed with COVID-19 in comparison with their counterparts who did not have mental illness, independently of known physical health risk factors for COVID-19.
“We did not anticipate that psychiatric history would be an independent risk factor for COVID-19. This finding appears robust, being observed in all age strata and in both sexes, and was substantial,” the authors write.
At present, “we don’t understand what the explanation is for the associations between COVID and mental illness. We are looking into this in more detail to try and understand better what subgroups are particularly vulnerable in this regard,” Harrison told Medscape Medical News.
“Ambitious” research
Commenting on the findings for Medscape Medical News, Roy H. Perlis, MD, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, said this is “an ambitious effort to understand the short-term consequences of COVID in terms of brain diseases.”
Perlis said he’s not particularly surprised by the increase in psychiatric diagnoses among COVID-19 patients.
“After COVID infection, people are more likely to get close medical follow-up than usual. They’re more likely to be accessing the healthcare system; after all, they’ve already had COVID, so they’re probably less fearful of seeing their doctor. But, that probably also means they’re more likely to get a new diagnosis of something like depression,” he said.
Dementia may be the clearest illustration of this, Perlis said. “It seems less likely that dementia develops a month after COVID; more likely, something that happens during the illness leads someone to be more likely to diagnose dementia later on,” he noted.
Perlis cautioned against being “unnecessarily alarmed” by the findings in this study.
“We know that rates of depression in the UK and the US, as in much of the world, are substantially elevated right now. Much of this is likely a consequence of the stress and disruption that accompanies the pandemic,” said Perlis.
The study was funded by the National Institute for Health Research. Harrison has disclosed no relevant financial relationships. One author is an employee of TriNetX. Perlis has received consulting fees for service on scientific advisory boards of Belle Artificial Intelligence, Burrage Capital, Genomind, Psy Therapeutics, Outermost Therapeutics, RID Ventures, and Takeda. He holds equity in Psy Therapeutics and Outermost Therapeutics.
This article first appeared on Medscape.com.
Late-onset epilepsy tied to a threefold increased dementia risk
Results of a retrospective analysis show that patients who develop epilepsy at age 67 or older have a threefold increased risk of subsequent dementia versus their counterparts without epilepsy.
“This is an exciting area, as we are finding that just as the risk of seizures is increased in neurodegenerative diseases, the risk of dementia is increased after late-onset epilepsy and seizures,” study investigator Emily L. Johnson, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview. “Several other cohort studies are finding similar results, including the Veterans’ Health Study and the Framingham Study,” she added.
The study was published online Oct. 23 in Neurology
Bidirectional relationship?
Previous research has established that dementia is a risk factor for epilepsy, but recent studies also suggest an increased risk of incident dementia among patients with adult-onset epilepsy. Several risk factors for late-onset epilepsy, including diabetes and hypertension, also are risk factors for dementia. However, the effect of late-onset epilepsy on dementia risk in patients with these comorbidities has not been clarified.
To investigate, the researchers examined data from the Atherosclerosis Risk in Communities (ARIC) study. Participants include Black and White men and women from four U.S. communities. Baseline visits in this longitudinal cohort study began between 1987 and 1989, and follow-up included seven additional visits and regular phone calls.
The investigators identified participants with late-onset epilepsy by searching for Medicare claims related to seizures or epilepsy filed between 1991 and 2015. Those with two or more such claims and age of onset of 67 years or greater were considered to have late-onset epilepsy. Participants with preexisting conditions such as brain tumors or multiple sclerosis were excluded.
ARIC participants who presented in person for visits 2, 4, 5, and 6 underwent cognitive testing with the Delayed Word Recall Test, the Digit Symbol Substitution Test, and the Word Fluency Test.
Testing at visits 5 and 6 also included other tests, such as the Mini-Mental State Examination, the Boston Naming test, and the Wechsler Memory Scale-III. Dr. Johnson and colleagues excluded data for visit 7 from the analysis because dementia adjudication was not yet complete.
The researchers identified participants with dementia using data from visits 5 and 6 and ascertained time of dementia onset through participant and informant interviews, phone calls, and hospital discharge data. Participants also were screened for mild cognitive impairment (MCI) at visits 5 and 6.
Data were analyzed using a Cox proportional hazards model and multinomial logistic regression. In subsequent analyses, researchers adjusted the data for age, sex, race, smoking status, alcohol use, hypertension, diabetes, body mass index (BMI), APOE4 status, and prevalent stroke.
The researchers found that of 9,033 study participants, 671 had late-onset epilepsy. The late-onset epilepsy group was older at baseline (56.5 vs. 55.1 years) and more likely to have hypertension (38.9% vs. 33.3%), diabetes (16.1% vs. 9.6%), and two alleles of APOE4 genotype (3.9% vs. 2.5%), compared with those without the disorder.
In all, 1,687 participants developed dementia during follow-up. The rate of incident dementia was 41.6% in participants with late-onset epilepsy and 16.8% in participants without late-onset epilepsy. The adjusted hazard ratio of subsequent dementia in participants with late-onset epilepsy versus those without the disorder was 3.05 (95% confidence interval, 2.65-3.51).
The median time to dementia ascertainment after late-onset epilepsy was 3.66 years.
Counterintuitive finding
The relationship between late-onset epilepsy and subsequent dementia was stronger in patients without stroke. The investigators offered a possible explanation for this counterintuitive finding. “We observed an interaction between [late-onset epilepsy] and stroke, with a lower (but still substantial) association between [late-onset epilepsy] and dementia in those with a history of stroke. This may be due to the known strong association between stroke and dementia, which may wash out the contributions of [late-onset epilepsy] to cognitive impairment,” the researchers wrote.
“There may also be under-capturing of dementia diagnoses among participants with stroke in the ascertainment from [Centers for Medicare & Medicaid Services] codes, as physicians may be reluctant to make a separate code for ‘dementia’ in those with cognitive impairment after stroke,” they added.
When the researchers restricted the analysis only to participants who attended visits 5 and 6 and had late-onset epilepsy ascertainment available, they found that the relative risk ratio for dementia at visit 6 was 2.90 (95% CI, 1.22-6.92; P = .009). The RRR for MCI was 0.97 (95% CI, 0.39-2.38; P = .803). The greater functional impairment in patients with late-onset epilepsy may explain the lack of a relationship between late-onset epilepsy and MCI.
“It will be important for neurologists to be aware of the possibility of cognitive impairment following late-onset epilepsy and to check in with patients and family members to see if there are concerns,” said Dr. Johnson.
“We should also be talking about the importance of lowering other risk factors for dementia by making sure cardiovascular risk factors are controlled and encouraging physical and cognitive activity,” she added.
The results require confirmation in a clinical population, the investigators noted. In addition, future research is necessary to clarify whether seizures directly increase the risk of dementia or whether shared neuropathology between epilepsy and dementia explains the risk.
“In the near future, I plan to enroll participants with late-onset epilepsy in an observational study to better understand factors that may contribute to cognitive change. Collaborations will be key as we seek to further understand what causes these changes and what could be done to prevent them,” Dr. Johnson added.
Strengths and weaknesses
In an accompanying editorial, W. Allen Hauser, MD, professor emeritus of neurology and epidemiology at Columbia University in New York, and colleagues noted that the findings support a bidirectional relationship between dementia and epilepsy, adding that accumulation of amyloid beta peptide is a plausible underlying pathophysiology that may explain this relationship.
Future research should clarify the effect of factors such as seizure type, seizure frequency, and age of onset on the risk of dementia among patients with epilepsy, the editorialists wrote. Such investigations could help elucidate the underlying mechanisms of these conditions and help to improve treatment, they added.
Commenting on the findings, Ilo Leppik, MD, professor of neurology and pharmacy at the University of Minnesota in Minneapolis described the research as “a very well-done study by qualified researchers in the field. … For the last century, medicine has unfortunately become compartmentalized by specialty and then subspecialty. The brain and disorders of the brain do not recognize these silos. … It is not a stretch of the known science to begin to understand that epilepsy and dementia have common anatomical and physiological underpinnings.”
The long period of prospectively gathering data and the measurement of cognitive function through various modalities are among the study’s great strengths, said Dr. Leppik. However, the study’s weakness is its reliance on Medicare claims data, which mainly would reflect convulsive seizures.
“What is missing is how many persons had subtle focal-unaware seizures that may not be identified unless a careful history is taken,” said Dr. Leppik. “Thus, this study likely underestimates the frequency of epilepsy.”
Neurologists who evaluate a person with early dementia should be on the lookout for a history of subtle seizures, said Dr. Leppik. Animal studies suggest treatment with levetiracetam or brivaracetam may slow the course of dementia, and a clinical study in participants with early dementia is underway.
“Treatment with an antiseizure drug may prove to be beneficial, especially if evidence for the presence of subtle epilepsy can be found,” Dr. Leppik added.
Greater collaboration between epileptologists and dementia specialists and larger studies of antiseizure drugs are necessary, he noted. “These studies can incorporate sophisticated structural and biochemical [analyses] to better identify the relationships between brain mechanisms that likely underlie both seizures and dementia. The ultimate promise is that early treatment of seizures may alter the course of dementia,” Dr. Leppik said.
The study by Dr. Johnson and colleagues was supported by a contract from the National Institute on Aging; ARIC from the National Heart, Lung, and Blood Institute; the National Institutes of Health; and the Department of Health & Human Services. The authors and Dr. Leppik have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Results of a retrospective analysis show that patients who develop epilepsy at age 67 or older have a threefold increased risk of subsequent dementia versus their counterparts without epilepsy.
“This is an exciting area, as we are finding that just as the risk of seizures is increased in neurodegenerative diseases, the risk of dementia is increased after late-onset epilepsy and seizures,” study investigator Emily L. Johnson, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview. “Several other cohort studies are finding similar results, including the Veterans’ Health Study and the Framingham Study,” she added.
The study was published online Oct. 23 in Neurology
Bidirectional relationship?
Previous research has established that dementia is a risk factor for epilepsy, but recent studies also suggest an increased risk of incident dementia among patients with adult-onset epilepsy. Several risk factors for late-onset epilepsy, including diabetes and hypertension, also are risk factors for dementia. However, the effect of late-onset epilepsy on dementia risk in patients with these comorbidities has not been clarified.
To investigate, the researchers examined data from the Atherosclerosis Risk in Communities (ARIC) study. Participants include Black and White men and women from four U.S. communities. Baseline visits in this longitudinal cohort study began between 1987 and 1989, and follow-up included seven additional visits and regular phone calls.
The investigators identified participants with late-onset epilepsy by searching for Medicare claims related to seizures or epilepsy filed between 1991 and 2015. Those with two or more such claims and age of onset of 67 years or greater were considered to have late-onset epilepsy. Participants with preexisting conditions such as brain tumors or multiple sclerosis were excluded.
ARIC participants who presented in person for visits 2, 4, 5, and 6 underwent cognitive testing with the Delayed Word Recall Test, the Digit Symbol Substitution Test, and the Word Fluency Test.
Testing at visits 5 and 6 also included other tests, such as the Mini-Mental State Examination, the Boston Naming test, and the Wechsler Memory Scale-III. Dr. Johnson and colleagues excluded data for visit 7 from the analysis because dementia adjudication was not yet complete.
The researchers identified participants with dementia using data from visits 5 and 6 and ascertained time of dementia onset through participant and informant interviews, phone calls, and hospital discharge data. Participants also were screened for mild cognitive impairment (MCI) at visits 5 and 6.
Data were analyzed using a Cox proportional hazards model and multinomial logistic regression. In subsequent analyses, researchers adjusted the data for age, sex, race, smoking status, alcohol use, hypertension, diabetes, body mass index (BMI), APOE4 status, and prevalent stroke.
The researchers found that of 9,033 study participants, 671 had late-onset epilepsy. The late-onset epilepsy group was older at baseline (56.5 vs. 55.1 years) and more likely to have hypertension (38.9% vs. 33.3%), diabetes (16.1% vs. 9.6%), and two alleles of APOE4 genotype (3.9% vs. 2.5%), compared with those without the disorder.
In all, 1,687 participants developed dementia during follow-up. The rate of incident dementia was 41.6% in participants with late-onset epilepsy and 16.8% in participants without late-onset epilepsy. The adjusted hazard ratio of subsequent dementia in participants with late-onset epilepsy versus those without the disorder was 3.05 (95% confidence interval, 2.65-3.51).
The median time to dementia ascertainment after late-onset epilepsy was 3.66 years.
Counterintuitive finding
The relationship between late-onset epilepsy and subsequent dementia was stronger in patients without stroke. The investigators offered a possible explanation for this counterintuitive finding. “We observed an interaction between [late-onset epilepsy] and stroke, with a lower (but still substantial) association between [late-onset epilepsy] and dementia in those with a history of stroke. This may be due to the known strong association between stroke and dementia, which may wash out the contributions of [late-onset epilepsy] to cognitive impairment,” the researchers wrote.
“There may also be under-capturing of dementia diagnoses among participants with stroke in the ascertainment from [Centers for Medicare & Medicaid Services] codes, as physicians may be reluctant to make a separate code for ‘dementia’ in those with cognitive impairment after stroke,” they added.
When the researchers restricted the analysis only to participants who attended visits 5 and 6 and had late-onset epilepsy ascertainment available, they found that the relative risk ratio for dementia at visit 6 was 2.90 (95% CI, 1.22-6.92; P = .009). The RRR for MCI was 0.97 (95% CI, 0.39-2.38; P = .803). The greater functional impairment in patients with late-onset epilepsy may explain the lack of a relationship between late-onset epilepsy and MCI.
“It will be important for neurologists to be aware of the possibility of cognitive impairment following late-onset epilepsy and to check in with patients and family members to see if there are concerns,” said Dr. Johnson.
“We should also be talking about the importance of lowering other risk factors for dementia by making sure cardiovascular risk factors are controlled and encouraging physical and cognitive activity,” she added.
The results require confirmation in a clinical population, the investigators noted. In addition, future research is necessary to clarify whether seizures directly increase the risk of dementia or whether shared neuropathology between epilepsy and dementia explains the risk.
“In the near future, I plan to enroll participants with late-onset epilepsy in an observational study to better understand factors that may contribute to cognitive change. Collaborations will be key as we seek to further understand what causes these changes and what could be done to prevent them,” Dr. Johnson added.
Strengths and weaknesses
In an accompanying editorial, W. Allen Hauser, MD, professor emeritus of neurology and epidemiology at Columbia University in New York, and colleagues noted that the findings support a bidirectional relationship between dementia and epilepsy, adding that accumulation of amyloid beta peptide is a plausible underlying pathophysiology that may explain this relationship.
Future research should clarify the effect of factors such as seizure type, seizure frequency, and age of onset on the risk of dementia among patients with epilepsy, the editorialists wrote. Such investigations could help elucidate the underlying mechanisms of these conditions and help to improve treatment, they added.
Commenting on the findings, Ilo Leppik, MD, professor of neurology and pharmacy at the University of Minnesota in Minneapolis described the research as “a very well-done study by qualified researchers in the field. … For the last century, medicine has unfortunately become compartmentalized by specialty and then subspecialty. The brain and disorders of the brain do not recognize these silos. … It is not a stretch of the known science to begin to understand that epilepsy and dementia have common anatomical and physiological underpinnings.”
The long period of prospectively gathering data and the measurement of cognitive function through various modalities are among the study’s great strengths, said Dr. Leppik. However, the study’s weakness is its reliance on Medicare claims data, which mainly would reflect convulsive seizures.
“What is missing is how many persons had subtle focal-unaware seizures that may not be identified unless a careful history is taken,” said Dr. Leppik. “Thus, this study likely underestimates the frequency of epilepsy.”
Neurologists who evaluate a person with early dementia should be on the lookout for a history of subtle seizures, said Dr. Leppik. Animal studies suggest treatment with levetiracetam or brivaracetam may slow the course of dementia, and a clinical study in participants with early dementia is underway.
“Treatment with an antiseizure drug may prove to be beneficial, especially if evidence for the presence of subtle epilepsy can be found,” Dr. Leppik added.
Greater collaboration between epileptologists and dementia specialists and larger studies of antiseizure drugs are necessary, he noted. “These studies can incorporate sophisticated structural and biochemical [analyses] to better identify the relationships between brain mechanisms that likely underlie both seizures and dementia. The ultimate promise is that early treatment of seizures may alter the course of dementia,” Dr. Leppik said.
The study by Dr. Johnson and colleagues was supported by a contract from the National Institute on Aging; ARIC from the National Heart, Lung, and Blood Institute; the National Institutes of Health; and the Department of Health & Human Services. The authors and Dr. Leppik have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Results of a retrospective analysis show that patients who develop epilepsy at age 67 or older have a threefold increased risk of subsequent dementia versus their counterparts without epilepsy.
“This is an exciting area, as we are finding that just as the risk of seizures is increased in neurodegenerative diseases, the risk of dementia is increased after late-onset epilepsy and seizures,” study investigator Emily L. Johnson, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview. “Several other cohort studies are finding similar results, including the Veterans’ Health Study and the Framingham Study,” she added.
The study was published online Oct. 23 in Neurology
Bidirectional relationship?
Previous research has established that dementia is a risk factor for epilepsy, but recent studies also suggest an increased risk of incident dementia among patients with adult-onset epilepsy. Several risk factors for late-onset epilepsy, including diabetes and hypertension, also are risk factors for dementia. However, the effect of late-onset epilepsy on dementia risk in patients with these comorbidities has not been clarified.
To investigate, the researchers examined data from the Atherosclerosis Risk in Communities (ARIC) study. Participants include Black and White men and women from four U.S. communities. Baseline visits in this longitudinal cohort study began between 1987 and 1989, and follow-up included seven additional visits and regular phone calls.
The investigators identified participants with late-onset epilepsy by searching for Medicare claims related to seizures or epilepsy filed between 1991 and 2015. Those with two or more such claims and age of onset of 67 years or greater were considered to have late-onset epilepsy. Participants with preexisting conditions such as brain tumors or multiple sclerosis were excluded.
ARIC participants who presented in person for visits 2, 4, 5, and 6 underwent cognitive testing with the Delayed Word Recall Test, the Digit Symbol Substitution Test, and the Word Fluency Test.
Testing at visits 5 and 6 also included other tests, such as the Mini-Mental State Examination, the Boston Naming test, and the Wechsler Memory Scale-III. Dr. Johnson and colleagues excluded data for visit 7 from the analysis because dementia adjudication was not yet complete.
The researchers identified participants with dementia using data from visits 5 and 6 and ascertained time of dementia onset through participant and informant interviews, phone calls, and hospital discharge data. Participants also were screened for mild cognitive impairment (MCI) at visits 5 and 6.
Data were analyzed using a Cox proportional hazards model and multinomial logistic regression. In subsequent analyses, researchers adjusted the data for age, sex, race, smoking status, alcohol use, hypertension, diabetes, body mass index (BMI), APOE4 status, and prevalent stroke.
The researchers found that of 9,033 study participants, 671 had late-onset epilepsy. The late-onset epilepsy group was older at baseline (56.5 vs. 55.1 years) and more likely to have hypertension (38.9% vs. 33.3%), diabetes (16.1% vs. 9.6%), and two alleles of APOE4 genotype (3.9% vs. 2.5%), compared with those without the disorder.
In all, 1,687 participants developed dementia during follow-up. The rate of incident dementia was 41.6% in participants with late-onset epilepsy and 16.8% in participants without late-onset epilepsy. The adjusted hazard ratio of subsequent dementia in participants with late-onset epilepsy versus those without the disorder was 3.05 (95% confidence interval, 2.65-3.51).
The median time to dementia ascertainment after late-onset epilepsy was 3.66 years.
Counterintuitive finding
The relationship between late-onset epilepsy and subsequent dementia was stronger in patients without stroke. The investigators offered a possible explanation for this counterintuitive finding. “We observed an interaction between [late-onset epilepsy] and stroke, with a lower (but still substantial) association between [late-onset epilepsy] and dementia in those with a history of stroke. This may be due to the known strong association between stroke and dementia, which may wash out the contributions of [late-onset epilepsy] to cognitive impairment,” the researchers wrote.
“There may also be under-capturing of dementia diagnoses among participants with stroke in the ascertainment from [Centers for Medicare & Medicaid Services] codes, as physicians may be reluctant to make a separate code for ‘dementia’ in those with cognitive impairment after stroke,” they added.
When the researchers restricted the analysis only to participants who attended visits 5 and 6 and had late-onset epilepsy ascertainment available, they found that the relative risk ratio for dementia at visit 6 was 2.90 (95% CI, 1.22-6.92; P = .009). The RRR for MCI was 0.97 (95% CI, 0.39-2.38; P = .803). The greater functional impairment in patients with late-onset epilepsy may explain the lack of a relationship between late-onset epilepsy and MCI.
“It will be important for neurologists to be aware of the possibility of cognitive impairment following late-onset epilepsy and to check in with patients and family members to see if there are concerns,” said Dr. Johnson.
“We should also be talking about the importance of lowering other risk factors for dementia by making sure cardiovascular risk factors are controlled and encouraging physical and cognitive activity,” she added.
The results require confirmation in a clinical population, the investigators noted. In addition, future research is necessary to clarify whether seizures directly increase the risk of dementia or whether shared neuropathology between epilepsy and dementia explains the risk.
“In the near future, I plan to enroll participants with late-onset epilepsy in an observational study to better understand factors that may contribute to cognitive change. Collaborations will be key as we seek to further understand what causes these changes and what could be done to prevent them,” Dr. Johnson added.
Strengths and weaknesses
In an accompanying editorial, W. Allen Hauser, MD, professor emeritus of neurology and epidemiology at Columbia University in New York, and colleagues noted that the findings support a bidirectional relationship between dementia and epilepsy, adding that accumulation of amyloid beta peptide is a plausible underlying pathophysiology that may explain this relationship.
Future research should clarify the effect of factors such as seizure type, seizure frequency, and age of onset on the risk of dementia among patients with epilepsy, the editorialists wrote. Such investigations could help elucidate the underlying mechanisms of these conditions and help to improve treatment, they added.
Commenting on the findings, Ilo Leppik, MD, professor of neurology and pharmacy at the University of Minnesota in Minneapolis described the research as “a very well-done study by qualified researchers in the field. … For the last century, medicine has unfortunately become compartmentalized by specialty and then subspecialty. The brain and disorders of the brain do not recognize these silos. … It is not a stretch of the known science to begin to understand that epilepsy and dementia have common anatomical and physiological underpinnings.”
The long period of prospectively gathering data and the measurement of cognitive function through various modalities are among the study’s great strengths, said Dr. Leppik. However, the study’s weakness is its reliance on Medicare claims data, which mainly would reflect convulsive seizures.
“What is missing is how many persons had subtle focal-unaware seizures that may not be identified unless a careful history is taken,” said Dr. Leppik. “Thus, this study likely underestimates the frequency of epilepsy.”
Neurologists who evaluate a person with early dementia should be on the lookout for a history of subtle seizures, said Dr. Leppik. Animal studies suggest treatment with levetiracetam or brivaracetam may slow the course of dementia, and a clinical study in participants with early dementia is underway.
“Treatment with an antiseizure drug may prove to be beneficial, especially if evidence for the presence of subtle epilepsy can be found,” Dr. Leppik added.
Greater collaboration between epileptologists and dementia specialists and larger studies of antiseizure drugs are necessary, he noted. “These studies can incorporate sophisticated structural and biochemical [analyses] to better identify the relationships between brain mechanisms that likely underlie both seizures and dementia. The ultimate promise is that early treatment of seizures may alter the course of dementia,” Dr. Leppik said.
The study by Dr. Johnson and colleagues was supported by a contract from the National Institute on Aging; ARIC from the National Heart, Lung, and Blood Institute; the National Institutes of Health; and the Department of Health & Human Services. The authors and Dr. Leppik have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM NEUROLOGY
Local hospitals still have a role in treating severe stroke
a new study has shown.
In the RACECAT trial, functional outcomes were similar for patients suspected of having a large-vessel occlusion stroke who were located in areas not currently served by a comprehensive stroke center, whether they were first taken to a local primary stroke center or whether they were transported over a longer distance to a comprehensive center.
“Under the particular conditions in our study where we had a very well-organized system, a ‘mothership’ transfer protocol for patients with suspected large-vessel occlusion has not proven superior over the ‘drip-and-ship’ protocol, but the opposite is also true,” lead investigator Marc Ribo, MD, concluded.
Dr. Ribo, assistant professor of neurology at Hospital Vall d’Hebron, Barcelona, presented the RACECAT results at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Dr. Ribo said in an interview that there is a feeling among the stroke community that patients with a suspected large-vessel occlusion should be transferred directly to a comprehensive stroke center capable of performing endovascular thrombectomy, even if there is a nearer, smaller primary stroke center where patients are usually taken first for thrombolysis.
“Many stroke neurologists believe we are losing time by sending patients with severe stroke to a local hospital and that we should skip this step, but this is controversial area,” he commented. “Our findings suggest that we should not automatically bypass local stroke centers.”
Dr. Ribo pointed out that the local centers performed very well in the study, with very fast “in/out” times for patients who were subsequently transferred for thrombectomy.
“On the basis of our results, we recommend that if a local stroke center can perform well like ours did – if they are within the time indicators for treating and transferring patients – then they should keep receiving these patients. But if they are not performing well in this regard, then they should probably be bypassed,” he commented.
The RACECAT trial was well received by stroke experts at an ESO-WSO 2020 press conference at which it was discussed.
Stefan Kiechl, MD, Medical University Innsbruck (Austria), described the trial as “outstanding,” adding: “It has addressed a very important question. It is a big achievement in stroke medicine.”
Patrik Michel, MD, Lausanne (Switzerland) University Hospital, said that “this is a very important and highly sophisticated trial in terms of design and execution. The message is that it doesn’t matter which pathway is used, but it is important to have a well-organized network with highly trained paramedics.”
RACECAT
The RACECAT trial was conducted in the Catalonia region of Spain. Twenty-seven hospitals participated, including 7 comprehensive stroke centers and 20 local stroke centers.
The trial included stroke patients with suspected large-vessel occlusion stroke, as determined on the basis of evaluation by paramedics using the criteria of a Rapid Arterial Occlusion Evaluation (RACE) scale score above 4 and on the basis of a call to a vascular neurologist. For inclusion in the study, patients had to be in a geographical area not served by a comprehensive stroke center and to have an estimated arrival time to a comprehensive center of less than 7 hours from symptom onset in order that thrombectomy would be possible.
Of 7,475 stroke code patients evaluated, 1,401 met the inclusion criteria and were randomly assigned to be transferred to a local hospital or to a comprehensive stroke center farther away.
Baseline characteristics were similar between the two groups. The patients had severe strokes with an average National Institutes of Health Stroke Scale score of 17. It was later confirmed that 46% of the patients enrolled in the study had a large-vessel occlusion stroke.
Results showed that time from symptom onset to hospital arrival was 142 minutes for those taken to a local center and 216 minutes for those taken to a comprehensive stroke center. Of those taken to a local hospital, 86% arrived within 4 hours of symptom onset and so were potential candidates for thrombolysis, compared with 76% of those taken to a comprehensive center.
Of the patients taken to a local hospital, 60% were given thrombolysis versus 43% of those taken immediately to a comprehensive center. On the other hand, 50% of patients who were taken directly to a comprehensive center underwent thrombectomy, compared with 40% who were first taken to a local center.
For patients who received thrombolysis, time to tissue plasminogen activator administration was 120 minutes for those treated at a local hospital versus 155 minutes for those taken directly to a comprehensive center.
For patients who received thrombectomy, time from symptom onset to groin puncture was 270 minutes if they were first taken to a local hospital and were then transferred, versus 214 minutes for those taken directly to the comprehensive center.
The primary efficacy endpoint was functional outcome using Modified Rankin Scale (mRS) shift analysis at 90 days for ischemic stroke patients. This showed a “completely flat” result, Dr. Ribo reported, with an adjusted hazard ratio of 1.029 for patients taken to a comprehensive center in comparison with those taken to a local center.
“There was absolutely no trend towards benefit in one group over the other,” he said.
What about hemorrhagic stroke?
The study also evaluated functional outcomes for the whole population enrolled. “If we make the decision just based on thrombectomy-eligible patients, we may harm the rest of the patients, so we did this study to look at the whole population of severe stroke patients,” Dr. Ribo said.
Of the study population, 25% of patients were found to have had a hemorrhagic stroke.
“The problem is, at the prehospital level, it is impossible to know if a patient is having a large-vessel occlusion ischemic stroke or a hemorrhagic stroke,” Dr. Ribo explained. “We have to make a decision for the whole population, and while a longer transport time to get to a comprehensive stroke center might help a patient with a large-vessel occlusion ischemic stroke, it might not be so appropriate for patients with a hemorrhagic stroke who need to have their blood pressure stabilized as soon as possible.”
For the whole population, the mRS shift analysis at 90 days was also neutral, with an aHR of 0.965.
When considering only patients with hemorrhagic stroke, the adjusted hazard ratio for the mRS shift analysis at 90 days was 1.216, which was still nonsignificant (95% confidence interval, 0.864-1.709). This included a nonsignificant increase in mortality among those taken directly to a comprehensive center.
“If we had better tools for a certain diagnosis in the field, then we could consider taking large-vessel occlusion ischemic stroke patients to a comprehensive center and hemorrhagic stroke patients to the local stroke center, but so far, we don’t have this option apart from a few places using mobile stroke units with CT scanners,” Dr. Ribo noted.
Transfer times to comprehensive centers in the study ranged from 30 minutes to 2.5 hours. “There might well be a difference in outcomes for short and long transfers, and we may be able to offer different transfer protocols in these different situations, and we are looking at that, but the study was only stopped in June, and we haven’t had a chance to analyze those results yet,” Dr. Ribo added.
Complications during transport occurred in 0.5% of those taken to a local hospital and in 1% of those taken directly to a comprehensive center. “We were concerned about complications with longer transfers, but these numbers are quite low. Intubations were very low – just one patient taken to a local center, versus three or four in the longer transfer group,” he added.
For both local and comprehensive centers, treatment times were impressive in the study. For local hospitals, the average in/out time was just 60 minutes for patients who went to a comprehensive center; for patients receiving thrombolysis, the average door to needle time was around 30 minutes.
Time to thrombectomy in the comprehensive center for patients transferred from a local hospital was also very fast, with an average door to groin puncture time of less than 40 minutes. “This shows we have a very well-oiled system,” Dr. Ribo said.
“There is always going to be a balance between a quicker time to thrombolysis by taking a patient to the closest hospital but a quicker time to thrombectomy if patients are taken straight to the comprehensive center,” he concluded. “But in our system, where we are achieving fast treatment and transfer times, our results show that patients had timely access to reperfusion therapies regardless of transfer protocol, and under these circumstances, it is fine for the emergency services to take stroke patients to the closest stroke center.”
Results applicable elsewhere?
During the discussion at an ESO-WSO 2020 press conference, other experts pointed out that the Catalonia group is a leader in this field, being the pioneers of the RACE score used in this study for paramedics to identify suspected large-vessel occlusions. This led to questions about the applicability of the results.
“The performance by paramedics was very good using the RACE scale, and the performance times were very impressive. Are these results applicable elsewhere?” Dr. Kiechl asked.
Dr. Ribo said the combination of the RACE score and a call with a vascular neurologist was of “great value” in identifying appropriate patients. Half of the patients selected in this way for the trial were confirmed to have a large-vessel occlusion. “That is a good result,” he added.
He noted that the performance of the local hospitals improved dramatically during the study. “They had an incentive to work on their times. They could have lost most of their stroke patients if their results came out worse. We told them they had an opportunity to show that they have a role in treating these patients, and they took that opportunity.”
Dr. Ribo said there were lessons here for those involved in acute stroke care. “When creating stroke transfer policies in local networks, the performances of individual centers need to be taken into account. If primary stroke centers are motivated and can work in a well-coordinated way and perform to within the recommended times, then they can keep receiving stroke code patients. This should be possible in most developed countries.”
Noting that the in/out time of 60 minutes at local hospitals was “very impressive,” Dr. Kiechl asked how such fast times were achieved.
Dr. Ribo responded that, to a great extent, this was because of ambulance staff. “We have trained the paramedics to anticipate a second transfer after delivering the patient to the local hospital so they can prepare for this rather than waiting for a second call.”
Dr. Ribo pointed out that there were other advantages in taking patients to local centers first. “For those that do not need to be transferred on, they will be closer to relatives. It is very difficult for the family if the patient is hundreds of miles away. And there may be a cost advantage. We did look at costs, but haven’t got that data yet.”
He said: “If local stroke centers do not treat so many stroke code patients, they will lose their expertise, and that will be detrimental to the remaining patients who are taken there. We want to try to maintain a good standard of stroke care across a decent spread of hospitals—not just a couple of major comprehensive centers,” he added.
Commenting on the study, Jesse Dawson, MD, University of Glasgow, who was chair of the plenary session at which the study was presented, said: “RACECAT is very interesting but needs a lot of thought to dissect. My takeaway is that we know that time to reperfusion is key, and we need to get these times as low as possible, but we don’t need to chase a particular care pathway. Thus, if your country/geography suits ‘drip and ship’ better, this is acceptable. If direct to endovascular is possible or you are close to such a center, then this is ideal. But within those paradigms, be as fast as possible.”
He added that results of the subgroups with regard to transfer time will be helpful.
The RACECAT study was funded by Fundacio Ictus Malaltia Vascular.
A version of this article originally appeared on Medscape.com.
a new study has shown.
In the RACECAT trial, functional outcomes were similar for patients suspected of having a large-vessel occlusion stroke who were located in areas not currently served by a comprehensive stroke center, whether they were first taken to a local primary stroke center or whether they were transported over a longer distance to a comprehensive center.
“Under the particular conditions in our study where we had a very well-organized system, a ‘mothership’ transfer protocol for patients with suspected large-vessel occlusion has not proven superior over the ‘drip-and-ship’ protocol, but the opposite is also true,” lead investigator Marc Ribo, MD, concluded.
Dr. Ribo, assistant professor of neurology at Hospital Vall d’Hebron, Barcelona, presented the RACECAT results at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Dr. Ribo said in an interview that there is a feeling among the stroke community that patients with a suspected large-vessel occlusion should be transferred directly to a comprehensive stroke center capable of performing endovascular thrombectomy, even if there is a nearer, smaller primary stroke center where patients are usually taken first for thrombolysis.
“Many stroke neurologists believe we are losing time by sending patients with severe stroke to a local hospital and that we should skip this step, but this is controversial area,” he commented. “Our findings suggest that we should not automatically bypass local stroke centers.”
Dr. Ribo pointed out that the local centers performed very well in the study, with very fast “in/out” times for patients who were subsequently transferred for thrombectomy.
“On the basis of our results, we recommend that if a local stroke center can perform well like ours did – if they are within the time indicators for treating and transferring patients – then they should keep receiving these patients. But if they are not performing well in this regard, then they should probably be bypassed,” he commented.
The RACECAT trial was well received by stroke experts at an ESO-WSO 2020 press conference at which it was discussed.
Stefan Kiechl, MD, Medical University Innsbruck (Austria), described the trial as “outstanding,” adding: “It has addressed a very important question. It is a big achievement in stroke medicine.”
Patrik Michel, MD, Lausanne (Switzerland) University Hospital, said that “this is a very important and highly sophisticated trial in terms of design and execution. The message is that it doesn’t matter which pathway is used, but it is important to have a well-organized network with highly trained paramedics.”
RACECAT
The RACECAT trial was conducted in the Catalonia region of Spain. Twenty-seven hospitals participated, including 7 comprehensive stroke centers and 20 local stroke centers.
The trial included stroke patients with suspected large-vessel occlusion stroke, as determined on the basis of evaluation by paramedics using the criteria of a Rapid Arterial Occlusion Evaluation (RACE) scale score above 4 and on the basis of a call to a vascular neurologist. For inclusion in the study, patients had to be in a geographical area not served by a comprehensive stroke center and to have an estimated arrival time to a comprehensive center of less than 7 hours from symptom onset in order that thrombectomy would be possible.
Of 7,475 stroke code patients evaluated, 1,401 met the inclusion criteria and were randomly assigned to be transferred to a local hospital or to a comprehensive stroke center farther away.
Baseline characteristics were similar between the two groups. The patients had severe strokes with an average National Institutes of Health Stroke Scale score of 17. It was later confirmed that 46% of the patients enrolled in the study had a large-vessel occlusion stroke.
Results showed that time from symptom onset to hospital arrival was 142 minutes for those taken to a local center and 216 minutes for those taken to a comprehensive stroke center. Of those taken to a local hospital, 86% arrived within 4 hours of symptom onset and so were potential candidates for thrombolysis, compared with 76% of those taken to a comprehensive center.
Of the patients taken to a local hospital, 60% were given thrombolysis versus 43% of those taken immediately to a comprehensive center. On the other hand, 50% of patients who were taken directly to a comprehensive center underwent thrombectomy, compared with 40% who were first taken to a local center.
For patients who received thrombolysis, time to tissue plasminogen activator administration was 120 minutes for those treated at a local hospital versus 155 minutes for those taken directly to a comprehensive center.
For patients who received thrombectomy, time from symptom onset to groin puncture was 270 minutes if they were first taken to a local hospital and were then transferred, versus 214 minutes for those taken directly to the comprehensive center.
The primary efficacy endpoint was functional outcome using Modified Rankin Scale (mRS) shift analysis at 90 days for ischemic stroke patients. This showed a “completely flat” result, Dr. Ribo reported, with an adjusted hazard ratio of 1.029 for patients taken to a comprehensive center in comparison with those taken to a local center.
“There was absolutely no trend towards benefit in one group over the other,” he said.
What about hemorrhagic stroke?
The study also evaluated functional outcomes for the whole population enrolled. “If we make the decision just based on thrombectomy-eligible patients, we may harm the rest of the patients, so we did this study to look at the whole population of severe stroke patients,” Dr. Ribo said.
Of the study population, 25% of patients were found to have had a hemorrhagic stroke.
“The problem is, at the prehospital level, it is impossible to know if a patient is having a large-vessel occlusion ischemic stroke or a hemorrhagic stroke,” Dr. Ribo explained. “We have to make a decision for the whole population, and while a longer transport time to get to a comprehensive stroke center might help a patient with a large-vessel occlusion ischemic stroke, it might not be so appropriate for patients with a hemorrhagic stroke who need to have their blood pressure stabilized as soon as possible.”
For the whole population, the mRS shift analysis at 90 days was also neutral, with an aHR of 0.965.
When considering only patients with hemorrhagic stroke, the adjusted hazard ratio for the mRS shift analysis at 90 days was 1.216, which was still nonsignificant (95% confidence interval, 0.864-1.709). This included a nonsignificant increase in mortality among those taken directly to a comprehensive center.
“If we had better tools for a certain diagnosis in the field, then we could consider taking large-vessel occlusion ischemic stroke patients to a comprehensive center and hemorrhagic stroke patients to the local stroke center, but so far, we don’t have this option apart from a few places using mobile stroke units with CT scanners,” Dr. Ribo noted.
Transfer times to comprehensive centers in the study ranged from 30 minutes to 2.5 hours. “There might well be a difference in outcomes for short and long transfers, and we may be able to offer different transfer protocols in these different situations, and we are looking at that, but the study was only stopped in June, and we haven’t had a chance to analyze those results yet,” Dr. Ribo added.
Complications during transport occurred in 0.5% of those taken to a local hospital and in 1% of those taken directly to a comprehensive center. “We were concerned about complications with longer transfers, but these numbers are quite low. Intubations were very low – just one patient taken to a local center, versus three or four in the longer transfer group,” he added.
For both local and comprehensive centers, treatment times were impressive in the study. For local hospitals, the average in/out time was just 60 minutes for patients who went to a comprehensive center; for patients receiving thrombolysis, the average door to needle time was around 30 minutes.
Time to thrombectomy in the comprehensive center for patients transferred from a local hospital was also very fast, with an average door to groin puncture time of less than 40 minutes. “This shows we have a very well-oiled system,” Dr. Ribo said.
“There is always going to be a balance between a quicker time to thrombolysis by taking a patient to the closest hospital but a quicker time to thrombectomy if patients are taken straight to the comprehensive center,” he concluded. “But in our system, where we are achieving fast treatment and transfer times, our results show that patients had timely access to reperfusion therapies regardless of transfer protocol, and under these circumstances, it is fine for the emergency services to take stroke patients to the closest stroke center.”
Results applicable elsewhere?
During the discussion at an ESO-WSO 2020 press conference, other experts pointed out that the Catalonia group is a leader in this field, being the pioneers of the RACE score used in this study for paramedics to identify suspected large-vessel occlusions. This led to questions about the applicability of the results.
“The performance by paramedics was very good using the RACE scale, and the performance times were very impressive. Are these results applicable elsewhere?” Dr. Kiechl asked.
Dr. Ribo said the combination of the RACE score and a call with a vascular neurologist was of “great value” in identifying appropriate patients. Half of the patients selected in this way for the trial were confirmed to have a large-vessel occlusion. “That is a good result,” he added.
He noted that the performance of the local hospitals improved dramatically during the study. “They had an incentive to work on their times. They could have lost most of their stroke patients if their results came out worse. We told them they had an opportunity to show that they have a role in treating these patients, and they took that opportunity.”
Dr. Ribo said there were lessons here for those involved in acute stroke care. “When creating stroke transfer policies in local networks, the performances of individual centers need to be taken into account. If primary stroke centers are motivated and can work in a well-coordinated way and perform to within the recommended times, then they can keep receiving stroke code patients. This should be possible in most developed countries.”
Noting that the in/out time of 60 minutes at local hospitals was “very impressive,” Dr. Kiechl asked how such fast times were achieved.
Dr. Ribo responded that, to a great extent, this was because of ambulance staff. “We have trained the paramedics to anticipate a second transfer after delivering the patient to the local hospital so they can prepare for this rather than waiting for a second call.”
Dr. Ribo pointed out that there were other advantages in taking patients to local centers first. “For those that do not need to be transferred on, they will be closer to relatives. It is very difficult for the family if the patient is hundreds of miles away. And there may be a cost advantage. We did look at costs, but haven’t got that data yet.”
He said: “If local stroke centers do not treat so many stroke code patients, they will lose their expertise, and that will be detrimental to the remaining patients who are taken there. We want to try to maintain a good standard of stroke care across a decent spread of hospitals—not just a couple of major comprehensive centers,” he added.
Commenting on the study, Jesse Dawson, MD, University of Glasgow, who was chair of the plenary session at which the study was presented, said: “RACECAT is very interesting but needs a lot of thought to dissect. My takeaway is that we know that time to reperfusion is key, and we need to get these times as low as possible, but we don’t need to chase a particular care pathway. Thus, if your country/geography suits ‘drip and ship’ better, this is acceptable. If direct to endovascular is possible or you are close to such a center, then this is ideal. But within those paradigms, be as fast as possible.”
He added that results of the subgroups with regard to transfer time will be helpful.
The RACECAT study was funded by Fundacio Ictus Malaltia Vascular.
A version of this article originally appeared on Medscape.com.
a new study has shown.
In the RACECAT trial, functional outcomes were similar for patients suspected of having a large-vessel occlusion stroke who were located in areas not currently served by a comprehensive stroke center, whether they were first taken to a local primary stroke center or whether they were transported over a longer distance to a comprehensive center.
“Under the particular conditions in our study where we had a very well-organized system, a ‘mothership’ transfer protocol for patients with suspected large-vessel occlusion has not proven superior over the ‘drip-and-ship’ protocol, but the opposite is also true,” lead investigator Marc Ribo, MD, concluded.
Dr. Ribo, assistant professor of neurology at Hospital Vall d’Hebron, Barcelona, presented the RACECAT results at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Dr. Ribo said in an interview that there is a feeling among the stroke community that patients with a suspected large-vessel occlusion should be transferred directly to a comprehensive stroke center capable of performing endovascular thrombectomy, even if there is a nearer, smaller primary stroke center where patients are usually taken first for thrombolysis.
“Many stroke neurologists believe we are losing time by sending patients with severe stroke to a local hospital and that we should skip this step, but this is controversial area,” he commented. “Our findings suggest that we should not automatically bypass local stroke centers.”
Dr. Ribo pointed out that the local centers performed very well in the study, with very fast “in/out” times for patients who were subsequently transferred for thrombectomy.
“On the basis of our results, we recommend that if a local stroke center can perform well like ours did – if they are within the time indicators for treating and transferring patients – then they should keep receiving these patients. But if they are not performing well in this regard, then they should probably be bypassed,” he commented.
The RACECAT trial was well received by stroke experts at an ESO-WSO 2020 press conference at which it was discussed.
Stefan Kiechl, MD, Medical University Innsbruck (Austria), described the trial as “outstanding,” adding: “It has addressed a very important question. It is a big achievement in stroke medicine.”
Patrik Michel, MD, Lausanne (Switzerland) University Hospital, said that “this is a very important and highly sophisticated trial in terms of design and execution. The message is that it doesn’t matter which pathway is used, but it is important to have a well-organized network with highly trained paramedics.”
RACECAT
The RACECAT trial was conducted in the Catalonia region of Spain. Twenty-seven hospitals participated, including 7 comprehensive stroke centers and 20 local stroke centers.
The trial included stroke patients with suspected large-vessel occlusion stroke, as determined on the basis of evaluation by paramedics using the criteria of a Rapid Arterial Occlusion Evaluation (RACE) scale score above 4 and on the basis of a call to a vascular neurologist. For inclusion in the study, patients had to be in a geographical area not served by a comprehensive stroke center and to have an estimated arrival time to a comprehensive center of less than 7 hours from symptom onset in order that thrombectomy would be possible.
Of 7,475 stroke code patients evaluated, 1,401 met the inclusion criteria and were randomly assigned to be transferred to a local hospital or to a comprehensive stroke center farther away.
Baseline characteristics were similar between the two groups. The patients had severe strokes with an average National Institutes of Health Stroke Scale score of 17. It was later confirmed that 46% of the patients enrolled in the study had a large-vessel occlusion stroke.
Results showed that time from symptom onset to hospital arrival was 142 minutes for those taken to a local center and 216 minutes for those taken to a comprehensive stroke center. Of those taken to a local hospital, 86% arrived within 4 hours of symptom onset and so were potential candidates for thrombolysis, compared with 76% of those taken to a comprehensive center.
Of the patients taken to a local hospital, 60% were given thrombolysis versus 43% of those taken immediately to a comprehensive center. On the other hand, 50% of patients who were taken directly to a comprehensive center underwent thrombectomy, compared with 40% who were first taken to a local center.
For patients who received thrombolysis, time to tissue plasminogen activator administration was 120 minutes for those treated at a local hospital versus 155 minutes for those taken directly to a comprehensive center.
For patients who received thrombectomy, time from symptom onset to groin puncture was 270 minutes if they were first taken to a local hospital and were then transferred, versus 214 minutes for those taken directly to the comprehensive center.
The primary efficacy endpoint was functional outcome using Modified Rankin Scale (mRS) shift analysis at 90 days for ischemic stroke patients. This showed a “completely flat” result, Dr. Ribo reported, with an adjusted hazard ratio of 1.029 for patients taken to a comprehensive center in comparison with those taken to a local center.
“There was absolutely no trend towards benefit in one group over the other,” he said.
What about hemorrhagic stroke?
The study also evaluated functional outcomes for the whole population enrolled. “If we make the decision just based on thrombectomy-eligible patients, we may harm the rest of the patients, so we did this study to look at the whole population of severe stroke patients,” Dr. Ribo said.
Of the study population, 25% of patients were found to have had a hemorrhagic stroke.
“The problem is, at the prehospital level, it is impossible to know if a patient is having a large-vessel occlusion ischemic stroke or a hemorrhagic stroke,” Dr. Ribo explained. “We have to make a decision for the whole population, and while a longer transport time to get to a comprehensive stroke center might help a patient with a large-vessel occlusion ischemic stroke, it might not be so appropriate for patients with a hemorrhagic stroke who need to have their blood pressure stabilized as soon as possible.”
For the whole population, the mRS shift analysis at 90 days was also neutral, with an aHR of 0.965.
When considering only patients with hemorrhagic stroke, the adjusted hazard ratio for the mRS shift analysis at 90 days was 1.216, which was still nonsignificant (95% confidence interval, 0.864-1.709). This included a nonsignificant increase in mortality among those taken directly to a comprehensive center.
“If we had better tools for a certain diagnosis in the field, then we could consider taking large-vessel occlusion ischemic stroke patients to a comprehensive center and hemorrhagic stroke patients to the local stroke center, but so far, we don’t have this option apart from a few places using mobile stroke units with CT scanners,” Dr. Ribo noted.
Transfer times to comprehensive centers in the study ranged from 30 minutes to 2.5 hours. “There might well be a difference in outcomes for short and long transfers, and we may be able to offer different transfer protocols in these different situations, and we are looking at that, but the study was only stopped in June, and we haven’t had a chance to analyze those results yet,” Dr. Ribo added.
Complications during transport occurred in 0.5% of those taken to a local hospital and in 1% of those taken directly to a comprehensive center. “We were concerned about complications with longer transfers, but these numbers are quite low. Intubations were very low – just one patient taken to a local center, versus three or four in the longer transfer group,” he added.
For both local and comprehensive centers, treatment times were impressive in the study. For local hospitals, the average in/out time was just 60 minutes for patients who went to a comprehensive center; for patients receiving thrombolysis, the average door to needle time was around 30 minutes.
Time to thrombectomy in the comprehensive center for patients transferred from a local hospital was also very fast, with an average door to groin puncture time of less than 40 minutes. “This shows we have a very well-oiled system,” Dr. Ribo said.
“There is always going to be a balance between a quicker time to thrombolysis by taking a patient to the closest hospital but a quicker time to thrombectomy if patients are taken straight to the comprehensive center,” he concluded. “But in our system, where we are achieving fast treatment and transfer times, our results show that patients had timely access to reperfusion therapies regardless of transfer protocol, and under these circumstances, it is fine for the emergency services to take stroke patients to the closest stroke center.”
Results applicable elsewhere?
During the discussion at an ESO-WSO 2020 press conference, other experts pointed out that the Catalonia group is a leader in this field, being the pioneers of the RACE score used in this study for paramedics to identify suspected large-vessel occlusions. This led to questions about the applicability of the results.
“The performance by paramedics was very good using the RACE scale, and the performance times were very impressive. Are these results applicable elsewhere?” Dr. Kiechl asked.
Dr. Ribo said the combination of the RACE score and a call with a vascular neurologist was of “great value” in identifying appropriate patients. Half of the patients selected in this way for the trial were confirmed to have a large-vessel occlusion. “That is a good result,” he added.
He noted that the performance of the local hospitals improved dramatically during the study. “They had an incentive to work on their times. They could have lost most of their stroke patients if their results came out worse. We told them they had an opportunity to show that they have a role in treating these patients, and they took that opportunity.”
Dr. Ribo said there were lessons here for those involved in acute stroke care. “When creating stroke transfer policies in local networks, the performances of individual centers need to be taken into account. If primary stroke centers are motivated and can work in a well-coordinated way and perform to within the recommended times, then they can keep receiving stroke code patients. This should be possible in most developed countries.”
Noting that the in/out time of 60 minutes at local hospitals was “very impressive,” Dr. Kiechl asked how such fast times were achieved.
Dr. Ribo responded that, to a great extent, this was because of ambulance staff. “We have trained the paramedics to anticipate a second transfer after delivering the patient to the local hospital so they can prepare for this rather than waiting for a second call.”
Dr. Ribo pointed out that there were other advantages in taking patients to local centers first. “For those that do not need to be transferred on, they will be closer to relatives. It is very difficult for the family if the patient is hundreds of miles away. And there may be a cost advantage. We did look at costs, but haven’t got that data yet.”
He said: “If local stroke centers do not treat so many stroke code patients, they will lose their expertise, and that will be detrimental to the remaining patients who are taken there. We want to try to maintain a good standard of stroke care across a decent spread of hospitals—not just a couple of major comprehensive centers,” he added.
Commenting on the study, Jesse Dawson, MD, University of Glasgow, who was chair of the plenary session at which the study was presented, said: “RACECAT is very interesting but needs a lot of thought to dissect. My takeaway is that we know that time to reperfusion is key, and we need to get these times as low as possible, but we don’t need to chase a particular care pathway. Thus, if your country/geography suits ‘drip and ship’ better, this is acceptable. If direct to endovascular is possible or you are close to such a center, then this is ideal. But within those paradigms, be as fast as possible.”
He added that results of the subgroups with regard to transfer time will be helpful.
The RACECAT study was funded by Fundacio Ictus Malaltia Vascular.
A version of this article originally appeared on Medscape.com.
FROM ESO-WSO 2020
What imaging can disclose about suspected stroke and its Tx
Stroke ranks second behind heart disease as the leading cause of mortality worldwide, accounting for 1 of every 19 deaths,1 and remains a serious cause of morbidity. Best practices in stroke diagnosis and management can seem elusive to front-line clinicians, for 2 reasons: the rate of proliferation and nuance in stroke medicine and the fact that the typical scope of primary care practice exists apart from much of the diagnostic tools and management schema provided in stroke centers.2 In this article, we describe and update the diagnosis of stroke and review imaging modalities, their nuances, and their application in practice.
Diagnosis of acute stroke
Acute stroke is diagnosed upon observation of new neurologic deficits and congruent neuroimaging. Some updated definitions favor a silent form of cerebral ischemia manifested by imaging pathology only; this form is not discussed in this article. Although there are several characteristically distinct stroke syndromes, there is no way to clinically distinguish ischemic pathology from hemorrhagic pathology.
Some common symptoms that should prompt evaluation for stroke are part of the American Stroke Association FAST mnemonic designed to promote public health awareness3-5:
f ace droopinga rm weaknesss peech difficultyt ime to call 911.
Other commonly reported stroke symptoms include unilateral weakness or numbness, confusion, word-finding difficulty, visual problems, difficulty ambulating, dizziness, loss of balance or coordination, and thunderclap headache. A stroke should also be considered in the presence of any new focal neurologic deficit.3,4
Stroke patients should be triaged by emergency medical services using a stroke screening scale, such as BE-FAST5 (a modification of FAST that adds balance and eye assessments); the Los Angeles Prehospital Stroke Screen (LAPSS)6,7; the Rapid Arterial oCclusion Evaluation (RACE)8; and the Cincinnati Prehospital Stroke Severity Scale (CP-SSS)9,10 (see “Stroke screening scales for early identification and triage"). Studies have not found that any single prehospital stroke scale is superior to the others for reliably predicting large-vessel occlusion; therefore, prehospital assessment is typically based on practice patterns in a given locale.11 A patient (or family member or caregiver) who seeks your care for stroke symptoms should be told to call 911 and get emergency transport to a health care facility that can capably administer intravenous (IV) thrombolysis.a
SIDEBAR
Stroke screening scales for early identification and triage
National Institutes of Health Stroke Scale
www.stroke.nih.gov/resources/scale.htm
FAST
www.stroke.org/en/help-and-support/resource-library/fast-materials
BE-FAST
www.ahajournals.org/doi/10.1161/STROKEAHA.116.015169
Los Angeles Prehospital Stroke Screen (LAPSS)
http://stroke.ucla.edu/workfiles/prehospital-screen.pdf
Rapid Artery Occlusion Evaluation (RACE)
www.mdcalc.com/rapid-arterial-occlusion-evaluation-race-scale-stroke
Cincinnati Prehospital Stroke Severity Scale (CP-SSS)
https://www.mdcalc.com/cincinnati-prehospital-stroke-severity-scale-cp-sss
First responders should elicit “last-known-normal” time; this critical information can aid in diagnosis and drive therapeutic options, especially if patients are unaccompanied at time of transport to a higher echelon of care. A point-of-care blood glucose test should be performed by emergency medical staff, with dextrose administered for a level < 45 mg/dL. Establishing IV access for fluids, medications, and contrast can be considered if it does not delay transport. A 12-lead electrocardiogram can also be considered, again, as long as it does not delay transport to a facility capable of providing definitive therapy. Notification by emergency services staff before arrival and transport of the patient to such a facility is the essential element of prehospital care, and should be prioritized above ancillary testing beyond the stroke assessment.14
Guidelines recommend use of the National Institutes of Health Stroke Scale (NIHSS; www.stroke.nih.gov/resources/scale.htm) for clinical evaluation upon arrival at the ED.15 Although no scale has been identified that can reliably predict large-vessel occlusion amenable to endovascular therapy (EVT), no other score has been found to outperform the NIHSS in achieving meaningful patient outcomes.16 Furthermore, NIHSS has been validated to track clinical changes in response to therapy, is widely utilized, and is free.
Continue to: A criticism of the NIHSS...
A criticism of the NIHSS is its bias toward left-hemispheric ischemic pathology.17 NIHSS includes 11 questions on a scale of 0 to 42; typically, a score < 4 is associated with a higher chance of a positive clinical outcome.18 There is no minimum or maximum NIHSS score that precludes treatment with thrombolysis or EVT.
Other commonly used scores in acute stroke include disability assessments. The modified Rankin scale, which is used most often, features a score of 0 (symptom-free) to 6 (death). A modified Rankin scale score of 0 or 1 is considered an indication of a favorable outcome after stroke.19 Note that these functional scores are not always part of an acute assessment but can be done early in the clinical course to gauge the response to treatment, and are collected for stroke-center certification.
Imaging modalities
Imaging is recommended within 20 minutes of arrival in the ED in a stroke patient who might be a candidate for thrombolysis or thrombectomy.3 There, imaging modalities commonly performed are noncontrast-enhanced head computed tomography (NCHCT); computed tomography (CT) angiography, with or without perfusion; and diffusion-weighted magnetic resonance imaging (MRI).20,21 In addition, more highly specialized imaging modalities are available for the evaluation of the stroke patient in specific, often limited, circumstances. All these modalities are described below and compared in the TABLE,20,21 using the ACR Appropriateness Criteria (of the American College of Radiology),21 which are guidelines for appropriate imaging of stroke, based on a clinical complaint. Separate recommendations and appraisals are offered by the most recent American Heart Association/American Stroke Association (AHA/ASA) guideline.3
NCHCT. This study should be performed within 20 minutes after arrival at the ED because it provides rapid assessment of intracerebral hemorrhage, can effectively corroborate the diagnosis of some stroke mimickers, and identifies some candidates for EVT or thrombolysis3,21,22 (typically, the decision to proceed with EVT is based on adjunct imaging studies discussed in a bit). Evaluation for intracerebral hemorrhage is required prior to administering thrombolysis. Ischemic changes can be seen with variable specificity and sensitivity on NCHCT, depending on how much time has passed since the original insult. In all historical trials, CT was the only imaging modality used in the diagnosis of acute ischemic stroke (AIS) that suggested benefit from IV thrombolysis.23-25
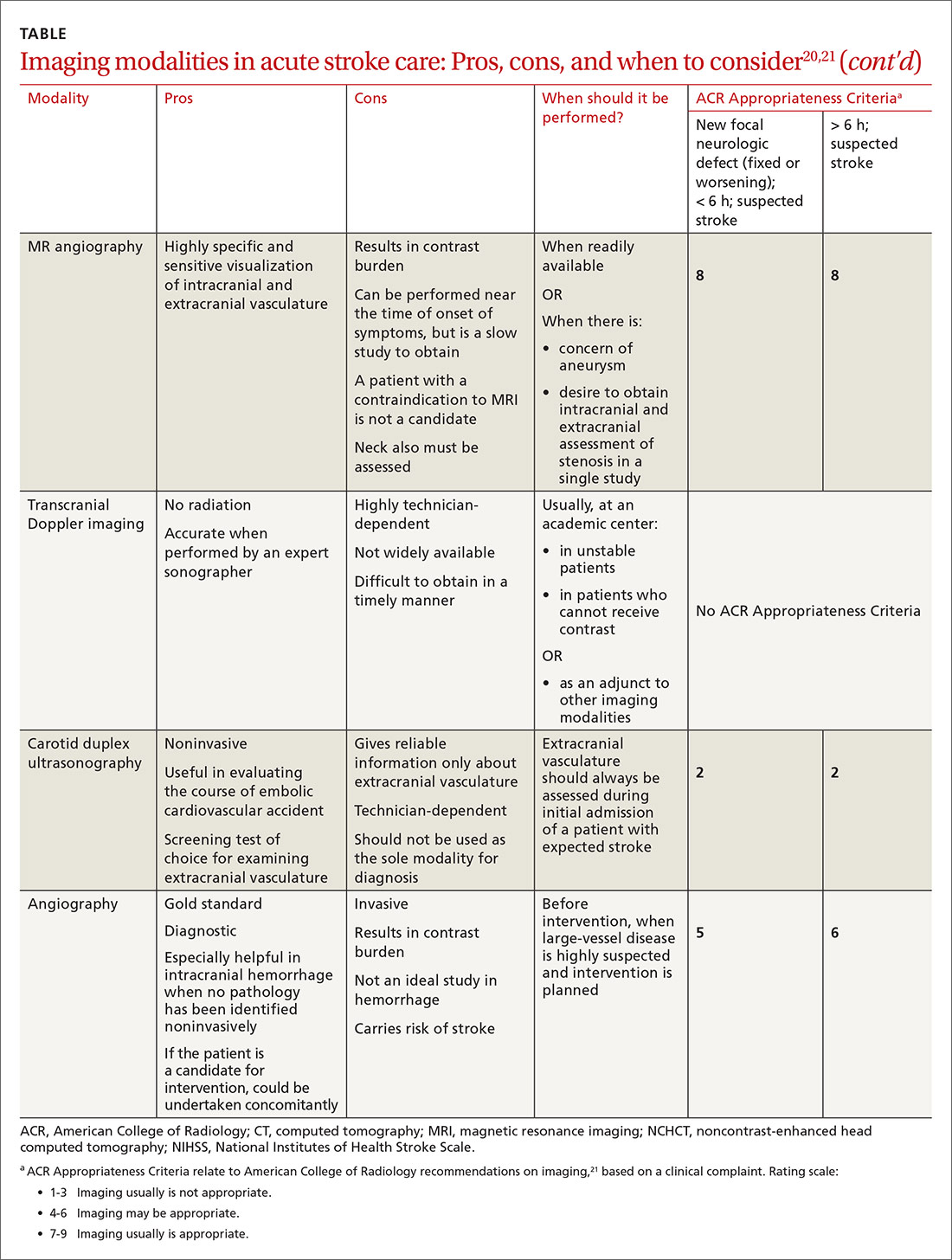
Acute, subacute, and chronic changes can be seen on NCHCT, although the modality has limited sensitivity for identifying AIS (ie, approximately 75% within 6 hours after the original insult):
- Acute findings on NCHCT include intracellular edema, which causes loss of the gray matter–white matter interface and effacement of the cortical sulci. This occurs as a result of increased cellular uptake of water in response to ischemia and cell death, resulting in a decreased density of tissue (hypoattenuation) in affected areas.
- Subacute changes appear in the 2- to 5-day window, including vasogenic edema with greater mass effect, hypoattenuation, and well-defined margins.3,20,21
- Chronic vascular findings on NCHCT include loss of brain tissue and hypoattenuation.
Continue to: NCHCT is typically performed...
NCHCT is typically performed in advance of other adjunct imaging modalities.3,20,21 Baseline NCHCT can be performed on patients with advanced kidney disease and those who have an indwelling metallic device.
CT angiography is performed with timed contrast, providing a 3-dimensional representation of the cerebral vasculature; the entire intracranial and extracranial vasculature, including the aortic arch, can be mapped in approximately 60 seconds. CT angiography is sensitive in identifying areas of stenosis > 50% and identifies clinically significant areas of stenosis up to approximately 90% of the time.26 For this reason, it is particularly helpful in identifying candidates for an interventional strategy beyond pharmacotherapeutic thrombolysis. In addition, CT angiography can visualize aneurysmal dilation and dissection, and help with the planning of interventions—specifically, the confident administration of thrombolysis or more specific planning for target lesions and EVT.
It also can help identify a host of vascular phenomena, such as arteriovenous malformations, Moyamoya disease (progressive arterial blockage within the basal ganglia and compensatory microvascularization), and some vasculopathies.20,27 In intracranial hemorrhage, CT with angiography can help evaluate for structural malformations and identify patients at risk of hematoma expansion.22
CT perfusion. Many stroke centers will perform a CT perfusion study,28 which encompasses as many as 3 different CT sequences:
- NCHCT
- vertex-to-arch angiography with contrast bolus
- administration of contrast and capture of a dynamic sequence through 1 or 2 slabs of tissue, allowing for the generation of maps of cerebral blood flow (CBF), mean transit time (MTT), and cerebral blood volume (CBV) of the entire cerebral vasculature.
The interplay of these 3 sequences drives characterization of lesions (ie, CBF = CBV/MTT). An infarct is characterized by low CBF, low CBV, and elevated MTT. In penumbral tissue, MTT is elevated but CBF is slightly decreased and CBV is normal or increased. Using CT perfusion, areas throughout the ischemic penumbra can be surveyed for favorable interventional characteristics.20,29
Continue to: A CT perfusion study adds...
A CT perfusion study adds at least 60 seconds to NCHCT. This modality can be useful in planning interventions and for stratifying appropriateness of reperfusion strategies in strokes of unknown duration.3,30 CT perfusion can be performed on any multidetector CT scan but (1) requires specialized software and expertise to interpret and (2) subjects the patient to a significant radiation dose, which, if incorrectly administered, can be considerably higher than intended.20,26,27
Diffusion-weighted MRI. This is the most sensitive study for demonstrating early ischemic changes; however, limitations include lack of availability, contraindication in patients with metallic indwelling implants, and duration of the study—although, at some stroke centers, diffusion-weighted MRI can be performed in ≤ 10 minutes.
MRI and NCHCT have comparable sensitivity in detecting intracranial hemorrhage. MRI is likely more sensitive in identifying areas of microhemorrhage: In diffusion-weighted MRI, the sensitivity of stroke detection increases to > 95%.31 The modality relies on the comparable movement of water through damaged vs normal neuronal tissue. Diffusion-weighted MRI does not require administration of concomitant contrast, which can be a benefit in patients who are allergic to gadolinium-based contrast agents or have advanced kidney disease that precludes the use of contrast. It typically does not result in adequate characterization of extracranial vasculature.
Other MRI modalities. These MRI extensions include magnetic resonance (MR) perfusion and MR angiography. Whereas diffusion-weighted MRI (discussed above) offers the most rapid and sensitive evaluation for ischemia, fluid-attenuated inversion recovery (FLAIR) imaging has been utilized as a comparator to isolated diffusion-weighted MRI to help determine stroke duration. FLAIR signal positivity typically occurs 6 to 24 hours after the initial insult but is negative in stroke that occurred < 3 hours earlier.32
MRI is limited, in terms of availability and increased study duration, especially when it comes to timely administration of thrombolysis. A benefit of this modality is less radiation and, as noted, superior sensitivity for ischemia. Diffusion-weighted MRI combined with MR perfusion analysis can help isolate areas of the ischemic penumbra. MR perfusion is performed for a similar reason as CT perfusion, although logistical execution across those modalities is significantly different. Considerations for choosing MR perfusion or CT perfusion should be made on an individual basis and based on available local resources and accepted local practice patterns.26
Continue to: In the subacute setting...
In the subacute setting, MR perfusion and MR angiography of the head and the neck are often performed to identify stenosis, dissection, and more subtle mimickers of cerebrovascular accident not ascertained on initial CT evaluation. These studies are typically performed well outside the window for thrombolysis or intervention.26 No guidelines specifically direct or recommend this practice pattern. The superior sensitivity and cerebral blood flow mapping of MR perfusion and MR angiography might be useful for validating a suspected diagnosis of ischemic stroke and providing phenotypic information about AIS events.
Transcranial Doppler imaging relies on bony windows to assess intracranial vascular flow, velocity, direction, and reactivity. This information can be utilized to diagnose stenosis or occlusion. This modality is principally used to evaluate for stenosis in the anterior circulation (sensitivity, 70%-90%; specificity, 90%-95%).20 Evaluation of the basilar, vertebral, and internal carotid arteries is less accurate (sensitivity, 55%-80%).20 Transcranial Doppler imaging is also used to assess for cerebral vasospasm after subarachnoid hemorrhage, monitor sickle cell disease patients’ overall risk for ischemic stroke, and augment thrombolysis. It is limited by the availability of an expert technician, and therefore is typically reserved for unstable patients or those who cannot receive contrast.20
Carotid duplex ultrasonography. A dynamic study such as duplex ultrasonography can be strongly considered for flow imaging of the extracranial carotids to evaluate for stenosis. Indications for carotid stenting or endarterectomy include 50% to 79% occlusion of the carotid artery on the same side as a recent transient ischemic attack or AIS. Carotid stenosis > 80% warrants consideration for intervention independent of a recent cerebrovascular accident. Interventions are typically performed 2 to 14 days after stroke.33 Although this study is of limited utility in the hyperacute setting, it is recommended within 24 hours after nondisabling stroke in the carotid territory, when (1) the patient is otherwise a candidate for a surgical or procedural intervention to address the stenosis and (2) none of the aforementioned studies that focus on neck vasculature have been performed.
Conventional (digital subtraction) angiography is the gold standard for mapping cerebrovascular disease because it is dynamic and highly accurate. It is, however, typically limited by the number of required personnel, its invasive nature, and the requirement for IV contrast. This study is performed during intra-arterial intervention techniques, including stent retrieval and intra-arterial thrombolysis.26
Impact of imaging on treatment
Imaging helps determine the cause and some characteristics of stroke, both of which can help determine therapy. Strokes can be broadly subcategorized as hemorrhagic or ischemic; recent studies suggest that 87% are ischemic.34 Knowledge of the historic details of the event, the patient (eg, known atrial fibrillation, anticoagulant use, history of falls), and findings on imaging can contribute to determine the cause of AIS, and can facilitate communication and consultation between the primary care physician and inpatient teams.35
Continue to: Best practices for stroke treatment...
Best practices for stroke treatment are based on the cause of the event.3 To identify the likely cause, the aforementioned characteristics are incorporated into one of the scoring systems, which seek to clarify either the cause or the phenotypic appearance of the AIS, which helps direct further testing and treatment. (The ASCOD36 and TOAST37 classification schemes are commonly used phenotypic and causative classifications, respectively.) Several (not all) of the broad phenotypic imaging patterns, with myriad clinical manifestations, are reviewed below. They include:
- Embolic stroke, which, classically, involves end circulation and therefore has cortical involvement. Typically, these originate from the heart or large extracranial arteries, and higher rates of atrial fibrillation and hypercoagulable states are implicated.
- Thrombotic stroke, which, typically, is from large vessels or small vessels, and occurs as a result of atherosclerosis. These strokes are more common at the origins or bifurcations of vessels. Symptoms of thrombotic stroke classically wax and wane slightly more frequently. Lacunar strokes are typically from thrombotic causes, although there are rare episodes of an embolic source contributing to a lacunar stroke syndrome.38
There is evidence for using MRI discrepancies between diffusion-weighted and FLAIR imaging to time AIS findings in so-called wake-up strokes.39 The rationale is that strokes < 4.5 hours old can be identified because they would have abnormal diffusion imaging components but normal findings with FLAIR. When these criteria were utilized in considering whether to treat with thrombolysis, there was a statistically significant improvement in 90-day modified Rankin scale (odds ratio = 1.61; 95% confidence interval, 1.09-2.36), but also an increased probability of death and intracerebral hemorrhage.39
A recent multicenter, randomized, open-label trial, with blinded outcomes assessment, showcased the efficacy of thrombectomy as an adjunct when ischemic brain territory was identified without frank infarction, as ascertained by CT perfusion within the anterior circulation. This trial showed that thrombectomy could be performed as long as 16 hours after the patient was last well-appearing and still result in an improved outcome with favorable imaging characteristics (on the modified Rankin scale, an ordinal score of 4 with medical therapy and an ordinal score of 3 with EVT [odds ratio = 2.77; 95% confidence interval, 1.63-4.70]).29 A 2018 multicenter, prospective, randomized trial with blinded assessment of endpoints extended this idea, demonstrating that, when there was mismatch of the clinical deficit (ie, high NIHSS score) and infarct volume (measured on diffusion-weighted MRI or CT perfusion), thrombectomy as late as 24 hours after the patient was last known to be well was beneficial for lesions in the anterior circulation—specifically, the intracranial internal carotid artery or the proximal middle cerebral artery.40
a Whether local emergency departments (EDs) should be bypassed in favor of a specialized stroke center is the subject of debate. The 2019 American Heart Association/American Stroke Association guidelines note the AHA’s Mission: Lifeline Stroke EMS algorithm, which bypasses the nearest ED in feared cases of large-vessel occlusion if travel to a comprehensive stroke center can be accomplished within 30 minutes of arrival at the scene. This is based on expert consensus.3,12,13
CORRESPONDENCE
Brian Ford, MD, 4301 Jones Bridge Road, Bethesda, MD; [email protected].
1. Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67-e492.
2. Darves B. Collaboration key to post-stroke follow-up. ACP Internist. October 2009. https://acpinternist.org/archives/2009/10/stroke.htm. Accessed September 22, 2020.
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50e344-e418.
4. Sacco RL, Kasner SE, Broderick JP, et al; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
5. Aroor S, Singh R, Goldstein LB. BE-FAST (Balance, Eyes, Face, Arm, Speech, Time): Reducing the proportion of strokes missed using the FAST mnemonic. 2017;48:479-481.
6. Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS). Stroke. 2000;31:71-76.
7. Llanes JN, Kidwell CS, Starkman S, et al. The Los Angeles Motor Scale (LAMS): a new measure to characterize stroke severity in the field. Prehosp Emerg Care. 2004;8:46-50.
8. Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014;45:87-91.
9. Katz BS, McMullan JT, Sucharew H, et al. Design and validation of a prehospital scale to predict stroke severity: Cincinnati Prehospital Stroke Severity Scale. Stroke. 2015;466:1508-1512.
10. Kummer BR, et al. External validation of the Cincinnati Prehospital Stroke Severity Scale. J Stroke Cerebrovasc Dis. 2016;25:1270-1274.
11. Beume L-A, Hieber M, Kaller CP, et al. Large vessel occlusion in acute stroke. Stroke. 2018;49:2323-2329.
12. Man S, Zhao X, Uchino K, et al. Comparison of acute ischemic stroke care and outcomes between comprehensive stroke centers and primary stroke centers in the United States. Circ Cardiovasc Qual Outcomes. 2018;11:e004512.
13. American Heart Association (Mission: Lifeline—Stroke). Emergency medical services acute stroke routing. 2020. www.heart.org/-/media/files/professional/quality-improvement/mission-lifeline/2_25_2020/ds15698-qi-ems-algorithm_update-2142020.pdf?la=en. Accessed October 8, 2020.
14. Glober NK, Sporer KA, Guluma KZ, et al. Acute stroke: current evidence-based recommendations for prehospital care. West J Emerg Med. 2016;17:104-128.
15. NIH stroke scale. Bethesda, MD: National Institute of Neurological Disorders and Stroke, National Institutes of Health. www.stroke.nih.gov/resources/scale.htm. Accessed October 10, 2020.
16. Smith EE, Kent DM, Bulsara KR, et al; . Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49:e111-e122.
17. Woo D, Broderick JP, Kothari RU, et al. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA Stroke Study Group. Stroke. 1999;30:2355-2359.
18. Adams HP Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53:126-131.
19. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091-1096.
20. Birenbaum D, Bancroft LW, Felsberg GJ. Imaging in acute stroke. West J Emerg Med. 2011;12:67-76.
21. Salmela MB, Mortazavi S, Jagadeesan BD, et al. ACR Appropriateness Criteria® Cerebrovascular Disease. J Am Coll Radiol. 2017;14:S34-S61.
22. Hemphill JC 3rd, Greenberg SM, Anderson CS, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032-60.
23. Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017-1025.
24. The Tissue plasminogen activator for acute ischemic stroke. N Engl J Med, 1995;333:1581-1587.
25. Albers GW, Clark WM, Madden KP, et al. ATLANTIS trial: results for patients treated within 3 hours of stroke onset. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. Stroke. 2002;33:493-495.
26. Khan R, Nael K, Erly W. Acute stroke imaging: what clinicians need to know. Am J Med. 2013;126:379-386.
27. Latchaw RE, Alberts MJ, Lev MH, et al; . Recommendations for managing of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646-3678.
28. Vagal A, Meganathan K, Kleindorfer DO, et al. Increasing use of computed tomographic perfusion and computed tomographic angiograms in acute ischemic stroke from 2006 to 2010. Stroke. 2014;45:1029-1034.
29. Albers GW, Marks MP, Kemp S, et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708-718.
30. Demeestere J, Wouters A, Christensen S, et al. Review of perfusion imaging in acute ischemic stroke: from time to tissue. Stroke. 2020;51:1017-1024.
31. Chalela JA, Kidwell CS, Nentwich LM, et al, Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293-298.
32. Aoki J, Kimura K, Iguchi Y, et al. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci. 2010;293:39-44.
33. Wabnitz AM, Turan TN. Symptomatic carotid artery stenosis: surgery, stenting, or medical therapy? Curr Treat Options Cardiovasc Med. 2017;19:62.
34. Muir KW, Santosh C. Imaging of acute stroke and transient ischaemic attack. J Neurol Neurosurg Psychiatry. 2005;76(suppl 3):iii19-iii28.
35. Cameron JI, Tsoi C, Marsella A.Optimizing stroke systems of care by enhancing transitions across care environments. Stroke. 2008;39:2637-2643.
36. Amarenco P, Bogousslavsky J, Caplan LR, et al. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping). Cerebrovasc Dis. 2013;36:1-5.
37. Adams HP Jr, Bendixen BH, Kappelle LJ. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35-41.
38. Cacciatore A, Russo LS Jr. Lacunar infarction as an embolic complication of cardiac and arch angiography. Stroke. 1991;22:1603-1605.
39. Thomalla G, Simonsen CZ, Boutitie F, et al; WAKE-UP Investigators. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611-622.
40. Nogueira RG, Jadhav AP, Haussen DC, et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11-21.
Stroke ranks second behind heart disease as the leading cause of mortality worldwide, accounting for 1 of every 19 deaths,1 and remains a serious cause of morbidity. Best practices in stroke diagnosis and management can seem elusive to front-line clinicians, for 2 reasons: the rate of proliferation and nuance in stroke medicine and the fact that the typical scope of primary care practice exists apart from much of the diagnostic tools and management schema provided in stroke centers.2 In this article, we describe and update the diagnosis of stroke and review imaging modalities, their nuances, and their application in practice.
Diagnosis of acute stroke
Acute stroke is diagnosed upon observation of new neurologic deficits and congruent neuroimaging. Some updated definitions favor a silent form of cerebral ischemia manifested by imaging pathology only; this form is not discussed in this article. Although there are several characteristically distinct stroke syndromes, there is no way to clinically distinguish ischemic pathology from hemorrhagic pathology.
Some common symptoms that should prompt evaluation for stroke are part of the American Stroke Association FAST mnemonic designed to promote public health awareness3-5:
f ace droopinga rm weaknesss peech difficultyt ime to call 911.
Other commonly reported stroke symptoms include unilateral weakness or numbness, confusion, word-finding difficulty, visual problems, difficulty ambulating, dizziness, loss of balance or coordination, and thunderclap headache. A stroke should also be considered in the presence of any new focal neurologic deficit.3,4
Stroke patients should be triaged by emergency medical services using a stroke screening scale, such as BE-FAST5 (a modification of FAST that adds balance and eye assessments); the Los Angeles Prehospital Stroke Screen (LAPSS)6,7; the Rapid Arterial oCclusion Evaluation (RACE)8; and the Cincinnati Prehospital Stroke Severity Scale (CP-SSS)9,10 (see “Stroke screening scales for early identification and triage"). Studies have not found that any single prehospital stroke scale is superior to the others for reliably predicting large-vessel occlusion; therefore, prehospital assessment is typically based on practice patterns in a given locale.11 A patient (or family member or caregiver) who seeks your care for stroke symptoms should be told to call 911 and get emergency transport to a health care facility that can capably administer intravenous (IV) thrombolysis.a
SIDEBAR
Stroke screening scales for early identification and triage
National Institutes of Health Stroke Scale
www.stroke.nih.gov/resources/scale.htm
FAST
www.stroke.org/en/help-and-support/resource-library/fast-materials
BE-FAST
www.ahajournals.org/doi/10.1161/STROKEAHA.116.015169
Los Angeles Prehospital Stroke Screen (LAPSS)
http://stroke.ucla.edu/workfiles/prehospital-screen.pdf
Rapid Artery Occlusion Evaluation (RACE)
www.mdcalc.com/rapid-arterial-occlusion-evaluation-race-scale-stroke
Cincinnati Prehospital Stroke Severity Scale (CP-SSS)
https://www.mdcalc.com/cincinnati-prehospital-stroke-severity-scale-cp-sss
First responders should elicit “last-known-normal” time; this critical information can aid in diagnosis and drive therapeutic options, especially if patients are unaccompanied at time of transport to a higher echelon of care. A point-of-care blood glucose test should be performed by emergency medical staff, with dextrose administered for a level < 45 mg/dL. Establishing IV access for fluids, medications, and contrast can be considered if it does not delay transport. A 12-lead electrocardiogram can also be considered, again, as long as it does not delay transport to a facility capable of providing definitive therapy. Notification by emergency services staff before arrival and transport of the patient to such a facility is the essential element of prehospital care, and should be prioritized above ancillary testing beyond the stroke assessment.14
Guidelines recommend use of the National Institutes of Health Stroke Scale (NIHSS; www.stroke.nih.gov/resources/scale.htm) for clinical evaluation upon arrival at the ED.15 Although no scale has been identified that can reliably predict large-vessel occlusion amenable to endovascular therapy (EVT), no other score has been found to outperform the NIHSS in achieving meaningful patient outcomes.16 Furthermore, NIHSS has been validated to track clinical changes in response to therapy, is widely utilized, and is free.
Continue to: A criticism of the NIHSS...
A criticism of the NIHSS is its bias toward left-hemispheric ischemic pathology.17 NIHSS includes 11 questions on a scale of 0 to 42; typically, a score < 4 is associated with a higher chance of a positive clinical outcome.18 There is no minimum or maximum NIHSS score that precludes treatment with thrombolysis or EVT.
Other commonly used scores in acute stroke include disability assessments. The modified Rankin scale, which is used most often, features a score of 0 (symptom-free) to 6 (death). A modified Rankin scale score of 0 or 1 is considered an indication of a favorable outcome after stroke.19 Note that these functional scores are not always part of an acute assessment but can be done early in the clinical course to gauge the response to treatment, and are collected for stroke-center certification.
Imaging modalities
Imaging is recommended within 20 minutes of arrival in the ED in a stroke patient who might be a candidate for thrombolysis or thrombectomy.3 There, imaging modalities commonly performed are noncontrast-enhanced head computed tomography (NCHCT); computed tomography (CT) angiography, with or without perfusion; and diffusion-weighted magnetic resonance imaging (MRI).20,21 In addition, more highly specialized imaging modalities are available for the evaluation of the stroke patient in specific, often limited, circumstances. All these modalities are described below and compared in the TABLE,20,21 using the ACR Appropriateness Criteria (of the American College of Radiology),21 which are guidelines for appropriate imaging of stroke, based on a clinical complaint. Separate recommendations and appraisals are offered by the most recent American Heart Association/American Stroke Association (AHA/ASA) guideline.3

NCHCT. This study should be performed within 20 minutes after arrival at the ED because it provides rapid assessment of intracerebral hemorrhage, can effectively corroborate the diagnosis of some stroke mimickers, and identifies some candidates for EVT or thrombolysis3,21,22 (typically, the decision to proceed with EVT is based on adjunct imaging studies discussed in a bit). Evaluation for intracerebral hemorrhage is required prior to administering thrombolysis. Ischemic changes can be seen with variable specificity and sensitivity on NCHCT, depending on how much time has passed since the original insult. In all historical trials, CT was the only imaging modality used in the diagnosis of acute ischemic stroke (AIS) that suggested benefit from IV thrombolysis.23-25

Acute, subacute, and chronic changes can be seen on NCHCT, although the modality has limited sensitivity for identifying AIS (ie, approximately 75% within 6 hours after the original insult):
- Acute findings on NCHCT include intracellular edema, which causes loss of the gray matter–white matter interface and effacement of the cortical sulci. This occurs as a result of increased cellular uptake of water in response to ischemia and cell death, resulting in a decreased density of tissue (hypoattenuation) in affected areas.
- Subacute changes appear in the 2- to 5-day window, including vasogenic edema with greater mass effect, hypoattenuation, and well-defined margins.3,20,21
- Chronic vascular findings on NCHCT include loss of brain tissue and hypoattenuation.
Continue to: NCHCT is typically performed...
NCHCT is typically performed in advance of other adjunct imaging modalities.3,20,21 Baseline NCHCT can be performed on patients with advanced kidney disease and those who have an indwelling metallic device.
CT angiography is performed with timed contrast, providing a 3-dimensional representation of the cerebral vasculature; the entire intracranial and extracranial vasculature, including the aortic arch, can be mapped in approximately 60 seconds. CT angiography is sensitive in identifying areas of stenosis > 50% and identifies clinically significant areas of stenosis up to approximately 90% of the time.26 For this reason, it is particularly helpful in identifying candidates for an interventional strategy beyond pharmacotherapeutic thrombolysis. In addition, CT angiography can visualize aneurysmal dilation and dissection, and help with the planning of interventions—specifically, the confident administration of thrombolysis or more specific planning for target lesions and EVT.
It also can help identify a host of vascular phenomena, such as arteriovenous malformations, Moyamoya disease (progressive arterial blockage within the basal ganglia and compensatory microvascularization), and some vasculopathies.20,27 In intracranial hemorrhage, CT with angiography can help evaluate for structural malformations and identify patients at risk of hematoma expansion.22
CT perfusion. Many stroke centers will perform a CT perfusion study,28 which encompasses as many as 3 different CT sequences:
- NCHCT
- vertex-to-arch angiography with contrast bolus
- administration of contrast and capture of a dynamic sequence through 1 or 2 slabs of tissue, allowing for the generation of maps of cerebral blood flow (CBF), mean transit time (MTT), and cerebral blood volume (CBV) of the entire cerebral vasculature.
The interplay of these 3 sequences drives characterization of lesions (ie, CBF = CBV/MTT). An infarct is characterized by low CBF, low CBV, and elevated MTT. In penumbral tissue, MTT is elevated but CBF is slightly decreased and CBV is normal or increased. Using CT perfusion, areas throughout the ischemic penumbra can be surveyed for favorable interventional characteristics.20,29
Continue to: A CT perfusion study adds...
A CT perfusion study adds at least 60 seconds to NCHCT. This modality can be useful in planning interventions and for stratifying appropriateness of reperfusion strategies in strokes of unknown duration.3,30 CT perfusion can be performed on any multidetector CT scan but (1) requires specialized software and expertise to interpret and (2) subjects the patient to a significant radiation dose, which, if incorrectly administered, can be considerably higher than intended.20,26,27
Diffusion-weighted MRI. This is the most sensitive study for demonstrating early ischemic changes; however, limitations include lack of availability, contraindication in patients with metallic indwelling implants, and duration of the study—although, at some stroke centers, diffusion-weighted MRI can be performed in ≤ 10 minutes.
MRI and NCHCT have comparable sensitivity in detecting intracranial hemorrhage. MRI is likely more sensitive in identifying areas of microhemorrhage: In diffusion-weighted MRI, the sensitivity of stroke detection increases to > 95%.31 The modality relies on the comparable movement of water through damaged vs normal neuronal tissue. Diffusion-weighted MRI does not require administration of concomitant contrast, which can be a benefit in patients who are allergic to gadolinium-based contrast agents or have advanced kidney disease that precludes the use of contrast. It typically does not result in adequate characterization of extracranial vasculature.
Other MRI modalities. These MRI extensions include magnetic resonance (MR) perfusion and MR angiography. Whereas diffusion-weighted MRI (discussed above) offers the most rapid and sensitive evaluation for ischemia, fluid-attenuated inversion recovery (FLAIR) imaging has been utilized as a comparator to isolated diffusion-weighted MRI to help determine stroke duration. FLAIR signal positivity typically occurs 6 to 24 hours after the initial insult but is negative in stroke that occurred < 3 hours earlier.32
MRI is limited, in terms of availability and increased study duration, especially when it comes to timely administration of thrombolysis. A benefit of this modality is less radiation and, as noted, superior sensitivity for ischemia. Diffusion-weighted MRI combined with MR perfusion analysis can help isolate areas of the ischemic penumbra. MR perfusion is performed for a similar reason as CT perfusion, although logistical execution across those modalities is significantly different. Considerations for choosing MR perfusion or CT perfusion should be made on an individual basis and based on available local resources and accepted local practice patterns.26
Continue to: In the subacute setting...
In the subacute setting, MR perfusion and MR angiography of the head and the neck are often performed to identify stenosis, dissection, and more subtle mimickers of cerebrovascular accident not ascertained on initial CT evaluation. These studies are typically performed well outside the window for thrombolysis or intervention.26 No guidelines specifically direct or recommend this practice pattern. The superior sensitivity and cerebral blood flow mapping of MR perfusion and MR angiography might be useful for validating a suspected diagnosis of ischemic stroke and providing phenotypic information about AIS events.
Transcranial Doppler imaging relies on bony windows to assess intracranial vascular flow, velocity, direction, and reactivity. This information can be utilized to diagnose stenosis or occlusion. This modality is principally used to evaluate for stenosis in the anterior circulation (sensitivity, 70%-90%; specificity, 90%-95%).20 Evaluation of the basilar, vertebral, and internal carotid arteries is less accurate (sensitivity, 55%-80%).20 Transcranial Doppler imaging is also used to assess for cerebral vasospasm after subarachnoid hemorrhage, monitor sickle cell disease patients’ overall risk for ischemic stroke, and augment thrombolysis. It is limited by the availability of an expert technician, and therefore is typically reserved for unstable patients or those who cannot receive contrast.20
Carotid duplex ultrasonography. A dynamic study such as duplex ultrasonography can be strongly considered for flow imaging of the extracranial carotids to evaluate for stenosis. Indications for carotid stenting or endarterectomy include 50% to 79% occlusion of the carotid artery on the same side as a recent transient ischemic attack or AIS. Carotid stenosis > 80% warrants consideration for intervention independent of a recent cerebrovascular accident. Interventions are typically performed 2 to 14 days after stroke.33 Although this study is of limited utility in the hyperacute setting, it is recommended within 24 hours after nondisabling stroke in the carotid territory, when (1) the patient is otherwise a candidate for a surgical or procedural intervention to address the stenosis and (2) none of the aforementioned studies that focus on neck vasculature have been performed.
Conventional (digital subtraction) angiography is the gold standard for mapping cerebrovascular disease because it is dynamic and highly accurate. It is, however, typically limited by the number of required personnel, its invasive nature, and the requirement for IV contrast. This study is performed during intra-arterial intervention techniques, including stent retrieval and intra-arterial thrombolysis.26
Impact of imaging on treatment
Imaging helps determine the cause and some characteristics of stroke, both of which can help determine therapy. Strokes can be broadly subcategorized as hemorrhagic or ischemic; recent studies suggest that 87% are ischemic.34 Knowledge of the historic details of the event, the patient (eg, known atrial fibrillation, anticoagulant use, history of falls), and findings on imaging can contribute to determine the cause of AIS, and can facilitate communication and consultation between the primary care physician and inpatient teams.35
Continue to: Best practices for stroke treatment...
Best practices for stroke treatment are based on the cause of the event.3 To identify the likely cause, the aforementioned characteristics are incorporated into one of the scoring systems, which seek to clarify either the cause or the phenotypic appearance of the AIS, which helps direct further testing and treatment. (The ASCOD36 and TOAST37 classification schemes are commonly used phenotypic and causative classifications, respectively.) Several (not all) of the broad phenotypic imaging patterns, with myriad clinical manifestations, are reviewed below. They include:
- Embolic stroke, which, classically, involves end circulation and therefore has cortical involvement. Typically, these originate from the heart or large extracranial arteries, and higher rates of atrial fibrillation and hypercoagulable states are implicated.
- Thrombotic stroke, which, typically, is from large vessels or small vessels, and occurs as a result of atherosclerosis. These strokes are more common at the origins or bifurcations of vessels. Symptoms of thrombotic stroke classically wax and wane slightly more frequently. Lacunar strokes are typically from thrombotic causes, although there are rare episodes of an embolic source contributing to a lacunar stroke syndrome.38
There is evidence for using MRI discrepancies between diffusion-weighted and FLAIR imaging to time AIS findings in so-called wake-up strokes.39 The rationale is that strokes < 4.5 hours old can be identified because they would have abnormal diffusion imaging components but normal findings with FLAIR. When these criteria were utilized in considering whether to treat with thrombolysis, there was a statistically significant improvement in 90-day modified Rankin scale (odds ratio = 1.61; 95% confidence interval, 1.09-2.36), but also an increased probability of death and intracerebral hemorrhage.39
A recent multicenter, randomized, open-label trial, with blinded outcomes assessment, showcased the efficacy of thrombectomy as an adjunct when ischemic brain territory was identified without frank infarction, as ascertained by CT perfusion within the anterior circulation. This trial showed that thrombectomy could be performed as long as 16 hours after the patient was last well-appearing and still result in an improved outcome with favorable imaging characteristics (on the modified Rankin scale, an ordinal score of 4 with medical therapy and an ordinal score of 3 with EVT [odds ratio = 2.77; 95% confidence interval, 1.63-4.70]).29 A 2018 multicenter, prospective, randomized trial with blinded assessment of endpoints extended this idea, demonstrating that, when there was mismatch of the clinical deficit (ie, high NIHSS score) and infarct volume (measured on diffusion-weighted MRI or CT perfusion), thrombectomy as late as 24 hours after the patient was last known to be well was beneficial for lesions in the anterior circulation—specifically, the intracranial internal carotid artery or the proximal middle cerebral artery.40
a Whether local emergency departments (EDs) should be bypassed in favor of a specialized stroke center is the subject of debate. The 2019 American Heart Association/American Stroke Association guidelines note the AHA’s Mission: Lifeline Stroke EMS algorithm, which bypasses the nearest ED in feared cases of large-vessel occlusion if travel to a comprehensive stroke center can be accomplished within 30 minutes of arrival at the scene. This is based on expert consensus.3,12,13
CORRESPONDENCE
Brian Ford, MD, 4301 Jones Bridge Road, Bethesda, MD; [email protected].
Stroke ranks second behind heart disease as the leading cause of mortality worldwide, accounting for 1 of every 19 deaths,1 and remains a serious cause of morbidity. Best practices in stroke diagnosis and management can seem elusive to front-line clinicians, for 2 reasons: the rate of proliferation and nuance in stroke medicine and the fact that the typical scope of primary care practice exists apart from much of the diagnostic tools and management schema provided in stroke centers.2 In this article, we describe and update the diagnosis of stroke and review imaging modalities, their nuances, and their application in practice.
Diagnosis of acute stroke
Acute stroke is diagnosed upon observation of new neurologic deficits and congruent neuroimaging. Some updated definitions favor a silent form of cerebral ischemia manifested by imaging pathology only; this form is not discussed in this article. Although there are several characteristically distinct stroke syndromes, there is no way to clinically distinguish ischemic pathology from hemorrhagic pathology.
Some common symptoms that should prompt evaluation for stroke are part of the American Stroke Association FAST mnemonic designed to promote public health awareness3-5:
f ace droopinga rm weaknesss peech difficultyt ime to call 911.
Other commonly reported stroke symptoms include unilateral weakness or numbness, confusion, word-finding difficulty, visual problems, difficulty ambulating, dizziness, loss of balance or coordination, and thunderclap headache. A stroke should also be considered in the presence of any new focal neurologic deficit.3,4
Stroke patients should be triaged by emergency medical services using a stroke screening scale, such as BE-FAST5 (a modification of FAST that adds balance and eye assessments); the Los Angeles Prehospital Stroke Screen (LAPSS)6,7; the Rapid Arterial oCclusion Evaluation (RACE)8; and the Cincinnati Prehospital Stroke Severity Scale (CP-SSS)9,10 (see “Stroke screening scales for early identification and triage"). Studies have not found that any single prehospital stroke scale is superior to the others for reliably predicting large-vessel occlusion; therefore, prehospital assessment is typically based on practice patterns in a given locale.11 A patient (or family member or caregiver) who seeks your care for stroke symptoms should be told to call 911 and get emergency transport to a health care facility that can capably administer intravenous (IV) thrombolysis.a
SIDEBAR
Stroke screening scales for early identification and triage
National Institutes of Health Stroke Scale
www.stroke.nih.gov/resources/scale.htm
FAST
www.stroke.org/en/help-and-support/resource-library/fast-materials
BE-FAST
www.ahajournals.org/doi/10.1161/STROKEAHA.116.015169
Los Angeles Prehospital Stroke Screen (LAPSS)
http://stroke.ucla.edu/workfiles/prehospital-screen.pdf
Rapid Artery Occlusion Evaluation (RACE)
www.mdcalc.com/rapid-arterial-occlusion-evaluation-race-scale-stroke
Cincinnati Prehospital Stroke Severity Scale (CP-SSS)
https://www.mdcalc.com/cincinnati-prehospital-stroke-severity-scale-cp-sss
First responders should elicit “last-known-normal” time; this critical information can aid in diagnosis and drive therapeutic options, especially if patients are unaccompanied at time of transport to a higher echelon of care. A point-of-care blood glucose test should be performed by emergency medical staff, with dextrose administered for a level < 45 mg/dL. Establishing IV access for fluids, medications, and contrast can be considered if it does not delay transport. A 12-lead electrocardiogram can also be considered, again, as long as it does not delay transport to a facility capable of providing definitive therapy. Notification by emergency services staff before arrival and transport of the patient to such a facility is the essential element of prehospital care, and should be prioritized above ancillary testing beyond the stroke assessment.14
Guidelines recommend use of the National Institutes of Health Stroke Scale (NIHSS; www.stroke.nih.gov/resources/scale.htm) for clinical evaluation upon arrival at the ED.15 Although no scale has been identified that can reliably predict large-vessel occlusion amenable to endovascular therapy (EVT), no other score has been found to outperform the NIHSS in achieving meaningful patient outcomes.16 Furthermore, NIHSS has been validated to track clinical changes in response to therapy, is widely utilized, and is free.
Continue to: A criticism of the NIHSS...
A criticism of the NIHSS is its bias toward left-hemispheric ischemic pathology.17 NIHSS includes 11 questions on a scale of 0 to 42; typically, a score < 4 is associated with a higher chance of a positive clinical outcome.18 There is no minimum or maximum NIHSS score that precludes treatment with thrombolysis or EVT.
Other commonly used scores in acute stroke include disability assessments. The modified Rankin scale, which is used most often, features a score of 0 (symptom-free) to 6 (death). A modified Rankin scale score of 0 or 1 is considered an indication of a favorable outcome after stroke.19 Note that these functional scores are not always part of an acute assessment but can be done early in the clinical course to gauge the response to treatment, and are collected for stroke-center certification.
Imaging modalities
Imaging is recommended within 20 minutes of arrival in the ED in a stroke patient who might be a candidate for thrombolysis or thrombectomy.3 There, imaging modalities commonly performed are noncontrast-enhanced head computed tomography (NCHCT); computed tomography (CT) angiography, with or without perfusion; and diffusion-weighted magnetic resonance imaging (MRI).20,21 In addition, more highly specialized imaging modalities are available for the evaluation of the stroke patient in specific, often limited, circumstances. All these modalities are described below and compared in the TABLE,20,21 using the ACR Appropriateness Criteria (of the American College of Radiology),21 which are guidelines for appropriate imaging of stroke, based on a clinical complaint. Separate recommendations and appraisals are offered by the most recent American Heart Association/American Stroke Association (AHA/ASA) guideline.3

NCHCT. This study should be performed within 20 minutes after arrival at the ED because it provides rapid assessment of intracerebral hemorrhage, can effectively corroborate the diagnosis of some stroke mimickers, and identifies some candidates for EVT or thrombolysis3,21,22 (typically, the decision to proceed with EVT is based on adjunct imaging studies discussed in a bit). Evaluation for intracerebral hemorrhage is required prior to administering thrombolysis. Ischemic changes can be seen with variable specificity and sensitivity on NCHCT, depending on how much time has passed since the original insult. In all historical trials, CT was the only imaging modality used in the diagnosis of acute ischemic stroke (AIS) that suggested benefit from IV thrombolysis.23-25

Acute, subacute, and chronic changes can be seen on NCHCT, although the modality has limited sensitivity for identifying AIS (ie, approximately 75% within 6 hours after the original insult):
- Acute findings on NCHCT include intracellular edema, which causes loss of the gray matter–white matter interface and effacement of the cortical sulci. This occurs as a result of increased cellular uptake of water in response to ischemia and cell death, resulting in a decreased density of tissue (hypoattenuation) in affected areas.
- Subacute changes appear in the 2- to 5-day window, including vasogenic edema with greater mass effect, hypoattenuation, and well-defined margins.3,20,21
- Chronic vascular findings on NCHCT include loss of brain tissue and hypoattenuation.
Continue to: NCHCT is typically performed...
NCHCT is typically performed in advance of other adjunct imaging modalities.3,20,21 Baseline NCHCT can be performed on patients with advanced kidney disease and those who have an indwelling metallic device.
CT angiography is performed with timed contrast, providing a 3-dimensional representation of the cerebral vasculature; the entire intracranial and extracranial vasculature, including the aortic arch, can be mapped in approximately 60 seconds. CT angiography is sensitive in identifying areas of stenosis > 50% and identifies clinically significant areas of stenosis up to approximately 90% of the time.26 For this reason, it is particularly helpful in identifying candidates for an interventional strategy beyond pharmacotherapeutic thrombolysis. In addition, CT angiography can visualize aneurysmal dilation and dissection, and help with the planning of interventions—specifically, the confident administration of thrombolysis or more specific planning for target lesions and EVT.
It also can help identify a host of vascular phenomena, such as arteriovenous malformations, Moyamoya disease (progressive arterial blockage within the basal ganglia and compensatory microvascularization), and some vasculopathies.20,27 In intracranial hemorrhage, CT with angiography can help evaluate for structural malformations and identify patients at risk of hematoma expansion.22
CT perfusion. Many stroke centers will perform a CT perfusion study,28 which encompasses as many as 3 different CT sequences:
- NCHCT
- vertex-to-arch angiography with contrast bolus
- administration of contrast and capture of a dynamic sequence through 1 or 2 slabs of tissue, allowing for the generation of maps of cerebral blood flow (CBF), mean transit time (MTT), and cerebral blood volume (CBV) of the entire cerebral vasculature.
The interplay of these 3 sequences drives characterization of lesions (ie, CBF = CBV/MTT). An infarct is characterized by low CBF, low CBV, and elevated MTT. In penumbral tissue, MTT is elevated but CBF is slightly decreased and CBV is normal or increased. Using CT perfusion, areas throughout the ischemic penumbra can be surveyed for favorable interventional characteristics.20,29
Continue to: A CT perfusion study adds...
A CT perfusion study adds at least 60 seconds to NCHCT. This modality can be useful in planning interventions and for stratifying appropriateness of reperfusion strategies in strokes of unknown duration.3,30 CT perfusion can be performed on any multidetector CT scan but (1) requires specialized software and expertise to interpret and (2) subjects the patient to a significant radiation dose, which, if incorrectly administered, can be considerably higher than intended.20,26,27
Diffusion-weighted MRI. This is the most sensitive study for demonstrating early ischemic changes; however, limitations include lack of availability, contraindication in patients with metallic indwelling implants, and duration of the study—although, at some stroke centers, diffusion-weighted MRI can be performed in ≤ 10 minutes.
MRI and NCHCT have comparable sensitivity in detecting intracranial hemorrhage. MRI is likely more sensitive in identifying areas of microhemorrhage: In diffusion-weighted MRI, the sensitivity of stroke detection increases to > 95%.31 The modality relies on the comparable movement of water through damaged vs normal neuronal tissue. Diffusion-weighted MRI does not require administration of concomitant contrast, which can be a benefit in patients who are allergic to gadolinium-based contrast agents or have advanced kidney disease that precludes the use of contrast. It typically does not result in adequate characterization of extracranial vasculature.
Other MRI modalities. These MRI extensions include magnetic resonance (MR) perfusion and MR angiography. Whereas diffusion-weighted MRI (discussed above) offers the most rapid and sensitive evaluation for ischemia, fluid-attenuated inversion recovery (FLAIR) imaging has been utilized as a comparator to isolated diffusion-weighted MRI to help determine stroke duration. FLAIR signal positivity typically occurs 6 to 24 hours after the initial insult but is negative in stroke that occurred < 3 hours earlier.32
MRI is limited, in terms of availability and increased study duration, especially when it comes to timely administration of thrombolysis. A benefit of this modality is less radiation and, as noted, superior sensitivity for ischemia. Diffusion-weighted MRI combined with MR perfusion analysis can help isolate areas of the ischemic penumbra. MR perfusion is performed for a similar reason as CT perfusion, although logistical execution across those modalities is significantly different. Considerations for choosing MR perfusion or CT perfusion should be made on an individual basis and based on available local resources and accepted local practice patterns.26
Continue to: In the subacute setting...
In the subacute setting, MR perfusion and MR angiography of the head and the neck are often performed to identify stenosis, dissection, and more subtle mimickers of cerebrovascular accident not ascertained on initial CT evaluation. These studies are typically performed well outside the window for thrombolysis or intervention.26 No guidelines specifically direct or recommend this practice pattern. The superior sensitivity and cerebral blood flow mapping of MR perfusion and MR angiography might be useful for validating a suspected diagnosis of ischemic stroke and providing phenotypic information about AIS events.
Transcranial Doppler imaging relies on bony windows to assess intracranial vascular flow, velocity, direction, and reactivity. This information can be utilized to diagnose stenosis or occlusion. This modality is principally used to evaluate for stenosis in the anterior circulation (sensitivity, 70%-90%; specificity, 90%-95%).20 Evaluation of the basilar, vertebral, and internal carotid arteries is less accurate (sensitivity, 55%-80%).20 Transcranial Doppler imaging is also used to assess for cerebral vasospasm after subarachnoid hemorrhage, monitor sickle cell disease patients’ overall risk for ischemic stroke, and augment thrombolysis. It is limited by the availability of an expert technician, and therefore is typically reserved for unstable patients or those who cannot receive contrast.20
Carotid duplex ultrasonography. A dynamic study such as duplex ultrasonography can be strongly considered for flow imaging of the extracranial carotids to evaluate for stenosis. Indications for carotid stenting or endarterectomy include 50% to 79% occlusion of the carotid artery on the same side as a recent transient ischemic attack or AIS. Carotid stenosis > 80% warrants consideration for intervention independent of a recent cerebrovascular accident. Interventions are typically performed 2 to 14 days after stroke.33 Although this study is of limited utility in the hyperacute setting, it is recommended within 24 hours after nondisabling stroke in the carotid territory, when (1) the patient is otherwise a candidate for a surgical or procedural intervention to address the stenosis and (2) none of the aforementioned studies that focus on neck vasculature have been performed.
Conventional (digital subtraction) angiography is the gold standard for mapping cerebrovascular disease because it is dynamic and highly accurate. It is, however, typically limited by the number of required personnel, its invasive nature, and the requirement for IV contrast. This study is performed during intra-arterial intervention techniques, including stent retrieval and intra-arterial thrombolysis.26
Impact of imaging on treatment
Imaging helps determine the cause and some characteristics of stroke, both of which can help determine therapy. Strokes can be broadly subcategorized as hemorrhagic or ischemic; recent studies suggest that 87% are ischemic.34 Knowledge of the historic details of the event, the patient (eg, known atrial fibrillation, anticoagulant use, history of falls), and findings on imaging can contribute to determine the cause of AIS, and can facilitate communication and consultation between the primary care physician and inpatient teams.35
Continue to: Best practices for stroke treatment...
Best practices for stroke treatment are based on the cause of the event.3 To identify the likely cause, the aforementioned characteristics are incorporated into one of the scoring systems, which seek to clarify either the cause or the phenotypic appearance of the AIS, which helps direct further testing and treatment. (The ASCOD36 and TOAST37 classification schemes are commonly used phenotypic and causative classifications, respectively.) Several (not all) of the broad phenotypic imaging patterns, with myriad clinical manifestations, are reviewed below. They include:
- Embolic stroke, which, classically, involves end circulation and therefore has cortical involvement. Typically, these originate from the heart or large extracranial arteries, and higher rates of atrial fibrillation and hypercoagulable states are implicated.
- Thrombotic stroke, which, typically, is from large vessels or small vessels, and occurs as a result of atherosclerosis. These strokes are more common at the origins or bifurcations of vessels. Symptoms of thrombotic stroke classically wax and wane slightly more frequently. Lacunar strokes are typically from thrombotic causes, although there are rare episodes of an embolic source contributing to a lacunar stroke syndrome.38
There is evidence for using MRI discrepancies between diffusion-weighted and FLAIR imaging to time AIS findings in so-called wake-up strokes.39 The rationale is that strokes < 4.5 hours old can be identified because they would have abnormal diffusion imaging components but normal findings with FLAIR. When these criteria were utilized in considering whether to treat with thrombolysis, there was a statistically significant improvement in 90-day modified Rankin scale (odds ratio = 1.61; 95% confidence interval, 1.09-2.36), but also an increased probability of death and intracerebral hemorrhage.39
A recent multicenter, randomized, open-label trial, with blinded outcomes assessment, showcased the efficacy of thrombectomy as an adjunct when ischemic brain territory was identified without frank infarction, as ascertained by CT perfusion within the anterior circulation. This trial showed that thrombectomy could be performed as long as 16 hours after the patient was last well-appearing and still result in an improved outcome with favorable imaging characteristics (on the modified Rankin scale, an ordinal score of 4 with medical therapy and an ordinal score of 3 with EVT [odds ratio = 2.77; 95% confidence interval, 1.63-4.70]).29 A 2018 multicenter, prospective, randomized trial with blinded assessment of endpoints extended this idea, demonstrating that, when there was mismatch of the clinical deficit (ie, high NIHSS score) and infarct volume (measured on diffusion-weighted MRI or CT perfusion), thrombectomy as late as 24 hours after the patient was last known to be well was beneficial for lesions in the anterior circulation—specifically, the intracranial internal carotid artery or the proximal middle cerebral artery.40
a Whether local emergency departments (EDs) should be bypassed in favor of a specialized stroke center is the subject of debate. The 2019 American Heart Association/American Stroke Association guidelines note the AHA’s Mission: Lifeline Stroke EMS algorithm, which bypasses the nearest ED in feared cases of large-vessel occlusion if travel to a comprehensive stroke center can be accomplished within 30 minutes of arrival at the scene. This is based on expert consensus.3,12,13
CORRESPONDENCE
Brian Ford, MD, 4301 Jones Bridge Road, Bethesda, MD; [email protected].
1. Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67-e492.
2. Darves B. Collaboration key to post-stroke follow-up. ACP Internist. October 2009. https://acpinternist.org/archives/2009/10/stroke.htm. Accessed September 22, 2020.
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50e344-e418.
4. Sacco RL, Kasner SE, Broderick JP, et al; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
5. Aroor S, Singh R, Goldstein LB. BE-FAST (Balance, Eyes, Face, Arm, Speech, Time): Reducing the proportion of strokes missed using the FAST mnemonic. 2017;48:479-481.
6. Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS). Stroke. 2000;31:71-76.
7. Llanes JN, Kidwell CS, Starkman S, et al. The Los Angeles Motor Scale (LAMS): a new measure to characterize stroke severity in the field. Prehosp Emerg Care. 2004;8:46-50.
8. Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014;45:87-91.
9. Katz BS, McMullan JT, Sucharew H, et al. Design and validation of a prehospital scale to predict stroke severity: Cincinnati Prehospital Stroke Severity Scale. Stroke. 2015;466:1508-1512.
10. Kummer BR, et al. External validation of the Cincinnati Prehospital Stroke Severity Scale. J Stroke Cerebrovasc Dis. 2016;25:1270-1274.
11. Beume L-A, Hieber M, Kaller CP, et al. Large vessel occlusion in acute stroke. Stroke. 2018;49:2323-2329.
12. Man S, Zhao X, Uchino K, et al. Comparison of acute ischemic stroke care and outcomes between comprehensive stroke centers and primary stroke centers in the United States. Circ Cardiovasc Qual Outcomes. 2018;11:e004512.
13. American Heart Association (Mission: Lifeline—Stroke). Emergency medical services acute stroke routing. 2020. www.heart.org/-/media/files/professional/quality-improvement/mission-lifeline/2_25_2020/ds15698-qi-ems-algorithm_update-2142020.pdf?la=en. Accessed October 8, 2020.
14. Glober NK, Sporer KA, Guluma KZ, et al. Acute stroke: current evidence-based recommendations for prehospital care. West J Emerg Med. 2016;17:104-128.
15. NIH stroke scale. Bethesda, MD: National Institute of Neurological Disorders and Stroke, National Institutes of Health. www.stroke.nih.gov/resources/scale.htm. Accessed October 10, 2020.
16. Smith EE, Kent DM, Bulsara KR, et al; . Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49:e111-e122.
17. Woo D, Broderick JP, Kothari RU, et al. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA Stroke Study Group. Stroke. 1999;30:2355-2359.
18. Adams HP Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53:126-131.
19. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091-1096.
20. Birenbaum D, Bancroft LW, Felsberg GJ. Imaging in acute stroke. West J Emerg Med. 2011;12:67-76.
21. Salmela MB, Mortazavi S, Jagadeesan BD, et al. ACR Appropriateness Criteria® Cerebrovascular Disease. J Am Coll Radiol. 2017;14:S34-S61.
22. Hemphill JC 3rd, Greenberg SM, Anderson CS, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032-60.
23. Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017-1025.
24. The Tissue plasminogen activator for acute ischemic stroke. N Engl J Med, 1995;333:1581-1587.
25. Albers GW, Clark WM, Madden KP, et al. ATLANTIS trial: results for patients treated within 3 hours of stroke onset. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. Stroke. 2002;33:493-495.
26. Khan R, Nael K, Erly W. Acute stroke imaging: what clinicians need to know. Am J Med. 2013;126:379-386.
27. Latchaw RE, Alberts MJ, Lev MH, et al; . Recommendations for managing of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646-3678.
28. Vagal A, Meganathan K, Kleindorfer DO, et al. Increasing use of computed tomographic perfusion and computed tomographic angiograms in acute ischemic stroke from 2006 to 2010. Stroke. 2014;45:1029-1034.
29. Albers GW, Marks MP, Kemp S, et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708-718.
30. Demeestere J, Wouters A, Christensen S, et al. Review of perfusion imaging in acute ischemic stroke: from time to tissue. Stroke. 2020;51:1017-1024.
31. Chalela JA, Kidwell CS, Nentwich LM, et al, Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293-298.
32. Aoki J, Kimura K, Iguchi Y, et al. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci. 2010;293:39-44.
33. Wabnitz AM, Turan TN. Symptomatic carotid artery stenosis: surgery, stenting, or medical therapy? Curr Treat Options Cardiovasc Med. 2017;19:62.
34. Muir KW, Santosh C. Imaging of acute stroke and transient ischaemic attack. J Neurol Neurosurg Psychiatry. 2005;76(suppl 3):iii19-iii28.
35. Cameron JI, Tsoi C, Marsella A.Optimizing stroke systems of care by enhancing transitions across care environments. Stroke. 2008;39:2637-2643.
36. Amarenco P, Bogousslavsky J, Caplan LR, et al. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping). Cerebrovasc Dis. 2013;36:1-5.
37. Adams HP Jr, Bendixen BH, Kappelle LJ. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35-41.
38. Cacciatore A, Russo LS Jr. Lacunar infarction as an embolic complication of cardiac and arch angiography. Stroke. 1991;22:1603-1605.
39. Thomalla G, Simonsen CZ, Boutitie F, et al; WAKE-UP Investigators. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611-622.
40. Nogueira RG, Jadhav AP, Haussen DC, et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11-21.
1. Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67-e492.
2. Darves B. Collaboration key to post-stroke follow-up. ACP Internist. October 2009. https://acpinternist.org/archives/2009/10/stroke.htm. Accessed September 22, 2020.
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50e344-e418.
4. Sacco RL, Kasner SE, Broderick JP, et al; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
5. Aroor S, Singh R, Goldstein LB. BE-FAST (Balance, Eyes, Face, Arm, Speech, Time): Reducing the proportion of strokes missed using the FAST mnemonic. 2017;48:479-481.
6. Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS). Stroke. 2000;31:71-76.
7. Llanes JN, Kidwell CS, Starkman S, et al. The Los Angeles Motor Scale (LAMS): a new measure to characterize stroke severity in the field. Prehosp Emerg Care. 2004;8:46-50.
8. Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014;45:87-91.
9. Katz BS, McMullan JT, Sucharew H, et al. Design and validation of a prehospital scale to predict stroke severity: Cincinnati Prehospital Stroke Severity Scale. Stroke. 2015;466:1508-1512.
10. Kummer BR, et al. External validation of the Cincinnati Prehospital Stroke Severity Scale. J Stroke Cerebrovasc Dis. 2016;25:1270-1274.
11. Beume L-A, Hieber M, Kaller CP, et al. Large vessel occlusion in acute stroke. Stroke. 2018;49:2323-2329.
12. Man S, Zhao X, Uchino K, et al. Comparison of acute ischemic stroke care and outcomes between comprehensive stroke centers and primary stroke centers in the United States. Circ Cardiovasc Qual Outcomes. 2018;11:e004512.
13. American Heart Association (Mission: Lifeline—Stroke). Emergency medical services acute stroke routing. 2020. www.heart.org/-/media/files/professional/quality-improvement/mission-lifeline/2_25_2020/ds15698-qi-ems-algorithm_update-2142020.pdf?la=en. Accessed October 8, 2020.
14. Glober NK, Sporer KA, Guluma KZ, et al. Acute stroke: current evidence-based recommendations for prehospital care. West J Emerg Med. 2016;17:104-128.
15. NIH stroke scale. Bethesda, MD: National Institute of Neurological Disorders and Stroke, National Institutes of Health. www.stroke.nih.gov/resources/scale.htm. Accessed October 10, 2020.
16. Smith EE, Kent DM, Bulsara KR, et al; . Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49:e111-e122.
17. Woo D, Broderick JP, Kothari RU, et al. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA Stroke Study Group. Stroke. 1999;30:2355-2359.
18. Adams HP Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53:126-131.
19. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091-1096.
20. Birenbaum D, Bancroft LW, Felsberg GJ. Imaging in acute stroke. West J Emerg Med. 2011;12:67-76.
21. Salmela MB, Mortazavi S, Jagadeesan BD, et al. ACR Appropriateness Criteria® Cerebrovascular Disease. J Am Coll Radiol. 2017;14:S34-S61.
22. Hemphill JC 3rd, Greenberg SM, Anderson CS, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032-60.
23. Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017-1025.
24. The Tissue plasminogen activator for acute ischemic stroke. N Engl J Med, 1995;333:1581-1587.
25. Albers GW, Clark WM, Madden KP, et al. ATLANTIS trial: results for patients treated within 3 hours of stroke onset. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. Stroke. 2002;33:493-495.
26. Khan R, Nael K, Erly W. Acute stroke imaging: what clinicians need to know. Am J Med. 2013;126:379-386.
27. Latchaw RE, Alberts MJ, Lev MH, et al; . Recommendations for managing of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646-3678.
28. Vagal A, Meganathan K, Kleindorfer DO, et al. Increasing use of computed tomographic perfusion and computed tomographic angiograms in acute ischemic stroke from 2006 to 2010. Stroke. 2014;45:1029-1034.
29. Albers GW, Marks MP, Kemp S, et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708-718.
30. Demeestere J, Wouters A, Christensen S, et al. Review of perfusion imaging in acute ischemic stroke: from time to tissue. Stroke. 2020;51:1017-1024.
31. Chalela JA, Kidwell CS, Nentwich LM, et al, Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293-298.
32. Aoki J, Kimura K, Iguchi Y, et al. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci. 2010;293:39-44.
33. Wabnitz AM, Turan TN. Symptomatic carotid artery stenosis: surgery, stenting, or medical therapy? Curr Treat Options Cardiovasc Med. 2017;19:62.
34. Muir KW, Santosh C. Imaging of acute stroke and transient ischaemic attack. J Neurol Neurosurg Psychiatry. 2005;76(suppl 3):iii19-iii28.
35. Cameron JI, Tsoi C, Marsella A.Optimizing stroke systems of care by enhancing transitions across care environments. Stroke. 2008;39:2637-2643.
36. Amarenco P, Bogousslavsky J, Caplan LR, et al. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping). Cerebrovasc Dis. 2013;36:1-5.
37. Adams HP Jr, Bendixen BH, Kappelle LJ. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35-41.
38. Cacciatore A, Russo LS Jr. Lacunar infarction as an embolic complication of cardiac and arch angiography. Stroke. 1991;22:1603-1605.
39. Thomalla G, Simonsen CZ, Boutitie F, et al; WAKE-UP Investigators. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611-622.
40. Nogueira RG, Jadhav AP, Haussen DC, et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11-21.
How to assess and relieve that perplexing rashless itch
Pruritus, defined as a sensation that induces a desire to scratch1 and classified as acute or chronic (lasting > 6 weeks),2 is one of the most common complaints among primary care patients: Approximately 1% of ambulatory visits in the United States are linked to pruritus.3
Chronic pruritus impairs quality of life; its impact has been compared to that of chronic pain.4 Treatment should therefore be instituted promptly. Although this condition might appear benign, chronic pruritus can be a symptom of a serious condition, as we describe here. When persistent pruritus is refractory to treatment, systemic causes should be fully explored.
In this article, we discuss the pathogenesis and management of pruritus without skin eruption in the adult nonpregnant patient. We also present practice recommendations to help you determine whether your patient’s pruritus is indicative of a serious systemic condition.
An incomplete understanding of the pathophysiology of pruritus
The pathophysiology of pruritus is not fully understood. It is generally recognized, however, that pruritus starts in the peripheral nerves located in the dermal–epidermal junction of the skin.5 The sensation is then transmitted along unmyelinated slow-conducting C fibers to the dorsal horn of the spinal cord.5,6 There are 2 types of C fibers that transmit the itch impulse6: A histamine-dependent type and a non-histamine-dependent type, which might explain why pruritus can be refractory to antihistamine treatment.6
Once the itch impulse has moved from the spinal cord, it travels along the spinothalamic tract up to the contralateral thalamus.1 From there, the impulse ascends to the cerebral cortex.1 In the cortex, the impulse triggers multiple areas of the brain, such as those responsible for sensation, motor function, reward, memory, and emotion.7
Several chemical mediators have been found to be peripheral and central inducers of pruritus: histamine, endogenous opioids, substance P, and serotonin.2 There are indications that certain receptors, such as mu-opioid receptors and kappa-opioid receptors, are key contributors to itch as well.2
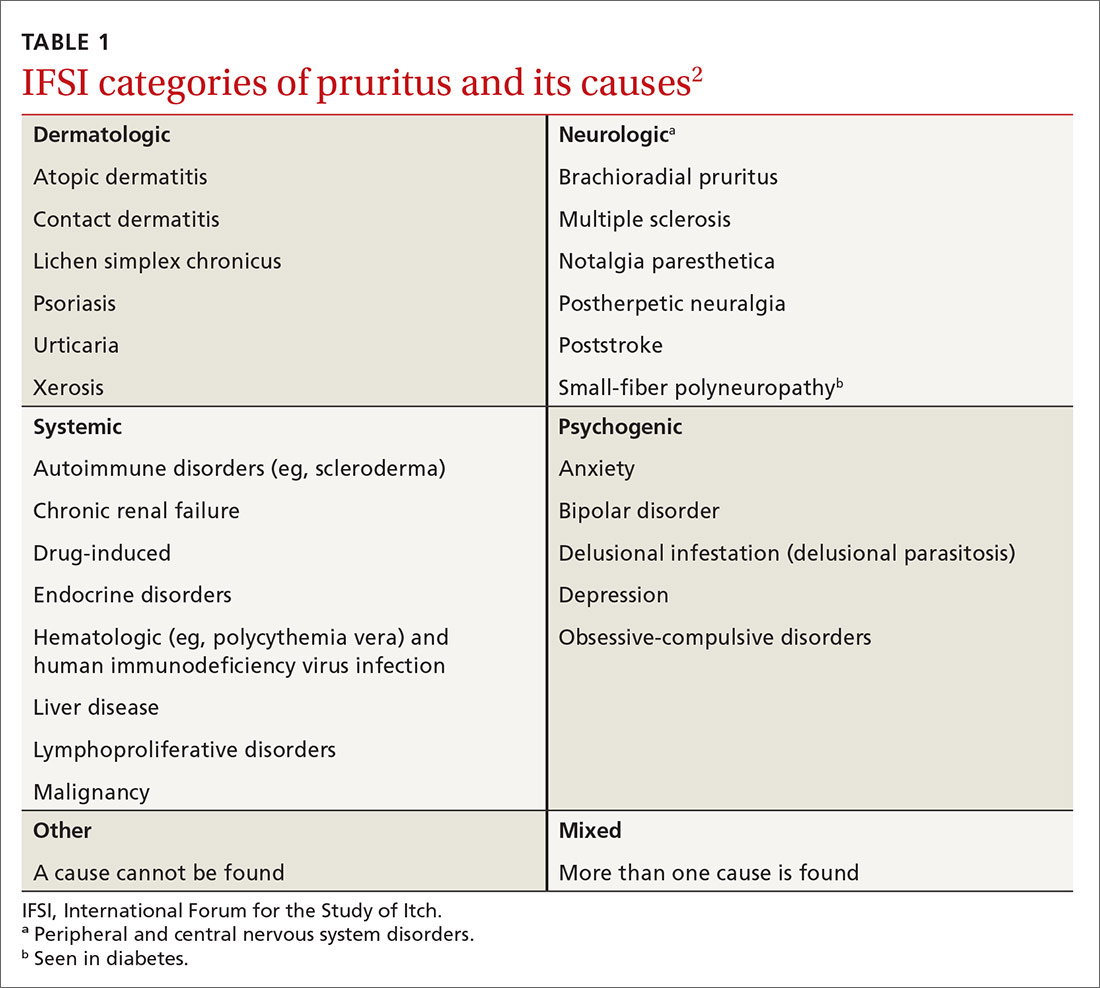
A diverse etiology
The International Forum for the Study of Itch (IFSI) has established 6 main categories of causes of pruritus(TABLE 1)2:
- dermatologic
- systemic
- neurologic
- psychogenic
- mixed
- other.
Continue to: In this review...
In this review, we focus on the work-up and management of 3 of those categories: systemic, neurologic, and psychogenic causes of pruritus.
Systemic causes
Research has shown that 14% to 24% of patients who seek the care of a dermatologist for chronic itch without skin lesions have a systemic illness.8
Renal disease. Approximately 40% of patients with end-stage renal disease who are on hemodialysis or peritoneal dialysis have uremic pruritus.2 The itch is mostly generalized but can be pronounced on the back. For most patients, the itch is worse at night, causing a major impact on quality of life.6
Liver disease. In hepatic disease, there is often impairment in the secretion of bile, which can lead to cholestatic pruritus.2 This condition commonly affects the hands and feet first; later, it becomes generalized.2 Cholestatic pruritus can be elicited by tight-fitting clothing. Relief is not achieved by scratching.9 This type of itch effects 70% of patients with primary biliary cirrhosis and 15% of patients with hepatitis C infection.9
Hematologic disorders. Pruritus is a hallmark symptom of polycythemia rubra vera. Almost 50% of patients with this disorder report pruritus that occurs after exposure to water9; aquagenic pruritus can precede the formal diagnosis of polycythemia rubra vera by years.2 It has been speculated that platelet aggregation in this disorder leads to release of serotonin and histamine, which, in turn, causes itch.9
Continue to: Endocrine disorders
Endocrine disorders. Approximately 4% to 11% of patients with thyrotoxicosis have pruritus.1 It has been suggested that vasodilation, increased skin temperature, and a decreased itch threshold from untreated Graves disease might be inciting factors.
Malignancy. In generalized chronic pruritus without a known cause, strongly consider the likelihood of underlying malignancy8,10; for 10% of these patients, their chronic pruritus is a paraneoplastic sign. Paraneoplastic pruritus is characterized as an itch that predates clinical onset, or occurs early in the course, of a malignancy.9 The condition is most strongly linked to cancers of the liver, gallbladder, biliary tract, hematologic system, and skin.11
Chronic pruritus affects 30% of patients with Hodgkin lymphoma.9 General pruritus can precede this diagnosis by months, even years.1 In Hodgkin lymphoma patients who are in remission, a return of pruritic symptoms can be a harbinger of recurrence.9
Neurologic causes
A recent study found that 8% to 15% of patients referred to a dermatology clinic for chronic pruritus without skin eruption had underlying neurologic pathology.12 Although the specific mechanisms of neuropathic itch are still poorly understood, it has been theorized that the itch emanates from neuronal damage, which can come from peripheral or central nervous system lesions.9
Brachioradial pruritus. There are divergent theories about the etiology of brachioradial pruritus. One hypothesis is that the condition is caused by cervical nerve-root impingement at the level of C5-C8 that leads to nerve damage2; another is that chronic exposure to sunlight causes injury to peripheral cutaneous nerves.2 Brachioradial pruritus is localized to the dorsolateral forearm; it can also involve the neck, back, shoulder, upper arm, and chest, unilaterally and bilaterally. This pruritus can be intermittent and become worse upon exposure to sunlight.2
Continue to: Notalgia paresthetica
Notalgia paresthetica. This condition might also cause neuropathic pruritus as a consequence of nerve impingement. The itch of notalgia paresthesia is located on the skin, medial to the scapular border on the upper or mid-back.2 It has been postulated that the itch is caused by nerve entrapment of the posterior rami of spinal nerves arising from T2-T6.9 However, another theory suggests that the itch is caused by damage to peripheral nerves.9 The itch of notalgia paresthetica can wax and wane.2
Poststroke pruritus. Brain lesions, most often caused by stroke, can cause neuropathic itch. One of the best-known syndromes related to poststroke itch is Wallenberg syndrome (ischemia from a lateral medullary infarction), which typically presents with itch, thermalgic hypoesthesia of the face, cerebellar dysfunction, nausea, and vomiting.7
Shingles. More than one-half of patients who develop postherpetic neuralgia as a consequence of a herpes zoster infection also develop neuropathic pruritus.9 It is thought that postherpetic pruritus shares a comparable pathophysiology with postherpetic neuralgia, in which neurons involved in itch stimuli become damaged.7
Diabetes mellitus. Pruritus from diabetes can be classified as systemic or neuropathic. Diabetes is one of the most common causes of small-fiber polyneuropathy, which can cause neuropathic pruritus.13
Multiple sclerosis. Central nervous system lesions that affect sensory pathways can lead to neuropathic itch in multiple sclerosis. Patients can have severe episodes of generalized pruritus. It has been hypothesized that the neuropathic itch in multiple sclerosis is induced by activation of artificial synapses in demyelinated areas.2
Continue to: Psychogenic pruritus
Psychogenic pruritus
Chronic pruritus can be a comorbidity of psychiatric illness. A retrospective study found that pruritus occurs in 32% to 42% of psychiatric inpatients.14 Depression, anxiety, bipolar disorders, obsessive–compulsive disorders, somatoform disorders, psychosis, and substance abuse all have a strong link to psychogenic excoriation.15 Psychogenic excoriation, which can cause secondary skin lesions, occurs in psychiatric patients who excessively pick and scratch normal skin because they perceive an itch sensation or have a delusion of infestation.2 Affected skin can be marked by scattered crusted lesions (FIGURE) anywhere on the body that the patient can reach—most commonly, the extremities.2
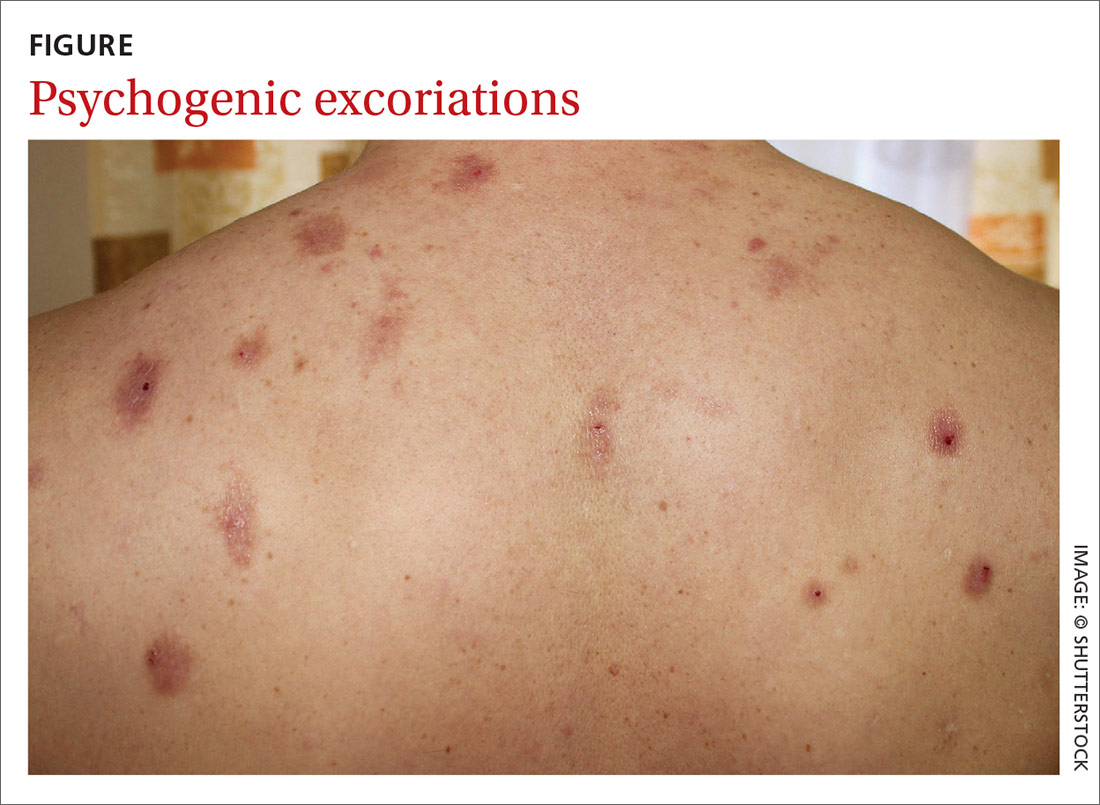
Delusion of infestation. Patients with a delusion of infestation have a strong belief that their body is infected by some kind of insect or microorganism.16 Before a diagnosis of delusion of infestation can be made, other organic causes must be excluded, including withdrawal from such substances as cocaine, amphetamines, and alcohol.16 Patients with a delusion of infestation can have, and maintain, a symptomatic response with continuing use of an atypical antipsychotic agent, including risperidone and olanzapine.17
Evaluation and diagnostic work-up
A thorough medical history, review of systems, medication review, social history, and family history are important when evaluating a patient with chronic pruritus.18 These items can be valuable in formulating a differential diagnosis, even before a physical examination.
Physical examination. The physical exam should include detailed inspection of the entire skin and hair18; such a comprehensive physical exam can determine whether the source of the itch is cutaneous.7 This, in turn, can help further narrow the differential diagnosis. It is crucial that the physical exam include palpation of the liver, spleen, lymph nodes, and thyroid for organomegaly,8 which could indicate a serious systemic condition, such as lymphoma.
The ice-pack sign—in which an ice pack is applied to the pruritic area, the patient experiences immediate relief of pruritus, and the itch returns soon after the ice pack is removed—is considered pathognomonic for brachioradial pruritus.19
Continue to: Chronic pruritus with abnormal findings...
Chronic pruritus with abnormal findings on the physical exam should prompt an initial work-up.18 Also consider an initial work-up for a patient with chronic pruritus whose symptom has not been relieved with conservative treatment.18
Laboratory testing. The initial laboratory work-up could include any of the following evaluations: complete blood count, measurement of thyroid-stimulating hormone, comprehensive metabolic panel (liver function, renal function, and the serum glucose level) and the erythrocyte sedimentation rate (TABLE 2).18 If warranted by the evaluation and physical exam, blood work can also include serologic studies for human immunodeficiency virus infection and hepatitis.17
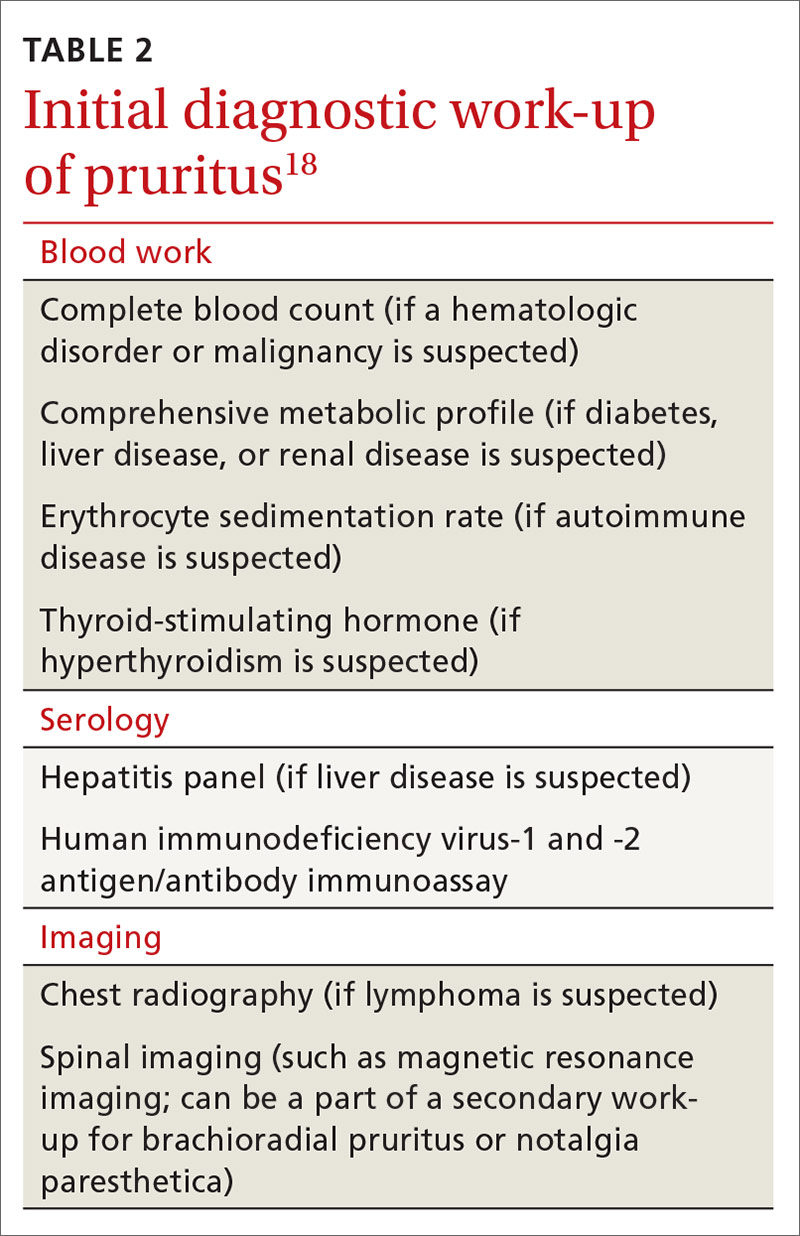
Imaging. Chest radiography should be performed if there is suspicion of malignancy, such as lymphoma.7 Although brachioradial pruritus and notalgia paresthetica have been postulated to be caused by impingement of spinal nerves, obtaining spinal imaging, such as magnetic resonance imaging, as part of the initial work-up is not recommended; because spinal images might not show evidence of spinal disease, obtaining spinal imaging is not a requirement before treating brachioradial pruritus and notalgia paresthetica. Do consider spinal imaging, however, for patients in whom brachioradial pruritus or notalgia paresthetica is suspected and conservative treatment has not produced a response.
Treatment: Nondrug approaches, topicals, systemic agents
Start conservatively. Treatment of pruritus should begin with behavior modification and nonpharmacotherapeutic options (TABLE 38). Educate the patient that scratching might cause secondary skin lesions; empowering them with that knowledge is sometimes enough to help break the scratching cycling—especially if the patient combines behavior modification with proper skin hydration with an emollient. To prevent secondary skin lesions through involuntary scratching, consider recommending that lesions be covered with an occlusive dressing or protective clothing.13

Stress has been shown to make chronic itch worse; therefore, stress-reduction activities, such as exercise, meditation, and yoga, might be helpful.20 For patients in whom pruritus has a psychological component, referral to a psychiatrist or psychologist might be therapeutic.
Continue to: When a patient complains...
When a patient complains of severe pruritus at first presentation, consider pharmacotherapy in conjunction with nonpharmacotherapeutic options. Several of the more effective topical therapies for pruritusa are listed in TABLE 4.20 Well-known systemic agents for this purpose are reviewed below and listed in TABLE 5.7
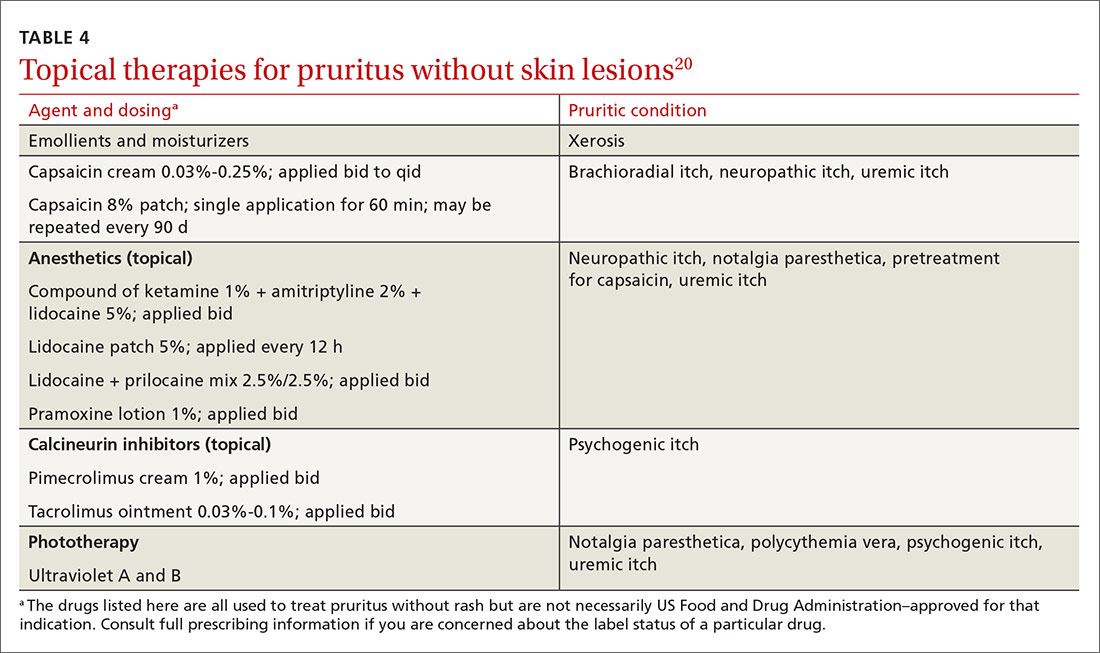
Systemic treatment
Antihistamines. A staple in the treatment of pruritus for many years, antihistamines are not effective for all causes; however, they are effective in treating paraneoplastic pruritus.20 First-generation antihistamines, with their sedating effect, can be useful for patients who experience generalized pruritus at night.20

Anticonvulsants. Gabapentin and pregabalin are analogs of the neurotransmitter gamma-aminobutyric acid.20 This drug class is helpful in neuropathic pruritus specifically caused by impingements, such as brachioradial pruritus and notalgia paresthetica.20 In addition, of all systemic therapies used to treat uremic pruritus, gabapentin has, in clinical trials, most consistently been found effective for uremic pruritus.6 (Note: Use renal dosing of gabapentin in patients with renal failure.)
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs; eg, fluvoxamine, paroxetine, and sertraline) might cause itch to subside by increasing the serotonin level, which, in turn, works to decrease inflammatory substances that cause itch.7 SSRIs have been used to treat patients with psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.7
Tricyclic antidepressants (eg, amitriptyline and doxepin) lessen the itch by antagonizing histamine receptors and through anticholinergic mechanisms. Tricyclics are best used in the treatment of psychogenic and nocturnal itch.7
Continue to: Mirtazapine...
Mirtazapine, a tetracyclic antidepressant, works in patients with uremic pruritus, psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.1
Substance P antagonist. Aprepitant, a neurokinin receptor I antagonist, is a newer agent that inhibits binding of the itch mediator substance P to the neurokinin receptor. The drug has been found helpful in patients with drug-induced, paraneoplastic, and brachioradial pruritus.7
Opioid-receptor agents. Naltrexone, as a mu opioid-receptor antagonist, has shown promise as a treatment for uremic pruritus and cholestatic pruritus. Nalfurafine, a kappa opioid-receptor agonist, is emerging as a possible therapy for uremic pruritus.7
Bile-acid sequestrants. A few small studies have shown that treatment with a bile-acid sequestrant, such as cholestyramine and ursodiol, induces moderate improvement in symptoms in patients with cholestatic pruritus.21
CORRESPONDENCE
Matasha Russell, MD, Department of Family and Community Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, 6431 Fannin Street, JJL 324, Houston, TX 77030; [email protected].
1. Tarikci N, E, S, et al. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. ScientificWorldJournal. 2015;2015:803752.
2. Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625-1634.
3. Silverberg JI, Kantor RW, Dalal P. A comprehensive conceptual model of the experience of chronic itch in adults. Am J Clin Dermatol. 2018;19:759-769.
4. Matterne U, Apfelbacher CJ, Vogelgsang L, et al. Incidence and determinants of chronic pruritus: a population based cohort study. Acta Derm Venereol. 2013;93:532-537.
5. Moses S. Pruritus. Am Fam Physician. 2003;68:1135-1142.
6. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383-391.
7. Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
8. Reamy BV, Bunt C. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
9. M. Current concepts of pathophysiology, epidemiology and classification of pruritus. Srp Arh Celok Lek. 2014;142:106-112.
10. Fett N, Haynes K, Propert KJ, et al. Five-year malignancy incidence in patients with chronic pruritus: a population-based cohort study aimed at limiting unnecessary screening practices. J Am Acad Dermatol. 2014;70:651-658.
11. Larson VA, Tang O, S, et al. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80:931-937.
12. Rosen JD, Fostini AC, Chan YH, et al. Cross-sectional study of clinical distinctions between neuropathic and inflammatory pruritus. J Am Acad Dermatol. 2018;79:1143-1144.
13. Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87-92.
14. Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol. 2010;90:395-400.
15. Jafferany M, Davari ME. Itch and psyche: psychiatric aspects of pruritus. Int J Dermatol. 2019;58:3-23.
16. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician. 2001;64:1873-1878.
17. Bewley AP, Lepping P, Freudenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol.2010;163:1-2.
18. Clerc C-J, Misery L. A literature review of senile pruritus: from diagnosis to treatment. Acta Derm Venereol. 2017;97:433-440.
19. Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
20. Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [version 1]. F1000Res. 2016;5 F1000 Faculty Rev–2042.
21. Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114-1123.
Pruritus, defined as a sensation that induces a desire to scratch1 and classified as acute or chronic (lasting > 6 weeks),2 is one of the most common complaints among primary care patients: Approximately 1% of ambulatory visits in the United States are linked to pruritus.3
Chronic pruritus impairs quality of life; its impact has been compared to that of chronic pain.4 Treatment should therefore be instituted promptly. Although this condition might appear benign, chronic pruritus can be a symptom of a serious condition, as we describe here. When persistent pruritus is refractory to treatment, systemic causes should be fully explored.
In this article, we discuss the pathogenesis and management of pruritus without skin eruption in the adult nonpregnant patient. We also present practice recommendations to help you determine whether your patient’s pruritus is indicative of a serious systemic condition.

An incomplete understanding of the pathophysiology of pruritus
The pathophysiology of pruritus is not fully understood. It is generally recognized, however, that pruritus starts in the peripheral nerves located in the dermal–epidermal junction of the skin.5 The sensation is then transmitted along unmyelinated slow-conducting C fibers to the dorsal horn of the spinal cord.5,6 There are 2 types of C fibers that transmit the itch impulse6: A histamine-dependent type and a non-histamine-dependent type, which might explain why pruritus can be refractory to antihistamine treatment.6
Once the itch impulse has moved from the spinal cord, it travels along the spinothalamic tract up to the contralateral thalamus.1 From there, the impulse ascends to the cerebral cortex.1 In the cortex, the impulse triggers multiple areas of the brain, such as those responsible for sensation, motor function, reward, memory, and emotion.7
Several chemical mediators have been found to be peripheral and central inducers of pruritus: histamine, endogenous opioids, substance P, and serotonin.2 There are indications that certain receptors, such as mu-opioid receptors and kappa-opioid receptors, are key contributors to itch as well.2

A diverse etiology
The International Forum for the Study of Itch (IFSI) has established 6 main categories of causes of pruritus(TABLE 1)2:
- dermatologic
- systemic
- neurologic
- psychogenic
- mixed
- other.
Continue to: In this review...
In this review, we focus on the work-up and management of 3 of those categories: systemic, neurologic, and psychogenic causes of pruritus.
Systemic causes
Research has shown that 14% to 24% of patients who seek the care of a dermatologist for chronic itch without skin lesions have a systemic illness.8
Renal disease. Approximately 40% of patients with end-stage renal disease who are on hemodialysis or peritoneal dialysis have uremic pruritus.2 The itch is mostly generalized but can be pronounced on the back. For most patients, the itch is worse at night, causing a major impact on quality of life.6
Liver disease. In hepatic disease, there is often impairment in the secretion of bile, which can lead to cholestatic pruritus.2 This condition commonly affects the hands and feet first; later, it becomes generalized.2 Cholestatic pruritus can be elicited by tight-fitting clothing. Relief is not achieved by scratching.9 This type of itch effects 70% of patients with primary biliary cirrhosis and 15% of patients with hepatitis C infection.9
Hematologic disorders. Pruritus is a hallmark symptom of polycythemia rubra vera. Almost 50% of patients with this disorder report pruritus that occurs after exposure to water9; aquagenic pruritus can precede the formal diagnosis of polycythemia rubra vera by years.2 It has been speculated that platelet aggregation in this disorder leads to release of serotonin and histamine, which, in turn, causes itch.9
Continue to: Endocrine disorders
Endocrine disorders. Approximately 4% to 11% of patients with thyrotoxicosis have pruritus.1 It has been suggested that vasodilation, increased skin temperature, and a decreased itch threshold from untreated Graves disease might be inciting factors.
Malignancy. In generalized chronic pruritus without a known cause, strongly consider the likelihood of underlying malignancy8,10; for 10% of these patients, their chronic pruritus is a paraneoplastic sign. Paraneoplastic pruritus is characterized as an itch that predates clinical onset, or occurs early in the course, of a malignancy.9 The condition is most strongly linked to cancers of the liver, gallbladder, biliary tract, hematologic system, and skin.11
Chronic pruritus affects 30% of patients with Hodgkin lymphoma.9 General pruritus can precede this diagnosis by months, even years.1 In Hodgkin lymphoma patients who are in remission, a return of pruritic symptoms can be a harbinger of recurrence.9
Neurologic causes
A recent study found that 8% to 15% of patients referred to a dermatology clinic for chronic pruritus without skin eruption had underlying neurologic pathology.12 Although the specific mechanisms of neuropathic itch are still poorly understood, it has been theorized that the itch emanates from neuronal damage, which can come from peripheral or central nervous system lesions.9
Brachioradial pruritus. There are divergent theories about the etiology of brachioradial pruritus. One hypothesis is that the condition is caused by cervical nerve-root impingement at the level of C5-C8 that leads to nerve damage2; another is that chronic exposure to sunlight causes injury to peripheral cutaneous nerves.2 Brachioradial pruritus is localized to the dorsolateral forearm; it can also involve the neck, back, shoulder, upper arm, and chest, unilaterally and bilaterally. This pruritus can be intermittent and become worse upon exposure to sunlight.2
Continue to: Notalgia paresthetica
Notalgia paresthetica. This condition might also cause neuropathic pruritus as a consequence of nerve impingement. The itch of notalgia paresthesia is located on the skin, medial to the scapular border on the upper or mid-back.2 It has been postulated that the itch is caused by nerve entrapment of the posterior rami of spinal nerves arising from T2-T6.9 However, another theory suggests that the itch is caused by damage to peripheral nerves.9 The itch of notalgia paresthetica can wax and wane.2
Poststroke pruritus. Brain lesions, most often caused by stroke, can cause neuropathic itch. One of the best-known syndromes related to poststroke itch is Wallenberg syndrome (ischemia from a lateral medullary infarction), which typically presents with itch, thermalgic hypoesthesia of the face, cerebellar dysfunction, nausea, and vomiting.7
Shingles. More than one-half of patients who develop postherpetic neuralgia as a consequence of a herpes zoster infection also develop neuropathic pruritus.9 It is thought that postherpetic pruritus shares a comparable pathophysiology with postherpetic neuralgia, in which neurons involved in itch stimuli become damaged.7
Diabetes mellitus. Pruritus from diabetes can be classified as systemic or neuropathic. Diabetes is one of the most common causes of small-fiber polyneuropathy, which can cause neuropathic pruritus.13
Multiple sclerosis. Central nervous system lesions that affect sensory pathways can lead to neuropathic itch in multiple sclerosis. Patients can have severe episodes of generalized pruritus. It has been hypothesized that the neuropathic itch in multiple sclerosis is induced by activation of artificial synapses in demyelinated areas.2
Continue to: Psychogenic pruritus
Psychogenic pruritus
Chronic pruritus can be a comorbidity of psychiatric illness. A retrospective study found that pruritus occurs in 32% to 42% of psychiatric inpatients.14 Depression, anxiety, bipolar disorders, obsessive–compulsive disorders, somatoform disorders, psychosis, and substance abuse all have a strong link to psychogenic excoriation.15 Psychogenic excoriation, which can cause secondary skin lesions, occurs in psychiatric patients who excessively pick and scratch normal skin because they perceive an itch sensation or have a delusion of infestation.2 Affected skin can be marked by scattered crusted lesions (FIGURE) anywhere on the body that the patient can reach—most commonly, the extremities.2

Delusion of infestation. Patients with a delusion of infestation have a strong belief that their body is infected by some kind of insect or microorganism.16 Before a diagnosis of delusion of infestation can be made, other organic causes must be excluded, including withdrawal from such substances as cocaine, amphetamines, and alcohol.16 Patients with a delusion of infestation can have, and maintain, a symptomatic response with continuing use of an atypical antipsychotic agent, including risperidone and olanzapine.17
Evaluation and diagnostic work-up
A thorough medical history, review of systems, medication review, social history, and family history are important when evaluating a patient with chronic pruritus.18 These items can be valuable in formulating a differential diagnosis, even before a physical examination.
Physical examination. The physical exam should include detailed inspection of the entire skin and hair18; such a comprehensive physical exam can determine whether the source of the itch is cutaneous.7 This, in turn, can help further narrow the differential diagnosis. It is crucial that the physical exam include palpation of the liver, spleen, lymph nodes, and thyroid for organomegaly,8 which could indicate a serious systemic condition, such as lymphoma.
The ice-pack sign—in which an ice pack is applied to the pruritic area, the patient experiences immediate relief of pruritus, and the itch returns soon after the ice pack is removed—is considered pathognomonic for brachioradial pruritus.19
Continue to: Chronic pruritus with abnormal findings...
Chronic pruritus with abnormal findings on the physical exam should prompt an initial work-up.18 Also consider an initial work-up for a patient with chronic pruritus whose symptom has not been relieved with conservative treatment.18
Laboratory testing. The initial laboratory work-up could include any of the following evaluations: complete blood count, measurement of thyroid-stimulating hormone, comprehensive metabolic panel (liver function, renal function, and the serum glucose level) and the erythrocyte sedimentation rate (TABLE 2).18 If warranted by the evaluation and physical exam, blood work can also include serologic studies for human immunodeficiency virus infection and hepatitis.17

Imaging. Chest radiography should be performed if there is suspicion of malignancy, such as lymphoma.7 Although brachioradial pruritus and notalgia paresthetica have been postulated to be caused by impingement of spinal nerves, obtaining spinal imaging, such as magnetic resonance imaging, as part of the initial work-up is not recommended; because spinal images might not show evidence of spinal disease, obtaining spinal imaging is not a requirement before treating brachioradial pruritus and notalgia paresthetica. Do consider spinal imaging, however, for patients in whom brachioradial pruritus or notalgia paresthetica is suspected and conservative treatment has not produced a response.
Treatment: Nondrug approaches, topicals, systemic agents
Start conservatively. Treatment of pruritus should begin with behavior modification and nonpharmacotherapeutic options (TABLE 38). Educate the patient that scratching might cause secondary skin lesions; empowering them with that knowledge is sometimes enough to help break the scratching cycling—especially if the patient combines behavior modification with proper skin hydration with an emollient. To prevent secondary skin lesions through involuntary scratching, consider recommending that lesions be covered with an occlusive dressing or protective clothing.13

Stress has been shown to make chronic itch worse; therefore, stress-reduction activities, such as exercise, meditation, and yoga, might be helpful.20 For patients in whom pruritus has a psychological component, referral to a psychiatrist or psychologist might be therapeutic.
Continue to: When a patient complains...
When a patient complains of severe pruritus at first presentation, consider pharmacotherapy in conjunction with nonpharmacotherapeutic options. Several of the more effective topical therapies for pruritusa are listed in TABLE 4.20 Well-known systemic agents for this purpose are reviewed below and listed in TABLE 5.7

Systemic treatment
Antihistamines. A staple in the treatment of pruritus for many years, antihistamines are not effective for all causes; however, they are effective in treating paraneoplastic pruritus.20 First-generation antihistamines, with their sedating effect, can be useful for patients who experience generalized pruritus at night.20

Anticonvulsants. Gabapentin and pregabalin are analogs of the neurotransmitter gamma-aminobutyric acid.20 This drug class is helpful in neuropathic pruritus specifically caused by impingements, such as brachioradial pruritus and notalgia paresthetica.20 In addition, of all systemic therapies used to treat uremic pruritus, gabapentin has, in clinical trials, most consistently been found effective for uremic pruritus.6 (Note: Use renal dosing of gabapentin in patients with renal failure.)
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs; eg, fluvoxamine, paroxetine, and sertraline) might cause itch to subside by increasing the serotonin level, which, in turn, works to decrease inflammatory substances that cause itch.7 SSRIs have been used to treat patients with psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.7
Tricyclic antidepressants (eg, amitriptyline and doxepin) lessen the itch by antagonizing histamine receptors and through anticholinergic mechanisms. Tricyclics are best used in the treatment of psychogenic and nocturnal itch.7
Continue to: Mirtazapine...
Mirtazapine, a tetracyclic antidepressant, works in patients with uremic pruritus, psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.1
Substance P antagonist. Aprepitant, a neurokinin receptor I antagonist, is a newer agent that inhibits binding of the itch mediator substance P to the neurokinin receptor. The drug has been found helpful in patients with drug-induced, paraneoplastic, and brachioradial pruritus.7
Opioid-receptor agents. Naltrexone, as a mu opioid-receptor antagonist, has shown promise as a treatment for uremic pruritus and cholestatic pruritus. Nalfurafine, a kappa opioid-receptor agonist, is emerging as a possible therapy for uremic pruritus.7
Bile-acid sequestrants. A few small studies have shown that treatment with a bile-acid sequestrant, such as cholestyramine and ursodiol, induces moderate improvement in symptoms in patients with cholestatic pruritus.21
CORRESPONDENCE
Matasha Russell, MD, Department of Family and Community Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, 6431 Fannin Street, JJL 324, Houston, TX 77030; [email protected].
Pruritus, defined as a sensation that induces a desire to scratch1 and classified as acute or chronic (lasting > 6 weeks),2 is one of the most common complaints among primary care patients: Approximately 1% of ambulatory visits in the United States are linked to pruritus.3
Chronic pruritus impairs quality of life; its impact has been compared to that of chronic pain.4 Treatment should therefore be instituted promptly. Although this condition might appear benign, chronic pruritus can be a symptom of a serious condition, as we describe here. When persistent pruritus is refractory to treatment, systemic causes should be fully explored.
In this article, we discuss the pathogenesis and management of pruritus without skin eruption in the adult nonpregnant patient. We also present practice recommendations to help you determine whether your patient’s pruritus is indicative of a serious systemic condition.

An incomplete understanding of the pathophysiology of pruritus
The pathophysiology of pruritus is not fully understood. It is generally recognized, however, that pruritus starts in the peripheral nerves located in the dermal–epidermal junction of the skin.5 The sensation is then transmitted along unmyelinated slow-conducting C fibers to the dorsal horn of the spinal cord.5,6 There are 2 types of C fibers that transmit the itch impulse6: A histamine-dependent type and a non-histamine-dependent type, which might explain why pruritus can be refractory to antihistamine treatment.6
Once the itch impulse has moved from the spinal cord, it travels along the spinothalamic tract up to the contralateral thalamus.1 From there, the impulse ascends to the cerebral cortex.1 In the cortex, the impulse triggers multiple areas of the brain, such as those responsible for sensation, motor function, reward, memory, and emotion.7
Several chemical mediators have been found to be peripheral and central inducers of pruritus: histamine, endogenous opioids, substance P, and serotonin.2 There are indications that certain receptors, such as mu-opioid receptors and kappa-opioid receptors, are key contributors to itch as well.2

A diverse etiology
The International Forum for the Study of Itch (IFSI) has established 6 main categories of causes of pruritus(TABLE 1)2:
- dermatologic
- systemic
- neurologic
- psychogenic
- mixed
- other.
Continue to: In this review...
In this review, we focus on the work-up and management of 3 of those categories: systemic, neurologic, and psychogenic causes of pruritus.
Systemic causes
Research has shown that 14% to 24% of patients who seek the care of a dermatologist for chronic itch without skin lesions have a systemic illness.8
Renal disease. Approximately 40% of patients with end-stage renal disease who are on hemodialysis or peritoneal dialysis have uremic pruritus.2 The itch is mostly generalized but can be pronounced on the back. For most patients, the itch is worse at night, causing a major impact on quality of life.6
Liver disease. In hepatic disease, there is often impairment in the secretion of bile, which can lead to cholestatic pruritus.2 This condition commonly affects the hands and feet first; later, it becomes generalized.2 Cholestatic pruritus can be elicited by tight-fitting clothing. Relief is not achieved by scratching.9 This type of itch effects 70% of patients with primary biliary cirrhosis and 15% of patients with hepatitis C infection.9
Hematologic disorders. Pruritus is a hallmark symptom of polycythemia rubra vera. Almost 50% of patients with this disorder report pruritus that occurs after exposure to water9; aquagenic pruritus can precede the formal diagnosis of polycythemia rubra vera by years.2 It has been speculated that platelet aggregation in this disorder leads to release of serotonin and histamine, which, in turn, causes itch.9
Continue to: Endocrine disorders
Endocrine disorders. Approximately 4% to 11% of patients with thyrotoxicosis have pruritus.1 It has been suggested that vasodilation, increased skin temperature, and a decreased itch threshold from untreated Graves disease might be inciting factors.
Malignancy. In generalized chronic pruritus without a known cause, strongly consider the likelihood of underlying malignancy8,10; for 10% of these patients, their chronic pruritus is a paraneoplastic sign. Paraneoplastic pruritus is characterized as an itch that predates clinical onset, or occurs early in the course, of a malignancy.9 The condition is most strongly linked to cancers of the liver, gallbladder, biliary tract, hematologic system, and skin.11
Chronic pruritus affects 30% of patients with Hodgkin lymphoma.9 General pruritus can precede this diagnosis by months, even years.1 In Hodgkin lymphoma patients who are in remission, a return of pruritic symptoms can be a harbinger of recurrence.9
Neurologic causes
A recent study found that 8% to 15% of patients referred to a dermatology clinic for chronic pruritus without skin eruption had underlying neurologic pathology.12 Although the specific mechanisms of neuropathic itch are still poorly understood, it has been theorized that the itch emanates from neuronal damage, which can come from peripheral or central nervous system lesions.9
Brachioradial pruritus. There are divergent theories about the etiology of brachioradial pruritus. One hypothesis is that the condition is caused by cervical nerve-root impingement at the level of C5-C8 that leads to nerve damage2; another is that chronic exposure to sunlight causes injury to peripheral cutaneous nerves.2 Brachioradial pruritus is localized to the dorsolateral forearm; it can also involve the neck, back, shoulder, upper arm, and chest, unilaterally and bilaterally. This pruritus can be intermittent and become worse upon exposure to sunlight.2
Continue to: Notalgia paresthetica
Notalgia paresthetica. This condition might also cause neuropathic pruritus as a consequence of nerve impingement. The itch of notalgia paresthesia is located on the skin, medial to the scapular border on the upper or mid-back.2 It has been postulated that the itch is caused by nerve entrapment of the posterior rami of spinal nerves arising from T2-T6.9 However, another theory suggests that the itch is caused by damage to peripheral nerves.9 The itch of notalgia paresthetica can wax and wane.2
Poststroke pruritus. Brain lesions, most often caused by stroke, can cause neuropathic itch. One of the best-known syndromes related to poststroke itch is Wallenberg syndrome (ischemia from a lateral medullary infarction), which typically presents with itch, thermalgic hypoesthesia of the face, cerebellar dysfunction, nausea, and vomiting.7
Shingles. More than one-half of patients who develop postherpetic neuralgia as a consequence of a herpes zoster infection also develop neuropathic pruritus.9 It is thought that postherpetic pruritus shares a comparable pathophysiology with postherpetic neuralgia, in which neurons involved in itch stimuli become damaged.7
Diabetes mellitus. Pruritus from diabetes can be classified as systemic or neuropathic. Diabetes is one of the most common causes of small-fiber polyneuropathy, which can cause neuropathic pruritus.13
Multiple sclerosis. Central nervous system lesions that affect sensory pathways can lead to neuropathic itch in multiple sclerosis. Patients can have severe episodes of generalized pruritus. It has been hypothesized that the neuropathic itch in multiple sclerosis is induced by activation of artificial synapses in demyelinated areas.2
Continue to: Psychogenic pruritus
Psychogenic pruritus
Chronic pruritus can be a comorbidity of psychiatric illness. A retrospective study found that pruritus occurs in 32% to 42% of psychiatric inpatients.14 Depression, anxiety, bipolar disorders, obsessive–compulsive disorders, somatoform disorders, psychosis, and substance abuse all have a strong link to psychogenic excoriation.15 Psychogenic excoriation, which can cause secondary skin lesions, occurs in psychiatric patients who excessively pick and scratch normal skin because they perceive an itch sensation or have a delusion of infestation.2 Affected skin can be marked by scattered crusted lesions (FIGURE) anywhere on the body that the patient can reach—most commonly, the extremities.2

Delusion of infestation. Patients with a delusion of infestation have a strong belief that their body is infected by some kind of insect or microorganism.16 Before a diagnosis of delusion of infestation can be made, other organic causes must be excluded, including withdrawal from such substances as cocaine, amphetamines, and alcohol.16 Patients with a delusion of infestation can have, and maintain, a symptomatic response with continuing use of an atypical antipsychotic agent, including risperidone and olanzapine.17
Evaluation and diagnostic work-up
A thorough medical history, review of systems, medication review, social history, and family history are important when evaluating a patient with chronic pruritus.18 These items can be valuable in formulating a differential diagnosis, even before a physical examination.
Physical examination. The physical exam should include detailed inspection of the entire skin and hair18; such a comprehensive physical exam can determine whether the source of the itch is cutaneous.7 This, in turn, can help further narrow the differential diagnosis. It is crucial that the physical exam include palpation of the liver, spleen, lymph nodes, and thyroid for organomegaly,8 which could indicate a serious systemic condition, such as lymphoma.
The ice-pack sign—in which an ice pack is applied to the pruritic area, the patient experiences immediate relief of pruritus, and the itch returns soon after the ice pack is removed—is considered pathognomonic for brachioradial pruritus.19
Continue to: Chronic pruritus with abnormal findings...
Chronic pruritus with abnormal findings on the physical exam should prompt an initial work-up.18 Also consider an initial work-up for a patient with chronic pruritus whose symptom has not been relieved with conservative treatment.18
Laboratory testing. The initial laboratory work-up could include any of the following evaluations: complete blood count, measurement of thyroid-stimulating hormone, comprehensive metabolic panel (liver function, renal function, and the serum glucose level) and the erythrocyte sedimentation rate (TABLE 2).18 If warranted by the evaluation and physical exam, blood work can also include serologic studies for human immunodeficiency virus infection and hepatitis.17

Imaging. Chest radiography should be performed if there is suspicion of malignancy, such as lymphoma.7 Although brachioradial pruritus and notalgia paresthetica have been postulated to be caused by impingement of spinal nerves, obtaining spinal imaging, such as magnetic resonance imaging, as part of the initial work-up is not recommended; because spinal images might not show evidence of spinal disease, obtaining spinal imaging is not a requirement before treating brachioradial pruritus and notalgia paresthetica. Do consider spinal imaging, however, for patients in whom brachioradial pruritus or notalgia paresthetica is suspected and conservative treatment has not produced a response.
Treatment: Nondrug approaches, topicals, systemic agents
Start conservatively. Treatment of pruritus should begin with behavior modification and nonpharmacotherapeutic options (TABLE 38). Educate the patient that scratching might cause secondary skin lesions; empowering them with that knowledge is sometimes enough to help break the scratching cycling—especially if the patient combines behavior modification with proper skin hydration with an emollient. To prevent secondary skin lesions through involuntary scratching, consider recommending that lesions be covered with an occlusive dressing or protective clothing.13

Stress has been shown to make chronic itch worse; therefore, stress-reduction activities, such as exercise, meditation, and yoga, might be helpful.20 For patients in whom pruritus has a psychological component, referral to a psychiatrist or psychologist might be therapeutic.
Continue to: When a patient complains...
When a patient complains of severe pruritus at first presentation, consider pharmacotherapy in conjunction with nonpharmacotherapeutic options. Several of the more effective topical therapies for pruritusa are listed in TABLE 4.20 Well-known systemic agents for this purpose are reviewed below and listed in TABLE 5.7

Systemic treatment
Antihistamines. A staple in the treatment of pruritus for many years, antihistamines are not effective for all causes; however, they are effective in treating paraneoplastic pruritus.20 First-generation antihistamines, with their sedating effect, can be useful for patients who experience generalized pruritus at night.20

Anticonvulsants. Gabapentin and pregabalin are analogs of the neurotransmitter gamma-aminobutyric acid.20 This drug class is helpful in neuropathic pruritus specifically caused by impingements, such as brachioradial pruritus and notalgia paresthetica.20 In addition, of all systemic therapies used to treat uremic pruritus, gabapentin has, in clinical trials, most consistently been found effective for uremic pruritus.6 (Note: Use renal dosing of gabapentin in patients with renal failure.)
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs; eg, fluvoxamine, paroxetine, and sertraline) might cause itch to subside by increasing the serotonin level, which, in turn, works to decrease inflammatory substances that cause itch.7 SSRIs have been used to treat patients with psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.7
Tricyclic antidepressants (eg, amitriptyline and doxepin) lessen the itch by antagonizing histamine receptors and through anticholinergic mechanisms. Tricyclics are best used in the treatment of psychogenic and nocturnal itch.7
Continue to: Mirtazapine...
Mirtazapine, a tetracyclic antidepressant, works in patients with uremic pruritus, psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.1
Substance P antagonist. Aprepitant, a neurokinin receptor I antagonist, is a newer agent that inhibits binding of the itch mediator substance P to the neurokinin receptor. The drug has been found helpful in patients with drug-induced, paraneoplastic, and brachioradial pruritus.7
Opioid-receptor agents. Naltrexone, as a mu opioid-receptor antagonist, has shown promise as a treatment for uremic pruritus and cholestatic pruritus. Nalfurafine, a kappa opioid-receptor agonist, is emerging as a possible therapy for uremic pruritus.7
Bile-acid sequestrants. A few small studies have shown that treatment with a bile-acid sequestrant, such as cholestyramine and ursodiol, induces moderate improvement in symptoms in patients with cholestatic pruritus.21
CORRESPONDENCE
Matasha Russell, MD, Department of Family and Community Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, 6431 Fannin Street, JJL 324, Houston, TX 77030; [email protected].
1. Tarikci N, E, S, et al. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. ScientificWorldJournal. 2015;2015:803752.
2. Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625-1634.
3. Silverberg JI, Kantor RW, Dalal P. A comprehensive conceptual model of the experience of chronic itch in adults. Am J Clin Dermatol. 2018;19:759-769.
4. Matterne U, Apfelbacher CJ, Vogelgsang L, et al. Incidence and determinants of chronic pruritus: a population based cohort study. Acta Derm Venereol. 2013;93:532-537.
5. Moses S. Pruritus. Am Fam Physician. 2003;68:1135-1142.
6. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383-391.
7. Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
8. Reamy BV, Bunt C. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
9. M. Current concepts of pathophysiology, epidemiology and classification of pruritus. Srp Arh Celok Lek. 2014;142:106-112.
10. Fett N, Haynes K, Propert KJ, et al. Five-year malignancy incidence in patients with chronic pruritus: a population-based cohort study aimed at limiting unnecessary screening practices. J Am Acad Dermatol. 2014;70:651-658.
11. Larson VA, Tang O, S, et al. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80:931-937.
12. Rosen JD, Fostini AC, Chan YH, et al. Cross-sectional study of clinical distinctions between neuropathic and inflammatory pruritus. J Am Acad Dermatol. 2018;79:1143-1144.
13. Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87-92.
14. Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol. 2010;90:395-400.
15. Jafferany M, Davari ME. Itch and psyche: psychiatric aspects of pruritus. Int J Dermatol. 2019;58:3-23.
16. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician. 2001;64:1873-1878.
17. Bewley AP, Lepping P, Freudenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol.2010;163:1-2.
18. Clerc C-J, Misery L. A literature review of senile pruritus: from diagnosis to treatment. Acta Derm Venereol. 2017;97:433-440.
19. Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
20. Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [version 1]. F1000Res. 2016;5 F1000 Faculty Rev–2042.
21. Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114-1123.
1. Tarikci N, E, S, et al. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. ScientificWorldJournal. 2015;2015:803752.
2. Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625-1634.
3. Silverberg JI, Kantor RW, Dalal P. A comprehensive conceptual model of the experience of chronic itch in adults. Am J Clin Dermatol. 2018;19:759-769.
4. Matterne U, Apfelbacher CJ, Vogelgsang L, et al. Incidence and determinants of chronic pruritus: a population based cohort study. Acta Derm Venereol. 2013;93:532-537.
5. Moses S. Pruritus. Am Fam Physician. 2003;68:1135-1142.
6. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383-391.
7. Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
8. Reamy BV, Bunt C. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
9. M. Current concepts of pathophysiology, epidemiology and classification of pruritus. Srp Arh Celok Lek. 2014;142:106-112.
10. Fett N, Haynes K, Propert KJ, et al. Five-year malignancy incidence in patients with chronic pruritus: a population-based cohort study aimed at limiting unnecessary screening practices. J Am Acad Dermatol. 2014;70:651-658.
11. Larson VA, Tang O, S, et al. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80:931-937.
12. Rosen JD, Fostini AC, Chan YH, et al. Cross-sectional study of clinical distinctions between neuropathic and inflammatory pruritus. J Am Acad Dermatol. 2018;79:1143-1144.
13. Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87-92.
14. Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol. 2010;90:395-400.
15. Jafferany M, Davari ME. Itch and psyche: psychiatric aspects of pruritus. Int J Dermatol. 2019;58:3-23.
16. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician. 2001;64:1873-1878.
17. Bewley AP, Lepping P, Freudenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol.2010;163:1-2.
18. Clerc C-J, Misery L. A literature review of senile pruritus: from diagnosis to treatment. Acta Derm Venereol. 2017;97:433-440.
19. Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
20. Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [version 1]. F1000Res. 2016;5 F1000 Faculty Rev–2042.
21. Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114-1123.
PRACTICE RECOMMENDATIONS
› Undertake a diagnostic work-up for systemic causes of pruritus in patients who have a chronic, generalized itch and abnormal findings on physical examination. C
› Prescribe gabapentin for its effectiveness in treating pruritus caused by uremic and neurologic itch. B
› Consider prescribing one of the bile-acid sequestrants in patients with cholestatic pruritus because these agents can provide moderate relief of the symptom. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Increasing ear pain and headache
A previously healthy 12-year-old boy with normal development presented to his primary care physician (PCP) with a 1-week history of moderate ear pain. He was given a diagnosis of acute otitis media (AOM) and prescribed a 7-day course of amoxicillin. Although the child’s history was otherwise unremarkable, the mother reported that she’d had a deep venous thrombosis and pulmonary embolism a year earlier.
The boy continued to experience intermittent left ear pain 2 weeks after completing his antibiotic treatment, leading the PCP to refer him to an otolaryngologist. An examination by the otolaryngologist revealed a cloudy, bulging tympanic membrane. The patient was prescribed amoxicillin/clavulanate and ofloxacin ear drops.
Two days later, he was admitted to the emergency department (ED) due to worsening left ear pain and a new-onset left-sided headache. His left tympanic membrane was normal, with no tenderness or erythema of the mastoid. His vital signs were normal. He was afebrile and discharged home.
A week later, he returned to the ED with worsening ear pain and severe persistent headache, which was localized in the left occipital, left frontal, and retro-orbital regions. He denied light or sound sensitivity, nausea, vomiting, or increased lacrimation. He was tearful on examination due to the pain. He had no meningismus and normal fundi. A neurologic examination was nonlateralizing. Laboratory tests showed a normal complete blood count but increased C-reactive protein at 113 mg/dL (normal, < 0.3 mg/dL) and an erythrocyte sedimentation rate of 88 mm/hr (normal, 0-20 mm/hr).
Magnetic resonance imaging was ordered (FIGURES 1A and 1B), and Neurosurgery and Otolaryngology were consulted.
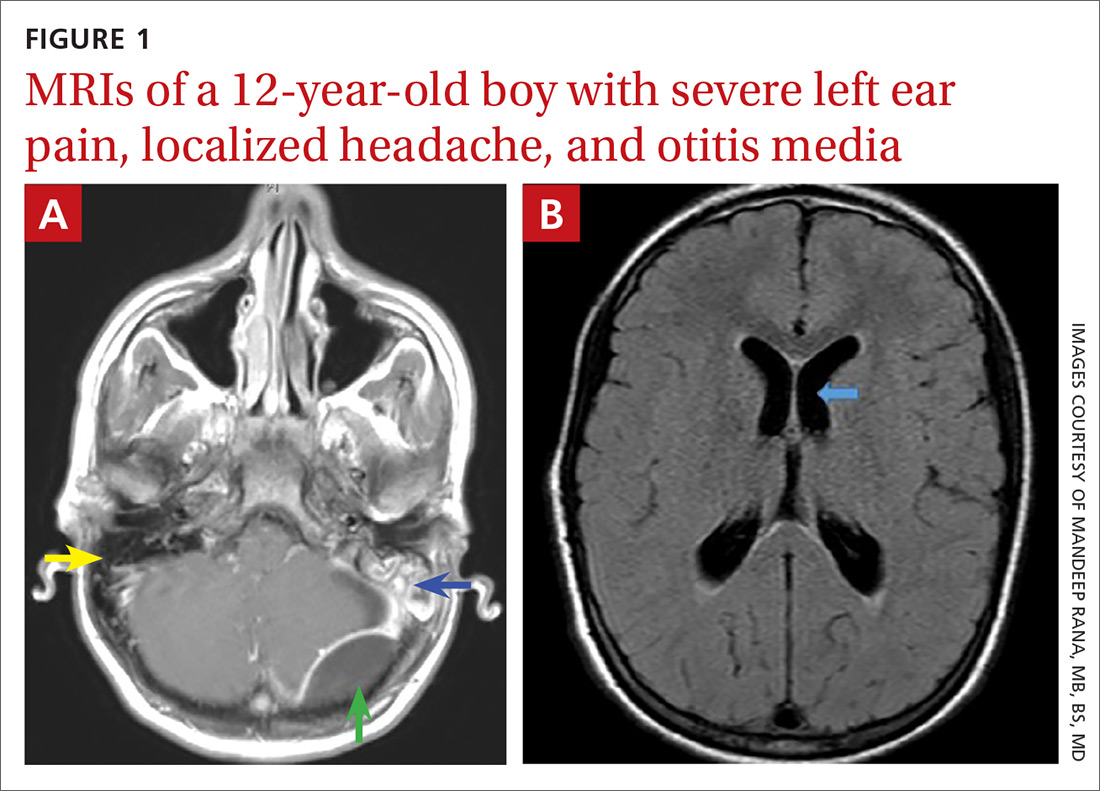
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Acute mastoiditis with epidural abscess
The contrast-enhanced cranial MRI scan (FIGURE 1A) revealed a case of acute mastoiditis with fluid in the left mastoid (blue arrow) and a large epidural abscess in the left posterior fossa (green arrow). The normal right mastoid was air-filled (yellow arrow). The T2-weighted MRI scan (FIGURE 1B) showed mild dilatation of the lateral ventricles (blue arrow) secondary to compression on the fourth ventricle by mass effect from the epidural abscess.
Acute mastoiditis—a complication of AOM—is an inflammatory process of mastoid air cells, which are contiguous to the middle ear cleft. In one large study of 61,783 inpatient children admitted with AOM, acute mastoiditis was reported as the most common complication in 1505 (2.4%) of the cases.1 The 2000-2012 national estimated incidence rate of pediatric mastoiditis has ranged from a high of 2.7 per 100,000 population in 2006 to a low of 1.8 per 100,000 in 2012.2 Clinical features of mastoiditis include localized mastoid tenderness, swelling, erythema, fluctuance, protrusion of the auricle, and ear pain.3
The clinical presentation of epidural abscess can be subtle with fever, headache, neck pain, and changes in mental status developing over several days.1 Focal deficits and seizures are relatively uncommon. In a review of 308 children with acute mastoiditis (3 with an epidural abscess), high-grade fever and high absolute neutrophil count and C-reactive protein levels were associated with the development of complications of mastoiditis, including hearing loss, sinus venous thrombosis, intracranial abscess, and cranial nerve palsies.4
Venous sinus thrombosis was part of the differential
When we were caring for this patient, the differential diagnosis included a cranial extension of AOM. Venous sinus thrombosis was also considered, given the family history of a hypercoagulable state. The patient did not have any features suggesting primary headache syndromes, such as migraine, tension type, or cluster headache.
The differential for a patient complaining of ear pain also includes postauricular lymphadenopathy, mumps, periauricular cellulitis (with and without otitis externa), perichondritis of the auricle, and tumors involving the mastoid bone.4
Continue to: Imaging and treatment
Imaging and treatment
Imaging of temporal bone is not recommended to make a diagnosis of mastoiditis in children with characteristic clinical findings. When imaging is needed, contrast-enhanced computed tomography (CT) is best to help visualize changes in temporal bone. If intracranial complications are suspected, cranial MRI with contrast or cranial CT with contrast can be ordered (depending on availability).5
Conservative management with intravenous antimicrobial therapy and middle ear drainage with myringotomy is indicated for a child with uncomplicated acute or subacute mastoiditis. For patients with suppurative extracranial or intracranial complications, aggressive surgical management is needed.5
Treatment for this patient included craniotomy, evacuation of the epidural abscess, and mastoidectomy. A culture obtained from the abscess showed Streptococcus intermedius. He was treated with broad-spectrum antibiotics, including ceftriaxone, vancomycin, and metronidazole. Within a week of surgery, he was discharged from the hospital and continued antibiotic treatment for 6 weeks via a peripherally inserted central catheter line.
1. Lavin JM, Rusher T, Shah RK. Complications of pediatric otitis media. Otolaryngol Head Neck Surg. 2016;154:366-370.
2. King LM, Bartoces M, Hersh AL, et al. National incidence of pediatric mastoiditis in the United States, 2000-2012: creating a baseline for public health surveillance. Pediatr Infect Dis J. 2019;38:e14-e16.
3. Pang LH, Barakate MS, Havas TE. Mastoiditis in a paediatric population: a review of 11 years’ experience in management. Int J Pediatr Otorhinolaryngol. 2009;73:1520.
4. Bilavsky E, Yarden-Bilavsky H, Samra Z, et al. Clinical, laboratory, and microbiological differences between children with simple or complicated mastoiditis. Int J Pediatr Otorhinolaryngol. 2009;73:1270-1273.
5. Chesney J, Black A, Choo D. What is the best practice for acute mastoiditis in children? Laryngoscope. 2014;124:1057-1059.
A previously healthy 12-year-old boy with normal development presented to his primary care physician (PCP) with a 1-week history of moderate ear pain. He was given a diagnosis of acute otitis media (AOM) and prescribed a 7-day course of amoxicillin. Although the child’s history was otherwise unremarkable, the mother reported that she’d had a deep venous thrombosis and pulmonary embolism a year earlier.
The boy continued to experience intermittent left ear pain 2 weeks after completing his antibiotic treatment, leading the PCP to refer him to an otolaryngologist. An examination by the otolaryngologist revealed a cloudy, bulging tympanic membrane. The patient was prescribed amoxicillin/clavulanate and ofloxacin ear drops.
Two days later, he was admitted to the emergency department (ED) due to worsening left ear pain and a new-onset left-sided headache. His left tympanic membrane was normal, with no tenderness or erythema of the mastoid. His vital signs were normal. He was afebrile and discharged home.
A week later, he returned to the ED with worsening ear pain and severe persistent headache, which was localized in the left occipital, left frontal, and retro-orbital regions. He denied light or sound sensitivity, nausea, vomiting, or increased lacrimation. He was tearful on examination due to the pain. He had no meningismus and normal fundi. A neurologic examination was nonlateralizing. Laboratory tests showed a normal complete blood count but increased C-reactive protein at 113 mg/dL (normal, < 0.3 mg/dL) and an erythrocyte sedimentation rate of 88 mm/hr (normal, 0-20 mm/hr).
Magnetic resonance imaging was ordered (FIGURES 1A and 1B), and Neurosurgery and Otolaryngology were consulted.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Acute mastoiditis with epidural abscess
The contrast-enhanced cranial MRI scan (FIGURE 1A) revealed a case of acute mastoiditis with fluid in the left mastoid (blue arrow) and a large epidural abscess in the left posterior fossa (green arrow). The normal right mastoid was air-filled (yellow arrow). The T2-weighted MRI scan (FIGURE 1B) showed mild dilatation of the lateral ventricles (blue arrow) secondary to compression on the fourth ventricle by mass effect from the epidural abscess.
Acute mastoiditis—a complication of AOM—is an inflammatory process of mastoid air cells, which are contiguous to the middle ear cleft. In one large study of 61,783 inpatient children admitted with AOM, acute mastoiditis was reported as the most common complication in 1505 (2.4%) of the cases.1 The 2000-2012 national estimated incidence rate of pediatric mastoiditis has ranged from a high of 2.7 per 100,000 population in 2006 to a low of 1.8 per 100,000 in 2012.2 Clinical features of mastoiditis include localized mastoid tenderness, swelling, erythema, fluctuance, protrusion of the auricle, and ear pain.3
The clinical presentation of epidural abscess can be subtle with fever, headache, neck pain, and changes in mental status developing over several days.1 Focal deficits and seizures are relatively uncommon. In a review of 308 children with acute mastoiditis (3 with an epidural abscess), high-grade fever and high absolute neutrophil count and C-reactive protein levels were associated with the development of complications of mastoiditis, including hearing loss, sinus venous thrombosis, intracranial abscess, and cranial nerve palsies.4
Venous sinus thrombosis was part of the differential
When we were caring for this patient, the differential diagnosis included a cranial extension of AOM. Venous sinus thrombosis was also considered, given the family history of a hypercoagulable state. The patient did not have any features suggesting primary headache syndromes, such as migraine, tension type, or cluster headache.
The differential for a patient complaining of ear pain also includes postauricular lymphadenopathy, mumps, periauricular cellulitis (with and without otitis externa), perichondritis of the auricle, and tumors involving the mastoid bone.4
Continue to: Imaging and treatment
Imaging and treatment
Imaging of temporal bone is not recommended to make a diagnosis of mastoiditis in children with characteristic clinical findings. When imaging is needed, contrast-enhanced computed tomography (CT) is best to help visualize changes in temporal bone. If intracranial complications are suspected, cranial MRI with contrast or cranial CT with contrast can be ordered (depending on availability).5
Conservative management with intravenous antimicrobial therapy and middle ear drainage with myringotomy is indicated for a child with uncomplicated acute or subacute mastoiditis. For patients with suppurative extracranial or intracranial complications, aggressive surgical management is needed.5
Treatment for this patient included craniotomy, evacuation of the epidural abscess, and mastoidectomy. A culture obtained from the abscess showed Streptococcus intermedius. He was treated with broad-spectrum antibiotics, including ceftriaxone, vancomycin, and metronidazole. Within a week of surgery, he was discharged from the hospital and continued antibiotic treatment for 6 weeks via a peripherally inserted central catheter line.
A previously healthy 12-year-old boy with normal development presented to his primary care physician (PCP) with a 1-week history of moderate ear pain. He was given a diagnosis of acute otitis media (AOM) and prescribed a 7-day course of amoxicillin. Although the child’s history was otherwise unremarkable, the mother reported that she’d had a deep venous thrombosis and pulmonary embolism a year earlier.
The boy continued to experience intermittent left ear pain 2 weeks after completing his antibiotic treatment, leading the PCP to refer him to an otolaryngologist. An examination by the otolaryngologist revealed a cloudy, bulging tympanic membrane. The patient was prescribed amoxicillin/clavulanate and ofloxacin ear drops.
Two days later, he was admitted to the emergency department (ED) due to worsening left ear pain and a new-onset left-sided headache. His left tympanic membrane was normal, with no tenderness or erythema of the mastoid. His vital signs were normal. He was afebrile and discharged home.
A week later, he returned to the ED with worsening ear pain and severe persistent headache, which was localized in the left occipital, left frontal, and retro-orbital regions. He denied light or sound sensitivity, nausea, vomiting, or increased lacrimation. He was tearful on examination due to the pain. He had no meningismus and normal fundi. A neurologic examination was nonlateralizing. Laboratory tests showed a normal complete blood count but increased C-reactive protein at 113 mg/dL (normal, < 0.3 mg/dL) and an erythrocyte sedimentation rate of 88 mm/hr (normal, 0-20 mm/hr).
Magnetic resonance imaging was ordered (FIGURES 1A and 1B), and Neurosurgery and Otolaryngology were consulted.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Acute mastoiditis with epidural abscess
The contrast-enhanced cranial MRI scan (FIGURE 1A) revealed a case of acute mastoiditis with fluid in the left mastoid (blue arrow) and a large epidural abscess in the left posterior fossa (green arrow). The normal right mastoid was air-filled (yellow arrow). The T2-weighted MRI scan (FIGURE 1B) showed mild dilatation of the lateral ventricles (blue arrow) secondary to compression on the fourth ventricle by mass effect from the epidural abscess.
Acute mastoiditis—a complication of AOM—is an inflammatory process of mastoid air cells, which are contiguous to the middle ear cleft. In one large study of 61,783 inpatient children admitted with AOM, acute mastoiditis was reported as the most common complication in 1505 (2.4%) of the cases.1 The 2000-2012 national estimated incidence rate of pediatric mastoiditis has ranged from a high of 2.7 per 100,000 population in 2006 to a low of 1.8 per 100,000 in 2012.2 Clinical features of mastoiditis include localized mastoid tenderness, swelling, erythema, fluctuance, protrusion of the auricle, and ear pain.3
The clinical presentation of epidural abscess can be subtle with fever, headache, neck pain, and changes in mental status developing over several days.1 Focal deficits and seizures are relatively uncommon. In a review of 308 children with acute mastoiditis (3 with an epidural abscess), high-grade fever and high absolute neutrophil count and C-reactive protein levels were associated with the development of complications of mastoiditis, including hearing loss, sinus venous thrombosis, intracranial abscess, and cranial nerve palsies.4
Venous sinus thrombosis was part of the differential
When we were caring for this patient, the differential diagnosis included a cranial extension of AOM. Venous sinus thrombosis was also considered, given the family history of a hypercoagulable state. The patient did not have any features suggesting primary headache syndromes, such as migraine, tension type, or cluster headache.
The differential for a patient complaining of ear pain also includes postauricular lymphadenopathy, mumps, periauricular cellulitis (with and without otitis externa), perichondritis of the auricle, and tumors involving the mastoid bone.4
Continue to: Imaging and treatment
Imaging and treatment
Imaging of temporal bone is not recommended to make a diagnosis of mastoiditis in children with characteristic clinical findings. When imaging is needed, contrast-enhanced computed tomography (CT) is best to help visualize changes in temporal bone. If intracranial complications are suspected, cranial MRI with contrast or cranial CT with contrast can be ordered (depending on availability).5
Conservative management with intravenous antimicrobial therapy and middle ear drainage with myringotomy is indicated for a child with uncomplicated acute or subacute mastoiditis. For patients with suppurative extracranial or intracranial complications, aggressive surgical management is needed.5
Treatment for this patient included craniotomy, evacuation of the epidural abscess, and mastoidectomy. A culture obtained from the abscess showed Streptococcus intermedius. He was treated with broad-spectrum antibiotics, including ceftriaxone, vancomycin, and metronidazole. Within a week of surgery, he was discharged from the hospital and continued antibiotic treatment for 6 weeks via a peripherally inserted central catheter line.
1. Lavin JM, Rusher T, Shah RK. Complications of pediatric otitis media. Otolaryngol Head Neck Surg. 2016;154:366-370.
2. King LM, Bartoces M, Hersh AL, et al. National incidence of pediatric mastoiditis in the United States, 2000-2012: creating a baseline for public health surveillance. Pediatr Infect Dis J. 2019;38:e14-e16.
3. Pang LH, Barakate MS, Havas TE. Mastoiditis in a paediatric population: a review of 11 years’ experience in management. Int J Pediatr Otorhinolaryngol. 2009;73:1520.
4. Bilavsky E, Yarden-Bilavsky H, Samra Z, et al. Clinical, laboratory, and microbiological differences between children with simple or complicated mastoiditis. Int J Pediatr Otorhinolaryngol. 2009;73:1270-1273.
5. Chesney J, Black A, Choo D. What is the best practice for acute mastoiditis in children? Laryngoscope. 2014;124:1057-1059.
1. Lavin JM, Rusher T, Shah RK. Complications of pediatric otitis media. Otolaryngol Head Neck Surg. 2016;154:366-370.
2. King LM, Bartoces M, Hersh AL, et al. National incidence of pediatric mastoiditis in the United States, 2000-2012: creating a baseline for public health surveillance. Pediatr Infect Dis J. 2019;38:e14-e16.
3. Pang LH, Barakate MS, Havas TE. Mastoiditis in a paediatric population: a review of 11 years’ experience in management. Int J Pediatr Otorhinolaryngol. 2009;73:1520.
4. Bilavsky E, Yarden-Bilavsky H, Samra Z, et al. Clinical, laboratory, and microbiological differences between children with simple or complicated mastoiditis. Int J Pediatr Otorhinolaryngol. 2009;73:1270-1273.
5. Chesney J, Black A, Choo D. What is the best practice for acute mastoiditis in children? Laryngoscope. 2014;124:1057-1059.
Gene-replacement therapy shows promise in X-linked myotubular myopathy
, according to research presented at the 2020 CNS-ICNA Conjoint Meeting, which was held virtually this year. The treatment also appears to improve patients’ motor function significantly and help them to achieve motor milestones.
The results come from a phase 1/2 study of two doses of AT132. Three of 17 patients who received the higher dose had fatal liver dysfunction. The researchers are investigating these cases and will communicate their findings.
X-linked myotubular myopathy is a rare and often fatal neuromuscular disease. Mutations in MTM1, which encodes the myotubularin enzyme that is required for the development and function of skeletal muscle, cause the disease, which affects about one in 50,000 to one in 40,000 newborn boys. The disease is associated with profound muscle weakness and impairment of neuromuscular and respiratory function. Patients with X-linked myotubular myopathy achieve motor milestones much later or not at all, and most require a ventilator or a feeding tube. The mortality by age 18 months is approximately 50%.
The ASPIRO trial
Investigators theorized that muscle tissue would be an appropriate therapeutic target because it does not display dystrophic or inflammatory changes in most patients. They identified adeno-associated virus AAV8 as a potential carrier for gene therapy, since it targets skeletal muscle effectively.
Nancy L. Kuntz, MD, an attending physician at Ann and Robert H. Lurie Children’s Hospital of Chicago, and colleagues conducted the ASPIRO trial to examine AT132 as a potential treatment for X-linked myotubular myopathy. Eligible patients were younger than 5 years or had previously enrolled in a natural history study of the disease, required ventilator support at baseline, and had no clinically significant underlying liver disease. Patients were randomly assigned to 1 × 1014 vg/kg of AAT132, 3 × 1014 vg/kg of AT132, or delayed treatment. Participants assigned to delayed treatment served as the study’s control group.
The study’s primary end points were safety and change in hours of daily ventilator support from baseline to week 24 after dosing. The investigators also examined a respiratory endpoint (i.e., maximal inspiratory pressure [MIP]) and neuromuscular endpoints (i.e., motor milestones, CHOP INTEND score, and muscle biopsy).
Treatment improved respiratory function
As of July 28, Dr. Kuntz and colleagues had enrolled 23 patients in the trial. Six participants received the lower dose of therapy, and 17 received the higher dose. Median age was 1.7 years for the low-dose group and 2.6 years for the high-dose group.
Patients assigned to receive the higher dose of therapy received treatment more recently than the low-dose group, and not all of the former have reached 48 weeks since treatment, said Dr. Kuntz. Fewer efficacy data are thus available for the high-dose group.
Each dose of AT132 was associated with a significantly greater decrease from baseline in least squares mean daily hours of ventilator dependence, compared with the control condition. At week 48, the mean reduction was approximately 19 hours/day for patients receiving 1 × 1014 vg/kg of AAT132 and approximately 13 hours per day for patients receiving 3 × 1014 vg/kg of AT132. The investigators did not perform a statistical comparison of the two doses because of differing protocols for ventilator weaning between groups. All six patients who received the lower dose achieved ventilator independence, as did one patient who received the higher dose.
In addition, all treated patients had significantly greater increases from baseline in least squares mean MIP, compared with controls. The mean increase was 45.7 cmH2O for the low-dose group, 46.1 cmH2O for the high-dose group, and −8.0 cmH2O for controls.
Before treatment, most patients had not achieved any of the motor milestones that investigators assessed. After treatment, five of six patients receiving the low dose achieved independent walking, as did one in 10 patients receiving the high dose. No controls achieved this milestone. Treated patients also had significantly greater increases from baseline in least squares mean CHOP INTEND scores, compared with controls. At least at one time point, five of six patients receiving the low dose, six of 10 patients receiving the high dose, and one control patient achieved the mean score observed in healthy infants.
Patients in both treatment arms had improvements in muscle pathology at weeks 24 and 48, including improvements in organelle localization and fiber size. In addition, patients in both treatment arms had continued detectable vector copies and myotubularin protein expression at both time points.
Deaths under investigation
In the low-dose group, one patient had four serious treatment-emergent adverse events, and in the high-dose group, eight patients had 27 serious treatment-emergent adverse events. The three patients in the high-dose group who developed fatal liver dysfunction were among the older, heavier patients in the study and, consequently, received among the highest total doses of treatment. These patients had evidence of likely preexisting intrahepatic cholestasis.
“This clinical trial is on hold pending discussions between regulatory agencies and the study sponsor regarding additional recruitment and the duration of follow-up,” said Dr. Kuntz.
Audentes Therapeutics, which is developing AT132, funded the trial. Dr. Kuntz had no conflicts of interest.
SOURCE: Bönnemann CG et al. CNS-ICNA 2020, Abstract P.62.
, according to research presented at the 2020 CNS-ICNA Conjoint Meeting, which was held virtually this year. The treatment also appears to improve patients’ motor function significantly and help them to achieve motor milestones.
The results come from a phase 1/2 study of two doses of AT132. Three of 17 patients who received the higher dose had fatal liver dysfunction. The researchers are investigating these cases and will communicate their findings.
X-linked myotubular myopathy is a rare and often fatal neuromuscular disease. Mutations in MTM1, which encodes the myotubularin enzyme that is required for the development and function of skeletal muscle, cause the disease, which affects about one in 50,000 to one in 40,000 newborn boys. The disease is associated with profound muscle weakness and impairment of neuromuscular and respiratory function. Patients with X-linked myotubular myopathy achieve motor milestones much later or not at all, and most require a ventilator or a feeding tube. The mortality by age 18 months is approximately 50%.
The ASPIRO trial
Investigators theorized that muscle tissue would be an appropriate therapeutic target because it does not display dystrophic or inflammatory changes in most patients. They identified adeno-associated virus AAV8 as a potential carrier for gene therapy, since it targets skeletal muscle effectively.
Nancy L. Kuntz, MD, an attending physician at Ann and Robert H. Lurie Children’s Hospital of Chicago, and colleagues conducted the ASPIRO trial to examine AT132 as a potential treatment for X-linked myotubular myopathy. Eligible patients were younger than 5 years or had previously enrolled in a natural history study of the disease, required ventilator support at baseline, and had no clinically significant underlying liver disease. Patients were randomly assigned to 1 × 1014 vg/kg of AAT132, 3 × 1014 vg/kg of AT132, or delayed treatment. Participants assigned to delayed treatment served as the study’s control group.
The study’s primary end points were safety and change in hours of daily ventilator support from baseline to week 24 after dosing. The investigators also examined a respiratory endpoint (i.e., maximal inspiratory pressure [MIP]) and neuromuscular endpoints (i.e., motor milestones, CHOP INTEND score, and muscle biopsy).
Treatment improved respiratory function
As of July 28, Dr. Kuntz and colleagues had enrolled 23 patients in the trial. Six participants received the lower dose of therapy, and 17 received the higher dose. Median age was 1.7 years for the low-dose group and 2.6 years for the high-dose group.
Patients assigned to receive the higher dose of therapy received treatment more recently than the low-dose group, and not all of the former have reached 48 weeks since treatment, said Dr. Kuntz. Fewer efficacy data are thus available for the high-dose group.
Each dose of AT132 was associated with a significantly greater decrease from baseline in least squares mean daily hours of ventilator dependence, compared with the control condition. At week 48, the mean reduction was approximately 19 hours/day for patients receiving 1 × 1014 vg/kg of AAT132 and approximately 13 hours per day for patients receiving 3 × 1014 vg/kg of AT132. The investigators did not perform a statistical comparison of the two doses because of differing protocols for ventilator weaning between groups. All six patients who received the lower dose achieved ventilator independence, as did one patient who received the higher dose.
In addition, all treated patients had significantly greater increases from baseline in least squares mean MIP, compared with controls. The mean increase was 45.7 cmH2O for the low-dose group, 46.1 cmH2O for the high-dose group, and −8.0 cmH2O for controls.
Before treatment, most patients had not achieved any of the motor milestones that investigators assessed. After treatment, five of six patients receiving the low dose achieved independent walking, as did one in 10 patients receiving the high dose. No controls achieved this milestone. Treated patients also had significantly greater increases from baseline in least squares mean CHOP INTEND scores, compared with controls. At least at one time point, five of six patients receiving the low dose, six of 10 patients receiving the high dose, and one control patient achieved the mean score observed in healthy infants.
Patients in both treatment arms had improvements in muscle pathology at weeks 24 and 48, including improvements in organelle localization and fiber size. In addition, patients in both treatment arms had continued detectable vector copies and myotubularin protein expression at both time points.
Deaths under investigation
In the low-dose group, one patient had four serious treatment-emergent adverse events, and in the high-dose group, eight patients had 27 serious treatment-emergent adverse events. The three patients in the high-dose group who developed fatal liver dysfunction were among the older, heavier patients in the study and, consequently, received among the highest total doses of treatment. These patients had evidence of likely preexisting intrahepatic cholestasis.
“This clinical trial is on hold pending discussions between regulatory agencies and the study sponsor regarding additional recruitment and the duration of follow-up,” said Dr. Kuntz.
Audentes Therapeutics, which is developing AT132, funded the trial. Dr. Kuntz had no conflicts of interest.
SOURCE: Bönnemann CG et al. CNS-ICNA 2020, Abstract P.62.
, according to research presented at the 2020 CNS-ICNA Conjoint Meeting, which was held virtually this year. The treatment also appears to improve patients’ motor function significantly and help them to achieve motor milestones.
The results come from a phase 1/2 study of two doses of AT132. Three of 17 patients who received the higher dose had fatal liver dysfunction. The researchers are investigating these cases and will communicate their findings.
X-linked myotubular myopathy is a rare and often fatal neuromuscular disease. Mutations in MTM1, which encodes the myotubularin enzyme that is required for the development and function of skeletal muscle, cause the disease, which affects about one in 50,000 to one in 40,000 newborn boys. The disease is associated with profound muscle weakness and impairment of neuromuscular and respiratory function. Patients with X-linked myotubular myopathy achieve motor milestones much later or not at all, and most require a ventilator or a feeding tube. The mortality by age 18 months is approximately 50%.
The ASPIRO trial
Investigators theorized that muscle tissue would be an appropriate therapeutic target because it does not display dystrophic or inflammatory changes in most patients. They identified adeno-associated virus AAV8 as a potential carrier for gene therapy, since it targets skeletal muscle effectively.
Nancy L. Kuntz, MD, an attending physician at Ann and Robert H. Lurie Children’s Hospital of Chicago, and colleagues conducted the ASPIRO trial to examine AT132 as a potential treatment for X-linked myotubular myopathy. Eligible patients were younger than 5 years or had previously enrolled in a natural history study of the disease, required ventilator support at baseline, and had no clinically significant underlying liver disease. Patients were randomly assigned to 1 × 1014 vg/kg of AAT132, 3 × 1014 vg/kg of AT132, or delayed treatment. Participants assigned to delayed treatment served as the study’s control group.
The study’s primary end points were safety and change in hours of daily ventilator support from baseline to week 24 after dosing. The investigators also examined a respiratory endpoint (i.e., maximal inspiratory pressure [MIP]) and neuromuscular endpoints (i.e., motor milestones, CHOP INTEND score, and muscle biopsy).
Treatment improved respiratory function
As of July 28, Dr. Kuntz and colleagues had enrolled 23 patients in the trial. Six participants received the lower dose of therapy, and 17 received the higher dose. Median age was 1.7 years for the low-dose group and 2.6 years for the high-dose group.
Patients assigned to receive the higher dose of therapy received treatment more recently than the low-dose group, and not all of the former have reached 48 weeks since treatment, said Dr. Kuntz. Fewer efficacy data are thus available for the high-dose group.
Each dose of AT132 was associated with a significantly greater decrease from baseline in least squares mean daily hours of ventilator dependence, compared with the control condition. At week 48, the mean reduction was approximately 19 hours/day for patients receiving 1 × 1014 vg/kg of AAT132 and approximately 13 hours per day for patients receiving 3 × 1014 vg/kg of AT132. The investigators did not perform a statistical comparison of the two doses because of differing protocols for ventilator weaning between groups. All six patients who received the lower dose achieved ventilator independence, as did one patient who received the higher dose.
In addition, all treated patients had significantly greater increases from baseline in least squares mean MIP, compared with controls. The mean increase was 45.7 cmH2O for the low-dose group, 46.1 cmH2O for the high-dose group, and −8.0 cmH2O for controls.
Before treatment, most patients had not achieved any of the motor milestones that investigators assessed. After treatment, five of six patients receiving the low dose achieved independent walking, as did one in 10 patients receiving the high dose. No controls achieved this milestone. Treated patients also had significantly greater increases from baseline in least squares mean CHOP INTEND scores, compared with controls. At least at one time point, five of six patients receiving the low dose, six of 10 patients receiving the high dose, and one control patient achieved the mean score observed in healthy infants.
Patients in both treatment arms had improvements in muscle pathology at weeks 24 and 48, including improvements in organelle localization and fiber size. In addition, patients in both treatment arms had continued detectable vector copies and myotubularin protein expression at both time points.
Deaths under investigation
In the low-dose group, one patient had four serious treatment-emergent adverse events, and in the high-dose group, eight patients had 27 serious treatment-emergent adverse events. The three patients in the high-dose group who developed fatal liver dysfunction were among the older, heavier patients in the study and, consequently, received among the highest total doses of treatment. These patients had evidence of likely preexisting intrahepatic cholestasis.
“This clinical trial is on hold pending discussions between regulatory agencies and the study sponsor regarding additional recruitment and the duration of follow-up,” said Dr. Kuntz.
Audentes Therapeutics, which is developing AT132, funded the trial. Dr. Kuntz had no conflicts of interest.
SOURCE: Bönnemann CG et al. CNS-ICNA 2020, Abstract P.62.
FROM CNS-ICNA 2020
Blood test for Alzheimer’s disease comes to the clinic
, according to C2N Diagnostics, the company behind the test’s development. The availability of the noninvasive, easily administered test is being called a milestone in the early detection and diagnosis of Alzheimer’s disease.
The blood test “introduces a new option for patients, families, and the medical community that have eagerly awaited innovative tools to address Alzheimer’s troubling problems,” Joel B. Braunstein, MD, MBA, CEO of C2N Diagnostics, said in a press release.
“This is really an important advance,” said Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation (ADDF), which partially funded the development of the test, in a separate press release.
“You can now walk into your doctor’s office to get a blood test to help detect Alzheimer’s disease,” said Dr. Fillit. “This test answers a critical need for less costly and accessible diagnostic testing in memory and dementia care.”
A word of caution
However, Maria C. Carrillo, PhD, chief science officer, Alzheimer’s Association, highlighted the need for caution. The test is “very new,” experts have only “limited information” about it, and it is only available by prescription from a healthcare provider for patients with cognitive impairment, said Dr. Carrillo.
“The test is not [Food and Drug Administration] approved and it does not, on its own, diagnose Alzheimer’s disease,” added Dr. Carrillo. “Without FDA review, healthcare providers lack the agency’s guidance for how to use it when making decisions about a person’s health or treatment.”
Dr. Carrillo also noted that the test has only been studied in a limited number of individuals and that few data are available regarding underrepresented populations.
“As a result, it is not clear how accurate or generalizable the results are for all individuals and populations,” she noted.
Another factor to consider, said Dr. Carrillo, is that the test is not covered by insurance, including Medicare and Medicaid.
How it works
The test (PrecivityAD) is for use in patients with cognitive impairment. It requires a very small blood sample – as little as a teaspoon – from the patient’s forearm. The physician sends the sample to C2N Diagnostic’s specialized laboratory, where it is analyzed using mass spectrometry to measure concentrations of amyloid beta 42 and 40 and to detect the presence of apolipoprotein E isoforms.
The lab report, which is sent to the patient’s physician, details biomarker levels and provides an overall combined score, known as the Amyloid Probability Score, to assess the likelihood of low, intermediate, or high levels of amyloid plaque in the brain.
The company reports that, on the basis of data from 686 patients older than 60 years who had subjective cognitive impairment or dementia, the test correctly identified brain amyloid plaque status, as determined by quantitative amyloid positron-emission tomography (PET) scans, in 86% of the patients. In the analysis, the area under the curve for the receiver operating characteristic was 0.88.
The company notes that the test, the results of which require interpretation by a health care provider, is an important new tool to aid physicians in the evaluation process.
The new blood test is currently available in 45 states, the District of Columbia, and Puerto Rico.
C2N Diagnostics is moving ahead with development of a brain health panel to detect multiple blood-based markers for Alzheimer’s disease to aid in disease staging, treatment monitoring, and differential diagnosis.
The ADDF believes the path to approval of treatments of Alzheimer’s disease starts with a better diagnosis, Dr. Fillit said in his organization’s press release.
“Investing in biomarker research has been a core goal for the ADDF because reliable, accessible, and affordable biomarkers for Alzheimer’s diagnosis are critical to our ability to find drugs to prevent, slow, and even cure the disease. Our funding helped bring the first PET scan to market and now has helped bring the first blood test to market,” he said.
In addition to the ADDF, the National Institutes of Health, the GHR Foundation, and the BrightFocus Foundation contributed funding for the development of the amyloid blood test.
A version of this article originally appeared on Medscape.com.
, according to C2N Diagnostics, the company behind the test’s development. The availability of the noninvasive, easily administered test is being called a milestone in the early detection and diagnosis of Alzheimer’s disease.
The blood test “introduces a new option for patients, families, and the medical community that have eagerly awaited innovative tools to address Alzheimer’s troubling problems,” Joel B. Braunstein, MD, MBA, CEO of C2N Diagnostics, said in a press release.
“This is really an important advance,” said Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation (ADDF), which partially funded the development of the test, in a separate press release.
“You can now walk into your doctor’s office to get a blood test to help detect Alzheimer’s disease,” said Dr. Fillit. “This test answers a critical need for less costly and accessible diagnostic testing in memory and dementia care.”
A word of caution
However, Maria C. Carrillo, PhD, chief science officer, Alzheimer’s Association, highlighted the need for caution. The test is “very new,” experts have only “limited information” about it, and it is only available by prescription from a healthcare provider for patients with cognitive impairment, said Dr. Carrillo.
“The test is not [Food and Drug Administration] approved and it does not, on its own, diagnose Alzheimer’s disease,” added Dr. Carrillo. “Without FDA review, healthcare providers lack the agency’s guidance for how to use it when making decisions about a person’s health or treatment.”
Dr. Carrillo also noted that the test has only been studied in a limited number of individuals and that few data are available regarding underrepresented populations.
“As a result, it is not clear how accurate or generalizable the results are for all individuals and populations,” she noted.
Another factor to consider, said Dr. Carrillo, is that the test is not covered by insurance, including Medicare and Medicaid.
How it works
The test (PrecivityAD) is for use in patients with cognitive impairment. It requires a very small blood sample – as little as a teaspoon – from the patient’s forearm. The physician sends the sample to C2N Diagnostic’s specialized laboratory, where it is analyzed using mass spectrometry to measure concentrations of amyloid beta 42 and 40 and to detect the presence of apolipoprotein E isoforms.
The lab report, which is sent to the patient’s physician, details biomarker levels and provides an overall combined score, known as the Amyloid Probability Score, to assess the likelihood of low, intermediate, or high levels of amyloid plaque in the brain.
The company reports that, on the basis of data from 686 patients older than 60 years who had subjective cognitive impairment or dementia, the test correctly identified brain amyloid plaque status, as determined by quantitative amyloid positron-emission tomography (PET) scans, in 86% of the patients. In the analysis, the area under the curve for the receiver operating characteristic was 0.88.
The company notes that the test, the results of which require interpretation by a health care provider, is an important new tool to aid physicians in the evaluation process.
The new blood test is currently available in 45 states, the District of Columbia, and Puerto Rico.
C2N Diagnostics is moving ahead with development of a brain health panel to detect multiple blood-based markers for Alzheimer’s disease to aid in disease staging, treatment monitoring, and differential diagnosis.
The ADDF believes the path to approval of treatments of Alzheimer’s disease starts with a better diagnosis, Dr. Fillit said in his organization’s press release.
“Investing in biomarker research has been a core goal for the ADDF because reliable, accessible, and affordable biomarkers for Alzheimer’s diagnosis are critical to our ability to find drugs to prevent, slow, and even cure the disease. Our funding helped bring the first PET scan to market and now has helped bring the first blood test to market,” he said.
In addition to the ADDF, the National Institutes of Health, the GHR Foundation, and the BrightFocus Foundation contributed funding for the development of the amyloid blood test.
A version of this article originally appeared on Medscape.com.
, according to C2N Diagnostics, the company behind the test’s development. The availability of the noninvasive, easily administered test is being called a milestone in the early detection and diagnosis of Alzheimer’s disease.
The blood test “introduces a new option for patients, families, and the medical community that have eagerly awaited innovative tools to address Alzheimer’s troubling problems,” Joel B. Braunstein, MD, MBA, CEO of C2N Diagnostics, said in a press release.
“This is really an important advance,” said Howard Fillit, MD, founding executive director and chief science officer of the Alzheimer’s Drug Discovery Foundation (ADDF), which partially funded the development of the test, in a separate press release.
“You can now walk into your doctor’s office to get a blood test to help detect Alzheimer’s disease,” said Dr. Fillit. “This test answers a critical need for less costly and accessible diagnostic testing in memory and dementia care.”
A word of caution
However, Maria C. Carrillo, PhD, chief science officer, Alzheimer’s Association, highlighted the need for caution. The test is “very new,” experts have only “limited information” about it, and it is only available by prescription from a healthcare provider for patients with cognitive impairment, said Dr. Carrillo.
“The test is not [Food and Drug Administration] approved and it does not, on its own, diagnose Alzheimer’s disease,” added Dr. Carrillo. “Without FDA review, healthcare providers lack the agency’s guidance for how to use it when making decisions about a person’s health or treatment.”
Dr. Carrillo also noted that the test has only been studied in a limited number of individuals and that few data are available regarding underrepresented populations.
“As a result, it is not clear how accurate or generalizable the results are for all individuals and populations,” she noted.
Another factor to consider, said Dr. Carrillo, is that the test is not covered by insurance, including Medicare and Medicaid.
How it works
The test (PrecivityAD) is for use in patients with cognitive impairment. It requires a very small blood sample – as little as a teaspoon – from the patient’s forearm. The physician sends the sample to C2N Diagnostic’s specialized laboratory, where it is analyzed using mass spectrometry to measure concentrations of amyloid beta 42 and 40 and to detect the presence of apolipoprotein E isoforms.
The lab report, which is sent to the patient’s physician, details biomarker levels and provides an overall combined score, known as the Amyloid Probability Score, to assess the likelihood of low, intermediate, or high levels of amyloid plaque in the brain.
The company reports that, on the basis of data from 686 patients older than 60 years who had subjective cognitive impairment or dementia, the test correctly identified brain amyloid plaque status, as determined by quantitative amyloid positron-emission tomography (PET) scans, in 86% of the patients. In the analysis, the area under the curve for the receiver operating characteristic was 0.88.
The company notes that the test, the results of which require interpretation by a health care provider, is an important new tool to aid physicians in the evaluation process.
The new blood test is currently available in 45 states, the District of Columbia, and Puerto Rico.
C2N Diagnostics is moving ahead with development of a brain health panel to detect multiple blood-based markers for Alzheimer’s disease to aid in disease staging, treatment monitoring, and differential diagnosis.
The ADDF believes the path to approval of treatments of Alzheimer’s disease starts with a better diagnosis, Dr. Fillit said in his organization’s press release.
“Investing in biomarker research has been a core goal for the ADDF because reliable, accessible, and affordable biomarkers for Alzheimer’s diagnosis are critical to our ability to find drugs to prevent, slow, and even cure the disease. Our funding helped bring the first PET scan to market and now has helped bring the first blood test to market,” he said.
In addition to the ADDF, the National Institutes of Health, the GHR Foundation, and the BrightFocus Foundation contributed funding for the development of the amyloid blood test.
A version of this article originally appeared on Medscape.com.