User login
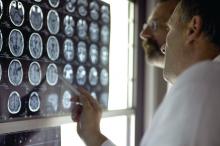
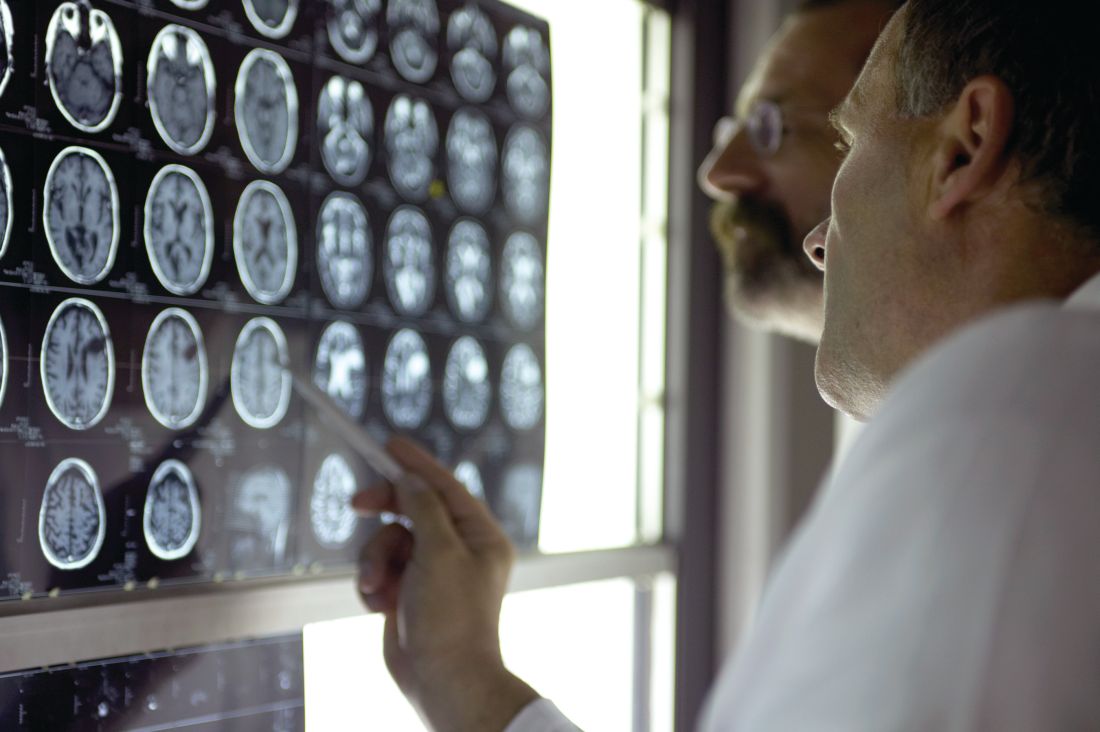
A skin test for Parkinson’s disease diagnosis?
a new study suggests. For the study, researchers used a chemical assay to detect clumping of the protein alpha-synuclein, a hallmark of Parkinson’s disease, in autopsy skin samples taken from patients who had Parkinson’s disease confirmed by brain pathology and from controls without the disease. The test showed a high degree of sensitivity and specificity for the diagnosis of Parkinson’s disease.
The study was published online in Movement Disorders.
“This test has a lot of promise,” said senior author Anumantha Kanthasamy, PhD, professor of biomedical sciences at Iowa State University in Ames. “At present there are no peripheral biomarkers for Parkinson’s disease. The current diagnosis is just based on symptoms, and the symptoms can be similar to many other neurological diseases,” he added. “It can take many years to establish a correct diagnosis and the accuracy is low even with experienced neurologists.”
If the current results can be replicated in samples from live patients and in those with very early stages of Parkinson’s disease, a skin test could allow early diagnosis and the possibility of starting preventive treatments to slow disease progression before symptoms develop too severely, the researchers suggest.
Sensitive and specific test
The blinded study used a seeding assay – used previously to detect misfolded proteins in prion diseases – to analyze 50 skin samples provided by the Arizona Study of Aging and Neurodegenerative Disorders/Brain and Body Donation Program based at Banner Sun Health Research Institute in Sun City.
Half of the skin samples came from patients with Parkinson’s disease and half came from people without neurologic disease. The protein assay correctly diagnosed 24 out of 25 patients with Parkinson’s disease and only one of the 25 controls had the protein clumping.
“At present, the only way to definitely diagnose Parkinson’s disease is on autopsy – by the detection of alpha-synuclein clumps [Lewy bodies] in the brain,” commented Charles Adler, MD, professor of neurology at Mayo Clinic Arizona in Scottsdale and a coinvestigator of the study. “In our research, we have also seen clumping of alpha-synuclein in many other organs including submandibular gland, colon, skin, heart, and stomach, but in terms of access, the skin is probably the easiest source.”
In this study, “we found this seeding assay for alpha-synuclein clumps to be extremely sensitive and specific in the diagnosis of Parkinson’s disease,” he added. “This is very valuable data as we have samples from patients with autopsy-validated Parkinson’s disease.”
A reliable biomarker?
The researchers are now starting a study in living patients with funding from the National Institutes of Health in which they will repeat the process comparing skin samples from patients with clinically diagnosed Parkinson’s disease and controls.
“We need to know whether analyzing alpha-synuclein clumping in skin biopsies from live patients with Parkinson’s disease would serve as a reliable biomarker for disease progression. Will clumping of this protein in skin samples increase over time and does it correspond with disease progression?” Dr. Adler said.
In future they are also hoping to test individuals who have not yet developed Parkinson’s disease but may have some prodromal type symptoms and to test whether this assay could measure a treatment effect of drug therapy.
Dr. Adler noted that they are currently conducting an autopsy study of skin samples from individuals who did not have clinical Parkinson’s disease when alive but in whom Lewy bodies have been found postmortem.
“This suggests that the disease pathology starts before Parkinson’s symptoms develop, and in the future, if we can diagnose Parkinson’s disease earlier then we may be able to stop progression,” he said.
“There is a long list of compounds that have been studied to try and slow progression but haven’t shown benefits, but by the time patients develop symptoms they already have significant disease and [have] lost most of their dopamine neurons,” he added. “If we could backtrack by 10 years, then these drugs may well make a difference.”
Dr. Adler also noted that currently more advanced patients may undergo invasive procedures such as deep brain stimulation or surgery. “It is of utmost importance that they have an accurate diagnosis before being subjected to such procedures.”
In addition, he pointed out that an accurate test would help the drug development process. “It is vitally important to enroll patients with an accurate diagnosis in clinical trials of new drugs. At present, a large percentage of patients in these trials may not actually have Parkinson’s disease, which makes it very difficult to show a treatment effect.”
Important step, but preliminary
Commenting on the research, James Beck, PhD, chief scientific officer of the Parkinson’s Foundation, said the study “is an important step toward the creation of a new way to potentially diagnose Parkinson’s disease.”
But he cautioned that this is a preliminary study. “To really confirm the possibility of using this approach for diagnosing Parkinson’s disease, a larger study will be necessary. And it will be important to test this in a population with early disease – the most difficult group to accurately diagnose.”
Also commenting on the findings, Beate Ritz, MD, PhD, an epidemiologist at UCLA Fielding School of Public Health in Los Angeles, who is part of a team also working on ways to measure abnormal alpha-synuclein to diagnose Parkinson’s disease, described the current study of skin samples as “pretty nifty.”
“Their research shows clearly that they can distinguish between patients with Parkinson’s disease and controls in this way,” she said. “The big advantage of this study is that they have brain pathology, so they know exactly which individuals had Parkinson’s disease.”
Dr. Ritz is working with Gal Bitan, PhD, from the UCLA Brain Research Institute on a potential blood test to measure abnormal alpha-synuclein.
Dr. Ritz explained that it is not possible to measure alpha-synuclein pathology in regular blood samples as it is expressed normally in red blood cells, but they are measuring the protein and its more toxic phosphorylated form from exosomes, which contain the waste discarded by cells using technology that determines the origin of these exosomes.
“Alpha-synuclein itself is not a problem. It is the way it misfolds that causes toxicity and disrupts the workings of the cell,” Dr. Ritz added. “In Parkinson’s disease, it is particularly toxic to dopaminergic neurons, and in multiple system atrophy, it is toxic to glial cells, so if we can identify the source of the protein then that could be helpful.”
The study was funded by the National Institutes of Health and the US Army Medical Research Materiel Command. The study authors, Dr. Beck, and Dr. Ritz have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a new study suggests. For the study, researchers used a chemical assay to detect clumping of the protein alpha-synuclein, a hallmark of Parkinson’s disease, in autopsy skin samples taken from patients who had Parkinson’s disease confirmed by brain pathology and from controls without the disease. The test showed a high degree of sensitivity and specificity for the diagnosis of Parkinson’s disease.
The study was published online in Movement Disorders.
“This test has a lot of promise,” said senior author Anumantha Kanthasamy, PhD, professor of biomedical sciences at Iowa State University in Ames. “At present there are no peripheral biomarkers for Parkinson’s disease. The current diagnosis is just based on symptoms, and the symptoms can be similar to many other neurological diseases,” he added. “It can take many years to establish a correct diagnosis and the accuracy is low even with experienced neurologists.”
If the current results can be replicated in samples from live patients and in those with very early stages of Parkinson’s disease, a skin test could allow early diagnosis and the possibility of starting preventive treatments to slow disease progression before symptoms develop too severely, the researchers suggest.
Sensitive and specific test
The blinded study used a seeding assay – used previously to detect misfolded proteins in prion diseases – to analyze 50 skin samples provided by the Arizona Study of Aging and Neurodegenerative Disorders/Brain and Body Donation Program based at Banner Sun Health Research Institute in Sun City.
Half of the skin samples came from patients with Parkinson’s disease and half came from people without neurologic disease. The protein assay correctly diagnosed 24 out of 25 patients with Parkinson’s disease and only one of the 25 controls had the protein clumping.
“At present, the only way to definitely diagnose Parkinson’s disease is on autopsy – by the detection of alpha-synuclein clumps [Lewy bodies] in the brain,” commented Charles Adler, MD, professor of neurology at Mayo Clinic Arizona in Scottsdale and a coinvestigator of the study. “In our research, we have also seen clumping of alpha-synuclein in many other organs including submandibular gland, colon, skin, heart, and stomach, but in terms of access, the skin is probably the easiest source.”
In this study, “we found this seeding assay for alpha-synuclein clumps to be extremely sensitive and specific in the diagnosis of Parkinson’s disease,” he added. “This is very valuable data as we have samples from patients with autopsy-validated Parkinson’s disease.”
A reliable biomarker?
The researchers are now starting a study in living patients with funding from the National Institutes of Health in which they will repeat the process comparing skin samples from patients with clinically diagnosed Parkinson’s disease and controls.
“We need to know whether analyzing alpha-synuclein clumping in skin biopsies from live patients with Parkinson’s disease would serve as a reliable biomarker for disease progression. Will clumping of this protein in skin samples increase over time and does it correspond with disease progression?” Dr. Adler said.
In future they are also hoping to test individuals who have not yet developed Parkinson’s disease but may have some prodromal type symptoms and to test whether this assay could measure a treatment effect of drug therapy.
Dr. Adler noted that they are currently conducting an autopsy study of skin samples from individuals who did not have clinical Parkinson’s disease when alive but in whom Lewy bodies have been found postmortem.
“This suggests that the disease pathology starts before Parkinson’s symptoms develop, and in the future, if we can diagnose Parkinson’s disease earlier then we may be able to stop progression,” he said.
“There is a long list of compounds that have been studied to try and slow progression but haven’t shown benefits, but by the time patients develop symptoms they already have significant disease and [have] lost most of their dopamine neurons,” he added. “If we could backtrack by 10 years, then these drugs may well make a difference.”
Dr. Adler also noted that currently more advanced patients may undergo invasive procedures such as deep brain stimulation or surgery. “It is of utmost importance that they have an accurate diagnosis before being subjected to such procedures.”
In addition, he pointed out that an accurate test would help the drug development process. “It is vitally important to enroll patients with an accurate diagnosis in clinical trials of new drugs. At present, a large percentage of patients in these trials may not actually have Parkinson’s disease, which makes it very difficult to show a treatment effect.”
Important step, but preliminary
Commenting on the research, James Beck, PhD, chief scientific officer of the Parkinson’s Foundation, said the study “is an important step toward the creation of a new way to potentially diagnose Parkinson’s disease.”
But he cautioned that this is a preliminary study. “To really confirm the possibility of using this approach for diagnosing Parkinson’s disease, a larger study will be necessary. And it will be important to test this in a population with early disease – the most difficult group to accurately diagnose.”
Also commenting on the findings, Beate Ritz, MD, PhD, an epidemiologist at UCLA Fielding School of Public Health in Los Angeles, who is part of a team also working on ways to measure abnormal alpha-synuclein to diagnose Parkinson’s disease, described the current study of skin samples as “pretty nifty.”
“Their research shows clearly that they can distinguish between patients with Parkinson’s disease and controls in this way,” she said. “The big advantage of this study is that they have brain pathology, so they know exactly which individuals had Parkinson’s disease.”
Dr. Ritz is working with Gal Bitan, PhD, from the UCLA Brain Research Institute on a potential blood test to measure abnormal alpha-synuclein.
Dr. Ritz explained that it is not possible to measure alpha-synuclein pathology in regular blood samples as it is expressed normally in red blood cells, but they are measuring the protein and its more toxic phosphorylated form from exosomes, which contain the waste discarded by cells using technology that determines the origin of these exosomes.
“Alpha-synuclein itself is not a problem. It is the way it misfolds that causes toxicity and disrupts the workings of the cell,” Dr. Ritz added. “In Parkinson’s disease, it is particularly toxic to dopaminergic neurons, and in multiple system atrophy, it is toxic to glial cells, so if we can identify the source of the protein then that could be helpful.”
The study was funded by the National Institutes of Health and the US Army Medical Research Materiel Command. The study authors, Dr. Beck, and Dr. Ritz have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a new study suggests. For the study, researchers used a chemical assay to detect clumping of the protein alpha-synuclein, a hallmark of Parkinson’s disease, in autopsy skin samples taken from patients who had Parkinson’s disease confirmed by brain pathology and from controls without the disease. The test showed a high degree of sensitivity and specificity for the diagnosis of Parkinson’s disease.
The study was published online in Movement Disorders.
“This test has a lot of promise,” said senior author Anumantha Kanthasamy, PhD, professor of biomedical sciences at Iowa State University in Ames. “At present there are no peripheral biomarkers for Parkinson’s disease. The current diagnosis is just based on symptoms, and the symptoms can be similar to many other neurological diseases,” he added. “It can take many years to establish a correct diagnosis and the accuracy is low even with experienced neurologists.”
If the current results can be replicated in samples from live patients and in those with very early stages of Parkinson’s disease, a skin test could allow early diagnosis and the possibility of starting preventive treatments to slow disease progression before symptoms develop too severely, the researchers suggest.
Sensitive and specific test
The blinded study used a seeding assay – used previously to detect misfolded proteins in prion diseases – to analyze 50 skin samples provided by the Arizona Study of Aging and Neurodegenerative Disorders/Brain and Body Donation Program based at Banner Sun Health Research Institute in Sun City.
Half of the skin samples came from patients with Parkinson’s disease and half came from people without neurologic disease. The protein assay correctly diagnosed 24 out of 25 patients with Parkinson’s disease and only one of the 25 controls had the protein clumping.
“At present, the only way to definitely diagnose Parkinson’s disease is on autopsy – by the detection of alpha-synuclein clumps [Lewy bodies] in the brain,” commented Charles Adler, MD, professor of neurology at Mayo Clinic Arizona in Scottsdale and a coinvestigator of the study. “In our research, we have also seen clumping of alpha-synuclein in many other organs including submandibular gland, colon, skin, heart, and stomach, but in terms of access, the skin is probably the easiest source.”
In this study, “we found this seeding assay for alpha-synuclein clumps to be extremely sensitive and specific in the diagnosis of Parkinson’s disease,” he added. “This is very valuable data as we have samples from patients with autopsy-validated Parkinson’s disease.”
A reliable biomarker?
The researchers are now starting a study in living patients with funding from the National Institutes of Health in which they will repeat the process comparing skin samples from patients with clinically diagnosed Parkinson’s disease and controls.
“We need to know whether analyzing alpha-synuclein clumping in skin biopsies from live patients with Parkinson’s disease would serve as a reliable biomarker for disease progression. Will clumping of this protein in skin samples increase over time and does it correspond with disease progression?” Dr. Adler said.
In future they are also hoping to test individuals who have not yet developed Parkinson’s disease but may have some prodromal type symptoms and to test whether this assay could measure a treatment effect of drug therapy.
Dr. Adler noted that they are currently conducting an autopsy study of skin samples from individuals who did not have clinical Parkinson’s disease when alive but in whom Lewy bodies have been found postmortem.
“This suggests that the disease pathology starts before Parkinson’s symptoms develop, and in the future, if we can diagnose Parkinson’s disease earlier then we may be able to stop progression,” he said.
“There is a long list of compounds that have been studied to try and slow progression but haven’t shown benefits, but by the time patients develop symptoms they already have significant disease and [have] lost most of their dopamine neurons,” he added. “If we could backtrack by 10 years, then these drugs may well make a difference.”
Dr. Adler also noted that currently more advanced patients may undergo invasive procedures such as deep brain stimulation or surgery. “It is of utmost importance that they have an accurate diagnosis before being subjected to such procedures.”
In addition, he pointed out that an accurate test would help the drug development process. “It is vitally important to enroll patients with an accurate diagnosis in clinical trials of new drugs. At present, a large percentage of patients in these trials may not actually have Parkinson’s disease, which makes it very difficult to show a treatment effect.”
Important step, but preliminary
Commenting on the research, James Beck, PhD, chief scientific officer of the Parkinson’s Foundation, said the study “is an important step toward the creation of a new way to potentially diagnose Parkinson’s disease.”
But he cautioned that this is a preliminary study. “To really confirm the possibility of using this approach for diagnosing Parkinson’s disease, a larger study will be necessary. And it will be important to test this in a population with early disease – the most difficult group to accurately diagnose.”
Also commenting on the findings, Beate Ritz, MD, PhD, an epidemiologist at UCLA Fielding School of Public Health in Los Angeles, who is part of a team also working on ways to measure abnormal alpha-synuclein to diagnose Parkinson’s disease, described the current study of skin samples as “pretty nifty.”
“Their research shows clearly that they can distinguish between patients with Parkinson’s disease and controls in this way,” she said. “The big advantage of this study is that they have brain pathology, so they know exactly which individuals had Parkinson’s disease.”
Dr. Ritz is working with Gal Bitan, PhD, from the UCLA Brain Research Institute on a potential blood test to measure abnormal alpha-synuclein.
Dr. Ritz explained that it is not possible to measure alpha-synuclein pathology in regular blood samples as it is expressed normally in red blood cells, but they are measuring the protein and its more toxic phosphorylated form from exosomes, which contain the waste discarded by cells using technology that determines the origin of these exosomes.
“Alpha-synuclein itself is not a problem. It is the way it misfolds that causes toxicity and disrupts the workings of the cell,” Dr. Ritz added. “In Parkinson’s disease, it is particularly toxic to dopaminergic neurons, and in multiple system atrophy, it is toxic to glial cells, so if we can identify the source of the protein then that could be helpful.”
The study was funded by the National Institutes of Health and the US Army Medical Research Materiel Command. The study authors, Dr. Beck, and Dr. Ritz have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM MOVEMENT DISORDERS
‘Landmark’ study pushed detection of covert consciousness in TBI
Compelling advances in the ability to detect signs of consciousness in unconscious patients who have experienced traumatic brain injury (TBI) are leading to unprecedented changes in the field. There is now hope of improving outcomes and even sparing lives of patients who may otherwise have been mistakenly assessed as having no chance of recovery.
That research, published in the New England Journal of Medicine in June 2019, linked the promising signals of consciousness in comatose patients, detected only on imaging, with remarkable outcomes a year later.
“This was a landmark study,” said Brian L. Edlow, MD, in a presentation on the issue of covert consciousness at the virtual annual meeting of the American Neurological Association.
“Importantly, it is the first compelling evidence that early detection of covert consciousness also predicts 1-year outcomes in the Glasgow Outcome Scale Extended (GOSE), showing that covert consciousness in the ICU appears to be relevant for predicting long-term outcomes,” said Dr. Edlow, who is associate director of the Center for Neurotechnology and Neurorecovery, Massachusetts General Hospital, in Boston.
The researchers showed that 15% of unconscious patients with acute brain injury in the study exhibited significant brain activity on EEG in response to stimuli that included verbal commands such as envisioning that they are playing tennis.
Although other studies have shown similar effects with task-based stimuli, the New England Journal of Medicine study further showed that a year later, the patients who had shown signs of covert consciousness, also called “cognitive motor dissociation” (CMD), were significantly more likely to have a good functional outcome, said the study’s senior author, Jan Claassen, MD, director of critical care neurology at Columbia University, New York, who also presented at the ANA session.
“Importantly, a year later after injury, we found that 44% of patients with CMD and only 14% of non-CMD patients had a good functional outcome, defined as a GOSE score indicating a state where they can at least take care of themselves for 8 hours in a day,” he said.
“[Whether] these patients in a CMD state represent a parallel state or a transitory state on the road to recovery remains to be shown,” he said.
Jennifer Frontera, MD, a professor in the department of neurology at NYU Langone Health in New York and comoderator of the session, agreed that the research is “remarkable.”
“Also,” she said, “it is practical, since many could potentially apply and validate his algorithms, since EEG technology is portable and widely available.”
Research has ushered in a ‘sea change’ in neurocritical care
The research has helped push forward recommendations on the treatment of unconscious patients, Dr. Edlow said. “This has led to a sea change in our field just over the last 2 years, with multiple guidelines published suggesting that it may be time for us to consider incorporating task-based fMRI and EEG techniques into our clinical assessment of patients with disorders of consciousness,” Dr. Edlow said.
Among those updating their recommendations was the American Academy of Neurology, which revised guidelines on practice parameters for patients in a persistent vegetative state. Those guidelines had not been updated since 1995.
Although concluding that “no diagnostic assessment procedure had moderate or strong evidence for use,” the guidelines acknowledge that “it is possible that a positive electromyographic (EMG) response to command, EEG reactivity to sensory stimuli, laser-evoked potentials, and the Perturbational Complexity Index can distinguish a minimally conscious state from vegetative state/unresponsive wakefulness syndrome (VS/UWS).”
Earlier this year, the European Academy of Neurology followed suit with updated guidelines of its own. In the EAN guideline, the academy’s Panel on Coma, Disorders of Consciousness recommends that task-based fMRI, EEG, and other advanced assessments be performed as part of a composite assessment of consciousness and that a patient’s best performance or highest level of consciousness on any of those tests should be a reflection of their diagnosis, Dr. Edlow explained.
“What this means is that our field is moving toward a multimodal assessment of consciousness in the ICU as well as beyond, in the subacute to chronic setting, whereby the behavioral exam, advanced DG, and advanced MRI methods all also contribute to the diagnosis of consciousness,” he said.
The standard for assessment of disorders of consciousness is the Coma Recovery Scale–Revised, with a 25-item scale for diagnosis, prediction of outcome, and assessment of potential treatment efficacy.
But much uncertainty can remain despite the assessment, Dr. Claassen said. “Behavioral assessments of patients with acute brain injury are challenging because examinations fluctuate, and there’s variability between assessors,” he said. “Nevertheless, patients and their families demand guidance from us.”
Dr. Edlow pointed out that the largest study to date of the causes of death among patients with TBI in the ICU underscores the need for better assessments.
The study of more than 600 patients at six level l trauma centers in Canada showed that 70% of patients who died in the ICU from TBI did so as the result of the withdrawal of life-sustaining therapy. However, only about a half (57%) had an unreactive pupil, and only about a quarter (23.7%) had evidence of herniation on CT, findings that are commonly associated with a poor prognosis.
“What emerges from this is that the manner in which the clinicians communicated the prognosis to families was a primary determinant of decisions to withdraw life-sustaining therapy,” Dr. Edlow said.
Negative response not necessarily conclusive
Dr. Edlow added a word of caution that the science is still far from perfect. He noted that, for 25% of healthy patients who are given a motor imagery task, neuroimaging might not show a response, implying that the lack of a signal may not be conclusive.
He described the case of a patient who was comatose at the time she was scanned on day 3 after injury and who showed no responses to language, music, or motor imagery during the MRI, yet a year later, she was functionally independent, back in the workforce, and had very few residual symptoms from her trauma.
“So if a patient does not show a response, that does not prove the patient is not conscious, and it does not prove that the patient is likely to have a poor outcome,” Dr. Edlow said. Such cases underscore the need for more advances in understanding the inner workings of brain injury.
Dr. Edlow and his colleagues are embarking on a trial of the effects of intravenous methylphenidate in targeting the stimulation of dopaminergic circuits within the subcortical ascending arousal network in patients with severe brain injuries.
“The scientific premise of the trial is that personalized brain network mapping in the ICU can identify patients whose connectomes are amenable to neuromodulation,” Dr. Edlow and his colleague report in an article in Neurocritical Care.
The trial, called STIMPACT (Stimulant Therapy Targeted to Individualized Connectivity Maps to Promote ReACTivation of Consciousness), is part of the newly launched Connectome-based Clinical Trial Platform, which the authors describe as “a new paradigm for developing and testing targeted therapies that promote early recovery of consciousness in the ICU.”
Such efforts are essential, given the high stakes of TBI outcomes, Dr. Edlow said.
“Let’s be clear about the stakes of an incorrect prognosis,” he said. “If we’re overly pessimistic, then a patient who could have potential for meaningful recovery will likely die in our ICU. On the other hand, if we are overly optimistic, then a patient could end up in a vegetative or minimally conscious state that he or she may never have found to be acceptable,” he said.
Access to technologies a ‘civil right?’
Some ethicists in the field are recommending that patients be given access to the advanced techniques as a civil right, similar to the rights described in the Convention on the Rights of Persons With Disabilities, which was adopted by the United Nations in 2008, Dr. Edlow noted.
“So the question that we as clinicians are going to face moving forward from an ethical standpoint is, if we have access to these techniques, is it an ethical obligation to offer them now?” he said.
Dr. Edlow underscored the need to consider the reality that “there are profound issues relating to resource allocation and access to these advanced techniques, but we’re going to have to consider this together as we move forward.”
Dr. Edlow has received funding from the National Institutes of Health. Dr. Claassen is a minority shareholder with ICE Neurosystems. Dr. Frontera has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Compelling advances in the ability to detect signs of consciousness in unconscious patients who have experienced traumatic brain injury (TBI) are leading to unprecedented changes in the field. There is now hope of improving outcomes and even sparing lives of patients who may otherwise have been mistakenly assessed as having no chance of recovery.
That research, published in the New England Journal of Medicine in June 2019, linked the promising signals of consciousness in comatose patients, detected only on imaging, with remarkable outcomes a year later.
“This was a landmark study,” said Brian L. Edlow, MD, in a presentation on the issue of covert consciousness at the virtual annual meeting of the American Neurological Association.
“Importantly, it is the first compelling evidence that early detection of covert consciousness also predicts 1-year outcomes in the Glasgow Outcome Scale Extended (GOSE), showing that covert consciousness in the ICU appears to be relevant for predicting long-term outcomes,” said Dr. Edlow, who is associate director of the Center for Neurotechnology and Neurorecovery, Massachusetts General Hospital, in Boston.
The researchers showed that 15% of unconscious patients with acute brain injury in the study exhibited significant brain activity on EEG in response to stimuli that included verbal commands such as envisioning that they are playing tennis.
Although other studies have shown similar effects with task-based stimuli, the New England Journal of Medicine study further showed that a year later, the patients who had shown signs of covert consciousness, also called “cognitive motor dissociation” (CMD), were significantly more likely to have a good functional outcome, said the study’s senior author, Jan Claassen, MD, director of critical care neurology at Columbia University, New York, who also presented at the ANA session.
“Importantly, a year later after injury, we found that 44% of patients with CMD and only 14% of non-CMD patients had a good functional outcome, defined as a GOSE score indicating a state where they can at least take care of themselves for 8 hours in a day,” he said.
“[Whether] these patients in a CMD state represent a parallel state or a transitory state on the road to recovery remains to be shown,” he said.
Jennifer Frontera, MD, a professor in the department of neurology at NYU Langone Health in New York and comoderator of the session, agreed that the research is “remarkable.”
“Also,” she said, “it is practical, since many could potentially apply and validate his algorithms, since EEG technology is portable and widely available.”
Research has ushered in a ‘sea change’ in neurocritical care
The research has helped push forward recommendations on the treatment of unconscious patients, Dr. Edlow said. “This has led to a sea change in our field just over the last 2 years, with multiple guidelines published suggesting that it may be time for us to consider incorporating task-based fMRI and EEG techniques into our clinical assessment of patients with disorders of consciousness,” Dr. Edlow said.
Among those updating their recommendations was the American Academy of Neurology, which revised guidelines on practice parameters for patients in a persistent vegetative state. Those guidelines had not been updated since 1995.
Although concluding that “no diagnostic assessment procedure had moderate or strong evidence for use,” the guidelines acknowledge that “it is possible that a positive electromyographic (EMG) response to command, EEG reactivity to sensory stimuli, laser-evoked potentials, and the Perturbational Complexity Index can distinguish a minimally conscious state from vegetative state/unresponsive wakefulness syndrome (VS/UWS).”
Earlier this year, the European Academy of Neurology followed suit with updated guidelines of its own. In the EAN guideline, the academy’s Panel on Coma, Disorders of Consciousness recommends that task-based fMRI, EEG, and other advanced assessments be performed as part of a composite assessment of consciousness and that a patient’s best performance or highest level of consciousness on any of those tests should be a reflection of their diagnosis, Dr. Edlow explained.
“What this means is that our field is moving toward a multimodal assessment of consciousness in the ICU as well as beyond, in the subacute to chronic setting, whereby the behavioral exam, advanced DG, and advanced MRI methods all also contribute to the diagnosis of consciousness,” he said.
The standard for assessment of disorders of consciousness is the Coma Recovery Scale–Revised, with a 25-item scale for diagnosis, prediction of outcome, and assessment of potential treatment efficacy.
But much uncertainty can remain despite the assessment, Dr. Claassen said. “Behavioral assessments of patients with acute brain injury are challenging because examinations fluctuate, and there’s variability between assessors,” he said. “Nevertheless, patients and their families demand guidance from us.”
Dr. Edlow pointed out that the largest study to date of the causes of death among patients with TBI in the ICU underscores the need for better assessments.
The study of more than 600 patients at six level l trauma centers in Canada showed that 70% of patients who died in the ICU from TBI did so as the result of the withdrawal of life-sustaining therapy. However, only about a half (57%) had an unreactive pupil, and only about a quarter (23.7%) had evidence of herniation on CT, findings that are commonly associated with a poor prognosis.
“What emerges from this is that the manner in which the clinicians communicated the prognosis to families was a primary determinant of decisions to withdraw life-sustaining therapy,” Dr. Edlow said.
Negative response not necessarily conclusive
Dr. Edlow added a word of caution that the science is still far from perfect. He noted that, for 25% of healthy patients who are given a motor imagery task, neuroimaging might not show a response, implying that the lack of a signal may not be conclusive.
He described the case of a patient who was comatose at the time she was scanned on day 3 after injury and who showed no responses to language, music, or motor imagery during the MRI, yet a year later, she was functionally independent, back in the workforce, and had very few residual symptoms from her trauma.
“So if a patient does not show a response, that does not prove the patient is not conscious, and it does not prove that the patient is likely to have a poor outcome,” Dr. Edlow said. Such cases underscore the need for more advances in understanding the inner workings of brain injury.
Dr. Edlow and his colleagues are embarking on a trial of the effects of intravenous methylphenidate in targeting the stimulation of dopaminergic circuits within the subcortical ascending arousal network in patients with severe brain injuries.
“The scientific premise of the trial is that personalized brain network mapping in the ICU can identify patients whose connectomes are amenable to neuromodulation,” Dr. Edlow and his colleague report in an article in Neurocritical Care.
The trial, called STIMPACT (Stimulant Therapy Targeted to Individualized Connectivity Maps to Promote ReACTivation of Consciousness), is part of the newly launched Connectome-based Clinical Trial Platform, which the authors describe as “a new paradigm for developing and testing targeted therapies that promote early recovery of consciousness in the ICU.”
Such efforts are essential, given the high stakes of TBI outcomes, Dr. Edlow said.
“Let’s be clear about the stakes of an incorrect prognosis,” he said. “If we’re overly pessimistic, then a patient who could have potential for meaningful recovery will likely die in our ICU. On the other hand, if we are overly optimistic, then a patient could end up in a vegetative or minimally conscious state that he or she may never have found to be acceptable,” he said.
Access to technologies a ‘civil right?’
Some ethicists in the field are recommending that patients be given access to the advanced techniques as a civil right, similar to the rights described in the Convention on the Rights of Persons With Disabilities, which was adopted by the United Nations in 2008, Dr. Edlow noted.
“So the question that we as clinicians are going to face moving forward from an ethical standpoint is, if we have access to these techniques, is it an ethical obligation to offer them now?” he said.
Dr. Edlow underscored the need to consider the reality that “there are profound issues relating to resource allocation and access to these advanced techniques, but we’re going to have to consider this together as we move forward.”
Dr. Edlow has received funding from the National Institutes of Health. Dr. Claassen is a minority shareholder with ICE Neurosystems. Dr. Frontera has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Compelling advances in the ability to detect signs of consciousness in unconscious patients who have experienced traumatic brain injury (TBI) are leading to unprecedented changes in the field. There is now hope of improving outcomes and even sparing lives of patients who may otherwise have been mistakenly assessed as having no chance of recovery.
That research, published in the New England Journal of Medicine in June 2019, linked the promising signals of consciousness in comatose patients, detected only on imaging, with remarkable outcomes a year later.
“This was a landmark study,” said Brian L. Edlow, MD, in a presentation on the issue of covert consciousness at the virtual annual meeting of the American Neurological Association.
“Importantly, it is the first compelling evidence that early detection of covert consciousness also predicts 1-year outcomes in the Glasgow Outcome Scale Extended (GOSE), showing that covert consciousness in the ICU appears to be relevant for predicting long-term outcomes,” said Dr. Edlow, who is associate director of the Center for Neurotechnology and Neurorecovery, Massachusetts General Hospital, in Boston.
The researchers showed that 15% of unconscious patients with acute brain injury in the study exhibited significant brain activity on EEG in response to stimuli that included verbal commands such as envisioning that they are playing tennis.
Although other studies have shown similar effects with task-based stimuli, the New England Journal of Medicine study further showed that a year later, the patients who had shown signs of covert consciousness, also called “cognitive motor dissociation” (CMD), were significantly more likely to have a good functional outcome, said the study’s senior author, Jan Claassen, MD, director of critical care neurology at Columbia University, New York, who also presented at the ANA session.
“Importantly, a year later after injury, we found that 44% of patients with CMD and only 14% of non-CMD patients had a good functional outcome, defined as a GOSE score indicating a state where they can at least take care of themselves for 8 hours in a day,” he said.
“[Whether] these patients in a CMD state represent a parallel state or a transitory state on the road to recovery remains to be shown,” he said.
Jennifer Frontera, MD, a professor in the department of neurology at NYU Langone Health in New York and comoderator of the session, agreed that the research is “remarkable.”
“Also,” she said, “it is practical, since many could potentially apply and validate his algorithms, since EEG technology is portable and widely available.”
Research has ushered in a ‘sea change’ in neurocritical care
The research has helped push forward recommendations on the treatment of unconscious patients, Dr. Edlow said. “This has led to a sea change in our field just over the last 2 years, with multiple guidelines published suggesting that it may be time for us to consider incorporating task-based fMRI and EEG techniques into our clinical assessment of patients with disorders of consciousness,” Dr. Edlow said.
Among those updating their recommendations was the American Academy of Neurology, which revised guidelines on practice parameters for patients in a persistent vegetative state. Those guidelines had not been updated since 1995.
Although concluding that “no diagnostic assessment procedure had moderate or strong evidence for use,” the guidelines acknowledge that “it is possible that a positive electromyographic (EMG) response to command, EEG reactivity to sensory stimuli, laser-evoked potentials, and the Perturbational Complexity Index can distinguish a minimally conscious state from vegetative state/unresponsive wakefulness syndrome (VS/UWS).”
Earlier this year, the European Academy of Neurology followed suit with updated guidelines of its own. In the EAN guideline, the academy’s Panel on Coma, Disorders of Consciousness recommends that task-based fMRI, EEG, and other advanced assessments be performed as part of a composite assessment of consciousness and that a patient’s best performance or highest level of consciousness on any of those tests should be a reflection of their diagnosis, Dr. Edlow explained.
“What this means is that our field is moving toward a multimodal assessment of consciousness in the ICU as well as beyond, in the subacute to chronic setting, whereby the behavioral exam, advanced DG, and advanced MRI methods all also contribute to the diagnosis of consciousness,” he said.
The standard for assessment of disorders of consciousness is the Coma Recovery Scale–Revised, with a 25-item scale for diagnosis, prediction of outcome, and assessment of potential treatment efficacy.
But much uncertainty can remain despite the assessment, Dr. Claassen said. “Behavioral assessments of patients with acute brain injury are challenging because examinations fluctuate, and there’s variability between assessors,” he said. “Nevertheless, patients and their families demand guidance from us.”
Dr. Edlow pointed out that the largest study to date of the causes of death among patients with TBI in the ICU underscores the need for better assessments.
The study of more than 600 patients at six level l trauma centers in Canada showed that 70% of patients who died in the ICU from TBI did so as the result of the withdrawal of life-sustaining therapy. However, only about a half (57%) had an unreactive pupil, and only about a quarter (23.7%) had evidence of herniation on CT, findings that are commonly associated with a poor prognosis.
“What emerges from this is that the manner in which the clinicians communicated the prognosis to families was a primary determinant of decisions to withdraw life-sustaining therapy,” Dr. Edlow said.
Negative response not necessarily conclusive
Dr. Edlow added a word of caution that the science is still far from perfect. He noted that, for 25% of healthy patients who are given a motor imagery task, neuroimaging might not show a response, implying that the lack of a signal may not be conclusive.
He described the case of a patient who was comatose at the time she was scanned on day 3 after injury and who showed no responses to language, music, or motor imagery during the MRI, yet a year later, she was functionally independent, back in the workforce, and had very few residual symptoms from her trauma.
“So if a patient does not show a response, that does not prove the patient is not conscious, and it does not prove that the patient is likely to have a poor outcome,” Dr. Edlow said. Such cases underscore the need for more advances in understanding the inner workings of brain injury.
Dr. Edlow and his colleagues are embarking on a trial of the effects of intravenous methylphenidate in targeting the stimulation of dopaminergic circuits within the subcortical ascending arousal network in patients with severe brain injuries.
“The scientific premise of the trial is that personalized brain network mapping in the ICU can identify patients whose connectomes are amenable to neuromodulation,” Dr. Edlow and his colleague report in an article in Neurocritical Care.
The trial, called STIMPACT (Stimulant Therapy Targeted to Individualized Connectivity Maps to Promote ReACTivation of Consciousness), is part of the newly launched Connectome-based Clinical Trial Platform, which the authors describe as “a new paradigm for developing and testing targeted therapies that promote early recovery of consciousness in the ICU.”
Such efforts are essential, given the high stakes of TBI outcomes, Dr. Edlow said.
“Let’s be clear about the stakes of an incorrect prognosis,” he said. “If we’re overly pessimistic, then a patient who could have potential for meaningful recovery will likely die in our ICU. On the other hand, if we are overly optimistic, then a patient could end up in a vegetative or minimally conscious state that he or she may never have found to be acceptable,” he said.
Access to technologies a ‘civil right?’
Some ethicists in the field are recommending that patients be given access to the advanced techniques as a civil right, similar to the rights described in the Convention on the Rights of Persons With Disabilities, which was adopted by the United Nations in 2008, Dr. Edlow noted.
“So the question that we as clinicians are going to face moving forward from an ethical standpoint is, if we have access to these techniques, is it an ethical obligation to offer them now?” he said.
Dr. Edlow underscored the need to consider the reality that “there are profound issues relating to resource allocation and access to these advanced techniques, but we’re going to have to consider this together as we move forward.”
Dr. Edlow has received funding from the National Institutes of Health. Dr. Claassen is a minority shareholder with ICE Neurosystems. Dr. Frontera has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ANA 2020
COVID-19 diagnosed on CTA scan in stroke patients
A routine scan used to evaluate some acute stroke patients can also detect SARS-CoV-2 infection in the upper lungs, a new study shows.
“As part of the stroke evaluation workup process, we were able to diagnose COVID-19 at the same time at no extra cost or additional workload,” lead author Charles Esenwa, MD, commented to Medscape Medical News. “This is an objective way to screen for COVID-19 in the acute stroke setting,” he added.
Esenwa is an assistant professor and a stroke neurologist at the Montefiore Medical Center/Albert Einstein College of Medicine in New York City.
He explained that, during the COVID-19 surge earlier this year, assessment of patients with severe acute stroke using computed tomography angiogram (CTA) scans – used to evaluate suitability for endovascular stroke therapy – also showed findings in the upper lung consistent with viral infection in some patients.
“We then assumed that these patients had COVID-19 and took extra precautions to keep them isolated and to protect staff involved in their care. It also allowed us to triage these patients more quickly than waiting for the COVID-19 swab test and arrange the most appropriate care for them,” Esenwa said.
The researchers have now gone back and analyzed their data on acute stroke patients who underwent CTA at their institution during the COVID-19 surge. They found that the changes identified in the lungs were highly specific for diagnosing SARS-CoV-2 infection.
The study was published online on Oct. 29 in Stroke.
“Stroke patients are normally screened for COVID-19 on hospitalization, but the swab test result can take several hours or longer to come back, and it is very useful for us to know if a patient could be infected,” Esenwa noted.
“When we do a CTA, we look at the blood vessels supplying the brain, but the scan also covers the top of the lung, as it starts at the aortic arch. We don’t normally look closely at that area, but we started to notice signs of active lung infection which could have been COVID-19,” he said. “For this paper, we went back to assess how accurate this approach actually was vs. the COVID-19 PCR test.”
The researchers report on 57 patients who presented to three Montefiore Health System hospitals in the Bronx, in New York City, with acute ischemic stroke and who underwent CTA of the head and neck in March and April 2020, the peak of the COVID-19 outbreak there. The patients also underwent PCR testing for COVID-19.
Results showed that 30 patients had a positive COVID-19 test result and that 27 had a negative result. Lung findings highly or very highly suspicious for COVID-19 pneumonia were identified during the CTA scan in 20 (67%) of the COVID-19–positive patients and in two (7%) of the COVID-19–negative patients.
These findings, when used in isolation, yielded a sensitivity of 0.67 and a specificity of 0.93. They had a positive predictive value of 0.19, a negative predictive value of 0.99, and accuracy of 0.92 for the diagnosis of COVID-19.
When apical lung assessment was combined with self-reported clinical symptoms of cough or dyspnea, sensitivity for the diagnosis of COVID-19 for patients presenting to the hospital for acute ischemic stroke increased to 0.83.
“We wondered whether looking at the whole lung would have found better results, but other studies which have done this actually found similar numbers to ours, so we think actually just looking at the top of the lungs, which can be seen in a stroke CTA, may be sufficient,” Esenwa said.
He emphasized the importance of establishing whether an acute stroke patient has COVID-19. “If we had a high suspicion of COVID-19 infection, we would take more precautions during any procedures, such as thrombectomy, and make sure to keep the patient isolated afterwards. It doesn’t necessarily affect the treatment given for stroke, but it affects the safety of the patients and everyone caring for them,” he commented.
Esenwa explained that intubation – which is sometime necessary during thrombectomy – can expose everyone in the room to aerosolized droplets. “So we would take much higher safety precautions if we thought the patient was COVID-19 positive,” he said.
“Early COVID-19 diagnosis also means patients can be given supportive treatment more quickly, admitted to ICU if appropriate, and we can all keep a close eye on pulmonary issues. So having that information is important in many ways,” he added.
Esenwa advises that any medical center that evaluates acute stroke patients for thrombectomy and is experiencing a COVID-19 surge can use this technique as a screening method for COVID-19.
He pointed out that the Montefiore Health System had a very high rate of COVID-19. That part of New York City was one of the worst hit areas of the world, and the CTA approach for identifying COVID-19 has been validated only in areas with such a high local incidence of COVID. If used in an area of lower prevalence, the accuracy would likely be less.
“We don’t know if this approach would work as well at times of low COVID-19 infection, where any lung findings would be more likely to be caused by other conditions, such as pneumonia due to other causes or congestive heart failure. So there would be more false positives,” Esenwa said.
“But when COVID-19 prevalence is high, the lung findings are much more likely to be a sign of COVID-19 infection. As COVID-19 numbers are now rising for a second time, it is likely to become a useful strategy again.”
The study was approved by the Albert Einstein College of Medicine/Montefiore Medical Center Institutional Review Board and had no external funding. Esenwa has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A routine scan used to evaluate some acute stroke patients can also detect SARS-CoV-2 infection in the upper lungs, a new study shows.
“As part of the stroke evaluation workup process, we were able to diagnose COVID-19 at the same time at no extra cost or additional workload,” lead author Charles Esenwa, MD, commented to Medscape Medical News. “This is an objective way to screen for COVID-19 in the acute stroke setting,” he added.
Esenwa is an assistant professor and a stroke neurologist at the Montefiore Medical Center/Albert Einstein College of Medicine in New York City.
He explained that, during the COVID-19 surge earlier this year, assessment of patients with severe acute stroke using computed tomography angiogram (CTA) scans – used to evaluate suitability for endovascular stroke therapy – also showed findings in the upper lung consistent with viral infection in some patients.
“We then assumed that these patients had COVID-19 and took extra precautions to keep them isolated and to protect staff involved in their care. It also allowed us to triage these patients more quickly than waiting for the COVID-19 swab test and arrange the most appropriate care for them,” Esenwa said.
The researchers have now gone back and analyzed their data on acute stroke patients who underwent CTA at their institution during the COVID-19 surge. They found that the changes identified in the lungs were highly specific for diagnosing SARS-CoV-2 infection.
The study was published online on Oct. 29 in Stroke.
“Stroke patients are normally screened for COVID-19 on hospitalization, but the swab test result can take several hours or longer to come back, and it is very useful for us to know if a patient could be infected,” Esenwa noted.
“When we do a CTA, we look at the blood vessels supplying the brain, but the scan also covers the top of the lung, as it starts at the aortic arch. We don’t normally look closely at that area, but we started to notice signs of active lung infection which could have been COVID-19,” he said. “For this paper, we went back to assess how accurate this approach actually was vs. the COVID-19 PCR test.”
The researchers report on 57 patients who presented to three Montefiore Health System hospitals in the Bronx, in New York City, with acute ischemic stroke and who underwent CTA of the head and neck in March and April 2020, the peak of the COVID-19 outbreak there. The patients also underwent PCR testing for COVID-19.
Results showed that 30 patients had a positive COVID-19 test result and that 27 had a negative result. Lung findings highly or very highly suspicious for COVID-19 pneumonia were identified during the CTA scan in 20 (67%) of the COVID-19–positive patients and in two (7%) of the COVID-19–negative patients.
These findings, when used in isolation, yielded a sensitivity of 0.67 and a specificity of 0.93. They had a positive predictive value of 0.19, a negative predictive value of 0.99, and accuracy of 0.92 for the diagnosis of COVID-19.
When apical lung assessment was combined with self-reported clinical symptoms of cough or dyspnea, sensitivity for the diagnosis of COVID-19 for patients presenting to the hospital for acute ischemic stroke increased to 0.83.
“We wondered whether looking at the whole lung would have found better results, but other studies which have done this actually found similar numbers to ours, so we think actually just looking at the top of the lungs, which can be seen in a stroke CTA, may be sufficient,” Esenwa said.
He emphasized the importance of establishing whether an acute stroke patient has COVID-19. “If we had a high suspicion of COVID-19 infection, we would take more precautions during any procedures, such as thrombectomy, and make sure to keep the patient isolated afterwards. It doesn’t necessarily affect the treatment given for stroke, but it affects the safety of the patients and everyone caring for them,” he commented.
Esenwa explained that intubation – which is sometime necessary during thrombectomy – can expose everyone in the room to aerosolized droplets. “So we would take much higher safety precautions if we thought the patient was COVID-19 positive,” he said.
“Early COVID-19 diagnosis also means patients can be given supportive treatment more quickly, admitted to ICU if appropriate, and we can all keep a close eye on pulmonary issues. So having that information is important in many ways,” he added.
Esenwa advises that any medical center that evaluates acute stroke patients for thrombectomy and is experiencing a COVID-19 surge can use this technique as a screening method for COVID-19.
He pointed out that the Montefiore Health System had a very high rate of COVID-19. That part of New York City was one of the worst hit areas of the world, and the CTA approach for identifying COVID-19 has been validated only in areas with such a high local incidence of COVID. If used in an area of lower prevalence, the accuracy would likely be less.
“We don’t know if this approach would work as well at times of low COVID-19 infection, where any lung findings would be more likely to be caused by other conditions, such as pneumonia due to other causes or congestive heart failure. So there would be more false positives,” Esenwa said.
“But when COVID-19 prevalence is high, the lung findings are much more likely to be a sign of COVID-19 infection. As COVID-19 numbers are now rising for a second time, it is likely to become a useful strategy again.”
The study was approved by the Albert Einstein College of Medicine/Montefiore Medical Center Institutional Review Board and had no external funding. Esenwa has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A routine scan used to evaluate some acute stroke patients can also detect SARS-CoV-2 infection in the upper lungs, a new study shows.
“As part of the stroke evaluation workup process, we were able to diagnose COVID-19 at the same time at no extra cost or additional workload,” lead author Charles Esenwa, MD, commented to Medscape Medical News. “This is an objective way to screen for COVID-19 in the acute stroke setting,” he added.
Esenwa is an assistant professor and a stroke neurologist at the Montefiore Medical Center/Albert Einstein College of Medicine in New York City.
He explained that, during the COVID-19 surge earlier this year, assessment of patients with severe acute stroke using computed tomography angiogram (CTA) scans – used to evaluate suitability for endovascular stroke therapy – also showed findings in the upper lung consistent with viral infection in some patients.
“We then assumed that these patients had COVID-19 and took extra precautions to keep them isolated and to protect staff involved in their care. It also allowed us to triage these patients more quickly than waiting for the COVID-19 swab test and arrange the most appropriate care for them,” Esenwa said.
The researchers have now gone back and analyzed their data on acute stroke patients who underwent CTA at their institution during the COVID-19 surge. They found that the changes identified in the lungs were highly specific for diagnosing SARS-CoV-2 infection.
The study was published online on Oct. 29 in Stroke.
“Stroke patients are normally screened for COVID-19 on hospitalization, but the swab test result can take several hours or longer to come back, and it is very useful for us to know if a patient could be infected,” Esenwa noted.
“When we do a CTA, we look at the blood vessels supplying the brain, but the scan also covers the top of the lung, as it starts at the aortic arch. We don’t normally look closely at that area, but we started to notice signs of active lung infection which could have been COVID-19,” he said. “For this paper, we went back to assess how accurate this approach actually was vs. the COVID-19 PCR test.”
The researchers report on 57 patients who presented to three Montefiore Health System hospitals in the Bronx, in New York City, with acute ischemic stroke and who underwent CTA of the head and neck in March and April 2020, the peak of the COVID-19 outbreak there. The patients also underwent PCR testing for COVID-19.
Results showed that 30 patients had a positive COVID-19 test result and that 27 had a negative result. Lung findings highly or very highly suspicious for COVID-19 pneumonia were identified during the CTA scan in 20 (67%) of the COVID-19–positive patients and in two (7%) of the COVID-19–negative patients.
These findings, when used in isolation, yielded a sensitivity of 0.67 and a specificity of 0.93. They had a positive predictive value of 0.19, a negative predictive value of 0.99, and accuracy of 0.92 for the diagnosis of COVID-19.
When apical lung assessment was combined with self-reported clinical symptoms of cough or dyspnea, sensitivity for the diagnosis of COVID-19 for patients presenting to the hospital for acute ischemic stroke increased to 0.83.
“We wondered whether looking at the whole lung would have found better results, but other studies which have done this actually found similar numbers to ours, so we think actually just looking at the top of the lungs, which can be seen in a stroke CTA, may be sufficient,” Esenwa said.
He emphasized the importance of establishing whether an acute stroke patient has COVID-19. “If we had a high suspicion of COVID-19 infection, we would take more precautions during any procedures, such as thrombectomy, and make sure to keep the patient isolated afterwards. It doesn’t necessarily affect the treatment given for stroke, but it affects the safety of the patients and everyone caring for them,” he commented.
Esenwa explained that intubation – which is sometime necessary during thrombectomy – can expose everyone in the room to aerosolized droplets. “So we would take much higher safety precautions if we thought the patient was COVID-19 positive,” he said.
“Early COVID-19 diagnosis also means patients can be given supportive treatment more quickly, admitted to ICU if appropriate, and we can all keep a close eye on pulmonary issues. So having that information is important in many ways,” he added.
Esenwa advises that any medical center that evaluates acute stroke patients for thrombectomy and is experiencing a COVID-19 surge can use this technique as a screening method for COVID-19.
He pointed out that the Montefiore Health System had a very high rate of COVID-19. That part of New York City was one of the worst hit areas of the world, and the CTA approach for identifying COVID-19 has been validated only in areas with such a high local incidence of COVID. If used in an area of lower prevalence, the accuracy would likely be less.
“We don’t know if this approach would work as well at times of low COVID-19 infection, where any lung findings would be more likely to be caused by other conditions, such as pneumonia due to other causes or congestive heart failure. So there would be more false positives,” Esenwa said.
“But when COVID-19 prevalence is high, the lung findings are much more likely to be a sign of COVID-19 infection. As COVID-19 numbers are now rising for a second time, it is likely to become a useful strategy again.”
The study was approved by the Albert Einstein College of Medicine/Montefiore Medical Center Institutional Review Board and had no external funding. Esenwa has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Inpatient opioid administration associated with postdischarge opioid use
Background: Efforts to reduce and monitor high-risk opioid prescribing have largely focused on outpatient prescribing with less empiric evaluation of inpatient administration. Little is known about the association of inpatient opioid administration and postdischarge opioid use.
Study design: Retrospective cohort.
Setting: 12 community and academic hospitals in Pennsylvania.
Synopsis: With electronic health record data from 2010-2014 to evaluate 148,068 opioid-naive patients aged 18 years and older, this study showed a relationship between inpatient opioid administration, specific patterns of inpatient opioid administration, and postdischarge opioid use. Specifically, inpatient opioid administration was associated with a 3.0% increase (95% CI, 2.8%-3.2%) in opioid use at 90 days post discharge. Additionally, inpatient opioid administration within 12 hours of hospital discharge was associated with a 3.6% increase (95% CI, 3.3%-3.9%) in opioid use at 90 days post discharge.
This observational study is prone to potential unmeasured confounders negating any clear causation. Rather, hospitalists should be aware of the increasing focus on inpatient opioid administration as it relates to outpatient opioid use, especially in the setting of the current opioid crisis.
Bottom line: Inpatient opioid administration and administration patterns are associated with 90-day postdischarge opioid use in opioid-naive patients.
Citation: Donohue JM et al. Patterns of opioid administration among opioid-naive inpatients and associations with postdischarge opioid use. Ann Intern Med. 2019 Jun 18:171(2):81-90.
Dr. Ledford is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: Efforts to reduce and monitor high-risk opioid prescribing have largely focused on outpatient prescribing with less empiric evaluation of inpatient administration. Little is known about the association of inpatient opioid administration and postdischarge opioid use.
Study design: Retrospective cohort.
Setting: 12 community and academic hospitals in Pennsylvania.
Synopsis: With electronic health record data from 2010-2014 to evaluate 148,068 opioid-naive patients aged 18 years and older, this study showed a relationship between inpatient opioid administration, specific patterns of inpatient opioid administration, and postdischarge opioid use. Specifically, inpatient opioid administration was associated with a 3.0% increase (95% CI, 2.8%-3.2%) in opioid use at 90 days post discharge. Additionally, inpatient opioid administration within 12 hours of hospital discharge was associated with a 3.6% increase (95% CI, 3.3%-3.9%) in opioid use at 90 days post discharge.
This observational study is prone to potential unmeasured confounders negating any clear causation. Rather, hospitalists should be aware of the increasing focus on inpatient opioid administration as it relates to outpatient opioid use, especially in the setting of the current opioid crisis.
Bottom line: Inpatient opioid administration and administration patterns are associated with 90-day postdischarge opioid use in opioid-naive patients.
Citation: Donohue JM et al. Patterns of opioid administration among opioid-naive inpatients and associations with postdischarge opioid use. Ann Intern Med. 2019 Jun 18:171(2):81-90.
Dr. Ledford is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
Background: Efforts to reduce and monitor high-risk opioid prescribing have largely focused on outpatient prescribing with less empiric evaluation of inpatient administration. Little is known about the association of inpatient opioid administration and postdischarge opioid use.
Study design: Retrospective cohort.
Setting: 12 community and academic hospitals in Pennsylvania.
Synopsis: With electronic health record data from 2010-2014 to evaluate 148,068 opioid-naive patients aged 18 years and older, this study showed a relationship between inpatient opioid administration, specific patterns of inpatient opioid administration, and postdischarge opioid use. Specifically, inpatient opioid administration was associated with a 3.0% increase (95% CI, 2.8%-3.2%) in opioid use at 90 days post discharge. Additionally, inpatient opioid administration within 12 hours of hospital discharge was associated with a 3.6% increase (95% CI, 3.3%-3.9%) in opioid use at 90 days post discharge.
This observational study is prone to potential unmeasured confounders negating any clear causation. Rather, hospitalists should be aware of the increasing focus on inpatient opioid administration as it relates to outpatient opioid use, especially in the setting of the current opioid crisis.
Bottom line: Inpatient opioid administration and administration patterns are associated with 90-day postdischarge opioid use in opioid-naive patients.
Citation: Donohue JM et al. Patterns of opioid administration among opioid-naive inpatients and associations with postdischarge opioid use. Ann Intern Med. 2019 Jun 18:171(2):81-90.
Dr. Ledford is a hospitalist at Vanderbilt University Medical Center, Nashville, Tenn.
ODC1 gene linked to newly described neurodevelopmental disorder
but it may be treated with diet modifications and available therapies, according to the researcher whose group first identified the disorder.
Lance Rodan, MD, of Boston Children’s Hospital and Harvard Medical School, reported on research into ODC1 gain-of-function disorder –named for ornithine decarboxylase 1, the rate-limiting enzyme involved in polyamine synthesis – in the Linda De Meirleir Neurometabolic award lecture at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year. Dr. Rodan and colleagues first described ODC1 disorder in a multicenter case series.
Dr. Rodan noted that dysregulated polyamine levels are associated with cancer, and that ODC1 is expressed “ubiquitously” throughout the body.
Pathophysiology and phenotypes
In an interview, he described the metabolic process more fully. “GI flora can produce putrescine, which is the polyamine that accumulates in excess in the ODC1 gain-of-function disorder. It is yet to be elucidated if decreasing putrescine production by GI flora and/or reducing dietary sources of putrescine may play a role in the management of this disorder.”
In the De Meirleir lecture, Dr. Rodan described four patients from his group’s published case series, all found to have heterozygous de novo variants in the ODC1 gene, along with a fifth patient reported by Caleb Bupp, MD, and colleagues at Michigan State University, East Lansing.
“There’s a recognizable phenotype to this disorder,” Dr. Rodan said. “These individuals have neurodevelopment abnormalities. They may have behavioral concerns. They have low-tone central hypertonia and macrocephaly.”
One of the most distinctive characteristics of ODC1 disorder is alopecia, he said, “which in almost everybody with this condition involves the eyebrows and eyelashes and in some individuals also involves the scalp hair.”
These patients also have what Dr. Rodan called “a common yet subtle facial gestalt.” That can include hypertelorism, spareness of the eyebrows and eyelashes, and a tubular- shaped nose with a short columella and a short philtrum.
They may also have abnormalities of the nails and cryptorchidism, and typically a prenatal history of polyhydramnios, he said.
MRI findings include prominent perivascular spaces, periventricular cysts, abnormal white matter and corpus callosum abnormalities, he said, adding that the fetal case MRI demonstrated subepidermal cysts, white matter cysts in the temporal pole, deficiency of the falx cerebri and abnormal white-matter signals.
Biochemical features of ODC1 disorder include increased N-acetylputrescine levels with normal spermine and spermidine levels, Dr. Rodan said. He also noted that Dr. Bupp’s group reported increased putrescine in fibroblasts and increased ODC1 protein levels in red blood cells.
Dr. Rodan also described possible molecular mechanisms in ODC1 disorder. One was the location of the ODC1 variants: all were reported closely located to truncating variants in the final exon of the ODC1 gene. This allows truncating proteins to survive, adding to the degradation that results in a net gain-of-function of ODC1 enzyme activity.
With regard to pathophysiology of ODC1 disorder, Dr. Rodan noted that research has implicated chronically elevated putrescine levels in the alopecia, a finding animal models support. “Since putrescine is a precursor for gamma-aminobutyric acid, it’s possible perturbed GABA levels may also be involved,” he said. Abnormal modulation N-methyl-D-aspirate receptors may also be involved, he said.
Another hypothesis purports that potential of elevated levels of toxic aldehydes/H2O2 similar to Snyder-Robinson syndrome, the better known polyamine-related neurometabolic disorder. “Along those lines, maybe there’s also a secondary mitochondrial or lysosomal dysfunction, but this is something that’s still being actively studied,” Dr. Rodan said.
Treatment
Because ODC1 disorder was only first described 2 years ago, research into treatment is nascent. “In terms of management, I think one of the more fundamental questions is whether this is more of a static developmental disorder or whether this actually represents a progressive degenerative disorder,” Dr. Rodan said.
One potential treatment that has been explored, he said, is difluoromethylornithine, a synthetic ODC1 inhibitor already Food and Drug Administration approved for African sleeping sickness and as a topical treatment for hirsutism. It is also the subject of ongoing clinical trials in colon cancer and neuroblastoma. Potential side effects include myelosuppression, seizures and hearing loss.
Dr. Rodan noted that a single-center study reported that difluoromethylornithine in a 3-year-old patient with ODC1 disorder reduced ODC protein activity and putrescine to control levels.
Other potential treatments include the natural ODC1 inhibitors agmatine and turmeric/curcumin, flagyl/rifaximin to decrease putrescine production in the gut, a low-dairy diet to lower putrescine levels, and antioxidants. “There could be a role for antioxidant stress similar to what is seen in Snyder-Robinson syndrome,” Dr. Rodan said.
Based on mouse studies, patients with ODC1 may be at risk of skin cancer, so regular skin checks along with sun protection should be part of management, he said. “This also raises the question of whether there should be surveillance for other types of cancer given the role of polyamine in various types of tumors.”
Dr. Rodan has no relevant financial relationships to disclose.
but it may be treated with diet modifications and available therapies, according to the researcher whose group first identified the disorder.
Lance Rodan, MD, of Boston Children’s Hospital and Harvard Medical School, reported on research into ODC1 gain-of-function disorder –named for ornithine decarboxylase 1, the rate-limiting enzyme involved in polyamine synthesis – in the Linda De Meirleir Neurometabolic award lecture at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year. Dr. Rodan and colleagues first described ODC1 disorder in a multicenter case series.
Dr. Rodan noted that dysregulated polyamine levels are associated with cancer, and that ODC1 is expressed “ubiquitously” throughout the body.
Pathophysiology and phenotypes
In an interview, he described the metabolic process more fully. “GI flora can produce putrescine, which is the polyamine that accumulates in excess in the ODC1 gain-of-function disorder. It is yet to be elucidated if decreasing putrescine production by GI flora and/or reducing dietary sources of putrescine may play a role in the management of this disorder.”
In the De Meirleir lecture, Dr. Rodan described four patients from his group’s published case series, all found to have heterozygous de novo variants in the ODC1 gene, along with a fifth patient reported by Caleb Bupp, MD, and colleagues at Michigan State University, East Lansing.
“There’s a recognizable phenotype to this disorder,” Dr. Rodan said. “These individuals have neurodevelopment abnormalities. They may have behavioral concerns. They have low-tone central hypertonia and macrocephaly.”
One of the most distinctive characteristics of ODC1 disorder is alopecia, he said, “which in almost everybody with this condition involves the eyebrows and eyelashes and in some individuals also involves the scalp hair.”
These patients also have what Dr. Rodan called “a common yet subtle facial gestalt.” That can include hypertelorism, spareness of the eyebrows and eyelashes, and a tubular- shaped nose with a short columella and a short philtrum.
They may also have abnormalities of the nails and cryptorchidism, and typically a prenatal history of polyhydramnios, he said.
MRI findings include prominent perivascular spaces, periventricular cysts, abnormal white matter and corpus callosum abnormalities, he said, adding that the fetal case MRI demonstrated subepidermal cysts, white matter cysts in the temporal pole, deficiency of the falx cerebri and abnormal white-matter signals.
Biochemical features of ODC1 disorder include increased N-acetylputrescine levels with normal spermine and spermidine levels, Dr. Rodan said. He also noted that Dr. Bupp’s group reported increased putrescine in fibroblasts and increased ODC1 protein levels in red blood cells.
Dr. Rodan also described possible molecular mechanisms in ODC1 disorder. One was the location of the ODC1 variants: all were reported closely located to truncating variants in the final exon of the ODC1 gene. This allows truncating proteins to survive, adding to the degradation that results in a net gain-of-function of ODC1 enzyme activity.
With regard to pathophysiology of ODC1 disorder, Dr. Rodan noted that research has implicated chronically elevated putrescine levels in the alopecia, a finding animal models support. “Since putrescine is a precursor for gamma-aminobutyric acid, it’s possible perturbed GABA levels may also be involved,” he said. Abnormal modulation N-methyl-D-aspirate receptors may also be involved, he said.
Another hypothesis purports that potential of elevated levels of toxic aldehydes/H2O2 similar to Snyder-Robinson syndrome, the better known polyamine-related neurometabolic disorder. “Along those lines, maybe there’s also a secondary mitochondrial or lysosomal dysfunction, but this is something that’s still being actively studied,” Dr. Rodan said.
Treatment
Because ODC1 disorder was only first described 2 years ago, research into treatment is nascent. “In terms of management, I think one of the more fundamental questions is whether this is more of a static developmental disorder or whether this actually represents a progressive degenerative disorder,” Dr. Rodan said.
One potential treatment that has been explored, he said, is difluoromethylornithine, a synthetic ODC1 inhibitor already Food and Drug Administration approved for African sleeping sickness and as a topical treatment for hirsutism. It is also the subject of ongoing clinical trials in colon cancer and neuroblastoma. Potential side effects include myelosuppression, seizures and hearing loss.
Dr. Rodan noted that a single-center study reported that difluoromethylornithine in a 3-year-old patient with ODC1 disorder reduced ODC protein activity and putrescine to control levels.
Other potential treatments include the natural ODC1 inhibitors agmatine and turmeric/curcumin, flagyl/rifaximin to decrease putrescine production in the gut, a low-dairy diet to lower putrescine levels, and antioxidants. “There could be a role for antioxidant stress similar to what is seen in Snyder-Robinson syndrome,” Dr. Rodan said.
Based on mouse studies, patients with ODC1 may be at risk of skin cancer, so regular skin checks along with sun protection should be part of management, he said. “This also raises the question of whether there should be surveillance for other types of cancer given the role of polyamine in various types of tumors.”
Dr. Rodan has no relevant financial relationships to disclose.
but it may be treated with diet modifications and available therapies, according to the researcher whose group first identified the disorder.
Lance Rodan, MD, of Boston Children’s Hospital and Harvard Medical School, reported on research into ODC1 gain-of-function disorder –named for ornithine decarboxylase 1, the rate-limiting enzyme involved in polyamine synthesis – in the Linda De Meirleir Neurometabolic award lecture at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year. Dr. Rodan and colleagues first described ODC1 disorder in a multicenter case series.
Dr. Rodan noted that dysregulated polyamine levels are associated with cancer, and that ODC1 is expressed “ubiquitously” throughout the body.
Pathophysiology and phenotypes
In an interview, he described the metabolic process more fully. “GI flora can produce putrescine, which is the polyamine that accumulates in excess in the ODC1 gain-of-function disorder. It is yet to be elucidated if decreasing putrescine production by GI flora and/or reducing dietary sources of putrescine may play a role in the management of this disorder.”
In the De Meirleir lecture, Dr. Rodan described four patients from his group’s published case series, all found to have heterozygous de novo variants in the ODC1 gene, along with a fifth patient reported by Caleb Bupp, MD, and colleagues at Michigan State University, East Lansing.
“There’s a recognizable phenotype to this disorder,” Dr. Rodan said. “These individuals have neurodevelopment abnormalities. They may have behavioral concerns. They have low-tone central hypertonia and macrocephaly.”
One of the most distinctive characteristics of ODC1 disorder is alopecia, he said, “which in almost everybody with this condition involves the eyebrows and eyelashes and in some individuals also involves the scalp hair.”
These patients also have what Dr. Rodan called “a common yet subtle facial gestalt.” That can include hypertelorism, spareness of the eyebrows and eyelashes, and a tubular- shaped nose with a short columella and a short philtrum.
They may also have abnormalities of the nails and cryptorchidism, and typically a prenatal history of polyhydramnios, he said.
MRI findings include prominent perivascular spaces, periventricular cysts, abnormal white matter and corpus callosum abnormalities, he said, adding that the fetal case MRI demonstrated subepidermal cysts, white matter cysts in the temporal pole, deficiency of the falx cerebri and abnormal white-matter signals.
Biochemical features of ODC1 disorder include increased N-acetylputrescine levels with normal spermine and spermidine levels, Dr. Rodan said. He also noted that Dr. Bupp’s group reported increased putrescine in fibroblasts and increased ODC1 protein levels in red blood cells.
Dr. Rodan also described possible molecular mechanisms in ODC1 disorder. One was the location of the ODC1 variants: all were reported closely located to truncating variants in the final exon of the ODC1 gene. This allows truncating proteins to survive, adding to the degradation that results in a net gain-of-function of ODC1 enzyme activity.
With regard to pathophysiology of ODC1 disorder, Dr. Rodan noted that research has implicated chronically elevated putrescine levels in the alopecia, a finding animal models support. “Since putrescine is a precursor for gamma-aminobutyric acid, it’s possible perturbed GABA levels may also be involved,” he said. Abnormal modulation N-methyl-D-aspirate receptors may also be involved, he said.
Another hypothesis purports that potential of elevated levels of toxic aldehydes/H2O2 similar to Snyder-Robinson syndrome, the better known polyamine-related neurometabolic disorder. “Along those lines, maybe there’s also a secondary mitochondrial or lysosomal dysfunction, but this is something that’s still being actively studied,” Dr. Rodan said.
Treatment
Because ODC1 disorder was only first described 2 years ago, research into treatment is nascent. “In terms of management, I think one of the more fundamental questions is whether this is more of a static developmental disorder or whether this actually represents a progressive degenerative disorder,” Dr. Rodan said.
One potential treatment that has been explored, he said, is difluoromethylornithine, a synthetic ODC1 inhibitor already Food and Drug Administration approved for African sleeping sickness and as a topical treatment for hirsutism. It is also the subject of ongoing clinical trials in colon cancer and neuroblastoma. Potential side effects include myelosuppression, seizures and hearing loss.
Dr. Rodan noted that a single-center study reported that difluoromethylornithine in a 3-year-old patient with ODC1 disorder reduced ODC protein activity and putrescine to control levels.
Other potential treatments include the natural ODC1 inhibitors agmatine and turmeric/curcumin, flagyl/rifaximin to decrease putrescine production in the gut, a low-dairy diet to lower putrescine levels, and antioxidants. “There could be a role for antioxidant stress similar to what is seen in Snyder-Robinson syndrome,” Dr. Rodan said.
Based on mouse studies, patients with ODC1 may be at risk of skin cancer, so regular skin checks along with sun protection should be part of management, he said. “This also raises the question of whether there should be surveillance for other types of cancer given the role of polyamine in various types of tumors.”
Dr. Rodan has no relevant financial relationships to disclose.
FROM CNS-ICNA 2020
Brain imaging reveals a neural basis for partisan politics
The differences between politically left- and right-leaning individuals may have neural underpinnings, results from a new brain imaging study suggest.
Investigators found that despite watching the same videos related to immigration policy, neural responses differed between liberals and conservatives.
“This divergence was strongest when the videos used language that highlighted threat, morality, and emotions, suggesting that certain words are more likely to drive polarized response,” lead researcher Yuan Chang Leong, PhD, a postdoctoral scholar in cognitive neuroscience at the University of California, Berkeley, told Medscape Medical News.
“The results suggest a neural basis for partisan biases in interpreting political messages, the effects these biases have on attitude change, and the type of language most likely to drive biased interpretations,” Leong added.
The study was published online Oct. 20 in Proceedings of the National Academy of Sciences.
Hardwired to disagree?
The researchers combined fMRI with semantic content analysis to investigate neural mechanisms that underlie the biased processing of political content.
They scanned 38 middle-aged men and women with liberal- or conservative-leaning immigration attitudes while the participants watched short news clips, campaign ads, and public speeches related to various immigration policies.
These policies included those that led to the United States–Mexico border wall, Deferred Action for Childhood Arrivals (DACA) protections for undocumented immigrants, the ban on refugees from majority-Muslim countries coming to the United States, and the cutting of federal funding to sanctuary cities.
After each video, participants rated on a scale of 1 to 5 how much they agreed with the general message of the video, the credibility of the information presented, and the extent to which the video made them likely to change their position and to support the policy in question.
The study revealed evidence of “neural polarization” – activity in the brain that diverges between people who hold liberal vs. conservative political views, the researchers reported.
Neural polarization was observed in the dorsomedial prefrontal cortex (DMPFC), a brain region associated with the interpretation of narrative content.
Neural polarization in this region intensified during moments in the videos that included risk-related and moral-emotional language, highlighting content features most likely to drive divergent interpretations between conservatives and liberals, they noted.
For a given individual, the closer that brain activity resembled that of the “average conservative” or “average liberal,” the more likely the person was to adopt that group’s position after watching the videos.
“We know that partisans respond differently to the same information. So in that sense, it’s not surprising to find that their brains respond differently as well,” Leong told Medscape Medical News.
“What we weren’t sure about was where in the brain we would find these differences, how neural differences were related to attitude change, and what type of content would be most likely to be associated with these differences,” he said.
Importantly, said Leong, these differences do not imply that people are hardwired to disagree. Rather, individual experiences and the media that is consumed likely contribute to neural polarization.
“ – for example, by framing messages to appeal to the core values of the respective voter,” he said.
Brain stimulation to alter political perception?
Reached for comment, Shaheen Lakhan, MD, PhD, neurologist in Newton, Mass., and executive director, Global Neuroscience Initiative Foundation, said the research “puts us one step closer to identifying how our brains interpret political information.”
The study, Lakhan noted, implicates a specific brain structure, the DMPFC, which is the “lens” in which information that gets into our brain is “viewed and acted on.”
“I trust that there will be plenty more work using a similar fMRI approach to tease out scenarios outside of immigration policy, as used in this study. Down the line, brain signatures through fMRI may be able to tell an individual’s political bent, and perhaps technologies like transcranial magnetic stimulation may be able to modulate our perceptions of political content, Shaheen said.
The research was supported by a grant from the Army Research Office. Leong and Lakhan have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The differences between politically left- and right-leaning individuals may have neural underpinnings, results from a new brain imaging study suggest.
Investigators found that despite watching the same videos related to immigration policy, neural responses differed between liberals and conservatives.
“This divergence was strongest when the videos used language that highlighted threat, morality, and emotions, suggesting that certain words are more likely to drive polarized response,” lead researcher Yuan Chang Leong, PhD, a postdoctoral scholar in cognitive neuroscience at the University of California, Berkeley, told Medscape Medical News.
“The results suggest a neural basis for partisan biases in interpreting political messages, the effects these biases have on attitude change, and the type of language most likely to drive biased interpretations,” Leong added.
The study was published online Oct. 20 in Proceedings of the National Academy of Sciences.
Hardwired to disagree?
The researchers combined fMRI with semantic content analysis to investigate neural mechanisms that underlie the biased processing of political content.
They scanned 38 middle-aged men and women with liberal- or conservative-leaning immigration attitudes while the participants watched short news clips, campaign ads, and public speeches related to various immigration policies.
These policies included those that led to the United States–Mexico border wall, Deferred Action for Childhood Arrivals (DACA) protections for undocumented immigrants, the ban on refugees from majority-Muslim countries coming to the United States, and the cutting of federal funding to sanctuary cities.
After each video, participants rated on a scale of 1 to 5 how much they agreed with the general message of the video, the credibility of the information presented, and the extent to which the video made them likely to change their position and to support the policy in question.
The study revealed evidence of “neural polarization” – activity in the brain that diverges between people who hold liberal vs. conservative political views, the researchers reported.
Neural polarization was observed in the dorsomedial prefrontal cortex (DMPFC), a brain region associated with the interpretation of narrative content.
Neural polarization in this region intensified during moments in the videos that included risk-related and moral-emotional language, highlighting content features most likely to drive divergent interpretations between conservatives and liberals, they noted.
For a given individual, the closer that brain activity resembled that of the “average conservative” or “average liberal,” the more likely the person was to adopt that group’s position after watching the videos.
“We know that partisans respond differently to the same information. So in that sense, it’s not surprising to find that their brains respond differently as well,” Leong told Medscape Medical News.
“What we weren’t sure about was where in the brain we would find these differences, how neural differences were related to attitude change, and what type of content would be most likely to be associated with these differences,” he said.
Importantly, said Leong, these differences do not imply that people are hardwired to disagree. Rather, individual experiences and the media that is consumed likely contribute to neural polarization.
“ – for example, by framing messages to appeal to the core values of the respective voter,” he said.
Brain stimulation to alter political perception?
Reached for comment, Shaheen Lakhan, MD, PhD, neurologist in Newton, Mass., and executive director, Global Neuroscience Initiative Foundation, said the research “puts us one step closer to identifying how our brains interpret political information.”
The study, Lakhan noted, implicates a specific brain structure, the DMPFC, which is the “lens” in which information that gets into our brain is “viewed and acted on.”
“I trust that there will be plenty more work using a similar fMRI approach to tease out scenarios outside of immigration policy, as used in this study. Down the line, brain signatures through fMRI may be able to tell an individual’s political bent, and perhaps technologies like transcranial magnetic stimulation may be able to modulate our perceptions of political content, Shaheen said.
The research was supported by a grant from the Army Research Office. Leong and Lakhan have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The differences between politically left- and right-leaning individuals may have neural underpinnings, results from a new brain imaging study suggest.
Investigators found that despite watching the same videos related to immigration policy, neural responses differed between liberals and conservatives.
“This divergence was strongest when the videos used language that highlighted threat, morality, and emotions, suggesting that certain words are more likely to drive polarized response,” lead researcher Yuan Chang Leong, PhD, a postdoctoral scholar in cognitive neuroscience at the University of California, Berkeley, told Medscape Medical News.
“The results suggest a neural basis for partisan biases in interpreting political messages, the effects these biases have on attitude change, and the type of language most likely to drive biased interpretations,” Leong added.
The study was published online Oct. 20 in Proceedings of the National Academy of Sciences.
Hardwired to disagree?
The researchers combined fMRI with semantic content analysis to investigate neural mechanisms that underlie the biased processing of political content.
They scanned 38 middle-aged men and women with liberal- or conservative-leaning immigration attitudes while the participants watched short news clips, campaign ads, and public speeches related to various immigration policies.
These policies included those that led to the United States–Mexico border wall, Deferred Action for Childhood Arrivals (DACA) protections for undocumented immigrants, the ban on refugees from majority-Muslim countries coming to the United States, and the cutting of federal funding to sanctuary cities.
After each video, participants rated on a scale of 1 to 5 how much they agreed with the general message of the video, the credibility of the information presented, and the extent to which the video made them likely to change their position and to support the policy in question.
The study revealed evidence of “neural polarization” – activity in the brain that diverges between people who hold liberal vs. conservative political views, the researchers reported.
Neural polarization was observed in the dorsomedial prefrontal cortex (DMPFC), a brain region associated with the interpretation of narrative content.
Neural polarization in this region intensified during moments in the videos that included risk-related and moral-emotional language, highlighting content features most likely to drive divergent interpretations between conservatives and liberals, they noted.
For a given individual, the closer that brain activity resembled that of the “average conservative” or “average liberal,” the more likely the person was to adopt that group’s position after watching the videos.
“We know that partisans respond differently to the same information. So in that sense, it’s not surprising to find that their brains respond differently as well,” Leong told Medscape Medical News.
“What we weren’t sure about was where in the brain we would find these differences, how neural differences were related to attitude change, and what type of content would be most likely to be associated with these differences,” he said.
Importantly, said Leong, these differences do not imply that people are hardwired to disagree. Rather, individual experiences and the media that is consumed likely contribute to neural polarization.
“ – for example, by framing messages to appeal to the core values of the respective voter,” he said.
Brain stimulation to alter political perception?
Reached for comment, Shaheen Lakhan, MD, PhD, neurologist in Newton, Mass., and executive director, Global Neuroscience Initiative Foundation, said the research “puts us one step closer to identifying how our brains interpret political information.”
The study, Lakhan noted, implicates a specific brain structure, the DMPFC, which is the “lens” in which information that gets into our brain is “viewed and acted on.”
“I trust that there will be plenty more work using a similar fMRI approach to tease out scenarios outside of immigration policy, as used in this study. Down the line, brain signatures through fMRI may be able to tell an individual’s political bent, and perhaps technologies like transcranial magnetic stimulation may be able to modulate our perceptions of political content, Shaheen said.
The research was supported by a grant from the Army Research Office. Leong and Lakhan have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Ataluren delays disease milestones in patients with nonsense mutation DMD
(nmDMD), according to study results presented at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year. Because so few patients in the study reached one of the negative pulmonary endpoints, longer follow-up will be needed to assess more conclusively the effect of ataluren on pulmonary function, said Francesco Bibbiani, MD, vice president of clinical development at PTC Therapeutics.
DMD is a rare and fatal neuromuscular disorder that causes progressive muscle weakness. Between 10% and 15% of patients with DMD have a nonsense mutation in the DMD gene. This mutation creates a premature stop codon that prevents the translation of a full-length dystrophin protein. Ataluren is designed to promote readthrough of this premature stop codon, thus enabling the production of a full-length dystrophin protein. An oral formulation of the drug has been approved in several European and South American countries.
Comparing treatment and standard of care
Study 019 was a phase 3, multicenter, open-label, long-term safety study of ataluren that enrolled international patients with nmDMD, most of whom had participated previously in a trial of ataluren. Dr. Bibbiani and colleagues conducted a post hoc analysis of Study 019 data to determine whether patients with nmDMD who received ataluren and standard of care for as long as 240 weeks had a different time to loss of ambulation and to decline of pulmonary function, compared with patients who received standard of care alone. Patients who were eligible to participate in Study 019 were male, had nmDMD, and had completed the blinded study drug treatment in a previous PTC-sponsored study. Treatment consisted of two 10-mg/kg doses and one 20-mg/kg dose of ataluren per day.
Dr. Bibbiani and colleagues used participants in the Cooperative International Neuromuscular Research Group Duchenne Natural History Study (CINRG DNHS) as a control group. CINRG DNHS was a prospective, longitudinal study of patients with DMD who received standard of care at 20 centers worldwide from 2006 to 2016. Dr. Bibbiani and colleagues used propensity-score matching to pair participants in this study with participants in Study 019. They matched patients with respect to age at onset of first symptoms, age at initiation of corticosteroid use, duration of deflazacort use, and duration of use of other corticosteroids. These factors are established predictors of disease progression in DMD.
Patients were eligible for inclusion in the post hoc analysis if they had available data for age, loss of ambulation, and the covariates selected for matching. Of 94 Study 019 participants, 60 were eligible for propensity-score matching with participants in CINRG DNHS. Forty-five nonambulatory patients were eligible for matching in the analysis of age at the decline in pulmonary function because data for age at loss of ambulation and for the three pulmonary endpoints measured were available for them. Thus, comparable population sizes were available for each analysis.
Treatment delayed disease milestones
Kaplan–Meier analysis indicated that the median age at various disease milestones was higher among patients who received ataluren and standard of care, compared with those who received standard of care alone. The median age at loss of ambulation was 15.5 years for Study 019 participants and 13.3 years for CINRG DNHS patients. The median age at predicted forced vital capacity (FVC) of less than 60% was 18.1 years for Study 019 participants and 15.8 years for CINRG DNHS participants. The median age at predicted FVC of less than 50% was 19.1 years for Study 019 participants and 17.9 years for CINRG DNHS participants. Finally, the median age at FVC of less than 1 L was not calculable for Study 019 participants and 23.8 years for CINRG DNHS participants.
The Study 019 and CINRG DNHS study groups are sponsored by PTC Therapeutics, which developed ataluren. Dr. Bibbiani is an employee of PTC Therapeutics.
SOURCE: McDonald C, et al. CNS-ICNA 2020. Abstract PL69.
(nmDMD), according to study results presented at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year. Because so few patients in the study reached one of the negative pulmonary endpoints, longer follow-up will be needed to assess more conclusively the effect of ataluren on pulmonary function, said Francesco Bibbiani, MD, vice president of clinical development at PTC Therapeutics.
DMD is a rare and fatal neuromuscular disorder that causes progressive muscle weakness. Between 10% and 15% of patients with DMD have a nonsense mutation in the DMD gene. This mutation creates a premature stop codon that prevents the translation of a full-length dystrophin protein. Ataluren is designed to promote readthrough of this premature stop codon, thus enabling the production of a full-length dystrophin protein. An oral formulation of the drug has been approved in several European and South American countries.
Comparing treatment and standard of care
Study 019 was a phase 3, multicenter, open-label, long-term safety study of ataluren that enrolled international patients with nmDMD, most of whom had participated previously in a trial of ataluren. Dr. Bibbiani and colleagues conducted a post hoc analysis of Study 019 data to determine whether patients with nmDMD who received ataluren and standard of care for as long as 240 weeks had a different time to loss of ambulation and to decline of pulmonary function, compared with patients who received standard of care alone. Patients who were eligible to participate in Study 019 were male, had nmDMD, and had completed the blinded study drug treatment in a previous PTC-sponsored study. Treatment consisted of two 10-mg/kg doses and one 20-mg/kg dose of ataluren per day.
Dr. Bibbiani and colleagues used participants in the Cooperative International Neuromuscular Research Group Duchenne Natural History Study (CINRG DNHS) as a control group. CINRG DNHS was a prospective, longitudinal study of patients with DMD who received standard of care at 20 centers worldwide from 2006 to 2016. Dr. Bibbiani and colleagues used propensity-score matching to pair participants in this study with participants in Study 019. They matched patients with respect to age at onset of first symptoms, age at initiation of corticosteroid use, duration of deflazacort use, and duration of use of other corticosteroids. These factors are established predictors of disease progression in DMD.
Patients were eligible for inclusion in the post hoc analysis if they had available data for age, loss of ambulation, and the covariates selected for matching. Of 94 Study 019 participants, 60 were eligible for propensity-score matching with participants in CINRG DNHS. Forty-five nonambulatory patients were eligible for matching in the analysis of age at the decline in pulmonary function because data for age at loss of ambulation and for the three pulmonary endpoints measured were available for them. Thus, comparable population sizes were available for each analysis.
Treatment delayed disease milestones
Kaplan–Meier analysis indicated that the median age at various disease milestones was higher among patients who received ataluren and standard of care, compared with those who received standard of care alone. The median age at loss of ambulation was 15.5 years for Study 019 participants and 13.3 years for CINRG DNHS patients. The median age at predicted forced vital capacity (FVC) of less than 60% was 18.1 years for Study 019 participants and 15.8 years for CINRG DNHS participants. The median age at predicted FVC of less than 50% was 19.1 years for Study 019 participants and 17.9 years for CINRG DNHS participants. Finally, the median age at FVC of less than 1 L was not calculable for Study 019 participants and 23.8 years for CINRG DNHS participants.
The Study 019 and CINRG DNHS study groups are sponsored by PTC Therapeutics, which developed ataluren. Dr. Bibbiani is an employee of PTC Therapeutics.
SOURCE: McDonald C, et al. CNS-ICNA 2020. Abstract PL69.
(nmDMD), according to study results presented at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year. Because so few patients in the study reached one of the negative pulmonary endpoints, longer follow-up will be needed to assess more conclusively the effect of ataluren on pulmonary function, said Francesco Bibbiani, MD, vice president of clinical development at PTC Therapeutics.
DMD is a rare and fatal neuromuscular disorder that causes progressive muscle weakness. Between 10% and 15% of patients with DMD have a nonsense mutation in the DMD gene. This mutation creates a premature stop codon that prevents the translation of a full-length dystrophin protein. Ataluren is designed to promote readthrough of this premature stop codon, thus enabling the production of a full-length dystrophin protein. An oral formulation of the drug has been approved in several European and South American countries.
Comparing treatment and standard of care
Study 019 was a phase 3, multicenter, open-label, long-term safety study of ataluren that enrolled international patients with nmDMD, most of whom had participated previously in a trial of ataluren. Dr. Bibbiani and colleagues conducted a post hoc analysis of Study 019 data to determine whether patients with nmDMD who received ataluren and standard of care for as long as 240 weeks had a different time to loss of ambulation and to decline of pulmonary function, compared with patients who received standard of care alone. Patients who were eligible to participate in Study 019 were male, had nmDMD, and had completed the blinded study drug treatment in a previous PTC-sponsored study. Treatment consisted of two 10-mg/kg doses and one 20-mg/kg dose of ataluren per day.
Dr. Bibbiani and colleagues used participants in the Cooperative International Neuromuscular Research Group Duchenne Natural History Study (CINRG DNHS) as a control group. CINRG DNHS was a prospective, longitudinal study of patients with DMD who received standard of care at 20 centers worldwide from 2006 to 2016. Dr. Bibbiani and colleagues used propensity-score matching to pair participants in this study with participants in Study 019. They matched patients with respect to age at onset of first symptoms, age at initiation of corticosteroid use, duration of deflazacort use, and duration of use of other corticosteroids. These factors are established predictors of disease progression in DMD.
Patients were eligible for inclusion in the post hoc analysis if they had available data for age, loss of ambulation, and the covariates selected for matching. Of 94 Study 019 participants, 60 were eligible for propensity-score matching with participants in CINRG DNHS. Forty-five nonambulatory patients were eligible for matching in the analysis of age at the decline in pulmonary function because data for age at loss of ambulation and for the three pulmonary endpoints measured were available for them. Thus, comparable population sizes were available for each analysis.
Treatment delayed disease milestones
Kaplan–Meier analysis indicated that the median age at various disease milestones was higher among patients who received ataluren and standard of care, compared with those who received standard of care alone. The median age at loss of ambulation was 15.5 years for Study 019 participants and 13.3 years for CINRG DNHS patients. The median age at predicted forced vital capacity (FVC) of less than 60% was 18.1 years for Study 019 participants and 15.8 years for CINRG DNHS participants. The median age at predicted FVC of less than 50% was 19.1 years for Study 019 participants and 17.9 years for CINRG DNHS participants. Finally, the median age at FVC of less than 1 L was not calculable for Study 019 participants and 23.8 years for CINRG DNHS participants.
The Study 019 and CINRG DNHS study groups are sponsored by PTC Therapeutics, which developed ataluren. Dr. Bibbiani is an employee of PTC Therapeutics.
SOURCE: McDonald C, et al. CNS-ICNA 2020. Abstract PL69.
FROM CNS-ICNA 2020
Newer DMTs are more effective than injectable DMTs in pediatric MS
Nevertheless, all DMTs reduce children’s annualized relapse rate (ARR), according to results presented at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year.
“Our study adds weight to the argument for an imminent shift in clinical practice toward the use of newer, more efficacious DMTs in the first instance,” said Omar Abdel-Mannan, MD, of Great Ormond Street Hospital in London. MRI activity continues among patients treated with DMTs, and the number of relapses is highest in the period following diagnosis. But because the effect of treatment on brain atrophy is greatest in the initial period of disease, “this time period may represent a critical therapeutic window for the use of highly effective therapies,” said Dr. Abdel-Mannan.
An examination of medical records
MS is much less prevalent among children than among adults. Compared with adults with MS, children with MS have a higher relapse rate and slower accumulation of disability. The individual response to DMTs is variable, said Dr. Abdel-Mannan. Furthermore, current standards of care for pediatric MS vary by center and are based on adult protocols.
Dr. Abdel-Mannan and colleagues conducted a retrospective study to evaluate the real-world effectiveness of the newer oral and infusion DMTs, compared with the older injectable DMTs, in children with relapsing-remitting MS. They examined data from seven tertiary pediatric neurology centers in the United Kingdom and identified patients under age 18 years with relapsing-remitting MS who were treated with DMTs between 2012 and 2018. The investigators reviewed clinical and paraclinical data retrospectively using electronic medical records. They compared patients’ ARR, new radiological activity, and Expanded Disability Status Scale score pretreatment and on treatment.
The researchers included 103 patients in their analysis. The population’s median age was 14 years. The ratio of girls to boys was approximately 3:1. Whites and other races/ethnicities accounted for approximately equal groups of patients. About one-third of patients presented with a clinically isolated syndrome (CIS) in the form of transverse myelitis or optic neuritis. Two-thirds presented with other CIS phenotypes. Almost all children had an abnormal MRI at onset.
Most patients initiated injectable DMTs
Of the 103 patients, 89 started treatment with an injectable (e.g., glatiramer or interferon) or an older DMT. Fourteen patients began treatment with a newer DMT (e.g., dimethyl fumarate, fingolimod, natalizumab, and alemtuzumab). Three of the 89 patients on an injectable DMT switched to another injectable DMT, and two of these patients later escalated to a newer DMT. Thirty-five of the 89 patients who initiated an injectable DMT were escalated immediately to a newer DMT. One of these patients later switched to another newer DMT. Two of the 14 patients who started on a newer DMT as their first drug switched to another newer DMT.
The investigators observed a reduction in ARR for all DMTs used during the study period. Nevertheless, a significant number of patients receiving injectable DMTs continued to relapse on treatment. Almost all patients receiving newer DMTs, however, had a reduction in relapses. When Dr. Abdel-Mannan and colleagues performed Kaplan–Meier survival analysis, they found that patients receiving newer DMTs had a longer time to first relapse and a longer time to switch treatment over 2 years, compared with patients receiving injectable DMTs. In addition, patients receiving newer DMTs had a longer time to develop new radiological activity, compared with patients receiving injectables. The analysis also indicated that the proportion of patients with new radiological activity was higher than the proportion who had clinical relapses and an Expanded Disability Status Scale score increase of more than 1 point over 2 years.
In all, 55 of the children receiving injectable DMTs and 18 of the patients receiving newer DMTs had side effects. The most commonly reported side effects were flulike symptoms and injection-site reactions. Five patients discontinued or switched their DMTs because of side effects. “Reassuringly, no pediatric-specific side effects were reported,” said Dr. Abdel-Mannan. The newer DMTs had similar short-term safety, tolerability, and side-effect profiles in these children as in adult patients.
The study was conducted on behalf of the UK Childhood Inflammatory Demyelination Network. Dr. Abdel-Mannan had no relevant disclosures.
SOURCE: Abdel-Mannan O et al. CNS-ICNA 2020, Abstract PL10.
Nevertheless, all DMTs reduce children’s annualized relapse rate (ARR), according to results presented at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year.
“Our study adds weight to the argument for an imminent shift in clinical practice toward the use of newer, more efficacious DMTs in the first instance,” said Omar Abdel-Mannan, MD, of Great Ormond Street Hospital in London. MRI activity continues among patients treated with DMTs, and the number of relapses is highest in the period following diagnosis. But because the effect of treatment on brain atrophy is greatest in the initial period of disease, “this time period may represent a critical therapeutic window for the use of highly effective therapies,” said Dr. Abdel-Mannan.
An examination of medical records
MS is much less prevalent among children than among adults. Compared with adults with MS, children with MS have a higher relapse rate and slower accumulation of disability. The individual response to DMTs is variable, said Dr. Abdel-Mannan. Furthermore, current standards of care for pediatric MS vary by center and are based on adult protocols.
Dr. Abdel-Mannan and colleagues conducted a retrospective study to evaluate the real-world effectiveness of the newer oral and infusion DMTs, compared with the older injectable DMTs, in children with relapsing-remitting MS. They examined data from seven tertiary pediatric neurology centers in the United Kingdom and identified patients under age 18 years with relapsing-remitting MS who were treated with DMTs between 2012 and 2018. The investigators reviewed clinical and paraclinical data retrospectively using electronic medical records. They compared patients’ ARR, new radiological activity, and Expanded Disability Status Scale score pretreatment and on treatment.
The researchers included 103 patients in their analysis. The population’s median age was 14 years. The ratio of girls to boys was approximately 3:1. Whites and other races/ethnicities accounted for approximately equal groups of patients. About one-third of patients presented with a clinically isolated syndrome (CIS) in the form of transverse myelitis or optic neuritis. Two-thirds presented with other CIS phenotypes. Almost all children had an abnormal MRI at onset.
Most patients initiated injectable DMTs
Of the 103 patients, 89 started treatment with an injectable (e.g., glatiramer or interferon) or an older DMT. Fourteen patients began treatment with a newer DMT (e.g., dimethyl fumarate, fingolimod, natalizumab, and alemtuzumab). Three of the 89 patients on an injectable DMT switched to another injectable DMT, and two of these patients later escalated to a newer DMT. Thirty-five of the 89 patients who initiated an injectable DMT were escalated immediately to a newer DMT. One of these patients later switched to another newer DMT. Two of the 14 patients who started on a newer DMT as their first drug switched to another newer DMT.
The investigators observed a reduction in ARR for all DMTs used during the study period. Nevertheless, a significant number of patients receiving injectable DMTs continued to relapse on treatment. Almost all patients receiving newer DMTs, however, had a reduction in relapses. When Dr. Abdel-Mannan and colleagues performed Kaplan–Meier survival analysis, they found that patients receiving newer DMTs had a longer time to first relapse and a longer time to switch treatment over 2 years, compared with patients receiving injectable DMTs. In addition, patients receiving newer DMTs had a longer time to develop new radiological activity, compared with patients receiving injectables. The analysis also indicated that the proportion of patients with new radiological activity was higher than the proportion who had clinical relapses and an Expanded Disability Status Scale score increase of more than 1 point over 2 years.
In all, 55 of the children receiving injectable DMTs and 18 of the patients receiving newer DMTs had side effects. The most commonly reported side effects were flulike symptoms and injection-site reactions. Five patients discontinued or switched their DMTs because of side effects. “Reassuringly, no pediatric-specific side effects were reported,” said Dr. Abdel-Mannan. The newer DMTs had similar short-term safety, tolerability, and side-effect profiles in these children as in adult patients.
The study was conducted on behalf of the UK Childhood Inflammatory Demyelination Network. Dr. Abdel-Mannan had no relevant disclosures.
SOURCE: Abdel-Mannan O et al. CNS-ICNA 2020, Abstract PL10.
Nevertheless, all DMTs reduce children’s annualized relapse rate (ARR), according to results presented at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year.
“Our study adds weight to the argument for an imminent shift in clinical practice toward the use of newer, more efficacious DMTs in the first instance,” said Omar Abdel-Mannan, MD, of Great Ormond Street Hospital in London. MRI activity continues among patients treated with DMTs, and the number of relapses is highest in the period following diagnosis. But because the effect of treatment on brain atrophy is greatest in the initial period of disease, “this time period may represent a critical therapeutic window for the use of highly effective therapies,” said Dr. Abdel-Mannan.
An examination of medical records
MS is much less prevalent among children than among adults. Compared with adults with MS, children with MS have a higher relapse rate and slower accumulation of disability. The individual response to DMTs is variable, said Dr. Abdel-Mannan. Furthermore, current standards of care for pediatric MS vary by center and are based on adult protocols.
Dr. Abdel-Mannan and colleagues conducted a retrospective study to evaluate the real-world effectiveness of the newer oral and infusion DMTs, compared with the older injectable DMTs, in children with relapsing-remitting MS. They examined data from seven tertiary pediatric neurology centers in the United Kingdom and identified patients under age 18 years with relapsing-remitting MS who were treated with DMTs between 2012 and 2018. The investigators reviewed clinical and paraclinical data retrospectively using electronic medical records. They compared patients’ ARR, new radiological activity, and Expanded Disability Status Scale score pretreatment and on treatment.
The researchers included 103 patients in their analysis. The population’s median age was 14 years. The ratio of girls to boys was approximately 3:1. Whites and other races/ethnicities accounted for approximately equal groups of patients. About one-third of patients presented with a clinically isolated syndrome (CIS) in the form of transverse myelitis or optic neuritis. Two-thirds presented with other CIS phenotypes. Almost all children had an abnormal MRI at onset.
Most patients initiated injectable DMTs
Of the 103 patients, 89 started treatment with an injectable (e.g., glatiramer or interferon) or an older DMT. Fourteen patients began treatment with a newer DMT (e.g., dimethyl fumarate, fingolimod, natalizumab, and alemtuzumab). Three of the 89 patients on an injectable DMT switched to another injectable DMT, and two of these patients later escalated to a newer DMT. Thirty-five of the 89 patients who initiated an injectable DMT were escalated immediately to a newer DMT. One of these patients later switched to another newer DMT. Two of the 14 patients who started on a newer DMT as their first drug switched to another newer DMT.
The investigators observed a reduction in ARR for all DMTs used during the study period. Nevertheless, a significant number of patients receiving injectable DMTs continued to relapse on treatment. Almost all patients receiving newer DMTs, however, had a reduction in relapses. When Dr. Abdel-Mannan and colleagues performed Kaplan–Meier survival analysis, they found that patients receiving newer DMTs had a longer time to first relapse and a longer time to switch treatment over 2 years, compared with patients receiving injectable DMTs. In addition, patients receiving newer DMTs had a longer time to develop new radiological activity, compared with patients receiving injectables. The analysis also indicated that the proportion of patients with new radiological activity was higher than the proportion who had clinical relapses and an Expanded Disability Status Scale score increase of more than 1 point over 2 years.
In all, 55 of the children receiving injectable DMTs and 18 of the patients receiving newer DMTs had side effects. The most commonly reported side effects were flulike symptoms and injection-site reactions. Five patients discontinued or switched their DMTs because of side effects. “Reassuringly, no pediatric-specific side effects were reported,” said Dr. Abdel-Mannan. The newer DMTs had similar short-term safety, tolerability, and side-effect profiles in these children as in adult patients.
The study was conducted on behalf of the UK Childhood Inflammatory Demyelination Network. Dr. Abdel-Mannan had no relevant disclosures.
SOURCE: Abdel-Mannan O et al. CNS-ICNA 2020, Abstract PL10.
FROM CNS-ICNA 2020
Study supports halting antiseizure medications after neonatal seizures
Maintaining antiseizure medication in infants who have had acute symptomatic neonatal seizures has been standard practice, but a prospective, observational, comparative effectiveness study calls that practice into question, providing evidence that discontinuing therapy at discharge poses no harm to children and has no effect on the development of epilepsies.
“,” said Hannah C. Glass, MDCM, MAS, of the University of California, San Francisco, Benioff Children’s Hospital, co-principal investigator, who presented results of the study at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year. Renee Shellhaas, MD, MS, clinical associate professor of pediatrics at C.S. Mott Children’s Hospital, University of Michigan, was the other co-principal investigator.
“Although other, smaller studies have suggested it is safe to discontinue antiseizure medication after resolution of acute symptomatic seizures, the practice of early discontinuation has been very variable and depends largely on individual provider preference,” Dr. Glass said in an interview. “In our study, two-thirds of newborns with acute symptomatic seizures were maintained on antiseizure medication at the time of hospital discharge. Thus, a change to early medication discontinuation represents a major shift.”
The study evaluated 270 infants at nine centers enrolled in the Neonatal Seizure Registry and born from July 2015 through March 2018. Inclusion criteria were acute symptomatic seizures that occurred at up to 44 weeks postmenstrual age. In this cohort, 36% of patients had antiseizure medication discontinued after a median of 6 days; the remainder stayed on antiseizure medication after discharge at a median of 4 months.
The patients were followed for 2 years. The primary outcome was functional development measured by the Warner Initial Development Evaluation of Adaptive and Functional Skills (WIDEA-FS) assessment. The secondary outcome was epilepsy defined by International League Against Epilepsy (ILAE) criteria. Follow-up consisted of phone calls and chart reviews at 12, 18, and 24 months.
“The primary outcome, functional development, was not significantly different between those children who were maintained on antiseizure medication as compared with those who were discontinued,” Dr. Glass said.
After propensity adjustment, the discontinued ASM group had an estimated WIDEA-FS score 4 points higher on average, she said. “The confidence intervals met our a priori noninferiority limit, indicating no harm to neurodevelopment for discontinuing antiseizure medication before discharge home from the neonatal seizure admission,” Dr. Glass noted.
The study also found that 13% of all participants developed epilepsy at a median of 8 months. “There was no significant difference in the frequency or timing of epilepsy between the two groups,” she said.
“We conclude there is no clear rationale for antiseizure medication maintenance,” Dr. Glass said. “There is no benefit to neurodevelopment, it prolongs the exposure to potentially harmful antiseizure medications, it does not significantly delay the onset of epilepsy, and the earliest-onset epilepsies occur in spite of antiseizure medication.”
The Patient-Centered Outcomes Research Institute (PCORI) and Pediatric Epilepsy Research Foundation funded the study. Dr. Glass has no other financial relationships to disclose.
SOURCE: Glass HC et al. CNS-ICNA 2020. Presentation PL58.
Maintaining antiseizure medication in infants who have had acute symptomatic neonatal seizures has been standard practice, but a prospective, observational, comparative effectiveness study calls that practice into question, providing evidence that discontinuing therapy at discharge poses no harm to children and has no effect on the development of epilepsies.
“,” said Hannah C. Glass, MDCM, MAS, of the University of California, San Francisco, Benioff Children’s Hospital, co-principal investigator, who presented results of the study at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year. Renee Shellhaas, MD, MS, clinical associate professor of pediatrics at C.S. Mott Children’s Hospital, University of Michigan, was the other co-principal investigator.
“Although other, smaller studies have suggested it is safe to discontinue antiseizure medication after resolution of acute symptomatic seizures, the practice of early discontinuation has been very variable and depends largely on individual provider preference,” Dr. Glass said in an interview. “In our study, two-thirds of newborns with acute symptomatic seizures were maintained on antiseizure medication at the time of hospital discharge. Thus, a change to early medication discontinuation represents a major shift.”
The study evaluated 270 infants at nine centers enrolled in the Neonatal Seizure Registry and born from July 2015 through March 2018. Inclusion criteria were acute symptomatic seizures that occurred at up to 44 weeks postmenstrual age. In this cohort, 36% of patients had antiseizure medication discontinued after a median of 6 days; the remainder stayed on antiseizure medication after discharge at a median of 4 months.
The patients were followed for 2 years. The primary outcome was functional development measured by the Warner Initial Development Evaluation of Adaptive and Functional Skills (WIDEA-FS) assessment. The secondary outcome was epilepsy defined by International League Against Epilepsy (ILAE) criteria. Follow-up consisted of phone calls and chart reviews at 12, 18, and 24 months.
“The primary outcome, functional development, was not significantly different between those children who were maintained on antiseizure medication as compared with those who were discontinued,” Dr. Glass said.
After propensity adjustment, the discontinued ASM group had an estimated WIDEA-FS score 4 points higher on average, she said. “The confidence intervals met our a priori noninferiority limit, indicating no harm to neurodevelopment for discontinuing antiseizure medication before discharge home from the neonatal seizure admission,” Dr. Glass noted.
The study also found that 13% of all participants developed epilepsy at a median of 8 months. “There was no significant difference in the frequency or timing of epilepsy between the two groups,” she said.
“We conclude there is no clear rationale for antiseizure medication maintenance,” Dr. Glass said. “There is no benefit to neurodevelopment, it prolongs the exposure to potentially harmful antiseizure medications, it does not significantly delay the onset of epilepsy, and the earliest-onset epilepsies occur in spite of antiseizure medication.”
The Patient-Centered Outcomes Research Institute (PCORI) and Pediatric Epilepsy Research Foundation funded the study. Dr. Glass has no other financial relationships to disclose.
SOURCE: Glass HC et al. CNS-ICNA 2020. Presentation PL58.
Maintaining antiseizure medication in infants who have had acute symptomatic neonatal seizures has been standard practice, but a prospective, observational, comparative effectiveness study calls that practice into question, providing evidence that discontinuing therapy at discharge poses no harm to children and has no effect on the development of epilepsies.
“,” said Hannah C. Glass, MDCM, MAS, of the University of California, San Francisco, Benioff Children’s Hospital, co-principal investigator, who presented results of the study at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year. Renee Shellhaas, MD, MS, clinical associate professor of pediatrics at C.S. Mott Children’s Hospital, University of Michigan, was the other co-principal investigator.
“Although other, smaller studies have suggested it is safe to discontinue antiseizure medication after resolution of acute symptomatic seizures, the practice of early discontinuation has been very variable and depends largely on individual provider preference,” Dr. Glass said in an interview. “In our study, two-thirds of newborns with acute symptomatic seizures were maintained on antiseizure medication at the time of hospital discharge. Thus, a change to early medication discontinuation represents a major shift.”
The study evaluated 270 infants at nine centers enrolled in the Neonatal Seizure Registry and born from July 2015 through March 2018. Inclusion criteria were acute symptomatic seizures that occurred at up to 44 weeks postmenstrual age. In this cohort, 36% of patients had antiseizure medication discontinued after a median of 6 days; the remainder stayed on antiseizure medication after discharge at a median of 4 months.
The patients were followed for 2 years. The primary outcome was functional development measured by the Warner Initial Development Evaluation of Adaptive and Functional Skills (WIDEA-FS) assessment. The secondary outcome was epilepsy defined by International League Against Epilepsy (ILAE) criteria. Follow-up consisted of phone calls and chart reviews at 12, 18, and 24 months.
“The primary outcome, functional development, was not significantly different between those children who were maintained on antiseizure medication as compared with those who were discontinued,” Dr. Glass said.
After propensity adjustment, the discontinued ASM group had an estimated WIDEA-FS score 4 points higher on average, she said. “The confidence intervals met our a priori noninferiority limit, indicating no harm to neurodevelopment for discontinuing antiseizure medication before discharge home from the neonatal seizure admission,” Dr. Glass noted.
The study also found that 13% of all participants developed epilepsy at a median of 8 months. “There was no significant difference in the frequency or timing of epilepsy between the two groups,” she said.
“We conclude there is no clear rationale for antiseizure medication maintenance,” Dr. Glass said. “There is no benefit to neurodevelopment, it prolongs the exposure to potentially harmful antiseizure medications, it does not significantly delay the onset of epilepsy, and the earliest-onset epilepsies occur in spite of antiseizure medication.”
The Patient-Centered Outcomes Research Institute (PCORI) and Pediatric Epilepsy Research Foundation funded the study. Dr. Glass has no other financial relationships to disclose.
SOURCE: Glass HC et al. CNS-ICNA 2020. Presentation PL58.
FROM CNS-ICNA 2020
Evaluating the impact of new pediatric brain tumor classifications
and that will have far-reaching implications for how clinicians diagnose and manage these rare and often debilitating malignancies, a leading European researcher reported at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year.
“These pediatric neuronal/glioneuronal tumors are quite heterogeneous in terms of the number of different tumors and subclasses of tumors going into these groups, but they have some molecular features in common,” said David T.W. Jones, PhD, of Hopp Children’s Cancer Center in Heidelberg, Germany. “Together they represent quite a sizable portion of all childhood brain tumors, so it’s important to recognize and understand them.”
Dr. Jones noted that updated WHO classifications would add six new descriptions to the category of mixed glioneuronal tumors and one to the list of neuronal tumors. A working group of the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy, known as cIMPACT-NOW, has recommended the expanded classifications for central nervous system tumors.
“The molecular understandings of pediatric neuro-glial tumors are critical in their management,” Roger Packer, MD, senior vice president of the Center for Neuroscience and Behavioral Health at Children’s National in Washington, said in an interview, especially as treatments targeting specific molecular structures emerge. “For those with tumors not amenable to safe, total resections, there’s little evidence that radiation or chemotherapy are effective, and molecular-targeted therapy, guided by the molecular genetic composition, increases the safe use of these new agents.”
Dr. Jones noted that “as a minimum” molecular diagnostics of pediatric low-grade glioneuronal and neuronal tumors should include a BRAF gene mutation and fusion status, as well as FGFR1 mutation plus fusion or rearrangement status.
“Ideally,” he added, “it should also have a broader copy number profile, whether that’s based on sequencing or SNP arrays or DNA methylation rate, a global DNA methylation profile to get those global molecular patterns, and also wider gene and RNA sequence to pick up some of those rarer alterations that may not be covered by targeted BRAF and FGFR1 mutations.”
The updated tumor classification will evolve to include novel tumor classes, as well as links or overlaps between the tumor classes and their characteristic underlying kinetic alterations, he noted. “Some of these profiling measures will actually be required to generate a fully WHO-compatible pathological diagnosis,” Dr. Jones said.
“This group of tumors are now just better molecularly characterized than it was 5 years ago, so in the last few years we’ve really made tremendous progress in understanding what alterations are driving some of these tumors,” he said. “That knowledge is now providing a basis for improved diagnosis and also for starting to plan more targeted treatment strategies.”
But, he added, there’s still a lot to learn about how these oncogenic mechanisms drive tumor pathogenesis. “What is the clinical costs when we really start getting down into defining these distinct molecular groups?” he said. “What are their different responses to treatment depending on different levels, where the MEKi [mitogen-activated protein kinase inhibitor] pathway might be activated and, for example, response to treatment of different subclasses of one tumor?”
Large, collaborative clinical studies will be needed to get those answers, he said.
“There are certainly some therapeutic opportunities arising in this group of tumors now, but in order to really translate those into a clinical benefit, we’re really going to need some careful planning of international studies because of the relative rarity of some of these groups,” he said.
Dr. Jones has no relevant financial relationships to disclose.
and that will have far-reaching implications for how clinicians diagnose and manage these rare and often debilitating malignancies, a leading European researcher reported at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year.
“These pediatric neuronal/glioneuronal tumors are quite heterogeneous in terms of the number of different tumors and subclasses of tumors going into these groups, but they have some molecular features in common,” said David T.W. Jones, PhD, of Hopp Children’s Cancer Center in Heidelberg, Germany. “Together they represent quite a sizable portion of all childhood brain tumors, so it’s important to recognize and understand them.”
Dr. Jones noted that updated WHO classifications would add six new descriptions to the category of mixed glioneuronal tumors and one to the list of neuronal tumors. A working group of the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy, known as cIMPACT-NOW, has recommended the expanded classifications for central nervous system tumors.
“The molecular understandings of pediatric neuro-glial tumors are critical in their management,” Roger Packer, MD, senior vice president of the Center for Neuroscience and Behavioral Health at Children’s National in Washington, said in an interview, especially as treatments targeting specific molecular structures emerge. “For those with tumors not amenable to safe, total resections, there’s little evidence that radiation or chemotherapy are effective, and molecular-targeted therapy, guided by the molecular genetic composition, increases the safe use of these new agents.”
Dr. Jones noted that “as a minimum” molecular diagnostics of pediatric low-grade glioneuronal and neuronal tumors should include a BRAF gene mutation and fusion status, as well as FGFR1 mutation plus fusion or rearrangement status.
“Ideally,” he added, “it should also have a broader copy number profile, whether that’s based on sequencing or SNP arrays or DNA methylation rate, a global DNA methylation profile to get those global molecular patterns, and also wider gene and RNA sequence to pick up some of those rarer alterations that may not be covered by targeted BRAF and FGFR1 mutations.”
The updated tumor classification will evolve to include novel tumor classes, as well as links or overlaps between the tumor classes and their characteristic underlying kinetic alterations, he noted. “Some of these profiling measures will actually be required to generate a fully WHO-compatible pathological diagnosis,” Dr. Jones said.
“This group of tumors are now just better molecularly characterized than it was 5 years ago, so in the last few years we’ve really made tremendous progress in understanding what alterations are driving some of these tumors,” he said. “That knowledge is now providing a basis for improved diagnosis and also for starting to plan more targeted treatment strategies.”
But, he added, there’s still a lot to learn about how these oncogenic mechanisms drive tumor pathogenesis. “What is the clinical costs when we really start getting down into defining these distinct molecular groups?” he said. “What are their different responses to treatment depending on different levels, where the MEKi [mitogen-activated protein kinase inhibitor] pathway might be activated and, for example, response to treatment of different subclasses of one tumor?”
Large, collaborative clinical studies will be needed to get those answers, he said.
“There are certainly some therapeutic opportunities arising in this group of tumors now, but in order to really translate those into a clinical benefit, we’re really going to need some careful planning of international studies because of the relative rarity of some of these groups,” he said.
Dr. Jones has no relevant financial relationships to disclose.
and that will have far-reaching implications for how clinicians diagnose and manage these rare and often debilitating malignancies, a leading European researcher reported at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year.
“These pediatric neuronal/glioneuronal tumors are quite heterogeneous in terms of the number of different tumors and subclasses of tumors going into these groups, but they have some molecular features in common,” said David T.W. Jones, PhD, of Hopp Children’s Cancer Center in Heidelberg, Germany. “Together they represent quite a sizable portion of all childhood brain tumors, so it’s important to recognize and understand them.”
Dr. Jones noted that updated WHO classifications would add six new descriptions to the category of mixed glioneuronal tumors and one to the list of neuronal tumors. A working group of the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy, known as cIMPACT-NOW, has recommended the expanded classifications for central nervous system tumors.
“The molecular understandings of pediatric neuro-glial tumors are critical in their management,” Roger Packer, MD, senior vice president of the Center for Neuroscience and Behavioral Health at Children’s National in Washington, said in an interview, especially as treatments targeting specific molecular structures emerge. “For those with tumors not amenable to safe, total resections, there’s little evidence that radiation or chemotherapy are effective, and molecular-targeted therapy, guided by the molecular genetic composition, increases the safe use of these new agents.”
Dr. Jones noted that “as a minimum” molecular diagnostics of pediatric low-grade glioneuronal and neuronal tumors should include a BRAF gene mutation and fusion status, as well as FGFR1 mutation plus fusion or rearrangement status.
“Ideally,” he added, “it should also have a broader copy number profile, whether that’s based on sequencing or SNP arrays or DNA methylation rate, a global DNA methylation profile to get those global molecular patterns, and also wider gene and RNA sequence to pick up some of those rarer alterations that may not be covered by targeted BRAF and FGFR1 mutations.”
The updated tumor classification will evolve to include novel tumor classes, as well as links or overlaps between the tumor classes and their characteristic underlying kinetic alterations, he noted. “Some of these profiling measures will actually be required to generate a fully WHO-compatible pathological diagnosis,” Dr. Jones said.
“This group of tumors are now just better molecularly characterized than it was 5 years ago, so in the last few years we’ve really made tremendous progress in understanding what alterations are driving some of these tumors,” he said. “That knowledge is now providing a basis for improved diagnosis and also for starting to plan more targeted treatment strategies.”
But, he added, there’s still a lot to learn about how these oncogenic mechanisms drive tumor pathogenesis. “What is the clinical costs when we really start getting down into defining these distinct molecular groups?” he said. “What are their different responses to treatment depending on different levels, where the MEKi [mitogen-activated protein kinase inhibitor] pathway might be activated and, for example, response to treatment of different subclasses of one tumor?”
Large, collaborative clinical studies will be needed to get those answers, he said.
“There are certainly some therapeutic opportunities arising in this group of tumors now, but in order to really translate those into a clinical benefit, we’re really going to need some careful planning of international studies because of the relative rarity of some of these groups,” he said.
Dr. Jones has no relevant financial relationships to disclose.
FROM CNS-ICNA 2020