User login
USPSTF round-up
In 2019, the US Preventive Services Task Force published 19 recommendation statements on 11 topics. Two of the topics are new; 9 are topics the Task Force had previously reviewed and has updated (TABLE 1). Three of these topics have been covered in Practice Alert podcasts (mdedge.com/familymedicine) and will not be discussed here: risk assessment, genetic counseling, and genetic testing for breast cancer susceptibility gene mutations (October 2019); medications to reduce the risk of breast cancer (December 2019); and preexposure prophylaxis to prevent HIV infections (January 2020).

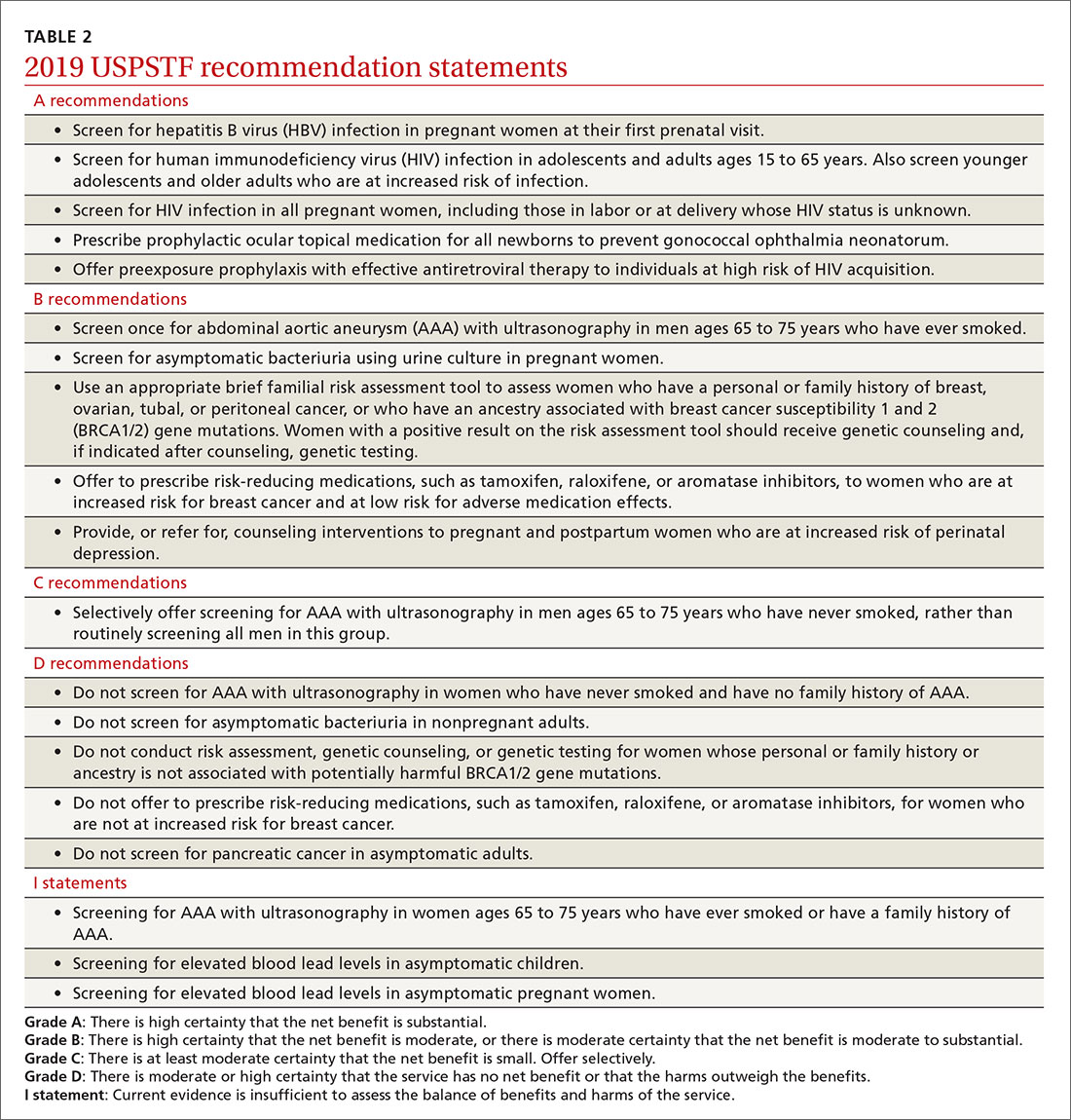
Of the 19 recommendation statements made in 2019 (TABLE 2), 5 were rated “A” and 5 were “B,” meaning the evidence shows that benefits outweigh harms and these interventions should be offered in primary care practice. There were 5 “D” recommendations for interventions that should not be offered because they are either ineffective or harms exceed benefits. There were 3 “I” statements on interventions having insufficient evidence on benefits or harms to warrant a recommendation. Only 1 recommendation was rated “C” (selectively offer based on individual factors); this assessment is the hardest one to interpret and implement. Keep in mind that all “A” and “B” recommendations must be covered by commercial health plans with no out-of-pocket cost to the patient (ie, no co-pay or deductible).
New recommendation on preventing perinatal depression
One of 2 new topics reviewed in 2019 was the prevention of perinatal depression. (As noted, the other on preexposure prophylaxis to prevent HIV infection has already been covered in a Practice Alert podcast.) The Task Force found that the prevalence of depression is estimated at 8.9% among pregnant women and 37% at any point in the first year postpartum.1
Depression during pregnancy and the postpartum period is associated with adverse effects on the mother and infant, including higher rates of suicide and suicidal ideation and thoughts of harming the infant.1 Women with perinatal depression are also more likely to exhibit significantly lower levels of positive maternal behaviors, such as praising and playing with their child,2 and higher rates of negative maternal behaviors.2 Perinatal depression is also associated with increased rates of preterm birth and low birth weight.3
Mothers with postpartum depression have higher rates of early termination of breast feeding and lower adherence for recommended child preventive services including vaccination.1 Children of mothers with perinatal depression develop more behavior problems, have lower cognitive functioning, and have an increased risk of psychiatric disorders than do children of mothers without this condition.4,5
A number of risk factors are associated with perinatal depression, but no screening tool was found to have enough predictive value to be recommended. In deciding who should receive an offer or referral for counseling, the Task Force recommends as a practical approach providing “counseling interventions to women with 1 or more of the following: a history of depression, current depressive symptoms (that do not reach a diagnostic threshold), certain socioeconomic risk factors such as low income or adolescent or single parenthood, recent intimate partner violence, or mental health-related factors such as elevated anxiety symptoms or a history of significant negative life events.”1
There is no conclusive evidence to guide timing of counseling interventions, but most studies reviewed started them in the second trimester. These studies included cognitive behavioral therapy and interpersonal therapy and involved counseling sessions that ranged from 4 to 20 sessions and lasted for 4 to 70 weeks. They involved group and individual sessions, mostly in-person visits, and were provided by a variety of health professionals.6
Continue to: The studies reviewed showed...
The studies reviewed showed that counseling interventions reduced the likelihood of developing depression symptoms by 39%, with a number needed to treat of 13.5.6 Studies that looked at pregnancy and maternal and infant clinical outcomes were mixed but usually found little to no difference with counseling.6 Even so, the Task Force felt that a reduction in depression itself was enough to warrant a “B” recommendation.
Screening for abdominal aortic aneurisms
Ultrasound is underused in screening for abdominal aortic aneurisms (AAA) and preventing death from their rupture. (See “Whom should you screen for abdominal aortic aneurysm?”) The prevalence of AAA is the United States is unknown; in other western countries it varies from 1.2% to 3.3% in men and is declining due to decreased rates of smoking, the primary risk factor.
The risk of AAA rupture is related to the size of the aneurism, and surgical repair (either endovascular or open repair) is usually reserved for lesions > 5.5 cm in diameter or for smaller ones that are rapidly increasing in size. The standard of care for most aneurysms < 5.5 cm is to periodically monitor growth using ultrasound.
The 2019 recommendations on AAA screening are essentially the same as those made in 2004; evaluation of new evidence supported the previous recommendations. The Task Force recommends one-time screening for men ages 65 to 75 years who have ever smoked (B recommendation). Selective screening is recommended for men in this age group who have never smoked, based mainly on personal factors such as a family history of AAA, the presence of other arterial aneurisms, and the number of risk factors for cardiovascular disease (C recommendation).
The Task Force recommends against screening women ages 65 to 75 years with no history of smoking or family history of AAA, while the evidence was felt to be insufficient to make a recommendation for women in this age range who have either risk factor. This is problematic for family physicians since women with these risk factors are at increased risk of AAA compared with women without risk factors.8 And aneurisms in women appear to rupture more frequently at smaller sizes, although at a later age than in men.8 Operative mortality is also higher in women than in men8 and there is no direct evidence that screening improves outcomes for women.
Continue to: Screening for asymptomatic bacteriuria
Screening for asymptomatic bacteriuria
The Task Force re-examined and reconfirmed its previous recommendations on screening for asymptomatic bacteriuria in adults. It recommends in favor of it for pregnant women, using a urine culture to screen, and against it for all other adults. There is good evidence that treating screen-detected asymptomatic bacteriuria in pregnant women reduces the incidence of pyelonephritis in pregnancy.
The Task Force made this a “B” recommendation based on a lower prevalence of pyelonephritis found in more recent studies, making the overall magnitude of benefits moderate. There is also good evidence that treating asymptomatic bacteriuria in nonpregnant adults offers no benefits.9 The Task Force has re-examined this topic 5 times since 1996 with essentially the same results.
Screening for elevated lead levels in children and pregnant women
In 2019 the Task Force changed its 2006 recommendation on screening for elevated lead levels. The earlier recommendation advised against screening both children ages 1 to 5 years and pregnant women at average risk for elevated blood lead levels. In 2006 the Task Force also felt that evidence was insufficient to make a recommendation regarding children ages 1 to 5 years at elevated risk.
The Task Force now believes the evidence is insufficient to make a recommendation for all children ages 1 to 5 years and for pregnant women, thus moving from a “D” to an “I” recommendation for children and pregnant women with average risk. Even though there is little evidence to support screening for elevated lead levels in children ages 1 to 5 years and in pregnant women, the Task Force apparently did not feel comfortable recommending against testing, given that the cutoff for elevated blood lead levels has been lowered from 10 to 5 mcg/dL and that other sources of lead may now be more prevalent than in 2006.10
Remember that the Medicaid Early and Periodic Screening, Diagnostic, and Treatment program requires that all children receive a blood lead test twice, at ages 12 and 24 months, and that previously unscreened children ages 36 to 72 months must be tested once.
Continue to: Additional updates with no recommendation changes
Additional updates with no recommendation changes
Four other topics were re-examined by the Task Force in 2019, resulting in no significant changes to recommendations (TABLE 2):
- Screen for hepatitis B infection in pregnant women at the first prenatal visit (A recommendation; updated from 2009).
- Screen for HIV infection in adolescents and adults ages 15 to 65 years, and in those younger and older who are at high risk, and during pregnancy (A recommendation; updated from 2013).
- Provide topical medication for all newborns to prevent gonococcal ophthalmia neonatorum (A recommendation; first recommendation in 1996, updated in 2005 and 2011).
- Avoid screening for pancreatic cancer in asymptomatic adults (D recommendation; updated from 2004).
Affirmation of USPSTF’s value
In only 1 out of 9 reassessments of past topics did the Task Force modify its previous recommendations in any significant way. This demonstrates that recommendations will usually stand the test of time if they are made using robust, evidence-based methods (that consider both benefits and harms) and they are not made when evidence is insufficient. That only 2 new topics could be addressed in 2019 may reflect a need for more resources for the Task Force.
1. USPSTF. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendation statement. 2019;321:580-587.
2. Lovejoy MC, Graczyk PA, O’Hare E, et al. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561-592.
3. Szegda K, Markenson G, Bertone-Johnson ER, et al. Depression during pregnancy: a risk factor for adverse neonatal outcomes? A critical review of the literature. J Matern Fetal Neonatal Med. 2014;27:960-967.
4. Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12:12-20.
5. Santos IS, Matijasevich A, Barros AJ, et al. Antenatal and postnatal maternal mood symptoms and psychiatric disorders in pre-school children from the 2004 Pelotas Birth Cohort. J Affect Disord. 2014;164:112-117.
6. O’Connor E, Senger CA, Henniger ML, et al. Interventions to prevent perinatal depression. Evidence report and systematic review for the US preventive services task force. JAMA. 2019;321:588-601.
7. USPSTF. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. 2019;322:2211-2218.
8. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238.
9. USPSTF. Owens DK, Davidson KW, Krist AH, et al. Screening for asymptomatic bacteriuria in adults: US Preventive Services Task Force recommendation statement. 2019;322:1188-1194.
10. USPSTF. Screening for elevated blood lead levels in children and pregnant women: US Preventive Services Task Force recommendation statement. 2019;321:1502-1509.
In 2019, the US Preventive Services Task Force published 19 recommendation statements on 11 topics. Two of the topics are new; 9 are topics the Task Force had previously reviewed and has updated (TABLE 1). Three of these topics have been covered in Practice Alert podcasts (mdedge.com/familymedicine) and will not be discussed here: risk assessment, genetic counseling, and genetic testing for breast cancer susceptibility gene mutations (October 2019); medications to reduce the risk of breast cancer (December 2019); and preexposure prophylaxis to prevent HIV infections (January 2020).

Of the 19 recommendation statements made in 2019 (TABLE 2), 5 were rated “A” and 5 were “B,” meaning the evidence shows that benefits outweigh harms and these interventions should be offered in primary care practice. There were 5 “D” recommendations for interventions that should not be offered because they are either ineffective or harms exceed benefits. There were 3 “I” statements on interventions having insufficient evidence on benefits or harms to warrant a recommendation. Only 1 recommendation was rated “C” (selectively offer based on individual factors); this assessment is the hardest one to interpret and implement. Keep in mind that all “A” and “B” recommendations must be covered by commercial health plans with no out-of-pocket cost to the patient (ie, no co-pay or deductible).

New recommendation on preventing perinatal depression
One of 2 new topics reviewed in 2019 was the prevention of perinatal depression. (As noted, the other on preexposure prophylaxis to prevent HIV infection has already been covered in a Practice Alert podcast.) The Task Force found that the prevalence of depression is estimated at 8.9% among pregnant women and 37% at any point in the first year postpartum.1
Depression during pregnancy and the postpartum period is associated with adverse effects on the mother and infant, including higher rates of suicide and suicidal ideation and thoughts of harming the infant.1 Women with perinatal depression are also more likely to exhibit significantly lower levels of positive maternal behaviors, such as praising and playing with their child,2 and higher rates of negative maternal behaviors.2 Perinatal depression is also associated with increased rates of preterm birth and low birth weight.3
Mothers with postpartum depression have higher rates of early termination of breast feeding and lower adherence for recommended child preventive services including vaccination.1 Children of mothers with perinatal depression develop more behavior problems, have lower cognitive functioning, and have an increased risk of psychiatric disorders than do children of mothers without this condition.4,5
A number of risk factors are associated with perinatal depression, but no screening tool was found to have enough predictive value to be recommended. In deciding who should receive an offer or referral for counseling, the Task Force recommends as a practical approach providing “counseling interventions to women with 1 or more of the following: a history of depression, current depressive symptoms (that do not reach a diagnostic threshold), certain socioeconomic risk factors such as low income or adolescent or single parenthood, recent intimate partner violence, or mental health-related factors such as elevated anxiety symptoms or a history of significant negative life events.”1
There is no conclusive evidence to guide timing of counseling interventions, but most studies reviewed started them in the second trimester. These studies included cognitive behavioral therapy and interpersonal therapy and involved counseling sessions that ranged from 4 to 20 sessions and lasted for 4 to 70 weeks. They involved group and individual sessions, mostly in-person visits, and were provided by a variety of health professionals.6
Continue to: The studies reviewed showed...
The studies reviewed showed that counseling interventions reduced the likelihood of developing depression symptoms by 39%, with a number needed to treat of 13.5.6 Studies that looked at pregnancy and maternal and infant clinical outcomes were mixed but usually found little to no difference with counseling.6 Even so, the Task Force felt that a reduction in depression itself was enough to warrant a “B” recommendation.
Screening for abdominal aortic aneurisms
Ultrasound is underused in screening for abdominal aortic aneurisms (AAA) and preventing death from their rupture. (See “Whom should you screen for abdominal aortic aneurysm?”) The prevalence of AAA is the United States is unknown; in other western countries it varies from 1.2% to 3.3% in men and is declining due to decreased rates of smoking, the primary risk factor.
The risk of AAA rupture is related to the size of the aneurism, and surgical repair (either endovascular or open repair) is usually reserved for lesions > 5.5 cm in diameter or for smaller ones that are rapidly increasing in size. The standard of care for most aneurysms < 5.5 cm is to periodically monitor growth using ultrasound.
The 2019 recommendations on AAA screening are essentially the same as those made in 2004; evaluation of new evidence supported the previous recommendations. The Task Force recommends one-time screening for men ages 65 to 75 years who have ever smoked (B recommendation). Selective screening is recommended for men in this age group who have never smoked, based mainly on personal factors such as a family history of AAA, the presence of other arterial aneurisms, and the number of risk factors for cardiovascular disease (C recommendation).
The Task Force recommends against screening women ages 65 to 75 years with no history of smoking or family history of AAA, while the evidence was felt to be insufficient to make a recommendation for women in this age range who have either risk factor. This is problematic for family physicians since women with these risk factors are at increased risk of AAA compared with women without risk factors.8 And aneurisms in women appear to rupture more frequently at smaller sizes, although at a later age than in men.8 Operative mortality is also higher in women than in men8 and there is no direct evidence that screening improves outcomes for women.
Continue to: Screening for asymptomatic bacteriuria
Screening for asymptomatic bacteriuria
The Task Force re-examined and reconfirmed its previous recommendations on screening for asymptomatic bacteriuria in adults. It recommends in favor of it for pregnant women, using a urine culture to screen, and against it for all other adults. There is good evidence that treating screen-detected asymptomatic bacteriuria in pregnant women reduces the incidence of pyelonephritis in pregnancy.
The Task Force made this a “B” recommendation based on a lower prevalence of pyelonephritis found in more recent studies, making the overall magnitude of benefits moderate. There is also good evidence that treating asymptomatic bacteriuria in nonpregnant adults offers no benefits.9 The Task Force has re-examined this topic 5 times since 1996 with essentially the same results.
Screening for elevated lead levels in children and pregnant women
In 2019 the Task Force changed its 2006 recommendation on screening for elevated lead levels. The earlier recommendation advised against screening both children ages 1 to 5 years and pregnant women at average risk for elevated blood lead levels. In 2006 the Task Force also felt that evidence was insufficient to make a recommendation regarding children ages 1 to 5 years at elevated risk.
The Task Force now believes the evidence is insufficient to make a recommendation for all children ages 1 to 5 years and for pregnant women, thus moving from a “D” to an “I” recommendation for children and pregnant women with average risk. Even though there is little evidence to support screening for elevated lead levels in children ages 1 to 5 years and in pregnant women, the Task Force apparently did not feel comfortable recommending against testing, given that the cutoff for elevated blood lead levels has been lowered from 10 to 5 mcg/dL and that other sources of lead may now be more prevalent than in 2006.10
Remember that the Medicaid Early and Periodic Screening, Diagnostic, and Treatment program requires that all children receive a blood lead test twice, at ages 12 and 24 months, and that previously unscreened children ages 36 to 72 months must be tested once.
Continue to: Additional updates with no recommendation changes
Additional updates with no recommendation changes
Four other topics were re-examined by the Task Force in 2019, resulting in no significant changes to recommendations (TABLE 2):
- Screen for hepatitis B infection in pregnant women at the first prenatal visit (A recommendation; updated from 2009).
- Screen for HIV infection in adolescents and adults ages 15 to 65 years, and in those younger and older who are at high risk, and during pregnancy (A recommendation; updated from 2013).
- Provide topical medication for all newborns to prevent gonococcal ophthalmia neonatorum (A recommendation; first recommendation in 1996, updated in 2005 and 2011).
- Avoid screening for pancreatic cancer in asymptomatic adults (D recommendation; updated from 2004).
Affirmation of USPSTF’s value
In only 1 out of 9 reassessments of past topics did the Task Force modify its previous recommendations in any significant way. This demonstrates that recommendations will usually stand the test of time if they are made using robust, evidence-based methods (that consider both benefits and harms) and they are not made when evidence is insufficient. That only 2 new topics could be addressed in 2019 may reflect a need for more resources for the Task Force.
In 2019, the US Preventive Services Task Force published 19 recommendation statements on 11 topics. Two of the topics are new; 9 are topics the Task Force had previously reviewed and has updated (TABLE 1). Three of these topics have been covered in Practice Alert podcasts (mdedge.com/familymedicine) and will not be discussed here: risk assessment, genetic counseling, and genetic testing for breast cancer susceptibility gene mutations (October 2019); medications to reduce the risk of breast cancer (December 2019); and preexposure prophylaxis to prevent HIV infections (January 2020).

Of the 19 recommendation statements made in 2019 (TABLE 2), 5 were rated “A” and 5 were “B,” meaning the evidence shows that benefits outweigh harms and these interventions should be offered in primary care practice. There were 5 “D” recommendations for interventions that should not be offered because they are either ineffective or harms exceed benefits. There were 3 “I” statements on interventions having insufficient evidence on benefits or harms to warrant a recommendation. Only 1 recommendation was rated “C” (selectively offer based on individual factors); this assessment is the hardest one to interpret and implement. Keep in mind that all “A” and “B” recommendations must be covered by commercial health plans with no out-of-pocket cost to the patient (ie, no co-pay or deductible).

New recommendation on preventing perinatal depression
One of 2 new topics reviewed in 2019 was the prevention of perinatal depression. (As noted, the other on preexposure prophylaxis to prevent HIV infection has already been covered in a Practice Alert podcast.) The Task Force found that the prevalence of depression is estimated at 8.9% among pregnant women and 37% at any point in the first year postpartum.1
Depression during pregnancy and the postpartum period is associated with adverse effects on the mother and infant, including higher rates of suicide and suicidal ideation and thoughts of harming the infant.1 Women with perinatal depression are also more likely to exhibit significantly lower levels of positive maternal behaviors, such as praising and playing with their child,2 and higher rates of negative maternal behaviors.2 Perinatal depression is also associated with increased rates of preterm birth and low birth weight.3
Mothers with postpartum depression have higher rates of early termination of breast feeding and lower adherence for recommended child preventive services including vaccination.1 Children of mothers with perinatal depression develop more behavior problems, have lower cognitive functioning, and have an increased risk of psychiatric disorders than do children of mothers without this condition.4,5
A number of risk factors are associated with perinatal depression, but no screening tool was found to have enough predictive value to be recommended. In deciding who should receive an offer or referral for counseling, the Task Force recommends as a practical approach providing “counseling interventions to women with 1 or more of the following: a history of depression, current depressive symptoms (that do not reach a diagnostic threshold), certain socioeconomic risk factors such as low income or adolescent or single parenthood, recent intimate partner violence, or mental health-related factors such as elevated anxiety symptoms or a history of significant negative life events.”1
There is no conclusive evidence to guide timing of counseling interventions, but most studies reviewed started them in the second trimester. These studies included cognitive behavioral therapy and interpersonal therapy and involved counseling sessions that ranged from 4 to 20 sessions and lasted for 4 to 70 weeks. They involved group and individual sessions, mostly in-person visits, and were provided by a variety of health professionals.6
Continue to: The studies reviewed showed...
The studies reviewed showed that counseling interventions reduced the likelihood of developing depression symptoms by 39%, with a number needed to treat of 13.5.6 Studies that looked at pregnancy and maternal and infant clinical outcomes were mixed but usually found little to no difference with counseling.6 Even so, the Task Force felt that a reduction in depression itself was enough to warrant a “B” recommendation.
Screening for abdominal aortic aneurisms
Ultrasound is underused in screening for abdominal aortic aneurisms (AAA) and preventing death from their rupture. (See “Whom should you screen for abdominal aortic aneurysm?”) The prevalence of AAA is the United States is unknown; in other western countries it varies from 1.2% to 3.3% in men and is declining due to decreased rates of smoking, the primary risk factor.
The risk of AAA rupture is related to the size of the aneurism, and surgical repair (either endovascular or open repair) is usually reserved for lesions > 5.5 cm in diameter or for smaller ones that are rapidly increasing in size. The standard of care for most aneurysms < 5.5 cm is to periodically monitor growth using ultrasound.
The 2019 recommendations on AAA screening are essentially the same as those made in 2004; evaluation of new evidence supported the previous recommendations. The Task Force recommends one-time screening for men ages 65 to 75 years who have ever smoked (B recommendation). Selective screening is recommended for men in this age group who have never smoked, based mainly on personal factors such as a family history of AAA, the presence of other arterial aneurisms, and the number of risk factors for cardiovascular disease (C recommendation).
The Task Force recommends against screening women ages 65 to 75 years with no history of smoking or family history of AAA, while the evidence was felt to be insufficient to make a recommendation for women in this age range who have either risk factor. This is problematic for family physicians since women with these risk factors are at increased risk of AAA compared with women without risk factors.8 And aneurisms in women appear to rupture more frequently at smaller sizes, although at a later age than in men.8 Operative mortality is also higher in women than in men8 and there is no direct evidence that screening improves outcomes for women.
Continue to: Screening for asymptomatic bacteriuria
Screening for asymptomatic bacteriuria
The Task Force re-examined and reconfirmed its previous recommendations on screening for asymptomatic bacteriuria in adults. It recommends in favor of it for pregnant women, using a urine culture to screen, and against it for all other adults. There is good evidence that treating screen-detected asymptomatic bacteriuria in pregnant women reduces the incidence of pyelonephritis in pregnancy.
The Task Force made this a “B” recommendation based on a lower prevalence of pyelonephritis found in more recent studies, making the overall magnitude of benefits moderate. There is also good evidence that treating asymptomatic bacteriuria in nonpregnant adults offers no benefits.9 The Task Force has re-examined this topic 5 times since 1996 with essentially the same results.
Screening for elevated lead levels in children and pregnant women
In 2019 the Task Force changed its 2006 recommendation on screening for elevated lead levels. The earlier recommendation advised against screening both children ages 1 to 5 years and pregnant women at average risk for elevated blood lead levels. In 2006 the Task Force also felt that evidence was insufficient to make a recommendation regarding children ages 1 to 5 years at elevated risk.
The Task Force now believes the evidence is insufficient to make a recommendation for all children ages 1 to 5 years and for pregnant women, thus moving from a “D” to an “I” recommendation for children and pregnant women with average risk. Even though there is little evidence to support screening for elevated lead levels in children ages 1 to 5 years and in pregnant women, the Task Force apparently did not feel comfortable recommending against testing, given that the cutoff for elevated blood lead levels has been lowered from 10 to 5 mcg/dL and that other sources of lead may now be more prevalent than in 2006.10
Remember that the Medicaid Early and Periodic Screening, Diagnostic, and Treatment program requires that all children receive a blood lead test twice, at ages 12 and 24 months, and that previously unscreened children ages 36 to 72 months must be tested once.
Continue to: Additional updates with no recommendation changes
Additional updates with no recommendation changes
Four other topics were re-examined by the Task Force in 2019, resulting in no significant changes to recommendations (TABLE 2):
- Screen for hepatitis B infection in pregnant women at the first prenatal visit (A recommendation; updated from 2009).
- Screen for HIV infection in adolescents and adults ages 15 to 65 years, and in those younger and older who are at high risk, and during pregnancy (A recommendation; updated from 2013).
- Provide topical medication for all newborns to prevent gonococcal ophthalmia neonatorum (A recommendation; first recommendation in 1996, updated in 2005 and 2011).
- Avoid screening for pancreatic cancer in asymptomatic adults (D recommendation; updated from 2004).
Affirmation of USPSTF’s value
In only 1 out of 9 reassessments of past topics did the Task Force modify its previous recommendations in any significant way. This demonstrates that recommendations will usually stand the test of time if they are made using robust, evidence-based methods (that consider both benefits and harms) and they are not made when evidence is insufficient. That only 2 new topics could be addressed in 2019 may reflect a need for more resources for the Task Force.
1. USPSTF. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendation statement. 2019;321:580-587.
2. Lovejoy MC, Graczyk PA, O’Hare E, et al. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561-592.
3. Szegda K, Markenson G, Bertone-Johnson ER, et al. Depression during pregnancy: a risk factor for adverse neonatal outcomes? A critical review of the literature. J Matern Fetal Neonatal Med. 2014;27:960-967.
4. Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12:12-20.
5. Santos IS, Matijasevich A, Barros AJ, et al. Antenatal and postnatal maternal mood symptoms and psychiatric disorders in pre-school children from the 2004 Pelotas Birth Cohort. J Affect Disord. 2014;164:112-117.
6. O’Connor E, Senger CA, Henniger ML, et al. Interventions to prevent perinatal depression. Evidence report and systematic review for the US preventive services task force. JAMA. 2019;321:588-601.
7. USPSTF. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. 2019;322:2211-2218.
8. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238.
9. USPSTF. Owens DK, Davidson KW, Krist AH, et al. Screening for asymptomatic bacteriuria in adults: US Preventive Services Task Force recommendation statement. 2019;322:1188-1194.
10. USPSTF. Screening for elevated blood lead levels in children and pregnant women: US Preventive Services Task Force recommendation statement. 2019;321:1502-1509.
1. USPSTF. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendation statement. 2019;321:580-587.
2. Lovejoy MC, Graczyk PA, O’Hare E, et al. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561-592.
3. Szegda K, Markenson G, Bertone-Johnson ER, et al. Depression during pregnancy: a risk factor for adverse neonatal outcomes? A critical review of the literature. J Matern Fetal Neonatal Med. 2014;27:960-967.
4. Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12:12-20.
5. Santos IS, Matijasevich A, Barros AJ, et al. Antenatal and postnatal maternal mood symptoms and psychiatric disorders in pre-school children from the 2004 Pelotas Birth Cohort. J Affect Disord. 2014;164:112-117.
6. O’Connor E, Senger CA, Henniger ML, et al. Interventions to prevent perinatal depression. Evidence report and systematic review for the US preventive services task force. JAMA. 2019;321:588-601.
7. USPSTF. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. 2019;322:2211-2218.
8. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238.
9. USPSTF. Owens DK, Davidson KW, Krist AH, et al. Screening for asymptomatic bacteriuria in adults: US Preventive Services Task Force recommendation statement. 2019;322:1188-1194.
10. USPSTF. Screening for elevated blood lead levels in children and pregnant women: US Preventive Services Task Force recommendation statement. 2019;321:1502-1509.
Silent brain infarcts found in 3% of AFib patients, tied to cognitive decline
Patients with atrial fibrillation, even those on oral anticoagulant therapy, developed clinically silent brain infarctions at a striking rate of close to 3% per year, according to results from SWISS-AF, a prospective of study of 1,227 Swiss patients followed with serial MR brain scans over a 2 year period.
The results also showed that these brain infarctions – which occurred in 68 (5.5%) of the atrial fibrillation (AFib) patients, including 58 (85%) who did not have any strokes or transient ischemic attacks during follow-up – appeared to represent enough pathology to link with a small but statistically significant decline in three separate cognitive measures, compared with patients who did not develop brain infarctions during follow-up.
“Cognitive decline may go unrecognized for a long time in clinical practice because usually no one tests for it,” plus “the absolute declines were small and probably not appreciable” in the everyday behavior of affected patients, David Conen, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. But “we were surprised to see a significant change after just 2 years. We expect much larger effects to develop over time,” he said during a press briefing.
Another key finding was that roughly half the patients had large cortical or noncortical infarcts, which usually have a thromboembolic cause, but the other half had small noncortical infarcts that likely have a different etiology involving the microvasculature. Causes for those small infarcts might include localized atherosclerotic disease or amyloidosis, proposed Dr. Conen, a cardiologist at McMaster University, Hamilton, Ont.
This finding also suggests that, as a consequence, anticoagulation alone may not be enough to prevent this brain damage in Afib patients. “It calls for a more comprehensive approach to prevention,” with attention to atherosclerotic cardiovascular disease risk factors in AFib patients, including interventions that address hypertension, diabetes, hyperlipidemia, and smoking cessation. “Anticoagulation in AFib patients is critical, but it also is not enough,” Dr. Conen said.
These data “are very important. The two pillars for taking care of AFib patients have traditionally been to manage the patient’s stroke risk and to treat symptoms. Dr. Conen’s data suggest that simply starting anticoagulation is not sufficient, and it stresses the importance of continued management of hypertension, diabetes, and other medical and social issues,” commented Fred Kusumoto, MD, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla.
“The risk factors associated with the development of cardiovascular disease are similar to those associated with the development of AFib and heart failure. It is important to understand the importance of managing hypertension, diabetes, and obesity; encouraging exercise and a healthy diet; and stopping smoking in all AFib patients as well as in the general population. Many clinicians have not emphasized the importance of continually addressing these behaviors,” Dr. Kusumoto said in an interview.
The SWISS-AF (Swiss Atrial Fibrillation Cohort) study enrolled 2,415 AFib patients at 14 Swiss centers during 2014-2017, and obtained both a baseline brain MR scan and baseline cognitive-test results for 1,737 patients (J Am Coll Cardiol. 2019 Mar;73[9]:989-99). Patients retook the cognitive tests annually, and 1,227 had a second MR brain scan after 2 years in the study, the cohort that supplied the data Dr. Conen presented. At baseline, these patients averaged 71 years of age, just over a quarter were women, and 90% were on an oral anticoagulant, with 84% on an oral anticoagulant at 2-year follow-up. Treatment split roughly equally between direct-acting oral anticoagulants and vitamin K antagonists like warfarin.
Among the 68 patients with evidence for an incident brain infarct after 2 years, 59 (87%) were on treatment with an OAC, and 51 (75%) who were both on treatment with a direct-acting oral anticoagulant and developed their brain infarct without also having a stroke or transient ischemic attack, which Dr. Conen called a “silent event.” The cognitive tests that showed statistically significant declines after 2 years in the patients with silent brain infarcts compared with those without a new infarct were the Trail Making Test parts A and B, and the animal-naming verbal fluency test. The two other tests applied were the Montreal Cognitive Assessment and the Digital Symbol Substitution Test.
Results from several prior studies also indicated a relationship between AFib and cognitive decline, but SWISS-AF is “the largest study to rigorously examine the incidence of silent brain infarcts in AFib patients,” commented Christine M. Albert, MD, chair of cardiology at the Smidt Heart Institute of Cedars-Sinai Medical Center in Los Angeles. “Silent infarcts could be the cause, at least in part, for the cognitive decline and dementia associated with AFib,” she noted. But divining the therapeutic implications of the finding will require further investigation that looks at factors such as the impact of anticoagulant type, other treatment that addresses AFib such as ablation and rate control, the duration and type of AFib, and the prevalence of hypertension and other stroke risk factors, she said as a designated discussant for Dr. Conen’s report.
SWISS-AF received no commercial funding. Dr. Conen has been a speaker on behalf of Servier. Dr. Kusumoto had no disclosures. Dr. Albert has been a consultant to Roche Diagnostics and has received research funding from Abbott, Roche Diagnostics, and St. Jude Medical.
Patients with atrial fibrillation, even those on oral anticoagulant therapy, developed clinically silent brain infarctions at a striking rate of close to 3% per year, according to results from SWISS-AF, a prospective of study of 1,227 Swiss patients followed with serial MR brain scans over a 2 year period.
The results also showed that these brain infarctions – which occurred in 68 (5.5%) of the atrial fibrillation (AFib) patients, including 58 (85%) who did not have any strokes or transient ischemic attacks during follow-up – appeared to represent enough pathology to link with a small but statistically significant decline in three separate cognitive measures, compared with patients who did not develop brain infarctions during follow-up.
“Cognitive decline may go unrecognized for a long time in clinical practice because usually no one tests for it,” plus “the absolute declines were small and probably not appreciable” in the everyday behavior of affected patients, David Conen, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. But “we were surprised to see a significant change after just 2 years. We expect much larger effects to develop over time,” he said during a press briefing.
Another key finding was that roughly half the patients had large cortical or noncortical infarcts, which usually have a thromboembolic cause, but the other half had small noncortical infarcts that likely have a different etiology involving the microvasculature. Causes for those small infarcts might include localized atherosclerotic disease or amyloidosis, proposed Dr. Conen, a cardiologist at McMaster University, Hamilton, Ont.
This finding also suggests that, as a consequence, anticoagulation alone may not be enough to prevent this brain damage in Afib patients. “It calls for a more comprehensive approach to prevention,” with attention to atherosclerotic cardiovascular disease risk factors in AFib patients, including interventions that address hypertension, diabetes, hyperlipidemia, and smoking cessation. “Anticoagulation in AFib patients is critical, but it also is not enough,” Dr. Conen said.
These data “are very important. The two pillars for taking care of AFib patients have traditionally been to manage the patient’s stroke risk and to treat symptoms. Dr. Conen’s data suggest that simply starting anticoagulation is not sufficient, and it stresses the importance of continued management of hypertension, diabetes, and other medical and social issues,” commented Fred Kusumoto, MD, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla.
“The risk factors associated with the development of cardiovascular disease are similar to those associated with the development of AFib and heart failure. It is important to understand the importance of managing hypertension, diabetes, and obesity; encouraging exercise and a healthy diet; and stopping smoking in all AFib patients as well as in the general population. Many clinicians have not emphasized the importance of continually addressing these behaviors,” Dr. Kusumoto said in an interview.
The SWISS-AF (Swiss Atrial Fibrillation Cohort) study enrolled 2,415 AFib patients at 14 Swiss centers during 2014-2017, and obtained both a baseline brain MR scan and baseline cognitive-test results for 1,737 patients (J Am Coll Cardiol. 2019 Mar;73[9]:989-99). Patients retook the cognitive tests annually, and 1,227 had a second MR brain scan after 2 years in the study, the cohort that supplied the data Dr. Conen presented. At baseline, these patients averaged 71 years of age, just over a quarter were women, and 90% were on an oral anticoagulant, with 84% on an oral anticoagulant at 2-year follow-up. Treatment split roughly equally between direct-acting oral anticoagulants and vitamin K antagonists like warfarin.
Among the 68 patients with evidence for an incident brain infarct after 2 years, 59 (87%) were on treatment with an OAC, and 51 (75%) who were both on treatment with a direct-acting oral anticoagulant and developed their brain infarct without also having a stroke or transient ischemic attack, which Dr. Conen called a “silent event.” The cognitive tests that showed statistically significant declines after 2 years in the patients with silent brain infarcts compared with those without a new infarct were the Trail Making Test parts A and B, and the animal-naming verbal fluency test. The two other tests applied were the Montreal Cognitive Assessment and the Digital Symbol Substitution Test.
Results from several prior studies also indicated a relationship between AFib and cognitive decline, but SWISS-AF is “the largest study to rigorously examine the incidence of silent brain infarcts in AFib patients,” commented Christine M. Albert, MD, chair of cardiology at the Smidt Heart Institute of Cedars-Sinai Medical Center in Los Angeles. “Silent infarcts could be the cause, at least in part, for the cognitive decline and dementia associated with AFib,” she noted. But divining the therapeutic implications of the finding will require further investigation that looks at factors such as the impact of anticoagulant type, other treatment that addresses AFib such as ablation and rate control, the duration and type of AFib, and the prevalence of hypertension and other stroke risk factors, she said as a designated discussant for Dr. Conen’s report.
SWISS-AF received no commercial funding. Dr. Conen has been a speaker on behalf of Servier. Dr. Kusumoto had no disclosures. Dr. Albert has been a consultant to Roche Diagnostics and has received research funding from Abbott, Roche Diagnostics, and St. Jude Medical.
Patients with atrial fibrillation, even those on oral anticoagulant therapy, developed clinically silent brain infarctions at a striking rate of close to 3% per year, according to results from SWISS-AF, a prospective of study of 1,227 Swiss patients followed with serial MR brain scans over a 2 year period.
The results also showed that these brain infarctions – which occurred in 68 (5.5%) of the atrial fibrillation (AFib) patients, including 58 (85%) who did not have any strokes or transient ischemic attacks during follow-up – appeared to represent enough pathology to link with a small but statistically significant decline in three separate cognitive measures, compared with patients who did not develop brain infarctions during follow-up.
“Cognitive decline may go unrecognized for a long time in clinical practice because usually no one tests for it,” plus “the absolute declines were small and probably not appreciable” in the everyday behavior of affected patients, David Conen, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. But “we were surprised to see a significant change after just 2 years. We expect much larger effects to develop over time,” he said during a press briefing.
Another key finding was that roughly half the patients had large cortical or noncortical infarcts, which usually have a thromboembolic cause, but the other half had small noncortical infarcts that likely have a different etiology involving the microvasculature. Causes for those small infarcts might include localized atherosclerotic disease or amyloidosis, proposed Dr. Conen, a cardiologist at McMaster University, Hamilton, Ont.
This finding also suggests that, as a consequence, anticoagulation alone may not be enough to prevent this brain damage in Afib patients. “It calls for a more comprehensive approach to prevention,” with attention to atherosclerotic cardiovascular disease risk factors in AFib patients, including interventions that address hypertension, diabetes, hyperlipidemia, and smoking cessation. “Anticoagulation in AFib patients is critical, but it also is not enough,” Dr. Conen said.
These data “are very important. The two pillars for taking care of AFib patients have traditionally been to manage the patient’s stroke risk and to treat symptoms. Dr. Conen’s data suggest that simply starting anticoagulation is not sufficient, and it stresses the importance of continued management of hypertension, diabetes, and other medical and social issues,” commented Fred Kusumoto, MD, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla.
“The risk factors associated with the development of cardiovascular disease are similar to those associated with the development of AFib and heart failure. It is important to understand the importance of managing hypertension, diabetes, and obesity; encouraging exercise and a healthy diet; and stopping smoking in all AFib patients as well as in the general population. Many clinicians have not emphasized the importance of continually addressing these behaviors,” Dr. Kusumoto said in an interview.
The SWISS-AF (Swiss Atrial Fibrillation Cohort) study enrolled 2,415 AFib patients at 14 Swiss centers during 2014-2017, and obtained both a baseline brain MR scan and baseline cognitive-test results for 1,737 patients (J Am Coll Cardiol. 2019 Mar;73[9]:989-99). Patients retook the cognitive tests annually, and 1,227 had a second MR brain scan after 2 years in the study, the cohort that supplied the data Dr. Conen presented. At baseline, these patients averaged 71 years of age, just over a quarter were women, and 90% were on an oral anticoagulant, with 84% on an oral anticoagulant at 2-year follow-up. Treatment split roughly equally between direct-acting oral anticoagulants and vitamin K antagonists like warfarin.
Among the 68 patients with evidence for an incident brain infarct after 2 years, 59 (87%) were on treatment with an OAC, and 51 (75%) who were both on treatment with a direct-acting oral anticoagulant and developed their brain infarct without also having a stroke or transient ischemic attack, which Dr. Conen called a “silent event.” The cognitive tests that showed statistically significant declines after 2 years in the patients with silent brain infarcts compared with those without a new infarct were the Trail Making Test parts A and B, and the animal-naming verbal fluency test. The two other tests applied were the Montreal Cognitive Assessment and the Digital Symbol Substitution Test.
Results from several prior studies also indicated a relationship between AFib and cognitive decline, but SWISS-AF is “the largest study to rigorously examine the incidence of silent brain infarcts in AFib patients,” commented Christine M. Albert, MD, chair of cardiology at the Smidt Heart Institute of Cedars-Sinai Medical Center in Los Angeles. “Silent infarcts could be the cause, at least in part, for the cognitive decline and dementia associated with AFib,” she noted. But divining the therapeutic implications of the finding will require further investigation that looks at factors such as the impact of anticoagulant type, other treatment that addresses AFib such as ablation and rate control, the duration and type of AFib, and the prevalence of hypertension and other stroke risk factors, she said as a designated discussant for Dr. Conen’s report.
SWISS-AF received no commercial funding. Dr. Conen has been a speaker on behalf of Servier. Dr. Kusumoto had no disclosures. Dr. Albert has been a consultant to Roche Diagnostics and has received research funding from Abbott, Roche Diagnostics, and St. Jude Medical.
FROM HEART RHYTHM 2020
Serum NfL in early MS can help predict clinical course
research suggests. The study showed that patients with higher sNfL within 5 years of MS diagnosis had a higher risk of long term-clinical disability and higher risk of developing progressive MS. The level of sNfL also predicted the rate of increase over time in the Expanded Disability Status Scale (EDSS).
Serum NfL levels can provide “useful information in both directions, adding to both an overall reassuring picture or worrying picture both at first presentation and then on subsequent visits,” said Simon Thebault, MBBCh, a neurology resident at the University of Ottawa and the Ottawa Hospital Research Institute, Canada.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Prognostication from day one
Many studies have shown a correlation between MS disease activity (clinical relapses, EDSS progression, MRI lesions) and elevated sNfL. Other studies have also looked at the prognostic value of NfL in serum and cerebrospinal fluid (CSF), but the data are limited by the lack of long-term biobanked samples and subsequent follow-up, Dr. Thebault explained.
The new study took advantage of the Ottawa MS biobank, which contains carefully frozen and stored samples from more than 3,000 patients with MS going back up to 25 years.
The team identified patients with serum collected within 5 years of first MS symptom onset (baseline) who were followed for a median of 18.9 years (range 15.0 to 27.0 years). They quantified levels of sNfL in 67 patients and 37 matched controls.
In patients with MS, the median baseline sNfL level was 10.1 pg/mL – 38.5% higher than the median level in controls (7.26 pg/mL, P = 0.004).
The baseline sNfL level was “most helpful as a sensitive predictive marker to rule out disease progression,” the researchers reported in their meeting abstract.
Patients with baseline sNfL levels less than 7.62 pg/mL were 4.3 times less likely to develop significant disability (EDSS score ≥ 4; P = 0.001) and 7.1 times less likely to develop progressive MS by end of follow-up (P = 0.054).
The most rapid disease progression was seen in patients with the highest baseline NfL levels (3rd-tertile, > 13.2 pg/mL). Higher baseline sNfL level was associated with faster rate of EDSS progression even after adjusting for confounders of age, sex, and disease-modifying treatment.
“We were able to show that serum neurofilament levels collected very early in the disease, usually at the time of first diagnosis, were predictive of the clinical progression [by EDSS score] and the risk of evolving to secondary progressive MS on average 19 years later,” Dr. Thebault said. A baseline level less than 7.6 pg/mL was “reassuring.”
“Prognostication in MS from day one is important,” he emphasized.
“If we know someone is on a bad trajectory, neurologists might recommend more aggressive therapies up front. Equally, if a patient has a very reassuring picture, then maybe it is more appropriate to start with safer treatments [the so called ‘platform therapies’] that may serve a patient well for many years, as they did for many in the years before higher-efficacy therapies were available,” Dr. Thebault said.
“In the hands of an expert MS neurologist who understands both the pearls and pitfalls of this test ... serum neurofilament is already a useful clinical tool, and we have implemented it in our daily practice in Ottawa,” he concluded.
Noteworthy study
Commenting on the study, Asaff Harel, MD, neurologist at Lenox Hill Hospital in New York City, said the findings in this study are “noteworthy, as there is a relative lack of effective prognostic biomarkers in the field of MS.”
“It remains to be seen whether this improves risk stratification of patients above what can be achieved by looking at other prognostic factors, such as age, gender, baseline EDSS, and severity and frequency of relapses during early disease course,” Dr. Harel cautioned.
“This was a relatively small study and further research is necessary,” Dr. Harel added. It’s also worth noting, he said, that out of the 67 patients who met criteria to be included in the study (i.e., those with blood samples taken during “early MS,” more than 15 years ago), almost half were lost to follow-up, which could potentially open the study to error.
It is also “unclear whether early NfL level is a better prognostic marker than severity of early disease course and baseline EDSS, both of which were not addressed in the study, and this will be interesting to determine in the future,” Dr. Harel commented.
Funding for the study was provided by The Ottawa Hospital Pilot Project Grant. Thebault and Harel have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
research suggests. The study showed that patients with higher sNfL within 5 years of MS diagnosis had a higher risk of long term-clinical disability and higher risk of developing progressive MS. The level of sNfL also predicted the rate of increase over time in the Expanded Disability Status Scale (EDSS).
Serum NfL levels can provide “useful information in both directions, adding to both an overall reassuring picture or worrying picture both at first presentation and then on subsequent visits,” said Simon Thebault, MBBCh, a neurology resident at the University of Ottawa and the Ottawa Hospital Research Institute, Canada.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Prognostication from day one
Many studies have shown a correlation between MS disease activity (clinical relapses, EDSS progression, MRI lesions) and elevated sNfL. Other studies have also looked at the prognostic value of NfL in serum and cerebrospinal fluid (CSF), but the data are limited by the lack of long-term biobanked samples and subsequent follow-up, Dr. Thebault explained.
The new study took advantage of the Ottawa MS biobank, which contains carefully frozen and stored samples from more than 3,000 patients with MS going back up to 25 years.
The team identified patients with serum collected within 5 years of first MS symptom onset (baseline) who were followed for a median of 18.9 years (range 15.0 to 27.0 years). They quantified levels of sNfL in 67 patients and 37 matched controls.
In patients with MS, the median baseline sNfL level was 10.1 pg/mL – 38.5% higher than the median level in controls (7.26 pg/mL, P = 0.004).
The baseline sNfL level was “most helpful as a sensitive predictive marker to rule out disease progression,” the researchers reported in their meeting abstract.
Patients with baseline sNfL levels less than 7.62 pg/mL were 4.3 times less likely to develop significant disability (EDSS score ≥ 4; P = 0.001) and 7.1 times less likely to develop progressive MS by end of follow-up (P = 0.054).
The most rapid disease progression was seen in patients with the highest baseline NfL levels (3rd-tertile, > 13.2 pg/mL). Higher baseline sNfL level was associated with faster rate of EDSS progression even after adjusting for confounders of age, sex, and disease-modifying treatment.
“We were able to show that serum neurofilament levels collected very early in the disease, usually at the time of first diagnosis, were predictive of the clinical progression [by EDSS score] and the risk of evolving to secondary progressive MS on average 19 years later,” Dr. Thebault said. A baseline level less than 7.6 pg/mL was “reassuring.”
“Prognostication in MS from day one is important,” he emphasized.
“If we know someone is on a bad trajectory, neurologists might recommend more aggressive therapies up front. Equally, if a patient has a very reassuring picture, then maybe it is more appropriate to start with safer treatments [the so called ‘platform therapies’] that may serve a patient well for many years, as they did for many in the years before higher-efficacy therapies were available,” Dr. Thebault said.
“In the hands of an expert MS neurologist who understands both the pearls and pitfalls of this test ... serum neurofilament is already a useful clinical tool, and we have implemented it in our daily practice in Ottawa,” he concluded.
Noteworthy study
Commenting on the study, Asaff Harel, MD, neurologist at Lenox Hill Hospital in New York City, said the findings in this study are “noteworthy, as there is a relative lack of effective prognostic biomarkers in the field of MS.”
“It remains to be seen whether this improves risk stratification of patients above what can be achieved by looking at other prognostic factors, such as age, gender, baseline EDSS, and severity and frequency of relapses during early disease course,” Dr. Harel cautioned.
“This was a relatively small study and further research is necessary,” Dr. Harel added. It’s also worth noting, he said, that out of the 67 patients who met criteria to be included in the study (i.e., those with blood samples taken during “early MS,” more than 15 years ago), almost half were lost to follow-up, which could potentially open the study to error.
It is also “unclear whether early NfL level is a better prognostic marker than severity of early disease course and baseline EDSS, both of which were not addressed in the study, and this will be interesting to determine in the future,” Dr. Harel commented.
Funding for the study was provided by The Ottawa Hospital Pilot Project Grant. Thebault and Harel have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
research suggests. The study showed that patients with higher sNfL within 5 years of MS diagnosis had a higher risk of long term-clinical disability and higher risk of developing progressive MS. The level of sNfL also predicted the rate of increase over time in the Expanded Disability Status Scale (EDSS).
Serum NfL levels can provide “useful information in both directions, adding to both an overall reassuring picture or worrying picture both at first presentation and then on subsequent visits,” said Simon Thebault, MBBCh, a neurology resident at the University of Ottawa and the Ottawa Hospital Research Institute, Canada.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Prognostication from day one
Many studies have shown a correlation between MS disease activity (clinical relapses, EDSS progression, MRI lesions) and elevated sNfL. Other studies have also looked at the prognostic value of NfL in serum and cerebrospinal fluid (CSF), but the data are limited by the lack of long-term biobanked samples and subsequent follow-up, Dr. Thebault explained.
The new study took advantage of the Ottawa MS biobank, which contains carefully frozen and stored samples from more than 3,000 patients with MS going back up to 25 years.
The team identified patients with serum collected within 5 years of first MS symptom onset (baseline) who were followed for a median of 18.9 years (range 15.0 to 27.0 years). They quantified levels of sNfL in 67 patients and 37 matched controls.
In patients with MS, the median baseline sNfL level was 10.1 pg/mL – 38.5% higher than the median level in controls (7.26 pg/mL, P = 0.004).
The baseline sNfL level was “most helpful as a sensitive predictive marker to rule out disease progression,” the researchers reported in their meeting abstract.
Patients with baseline sNfL levels less than 7.62 pg/mL were 4.3 times less likely to develop significant disability (EDSS score ≥ 4; P = 0.001) and 7.1 times less likely to develop progressive MS by end of follow-up (P = 0.054).
The most rapid disease progression was seen in patients with the highest baseline NfL levels (3rd-tertile, > 13.2 pg/mL). Higher baseline sNfL level was associated with faster rate of EDSS progression even after adjusting for confounders of age, sex, and disease-modifying treatment.
“We were able to show that serum neurofilament levels collected very early in the disease, usually at the time of first diagnosis, were predictive of the clinical progression [by EDSS score] and the risk of evolving to secondary progressive MS on average 19 years later,” Dr. Thebault said. A baseline level less than 7.6 pg/mL was “reassuring.”
“Prognostication in MS from day one is important,” he emphasized.
“If we know someone is on a bad trajectory, neurologists might recommend more aggressive therapies up front. Equally, if a patient has a very reassuring picture, then maybe it is more appropriate to start with safer treatments [the so called ‘platform therapies’] that may serve a patient well for many years, as they did for many in the years before higher-efficacy therapies were available,” Dr. Thebault said.
“In the hands of an expert MS neurologist who understands both the pearls and pitfalls of this test ... serum neurofilament is already a useful clinical tool, and we have implemented it in our daily practice in Ottawa,” he concluded.
Noteworthy study
Commenting on the study, Asaff Harel, MD, neurologist at Lenox Hill Hospital in New York City, said the findings in this study are “noteworthy, as there is a relative lack of effective prognostic biomarkers in the field of MS.”
“It remains to be seen whether this improves risk stratification of patients above what can be achieved by looking at other prognostic factors, such as age, gender, baseline EDSS, and severity and frequency of relapses during early disease course,” Dr. Harel cautioned.
“This was a relatively small study and further research is necessary,” Dr. Harel added. It’s also worth noting, he said, that out of the 67 patients who met criteria to be included in the study (i.e., those with blood samples taken during “early MS,” more than 15 years ago), almost half were lost to follow-up, which could potentially open the study to error.
It is also “unclear whether early NfL level is a better prognostic marker than severity of early disease course and baseline EDSS, both of which were not addressed in the study, and this will be interesting to determine in the future,” Dr. Harel commented.
Funding for the study was provided by The Ottawa Hospital Pilot Project Grant. Thebault and Harel have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with epilepsy may underreport seizures, survey finds
according to survey results presented online as part of the 2020 American Academy of Neurology Science Highlights.
Clinicians, for their part, may underestimate the number of seizures that go unreported. This disconnect may contribute to complacency about epilepsy treatment regimens among patients, caregivers, and health care professionals (HCPs), despite continuations in seizures. “Improved reporting of all seizure occurrences and more frequent discussion of potential treatment changes, initiated by all groups, may be needed to optimize treatment outcomes,” said Patricia E. Penovich, MD, a neurologist at Minnesota Epilepsy Group in St. Paul, and colleagues.
To evaluate treatment complacency among adult patients with epilepsy, caregivers, and HCPs, Dr. Penovich and collaborators analyzed data from the STEP survey (Seize the Truth about Epilepsy Perceptions), which was conducted between February and March 2019. In all, 400 adults with epilepsy, 201 caregivers, and 258 HCPs completed the survey. The HCPs included 96 epileptologists, 112 general neurologists, and 50 nurse practitioners or physician assistants.
Patients had an average epilepsy duration of 16 years, and 58% were on at least their third antiepileptic drug (AED). In the past year, 52% of patients had 1-9 seizures, and 31% had 10 or more seizures. “Patients estimated reporting 45% of their seizures to their HCPs, and for the seizures not reported, 57% provided reasoning that they were not serious enough to mention,” reported Dr. Penovich and colleagues. “Alternatively, HCPs estimated that patients report 73% of seizures.”
Survey participants most frequently selected HCPs as the ones to initiate conversations about changing AEDs or increasing dosage. “Patient-initiated discussions were reported by 39% of patients for changing AEDs and 27% of patients for increasing AED dosage; 25% of patients reported they were likely to ask their HCP about changing treatments in the next 12 months,” the authors said. Discussion of vagus nerve stimulation was reported by 21% of HCPs, and 10% reported discussion of responsive neurostimulation. HCPs also discussed surgical options such as hemispherectomy (3%), corpus callosotomy and multiple subpial transection (4%), lobe resection (8%), and lesionectomy (11%).
Among patients with 13 or more seizures per year, 27% reported referral to an epilepsy center. Most survey participants – 61% of patients and HCPs and 68% of caregivers – “reported a desire for a treatment map that tells patients to see an epileptologist/specialist as soon as they have symptoms,” the researchers said.
“What we would like to think is that we are getting the whole scoop and the honest scoop” about seizure activity, Dr. Penovich said in an interview. “What this shows us is that that’s probably not always true. Some health care providers understand that the patients do not tell them everything,” but the degree of seizure underreporting may be surprising.
Dr. Penovich has seen this phenomenon in practice. In some cases, caregivers return to the office to explain that a patient did not report all of their seizures. Other patients may omit entire days of seizures in their diaries as an oversight. In addition, patients may not report seizures to avoid having a driver’s license revoked. In some instances, clinicians may not take the time to discuss seizure activity in detail.
Having an accurate picture of seizure activity is an “important part of working with our patients, particularly when we are trying to get them to the point of being seizure free,” said Dr. Penovich.
Failing a first or second AED indicates a greater likelihood that medication will not stop a patient’s seizures, “but it does not mean that you will not be controlled,” Dr. Penovich said. More medications, surgical options, and investigative treatments have become available. Still, AED trials should not prevent a timely referral to an epilepsy center. “You don’t need to go through 10 or 15 before you get referred” to an epilepsy center, she said.
The STEP survey was conducted by Kantar Health on behalf of SK Life Science. Dr. Penovich has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from SK Life Science, Neurelis, GW Pharmaceuticals, Engage Therapeutics, and UCB Pharma. A coauthor was employed by Kantar Health. Other coauthors disclosed compensation from SK Life Science and various pharmaceutical companies.
SOURCE: Penovich PE et al. AAN 2020, Abstract S59.007.
according to survey results presented online as part of the 2020 American Academy of Neurology Science Highlights.
Clinicians, for their part, may underestimate the number of seizures that go unreported. This disconnect may contribute to complacency about epilepsy treatment regimens among patients, caregivers, and health care professionals (HCPs), despite continuations in seizures. “Improved reporting of all seizure occurrences and more frequent discussion of potential treatment changes, initiated by all groups, may be needed to optimize treatment outcomes,” said Patricia E. Penovich, MD, a neurologist at Minnesota Epilepsy Group in St. Paul, and colleagues.
To evaluate treatment complacency among adult patients with epilepsy, caregivers, and HCPs, Dr. Penovich and collaborators analyzed data from the STEP survey (Seize the Truth about Epilepsy Perceptions), which was conducted between February and March 2019. In all, 400 adults with epilepsy, 201 caregivers, and 258 HCPs completed the survey. The HCPs included 96 epileptologists, 112 general neurologists, and 50 nurse practitioners or physician assistants.
Patients had an average epilepsy duration of 16 years, and 58% were on at least their third antiepileptic drug (AED). In the past year, 52% of patients had 1-9 seizures, and 31% had 10 or more seizures. “Patients estimated reporting 45% of their seizures to their HCPs, and for the seizures not reported, 57% provided reasoning that they were not serious enough to mention,” reported Dr. Penovich and colleagues. “Alternatively, HCPs estimated that patients report 73% of seizures.”
Survey participants most frequently selected HCPs as the ones to initiate conversations about changing AEDs or increasing dosage. “Patient-initiated discussions were reported by 39% of patients for changing AEDs and 27% of patients for increasing AED dosage; 25% of patients reported they were likely to ask their HCP about changing treatments in the next 12 months,” the authors said. Discussion of vagus nerve stimulation was reported by 21% of HCPs, and 10% reported discussion of responsive neurostimulation. HCPs also discussed surgical options such as hemispherectomy (3%), corpus callosotomy and multiple subpial transection (4%), lobe resection (8%), and lesionectomy (11%).
Among patients with 13 or more seizures per year, 27% reported referral to an epilepsy center. Most survey participants – 61% of patients and HCPs and 68% of caregivers – “reported a desire for a treatment map that tells patients to see an epileptologist/specialist as soon as they have symptoms,” the researchers said.
“What we would like to think is that we are getting the whole scoop and the honest scoop” about seizure activity, Dr. Penovich said in an interview. “What this shows us is that that’s probably not always true. Some health care providers understand that the patients do not tell them everything,” but the degree of seizure underreporting may be surprising.
Dr. Penovich has seen this phenomenon in practice. In some cases, caregivers return to the office to explain that a patient did not report all of their seizures. Other patients may omit entire days of seizures in their diaries as an oversight. In addition, patients may not report seizures to avoid having a driver’s license revoked. In some instances, clinicians may not take the time to discuss seizure activity in detail.
Having an accurate picture of seizure activity is an “important part of working with our patients, particularly when we are trying to get them to the point of being seizure free,” said Dr. Penovich.
Failing a first or second AED indicates a greater likelihood that medication will not stop a patient’s seizures, “but it does not mean that you will not be controlled,” Dr. Penovich said. More medications, surgical options, and investigative treatments have become available. Still, AED trials should not prevent a timely referral to an epilepsy center. “You don’t need to go through 10 or 15 before you get referred” to an epilepsy center, she said.
The STEP survey was conducted by Kantar Health on behalf of SK Life Science. Dr. Penovich has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from SK Life Science, Neurelis, GW Pharmaceuticals, Engage Therapeutics, and UCB Pharma. A coauthor was employed by Kantar Health. Other coauthors disclosed compensation from SK Life Science and various pharmaceutical companies.
SOURCE: Penovich PE et al. AAN 2020, Abstract S59.007.
according to survey results presented online as part of the 2020 American Academy of Neurology Science Highlights.
Clinicians, for their part, may underestimate the number of seizures that go unreported. This disconnect may contribute to complacency about epilepsy treatment regimens among patients, caregivers, and health care professionals (HCPs), despite continuations in seizures. “Improved reporting of all seizure occurrences and more frequent discussion of potential treatment changes, initiated by all groups, may be needed to optimize treatment outcomes,” said Patricia E. Penovich, MD, a neurologist at Minnesota Epilepsy Group in St. Paul, and colleagues.
To evaluate treatment complacency among adult patients with epilepsy, caregivers, and HCPs, Dr. Penovich and collaborators analyzed data from the STEP survey (Seize the Truth about Epilepsy Perceptions), which was conducted between February and March 2019. In all, 400 adults with epilepsy, 201 caregivers, and 258 HCPs completed the survey. The HCPs included 96 epileptologists, 112 general neurologists, and 50 nurse practitioners or physician assistants.
Patients had an average epilepsy duration of 16 years, and 58% were on at least their third antiepileptic drug (AED). In the past year, 52% of patients had 1-9 seizures, and 31% had 10 or more seizures. “Patients estimated reporting 45% of their seizures to their HCPs, and for the seizures not reported, 57% provided reasoning that they were not serious enough to mention,” reported Dr. Penovich and colleagues. “Alternatively, HCPs estimated that patients report 73% of seizures.”
Survey participants most frequently selected HCPs as the ones to initiate conversations about changing AEDs or increasing dosage. “Patient-initiated discussions were reported by 39% of patients for changing AEDs and 27% of patients for increasing AED dosage; 25% of patients reported they were likely to ask their HCP about changing treatments in the next 12 months,” the authors said. Discussion of vagus nerve stimulation was reported by 21% of HCPs, and 10% reported discussion of responsive neurostimulation. HCPs also discussed surgical options such as hemispherectomy (3%), corpus callosotomy and multiple subpial transection (4%), lobe resection (8%), and lesionectomy (11%).
Among patients with 13 or more seizures per year, 27% reported referral to an epilepsy center. Most survey participants – 61% of patients and HCPs and 68% of caregivers – “reported a desire for a treatment map that tells patients to see an epileptologist/specialist as soon as they have symptoms,” the researchers said.
“What we would like to think is that we are getting the whole scoop and the honest scoop” about seizure activity, Dr. Penovich said in an interview. “What this shows us is that that’s probably not always true. Some health care providers understand that the patients do not tell them everything,” but the degree of seizure underreporting may be surprising.
Dr. Penovich has seen this phenomenon in practice. In some cases, caregivers return to the office to explain that a patient did not report all of their seizures. Other patients may omit entire days of seizures in their diaries as an oversight. In addition, patients may not report seizures to avoid having a driver’s license revoked. In some instances, clinicians may not take the time to discuss seizure activity in detail.
Having an accurate picture of seizure activity is an “important part of working with our patients, particularly when we are trying to get them to the point of being seizure free,” said Dr. Penovich.
Failing a first or second AED indicates a greater likelihood that medication will not stop a patient’s seizures, “but it does not mean that you will not be controlled,” Dr. Penovich said. More medications, surgical options, and investigative treatments have become available. Still, AED trials should not prevent a timely referral to an epilepsy center. “You don’t need to go through 10 or 15 before you get referred” to an epilepsy center, she said.
The STEP survey was conducted by Kantar Health on behalf of SK Life Science. Dr. Penovich has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from SK Life Science, Neurelis, GW Pharmaceuticals, Engage Therapeutics, and UCB Pharma. A coauthor was employed by Kantar Health. Other coauthors disclosed compensation from SK Life Science and various pharmaceutical companies.
SOURCE: Penovich PE et al. AAN 2020, Abstract S59.007.
FROM AAN 2020
Statins and ICH: new meta-analysis
The meta-analysis was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Coauthor Abhi Pandhi, MD, the University of Tennessee Health Science Center, Memphis, explained that some previous studies have suggested that statin therapy may be associated with an increased risk for ICH, especially at higher doses. Other studies, however, have failed to confirm this and have shown an increase in cardiovascular events if statins are stopped.
To look further into this issue, Dr. Pandhi and colleagues conducted a meta-analysis of 19 clinical studies involving patients who had a history of cardiovascular or cerebrovascular events and who had been treated with statins. A total of 35,842 patients were included.
Results showed that statin use was not significantly associated with the risk for combined primary and secondary ICH (relative risk, 1.03; 95% confidence interval, 0.85–1.08). But the risk for cerebral ischemia (stroke and transient ischemic attack) was significantly lower in those who received statins (RR, 0.79; 95% CI, 0.61–0.87).
“Overall, we found no effect of statins on the risk of ICH, and benefits on reducing ischemic events are clear,” Dr. Pandhi said.
Increased secondary ICH?
However, a sensitivity analysis showed a trend toward a higher risk for secondary ICH among those who were assigned to statin treatment (odds ratio, 1.87; 95% CI, 0.91–3.86).
“While this may suggest an increased risk of secondary ICH, when we look at the big picture, putting all the data together, and given that ischemic events are far more common than ICH, the risk of stopping statins and losing the protection against ischemic events is probably greater than any harm even in patients with underlying risk factors for ICH,” Dr. Pandhi concluded.
Commenting on the study, Michael Szarek, PhD, who has also conducted research in this field, said: “The results of this meta-analysis appear to be consistent with individual randomized trials of statins in patients with cerebrovascular disease that have shown clear benefit in terms of ischemic stroke or TIA and potential harm in terms of hemorrhagic stroke.”
Dr. Szarek is chair and professor in the Department of Epidemiology and Biostatistics at the SUNY Downstate Health Sciences University, New York City.
“However, the much greater frequency of ischemic events, coupled with benefits in coronary and peripheral vascular territories, suggest the risk/benefit of statin treatment remains favorable in this patient population, with the possible exception of patients with a history of hemorrhagic stroke,” he added.
Also commenting on this latest meta-analysis, Pamela Rist, ScD, associate epidemiologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, Boston, who has also authored studies in this area, said the results of this study seem similar to prior results from meta-analyses of clinical trials.
“It will be interesting to see the full manuscript to learn more about the sensitivity analyses they conducted and why they may have observed a nonsignificant increased risk of secondary ICH among some individuals using statins,” Dr. Rist added.
“Based on prior published meta-analyses of statin use and ICH, any potential increase in risk of hemorrhagic stroke is probably outweighed by the reduction in ischemic stroke and other cardiovascular events,” she concluded.
Dr. Pandhi has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Ishfaq A et al. AAN 2020. Abstract S9.010.
The meta-analysis was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Coauthor Abhi Pandhi, MD, the University of Tennessee Health Science Center, Memphis, explained that some previous studies have suggested that statin therapy may be associated with an increased risk for ICH, especially at higher doses. Other studies, however, have failed to confirm this and have shown an increase in cardiovascular events if statins are stopped.
To look further into this issue, Dr. Pandhi and colleagues conducted a meta-analysis of 19 clinical studies involving patients who had a history of cardiovascular or cerebrovascular events and who had been treated with statins. A total of 35,842 patients were included.
Results showed that statin use was not significantly associated with the risk for combined primary and secondary ICH (relative risk, 1.03; 95% confidence interval, 0.85–1.08). But the risk for cerebral ischemia (stroke and transient ischemic attack) was significantly lower in those who received statins (RR, 0.79; 95% CI, 0.61–0.87).
“Overall, we found no effect of statins on the risk of ICH, and benefits on reducing ischemic events are clear,” Dr. Pandhi said.
Increased secondary ICH?
However, a sensitivity analysis showed a trend toward a higher risk for secondary ICH among those who were assigned to statin treatment (odds ratio, 1.87; 95% CI, 0.91–3.86).
“While this may suggest an increased risk of secondary ICH, when we look at the big picture, putting all the data together, and given that ischemic events are far more common than ICH, the risk of stopping statins and losing the protection against ischemic events is probably greater than any harm even in patients with underlying risk factors for ICH,” Dr. Pandhi concluded.
Commenting on the study, Michael Szarek, PhD, who has also conducted research in this field, said: “The results of this meta-analysis appear to be consistent with individual randomized trials of statins in patients with cerebrovascular disease that have shown clear benefit in terms of ischemic stroke or TIA and potential harm in terms of hemorrhagic stroke.”
Dr. Szarek is chair and professor in the Department of Epidemiology and Biostatistics at the SUNY Downstate Health Sciences University, New York City.
“However, the much greater frequency of ischemic events, coupled with benefits in coronary and peripheral vascular territories, suggest the risk/benefit of statin treatment remains favorable in this patient population, with the possible exception of patients with a history of hemorrhagic stroke,” he added.
Also commenting on this latest meta-analysis, Pamela Rist, ScD, associate epidemiologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, Boston, who has also authored studies in this area, said the results of this study seem similar to prior results from meta-analyses of clinical trials.
“It will be interesting to see the full manuscript to learn more about the sensitivity analyses they conducted and why they may have observed a nonsignificant increased risk of secondary ICH among some individuals using statins,” Dr. Rist added.
“Based on prior published meta-analyses of statin use and ICH, any potential increase in risk of hemorrhagic stroke is probably outweighed by the reduction in ischemic stroke and other cardiovascular events,” she concluded.
Dr. Pandhi has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Ishfaq A et al. AAN 2020. Abstract S9.010.
The meta-analysis was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Coauthor Abhi Pandhi, MD, the University of Tennessee Health Science Center, Memphis, explained that some previous studies have suggested that statin therapy may be associated with an increased risk for ICH, especially at higher doses. Other studies, however, have failed to confirm this and have shown an increase in cardiovascular events if statins are stopped.
To look further into this issue, Dr. Pandhi and colleagues conducted a meta-analysis of 19 clinical studies involving patients who had a history of cardiovascular or cerebrovascular events and who had been treated with statins. A total of 35,842 patients were included.
Results showed that statin use was not significantly associated with the risk for combined primary and secondary ICH (relative risk, 1.03; 95% confidence interval, 0.85–1.08). But the risk for cerebral ischemia (stroke and transient ischemic attack) was significantly lower in those who received statins (RR, 0.79; 95% CI, 0.61–0.87).
“Overall, we found no effect of statins on the risk of ICH, and benefits on reducing ischemic events are clear,” Dr. Pandhi said.
Increased secondary ICH?
However, a sensitivity analysis showed a trend toward a higher risk for secondary ICH among those who were assigned to statin treatment (odds ratio, 1.87; 95% CI, 0.91–3.86).
“While this may suggest an increased risk of secondary ICH, when we look at the big picture, putting all the data together, and given that ischemic events are far more common than ICH, the risk of stopping statins and losing the protection against ischemic events is probably greater than any harm even in patients with underlying risk factors for ICH,” Dr. Pandhi concluded.
Commenting on the study, Michael Szarek, PhD, who has also conducted research in this field, said: “The results of this meta-analysis appear to be consistent with individual randomized trials of statins in patients with cerebrovascular disease that have shown clear benefit in terms of ischemic stroke or TIA and potential harm in terms of hemorrhagic stroke.”
Dr. Szarek is chair and professor in the Department of Epidemiology and Biostatistics at the SUNY Downstate Health Sciences University, New York City.
“However, the much greater frequency of ischemic events, coupled with benefits in coronary and peripheral vascular territories, suggest the risk/benefit of statin treatment remains favorable in this patient population, with the possible exception of patients with a history of hemorrhagic stroke,” he added.
Also commenting on this latest meta-analysis, Pamela Rist, ScD, associate epidemiologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, Boston, who has also authored studies in this area, said the results of this study seem similar to prior results from meta-analyses of clinical trials.
“It will be interesting to see the full manuscript to learn more about the sensitivity analyses they conducted and why they may have observed a nonsignificant increased risk of secondary ICH among some individuals using statins,” Dr. Rist added.
“Based on prior published meta-analyses of statin use and ICH, any potential increase in risk of hemorrhagic stroke is probably outweighed by the reduction in ischemic stroke and other cardiovascular events,” she concluded.
Dr. Pandhi has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Ishfaq A et al. AAN 2020. Abstract S9.010.
AAN 2020
Initial high-efficacy MS therapy tied to less disability later
new research suggests. However, there is a trade-off: In this study of nearly 300 patients, those treated with initial HET experienced more disease activity in the first 2 years than other participants.
The HET benefit emerged between 2 and 10 years into the study. For example, the mean Expanded Disability Status Scale (EDSS) scores were significantly lower at 6 years in the early, aggressive treatment group than in the later HET group (2.4 vs 3.3, respectively).
“Treatment decisions made around the time of diagnosis will affect long-term outcomes,” said lead author Anna He, MBBS, currently with the Department of Clinical Neuroscience, Karolinska Institute, Stockholm, and the UCL Queen Square Institute of Neurology in London.
Using the most efficacious disease-modifying therapies from the start minimizes disability, “whereas those patients escalating to high-efficacy disease-modifying therapies later do not seem to catch up to those who commenced earlier,” Dr. He said.
“Patients and clinicians should be aware of this when choosing treatment in early MS,” she added.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Patient-centered outcome
Instead of measures of brain volume, lesion count, serum neurofilament, or other biomarkers that are mainly of interest to clinicians and scientists, “the main outcome of interest to our patients is their disability,” Dr. He said. “The first question they ask at diagnosis is usually along the lines of: ‘What will my disability be in 10 years?’ ”
“This is what matters to patients and is fundamentally what motivated this study,” Dr. He added.
The investigators searched international MS registries for patients with relapsing-remitting MS starting HET, which included rituximab, ocrelizumab, mitoxantrone, alemtuzumab, or natalizumab.
They compared 117 participants who started HET within the first 2 years of clinical disease onset (the early group) with 181 participants who started HET after more than 4 years (the late group). All were followed for a median of 7.4 years (range, 6.4 to 8.6 years).
Difference in EDSS scores from baseline was the primary outcome. Both cohorts began the study with a mean EDSS score of 2.4, but between-group differences were significant at 10 years.
The secondary outcome of cumulative hazard of disability progression was higher in the early-treatment group from baseline to 2 years. Between the period of 2 and 10 years, the inverse was true.
In patients with highly active MS, “early exposure to high efficacy therapies is recommended,” Dr. He noted.
“We can already affect our patients’ lives enormously by utilizing our current toolbox in the most optimal way. It is our task to optimize this in a data-driven manner.”
Going forward, Dr. He plans to look at other outcomes, including patient-reported quality of life and health economic measures, and to take a different approach to future research.
Rather than assess MS outcomes from a disease-biology perspective, “I will be looking at MS outcomes from the perspective of its key stakeholders—the individual and society,” and the factors that influence them, Dr. He said.
Confirmatory evidence?
Commenting on the findings, Robert Gross, MD, a neurologist at the Rocky Mountain MS Center at the University of Colorado Denver in Aurora, said it is “hard to believe we are still having this debate” about earlier versus later HET.
There are now “numerous studies, including head-to-head trials and large cohort studies, showing superiority of highly efficacious agents to older disease-modifying therapies of more limited efficacy, as well as better outcomes with early versus delayed use of high-efficacy therapy,” said Dr. Gross, who was not involved with the current research.
“This study further adds to the evidence that we should be preferentially starting folks with relapsing-remitting MS right away on high-efficacy therapy, rather than waiting for relapses and disease progression to occur,” he added.
Drs. He and Gross have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
new research suggests. However, there is a trade-off: In this study of nearly 300 patients, those treated with initial HET experienced more disease activity in the first 2 years than other participants.
The HET benefit emerged between 2 and 10 years into the study. For example, the mean Expanded Disability Status Scale (EDSS) scores were significantly lower at 6 years in the early, aggressive treatment group than in the later HET group (2.4 vs 3.3, respectively).
“Treatment decisions made around the time of diagnosis will affect long-term outcomes,” said lead author Anna He, MBBS, currently with the Department of Clinical Neuroscience, Karolinska Institute, Stockholm, and the UCL Queen Square Institute of Neurology in London.
Using the most efficacious disease-modifying therapies from the start minimizes disability, “whereas those patients escalating to high-efficacy disease-modifying therapies later do not seem to catch up to those who commenced earlier,” Dr. He said.
“Patients and clinicians should be aware of this when choosing treatment in early MS,” she added.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Patient-centered outcome
Instead of measures of brain volume, lesion count, serum neurofilament, or other biomarkers that are mainly of interest to clinicians and scientists, “the main outcome of interest to our patients is their disability,” Dr. He said. “The first question they ask at diagnosis is usually along the lines of: ‘What will my disability be in 10 years?’ ”
“This is what matters to patients and is fundamentally what motivated this study,” Dr. He added.
The investigators searched international MS registries for patients with relapsing-remitting MS starting HET, which included rituximab, ocrelizumab, mitoxantrone, alemtuzumab, or natalizumab.
They compared 117 participants who started HET within the first 2 years of clinical disease onset (the early group) with 181 participants who started HET after more than 4 years (the late group). All were followed for a median of 7.4 years (range, 6.4 to 8.6 years).
Difference in EDSS scores from baseline was the primary outcome. Both cohorts began the study with a mean EDSS score of 2.4, but between-group differences were significant at 10 years.
The secondary outcome of cumulative hazard of disability progression was higher in the early-treatment group from baseline to 2 years. Between the period of 2 and 10 years, the inverse was true.
In patients with highly active MS, “early exposure to high efficacy therapies is recommended,” Dr. He noted.
“We can already affect our patients’ lives enormously by utilizing our current toolbox in the most optimal way. It is our task to optimize this in a data-driven manner.”
Going forward, Dr. He plans to look at other outcomes, including patient-reported quality of life and health economic measures, and to take a different approach to future research.
Rather than assess MS outcomes from a disease-biology perspective, “I will be looking at MS outcomes from the perspective of its key stakeholders—the individual and society,” and the factors that influence them, Dr. He said.
Confirmatory evidence?
Commenting on the findings, Robert Gross, MD, a neurologist at the Rocky Mountain MS Center at the University of Colorado Denver in Aurora, said it is “hard to believe we are still having this debate” about earlier versus later HET.
There are now “numerous studies, including head-to-head trials and large cohort studies, showing superiority of highly efficacious agents to older disease-modifying therapies of more limited efficacy, as well as better outcomes with early versus delayed use of high-efficacy therapy,” said Dr. Gross, who was not involved with the current research.
“This study further adds to the evidence that we should be preferentially starting folks with relapsing-remitting MS right away on high-efficacy therapy, rather than waiting for relapses and disease progression to occur,” he added.
Drs. He and Gross have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
new research suggests. However, there is a trade-off: In this study of nearly 300 patients, those treated with initial HET experienced more disease activity in the first 2 years than other participants.
The HET benefit emerged between 2 and 10 years into the study. For example, the mean Expanded Disability Status Scale (EDSS) scores were significantly lower at 6 years in the early, aggressive treatment group than in the later HET group (2.4 vs 3.3, respectively).
“Treatment decisions made around the time of diagnosis will affect long-term outcomes,” said lead author Anna He, MBBS, currently with the Department of Clinical Neuroscience, Karolinska Institute, Stockholm, and the UCL Queen Square Institute of Neurology in London.
Using the most efficacious disease-modifying therapies from the start minimizes disability, “whereas those patients escalating to high-efficacy disease-modifying therapies later do not seem to catch up to those who commenced earlier,” Dr. He said.
“Patients and clinicians should be aware of this when choosing treatment in early MS,” she added.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Patient-centered outcome
Instead of measures of brain volume, lesion count, serum neurofilament, or other biomarkers that are mainly of interest to clinicians and scientists, “the main outcome of interest to our patients is their disability,” Dr. He said. “The first question they ask at diagnosis is usually along the lines of: ‘What will my disability be in 10 years?’ ”
“This is what matters to patients and is fundamentally what motivated this study,” Dr. He added.
The investigators searched international MS registries for patients with relapsing-remitting MS starting HET, which included rituximab, ocrelizumab, mitoxantrone, alemtuzumab, or natalizumab.
They compared 117 participants who started HET within the first 2 years of clinical disease onset (the early group) with 181 participants who started HET after more than 4 years (the late group). All were followed for a median of 7.4 years (range, 6.4 to 8.6 years).
Difference in EDSS scores from baseline was the primary outcome. Both cohorts began the study with a mean EDSS score of 2.4, but between-group differences were significant at 10 years.
The secondary outcome of cumulative hazard of disability progression was higher in the early-treatment group from baseline to 2 years. Between the period of 2 and 10 years, the inverse was true.
In patients with highly active MS, “early exposure to high efficacy therapies is recommended,” Dr. He noted.
“We can already affect our patients’ lives enormously by utilizing our current toolbox in the most optimal way. It is our task to optimize this in a data-driven manner.”
Going forward, Dr. He plans to look at other outcomes, including patient-reported quality of life and health economic measures, and to take a different approach to future research.
Rather than assess MS outcomes from a disease-biology perspective, “I will be looking at MS outcomes from the perspective of its key stakeholders—the individual and society,” and the factors that influence them, Dr. He said.
Confirmatory evidence?
Commenting on the findings, Robert Gross, MD, a neurologist at the Rocky Mountain MS Center at the University of Colorado Denver in Aurora, said it is “hard to believe we are still having this debate” about earlier versus later HET.
There are now “numerous studies, including head-to-head trials and large cohort studies, showing superiority of highly efficacious agents to older disease-modifying therapies of more limited efficacy, as well as better outcomes with early versus delayed use of high-efficacy therapy,” said Dr. Gross, who was not involved with the current research.
“This study further adds to the evidence that we should be preferentially starting folks with relapsing-remitting MS right away on high-efficacy therapy, rather than waiting for relapses and disease progression to occur,” he added.
Drs. He and Gross have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Diastolic dysfunction is a common risk factor for cognitive decline
a new study suggests. “We found people with worsening diastolic dysfunction have more white matter hyperintensities on brain imaging and greater difficulty with executive functioning, suggesting that diastolic dysfunction is a common modifiable risk factor for cognitive impairment,” said lead author Alicia S. Parker, MD. Dr. Parker is assistant professor of cognitive and behavioral neurology at the Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases, University of Texas Health, San Antonio.
“This is an entirely new finding. While there have been some small studies suggesting a link between diastolic dysfunction and a reduction in working memory, this is by far the largest dataset on this topic and the first study that has included brain imaging and neuropsychological measures,” she said.
“Diastolic dysfunction is very common in the older population, and we need to do more to find it and treat it to help prevent or reduce cognitive decline,” Dr. Parker added.
This research is being presented online as part of the 2020 American Academy of Neurology Science Highlights.
Dr. Parker explained that systolic dysfunction is known to have a major effect on cardiovascular outcomes and has been found to be associated with cognitive decline. Proposed mechanisms for cognitive decline in patients with systolic dysfunction include low cardiac output, embolic infarctions, and hypoxic changes, among others.
“There is increasing interest in analyzing the influence of diastolic dysfunction on cardiovascular outcomes, and the effects of diastolic dysfunction on cognition are not currently well delineated, which this study seeks to address,” she added.
“While these results are new, they are not surprising. In general, we are finding more and more that heart health is connected to brain health,” she commented.
Dr. Parker and her colleagues started the current research after noticing in clinic that among patients with significant diastolic dysfunction, there were often changes on brain MRI imaging, and the patients often had trouble with executive function. “The effect of diastolic dysfunction on cognition has not been well characterized, so we wanted to look at this,” she said.
The investigators analyzed data from the Framingham Heart Study Offspring Cohort at examination 8, collected between 2005 and 2008. The study sample included 1,438 individuals older than 55 years who had undergone neuropsychological assessment and echocardiographic diastolic measurement. Systolic measurements were normal for the participants, and they did not currently have dementia, stroke, or other neurologic illness.
Results showed that increasing E/E’ ratio (the ratio of mitral peak velocity of early filling to early diastolic mitral annular velocity) indicated increasing diastolic dysfunction and was associated with an increase in the incidence of mild cognitive impairment (hazard ratio, 1.29; 95% confidence interval, 1.01-1.66; P < .043).
An increased E/E’ ratio was associated with increased executive function impairment in the “similarities” (beta, –0.29; P < .002) and “phonemic fluency” (–1.28; P < .001) tasks.
Participants with moderate to severe diastolic dysfunction were more impaired with respect to both similarities (–0.62; P < .046) and phonemic fluency (–2.60; P < .023).
Data from 1,217 participants showed that among those with mild diastolic dysfunction, there was a trend toward an increase in white matter hyperintensities (0.11; P < .105). For participants with moderate to severe diastolic dysfunction, white matter hyperintensities were increased (0.30; P < 0.001).
The results were unchanged after the researchers adjusted for many other predictors of cognitive decline affecting diastolic function.
The researchers conclude: “As cerebral small vessel disease clinically presents with executive dysfunction, these results align well.” They add that replication in additional cohorts and analyses of cognition in treatment trials of diastolic dysfunction are warranted.
Earlier interventions
Commenting on the study, Marco R. Di Tullio, MD, professor of medicine and Columbia University Medical Center, New York City, who is also studying the relationship between subclinical cardiac abnormalities and cognition, said: “This is a promising area of research, as it might allow us to uncover novel risk factors for cognitive decline at an early stage, before the development of clinically manifest cardiac disease, which might allow earlier interventions to decrease or delay the onset of cognitive decline.”
Dr. Di Tullio added that he would like to know more about the interaction between diastolic dysfunction, MRI abnormalities, and cognitive impairment risk. “In this study, MRI abnormalities and cognitive impairment are treated as separate outcomes, with diastolic dysfunction being the exposure for each of them. An additional analysis of the association between diastolic dysfunction and cognitive impairment stratified by presence or absence of brain MRI findings would have been interesting.”
Dr. Parker responded that this is an area of investigation. “We suspect that our cognitive findings would not be explained by any one MRI measure, though a comprehensive examination of MRI findings would be of benefit. The thought that there may be a reversible cardiac abnormality that does not have a structural brain imaging correlate on MRI is an interesting possibility,” she said.
Dr. Di Tullio also pointed out that at present, there is no specific treatment for diastolic dysfunction other than to address some the conditions that predispose to it, such as hypertension and atrial fibrillation.
“We completely agree that specific treatments are an area of investigation and that treatment is therefore targeted at associated modifiable conditions,” Dr. Parker replied.
With regard to more specific estimates of the prevalence of diastolic dysfunction, Dr. Parker cites another Framingham analysis that involved 2,355 persons without any prevalent cardiovascular conditions. That study found that diastolic dysfunction was rare until 50 years of age and then gradually increased with age.
About 5% of people in their 50s had mild diastolic dysfunction, and about 3% had moderate to severe diastolic dysfunction. Among persons in their 60s, about 18% had mild and 5% had severe diastolic dysfunction. Among persons in their 70s, mild diastolic dysfunction occurred in 35%, and moderate to severe disease was present in 18%; and in persons older than 80 years, nearly half had mild and about 20% had moderate to severe diastolic dysfunction.
Dr. Parker has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
a new study suggests. “We found people with worsening diastolic dysfunction have more white matter hyperintensities on brain imaging and greater difficulty with executive functioning, suggesting that diastolic dysfunction is a common modifiable risk factor for cognitive impairment,” said lead author Alicia S. Parker, MD. Dr. Parker is assistant professor of cognitive and behavioral neurology at the Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases, University of Texas Health, San Antonio.
“This is an entirely new finding. While there have been some small studies suggesting a link between diastolic dysfunction and a reduction in working memory, this is by far the largest dataset on this topic and the first study that has included brain imaging and neuropsychological measures,” she said.
“Diastolic dysfunction is very common in the older population, and we need to do more to find it and treat it to help prevent or reduce cognitive decline,” Dr. Parker added.
This research is being presented online as part of the 2020 American Academy of Neurology Science Highlights.
Dr. Parker explained that systolic dysfunction is known to have a major effect on cardiovascular outcomes and has been found to be associated with cognitive decline. Proposed mechanisms for cognitive decline in patients with systolic dysfunction include low cardiac output, embolic infarctions, and hypoxic changes, among others.
“There is increasing interest in analyzing the influence of diastolic dysfunction on cardiovascular outcomes, and the effects of diastolic dysfunction on cognition are not currently well delineated, which this study seeks to address,” she added.
“While these results are new, they are not surprising. In general, we are finding more and more that heart health is connected to brain health,” she commented.
Dr. Parker and her colleagues started the current research after noticing in clinic that among patients with significant diastolic dysfunction, there were often changes on brain MRI imaging, and the patients often had trouble with executive function. “The effect of diastolic dysfunction on cognition has not been well characterized, so we wanted to look at this,” she said.
The investigators analyzed data from the Framingham Heart Study Offspring Cohort at examination 8, collected between 2005 and 2008. The study sample included 1,438 individuals older than 55 years who had undergone neuropsychological assessment and echocardiographic diastolic measurement. Systolic measurements were normal for the participants, and they did not currently have dementia, stroke, or other neurologic illness.
Results showed that increasing E/E’ ratio (the ratio of mitral peak velocity of early filling to early diastolic mitral annular velocity) indicated increasing diastolic dysfunction and was associated with an increase in the incidence of mild cognitive impairment (hazard ratio, 1.29; 95% confidence interval, 1.01-1.66; P < .043).
An increased E/E’ ratio was associated with increased executive function impairment in the “similarities” (beta, –0.29; P < .002) and “phonemic fluency” (–1.28; P < .001) tasks.
Participants with moderate to severe diastolic dysfunction were more impaired with respect to both similarities (–0.62; P < .046) and phonemic fluency (–2.60; P < .023).
Data from 1,217 participants showed that among those with mild diastolic dysfunction, there was a trend toward an increase in white matter hyperintensities (0.11; P < .105). For participants with moderate to severe diastolic dysfunction, white matter hyperintensities were increased (0.30; P < 0.001).
The results were unchanged after the researchers adjusted for many other predictors of cognitive decline affecting diastolic function.
The researchers conclude: “As cerebral small vessel disease clinically presents with executive dysfunction, these results align well.” They add that replication in additional cohorts and analyses of cognition in treatment trials of diastolic dysfunction are warranted.
Earlier interventions
Commenting on the study, Marco R. Di Tullio, MD, professor of medicine and Columbia University Medical Center, New York City, who is also studying the relationship between subclinical cardiac abnormalities and cognition, said: “This is a promising area of research, as it might allow us to uncover novel risk factors for cognitive decline at an early stage, before the development of clinically manifest cardiac disease, which might allow earlier interventions to decrease or delay the onset of cognitive decline.”
Dr. Di Tullio added that he would like to know more about the interaction between diastolic dysfunction, MRI abnormalities, and cognitive impairment risk. “In this study, MRI abnormalities and cognitive impairment are treated as separate outcomes, with diastolic dysfunction being the exposure for each of them. An additional analysis of the association between diastolic dysfunction and cognitive impairment stratified by presence or absence of brain MRI findings would have been interesting.”
Dr. Parker responded that this is an area of investigation. “We suspect that our cognitive findings would not be explained by any one MRI measure, though a comprehensive examination of MRI findings would be of benefit. The thought that there may be a reversible cardiac abnormality that does not have a structural brain imaging correlate on MRI is an interesting possibility,” she said.
Dr. Di Tullio also pointed out that at present, there is no specific treatment for diastolic dysfunction other than to address some the conditions that predispose to it, such as hypertension and atrial fibrillation.
“We completely agree that specific treatments are an area of investigation and that treatment is therefore targeted at associated modifiable conditions,” Dr. Parker replied.
With regard to more specific estimates of the prevalence of diastolic dysfunction, Dr. Parker cites another Framingham analysis that involved 2,355 persons without any prevalent cardiovascular conditions. That study found that diastolic dysfunction was rare until 50 years of age and then gradually increased with age.
About 5% of people in their 50s had mild diastolic dysfunction, and about 3% had moderate to severe diastolic dysfunction. Among persons in their 60s, about 18% had mild and 5% had severe diastolic dysfunction. Among persons in their 70s, mild diastolic dysfunction occurred in 35%, and moderate to severe disease was present in 18%; and in persons older than 80 years, nearly half had mild and about 20% had moderate to severe diastolic dysfunction.
Dr. Parker has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
a new study suggests. “We found people with worsening diastolic dysfunction have more white matter hyperintensities on brain imaging and greater difficulty with executive functioning, suggesting that diastolic dysfunction is a common modifiable risk factor for cognitive impairment,” said lead author Alicia S. Parker, MD. Dr. Parker is assistant professor of cognitive and behavioral neurology at the Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases, University of Texas Health, San Antonio.
“This is an entirely new finding. While there have been some small studies suggesting a link between diastolic dysfunction and a reduction in working memory, this is by far the largest dataset on this topic and the first study that has included brain imaging and neuropsychological measures,” she said.
“Diastolic dysfunction is very common in the older population, and we need to do more to find it and treat it to help prevent or reduce cognitive decline,” Dr. Parker added.
This research is being presented online as part of the 2020 American Academy of Neurology Science Highlights.
Dr. Parker explained that systolic dysfunction is known to have a major effect on cardiovascular outcomes and has been found to be associated with cognitive decline. Proposed mechanisms for cognitive decline in patients with systolic dysfunction include low cardiac output, embolic infarctions, and hypoxic changes, among others.
“There is increasing interest in analyzing the influence of diastolic dysfunction on cardiovascular outcomes, and the effects of diastolic dysfunction on cognition are not currently well delineated, which this study seeks to address,” she added.
“While these results are new, they are not surprising. In general, we are finding more and more that heart health is connected to brain health,” she commented.
Dr. Parker and her colleagues started the current research after noticing in clinic that among patients with significant diastolic dysfunction, there were often changes on brain MRI imaging, and the patients often had trouble with executive function. “The effect of diastolic dysfunction on cognition has not been well characterized, so we wanted to look at this,” she said.
The investigators analyzed data from the Framingham Heart Study Offspring Cohort at examination 8, collected between 2005 and 2008. The study sample included 1,438 individuals older than 55 years who had undergone neuropsychological assessment and echocardiographic diastolic measurement. Systolic measurements were normal for the participants, and they did not currently have dementia, stroke, or other neurologic illness.
Results showed that increasing E/E’ ratio (the ratio of mitral peak velocity of early filling to early diastolic mitral annular velocity) indicated increasing diastolic dysfunction and was associated with an increase in the incidence of mild cognitive impairment (hazard ratio, 1.29; 95% confidence interval, 1.01-1.66; P < .043).
An increased E/E’ ratio was associated with increased executive function impairment in the “similarities” (beta, –0.29; P < .002) and “phonemic fluency” (–1.28; P < .001) tasks.
Participants with moderate to severe diastolic dysfunction were more impaired with respect to both similarities (–0.62; P < .046) and phonemic fluency (–2.60; P < .023).
Data from 1,217 participants showed that among those with mild diastolic dysfunction, there was a trend toward an increase in white matter hyperintensities (0.11; P < .105). For participants with moderate to severe diastolic dysfunction, white matter hyperintensities were increased (0.30; P < 0.001).
The results were unchanged after the researchers adjusted for many other predictors of cognitive decline affecting diastolic function.
The researchers conclude: “As cerebral small vessel disease clinically presents with executive dysfunction, these results align well.” They add that replication in additional cohorts and analyses of cognition in treatment trials of diastolic dysfunction are warranted.
Earlier interventions
Commenting on the study, Marco R. Di Tullio, MD, professor of medicine and Columbia University Medical Center, New York City, who is also studying the relationship between subclinical cardiac abnormalities and cognition, said: “This is a promising area of research, as it might allow us to uncover novel risk factors for cognitive decline at an early stage, before the development of clinically manifest cardiac disease, which might allow earlier interventions to decrease or delay the onset of cognitive decline.”
Dr. Di Tullio added that he would like to know more about the interaction between diastolic dysfunction, MRI abnormalities, and cognitive impairment risk. “In this study, MRI abnormalities and cognitive impairment are treated as separate outcomes, with diastolic dysfunction being the exposure for each of them. An additional analysis of the association between diastolic dysfunction and cognitive impairment stratified by presence or absence of brain MRI findings would have been interesting.”
Dr. Parker responded that this is an area of investigation. “We suspect that our cognitive findings would not be explained by any one MRI measure, though a comprehensive examination of MRI findings would be of benefit. The thought that there may be a reversible cardiac abnormality that does not have a structural brain imaging correlate on MRI is an interesting possibility,” she said.
Dr. Di Tullio also pointed out that at present, there is no specific treatment for diastolic dysfunction other than to address some the conditions that predispose to it, such as hypertension and atrial fibrillation.
“We completely agree that specific treatments are an area of investigation and that treatment is therefore targeted at associated modifiable conditions,” Dr. Parker replied.
With regard to more specific estimates of the prevalence of diastolic dysfunction, Dr. Parker cites another Framingham analysis that involved 2,355 persons without any prevalent cardiovascular conditions. That study found that diastolic dysfunction was rare until 50 years of age and then gradually increased with age.
About 5% of people in their 50s had mild diastolic dysfunction, and about 3% had moderate to severe diastolic dysfunction. Among persons in their 60s, about 18% had mild and 5% had severe diastolic dysfunction. Among persons in their 70s, mild diastolic dysfunction occurred in 35%, and moderate to severe disease was present in 18%; and in persons older than 80 years, nearly half had mild and about 20% had moderate to severe diastolic dysfunction.
Dr. Parker has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
FOURIER: Evolocumab follow-up shows no cognitive adverse effects
Treatment with a PCSK9 inhibitor, as well as achieving dramatically lowered cholesterol levels, did not mess with patients’ minds. Results from a cognition self-assessment completed by more than 22,000 patients when they finished participation in the FOURIER pivotal outcomes trial for evolocumab showed no signal of mental harm from either treatment with this PCSK9 inhibitor or from reaching a serum level of low-density lipoprotein cholesterol (LDL-C) of less than 20 mg/dL.
“We observed that patients treated with evolocumab, as well as those who achieved progressively very low LDL-C at 4 weeks in the FOURIER trial, had similar self-reported cognition in comparison with those receiving placebo and those with higher achieved LDL-C levels,” wrote a team of researchers from the trial in an article published online on May 4 (J Am Coll Cardiol. 2020 May 12;75[18]: 2283-93). “These data confirm the neurocognitive safety of intensive LDL-C reduction with evolocumab while reducing recurrent CV [cardiovascular] events in high-risk patients, and suggest that very low achieved LDL-C levels may be safely targeted for high-risk patients.”
The findings added to prior results documenting the cognitive safety of evolocumab (Repatha) from a much smaller FOURIER substudy that involved more intensive testing, the EBBINGHAUS (Evaluating PCSK9 Binding Antibody Influence on Cognitive Health in High Cardiovascular Risk Subjects) study with 1,204 patients drawn from the broader study and tested after a median 19 months on treatment (N Engl J Med. 2017 Aug 17;377[17]: 633-43), as well as reports of neurocognitive safety for the other U.S. approved PCSK9 (proprotein convertase subtilisin kexin 9) inhibitor, alirocumab (Praluent) (N Engl J Med. 2015 Apr 16;372[16]:1489-99), various statins (J Gen Intern Med. 2015 Mar;30[3]: 348-58), and a third type of LDL-C–lowering agent, ezetimibe (JAMA Cardiol. 2017 May;2[5]:547-55).
Despite this evidence from across several drug classes that all cut LDL-C a long-standing but unsubstantiated belief persists among some that lipid lowering, especially by statins, blunts mental function, misinformation that’s easy to find on the Internet. “I estimate that about 20% of patients prescribed a statin won’t take it because of something they’ve heard” including that statins make you stupid. “It’s hard to undo that,” said Robert P. Giugliano, MD, a cardiologist at Brigham and Women’s Hospital in Boston and senior author for the new FOURIER study as well as for EBBINGHAUS. The same stigma has not gained nearly as much traction for PCSK9 inhibitors, however, and Dr. Giugliano said he has also recently sensed what may be a downtrend in statin apprehension.
“The information added by this study is very important,” commented Massimo R. Mannarino, MD, an atherosclerotic disease researcher at the University of Perugia (Italy). “The prejudice and misinformation regarding possible side effects of statins among patients and also some physicians unfortunately remains very strong today,” he said in an interview. “My impression is that PCSK9 inhibitors are less affected by this negative bias and are seen as a safer alternative to statins.” Concerns about PCSK9 inhibitors have especially focused on “the possible risks from very low cholesterol levels on the brain.” The evidence from both studies and clinical experience “allows for a very positive opinion about the efficacy and safety of PCSK9 inhibitors, although the long-term effects still require a few more years of observation,” said Dr. Mannarino, who led a review of the evidence that clears this class from links to neurocognitive loss (J Clin Lipid. 2018 Sep 1;12[5]:1123-32).
FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) randomized 27,564 patients with atherosclerotic cardiovascular disease and elevated LDL cholesterol despite maximally tolerated standard treatment. Treatment with evolocumab for a median of 2.2 years resulted in a statistically significant 15% reduction in the study’s primary efficacy endpoint, compared with placebo (N Engl J Med. 2017 May 4;376[18]:1713-22), and led to the drug receiving an indication for lowering rates of MI, stroke, and symptom-driven coronary revascularization.
The prespecified substudy reported by Dr. Giugliano and his associates focused on a 23-question, validated, self-assessment survey of cognitive function completed by 22,655 of the FOURIER patients (82%). The more than 4,900 other patients in the study who did not complete the survey had modestly higher prevalence rates of various comorbidities at baseline, and also higher rates of adverse outcomes during follow-up, and in many cases these adverse outcome may have contributed to these patients not being able to complete their end-of-study cognitive assessment. For example, almost a quarter of the patients who did not complete their end-of-study cognitive assessment failed to do so because they had already died.
Overall, the prevalence of patients indicating a cognitive decline was virtually identical among 11,363 patients who had been maintained on evolocumab, with a 3.7% rate, and the 11,292 patients in the placebo group, with a 3.6% rate. When analyzed by achieved level of LDL-C after 4 weeks on treatment, the 2,338 patients with a level below 20 mg/dL had a 3.8% rate of self-reported cognitive loss, compared with a 4.5% rate among 3,613 patients who had an LDL-C level of at least 100 mg/dL when measured 4 weeks into the study.
One of the strengths of the new cognitive analysis is that, although it did not use the more sophisticated assessment tests employed on fewer patients in the EBBINGHAUS substudy, it used the Everyday Cognition scale (Neuropsychiatry. 2008 Jul;22[4]: 531-44). “We asked patients what they have experienced, and in the end that is what’s important, so this adds to the neurocognitive testing,” run in EBBINGHAUS, Dr. Giugliano said in an interview.
“The neurocognitive results in the present study were self-reported, and that might be a limitation, as it is less specific and objective, but it is also a strength, as it could be more sensitive” especially for a “nocebo effect common to all lipid-lowering drugs linked to the bad reputation historically attributed to statins,” Dr. Mannarino said.
Should the new FOURIER data “be interpreted as definitive evidence that intensive LDL-C lowering with PCSK9 monoclonal antibodies has no major harmful cognitive effects, at least over a period of 3 years? The answer appears to be a qualified yes, but with three important caveats,” Jennifer G. Robinson, MD, a professor of epidemiology at the University of Iowa College of Public Health in Iowa City, said in an editorial that accompanied the new report (J Am Coll Cardiol. 2020 May 12;75[18]:2294-6). Her three caveats are the missing 18% of patients who never took the end-of-study assessment, the relative paucity of patients at very advanced age in FOURIER, in which patients averaged 62.5 years old, and the exclusion from FOURIER of patients with a history of hemorrhagic stroke. Dr. Robinson also cited the 2.2 year median follow-up as leaving unsettled the potential cognitive impact of longer treatment.
In response, Dr. Giugliano noted that the very large size of FOURIER and the 22,655 patients who completed their survey provided substantial numbers of patients to address some of these concerns in robust subgroup analyses. For example, the new report showed no signal of excess cognitive complaints with evolocumab treatment among 1,999 patients who were at least 75 years old when entering the study, or in more than 5,000 patients with a history of cerebrovascular disease at baseline, or in 1,990 patients with a history of a nonstroke neurologic disease. In addition, while he conceded that the 18% of patients not accounted for in the new study placed some limits on generalizability of the findings, he also maintained that this unavoidable failure to collect data from a modest percentage of patients doesn’t scuttle the overarching signal of cognitive safety for most patients. And regarding the duration of treatment monitored, he noted that 5-year follow-up cognitive assessments are planned.
FOURIER was sponsored by Amgen, the company that markets evolocumab (Repatha). Dr. Giugliano has received personal fees and research support from Amgen and from several other companies. Dr. Mannarino had no disclosures. Dr. Robinson has been a consultant to The Medicines Company, Novartis, and Pfizer, and she has received research funding to her institution from Amgen and several other companies.
SOURCE: Gencer B et al. J Am Coll Cardiol. 2020 May 12;75[18]:2283-93.
Correction: Dr. Giugliano's name was misspelled in an earlier version of this article.
Treatment with a PCSK9 inhibitor, as well as achieving dramatically lowered cholesterol levels, did not mess with patients’ minds. Results from a cognition self-assessment completed by more than 22,000 patients when they finished participation in the FOURIER pivotal outcomes trial for evolocumab showed no signal of mental harm from either treatment with this PCSK9 inhibitor or from reaching a serum level of low-density lipoprotein cholesterol (LDL-C) of less than 20 mg/dL.
“We observed that patients treated with evolocumab, as well as those who achieved progressively very low LDL-C at 4 weeks in the FOURIER trial, had similar self-reported cognition in comparison with those receiving placebo and those with higher achieved LDL-C levels,” wrote a team of researchers from the trial in an article published online on May 4 (J Am Coll Cardiol. 2020 May 12;75[18]: 2283-93). “These data confirm the neurocognitive safety of intensive LDL-C reduction with evolocumab while reducing recurrent CV [cardiovascular] events in high-risk patients, and suggest that very low achieved LDL-C levels may be safely targeted for high-risk patients.”
The findings added to prior results documenting the cognitive safety of evolocumab (Repatha) from a much smaller FOURIER substudy that involved more intensive testing, the EBBINGHAUS (Evaluating PCSK9 Binding Antibody Influence on Cognitive Health in High Cardiovascular Risk Subjects) study with 1,204 patients drawn from the broader study and tested after a median 19 months on treatment (N Engl J Med. 2017 Aug 17;377[17]: 633-43), as well as reports of neurocognitive safety for the other U.S. approved PCSK9 (proprotein convertase subtilisin kexin 9) inhibitor, alirocumab (Praluent) (N Engl J Med. 2015 Apr 16;372[16]:1489-99), various statins (J Gen Intern Med. 2015 Mar;30[3]: 348-58), and a third type of LDL-C–lowering agent, ezetimibe (JAMA Cardiol. 2017 May;2[5]:547-55).
Despite this evidence from across several drug classes that all cut LDL-C a long-standing but unsubstantiated belief persists among some that lipid lowering, especially by statins, blunts mental function, misinformation that’s easy to find on the Internet. “I estimate that about 20% of patients prescribed a statin won’t take it because of something they’ve heard” including that statins make you stupid. “It’s hard to undo that,” said Robert P. Giugliano, MD, a cardiologist at Brigham and Women’s Hospital in Boston and senior author for the new FOURIER study as well as for EBBINGHAUS. The same stigma has not gained nearly as much traction for PCSK9 inhibitors, however, and Dr. Giugliano said he has also recently sensed what may be a downtrend in statin apprehension.
“The information added by this study is very important,” commented Massimo R. Mannarino, MD, an atherosclerotic disease researcher at the University of Perugia (Italy). “The prejudice and misinformation regarding possible side effects of statins among patients and also some physicians unfortunately remains very strong today,” he said in an interview. “My impression is that PCSK9 inhibitors are less affected by this negative bias and are seen as a safer alternative to statins.” Concerns about PCSK9 inhibitors have especially focused on “the possible risks from very low cholesterol levels on the brain.” The evidence from both studies and clinical experience “allows for a very positive opinion about the efficacy and safety of PCSK9 inhibitors, although the long-term effects still require a few more years of observation,” said Dr. Mannarino, who led a review of the evidence that clears this class from links to neurocognitive loss (J Clin Lipid. 2018 Sep 1;12[5]:1123-32).
FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) randomized 27,564 patients with atherosclerotic cardiovascular disease and elevated LDL cholesterol despite maximally tolerated standard treatment. Treatment with evolocumab for a median of 2.2 years resulted in a statistically significant 15% reduction in the study’s primary efficacy endpoint, compared with placebo (N Engl J Med. 2017 May 4;376[18]:1713-22), and led to the drug receiving an indication for lowering rates of MI, stroke, and symptom-driven coronary revascularization.
The prespecified substudy reported by Dr. Giugliano and his associates focused on a 23-question, validated, self-assessment survey of cognitive function completed by 22,655 of the FOURIER patients (82%). The more than 4,900 other patients in the study who did not complete the survey had modestly higher prevalence rates of various comorbidities at baseline, and also higher rates of adverse outcomes during follow-up, and in many cases these adverse outcome may have contributed to these patients not being able to complete their end-of-study cognitive assessment. For example, almost a quarter of the patients who did not complete their end-of-study cognitive assessment failed to do so because they had already died.
Overall, the prevalence of patients indicating a cognitive decline was virtually identical among 11,363 patients who had been maintained on evolocumab, with a 3.7% rate, and the 11,292 patients in the placebo group, with a 3.6% rate. When analyzed by achieved level of LDL-C after 4 weeks on treatment, the 2,338 patients with a level below 20 mg/dL had a 3.8% rate of self-reported cognitive loss, compared with a 4.5% rate among 3,613 patients who had an LDL-C level of at least 100 mg/dL when measured 4 weeks into the study.
One of the strengths of the new cognitive analysis is that, although it did not use the more sophisticated assessment tests employed on fewer patients in the EBBINGHAUS substudy, it used the Everyday Cognition scale (Neuropsychiatry. 2008 Jul;22[4]: 531-44). “We asked patients what they have experienced, and in the end that is what’s important, so this adds to the neurocognitive testing,” run in EBBINGHAUS, Dr. Giugliano said in an interview.
“The neurocognitive results in the present study were self-reported, and that might be a limitation, as it is less specific and objective, but it is also a strength, as it could be more sensitive” especially for a “nocebo effect common to all lipid-lowering drugs linked to the bad reputation historically attributed to statins,” Dr. Mannarino said.
Should the new FOURIER data “be interpreted as definitive evidence that intensive LDL-C lowering with PCSK9 monoclonal antibodies has no major harmful cognitive effects, at least over a period of 3 years? The answer appears to be a qualified yes, but with three important caveats,” Jennifer G. Robinson, MD, a professor of epidemiology at the University of Iowa College of Public Health in Iowa City, said in an editorial that accompanied the new report (J Am Coll Cardiol. 2020 May 12;75[18]:2294-6). Her three caveats are the missing 18% of patients who never took the end-of-study assessment, the relative paucity of patients at very advanced age in FOURIER, in which patients averaged 62.5 years old, and the exclusion from FOURIER of patients with a history of hemorrhagic stroke. Dr. Robinson also cited the 2.2 year median follow-up as leaving unsettled the potential cognitive impact of longer treatment.
In response, Dr. Giugliano noted that the very large size of FOURIER and the 22,655 patients who completed their survey provided substantial numbers of patients to address some of these concerns in robust subgroup analyses. For example, the new report showed no signal of excess cognitive complaints with evolocumab treatment among 1,999 patients who were at least 75 years old when entering the study, or in more than 5,000 patients with a history of cerebrovascular disease at baseline, or in 1,990 patients with a history of a nonstroke neurologic disease. In addition, while he conceded that the 18% of patients not accounted for in the new study placed some limits on generalizability of the findings, he also maintained that this unavoidable failure to collect data from a modest percentage of patients doesn’t scuttle the overarching signal of cognitive safety for most patients. And regarding the duration of treatment monitored, he noted that 5-year follow-up cognitive assessments are planned.
FOURIER was sponsored by Amgen, the company that markets evolocumab (Repatha). Dr. Giugliano has received personal fees and research support from Amgen and from several other companies. Dr. Mannarino had no disclosures. Dr. Robinson has been a consultant to The Medicines Company, Novartis, and Pfizer, and she has received research funding to her institution from Amgen and several other companies.
SOURCE: Gencer B et al. J Am Coll Cardiol. 2020 May 12;75[18]:2283-93.
Correction: Dr. Giugliano's name was misspelled in an earlier version of this article.
Treatment with a PCSK9 inhibitor, as well as achieving dramatically lowered cholesterol levels, did not mess with patients’ minds. Results from a cognition self-assessment completed by more than 22,000 patients when they finished participation in the FOURIER pivotal outcomes trial for evolocumab showed no signal of mental harm from either treatment with this PCSK9 inhibitor or from reaching a serum level of low-density lipoprotein cholesterol (LDL-C) of less than 20 mg/dL.
“We observed that patients treated with evolocumab, as well as those who achieved progressively very low LDL-C at 4 weeks in the FOURIER trial, had similar self-reported cognition in comparison with those receiving placebo and those with higher achieved LDL-C levels,” wrote a team of researchers from the trial in an article published online on May 4 (J Am Coll Cardiol. 2020 May 12;75[18]: 2283-93). “These data confirm the neurocognitive safety of intensive LDL-C reduction with evolocumab while reducing recurrent CV [cardiovascular] events in high-risk patients, and suggest that very low achieved LDL-C levels may be safely targeted for high-risk patients.”
The findings added to prior results documenting the cognitive safety of evolocumab (Repatha) from a much smaller FOURIER substudy that involved more intensive testing, the EBBINGHAUS (Evaluating PCSK9 Binding Antibody Influence on Cognitive Health in High Cardiovascular Risk Subjects) study with 1,204 patients drawn from the broader study and tested after a median 19 months on treatment (N Engl J Med. 2017 Aug 17;377[17]: 633-43), as well as reports of neurocognitive safety for the other U.S. approved PCSK9 (proprotein convertase subtilisin kexin 9) inhibitor, alirocumab (Praluent) (N Engl J Med. 2015 Apr 16;372[16]:1489-99), various statins (J Gen Intern Med. 2015 Mar;30[3]: 348-58), and a third type of LDL-C–lowering agent, ezetimibe (JAMA Cardiol. 2017 May;2[5]:547-55).
Despite this evidence from across several drug classes that all cut LDL-C a long-standing but unsubstantiated belief persists among some that lipid lowering, especially by statins, blunts mental function, misinformation that’s easy to find on the Internet. “I estimate that about 20% of patients prescribed a statin won’t take it because of something they’ve heard” including that statins make you stupid. “It’s hard to undo that,” said Robert P. Giugliano, MD, a cardiologist at Brigham and Women’s Hospital in Boston and senior author for the new FOURIER study as well as for EBBINGHAUS. The same stigma has not gained nearly as much traction for PCSK9 inhibitors, however, and Dr. Giugliano said he has also recently sensed what may be a downtrend in statin apprehension.
“The information added by this study is very important,” commented Massimo R. Mannarino, MD, an atherosclerotic disease researcher at the University of Perugia (Italy). “The prejudice and misinformation regarding possible side effects of statins among patients and also some physicians unfortunately remains very strong today,” he said in an interview. “My impression is that PCSK9 inhibitors are less affected by this negative bias and are seen as a safer alternative to statins.” Concerns about PCSK9 inhibitors have especially focused on “the possible risks from very low cholesterol levels on the brain.” The evidence from both studies and clinical experience “allows for a very positive opinion about the efficacy and safety of PCSK9 inhibitors, although the long-term effects still require a few more years of observation,” said Dr. Mannarino, who led a review of the evidence that clears this class from links to neurocognitive loss (J Clin Lipid. 2018 Sep 1;12[5]:1123-32).
FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) randomized 27,564 patients with atherosclerotic cardiovascular disease and elevated LDL cholesterol despite maximally tolerated standard treatment. Treatment with evolocumab for a median of 2.2 years resulted in a statistically significant 15% reduction in the study’s primary efficacy endpoint, compared with placebo (N Engl J Med. 2017 May 4;376[18]:1713-22), and led to the drug receiving an indication for lowering rates of MI, stroke, and symptom-driven coronary revascularization.
The prespecified substudy reported by Dr. Giugliano and his associates focused on a 23-question, validated, self-assessment survey of cognitive function completed by 22,655 of the FOURIER patients (82%). The more than 4,900 other patients in the study who did not complete the survey had modestly higher prevalence rates of various comorbidities at baseline, and also higher rates of adverse outcomes during follow-up, and in many cases these adverse outcome may have contributed to these patients not being able to complete their end-of-study cognitive assessment. For example, almost a quarter of the patients who did not complete their end-of-study cognitive assessment failed to do so because they had already died.
Overall, the prevalence of patients indicating a cognitive decline was virtually identical among 11,363 patients who had been maintained on evolocumab, with a 3.7% rate, and the 11,292 patients in the placebo group, with a 3.6% rate. When analyzed by achieved level of LDL-C after 4 weeks on treatment, the 2,338 patients with a level below 20 mg/dL had a 3.8% rate of self-reported cognitive loss, compared with a 4.5% rate among 3,613 patients who had an LDL-C level of at least 100 mg/dL when measured 4 weeks into the study.
One of the strengths of the new cognitive analysis is that, although it did not use the more sophisticated assessment tests employed on fewer patients in the EBBINGHAUS substudy, it used the Everyday Cognition scale (Neuropsychiatry. 2008 Jul;22[4]: 531-44). “We asked patients what they have experienced, and in the end that is what’s important, so this adds to the neurocognitive testing,” run in EBBINGHAUS, Dr. Giugliano said in an interview.
“The neurocognitive results in the present study were self-reported, and that might be a limitation, as it is less specific and objective, but it is also a strength, as it could be more sensitive” especially for a “nocebo effect common to all lipid-lowering drugs linked to the bad reputation historically attributed to statins,” Dr. Mannarino said.
Should the new FOURIER data “be interpreted as definitive evidence that intensive LDL-C lowering with PCSK9 monoclonal antibodies has no major harmful cognitive effects, at least over a period of 3 years? The answer appears to be a qualified yes, but with three important caveats,” Jennifer G. Robinson, MD, a professor of epidemiology at the University of Iowa College of Public Health in Iowa City, said in an editorial that accompanied the new report (J Am Coll Cardiol. 2020 May 12;75[18]:2294-6). Her three caveats are the missing 18% of patients who never took the end-of-study assessment, the relative paucity of patients at very advanced age in FOURIER, in which patients averaged 62.5 years old, and the exclusion from FOURIER of patients with a history of hemorrhagic stroke. Dr. Robinson also cited the 2.2 year median follow-up as leaving unsettled the potential cognitive impact of longer treatment.
In response, Dr. Giugliano noted that the very large size of FOURIER and the 22,655 patients who completed their survey provided substantial numbers of patients to address some of these concerns in robust subgroup analyses. For example, the new report showed no signal of excess cognitive complaints with evolocumab treatment among 1,999 patients who were at least 75 years old when entering the study, or in more than 5,000 patients with a history of cerebrovascular disease at baseline, or in 1,990 patients with a history of a nonstroke neurologic disease. In addition, while he conceded that the 18% of patients not accounted for in the new study placed some limits on generalizability of the findings, he also maintained that this unavoidable failure to collect data from a modest percentage of patients doesn’t scuttle the overarching signal of cognitive safety for most patients. And regarding the duration of treatment monitored, he noted that 5-year follow-up cognitive assessments are planned.
FOURIER was sponsored by Amgen, the company that markets evolocumab (Repatha). Dr. Giugliano has received personal fees and research support from Amgen and from several other companies. Dr. Mannarino had no disclosures. Dr. Robinson has been a consultant to The Medicines Company, Novartis, and Pfizer, and she has received research funding to her institution from Amgen and several other companies.
SOURCE: Gencer B et al. J Am Coll Cardiol. 2020 May 12;75[18]:2283-93.
Correction: Dr. Giugliano's name was misspelled in an earlier version of this article.
FROM JACC
Key clinical point: A cognition survey of a large number of trial participants showed no signal of adverse effects from evolocumab treatment.
Major finding: Survey results showed cognitive compromise in 3.7% of patients on evolocumab and in 3.6% control patients on placebo.
Study details:
Disclosures: FOURIER was sponsored by Amgen, the company that markets evolocumab (Repatha). Dr. Guigliano has received personal fees and research support from Amgen and from several other companies.
Source: Gencer B et al. J Am Coll Cardiol. 2020 May 12;75[18]:2283-93.
Cautionary findings on acquired immunodeficiency from anti-CD20 MS therapy
, Brandi L. Vollmer, MPH, reported online as part of the 2020 American Academy of Neurology Science Highlights.
The hypogammaglobulinemia was preceded by an IgM of 40 mg/dL or less in 35% of cases and was accompanied by concurrent development of low IgM in another 39%, added Ms. Vollmer, a professional research assistant at the Rocky Mountain Multiple Sclerosis Center at Anschutz Medical Campus, University of Colorado, Denver.
She presented a retrospective study of 527 randomly selected MS patients and another 17 with neuromyelitis optica spectrum disorder who averaged 44 years of age and a 9.2-year disease duration upon commencing rituximab (Rituxan) with close laboratory monitoring. Their mean cumulative rituximab dose during a mean 30.2 months of therapy was 3,312 mg. Ninety-six MS patients eventually switched to ocrelizumab (Ocrevus), accumulating a total dose of 1,175 mg of that anti-CD20 humanized monoclonal antibody.
Absolute lymphocyte count dropped to 500 cells/mm3 or lower in 10.4% of patients at a mean of 11.3 months into anti-CD20 therapy. Low immunoglobulins came later: The mean time to onset of low IgM in affected patients was 19.7 months, and hypogammaglobulinemia, as defined by an IgG of 500 mg/dL or less, occurred at a mean of 36.1 months. Higher cumulative doses of anti-CD20 agents were associated with increased likelihood of hypogammaglobulinemia.
Asked to comment on the research findings, neurologist Nida Laurin, MD, said the Colorado study provides helpful insights into the timing of onset of acquired immunodeficiency in patients on B-cell-targeted therapy.
“This paper informs us that we should monitor our patients much closer for signs of hypogammaglobulinemia and lymphopenia starting with year 2 on therapy, and switch treatment when the threshold is reached. I do expect production of gamma globulins and lymphocytes to recover with discontinuation of anti-CD20 therapy, maybe over a period of 6-10 months. It might also recover with lower-dose therapy because the effect on B cells is dose-dependent,” observed Dr. Laurin, an MS specialist at the Banner Health–University Medicine Neuroscience Institute in Phoenix and the University of Arizona in Tucson.
Her colleague Barry Hendin, MD, noted that there is no consensus regarding the best response to all these changes.
“Some clinicians add IVIG, some change therapies, and some observe only,” said Dr. Hendin, a neurologist at Banner Health–University Medical Center, Phoenix, and clinical professor of neurology at the University of Arizona in Tucson.
However, Dr. Laurin asserted that it would be a mistake for physicians and patients to shrug off anti-CD20 therapy–induced lymphopenia in light of studies demonstrating that lymphopenia and older age are two main risk factors for progressive multifocal leukoencephalopathy in patients on disease-modifying therapies.
“More cases of PML can be expected with continuous use of anti-CD20 therapies if lymphopenia is ignored,” she cautioned.
Depressed levels of IgM and IgG have been associated with increased risk of serious infections. In light of the COVID-19 pandemic and the eventual prospect of a vaccine, it is especially important to avoid putting patients with MS in harm’s way via treatment-induced acquired immunodeficiency, Dr. Laurin said.
Ms. Vollmer reported having no financial conflicts regarding her study. Dr. Laurin reported serving as a speaker or consultant for Alexion, Allergan, Biogen, Bristol-Myers Squibb, EMD Serono, Genentech, Lundbeck, and Sanofi Genzyme. Dr. Hendin serves as a consultant to Biogen, Genentech, Genzyme, EMD Serono, Novartis, and Bristol-Myers Squibb.
SOURCE: Vollmer BL et al. AAN 2020. Abstract S29.002.
, Brandi L. Vollmer, MPH, reported online as part of the 2020 American Academy of Neurology Science Highlights.
The hypogammaglobulinemia was preceded by an IgM of 40 mg/dL or less in 35% of cases and was accompanied by concurrent development of low IgM in another 39%, added Ms. Vollmer, a professional research assistant at the Rocky Mountain Multiple Sclerosis Center at Anschutz Medical Campus, University of Colorado, Denver.
She presented a retrospective study of 527 randomly selected MS patients and another 17 with neuromyelitis optica spectrum disorder who averaged 44 years of age and a 9.2-year disease duration upon commencing rituximab (Rituxan) with close laboratory monitoring. Their mean cumulative rituximab dose during a mean 30.2 months of therapy was 3,312 mg. Ninety-six MS patients eventually switched to ocrelizumab (Ocrevus), accumulating a total dose of 1,175 mg of that anti-CD20 humanized monoclonal antibody.
Absolute lymphocyte count dropped to 500 cells/mm3 or lower in 10.4% of patients at a mean of 11.3 months into anti-CD20 therapy. Low immunoglobulins came later: The mean time to onset of low IgM in affected patients was 19.7 months, and hypogammaglobulinemia, as defined by an IgG of 500 mg/dL or less, occurred at a mean of 36.1 months. Higher cumulative doses of anti-CD20 agents were associated with increased likelihood of hypogammaglobulinemia.
Asked to comment on the research findings, neurologist Nida Laurin, MD, said the Colorado study provides helpful insights into the timing of onset of acquired immunodeficiency in patients on B-cell-targeted therapy.
“This paper informs us that we should monitor our patients much closer for signs of hypogammaglobulinemia and lymphopenia starting with year 2 on therapy, and switch treatment when the threshold is reached. I do expect production of gamma globulins and lymphocytes to recover with discontinuation of anti-CD20 therapy, maybe over a period of 6-10 months. It might also recover with lower-dose therapy because the effect on B cells is dose-dependent,” observed Dr. Laurin, an MS specialist at the Banner Health–University Medicine Neuroscience Institute in Phoenix and the University of Arizona in Tucson.
Her colleague Barry Hendin, MD, noted that there is no consensus regarding the best response to all these changes.
“Some clinicians add IVIG, some change therapies, and some observe only,” said Dr. Hendin, a neurologist at Banner Health–University Medical Center, Phoenix, and clinical professor of neurology at the University of Arizona in Tucson.
However, Dr. Laurin asserted that it would be a mistake for physicians and patients to shrug off anti-CD20 therapy–induced lymphopenia in light of studies demonstrating that lymphopenia and older age are two main risk factors for progressive multifocal leukoencephalopathy in patients on disease-modifying therapies.
“More cases of PML can be expected with continuous use of anti-CD20 therapies if lymphopenia is ignored,” she cautioned.
Depressed levels of IgM and IgG have been associated with increased risk of serious infections. In light of the COVID-19 pandemic and the eventual prospect of a vaccine, it is especially important to avoid putting patients with MS in harm’s way via treatment-induced acquired immunodeficiency, Dr. Laurin said.
Ms. Vollmer reported having no financial conflicts regarding her study. Dr. Laurin reported serving as a speaker or consultant for Alexion, Allergan, Biogen, Bristol-Myers Squibb, EMD Serono, Genentech, Lundbeck, and Sanofi Genzyme. Dr. Hendin serves as a consultant to Biogen, Genentech, Genzyme, EMD Serono, Novartis, and Bristol-Myers Squibb.
SOURCE: Vollmer BL et al. AAN 2020. Abstract S29.002.
, Brandi L. Vollmer, MPH, reported online as part of the 2020 American Academy of Neurology Science Highlights.
The hypogammaglobulinemia was preceded by an IgM of 40 mg/dL or less in 35% of cases and was accompanied by concurrent development of low IgM in another 39%, added Ms. Vollmer, a professional research assistant at the Rocky Mountain Multiple Sclerosis Center at Anschutz Medical Campus, University of Colorado, Denver.
She presented a retrospective study of 527 randomly selected MS patients and another 17 with neuromyelitis optica spectrum disorder who averaged 44 years of age and a 9.2-year disease duration upon commencing rituximab (Rituxan) with close laboratory monitoring. Their mean cumulative rituximab dose during a mean 30.2 months of therapy was 3,312 mg. Ninety-six MS patients eventually switched to ocrelizumab (Ocrevus), accumulating a total dose of 1,175 mg of that anti-CD20 humanized monoclonal antibody.
Absolute lymphocyte count dropped to 500 cells/mm3 or lower in 10.4% of patients at a mean of 11.3 months into anti-CD20 therapy. Low immunoglobulins came later: The mean time to onset of low IgM in affected patients was 19.7 months, and hypogammaglobulinemia, as defined by an IgG of 500 mg/dL or less, occurred at a mean of 36.1 months. Higher cumulative doses of anti-CD20 agents were associated with increased likelihood of hypogammaglobulinemia.
Asked to comment on the research findings, neurologist Nida Laurin, MD, said the Colorado study provides helpful insights into the timing of onset of acquired immunodeficiency in patients on B-cell-targeted therapy.
“This paper informs us that we should monitor our patients much closer for signs of hypogammaglobulinemia and lymphopenia starting with year 2 on therapy, and switch treatment when the threshold is reached. I do expect production of gamma globulins and lymphocytes to recover with discontinuation of anti-CD20 therapy, maybe over a period of 6-10 months. It might also recover with lower-dose therapy because the effect on B cells is dose-dependent,” observed Dr. Laurin, an MS specialist at the Banner Health–University Medicine Neuroscience Institute in Phoenix and the University of Arizona in Tucson.
Her colleague Barry Hendin, MD, noted that there is no consensus regarding the best response to all these changes.
“Some clinicians add IVIG, some change therapies, and some observe only,” said Dr. Hendin, a neurologist at Banner Health–University Medical Center, Phoenix, and clinical professor of neurology at the University of Arizona in Tucson.
However, Dr. Laurin asserted that it would be a mistake for physicians and patients to shrug off anti-CD20 therapy–induced lymphopenia in light of studies demonstrating that lymphopenia and older age are two main risk factors for progressive multifocal leukoencephalopathy in patients on disease-modifying therapies.
“More cases of PML can be expected with continuous use of anti-CD20 therapies if lymphopenia is ignored,” she cautioned.
Depressed levels of IgM and IgG have been associated with increased risk of serious infections. In light of the COVID-19 pandemic and the eventual prospect of a vaccine, it is especially important to avoid putting patients with MS in harm’s way via treatment-induced acquired immunodeficiency, Dr. Laurin said.
Ms. Vollmer reported having no financial conflicts regarding her study. Dr. Laurin reported serving as a speaker or consultant for Alexion, Allergan, Biogen, Bristol-Myers Squibb, EMD Serono, Genentech, Lundbeck, and Sanofi Genzyme. Dr. Hendin serves as a consultant to Biogen, Genentech, Genzyme, EMD Serono, Novartis, and Bristol-Myers Squibb.
SOURCE: Vollmer BL et al. AAN 2020. Abstract S29.002.
REPORTING FROM AAN 2020
Five-year siponimod data support early MS treatment
Among and had a lower annualized relapse rate up to 5 years later, compared with patients who initially received placebo and switched to siponimod. This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Benefits of siponimod gained during the controlled period were “sustained for up to 5 years, suggesting a continuous effect of siponimod and underlining the advantages of early treatment initiation with siponimod,” the researchers said. Incidence rates of adverse events during the extension study were consistent with those during the controlled treatment period.
The results “highlight the critical importance of early treatment intervention ... to ensure the best possible long-term outcomes for patients with MS who are experiencing progression,” study investigator Bruce Cree, MD, PhD, said in a news release.
“It’s never too early to stay ahead of progression in MS, since the early identification of physical and cognitive changes – even subtle ones – can indicate MS disease progression and therefore allow for timely intervention.” said Dr. Cree, who is clinical research director and George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California, San Francisco.
Siponimod, marketed as Mayzent, is a sphingosine 1-phosphate receptor modulator that selectively binds to S1P1 and S1P5 receptors. The oral drug was approved by the Food and Drug Administration in 2019 for the treatment of relapsing forms of MS, including clinically isolated syndrome, relapsing remitting disease, and active secondary progressive disease in adults.
To assess the long-term efficacy and safety of siponimod in patients with secondary progressive MS, Dr. Cree and colleagues analyzed data from patients in the controlled and extension parts of the EXPAND trial. Patients could have had been in the study for as long as 5 years at the data cutoff in April 2019. Efficacy analyses included time to 3-month confirmed disability progression on the Expanded Disability Status Scale (EDSS), time to 6-month confirmed disability progression, time to 6-month confirmed worsening of 4 or more points on the Symbol Digit Modalities Test (SDMT), and annualized relapse rate. In EXPAND, the researchers defined confirmed disability progression as a 1-point increase in EDSS if the baseline score was 3.0-5.0, or a 0.5-point increase if the baseline score was 5.5-6.5.
“Of the 1,224 (74% of 1,651 randomized) patients entering the extension, 878 (72%) were ongoing,” the researchers reported. Patients who received siponimod continuously were less likely to experience 3-month confirmed disability progression and 6-month confirmed disability progression, relative to patients who switched from placebo. In addition, patients who received continuous siponimod treatment had a prolonged time to 6-month confirmed disability progression, compared with patients who switched from placebo. For the 25th percentile of patients, continuous siponimod treatment corresponded to a delay of 54% (21 months vs. 13.6 months). Risk of worsening on the SDMT was reduced by 23% in the continuous siponimod–treatment group. For the 25th percentile of patients, this reduced risk corresponded to a delay of 62% (29.6 months vs. 18.3 months). ARR was 0.054 in the continuous-siponimod group, compared with 0.097 in the group that switched to siponimod from placebo, a reduction of 52%.
Dr. Cree has received personal compensation from Novartis, which markets siponimod, as well as Akili, Alexion, Atara, Biogen, EMD Serono, and TG Therapeutics. His coauthors reported receiving research support and personal compensation from Novartis and other pharmaceutical companies. Several coauthors were Novartis employees.
SOURCE: Kappos L et al. AAN 2020, Abstract S40.003.
Among and had a lower annualized relapse rate up to 5 years later, compared with patients who initially received placebo and switched to siponimod. This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Benefits of siponimod gained during the controlled period were “sustained for up to 5 years, suggesting a continuous effect of siponimod and underlining the advantages of early treatment initiation with siponimod,” the researchers said. Incidence rates of adverse events during the extension study were consistent with those during the controlled treatment period.
The results “highlight the critical importance of early treatment intervention ... to ensure the best possible long-term outcomes for patients with MS who are experiencing progression,” study investigator Bruce Cree, MD, PhD, said in a news release.
“It’s never too early to stay ahead of progression in MS, since the early identification of physical and cognitive changes – even subtle ones – can indicate MS disease progression and therefore allow for timely intervention.” said Dr. Cree, who is clinical research director and George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California, San Francisco.
Siponimod, marketed as Mayzent, is a sphingosine 1-phosphate receptor modulator that selectively binds to S1P1 and S1P5 receptors. The oral drug was approved by the Food and Drug Administration in 2019 for the treatment of relapsing forms of MS, including clinically isolated syndrome, relapsing remitting disease, and active secondary progressive disease in adults.
To assess the long-term efficacy and safety of siponimod in patients with secondary progressive MS, Dr. Cree and colleagues analyzed data from patients in the controlled and extension parts of the EXPAND trial. Patients could have had been in the study for as long as 5 years at the data cutoff in April 2019. Efficacy analyses included time to 3-month confirmed disability progression on the Expanded Disability Status Scale (EDSS), time to 6-month confirmed disability progression, time to 6-month confirmed worsening of 4 or more points on the Symbol Digit Modalities Test (SDMT), and annualized relapse rate. In EXPAND, the researchers defined confirmed disability progression as a 1-point increase in EDSS if the baseline score was 3.0-5.0, or a 0.5-point increase if the baseline score was 5.5-6.5.
“Of the 1,224 (74% of 1,651 randomized) patients entering the extension, 878 (72%) were ongoing,” the researchers reported. Patients who received siponimod continuously were less likely to experience 3-month confirmed disability progression and 6-month confirmed disability progression, relative to patients who switched from placebo. In addition, patients who received continuous siponimod treatment had a prolonged time to 6-month confirmed disability progression, compared with patients who switched from placebo. For the 25th percentile of patients, continuous siponimod treatment corresponded to a delay of 54% (21 months vs. 13.6 months). Risk of worsening on the SDMT was reduced by 23% in the continuous siponimod–treatment group. For the 25th percentile of patients, this reduced risk corresponded to a delay of 62% (29.6 months vs. 18.3 months). ARR was 0.054 in the continuous-siponimod group, compared with 0.097 in the group that switched to siponimod from placebo, a reduction of 52%.
Dr. Cree has received personal compensation from Novartis, which markets siponimod, as well as Akili, Alexion, Atara, Biogen, EMD Serono, and TG Therapeutics. His coauthors reported receiving research support and personal compensation from Novartis and other pharmaceutical companies. Several coauthors were Novartis employees.
SOURCE: Kappos L et al. AAN 2020, Abstract S40.003.
Among and had a lower annualized relapse rate up to 5 years later, compared with patients who initially received placebo and switched to siponimod. This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Benefits of siponimod gained during the controlled period were “sustained for up to 5 years, suggesting a continuous effect of siponimod and underlining the advantages of early treatment initiation with siponimod,” the researchers said. Incidence rates of adverse events during the extension study were consistent with those during the controlled treatment period.
The results “highlight the critical importance of early treatment intervention ... to ensure the best possible long-term outcomes for patients with MS who are experiencing progression,” study investigator Bruce Cree, MD, PhD, said in a news release.
“It’s never too early to stay ahead of progression in MS, since the early identification of physical and cognitive changes – even subtle ones – can indicate MS disease progression and therefore allow for timely intervention.” said Dr. Cree, who is clinical research director and George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California, San Francisco.
Siponimod, marketed as Mayzent, is a sphingosine 1-phosphate receptor modulator that selectively binds to S1P1 and S1P5 receptors. The oral drug was approved by the Food and Drug Administration in 2019 for the treatment of relapsing forms of MS, including clinically isolated syndrome, relapsing remitting disease, and active secondary progressive disease in adults.
To assess the long-term efficacy and safety of siponimod in patients with secondary progressive MS, Dr. Cree and colleagues analyzed data from patients in the controlled and extension parts of the EXPAND trial. Patients could have had been in the study for as long as 5 years at the data cutoff in April 2019. Efficacy analyses included time to 3-month confirmed disability progression on the Expanded Disability Status Scale (EDSS), time to 6-month confirmed disability progression, time to 6-month confirmed worsening of 4 or more points on the Symbol Digit Modalities Test (SDMT), and annualized relapse rate. In EXPAND, the researchers defined confirmed disability progression as a 1-point increase in EDSS if the baseline score was 3.0-5.0, or a 0.5-point increase if the baseline score was 5.5-6.5.
“Of the 1,224 (74% of 1,651 randomized) patients entering the extension, 878 (72%) were ongoing,” the researchers reported. Patients who received siponimod continuously were less likely to experience 3-month confirmed disability progression and 6-month confirmed disability progression, relative to patients who switched from placebo. In addition, patients who received continuous siponimod treatment had a prolonged time to 6-month confirmed disability progression, compared with patients who switched from placebo. For the 25th percentile of patients, continuous siponimod treatment corresponded to a delay of 54% (21 months vs. 13.6 months). Risk of worsening on the SDMT was reduced by 23% in the continuous siponimod–treatment group. For the 25th percentile of patients, this reduced risk corresponded to a delay of 62% (29.6 months vs. 18.3 months). ARR was 0.054 in the continuous-siponimod group, compared with 0.097 in the group that switched to siponimod from placebo, a reduction of 52%.
Dr. Cree has received personal compensation from Novartis, which markets siponimod, as well as Akili, Alexion, Atara, Biogen, EMD Serono, and TG Therapeutics. His coauthors reported receiving research support and personal compensation from Novartis and other pharmaceutical companies. Several coauthors were Novartis employees.
SOURCE: Kappos L et al. AAN 2020, Abstract S40.003.
FROM AAN 2020











