User login
An eConsults Program to Improve Patient Access to Specialty Care in an Academic Health System
From the Department of Medicine, University of California, Irvine, Orange, CA.
Abstract
Background: Orange County’s residents have difficulty accessing timely, quality, affordable specialty care services. As the county’s only academic health system, the University of California, Irvine (UCI) aimed to improve specialty care access for the communities it serves by implementing an electronic consultations (eConsults) program that allows primary care providers (PCPs) to efficiently receive specialist recommendations on referral problems that do not require an in-person evaluation.
Objective: To implement an eConsults program at the UCI that enhances access to and the delivery of coordinated specialty care for lower-complexity referral problems.
Methods: We developed custom solutions to integrate eConsults into UCI’s 2 electronic health record platforms. The impact of the eConsults program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of submitted eConsult requests per PCP, the number of completed responses per specialty, and the response time for eConsult requests. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback.
Results: Over 4.5 years, more than 1400 successful eConsults have been completed, and the program has expanded to 17 specialties. The average turnaround time for an eConsult response across all specialties was 1 business day. Moreover, more than 50% of the eConsults received specialty responses within the same day of the eConsult request. Most important, about 80% of eConsult requests were addressed without the need for an in-office visit with a specialist.
Conclusion: The enhanced access to and the delivery of coordinated specialty care provided by eConsults resulted in improved efficiency and specialty access, while likely reducing costs and improving patient satisfaction. The improved communication and collaboration among providers with eConsults has also led to overwhelmingly positive feedback from both PCPs and specialists.
Keywords: electronic consultation; access to care; primary care; specialty referral; telehealth.
Orange County’s growing, aging, and diverse population is driving an increased demand for health care.1 But with the county’s high cost of living and worsening shortage of physicians,1-3 many of its residents are struggling to access timely, quality, affordable care. Access to specialty care services is especially frustrating for many patients and their providers, both primary care providers (PCPs) and specialists, due to problems with the referral process. Many patients experience increased wait times for a visit with a specialist due to poor communication between providers, insufficient guidance on the information or diagnostic results needed by specialists, and lack of care coordination.4-6 One promising approach to overcome these challenges is the use of an electronic consultation, or eConsult, in place of a standard in-person referral. An eConsult is an asynchronous, non-face-to-face, provider-to-provider exchange using a secure electronic communication platform. For appropriate referral problems, the patient is able to receive timely access to specialist expertise through electronic referral by their PCP,7-9 and avoid the time and costs associated with a visit to the specialist,10,11 such as travel, missed work, co-pays, and child-care expenses. Clinical questions addressed using an eConsult system subsequently free up office visit appointment slots, improving access for patients requiring in-office evaluation.8,12
Orange County’s only academic health system, the University of California, Irvine (UCI), serves a population of 3.5 million, and its principal priority is providing the communities in the county (which is the sixth largest in United States) and the surrounding region with the highest quality health care possible. Thus, UCI aimed to improve its referral processes and provide timely access to specialty care for its patients by implementing an eConsults program that allows PCPs to efficiently receive specialist recommendations on referral problems that do not require the specialist to evaluate the patient in person. This report describes our experiences with developing and implementing a custom-built eConsults workflow in UCI’s prior electronic health record (EHR) platform, Allscripts, and subsequently transitioning our mature eConsults program to a new EHR system when UCI adopted Epic. UCI is likely the only academic medical center to have experience in successfully implementing eConsults into 2 different EHR systems.
Setting
UCI’s medical center is a 417-bed acute care hospital providing tertiary and quaternary care, ambulatory and specialty medical clinics, behavioral health care, and rehabilitation services. It is located in Orange, CA, and serves a diverse population of 3.5 million persons with broad health care needs. With more than 400 specialty and primary care physicians, UCI offers a full scope of acute and general care services. It is also the primary teaching location for UCI medical and nursing students, medical residents, and fellows, and is home to Orange County’s only adult Level I and pediatric Level II trauma centers and the regional burn center.
eConsults Program
We designed the initial eConsults program within UCI’s Allscripts EHR platform. Our information technology (IT) build team developed unique “documents-based” eConsults workflows that simplified the process of initiating requests directly from the EHR and facilitated rapid responses from participating specialties. The requesting provider’s eConsults interface was user-friendly, and referring providers were able to initiate an eConsult easily by selecting the customized eConsult icon from the Allscripts main toolbar. To ensure that all relevant information is provided to the specialists, condition-specific templates are embedded in the requesting provider’s eConsults workflow that allow PCPs to enter a focused, patient-specific clinical question and provide guidance on recommended patient information (eg, health history, laboratory results, and digital images) that may help the specialist provide an informed response. The eConsult templates were adapted from standardized forms developed by partner University of California Health Systems in an initiative funded by the University of California Center for Health Quality and Innovation.
To facilitate timely responses from specialists, an innovative notification system was created in the responding provider’s eConsults workflow to automatically send an email to participating specialists when a new eConsult is requested. The responding provider’s workflow also includes an option for the specialist to decline the eConsult if the case is deemed too complex to be addressed electronically. For every completed eConsult that does not result in an in-person patient evaluation, the requesting provider and responding specialist each receives a modest reimbursement, which was initially paid by UCI Health System funds.
Implementation
The design and implementation of the eConsults program began in November 2014, and was guided by a steering committee that included the chair of the department of medicine, chief medical information officer, primary care and specialty physician leads, IT build team, and a project manager. Early on, members of this committee engaged UCI leadership to affirm support for the program and obtain the IT resources needed to build the eConsults workflow. Regular steering committee meetings were established to discuss the design of the workflow, adapt the clinical content of the referral templates, and develop a provider reimbursement plan. After completion of the workflow build, the eConsults system was tested to identify failure points and obtain feedback from users. Prior to going live, the eConsults program was publicized by members of the steering committee through meetings with primary care groups and email communications. Committee members also hosted in-person training and orientation sessions with PCPs and participating specialists, and distributed tip sheets summarizing the steps to complete the PCP and specialist eConsult workflows.
The eConsults workflow build, testing, and launch were completed within 5 months (April 2015; Figure 1). eConsults went live in the 3 initial specialties (endocrinology, cardiology, and rheumatology) that were interested in participating in the first wave of the program. UCI’s eConsults service has subsequently expanded to 17 total specialties (allergy, cardiology, dermatology, endocrinology, gastroenterology, geriatrics, gynecology, hematology, hepatology, infectious disease, nephrology, neurology, palliative care, psychiatry, pulmonary, rheumatology, and sports medicine).
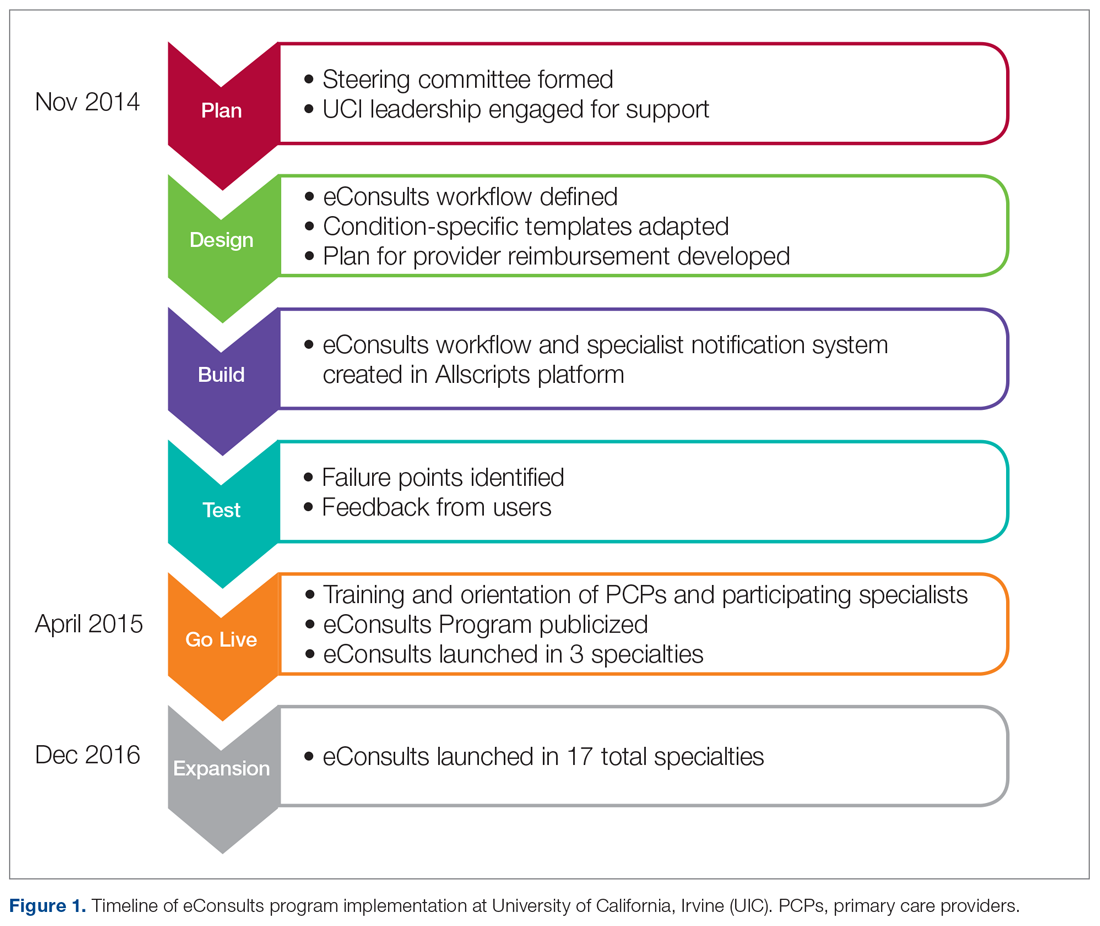
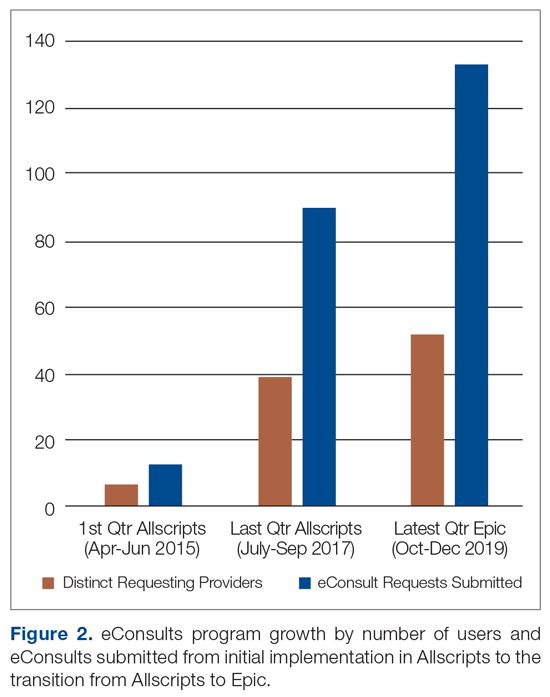
Two and half years after the eConsults program was implemented in Allscripts, UCI adopted a new EHR platform, Epic. By this time, the eConsults service had grown into a mature program with greater numbers of PCP users and submitted eConsults (Figure 2). Using our experience with the Allscripts build, our IT team was able to efficiently transition the eConsults service to the new EHR system. In contrast to the “documents-based” eConsult workflows on Allscripts, our IT team utilized an “orders-based” strategy on Epic, which followed a more traditional approach to requesting a consultation. We re-launched the service in Epic within 3 months (February 2018). However, both platforms utilized user-friendly workflows to achieve similar goals, and the program has continued to grow with respect to the number of users and eConsults.
Measurement/Analysis
The impact of the program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of PCP users, the number of submitted eConsult requests per PCP, and the number of requests per specialty. The response time for eConsult requests and the self-reported amount of time spent by specialists on the response were also tracked. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback. Provider satisfaction was primarily obtained by soliciting feedback from individual eConsult users.
Implementation of this eConsults program constituted a quality improvement activity and did not require Institutional Review Board review.
Results
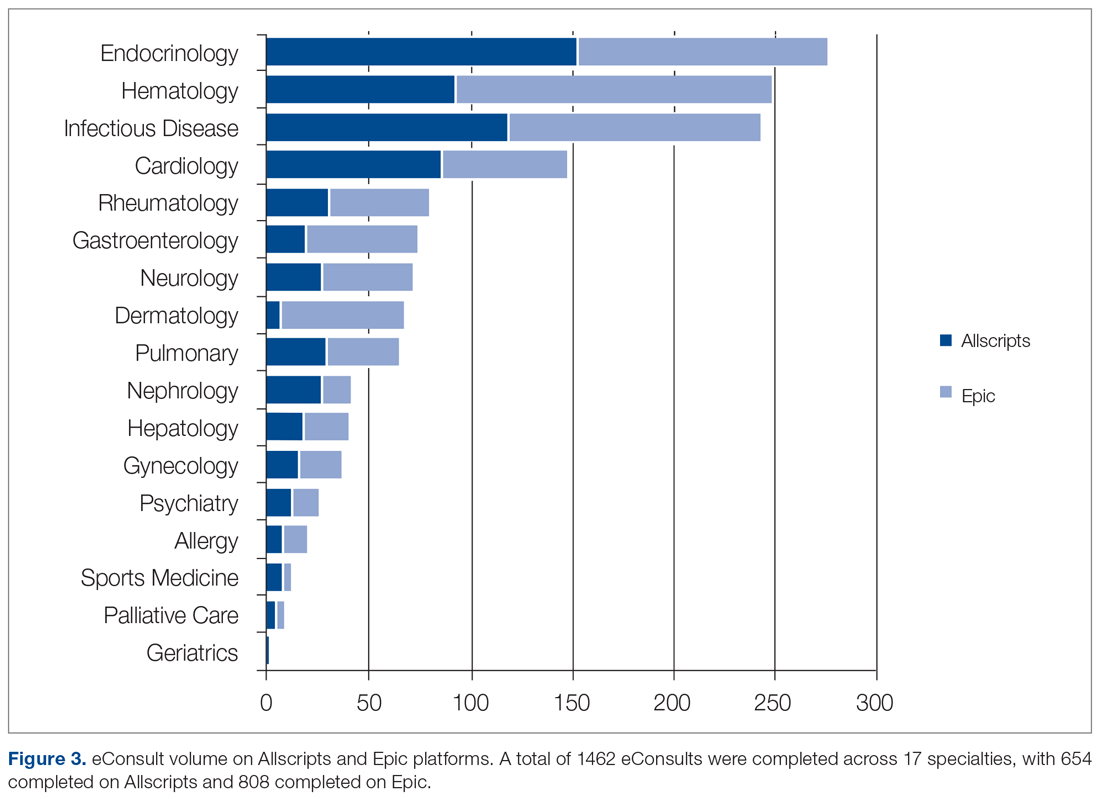
Since the program was launched in April 2015, more than 1400 eConsults have been completed across 17 specialties (Figure 3). There were 654 completed eConsults on the Allscripts platform, and 808 eConsults have been completed using the Epic platform to date. The busiest eConsult specialties were endocrinology (receiving 276, or 19%, of the eConsults requests), hematology (receiving 249 requests, or 17%), infectious disease (receiving 244 requests, or 17% ), and cardiology (receiving 148 requests, or 10%).
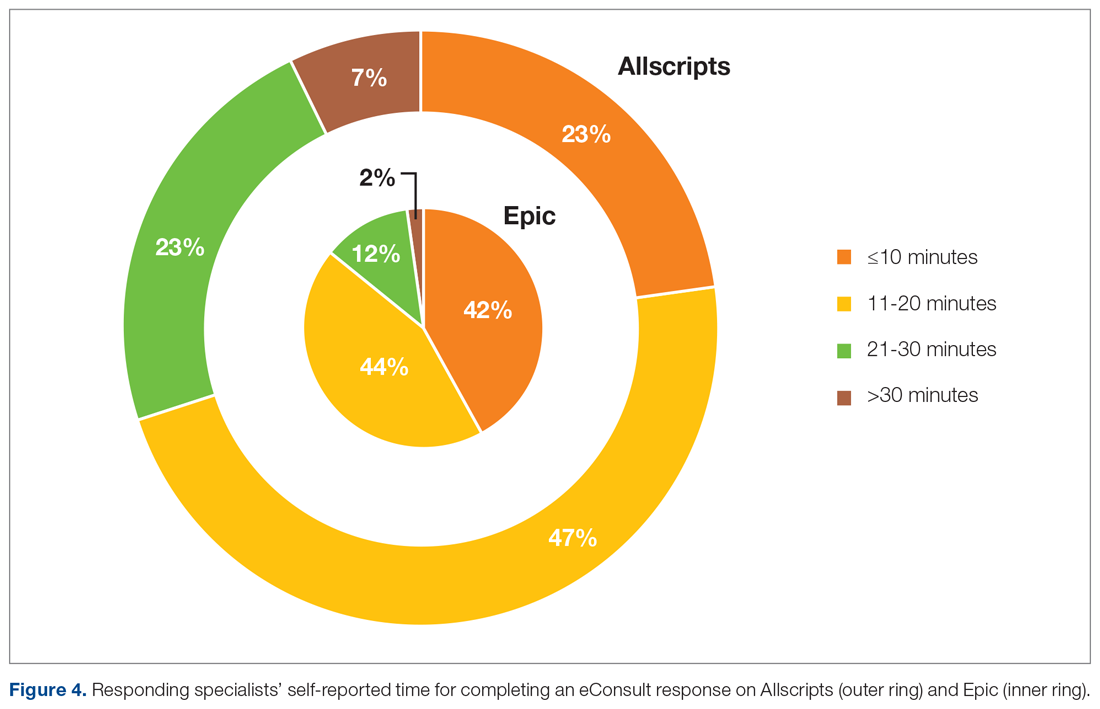
The self-reported amount of time specialists spent on the response was different between the 2 EHR systems (Figure 4). On Allscripts, specialists reported that 23% of eConsults took 10 minutes or less to complete, 47% took 11 to 20 minutes, 23% took 21 to 30 minutes, and 7% took more than 30 minutes. On Epic, specialists reported that 42% of eConsults took 10 minutes or less to complete, 44% took 11 to 20 minutes, 12% took 21 to 30 minutes, and 2% took more than 30 minutes. This difference in time spent fielding eConsults likely represents the subtle nuances between Allscripts’ “documents-based” and Epic’s “orders-based” workflows.
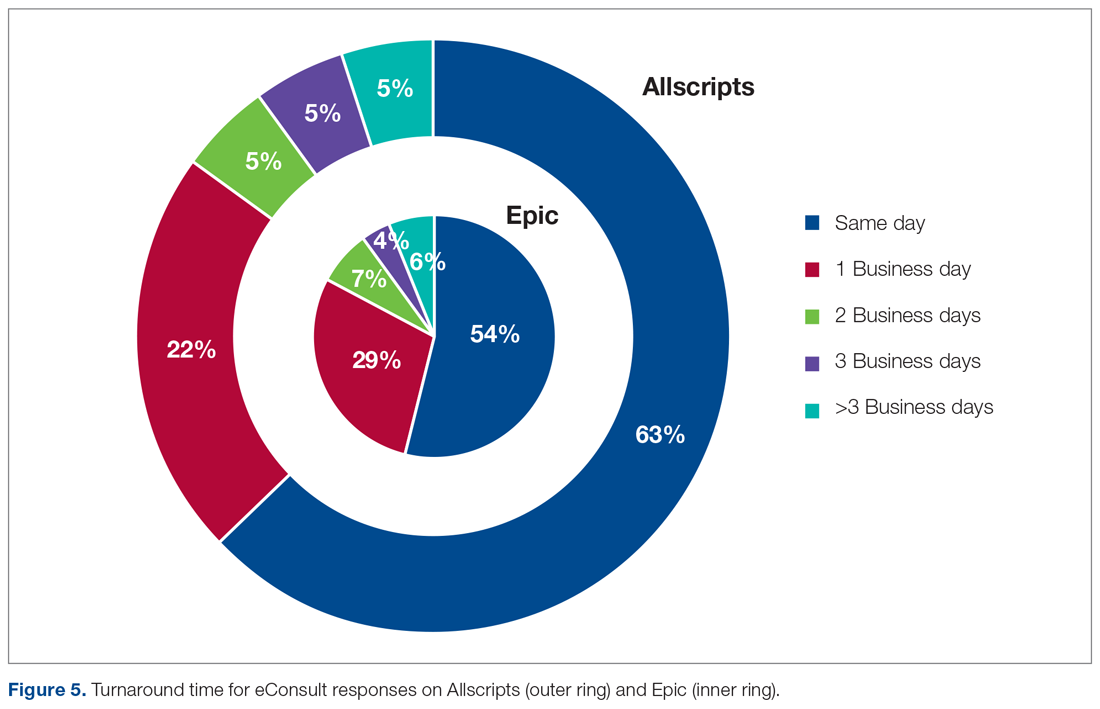
As a result of the automated notification system that was introduced early in the eConsults implementation process on Allscripts, the specialty response times were much faster than the expected 3 business days’ turnaround goal instituted by the Center for Health Quality and Innovation initiative, regardless of the EHR platform used. In fact, the average turnaround time for an eConsult response across all specialties was 1 business day, which was similar for both EHR systems (Figure 5). Furthermore, more than 50% of the eConsults on both EHR systems received specialist responses within the same day of the eConsult request (63% on Allscripts, 54% on Epic). There was a small decrease in the percentage of same-day responses when we transitioned to Epic, likely because the functionality of an automated notification email could not be restored in Epic. Regardless, the specialty response times on Epic remained expeditious, likely because the automated notifications on Allscripts instilled good practices for the specialists, and regularly checking for new eConsult requests became an ingrained behavior.
Our most important finding was that approximately 80% of eConsult requests were addressed without the need for an in-office visit with a specialist. This measure was similar for both EHR platforms (83% on Allscripts and 78% on Epic).
Provider feedback has been overwhelmingly positive. PCPs are impressed with the robust educational content of the eConsult responses, since the goal for specialists is to justify their recommendations. Specialists appreciate the convenience and efficiency that eConsults offer, as well as the improved communication and collaboration among physicians. eConsults have been especially beneficial to PCPs at UCI’s Family Health Centers, who are now able to receive subspecialty consultations from UCI specialists despite insurance barriers.
Discussion
Our eConsults program uniquely contrasts with other programs because UCI is likely the only academic medical center to have experience in successfully incorporating eConsults into 2 different EHR systems: initial development of the eConsults workflow in UCI’s existing Allscripts EHR platform, and subsequently transitioning a mature eConsults program to a new EHR system when the institution adopted Epic.
We measured the impact of the eConsults program on access to care by the response time for eConsult requests and the percentage of eConsults that averted an in-office visit with a specialist. We found that the eConsults program at UCI provided our PCPs access to specialist consultations in a timely manner, with much shorter response times than standard in-person referrals. The average turnaround time for an eConsult response we reported is consistent with findings from other studies.12-15 Additionally, our program was able to address about 80% of its eConsults electronically, helping to reduce unnecessary in-person specialist referrals. In the literature, the percentage of eConsults that avoided an in-person specialist visit varies widely.8,12-16
We reported very positive feedback from both PCPs and specialists on UCI’s eConsults service. Similarly, other studies described PCP satisfaction with their respective eConsults programs to be uniformly high,8,9,13,14,17-19 though some reported that the level of satisfaction among specialists was more varied.18-21
Lessons Learned
The successful design and implementation of our eConsults program began with assembling the right clinical champions and technology partners for our steering committee. Establishing regular steering committee meetings helped maintain an appropriate timeline for completion of different aspects of the project. Engaging support from UCI’s leadership also provided us with a dedicated IT team that helped us with the build, training resources, troubleshooting issues, and reporting for the project.
Our experience with implementing the eConsults program on 2 different EHR systems highlighted the importance of creating efficient, user-friendly workflows to foster provider adoption and achieve sustainability. Allscripts’ open platform gave our IT team the ability to create a homegrown solution to implementing an eConsult model that was simple and easy to use. The Epic platform’s interoperability allowed us to leverage our learnings from the Allscripts build to efficiently implement eConsults in Epic.
We also found that providing modest incentive payments or reimbursements to both PCPs and specialists for each completed eConsult helps with both adoption and program sustainability. Initially, credit for the eConsult work was paid by internal UCI Health System funds. Two payers, UC Care (a preferred provider organization plan created just for the University of California) and more recently, the Centers for Medicare & Medicaid Services, have agreed to reimburse for outpatient eConsults. Securing additional payers for reimbursement of the eConsult service will not only ensure the program’s long-term sustainability, but also represents an acknowledgment of the value of eConsults in supporting access to care.
Applicability
Other health care settings that are experiencing issues with specialty care access can successfully implement their own eConsults program by employing strategies similar to those described in this report—assembling the right team, creating user-friendly workflows, and providing incentives. Our advice for successful implementation is to clearly communicate your goals to all involved, including primary care, specialists, leadership, and IT partners, and establish with these stakeholders the appropriate support and resources needed to facilitate the development of the program and overcome any barriers to adoption.
Current Status and Future Directions
Our future plans include continuing to optimize the Epic eConsult backend build and workflows using our experience in Allscripts. We have implemented eConsult workflows for use by graduate medical education trainees and advanced practice providers, with attending supervision. Further work is in progress to optimize these workflows, which will allow for appropriate education and supervision without delaying care. Furthermore, we plan to expand the program to include inpatient-to-inpatient and emergency department-to-inpatient eConsults. We will continue to expand the eConsults program by offering additional specialties, engage providers to encourage ongoing participation, and maximize PCP use by continuing to market the program through regular newsletters and email communications. Finally, the eConsults has served as an effective, important resource in the current era of COVID-19 in several ways: it allows for optimization of specialty input in patient care delivery without subjecting more health care workers to unnecessary exposure; saves on utilization of precious personal protective equipment; and enhances our ability to deal with a potential surge by providing access to specialists remotely and electronically all hours of the day, thus expanding care to the evening and weekend hours.
Acknowledgment: The authors thank our steering committee members (Dr. Ralph Cygan, Dr. Andrew Reikes, Dr. Byron Allen, Dr. George Lawry) and IT build team (Lori Bocchicchio, Meghan van Witsen, Jaymee Zillgitt, Tanya Sickles, Dennis Hoang, Jeanette Lisak-Phillips) for their contributions in the design and implementation of our eConsults program. We also thank additional team members Kurt McArthur and Neaktisia Lee for their assistance with generating reports, and Kathy LaPierre, Jennifer Rios, and Debra Webb Torres for their guidance with compliance and billing issues.
Corresponding author: Alpesh N. Amin, MD, MBA, University of California, Irvine, 101 The City Drive South, Building 26, Room 1000, ZC-4076H, Orange, CA 92868; [email protected].
Financial disclosures: None.
1. County of Orange, Health Care Agency, Public Health Services. Orange County Health Profile 2013.
2. Coffman JM, Fix M Ko, M. California physician supply and distribution: headed for a drought? California Health Care Foundation, June 2018.
3. Spetz J, Coffman J, Geyn I. California’s primary care workforce: forecasted supply, demand, and pipeline of trainees, 2016-2030. Healthforce Center at the University of California, San Francisco, August 2017.
4. Gandhi TK, Sittig DF, Franklin M, et al. Communication breakdown in the outpatient referral process. J Gen Intern Med. 2000;15:626-631.
5. McPhee SJ, Lo B, Saika GY, Meltzer R. How good is communication between primary care physicians and subspecialty consultants? Arch Intern Med. 1984;144:1265-1268.
6. Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89:39-68.
7. Wrenn K, Catschegn S, Cruz M, et al. Analysis of an electronic consultation program at an academic medical centre: Primary care provider questions, specialist responses, and primary care provider actions. J Telemed Telecare. 2017;23: 217-224.
8. Gleason N, Prasad PA, Ackerman S, et al. Adoption and impact of an eConsult system in a fee-for-service setting. Healthc (Amst). 2017;5(1-2):40-45.
9. Stoves J, Connolly J, Cheung CK, et al. Electronic consultation as an alternative to hospital referral for patients with chronic kidney disease: a novel application for networked electronic health records to improve the accessibility and efficiency of healthcare. Qual Saf Health Care. 2010;19: e54.
10. Datta SK, Warshaw EM, Edison KE, et al. Cost and utility analysis of a store-and-forward teledermatology referral system: a randomized clinical trial. JAMA Dermatol. 2015;151:1323-1329.
11. Liddy C, Drosinis P, Deri Armstrong C, et al. What are the cost savings associated with providing access to specialist care through the Champlain BASE eConsult service? A costing evaluation. BMJ Open. 2016;6:e010920.
12. Barnett ML, Yee HF Jr, Mehrotra A, Giboney P. Los Angeles safety-net program eConsult system was rapidly adopted and decreased wait times to see specialists. Health Aff. 2017;36:492-499.
13. Malagrino GD, Chaudhry R, Gardner M, et al. A study of 6,000 electronic specialty consultations for person-centered care at The Mayo Clinic. Int J Person Centered Med. 2012;2:458-466.
14. Keely E, Liddy C, Afkham A. Utilization, benefits, and impact of an e-consultation service across diverse specialties and primary care providers. Telemed J E Health. 2013;19:733-738.
15. Scherpbier-de Haan ND, van Gelder VA, Van Weel C, et al. Initial implementation of a web-based consultation process for patients with chronic kidney disease. Ann Fam Med. 2013;11:151-156.
16. Palen TE, Price D, Shetterly S, Wallace KB. Comparing virtual consults to traditional consults using an electronic health record: an observational case-control study. BMC Med Inform Decis Mak. 2012;12:65.
17. Liddy C, Afkham A, Drosinis P, et al. Impact of and satisfaction with a new eConsult service: a mixed methods study of primary care providers. J Am Board Fam Med. 2015;28:394-403.
18. Angstman KB, Adamson SC, Furst JW, et al. Provider satisfaction with virtual specialist consultations in a family medicine department. Health Care Manag (Frederick). 2009;28:14-18.
19. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA health care system. Fed Pract. 2014; 31:26–31.
20. Keely E, Williams R, Epstein G, et al. Specialist perspectives on Ontario Provincial electronic consultation services. Telemed J E Health. 2019;25:3-10.
21. Kim-Hwang JE, Chen AH, Bell DS, et al. Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25:1123-1128.
From the Department of Medicine, University of California, Irvine, Orange, CA.
Abstract
Background: Orange County’s residents have difficulty accessing timely, quality, affordable specialty care services. As the county’s only academic health system, the University of California, Irvine (UCI) aimed to improve specialty care access for the communities it serves by implementing an electronic consultations (eConsults) program that allows primary care providers (PCPs) to efficiently receive specialist recommendations on referral problems that do not require an in-person evaluation.
Objective: To implement an eConsults program at the UCI that enhances access to and the delivery of coordinated specialty care for lower-complexity referral problems.
Methods: We developed custom solutions to integrate eConsults into UCI’s 2 electronic health record platforms. The impact of the eConsults program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of submitted eConsult requests per PCP, the number of completed responses per specialty, and the response time for eConsult requests. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback.
Results: Over 4.5 years, more than 1400 successful eConsults have been completed, and the program has expanded to 17 specialties. The average turnaround time for an eConsult response across all specialties was 1 business day. Moreover, more than 50% of the eConsults received specialty responses within the same day of the eConsult request. Most important, about 80% of eConsult requests were addressed without the need for an in-office visit with a specialist.
Conclusion: The enhanced access to and the delivery of coordinated specialty care provided by eConsults resulted in improved efficiency and specialty access, while likely reducing costs and improving patient satisfaction. The improved communication and collaboration among providers with eConsults has also led to overwhelmingly positive feedback from both PCPs and specialists.
Keywords: electronic consultation; access to care; primary care; specialty referral; telehealth.
Orange County’s growing, aging, and diverse population is driving an increased demand for health care.1 But with the county’s high cost of living and worsening shortage of physicians,1-3 many of its residents are struggling to access timely, quality, affordable care. Access to specialty care services is especially frustrating for many patients and their providers, both primary care providers (PCPs) and specialists, due to problems with the referral process. Many patients experience increased wait times for a visit with a specialist due to poor communication between providers, insufficient guidance on the information or diagnostic results needed by specialists, and lack of care coordination.4-6 One promising approach to overcome these challenges is the use of an electronic consultation, or eConsult, in place of a standard in-person referral. An eConsult is an asynchronous, non-face-to-face, provider-to-provider exchange using a secure electronic communication platform. For appropriate referral problems, the patient is able to receive timely access to specialist expertise through electronic referral by their PCP,7-9 and avoid the time and costs associated with a visit to the specialist,10,11 such as travel, missed work, co-pays, and child-care expenses. Clinical questions addressed using an eConsult system subsequently free up office visit appointment slots, improving access for patients requiring in-office evaluation.8,12
Orange County’s only academic health system, the University of California, Irvine (UCI), serves a population of 3.5 million, and its principal priority is providing the communities in the county (which is the sixth largest in United States) and the surrounding region with the highest quality health care possible. Thus, UCI aimed to improve its referral processes and provide timely access to specialty care for its patients by implementing an eConsults program that allows PCPs to efficiently receive specialist recommendations on referral problems that do not require the specialist to evaluate the patient in person. This report describes our experiences with developing and implementing a custom-built eConsults workflow in UCI’s prior electronic health record (EHR) platform, Allscripts, and subsequently transitioning our mature eConsults program to a new EHR system when UCI adopted Epic. UCI is likely the only academic medical center to have experience in successfully implementing eConsults into 2 different EHR systems.
Setting
UCI’s medical center is a 417-bed acute care hospital providing tertiary and quaternary care, ambulatory and specialty medical clinics, behavioral health care, and rehabilitation services. It is located in Orange, CA, and serves a diverse population of 3.5 million persons with broad health care needs. With more than 400 specialty and primary care physicians, UCI offers a full scope of acute and general care services. It is also the primary teaching location for UCI medical and nursing students, medical residents, and fellows, and is home to Orange County’s only adult Level I and pediatric Level II trauma centers and the regional burn center.
eConsults Program
We designed the initial eConsults program within UCI’s Allscripts EHR platform. Our information technology (IT) build team developed unique “documents-based” eConsults workflows that simplified the process of initiating requests directly from the EHR and facilitated rapid responses from participating specialties. The requesting provider’s eConsults interface was user-friendly, and referring providers were able to initiate an eConsult easily by selecting the customized eConsult icon from the Allscripts main toolbar. To ensure that all relevant information is provided to the specialists, condition-specific templates are embedded in the requesting provider’s eConsults workflow that allow PCPs to enter a focused, patient-specific clinical question and provide guidance on recommended patient information (eg, health history, laboratory results, and digital images) that may help the specialist provide an informed response. The eConsult templates were adapted from standardized forms developed by partner University of California Health Systems in an initiative funded by the University of California Center for Health Quality and Innovation.
To facilitate timely responses from specialists, an innovative notification system was created in the responding provider’s eConsults workflow to automatically send an email to participating specialists when a new eConsult is requested. The responding provider’s workflow also includes an option for the specialist to decline the eConsult if the case is deemed too complex to be addressed electronically. For every completed eConsult that does not result in an in-person patient evaluation, the requesting provider and responding specialist each receives a modest reimbursement, which was initially paid by UCI Health System funds.
Implementation
The design and implementation of the eConsults program began in November 2014, and was guided by a steering committee that included the chair of the department of medicine, chief medical information officer, primary care and specialty physician leads, IT build team, and a project manager. Early on, members of this committee engaged UCI leadership to affirm support for the program and obtain the IT resources needed to build the eConsults workflow. Regular steering committee meetings were established to discuss the design of the workflow, adapt the clinical content of the referral templates, and develop a provider reimbursement plan. After completion of the workflow build, the eConsults system was tested to identify failure points and obtain feedback from users. Prior to going live, the eConsults program was publicized by members of the steering committee through meetings with primary care groups and email communications. Committee members also hosted in-person training and orientation sessions with PCPs and participating specialists, and distributed tip sheets summarizing the steps to complete the PCP and specialist eConsult workflows.
The eConsults workflow build, testing, and launch were completed within 5 months (April 2015; Figure 1). eConsults went live in the 3 initial specialties (endocrinology, cardiology, and rheumatology) that were interested in participating in the first wave of the program. UCI’s eConsults service has subsequently expanded to 17 total specialties (allergy, cardiology, dermatology, endocrinology, gastroenterology, geriatrics, gynecology, hematology, hepatology, infectious disease, nephrology, neurology, palliative care, psychiatry, pulmonary, rheumatology, and sports medicine).

Two and half years after the eConsults program was implemented in Allscripts, UCI adopted a new EHR platform, Epic. By this time, the eConsults service had grown into a mature program with greater numbers of PCP users and submitted eConsults (Figure 2). Using our experience with the Allscripts build, our IT team was able to efficiently transition the eConsults service to the new EHR system. In contrast to the “documents-based” eConsult workflows on Allscripts, our IT team utilized an “orders-based” strategy on Epic, which followed a more traditional approach to requesting a consultation. We re-launched the service in Epic within 3 months (February 2018). However, both platforms utilized user-friendly workflows to achieve similar goals, and the program has continued to grow with respect to the number of users and eConsults.

Measurement/Analysis
The impact of the program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of PCP users, the number of submitted eConsult requests per PCP, and the number of requests per specialty. The response time for eConsult requests and the self-reported amount of time spent by specialists on the response were also tracked. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback. Provider satisfaction was primarily obtained by soliciting feedback from individual eConsult users.
Implementation of this eConsults program constituted a quality improvement activity and did not require Institutional Review Board review.
Results
Since the program was launched in April 2015, more than 1400 eConsults have been completed across 17 specialties (Figure 3). There were 654 completed eConsults on the Allscripts platform, and 808 eConsults have been completed using the Epic platform to date. The busiest eConsult specialties were endocrinology (receiving 276, or 19%, of the eConsults requests), hematology (receiving 249 requests, or 17%), infectious disease (receiving 244 requests, or 17% ), and cardiology (receiving 148 requests, or 10%).

The self-reported amount of time specialists spent on the response was different between the 2 EHR systems (Figure 4). On Allscripts, specialists reported that 23% of eConsults took 10 minutes or less to complete, 47% took 11 to 20 minutes, 23% took 21 to 30 minutes, and 7% took more than 30 minutes. On Epic, specialists reported that 42% of eConsults took 10 minutes or less to complete, 44% took 11 to 20 minutes, 12% took 21 to 30 minutes, and 2% took more than 30 minutes. This difference in time spent fielding eConsults likely represents the subtle nuances between Allscripts’ “documents-based” and Epic’s “orders-based” workflows.

As a result of the automated notification system that was introduced early in the eConsults implementation process on Allscripts, the specialty response times were much faster than the expected 3 business days’ turnaround goal instituted by the Center for Health Quality and Innovation initiative, regardless of the EHR platform used. In fact, the average turnaround time for an eConsult response across all specialties was 1 business day, which was similar for both EHR systems (Figure 5). Furthermore, more than 50% of the eConsults on both EHR systems received specialist responses within the same day of the eConsult request (63% on Allscripts, 54% on Epic). There was a small decrease in the percentage of same-day responses when we transitioned to Epic, likely because the functionality of an automated notification email could not be restored in Epic. Regardless, the specialty response times on Epic remained expeditious, likely because the automated notifications on Allscripts instilled good practices for the specialists, and regularly checking for new eConsult requests became an ingrained behavior.

Our most important finding was that approximately 80% of eConsult requests were addressed without the need for an in-office visit with a specialist. This measure was similar for both EHR platforms (83% on Allscripts and 78% on Epic).
Provider feedback has been overwhelmingly positive. PCPs are impressed with the robust educational content of the eConsult responses, since the goal for specialists is to justify their recommendations. Specialists appreciate the convenience and efficiency that eConsults offer, as well as the improved communication and collaboration among physicians. eConsults have been especially beneficial to PCPs at UCI’s Family Health Centers, who are now able to receive subspecialty consultations from UCI specialists despite insurance barriers.
Discussion
Our eConsults program uniquely contrasts with other programs because UCI is likely the only academic medical center to have experience in successfully incorporating eConsults into 2 different EHR systems: initial development of the eConsults workflow in UCI’s existing Allscripts EHR platform, and subsequently transitioning a mature eConsults program to a new EHR system when the institution adopted Epic.
We measured the impact of the eConsults program on access to care by the response time for eConsult requests and the percentage of eConsults that averted an in-office visit with a specialist. We found that the eConsults program at UCI provided our PCPs access to specialist consultations in a timely manner, with much shorter response times than standard in-person referrals. The average turnaround time for an eConsult response we reported is consistent with findings from other studies.12-15 Additionally, our program was able to address about 80% of its eConsults electronically, helping to reduce unnecessary in-person specialist referrals. In the literature, the percentage of eConsults that avoided an in-person specialist visit varies widely.8,12-16
We reported very positive feedback from both PCPs and specialists on UCI’s eConsults service. Similarly, other studies described PCP satisfaction with their respective eConsults programs to be uniformly high,8,9,13,14,17-19 though some reported that the level of satisfaction among specialists was more varied.18-21
Lessons Learned
The successful design and implementation of our eConsults program began with assembling the right clinical champions and technology partners for our steering committee. Establishing regular steering committee meetings helped maintain an appropriate timeline for completion of different aspects of the project. Engaging support from UCI’s leadership also provided us with a dedicated IT team that helped us with the build, training resources, troubleshooting issues, and reporting for the project.
Our experience with implementing the eConsults program on 2 different EHR systems highlighted the importance of creating efficient, user-friendly workflows to foster provider adoption and achieve sustainability. Allscripts’ open platform gave our IT team the ability to create a homegrown solution to implementing an eConsult model that was simple and easy to use. The Epic platform’s interoperability allowed us to leverage our learnings from the Allscripts build to efficiently implement eConsults in Epic.
We also found that providing modest incentive payments or reimbursements to both PCPs and specialists for each completed eConsult helps with both adoption and program sustainability. Initially, credit for the eConsult work was paid by internal UCI Health System funds. Two payers, UC Care (a preferred provider organization plan created just for the University of California) and more recently, the Centers for Medicare & Medicaid Services, have agreed to reimburse for outpatient eConsults. Securing additional payers for reimbursement of the eConsult service will not only ensure the program’s long-term sustainability, but also represents an acknowledgment of the value of eConsults in supporting access to care.
Applicability
Other health care settings that are experiencing issues with specialty care access can successfully implement their own eConsults program by employing strategies similar to those described in this report—assembling the right team, creating user-friendly workflows, and providing incentives. Our advice for successful implementation is to clearly communicate your goals to all involved, including primary care, specialists, leadership, and IT partners, and establish with these stakeholders the appropriate support and resources needed to facilitate the development of the program and overcome any barriers to adoption.
Current Status and Future Directions
Our future plans include continuing to optimize the Epic eConsult backend build and workflows using our experience in Allscripts. We have implemented eConsult workflows for use by graduate medical education trainees and advanced practice providers, with attending supervision. Further work is in progress to optimize these workflows, which will allow for appropriate education and supervision without delaying care. Furthermore, we plan to expand the program to include inpatient-to-inpatient and emergency department-to-inpatient eConsults. We will continue to expand the eConsults program by offering additional specialties, engage providers to encourage ongoing participation, and maximize PCP use by continuing to market the program through regular newsletters and email communications. Finally, the eConsults has served as an effective, important resource in the current era of COVID-19 in several ways: it allows for optimization of specialty input in patient care delivery without subjecting more health care workers to unnecessary exposure; saves on utilization of precious personal protective equipment; and enhances our ability to deal with a potential surge by providing access to specialists remotely and electronically all hours of the day, thus expanding care to the evening and weekend hours.
Acknowledgment: The authors thank our steering committee members (Dr. Ralph Cygan, Dr. Andrew Reikes, Dr. Byron Allen, Dr. George Lawry) and IT build team (Lori Bocchicchio, Meghan van Witsen, Jaymee Zillgitt, Tanya Sickles, Dennis Hoang, Jeanette Lisak-Phillips) for their contributions in the design and implementation of our eConsults program. We also thank additional team members Kurt McArthur and Neaktisia Lee for their assistance with generating reports, and Kathy LaPierre, Jennifer Rios, and Debra Webb Torres for their guidance with compliance and billing issues.
Corresponding author: Alpesh N. Amin, MD, MBA, University of California, Irvine, 101 The City Drive South, Building 26, Room 1000, ZC-4076H, Orange, CA 92868; [email protected].
Financial disclosures: None.
From the Department of Medicine, University of California, Irvine, Orange, CA.
Abstract
Background: Orange County’s residents have difficulty accessing timely, quality, affordable specialty care services. As the county’s only academic health system, the University of California, Irvine (UCI) aimed to improve specialty care access for the communities it serves by implementing an electronic consultations (eConsults) program that allows primary care providers (PCPs) to efficiently receive specialist recommendations on referral problems that do not require an in-person evaluation.
Objective: To implement an eConsults program at the UCI that enhances access to and the delivery of coordinated specialty care for lower-complexity referral problems.
Methods: We developed custom solutions to integrate eConsults into UCI’s 2 electronic health record platforms. The impact of the eConsults program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of submitted eConsult requests per PCP, the number of completed responses per specialty, and the response time for eConsult requests. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback.
Results: Over 4.5 years, more than 1400 successful eConsults have been completed, and the program has expanded to 17 specialties. The average turnaround time for an eConsult response across all specialties was 1 business day. Moreover, more than 50% of the eConsults received specialty responses within the same day of the eConsult request. Most important, about 80% of eConsult requests were addressed without the need for an in-office visit with a specialist.
Conclusion: The enhanced access to and the delivery of coordinated specialty care provided by eConsults resulted in improved efficiency and specialty access, while likely reducing costs and improving patient satisfaction. The improved communication and collaboration among providers with eConsults has also led to overwhelmingly positive feedback from both PCPs and specialists.
Keywords: electronic consultation; access to care; primary care; specialty referral; telehealth.
Orange County’s growing, aging, and diverse population is driving an increased demand for health care.1 But with the county’s high cost of living and worsening shortage of physicians,1-3 many of its residents are struggling to access timely, quality, affordable care. Access to specialty care services is especially frustrating for many patients and their providers, both primary care providers (PCPs) and specialists, due to problems with the referral process. Many patients experience increased wait times for a visit with a specialist due to poor communication between providers, insufficient guidance on the information or diagnostic results needed by specialists, and lack of care coordination.4-6 One promising approach to overcome these challenges is the use of an electronic consultation, or eConsult, in place of a standard in-person referral. An eConsult is an asynchronous, non-face-to-face, provider-to-provider exchange using a secure electronic communication platform. For appropriate referral problems, the patient is able to receive timely access to specialist expertise through electronic referral by their PCP,7-9 and avoid the time and costs associated with a visit to the specialist,10,11 such as travel, missed work, co-pays, and child-care expenses. Clinical questions addressed using an eConsult system subsequently free up office visit appointment slots, improving access for patients requiring in-office evaluation.8,12
Orange County’s only academic health system, the University of California, Irvine (UCI), serves a population of 3.5 million, and its principal priority is providing the communities in the county (which is the sixth largest in United States) and the surrounding region with the highest quality health care possible. Thus, UCI aimed to improve its referral processes and provide timely access to specialty care for its patients by implementing an eConsults program that allows PCPs to efficiently receive specialist recommendations on referral problems that do not require the specialist to evaluate the patient in person. This report describes our experiences with developing and implementing a custom-built eConsults workflow in UCI’s prior electronic health record (EHR) platform, Allscripts, and subsequently transitioning our mature eConsults program to a new EHR system when UCI adopted Epic. UCI is likely the only academic medical center to have experience in successfully implementing eConsults into 2 different EHR systems.
Setting
UCI’s medical center is a 417-bed acute care hospital providing tertiary and quaternary care, ambulatory and specialty medical clinics, behavioral health care, and rehabilitation services. It is located in Orange, CA, and serves a diverse population of 3.5 million persons with broad health care needs. With more than 400 specialty and primary care physicians, UCI offers a full scope of acute and general care services. It is also the primary teaching location for UCI medical and nursing students, medical residents, and fellows, and is home to Orange County’s only adult Level I and pediatric Level II trauma centers and the regional burn center.
eConsults Program
We designed the initial eConsults program within UCI’s Allscripts EHR platform. Our information technology (IT) build team developed unique “documents-based” eConsults workflows that simplified the process of initiating requests directly from the EHR and facilitated rapid responses from participating specialties. The requesting provider’s eConsults interface was user-friendly, and referring providers were able to initiate an eConsult easily by selecting the customized eConsult icon from the Allscripts main toolbar. To ensure that all relevant information is provided to the specialists, condition-specific templates are embedded in the requesting provider’s eConsults workflow that allow PCPs to enter a focused, patient-specific clinical question and provide guidance on recommended patient information (eg, health history, laboratory results, and digital images) that may help the specialist provide an informed response. The eConsult templates were adapted from standardized forms developed by partner University of California Health Systems in an initiative funded by the University of California Center for Health Quality and Innovation.
To facilitate timely responses from specialists, an innovative notification system was created in the responding provider’s eConsults workflow to automatically send an email to participating specialists when a new eConsult is requested. The responding provider’s workflow also includes an option for the specialist to decline the eConsult if the case is deemed too complex to be addressed electronically. For every completed eConsult that does not result in an in-person patient evaluation, the requesting provider and responding specialist each receives a modest reimbursement, which was initially paid by UCI Health System funds.
Implementation
The design and implementation of the eConsults program began in November 2014, and was guided by a steering committee that included the chair of the department of medicine, chief medical information officer, primary care and specialty physician leads, IT build team, and a project manager. Early on, members of this committee engaged UCI leadership to affirm support for the program and obtain the IT resources needed to build the eConsults workflow. Regular steering committee meetings were established to discuss the design of the workflow, adapt the clinical content of the referral templates, and develop a provider reimbursement plan. After completion of the workflow build, the eConsults system was tested to identify failure points and obtain feedback from users. Prior to going live, the eConsults program was publicized by members of the steering committee through meetings with primary care groups and email communications. Committee members also hosted in-person training and orientation sessions with PCPs and participating specialists, and distributed tip sheets summarizing the steps to complete the PCP and specialist eConsult workflows.
The eConsults workflow build, testing, and launch were completed within 5 months (April 2015; Figure 1). eConsults went live in the 3 initial specialties (endocrinology, cardiology, and rheumatology) that were interested in participating in the first wave of the program. UCI’s eConsults service has subsequently expanded to 17 total specialties (allergy, cardiology, dermatology, endocrinology, gastroenterology, geriatrics, gynecology, hematology, hepatology, infectious disease, nephrology, neurology, palliative care, psychiatry, pulmonary, rheumatology, and sports medicine).

Two and half years after the eConsults program was implemented in Allscripts, UCI adopted a new EHR platform, Epic. By this time, the eConsults service had grown into a mature program with greater numbers of PCP users and submitted eConsults (Figure 2). Using our experience with the Allscripts build, our IT team was able to efficiently transition the eConsults service to the new EHR system. In contrast to the “documents-based” eConsult workflows on Allscripts, our IT team utilized an “orders-based” strategy on Epic, which followed a more traditional approach to requesting a consultation. We re-launched the service in Epic within 3 months (February 2018). However, both platforms utilized user-friendly workflows to achieve similar goals, and the program has continued to grow with respect to the number of users and eConsults.

Measurement/Analysis
The impact of the program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of PCP users, the number of submitted eConsult requests per PCP, and the number of requests per specialty. The response time for eConsult requests and the self-reported amount of time spent by specialists on the response were also tracked. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback. Provider satisfaction was primarily obtained by soliciting feedback from individual eConsult users.
Implementation of this eConsults program constituted a quality improvement activity and did not require Institutional Review Board review.
Results
Since the program was launched in April 2015, more than 1400 eConsults have been completed across 17 specialties (Figure 3). There were 654 completed eConsults on the Allscripts platform, and 808 eConsults have been completed using the Epic platform to date. The busiest eConsult specialties were endocrinology (receiving 276, or 19%, of the eConsults requests), hematology (receiving 249 requests, or 17%), infectious disease (receiving 244 requests, or 17% ), and cardiology (receiving 148 requests, or 10%).

The self-reported amount of time specialists spent on the response was different between the 2 EHR systems (Figure 4). On Allscripts, specialists reported that 23% of eConsults took 10 minutes or less to complete, 47% took 11 to 20 minutes, 23% took 21 to 30 minutes, and 7% took more than 30 minutes. On Epic, specialists reported that 42% of eConsults took 10 minutes or less to complete, 44% took 11 to 20 minutes, 12% took 21 to 30 minutes, and 2% took more than 30 minutes. This difference in time spent fielding eConsults likely represents the subtle nuances between Allscripts’ “documents-based” and Epic’s “orders-based” workflows.

As a result of the automated notification system that was introduced early in the eConsults implementation process on Allscripts, the specialty response times were much faster than the expected 3 business days’ turnaround goal instituted by the Center for Health Quality and Innovation initiative, regardless of the EHR platform used. In fact, the average turnaround time for an eConsult response across all specialties was 1 business day, which was similar for both EHR systems (Figure 5). Furthermore, more than 50% of the eConsults on both EHR systems received specialist responses within the same day of the eConsult request (63% on Allscripts, 54% on Epic). There was a small decrease in the percentage of same-day responses when we transitioned to Epic, likely because the functionality of an automated notification email could not be restored in Epic. Regardless, the specialty response times on Epic remained expeditious, likely because the automated notifications on Allscripts instilled good practices for the specialists, and regularly checking for new eConsult requests became an ingrained behavior.

Our most important finding was that approximately 80% of eConsult requests were addressed without the need for an in-office visit with a specialist. This measure was similar for both EHR platforms (83% on Allscripts and 78% on Epic).
Provider feedback has been overwhelmingly positive. PCPs are impressed with the robust educational content of the eConsult responses, since the goal for specialists is to justify their recommendations. Specialists appreciate the convenience and efficiency that eConsults offer, as well as the improved communication and collaboration among physicians. eConsults have been especially beneficial to PCPs at UCI’s Family Health Centers, who are now able to receive subspecialty consultations from UCI specialists despite insurance barriers.
Discussion
Our eConsults program uniquely contrasts with other programs because UCI is likely the only academic medical center to have experience in successfully incorporating eConsults into 2 different EHR systems: initial development of the eConsults workflow in UCI’s existing Allscripts EHR platform, and subsequently transitioning a mature eConsults program to a new EHR system when the institution adopted Epic.
We measured the impact of the eConsults program on access to care by the response time for eConsult requests and the percentage of eConsults that averted an in-office visit with a specialist. We found that the eConsults program at UCI provided our PCPs access to specialist consultations in a timely manner, with much shorter response times than standard in-person referrals. The average turnaround time for an eConsult response we reported is consistent with findings from other studies.12-15 Additionally, our program was able to address about 80% of its eConsults electronically, helping to reduce unnecessary in-person specialist referrals. In the literature, the percentage of eConsults that avoided an in-person specialist visit varies widely.8,12-16
We reported very positive feedback from both PCPs and specialists on UCI’s eConsults service. Similarly, other studies described PCP satisfaction with their respective eConsults programs to be uniformly high,8,9,13,14,17-19 though some reported that the level of satisfaction among specialists was more varied.18-21
Lessons Learned
The successful design and implementation of our eConsults program began with assembling the right clinical champions and technology partners for our steering committee. Establishing regular steering committee meetings helped maintain an appropriate timeline for completion of different aspects of the project. Engaging support from UCI’s leadership also provided us with a dedicated IT team that helped us with the build, training resources, troubleshooting issues, and reporting for the project.
Our experience with implementing the eConsults program on 2 different EHR systems highlighted the importance of creating efficient, user-friendly workflows to foster provider adoption and achieve sustainability. Allscripts’ open platform gave our IT team the ability to create a homegrown solution to implementing an eConsult model that was simple and easy to use. The Epic platform’s interoperability allowed us to leverage our learnings from the Allscripts build to efficiently implement eConsults in Epic.
We also found that providing modest incentive payments or reimbursements to both PCPs and specialists for each completed eConsult helps with both adoption and program sustainability. Initially, credit for the eConsult work was paid by internal UCI Health System funds. Two payers, UC Care (a preferred provider organization plan created just for the University of California) and more recently, the Centers for Medicare & Medicaid Services, have agreed to reimburse for outpatient eConsults. Securing additional payers for reimbursement of the eConsult service will not only ensure the program’s long-term sustainability, but also represents an acknowledgment of the value of eConsults in supporting access to care.
Applicability
Other health care settings that are experiencing issues with specialty care access can successfully implement their own eConsults program by employing strategies similar to those described in this report—assembling the right team, creating user-friendly workflows, and providing incentives. Our advice for successful implementation is to clearly communicate your goals to all involved, including primary care, specialists, leadership, and IT partners, and establish with these stakeholders the appropriate support and resources needed to facilitate the development of the program and overcome any barriers to adoption.
Current Status and Future Directions
Our future plans include continuing to optimize the Epic eConsult backend build and workflows using our experience in Allscripts. We have implemented eConsult workflows for use by graduate medical education trainees and advanced practice providers, with attending supervision. Further work is in progress to optimize these workflows, which will allow for appropriate education and supervision without delaying care. Furthermore, we plan to expand the program to include inpatient-to-inpatient and emergency department-to-inpatient eConsults. We will continue to expand the eConsults program by offering additional specialties, engage providers to encourage ongoing participation, and maximize PCP use by continuing to market the program through regular newsletters and email communications. Finally, the eConsults has served as an effective, important resource in the current era of COVID-19 in several ways: it allows for optimization of specialty input in patient care delivery without subjecting more health care workers to unnecessary exposure; saves on utilization of precious personal protective equipment; and enhances our ability to deal with a potential surge by providing access to specialists remotely and electronically all hours of the day, thus expanding care to the evening and weekend hours.
Acknowledgment: The authors thank our steering committee members (Dr. Ralph Cygan, Dr. Andrew Reikes, Dr. Byron Allen, Dr. George Lawry) and IT build team (Lori Bocchicchio, Meghan van Witsen, Jaymee Zillgitt, Tanya Sickles, Dennis Hoang, Jeanette Lisak-Phillips) for their contributions in the design and implementation of our eConsults program. We also thank additional team members Kurt McArthur and Neaktisia Lee for their assistance with generating reports, and Kathy LaPierre, Jennifer Rios, and Debra Webb Torres for their guidance with compliance and billing issues.
Corresponding author: Alpesh N. Amin, MD, MBA, University of California, Irvine, 101 The City Drive South, Building 26, Room 1000, ZC-4076H, Orange, CA 92868; [email protected].
Financial disclosures: None.
1. County of Orange, Health Care Agency, Public Health Services. Orange County Health Profile 2013.
2. Coffman JM, Fix M Ko, M. California physician supply and distribution: headed for a drought? California Health Care Foundation, June 2018.
3. Spetz J, Coffman J, Geyn I. California’s primary care workforce: forecasted supply, demand, and pipeline of trainees, 2016-2030. Healthforce Center at the University of California, San Francisco, August 2017.
4. Gandhi TK, Sittig DF, Franklin M, et al. Communication breakdown in the outpatient referral process. J Gen Intern Med. 2000;15:626-631.
5. McPhee SJ, Lo B, Saika GY, Meltzer R. How good is communication between primary care physicians and subspecialty consultants? Arch Intern Med. 1984;144:1265-1268.
6. Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89:39-68.
7. Wrenn K, Catschegn S, Cruz M, et al. Analysis of an electronic consultation program at an academic medical centre: Primary care provider questions, specialist responses, and primary care provider actions. J Telemed Telecare. 2017;23: 217-224.
8. Gleason N, Prasad PA, Ackerman S, et al. Adoption and impact of an eConsult system in a fee-for-service setting. Healthc (Amst). 2017;5(1-2):40-45.
9. Stoves J, Connolly J, Cheung CK, et al. Electronic consultation as an alternative to hospital referral for patients with chronic kidney disease: a novel application for networked electronic health records to improve the accessibility and efficiency of healthcare. Qual Saf Health Care. 2010;19: e54.
10. Datta SK, Warshaw EM, Edison KE, et al. Cost and utility analysis of a store-and-forward teledermatology referral system: a randomized clinical trial. JAMA Dermatol. 2015;151:1323-1329.
11. Liddy C, Drosinis P, Deri Armstrong C, et al. What are the cost savings associated with providing access to specialist care through the Champlain BASE eConsult service? A costing evaluation. BMJ Open. 2016;6:e010920.
12. Barnett ML, Yee HF Jr, Mehrotra A, Giboney P. Los Angeles safety-net program eConsult system was rapidly adopted and decreased wait times to see specialists. Health Aff. 2017;36:492-499.
13. Malagrino GD, Chaudhry R, Gardner M, et al. A study of 6,000 electronic specialty consultations for person-centered care at The Mayo Clinic. Int J Person Centered Med. 2012;2:458-466.
14. Keely E, Liddy C, Afkham A. Utilization, benefits, and impact of an e-consultation service across diverse specialties and primary care providers. Telemed J E Health. 2013;19:733-738.
15. Scherpbier-de Haan ND, van Gelder VA, Van Weel C, et al. Initial implementation of a web-based consultation process for patients with chronic kidney disease. Ann Fam Med. 2013;11:151-156.
16. Palen TE, Price D, Shetterly S, Wallace KB. Comparing virtual consults to traditional consults using an electronic health record: an observational case-control study. BMC Med Inform Decis Mak. 2012;12:65.
17. Liddy C, Afkham A, Drosinis P, et al. Impact of and satisfaction with a new eConsult service: a mixed methods study of primary care providers. J Am Board Fam Med. 2015;28:394-403.
18. Angstman KB, Adamson SC, Furst JW, et al. Provider satisfaction with virtual specialist consultations in a family medicine department. Health Care Manag (Frederick). 2009;28:14-18.
19. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA health care system. Fed Pract. 2014; 31:26–31.
20. Keely E, Williams R, Epstein G, et al. Specialist perspectives on Ontario Provincial electronic consultation services. Telemed J E Health. 2019;25:3-10.
21. Kim-Hwang JE, Chen AH, Bell DS, et al. Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25:1123-1128.
1. County of Orange, Health Care Agency, Public Health Services. Orange County Health Profile 2013.
2. Coffman JM, Fix M Ko, M. California physician supply and distribution: headed for a drought? California Health Care Foundation, June 2018.
3. Spetz J, Coffman J, Geyn I. California’s primary care workforce: forecasted supply, demand, and pipeline of trainees, 2016-2030. Healthforce Center at the University of California, San Francisco, August 2017.
4. Gandhi TK, Sittig DF, Franklin M, et al. Communication breakdown in the outpatient referral process. J Gen Intern Med. 2000;15:626-631.
5. McPhee SJ, Lo B, Saika GY, Meltzer R. How good is communication between primary care physicians and subspecialty consultants? Arch Intern Med. 1984;144:1265-1268.
6. Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89:39-68.
7. Wrenn K, Catschegn S, Cruz M, et al. Analysis of an electronic consultation program at an academic medical centre: Primary care provider questions, specialist responses, and primary care provider actions. J Telemed Telecare. 2017;23: 217-224.
8. Gleason N, Prasad PA, Ackerman S, et al. Adoption and impact of an eConsult system in a fee-for-service setting. Healthc (Amst). 2017;5(1-2):40-45.
9. Stoves J, Connolly J, Cheung CK, et al. Electronic consultation as an alternative to hospital referral for patients with chronic kidney disease: a novel application for networked electronic health records to improve the accessibility and efficiency of healthcare. Qual Saf Health Care. 2010;19: e54.
10. Datta SK, Warshaw EM, Edison KE, et al. Cost and utility analysis of a store-and-forward teledermatology referral system: a randomized clinical trial. JAMA Dermatol. 2015;151:1323-1329.
11. Liddy C, Drosinis P, Deri Armstrong C, et al. What are the cost savings associated with providing access to specialist care through the Champlain BASE eConsult service? A costing evaluation. BMJ Open. 2016;6:e010920.
12. Barnett ML, Yee HF Jr, Mehrotra A, Giboney P. Los Angeles safety-net program eConsult system was rapidly adopted and decreased wait times to see specialists. Health Aff. 2017;36:492-499.
13. Malagrino GD, Chaudhry R, Gardner M, et al. A study of 6,000 electronic specialty consultations for person-centered care at The Mayo Clinic. Int J Person Centered Med. 2012;2:458-466.
14. Keely E, Liddy C, Afkham A. Utilization, benefits, and impact of an e-consultation service across diverse specialties and primary care providers. Telemed J E Health. 2013;19:733-738.
15. Scherpbier-de Haan ND, van Gelder VA, Van Weel C, et al. Initial implementation of a web-based consultation process for patients with chronic kidney disease. Ann Fam Med. 2013;11:151-156.
16. Palen TE, Price D, Shetterly S, Wallace KB. Comparing virtual consults to traditional consults using an electronic health record: an observational case-control study. BMC Med Inform Decis Mak. 2012;12:65.
17. Liddy C, Afkham A, Drosinis P, et al. Impact of and satisfaction with a new eConsult service: a mixed methods study of primary care providers. J Am Board Fam Med. 2015;28:394-403.
18. Angstman KB, Adamson SC, Furst JW, et al. Provider satisfaction with virtual specialist consultations in a family medicine department. Health Care Manag (Frederick). 2009;28:14-18.
19. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA health care system. Fed Pract. 2014; 31:26–31.
20. Keely E, Williams R, Epstein G, et al. Specialist perspectives on Ontario Provincial electronic consultation services. Telemed J E Health. 2019;25:3-10.
21. Kim-Hwang JE, Chen AH, Bell DS, et al. Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25:1123-1128.
Framingham risk score may also predict cognitive decline
“In the absence of effective treatments for dementia, we need to monitor and control cardiovascular risk burden as a way to maintain patient’s cognitive health as they age,” said Weili Xu, PhD, Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin, China, in a press release.
“Given the progressive increase in the number of dementia cases worldwide, our findings have both clinical and public health relevance.”
Dr. Xu and first author Ruixue Song, MSc, also from Tianjin Medical University, published their findings online ahead of print May 18 in the Journal of the American College of Cardiology.
The World Health Organization projects that up to 82 million people will have dementia by 2050. Given the lack of effective treatments for dementia, identifying modifiable risk factors for cognitive decline and aggressively managing them is an increasingly appealing strategy.
Assessing cardiovascular risk and cognition
The researchers followed 1,588 dementia-free participants from the Rush Memory and Aging Project for 21 years (median, 5.8 years). FGCRS was assessed at baseline and categorized into tertiles (lowest, middle, and highest). Mean age of the studied population was 79.5 years, 75.8% of participants were female, and mean Framingham score was 15.6 (range, 4 to 28).
Annual evaluations included assessment of episodic memory (memory of everyday events), semantic memory (long-term memory), working memory (short-term memory), visuospatial ability (capacity to identify visual and spatial relationships among objects), and perceptual speed (ability to accurately and completely compare letters, numbers, objects, pictures, or patterns) using 19 tests to derive a composite score.
A subsample (n = 378) of participants underwent MRI, and structural total and regional brain volumes were estimated.
Linear regression was used to estimate beta-coefficients for the relationship between cardiovascular risk burden at baseline and longitudinally. If the beta-coefficient is negative, the interpretation is that for every 1-unit increase in the predictor variable (FGCRS), the outcome variable (cognitive function) will decrease by the beta-coefficient value.
At baseline, higher FGCRS was related to small but consistent (although not usually statistically significant) decreases in hippocampal volume, gray matter, and total brain volume.
Considered longitudinally, participants in the highest-risk tertile of FGCRS experienced faster decline in global cognition (beta = −0.019), episodic memory (beta = −0.023), working memory (beta = −0.021), and perceptual speed (beta = −0.027) during follow-up (P < .05 for all) than those in the lowest-risk tertile.
The declines in semantic memory (beta = –0.012) and visuospatial ability (beta = –0.010) did not reach statistical significance.
Bringing dementia prevention into the exam room early
Commenting on the research, Costantino Iadecola, MD, director of the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine in New York City, said the study has immediate clinical usefulness.
“The link between the cardiovascular risk factors and dementia is well known, but in your doctor’s office, that link is not seen. If your GP or cardiologist sees you with high blood pressure, he’s not immediately going to think about the risk of dementia 20 years later,” said Dr. Iadecola.
“What this study does is it directly links a simple score that’s commonly used to assess cardiovascular risk to dementia risk, which can be used to counsel patients and, hopefully, reduce the risk of both cardiovascular disease and cognitive disorders.”
Dr. Iadecola wrote an editorial together with Neal S. Parikh, MD, MS, also from Weill Cornell Medicine, that accompanied the findings of the trial.
Even neurologists sometimes fail to make the connection between vascular risk and dementia, he said. “They think that by making a stroke patient move their hand better, they’re treating them, but 30% of stroke patients get dementia 6 or 8 months later and they’re missing this link between cerebrovascular pathology and dementia.
Dr. Iadecola is one of 26 experts who authored the recent Berlin Manifesto, an effort led by Vladimir Hachinski, MD, professor of neurology and epidemiology at Western University in Ontario, Canada, to raise awareness of the link between cardiovascular and brain health.
Dr. Hachinski coined the term “brain attack” and devised the Hachinski Ischemic Score that remains the standard for identifying a vascular component of cognitive impairment.
The current study has some strengths and limitations, noted Dr. Iadecola. The average age of participants was 80 years, which is appropriate given the high risk for cognitive decline at this age, but the generalizability of the study may be limited given that most participants were white women.
Going forward, he said, rigorous studies are needed to confirm these findings and to determine how to best prevent dementia through treatment of individual cardiovascular risk factors.
Dr. Xu has received grants from nonindustry entities, including the Swedish Research Council and the National Natural Science Foundation of China. The study was funded by the European Union’s Horizon 320230 research and innovation program. Dr. Iadecola is a member of the scientific advisory board for Broadview Ventures.
This article appeared on Medscape.com.
“In the absence of effective treatments for dementia, we need to monitor and control cardiovascular risk burden as a way to maintain patient’s cognitive health as they age,” said Weili Xu, PhD, Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin, China, in a press release.
“Given the progressive increase in the number of dementia cases worldwide, our findings have both clinical and public health relevance.”
Dr. Xu and first author Ruixue Song, MSc, also from Tianjin Medical University, published their findings online ahead of print May 18 in the Journal of the American College of Cardiology.
The World Health Organization projects that up to 82 million people will have dementia by 2050. Given the lack of effective treatments for dementia, identifying modifiable risk factors for cognitive decline and aggressively managing them is an increasingly appealing strategy.
Assessing cardiovascular risk and cognition
The researchers followed 1,588 dementia-free participants from the Rush Memory and Aging Project for 21 years (median, 5.8 years). FGCRS was assessed at baseline and categorized into tertiles (lowest, middle, and highest). Mean age of the studied population was 79.5 years, 75.8% of participants were female, and mean Framingham score was 15.6 (range, 4 to 28).
Annual evaluations included assessment of episodic memory (memory of everyday events), semantic memory (long-term memory), working memory (short-term memory), visuospatial ability (capacity to identify visual and spatial relationships among objects), and perceptual speed (ability to accurately and completely compare letters, numbers, objects, pictures, or patterns) using 19 tests to derive a composite score.
A subsample (n = 378) of participants underwent MRI, and structural total and regional brain volumes were estimated.
Linear regression was used to estimate beta-coefficients for the relationship between cardiovascular risk burden at baseline and longitudinally. If the beta-coefficient is negative, the interpretation is that for every 1-unit increase in the predictor variable (FGCRS), the outcome variable (cognitive function) will decrease by the beta-coefficient value.
At baseline, higher FGCRS was related to small but consistent (although not usually statistically significant) decreases in hippocampal volume, gray matter, and total brain volume.
Considered longitudinally, participants in the highest-risk tertile of FGCRS experienced faster decline in global cognition (beta = −0.019), episodic memory (beta = −0.023), working memory (beta = −0.021), and perceptual speed (beta = −0.027) during follow-up (P < .05 for all) than those in the lowest-risk tertile.
The declines in semantic memory (beta = –0.012) and visuospatial ability (beta = –0.010) did not reach statistical significance.
Bringing dementia prevention into the exam room early
Commenting on the research, Costantino Iadecola, MD, director of the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine in New York City, said the study has immediate clinical usefulness.
“The link between the cardiovascular risk factors and dementia is well known, but in your doctor’s office, that link is not seen. If your GP or cardiologist sees you with high blood pressure, he’s not immediately going to think about the risk of dementia 20 years later,” said Dr. Iadecola.
“What this study does is it directly links a simple score that’s commonly used to assess cardiovascular risk to dementia risk, which can be used to counsel patients and, hopefully, reduce the risk of both cardiovascular disease and cognitive disorders.”
Dr. Iadecola wrote an editorial together with Neal S. Parikh, MD, MS, also from Weill Cornell Medicine, that accompanied the findings of the trial.
Even neurologists sometimes fail to make the connection between vascular risk and dementia, he said. “They think that by making a stroke patient move their hand better, they’re treating them, but 30% of stroke patients get dementia 6 or 8 months later and they’re missing this link between cerebrovascular pathology and dementia.
Dr. Iadecola is one of 26 experts who authored the recent Berlin Manifesto, an effort led by Vladimir Hachinski, MD, professor of neurology and epidemiology at Western University in Ontario, Canada, to raise awareness of the link between cardiovascular and brain health.
Dr. Hachinski coined the term “brain attack” and devised the Hachinski Ischemic Score that remains the standard for identifying a vascular component of cognitive impairment.
The current study has some strengths and limitations, noted Dr. Iadecola. The average age of participants was 80 years, which is appropriate given the high risk for cognitive decline at this age, but the generalizability of the study may be limited given that most participants were white women.
Going forward, he said, rigorous studies are needed to confirm these findings and to determine how to best prevent dementia through treatment of individual cardiovascular risk factors.
Dr. Xu has received grants from nonindustry entities, including the Swedish Research Council and the National Natural Science Foundation of China. The study was funded by the European Union’s Horizon 320230 research and innovation program. Dr. Iadecola is a member of the scientific advisory board for Broadview Ventures.
This article appeared on Medscape.com.
“In the absence of effective treatments for dementia, we need to monitor and control cardiovascular risk burden as a way to maintain patient’s cognitive health as they age,” said Weili Xu, PhD, Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin, China, in a press release.
“Given the progressive increase in the number of dementia cases worldwide, our findings have both clinical and public health relevance.”
Dr. Xu and first author Ruixue Song, MSc, also from Tianjin Medical University, published their findings online ahead of print May 18 in the Journal of the American College of Cardiology.
The World Health Organization projects that up to 82 million people will have dementia by 2050. Given the lack of effective treatments for dementia, identifying modifiable risk factors for cognitive decline and aggressively managing them is an increasingly appealing strategy.
Assessing cardiovascular risk and cognition
The researchers followed 1,588 dementia-free participants from the Rush Memory and Aging Project for 21 years (median, 5.8 years). FGCRS was assessed at baseline and categorized into tertiles (lowest, middle, and highest). Mean age of the studied population was 79.5 years, 75.8% of participants were female, and mean Framingham score was 15.6 (range, 4 to 28).
Annual evaluations included assessment of episodic memory (memory of everyday events), semantic memory (long-term memory), working memory (short-term memory), visuospatial ability (capacity to identify visual and spatial relationships among objects), and perceptual speed (ability to accurately and completely compare letters, numbers, objects, pictures, or patterns) using 19 tests to derive a composite score.
A subsample (n = 378) of participants underwent MRI, and structural total and regional brain volumes were estimated.
Linear regression was used to estimate beta-coefficients for the relationship between cardiovascular risk burden at baseline and longitudinally. If the beta-coefficient is negative, the interpretation is that for every 1-unit increase in the predictor variable (FGCRS), the outcome variable (cognitive function) will decrease by the beta-coefficient value.
At baseline, higher FGCRS was related to small but consistent (although not usually statistically significant) decreases in hippocampal volume, gray matter, and total brain volume.
Considered longitudinally, participants in the highest-risk tertile of FGCRS experienced faster decline in global cognition (beta = −0.019), episodic memory (beta = −0.023), working memory (beta = −0.021), and perceptual speed (beta = −0.027) during follow-up (P < .05 for all) than those in the lowest-risk tertile.
The declines in semantic memory (beta = –0.012) and visuospatial ability (beta = –0.010) did not reach statistical significance.
Bringing dementia prevention into the exam room early
Commenting on the research, Costantino Iadecola, MD, director of the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine in New York City, said the study has immediate clinical usefulness.
“The link between the cardiovascular risk factors and dementia is well known, but in your doctor’s office, that link is not seen. If your GP or cardiologist sees you with high blood pressure, he’s not immediately going to think about the risk of dementia 20 years later,” said Dr. Iadecola.
“What this study does is it directly links a simple score that’s commonly used to assess cardiovascular risk to dementia risk, which can be used to counsel patients and, hopefully, reduce the risk of both cardiovascular disease and cognitive disorders.”
Dr. Iadecola wrote an editorial together with Neal S. Parikh, MD, MS, also from Weill Cornell Medicine, that accompanied the findings of the trial.
Even neurologists sometimes fail to make the connection between vascular risk and dementia, he said. “They think that by making a stroke patient move their hand better, they’re treating them, but 30% of stroke patients get dementia 6 or 8 months later and they’re missing this link between cerebrovascular pathology and dementia.
Dr. Iadecola is one of 26 experts who authored the recent Berlin Manifesto, an effort led by Vladimir Hachinski, MD, professor of neurology and epidemiology at Western University in Ontario, Canada, to raise awareness of the link between cardiovascular and brain health.
Dr. Hachinski coined the term “brain attack” and devised the Hachinski Ischemic Score that remains the standard for identifying a vascular component of cognitive impairment.
The current study has some strengths and limitations, noted Dr. Iadecola. The average age of participants was 80 years, which is appropriate given the high risk for cognitive decline at this age, but the generalizability of the study may be limited given that most participants were white women.
Going forward, he said, rigorous studies are needed to confirm these findings and to determine how to best prevent dementia through treatment of individual cardiovascular risk factors.
Dr. Xu has received grants from nonindustry entities, including the Swedish Research Council and the National Natural Science Foundation of China. The study was funded by the European Union’s Horizon 320230 research and innovation program. Dr. Iadecola is a member of the scientific advisory board for Broadview Ventures.
This article appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
AHA offers advice on prehospital acute stroke triage amid COVID-19
A key goal is to ensure timely transfer of patients while minimizing the risk of infectious exposure for EMS personnel, coworkers, and other patients, the writing group says.
“Acute ischemic stroke is still a highly devastating disease and the Time Is Brain paradigm remains true during the COVID-19 pandemic as well,” said writing group chair Mayank Goyal, MD, of the University of Calgary (Alta.)
“We have highly effective and proven treatments available. As such, treatment delays due to additional screening requirements and personal protection equipment (PPE) should be kept at a minimum,” Dr. Goyal said.
“Practicing COVID-19 stroke work flows, through simulation training, can help to reduce treatment delays, minimize the risk of infectious exposure for patients and staff, and help alleviate stress,” he added.
A new layer of complexity
The guidance statement, Prehospital Triage of Acute Stroke Patients During the COVID-19 Pandemic, was published online May 13 in the journal Stroke.
“The need to limit infectious spread during the COVID-19 pandemic has added a new layer of complexity to prehospital stroke triage and transfer,” the writing group noted. “Timely and enhanced” communication between EMS, hospitals, and local coordinating authorities are critical, especially ambulance-and facility-based telestroke networks, they wrote.
The main factors to guide the triage decision are the likelihood of a large vessel occlusion; the magnitude of additional delays because of interhospital transfer and work flow efficiency at the primary stroke center or acute stroke ready hospital; the need for advanced critical care resources; and the available bed, staff, and PPE resources at the hospitals.
The group said it “seems reasonable” to lower the threshold to bypass hospitals that can’t provide acute stroke treatment in favor of transporting to a hospital that is “stroke ready,” particularly in patients likely to require advanced care. They cautioned, however, that taking all acute stroke patients to a comprehensive stroke center could overwhelm these centers and lead to clustering of COVID-19 patients.
They said it is equally important to ensure “necessary transfers” of stroke patients who would benefit from endovascular therapy or neurocritical care and avoid unnecessary patient transfers. “Doing so will likely require local hospital boards and health care authorities to collaborate and establish local guidelines and protocols,” the writing group said.
“During the COVID-19 pandemic, it is more important than ever to ensure that stroke patients are taken to the right hospital that can meet their urgent needs at the outset,” Dr. Goyal commented in an AHA news release.
The writing group emphasized that the principles put forth in the document are intended as suggestions rather than strict rules and will be adapted and updated to meet the evolving needs during the COVID-19 crisis and future pandemics.
“The process of improving stroke work flow and getting the correct patient to the correct hospital fast is dependent on training, protocols, simulation, technology, and – probably most importantly – teamwork. These principles are extremely important during the current pandemic but will be useful in improving stroke care afterwards as well,” Dr. Goyal said.
This research had no commercial funding. Members of the writing committee are on several AHA/ASA Council Science Subcommittees, including the Emergency Neurovascular Care, the Telestroke, and the Neurovascular Intervention committees. Goyal is a consultant for Medtronic, Stryker, Microvention, GE Healthcare, and Mentice. A complete list of author disclosures is available with the original article.
This article first appeared on Medscape.com.
A key goal is to ensure timely transfer of patients while minimizing the risk of infectious exposure for EMS personnel, coworkers, and other patients, the writing group says.
“Acute ischemic stroke is still a highly devastating disease and the Time Is Brain paradigm remains true during the COVID-19 pandemic as well,” said writing group chair Mayank Goyal, MD, of the University of Calgary (Alta.)
“We have highly effective and proven treatments available. As such, treatment delays due to additional screening requirements and personal protection equipment (PPE) should be kept at a minimum,” Dr. Goyal said.
“Practicing COVID-19 stroke work flows, through simulation training, can help to reduce treatment delays, minimize the risk of infectious exposure for patients and staff, and help alleviate stress,” he added.
A new layer of complexity
The guidance statement, Prehospital Triage of Acute Stroke Patients During the COVID-19 Pandemic, was published online May 13 in the journal Stroke.
“The need to limit infectious spread during the COVID-19 pandemic has added a new layer of complexity to prehospital stroke triage and transfer,” the writing group noted. “Timely and enhanced” communication between EMS, hospitals, and local coordinating authorities are critical, especially ambulance-and facility-based telestroke networks, they wrote.
The main factors to guide the triage decision are the likelihood of a large vessel occlusion; the magnitude of additional delays because of interhospital transfer and work flow efficiency at the primary stroke center or acute stroke ready hospital; the need for advanced critical care resources; and the available bed, staff, and PPE resources at the hospitals.
The group said it “seems reasonable” to lower the threshold to bypass hospitals that can’t provide acute stroke treatment in favor of transporting to a hospital that is “stroke ready,” particularly in patients likely to require advanced care. They cautioned, however, that taking all acute stroke patients to a comprehensive stroke center could overwhelm these centers and lead to clustering of COVID-19 patients.
They said it is equally important to ensure “necessary transfers” of stroke patients who would benefit from endovascular therapy or neurocritical care and avoid unnecessary patient transfers. “Doing so will likely require local hospital boards and health care authorities to collaborate and establish local guidelines and protocols,” the writing group said.
“During the COVID-19 pandemic, it is more important than ever to ensure that stroke patients are taken to the right hospital that can meet their urgent needs at the outset,” Dr. Goyal commented in an AHA news release.
The writing group emphasized that the principles put forth in the document are intended as suggestions rather than strict rules and will be adapted and updated to meet the evolving needs during the COVID-19 crisis and future pandemics.
“The process of improving stroke work flow and getting the correct patient to the correct hospital fast is dependent on training, protocols, simulation, technology, and – probably most importantly – teamwork. These principles are extremely important during the current pandemic but will be useful in improving stroke care afterwards as well,” Dr. Goyal said.
This research had no commercial funding. Members of the writing committee are on several AHA/ASA Council Science Subcommittees, including the Emergency Neurovascular Care, the Telestroke, and the Neurovascular Intervention committees. Goyal is a consultant for Medtronic, Stryker, Microvention, GE Healthcare, and Mentice. A complete list of author disclosures is available with the original article.
This article first appeared on Medscape.com.
A key goal is to ensure timely transfer of patients while minimizing the risk of infectious exposure for EMS personnel, coworkers, and other patients, the writing group says.
“Acute ischemic stroke is still a highly devastating disease and the Time Is Brain paradigm remains true during the COVID-19 pandemic as well,” said writing group chair Mayank Goyal, MD, of the University of Calgary (Alta.)
“We have highly effective and proven treatments available. As such, treatment delays due to additional screening requirements and personal protection equipment (PPE) should be kept at a minimum,” Dr. Goyal said.
“Practicing COVID-19 stroke work flows, through simulation training, can help to reduce treatment delays, minimize the risk of infectious exposure for patients and staff, and help alleviate stress,” he added.
A new layer of complexity
The guidance statement, Prehospital Triage of Acute Stroke Patients During the COVID-19 Pandemic, was published online May 13 in the journal Stroke.
“The need to limit infectious spread during the COVID-19 pandemic has added a new layer of complexity to prehospital stroke triage and transfer,” the writing group noted. “Timely and enhanced” communication between EMS, hospitals, and local coordinating authorities are critical, especially ambulance-and facility-based telestroke networks, they wrote.
The main factors to guide the triage decision are the likelihood of a large vessel occlusion; the magnitude of additional delays because of interhospital transfer and work flow efficiency at the primary stroke center or acute stroke ready hospital; the need for advanced critical care resources; and the available bed, staff, and PPE resources at the hospitals.
The group said it “seems reasonable” to lower the threshold to bypass hospitals that can’t provide acute stroke treatment in favor of transporting to a hospital that is “stroke ready,” particularly in patients likely to require advanced care. They cautioned, however, that taking all acute stroke patients to a comprehensive stroke center could overwhelm these centers and lead to clustering of COVID-19 patients.
They said it is equally important to ensure “necessary transfers” of stroke patients who would benefit from endovascular therapy or neurocritical care and avoid unnecessary patient transfers. “Doing so will likely require local hospital boards and health care authorities to collaborate and establish local guidelines and protocols,” the writing group said.
“During the COVID-19 pandemic, it is more important than ever to ensure that stroke patients are taken to the right hospital that can meet their urgent needs at the outset,” Dr. Goyal commented in an AHA news release.
The writing group emphasized that the principles put forth in the document are intended as suggestions rather than strict rules and will be adapted and updated to meet the evolving needs during the COVID-19 crisis and future pandemics.
“The process of improving stroke work flow and getting the correct patient to the correct hospital fast is dependent on training, protocols, simulation, technology, and – probably most importantly – teamwork. These principles are extremely important during the current pandemic but will be useful in improving stroke care afterwards as well,” Dr. Goyal said.
This research had no commercial funding. Members of the writing committee are on several AHA/ASA Council Science Subcommittees, including the Emergency Neurovascular Care, the Telestroke, and the Neurovascular Intervention committees. Goyal is a consultant for Medtronic, Stryker, Microvention, GE Healthcare, and Mentice. A complete list of author disclosures is available with the original article.
This article first appeared on Medscape.com.
AAN publishes ethical guidance on patient care during the pandemic
The document, which was published online May 15 in Neurology, reviews adaptations to the inpatient and outpatient settings and addresses the need to develop protocols for the allocation of scarce medical resources. The guidance is the product of a joint committee of the AAN, the American Neurological Association, the Child Neurology Society, and the Neurocritical Care Society Ethics Committee.
“Now is one of the most challenging times of our careers as neurologists,” said James C. Stevens, MD, president of the AAN, in a press release. “Clinics and hospitals are adapting to caring for the most ill, managing scarce resources, and trying to protect people without the disease. As neurologists, we must continue to adapt our daily practice, continue to care for our most ill neurology patients, and help contribute to the care of those afflicted with COVID-19.”
The role of telehealth
The authors recommended that ordinary appointments be held using telehealth, which, they say, already has become part of patient care. Telehealth enables neurologists to continue providing care while reducing the risk of exposure to and spread of SARS-CoV-2. The disadvantages of telehealth are that it limits physical examinations and behavioral health examinations, the authors acknowledged. “Each clinician should decide, in concert with his or her patient, if an in-person evaluation warrants the risk of an encounter,” according to the guidance.
Neurologists also should advise their patients that their neurologic condition could affect their relative risk of hospitalization and death resulting from COVID-19. Patients with multiple sclerosis or myasthenia gravis, for example, may be receiving corticosteroids or immunomodulatory therapies that make them more vulnerable to COVID-19 infection. “Even if desired services are available, neurologists and their patients ought to consider whether their care plans can safely be delayed in order to mitigate risk,” wrote the authors. Neurologists must try to maintain the customary standard of care, however, for patients with neurologic disease severe enough to warrant hospitalization, such as stroke or epilepsy.
The potential need for triage
Resources such as ventilators and ICU beds are limited, and health care facilities have had to triage them during the pandemic. Patients with a neurologic disease that decreases their likelihood of survival from a respiratory illness may not be offered these resources. Neurologists should discuss with patients and decision makers the ways in which reduced resources might affect patient care. Neurologists must “be aware of the burden of disease in their local community and how healthcare leaders plan on coping with a surge,” according to the guidance.
Advance directives, which should be a standard part of clinical care, take on increased importance during the pandemic. Patients who have not completed advance care planning documents should be encouraged to do so, according to the authors. These documents include patients’ preferences for “do not attempt resuscitation” status. Nevertheless, “we must assure patients with chronic illness that diminished resources in this healthcare crisis will not restrict their access to comfort and palliative care,” the document states.
Scarce resource allocation protocols
In the event that a surge in patients overwhelms a hospital’s contingencies and forces it to operate in crisis mode, it should have a scarce resource allocation protocol in place.
“This will surely be the most challenging aspect of patient care during this pandemic public health emergency,” wrote the authors. To ensure transparency and to mitigate the emotional effect of these decisions on patients and clinicians, scarce resource allocation protocols should be developed by teams that include intensivists, clinical ethicists, and nursing representatives who are not directly involved in the care of the critically ill patients. The goal of these protocols is to maximize the number of lives saved. They generally include an initial patient assessment followed by regular reevaluations to determine whether patients using scarce resources are benefiting less than other patients who need the same resources. The protocols should consider not only patients with COVID-19 infection, but also patients with stroke, traumatic injury, influenza, and heart failure who may need the same resources. Race, gender, ethnicity, socioeconomics, and perceived social worth should not influence care decisions, according to the guidance. Validated mortality prediction scales, such as the Glasgow Outcome Scale, can contribute to care decisions. Obtaining community input into these protocols will ensure trust in the health care system.
“If the situation necessitates hard decisions, we need to be fair, objective, transparent, and adamantly preserve our professional integrity,” wrote the authors. “Through it all, we owe it to our patients and families, as well as ourselves, to maintain our own health and wellness.”
The guidance was developed without funding, and the authors reported no relevant disclosures.
SOURCE: Rubin MA et al. Neurology. 2020 May 15. doi: 10.1212/WNL.0000000000009744.
The document, which was published online May 15 in Neurology, reviews adaptations to the inpatient and outpatient settings and addresses the need to develop protocols for the allocation of scarce medical resources. The guidance is the product of a joint committee of the AAN, the American Neurological Association, the Child Neurology Society, and the Neurocritical Care Society Ethics Committee.
“Now is one of the most challenging times of our careers as neurologists,” said James C. Stevens, MD, president of the AAN, in a press release. “Clinics and hospitals are adapting to caring for the most ill, managing scarce resources, and trying to protect people without the disease. As neurologists, we must continue to adapt our daily practice, continue to care for our most ill neurology patients, and help contribute to the care of those afflicted with COVID-19.”
The role of telehealth
The authors recommended that ordinary appointments be held using telehealth, which, they say, already has become part of patient care. Telehealth enables neurologists to continue providing care while reducing the risk of exposure to and spread of SARS-CoV-2. The disadvantages of telehealth are that it limits physical examinations and behavioral health examinations, the authors acknowledged. “Each clinician should decide, in concert with his or her patient, if an in-person evaluation warrants the risk of an encounter,” according to the guidance.
Neurologists also should advise their patients that their neurologic condition could affect their relative risk of hospitalization and death resulting from COVID-19. Patients with multiple sclerosis or myasthenia gravis, for example, may be receiving corticosteroids or immunomodulatory therapies that make them more vulnerable to COVID-19 infection. “Even if desired services are available, neurologists and their patients ought to consider whether their care plans can safely be delayed in order to mitigate risk,” wrote the authors. Neurologists must try to maintain the customary standard of care, however, for patients with neurologic disease severe enough to warrant hospitalization, such as stroke or epilepsy.
The potential need for triage
Resources such as ventilators and ICU beds are limited, and health care facilities have had to triage them during the pandemic. Patients with a neurologic disease that decreases their likelihood of survival from a respiratory illness may not be offered these resources. Neurologists should discuss with patients and decision makers the ways in which reduced resources might affect patient care. Neurologists must “be aware of the burden of disease in their local community and how healthcare leaders plan on coping with a surge,” according to the guidance.
Advance directives, which should be a standard part of clinical care, take on increased importance during the pandemic. Patients who have not completed advance care planning documents should be encouraged to do so, according to the authors. These documents include patients’ preferences for “do not attempt resuscitation” status. Nevertheless, “we must assure patients with chronic illness that diminished resources in this healthcare crisis will not restrict their access to comfort and palliative care,” the document states.
Scarce resource allocation protocols
In the event that a surge in patients overwhelms a hospital’s contingencies and forces it to operate in crisis mode, it should have a scarce resource allocation protocol in place.
“This will surely be the most challenging aspect of patient care during this pandemic public health emergency,” wrote the authors. To ensure transparency and to mitigate the emotional effect of these decisions on patients and clinicians, scarce resource allocation protocols should be developed by teams that include intensivists, clinical ethicists, and nursing representatives who are not directly involved in the care of the critically ill patients. The goal of these protocols is to maximize the number of lives saved. They generally include an initial patient assessment followed by regular reevaluations to determine whether patients using scarce resources are benefiting less than other patients who need the same resources. The protocols should consider not only patients with COVID-19 infection, but also patients with stroke, traumatic injury, influenza, and heart failure who may need the same resources. Race, gender, ethnicity, socioeconomics, and perceived social worth should not influence care decisions, according to the guidance. Validated mortality prediction scales, such as the Glasgow Outcome Scale, can contribute to care decisions. Obtaining community input into these protocols will ensure trust in the health care system.
“If the situation necessitates hard decisions, we need to be fair, objective, transparent, and adamantly preserve our professional integrity,” wrote the authors. “Through it all, we owe it to our patients and families, as well as ourselves, to maintain our own health and wellness.”
The guidance was developed without funding, and the authors reported no relevant disclosures.
SOURCE: Rubin MA et al. Neurology. 2020 May 15. doi: 10.1212/WNL.0000000000009744.
The document, which was published online May 15 in Neurology, reviews adaptations to the inpatient and outpatient settings and addresses the need to develop protocols for the allocation of scarce medical resources. The guidance is the product of a joint committee of the AAN, the American Neurological Association, the Child Neurology Society, and the Neurocritical Care Society Ethics Committee.
“Now is one of the most challenging times of our careers as neurologists,” said James C. Stevens, MD, president of the AAN, in a press release. “Clinics and hospitals are adapting to caring for the most ill, managing scarce resources, and trying to protect people without the disease. As neurologists, we must continue to adapt our daily practice, continue to care for our most ill neurology patients, and help contribute to the care of those afflicted with COVID-19.”
The role of telehealth
The authors recommended that ordinary appointments be held using telehealth, which, they say, already has become part of patient care. Telehealth enables neurologists to continue providing care while reducing the risk of exposure to and spread of SARS-CoV-2. The disadvantages of telehealth are that it limits physical examinations and behavioral health examinations, the authors acknowledged. “Each clinician should decide, in concert with his or her patient, if an in-person evaluation warrants the risk of an encounter,” according to the guidance.
Neurologists also should advise their patients that their neurologic condition could affect their relative risk of hospitalization and death resulting from COVID-19. Patients with multiple sclerosis or myasthenia gravis, for example, may be receiving corticosteroids or immunomodulatory therapies that make them more vulnerable to COVID-19 infection. “Even if desired services are available, neurologists and their patients ought to consider whether their care plans can safely be delayed in order to mitigate risk,” wrote the authors. Neurologists must try to maintain the customary standard of care, however, for patients with neurologic disease severe enough to warrant hospitalization, such as stroke or epilepsy.
The potential need for triage
Resources such as ventilators and ICU beds are limited, and health care facilities have had to triage them during the pandemic. Patients with a neurologic disease that decreases their likelihood of survival from a respiratory illness may not be offered these resources. Neurologists should discuss with patients and decision makers the ways in which reduced resources might affect patient care. Neurologists must “be aware of the burden of disease in their local community and how healthcare leaders plan on coping with a surge,” according to the guidance.
Advance directives, which should be a standard part of clinical care, take on increased importance during the pandemic. Patients who have not completed advance care planning documents should be encouraged to do so, according to the authors. These documents include patients’ preferences for “do not attempt resuscitation” status. Nevertheless, “we must assure patients with chronic illness that diminished resources in this healthcare crisis will not restrict their access to comfort and palliative care,” the document states.
Scarce resource allocation protocols
In the event that a surge in patients overwhelms a hospital’s contingencies and forces it to operate in crisis mode, it should have a scarce resource allocation protocol in place.
“This will surely be the most challenging aspect of patient care during this pandemic public health emergency,” wrote the authors. To ensure transparency and to mitigate the emotional effect of these decisions on patients and clinicians, scarce resource allocation protocols should be developed by teams that include intensivists, clinical ethicists, and nursing representatives who are not directly involved in the care of the critically ill patients. The goal of these protocols is to maximize the number of lives saved. They generally include an initial patient assessment followed by regular reevaluations to determine whether patients using scarce resources are benefiting less than other patients who need the same resources. The protocols should consider not only patients with COVID-19 infection, but also patients with stroke, traumatic injury, influenza, and heart failure who may need the same resources. Race, gender, ethnicity, socioeconomics, and perceived social worth should not influence care decisions, according to the guidance. Validated mortality prediction scales, such as the Glasgow Outcome Scale, can contribute to care decisions. Obtaining community input into these protocols will ensure trust in the health care system.
“If the situation necessitates hard decisions, we need to be fair, objective, transparent, and adamantly preserve our professional integrity,” wrote the authors. “Through it all, we owe it to our patients and families, as well as ourselves, to maintain our own health and wellness.”
The guidance was developed without funding, and the authors reported no relevant disclosures.
SOURCE: Rubin MA et al. Neurology. 2020 May 15. doi: 10.1212/WNL.0000000000009744.
FROM NEUROLOGY
Blood pressure lowering lessens risk of dementia, cognitive decline
“Although observational studies report hypertension to be an important risk factor for dementia, the benefit of blood pressure lowering on dementia or cognitive impairment in clinical trials is modest and lower than the risk reduction for stroke,” wrote Diarmaid Hughes, MB, of the NUI Galway and Saolta University Hospital Group in Galway, Ireland, and coauthors. They added, however, that “these findings have the potential to inform public health strategies to reduce the burden of dementia globally.” The study was published online ahead of print May 19 in JAMA.
A rich data set
To assess the relationship between lowering blood pressure and cognitive issues, the researchers performed a systemic search of randomized, clinical trials that compared blood pressure lowering via antihypertensive agents with a control, had at least 1 year of follow-up, included more than 1,000 participants, and reported on either dementia, cognitive impairment, cognitive decline, or a change in cognitive test scores as outcomes. Of the 14 studies deemed eligible, 12 reported either the incidence of dementia (n = 9) or a composite of dementia or cognitive impairment (n = 3) at follow-up and thus were included in the primary meta-analysis. The other two studies were used for secondary outcomes only.
The studies included 96,158 participants in total – 42.2% were women – and their mean age was 69 years. At baseline, participants’ mean systolic blood pressure was 154 mm Hg and their mean diastolic blood pressure was 83.3 mm Hg. The mean duration of follow-up was 49.24 months.
In the 12 trials that reported dementia or cognitive impairment, blood pressure lowering via antihypertensive agents, compared with control, was significantly associated with a reduction in those two outcomes (7.0% vs. 7.5% over a mean trial follow-up of 4.1 years; odds ratio, 0.93; 95% confidence interval, 0.88-0.98; absolute risk reduction, 0.39%; 95% CI, 0.09%-0.68%). Blood pressure lowering, compared with control, was also significantly associated with a reduction in cognitive decline (20.2% vs. 21.1% over a mean trial follow-up of 4.1 years; OR, 0.93; 95% CI, 0.88-0.99; ARR, 0.71%; 95% CI, 0.19%-1.2%) in the eight trials that reported it as an outcome. An analysis of the eight trials that reported a change in cognitive scores did not find a significant association between that outcome and blood pressure lowering.
Subpopulations should be examined
“This is a very broad brush stroke study, albeit a definitive one,” Richard J. Caselli, MD, of the Mayo Clinic in Phoenix said in an interview. “With all the thousands of people in this meta-analysis, there are going to be subpopulations of patients with certain characteristics or common conditions in which blood pressure lowering might have a bigger or a lesser impact on their risk factor. Is there a difference between certain racial groups? Does it matter what antihypertensive strategies are used? You can look at the interactions between blood pressure lowering and other conditions: diabetes, head injuries, air pollution, certain genetic risk factors. There are a number of additional findings that could come from a very rich data set like this.”
The authors acknowledged their study’s limitations, including the challenges of performing a meta-analysis of studies that drew from different populations and had potentially different definitions of dementia, cognitive impairment, and cognitive decline outcomes. In addition, the low incidence of dementia across clinical trials limited the researchers, and its underdetection in trials and the potential of survivor bias for healthier participants with blood pressure reductions were noted as “unmeasured sources of potential error.”
Three authors reported receiving grants or personal fees from the Wellcome Trust and the Health Research Board, the Chief Scientist Office, and Bayer AG, respectively.
SOURCE: Hughes D et al. JAMA. 2020 May 19. doi: 10.1001/jama.2020.4249.
“Although observational studies report hypertension to be an important risk factor for dementia, the benefit of blood pressure lowering on dementia or cognitive impairment in clinical trials is modest and lower than the risk reduction for stroke,” wrote Diarmaid Hughes, MB, of the NUI Galway and Saolta University Hospital Group in Galway, Ireland, and coauthors. They added, however, that “these findings have the potential to inform public health strategies to reduce the burden of dementia globally.” The study was published online ahead of print May 19 in JAMA.
A rich data set
To assess the relationship between lowering blood pressure and cognitive issues, the researchers performed a systemic search of randomized, clinical trials that compared blood pressure lowering via antihypertensive agents with a control, had at least 1 year of follow-up, included more than 1,000 participants, and reported on either dementia, cognitive impairment, cognitive decline, or a change in cognitive test scores as outcomes. Of the 14 studies deemed eligible, 12 reported either the incidence of dementia (n = 9) or a composite of dementia or cognitive impairment (n = 3) at follow-up and thus were included in the primary meta-analysis. The other two studies were used for secondary outcomes only.
The studies included 96,158 participants in total – 42.2% were women – and their mean age was 69 years. At baseline, participants’ mean systolic blood pressure was 154 mm Hg and their mean diastolic blood pressure was 83.3 mm Hg. The mean duration of follow-up was 49.24 months.
In the 12 trials that reported dementia or cognitive impairment, blood pressure lowering via antihypertensive agents, compared with control, was significantly associated with a reduction in those two outcomes (7.0% vs. 7.5% over a mean trial follow-up of 4.1 years; odds ratio, 0.93; 95% confidence interval, 0.88-0.98; absolute risk reduction, 0.39%; 95% CI, 0.09%-0.68%). Blood pressure lowering, compared with control, was also significantly associated with a reduction in cognitive decline (20.2% vs. 21.1% over a mean trial follow-up of 4.1 years; OR, 0.93; 95% CI, 0.88-0.99; ARR, 0.71%; 95% CI, 0.19%-1.2%) in the eight trials that reported it as an outcome. An analysis of the eight trials that reported a change in cognitive scores did not find a significant association between that outcome and blood pressure lowering.
Subpopulations should be examined
“This is a very broad brush stroke study, albeit a definitive one,” Richard J. Caselli, MD, of the Mayo Clinic in Phoenix said in an interview. “With all the thousands of people in this meta-analysis, there are going to be subpopulations of patients with certain characteristics or common conditions in which blood pressure lowering might have a bigger or a lesser impact on their risk factor. Is there a difference between certain racial groups? Does it matter what antihypertensive strategies are used? You can look at the interactions between blood pressure lowering and other conditions: diabetes, head injuries, air pollution, certain genetic risk factors. There are a number of additional findings that could come from a very rich data set like this.”
The authors acknowledged their study’s limitations, including the challenges of performing a meta-analysis of studies that drew from different populations and had potentially different definitions of dementia, cognitive impairment, and cognitive decline outcomes. In addition, the low incidence of dementia across clinical trials limited the researchers, and its underdetection in trials and the potential of survivor bias for healthier participants with blood pressure reductions were noted as “unmeasured sources of potential error.”
Three authors reported receiving grants or personal fees from the Wellcome Trust and the Health Research Board, the Chief Scientist Office, and Bayer AG, respectively.
SOURCE: Hughes D et al. JAMA. 2020 May 19. doi: 10.1001/jama.2020.4249.
“Although observational studies report hypertension to be an important risk factor for dementia, the benefit of blood pressure lowering on dementia or cognitive impairment in clinical trials is modest and lower than the risk reduction for stroke,” wrote Diarmaid Hughes, MB, of the NUI Galway and Saolta University Hospital Group in Galway, Ireland, and coauthors. They added, however, that “these findings have the potential to inform public health strategies to reduce the burden of dementia globally.” The study was published online ahead of print May 19 in JAMA.
A rich data set
To assess the relationship between lowering blood pressure and cognitive issues, the researchers performed a systemic search of randomized, clinical trials that compared blood pressure lowering via antihypertensive agents with a control, had at least 1 year of follow-up, included more than 1,000 participants, and reported on either dementia, cognitive impairment, cognitive decline, or a change in cognitive test scores as outcomes. Of the 14 studies deemed eligible, 12 reported either the incidence of dementia (n = 9) or a composite of dementia or cognitive impairment (n = 3) at follow-up and thus were included in the primary meta-analysis. The other two studies were used for secondary outcomes only.
The studies included 96,158 participants in total – 42.2% were women – and their mean age was 69 years. At baseline, participants’ mean systolic blood pressure was 154 mm Hg and their mean diastolic blood pressure was 83.3 mm Hg. The mean duration of follow-up was 49.24 months.
In the 12 trials that reported dementia or cognitive impairment, blood pressure lowering via antihypertensive agents, compared with control, was significantly associated with a reduction in those two outcomes (7.0% vs. 7.5% over a mean trial follow-up of 4.1 years; odds ratio, 0.93; 95% confidence interval, 0.88-0.98; absolute risk reduction, 0.39%; 95% CI, 0.09%-0.68%). Blood pressure lowering, compared with control, was also significantly associated with a reduction in cognitive decline (20.2% vs. 21.1% over a mean trial follow-up of 4.1 years; OR, 0.93; 95% CI, 0.88-0.99; ARR, 0.71%; 95% CI, 0.19%-1.2%) in the eight trials that reported it as an outcome. An analysis of the eight trials that reported a change in cognitive scores did not find a significant association between that outcome and blood pressure lowering.
Subpopulations should be examined
“This is a very broad brush stroke study, albeit a definitive one,” Richard J. Caselli, MD, of the Mayo Clinic in Phoenix said in an interview. “With all the thousands of people in this meta-analysis, there are going to be subpopulations of patients with certain characteristics or common conditions in which blood pressure lowering might have a bigger or a lesser impact on their risk factor. Is there a difference between certain racial groups? Does it matter what antihypertensive strategies are used? You can look at the interactions between blood pressure lowering and other conditions: diabetes, head injuries, air pollution, certain genetic risk factors. There are a number of additional findings that could come from a very rich data set like this.”
The authors acknowledged their study’s limitations, including the challenges of performing a meta-analysis of studies that drew from different populations and had potentially different definitions of dementia, cognitive impairment, and cognitive decline outcomes. In addition, the low incidence of dementia across clinical trials limited the researchers, and its underdetection in trials and the potential of survivor bias for healthier participants with blood pressure reductions were noted as “unmeasured sources of potential error.”
Three authors reported receiving grants or personal fees from the Wellcome Trust and the Health Research Board, the Chief Scientist Office, and Bayer AG, respectively.
SOURCE: Hughes D et al. JAMA. 2020 May 19. doi: 10.1001/jama.2020.4249.
FROM JAMA
Frontal lobe glucose abnormalities may indicate increased SUDEP risk
, new research suggests.
“The data provide initial evidence that hypometabolism in certain parts of the frontal cortex may be associated with higher SUDEP risk,” said lead author Maysaa M. Basha, MD, associate professor of neurology and director of the Adult Comprehensive Epilepsy Program, Wayne State University/Detroit Medical Center, in Michigan.
If this research is validated, “it potentially can be used to screen patients for higher SUDEP risk,” she said. The idea is to identify those at high risk and then reduce that risk with more aggressive management of seizures or closer monitoring in certain cases, she added.
The research is being presented online as part of the 2020 American Academy of Neurology (AAN) Science Highlights.
Hypometabolism
Dr. Basha and colleagues were encouraged to pursue this new line of research after a pilot [18F]fluorodeoxyglucose positron-emission tomography (FDG-PET) study revealed frontal lobe hypometabolism among patients who subsequently died.
“We wanted to determine if such a metabolic abnormality is associated with SUDEP risk,” said Dr. Basha. She noted that no PET studies have addressed this question, only MRI studies.
In this new study, researchers aimed to identify specific patterns of objectively detected brain glucose metabolic abnormalities in patients with refractory focal epilepsy who were at risk for SUDEP.
The study included 80 patients (45 female patients) aged 16 to 61 years (mean age, 37 years) who underwent FDG-PET as part of their presurgical evaluation for epilepsy surgery. Patients with large brain lesions, such as an infarct or a large tumor, were excluded from the study; such lesions can affect the accuracy of an objective PET analysis, explained Dr. Basha.
The researchers assessed risk for SUDEP using the seven-item SUDEP inventory (SUDEP-7), which was developed as a marker of clinical SUDEP risk. The 0- to 10-point scale is used to evaluate the frequency of tonic-clonic and other seizures, the duration of epilepsy, the use of antiepileptic drugs, and intellectual disability.
The researchers calculated SUDEP-7 inventory scores as closely as possible to FDG-PET assessments. The mean score in the patient population was 3.6.
The investigators divided participants into two subgroups: 22 patients had a SUDEP score of 5 or greater; and 58 had a score of less than 5 (higher scores indicate higher risk for SUDEP).
The researchers compared PET scans of each of these subgroups to PET scans from healthy adults to determine whether they showed common areas of metabolic abnormality. For this, they used an image analytic software program called Statistical Parametric Mapping, which compares group values of metabolic activity measured in small units of the brain (voxels) with statistical methods.
The analysis showed that the higher-risk group displayed a common pattern of hypometabolism in certain brain areas.
“The epilepsy patient subgroup with high SUDEP risk showed areas of decreased metabolism, as compared to the control group, in portions of the frontal cortex,” said Dr. Basha. “The statistically most significant decreases were in the right frontal lobe area—both lateral convexity and medial cortex.”
Dr. Basha added that these group abnormalities were “remarkably similar” to the individual metabolic abnormalities found in the four SUDEP patients in the previous pilot study who underwent PET scanning and who subsequently died.
A similar group analysis showed that the group at low SUDEP risk displayed no common metabolic abnormalities.
MRI findings were normal for 40 patients.
Dr. Basha and colleagues believe that “this is the first PET study assessing the metabolic correlates of SUDEP risk on the group level.”
Common feature
Interictal glucose hypometabolism is “common in and around epileptic foci,” noted Dr. Basha. However, this could extend into nonepileptic regions—for example, to remote connected regions where seizures can spread from the primary focus and into subcortical gray matter structures, such the thalamus.
Some of these metabolic abnormalities may indicate subtle, microscopic, structural abnormalities in the affected brain, said Dr. Basha.
Abnormalities that are induced by epilepsy and that result from purely metabolic changes could be partly or fully reversed if seizures are controlled on a long-term basis, she said. “Some metabolic abnormalities can be reversed after better seizure control with antiepileptic drugs, epileptic surgery, or other antiepileptic treatment,” she said.
It’s “quite possible” that the same brain pattern would be evident in children with epilepsy, although her team has not performed the same analysis in a younger pediatric group, said Dr. Basha. She noted that it would be unethical to administer PET scans, which involve radiation, to young, healthy control persons.
It’s too early to recommend that all epilepsy patients undergo FDG-PET scanning to see whether this pattern of brain glucose hypometabolism is present, said Dr. Basha. “But if this is proven to be a good biomarker, the next step would be a prospective study” to see whether this brain marker is a true signal of SUDEP risk.
“I don’t think our single study would do that, but ultimately, that would be the goal,” she added.
One more piece of the SUDEP puzzle
Commenting on the study, William Davis Gaillard, MD, president of the American Epilepsy Society and chief of neurology, Children’s National Medical Center, Chevy Chase, Maryland, said this new information provides one more piece of the SUDEP puzzle but doesn’t complete the picture.
The study authors assessed PET scans of a group of patients and found common abnormalities that implicate the right medial frontal cortex. “That’s a pretty reasonable method” of investigation, said Dr. Gaillard.
“The challenge is that they’re looking at people they believe have a risk of SUDEP as opposed to people who died,” said Dr. Gaillard.
But he agreed that the results might signal “a biomarker” that “allows you to identify who’s at high risk, and then you may be able to intervene to save them.”
It’s not clear that people with frontal lobe epilepsy are at greater risk for SUDEP than those with temporal lobe epilepsy, he said.
“What you don’t know is whether this represents people with a seizure focus in that area or this represents a common network implicated in people with diverse forms of focal epilepsy; so you need to do some more work,” he said.
Dr. Gaillard pointed out that other research has implicated regions other than the mesial frontal cortex in SUDEP risk. These regions include the insula, the amygdala, the hippocampus, and the brain stem.
He also noted that the SUDEP-7, which has not been thoroughly validated, is designed for use only in adults.
In his own practice, he asks patients about the frequency of tonic-clonic seizures and whether they occur at night. The number of antiepileptic medications a patient takes reflects the difficulty of controlling seizures and may not be “an independent variable for risk,” said Dr. Gaillard.
“It’s clear one needs a better assessment and better idea of who is at risk,” he said.
The researchers have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Basha A et al. AAN 2020. Abstract P5.001.
, new research suggests.
“The data provide initial evidence that hypometabolism in certain parts of the frontal cortex may be associated with higher SUDEP risk,” said lead author Maysaa M. Basha, MD, associate professor of neurology and director of the Adult Comprehensive Epilepsy Program, Wayne State University/Detroit Medical Center, in Michigan.
If this research is validated, “it potentially can be used to screen patients for higher SUDEP risk,” she said. The idea is to identify those at high risk and then reduce that risk with more aggressive management of seizures or closer monitoring in certain cases, she added.
The research is being presented online as part of the 2020 American Academy of Neurology (AAN) Science Highlights.
Hypometabolism
Dr. Basha and colleagues were encouraged to pursue this new line of research after a pilot [18F]fluorodeoxyglucose positron-emission tomography (FDG-PET) study revealed frontal lobe hypometabolism among patients who subsequently died.
“We wanted to determine if such a metabolic abnormality is associated with SUDEP risk,” said Dr. Basha. She noted that no PET studies have addressed this question, only MRI studies.
In this new study, researchers aimed to identify specific patterns of objectively detected brain glucose metabolic abnormalities in patients with refractory focal epilepsy who were at risk for SUDEP.
The study included 80 patients (45 female patients) aged 16 to 61 years (mean age, 37 years) who underwent FDG-PET as part of their presurgical evaluation for epilepsy surgery. Patients with large brain lesions, such as an infarct or a large tumor, were excluded from the study; such lesions can affect the accuracy of an objective PET analysis, explained Dr. Basha.
The researchers assessed risk for SUDEP using the seven-item SUDEP inventory (SUDEP-7), which was developed as a marker of clinical SUDEP risk. The 0- to 10-point scale is used to evaluate the frequency of tonic-clonic and other seizures, the duration of epilepsy, the use of antiepileptic drugs, and intellectual disability.
The researchers calculated SUDEP-7 inventory scores as closely as possible to FDG-PET assessments. The mean score in the patient population was 3.6.
The investigators divided participants into two subgroups: 22 patients had a SUDEP score of 5 or greater; and 58 had a score of less than 5 (higher scores indicate higher risk for SUDEP).
The researchers compared PET scans of each of these subgroups to PET scans from healthy adults to determine whether they showed common areas of metabolic abnormality. For this, they used an image analytic software program called Statistical Parametric Mapping, which compares group values of metabolic activity measured in small units of the brain (voxels) with statistical methods.
The analysis showed that the higher-risk group displayed a common pattern of hypometabolism in certain brain areas.
“The epilepsy patient subgroup with high SUDEP risk showed areas of decreased metabolism, as compared to the control group, in portions of the frontal cortex,” said Dr. Basha. “The statistically most significant decreases were in the right frontal lobe area—both lateral convexity and medial cortex.”
Dr. Basha added that these group abnormalities were “remarkably similar” to the individual metabolic abnormalities found in the four SUDEP patients in the previous pilot study who underwent PET scanning and who subsequently died.
A similar group analysis showed that the group at low SUDEP risk displayed no common metabolic abnormalities.
MRI findings were normal for 40 patients.
Dr. Basha and colleagues believe that “this is the first PET study assessing the metabolic correlates of SUDEP risk on the group level.”
Common feature
Interictal glucose hypometabolism is “common in and around epileptic foci,” noted Dr. Basha. However, this could extend into nonepileptic regions—for example, to remote connected regions where seizures can spread from the primary focus and into subcortical gray matter structures, such the thalamus.
Some of these metabolic abnormalities may indicate subtle, microscopic, structural abnormalities in the affected brain, said Dr. Basha.
Abnormalities that are induced by epilepsy and that result from purely metabolic changes could be partly or fully reversed if seizures are controlled on a long-term basis, she said. “Some metabolic abnormalities can be reversed after better seizure control with antiepileptic drugs, epileptic surgery, or other antiepileptic treatment,” she said.
It’s “quite possible” that the same brain pattern would be evident in children with epilepsy, although her team has not performed the same analysis in a younger pediatric group, said Dr. Basha. She noted that it would be unethical to administer PET scans, which involve radiation, to young, healthy control persons.
It’s too early to recommend that all epilepsy patients undergo FDG-PET scanning to see whether this pattern of brain glucose hypometabolism is present, said Dr. Basha. “But if this is proven to be a good biomarker, the next step would be a prospective study” to see whether this brain marker is a true signal of SUDEP risk.
“I don’t think our single study would do that, but ultimately, that would be the goal,” she added.
One more piece of the SUDEP puzzle
Commenting on the study, William Davis Gaillard, MD, president of the American Epilepsy Society and chief of neurology, Children’s National Medical Center, Chevy Chase, Maryland, said this new information provides one more piece of the SUDEP puzzle but doesn’t complete the picture.
The study authors assessed PET scans of a group of patients and found common abnormalities that implicate the right medial frontal cortex. “That’s a pretty reasonable method” of investigation, said Dr. Gaillard.
“The challenge is that they’re looking at people they believe have a risk of SUDEP as opposed to people who died,” said Dr. Gaillard.
But he agreed that the results might signal “a biomarker” that “allows you to identify who’s at high risk, and then you may be able to intervene to save them.”
It’s not clear that people with frontal lobe epilepsy are at greater risk for SUDEP than those with temporal lobe epilepsy, he said.
“What you don’t know is whether this represents people with a seizure focus in that area or this represents a common network implicated in people with diverse forms of focal epilepsy; so you need to do some more work,” he said.
Dr. Gaillard pointed out that other research has implicated regions other than the mesial frontal cortex in SUDEP risk. These regions include the insula, the amygdala, the hippocampus, and the brain stem.
He also noted that the SUDEP-7, which has not been thoroughly validated, is designed for use only in adults.
In his own practice, he asks patients about the frequency of tonic-clonic seizures and whether they occur at night. The number of antiepileptic medications a patient takes reflects the difficulty of controlling seizures and may not be “an independent variable for risk,” said Dr. Gaillard.
“It’s clear one needs a better assessment and better idea of who is at risk,” he said.
The researchers have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Basha A et al. AAN 2020. Abstract P5.001.
, new research suggests.
“The data provide initial evidence that hypometabolism in certain parts of the frontal cortex may be associated with higher SUDEP risk,” said lead author Maysaa M. Basha, MD, associate professor of neurology and director of the Adult Comprehensive Epilepsy Program, Wayne State University/Detroit Medical Center, in Michigan.
If this research is validated, “it potentially can be used to screen patients for higher SUDEP risk,” she said. The idea is to identify those at high risk and then reduce that risk with more aggressive management of seizures or closer monitoring in certain cases, she added.
The research is being presented online as part of the 2020 American Academy of Neurology (AAN) Science Highlights.
Hypometabolism
Dr. Basha and colleagues were encouraged to pursue this new line of research after a pilot [18F]fluorodeoxyglucose positron-emission tomography (FDG-PET) study revealed frontal lobe hypometabolism among patients who subsequently died.
“We wanted to determine if such a metabolic abnormality is associated with SUDEP risk,” said Dr. Basha. She noted that no PET studies have addressed this question, only MRI studies.
In this new study, researchers aimed to identify specific patterns of objectively detected brain glucose metabolic abnormalities in patients with refractory focal epilepsy who were at risk for SUDEP.
The study included 80 patients (45 female patients) aged 16 to 61 years (mean age, 37 years) who underwent FDG-PET as part of their presurgical evaluation for epilepsy surgery. Patients with large brain lesions, such as an infarct or a large tumor, were excluded from the study; such lesions can affect the accuracy of an objective PET analysis, explained Dr. Basha.
The researchers assessed risk for SUDEP using the seven-item SUDEP inventory (SUDEP-7), which was developed as a marker of clinical SUDEP risk. The 0- to 10-point scale is used to evaluate the frequency of tonic-clonic and other seizures, the duration of epilepsy, the use of antiepileptic drugs, and intellectual disability.
The researchers calculated SUDEP-7 inventory scores as closely as possible to FDG-PET assessments. The mean score in the patient population was 3.6.
The investigators divided participants into two subgroups: 22 patients had a SUDEP score of 5 or greater; and 58 had a score of less than 5 (higher scores indicate higher risk for SUDEP).
The researchers compared PET scans of each of these subgroups to PET scans from healthy adults to determine whether they showed common areas of metabolic abnormality. For this, they used an image analytic software program called Statistical Parametric Mapping, which compares group values of metabolic activity measured in small units of the brain (voxels) with statistical methods.
The analysis showed that the higher-risk group displayed a common pattern of hypometabolism in certain brain areas.
“The epilepsy patient subgroup with high SUDEP risk showed areas of decreased metabolism, as compared to the control group, in portions of the frontal cortex,” said Dr. Basha. “The statistically most significant decreases were in the right frontal lobe area—both lateral convexity and medial cortex.”
Dr. Basha added that these group abnormalities were “remarkably similar” to the individual metabolic abnormalities found in the four SUDEP patients in the previous pilot study who underwent PET scanning and who subsequently died.
A similar group analysis showed that the group at low SUDEP risk displayed no common metabolic abnormalities.
MRI findings were normal for 40 patients.
Dr. Basha and colleagues believe that “this is the first PET study assessing the metabolic correlates of SUDEP risk on the group level.”
Common feature
Interictal glucose hypometabolism is “common in and around epileptic foci,” noted Dr. Basha. However, this could extend into nonepileptic regions—for example, to remote connected regions where seizures can spread from the primary focus and into subcortical gray matter structures, such the thalamus.
Some of these metabolic abnormalities may indicate subtle, microscopic, structural abnormalities in the affected brain, said Dr. Basha.
Abnormalities that are induced by epilepsy and that result from purely metabolic changes could be partly or fully reversed if seizures are controlled on a long-term basis, she said. “Some metabolic abnormalities can be reversed after better seizure control with antiepileptic drugs, epileptic surgery, or other antiepileptic treatment,” she said.
It’s “quite possible” that the same brain pattern would be evident in children with epilepsy, although her team has not performed the same analysis in a younger pediatric group, said Dr. Basha. She noted that it would be unethical to administer PET scans, which involve radiation, to young, healthy control persons.
It’s too early to recommend that all epilepsy patients undergo FDG-PET scanning to see whether this pattern of brain glucose hypometabolism is present, said Dr. Basha. “But if this is proven to be a good biomarker, the next step would be a prospective study” to see whether this brain marker is a true signal of SUDEP risk.
“I don’t think our single study would do that, but ultimately, that would be the goal,” she added.
One more piece of the SUDEP puzzle
Commenting on the study, William Davis Gaillard, MD, president of the American Epilepsy Society and chief of neurology, Children’s National Medical Center, Chevy Chase, Maryland, said this new information provides one more piece of the SUDEP puzzle but doesn’t complete the picture.
The study authors assessed PET scans of a group of patients and found common abnormalities that implicate the right medial frontal cortex. “That’s a pretty reasonable method” of investigation, said Dr. Gaillard.
“The challenge is that they’re looking at people they believe have a risk of SUDEP as opposed to people who died,” said Dr. Gaillard.
But he agreed that the results might signal “a biomarker” that “allows you to identify who’s at high risk, and then you may be able to intervene to save them.”
It’s not clear that people with frontal lobe epilepsy are at greater risk for SUDEP than those with temporal lobe epilepsy, he said.
“What you don’t know is whether this represents people with a seizure focus in that area or this represents a common network implicated in people with diverse forms of focal epilepsy; so you need to do some more work,” he said.
Dr. Gaillard pointed out that other research has implicated regions other than the mesial frontal cortex in SUDEP risk. These regions include the insula, the amygdala, the hippocampus, and the brain stem.
He also noted that the SUDEP-7, which has not been thoroughly validated, is designed for use only in adults.
In his own practice, he asks patients about the frequency of tonic-clonic seizures and whether they occur at night. The number of antiepileptic medications a patient takes reflects the difficulty of controlling seizures and may not be “an independent variable for risk,” said Dr. Gaillard.
“It’s clear one needs a better assessment and better idea of who is at risk,” he said.
The researchers have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Basha A et al. AAN 2020. Abstract P5.001.
Yoga is a good adjunct to migraine therapy
in Neurology.
The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
in Neurology.

The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
in Neurology.

The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
FROM NEUROLOGY
Incidental finding on brain MRI seen in 5% of older patients
New research shows that Knowing the expected prevalence of such incidental findings in the older general population is “extremely useful” for both researchers and clinicians, said study co-author Sarah Elisabeth Keuss, MBChB, clinical research associate, Dementia Research Centre, UCL Queen Square Institute of Neurology, London, UK.
“In research, the knowledge helps to inform study protocols regarding how to manage incidental findings and enables study participants to be appropriately informed,” said Dr. Keuss. Greater awareness also helps clinicians make decisions about whether or not to scan a patient, she said, adding that imaging is increasingly available to them. It allows clinicians to counsel patients regarding the probability of an incidental finding and balance that risk against the potential benefits of having a test.
The research is being presented online as part of the American Academy of Neurology 2020 Science Highlights. The incidental findings also were published last year in BMJ Open.
The new findings are from the first wave of data collection for the Insight 46 study, a neuroimaging substudy of the MRC National Survey of Health and Development (NSHD) 1946 British birth cohort, a broadly representative sample of the population born in mainland Britain during 1946. The research uses detailed brain imaging, cognitive testing, and blood and other biomarkers to investigate genetic and life-course factors associated with Alzheimer’s disease and cerebrovascular disease.
The current study included 502 individuals, aged about 71 years at the time of the analysis, and 49% were women. Almost all (93.8%) participants underwent 1-day MRI scans. Some 4.5% of these participants had an incidental finding of brain abnormality as per a prespecified standardized protocol.
Suspected vascular malformations were present in 1.9%, and suspected intracranial mass lesions were present in 1.5%. The single most common vascular abnormality was a suspected cerebral aneurysm, which affected 1.1% of participants.
Suspected meningiomas were the most common intracranial lesion, affecting 0.6% of study participants.
Action plan
Participants and their primary care provider were informed of findings “that were deemed to be potentially serious, or life-threatening, or could have a major impact on quality of life,” said Dr. Keuss. Relevant experts “came up with a recommended clinical action plan to help the primary care provider decide what should be the next course of action with regard to investigation or referral to another specialist,” said Dr. Keuss.
The new results are important for clinical decision-making, said Dr. Keuss. “Clinicians should consider the possibility of detecting an incidental finding whenever they’re requesting a brain scan. They should balance that risk against the possible benefits of recommending a test.”
The prevalence of incidental findings on MRI reported in the literature varies because of different methods used to review scans. “However, comparing our study with similar studies, the prevalence of the key findings with regard to aneurysms and intracranial mass lesions are very similar,” said Dr. Keuss.
Dr. Keuss and colleagues do not recommend all elderly patients get a brain scan.
“We don’t know what the long-term consequences are of being informed you have an incidental finding of an abnormality; we don’t know if it improves their outcome, and it potentially could cause anxiety,” said Dr. Keuss.
Psychological impact
The researchers have not looked at the psychological impact of negative findings on study participants, but they could do so at a later date.
“It would be very important to look into that given the potential to cause anxiety,” said Dr. Keuss. “It’s important to find out the potential negative consequences to inform researchers in future about how best to manage these findings.”
From blood tests, the analysis found that more than a third (34.6%) of participants had at least one related abnormality. The most common of these were kidney impairment (about 9%), thyroid function abnormalities (between 4% and 5%), anemia (about 4%), and low vitamin B12 levels (about 3%).
However, few of these reached the prespecified threshold for urgent action, and Dr. Keuss noted these findings were not the focus of her AAN presentation.
A strength of the study was that participants were almost the exact same age.
Important issue
Commenting on the research, David S. Liebeskind, MD, professor of neurology and director, Neurovascular Imaging Research Core, University of California, Los Angeles, said it raises “a very interesting” and “important” public health issue.
“The question is whether we do things based around individual symptomatic status, or at a larger level in terms of public health, screening the larger population to figure out who is at risk for any particular disease or disorder.”
From the standpoint of imaging technologies like MRI that show details about brain structures, experts debate whether the population should be screened “before something occurs,” said Dr. Liebeskind. “Imaging has the capacity to tell us a tremendous amount; whether this implies we should therefore image everybody is a larger public health question.”
The issue is “fraught with a lot of difficulty and complexity” as treatment paradigms tend to be “built around symptomatic status,” he said. “When we sit in the office or with a patient at the bedside, we usually focus on that individual patient and not necessarily on the larger public.”
Dr. Liebeskind noted that the question of whether to put the emphasis on the individual patient or the public at large is also being discussed during the current COVID-19 pandemic.
He wasn’t surprised that the study uncovered incidental findings in almost 5% of the sample. “If you take an 80-year-old and study their brain, a good chunk, if not half or more, will have some abnormality,” he said.
Drs. Keuss and Liebeskind have reported no relevant financial relationships.
This article first appeared on Medscape.com.
New research shows that Knowing the expected prevalence of such incidental findings in the older general population is “extremely useful” for both researchers and clinicians, said study co-author Sarah Elisabeth Keuss, MBChB, clinical research associate, Dementia Research Centre, UCL Queen Square Institute of Neurology, London, UK.
“In research, the knowledge helps to inform study protocols regarding how to manage incidental findings and enables study participants to be appropriately informed,” said Dr. Keuss. Greater awareness also helps clinicians make decisions about whether or not to scan a patient, she said, adding that imaging is increasingly available to them. It allows clinicians to counsel patients regarding the probability of an incidental finding and balance that risk against the potential benefits of having a test.
The research is being presented online as part of the American Academy of Neurology 2020 Science Highlights. The incidental findings also were published last year in BMJ Open.
The new findings are from the first wave of data collection for the Insight 46 study, a neuroimaging substudy of the MRC National Survey of Health and Development (NSHD) 1946 British birth cohort, a broadly representative sample of the population born in mainland Britain during 1946. The research uses detailed brain imaging, cognitive testing, and blood and other biomarkers to investigate genetic and life-course factors associated with Alzheimer’s disease and cerebrovascular disease.
The current study included 502 individuals, aged about 71 years at the time of the analysis, and 49% were women. Almost all (93.8%) participants underwent 1-day MRI scans. Some 4.5% of these participants had an incidental finding of brain abnormality as per a prespecified standardized protocol.
Suspected vascular malformations were present in 1.9%, and suspected intracranial mass lesions were present in 1.5%. The single most common vascular abnormality was a suspected cerebral aneurysm, which affected 1.1% of participants.
Suspected meningiomas were the most common intracranial lesion, affecting 0.6% of study participants.
Action plan
Participants and their primary care provider were informed of findings “that were deemed to be potentially serious, or life-threatening, or could have a major impact on quality of life,” said Dr. Keuss. Relevant experts “came up with a recommended clinical action plan to help the primary care provider decide what should be the next course of action with regard to investigation or referral to another specialist,” said Dr. Keuss.
The new results are important for clinical decision-making, said Dr. Keuss. “Clinicians should consider the possibility of detecting an incidental finding whenever they’re requesting a brain scan. They should balance that risk against the possible benefits of recommending a test.”
The prevalence of incidental findings on MRI reported in the literature varies because of different methods used to review scans. “However, comparing our study with similar studies, the prevalence of the key findings with regard to aneurysms and intracranial mass lesions are very similar,” said Dr. Keuss.
Dr. Keuss and colleagues do not recommend all elderly patients get a brain scan.
“We don’t know what the long-term consequences are of being informed you have an incidental finding of an abnormality; we don’t know if it improves their outcome, and it potentially could cause anxiety,” said Dr. Keuss.
Psychological impact
The researchers have not looked at the psychological impact of negative findings on study participants, but they could do so at a later date.
“It would be very important to look into that given the potential to cause anxiety,” said Dr. Keuss. “It’s important to find out the potential negative consequences to inform researchers in future about how best to manage these findings.”
From blood tests, the analysis found that more than a third (34.6%) of participants had at least one related abnormality. The most common of these were kidney impairment (about 9%), thyroid function abnormalities (between 4% and 5%), anemia (about 4%), and low vitamin B12 levels (about 3%).
However, few of these reached the prespecified threshold for urgent action, and Dr. Keuss noted these findings were not the focus of her AAN presentation.
A strength of the study was that participants were almost the exact same age.
Important issue
Commenting on the research, David S. Liebeskind, MD, professor of neurology and director, Neurovascular Imaging Research Core, University of California, Los Angeles, said it raises “a very interesting” and “important” public health issue.
“The question is whether we do things based around individual symptomatic status, or at a larger level in terms of public health, screening the larger population to figure out who is at risk for any particular disease or disorder.”
From the standpoint of imaging technologies like MRI that show details about brain structures, experts debate whether the population should be screened “before something occurs,” said Dr. Liebeskind. “Imaging has the capacity to tell us a tremendous amount; whether this implies we should therefore image everybody is a larger public health question.”
The issue is “fraught with a lot of difficulty and complexity” as treatment paradigms tend to be “built around symptomatic status,” he said. “When we sit in the office or with a patient at the bedside, we usually focus on that individual patient and not necessarily on the larger public.”
Dr. Liebeskind noted that the question of whether to put the emphasis on the individual patient or the public at large is also being discussed during the current COVID-19 pandemic.
He wasn’t surprised that the study uncovered incidental findings in almost 5% of the sample. “If you take an 80-year-old and study their brain, a good chunk, if not half or more, will have some abnormality,” he said.
Drs. Keuss and Liebeskind have reported no relevant financial relationships.
This article first appeared on Medscape.com.
New research shows that Knowing the expected prevalence of such incidental findings in the older general population is “extremely useful” for both researchers and clinicians, said study co-author Sarah Elisabeth Keuss, MBChB, clinical research associate, Dementia Research Centre, UCL Queen Square Institute of Neurology, London, UK.
“In research, the knowledge helps to inform study protocols regarding how to manage incidental findings and enables study participants to be appropriately informed,” said Dr. Keuss. Greater awareness also helps clinicians make decisions about whether or not to scan a patient, she said, adding that imaging is increasingly available to them. It allows clinicians to counsel patients regarding the probability of an incidental finding and balance that risk against the potential benefits of having a test.
The research is being presented online as part of the American Academy of Neurology 2020 Science Highlights. The incidental findings also were published last year in BMJ Open.
The new findings are from the first wave of data collection for the Insight 46 study, a neuroimaging substudy of the MRC National Survey of Health and Development (NSHD) 1946 British birth cohort, a broadly representative sample of the population born in mainland Britain during 1946. The research uses detailed brain imaging, cognitive testing, and blood and other biomarkers to investigate genetic and life-course factors associated with Alzheimer’s disease and cerebrovascular disease.
The current study included 502 individuals, aged about 71 years at the time of the analysis, and 49% were women. Almost all (93.8%) participants underwent 1-day MRI scans. Some 4.5% of these participants had an incidental finding of brain abnormality as per a prespecified standardized protocol.
Suspected vascular malformations were present in 1.9%, and suspected intracranial mass lesions were present in 1.5%. The single most common vascular abnormality was a suspected cerebral aneurysm, which affected 1.1% of participants.
Suspected meningiomas were the most common intracranial lesion, affecting 0.6% of study participants.
Action plan
Participants and their primary care provider were informed of findings “that were deemed to be potentially serious, or life-threatening, or could have a major impact on quality of life,” said Dr. Keuss. Relevant experts “came up with a recommended clinical action plan to help the primary care provider decide what should be the next course of action with regard to investigation or referral to another specialist,” said Dr. Keuss.
The new results are important for clinical decision-making, said Dr. Keuss. “Clinicians should consider the possibility of detecting an incidental finding whenever they’re requesting a brain scan. They should balance that risk against the possible benefits of recommending a test.”
The prevalence of incidental findings on MRI reported in the literature varies because of different methods used to review scans. “However, comparing our study with similar studies, the prevalence of the key findings with regard to aneurysms and intracranial mass lesions are very similar,” said Dr. Keuss.
Dr. Keuss and colleagues do not recommend all elderly patients get a brain scan.
“We don’t know what the long-term consequences are of being informed you have an incidental finding of an abnormality; we don’t know if it improves their outcome, and it potentially could cause anxiety,” said Dr. Keuss.
Psychological impact
The researchers have not looked at the psychological impact of negative findings on study participants, but they could do so at a later date.
“It would be very important to look into that given the potential to cause anxiety,” said Dr. Keuss. “It’s important to find out the potential negative consequences to inform researchers in future about how best to manage these findings.”
From blood tests, the analysis found that more than a third (34.6%) of participants had at least one related abnormality. The most common of these were kidney impairment (about 9%), thyroid function abnormalities (between 4% and 5%), anemia (about 4%), and low vitamin B12 levels (about 3%).
However, few of these reached the prespecified threshold for urgent action, and Dr. Keuss noted these findings were not the focus of her AAN presentation.
A strength of the study was that participants were almost the exact same age.
Important issue
Commenting on the research, David S. Liebeskind, MD, professor of neurology and director, Neurovascular Imaging Research Core, University of California, Los Angeles, said it raises “a very interesting” and “important” public health issue.
“The question is whether we do things based around individual symptomatic status, or at a larger level in terms of public health, screening the larger population to figure out who is at risk for any particular disease or disorder.”
From the standpoint of imaging technologies like MRI that show details about brain structures, experts debate whether the population should be screened “before something occurs,” said Dr. Liebeskind. “Imaging has the capacity to tell us a tremendous amount; whether this implies we should therefore image everybody is a larger public health question.”
The issue is “fraught with a lot of difficulty and complexity” as treatment paradigms tend to be “built around symptomatic status,” he said. “When we sit in the office or with a patient at the bedside, we usually focus on that individual patient and not necessarily on the larger public.”
Dr. Liebeskind noted that the question of whether to put the emphasis on the individual patient or the public at large is also being discussed during the current COVID-19 pandemic.
He wasn’t surprised that the study uncovered incidental findings in almost 5% of the sample. “If you take an 80-year-old and study their brain, a good chunk, if not half or more, will have some abnormality,” he said.
Drs. Keuss and Liebeskind have reported no relevant financial relationships.
This article first appeared on Medscape.com.
New ‘atlas’ maps links between mental disorders, physical illnesses
Mental illnesses are associated with a significantly increased risk of subsequent physical diseases, new research shows.
An international team of researchers has created an “atlas” that maps the relationship between specific mental disorders and the risk of subsequent physical illnesses.
The researchers found that, following the diagnosis of a mental disorder, psychiatric patients are significantly more likely than the general population to develop potentially life-threatening conditions, including heart disease and stroke.
These findings, the investigators noted, highlight the need for better medical care in this vulnerable population. They have created a website with detailed information about the risks of specific physical ailments and the link to particular mental disorders.
“We found that women with anxiety disorders have a 50% increased risk of developing a heart condition or stroke – over 15 years, one in three women with anxiety disorders will develop these medical disorders,” lead investigator John McGrath, MD, PhD, University of Queensland’s Brain Institute, Brisbane, Australia, and Aarhus (Denmark) University, said in a statement.
“We also looked at men with substance use disorders such as alcohol-related disorders and found they have a 400% increased risk of gut or liver disorders, while over 15 years, one in five of them will develop gut or liver conditions,” he added.
The study was published in the New England Journal of Medicine.
New ‘atlas’
It’s well known that patients with mental disorders have decreased quality of life, increased health care utilization, and a shorter life expectancy than individuals in the general population – about 10 years for men and 7 years for women.
However, the investigators noted, previous research examining the relationship between mental disorders and medical conditions only focused on “particular pairs or a small set of mental disorders and medical conditions.”
“We needed a comprehensive study to map the links between different types of mental disorders versus different types of general medical conditions. Our study has provided this atlas,” Dr. McGrath said in an interview.
The clinical utility of such a map could provide comprehensive data on relative and absolute risks of various medical conditions after a diagnosis of a mental disorder. This information, the researchers noted, would “help clinicians and health care planners identify the primary prevention needs of their patients.”
The study included 5.9 million people born in Denmark between 1900 and 2015 and followed them from 2000 to 2016, a total of 83.9 million person-years. The researchers followed patients for up to 17 years (2000-2016) for medical diagnoses and up to 48 years (1969-2016) for diagnoses of mental disorders.
The study’s large sample size allowed investigators to assess 10 broad types of mental disorders and 9 broad categories of medical conditions that encompassed 31 specific conditions.
Categories of medical conditions included circulatory, endocrine, pulmonary, gastrointestinal, urogenital, musculoskeletal, hematologic, neurologic, and cancer. Mental disorder categories included organic disorders such as Alzheimer’s, substance abuse disorders, schizophrenia, mood disorders, neurotic disorders, eating disorders, personality disorders, developmental disorders, behavioral/emotional disorders, and intellectual disabilities.
The researchers estimated associations between 90 pairs of mental disorders and broad-category medical conditions, as well as 310 pairs of mental disorders and specific medical conditions.
‘Curious’ finding
Individuals with mental disorders showed a higher risk of medical conditions in 76 out of 90 specific mental disorder–medical condition pairs.
After adjusting for sex, age, calendar time, and previous coexisting mental disorders, the median hazard ratio for a subsequent medical condition was 1.37 in patients with a mental disorder.
The lowest HR was 0.82 for organic mental disorders and the broad category of cancer (95% confidence interval, 0.80-0.84), and the highest was 3.62 for eating disorders and urogenital conditions (95% CI, 3.11-4.22). On the other hand, schizophrenia was associated with a reduced risk of developing musculoskeletal conditions (HR, 0.87; 95% CI, 0.84-0.91).
Dr. McGrath described this finding as “curious” and speculated it “may be related to underlying genetic risk factors.”
compared with the matched reference group without a mood disorder (40.9% vs. 32.6%, respectively).
The risk of developing subsequent medical conditions after a mental disorder diagnosis did not remain steady over time. For instance, although mood disorders were associated with an increased risk of developing circulatory problems (HR, 1.32; 95% CI, 1.31-1.34), the highest risk occurred during the first 6 months following diagnosis and gradually decreased over the next 15 years (HR, 2.39; 95% CI, 2.29-2.48 and HR, 1.18; 95% CI, 1.17-1.20, respectively).
“Many people with mental disorders have unhealthy lifestyle, including low exercise, poor diet, smoking, and alcohol, which may account for the increased risk of physical illness, and also they may not seek and/or may not get quick treatment for their health conditions,” said Dr. McGrath.
Additionally, “perhaps some genetic and early life exposures, such as trauma, may increase the risk of both medical conditions and mental disorders,” he added. “We need better treatments for mental disorders, so that they do not slip into unemployment or poverty.”
A strong case
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto and head of the mood disorders psychopharmacology unit, University Health Network, said that the research “really makes a strong case for the fact that persons who have mental disorders are at higher risk of chronic diseases, and it’s the chronic diseases that decrease their lifespan.”
Dr. McIntyre, who is also director of the Depression and Bipolar Support Alliance, said that the “takeaway message is that mental disorders are not just brain disorders but are multisystem disorders.”
For this reason, “the most appropriate way to provide care would be to provide a holistic approach to treat and prevent the chronic diseases that lead to increase in mortality,” recommended Dr. McIntyre, who was not involved with the current study.
The study was supported by grants from the Danish National Research Foundation, the National Health and Medical Research Council, the Novo Nordisk Foundation , the European Union’s Horizon 2020 Research and Innovation Program, the Aarhus University Research Foundation, the Lundbeck Foundation, the National Institutes of Health, the European Commission, Helsefonden, the Danish Council for Independent Research, the Independent Research Fund Denmark, the National Health and Medical Research Council of Australia, and the National Institute on Drug Abuse.
Dr. McGrath has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. McIntyre reports receiving grants from Stanley Medical Research Institute; the Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/Chinese National Natural Research Foundation; and receiving speaking/consultation fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, and Minerva.
A version of this article originally appeared on Medscape.com.
Mental illnesses are associated with a significantly increased risk of subsequent physical diseases, new research shows.
An international team of researchers has created an “atlas” that maps the relationship between specific mental disorders and the risk of subsequent physical illnesses.
The researchers found that, following the diagnosis of a mental disorder, psychiatric patients are significantly more likely than the general population to develop potentially life-threatening conditions, including heart disease and stroke.
These findings, the investigators noted, highlight the need for better medical care in this vulnerable population. They have created a website with detailed information about the risks of specific physical ailments and the link to particular mental disorders.
“We found that women with anxiety disorders have a 50% increased risk of developing a heart condition or stroke – over 15 years, one in three women with anxiety disorders will develop these medical disorders,” lead investigator John McGrath, MD, PhD, University of Queensland’s Brain Institute, Brisbane, Australia, and Aarhus (Denmark) University, said in a statement.
“We also looked at men with substance use disorders such as alcohol-related disorders and found they have a 400% increased risk of gut or liver disorders, while over 15 years, one in five of them will develop gut or liver conditions,” he added.
The study was published in the New England Journal of Medicine.
New ‘atlas’
It’s well known that patients with mental disorders have decreased quality of life, increased health care utilization, and a shorter life expectancy than individuals in the general population – about 10 years for men and 7 years for women.
However, the investigators noted, previous research examining the relationship between mental disorders and medical conditions only focused on “particular pairs or a small set of mental disorders and medical conditions.”
“We needed a comprehensive study to map the links between different types of mental disorders versus different types of general medical conditions. Our study has provided this atlas,” Dr. McGrath said in an interview.
The clinical utility of such a map could provide comprehensive data on relative and absolute risks of various medical conditions after a diagnosis of a mental disorder. This information, the researchers noted, would “help clinicians and health care planners identify the primary prevention needs of their patients.”
The study included 5.9 million people born in Denmark between 1900 and 2015 and followed them from 2000 to 2016, a total of 83.9 million person-years. The researchers followed patients for up to 17 years (2000-2016) for medical diagnoses and up to 48 years (1969-2016) for diagnoses of mental disorders.
The study’s large sample size allowed investigators to assess 10 broad types of mental disorders and 9 broad categories of medical conditions that encompassed 31 specific conditions.
Categories of medical conditions included circulatory, endocrine, pulmonary, gastrointestinal, urogenital, musculoskeletal, hematologic, neurologic, and cancer. Mental disorder categories included organic disorders such as Alzheimer’s, substance abuse disorders, schizophrenia, mood disorders, neurotic disorders, eating disorders, personality disorders, developmental disorders, behavioral/emotional disorders, and intellectual disabilities.
The researchers estimated associations between 90 pairs of mental disorders and broad-category medical conditions, as well as 310 pairs of mental disorders and specific medical conditions.
‘Curious’ finding
Individuals with mental disorders showed a higher risk of medical conditions in 76 out of 90 specific mental disorder–medical condition pairs.
After adjusting for sex, age, calendar time, and previous coexisting mental disorders, the median hazard ratio for a subsequent medical condition was 1.37 in patients with a mental disorder.
The lowest HR was 0.82 for organic mental disorders and the broad category of cancer (95% confidence interval, 0.80-0.84), and the highest was 3.62 for eating disorders and urogenital conditions (95% CI, 3.11-4.22). On the other hand, schizophrenia was associated with a reduced risk of developing musculoskeletal conditions (HR, 0.87; 95% CI, 0.84-0.91).
Dr. McGrath described this finding as “curious” and speculated it “may be related to underlying genetic risk factors.”
compared with the matched reference group without a mood disorder (40.9% vs. 32.6%, respectively).
The risk of developing subsequent medical conditions after a mental disorder diagnosis did not remain steady over time. For instance, although mood disorders were associated with an increased risk of developing circulatory problems (HR, 1.32; 95% CI, 1.31-1.34), the highest risk occurred during the first 6 months following diagnosis and gradually decreased over the next 15 years (HR, 2.39; 95% CI, 2.29-2.48 and HR, 1.18; 95% CI, 1.17-1.20, respectively).
“Many people with mental disorders have unhealthy lifestyle, including low exercise, poor diet, smoking, and alcohol, which may account for the increased risk of physical illness, and also they may not seek and/or may not get quick treatment for their health conditions,” said Dr. McGrath.
Additionally, “perhaps some genetic and early life exposures, such as trauma, may increase the risk of both medical conditions and mental disorders,” he added. “We need better treatments for mental disorders, so that they do not slip into unemployment or poverty.”
A strong case
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto and head of the mood disorders psychopharmacology unit, University Health Network, said that the research “really makes a strong case for the fact that persons who have mental disorders are at higher risk of chronic diseases, and it’s the chronic diseases that decrease their lifespan.”
Dr. McIntyre, who is also director of the Depression and Bipolar Support Alliance, said that the “takeaway message is that mental disorders are not just brain disorders but are multisystem disorders.”
For this reason, “the most appropriate way to provide care would be to provide a holistic approach to treat and prevent the chronic diseases that lead to increase in mortality,” recommended Dr. McIntyre, who was not involved with the current study.
The study was supported by grants from the Danish National Research Foundation, the National Health and Medical Research Council, the Novo Nordisk Foundation , the European Union’s Horizon 2020 Research and Innovation Program, the Aarhus University Research Foundation, the Lundbeck Foundation, the National Institutes of Health, the European Commission, Helsefonden, the Danish Council for Independent Research, the Independent Research Fund Denmark, the National Health and Medical Research Council of Australia, and the National Institute on Drug Abuse.
Dr. McGrath has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. McIntyre reports receiving grants from Stanley Medical Research Institute; the Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/Chinese National Natural Research Foundation; and receiving speaking/consultation fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, and Minerva.
A version of this article originally appeared on Medscape.com.
Mental illnesses are associated with a significantly increased risk of subsequent physical diseases, new research shows.
An international team of researchers has created an “atlas” that maps the relationship between specific mental disorders and the risk of subsequent physical illnesses.
The researchers found that, following the diagnosis of a mental disorder, psychiatric patients are significantly more likely than the general population to develop potentially life-threatening conditions, including heart disease and stroke.
These findings, the investigators noted, highlight the need for better medical care in this vulnerable population. They have created a website with detailed information about the risks of specific physical ailments and the link to particular mental disorders.
“We found that women with anxiety disorders have a 50% increased risk of developing a heart condition or stroke – over 15 years, one in three women with anxiety disorders will develop these medical disorders,” lead investigator John McGrath, MD, PhD, University of Queensland’s Brain Institute, Brisbane, Australia, and Aarhus (Denmark) University, said in a statement.
“We also looked at men with substance use disorders such as alcohol-related disorders and found they have a 400% increased risk of gut or liver disorders, while over 15 years, one in five of them will develop gut or liver conditions,” he added.
The study was published in the New England Journal of Medicine.
New ‘atlas’
It’s well known that patients with mental disorders have decreased quality of life, increased health care utilization, and a shorter life expectancy than individuals in the general population – about 10 years for men and 7 years for women.
However, the investigators noted, previous research examining the relationship between mental disorders and medical conditions only focused on “particular pairs or a small set of mental disorders and medical conditions.”
“We needed a comprehensive study to map the links between different types of mental disorders versus different types of general medical conditions. Our study has provided this atlas,” Dr. McGrath said in an interview.
The clinical utility of such a map could provide comprehensive data on relative and absolute risks of various medical conditions after a diagnosis of a mental disorder. This information, the researchers noted, would “help clinicians and health care planners identify the primary prevention needs of their patients.”
The study included 5.9 million people born in Denmark between 1900 and 2015 and followed them from 2000 to 2016, a total of 83.9 million person-years. The researchers followed patients for up to 17 years (2000-2016) for medical diagnoses and up to 48 years (1969-2016) for diagnoses of mental disorders.
The study’s large sample size allowed investigators to assess 10 broad types of mental disorders and 9 broad categories of medical conditions that encompassed 31 specific conditions.
Categories of medical conditions included circulatory, endocrine, pulmonary, gastrointestinal, urogenital, musculoskeletal, hematologic, neurologic, and cancer. Mental disorder categories included organic disorders such as Alzheimer’s, substance abuse disorders, schizophrenia, mood disorders, neurotic disorders, eating disorders, personality disorders, developmental disorders, behavioral/emotional disorders, and intellectual disabilities.
The researchers estimated associations between 90 pairs of mental disorders and broad-category medical conditions, as well as 310 pairs of mental disorders and specific medical conditions.
‘Curious’ finding
Individuals with mental disorders showed a higher risk of medical conditions in 76 out of 90 specific mental disorder–medical condition pairs.
After adjusting for sex, age, calendar time, and previous coexisting mental disorders, the median hazard ratio for a subsequent medical condition was 1.37 in patients with a mental disorder.
The lowest HR was 0.82 for organic mental disorders and the broad category of cancer (95% confidence interval, 0.80-0.84), and the highest was 3.62 for eating disorders and urogenital conditions (95% CI, 3.11-4.22). On the other hand, schizophrenia was associated with a reduced risk of developing musculoskeletal conditions (HR, 0.87; 95% CI, 0.84-0.91).
Dr. McGrath described this finding as “curious” and speculated it “may be related to underlying genetic risk factors.”
compared with the matched reference group without a mood disorder (40.9% vs. 32.6%, respectively).
The risk of developing subsequent medical conditions after a mental disorder diagnosis did not remain steady over time. For instance, although mood disorders were associated with an increased risk of developing circulatory problems (HR, 1.32; 95% CI, 1.31-1.34), the highest risk occurred during the first 6 months following diagnosis and gradually decreased over the next 15 years (HR, 2.39; 95% CI, 2.29-2.48 and HR, 1.18; 95% CI, 1.17-1.20, respectively).
“Many people with mental disorders have unhealthy lifestyle, including low exercise, poor diet, smoking, and alcohol, which may account for the increased risk of physical illness, and also they may not seek and/or may not get quick treatment for their health conditions,” said Dr. McGrath.
Additionally, “perhaps some genetic and early life exposures, such as trauma, may increase the risk of both medical conditions and mental disorders,” he added. “We need better treatments for mental disorders, so that they do not slip into unemployment or poverty.”
A strong case
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto and head of the mood disorders psychopharmacology unit, University Health Network, said that the research “really makes a strong case for the fact that persons who have mental disorders are at higher risk of chronic diseases, and it’s the chronic diseases that decrease their lifespan.”
Dr. McIntyre, who is also director of the Depression and Bipolar Support Alliance, said that the “takeaway message is that mental disorders are not just brain disorders but are multisystem disorders.”
For this reason, “the most appropriate way to provide care would be to provide a holistic approach to treat and prevent the chronic diseases that lead to increase in mortality,” recommended Dr. McIntyre, who was not involved with the current study.
The study was supported by grants from the Danish National Research Foundation, the National Health and Medical Research Council, the Novo Nordisk Foundation , the European Union’s Horizon 2020 Research and Innovation Program, the Aarhus University Research Foundation, the Lundbeck Foundation, the National Institutes of Health, the European Commission, Helsefonden, the Danish Council for Independent Research, the Independent Research Fund Denmark, the National Health and Medical Research Council of Australia, and the National Institute on Drug Abuse.
Dr. McGrath has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. McIntyre reports receiving grants from Stanley Medical Research Institute; the Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/Chinese National Natural Research Foundation; and receiving speaking/consultation fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, and Minerva.
A version of this article originally appeared on Medscape.com.
Depression linked to neuro dysfunction, brain lesions in MS
Depression is associated with decreased neurologic function and new brain lesions in patients with multiple sclerosis (MS), new research suggests.
In an observational study of more than 2500 patients with relapsing-remitting MS (RRMS), participants with self-reported depression were more likely to have worse scores on neuroperformance measures, such as processing speed tests, than their peers without depression.
At baseline, the group with depression also had greater odds of having at least one new contrast-enhancing lesion on MRI.
“Our results suggest that depression is not merely a reactive symptom but indicates increased risk of future MS disease activity,” the investigators note.
Lead author Jenny Feng, MD, clinical associate at the Mellen Center for MS Treatment and Research at Cleveland Clinic, added that depression should be routinely screened for in all patients with MS, something done routinely at her center.
“Every single patient that comes through the door with newly diagnosed MS, we refer to neuropsychology to screen for depression; and if there is depression, then we actively manage it because it does have an effect” on patients, she told Medscape Medical News.
“Depression isn’t just a neuropsychiatric disease,” Feng added. As shown in their study, “it may have effects on MS, especially with regards to performance in neurological function testing.”
The research is presented on AAN.com as part of the American Academy of Neurology 2020 Science Highlights. Because of the COVID-19 pandemic, the AAN had to cancel its 2020 annual meeting.
Associations Have Been “Unclear”
Although inflammatory, psychosocial, and neurodegenerative factors “have been hypothesized as etiologies” for why depression is commonly found in patients with MS,
For the current study, they assessed data from the Partners Advancing Technology and Health Solutions (MS-PATHS) database, an ongoing collaborative network of seven MS centers in the United States and three in Europe.
MS disease history and MRI data were examined, as well as 12-month scores on neuroperformance tests measuring processing speed (Symbol Digit Modalities Test), walking speed (Timed 25-Foot Walk), and manual dexterity (Nine-Hole Peg Test).
Patient-reported outcomes (PROs), as measured with the Quality of Life in Neurological Disorders (Neuro-QoL) and patient-determined disease steps, were also assessed. Depression was defined as a depression T score at baseline greater than “the 50th percentile” on the Neuro-QoL.
In the patient sample, 1333 of the participants with RRMS were classified as “not depressed” (73.7% women; mean age, 45.6 years; disease duration, 13.7 years) while 1172 were “depressed” (78.4% women; mean age, 45.9 years; disease duration, 14.3 years).
“To balance for baseline variances in the observational cohort between group with depression and group without depression, propensity score analysis was used to adjust for potential confounding factors,” the investigators report.
Worse Performance
After adjustment for baseline covariates, results showed that the depressed patients performed worse on the walking speed test (0.48; 95% confidence interval, 0.038-0.918) and processing speed test (–1.899; 95% CI, –3.548 to –0.250).
The depressed group also had increased odds at baseline of having new contrast-enhancing lesions (odds ratio, 5.89; 95% CI, 2.236-15.517). This demonstrated an “association of depression and neuroinflammatory activity” in the central nervous system, the investigators note.
At 12 months, processing speed continued to be worse in the depressed group (–1.68; 95% CI, –3.254 to –0.105).
There were trends, albeit insignificant, for decreased walking speed scores at 12 months and for decreased manual dexterity scores at both baseline and at 12 months for the participants who were depressed.
Interestingly, there were “no significant differences in PROs at month 12, despite worsening neuroperformance,” the investigators report.
“This means that patients themselves may not even realize that they were getting worse,” Feng said.
Underpowered Study?
Further results showed nonsignificant trends for increased T2 lesion volume and white matter fraction and decreased brain volume, gray matter fraction, and cortical gray matter volume at baseline and at 12 months in the depressed group.
The researchers note that study limitations include the unavailability of information on treatment compliance for depression or date of depression onset.
Feng added that because this was an observational study, other missing data included depression status for some patients at year 1 and some MRI metrics.
“So this may have been underpowered to detect some of the results. The power may have been inadequate to detect all changes,” she said.
The investigators write that future research should assess larger sample sizes with longer follow-ups and should use more advanced MRI measures, such as diffusion tensor imaging or functional MRI.
In addition, they will continue examining data from MS-PATHS. “With the newest data cut, we have new patients that we can analyze. So perhaps that can provide sufficient power to detect [more MRI] changes,” Feng said.
Unusual, Intriguing Findings
Commenting on the study for Medscape Medical News, Mark Freedman, MD, professor of neurology at the University of Ottawa and director of the Multiple Sclerosis Research Clinic at the Ottawa Hospital Research Institute, noted that he wasn’t terribly surprised” by the overall findings.
“We’ve known for years that patients who are depressed don’t do as well on our performance methods,” said Freedman, who was not involved with the research.
However, the current investigators “took a huge number of patients in this multicenter study and started using some of the statistical methods we’ve seen in the use of real-world evidence,” he noted.
“So you’re looking at some outcome measures and you have to ask yourself, ‘Why would it influence that?’ and ‘Did it happen by chance or not?’ And you ask why it is that depressed people might actually have more lesions on their MRI, which is something that is unusual,” Freedman said.
“When you start to look at this, even when you’re trying to standardize things for the differences that we know of, there are some stuff that comes out as intriguing. In general, I think those depressed patients did worse on several outcome measures that one would say, ‘That’s somewhat surprising.’ That’s why this group was very careful to not conclude absolutely that depression drives this disease. But it was consistently trending in the direction that it looks like there was more inflammatory activity in these people,” he said.
He echoed the investigators’ note that drug adherence and which depression treatment was used wasn’t controlled for; and he added that depression in the study was not based on receiving a diagnosis of clinical depression but on self-report.
Still, the patients classified as depressed “did worse. They didn’t walk as fast, which was interesting; and we know that cognitive performance is often damped because of poor concentration. But how do you get worse MRIs? This study is raising a question and [the researchers] conclude that it may be that depression might be an independent factor” for that outcome, Freedman said.
“It might be that you could get more out of a particular [MS] medicine if you pay attention to depression; and if that’s the investigators’ conclusion, and I think it is, then I certainly agree with it.”
Freedman noted that, instead of a blanket recommendation that all patients with MS should be screened for depression, he thinks clinicians, especially those at smaller centers, should focus on what’s best for treating all aspects of an individual patient.
“Don’t try to manage them if you’re not going to manage the entire picture. Looking at depression and mood and other things is very important. And if you have the capacity for an official screening, I think it’s wonderful; but not everybody does,” he said.
Feng and Freedman have disclosed no relevant financial relationships. Freedman is currently a member of the Medscape Neurology Advisory Board.
This article appeared on Medscape.com.
Depression is associated with decreased neurologic function and new brain lesions in patients with multiple sclerosis (MS), new research suggests.
In an observational study of more than 2500 patients with relapsing-remitting MS (RRMS), participants with self-reported depression were more likely to have worse scores on neuroperformance measures, such as processing speed tests, than their peers without depression.
At baseline, the group with depression also had greater odds of having at least one new contrast-enhancing lesion on MRI.
“Our results suggest that depression is not merely a reactive symptom but indicates increased risk of future MS disease activity,” the investigators note.
Lead author Jenny Feng, MD, clinical associate at the Mellen Center for MS Treatment and Research at Cleveland Clinic, added that depression should be routinely screened for in all patients with MS, something done routinely at her center.
“Every single patient that comes through the door with newly diagnosed MS, we refer to neuropsychology to screen for depression; and if there is depression, then we actively manage it because it does have an effect” on patients, she told Medscape Medical News.
“Depression isn’t just a neuropsychiatric disease,” Feng added. As shown in their study, “it may have effects on MS, especially with regards to performance in neurological function testing.”
The research is presented on AAN.com as part of the American Academy of Neurology 2020 Science Highlights. Because of the COVID-19 pandemic, the AAN had to cancel its 2020 annual meeting.
Associations Have Been “Unclear”
Although inflammatory, psychosocial, and neurodegenerative factors “have been hypothesized as etiologies” for why depression is commonly found in patients with MS,
For the current study, they assessed data from the Partners Advancing Technology and Health Solutions (MS-PATHS) database, an ongoing collaborative network of seven MS centers in the United States and three in Europe.
MS disease history and MRI data were examined, as well as 12-month scores on neuroperformance tests measuring processing speed (Symbol Digit Modalities Test), walking speed (Timed 25-Foot Walk), and manual dexterity (Nine-Hole Peg Test).
Patient-reported outcomes (PROs), as measured with the Quality of Life in Neurological Disorders (Neuro-QoL) and patient-determined disease steps, were also assessed. Depression was defined as a depression T score at baseline greater than “the 50th percentile” on the Neuro-QoL.
In the patient sample, 1333 of the participants with RRMS were classified as “not depressed” (73.7% women; mean age, 45.6 years; disease duration, 13.7 years) while 1172 were “depressed” (78.4% women; mean age, 45.9 years; disease duration, 14.3 years).
“To balance for baseline variances in the observational cohort between group with depression and group without depression, propensity score analysis was used to adjust for potential confounding factors,” the investigators report.
Worse Performance
After adjustment for baseline covariates, results showed that the depressed patients performed worse on the walking speed test (0.48; 95% confidence interval, 0.038-0.918) and processing speed test (–1.899; 95% CI, –3.548 to –0.250).
The depressed group also had increased odds at baseline of having new contrast-enhancing lesions (odds ratio, 5.89; 95% CI, 2.236-15.517). This demonstrated an “association of depression and neuroinflammatory activity” in the central nervous system, the investigators note.
At 12 months, processing speed continued to be worse in the depressed group (–1.68; 95% CI, –3.254 to –0.105).
There were trends, albeit insignificant, for decreased walking speed scores at 12 months and for decreased manual dexterity scores at both baseline and at 12 months for the participants who were depressed.
Interestingly, there were “no significant differences in PROs at month 12, despite worsening neuroperformance,” the investigators report.
“This means that patients themselves may not even realize that they were getting worse,” Feng said.
Underpowered Study?
Further results showed nonsignificant trends for increased T2 lesion volume and white matter fraction and decreased brain volume, gray matter fraction, and cortical gray matter volume at baseline and at 12 months in the depressed group.
The researchers note that study limitations include the unavailability of information on treatment compliance for depression or date of depression onset.
Feng added that because this was an observational study, other missing data included depression status for some patients at year 1 and some MRI metrics.
“So this may have been underpowered to detect some of the results. The power may have been inadequate to detect all changes,” she said.
The investigators write that future research should assess larger sample sizes with longer follow-ups and should use more advanced MRI measures, such as diffusion tensor imaging or functional MRI.
In addition, they will continue examining data from MS-PATHS. “With the newest data cut, we have new patients that we can analyze. So perhaps that can provide sufficient power to detect [more MRI] changes,” Feng said.
Unusual, Intriguing Findings
Commenting on the study for Medscape Medical News, Mark Freedman, MD, professor of neurology at the University of Ottawa and director of the Multiple Sclerosis Research Clinic at the Ottawa Hospital Research Institute, noted that he wasn’t terribly surprised” by the overall findings.
“We’ve known for years that patients who are depressed don’t do as well on our performance methods,” said Freedman, who was not involved with the research.
However, the current investigators “took a huge number of patients in this multicenter study and started using some of the statistical methods we’ve seen in the use of real-world evidence,” he noted.
“So you’re looking at some outcome measures and you have to ask yourself, ‘Why would it influence that?’ and ‘Did it happen by chance or not?’ And you ask why it is that depressed people might actually have more lesions on their MRI, which is something that is unusual,” Freedman said.
“When you start to look at this, even when you’re trying to standardize things for the differences that we know of, there are some stuff that comes out as intriguing. In general, I think those depressed patients did worse on several outcome measures that one would say, ‘That’s somewhat surprising.’ That’s why this group was very careful to not conclude absolutely that depression drives this disease. But it was consistently trending in the direction that it looks like there was more inflammatory activity in these people,” he said.
He echoed the investigators’ note that drug adherence and which depression treatment was used wasn’t controlled for; and he added that depression in the study was not based on receiving a diagnosis of clinical depression but on self-report.
Still, the patients classified as depressed “did worse. They didn’t walk as fast, which was interesting; and we know that cognitive performance is often damped because of poor concentration. But how do you get worse MRIs? This study is raising a question and [the researchers] conclude that it may be that depression might be an independent factor” for that outcome, Freedman said.
“It might be that you could get more out of a particular [MS] medicine if you pay attention to depression; and if that’s the investigators’ conclusion, and I think it is, then I certainly agree with it.”
Freedman noted that, instead of a blanket recommendation that all patients with MS should be screened for depression, he thinks clinicians, especially those at smaller centers, should focus on what’s best for treating all aspects of an individual patient.
“Don’t try to manage them if you’re not going to manage the entire picture. Looking at depression and mood and other things is very important. And if you have the capacity for an official screening, I think it’s wonderful; but not everybody does,” he said.
Feng and Freedman have disclosed no relevant financial relationships. Freedman is currently a member of the Medscape Neurology Advisory Board.
This article appeared on Medscape.com.
Depression is associated with decreased neurologic function and new brain lesions in patients with multiple sclerosis (MS), new research suggests.
In an observational study of more than 2500 patients with relapsing-remitting MS (RRMS), participants with self-reported depression were more likely to have worse scores on neuroperformance measures, such as processing speed tests, than their peers without depression.
At baseline, the group with depression also had greater odds of having at least one new contrast-enhancing lesion on MRI.
“Our results suggest that depression is not merely a reactive symptom but indicates increased risk of future MS disease activity,” the investigators note.
Lead author Jenny Feng, MD, clinical associate at the Mellen Center for MS Treatment and Research at Cleveland Clinic, added that depression should be routinely screened for in all patients with MS, something done routinely at her center.
“Every single patient that comes through the door with newly diagnosed MS, we refer to neuropsychology to screen for depression; and if there is depression, then we actively manage it because it does have an effect” on patients, she told Medscape Medical News.
“Depression isn’t just a neuropsychiatric disease,” Feng added. As shown in their study, “it may have effects on MS, especially with regards to performance in neurological function testing.”
The research is presented on AAN.com as part of the American Academy of Neurology 2020 Science Highlights. Because of the COVID-19 pandemic, the AAN had to cancel its 2020 annual meeting.
Associations Have Been “Unclear”
Although inflammatory, psychosocial, and neurodegenerative factors “have been hypothesized as etiologies” for why depression is commonly found in patients with MS,
For the current study, they assessed data from the Partners Advancing Technology and Health Solutions (MS-PATHS) database, an ongoing collaborative network of seven MS centers in the United States and three in Europe.
MS disease history and MRI data were examined, as well as 12-month scores on neuroperformance tests measuring processing speed (Symbol Digit Modalities Test), walking speed (Timed 25-Foot Walk), and manual dexterity (Nine-Hole Peg Test).
Patient-reported outcomes (PROs), as measured with the Quality of Life in Neurological Disorders (Neuro-QoL) and patient-determined disease steps, were also assessed. Depression was defined as a depression T score at baseline greater than “the 50th percentile” on the Neuro-QoL.
In the patient sample, 1333 of the participants with RRMS were classified as “not depressed” (73.7% women; mean age, 45.6 years; disease duration, 13.7 years) while 1172 were “depressed” (78.4% women; mean age, 45.9 years; disease duration, 14.3 years).
“To balance for baseline variances in the observational cohort between group with depression and group without depression, propensity score analysis was used to adjust for potential confounding factors,” the investigators report.
Worse Performance
After adjustment for baseline covariates, results showed that the depressed patients performed worse on the walking speed test (0.48; 95% confidence interval, 0.038-0.918) and processing speed test (–1.899; 95% CI, –3.548 to –0.250).
The depressed group also had increased odds at baseline of having new contrast-enhancing lesions (odds ratio, 5.89; 95% CI, 2.236-15.517). This demonstrated an “association of depression and neuroinflammatory activity” in the central nervous system, the investigators note.
At 12 months, processing speed continued to be worse in the depressed group (–1.68; 95% CI, –3.254 to –0.105).
There were trends, albeit insignificant, for decreased walking speed scores at 12 months and for decreased manual dexterity scores at both baseline and at 12 months for the participants who were depressed.
Interestingly, there were “no significant differences in PROs at month 12, despite worsening neuroperformance,” the investigators report.
“This means that patients themselves may not even realize that they were getting worse,” Feng said.
Underpowered Study?
Further results showed nonsignificant trends for increased T2 lesion volume and white matter fraction and decreased brain volume, gray matter fraction, and cortical gray matter volume at baseline and at 12 months in the depressed group.
The researchers note that study limitations include the unavailability of information on treatment compliance for depression or date of depression onset.
Feng added that because this was an observational study, other missing data included depression status for some patients at year 1 and some MRI metrics.
“So this may have been underpowered to detect some of the results. The power may have been inadequate to detect all changes,” she said.
The investigators write that future research should assess larger sample sizes with longer follow-ups and should use more advanced MRI measures, such as diffusion tensor imaging or functional MRI.
In addition, they will continue examining data from MS-PATHS. “With the newest data cut, we have new patients that we can analyze. So perhaps that can provide sufficient power to detect [more MRI] changes,” Feng said.
Unusual, Intriguing Findings
Commenting on the study for Medscape Medical News, Mark Freedman, MD, professor of neurology at the University of Ottawa and director of the Multiple Sclerosis Research Clinic at the Ottawa Hospital Research Institute, noted that he wasn’t terribly surprised” by the overall findings.
“We’ve known for years that patients who are depressed don’t do as well on our performance methods,” said Freedman, who was not involved with the research.
However, the current investigators “took a huge number of patients in this multicenter study and started using some of the statistical methods we’ve seen in the use of real-world evidence,” he noted.
“So you’re looking at some outcome measures and you have to ask yourself, ‘Why would it influence that?’ and ‘Did it happen by chance or not?’ And you ask why it is that depressed people might actually have more lesions on their MRI, which is something that is unusual,” Freedman said.
“When you start to look at this, even when you’re trying to standardize things for the differences that we know of, there are some stuff that comes out as intriguing. In general, I think those depressed patients did worse on several outcome measures that one would say, ‘That’s somewhat surprising.’ That’s why this group was very careful to not conclude absolutely that depression drives this disease. But it was consistently trending in the direction that it looks like there was more inflammatory activity in these people,” he said.
He echoed the investigators’ note that drug adherence and which depression treatment was used wasn’t controlled for; and he added that depression in the study was not based on receiving a diagnosis of clinical depression but on self-report.
Still, the patients classified as depressed “did worse. They didn’t walk as fast, which was interesting; and we know that cognitive performance is often damped because of poor concentration. But how do you get worse MRIs? This study is raising a question and [the researchers] conclude that it may be that depression might be an independent factor” for that outcome, Freedman said.
“It might be that you could get more out of a particular [MS] medicine if you pay attention to depression; and if that’s the investigators’ conclusion, and I think it is, then I certainly agree with it.”
Freedman noted that, instead of a blanket recommendation that all patients with MS should be screened for depression, he thinks clinicians, especially those at smaller centers, should focus on what’s best for treating all aspects of an individual patient.
“Don’t try to manage them if you’re not going to manage the entire picture. Looking at depression and mood and other things is very important. And if you have the capacity for an official screening, I think it’s wonderful; but not everybody does,” he said.
Feng and Freedman have disclosed no relevant financial relationships. Freedman is currently a member of the Medscape Neurology Advisory Board.
This article appeared on Medscape.com.