User login
New Clues on How Blast Exposure May Lead to Alzheimer’s Disease
In October 2023, Robert Card — a grenade instructor in the Army Reserve — shot and killed 18 people in Maine, before turning the gun on himself. As reported by The New York Times, his family said that he had become increasingly erratic and violent during the months before the rampage.
A postmortem conducted by the Chronic Traumatic Encephalopathy (CTE) Center at Boston University found “significant evidence of traumatic brain injuries” [TBIs] and “significant degeneration, axonal and myelin loss, inflammation, and small blood vessel injury” in the white matter, the center’s director, Ann McKee, MD, said in a press release. “These findings align with our previous studies on the effects of blast injury in humans and experimental models.”
Members of the military, such as Mr. Card, are exposed to blasts from repeated firing of heavy weapons not only during combat but also during training.
A higher index of suspicion for dementia or Alzheimer’s disease may be warranted in patients with a history of blast exposure or subconcussive brain injury who present with cognitive issues, according to experts interviewed.
In 2022, the US Department of Defense (DOD) launched its Warfighter Brain Health Initiative with the aim of “optimizing service member brain health and countering traumatic brain injuries.”
In April 2024, the Blast Overpressure Safety Act was introduced in the Senate to require the DOD to enact better blast screening, tracking, prevention, and treatment. The DOD initiated 26 blast overpressure studies.
Heather Snyder, PhD, Alzheimer’s Association vice president of Medical and Scientific Relations, said that an important component of that research involves “the need to study the difference between TBI-caused dementia and dementia caused independently” and “the need to study biomarkers to better understand the long-term consequences of TBI.”
What Is the Underlying Biology?
Dr. Snyder was the lead author of a white paper produced by the Alzheimer’s Association in 2018 on military-related risk factors for Alzheimer’s disease and related dementias. “There is a lot of work trying to understand the effect of pure blast waves on the brain, as opposed to the actual impact of the injury,” she said.
The white paper speculated that blast exposure may be analogous to subconcussive brain injury in athletes where there are no obvious immediate clinical symptoms or neurological dysfunction but which can cause cumulative injury and functional impairment over time.
“We are also trying to understand the underlying biology around brain changes, such as accumulation of tau and amyloid and other specific markers related to brain changes in Alzheimer’s disease,” said Dr. Snyder, chair of the Peer Reviewed Alzheimer’s Research Program Programmatic Panel for Alzheimer’s Disease/Alzheimer’s Disease and Related Dementias and TBI.
Common Biomarker Signatures
A recent study in Neurology comparing 51 veterans with mild TBI (mTBI) with 85 veterans and civilians with no lifetime history of TBI is among the first to explore these biomarker changes in human beings.
“Our findings suggest that chronic neuropathologic processes associated with blast mTBI share properties in common with pathogenic processes that are precursors to Alzheimer’s disease onset,” said coauthor Elaine R. Peskind, MD, professor of psychiatry and behavioral sciences, University of Washington, Seattle.
The largely male participants were a mean age of 34 years and underwent standardized clinical and neuropsychological testing as well as lumbar puncture to collect cerebrospinal fluid (CSF). The mTBI group had experienced at least one war zone blast or combined blast/impact that met criteria for mTBI, but 91% had more than one blast mTBI, and the study took place over 13 years.
The researchers found that the mTBI group “had biomarker signatures in common with the earliest stages of Alzheimer’s disease,” said Dr. Peskind.
For example, at age 50, they had lower mean levels of CSF amyloid beta 42 (Abeta42), the earliest marker of brain parenchymal Abeta deposition, compared with the control group (154 pg/mL and 1864 pg/mL lower, respectively).
High CSF phosphorylated tau181 (p-tau181) and total tau are established biomarkers for Alzheimer’s disease. However, levels of these biomarkers remained “relatively constant with age” in participants with mTBI but were higher in older ages for the non-TBI group.
The mTBI group also showed worse cognitive performance at older ages (P < .08). Poorer verbal memory and verbal fluency performance were associated with lower CSF Abeta42 in older participants (P ≤ .05).
In Alzheimer’s disease, a reduction in CSF Abeta42 may occur up to 20 years before the onset of clinical symptoms, according to Dr. Peskind. “But what we don’t know from this study is what this means, as total tau protein and p-tau181 in the CSF were also low, which isn’t entirely typical in the picture of preclinical Alzheimer’s disease,” she said. However, changes in total tau and p-tau181 lag behind changes in Abeta42.
Is Impaired Clearance the Culprit?
Coauthor Jeffrey Iliff, PhD, professor, University of Washington Department of Psychiatry and Behavioral Sciences and University of Washington Department of Neurology, Seattle, elaborated.
“In the setting of Alzheimer’s disease, a signature of the disease is reduced CSF Abeta42, which is thought to reflect that much of the amyloid gets ‘stuck’ in the brain in the form of amyloid plaques,” he said. “There are usually higher levels of phosphorylated tau and total tau, which are thought to reflect the presence of tau tangles and degeneration of neurons in the brain. But in this study, all of those were lowered, which is not exactly an Alzheimer’s disease profile.”
Dr. Iliff, associate director for research, VA Northwest Mental Illness Research, Education, and Clinical Center at VA Puget Sound Health Care System, Seattle, suggested that the culprit may be impairment in the brain’s glymphatic system. “Recently described biological research supports [the concept of] clearance of waste out of the brain during sleep via the glymphatic system, with amyloid and tau being cleared from the brain interstitium during sleep.”
A recent hypothesis is that blast TBI impairs that process. “This is why we see less of those proteins in the CSF. They’re not being cleared, which might contribute downstream to the clumping up of protein in the brain,” he suggested.
The evidence base corroborating that hypothesis is in its infancy; however, new research conducted by Dr. Iliff and his colleagues sheds light on this potential mechanism.
In blast TBI, energy from the explosion and resulting overpressure wave are “transmitted through the brain, which causes tissues of different densities — such as gray and white matter — to accelerate at different rates,” according to Dr. Iliff. This results in the shearing and stretching of brain tissue, leading to a “diffuse pattern of tissue damage.”
It is known that blast TBI has clinical overlap and associations with posttraumatic stress disorder (PTSD), depression, and persistent neurobehavioral symptoms; that veterans with a history of TBI are more than twice as likely to die by suicide than veterans with no TBI history; and that TBI may increase the risk for Alzheimer’s disease and related dementing disorders, as well as CTE.
The missing link may be the glymphatic system — a “brain-wide network of perivascular pathways, along which CSF and interstitial fluid (ISF) exchange, supporting the clearance of interstitial solutes, including amyloid-beta.”
Dr. Iliff and his group previously found that glymphatic function is “markedly and chronically impaired” following impact TBI in mice and that this impairment is associated with the mislocalization of astroglial aquaporin 4 (AQP4), a water channel that lines perivascular spaces and plays a role in healthy glymphatic exchange.
In their new study, the researchers examined both the expression and the localization of AQP4 in the human postmortem frontal cortex and found “distinct laminar differences” in AQP4 expression following blast exposure. They observed similar changes as well as impairment of glymphatic function, which emerged 28 days following blast injury in a mouse model of repetitive blast mTBI.
And in a cohort of veterans with blast mTBI, blast exposure was found to be associated with an increased burden of frontal cortical MRI-visible perivascular spaces — a “putative neuroimaging marker” of glymphatic perivascular dysfunction.
The earlier Neurology study “showed impairment of biomarkers in the CSF, but the new study showed ‘why’ or ‘how’ these biomarkers are impaired, which is via impairment of the glymphatic clearance process,” Dr. Iliff explained.
Veterans Especially Vulnerable
Dr. Peskind, co-director of the VA Northwest Mental Illness Research, Education and Clinical Center, VA Puget Sound Health Care System, noted that while the veterans in the earlier study had at least one TBI, the average number was 20, and it was more common to have more than 50 mTBIs than to have a single one.
“These were highly exposed combat vets,” she said. “And that number doesn’t even account for subconcussive exposure to blasts, which now appear to cause detectable brain damage, even in the absence of a diagnosable TBI.”
The Maine shooter, Mr. Card, had not seen combat and was not assessed for TBI during a psychiatric hospitalization, according to The New York Times.
Dr. Peskind added that this type of blast damage is likely specific to individuals in the military. “It isn’t the sound that causes the damage,” she explained. “It’s the blast wave, the pressure wave, and there aren’t a lot of other occupations that have those types of occupational exposures.”
Dr. Snyder added that the majority of blast TBIs have been studied in military personnel, and she is not aware of studies that have looked at blast injuries in other industries, such as demolition or mining, to see if they have the same type of biologic consequences.
Dr. Snyder hopes that the researchers will follow the participants in the Neurology study and continue looking at specific markers related to Alzheimer’s disease brain changes. What the research so far shows “is that, at an earlier age, we’re starting to see those markers changing, suggesting that the underlying biology in people with mild blast TBI is similar to the underlying biology in Alzheimer’s disease as well.”
Michael Alosco, PhD, associate professor and vice chair of research, department of neurology, Boston University Chobanian & Avedisian School of Medicine, called the issue of blast exposure and TBI “a very complex and nuanced topic,” especially because TBI is “considered a risk factor of Alzheimer’s disease” and “different types of TBIs could trigger distinct pathophysiologic processes; however, the long-term impact of repetitive blast TBIs on neurodegenerative disease changes remains unknown.”
He coauthored an editorial on the earlier Neurology study that noted its limitations, such as a small sample size and lack of consideration of lifestyle and health factors but acknowledged that the “findings provide preliminary evidence that repetitive blast exposures might influence beta-amyloid accumulation.”
Clinical Implications
For Dr. Peskind, the “inflection point” was seeing lower CSF Abeta42, about 20 years earlier than ages 60 and 70, which is more typical in cognitively normal community volunteers.
But she described herself as “loath to say that veterans or service members have a 20-year acceleration of risk of Alzheimer’s disease,” adding, “I don’t want to scare the heck out of our service members of veterans.” Although “this is what we fear, we’re not ready to say it for sure yet because we need to do more work. Nevertheless, it does increase the index of suspicion.”
The clinical take-home messages are not unique to service members or veterans or people with a history of head injuries or a genetic predisposition to Alzheimer’s disease, she emphasized. “If anyone of any age or occupation comes in with cognitive issues, such as [impaired] memory or executive function, they deserve a workup for dementing disorders.” Frontotemporal dementia, for example, can present earlier than Alzheimer’s disease typically does.
Common comorbidities with TBI are PTSD and obstructive sleep apnea (OSA), which can also cause cognitive issues and are also risk factors for dementia.
Dr. Iliff agreed. “If you see a veteran with a history of PTSD, a history of blast TBI, and a history of OSA or some combination of those three, I recommend having a higher index of suspicion [for potential dementia] than for an average person without any of these, even at a younger age than one would ordinarily expect.”
Of all of these factors, the only truly directly modifiable one is sleep disruption, including that caused by OSA or sleep disorders related to PTSD, he added. “Epidemiologic data suggest a connection particularly between midlife sleep disruption and the risk of dementia and Alzheimer’s disease, and so it’s worth thinking about sleep as a modifiable risk factor even as early as the 40s and 50s, whether the patient is or isn’t a veteran.”
Dr. Peskind recommended asking patients, “Do they snore? Do they thrash about during sleep? Do they have trauma nightmares? This will inform the type of intervention required.”
Dr. Alosco added that there is no known “safe” threshold of exposure to blasts, and that thresholds are “unclear, particularly at the individual level.” In American football, there is a dose-response relationship between years of play and risk for later-life neurological disorder. “The best way to mitigate risk is to limit cumulative exposure,” he said.
The study by Li and colleagues was funded by grant funding from the Department of Veterans Affairs Rehabilitation Research and Development Service and the University of Washington Friends of Alzheimer’s Research. Other sources of funding to individual researchers are listed in the original paper. The study by Braun and colleagues was supported by the National Heart, Lung and Blood Institute; the Department of Veterans Affairs Rehabilitation Research and Development Service; and the National Institute on Aging. The white paper included studies that received funding from numerous sources, including the National Institutes of Health and the DOD. Dr. Iliff serves as the chair of the Scientific Advisory Board for Applied Cognition Inc., from which he receives compensation and in which he holds an equity stake. In the last year, he served as a paid consultant to Gryphon Biosciences. Dr. Peskind has served as a paid consultant to the companies Genentech, Roche, and Alpha Cognition. Dr. Alosco was supported by grant funding from the NIH; he received research support from Rainwater Charitable Foundation Inc., and Life Molecular Imaging Inc.; he has received a single honorarium from the Michael J. Fox Foundation for services unrelated to this editorial; and he received royalties from Oxford University Press Inc. The other authors’ disclosures are listed in the original papers.
A version of this article appeared on Medscape.com.
In October 2023, Robert Card — a grenade instructor in the Army Reserve — shot and killed 18 people in Maine, before turning the gun on himself. As reported by The New York Times, his family said that he had become increasingly erratic and violent during the months before the rampage.
A postmortem conducted by the Chronic Traumatic Encephalopathy (CTE) Center at Boston University found “significant evidence of traumatic brain injuries” [TBIs] and “significant degeneration, axonal and myelin loss, inflammation, and small blood vessel injury” in the white matter, the center’s director, Ann McKee, MD, said in a press release. “These findings align with our previous studies on the effects of blast injury in humans and experimental models.”
Members of the military, such as Mr. Card, are exposed to blasts from repeated firing of heavy weapons not only during combat but also during training.
A higher index of suspicion for dementia or Alzheimer’s disease may be warranted in patients with a history of blast exposure or subconcussive brain injury who present with cognitive issues, according to experts interviewed.
In 2022, the US Department of Defense (DOD) launched its Warfighter Brain Health Initiative with the aim of “optimizing service member brain health and countering traumatic brain injuries.”
In April 2024, the Blast Overpressure Safety Act was introduced in the Senate to require the DOD to enact better blast screening, tracking, prevention, and treatment. The DOD initiated 26 blast overpressure studies.
Heather Snyder, PhD, Alzheimer’s Association vice president of Medical and Scientific Relations, said that an important component of that research involves “the need to study the difference between TBI-caused dementia and dementia caused independently” and “the need to study biomarkers to better understand the long-term consequences of TBI.”
What Is the Underlying Biology?
Dr. Snyder was the lead author of a white paper produced by the Alzheimer’s Association in 2018 on military-related risk factors for Alzheimer’s disease and related dementias. “There is a lot of work trying to understand the effect of pure blast waves on the brain, as opposed to the actual impact of the injury,” she said.
The white paper speculated that blast exposure may be analogous to subconcussive brain injury in athletes where there are no obvious immediate clinical symptoms or neurological dysfunction but which can cause cumulative injury and functional impairment over time.
“We are also trying to understand the underlying biology around brain changes, such as accumulation of tau and amyloid and other specific markers related to brain changes in Alzheimer’s disease,” said Dr. Snyder, chair of the Peer Reviewed Alzheimer’s Research Program Programmatic Panel for Alzheimer’s Disease/Alzheimer’s Disease and Related Dementias and TBI.
Common Biomarker Signatures
A recent study in Neurology comparing 51 veterans with mild TBI (mTBI) with 85 veterans and civilians with no lifetime history of TBI is among the first to explore these biomarker changes in human beings.
“Our findings suggest that chronic neuropathologic processes associated with blast mTBI share properties in common with pathogenic processes that are precursors to Alzheimer’s disease onset,” said coauthor Elaine R. Peskind, MD, professor of psychiatry and behavioral sciences, University of Washington, Seattle.
The largely male participants were a mean age of 34 years and underwent standardized clinical and neuropsychological testing as well as lumbar puncture to collect cerebrospinal fluid (CSF). The mTBI group had experienced at least one war zone blast or combined blast/impact that met criteria for mTBI, but 91% had more than one blast mTBI, and the study took place over 13 years.
The researchers found that the mTBI group “had biomarker signatures in common with the earliest stages of Alzheimer’s disease,” said Dr. Peskind.
For example, at age 50, they had lower mean levels of CSF amyloid beta 42 (Abeta42), the earliest marker of brain parenchymal Abeta deposition, compared with the control group (154 pg/mL and 1864 pg/mL lower, respectively).
High CSF phosphorylated tau181 (p-tau181) and total tau are established biomarkers for Alzheimer’s disease. However, levels of these biomarkers remained “relatively constant with age” in participants with mTBI but were higher in older ages for the non-TBI group.
The mTBI group also showed worse cognitive performance at older ages (P < .08). Poorer verbal memory and verbal fluency performance were associated with lower CSF Abeta42 in older participants (P ≤ .05).
In Alzheimer’s disease, a reduction in CSF Abeta42 may occur up to 20 years before the onset of clinical symptoms, according to Dr. Peskind. “But what we don’t know from this study is what this means, as total tau protein and p-tau181 in the CSF were also low, which isn’t entirely typical in the picture of preclinical Alzheimer’s disease,” she said. However, changes in total tau and p-tau181 lag behind changes in Abeta42.
Is Impaired Clearance the Culprit?
Coauthor Jeffrey Iliff, PhD, professor, University of Washington Department of Psychiatry and Behavioral Sciences and University of Washington Department of Neurology, Seattle, elaborated.
“In the setting of Alzheimer’s disease, a signature of the disease is reduced CSF Abeta42, which is thought to reflect that much of the amyloid gets ‘stuck’ in the brain in the form of amyloid plaques,” he said. “There are usually higher levels of phosphorylated tau and total tau, which are thought to reflect the presence of tau tangles and degeneration of neurons in the brain. But in this study, all of those were lowered, which is not exactly an Alzheimer’s disease profile.”
Dr. Iliff, associate director for research, VA Northwest Mental Illness Research, Education, and Clinical Center at VA Puget Sound Health Care System, Seattle, suggested that the culprit may be impairment in the brain’s glymphatic system. “Recently described biological research supports [the concept of] clearance of waste out of the brain during sleep via the glymphatic system, with amyloid and tau being cleared from the brain interstitium during sleep.”
A recent hypothesis is that blast TBI impairs that process. “This is why we see less of those proteins in the CSF. They’re not being cleared, which might contribute downstream to the clumping up of protein in the brain,” he suggested.
The evidence base corroborating that hypothesis is in its infancy; however, new research conducted by Dr. Iliff and his colleagues sheds light on this potential mechanism.
In blast TBI, energy from the explosion and resulting overpressure wave are “transmitted through the brain, which causes tissues of different densities — such as gray and white matter — to accelerate at different rates,” according to Dr. Iliff. This results in the shearing and stretching of brain tissue, leading to a “diffuse pattern of tissue damage.”
It is known that blast TBI has clinical overlap and associations with posttraumatic stress disorder (PTSD), depression, and persistent neurobehavioral symptoms; that veterans with a history of TBI are more than twice as likely to die by suicide than veterans with no TBI history; and that TBI may increase the risk for Alzheimer’s disease and related dementing disorders, as well as CTE.
The missing link may be the glymphatic system — a “brain-wide network of perivascular pathways, along which CSF and interstitial fluid (ISF) exchange, supporting the clearance of interstitial solutes, including amyloid-beta.”
Dr. Iliff and his group previously found that glymphatic function is “markedly and chronically impaired” following impact TBI in mice and that this impairment is associated with the mislocalization of astroglial aquaporin 4 (AQP4), a water channel that lines perivascular spaces and plays a role in healthy glymphatic exchange.
In their new study, the researchers examined both the expression and the localization of AQP4 in the human postmortem frontal cortex and found “distinct laminar differences” in AQP4 expression following blast exposure. They observed similar changes as well as impairment of glymphatic function, which emerged 28 days following blast injury in a mouse model of repetitive blast mTBI.
And in a cohort of veterans with blast mTBI, blast exposure was found to be associated with an increased burden of frontal cortical MRI-visible perivascular spaces — a “putative neuroimaging marker” of glymphatic perivascular dysfunction.
The earlier Neurology study “showed impairment of biomarkers in the CSF, but the new study showed ‘why’ or ‘how’ these biomarkers are impaired, which is via impairment of the glymphatic clearance process,” Dr. Iliff explained.
Veterans Especially Vulnerable
Dr. Peskind, co-director of the VA Northwest Mental Illness Research, Education and Clinical Center, VA Puget Sound Health Care System, noted that while the veterans in the earlier study had at least one TBI, the average number was 20, and it was more common to have more than 50 mTBIs than to have a single one.
“These were highly exposed combat vets,” she said. “And that number doesn’t even account for subconcussive exposure to blasts, which now appear to cause detectable brain damage, even in the absence of a diagnosable TBI.”
The Maine shooter, Mr. Card, had not seen combat and was not assessed for TBI during a psychiatric hospitalization, according to The New York Times.
Dr. Peskind added that this type of blast damage is likely specific to individuals in the military. “It isn’t the sound that causes the damage,” she explained. “It’s the blast wave, the pressure wave, and there aren’t a lot of other occupations that have those types of occupational exposures.”
Dr. Snyder added that the majority of blast TBIs have been studied in military personnel, and she is not aware of studies that have looked at blast injuries in other industries, such as demolition or mining, to see if they have the same type of biologic consequences.
Dr. Snyder hopes that the researchers will follow the participants in the Neurology study and continue looking at specific markers related to Alzheimer’s disease brain changes. What the research so far shows “is that, at an earlier age, we’re starting to see those markers changing, suggesting that the underlying biology in people with mild blast TBI is similar to the underlying biology in Alzheimer’s disease as well.”
Michael Alosco, PhD, associate professor and vice chair of research, department of neurology, Boston University Chobanian & Avedisian School of Medicine, called the issue of blast exposure and TBI “a very complex and nuanced topic,” especially because TBI is “considered a risk factor of Alzheimer’s disease” and “different types of TBIs could trigger distinct pathophysiologic processes; however, the long-term impact of repetitive blast TBIs on neurodegenerative disease changes remains unknown.”
He coauthored an editorial on the earlier Neurology study that noted its limitations, such as a small sample size and lack of consideration of lifestyle and health factors but acknowledged that the “findings provide preliminary evidence that repetitive blast exposures might influence beta-amyloid accumulation.”
Clinical Implications
For Dr. Peskind, the “inflection point” was seeing lower CSF Abeta42, about 20 years earlier than ages 60 and 70, which is more typical in cognitively normal community volunteers.
But she described herself as “loath to say that veterans or service members have a 20-year acceleration of risk of Alzheimer’s disease,” adding, “I don’t want to scare the heck out of our service members of veterans.” Although “this is what we fear, we’re not ready to say it for sure yet because we need to do more work. Nevertheless, it does increase the index of suspicion.”
The clinical take-home messages are not unique to service members or veterans or people with a history of head injuries or a genetic predisposition to Alzheimer’s disease, she emphasized. “If anyone of any age or occupation comes in with cognitive issues, such as [impaired] memory or executive function, they deserve a workup for dementing disorders.” Frontotemporal dementia, for example, can present earlier than Alzheimer’s disease typically does.
Common comorbidities with TBI are PTSD and obstructive sleep apnea (OSA), which can also cause cognitive issues and are also risk factors for dementia.
Dr. Iliff agreed. “If you see a veteran with a history of PTSD, a history of blast TBI, and a history of OSA or some combination of those three, I recommend having a higher index of suspicion [for potential dementia] than for an average person without any of these, even at a younger age than one would ordinarily expect.”
Of all of these factors, the only truly directly modifiable one is sleep disruption, including that caused by OSA or sleep disorders related to PTSD, he added. “Epidemiologic data suggest a connection particularly between midlife sleep disruption and the risk of dementia and Alzheimer’s disease, and so it’s worth thinking about sleep as a modifiable risk factor even as early as the 40s and 50s, whether the patient is or isn’t a veteran.”
Dr. Peskind recommended asking patients, “Do they snore? Do they thrash about during sleep? Do they have trauma nightmares? This will inform the type of intervention required.”
Dr. Alosco added that there is no known “safe” threshold of exposure to blasts, and that thresholds are “unclear, particularly at the individual level.” In American football, there is a dose-response relationship between years of play and risk for later-life neurological disorder. “The best way to mitigate risk is to limit cumulative exposure,” he said.
The study by Li and colleagues was funded by grant funding from the Department of Veterans Affairs Rehabilitation Research and Development Service and the University of Washington Friends of Alzheimer’s Research. Other sources of funding to individual researchers are listed in the original paper. The study by Braun and colleagues was supported by the National Heart, Lung and Blood Institute; the Department of Veterans Affairs Rehabilitation Research and Development Service; and the National Institute on Aging. The white paper included studies that received funding from numerous sources, including the National Institutes of Health and the DOD. Dr. Iliff serves as the chair of the Scientific Advisory Board for Applied Cognition Inc., from which he receives compensation and in which he holds an equity stake. In the last year, he served as a paid consultant to Gryphon Biosciences. Dr. Peskind has served as a paid consultant to the companies Genentech, Roche, and Alpha Cognition. Dr. Alosco was supported by grant funding from the NIH; he received research support from Rainwater Charitable Foundation Inc., and Life Molecular Imaging Inc.; he has received a single honorarium from the Michael J. Fox Foundation for services unrelated to this editorial; and he received royalties from Oxford University Press Inc. The other authors’ disclosures are listed in the original papers.
A version of this article appeared on Medscape.com.
In October 2023, Robert Card — a grenade instructor in the Army Reserve — shot and killed 18 people in Maine, before turning the gun on himself. As reported by The New York Times, his family said that he had become increasingly erratic and violent during the months before the rampage.
A postmortem conducted by the Chronic Traumatic Encephalopathy (CTE) Center at Boston University found “significant evidence of traumatic brain injuries” [TBIs] and “significant degeneration, axonal and myelin loss, inflammation, and small blood vessel injury” in the white matter, the center’s director, Ann McKee, MD, said in a press release. “These findings align with our previous studies on the effects of blast injury in humans and experimental models.”
Members of the military, such as Mr. Card, are exposed to blasts from repeated firing of heavy weapons not only during combat but also during training.
A higher index of suspicion for dementia or Alzheimer’s disease may be warranted in patients with a history of blast exposure or subconcussive brain injury who present with cognitive issues, according to experts interviewed.
In 2022, the US Department of Defense (DOD) launched its Warfighter Brain Health Initiative with the aim of “optimizing service member brain health and countering traumatic brain injuries.”
In April 2024, the Blast Overpressure Safety Act was introduced in the Senate to require the DOD to enact better blast screening, tracking, prevention, and treatment. The DOD initiated 26 blast overpressure studies.
Heather Snyder, PhD, Alzheimer’s Association vice president of Medical and Scientific Relations, said that an important component of that research involves “the need to study the difference between TBI-caused dementia and dementia caused independently” and “the need to study biomarkers to better understand the long-term consequences of TBI.”
What Is the Underlying Biology?
Dr. Snyder was the lead author of a white paper produced by the Alzheimer’s Association in 2018 on military-related risk factors for Alzheimer’s disease and related dementias. “There is a lot of work trying to understand the effect of pure blast waves on the brain, as opposed to the actual impact of the injury,” she said.
The white paper speculated that blast exposure may be analogous to subconcussive brain injury in athletes where there are no obvious immediate clinical symptoms or neurological dysfunction but which can cause cumulative injury and functional impairment over time.
“We are also trying to understand the underlying biology around brain changes, such as accumulation of tau and amyloid and other specific markers related to brain changes in Alzheimer’s disease,” said Dr. Snyder, chair of the Peer Reviewed Alzheimer’s Research Program Programmatic Panel for Alzheimer’s Disease/Alzheimer’s Disease and Related Dementias and TBI.
Common Biomarker Signatures
A recent study in Neurology comparing 51 veterans with mild TBI (mTBI) with 85 veterans and civilians with no lifetime history of TBI is among the first to explore these biomarker changes in human beings.
“Our findings suggest that chronic neuropathologic processes associated with blast mTBI share properties in common with pathogenic processes that are precursors to Alzheimer’s disease onset,” said coauthor Elaine R. Peskind, MD, professor of psychiatry and behavioral sciences, University of Washington, Seattle.
The largely male participants were a mean age of 34 years and underwent standardized clinical and neuropsychological testing as well as lumbar puncture to collect cerebrospinal fluid (CSF). The mTBI group had experienced at least one war zone blast or combined blast/impact that met criteria for mTBI, but 91% had more than one blast mTBI, and the study took place over 13 years.
The researchers found that the mTBI group “had biomarker signatures in common with the earliest stages of Alzheimer’s disease,” said Dr. Peskind.
For example, at age 50, they had lower mean levels of CSF amyloid beta 42 (Abeta42), the earliest marker of brain parenchymal Abeta deposition, compared with the control group (154 pg/mL and 1864 pg/mL lower, respectively).
High CSF phosphorylated tau181 (p-tau181) and total tau are established biomarkers for Alzheimer’s disease. However, levels of these biomarkers remained “relatively constant with age” in participants with mTBI but were higher in older ages for the non-TBI group.
The mTBI group also showed worse cognitive performance at older ages (P < .08). Poorer verbal memory and verbal fluency performance were associated with lower CSF Abeta42 in older participants (P ≤ .05).
In Alzheimer’s disease, a reduction in CSF Abeta42 may occur up to 20 years before the onset of clinical symptoms, according to Dr. Peskind. “But what we don’t know from this study is what this means, as total tau protein and p-tau181 in the CSF were also low, which isn’t entirely typical in the picture of preclinical Alzheimer’s disease,” she said. However, changes in total tau and p-tau181 lag behind changes in Abeta42.
Is Impaired Clearance the Culprit?
Coauthor Jeffrey Iliff, PhD, professor, University of Washington Department of Psychiatry and Behavioral Sciences and University of Washington Department of Neurology, Seattle, elaborated.
“In the setting of Alzheimer’s disease, a signature of the disease is reduced CSF Abeta42, which is thought to reflect that much of the amyloid gets ‘stuck’ in the brain in the form of amyloid plaques,” he said. “There are usually higher levels of phosphorylated tau and total tau, which are thought to reflect the presence of tau tangles and degeneration of neurons in the brain. But in this study, all of those were lowered, which is not exactly an Alzheimer’s disease profile.”
Dr. Iliff, associate director for research, VA Northwest Mental Illness Research, Education, and Clinical Center at VA Puget Sound Health Care System, Seattle, suggested that the culprit may be impairment in the brain’s glymphatic system. “Recently described biological research supports [the concept of] clearance of waste out of the brain during sleep via the glymphatic system, with amyloid and tau being cleared from the brain interstitium during sleep.”
A recent hypothesis is that blast TBI impairs that process. “This is why we see less of those proteins in the CSF. They’re not being cleared, which might contribute downstream to the clumping up of protein in the brain,” he suggested.
The evidence base corroborating that hypothesis is in its infancy; however, new research conducted by Dr. Iliff and his colleagues sheds light on this potential mechanism.
In blast TBI, energy from the explosion and resulting overpressure wave are “transmitted through the brain, which causes tissues of different densities — such as gray and white matter — to accelerate at different rates,” according to Dr. Iliff. This results in the shearing and stretching of brain tissue, leading to a “diffuse pattern of tissue damage.”
It is known that blast TBI has clinical overlap and associations with posttraumatic stress disorder (PTSD), depression, and persistent neurobehavioral symptoms; that veterans with a history of TBI are more than twice as likely to die by suicide than veterans with no TBI history; and that TBI may increase the risk for Alzheimer’s disease and related dementing disorders, as well as CTE.
The missing link may be the glymphatic system — a “brain-wide network of perivascular pathways, along which CSF and interstitial fluid (ISF) exchange, supporting the clearance of interstitial solutes, including amyloid-beta.”
Dr. Iliff and his group previously found that glymphatic function is “markedly and chronically impaired” following impact TBI in mice and that this impairment is associated with the mislocalization of astroglial aquaporin 4 (AQP4), a water channel that lines perivascular spaces and plays a role in healthy glymphatic exchange.
In their new study, the researchers examined both the expression and the localization of AQP4 in the human postmortem frontal cortex and found “distinct laminar differences” in AQP4 expression following blast exposure. They observed similar changes as well as impairment of glymphatic function, which emerged 28 days following blast injury in a mouse model of repetitive blast mTBI.
And in a cohort of veterans with blast mTBI, blast exposure was found to be associated with an increased burden of frontal cortical MRI-visible perivascular spaces — a “putative neuroimaging marker” of glymphatic perivascular dysfunction.
The earlier Neurology study “showed impairment of biomarkers in the CSF, but the new study showed ‘why’ or ‘how’ these biomarkers are impaired, which is via impairment of the glymphatic clearance process,” Dr. Iliff explained.
Veterans Especially Vulnerable
Dr. Peskind, co-director of the VA Northwest Mental Illness Research, Education and Clinical Center, VA Puget Sound Health Care System, noted that while the veterans in the earlier study had at least one TBI, the average number was 20, and it was more common to have more than 50 mTBIs than to have a single one.
“These were highly exposed combat vets,” she said. “And that number doesn’t even account for subconcussive exposure to blasts, which now appear to cause detectable brain damage, even in the absence of a diagnosable TBI.”
The Maine shooter, Mr. Card, had not seen combat and was not assessed for TBI during a psychiatric hospitalization, according to The New York Times.
Dr. Peskind added that this type of blast damage is likely specific to individuals in the military. “It isn’t the sound that causes the damage,” she explained. “It’s the blast wave, the pressure wave, and there aren’t a lot of other occupations that have those types of occupational exposures.”
Dr. Snyder added that the majority of blast TBIs have been studied in military personnel, and she is not aware of studies that have looked at blast injuries in other industries, such as demolition or mining, to see if they have the same type of biologic consequences.
Dr. Snyder hopes that the researchers will follow the participants in the Neurology study and continue looking at specific markers related to Alzheimer’s disease brain changes. What the research so far shows “is that, at an earlier age, we’re starting to see those markers changing, suggesting that the underlying biology in people with mild blast TBI is similar to the underlying biology in Alzheimer’s disease as well.”
Michael Alosco, PhD, associate professor and vice chair of research, department of neurology, Boston University Chobanian & Avedisian School of Medicine, called the issue of blast exposure and TBI “a very complex and nuanced topic,” especially because TBI is “considered a risk factor of Alzheimer’s disease” and “different types of TBIs could trigger distinct pathophysiologic processes; however, the long-term impact of repetitive blast TBIs on neurodegenerative disease changes remains unknown.”
He coauthored an editorial on the earlier Neurology study that noted its limitations, such as a small sample size and lack of consideration of lifestyle and health factors but acknowledged that the “findings provide preliminary evidence that repetitive blast exposures might influence beta-amyloid accumulation.”
Clinical Implications
For Dr. Peskind, the “inflection point” was seeing lower CSF Abeta42, about 20 years earlier than ages 60 and 70, which is more typical in cognitively normal community volunteers.
But she described herself as “loath to say that veterans or service members have a 20-year acceleration of risk of Alzheimer’s disease,” adding, “I don’t want to scare the heck out of our service members of veterans.” Although “this is what we fear, we’re not ready to say it for sure yet because we need to do more work. Nevertheless, it does increase the index of suspicion.”
The clinical take-home messages are not unique to service members or veterans or people with a history of head injuries or a genetic predisposition to Alzheimer’s disease, she emphasized. “If anyone of any age or occupation comes in with cognitive issues, such as [impaired] memory or executive function, they deserve a workup for dementing disorders.” Frontotemporal dementia, for example, can present earlier than Alzheimer’s disease typically does.
Common comorbidities with TBI are PTSD and obstructive sleep apnea (OSA), which can also cause cognitive issues and are also risk factors for dementia.
Dr. Iliff agreed. “If you see a veteran with a history of PTSD, a history of blast TBI, and a history of OSA or some combination of those three, I recommend having a higher index of suspicion [for potential dementia] than for an average person without any of these, even at a younger age than one would ordinarily expect.”
Of all of these factors, the only truly directly modifiable one is sleep disruption, including that caused by OSA or sleep disorders related to PTSD, he added. “Epidemiologic data suggest a connection particularly between midlife sleep disruption and the risk of dementia and Alzheimer’s disease, and so it’s worth thinking about sleep as a modifiable risk factor even as early as the 40s and 50s, whether the patient is or isn’t a veteran.”
Dr. Peskind recommended asking patients, “Do they snore? Do they thrash about during sleep? Do they have trauma nightmares? This will inform the type of intervention required.”
Dr. Alosco added that there is no known “safe” threshold of exposure to blasts, and that thresholds are “unclear, particularly at the individual level.” In American football, there is a dose-response relationship between years of play and risk for later-life neurological disorder. “The best way to mitigate risk is to limit cumulative exposure,” he said.
The study by Li and colleagues was funded by grant funding from the Department of Veterans Affairs Rehabilitation Research and Development Service and the University of Washington Friends of Alzheimer’s Research. Other sources of funding to individual researchers are listed in the original paper. The study by Braun and colleagues was supported by the National Heart, Lung and Blood Institute; the Department of Veterans Affairs Rehabilitation Research and Development Service; and the National Institute on Aging. The white paper included studies that received funding from numerous sources, including the National Institutes of Health and the DOD. Dr. Iliff serves as the chair of the Scientific Advisory Board for Applied Cognition Inc., from which he receives compensation and in which he holds an equity stake. In the last year, he served as a paid consultant to Gryphon Biosciences. Dr. Peskind has served as a paid consultant to the companies Genentech, Roche, and Alpha Cognition. Dr. Alosco was supported by grant funding from the NIH; he received research support from Rainwater Charitable Foundation Inc., and Life Molecular Imaging Inc.; he has received a single honorarium from the Michael J. Fox Foundation for services unrelated to this editorial; and he received royalties from Oxford University Press Inc. The other authors’ disclosures are listed in the original papers.
A version of this article appeared on Medscape.com.
Are Primary Care Physicians the Answer to the US Headache Neurologist Shortage?
SAN DIEGO —
It is estimated that about 4 million PCP office visits annually are headache related, and that 52.8% of all migraine encounters occur in primary care settings.
However, PCPs aren’t always adequately trained in headache management and referral times to specialist care can be lengthy.
Data published in Headache show only 564 accredited headache specialists practice in the United States, but at least 3700 headache specialists are needed to treat those affected by migraine, with even more needed to address other disabling headache types such as tension-type headache and cluster headache. To keep up with population growth, it is estimated that the United States will require 4500 headache specialists by 2040.
First Contact
To tackle this specialist shortfall, the AHS developed the First Contact program with the aim of improving headache education in primary care and help alleviate at least some of the demand for specialist care.
The national program was rolled out in 2020 and 2021. The educational symposia were delivered to PCPs at multiple locations across the country. The initiative also included a comprehensive website with numerous support resources.
After participating in the initiative, attendees were surveyed about the value of the program, and the results were subsequently analyzed and presented at the annual meeting of the American Headache Society.
The analysis included 636 survey respondents, a 38% response rate. Almost all participants (96%) were MDs and DOs. The remainder included nurse practitioners, physician assistants, and dentists.
About 85.6% of respondents reported being completely or very confident in their ability to recognize and accurately diagnose headache disorders, and 81.3% said they were completely or very confident in their ability to create tailored treatment plans.
Just over 90% of participants reported they would implement practice changes as a result of the program. The most commonly cited change was the use of diagnostic tools such as the three-question Migraine ID screener, followed closely by consideration of prescribing triptans and reducing the use of unnecessary neuroimaging.
“Overall, there was a positive response to this type of educational programming and interest in ongoing education in addressing headache disorders with both pharmaceutical and non-pharmaceutical treatment options,” said Nisha Malhotra, MD, a resident at New York University (NYU) Langone Health, New York City, who presented the findings at the conference.
The fact that so many general practitioners were keen to use this easy-to-use screen [Migraine ID screener], which can pick up about 90% of people with migraine, is “great,” said study investigator Mia Minen, MD, associate professor and chief of headache research at NYU Langone Health. “I’m pleased primary care providers said they were considering implementing this simple tool.”
However, respondents also cited barriers to change. These included cost constraints (48.9%), insurance reimbursement issues (48.6%), and lack of time (45.3%). Dr. Malhotra noted these concerns are primarily related to workflow rather than knowledge gaps or lack of training.
“This is exciting in that there doesn’t seem to be an issue with education primarily but rather with the logistical issues that exist in the workflow in a primary care setting,” said Dr. Malhotra.
Participants also noted the need for other improvements. For example, they expressed interest in differentiating migraine from other headache types and having a better understanding of how and when to refer to specialists, said Dr. Malhotra.
These practitioners also want to know more about treatment options beyond first-line medications. “They were interested in understanding more advanced medication treatment options beyond just the typical triptan,” said Dr. Malhotra.
In addition, they want to become more skilled in non-pharmaceutical options such as occipital nerve blocks and in massage, acupuncture, and other complementary forms of migraine management, she said.
The study may be vulnerable to sampling bias as survey participants had just attended an educational symposium on headaches. “They were already, to some degree, interested in improving their knowledge on headache,” said Dr. Malhotra.
Another study limitation was that researchers didn’t conduct a pre-survey analysis to determine changes as a result of the symposia. And as the survey was conducted so close to the symposium, “it’s difficult to draw conclusions on the long-term effects,” she added.
“That being said, First Contact is one of the first national initiatives for primary care education, and thus far, it has been very well received.”
The next step is to continue expanding the program and to create a First Contact for women and First Contact for pediatrics, said Dr. Minen.
Improved Diagnosis, Better Care
Commenting on the initiative, Juliana VanderPluym, MD, a headache specialist at the Mayo Clinic, Phoenix, who co-chaired the session where the survey results were presented, said it helps address the supply-demand imbalance in headache healthcare.
“Many, many people have headache disorders, and very few people are technically headache specialists, so we have to rely on our colleagues in primary care to help address the great need that’s out there for patients with headache disorders.”
Too many patients don’t get a proper diagnosis or appropriate treatment, said Dr. VanderPluym, so as time passes, “diseases can become more chronic and more refractory, and it affects people’s quality of life and productivity.”
The First Contact program, she said, helps increase providers’ comfort and confidence that they are providing the best patient care possible and lead to a reduction in the need for specialist referrals.
Dr. Minen serves on the First Contact advisory board.
A version of this article appeared on Medscape.com.
SAN DIEGO —
It is estimated that about 4 million PCP office visits annually are headache related, and that 52.8% of all migraine encounters occur in primary care settings.
However, PCPs aren’t always adequately trained in headache management and referral times to specialist care can be lengthy.
Data published in Headache show only 564 accredited headache specialists practice in the United States, but at least 3700 headache specialists are needed to treat those affected by migraine, with even more needed to address other disabling headache types such as tension-type headache and cluster headache. To keep up with population growth, it is estimated that the United States will require 4500 headache specialists by 2040.
First Contact
To tackle this specialist shortfall, the AHS developed the First Contact program with the aim of improving headache education in primary care and help alleviate at least some of the demand for specialist care.
The national program was rolled out in 2020 and 2021. The educational symposia were delivered to PCPs at multiple locations across the country. The initiative also included a comprehensive website with numerous support resources.
After participating in the initiative, attendees were surveyed about the value of the program, and the results were subsequently analyzed and presented at the annual meeting of the American Headache Society.
The analysis included 636 survey respondents, a 38% response rate. Almost all participants (96%) were MDs and DOs. The remainder included nurse practitioners, physician assistants, and dentists.
About 85.6% of respondents reported being completely or very confident in their ability to recognize and accurately diagnose headache disorders, and 81.3% said they were completely or very confident in their ability to create tailored treatment plans.
Just over 90% of participants reported they would implement practice changes as a result of the program. The most commonly cited change was the use of diagnostic tools such as the three-question Migraine ID screener, followed closely by consideration of prescribing triptans and reducing the use of unnecessary neuroimaging.
“Overall, there was a positive response to this type of educational programming and interest in ongoing education in addressing headache disorders with both pharmaceutical and non-pharmaceutical treatment options,” said Nisha Malhotra, MD, a resident at New York University (NYU) Langone Health, New York City, who presented the findings at the conference.
The fact that so many general practitioners were keen to use this easy-to-use screen [Migraine ID screener], which can pick up about 90% of people with migraine, is “great,” said study investigator Mia Minen, MD, associate professor and chief of headache research at NYU Langone Health. “I’m pleased primary care providers said they were considering implementing this simple tool.”
However, respondents also cited barriers to change. These included cost constraints (48.9%), insurance reimbursement issues (48.6%), and lack of time (45.3%). Dr. Malhotra noted these concerns are primarily related to workflow rather than knowledge gaps or lack of training.
“This is exciting in that there doesn’t seem to be an issue with education primarily but rather with the logistical issues that exist in the workflow in a primary care setting,” said Dr. Malhotra.
Participants also noted the need for other improvements. For example, they expressed interest in differentiating migraine from other headache types and having a better understanding of how and when to refer to specialists, said Dr. Malhotra.
These practitioners also want to know more about treatment options beyond first-line medications. “They were interested in understanding more advanced medication treatment options beyond just the typical triptan,” said Dr. Malhotra.
In addition, they want to become more skilled in non-pharmaceutical options such as occipital nerve blocks and in massage, acupuncture, and other complementary forms of migraine management, she said.
The study may be vulnerable to sampling bias as survey participants had just attended an educational symposium on headaches. “They were already, to some degree, interested in improving their knowledge on headache,” said Dr. Malhotra.
Another study limitation was that researchers didn’t conduct a pre-survey analysis to determine changes as a result of the symposia. And as the survey was conducted so close to the symposium, “it’s difficult to draw conclusions on the long-term effects,” she added.
“That being said, First Contact is one of the first national initiatives for primary care education, and thus far, it has been very well received.”
The next step is to continue expanding the program and to create a First Contact for women and First Contact for pediatrics, said Dr. Minen.
Improved Diagnosis, Better Care
Commenting on the initiative, Juliana VanderPluym, MD, a headache specialist at the Mayo Clinic, Phoenix, who co-chaired the session where the survey results were presented, said it helps address the supply-demand imbalance in headache healthcare.
“Many, many people have headache disorders, and very few people are technically headache specialists, so we have to rely on our colleagues in primary care to help address the great need that’s out there for patients with headache disorders.”
Too many patients don’t get a proper diagnosis or appropriate treatment, said Dr. VanderPluym, so as time passes, “diseases can become more chronic and more refractory, and it affects people’s quality of life and productivity.”
The First Contact program, she said, helps increase providers’ comfort and confidence that they are providing the best patient care possible and lead to a reduction in the need for specialist referrals.
Dr. Minen serves on the First Contact advisory board.
A version of this article appeared on Medscape.com.
SAN DIEGO —
It is estimated that about 4 million PCP office visits annually are headache related, and that 52.8% of all migraine encounters occur in primary care settings.
However, PCPs aren’t always adequately trained in headache management and referral times to specialist care can be lengthy.
Data published in Headache show only 564 accredited headache specialists practice in the United States, but at least 3700 headache specialists are needed to treat those affected by migraine, with even more needed to address other disabling headache types such as tension-type headache and cluster headache. To keep up with population growth, it is estimated that the United States will require 4500 headache specialists by 2040.
First Contact
To tackle this specialist shortfall, the AHS developed the First Contact program with the aim of improving headache education in primary care and help alleviate at least some of the demand for specialist care.
The national program was rolled out in 2020 and 2021. The educational symposia were delivered to PCPs at multiple locations across the country. The initiative also included a comprehensive website with numerous support resources.
After participating in the initiative, attendees were surveyed about the value of the program, and the results were subsequently analyzed and presented at the annual meeting of the American Headache Society.
The analysis included 636 survey respondents, a 38% response rate. Almost all participants (96%) were MDs and DOs. The remainder included nurse practitioners, physician assistants, and dentists.
About 85.6% of respondents reported being completely or very confident in their ability to recognize and accurately diagnose headache disorders, and 81.3% said they were completely or very confident in their ability to create tailored treatment plans.
Just over 90% of participants reported they would implement practice changes as a result of the program. The most commonly cited change was the use of diagnostic tools such as the three-question Migraine ID screener, followed closely by consideration of prescribing triptans and reducing the use of unnecessary neuroimaging.
“Overall, there was a positive response to this type of educational programming and interest in ongoing education in addressing headache disorders with both pharmaceutical and non-pharmaceutical treatment options,” said Nisha Malhotra, MD, a resident at New York University (NYU) Langone Health, New York City, who presented the findings at the conference.
The fact that so many general practitioners were keen to use this easy-to-use screen [Migraine ID screener], which can pick up about 90% of people with migraine, is “great,” said study investigator Mia Minen, MD, associate professor and chief of headache research at NYU Langone Health. “I’m pleased primary care providers said they were considering implementing this simple tool.”
However, respondents also cited barriers to change. These included cost constraints (48.9%), insurance reimbursement issues (48.6%), and lack of time (45.3%). Dr. Malhotra noted these concerns are primarily related to workflow rather than knowledge gaps or lack of training.
“This is exciting in that there doesn’t seem to be an issue with education primarily but rather with the logistical issues that exist in the workflow in a primary care setting,” said Dr. Malhotra.
Participants also noted the need for other improvements. For example, they expressed interest in differentiating migraine from other headache types and having a better understanding of how and when to refer to specialists, said Dr. Malhotra.
These practitioners also want to know more about treatment options beyond first-line medications. “They were interested in understanding more advanced medication treatment options beyond just the typical triptan,” said Dr. Malhotra.
In addition, they want to become more skilled in non-pharmaceutical options such as occipital nerve blocks and in massage, acupuncture, and other complementary forms of migraine management, she said.
The study may be vulnerable to sampling bias as survey participants had just attended an educational symposium on headaches. “They were already, to some degree, interested in improving their knowledge on headache,” said Dr. Malhotra.
Another study limitation was that researchers didn’t conduct a pre-survey analysis to determine changes as a result of the symposia. And as the survey was conducted so close to the symposium, “it’s difficult to draw conclusions on the long-term effects,” she added.
“That being said, First Contact is one of the first national initiatives for primary care education, and thus far, it has been very well received.”
The next step is to continue expanding the program and to create a First Contact for women and First Contact for pediatrics, said Dr. Minen.
Improved Diagnosis, Better Care
Commenting on the initiative, Juliana VanderPluym, MD, a headache specialist at the Mayo Clinic, Phoenix, who co-chaired the session where the survey results were presented, said it helps address the supply-demand imbalance in headache healthcare.
“Many, many people have headache disorders, and very few people are technically headache specialists, so we have to rely on our colleagues in primary care to help address the great need that’s out there for patients with headache disorders.”
Too many patients don’t get a proper diagnosis or appropriate treatment, said Dr. VanderPluym, so as time passes, “diseases can become more chronic and more refractory, and it affects people’s quality of life and productivity.”
The First Contact program, she said, helps increase providers’ comfort and confidence that they are providing the best patient care possible and lead to a reduction in the need for specialist referrals.
Dr. Minen serves on the First Contact advisory board.
A version of this article appeared on Medscape.com.
FROM AHS 2024
Vision Impairment Tied to Higher Dementia Risk in Older Adults
TOPLINE:
; a decline in contrast sensitivity over time also correlates with the risk of developing dementia.
METHODOLOGY:
- Researchers conducted a longitudinal study to analyze the association of visual function with the risk for dementia in 2159 men and women (mean age, 77.9 years; 54% women) included from the National Health and Aging Trends Study between 2021 and 2022.
- All participants were free from dementia at baseline and underwent visual assessment while wearing their usual glasses or contact lenses.
- Distance and near visual acuity were measured as the log minimum angle of resolution (logMAR) units where higher values indicated worse visual acuity; contrast sensitivity was measured as the log contrast sensitivity (logCS) units where lower values represented worse outcomes.
- Dementia status was determined by a medical diagnosis, a dementia score of 2 or more, or poor performance on cognitive testing.
TAKEAWAY:
- Over the 1-year follow-up period, 192 adults (6.6%) developed dementia.
- Worsening of distant and near vision by 0.1 logMAR increased the risk for dementia by 8% (P = .01) and 7% (P = .02), respectively.
- Each 0.1 logCS decline in baseline contrast sensitivity increased the risk for dementia by 9% (P = .003).
- A yearly decline in contrast sensitivity by 0.1 logCS increased the likelihood of dementia by 14% (P = .007).
- Changes in distant and near vision over time did not show a significant association with risk for dementia (P = .58 and P = .79, respectively).
IN PRACTICE:
“Visual function, especially contrast sensitivity, might be a risk factor for developing dementia,” the authors wrote. “Early vision screening may help identify adults at higher risk of dementia, allowing for timely interventions.”
SOURCE:
The study was led by Louay Almidani, MD, MSc, of the Wilmer Eye Institute at the Johns Hopkins University School of Medicine, in Baltimore, and was published online in the American Journal of Ophthalmology.
LIMITATIONS:
The study had a limited follow-up period of 1 year and may not have captured the long-term association between visual impairment and the risk for dementia. Moreover, the researchers did not consider other visual function measures such as depth perception and visual field, which might have affected the results.
DISCLOSURES:
The study did not have any funding source. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
; a decline in contrast sensitivity over time also correlates with the risk of developing dementia.
METHODOLOGY:
- Researchers conducted a longitudinal study to analyze the association of visual function with the risk for dementia in 2159 men and women (mean age, 77.9 years; 54% women) included from the National Health and Aging Trends Study between 2021 and 2022.
- All participants were free from dementia at baseline and underwent visual assessment while wearing their usual glasses or contact lenses.
- Distance and near visual acuity were measured as the log minimum angle of resolution (logMAR) units where higher values indicated worse visual acuity; contrast sensitivity was measured as the log contrast sensitivity (logCS) units where lower values represented worse outcomes.
- Dementia status was determined by a medical diagnosis, a dementia score of 2 or more, or poor performance on cognitive testing.
TAKEAWAY:
- Over the 1-year follow-up period, 192 adults (6.6%) developed dementia.
- Worsening of distant and near vision by 0.1 logMAR increased the risk for dementia by 8% (P = .01) and 7% (P = .02), respectively.
- Each 0.1 logCS decline in baseline contrast sensitivity increased the risk for dementia by 9% (P = .003).
- A yearly decline in contrast sensitivity by 0.1 logCS increased the likelihood of dementia by 14% (P = .007).
- Changes in distant and near vision over time did not show a significant association with risk for dementia (P = .58 and P = .79, respectively).
IN PRACTICE:
“Visual function, especially contrast sensitivity, might be a risk factor for developing dementia,” the authors wrote. “Early vision screening may help identify adults at higher risk of dementia, allowing for timely interventions.”
SOURCE:
The study was led by Louay Almidani, MD, MSc, of the Wilmer Eye Institute at the Johns Hopkins University School of Medicine, in Baltimore, and was published online in the American Journal of Ophthalmology.
LIMITATIONS:
The study had a limited follow-up period of 1 year and may not have captured the long-term association between visual impairment and the risk for dementia. Moreover, the researchers did not consider other visual function measures such as depth perception and visual field, which might have affected the results.
DISCLOSURES:
The study did not have any funding source. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
; a decline in contrast sensitivity over time also correlates with the risk of developing dementia.
METHODOLOGY:
- Researchers conducted a longitudinal study to analyze the association of visual function with the risk for dementia in 2159 men and women (mean age, 77.9 years; 54% women) included from the National Health and Aging Trends Study between 2021 and 2022.
- All participants were free from dementia at baseline and underwent visual assessment while wearing their usual glasses or contact lenses.
- Distance and near visual acuity were measured as the log minimum angle of resolution (logMAR) units where higher values indicated worse visual acuity; contrast sensitivity was measured as the log contrast sensitivity (logCS) units where lower values represented worse outcomes.
- Dementia status was determined by a medical diagnosis, a dementia score of 2 or more, or poor performance on cognitive testing.
TAKEAWAY:
- Over the 1-year follow-up period, 192 adults (6.6%) developed dementia.
- Worsening of distant and near vision by 0.1 logMAR increased the risk for dementia by 8% (P = .01) and 7% (P = .02), respectively.
- Each 0.1 logCS decline in baseline contrast sensitivity increased the risk for dementia by 9% (P = .003).
- A yearly decline in contrast sensitivity by 0.1 logCS increased the likelihood of dementia by 14% (P = .007).
- Changes in distant and near vision over time did not show a significant association with risk for dementia (P = .58 and P = .79, respectively).
IN PRACTICE:
“Visual function, especially contrast sensitivity, might be a risk factor for developing dementia,” the authors wrote. “Early vision screening may help identify adults at higher risk of dementia, allowing for timely interventions.”
SOURCE:
The study was led by Louay Almidani, MD, MSc, of the Wilmer Eye Institute at the Johns Hopkins University School of Medicine, in Baltimore, and was published online in the American Journal of Ophthalmology.
LIMITATIONS:
The study had a limited follow-up period of 1 year and may not have captured the long-term association between visual impairment and the risk for dementia. Moreover, the researchers did not consider other visual function measures such as depth perception and visual field, which might have affected the results.
DISCLOSURES:
The study did not have any funding source. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Uncommon Locations for Brain Herniations Into Arachnoid Granulations: 5 Cases and Literature Review
The circulation of cerebrospinal fluid (CSF) is crucial for maintaining homeostasis for the optimal functioning of the multiple complex activities of the brain and spinal cord, including the disposal of metabolic waste products of brain and spinal cord activity into the cerebral venous drainage. Throughout the brain, the arachnoid mater forms small outpouchings or diverticula that penetrate the dura mater and communicate with the dural venous sinuses. These outpuchings are called arachnoid granulations or arachnoid villi, and most are found within the dural sinuses, primarily in the transverse sinuses and superior sagittal sinus, but can occasionally be seen extending into the inner table of the calvarium.1,2
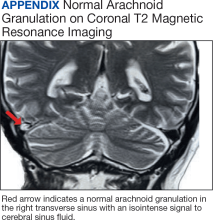
The amount of arachnoid granulations seen in bone, particularly around the superior sagittal sinus, may increase with age.2 Arachnoid granulations are generally small but the largest ones can be seen on gross examination during intracranial procedures or autopsy.3 Magnetic resonance imaging (MRI) can detect arachnoid granulations, which are characterized as T1 hypointense and T2 hyperintense (CSF isointense), well-circumscribed, small, nonenhancing masses within the dural sinuses or in the diploic space (Figure 1). Even small arachnoid granulations < 1 mm in length can be detected.2
Smaller arachnoid granulations have been described histologically as entirely covered by a dural membrane, thus creating a subdural space that separates the body of the arachnoid granulation from the lumen of the accompanying venous sinus.4 However, larger arachnoid granulations may not be completely covered by a dural membrane, thus creating a point of contact between the arachnoid granulation and the venous sinus.4 Larger arachnoid granulations are normally filled with CSF, and their signal characteristics are similar to CSF on imaging.5,6 Arachnoid granulations also often contain vessels draining into the adjacent venous sinus.5,6
When larger arachnoid granulations are present, they may permit the protrusion of herniated brain tissue. There has been an increasing number of reports of these brain herniations into arachnoid granulations (BHAGs) in the literature.7-10 While these herniations have been associated with nonspecific neurologic symptoms like tinnitus and idiopathic intracranial hypertension, their true clinical significance remains undetermined.10,11 This article presents 5 cases of BHAG, discusses their clinical presentations and image findings, and reviews the current literature.
Case 1
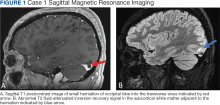
A 30-year-old male with a history of multiple traumatic brain injuries presented for evaluation of seizures. The patient described the semiology of the seizures as a bright, colorful light in his right visual field, followed by loss of vision, then loss of awareness and full body convulsion. The semiology of this patient’s seizures was consistent with left temporo-occipital lobe seizure. The only abnormality seen in the brain MRI was the herniation of brain parenchyma originating from the occipital lobe into the transverse sinus, presumably through an arachnoid granulation (Figure 1). An electroencephalogram (EEG) was unremarkable, though the semiology of the seizure historically described by the patient was localized to the area of BHAG. The patient is currently taking antiseizure medications and has experienced no additional seizures.
Case 2
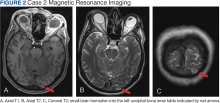
A male aged 53 years with a history of peripheral artery disease presented with a 6-month history of headaches and dizziness. The patient reported the onset of visual aura to his right visual field, starting as a fingernail-sized scintillating kaleidoscope light that would gradually increase in size to a round shape with fading kaleidoscope colors. This episode would last for a few minutes and was immediately followed by a headache. There was no alteration of consciousness during visual aura, although sometimes the patient would have right-sided scalp tingling. These episodes were often unprovoked, but occasionally triggered by bright lights. A single routine EEG was unremarkable. The patient reported headaches without aura, but not aura without headaches, which made occipital lobe seizure less likely. MRI demonstrated a small herniation of brain parenchyma into the inner table of the left occipital bone (Figure 2). The patient was diagnosed with migraine with aura, and the semiology of the visual aura corresponded to the location of the herniation in the left occipital region.
Case 3
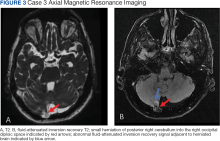
A 77-year-old male with a history of left ear diving injury presented with left-sided asymmetric hearing loss and word recognition difficulty for several years. MRI obtained as part of his work-up to evaluate for possible schwannoma of the eighth left cranial nerve instead demonstrated an incidental right cerebellar herniation within an arachnoid granulation into the diploic space of the occipital bone (Figure 3). The BHAG for this patient appeared to be an incidental finding unrelated to his asymmetric hearing loss.
Case 4
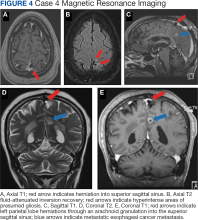
A male aged 62 years with a history of metastatic esophageal cancer, substance abuse, and a prior presumed alcohol withdrawal seizure underwent an MRI for evaluation of brain metastasis after presenting to the hospital with confusion 1 day after starting chemotherapy (Figure 4). Nine years prior, the patient had an isolated generalized tonic-clonic seizure approximately 72 hours following a period of alcohol cessation. The MRI demonstrated an incidental left parasagittal herniation of left parietal lobe tissue through an arachnoid granulation into the superior sagittal sinus, in addition to metastatic brain lesions. An EEG showed mild encephalopathy without evidence of seizures. It was determined that the patient's confusion was most likely due to toxic-metabolic encephalopathy from chemotherapy.
Case 5
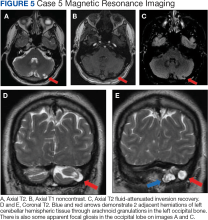
A 51-year-old male presented with worsening headache severity and frequency. He had a history of chronic headaches for about 20 years that occurred annually, but were now occurring twice weekly. The headaches often started with a left eye visual aura followed by pressure in the left eye, left frontal region, and left ear, with at times a cervicogenic component. No cervical spine imaging was available. An MRI revealed 2 small adjacent areas of cerebellar herniation into arachnoid granulations in the left occipital bone (Figure 5).
Discussion
Arachnoid granulations appear very early in life, although they are uncommon before age 2 years.2 Classically, they have been understood to act as 1-way valves permitting the outflow of CSF from the subarachnoid space to the dural venous sinuses. However, increasing evidence shows they may only play a minor role in that process.12 The structure of arachnoid granulations is being reexamined. A recent microscopy study demonstrated structural heterogeneity with a fine, porous lining that permits flow.13 Additionally, associated immune components in the microenvironment suggests that arachnoid granulations may function similarly to lymph nodes as part of a central nervous system lymphatic network.13 Evidence is lacking for arachnoid granulations being the primary route of CSF outflow, and newer models include CSF exit pathways along the cranial nerves and drainage through lymphatics within the dura mater.12
New MRI systems have demonstrated that the prevalence of arachnoid granulations increases with age. One study found that all subjects in the aged 40 years cohort had detectable arachnoid granulations on images obtained with a 3T MRI system, with the main site being the superior sagittal sinus.2 The prevalence increased until age 40 years and then noticeably decreased. Not only did the prevalence increase in this pattern, but the total number of detectable arachnoid granulations followed a similar pattern.2 In addition, the detectable arachnoid granulations tend to be larger in older patients. Arachnoid granulations are very common in adults, but little is known about when and why brain tissue herniates through these structures.
This case series illustrates how a small amount of adult cerebral or cerebellar matter in large arachnoid granulations can herniate into the dural sinuses and diploic space. Although arachnoid granulations extending into the dural sinuses and diploic space are a relatively common finding on MRI, BHAGs are rare in these locations.1,2,8 Improved spatial resolution afforded by newer high-field scanners with thinner sections, such as very thin (1 mm) T1- and heavily T2-weighted 3 dimensional sequences may lead to increased detection of BHAG. Some of these herniations are small and may be easily missed or confused for normal arachnoid granulations on 3 to 5 mm thickness MRIs.
Despite increased recognition, it is still uncertain to what degree these herniations contribute to the clinical presentations. Associated neurologic symptoms may include seizures, headaches, tinnitus, syncope, and increased intracranial pressure.7-10
Three cases presented in this article demonstrated abnormal signals adjacent to the herniated brain, presumably due to dysplasia of gliotic tissue. In 1 study, parenchymal signal and structural changes occurred in about one-half of the reported BHAG, all of which were cerebellar herniations.7 In Case 1, the herniation and adjacent abnormal MRI signal corresponded to localization of the seizure semiology as obtained from patient history, strongly suggesting the BHAG played a role in the presentation. Signal abnormality accompanying an adjacent BHAG may suggest a higher likelihood that the BHAG has clinical relevance. However, the patient in Case 2 had a visual aura that corresponded to the BHAG location, so a signal abnormality may not be necessary for a patient to develop symptoms. Case 1 also included a history of documented traumatic brain injuries, suggesting that perhaps head trauma may facilitate BHAG development. Regardless, there is likely also a congenital component to their formation, as BHAG has been observed in the pediatric population.14
The patient's asymmetric left-sided hearing loss in Case 3 appeared unrelated to the BHAG as its location was in the contralateral cerebellar region and did not correspond to the patient’s clinical findings. The patient in Case 4 had a limited history regarding localization details of their prior presumed alcohol withdrawal seizure, such as head movements, eye deviation, or lateralized onset of convulsions. Given this limited data, it is unclear whether their prior seizure could have been related to BHAG or not. The patient in case 5 reported worsening headaches on the left side of his head, which corresponded to BHAG occurring on the left side. However, given that the increased T2 signal occurred in the left cerebellar hemisphere with BHAG in the left occipital bone, the occipital cortex was not involved. In this case, the BHAG would not explain the patient’s visual aura as such a lesion would have been expected in the right occipital cortex rather than its actual location in this patient’s left cerebellar hemisphere.
CONCLUSIONS
Understanding the clinical impact of brain herniations is important because they are probably more common than previously thought. Improved MRI capabilities suggest that more BHAG will be detected moving forward as radiologists interpret images with higher resolution and thinner slices. Until its significance is fully understood, BHAG will continue to complicate the diagnosis of patients with neurologic complaints whose brain MRIs and EEGs are otherwise unremarkable.
There have been no cases of surgical BHAG intervention and pathology analysis that would help determine their clinical significance. A related entity, temporal lobe encephalocele, has been linked to focal temporal lobe epilepsy, which has demonstrated significant symptom improvement following surgical correction.15 However, encephaloceles have been distinguished from BHAG in part because they do not necessarily herniate through an arachnoid granulation.8 BHAG has only begun to be characterized in detail over the last decade, so more research is needed to understand how it develops and what clinical significance it truly holds.
1. Ikushima I, Korogi Y, Makita O, et al. MRI of arachnoid granulations within the dural sinuses using a FLAIR pulse sequence. Br J Radiol. 1999;72(863):1046-1051. doi:10.1259/bjr.72.863.10700819
2. Rados M, Zivko M, Perisa A, Oreskovic D, Klarica M. No arachnoid granulations-no problems: number, size, and distribution of arachnoid granulations from birth to 80 years of age. Front Aging Neurosci. 2021;13:698865. doi:10.3389/fnagi.2021.698865
3. Grossman CB, Potts DG. Arachnoid granulations: radiology and anatomy. Radiology. 1974;113(1):95-100. doi:10.1148/113.1.95
4. Wolpow ER, Schaumburg HH. Structure of the human arachnoid granulation. J Neurosurg. 1972;37(6):724-727. doi:10.3171/jns.1972.37.6.0724
5. Leach JL, Jones BV, Tomsick TA, Stewart CA, Balko MG. Normal appearance of arachnoid granulations on contrast-enhanced CT and MR of the brain: differentiation from dural sinus disease. AJNR Am J Neuroradiol. 1996;17(8):1523-1532.
6. Roche J, Warner D. Arachnoid granulations in the transverse and sigmoid sinuses: CT, MR, and MR angiographic appearance of a normal anatomic variation. AJNR Am J Neuroradiol. 1996;17(4):677-683.
7. Malekzadehlashkariani S, Wanke I, Rufenacht DA, San Millan D. Brain herniations into arachnoid granulations: about 68 cases in 38 patients and review of the literature. Neuroradiology. 2016;58(5):443-457. doi:10.1007/s00234-016-1662-5
8. Battal B, Castillo M. Brain herniations into the dural venous sinuses or calvarium: MRI of a recently recognized entity. Neuroradiol J. 2014;27(1):55-62. doi:10.15274/NRJ-2014-10006
9. Liebo GB, Lane JJ, Van Gompel JJ, Eckel LJ, Schwartz KM, Lehman VT. Brain herniation into arachnoid granulations: clinical and neuroimaging features. J Neuroimaging. 2016;26(6):592-598. doi:10.1111/jon.12366
10. Smith ER, Caton MT, Villanueva-Meyer JE, et al. Brain herniation (encephalocele) into arachnoid granulations: Prevalence and association with pulsatile tinnitus and idiopathic intracranial hypertension. Neuroradiology. 2022;64(9):1747-1754.
11. Battal B, Hamcan S, Akgun V, et al. Brain herniations into the dural venous sinus or calvarium: MRI findings, possible causes and clinical significance. Eur Radiol. 2016;26(6):1723-1731.
12. Proulx ST. Cerebrospinal fluid outflow: A review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol Life Sci. 2021;78(6):2429-2457.
13. Shah T, Leurgans SE, Mehta RI, et al. Arachnoid granulations are lymphatic conduits that communicate with bone marrow and dura-arachnoid stroma. J Exp Med. 2023;220(2).
14. Sade R, Ogul H, Polat G, Pirimoglu B, Kantarci M. Brain herniation into the transverse sinuses’ arachnoid granulations in the pediatric population investigated with 3 T MRI. Acta Neurol Belg. 2019;119(2):225-231.
15. Saavalainen T, Jutila L, Mervaala E, Kalviainen R, Vanninen R, Immonen A. Temporal anteroinferior encephalocele: An underrecognized etiology of temporal lobe epilepsy? Neurology. 2015;85(17):1467-1474.
The circulation of cerebrospinal fluid (CSF) is crucial for maintaining homeostasis for the optimal functioning of the multiple complex activities of the brain and spinal cord, including the disposal of metabolic waste products of brain and spinal cord activity into the cerebral venous drainage. Throughout the brain, the arachnoid mater forms small outpouchings or diverticula that penetrate the dura mater and communicate with the dural venous sinuses. These outpuchings are called arachnoid granulations or arachnoid villi, and most are found within the dural sinuses, primarily in the transverse sinuses and superior sagittal sinus, but can occasionally be seen extending into the inner table of the calvarium.1,2
The amount of arachnoid granulations seen in bone, particularly around the superior sagittal sinus, may increase with age.2 Arachnoid granulations are generally small but the largest ones can be seen on gross examination during intracranial procedures or autopsy.3 Magnetic resonance imaging (MRI) can detect arachnoid granulations, which are characterized as T1 hypointense and T2 hyperintense (CSF isointense), well-circumscribed, small, nonenhancing masses within the dural sinuses or in the diploic space (Figure 1). Even small arachnoid granulations < 1 mm in length can be detected.2
Smaller arachnoid granulations have been described histologically as entirely covered by a dural membrane, thus creating a subdural space that separates the body of the arachnoid granulation from the lumen of the accompanying venous sinus.4 However, larger arachnoid granulations may not be completely covered by a dural membrane, thus creating a point of contact between the arachnoid granulation and the venous sinus.4 Larger arachnoid granulations are normally filled with CSF, and their signal characteristics are similar to CSF on imaging.5,6 Arachnoid granulations also often contain vessels draining into the adjacent venous sinus.5,6
When larger arachnoid granulations are present, they may permit the protrusion of herniated brain tissue. There has been an increasing number of reports of these brain herniations into arachnoid granulations (BHAGs) in the literature.7-10 While these herniations have been associated with nonspecific neurologic symptoms like tinnitus and idiopathic intracranial hypertension, their true clinical significance remains undetermined.10,11 This article presents 5 cases of BHAG, discusses their clinical presentations and image findings, and reviews the current literature.
Case 1
A 30-year-old male with a history of multiple traumatic brain injuries presented for evaluation of seizures. The patient described the semiology of the seizures as a bright, colorful light in his right visual field, followed by loss of vision, then loss of awareness and full body convulsion. The semiology of this patient’s seizures was consistent with left temporo-occipital lobe seizure. The only abnormality seen in the brain MRI was the herniation of brain parenchyma originating from the occipital lobe into the transverse sinus, presumably through an arachnoid granulation (Figure 1). An electroencephalogram (EEG) was unremarkable, though the semiology of the seizure historically described by the patient was localized to the area of BHAG. The patient is currently taking antiseizure medications and has experienced no additional seizures.
Case 2
A male aged 53 years with a history of peripheral artery disease presented with a 6-month history of headaches and dizziness. The patient reported the onset of visual aura to his right visual field, starting as a fingernail-sized scintillating kaleidoscope light that would gradually increase in size to a round shape with fading kaleidoscope colors. This episode would last for a few minutes and was immediately followed by a headache. There was no alteration of consciousness during visual aura, although sometimes the patient would have right-sided scalp tingling. These episodes were often unprovoked, but occasionally triggered by bright lights. A single routine EEG was unremarkable. The patient reported headaches without aura, but not aura without headaches, which made occipital lobe seizure less likely. MRI demonstrated a small herniation of brain parenchyma into the inner table of the left occipital bone (Figure 2). The patient was diagnosed with migraine with aura, and the semiology of the visual aura corresponded to the location of the herniation in the left occipital region.
Case 3
A 77-year-old male with a history of left ear diving injury presented with left-sided asymmetric hearing loss and word recognition difficulty for several years. MRI obtained as part of his work-up to evaluate for possible schwannoma of the eighth left cranial nerve instead demonstrated an incidental right cerebellar herniation within an arachnoid granulation into the diploic space of the occipital bone (Figure 3). The BHAG for this patient appeared to be an incidental finding unrelated to his asymmetric hearing loss.
Case 4
A male aged 62 years with a history of metastatic esophageal cancer, substance abuse, and a prior presumed alcohol withdrawal seizure underwent an MRI for evaluation of brain metastasis after presenting to the hospital with confusion 1 day after starting chemotherapy (Figure 4). Nine years prior, the patient had an isolated generalized tonic-clonic seizure approximately 72 hours following a period of alcohol cessation. The MRI demonstrated an incidental left parasagittal herniation of left parietal lobe tissue through an arachnoid granulation into the superior sagittal sinus, in addition to metastatic brain lesions. An EEG showed mild encephalopathy without evidence of seizures. It was determined that the patient's confusion was most likely due to toxic-metabolic encephalopathy from chemotherapy.
Case 5
A 51-year-old male presented with worsening headache severity and frequency. He had a history of chronic headaches for about 20 years that occurred annually, but were now occurring twice weekly. The headaches often started with a left eye visual aura followed by pressure in the left eye, left frontal region, and left ear, with at times a cervicogenic component. No cervical spine imaging was available. An MRI revealed 2 small adjacent areas of cerebellar herniation into arachnoid granulations in the left occipital bone (Figure 5).
Discussion
Arachnoid granulations appear very early in life, although they are uncommon before age 2 years.2 Classically, they have been understood to act as 1-way valves permitting the outflow of CSF from the subarachnoid space to the dural venous sinuses. However, increasing evidence shows they may only play a minor role in that process.12 The structure of arachnoid granulations is being reexamined. A recent microscopy study demonstrated structural heterogeneity with a fine, porous lining that permits flow.13 Additionally, associated immune components in the microenvironment suggests that arachnoid granulations may function similarly to lymph nodes as part of a central nervous system lymphatic network.13 Evidence is lacking for arachnoid granulations being the primary route of CSF outflow, and newer models include CSF exit pathways along the cranial nerves and drainage through lymphatics within the dura mater.12
New MRI systems have demonstrated that the prevalence of arachnoid granulations increases with age. One study found that all subjects in the aged 40 years cohort had detectable arachnoid granulations on images obtained with a 3T MRI system, with the main site being the superior sagittal sinus.2 The prevalence increased until age 40 years and then noticeably decreased. Not only did the prevalence increase in this pattern, but the total number of detectable arachnoid granulations followed a similar pattern.2 In addition, the detectable arachnoid granulations tend to be larger in older patients. Arachnoid granulations are very common in adults, but little is known about when and why brain tissue herniates through these structures.
This case series illustrates how a small amount of adult cerebral or cerebellar matter in large arachnoid granulations can herniate into the dural sinuses and diploic space. Although arachnoid granulations extending into the dural sinuses and diploic space are a relatively common finding on MRI, BHAGs are rare in these locations.1,2,8 Improved spatial resolution afforded by newer high-field scanners with thinner sections, such as very thin (1 mm) T1- and heavily T2-weighted 3 dimensional sequences may lead to increased detection of BHAG. Some of these herniations are small and may be easily missed or confused for normal arachnoid granulations on 3 to 5 mm thickness MRIs.
Despite increased recognition, it is still uncertain to what degree these herniations contribute to the clinical presentations. Associated neurologic symptoms may include seizures, headaches, tinnitus, syncope, and increased intracranial pressure.7-10
Three cases presented in this article demonstrated abnormal signals adjacent to the herniated brain, presumably due to dysplasia of gliotic tissue. In 1 study, parenchymal signal and structural changes occurred in about one-half of the reported BHAG, all of which were cerebellar herniations.7 In Case 1, the herniation and adjacent abnormal MRI signal corresponded to localization of the seizure semiology as obtained from patient history, strongly suggesting the BHAG played a role in the presentation. Signal abnormality accompanying an adjacent BHAG may suggest a higher likelihood that the BHAG has clinical relevance. However, the patient in Case 2 had a visual aura that corresponded to the BHAG location, so a signal abnormality may not be necessary for a patient to develop symptoms. Case 1 also included a history of documented traumatic brain injuries, suggesting that perhaps head trauma may facilitate BHAG development. Regardless, there is likely also a congenital component to their formation, as BHAG has been observed in the pediatric population.14
The patient's asymmetric left-sided hearing loss in Case 3 appeared unrelated to the BHAG as its location was in the contralateral cerebellar region and did not correspond to the patient’s clinical findings. The patient in Case 4 had a limited history regarding localization details of their prior presumed alcohol withdrawal seizure, such as head movements, eye deviation, or lateralized onset of convulsions. Given this limited data, it is unclear whether their prior seizure could have been related to BHAG or not. The patient in case 5 reported worsening headaches on the left side of his head, which corresponded to BHAG occurring on the left side. However, given that the increased T2 signal occurred in the left cerebellar hemisphere with BHAG in the left occipital bone, the occipital cortex was not involved. In this case, the BHAG would not explain the patient’s visual aura as such a lesion would have been expected in the right occipital cortex rather than its actual location in this patient’s left cerebellar hemisphere.
CONCLUSIONS
Understanding the clinical impact of brain herniations is important because they are probably more common than previously thought. Improved MRI capabilities suggest that more BHAG will be detected moving forward as radiologists interpret images with higher resolution and thinner slices. Until its significance is fully understood, BHAG will continue to complicate the diagnosis of patients with neurologic complaints whose brain MRIs and EEGs are otherwise unremarkable.
There have been no cases of surgical BHAG intervention and pathology analysis that would help determine their clinical significance. A related entity, temporal lobe encephalocele, has been linked to focal temporal lobe epilepsy, which has demonstrated significant symptom improvement following surgical correction.15 However, encephaloceles have been distinguished from BHAG in part because they do not necessarily herniate through an arachnoid granulation.8 BHAG has only begun to be characterized in detail over the last decade, so more research is needed to understand how it develops and what clinical significance it truly holds.
The circulation of cerebrospinal fluid (CSF) is crucial for maintaining homeostasis for the optimal functioning of the multiple complex activities of the brain and spinal cord, including the disposal of metabolic waste products of brain and spinal cord activity into the cerebral venous drainage. Throughout the brain, the arachnoid mater forms small outpouchings or diverticula that penetrate the dura mater and communicate with the dural venous sinuses. These outpuchings are called arachnoid granulations or arachnoid villi, and most are found within the dural sinuses, primarily in the transverse sinuses and superior sagittal sinus, but can occasionally be seen extending into the inner table of the calvarium.1,2
The amount of arachnoid granulations seen in bone, particularly around the superior sagittal sinus, may increase with age.2 Arachnoid granulations are generally small but the largest ones can be seen on gross examination during intracranial procedures or autopsy.3 Magnetic resonance imaging (MRI) can detect arachnoid granulations, which are characterized as T1 hypointense and T2 hyperintense (CSF isointense), well-circumscribed, small, nonenhancing masses within the dural sinuses or in the diploic space (Figure 1). Even small arachnoid granulations < 1 mm in length can be detected.2
Smaller arachnoid granulations have been described histologically as entirely covered by a dural membrane, thus creating a subdural space that separates the body of the arachnoid granulation from the lumen of the accompanying venous sinus.4 However, larger arachnoid granulations may not be completely covered by a dural membrane, thus creating a point of contact between the arachnoid granulation and the venous sinus.4 Larger arachnoid granulations are normally filled with CSF, and their signal characteristics are similar to CSF on imaging.5,6 Arachnoid granulations also often contain vessels draining into the adjacent venous sinus.5,6
When larger arachnoid granulations are present, they may permit the protrusion of herniated brain tissue. There has been an increasing number of reports of these brain herniations into arachnoid granulations (BHAGs) in the literature.7-10 While these herniations have been associated with nonspecific neurologic symptoms like tinnitus and idiopathic intracranial hypertension, their true clinical significance remains undetermined.10,11 This article presents 5 cases of BHAG, discusses their clinical presentations and image findings, and reviews the current literature.
Case 1
A 30-year-old male with a history of multiple traumatic brain injuries presented for evaluation of seizures. The patient described the semiology of the seizures as a bright, colorful light in his right visual field, followed by loss of vision, then loss of awareness and full body convulsion. The semiology of this patient’s seizures was consistent with left temporo-occipital lobe seizure. The only abnormality seen in the brain MRI was the herniation of brain parenchyma originating from the occipital lobe into the transverse sinus, presumably through an arachnoid granulation (Figure 1). An electroencephalogram (EEG) was unremarkable, though the semiology of the seizure historically described by the patient was localized to the area of BHAG. The patient is currently taking antiseizure medications and has experienced no additional seizures.
Case 2
A male aged 53 years with a history of peripheral artery disease presented with a 6-month history of headaches and dizziness. The patient reported the onset of visual aura to his right visual field, starting as a fingernail-sized scintillating kaleidoscope light that would gradually increase in size to a round shape with fading kaleidoscope colors. This episode would last for a few minutes and was immediately followed by a headache. There was no alteration of consciousness during visual aura, although sometimes the patient would have right-sided scalp tingling. These episodes were often unprovoked, but occasionally triggered by bright lights. A single routine EEG was unremarkable. The patient reported headaches without aura, but not aura without headaches, which made occipital lobe seizure less likely. MRI demonstrated a small herniation of brain parenchyma into the inner table of the left occipital bone (Figure 2). The patient was diagnosed with migraine with aura, and the semiology of the visual aura corresponded to the location of the herniation in the left occipital region.
Case 3
A 77-year-old male with a history of left ear diving injury presented with left-sided asymmetric hearing loss and word recognition difficulty for several years. MRI obtained as part of his work-up to evaluate for possible schwannoma of the eighth left cranial nerve instead demonstrated an incidental right cerebellar herniation within an arachnoid granulation into the diploic space of the occipital bone (Figure 3). The BHAG for this patient appeared to be an incidental finding unrelated to his asymmetric hearing loss.
Case 4
A male aged 62 years with a history of metastatic esophageal cancer, substance abuse, and a prior presumed alcohol withdrawal seizure underwent an MRI for evaluation of brain metastasis after presenting to the hospital with confusion 1 day after starting chemotherapy (Figure 4). Nine years prior, the patient had an isolated generalized tonic-clonic seizure approximately 72 hours following a period of alcohol cessation. The MRI demonstrated an incidental left parasagittal herniation of left parietal lobe tissue through an arachnoid granulation into the superior sagittal sinus, in addition to metastatic brain lesions. An EEG showed mild encephalopathy without evidence of seizures. It was determined that the patient's confusion was most likely due to toxic-metabolic encephalopathy from chemotherapy.
Case 5
A 51-year-old male presented with worsening headache severity and frequency. He had a history of chronic headaches for about 20 years that occurred annually, but were now occurring twice weekly. The headaches often started with a left eye visual aura followed by pressure in the left eye, left frontal region, and left ear, with at times a cervicogenic component. No cervical spine imaging was available. An MRI revealed 2 small adjacent areas of cerebellar herniation into arachnoid granulations in the left occipital bone (Figure 5).
Discussion
Arachnoid granulations appear very early in life, although they are uncommon before age 2 years.2 Classically, they have been understood to act as 1-way valves permitting the outflow of CSF from the subarachnoid space to the dural venous sinuses. However, increasing evidence shows they may only play a minor role in that process.12 The structure of arachnoid granulations is being reexamined. A recent microscopy study demonstrated structural heterogeneity with a fine, porous lining that permits flow.13 Additionally, associated immune components in the microenvironment suggests that arachnoid granulations may function similarly to lymph nodes as part of a central nervous system lymphatic network.13 Evidence is lacking for arachnoid granulations being the primary route of CSF outflow, and newer models include CSF exit pathways along the cranial nerves and drainage through lymphatics within the dura mater.12
New MRI systems have demonstrated that the prevalence of arachnoid granulations increases with age. One study found that all subjects in the aged 40 years cohort had detectable arachnoid granulations on images obtained with a 3T MRI system, with the main site being the superior sagittal sinus.2 The prevalence increased until age 40 years and then noticeably decreased. Not only did the prevalence increase in this pattern, but the total number of detectable arachnoid granulations followed a similar pattern.2 In addition, the detectable arachnoid granulations tend to be larger in older patients. Arachnoid granulations are very common in adults, but little is known about when and why brain tissue herniates through these structures.
This case series illustrates how a small amount of adult cerebral or cerebellar matter in large arachnoid granulations can herniate into the dural sinuses and diploic space. Although arachnoid granulations extending into the dural sinuses and diploic space are a relatively common finding on MRI, BHAGs are rare in these locations.1,2,8 Improved spatial resolution afforded by newer high-field scanners with thinner sections, such as very thin (1 mm) T1- and heavily T2-weighted 3 dimensional sequences may lead to increased detection of BHAG. Some of these herniations are small and may be easily missed or confused for normal arachnoid granulations on 3 to 5 mm thickness MRIs.
Despite increased recognition, it is still uncertain to what degree these herniations contribute to the clinical presentations. Associated neurologic symptoms may include seizures, headaches, tinnitus, syncope, and increased intracranial pressure.7-10
Three cases presented in this article demonstrated abnormal signals adjacent to the herniated brain, presumably due to dysplasia of gliotic tissue. In 1 study, parenchymal signal and structural changes occurred in about one-half of the reported BHAG, all of which were cerebellar herniations.7 In Case 1, the herniation and adjacent abnormal MRI signal corresponded to localization of the seizure semiology as obtained from patient history, strongly suggesting the BHAG played a role in the presentation. Signal abnormality accompanying an adjacent BHAG may suggest a higher likelihood that the BHAG has clinical relevance. However, the patient in Case 2 had a visual aura that corresponded to the BHAG location, so a signal abnormality may not be necessary for a patient to develop symptoms. Case 1 also included a history of documented traumatic brain injuries, suggesting that perhaps head trauma may facilitate BHAG development. Regardless, there is likely also a congenital component to their formation, as BHAG has been observed in the pediatric population.14
The patient's asymmetric left-sided hearing loss in Case 3 appeared unrelated to the BHAG as its location was in the contralateral cerebellar region and did not correspond to the patient’s clinical findings. The patient in Case 4 had a limited history regarding localization details of their prior presumed alcohol withdrawal seizure, such as head movements, eye deviation, or lateralized onset of convulsions. Given this limited data, it is unclear whether their prior seizure could have been related to BHAG or not. The patient in case 5 reported worsening headaches on the left side of his head, which corresponded to BHAG occurring on the left side. However, given that the increased T2 signal occurred in the left cerebellar hemisphere with BHAG in the left occipital bone, the occipital cortex was not involved. In this case, the BHAG would not explain the patient’s visual aura as such a lesion would have been expected in the right occipital cortex rather than its actual location in this patient’s left cerebellar hemisphere.
CONCLUSIONS
Understanding the clinical impact of brain herniations is important because they are probably more common than previously thought. Improved MRI capabilities suggest that more BHAG will be detected moving forward as radiologists interpret images with higher resolution and thinner slices. Until its significance is fully understood, BHAG will continue to complicate the diagnosis of patients with neurologic complaints whose brain MRIs and EEGs are otherwise unremarkable.
There have been no cases of surgical BHAG intervention and pathology analysis that would help determine their clinical significance. A related entity, temporal lobe encephalocele, has been linked to focal temporal lobe epilepsy, which has demonstrated significant symptom improvement following surgical correction.15 However, encephaloceles have been distinguished from BHAG in part because they do not necessarily herniate through an arachnoid granulation.8 BHAG has only begun to be characterized in detail over the last decade, so more research is needed to understand how it develops and what clinical significance it truly holds.
1. Ikushima I, Korogi Y, Makita O, et al. MRI of arachnoid granulations within the dural sinuses using a FLAIR pulse sequence. Br J Radiol. 1999;72(863):1046-1051. doi:10.1259/bjr.72.863.10700819
2. Rados M, Zivko M, Perisa A, Oreskovic D, Klarica M. No arachnoid granulations-no problems: number, size, and distribution of arachnoid granulations from birth to 80 years of age. Front Aging Neurosci. 2021;13:698865. doi:10.3389/fnagi.2021.698865
3. Grossman CB, Potts DG. Arachnoid granulations: radiology and anatomy. Radiology. 1974;113(1):95-100. doi:10.1148/113.1.95
4. Wolpow ER, Schaumburg HH. Structure of the human arachnoid granulation. J Neurosurg. 1972;37(6):724-727. doi:10.3171/jns.1972.37.6.0724
5. Leach JL, Jones BV, Tomsick TA, Stewart CA, Balko MG. Normal appearance of arachnoid granulations on contrast-enhanced CT and MR of the brain: differentiation from dural sinus disease. AJNR Am J Neuroradiol. 1996;17(8):1523-1532.
6. Roche J, Warner D. Arachnoid granulations in the transverse and sigmoid sinuses: CT, MR, and MR angiographic appearance of a normal anatomic variation. AJNR Am J Neuroradiol. 1996;17(4):677-683.
7. Malekzadehlashkariani S, Wanke I, Rufenacht DA, San Millan D. Brain herniations into arachnoid granulations: about 68 cases in 38 patients and review of the literature. Neuroradiology. 2016;58(5):443-457. doi:10.1007/s00234-016-1662-5
8. Battal B, Castillo M. Brain herniations into the dural venous sinuses or calvarium: MRI of a recently recognized entity. Neuroradiol J. 2014;27(1):55-62. doi:10.15274/NRJ-2014-10006
9. Liebo GB, Lane JJ, Van Gompel JJ, Eckel LJ, Schwartz KM, Lehman VT. Brain herniation into arachnoid granulations: clinical and neuroimaging features. J Neuroimaging. 2016;26(6):592-598. doi:10.1111/jon.12366
10. Smith ER, Caton MT, Villanueva-Meyer JE, et al. Brain herniation (encephalocele) into arachnoid granulations: Prevalence and association with pulsatile tinnitus and idiopathic intracranial hypertension. Neuroradiology. 2022;64(9):1747-1754.
11. Battal B, Hamcan S, Akgun V, et al. Brain herniations into the dural venous sinus or calvarium: MRI findings, possible causes and clinical significance. Eur Radiol. 2016;26(6):1723-1731.
12. Proulx ST. Cerebrospinal fluid outflow: A review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol Life Sci. 2021;78(6):2429-2457.
13. Shah T, Leurgans SE, Mehta RI, et al. Arachnoid granulations are lymphatic conduits that communicate with bone marrow and dura-arachnoid stroma. J Exp Med. 2023;220(2).
14. Sade R, Ogul H, Polat G, Pirimoglu B, Kantarci M. Brain herniation into the transverse sinuses’ arachnoid granulations in the pediatric population investigated with 3 T MRI. Acta Neurol Belg. 2019;119(2):225-231.
15. Saavalainen T, Jutila L, Mervaala E, Kalviainen R, Vanninen R, Immonen A. Temporal anteroinferior encephalocele: An underrecognized etiology of temporal lobe epilepsy? Neurology. 2015;85(17):1467-1474.
1. Ikushima I, Korogi Y, Makita O, et al. MRI of arachnoid granulations within the dural sinuses using a FLAIR pulse sequence. Br J Radiol. 1999;72(863):1046-1051. doi:10.1259/bjr.72.863.10700819
2. Rados M, Zivko M, Perisa A, Oreskovic D, Klarica M. No arachnoid granulations-no problems: number, size, and distribution of arachnoid granulations from birth to 80 years of age. Front Aging Neurosci. 2021;13:698865. doi:10.3389/fnagi.2021.698865
3. Grossman CB, Potts DG. Arachnoid granulations: radiology and anatomy. Radiology. 1974;113(1):95-100. doi:10.1148/113.1.95
4. Wolpow ER, Schaumburg HH. Structure of the human arachnoid granulation. J Neurosurg. 1972;37(6):724-727. doi:10.3171/jns.1972.37.6.0724
5. Leach JL, Jones BV, Tomsick TA, Stewart CA, Balko MG. Normal appearance of arachnoid granulations on contrast-enhanced CT and MR of the brain: differentiation from dural sinus disease. AJNR Am J Neuroradiol. 1996;17(8):1523-1532.
6. Roche J, Warner D. Arachnoid granulations in the transverse and sigmoid sinuses: CT, MR, and MR angiographic appearance of a normal anatomic variation. AJNR Am J Neuroradiol. 1996;17(4):677-683.
7. Malekzadehlashkariani S, Wanke I, Rufenacht DA, San Millan D. Brain herniations into arachnoid granulations: about 68 cases in 38 patients and review of the literature. Neuroradiology. 2016;58(5):443-457. doi:10.1007/s00234-016-1662-5
8. Battal B, Castillo M. Brain herniations into the dural venous sinuses or calvarium: MRI of a recently recognized entity. Neuroradiol J. 2014;27(1):55-62. doi:10.15274/NRJ-2014-10006
9. Liebo GB, Lane JJ, Van Gompel JJ, Eckel LJ, Schwartz KM, Lehman VT. Brain herniation into arachnoid granulations: clinical and neuroimaging features. J Neuroimaging. 2016;26(6):592-598. doi:10.1111/jon.12366
10. Smith ER, Caton MT, Villanueva-Meyer JE, et al. Brain herniation (encephalocele) into arachnoid granulations: Prevalence and association with pulsatile tinnitus and idiopathic intracranial hypertension. Neuroradiology. 2022;64(9):1747-1754.
11. Battal B, Hamcan S, Akgun V, et al. Brain herniations into the dural venous sinus or calvarium: MRI findings, possible causes and clinical significance. Eur Radiol. 2016;26(6):1723-1731.
12. Proulx ST. Cerebrospinal fluid outflow: A review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol Life Sci. 2021;78(6):2429-2457.
13. Shah T, Leurgans SE, Mehta RI, et al. Arachnoid granulations are lymphatic conduits that communicate with bone marrow and dura-arachnoid stroma. J Exp Med. 2023;220(2).
14. Sade R, Ogul H, Polat G, Pirimoglu B, Kantarci M. Brain herniation into the transverse sinuses’ arachnoid granulations in the pediatric population investigated with 3 T MRI. Acta Neurol Belg. 2019;119(2):225-231.
15. Saavalainen T, Jutila L, Mervaala E, Kalviainen R, Vanninen R, Immonen A. Temporal anteroinferior encephalocele: An underrecognized etiology of temporal lobe epilepsy? Neurology. 2015;85(17):1467-1474.
Why Don’t Migraine Patients Seek Treatment?
SAN DIEGO — results of a recent survey showed.
Participants cited concerns that their complaints would be dismissed, a belief that healthcare providers could offer no additional help, and a prior unsuccessful clinician visit as reasons for not seeking care. Survey respondents saw an average of four clinicians before finally receiving a diagnosis.
“I was shocked that a third of patients were reluctant to seek care,” said study investigator Elizabeth K. Seng, PhD, associate professor, Ferkauf Graduate School of Psychology, Yeshiva University, and research associate professor, department of neurology, Albert Einstein College of Medicine, both in New York City. “That just shows a much higher level of medical distress than I expected from this community of people who are obviously suffering from this significant neurologic disease.”
The findings were presented at the annual meeting of the American Headache Society.
‘Significant Disease’
The study included 500 adults with migraine (mean age, 40 years) who signed up for a patient support group sponsored by Eli Lilly and completed a comprehensive survey. Respondents were mostly female, White, non-Hispanic, and well-educated individuals.
Half of participants had episodic migraines, and half had chronic migraines; 46% reported experiencing anxiety and 33% reported depression.
Almost all respondents had initiated treatment with a first calcitonin gene-related peptide (CGRP) monoclonal antibody.
“These are people who have significant enough disease that eventually they needed our top-tier preventive medication,” Dr. Seng said.
Participants answered a variety of questions pertaining to disease factors and treatment seeking. Just over 70% said they suspected they had migraine prior to diagnosis, “which means for almost 30%, it was a surprise when they received the diagnosis,” said Dr. Seng.
Nearly 40% reported that a relative first suggested they may have migraine, and 33% suspected it themselves. Only 17.4% said a healthcare provider suggested they may have the condition.
Almost a third of respondents (30.5%) reported they were reluctant to seek medical help.
“Some said they didn’t think their physician could do anything more than they were already doing for themselves, or that they’d be taken seriously, or they had had talked to doctors before and this wasn’t helpful,” said Dr. Seng.
These responses speak to the need for better public health messaging, she said. “People have this idea that migraine attacks aren’t a big deal when, in fact, these attacks area big deal and certainly deserve treatment.”
Family and friends were participants’ most common source of information on migraine, followed by the Internet. “This highlights the importance of getting migraine-related information out there so that when people talk to their friends and family, they’re receiving accurate information,” said Dr. Seng.
When asked about the path to a diagnosis, respondents reported consulting an average of four providers before receiving an accurate diagnosis. “That’s pretty remarkable,” Dr. Seng said.
An increase in frequency or severity of migraine attacks or attacks that interfered with work or school “pushed people over the threshold to seek care,” Dr. Seng said.
A subset of patients was asked about the factors they believed could help with migraine attacks. Of these, 80% cited diet and 70% stress reduction. Supplements, exercise, and relaxation techniques were cited much less frequently, said Dr. Seng.
The mean age of respondents’ migraine diagnosis was 26 years, so there was about 18 years from the time of diagnosis to participation in the survey, which could introduce recall bias. Other potential limitations included the fact that the survey had no open-ended questions, and men and ethnic minorities were underrepresented.
Useful Data
Commenting on the study findings, Nina Riggins, MD, PhD, president, Brain Performance Center and Research Institute, and director of the Headache Center at The Neuron Clinic, San Diego, California, said the survey findings are “very useful” and highlight “significant opportunities for improvement in migraine education for clinicians and people living with migraine disease.”
The fact that participants reported consulting an average of four healthcare providers before receiving an accurate diagnosis underscores the importance of providing clinicians with tools to identify migraine, she said.
This is especially relevant as new migraine therapies that may improve efficacy and have fewer side effects become available, she added.
“It would be interesting to see in future studies if migraine recognition by non-headache specialists improved after CGRP-blocking medications for migraine management became available,” said Dr. Riggins, who is cochair of the AHS First Contact program which is aimed at improving headache management in primary care.
She added that she and her colleagues will keep these survey results in mind when creating future educational materials for clinicians.
The study was supported by Eli Lily. Dr. Seng is a consultant for GlaxoSmithKline, Theranica, and Abbvie, and receives research support from the National Institutes of Health, National Center for Complementary and Integrative Health, National Institute of Neurological Disorders and Stroke, Veterans Health Administration, Cystic Fibrosis Foundation, and the American Heart Association. Dr. Riggins reported no relevant conflicts.
A version of this article appeared on Medscape.com.
SAN DIEGO — results of a recent survey showed.
Participants cited concerns that their complaints would be dismissed, a belief that healthcare providers could offer no additional help, and a prior unsuccessful clinician visit as reasons for not seeking care. Survey respondents saw an average of four clinicians before finally receiving a diagnosis.
“I was shocked that a third of patients were reluctant to seek care,” said study investigator Elizabeth K. Seng, PhD, associate professor, Ferkauf Graduate School of Psychology, Yeshiva University, and research associate professor, department of neurology, Albert Einstein College of Medicine, both in New York City. “That just shows a much higher level of medical distress than I expected from this community of people who are obviously suffering from this significant neurologic disease.”
The findings were presented at the annual meeting of the American Headache Society.
‘Significant Disease’
The study included 500 adults with migraine (mean age, 40 years) who signed up for a patient support group sponsored by Eli Lilly and completed a comprehensive survey. Respondents were mostly female, White, non-Hispanic, and well-educated individuals.
Half of participants had episodic migraines, and half had chronic migraines; 46% reported experiencing anxiety and 33% reported depression.
Almost all respondents had initiated treatment with a first calcitonin gene-related peptide (CGRP) monoclonal antibody.
“These are people who have significant enough disease that eventually they needed our top-tier preventive medication,” Dr. Seng said.
Participants answered a variety of questions pertaining to disease factors and treatment seeking. Just over 70% said they suspected they had migraine prior to diagnosis, “which means for almost 30%, it was a surprise when they received the diagnosis,” said Dr. Seng.
Nearly 40% reported that a relative first suggested they may have migraine, and 33% suspected it themselves. Only 17.4% said a healthcare provider suggested they may have the condition.
Almost a third of respondents (30.5%) reported they were reluctant to seek medical help.
“Some said they didn’t think their physician could do anything more than they were already doing for themselves, or that they’d be taken seriously, or they had had talked to doctors before and this wasn’t helpful,” said Dr. Seng.
These responses speak to the need for better public health messaging, she said. “People have this idea that migraine attacks aren’t a big deal when, in fact, these attacks area big deal and certainly deserve treatment.”
Family and friends were participants’ most common source of information on migraine, followed by the Internet. “This highlights the importance of getting migraine-related information out there so that when people talk to their friends and family, they’re receiving accurate information,” said Dr. Seng.
When asked about the path to a diagnosis, respondents reported consulting an average of four providers before receiving an accurate diagnosis. “That’s pretty remarkable,” Dr. Seng said.
An increase in frequency or severity of migraine attacks or attacks that interfered with work or school “pushed people over the threshold to seek care,” Dr. Seng said.
A subset of patients was asked about the factors they believed could help with migraine attacks. Of these, 80% cited diet and 70% stress reduction. Supplements, exercise, and relaxation techniques were cited much less frequently, said Dr. Seng.
The mean age of respondents’ migraine diagnosis was 26 years, so there was about 18 years from the time of diagnosis to participation in the survey, which could introduce recall bias. Other potential limitations included the fact that the survey had no open-ended questions, and men and ethnic minorities were underrepresented.
Useful Data
Commenting on the study findings, Nina Riggins, MD, PhD, president, Brain Performance Center and Research Institute, and director of the Headache Center at The Neuron Clinic, San Diego, California, said the survey findings are “very useful” and highlight “significant opportunities for improvement in migraine education for clinicians and people living with migraine disease.”
The fact that participants reported consulting an average of four healthcare providers before receiving an accurate diagnosis underscores the importance of providing clinicians with tools to identify migraine, she said.
This is especially relevant as new migraine therapies that may improve efficacy and have fewer side effects become available, she added.
“It would be interesting to see in future studies if migraine recognition by non-headache specialists improved after CGRP-blocking medications for migraine management became available,” said Dr. Riggins, who is cochair of the AHS First Contact program which is aimed at improving headache management in primary care.
She added that she and her colleagues will keep these survey results in mind when creating future educational materials for clinicians.
The study was supported by Eli Lily. Dr. Seng is a consultant for GlaxoSmithKline, Theranica, and Abbvie, and receives research support from the National Institutes of Health, National Center for Complementary and Integrative Health, National Institute of Neurological Disorders and Stroke, Veterans Health Administration, Cystic Fibrosis Foundation, and the American Heart Association. Dr. Riggins reported no relevant conflicts.
A version of this article appeared on Medscape.com.
SAN DIEGO — results of a recent survey showed.
Participants cited concerns that their complaints would be dismissed, a belief that healthcare providers could offer no additional help, and a prior unsuccessful clinician visit as reasons for not seeking care. Survey respondents saw an average of four clinicians before finally receiving a diagnosis.
“I was shocked that a third of patients were reluctant to seek care,” said study investigator Elizabeth K. Seng, PhD, associate professor, Ferkauf Graduate School of Psychology, Yeshiva University, and research associate professor, department of neurology, Albert Einstein College of Medicine, both in New York City. “That just shows a much higher level of medical distress than I expected from this community of people who are obviously suffering from this significant neurologic disease.”
The findings were presented at the annual meeting of the American Headache Society.
‘Significant Disease’
The study included 500 adults with migraine (mean age, 40 years) who signed up for a patient support group sponsored by Eli Lilly and completed a comprehensive survey. Respondents were mostly female, White, non-Hispanic, and well-educated individuals.
Half of participants had episodic migraines, and half had chronic migraines; 46% reported experiencing anxiety and 33% reported depression.
Almost all respondents had initiated treatment with a first calcitonin gene-related peptide (CGRP) monoclonal antibody.
“These are people who have significant enough disease that eventually they needed our top-tier preventive medication,” Dr. Seng said.
Participants answered a variety of questions pertaining to disease factors and treatment seeking. Just over 70% said they suspected they had migraine prior to diagnosis, “which means for almost 30%, it was a surprise when they received the diagnosis,” said Dr. Seng.
Nearly 40% reported that a relative first suggested they may have migraine, and 33% suspected it themselves. Only 17.4% said a healthcare provider suggested they may have the condition.
Almost a third of respondents (30.5%) reported they were reluctant to seek medical help.
“Some said they didn’t think their physician could do anything more than they were already doing for themselves, or that they’d be taken seriously, or they had had talked to doctors before and this wasn’t helpful,” said Dr. Seng.
These responses speak to the need for better public health messaging, she said. “People have this idea that migraine attacks aren’t a big deal when, in fact, these attacks area big deal and certainly deserve treatment.”
Family and friends were participants’ most common source of information on migraine, followed by the Internet. “This highlights the importance of getting migraine-related information out there so that when people talk to their friends and family, they’re receiving accurate information,” said Dr. Seng.
When asked about the path to a diagnosis, respondents reported consulting an average of four providers before receiving an accurate diagnosis. “That’s pretty remarkable,” Dr. Seng said.
An increase in frequency or severity of migraine attacks or attacks that interfered with work or school “pushed people over the threshold to seek care,” Dr. Seng said.
A subset of patients was asked about the factors they believed could help with migraine attacks. Of these, 80% cited diet and 70% stress reduction. Supplements, exercise, and relaxation techniques were cited much less frequently, said Dr. Seng.
The mean age of respondents’ migraine diagnosis was 26 years, so there was about 18 years from the time of diagnosis to participation in the survey, which could introduce recall bias. Other potential limitations included the fact that the survey had no open-ended questions, and men and ethnic minorities were underrepresented.
Useful Data
Commenting on the study findings, Nina Riggins, MD, PhD, president, Brain Performance Center and Research Institute, and director of the Headache Center at The Neuron Clinic, San Diego, California, said the survey findings are “very useful” and highlight “significant opportunities for improvement in migraine education for clinicians and people living with migraine disease.”
The fact that participants reported consulting an average of four healthcare providers before receiving an accurate diagnosis underscores the importance of providing clinicians with tools to identify migraine, she said.
This is especially relevant as new migraine therapies that may improve efficacy and have fewer side effects become available, she added.
“It would be interesting to see in future studies if migraine recognition by non-headache specialists improved after CGRP-blocking medications for migraine management became available,” said Dr. Riggins, who is cochair of the AHS First Contact program which is aimed at improving headache management in primary care.
She added that she and her colleagues will keep these survey results in mind when creating future educational materials for clinicians.
The study was supported by Eli Lily. Dr. Seng is a consultant for GlaxoSmithKline, Theranica, and Abbvie, and receives research support from the National Institutes of Health, National Center for Complementary and Integrative Health, National Institute of Neurological Disorders and Stroke, Veterans Health Administration, Cystic Fibrosis Foundation, and the American Heart Association. Dr. Riggins reported no relevant conflicts.
A version of this article appeared on Medscape.com.
FROM AHS 2024
Intensive Lifestyle Changes May Counter Early Alzheimer’s Symptoms
, in what authors said is the first randomized controlled trial of intensive lifestyle modification for patients diagnosed with Alzheimer’s disease. Results could help physicians address patients at risk of Alzheimer’s disease who reject relevant testing because they believe nothing can forestall development of the disease, the authors added. The study was published online in Alzheimer’s Research & Therapy.
Although technology allows probable Alzheimer’s disease diagnosis years before clinical symptoms appear, wrote investigators led by Dean Ornish, MD, of the Preventive Medicine Research Institute in Sausalito, California, “many people do not want to know if they are likely to get Alzheimer’s disease if they do not believe they can do anything about it. If intensive lifestyle changes may cause improvement in cognition and function in MCI or early dementia due to Alzheimer’s disease, then it is reasonable to think that these lifestyle changes may also help to prevent MCI or early dementia due to Alzheimer’s disease.” As with cardiovascular disease, the authors added, preventing Alzheimer’s disease might require less intensive lifestyle modifications than treating it.
Study Methodology
Investigators randomized 26 patients with Montréal Cognitive Assessment scores of 18 or higher to an intensive intervention involving nutrition, exercise, and stress management techniques. To improve adherence, the protocol included participants’ spouses or caregivers.
Two patients, both in the treatment group, withdrew over logistical concerns.
After 20 weeks, treated patients exhibited statistically significant differences in several key measures versus a 25-patient usual-care control group. Scores that improved in the intervention group and worsened among controls included the following:
- Clinical Global Impression of Change (CGIC, P = .001)
- Clinical Dementia Rating-Global (CDR-Global, -0.04, P = .037)
- Clinical Dementia Rating Sum of Boxes (CDR-SB, +0.08, P = .032)
- Alzheimer’s Disease Assessment Scale (ADAS-Cog, -1.01, P = .053)
The validity of these changes in cognition and function, and possible biological mechanisms of improvement, were supported by statistically significant improvements in several clinically relevant biomarkers versus controls, the investigators wrote. These biomarkers included Abeta42/40 ratio, HbA1c, insulin, and glycoprotein acetylation. “This information may also help in predicting which patients are more likely to show improvements in cognition and function by making these intensive lifestyle changes,” the authors added.
In primary analysis, the degree of lifestyle changes required to stop progression of MCI ranged from 71.4% (ADAS-Cog) to 120.6% (CDR-SB). “This helps to explain why other studies of less intensive lifestyle interventions may not have been sufficient to stop deterioration or improve cognition and function,” the authors wrote. Moreover, they added, variable adherence might explain why in the intervention group, 10 patients improved their CGIC scores, while the rest held static or worsened.
Caveats
Alzheimer’s Association Vice President of Medical and Scientific Relations Heather M. Snyder, PhD, said, “This is an interesting paper in an important area of research and adds to the growing body of literature on how behavior or lifestyle may be related to cognitive decline. However, because this is a small phase 2 study, it is important for this or similar work to be done in larger, more diverse populations and over a longer duration of the intervention.” She was not involved with the study but was asked to comment.
Investigators chose the 20-week duration, they explained, because control-group patients likely would not refrain from trying the lifestyle intervention beyond that timeframe. Perhaps more importantly, challenges created by the COVID-19 pandemic required researchers to cut planned enrollment in half, eliminate planned MRI and amyloid PET scans, and reduce the number of cognition and function tests.
Such shortcomings limit what neurologists can glean and generalize from the study, said Dr. Snyder. “That said,” she added, “it does demonstrate the potential of an intensive behavior/lifestyle intervention, and the importance of this sort of research in Alzheimer’s and dementia.” Although the complexity of the interventions makes these studies challenging, she added, “it is important that we continue to advance larger, longer studies in more representative study populations to develop specific recommendations.”
Further Study
The Alzheimer’s Association’s U.S. POINTER study is the first large-scale study in the United States to explore the impact of comprehensive lifestyle changes on cognitive health. About 2000 older adults at risk for cognitive decline are participating, from diverse locations across the country. More than 25% of participants come from groups typically underrepresented in dementia research, said Dr. Snyder. Initial results are expected in summer 2025.
Future research also should explore reasons (beyond adherence) why some patients respond to lifestyle interventions better than others, and the potential synergy of lifestyle changes with drug therapies, wrote Dr. Ornish and colleagues.
“For now,” said Dr. Snyder, “there is an opportunity for providers to incorporate or expand messaging with their patients and families about the habits that they can incorporate into their daily lives. The Alzheimer’s Association offers 10 Healthy Habits for Your Brain — everyday actions that can make a difference for your brain health.”
Investigators received study funding from more than two dozen charitable foundations and other organizations. Dr. Snyder is a full-time employee of the Alzheimer’s Association and in this role, serves on the leadership team of the U.S. POINTER study. Her partner works for Abbott in an unrelated field.
, in what authors said is the first randomized controlled trial of intensive lifestyle modification for patients diagnosed with Alzheimer’s disease. Results could help physicians address patients at risk of Alzheimer’s disease who reject relevant testing because they believe nothing can forestall development of the disease, the authors added. The study was published online in Alzheimer’s Research & Therapy.
Although technology allows probable Alzheimer’s disease diagnosis years before clinical symptoms appear, wrote investigators led by Dean Ornish, MD, of the Preventive Medicine Research Institute in Sausalito, California, “many people do not want to know if they are likely to get Alzheimer’s disease if they do not believe they can do anything about it. If intensive lifestyle changes may cause improvement in cognition and function in MCI or early dementia due to Alzheimer’s disease, then it is reasonable to think that these lifestyle changes may also help to prevent MCI or early dementia due to Alzheimer’s disease.” As with cardiovascular disease, the authors added, preventing Alzheimer’s disease might require less intensive lifestyle modifications than treating it.
Study Methodology
Investigators randomized 26 patients with Montréal Cognitive Assessment scores of 18 or higher to an intensive intervention involving nutrition, exercise, and stress management techniques. To improve adherence, the protocol included participants’ spouses or caregivers.
Two patients, both in the treatment group, withdrew over logistical concerns.
After 20 weeks, treated patients exhibited statistically significant differences in several key measures versus a 25-patient usual-care control group. Scores that improved in the intervention group and worsened among controls included the following:
- Clinical Global Impression of Change (CGIC, P = .001)
- Clinical Dementia Rating-Global (CDR-Global, -0.04, P = .037)
- Clinical Dementia Rating Sum of Boxes (CDR-SB, +0.08, P = .032)
- Alzheimer’s Disease Assessment Scale (ADAS-Cog, -1.01, P = .053)
The validity of these changes in cognition and function, and possible biological mechanisms of improvement, were supported by statistically significant improvements in several clinically relevant biomarkers versus controls, the investigators wrote. These biomarkers included Abeta42/40 ratio, HbA1c, insulin, and glycoprotein acetylation. “This information may also help in predicting which patients are more likely to show improvements in cognition and function by making these intensive lifestyle changes,” the authors added.
In primary analysis, the degree of lifestyle changes required to stop progression of MCI ranged from 71.4% (ADAS-Cog) to 120.6% (CDR-SB). “This helps to explain why other studies of less intensive lifestyle interventions may not have been sufficient to stop deterioration or improve cognition and function,” the authors wrote. Moreover, they added, variable adherence might explain why in the intervention group, 10 patients improved their CGIC scores, while the rest held static or worsened.
Caveats
Alzheimer’s Association Vice President of Medical and Scientific Relations Heather M. Snyder, PhD, said, “This is an interesting paper in an important area of research and adds to the growing body of literature on how behavior or lifestyle may be related to cognitive decline. However, because this is a small phase 2 study, it is important for this or similar work to be done in larger, more diverse populations and over a longer duration of the intervention.” She was not involved with the study but was asked to comment.
Investigators chose the 20-week duration, they explained, because control-group patients likely would not refrain from trying the lifestyle intervention beyond that timeframe. Perhaps more importantly, challenges created by the COVID-19 pandemic required researchers to cut planned enrollment in half, eliminate planned MRI and amyloid PET scans, and reduce the number of cognition and function tests.
Such shortcomings limit what neurologists can glean and generalize from the study, said Dr. Snyder. “That said,” she added, “it does demonstrate the potential of an intensive behavior/lifestyle intervention, and the importance of this sort of research in Alzheimer’s and dementia.” Although the complexity of the interventions makes these studies challenging, she added, “it is important that we continue to advance larger, longer studies in more representative study populations to develop specific recommendations.”
Further Study
The Alzheimer’s Association’s U.S. POINTER study is the first large-scale study in the United States to explore the impact of comprehensive lifestyle changes on cognitive health. About 2000 older adults at risk for cognitive decline are participating, from diverse locations across the country. More than 25% of participants come from groups typically underrepresented in dementia research, said Dr. Snyder. Initial results are expected in summer 2025.
Future research also should explore reasons (beyond adherence) why some patients respond to lifestyle interventions better than others, and the potential synergy of lifestyle changes with drug therapies, wrote Dr. Ornish and colleagues.
“For now,” said Dr. Snyder, “there is an opportunity for providers to incorporate or expand messaging with their patients and families about the habits that they can incorporate into their daily lives. The Alzheimer’s Association offers 10 Healthy Habits for Your Brain — everyday actions that can make a difference for your brain health.”
Investigators received study funding from more than two dozen charitable foundations and other organizations. Dr. Snyder is a full-time employee of the Alzheimer’s Association and in this role, serves on the leadership team of the U.S. POINTER study. Her partner works for Abbott in an unrelated field.
, in what authors said is the first randomized controlled trial of intensive lifestyle modification for patients diagnosed with Alzheimer’s disease. Results could help physicians address patients at risk of Alzheimer’s disease who reject relevant testing because they believe nothing can forestall development of the disease, the authors added. The study was published online in Alzheimer’s Research & Therapy.
Although technology allows probable Alzheimer’s disease diagnosis years before clinical symptoms appear, wrote investigators led by Dean Ornish, MD, of the Preventive Medicine Research Institute in Sausalito, California, “many people do not want to know if they are likely to get Alzheimer’s disease if they do not believe they can do anything about it. If intensive lifestyle changes may cause improvement in cognition and function in MCI or early dementia due to Alzheimer’s disease, then it is reasonable to think that these lifestyle changes may also help to prevent MCI or early dementia due to Alzheimer’s disease.” As with cardiovascular disease, the authors added, preventing Alzheimer’s disease might require less intensive lifestyle modifications than treating it.
Study Methodology
Investigators randomized 26 patients with Montréal Cognitive Assessment scores of 18 or higher to an intensive intervention involving nutrition, exercise, and stress management techniques. To improve adherence, the protocol included participants’ spouses or caregivers.
Two patients, both in the treatment group, withdrew over logistical concerns.
After 20 weeks, treated patients exhibited statistically significant differences in several key measures versus a 25-patient usual-care control group. Scores that improved in the intervention group and worsened among controls included the following:
- Clinical Global Impression of Change (CGIC, P = .001)
- Clinical Dementia Rating-Global (CDR-Global, -0.04, P = .037)
- Clinical Dementia Rating Sum of Boxes (CDR-SB, +0.08, P = .032)
- Alzheimer’s Disease Assessment Scale (ADAS-Cog, -1.01, P = .053)
The validity of these changes in cognition and function, and possible biological mechanisms of improvement, were supported by statistically significant improvements in several clinically relevant biomarkers versus controls, the investigators wrote. These biomarkers included Abeta42/40 ratio, HbA1c, insulin, and glycoprotein acetylation. “This information may also help in predicting which patients are more likely to show improvements in cognition and function by making these intensive lifestyle changes,” the authors added.
In primary analysis, the degree of lifestyle changes required to stop progression of MCI ranged from 71.4% (ADAS-Cog) to 120.6% (CDR-SB). “This helps to explain why other studies of less intensive lifestyle interventions may not have been sufficient to stop deterioration or improve cognition and function,” the authors wrote. Moreover, they added, variable adherence might explain why in the intervention group, 10 patients improved their CGIC scores, while the rest held static or worsened.
Caveats
Alzheimer’s Association Vice President of Medical and Scientific Relations Heather M. Snyder, PhD, said, “This is an interesting paper in an important area of research and adds to the growing body of literature on how behavior or lifestyle may be related to cognitive decline. However, because this is a small phase 2 study, it is important for this or similar work to be done in larger, more diverse populations and over a longer duration of the intervention.” She was not involved with the study but was asked to comment.
Investigators chose the 20-week duration, they explained, because control-group patients likely would not refrain from trying the lifestyle intervention beyond that timeframe. Perhaps more importantly, challenges created by the COVID-19 pandemic required researchers to cut planned enrollment in half, eliminate planned MRI and amyloid PET scans, and reduce the number of cognition and function tests.
Such shortcomings limit what neurologists can glean and generalize from the study, said Dr. Snyder. “That said,” she added, “it does demonstrate the potential of an intensive behavior/lifestyle intervention, and the importance of this sort of research in Alzheimer’s and dementia.” Although the complexity of the interventions makes these studies challenging, she added, “it is important that we continue to advance larger, longer studies in more representative study populations to develop specific recommendations.”
Further Study
The Alzheimer’s Association’s U.S. POINTER study is the first large-scale study in the United States to explore the impact of comprehensive lifestyle changes on cognitive health. About 2000 older adults at risk for cognitive decline are participating, from diverse locations across the country. More than 25% of participants come from groups typically underrepresented in dementia research, said Dr. Snyder. Initial results are expected in summer 2025.
Future research also should explore reasons (beyond adherence) why some patients respond to lifestyle interventions better than others, and the potential synergy of lifestyle changes with drug therapies, wrote Dr. Ornish and colleagues.
“For now,” said Dr. Snyder, “there is an opportunity for providers to incorporate or expand messaging with their patients and families about the habits that they can incorporate into their daily lives. The Alzheimer’s Association offers 10 Healthy Habits for Your Brain — everyday actions that can make a difference for your brain health.”
Investigators received study funding from more than two dozen charitable foundations and other organizations. Dr. Snyder is a full-time employee of the Alzheimer’s Association and in this role, serves on the leadership team of the U.S. POINTER study. Her partner works for Abbott in an unrelated field.
FROM ALZHEIMER’S RESEARCH & THERAPY
Lidocaine Effective Against Pediatric Migraine
SAN DIEGO — The treatment has long been used in adults, and frequently in children on the strength of observational evidence.
Prior Research
Most of the studies have been conducted in adults, and these were often in specific settings like the emergency department for status migrainosus, while outpatient studies were generally conducted in chronic migraine, according to presenting author Christina Szperka, MD. “The assumptions were a little bit different,” Dr. Szperka, director of the pediatric headache program at Children’s Hospital of Philadelphia, said in an interview.
Retrospective studies are also fraught with bias. “We’ve tried to look at retrospective data. People don’t necessarily report how they’re doing unless they come back, and so you lose a huge portion of kids,” said Dr. Szperka, who presented the research at the annual meeting of the American Headache Society.
“From a clinical perspective, I think it gives us additional evidence that what we’re doing makes a difference, and I think that will help us in terms of insurance coverage, because that’s really been a major barrier,” said Dr. Szperka.
The study also opens other avenues for research. “Just doing the greater occipital nerves only reduces the pain so much. So what’s the next step? Do I study additional injections? Do I do a study where I compare different medications?”
She previously conducted a study of how providers were using lidocaine injections, and “there was a large amount of variability, both in terms of what nerves are being injected, what medications they were using, the patient population, et cetera,” said Dr. Szperka. Previous observational studies have suggested efficacy in pediatric populations for transition and prevention of migraine, new daily persistent headache, posttraumatic headache, and post-shunt occipital neuralgia.
A Randomized, Controlled Trial
In the new study, 58 adolescents aged 7 to 21 (mean age, 16.0 years; 44 female) were initially treated with lidocaine cream. The patients were “relatively refractory,” said Dr. Szperka, with 25 having received intravenous medications and 6 having been inpatients. After 30 minutes, if they still had pain and consented to further treatment, Dr. Szperka performed bilateral greater occipital nerve injections with lidocaine or a saline placebo, and did additional injections after 30 minutes if there wasn’t sufficient improvement.
There was no significant change in pain after the lidocaine cream treatment, and all patients proceeded to be randomized to lidocaine or placebo injections. The primary outcome of 30-minute reduction in pain score ranked 0-10 favored the lidocaine group (2.3 vs 1.1; P = .013). There was a 2-point reduction in pain scores in 69% of the lidocaine group and 34% of the saline group (P = .009) and a higher frequency of pain relief from moderate/severe to no pain or mild (52% versus 24%; P = .03). There was no significant difference in pain freedom.
After 24 hours, the treatment group was more likely to experience pain relief from moderate/severe to no pain or mild (24% vs 3%; P = .05) and to be free from associated symptoms (48% vs 21%; P = .027). Pain at the injection site was significantly higher in the placebo group (5.4 vs 3.2), prompting a change in plans for future trials. “I don’t think I would do saline again, because I think it hurt them, and I don’t want to cause them harm,” said Dr. Szperka.
Adverse events were common, with all but one patient in the study experiencing at least one. “I think this is a couple of things: One, kids don’t like needles in their head. Nerve blocks hurt. And so it was not surprising in some ways that we had a very high rate of adverse events. We also consented them, and that had a long wait period, and there’s a lot of anxiety in the room. However, most of the adverse events were mild,” said Dr. Szperka.
Important Research in an Understudied Population
Laine Greene, MD, who moderated the session, was asked for comment. “I think it’s an important study. Occipital nerve blocks have been used for a long period of time in management of migraine and other headache disorders. The quality of the evidence has always been brought into question, especially from payers, but also a very important aspect to this is that a lot of clinical trials over time have not specifically been done in children or adolescents, so any work that is done in that age category is significantly helpful to advancing therapeutics,” said Dr. Greene, associate professor of neurology at Mayo Clinic Arizona.
Dr. Szperka has consulted for AbbVie and Teva, and serves on data safety and monitoring boards for Eli Lilly and Upsher-Smith. She has been a principal investigator in trials sponsored by Abbvie, Amgen, Biohaven/Pfizer, Teva, and Theranica. Dr. Greene has no relevant financial disclosures.
SAN DIEGO — The treatment has long been used in adults, and frequently in children on the strength of observational evidence.
Prior Research
Most of the studies have been conducted in adults, and these were often in specific settings like the emergency department for status migrainosus, while outpatient studies were generally conducted in chronic migraine, according to presenting author Christina Szperka, MD. “The assumptions were a little bit different,” Dr. Szperka, director of the pediatric headache program at Children’s Hospital of Philadelphia, said in an interview.
Retrospective studies are also fraught with bias. “We’ve tried to look at retrospective data. People don’t necessarily report how they’re doing unless they come back, and so you lose a huge portion of kids,” said Dr. Szperka, who presented the research at the annual meeting of the American Headache Society.
“From a clinical perspective, I think it gives us additional evidence that what we’re doing makes a difference, and I think that will help us in terms of insurance coverage, because that’s really been a major barrier,” said Dr. Szperka.
The study also opens other avenues for research. “Just doing the greater occipital nerves only reduces the pain so much. So what’s the next step? Do I study additional injections? Do I do a study where I compare different medications?”
She previously conducted a study of how providers were using lidocaine injections, and “there was a large amount of variability, both in terms of what nerves are being injected, what medications they were using, the patient population, et cetera,” said Dr. Szperka. Previous observational studies have suggested efficacy in pediatric populations for transition and prevention of migraine, new daily persistent headache, posttraumatic headache, and post-shunt occipital neuralgia.
A Randomized, Controlled Trial
In the new study, 58 adolescents aged 7 to 21 (mean age, 16.0 years; 44 female) were initially treated with lidocaine cream. The patients were “relatively refractory,” said Dr. Szperka, with 25 having received intravenous medications and 6 having been inpatients. After 30 minutes, if they still had pain and consented to further treatment, Dr. Szperka performed bilateral greater occipital nerve injections with lidocaine or a saline placebo, and did additional injections after 30 minutes if there wasn’t sufficient improvement.
There was no significant change in pain after the lidocaine cream treatment, and all patients proceeded to be randomized to lidocaine or placebo injections. The primary outcome of 30-minute reduction in pain score ranked 0-10 favored the lidocaine group (2.3 vs 1.1; P = .013). There was a 2-point reduction in pain scores in 69% of the lidocaine group and 34% of the saline group (P = .009) and a higher frequency of pain relief from moderate/severe to no pain or mild (52% versus 24%; P = .03). There was no significant difference in pain freedom.
After 24 hours, the treatment group was more likely to experience pain relief from moderate/severe to no pain or mild (24% vs 3%; P = .05) and to be free from associated symptoms (48% vs 21%; P = .027). Pain at the injection site was significantly higher in the placebo group (5.4 vs 3.2), prompting a change in plans for future trials. “I don’t think I would do saline again, because I think it hurt them, and I don’t want to cause them harm,” said Dr. Szperka.
Adverse events were common, with all but one patient in the study experiencing at least one. “I think this is a couple of things: One, kids don’t like needles in their head. Nerve blocks hurt. And so it was not surprising in some ways that we had a very high rate of adverse events. We also consented them, and that had a long wait period, and there’s a lot of anxiety in the room. However, most of the adverse events were mild,” said Dr. Szperka.
Important Research in an Understudied Population
Laine Greene, MD, who moderated the session, was asked for comment. “I think it’s an important study. Occipital nerve blocks have been used for a long period of time in management of migraine and other headache disorders. The quality of the evidence has always been brought into question, especially from payers, but also a very important aspect to this is that a lot of clinical trials over time have not specifically been done in children or adolescents, so any work that is done in that age category is significantly helpful to advancing therapeutics,” said Dr. Greene, associate professor of neurology at Mayo Clinic Arizona.
Dr. Szperka has consulted for AbbVie and Teva, and serves on data safety and monitoring boards for Eli Lilly and Upsher-Smith. She has been a principal investigator in trials sponsored by Abbvie, Amgen, Biohaven/Pfizer, Teva, and Theranica. Dr. Greene has no relevant financial disclosures.
SAN DIEGO — The treatment has long been used in adults, and frequently in children on the strength of observational evidence.
Prior Research
Most of the studies have been conducted in adults, and these were often in specific settings like the emergency department for status migrainosus, while outpatient studies were generally conducted in chronic migraine, according to presenting author Christina Szperka, MD. “The assumptions were a little bit different,” Dr. Szperka, director of the pediatric headache program at Children’s Hospital of Philadelphia, said in an interview.
Retrospective studies are also fraught with bias. “We’ve tried to look at retrospective data. People don’t necessarily report how they’re doing unless they come back, and so you lose a huge portion of kids,” said Dr. Szperka, who presented the research at the annual meeting of the American Headache Society.
“From a clinical perspective, I think it gives us additional evidence that what we’re doing makes a difference, and I think that will help us in terms of insurance coverage, because that’s really been a major barrier,” said Dr. Szperka.
The study also opens other avenues for research. “Just doing the greater occipital nerves only reduces the pain so much. So what’s the next step? Do I study additional injections? Do I do a study where I compare different medications?”
She previously conducted a study of how providers were using lidocaine injections, and “there was a large amount of variability, both in terms of what nerves are being injected, what medications they were using, the patient population, et cetera,” said Dr. Szperka. Previous observational studies have suggested efficacy in pediatric populations for transition and prevention of migraine, new daily persistent headache, posttraumatic headache, and post-shunt occipital neuralgia.
A Randomized, Controlled Trial
In the new study, 58 adolescents aged 7 to 21 (mean age, 16.0 years; 44 female) were initially treated with lidocaine cream. The patients were “relatively refractory,” said Dr. Szperka, with 25 having received intravenous medications and 6 having been inpatients. After 30 minutes, if they still had pain and consented to further treatment, Dr. Szperka performed bilateral greater occipital nerve injections with lidocaine or a saline placebo, and did additional injections after 30 minutes if there wasn’t sufficient improvement.
There was no significant change in pain after the lidocaine cream treatment, and all patients proceeded to be randomized to lidocaine or placebo injections. The primary outcome of 30-minute reduction in pain score ranked 0-10 favored the lidocaine group (2.3 vs 1.1; P = .013). There was a 2-point reduction in pain scores in 69% of the lidocaine group and 34% of the saline group (P = .009) and a higher frequency of pain relief from moderate/severe to no pain or mild (52% versus 24%; P = .03). There was no significant difference in pain freedom.
After 24 hours, the treatment group was more likely to experience pain relief from moderate/severe to no pain or mild (24% vs 3%; P = .05) and to be free from associated symptoms (48% vs 21%; P = .027). Pain at the injection site was significantly higher in the placebo group (5.4 vs 3.2), prompting a change in plans for future trials. “I don’t think I would do saline again, because I think it hurt them, and I don’t want to cause them harm,” said Dr. Szperka.
Adverse events were common, with all but one patient in the study experiencing at least one. “I think this is a couple of things: One, kids don’t like needles in their head. Nerve blocks hurt. And so it was not surprising in some ways that we had a very high rate of adverse events. We also consented them, and that had a long wait period, and there’s a lot of anxiety in the room. However, most of the adverse events were mild,” said Dr. Szperka.
Important Research in an Understudied Population
Laine Greene, MD, who moderated the session, was asked for comment. “I think it’s an important study. Occipital nerve blocks have been used for a long period of time in management of migraine and other headache disorders. The quality of the evidence has always been brought into question, especially from payers, but also a very important aspect to this is that a lot of clinical trials over time have not specifically been done in children or adolescents, so any work that is done in that age category is significantly helpful to advancing therapeutics,” said Dr. Greene, associate professor of neurology at Mayo Clinic Arizona.
Dr. Szperka has consulted for AbbVie and Teva, and serves on data safety and monitoring boards for Eli Lilly and Upsher-Smith. She has been a principal investigator in trials sponsored by Abbvie, Amgen, Biohaven/Pfizer, Teva, and Theranica. Dr. Greene has no relevant financial disclosures.
FROM AHS 2024
Genetic Test Combo May Help Identify Global Development Delay
, a new study suggests.
Researchers, led by Jiamei Zhang, MS, Department of Rehabilitation Medicine, Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China, in a multicenter, prospective cohort study enrolled patients ages 12 to 60 months with GDD from six centers in China from July 2020 through August 2023. Participants underwent trio whole exome sequencing (trio-WES) paired with copy number variation sequencing (CNV-seq).
“To the best of our knowledge, this study represents the largest prospective examination of combined genetic testing methods in a GDD cohort,” the authors reported in JAMA Network Open.
GDD is a common neurodevelopmental disorder, marked by cognitive impairment, and affects about 1% of children, the paper states. Most children with GDD develop intellectual disability (ID) after 5 years of age, with implications for quality of life, their physical abilities, and social functioning. Early and accurate diagnosis followed by appropriately targeted treatment is critical, but lacking. Researchers note that there is lack of consensus among health care professionals on whether genetic testing is necessary.
Genetics are known to play a significant role in pathogenesis of GDD, but definitive biomarkers have been elusive.
Positive Detection Rate of 61%
In this study, the combined use of trio-WES with CNV-seq in children with early-stage GDD resulted in a positive detection rate of 61%, a significant improvement over performing individual tests, “enhancing the positive detection rate by 18%-40%,” the researchers wrote. The combined approach also saves families time and costs, they note, while leading to more comprehensive genetic analysis and fewer missed diagnoses.
The combined approach also addressed the limitations of trio-WES and CNV-seq used alone, the authors wrote. Because of technological constraints, trio-WES may miss 55% of CNV variations, and CNV-seq has a missed diagnosis rate of 3%.
The study included 434 patients with GDD (60% male; average age, 25 months) with diverse degrees of cognitive impairment: mild (23%); moderate (32%); severe (28%); and profound (17%).
Three characteristics were linked with higher likelihood of having genetic variants: Craniofacial abnormalities (odds ratio [OR], 2.27; 95% confidence interval [CI], 1.45-3.56); moderate or severe cognitive impairment (OR, 1.69; 95% CI, 1.05-2.70); and age between 12 and 24 months (OR, 1.57; 95% CI, 1.05-2.35).
Dopaminergic Pathway Promising for Treatment
Researchers also discovered that GDD-related genes were primarily enriched in lysosome, dopaminergic synapse, and lysine degradation pathways. Dopaminergic synapse emerged as a significant pathway linked with GDD.
“In this cohort study, our findings support the correlation between dopaminergic synapse and cognitive impairment, as substantiated by prior research and animal models. Therefore, targeting the dopaminergic pathway holds promise for treating GDD and ID,” the authors wrote.
However, the authors note in the limitations that they used only a subset of 100 patients with GDD to measure dopamine concentration.
“Expanding the sample size and conducting in vivo and in vitro experiments are necessary steps to verify whether dopamine can be targeted for clinical precision medical intervention in patients with GDD,” they wrote.
The authors reported no relevant financial relationships.
, a new study suggests.
Researchers, led by Jiamei Zhang, MS, Department of Rehabilitation Medicine, Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China, in a multicenter, prospective cohort study enrolled patients ages 12 to 60 months with GDD from six centers in China from July 2020 through August 2023. Participants underwent trio whole exome sequencing (trio-WES) paired with copy number variation sequencing (CNV-seq).
“To the best of our knowledge, this study represents the largest prospective examination of combined genetic testing methods in a GDD cohort,” the authors reported in JAMA Network Open.
GDD is a common neurodevelopmental disorder, marked by cognitive impairment, and affects about 1% of children, the paper states. Most children with GDD develop intellectual disability (ID) after 5 years of age, with implications for quality of life, their physical abilities, and social functioning. Early and accurate diagnosis followed by appropriately targeted treatment is critical, but lacking. Researchers note that there is lack of consensus among health care professionals on whether genetic testing is necessary.
Genetics are known to play a significant role in pathogenesis of GDD, but definitive biomarkers have been elusive.
Positive Detection Rate of 61%
In this study, the combined use of trio-WES with CNV-seq in children with early-stage GDD resulted in a positive detection rate of 61%, a significant improvement over performing individual tests, “enhancing the positive detection rate by 18%-40%,” the researchers wrote. The combined approach also saves families time and costs, they note, while leading to more comprehensive genetic analysis and fewer missed diagnoses.
The combined approach also addressed the limitations of trio-WES and CNV-seq used alone, the authors wrote. Because of technological constraints, trio-WES may miss 55% of CNV variations, and CNV-seq has a missed diagnosis rate of 3%.
The study included 434 patients with GDD (60% male; average age, 25 months) with diverse degrees of cognitive impairment: mild (23%); moderate (32%); severe (28%); and profound (17%).
Three characteristics were linked with higher likelihood of having genetic variants: Craniofacial abnormalities (odds ratio [OR], 2.27; 95% confidence interval [CI], 1.45-3.56); moderate or severe cognitive impairment (OR, 1.69; 95% CI, 1.05-2.70); and age between 12 and 24 months (OR, 1.57; 95% CI, 1.05-2.35).
Dopaminergic Pathway Promising for Treatment
Researchers also discovered that GDD-related genes were primarily enriched in lysosome, dopaminergic synapse, and lysine degradation pathways. Dopaminergic synapse emerged as a significant pathway linked with GDD.
“In this cohort study, our findings support the correlation between dopaminergic synapse and cognitive impairment, as substantiated by prior research and animal models. Therefore, targeting the dopaminergic pathway holds promise for treating GDD and ID,” the authors wrote.
However, the authors note in the limitations that they used only a subset of 100 patients with GDD to measure dopamine concentration.
“Expanding the sample size and conducting in vivo and in vitro experiments are necessary steps to verify whether dopamine can be targeted for clinical precision medical intervention in patients with GDD,” they wrote.
The authors reported no relevant financial relationships.
, a new study suggests.
Researchers, led by Jiamei Zhang, MS, Department of Rehabilitation Medicine, Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China, in a multicenter, prospective cohort study enrolled patients ages 12 to 60 months with GDD from six centers in China from July 2020 through August 2023. Participants underwent trio whole exome sequencing (trio-WES) paired with copy number variation sequencing (CNV-seq).
“To the best of our knowledge, this study represents the largest prospective examination of combined genetic testing methods in a GDD cohort,” the authors reported in JAMA Network Open.
GDD is a common neurodevelopmental disorder, marked by cognitive impairment, and affects about 1% of children, the paper states. Most children with GDD develop intellectual disability (ID) after 5 years of age, with implications for quality of life, their physical abilities, and social functioning. Early and accurate diagnosis followed by appropriately targeted treatment is critical, but lacking. Researchers note that there is lack of consensus among health care professionals on whether genetic testing is necessary.
Genetics are known to play a significant role in pathogenesis of GDD, but definitive biomarkers have been elusive.
Positive Detection Rate of 61%
In this study, the combined use of trio-WES with CNV-seq in children with early-stage GDD resulted in a positive detection rate of 61%, a significant improvement over performing individual tests, “enhancing the positive detection rate by 18%-40%,” the researchers wrote. The combined approach also saves families time and costs, they note, while leading to more comprehensive genetic analysis and fewer missed diagnoses.
The combined approach also addressed the limitations of trio-WES and CNV-seq used alone, the authors wrote. Because of technological constraints, trio-WES may miss 55% of CNV variations, and CNV-seq has a missed diagnosis rate of 3%.
The study included 434 patients with GDD (60% male; average age, 25 months) with diverse degrees of cognitive impairment: mild (23%); moderate (32%); severe (28%); and profound (17%).
Three characteristics were linked with higher likelihood of having genetic variants: Craniofacial abnormalities (odds ratio [OR], 2.27; 95% confidence interval [CI], 1.45-3.56); moderate or severe cognitive impairment (OR, 1.69; 95% CI, 1.05-2.70); and age between 12 and 24 months (OR, 1.57; 95% CI, 1.05-2.35).
Dopaminergic Pathway Promising for Treatment
Researchers also discovered that GDD-related genes were primarily enriched in lysosome, dopaminergic synapse, and lysine degradation pathways. Dopaminergic synapse emerged as a significant pathway linked with GDD.
“In this cohort study, our findings support the correlation between dopaminergic synapse and cognitive impairment, as substantiated by prior research and animal models. Therefore, targeting the dopaminergic pathway holds promise for treating GDD and ID,” the authors wrote.
However, the authors note in the limitations that they used only a subset of 100 patients with GDD to measure dopamine concentration.
“Expanding the sample size and conducting in vivo and in vitro experiments are necessary steps to verify whether dopamine can be targeted for clinical precision medical intervention in patients with GDD,” they wrote.
The authors reported no relevant financial relationships.
FROM JAMA NETWORK OPEN
What Toxic Stress Can Do to Health
We recently shared a clinical case drawn from a family medicine practice about the effect of adverse childhood experiences (ACEs) on health. The widespread epidemiology and significant health consequences require a focus on the prevention and management of ACEs.
The Centers for Disease Control and Prevention published an important monograph on ACEs in 2019. Although it is evidence based, most of the interventions recommended to reduce ACEs and their sequelae are larger policy and public health efforts that go well beyond the clinician’s office. Important highlights from these recommended strategies to reduce ACEs include:
- Strengthen economic support for families through policies such as the earned income tax credit and child tax credit.
- Establish routine parental work/shift times to optimize cognitive outcomes in children.
- Promote social norms for healthy families through public health campaigns and legislative efforts to reduce corporal punishment of children. Bystander training that targets boys and men has also proven effective in reducing sexual violence.
- Facilitate early in-home visitation for at-risk families as well as high-quality childcare.
- Employ social-emotional learning approaches for children and adolescents, which can improve aggressive or violent behavior, rates of substance use, and academic success.
- Connect youth to after-school programs featuring caring adults.
But clinicians still play a vital role in the prevention and management of ACEs among their patients. Akin to gathering a patient’s past medical history or family history is initiating universal ACE screening in practice and exploring related topics in conversation.
The ACEs Aware initiative in California provides a comprehensive ACE screening clinical workflow to help implement these conversations in practice, including the assessment of associated health conditions and their appropriate clinical follow-up. While it is encouraged to universally screen patients, the key screenings to prioritize for the pediatric population are “parental depression, severe stress, unhealthy drug use, domestic violence, harsh punishment, [and] food insecurity.” Moreover, a systematic review by Steen and colleagues shared insight into newer interpretations of ACE screening which relate trauma to “[...] community violence, poverty, housing instability, structural racism, environmental blight, and climate change.”
These exposures are now being investigated for a connection to the toxic stress response. In the long term, this genetic regulatory mechanism can be affected by “high doses of cumulative adversity experienced during critical and sensitive periods of early life development — without the buffering protections of trusted, nurturing caregivers and safe, stable environments.” This micro and macro lens fosters a deeper clinician understanding of a patient’s trauma origin and can better guide appropriate clinical follow-up.
ACE-associated health conditions can be neurologic, endocrine, metabolic, or immune system–related. Early diagnosis and treatment of these conditions can help prevent long-term health care complications, costly for both patient and the health care system.
The ACEs Aware Stress Buster wheel highlights seven targets to strategize stress regulation. This wheel can be used to identify existing protective factors for patients and track treatment progress, which may buffer the negative impact of stressors and contribute to health and resilience.
The burden of universal screenings in primary care is high. Without ACE screening, however, the opportunity to address downstream health effects from toxic stress may be lost. Dubowitz and colleagues suggest ways to successfully incorporate ACE screenings in clinical workflow:
- Utilize technology to implement a streamlined referral processing/tracking system.
- Train clinicians to respond competently to positive ACE screens.
- Gather in-network and community-based resources for patients.
In addition, prioritize screening for families with children younger than 6 years of age to begin interventions as early as possible. Primary care clinicians have the unique opportunity to provide appropriate intervention over continual care. An intervention as simple as encouraging pediatric patient involvement in after-school programs may mitigate toxic stress and prevent the development of an ACE-associated health condition.
Dr. Vega, Health Sciences Clinical Professor, Family Medicine, University of California, Irvine, disclosed ties with McNeil Pharmaceuticals. Alejandra Hurtado, MD candidate, University of California, Irvine School of Medicine, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
We recently shared a clinical case drawn from a family medicine practice about the effect of adverse childhood experiences (ACEs) on health. The widespread epidemiology and significant health consequences require a focus on the prevention and management of ACEs.
The Centers for Disease Control and Prevention published an important monograph on ACEs in 2019. Although it is evidence based, most of the interventions recommended to reduce ACEs and their sequelae are larger policy and public health efforts that go well beyond the clinician’s office. Important highlights from these recommended strategies to reduce ACEs include:
- Strengthen economic support for families through policies such as the earned income tax credit and child tax credit.
- Establish routine parental work/shift times to optimize cognitive outcomes in children.
- Promote social norms for healthy families through public health campaigns and legislative efforts to reduce corporal punishment of children. Bystander training that targets boys and men has also proven effective in reducing sexual violence.
- Facilitate early in-home visitation for at-risk families as well as high-quality childcare.
- Employ social-emotional learning approaches for children and adolescents, which can improve aggressive or violent behavior, rates of substance use, and academic success.
- Connect youth to after-school programs featuring caring adults.
But clinicians still play a vital role in the prevention and management of ACEs among their patients. Akin to gathering a patient’s past medical history or family history is initiating universal ACE screening in practice and exploring related topics in conversation.
The ACEs Aware initiative in California provides a comprehensive ACE screening clinical workflow to help implement these conversations in practice, including the assessment of associated health conditions and their appropriate clinical follow-up. While it is encouraged to universally screen patients, the key screenings to prioritize for the pediatric population are “parental depression, severe stress, unhealthy drug use, domestic violence, harsh punishment, [and] food insecurity.” Moreover, a systematic review by Steen and colleagues shared insight into newer interpretations of ACE screening which relate trauma to “[...] community violence, poverty, housing instability, structural racism, environmental blight, and climate change.”
These exposures are now being investigated for a connection to the toxic stress response. In the long term, this genetic regulatory mechanism can be affected by “high doses of cumulative adversity experienced during critical and sensitive periods of early life development — without the buffering protections of trusted, nurturing caregivers and safe, stable environments.” This micro and macro lens fosters a deeper clinician understanding of a patient’s trauma origin and can better guide appropriate clinical follow-up.
ACE-associated health conditions can be neurologic, endocrine, metabolic, or immune system–related. Early diagnosis and treatment of these conditions can help prevent long-term health care complications, costly for both patient and the health care system.
The ACEs Aware Stress Buster wheel highlights seven targets to strategize stress regulation. This wheel can be used to identify existing protective factors for patients and track treatment progress, which may buffer the negative impact of stressors and contribute to health and resilience.
The burden of universal screenings in primary care is high. Without ACE screening, however, the opportunity to address downstream health effects from toxic stress may be lost. Dubowitz and colleagues suggest ways to successfully incorporate ACE screenings in clinical workflow:
- Utilize technology to implement a streamlined referral processing/tracking system.
- Train clinicians to respond competently to positive ACE screens.
- Gather in-network and community-based resources for patients.
In addition, prioritize screening for families with children younger than 6 years of age to begin interventions as early as possible. Primary care clinicians have the unique opportunity to provide appropriate intervention over continual care. An intervention as simple as encouraging pediatric patient involvement in after-school programs may mitigate toxic stress and prevent the development of an ACE-associated health condition.
Dr. Vega, Health Sciences Clinical Professor, Family Medicine, University of California, Irvine, disclosed ties with McNeil Pharmaceuticals. Alejandra Hurtado, MD candidate, University of California, Irvine School of Medicine, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
We recently shared a clinical case drawn from a family medicine practice about the effect of adverse childhood experiences (ACEs) on health. The widespread epidemiology and significant health consequences require a focus on the prevention and management of ACEs.
The Centers for Disease Control and Prevention published an important monograph on ACEs in 2019. Although it is evidence based, most of the interventions recommended to reduce ACEs and their sequelae are larger policy and public health efforts that go well beyond the clinician’s office. Important highlights from these recommended strategies to reduce ACEs include:
- Strengthen economic support for families through policies such as the earned income tax credit and child tax credit.
- Establish routine parental work/shift times to optimize cognitive outcomes in children.
- Promote social norms for healthy families through public health campaigns and legislative efforts to reduce corporal punishment of children. Bystander training that targets boys and men has also proven effective in reducing sexual violence.
- Facilitate early in-home visitation for at-risk families as well as high-quality childcare.
- Employ social-emotional learning approaches for children and adolescents, which can improve aggressive or violent behavior, rates of substance use, and academic success.
- Connect youth to after-school programs featuring caring adults.
But clinicians still play a vital role in the prevention and management of ACEs among their patients. Akin to gathering a patient’s past medical history or family history is initiating universal ACE screening in practice and exploring related topics in conversation.
The ACEs Aware initiative in California provides a comprehensive ACE screening clinical workflow to help implement these conversations in practice, including the assessment of associated health conditions and their appropriate clinical follow-up. While it is encouraged to universally screen patients, the key screenings to prioritize for the pediatric population are “parental depression, severe stress, unhealthy drug use, domestic violence, harsh punishment, [and] food insecurity.” Moreover, a systematic review by Steen and colleagues shared insight into newer interpretations of ACE screening which relate trauma to “[...] community violence, poverty, housing instability, structural racism, environmental blight, and climate change.”
These exposures are now being investigated for a connection to the toxic stress response. In the long term, this genetic regulatory mechanism can be affected by “high doses of cumulative adversity experienced during critical and sensitive periods of early life development — without the buffering protections of trusted, nurturing caregivers and safe, stable environments.” This micro and macro lens fosters a deeper clinician understanding of a patient’s trauma origin and can better guide appropriate clinical follow-up.
ACE-associated health conditions can be neurologic, endocrine, metabolic, or immune system–related. Early diagnosis and treatment of these conditions can help prevent long-term health care complications, costly for both patient and the health care system.
The ACEs Aware Stress Buster wheel highlights seven targets to strategize stress regulation. This wheel can be used to identify existing protective factors for patients and track treatment progress, which may buffer the negative impact of stressors and contribute to health and resilience.
The burden of universal screenings in primary care is high. Without ACE screening, however, the opportunity to address downstream health effects from toxic stress may be lost. Dubowitz and colleagues suggest ways to successfully incorporate ACE screenings in clinical workflow:
- Utilize technology to implement a streamlined referral processing/tracking system.
- Train clinicians to respond competently to positive ACE screens.
- Gather in-network and community-based resources for patients.
In addition, prioritize screening for families with children younger than 6 years of age to begin interventions as early as possible. Primary care clinicians have the unique opportunity to provide appropriate intervention over continual care. An intervention as simple as encouraging pediatric patient involvement in after-school programs may mitigate toxic stress and prevent the development of an ACE-associated health condition.
Dr. Vega, Health Sciences Clinical Professor, Family Medicine, University of California, Irvine, disclosed ties with McNeil Pharmaceuticals. Alejandra Hurtado, MD candidate, University of California, Irvine School of Medicine, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Anticoagulation Shows No Benefit in Preventing Second Stroke
BOSTON — Patients who have had a stroke are thought to be at a higher risk for another one, but oral anticoagulation with edoxaban led to no discernible reduction in the risk for a second stroke, and the risk for major bleeding was more than quadruple the risk with no anticoagulation, a subanalysis of a major European trial has shown.
“There is no interaction between prior stroke or TIA [transient ischemic attack] and the treatment effect, and this is true for the primary outcome and the safety outcome,” Paulus Kirchoff, MD, director of cardiology at the University Heart and Vascular Center in Hamburg, Germany, said during his presentation of a subanalysis of the NOAH-AFNET 6 trial at the annual meeting of the Heart Rhythm Society (HRS) 2024. However, “there is a signal for more safety events in patients randomized to anticoagulation with a prior stroke.”
The subanalysis involved 253 patients who had had a stroke or TIA and who had device-detected atrial fibrillation (AF) from the overall NOAH-AFNET 6 population of 2536 patients, which enrolled patients 65 years and older with at least one additional CHA2DS-VASc risk factor and patients 75 years and older with device-detected subclinical AF episodes of at least 6 minutes. Patients were randomized to either edoxaban or no anticoagulation, but 53.9% of the no-anticoagulation group was taking aspirin at trial enrollment. Anticoagulation with edoxaban was shown to have no significant impact on stroke rates or other cardiovascular outcomes.
Subanalysis Results
In the subanalysis, a composite of stroke, systemic embolism, and cardiovascular death — the primary outcome — was similar in the edoxaban and no-anticoagulation groups (14/122 patients [11.5%] vs 16/131 patients [12.2%]; 5.7% vs 6.3% per patient-year).
The rate of recurrent stroke was also similar in the edoxaban and no-anticoagulation groups (4 of 122 patients [3.3%] vs 6 of 131 patients [4.6%]; 1.6% vs 2.3% per patient-year). And there were eight cardiovascular deaths in each group.
However, edoxaban patients had significantly higher rates of major bleeding.
“This is a subanalysis, so what we see in terms of the number of patients with events is not powered for a definitive answer, but we do see that there were 10 major bleeds in the group of patients with a prior stroke or TIA in NOAH,” Dr. Kirchoff reported. “Eight of those 10 major bleeds occurred in patients randomized to edoxaban.”
Results from the NOAH-AFNET 6 trial have been compared with those from the ARTESiA trial, which compared apixaban anticoagulation with aspirin in patients with subclinical AF and was also presented at HRS 2024. ARTESiA showed that apixaban significantly lowered the risk for stroke and systemic embolism.
“In ARTESiA, everyone was on aspirin when they were randomized to no anticoagulation; in NOAH, only about half were on aspirin,” Dr. Kirchoff said.
Both studies had similar outcomes for cardiovascular death in the anticoagulation and no-anticoagulation groups. “It’s not significant; it may be chance, but it’s definitely not the reduction in death that we have seen in the anticoagulant trials,” Dr. Kirchoff said. “When you look at the meta-analyses of the early anticoagulation trials, there’s a one third reduction in death, and here we’re talking about a smaller reduction.”
This research points to a need for a better way to evaluate stroke risk. “We need new markers,” Dr. Kirchoff said. “Some of them may be in the blood or imaging, genetics maybe, and one thing that really emerges from my perspective is that we now have the first evidence to suggest that patients with a very low atrial fibrillation burden have a low stroke rate.”
More research is needed to better understand AF characteristics and stroke risk, he said.
AF Care Enters a ‘Gray Zone’
The NOAH-AFNET 6 results, coupled with those from ARTESiA, are changing the paradigm for anticoagulation in patients with stroke, said Taya Glotzer, MD, an electrophysiologist at the Hackensack University Medical Center in Hackensack, New Jersey, who compiled her own analysis of the studies’ outcomes.
“In ARTESiA, the stroke reduction was only 0.44% a year, with a number needed to treat of 250,” she said. “In the NOAH-AFNET 6 main trial, the stroke reduction was 0.2%, with the number needed to treat of 500, and in the NOAH prior stroke patients, there was a 0.7% reduction, with a number needed to treat of 143.”
None of these trials would meet the standard for a class 1 recommendation for anticoagulation with a reduction of even 1%-2% per year, she noted, but they do show that the stroke rate “is very, very low” in prior patients with stroke.
“Prior to 2024, we knew what was black and white; we knew who to anticoagulate and who not to anticoagulate. And now we are in a gray zone, trying to balance the risk of stroke and bleeding. We have to individualize or hope for substudies, perhaps using the CHA2DS-VASc score or other information about the left atrium, to help us make decisions in these patients. It’s not just going to be black and white,” she said.
Dr. Kirchoff had no relevant financial relationships to disclose. Dr. Glotzer disclosed financial relationships with Medtronic, Abbott, Boston Scientific, and MediaSphere Medical.
A version of this article first appeared on Medscape.com.
BOSTON — Patients who have had a stroke are thought to be at a higher risk for another one, but oral anticoagulation with edoxaban led to no discernible reduction in the risk for a second stroke, and the risk for major bleeding was more than quadruple the risk with no anticoagulation, a subanalysis of a major European trial has shown.
“There is no interaction between prior stroke or TIA [transient ischemic attack] and the treatment effect, and this is true for the primary outcome and the safety outcome,” Paulus Kirchoff, MD, director of cardiology at the University Heart and Vascular Center in Hamburg, Germany, said during his presentation of a subanalysis of the NOAH-AFNET 6 trial at the annual meeting of the Heart Rhythm Society (HRS) 2024. However, “there is a signal for more safety events in patients randomized to anticoagulation with a prior stroke.”
The subanalysis involved 253 patients who had had a stroke or TIA and who had device-detected atrial fibrillation (AF) from the overall NOAH-AFNET 6 population of 2536 patients, which enrolled patients 65 years and older with at least one additional CHA2DS-VASc risk factor and patients 75 years and older with device-detected subclinical AF episodes of at least 6 minutes. Patients were randomized to either edoxaban or no anticoagulation, but 53.9% of the no-anticoagulation group was taking aspirin at trial enrollment. Anticoagulation with edoxaban was shown to have no significant impact on stroke rates or other cardiovascular outcomes.
Subanalysis Results
In the subanalysis, a composite of stroke, systemic embolism, and cardiovascular death — the primary outcome — was similar in the edoxaban and no-anticoagulation groups (14/122 patients [11.5%] vs 16/131 patients [12.2%]; 5.7% vs 6.3% per patient-year).
The rate of recurrent stroke was also similar in the edoxaban and no-anticoagulation groups (4 of 122 patients [3.3%] vs 6 of 131 patients [4.6%]; 1.6% vs 2.3% per patient-year). And there were eight cardiovascular deaths in each group.
However, edoxaban patients had significantly higher rates of major bleeding.
“This is a subanalysis, so what we see in terms of the number of patients with events is not powered for a definitive answer, but we do see that there were 10 major bleeds in the group of patients with a prior stroke or TIA in NOAH,” Dr. Kirchoff reported. “Eight of those 10 major bleeds occurred in patients randomized to edoxaban.”
Results from the NOAH-AFNET 6 trial have been compared with those from the ARTESiA trial, which compared apixaban anticoagulation with aspirin in patients with subclinical AF and was also presented at HRS 2024. ARTESiA showed that apixaban significantly lowered the risk for stroke and systemic embolism.
“In ARTESiA, everyone was on aspirin when they were randomized to no anticoagulation; in NOAH, only about half were on aspirin,” Dr. Kirchoff said.
Both studies had similar outcomes for cardiovascular death in the anticoagulation and no-anticoagulation groups. “It’s not significant; it may be chance, but it’s definitely not the reduction in death that we have seen in the anticoagulant trials,” Dr. Kirchoff said. “When you look at the meta-analyses of the early anticoagulation trials, there’s a one third reduction in death, and here we’re talking about a smaller reduction.”
This research points to a need for a better way to evaluate stroke risk. “We need new markers,” Dr. Kirchoff said. “Some of them may be in the blood or imaging, genetics maybe, and one thing that really emerges from my perspective is that we now have the first evidence to suggest that patients with a very low atrial fibrillation burden have a low stroke rate.”
More research is needed to better understand AF characteristics and stroke risk, he said.
AF Care Enters a ‘Gray Zone’
The NOAH-AFNET 6 results, coupled with those from ARTESiA, are changing the paradigm for anticoagulation in patients with stroke, said Taya Glotzer, MD, an electrophysiologist at the Hackensack University Medical Center in Hackensack, New Jersey, who compiled her own analysis of the studies’ outcomes.
“In ARTESiA, the stroke reduction was only 0.44% a year, with a number needed to treat of 250,” she said. “In the NOAH-AFNET 6 main trial, the stroke reduction was 0.2%, with the number needed to treat of 500, and in the NOAH prior stroke patients, there was a 0.7% reduction, with a number needed to treat of 143.”
None of these trials would meet the standard for a class 1 recommendation for anticoagulation with a reduction of even 1%-2% per year, she noted, but they do show that the stroke rate “is very, very low” in prior patients with stroke.
“Prior to 2024, we knew what was black and white; we knew who to anticoagulate and who not to anticoagulate. And now we are in a gray zone, trying to balance the risk of stroke and bleeding. We have to individualize or hope for substudies, perhaps using the CHA2DS-VASc score or other information about the left atrium, to help us make decisions in these patients. It’s not just going to be black and white,” she said.
Dr. Kirchoff had no relevant financial relationships to disclose. Dr. Glotzer disclosed financial relationships with Medtronic, Abbott, Boston Scientific, and MediaSphere Medical.
A version of this article first appeared on Medscape.com.
BOSTON — Patients who have had a stroke are thought to be at a higher risk for another one, but oral anticoagulation with edoxaban led to no discernible reduction in the risk for a second stroke, and the risk for major bleeding was more than quadruple the risk with no anticoagulation, a subanalysis of a major European trial has shown.
“There is no interaction between prior stroke or TIA [transient ischemic attack] and the treatment effect, and this is true for the primary outcome and the safety outcome,” Paulus Kirchoff, MD, director of cardiology at the University Heart and Vascular Center in Hamburg, Germany, said during his presentation of a subanalysis of the NOAH-AFNET 6 trial at the annual meeting of the Heart Rhythm Society (HRS) 2024. However, “there is a signal for more safety events in patients randomized to anticoagulation with a prior stroke.”
The subanalysis involved 253 patients who had had a stroke or TIA and who had device-detected atrial fibrillation (AF) from the overall NOAH-AFNET 6 population of 2536 patients, which enrolled patients 65 years and older with at least one additional CHA2DS-VASc risk factor and patients 75 years and older with device-detected subclinical AF episodes of at least 6 minutes. Patients were randomized to either edoxaban or no anticoagulation, but 53.9% of the no-anticoagulation group was taking aspirin at trial enrollment. Anticoagulation with edoxaban was shown to have no significant impact on stroke rates or other cardiovascular outcomes.
Subanalysis Results
In the subanalysis, a composite of stroke, systemic embolism, and cardiovascular death — the primary outcome — was similar in the edoxaban and no-anticoagulation groups (14/122 patients [11.5%] vs 16/131 patients [12.2%]; 5.7% vs 6.3% per patient-year).
The rate of recurrent stroke was also similar in the edoxaban and no-anticoagulation groups (4 of 122 patients [3.3%] vs 6 of 131 patients [4.6%]; 1.6% vs 2.3% per patient-year). And there were eight cardiovascular deaths in each group.
However, edoxaban patients had significantly higher rates of major bleeding.
“This is a subanalysis, so what we see in terms of the number of patients with events is not powered for a definitive answer, but we do see that there were 10 major bleeds in the group of patients with a prior stroke or TIA in NOAH,” Dr. Kirchoff reported. “Eight of those 10 major bleeds occurred in patients randomized to edoxaban.”
Results from the NOAH-AFNET 6 trial have been compared with those from the ARTESiA trial, which compared apixaban anticoagulation with aspirin in patients with subclinical AF and was also presented at HRS 2024. ARTESiA showed that apixaban significantly lowered the risk for stroke and systemic embolism.
“In ARTESiA, everyone was on aspirin when they were randomized to no anticoagulation; in NOAH, only about half were on aspirin,” Dr. Kirchoff said.
Both studies had similar outcomes for cardiovascular death in the anticoagulation and no-anticoagulation groups. “It’s not significant; it may be chance, but it’s definitely not the reduction in death that we have seen in the anticoagulant trials,” Dr. Kirchoff said. “When you look at the meta-analyses of the early anticoagulation trials, there’s a one third reduction in death, and here we’re talking about a smaller reduction.”
This research points to a need for a better way to evaluate stroke risk. “We need new markers,” Dr. Kirchoff said. “Some of them may be in the blood or imaging, genetics maybe, and one thing that really emerges from my perspective is that we now have the first evidence to suggest that patients with a very low atrial fibrillation burden have a low stroke rate.”
More research is needed to better understand AF characteristics and stroke risk, he said.
AF Care Enters a ‘Gray Zone’
The NOAH-AFNET 6 results, coupled with those from ARTESiA, are changing the paradigm for anticoagulation in patients with stroke, said Taya Glotzer, MD, an electrophysiologist at the Hackensack University Medical Center in Hackensack, New Jersey, who compiled her own analysis of the studies’ outcomes.
“In ARTESiA, the stroke reduction was only 0.44% a year, with a number needed to treat of 250,” she said. “In the NOAH-AFNET 6 main trial, the stroke reduction was 0.2%, with the number needed to treat of 500, and in the NOAH prior stroke patients, there was a 0.7% reduction, with a number needed to treat of 143.”
None of these trials would meet the standard for a class 1 recommendation for anticoagulation with a reduction of even 1%-2% per year, she noted, but they do show that the stroke rate “is very, very low” in prior patients with stroke.
“Prior to 2024, we knew what was black and white; we knew who to anticoagulate and who not to anticoagulate. And now we are in a gray zone, trying to balance the risk of stroke and bleeding. We have to individualize or hope for substudies, perhaps using the CHA2DS-VASc score or other information about the left atrium, to help us make decisions in these patients. It’s not just going to be black and white,” she said.
Dr. Kirchoff had no relevant financial relationships to disclose. Dr. Glotzer disclosed financial relationships with Medtronic, Abbott, Boston Scientific, and MediaSphere Medical.
A version of this article first appeared on Medscape.com.
FROM HRS 2024