User login
Donepezil Shows Promise in TBI Recovery
TOPLINE:
Donepezil was associated with improved verbal memory and enhanced recall and processing speed, compared with placebo, in patients with severe traumatic brain injury (TBI), with a favorable safety profile despite mild to moderate gastrointestinal side effects.
METHODOLOGY:
- A four-site, randomized, parallel-group, double-blind, placebo-controlled, 10-week clinical trial (MEMRI-TBI-D) was conducted between 2013 and 2019 to evaluate the efficacy of donepezil for verbal memory impairments following severe TBI.
- 75 adults (75% men; mean age, 37 years) with complicated mild, moderate, or severe nonpenetrating TBI at least 6 months prior to study participation were included and randomly assigned to receive donepezil (n = 37) or placebo (n = 38).
- Participants received 5 mg donepezil daily or matching placebo for 2 weeks, then donepezil at 10 mg daily or matching placebo for 8 weeks; treatment was discontinued at 10 weeks, with an additional 4-week observation period.
- Verbal memory was assessed using the Hopkins Verbal Learning Test–Revised (HVLT-R). The primary outcome measure was verbal learning, evaluated through the HVLT-R total recall (ie, Total Trials 1-3) score.
TAKEAWAY:
- Compared with placebo, donepezil was associated with significantly greater improvements in verbal learning in both modified intent-to-treat and per-protocol analyses (P = .034 and .036, respectively).
- Treatment-responder rates were significantly higher in the donepezil group than in the placebo group (42 vs 18%; P = .03), with donepezil responders showing significant improvements in delayed recall and processing speed.
- Although there were no serious adverse events in either group, treatment-emergent adverse events were significantly more common in the donepezil group vs placebo (46% vs 8%; P < .001). No serious adverse events occurred in either group.
- Diarrhea and nausea were significantly more common in the donepezil group than in the placebo group (Fisher’s exact test: diarrhea, P = .03; nausea, P = .01).
IN PRACTICE:
“This study demonstrates the efficacy of donepezil on severe, persistent verbal memory impairments after predominantly severe TBI, with significant benefit for a subset of persons with such injuries, as well as a relatively favorable safety and tolerability profile,” the investigators wrote.
SOURCE:
The study was led by David B. Arciniegas, MD, University of Colorado School of Medicine, Aurora. It was published online in The Journal of Neuropsychiatry and Clinical Neurosciences.
LIMITATIONS:
The study included a relatively small sample with predominantly severe TBI requiring hospitalization and inpatient rehabilitation. The sample characteristics limit the generalizability of the findings to persons with other severities of TBI, other types of memory impairments, or more complex neuropsychiatric presentations. The study population had an average of 14 years of education, making generalizability to individuals with lower education levels uncertain. Additionally, while measures of information processing speed and immediate auditory attention were included, specific measures of sustained or selective attention were not, making it difficult to rule out improvements in higher-level attention as potential contributors to the observed verbal memory performance improvements.
DISCLOSURES:
The study was funded by the National Institute on Disability, Independent Living, and Rehabilitation Research, with in-kind support from TIRR Memorial Hermann. Four authors disclosed various financial and professional affiliations, including advisory roles with pharmaceutical and diagnostic companies, support from institutional awards, and involvement in programs funded by external organizations. One author served as the editor of The Journal of Neuropsychiatry and Clinical Neurosciences, with an independent editor overseeing the review and publication process for this article.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Donepezil was associated with improved verbal memory and enhanced recall and processing speed, compared with placebo, in patients with severe traumatic brain injury (TBI), with a favorable safety profile despite mild to moderate gastrointestinal side effects.
METHODOLOGY:
- A four-site, randomized, parallel-group, double-blind, placebo-controlled, 10-week clinical trial (MEMRI-TBI-D) was conducted between 2013 and 2019 to evaluate the efficacy of donepezil for verbal memory impairments following severe TBI.
- 75 adults (75% men; mean age, 37 years) with complicated mild, moderate, or severe nonpenetrating TBI at least 6 months prior to study participation were included and randomly assigned to receive donepezil (n = 37) or placebo (n = 38).
- Participants received 5 mg donepezil daily or matching placebo for 2 weeks, then donepezil at 10 mg daily or matching placebo for 8 weeks; treatment was discontinued at 10 weeks, with an additional 4-week observation period.
- Verbal memory was assessed using the Hopkins Verbal Learning Test–Revised (HVLT-R). The primary outcome measure was verbal learning, evaluated through the HVLT-R total recall (ie, Total Trials 1-3) score.
TAKEAWAY:
- Compared with placebo, donepezil was associated with significantly greater improvements in verbal learning in both modified intent-to-treat and per-protocol analyses (P = .034 and .036, respectively).
- Treatment-responder rates were significantly higher in the donepezil group than in the placebo group (42 vs 18%; P = .03), with donepezil responders showing significant improvements in delayed recall and processing speed.
- Although there were no serious adverse events in either group, treatment-emergent adverse events were significantly more common in the donepezil group vs placebo (46% vs 8%; P < .001). No serious adverse events occurred in either group.
- Diarrhea and nausea were significantly more common in the donepezil group than in the placebo group (Fisher’s exact test: diarrhea, P = .03; nausea, P = .01).
IN PRACTICE:
“This study demonstrates the efficacy of donepezil on severe, persistent verbal memory impairments after predominantly severe TBI, with significant benefit for a subset of persons with such injuries, as well as a relatively favorable safety and tolerability profile,” the investigators wrote.
SOURCE:
The study was led by David B. Arciniegas, MD, University of Colorado School of Medicine, Aurora. It was published online in The Journal of Neuropsychiatry and Clinical Neurosciences.
LIMITATIONS:
The study included a relatively small sample with predominantly severe TBI requiring hospitalization and inpatient rehabilitation. The sample characteristics limit the generalizability of the findings to persons with other severities of TBI, other types of memory impairments, or more complex neuropsychiatric presentations. The study population had an average of 14 years of education, making generalizability to individuals with lower education levels uncertain. Additionally, while measures of information processing speed and immediate auditory attention were included, specific measures of sustained or selective attention were not, making it difficult to rule out improvements in higher-level attention as potential contributors to the observed verbal memory performance improvements.
DISCLOSURES:
The study was funded by the National Institute on Disability, Independent Living, and Rehabilitation Research, with in-kind support from TIRR Memorial Hermann. Four authors disclosed various financial and professional affiliations, including advisory roles with pharmaceutical and diagnostic companies, support from institutional awards, and involvement in programs funded by external organizations. One author served as the editor of The Journal of Neuropsychiatry and Clinical Neurosciences, with an independent editor overseeing the review and publication process for this article.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Donepezil was associated with improved verbal memory and enhanced recall and processing speed, compared with placebo, in patients with severe traumatic brain injury (TBI), with a favorable safety profile despite mild to moderate gastrointestinal side effects.
METHODOLOGY:
- A four-site, randomized, parallel-group, double-blind, placebo-controlled, 10-week clinical trial (MEMRI-TBI-D) was conducted between 2013 and 2019 to evaluate the efficacy of donepezil for verbal memory impairments following severe TBI.
- 75 adults (75% men; mean age, 37 years) with complicated mild, moderate, or severe nonpenetrating TBI at least 6 months prior to study participation were included and randomly assigned to receive donepezil (n = 37) or placebo (n = 38).
- Participants received 5 mg donepezil daily or matching placebo for 2 weeks, then donepezil at 10 mg daily or matching placebo for 8 weeks; treatment was discontinued at 10 weeks, with an additional 4-week observation period.
- Verbal memory was assessed using the Hopkins Verbal Learning Test–Revised (HVLT-R). The primary outcome measure was verbal learning, evaluated through the HVLT-R total recall (ie, Total Trials 1-3) score.
TAKEAWAY:
- Compared with placebo, donepezil was associated with significantly greater improvements in verbal learning in both modified intent-to-treat and per-protocol analyses (P = .034 and .036, respectively).
- Treatment-responder rates were significantly higher in the donepezil group than in the placebo group (42 vs 18%; P = .03), with donepezil responders showing significant improvements in delayed recall and processing speed.
- Although there were no serious adverse events in either group, treatment-emergent adverse events were significantly more common in the donepezil group vs placebo (46% vs 8%; P < .001). No serious adverse events occurred in either group.
- Diarrhea and nausea were significantly more common in the donepezil group than in the placebo group (Fisher’s exact test: diarrhea, P = .03; nausea, P = .01).
IN PRACTICE:
“This study demonstrates the efficacy of donepezil on severe, persistent verbal memory impairments after predominantly severe TBI, with significant benefit for a subset of persons with such injuries, as well as a relatively favorable safety and tolerability profile,” the investigators wrote.
SOURCE:
The study was led by David B. Arciniegas, MD, University of Colorado School of Medicine, Aurora. It was published online in The Journal of Neuropsychiatry and Clinical Neurosciences.
LIMITATIONS:
The study included a relatively small sample with predominantly severe TBI requiring hospitalization and inpatient rehabilitation. The sample characteristics limit the generalizability of the findings to persons with other severities of TBI, other types of memory impairments, or more complex neuropsychiatric presentations. The study population had an average of 14 years of education, making generalizability to individuals with lower education levels uncertain. Additionally, while measures of information processing speed and immediate auditory attention were included, specific measures of sustained or selective attention were not, making it difficult to rule out improvements in higher-level attention as potential contributors to the observed verbal memory performance improvements.
DISCLOSURES:
The study was funded by the National Institute on Disability, Independent Living, and Rehabilitation Research, with in-kind support from TIRR Memorial Hermann. Four authors disclosed various financial and professional affiliations, including advisory roles with pharmaceutical and diagnostic companies, support from institutional awards, and involvement in programs funded by external organizations. One author served as the editor of The Journal of Neuropsychiatry and Clinical Neurosciences, with an independent editor overseeing the review and publication process for this article.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Brain Changes in Youth Who Use Substances: Cause or Effect?
A widely accepted assumption in the addiction field is that neuroanatomical changes observed in young people who use alcohol or other substances are largely the consequence of exposure to these substances.
But a new study suggests that neuroanatomical features in children, including greater whole brain and cortical volumes, are evident before exposure to any substances.
The investigators, led by Alex P. Miller, PhD, assistant professor, Department of Psychiatry, Indiana University, Indianapolis, noted that the findings add to a growing body of work that suggests
The findings were published online in JAMA Network Open.
Neuroanatomy a Predisposing Risk Factor?
Earlier research showed that substance use is associated with lower gray matter volume, thinner cortex, and less white matter integrity. While it has been widely thought that these changes were induced by the use of alcohol or illicit drugs, recent longitudinal and genetic studies suggest that the neuroanatomical changes may also be predisposing risk factors for substance use.
To better understand the issue, investigators analyzed data on 9804 children (mean baseline age, 9.9 years; 53% men; 76% White) at 22 US sites enrolled in the Adolescent Brain Cognitive Development (ABCD) Study that’s examining brain and behavioral development from middle childhood to young adulthood.
The researchers collected information on the use of alcohol, nicotine, cannabis, and other illicit substances from in-person interviews at baseline and years 1, 2, and 3, as well as interim phone interviews at 6, 18, and 30 months. MRI scans provided extensive brain structural data, including global and regional cortical volume, thickness, surface area, sulcal depth, and subcortical volume.
Of the total, 3460 participants (35%) initiated substance use before age 15, with 90% reporting alcohol use initiation. There was considerable overlap between initiation of alcohol, nicotine, and cannabis.
The researchers tested whether baseline neuroanatomical variability was associated with any substance use initiation before or up to 3 years following initial neuroimaging scans. Study covariates included baseline age, sex, pubertal status, familial relationship (eg, sibling or twin), and prenatal substance exposures. Researchers didn’t control for sociodemographic characteristics as these could influence associations.
Significant Brain Differences
Compared with no substance use initiation, any substance use initiation was associated with larger global neuroanatomical indices, including whole brain (beta = 0.05; P = 2.80 × 10–8), total intracranial (beta = 0.04; P = 3.49 × 10−6), cortical (beta = 0.05; P = 4.31 × 10–8), and subcortical volumes (beta = 0.05; P = 4.39 × 10–8), as well as greater total cortical surface area (beta = 0.04; P = 6.05 × 10–7).
The direction of associations between cortical thickness and substance use initiation was regionally specific; any substance use initiation was characterized by thinner cortex in all frontal regions (eg, rostral middle frontal gyrus, beta = −0.03; P = 6.99 × 10–6), but thicker cortex in all other lobes. It was also associated with larger regional brain volumes, deeper regional sulci, and differences in regional cortical surface area.
The authors noted total cortical thickness peaks at age 1.7 years and steadily declines throughout life. By contrast, subcortical volumes peak at 14.4 years of age and generally remain stable before steep later life declines.
Secondary analyses compared initiation of the three most commonly used substances in early adolescence (alcohol, nicotine, and cannabis) with no substance use.
Findings for alcohol largely mirrored those for any substance use. However, the study uncovered additional significant associations, including greater left lateral occipital volume and bilateral para-hippocampal gyri cortical thickness and less bilateral superior frontal gyri cortical thickness.
Nicotine use was associated with lower right superior frontal gyrus volume and deeper left lateral orbitofrontal cortex sulci. And cannabis use was associated with thinner left precentral gyrus and lower right inferior parietal gyrus and right caudate volumes.
The authors noted results for nicotine and cannabis may not have had adequate statistical power, and small effects suggest these findings aren’t clinically informative for individuals. However, they wrote, “They do inform and challenge current theoretical models of addiction.”
Associations Precede Substance Use
A post hoc analysis further challenges current models of addiction. When researchers looked only at the 1203 youth who initiated substance use after the baseline neuroimaging session, they found most associations preceded substance use.
“That regional associations may precede substance use initiation, including less cortical thickness in the right rostral middle frontal gyrus, challenges predominant interpretations that these associations arise largely due to neurotoxic consequences of exposure and increases the plausibility that these features may, at least partially, reflect markers of predispositional risk,” wrote the authors.
A study limitation was that unmeasured confounders and undetected systemic differences in missing data may have influenced associations. Sociodemographic, environmental, and genetic variables that were not included as covariates are likely associated with both neuroanatomical variability and substance use initiation and may moderate associations between them, said the authors.
The ABCD Study provides “a robust and large database of longitudinal data” that goes beyond previous neuroimaging research “to understand the bidirectional relationship between brain structure and substance use,” Miller said in a press release.
“The hope is that these types of studies, in conjunction with other data on environmental exposures and genetic risk, could help change how we think about the development of substance use disorders and inform more accurate models of addiction moving forward,” Miller said.
Reevaluating Causal Assumptions
In an accompanying editorial, Felix Pichardo, MA, and Sylia Wilson, PhD, from the Institute of Child Development, University of Minnesota, Minneapolis, suggested that it may be time to “reevaluate the causal assumptions that underlie brain disease models of addiction” and the mechanisms by which it develops, persists, and becomes harmful.
Neurotoxic effects of substances are central to current brain disease models of addiction, wrote Pichardo and Wilson. “Substance exposure is thought to affect cortical and subcortical regions that support interrelated systems, resulting in desensitization of reward-related processing, increased stress that prompts cravings, negative emotions when cravings are unsated, and weakening of cognitive control abilities that leads to repeated returns to use.”
The editorial writers praised the ABCD Study for its large sample size for providing a level of precision, statistical accuracy, and ability to identify both larger and smaller effects, which are critical for addiction research.
Unlike most addiction research that relies on cross-sectional designs, the current study used longitudinal assessments, which is another of its strengths, they noted.
“Longitudinal study designs like in the ABCD Study are fundamental for establishing temporal ordering across constructs, which is important because establishing temporal precedence is a key step in determining causal links and underlying mechanisms.”
The inclusion of several genetically informative components, such as the family study design, nested twin subsamples, and DNA collection, “allows researchers to extend beyond temporal precedence toward increased causal inference and identification of mechanisms,” they added.
The study received support from the National Institutes of Health. The study authors and editorial writers had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
A widely accepted assumption in the addiction field is that neuroanatomical changes observed in young people who use alcohol or other substances are largely the consequence of exposure to these substances.
But a new study suggests that neuroanatomical features in children, including greater whole brain and cortical volumes, are evident before exposure to any substances.
The investigators, led by Alex P. Miller, PhD, assistant professor, Department of Psychiatry, Indiana University, Indianapolis, noted that the findings add to a growing body of work that suggests
The findings were published online in JAMA Network Open.
Neuroanatomy a Predisposing Risk Factor?
Earlier research showed that substance use is associated with lower gray matter volume, thinner cortex, and less white matter integrity. While it has been widely thought that these changes were induced by the use of alcohol or illicit drugs, recent longitudinal and genetic studies suggest that the neuroanatomical changes may also be predisposing risk factors for substance use.
To better understand the issue, investigators analyzed data on 9804 children (mean baseline age, 9.9 years; 53% men; 76% White) at 22 US sites enrolled in the Adolescent Brain Cognitive Development (ABCD) Study that’s examining brain and behavioral development from middle childhood to young adulthood.
The researchers collected information on the use of alcohol, nicotine, cannabis, and other illicit substances from in-person interviews at baseline and years 1, 2, and 3, as well as interim phone interviews at 6, 18, and 30 months. MRI scans provided extensive brain structural data, including global and regional cortical volume, thickness, surface area, sulcal depth, and subcortical volume.
Of the total, 3460 participants (35%) initiated substance use before age 15, with 90% reporting alcohol use initiation. There was considerable overlap between initiation of alcohol, nicotine, and cannabis.
The researchers tested whether baseline neuroanatomical variability was associated with any substance use initiation before or up to 3 years following initial neuroimaging scans. Study covariates included baseline age, sex, pubertal status, familial relationship (eg, sibling or twin), and prenatal substance exposures. Researchers didn’t control for sociodemographic characteristics as these could influence associations.
Significant Brain Differences
Compared with no substance use initiation, any substance use initiation was associated with larger global neuroanatomical indices, including whole brain (beta = 0.05; P = 2.80 × 10–8), total intracranial (beta = 0.04; P = 3.49 × 10−6), cortical (beta = 0.05; P = 4.31 × 10–8), and subcortical volumes (beta = 0.05; P = 4.39 × 10–8), as well as greater total cortical surface area (beta = 0.04; P = 6.05 × 10–7).
The direction of associations between cortical thickness and substance use initiation was regionally specific; any substance use initiation was characterized by thinner cortex in all frontal regions (eg, rostral middle frontal gyrus, beta = −0.03; P = 6.99 × 10–6), but thicker cortex in all other lobes. It was also associated with larger regional brain volumes, deeper regional sulci, and differences in regional cortical surface area.
The authors noted total cortical thickness peaks at age 1.7 years and steadily declines throughout life. By contrast, subcortical volumes peak at 14.4 years of age and generally remain stable before steep later life declines.
Secondary analyses compared initiation of the three most commonly used substances in early adolescence (alcohol, nicotine, and cannabis) with no substance use.
Findings for alcohol largely mirrored those for any substance use. However, the study uncovered additional significant associations, including greater left lateral occipital volume and bilateral para-hippocampal gyri cortical thickness and less bilateral superior frontal gyri cortical thickness.
Nicotine use was associated with lower right superior frontal gyrus volume and deeper left lateral orbitofrontal cortex sulci. And cannabis use was associated with thinner left precentral gyrus and lower right inferior parietal gyrus and right caudate volumes.
The authors noted results for nicotine and cannabis may not have had adequate statistical power, and small effects suggest these findings aren’t clinically informative for individuals. However, they wrote, “They do inform and challenge current theoretical models of addiction.”
Associations Precede Substance Use
A post hoc analysis further challenges current models of addiction. When researchers looked only at the 1203 youth who initiated substance use after the baseline neuroimaging session, they found most associations preceded substance use.
“That regional associations may precede substance use initiation, including less cortical thickness in the right rostral middle frontal gyrus, challenges predominant interpretations that these associations arise largely due to neurotoxic consequences of exposure and increases the plausibility that these features may, at least partially, reflect markers of predispositional risk,” wrote the authors.
A study limitation was that unmeasured confounders and undetected systemic differences in missing data may have influenced associations. Sociodemographic, environmental, and genetic variables that were not included as covariates are likely associated with both neuroanatomical variability and substance use initiation and may moderate associations between them, said the authors.
The ABCD Study provides “a robust and large database of longitudinal data” that goes beyond previous neuroimaging research “to understand the bidirectional relationship between brain structure and substance use,” Miller said in a press release.
“The hope is that these types of studies, in conjunction with other data on environmental exposures and genetic risk, could help change how we think about the development of substance use disorders and inform more accurate models of addiction moving forward,” Miller said.
Reevaluating Causal Assumptions
In an accompanying editorial, Felix Pichardo, MA, and Sylia Wilson, PhD, from the Institute of Child Development, University of Minnesota, Minneapolis, suggested that it may be time to “reevaluate the causal assumptions that underlie brain disease models of addiction” and the mechanisms by which it develops, persists, and becomes harmful.
Neurotoxic effects of substances are central to current brain disease models of addiction, wrote Pichardo and Wilson. “Substance exposure is thought to affect cortical and subcortical regions that support interrelated systems, resulting in desensitization of reward-related processing, increased stress that prompts cravings, negative emotions when cravings are unsated, and weakening of cognitive control abilities that leads to repeated returns to use.”
The editorial writers praised the ABCD Study for its large sample size for providing a level of precision, statistical accuracy, and ability to identify both larger and smaller effects, which are critical for addiction research.
Unlike most addiction research that relies on cross-sectional designs, the current study used longitudinal assessments, which is another of its strengths, they noted.
“Longitudinal study designs like in the ABCD Study are fundamental for establishing temporal ordering across constructs, which is important because establishing temporal precedence is a key step in determining causal links and underlying mechanisms.”
The inclusion of several genetically informative components, such as the family study design, nested twin subsamples, and DNA collection, “allows researchers to extend beyond temporal precedence toward increased causal inference and identification of mechanisms,” they added.
The study received support from the National Institutes of Health. The study authors and editorial writers had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
A widely accepted assumption in the addiction field is that neuroanatomical changes observed in young people who use alcohol or other substances are largely the consequence of exposure to these substances.
But a new study suggests that neuroanatomical features in children, including greater whole brain and cortical volumes, are evident before exposure to any substances.
The investigators, led by Alex P. Miller, PhD, assistant professor, Department of Psychiatry, Indiana University, Indianapolis, noted that the findings add to a growing body of work that suggests
The findings were published online in JAMA Network Open.
Neuroanatomy a Predisposing Risk Factor?
Earlier research showed that substance use is associated with lower gray matter volume, thinner cortex, and less white matter integrity. While it has been widely thought that these changes were induced by the use of alcohol or illicit drugs, recent longitudinal and genetic studies suggest that the neuroanatomical changes may also be predisposing risk factors for substance use.
To better understand the issue, investigators analyzed data on 9804 children (mean baseline age, 9.9 years; 53% men; 76% White) at 22 US sites enrolled in the Adolescent Brain Cognitive Development (ABCD) Study that’s examining brain and behavioral development from middle childhood to young adulthood.
The researchers collected information on the use of alcohol, nicotine, cannabis, and other illicit substances from in-person interviews at baseline and years 1, 2, and 3, as well as interim phone interviews at 6, 18, and 30 months. MRI scans provided extensive brain structural data, including global and regional cortical volume, thickness, surface area, sulcal depth, and subcortical volume.
Of the total, 3460 participants (35%) initiated substance use before age 15, with 90% reporting alcohol use initiation. There was considerable overlap between initiation of alcohol, nicotine, and cannabis.
The researchers tested whether baseline neuroanatomical variability was associated with any substance use initiation before or up to 3 years following initial neuroimaging scans. Study covariates included baseline age, sex, pubertal status, familial relationship (eg, sibling or twin), and prenatal substance exposures. Researchers didn’t control for sociodemographic characteristics as these could influence associations.
Significant Brain Differences
Compared with no substance use initiation, any substance use initiation was associated with larger global neuroanatomical indices, including whole brain (beta = 0.05; P = 2.80 × 10–8), total intracranial (beta = 0.04; P = 3.49 × 10−6), cortical (beta = 0.05; P = 4.31 × 10–8), and subcortical volumes (beta = 0.05; P = 4.39 × 10–8), as well as greater total cortical surface area (beta = 0.04; P = 6.05 × 10–7).
The direction of associations between cortical thickness and substance use initiation was regionally specific; any substance use initiation was characterized by thinner cortex in all frontal regions (eg, rostral middle frontal gyrus, beta = −0.03; P = 6.99 × 10–6), but thicker cortex in all other lobes. It was also associated with larger regional brain volumes, deeper regional sulci, and differences in regional cortical surface area.
The authors noted total cortical thickness peaks at age 1.7 years and steadily declines throughout life. By contrast, subcortical volumes peak at 14.4 years of age and generally remain stable before steep later life declines.
Secondary analyses compared initiation of the three most commonly used substances in early adolescence (alcohol, nicotine, and cannabis) with no substance use.
Findings for alcohol largely mirrored those for any substance use. However, the study uncovered additional significant associations, including greater left lateral occipital volume and bilateral para-hippocampal gyri cortical thickness and less bilateral superior frontal gyri cortical thickness.
Nicotine use was associated with lower right superior frontal gyrus volume and deeper left lateral orbitofrontal cortex sulci. And cannabis use was associated with thinner left precentral gyrus and lower right inferior parietal gyrus and right caudate volumes.
The authors noted results for nicotine and cannabis may not have had adequate statistical power, and small effects suggest these findings aren’t clinically informative for individuals. However, they wrote, “They do inform and challenge current theoretical models of addiction.”
Associations Precede Substance Use
A post hoc analysis further challenges current models of addiction. When researchers looked only at the 1203 youth who initiated substance use after the baseline neuroimaging session, they found most associations preceded substance use.
“That regional associations may precede substance use initiation, including less cortical thickness in the right rostral middle frontal gyrus, challenges predominant interpretations that these associations arise largely due to neurotoxic consequences of exposure and increases the plausibility that these features may, at least partially, reflect markers of predispositional risk,” wrote the authors.
A study limitation was that unmeasured confounders and undetected systemic differences in missing data may have influenced associations. Sociodemographic, environmental, and genetic variables that were not included as covariates are likely associated with both neuroanatomical variability and substance use initiation and may moderate associations between them, said the authors.
The ABCD Study provides “a robust and large database of longitudinal data” that goes beyond previous neuroimaging research “to understand the bidirectional relationship between brain structure and substance use,” Miller said in a press release.
“The hope is that these types of studies, in conjunction with other data on environmental exposures and genetic risk, could help change how we think about the development of substance use disorders and inform more accurate models of addiction moving forward,” Miller said.
Reevaluating Causal Assumptions
In an accompanying editorial, Felix Pichardo, MA, and Sylia Wilson, PhD, from the Institute of Child Development, University of Minnesota, Minneapolis, suggested that it may be time to “reevaluate the causal assumptions that underlie brain disease models of addiction” and the mechanisms by which it develops, persists, and becomes harmful.
Neurotoxic effects of substances are central to current brain disease models of addiction, wrote Pichardo and Wilson. “Substance exposure is thought to affect cortical and subcortical regions that support interrelated systems, resulting in desensitization of reward-related processing, increased stress that prompts cravings, negative emotions when cravings are unsated, and weakening of cognitive control abilities that leads to repeated returns to use.”
The editorial writers praised the ABCD Study for its large sample size for providing a level of precision, statistical accuracy, and ability to identify both larger and smaller effects, which are critical for addiction research.
Unlike most addiction research that relies on cross-sectional designs, the current study used longitudinal assessments, which is another of its strengths, they noted.
“Longitudinal study designs like in the ABCD Study are fundamental for establishing temporal ordering across constructs, which is important because establishing temporal precedence is a key step in determining causal links and underlying mechanisms.”
The inclusion of several genetically informative components, such as the family study design, nested twin subsamples, and DNA collection, “allows researchers to extend beyond temporal precedence toward increased causal inference and identification of mechanisms,” they added.
The study received support from the National Institutes of Health. The study authors and editorial writers had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
AI Shows Early Promise in Detecting Infantile Spasms
LOS ANGELES — according to a new study.
Infants with the condition can have poor outcomes with even small delays in diagnosis and ensuing treatment, potentially leading to intellectual disability, autism, and worse epilepsy. “It’s super important to start the treatment early, but oftentimes, these symptoms are just misrecognized by primary care or ER physicians. It takes a long time to diagnose,” said Gadi Miron, MD, who presented the study at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
What Is This? What Should I Do?
Parents who observe unusual behavior often seek advice from friends and family members and receive false reassurance that such behavior isn’t unusual. Even physicians may contribute if they are unaware of infantile spasms, which is a rare disorder. “And then again, they get false reassurance, and because of that false reassurance, you get a diagnostic delay,” said Shaun Hussain, MD, who was asked to comment on the study.
The timing and frequency of infantile spasms create challenges for diagnosis. They only last about 1 second, and they tend to cluster in the morning. By the time a caregiver brings an infant to a healthcare provider, they may have trouble describing the behavior. “Parents are struggling to describe what they saw, and it often just does not resonate, or doesn’t make the healthcare provider think about infantile spasms,” said Hussain.
The idea to employ AI came from looking at videos of infants on YouTube and the realization that many patients upload them in an effort to seek advice. “So many parents upload these videos and ask in the comments, ‘What is this? What should I do? Can somebody help me?’ said Miron, who is a neurologist and researcher at Charité — Universitätsmedizin Berlin in Germany.
AI and Video Can Aid Diagnosis
The researchers built a model that they trained to recognize epileptic spasms using openly available YouTube videos, including 141 infants, 991 recorded seizures, and 597 non-seizure video segments, along with a non-seizure cohort of 127 infants with an accompanying 1385 video segments.
Each video segment was reviewed by two specialists, and they had to agree for it to be counted as an epileptic spasm.
The model detected epileptic seizures with an area under the curve (AUC) of 0.96. It had a sensitivity of 82%, specificity of 90%, and accuracy of 85% when applied to the training set.
The researchers then tested it against three validation sets. In the first, a smartphone-based set retrieved from TikTok of 26 infants with 70 epileptic spasms and 31 non-seizure 5-second video segments, the model had an AUC of 0.98, a sensitivity of 89%, a specificity of 100%, and an accuracy of 92%.
A second smartphone-based set of 67 infants, drawn from YouTube, showed a false detection rate of 0.75% (five detections out of 666 video segments). A third dataset collected from in-hospital EEG monitoring of 21 infants without seizures revealed a false-positive rate of 3.4% (365 of 10,860 video segments).
The group is now developing an app that will allow parents to upload videos that can be analyzed using the model. Physicians can then view the video and determine if there is suspicion of a seizure.
Miron also believes that this approach could find use in other types of seizures and populations, including older children and adults. “We have actually built some models for detection of seizures for videos in adults as well. Looking more towards the future, I’m sure AI will be used to analyze videos of other neurological disorders with motor symptoms [such as] movement disorders and gait,” he said.
Encouraging Early Research
Hussain, who is a professor of pediatrics at UCLA Health, lauded the work generally but emphasized that it is still in the early stage. “Their comparison was a relatively easy one. They’re just comparing normal versus infantile spasms, and they’re looking at the seizure versus normal behavior. Usually, the distinction is much harder in that there are kids who are having behaviors that are maybe other types of seizures, which is much harder to distinguish from infantile spasms, in contrast to just normal behaviors. The other mimic of infantile spasms is things like infant heartburn. Those kids will often have some posturing, and they often will be in pain. They might cry. That’s something that infantile spasms will often generate, so that’s why there’s a lot of confusion between those two,” said Hussain.
He noted that there have been efforts to raise awareness of infantile spasms among physicians and the general public, but that hasn’t reduced the increased detection.
Another Resource
In fact, parents with suspicions often go to social media sites like YouTube and a Facebook group dedicated to infantile spasms. “You can Google infantile spasms, and you’ll see examples of weird behaviors, and then you’ll look in the comments, and you’ll see this commenter said: ‘These could be infantile spasms. You should go to a children’s hospital. Don’t leave until you get an EEG to make sure that these are not seizures. There’s all kinds of great advice there, and it really shouldn’t be the situation where to get the best care, you need to go on YouTube,’ ” said Hussain.
Miron and Hussain had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
LOS ANGELES — according to a new study.
Infants with the condition can have poor outcomes with even small delays in diagnosis and ensuing treatment, potentially leading to intellectual disability, autism, and worse epilepsy. “It’s super important to start the treatment early, but oftentimes, these symptoms are just misrecognized by primary care or ER physicians. It takes a long time to diagnose,” said Gadi Miron, MD, who presented the study at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
What Is This? What Should I Do?
Parents who observe unusual behavior often seek advice from friends and family members and receive false reassurance that such behavior isn’t unusual. Even physicians may contribute if they are unaware of infantile spasms, which is a rare disorder. “And then again, they get false reassurance, and because of that false reassurance, you get a diagnostic delay,” said Shaun Hussain, MD, who was asked to comment on the study.
The timing and frequency of infantile spasms create challenges for diagnosis. They only last about 1 second, and they tend to cluster in the morning. By the time a caregiver brings an infant to a healthcare provider, they may have trouble describing the behavior. “Parents are struggling to describe what they saw, and it often just does not resonate, or doesn’t make the healthcare provider think about infantile spasms,” said Hussain.
The idea to employ AI came from looking at videos of infants on YouTube and the realization that many patients upload them in an effort to seek advice. “So many parents upload these videos and ask in the comments, ‘What is this? What should I do? Can somebody help me?’ said Miron, who is a neurologist and researcher at Charité — Universitätsmedizin Berlin in Germany.
AI and Video Can Aid Diagnosis
The researchers built a model that they trained to recognize epileptic spasms using openly available YouTube videos, including 141 infants, 991 recorded seizures, and 597 non-seizure video segments, along with a non-seizure cohort of 127 infants with an accompanying 1385 video segments.
Each video segment was reviewed by two specialists, and they had to agree for it to be counted as an epileptic spasm.
The model detected epileptic seizures with an area under the curve (AUC) of 0.96. It had a sensitivity of 82%, specificity of 90%, and accuracy of 85% when applied to the training set.
The researchers then tested it against three validation sets. In the first, a smartphone-based set retrieved from TikTok of 26 infants with 70 epileptic spasms and 31 non-seizure 5-second video segments, the model had an AUC of 0.98, a sensitivity of 89%, a specificity of 100%, and an accuracy of 92%.
A second smartphone-based set of 67 infants, drawn from YouTube, showed a false detection rate of 0.75% (five detections out of 666 video segments). A third dataset collected from in-hospital EEG monitoring of 21 infants without seizures revealed a false-positive rate of 3.4% (365 of 10,860 video segments).
The group is now developing an app that will allow parents to upload videos that can be analyzed using the model. Physicians can then view the video and determine if there is suspicion of a seizure.
Miron also believes that this approach could find use in other types of seizures and populations, including older children and adults. “We have actually built some models for detection of seizures for videos in adults as well. Looking more towards the future, I’m sure AI will be used to analyze videos of other neurological disorders with motor symptoms [such as] movement disorders and gait,” he said.
Encouraging Early Research
Hussain, who is a professor of pediatrics at UCLA Health, lauded the work generally but emphasized that it is still in the early stage. “Their comparison was a relatively easy one. They’re just comparing normal versus infantile spasms, and they’re looking at the seizure versus normal behavior. Usually, the distinction is much harder in that there are kids who are having behaviors that are maybe other types of seizures, which is much harder to distinguish from infantile spasms, in contrast to just normal behaviors. The other mimic of infantile spasms is things like infant heartburn. Those kids will often have some posturing, and they often will be in pain. They might cry. That’s something that infantile spasms will often generate, so that’s why there’s a lot of confusion between those two,” said Hussain.
He noted that there have been efforts to raise awareness of infantile spasms among physicians and the general public, but that hasn’t reduced the increased detection.
Another Resource
In fact, parents with suspicions often go to social media sites like YouTube and a Facebook group dedicated to infantile spasms. “You can Google infantile spasms, and you’ll see examples of weird behaviors, and then you’ll look in the comments, and you’ll see this commenter said: ‘These could be infantile spasms. You should go to a children’s hospital. Don’t leave until you get an EEG to make sure that these are not seizures. There’s all kinds of great advice there, and it really shouldn’t be the situation where to get the best care, you need to go on YouTube,’ ” said Hussain.
Miron and Hussain had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
LOS ANGELES — according to a new study.
Infants with the condition can have poor outcomes with even small delays in diagnosis and ensuing treatment, potentially leading to intellectual disability, autism, and worse epilepsy. “It’s super important to start the treatment early, but oftentimes, these symptoms are just misrecognized by primary care or ER physicians. It takes a long time to diagnose,” said Gadi Miron, MD, who presented the study at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
What Is This? What Should I Do?
Parents who observe unusual behavior often seek advice from friends and family members and receive false reassurance that such behavior isn’t unusual. Even physicians may contribute if they are unaware of infantile spasms, which is a rare disorder. “And then again, they get false reassurance, and because of that false reassurance, you get a diagnostic delay,” said Shaun Hussain, MD, who was asked to comment on the study.
The timing and frequency of infantile spasms create challenges for diagnosis. They only last about 1 second, and they tend to cluster in the morning. By the time a caregiver brings an infant to a healthcare provider, they may have trouble describing the behavior. “Parents are struggling to describe what they saw, and it often just does not resonate, or doesn’t make the healthcare provider think about infantile spasms,” said Hussain.
The idea to employ AI came from looking at videos of infants on YouTube and the realization that many patients upload them in an effort to seek advice. “So many parents upload these videos and ask in the comments, ‘What is this? What should I do? Can somebody help me?’ said Miron, who is a neurologist and researcher at Charité — Universitätsmedizin Berlin in Germany.
AI and Video Can Aid Diagnosis
The researchers built a model that they trained to recognize epileptic spasms using openly available YouTube videos, including 141 infants, 991 recorded seizures, and 597 non-seizure video segments, along with a non-seizure cohort of 127 infants with an accompanying 1385 video segments.
Each video segment was reviewed by two specialists, and they had to agree for it to be counted as an epileptic spasm.
The model detected epileptic seizures with an area under the curve (AUC) of 0.96. It had a sensitivity of 82%, specificity of 90%, and accuracy of 85% when applied to the training set.
The researchers then tested it against three validation sets. In the first, a smartphone-based set retrieved from TikTok of 26 infants with 70 epileptic spasms and 31 non-seizure 5-second video segments, the model had an AUC of 0.98, a sensitivity of 89%, a specificity of 100%, and an accuracy of 92%.
A second smartphone-based set of 67 infants, drawn from YouTube, showed a false detection rate of 0.75% (five detections out of 666 video segments). A third dataset collected from in-hospital EEG monitoring of 21 infants without seizures revealed a false-positive rate of 3.4% (365 of 10,860 video segments).
The group is now developing an app that will allow parents to upload videos that can be analyzed using the model. Physicians can then view the video and determine if there is suspicion of a seizure.
Miron also believes that this approach could find use in other types of seizures and populations, including older children and adults. “We have actually built some models for detection of seizures for videos in adults as well. Looking more towards the future, I’m sure AI will be used to analyze videos of other neurological disorders with motor symptoms [such as] movement disorders and gait,” he said.
Encouraging Early Research
Hussain, who is a professor of pediatrics at UCLA Health, lauded the work generally but emphasized that it is still in the early stage. “Their comparison was a relatively easy one. They’re just comparing normal versus infantile spasms, and they’re looking at the seizure versus normal behavior. Usually, the distinction is much harder in that there are kids who are having behaviors that are maybe other types of seizures, which is much harder to distinguish from infantile spasms, in contrast to just normal behaviors. The other mimic of infantile spasms is things like infant heartburn. Those kids will often have some posturing, and they often will be in pain. They might cry. That’s something that infantile spasms will often generate, so that’s why there’s a lot of confusion between those two,” said Hussain.
He noted that there have been efforts to raise awareness of infantile spasms among physicians and the general public, but that hasn’t reduced the increased detection.
Another Resource
In fact, parents with suspicions often go to social media sites like YouTube and a Facebook group dedicated to infantile spasms. “You can Google infantile spasms, and you’ll see examples of weird behaviors, and then you’ll look in the comments, and you’ll see this commenter said: ‘These could be infantile spasms. You should go to a children’s hospital. Don’t leave until you get an EEG to make sure that these are not seizures. There’s all kinds of great advice there, and it really shouldn’t be the situation where to get the best care, you need to go on YouTube,’ ” said Hussain.
Miron and Hussain had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM AES 2024
Urinary Metals Linked to Increased Dementia Risk
TOPLINE:
METHODOLOGY:
- This multicenter prospective cohort study included 6303 participants from six US study centers from 2000 to 2002, with follow-up through 2018.
- Participants were aged 45-84 years (median age at baseline, 60 years; 52% women) and were free of diagnosed cardiovascular disease.
- Researchers measured urinary levels of arsenic, cadmium, cobalt, copper, lead, manganese, tungsten, uranium, and zinc.
- Neuropsychological assessments included the Digit Symbol Coding, Cognitive Abilities Screening Instrument, and Digit Span tests.
- The median follow-up duration was 11.7 years for participants with dementia and 16.8 years for those without; 559 cases of dementia were identified during the study.
TAKEAWAY:
- Lower Digit Symbol Coding scores were associated with higher urinary concentrations of arsenic (mean difference [MD] in score per interquartile range [IQR] increase, –0.03), cobalt (MD per IQR increase, –0.05), copper (MD per IQR increase, –0.05), uranium (MD per IQR increase, –0.04), and zinc (MD per IQR increase, –0.03).
- Effects for cobalt, uranium, and zinc were stronger in apolipoprotein epsilon 4 allele (APOE4) carriers vs noncarriers.
- Higher urinary levels of copper were associated with lower Digit Span scores (MD, –0.043) and elevated levels of copper (MD, –0.028) and zinc (MD, –0.024) were associated with lower global cognitive scores.
- Individuals with urinary levels of the nine-metal mixture at the 95th percentile had a 71% higher risk for dementia compared to those with levels at the 25th percentile, with the risk more pronounced in APOE4 carriers than in noncarriers (MD, –0.30 vs –0.10, respectively).
IN PRACTICE:
“We found an inverse association of essential and nonessential metals in urine, both individually and as a mixture, with the speed of mental operations, as well as a positive association of urinary metal levels with dementia risk. As metal exposure and levels in the body are modifiable, these findings could inform early screening and precision interventions for dementia prevention based on individuals’ metal exposure and genetic profiles,” the investigators wrote.
SOURCE:
The study was led by Arce Domingo-Relloso, PhD, Columbia University Mailman School of Public Health, New York City. It was published online in JAMA Network Open.
LIMITATIONS:
Data may have been missed for patients with dementia who were never hospitalized, died, or were lost to follow-up. The dementia diagnosis included nonspecific International Classification of Diseases codes, potentially leading to false-positive reports. In addition, the sample size was not sufficient to evaluate the associations between metal exposure and cognitive test scores for carriers of two APOE4 alleles.
DISCLOSURES:
The study was supported by the National Heart, Lung, and Blood Institute. Several authors reported receiving grants from the National Institutes of Health and consulting fees, editorial stipends, teaching fees, or unrelated grant funding from various sources, which are fully listed in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- This multicenter prospective cohort study included 6303 participants from six US study centers from 2000 to 2002, with follow-up through 2018.
- Participants were aged 45-84 years (median age at baseline, 60 years; 52% women) and were free of diagnosed cardiovascular disease.
- Researchers measured urinary levels of arsenic, cadmium, cobalt, copper, lead, manganese, tungsten, uranium, and zinc.
- Neuropsychological assessments included the Digit Symbol Coding, Cognitive Abilities Screening Instrument, and Digit Span tests.
- The median follow-up duration was 11.7 years for participants with dementia and 16.8 years for those without; 559 cases of dementia were identified during the study.
TAKEAWAY:
- Lower Digit Symbol Coding scores were associated with higher urinary concentrations of arsenic (mean difference [MD] in score per interquartile range [IQR] increase, –0.03), cobalt (MD per IQR increase, –0.05), copper (MD per IQR increase, –0.05), uranium (MD per IQR increase, –0.04), and zinc (MD per IQR increase, –0.03).
- Effects for cobalt, uranium, and zinc were stronger in apolipoprotein epsilon 4 allele (APOE4) carriers vs noncarriers.
- Higher urinary levels of copper were associated with lower Digit Span scores (MD, –0.043) and elevated levels of copper (MD, –0.028) and zinc (MD, –0.024) were associated with lower global cognitive scores.
- Individuals with urinary levels of the nine-metal mixture at the 95th percentile had a 71% higher risk for dementia compared to those with levels at the 25th percentile, with the risk more pronounced in APOE4 carriers than in noncarriers (MD, –0.30 vs –0.10, respectively).
IN PRACTICE:
“We found an inverse association of essential and nonessential metals in urine, both individually and as a mixture, with the speed of mental operations, as well as a positive association of urinary metal levels with dementia risk. As metal exposure and levels in the body are modifiable, these findings could inform early screening and precision interventions for dementia prevention based on individuals’ metal exposure and genetic profiles,” the investigators wrote.
SOURCE:
The study was led by Arce Domingo-Relloso, PhD, Columbia University Mailman School of Public Health, New York City. It was published online in JAMA Network Open.
LIMITATIONS:
Data may have been missed for patients with dementia who were never hospitalized, died, or were lost to follow-up. The dementia diagnosis included nonspecific International Classification of Diseases codes, potentially leading to false-positive reports. In addition, the sample size was not sufficient to evaluate the associations between metal exposure and cognitive test scores for carriers of two APOE4 alleles.
DISCLOSURES:
The study was supported by the National Heart, Lung, and Blood Institute. Several authors reported receiving grants from the National Institutes of Health and consulting fees, editorial stipends, teaching fees, or unrelated grant funding from various sources, which are fully listed in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- This multicenter prospective cohort study included 6303 participants from six US study centers from 2000 to 2002, with follow-up through 2018.
- Participants were aged 45-84 years (median age at baseline, 60 years; 52% women) and were free of diagnosed cardiovascular disease.
- Researchers measured urinary levels of arsenic, cadmium, cobalt, copper, lead, manganese, tungsten, uranium, and zinc.
- Neuropsychological assessments included the Digit Symbol Coding, Cognitive Abilities Screening Instrument, and Digit Span tests.
- The median follow-up duration was 11.7 years for participants with dementia and 16.8 years for those without; 559 cases of dementia were identified during the study.
TAKEAWAY:
- Lower Digit Symbol Coding scores were associated with higher urinary concentrations of arsenic (mean difference [MD] in score per interquartile range [IQR] increase, –0.03), cobalt (MD per IQR increase, –0.05), copper (MD per IQR increase, –0.05), uranium (MD per IQR increase, –0.04), and zinc (MD per IQR increase, –0.03).
- Effects for cobalt, uranium, and zinc were stronger in apolipoprotein epsilon 4 allele (APOE4) carriers vs noncarriers.
- Higher urinary levels of copper were associated with lower Digit Span scores (MD, –0.043) and elevated levels of copper (MD, –0.028) and zinc (MD, –0.024) were associated with lower global cognitive scores.
- Individuals with urinary levels of the nine-metal mixture at the 95th percentile had a 71% higher risk for dementia compared to those with levels at the 25th percentile, with the risk more pronounced in APOE4 carriers than in noncarriers (MD, –0.30 vs –0.10, respectively).
IN PRACTICE:
“We found an inverse association of essential and nonessential metals in urine, both individually and as a mixture, with the speed of mental operations, as well as a positive association of urinary metal levels with dementia risk. As metal exposure and levels in the body are modifiable, these findings could inform early screening and precision interventions for dementia prevention based on individuals’ metal exposure and genetic profiles,” the investigators wrote.
SOURCE:
The study was led by Arce Domingo-Relloso, PhD, Columbia University Mailman School of Public Health, New York City. It was published online in JAMA Network Open.
LIMITATIONS:
Data may have been missed for patients with dementia who were never hospitalized, died, or were lost to follow-up. The dementia diagnosis included nonspecific International Classification of Diseases codes, potentially leading to false-positive reports. In addition, the sample size was not sufficient to evaluate the associations between metal exposure and cognitive test scores for carriers of two APOE4 alleles.
DISCLOSURES:
The study was supported by the National Heart, Lung, and Blood Institute. Several authors reported receiving grants from the National Institutes of Health and consulting fees, editorial stipends, teaching fees, or unrelated grant funding from various sources, which are fully listed in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Common Gut Infection Tied to Alzheimer’s Disease
Researchers are gaining new insight into the relationship between the human cytomegalovirus (HCMV), a common herpes virus found in the gut, and the immune response associated with CD83 antibody in some individuals with Alzheimer’s disease (AD).
Using tissue samples from deceased donors with AD, the study showed CD83-positive (CD83+) microglia in the superior frontal gyrus (SFG) are significantly associated with elevated immunoglobulin gamma 4 (IgG4) and HCMV in the transverse colon (TC), increased anti-HCMV IgG4 in the cerebrospinal fluid (CSF), and both HCMV and IgG4 in the SFG and vagus nerve.
“Our results indicate a complex, cross-tissue interaction between HCMV and the host adaptive immune response associated with CD83+ microglia in persons with AD,” noted the investigators, including Benjamin P. Readhead, MBBS, research associate professor, ASU-Banner Neurodegenerative Disease Research Center, Arizona State University, Tempe.
The results suggest antiviral therapy in patients with biomarker evidence of HCMV, IgG4, or CD83+ microglia might ward off dementia.
“We’re preparing to conduct a clinical trial to evaluate whether careful use of existing antivirals might be clinically helpful in preventing or slowing progression of CD83+ associated Alzheimer’s disease,” Readhead said in an interview.
The study was published on December 19, 2024, in Alzheimer’s & Dementia.
Vagus Nerve a Potential Pathway?
CMV is a common virus. In the United States, nearly one in three children are already infected with CMV by age 5 years. Over half the adults have been infected with CMV by age 40 years, the Centers for Disease Control and Prevention reported.
It is typically passed through bodily fluids and spread only when the virus is active. It’s not considered a sexually transmitted disease.
Compared with other IgG subclasses, IgG4 is believed to be a less inflammatory, and therefore less damaging, immune response. But this response may be less effective at clearing infections and allow invasion of HCMV into the brain.
The researchers previously found a CD83+ microglial subtype in the SFG of 47% of brain donors with AD vs 25% of unaffected control individuals. They reported this subtype is associated with increased IgG4 in the TC.
The current analysis extends investigations of the potential etiology and clinicopathologic relevance of CD83+ microglia in the context of AD.
Researchers conducted experiments using donated tissue samples from deceased patients with AD and control individuals. Sources for these samples included the Banner cohort, for whom classifications for the presence of CD83+ microglia were available, as were tissue samples from the SFG, TC, and vagus nerve, and the Religious Orders Study and Rush Memory and Aging Project (ROSMAP), in which participants without known dementia are evaluated annually.
From the Banner cohort, researchers completed immunohistochemistry (IHC) studies on 34 SFG samples (21 AD and 13 control individuals) and included 25 TC samples (13 AD and 12 control individuals) and 8 vagal nerve samples (6 AD and 2 control individuals) in the study. From the ROSMAP cohort, they completed IHC studies on 27 prefrontal cortex samples from individuals with AD.
They carefully selected these samples to ensure matching for critical factors such as postmortem interval, age, and sex, as well as other relevant covariates, said the authors.
The study verified that CD83+ microglia are associated with IgG4 and HCMV in the TC and showed a significant association between CD83+ microglia and IgG4 immunoreactivity in the TC.
Investigators confirmed HCMV positivity in all nine CD83+ TC samples evaluated and in one CD83– TC sample, indicating a strong positive association between HCMV within the TC and CD83+ microglia within the SFG.
HCMV IgG seroprevalence is common, varies by age and comorbidity, and is present in 79% of 85-year-olds, the investigators noted. “Despite this, we note that HCMV presence in the TC was not ubiquitous and was significantly associated with CD83+ microglia and HCMV in the SFG,” they wrote.
This observation, they added, “may help reconcile how a common pathogen might contribute to a disease that most individuals do not develop.”
The experiments also uncovered increased anti-HCMV IgG4 in the CSF and evidence of HCMV and IgG4 in the vagus nerve.
“Overall, the histochemical staining patterns observed in TC, SFG, and vagus nerve of CD83+ subjects are consistent with active HCMV infection,” the investigators wrote. “Taken together, these results indicate a multiorgan presence of IgG4 and HCMV in subjects with CD83+ microglia within the SFG,” they added.
Accelerated AD Pathology
The team showed HCMV infection accelerates production of two pathologic features of AD — amyloid beta (Abeta) and tau — and causes neuronal death. “We observed high, positive correlations between the abundance of HCMV, and both Abeta42 and pTau-212,” they wrote.
As HCMV histochemistry is consistent with an active HCMV infection, the findings “may indicate an opportunity for the administration of antiviral therapy in subjects with AD and biomarker evidence of HCMV, IgG4, or CD83+ microglia,” they added.
In addition to planning a clinical trial of existing antivirals, the research team is developing a blood test that can help identify patients with an active HCMV infection who might benefit from such an intervention, said Readhead.
But he emphasized that the research is still in its infancy. “Our study is best understood as a series of interesting scientific findings that warrant further exploration, replication, and validation in additional study populations.”
Although it’s too early for the study to impact practice, “we’re motivated to understand whether these findings have implications for clinical care,” he added.
Tipping the Balance
A number of experts have weighed in on the research via the Science Media Center, an independent forum featuring the voices and views on science news from experts in the field.
Andrew Doig, PhD, professor, Division of Neuroscience, University of Manchester in England, said the new work supports the hypothesis that HCMV might be a trigger that tips the balance from a healthy brain to one with dementia. “If so, antiviral drugs against HCMV might be beneficial in reducing the risk of AD.”
Doig noted newly approved drugs for AD are expensive, provide only a small benefit, and have significant risks, such as causing brain hemorrhages. “Antiviral drugs are an attractive alternative that are well worth exploring.”
Richard Oakley, PhD, associate director of research and innovation, Alzheimer’s Society, cautioned the study only established a connection and didn’t directly show the virus leads to AD. “Also, the virus is not found in the brain of everyone with Alzheimer’s disease, the most common form of dementia.”
The significance of the new findings is “far from clear,” commented William McEwan, PhD, group leader at the UK Dementia Research Institute at Cambridge, England. “The study does not address how common this infection is in people without Alzheimer’s and therefore cannot by itself suggest that HCMV infection, or the associated immune response, is a driver of disease.”
The experts agreed follow-up research is needed to confirm these new findings and understand what they mean.
The study received support from the National Institute on Aging, National Institutes of Health, Global Lyme Alliance, National Institute of Neurological Disorders and Stroke, Arizona Alzheimer’s Consortium, The Benter Foundation, and NOMIS Stiftung. Readhead is a coinventor on a patent application for an IgG4-based peripheral biomarker for the detection of CD83+ microglia. Doig is a founder, director, and consultant for PharmaKure, which works on AD drugs and diagnostics, although not on viruses. He has cowritten a review on Viral Involvement in Alzheimer’s Disease. McEwan reported receiving research funding from Takeda Pharmaceuticals and is a founder and consultant to Trimtech Therapeutics.
A version of this article first appeared on Medscape.com.
Researchers are gaining new insight into the relationship between the human cytomegalovirus (HCMV), a common herpes virus found in the gut, and the immune response associated with CD83 antibody in some individuals with Alzheimer’s disease (AD).
Using tissue samples from deceased donors with AD, the study showed CD83-positive (CD83+) microglia in the superior frontal gyrus (SFG) are significantly associated with elevated immunoglobulin gamma 4 (IgG4) and HCMV in the transverse colon (TC), increased anti-HCMV IgG4 in the cerebrospinal fluid (CSF), and both HCMV and IgG4 in the SFG and vagus nerve.
“Our results indicate a complex, cross-tissue interaction between HCMV and the host adaptive immune response associated with CD83+ microglia in persons with AD,” noted the investigators, including Benjamin P. Readhead, MBBS, research associate professor, ASU-Banner Neurodegenerative Disease Research Center, Arizona State University, Tempe.
The results suggest antiviral therapy in patients with biomarker evidence of HCMV, IgG4, or CD83+ microglia might ward off dementia.
“We’re preparing to conduct a clinical trial to evaluate whether careful use of existing antivirals might be clinically helpful in preventing or slowing progression of CD83+ associated Alzheimer’s disease,” Readhead said in an interview.
The study was published on December 19, 2024, in Alzheimer’s & Dementia.
Vagus Nerve a Potential Pathway?
CMV is a common virus. In the United States, nearly one in three children are already infected with CMV by age 5 years. Over half the adults have been infected with CMV by age 40 years, the Centers for Disease Control and Prevention reported.
It is typically passed through bodily fluids and spread only when the virus is active. It’s not considered a sexually transmitted disease.
Compared with other IgG subclasses, IgG4 is believed to be a less inflammatory, and therefore less damaging, immune response. But this response may be less effective at clearing infections and allow invasion of HCMV into the brain.
The researchers previously found a CD83+ microglial subtype in the SFG of 47% of brain donors with AD vs 25% of unaffected control individuals. They reported this subtype is associated with increased IgG4 in the TC.
The current analysis extends investigations of the potential etiology and clinicopathologic relevance of CD83+ microglia in the context of AD.
Researchers conducted experiments using donated tissue samples from deceased patients with AD and control individuals. Sources for these samples included the Banner cohort, for whom classifications for the presence of CD83+ microglia were available, as were tissue samples from the SFG, TC, and vagus nerve, and the Religious Orders Study and Rush Memory and Aging Project (ROSMAP), in which participants without known dementia are evaluated annually.
From the Banner cohort, researchers completed immunohistochemistry (IHC) studies on 34 SFG samples (21 AD and 13 control individuals) and included 25 TC samples (13 AD and 12 control individuals) and 8 vagal nerve samples (6 AD and 2 control individuals) in the study. From the ROSMAP cohort, they completed IHC studies on 27 prefrontal cortex samples from individuals with AD.
They carefully selected these samples to ensure matching for critical factors such as postmortem interval, age, and sex, as well as other relevant covariates, said the authors.
The study verified that CD83+ microglia are associated with IgG4 and HCMV in the TC and showed a significant association between CD83+ microglia and IgG4 immunoreactivity in the TC.
Investigators confirmed HCMV positivity in all nine CD83+ TC samples evaluated and in one CD83– TC sample, indicating a strong positive association between HCMV within the TC and CD83+ microglia within the SFG.
HCMV IgG seroprevalence is common, varies by age and comorbidity, and is present in 79% of 85-year-olds, the investigators noted. “Despite this, we note that HCMV presence in the TC was not ubiquitous and was significantly associated with CD83+ microglia and HCMV in the SFG,” they wrote.
This observation, they added, “may help reconcile how a common pathogen might contribute to a disease that most individuals do not develop.”
The experiments also uncovered increased anti-HCMV IgG4 in the CSF and evidence of HCMV and IgG4 in the vagus nerve.
“Overall, the histochemical staining patterns observed in TC, SFG, and vagus nerve of CD83+ subjects are consistent with active HCMV infection,” the investigators wrote. “Taken together, these results indicate a multiorgan presence of IgG4 and HCMV in subjects with CD83+ microglia within the SFG,” they added.
Accelerated AD Pathology
The team showed HCMV infection accelerates production of two pathologic features of AD — amyloid beta (Abeta) and tau — and causes neuronal death. “We observed high, positive correlations between the abundance of HCMV, and both Abeta42 and pTau-212,” they wrote.
As HCMV histochemistry is consistent with an active HCMV infection, the findings “may indicate an opportunity for the administration of antiviral therapy in subjects with AD and biomarker evidence of HCMV, IgG4, or CD83+ microglia,” they added.
In addition to planning a clinical trial of existing antivirals, the research team is developing a blood test that can help identify patients with an active HCMV infection who might benefit from such an intervention, said Readhead.
But he emphasized that the research is still in its infancy. “Our study is best understood as a series of interesting scientific findings that warrant further exploration, replication, and validation in additional study populations.”
Although it’s too early for the study to impact practice, “we’re motivated to understand whether these findings have implications for clinical care,” he added.
Tipping the Balance
A number of experts have weighed in on the research via the Science Media Center, an independent forum featuring the voices and views on science news from experts in the field.
Andrew Doig, PhD, professor, Division of Neuroscience, University of Manchester in England, said the new work supports the hypothesis that HCMV might be a trigger that tips the balance from a healthy brain to one with dementia. “If so, antiviral drugs against HCMV might be beneficial in reducing the risk of AD.”
Doig noted newly approved drugs for AD are expensive, provide only a small benefit, and have significant risks, such as causing brain hemorrhages. “Antiviral drugs are an attractive alternative that are well worth exploring.”
Richard Oakley, PhD, associate director of research and innovation, Alzheimer’s Society, cautioned the study only established a connection and didn’t directly show the virus leads to AD. “Also, the virus is not found in the brain of everyone with Alzheimer’s disease, the most common form of dementia.”
The significance of the new findings is “far from clear,” commented William McEwan, PhD, group leader at the UK Dementia Research Institute at Cambridge, England. “The study does not address how common this infection is in people without Alzheimer’s and therefore cannot by itself suggest that HCMV infection, or the associated immune response, is a driver of disease.”
The experts agreed follow-up research is needed to confirm these new findings and understand what they mean.
The study received support from the National Institute on Aging, National Institutes of Health, Global Lyme Alliance, National Institute of Neurological Disorders and Stroke, Arizona Alzheimer’s Consortium, The Benter Foundation, and NOMIS Stiftung. Readhead is a coinventor on a patent application for an IgG4-based peripheral biomarker for the detection of CD83+ microglia. Doig is a founder, director, and consultant for PharmaKure, which works on AD drugs and diagnostics, although not on viruses. He has cowritten a review on Viral Involvement in Alzheimer’s Disease. McEwan reported receiving research funding from Takeda Pharmaceuticals and is a founder and consultant to Trimtech Therapeutics.
A version of this article first appeared on Medscape.com.
Researchers are gaining new insight into the relationship between the human cytomegalovirus (HCMV), a common herpes virus found in the gut, and the immune response associated with CD83 antibody in some individuals with Alzheimer’s disease (AD).
Using tissue samples from deceased donors with AD, the study showed CD83-positive (CD83+) microglia in the superior frontal gyrus (SFG) are significantly associated with elevated immunoglobulin gamma 4 (IgG4) and HCMV in the transverse colon (TC), increased anti-HCMV IgG4 in the cerebrospinal fluid (CSF), and both HCMV and IgG4 in the SFG and vagus nerve.
“Our results indicate a complex, cross-tissue interaction between HCMV and the host adaptive immune response associated with CD83+ microglia in persons with AD,” noted the investigators, including Benjamin P. Readhead, MBBS, research associate professor, ASU-Banner Neurodegenerative Disease Research Center, Arizona State University, Tempe.
The results suggest antiviral therapy in patients with biomarker evidence of HCMV, IgG4, or CD83+ microglia might ward off dementia.
“We’re preparing to conduct a clinical trial to evaluate whether careful use of existing antivirals might be clinically helpful in preventing or slowing progression of CD83+ associated Alzheimer’s disease,” Readhead said in an interview.
The study was published on December 19, 2024, in Alzheimer’s & Dementia.
Vagus Nerve a Potential Pathway?
CMV is a common virus. In the United States, nearly one in three children are already infected with CMV by age 5 years. Over half the adults have been infected with CMV by age 40 years, the Centers for Disease Control and Prevention reported.
It is typically passed through bodily fluids and spread only when the virus is active. It’s not considered a sexually transmitted disease.
Compared with other IgG subclasses, IgG4 is believed to be a less inflammatory, and therefore less damaging, immune response. But this response may be less effective at clearing infections and allow invasion of HCMV into the brain.
The researchers previously found a CD83+ microglial subtype in the SFG of 47% of brain donors with AD vs 25% of unaffected control individuals. They reported this subtype is associated with increased IgG4 in the TC.
The current analysis extends investigations of the potential etiology and clinicopathologic relevance of CD83+ microglia in the context of AD.
Researchers conducted experiments using donated tissue samples from deceased patients with AD and control individuals. Sources for these samples included the Banner cohort, for whom classifications for the presence of CD83+ microglia were available, as were tissue samples from the SFG, TC, and vagus nerve, and the Religious Orders Study and Rush Memory and Aging Project (ROSMAP), in which participants without known dementia are evaluated annually.
From the Banner cohort, researchers completed immunohistochemistry (IHC) studies on 34 SFG samples (21 AD and 13 control individuals) and included 25 TC samples (13 AD and 12 control individuals) and 8 vagal nerve samples (6 AD and 2 control individuals) in the study. From the ROSMAP cohort, they completed IHC studies on 27 prefrontal cortex samples from individuals with AD.
They carefully selected these samples to ensure matching for critical factors such as postmortem interval, age, and sex, as well as other relevant covariates, said the authors.
The study verified that CD83+ microglia are associated with IgG4 and HCMV in the TC and showed a significant association between CD83+ microglia and IgG4 immunoreactivity in the TC.
Investigators confirmed HCMV positivity in all nine CD83+ TC samples evaluated and in one CD83– TC sample, indicating a strong positive association between HCMV within the TC and CD83+ microglia within the SFG.
HCMV IgG seroprevalence is common, varies by age and comorbidity, and is present in 79% of 85-year-olds, the investigators noted. “Despite this, we note that HCMV presence in the TC was not ubiquitous and was significantly associated with CD83+ microglia and HCMV in the SFG,” they wrote.
This observation, they added, “may help reconcile how a common pathogen might contribute to a disease that most individuals do not develop.”
The experiments also uncovered increased anti-HCMV IgG4 in the CSF and evidence of HCMV and IgG4 in the vagus nerve.
“Overall, the histochemical staining patterns observed in TC, SFG, and vagus nerve of CD83+ subjects are consistent with active HCMV infection,” the investigators wrote. “Taken together, these results indicate a multiorgan presence of IgG4 and HCMV in subjects with CD83+ microglia within the SFG,” they added.
Accelerated AD Pathology
The team showed HCMV infection accelerates production of two pathologic features of AD — amyloid beta (Abeta) and tau — and causes neuronal death. “We observed high, positive correlations between the abundance of HCMV, and both Abeta42 and pTau-212,” they wrote.
As HCMV histochemistry is consistent with an active HCMV infection, the findings “may indicate an opportunity for the administration of antiviral therapy in subjects with AD and biomarker evidence of HCMV, IgG4, or CD83+ microglia,” they added.
In addition to planning a clinical trial of existing antivirals, the research team is developing a blood test that can help identify patients with an active HCMV infection who might benefit from such an intervention, said Readhead.
But he emphasized that the research is still in its infancy. “Our study is best understood as a series of interesting scientific findings that warrant further exploration, replication, and validation in additional study populations.”
Although it’s too early for the study to impact practice, “we’re motivated to understand whether these findings have implications for clinical care,” he added.
Tipping the Balance
A number of experts have weighed in on the research via the Science Media Center, an independent forum featuring the voices and views on science news from experts in the field.
Andrew Doig, PhD, professor, Division of Neuroscience, University of Manchester in England, said the new work supports the hypothesis that HCMV might be a trigger that tips the balance from a healthy brain to one with dementia. “If so, antiviral drugs against HCMV might be beneficial in reducing the risk of AD.”
Doig noted newly approved drugs for AD are expensive, provide only a small benefit, and have significant risks, such as causing brain hemorrhages. “Antiviral drugs are an attractive alternative that are well worth exploring.”
Richard Oakley, PhD, associate director of research and innovation, Alzheimer’s Society, cautioned the study only established a connection and didn’t directly show the virus leads to AD. “Also, the virus is not found in the brain of everyone with Alzheimer’s disease, the most common form of dementia.”
The significance of the new findings is “far from clear,” commented William McEwan, PhD, group leader at the UK Dementia Research Institute at Cambridge, England. “The study does not address how common this infection is in people without Alzheimer’s and therefore cannot by itself suggest that HCMV infection, or the associated immune response, is a driver of disease.”
The experts agreed follow-up research is needed to confirm these new findings and understand what they mean.
The study received support from the National Institute on Aging, National Institutes of Health, Global Lyme Alliance, National Institute of Neurological Disorders and Stroke, Arizona Alzheimer’s Consortium, The Benter Foundation, and NOMIS Stiftung. Readhead is a coinventor on a patent application for an IgG4-based peripheral biomarker for the detection of CD83+ microglia. Doig is a founder, director, and consultant for PharmaKure, which works on AD drugs and diagnostics, although not on viruses. He has cowritten a review on Viral Involvement in Alzheimer’s Disease. McEwan reported receiving research funding from Takeda Pharmaceuticals and is a founder and consultant to Trimtech Therapeutics.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S & DEMENTIA
Study Supports Pediatric Concussion Management Approach
“With that result, it means we don’t need to change management protocols” depending on the cause of the concussion, study author Andrée-Anne Ledoux, PhD, a scientist at Children’s Hospital of Eastern Ontario Research Institute in Ottawa, Ontario, Canada, said in an interview. “That’s kind of good news. We’re applying the right management protocols with them.”
The data were published on December 4 in JAMA Network Open.
Secondary Analysis
The results stem from a planned secondary analysis of the prospective Predicting and Preventing Postconcussive Problems in Pediatrics study. Conducted from August 2013 to June 2015 at nine pediatric emergency departments in Canada, it included children of different ages (5 to < 18 years), genders, demographic characteristics, and comorbidities. All participants had a concussion.
The secondary analysis focused on study participants who were aged 5-12 years and had presented within 48 hours of injury. The primary outcome was symptom change, which was defined as current ratings minus preinjury ratings, across time (1, 2, 4, 8, and 12 weeks), measured using the Post-Concussion Symptom Inventory.
No significant differences in postinjury recovery curves were found between participants with sport-related concussions (SRC) and those with non-SRC. The latter injuries resulted from causes such as falls and objects dropped on heads. SRC and non-SRC showed a nonlinear association with time, with symptoms decreasing over time.
Perhaps surprisingly, the researchers also reported a higher rate of persisting symptoms after concussion (PSAC) following limited contact sports than following contact sports such as hockey, soccer, rugby, lacrosse, and football. Limited contact sports include activities such as bicycling, horseback riding, tobogganing, gymnastics, and cheerleading.
This finding suggests that the management of SRC may not require distinct strategies based on sports classification, the researchers wrote. “Instead, it may be more appropriate for clinicians to consider the specific dynamics of the activity, such as velocity and risk of falls from heights. This nuanced perspective can aid in assessing the likelihood of persisting symptoms.” The researchers urged more investigation of this question. “A larger sample with more information on injury height and velocity would be required to confirm whether an association exists.”
In addition, the researchers cited guidelines that include a recommendation for a gradual return to low to moderate physical and cognitive activity starting 24-48 hours after a concussion at a level that does not result in recurrence or exacerbation of symptoms.
“Children do need to return to their lives. They need to return to school,” said Ledoux. “They can have accommodations while they return to school, but just returning to school has huge benefits because you’re reintegrating the child into their typical lifestyle and socialization as well.”
A potential limitation of the study was its reliance on participants who had been seen in emergency departments and thus may have been experiencing more intense symptoms than those seen elsewhere.
The researchers also excluded cases of concussion resulting from assaults and motor vehicle crashes. This decision may explain why they didn’t reproduce the previous observation that patients with SRC tended to recover faster than those with concussions from other causes.
Injuries resulting from assaults and motor vehicle crashes can involve damage beyond concussions, Ledoux said. Including these cases would not allow for an apples-to-apples comparison of SRC and non-SRC.
‘Don’t Cocoon Kids’
The authors of an accompanying editorial wrote that the researchers had done “a beautiful job highlighting this important nuance.” Noncontact sports with seemingly little risk “actually carry substantial risks when one imagines the high-impact forces that can occur with a fall from height, albeit rare,” Scott Zuckerman, MD, MPH, assistant professor of neurological surgery at Vanderbilt University Medical Center, Nashville, Tennessee, and colleagues wrote.
The new analysis suggests a need to rethink a “somewhat archaic way of classifying sport risk, which may oversimplify how we categorize risk of brain and spine injuries.”
The commentary also noted how the researchers used the term PSAC to describe lingering symptoms instead of more widely used terms like “persistent postconcussive symptoms” or “postconcussive syndrome.”
“These traditional terms often connote a permanent syndrome or assumption that the concussion itself is solely responsible for 100% of symptoms, which can be harmful to a patient’s recovery,” the editorialists wrote. “Conversely, PSAC offers room for the clinician to discuss how other causes may be maintaining, magnifying, or mimicking concussion symptoms.”
Commenting on the findings, Richard Figler, MD, an orthopedic surgeon at the Cleveland Clinic, Cleveland, praised the researchers for addressing concussion in younger children, a field in which little research has been conducted. The research supports the current approaches to treatment. The approach has shifted toward easing children quickly and safely back into normal routines. “We don’t cocoon kids. We don’t send them to dark rooms,” Figler added.
He also commended the researchers’ decision to examine data about concussions linked to limited contact sports. In contact sports, participants may be more likely to anticipate and prepare for a hit. That’s not the case with injuries sustained in limited contact sports.
“Dodgeball is basically a sucker punch. That’s why these kids have so many concussions,” said Figler. “They typically don’t see the ball coming, or they can’t get out of the way, and they can’t tense themselves to take that blow.”
The Predicting and Preventing Postconcussive Problems in Pediatrics study was funded by the Canadian Institutes of Health Research and the Canadian Institutes of Health Research-Ontario Neurotrauma Foundation Mild Traumatic Brain Injury Team. Ledoux reported receiving grants from the Children’s Hospital of Eastern Ontario Foundation, Ontario Brain Institute, and University of Ottawa Brain and Mind Research Institute. She received nonfinancial support from Mobio Interactive outside the submitted work. Zuckerman reported receiving personal fees from the National Football League and Medtronic outside the submitted work. Figler had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
“With that result, it means we don’t need to change management protocols” depending on the cause of the concussion, study author Andrée-Anne Ledoux, PhD, a scientist at Children’s Hospital of Eastern Ontario Research Institute in Ottawa, Ontario, Canada, said in an interview. “That’s kind of good news. We’re applying the right management protocols with them.”
The data were published on December 4 in JAMA Network Open.
Secondary Analysis
The results stem from a planned secondary analysis of the prospective Predicting and Preventing Postconcussive Problems in Pediatrics study. Conducted from August 2013 to June 2015 at nine pediatric emergency departments in Canada, it included children of different ages (5 to < 18 years), genders, demographic characteristics, and comorbidities. All participants had a concussion.
The secondary analysis focused on study participants who were aged 5-12 years and had presented within 48 hours of injury. The primary outcome was symptom change, which was defined as current ratings minus preinjury ratings, across time (1, 2, 4, 8, and 12 weeks), measured using the Post-Concussion Symptom Inventory.
No significant differences in postinjury recovery curves were found between participants with sport-related concussions (SRC) and those with non-SRC. The latter injuries resulted from causes such as falls and objects dropped on heads. SRC and non-SRC showed a nonlinear association with time, with symptoms decreasing over time.
Perhaps surprisingly, the researchers also reported a higher rate of persisting symptoms after concussion (PSAC) following limited contact sports than following contact sports such as hockey, soccer, rugby, lacrosse, and football. Limited contact sports include activities such as bicycling, horseback riding, tobogganing, gymnastics, and cheerleading.
This finding suggests that the management of SRC may not require distinct strategies based on sports classification, the researchers wrote. “Instead, it may be more appropriate for clinicians to consider the specific dynamics of the activity, such as velocity and risk of falls from heights. This nuanced perspective can aid in assessing the likelihood of persisting symptoms.” The researchers urged more investigation of this question. “A larger sample with more information on injury height and velocity would be required to confirm whether an association exists.”
In addition, the researchers cited guidelines that include a recommendation for a gradual return to low to moderate physical and cognitive activity starting 24-48 hours after a concussion at a level that does not result in recurrence or exacerbation of symptoms.
“Children do need to return to their lives. They need to return to school,” said Ledoux. “They can have accommodations while they return to school, but just returning to school has huge benefits because you’re reintegrating the child into their typical lifestyle and socialization as well.”
A potential limitation of the study was its reliance on participants who had been seen in emergency departments and thus may have been experiencing more intense symptoms than those seen elsewhere.
The researchers also excluded cases of concussion resulting from assaults and motor vehicle crashes. This decision may explain why they didn’t reproduce the previous observation that patients with SRC tended to recover faster than those with concussions from other causes.
Injuries resulting from assaults and motor vehicle crashes can involve damage beyond concussions, Ledoux said. Including these cases would not allow for an apples-to-apples comparison of SRC and non-SRC.
‘Don’t Cocoon Kids’
The authors of an accompanying editorial wrote that the researchers had done “a beautiful job highlighting this important nuance.” Noncontact sports with seemingly little risk “actually carry substantial risks when one imagines the high-impact forces that can occur with a fall from height, albeit rare,” Scott Zuckerman, MD, MPH, assistant professor of neurological surgery at Vanderbilt University Medical Center, Nashville, Tennessee, and colleagues wrote.
The new analysis suggests a need to rethink a “somewhat archaic way of classifying sport risk, which may oversimplify how we categorize risk of brain and spine injuries.”
The commentary also noted how the researchers used the term PSAC to describe lingering symptoms instead of more widely used terms like “persistent postconcussive symptoms” or “postconcussive syndrome.”
“These traditional terms often connote a permanent syndrome or assumption that the concussion itself is solely responsible for 100% of symptoms, which can be harmful to a patient’s recovery,” the editorialists wrote. “Conversely, PSAC offers room for the clinician to discuss how other causes may be maintaining, magnifying, or mimicking concussion symptoms.”
Commenting on the findings, Richard Figler, MD, an orthopedic surgeon at the Cleveland Clinic, Cleveland, praised the researchers for addressing concussion in younger children, a field in which little research has been conducted. The research supports the current approaches to treatment. The approach has shifted toward easing children quickly and safely back into normal routines. “We don’t cocoon kids. We don’t send them to dark rooms,” Figler added.
He also commended the researchers’ decision to examine data about concussions linked to limited contact sports. In contact sports, participants may be more likely to anticipate and prepare for a hit. That’s not the case with injuries sustained in limited contact sports.
“Dodgeball is basically a sucker punch. That’s why these kids have so many concussions,” said Figler. “They typically don’t see the ball coming, or they can’t get out of the way, and they can’t tense themselves to take that blow.”
The Predicting and Preventing Postconcussive Problems in Pediatrics study was funded by the Canadian Institutes of Health Research and the Canadian Institutes of Health Research-Ontario Neurotrauma Foundation Mild Traumatic Brain Injury Team. Ledoux reported receiving grants from the Children’s Hospital of Eastern Ontario Foundation, Ontario Brain Institute, and University of Ottawa Brain and Mind Research Institute. She received nonfinancial support from Mobio Interactive outside the submitted work. Zuckerman reported receiving personal fees from the National Football League and Medtronic outside the submitted work. Figler had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
“With that result, it means we don’t need to change management protocols” depending on the cause of the concussion, study author Andrée-Anne Ledoux, PhD, a scientist at Children’s Hospital of Eastern Ontario Research Institute in Ottawa, Ontario, Canada, said in an interview. “That’s kind of good news. We’re applying the right management protocols with them.”
The data were published on December 4 in JAMA Network Open.
Secondary Analysis
The results stem from a planned secondary analysis of the prospective Predicting and Preventing Postconcussive Problems in Pediatrics study. Conducted from August 2013 to June 2015 at nine pediatric emergency departments in Canada, it included children of different ages (5 to < 18 years), genders, demographic characteristics, and comorbidities. All participants had a concussion.
The secondary analysis focused on study participants who were aged 5-12 years and had presented within 48 hours of injury. The primary outcome was symptom change, which was defined as current ratings minus preinjury ratings, across time (1, 2, 4, 8, and 12 weeks), measured using the Post-Concussion Symptom Inventory.
No significant differences in postinjury recovery curves were found between participants with sport-related concussions (SRC) and those with non-SRC. The latter injuries resulted from causes such as falls and objects dropped on heads. SRC and non-SRC showed a nonlinear association with time, with symptoms decreasing over time.
Perhaps surprisingly, the researchers also reported a higher rate of persisting symptoms after concussion (PSAC) following limited contact sports than following contact sports such as hockey, soccer, rugby, lacrosse, and football. Limited contact sports include activities such as bicycling, horseback riding, tobogganing, gymnastics, and cheerleading.
This finding suggests that the management of SRC may not require distinct strategies based on sports classification, the researchers wrote. “Instead, it may be more appropriate for clinicians to consider the specific dynamics of the activity, such as velocity and risk of falls from heights. This nuanced perspective can aid in assessing the likelihood of persisting symptoms.” The researchers urged more investigation of this question. “A larger sample with more information on injury height and velocity would be required to confirm whether an association exists.”
In addition, the researchers cited guidelines that include a recommendation for a gradual return to low to moderate physical and cognitive activity starting 24-48 hours after a concussion at a level that does not result in recurrence or exacerbation of symptoms.
“Children do need to return to their lives. They need to return to school,” said Ledoux. “They can have accommodations while they return to school, but just returning to school has huge benefits because you’re reintegrating the child into their typical lifestyle and socialization as well.”
A potential limitation of the study was its reliance on participants who had been seen in emergency departments and thus may have been experiencing more intense symptoms than those seen elsewhere.
The researchers also excluded cases of concussion resulting from assaults and motor vehicle crashes. This decision may explain why they didn’t reproduce the previous observation that patients with SRC tended to recover faster than those with concussions from other causes.
Injuries resulting from assaults and motor vehicle crashes can involve damage beyond concussions, Ledoux said. Including these cases would not allow for an apples-to-apples comparison of SRC and non-SRC.
‘Don’t Cocoon Kids’
The authors of an accompanying editorial wrote that the researchers had done “a beautiful job highlighting this important nuance.” Noncontact sports with seemingly little risk “actually carry substantial risks when one imagines the high-impact forces that can occur with a fall from height, albeit rare,” Scott Zuckerman, MD, MPH, assistant professor of neurological surgery at Vanderbilt University Medical Center, Nashville, Tennessee, and colleagues wrote.
The new analysis suggests a need to rethink a “somewhat archaic way of classifying sport risk, which may oversimplify how we categorize risk of brain and spine injuries.”
The commentary also noted how the researchers used the term PSAC to describe lingering symptoms instead of more widely used terms like “persistent postconcussive symptoms” or “postconcussive syndrome.”
“These traditional terms often connote a permanent syndrome or assumption that the concussion itself is solely responsible for 100% of symptoms, which can be harmful to a patient’s recovery,” the editorialists wrote. “Conversely, PSAC offers room for the clinician to discuss how other causes may be maintaining, magnifying, or mimicking concussion symptoms.”
Commenting on the findings, Richard Figler, MD, an orthopedic surgeon at the Cleveland Clinic, Cleveland, praised the researchers for addressing concussion in younger children, a field in which little research has been conducted. The research supports the current approaches to treatment. The approach has shifted toward easing children quickly and safely back into normal routines. “We don’t cocoon kids. We don’t send them to dark rooms,” Figler added.
He also commended the researchers’ decision to examine data about concussions linked to limited contact sports. In contact sports, participants may be more likely to anticipate and prepare for a hit. That’s not the case with injuries sustained in limited contact sports.
“Dodgeball is basically a sucker punch. That’s why these kids have so many concussions,” said Figler. “They typically don’t see the ball coming, or they can’t get out of the way, and they can’t tense themselves to take that blow.”
The Predicting and Preventing Postconcussive Problems in Pediatrics study was funded by the Canadian Institutes of Health Research and the Canadian Institutes of Health Research-Ontario Neurotrauma Foundation Mild Traumatic Brain Injury Team. Ledoux reported receiving grants from the Children’s Hospital of Eastern Ontario Foundation, Ontario Brain Institute, and University of Ottawa Brain and Mind Research Institute. She received nonfinancial support from Mobio Interactive outside the submitted work. Zuckerman reported receiving personal fees from the National Football League and Medtronic outside the submitted work. Figler had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Total Intravenous Anesthesia Enables Earlier Facial Nerve Monitoring Than Sevoflurane in Ear Surgery
TOPLINE:
Total intravenous anesthesia (TIVA) enables earlier intraoperative monitoring of facial nerve activity than sevoflurane anesthesia during ear surgery, with reduced patient-ventilator dyssynchrony and fewer requirements for postoperative antiemetics.
METHODOLOGY:
- Researchers evaluated the difference in the timeliness of intraoperative monitoring of facial nerve activity during ear surgery with TIVA vs sevoflurane anesthesia.
- They included 98 patients aged 18-74 years undergoing ear surgery between November 2021 and November 2022; patients were randomly assigned to receive either TIVA or sevoflurane during the procedure. Of these, 92 were included in the final analysis.
- Neuromuscular function was monitored quantitatively throughout anesthesia with train-of-four counts and train-of-four ratios.
- The time from the administration of rocuronium to the start of facial nerve monitoring was recorded.
- The primary outcome measure focused on the recovery index, defined as the time interval between a train-of-four ratio of 0.25 and 0.75; the key secondary outcome was the time to reach a train-of-four ratio of 0.25 from rocuronium administration.
TAKEAWAY:
- The time to reach a train-of-four ratio of 0.25 was achieved earlier with TIVA than with sevoflurane (34 minutes vs 51 minutes; P < .001).
- Patient-ventilator dyssynchrony occurred less frequently in the TIVA group than in the sevoflurane group (15% vs 39%; P = .01).
- Postoperative requests for antiemetics were less frequent in the TIVA group than in the sevoflurane group (2% vs 17%; P = .03).
IN PRACTICE:
“We suggest that TIVA may be a better choice than sevoflurane anesthesia to meet an earlier request” for intraoperative facial nerve monitoring by surgeons, the study authors wrote.
SOURCE:
The study was led by Yu Jeong Bang, MD, of the Department of Anesthesiology and Pain Medicine at Sungkyunkwan University School of Medicine, in Seoul, Republic of Korea. It was published online on November 27, 2024, in The Canadian Journal of Anesthesia.
LIMITATIONS:
A careful interpretation of results may be necessary when clinicians use balanced anesthesia, such as sevoflurane with adjuvants like opioids or nonopioids. The feasibility of intraoperative facial nerve monitoring was decided by the surgeon during surgery, and the lowest stimulation intensity threshold for electromyography amplitude was not detected, as it was not the focus of this study. Although patients requiring intraoperative facial nerve monitoring during ear surgery were enrolled, some did not undergo the procedure based on the surgeon’s judgment.
DISCLOSURES:
This study did not receive any funding. The authors disclosed no relevant conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Total intravenous anesthesia (TIVA) enables earlier intraoperative monitoring of facial nerve activity than sevoflurane anesthesia during ear surgery, with reduced patient-ventilator dyssynchrony and fewer requirements for postoperative antiemetics.
METHODOLOGY:
- Researchers evaluated the difference in the timeliness of intraoperative monitoring of facial nerve activity during ear surgery with TIVA vs sevoflurane anesthesia.
- They included 98 patients aged 18-74 years undergoing ear surgery between November 2021 and November 2022; patients were randomly assigned to receive either TIVA or sevoflurane during the procedure. Of these, 92 were included in the final analysis.
- Neuromuscular function was monitored quantitatively throughout anesthesia with train-of-four counts and train-of-four ratios.
- The time from the administration of rocuronium to the start of facial nerve monitoring was recorded.
- The primary outcome measure focused on the recovery index, defined as the time interval between a train-of-four ratio of 0.25 and 0.75; the key secondary outcome was the time to reach a train-of-four ratio of 0.25 from rocuronium administration.
TAKEAWAY:
- The time to reach a train-of-four ratio of 0.25 was achieved earlier with TIVA than with sevoflurane (34 minutes vs 51 minutes; P < .001).
- Patient-ventilator dyssynchrony occurred less frequently in the TIVA group than in the sevoflurane group (15% vs 39%; P = .01).
- Postoperative requests for antiemetics were less frequent in the TIVA group than in the sevoflurane group (2% vs 17%; P = .03).
IN PRACTICE:
“We suggest that TIVA may be a better choice than sevoflurane anesthesia to meet an earlier request” for intraoperative facial nerve monitoring by surgeons, the study authors wrote.
SOURCE:
The study was led by Yu Jeong Bang, MD, of the Department of Anesthesiology and Pain Medicine at Sungkyunkwan University School of Medicine, in Seoul, Republic of Korea. It was published online on November 27, 2024, in The Canadian Journal of Anesthesia.
LIMITATIONS:
A careful interpretation of results may be necessary when clinicians use balanced anesthesia, such as sevoflurane with adjuvants like opioids or nonopioids. The feasibility of intraoperative facial nerve monitoring was decided by the surgeon during surgery, and the lowest stimulation intensity threshold for electromyography amplitude was not detected, as it was not the focus of this study. Although patients requiring intraoperative facial nerve monitoring during ear surgery were enrolled, some did not undergo the procedure based on the surgeon’s judgment.
DISCLOSURES:
This study did not receive any funding. The authors disclosed no relevant conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Total intravenous anesthesia (TIVA) enables earlier intraoperative monitoring of facial nerve activity than sevoflurane anesthesia during ear surgery, with reduced patient-ventilator dyssynchrony and fewer requirements for postoperative antiemetics.
METHODOLOGY:
- Researchers evaluated the difference in the timeliness of intraoperative monitoring of facial nerve activity during ear surgery with TIVA vs sevoflurane anesthesia.
- They included 98 patients aged 18-74 years undergoing ear surgery between November 2021 and November 2022; patients were randomly assigned to receive either TIVA or sevoflurane during the procedure. Of these, 92 were included in the final analysis.
- Neuromuscular function was monitored quantitatively throughout anesthesia with train-of-four counts and train-of-four ratios.
- The time from the administration of rocuronium to the start of facial nerve monitoring was recorded.
- The primary outcome measure focused on the recovery index, defined as the time interval between a train-of-four ratio of 0.25 and 0.75; the key secondary outcome was the time to reach a train-of-four ratio of 0.25 from rocuronium administration.
TAKEAWAY:
- The time to reach a train-of-four ratio of 0.25 was achieved earlier with TIVA than with sevoflurane (34 minutes vs 51 minutes; P < .001).
- Patient-ventilator dyssynchrony occurred less frequently in the TIVA group than in the sevoflurane group (15% vs 39%; P = .01).
- Postoperative requests for antiemetics were less frequent in the TIVA group than in the sevoflurane group (2% vs 17%; P = .03).
IN PRACTICE:
“We suggest that TIVA may be a better choice than sevoflurane anesthesia to meet an earlier request” for intraoperative facial nerve monitoring by surgeons, the study authors wrote.
SOURCE:
The study was led by Yu Jeong Bang, MD, of the Department of Anesthesiology and Pain Medicine at Sungkyunkwan University School of Medicine, in Seoul, Republic of Korea. It was published online on November 27, 2024, in The Canadian Journal of Anesthesia.
LIMITATIONS:
A careful interpretation of results may be necessary when clinicians use balanced anesthesia, such as sevoflurane with adjuvants like opioids or nonopioids. The feasibility of intraoperative facial nerve monitoring was decided by the surgeon during surgery, and the lowest stimulation intensity threshold for electromyography amplitude was not detected, as it was not the focus of this study. Although patients requiring intraoperative facial nerve monitoring during ear surgery were enrolled, some did not undergo the procedure based on the surgeon’s judgment.
DISCLOSURES:
This study did not receive any funding. The authors disclosed no relevant conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Health Impacts of Micro- and Nanoplastics
In preparation for a future international treaty aimed at reducing plastic pollution, the French Parliamentary Office for the Evaluation of Scientific and Technological Choices presented the conclusions of a public hearing on the impact of plastics on various aspects of human health.
Increased Global Plastic Production
Philippe Bolo, a member of the French Democratic Party and the rapporteur for the public mission on the health impacts of plastics, spoke about the latest round of treaty negotiations, held from November 25 to December 1 in South Korea, attended by leading French and global experts about the impact of plastics on human health.
The hearing highlighted a sharp increase in plastic production. “It has doubled in the last 20 years and is expected to exceed 500 million tons in 2024,” Bolo said. This is about 60 kg per person. According to projections from the Organization for Economic Co-operation and Development, on its current trajectory, plastic production will reach 750 million tons by 2040 and surpass 1 billion tons before 2050, he said.
Minimal Plastic Waste Recycling
Around one third (32%) of plastics are used for packaging. “Therefore, most plastic production is still intended for single-use purposes,” he said. Plastic waste follows a similar growth trajectory, with volumes expected to rise from 360 million tons in 2020 to 617 million tons by 2040 unless action is taken. Very little of this waste is recycled, even in the most countries that are most advanced in terms of collection, sorting, and processing.
In France, for example, in 2018, only 0.6 million tons of the 3.6 million tons of plastic waste produced was truly recycled. This is less than one fifth (17%). Globally, less than 10% of plastic waste is recycled. In 2020, plastic waste that ended up in the environment represented 81 million tons, or 22% of the total. “Beyond waste, this leads to pollution by microplastics and nanoplastics, resulting from their fragmentation. All environments are affected: Seas, rivers, soils, air, and even living organisms,” Bolo said.
Methodological Challenges
However, measuring the impact of plastics on health faces methodological difficulties due to the wide variety of composition, size, and shape of plastics. Nevertheless, the French Standardization Association (Association Française de Normalisation) has conducted work to establish a characterization standard for microplastics in water, which serves as an international reference.
“It is also very difficult to know what we are ingesting,” Bolo said. “A study conducted in 2019 estimated that the average human absorbs 5 grams of plastics per week, the equivalent of a credit card.» Since then, other studies have revised this estimate downward, but no consensus has been reached.
A recent study across 109 countries, both industrialized and developing, found significant exposure, estimated at 500 mg/d, particularly in Southeast Asian countries, where it was due mainly to seafood consumption.
A study concluded that plastic water bottles contain 240,000 particles per liter, 90% of which are nanoplastics. These nanoparticles can pass through the intestinal barrier to enter the bloodstream and reach several organs including the heart, brain, and placenta, as well as the fetus.
Changes to the Microbiome
Microplastics also accumulate in organs. Thus, the amount of plastic in the lungs increases with age, suggesting that particles may persist in the body without being eliminated. The health consequences of this are still poorly understood, but exposure to plastics appears to cause changes in the composition of the intestinal microbiota. Pathobionts (commensal bacteria with harmful potential) have been found in both adults and children, which could contribute to dysbiosis of the gut microbiome. Furthermore, a decrease in butyrate, a short-chain fatty acid beneficial to health, has been observed in children’s intestines.
Inhaled nanoplastics may disrupt the mucociliary clearance mechanisms of the respiratory system. The toxicity of inhaled plastic particles was demonstrated as early as the 1970s among workers in the flocking industry. Some developed lung function impairments, shortness of breath, inflammation, fibrosis, and even lung cancer. Similar symptoms have been observed in workers in the textile and polyvinyl chloride industries.
A study published recently in The New England Journal of Medicine measured the amount of microplastics collected from carotid plaque of more than 300 patients who had undergone carotid endarterectomy for asymptomatic carotid artery disease. It found a 4.53 times higher risk for the primary endpoint, a composite of myocardial infarction, stroke, and all-cause mortality, among individuals with microplastics and nanoplastics in plaque compared with those without.
Health Affects High
The danger of plastics is also directly linked to the chemical substances they contain. A general scientific review looked at the health impacts of three chemicals used almost exclusively in plastics: Polybromodiphenyl ethers (PBDEs), used as flame retardants in textiles or electronics; bisphenol A (BPA), used in the lining of cans and bottles; and phthalates, particularly diethylhexyl phthalate (DEHP), used to make plastics more flexible.
The review highlighted strong epidemiological evidence linking fetal exposure to PBDEs during pregnancy to low birth weight and later exposure to delayed or impaired cognitive development in children and even a loss of IQ. Statistically significant evidence of disruption of thyroid function in adults was also found.
BPA is linked to genital malformations in female newborns exposed to BPA in utero, type 2 diabetes in adults, insulin resistance, and polycystic ovary syndrome in women. BPA exposure also increases the risk for obesity and hypertension in both children and adults, as well as the risk for cardiovascular disease in adults.
Finally, the review established links between exposure to DEHP and miscarriages, genital malformations in male newborns, delayed or impaired cognitive development in children, loss of IQ, delayed psychomotor development, early puberty in young girls, and endometriosis in young women. DEHP exposure also has multiple effects on cardiometabolic health, including insulin resistance, obesity, and elevated blood pressure.
The economic costs associated with the health impacts of these three substances have been estimated at $675 billion in the United States.
Bolo said that the solution to this plastic pollution is necessarily international. “We need an ambitious and legally binding treaty to reduce plastic production,” he said. “The damage is already done; we need to act to protect human health,” he concluded. The parliamentary office has made nine recommendations to the treaty negotiators.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
In preparation for a future international treaty aimed at reducing plastic pollution, the French Parliamentary Office for the Evaluation of Scientific and Technological Choices presented the conclusions of a public hearing on the impact of plastics on various aspects of human health.
Increased Global Plastic Production
Philippe Bolo, a member of the French Democratic Party and the rapporteur for the public mission on the health impacts of plastics, spoke about the latest round of treaty negotiations, held from November 25 to December 1 in South Korea, attended by leading French and global experts about the impact of plastics on human health.
The hearing highlighted a sharp increase in plastic production. “It has doubled in the last 20 years and is expected to exceed 500 million tons in 2024,” Bolo said. This is about 60 kg per person. According to projections from the Organization for Economic Co-operation and Development, on its current trajectory, plastic production will reach 750 million tons by 2040 and surpass 1 billion tons before 2050, he said.
Minimal Plastic Waste Recycling
Around one third (32%) of plastics are used for packaging. “Therefore, most plastic production is still intended for single-use purposes,” he said. Plastic waste follows a similar growth trajectory, with volumes expected to rise from 360 million tons in 2020 to 617 million tons by 2040 unless action is taken. Very little of this waste is recycled, even in the most countries that are most advanced in terms of collection, sorting, and processing.
In France, for example, in 2018, only 0.6 million tons of the 3.6 million tons of plastic waste produced was truly recycled. This is less than one fifth (17%). Globally, less than 10% of plastic waste is recycled. In 2020, plastic waste that ended up in the environment represented 81 million tons, or 22% of the total. “Beyond waste, this leads to pollution by microplastics and nanoplastics, resulting from their fragmentation. All environments are affected: Seas, rivers, soils, air, and even living organisms,” Bolo said.
Methodological Challenges
However, measuring the impact of plastics on health faces methodological difficulties due to the wide variety of composition, size, and shape of plastics. Nevertheless, the French Standardization Association (Association Française de Normalisation) has conducted work to establish a characterization standard for microplastics in water, which serves as an international reference.
“It is also very difficult to know what we are ingesting,” Bolo said. “A study conducted in 2019 estimated that the average human absorbs 5 grams of plastics per week, the equivalent of a credit card.» Since then, other studies have revised this estimate downward, but no consensus has been reached.
A recent study across 109 countries, both industrialized and developing, found significant exposure, estimated at 500 mg/d, particularly in Southeast Asian countries, where it was due mainly to seafood consumption.
A study concluded that plastic water bottles contain 240,000 particles per liter, 90% of which are nanoplastics. These nanoparticles can pass through the intestinal barrier to enter the bloodstream and reach several organs including the heart, brain, and placenta, as well as the fetus.
Changes to the Microbiome
Microplastics also accumulate in organs. Thus, the amount of plastic in the lungs increases with age, suggesting that particles may persist in the body without being eliminated. The health consequences of this are still poorly understood, but exposure to plastics appears to cause changes in the composition of the intestinal microbiota. Pathobionts (commensal bacteria with harmful potential) have been found in both adults and children, which could contribute to dysbiosis of the gut microbiome. Furthermore, a decrease in butyrate, a short-chain fatty acid beneficial to health, has been observed in children’s intestines.
Inhaled nanoplastics may disrupt the mucociliary clearance mechanisms of the respiratory system. The toxicity of inhaled plastic particles was demonstrated as early as the 1970s among workers in the flocking industry. Some developed lung function impairments, shortness of breath, inflammation, fibrosis, and even lung cancer. Similar symptoms have been observed in workers in the textile and polyvinyl chloride industries.
A study published recently in The New England Journal of Medicine measured the amount of microplastics collected from carotid plaque of more than 300 patients who had undergone carotid endarterectomy for asymptomatic carotid artery disease. It found a 4.53 times higher risk for the primary endpoint, a composite of myocardial infarction, stroke, and all-cause mortality, among individuals with microplastics and nanoplastics in plaque compared with those without.
Health Affects High
The danger of plastics is also directly linked to the chemical substances they contain. A general scientific review looked at the health impacts of three chemicals used almost exclusively in plastics: Polybromodiphenyl ethers (PBDEs), used as flame retardants in textiles or electronics; bisphenol A (BPA), used in the lining of cans and bottles; and phthalates, particularly diethylhexyl phthalate (DEHP), used to make plastics more flexible.
The review highlighted strong epidemiological evidence linking fetal exposure to PBDEs during pregnancy to low birth weight and later exposure to delayed or impaired cognitive development in children and even a loss of IQ. Statistically significant evidence of disruption of thyroid function in adults was also found.
BPA is linked to genital malformations in female newborns exposed to BPA in utero, type 2 diabetes in adults, insulin resistance, and polycystic ovary syndrome in women. BPA exposure also increases the risk for obesity and hypertension in both children and adults, as well as the risk for cardiovascular disease in adults.
Finally, the review established links between exposure to DEHP and miscarriages, genital malformations in male newborns, delayed or impaired cognitive development in children, loss of IQ, delayed psychomotor development, early puberty in young girls, and endometriosis in young women. DEHP exposure also has multiple effects on cardiometabolic health, including insulin resistance, obesity, and elevated blood pressure.
The economic costs associated with the health impacts of these three substances have been estimated at $675 billion in the United States.
Bolo said that the solution to this plastic pollution is necessarily international. “We need an ambitious and legally binding treaty to reduce plastic production,” he said. “The damage is already done; we need to act to protect human health,” he concluded. The parliamentary office has made nine recommendations to the treaty negotiators.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
In preparation for a future international treaty aimed at reducing plastic pollution, the French Parliamentary Office for the Evaluation of Scientific and Technological Choices presented the conclusions of a public hearing on the impact of plastics on various aspects of human health.
Increased Global Plastic Production
Philippe Bolo, a member of the French Democratic Party and the rapporteur for the public mission on the health impacts of plastics, spoke about the latest round of treaty negotiations, held from November 25 to December 1 in South Korea, attended by leading French and global experts about the impact of plastics on human health.
The hearing highlighted a sharp increase in plastic production. “It has doubled in the last 20 years and is expected to exceed 500 million tons in 2024,” Bolo said. This is about 60 kg per person. According to projections from the Organization for Economic Co-operation and Development, on its current trajectory, plastic production will reach 750 million tons by 2040 and surpass 1 billion tons before 2050, he said.
Minimal Plastic Waste Recycling
Around one third (32%) of plastics are used for packaging. “Therefore, most plastic production is still intended for single-use purposes,” he said. Plastic waste follows a similar growth trajectory, with volumes expected to rise from 360 million tons in 2020 to 617 million tons by 2040 unless action is taken. Very little of this waste is recycled, even in the most countries that are most advanced in terms of collection, sorting, and processing.
In France, for example, in 2018, only 0.6 million tons of the 3.6 million tons of plastic waste produced was truly recycled. This is less than one fifth (17%). Globally, less than 10% of plastic waste is recycled. In 2020, plastic waste that ended up in the environment represented 81 million tons, or 22% of the total. “Beyond waste, this leads to pollution by microplastics and nanoplastics, resulting from their fragmentation. All environments are affected: Seas, rivers, soils, air, and even living organisms,” Bolo said.
Methodological Challenges
However, measuring the impact of plastics on health faces methodological difficulties due to the wide variety of composition, size, and shape of plastics. Nevertheless, the French Standardization Association (Association Française de Normalisation) has conducted work to establish a characterization standard for microplastics in water, which serves as an international reference.
“It is also very difficult to know what we are ingesting,” Bolo said. “A study conducted in 2019 estimated that the average human absorbs 5 grams of plastics per week, the equivalent of a credit card.» Since then, other studies have revised this estimate downward, but no consensus has been reached.
A recent study across 109 countries, both industrialized and developing, found significant exposure, estimated at 500 mg/d, particularly in Southeast Asian countries, where it was due mainly to seafood consumption.
A study concluded that plastic water bottles contain 240,000 particles per liter, 90% of which are nanoplastics. These nanoparticles can pass through the intestinal barrier to enter the bloodstream and reach several organs including the heart, brain, and placenta, as well as the fetus.
Changes to the Microbiome
Microplastics also accumulate in organs. Thus, the amount of plastic in the lungs increases with age, suggesting that particles may persist in the body without being eliminated. The health consequences of this are still poorly understood, but exposure to plastics appears to cause changes in the composition of the intestinal microbiota. Pathobionts (commensal bacteria with harmful potential) have been found in both adults and children, which could contribute to dysbiosis of the gut microbiome. Furthermore, a decrease in butyrate, a short-chain fatty acid beneficial to health, has been observed in children’s intestines.
Inhaled nanoplastics may disrupt the mucociliary clearance mechanisms of the respiratory system. The toxicity of inhaled plastic particles was demonstrated as early as the 1970s among workers in the flocking industry. Some developed lung function impairments, shortness of breath, inflammation, fibrosis, and even lung cancer. Similar symptoms have been observed in workers in the textile and polyvinyl chloride industries.
A study published recently in The New England Journal of Medicine measured the amount of microplastics collected from carotid plaque of more than 300 patients who had undergone carotid endarterectomy for asymptomatic carotid artery disease. It found a 4.53 times higher risk for the primary endpoint, a composite of myocardial infarction, stroke, and all-cause mortality, among individuals with microplastics and nanoplastics in plaque compared with those without.
Health Affects High
The danger of plastics is also directly linked to the chemical substances they contain. A general scientific review looked at the health impacts of three chemicals used almost exclusively in plastics: Polybromodiphenyl ethers (PBDEs), used as flame retardants in textiles or electronics; bisphenol A (BPA), used in the lining of cans and bottles; and phthalates, particularly diethylhexyl phthalate (DEHP), used to make plastics more flexible.
The review highlighted strong epidemiological evidence linking fetal exposure to PBDEs during pregnancy to low birth weight and later exposure to delayed or impaired cognitive development in children and even a loss of IQ. Statistically significant evidence of disruption of thyroid function in adults was also found.
BPA is linked to genital malformations in female newborns exposed to BPA in utero, type 2 diabetes in adults, insulin resistance, and polycystic ovary syndrome in women. BPA exposure also increases the risk for obesity and hypertension in both children and adults, as well as the risk for cardiovascular disease in adults.
Finally, the review established links between exposure to DEHP and miscarriages, genital malformations in male newborns, delayed or impaired cognitive development in children, loss of IQ, delayed psychomotor development, early puberty in young girls, and endometriosis in young women. DEHP exposure also has multiple effects on cardiometabolic health, including insulin resistance, obesity, and elevated blood pressure.
The economic costs associated with the health impacts of these three substances have been estimated at $675 billion in the United States.
Bolo said that the solution to this plastic pollution is necessarily international. “We need an ambitious and legally binding treaty to reduce plastic production,” he said. “The damage is already done; we need to act to protect human health,” he concluded. The parliamentary office has made nine recommendations to the treaty negotiators.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
High-Volume Burn Resuscitation Increases Neurologic Risk
TOPLINE:
METHODOLOGY:
- Researchers conducted a single-center review of 5176 patients with burn injuries who were admitted to a verified American Burn Association center (2003-2017); 622 of them underwent head CT within 96 hours of admission, and 83 showed intracranial abnormalities.
- Of 42 patients (mean age, 49.7 years; 80.5% men) who were admitted within 24 hours of burn, 30 patients received < 200 mL/kg and 11 received > 200 mL/kg of total resuscitation fluids, with a median total body surface area (TBSA) of 20.0.
- The primary outcome assessed was the worsening of neurologic findings on imaging related to the volume of the resuscitation fluid administered; the secondary outcomes were the incidence of new or worsening intracranial abnormalities, including hemorrhage, edema, ischemia, or infarction.
TAKEAWAY:
- Neurologic findings worsened in 47.6% patients receiving < 200 mL/kg of fluid resuscitation and 85.7% of those receiving > 200 mL/kg (P =.064).
- Repeat imaging was performed in 21 (70.0%) patients receiving < 200 mL/kg and 7 (63.6%) patients receiving > 200 mL/kg of resuscitation who underwent follow-up imaging.
- The median TBSA was 16.5 in the < 200 mL/kg group and 53.2 in the > 200 mL/kg group (P <.001).
- Intracranial abnormalities were found in 31.3% patients with hemorrhage, 18.8% with worsening edema, and 43.8% with ischemia or infarction.
IN PRACTICE:
“Patients who received over 200 mL/kg of resuscitation had an increased progression of intracranial abnormalities when compared with patients receiving less volume resuscitation,” the authors wrote. “Neurologic changes prompting imaging in burn patients may be undetectable, and our study further highlights the need for routine evaluation with neurologic imaging when undergoing large-volume resuscitations.”
SOURCE:
The study was led by Connor L. Kenney, MD, Brooke Army Medical Center, San Antonio, and was published online on November 07, 2024, in the Journal of Surgical Research.
LIMITATIONS:
Study limitations included a small patient sample and unclear guidelines for obtaining head CT scans, making it difficult to distinguish between trauma-related brain changes and disease progression. Additionally, the study lacked data on hypotensive episodes and long-term neurologic outcomes.
DISCLOSURES:
This study did not receive any specific funding. The authors declared no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a single-center review of 5176 patients with burn injuries who were admitted to a verified American Burn Association center (2003-2017); 622 of them underwent head CT within 96 hours of admission, and 83 showed intracranial abnormalities.
- Of 42 patients (mean age, 49.7 years; 80.5% men) who were admitted within 24 hours of burn, 30 patients received < 200 mL/kg and 11 received > 200 mL/kg of total resuscitation fluids, with a median total body surface area (TBSA) of 20.0.
- The primary outcome assessed was the worsening of neurologic findings on imaging related to the volume of the resuscitation fluid administered; the secondary outcomes were the incidence of new or worsening intracranial abnormalities, including hemorrhage, edema, ischemia, or infarction.
TAKEAWAY:
- Neurologic findings worsened in 47.6% patients receiving < 200 mL/kg of fluid resuscitation and 85.7% of those receiving > 200 mL/kg (P =.064).
- Repeat imaging was performed in 21 (70.0%) patients receiving < 200 mL/kg and 7 (63.6%) patients receiving > 200 mL/kg of resuscitation who underwent follow-up imaging.
- The median TBSA was 16.5 in the < 200 mL/kg group and 53.2 in the > 200 mL/kg group (P <.001).
- Intracranial abnormalities were found in 31.3% patients with hemorrhage, 18.8% with worsening edema, and 43.8% with ischemia or infarction.
IN PRACTICE:
“Patients who received over 200 mL/kg of resuscitation had an increased progression of intracranial abnormalities when compared with patients receiving less volume resuscitation,” the authors wrote. “Neurologic changes prompting imaging in burn patients may be undetectable, and our study further highlights the need for routine evaluation with neurologic imaging when undergoing large-volume resuscitations.”
SOURCE:
The study was led by Connor L. Kenney, MD, Brooke Army Medical Center, San Antonio, and was published online on November 07, 2024, in the Journal of Surgical Research.
LIMITATIONS:
Study limitations included a small patient sample and unclear guidelines for obtaining head CT scans, making it difficult to distinguish between trauma-related brain changes and disease progression. Additionally, the study lacked data on hypotensive episodes and long-term neurologic outcomes.
DISCLOSURES:
This study did not receive any specific funding. The authors declared no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a single-center review of 5176 patients with burn injuries who were admitted to a verified American Burn Association center (2003-2017); 622 of them underwent head CT within 96 hours of admission, and 83 showed intracranial abnormalities.
- Of 42 patients (mean age, 49.7 years; 80.5% men) who were admitted within 24 hours of burn, 30 patients received < 200 mL/kg and 11 received > 200 mL/kg of total resuscitation fluids, with a median total body surface area (TBSA) of 20.0.
- The primary outcome assessed was the worsening of neurologic findings on imaging related to the volume of the resuscitation fluid administered; the secondary outcomes were the incidence of new or worsening intracranial abnormalities, including hemorrhage, edema, ischemia, or infarction.
TAKEAWAY:
- Neurologic findings worsened in 47.6% patients receiving < 200 mL/kg of fluid resuscitation and 85.7% of those receiving > 200 mL/kg (P =.064).
- Repeat imaging was performed in 21 (70.0%) patients receiving < 200 mL/kg and 7 (63.6%) patients receiving > 200 mL/kg of resuscitation who underwent follow-up imaging.
- The median TBSA was 16.5 in the < 200 mL/kg group and 53.2 in the > 200 mL/kg group (P <.001).
- Intracranial abnormalities were found in 31.3% patients with hemorrhage, 18.8% with worsening edema, and 43.8% with ischemia or infarction.
IN PRACTICE:
“Patients who received over 200 mL/kg of resuscitation had an increased progression of intracranial abnormalities when compared with patients receiving less volume resuscitation,” the authors wrote. “Neurologic changes prompting imaging in burn patients may be undetectable, and our study further highlights the need for routine evaluation with neurologic imaging when undergoing large-volume resuscitations.”
SOURCE:
The study was led by Connor L. Kenney, MD, Brooke Army Medical Center, San Antonio, and was published online on November 07, 2024, in the Journal of Surgical Research.
LIMITATIONS:
Study limitations included a small patient sample and unclear guidelines for obtaining head CT scans, making it difficult to distinguish between trauma-related brain changes and disease progression. Additionally, the study lacked data on hypotensive episodes and long-term neurologic outcomes.
DISCLOSURES:
This study did not receive any specific funding. The authors declared no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Whipple Disease With Central Nervous System Involvement
Whipple Disease With Central Nervous System Involvement
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.
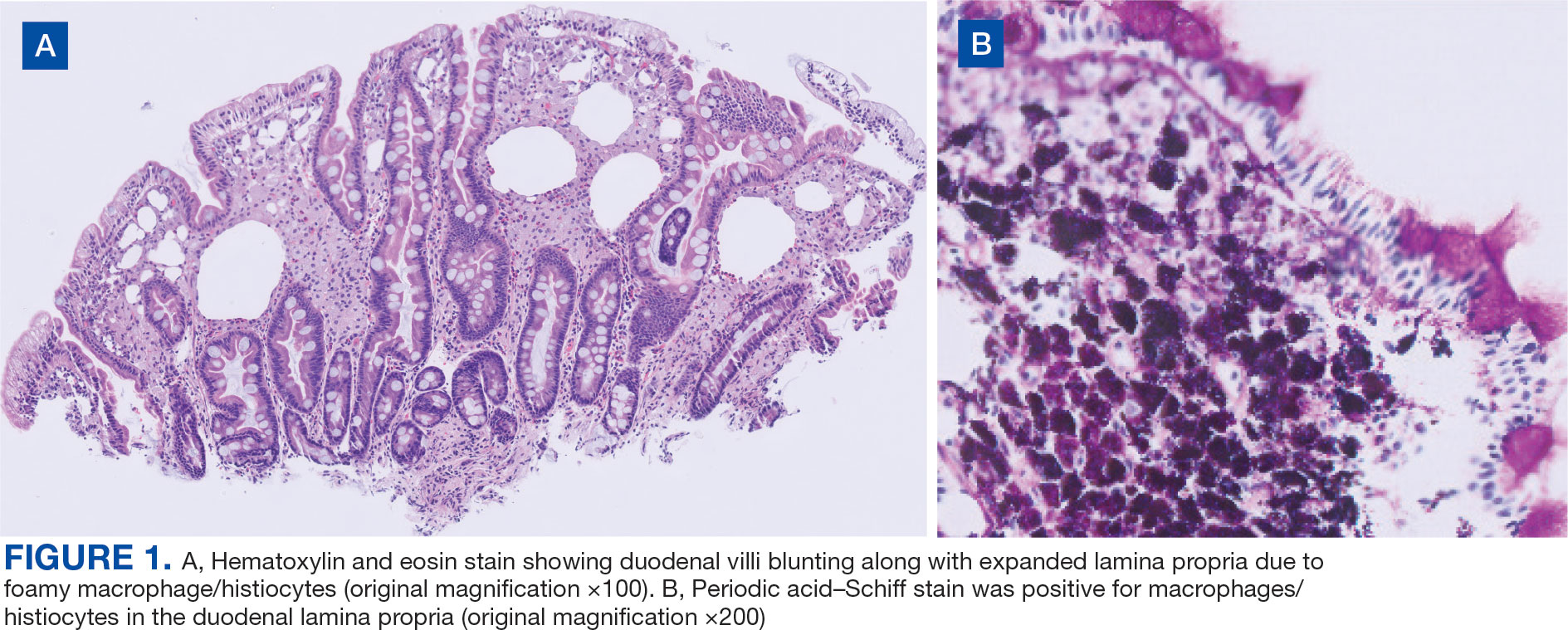
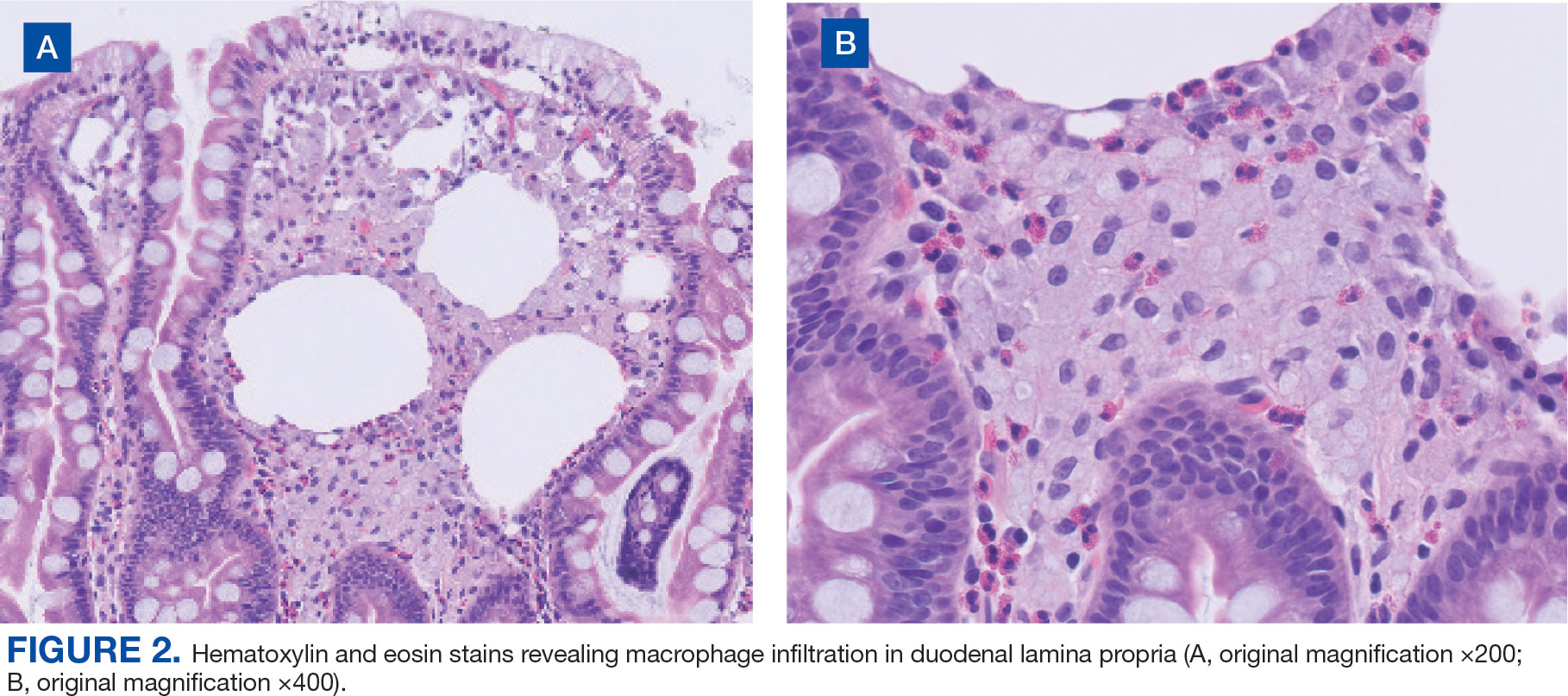
We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.


We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.


We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
Whipple Disease With Central Nervous System Involvement
Whipple Disease With Central Nervous System Involvement