User login
Smart Mattress to Reduce SUDEP?
LOS ANGELES — says one of the experts involved in its development.
When used along with a seizure detection device, Jong Woo Lee, MD, PhD, associate professor of neurology, Harvard Medical School, and Brigham and Women’s Hospital, both in Boston, Massachusetts, estimates the smart mattress could cut SUDEP by more than 50%.
In addition, early results from an observational study are backing this up, he said.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
Most SUDEP Cases Found Face Down
SUDEP is the leading cause of death in children with epilepsy and in otherwise healthy adult patients with epilepsy. When his fifth patient died of SUDEP, Lee decided it was time to try to tackle the high mortality rate associated with these unexpected deaths. “I desperately wanted to help, ” he said.
About 70% of SUDEP occurs during sleep, and victims are found face down, or in the prone position, 90% of the time, said Lee.
“Of course, the best way to prevent SUDEP is not to have a seizure, but once you have a seizure and once you’re face down, your risk for death goes up by somewhere between 30 and 100 times,” he explained.
Lee was convinced SUDEP could be prevented by simple interventions that stimulate the patient and turn them over. He noted the incidence of sudden infant death syndrome, “which has similar characteristics” to SUDEP, has been reduced by up to 75% through campaigns that simply advise placing babies on their backs.
“Most of SUDEP happens because your arousal system is knocked out and you just don’t take the breath that you’re supposed to. Just the act of turning people over and vibrating the bed will stimulate them,” he said.
However, it’s crucial that this be done quickly, said Lee. “When you look at patients who died on video and see the EEGs, everybody took their last breath within 3 minutes.”
Because the window of opportunity is so short, “we think that seizure detection devices alone are not going to really be effective because you just can’t get there or react within those 3 minutes.”
There are currently no products that detect the prone position or have the ability to reposition a patient quickly into the recovery sideways position.
Lee and his colleagues developed a smart system that can be embedded in a mattress that detects when someone is having a seizure, determines if that person is face down, and if so, safely stimulates and repositions them.
The mattress is made up of a series of programmable inflatable blocks or “cells” that have pressure, vibration, temperature, and humidity sensors embedded within. “Based on the pressure readings, we can figure out whether the patient is right side up, on their right side, on their left side, or face down,” said Lee.
If the person is face down, he or she can be repositioned within a matter of seconds. “Each of the cells can lift 1000 pounds,” he said. The mattress is “very comfortable,” said Lee, who has tried it out himself.
Eighteen normative control participants have been enrolled for development and training purposes. To date, 10 of these individuals, aged 18-53 years, weighing 100-182 lb, and with a height of 5 ft 2 in to 6 ft 1 in, underwent extensive formal testing on the prototype bed.
Researchers found the mattress responded quickly to different body positions and weights. “We were able to reposition everybody in around 20 seconds,” said Lee.
The overall accuracy of detecting the prone position was 96.8%. There were no cases of a supine or prone position being mistaken for each other.
Researchers are refining the algorithm to improve the accuracy for detecting the prone position and expect to have a completely functional prototype within a few years.
Big Step Forward
Commenting on the research, Daniel M. Goldenholz, MD, PhD, assistant professor, Division of Epilepsy, Harvard Beth Israel Deaconess Medical Center, Boston, said the study “is a big step forward in the race to provide an actionable tool to prevent SUDEP.”
The technology “appears to mostly be doing what it’s intended to do, with relatively minor technical errors being made,” he said.
However, it is not clear if this technology can truly save lives, said Goldenholz. “The data we have suggests that lying face down in bed after a seizure is correlated with SUDEP, but that does not mean that if we can simply flip people over, they for sure won’t die.”
Even if the new technology “works perfectly,” it’s still an open question, said Goldenholz. If it does save lives, “this will be a major breakthrough, and one that has been needed for a long time.”
However, even if it does not, he congratulates the team for trying to determine if reducing the prone position can help prevent SUDEP. He would like to see more “high-risk, high-reward” studies in the epilepsy field. “We are in so much need of new innovations.”
He said he was “personally very inspired” by this work. “People are dying from this terrible disease, and this team is building what they hope might save lives.”
The study was funded by the National Institutes of Health. The mattress is being developed by Soterya. Lee reported no equity in Soterya. Goldenholz reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
LOS ANGELES — says one of the experts involved in its development.
When used along with a seizure detection device, Jong Woo Lee, MD, PhD, associate professor of neurology, Harvard Medical School, and Brigham and Women’s Hospital, both in Boston, Massachusetts, estimates the smart mattress could cut SUDEP by more than 50%.
In addition, early results from an observational study are backing this up, he said.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
Most SUDEP Cases Found Face Down
SUDEP is the leading cause of death in children with epilepsy and in otherwise healthy adult patients with epilepsy. When his fifth patient died of SUDEP, Lee decided it was time to try to tackle the high mortality rate associated with these unexpected deaths. “I desperately wanted to help, ” he said.
About 70% of SUDEP occurs during sleep, and victims are found face down, or in the prone position, 90% of the time, said Lee.
“Of course, the best way to prevent SUDEP is not to have a seizure, but once you have a seizure and once you’re face down, your risk for death goes up by somewhere between 30 and 100 times,” he explained.
Lee was convinced SUDEP could be prevented by simple interventions that stimulate the patient and turn them over. He noted the incidence of sudden infant death syndrome, “which has similar characteristics” to SUDEP, has been reduced by up to 75% through campaigns that simply advise placing babies on their backs.
“Most of SUDEP happens because your arousal system is knocked out and you just don’t take the breath that you’re supposed to. Just the act of turning people over and vibrating the bed will stimulate them,” he said.
However, it’s crucial that this be done quickly, said Lee. “When you look at patients who died on video and see the EEGs, everybody took their last breath within 3 minutes.”
Because the window of opportunity is so short, “we think that seizure detection devices alone are not going to really be effective because you just can’t get there or react within those 3 minutes.”
There are currently no products that detect the prone position or have the ability to reposition a patient quickly into the recovery sideways position.
Lee and his colleagues developed a smart system that can be embedded in a mattress that detects when someone is having a seizure, determines if that person is face down, and if so, safely stimulates and repositions them.
The mattress is made up of a series of programmable inflatable blocks or “cells” that have pressure, vibration, temperature, and humidity sensors embedded within. “Based on the pressure readings, we can figure out whether the patient is right side up, on their right side, on their left side, or face down,” said Lee.
If the person is face down, he or she can be repositioned within a matter of seconds. “Each of the cells can lift 1000 pounds,” he said. The mattress is “very comfortable,” said Lee, who has tried it out himself.
Eighteen normative control participants have been enrolled for development and training purposes. To date, 10 of these individuals, aged 18-53 years, weighing 100-182 lb, and with a height of 5 ft 2 in to 6 ft 1 in, underwent extensive formal testing on the prototype bed.
Researchers found the mattress responded quickly to different body positions and weights. “We were able to reposition everybody in around 20 seconds,” said Lee.
The overall accuracy of detecting the prone position was 96.8%. There were no cases of a supine or prone position being mistaken for each other.
Researchers are refining the algorithm to improve the accuracy for detecting the prone position and expect to have a completely functional prototype within a few years.
Big Step Forward
Commenting on the research, Daniel M. Goldenholz, MD, PhD, assistant professor, Division of Epilepsy, Harvard Beth Israel Deaconess Medical Center, Boston, said the study “is a big step forward in the race to provide an actionable tool to prevent SUDEP.”
The technology “appears to mostly be doing what it’s intended to do, with relatively minor technical errors being made,” he said.
However, it is not clear if this technology can truly save lives, said Goldenholz. “The data we have suggests that lying face down in bed after a seizure is correlated with SUDEP, but that does not mean that if we can simply flip people over, they for sure won’t die.”
Even if the new technology “works perfectly,” it’s still an open question, said Goldenholz. If it does save lives, “this will be a major breakthrough, and one that has been needed for a long time.”
However, even if it does not, he congratulates the team for trying to determine if reducing the prone position can help prevent SUDEP. He would like to see more “high-risk, high-reward” studies in the epilepsy field. “We are in so much need of new innovations.”
He said he was “personally very inspired” by this work. “People are dying from this terrible disease, and this team is building what they hope might save lives.”
The study was funded by the National Institutes of Health. The mattress is being developed by Soterya. Lee reported no equity in Soterya. Goldenholz reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
LOS ANGELES — says one of the experts involved in its development.
When used along with a seizure detection device, Jong Woo Lee, MD, PhD, associate professor of neurology, Harvard Medical School, and Brigham and Women’s Hospital, both in Boston, Massachusetts, estimates the smart mattress could cut SUDEP by more than 50%.
In addition, early results from an observational study are backing this up, he said.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
Most SUDEP Cases Found Face Down
SUDEP is the leading cause of death in children with epilepsy and in otherwise healthy adult patients with epilepsy. When his fifth patient died of SUDEP, Lee decided it was time to try to tackle the high mortality rate associated with these unexpected deaths. “I desperately wanted to help, ” he said.
About 70% of SUDEP occurs during sleep, and victims are found face down, or in the prone position, 90% of the time, said Lee.
“Of course, the best way to prevent SUDEP is not to have a seizure, but once you have a seizure and once you’re face down, your risk for death goes up by somewhere between 30 and 100 times,” he explained.
Lee was convinced SUDEP could be prevented by simple interventions that stimulate the patient and turn them over. He noted the incidence of sudden infant death syndrome, “which has similar characteristics” to SUDEP, has been reduced by up to 75% through campaigns that simply advise placing babies on their backs.
“Most of SUDEP happens because your arousal system is knocked out and you just don’t take the breath that you’re supposed to. Just the act of turning people over and vibrating the bed will stimulate them,” he said.
However, it’s crucial that this be done quickly, said Lee. “When you look at patients who died on video and see the EEGs, everybody took their last breath within 3 minutes.”
Because the window of opportunity is so short, “we think that seizure detection devices alone are not going to really be effective because you just can’t get there or react within those 3 minutes.”
There are currently no products that detect the prone position or have the ability to reposition a patient quickly into the recovery sideways position.
Lee and his colleagues developed a smart system that can be embedded in a mattress that detects when someone is having a seizure, determines if that person is face down, and if so, safely stimulates and repositions them.
The mattress is made up of a series of programmable inflatable blocks or “cells” that have pressure, vibration, temperature, and humidity sensors embedded within. “Based on the pressure readings, we can figure out whether the patient is right side up, on their right side, on their left side, or face down,” said Lee.
If the person is face down, he or she can be repositioned within a matter of seconds. “Each of the cells can lift 1000 pounds,” he said. The mattress is “very comfortable,” said Lee, who has tried it out himself.
Eighteen normative control participants have been enrolled for development and training purposes. To date, 10 of these individuals, aged 18-53 years, weighing 100-182 lb, and with a height of 5 ft 2 in to 6 ft 1 in, underwent extensive formal testing on the prototype bed.
Researchers found the mattress responded quickly to different body positions and weights. “We were able to reposition everybody in around 20 seconds,” said Lee.
The overall accuracy of detecting the prone position was 96.8%. There were no cases of a supine or prone position being mistaken for each other.
Researchers are refining the algorithm to improve the accuracy for detecting the prone position and expect to have a completely functional prototype within a few years.
Big Step Forward
Commenting on the research, Daniel M. Goldenholz, MD, PhD, assistant professor, Division of Epilepsy, Harvard Beth Israel Deaconess Medical Center, Boston, said the study “is a big step forward in the race to provide an actionable tool to prevent SUDEP.”
The technology “appears to mostly be doing what it’s intended to do, with relatively minor technical errors being made,” he said.
However, it is not clear if this technology can truly save lives, said Goldenholz. “The data we have suggests that lying face down in bed after a seizure is correlated with SUDEP, but that does not mean that if we can simply flip people over, they for sure won’t die.”
Even if the new technology “works perfectly,” it’s still an open question, said Goldenholz. If it does save lives, “this will be a major breakthrough, and one that has been needed for a long time.”
However, even if it does not, he congratulates the team for trying to determine if reducing the prone position can help prevent SUDEP. He would like to see more “high-risk, high-reward” studies in the epilepsy field. “We are in so much need of new innovations.”
He said he was “personally very inspired” by this work. “People are dying from this terrible disease, and this team is building what they hope might save lives.”
The study was funded by the National Institutes of Health. The mattress is being developed by Soterya. Lee reported no equity in Soterya. Goldenholz reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM AES 2024
Sleep Apnea Linked to Heightened Mortality in Epilepsy
LOS ANGELES — according to a new analysis of over 2 million patient-years drawn from the Komodo Health Claims Database.
“A 10-year-old with uncontrolled epilepsy and central sleep apnea is about 200 times more likely to die than a general population 10-year-old. That’s comparable to a 10-year-old with {epilepsy and} congestive heart failure. Noncentral sleep apnea is comparable to being paralyzed. It’s a huge risk factor,” said poster presenter Dan Lloyd, advanced analytics lead at UCB, which sponsored the research.
The ordering of sleep apnea tests for patients with epilepsy is widely variable, according to Stefanie Dedeurwaerdere, PhD, who is the innovation and value creation lead at UCB. “Some doctors do that as a general practice, and some don’t. There’s no coherency in the way these studies are requested for epilepsy patients. We want to create some awareness around this topic,” she said, and added that treatment of sleep apnea may improve epileptic seizures.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
The study included mortality rates between January 2018 and December 2022, with a total of 2,355,410 patient-years and 968,993 patients, with an age distribution of 19.1% age 1 to less than 18 years, 23.7% age 18-35 years, and 57.2% age 36 years or older. Sleep apnea prevalences were 0.7% for central sleep apnea (CSA), 14.0% for obstructive sleep apnea (OSA), and 85.3% with no sleep apnea.
Among those aged 1-18 years, the standardized mortality ratio (SMR) for those with uncontrolled epilepsy was 27.7. For those with comorbid OSA, the SMR was 74.2, and for comorbid CSA, the SMR was 135.9. The association was less pronounced in older groups, dipping to 7.0, 11.3, and 19.5 in those aged 18-35 years, and 3.3, 3.1, and 2.8 among those aged 36 years or older.
Among the 1-18 age group, SMRs for other comorbidities included 132.3 for heart failure, 74.9 for hemiplegia/paraplegia, 55.3 for cerebrovascular disease, and 44.6 for chronic pulmonary disease.
Asked for comment, Gordon Buchanan, MD, PhD, welcomed the new work. “The results did not surprise me. I study sleep, epilepsy, and [sudden unexplained death in epilepsy (SUDEP)] in particular ... and every time I speak on these topics, someone asks me about risk of SUDEP in patients with sleep apnea. It’s great to finally have some data,” said Buchanan, a professor of neurology at the University of Iowa, Iowa City.
The authors found that patients undergoing continuous positive airway pressure (CPAP)/bi-level positive airway pressure therapy had a higher mortality risk than those not undergoing CPAP therapy but cautioned that uncontrolled confounders may be contributing to the effect.
Buchanan wondered if treatment with CPAP would be associated with a decreased mortality risk. “I think that would be interesting, but I know that, especially in children, it can be difficult to get them to remain compliant with CPAP. I think that would be interesting to know, if pushing harder to get the kids to comply with CPAP would reduce mortality,” he said.
The specific finding of heightened mortality associated with CSA is interesting, according to Buchanan. “We think of seizures propagating through the brain, maybe through direct synaptic connections or through spreading depolarization. So I think it would make sense that it would hit central regions that would then lead to sleep apnea.”
The relationship between OSA and epilepsy is likely complex. Epilepsy medications and special diets may influence body composition, which could in turn affect the risk for OSA, as could medications associated with psychiatric comorbidities, according to Buchanan.
The study is retrospective and based on claims data. It does not prove causation, and claims data do not fully capture mortality, which may lead to conservative SMR estimates. The researchers did not control for socioeconomic status, treatment status, and other comorbidities or conditions.
Lloyd and Dedeurwaerdere are employees of UCB, which sponsored the study. Buchanan had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
LOS ANGELES — according to a new analysis of over 2 million patient-years drawn from the Komodo Health Claims Database.
“A 10-year-old with uncontrolled epilepsy and central sleep apnea is about 200 times more likely to die than a general population 10-year-old. That’s comparable to a 10-year-old with {epilepsy and} congestive heart failure. Noncentral sleep apnea is comparable to being paralyzed. It’s a huge risk factor,” said poster presenter Dan Lloyd, advanced analytics lead at UCB, which sponsored the research.
The ordering of sleep apnea tests for patients with epilepsy is widely variable, according to Stefanie Dedeurwaerdere, PhD, who is the innovation and value creation lead at UCB. “Some doctors do that as a general practice, and some don’t. There’s no coherency in the way these studies are requested for epilepsy patients. We want to create some awareness around this topic,” she said, and added that treatment of sleep apnea may improve epileptic seizures.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
The study included mortality rates between January 2018 and December 2022, with a total of 2,355,410 patient-years and 968,993 patients, with an age distribution of 19.1% age 1 to less than 18 years, 23.7% age 18-35 years, and 57.2% age 36 years or older. Sleep apnea prevalences were 0.7% for central sleep apnea (CSA), 14.0% for obstructive sleep apnea (OSA), and 85.3% with no sleep apnea.
Among those aged 1-18 years, the standardized mortality ratio (SMR) for those with uncontrolled epilepsy was 27.7. For those with comorbid OSA, the SMR was 74.2, and for comorbid CSA, the SMR was 135.9. The association was less pronounced in older groups, dipping to 7.0, 11.3, and 19.5 in those aged 18-35 years, and 3.3, 3.1, and 2.8 among those aged 36 years or older.
Among the 1-18 age group, SMRs for other comorbidities included 132.3 for heart failure, 74.9 for hemiplegia/paraplegia, 55.3 for cerebrovascular disease, and 44.6 for chronic pulmonary disease.
Asked for comment, Gordon Buchanan, MD, PhD, welcomed the new work. “The results did not surprise me. I study sleep, epilepsy, and [sudden unexplained death in epilepsy (SUDEP)] in particular ... and every time I speak on these topics, someone asks me about risk of SUDEP in patients with sleep apnea. It’s great to finally have some data,” said Buchanan, a professor of neurology at the University of Iowa, Iowa City.
The authors found that patients undergoing continuous positive airway pressure (CPAP)/bi-level positive airway pressure therapy had a higher mortality risk than those not undergoing CPAP therapy but cautioned that uncontrolled confounders may be contributing to the effect.
Buchanan wondered if treatment with CPAP would be associated with a decreased mortality risk. “I think that would be interesting, but I know that, especially in children, it can be difficult to get them to remain compliant with CPAP. I think that would be interesting to know, if pushing harder to get the kids to comply with CPAP would reduce mortality,” he said.
The specific finding of heightened mortality associated with CSA is interesting, according to Buchanan. “We think of seizures propagating through the brain, maybe through direct synaptic connections or through spreading depolarization. So I think it would make sense that it would hit central regions that would then lead to sleep apnea.”
The relationship between OSA and epilepsy is likely complex. Epilepsy medications and special diets may influence body composition, which could in turn affect the risk for OSA, as could medications associated with psychiatric comorbidities, according to Buchanan.
The study is retrospective and based on claims data. It does not prove causation, and claims data do not fully capture mortality, which may lead to conservative SMR estimates. The researchers did not control for socioeconomic status, treatment status, and other comorbidities or conditions.
Lloyd and Dedeurwaerdere are employees of UCB, which sponsored the study. Buchanan had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
LOS ANGELES — according to a new analysis of over 2 million patient-years drawn from the Komodo Health Claims Database.
“A 10-year-old with uncontrolled epilepsy and central sleep apnea is about 200 times more likely to die than a general population 10-year-old. That’s comparable to a 10-year-old with {epilepsy and} congestive heart failure. Noncentral sleep apnea is comparable to being paralyzed. It’s a huge risk factor,” said poster presenter Dan Lloyd, advanced analytics lead at UCB, which sponsored the research.
The ordering of sleep apnea tests for patients with epilepsy is widely variable, according to Stefanie Dedeurwaerdere, PhD, who is the innovation and value creation lead at UCB. “Some doctors do that as a general practice, and some don’t. There’s no coherency in the way these studies are requested for epilepsy patients. We want to create some awareness around this topic,” she said, and added that treatment of sleep apnea may improve epileptic seizures.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
The study included mortality rates between January 2018 and December 2022, with a total of 2,355,410 patient-years and 968,993 patients, with an age distribution of 19.1% age 1 to less than 18 years, 23.7% age 18-35 years, and 57.2% age 36 years or older. Sleep apnea prevalences were 0.7% for central sleep apnea (CSA), 14.0% for obstructive sleep apnea (OSA), and 85.3% with no sleep apnea.
Among those aged 1-18 years, the standardized mortality ratio (SMR) for those with uncontrolled epilepsy was 27.7. For those with comorbid OSA, the SMR was 74.2, and for comorbid CSA, the SMR was 135.9. The association was less pronounced in older groups, dipping to 7.0, 11.3, and 19.5 in those aged 18-35 years, and 3.3, 3.1, and 2.8 among those aged 36 years or older.
Among the 1-18 age group, SMRs for other comorbidities included 132.3 for heart failure, 74.9 for hemiplegia/paraplegia, 55.3 for cerebrovascular disease, and 44.6 for chronic pulmonary disease.
Asked for comment, Gordon Buchanan, MD, PhD, welcomed the new work. “The results did not surprise me. I study sleep, epilepsy, and [sudden unexplained death in epilepsy (SUDEP)] in particular ... and every time I speak on these topics, someone asks me about risk of SUDEP in patients with sleep apnea. It’s great to finally have some data,” said Buchanan, a professor of neurology at the University of Iowa, Iowa City.
The authors found that patients undergoing continuous positive airway pressure (CPAP)/bi-level positive airway pressure therapy had a higher mortality risk than those not undergoing CPAP therapy but cautioned that uncontrolled confounders may be contributing to the effect.
Buchanan wondered if treatment with CPAP would be associated with a decreased mortality risk. “I think that would be interesting, but I know that, especially in children, it can be difficult to get them to remain compliant with CPAP. I think that would be interesting to know, if pushing harder to get the kids to comply with CPAP would reduce mortality,” he said.
The specific finding of heightened mortality associated with CSA is interesting, according to Buchanan. “We think of seizures propagating through the brain, maybe through direct synaptic connections or through spreading depolarization. So I think it would make sense that it would hit central regions that would then lead to sleep apnea.”
The relationship between OSA and epilepsy is likely complex. Epilepsy medications and special diets may influence body composition, which could in turn affect the risk for OSA, as could medications associated with psychiatric comorbidities, according to Buchanan.
The study is retrospective and based on claims data. It does not prove causation, and claims data do not fully capture mortality, which may lead to conservative SMR estimates. The researchers did not control for socioeconomic status, treatment status, and other comorbidities or conditions.
Lloyd and Dedeurwaerdere are employees of UCB, which sponsored the study. Buchanan had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
FROM AES 2024
Agranulocytosis and Aseptic Meningitis Induced by Sulfamethoxazole-Trimethoprim
Agranulocytosis and Aseptic Meningitis Induced by Sulfamethoxazole-Trimethoprim
Acute agranulocytosis and aseptic meningitis are serious adverse effects (AEs) associated with sulfamethoxazole-trimethoprim. Acute agranulocytosis is a rare, potentially life-threatening blood dyscrasia characterized by a neutrophil count of < 500 cells per μL, with no relevant decrease in hemoglobin or platelet levels.1 Patients with agranulocytosis may be asymptomatic or experience severe sore throat, pharyngitis, or tonsillitis in combination with high fever, rigors, headaches, or malaise. These AEs are commonly classified as idiosyncratic and, in most cases, attributable to medications. If drug-induced agranulocytosis is suspected, the patient should discontinue the medication immediately.1
Meningitis is an inflammatory disease typically caused by viral or bacterial infections; however, it may also be attributed to medications or malignancy. Inflammation of the meninges with a negative bacterial cerebrospinal fluid culture is classified as aseptic meningitis. Distinguishing between aseptic and bacterial meningitis is crucial due to differences in illness severity, treatment options, and prognosis.2 Symptoms of meningitis may include fever, headache, nuchal rigidity, nausea, or vomiting.3 Several classes of medications can cause drug-induced aseptic meningitis (DIAM), but the most commonly reported antibiotic is sulfamethoxazole-trimethoprim.
DIAM is more prevalent in immunocompromised patients, such as those with a history of HIV/AIDS, organ transplant, collagen vascular disease, or malignancy, who may be prescribed sulfamethoxazoletrimethoprim for prophylaxis or treatment of infection.2 The case described in this article serves as a distinctive example of acute agranulocytosis complicated with aseptic meningitis caused by sulfamethoxazole-trimethoprim in an immunocompetent patient.
Case Presentation
A healthy male veteran aged 39 years presented to the Fargo Veterans Affairs Medical Center emergency department (ED) for worsening left testicular pain and increased urinary urgency and frequency for about 48 hours. The patient had no known medication allergies, was current on vaccinations, and his only relevant prescription was valacyclovir for herpes labialis. The evaluation included urinalysis, blood tests, and scrotal ultrasound. The urinalysis, blood tests, and vitals were unremarkable for any signs of systemic infection. The scrotal ultrasound was significant for left focal area of abnormal echogenicity with absent blood flow in the superior left testicle and a significant increase in blood flow around the left epididymis. Mild swelling in the left epididymis was present, with no significant testicular or scrotal swelling or skin changes observed. Urology was consulted and prescribed oral sulfamethoxazole-trimethoprim 800-160 mg every 12 hours for 30 days for the treatment of left epididymo-orchitis.
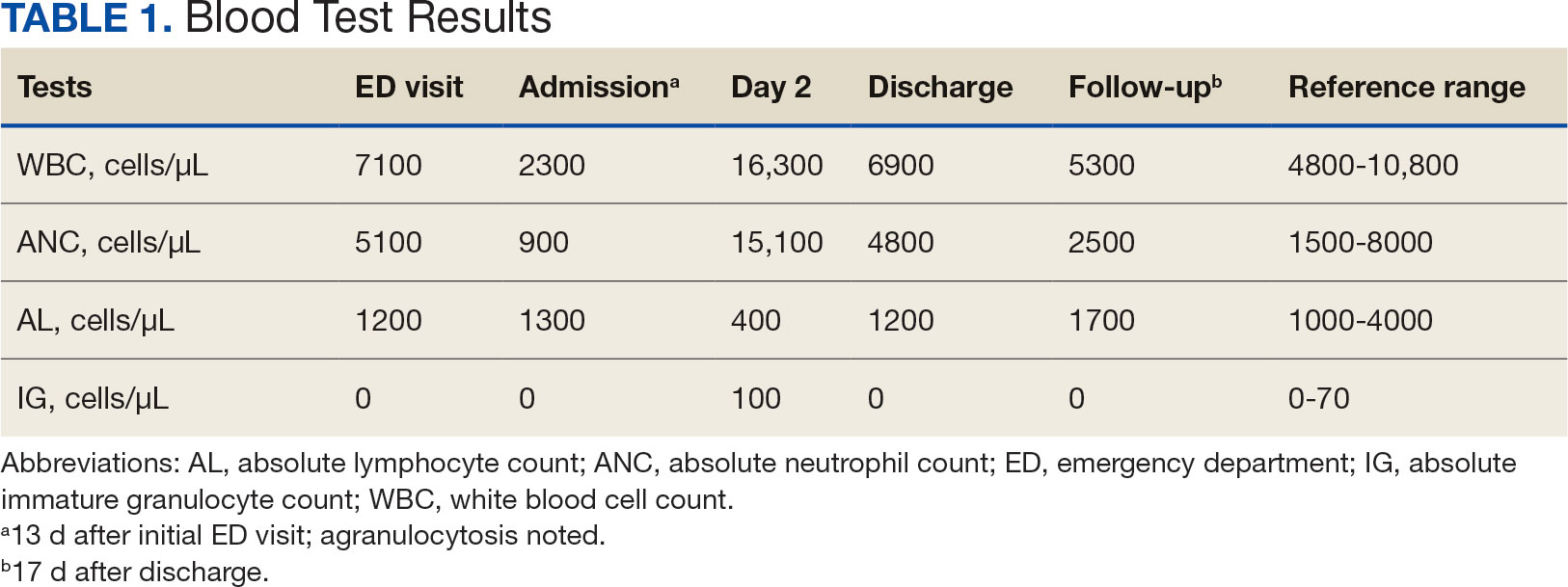
The patient returned to the ED 2 weeks later with fever, chills, headache, generalized body aches, urinary retention, loose stools, and nonspecific chest pressure. A serum blood test revealed significant neutropenia and leukopenia. The patient was admitted for observation, and sulfamethoxazole-trimethoprim was discontinued. The patient received sodium chloride intravenous (IV) fluid, oral potassium chloride supplementation, IV ondansetron, and analgesics, including oral acetaminophen, oral ibuprofen, and IV hydromorphone as needed. Repeated laboratory tests were completed with no specific findings; serum laboratory work, urinalysis, chest and abdominal X-rays, and echocardiogram were all unremarkable. The patient’s neutrophil count dropped from 5100 cells/µL at the initial ED presentation to 900 cells/µL (reference range, 1500-8000 cells/µL) (Table 1). Agranulocytosis quickly resolved after antibiotic discontinuation.
Upon further investigation, the patient took the prescribed sulfamethoxazole-trimethoprim for 10 days before stopping due to the resolution of testicular pain and epididymal swelling. The patient reported initial AEs of loose stools and generalized myalgias when first taking the medication. The patient restarted the antibiotic to complete the course of therapy after not taking it for 2 days. Within 20 minutes of restarting the medication, he experienced myalgias with pruritus, prompting him to return to the ED. Agranulocytosis and aseptic meningitis developed within 12 days after he was prescribed sulfamethoxazole-trimethoprim, though the exact timeframe is unknown.
The patient’s symptoms, except for a persistent headache, resolved during hospitalization. Infectious disease was consulted, and a lumbar puncture was performed due to prior fever and ongoing headaches to rule out a treatable cause of meningitis. The lumbar puncture showed clear spinal fluid, an elevated white blood cell count with neutrophil predominance, and normal protein and glucose levels. Cultures showed no aerobic, anaerobic, or fungal organisms. Herpes virus simplex and Lyme disease testing was not completed during hospitalization. Respiratory panel, legionella, and hepatitis A, B, and C tests were negative (Table 2). The negative laboratory test results strengthened the suspicion of aseptic meningitis caused by sulfamethoxazole-trimethoprim. The neurology consult recommended no additional treatments or tests.

The patient spontaneously recovered 2 days later, with a normalized complete blood count and resolution of headache. Repeat scrotal ultrasounds showed resolution of the left testicle findings. The patient was discharged and seen for a follow-up 14 days later. The final diagnosis requiring hospitalization was aseptic meningitis secondary to a sulfamethoxazole-trimethoprim.
Discussion
Sulfamethoxazole-trimethoprim is a commonly prescribed antibiotic for urinary tract infections, pneumocystis pneumonia, pneumocystis pneumonia prophylaxis, and methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Empiric antibiotics for epididymo-orchitis caused by enteric organisms include levofloxacin or ofloxacin; however sulfamethoxazole-trimethoprim may be considered as alternative.5,6 Agranulocytosis induced by sulfamethoxazole-trimethoprim may occur due to the inhibition on folic acid metabolism, which makes the highly proliferating cells of the hematopoietic system more susceptible to neutropenia. Agranulocytosis typically occurs within 7 days of treatment initiation and generally resolves within a month after discontinuation of the offending agent.7 In this case, agranulocytosis resolved overnight, resulting in leukocytosis. One explanation for the rapid increase in white blood cell count may be the concurrent diagnosis of aseptic meningitis. Alternatively, the patient’s health and immunocompetence may have helped generate an adequate immune response. Medication-induced agranulocytosis is often a diagnosis of exclusion because it is typically difficult to diagnose.7 In more severe or complicated cases of agranulocytosis, filgrastim may be indicated.1
Sulfamethoxazole-trimethoprim is a lipophilic small-molecule medication that can cross the blood-brain barrier and penetrate the tissues of the bone, prostate, and central nervous system.8 Specifically, the medication can pass into the cerebrospinal fluid regardless of meningeal inflammation.9 The exact mechanism for aseptic meningitis caused by sulfamethoxazole-trimethoprim is unknown; however, it may accumulate in the choroid plexus, causing destructive inflammation of small blood vessels and resulting in aseptic meningitis.10 The onset of aseptic meningitis can vary from 10 minutes to 10 days after initiation of the medication. Pre-exposure to the medication typically results in earlier onset of symptoms, though patients do not need to have previously taken the medication to develop aseptic meningitis. Patients generally become afebrile with resolution of headache and mental status changes within 48 to 72 hours after stopping the medication or after about 5 to 7 half-lives of the medication are eliminated.11 Some patients may continue to experience worsening symptoms after discontinuation because the medication is already absorbed into the serum and tissues.
DIAM is an uncommon drug-induced hypersensitivity AE of the central nervous system. Diagnosing aseptic meningitis caused by sulfamethoxazole-trimethoprim can be challenging, as antibiotics are given to treat suspected infections, and the symptoms of aseptic meningitis can be hard to differentiate from those of infectious meningitis.11 Close monitoring between the initiation of the medication and the onset of clinical symptoms is necessary to assist in distinguishing between aseptic and infectious meningitis.3 If the causative agent is not discontinued, symptoms can quickly worsen, progressing from fever and headache to confusion, coma, and respiratory depression. A DIAM diagnosis can only be made with resolution of aseptic meningitis after stopping the contributory agent. If appropriate, this can be proven by rechallenging the medication in a controlled setting. The usual treatment for aseptic meningitis is supportive care, including hydration, antiemetics, electrolyte supplementation, and adequate analgesia.3
Differential diagnoses in this case included viral infection, meningitis, and allergic reaction to sulfamethoxazole-trimethoprim. The patient reported history of experiencing symptoms after restarting his antibiotic, raising strong suspicion for DIAM. Initiation of this antibiotic was the only recent medication change noted. Laboratory testing for infectious agents yielded negative results, including tests for aerobic and anaerobic bacteria as well as viral and fungal infections. A lumbar puncture and cerebrospinal fluid culture was clear, with no organisms shown on gram stain. Bacterial or viral meningitis was presumed less likely due to the duration of symptoms, correlation of symptoms coinciding with restarting the antibiotic, and negative cerebrospinal fluid culture results.
It was concluded that agranulocytosis and aseptic meningitis were likely induced by sulfamethoxazole-trimethoprim as supported by the improvement upon discontinuing the medication and subsequent worsening upon restarting it. Concurrent agranulocytosis and aseptic meningitis is rare, and there is typically no correlation between the 2 reactions. Since agranulocytosis may be asymptomatic, this case highlights the need to monitor blood cell counts in patients using sulfamethoxazole-trimethoprim. The possibility of DIAM should be considered in patients presenting with flu-like symptoms, and a lumbar puncture may be collected to rule out aseptic meningitis if the patient’s AEs are severe following the initiation of an antibiotic, particularly in immunosuppressed patients taking sulfamethoxazole-trimethoprim. This case is unusual because the patient was healthy and immunocompetent.
This case may not be generalizable and may be difficult to compare to other cases. Every case has patient-specific factors affecting subjective information, including the patient’s baseline, severity of symptoms, and treatment options. This report was based on a single patient case and corresponding results may be found in similar patient cases.
Conclusions
This case emphasizes the rare but serious AEs of acute agranulocytosis complicated with aseptic meningitis after prescribed sulfamethoxazole-trimethoprim. This is a unique case of an immunocompetent patient developing both agranulocytosis and aseptic meningitis after restarting the antibiotic to complete therapy. This case highlights the importance of monitoring blood cell counts and monitoring for signs and symptoms of aseptic meningitis, even during short courses of therapy. Further research is needed to recognize characteristics that may increase the risk for these AEs and to develop strategies for their prevention.
- Garbe E. Non-chemotherapy drug-induced agranulocytosis. Expert Opin Drug Saf. 2007;6(3):323-335. doi:10.1517/14740338.6.3.323
- Jha P, Stromich J, Cohen M, Wainaina JN. A rare complication of trimethoprim-sulfamethoxazole: drug induced aseptic meningitis. Case Rep Infect Dis. 2016;2016:3879406. doi:10.1155/2016/3879406
- Hopkins S, Jolles S. Drug-induced aseptic meningitis. Expert Opin Drug Saf. 2005;4(2):285-297. doi:10.1517/14740338.4.2.285
- Jarrin I, Sellier P, Lopes A, et al. Etiologies and management of aseptic meningitis in patients admitted to an internal medicine department. Medicine (Baltimore). 2016;95(2):e2372. doi:10.1097/MD.0000000000002372
- Street EJ, Justice ED, Kopa Z, et al. The 2016 European guideline on the management of epididymo-orchitis. Int J STD AIDS. 2017;28(8):744-749. doi:10.1177/0956462417699356
- Kbirou A, Alafifi M, Sayah M, Dakir M, Debbagh A, Aboutaieb R. Acute orchiepididymitis: epidemiological and clinical aspects: an analysis of 152 cases. Ann Med Surg (Lond). 2022;75:103335. doi:10.1016/j.amsu.2022.103335
- Rattay B, Benndorf RA. Drug-induced idiosyncratic agranulocytosis - infrequent but dangerous. Front Pharmacol. 2021;12:727717. doi:10.3389/fphar.2021.727717
- Elmedani S, Albayati A, Udongwo N, Odak M, Khawaja S. Trimethoprim-sulfamethoxazole-induced aseptic meningitis: a new approach. Cureus. 2021;13(6):e15869. doi:10.7759/cureus.15869
- Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858-883. doi:10.1128/CMR.00007-10
- Moris G, Garcia-Monco JC. The challenge of drug-induced aseptic meningitis. Arch Intern Med. 1999;159(11):1185- 1194. doi:10.1001/archinte.159.11.1185
- Bruner KE, Coop CA, White KM. Trimethoprim-sulfamethoxazole-induced aseptic meningitis-not just another sulfa allergy. Ann Allergy Asthma Immunol. 2014;113(5):520-526. doi:10.1016/j.anai.2014.08.006
Acute agranulocytosis and aseptic meningitis are serious adverse effects (AEs) associated with sulfamethoxazole-trimethoprim. Acute agranulocytosis is a rare, potentially life-threatening blood dyscrasia characterized by a neutrophil count of < 500 cells per μL, with no relevant decrease in hemoglobin or platelet levels.1 Patients with agranulocytosis may be asymptomatic or experience severe sore throat, pharyngitis, or tonsillitis in combination with high fever, rigors, headaches, or malaise. These AEs are commonly classified as idiosyncratic and, in most cases, attributable to medications. If drug-induced agranulocytosis is suspected, the patient should discontinue the medication immediately.1
Meningitis is an inflammatory disease typically caused by viral or bacterial infections; however, it may also be attributed to medications or malignancy. Inflammation of the meninges with a negative bacterial cerebrospinal fluid culture is classified as aseptic meningitis. Distinguishing between aseptic and bacterial meningitis is crucial due to differences in illness severity, treatment options, and prognosis.2 Symptoms of meningitis may include fever, headache, nuchal rigidity, nausea, or vomiting.3 Several classes of medications can cause drug-induced aseptic meningitis (DIAM), but the most commonly reported antibiotic is sulfamethoxazole-trimethoprim.
DIAM is more prevalent in immunocompromised patients, such as those with a history of HIV/AIDS, organ transplant, collagen vascular disease, or malignancy, who may be prescribed sulfamethoxazoletrimethoprim for prophylaxis or treatment of infection.2 The case described in this article serves as a distinctive example of acute agranulocytosis complicated with aseptic meningitis caused by sulfamethoxazole-trimethoprim in an immunocompetent patient.
Case Presentation
A healthy male veteran aged 39 years presented to the Fargo Veterans Affairs Medical Center emergency department (ED) for worsening left testicular pain and increased urinary urgency and frequency for about 48 hours. The patient had no known medication allergies, was current on vaccinations, and his only relevant prescription was valacyclovir for herpes labialis. The evaluation included urinalysis, blood tests, and scrotal ultrasound. The urinalysis, blood tests, and vitals were unremarkable for any signs of systemic infection. The scrotal ultrasound was significant for left focal area of abnormal echogenicity with absent blood flow in the superior left testicle and a significant increase in blood flow around the left epididymis. Mild swelling in the left epididymis was present, with no significant testicular or scrotal swelling or skin changes observed. Urology was consulted and prescribed oral sulfamethoxazole-trimethoprim 800-160 mg every 12 hours for 30 days for the treatment of left epididymo-orchitis.
The patient returned to the ED 2 weeks later with fever, chills, headache, generalized body aches, urinary retention, loose stools, and nonspecific chest pressure. A serum blood test revealed significant neutropenia and leukopenia. The patient was admitted for observation, and sulfamethoxazole-trimethoprim was discontinued. The patient received sodium chloride intravenous (IV) fluid, oral potassium chloride supplementation, IV ondansetron, and analgesics, including oral acetaminophen, oral ibuprofen, and IV hydromorphone as needed. Repeated laboratory tests were completed with no specific findings; serum laboratory work, urinalysis, chest and abdominal X-rays, and echocardiogram were all unremarkable. The patient’s neutrophil count dropped from 5100 cells/µL at the initial ED presentation to 900 cells/µL (reference range, 1500-8000 cells/µL) (Table 1). Agranulocytosis quickly resolved after antibiotic discontinuation.

Upon further investigation, the patient took the prescribed sulfamethoxazole-trimethoprim for 10 days before stopping due to the resolution of testicular pain and epididymal swelling. The patient reported initial AEs of loose stools and generalized myalgias when first taking the medication. The patient restarted the antibiotic to complete the course of therapy after not taking it for 2 days. Within 20 minutes of restarting the medication, he experienced myalgias with pruritus, prompting him to return to the ED. Agranulocytosis and aseptic meningitis developed within 12 days after he was prescribed sulfamethoxazole-trimethoprim, though the exact timeframe is unknown.
The patient’s symptoms, except for a persistent headache, resolved during hospitalization. Infectious disease was consulted, and a lumbar puncture was performed due to prior fever and ongoing headaches to rule out a treatable cause of meningitis. The lumbar puncture showed clear spinal fluid, an elevated white blood cell count with neutrophil predominance, and normal protein and glucose levels. Cultures showed no aerobic, anaerobic, or fungal organisms. Herpes virus simplex and Lyme disease testing was not completed during hospitalization. Respiratory panel, legionella, and hepatitis A, B, and C tests were negative (Table 2). The negative laboratory test results strengthened the suspicion of aseptic meningitis caused by sulfamethoxazole-trimethoprim. The neurology consult recommended no additional treatments or tests.

The patient spontaneously recovered 2 days later, with a normalized complete blood count and resolution of headache. Repeat scrotal ultrasounds showed resolution of the left testicle findings. The patient was discharged and seen for a follow-up 14 days later. The final diagnosis requiring hospitalization was aseptic meningitis secondary to a sulfamethoxazole-trimethoprim.
Discussion
Sulfamethoxazole-trimethoprim is a commonly prescribed antibiotic for urinary tract infections, pneumocystis pneumonia, pneumocystis pneumonia prophylaxis, and methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Empiric antibiotics for epididymo-orchitis caused by enteric organisms include levofloxacin or ofloxacin; however sulfamethoxazole-trimethoprim may be considered as alternative.5,6 Agranulocytosis induced by sulfamethoxazole-trimethoprim may occur due to the inhibition on folic acid metabolism, which makes the highly proliferating cells of the hematopoietic system more susceptible to neutropenia. Agranulocytosis typically occurs within 7 days of treatment initiation and generally resolves within a month after discontinuation of the offending agent.7 In this case, agranulocytosis resolved overnight, resulting in leukocytosis. One explanation for the rapid increase in white blood cell count may be the concurrent diagnosis of aseptic meningitis. Alternatively, the patient’s health and immunocompetence may have helped generate an adequate immune response. Medication-induced agranulocytosis is often a diagnosis of exclusion because it is typically difficult to diagnose.7 In more severe or complicated cases of agranulocytosis, filgrastim may be indicated.1
Sulfamethoxazole-trimethoprim is a lipophilic small-molecule medication that can cross the blood-brain barrier and penetrate the tissues of the bone, prostate, and central nervous system.8 Specifically, the medication can pass into the cerebrospinal fluid regardless of meningeal inflammation.9 The exact mechanism for aseptic meningitis caused by sulfamethoxazole-trimethoprim is unknown; however, it may accumulate in the choroid plexus, causing destructive inflammation of small blood vessels and resulting in aseptic meningitis.10 The onset of aseptic meningitis can vary from 10 minutes to 10 days after initiation of the medication. Pre-exposure to the medication typically results in earlier onset of symptoms, though patients do not need to have previously taken the medication to develop aseptic meningitis. Patients generally become afebrile with resolution of headache and mental status changes within 48 to 72 hours after stopping the medication or after about 5 to 7 half-lives of the medication are eliminated.11 Some patients may continue to experience worsening symptoms after discontinuation because the medication is already absorbed into the serum and tissues.
DIAM is an uncommon drug-induced hypersensitivity AE of the central nervous system. Diagnosing aseptic meningitis caused by sulfamethoxazole-trimethoprim can be challenging, as antibiotics are given to treat suspected infections, and the symptoms of aseptic meningitis can be hard to differentiate from those of infectious meningitis.11 Close monitoring between the initiation of the medication and the onset of clinical symptoms is necessary to assist in distinguishing between aseptic and infectious meningitis.3 If the causative agent is not discontinued, symptoms can quickly worsen, progressing from fever and headache to confusion, coma, and respiratory depression. A DIAM diagnosis can only be made with resolution of aseptic meningitis after stopping the contributory agent. If appropriate, this can be proven by rechallenging the medication in a controlled setting. The usual treatment for aseptic meningitis is supportive care, including hydration, antiemetics, electrolyte supplementation, and adequate analgesia.3
Differential diagnoses in this case included viral infection, meningitis, and allergic reaction to sulfamethoxazole-trimethoprim. The patient reported history of experiencing symptoms after restarting his antibiotic, raising strong suspicion for DIAM. Initiation of this antibiotic was the only recent medication change noted. Laboratory testing for infectious agents yielded negative results, including tests for aerobic and anaerobic bacteria as well as viral and fungal infections. A lumbar puncture and cerebrospinal fluid culture was clear, with no organisms shown on gram stain. Bacterial or viral meningitis was presumed less likely due to the duration of symptoms, correlation of symptoms coinciding with restarting the antibiotic, and negative cerebrospinal fluid culture results.
It was concluded that agranulocytosis and aseptic meningitis were likely induced by sulfamethoxazole-trimethoprim as supported by the improvement upon discontinuing the medication and subsequent worsening upon restarting it. Concurrent agranulocytosis and aseptic meningitis is rare, and there is typically no correlation between the 2 reactions. Since agranulocytosis may be asymptomatic, this case highlights the need to monitor blood cell counts in patients using sulfamethoxazole-trimethoprim. The possibility of DIAM should be considered in patients presenting with flu-like symptoms, and a lumbar puncture may be collected to rule out aseptic meningitis if the patient’s AEs are severe following the initiation of an antibiotic, particularly in immunosuppressed patients taking sulfamethoxazole-trimethoprim. This case is unusual because the patient was healthy and immunocompetent.
This case may not be generalizable and may be difficult to compare to other cases. Every case has patient-specific factors affecting subjective information, including the patient’s baseline, severity of symptoms, and treatment options. This report was based on a single patient case and corresponding results may be found in similar patient cases.
Conclusions
This case emphasizes the rare but serious AEs of acute agranulocytosis complicated with aseptic meningitis after prescribed sulfamethoxazole-trimethoprim. This is a unique case of an immunocompetent patient developing both agranulocytosis and aseptic meningitis after restarting the antibiotic to complete therapy. This case highlights the importance of monitoring blood cell counts and monitoring for signs and symptoms of aseptic meningitis, even during short courses of therapy. Further research is needed to recognize characteristics that may increase the risk for these AEs and to develop strategies for their prevention.
Acute agranulocytosis and aseptic meningitis are serious adverse effects (AEs) associated with sulfamethoxazole-trimethoprim. Acute agranulocytosis is a rare, potentially life-threatening blood dyscrasia characterized by a neutrophil count of < 500 cells per μL, with no relevant decrease in hemoglobin or platelet levels.1 Patients with agranulocytosis may be asymptomatic or experience severe sore throat, pharyngitis, or tonsillitis in combination with high fever, rigors, headaches, or malaise. These AEs are commonly classified as idiosyncratic and, in most cases, attributable to medications. If drug-induced agranulocytosis is suspected, the patient should discontinue the medication immediately.1
Meningitis is an inflammatory disease typically caused by viral or bacterial infections; however, it may also be attributed to medications or malignancy. Inflammation of the meninges with a negative bacterial cerebrospinal fluid culture is classified as aseptic meningitis. Distinguishing between aseptic and bacterial meningitis is crucial due to differences in illness severity, treatment options, and prognosis.2 Symptoms of meningitis may include fever, headache, nuchal rigidity, nausea, or vomiting.3 Several classes of medications can cause drug-induced aseptic meningitis (DIAM), but the most commonly reported antibiotic is sulfamethoxazole-trimethoprim.
DIAM is more prevalent in immunocompromised patients, such as those with a history of HIV/AIDS, organ transplant, collagen vascular disease, or malignancy, who may be prescribed sulfamethoxazoletrimethoprim for prophylaxis or treatment of infection.2 The case described in this article serves as a distinctive example of acute agranulocytosis complicated with aseptic meningitis caused by sulfamethoxazole-trimethoprim in an immunocompetent patient.
Case Presentation
A healthy male veteran aged 39 years presented to the Fargo Veterans Affairs Medical Center emergency department (ED) for worsening left testicular pain and increased urinary urgency and frequency for about 48 hours. The patient had no known medication allergies, was current on vaccinations, and his only relevant prescription was valacyclovir for herpes labialis. The evaluation included urinalysis, blood tests, and scrotal ultrasound. The urinalysis, blood tests, and vitals were unremarkable for any signs of systemic infection. The scrotal ultrasound was significant for left focal area of abnormal echogenicity with absent blood flow in the superior left testicle and a significant increase in blood flow around the left epididymis. Mild swelling in the left epididymis was present, with no significant testicular or scrotal swelling or skin changes observed. Urology was consulted and prescribed oral sulfamethoxazole-trimethoprim 800-160 mg every 12 hours for 30 days for the treatment of left epididymo-orchitis.
The patient returned to the ED 2 weeks later with fever, chills, headache, generalized body aches, urinary retention, loose stools, and nonspecific chest pressure. A serum blood test revealed significant neutropenia and leukopenia. The patient was admitted for observation, and sulfamethoxazole-trimethoprim was discontinued. The patient received sodium chloride intravenous (IV) fluid, oral potassium chloride supplementation, IV ondansetron, and analgesics, including oral acetaminophen, oral ibuprofen, and IV hydromorphone as needed. Repeated laboratory tests were completed with no specific findings; serum laboratory work, urinalysis, chest and abdominal X-rays, and echocardiogram were all unremarkable. The patient’s neutrophil count dropped from 5100 cells/µL at the initial ED presentation to 900 cells/µL (reference range, 1500-8000 cells/µL) (Table 1). Agranulocytosis quickly resolved after antibiotic discontinuation.

Upon further investigation, the patient took the prescribed sulfamethoxazole-trimethoprim for 10 days before stopping due to the resolution of testicular pain and epididymal swelling. The patient reported initial AEs of loose stools and generalized myalgias when first taking the medication. The patient restarted the antibiotic to complete the course of therapy after not taking it for 2 days. Within 20 minutes of restarting the medication, he experienced myalgias with pruritus, prompting him to return to the ED. Agranulocytosis and aseptic meningitis developed within 12 days after he was prescribed sulfamethoxazole-trimethoprim, though the exact timeframe is unknown.
The patient’s symptoms, except for a persistent headache, resolved during hospitalization. Infectious disease was consulted, and a lumbar puncture was performed due to prior fever and ongoing headaches to rule out a treatable cause of meningitis. The lumbar puncture showed clear spinal fluid, an elevated white blood cell count with neutrophil predominance, and normal protein and glucose levels. Cultures showed no aerobic, anaerobic, or fungal organisms. Herpes virus simplex and Lyme disease testing was not completed during hospitalization. Respiratory panel, legionella, and hepatitis A, B, and C tests were negative (Table 2). The negative laboratory test results strengthened the suspicion of aseptic meningitis caused by sulfamethoxazole-trimethoprim. The neurology consult recommended no additional treatments or tests.

The patient spontaneously recovered 2 days later, with a normalized complete blood count and resolution of headache. Repeat scrotal ultrasounds showed resolution of the left testicle findings. The patient was discharged and seen for a follow-up 14 days later. The final diagnosis requiring hospitalization was aseptic meningitis secondary to a sulfamethoxazole-trimethoprim.
Discussion
Sulfamethoxazole-trimethoprim is a commonly prescribed antibiotic for urinary tract infections, pneumocystis pneumonia, pneumocystis pneumonia prophylaxis, and methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Empiric antibiotics for epididymo-orchitis caused by enteric organisms include levofloxacin or ofloxacin; however sulfamethoxazole-trimethoprim may be considered as alternative.5,6 Agranulocytosis induced by sulfamethoxazole-trimethoprim may occur due to the inhibition on folic acid metabolism, which makes the highly proliferating cells of the hematopoietic system more susceptible to neutropenia. Agranulocytosis typically occurs within 7 days of treatment initiation and generally resolves within a month after discontinuation of the offending agent.7 In this case, agranulocytosis resolved overnight, resulting in leukocytosis. One explanation for the rapid increase in white blood cell count may be the concurrent diagnosis of aseptic meningitis. Alternatively, the patient’s health and immunocompetence may have helped generate an adequate immune response. Medication-induced agranulocytosis is often a diagnosis of exclusion because it is typically difficult to diagnose.7 In more severe or complicated cases of agranulocytosis, filgrastim may be indicated.1
Sulfamethoxazole-trimethoprim is a lipophilic small-molecule medication that can cross the blood-brain barrier and penetrate the tissues of the bone, prostate, and central nervous system.8 Specifically, the medication can pass into the cerebrospinal fluid regardless of meningeal inflammation.9 The exact mechanism for aseptic meningitis caused by sulfamethoxazole-trimethoprim is unknown; however, it may accumulate in the choroid plexus, causing destructive inflammation of small blood vessels and resulting in aseptic meningitis.10 The onset of aseptic meningitis can vary from 10 minutes to 10 days after initiation of the medication. Pre-exposure to the medication typically results in earlier onset of symptoms, though patients do not need to have previously taken the medication to develop aseptic meningitis. Patients generally become afebrile with resolution of headache and mental status changes within 48 to 72 hours after stopping the medication or after about 5 to 7 half-lives of the medication are eliminated.11 Some patients may continue to experience worsening symptoms after discontinuation because the medication is already absorbed into the serum and tissues.
DIAM is an uncommon drug-induced hypersensitivity AE of the central nervous system. Diagnosing aseptic meningitis caused by sulfamethoxazole-trimethoprim can be challenging, as antibiotics are given to treat suspected infections, and the symptoms of aseptic meningitis can be hard to differentiate from those of infectious meningitis.11 Close monitoring between the initiation of the medication and the onset of clinical symptoms is necessary to assist in distinguishing between aseptic and infectious meningitis.3 If the causative agent is not discontinued, symptoms can quickly worsen, progressing from fever and headache to confusion, coma, and respiratory depression. A DIAM diagnosis can only be made with resolution of aseptic meningitis after stopping the contributory agent. If appropriate, this can be proven by rechallenging the medication in a controlled setting. The usual treatment for aseptic meningitis is supportive care, including hydration, antiemetics, electrolyte supplementation, and adequate analgesia.3
Differential diagnoses in this case included viral infection, meningitis, and allergic reaction to sulfamethoxazole-trimethoprim. The patient reported history of experiencing symptoms after restarting his antibiotic, raising strong suspicion for DIAM. Initiation of this antibiotic was the only recent medication change noted. Laboratory testing for infectious agents yielded negative results, including tests for aerobic and anaerobic bacteria as well as viral and fungal infections. A lumbar puncture and cerebrospinal fluid culture was clear, with no organisms shown on gram stain. Bacterial or viral meningitis was presumed less likely due to the duration of symptoms, correlation of symptoms coinciding with restarting the antibiotic, and negative cerebrospinal fluid culture results.
It was concluded that agranulocytosis and aseptic meningitis were likely induced by sulfamethoxazole-trimethoprim as supported by the improvement upon discontinuing the medication and subsequent worsening upon restarting it. Concurrent agranulocytosis and aseptic meningitis is rare, and there is typically no correlation between the 2 reactions. Since agranulocytosis may be asymptomatic, this case highlights the need to monitor blood cell counts in patients using sulfamethoxazole-trimethoprim. The possibility of DIAM should be considered in patients presenting with flu-like symptoms, and a lumbar puncture may be collected to rule out aseptic meningitis if the patient’s AEs are severe following the initiation of an antibiotic, particularly in immunosuppressed patients taking sulfamethoxazole-trimethoprim. This case is unusual because the patient was healthy and immunocompetent.
This case may not be generalizable and may be difficult to compare to other cases. Every case has patient-specific factors affecting subjective information, including the patient’s baseline, severity of symptoms, and treatment options. This report was based on a single patient case and corresponding results may be found in similar patient cases.
Conclusions
This case emphasizes the rare but serious AEs of acute agranulocytosis complicated with aseptic meningitis after prescribed sulfamethoxazole-trimethoprim. This is a unique case of an immunocompetent patient developing both agranulocytosis and aseptic meningitis after restarting the antibiotic to complete therapy. This case highlights the importance of monitoring blood cell counts and monitoring for signs and symptoms of aseptic meningitis, even during short courses of therapy. Further research is needed to recognize characteristics that may increase the risk for these AEs and to develop strategies for their prevention.
- Garbe E. Non-chemotherapy drug-induced agranulocytosis. Expert Opin Drug Saf. 2007;6(3):323-335. doi:10.1517/14740338.6.3.323
- Jha P, Stromich J, Cohen M, Wainaina JN. A rare complication of trimethoprim-sulfamethoxazole: drug induced aseptic meningitis. Case Rep Infect Dis. 2016;2016:3879406. doi:10.1155/2016/3879406
- Hopkins S, Jolles S. Drug-induced aseptic meningitis. Expert Opin Drug Saf. 2005;4(2):285-297. doi:10.1517/14740338.4.2.285
- Jarrin I, Sellier P, Lopes A, et al. Etiologies and management of aseptic meningitis in patients admitted to an internal medicine department. Medicine (Baltimore). 2016;95(2):e2372. doi:10.1097/MD.0000000000002372
- Street EJ, Justice ED, Kopa Z, et al. The 2016 European guideline on the management of epididymo-orchitis. Int J STD AIDS. 2017;28(8):744-749. doi:10.1177/0956462417699356
- Kbirou A, Alafifi M, Sayah M, Dakir M, Debbagh A, Aboutaieb R. Acute orchiepididymitis: epidemiological and clinical aspects: an analysis of 152 cases. Ann Med Surg (Lond). 2022;75:103335. doi:10.1016/j.amsu.2022.103335
- Rattay B, Benndorf RA. Drug-induced idiosyncratic agranulocytosis - infrequent but dangerous. Front Pharmacol. 2021;12:727717. doi:10.3389/fphar.2021.727717
- Elmedani S, Albayati A, Udongwo N, Odak M, Khawaja S. Trimethoprim-sulfamethoxazole-induced aseptic meningitis: a new approach. Cureus. 2021;13(6):e15869. doi:10.7759/cureus.15869
- Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858-883. doi:10.1128/CMR.00007-10
- Moris G, Garcia-Monco JC. The challenge of drug-induced aseptic meningitis. Arch Intern Med. 1999;159(11):1185- 1194. doi:10.1001/archinte.159.11.1185
- Bruner KE, Coop CA, White KM. Trimethoprim-sulfamethoxazole-induced aseptic meningitis-not just another sulfa allergy. Ann Allergy Asthma Immunol. 2014;113(5):520-526. doi:10.1016/j.anai.2014.08.006
- Garbe E. Non-chemotherapy drug-induced agranulocytosis. Expert Opin Drug Saf. 2007;6(3):323-335. doi:10.1517/14740338.6.3.323
- Jha P, Stromich J, Cohen M, Wainaina JN. A rare complication of trimethoprim-sulfamethoxazole: drug induced aseptic meningitis. Case Rep Infect Dis. 2016;2016:3879406. doi:10.1155/2016/3879406
- Hopkins S, Jolles S. Drug-induced aseptic meningitis. Expert Opin Drug Saf. 2005;4(2):285-297. doi:10.1517/14740338.4.2.285
- Jarrin I, Sellier P, Lopes A, et al. Etiologies and management of aseptic meningitis in patients admitted to an internal medicine department. Medicine (Baltimore). 2016;95(2):e2372. doi:10.1097/MD.0000000000002372
- Street EJ, Justice ED, Kopa Z, et al. The 2016 European guideline on the management of epididymo-orchitis. Int J STD AIDS. 2017;28(8):744-749. doi:10.1177/0956462417699356
- Kbirou A, Alafifi M, Sayah M, Dakir M, Debbagh A, Aboutaieb R. Acute orchiepididymitis: epidemiological and clinical aspects: an analysis of 152 cases. Ann Med Surg (Lond). 2022;75:103335. doi:10.1016/j.amsu.2022.103335
- Rattay B, Benndorf RA. Drug-induced idiosyncratic agranulocytosis - infrequent but dangerous. Front Pharmacol. 2021;12:727717. doi:10.3389/fphar.2021.727717
- Elmedani S, Albayati A, Udongwo N, Odak M, Khawaja S. Trimethoprim-sulfamethoxazole-induced aseptic meningitis: a new approach. Cureus. 2021;13(6):e15869. doi:10.7759/cureus.15869
- Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858-883. doi:10.1128/CMR.00007-10
- Moris G, Garcia-Monco JC. The challenge of drug-induced aseptic meningitis. Arch Intern Med. 1999;159(11):1185- 1194. doi:10.1001/archinte.159.11.1185
- Bruner KE, Coop CA, White KM. Trimethoprim-sulfamethoxazole-induced aseptic meningitis-not just another sulfa allergy. Ann Allergy Asthma Immunol. 2014;113(5):520-526. doi:10.1016/j.anai.2014.08.006
Agranulocytosis and Aseptic Meningitis Induced by Sulfamethoxazole-Trimethoprim
Agranulocytosis and Aseptic Meningitis Induced by Sulfamethoxazole-Trimethoprim
Pharmacist-Driven Deprescribing to Reduce Anticholinergic Burden in Veterans With Dementia
Pharmacist-Driven Deprescribing to Reduce Anticholinergic Burden in Veterans With Dementia
Anticholinergic medications block the activity of the neurotransmitter acetylcholine by binding to either muscarinic or nicotinic receptors in both the peripheral and central nervous system. Anticholinergic medications typically refer to antimuscarinic medications and have been prescribed to treat a variety of conditions common in older adults, including overactive bladder, allergies, muscle spasms, and sleep disorders.1,2 Since muscarinic receptors are present throughout the body, anticholinergic medications are associated with many adverse effects (AEs), including constipation, urinary retention, xerostomia, and delirium. Older adults are more sensitive to these AEs due to physiological changes associated with aging.1
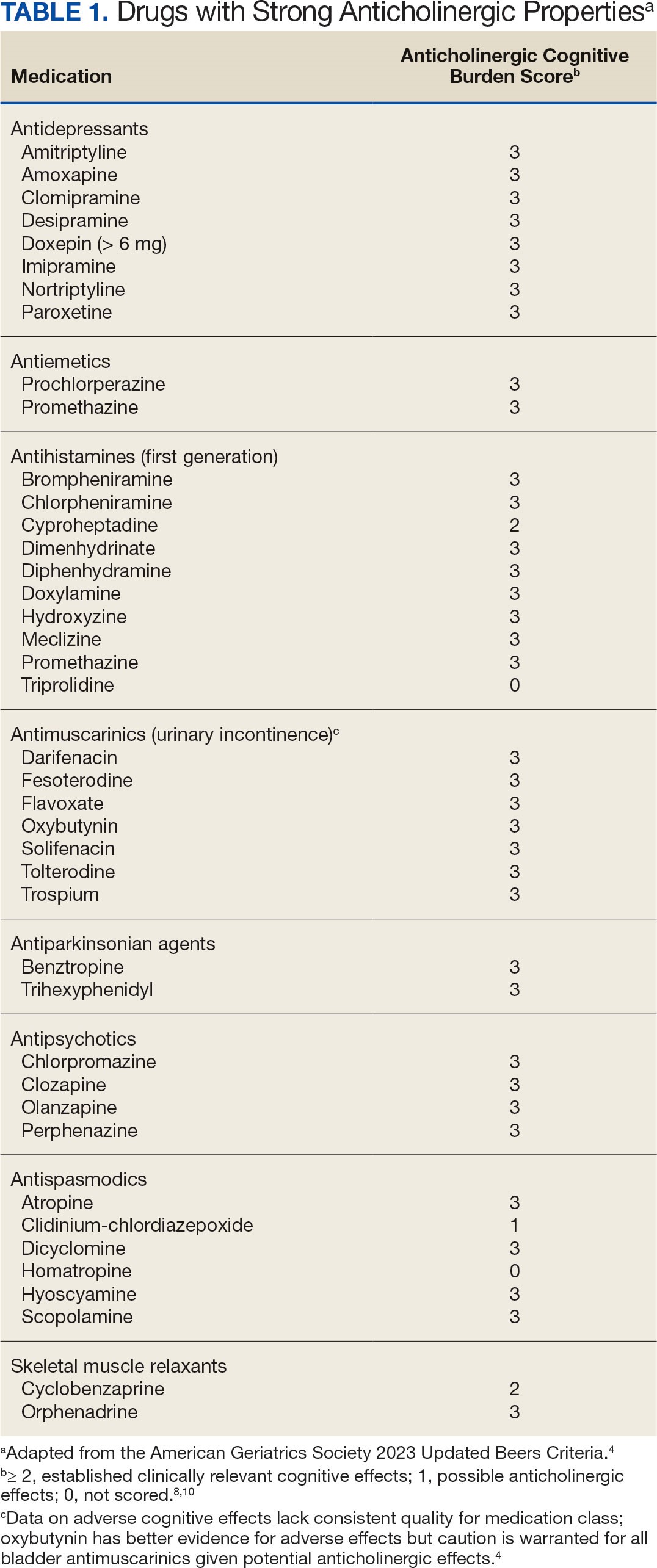
The American Geriatric Society Beers Criteria for Potentially Inappropriate Medications Use in Older Adults identifies drugs with strong anticholinergic properties. The Beers Criteria strongly recommends avoiding these medications in patients with dementia or cognitive impairment due to the risk of central nervous system AEs. In the updated 2023 Beers Criteria, the rationale was expanded to recognize the risks of the cumulative anticholinergic burden associated with concurrent anticholinergic use.3,4
Given the prevalent use of anticholinergic medications in older adults, there has been significant research demonstrating their AEs, specifically delirium and cognitive impairment in geriatric patients. A systematic review of 14 articles conducted in 7 different countries of patients with median age of 76.4 to 86.1 years reviewed clinical outcomes of anticholinergic use in patients with dementia. Five studies found anticholinergics were associated with increased all-cause mortality in patients with dementia, and 3 studies found anticholinergics were associated with longer hospital stays. Other studies found that anticholinergics were associated with delirium and reduced health-related quality of life.5
About 35% of veterans with dementia have been prescribed a medication regimen with a high anticholinergic burden.6 In 2018, the US Department of Veterans Affairs (VA) Pharmacy Benfits Management Center for Medical Safety completed a centrally aggregated medication use evaluation (CAMUE) to assess the appropriateness of anticholinergic medication use in patients with dementia. The retrospective chart review included 1094 veterans from 19 sites. Overall, about 15% of the veterans experienced new falls, delirium, or worsening dementia within 30 days of starting an anticholinergic medication. Furthermore, < 40% had documentation of a nonanticholinergic alternative medication trial, and < 20% had documented nonpharmacologic therapy. The documentation of risk-benefit assessment acknowledging the risks of anticholinergic medication use in veterans with dementia occurred only about 13% of the time. The CAMUE concluded that the risks of initiating an anticholinergic medication in veterans with dementia are likely underdocumented and possibly under considered by prescribers.7
Developed within the Veterans Health Administration (VHA), VIONE (Vital, Important, Optional, Not Indicated, Every medication has an indication) is a medication management methodology that aims to reduce polypharmacy and improve patient safety consistent with high-reliability organizations. Since it launched in 2016, VIONE has gradually been implemented at many VHA facilities. The VIONE deprescribing dashboard had not been used at the VA Louisville Healthcare System prior to this quality improvement project.
This dashboard uses the Beers Criteria to identify potentially inappropriate anticholinergic medications. It uses the Anticholinergic Cognitive Burden (ACB) scale to calculate the cumulative anticholinergic risk for each patient. Medications with an ACB score of 2 or 3 have clinically relevant cognitive effects such as delirium and dementia (Table 1). For each point increase in total ACB score, a decline in mini-mental state examination score of 0.33 points over 2 years has been shown. Each point increase has also been correlated with a 26% increase in risk of death.8-10
Methods
The purpose of this quality improvement project was to determine the impact of pharmacist-driven deprescribing on the anticholinergic burden in veterans with dementia at VA Louisville Healthcare System. Data were obtained through the Computerized Patient Record System (CPRS) and VIONE deprescribing dashboard and entered in a secure Microsoft Excel spreadsheet. Pharmacist deprescribing steps were entered as CPRS progress notes. A deprescribing note template was created, and 11 templates with indication-specific recommendations were created for each anticholinergic indication identified (contact authors for deprescribing note template examples). Usage of anticholinergic medications was reexamined 3 months after the deprescribing note was entered.
Eligible patients identified in the VIONE deprescribing dashboard had an outpatient order for a medication with strong anticholinergic properties as identified using the Beers Criteria and were aged ≥ 65 years. Patients also had to be diagnosed with dementia or cognitive impairment. Patients were excluded if they were receiving hospice care or if the anticholinergic medication was from a non-VA prescriber or filled at a non-VA pharmacy. The VIONE deprescribing dashboard also excluded skeletal muscle relaxants if the patient had a spinal cord-related visit in the previous 2 years, first-generation antihistamines if the patient had a vertigo diagnosis, hydroxyzine if the indication was for anxiety, trospium if the indication was for overactive bladder, and antipsychotics if the patient had been diagnosed with schizophrenia or bipolar disorder. The following were included in the deprescribing recommendations if the dashboard identified the patient due to receiving a second strongly anticholinergic medication: first generation antihistamines if the patient was diagnosed with vertigo and hydroxyzine if the indication is for anxiety.
Each eligible patient received a focused medication review by a pharmacist via electronic chart review and a templated CPRS progress note with patient-specific recommendations. The prescriber and the patient’s primary care practitioner were recommended to perform a patient-specific risk-benefit assessment, deprescribe potentially inappropriate anticholinergic medications, and consider nonanticholinergic alternatives (both pharmacologic and nonpharmacologic). Data collected included baseline age, sex, prespecified comorbidities (type of dementia, cognitive impairment, delirium, benign prostatic hyperplasia/lower urinary tract symptoms), duration of prescribed anticholinergic medication, indication and deprescribing rate for each anticholinergic agent, and concurrent dementia medications (acetylcholinesterase inhibitors, memantine, or both).
The primary outcome was the number of patients that had = 1 medication with strong anticholinergic properties deprescribed. Deprescribing was defined as medication discontinuation or reduction of total daily dose. Secondary outcomes were the mean change in ACB scale, the number of patients with dose tapering, documented patient-specific risk-benefit assessment, and initiated nonanticholinergic alternative per pharmacist recommendation.
Results
The VIONE deprescribing dashboard identified 121 patients; 45 were excluded for non-VA prescriber or pharmacy, and 8 patients were excluded for other reasons. Sixty-eight patients were included in the deprescribing initiative. The mean age was 73.4 years (range, 67-93), 65 (96%) were male, and 34 (50%) had unspecified dementia (Table 2). Thirty-one patients (46%) had concurrent cholinesterase inhibitor prescriptions for dementia. The median duration of use of a strong anticholinergic medication was 11 months.
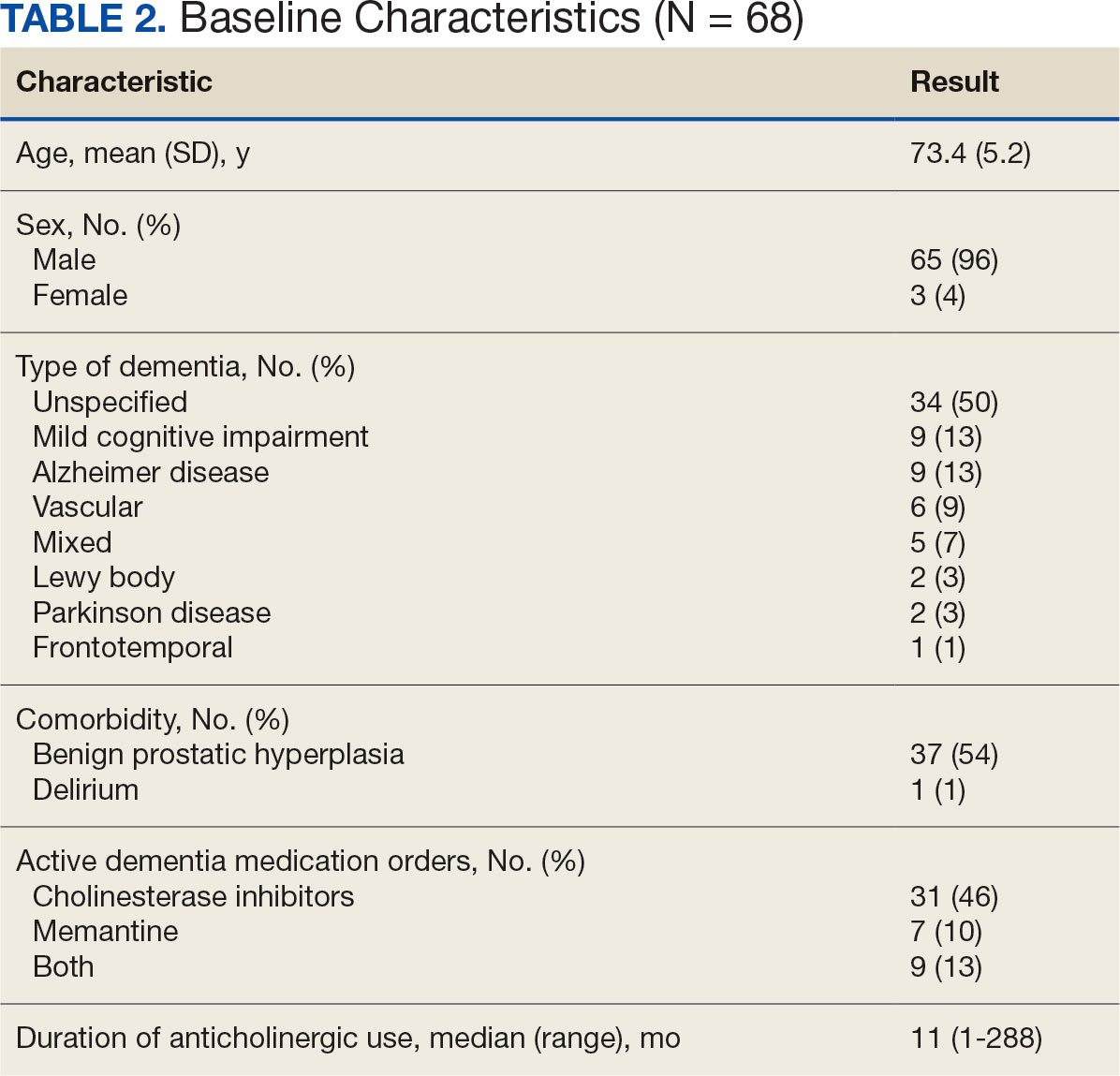
Twenty-nine patients (43%) had ≥ 1 medication with strong anticholinergic properties deprescribed. Anticholinergic medication was discontinued for 26 patients, and the dose was decreased for 3 patients. ACB score fell by a mean of 1.1 per patient. There was an increase in the documented risk-benefit assessment for anticholinergic medications from a baseline of 4 (6%) to 19 (28%) 3 months after the deprescribing note. Cyclobenzaprine, paroxetine, and oxybutynin were deprescribed the most, and amitriptyline had the lowest rate of deprescribing (Table 3). Thirty patients (44%) had a pharmacologic, nonanticholinergic alternative initiated per pharmacist recommendation, and 6 patients (9%) had a nonpharmacologic alternative initiated per pharmacist recommendation.
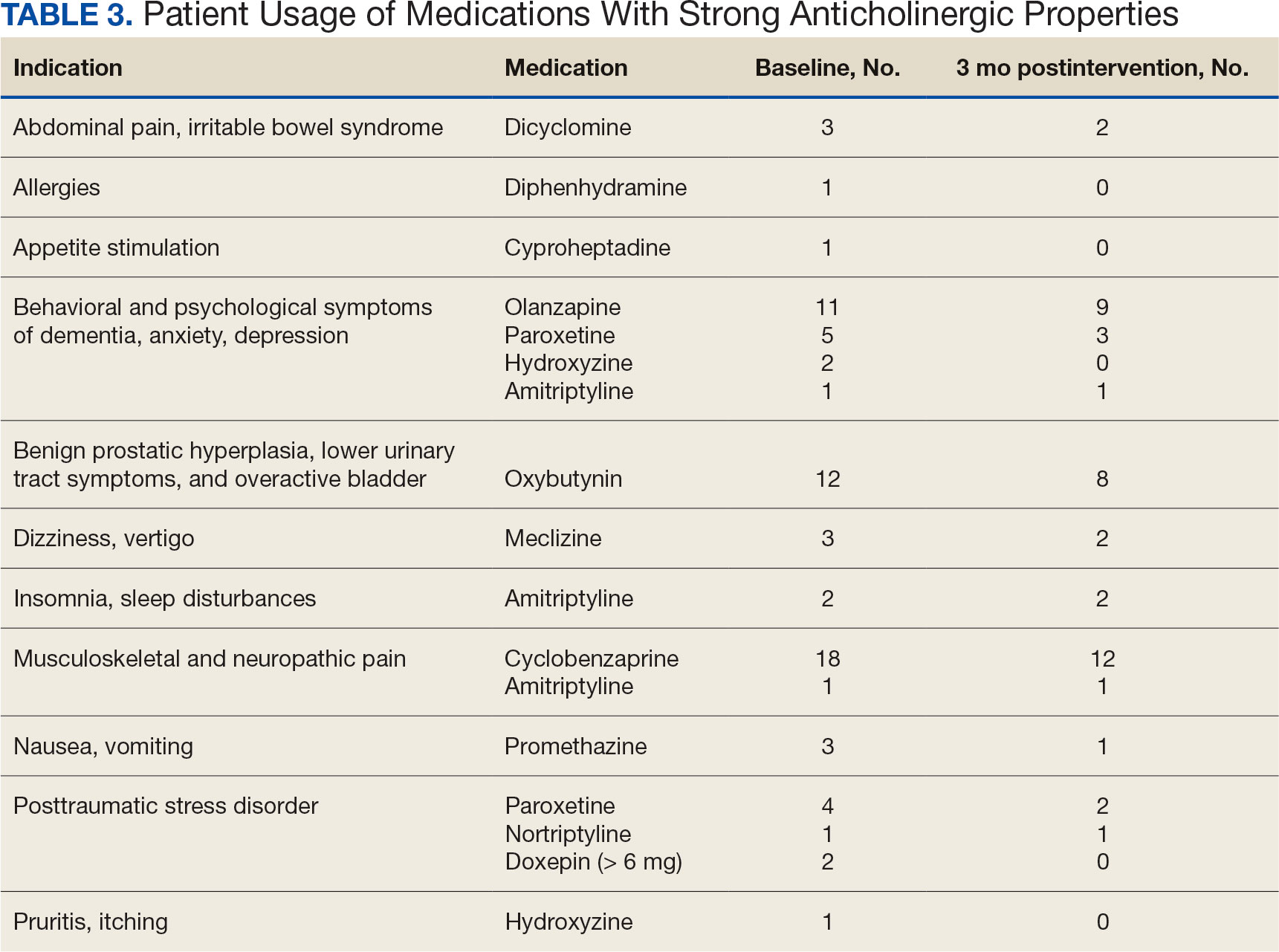
Discussion
This quality improvement project suggests that with the use of population health management tools such as the VIONE deprescribing dashboard, pharmacists can help identify and deprescribe strong anticholinergic medications in patients with cognitive impairment or dementia. Pharmacists can also aid in deprescribing through evidence-based recommendations to guide risk-benefit discussion and consider safer, nonanticholinergic alternatives. The authors were able to help reduce anticholinergic cognitive burden in 43% of patients in this sample. The mean 1.1 ACB score reduction was considered clinically significant based on prior studies that found that each 1-point increase in ACB score correlated with declined cognition and increased mortality.8,10 The VIONE deprescribing dashboard provided real-time patient data and helped target patients at the highest risk of anticholinergic AEs. The creation of the note templates based on the indication helped streamline recommendations. Typically, the prescriber addressed the recommendations at a routine follow-up appointment. The deprescribing method used in this project was time-efficient and could be easily replicated once the CPRS note templates were created. Future deprescribing projects could consider more direct pharmacist intervention and medication management.
Limitations
There was no direct assessment of clinical outcomes such as change in cognition using cognitive function tests. However, multiple studies have demonstrated AEs associated with strong anticholinergic medication use and additive anticholinergic burden in patients with dementia or cognitive impairment.1,5 Also, the 3-month follow-up period was relatively short. The pharmacist’s deprescribing recommendations may have been accepted after 3 months, or patients could have restarted their anticholinergic medications. Longer follow-up time could provide more robust results and conclusions. Thirdly, there was no formal definition of what constituted a risk-benefit assessment of anticholinergic medications. The risk-benefit assessment was determined at the discretion of the authors, which was subjective and allowed for bias. Finally, 6 patients died during the 3-month follow-up. The data for these patients were included in the baseline characteristics but not in the study outcomes. If these patients had been excluded from the results, a higher percentage of patients (47%) would have had ≥ 1 anticholinergic medication deprescribed.
Conclusions
In collaboration with the interdisciplinary team, pharmacist recommendations resulted in deprescribing of anticholinergic medications in veterans with dementia or cognitive impairment. The VIONE deprescribing dashboard, an easily accessible population health management tool, can identify patients prescribed potentially inappropriate medications and help target patients at the highest risk of anticholinergic AEs. To prevent worsening cognitive impairment, delirium, falls, and other AEs, this deprescribing initiative can be replicated at other VHA facilities. Future projects could have a longer follow-up period, incorporate more direct pharmacist intervention, and assess clinical outcomes of deprescribing.
- Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Saf. 2016;7(5):217-224. doi:10.1177/2042098616658399
- Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain - how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151-159. doi:10.1111/bcpt.12140
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Wang K, Alan J, Page AT, Dimopoulos E, Etherton-Beer C. Anticholinergics and clinical outcomes amongst people with pre-existing dementia: a systematic review. Maturitas. 2021;151:1-14. doi:10.1016/j.maturitas.2021.06.004
- Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
- McCarren M, Burk M, Carico R, Glassman P, Good CB, Cunningham F. Design of a centrally aggregated medication use evaluation (CAMUE): anticholinergics in dementia. Presented at: 2019 HSR&D/QUERI National Conference; October 29-31, 2019; Washington, DC. https://www.hsrd.research.va.gov/meetings/2019/abstract-display.cfm?AbsNum=4027
- Boustani, M, Campbell, N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311-320. doi:10.2217/1745509.x
- Constantino-Corpuz JK, Alonso MTD. Assessment of a medication deprescribing tool on polypharmacy and cost avoidance. Fed Pract. 2021;38(7):332-336. doi:10.12788/fp.0146
- Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477-1483. doi:10.1111/j.1532-5415.2011.03491.x
Anticholinergic medications block the activity of the neurotransmitter acetylcholine by binding to either muscarinic or nicotinic receptors in both the peripheral and central nervous system. Anticholinergic medications typically refer to antimuscarinic medications and have been prescribed to treat a variety of conditions common in older adults, including overactive bladder, allergies, muscle spasms, and sleep disorders.1,2 Since muscarinic receptors are present throughout the body, anticholinergic medications are associated with many adverse effects (AEs), including constipation, urinary retention, xerostomia, and delirium. Older adults are more sensitive to these AEs due to physiological changes associated with aging.1
The American Geriatric Society Beers Criteria for Potentially Inappropriate Medications Use in Older Adults identifies drugs with strong anticholinergic properties. The Beers Criteria strongly recommends avoiding these medications in patients with dementia or cognitive impairment due to the risk of central nervous system AEs. In the updated 2023 Beers Criteria, the rationale was expanded to recognize the risks of the cumulative anticholinergic burden associated with concurrent anticholinergic use.3,4
Given the prevalent use of anticholinergic medications in older adults, there has been significant research demonstrating their AEs, specifically delirium and cognitive impairment in geriatric patients. A systematic review of 14 articles conducted in 7 different countries of patients with median age of 76.4 to 86.1 years reviewed clinical outcomes of anticholinergic use in patients with dementia. Five studies found anticholinergics were associated with increased all-cause mortality in patients with dementia, and 3 studies found anticholinergics were associated with longer hospital stays. Other studies found that anticholinergics were associated with delirium and reduced health-related quality of life.5
About 35% of veterans with dementia have been prescribed a medication regimen with a high anticholinergic burden.6 In 2018, the US Department of Veterans Affairs (VA) Pharmacy Benfits Management Center for Medical Safety completed a centrally aggregated medication use evaluation (CAMUE) to assess the appropriateness of anticholinergic medication use in patients with dementia. The retrospective chart review included 1094 veterans from 19 sites. Overall, about 15% of the veterans experienced new falls, delirium, or worsening dementia within 30 days of starting an anticholinergic medication. Furthermore, < 40% had documentation of a nonanticholinergic alternative medication trial, and < 20% had documented nonpharmacologic therapy. The documentation of risk-benefit assessment acknowledging the risks of anticholinergic medication use in veterans with dementia occurred only about 13% of the time. The CAMUE concluded that the risks of initiating an anticholinergic medication in veterans with dementia are likely underdocumented and possibly under considered by prescribers.7
Developed within the Veterans Health Administration (VHA), VIONE (Vital, Important, Optional, Not Indicated, Every medication has an indication) is a medication management methodology that aims to reduce polypharmacy and improve patient safety consistent with high-reliability organizations. Since it launched in 2016, VIONE has gradually been implemented at many VHA facilities. The VIONE deprescribing dashboard had not been used at the VA Louisville Healthcare System prior to this quality improvement project.
This dashboard uses the Beers Criteria to identify potentially inappropriate anticholinergic medications. It uses the Anticholinergic Cognitive Burden (ACB) scale to calculate the cumulative anticholinergic risk for each patient. Medications with an ACB score of 2 or 3 have clinically relevant cognitive effects such as delirium and dementia (Table 1). For each point increase in total ACB score, a decline in mini-mental state examination score of 0.33 points over 2 years has been shown. Each point increase has also been correlated with a 26% increase in risk of death.8-10

Methods
The purpose of this quality improvement project was to determine the impact of pharmacist-driven deprescribing on the anticholinergic burden in veterans with dementia at VA Louisville Healthcare System. Data were obtained through the Computerized Patient Record System (CPRS) and VIONE deprescribing dashboard and entered in a secure Microsoft Excel spreadsheet. Pharmacist deprescribing steps were entered as CPRS progress notes. A deprescribing note template was created, and 11 templates with indication-specific recommendations were created for each anticholinergic indication identified (contact authors for deprescribing note template examples). Usage of anticholinergic medications was reexamined 3 months after the deprescribing note was entered.
Eligible patients identified in the VIONE deprescribing dashboard had an outpatient order for a medication with strong anticholinergic properties as identified using the Beers Criteria and were aged ≥ 65 years. Patients also had to be diagnosed with dementia or cognitive impairment. Patients were excluded if they were receiving hospice care or if the anticholinergic medication was from a non-VA prescriber or filled at a non-VA pharmacy. The VIONE deprescribing dashboard also excluded skeletal muscle relaxants if the patient had a spinal cord-related visit in the previous 2 years, first-generation antihistamines if the patient had a vertigo diagnosis, hydroxyzine if the indication was for anxiety, trospium if the indication was for overactive bladder, and antipsychotics if the patient had been diagnosed with schizophrenia or bipolar disorder. The following were included in the deprescribing recommendations if the dashboard identified the patient due to receiving a second strongly anticholinergic medication: first generation antihistamines if the patient was diagnosed with vertigo and hydroxyzine if the indication is for anxiety.
Each eligible patient received a focused medication review by a pharmacist via electronic chart review and a templated CPRS progress note with patient-specific recommendations. The prescriber and the patient’s primary care practitioner were recommended to perform a patient-specific risk-benefit assessment, deprescribe potentially inappropriate anticholinergic medications, and consider nonanticholinergic alternatives (both pharmacologic and nonpharmacologic). Data collected included baseline age, sex, prespecified comorbidities (type of dementia, cognitive impairment, delirium, benign prostatic hyperplasia/lower urinary tract symptoms), duration of prescribed anticholinergic medication, indication and deprescribing rate for each anticholinergic agent, and concurrent dementia medications (acetylcholinesterase inhibitors, memantine, or both).
The primary outcome was the number of patients that had = 1 medication with strong anticholinergic properties deprescribed. Deprescribing was defined as medication discontinuation or reduction of total daily dose. Secondary outcomes were the mean change in ACB scale, the number of patients with dose tapering, documented patient-specific risk-benefit assessment, and initiated nonanticholinergic alternative per pharmacist recommendation.
Results
The VIONE deprescribing dashboard identified 121 patients; 45 were excluded for non-VA prescriber or pharmacy, and 8 patients were excluded for other reasons. Sixty-eight patients were included in the deprescribing initiative. The mean age was 73.4 years (range, 67-93), 65 (96%) were male, and 34 (50%) had unspecified dementia (Table 2). Thirty-one patients (46%) had concurrent cholinesterase inhibitor prescriptions for dementia. The median duration of use of a strong anticholinergic medication was 11 months.

Twenty-nine patients (43%) had ≥ 1 medication with strong anticholinergic properties deprescribed. Anticholinergic medication was discontinued for 26 patients, and the dose was decreased for 3 patients. ACB score fell by a mean of 1.1 per patient. There was an increase in the documented risk-benefit assessment for anticholinergic medications from a baseline of 4 (6%) to 19 (28%) 3 months after the deprescribing note. Cyclobenzaprine, paroxetine, and oxybutynin were deprescribed the most, and amitriptyline had the lowest rate of deprescribing (Table 3). Thirty patients (44%) had a pharmacologic, nonanticholinergic alternative initiated per pharmacist recommendation, and 6 patients (9%) had a nonpharmacologic alternative initiated per pharmacist recommendation.

Discussion
This quality improvement project suggests that with the use of population health management tools such as the VIONE deprescribing dashboard, pharmacists can help identify and deprescribe strong anticholinergic medications in patients with cognitive impairment or dementia. Pharmacists can also aid in deprescribing through evidence-based recommendations to guide risk-benefit discussion and consider safer, nonanticholinergic alternatives. The authors were able to help reduce anticholinergic cognitive burden in 43% of patients in this sample. The mean 1.1 ACB score reduction was considered clinically significant based on prior studies that found that each 1-point increase in ACB score correlated with declined cognition and increased mortality.8,10 The VIONE deprescribing dashboard provided real-time patient data and helped target patients at the highest risk of anticholinergic AEs. The creation of the note templates based on the indication helped streamline recommendations. Typically, the prescriber addressed the recommendations at a routine follow-up appointment. The deprescribing method used in this project was time-efficient and could be easily replicated once the CPRS note templates were created. Future deprescribing projects could consider more direct pharmacist intervention and medication management.
Limitations
There was no direct assessment of clinical outcomes such as change in cognition using cognitive function tests. However, multiple studies have demonstrated AEs associated with strong anticholinergic medication use and additive anticholinergic burden in patients with dementia or cognitive impairment.1,5 Also, the 3-month follow-up period was relatively short. The pharmacist’s deprescribing recommendations may have been accepted after 3 months, or patients could have restarted their anticholinergic medications. Longer follow-up time could provide more robust results and conclusions. Thirdly, there was no formal definition of what constituted a risk-benefit assessment of anticholinergic medications. The risk-benefit assessment was determined at the discretion of the authors, which was subjective and allowed for bias. Finally, 6 patients died during the 3-month follow-up. The data for these patients were included in the baseline characteristics but not in the study outcomes. If these patients had been excluded from the results, a higher percentage of patients (47%) would have had ≥ 1 anticholinergic medication deprescribed.
Conclusions
In collaboration with the interdisciplinary team, pharmacist recommendations resulted in deprescribing of anticholinergic medications in veterans with dementia or cognitive impairment. The VIONE deprescribing dashboard, an easily accessible population health management tool, can identify patients prescribed potentially inappropriate medications and help target patients at the highest risk of anticholinergic AEs. To prevent worsening cognitive impairment, delirium, falls, and other AEs, this deprescribing initiative can be replicated at other VHA facilities. Future projects could have a longer follow-up period, incorporate more direct pharmacist intervention, and assess clinical outcomes of deprescribing.
Anticholinergic medications block the activity of the neurotransmitter acetylcholine by binding to either muscarinic or nicotinic receptors in both the peripheral and central nervous system. Anticholinergic medications typically refer to antimuscarinic medications and have been prescribed to treat a variety of conditions common in older adults, including overactive bladder, allergies, muscle spasms, and sleep disorders.1,2 Since muscarinic receptors are present throughout the body, anticholinergic medications are associated with many adverse effects (AEs), including constipation, urinary retention, xerostomia, and delirium. Older adults are more sensitive to these AEs due to physiological changes associated with aging.1
The American Geriatric Society Beers Criteria for Potentially Inappropriate Medications Use in Older Adults identifies drugs with strong anticholinergic properties. The Beers Criteria strongly recommends avoiding these medications in patients with dementia or cognitive impairment due to the risk of central nervous system AEs. In the updated 2023 Beers Criteria, the rationale was expanded to recognize the risks of the cumulative anticholinergic burden associated with concurrent anticholinergic use.3,4
Given the prevalent use of anticholinergic medications in older adults, there has been significant research demonstrating their AEs, specifically delirium and cognitive impairment in geriatric patients. A systematic review of 14 articles conducted in 7 different countries of patients with median age of 76.4 to 86.1 years reviewed clinical outcomes of anticholinergic use in patients with dementia. Five studies found anticholinergics were associated with increased all-cause mortality in patients with dementia, and 3 studies found anticholinergics were associated with longer hospital stays. Other studies found that anticholinergics were associated with delirium and reduced health-related quality of life.5
About 35% of veterans with dementia have been prescribed a medication regimen with a high anticholinergic burden.6 In 2018, the US Department of Veterans Affairs (VA) Pharmacy Benfits Management Center for Medical Safety completed a centrally aggregated medication use evaluation (CAMUE) to assess the appropriateness of anticholinergic medication use in patients with dementia. The retrospective chart review included 1094 veterans from 19 sites. Overall, about 15% of the veterans experienced new falls, delirium, or worsening dementia within 30 days of starting an anticholinergic medication. Furthermore, < 40% had documentation of a nonanticholinergic alternative medication trial, and < 20% had documented nonpharmacologic therapy. The documentation of risk-benefit assessment acknowledging the risks of anticholinergic medication use in veterans with dementia occurred only about 13% of the time. The CAMUE concluded that the risks of initiating an anticholinergic medication in veterans with dementia are likely underdocumented and possibly under considered by prescribers.7
Developed within the Veterans Health Administration (VHA), VIONE (Vital, Important, Optional, Not Indicated, Every medication has an indication) is a medication management methodology that aims to reduce polypharmacy and improve patient safety consistent with high-reliability organizations. Since it launched in 2016, VIONE has gradually been implemented at many VHA facilities. The VIONE deprescribing dashboard had not been used at the VA Louisville Healthcare System prior to this quality improvement project.
This dashboard uses the Beers Criteria to identify potentially inappropriate anticholinergic medications. It uses the Anticholinergic Cognitive Burden (ACB) scale to calculate the cumulative anticholinergic risk for each patient. Medications with an ACB score of 2 or 3 have clinically relevant cognitive effects such as delirium and dementia (Table 1). For each point increase in total ACB score, a decline in mini-mental state examination score of 0.33 points over 2 years has been shown. Each point increase has also been correlated with a 26% increase in risk of death.8-10

Methods
The purpose of this quality improvement project was to determine the impact of pharmacist-driven deprescribing on the anticholinergic burden in veterans with dementia at VA Louisville Healthcare System. Data were obtained through the Computerized Patient Record System (CPRS) and VIONE deprescribing dashboard and entered in a secure Microsoft Excel spreadsheet. Pharmacist deprescribing steps were entered as CPRS progress notes. A deprescribing note template was created, and 11 templates with indication-specific recommendations were created for each anticholinergic indication identified (contact authors for deprescribing note template examples). Usage of anticholinergic medications was reexamined 3 months after the deprescribing note was entered.
Eligible patients identified in the VIONE deprescribing dashboard had an outpatient order for a medication with strong anticholinergic properties as identified using the Beers Criteria and were aged ≥ 65 years. Patients also had to be diagnosed with dementia or cognitive impairment. Patients were excluded if they were receiving hospice care or if the anticholinergic medication was from a non-VA prescriber or filled at a non-VA pharmacy. The VIONE deprescribing dashboard also excluded skeletal muscle relaxants if the patient had a spinal cord-related visit in the previous 2 years, first-generation antihistamines if the patient had a vertigo diagnosis, hydroxyzine if the indication was for anxiety, trospium if the indication was for overactive bladder, and antipsychotics if the patient had been diagnosed with schizophrenia or bipolar disorder. The following were included in the deprescribing recommendations if the dashboard identified the patient due to receiving a second strongly anticholinergic medication: first generation antihistamines if the patient was diagnosed with vertigo and hydroxyzine if the indication is for anxiety.
Each eligible patient received a focused medication review by a pharmacist via electronic chart review and a templated CPRS progress note with patient-specific recommendations. The prescriber and the patient’s primary care practitioner were recommended to perform a patient-specific risk-benefit assessment, deprescribe potentially inappropriate anticholinergic medications, and consider nonanticholinergic alternatives (both pharmacologic and nonpharmacologic). Data collected included baseline age, sex, prespecified comorbidities (type of dementia, cognitive impairment, delirium, benign prostatic hyperplasia/lower urinary tract symptoms), duration of prescribed anticholinergic medication, indication and deprescribing rate for each anticholinergic agent, and concurrent dementia medications (acetylcholinesterase inhibitors, memantine, or both).
The primary outcome was the number of patients that had = 1 medication with strong anticholinergic properties deprescribed. Deprescribing was defined as medication discontinuation or reduction of total daily dose. Secondary outcomes were the mean change in ACB scale, the number of patients with dose tapering, documented patient-specific risk-benefit assessment, and initiated nonanticholinergic alternative per pharmacist recommendation.
Results
The VIONE deprescribing dashboard identified 121 patients; 45 were excluded for non-VA prescriber or pharmacy, and 8 patients were excluded for other reasons. Sixty-eight patients were included in the deprescribing initiative. The mean age was 73.4 years (range, 67-93), 65 (96%) were male, and 34 (50%) had unspecified dementia (Table 2). Thirty-one patients (46%) had concurrent cholinesterase inhibitor prescriptions for dementia. The median duration of use of a strong anticholinergic medication was 11 months.

Twenty-nine patients (43%) had ≥ 1 medication with strong anticholinergic properties deprescribed. Anticholinergic medication was discontinued for 26 patients, and the dose was decreased for 3 patients. ACB score fell by a mean of 1.1 per patient. There was an increase in the documented risk-benefit assessment for anticholinergic medications from a baseline of 4 (6%) to 19 (28%) 3 months after the deprescribing note. Cyclobenzaprine, paroxetine, and oxybutynin were deprescribed the most, and amitriptyline had the lowest rate of deprescribing (Table 3). Thirty patients (44%) had a pharmacologic, nonanticholinergic alternative initiated per pharmacist recommendation, and 6 patients (9%) had a nonpharmacologic alternative initiated per pharmacist recommendation.

Discussion
This quality improvement project suggests that with the use of population health management tools such as the VIONE deprescribing dashboard, pharmacists can help identify and deprescribe strong anticholinergic medications in patients with cognitive impairment or dementia. Pharmacists can also aid in deprescribing through evidence-based recommendations to guide risk-benefit discussion and consider safer, nonanticholinergic alternatives. The authors were able to help reduce anticholinergic cognitive burden in 43% of patients in this sample. The mean 1.1 ACB score reduction was considered clinically significant based on prior studies that found that each 1-point increase in ACB score correlated with declined cognition and increased mortality.8,10 The VIONE deprescribing dashboard provided real-time patient data and helped target patients at the highest risk of anticholinergic AEs. The creation of the note templates based on the indication helped streamline recommendations. Typically, the prescriber addressed the recommendations at a routine follow-up appointment. The deprescribing method used in this project was time-efficient and could be easily replicated once the CPRS note templates were created. Future deprescribing projects could consider more direct pharmacist intervention and medication management.
Limitations
There was no direct assessment of clinical outcomes such as change in cognition using cognitive function tests. However, multiple studies have demonstrated AEs associated with strong anticholinergic medication use and additive anticholinergic burden in patients with dementia or cognitive impairment.1,5 Also, the 3-month follow-up period was relatively short. The pharmacist’s deprescribing recommendations may have been accepted after 3 months, or patients could have restarted their anticholinergic medications. Longer follow-up time could provide more robust results and conclusions. Thirdly, there was no formal definition of what constituted a risk-benefit assessment of anticholinergic medications. The risk-benefit assessment was determined at the discretion of the authors, which was subjective and allowed for bias. Finally, 6 patients died during the 3-month follow-up. The data for these patients were included in the baseline characteristics but not in the study outcomes. If these patients had been excluded from the results, a higher percentage of patients (47%) would have had ≥ 1 anticholinergic medication deprescribed.
Conclusions
In collaboration with the interdisciplinary team, pharmacist recommendations resulted in deprescribing of anticholinergic medications in veterans with dementia or cognitive impairment. The VIONE deprescribing dashboard, an easily accessible population health management tool, can identify patients prescribed potentially inappropriate medications and help target patients at the highest risk of anticholinergic AEs. To prevent worsening cognitive impairment, delirium, falls, and other AEs, this deprescribing initiative can be replicated at other VHA facilities. Future projects could have a longer follow-up period, incorporate more direct pharmacist intervention, and assess clinical outcomes of deprescribing.
- Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Saf. 2016;7(5):217-224. doi:10.1177/2042098616658399
- Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain - how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151-159. doi:10.1111/bcpt.12140
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Wang K, Alan J, Page AT, Dimopoulos E, Etherton-Beer C. Anticholinergics and clinical outcomes amongst people with pre-existing dementia: a systematic review. Maturitas. 2021;151:1-14. doi:10.1016/j.maturitas.2021.06.004
- Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
- McCarren M, Burk M, Carico R, Glassman P, Good CB, Cunningham F. Design of a centrally aggregated medication use evaluation (CAMUE): anticholinergics in dementia. Presented at: 2019 HSR&D/QUERI National Conference; October 29-31, 2019; Washington, DC. https://www.hsrd.research.va.gov/meetings/2019/abstract-display.cfm?AbsNum=4027
- Boustani, M, Campbell, N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311-320. doi:10.2217/1745509.x
- Constantino-Corpuz JK, Alonso MTD. Assessment of a medication deprescribing tool on polypharmacy and cost avoidance. Fed Pract. 2021;38(7):332-336. doi:10.12788/fp.0146
- Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477-1483. doi:10.1111/j.1532-5415.2011.03491.x
- Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Saf. 2016;7(5):217-224. doi:10.1177/2042098616658399
- Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain - how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151-159. doi:10.1111/bcpt.12140
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Wang K, Alan J, Page AT, Dimopoulos E, Etherton-Beer C. Anticholinergics and clinical outcomes amongst people with pre-existing dementia: a systematic review. Maturitas. 2021;151:1-14. doi:10.1016/j.maturitas.2021.06.004
- Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
- McCarren M, Burk M, Carico R, Glassman P, Good CB, Cunningham F. Design of a centrally aggregated medication use evaluation (CAMUE): anticholinergics in dementia. Presented at: 2019 HSR&D/QUERI National Conference; October 29-31, 2019; Washington, DC. https://www.hsrd.research.va.gov/meetings/2019/abstract-display.cfm?AbsNum=4027
- Boustani, M, Campbell, N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311-320. doi:10.2217/1745509.x
- Constantino-Corpuz JK, Alonso MTD. Assessment of a medication deprescribing tool on polypharmacy and cost avoidance. Fed Pract. 2021;38(7):332-336. doi:10.12788/fp.0146
- Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477-1483. doi:10.1111/j.1532-5415.2011.03491.x
Pharmacist-Driven Deprescribing to Reduce Anticholinergic Burden in Veterans With Dementia
Pharmacist-Driven Deprescribing to Reduce Anticholinergic Burden in Veterans With Dementia
Common Herbicide a Player in Neurodegeneration?
new research showed.
Researchers found that glyphosate exposure even at regulated levels was associated with increased neuroinflammation and accelerated Alzheimer’s disease–like pathology in mice — an effect that persisted 6 months after a recovery period when exposure was stopped.
“More research is needed to understand the consequences of glyphosate exposure to the brain in humans and to understand the appropriate dose of exposure to limit detrimental outcomes,” said co–senior author Ramon Velazquez, PhD, with Arizona State University, Tempe.
The study was published online in The Journal of Neuroinflammation.
Persistent Accumulation Within the Brain
Glyphosate is the most heavily applied herbicide in the United States, with roughly 300 million pounds used annually in agricultural communities throughout the United States. It is also used for weed control in parks, residential areas, and personal gardens.
The Environmental Protection Agency (EPA) has determined that glyphosate poses no risks to human health when used as directed. But the World Health Organization’s International Agency for Research on Cancer disagrees, classifying the herbicide as “possibly carcinogenic to humans.”
In addition to the possible cancer risk, multiple reports have also suggested potential harmful effects of glyphosate exposure on the brain.
In earlier work, Velazquez and colleagues showed that glyphosate crosses the blood-brain barrier and infiltrates the brains of mice, contributing to neuroinflammation and other detrimental effects on brain function.
In their latest study, they examined the long-term effects of glyphosate exposure on neuroinflammation and Alzheimer’s disease–like pathology using a mouse model.
They dosed 4.5-month-old mice genetically predisposed to Alzheimer’s disease and non-transgenic control mice with either 0, 50, or 500 mg/kg of glyphosate daily for 13 weeks followed by a 6-month recovery period.
The high dose is similar to levels used in earlier research, and the low dose is close to the limit used to establish the current EPA acceptable dose in humans.
Glyphosate’s metabolite, aminomethylphosphonic acid, was detectable and persisted in mouse brain tissue even 6 months after exposure ceased, the researchers reported.
Additionally, there was a significant increase in soluble and insoluble fractions of amyloid-beta (Abeta), Abeta42 plaque load and plaque size, and phosphorylated tau at Threonine 181 and Serine 396 in hippocampus and cortex brain tissue from glyphosate-exposed mice, “highlighting an exacerbation of hallmark Alzheimer’s disease–like proteinopathies,” they noted.
Glyphosate exposure was also associated with significant elevations in both pro- and anti-inflammatory cytokines and chemokines in brain tissue of transgenic and normal mice and in peripheral blood plasma of transgenic mice.
Glyphosate-exposed transgenic mice also showed heightened anxiety-like behaviors and reduced survival.
“These findings highlight that many chemicals we regularly encounter, previously considered safe, may pose potential health risks,” co–senior author Patrick Pirrotte, PhD, with the Translational Genomics Research Institute, Phoenix, Arizona, said in a statement.
“However, further research is needed to fully assess the public health impact and identify safer alternatives,” Pirrotte added.
Funding for the study was provided by the National Institutes on Aging, National Cancer Institute and the Arizona State University (ASU) Biodesign Institute. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
new research showed.
Researchers found that glyphosate exposure even at regulated levels was associated with increased neuroinflammation and accelerated Alzheimer’s disease–like pathology in mice — an effect that persisted 6 months after a recovery period when exposure was stopped.
“More research is needed to understand the consequences of glyphosate exposure to the brain in humans and to understand the appropriate dose of exposure to limit detrimental outcomes,” said co–senior author Ramon Velazquez, PhD, with Arizona State University, Tempe.
The study was published online in The Journal of Neuroinflammation.
Persistent Accumulation Within the Brain
Glyphosate is the most heavily applied herbicide in the United States, with roughly 300 million pounds used annually in agricultural communities throughout the United States. It is also used for weed control in parks, residential areas, and personal gardens.
The Environmental Protection Agency (EPA) has determined that glyphosate poses no risks to human health when used as directed. But the World Health Organization’s International Agency for Research on Cancer disagrees, classifying the herbicide as “possibly carcinogenic to humans.”
In addition to the possible cancer risk, multiple reports have also suggested potential harmful effects of glyphosate exposure on the brain.
In earlier work, Velazquez and colleagues showed that glyphosate crosses the blood-brain barrier and infiltrates the brains of mice, contributing to neuroinflammation and other detrimental effects on brain function.
In their latest study, they examined the long-term effects of glyphosate exposure on neuroinflammation and Alzheimer’s disease–like pathology using a mouse model.
They dosed 4.5-month-old mice genetically predisposed to Alzheimer’s disease and non-transgenic control mice with either 0, 50, or 500 mg/kg of glyphosate daily for 13 weeks followed by a 6-month recovery period.
The high dose is similar to levels used in earlier research, and the low dose is close to the limit used to establish the current EPA acceptable dose in humans.
Glyphosate’s metabolite, aminomethylphosphonic acid, was detectable and persisted in mouse brain tissue even 6 months after exposure ceased, the researchers reported.
Additionally, there was a significant increase in soluble and insoluble fractions of amyloid-beta (Abeta), Abeta42 plaque load and plaque size, and phosphorylated tau at Threonine 181 and Serine 396 in hippocampus and cortex brain tissue from glyphosate-exposed mice, “highlighting an exacerbation of hallmark Alzheimer’s disease–like proteinopathies,” they noted.
Glyphosate exposure was also associated with significant elevations in both pro- and anti-inflammatory cytokines and chemokines in brain tissue of transgenic and normal mice and in peripheral blood plasma of transgenic mice.
Glyphosate-exposed transgenic mice also showed heightened anxiety-like behaviors and reduced survival.
“These findings highlight that many chemicals we regularly encounter, previously considered safe, may pose potential health risks,” co–senior author Patrick Pirrotte, PhD, with the Translational Genomics Research Institute, Phoenix, Arizona, said in a statement.
“However, further research is needed to fully assess the public health impact and identify safer alternatives,” Pirrotte added.
Funding for the study was provided by the National Institutes on Aging, National Cancer Institute and the Arizona State University (ASU) Biodesign Institute. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
new research showed.
Researchers found that glyphosate exposure even at regulated levels was associated with increased neuroinflammation and accelerated Alzheimer’s disease–like pathology in mice — an effect that persisted 6 months after a recovery period when exposure was stopped.
“More research is needed to understand the consequences of glyphosate exposure to the brain in humans and to understand the appropriate dose of exposure to limit detrimental outcomes,” said co–senior author Ramon Velazquez, PhD, with Arizona State University, Tempe.
The study was published online in The Journal of Neuroinflammation.
Persistent Accumulation Within the Brain
Glyphosate is the most heavily applied herbicide in the United States, with roughly 300 million pounds used annually in agricultural communities throughout the United States. It is also used for weed control in parks, residential areas, and personal gardens.
The Environmental Protection Agency (EPA) has determined that glyphosate poses no risks to human health when used as directed. But the World Health Organization’s International Agency for Research on Cancer disagrees, classifying the herbicide as “possibly carcinogenic to humans.”
In addition to the possible cancer risk, multiple reports have also suggested potential harmful effects of glyphosate exposure on the brain.
In earlier work, Velazquez and colleagues showed that glyphosate crosses the blood-brain barrier and infiltrates the brains of mice, contributing to neuroinflammation and other detrimental effects on brain function.
In their latest study, they examined the long-term effects of glyphosate exposure on neuroinflammation and Alzheimer’s disease–like pathology using a mouse model.
They dosed 4.5-month-old mice genetically predisposed to Alzheimer’s disease and non-transgenic control mice with either 0, 50, or 500 mg/kg of glyphosate daily for 13 weeks followed by a 6-month recovery period.
The high dose is similar to levels used in earlier research, and the low dose is close to the limit used to establish the current EPA acceptable dose in humans.
Glyphosate’s metabolite, aminomethylphosphonic acid, was detectable and persisted in mouse brain tissue even 6 months after exposure ceased, the researchers reported.
Additionally, there was a significant increase in soluble and insoluble fractions of amyloid-beta (Abeta), Abeta42 plaque load and plaque size, and phosphorylated tau at Threonine 181 and Serine 396 in hippocampus and cortex brain tissue from glyphosate-exposed mice, “highlighting an exacerbation of hallmark Alzheimer’s disease–like proteinopathies,” they noted.
Glyphosate exposure was also associated with significant elevations in both pro- and anti-inflammatory cytokines and chemokines in brain tissue of transgenic and normal mice and in peripheral blood plasma of transgenic mice.
Glyphosate-exposed transgenic mice also showed heightened anxiety-like behaviors and reduced survival.
“These findings highlight that many chemicals we regularly encounter, previously considered safe, may pose potential health risks,” co–senior author Patrick Pirrotte, PhD, with the Translational Genomics Research Institute, Phoenix, Arizona, said in a statement.
“However, further research is needed to fully assess the public health impact and identify safer alternatives,” Pirrotte added.
Funding for the study was provided by the National Institutes on Aging, National Cancer Institute and the Arizona State University (ASU) Biodesign Institute. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF NEUROINFLAMMATION
BCG Vaccine May Protect Against Long COVID Symptoms
TOPLINE:
METHODOLOGY:
- A phase 3 clinical trial initiated in early 2020 investigated the effect of the BCG vaccine injected during active infection on COVID-19 progression in adults with mild or moderate COVID-19. The current study summarizes the 6- and 12-month follow-up data with a focus on long-COVID symptoms.
- Patients who tested positive for severe acute respiratory syndrome coronavirus 2 were randomly assigned to receive either 0.1 mL of intradermal BCG (n = 191) or 0.9% saline placebo (n = 202) within 14 days of symptom onset and were followed up at 7, 14, 21, and 45 days and at 6 and 12 months postinjection.
- Overall, 157 BCG (median age, 40 years; 54.1% women) and 142 placebo (median age, 41 years; 65.5% women) recipients completed the 6-month follow-up, and 97 BCG (median age, 37 years; 49.5% women) and 95 placebo (median age, 40 years; 67.4% women) recipients completed the 12-month follow-up.
- The researchers primarily assessed the effect of the BCG vaccine on the development of the symptoms of long COVID at 6 and 12 months.
TAKEAWAY:
- Hearing problems were less frequent among BCG recipients at 6 months compared with those who received placebo (odds ratio [OR], 0.26; 95% CI, 0.045-1.0; P = .044).
- At 12 months, participants who received the BCG vaccine exhibited fewer issues with sleeping (P = .027), concentration (P = .009), memory (P = .009), and vision (P = .022) along with a lower long-COVID score (one-sided Wilcoxon test, P = .002) than those who received placebo.
- At 6 months, BCG demonstrated a sex-specific paradoxical effect on hair loss, decreasing it in men (P = .031), while causing a slight, though statistically nonsignificant, increase in women.
- Male sex was the strongest predictive factor for long COVID, cognitive dysfunction, and cardiopulmonary scores at both follow-up assessments.
IN PRACTICE:
“[The study] findings suggest that BCG immunotherapy for an existing ailment may be superior to prophylaxis in healthy individuals,” the authors wrote.
SOURCE:
The study was led by Mehrsa Jalalizadeh and Keini Buosi, UroScience, State University of Campinas, Unicamp, São Paulo, Brazil. It was published online on November 19, 2024, in the Journal of Internal Medicine.
LIMITATIONS:
Previous mycobacterial exposure was not tested among the study participants. A notable loss to follow-up, particularly at 12 months, may have introduced bias into the results.
DISCLOSURES:
The study was supported by the Coordination for the Improvement of Higher Education Personnel, Federal Government of Brazil, the General Coordination of the National Immunization Program, Ministry of Health (Brazil), and the National Council for Scientific and Technological Development-Research Productivity. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A phase 3 clinical trial initiated in early 2020 investigated the effect of the BCG vaccine injected during active infection on COVID-19 progression in adults with mild or moderate COVID-19. The current study summarizes the 6- and 12-month follow-up data with a focus on long-COVID symptoms.
- Patients who tested positive for severe acute respiratory syndrome coronavirus 2 were randomly assigned to receive either 0.1 mL of intradermal BCG (n = 191) or 0.9% saline placebo (n = 202) within 14 days of symptom onset and were followed up at 7, 14, 21, and 45 days and at 6 and 12 months postinjection.
- Overall, 157 BCG (median age, 40 years; 54.1% women) and 142 placebo (median age, 41 years; 65.5% women) recipients completed the 6-month follow-up, and 97 BCG (median age, 37 years; 49.5% women) and 95 placebo (median age, 40 years; 67.4% women) recipients completed the 12-month follow-up.
- The researchers primarily assessed the effect of the BCG vaccine on the development of the symptoms of long COVID at 6 and 12 months.
TAKEAWAY:
- Hearing problems were less frequent among BCG recipients at 6 months compared with those who received placebo (odds ratio [OR], 0.26; 95% CI, 0.045-1.0; P = .044).
- At 12 months, participants who received the BCG vaccine exhibited fewer issues with sleeping (P = .027), concentration (P = .009), memory (P = .009), and vision (P = .022) along with a lower long-COVID score (one-sided Wilcoxon test, P = .002) than those who received placebo.
- At 6 months, BCG demonstrated a sex-specific paradoxical effect on hair loss, decreasing it in men (P = .031), while causing a slight, though statistically nonsignificant, increase in women.
- Male sex was the strongest predictive factor for long COVID, cognitive dysfunction, and cardiopulmonary scores at both follow-up assessments.
IN PRACTICE:
“[The study] findings suggest that BCG immunotherapy for an existing ailment may be superior to prophylaxis in healthy individuals,” the authors wrote.
SOURCE:
The study was led by Mehrsa Jalalizadeh and Keini Buosi, UroScience, State University of Campinas, Unicamp, São Paulo, Brazil. It was published online on November 19, 2024, in the Journal of Internal Medicine.
LIMITATIONS:
Previous mycobacterial exposure was not tested among the study participants. A notable loss to follow-up, particularly at 12 months, may have introduced bias into the results.
DISCLOSURES:
The study was supported by the Coordination for the Improvement of Higher Education Personnel, Federal Government of Brazil, the General Coordination of the National Immunization Program, Ministry of Health (Brazil), and the National Council for Scientific and Technological Development-Research Productivity. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A phase 3 clinical trial initiated in early 2020 investigated the effect of the BCG vaccine injected during active infection on COVID-19 progression in adults with mild or moderate COVID-19. The current study summarizes the 6- and 12-month follow-up data with a focus on long-COVID symptoms.
- Patients who tested positive for severe acute respiratory syndrome coronavirus 2 were randomly assigned to receive either 0.1 mL of intradermal BCG (n = 191) or 0.9% saline placebo (n = 202) within 14 days of symptom onset and were followed up at 7, 14, 21, and 45 days and at 6 and 12 months postinjection.
- Overall, 157 BCG (median age, 40 years; 54.1% women) and 142 placebo (median age, 41 years; 65.5% women) recipients completed the 6-month follow-up, and 97 BCG (median age, 37 years; 49.5% women) and 95 placebo (median age, 40 years; 67.4% women) recipients completed the 12-month follow-up.
- The researchers primarily assessed the effect of the BCG vaccine on the development of the symptoms of long COVID at 6 and 12 months.
TAKEAWAY:
- Hearing problems were less frequent among BCG recipients at 6 months compared with those who received placebo (odds ratio [OR], 0.26; 95% CI, 0.045-1.0; P = .044).
- At 12 months, participants who received the BCG vaccine exhibited fewer issues with sleeping (P = .027), concentration (P = .009), memory (P = .009), and vision (P = .022) along with a lower long-COVID score (one-sided Wilcoxon test, P = .002) than those who received placebo.
- At 6 months, BCG demonstrated a sex-specific paradoxical effect on hair loss, decreasing it in men (P = .031), while causing a slight, though statistically nonsignificant, increase in women.
- Male sex was the strongest predictive factor for long COVID, cognitive dysfunction, and cardiopulmonary scores at both follow-up assessments.
IN PRACTICE:
“[The study] findings suggest that BCG immunotherapy for an existing ailment may be superior to prophylaxis in healthy individuals,” the authors wrote.
SOURCE:
The study was led by Mehrsa Jalalizadeh and Keini Buosi, UroScience, State University of Campinas, Unicamp, São Paulo, Brazil. It was published online on November 19, 2024, in the Journal of Internal Medicine.
LIMITATIONS:
Previous mycobacterial exposure was not tested among the study participants. A notable loss to follow-up, particularly at 12 months, may have introduced bias into the results.
DISCLOSURES:
The study was supported by the Coordination for the Improvement of Higher Education Personnel, Federal Government of Brazil, the General Coordination of the National Immunization Program, Ministry of Health (Brazil), and the National Council for Scientific and Technological Development-Research Productivity. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
How Metals Affect the Brain
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.
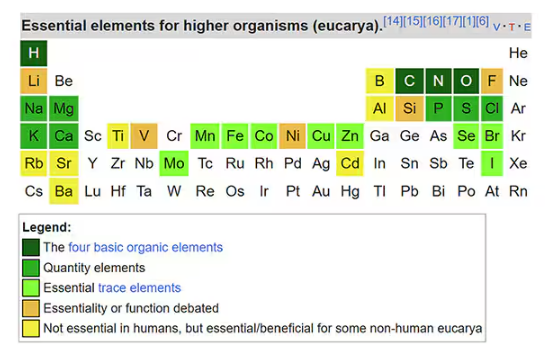
Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.
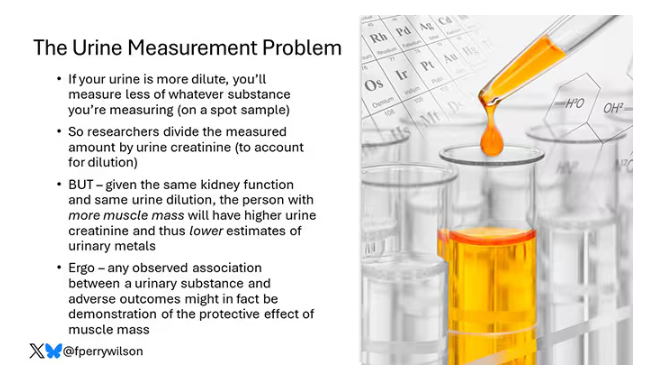
This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.
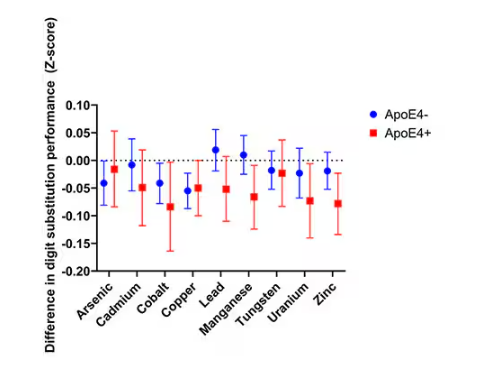
Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.
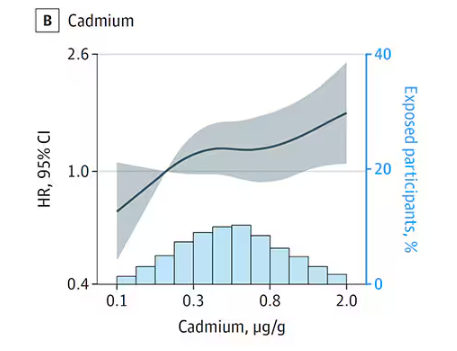
We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).
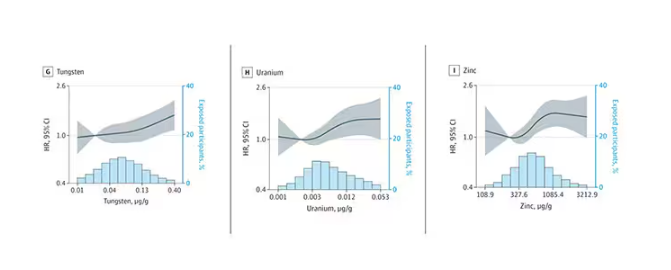
But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.
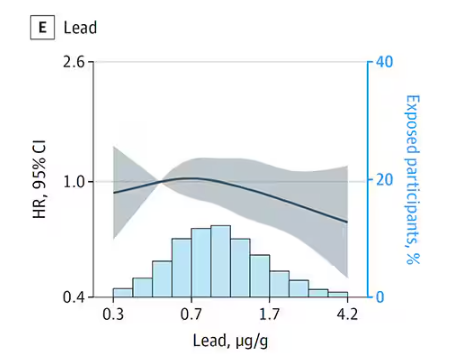
This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.
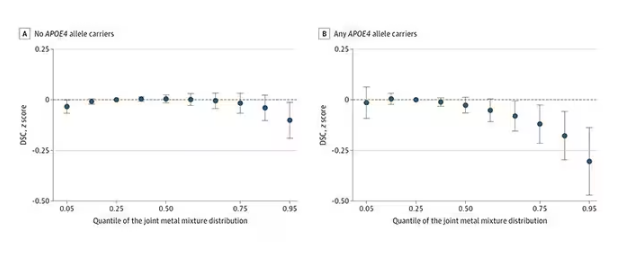
So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.

Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.

This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.

Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.

We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).

But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.

This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.

So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.

Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.

This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.

Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.

We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).

But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.

This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.

So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Microplastics Have Been Found in the Human Brain. Now What?
In a recent case series study that examined olfactory bulb tissue from deceased individuals, 8 of the 15 decedent brains showed the presence of microplastics, most commonly polypropylene, a plastic typically used in food packaging and water bottles.
Measuring less than 5 mm in size, microplastics are formed over time as plastic materials break down but don’t biodegrade. Exposure to these substances can come through food, air, and skin absorption.
While scientists are learning more about how these substances are absorbed by the body, questions remain about how much exposure is safe, what effect — if any — microplastics could have on brain function, and what clinicians should tell their patients.
What Are the Major Health Concerns?
The Plastic Health Council estimates that more than 500 million metric tons of plastic are produced worldwide each year. In addition, it reports that plastic products can contain more than 16,000 chemicals, about a quarter of which have been found to be hazardous to human health and the environment. Microplastics and nanoplastics can enter the body through the air, in food, or absorption through the skin.
A study published in March showed that patients with carotid plaques and the presence of microplastics and nanoplastics were at an increased risk for death or major cardiovascular events.
Other studies have shown a link between these substances and placental inflammation and preterm births, reduced male fertility, and endocrine disruption — as well as accelerated spread of cancer cells in the gut.
There is also evidence suggesting that microplastics may facilitate the development of antibiotic resistance in bacteria and could contribute to the rise in food allergies.
And now, Thais Mauad, MD, PhD, and colleagues have found the substances in the brain.
How Is the Brain Affected?
The investigators examined olfactory bulb tissues from 15 deceased Sao Paulo, Brazil, residents ranging in age from 33 to 100 years who underwent routine coroner autopsies. All but three of the participants were men.
Exclusion criteria included having undergone previous neurosurgical interventions. The tissues were analyzed using micro–Fourier transform infrared spectroscopy (µFTIR).
In addition, the researchers practiced a “plastic-free approach” in their analysis, which included using filters and covering glassware and samples with aluminum foil.
Study findings showed microplastics in 8 of the 15 participants — including in the centenarian. In total, there were 16 synthetic polymer particles and fibers detected, with up to four microplastics detected per olfactory bulb. Polypropylene was the most common polymer found (44%), followed by polyamide, nylon, and polyethylene vinyl acetate. These substances are commonly used in a wide range of products, including food packaging, textiles, kitchen utensils, medical devices, and adhesives.
The microplastic particles ranged in length from 5.5 to 26 microns (one millionth of a meter), with a width that ranged from 3 to 25 microns. The mean fiber length and width was 21 and 4 microns, respectively. For comparison, the diameter of one human hair averages about 70 microns, according to the US Food and Drug Administration (FDA).
“To our knowledge, this is the first study in which the presence of microplastics in the human brain was identified and characterized using µFTIR,” the researchers wrote.
How Do Microplastics Reach the Brain?
Although the possibility of microplastics crossing the blood-brain barrier has been questioned, senior investigator Mauad, associate professor in the Department of Pathology, the University of Sao Paulo in Brazil, noted that the olfactory pathway could offer an entry route through inhalation of the particles.
This means that “breathing within indoor environments could be a major source of plastic pollution in the brain,” she said in a press release.
“With much smaller nanoplastics entering the body with greater ease, the total level of plastic particles may be much higher. What is worrying is the capacity of such particles to be internalized by cells and alter how our bodies function,” she added.
Mauad said that although questions remain regarding the health implications of their findings, some animal studies have shown that the presence of microplastics in the brain is linked to neurotoxic effects, including oxidative stress.
In addition, exposure to particulate matter has been linked previously to such neurologic conditions as dementia and neurodegenerative conditions such as Parkinson’s disease “seem to have a connection with nasal abnormalities as initial symptoms,” the investigators noted.
While the olfactory pathway appears to be a likely route of exposure the researchers noted that other potential entry routes, including through blood circulation, may also be involved.
The research suggests that inhaling microplastics while indoors may be unavoidable, Mauad said, making it unlikely individuals can eliminate exposure to these substances.
“Everything that surrounds us is plastic. So we can’t really get rid of it,” she said.
Are Microplastics Regulated?
The most effective solution would be stricter regulations, Mauad said.
“The industry has chosen to sell many things in plastic, and I think this has to change. We need more policies to decrease plastic production — especially single-use plastic,” she said.
Federal, state, and local regulations for microplastics are “virtually nonexistent,” reported the Interstate Technology and Regulatory Council (ITRC), a state-led coalition that produces documents and trainings related to regulatory issues.
In 2021, the ITRC sent a survey to all US states asking about microplastics regulations. Of the 26 states that responded, only 4 said they had conducted sampling for microplastics. None of the responders indicated they had established any criteria or standards for microplastics, although eight states indicated they had plans to pursue them in the future.
Although federal regulations include the Microbead-Free Waters Act of 2015 and the Save Our Seas Act 2.0, the rules don’t directly pertain to microplastics.
There are also no regulations currently in place regarding microplastics or nanoplastics in food. A report issued in July by the FDA claimed that “the overall scientific evidence does not demonstrate that levels of microplastics or nanoplastics found in foods pose a risk to human health.”
International efforts to regulate microplastics are much further along. First created in 2022, the treaty would forge an international, legally binding agreement.
While it is a step in the right direction, the Plastic Health Council has cautioned about “the omission of measures in draft provisions that fully address the impact of plastic pollution on human health.” The treaty should reduce plastic production, eliminate single-use plastic items, and call for testing of all chemicals in plastics, the council argues.
The final round of negotiations for the UN Global Plastic Treaty is set for completion before the end of the year.
What Should Clinicians Know?
Much remains unknown about the potential health effects of microplastic exposure. So how can clinicians respond to questions from concerned patients?
“We don’t yet have enough evidence about the plastic particle itself, like those highlighted in the current study — and even more so when it comes to nanoplastics, which are a thousand times smaller,” said Phoebe Stapleton, PhD, associated professor in the Department of Pharmacology and Toxicology at the Ernest Mario School of Pharmacy at Rutgers University, Piscataway, New Jersey.
“But we do have a lot of evidence about the chemicals that are used to make plastics, and we’ve already seen regulation there from the EPA. That’s one conversation that clinicians could have with patients: about those chemicals,” she added.
Stapleton recommended clinicians stay current on the latest research and be ready to respond should a patient raise the issue. She also noted the importance of exercising caution when interpreting these new findings.
While the study is important — especially because it highlights inhalation as a viable route of entry — exposure through the olfactory area is still just a theory and hasn’t yet been fully proven.
In addition, Stapleton wonders whether there are tissues where these substances are not found. A discovery like that “would be really exciting because that means that that tissue has mechanisms protecting it, and maybe, we could learn more about how to keep microplastics out,” she said.
She would also like to see more studies on specific adverse health effects from microplastics in the body.
Mauad agreed.
“That’s the next set of questions: What are the toxicities or lack thereof in those tissues? That will give us more information as it pertains to human health. It doesn’t feel good to know they’re in our tissues, but we still don’t have a real understanding of what they’re doing when they’re there,” she said.
The current study was funded by the Alexander von Humboldt Foundation and by grants from the Brazilian Research Council and the Soa State Research Agency. It was also funded by the Plastic Soup Foundation — which, together with A Plastic Planet, forms the Plastic Health Council. The investigators and Stapleton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a recent case series study that examined olfactory bulb tissue from deceased individuals, 8 of the 15 decedent brains showed the presence of microplastics, most commonly polypropylene, a plastic typically used in food packaging and water bottles.
Measuring less than 5 mm in size, microplastics are formed over time as plastic materials break down but don’t biodegrade. Exposure to these substances can come through food, air, and skin absorption.
While scientists are learning more about how these substances are absorbed by the body, questions remain about how much exposure is safe, what effect — if any — microplastics could have on brain function, and what clinicians should tell their patients.
What Are the Major Health Concerns?
The Plastic Health Council estimates that more than 500 million metric tons of plastic are produced worldwide each year. In addition, it reports that plastic products can contain more than 16,000 chemicals, about a quarter of which have been found to be hazardous to human health and the environment. Microplastics and nanoplastics can enter the body through the air, in food, or absorption through the skin.
A study published in March showed that patients with carotid plaques and the presence of microplastics and nanoplastics were at an increased risk for death or major cardiovascular events.
Other studies have shown a link between these substances and placental inflammation and preterm births, reduced male fertility, and endocrine disruption — as well as accelerated spread of cancer cells in the gut.
There is also evidence suggesting that microplastics may facilitate the development of antibiotic resistance in bacteria and could contribute to the rise in food allergies.
And now, Thais Mauad, MD, PhD, and colleagues have found the substances in the brain.
How Is the Brain Affected?
The investigators examined olfactory bulb tissues from 15 deceased Sao Paulo, Brazil, residents ranging in age from 33 to 100 years who underwent routine coroner autopsies. All but three of the participants were men.
Exclusion criteria included having undergone previous neurosurgical interventions. The tissues were analyzed using micro–Fourier transform infrared spectroscopy (µFTIR).
In addition, the researchers practiced a “plastic-free approach” in their analysis, which included using filters and covering glassware and samples with aluminum foil.
Study findings showed microplastics in 8 of the 15 participants — including in the centenarian. In total, there were 16 synthetic polymer particles and fibers detected, with up to four microplastics detected per olfactory bulb. Polypropylene was the most common polymer found (44%), followed by polyamide, nylon, and polyethylene vinyl acetate. These substances are commonly used in a wide range of products, including food packaging, textiles, kitchen utensils, medical devices, and adhesives.
The microplastic particles ranged in length from 5.5 to 26 microns (one millionth of a meter), with a width that ranged from 3 to 25 microns. The mean fiber length and width was 21 and 4 microns, respectively. For comparison, the diameter of one human hair averages about 70 microns, according to the US Food and Drug Administration (FDA).
“To our knowledge, this is the first study in which the presence of microplastics in the human brain was identified and characterized using µFTIR,” the researchers wrote.
How Do Microplastics Reach the Brain?
Although the possibility of microplastics crossing the blood-brain barrier has been questioned, senior investigator Mauad, associate professor in the Department of Pathology, the University of Sao Paulo in Brazil, noted that the olfactory pathway could offer an entry route through inhalation of the particles.
This means that “breathing within indoor environments could be a major source of plastic pollution in the brain,” she said in a press release.
“With much smaller nanoplastics entering the body with greater ease, the total level of plastic particles may be much higher. What is worrying is the capacity of such particles to be internalized by cells and alter how our bodies function,” she added.
Mauad said that although questions remain regarding the health implications of their findings, some animal studies have shown that the presence of microplastics in the brain is linked to neurotoxic effects, including oxidative stress.
In addition, exposure to particulate matter has been linked previously to such neurologic conditions as dementia and neurodegenerative conditions such as Parkinson’s disease “seem to have a connection with nasal abnormalities as initial symptoms,” the investigators noted.
While the olfactory pathway appears to be a likely route of exposure the researchers noted that other potential entry routes, including through blood circulation, may also be involved.
The research suggests that inhaling microplastics while indoors may be unavoidable, Mauad said, making it unlikely individuals can eliminate exposure to these substances.
“Everything that surrounds us is plastic. So we can’t really get rid of it,” she said.
Are Microplastics Regulated?
The most effective solution would be stricter regulations, Mauad said.
“The industry has chosen to sell many things in plastic, and I think this has to change. We need more policies to decrease plastic production — especially single-use plastic,” she said.
Federal, state, and local regulations for microplastics are “virtually nonexistent,” reported the Interstate Technology and Regulatory Council (ITRC), a state-led coalition that produces documents and trainings related to regulatory issues.
In 2021, the ITRC sent a survey to all US states asking about microplastics regulations. Of the 26 states that responded, only 4 said they had conducted sampling for microplastics. None of the responders indicated they had established any criteria or standards for microplastics, although eight states indicated they had plans to pursue them in the future.
Although federal regulations include the Microbead-Free Waters Act of 2015 and the Save Our Seas Act 2.0, the rules don’t directly pertain to microplastics.
There are also no regulations currently in place regarding microplastics or nanoplastics in food. A report issued in July by the FDA claimed that “the overall scientific evidence does not demonstrate that levels of microplastics or nanoplastics found in foods pose a risk to human health.”
International efforts to regulate microplastics are much further along. First created in 2022, the treaty would forge an international, legally binding agreement.
While it is a step in the right direction, the Plastic Health Council has cautioned about “the omission of measures in draft provisions that fully address the impact of plastic pollution on human health.” The treaty should reduce plastic production, eliminate single-use plastic items, and call for testing of all chemicals in plastics, the council argues.
The final round of negotiations for the UN Global Plastic Treaty is set for completion before the end of the year.
What Should Clinicians Know?
Much remains unknown about the potential health effects of microplastic exposure. So how can clinicians respond to questions from concerned patients?
“We don’t yet have enough evidence about the plastic particle itself, like those highlighted in the current study — and even more so when it comes to nanoplastics, which are a thousand times smaller,” said Phoebe Stapleton, PhD, associated professor in the Department of Pharmacology and Toxicology at the Ernest Mario School of Pharmacy at Rutgers University, Piscataway, New Jersey.
“But we do have a lot of evidence about the chemicals that are used to make plastics, and we’ve already seen regulation there from the EPA. That’s one conversation that clinicians could have with patients: about those chemicals,” she added.
Stapleton recommended clinicians stay current on the latest research and be ready to respond should a patient raise the issue. She also noted the importance of exercising caution when interpreting these new findings.
While the study is important — especially because it highlights inhalation as a viable route of entry — exposure through the olfactory area is still just a theory and hasn’t yet been fully proven.
In addition, Stapleton wonders whether there are tissues where these substances are not found. A discovery like that “would be really exciting because that means that that tissue has mechanisms protecting it, and maybe, we could learn more about how to keep microplastics out,” she said.
She would also like to see more studies on specific adverse health effects from microplastics in the body.
Mauad agreed.
“That’s the next set of questions: What are the toxicities or lack thereof in those tissues? That will give us more information as it pertains to human health. It doesn’t feel good to know they’re in our tissues, but we still don’t have a real understanding of what they’re doing when they’re there,” she said.
The current study was funded by the Alexander von Humboldt Foundation and by grants from the Brazilian Research Council and the Soa State Research Agency. It was also funded by the Plastic Soup Foundation — which, together with A Plastic Planet, forms the Plastic Health Council. The investigators and Stapleton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a recent case series study that examined olfactory bulb tissue from deceased individuals, 8 of the 15 decedent brains showed the presence of microplastics, most commonly polypropylene, a plastic typically used in food packaging and water bottles.
Measuring less than 5 mm in size, microplastics are formed over time as plastic materials break down but don’t biodegrade. Exposure to these substances can come through food, air, and skin absorption.
While scientists are learning more about how these substances are absorbed by the body, questions remain about how much exposure is safe, what effect — if any — microplastics could have on brain function, and what clinicians should tell their patients.
What Are the Major Health Concerns?
The Plastic Health Council estimates that more than 500 million metric tons of plastic are produced worldwide each year. In addition, it reports that plastic products can contain more than 16,000 chemicals, about a quarter of which have been found to be hazardous to human health and the environment. Microplastics and nanoplastics can enter the body through the air, in food, or absorption through the skin.
A study published in March showed that patients with carotid plaques and the presence of microplastics and nanoplastics were at an increased risk for death or major cardiovascular events.
Other studies have shown a link between these substances and placental inflammation and preterm births, reduced male fertility, and endocrine disruption — as well as accelerated spread of cancer cells in the gut.
There is also evidence suggesting that microplastics may facilitate the development of antibiotic resistance in bacteria and could contribute to the rise in food allergies.
And now, Thais Mauad, MD, PhD, and colleagues have found the substances in the brain.
How Is the Brain Affected?
The investigators examined olfactory bulb tissues from 15 deceased Sao Paulo, Brazil, residents ranging in age from 33 to 100 years who underwent routine coroner autopsies. All but three of the participants were men.
Exclusion criteria included having undergone previous neurosurgical interventions. The tissues were analyzed using micro–Fourier transform infrared spectroscopy (µFTIR).
In addition, the researchers practiced a “plastic-free approach” in their analysis, which included using filters and covering glassware and samples with aluminum foil.
Study findings showed microplastics in 8 of the 15 participants — including in the centenarian. In total, there were 16 synthetic polymer particles and fibers detected, with up to four microplastics detected per olfactory bulb. Polypropylene was the most common polymer found (44%), followed by polyamide, nylon, and polyethylene vinyl acetate. These substances are commonly used in a wide range of products, including food packaging, textiles, kitchen utensils, medical devices, and adhesives.
The microplastic particles ranged in length from 5.5 to 26 microns (one millionth of a meter), with a width that ranged from 3 to 25 microns. The mean fiber length and width was 21 and 4 microns, respectively. For comparison, the diameter of one human hair averages about 70 microns, according to the US Food and Drug Administration (FDA).
“To our knowledge, this is the first study in which the presence of microplastics in the human brain was identified and characterized using µFTIR,” the researchers wrote.
How Do Microplastics Reach the Brain?
Although the possibility of microplastics crossing the blood-brain barrier has been questioned, senior investigator Mauad, associate professor in the Department of Pathology, the University of Sao Paulo in Brazil, noted that the olfactory pathway could offer an entry route through inhalation of the particles.
This means that “breathing within indoor environments could be a major source of plastic pollution in the brain,” she said in a press release.
“With much smaller nanoplastics entering the body with greater ease, the total level of plastic particles may be much higher. What is worrying is the capacity of such particles to be internalized by cells and alter how our bodies function,” she added.
Mauad said that although questions remain regarding the health implications of their findings, some animal studies have shown that the presence of microplastics in the brain is linked to neurotoxic effects, including oxidative stress.
In addition, exposure to particulate matter has been linked previously to such neurologic conditions as dementia and neurodegenerative conditions such as Parkinson’s disease “seem to have a connection with nasal abnormalities as initial symptoms,” the investigators noted.
While the olfactory pathway appears to be a likely route of exposure the researchers noted that other potential entry routes, including through blood circulation, may also be involved.
The research suggests that inhaling microplastics while indoors may be unavoidable, Mauad said, making it unlikely individuals can eliminate exposure to these substances.
“Everything that surrounds us is plastic. So we can’t really get rid of it,” she said.
Are Microplastics Regulated?
The most effective solution would be stricter regulations, Mauad said.
“The industry has chosen to sell many things in plastic, and I think this has to change. We need more policies to decrease plastic production — especially single-use plastic,” she said.
Federal, state, and local regulations for microplastics are “virtually nonexistent,” reported the Interstate Technology and Regulatory Council (ITRC), a state-led coalition that produces documents and trainings related to regulatory issues.
In 2021, the ITRC sent a survey to all US states asking about microplastics regulations. Of the 26 states that responded, only 4 said they had conducted sampling for microplastics. None of the responders indicated they had established any criteria or standards for microplastics, although eight states indicated they had plans to pursue them in the future.
Although federal regulations include the Microbead-Free Waters Act of 2015 and the Save Our Seas Act 2.0, the rules don’t directly pertain to microplastics.
There are also no regulations currently in place regarding microplastics or nanoplastics in food. A report issued in July by the FDA claimed that “the overall scientific evidence does not demonstrate that levels of microplastics or nanoplastics found in foods pose a risk to human health.”
International efforts to regulate microplastics are much further along. First created in 2022, the treaty would forge an international, legally binding agreement.
While it is a step in the right direction, the Plastic Health Council has cautioned about “the omission of measures in draft provisions that fully address the impact of plastic pollution on human health.” The treaty should reduce plastic production, eliminate single-use plastic items, and call for testing of all chemicals in plastics, the council argues.
The final round of negotiations for the UN Global Plastic Treaty is set for completion before the end of the year.
What Should Clinicians Know?
Much remains unknown about the potential health effects of microplastic exposure. So how can clinicians respond to questions from concerned patients?
“We don’t yet have enough evidence about the plastic particle itself, like those highlighted in the current study — and even more so when it comes to nanoplastics, which are a thousand times smaller,” said Phoebe Stapleton, PhD, associated professor in the Department of Pharmacology and Toxicology at the Ernest Mario School of Pharmacy at Rutgers University, Piscataway, New Jersey.
“But we do have a lot of evidence about the chemicals that are used to make plastics, and we’ve already seen regulation there from the EPA. That’s one conversation that clinicians could have with patients: about those chemicals,” she added.
Stapleton recommended clinicians stay current on the latest research and be ready to respond should a patient raise the issue. She also noted the importance of exercising caution when interpreting these new findings.
While the study is important — especially because it highlights inhalation as a viable route of entry — exposure through the olfactory area is still just a theory and hasn’t yet been fully proven.
In addition, Stapleton wonders whether there are tissues where these substances are not found. A discovery like that “would be really exciting because that means that that tissue has mechanisms protecting it, and maybe, we could learn more about how to keep microplastics out,” she said.
She would also like to see more studies on specific adverse health effects from microplastics in the body.
Mauad agreed.
“That’s the next set of questions: What are the toxicities or lack thereof in those tissues? That will give us more information as it pertains to human health. It doesn’t feel good to know they’re in our tissues, but we still don’t have a real understanding of what they’re doing when they’re there,” she said.
The current study was funded by the Alexander von Humboldt Foundation and by grants from the Brazilian Research Council and the Soa State Research Agency. It was also funded by the Plastic Soup Foundation — which, together with A Plastic Planet, forms the Plastic Health Council. The investigators and Stapleton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Six Updates on Stroke Management
This video transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener, from the Faculty of Medicine at the University Duisburg-Essen in Germany. In this video, I would like to cover six publications on stroke, which were published this fall.
The Best Thrombolytic?
Let me start with systemic thrombolysis. We now have two thrombolytic agents available. One is the well-known alteplase, and newly approved for the treatment of stroke is tenecteplase. The ATTEST-2 study in the United Kingdom, published in The Lancet Neurology, compared tenecteplase 0.25 mg/kg body weight as a bolus with alteplase 0.9 mg/kg body weight as an infusion over 60 minutes in the 4.5-hour time window in 1777 patients with ischemic stroke.
There was no significant difference between the two thrombolytics for the primary endpoint of modified Rankin Scale score after 90 days. There was also no difference with respect to mortality, intracranial bleeding, or extracranial bleeding.
We finally have 11 randomized controlled trials that compared tenecteplase and alteplase in acute ischemic stroke. A meta-analysis of these randomized trials was published in Neurology. The analysis included 3700 patients treated with tenecteplase and 3700 patients treated with alteplase. For the primary endpoint, excellent functional outcome defined as modified Rankin Scale score 0-1 after 90 days, there was a significant benefit for tenecteplase (relative risk, 1.05), but the absolute difference was very small, at 3%. There was no difference in mortality or bleeding complications.
In conclusion, I think both substances are great. They are effective. Tenecteplase is most probably the drug which should be used in people who have to transfer from a primary stroke center to a dedicated stroke center that provides thrombectomy. Otherwise, I think it’s a choice of the physician as to which thrombolytic agent to use.
Mobile Stroke Units
A highly debated topic is mobile stroke units. These stroke units have a CT scanner and laboratory on board, and this makes it possible to perform thrombolysis on the way to the hospital. A retrospective, observational study collected data between 2018 and 2023, and included 19,400 patients with acute stroke, of whom 1237, or 6.4%, were treated in a mobile stroke unit. This study was published in JAMA Neurology.
The modified Rankin Scale score at the time of discharge was better in patients treated with a mobile stroke unit, but the absolute benefit was only 0.03 points on the modified Rankin Scale. The question is whether this is cost-effective, and can we really do this at times when there is a traumatic shortage of physicians and nursing staff in the hospital?
DOAC Reversal Agents
Oral anticoagulation, as you know, is usually considered a contraindication for systemic thrombolysis. Idarucizumab, a monoclonal antibody, was developed to reverse the biological activity of dabigatran and then allow systemic thrombolysis.
A recent publication in Neurology analyzed 13 cohort studies with 553 stroke patients on dabigatran who received idarucizumab prior to systemic thrombolysis, and the rate of intracranial hemorrhage was 4%. This means it’s obviously possible to perform thrombolysis when the activity of dabigatran is neutralized by idarucizumab.
Unfortunately, until today, we have no data on whether this can also be done with andexanet alfa in people who are treated with a factor Xa inhibitor like, for example, apixaban, rivaroxaban, or edoxaban.
Anticoagulation in ESUS
My next topic is ESUS, or embolic stroke of undetermined source. We have four large randomized trials and three smaller trials that compared antiplatelet therapy with DOACs in patients with ESUS. A group in Neurology published a meta-analysis of seven randomized controlled studies with, altogether, 14,800 patients with ESUS.
The comparison between antiplatelet therapy and anticoagulants showed no difference for recurrent ischemic stroke, and also not for major subgroups. This means that people with ESUS should receive antiplatelet therapy, most probably aspirin.
Anticoagulation Post–Ischemic Stroke With AF
My final topic is the optimal time to start anticoagulation in people with atrial fibrillation who suffer an ischemic stroke. The OPTIMAS study, published in The Lancet, randomized 3650 patients who were anticoagulated with DOACs early (which means less than 4 days) or delayed (between 7 and 14 days). There was no difference in the primary endpoint, which was recurrent ischemic stroke, intracranial hemorrhage, or systemic embolism at 90 days.
The conclusion is that, in most cases, we can probably initiate anticoagulation in people with ischemic stroke and atrial fibrillation within the first 4 days.
Dear colleagues, this is an exciting time for the stroke field. I presented six new studies that have impact, I think, on the management of patients with ischemic stroke.
Dr. Diener is a professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen in Germany. He reported conflicts of interest with Abbott, AbbVie, Boehringer Ingelheim, Lundbeck, Novartis, Orion Pharma, Teva, WebMD, and The German Research Council. He also serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener, from the Faculty of Medicine at the University Duisburg-Essen in Germany. In this video, I would like to cover six publications on stroke, which were published this fall.
The Best Thrombolytic?
Let me start with systemic thrombolysis. We now have two thrombolytic agents available. One is the well-known alteplase, and newly approved for the treatment of stroke is tenecteplase. The ATTEST-2 study in the United Kingdom, published in The Lancet Neurology, compared tenecteplase 0.25 mg/kg body weight as a bolus with alteplase 0.9 mg/kg body weight as an infusion over 60 minutes in the 4.5-hour time window in 1777 patients with ischemic stroke.
There was no significant difference between the two thrombolytics for the primary endpoint of modified Rankin Scale score after 90 days. There was also no difference with respect to mortality, intracranial bleeding, or extracranial bleeding.
We finally have 11 randomized controlled trials that compared tenecteplase and alteplase in acute ischemic stroke. A meta-analysis of these randomized trials was published in Neurology. The analysis included 3700 patients treated with tenecteplase and 3700 patients treated with alteplase. For the primary endpoint, excellent functional outcome defined as modified Rankin Scale score 0-1 after 90 days, there was a significant benefit for tenecteplase (relative risk, 1.05), but the absolute difference was very small, at 3%. There was no difference in mortality or bleeding complications.
In conclusion, I think both substances are great. They are effective. Tenecteplase is most probably the drug which should be used in people who have to transfer from a primary stroke center to a dedicated stroke center that provides thrombectomy. Otherwise, I think it’s a choice of the physician as to which thrombolytic agent to use.
Mobile Stroke Units
A highly debated topic is mobile stroke units. These stroke units have a CT scanner and laboratory on board, and this makes it possible to perform thrombolysis on the way to the hospital. A retrospective, observational study collected data between 2018 and 2023, and included 19,400 patients with acute stroke, of whom 1237, or 6.4%, were treated in a mobile stroke unit. This study was published in JAMA Neurology.
The modified Rankin Scale score at the time of discharge was better in patients treated with a mobile stroke unit, but the absolute benefit was only 0.03 points on the modified Rankin Scale. The question is whether this is cost-effective, and can we really do this at times when there is a traumatic shortage of physicians and nursing staff in the hospital?
DOAC Reversal Agents
Oral anticoagulation, as you know, is usually considered a contraindication for systemic thrombolysis. Idarucizumab, a monoclonal antibody, was developed to reverse the biological activity of dabigatran and then allow systemic thrombolysis.
A recent publication in Neurology analyzed 13 cohort studies with 553 stroke patients on dabigatran who received idarucizumab prior to systemic thrombolysis, and the rate of intracranial hemorrhage was 4%. This means it’s obviously possible to perform thrombolysis when the activity of dabigatran is neutralized by idarucizumab.
Unfortunately, until today, we have no data on whether this can also be done with andexanet alfa in people who are treated with a factor Xa inhibitor like, for example, apixaban, rivaroxaban, or edoxaban.
Anticoagulation in ESUS
My next topic is ESUS, or embolic stroke of undetermined source. We have four large randomized trials and three smaller trials that compared antiplatelet therapy with DOACs in patients with ESUS. A group in Neurology published a meta-analysis of seven randomized controlled studies with, altogether, 14,800 patients with ESUS.
The comparison between antiplatelet therapy and anticoagulants showed no difference for recurrent ischemic stroke, and also not for major subgroups. This means that people with ESUS should receive antiplatelet therapy, most probably aspirin.
Anticoagulation Post–Ischemic Stroke With AF
My final topic is the optimal time to start anticoagulation in people with atrial fibrillation who suffer an ischemic stroke. The OPTIMAS study, published in The Lancet, randomized 3650 patients who were anticoagulated with DOACs early (which means less than 4 days) or delayed (between 7 and 14 days). There was no difference in the primary endpoint, which was recurrent ischemic stroke, intracranial hemorrhage, or systemic embolism at 90 days.
The conclusion is that, in most cases, we can probably initiate anticoagulation in people with ischemic stroke and atrial fibrillation within the first 4 days.
Dear colleagues, this is an exciting time for the stroke field. I presented six new studies that have impact, I think, on the management of patients with ischemic stroke.
Dr. Diener is a professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen in Germany. He reported conflicts of interest with Abbott, AbbVie, Boehringer Ingelheim, Lundbeck, Novartis, Orion Pharma, Teva, WebMD, and The German Research Council. He also serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener, from the Faculty of Medicine at the University Duisburg-Essen in Germany. In this video, I would like to cover six publications on stroke, which were published this fall.
The Best Thrombolytic?
Let me start with systemic thrombolysis. We now have two thrombolytic agents available. One is the well-known alteplase, and newly approved for the treatment of stroke is tenecteplase. The ATTEST-2 study in the United Kingdom, published in The Lancet Neurology, compared tenecteplase 0.25 mg/kg body weight as a bolus with alteplase 0.9 mg/kg body weight as an infusion over 60 minutes in the 4.5-hour time window in 1777 patients with ischemic stroke.
There was no significant difference between the two thrombolytics for the primary endpoint of modified Rankin Scale score after 90 days. There was also no difference with respect to mortality, intracranial bleeding, or extracranial bleeding.
We finally have 11 randomized controlled trials that compared tenecteplase and alteplase in acute ischemic stroke. A meta-analysis of these randomized trials was published in Neurology. The analysis included 3700 patients treated with tenecteplase and 3700 patients treated with alteplase. For the primary endpoint, excellent functional outcome defined as modified Rankin Scale score 0-1 after 90 days, there was a significant benefit for tenecteplase (relative risk, 1.05), but the absolute difference was very small, at 3%. There was no difference in mortality or bleeding complications.
In conclusion, I think both substances are great. They are effective. Tenecteplase is most probably the drug which should be used in people who have to transfer from a primary stroke center to a dedicated stroke center that provides thrombectomy. Otherwise, I think it’s a choice of the physician as to which thrombolytic agent to use.
Mobile Stroke Units
A highly debated topic is mobile stroke units. These stroke units have a CT scanner and laboratory on board, and this makes it possible to perform thrombolysis on the way to the hospital. A retrospective, observational study collected data between 2018 and 2023, and included 19,400 patients with acute stroke, of whom 1237, or 6.4%, were treated in a mobile stroke unit. This study was published in JAMA Neurology.
The modified Rankin Scale score at the time of discharge was better in patients treated with a mobile stroke unit, but the absolute benefit was only 0.03 points on the modified Rankin Scale. The question is whether this is cost-effective, and can we really do this at times when there is a traumatic shortage of physicians and nursing staff in the hospital?
DOAC Reversal Agents
Oral anticoagulation, as you know, is usually considered a contraindication for systemic thrombolysis. Idarucizumab, a monoclonal antibody, was developed to reverse the biological activity of dabigatran and then allow systemic thrombolysis.
A recent publication in Neurology analyzed 13 cohort studies with 553 stroke patients on dabigatran who received idarucizumab prior to systemic thrombolysis, and the rate of intracranial hemorrhage was 4%. This means it’s obviously possible to perform thrombolysis when the activity of dabigatran is neutralized by idarucizumab.
Unfortunately, until today, we have no data on whether this can also be done with andexanet alfa in people who are treated with a factor Xa inhibitor like, for example, apixaban, rivaroxaban, or edoxaban.
Anticoagulation in ESUS
My next topic is ESUS, or embolic stroke of undetermined source. We have four large randomized trials and three smaller trials that compared antiplatelet therapy with DOACs in patients with ESUS. A group in Neurology published a meta-analysis of seven randomized controlled studies with, altogether, 14,800 patients with ESUS.
The comparison between antiplatelet therapy and anticoagulants showed no difference for recurrent ischemic stroke, and also not for major subgroups. This means that people with ESUS should receive antiplatelet therapy, most probably aspirin.
Anticoagulation Post–Ischemic Stroke With AF
My final topic is the optimal time to start anticoagulation in people with atrial fibrillation who suffer an ischemic stroke. The OPTIMAS study, published in The Lancet, randomized 3650 patients who were anticoagulated with DOACs early (which means less than 4 days) or delayed (between 7 and 14 days). There was no difference in the primary endpoint, which was recurrent ischemic stroke, intracranial hemorrhage, or systemic embolism at 90 days.
The conclusion is that, in most cases, we can probably initiate anticoagulation in people with ischemic stroke and atrial fibrillation within the first 4 days.
Dear colleagues, this is an exciting time for the stroke field. I presented six new studies that have impact, I think, on the management of patients with ischemic stroke.
Dr. Diener is a professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen in Germany. He reported conflicts of interest with Abbott, AbbVie, Boehringer Ingelheim, Lundbeck, Novartis, Orion Pharma, Teva, WebMD, and The German Research Council. He also serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs.
A version of this article first appeared on Medscape.com.
Three Vascular Risk Factors May Up Severe Stroke Risk
TOPLINE:
, a global study shows.
METHODOLOGY:
- The INTERSTROKE case-control study included nearly 27,000 participants, half of whom had a first acute stroke (ischemic or hemorrhagic) and the other half acting as age- and sex-matched controls.
- Participants (mean age, 62 years; 40% women) were recruited across 142 centers in 32 countries between 2007 and 2015. Baseline demographics and lifestyle risk factors for stroke were gathered using standardized questionnaires
- Modified Rankin Scale (mRS) scores measured within 72 hours of hospital admission were used to classify stroke severity (0-3, nonsevere stroke; 4-6, severe stroke).
TAKEAWAY:
- Among the participants with acute stroke, 64% had nonsevere stroke and 36% had severe stroke, based on the mRS.
- Hypertension, atrial fibrillation, and smoking showed a significantly stronger association with severe stroke than with nonsevere stroke (odds ratios [ORs], 3.21 vs 2.87, 4.70 vs 3.61, and 1.87 vs 1.65, respectively; all P < .001).
- A high waist-to-hip ratio showed a stronger association with nonsevere stroke than with severe stroke (OR, 1.37 vs 1.11, respectively; P < .001).
- Diabetes, poor diet, physical inactivity, and stress were linked to increased odds of both severe and nonsevere stroke, whereas alcohol consumption and high apolipoprotein B levels were linked to higher odds of only nonsevere stroke. No significant differences in odds were observed between stroke severities in matched individuals.
IN PRACTICE:
“Our findings emphasize the importance of controlling high blood pressure, which is the most important modifiable risk factor for stroke globally,” lead author Catriona Reddin, MB BCh, BAO, MSc, School of Medicine, University of Galway, in Ireland, said in a press release.
SOURCE:
The study was published online in Neurology.
LIMITATIONS:
The study limitations included potential unmeasured confounders; reliance on the mRS score, which may have underestimated stroke severity; and challenges with recruiting patients with severe stroke in a case-control study. Smoking-related comorbidities and regional or sex-related variations in alcohol intake may also have influenced the results.
DISCLOSURES:
The study was funded by various organizations, including health research councils and foundations from Canada, Sweden, and Scotland, and pharmaceutical companies such as AstraZeneca, Boehringer Ingelheim, Pfizer, and MSD. One investigator reported receiving funding from the Irish Clinical Academic Training Programme, the Wellcome Trust and the Health Research Board, the Health Service Executive, National Doctors Training and Planning, and the Health and Social Care, Research and Development Division in Northern Ireland. No other conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
, a global study shows.
METHODOLOGY:
- The INTERSTROKE case-control study included nearly 27,000 participants, half of whom had a first acute stroke (ischemic or hemorrhagic) and the other half acting as age- and sex-matched controls.
- Participants (mean age, 62 years; 40% women) were recruited across 142 centers in 32 countries between 2007 and 2015. Baseline demographics and lifestyle risk factors for stroke were gathered using standardized questionnaires
- Modified Rankin Scale (mRS) scores measured within 72 hours of hospital admission were used to classify stroke severity (0-3, nonsevere stroke; 4-6, severe stroke).
TAKEAWAY:
- Among the participants with acute stroke, 64% had nonsevere stroke and 36% had severe stroke, based on the mRS.
- Hypertension, atrial fibrillation, and smoking showed a significantly stronger association with severe stroke than with nonsevere stroke (odds ratios [ORs], 3.21 vs 2.87, 4.70 vs 3.61, and 1.87 vs 1.65, respectively; all P < .001).
- A high waist-to-hip ratio showed a stronger association with nonsevere stroke than with severe stroke (OR, 1.37 vs 1.11, respectively; P < .001).
- Diabetes, poor diet, physical inactivity, and stress were linked to increased odds of both severe and nonsevere stroke, whereas alcohol consumption and high apolipoprotein B levels were linked to higher odds of only nonsevere stroke. No significant differences in odds were observed between stroke severities in matched individuals.
IN PRACTICE:
“Our findings emphasize the importance of controlling high blood pressure, which is the most important modifiable risk factor for stroke globally,” lead author Catriona Reddin, MB BCh, BAO, MSc, School of Medicine, University of Galway, in Ireland, said in a press release.
SOURCE:
The study was published online in Neurology.
LIMITATIONS:
The study limitations included potential unmeasured confounders; reliance on the mRS score, which may have underestimated stroke severity; and challenges with recruiting patients with severe stroke in a case-control study. Smoking-related comorbidities and regional or sex-related variations in alcohol intake may also have influenced the results.
DISCLOSURES:
The study was funded by various organizations, including health research councils and foundations from Canada, Sweden, and Scotland, and pharmaceutical companies such as AstraZeneca, Boehringer Ingelheim, Pfizer, and MSD. One investigator reported receiving funding from the Irish Clinical Academic Training Programme, the Wellcome Trust and the Health Research Board, the Health Service Executive, National Doctors Training and Planning, and the Health and Social Care, Research and Development Division in Northern Ireland. No other conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
, a global study shows.
METHODOLOGY:
- The INTERSTROKE case-control study included nearly 27,000 participants, half of whom had a first acute stroke (ischemic or hemorrhagic) and the other half acting as age- and sex-matched controls.
- Participants (mean age, 62 years; 40% women) were recruited across 142 centers in 32 countries between 2007 and 2015. Baseline demographics and lifestyle risk factors for stroke were gathered using standardized questionnaires
- Modified Rankin Scale (mRS) scores measured within 72 hours of hospital admission were used to classify stroke severity (0-3, nonsevere stroke; 4-6, severe stroke).
TAKEAWAY:
- Among the participants with acute stroke, 64% had nonsevere stroke and 36% had severe stroke, based on the mRS.
- Hypertension, atrial fibrillation, and smoking showed a significantly stronger association with severe stroke than with nonsevere stroke (odds ratios [ORs], 3.21 vs 2.87, 4.70 vs 3.61, and 1.87 vs 1.65, respectively; all P < .001).
- A high waist-to-hip ratio showed a stronger association with nonsevere stroke than with severe stroke (OR, 1.37 vs 1.11, respectively; P < .001).
- Diabetes, poor diet, physical inactivity, and stress were linked to increased odds of both severe and nonsevere stroke, whereas alcohol consumption and high apolipoprotein B levels were linked to higher odds of only nonsevere stroke. No significant differences in odds were observed between stroke severities in matched individuals.
IN PRACTICE:
“Our findings emphasize the importance of controlling high blood pressure, which is the most important modifiable risk factor for stroke globally,” lead author Catriona Reddin, MB BCh, BAO, MSc, School of Medicine, University of Galway, in Ireland, said in a press release.
SOURCE:
The study was published online in Neurology.
LIMITATIONS:
The study limitations included potential unmeasured confounders; reliance on the mRS score, which may have underestimated stroke severity; and challenges with recruiting patients with severe stroke in a case-control study. Smoking-related comorbidities and regional or sex-related variations in alcohol intake may also have influenced the results.
DISCLOSURES:
The study was funded by various organizations, including health research councils and foundations from Canada, Sweden, and Scotland, and pharmaceutical companies such as AstraZeneca, Boehringer Ingelheim, Pfizer, and MSD. One investigator reported receiving funding from the Irish Clinical Academic Training Programme, the Wellcome Trust and the Health Research Board, the Health Service Executive, National Doctors Training and Planning, and the Health and Social Care, Research and Development Division in Northern Ireland. No other conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
