User login
Intensive insulin added no benefit for hyperglycemia after ischemic stroke
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
REPORTING FROM ISC 2019
Key clinical point: Aggressive insulin management of hyperglycemia following an ischemic stroke gave no clinical benefit, compared with a standard approach.
Major finding: After 90 days, favorable outcomes occurred in 21% of patients on aggressive insulin treatment and 22% on standard treatment.
Study details: SHINE, a multicenter, randomized trial with 1,151 acute ischemic stroke patients.
Disclosures: SHINE received no commercial funding. Dr. Johnston had no disclosures.
Source: Johnston KC et al. ISC 2019, Abstract LB1.
Novel plasma biomarkers may predict preclinical Alzheimer’s disease
, researchers reported in Science Advances.
“To our knowledge, this is the first time that a multianalyte plasma biomarker panel for an Alzheimer’s disease–related phenotype has been found and independently replicated by a nontargeted mass spectrometry approach,” said Nicholas J. Ashton, PhD, of King’s College London and the University of Gothenburg in Sweden, and his research colleagues.
Blood-based measures that predict amyloid-beta burden in preclinical Alzheimer’s disease have the potential to help investigators conduct clinical trials and aid in diagnostic management. However, this novel approach needs to be validated and translated “to a simpler automated platform suitable for wider utility,” the investigators noted. In addition, it is unclear whether their classifier can track changes in amyloid-beta or differentiate between other diseases with amyloid-beta pathology.
Advances in mass spectrometry technology have renewed interest in the analysis of plasma proteins in patients with various diseases. To assess whether proteomic discovery in plasma can help predict amyloid-beta burden in preclinical Alzheimer’s disease, Dr. Ashton and his colleagues studied 238 cognitively unimpaired individuals from the Australian Imaging, Biomarker and Lifestyle Flagship Study of Ageing (AIBL) and the Kerr Anglican Retirement Village Initiative in Ageing Health (KARVIAH). The participants had undergone PET to determine their amyloid-beta status. In the AIBL cohort (n = 144), 100 participants were amyloid-beta negative, and 44 were amyloid-beta positive. In the KARVIAH cohort (n = 94), 59 participants were amyloid-beta negative, and 35 were amyloid-beta positive. There were significantly more APOE4 carriers in the amyloid-beta–positive groups than in the amyloid-beta–negative groups. In addition, the amyloid-beta–positive groups tended to be older.
A support vector machine analysis created classifiers predicting amyloid-beta positivity in the AIBL cohort using demographics, proteins, or both. The researchers then tested each classifier in the KARVIAH dataset to identify which model best predicted amyloid-beta positivity. The optimal model included 10 protein features (prothrombin, adhesion G protein–coupled receptor, amyloid-beta A4 protein, NGN2, DNAH10, REST, NfL, RPS6KA3, GPSM2, FHAD1) and two demographic features (APOE4 count and age).
The classifier achieved a testing area under the receiver operator characteristic curve of 0.891 in the KARVIAH cohort to predict amyloid-beta positivity in cognitively unimpaired individuals with a sensitivity of 0.78 and specificity of 0.77.
The 10 protein features “represent a diverse array of pathways,” and the highest ranked feature was the serine protease prothrombin, which is a precursor to thrombin, the authors noted. “Multiple lines of evidence support that cerebrovascular disease may play a role in AD and that amyloid-beta may be involved in thrombosis, fibrinolysis, and inflammation via its interaction with the coagulation cascade,” the researchers wrote.
Two of the biomarkers – amyloid-beta A4 protein and NfL – have been examined in prior research and had a greater effect size in a secondary analysis that included participants with mild cognitive impairment and Alzheimer’s disease. This finding confirms “their connection with the more established disease state,” Dr. Ashton and colleagues said. In the secondary analysis, the optimal classifier included one demographic factor (APOE4 count) and nine protein features, eight of which also were used in the cognitively unimpaired classifier.
The study was funded in part by the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, and many authors reported additional research support from various institutions. One author is an employee of Johnson & Johnson and a named inventor on unrelated biomarker intellectual property owned by Proteome Science and King’s College London.
SOURCE: Ashton NJ et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aau7220.
, researchers reported in Science Advances.
“To our knowledge, this is the first time that a multianalyte plasma biomarker panel for an Alzheimer’s disease–related phenotype has been found and independently replicated by a nontargeted mass spectrometry approach,” said Nicholas J. Ashton, PhD, of King’s College London and the University of Gothenburg in Sweden, and his research colleagues.
Blood-based measures that predict amyloid-beta burden in preclinical Alzheimer’s disease have the potential to help investigators conduct clinical trials and aid in diagnostic management. However, this novel approach needs to be validated and translated “to a simpler automated platform suitable for wider utility,” the investigators noted. In addition, it is unclear whether their classifier can track changes in amyloid-beta or differentiate between other diseases with amyloid-beta pathology.
Advances in mass spectrometry technology have renewed interest in the analysis of plasma proteins in patients with various diseases. To assess whether proteomic discovery in plasma can help predict amyloid-beta burden in preclinical Alzheimer’s disease, Dr. Ashton and his colleagues studied 238 cognitively unimpaired individuals from the Australian Imaging, Biomarker and Lifestyle Flagship Study of Ageing (AIBL) and the Kerr Anglican Retirement Village Initiative in Ageing Health (KARVIAH). The participants had undergone PET to determine their amyloid-beta status. In the AIBL cohort (n = 144), 100 participants were amyloid-beta negative, and 44 were amyloid-beta positive. In the KARVIAH cohort (n = 94), 59 participants were amyloid-beta negative, and 35 were amyloid-beta positive. There were significantly more APOE4 carriers in the amyloid-beta–positive groups than in the amyloid-beta–negative groups. In addition, the amyloid-beta–positive groups tended to be older.
A support vector machine analysis created classifiers predicting amyloid-beta positivity in the AIBL cohort using demographics, proteins, or both. The researchers then tested each classifier in the KARVIAH dataset to identify which model best predicted amyloid-beta positivity. The optimal model included 10 protein features (prothrombin, adhesion G protein–coupled receptor, amyloid-beta A4 protein, NGN2, DNAH10, REST, NfL, RPS6KA3, GPSM2, FHAD1) and two demographic features (APOE4 count and age).
The classifier achieved a testing area under the receiver operator characteristic curve of 0.891 in the KARVIAH cohort to predict amyloid-beta positivity in cognitively unimpaired individuals with a sensitivity of 0.78 and specificity of 0.77.
The 10 protein features “represent a diverse array of pathways,” and the highest ranked feature was the serine protease prothrombin, which is a precursor to thrombin, the authors noted. “Multiple lines of evidence support that cerebrovascular disease may play a role in AD and that amyloid-beta may be involved in thrombosis, fibrinolysis, and inflammation via its interaction with the coagulation cascade,” the researchers wrote.
Two of the biomarkers – amyloid-beta A4 protein and NfL – have been examined in prior research and had a greater effect size in a secondary analysis that included participants with mild cognitive impairment and Alzheimer’s disease. This finding confirms “their connection with the more established disease state,” Dr. Ashton and colleagues said. In the secondary analysis, the optimal classifier included one demographic factor (APOE4 count) and nine protein features, eight of which also were used in the cognitively unimpaired classifier.
The study was funded in part by the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, and many authors reported additional research support from various institutions. One author is an employee of Johnson & Johnson and a named inventor on unrelated biomarker intellectual property owned by Proteome Science and King’s College London.
SOURCE: Ashton NJ et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aau7220.
, researchers reported in Science Advances.
“To our knowledge, this is the first time that a multianalyte plasma biomarker panel for an Alzheimer’s disease–related phenotype has been found and independently replicated by a nontargeted mass spectrometry approach,” said Nicholas J. Ashton, PhD, of King’s College London and the University of Gothenburg in Sweden, and his research colleagues.
Blood-based measures that predict amyloid-beta burden in preclinical Alzheimer’s disease have the potential to help investigators conduct clinical trials and aid in diagnostic management. However, this novel approach needs to be validated and translated “to a simpler automated platform suitable for wider utility,” the investigators noted. In addition, it is unclear whether their classifier can track changes in amyloid-beta or differentiate between other diseases with amyloid-beta pathology.
Advances in mass spectrometry technology have renewed interest in the analysis of plasma proteins in patients with various diseases. To assess whether proteomic discovery in plasma can help predict amyloid-beta burden in preclinical Alzheimer’s disease, Dr. Ashton and his colleagues studied 238 cognitively unimpaired individuals from the Australian Imaging, Biomarker and Lifestyle Flagship Study of Ageing (AIBL) and the Kerr Anglican Retirement Village Initiative in Ageing Health (KARVIAH). The participants had undergone PET to determine their amyloid-beta status. In the AIBL cohort (n = 144), 100 participants were amyloid-beta negative, and 44 were amyloid-beta positive. In the KARVIAH cohort (n = 94), 59 participants were amyloid-beta negative, and 35 were amyloid-beta positive. There were significantly more APOE4 carriers in the amyloid-beta–positive groups than in the amyloid-beta–negative groups. In addition, the amyloid-beta–positive groups tended to be older.
A support vector machine analysis created classifiers predicting amyloid-beta positivity in the AIBL cohort using demographics, proteins, or both. The researchers then tested each classifier in the KARVIAH dataset to identify which model best predicted amyloid-beta positivity. The optimal model included 10 protein features (prothrombin, adhesion G protein–coupled receptor, amyloid-beta A4 protein, NGN2, DNAH10, REST, NfL, RPS6KA3, GPSM2, FHAD1) and two demographic features (APOE4 count and age).
The classifier achieved a testing area under the receiver operator characteristic curve of 0.891 in the KARVIAH cohort to predict amyloid-beta positivity in cognitively unimpaired individuals with a sensitivity of 0.78 and specificity of 0.77.
The 10 protein features “represent a diverse array of pathways,” and the highest ranked feature was the serine protease prothrombin, which is a precursor to thrombin, the authors noted. “Multiple lines of evidence support that cerebrovascular disease may play a role in AD and that amyloid-beta may be involved in thrombosis, fibrinolysis, and inflammation via its interaction with the coagulation cascade,” the researchers wrote.
Two of the biomarkers – amyloid-beta A4 protein and NfL – have been examined in prior research and had a greater effect size in a secondary analysis that included participants with mild cognitive impairment and Alzheimer’s disease. This finding confirms “their connection with the more established disease state,” Dr. Ashton and colleagues said. In the secondary analysis, the optimal classifier included one demographic factor (APOE4 count) and nine protein features, eight of which also were used in the cognitively unimpaired classifier.
The study was funded in part by the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, and many authors reported additional research support from various institutions. One author is an employee of Johnson & Johnson and a named inventor on unrelated biomarker intellectual property owned by Proteome Science and King’s College London.
SOURCE: Ashton NJ et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aau7220.
FROM SCIENCE ADVANCES
Key clinical point: Blood-based measures that predict amyloid-beta burden in preclinical Alzheimer’s disease have the potential to help investigators conduct clinical trials and aid in diagnostic management.
Major finding: A classifier developed using plasma proteomic analysis achieved an area under the receiver operator characteristic curve of 0.891.
Study details: An analysis of data from 238 cognitively unimpaired individuals from the Australian Imaging, Biomarker and Lifestyle Flagship Study of Ageing (AIBL) and the Kerr Anglican Retirement Village Initiative in Ageing Health (KARVIAH).
Disclosures: The study was funded in part by the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, and many authors reported additional research support from various institutions. One author is an employee of Johnson & Johnson and a named inventor on unrelated biomarker intellectual property owned by Proteome Science and King’s College London.
Source: Ashton NJ et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aau7220.
Functional MRI detects consciousness after brain damage
Functional MRI can measure patterns of connectivity to determine levels of consciousness in nonresponsive patients with brain injury, according to results from a multicenter, cross-sectional, observational study.
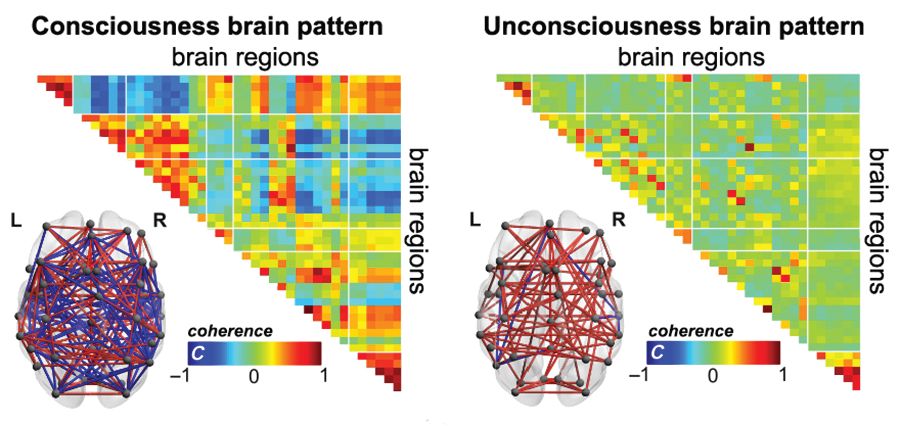
Blood oxygen level–dependent (BOLD) fMRI showed that brain-wide coordination patterns of high complexity became increasingly common moving from unresponsive patients to those with minimal consciousness to healthy individuals, reported lead author Athena Demertzi, PhD, of GIGA Research Institute at the University of Liège in Belgium, and her colleagues.
“Finding reliable markers indicating the presence or absence of consciousness represents an outstanding open problem for science,” the investigators wrote in Science Advances.
In medicine, an fMRI-based measure of consciousness could supplement behavioral assessments of awareness and guide therapeutic strategies; more broadly, image-based markers could help elucidate the nature of consciousness itself.
“We postulate that consciousness has specific characteristics that are based on the temporal dynamics of ongoing brain activity and its coordination over distant cortical regions,” the investigators wrote. “Our hypothesis stems from the common stance of various contemporary theories which propose that consciousness relates to a dynamic process of self-sustained, coordinated brain-scale activity assisting the tuning to a constantly evolving environment, rather than in static descriptions of brain function.”
There is a need for a reliable way of distinguishing consciousness from unconscious states, the investigators said. “Given that nonresponsiveness can be associated with a variety of brain lesions, varying levels of vigilance, and covert cognition, we highlight the need to determine a common set of features capable of accounting for the capacity to sustain conscious experience.”
To search for patterns of brain signal coordination that correlate with consciousness, four independent research centers performed BOLD fMRI scans of participants at rest or under anesthesia with propofol. Of 159 total participants, 47 were healthy individuals and 112 were patients in a vegetative state/with unresponsive wakefulness syndrome (UWS) or in a minimally conscious state (MCS), based on standardized behavioral assessments. The main data analysis, which included 125 participants, assessed BOLD fMRI signal coordination between six brain networks known to have roles in cognitive and functional processes.
The researchers’ analysis revealed four distinct and recurring brain-wide coordination patterns ranging on a scale from highest activity (pattern 1) to lowest activity (pattern 4). Pattern 1, which exhibited most long-distance edges, spatial complexity, efficiency, and community structure, became increasingly common when moving from UWS patients to MCS patients to healthy control individuals (UWS < MCS < HC, rho = 0.7, Spearman rank correlation between rate and group, P less than 1 x 10-16).
In contrast, pattern 4, characterized by low interareal coordination, showed an inverse trend; it became less common when moving from vegetative patients to healthy individuals (UWS > MCS > HC, Spearman rank correlation between rate and group, rho = –0.6, P less than 1 x 10-11). Although patterns 2 and 3 occurred with equal frequency across all groups, the investigators noted that switching between patterns was most common and predictably sequential in healthy individuals, versus patients with UWS, who were least likely to switch patterns. A total of 23 patients who were scanned under propofol anesthesia were equally likely to exhibit pattern 4, regardless of health status, suggesting that pattern 4 depends upon fixed anatomical pathways. Results were not affected by scanning site or other patient characteristics, such as age, gender, etiology, or chronicity.
“We conclude that these patterns of transient brain signal coordination are characteristic of conscious and unconscious brain states,” the investigators wrote, “warranting future research concerning their relationship to ongoing conscious content, and the possibility of modifying their prevalence by external perturbations, both in healthy and pathological individuals, as well as across species.”
The study was funded by a James S. McDonnell Foundation Collaborative Activity Award, INSERM, the Belgian National Funds for Scientific Research, the Canada Excellence Research Chairs program, and others. The authors declared having no conflicts of interest.
SOURCE: Demertzi A et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aat7603.
Functional MRI can measure patterns of connectivity to determine levels of consciousness in nonresponsive patients with brain injury, according to results from a multicenter, cross-sectional, observational study.

Blood oxygen level–dependent (BOLD) fMRI showed that brain-wide coordination patterns of high complexity became increasingly common moving from unresponsive patients to those with minimal consciousness to healthy individuals, reported lead author Athena Demertzi, PhD, of GIGA Research Institute at the University of Liège in Belgium, and her colleagues.
“Finding reliable markers indicating the presence or absence of consciousness represents an outstanding open problem for science,” the investigators wrote in Science Advances.
In medicine, an fMRI-based measure of consciousness could supplement behavioral assessments of awareness and guide therapeutic strategies; more broadly, image-based markers could help elucidate the nature of consciousness itself.
“We postulate that consciousness has specific characteristics that are based on the temporal dynamics of ongoing brain activity and its coordination over distant cortical regions,” the investigators wrote. “Our hypothesis stems from the common stance of various contemporary theories which propose that consciousness relates to a dynamic process of self-sustained, coordinated brain-scale activity assisting the tuning to a constantly evolving environment, rather than in static descriptions of brain function.”
There is a need for a reliable way of distinguishing consciousness from unconscious states, the investigators said. “Given that nonresponsiveness can be associated with a variety of brain lesions, varying levels of vigilance, and covert cognition, we highlight the need to determine a common set of features capable of accounting for the capacity to sustain conscious experience.”
To search for patterns of brain signal coordination that correlate with consciousness, four independent research centers performed BOLD fMRI scans of participants at rest or under anesthesia with propofol. Of 159 total participants, 47 were healthy individuals and 112 were patients in a vegetative state/with unresponsive wakefulness syndrome (UWS) or in a minimally conscious state (MCS), based on standardized behavioral assessments. The main data analysis, which included 125 participants, assessed BOLD fMRI signal coordination between six brain networks known to have roles in cognitive and functional processes.
The researchers’ analysis revealed four distinct and recurring brain-wide coordination patterns ranging on a scale from highest activity (pattern 1) to lowest activity (pattern 4). Pattern 1, which exhibited most long-distance edges, spatial complexity, efficiency, and community structure, became increasingly common when moving from UWS patients to MCS patients to healthy control individuals (UWS < MCS < HC, rho = 0.7, Spearman rank correlation between rate and group, P less than 1 x 10-16).
In contrast, pattern 4, characterized by low interareal coordination, showed an inverse trend; it became less common when moving from vegetative patients to healthy individuals (UWS > MCS > HC, Spearman rank correlation between rate and group, rho = –0.6, P less than 1 x 10-11). Although patterns 2 and 3 occurred with equal frequency across all groups, the investigators noted that switching between patterns was most common and predictably sequential in healthy individuals, versus patients with UWS, who were least likely to switch patterns. A total of 23 patients who were scanned under propofol anesthesia were equally likely to exhibit pattern 4, regardless of health status, suggesting that pattern 4 depends upon fixed anatomical pathways. Results were not affected by scanning site or other patient characteristics, such as age, gender, etiology, or chronicity.
“We conclude that these patterns of transient brain signal coordination are characteristic of conscious and unconscious brain states,” the investigators wrote, “warranting future research concerning their relationship to ongoing conscious content, and the possibility of modifying their prevalence by external perturbations, both in healthy and pathological individuals, as well as across species.”
The study was funded by a James S. McDonnell Foundation Collaborative Activity Award, INSERM, the Belgian National Funds for Scientific Research, the Canada Excellence Research Chairs program, and others. The authors declared having no conflicts of interest.
SOURCE: Demertzi A et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aat7603.
Functional MRI can measure patterns of connectivity to determine levels of consciousness in nonresponsive patients with brain injury, according to results from a multicenter, cross-sectional, observational study.

Blood oxygen level–dependent (BOLD) fMRI showed that brain-wide coordination patterns of high complexity became increasingly common moving from unresponsive patients to those with minimal consciousness to healthy individuals, reported lead author Athena Demertzi, PhD, of GIGA Research Institute at the University of Liège in Belgium, and her colleagues.
“Finding reliable markers indicating the presence or absence of consciousness represents an outstanding open problem for science,” the investigators wrote in Science Advances.
In medicine, an fMRI-based measure of consciousness could supplement behavioral assessments of awareness and guide therapeutic strategies; more broadly, image-based markers could help elucidate the nature of consciousness itself.
“We postulate that consciousness has specific characteristics that are based on the temporal dynamics of ongoing brain activity and its coordination over distant cortical regions,” the investigators wrote. “Our hypothesis stems from the common stance of various contemporary theories which propose that consciousness relates to a dynamic process of self-sustained, coordinated brain-scale activity assisting the tuning to a constantly evolving environment, rather than in static descriptions of brain function.”
There is a need for a reliable way of distinguishing consciousness from unconscious states, the investigators said. “Given that nonresponsiveness can be associated with a variety of brain lesions, varying levels of vigilance, and covert cognition, we highlight the need to determine a common set of features capable of accounting for the capacity to sustain conscious experience.”
To search for patterns of brain signal coordination that correlate with consciousness, four independent research centers performed BOLD fMRI scans of participants at rest or under anesthesia with propofol. Of 159 total participants, 47 were healthy individuals and 112 were patients in a vegetative state/with unresponsive wakefulness syndrome (UWS) or in a minimally conscious state (MCS), based on standardized behavioral assessments. The main data analysis, which included 125 participants, assessed BOLD fMRI signal coordination between six brain networks known to have roles in cognitive and functional processes.
The researchers’ analysis revealed four distinct and recurring brain-wide coordination patterns ranging on a scale from highest activity (pattern 1) to lowest activity (pattern 4). Pattern 1, which exhibited most long-distance edges, spatial complexity, efficiency, and community structure, became increasingly common when moving from UWS patients to MCS patients to healthy control individuals (UWS < MCS < HC, rho = 0.7, Spearman rank correlation between rate and group, P less than 1 x 10-16).
In contrast, pattern 4, characterized by low interareal coordination, showed an inverse trend; it became less common when moving from vegetative patients to healthy individuals (UWS > MCS > HC, Spearman rank correlation between rate and group, rho = –0.6, P less than 1 x 10-11). Although patterns 2 and 3 occurred with equal frequency across all groups, the investigators noted that switching between patterns was most common and predictably sequential in healthy individuals, versus patients with UWS, who were least likely to switch patterns. A total of 23 patients who were scanned under propofol anesthesia were equally likely to exhibit pattern 4, regardless of health status, suggesting that pattern 4 depends upon fixed anatomical pathways. Results were not affected by scanning site or other patient characteristics, such as age, gender, etiology, or chronicity.
“We conclude that these patterns of transient brain signal coordination are characteristic of conscious and unconscious brain states,” the investigators wrote, “warranting future research concerning their relationship to ongoing conscious content, and the possibility of modifying their prevalence by external perturbations, both in healthy and pathological individuals, as well as across species.”
The study was funded by a James S. McDonnell Foundation Collaborative Activity Award, INSERM, the Belgian National Funds for Scientific Research, the Canada Excellence Research Chairs program, and others. The authors declared having no conflicts of interest.
SOURCE: Demertzi A et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aat7603.
FROM SCIENCE ADVANCES
Key clinical point:
Major finding: A brain-wide coordination pattern of high complexity became increasingly common when moving from patients with unresponsive wakefulness syndrome (UWS) to patients in a minimally conscious state (MCS) to healthy control individuals.
Study details: A study involving blood oxygen level–dependent (BOLD) fMRI scans at rest or under anesthesia in 159 participants at four independent research facilities.
Disclosures: The study was funded by a James S. McDonnell Foundation Collaborative Activity Award, INSERM, the Belgian National Funds for Scientific Research, the Canada Excellence Research Chairs program, and others. The authors declared having no conflicts of interest.
Source: Demertzi A et al. Sci Adv. 2019 Feb 6. doi: 10.1126/sciadv.aat7603.
American football and CTE: Is a racial divide inevitable?
Evidence that American football can lead to chronic traumatic encephalopathy (CTE), continues to grow. As a result, some parents are opting to sign their sons up for other sports.
In the 2017-2018 school year, 6.6% fewer high school athletes participated in tackle football than did 8 years before according to the National Federation of State High School Associations.
Many black parents encourage their sons to play football as a way to protect them gang activity. In addition, the sport can be their sole option for securing a college education for their children, an article in the Atlantic said. A recent survey of 50,000 8th-, 10th-, and 12th-grade students found that tackle football is predominantly the domain of black youth.
“This divergence paints a troubling picture of how economic opportunity – or a lack thereof – governs which boys are incentivized to put their body and brain at risk to play. Depending on where families live, and what other options are available to them, they see either a game that is too violent to consider or one that is necessary and important, if risky. Millions of Americans still watch football; NFL ratings were up this season,” Alana Semuels wrote in the article. “That a distinct portion of families won’t let their children play creates a disturbing future for the country’s most popular game.”
“Without a reversal in economic fortunes for poor communities across the country, football could one day become a sport played almost exclusively by black athletes, while still enjoyed by everyone. Black athletes – who already make up the majority of players in the most dangerous on-field positions – would continue to suffer from long-term brain damage, their life cut short by dementia and the scourge of CTE,” she wrote.
Meanwhile, numerous outlets reported that Super Bowl LIII garnered the lowest ratings since 2008.
Psychiatric hospital set to close
In both Kansas and Missouri, a shortage in mental health care has become evident, according to an article in the Kansas City Star. And now the Two Rivers Behavioral Health System, a private psychiatric hospital in southeast Kansas City, Mo., is closing its doors. The result will be a loss of 129 jobs and 105 fewer mental health beds in the city.
Patients currently in the facility will be relocated, and their care will continue. But for those who come after, care will now be tougher to find.
Two Rivers, owned by Pennsylvania-based Universal Health Services, treats children and adults. It had 2,347 discharges in 2017 and almost $28 million in revenue but had a net loss of about $3.4 million. The facility has been under scrutiny in the past two decades over its treatment of patients, with accusations about the bolstering of false memories concerning involvements in satanic cults and the treatment of a convicted sex offender who assaulted another patient. The most recent state inspection showed that Two Rivers had failed to provide a safe environment for six patients who were considered suicide risks. The patients had unsupervised access to the nurses’ station, as well as access to pens that could have been used for stabbing and a charging cord that could have been used for strangulation.
In an interview with the Star, Mark Stringer, director of the Missouri Department of Mental Health, said private psychiatric hospitals like Two Rivers are finding it harder to keep functioning, partly because of nursing shortages. Private facilities are not subsidized like state mental hospitals and are unable to secure staff from other facilities.
“There is a general worry about the availability of psychiatric services for people in crisis; there’s just no doubt about that,” Mr. Stringer said. “The loss of beds certainly hurts.”
New center offers ‘kind patient care’
In Nashville, Tenn., a new mental illness crisis treatment center is open. The center offers a 24/7 option for those with mental health issues who have run afoul of the law. Instead of incarceration, they can receive treatment, the Tennessean reported.
Estimates are that more than 1 million residents of Tennessee aged 18 years and older have a mental health or substance use disorder. About 25% of those residents having a serious mental health illness.
The new facility includes a crisis walk-in center and a unit where those in the throes of a mental health crisis can seek care. A goal is to get people suffering from an urgent mental illness crisis connected to help faster, especially when they come into contact with police.
“It’s very important to come to a place that’s going to get you help,” Bonnie Kelly said in the article. Ms. Kelly, who reportedly has bipolar disorder, has been arrested several times for disorderly conduct tied to her condition. “It means everything. It is good, kind patient care, rather than just getting you out of the way.”
Aside from benefiting those in need of mental health care, the center will ease the strain on Nashville police, who currently spend more than 5,000 hours each year responding to mental health–related calls. The officers must remain with the person until transfer to a jail or mental health facility is done.
“As a city, we are recognizing that there is a need, and we are investing in that,” East Precinct Commander David Imhof said in the article. “We are helping a population that has had no voice in the past.” Right now, fewer than 60% of patients discharged from state mental health facilities receive any sort of coverage. The result can be cycles of release, arrest, and incarceration.
Agency aims to protect patients
The Oregon Health Authority has stepped in to prevent numerous state-funded mental health facilities run by the same contractor from booting out patients with severe mental health problems.
The contractor is Kepro, a Pennsylvania-based company. Since December, the health authority has reversed decisions to release 17 patients, according to an article in the Oregonian. The harder line follows revelations by the newspaper of serious harm to patients who had been released before they were capable of caring for themselves.
Kepro was hired by the health authority and paid $27 million to evaluate the medical needs of mental health patients in Oregon. As part of the evaluation, 215 of 250 patients were deemed unqualified to remain in care.
One was Ruane Oliverio, who has schizophrenia, who was kicked out of a locked facility in Portland last June. Clinicians had warned against her release, insisting that her mental state remained too vulnerable. After being hospitalized multiple times, she was sent to the Oregon State Hospital, the highest and most expensive level of care. She was one of those targeted for release. This decision was reversed, and she continues to receive care.
Coalition seeks mental health care for refugees
A new coalition called Matters Involving Neuro-Disorders, or MIND, is trying to help refugees with mental health conditions. The effort is a response to several mental health-related deaths of refugees during 2014-2016, a video produced by the San Diego Union-Tribune said.
“Refugees are brought to this country to help them rebuild their lives,” said Justin Mudekereza, executive director of New Neighbor Relief, a nonprofit organization dedicated to helping refugees adjust to their new lives in the United States. “They have gone through a lot in their countries, then from there, they went to refugee camps, where they spend 15-20 years or more before they got a chance to come to this country.”
Sheila S. Mitra-Sarkar, PhD, of the Institute of Public Urban Affairs at San Diego State University, described the need for a “comprehensive solution” to help refugees adapt to their new society, learn English, find housing and employment, and thrive.
“When I see a patient or someone who seems to have a psychological issue ... I look at everything that goes around them,” said John C. Kuek, PhD, of La Maestra Community Health Centers in San Diego. “I’m looking at the housing issue, the employment issue, and translational issue – meaning they have some family back home and they have a live family here to care for.”
Evidence that American football can lead to chronic traumatic encephalopathy (CTE), continues to grow. As a result, some parents are opting to sign their sons up for other sports.
In the 2017-2018 school year, 6.6% fewer high school athletes participated in tackle football than did 8 years before according to the National Federation of State High School Associations.
Many black parents encourage their sons to play football as a way to protect them gang activity. In addition, the sport can be their sole option for securing a college education for their children, an article in the Atlantic said. A recent survey of 50,000 8th-, 10th-, and 12th-grade students found that tackle football is predominantly the domain of black youth.
“This divergence paints a troubling picture of how economic opportunity – or a lack thereof – governs which boys are incentivized to put their body and brain at risk to play. Depending on where families live, and what other options are available to them, they see either a game that is too violent to consider or one that is necessary and important, if risky. Millions of Americans still watch football; NFL ratings were up this season,” Alana Semuels wrote in the article. “That a distinct portion of families won’t let their children play creates a disturbing future for the country’s most popular game.”
“Without a reversal in economic fortunes for poor communities across the country, football could one day become a sport played almost exclusively by black athletes, while still enjoyed by everyone. Black athletes – who already make up the majority of players in the most dangerous on-field positions – would continue to suffer from long-term brain damage, their life cut short by dementia and the scourge of CTE,” she wrote.
Meanwhile, numerous outlets reported that Super Bowl LIII garnered the lowest ratings since 2008.
Psychiatric hospital set to close
In both Kansas and Missouri, a shortage in mental health care has become evident, according to an article in the Kansas City Star. And now the Two Rivers Behavioral Health System, a private psychiatric hospital in southeast Kansas City, Mo., is closing its doors. The result will be a loss of 129 jobs and 105 fewer mental health beds in the city.
Patients currently in the facility will be relocated, and their care will continue. But for those who come after, care will now be tougher to find.
Two Rivers, owned by Pennsylvania-based Universal Health Services, treats children and adults. It had 2,347 discharges in 2017 and almost $28 million in revenue but had a net loss of about $3.4 million. The facility has been under scrutiny in the past two decades over its treatment of patients, with accusations about the bolstering of false memories concerning involvements in satanic cults and the treatment of a convicted sex offender who assaulted another patient. The most recent state inspection showed that Two Rivers had failed to provide a safe environment for six patients who were considered suicide risks. The patients had unsupervised access to the nurses’ station, as well as access to pens that could have been used for stabbing and a charging cord that could have been used for strangulation.
In an interview with the Star, Mark Stringer, director of the Missouri Department of Mental Health, said private psychiatric hospitals like Two Rivers are finding it harder to keep functioning, partly because of nursing shortages. Private facilities are not subsidized like state mental hospitals and are unable to secure staff from other facilities.
“There is a general worry about the availability of psychiatric services for people in crisis; there’s just no doubt about that,” Mr. Stringer said. “The loss of beds certainly hurts.”
New center offers ‘kind patient care’
In Nashville, Tenn., a new mental illness crisis treatment center is open. The center offers a 24/7 option for those with mental health issues who have run afoul of the law. Instead of incarceration, they can receive treatment, the Tennessean reported.
Estimates are that more than 1 million residents of Tennessee aged 18 years and older have a mental health or substance use disorder. About 25% of those residents having a serious mental health illness.
The new facility includes a crisis walk-in center and a unit where those in the throes of a mental health crisis can seek care. A goal is to get people suffering from an urgent mental illness crisis connected to help faster, especially when they come into contact with police.
“It’s very important to come to a place that’s going to get you help,” Bonnie Kelly said in the article. Ms. Kelly, who reportedly has bipolar disorder, has been arrested several times for disorderly conduct tied to her condition. “It means everything. It is good, kind patient care, rather than just getting you out of the way.”
Aside from benefiting those in need of mental health care, the center will ease the strain on Nashville police, who currently spend more than 5,000 hours each year responding to mental health–related calls. The officers must remain with the person until transfer to a jail or mental health facility is done.
“As a city, we are recognizing that there is a need, and we are investing in that,” East Precinct Commander David Imhof said in the article. “We are helping a population that has had no voice in the past.” Right now, fewer than 60% of patients discharged from state mental health facilities receive any sort of coverage. The result can be cycles of release, arrest, and incarceration.
Agency aims to protect patients
The Oregon Health Authority has stepped in to prevent numerous state-funded mental health facilities run by the same contractor from booting out patients with severe mental health problems.
The contractor is Kepro, a Pennsylvania-based company. Since December, the health authority has reversed decisions to release 17 patients, according to an article in the Oregonian. The harder line follows revelations by the newspaper of serious harm to patients who had been released before they were capable of caring for themselves.
Kepro was hired by the health authority and paid $27 million to evaluate the medical needs of mental health patients in Oregon. As part of the evaluation, 215 of 250 patients were deemed unqualified to remain in care.
One was Ruane Oliverio, who has schizophrenia, who was kicked out of a locked facility in Portland last June. Clinicians had warned against her release, insisting that her mental state remained too vulnerable. After being hospitalized multiple times, she was sent to the Oregon State Hospital, the highest and most expensive level of care. She was one of those targeted for release. This decision was reversed, and she continues to receive care.
Coalition seeks mental health care for refugees
A new coalition called Matters Involving Neuro-Disorders, or MIND, is trying to help refugees with mental health conditions. The effort is a response to several mental health-related deaths of refugees during 2014-2016, a video produced by the San Diego Union-Tribune said.
“Refugees are brought to this country to help them rebuild their lives,” said Justin Mudekereza, executive director of New Neighbor Relief, a nonprofit organization dedicated to helping refugees adjust to their new lives in the United States. “They have gone through a lot in their countries, then from there, they went to refugee camps, where they spend 15-20 years or more before they got a chance to come to this country.”
Sheila S. Mitra-Sarkar, PhD, of the Institute of Public Urban Affairs at San Diego State University, described the need for a “comprehensive solution” to help refugees adapt to their new society, learn English, find housing and employment, and thrive.
“When I see a patient or someone who seems to have a psychological issue ... I look at everything that goes around them,” said John C. Kuek, PhD, of La Maestra Community Health Centers in San Diego. “I’m looking at the housing issue, the employment issue, and translational issue – meaning they have some family back home and they have a live family here to care for.”
Evidence that American football can lead to chronic traumatic encephalopathy (CTE), continues to grow. As a result, some parents are opting to sign their sons up for other sports.
In the 2017-2018 school year, 6.6% fewer high school athletes participated in tackle football than did 8 years before according to the National Federation of State High School Associations.
Many black parents encourage their sons to play football as a way to protect them gang activity. In addition, the sport can be their sole option for securing a college education for their children, an article in the Atlantic said. A recent survey of 50,000 8th-, 10th-, and 12th-grade students found that tackle football is predominantly the domain of black youth.
“This divergence paints a troubling picture of how economic opportunity – or a lack thereof – governs which boys are incentivized to put their body and brain at risk to play. Depending on where families live, and what other options are available to them, they see either a game that is too violent to consider or one that is necessary and important, if risky. Millions of Americans still watch football; NFL ratings were up this season,” Alana Semuels wrote in the article. “That a distinct portion of families won’t let their children play creates a disturbing future for the country’s most popular game.”
“Without a reversal in economic fortunes for poor communities across the country, football could one day become a sport played almost exclusively by black athletes, while still enjoyed by everyone. Black athletes – who already make up the majority of players in the most dangerous on-field positions – would continue to suffer from long-term brain damage, their life cut short by dementia and the scourge of CTE,” she wrote.
Meanwhile, numerous outlets reported that Super Bowl LIII garnered the lowest ratings since 2008.
Psychiatric hospital set to close
In both Kansas and Missouri, a shortage in mental health care has become evident, according to an article in the Kansas City Star. And now the Two Rivers Behavioral Health System, a private psychiatric hospital in southeast Kansas City, Mo., is closing its doors. The result will be a loss of 129 jobs and 105 fewer mental health beds in the city.
Patients currently in the facility will be relocated, and their care will continue. But for those who come after, care will now be tougher to find.
Two Rivers, owned by Pennsylvania-based Universal Health Services, treats children and adults. It had 2,347 discharges in 2017 and almost $28 million in revenue but had a net loss of about $3.4 million. The facility has been under scrutiny in the past two decades over its treatment of patients, with accusations about the bolstering of false memories concerning involvements in satanic cults and the treatment of a convicted sex offender who assaulted another patient. The most recent state inspection showed that Two Rivers had failed to provide a safe environment for six patients who were considered suicide risks. The patients had unsupervised access to the nurses’ station, as well as access to pens that could have been used for stabbing and a charging cord that could have been used for strangulation.
In an interview with the Star, Mark Stringer, director of the Missouri Department of Mental Health, said private psychiatric hospitals like Two Rivers are finding it harder to keep functioning, partly because of nursing shortages. Private facilities are not subsidized like state mental hospitals and are unable to secure staff from other facilities.
“There is a general worry about the availability of psychiatric services for people in crisis; there’s just no doubt about that,” Mr. Stringer said. “The loss of beds certainly hurts.”
New center offers ‘kind patient care’
In Nashville, Tenn., a new mental illness crisis treatment center is open. The center offers a 24/7 option for those with mental health issues who have run afoul of the law. Instead of incarceration, they can receive treatment, the Tennessean reported.
Estimates are that more than 1 million residents of Tennessee aged 18 years and older have a mental health or substance use disorder. About 25% of those residents having a serious mental health illness.
The new facility includes a crisis walk-in center and a unit where those in the throes of a mental health crisis can seek care. A goal is to get people suffering from an urgent mental illness crisis connected to help faster, especially when they come into contact with police.
“It’s very important to come to a place that’s going to get you help,” Bonnie Kelly said in the article. Ms. Kelly, who reportedly has bipolar disorder, has been arrested several times for disorderly conduct tied to her condition. “It means everything. It is good, kind patient care, rather than just getting you out of the way.”
Aside from benefiting those in need of mental health care, the center will ease the strain on Nashville police, who currently spend more than 5,000 hours each year responding to mental health–related calls. The officers must remain with the person until transfer to a jail or mental health facility is done.
“As a city, we are recognizing that there is a need, and we are investing in that,” East Precinct Commander David Imhof said in the article. “We are helping a population that has had no voice in the past.” Right now, fewer than 60% of patients discharged from state mental health facilities receive any sort of coverage. The result can be cycles of release, arrest, and incarceration.
Agency aims to protect patients
The Oregon Health Authority has stepped in to prevent numerous state-funded mental health facilities run by the same contractor from booting out patients with severe mental health problems.
The contractor is Kepro, a Pennsylvania-based company. Since December, the health authority has reversed decisions to release 17 patients, according to an article in the Oregonian. The harder line follows revelations by the newspaper of serious harm to patients who had been released before they were capable of caring for themselves.
Kepro was hired by the health authority and paid $27 million to evaluate the medical needs of mental health patients in Oregon. As part of the evaluation, 215 of 250 patients were deemed unqualified to remain in care.
One was Ruane Oliverio, who has schizophrenia, who was kicked out of a locked facility in Portland last June. Clinicians had warned against her release, insisting that her mental state remained too vulnerable. After being hospitalized multiple times, she was sent to the Oregon State Hospital, the highest and most expensive level of care. She was one of those targeted for release. This decision was reversed, and she continues to receive care.
Coalition seeks mental health care for refugees
A new coalition called Matters Involving Neuro-Disorders, or MIND, is trying to help refugees with mental health conditions. The effort is a response to several mental health-related deaths of refugees during 2014-2016, a video produced by the San Diego Union-Tribune said.
“Refugees are brought to this country to help them rebuild their lives,” said Justin Mudekereza, executive director of New Neighbor Relief, a nonprofit organization dedicated to helping refugees adjust to their new lives in the United States. “They have gone through a lot in their countries, then from there, they went to refugee camps, where they spend 15-20 years or more before they got a chance to come to this country.”
Sheila S. Mitra-Sarkar, PhD, of the Institute of Public Urban Affairs at San Diego State University, described the need for a “comprehensive solution” to help refugees adapt to their new society, learn English, find housing and employment, and thrive.
“When I see a patient or someone who seems to have a psychological issue ... I look at everything that goes around them,” said John C. Kuek, PhD, of La Maestra Community Health Centers in San Diego. “I’m looking at the housing issue, the employment issue, and translational issue – meaning they have some family back home and they have a live family here to care for.”
Researchers compare focused ultrasound and DBS for essential tremor
LAS VEGAS – according to two presentations delivered at the annual meeting of the North American Neuromodulation Society. The techniques’ surgical procedures, associated risks, and adverse event profiles may influence neurologists and patients in their choice of treatment.
FUS allows neurosurgeons to apply thermal ablation to create a lesion on the thalamus. MRI guidance enables precise control of the lesion location (within approximately 1 mm) and of the treatment intensity. The surgery can be performed with high-resolution stereotactic framing.
DBS entails the surgical implantation of a neurostimulator and attached leads and electrodes. The neurosurgeon drills a hole of approximately 14 mm in diameter into the skull so that the electrode can be inserted stereotactically while the patient is awake or asleep. The neurostimulator is installed separately.
Both treatments provide functional benefits
In 2016, W. Jeff Elias, MD, director of stereotactic and functional neurosurgery at the University of Virginia in Charlottesville, and his colleagues published the results of a randomized controlled trial that compared FUS with sham treatment in 76 patients with essential tremor. At three months, hand tremor had improved by approximately 50% among treated patients, but controls had no significant benefit(N Engl J Med. 2016 Aug 25;375[8]:730-9). The improvement among treated patients was maintained for 12 months. Disability and quality of life also improved after FUS.
A study by Schuurman et al. published in 2000 (N Engl J Med. 2000 Feb 17;342[7]:461-8) showed that DBS and FUS had similar efficacy at 1 year, said Kathryn L. Holloway, MD, professor of neurosurgery at Virginia Commonwealth University in Richmond. It included 45 patients with Parkinson’s disease, 13 with essential tremor, and 10 with multiple sclerosis who were randomized 1:1 to FUS or DBS. The primary outcome was activities of daily living, and blinded physicians assessed patient videos. Most of the patients who improved had received DBS, and most of the ones who worsened had received FUS, said Dr. Holloway. Among patients with essential tremor, tremor improved by between 94% and 100% with either treatment.
To find more recent data about these treatments, Dr. Holloway searched the literature for studies of FUS or DBS for essential tremor. She analyzed only studies that included unselected populations, blinded evaluations within 1 or 2 years of surgery, and tremor scores for the treated side. She found two studies of FUS, including Dr. Elias’s 2016 trial and a 2018 follow-up (Ann Neurol. 2018 Jan;83[1]:107-14). Dr. Holloway also identified three trials of DBS.
In these studies, reduction of hand tremor was 55% with FUS and between 63% and 69% with DBS. Reduction of postural tremor was approximately 72% with FUS and approximately 67% with DBS. Reduction of action tremor was about 52% with FUS and between 65% and 71% with DBS. Overall, DBS appears to be more effective, said Dr. Holloway.
A 2015 study (Mov Disord. 2015 Dec;30[14]:1937-43) that compared bilateral DBS, unilateral DBS, and unilateral FUS for essential tremor indicated that the treatments provide similar benefits on hand tremor, disability, and quality of life, said Dr. Elias. FUS is inferior to DBS, however, for total tremor and axial tremor.
Furthermore, the efficacy of FUS wanes over time, said Dr. Elias. He and his colleagues conducted a pilot study of 15 patients with essential tremor who received FUS (N Engl J Med. 2013 Aug 15;369[7]:640-8). At 6 years, 6 of 13 patients whose data were available still had a 50% improvement in tremor. “Some went on to [receive] DBS,” said Dr. Elias. “Functional improvements persisted more than the tremor improvement.”
Adverse events
In their 2016 trial of FUS, Dr. Elias and his colleagues observed 210 adverse events, which is approximately “what you would expect with a modern day, FDA-monitored clinical trial.” Sensory effects and gait disturbance accounted for most of the thalamotomy-related adverse events. Sensory problems such as numbness or parestheisa persisted at 1 year in 14% of treated patients, and gait disturbance persisted at 1 year in 9%. The investigators did not observe any hemorrhages, infections, or cavitation-related effects from FUS.
In a 2018 analysis of five clinical trials of FUS for essential tremor, Fishman et al. found that 79% of adverse events were mild and 1% were severe (Mov Disord. 2018 May;33[5]:843-7). The risk of a severe adverse event therefore can be considered low, and it may decrease as neurosurgeons gain experience with the procedure, said Dr. Elias.
In the 2000 Schuurman et al. study, the researchers observed significantly fewer adverse events overall among patients with Parkinson’s disease or essential tremor who received DBS, compared with patients who received FUS. Cognitive deterioration, severe dysarthria, and severe ataxia were more common in the FUS group than in the DBS group. Dr. Holloway’s analysis of adverse events in the five more recent trials that she identified yielded similar results.
Although MRI-guided FUS is a precise way to make lesions, functional areas in the thalamus overlap, which makes it more difficult to target only the intended region, said Dr. Holloway. The functional overlap thus increases the risk of adverse events (e.g., sensory impairments, dysarthria, or ataxia). The adverse events that result from FUS may last as long as a year. “Patients will put up anything for about a month after surgery, and then they start to get annoyed,” said Dr. Holloway.
In addition, Schuurman et al. found that FUS entailed a greater risk of permanent side effects, compared with DBS. “That’s the key point here,” said Dr. Holloway. Most of the adverse effects in the DBS group were resolved by adjusting or turning off the stimulator. Hardware issues resulting from DBS are frustrating, but reversible, but a patient with an adverse event after FUS often is “stuck with it,” said Dr. Holloway. The Schuurman et al. data indicated that, in terms of adverse events, “thalamotomy was inferior to DBS,” she added.
Implantation of DBS entails the risks inherent to surgeries that open the skull (such as seizures, air embolism, and hemorrhage). DBS entails a 2% risk of hemorrhage or infection, said Dr. Elias. Furthermore, as much as 15% of patients who undergo DBS implantation require additional surgery.
“FUS is not going to cause a life-threatening hemorrhage, but DBS certainly can,” said Dr. Holloway.
Managing disease progression
Essential tremor is a progressive disease, and older patients are more likely to have exponential progression than linear progression. Data, such as those published by Zhang et al. (J Neurosurg. 2010 Jun;112[6]:1271-6), indicate that DBS can “keep up with the progression of the disease,” said Dr. Holloway. The authors found that tremor scores did not change significantly over approximately 5 years when patients with essential tremor who had received DBS implantation had periodic assessments and increases in stimulation parameters when appropriate.
If a patient with essential tremor undergoes FUS thalamotomy and has subsequent disease progression, DBS may be considered for reducing tremor, said Dr. Holloway. Most adverse events resulting from DBS implantation are reversible with adjustment of the stimulation parameters. A second thalamotomy, however, could cause severe dysarthria and other irreversible adverse events. “Only DBS can safely address tremor progression,” said Dr. Holloway.
LAS VEGAS – according to two presentations delivered at the annual meeting of the North American Neuromodulation Society. The techniques’ surgical procedures, associated risks, and adverse event profiles may influence neurologists and patients in their choice of treatment.
FUS allows neurosurgeons to apply thermal ablation to create a lesion on the thalamus. MRI guidance enables precise control of the lesion location (within approximately 1 mm) and of the treatment intensity. The surgery can be performed with high-resolution stereotactic framing.
DBS entails the surgical implantation of a neurostimulator and attached leads and electrodes. The neurosurgeon drills a hole of approximately 14 mm in diameter into the skull so that the electrode can be inserted stereotactically while the patient is awake or asleep. The neurostimulator is installed separately.
Both treatments provide functional benefits
In 2016, W. Jeff Elias, MD, director of stereotactic and functional neurosurgery at the University of Virginia in Charlottesville, and his colleagues published the results of a randomized controlled trial that compared FUS with sham treatment in 76 patients with essential tremor. At three months, hand tremor had improved by approximately 50% among treated patients, but controls had no significant benefit(N Engl J Med. 2016 Aug 25;375[8]:730-9). The improvement among treated patients was maintained for 12 months. Disability and quality of life also improved after FUS.
A study by Schuurman et al. published in 2000 (N Engl J Med. 2000 Feb 17;342[7]:461-8) showed that DBS and FUS had similar efficacy at 1 year, said Kathryn L. Holloway, MD, professor of neurosurgery at Virginia Commonwealth University in Richmond. It included 45 patients with Parkinson’s disease, 13 with essential tremor, and 10 with multiple sclerosis who were randomized 1:1 to FUS or DBS. The primary outcome was activities of daily living, and blinded physicians assessed patient videos. Most of the patients who improved had received DBS, and most of the ones who worsened had received FUS, said Dr. Holloway. Among patients with essential tremor, tremor improved by between 94% and 100% with either treatment.
To find more recent data about these treatments, Dr. Holloway searched the literature for studies of FUS or DBS for essential tremor. She analyzed only studies that included unselected populations, blinded evaluations within 1 or 2 years of surgery, and tremor scores for the treated side. She found two studies of FUS, including Dr. Elias’s 2016 trial and a 2018 follow-up (Ann Neurol. 2018 Jan;83[1]:107-14). Dr. Holloway also identified three trials of DBS.
In these studies, reduction of hand tremor was 55% with FUS and between 63% and 69% with DBS. Reduction of postural tremor was approximately 72% with FUS and approximately 67% with DBS. Reduction of action tremor was about 52% with FUS and between 65% and 71% with DBS. Overall, DBS appears to be more effective, said Dr. Holloway.
A 2015 study (Mov Disord. 2015 Dec;30[14]:1937-43) that compared bilateral DBS, unilateral DBS, and unilateral FUS for essential tremor indicated that the treatments provide similar benefits on hand tremor, disability, and quality of life, said Dr. Elias. FUS is inferior to DBS, however, for total tremor and axial tremor.
Furthermore, the efficacy of FUS wanes over time, said Dr. Elias. He and his colleagues conducted a pilot study of 15 patients with essential tremor who received FUS (N Engl J Med. 2013 Aug 15;369[7]:640-8). At 6 years, 6 of 13 patients whose data were available still had a 50% improvement in tremor. “Some went on to [receive] DBS,” said Dr. Elias. “Functional improvements persisted more than the tremor improvement.”
Adverse events
In their 2016 trial of FUS, Dr. Elias and his colleagues observed 210 adverse events, which is approximately “what you would expect with a modern day, FDA-monitored clinical trial.” Sensory effects and gait disturbance accounted for most of the thalamotomy-related adverse events. Sensory problems such as numbness or parestheisa persisted at 1 year in 14% of treated patients, and gait disturbance persisted at 1 year in 9%. The investigators did not observe any hemorrhages, infections, or cavitation-related effects from FUS.
In a 2018 analysis of five clinical trials of FUS for essential tremor, Fishman et al. found that 79% of adverse events were mild and 1% were severe (Mov Disord. 2018 May;33[5]:843-7). The risk of a severe adverse event therefore can be considered low, and it may decrease as neurosurgeons gain experience with the procedure, said Dr. Elias.
In the 2000 Schuurman et al. study, the researchers observed significantly fewer adverse events overall among patients with Parkinson’s disease or essential tremor who received DBS, compared with patients who received FUS. Cognitive deterioration, severe dysarthria, and severe ataxia were more common in the FUS group than in the DBS group. Dr. Holloway’s analysis of adverse events in the five more recent trials that she identified yielded similar results.
Although MRI-guided FUS is a precise way to make lesions, functional areas in the thalamus overlap, which makes it more difficult to target only the intended region, said Dr. Holloway. The functional overlap thus increases the risk of adverse events (e.g., sensory impairments, dysarthria, or ataxia). The adverse events that result from FUS may last as long as a year. “Patients will put up anything for about a month after surgery, and then they start to get annoyed,” said Dr. Holloway.
In addition, Schuurman et al. found that FUS entailed a greater risk of permanent side effects, compared with DBS. “That’s the key point here,” said Dr. Holloway. Most of the adverse effects in the DBS group were resolved by adjusting or turning off the stimulator. Hardware issues resulting from DBS are frustrating, but reversible, but a patient with an adverse event after FUS often is “stuck with it,” said Dr. Holloway. The Schuurman et al. data indicated that, in terms of adverse events, “thalamotomy was inferior to DBS,” she added.
Implantation of DBS entails the risks inherent to surgeries that open the skull (such as seizures, air embolism, and hemorrhage). DBS entails a 2% risk of hemorrhage or infection, said Dr. Elias. Furthermore, as much as 15% of patients who undergo DBS implantation require additional surgery.
“FUS is not going to cause a life-threatening hemorrhage, but DBS certainly can,” said Dr. Holloway.
Managing disease progression
Essential tremor is a progressive disease, and older patients are more likely to have exponential progression than linear progression. Data, such as those published by Zhang et al. (J Neurosurg. 2010 Jun;112[6]:1271-6), indicate that DBS can “keep up with the progression of the disease,” said Dr. Holloway. The authors found that tremor scores did not change significantly over approximately 5 years when patients with essential tremor who had received DBS implantation had periodic assessments and increases in stimulation parameters when appropriate.
If a patient with essential tremor undergoes FUS thalamotomy and has subsequent disease progression, DBS may be considered for reducing tremor, said Dr. Holloway. Most adverse events resulting from DBS implantation are reversible with adjustment of the stimulation parameters. A second thalamotomy, however, could cause severe dysarthria and other irreversible adverse events. “Only DBS can safely address tremor progression,” said Dr. Holloway.
LAS VEGAS – according to two presentations delivered at the annual meeting of the North American Neuromodulation Society. The techniques’ surgical procedures, associated risks, and adverse event profiles may influence neurologists and patients in their choice of treatment.
FUS allows neurosurgeons to apply thermal ablation to create a lesion on the thalamus. MRI guidance enables precise control of the lesion location (within approximately 1 mm) and of the treatment intensity. The surgery can be performed with high-resolution stereotactic framing.
DBS entails the surgical implantation of a neurostimulator and attached leads and electrodes. The neurosurgeon drills a hole of approximately 14 mm in diameter into the skull so that the electrode can be inserted stereotactically while the patient is awake or asleep. The neurostimulator is installed separately.
Both treatments provide functional benefits
In 2016, W. Jeff Elias, MD, director of stereotactic and functional neurosurgery at the University of Virginia in Charlottesville, and his colleagues published the results of a randomized controlled trial that compared FUS with sham treatment in 76 patients with essential tremor. At three months, hand tremor had improved by approximately 50% among treated patients, but controls had no significant benefit(N Engl J Med. 2016 Aug 25;375[8]:730-9). The improvement among treated patients was maintained for 12 months. Disability and quality of life also improved after FUS.
A study by Schuurman et al. published in 2000 (N Engl J Med. 2000 Feb 17;342[7]:461-8) showed that DBS and FUS had similar efficacy at 1 year, said Kathryn L. Holloway, MD, professor of neurosurgery at Virginia Commonwealth University in Richmond. It included 45 patients with Parkinson’s disease, 13 with essential tremor, and 10 with multiple sclerosis who were randomized 1:1 to FUS or DBS. The primary outcome was activities of daily living, and blinded physicians assessed patient videos. Most of the patients who improved had received DBS, and most of the ones who worsened had received FUS, said Dr. Holloway. Among patients with essential tremor, tremor improved by between 94% and 100% with either treatment.
To find more recent data about these treatments, Dr. Holloway searched the literature for studies of FUS or DBS for essential tremor. She analyzed only studies that included unselected populations, blinded evaluations within 1 or 2 years of surgery, and tremor scores for the treated side. She found two studies of FUS, including Dr. Elias’s 2016 trial and a 2018 follow-up (Ann Neurol. 2018 Jan;83[1]:107-14). Dr. Holloway also identified three trials of DBS.
In these studies, reduction of hand tremor was 55% with FUS and between 63% and 69% with DBS. Reduction of postural tremor was approximately 72% with FUS and approximately 67% with DBS. Reduction of action tremor was about 52% with FUS and between 65% and 71% with DBS. Overall, DBS appears to be more effective, said Dr. Holloway.
A 2015 study (Mov Disord. 2015 Dec;30[14]:1937-43) that compared bilateral DBS, unilateral DBS, and unilateral FUS for essential tremor indicated that the treatments provide similar benefits on hand tremor, disability, and quality of life, said Dr. Elias. FUS is inferior to DBS, however, for total tremor and axial tremor.
Furthermore, the efficacy of FUS wanes over time, said Dr. Elias. He and his colleagues conducted a pilot study of 15 patients with essential tremor who received FUS (N Engl J Med. 2013 Aug 15;369[7]:640-8). At 6 years, 6 of 13 patients whose data were available still had a 50% improvement in tremor. “Some went on to [receive] DBS,” said Dr. Elias. “Functional improvements persisted more than the tremor improvement.”
Adverse events
In their 2016 trial of FUS, Dr. Elias and his colleagues observed 210 adverse events, which is approximately “what you would expect with a modern day, FDA-monitored clinical trial.” Sensory effects and gait disturbance accounted for most of the thalamotomy-related adverse events. Sensory problems such as numbness or parestheisa persisted at 1 year in 14% of treated patients, and gait disturbance persisted at 1 year in 9%. The investigators did not observe any hemorrhages, infections, or cavitation-related effects from FUS.
In a 2018 analysis of five clinical trials of FUS for essential tremor, Fishman et al. found that 79% of adverse events were mild and 1% were severe (Mov Disord. 2018 May;33[5]:843-7). The risk of a severe adverse event therefore can be considered low, and it may decrease as neurosurgeons gain experience with the procedure, said Dr. Elias.
In the 2000 Schuurman et al. study, the researchers observed significantly fewer adverse events overall among patients with Parkinson’s disease or essential tremor who received DBS, compared with patients who received FUS. Cognitive deterioration, severe dysarthria, and severe ataxia were more common in the FUS group than in the DBS group. Dr. Holloway’s analysis of adverse events in the five more recent trials that she identified yielded similar results.
Although MRI-guided FUS is a precise way to make lesions, functional areas in the thalamus overlap, which makes it more difficult to target only the intended region, said Dr. Holloway. The functional overlap thus increases the risk of adverse events (e.g., sensory impairments, dysarthria, or ataxia). The adverse events that result from FUS may last as long as a year. “Patients will put up anything for about a month after surgery, and then they start to get annoyed,” said Dr. Holloway.
In addition, Schuurman et al. found that FUS entailed a greater risk of permanent side effects, compared with DBS. “That’s the key point here,” said Dr. Holloway. Most of the adverse effects in the DBS group were resolved by adjusting or turning off the stimulator. Hardware issues resulting from DBS are frustrating, but reversible, but a patient with an adverse event after FUS often is “stuck with it,” said Dr. Holloway. The Schuurman et al. data indicated that, in terms of adverse events, “thalamotomy was inferior to DBS,” she added.
Implantation of DBS entails the risks inherent to surgeries that open the skull (such as seizures, air embolism, and hemorrhage). DBS entails a 2% risk of hemorrhage or infection, said Dr. Elias. Furthermore, as much as 15% of patients who undergo DBS implantation require additional surgery.
“FUS is not going to cause a life-threatening hemorrhage, but DBS certainly can,” said Dr. Holloway.
Managing disease progression
Essential tremor is a progressive disease, and older patients are more likely to have exponential progression than linear progression. Data, such as those published by Zhang et al. (J Neurosurg. 2010 Jun;112[6]:1271-6), indicate that DBS can “keep up with the progression of the disease,” said Dr. Holloway. The authors found that tremor scores did not change significantly over approximately 5 years when patients with essential tremor who had received DBS implantation had periodic assessments and increases in stimulation parameters when appropriate.
If a patient with essential tremor undergoes FUS thalamotomy and has subsequent disease progression, DBS may be considered for reducing tremor, said Dr. Holloway. Most adverse events resulting from DBS implantation are reversible with adjustment of the stimulation parameters. A second thalamotomy, however, could cause severe dysarthria and other irreversible adverse events. “Only DBS can safely address tremor progression,” said Dr. Holloway.
REPORTING FROM NANS 2019
Statins cut vascular events in elderly patients
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
FROM THE LANCET
Key clinical point: but patients older than 75 years with occlusive vascular disease have a smaller reduction in major coronary events.
Major finding: Major vascular coronary events were reduced by 24% (risk ratio, 0.76; 95% confidence interval, 0.73-0.79) with a decrease in the reduction of coronary events among patients older than 75 years. Study details: A meta-analysis of 28 trials with 186,854 individuals undergoing statin therapy from the Cholesterol Treatment Trialists’ Collaboration.
Disclosures: This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
Source: Fulcher J et al. Lancet. 2019;393:407-15.
When is it safe to resume anticoagulation in my patient with hemorrhagic stroke?
Balancing risk is critical to decision making
Department of Medicine, Massachusetts General Hospital, Boston
Case
A 75 year-old woman with a history of hypertension, diabetes mellitus, heart failure and nonvalvular atrial fibrillation (CHA2DS2-VASc score, 8) on anticoagulation is admitted with weakness and dysarthria. Exam is notable for hypertension and right-sided hemiparesis. CT of the head shows an intraparenchymal hemorrhage in the left putamen. Her anticoagulation is reversed and blood pressure well controlled. She is discharged 12 days later.
Brief overview of the issue
Intracranial hemorrhage (ICH) is the second most common cause of stroke and is associated with high morbidity and mortality.1 It is estimated that 10%-15% of spontaneous ICH cases occur in patients on therapeutic anticoagulation for atrial fibrillation.2 As our population ages and more people develop atrial fibrillation, anticoagulation for primary or secondary prevention of embolic stroke also will likely increase, placing more people at risk for ICH. Even stringently controlled therapeutic international normalized ratios (INRs) between 2 and 3 may double the risk of ICH.3
Patients with ICH require close monitoring and treatment, including blood pressure control, reversal of anticoagulation, reduction of intracranial pressure and, at times, neurosurgery.4 Although anticoagulation is discontinued and reversed at the onset of ICH, no clear consensus exists as to when it is safe to resume it. Although anticoagulation decreases the risk of stroke/thromboembolism, it may also increase the amount of bleeding associated with the initial ICH or lead to its recurrence.
Factors that may contribute to rebleeding include uncontrolled hypertension, advanced age, time to resumption of anticoagulation, and lobar location of ICH (i.e., in cerebral cortex and/or underlying white matter).5 Traditionally, lobar ICH has high incidence of cerebral amyloid angiopathy and has been associated with higher bleeding rates than has deep ICH (i.e., involving the thalami, basal ganglia, cerebellum, or brainstem) where cerebral amyloid angiopathy is rare and ICH is usually from hypertensive vessel disease. However, in patients with active thromboembolic disease, high-risk atrial fibrillation, and mechanical valves, withholding anticoagulation could place them at high risk of stroke.
Two questions should be addressed in the case presented: Is it safe to restart therapeutic anticoagulation; and if so, what is the optimal time interval between ICH and reinitiation of anticoagulation?
Overview of the data
There is limited guidance from major professional societies regarding the reinitiation of anticoagulation and the optimal timing of safely resuming anticoagulation in patients with prior ICH.
Current European Stroke Organization guidelines provide no specific recommendations for anticoagulation resumption after ICH.7 The American Heart Association/American Stroke Association guideline has a class IIA (weak) recommendation to avoid anticoagulation in spontaneous lobar ICH and a class IIB (very weak) recommendation to consider resuming anticoagulation in nonlobar ICH on a case-by-case basis.4
Two recent meta-analyses have examined outcomes of resuming anticoagulation after ICH. In a meta-analysis of 5,300 patients with nonlobar ICH involving eight retrospective studies, Murthy et al. evaluated the risk of thromboembolic events (described as a composite outcome of MI and stroke) and the risk of recurrent ICH.8 They reported that resumption of therapeutic anticoagulation was associated with a decrease in the rate of thromboembolic events (6.7% vs. 17.6%; risk ratio, 0.35; 95% confidence interval, 0.25-0.45) with no significant change in the rate of repeat ICH (8.7% vs. 7.8%).
A second meta-analysis of three retrospective trials conducted by Biffi et al. examined anticoagulation resumption in 1,012 patients with ICH solely in the setting of thromboprophylaxis for nonvalvular atrial fibrillation.9 Reinitiation of anticoagulation after ICH was associated with decreased mortality (hazard ratio, 0.27; 95% CI, 0.19-0.40; P less than .0001), improved functional outcome (HR, 4.15; 95% CI, 2.92-5.90; P less than .0001), and reduction in all-cause stroke recurrence (HR 0.47; 95% CI, 0.36-0.64; P less than .0001). There was no significant difference in the rate of recurrent ICH when anticoagulation was resumed. Despite the notion that patients with cerebral amyloid angiopathy are at high risk of rebleeding, this positive association still held irrespective of lobar vs. nonlobar location of ICH.
Collectively, these studies suggest that resumption of anticoagulation may be effective in decreasing the rates of thromboembolism, as well as provide a functional and mortality benefit without increasing the risk of rebleeding, irrespective of the location of the bleed.
Less is known about the optimal timing of resumption of therapeutic anticoagulation, with data ranging from 72 hours to 30 weeks.10 The American Heart Association/American Stroke Association has a class IIB (very weak) recommendation to avoid anticoagulation for at least 4 weeks in patients without mechanical heart valves.4 The median time to resumption of therapeutic anticoagulation in aforementioned meta-analyses ranged from 10 to 44 days.8,9
A recent observational study of 2,619 ICH survivors explored the relationship between the timing of reinitiation of anticoagulation and the incidence of thrombotic events (defined as ischemic stroke or death because of MI or systemic arterial thromboembolism) and hemorrhagic events (defined as recurrent ICH or bleeding event leading to death) occurring at least 28 days after initial ICH in patients with atrial fibrillation.11
A decrease in thrombotic events was demonstrated if anticoagulation was started 4-16 weeks after ICH. However, when anticoagulation was started more than 16 weeks after ICH, no benefit was seen. Additionally, there was no significant difference in hemorrhagic events between men and women who resumed anticoagulation. In patients with high venous thromboembolism risk based on CHA2DS2-VASc score, resumption of anticoagulation was associated with a decreased predicted incidence of vascular death and nonfatal stroke, with the greatest benefit observed when anticoagulation was started at 7-8 weeks after ICH.
Unfortunately, published literature to date on anticoagulation after ICH is based entirely on retrospective studies – not randomized, controlled studies – making it more likely that anticoagulation would have been resumed in healthier patients, not those left debilitated by the ICH.
Furthermore, information on the location and size of the hemorrhages – which may serve as another confounding factor – often has not been reported. This is important since patients with smaller hemorrhages in less precarious areas also may be more likely to have resumption of anticoagulation. Another limitation of the current literature is that warfarin is the most common anticoagulant studied, with few studies involving the increasingly prescribed newer direct oral anticoagulants. It is also important to stress that a causal relationship between use of anticoagulants and certain outcomes or adverse effects following ICH may be more difficult to invoke in the absence of randomized controlled study designs.
Application of the data to our patient
Resumption of anticoagulation in our patient with ICH requires balancing the risk of hemorrhage expansion and recurrent ICH with the risk of thromboembolic disease.
Our patient is at higher risk of bleeding because of her advanced age, but adequate control of her blood pressure and nonlobar location of her ICH in the basal ganglia also may decrease her risk of recurrent ICH. Her high CHA2DS2-VASc score places her at high risk of thromboembolic event and stroke, making it more likely for reinitiation of anticoagulation to confer a mortality benefit.
Based on AHA guidelines,4 we should wait at least 4 weeks, or possibly wait until weeks 7-8 after ICH when the greatest benefit may be expected based on prediction models.11
Bottom line
It would likely be safe to resume anticoagulation 4-8 weeks after ICH in our patient.
Dr. Gibson, Dr. Restrepo, Dr. Sasidhara, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. An SJ et al. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. 2017 Jan;19:3-10.
2. Horstmann S et al. Intracerebral hemorrhage during anticoagulation with vitamin K antagonists: a consecutive observational study. J Neurol. 2013 Aug;260:2046-51.
3. Rosand J et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004 Apr 26;164:880-4.
4. Hemphill JC et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015 Jul;46:2032-60.
5. Aguillar MI et al. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148-59.
6. Hill MD et al. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123-7.
7. Steiner T et al. European Stroke Organization (ESO) guidelines for the management of spontaneous cerebral hemorrhage. Int J Stroke. 2014;9:840-55.
8. Murthy SB et al. Restarting anticoagulation therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017 Jun;48:1594-600.
9. Biffi A et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017 Nov;82:755-65.
10. Witt DM. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;206:620-4.
11. Pennlert J et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017 Feb;48:314-20.
Key Points
- Robust scientific data on when to resume anticoagulation after ICH does not exist.
- Retrospective studies have shown that anticoagulation resumption after 4-8 weeks decreases the risk of thromboembolic events, decreases mortality, and improves functional status following ICH with no significant change in the risk of its recurrence.
- Prospective, randomized controlled trials are needed to explore risks/benefits of anticoagulation resumption and better define its optimal timing in relation to ICH.
Quiz
Which of the following is false regarding ICH?
A. Lobar ICHs are usually associated with cerebral amyloid angiopathy which are prone to bleeding.
B. Randomized, controlled studies have helped guide the decision as to when to resume anticoagulation in patients with ICH.
C. Current guidelines suggest deferring therapeutic anticoagulation for at least 4 weeks following ICH.
D. Resumption of anticoagulation after 4-8 weeks does not lead to increased risk of rebleeding in patients with prior ICH.
The false answer is B: Current recommendations regarding resumption of anticoagulation in patients with ICH are based solely on retrospective observational studies; there are no randomized, control trials to date.
A is true: In contrast to hypertensive vessel disease associated with deep ICH, lobar hemorrhages are usually associated with cerebral amyloid angiopathy, which are more prone to bleeding.
C is true: The AHA/ASA has a class IIB recommendation to avoid anticoagulation for at least 4 weeks after ICH in patients without mechanical heart valves.
D is true: Several studies have shown that resumption of anticoagulation 4-8 weeks after ICH does not increase the risk of rebleeding.
Balancing risk is critical to decision making
Balancing risk is critical to decision making
Department of Medicine, Massachusetts General Hospital, Boston
Case
A 75 year-old woman with a history of hypertension, diabetes mellitus, heart failure and nonvalvular atrial fibrillation (CHA2DS2-VASc score, 8) on anticoagulation is admitted with weakness and dysarthria. Exam is notable for hypertension and right-sided hemiparesis. CT of the head shows an intraparenchymal hemorrhage in the left putamen. Her anticoagulation is reversed and blood pressure well controlled. She is discharged 12 days later.
Brief overview of the issue
Intracranial hemorrhage (ICH) is the second most common cause of stroke and is associated with high morbidity and mortality.1 It is estimated that 10%-15% of spontaneous ICH cases occur in patients on therapeutic anticoagulation for atrial fibrillation.2 As our population ages and more people develop atrial fibrillation, anticoagulation for primary or secondary prevention of embolic stroke also will likely increase, placing more people at risk for ICH. Even stringently controlled therapeutic international normalized ratios (INRs) between 2 and 3 may double the risk of ICH.3
Patients with ICH require close monitoring and treatment, including blood pressure control, reversal of anticoagulation, reduction of intracranial pressure and, at times, neurosurgery.4 Although anticoagulation is discontinued and reversed at the onset of ICH, no clear consensus exists as to when it is safe to resume it. Although anticoagulation decreases the risk of stroke/thromboembolism, it may also increase the amount of bleeding associated with the initial ICH or lead to its recurrence.
Factors that may contribute to rebleeding include uncontrolled hypertension, advanced age, time to resumption of anticoagulation, and lobar location of ICH (i.e., in cerebral cortex and/or underlying white matter).5 Traditionally, lobar ICH has high incidence of cerebral amyloid angiopathy and has been associated with higher bleeding rates than has deep ICH (i.e., involving the thalami, basal ganglia, cerebellum, or brainstem) where cerebral amyloid angiopathy is rare and ICH is usually from hypertensive vessel disease. However, in patients with active thromboembolic disease, high-risk atrial fibrillation, and mechanical valves, withholding anticoagulation could place them at high risk of stroke.
Two questions should be addressed in the case presented: Is it safe to restart therapeutic anticoagulation; and if so, what is the optimal time interval between ICH and reinitiation of anticoagulation?
Overview of the data
There is limited guidance from major professional societies regarding the reinitiation of anticoagulation and the optimal timing of safely resuming anticoagulation in patients with prior ICH.
Current European Stroke Organization guidelines provide no specific recommendations for anticoagulation resumption after ICH.7 The American Heart Association/American Stroke Association guideline has a class IIA (weak) recommendation to avoid anticoagulation in spontaneous lobar ICH and a class IIB (very weak) recommendation to consider resuming anticoagulation in nonlobar ICH on a case-by-case basis.4
Two recent meta-analyses have examined outcomes of resuming anticoagulation after ICH. In a meta-analysis of 5,300 patients with nonlobar ICH involving eight retrospective studies, Murthy et al. evaluated the risk of thromboembolic events (described as a composite outcome of MI and stroke) and the risk of recurrent ICH.8 They reported that resumption of therapeutic anticoagulation was associated with a decrease in the rate of thromboembolic events (6.7% vs. 17.6%; risk ratio, 0.35; 95% confidence interval, 0.25-0.45) with no significant change in the rate of repeat ICH (8.7% vs. 7.8%).
A second meta-analysis of three retrospective trials conducted by Biffi et al. examined anticoagulation resumption in 1,012 patients with ICH solely in the setting of thromboprophylaxis for nonvalvular atrial fibrillation.9 Reinitiation of anticoagulation after ICH was associated with decreased mortality (hazard ratio, 0.27; 95% CI, 0.19-0.40; P less than .0001), improved functional outcome (HR, 4.15; 95% CI, 2.92-5.90; P less than .0001), and reduction in all-cause stroke recurrence (HR 0.47; 95% CI, 0.36-0.64; P less than .0001). There was no significant difference in the rate of recurrent ICH when anticoagulation was resumed. Despite the notion that patients with cerebral amyloid angiopathy are at high risk of rebleeding, this positive association still held irrespective of lobar vs. nonlobar location of ICH.
Collectively, these studies suggest that resumption of anticoagulation may be effective in decreasing the rates of thromboembolism, as well as provide a functional and mortality benefit without increasing the risk of rebleeding, irrespective of the location of the bleed.
Less is known about the optimal timing of resumption of therapeutic anticoagulation, with data ranging from 72 hours to 30 weeks.10 The American Heart Association/American Stroke Association has a class IIB (very weak) recommendation to avoid anticoagulation for at least 4 weeks in patients without mechanical heart valves.4 The median time to resumption of therapeutic anticoagulation in aforementioned meta-analyses ranged from 10 to 44 days.8,9
A recent observational study of 2,619 ICH survivors explored the relationship between the timing of reinitiation of anticoagulation and the incidence of thrombotic events (defined as ischemic stroke or death because of MI or systemic arterial thromboembolism) and hemorrhagic events (defined as recurrent ICH or bleeding event leading to death) occurring at least 28 days after initial ICH in patients with atrial fibrillation.11
A decrease in thrombotic events was demonstrated if anticoagulation was started 4-16 weeks after ICH. However, when anticoagulation was started more than 16 weeks after ICH, no benefit was seen. Additionally, there was no significant difference in hemorrhagic events between men and women who resumed anticoagulation. In patients with high venous thromboembolism risk based on CHA2DS2-VASc score, resumption of anticoagulation was associated with a decreased predicted incidence of vascular death and nonfatal stroke, with the greatest benefit observed when anticoagulation was started at 7-8 weeks after ICH.
Unfortunately, published literature to date on anticoagulation after ICH is based entirely on retrospective studies – not randomized, controlled studies – making it more likely that anticoagulation would have been resumed in healthier patients, not those left debilitated by the ICH.
Furthermore, information on the location and size of the hemorrhages – which may serve as another confounding factor – often has not been reported. This is important since patients with smaller hemorrhages in less precarious areas also may be more likely to have resumption of anticoagulation. Another limitation of the current literature is that warfarin is the most common anticoagulant studied, with few studies involving the increasingly prescribed newer direct oral anticoagulants. It is also important to stress that a causal relationship between use of anticoagulants and certain outcomes or adverse effects following ICH may be more difficult to invoke in the absence of randomized controlled study designs.
Application of the data to our patient
Resumption of anticoagulation in our patient with ICH requires balancing the risk of hemorrhage expansion and recurrent ICH with the risk of thromboembolic disease.
Our patient is at higher risk of bleeding because of her advanced age, but adequate control of her blood pressure and nonlobar location of her ICH in the basal ganglia also may decrease her risk of recurrent ICH. Her high CHA2DS2-VASc score places her at high risk of thromboembolic event and stroke, making it more likely for reinitiation of anticoagulation to confer a mortality benefit.
Based on AHA guidelines,4 we should wait at least 4 weeks, or possibly wait until weeks 7-8 after ICH when the greatest benefit may be expected based on prediction models.11
Bottom line
It would likely be safe to resume anticoagulation 4-8 weeks after ICH in our patient.
Dr. Gibson, Dr. Restrepo, Dr. Sasidhara, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. An SJ et al. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. 2017 Jan;19:3-10.
2. Horstmann S et al. Intracerebral hemorrhage during anticoagulation with vitamin K antagonists: a consecutive observational study. J Neurol. 2013 Aug;260:2046-51.
3. Rosand J et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004 Apr 26;164:880-4.
4. Hemphill JC et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015 Jul;46:2032-60.
5. Aguillar MI et al. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148-59.
6. Hill MD et al. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123-7.
7. Steiner T et al. European Stroke Organization (ESO) guidelines for the management of spontaneous cerebral hemorrhage. Int J Stroke. 2014;9:840-55.
8. Murthy SB et al. Restarting anticoagulation therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017 Jun;48:1594-600.
9. Biffi A et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017 Nov;82:755-65.
10. Witt DM. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;206:620-4.
11. Pennlert J et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017 Feb;48:314-20.
Key Points
- Robust scientific data on when to resume anticoagulation after ICH does not exist.
- Retrospective studies have shown that anticoagulation resumption after 4-8 weeks decreases the risk of thromboembolic events, decreases mortality, and improves functional status following ICH with no significant change in the risk of its recurrence.
- Prospective, randomized controlled trials are needed to explore risks/benefits of anticoagulation resumption and better define its optimal timing in relation to ICH.
Quiz
Which of the following is false regarding ICH?
A. Lobar ICHs are usually associated with cerebral amyloid angiopathy which are prone to bleeding.
B. Randomized, controlled studies have helped guide the decision as to when to resume anticoagulation in patients with ICH.
C. Current guidelines suggest deferring therapeutic anticoagulation for at least 4 weeks following ICH.
D. Resumption of anticoagulation after 4-8 weeks does not lead to increased risk of rebleeding in patients with prior ICH.
The false answer is B: Current recommendations regarding resumption of anticoagulation in patients with ICH are based solely on retrospective observational studies; there are no randomized, control trials to date.
A is true: In contrast to hypertensive vessel disease associated with deep ICH, lobar hemorrhages are usually associated with cerebral amyloid angiopathy, which are more prone to bleeding.
C is true: The AHA/ASA has a class IIB recommendation to avoid anticoagulation for at least 4 weeks after ICH in patients without mechanical heart valves.
D is true: Several studies have shown that resumption of anticoagulation 4-8 weeks after ICH does not increase the risk of rebleeding.
Department of Medicine, Massachusetts General Hospital, Boston
Case
A 75 year-old woman with a history of hypertension, diabetes mellitus, heart failure and nonvalvular atrial fibrillation (CHA2DS2-VASc score, 8) on anticoagulation is admitted with weakness and dysarthria. Exam is notable for hypertension and right-sided hemiparesis. CT of the head shows an intraparenchymal hemorrhage in the left putamen. Her anticoagulation is reversed and blood pressure well controlled. She is discharged 12 days later.
Brief overview of the issue
Intracranial hemorrhage (ICH) is the second most common cause of stroke and is associated with high morbidity and mortality.1 It is estimated that 10%-15% of spontaneous ICH cases occur in patients on therapeutic anticoagulation for atrial fibrillation.2 As our population ages and more people develop atrial fibrillation, anticoagulation for primary or secondary prevention of embolic stroke also will likely increase, placing more people at risk for ICH. Even stringently controlled therapeutic international normalized ratios (INRs) between 2 and 3 may double the risk of ICH.3
Patients with ICH require close monitoring and treatment, including blood pressure control, reversal of anticoagulation, reduction of intracranial pressure and, at times, neurosurgery.4 Although anticoagulation is discontinued and reversed at the onset of ICH, no clear consensus exists as to when it is safe to resume it. Although anticoagulation decreases the risk of stroke/thromboembolism, it may also increase the amount of bleeding associated with the initial ICH or lead to its recurrence.
Factors that may contribute to rebleeding include uncontrolled hypertension, advanced age, time to resumption of anticoagulation, and lobar location of ICH (i.e., in cerebral cortex and/or underlying white matter).5 Traditionally, lobar ICH has high incidence of cerebral amyloid angiopathy and has been associated with higher bleeding rates than has deep ICH (i.e., involving the thalami, basal ganglia, cerebellum, or brainstem) where cerebral amyloid angiopathy is rare and ICH is usually from hypertensive vessel disease. However, in patients with active thromboembolic disease, high-risk atrial fibrillation, and mechanical valves, withholding anticoagulation could place them at high risk of stroke.
Two questions should be addressed in the case presented: Is it safe to restart therapeutic anticoagulation; and if so, what is the optimal time interval between ICH and reinitiation of anticoagulation?
Overview of the data
There is limited guidance from major professional societies regarding the reinitiation of anticoagulation and the optimal timing of safely resuming anticoagulation in patients with prior ICH.
Current European Stroke Organization guidelines provide no specific recommendations for anticoagulation resumption after ICH.7 The American Heart Association/American Stroke Association guideline has a class IIA (weak) recommendation to avoid anticoagulation in spontaneous lobar ICH and a class IIB (very weak) recommendation to consider resuming anticoagulation in nonlobar ICH on a case-by-case basis.4
Two recent meta-analyses have examined outcomes of resuming anticoagulation after ICH. In a meta-analysis of 5,300 patients with nonlobar ICH involving eight retrospective studies, Murthy et al. evaluated the risk of thromboembolic events (described as a composite outcome of MI and stroke) and the risk of recurrent ICH.8 They reported that resumption of therapeutic anticoagulation was associated with a decrease in the rate of thromboembolic events (6.7% vs. 17.6%; risk ratio, 0.35; 95% confidence interval, 0.25-0.45) with no significant change in the rate of repeat ICH (8.7% vs. 7.8%).
A second meta-analysis of three retrospective trials conducted by Biffi et al. examined anticoagulation resumption in 1,012 patients with ICH solely in the setting of thromboprophylaxis for nonvalvular atrial fibrillation.9 Reinitiation of anticoagulation after ICH was associated with decreased mortality (hazard ratio, 0.27; 95% CI, 0.19-0.40; P less than .0001), improved functional outcome (HR, 4.15; 95% CI, 2.92-5.90; P less than .0001), and reduction in all-cause stroke recurrence (HR 0.47; 95% CI, 0.36-0.64; P less than .0001). There was no significant difference in the rate of recurrent ICH when anticoagulation was resumed. Despite the notion that patients with cerebral amyloid angiopathy are at high risk of rebleeding, this positive association still held irrespective of lobar vs. nonlobar location of ICH.
Collectively, these studies suggest that resumption of anticoagulation may be effective in decreasing the rates of thromboembolism, as well as provide a functional and mortality benefit without increasing the risk of rebleeding, irrespective of the location of the bleed.
Less is known about the optimal timing of resumption of therapeutic anticoagulation, with data ranging from 72 hours to 30 weeks.10 The American Heart Association/American Stroke Association has a class IIB (very weak) recommendation to avoid anticoagulation for at least 4 weeks in patients without mechanical heart valves.4 The median time to resumption of therapeutic anticoagulation in aforementioned meta-analyses ranged from 10 to 44 days.8,9
A recent observational study of 2,619 ICH survivors explored the relationship between the timing of reinitiation of anticoagulation and the incidence of thrombotic events (defined as ischemic stroke or death because of MI or systemic arterial thromboembolism) and hemorrhagic events (defined as recurrent ICH or bleeding event leading to death) occurring at least 28 days after initial ICH in patients with atrial fibrillation.11
A decrease in thrombotic events was demonstrated if anticoagulation was started 4-16 weeks after ICH. However, when anticoagulation was started more than 16 weeks after ICH, no benefit was seen. Additionally, there was no significant difference in hemorrhagic events between men and women who resumed anticoagulation. In patients with high venous thromboembolism risk based on CHA2DS2-VASc score, resumption of anticoagulation was associated with a decreased predicted incidence of vascular death and nonfatal stroke, with the greatest benefit observed when anticoagulation was started at 7-8 weeks after ICH.
Unfortunately, published literature to date on anticoagulation after ICH is based entirely on retrospective studies – not randomized, controlled studies – making it more likely that anticoagulation would have been resumed in healthier patients, not those left debilitated by the ICH.
Furthermore, information on the location and size of the hemorrhages – which may serve as another confounding factor – often has not been reported. This is important since patients with smaller hemorrhages in less precarious areas also may be more likely to have resumption of anticoagulation. Another limitation of the current literature is that warfarin is the most common anticoagulant studied, with few studies involving the increasingly prescribed newer direct oral anticoagulants. It is also important to stress that a causal relationship between use of anticoagulants and certain outcomes or adverse effects following ICH may be more difficult to invoke in the absence of randomized controlled study designs.
Application of the data to our patient
Resumption of anticoagulation in our patient with ICH requires balancing the risk of hemorrhage expansion and recurrent ICH with the risk of thromboembolic disease.
Our patient is at higher risk of bleeding because of her advanced age, but adequate control of her blood pressure and nonlobar location of her ICH in the basal ganglia also may decrease her risk of recurrent ICH. Her high CHA2DS2-VASc score places her at high risk of thromboembolic event and stroke, making it more likely for reinitiation of anticoagulation to confer a mortality benefit.
Based on AHA guidelines,4 we should wait at least 4 weeks, or possibly wait until weeks 7-8 after ICH when the greatest benefit may be expected based on prediction models.11
Bottom line
It would likely be safe to resume anticoagulation 4-8 weeks after ICH in our patient.
Dr. Gibson, Dr. Restrepo, Dr. Sasidhara, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. An SJ et al. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. 2017 Jan;19:3-10.
2. Horstmann S et al. Intracerebral hemorrhage during anticoagulation with vitamin K antagonists: a consecutive observational study. J Neurol. 2013 Aug;260:2046-51.
3. Rosand J et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004 Apr 26;164:880-4.
4. Hemphill JC et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015 Jul;46:2032-60.
5. Aguillar MI et al. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148-59.
6. Hill MD et al. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123-7.
7. Steiner T et al. European Stroke Organization (ESO) guidelines for the management of spontaneous cerebral hemorrhage. Int J Stroke. 2014;9:840-55.
8. Murthy SB et al. Restarting anticoagulation therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017 Jun;48:1594-600.
9. Biffi A et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017 Nov;82:755-65.
10. Witt DM. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;206:620-4.
11. Pennlert J et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017 Feb;48:314-20.
Key Points
- Robust scientific data on when to resume anticoagulation after ICH does not exist.
- Retrospective studies have shown that anticoagulation resumption after 4-8 weeks decreases the risk of thromboembolic events, decreases mortality, and improves functional status following ICH with no significant change in the risk of its recurrence.
- Prospective, randomized controlled trials are needed to explore risks/benefits of anticoagulation resumption and better define its optimal timing in relation to ICH.
Quiz
Which of the following is false regarding ICH?
A. Lobar ICHs are usually associated with cerebral amyloid angiopathy which are prone to bleeding.
B. Randomized, controlled studies have helped guide the decision as to when to resume anticoagulation in patients with ICH.
C. Current guidelines suggest deferring therapeutic anticoagulation for at least 4 weeks following ICH.
D. Resumption of anticoagulation after 4-8 weeks does not lead to increased risk of rebleeding in patients with prior ICH.
The false answer is B: Current recommendations regarding resumption of anticoagulation in patients with ICH are based solely on retrospective observational studies; there are no randomized, control trials to date.
A is true: In contrast to hypertensive vessel disease associated with deep ICH, lobar hemorrhages are usually associated with cerebral amyloid angiopathy, which are more prone to bleeding.
C is true: The AHA/ASA has a class IIB recommendation to avoid anticoagulation for at least 4 weeks after ICH in patients without mechanical heart valves.
D is true: Several studies have shown that resumption of anticoagulation 4-8 weeks after ICH does not increase the risk of rebleeding.
Mild aerobic exercise speeds sports concussion recovery
Mild aerobic exercise significantly shortened recovery time from sports-related concussion in adolescent athletes, compared with a stretching program in a randomized trial of 103 participants.
Sports-related concussion (SRC) remains a major public health problem with no effective treatment, wrote John J. Leddy, MD, of the State University of New York at Buffalo, and his colleagues.
Exercise tolerance after SRC has not been well studied. However, given the demonstrated benefits of aerobic exercise training on autonomic nervous system regulation, cerebral blood flow regulation, cardiovascular physiology, and brain neuroplasticity, the researchers hypothesized that exercise at a level that does not exacerbate symptoms might facilitate recovery in concussion patients.
In a study published in JAMA Pediatrics, the researchers randomized 103 adolescent athletes aged 13-18 years to a program of subsymptom aerobic exercise or a placebo stretching program. The participants were enrolled in the study within 10 days of an SRC, and were followed for 30 days or until recovery.
Athletes in the aerobic exercise group recovered in a median of 13 days, compared with 17 days for those in the stretching group (P = .009). Recovery was defined as “symptom resolution to normal,” based on normal physical and neurological examinations, “further confirmed by demonstration of the ability to exercise to exhaustion without exacerbation of symptoms” according to the Buffalo Concussion Treadmill Test, the researchers wrote.
No demographic differences or difference in previous concussions, time from injury until treatment, initial symptom severity score, initial exercise treadmill test, or physical exam were noted between the groups.
The average age of the participants was 15 years, 47% were female. The athletes performed the aerobic exercise or stretching programs approximately 20 minutes per day, and reported their daily symptoms and compliance via a website. The aerobic exercise consisted of walking or jogging on a treadmill or outdoors, or riding a stationary bike while wearing a heart rate monitor to maintain a target heart rate. The target heart rate was calculated as 80% of the heart rate at symptom exacerbation during the Buffalo Concussion Treadmill Test at each participant’s initial visit.
No adverse events related to the exercise intervention were reported, which supports the safety of subsymptom threshhold exercise, in the study population, Dr. Leddy and his associates noted.
The researchers also found lower rates of persistent symptoms at 1 month in the exercise group, compared with the stretching group (two participants vs. seven participants), but this difference was not statistically significant.
The study findings were limited by several factors, including the unblinded design and failure to address the mechanism of action for the effects of exercise. In addition, the results are not generalizable to younger children or other demographic groups, including those with concussions from causes other than sports and adults with heart conditions, the researchers noted.
However, “the results of this study should give clinicians confidence that moderate levels of physical activity, including prescribed subsymptom threshold aerobic exercise, after the first 48 hours following SRC can safely and significantly speed recovery,” Dr. Leddy and his associates concluded.
The study was supported by grants from the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Leddy JJ et al. JAMA Pediatr. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.4397.
In 2009 and 2010, the culture of sports concussion care began to shift with the publication of an initial study by Leddy et al. on the use of exercise at subsymptom levels as part of concussion rehabilitation, Sara P. D. Chrisman, MD, MPH, wrote in an accompanying editorial. Previous guidelines had emphasized total avoidance of physical activity, as well as avoidance of screen time and social activity, until patients were asymptomatic; however, “no definition was provided for the term asymptomatic, and no time limits were placed on rest, and as a result, rest often continued for weeks or months,” Dr. Chrisman said. Additional research over the past decade supported the potential value of moderate exercise, and the 2016 meeting of the Concussion in Sport Group resulted in recommendations limiting rest to 24-48 hours, which prompted further studies of exercise intervention.
The current study by Leddy et al. is a clinical trial using exercise “to treat acute concussion with a goal of reducing symptom duration,” she said. Despite the study’s limitations, including the inability to estimate how much exercise was needed to achieve the treatment outcome, “this is a landmark study that may shift the standard of care toward the use of rehabilitative exercise to decrease the duration of concussion symptoms.
“Future studies will need to explore the limits of exercise treatment for concussion,” and should address questions including the timing, intensity, and duration of exercise and whether the strategy is appropriate for other populations, such as those with mental health comorbidities, Dr. Chrisman concluded.
Dr. Chrisman is at the Center for Child Health, Behavior, and Development, Seattle Children’s Research Institute. These comments are from her editorial accompanying the article by Leddy et al. (JAMA Pedatr. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.5281). She had no financial conflicts to disclose.
In 2009 and 2010, the culture of sports concussion care began to shift with the publication of an initial study by Leddy et al. on the use of exercise at subsymptom levels as part of concussion rehabilitation, Sara P. D. Chrisman, MD, MPH, wrote in an accompanying editorial. Previous guidelines had emphasized total avoidance of physical activity, as well as avoidance of screen time and social activity, until patients were asymptomatic; however, “no definition was provided for the term asymptomatic, and no time limits were placed on rest, and as a result, rest often continued for weeks or months,” Dr. Chrisman said. Additional research over the past decade supported the potential value of moderate exercise, and the 2016 meeting of the Concussion in Sport Group resulted in recommendations limiting rest to 24-48 hours, which prompted further studies of exercise intervention.
The current study by Leddy et al. is a clinical trial using exercise “to treat acute concussion with a goal of reducing symptom duration,” she said. Despite the study’s limitations, including the inability to estimate how much exercise was needed to achieve the treatment outcome, “this is a landmark study that may shift the standard of care toward the use of rehabilitative exercise to decrease the duration of concussion symptoms.
“Future studies will need to explore the limits of exercise treatment for concussion,” and should address questions including the timing, intensity, and duration of exercise and whether the strategy is appropriate for other populations, such as those with mental health comorbidities, Dr. Chrisman concluded.
Dr. Chrisman is at the Center for Child Health, Behavior, and Development, Seattle Children’s Research Institute. These comments are from her editorial accompanying the article by Leddy et al. (JAMA Pedatr. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.5281). She had no financial conflicts to disclose.
In 2009 and 2010, the culture of sports concussion care began to shift with the publication of an initial study by Leddy et al. on the use of exercise at subsymptom levels as part of concussion rehabilitation, Sara P. D. Chrisman, MD, MPH, wrote in an accompanying editorial. Previous guidelines had emphasized total avoidance of physical activity, as well as avoidance of screen time and social activity, until patients were asymptomatic; however, “no definition was provided for the term asymptomatic, and no time limits were placed on rest, and as a result, rest often continued for weeks or months,” Dr. Chrisman said. Additional research over the past decade supported the potential value of moderate exercise, and the 2016 meeting of the Concussion in Sport Group resulted in recommendations limiting rest to 24-48 hours, which prompted further studies of exercise intervention.
The current study by Leddy et al. is a clinical trial using exercise “to treat acute concussion with a goal of reducing symptom duration,” she said. Despite the study’s limitations, including the inability to estimate how much exercise was needed to achieve the treatment outcome, “this is a landmark study that may shift the standard of care toward the use of rehabilitative exercise to decrease the duration of concussion symptoms.
“Future studies will need to explore the limits of exercise treatment for concussion,” and should address questions including the timing, intensity, and duration of exercise and whether the strategy is appropriate for other populations, such as those with mental health comorbidities, Dr. Chrisman concluded.
Dr. Chrisman is at the Center for Child Health, Behavior, and Development, Seattle Children’s Research Institute. These comments are from her editorial accompanying the article by Leddy et al. (JAMA Pedatr. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.5281). She had no financial conflicts to disclose.
Mild aerobic exercise significantly shortened recovery time from sports-related concussion in adolescent athletes, compared with a stretching program in a randomized trial of 103 participants.
Sports-related concussion (SRC) remains a major public health problem with no effective treatment, wrote John J. Leddy, MD, of the State University of New York at Buffalo, and his colleagues.
Exercise tolerance after SRC has not been well studied. However, given the demonstrated benefits of aerobic exercise training on autonomic nervous system regulation, cerebral blood flow regulation, cardiovascular physiology, and brain neuroplasticity, the researchers hypothesized that exercise at a level that does not exacerbate symptoms might facilitate recovery in concussion patients.
In a study published in JAMA Pediatrics, the researchers randomized 103 adolescent athletes aged 13-18 years to a program of subsymptom aerobic exercise or a placebo stretching program. The participants were enrolled in the study within 10 days of an SRC, and were followed for 30 days or until recovery.
Athletes in the aerobic exercise group recovered in a median of 13 days, compared with 17 days for those in the stretching group (P = .009). Recovery was defined as “symptom resolution to normal,” based on normal physical and neurological examinations, “further confirmed by demonstration of the ability to exercise to exhaustion without exacerbation of symptoms” according to the Buffalo Concussion Treadmill Test, the researchers wrote.
No demographic differences or difference in previous concussions, time from injury until treatment, initial symptom severity score, initial exercise treadmill test, or physical exam were noted between the groups.
The average age of the participants was 15 years, 47% were female. The athletes performed the aerobic exercise or stretching programs approximately 20 minutes per day, and reported their daily symptoms and compliance via a website. The aerobic exercise consisted of walking or jogging on a treadmill or outdoors, or riding a stationary bike while wearing a heart rate monitor to maintain a target heart rate. The target heart rate was calculated as 80% of the heart rate at symptom exacerbation during the Buffalo Concussion Treadmill Test at each participant’s initial visit.
No adverse events related to the exercise intervention were reported, which supports the safety of subsymptom threshhold exercise, in the study population, Dr. Leddy and his associates noted.
The researchers also found lower rates of persistent symptoms at 1 month in the exercise group, compared with the stretching group (two participants vs. seven participants), but this difference was not statistically significant.
The study findings were limited by several factors, including the unblinded design and failure to address the mechanism of action for the effects of exercise. In addition, the results are not generalizable to younger children or other demographic groups, including those with concussions from causes other than sports and adults with heart conditions, the researchers noted.
However, “the results of this study should give clinicians confidence that moderate levels of physical activity, including prescribed subsymptom threshold aerobic exercise, after the first 48 hours following SRC can safely and significantly speed recovery,” Dr. Leddy and his associates concluded.
The study was supported by grants from the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Leddy JJ et al. JAMA Pediatr. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.4397.
Mild aerobic exercise significantly shortened recovery time from sports-related concussion in adolescent athletes, compared with a stretching program in a randomized trial of 103 participants.
Sports-related concussion (SRC) remains a major public health problem with no effective treatment, wrote John J. Leddy, MD, of the State University of New York at Buffalo, and his colleagues.
Exercise tolerance after SRC has not been well studied. However, given the demonstrated benefits of aerobic exercise training on autonomic nervous system regulation, cerebral blood flow regulation, cardiovascular physiology, and brain neuroplasticity, the researchers hypothesized that exercise at a level that does not exacerbate symptoms might facilitate recovery in concussion patients.
In a study published in JAMA Pediatrics, the researchers randomized 103 adolescent athletes aged 13-18 years to a program of subsymptom aerobic exercise or a placebo stretching program. The participants were enrolled in the study within 10 days of an SRC, and were followed for 30 days or until recovery.
Athletes in the aerobic exercise group recovered in a median of 13 days, compared with 17 days for those in the stretching group (P = .009). Recovery was defined as “symptom resolution to normal,” based on normal physical and neurological examinations, “further confirmed by demonstration of the ability to exercise to exhaustion without exacerbation of symptoms” according to the Buffalo Concussion Treadmill Test, the researchers wrote.
No demographic differences or difference in previous concussions, time from injury until treatment, initial symptom severity score, initial exercise treadmill test, or physical exam were noted between the groups.
The average age of the participants was 15 years, 47% were female. The athletes performed the aerobic exercise or stretching programs approximately 20 minutes per day, and reported their daily symptoms and compliance via a website. The aerobic exercise consisted of walking or jogging on a treadmill or outdoors, or riding a stationary bike while wearing a heart rate monitor to maintain a target heart rate. The target heart rate was calculated as 80% of the heart rate at symptom exacerbation during the Buffalo Concussion Treadmill Test at each participant’s initial visit.
No adverse events related to the exercise intervention were reported, which supports the safety of subsymptom threshhold exercise, in the study population, Dr. Leddy and his associates noted.
The researchers also found lower rates of persistent symptoms at 1 month in the exercise group, compared with the stretching group (two participants vs. seven participants), but this difference was not statistically significant.
The study findings were limited by several factors, including the unblinded design and failure to address the mechanism of action for the effects of exercise. In addition, the results are not generalizable to younger children or other demographic groups, including those with concussions from causes other than sports and adults with heart conditions, the researchers noted.
However, “the results of this study should give clinicians confidence that moderate levels of physical activity, including prescribed subsymptom threshold aerobic exercise, after the first 48 hours following SRC can safely and significantly speed recovery,” Dr. Leddy and his associates concluded.
The study was supported by grants from the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Leddy JJ et al. JAMA Pediatr. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.4397.
FROM JAMA PEDIATRICS
Key clinical point:
Major finding: Teen athletes who performed aerobic exercise recovered from sports-related concussions in 13 days, compared with 17 days for those in a placebo-stretching group.
Study details: The data come from a randomized trial of 103 athletes aged 13-18 years.
Disclosures: The study was supported by grants from the National Institutes of Health. The researchers had no financial conflicts to disclose.
Source: Leddy JJ et al. JAMA Pediatr. 2019 Feb 4. doi: 10.1001/jamapediatrics.2018.4397.
Click for Credit: Missed HIV screening opps; aspirin & preeclampsia; more
Here are 5 articles from the February issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term lung function better predicts mortality risk in SSc
To take the posttest, go to: https://bit.ly/2RrRuIY
Expires November 26, 2019
2. Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
To take the posttest, go to: https://bit.ly/2TvDH5G
Expires November 28, 2019
3. Three commonly used quick cognitive assessments often yield flawed results
To take the posttest, go to: https://bit.ly/2G1qkHn
Expires November 28, 2019
4. Missed HIV screening opportunities found among subsequently infected youth
To take the posttest, go to: https://bit.ly/2HGa8Nm
Expires November 29, 2019
5. Aspirin appears underused to prevent preeclampsia in SLE patients
To take the posttest, go to: https://bit.ly/2G0dU2v
Expires January 2, 2019
Here are 5 articles from the February issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term lung function better predicts mortality risk in SSc
To take the posttest, go to: https://bit.ly/2RrRuIY
Expires November 26, 2019
2. Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
To take the posttest, go to: https://bit.ly/2TvDH5G
Expires November 28, 2019
3. Three commonly used quick cognitive assessments often yield flawed results
To take the posttest, go to: https://bit.ly/2G1qkHn
Expires November 28, 2019
4. Missed HIV screening opportunities found among subsequently infected youth
To take the posttest, go to: https://bit.ly/2HGa8Nm
Expires November 29, 2019
5. Aspirin appears underused to prevent preeclampsia in SLE patients
To take the posttest, go to: https://bit.ly/2G0dU2v
Expires January 2, 2019
Here are 5 articles from the February issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Short-term lung function better predicts mortality risk in SSc
To take the posttest, go to: https://bit.ly/2RrRuIY
Expires November 26, 2019
2. Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
To take the posttest, go to: https://bit.ly/2TvDH5G
Expires November 28, 2019
3. Three commonly used quick cognitive assessments often yield flawed results
To take the posttest, go to: https://bit.ly/2G1qkHn
Expires November 28, 2019
4. Missed HIV screening opportunities found among subsequently infected youth
To take the posttest, go to: https://bit.ly/2HGa8Nm
Expires November 29, 2019
5. Aspirin appears underused to prevent preeclampsia in SLE patients
To take the posttest, go to: https://bit.ly/2G0dU2v
Expires January 2, 2019
Mapping the Pathway of Pain
What makes us pull a hand away from a hot stove or flinch at a pinprick? Researchers from the National Center for Complementary and Integrative Health say they have identified activity in the brain that governs these reactions.
Alexander Chesler, PhD, senior author of the study, says we already know a lot about local spinal cord circuits for simple reflexive responses, but “the mechanisms underlying more complex behaviors remain poorly understood.”
Using heat as the source of discomfort in their experiments, the researchers found a predictable sequence of behaviors—likened to the sequence of responding to walking cautiously on a hot beach, then hopping as the heat intensifies, then running to a water source. “This kind of ‘feed-forward’ circuitry is unique because it is an upward spiral,” says Arnab Barik, PhD, one of the study authors. “The more this pathway is activated by harmful activity, the more it reacts, leading to dramatic behavioral responses.”
The experiments showed that the parts of the brainstem involved in this circuit are the parabrachial nucleus (PBNI) and the dorsal reticular formation in the medulla (MdD). Standing on a hot surface activated a group of nerve cells in the PBNI, triggering escape responses through connections to the MdD. Interestingly, the PBNI cells express a gene that codes for substances that also contribute to multiple disease processes.
“Our data provide evidence that the PBNI produces streams of information with distinct functional significance,” says Arnab Barik, PhD, one of the study authors. “The brainstem-spinal cord pathway identified in this study selectively controls pain response and elicits appropriate behaviors based on sensory input.”
Further investigation, the researchers say, can help us understand how pain is encoded in the brain. The study findings may also offer opportunities to understand how the body becomes dysregulated during chronic pain.
What makes us pull a hand away from a hot stove or flinch at a pinprick? Researchers from the National Center for Complementary and Integrative Health say they have identified activity in the brain that governs these reactions.
Alexander Chesler, PhD, senior author of the study, says we already know a lot about local spinal cord circuits for simple reflexive responses, but “the mechanisms underlying more complex behaviors remain poorly understood.”
Using heat as the source of discomfort in their experiments, the researchers found a predictable sequence of behaviors—likened to the sequence of responding to walking cautiously on a hot beach, then hopping as the heat intensifies, then running to a water source. “This kind of ‘feed-forward’ circuitry is unique because it is an upward spiral,” says Arnab Barik, PhD, one of the study authors. “The more this pathway is activated by harmful activity, the more it reacts, leading to dramatic behavioral responses.”
The experiments showed that the parts of the brainstem involved in this circuit are the parabrachial nucleus (PBNI) and the dorsal reticular formation in the medulla (MdD). Standing on a hot surface activated a group of nerve cells in the PBNI, triggering escape responses through connections to the MdD. Interestingly, the PBNI cells express a gene that codes for substances that also contribute to multiple disease processes.
“Our data provide evidence that the PBNI produces streams of information with distinct functional significance,” says Arnab Barik, PhD, one of the study authors. “The brainstem-spinal cord pathway identified in this study selectively controls pain response and elicits appropriate behaviors based on sensory input.”
Further investigation, the researchers say, can help us understand how pain is encoded in the brain. The study findings may also offer opportunities to understand how the body becomes dysregulated during chronic pain.
What makes us pull a hand away from a hot stove or flinch at a pinprick? Researchers from the National Center for Complementary and Integrative Health say they have identified activity in the brain that governs these reactions.
Alexander Chesler, PhD, senior author of the study, says we already know a lot about local spinal cord circuits for simple reflexive responses, but “the mechanisms underlying more complex behaviors remain poorly understood.”
Using heat as the source of discomfort in their experiments, the researchers found a predictable sequence of behaviors—likened to the sequence of responding to walking cautiously on a hot beach, then hopping as the heat intensifies, then running to a water source. “This kind of ‘feed-forward’ circuitry is unique because it is an upward spiral,” says Arnab Barik, PhD, one of the study authors. “The more this pathway is activated by harmful activity, the more it reacts, leading to dramatic behavioral responses.”
The experiments showed that the parts of the brainstem involved in this circuit are the parabrachial nucleus (PBNI) and the dorsal reticular formation in the medulla (MdD). Standing on a hot surface activated a group of nerve cells in the PBNI, triggering escape responses through connections to the MdD. Interestingly, the PBNI cells express a gene that codes for substances that also contribute to multiple disease processes.
“Our data provide evidence that the PBNI produces streams of information with distinct functional significance,” says Arnab Barik, PhD, one of the study authors. “The brainstem-spinal cord pathway identified in this study selectively controls pain response and elicits appropriate behaviors based on sensory input.”
Further investigation, the researchers say, can help us understand how pain is encoded in the brain. The study findings may also offer opportunities to understand how the body becomes dysregulated during chronic pain.