User login
History of depression, stress tied to Alzheimer’s, MCI risk
TOPLINE:
compared with those without either condition, a new study found.
METHODOLOGY:
- Longitudinal cohort study of 1,362,548 people with records in the Region Stockholm administrative health care database with a diagnosis of stress-induced exhaustion disorder (SED), depression, or both between 2012 and 2013.
- Cohort followed for diagnosis of MCI or AD between 2014 and 2022.
TAKEAWAY:
- SED diagnosed in 0.3%, depression in 2.9% and both SED and depression in 0.1%
- Compared with people without SED or depression, AD risk was more than double in patients with SED (adjusted odds ratio [aOR], 2.45; 99% confidence interval [CI], 1.22-4.91) or depression (aOR, 2.32; 99% CI, 1.85-2.90) and four times higher in patients with both SED and depression (aOR, 4.00; 99% CI, 1.67-9.58)
- Risk for MCI was also higher in people with SED (aOR, 1.87; 99% CI,1.20-2.91), depression (aOR, 2.85; 99% CI, 2.53-3.22) or both SED and depression (aOR, 3.87; 99% CI, 2.39-6.27) vs patients with no history of SED or depression.
- Only patients with depression had a higher risk for another dementia type (aOR, 2.39; 99% CI, 1.92-2.96).
IN PRACTICE:
“Future studies should examine the possibility that symptoms of depression and/or chronic stress could be prodromal symptoms of dementia rather than risk factors,” study authors wrote.
SOURCE:
The study was conducted by Johanna Wallensten, doctoral student, department of clinical sciences, Danderyd Hospital, Stockholm, and colleagues and funded by the Karolinska Institute. It was published online in Alzheimer’s Research and Therapy.
LIMITATIONS:
Use of a health care registry could have led to over- or underestimation of depression, MCI and AD. The study probably captures most people with depression but not most people with depressive symptoms.
DISCLOSURES:
The authors reported no relevant conflicts.
A version of this article appeared on Medscape.com.
TOPLINE:
compared with those without either condition, a new study found.
METHODOLOGY:
- Longitudinal cohort study of 1,362,548 people with records in the Region Stockholm administrative health care database with a diagnosis of stress-induced exhaustion disorder (SED), depression, or both between 2012 and 2013.
- Cohort followed for diagnosis of MCI or AD between 2014 and 2022.
TAKEAWAY:
- SED diagnosed in 0.3%, depression in 2.9% and both SED and depression in 0.1%
- Compared with people without SED or depression, AD risk was more than double in patients with SED (adjusted odds ratio [aOR], 2.45; 99% confidence interval [CI], 1.22-4.91) or depression (aOR, 2.32; 99% CI, 1.85-2.90) and four times higher in patients with both SED and depression (aOR, 4.00; 99% CI, 1.67-9.58)
- Risk for MCI was also higher in people with SED (aOR, 1.87; 99% CI,1.20-2.91), depression (aOR, 2.85; 99% CI, 2.53-3.22) or both SED and depression (aOR, 3.87; 99% CI, 2.39-6.27) vs patients with no history of SED or depression.
- Only patients with depression had a higher risk for another dementia type (aOR, 2.39; 99% CI, 1.92-2.96).
IN PRACTICE:
“Future studies should examine the possibility that symptoms of depression and/or chronic stress could be prodromal symptoms of dementia rather than risk factors,” study authors wrote.
SOURCE:
The study was conducted by Johanna Wallensten, doctoral student, department of clinical sciences, Danderyd Hospital, Stockholm, and colleagues and funded by the Karolinska Institute. It was published online in Alzheimer’s Research and Therapy.
LIMITATIONS:
Use of a health care registry could have led to over- or underestimation of depression, MCI and AD. The study probably captures most people with depression but not most people with depressive symptoms.
DISCLOSURES:
The authors reported no relevant conflicts.
A version of this article appeared on Medscape.com.
TOPLINE:
compared with those without either condition, a new study found.
METHODOLOGY:
- Longitudinal cohort study of 1,362,548 people with records in the Region Stockholm administrative health care database with a diagnosis of stress-induced exhaustion disorder (SED), depression, or both between 2012 and 2013.
- Cohort followed for diagnosis of MCI or AD between 2014 and 2022.
TAKEAWAY:
- SED diagnosed in 0.3%, depression in 2.9% and both SED and depression in 0.1%
- Compared with people without SED or depression, AD risk was more than double in patients with SED (adjusted odds ratio [aOR], 2.45; 99% confidence interval [CI], 1.22-4.91) or depression (aOR, 2.32; 99% CI, 1.85-2.90) and four times higher in patients with both SED and depression (aOR, 4.00; 99% CI, 1.67-9.58)
- Risk for MCI was also higher in people with SED (aOR, 1.87; 99% CI,1.20-2.91), depression (aOR, 2.85; 99% CI, 2.53-3.22) or both SED and depression (aOR, 3.87; 99% CI, 2.39-6.27) vs patients with no history of SED or depression.
- Only patients with depression had a higher risk for another dementia type (aOR, 2.39; 99% CI, 1.92-2.96).
IN PRACTICE:
“Future studies should examine the possibility that symptoms of depression and/or chronic stress could be prodromal symptoms of dementia rather than risk factors,” study authors wrote.
SOURCE:
The study was conducted by Johanna Wallensten, doctoral student, department of clinical sciences, Danderyd Hospital, Stockholm, and colleagues and funded by the Karolinska Institute. It was published online in Alzheimer’s Research and Therapy.
LIMITATIONS:
Use of a health care registry could have led to over- or underestimation of depression, MCI and AD. The study probably captures most people with depression but not most people with depressive symptoms.
DISCLOSURES:
The authors reported no relevant conflicts.
A version of this article appeared on Medscape.com.
Hyperbaric oxygen therapy for traumatic brain injury: Promising or wishful thinking?
A recent review by Hadanny and colleagues recommends hyperbaric oxygen therapy (HBOT) for acute moderate to severe traumatic brain injury (TBI) and selected patients with prolonged postconcussive syndrome.
This article piqued my curiosity because I trained in HBOT more than 20 years ago. As a passionate scuba diver, my motivation was to master treatment for air embolism and decompression illness. Thankfully, these diving accidents are rare. However, I used HBOT for nonhealing wounds, and its efficacy was sometimes remarkable.
Paradoxical results with oxygen therapy
Although it may seem self-evident that “more oxygen is better” for medical illness, this is not necessarily true. I recently interviewed Ola Didrik Saugstad, MD, who demonstrated that the traditional practice of resuscitating newborns with 100% oxygen was more toxic than resuscitation with air (which contains 21% oxygen). His counterintuitive discovery led to a lifesaving change in the international newborn resuscitation guidelines.
The Food and Drug Administration has approved HBOT for a wide variety of conditions, but some practitioners enthusiastically promote it for off-label indications. These include antiaging, autism, multiple sclerosis, and the aforementioned TBI.
More than 50 years ago, HBOT was proposed for stroke, another disorder where the brain has been deprived of oxygen. Despite obvious logic, clinical trials have been unconvincing. The FDA has not approved HBOT for stroke.
HBOT in practice
During HBOT, the patient breathes 100% oxygen while the whole body is pressurized within a hyperbaric chamber. The chamber’s construction allows pressures above normal sea level of 1.0 atmosphere absolute (ATA). For example, The U.S. Navy Treatment Table for decompression sickness recommends 100% oxygen at 2.8 ATA. Chambers may hold one or more patients at a time.
The frequency of therapy varies but often consists of 20-60 sessions lasting 90-120 minutes. For off-label use like TBI, patients usually pay out of pocket. Given the multiple treatments, costs can add up.
Inconsistent evidence and sham controls
The unwieldy 33-page evidence review by Hadanny and colleagues cites multiple studies supporting HBOT for TBI. However, many, if not all, suffer from methodological flaws. These include vague inclusion criteria, lack of a control group, small patient numbers, treatment at different times since injury, poorly defined or varying HBOT protocols, varying outcome measures, and superficial results analysis.
A sham or control arm is essential for HBOT research trials, given the potential placebo effect of placing a human being inside a large, high-tech, sealed tube for an hour or more. In some sham-controlled studies, which consisted of low-pressure oxygen (that is, 1.3 ATA as sham vs. 2.4 ATA as treatment), all groups experienced symptom improvement. The review authors argue that the low-dose HBOT sham arms were biologically active and that the improvements seen mean that both high- and low-dose HBOT is therapeutic. The alternative explanation is that the placebo effect accounted for improvement in both groups.
The late Michael Bennett, a world authority on hyperbaric and underwater medicine, doubted that conventional HBOT sham controls could genuinely have a therapeutic effect, and I agree. The upcoming HOT-POCS trial (discussed below) should answer the question more definitively.
Mechanisms of action and safety
Mechanisms of benefit for HBOT include increased oxygen availability and angiogenesis. Animal research suggests that it may reduce secondary cell death from TBI, through stabilization of the blood-brain barrier and inflammation reduction.
HBOT is generally safe and well tolerated. A retrospective analysis of 1.5 million outpatient hyperbaric treatments revealed that less than 1% were associated with adverse events. The most common were ear and sinus barotrauma. Because HBOT uses increased air pressure, patients must equalize their ears and sinuses. Those who cannot because of altered consciousness, anatomical defects, or congestion must undergo myringotomy or terminate therapy. Claustrophobia was the second most common adverse effect. Convulsions and tension pneumocephalus were rare.
Perhaps the most concerning risk of HBOT for patients with TBI is the potential waste of human and financial resources.
Desperate physicians and patients
As a neurologist who regularly treats patients with TBI, I share the review authors’ frustration regarding the limited efficacy of available treatments. However, the suboptimal efficacy of currently available therapy is insufficient justification to recommend HBOT.
With respect to chronic TBI, it is difficult to imagine how HBOT could reverse brain injury that has been present for months or years. No other therapy exists that reliably encourages neuronal regeneration or prevents the development of posttraumatic epilepsy.
Frank Conidi, MD, a board-certified sports neurologist and headache specialist, shared his thoughts via email. He agrees that HBOT may have a role in TBI, but after reviewing Hadanny and colleagues’ paper, he concluded that there is insufficient evidence for the use of HBOT in all forms of TBI. He would like to see large multicenter, well-designed studies with standardized pressures and duration and a standard definition of the various types of head injury.
Ongoing research
There are at least five ongoing trials on HBOT for TBI or postconcussive syndrome, including the well-designed placebo-controlled HOT-POCS study. The latter has a novel placebo gas system that addresses Hadanny and colleagues’ contention that even low-dose HBOT might be effective.
The placebo arm in HOT-POCS mimics the HBO environment but provides only 0.21 ATA of oxygen, the same as room air. The active arm provides 100% oxygen at 2.0 ATA. If patients in both arms improve, the benefit will be caused by a placebo response, not HBOT.
Conflict of interest
Another concern with the review is that all three authors are affiliated with Aviv Scientific. This company has an exclusive partnership with the world’s largest hyperbaric medicine and research facility, the Sagol Center at Shamir Medical Center in Be’er Ya’akov, Israel.
This conflict of interest does not a priori invalidate their conclusions. However, official HBOT guidelines from a leading organization like the Undersea and Hyperbaric Medicine Society or the American Academy of Neurology would be preferable.
Conclusion
There is an urgent unmet need for more effective treatments for postconcussive syndrome and chronic TBI.
The review authors’ recommendations for HBOT seem premature. They are arguably a disservice to the many desperate patients and their families who will be tempted to expend valuable resources of time and money for an appealing but unproven therapy. Appropriately designed placebo-controlled studies such as HOT-POCS will help separate fact from wishful thinking.
Dr. Wilner is associate professor of neurology at University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
A recent review by Hadanny and colleagues recommends hyperbaric oxygen therapy (HBOT) for acute moderate to severe traumatic brain injury (TBI) and selected patients with prolonged postconcussive syndrome.
This article piqued my curiosity because I trained in HBOT more than 20 years ago. As a passionate scuba diver, my motivation was to master treatment for air embolism and decompression illness. Thankfully, these diving accidents are rare. However, I used HBOT for nonhealing wounds, and its efficacy was sometimes remarkable.
Paradoxical results with oxygen therapy
Although it may seem self-evident that “more oxygen is better” for medical illness, this is not necessarily true. I recently interviewed Ola Didrik Saugstad, MD, who demonstrated that the traditional practice of resuscitating newborns with 100% oxygen was more toxic than resuscitation with air (which contains 21% oxygen). His counterintuitive discovery led to a lifesaving change in the international newborn resuscitation guidelines.
The Food and Drug Administration has approved HBOT for a wide variety of conditions, but some practitioners enthusiastically promote it for off-label indications. These include antiaging, autism, multiple sclerosis, and the aforementioned TBI.
More than 50 years ago, HBOT was proposed for stroke, another disorder where the brain has been deprived of oxygen. Despite obvious logic, clinical trials have been unconvincing. The FDA has not approved HBOT for stroke.
HBOT in practice
During HBOT, the patient breathes 100% oxygen while the whole body is pressurized within a hyperbaric chamber. The chamber’s construction allows pressures above normal sea level of 1.0 atmosphere absolute (ATA). For example, The U.S. Navy Treatment Table for decompression sickness recommends 100% oxygen at 2.8 ATA. Chambers may hold one or more patients at a time.
The frequency of therapy varies but often consists of 20-60 sessions lasting 90-120 minutes. For off-label use like TBI, patients usually pay out of pocket. Given the multiple treatments, costs can add up.
Inconsistent evidence and sham controls
The unwieldy 33-page evidence review by Hadanny and colleagues cites multiple studies supporting HBOT for TBI. However, many, if not all, suffer from methodological flaws. These include vague inclusion criteria, lack of a control group, small patient numbers, treatment at different times since injury, poorly defined or varying HBOT protocols, varying outcome measures, and superficial results analysis.
A sham or control arm is essential for HBOT research trials, given the potential placebo effect of placing a human being inside a large, high-tech, sealed tube for an hour or more. In some sham-controlled studies, which consisted of low-pressure oxygen (that is, 1.3 ATA as sham vs. 2.4 ATA as treatment), all groups experienced symptom improvement. The review authors argue that the low-dose HBOT sham arms were biologically active and that the improvements seen mean that both high- and low-dose HBOT is therapeutic. The alternative explanation is that the placebo effect accounted for improvement in both groups.
The late Michael Bennett, a world authority on hyperbaric and underwater medicine, doubted that conventional HBOT sham controls could genuinely have a therapeutic effect, and I agree. The upcoming HOT-POCS trial (discussed below) should answer the question more definitively.
Mechanisms of action and safety
Mechanisms of benefit for HBOT include increased oxygen availability and angiogenesis. Animal research suggests that it may reduce secondary cell death from TBI, through stabilization of the blood-brain barrier and inflammation reduction.
HBOT is generally safe and well tolerated. A retrospective analysis of 1.5 million outpatient hyperbaric treatments revealed that less than 1% were associated with adverse events. The most common were ear and sinus barotrauma. Because HBOT uses increased air pressure, patients must equalize their ears and sinuses. Those who cannot because of altered consciousness, anatomical defects, or congestion must undergo myringotomy or terminate therapy. Claustrophobia was the second most common adverse effect. Convulsions and tension pneumocephalus were rare.
Perhaps the most concerning risk of HBOT for patients with TBI is the potential waste of human and financial resources.
Desperate physicians and patients
As a neurologist who regularly treats patients with TBI, I share the review authors’ frustration regarding the limited efficacy of available treatments. However, the suboptimal efficacy of currently available therapy is insufficient justification to recommend HBOT.
With respect to chronic TBI, it is difficult to imagine how HBOT could reverse brain injury that has been present for months or years. No other therapy exists that reliably encourages neuronal regeneration or prevents the development of posttraumatic epilepsy.
Frank Conidi, MD, a board-certified sports neurologist and headache specialist, shared his thoughts via email. He agrees that HBOT may have a role in TBI, but after reviewing Hadanny and colleagues’ paper, he concluded that there is insufficient evidence for the use of HBOT in all forms of TBI. He would like to see large multicenter, well-designed studies with standardized pressures and duration and a standard definition of the various types of head injury.
Ongoing research
There are at least five ongoing trials on HBOT for TBI or postconcussive syndrome, including the well-designed placebo-controlled HOT-POCS study. The latter has a novel placebo gas system that addresses Hadanny and colleagues’ contention that even low-dose HBOT might be effective.
The placebo arm in HOT-POCS mimics the HBO environment but provides only 0.21 ATA of oxygen, the same as room air. The active arm provides 100% oxygen at 2.0 ATA. If patients in both arms improve, the benefit will be caused by a placebo response, not HBOT.
Conflict of interest
Another concern with the review is that all three authors are affiliated with Aviv Scientific. This company has an exclusive partnership with the world’s largest hyperbaric medicine and research facility, the Sagol Center at Shamir Medical Center in Be’er Ya’akov, Israel.
This conflict of interest does not a priori invalidate their conclusions. However, official HBOT guidelines from a leading organization like the Undersea and Hyperbaric Medicine Society or the American Academy of Neurology would be preferable.
Conclusion
There is an urgent unmet need for more effective treatments for postconcussive syndrome and chronic TBI.
The review authors’ recommendations for HBOT seem premature. They are arguably a disservice to the many desperate patients and their families who will be tempted to expend valuable resources of time and money for an appealing but unproven therapy. Appropriately designed placebo-controlled studies such as HOT-POCS will help separate fact from wishful thinking.
Dr. Wilner is associate professor of neurology at University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
A recent review by Hadanny and colleagues recommends hyperbaric oxygen therapy (HBOT) for acute moderate to severe traumatic brain injury (TBI) and selected patients with prolonged postconcussive syndrome.
This article piqued my curiosity because I trained in HBOT more than 20 years ago. As a passionate scuba diver, my motivation was to master treatment for air embolism and decompression illness. Thankfully, these diving accidents are rare. However, I used HBOT for nonhealing wounds, and its efficacy was sometimes remarkable.
Paradoxical results with oxygen therapy
Although it may seem self-evident that “more oxygen is better” for medical illness, this is not necessarily true. I recently interviewed Ola Didrik Saugstad, MD, who demonstrated that the traditional practice of resuscitating newborns with 100% oxygen was more toxic than resuscitation with air (which contains 21% oxygen). His counterintuitive discovery led to a lifesaving change in the international newborn resuscitation guidelines.
The Food and Drug Administration has approved HBOT for a wide variety of conditions, but some practitioners enthusiastically promote it for off-label indications. These include antiaging, autism, multiple sclerosis, and the aforementioned TBI.
More than 50 years ago, HBOT was proposed for stroke, another disorder where the brain has been deprived of oxygen. Despite obvious logic, clinical trials have been unconvincing. The FDA has not approved HBOT for stroke.
HBOT in practice
During HBOT, the patient breathes 100% oxygen while the whole body is pressurized within a hyperbaric chamber. The chamber’s construction allows pressures above normal sea level of 1.0 atmosphere absolute (ATA). For example, The U.S. Navy Treatment Table for decompression sickness recommends 100% oxygen at 2.8 ATA. Chambers may hold one or more patients at a time.
The frequency of therapy varies but often consists of 20-60 sessions lasting 90-120 minutes. For off-label use like TBI, patients usually pay out of pocket. Given the multiple treatments, costs can add up.
Inconsistent evidence and sham controls
The unwieldy 33-page evidence review by Hadanny and colleagues cites multiple studies supporting HBOT for TBI. However, many, if not all, suffer from methodological flaws. These include vague inclusion criteria, lack of a control group, small patient numbers, treatment at different times since injury, poorly defined or varying HBOT protocols, varying outcome measures, and superficial results analysis.
A sham or control arm is essential for HBOT research trials, given the potential placebo effect of placing a human being inside a large, high-tech, sealed tube for an hour or more. In some sham-controlled studies, which consisted of low-pressure oxygen (that is, 1.3 ATA as sham vs. 2.4 ATA as treatment), all groups experienced symptom improvement. The review authors argue that the low-dose HBOT sham arms were biologically active and that the improvements seen mean that both high- and low-dose HBOT is therapeutic. The alternative explanation is that the placebo effect accounted for improvement in both groups.
The late Michael Bennett, a world authority on hyperbaric and underwater medicine, doubted that conventional HBOT sham controls could genuinely have a therapeutic effect, and I agree. The upcoming HOT-POCS trial (discussed below) should answer the question more definitively.
Mechanisms of action and safety
Mechanisms of benefit for HBOT include increased oxygen availability and angiogenesis. Animal research suggests that it may reduce secondary cell death from TBI, through stabilization of the blood-brain barrier and inflammation reduction.
HBOT is generally safe and well tolerated. A retrospective analysis of 1.5 million outpatient hyperbaric treatments revealed that less than 1% were associated with adverse events. The most common were ear and sinus barotrauma. Because HBOT uses increased air pressure, patients must equalize their ears and sinuses. Those who cannot because of altered consciousness, anatomical defects, or congestion must undergo myringotomy or terminate therapy. Claustrophobia was the second most common adverse effect. Convulsions and tension pneumocephalus were rare.
Perhaps the most concerning risk of HBOT for patients with TBI is the potential waste of human and financial resources.
Desperate physicians and patients
As a neurologist who regularly treats patients with TBI, I share the review authors’ frustration regarding the limited efficacy of available treatments. However, the suboptimal efficacy of currently available therapy is insufficient justification to recommend HBOT.
With respect to chronic TBI, it is difficult to imagine how HBOT could reverse brain injury that has been present for months or years. No other therapy exists that reliably encourages neuronal regeneration or prevents the development of posttraumatic epilepsy.
Frank Conidi, MD, a board-certified sports neurologist and headache specialist, shared his thoughts via email. He agrees that HBOT may have a role in TBI, but after reviewing Hadanny and colleagues’ paper, he concluded that there is insufficient evidence for the use of HBOT in all forms of TBI. He would like to see large multicenter, well-designed studies with standardized pressures and duration and a standard definition of the various types of head injury.
Ongoing research
There are at least five ongoing trials on HBOT for TBI or postconcussive syndrome, including the well-designed placebo-controlled HOT-POCS study. The latter has a novel placebo gas system that addresses Hadanny and colleagues’ contention that even low-dose HBOT might be effective.
The placebo arm in HOT-POCS mimics the HBO environment but provides only 0.21 ATA of oxygen, the same as room air. The active arm provides 100% oxygen at 2.0 ATA. If patients in both arms improve, the benefit will be caused by a placebo response, not HBOT.
Conflict of interest
Another concern with the review is that all three authors are affiliated with Aviv Scientific. This company has an exclusive partnership with the world’s largest hyperbaric medicine and research facility, the Sagol Center at Shamir Medical Center in Be’er Ya’akov, Israel.
This conflict of interest does not a priori invalidate their conclusions. However, official HBOT guidelines from a leading organization like the Undersea and Hyperbaric Medicine Society or the American Academy of Neurology would be preferable.
Conclusion
There is an urgent unmet need for more effective treatments for postconcussive syndrome and chronic TBI.
The review authors’ recommendations for HBOT seem premature. They are arguably a disservice to the many desperate patients and their families who will be tempted to expend valuable resources of time and money for an appealing but unproven therapy. Appropriately designed placebo-controlled studies such as HOT-POCS will help separate fact from wishful thinking.
Dr. Wilner is associate professor of neurology at University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
Loneliness tied to increased risk for Parkinson’s disease
TOPLINE:
Loneliness is associated with a higher risk of developing Parkinson’s disease (PD) across demographic groups and independent of other risk factors, data from nearly 500,000 U.K. adults suggest.
METHODOLOGY:
- Loneliness is associated with illness and death, including higher risk of neurodegenerative diseases, but no study has examined whether the association between loneliness and detrimental outcomes extends to PD.
- The current analysis included 491,603 U.K. Biobank participants (mean age, 56; 54% women) without a diagnosis of PD at baseline.
- Loneliness was assessed by a single question at baseline and incident PD was ascertained via health records over 15 years.
- Researchers assessed whether the association between loneliness and PD was moderated by age, sex, or genetic risk and whether the association was accounted for by sociodemographic factors; behavioral, mental, physical, or social factors; or genetic risk.
TAKEAWAY:
- Roughly 19% of the cohort reported being lonely. Compared with those who were not lonely, those who did report being lonely were slightly younger and were more likely to be women. They also had fewer resources, more health risk behaviors (current smoker and physically inactive), and worse physical and mental health.
- Over 15+ years of follow-up, 2,822 participants developed PD (incidence rate: 47 per 100,000 person-years). Compared with those who did not develop PD, those who did were older and more likely to be male, former smokers, have higher BMI and PD polygenetic risk score, and to have diabetes, hypertension, myocardial infarction or stroke, anxiety, or depression.
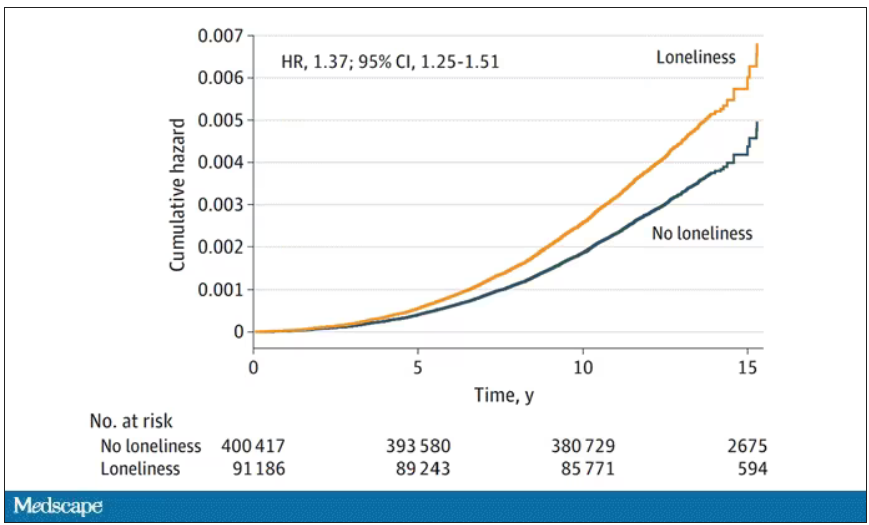
- In the primary analysis, individuals who reported being lonely had a higher risk for PD (hazard ratio, 1.37) – an association that remained after accounting for demographic and socioeconomic status, social isolation, PD polygenetic risk score, smoking, physical activity, BMI, diabetes, hypertension, stroke, myocardial infarction, depression, and having ever seen a psychiatrist (fully adjusted HR, 1.25).
- The association between loneliness and incident PD was not moderated by sex, age, or polygenetic risk score.
- Contrary to expectations for a prodromal syndrome, loneliness was not associated with incident PD in the first 5 years after baseline but was associated with PD risk in the subsequent 10 years of follow-up (HR, 1.32).
IN PRACTICE:
“Our findings complement other evidence that loneliness is a psychosocial determinant of health associated with increased risk of morbidity and mortality [and] supports recent calls for the protective and healing effects of personally meaningful social connection,” the authors write.
SOURCE:
The study, with first author Antonio Terracciano, PhD, of Florida State University College of Medicine, Tallahassee, was published online in JAMA Neurology.
LIMITATIONS:
This observational study could not determine causality or whether reverse causality could explain the association. Loneliness was assessed by a single yes/no question. PD diagnosis relied on hospital admission and death records and may have missed early PD diagnoses.
DISCLOSURES:
Funding for the study was provided by the National Institutes of Health and National Institute on Aging. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Loneliness is associated with a higher risk of developing Parkinson’s disease (PD) across demographic groups and independent of other risk factors, data from nearly 500,000 U.K. adults suggest.
METHODOLOGY:
- Loneliness is associated with illness and death, including higher risk of neurodegenerative diseases, but no study has examined whether the association between loneliness and detrimental outcomes extends to PD.
- The current analysis included 491,603 U.K. Biobank participants (mean age, 56; 54% women) without a diagnosis of PD at baseline.
- Loneliness was assessed by a single question at baseline and incident PD was ascertained via health records over 15 years.
- Researchers assessed whether the association between loneliness and PD was moderated by age, sex, or genetic risk and whether the association was accounted for by sociodemographic factors; behavioral, mental, physical, or social factors; or genetic risk.
TAKEAWAY:
- Roughly 19% of the cohort reported being lonely. Compared with those who were not lonely, those who did report being lonely were slightly younger and were more likely to be women. They also had fewer resources, more health risk behaviors (current smoker and physically inactive), and worse physical and mental health.
- Over 15+ years of follow-up, 2,822 participants developed PD (incidence rate: 47 per 100,000 person-years). Compared with those who did not develop PD, those who did were older and more likely to be male, former smokers, have higher BMI and PD polygenetic risk score, and to have diabetes, hypertension, myocardial infarction or stroke, anxiety, or depression.
- In the primary analysis, individuals who reported being lonely had a higher risk for PD (hazard ratio, 1.37) – an association that remained after accounting for demographic and socioeconomic status, social isolation, PD polygenetic risk score, smoking, physical activity, BMI, diabetes, hypertension, stroke, myocardial infarction, depression, and having ever seen a psychiatrist (fully adjusted HR, 1.25).
- The association between loneliness and incident PD was not moderated by sex, age, or polygenetic risk score.
- Contrary to expectations for a prodromal syndrome, loneliness was not associated with incident PD in the first 5 years after baseline but was associated with PD risk in the subsequent 10 years of follow-up (HR, 1.32).
IN PRACTICE:
“Our findings complement other evidence that loneliness is a psychosocial determinant of health associated with increased risk of morbidity and mortality [and] supports recent calls for the protective and healing effects of personally meaningful social connection,” the authors write.
SOURCE:
The study, with first author Antonio Terracciano, PhD, of Florida State University College of Medicine, Tallahassee, was published online in JAMA Neurology.
LIMITATIONS:
This observational study could not determine causality or whether reverse causality could explain the association. Loneliness was assessed by a single yes/no question. PD diagnosis relied on hospital admission and death records and may have missed early PD diagnoses.
DISCLOSURES:
Funding for the study was provided by the National Institutes of Health and National Institute on Aging. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Loneliness is associated with a higher risk of developing Parkinson’s disease (PD) across demographic groups and independent of other risk factors, data from nearly 500,000 U.K. adults suggest.
METHODOLOGY:
- Loneliness is associated with illness and death, including higher risk of neurodegenerative diseases, but no study has examined whether the association between loneliness and detrimental outcomes extends to PD.
- The current analysis included 491,603 U.K. Biobank participants (mean age, 56; 54% women) without a diagnosis of PD at baseline.
- Loneliness was assessed by a single question at baseline and incident PD was ascertained via health records over 15 years.
- Researchers assessed whether the association between loneliness and PD was moderated by age, sex, or genetic risk and whether the association was accounted for by sociodemographic factors; behavioral, mental, physical, or social factors; or genetic risk.
TAKEAWAY:
- Roughly 19% of the cohort reported being lonely. Compared with those who were not lonely, those who did report being lonely were slightly younger and were more likely to be women. They also had fewer resources, more health risk behaviors (current smoker and physically inactive), and worse physical and mental health.
- Over 15+ years of follow-up, 2,822 participants developed PD (incidence rate: 47 per 100,000 person-years). Compared with those who did not develop PD, those who did were older and more likely to be male, former smokers, have higher BMI and PD polygenetic risk score, and to have diabetes, hypertension, myocardial infarction or stroke, anxiety, or depression.
- In the primary analysis, individuals who reported being lonely had a higher risk for PD (hazard ratio, 1.37) – an association that remained after accounting for demographic and socioeconomic status, social isolation, PD polygenetic risk score, smoking, physical activity, BMI, diabetes, hypertension, stroke, myocardial infarction, depression, and having ever seen a psychiatrist (fully adjusted HR, 1.25).
- The association between loneliness and incident PD was not moderated by sex, age, or polygenetic risk score.
- Contrary to expectations for a prodromal syndrome, loneliness was not associated with incident PD in the first 5 years after baseline but was associated with PD risk in the subsequent 10 years of follow-up (HR, 1.32).
IN PRACTICE:
“Our findings complement other evidence that loneliness is a psychosocial determinant of health associated with increased risk of morbidity and mortality [and] supports recent calls for the protective and healing effects of personally meaningful social connection,” the authors write.
SOURCE:
The study, with first author Antonio Terracciano, PhD, of Florida State University College of Medicine, Tallahassee, was published online in JAMA Neurology.
LIMITATIONS:
This observational study could not determine causality or whether reverse causality could explain the association. Loneliness was assessed by a single yes/no question. PD diagnosis relied on hospital admission and death records and may have missed early PD diagnoses.
DISCLOSURES:
Funding for the study was provided by the National Institutes of Health and National Institute on Aging. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The surprising link between loneliness and Parkinson’s disease
This transcript has been edited for clarity.
On May 3, 2023, Surgeon General Vivek Murthy issued an advisory raising an alarm about what he called an “epidemic of loneliness” in the United States.
Now, I am not saying that Vivek Murthy read my book, “How Medicine Works and When It Doesn’t” – released in January and available in bookstores now – where, in chapter 11, I call attention to the problem of loneliness and its relationship to the exponential rise in deaths of despair. But Vivek, if you did, let me know. I could use the publicity.
No, of course the idea that loneliness is a public health issue is not new, but I’m glad to see it finally getting attention. At this point, studies have linked loneliness to heart disease, stroke, dementia, and premature death.
The UK Biobank is really a treasure trove of data for epidemiologists. I must see three to four studies a week coming out of this mega-dataset. This one, appearing in JAMA Neurology, caught my eye for its focus specifically on loneliness as a risk factor – something I’m hoping to see more of in the future.
The study examines data from just under 500,000 individuals in the United Kingdom who answered a survey including the question “Do you often feel lonely?” between 2006 and 2010; 18.4% of people answered yes. Individuals’ electronic health record data were then monitored over time to see who would get a new diagnosis code consistent with Parkinson’s disease. Through 2021, 2822 people did – that’s just over half a percent.
So, now we do the statistics thing. Of the nonlonely folks, 2,273 went on to develop Parkinson’s disease. Of those who said they often feel lonely, 549 people did. The raw numbers here, to be honest, aren’t that compelling. Lonely people had an absolute risk for Parkinson’s disease about 0.03% higher than that of nonlonely people. Put another way, you’d need to take over 3,000 lonely souls and make them not lonely to prevent 1 case of Parkinson’s disease.
Still, the costs of loneliness are not measured exclusively in Parkinson’s disease, and I would argue that the real risks here come from other sources: alcohol abuse, drug abuse, and suicide. Nevertheless, the weak but significant association with Parkinson’s disease reminds us that loneliness is a neurologic phenomenon. There is something about social connection that affects our brain in a way that is not just spiritual; it is actually biological.
Of course, people who say they are often lonely are different in other ways from people who report not being lonely. Lonely people, in this dataset, were younger, more likely to be female, less likely to have a college degree, in worse physical health, and engaged in more high-risk health behaviors like smoking.
The authors adjusted for all of these factors and found that, on the relative scale, lonely people were still about 20%-30% more likely to develop Parkinson’s disease.
So, what do we do about this? There is no pill for loneliness, and God help us if there ever is. Recognizing the problem is a good start. But there are some policy things we can do to reduce loneliness. We can invest in public spaces that bring people together – parks, museums, libraries – and public transportation. We can deal with tech companies that are so optimized at capturing our attention that we cease to engage with other humans. And, individually, we can just reach out a bit more. We’ve spent the past few pandemic years with our attention focused sharply inward. It’s time to look out again.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
On May 3, 2023, Surgeon General Vivek Murthy issued an advisory raising an alarm about what he called an “epidemic of loneliness” in the United States.
Now, I am not saying that Vivek Murthy read my book, “How Medicine Works and When It Doesn’t” – released in January and available in bookstores now – where, in chapter 11, I call attention to the problem of loneliness and its relationship to the exponential rise in deaths of despair. But Vivek, if you did, let me know. I could use the publicity.
No, of course the idea that loneliness is a public health issue is not new, but I’m glad to see it finally getting attention. At this point, studies have linked loneliness to heart disease, stroke, dementia, and premature death.
The UK Biobank is really a treasure trove of data for epidemiologists. I must see three to four studies a week coming out of this mega-dataset. This one, appearing in JAMA Neurology, caught my eye for its focus specifically on loneliness as a risk factor – something I’m hoping to see more of in the future.
The study examines data from just under 500,000 individuals in the United Kingdom who answered a survey including the question “Do you often feel lonely?” between 2006 and 2010; 18.4% of people answered yes. Individuals’ electronic health record data were then monitored over time to see who would get a new diagnosis code consistent with Parkinson’s disease. Through 2021, 2822 people did – that’s just over half a percent.
So, now we do the statistics thing. Of the nonlonely folks, 2,273 went on to develop Parkinson’s disease. Of those who said they often feel lonely, 549 people did. The raw numbers here, to be honest, aren’t that compelling. Lonely people had an absolute risk for Parkinson’s disease about 0.03% higher than that of nonlonely people. Put another way, you’d need to take over 3,000 lonely souls and make them not lonely to prevent 1 case of Parkinson’s disease.
Still, the costs of loneliness are not measured exclusively in Parkinson’s disease, and I would argue that the real risks here come from other sources: alcohol abuse, drug abuse, and suicide. Nevertheless, the weak but significant association with Parkinson’s disease reminds us that loneliness is a neurologic phenomenon. There is something about social connection that affects our brain in a way that is not just spiritual; it is actually biological.
Of course, people who say they are often lonely are different in other ways from people who report not being lonely. Lonely people, in this dataset, were younger, more likely to be female, less likely to have a college degree, in worse physical health, and engaged in more high-risk health behaviors like smoking.
The authors adjusted for all of these factors and found that, on the relative scale, lonely people were still about 20%-30% more likely to develop Parkinson’s disease.
So, what do we do about this? There is no pill for loneliness, and God help us if there ever is. Recognizing the problem is a good start. But there are some policy things we can do to reduce loneliness. We can invest in public spaces that bring people together – parks, museums, libraries – and public transportation. We can deal with tech companies that are so optimized at capturing our attention that we cease to engage with other humans. And, individually, we can just reach out a bit more. We’ve spent the past few pandemic years with our attention focused sharply inward. It’s time to look out again.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
On May 3, 2023, Surgeon General Vivek Murthy issued an advisory raising an alarm about what he called an “epidemic of loneliness” in the United States.
Now, I am not saying that Vivek Murthy read my book, “How Medicine Works and When It Doesn’t” – released in January and available in bookstores now – where, in chapter 11, I call attention to the problem of loneliness and its relationship to the exponential rise in deaths of despair. But Vivek, if you did, let me know. I could use the publicity.
No, of course the idea that loneliness is a public health issue is not new, but I’m glad to see it finally getting attention. At this point, studies have linked loneliness to heart disease, stroke, dementia, and premature death.
The UK Biobank is really a treasure trove of data for epidemiologists. I must see three to four studies a week coming out of this mega-dataset. This one, appearing in JAMA Neurology, caught my eye for its focus specifically on loneliness as a risk factor – something I’m hoping to see more of in the future.
The study examines data from just under 500,000 individuals in the United Kingdom who answered a survey including the question “Do you often feel lonely?” between 2006 and 2010; 18.4% of people answered yes. Individuals’ electronic health record data were then monitored over time to see who would get a new diagnosis code consistent with Parkinson’s disease. Through 2021, 2822 people did – that’s just over half a percent.
So, now we do the statistics thing. Of the nonlonely folks, 2,273 went on to develop Parkinson’s disease. Of those who said they often feel lonely, 549 people did. The raw numbers here, to be honest, aren’t that compelling. Lonely people had an absolute risk for Parkinson’s disease about 0.03% higher than that of nonlonely people. Put another way, you’d need to take over 3,000 lonely souls and make them not lonely to prevent 1 case of Parkinson’s disease.
Still, the costs of loneliness are not measured exclusively in Parkinson’s disease, and I would argue that the real risks here come from other sources: alcohol abuse, drug abuse, and suicide. Nevertheless, the weak but significant association with Parkinson’s disease reminds us that loneliness is a neurologic phenomenon. There is something about social connection that affects our brain in a way that is not just spiritual; it is actually biological.
Of course, people who say they are often lonely are different in other ways from people who report not being lonely. Lonely people, in this dataset, were younger, more likely to be female, less likely to have a college degree, in worse physical health, and engaged in more high-risk health behaviors like smoking.
The authors adjusted for all of these factors and found that, on the relative scale, lonely people were still about 20%-30% more likely to develop Parkinson’s disease.
So, what do we do about this? There is no pill for loneliness, and God help us if there ever is. Recognizing the problem is a good start. But there are some policy things we can do to reduce loneliness. We can invest in public spaces that bring people together – parks, museums, libraries – and public transportation. We can deal with tech companies that are so optimized at capturing our attention that we cease to engage with other humans. And, individually, we can just reach out a bit more. We’ve spent the past few pandemic years with our attention focused sharply inward. It’s time to look out again.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Data Trends 2023: Migraine and Headache
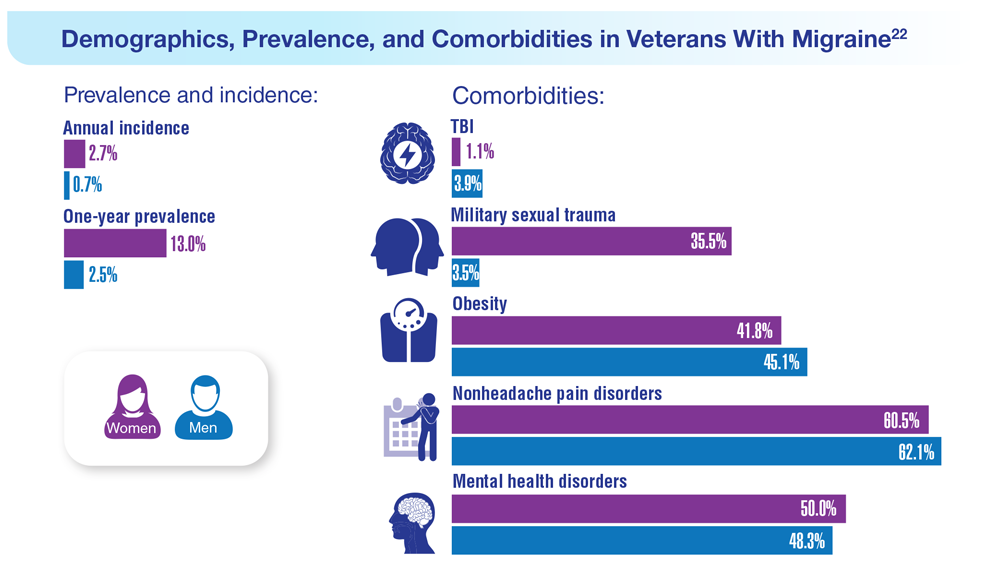
22. Seng EK et al. Neurology. 2022;99(18):e1979-e1992. doi:10.1212/WNL.0000000000200888
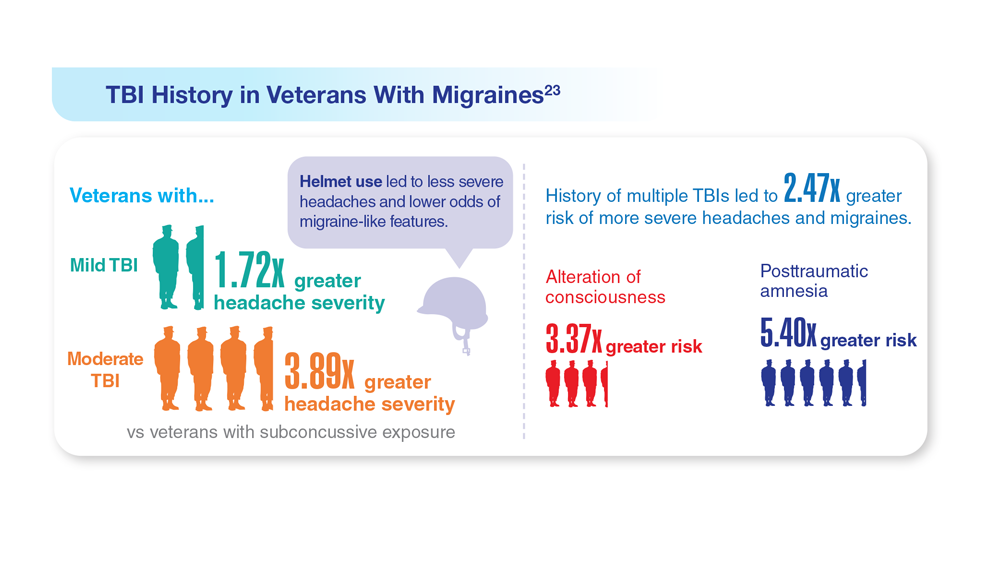
23. Coffman C et al. Neurology. 2022;99(2):e187-e198. doi:10.1212/WNL.0000000000200518
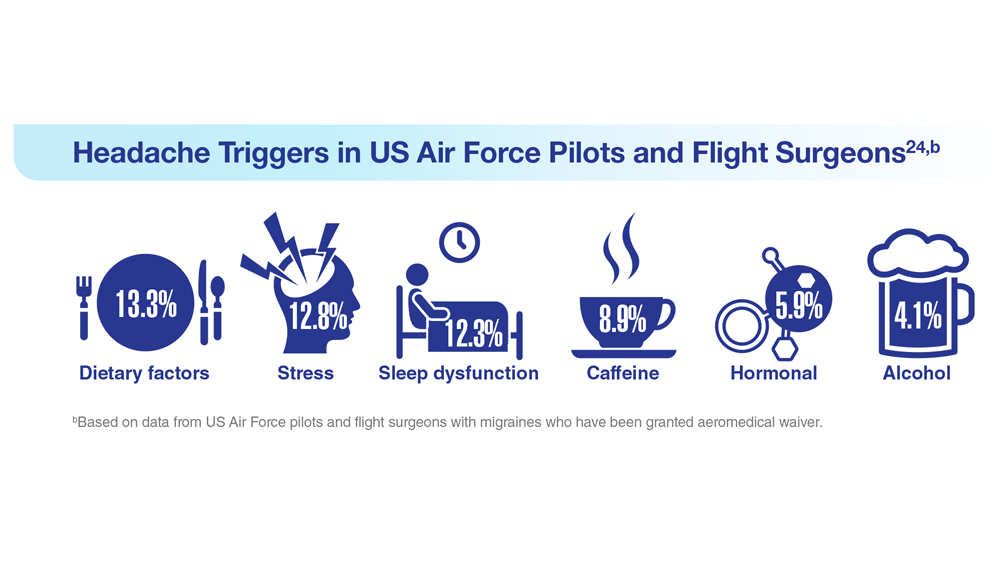
24. Hesselbrock RR et al. Aerosp Med Hum Perform. 2022;93(1):26-31. doi:10.3357/amhp.5980.2022
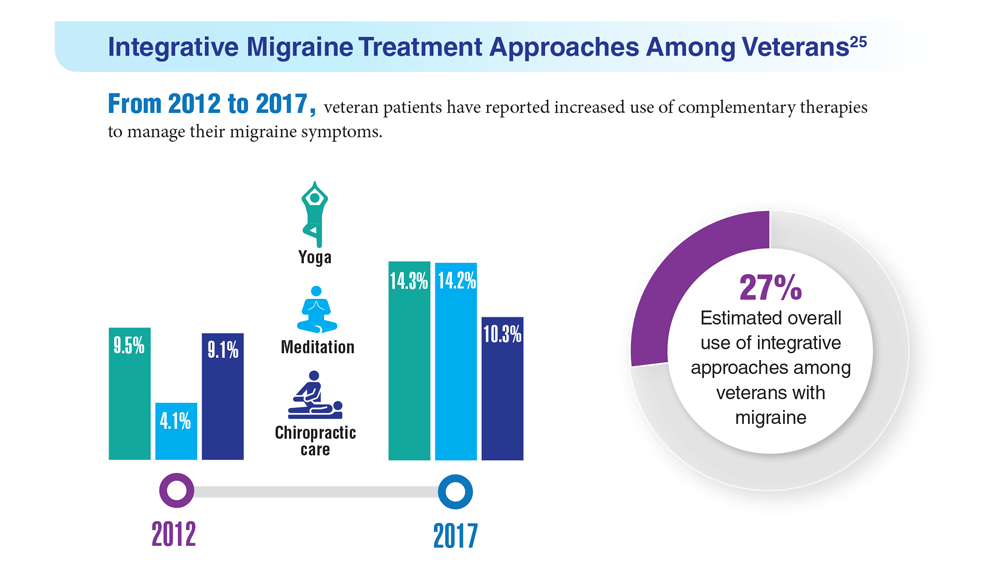
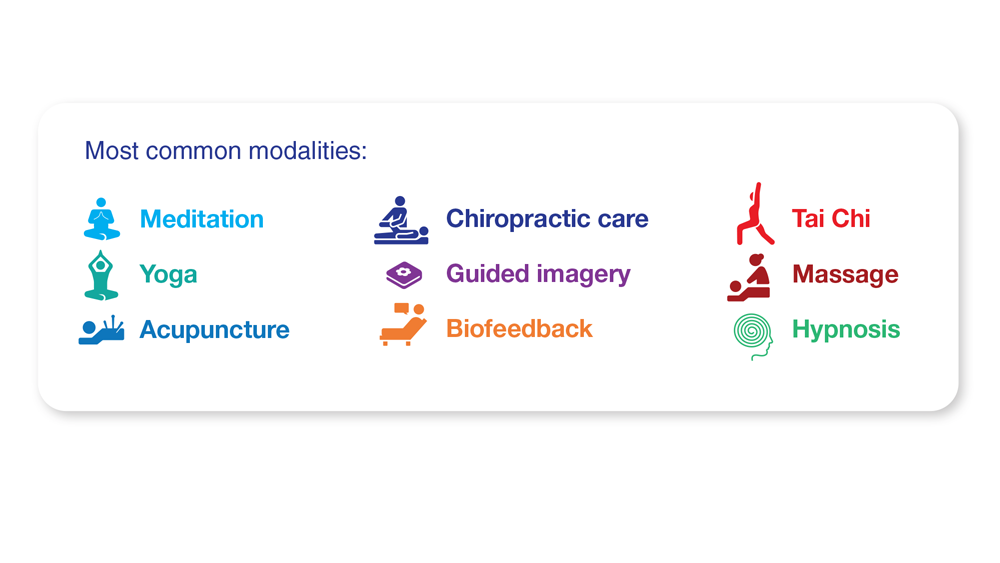
25. Kuruvilla DE et al. BMC Complement Med Ther. 2022;22(1):22. doi:10.1186/s12906-022-03511-6
22. Seng EK et al. Neurology. 2022;99(18):e1979-e1992. doi:10.1212/WNL.0000000000200888
23. Coffman C et al. Neurology. 2022;99(2):e187-e198. doi:10.1212/WNL.0000000000200518
24. Hesselbrock RR et al. Aerosp Med Hum Perform. 2022;93(1):26-31. doi:10.3357/amhp.5980.2022
25. Kuruvilla DE et al. BMC Complement Med Ther. 2022;22(1):22. doi:10.1186/s12906-022-03511-6
22. Seng EK et al. Neurology. 2022;99(18):e1979-e1992. doi:10.1212/WNL.0000000000200888
23. Coffman C et al. Neurology. 2022;99(2):e187-e198. doi:10.1212/WNL.0000000000200518
24. Hesselbrock RR et al. Aerosp Med Hum Perform. 2022;93(1):26-31. doi:10.3357/amhp.5980.2022
25. Kuruvilla DE et al. BMC Complement Med Ther. 2022;22(1):22. doi:10.1186/s12906-022-03511-6
Data Trends 2023: Parkinson’s Disease
17. US Department of Veterans Affairs, Office of Research and Development.
Parkinson’s disease. Updated October 28, 2021. Accessed May 5, 2023.
https://www.research.va.gov/topics/parkinsons.cfm
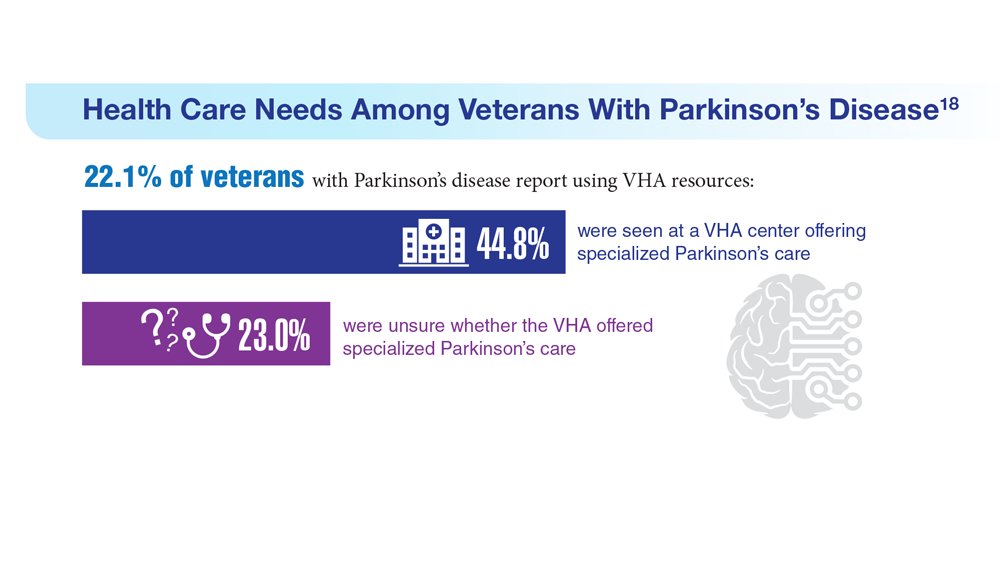
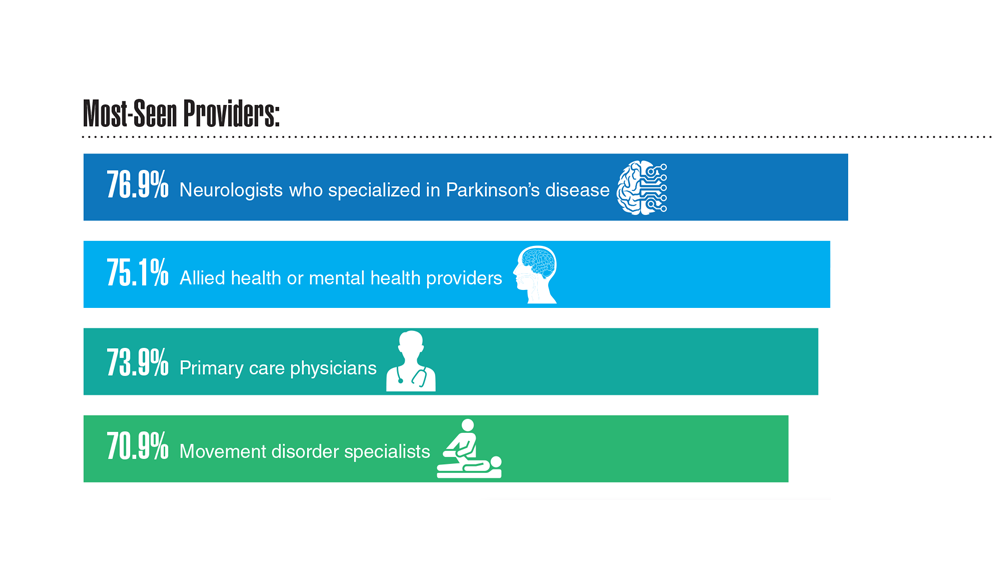
18. Feeney M et al. Front Neurol. 2022;13:924999. doi:10.3389/fneur.2022.924999
19. Heronemus M et al. Parkinsonism Relat Disord. 2022;105:58-61. doi:10.1016/j.parkreldis.2022.11.003
20. Nejtek VA et al. PLoS One. 2021;16(11):e0258851. doi:10.1371/journal.pone.0258851
21. Yang Y et al. Dement Neurocogn Disord. 2016;15(3):75-81. doi:10.12779/dnd.2016.15.3.75
17. US Department of Veterans Affairs, Office of Research and Development.
Parkinson’s disease. Updated October 28, 2021. Accessed May 5, 2023.
https://www.research.va.gov/topics/parkinsons.cfm
18. Feeney M et al. Front Neurol. 2022;13:924999. doi:10.3389/fneur.2022.924999
19. Heronemus M et al. Parkinsonism Relat Disord. 2022;105:58-61. doi:10.1016/j.parkreldis.2022.11.003
20. Nejtek VA et al. PLoS One. 2021;16(11):e0258851. doi:10.1371/journal.pone.0258851
21. Yang Y et al. Dement Neurocogn Disord. 2016;15(3):75-81. doi:10.12779/dnd.2016.15.3.75
17. US Department of Veterans Affairs, Office of Research and Development.
Parkinson’s disease. Updated October 28, 2021. Accessed May 5, 2023.
https://www.research.va.gov/topics/parkinsons.cfm
18. Feeney M et al. Front Neurol. 2022;13:924999. doi:10.3389/fneur.2022.924999
19. Heronemus M et al. Parkinsonism Relat Disord. 2022;105:58-61. doi:10.1016/j.parkreldis.2022.11.003
20. Nejtek VA et al. PLoS One. 2021;16(11):e0258851. doi:10.1371/journal.pone.0258851
21. Yang Y et al. Dement Neurocogn Disord. 2016;15(3):75-81. doi:10.12779/dnd.2016.15.3.75
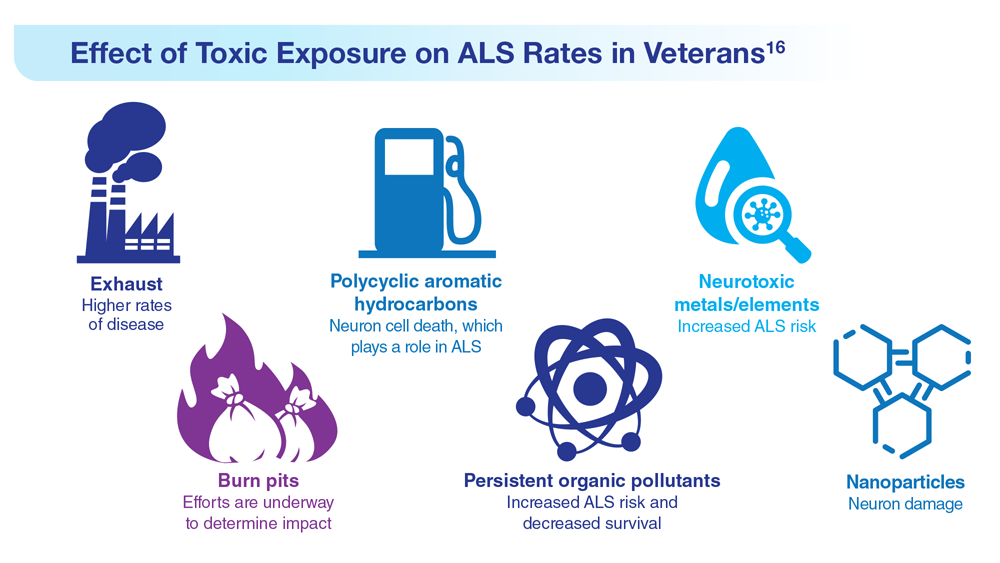
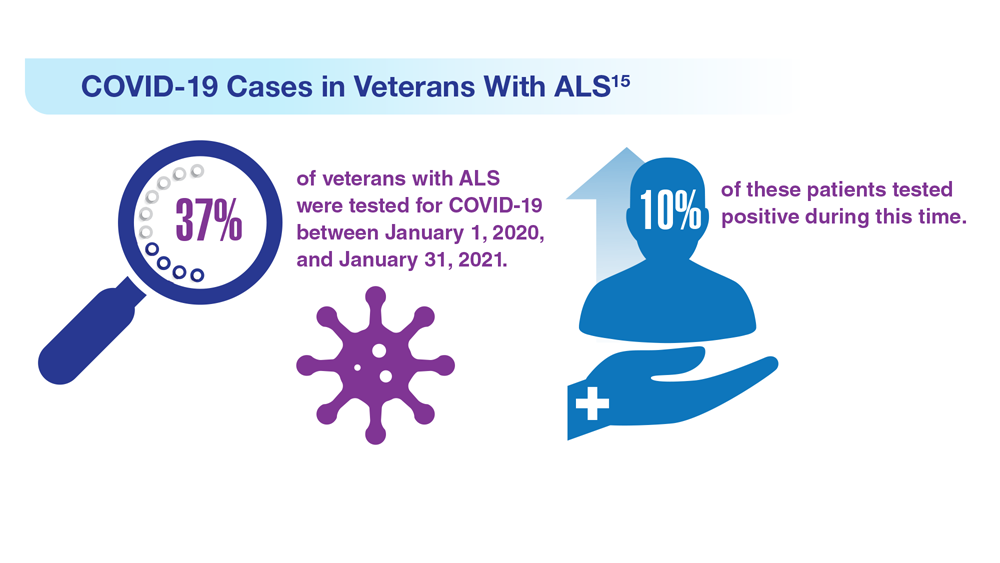
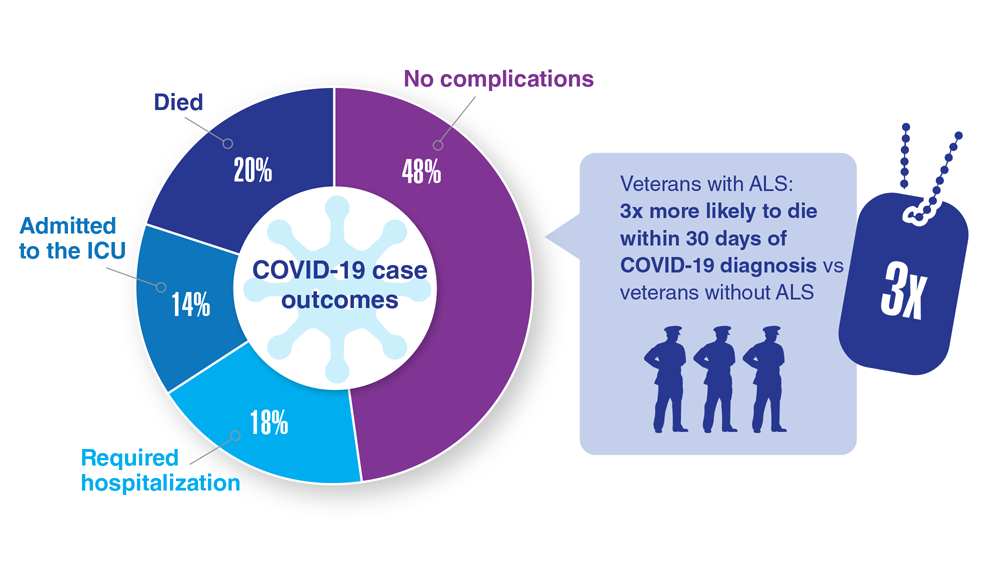
Data Trends 2023: Amyotrophic Lateral Sclerosis (ALS)
12. The ALS Association. ALS in the military. https://www.als.org/navigating-als/military-veterans/ALS-in-the-Military
13. McKay KA et al. Acta Neurol Scand. 2021;143(1):39-50. doi:10.1111/ane.13345
14. Lund EM et al. Muscle Nerve. 2021;63(6):807-811. doi:10.1002/mus.27181
15. Galea MD et al. Muscle Nerve. 2021;64(4):E18-E20. doi:10.1002/mus.27373
16. Re DB et al. J Neurol. 2022;269(5):2359-2377. doi:10.1007/s00415-021-10928-5
12. The ALS Association. ALS in the military. https://www.als.org/navigating-als/military-veterans/ALS-in-the-Military
13. McKay KA et al. Acta Neurol Scand. 2021;143(1):39-50. doi:10.1111/ane.13345
14. Lund EM et al. Muscle Nerve. 2021;63(6):807-811. doi:10.1002/mus.27181
15. Galea MD et al. Muscle Nerve. 2021;64(4):E18-E20. doi:10.1002/mus.27373
16. Re DB et al. J Neurol. 2022;269(5):2359-2377. doi:10.1007/s00415-021-10928-5
12. The ALS Association. ALS in the military. https://www.als.org/navigating-als/military-veterans/ALS-in-the-Military
13. McKay KA et al. Acta Neurol Scand. 2021;143(1):39-50. doi:10.1111/ane.13345
14. Lund EM et al. Muscle Nerve. 2021;63(6):807-811. doi:10.1002/mus.27181
15. Galea MD et al. Muscle Nerve. 2021;64(4):E18-E20. doi:10.1002/mus.27373
16. Re DB et al. J Neurol. 2022;269(5):2359-2377. doi:10.1007/s00415-021-10928-5
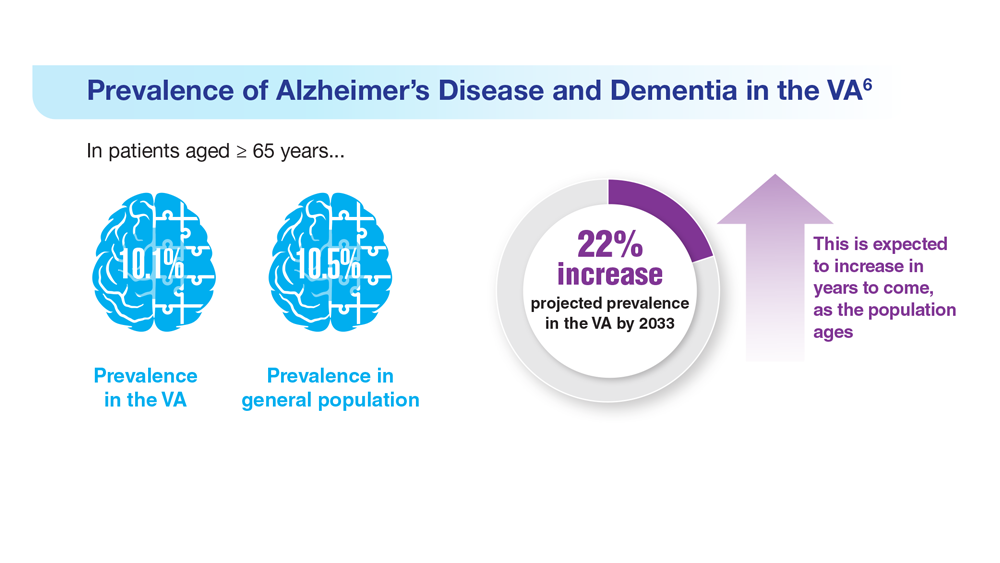
Data Trends 2023: Alzheimer’s and Dementia
6. Zhu CW, Sano M. Front Psychiatry. 2021;12:610334. doi:10.3389/fpsyt.2021.610334
7. Nianogo RA et al. JAMA Neurol. 2022;79(6):584-591. doi:10.1001/jamaneurol.2022.0976
8. Logue MW et al. Alzheimers Dement. 2022 Dec 22. Online ahead of print. doi:10.1002/alz.12870
9. Kempuraj D et al. Clin Ther. 2020;42(6):974-982. doi:10.1016/j.clinthera.2020.02.018
10. Martinez S et al. JAMA Neurol. 2021;78(4):473-477. doi:10.1001/jamaneurol.2020.5011
11. Verger A et al. Eur J Nucl Med Mol Imaging. 2023;50(6):1553-1555. doi:10.1007/s00259-023-06177-5
6. Zhu CW, Sano M. Front Psychiatry. 2021;12:610334. doi:10.3389/fpsyt.2021.610334
7. Nianogo RA et al. JAMA Neurol. 2022;79(6):584-591. doi:10.1001/jamaneurol.2022.0976
8. Logue MW et al. Alzheimers Dement. 2022 Dec 22. Online ahead of print. doi:10.1002/alz.12870
9. Kempuraj D et al. Clin Ther. 2020;42(6):974-982. doi:10.1016/j.clinthera.2020.02.018
10. Martinez S et al. JAMA Neurol. 2021;78(4):473-477. doi:10.1001/jamaneurol.2020.5011
11. Verger A et al. Eur J Nucl Med Mol Imaging. 2023;50(6):1553-1555. doi:10.1007/s00259-023-06177-5
6. Zhu CW, Sano M. Front Psychiatry. 2021;12:610334. doi:10.3389/fpsyt.2021.610334
7. Nianogo RA et al. JAMA Neurol. 2022;79(6):584-591. doi:10.1001/jamaneurol.2022.0976
8. Logue MW et al. Alzheimers Dement. 2022 Dec 22. Online ahead of print. doi:10.1002/alz.12870
9. Kempuraj D et al. Clin Ther. 2020;42(6):974-982. doi:10.1016/j.clinthera.2020.02.018
10. Martinez S et al. JAMA Neurol. 2021;78(4):473-477. doi:10.1001/jamaneurol.2020.5011
11. Verger A et al. Eur J Nucl Med Mol Imaging. 2023;50(6):1553-1555. doi:10.1007/s00259-023-06177-5
Federal Health Care Data Trends 2023
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner, highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner, highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner, highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Limb Loss and Prostheses
- Neurology
- Cardiology
- Mental Health
- Diabetes
- Rheumatoid Arthritis
- Respiratory illnesses
- Women's Health
- HPV and Related Cancers
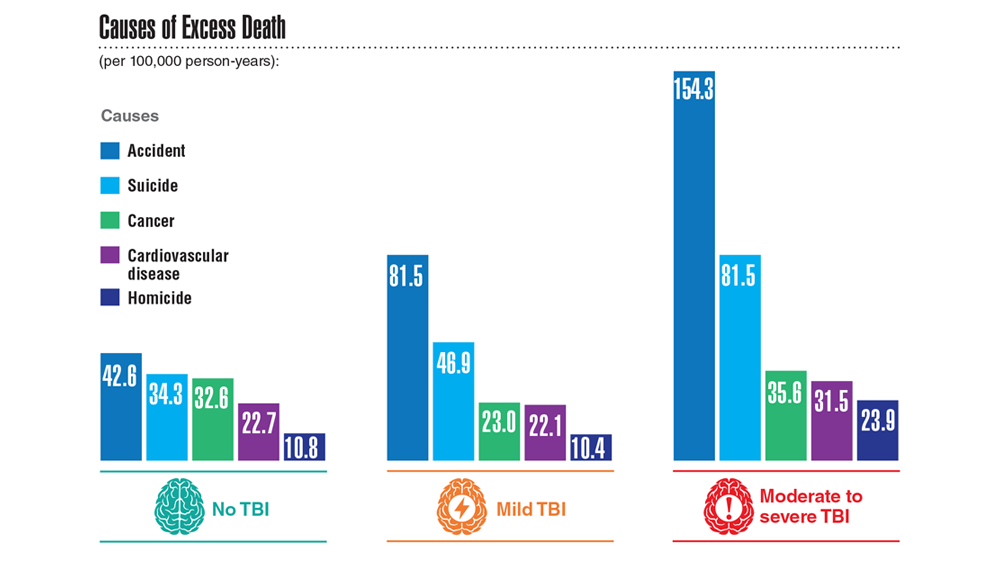
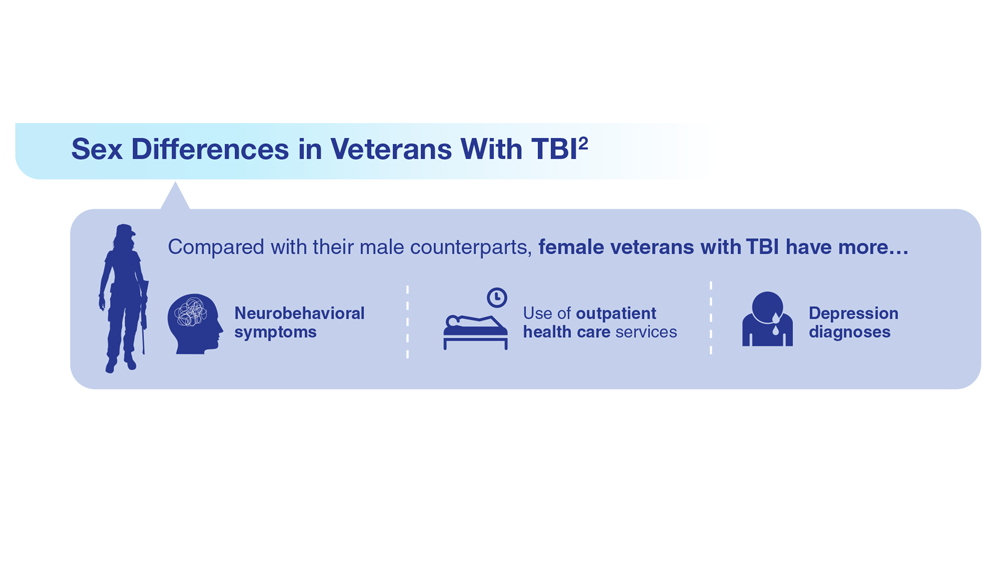
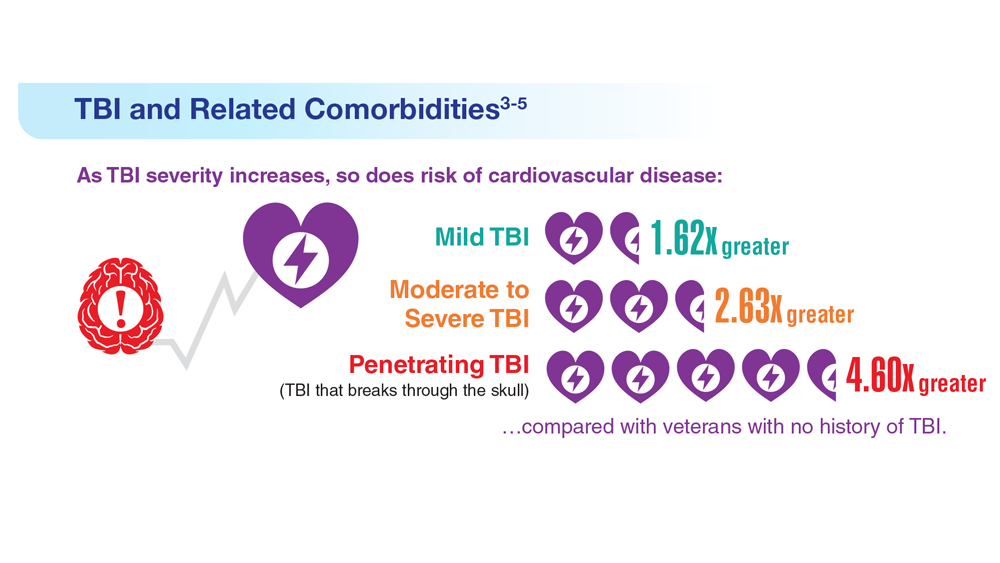
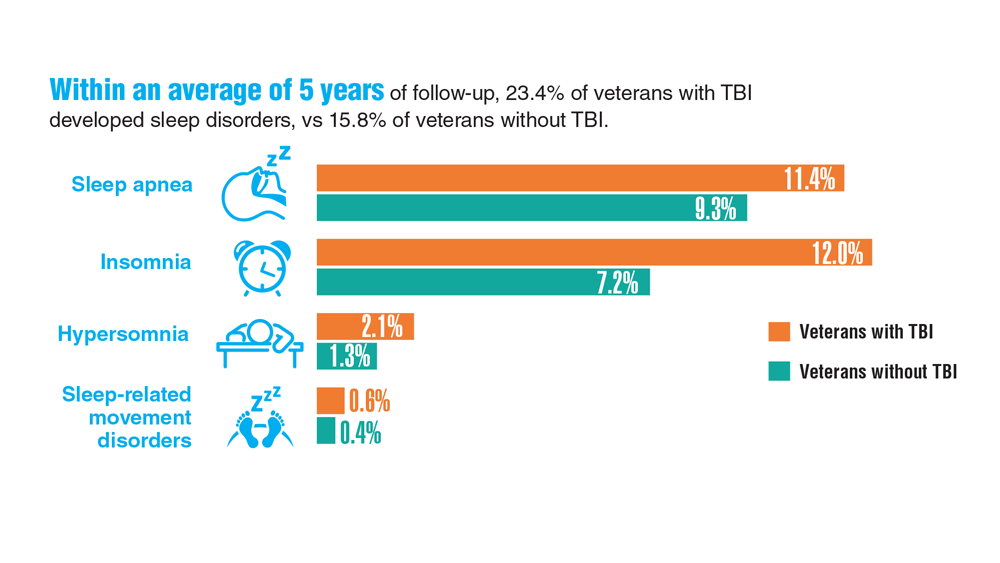
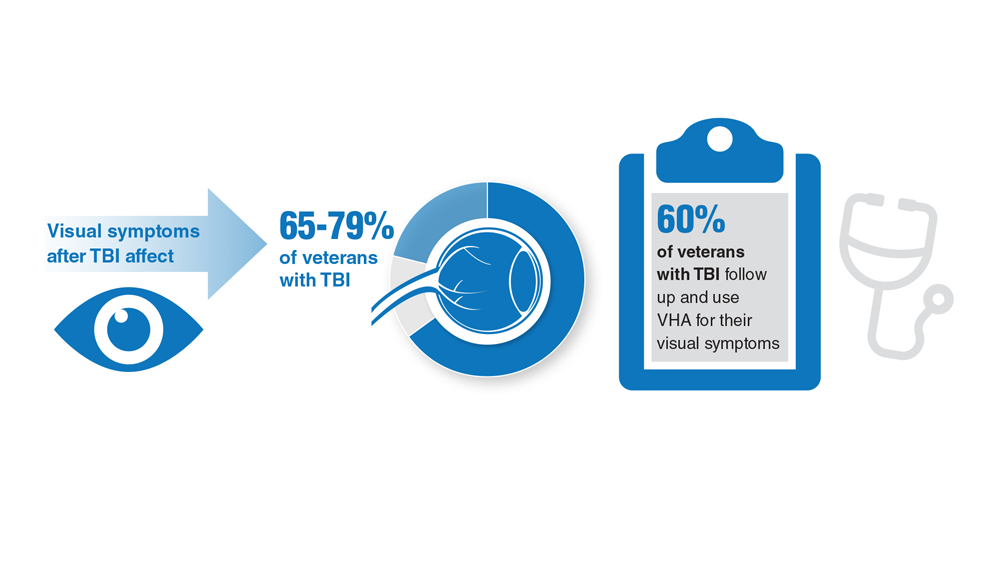
Data Trends 2023: Traumatic Brain Injury
- Howard JT et al. JAMA Netw Open. 2022;5(2):e2148150. doi:10.1001/jamanetworkopen.2021.48150
- Cogan AM et al. PM R. 2020;12(3):301-314. doi:10.1002/pmrj.12237
- Stewart IJ et al. JAMA Neurol. 2022;79(11):1122-1129. doi:10.1001/jamaneurol.2022.2682
- Leng Y et al. Neurology. 2021;96(13):e1792-e1799. doi:10.1212/WNL.0000000000011656
- Winkler SL et al. Optom Vis Sci. 2022;99(1):3-8. doi:10.1097/OPX.0000000000001824
- Howard JT et al. JAMA Netw Open. 2022;5(2):e2148150. doi:10.1001/jamanetworkopen.2021.48150
- Cogan AM et al. PM R. 2020;12(3):301-314. doi:10.1002/pmrj.12237
- Stewart IJ et al. JAMA Neurol. 2022;79(11):1122-1129. doi:10.1001/jamaneurol.2022.2682
- Leng Y et al. Neurology. 2021;96(13):e1792-e1799. doi:10.1212/WNL.0000000000011656
- Winkler SL et al. Optom Vis Sci. 2022;99(1):3-8. doi:10.1097/OPX.0000000000001824
- Howard JT et al. JAMA Netw Open. 2022;5(2):e2148150. doi:10.1001/jamanetworkopen.2021.48150
- Cogan AM et al. PM R. 2020;12(3):301-314. doi:10.1002/pmrj.12237
- Stewart IJ et al. JAMA Neurol. 2022;79(11):1122-1129. doi:10.1001/jamaneurol.2022.2682
- Leng Y et al. Neurology. 2021;96(13):e1792-e1799. doi:10.1212/WNL.0000000000011656
- Winkler SL et al. Optom Vis Sci. 2022;99(1):3-8. doi:10.1097/OPX.0000000000001824