User login
Skin imaging working group releases first guidelines for AI algorithms used in dermatology
The
The guidelines, published in JAMA Dermatology on Dec. 1, 2021, contain a broad range of recommendations stakeholders should consider when developing and assessing image-based AI algorithms in dermatology. The recommendations are divided into categories of data, technique, technical assessment, and application. ISIC is “an academia and industry partnership designed to facilitate the application of digital skin imaging to help reduce melanoma mortality,” and is organized into different working groups, including the AI working group, according to its website.
“Our goal with these guidelines was to create higher-quality reporting of dataset and algorithm characteristics for dermatology AI,” first author Roxana Daneshjou, MD, PhD, clinical scholar in dermatology, in the department of dermatology at Stanford (Calif.) University, said in an interview. “We hope these guidelines also aid regulatory bodies around the world when they are assessing algorithms to be used in dermatology.”
Recommendations for data
The authors recommended that datasets used by AI algorithms have image descriptions and details on image artifacts. “For photography, these include the type of camera used; whether images were taken under standardized or varying conditions; whether they were taken by professional photographers, laymen, or health care professionals; and image quality,” they wrote. They also recommended that developers include in an image description the type of lighting used and whether the photo contains pen markings, hair, tattoos, injuries, surgical effects, or other “physical perturbations.”
Exchangeable image file format data obtained from the camera, and preprocessing procedures like color normalization and “postprocessing” of images, such as filtering, should also be disclosed. In addition, developers should disclose and justify inclusion of images that have been created by an algorithm within a dataset. Any public images used in the datasets should have references, and privately used images should be made public where possible, the authors said.
The ISIC working group guidelines also provided recommendations for patient-level metadata. Each image should include a patient’s geographical location and medical center they visited as well as their age, sex and gender, ethnicity and/or race, and skin tone. Dr. Daneshjou said this was one area where she and her colleagues found a lack of transparency in AI datasets in algorithms in a recent review. “We found that many AI papers provided sparse details about the images used to train and test their algorithms,” Dr. Daneshjou explained. “For example, only 7 out of 70 papers had any information about the skin tones in the images used for developing and/or testing AI algorithms. Understanding the diversity of images used to train and test algorithms is important because algorithms that are developed on images of predominantly white skin likely won’t work as well on Black and brown skin.”
The guideline authors also asked algorithm developers to describe the limitations of not including patient-level metadata information when it is incomplete or unavailable. In addition, “we ask that algorithm developers comment on potential biases of their algorithms,” Dr. Daneshjou said. “For example, an algorithm based only on telemedicine images may not capture the full range of diseases seen within an in-person clinic.”
When describing their AI algorithm, developers should detail their reasoning for the dataset size and partitions, inclusion and exclusion criteria for images, and use of any external samples for test sets. “Authors should consider any differences between the image characteristics used for algorithm development and those that might be encountered in the real world,” the guidelines stated.
Recommendations for technique
How the images in a dataset are labeled is a unique challenge in developing AI algorithms for dermatology, the authors noted. Developers should use histopathological diagnosis in their labeling, but this can sometimes result in label noise.
“Many of the AI algorithms in dermatology use supervised learning, which requires labeled examples to help the algorithm ‘learn’ features for discriminating between lesions. We found that some papers use consensus labeling – dermatologists providing a label – to label skin cancers; however, the standard for diagnosing skin cancer is using histopathology from a biopsy,” she said. “Dermatologists can biopsy seven to eight suspected melanomas before discovering a true melanoma, so dermatologist labeling of skin cancers is prone to label noise.”
ISIC’s guidelines stated a gold standard of labeling for dermatologic images is one area that still needs future research, but currently, “diagnoses, labels and diagnostic groups used in data repositories as well as public ontologies” such as ICD-11, AnatomyMapper, and SNOMED-CT should be included in dermatologic image datasets.
AI developers should also provide a detailed description of their algorithm, which includes methods, work flows, mathematical formulas as well as the generalizability of the algorithm across more than one dataset.
Recommendations for technical assessment
“Another important recommendation is that algorithm developers should provide a way for algorithms to be publicly evaluable by researchers,” Dr. Daneshjou said. “Many dermatology AI algorithms do not share either their data or their algorithm. Algorithm sharing is important for assessing reproducibility and robustness.”
Google’s recently announced AI-powered dermatology assistant tool, for example, “has made claims about its accuracy and ability to diagnose skin disease at a dermatologist level, but there is no way for researchers to independently test these claims,” she said. Other options like Model Dermatology, developed by Seung Seog Han, MD, PhD, of the Dermatology Clinic in Seoul, South Korea, and colleagues, offer an application programming interface “that allows researchers to test the algorithm,” Dr. Daneshjou said. “This kind of openness is key for assessing algorithm robustness.”
Developers should also note in their algorithm explanations how performance markers and benchmarks would translate to proposed clinical application. “In this context,” the use case – the context in which the AI application is being used – “should be clearly described – who are the intended users and under what clinical scenario are they using the algorithm,” the authors wrote.
Recommendations for application
The guidelines note that use case for the model should also be described by the AI developers. “Our checklist includes delineating use cases for algorithms and describing what use cases may be within the scope of the algorithm versus which use cases are out of scope,” Dr. Daneshjou said. “For example, an algorithm developed to provide decision support to dermatologists, with a human in the loop, may not be accurate enough to release directly to consumers.”
As the goal of AI algorithms in dermatology is eventual implementation for clinicians and patients, the authors asked developers to consider shortcomings and potential harms of the algorithm during implementation. “Ethical considerations and impact on vulnerable populations should also be considered and discussed,” they wrote. An algorithm “suggesting aesthetic medical treatments may have negative effects given the biased nature of beauty standards,” and “an algorithm that diagnoses basal cell carcinomas but lacks any pigmented basal cell carcinomas, which are more often seen in skin of color, will not perform equitably across populations.”
Prior to implementing an AI algorithm, the ISIC working group recommended developers perform prospective clinical trials for validation. Checklists and guidelines like SPIRIT-AI and CONSORT-AI “provide guidance on how to design clinical trials to test AI algorithms,” Dr. Daneshjou said.
After implementation, “I believe we need additional research in how we monitor algorithms after they are deployed clinically, Dr. Daneshjou said. “Currently there are no [Food and Drug Administration]–approved AI algorithms in dermatology; however, there are several applications that have CE mark in Europe, and there are no mechanisms for postmarket surveillance there.
'Timely' recommendations
Commenting on the ISIC working group guidelines, Justin M. Ko, MD, MBA, director and chief of medical dermatology for Stanford Health Care, who was not involved with the work, said that the recommendations are timely and provide “a framework for a ‘common language’ around AI datasets specifically tailored to dermatology.” Dr. Ko, chair of the American Academy of Dermatology’s Ad Hoc Task Force on Augmented Intelligence, noted the work by Dr. Daneshjou and colleagues “is consistent with and builds further details” on the position statement released by the AAD AI task force in 2019.
“As machine-learning capabilities and commercial efforts continue to mature, it becomes increasingly important that we are able to ‘look under the hood,’ and evaluate all the critical factors that influence development of these capabilities,” he said in an interview. “A standard set of reporting guidelines not only allows for transparency in evaluating data and performance of models and algorithms, but also forces the consideration of issues of equity, fairness, mitigation of bias, and clinically meaningful outcomes.”
One concern is the impact of AI algorithms on societal or health systems, he noted, which is brought up in the guidelines. “The last thing we would want is the development of robust AI systems that exacerbate access challenges, or generate patient anxiety/worry, or drive low-value utilization, or adds to care team burden, or create a technological barrier to care, or increases inequity in dermatologic care,” he said.
In developing AI algorithms for dermatology, a “major practical issue” is how performance on paper will translate to real-world use, Dr. Ko explained, and the ISIC guidelines “provide a critical step in empowering clinicians, practices, and our field to shape the advent of the AI and augmented intelligence tools and systems to promote and enhance meaningful clinical outcomes, and augment the core patient-clinician relationship and ensure they are grounded in principles of fairness, equity and transparency.”
This research was funded by awards and grants to individual authors from the Charina Fund, a Google Research Award, Melanoma Research Alliance, National Health and Medical Research Council, National Institutes of Health/National Cancer Institute, National Science Foundation, and the Department of Veterans Affairs. The authors disclosed relationships with governmental entities, pharmaceutical companies, technology startups, medical publishers, charitable trusts, consulting firms, dermatology training companies, providers of medical devices, manufacturers of dermatologic products, and other organizations related to the paper in the form of supplied equipment, having founded a company; receiving grants, patents, or personal fees; holding shares; and medical reporting. Dr. Ko reported that he serves as a clinical advisor for Skin Analytics, and has an ongoing research collaboration with Google.
The
The guidelines, published in JAMA Dermatology on Dec. 1, 2021, contain a broad range of recommendations stakeholders should consider when developing and assessing image-based AI algorithms in dermatology. The recommendations are divided into categories of data, technique, technical assessment, and application. ISIC is “an academia and industry partnership designed to facilitate the application of digital skin imaging to help reduce melanoma mortality,” and is organized into different working groups, including the AI working group, according to its website.
“Our goal with these guidelines was to create higher-quality reporting of dataset and algorithm characteristics for dermatology AI,” first author Roxana Daneshjou, MD, PhD, clinical scholar in dermatology, in the department of dermatology at Stanford (Calif.) University, said in an interview. “We hope these guidelines also aid regulatory bodies around the world when they are assessing algorithms to be used in dermatology.”
Recommendations for data
The authors recommended that datasets used by AI algorithms have image descriptions and details on image artifacts. “For photography, these include the type of camera used; whether images were taken under standardized or varying conditions; whether they were taken by professional photographers, laymen, or health care professionals; and image quality,” they wrote. They also recommended that developers include in an image description the type of lighting used and whether the photo contains pen markings, hair, tattoos, injuries, surgical effects, or other “physical perturbations.”
Exchangeable image file format data obtained from the camera, and preprocessing procedures like color normalization and “postprocessing” of images, such as filtering, should also be disclosed. In addition, developers should disclose and justify inclusion of images that have been created by an algorithm within a dataset. Any public images used in the datasets should have references, and privately used images should be made public where possible, the authors said.
The ISIC working group guidelines also provided recommendations for patient-level metadata. Each image should include a patient’s geographical location and medical center they visited as well as their age, sex and gender, ethnicity and/or race, and skin tone. Dr. Daneshjou said this was one area where she and her colleagues found a lack of transparency in AI datasets in algorithms in a recent review. “We found that many AI papers provided sparse details about the images used to train and test their algorithms,” Dr. Daneshjou explained. “For example, only 7 out of 70 papers had any information about the skin tones in the images used for developing and/or testing AI algorithms. Understanding the diversity of images used to train and test algorithms is important because algorithms that are developed on images of predominantly white skin likely won’t work as well on Black and brown skin.”
The guideline authors also asked algorithm developers to describe the limitations of not including patient-level metadata information when it is incomplete or unavailable. In addition, “we ask that algorithm developers comment on potential biases of their algorithms,” Dr. Daneshjou said. “For example, an algorithm based only on telemedicine images may not capture the full range of diseases seen within an in-person clinic.”
When describing their AI algorithm, developers should detail their reasoning for the dataset size and partitions, inclusion and exclusion criteria for images, and use of any external samples for test sets. “Authors should consider any differences between the image characteristics used for algorithm development and those that might be encountered in the real world,” the guidelines stated.
Recommendations for technique
How the images in a dataset are labeled is a unique challenge in developing AI algorithms for dermatology, the authors noted. Developers should use histopathological diagnosis in their labeling, but this can sometimes result in label noise.
“Many of the AI algorithms in dermatology use supervised learning, which requires labeled examples to help the algorithm ‘learn’ features for discriminating between lesions. We found that some papers use consensus labeling – dermatologists providing a label – to label skin cancers; however, the standard for diagnosing skin cancer is using histopathology from a biopsy,” she said. “Dermatologists can biopsy seven to eight suspected melanomas before discovering a true melanoma, so dermatologist labeling of skin cancers is prone to label noise.”
ISIC’s guidelines stated a gold standard of labeling for dermatologic images is one area that still needs future research, but currently, “diagnoses, labels and diagnostic groups used in data repositories as well as public ontologies” such as ICD-11, AnatomyMapper, and SNOMED-CT should be included in dermatologic image datasets.
AI developers should also provide a detailed description of their algorithm, which includes methods, work flows, mathematical formulas as well as the generalizability of the algorithm across more than one dataset.
Recommendations for technical assessment
“Another important recommendation is that algorithm developers should provide a way for algorithms to be publicly evaluable by researchers,” Dr. Daneshjou said. “Many dermatology AI algorithms do not share either their data or their algorithm. Algorithm sharing is important for assessing reproducibility and robustness.”
Google’s recently announced AI-powered dermatology assistant tool, for example, “has made claims about its accuracy and ability to diagnose skin disease at a dermatologist level, but there is no way for researchers to independently test these claims,” she said. Other options like Model Dermatology, developed by Seung Seog Han, MD, PhD, of the Dermatology Clinic in Seoul, South Korea, and colleagues, offer an application programming interface “that allows researchers to test the algorithm,” Dr. Daneshjou said. “This kind of openness is key for assessing algorithm robustness.”
Developers should also note in their algorithm explanations how performance markers and benchmarks would translate to proposed clinical application. “In this context,” the use case – the context in which the AI application is being used – “should be clearly described – who are the intended users and under what clinical scenario are they using the algorithm,” the authors wrote.
Recommendations for application
The guidelines note that use case for the model should also be described by the AI developers. “Our checklist includes delineating use cases for algorithms and describing what use cases may be within the scope of the algorithm versus which use cases are out of scope,” Dr. Daneshjou said. “For example, an algorithm developed to provide decision support to dermatologists, with a human in the loop, may not be accurate enough to release directly to consumers.”
As the goal of AI algorithms in dermatology is eventual implementation for clinicians and patients, the authors asked developers to consider shortcomings and potential harms of the algorithm during implementation. “Ethical considerations and impact on vulnerable populations should also be considered and discussed,” they wrote. An algorithm “suggesting aesthetic medical treatments may have negative effects given the biased nature of beauty standards,” and “an algorithm that diagnoses basal cell carcinomas but lacks any pigmented basal cell carcinomas, which are more often seen in skin of color, will not perform equitably across populations.”
Prior to implementing an AI algorithm, the ISIC working group recommended developers perform prospective clinical trials for validation. Checklists and guidelines like SPIRIT-AI and CONSORT-AI “provide guidance on how to design clinical trials to test AI algorithms,” Dr. Daneshjou said.
After implementation, “I believe we need additional research in how we monitor algorithms after they are deployed clinically, Dr. Daneshjou said. “Currently there are no [Food and Drug Administration]–approved AI algorithms in dermatology; however, there are several applications that have CE mark in Europe, and there are no mechanisms for postmarket surveillance there.
'Timely' recommendations
Commenting on the ISIC working group guidelines, Justin M. Ko, MD, MBA, director and chief of medical dermatology for Stanford Health Care, who was not involved with the work, said that the recommendations are timely and provide “a framework for a ‘common language’ around AI datasets specifically tailored to dermatology.” Dr. Ko, chair of the American Academy of Dermatology’s Ad Hoc Task Force on Augmented Intelligence, noted the work by Dr. Daneshjou and colleagues “is consistent with and builds further details” on the position statement released by the AAD AI task force in 2019.
“As machine-learning capabilities and commercial efforts continue to mature, it becomes increasingly important that we are able to ‘look under the hood,’ and evaluate all the critical factors that influence development of these capabilities,” he said in an interview. “A standard set of reporting guidelines not only allows for transparency in evaluating data and performance of models and algorithms, but also forces the consideration of issues of equity, fairness, mitigation of bias, and clinically meaningful outcomes.”
One concern is the impact of AI algorithms on societal or health systems, he noted, which is brought up in the guidelines. “The last thing we would want is the development of robust AI systems that exacerbate access challenges, or generate patient anxiety/worry, or drive low-value utilization, or adds to care team burden, or create a technological barrier to care, or increases inequity in dermatologic care,” he said.
In developing AI algorithms for dermatology, a “major practical issue” is how performance on paper will translate to real-world use, Dr. Ko explained, and the ISIC guidelines “provide a critical step in empowering clinicians, practices, and our field to shape the advent of the AI and augmented intelligence tools and systems to promote and enhance meaningful clinical outcomes, and augment the core patient-clinician relationship and ensure they are grounded in principles of fairness, equity and transparency.”
This research was funded by awards and grants to individual authors from the Charina Fund, a Google Research Award, Melanoma Research Alliance, National Health and Medical Research Council, National Institutes of Health/National Cancer Institute, National Science Foundation, and the Department of Veterans Affairs. The authors disclosed relationships with governmental entities, pharmaceutical companies, technology startups, medical publishers, charitable trusts, consulting firms, dermatology training companies, providers of medical devices, manufacturers of dermatologic products, and other organizations related to the paper in the form of supplied equipment, having founded a company; receiving grants, patents, or personal fees; holding shares; and medical reporting. Dr. Ko reported that he serves as a clinical advisor for Skin Analytics, and has an ongoing research collaboration with Google.
The
The guidelines, published in JAMA Dermatology on Dec. 1, 2021, contain a broad range of recommendations stakeholders should consider when developing and assessing image-based AI algorithms in dermatology. The recommendations are divided into categories of data, technique, technical assessment, and application. ISIC is “an academia and industry partnership designed to facilitate the application of digital skin imaging to help reduce melanoma mortality,” and is organized into different working groups, including the AI working group, according to its website.
“Our goal with these guidelines was to create higher-quality reporting of dataset and algorithm characteristics for dermatology AI,” first author Roxana Daneshjou, MD, PhD, clinical scholar in dermatology, in the department of dermatology at Stanford (Calif.) University, said in an interview. “We hope these guidelines also aid regulatory bodies around the world when they are assessing algorithms to be used in dermatology.”
Recommendations for data
The authors recommended that datasets used by AI algorithms have image descriptions and details on image artifacts. “For photography, these include the type of camera used; whether images were taken under standardized or varying conditions; whether they were taken by professional photographers, laymen, or health care professionals; and image quality,” they wrote. They also recommended that developers include in an image description the type of lighting used and whether the photo contains pen markings, hair, tattoos, injuries, surgical effects, or other “physical perturbations.”
Exchangeable image file format data obtained from the camera, and preprocessing procedures like color normalization and “postprocessing” of images, such as filtering, should also be disclosed. In addition, developers should disclose and justify inclusion of images that have been created by an algorithm within a dataset. Any public images used in the datasets should have references, and privately used images should be made public where possible, the authors said.
The ISIC working group guidelines also provided recommendations for patient-level metadata. Each image should include a patient’s geographical location and medical center they visited as well as their age, sex and gender, ethnicity and/or race, and skin tone. Dr. Daneshjou said this was one area where she and her colleagues found a lack of transparency in AI datasets in algorithms in a recent review. “We found that many AI papers provided sparse details about the images used to train and test their algorithms,” Dr. Daneshjou explained. “For example, only 7 out of 70 papers had any information about the skin tones in the images used for developing and/or testing AI algorithms. Understanding the diversity of images used to train and test algorithms is important because algorithms that are developed on images of predominantly white skin likely won’t work as well on Black and brown skin.”
The guideline authors also asked algorithm developers to describe the limitations of not including patient-level metadata information when it is incomplete or unavailable. In addition, “we ask that algorithm developers comment on potential biases of their algorithms,” Dr. Daneshjou said. “For example, an algorithm based only on telemedicine images may not capture the full range of diseases seen within an in-person clinic.”
When describing their AI algorithm, developers should detail their reasoning for the dataset size and partitions, inclusion and exclusion criteria for images, and use of any external samples for test sets. “Authors should consider any differences between the image characteristics used for algorithm development and those that might be encountered in the real world,” the guidelines stated.
Recommendations for technique
How the images in a dataset are labeled is a unique challenge in developing AI algorithms for dermatology, the authors noted. Developers should use histopathological diagnosis in their labeling, but this can sometimes result in label noise.
“Many of the AI algorithms in dermatology use supervised learning, which requires labeled examples to help the algorithm ‘learn’ features for discriminating between lesions. We found that some papers use consensus labeling – dermatologists providing a label – to label skin cancers; however, the standard for diagnosing skin cancer is using histopathology from a biopsy,” she said. “Dermatologists can biopsy seven to eight suspected melanomas before discovering a true melanoma, so dermatologist labeling of skin cancers is prone to label noise.”
ISIC’s guidelines stated a gold standard of labeling for dermatologic images is one area that still needs future research, but currently, “diagnoses, labels and diagnostic groups used in data repositories as well as public ontologies” such as ICD-11, AnatomyMapper, and SNOMED-CT should be included in dermatologic image datasets.
AI developers should also provide a detailed description of their algorithm, which includes methods, work flows, mathematical formulas as well as the generalizability of the algorithm across more than one dataset.
Recommendations for technical assessment
“Another important recommendation is that algorithm developers should provide a way for algorithms to be publicly evaluable by researchers,” Dr. Daneshjou said. “Many dermatology AI algorithms do not share either their data or their algorithm. Algorithm sharing is important for assessing reproducibility and robustness.”
Google’s recently announced AI-powered dermatology assistant tool, for example, “has made claims about its accuracy and ability to diagnose skin disease at a dermatologist level, but there is no way for researchers to independently test these claims,” she said. Other options like Model Dermatology, developed by Seung Seog Han, MD, PhD, of the Dermatology Clinic in Seoul, South Korea, and colleagues, offer an application programming interface “that allows researchers to test the algorithm,” Dr. Daneshjou said. “This kind of openness is key for assessing algorithm robustness.”
Developers should also note in their algorithm explanations how performance markers and benchmarks would translate to proposed clinical application. “In this context,” the use case – the context in which the AI application is being used – “should be clearly described – who are the intended users and under what clinical scenario are they using the algorithm,” the authors wrote.
Recommendations for application
The guidelines note that use case for the model should also be described by the AI developers. “Our checklist includes delineating use cases for algorithms and describing what use cases may be within the scope of the algorithm versus which use cases are out of scope,” Dr. Daneshjou said. “For example, an algorithm developed to provide decision support to dermatologists, with a human in the loop, may not be accurate enough to release directly to consumers.”
As the goal of AI algorithms in dermatology is eventual implementation for clinicians and patients, the authors asked developers to consider shortcomings and potential harms of the algorithm during implementation. “Ethical considerations and impact on vulnerable populations should also be considered and discussed,” they wrote. An algorithm “suggesting aesthetic medical treatments may have negative effects given the biased nature of beauty standards,” and “an algorithm that diagnoses basal cell carcinomas but lacks any pigmented basal cell carcinomas, which are more often seen in skin of color, will not perform equitably across populations.”
Prior to implementing an AI algorithm, the ISIC working group recommended developers perform prospective clinical trials for validation. Checklists and guidelines like SPIRIT-AI and CONSORT-AI “provide guidance on how to design clinical trials to test AI algorithms,” Dr. Daneshjou said.
After implementation, “I believe we need additional research in how we monitor algorithms after they are deployed clinically, Dr. Daneshjou said. “Currently there are no [Food and Drug Administration]–approved AI algorithms in dermatology; however, there are several applications that have CE mark in Europe, and there are no mechanisms for postmarket surveillance there.
'Timely' recommendations
Commenting on the ISIC working group guidelines, Justin M. Ko, MD, MBA, director and chief of medical dermatology for Stanford Health Care, who was not involved with the work, said that the recommendations are timely and provide “a framework for a ‘common language’ around AI datasets specifically tailored to dermatology.” Dr. Ko, chair of the American Academy of Dermatology’s Ad Hoc Task Force on Augmented Intelligence, noted the work by Dr. Daneshjou and colleagues “is consistent with and builds further details” on the position statement released by the AAD AI task force in 2019.
“As machine-learning capabilities and commercial efforts continue to mature, it becomes increasingly important that we are able to ‘look under the hood,’ and evaluate all the critical factors that influence development of these capabilities,” he said in an interview. “A standard set of reporting guidelines not only allows for transparency in evaluating data and performance of models and algorithms, but also forces the consideration of issues of equity, fairness, mitigation of bias, and clinically meaningful outcomes.”
One concern is the impact of AI algorithms on societal or health systems, he noted, which is brought up in the guidelines. “The last thing we would want is the development of robust AI systems that exacerbate access challenges, or generate patient anxiety/worry, or drive low-value utilization, or adds to care team burden, or create a technological barrier to care, or increases inequity in dermatologic care,” he said.
In developing AI algorithms for dermatology, a “major practical issue” is how performance on paper will translate to real-world use, Dr. Ko explained, and the ISIC guidelines “provide a critical step in empowering clinicians, practices, and our field to shape the advent of the AI and augmented intelligence tools and systems to promote and enhance meaningful clinical outcomes, and augment the core patient-clinician relationship and ensure they are grounded in principles of fairness, equity and transparency.”
This research was funded by awards and grants to individual authors from the Charina Fund, a Google Research Award, Melanoma Research Alliance, National Health and Medical Research Council, National Institutes of Health/National Cancer Institute, National Science Foundation, and the Department of Veterans Affairs. The authors disclosed relationships with governmental entities, pharmaceutical companies, technology startups, medical publishers, charitable trusts, consulting firms, dermatology training companies, providers of medical devices, manufacturers of dermatologic products, and other organizations related to the paper in the form of supplied equipment, having founded a company; receiving grants, patents, or personal fees; holding shares; and medical reporting. Dr. Ko reported that he serves as a clinical advisor for Skin Analytics, and has an ongoing research collaboration with Google.
FROM JAMA DERMATOLOGY
Elevated mortality seen in Merkel cell patients from rural areas
This paradox was discovered in an analysis of data from the Surveillance, Epidemiology, and End Results (SEER) Program that primary author Bryan T. Carroll, MD, PhD, and colleagues presented during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery.
“MCC is a rare and aggressive neoplasm of the skin with high mortality,” said coauthor Emma Larson, MD, a dermatology clinical research fellow at University Hospitals of Cleveland. “Previous studies have demonstrated that MCC survival is lower in low–dermatologist density areas. Associations are difficult to characterize without historical staging data aggregated from large registries. We hypothesized that decreased MCC survival is associated with rural counties.”
The researchers used 18 registries from the November 2019 SEER database to retrospectively evaluate adults who were diagnosed with MCC between 2004 and 2015 as confirmed by positive histology. Study endpoints were SEER historic stage at diagnosis and 5-year survival. MCC cases were stratified by 2013 USDA urban-rural continuum codes, which defines metropolitan counties as those with a population of 1 million or more, urban counties as those with a population of less than 1 million, and rural counties as nonmetropolitan counties not adjacent to a metropolitan area.
A total of 6,291 cases with a mean age of 75 years were included in the final analysis: 3,750 from metro areas, 2,235 from urban areas, and 306 from rural areas. A higher proportion of MCC patients from rural areas were male (69% vs. 62% from metro areas and 64% from urban areas) and white (97% vs. 95% and 96%, respectively). “This may contribute to differences in MCC care,” Dr. Larson said. “However, we also found that there is an increased incidence of locally staged disease in rural areas (51%) than in metro (44%) or urban (45%) areas (P = .02). In addition, fewer lymph node surgeries were performed in rural (50%) and urban (51%) areas than in metro areas (45%; P = .01).”
Overall survival was worse among patients in rural areas (a mean of 34 months), compared with those in urban (a mean of 41 months) and metro areas (a mean of 47 months; P = .02). “This may be due to the fact that rural counties have the higher risk factors for MCC incidence and death, but when we account for the confounders, including sex, age, race, and MCC stage, we still found a difference in overall survival in rural counties, compared to metro and urban counties,” Dr. Larson said.
Dr. Carroll, an associate professor of dermatology at University Hospitals of Cleveland, characterized the finding as “not what you’d expect with a higher incidence of local disease. Therefore, there is the potential for mis-staging in rural counties, where we did see that the interrogation of lymph nodes was done less frequently than in urban centers, which were more aligned with National Comprehensive Cancer Network guidelines during this time period. Still, after correction, rural location is still associated with a higher MCC mortality. There is a need for us to further interrogate what the causes are for this disparity in care between rural and urban centers.”
The other study authors were Dustin DeMeo and Christian Scheufele, MD. The researchers reported having no relevant financial disclosures.
This paradox was discovered in an analysis of data from the Surveillance, Epidemiology, and End Results (SEER) Program that primary author Bryan T. Carroll, MD, PhD, and colleagues presented during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery.
“MCC is a rare and aggressive neoplasm of the skin with high mortality,” said coauthor Emma Larson, MD, a dermatology clinical research fellow at University Hospitals of Cleveland. “Previous studies have demonstrated that MCC survival is lower in low–dermatologist density areas. Associations are difficult to characterize without historical staging data aggregated from large registries. We hypothesized that decreased MCC survival is associated with rural counties.”
The researchers used 18 registries from the November 2019 SEER database to retrospectively evaluate adults who were diagnosed with MCC between 2004 and 2015 as confirmed by positive histology. Study endpoints were SEER historic stage at diagnosis and 5-year survival. MCC cases were stratified by 2013 USDA urban-rural continuum codes, which defines metropolitan counties as those with a population of 1 million or more, urban counties as those with a population of less than 1 million, and rural counties as nonmetropolitan counties not adjacent to a metropolitan area.
A total of 6,291 cases with a mean age of 75 years were included in the final analysis: 3,750 from metro areas, 2,235 from urban areas, and 306 from rural areas. A higher proportion of MCC patients from rural areas were male (69% vs. 62% from metro areas and 64% from urban areas) and white (97% vs. 95% and 96%, respectively). “This may contribute to differences in MCC care,” Dr. Larson said. “However, we also found that there is an increased incidence of locally staged disease in rural areas (51%) than in metro (44%) or urban (45%) areas (P = .02). In addition, fewer lymph node surgeries were performed in rural (50%) and urban (51%) areas than in metro areas (45%; P = .01).”
Overall survival was worse among patients in rural areas (a mean of 34 months), compared with those in urban (a mean of 41 months) and metro areas (a mean of 47 months; P = .02). “This may be due to the fact that rural counties have the higher risk factors for MCC incidence and death, but when we account for the confounders, including sex, age, race, and MCC stage, we still found a difference in overall survival in rural counties, compared to metro and urban counties,” Dr. Larson said.
Dr. Carroll, an associate professor of dermatology at University Hospitals of Cleveland, characterized the finding as “not what you’d expect with a higher incidence of local disease. Therefore, there is the potential for mis-staging in rural counties, where we did see that the interrogation of lymph nodes was done less frequently than in urban centers, which were more aligned with National Comprehensive Cancer Network guidelines during this time period. Still, after correction, rural location is still associated with a higher MCC mortality. There is a need for us to further interrogate what the causes are for this disparity in care between rural and urban centers.”
The other study authors were Dustin DeMeo and Christian Scheufele, MD. The researchers reported having no relevant financial disclosures.
This paradox was discovered in an analysis of data from the Surveillance, Epidemiology, and End Results (SEER) Program that primary author Bryan T. Carroll, MD, PhD, and colleagues presented during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery.
“MCC is a rare and aggressive neoplasm of the skin with high mortality,” said coauthor Emma Larson, MD, a dermatology clinical research fellow at University Hospitals of Cleveland. “Previous studies have demonstrated that MCC survival is lower in low–dermatologist density areas. Associations are difficult to characterize without historical staging data aggregated from large registries. We hypothesized that decreased MCC survival is associated with rural counties.”
The researchers used 18 registries from the November 2019 SEER database to retrospectively evaluate adults who were diagnosed with MCC between 2004 and 2015 as confirmed by positive histology. Study endpoints were SEER historic stage at diagnosis and 5-year survival. MCC cases were stratified by 2013 USDA urban-rural continuum codes, which defines metropolitan counties as those with a population of 1 million or more, urban counties as those with a population of less than 1 million, and rural counties as nonmetropolitan counties not adjacent to a metropolitan area.
A total of 6,291 cases with a mean age of 75 years were included in the final analysis: 3,750 from metro areas, 2,235 from urban areas, and 306 from rural areas. A higher proportion of MCC patients from rural areas were male (69% vs. 62% from metro areas and 64% from urban areas) and white (97% vs. 95% and 96%, respectively). “This may contribute to differences in MCC care,” Dr. Larson said. “However, we also found that there is an increased incidence of locally staged disease in rural areas (51%) than in metro (44%) or urban (45%) areas (P = .02). In addition, fewer lymph node surgeries were performed in rural (50%) and urban (51%) areas than in metro areas (45%; P = .01).”
Overall survival was worse among patients in rural areas (a mean of 34 months), compared with those in urban (a mean of 41 months) and metro areas (a mean of 47 months; P = .02). “This may be due to the fact that rural counties have the higher risk factors for MCC incidence and death, but when we account for the confounders, including sex, age, race, and MCC stage, we still found a difference in overall survival in rural counties, compared to metro and urban counties,” Dr. Larson said.
Dr. Carroll, an associate professor of dermatology at University Hospitals of Cleveland, characterized the finding as “not what you’d expect with a higher incidence of local disease. Therefore, there is the potential for mis-staging in rural counties, where we did see that the interrogation of lymph nodes was done less frequently than in urban centers, which were more aligned with National Comprehensive Cancer Network guidelines during this time period. Still, after correction, rural location is still associated with a higher MCC mortality. There is a need for us to further interrogate what the causes are for this disparity in care between rural and urban centers.”
The other study authors were Dustin DeMeo and Christian Scheufele, MD. The researchers reported having no relevant financial disclosures.
FROM ASDS 2021
Multiple Lesions With Recurrent Bleeding
The Diagnosis: Nevoid Basal Cell Carcinoma Syndrome
Nevoid basal cell carcinoma syndrome (NBCCS), also known as Gorlin syndrome, is a rare autosomal-dominant disorder that increases the risk for developing various carcinomas and affects multiple organ systems. Nevoid basal cell carcinoma syndrome is estimated at 1 per 40,000 to 60,000 individuals with no sexual predilection.1,2 Pathogenesis of NBCCS occurs through molecular alterations in the dormant hedgehog signaling pathway, causing constitutive signaling activity and a loss of function in the tumor suppressor patched 1 gene, PTCH1. As a result, the inhibition of smoothened oncogenes is released, Gli proteins are activated, and the hedgehog signaling pathway is no longer quiescent.2 Additional loss of function in the suppressor of fused homolog protein, a negative regulator of the hedgehog pathway, allows for further tumor proliferation. The crucial role these genes play in the hedgehog signaling pathway and their mutation association with NBCCS allows for molecular confirmation in the diagnosis of NBCCS. Allelic losses at the PTCH1 gene site are thought to occur in approximately 70% of NBCCS patients.2
Diagnosis of NBCCS is based on genetic testing to examine pathogenic gene variants, notably in the PTCH1 gene, and identification of characteristic clinical findings.2 Diagnosis of NBCCS requires either 2 minor suggestive criteria and 1 major suggestive criterion, 2 major suggestive criteria and 1 minor suggestive criterion, or 1 major suggestive criterion with molecular confirmation. The presence of basal cell carcinomas (BCCs) before 20 years of age or an excessive numbers of BCCs, keratocystic odontogenic tumors (KOTs), palmar or plantar pitting, and first-degree relatives with NBCCS are classified as major suggestive criteria.2 Nevoid basal cell carcinoma syndrome patients typically have BCCs that crust, ulcerate, or bleed. Minor suggestive criteria for NBCCS are rib abnormalities, skeletal malformations, macrocephaly, cleft lip or palate, and desmoplastic medulloblastoma.2-4 Suppressor of fused homolog protein mutations may increase the risk for desmoplastic medulloblastoma in NBCCS patients. Our patient had 4 of the major suggestive criteria, including a history of KOTs, multiple BCCs, first-degree relatives with NBCCS, and palmar or plantar pitting (bottom quiz image), while having 1 minor suggestive criterion of frontal bossing.
Patients with NBCCS have high phenotypic variability, as their skin carcinomas do not have the classic features of pearly surfaces or corkscrew telangiectasia that typically are associated with BCCs.1 Basal cell carcinomas in NBCCS-affected individuals usually are indistinguishable from sporadic lesions that arise in sun-exposed areas, making NBCCS difficult to diagnose. These sporadic lesions often are misdiagnosed as psoriatic or eczematous lesions, and additional subsequent examination is required. The findings of multiple papules and plaques spanning the body as well as lesions with rolled borders and ulcerated bases, indicative of BCCs, aid dermatologists in distinguishing benign lesions from those of NBCCS.1
Additional differential diagnoses are required to distinguish NBCCS from other similar inherited skin disorders that are characterized by BCCs. The presence of multiple incidental BCCs early in life remains a histopathologic clue for NBCCS diagnosis, as opposed to Rombo syndrome, in which BCCs often develop in adulthood.2,4 In addition, although both Bazex syndrome and Muir-Torre syndrome are characterized by the early onset of BCCs, the lack of skeletal abnormalities and palmar and plantar pitting distinguish these entities from NBCCS.2,4 Furthermore, though psoriasis also can present on the scalp, clinical presentation often includes well-demarcated and symmetric plaques that are erythematous and silvery, all of which were not present in our patient and typically are not seen in NBCCS.5
The recommended treatment of NBCCS is vismodegib, a specific oncogene inhibitor. This medication suppresses the hedgehog signaling pathway by inhibiting smoothened oncogenes and downstream target molecules, thereby decreasing tumor proliferation.6 In doing so, vismodegib inhibits the development of new BCCs while reducing the burden of present ones. Additionally, vismodegib appears to effectively treat KOTs. If successful, this medication may be able to suppress KOTs in patients with NBCCS and thus facilitate surgery.6 Additional hedgehog inhibitors include patidegib, sonidegib, and itraconazole. Patidegib gel 2% currently is in phase 3 clinical trials for evaluation of efficacy and safety in treatment of NBCCS. Sonidegib is approved for the treatment of locally advanced BCCs in the United States and the European Union and for both locally advanced BCCs and metastatic BCCs in Switzerland and Australia.7 Further research is needed before recommending antifungal itraconazole for NBCCS clinical use.8 Other medications for localized areas include topical application of 5-fluorouracil and imiquimod.2
- Sangehra R, Grewal P. Gorlin syndrome presentation and the importance of differential diagnosis of skin cancer: a case report. J Pharm Pharm Sci. 2018;21:222-224.
- Bresler S, Padwa B, Granter S. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Head Neck Pathol. 2016;10:119-124.
- Fujii K, Miyashita T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): update and literature review. Pediatr Int. 2014;56:667-674.
- Evans G, Farndon P. Nevoid basal cell carcinoma syndrome. GeneReviews [Internet]. University of Washington; 1993-2020.
- Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63:278-285.
- Booms P, Harth M, Sader R, et al. Vismodegib hedgehog-signaling inhibition and treatment of basal cell carcinomas as well as keratocystic odontogenic tumors in Gorlin syndrome. Ann Maxillofac Surg. 2015;5:14-19.
- Gutzmer R, Soloon J. Hedgehog pathway inhibition for the treatment of basal cell carcinoma. Target Oncol. 2019;14:253-267.
- Leavitt E, Lask G, Martin S. Sonic hedgehog pathway inhibition in the treatment of advanced basal cell carcinoma. Curr Treat Options Oncol. 2019;20:84.
The Diagnosis: Nevoid Basal Cell Carcinoma Syndrome
Nevoid basal cell carcinoma syndrome (NBCCS), also known as Gorlin syndrome, is a rare autosomal-dominant disorder that increases the risk for developing various carcinomas and affects multiple organ systems. Nevoid basal cell carcinoma syndrome is estimated at 1 per 40,000 to 60,000 individuals with no sexual predilection.1,2 Pathogenesis of NBCCS occurs through molecular alterations in the dormant hedgehog signaling pathway, causing constitutive signaling activity and a loss of function in the tumor suppressor patched 1 gene, PTCH1. As a result, the inhibition of smoothened oncogenes is released, Gli proteins are activated, and the hedgehog signaling pathway is no longer quiescent.2 Additional loss of function in the suppressor of fused homolog protein, a negative regulator of the hedgehog pathway, allows for further tumor proliferation. The crucial role these genes play in the hedgehog signaling pathway and their mutation association with NBCCS allows for molecular confirmation in the diagnosis of NBCCS. Allelic losses at the PTCH1 gene site are thought to occur in approximately 70% of NBCCS patients.2
Diagnosis of NBCCS is based on genetic testing to examine pathogenic gene variants, notably in the PTCH1 gene, and identification of characteristic clinical findings.2 Diagnosis of NBCCS requires either 2 minor suggestive criteria and 1 major suggestive criterion, 2 major suggestive criteria and 1 minor suggestive criterion, or 1 major suggestive criterion with molecular confirmation. The presence of basal cell carcinomas (BCCs) before 20 years of age or an excessive numbers of BCCs, keratocystic odontogenic tumors (KOTs), palmar or plantar pitting, and first-degree relatives with NBCCS are classified as major suggestive criteria.2 Nevoid basal cell carcinoma syndrome patients typically have BCCs that crust, ulcerate, or bleed. Minor suggestive criteria for NBCCS are rib abnormalities, skeletal malformations, macrocephaly, cleft lip or palate, and desmoplastic medulloblastoma.2-4 Suppressor of fused homolog protein mutations may increase the risk for desmoplastic medulloblastoma in NBCCS patients. Our patient had 4 of the major suggestive criteria, including a history of KOTs, multiple BCCs, first-degree relatives with NBCCS, and palmar or plantar pitting (bottom quiz image), while having 1 minor suggestive criterion of frontal bossing.
Patients with NBCCS have high phenotypic variability, as their skin carcinomas do not have the classic features of pearly surfaces or corkscrew telangiectasia that typically are associated with BCCs.1 Basal cell carcinomas in NBCCS-affected individuals usually are indistinguishable from sporadic lesions that arise in sun-exposed areas, making NBCCS difficult to diagnose. These sporadic lesions often are misdiagnosed as psoriatic or eczematous lesions, and additional subsequent examination is required. The findings of multiple papules and plaques spanning the body as well as lesions with rolled borders and ulcerated bases, indicative of BCCs, aid dermatologists in distinguishing benign lesions from those of NBCCS.1
Additional differential diagnoses are required to distinguish NBCCS from other similar inherited skin disorders that are characterized by BCCs. The presence of multiple incidental BCCs early in life remains a histopathologic clue for NBCCS diagnosis, as opposed to Rombo syndrome, in which BCCs often develop in adulthood.2,4 In addition, although both Bazex syndrome and Muir-Torre syndrome are characterized by the early onset of BCCs, the lack of skeletal abnormalities and palmar and plantar pitting distinguish these entities from NBCCS.2,4 Furthermore, though psoriasis also can present on the scalp, clinical presentation often includes well-demarcated and symmetric plaques that are erythematous and silvery, all of which were not present in our patient and typically are not seen in NBCCS.5
The recommended treatment of NBCCS is vismodegib, a specific oncogene inhibitor. This medication suppresses the hedgehog signaling pathway by inhibiting smoothened oncogenes and downstream target molecules, thereby decreasing tumor proliferation.6 In doing so, vismodegib inhibits the development of new BCCs while reducing the burden of present ones. Additionally, vismodegib appears to effectively treat KOTs. If successful, this medication may be able to suppress KOTs in patients with NBCCS and thus facilitate surgery.6 Additional hedgehog inhibitors include patidegib, sonidegib, and itraconazole. Patidegib gel 2% currently is in phase 3 clinical trials for evaluation of efficacy and safety in treatment of NBCCS. Sonidegib is approved for the treatment of locally advanced BCCs in the United States and the European Union and for both locally advanced BCCs and metastatic BCCs in Switzerland and Australia.7 Further research is needed before recommending antifungal itraconazole for NBCCS clinical use.8 Other medications for localized areas include topical application of 5-fluorouracil and imiquimod.2
The Diagnosis: Nevoid Basal Cell Carcinoma Syndrome
Nevoid basal cell carcinoma syndrome (NBCCS), also known as Gorlin syndrome, is a rare autosomal-dominant disorder that increases the risk for developing various carcinomas and affects multiple organ systems. Nevoid basal cell carcinoma syndrome is estimated at 1 per 40,000 to 60,000 individuals with no sexual predilection.1,2 Pathogenesis of NBCCS occurs through molecular alterations in the dormant hedgehog signaling pathway, causing constitutive signaling activity and a loss of function in the tumor suppressor patched 1 gene, PTCH1. As a result, the inhibition of smoothened oncogenes is released, Gli proteins are activated, and the hedgehog signaling pathway is no longer quiescent.2 Additional loss of function in the suppressor of fused homolog protein, a negative regulator of the hedgehog pathway, allows for further tumor proliferation. The crucial role these genes play in the hedgehog signaling pathway and their mutation association with NBCCS allows for molecular confirmation in the diagnosis of NBCCS. Allelic losses at the PTCH1 gene site are thought to occur in approximately 70% of NBCCS patients.2
Diagnosis of NBCCS is based on genetic testing to examine pathogenic gene variants, notably in the PTCH1 gene, and identification of characteristic clinical findings.2 Diagnosis of NBCCS requires either 2 minor suggestive criteria and 1 major suggestive criterion, 2 major suggestive criteria and 1 minor suggestive criterion, or 1 major suggestive criterion with molecular confirmation. The presence of basal cell carcinomas (BCCs) before 20 years of age or an excessive numbers of BCCs, keratocystic odontogenic tumors (KOTs), palmar or plantar pitting, and first-degree relatives with NBCCS are classified as major suggestive criteria.2 Nevoid basal cell carcinoma syndrome patients typically have BCCs that crust, ulcerate, or bleed. Minor suggestive criteria for NBCCS are rib abnormalities, skeletal malformations, macrocephaly, cleft lip or palate, and desmoplastic medulloblastoma.2-4 Suppressor of fused homolog protein mutations may increase the risk for desmoplastic medulloblastoma in NBCCS patients. Our patient had 4 of the major suggestive criteria, including a history of KOTs, multiple BCCs, first-degree relatives with NBCCS, and palmar or plantar pitting (bottom quiz image), while having 1 minor suggestive criterion of frontal bossing.
Patients with NBCCS have high phenotypic variability, as their skin carcinomas do not have the classic features of pearly surfaces or corkscrew telangiectasia that typically are associated with BCCs.1 Basal cell carcinomas in NBCCS-affected individuals usually are indistinguishable from sporadic lesions that arise in sun-exposed areas, making NBCCS difficult to diagnose. These sporadic lesions often are misdiagnosed as psoriatic or eczematous lesions, and additional subsequent examination is required. The findings of multiple papules and plaques spanning the body as well as lesions with rolled borders and ulcerated bases, indicative of BCCs, aid dermatologists in distinguishing benign lesions from those of NBCCS.1
Additional differential diagnoses are required to distinguish NBCCS from other similar inherited skin disorders that are characterized by BCCs. The presence of multiple incidental BCCs early in life remains a histopathologic clue for NBCCS diagnosis, as opposed to Rombo syndrome, in which BCCs often develop in adulthood.2,4 In addition, although both Bazex syndrome and Muir-Torre syndrome are characterized by the early onset of BCCs, the lack of skeletal abnormalities and palmar and plantar pitting distinguish these entities from NBCCS.2,4 Furthermore, though psoriasis also can present on the scalp, clinical presentation often includes well-demarcated and symmetric plaques that are erythematous and silvery, all of which were not present in our patient and typically are not seen in NBCCS.5
The recommended treatment of NBCCS is vismodegib, a specific oncogene inhibitor. This medication suppresses the hedgehog signaling pathway by inhibiting smoothened oncogenes and downstream target molecules, thereby decreasing tumor proliferation.6 In doing so, vismodegib inhibits the development of new BCCs while reducing the burden of present ones. Additionally, vismodegib appears to effectively treat KOTs. If successful, this medication may be able to suppress KOTs in patients with NBCCS and thus facilitate surgery.6 Additional hedgehog inhibitors include patidegib, sonidegib, and itraconazole. Patidegib gel 2% currently is in phase 3 clinical trials for evaluation of efficacy and safety in treatment of NBCCS. Sonidegib is approved for the treatment of locally advanced BCCs in the United States and the European Union and for both locally advanced BCCs and metastatic BCCs in Switzerland and Australia.7 Further research is needed before recommending antifungal itraconazole for NBCCS clinical use.8 Other medications for localized areas include topical application of 5-fluorouracil and imiquimod.2
- Sangehra R, Grewal P. Gorlin syndrome presentation and the importance of differential diagnosis of skin cancer: a case report. J Pharm Pharm Sci. 2018;21:222-224.
- Bresler S, Padwa B, Granter S. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Head Neck Pathol. 2016;10:119-124.
- Fujii K, Miyashita T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): update and literature review. Pediatr Int. 2014;56:667-674.
- Evans G, Farndon P. Nevoid basal cell carcinoma syndrome. GeneReviews [Internet]. University of Washington; 1993-2020.
- Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63:278-285.
- Booms P, Harth M, Sader R, et al. Vismodegib hedgehog-signaling inhibition and treatment of basal cell carcinomas as well as keratocystic odontogenic tumors in Gorlin syndrome. Ann Maxillofac Surg. 2015;5:14-19.
- Gutzmer R, Soloon J. Hedgehog pathway inhibition for the treatment of basal cell carcinoma. Target Oncol. 2019;14:253-267.
- Leavitt E, Lask G, Martin S. Sonic hedgehog pathway inhibition in the treatment of advanced basal cell carcinoma. Curr Treat Options Oncol. 2019;20:84.
- Sangehra R, Grewal P. Gorlin syndrome presentation and the importance of differential diagnosis of skin cancer: a case report. J Pharm Pharm Sci. 2018;21:222-224.
- Bresler S, Padwa B, Granter S. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Head Neck Pathol. 2016;10:119-124.
- Fujii K, Miyashita T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): update and literature review. Pediatr Int. 2014;56:667-674.
- Evans G, Farndon P. Nevoid basal cell carcinoma syndrome. GeneReviews [Internet]. University of Washington; 1993-2020.
- Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63:278-285.
- Booms P, Harth M, Sader R, et al. Vismodegib hedgehog-signaling inhibition and treatment of basal cell carcinomas as well as keratocystic odontogenic tumors in Gorlin syndrome. Ann Maxillofac Surg. 2015;5:14-19.
- Gutzmer R, Soloon J. Hedgehog pathway inhibition for the treatment of basal cell carcinoma. Target Oncol. 2019;14:253-267.
- Leavitt E, Lask G, Martin S. Sonic hedgehog pathway inhibition in the treatment of advanced basal cell carcinoma. Curr Treat Options Oncol. 2019;20:84.

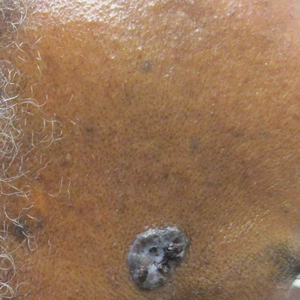
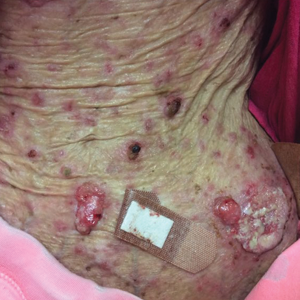
A 63-year-old man with frontal bossing and a history of jaw cysts presented with numerous lesions on the scalp, trunk, and legs with recurrent bleeding. Both of his siblings had similar findings. Many lesions had been present for at least 40 years. Physical examination revealed a large, irregular, atrophic, hyperpigmented plaque on the central scalp with multiple translucent hyperpigmented papules at the periphery (top). Similar papules and plaques were present at the temples, around the waist, and on the distal lower extremities, leading to surgical excision of the largest leg lesions. In addition, there were many pinpoint pits on both palms (bottom). A biopsy was submitted for review, which confirmed basal cell carcinomas on the scalp.
AI: Skin of color underrepresented in datasets used to identify skin cancer
An in the databases, researchers in the United Kingdom report.
Out of 106,950 skin lesions documented in 21 open-access databases and 17 open-access atlases identified by David Wen, BMBCh, from the University of Oxford (England), and colleagues, 2,436 images contained information on Fitzpatrick skin type. Of these, “only 10 images were from individuals with Fitzpatrick skin type V, and only a single image was from an individual with Fitzpatrick skin type VI,” the researchers said. “The ethnicity of these individuals was either Brazilian or unknown.”
In two datasets containing 1,585 images with ethnicity data, “no images were from individuals with an African, Afro-Caribbean, or South Asian background,” Dr. Wen and colleagues noted. “Coupled with the geographical origins of datasets, there was massive under-representation of skin lesion images from darker-skinned populations.”
The results of their systematic review were presented at the National Cancer Research Institute Festival and published on Nov. 9, 2021, in The Lancet Digital Health. To the best of their knowledge, they wrote, this is “the first systematic review of publicly available skin lesion images comprising predominantly dermoscopic and macroscopic images available through open access datasets and atlases.”
Overall, 11 of 14 datasets (79%) were from North America, Europe, or Oceania among datasets with information on country of origin, the researchers said. Either dermoscopic images or macroscopic photographs were the only types of images available in 19 of 21 (91%) datasets. There was some variation in the clinical information available, with 81,662 images (76.4%) containing information on age, 82,848 images (77.5%) having information on gender, and 79,561 images having information about body site (74.4%).
The researchers explained that these datasets might be of limited use in a real-world setting where the images aren’t representative of the population. Artificial intelligence (AI) programs that train using images of patients with one skin type, for example, can potentially misdiagnose patients of another skin type, they said.
“AI programs hold a lot of potential for diagnosing skin cancer because it can look at pictures and quickly and cost-effectively evaluate any worrying spots on the skin,” Dr. Wen said in a press release from the NCRI Festival. “However, it’s important to know about the images and patients used to develop programs, as these influence which groups of people the programs will be most effective for in real-life settings. Research has shown that programs trained on images taken from people with lighter skin types only might not be as accurate for people with darker skin, and vice versa.”
There was also “limited information on who, how and why the images were taken,” Dr. Wen said in the release. “This has implications for the programs developed from these images, due to uncertainty around how they may perform in different groups of people, especially in those who aren’t well represented in datasets, such as those with darker skin. This can potentially lead to the exclusion or even harm of these groups from AI technologies.”
While there are no current guidelines for developing skin image datasets, quality standards are needed, according to the researchers.
“Ensuring equitable digital health includes building unbiased, representative datasets to ensure that the algorithms that are created benefit people of all backgrounds and skin types,” they concluded in the study.
Neil Steven, MBBS, MA, PhD, FRCP, an NCRI Skin Group member who was not involved with the research, stated in the press release that the results from the study by Dr. Wen and colleagues “raise concerns about the ability of AI to assist in skin cancer diagnosis, especially in a global context.”
“I hope this work will continue and help ensure that the progress we make in using AI in medicine will benefit all patients, recognizing that human skin color is highly diverse,” said Dr. Steven, honorary consultant in medical oncology at University Hospitals Birmingham (England) NHS Foundation Trust.
‘We need more images of everybody’
Dermatologist Adewole Adamson, MD, MPP, assistant professor in the department of internal medicine (division of dermatology) at the University of Texas at Austin, said in an interview that a “major potential downside” of algorithms not trained on diverse datasets is the potential for incorrect diagnoses.
“The harms of algorithms used for diagnostic purposes in the skin can be particularly significant because of the scalability of this technology. A lot of thought needs to be put into how these algorithms are developed and tested,” said Dr. Adamson, who reviewed the manuscript of The Lancet Digital Health study but was not involved with the research.
He referred to the results of a recently published study in JAMA Dermatology, which found that only 10% of studies used to develop or test deep-learning algorithms contained metadata on skin tone. “Furthermore, most datasets are from countries where darker skin types are not represented. [These] algorithms therefore likely underperform on people of darker skin types and thus, users should be wary,” Dr. Adamson said.
A consensus guideline should be developed for public AI algorithms, he said, which should have metadata containing information on sex, race/ethnicity, geographic location, skin type, and part of the body. “This distribution should also be reported in any publication of an algorithm so that users can see if the distribution of the population in the training data mirrors that of the population in which it is intended to be used,” he added.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was not involved with the research, said that, while this issue of underrepresentation has been known in dermatology for some time, the strength of the Lancet study is that it is a large study, with a message of “we need more images of everybody.”
“This is probably the broadest study looking at every possible accessible resource and taking an organized approach,” Dr. Friedman said in an interview. “But I think it also raises some important points about how we think about skin tones and how we refer to them as well with respect to misusing classification schemes that we currently have.”
While using ethnicity data and certain Fitzpatrick skin types as a proxy for darker skin is a limitation of the metadata the study authors had available, it also highlights “a broader problem with respect to lexicon regarding skin tone,” he explained.
“Skin does not have a race, it doesn’t have an ethnicity,” Dr. Friedman said.
A dataset that contains not only different skin tones but how different dermatologic conditions look across skin tones is important. “If you just look at one photo of one skin tone, you missed the fact that clinical presentations can be so polymorphic, especially because of different skin tones,” Dr. Friedman said.
“We need to keep pushing this message to ensure that images keep getting collected. We [need to] ensure that there’s quality control with these images and that we’re disseminating them in a way that everyone has access, both from self-learning, but also to teach others,” said Dr. Friedman, coeditor of a recently introduced dermatology atlas showing skin conditions in different skin tones.
Adamson reports no relevant financial relationships. Dr. Friedman is a coeditor of a dermatology atlas supported by Allergan Aesthetics and SkinBetter Science. This study was funded by NHSX and the Health Foundation. Three authors reported being paid employees of Databiology at the time of the study. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An in the databases, researchers in the United Kingdom report.
Out of 106,950 skin lesions documented in 21 open-access databases and 17 open-access atlases identified by David Wen, BMBCh, from the University of Oxford (England), and colleagues, 2,436 images contained information on Fitzpatrick skin type. Of these, “only 10 images were from individuals with Fitzpatrick skin type V, and only a single image was from an individual with Fitzpatrick skin type VI,” the researchers said. “The ethnicity of these individuals was either Brazilian or unknown.”
In two datasets containing 1,585 images with ethnicity data, “no images were from individuals with an African, Afro-Caribbean, or South Asian background,” Dr. Wen and colleagues noted. “Coupled with the geographical origins of datasets, there was massive under-representation of skin lesion images from darker-skinned populations.”
The results of their systematic review were presented at the National Cancer Research Institute Festival and published on Nov. 9, 2021, in The Lancet Digital Health. To the best of their knowledge, they wrote, this is “the first systematic review of publicly available skin lesion images comprising predominantly dermoscopic and macroscopic images available through open access datasets and atlases.”
Overall, 11 of 14 datasets (79%) were from North America, Europe, or Oceania among datasets with information on country of origin, the researchers said. Either dermoscopic images or macroscopic photographs were the only types of images available in 19 of 21 (91%) datasets. There was some variation in the clinical information available, with 81,662 images (76.4%) containing information on age, 82,848 images (77.5%) having information on gender, and 79,561 images having information about body site (74.4%).
The researchers explained that these datasets might be of limited use in a real-world setting where the images aren’t representative of the population. Artificial intelligence (AI) programs that train using images of patients with one skin type, for example, can potentially misdiagnose patients of another skin type, they said.
“AI programs hold a lot of potential for diagnosing skin cancer because it can look at pictures and quickly and cost-effectively evaluate any worrying spots on the skin,” Dr. Wen said in a press release from the NCRI Festival. “However, it’s important to know about the images and patients used to develop programs, as these influence which groups of people the programs will be most effective for in real-life settings. Research has shown that programs trained on images taken from people with lighter skin types only might not be as accurate for people with darker skin, and vice versa.”
There was also “limited information on who, how and why the images were taken,” Dr. Wen said in the release. “This has implications for the programs developed from these images, due to uncertainty around how they may perform in different groups of people, especially in those who aren’t well represented in datasets, such as those with darker skin. This can potentially lead to the exclusion or even harm of these groups from AI technologies.”
While there are no current guidelines for developing skin image datasets, quality standards are needed, according to the researchers.
“Ensuring equitable digital health includes building unbiased, representative datasets to ensure that the algorithms that are created benefit people of all backgrounds and skin types,” they concluded in the study.
Neil Steven, MBBS, MA, PhD, FRCP, an NCRI Skin Group member who was not involved with the research, stated in the press release that the results from the study by Dr. Wen and colleagues “raise concerns about the ability of AI to assist in skin cancer diagnosis, especially in a global context.”
“I hope this work will continue and help ensure that the progress we make in using AI in medicine will benefit all patients, recognizing that human skin color is highly diverse,” said Dr. Steven, honorary consultant in medical oncology at University Hospitals Birmingham (England) NHS Foundation Trust.
‘We need more images of everybody’
Dermatologist Adewole Adamson, MD, MPP, assistant professor in the department of internal medicine (division of dermatology) at the University of Texas at Austin, said in an interview that a “major potential downside” of algorithms not trained on diverse datasets is the potential for incorrect diagnoses.
“The harms of algorithms used for diagnostic purposes in the skin can be particularly significant because of the scalability of this technology. A lot of thought needs to be put into how these algorithms are developed and tested,” said Dr. Adamson, who reviewed the manuscript of The Lancet Digital Health study but was not involved with the research.
He referred to the results of a recently published study in JAMA Dermatology, which found that only 10% of studies used to develop or test deep-learning algorithms contained metadata on skin tone. “Furthermore, most datasets are from countries where darker skin types are not represented. [These] algorithms therefore likely underperform on people of darker skin types and thus, users should be wary,” Dr. Adamson said.
A consensus guideline should be developed for public AI algorithms, he said, which should have metadata containing information on sex, race/ethnicity, geographic location, skin type, and part of the body. “This distribution should also be reported in any publication of an algorithm so that users can see if the distribution of the population in the training data mirrors that of the population in which it is intended to be used,” he added.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was not involved with the research, said that, while this issue of underrepresentation has been known in dermatology for some time, the strength of the Lancet study is that it is a large study, with a message of “we need more images of everybody.”
“This is probably the broadest study looking at every possible accessible resource and taking an organized approach,” Dr. Friedman said in an interview. “But I think it also raises some important points about how we think about skin tones and how we refer to them as well with respect to misusing classification schemes that we currently have.”
While using ethnicity data and certain Fitzpatrick skin types as a proxy for darker skin is a limitation of the metadata the study authors had available, it also highlights “a broader problem with respect to lexicon regarding skin tone,” he explained.
“Skin does not have a race, it doesn’t have an ethnicity,” Dr. Friedman said.
A dataset that contains not only different skin tones but how different dermatologic conditions look across skin tones is important. “If you just look at one photo of one skin tone, you missed the fact that clinical presentations can be so polymorphic, especially because of different skin tones,” Dr. Friedman said.
“We need to keep pushing this message to ensure that images keep getting collected. We [need to] ensure that there’s quality control with these images and that we’re disseminating them in a way that everyone has access, both from self-learning, but also to teach others,” said Dr. Friedman, coeditor of a recently introduced dermatology atlas showing skin conditions in different skin tones.
Adamson reports no relevant financial relationships. Dr. Friedman is a coeditor of a dermatology atlas supported by Allergan Aesthetics and SkinBetter Science. This study was funded by NHSX and the Health Foundation. Three authors reported being paid employees of Databiology at the time of the study. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An in the databases, researchers in the United Kingdom report.
Out of 106,950 skin lesions documented in 21 open-access databases and 17 open-access atlases identified by David Wen, BMBCh, from the University of Oxford (England), and colleagues, 2,436 images contained information on Fitzpatrick skin type. Of these, “only 10 images were from individuals with Fitzpatrick skin type V, and only a single image was from an individual with Fitzpatrick skin type VI,” the researchers said. “The ethnicity of these individuals was either Brazilian or unknown.”
In two datasets containing 1,585 images with ethnicity data, “no images were from individuals with an African, Afro-Caribbean, or South Asian background,” Dr. Wen and colleagues noted. “Coupled with the geographical origins of datasets, there was massive under-representation of skin lesion images from darker-skinned populations.”
The results of their systematic review were presented at the National Cancer Research Institute Festival and published on Nov. 9, 2021, in The Lancet Digital Health. To the best of their knowledge, they wrote, this is “the first systematic review of publicly available skin lesion images comprising predominantly dermoscopic and macroscopic images available through open access datasets and atlases.”
Overall, 11 of 14 datasets (79%) were from North America, Europe, or Oceania among datasets with information on country of origin, the researchers said. Either dermoscopic images or macroscopic photographs were the only types of images available in 19 of 21 (91%) datasets. There was some variation in the clinical information available, with 81,662 images (76.4%) containing information on age, 82,848 images (77.5%) having information on gender, and 79,561 images having information about body site (74.4%).
The researchers explained that these datasets might be of limited use in a real-world setting where the images aren’t representative of the population. Artificial intelligence (AI) programs that train using images of patients with one skin type, for example, can potentially misdiagnose patients of another skin type, they said.
“AI programs hold a lot of potential for diagnosing skin cancer because it can look at pictures and quickly and cost-effectively evaluate any worrying spots on the skin,” Dr. Wen said in a press release from the NCRI Festival. “However, it’s important to know about the images and patients used to develop programs, as these influence which groups of people the programs will be most effective for in real-life settings. Research has shown that programs trained on images taken from people with lighter skin types only might not be as accurate for people with darker skin, and vice versa.”
There was also “limited information on who, how and why the images were taken,” Dr. Wen said in the release. “This has implications for the programs developed from these images, due to uncertainty around how they may perform in different groups of people, especially in those who aren’t well represented in datasets, such as those with darker skin. This can potentially lead to the exclusion or even harm of these groups from AI technologies.”
While there are no current guidelines for developing skin image datasets, quality standards are needed, according to the researchers.
“Ensuring equitable digital health includes building unbiased, representative datasets to ensure that the algorithms that are created benefit people of all backgrounds and skin types,” they concluded in the study.
Neil Steven, MBBS, MA, PhD, FRCP, an NCRI Skin Group member who was not involved with the research, stated in the press release that the results from the study by Dr. Wen and colleagues “raise concerns about the ability of AI to assist in skin cancer diagnosis, especially in a global context.”
“I hope this work will continue and help ensure that the progress we make in using AI in medicine will benefit all patients, recognizing that human skin color is highly diverse,” said Dr. Steven, honorary consultant in medical oncology at University Hospitals Birmingham (England) NHS Foundation Trust.
‘We need more images of everybody’
Dermatologist Adewole Adamson, MD, MPP, assistant professor in the department of internal medicine (division of dermatology) at the University of Texas at Austin, said in an interview that a “major potential downside” of algorithms not trained on diverse datasets is the potential for incorrect diagnoses.
“The harms of algorithms used for diagnostic purposes in the skin can be particularly significant because of the scalability of this technology. A lot of thought needs to be put into how these algorithms are developed and tested,” said Dr. Adamson, who reviewed the manuscript of The Lancet Digital Health study but was not involved with the research.
He referred to the results of a recently published study in JAMA Dermatology, which found that only 10% of studies used to develop or test deep-learning algorithms contained metadata on skin tone. “Furthermore, most datasets are from countries where darker skin types are not represented. [These] algorithms therefore likely underperform on people of darker skin types and thus, users should be wary,” Dr. Adamson said.
A consensus guideline should be developed for public AI algorithms, he said, which should have metadata containing information on sex, race/ethnicity, geographic location, skin type, and part of the body. “This distribution should also be reported in any publication of an algorithm so that users can see if the distribution of the population in the training data mirrors that of the population in which it is intended to be used,” he added.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was not involved with the research, said that, while this issue of underrepresentation has been known in dermatology for some time, the strength of the Lancet study is that it is a large study, with a message of “we need more images of everybody.”
“This is probably the broadest study looking at every possible accessible resource and taking an organized approach,” Dr. Friedman said in an interview. “But I think it also raises some important points about how we think about skin tones and how we refer to them as well with respect to misusing classification schemes that we currently have.”
While using ethnicity data and certain Fitzpatrick skin types as a proxy for darker skin is a limitation of the metadata the study authors had available, it also highlights “a broader problem with respect to lexicon regarding skin tone,” he explained.
“Skin does not have a race, it doesn’t have an ethnicity,” Dr. Friedman said.
A dataset that contains not only different skin tones but how different dermatologic conditions look across skin tones is important. “If you just look at one photo of one skin tone, you missed the fact that clinical presentations can be so polymorphic, especially because of different skin tones,” Dr. Friedman said.
“We need to keep pushing this message to ensure that images keep getting collected. We [need to] ensure that there’s quality control with these images and that we’re disseminating them in a way that everyone has access, both from self-learning, but also to teach others,” said Dr. Friedman, coeditor of a recently introduced dermatology atlas showing skin conditions in different skin tones.
Adamson reports no relevant financial relationships. Dr. Friedman is a coeditor of a dermatology atlas supported by Allergan Aesthetics and SkinBetter Science. This study was funded by NHSX and the Health Foundation. Three authors reported being paid employees of Databiology at the time of the study. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Does the use of frankincense make sense in dermatology?
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
Early Pilomatrix Carcinoma: A Case Report With Emphasis on Molecular Pathology and Review of the Literature
Pilomatrix carcinoma is a rare adnexal tumor with origin from the germinative matrical cells of the hair follicle. Clinically, it presents as a solitary lesion commonly found in the head and neck region as well as the upper back. The tumors cannot be distinguished by their clinical appearance only and frequently are mistaken for cysts. Histopathologic examination provides the definitive diagnosis in most cases. These carcinomas are aggressive neoplasms with a high probability of local recurrence and distant metastasis. Assessment of the Wnt signaling pathway components such as β-catenin, lymphoid enhancer-binding factor 1 (LEF-1), and caudal-related homeobox transcription factor 2 (CDX-2) potentially can be used for diagnostic purposes and targeted therapy.
We report a rare and unique case of early pilomatrix carcinoma with intralesional melanocytes. We review the molecular pathology and pathogenesis of these carcinomas as well as the significance of early diagnosis.
Case Report
A 73-year-old man with a history of extensive sun exposure presented with a 1-cm, raised, rapidly growing, slightly irregular, purple lesion on the right forearm of 3 months’ duration with tendency to bleed. He did not have a history of skin cancers and was otherwise healthy. Excision was recommended due to the progressive and rapid growth of the lesion.
Histopathologic Findings—Gross examination revealed a 0.9×0.7-cm, raised, slightly irregular lesion located 1 mm away from the closest peripheral margin. Histologically, the lesion was a relatively circumscribed, dermal-based basaloid neoplasm with slightly ill-defined edges involving the superficial and deep dermis (Figure 1A). The neoplasm was formed predominantly of sheets of basaloid cells and small nests of ghost cells, in addition to some squamoid and transitional cells (Figure 1B). The basaloid cells exhibited severe nuclear atypia, pleomorphism, increased nuclear to cytoplasmic ratio (Figure 1C), minimal to moderate amounts of eosinophilic cytoplasm, enlarged nuclei, prominent nucleoli, and coarse chromatin pattern. Abundant mitotic activity and apoptotic bodies were present as well as focal area of central necrosis (Figure 1C). Also, melanophages and a multinucleated giant cell reaction was noted. Elastic trichrome special stain highlighted focal infiltration of the neoplastic cells into the adjacent desmoplastic stroma. Melanin stain was negative for melanin pigment within the neoplasm. Given the presence of severely atypical basaloid cells along with ghost cells indicating matrical differentiation, a diagnosis of pilomatrix carcinoma was rendered.
Immunohistochemistry—The neoplastic cells were diffusely positive for p63, CDX-2 (Figure 2A), β-catenin (Figure 2B), and CD10 (Figure 2C), and focally and weakly positive for cytokeratin (CK) 5, BerEP4 (staining the tumor periphery), androgen receptor, and CK18 (a low-molecular-weight keratin). They were negative for monoclonal carcinoembryonic antigen, epithelial membrane antigen, CK7, CK20, CD34, SOX-10, CD56, synaptophysin, and chromogranin. Cytokeratin 14 was positive in the squamoid cells but negative in the basaloid cells. SOX-10 and melanoma cocktail immunostains demonstrated few intralesional dendritic melanocytes.

Comment
Pilomatrix carcinoma is a rare malignant cutaneous adnexal neoplasm with origin from the germinative matrix of the hair bulb region of hair follicles. Pilomatrix carcinoma was first reported in 1980.1,2 These tumors are characterized by rapid growth and aggressive behavior. Their benign counterpart, pilomatrixoma, is a slow-growing, dermal or subcutaneous tumor that rarely recurs after complete excision.
As with pilomatrixoma, pilomatrix carcinomas are asymptomatic and present as solitary dermal or subcutaneous masses3,4 that most commonly are found in the posterior neck, upper back, and preauricular regions of middle-aged or elderly adults with male predominance.5 They range in size from 0.5 to 20 cm with a mean of 4 cm that is slightly larger than pilomatrixoma. Pilomatrix carcinomas predominantly are firm tumors with or without cystic components, and they exhibit a high probability of recurrence and have risk for distant metastasis.6-15
The differential diagnosis includes epidermal cysts, pilomatrixoma, basal cell carcinoma with matrical differentiation, trichoblastoma/trichoblastic carcinoma, and trichilemmal carcinoma. Pilomatrix carcinomas frequently are mistaken for epidermal cysts on clinical examination. Such a distinction can be easily resolved by histopathologic evaluation. The more challenging differential diagnosis is with pilomatrixoma. Histologically, pilomatrixomas consist of a distinct population of cells including basaloid, squamoid, transitional, and shadow cells in variable proportions. The basaloid cells transition to shadow cells in an organized zonal fashion.16 Compared to pilomatrixomas, pilomatrix carcinomas often show predominance of the basaloid cells; marked cytologic atypia and pleomorphism; numerous mitotic figures; deep infiltrative pattern into subcutaneous fat, fascia, and skeletal muscle; stromal desmoplasia; necrosis; and neurovascular invasion (Tables 1 and 2). Furthermore, the shadow cells tend to form a small nested pattern in pilomatrix carcinoma instead of the flat sheetlike pattern usually observed in pilomatrixoma.16 Basal cell carcinoma with matrical differentiation can pose a diagnostic challenge in the differential diagnosis; basal cell carcinoma usually exhibits a peripheral palisade of the basaloid cells accompanied by retraction spaces separating the tumor from the stroma. Trichoblastoma/trichoblastic carcinoma with matrical differentiation can be distinguished by its exuberant stroma, prominent primitive hair follicles, and papillary mesenchymal bodies. Trichilemmal carcinomas are recognized by their connection to the overlying epidermis, peripheral palisading, and presence of clear cells, while pilomatrix carcinoma lacks connection to the surface epithelium.

Immunohistochemical stains have little to no role in the differential diagnosis, and morphology is the mainstay in making the diagnosis. Rarely, pilomatrix carcinoma can be confused with poorly differentiated sebaceous carcinoma and poorly differentiated squamous cell carcinoma. Although careful scrutiny of the histologic features may help identify mature sebocytes in sebaceous carcinoma, evidence of keratinization in squamous cell carcinoma and ghost cells in pilomatrix carcinoma, using a panel of immunohistochemical stains can be helpful in reaching the final diagnosis (Table 3).

The development of hair matrix tumors have been known to harbor mutations in exon 3 of the catenin beta-1 gene, CTNNB1, that encodes for β-catenin, a downstream effector in the Wnt signaling pathway responsible for differentiation, proliferation, and adhesion of epithelial stem cells.17-21 In a study conducted by Kazakov et al,22 DNA was extracted from 86 lesions: 4 were pilomatrixomas and 1 was a pilomatrix carcinoma. A polymerase chain reaction assay revealed 8 pathogenic variants of the β-catenin gene. D32Y (CTNNB1):c.94G>T (p.Asp32Tyr) and G34R (CTNNB1):c.100G>C (p.Gly34Arg) were the mutations present in pilomatrixoma and pilomatrix carcinoma, respectively.22 In addition, there are several proteins that are part of the Wnt pathway in addition to β-catenin—LEF-1 and CDX-2.
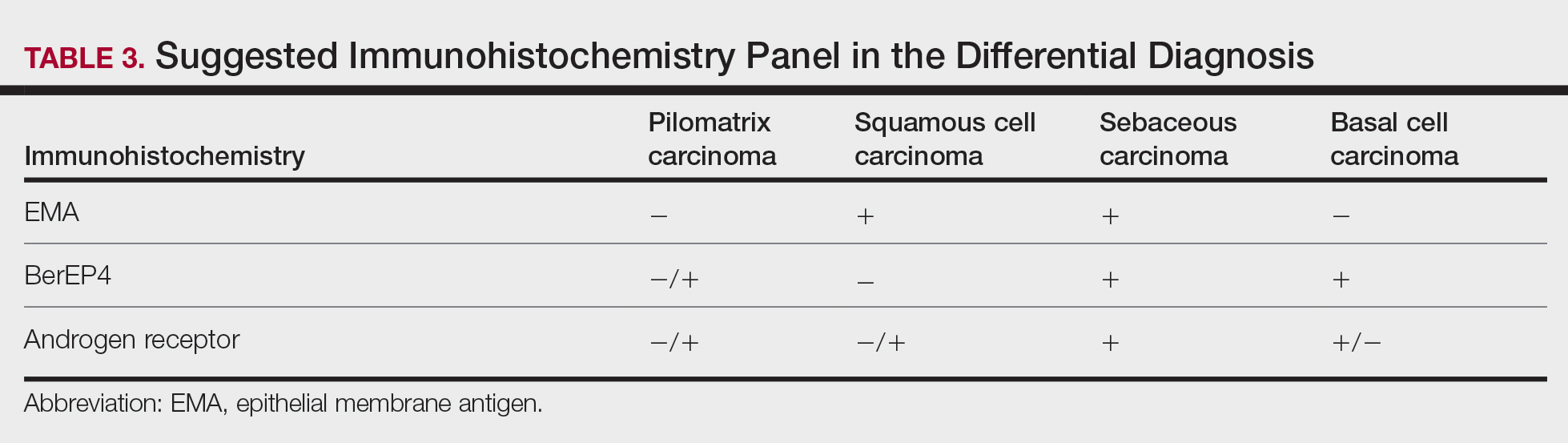
Tumminello and Hosler23 found that pilomatrixomas and pilomatrix carcinomas were positive for CDX-2, β-catenin, and LEF-1 by immunohistochemistry. These downstream molecules in the Wnt signaling pathway could have the potential to be used as diagnostic and prognostic markers.2,13,15,23
Although the pathogenesis is unclear, there are 2 possible mechanisms by which pilomatrix carcinomas develop. They can either arise as de novo tumors, or it is possible that initial mutations in β-catenin result in the formation of pilomatrixomas at an early age that may undergo malignant transformation in elderly patients over time with additional mutations.2
Our case was strongly and diffusely positive for β-catenin in a nuclear and cytoplasmic pattern and CDX-2 in a nuclear pattern, supporting the role of the Wnt signaling pathway in such tumors. Furthermore, our case demonstrated the presence of few intralesional normal dendritic melanocytes, a rare finding1,24,25 but not unexpected, as melanocytes normally are present within the hair follicle matrix.
Pilomatrix carcinomas are aggressive tumors with a high risk for local recurrence and tendency for metastasis. In a study of 13 cases of pilomatrix carcinomas, Herrmann et al13 found that metastasis was significantly associated with local tumor recurrence (P<.0413). They concluded that the combination of overall high local recurrence and metastatic rates of pilomatrix carcinoma as well as documented tumor-related deaths would warrant continued patient follow-up, especially for recurrent tumors.13 Rapid growth of a tumor, either de novo or following several months of stable size, should alert physicians to perform a diagnostic biopsy.
Management options of pilomatrix carcinoma include surgery or radiation with close follow-up. The most widely reported treatment of pilomatrix carcinoma is wide local excision with histologically confirmed clear margins. Mohs micrographic surgery is an excellent treatment option.2,13-15 Adjuvant radiation therapy may be necessary following excision. Currently there is no consensus on surgical management, and standard excisional margins have not been defined.26 Jones et al2 concluded that complete excision with wide margins likely is curative, with decreased rates of recurrence, and better awareness of this carcinoma would lead to appropriate treatment while avoiding unnecessary diagnostic tests.2
Conclusion
We report an exceptionally unique case of early pilomatrix carcinoma with a discussion on the pathogenesis and molecular pathology of hair matrix tumors. A large cohort of patients with longer follow-up periods and better molecular characterization is essential in drawing accurate information about their prognosis, identifying molecular markers that can be used as therapeutic targets, and determining ideal management strategy.
- Jani P, Chetty R, Ghazarian DM. An unusual composite pilomatrix carcinoma with intralesional melanocytes: differential diagnosis, immunohistochemical evaluation, and review of the literature. Am J Dermatopathol. 2008;30:174-177.
- Jones C, Twoon M, Ho W, et al. Pilomatrix carcinoma: 12-year experience and review of the literature. J Cutan Pathol. 2018;45:33-38.
- Forbis R, Helwig EB. Pilomatrixoma (calcifying epithelioma). Arch Dermatol. 1961;83:606.
- Elder D, Elenitsas R, Ragsdale BD. Tumors of epidermal appendages. In: Elder D, Elenitsas R, Jaworsky C, eds. Lever’s Histopathology of the Skin. 8th ed. Lippincott Raven; 1997:757-759.
- Aherne NJ, Fitzpatrick DA, Gibbons D, et al. Pilomatrix carcinoma presenting as an extra axial mass: clinicopathological features. Diagn Pathol. 2008;3:47.
- Papadakis M, de Bree E, Floros N, et al. Pilomatrix carcinoma: more malignant biological behavior than was considered in the past. Mol Clin Oncol. 2017;6:415-418.
- LeBoit PE, Parslow TG, Choy SH. Hair matrix differentiation: occurrence in lesions other than pilomatricoma. Am J Dermatopathol. 1987;9:399-405.
- Campoy F, Stiefel P, Stiefel E, et al. Pilomatrix carcinoma: role played by MR imaging. Neuroradiology. 1989;31:196-198.
- Tateyama H, Eimoto T, Tada T, et al. Malignant pilomatricoma: an immunohistochemical study with antihair keratin antibody. Cancer. 1992;69:127-132.
- O’Donovan DG, Freemont AJ, Adams JE, et al. Malignant pilomatrixoma with bone metastasis. Histopathology. 1993;23:385-386.
- Cross P, Richmond I, Wells S, et al. Malignant pilomatrixoma with bone metastasis. Histopathology. 1994;24:499-500.
- Niedermeyer HP, Peris K, Höfler H. Pilomatrix carcinoma with multiple visceral metastases: report of a case. Cancer. 1996;77:1311-1314.
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.
- Xing L, Marzolf SA, Vandergriff T, et al. Facial pilomatrix carcinomas treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:253-255.
- Fernandez-Flores A, Cassarino DS. Sarcomatoid pilomatrix carcinoma. J Cutan Pathol. 2018;45:508-514.
- Sau P, Lupton GP, Graham JH. Pilomatrix carcinoma. Cancer. 1993;71:2491-2498.
- Chan E, Gat U, McNiff JM, et al. A common human skin tumour is caused by activating mutations in β-catenin. Nat Genet. 1999;21:410-413.
- Huelsken J, Vogel R, Erdmann B, et al. β-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533-545.
- Kikuchi A. Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci. 2003;94:225-229.
- Durand M, Moles J. Beta-catenin mutations in a common skin cancer: pilomatricoma. Bull Cancer. 1999;86:725-726.
- Lazar AJF, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J Cutan Pathol. 2005;32:148-157.
- Kazakov DV, Sima R, Vanecek T, et al. Mutations in exon 3 of the CTNNB1 gene (β-catenin gene) in cutaneous adnexal tumors. Am J Dermatopathol. 2009;31:248-255.
- Tumminello K, Hosler GA. CDX2 and LEF-1 expression in pilomatrical tumors and their utility in the diagnosis of pilomatrical carcinoma. J Cutan Pathol. 2018;45:318-324.
- Rodic´ N, Taube JM, Manson P, et al Locally invasive dermal squamomelanocytic tumor with matrical differentiation: a peculiar case with review of the literature. Am J Dermatopathol. 2013;35:E72-E76.
- Perez C, Debbaneh M, Cassarino D. Preference for the term pilomatrical carcinoma with melanocytic hyperplasia: letter to the editor. J Cutan Pathol. 2017;44:655-657.
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.
Pilomatrix carcinoma is a rare adnexal tumor with origin from the germinative matrical cells of the hair follicle. Clinically, it presents as a solitary lesion commonly found in the head and neck region as well as the upper back. The tumors cannot be distinguished by their clinical appearance only and frequently are mistaken for cysts. Histopathologic examination provides the definitive diagnosis in most cases. These carcinomas are aggressive neoplasms with a high probability of local recurrence and distant metastasis. Assessment of the Wnt signaling pathway components such as β-catenin, lymphoid enhancer-binding factor 1 (LEF-1), and caudal-related homeobox transcription factor 2 (CDX-2) potentially can be used for diagnostic purposes and targeted therapy.
We report a rare and unique case of early pilomatrix carcinoma with intralesional melanocytes. We review the molecular pathology and pathogenesis of these carcinomas as well as the significance of early diagnosis.
Case Report
A 73-year-old man with a history of extensive sun exposure presented with a 1-cm, raised, rapidly growing, slightly irregular, purple lesion on the right forearm of 3 months’ duration with tendency to bleed. He did not have a history of skin cancers and was otherwise healthy. Excision was recommended due to the progressive and rapid growth of the lesion.
Histopathologic Findings—Gross examination revealed a 0.9×0.7-cm, raised, slightly irregular lesion located 1 mm away from the closest peripheral margin. Histologically, the lesion was a relatively circumscribed, dermal-based basaloid neoplasm with slightly ill-defined edges involving the superficial and deep dermis (Figure 1A). The neoplasm was formed predominantly of sheets of basaloid cells and small nests of ghost cells, in addition to some squamoid and transitional cells (Figure 1B). The basaloid cells exhibited severe nuclear atypia, pleomorphism, increased nuclear to cytoplasmic ratio (Figure 1C), minimal to moderate amounts of eosinophilic cytoplasm, enlarged nuclei, prominent nucleoli, and coarse chromatin pattern. Abundant mitotic activity and apoptotic bodies were present as well as focal area of central necrosis (Figure 1C). Also, melanophages and a multinucleated giant cell reaction was noted. Elastic trichrome special stain highlighted focal infiltration of the neoplastic cells into the adjacent desmoplastic stroma. Melanin stain was negative for melanin pigment within the neoplasm. Given the presence of severely atypical basaloid cells along with ghost cells indicating matrical differentiation, a diagnosis of pilomatrix carcinoma was rendered.

Immunohistochemistry—The neoplastic cells were diffusely positive for p63, CDX-2 (Figure 2A), β-catenin (Figure 2B), and CD10 (Figure 2C), and focally and weakly positive for cytokeratin (CK) 5, BerEP4 (staining the tumor periphery), androgen receptor, and CK18 (a low-molecular-weight keratin). They were negative for monoclonal carcinoembryonic antigen, epithelial membrane antigen, CK7, CK20, CD34, SOX-10, CD56, synaptophysin, and chromogranin. Cytokeratin 14 was positive in the squamoid cells but negative in the basaloid cells. SOX-10 and melanoma cocktail immunostains demonstrated few intralesional dendritic melanocytes.

Comment
Pilomatrix carcinoma is a rare malignant cutaneous adnexal neoplasm with origin from the germinative matrix of the hair bulb region of hair follicles. Pilomatrix carcinoma was first reported in 1980.1,2 These tumors are characterized by rapid growth and aggressive behavior. Their benign counterpart, pilomatrixoma, is a slow-growing, dermal or subcutaneous tumor that rarely recurs after complete excision.
As with pilomatrixoma, pilomatrix carcinomas are asymptomatic and present as solitary dermal or subcutaneous masses3,4 that most commonly are found in the posterior neck, upper back, and preauricular regions of middle-aged or elderly adults with male predominance.5 They range in size from 0.5 to 20 cm with a mean of 4 cm that is slightly larger than pilomatrixoma. Pilomatrix carcinomas predominantly are firm tumors with or without cystic components, and they exhibit a high probability of recurrence and have risk for distant metastasis.6-15
The differential diagnosis includes epidermal cysts, pilomatrixoma, basal cell carcinoma with matrical differentiation, trichoblastoma/trichoblastic carcinoma, and trichilemmal carcinoma. Pilomatrix carcinomas frequently are mistaken for epidermal cysts on clinical examination. Such a distinction can be easily resolved by histopathologic evaluation. The more challenging differential diagnosis is with pilomatrixoma. Histologically, pilomatrixomas consist of a distinct population of cells including basaloid, squamoid, transitional, and shadow cells in variable proportions. The basaloid cells transition to shadow cells in an organized zonal fashion.16 Compared to pilomatrixomas, pilomatrix carcinomas often show predominance of the basaloid cells; marked cytologic atypia and pleomorphism; numerous mitotic figures; deep infiltrative pattern into subcutaneous fat, fascia, and skeletal muscle; stromal desmoplasia; necrosis; and neurovascular invasion (Tables 1 and 2). Furthermore, the shadow cells tend to form a small nested pattern in pilomatrix carcinoma instead of the flat sheetlike pattern usually observed in pilomatrixoma.16 Basal cell carcinoma with matrical differentiation can pose a diagnostic challenge in the differential diagnosis; basal cell carcinoma usually exhibits a peripheral palisade of the basaloid cells accompanied by retraction spaces separating the tumor from the stroma. Trichoblastoma/trichoblastic carcinoma with matrical differentiation can be distinguished by its exuberant stroma, prominent primitive hair follicles, and papillary mesenchymal bodies. Trichilemmal carcinomas are recognized by their connection to the overlying epidermis, peripheral palisading, and presence of clear cells, while pilomatrix carcinoma lacks connection to the surface epithelium.

Immunohistochemical stains have little to no role in the differential diagnosis, and morphology is the mainstay in making the diagnosis. Rarely, pilomatrix carcinoma can be confused with poorly differentiated sebaceous carcinoma and poorly differentiated squamous cell carcinoma. Although careful scrutiny of the histologic features may help identify mature sebocytes in sebaceous carcinoma, evidence of keratinization in squamous cell carcinoma and ghost cells in pilomatrix carcinoma, using a panel of immunohistochemical stains can be helpful in reaching the final diagnosis (Table 3).

The development of hair matrix tumors have been known to harbor mutations in exon 3 of the catenin beta-1 gene, CTNNB1, that encodes for β-catenin, a downstream effector in the Wnt signaling pathway responsible for differentiation, proliferation, and adhesion of epithelial stem cells.17-21 In a study conducted by Kazakov et al,22 DNA was extracted from 86 lesions: 4 were pilomatrixomas and 1 was a pilomatrix carcinoma. A polymerase chain reaction assay revealed 8 pathogenic variants of the β-catenin gene. D32Y (CTNNB1):c.94G>T (p.Asp32Tyr) and G34R (CTNNB1):c.100G>C (p.Gly34Arg) were the mutations present in pilomatrixoma and pilomatrix carcinoma, respectively.22 In addition, there are several proteins that are part of the Wnt pathway in addition to β-catenin—LEF-1 and CDX-2.

Tumminello and Hosler23 found that pilomatrixomas and pilomatrix carcinomas were positive for CDX-2, β-catenin, and LEF-1 by immunohistochemistry. These downstream molecules in the Wnt signaling pathway could have the potential to be used as diagnostic and prognostic markers.2,13,15,23
Although the pathogenesis is unclear, there are 2 possible mechanisms by which pilomatrix carcinomas develop. They can either arise as de novo tumors, or it is possible that initial mutations in β-catenin result in the formation of pilomatrixomas at an early age that may undergo malignant transformation in elderly patients over time with additional mutations.2
Our case was strongly and diffusely positive for β-catenin in a nuclear and cytoplasmic pattern and CDX-2 in a nuclear pattern, supporting the role of the Wnt signaling pathway in such tumors. Furthermore, our case demonstrated the presence of few intralesional normal dendritic melanocytes, a rare finding1,24,25 but not unexpected, as melanocytes normally are present within the hair follicle matrix.
Pilomatrix carcinomas are aggressive tumors with a high risk for local recurrence and tendency for metastasis. In a study of 13 cases of pilomatrix carcinomas, Herrmann et al13 found that metastasis was significantly associated with local tumor recurrence (P<.0413). They concluded that the combination of overall high local recurrence and metastatic rates of pilomatrix carcinoma as well as documented tumor-related deaths would warrant continued patient follow-up, especially for recurrent tumors.13 Rapid growth of a tumor, either de novo or following several months of stable size, should alert physicians to perform a diagnostic biopsy.
Management options of pilomatrix carcinoma include surgery or radiation with close follow-up. The most widely reported treatment of pilomatrix carcinoma is wide local excision with histologically confirmed clear margins. Mohs micrographic surgery is an excellent treatment option.2,13-15 Adjuvant radiation therapy may be necessary following excision. Currently there is no consensus on surgical management, and standard excisional margins have not been defined.26 Jones et al2 concluded that complete excision with wide margins likely is curative, with decreased rates of recurrence, and better awareness of this carcinoma would lead to appropriate treatment while avoiding unnecessary diagnostic tests.2
Conclusion
We report an exceptionally unique case of early pilomatrix carcinoma with a discussion on the pathogenesis and molecular pathology of hair matrix tumors. A large cohort of patients with longer follow-up periods and better molecular characterization is essential in drawing accurate information about their prognosis, identifying molecular markers that can be used as therapeutic targets, and determining ideal management strategy.
Pilomatrix carcinoma is a rare adnexal tumor with origin from the germinative matrical cells of the hair follicle. Clinically, it presents as a solitary lesion commonly found in the head and neck region as well as the upper back. The tumors cannot be distinguished by their clinical appearance only and frequently are mistaken for cysts. Histopathologic examination provides the definitive diagnosis in most cases. These carcinomas are aggressive neoplasms with a high probability of local recurrence and distant metastasis. Assessment of the Wnt signaling pathway components such as β-catenin, lymphoid enhancer-binding factor 1 (LEF-1), and caudal-related homeobox transcription factor 2 (CDX-2) potentially can be used for diagnostic purposes and targeted therapy.
We report a rare and unique case of early pilomatrix carcinoma with intralesional melanocytes. We review the molecular pathology and pathogenesis of these carcinomas as well as the significance of early diagnosis.
Case Report
A 73-year-old man with a history of extensive sun exposure presented with a 1-cm, raised, rapidly growing, slightly irregular, purple lesion on the right forearm of 3 months’ duration with tendency to bleed. He did not have a history of skin cancers and was otherwise healthy. Excision was recommended due to the progressive and rapid growth of the lesion.
Histopathologic Findings—Gross examination revealed a 0.9×0.7-cm, raised, slightly irregular lesion located 1 mm away from the closest peripheral margin. Histologically, the lesion was a relatively circumscribed, dermal-based basaloid neoplasm with slightly ill-defined edges involving the superficial and deep dermis (Figure 1A). The neoplasm was formed predominantly of sheets of basaloid cells and small nests of ghost cells, in addition to some squamoid and transitional cells (Figure 1B). The basaloid cells exhibited severe nuclear atypia, pleomorphism, increased nuclear to cytoplasmic ratio (Figure 1C), minimal to moderate amounts of eosinophilic cytoplasm, enlarged nuclei, prominent nucleoli, and coarse chromatin pattern. Abundant mitotic activity and apoptotic bodies were present as well as focal area of central necrosis (Figure 1C). Also, melanophages and a multinucleated giant cell reaction was noted. Elastic trichrome special stain highlighted focal infiltration of the neoplastic cells into the adjacent desmoplastic stroma. Melanin stain was negative for melanin pigment within the neoplasm. Given the presence of severely atypical basaloid cells along with ghost cells indicating matrical differentiation, a diagnosis of pilomatrix carcinoma was rendered.

Immunohistochemistry—The neoplastic cells were diffusely positive for p63, CDX-2 (Figure 2A), β-catenin (Figure 2B), and CD10 (Figure 2C), and focally and weakly positive for cytokeratin (CK) 5, BerEP4 (staining the tumor periphery), androgen receptor, and CK18 (a low-molecular-weight keratin). They were negative for monoclonal carcinoembryonic antigen, epithelial membrane antigen, CK7, CK20, CD34, SOX-10, CD56, synaptophysin, and chromogranin. Cytokeratin 14 was positive in the squamoid cells but negative in the basaloid cells. SOX-10 and melanoma cocktail immunostains demonstrated few intralesional dendritic melanocytes.

Comment
Pilomatrix carcinoma is a rare malignant cutaneous adnexal neoplasm with origin from the germinative matrix of the hair bulb region of hair follicles. Pilomatrix carcinoma was first reported in 1980.1,2 These tumors are characterized by rapid growth and aggressive behavior. Their benign counterpart, pilomatrixoma, is a slow-growing, dermal or subcutaneous tumor that rarely recurs after complete excision.
As with pilomatrixoma, pilomatrix carcinomas are asymptomatic and present as solitary dermal or subcutaneous masses3,4 that most commonly are found in the posterior neck, upper back, and preauricular regions of middle-aged or elderly adults with male predominance.5 They range in size from 0.5 to 20 cm with a mean of 4 cm that is slightly larger than pilomatrixoma. Pilomatrix carcinomas predominantly are firm tumors with or without cystic components, and they exhibit a high probability of recurrence and have risk for distant metastasis.6-15
The differential diagnosis includes epidermal cysts, pilomatrixoma, basal cell carcinoma with matrical differentiation, trichoblastoma/trichoblastic carcinoma, and trichilemmal carcinoma. Pilomatrix carcinomas frequently are mistaken for epidermal cysts on clinical examination. Such a distinction can be easily resolved by histopathologic evaluation. The more challenging differential diagnosis is with pilomatrixoma. Histologically, pilomatrixomas consist of a distinct population of cells including basaloid, squamoid, transitional, and shadow cells in variable proportions. The basaloid cells transition to shadow cells in an organized zonal fashion.16 Compared to pilomatrixomas, pilomatrix carcinomas often show predominance of the basaloid cells; marked cytologic atypia and pleomorphism; numerous mitotic figures; deep infiltrative pattern into subcutaneous fat, fascia, and skeletal muscle; stromal desmoplasia; necrosis; and neurovascular invasion (Tables 1 and 2). Furthermore, the shadow cells tend to form a small nested pattern in pilomatrix carcinoma instead of the flat sheetlike pattern usually observed in pilomatrixoma.16 Basal cell carcinoma with matrical differentiation can pose a diagnostic challenge in the differential diagnosis; basal cell carcinoma usually exhibits a peripheral palisade of the basaloid cells accompanied by retraction spaces separating the tumor from the stroma. Trichoblastoma/trichoblastic carcinoma with matrical differentiation can be distinguished by its exuberant stroma, prominent primitive hair follicles, and papillary mesenchymal bodies. Trichilemmal carcinomas are recognized by their connection to the overlying epidermis, peripheral palisading, and presence of clear cells, while pilomatrix carcinoma lacks connection to the surface epithelium.

Immunohistochemical stains have little to no role in the differential diagnosis, and morphology is the mainstay in making the diagnosis. Rarely, pilomatrix carcinoma can be confused with poorly differentiated sebaceous carcinoma and poorly differentiated squamous cell carcinoma. Although careful scrutiny of the histologic features may help identify mature sebocytes in sebaceous carcinoma, evidence of keratinization in squamous cell carcinoma and ghost cells in pilomatrix carcinoma, using a panel of immunohistochemical stains can be helpful in reaching the final diagnosis (Table 3).

The development of hair matrix tumors have been known to harbor mutations in exon 3 of the catenin beta-1 gene, CTNNB1, that encodes for β-catenin, a downstream effector in the Wnt signaling pathway responsible for differentiation, proliferation, and adhesion of epithelial stem cells.17-21 In a study conducted by Kazakov et al,22 DNA was extracted from 86 lesions: 4 were pilomatrixomas and 1 was a pilomatrix carcinoma. A polymerase chain reaction assay revealed 8 pathogenic variants of the β-catenin gene. D32Y (CTNNB1):c.94G>T (p.Asp32Tyr) and G34R (CTNNB1):c.100G>C (p.Gly34Arg) were the mutations present in pilomatrixoma and pilomatrix carcinoma, respectively.22 In addition, there are several proteins that are part of the Wnt pathway in addition to β-catenin—LEF-1 and CDX-2.

Tumminello and Hosler23 found that pilomatrixomas and pilomatrix carcinomas were positive for CDX-2, β-catenin, and LEF-1 by immunohistochemistry. These downstream molecules in the Wnt signaling pathway could have the potential to be used as diagnostic and prognostic markers.2,13,15,23
Although the pathogenesis is unclear, there are 2 possible mechanisms by which pilomatrix carcinomas develop. They can either arise as de novo tumors, or it is possible that initial mutations in β-catenin result in the formation of pilomatrixomas at an early age that may undergo malignant transformation in elderly patients over time with additional mutations.2
Our case was strongly and diffusely positive for β-catenin in a nuclear and cytoplasmic pattern and CDX-2 in a nuclear pattern, supporting the role of the Wnt signaling pathway in such tumors. Furthermore, our case demonstrated the presence of few intralesional normal dendritic melanocytes, a rare finding1,24,25 but not unexpected, as melanocytes normally are present within the hair follicle matrix.
Pilomatrix carcinomas are aggressive tumors with a high risk for local recurrence and tendency for metastasis. In a study of 13 cases of pilomatrix carcinomas, Herrmann et al13 found that metastasis was significantly associated with local tumor recurrence (P<.0413). They concluded that the combination of overall high local recurrence and metastatic rates of pilomatrix carcinoma as well as documented tumor-related deaths would warrant continued patient follow-up, especially for recurrent tumors.13 Rapid growth of a tumor, either de novo or following several months of stable size, should alert physicians to perform a diagnostic biopsy.
Management options of pilomatrix carcinoma include surgery or radiation with close follow-up. The most widely reported treatment of pilomatrix carcinoma is wide local excision with histologically confirmed clear margins. Mohs micrographic surgery is an excellent treatment option.2,13-15 Adjuvant radiation therapy may be necessary following excision. Currently there is no consensus on surgical management, and standard excisional margins have not been defined.26 Jones et al2 concluded that complete excision with wide margins likely is curative, with decreased rates of recurrence, and better awareness of this carcinoma would lead to appropriate treatment while avoiding unnecessary diagnostic tests.2
Conclusion
We report an exceptionally unique case of early pilomatrix carcinoma with a discussion on the pathogenesis and molecular pathology of hair matrix tumors. A large cohort of patients with longer follow-up periods and better molecular characterization is essential in drawing accurate information about their prognosis, identifying molecular markers that can be used as therapeutic targets, and determining ideal management strategy.
- Jani P, Chetty R, Ghazarian DM. An unusual composite pilomatrix carcinoma with intralesional melanocytes: differential diagnosis, immunohistochemical evaluation, and review of the literature. Am J Dermatopathol. 2008;30:174-177.
- Jones C, Twoon M, Ho W, et al. Pilomatrix carcinoma: 12-year experience and review of the literature. J Cutan Pathol. 2018;45:33-38.
- Forbis R, Helwig EB. Pilomatrixoma (calcifying epithelioma). Arch Dermatol. 1961;83:606.
- Elder D, Elenitsas R, Ragsdale BD. Tumors of epidermal appendages. In: Elder D, Elenitsas R, Jaworsky C, eds. Lever’s Histopathology of the Skin. 8th ed. Lippincott Raven; 1997:757-759.
- Aherne NJ, Fitzpatrick DA, Gibbons D, et al. Pilomatrix carcinoma presenting as an extra axial mass: clinicopathological features. Diagn Pathol. 2008;3:47.
- Papadakis M, de Bree E, Floros N, et al. Pilomatrix carcinoma: more malignant biological behavior than was considered in the past. Mol Clin Oncol. 2017;6:415-418.
- LeBoit PE, Parslow TG, Choy SH. Hair matrix differentiation: occurrence in lesions other than pilomatricoma. Am J Dermatopathol. 1987;9:399-405.
- Campoy F, Stiefel P, Stiefel E, et al. Pilomatrix carcinoma: role played by MR imaging. Neuroradiology. 1989;31:196-198.
- Tateyama H, Eimoto T, Tada T, et al. Malignant pilomatricoma: an immunohistochemical study with antihair keratin antibody. Cancer. 1992;69:127-132.
- O’Donovan DG, Freemont AJ, Adams JE, et al. Malignant pilomatrixoma with bone metastasis. Histopathology. 1993;23:385-386.
- Cross P, Richmond I, Wells S, et al. Malignant pilomatrixoma with bone metastasis. Histopathology. 1994;24:499-500.
- Niedermeyer HP, Peris K, Höfler H. Pilomatrix carcinoma with multiple visceral metastases: report of a case. Cancer. 1996;77:1311-1314.
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.
- Xing L, Marzolf SA, Vandergriff T, et al. Facial pilomatrix carcinomas treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:253-255.
- Fernandez-Flores A, Cassarino DS. Sarcomatoid pilomatrix carcinoma. J Cutan Pathol. 2018;45:508-514.
- Sau P, Lupton GP, Graham JH. Pilomatrix carcinoma. Cancer. 1993;71:2491-2498.
- Chan E, Gat U, McNiff JM, et al. A common human skin tumour is caused by activating mutations in β-catenin. Nat Genet. 1999;21:410-413.
- Huelsken J, Vogel R, Erdmann B, et al. β-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533-545.
- Kikuchi A. Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci. 2003;94:225-229.
- Durand M, Moles J. Beta-catenin mutations in a common skin cancer: pilomatricoma. Bull Cancer. 1999;86:725-726.
- Lazar AJF, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J Cutan Pathol. 2005;32:148-157.
- Kazakov DV, Sima R, Vanecek T, et al. Mutations in exon 3 of the CTNNB1 gene (β-catenin gene) in cutaneous adnexal tumors. Am J Dermatopathol. 2009;31:248-255.
- Tumminello K, Hosler GA. CDX2 and LEF-1 expression in pilomatrical tumors and their utility in the diagnosis of pilomatrical carcinoma. J Cutan Pathol. 2018;45:318-324.
- Rodic´ N, Taube JM, Manson P, et al Locally invasive dermal squamomelanocytic tumor with matrical differentiation: a peculiar case with review of the literature. Am J Dermatopathol. 2013;35:E72-E76.
- Perez C, Debbaneh M, Cassarino D. Preference for the term pilomatrical carcinoma with melanocytic hyperplasia: letter to the editor. J Cutan Pathol. 2017;44:655-657.
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.
- Jani P, Chetty R, Ghazarian DM. An unusual composite pilomatrix carcinoma with intralesional melanocytes: differential diagnosis, immunohistochemical evaluation, and review of the literature. Am J Dermatopathol. 2008;30:174-177.
- Jones C, Twoon M, Ho W, et al. Pilomatrix carcinoma: 12-year experience and review of the literature. J Cutan Pathol. 2018;45:33-38.
- Forbis R, Helwig EB. Pilomatrixoma (calcifying epithelioma). Arch Dermatol. 1961;83:606.
- Elder D, Elenitsas R, Ragsdale BD. Tumors of epidermal appendages. In: Elder D, Elenitsas R, Jaworsky C, eds. Lever’s Histopathology of the Skin. 8th ed. Lippincott Raven; 1997:757-759.
- Aherne NJ, Fitzpatrick DA, Gibbons D, et al. Pilomatrix carcinoma presenting as an extra axial mass: clinicopathological features. Diagn Pathol. 2008;3:47.
- Papadakis M, de Bree E, Floros N, et al. Pilomatrix carcinoma: more malignant biological behavior than was considered in the past. Mol Clin Oncol. 2017;6:415-418.
- LeBoit PE, Parslow TG, Choy SH. Hair matrix differentiation: occurrence in lesions other than pilomatricoma. Am J Dermatopathol. 1987;9:399-405.
- Campoy F, Stiefel P, Stiefel E, et al. Pilomatrix carcinoma: role played by MR imaging. Neuroradiology. 1989;31:196-198.
- Tateyama H, Eimoto T, Tada T, et al. Malignant pilomatricoma: an immunohistochemical study with antihair keratin antibody. Cancer. 1992;69:127-132.
- O’Donovan DG, Freemont AJ, Adams JE, et al. Malignant pilomatrixoma with bone metastasis. Histopathology. 1993;23:385-386.
- Cross P, Richmond I, Wells S, et al. Malignant pilomatrixoma with bone metastasis. Histopathology. 1994;24:499-500.
- Niedermeyer HP, Peris K, Höfler H. Pilomatrix carcinoma with multiple visceral metastases: report of a case. Cancer. 1996;77:1311-1314.
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.
- Xing L, Marzolf SA, Vandergriff T, et al. Facial pilomatrix carcinomas treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:253-255.
- Fernandez-Flores A, Cassarino DS. Sarcomatoid pilomatrix carcinoma. J Cutan Pathol. 2018;45:508-514.
- Sau P, Lupton GP, Graham JH. Pilomatrix carcinoma. Cancer. 1993;71:2491-2498.
- Chan E, Gat U, McNiff JM, et al. A common human skin tumour is caused by activating mutations in β-catenin. Nat Genet. 1999;21:410-413.
- Huelsken J, Vogel R, Erdmann B, et al. β-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533-545.
- Kikuchi A. Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci. 2003;94:225-229.
- Durand M, Moles J. Beta-catenin mutations in a common skin cancer: pilomatricoma. Bull Cancer. 1999;86:725-726.
- Lazar AJF, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J Cutan Pathol. 2005;32:148-157.
- Kazakov DV, Sima R, Vanecek T, et al. Mutations in exon 3 of the CTNNB1 gene (β-catenin gene) in cutaneous adnexal tumors. Am J Dermatopathol. 2009;31:248-255.
- Tumminello K, Hosler GA. CDX2 and LEF-1 expression in pilomatrical tumors and their utility in the diagnosis of pilomatrical carcinoma. J Cutan Pathol. 2018;45:318-324.
- Rodic´ N, Taube JM, Manson P, et al Locally invasive dermal squamomelanocytic tumor with matrical differentiation: a peculiar case with review of the literature. Am J Dermatopathol. 2013;35:E72-E76.
- Perez C, Debbaneh M, Cassarino D. Preference for the term pilomatrical carcinoma with melanocytic hyperplasia: letter to the editor. J Cutan Pathol. 2017;44:655-657.
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.
Practice Points
- Clinicians and pathologists should be aware of pilomatrix carcinoma to facilitate early detection.
- Early diagnosis and prompt treatment of pilomatrix carcinoma is crucial in lowering recurrence rate and avoiding a poor outcome.
- Caudal-related homeobox transcription factor 2 and β-catenin components of the Wnt signaling pathway play an important role in the pathogenesis of pilomatrix carcinoma.
- Although controversial, wide local excision is the treatment of choice for pilomatrix carcinoma.
TANS Syndrome: Tanorexia, Anorexia, and Nonmelanoma Skin Cancer
The term tanorexia describes compulsive use of a tanning bed, a disorder often identified in White patients. This compulsion is driven by underlying psychological distress that typically correlates with another psychiatric disorder, such as anxiety, body dysmorphic disorder, or an eating disorder. 1 Severe anorexia combined with excessive indoor tanning led to a notable burden of cutaneous squamous cell carcinomas (SCCs) and keratoacanthomas in one of our patients. We discuss the management and approach to patient care in this difficult situation, which we have coined TANS syndrome (for T anorexia, A norexia, and N onmelanoma s kin cancer).
A Patient With TANS Syndrome
A 35-year-old cachectic woman, who appeared much older than her chronologic age, presented for management of numerous painful bleeding skin lesions. Diffuse, erythematous, tender nodules with central keratotic cores, some several centimeters in diameter, were scattered on the abdomen, chest, and extremities (Figure 1); similar lesions were noted on the neck (Figure 2). Numerous erythematous scaly papules and plaques consistent with actinic keratoses were noted throughout the body.
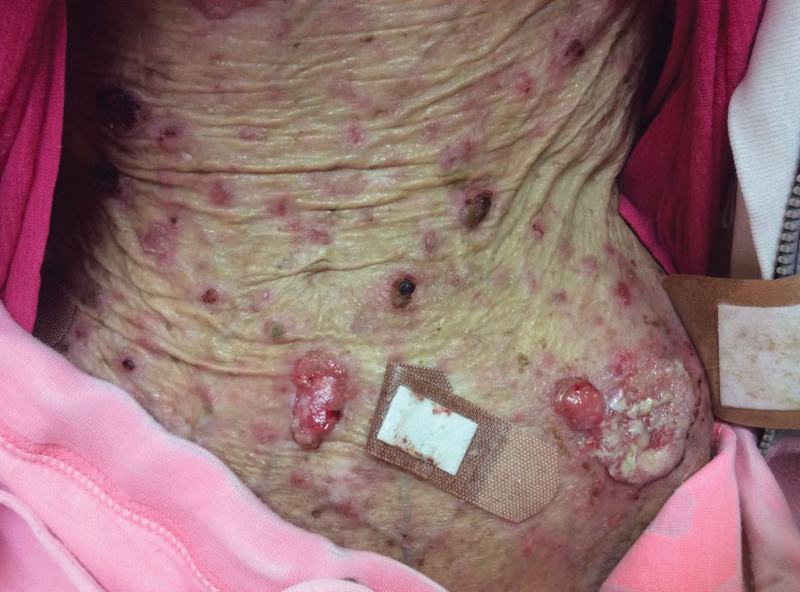
The patient reported that the cutaneous SCCs presented over the last few years, whereas her eating disorder began in adolescence and persisted despite multiple intensive outpatient and inpatient programs. The patient adamantly refused repeat hospitalization, against repeated suggestions by health care providers and her family. Comorbidities related to her anorexia included severe renal insufficiency, iron deficiency anemia, hypertriglyceridemia, kwashiorkor, and pellagra.
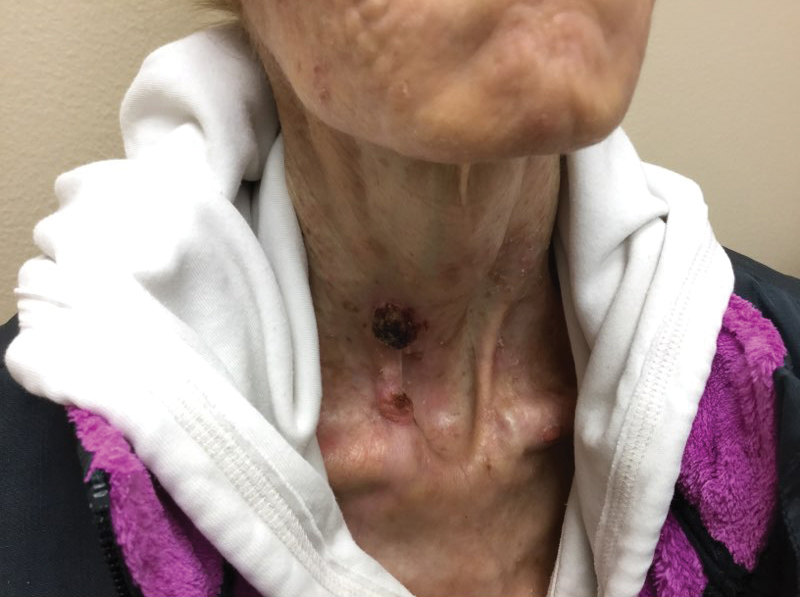
Within the last year, the patient had several biopsies showing SCC, keratoacanthoma type. The largest tumors had been treated by Mohs micrographic surgery, excision, and electrodesiccation or curettage. Adjuvant therapy over the last 2 years consisted of tazarotene cream 0.1%, imiquimod cream 5%, oral nicotinamide 500 mg twice daily, and acitretin 10 to 20 mg daily. Human papillomavirus 9-valent vaccine, recombinant, also had been tried as a chemopreventive and treatment, based on a published report of 2 patients in whom keratinocytic carcinomas decreased after such vaccination.2 The dose of acitretin was kept low because of the patient’s severe renal insufficiency and lack of supporting data for its use in this setting. Despite these modalities, our patient continued to develop new cutaneous SCCs.
We considered starting intralesional methotrexate but deferred this course of action, given the patient’s deteriorating renal function. Our plan was to initiate intralesional 5-fluorouracil; however, the patient was admitted to the hospital and subsequently died due to cardiovascular complications of anorexia.
UV Radiation in the Setting of Immune Compromise
Habitual tanning bed use has been recognized as a psychologic addiction.3,4 After exposure to UV radiation, damaged DNA upregulates pro-opiomelanocortin, which posttranslationally generates β-endorphins to elevate mood.3,5
Tanning beds deliver a higher dose of UVA radiation than UVB radiation and cause darkening of pigmentation by oxidation of preformed melanin and redistribution of melanosomes.3 UVA radiation (320–400 nm) emitted from a tanning bed is 10- to 15-times higher than the radiation emitted by the midday sun and causes DNA damage through generation of reactive oxygen species. UVA penetrates the dermis; its harmful effect on DNA contributes to the pathogenesis of melanoma.
UVB radiation (290–320 nm) is mainly restricted to the epidermis and is largely responsible for erythema of the skin. UVB specifically causes direct damage to DNA by forming pyrimidine dimers, superficially causing sunburn. Excessive exposure to UVB radiation increases the risk for nonmelanoma skin cancer.6
Severe starvation and chronic malnutrition, as seen in anorexia nervosa, also are known to lead to immunosuppression.7 Exposure to UV radiation has been shown to impair the function of antigen-presenting cells, cytokines, and suppressor T cells, and is classified as a Group 1 carcinogen by the World Health Organization.3,8 Combining a compromised immune system in anorexia with DNA damage from frequent indoor tanning provides a dangerous milieu for carcinogenesis.8 Without immune surveillance, as occurs with adequate nutrition, treatment of cutaneous SCC is, at best, challenging.
Primary care physicians, dermatologists, psychiatrists, nutritionists, and public health officials should educate high-risk patients to prevent TANS syndrome.
- Petit A, Karila L, Chalmin F, et al. Phenomenology and psychopathology of excessive indoor tanning. Int J Dermatol. 2014;53:664-672. doi:10.1111/ijd.12336
- Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi:10.1001/jamadermatol.2016.5703
- Madigan LM, Lim HW. Tanning beds: impact on health, and recent regulations. Clin Dermatol. 2016;34:640-648. doi:10.1016/j.clindermatol.2016.05.016
- Schwebel DC. Adolescent tanning, disordered eating, and risk taking. J Dev Behav Pediatr. 2014;35:225-227. doi:10.1097/DBP.0000000000000045
- Friedman B, English JC 3rd, Ferris LK. Indoor tanning, skin cancer and the young female patient: a review of the literature. J Pediatr Adolesc Gynecol. 2015;28:275-283. doi:10.1016/j.jpag.2014.07.015
- Armstrong BK, Kricker A. Epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Hanachi M, Bohem V, Bemer P, et al. Negative role of malnutrition in cell-mediated immune response: Pneumocystis jirovecii pneumonia (PCP) in a severely malnourished, HIV-negative patient with anorexia nervosa. Clin Nutr ESPEN. 2018;25:163-165. doi:10.1016/j.clnesp.2018.03.121
- Schwarz T, Beissert S. Milestones in photoimmunology. J Invest Dermatol. 2013;133:E7-E10. doi:10.1038/skinbio.2013.177
The term tanorexia describes compulsive use of a tanning bed, a disorder often identified in White patients. This compulsion is driven by underlying psychological distress that typically correlates with another psychiatric disorder, such as anxiety, body dysmorphic disorder, or an eating disorder. 1 Severe anorexia combined with excessive indoor tanning led to a notable burden of cutaneous squamous cell carcinomas (SCCs) and keratoacanthomas in one of our patients. We discuss the management and approach to patient care in this difficult situation, which we have coined TANS syndrome (for T anorexia, A norexia, and N onmelanoma s kin cancer).
A Patient With TANS Syndrome
A 35-year-old cachectic woman, who appeared much older than her chronologic age, presented for management of numerous painful bleeding skin lesions. Diffuse, erythematous, tender nodules with central keratotic cores, some several centimeters in diameter, were scattered on the abdomen, chest, and extremities (Figure 1); similar lesions were noted on the neck (Figure 2). Numerous erythematous scaly papules and plaques consistent with actinic keratoses were noted throughout the body.

The patient reported that the cutaneous SCCs presented over the last few years, whereas her eating disorder began in adolescence and persisted despite multiple intensive outpatient and inpatient programs. The patient adamantly refused repeat hospitalization, against repeated suggestions by health care providers and her family. Comorbidities related to her anorexia included severe renal insufficiency, iron deficiency anemia, hypertriglyceridemia, kwashiorkor, and pellagra.

Within the last year, the patient had several biopsies showing SCC, keratoacanthoma type. The largest tumors had been treated by Mohs micrographic surgery, excision, and electrodesiccation or curettage. Adjuvant therapy over the last 2 years consisted of tazarotene cream 0.1%, imiquimod cream 5%, oral nicotinamide 500 mg twice daily, and acitretin 10 to 20 mg daily. Human papillomavirus 9-valent vaccine, recombinant, also had been tried as a chemopreventive and treatment, based on a published report of 2 patients in whom keratinocytic carcinomas decreased after such vaccination.2 The dose of acitretin was kept low because of the patient’s severe renal insufficiency and lack of supporting data for its use in this setting. Despite these modalities, our patient continued to develop new cutaneous SCCs.
We considered starting intralesional methotrexate but deferred this course of action, given the patient’s deteriorating renal function. Our plan was to initiate intralesional 5-fluorouracil; however, the patient was admitted to the hospital and subsequently died due to cardiovascular complications of anorexia.
UV Radiation in the Setting of Immune Compromise
Habitual tanning bed use has been recognized as a psychologic addiction.3,4 After exposure to UV radiation, damaged DNA upregulates pro-opiomelanocortin, which posttranslationally generates β-endorphins to elevate mood.3,5
Tanning beds deliver a higher dose of UVA radiation than UVB radiation and cause darkening of pigmentation by oxidation of preformed melanin and redistribution of melanosomes.3 UVA radiation (320–400 nm) emitted from a tanning bed is 10- to 15-times higher than the radiation emitted by the midday sun and causes DNA damage through generation of reactive oxygen species. UVA penetrates the dermis; its harmful effect on DNA contributes to the pathogenesis of melanoma.
UVB radiation (290–320 nm) is mainly restricted to the epidermis and is largely responsible for erythema of the skin. UVB specifically causes direct damage to DNA by forming pyrimidine dimers, superficially causing sunburn. Excessive exposure to UVB radiation increases the risk for nonmelanoma skin cancer.6
Severe starvation and chronic malnutrition, as seen in anorexia nervosa, also are known to lead to immunosuppression.7 Exposure to UV radiation has been shown to impair the function of antigen-presenting cells, cytokines, and suppressor T cells, and is classified as a Group 1 carcinogen by the World Health Organization.3,8 Combining a compromised immune system in anorexia with DNA damage from frequent indoor tanning provides a dangerous milieu for carcinogenesis.8 Without immune surveillance, as occurs with adequate nutrition, treatment of cutaneous SCC is, at best, challenging.
Primary care physicians, dermatologists, psychiatrists, nutritionists, and public health officials should educate high-risk patients to prevent TANS syndrome.
The term tanorexia describes compulsive use of a tanning bed, a disorder often identified in White patients. This compulsion is driven by underlying psychological distress that typically correlates with another psychiatric disorder, such as anxiety, body dysmorphic disorder, or an eating disorder. 1 Severe anorexia combined with excessive indoor tanning led to a notable burden of cutaneous squamous cell carcinomas (SCCs) and keratoacanthomas in one of our patients. We discuss the management and approach to patient care in this difficult situation, which we have coined TANS syndrome (for T anorexia, A norexia, and N onmelanoma s kin cancer).
A Patient With TANS Syndrome
A 35-year-old cachectic woman, who appeared much older than her chronologic age, presented for management of numerous painful bleeding skin lesions. Diffuse, erythematous, tender nodules with central keratotic cores, some several centimeters in diameter, were scattered on the abdomen, chest, and extremities (Figure 1); similar lesions were noted on the neck (Figure 2). Numerous erythematous scaly papules and plaques consistent with actinic keratoses were noted throughout the body.

The patient reported that the cutaneous SCCs presented over the last few years, whereas her eating disorder began in adolescence and persisted despite multiple intensive outpatient and inpatient programs. The patient adamantly refused repeat hospitalization, against repeated suggestions by health care providers and her family. Comorbidities related to her anorexia included severe renal insufficiency, iron deficiency anemia, hypertriglyceridemia, kwashiorkor, and pellagra.

Within the last year, the patient had several biopsies showing SCC, keratoacanthoma type. The largest tumors had been treated by Mohs micrographic surgery, excision, and electrodesiccation or curettage. Adjuvant therapy over the last 2 years consisted of tazarotene cream 0.1%, imiquimod cream 5%, oral nicotinamide 500 mg twice daily, and acitretin 10 to 20 mg daily. Human papillomavirus 9-valent vaccine, recombinant, also had been tried as a chemopreventive and treatment, based on a published report of 2 patients in whom keratinocytic carcinomas decreased after such vaccination.2 The dose of acitretin was kept low because of the patient’s severe renal insufficiency and lack of supporting data for its use in this setting. Despite these modalities, our patient continued to develop new cutaneous SCCs.
We considered starting intralesional methotrexate but deferred this course of action, given the patient’s deteriorating renal function. Our plan was to initiate intralesional 5-fluorouracil; however, the patient was admitted to the hospital and subsequently died due to cardiovascular complications of anorexia.
UV Radiation in the Setting of Immune Compromise
Habitual tanning bed use has been recognized as a psychologic addiction.3,4 After exposure to UV radiation, damaged DNA upregulates pro-opiomelanocortin, which posttranslationally generates β-endorphins to elevate mood.3,5
Tanning beds deliver a higher dose of UVA radiation than UVB radiation and cause darkening of pigmentation by oxidation of preformed melanin and redistribution of melanosomes.3 UVA radiation (320–400 nm) emitted from a tanning bed is 10- to 15-times higher than the radiation emitted by the midday sun and causes DNA damage through generation of reactive oxygen species. UVA penetrates the dermis; its harmful effect on DNA contributes to the pathogenesis of melanoma.
UVB radiation (290–320 nm) is mainly restricted to the epidermis and is largely responsible for erythema of the skin. UVB specifically causes direct damage to DNA by forming pyrimidine dimers, superficially causing sunburn. Excessive exposure to UVB radiation increases the risk for nonmelanoma skin cancer.6
Severe starvation and chronic malnutrition, as seen in anorexia nervosa, also are known to lead to immunosuppression.7 Exposure to UV radiation has been shown to impair the function of antigen-presenting cells, cytokines, and suppressor T cells, and is classified as a Group 1 carcinogen by the World Health Organization.3,8 Combining a compromised immune system in anorexia with DNA damage from frequent indoor tanning provides a dangerous milieu for carcinogenesis.8 Without immune surveillance, as occurs with adequate nutrition, treatment of cutaneous SCC is, at best, challenging.
Primary care physicians, dermatologists, psychiatrists, nutritionists, and public health officials should educate high-risk patients to prevent TANS syndrome.
- Petit A, Karila L, Chalmin F, et al. Phenomenology and psychopathology of excessive indoor tanning. Int J Dermatol. 2014;53:664-672. doi:10.1111/ijd.12336
- Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi:10.1001/jamadermatol.2016.5703
- Madigan LM, Lim HW. Tanning beds: impact on health, and recent regulations. Clin Dermatol. 2016;34:640-648. doi:10.1016/j.clindermatol.2016.05.016
- Schwebel DC. Adolescent tanning, disordered eating, and risk taking. J Dev Behav Pediatr. 2014;35:225-227. doi:10.1097/DBP.0000000000000045
- Friedman B, English JC 3rd, Ferris LK. Indoor tanning, skin cancer and the young female patient: a review of the literature. J Pediatr Adolesc Gynecol. 2015;28:275-283. doi:10.1016/j.jpag.2014.07.015
- Armstrong BK, Kricker A. Epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Hanachi M, Bohem V, Bemer P, et al. Negative role of malnutrition in cell-mediated immune response: Pneumocystis jirovecii pneumonia (PCP) in a severely malnourished, HIV-negative patient with anorexia nervosa. Clin Nutr ESPEN. 2018;25:163-165. doi:10.1016/j.clnesp.2018.03.121
- Schwarz T, Beissert S. Milestones in photoimmunology. J Invest Dermatol. 2013;133:E7-E10. doi:10.1038/skinbio.2013.177
- Petit A, Karila L, Chalmin F, et al. Phenomenology and psychopathology of excessive indoor tanning. Int J Dermatol. 2014;53:664-672. doi:10.1111/ijd.12336
- Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi:10.1001/jamadermatol.2016.5703
- Madigan LM, Lim HW. Tanning beds: impact on health, and recent regulations. Clin Dermatol. 2016;34:640-648. doi:10.1016/j.clindermatol.2016.05.016
- Schwebel DC. Adolescent tanning, disordered eating, and risk taking. J Dev Behav Pediatr. 2014;35:225-227. doi:10.1097/DBP.0000000000000045
- Friedman B, English JC 3rd, Ferris LK. Indoor tanning, skin cancer and the young female patient: a review of the literature. J Pediatr Adolesc Gynecol. 2015;28:275-283. doi:10.1016/j.jpag.2014.07.015
- Armstrong BK, Kricker A. Epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Hanachi M, Bohem V, Bemer P, et al. Negative role of malnutrition in cell-mediated immune response: Pneumocystis jirovecii pneumonia (PCP) in a severely malnourished, HIV-negative patient with anorexia nervosa. Clin Nutr ESPEN. 2018;25:163-165. doi:10.1016/j.clnesp.2018.03.121
- Schwarz T, Beissert S. Milestones in photoimmunology. J Invest Dermatol. 2013;133:E7-E10. doi:10.1038/skinbio.2013.177
Practice Points
- Primary care physicians, dermatologists, psychiatrists, nutritionists, and public health officials should educate high-risk patients to prevent TANS syndrome.
- Combining a compromised immune system in anorexia with DNA damage from frequent indoor tanning provides a dangerous milieu for carcinogenesis.
- Comorbidities related to TANS syndrome make it challenging to effectively treat cutaneous squamous cell carcinoma.
In and out surgeries become the norm during pandemic
Urologist Ronney Abaza, MD, a robotic surgery specialist in Dublin, Ohio, and colleagues, reviewed robotic surgeries at their hospital during COVID-19 restrictions on surgery in Ohio between March 17 and June 5, 2020, and compared them with robotic procedures before COVID-19 and after restrictions were lifted. They published their results in Urology.
Since 2016, the hospital has offered the option of same-day discharge (SDD) to all robotic urologic surgery patients, regardless of procedure or patient-specific factors.
Among patients who had surgery during COVID-19 restrictions, 98% (87/89 patients) opted for SDD versus 52% in the group having surgery before the restrictions (P < .00001). After the COVID-19 surgery restrictions were lifted, the higher rate of SDD remained at 98%.
“There were no differences in 30-day complications or readmissions between SDD and overnight patients,” the authors write.
The right patient, the right motivation for successful surgery
Brian Lane, MD, PhD, a urologic oncologist with Spectrum Health in Grand Rapids, Michigan, told this news organization that, for nephrectomies, uptake of same-day discharge will continue to be slow.
“You have to have the right patient, the right patient motivation, and the surgery has to go smoothly,” he said. “If you start sending everyone home the same day, you will certainly see readmissions,” he said.
Dr. Lane is part of the Michigan Urologic Surgery Improvement Collaborative and he said the group recently looked at same-day discharge outcomes after robotic prostatectomies with SDD as compared with 1-2 nights in the hospital.
The work has not yet been published but, “There was a slight signal that there were increased readmissions with same-day discharge vs. 0-1 day,” he said.
A paper on outcomes of same-day discharge in total knee arthroplasty in the Journal of Bone & Joint Surgery found a higher risk of perioperative complications “including component failure, surgical site infection, knee stiffness, and deep vein thrombosis.” Researchers compared outcomes between 4,391 patients who underwent outpatient TKA and 128,951 patients who underwent inpatient TKA.
But for other many surgeries, same-day discharge numbers are increasing without worsening outcomes.
A paper in the Journal of Robotic Surgery found that same-day discharge following robotic-assisted endometrial cancer staging is “safe and feasible.”
Stephen Bradley, MD, MPH, with the Minneapolis Heart Institute in Minneapolis, and colleagues write in the Journal of the American College of Cardiology: Cardiovascular Interventions that they found a large increase in the use of same-day discharge after elective percutaneous coronary intervention (PCI) was not associated with worse 30-day mortality rates or readmission.
In that study, 114,461 patients were discharged the same day they underwent PCI. The proportion of patients who had a same-day discharge increased from 4.5% in 2009 to 28.6% in the fourth quarter of 2017.
Risk-adjusted 30-day mortality did not change in that time, while risk-adjusted rehospitalization decreased over time and more quickly when patients had same-day discharge.
Deepak L. Bhatt, MD, MPH, and Jonathan G. Sung, MBCHB, both of Brigham and Women’s Hospital Heart & Vascular Center, Harvard Medical School, Boston, wrote in an accompanying article that, “Advances in the devices and techniques of PCI have improved the safety and efficacy of the procedure. In selected patients, same-day discharge has become possible, and overnight in-hospital observation can be avoided. By reducing unnecessary hospital stays, both patients and hospitals could benefit.”
Evan Garden, a medical student at Icahn School of Medicine at Mount Sinai in New York, presented findings at the American Urological Association 2021 annual meeting that show patients selected for same-day discharge after partial or radical nephrectomy did not have increased rates of postoperative complications or readmissions in the immediate postoperative period, compared with standard discharge of 1-3 days.
Case studies in nephrectomy
While several case studies have looked at the feasibility and safety of performing partial and radical nephrectomy with same-day discharge in select cases, “this topic has not been addressed on a national level,” Mr. Garden said.
Few patients who have partial or radical nephrectomies have same-day discharges. The researchers found that fewer than 1% of patients who have either procedure in the sample studied were discharged the same day.
Researchers used the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database, a nationally representative deidentified database that prospectively tracks patient characteristics and 30-day perioperative outcomes for major inpatient and outpatient surgical procedures at more than 700 hospitals.
They extracted all minimally invasive partial and radical nephrectomies from 2012 to 2019 and refined the cohort to 28,140 patients who were theoretically eligible for same-day discharge: Of those, 237 (0.8%) had SSD, and 27,903 (99.2%) had a standard-length discharge (SLD).
The team found that there were no differences in 30-day complications or readmissions between same-day discharge (Clavien-Dindo [CD] I/II, 4.22%; CD III, 0%; CD IV, 1.27%; readmission, 4.64%); and SLD (CD I/II, 4.11%; CD III, 0.95%; CD IV, 0.79%; readmission, 3.90%; all P > .05).
Controlling for demographic and clinical variables, SDD was not associated with greater risk of 30-day complications or readmissions (CD I/II: odds ratio, 1.08; 95% confidence interval, 0.57-2.048; P = .813; CD IV: OR 1.699; 95% CI, 0.537-5.375; P = .367; readmission: OR, 1.254; 95% CI, 0.681-2.31; P = .467).
Mr. Garden and coauthors report no relevant financial relationships.
Dr. Lane reports no relevant financial relationships.
Urologist Ronney Abaza, MD, a robotic surgery specialist in Dublin, Ohio, and colleagues, reviewed robotic surgeries at their hospital during COVID-19 restrictions on surgery in Ohio between March 17 and June 5, 2020, and compared them with robotic procedures before COVID-19 and after restrictions were lifted. They published their results in Urology.
Since 2016, the hospital has offered the option of same-day discharge (SDD) to all robotic urologic surgery patients, regardless of procedure or patient-specific factors.
Among patients who had surgery during COVID-19 restrictions, 98% (87/89 patients) opted for SDD versus 52% in the group having surgery before the restrictions (P < .00001). After the COVID-19 surgery restrictions were lifted, the higher rate of SDD remained at 98%.
“There were no differences in 30-day complications or readmissions between SDD and overnight patients,” the authors write.
The right patient, the right motivation for successful surgery
Brian Lane, MD, PhD, a urologic oncologist with Spectrum Health in Grand Rapids, Michigan, told this news organization that, for nephrectomies, uptake of same-day discharge will continue to be slow.
“You have to have the right patient, the right patient motivation, and the surgery has to go smoothly,” he said. “If you start sending everyone home the same day, you will certainly see readmissions,” he said.
Dr. Lane is part of the Michigan Urologic Surgery Improvement Collaborative and he said the group recently looked at same-day discharge outcomes after robotic prostatectomies with SDD as compared with 1-2 nights in the hospital.
The work has not yet been published but, “There was a slight signal that there were increased readmissions with same-day discharge vs. 0-1 day,” he said.
A paper on outcomes of same-day discharge in total knee arthroplasty in the Journal of Bone & Joint Surgery found a higher risk of perioperative complications “including component failure, surgical site infection, knee stiffness, and deep vein thrombosis.” Researchers compared outcomes between 4,391 patients who underwent outpatient TKA and 128,951 patients who underwent inpatient TKA.
But for other many surgeries, same-day discharge numbers are increasing without worsening outcomes.
A paper in the Journal of Robotic Surgery found that same-day discharge following robotic-assisted endometrial cancer staging is “safe and feasible.”
Stephen Bradley, MD, MPH, with the Minneapolis Heart Institute in Minneapolis, and colleagues write in the Journal of the American College of Cardiology: Cardiovascular Interventions that they found a large increase in the use of same-day discharge after elective percutaneous coronary intervention (PCI) was not associated with worse 30-day mortality rates or readmission.
In that study, 114,461 patients were discharged the same day they underwent PCI. The proportion of patients who had a same-day discharge increased from 4.5% in 2009 to 28.6% in the fourth quarter of 2017.
Risk-adjusted 30-day mortality did not change in that time, while risk-adjusted rehospitalization decreased over time and more quickly when patients had same-day discharge.
Deepak L. Bhatt, MD, MPH, and Jonathan G. Sung, MBCHB, both of Brigham and Women’s Hospital Heart & Vascular Center, Harvard Medical School, Boston, wrote in an accompanying article that, “Advances in the devices and techniques of PCI have improved the safety and efficacy of the procedure. In selected patients, same-day discharge has become possible, and overnight in-hospital observation can be avoided. By reducing unnecessary hospital stays, both patients and hospitals could benefit.”
Evan Garden, a medical student at Icahn School of Medicine at Mount Sinai in New York, presented findings at the American Urological Association 2021 annual meeting that show patients selected for same-day discharge after partial or radical nephrectomy did not have increased rates of postoperative complications or readmissions in the immediate postoperative period, compared with standard discharge of 1-3 days.
Case studies in nephrectomy
While several case studies have looked at the feasibility and safety of performing partial and radical nephrectomy with same-day discharge in select cases, “this topic has not been addressed on a national level,” Mr. Garden said.
Few patients who have partial or radical nephrectomies have same-day discharges. The researchers found that fewer than 1% of patients who have either procedure in the sample studied were discharged the same day.
Researchers used the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database, a nationally representative deidentified database that prospectively tracks patient characteristics and 30-day perioperative outcomes for major inpatient and outpatient surgical procedures at more than 700 hospitals.
They extracted all minimally invasive partial and radical nephrectomies from 2012 to 2019 and refined the cohort to 28,140 patients who were theoretically eligible for same-day discharge: Of those, 237 (0.8%) had SSD, and 27,903 (99.2%) had a standard-length discharge (SLD).
The team found that there were no differences in 30-day complications or readmissions between same-day discharge (Clavien-Dindo [CD] I/II, 4.22%; CD III, 0%; CD IV, 1.27%; readmission, 4.64%); and SLD (CD I/II, 4.11%; CD III, 0.95%; CD IV, 0.79%; readmission, 3.90%; all P > .05).
Controlling for demographic and clinical variables, SDD was not associated with greater risk of 30-day complications or readmissions (CD I/II: odds ratio, 1.08; 95% confidence interval, 0.57-2.048; P = .813; CD IV: OR 1.699; 95% CI, 0.537-5.375; P = .367; readmission: OR, 1.254; 95% CI, 0.681-2.31; P = .467).
Mr. Garden and coauthors report no relevant financial relationships.
Dr. Lane reports no relevant financial relationships.
Urologist Ronney Abaza, MD, a robotic surgery specialist in Dublin, Ohio, and colleagues, reviewed robotic surgeries at their hospital during COVID-19 restrictions on surgery in Ohio between March 17 and June 5, 2020, and compared them with robotic procedures before COVID-19 and after restrictions were lifted. They published their results in Urology.
Since 2016, the hospital has offered the option of same-day discharge (SDD) to all robotic urologic surgery patients, regardless of procedure or patient-specific factors.
Among patients who had surgery during COVID-19 restrictions, 98% (87/89 patients) opted for SDD versus 52% in the group having surgery before the restrictions (P < .00001). After the COVID-19 surgery restrictions were lifted, the higher rate of SDD remained at 98%.
“There were no differences in 30-day complications or readmissions between SDD and overnight patients,” the authors write.
The right patient, the right motivation for successful surgery
Brian Lane, MD, PhD, a urologic oncologist with Spectrum Health in Grand Rapids, Michigan, told this news organization that, for nephrectomies, uptake of same-day discharge will continue to be slow.
“You have to have the right patient, the right patient motivation, and the surgery has to go smoothly,” he said. “If you start sending everyone home the same day, you will certainly see readmissions,” he said.
Dr. Lane is part of the Michigan Urologic Surgery Improvement Collaborative and he said the group recently looked at same-day discharge outcomes after robotic prostatectomies with SDD as compared with 1-2 nights in the hospital.
The work has not yet been published but, “There was a slight signal that there were increased readmissions with same-day discharge vs. 0-1 day,” he said.
A paper on outcomes of same-day discharge in total knee arthroplasty in the Journal of Bone & Joint Surgery found a higher risk of perioperative complications “including component failure, surgical site infection, knee stiffness, and deep vein thrombosis.” Researchers compared outcomes between 4,391 patients who underwent outpatient TKA and 128,951 patients who underwent inpatient TKA.
But for other many surgeries, same-day discharge numbers are increasing without worsening outcomes.
A paper in the Journal of Robotic Surgery found that same-day discharge following robotic-assisted endometrial cancer staging is “safe and feasible.”
Stephen Bradley, MD, MPH, with the Minneapolis Heart Institute in Minneapolis, and colleagues write in the Journal of the American College of Cardiology: Cardiovascular Interventions that they found a large increase in the use of same-day discharge after elective percutaneous coronary intervention (PCI) was not associated with worse 30-day mortality rates or readmission.
In that study, 114,461 patients were discharged the same day they underwent PCI. The proportion of patients who had a same-day discharge increased from 4.5% in 2009 to 28.6% in the fourth quarter of 2017.
Risk-adjusted 30-day mortality did not change in that time, while risk-adjusted rehospitalization decreased over time and more quickly when patients had same-day discharge.
Deepak L. Bhatt, MD, MPH, and Jonathan G. Sung, MBCHB, both of Brigham and Women’s Hospital Heart & Vascular Center, Harvard Medical School, Boston, wrote in an accompanying article that, “Advances in the devices and techniques of PCI have improved the safety and efficacy of the procedure. In selected patients, same-day discharge has become possible, and overnight in-hospital observation can be avoided. By reducing unnecessary hospital stays, both patients and hospitals could benefit.”
Evan Garden, a medical student at Icahn School of Medicine at Mount Sinai in New York, presented findings at the American Urological Association 2021 annual meeting that show patients selected for same-day discharge after partial or radical nephrectomy did not have increased rates of postoperative complications or readmissions in the immediate postoperative period, compared with standard discharge of 1-3 days.
Case studies in nephrectomy
While several case studies have looked at the feasibility and safety of performing partial and radical nephrectomy with same-day discharge in select cases, “this topic has not been addressed on a national level,” Mr. Garden said.
Few patients who have partial or radical nephrectomies have same-day discharges. The researchers found that fewer than 1% of patients who have either procedure in the sample studied were discharged the same day.
Researchers used the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database, a nationally representative deidentified database that prospectively tracks patient characteristics and 30-day perioperative outcomes for major inpatient and outpatient surgical procedures at more than 700 hospitals.
They extracted all minimally invasive partial and radical nephrectomies from 2012 to 2019 and refined the cohort to 28,140 patients who were theoretically eligible for same-day discharge: Of those, 237 (0.8%) had SSD, and 27,903 (99.2%) had a standard-length discharge (SLD).
The team found that there were no differences in 30-day complications or readmissions between same-day discharge (Clavien-Dindo [CD] I/II, 4.22%; CD III, 0%; CD IV, 1.27%; readmission, 4.64%); and SLD (CD I/II, 4.11%; CD III, 0.95%; CD IV, 0.79%; readmission, 3.90%; all P > .05).
Controlling for demographic and clinical variables, SDD was not associated with greater risk of 30-day complications or readmissions (CD I/II: odds ratio, 1.08; 95% confidence interval, 0.57-2.048; P = .813; CD IV: OR 1.699; 95% CI, 0.537-5.375; P = .367; readmission: OR, 1.254; 95% CI, 0.681-2.31; P = .467).
Mr. Garden and coauthors report no relevant financial relationships.
Dr. Lane reports no relevant financial relationships.
Sunscreen, other sun-protective habits not linked with poorer bone health, fractures
Using , according to a new study that included more than 3,000 men and women.
“We have objective data for the first time, and in a large-scale representative population of the U.S. adults, to indicate sun protection is not associated with negative bone-related outcomes,” said study lead author Mohsen Afarideh, MD, MPH, a postdoctoral research fellow at the autoimmune skin diseases unit at the University of Pennsylvania, Philadelphia.
The study, published online in JAMA Dermatology, goes a step further than previous research by others that has found sunscreen use does not compromise vitamin D synthesis and has little effect on circulating 25-hydroxyvitamin D levels.
In the new study, researchers looked at three sun-protective behaviors – sunscreen use, staying in the shade, wearing long sleeves – and their effects on bone mineral density and the risk of fractures.
While the effects of sun-protective habits on blood levels of vitamin D and BMD scores are important, ‘’what we are more interested to know is if the sun-protective behaviors actually cause or increase the risk of fracture,” Dr. Afarideh said in an interview. “The answer to that is a firm ‘No.’ These data are very reassuring and will help clinicians to keep recommending sun protection to the public.”
Study details
Dr. Afarideh and his colleagues from the Mayo Clinic in Rochester, Minn., looked at data from the National Health and Nutrition Examination Survey (NHANES) from 2017 to 2018, obtaining final information on 3,403 men and women, ages 20-59, who completed a dermatology questionnaire The men and women reported on the three sun-protective habits, and noted whether they followed these practices always or most of the time, sometimes, or never or rarely.
The frequency of the three behaviors was not widespread. Frequent staying in the shade was reported by 31.6% of the sample, wearing long sleeves by 11.8%, and sunscreen use by 26.1%.
The researchers also had data on the participants’ bone mineral density (BMD) scores along with dietary information such as milk consumption, vitamin D supplement use, taking steroid drugs, and exercise activity.
“Moderate sunscreen use was linked with a slightly lower lumbar BMD score,” Dr. Afarideh said, which was “the only significant association that could be interpreted as concerning.” And this was more likely to be seen in older respondents, he said.
However, otherwise they found the practice of the three behaviors was not associated with lower total or site-specific BMD z scores, nor was it linked with an increased risk of osteoporotic fractures. (The BMD z score compares an individual’s bone density to the average bone density of someone their same age and gender.)
The focus on fracture risk is the more important outcome, Dr. Afarideh said. And they found no increased risk overall of osteoporotic fractures in those who practiced sun-protective behaviors.
Moderate to frequent staying in the shade was actually linked with a reduced prevalence of spine fractures in the multivariate model (odds ratio, 0.19; 95% confidence interval, 0.04-0.86, P = .02). The researchers say that may be attributable to these respondents also being careful in other areas of life, such as avoiding falls and not participating in high-risk activities that would increase the chance of fractures. “However, this is just an assumption,” Dr. Afarideh said.
Expert perspectives
Other dermatologists not involved in the new research said the study results provide some “real-world” information that’s valuable for clinicians to share with patients.
“I think this is an important study on multiple levels,” said Henry W. Lim, MD, a former president of the American Academy of Dermatology who is a member of the department of dermatology and senior vice president of academic affairs at Henry Ford Health System, Detroit. “It is a well-done study, involving a large number. It is a real-life situation, asking people their photo protective behaviors and then looking at their bone mineral density.” The bottom line, he said: “Bone health is not affected by photo protection habits in real life.”
The findings are important but not surprising, said Antony R. Young, PhD, emeritus professor of experimental photobiology at St. John’s Institute of Dermatology, King’s College, London, who has researched sunscreens and vitamin D status. “My study showed that correct sunscreen use, albeit with a relatively low SPF of 15, did prevent sunburn in a high UVR [ultraviolet radiation] environment but did allow very good vitamin D synthesis. I think this is because the necessary dose of UVB is very low.”
Michele Green, MD, a New York dermatologist and clinical staff member at Lenox Hill Hospital there, said she often hears concerns about bone health from patients. “Every week, patients ask, ‘Why would I wear sunblock? Don’t I need sun for bone health? Don’t I need it for vitamin D?’’’
Now, she said, ‘’Dermatologists can point to the study and say ‘Don’t worry.’ It clarifies that using sunscreen won’t cause you to have osteoporosis.’’
Dr. Afarideh, who was a postdoctoral research fellow at the Mayo Clinic, and his coauthors, Megha M. Tollefson, MD, and Julio C. Sartori-Valinotti, of the Mayo Clinic, and Dr. Green had no disclosures. Dr. Lim and Dr. Young consult for the sunscreen industry.
Using , according to a new study that included more than 3,000 men and women.
“We have objective data for the first time, and in a large-scale representative population of the U.S. adults, to indicate sun protection is not associated with negative bone-related outcomes,” said study lead author Mohsen Afarideh, MD, MPH, a postdoctoral research fellow at the autoimmune skin diseases unit at the University of Pennsylvania, Philadelphia.
The study, published online in JAMA Dermatology, goes a step further than previous research by others that has found sunscreen use does not compromise vitamin D synthesis and has little effect on circulating 25-hydroxyvitamin D levels.
In the new study, researchers looked at three sun-protective behaviors – sunscreen use, staying in the shade, wearing long sleeves – and their effects on bone mineral density and the risk of fractures.
While the effects of sun-protective habits on blood levels of vitamin D and BMD scores are important, ‘’what we are more interested to know is if the sun-protective behaviors actually cause or increase the risk of fracture,” Dr. Afarideh said in an interview. “The answer to that is a firm ‘No.’ These data are very reassuring and will help clinicians to keep recommending sun protection to the public.”
Study details
Dr. Afarideh and his colleagues from the Mayo Clinic in Rochester, Minn., looked at data from the National Health and Nutrition Examination Survey (NHANES) from 2017 to 2018, obtaining final information on 3,403 men and women, ages 20-59, who completed a dermatology questionnaire The men and women reported on the three sun-protective habits, and noted whether they followed these practices always or most of the time, sometimes, or never or rarely.
The frequency of the three behaviors was not widespread. Frequent staying in the shade was reported by 31.6% of the sample, wearing long sleeves by 11.8%, and sunscreen use by 26.1%.
The researchers also had data on the participants’ bone mineral density (BMD) scores along with dietary information such as milk consumption, vitamin D supplement use, taking steroid drugs, and exercise activity.
“Moderate sunscreen use was linked with a slightly lower lumbar BMD score,” Dr. Afarideh said, which was “the only significant association that could be interpreted as concerning.” And this was more likely to be seen in older respondents, he said.
However, otherwise they found the practice of the three behaviors was not associated with lower total or site-specific BMD z scores, nor was it linked with an increased risk of osteoporotic fractures. (The BMD z score compares an individual’s bone density to the average bone density of someone their same age and gender.)
The focus on fracture risk is the more important outcome, Dr. Afarideh said. And they found no increased risk overall of osteoporotic fractures in those who practiced sun-protective behaviors.
Moderate to frequent staying in the shade was actually linked with a reduced prevalence of spine fractures in the multivariate model (odds ratio, 0.19; 95% confidence interval, 0.04-0.86, P = .02). The researchers say that may be attributable to these respondents also being careful in other areas of life, such as avoiding falls and not participating in high-risk activities that would increase the chance of fractures. “However, this is just an assumption,” Dr. Afarideh said.
Expert perspectives
Other dermatologists not involved in the new research said the study results provide some “real-world” information that’s valuable for clinicians to share with patients.
“I think this is an important study on multiple levels,” said Henry W. Lim, MD, a former president of the American Academy of Dermatology who is a member of the department of dermatology and senior vice president of academic affairs at Henry Ford Health System, Detroit. “It is a well-done study, involving a large number. It is a real-life situation, asking people their photo protective behaviors and then looking at their bone mineral density.” The bottom line, he said: “Bone health is not affected by photo protection habits in real life.”
The findings are important but not surprising, said Antony R. Young, PhD, emeritus professor of experimental photobiology at St. John’s Institute of Dermatology, King’s College, London, who has researched sunscreens and vitamin D status. “My study showed that correct sunscreen use, albeit with a relatively low SPF of 15, did prevent sunburn in a high UVR [ultraviolet radiation] environment but did allow very good vitamin D synthesis. I think this is because the necessary dose of UVB is very low.”
Michele Green, MD, a New York dermatologist and clinical staff member at Lenox Hill Hospital there, said she often hears concerns about bone health from patients. “Every week, patients ask, ‘Why would I wear sunblock? Don’t I need sun for bone health? Don’t I need it for vitamin D?’’’
Now, she said, ‘’Dermatologists can point to the study and say ‘Don’t worry.’ It clarifies that using sunscreen won’t cause you to have osteoporosis.’’
Dr. Afarideh, who was a postdoctoral research fellow at the Mayo Clinic, and his coauthors, Megha M. Tollefson, MD, and Julio C. Sartori-Valinotti, of the Mayo Clinic, and Dr. Green had no disclosures. Dr. Lim and Dr. Young consult for the sunscreen industry.
Using , according to a new study that included more than 3,000 men and women.
“We have objective data for the first time, and in a large-scale representative population of the U.S. adults, to indicate sun protection is not associated with negative bone-related outcomes,” said study lead author Mohsen Afarideh, MD, MPH, a postdoctoral research fellow at the autoimmune skin diseases unit at the University of Pennsylvania, Philadelphia.
The study, published online in JAMA Dermatology, goes a step further than previous research by others that has found sunscreen use does not compromise vitamin D synthesis and has little effect on circulating 25-hydroxyvitamin D levels.
In the new study, researchers looked at three sun-protective behaviors – sunscreen use, staying in the shade, wearing long sleeves – and their effects on bone mineral density and the risk of fractures.
While the effects of sun-protective habits on blood levels of vitamin D and BMD scores are important, ‘’what we are more interested to know is if the sun-protective behaviors actually cause or increase the risk of fracture,” Dr. Afarideh said in an interview. “The answer to that is a firm ‘No.’ These data are very reassuring and will help clinicians to keep recommending sun protection to the public.”
Study details
Dr. Afarideh and his colleagues from the Mayo Clinic in Rochester, Minn., looked at data from the National Health and Nutrition Examination Survey (NHANES) from 2017 to 2018, obtaining final information on 3,403 men and women, ages 20-59, who completed a dermatology questionnaire The men and women reported on the three sun-protective habits, and noted whether they followed these practices always or most of the time, sometimes, or never or rarely.
The frequency of the three behaviors was not widespread. Frequent staying in the shade was reported by 31.6% of the sample, wearing long sleeves by 11.8%, and sunscreen use by 26.1%.
The researchers also had data on the participants’ bone mineral density (BMD) scores along with dietary information such as milk consumption, vitamin D supplement use, taking steroid drugs, and exercise activity.
“Moderate sunscreen use was linked with a slightly lower lumbar BMD score,” Dr. Afarideh said, which was “the only significant association that could be interpreted as concerning.” And this was more likely to be seen in older respondents, he said.
However, otherwise they found the practice of the three behaviors was not associated with lower total or site-specific BMD z scores, nor was it linked with an increased risk of osteoporotic fractures. (The BMD z score compares an individual’s bone density to the average bone density of someone their same age and gender.)
The focus on fracture risk is the more important outcome, Dr. Afarideh said. And they found no increased risk overall of osteoporotic fractures in those who practiced sun-protective behaviors.
Moderate to frequent staying in the shade was actually linked with a reduced prevalence of spine fractures in the multivariate model (odds ratio, 0.19; 95% confidence interval, 0.04-0.86, P = .02). The researchers say that may be attributable to these respondents also being careful in other areas of life, such as avoiding falls and not participating in high-risk activities that would increase the chance of fractures. “However, this is just an assumption,” Dr. Afarideh said.
Expert perspectives
Other dermatologists not involved in the new research said the study results provide some “real-world” information that’s valuable for clinicians to share with patients.
“I think this is an important study on multiple levels,” said Henry W. Lim, MD, a former president of the American Academy of Dermatology who is a member of the department of dermatology and senior vice president of academic affairs at Henry Ford Health System, Detroit. “It is a well-done study, involving a large number. It is a real-life situation, asking people their photo protective behaviors and then looking at their bone mineral density.” The bottom line, he said: “Bone health is not affected by photo protection habits in real life.”
The findings are important but not surprising, said Antony R. Young, PhD, emeritus professor of experimental photobiology at St. John’s Institute of Dermatology, King’s College, London, who has researched sunscreens and vitamin D status. “My study showed that correct sunscreen use, albeit with a relatively low SPF of 15, did prevent sunburn in a high UVR [ultraviolet radiation] environment but did allow very good vitamin D synthesis. I think this is because the necessary dose of UVB is very low.”
Michele Green, MD, a New York dermatologist and clinical staff member at Lenox Hill Hospital there, said she often hears concerns about bone health from patients. “Every week, patients ask, ‘Why would I wear sunblock? Don’t I need sun for bone health? Don’t I need it for vitamin D?’’’
Now, she said, ‘’Dermatologists can point to the study and say ‘Don’t worry.’ It clarifies that using sunscreen won’t cause you to have osteoporosis.’’
Dr. Afarideh, who was a postdoctoral research fellow at the Mayo Clinic, and his coauthors, Megha M. Tollefson, MD, and Julio C. Sartori-Valinotti, of the Mayo Clinic, and Dr. Green had no disclosures. Dr. Lim and Dr. Young consult for the sunscreen industry.
FROM JAMA DERMATOLOGY
Some diuretics tied to increased skin cancer risk
The findings were originally reported in two Danish case-control studies in which physicians reported a fourfold increased risk of squamous cell carcinoma, and a moderate increased risk of basal cell carcinoma and cutaneous malignant melanoma in patients who used hydrochlorothiazide long-term.
And, while the new study did not find an increased risk of basal cell carcinoma and cutaneous malignant melanoma among long-term users of hydrochlorothiazide, they suggest that bendroflumethiazide “may be a safer alternative for patients at increased risk of skin cancer.” The long-term use of indapamide was associated with a moderately increased risk of cutaneous malignant melanoma but did not alter the risk of either squamous cell or basal cell carcinoma
“Our results suggest that bendroflumethiazide may be a safer alternative to hydrochlorothiazide and indapamide, especially for patients at increased risk of skin cancer, but future studies are needed to rule out a causal association between bendroflumethiazide and cutaneous malignant melanoma,” wrote authors who were led by Christoph R. Meier, PhD, a professor in pharmacy with University Hospital Basel (Switzerland) and a contributor to the Boston Collaborative Drug Surveillance Program.
This study adds to existing evidence that there is a dose-dependent increased risk of squamous cell carcinoma in users of high cumulative doses of hydrochlorothiazide, compared with non–hydrochlorothiazide users.
The study, an observational cohort study, was published earlier this year. It is based on data from the U.K.-based Clinical Practice Research Datalink. It included 271,154 new users of thiazides and thiazidelike diuretics, the majority at 87.6% having been prescribed bendroflumethiazide, 5.8% indapamide, and 3.6% hydrochlorothiazide. Outcomes were compared to those observed in 275,263 users of calcium channel blockers.
“The three primary outcomes of interest were a first-time diagnosis of cutaneous malignant melanoma, basal cell carcinoma, or squamous cell carcinoma,” the authors wrote.
Incidence rates and incidence rate ratios were estimated for both short-term and long-term users of thiazidelike diuretics and calcium channel blockers, while a propensity score (PS) analysis was done in order to control for 23 baseline covariates. The mean follow-up after PS weighting was 3.9 years for indapamide users and 5.5 years for hydrochlorothiazide users. Overall, the incidence rate ratios of squamous cell carcinoma were not markedly increased for either short-term or long-term users of thiazidelike diuretics, the authors reported.
In contrast, the incidence rate ratios of squamous cell carcinoma for hydrochlorothiazide users were increased by 29% for short-term users at an IRR of 1.29 while they were increased by almost twofold for long-term hydrochlorothiazide users at an IRR of 1.95.
Long-term use of hydrochlorothiazide was again associated with a 64% increased risk of basal cell carcinoma, compared with users of a renin-angiotensin inhibitor at a weighted IRR of 1.64.
In contrast, weighted incident rate ratios for basal cell carcinoma for both short-term and long-term thiazide users were not significantly different and results were similar for patients who took hydrochlorothiazide, indapamide, or bendroflumethiazide.
Weighted overall incident rate ratios for cutaneous malignant melanoma were not significantly different for either short-term or long-term users of thiazidelike diuretics, compared with calcium channel blocker users.
However, there was a 43% increased risk of cutaneous malignant melanoma among long-term indapamide users at a weighted IRR of 1.43, compared with calcium channel blocker users, the authors reported.
“Given the biological plausibility and the severe clinical implications of cutaneous malignant melanoma, this finding should be considered carefully,” they cautioned.
Limitations to the study include the fact that the database analyzed does not have information on sun exposure, skin characteristics, or socioeconomic status which may affect the amount of sun exposure participants received.
The authors had no conflicts of interest to declare.
The findings were originally reported in two Danish case-control studies in which physicians reported a fourfold increased risk of squamous cell carcinoma, and a moderate increased risk of basal cell carcinoma and cutaneous malignant melanoma in patients who used hydrochlorothiazide long-term.
And, while the new study did not find an increased risk of basal cell carcinoma and cutaneous malignant melanoma among long-term users of hydrochlorothiazide, they suggest that bendroflumethiazide “may be a safer alternative for patients at increased risk of skin cancer.” The long-term use of indapamide was associated with a moderately increased risk of cutaneous malignant melanoma but did not alter the risk of either squamous cell or basal cell carcinoma
“Our results suggest that bendroflumethiazide may be a safer alternative to hydrochlorothiazide and indapamide, especially for patients at increased risk of skin cancer, but future studies are needed to rule out a causal association between bendroflumethiazide and cutaneous malignant melanoma,” wrote authors who were led by Christoph R. Meier, PhD, a professor in pharmacy with University Hospital Basel (Switzerland) and a contributor to the Boston Collaborative Drug Surveillance Program.
This study adds to existing evidence that there is a dose-dependent increased risk of squamous cell carcinoma in users of high cumulative doses of hydrochlorothiazide, compared with non–hydrochlorothiazide users.
The study, an observational cohort study, was published earlier this year. It is based on data from the U.K.-based Clinical Practice Research Datalink. It included 271,154 new users of thiazides and thiazidelike diuretics, the majority at 87.6% having been prescribed bendroflumethiazide, 5.8% indapamide, and 3.6% hydrochlorothiazide. Outcomes were compared to those observed in 275,263 users of calcium channel blockers.
“The three primary outcomes of interest were a first-time diagnosis of cutaneous malignant melanoma, basal cell carcinoma, or squamous cell carcinoma,” the authors wrote.
Incidence rates and incidence rate ratios were estimated for both short-term and long-term users of thiazidelike diuretics and calcium channel blockers, while a propensity score (PS) analysis was done in order to control for 23 baseline covariates. The mean follow-up after PS weighting was 3.9 years for indapamide users and 5.5 years for hydrochlorothiazide users. Overall, the incidence rate ratios of squamous cell carcinoma were not markedly increased for either short-term or long-term users of thiazidelike diuretics, the authors reported.
In contrast, the incidence rate ratios of squamous cell carcinoma for hydrochlorothiazide users were increased by 29% for short-term users at an IRR of 1.29 while they were increased by almost twofold for long-term hydrochlorothiazide users at an IRR of 1.95.
Long-term use of hydrochlorothiazide was again associated with a 64% increased risk of basal cell carcinoma, compared with users of a renin-angiotensin inhibitor at a weighted IRR of 1.64.
In contrast, weighted incident rate ratios for basal cell carcinoma for both short-term and long-term thiazide users were not significantly different and results were similar for patients who took hydrochlorothiazide, indapamide, or bendroflumethiazide.
Weighted overall incident rate ratios for cutaneous malignant melanoma were not significantly different for either short-term or long-term users of thiazidelike diuretics, compared with calcium channel blocker users.
However, there was a 43% increased risk of cutaneous malignant melanoma among long-term indapamide users at a weighted IRR of 1.43, compared with calcium channel blocker users, the authors reported.
“Given the biological plausibility and the severe clinical implications of cutaneous malignant melanoma, this finding should be considered carefully,” they cautioned.
Limitations to the study include the fact that the database analyzed does not have information on sun exposure, skin characteristics, or socioeconomic status which may affect the amount of sun exposure participants received.
The authors had no conflicts of interest to declare.
The findings were originally reported in two Danish case-control studies in which physicians reported a fourfold increased risk of squamous cell carcinoma, and a moderate increased risk of basal cell carcinoma and cutaneous malignant melanoma in patients who used hydrochlorothiazide long-term.
And, while the new study did not find an increased risk of basal cell carcinoma and cutaneous malignant melanoma among long-term users of hydrochlorothiazide, they suggest that bendroflumethiazide “may be a safer alternative for patients at increased risk of skin cancer.” The long-term use of indapamide was associated with a moderately increased risk of cutaneous malignant melanoma but did not alter the risk of either squamous cell or basal cell carcinoma
“Our results suggest that bendroflumethiazide may be a safer alternative to hydrochlorothiazide and indapamide, especially for patients at increased risk of skin cancer, but future studies are needed to rule out a causal association between bendroflumethiazide and cutaneous malignant melanoma,” wrote authors who were led by Christoph R. Meier, PhD, a professor in pharmacy with University Hospital Basel (Switzerland) and a contributor to the Boston Collaborative Drug Surveillance Program.
This study adds to existing evidence that there is a dose-dependent increased risk of squamous cell carcinoma in users of high cumulative doses of hydrochlorothiazide, compared with non–hydrochlorothiazide users.
The study, an observational cohort study, was published earlier this year. It is based on data from the U.K.-based Clinical Practice Research Datalink. It included 271,154 new users of thiazides and thiazidelike diuretics, the majority at 87.6% having been prescribed bendroflumethiazide, 5.8% indapamide, and 3.6% hydrochlorothiazide. Outcomes were compared to those observed in 275,263 users of calcium channel blockers.
“The three primary outcomes of interest were a first-time diagnosis of cutaneous malignant melanoma, basal cell carcinoma, or squamous cell carcinoma,” the authors wrote.
Incidence rates and incidence rate ratios were estimated for both short-term and long-term users of thiazidelike diuretics and calcium channel blockers, while a propensity score (PS) analysis was done in order to control for 23 baseline covariates. The mean follow-up after PS weighting was 3.9 years for indapamide users and 5.5 years for hydrochlorothiazide users. Overall, the incidence rate ratios of squamous cell carcinoma were not markedly increased for either short-term or long-term users of thiazidelike diuretics, the authors reported.
In contrast, the incidence rate ratios of squamous cell carcinoma for hydrochlorothiazide users were increased by 29% for short-term users at an IRR of 1.29 while they were increased by almost twofold for long-term hydrochlorothiazide users at an IRR of 1.95.
Long-term use of hydrochlorothiazide was again associated with a 64% increased risk of basal cell carcinoma, compared with users of a renin-angiotensin inhibitor at a weighted IRR of 1.64.
In contrast, weighted incident rate ratios for basal cell carcinoma for both short-term and long-term thiazide users were not significantly different and results were similar for patients who took hydrochlorothiazide, indapamide, or bendroflumethiazide.
Weighted overall incident rate ratios for cutaneous malignant melanoma were not significantly different for either short-term or long-term users of thiazidelike diuretics, compared with calcium channel blocker users.
However, there was a 43% increased risk of cutaneous malignant melanoma among long-term indapamide users at a weighted IRR of 1.43, compared with calcium channel blocker users, the authors reported.
“Given the biological plausibility and the severe clinical implications of cutaneous malignant melanoma, this finding should be considered carefully,” they cautioned.
Limitations to the study include the fact that the database analyzed does not have information on sun exposure, skin characteristics, or socioeconomic status which may affect the amount of sun exposure participants received.
The authors had no conflicts of interest to declare.
FROM BRITISH JOURNAL OF DERMATOLOGY