User login
No increase in autism risk with prenatal Tdap
A retrospective cohort study in more than 80,000 children has found no evidence of an increased risk of autism spectrum disorder associated with prenatal tetanus, diphtheria, and acellular pertussis (Tdap) immunization.
Of 81,993 children born between 2011 and 2014, 1,341 children (1.6%) were diagnosed with autism spectrum disorder. The incidence of autism spectrum disorder was 3.78 per 1,000 person-years in the Tdap-vaccinated group, and 4.05 per 1,000 person years in the unvaccinated group, representing an unadjusted hazard ratio of 0.98 and an adjusted hazard ratio of 0.85. This was consistent across all the birth cohorts.
Prenatal immunization rates with the prenatal Tdap vaccine ranged from 26% of the 2012 birth cohort to 79% of the 2014 birth cohort, and mean gestational age at vaccination was 28 weeks.
Tracy A. Becerra-Culqui, PhD, MPH, and colleagues of the department of research and evaluation at Kaiser Permanente Southern California, Pasadena, said this was the first study to look at the risk of autism spectrum disorder after maternal exposure to the Tdap vaccine, to their knowledge. “Our results potentially indicate that the maternal Tdap vaccine affects immune trajectories protecting infants against infections that would otherwise lead to neurodevelopmental alterations.”
They highlighted several strengths of their study. One was that maternal Tdap vaccination and information on autism spectrum disorder both were derived from EHRs and therefore not subject to recall bias. The study, published online in Pediatrics, also included children diagnosed with autism spectrum disorder from age 1 year onwards, reflecting the latest evidence on screening and diagnosis of autism.
“Our weighting procedures enabled us to balance the Tdap-exposed and -unexposed groups to compare two populations that were comparable in important measured confounding factors,” Dr. Becerra-Culqui and associates noted.
The investigators found that women who received the Tdap vaccine during pregnancy were more likely to be Asian American or Pacific Islander, to have a bachelor’s degree or higher, be nulliparous, to have also been vaccinated prenatally against influenza, and to deliver at term, compared with unvaccinated women.
However the authors did note that their follow-up was limited to 6.5 years for the earliest birth cohort, and 3.5 years for the latest cohort, so they may not have picked up children who received a later diagnosis of autism spectrum disorder.
The study was supported by Kaiser Permanente Southern California. Five authors declared funding from GlaxoSmithKline, Bayer AG, or the Centers for Disease Control and Prevention for unrelated or separate studies.
SOURCE: Becerra-Culqui T et al. Pediatrics. 2018;142(3):e20180120.
A retrospective cohort study in more than 80,000 children has found no evidence of an increased risk of autism spectrum disorder associated with prenatal tetanus, diphtheria, and acellular pertussis (Tdap) immunization.
Of 81,993 children born between 2011 and 2014, 1,341 children (1.6%) were diagnosed with autism spectrum disorder. The incidence of autism spectrum disorder was 3.78 per 1,000 person-years in the Tdap-vaccinated group, and 4.05 per 1,000 person years in the unvaccinated group, representing an unadjusted hazard ratio of 0.98 and an adjusted hazard ratio of 0.85. This was consistent across all the birth cohorts.
Prenatal immunization rates with the prenatal Tdap vaccine ranged from 26% of the 2012 birth cohort to 79% of the 2014 birth cohort, and mean gestational age at vaccination was 28 weeks.
Tracy A. Becerra-Culqui, PhD, MPH, and colleagues of the department of research and evaluation at Kaiser Permanente Southern California, Pasadena, said this was the first study to look at the risk of autism spectrum disorder after maternal exposure to the Tdap vaccine, to their knowledge. “Our results potentially indicate that the maternal Tdap vaccine affects immune trajectories protecting infants against infections that would otherwise lead to neurodevelopmental alterations.”
They highlighted several strengths of their study. One was that maternal Tdap vaccination and information on autism spectrum disorder both were derived from EHRs and therefore not subject to recall bias. The study, published online in Pediatrics, also included children diagnosed with autism spectrum disorder from age 1 year onwards, reflecting the latest evidence on screening and diagnosis of autism.
“Our weighting procedures enabled us to balance the Tdap-exposed and -unexposed groups to compare two populations that were comparable in important measured confounding factors,” Dr. Becerra-Culqui and associates noted.
The investigators found that women who received the Tdap vaccine during pregnancy were more likely to be Asian American or Pacific Islander, to have a bachelor’s degree or higher, be nulliparous, to have also been vaccinated prenatally against influenza, and to deliver at term, compared with unvaccinated women.
However the authors did note that their follow-up was limited to 6.5 years for the earliest birth cohort, and 3.5 years for the latest cohort, so they may not have picked up children who received a later diagnosis of autism spectrum disorder.
The study was supported by Kaiser Permanente Southern California. Five authors declared funding from GlaxoSmithKline, Bayer AG, or the Centers for Disease Control and Prevention for unrelated or separate studies.
SOURCE: Becerra-Culqui T et al. Pediatrics. 2018;142(3):e20180120.
A retrospective cohort study in more than 80,000 children has found no evidence of an increased risk of autism spectrum disorder associated with prenatal tetanus, diphtheria, and acellular pertussis (Tdap) immunization.
Of 81,993 children born between 2011 and 2014, 1,341 children (1.6%) were diagnosed with autism spectrum disorder. The incidence of autism spectrum disorder was 3.78 per 1,000 person-years in the Tdap-vaccinated group, and 4.05 per 1,000 person years in the unvaccinated group, representing an unadjusted hazard ratio of 0.98 and an adjusted hazard ratio of 0.85. This was consistent across all the birth cohorts.
Prenatal immunization rates with the prenatal Tdap vaccine ranged from 26% of the 2012 birth cohort to 79% of the 2014 birth cohort, and mean gestational age at vaccination was 28 weeks.
Tracy A. Becerra-Culqui, PhD, MPH, and colleagues of the department of research and evaluation at Kaiser Permanente Southern California, Pasadena, said this was the first study to look at the risk of autism spectrum disorder after maternal exposure to the Tdap vaccine, to their knowledge. “Our results potentially indicate that the maternal Tdap vaccine affects immune trajectories protecting infants against infections that would otherwise lead to neurodevelopmental alterations.”
They highlighted several strengths of their study. One was that maternal Tdap vaccination and information on autism spectrum disorder both were derived from EHRs and therefore not subject to recall bias. The study, published online in Pediatrics, also included children diagnosed with autism spectrum disorder from age 1 year onwards, reflecting the latest evidence on screening and diagnosis of autism.
“Our weighting procedures enabled us to balance the Tdap-exposed and -unexposed groups to compare two populations that were comparable in important measured confounding factors,” Dr. Becerra-Culqui and associates noted.
The investigators found that women who received the Tdap vaccine during pregnancy were more likely to be Asian American or Pacific Islander, to have a bachelor’s degree or higher, be nulliparous, to have also been vaccinated prenatally against influenza, and to deliver at term, compared with unvaccinated women.
However the authors did note that their follow-up was limited to 6.5 years for the earliest birth cohort, and 3.5 years for the latest cohort, so they may not have picked up children who received a later diagnosis of autism spectrum disorder.
The study was supported by Kaiser Permanente Southern California. Five authors declared funding from GlaxoSmithKline, Bayer AG, or the Centers for Disease Control and Prevention for unrelated or separate studies.
SOURCE: Becerra-Culqui T et al. Pediatrics. 2018;142(3):e20180120.
FROM PEDIATRICS
Key clinical point:
Major finding: The adjusted hazard ratio for autism spectrum disorder in children exposed to the prenatal Tdap vaccine is 0.98, compared with unvaccinated children.
Study details: A retrospective cohort study in 81,993 children exposed to the prenatal Tdap vaccine.
Disclosures: The study was supported by Kaiser Permanente Southern California. Five authors declared funding from GlaxoSmithKline, Bayer AG, or the Centers for Disease Control and Prevention for unrelated or separate studies.
Source: Becerra-Culqui T et al. Pediatrics. 2018;142(3):e20180120.
More deliveries now include opioid use disorder
according to the Centers for Disease Control and Prevention.
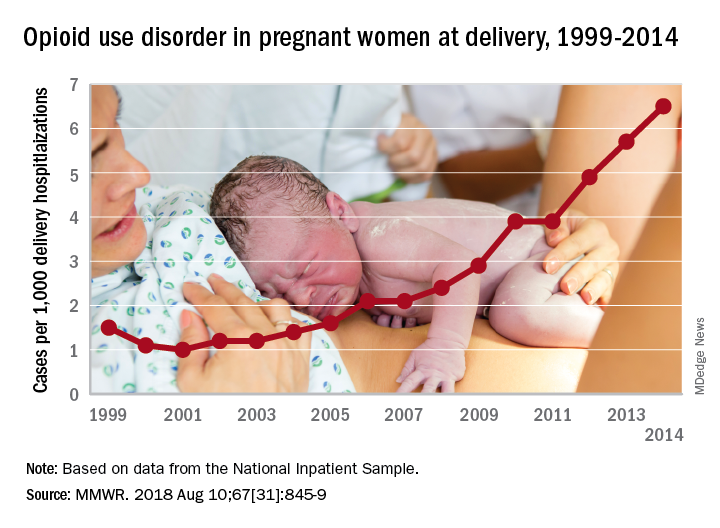
The national prevalence of opioid use disorder increased by 333% as it went from 1.5 cases per 1,000 delivery hospitalizations in 1999 to 6.5 cases per 1,000 in 2014. At the state level, there were significant increases in all 28 states with data available for at least 3 consecutive years during the study period, Sarah C. Haight, MPH, and her associates at the CDC in Atlanta said in the Morbidity and Mortality Weekly Report.
Average annual rate changes for those states ranged from a low of 0.01 per 1,000 delivery hospitalizations per year in California to 5.37 per year in Vermont, with the national rate change coming in at 0.39 per year. Of the 14 states with data available in 1999, Iowa had the lowest rate at 0.1 per 1,000 deliveries and Maryland had the highest at 8.2. In 2014, when data were available for 26 states and the District of Columbia, the highest rate was Vermont’s 48.6 per 1,000 deliveries and the lowest was 0.7 in Washington, D.C., the investigators reported.
Although “increasing trends might represent actual increases in prevalence or improved screening and diagnosis,” Ms. Haight and her associates added that “these estimates also correlate with state opioid prescribing rates in the general population. West Virginia, for example, had a prescribing rate estimated at 138 opioid prescriptions per 100 persons in 2012.”
“These findings illustrate the devastating impact of the opioid epidemic on families across the U.S., including on the very youngest,” said CDC Director Robert R. Redfield, MD. “Untreated opioid use disorder during pregnancy can lead to heartbreaking results. Each case represents a mother, a child, and a family in need of continued treatment and support.”
Data for the analysis came from the Agency for Healthcare Research and Quality’s National Inpatient Sample and State Inpatient Databases.
SOURCE: Haight SC et al. MMWR. 2018 Aug 10;67[31]:845-9.
according to the Centers for Disease Control and Prevention.

The national prevalence of opioid use disorder increased by 333% as it went from 1.5 cases per 1,000 delivery hospitalizations in 1999 to 6.5 cases per 1,000 in 2014. At the state level, there were significant increases in all 28 states with data available for at least 3 consecutive years during the study period, Sarah C. Haight, MPH, and her associates at the CDC in Atlanta said in the Morbidity and Mortality Weekly Report.
Average annual rate changes for those states ranged from a low of 0.01 per 1,000 delivery hospitalizations per year in California to 5.37 per year in Vermont, with the national rate change coming in at 0.39 per year. Of the 14 states with data available in 1999, Iowa had the lowest rate at 0.1 per 1,000 deliveries and Maryland had the highest at 8.2. In 2014, when data were available for 26 states and the District of Columbia, the highest rate was Vermont’s 48.6 per 1,000 deliveries and the lowest was 0.7 in Washington, D.C., the investigators reported.
Although “increasing trends might represent actual increases in prevalence or improved screening and diagnosis,” Ms. Haight and her associates added that “these estimates also correlate with state opioid prescribing rates in the general population. West Virginia, for example, had a prescribing rate estimated at 138 opioid prescriptions per 100 persons in 2012.”
“These findings illustrate the devastating impact of the opioid epidemic on families across the U.S., including on the very youngest,” said CDC Director Robert R. Redfield, MD. “Untreated opioid use disorder during pregnancy can lead to heartbreaking results. Each case represents a mother, a child, and a family in need of continued treatment and support.”
Data for the analysis came from the Agency for Healthcare Research and Quality’s National Inpatient Sample and State Inpatient Databases.
SOURCE: Haight SC et al. MMWR. 2018 Aug 10;67[31]:845-9.
according to the Centers for Disease Control and Prevention.

The national prevalence of opioid use disorder increased by 333% as it went from 1.5 cases per 1,000 delivery hospitalizations in 1999 to 6.5 cases per 1,000 in 2014. At the state level, there were significant increases in all 28 states with data available for at least 3 consecutive years during the study period, Sarah C. Haight, MPH, and her associates at the CDC in Atlanta said in the Morbidity and Mortality Weekly Report.
Average annual rate changes for those states ranged from a low of 0.01 per 1,000 delivery hospitalizations per year in California to 5.37 per year in Vermont, with the national rate change coming in at 0.39 per year. Of the 14 states with data available in 1999, Iowa had the lowest rate at 0.1 per 1,000 deliveries and Maryland had the highest at 8.2. In 2014, when data were available for 26 states and the District of Columbia, the highest rate was Vermont’s 48.6 per 1,000 deliveries and the lowest was 0.7 in Washington, D.C., the investigators reported.
Although “increasing trends might represent actual increases in prevalence or improved screening and diagnosis,” Ms. Haight and her associates added that “these estimates also correlate with state opioid prescribing rates in the general population. West Virginia, for example, had a prescribing rate estimated at 138 opioid prescriptions per 100 persons in 2012.”
“These findings illustrate the devastating impact of the opioid epidemic on families across the U.S., including on the very youngest,” said CDC Director Robert R. Redfield, MD. “Untreated opioid use disorder during pregnancy can lead to heartbreaking results. Each case represents a mother, a child, and a family in need of continued treatment and support.”
Data for the analysis came from the Agency for Healthcare Research and Quality’s National Inpatient Sample and State Inpatient Databases.
SOURCE: Haight SC et al. MMWR. 2018 Aug 10;67[31]:845-9.
FROM MMWR
Labor induction at 39 weeks reduced cesarean rate for low-risk, first-time mothers
Nulliparous women who were induced at 39 weeks had the same relative risk of adverse perinatal outcomes but a lower risk of a cesarean delivery, compared with women who received expectant management, results that researchers say contrast traditional recommendations for perinatal care, according to study from the New England Journal of Medicine.
“These findings contradict the conclusions of multiple observational studies that have suggested that labor induction is associated with an increased risk of adverse maternal and perinatal outcomes,” William A. Grobman, MD, the Arthur Hale Curtis, MD, Professor of Obstetrics and Gynecology at Northwestern University in Chicago, and his colleagues wrote. “These studies, however, compared women who underwent labor induction with those who had spontaneous labor, which is not a comparison that is useful to guide clinical decision making.”
Dr. Grobman and his colleagues evaluated the deliveries of 3,062 women who underwent labor induction between 39 weeks of gestation and 39 weeks and 4 days of gestation, and compared them with outcomes of 3,044 women who received expectant management until 40 weeks and 5 days of gestation. Women in both groups had a singleton fetus, no indication of early delivery, and did not plan on delivering by C-section. The participants were assessed again at about 38 weeks of gestation and randomly assigned to receive labor induction or expectant management as part of a multicenter randomized, controlled, parallel-group, unmasked trial in 41 maternal-fetal medicine departments in hospitals participating in the Eunice Kennedy Shriver National Institute of Child Health and Human Development network screened between March 2014, and August 2017.
Primary perinatal outcomes and components were defined as perinatal death, respiratory support, an Apgar score of 3 or less at 5 minutes, hypoxic-ischemic encephalopathy, seizure, infection, meconium aspiration syndrome, birth trauma, intracranial or subgaleal hemorrhage, or hypotension that requires vasopressor support. The principal secondary outcome was cesarean delivery, but other secondary outcomes included neonatal or intensive care, infection, postpartum hospital stay, and hypertension, among others.
Dr. Grobman and his colleagues found 132 (4.3%) of neonates in the induction group and 164 (5.4%) in the expectant-management group experienced a primary composite outcome (relative risk, 0.80; 95% confidence interval, 0.64-1.00; P = .049).
Regarding secondary outcomes, there was a significantly lower risk of cesarean delivery in the induction group, with 18.6% of women undergoing a cesarean delivery, compared with 22.2% of women in the expectant-management group (RR, 0.84; 95% CI, 0.76-0.93; P less than .001). Women in the labor induction group had a significantly lower relative risk of hypertensive disorders of pregnancy (9.1%), compared with the expectant-management (14.1%) group (RR, 0.64; 95% CI, 0.56-0.74; P less than .001). The investigators said women who underwent induced labor had lower 10-point Likert scale scores, were more likely to have “extensions of the uterine incision during cesarean delivery,” perceived they had “more control” during delivery, and had a shorter postpartum stay in the hospital, compared with women who received expectant management. However, women in the induced labor group also had a longer stay in the labor and delivery units, they said.
The researchers noted the limitations in this study, which included its unmasked design, lack of power to detect infrequent outcome differences, and the lack of information surrounding labor induction at 39 weeks in low-risk nulliparous women.
“These results suggest that policies aimed at the avoidance of elective labor induction among low-risk nulliparous women at 39 weeks of gestation are unlikely to reduce the rate of cesarean delivery on a population level; the trial provides information that can be incorporated into discussions that rely on principles of shared decision making,” Dr. Grobman and his colleagues wrote.
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Silver reports receiving personal fees from Gestavision. The other authors report no relevant financial disclosures.
SOURCE: Grobman WA et al. N Engl J Med. 2018 Aug 9. doi: 10.1056/NEJMoa1800566.
Of the more than 50,000 women screened for the study by Grobman et al., there were more than 44,000 women excluded and more than 16,000 did not participate in the trial. Further, the study participants tended to be younger and comprised more black or Hispanic women than the general population of mothers in the United States, Michael F. Greene, MD, said in a related editorial.
“Readers can only speculate as to why so many women declined to participate in the trial and what implications the demographics of the participants may have for the generalizability of the trial results and the acceptability of elective induction of labor at 39 weeks among women in the United States more generally,” Dr. Greene said. “If induction at 39 weeks becomes a widely popular option, busy obstetrical centers will need to find new ways to accommodate larger numbers of women with longer lengths of stay in the labor and delivery unit.”
Nevertheless, the study reflects a “public preference for a less interventionist approach” to delivery, Dr. Greene said, and the interest is backed by available data. He cited a meta-analysis of 20 randomized trials that found inducing labor at 39 weeks may reduce perinatal morality while not increasing the risk of operative deliveries. Specifically, he noted a randomized trial from the United Kingdom found induction of labor among 619 women at 39 weeks who were at least 35 years old did not affect the participants’ perception of delivery or increase the number of operative deliveries.
“These results across multiple obstetrical centers in the United States, however, should reassure women that elective induction of labor at 39 weeks is a reasonable choice that is very unlikely to result in poorer obstetrical outcomes,” he said.
Dr. Greene is chief of obstetrics and gynecology at Massachusetts General Hospital in Boston. He reported no relevant conflicts of interest. These comments summarize his editorial accompanying the article by Dr. Grobman and his associates ( N Engl J Med. 2018 Aug 9;379[6]:580-1 ).
Of the more than 50,000 women screened for the study by Grobman et al., there were more than 44,000 women excluded and more than 16,000 did not participate in the trial. Further, the study participants tended to be younger and comprised more black or Hispanic women than the general population of mothers in the United States, Michael F. Greene, MD, said in a related editorial.
“Readers can only speculate as to why so many women declined to participate in the trial and what implications the demographics of the participants may have for the generalizability of the trial results and the acceptability of elective induction of labor at 39 weeks among women in the United States more generally,” Dr. Greene said. “If induction at 39 weeks becomes a widely popular option, busy obstetrical centers will need to find new ways to accommodate larger numbers of women with longer lengths of stay in the labor and delivery unit.”
Nevertheless, the study reflects a “public preference for a less interventionist approach” to delivery, Dr. Greene said, and the interest is backed by available data. He cited a meta-analysis of 20 randomized trials that found inducing labor at 39 weeks may reduce perinatal morality while not increasing the risk of operative deliveries. Specifically, he noted a randomized trial from the United Kingdom found induction of labor among 619 women at 39 weeks who were at least 35 years old did not affect the participants’ perception of delivery or increase the number of operative deliveries.
“These results across multiple obstetrical centers in the United States, however, should reassure women that elective induction of labor at 39 weeks is a reasonable choice that is very unlikely to result in poorer obstetrical outcomes,” he said.
Dr. Greene is chief of obstetrics and gynecology at Massachusetts General Hospital in Boston. He reported no relevant conflicts of interest. These comments summarize his editorial accompanying the article by Dr. Grobman and his associates ( N Engl J Med. 2018 Aug 9;379[6]:580-1 ).
Of the more than 50,000 women screened for the study by Grobman et al., there were more than 44,000 women excluded and more than 16,000 did not participate in the trial. Further, the study participants tended to be younger and comprised more black or Hispanic women than the general population of mothers in the United States, Michael F. Greene, MD, said in a related editorial.
“Readers can only speculate as to why so many women declined to participate in the trial and what implications the demographics of the participants may have for the generalizability of the trial results and the acceptability of elective induction of labor at 39 weeks among women in the United States more generally,” Dr. Greene said. “If induction at 39 weeks becomes a widely popular option, busy obstetrical centers will need to find new ways to accommodate larger numbers of women with longer lengths of stay in the labor and delivery unit.”
Nevertheless, the study reflects a “public preference for a less interventionist approach” to delivery, Dr. Greene said, and the interest is backed by available data. He cited a meta-analysis of 20 randomized trials that found inducing labor at 39 weeks may reduce perinatal morality while not increasing the risk of operative deliveries. Specifically, he noted a randomized trial from the United Kingdom found induction of labor among 619 women at 39 weeks who were at least 35 years old did not affect the participants’ perception of delivery or increase the number of operative deliveries.
“These results across multiple obstetrical centers in the United States, however, should reassure women that elective induction of labor at 39 weeks is a reasonable choice that is very unlikely to result in poorer obstetrical outcomes,” he said.
Dr. Greene is chief of obstetrics and gynecology at Massachusetts General Hospital in Boston. He reported no relevant conflicts of interest. These comments summarize his editorial accompanying the article by Dr. Grobman and his associates ( N Engl J Med. 2018 Aug 9;379[6]:580-1 ).
Nulliparous women who were induced at 39 weeks had the same relative risk of adverse perinatal outcomes but a lower risk of a cesarean delivery, compared with women who received expectant management, results that researchers say contrast traditional recommendations for perinatal care, according to study from the New England Journal of Medicine.
“These findings contradict the conclusions of multiple observational studies that have suggested that labor induction is associated with an increased risk of adverse maternal and perinatal outcomes,” William A. Grobman, MD, the Arthur Hale Curtis, MD, Professor of Obstetrics and Gynecology at Northwestern University in Chicago, and his colleagues wrote. “These studies, however, compared women who underwent labor induction with those who had spontaneous labor, which is not a comparison that is useful to guide clinical decision making.”
Dr. Grobman and his colleagues evaluated the deliveries of 3,062 women who underwent labor induction between 39 weeks of gestation and 39 weeks and 4 days of gestation, and compared them with outcomes of 3,044 women who received expectant management until 40 weeks and 5 days of gestation. Women in both groups had a singleton fetus, no indication of early delivery, and did not plan on delivering by C-section. The participants were assessed again at about 38 weeks of gestation and randomly assigned to receive labor induction or expectant management as part of a multicenter randomized, controlled, parallel-group, unmasked trial in 41 maternal-fetal medicine departments in hospitals participating in the Eunice Kennedy Shriver National Institute of Child Health and Human Development network screened between March 2014, and August 2017.
Primary perinatal outcomes and components were defined as perinatal death, respiratory support, an Apgar score of 3 or less at 5 minutes, hypoxic-ischemic encephalopathy, seizure, infection, meconium aspiration syndrome, birth trauma, intracranial or subgaleal hemorrhage, or hypotension that requires vasopressor support. The principal secondary outcome was cesarean delivery, but other secondary outcomes included neonatal or intensive care, infection, postpartum hospital stay, and hypertension, among others.
Dr. Grobman and his colleagues found 132 (4.3%) of neonates in the induction group and 164 (5.4%) in the expectant-management group experienced a primary composite outcome (relative risk, 0.80; 95% confidence interval, 0.64-1.00; P = .049).
Regarding secondary outcomes, there was a significantly lower risk of cesarean delivery in the induction group, with 18.6% of women undergoing a cesarean delivery, compared with 22.2% of women in the expectant-management group (RR, 0.84; 95% CI, 0.76-0.93; P less than .001). Women in the labor induction group had a significantly lower relative risk of hypertensive disorders of pregnancy (9.1%), compared with the expectant-management (14.1%) group (RR, 0.64; 95% CI, 0.56-0.74; P less than .001). The investigators said women who underwent induced labor had lower 10-point Likert scale scores, were more likely to have “extensions of the uterine incision during cesarean delivery,” perceived they had “more control” during delivery, and had a shorter postpartum stay in the hospital, compared with women who received expectant management. However, women in the induced labor group also had a longer stay in the labor and delivery units, they said.
The researchers noted the limitations in this study, which included its unmasked design, lack of power to detect infrequent outcome differences, and the lack of information surrounding labor induction at 39 weeks in low-risk nulliparous women.
“These results suggest that policies aimed at the avoidance of elective labor induction among low-risk nulliparous women at 39 weeks of gestation are unlikely to reduce the rate of cesarean delivery on a population level; the trial provides information that can be incorporated into discussions that rely on principles of shared decision making,” Dr. Grobman and his colleagues wrote.
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Silver reports receiving personal fees from Gestavision. The other authors report no relevant financial disclosures.
SOURCE: Grobman WA et al. N Engl J Med. 2018 Aug 9. doi: 10.1056/NEJMoa1800566.
Nulliparous women who were induced at 39 weeks had the same relative risk of adverse perinatal outcomes but a lower risk of a cesarean delivery, compared with women who received expectant management, results that researchers say contrast traditional recommendations for perinatal care, according to study from the New England Journal of Medicine.
“These findings contradict the conclusions of multiple observational studies that have suggested that labor induction is associated with an increased risk of adverse maternal and perinatal outcomes,” William A. Grobman, MD, the Arthur Hale Curtis, MD, Professor of Obstetrics and Gynecology at Northwestern University in Chicago, and his colleagues wrote. “These studies, however, compared women who underwent labor induction with those who had spontaneous labor, which is not a comparison that is useful to guide clinical decision making.”
Dr. Grobman and his colleagues evaluated the deliveries of 3,062 women who underwent labor induction between 39 weeks of gestation and 39 weeks and 4 days of gestation, and compared them with outcomes of 3,044 women who received expectant management until 40 weeks and 5 days of gestation. Women in both groups had a singleton fetus, no indication of early delivery, and did not plan on delivering by C-section. The participants were assessed again at about 38 weeks of gestation and randomly assigned to receive labor induction or expectant management as part of a multicenter randomized, controlled, parallel-group, unmasked trial in 41 maternal-fetal medicine departments in hospitals participating in the Eunice Kennedy Shriver National Institute of Child Health and Human Development network screened between March 2014, and August 2017.
Primary perinatal outcomes and components were defined as perinatal death, respiratory support, an Apgar score of 3 or less at 5 minutes, hypoxic-ischemic encephalopathy, seizure, infection, meconium aspiration syndrome, birth trauma, intracranial or subgaleal hemorrhage, or hypotension that requires vasopressor support. The principal secondary outcome was cesarean delivery, but other secondary outcomes included neonatal or intensive care, infection, postpartum hospital stay, and hypertension, among others.
Dr. Grobman and his colleagues found 132 (4.3%) of neonates in the induction group and 164 (5.4%) in the expectant-management group experienced a primary composite outcome (relative risk, 0.80; 95% confidence interval, 0.64-1.00; P = .049).
Regarding secondary outcomes, there was a significantly lower risk of cesarean delivery in the induction group, with 18.6% of women undergoing a cesarean delivery, compared with 22.2% of women in the expectant-management group (RR, 0.84; 95% CI, 0.76-0.93; P less than .001). Women in the labor induction group had a significantly lower relative risk of hypertensive disorders of pregnancy (9.1%), compared with the expectant-management (14.1%) group (RR, 0.64; 95% CI, 0.56-0.74; P less than .001). The investigators said women who underwent induced labor had lower 10-point Likert scale scores, were more likely to have “extensions of the uterine incision during cesarean delivery,” perceived they had “more control” during delivery, and had a shorter postpartum stay in the hospital, compared with women who received expectant management. However, women in the induced labor group also had a longer stay in the labor and delivery units, they said.
The researchers noted the limitations in this study, which included its unmasked design, lack of power to detect infrequent outcome differences, and the lack of information surrounding labor induction at 39 weeks in low-risk nulliparous women.
“These results suggest that policies aimed at the avoidance of elective labor induction among low-risk nulliparous women at 39 weeks of gestation are unlikely to reduce the rate of cesarean delivery on a population level; the trial provides information that can be incorporated into discussions that rely on principles of shared decision making,” Dr. Grobman and his colleagues wrote.
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Silver reports receiving personal fees from Gestavision. The other authors report no relevant financial disclosures.
SOURCE: Grobman WA et al. N Engl J Med. 2018 Aug 9. doi: 10.1056/NEJMoa1800566.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: 18.6% of women in the induced labor group underwent cesarean delivery, compared with 22.2% in the expectant management group.
Study details: A multicenter randomized, controlled, parallel-group, unmasked trial of 6,106 women from 41 maternal-fetal medicine departments in hospitals participating in the Eunice Kennedy Shriver National Institute of Child Health and Human Development network screened between March 2014 and August 2017.
Disclosures: This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Silver reports receiving personal fees from Gestavision. The other authors report no relevant financial disclosures..
Source: Grobman WA et al. N Engl J Med. 2018 Aug 9. doi: 10.1056/NEJMoa1800566.
Maternal obesity plus diabetes lead to psychiatric disorders in offspring
Women who were obese and had diabetes before becoming pregnant were sixfold more likely to have children with psychiatric and neurodevelopmental disorders by age 11 years, as compared to women with normal body mass indexes (BMIs), based on results of a large, prospective, population-based, cohort study published in Pediatrics.
The risks to offspring whose mothers were obese and had pregestational diabetes mellitus (PGDM) were far greater than the risks seen when mothers had either condition alone or had gestational diabetes mellitus (GDM) in the study, reported Linghua Kong of the Karolinska Institute, Stockholm, and colleagues. The study is based on data from various national registries in Finland regarding 649,043 live births during 2004-2014 and data regarding psychiatric diagnoses from the Finnish Care Registers for Health Care.
Of the children in the cohort, 7.67% had mothers who were obese and 3.66% had mothers who were severely obese based on standard World Health Organization criteria; mothers had PGDM in 0.62% of the births and GDM in 15.7% of the births.
Overall, 5.4% of the children were diagnosed with a psychiatric disorder by age 11 years.
Compared with children born to mothers of normal weight (BMI less than 25 kg/m2), those born to mothers with severe maternal obesity alone (BMI greater than 35) had higher rates of developmental disorders or speech, language, motor, and scholastic skills (hazard ratio, 1.69; 95% confidence interval 1.54-1.86); ADHD and/or conduct disorder (HR, 1.88; 95% CI, 1.58-2.23); and psychosis and mood and anxiety disorders (HR, 1.67; 95% CI, 1.31-2.13). Increased risk of psychiatric disorders were only slightly statistically significant in the offspring of women with severe obesity and GDM.
The risks were significantly elevated, however, for children born to obese women who also had PGDM. The hazard ratio for autism spectrum disorder was 6.49 (95% CI, 3.08-13.69), and the HR for ADHD and/or conduct disorder was 6.03 (95% CI, 3.23-11.24). The risks were fourfold higher for mixed disorders of emotions and conduct, disorders of social function, and tics (HR, 4.29; 95% CI, 2.14-8.60).
Limitations of the study included basing results on shorter follow-up times for those born later in the study period, grouping of offspring’s disorder diagnoses, basing the definition of PGDM on insulin prescription, and using BMI measurements taken at only one time point during pregnancy.
The researchers were supported by the National Institute for Health and Welfare: Drugs and Pregnancy project, the Swedish Research Council, the regional agreement on medical training and clinical research between Stockholm County Council and Karolinska Institutet Stockholm County Council, the China Scholarship Council, and the Swedish Brain Foundation.
SOURCE: Kong L et al. Pediatrics. 2018 Sep;142(3):1-11.
Women who were obese and had diabetes before becoming pregnant were sixfold more likely to have children with psychiatric and neurodevelopmental disorders by age 11 years, as compared to women with normal body mass indexes (BMIs), based on results of a large, prospective, population-based, cohort study published in Pediatrics.
The risks to offspring whose mothers were obese and had pregestational diabetes mellitus (PGDM) were far greater than the risks seen when mothers had either condition alone or had gestational diabetes mellitus (GDM) in the study, reported Linghua Kong of the Karolinska Institute, Stockholm, and colleagues. The study is based on data from various national registries in Finland regarding 649,043 live births during 2004-2014 and data regarding psychiatric diagnoses from the Finnish Care Registers for Health Care.
Of the children in the cohort, 7.67% had mothers who were obese and 3.66% had mothers who were severely obese based on standard World Health Organization criteria; mothers had PGDM in 0.62% of the births and GDM in 15.7% of the births.
Overall, 5.4% of the children were diagnosed with a psychiatric disorder by age 11 years.
Compared with children born to mothers of normal weight (BMI less than 25 kg/m2), those born to mothers with severe maternal obesity alone (BMI greater than 35) had higher rates of developmental disorders or speech, language, motor, and scholastic skills (hazard ratio, 1.69; 95% confidence interval 1.54-1.86); ADHD and/or conduct disorder (HR, 1.88; 95% CI, 1.58-2.23); and psychosis and mood and anxiety disorders (HR, 1.67; 95% CI, 1.31-2.13). Increased risk of psychiatric disorders were only slightly statistically significant in the offspring of women with severe obesity and GDM.
The risks were significantly elevated, however, for children born to obese women who also had PGDM. The hazard ratio for autism spectrum disorder was 6.49 (95% CI, 3.08-13.69), and the HR for ADHD and/or conduct disorder was 6.03 (95% CI, 3.23-11.24). The risks were fourfold higher for mixed disorders of emotions and conduct, disorders of social function, and tics (HR, 4.29; 95% CI, 2.14-8.60).
Limitations of the study included basing results on shorter follow-up times for those born later in the study period, grouping of offspring’s disorder diagnoses, basing the definition of PGDM on insulin prescription, and using BMI measurements taken at only one time point during pregnancy.
The researchers were supported by the National Institute for Health and Welfare: Drugs and Pregnancy project, the Swedish Research Council, the regional agreement on medical training and clinical research between Stockholm County Council and Karolinska Institutet Stockholm County Council, the China Scholarship Council, and the Swedish Brain Foundation.
SOURCE: Kong L et al. Pediatrics. 2018 Sep;142(3):1-11.
Women who were obese and had diabetes before becoming pregnant were sixfold more likely to have children with psychiatric and neurodevelopmental disorders by age 11 years, as compared to women with normal body mass indexes (BMIs), based on results of a large, prospective, population-based, cohort study published in Pediatrics.
The risks to offspring whose mothers were obese and had pregestational diabetes mellitus (PGDM) were far greater than the risks seen when mothers had either condition alone or had gestational diabetes mellitus (GDM) in the study, reported Linghua Kong of the Karolinska Institute, Stockholm, and colleagues. The study is based on data from various national registries in Finland regarding 649,043 live births during 2004-2014 and data regarding psychiatric diagnoses from the Finnish Care Registers for Health Care.
Of the children in the cohort, 7.67% had mothers who were obese and 3.66% had mothers who were severely obese based on standard World Health Organization criteria; mothers had PGDM in 0.62% of the births and GDM in 15.7% of the births.
Overall, 5.4% of the children were diagnosed with a psychiatric disorder by age 11 years.
Compared with children born to mothers of normal weight (BMI less than 25 kg/m2), those born to mothers with severe maternal obesity alone (BMI greater than 35) had higher rates of developmental disorders or speech, language, motor, and scholastic skills (hazard ratio, 1.69; 95% confidence interval 1.54-1.86); ADHD and/or conduct disorder (HR, 1.88; 95% CI, 1.58-2.23); and psychosis and mood and anxiety disorders (HR, 1.67; 95% CI, 1.31-2.13). Increased risk of psychiatric disorders were only slightly statistically significant in the offspring of women with severe obesity and GDM.
The risks were significantly elevated, however, for children born to obese women who also had PGDM. The hazard ratio for autism spectrum disorder was 6.49 (95% CI, 3.08-13.69), and the HR for ADHD and/or conduct disorder was 6.03 (95% CI, 3.23-11.24). The risks were fourfold higher for mixed disorders of emotions and conduct, disorders of social function, and tics (HR, 4.29; 95% CI, 2.14-8.60).
Limitations of the study included basing results on shorter follow-up times for those born later in the study period, grouping of offspring’s disorder diagnoses, basing the definition of PGDM on insulin prescription, and using BMI measurements taken at only one time point during pregnancy.
The researchers were supported by the National Institute for Health and Welfare: Drugs and Pregnancy project, the Swedish Research Council, the regional agreement on medical training and clinical research between Stockholm County Council and Karolinska Institutet Stockholm County Council, the China Scholarship Council, and the Swedish Brain Foundation.
SOURCE: Kong L et al. Pediatrics. 2018 Sep;142(3):1-11.
FROM PEDIATRICS
1 in 7 Zika-exposed babies have at least one health problem related to the virus
About 14% of 1-year-olds with prenatal Zika virus exposure show at least one health problem probably related to the virus, according to a study published in Morbidity and Mortality Weekly Report.
Many of the problems are brain and eye abnormalities, which occurred at 30 times the 0.16% background rate among unexposed babies, Margaret Honein, PhD, and colleagues reported.
In a press briefing, Dr. Honein, chief of the Birth Defects Branch at the National Center on Birth Defects and Developmental Disabilities, described findings from the U.S. Zika Pregnancy and Infant Registry (USZPIR).
“Today’s report is the largest to date with long-term outcomes of babies born to mothers with [lab-confirmed] Zika infections, and the first published data on children 1 year or older from the ongoing surveillance network,” Dr. Honein said. It “clearly shows that the Zika story is not over, especially for the children and families who are affected by it.”
USZPIR is monitoring the outcomes of 7,300 pregnancies with lab-confirmed Zika infection. From these, 4,800 babies were born in the U.S. territories and freely associated states, had reached the age of 1 year by Feb. 1, 2018, and were included in the study.
In addition to clinical outcomes, the investigators looked at how many babies received the recommended evaluations, including neuroimaging, hearing screens, opthalmologic exams, developmental screening, and physical exams.
Almost all (95%) had at least one exam in the first 2 weeks of life; 76% had at least one developmental screening; 60% had postnatal neuroimaging; 48% at least one hearing exam; and 36% at least one eye exam by a specialist, the investigators found.
Findings that many didn’t get all the recommended health screenings are concerning, CDC Director Robert Redfield, MD. said during the briefing. “We are still learning about the full range of long-term health problems these babies could face. We thank clinicians for their continued commitment to conduct all necessary tests and evaluations to ensure appropriate care.”
Zika-associated birth defects occurred 203 babies (14%). Another 136 (9%) had at least one neurodevelopmental abnormality possibly associated with congenital Zika virus infection, and 20 (1%) had both. Most babies (1,386; 96%) did not have microcephaly detected at birth. But there was some “misclassification” of the condition, the investigators found. “Five infants had microcephaly at birth with brain or eye anomalies identified at birth; 59 had microcephaly at birth with no brain or eye anomalies identified at birth; and 20 infants did not have microcephaly identified at birth but had postnatal identification of microcephaly.”
Neurodevelopmental abnormalities possibly associated with Zika occurred in 136 (9%) of the cohort; 116 (8%) had no Zika-associated birth defects. Among these, half (58) had only possible developmental delay.
Zika transmission appears to be slowing, Lyle Peterson, MD, said during the press briefing, with no cases in the continental U.S. since 2017. That year, there were two cases in Florida and five in Texas. However, it is now endemic in many regions. Everyone should continue to take precautions against mosquito bites, he urged.
The MMWR also included updated guidance for men who are planning a pregnancy with a partner and may have been exposed to Zika.
CDC now recommends that these men wait at least 3 months after onset of Zika symptoms or any possible exposure, including travel to or living in a risk area. Past guidance recommended a 6-month waiting period. The new recommendation reflects emerging data suggesting that the risk of infectious Zika in semen declines during the 3 months after symptom onset.
Men who want to avoid passing Zika through sex should abstain for 3 months, or use a condom every time they have sex, the new recommendation said.
“All other Zika guidance remains unchanged,” the guidelines note. “Men with possible Zika virus exposure whose partner is pregnant should use condoms or the couple should not have sex for the entire pregnancy to reduce the risk of transmission.”
SOURCE: Honein, MA et al. MMWR 2018; 67: 1-10.
About 14% of 1-year-olds with prenatal Zika virus exposure show at least one health problem probably related to the virus, according to a study published in Morbidity and Mortality Weekly Report.
Many of the problems are brain and eye abnormalities, which occurred at 30 times the 0.16% background rate among unexposed babies, Margaret Honein, PhD, and colleagues reported.
In a press briefing, Dr. Honein, chief of the Birth Defects Branch at the National Center on Birth Defects and Developmental Disabilities, described findings from the U.S. Zika Pregnancy and Infant Registry (USZPIR).
“Today’s report is the largest to date with long-term outcomes of babies born to mothers with [lab-confirmed] Zika infections, and the first published data on children 1 year or older from the ongoing surveillance network,” Dr. Honein said. It “clearly shows that the Zika story is not over, especially for the children and families who are affected by it.”
USZPIR is monitoring the outcomes of 7,300 pregnancies with lab-confirmed Zika infection. From these, 4,800 babies were born in the U.S. territories and freely associated states, had reached the age of 1 year by Feb. 1, 2018, and were included in the study.
In addition to clinical outcomes, the investigators looked at how many babies received the recommended evaluations, including neuroimaging, hearing screens, opthalmologic exams, developmental screening, and physical exams.
Almost all (95%) had at least one exam in the first 2 weeks of life; 76% had at least one developmental screening; 60% had postnatal neuroimaging; 48% at least one hearing exam; and 36% at least one eye exam by a specialist, the investigators found.
Findings that many didn’t get all the recommended health screenings are concerning, CDC Director Robert Redfield, MD. said during the briefing. “We are still learning about the full range of long-term health problems these babies could face. We thank clinicians for their continued commitment to conduct all necessary tests and evaluations to ensure appropriate care.”
Zika-associated birth defects occurred 203 babies (14%). Another 136 (9%) had at least one neurodevelopmental abnormality possibly associated with congenital Zika virus infection, and 20 (1%) had both. Most babies (1,386; 96%) did not have microcephaly detected at birth. But there was some “misclassification” of the condition, the investigators found. “Five infants had microcephaly at birth with brain or eye anomalies identified at birth; 59 had microcephaly at birth with no brain or eye anomalies identified at birth; and 20 infants did not have microcephaly identified at birth but had postnatal identification of microcephaly.”
Neurodevelopmental abnormalities possibly associated with Zika occurred in 136 (9%) of the cohort; 116 (8%) had no Zika-associated birth defects. Among these, half (58) had only possible developmental delay.
Zika transmission appears to be slowing, Lyle Peterson, MD, said during the press briefing, with no cases in the continental U.S. since 2017. That year, there were two cases in Florida and five in Texas. However, it is now endemic in many regions. Everyone should continue to take precautions against mosquito bites, he urged.
The MMWR also included updated guidance for men who are planning a pregnancy with a partner and may have been exposed to Zika.
CDC now recommends that these men wait at least 3 months after onset of Zika symptoms or any possible exposure, including travel to or living in a risk area. Past guidance recommended a 6-month waiting period. The new recommendation reflects emerging data suggesting that the risk of infectious Zika in semen declines during the 3 months after symptom onset.
Men who want to avoid passing Zika through sex should abstain for 3 months, or use a condom every time they have sex, the new recommendation said.
“All other Zika guidance remains unchanged,” the guidelines note. “Men with possible Zika virus exposure whose partner is pregnant should use condoms or the couple should not have sex for the entire pregnancy to reduce the risk of transmission.”
SOURCE: Honein, MA et al. MMWR 2018; 67: 1-10.
About 14% of 1-year-olds with prenatal Zika virus exposure show at least one health problem probably related to the virus, according to a study published in Morbidity and Mortality Weekly Report.
Many of the problems are brain and eye abnormalities, which occurred at 30 times the 0.16% background rate among unexposed babies, Margaret Honein, PhD, and colleagues reported.
In a press briefing, Dr. Honein, chief of the Birth Defects Branch at the National Center on Birth Defects and Developmental Disabilities, described findings from the U.S. Zika Pregnancy and Infant Registry (USZPIR).
“Today’s report is the largest to date with long-term outcomes of babies born to mothers with [lab-confirmed] Zika infections, and the first published data on children 1 year or older from the ongoing surveillance network,” Dr. Honein said. It “clearly shows that the Zika story is not over, especially for the children and families who are affected by it.”
USZPIR is monitoring the outcomes of 7,300 pregnancies with lab-confirmed Zika infection. From these, 4,800 babies were born in the U.S. territories and freely associated states, had reached the age of 1 year by Feb. 1, 2018, and were included in the study.
In addition to clinical outcomes, the investigators looked at how many babies received the recommended evaluations, including neuroimaging, hearing screens, opthalmologic exams, developmental screening, and physical exams.
Almost all (95%) had at least one exam in the first 2 weeks of life; 76% had at least one developmental screening; 60% had postnatal neuroimaging; 48% at least one hearing exam; and 36% at least one eye exam by a specialist, the investigators found.
Findings that many didn’t get all the recommended health screenings are concerning, CDC Director Robert Redfield, MD. said during the briefing. “We are still learning about the full range of long-term health problems these babies could face. We thank clinicians for their continued commitment to conduct all necessary tests and evaluations to ensure appropriate care.”
Zika-associated birth defects occurred 203 babies (14%). Another 136 (9%) had at least one neurodevelopmental abnormality possibly associated with congenital Zika virus infection, and 20 (1%) had both. Most babies (1,386; 96%) did not have microcephaly detected at birth. But there was some “misclassification” of the condition, the investigators found. “Five infants had microcephaly at birth with brain or eye anomalies identified at birth; 59 had microcephaly at birth with no brain or eye anomalies identified at birth; and 20 infants did not have microcephaly identified at birth but had postnatal identification of microcephaly.”
Neurodevelopmental abnormalities possibly associated with Zika occurred in 136 (9%) of the cohort; 116 (8%) had no Zika-associated birth defects. Among these, half (58) had only possible developmental delay.
Zika transmission appears to be slowing, Lyle Peterson, MD, said during the press briefing, with no cases in the continental U.S. since 2017. That year, there were two cases in Florida and five in Texas. However, it is now endemic in many regions. Everyone should continue to take precautions against mosquito bites, he urged.
The MMWR also included updated guidance for men who are planning a pregnancy with a partner and may have been exposed to Zika.
CDC now recommends that these men wait at least 3 months after onset of Zika symptoms or any possible exposure, including travel to or living in a risk area. Past guidance recommended a 6-month waiting period. The new recommendation reflects emerging data suggesting that the risk of infectious Zika in semen declines during the 3 months after symptom onset.
Men who want to avoid passing Zika through sex should abstain for 3 months, or use a condom every time they have sex, the new recommendation said.
“All other Zika guidance remains unchanged,” the guidelines note. “Men with possible Zika virus exposure whose partner is pregnant should use condoms or the couple should not have sex for the entire pregnancy to reduce the risk of transmission.”
SOURCE: Honein, MA et al. MMWR 2018; 67: 1-10.
FROM MMWR
Key clinical point: Zika-related health problems are present in a substantial number of prenatally exposed babies.
Major finding: Problems occurred in 14% of 4,800 included in a national registry.
Study details: USZPIR is monitoring the outcomes of 7,300 pregnancies with lab-confirmed Zika infection. Disclosures: No relevant conflicts of interest were disclosed.
Source: Honein, MA et al. MMWR 2018; 67: 1-10.
MOC: ACOG’s role in developing a solution to the heated controversy

The American Board of Medical Specialties (ABMS) has decided to trade the phrase “maintenance of certification” (MOC) for “continuing board certification,” a seemingly minor change that has an important backstory. This is the story of how the physician community flexed its collective muscle and how the American College of Obstetricians and Gynecologists (ACOG) helped broker an important détente and pathway in a highly contentious issue.
Founded in 1933 as a nonprofit organization dedicated to maintaining high uniform standards among physicians, the ABMS and many of its specialty boards have found themselves, for more than a decade, under heavy fire from physicians (especially family physicians, internists, and surgeons), their 24 subspecialties, and the state medical societies representing them.
The ObGyn experience with the American Board of Obstetrics and Gynecology (ABOG), however, is better for a number of reasons. Historically, ABOG and ACOG have worked closely together, which is an anomaly among boards as many boards have an arms-length or even an antagonistic relationship with their specialty society.
The discussion below outlines physician concerns with the ABMS and related boards and describes efforts to address and rebuild the continuing board certification process.
Direct and indirect costs
Physicians are very concerned with the costs involved in MOC. Measurable costs include testing fees, while indirect costs include time, stress, travel to test centers, and threats to livelihood for failing a high-stakes examination. Physicians want the high-stakes exam eliminated.
Relevance to practice
Physicians often feel that the MOC has little relevance to their practice, which fuels a sense of resentment toward boards that they believe are dominated by physicians who no longer practice. Subspecialists feel farther away from general practice and the base exams. Generalists feel that the exams miss the points of their daily practice.
Lack of data to show improved quality of care
Physicians want to know that the MOC is worth their time, effort, and money because it improves patient care. To date, however, empirical or clinical data on patient outcomes are absent or ambiguous; most studies lack high-level data or do not investigate the MOC requirements. Physicians want to know what the best MOC practices are, what improves care, and that practices that make no difference will be discarded. In addition, they want timely knowledge alerts when evidence changes.
Relationship to licensing, employment, privileging, credentialing, and reimbursement
Hospitals, insurers, and states increasingly—and inappropriately—use board certification as the primary (sometimes only) default measure of a physician’s fitness for patient care. Physicians without board certification often are denied hospital privileges, inclusion in insurance panels, and even medical licenses. This changes certification from a voluntary physician self-improvement exercise into a can’t-earn-a-living-without-it cudgel.
Variation
Boards vary significantly in their MOC requirements and costs. The importance of an equal standard across all boards is a clear theme among physician concerns.
Role and authority of the ABMS and related boards
Many physicians are frustrated with the perceived autocratic nature of their boards—boards that lack transparency, do not solicit or allow input from practicing physicians, and are unresponsive to physician concerns.
According to Susan Ramin, MD, ABOG Associate Executive Director, ABOG is leading in a number of these areas, including:
- rapidly disseminating clinical information on emerging topics, such as Zika virus infection and opioid misuse
- offering physician choice of testing categories
- exempting high scorers from the secured written exam, which saved physicians a total of $881,000 in exam fees
- crediting physicians for what they already are doing, including serving on maternal mortality review committees, participating in registries, and participating in the Alliance for Innovation on Maternal Health (AIM)
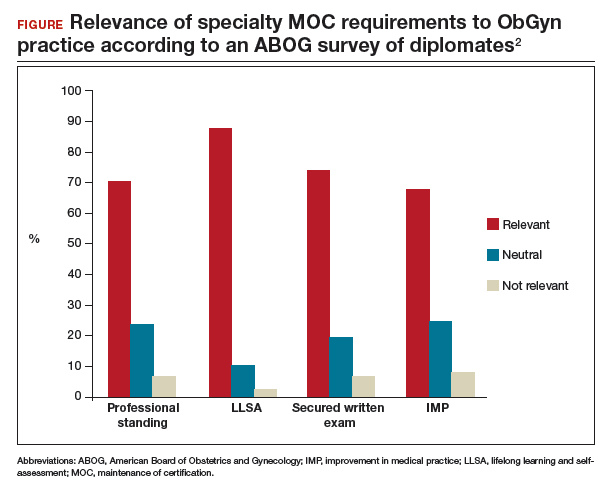
- providing Lifelong Learning and Self-Assessment (LLSA) articles that, according to 90% of diplomates surveyed, are beneficial to their clinical practice (FIGURE).1,2
Our colleague physicians are not so lucky. In a 2015 New England Journal of Medicine Perspective, one physician called out the American Board of Internal Medicine as “a private, self-appointed certifying organization,” a not-for-profit organization that has “grown into a $55-million-per-year business.”3 He concluded that “many physicians are waking up to the fact that our profession is increasingly controlled by people not directly involved in patient care who have lost contact with the realities of day-to-day clinical practice.”3
State and society responses to MOC requirements
Frustration with an inability to resolve these concerns has grown steadily, bubbling over into state governments. The American Medical Association developed “model state legislation intended to prohibit hospitals, health care insurers, and state boards of medicine and osteopathic medicine from requiring participation in MOC processes as a condition of credentialing, privileging, insurance panel participation, licensure, or licensure renewal.”4
Some states are proposing or have enacted legislation that prohibits the use of MOC as a criterion for licensure, privileging, employment, reimbursement, and/or insurance panel participation. Eight states (Arizona, Georgia, Kentucky, Maryland, Maine, Missouri, Oklahoma, Tennessee) have enacted laws to prohibit the use of MOC for initial and renewal licensure decisions. Many states are actively considering MOC-related legislation, including Alaska, Florida, Iowa, Indiana, Maryland, Massachusetts, Michigan, Missouri, New Hampshire, New York, Ohio, Oklahoma, Rhode Island, South Carolina, Tennessee, Utah, Washington, and Wisconsin.
Legislation is not the only outlet for physician frustration. Some medical specialty societies are considering dropping board certification as a membership requirement; physicians are exploring developing alternative boards; and some physicians are defying the board certification requirement altogether, with thousands signing anti-MOC petitions.
ACOG asserts importance of maintaining self-regulation
While other specialties are actively advocating state legislation, ACOG and ABOG have worked together to oppose state legislation, believing that physician self-regulation is paramount. In fact, in 2017, ACOG and ABOG issued a joint statement urging state lawmakers to “not interfere with our decades of successful self-regulation and to realize that each medical society has its own experience with its MOC program.”5
Negotiations lead to new initiative
This brings us to an interesting situation. ACOG’s Executive Vice President and CEO Hal Lawrence III, MD, was tapped (in his position as Chair of the Specialty Society CEO Consortium) to represent physician specialties in negotiations and discussions with the boards, which were represented by Lois Nora, MD, JD, President and CEO of the ABMS, and state medical societies, represented by Donald Palmisano Jr, JD, Executive Director and CEO of the Medical Association of Georgia. Many state medical societies, boards, and physician specialty organizations participated in these meetings.
Throughout months of debate, Dr. Lawrence urged his colleagues to stay at the table and do the hard work of reaching an agreement, rather than ask politicians to solve medicine’s problems. This approach was leveraged by the serious efforts and threats of state legislation, which brought the boards to the table. In August 2017, 41 state medical societies and 33 national medical specialty societies wrote to Dr. Nora expressing their concerns that “professional self-regulation is under attack. Concerns regarding the usefulness of the high-stakes exam, the exorbitant costs of the MOC process, and the lack of transparent communication from the certifying boards have led to damaging the MOC brand, and creating state-based attacks on the MOC process.”6
In December 2017, Dr. Lawrence and Mr. Palmisano led a meeting of principals from the national medical specialty societies and state medical societies with leaders of ABMS and 8 specialty boards, including ABOG, an opportunity to secure meaningful change. Dr. Lawrence began by stressing that the interests of physicians and patients would be best served by all parties coming together and collaborating on a meaningful solution, to repair trust and preserve physician self-regulation.
Dr. Ramin presented ABOG’s approach to continuous certification, lifelong learning, and self-assessment. The American Board of Urology and the American Board of Psychiatry and Neurology indicated that they were basing important changes in their MOC process on ABOG’s work, including using 5 modules (1 general and 4 specific to the physician’s practice) and multiple open-book mini-exams based on selected journal articles as an alternative to the 10-year MOC exam.
The Vision Initiative. At that meeting and others, the ABMS and other boards heard physicians’ candid and sometimes blunt concerns. Dr. Nora spoke to the recently announced Continuing Board Certification: Vision for the Future program, also known as the “Vision Initiative,” a process designed to fundamentally rebuild the continuing certification process with input and guidance from practicing physicians. Physician response seemed uniform: Seeing is believing.
Importantly, all participants at the December meeting agreed to work together to rebuild trust and ensure professionalism and professional self-regulation, reflected in this Statement of Shared Purpose:
ABMS certifying boards and national medical specialty societies will collaborate to resolve differences in the process of ongoing certification and to fulfill the principles of professional self-regulation, achieving appropriate standardization, and assuring that ongoing certification is relevant to the practices of physicians without undue burden. Furthermore, the boards and societies, and their organizations (ABMS and CMSS [Council of Medical Specialty Societies]), will undertake necessary changes in a timely manner, and will commit to ongoing communication with state medical associations to solicit their input.4
Two ObGyns participating in the Vision Initiative are Haywood Brown, MD, ACOG’s Immediate Past President, and George Wendel, MD, ABOG’s Executive Director. The Vision Initiative is composed of 3 parts. Part 1, Organization, is complete. The committee is currently working on part 2, Envisioning the Future, an information-gathering component that includes physician surveys, hearings, open solicited input, and identifying new and better approaches. After the final report is delivered to the ABMS in February 2019, part 3, Implementation, will begin.
The Vision Initiative offers physicians an important opportunity to help shape the future of continuing education and certification. ObGyns and other physicians should consider reviewing and commenting on the draft report, due in November, during the public comment period. Visit https://visioninitiative.org for more information and to sign up for email updates.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American Board of Obstetrics and Gynecology. From pilot to permanent: ABOG's program offering an innovative pathway integrating lifelong learning and self-assessment and external assessment is approved. https://www.abog.org/new/ABOG_PilotToPermanent.aspx. Accessed July 6, 2018.
- Ramin S. American Board of Obstetrics and Gynecology MOC program. PowerPoint presentation; December 4, 2017.
- Teirstein PS. Boarded to death--why maintenance of certification is bad for doctors and patients. N Engl J Med. 2015;372(2):106-108.
- AMA Council on Medical Education. Executive summary. 2017. https://www.ama-assn.org/sites/default/files/media-browser/public/council-on-med-ed/a18-cme-02.pdf. Accessed July 6, 2018.
- American College of Obstetricians and Gynecologists. ACOG-ABOG joint statement: political interference in physician maintenance of skills threatens women's health care. https://www.acog.org/-/media/Departments/State-Legislative-Activities/2017ACOG-ABMS-MOC-Statement.pdf?dmc=1&ts=20180706T1615538746. Accessed July 6, 2018.
- Letter to Lois Nora, MD, JD. August 18, 2017. https://www.mainemed.com/sites/default/files/content/MOC%20Letter%20082117.pdf. Accessed July 6, 2018.

The American Board of Medical Specialties (ABMS) has decided to trade the phrase “maintenance of certification” (MOC) for “continuing board certification,” a seemingly minor change that has an important backstory. This is the story of how the physician community flexed its collective muscle and how the American College of Obstetricians and Gynecologists (ACOG) helped broker an important détente and pathway in a highly contentious issue.
Founded in 1933 as a nonprofit organization dedicated to maintaining high uniform standards among physicians, the ABMS and many of its specialty boards have found themselves, for more than a decade, under heavy fire from physicians (especially family physicians, internists, and surgeons), their 24 subspecialties, and the state medical societies representing them.
The ObGyn experience with the American Board of Obstetrics and Gynecology (ABOG), however, is better for a number of reasons. Historically, ABOG and ACOG have worked closely together, which is an anomaly among boards as many boards have an arms-length or even an antagonistic relationship with their specialty society.
The discussion below outlines physician concerns with the ABMS and related boards and describes efforts to address and rebuild the continuing board certification process.
Direct and indirect costs
Physicians are very concerned with the costs involved in MOC. Measurable costs include testing fees, while indirect costs include time, stress, travel to test centers, and threats to livelihood for failing a high-stakes examination. Physicians want the high-stakes exam eliminated.
Relevance to practice
Physicians often feel that the MOC has little relevance to their practice, which fuels a sense of resentment toward boards that they believe are dominated by physicians who no longer practice. Subspecialists feel farther away from general practice and the base exams. Generalists feel that the exams miss the points of their daily practice.
Lack of data to show improved quality of care
Physicians want to know that the MOC is worth their time, effort, and money because it improves patient care. To date, however, empirical or clinical data on patient outcomes are absent or ambiguous; most studies lack high-level data or do not investigate the MOC requirements. Physicians want to know what the best MOC practices are, what improves care, and that practices that make no difference will be discarded. In addition, they want timely knowledge alerts when evidence changes.
Relationship to licensing, employment, privileging, credentialing, and reimbursement
Hospitals, insurers, and states increasingly—and inappropriately—use board certification as the primary (sometimes only) default measure of a physician’s fitness for patient care. Physicians without board certification often are denied hospital privileges, inclusion in insurance panels, and even medical licenses. This changes certification from a voluntary physician self-improvement exercise into a can’t-earn-a-living-without-it cudgel.
Variation
Boards vary significantly in their MOC requirements and costs. The importance of an equal standard across all boards is a clear theme among physician concerns.
Role and authority of the ABMS and related boards
Many physicians are frustrated with the perceived autocratic nature of their boards—boards that lack transparency, do not solicit or allow input from practicing physicians, and are unresponsive to physician concerns.
According to Susan Ramin, MD, ABOG Associate Executive Director, ABOG is leading in a number of these areas, including:
- rapidly disseminating clinical information on emerging topics, such as Zika virus infection and opioid misuse
- offering physician choice of testing categories
- exempting high scorers from the secured written exam, which saved physicians a total of $881,000 in exam fees
- crediting physicians for what they already are doing, including serving on maternal mortality review committees, participating in registries, and participating in the Alliance for Innovation on Maternal Health (AIM)
- providing Lifelong Learning and Self-Assessment (LLSA) articles that, according to 90% of diplomates surveyed, are beneficial to their clinical practice (FIGURE).1,2

Our colleague physicians are not so lucky. In a 2015 New England Journal of Medicine Perspective, one physician called out the American Board of Internal Medicine as “a private, self-appointed certifying organization,” a not-for-profit organization that has “grown into a $55-million-per-year business.”3 He concluded that “many physicians are waking up to the fact that our profession is increasingly controlled by people not directly involved in patient care who have lost contact with the realities of day-to-day clinical practice.”3
State and society responses to MOC requirements
Frustration with an inability to resolve these concerns has grown steadily, bubbling over into state governments. The American Medical Association developed “model state legislation intended to prohibit hospitals, health care insurers, and state boards of medicine and osteopathic medicine from requiring participation in MOC processes as a condition of credentialing, privileging, insurance panel participation, licensure, or licensure renewal.”4
Some states are proposing or have enacted legislation that prohibits the use of MOC as a criterion for licensure, privileging, employment, reimbursement, and/or insurance panel participation. Eight states (Arizona, Georgia, Kentucky, Maryland, Maine, Missouri, Oklahoma, Tennessee) have enacted laws to prohibit the use of MOC for initial and renewal licensure decisions. Many states are actively considering MOC-related legislation, including Alaska, Florida, Iowa, Indiana, Maryland, Massachusetts, Michigan, Missouri, New Hampshire, New York, Ohio, Oklahoma, Rhode Island, South Carolina, Tennessee, Utah, Washington, and Wisconsin.
Legislation is not the only outlet for physician frustration. Some medical specialty societies are considering dropping board certification as a membership requirement; physicians are exploring developing alternative boards; and some physicians are defying the board certification requirement altogether, with thousands signing anti-MOC petitions.
ACOG asserts importance of maintaining self-regulation
While other specialties are actively advocating state legislation, ACOG and ABOG have worked together to oppose state legislation, believing that physician self-regulation is paramount. In fact, in 2017, ACOG and ABOG issued a joint statement urging state lawmakers to “not interfere with our decades of successful self-regulation and to realize that each medical society has its own experience with its MOC program.”5
Negotiations lead to new initiative
This brings us to an interesting situation. ACOG’s Executive Vice President and CEO Hal Lawrence III, MD, was tapped (in his position as Chair of the Specialty Society CEO Consortium) to represent physician specialties in negotiations and discussions with the boards, which were represented by Lois Nora, MD, JD, President and CEO of the ABMS, and state medical societies, represented by Donald Palmisano Jr, JD, Executive Director and CEO of the Medical Association of Georgia. Many state medical societies, boards, and physician specialty organizations participated in these meetings.
Throughout months of debate, Dr. Lawrence urged his colleagues to stay at the table and do the hard work of reaching an agreement, rather than ask politicians to solve medicine’s problems. This approach was leveraged by the serious efforts and threats of state legislation, which brought the boards to the table. In August 2017, 41 state medical societies and 33 national medical specialty societies wrote to Dr. Nora expressing their concerns that “professional self-regulation is under attack. Concerns regarding the usefulness of the high-stakes exam, the exorbitant costs of the MOC process, and the lack of transparent communication from the certifying boards have led to damaging the MOC brand, and creating state-based attacks on the MOC process.”6
In December 2017, Dr. Lawrence and Mr. Palmisano led a meeting of principals from the national medical specialty societies and state medical societies with leaders of ABMS and 8 specialty boards, including ABOG, an opportunity to secure meaningful change. Dr. Lawrence began by stressing that the interests of physicians and patients would be best served by all parties coming together and collaborating on a meaningful solution, to repair trust and preserve physician self-regulation.
Dr. Ramin presented ABOG’s approach to continuous certification, lifelong learning, and self-assessment. The American Board of Urology and the American Board of Psychiatry and Neurology indicated that they were basing important changes in their MOC process on ABOG’s work, including using 5 modules (1 general and 4 specific to the physician’s practice) and multiple open-book mini-exams based on selected journal articles as an alternative to the 10-year MOC exam.
The Vision Initiative. At that meeting and others, the ABMS and other boards heard physicians’ candid and sometimes blunt concerns. Dr. Nora spoke to the recently announced Continuing Board Certification: Vision for the Future program, also known as the “Vision Initiative,” a process designed to fundamentally rebuild the continuing certification process with input and guidance from practicing physicians. Physician response seemed uniform: Seeing is believing.
Importantly, all participants at the December meeting agreed to work together to rebuild trust and ensure professionalism and professional self-regulation, reflected in this Statement of Shared Purpose:
ABMS certifying boards and national medical specialty societies will collaborate to resolve differences in the process of ongoing certification and to fulfill the principles of professional self-regulation, achieving appropriate standardization, and assuring that ongoing certification is relevant to the practices of physicians without undue burden. Furthermore, the boards and societies, and their organizations (ABMS and CMSS [Council of Medical Specialty Societies]), will undertake necessary changes in a timely manner, and will commit to ongoing communication with state medical associations to solicit their input.4
Two ObGyns participating in the Vision Initiative are Haywood Brown, MD, ACOG’s Immediate Past President, and George Wendel, MD, ABOG’s Executive Director. The Vision Initiative is composed of 3 parts. Part 1, Organization, is complete. The committee is currently working on part 2, Envisioning the Future, an information-gathering component that includes physician surveys, hearings, open solicited input, and identifying new and better approaches. After the final report is delivered to the ABMS in February 2019, part 3, Implementation, will begin.
The Vision Initiative offers physicians an important opportunity to help shape the future of continuing education and certification. ObGyns and other physicians should consider reviewing and commenting on the draft report, due in November, during the public comment period. Visit https://visioninitiative.org for more information and to sign up for email updates.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

The American Board of Medical Specialties (ABMS) has decided to trade the phrase “maintenance of certification” (MOC) for “continuing board certification,” a seemingly minor change that has an important backstory. This is the story of how the physician community flexed its collective muscle and how the American College of Obstetricians and Gynecologists (ACOG) helped broker an important détente and pathway in a highly contentious issue.
Founded in 1933 as a nonprofit organization dedicated to maintaining high uniform standards among physicians, the ABMS and many of its specialty boards have found themselves, for more than a decade, under heavy fire from physicians (especially family physicians, internists, and surgeons), their 24 subspecialties, and the state medical societies representing them.
The ObGyn experience with the American Board of Obstetrics and Gynecology (ABOG), however, is better for a number of reasons. Historically, ABOG and ACOG have worked closely together, which is an anomaly among boards as many boards have an arms-length or even an antagonistic relationship with their specialty society.
The discussion below outlines physician concerns with the ABMS and related boards and describes efforts to address and rebuild the continuing board certification process.
Direct and indirect costs
Physicians are very concerned with the costs involved in MOC. Measurable costs include testing fees, while indirect costs include time, stress, travel to test centers, and threats to livelihood for failing a high-stakes examination. Physicians want the high-stakes exam eliminated.
Relevance to practice
Physicians often feel that the MOC has little relevance to their practice, which fuels a sense of resentment toward boards that they believe are dominated by physicians who no longer practice. Subspecialists feel farther away from general practice and the base exams. Generalists feel that the exams miss the points of their daily practice.
Lack of data to show improved quality of care
Physicians want to know that the MOC is worth their time, effort, and money because it improves patient care. To date, however, empirical or clinical data on patient outcomes are absent or ambiguous; most studies lack high-level data or do not investigate the MOC requirements. Physicians want to know what the best MOC practices are, what improves care, and that practices that make no difference will be discarded. In addition, they want timely knowledge alerts when evidence changes.
Relationship to licensing, employment, privileging, credentialing, and reimbursement
Hospitals, insurers, and states increasingly—and inappropriately—use board certification as the primary (sometimes only) default measure of a physician’s fitness for patient care. Physicians without board certification often are denied hospital privileges, inclusion in insurance panels, and even medical licenses. This changes certification from a voluntary physician self-improvement exercise into a can’t-earn-a-living-without-it cudgel.
Variation
Boards vary significantly in their MOC requirements and costs. The importance of an equal standard across all boards is a clear theme among physician concerns.
Role and authority of the ABMS and related boards
Many physicians are frustrated with the perceived autocratic nature of their boards—boards that lack transparency, do not solicit or allow input from practicing physicians, and are unresponsive to physician concerns.
According to Susan Ramin, MD, ABOG Associate Executive Director, ABOG is leading in a number of these areas, including:
- rapidly disseminating clinical information on emerging topics, such as Zika virus infection and opioid misuse
- offering physician choice of testing categories
- exempting high scorers from the secured written exam, which saved physicians a total of $881,000 in exam fees
- crediting physicians for what they already are doing, including serving on maternal mortality review committees, participating in registries, and participating in the Alliance for Innovation on Maternal Health (AIM)
- providing Lifelong Learning and Self-Assessment (LLSA) articles that, according to 90% of diplomates surveyed, are beneficial to their clinical practice (FIGURE).1,2

Our colleague physicians are not so lucky. In a 2015 New England Journal of Medicine Perspective, one physician called out the American Board of Internal Medicine as “a private, self-appointed certifying organization,” a not-for-profit organization that has “grown into a $55-million-per-year business.”3 He concluded that “many physicians are waking up to the fact that our profession is increasingly controlled by people not directly involved in patient care who have lost contact with the realities of day-to-day clinical practice.”3
State and society responses to MOC requirements
Frustration with an inability to resolve these concerns has grown steadily, bubbling over into state governments. The American Medical Association developed “model state legislation intended to prohibit hospitals, health care insurers, and state boards of medicine and osteopathic medicine from requiring participation in MOC processes as a condition of credentialing, privileging, insurance panel participation, licensure, or licensure renewal.”4
Some states are proposing or have enacted legislation that prohibits the use of MOC as a criterion for licensure, privileging, employment, reimbursement, and/or insurance panel participation. Eight states (Arizona, Georgia, Kentucky, Maryland, Maine, Missouri, Oklahoma, Tennessee) have enacted laws to prohibit the use of MOC for initial and renewal licensure decisions. Many states are actively considering MOC-related legislation, including Alaska, Florida, Iowa, Indiana, Maryland, Massachusetts, Michigan, Missouri, New Hampshire, New York, Ohio, Oklahoma, Rhode Island, South Carolina, Tennessee, Utah, Washington, and Wisconsin.
Legislation is not the only outlet for physician frustration. Some medical specialty societies are considering dropping board certification as a membership requirement; physicians are exploring developing alternative boards; and some physicians are defying the board certification requirement altogether, with thousands signing anti-MOC petitions.
ACOG asserts importance of maintaining self-regulation
While other specialties are actively advocating state legislation, ACOG and ABOG have worked together to oppose state legislation, believing that physician self-regulation is paramount. In fact, in 2017, ACOG and ABOG issued a joint statement urging state lawmakers to “not interfere with our decades of successful self-regulation and to realize that each medical society has its own experience with its MOC program.”5
Negotiations lead to new initiative
This brings us to an interesting situation. ACOG’s Executive Vice President and CEO Hal Lawrence III, MD, was tapped (in his position as Chair of the Specialty Society CEO Consortium) to represent physician specialties in negotiations and discussions with the boards, which were represented by Lois Nora, MD, JD, President and CEO of the ABMS, and state medical societies, represented by Donald Palmisano Jr, JD, Executive Director and CEO of the Medical Association of Georgia. Many state medical societies, boards, and physician specialty organizations participated in these meetings.
Throughout months of debate, Dr. Lawrence urged his colleagues to stay at the table and do the hard work of reaching an agreement, rather than ask politicians to solve medicine’s problems. This approach was leveraged by the serious efforts and threats of state legislation, which brought the boards to the table. In August 2017, 41 state medical societies and 33 national medical specialty societies wrote to Dr. Nora expressing their concerns that “professional self-regulation is under attack. Concerns regarding the usefulness of the high-stakes exam, the exorbitant costs of the MOC process, and the lack of transparent communication from the certifying boards have led to damaging the MOC brand, and creating state-based attacks on the MOC process.”6
In December 2017, Dr. Lawrence and Mr. Palmisano led a meeting of principals from the national medical specialty societies and state medical societies with leaders of ABMS and 8 specialty boards, including ABOG, an opportunity to secure meaningful change. Dr. Lawrence began by stressing that the interests of physicians and patients would be best served by all parties coming together and collaborating on a meaningful solution, to repair trust and preserve physician self-regulation.
Dr. Ramin presented ABOG’s approach to continuous certification, lifelong learning, and self-assessment. The American Board of Urology and the American Board of Psychiatry and Neurology indicated that they were basing important changes in their MOC process on ABOG’s work, including using 5 modules (1 general and 4 specific to the physician’s practice) and multiple open-book mini-exams based on selected journal articles as an alternative to the 10-year MOC exam.
The Vision Initiative. At that meeting and others, the ABMS and other boards heard physicians’ candid and sometimes blunt concerns. Dr. Nora spoke to the recently announced Continuing Board Certification: Vision for the Future program, also known as the “Vision Initiative,” a process designed to fundamentally rebuild the continuing certification process with input and guidance from practicing physicians. Physician response seemed uniform: Seeing is believing.
Importantly, all participants at the December meeting agreed to work together to rebuild trust and ensure professionalism and professional self-regulation, reflected in this Statement of Shared Purpose:
ABMS certifying boards and national medical specialty societies will collaborate to resolve differences in the process of ongoing certification and to fulfill the principles of professional self-regulation, achieving appropriate standardization, and assuring that ongoing certification is relevant to the practices of physicians without undue burden. Furthermore, the boards and societies, and their organizations (ABMS and CMSS [Council of Medical Specialty Societies]), will undertake necessary changes in a timely manner, and will commit to ongoing communication with state medical associations to solicit their input.4
Two ObGyns participating in the Vision Initiative are Haywood Brown, MD, ACOG’s Immediate Past President, and George Wendel, MD, ABOG’s Executive Director. The Vision Initiative is composed of 3 parts. Part 1, Organization, is complete. The committee is currently working on part 2, Envisioning the Future, an information-gathering component that includes physician surveys, hearings, open solicited input, and identifying new and better approaches. After the final report is delivered to the ABMS in February 2019, part 3, Implementation, will begin.
The Vision Initiative offers physicians an important opportunity to help shape the future of continuing education and certification. ObGyns and other physicians should consider reviewing and commenting on the draft report, due in November, during the public comment period. Visit https://visioninitiative.org for more information and to sign up for email updates.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American Board of Obstetrics and Gynecology. From pilot to permanent: ABOG's program offering an innovative pathway integrating lifelong learning and self-assessment and external assessment is approved. https://www.abog.org/new/ABOG_PilotToPermanent.aspx. Accessed July 6, 2018.
- Ramin S. American Board of Obstetrics and Gynecology MOC program. PowerPoint presentation; December 4, 2017.
- Teirstein PS. Boarded to death--why maintenance of certification is bad for doctors and patients. N Engl J Med. 2015;372(2):106-108.
- AMA Council on Medical Education. Executive summary. 2017. https://www.ama-assn.org/sites/default/files/media-browser/public/council-on-med-ed/a18-cme-02.pdf. Accessed July 6, 2018.
- American College of Obstetricians and Gynecologists. ACOG-ABOG joint statement: political interference in physician maintenance of skills threatens women's health care. https://www.acog.org/-/media/Departments/State-Legislative-Activities/2017ACOG-ABMS-MOC-Statement.pdf?dmc=1&ts=20180706T1615538746. Accessed July 6, 2018.
- Letter to Lois Nora, MD, JD. August 18, 2017. https://www.mainemed.com/sites/default/files/content/MOC%20Letter%20082117.pdf. Accessed July 6, 2018.
- American Board of Obstetrics and Gynecology. From pilot to permanent: ABOG's program offering an innovative pathway integrating lifelong learning and self-assessment and external assessment is approved. https://www.abog.org/new/ABOG_PilotToPermanent.aspx. Accessed July 6, 2018.
- Ramin S. American Board of Obstetrics and Gynecology MOC program. PowerPoint presentation; December 4, 2017.
- Teirstein PS. Boarded to death--why maintenance of certification is bad for doctors and patients. N Engl J Med. 2015;372(2):106-108.
- AMA Council on Medical Education. Executive summary. 2017. https://www.ama-assn.org/sites/default/files/media-browser/public/council-on-med-ed/a18-cme-02.pdf. Accessed July 6, 2018.
- American College of Obstetricians and Gynecologists. ACOG-ABOG joint statement: political interference in physician maintenance of skills threatens women's health care. https://www.acog.org/-/media/Departments/State-Legislative-Activities/2017ACOG-ABMS-MOC-Statement.pdf?dmc=1&ts=20180706T1615538746. Accessed July 6, 2018.
- Letter to Lois Nora, MD, JD. August 18, 2017. https://www.mainemed.com/sites/default/files/content/MOC%20Letter%20082117.pdf. Accessed July 6, 2018.
CDC apps specific for ObGyns
The Centers for Disease Control and Prevention (CDC) is a US federal agency under the Department of Health and Human Services. It is the nation’s leading public health institute. Its main goal is to save lives and protect people from health, safety, and security threats. The CDC website lists 25 no-cost applications that the agency has developed: https://www.cdc.gov/mobile/mobileapp.html.
This review will focus on 3 CDC apps (Table) that I feel are useful to ObGyn health care providers: Prevent Group B Strep (GBS), STD Tx Guide, and US Medical Eligibility Criteria for Contraceptive Use. In fact, in an evaluation of contraception apps for providers of family planning services, US Medical Eligibility Criteria for Contraceptive Use was one of the highest scoring apps.1 I will evaluate each app by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).2 I commend the CDC for developing these useful tools to assist health care providers.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Perry R, Lunde B, Chen KT. An evaluation of contraception mobile applications for providers of family planning services. Contraception. 2016;93(6):539-544.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
The Centers for Disease Control and Prevention (CDC) is a US federal agency under the Department of Health and Human Services. It is the nation’s leading public health institute. Its main goal is to save lives and protect people from health, safety, and security threats. The CDC website lists 25 no-cost applications that the agency has developed: https://www.cdc.gov/mobile/mobileapp.html.
This review will focus on 3 CDC apps (Table) that I feel are useful to ObGyn health care providers: Prevent Group B Strep (GBS), STD Tx Guide, and US Medical Eligibility Criteria for Contraceptive Use. In fact, in an evaluation of contraception apps for providers of family planning services, US Medical Eligibility Criteria for Contraceptive Use was one of the highest scoring apps.1 I will evaluate each app by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).2 I commend the CDC for developing these useful tools to assist health care providers.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The Centers for Disease Control and Prevention (CDC) is a US federal agency under the Department of Health and Human Services. It is the nation’s leading public health institute. Its main goal is to save lives and protect people from health, safety, and security threats. The CDC website lists 25 no-cost applications that the agency has developed: https://www.cdc.gov/mobile/mobileapp.html.
This review will focus on 3 CDC apps (Table) that I feel are useful to ObGyn health care providers: Prevent Group B Strep (GBS), STD Tx Guide, and US Medical Eligibility Criteria for Contraceptive Use. In fact, in an evaluation of contraception apps for providers of family planning services, US Medical Eligibility Criteria for Contraceptive Use was one of the highest scoring apps.1 I will evaluate each app by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).2 I commend the CDC for developing these useful tools to assist health care providers.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Perry R, Lunde B, Chen KT. An evaluation of contraception mobile applications for providers of family planning services. Contraception. 2016;93(6):539-544.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
- Perry R, Lunde B, Chen KT. An evaluation of contraception mobile applications for providers of family planning services. Contraception. 2016;93(6):539-544.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
IN THIS ARTICLE
- Details on recommended apps
Cervical screening recommendations do not cover all circumstances
Cervical screening recommendations do not cover all circumstances
Starting cervical cancer screening at age 21 does not necessarily take into account the fact that we are seeing youngsters initiating sexual activity as young as age 9. We obviously see pregnancies early as well. Waiting to screen until age 21, therefore, may cause us to miss the development of high-grade lesions and cervical cancer. As you know, cases in the literature report instances of invasive cancer with first Pap test at age 21. Also, human papillomavirus (HPV) is spread by sexual activity, with the squamous columnar junction more susceptible to infection at a young age.
Recommendations regarding cervical cancer screening for older women also should take into account new sexual partners. Currently, both men and women are living longer and are remarrying or are sexually active with multiple partners. The fact that older women are desiring hormone replacement for vaginal lubrication and dyspareunia shows that they are sexually active even in their late 70s. I believe that the incidence of HPV infection to cervical, vaginal, and vulvar tissue will be increasing as a result.
In an age in which primary care physicians do not have time to perform Pap tests or vaginal, cervical, and vulvar exams because they are overwhelmed with keeping up with patients’ major medical issues is a misunderstanding regarding current recommendations for Pap test screening.
Elizabeth Reinoehl-McClaskey, DO
Onley, Virginia
Dr. Einstein responds
Sexual behavior can start early, but this does not lead to cancer. When we screen, we are looking for cancer, not HPV infection, which is quite common in women and men younger than age 21. Also, one might question whether current screening techniques pick up early-onset tumors. Regarding older women, sexual activity and the rate of older women getting cervical cancer should be considered in future guidelines.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Cervical screening recommendations do not cover all circumstances
Starting cervical cancer screening at age 21 does not necessarily take into account the fact that we are seeing youngsters initiating sexual activity as young as age 9. We obviously see pregnancies early as well. Waiting to screen until age 21, therefore, may cause us to miss the development of high-grade lesions and cervical cancer. As you know, cases in the literature report instances of invasive cancer with first Pap test at age 21. Also, human papillomavirus (HPV) is spread by sexual activity, with the squamous columnar junction more susceptible to infection at a young age.
Recommendations regarding cervical cancer screening for older women also should take into account new sexual partners. Currently, both men and women are living longer and are remarrying or are sexually active with multiple partners. The fact that older women are desiring hormone replacement for vaginal lubrication and dyspareunia shows that they are sexually active even in their late 70s. I believe that the incidence of HPV infection to cervical, vaginal, and vulvar tissue will be increasing as a result.
In an age in which primary care physicians do not have time to perform Pap tests or vaginal, cervical, and vulvar exams because they are overwhelmed with keeping up with patients’ major medical issues is a misunderstanding regarding current recommendations for Pap test screening.
Elizabeth Reinoehl-McClaskey, DO
Onley, Virginia
Dr. Einstein responds
Sexual behavior can start early, but this does not lead to cancer. When we screen, we are looking for cancer, not HPV infection, which is quite common in women and men younger than age 21. Also, one might question whether current screening techniques pick up early-onset tumors. Regarding older women, sexual activity and the rate of older women getting cervical cancer should be considered in future guidelines.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Cervical screening recommendations do not cover all circumstances
Starting cervical cancer screening at age 21 does not necessarily take into account the fact that we are seeing youngsters initiating sexual activity as young as age 9. We obviously see pregnancies early as well. Waiting to screen until age 21, therefore, may cause us to miss the development of high-grade lesions and cervical cancer. As you know, cases in the literature report instances of invasive cancer with first Pap test at age 21. Also, human papillomavirus (HPV) is spread by sexual activity, with the squamous columnar junction more susceptible to infection at a young age.
Recommendations regarding cervical cancer screening for older women also should take into account new sexual partners. Currently, both men and women are living longer and are remarrying or are sexually active with multiple partners. The fact that older women are desiring hormone replacement for vaginal lubrication and dyspareunia shows that they are sexually active even in their late 70s. I believe that the incidence of HPV infection to cervical, vaginal, and vulvar tissue will be increasing as a result.
In an age in which primary care physicians do not have time to perform Pap tests or vaginal, cervical, and vulvar exams because they are overwhelmed with keeping up with patients’ major medical issues is a misunderstanding regarding current recommendations for Pap test screening.
Elizabeth Reinoehl-McClaskey, DO
Onley, Virginia
Dr. Einstein responds
Sexual behavior can start early, but this does not lead to cancer. When we screen, we are looking for cancer, not HPV infection, which is quite common in women and men younger than age 21. Also, one might question whether current screening techniques pick up early-onset tumors. Regarding older women, sexual activity and the rate of older women getting cervical cancer should be considered in future guidelines.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Midwife-physician alliance benefits women
Midwife-physician alliance benefits women
I want to thank Dr. Barbieri for the introduction to his April editorial in which he states that the “trusted nurse midwife asks you to consult on her patient.” Where I practice (in a large suburb of Kansas with a hospital where more than 5,000 babies are delivered yearly), there is a serious lack of midwives and an even greater lack of physicians to support them. As the co-owner of an independently owned nurse-midwife practice, after losing our collaborating physician, we were unable to secure collaboration from any other group, despite our cesarean delivery rate of 5%, vaginal birth after cesarean success rate of 87%, and chorioamnionitis rate of 0%. Please continue to educate your readers on the benefit to women when all obstetric providers work together.
Julie Gorenc, CNM
Lenexa, Kansas
Dr. Barbieri responds
I thank Ms. Gorenc for her support of OBG Management and share her concern about optimizing obstetric care. Given the pending shortage of clinicians, we will need all experienced clinicians to work together to ensure access to high-quality obstetric care. My observation is that many obstetricians are concerned about liability issues that can be associated with coverage of other clinicians, including nurse midwives. The quality of obstetric care and collaboration would be enhanced if our medical tort system could evolve to a “just culture,” ending the “blame and shame” associated with tort litigation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Midwife-physician alliance benefits women
I want to thank Dr. Barbieri for the introduction to his April editorial in which he states that the “trusted nurse midwife asks you to consult on her patient.” Where I practice (in a large suburb of Kansas with a hospital where more than 5,000 babies are delivered yearly), there is a serious lack of midwives and an even greater lack of physicians to support them. As the co-owner of an independently owned nurse-midwife practice, after losing our collaborating physician, we were unable to secure collaboration from any other group, despite our cesarean delivery rate of 5%, vaginal birth after cesarean success rate of 87%, and chorioamnionitis rate of 0%. Please continue to educate your readers on the benefit to women when all obstetric providers work together.
Julie Gorenc, CNM
Lenexa, Kansas
Dr. Barbieri responds
I thank Ms. Gorenc for her support of OBG Management and share her concern about optimizing obstetric care. Given the pending shortage of clinicians, we will need all experienced clinicians to work together to ensure access to high-quality obstetric care. My observation is that many obstetricians are concerned about liability issues that can be associated with coverage of other clinicians, including nurse midwives. The quality of obstetric care and collaboration would be enhanced if our medical tort system could evolve to a “just culture,” ending the “blame and shame” associated with tort litigation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Midwife-physician alliance benefits women
I want to thank Dr. Barbieri for the introduction to his April editorial in which he states that the “trusted nurse midwife asks you to consult on her patient.” Where I practice (in a large suburb of Kansas with a hospital where more than 5,000 babies are delivered yearly), there is a serious lack of midwives and an even greater lack of physicians to support them. As the co-owner of an independently owned nurse-midwife practice, after losing our collaborating physician, we were unable to secure collaboration from any other group, despite our cesarean delivery rate of 5%, vaginal birth after cesarean success rate of 87%, and chorioamnionitis rate of 0%. Please continue to educate your readers on the benefit to women when all obstetric providers work together.
Julie Gorenc, CNM
Lenexa, Kansas
Dr. Barbieri responds
I thank Ms. Gorenc for her support of OBG Management and share her concern about optimizing obstetric care. Given the pending shortage of clinicians, we will need all experienced clinicians to work together to ensure access to high-quality obstetric care. My observation is that many obstetricians are concerned about liability issues that can be associated with coverage of other clinicians, including nurse midwives. The quality of obstetric care and collaboration would be enhanced if our medical tort system could evolve to a “just culture,” ending the “blame and shame” associated with tort litigation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Diagnostics company asserts medical and pathology groups prefer cotesting for cervical cancer screening
Diagnostics company asserts medical and pathology groups prefer cotesting for cervical cancer screening
We are concerned about Dr. Wright’s March 2018 gynecologic cancer coverage of US Preventive Services Task Force (USPSTF) screening guidelines for cervical cancer.
The article suggests that draft USPSTF cervical cancer guidelines issued in September 2017 are final when in fact that is not the case. The USPSTF issued draft guidelines in late 2017, butfinal publication is pending USPSTFrevisions in response to submitted public comments. This means that, for now, existing USPSTF guidelines remain in place, and these guidelines clearly recommend cotesting (high-risk HPV and cytology/Pap) in women 30 to 65 years of age every 5 years as an appropriate screening modality, in alignment with the American College of Obstetricians and Gynecologists, the American Society for Colposcopy and Cervical Pathology, and the American Cancer Society, among others.
It is also notable that the proposed USPSTF guidelines have been met with sharp resistance. ACOG, as well as several organizations, including the American Society of Clinical Pathology, American Society of Cytopathology, the American Society for Cytotechnology, the College of American Pathologists, the International Academy of Cytology, and the Papanicolaou Society of Cytopathology, cite concerns with the proposed USPSTF guidelines and continue to argue in favor of cotesting in women 30 to 65 years of age.1,2
We also fear that Dr. Wright may have provided data out of context. For instance, he notes that the USPSTF, in its draft guidelines, found that cotesting increased the number of follow-up tests but did not increase detection of CIN3+ in a decision model. Yet, the USPSTF analysis overrelied on research from European populations (not representative of the US cervical cancer experience) and excluded peer-reviewed data of women in the United States, which clearly shows that HPV-Pap together catches more cervical cancers than either Pap or HPV alone.3
D.P. Alagia, MD, and Harvey W. Kaufman, MD, MBA
Quest Diagnostics
Madison, New Jersey
Dr. Wright responds
I thank Drs. Alagia and Kaufman for their interest in the work and their comments regarding the USPSTF cervical cancer guidelines. As stated in the article, the USPSTF recommendations are currently in draft form and subject to revision based on public comment. The guidelines are a synthesis of best available evidence and are meant to weigh the benefits and harms of various cervical cancer screening strategies. The recommendations are based in part on simulation modeling that incorporates available evidence and projects the long-term effects of multiple rounds of screening. While the decision models incorporated a large amount of data and were robust in a variety of sensitivity analyses, as with all decision analyses, they are limited by the underlying assumptions utilized in the model. Over the last 2 decades, screening practices for cervical cancer have dramatically shifted. Highlighting the USPSTF draft guidelines was meant to raise awareness among clinicians and policy makers of the evolving role of high-risk HPV testing, either alone or in combination with cytology, as a screening modality for cervical cancer.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American College of Obstetricians and Gynecologists. Leading women’s health care groups issue joint statement on USPSTF draft cervical cancer screening recommendations. September 13, 2017. https://www.acog.org/About-ACOG/News-Room/Statements/2017/Leading-Womens-Health-Care-Groups-Issue-Joint-Statement-on-USPSTF. Accessed July 5, 2018.
- Cytopathology Education and Technology Consortium. Response to new USPSTF guidelines for cervical cancer screening. October 2, 2017. https://s3.amazonaws.com/ascpcdn/static/ONELab/pdf/2017/CETC+-USPSTF+Letter+10-2-17.PDF. Accessed July 5, 2018.
- Blatt AJ, Kennedy R, Luff RD, Austin RM, Rabin DS. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015;123:282–288.
Diagnostics company asserts medical and pathology groups prefer cotesting for cervical cancer screening
We are concerned about Dr. Wright’s March 2018 gynecologic cancer coverage of US Preventive Services Task Force (USPSTF) screening guidelines for cervical cancer.
The article suggests that draft USPSTF cervical cancer guidelines issued in September 2017 are final when in fact that is not the case. The USPSTF issued draft guidelines in late 2017, butfinal publication is pending USPSTFrevisions in response to submitted public comments. This means that, for now, existing USPSTF guidelines remain in place, and these guidelines clearly recommend cotesting (high-risk HPV and cytology/Pap) in women 30 to 65 years of age every 5 years as an appropriate screening modality, in alignment with the American College of Obstetricians and Gynecologists, the American Society for Colposcopy and Cervical Pathology, and the American Cancer Society, among others.
It is also notable that the proposed USPSTF guidelines have been met with sharp resistance. ACOG, as well as several organizations, including the American Society of Clinical Pathology, American Society of Cytopathology, the American Society for Cytotechnology, the College of American Pathologists, the International Academy of Cytology, and the Papanicolaou Society of Cytopathology, cite concerns with the proposed USPSTF guidelines and continue to argue in favor of cotesting in women 30 to 65 years of age.1,2
We also fear that Dr. Wright may have provided data out of context. For instance, he notes that the USPSTF, in its draft guidelines, found that cotesting increased the number of follow-up tests but did not increase detection of CIN3+ in a decision model. Yet, the USPSTF analysis overrelied on research from European populations (not representative of the US cervical cancer experience) and excluded peer-reviewed data of women in the United States, which clearly shows that HPV-Pap together catches more cervical cancers than either Pap or HPV alone.3
D.P. Alagia, MD, and Harvey W. Kaufman, MD, MBA
Quest Diagnostics
Madison, New Jersey
Dr. Wright responds
I thank Drs. Alagia and Kaufman for their interest in the work and their comments regarding the USPSTF cervical cancer guidelines. As stated in the article, the USPSTF recommendations are currently in draft form and subject to revision based on public comment. The guidelines are a synthesis of best available evidence and are meant to weigh the benefits and harms of various cervical cancer screening strategies. The recommendations are based in part on simulation modeling that incorporates available evidence and projects the long-term effects of multiple rounds of screening. While the decision models incorporated a large amount of data and were robust in a variety of sensitivity analyses, as with all decision analyses, they are limited by the underlying assumptions utilized in the model. Over the last 2 decades, screening practices for cervical cancer have dramatically shifted. Highlighting the USPSTF draft guidelines was meant to raise awareness among clinicians and policy makers of the evolving role of high-risk HPV testing, either alone or in combination with cytology, as a screening modality for cervical cancer.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Diagnostics company asserts medical and pathology groups prefer cotesting for cervical cancer screening
We are concerned about Dr. Wright’s March 2018 gynecologic cancer coverage of US Preventive Services Task Force (USPSTF) screening guidelines for cervical cancer.
The article suggests that draft USPSTF cervical cancer guidelines issued in September 2017 are final when in fact that is not the case. The USPSTF issued draft guidelines in late 2017, butfinal publication is pending USPSTFrevisions in response to submitted public comments. This means that, for now, existing USPSTF guidelines remain in place, and these guidelines clearly recommend cotesting (high-risk HPV and cytology/Pap) in women 30 to 65 years of age every 5 years as an appropriate screening modality, in alignment with the American College of Obstetricians and Gynecologists, the American Society for Colposcopy and Cervical Pathology, and the American Cancer Society, among others.
It is also notable that the proposed USPSTF guidelines have been met with sharp resistance. ACOG, as well as several organizations, including the American Society of Clinical Pathology, American Society of Cytopathology, the American Society for Cytotechnology, the College of American Pathologists, the International Academy of Cytology, and the Papanicolaou Society of Cytopathology, cite concerns with the proposed USPSTF guidelines and continue to argue in favor of cotesting in women 30 to 65 years of age.1,2
We also fear that Dr. Wright may have provided data out of context. For instance, he notes that the USPSTF, in its draft guidelines, found that cotesting increased the number of follow-up tests but did not increase detection of CIN3+ in a decision model. Yet, the USPSTF analysis overrelied on research from European populations (not representative of the US cervical cancer experience) and excluded peer-reviewed data of women in the United States, which clearly shows that HPV-Pap together catches more cervical cancers than either Pap or HPV alone.3
D.P. Alagia, MD, and Harvey W. Kaufman, MD, MBA
Quest Diagnostics
Madison, New Jersey
Dr. Wright responds
I thank Drs. Alagia and Kaufman for their interest in the work and their comments regarding the USPSTF cervical cancer guidelines. As stated in the article, the USPSTF recommendations are currently in draft form and subject to revision based on public comment. The guidelines are a synthesis of best available evidence and are meant to weigh the benefits and harms of various cervical cancer screening strategies. The recommendations are based in part on simulation modeling that incorporates available evidence and projects the long-term effects of multiple rounds of screening. While the decision models incorporated a large amount of data and were robust in a variety of sensitivity analyses, as with all decision analyses, they are limited by the underlying assumptions utilized in the model. Over the last 2 decades, screening practices for cervical cancer have dramatically shifted. Highlighting the USPSTF draft guidelines was meant to raise awareness among clinicians and policy makers of the evolving role of high-risk HPV testing, either alone or in combination with cytology, as a screening modality for cervical cancer.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American College of Obstetricians and Gynecologists. Leading women’s health care groups issue joint statement on USPSTF draft cervical cancer screening recommendations. September 13, 2017. https://www.acog.org/About-ACOG/News-Room/Statements/2017/Leading-Womens-Health-Care-Groups-Issue-Joint-Statement-on-USPSTF. Accessed July 5, 2018.
- Cytopathology Education and Technology Consortium. Response to new USPSTF guidelines for cervical cancer screening. October 2, 2017. https://s3.amazonaws.com/ascpcdn/static/ONELab/pdf/2017/CETC+-USPSTF+Letter+10-2-17.PDF. Accessed July 5, 2018.
- Blatt AJ, Kennedy R, Luff RD, Austin RM, Rabin DS. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015;123:282–288.
- American College of Obstetricians and Gynecologists. Leading women’s health care groups issue joint statement on USPSTF draft cervical cancer screening recommendations. September 13, 2017. https://www.acog.org/About-ACOG/News-Room/Statements/2017/Leading-Womens-Health-Care-Groups-Issue-Joint-Statement-on-USPSTF. Accessed July 5, 2018.
- Cytopathology Education and Technology Consortium. Response to new USPSTF guidelines for cervical cancer screening. October 2, 2017. https://s3.amazonaws.com/ascpcdn/static/ONELab/pdf/2017/CETC+-USPSTF+Letter+10-2-17.PDF. Accessed July 5, 2018.
- Blatt AJ, Kennedy R, Luff RD, Austin RM, Rabin DS. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015;123:282–288.








