User login
2018 Update on prenatal carrier screening
Prenatal care has long included carrier screening for genetic diseases, such as cystic fibrosis and Tay-Sachs disease. Recently, advances in genetics technologies led to the development of multiplex panels that can be used to test for hundreds of genetic disorders simultaneously, and can be used to assess carrier status for expectant couples or those planning a pregnancy. Although such screening covers many more conditions than those recommended in traditional guidelines, the benefit of expanded carrier screening (ECS) over standard gene-by-gene testing is not clear.
In this Update, I review recent ECS research that can be helpful to those who practice reproductive endocrinology and infertility medicine, maternal–fetal medicine, and general ObGyn. This research considered some of the many complexities of ECS:
- number and type of severe autosomal recessive conditions identified by an ECS panel, or by panethnic screening for 3 common conditions (cystic fibrosis, fragile X syndrome, spinal muscular atrophy)
- whether the disorders covered by ECS panels meet recommended criteria regarding severity, prevalence, and test accuracy
- women’s thoughts and perspectives on ECS
- whether the marketing materials disseminated by commercial providers of ECS are accurate and balanced.
Genetic diseases identified by expanded carrier screening
Haque IS, Lazarin GA, Kang HP, Evans EA, Goldberg JD, Wapner RJ. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316(7):734-742.
Screening during pregnancy to determine if one or both parents are carriers of genetic disorders historically has involved testing for a limited number of conditions, such as cystic fibrosis, hemoglobinopathies, and Tay-Sachs disease. Patients usually are offered testing for 1 or 2 disorders, with test choices primarily based on patient race and ethnicity. Unfortunately, ancestry-based screening may result in inequitable distribution of genetic testing and resources, as it has significant limitations in our increasingly multicultural society, which includes many people of uncertain or mixed race and ethnicity.
Advantages of expanded carrier screening
Several commercial laboratories now offer ECS. Haque and colleagues used data from one of these laboratories and modeled the predicted number of potentially affected fetuses that would be identified with traditional, ethnicity-based screening as compared with ECS. In one of their hypothetical cohorts, of Northern European couples, traditional screening would identify 55 affected fetuses per 100,000 (1 in 1,800), and ECS would identify 159 per 100,000 (almost 3 times more). The numbers identified with ECS varied with race or ethnicity and ranged from 94 per 100,000 (about 1 in 1,000) for Hispanic couples to 392 per 100,000 (about 1 in 250) for Ashkenazi Jewish couples.
In Australia, Archibald and colleagues conducted a similar study, of panethnic screening of 12,000 women for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy.1 The number of affected fetuses identified was about 1 per 1,000 screened couples--not much different from the ECS number, though comparison is difficult given the likely very different racial and ethnic backgrounds of the 2 cohorts.
Although these data suggest ECS increases detection of genetic disorders, and it seems almost self-evident that more screening is better, there are concerns about ECS.2 Traditional carrier screening methods focus on conditions that significantly affect quality of life--owing to cognitive or physical disabilities or required lifelong medical therapies--and that have a fetal, neonatal, or early-childhood onset and well-defined phenotype. In ECS panels, additional conditions may vary significantly in severity or age of onset. Although some genetic variants on ECS panels have a consistent phenotype, the natural history of others is less well understood. Panels often include conditions for which carrier screening of the general population is not recommended by current guidelines--for example, hemochromatosis and factor V Leiden. Moreover, almost by definition, ECS panels include rare conditions for which the natural history may not be well understood, and the carrier frequency as well as the proportion of condition-causing variants that can be detected may be unclear, leaving the residual risk unknown.
This study provides additional information on the number and type of conditions that can be detected with ECS in different populations. Although ever larger panels can detect more conditions, the veracity of the results and the types of conditions detected are important considerations as providers and patients weigh the risks and benefits of this screening.
Read about the ideal expanded carrier screening panel.
The ideal expanded carrier screening panel
Stevens B, Krstic N, Jones M, Murphy L, Hoskovec J. Finding middle ground in constructing a clinically useful expanded carrier screening panel. Obstet Gynecol. 2017;130(2):279-284.
Both the American College of Obstetricians and Gynecologists (ACOG) and the American College of Medical Genetics and Genomics (ACMG) have proposed criteria for including specific disorders on ECS panels.3,4 These criteria consider disorder characteristics, such as carrier prevalence, which should be at least 1 in 100; severity; early-childhood onset; and complete penetrance. In addition, they consider test characteristics, such as sensitivity, which should be at least 70%.
Details of the study
Stevens and colleagues evaluated the ECS panels offered by 6 commercial laboratories in the United States. They found that only 27% of included conditions met the recommended criteria, and concluded that these panels are putting patients at risk for undue anxiety, and that time and money are being spent on follow-up testing for rare and mild conditions for which the benefits of testing are unclear or unlikely. The potential benefits of the extra screening should be weighed against the significant resulting harms.
Across the 6 ECS panels, 96 conditions met the criteria. As some laboratories allow providers to customize their panels, members of my practice, after reviewing this thought-provoking article, agreed we should create a custom panel that includes only these 96 conditions. Unfortunately, no commercial laboratory includes all 96 conditions, so it is not feasible to create an "ideal" panel at this time.
Arguments favoring ECS include its low cost and the efficiency of screening with multigene panels. In a 2013 study, however, 24% of patients were identified as carriers, and in most cases this finding led to screening for the reproductive partner as well.5 If the rate of detection of the disorder is low, the utility of screening with the same panel may be limited, and couples may require more extensive testing, such as gene sequencing, which is far more expensive. These findings and the additional testing also will increase the need for genetic counseling, and may lead to invasive prenatal diagnostic testing with further increases in costs. If counseling and prenatal testing yield improved outcomes--increased detection of important findings--the benefit will justify the higher costs. However, if the increased costs are largely generated chasing down and explaining findings that are not important to patients or providers, the costs may be incurred without benefit.
For practices that want to offer ECS, it is important to consider the type of conditions on a given laboratory's panel. Panels that include more conditions will detect at least one condition in more patients. As each positive test requires follow-up (typically partner testing), careful consideration should be given up-front to which test is used.
Read about the pregnant women’s perspectives on ECS.
Pregnant women's perspectives on expanded carrier screening
Propst L, Connor G, Hinton M, Poorvu T, Dungan J. Pregnant women's perspectives on expanded carrier screening [published online February 23, 2018]. J Genet Couns. doi:10.1007/s10897-018-0232-x.
Although several authors have discussed ECS detection rates, less has been reported on how women perceive ECS or how they elect or decline screening. Studies have found that the decision to undergo screening for cystic fibrosis is influenced by factors that include age, sex, ethnicity, socioeconomic status, lack of family history, cost, fear of a blood test, lack of knowledge about the condition, already having children, wanting to avoid having a disabled child, abortion preferences, and feeling pressured by health care providers.6,7 Propst and colleagues asked women for their perspectives on ECS, on electing or declining screening, and on any anxiety associated with their decision.
Details of the study
Women who declined ECS said they did so because they:
- had no family history
- knew there was a very small chance their partner carried the same condition
- would not change the course of their pregnancy on the basis of the test results.
Women who elected ECS said they did so because they wanted to:
- know their risk of having a child with a genetic condition
- have all available information about their genetic risks
- be able to make decisions about continuing or terminating their pregnancy.
Women also were asked what they would do if they discovered their fetus had a genetic disorder. About 42% said they were unsure what they would do, 34% said they would continue their pregnancy and prepare for the birth of an affected child, and 24% said they likely would terminate their pregnancy.
The most common reason women gave for declining ECS was that they had no family history. However, ECS is not a good option for women with a positive family history, as they need genetic counseling and specific consideration of their own risks and what testing should be done. The majority of couples who have a child with a genetic disease have no other family history of the disorder. In a study of reproductive carrier screening in Australia, 88% of carriers had no family history.1 Careful pretest counseling is needed to explain the distinction between, on one hand, genetic counseling and testing for those with a family history of genetic disease and, on the other hand, population screening performed to identify unsuspecting individuals who are healthy carriers of genetic disorders.
Another crucial point about carrier screening is the need to consider how its results will be used, and what options the carrier couple will have. For women who are pregnant when a risk is identified, options include expectant management, with diagnosis after birth, or prenatal diagnosis with termination of an affected fetus, out-adoption of an affected fetus, or expectant management with preparation for caring for an affected child. For women who are not pregnant when they have ECS, additional options include use of a gamete (ovum or sperm) donor to achieve pregnancy, or preimplantation genetic diagnosis with implantation of only unaffected embryos.
Different pregnant women may have very different preferences regarding genetic testing. Although many are unsure how they would proceed following the diagnosis of a fetal genetic disorder, it is important to carefully explain their options before any testing is done.
Read about the marketing of ECS.
Marketing of expanded carrier screening
Chokoshvili D, Borry P, Vears DF. A systematic analysis of online marketing materials used by providers of expanded carrier screening [published online December 14, 2017]. Genet Med. doi:10.1038/gim.2017.222.
Prenatal carrier screening can be helpful to women and their families, but it is also a high-volume, lucrative business, with many commercial laboratories competing for the growing ECS market. Professional medical societies recommend making all screening candidates aware of the purpose, characteristics, and limitations of the tests, and of the potential significance of their results. As becoming familiar and comfortable with the tests and explaining them to each patient can be time-consuming, and daunting, many busy clinicians have started relying on marketing materials and other information from the commercial laboratories. Therefore analysis of the accuracy of such materials is in order.
Details of the study
Chokoshvili and colleagues performed a systematic analysis of the quality and accuracy of online marketing materials for ECS. They identified 18 providers: 16 commercial laboratories and 2 medical services providers. All described ECS as a useful tool for family planning, and some were very directive in stating that this testing is "one of the most important steps in preparing for parenthood." In their materials, most of the companies cover some limitations, such as residual risk, but none of the commercial laboratories indicate that ECS can overestimate risk (many variants have incomplete penetrance, meaning that some individuals with a positive test result may in fact be asymptomatic throughout their lifetime).
In addition, whereas a large amount of the marketing materials implies the test was developed in line with professional recommendations, none in fact complies with ACOG and ACMG guidance. Finally, though some of the online information provided by laboratories can be helpful, it is important for clinicians to remember that reproductive genetic counseling should be nondirective and balanced. Carrier testing should be based on patient (not provider) values regarding reproductive autonomy.
Determining that a woman carries a genetic disorder in the preconception period allows more time to evaluate her reproductive partner. If both partners in the couple carry the same genetic disorder, there are more options available to avoid an affected pregnancy. These options include the use of an ovum or sperm donor, or use of preimplantation genetic diagnosis on embryos conceived through in vitro fertilization. While obstetric providers commonly offer carrier screening, and most women are only screened during pregnancy, such genetic testing should be part of pregnancy planning. When gyn providers see patients who are considering a pregnancy, he or she should discuss the options of expanded carrier screening, or ethnicity-based screening.
Summary
ECS increasingly is being adopted into clinical practice. According to ACOG, traditional ethnicity-based screening, panethnic screening (the same limited panel of tests for all patients), and ECS are all acceptable alternatives for prenatal carrier screening.3 For providers who offer ECS, it is important to have a good understanding of each selected test and its limitations. Providers should have a plan for following up patients who have positive test results; this plan may include having genetic counseling and prenatal genetic diagnostic testing in place. Although treatment is available for a few genetic conditions, for the large majority, prenatal screening has not been proved to lead to improved therapeutic options. Providers should try to make sure that patients do not have unrealistic expectations of the outcomes of carrier screening.
Laboratories' educational materials can be useful, but clinicians must carefully assess them before recommending them to patients. Some commercial laboratory information is helpful and balanced; other information is directive or even coercive. Nonbiased information on prenatal genetic testing, for both patients and clinicians, is available in the Genetic Education Modules offered by the Perinatal Quality Foundation (https://www.perinatalquality.org).
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Archibald AD, Smith MJ, Burgess T, et al. Reproductive genetic carrier screening for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy in Australia: outcomes of 12,000 tests [published online October 26, 2017; published correction appears in Genet Med. 2018. doi:10.1038/gim.2017.266]. Genet Med. doi:10.1038/gim.2017.134.
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125(3):653–662.
- Committee on Genetics. Committee opinion no. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129(3):e35–e40.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15(6):482–483.
- Lazarin GA, Haque IS, Nazareth S, et al. An empirical estimate of carrier frequencies for 400+ causal Mendelian variants: results from an ethnically diverse clinical sample of 23,453 individuals. Genet Med. 2013;15(3):178–186.
- Chen LS, Goodson P. Factors affecting decisions to accept or decline cystic fibrosis carrier testing/screening: a theory-guided systematic review. Genet Med. 2007;9(7):442–450.
- Ioannou L, McClaren BJ, Massie J, et al. Population-based carrier screening for cystic fibrosis: a systematic review of 23 years of research. Genet Med. 2014;16(3):207-216.
Prenatal care has long included carrier screening for genetic diseases, such as cystic fibrosis and Tay-Sachs disease. Recently, advances in genetics technologies led to the development of multiplex panels that can be used to test for hundreds of genetic disorders simultaneously, and can be used to assess carrier status for expectant couples or those planning a pregnancy. Although such screening covers many more conditions than those recommended in traditional guidelines, the benefit of expanded carrier screening (ECS) over standard gene-by-gene testing is not clear.
In this Update, I review recent ECS research that can be helpful to those who practice reproductive endocrinology and infertility medicine, maternal–fetal medicine, and general ObGyn. This research considered some of the many complexities of ECS:
- number and type of severe autosomal recessive conditions identified by an ECS panel, or by panethnic screening for 3 common conditions (cystic fibrosis, fragile X syndrome, spinal muscular atrophy)
- whether the disorders covered by ECS panels meet recommended criteria regarding severity, prevalence, and test accuracy
- women’s thoughts and perspectives on ECS
- whether the marketing materials disseminated by commercial providers of ECS are accurate and balanced.
Genetic diseases identified by expanded carrier screening
Haque IS, Lazarin GA, Kang HP, Evans EA, Goldberg JD, Wapner RJ. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316(7):734-742.
Screening during pregnancy to determine if one or both parents are carriers of genetic disorders historically has involved testing for a limited number of conditions, such as cystic fibrosis, hemoglobinopathies, and Tay-Sachs disease. Patients usually are offered testing for 1 or 2 disorders, with test choices primarily based on patient race and ethnicity. Unfortunately, ancestry-based screening may result in inequitable distribution of genetic testing and resources, as it has significant limitations in our increasingly multicultural society, which includes many people of uncertain or mixed race and ethnicity.
Advantages of expanded carrier screening
Several commercial laboratories now offer ECS. Haque and colleagues used data from one of these laboratories and modeled the predicted number of potentially affected fetuses that would be identified with traditional, ethnicity-based screening as compared with ECS. In one of their hypothetical cohorts, of Northern European couples, traditional screening would identify 55 affected fetuses per 100,000 (1 in 1,800), and ECS would identify 159 per 100,000 (almost 3 times more). The numbers identified with ECS varied with race or ethnicity and ranged from 94 per 100,000 (about 1 in 1,000) for Hispanic couples to 392 per 100,000 (about 1 in 250) for Ashkenazi Jewish couples.
In Australia, Archibald and colleagues conducted a similar study, of panethnic screening of 12,000 women for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy.1 The number of affected fetuses identified was about 1 per 1,000 screened couples--not much different from the ECS number, though comparison is difficult given the likely very different racial and ethnic backgrounds of the 2 cohorts.
Although these data suggest ECS increases detection of genetic disorders, and it seems almost self-evident that more screening is better, there are concerns about ECS.2 Traditional carrier screening methods focus on conditions that significantly affect quality of life--owing to cognitive or physical disabilities or required lifelong medical therapies--and that have a fetal, neonatal, or early-childhood onset and well-defined phenotype. In ECS panels, additional conditions may vary significantly in severity or age of onset. Although some genetic variants on ECS panels have a consistent phenotype, the natural history of others is less well understood. Panels often include conditions for which carrier screening of the general population is not recommended by current guidelines--for example, hemochromatosis and factor V Leiden. Moreover, almost by definition, ECS panels include rare conditions for which the natural history may not be well understood, and the carrier frequency as well as the proportion of condition-causing variants that can be detected may be unclear, leaving the residual risk unknown.
This study provides additional information on the number and type of conditions that can be detected with ECS in different populations. Although ever larger panels can detect more conditions, the veracity of the results and the types of conditions detected are important considerations as providers and patients weigh the risks and benefits of this screening.
Read about the ideal expanded carrier screening panel.
The ideal expanded carrier screening panel
Stevens B, Krstic N, Jones M, Murphy L, Hoskovec J. Finding middle ground in constructing a clinically useful expanded carrier screening panel. Obstet Gynecol. 2017;130(2):279-284.
Both the American College of Obstetricians and Gynecologists (ACOG) and the American College of Medical Genetics and Genomics (ACMG) have proposed criteria for including specific disorders on ECS panels.3,4 These criteria consider disorder characteristics, such as carrier prevalence, which should be at least 1 in 100; severity; early-childhood onset; and complete penetrance. In addition, they consider test characteristics, such as sensitivity, which should be at least 70%.
Details of the study
Stevens and colleagues evaluated the ECS panels offered by 6 commercial laboratories in the United States. They found that only 27% of included conditions met the recommended criteria, and concluded that these panels are putting patients at risk for undue anxiety, and that time and money are being spent on follow-up testing for rare and mild conditions for which the benefits of testing are unclear or unlikely. The potential benefits of the extra screening should be weighed against the significant resulting harms.
Across the 6 ECS panels, 96 conditions met the criteria. As some laboratories allow providers to customize their panels, members of my practice, after reviewing this thought-provoking article, agreed we should create a custom panel that includes only these 96 conditions. Unfortunately, no commercial laboratory includes all 96 conditions, so it is not feasible to create an "ideal" panel at this time.
Arguments favoring ECS include its low cost and the efficiency of screening with multigene panels. In a 2013 study, however, 24% of patients were identified as carriers, and in most cases this finding led to screening for the reproductive partner as well.5 If the rate of detection of the disorder is low, the utility of screening with the same panel may be limited, and couples may require more extensive testing, such as gene sequencing, which is far more expensive. These findings and the additional testing also will increase the need for genetic counseling, and may lead to invasive prenatal diagnostic testing with further increases in costs. If counseling and prenatal testing yield improved outcomes--increased detection of important findings--the benefit will justify the higher costs. However, if the increased costs are largely generated chasing down and explaining findings that are not important to patients or providers, the costs may be incurred without benefit.
For practices that want to offer ECS, it is important to consider the type of conditions on a given laboratory's panel. Panels that include more conditions will detect at least one condition in more patients. As each positive test requires follow-up (typically partner testing), careful consideration should be given up-front to which test is used.
Read about the pregnant women’s perspectives on ECS.
Pregnant women's perspectives on expanded carrier screening
Propst L, Connor G, Hinton M, Poorvu T, Dungan J. Pregnant women's perspectives on expanded carrier screening [published online February 23, 2018]. J Genet Couns. doi:10.1007/s10897-018-0232-x.
Although several authors have discussed ECS detection rates, less has been reported on how women perceive ECS or how they elect or decline screening. Studies have found that the decision to undergo screening for cystic fibrosis is influenced by factors that include age, sex, ethnicity, socioeconomic status, lack of family history, cost, fear of a blood test, lack of knowledge about the condition, already having children, wanting to avoid having a disabled child, abortion preferences, and feeling pressured by health care providers.6,7 Propst and colleagues asked women for their perspectives on ECS, on electing or declining screening, and on any anxiety associated with their decision.
Details of the study
Women who declined ECS said they did so because they:
- had no family history
- knew there was a very small chance their partner carried the same condition
- would not change the course of their pregnancy on the basis of the test results.
Women who elected ECS said they did so because they wanted to:
- know their risk of having a child with a genetic condition
- have all available information about their genetic risks
- be able to make decisions about continuing or terminating their pregnancy.
Women also were asked what they would do if they discovered their fetus had a genetic disorder. About 42% said they were unsure what they would do, 34% said they would continue their pregnancy and prepare for the birth of an affected child, and 24% said they likely would terminate their pregnancy.
The most common reason women gave for declining ECS was that they had no family history. However, ECS is not a good option for women with a positive family history, as they need genetic counseling and specific consideration of their own risks and what testing should be done. The majority of couples who have a child with a genetic disease have no other family history of the disorder. In a study of reproductive carrier screening in Australia, 88% of carriers had no family history.1 Careful pretest counseling is needed to explain the distinction between, on one hand, genetic counseling and testing for those with a family history of genetic disease and, on the other hand, population screening performed to identify unsuspecting individuals who are healthy carriers of genetic disorders.
Another crucial point about carrier screening is the need to consider how its results will be used, and what options the carrier couple will have. For women who are pregnant when a risk is identified, options include expectant management, with diagnosis after birth, or prenatal diagnosis with termination of an affected fetus, out-adoption of an affected fetus, or expectant management with preparation for caring for an affected child. For women who are not pregnant when they have ECS, additional options include use of a gamete (ovum or sperm) donor to achieve pregnancy, or preimplantation genetic diagnosis with implantation of only unaffected embryos.
Different pregnant women may have very different preferences regarding genetic testing. Although many are unsure how they would proceed following the diagnosis of a fetal genetic disorder, it is important to carefully explain their options before any testing is done.
Read about the marketing of ECS.
Marketing of expanded carrier screening
Chokoshvili D, Borry P, Vears DF. A systematic analysis of online marketing materials used by providers of expanded carrier screening [published online December 14, 2017]. Genet Med. doi:10.1038/gim.2017.222.
Prenatal carrier screening can be helpful to women and their families, but it is also a high-volume, lucrative business, with many commercial laboratories competing for the growing ECS market. Professional medical societies recommend making all screening candidates aware of the purpose, characteristics, and limitations of the tests, and of the potential significance of their results. As becoming familiar and comfortable with the tests and explaining them to each patient can be time-consuming, and daunting, many busy clinicians have started relying on marketing materials and other information from the commercial laboratories. Therefore analysis of the accuracy of such materials is in order.
Details of the study
Chokoshvili and colleagues performed a systematic analysis of the quality and accuracy of online marketing materials for ECS. They identified 18 providers: 16 commercial laboratories and 2 medical services providers. All described ECS as a useful tool for family planning, and some were very directive in stating that this testing is "one of the most important steps in preparing for parenthood." In their materials, most of the companies cover some limitations, such as residual risk, but none of the commercial laboratories indicate that ECS can overestimate risk (many variants have incomplete penetrance, meaning that some individuals with a positive test result may in fact be asymptomatic throughout their lifetime).
In addition, whereas a large amount of the marketing materials implies the test was developed in line with professional recommendations, none in fact complies with ACOG and ACMG guidance. Finally, though some of the online information provided by laboratories can be helpful, it is important for clinicians to remember that reproductive genetic counseling should be nondirective and balanced. Carrier testing should be based on patient (not provider) values regarding reproductive autonomy.
Determining that a woman carries a genetic disorder in the preconception period allows more time to evaluate her reproductive partner. If both partners in the couple carry the same genetic disorder, there are more options available to avoid an affected pregnancy. These options include the use of an ovum or sperm donor, or use of preimplantation genetic diagnosis on embryos conceived through in vitro fertilization. While obstetric providers commonly offer carrier screening, and most women are only screened during pregnancy, such genetic testing should be part of pregnancy planning. When gyn providers see patients who are considering a pregnancy, he or she should discuss the options of expanded carrier screening, or ethnicity-based screening.
Summary
ECS increasingly is being adopted into clinical practice. According to ACOG, traditional ethnicity-based screening, panethnic screening (the same limited panel of tests for all patients), and ECS are all acceptable alternatives for prenatal carrier screening.3 For providers who offer ECS, it is important to have a good understanding of each selected test and its limitations. Providers should have a plan for following up patients who have positive test results; this plan may include having genetic counseling and prenatal genetic diagnostic testing in place. Although treatment is available for a few genetic conditions, for the large majority, prenatal screening has not been proved to lead to improved therapeutic options. Providers should try to make sure that patients do not have unrealistic expectations of the outcomes of carrier screening.
Laboratories' educational materials can be useful, but clinicians must carefully assess them before recommending them to patients. Some commercial laboratory information is helpful and balanced; other information is directive or even coercive. Nonbiased information on prenatal genetic testing, for both patients and clinicians, is available in the Genetic Education Modules offered by the Perinatal Quality Foundation (https://www.perinatalquality.org).
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Prenatal care has long included carrier screening for genetic diseases, such as cystic fibrosis and Tay-Sachs disease. Recently, advances in genetics technologies led to the development of multiplex panels that can be used to test for hundreds of genetic disorders simultaneously, and can be used to assess carrier status for expectant couples or those planning a pregnancy. Although such screening covers many more conditions than those recommended in traditional guidelines, the benefit of expanded carrier screening (ECS) over standard gene-by-gene testing is not clear.
In this Update, I review recent ECS research that can be helpful to those who practice reproductive endocrinology and infertility medicine, maternal–fetal medicine, and general ObGyn. This research considered some of the many complexities of ECS:
- number and type of severe autosomal recessive conditions identified by an ECS panel, or by panethnic screening for 3 common conditions (cystic fibrosis, fragile X syndrome, spinal muscular atrophy)
- whether the disorders covered by ECS panels meet recommended criteria regarding severity, prevalence, and test accuracy
- women’s thoughts and perspectives on ECS
- whether the marketing materials disseminated by commercial providers of ECS are accurate and balanced.
Genetic diseases identified by expanded carrier screening
Haque IS, Lazarin GA, Kang HP, Evans EA, Goldberg JD, Wapner RJ. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316(7):734-742.
Screening during pregnancy to determine if one or both parents are carriers of genetic disorders historically has involved testing for a limited number of conditions, such as cystic fibrosis, hemoglobinopathies, and Tay-Sachs disease. Patients usually are offered testing for 1 or 2 disorders, with test choices primarily based on patient race and ethnicity. Unfortunately, ancestry-based screening may result in inequitable distribution of genetic testing and resources, as it has significant limitations in our increasingly multicultural society, which includes many people of uncertain or mixed race and ethnicity.
Advantages of expanded carrier screening
Several commercial laboratories now offer ECS. Haque and colleagues used data from one of these laboratories and modeled the predicted number of potentially affected fetuses that would be identified with traditional, ethnicity-based screening as compared with ECS. In one of their hypothetical cohorts, of Northern European couples, traditional screening would identify 55 affected fetuses per 100,000 (1 in 1,800), and ECS would identify 159 per 100,000 (almost 3 times more). The numbers identified with ECS varied with race or ethnicity and ranged from 94 per 100,000 (about 1 in 1,000) for Hispanic couples to 392 per 100,000 (about 1 in 250) for Ashkenazi Jewish couples.
In Australia, Archibald and colleagues conducted a similar study, of panethnic screening of 12,000 women for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy.1 The number of affected fetuses identified was about 1 per 1,000 screened couples--not much different from the ECS number, though comparison is difficult given the likely very different racial and ethnic backgrounds of the 2 cohorts.
Although these data suggest ECS increases detection of genetic disorders, and it seems almost self-evident that more screening is better, there are concerns about ECS.2 Traditional carrier screening methods focus on conditions that significantly affect quality of life--owing to cognitive or physical disabilities or required lifelong medical therapies--and that have a fetal, neonatal, or early-childhood onset and well-defined phenotype. In ECS panels, additional conditions may vary significantly in severity or age of onset. Although some genetic variants on ECS panels have a consistent phenotype, the natural history of others is less well understood. Panels often include conditions for which carrier screening of the general population is not recommended by current guidelines--for example, hemochromatosis and factor V Leiden. Moreover, almost by definition, ECS panels include rare conditions for which the natural history may not be well understood, and the carrier frequency as well as the proportion of condition-causing variants that can be detected may be unclear, leaving the residual risk unknown.
This study provides additional information on the number and type of conditions that can be detected with ECS in different populations. Although ever larger panels can detect more conditions, the veracity of the results and the types of conditions detected are important considerations as providers and patients weigh the risks and benefits of this screening.
Read about the ideal expanded carrier screening panel.
The ideal expanded carrier screening panel
Stevens B, Krstic N, Jones M, Murphy L, Hoskovec J. Finding middle ground in constructing a clinically useful expanded carrier screening panel. Obstet Gynecol. 2017;130(2):279-284.
Both the American College of Obstetricians and Gynecologists (ACOG) and the American College of Medical Genetics and Genomics (ACMG) have proposed criteria for including specific disorders on ECS panels.3,4 These criteria consider disorder characteristics, such as carrier prevalence, which should be at least 1 in 100; severity; early-childhood onset; and complete penetrance. In addition, they consider test characteristics, such as sensitivity, which should be at least 70%.
Details of the study
Stevens and colleagues evaluated the ECS panels offered by 6 commercial laboratories in the United States. They found that only 27% of included conditions met the recommended criteria, and concluded that these panels are putting patients at risk for undue anxiety, and that time and money are being spent on follow-up testing for rare and mild conditions for which the benefits of testing are unclear or unlikely. The potential benefits of the extra screening should be weighed against the significant resulting harms.
Across the 6 ECS panels, 96 conditions met the criteria. As some laboratories allow providers to customize their panels, members of my practice, after reviewing this thought-provoking article, agreed we should create a custom panel that includes only these 96 conditions. Unfortunately, no commercial laboratory includes all 96 conditions, so it is not feasible to create an "ideal" panel at this time.
Arguments favoring ECS include its low cost and the efficiency of screening with multigene panels. In a 2013 study, however, 24% of patients were identified as carriers, and in most cases this finding led to screening for the reproductive partner as well.5 If the rate of detection of the disorder is low, the utility of screening with the same panel may be limited, and couples may require more extensive testing, such as gene sequencing, which is far more expensive. These findings and the additional testing also will increase the need for genetic counseling, and may lead to invasive prenatal diagnostic testing with further increases in costs. If counseling and prenatal testing yield improved outcomes--increased detection of important findings--the benefit will justify the higher costs. However, if the increased costs are largely generated chasing down and explaining findings that are not important to patients or providers, the costs may be incurred without benefit.
For practices that want to offer ECS, it is important to consider the type of conditions on a given laboratory's panel. Panels that include more conditions will detect at least one condition in more patients. As each positive test requires follow-up (typically partner testing), careful consideration should be given up-front to which test is used.
Read about the pregnant women’s perspectives on ECS.
Pregnant women's perspectives on expanded carrier screening
Propst L, Connor G, Hinton M, Poorvu T, Dungan J. Pregnant women's perspectives on expanded carrier screening [published online February 23, 2018]. J Genet Couns. doi:10.1007/s10897-018-0232-x.
Although several authors have discussed ECS detection rates, less has been reported on how women perceive ECS or how they elect or decline screening. Studies have found that the decision to undergo screening for cystic fibrosis is influenced by factors that include age, sex, ethnicity, socioeconomic status, lack of family history, cost, fear of a blood test, lack of knowledge about the condition, already having children, wanting to avoid having a disabled child, abortion preferences, and feeling pressured by health care providers.6,7 Propst and colleagues asked women for their perspectives on ECS, on electing or declining screening, and on any anxiety associated with their decision.
Details of the study
Women who declined ECS said they did so because they:
- had no family history
- knew there was a very small chance their partner carried the same condition
- would not change the course of their pregnancy on the basis of the test results.
Women who elected ECS said they did so because they wanted to:
- know their risk of having a child with a genetic condition
- have all available information about their genetic risks
- be able to make decisions about continuing or terminating their pregnancy.
Women also were asked what they would do if they discovered their fetus had a genetic disorder. About 42% said they were unsure what they would do, 34% said they would continue their pregnancy and prepare for the birth of an affected child, and 24% said they likely would terminate their pregnancy.
The most common reason women gave for declining ECS was that they had no family history. However, ECS is not a good option for women with a positive family history, as they need genetic counseling and specific consideration of their own risks and what testing should be done. The majority of couples who have a child with a genetic disease have no other family history of the disorder. In a study of reproductive carrier screening in Australia, 88% of carriers had no family history.1 Careful pretest counseling is needed to explain the distinction between, on one hand, genetic counseling and testing for those with a family history of genetic disease and, on the other hand, population screening performed to identify unsuspecting individuals who are healthy carriers of genetic disorders.
Another crucial point about carrier screening is the need to consider how its results will be used, and what options the carrier couple will have. For women who are pregnant when a risk is identified, options include expectant management, with diagnosis after birth, or prenatal diagnosis with termination of an affected fetus, out-adoption of an affected fetus, or expectant management with preparation for caring for an affected child. For women who are not pregnant when they have ECS, additional options include use of a gamete (ovum or sperm) donor to achieve pregnancy, or preimplantation genetic diagnosis with implantation of only unaffected embryos.
Different pregnant women may have very different preferences regarding genetic testing. Although many are unsure how they would proceed following the diagnosis of a fetal genetic disorder, it is important to carefully explain their options before any testing is done.
Read about the marketing of ECS.
Marketing of expanded carrier screening
Chokoshvili D, Borry P, Vears DF. A systematic analysis of online marketing materials used by providers of expanded carrier screening [published online December 14, 2017]. Genet Med. doi:10.1038/gim.2017.222.
Prenatal carrier screening can be helpful to women and their families, but it is also a high-volume, lucrative business, with many commercial laboratories competing for the growing ECS market. Professional medical societies recommend making all screening candidates aware of the purpose, characteristics, and limitations of the tests, and of the potential significance of their results. As becoming familiar and comfortable with the tests and explaining them to each patient can be time-consuming, and daunting, many busy clinicians have started relying on marketing materials and other information from the commercial laboratories. Therefore analysis of the accuracy of such materials is in order.
Details of the study
Chokoshvili and colleagues performed a systematic analysis of the quality and accuracy of online marketing materials for ECS. They identified 18 providers: 16 commercial laboratories and 2 medical services providers. All described ECS as a useful tool for family planning, and some were very directive in stating that this testing is "one of the most important steps in preparing for parenthood." In their materials, most of the companies cover some limitations, such as residual risk, but none of the commercial laboratories indicate that ECS can overestimate risk (many variants have incomplete penetrance, meaning that some individuals with a positive test result may in fact be asymptomatic throughout their lifetime).
In addition, whereas a large amount of the marketing materials implies the test was developed in line with professional recommendations, none in fact complies with ACOG and ACMG guidance. Finally, though some of the online information provided by laboratories can be helpful, it is important for clinicians to remember that reproductive genetic counseling should be nondirective and balanced. Carrier testing should be based on patient (not provider) values regarding reproductive autonomy.
Determining that a woman carries a genetic disorder in the preconception period allows more time to evaluate her reproductive partner. If both partners in the couple carry the same genetic disorder, there are more options available to avoid an affected pregnancy. These options include the use of an ovum or sperm donor, or use of preimplantation genetic diagnosis on embryos conceived through in vitro fertilization. While obstetric providers commonly offer carrier screening, and most women are only screened during pregnancy, such genetic testing should be part of pregnancy planning. When gyn providers see patients who are considering a pregnancy, he or she should discuss the options of expanded carrier screening, or ethnicity-based screening.
Summary
ECS increasingly is being adopted into clinical practice. According to ACOG, traditional ethnicity-based screening, panethnic screening (the same limited panel of tests for all patients), and ECS are all acceptable alternatives for prenatal carrier screening.3 For providers who offer ECS, it is important to have a good understanding of each selected test and its limitations. Providers should have a plan for following up patients who have positive test results; this plan may include having genetic counseling and prenatal genetic diagnostic testing in place. Although treatment is available for a few genetic conditions, for the large majority, prenatal screening has not been proved to lead to improved therapeutic options. Providers should try to make sure that patients do not have unrealistic expectations of the outcomes of carrier screening.
Laboratories' educational materials can be useful, but clinicians must carefully assess them before recommending them to patients. Some commercial laboratory information is helpful and balanced; other information is directive or even coercive. Nonbiased information on prenatal genetic testing, for both patients and clinicians, is available in the Genetic Education Modules offered by the Perinatal Quality Foundation (https://www.perinatalquality.org).
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Archibald AD, Smith MJ, Burgess T, et al. Reproductive genetic carrier screening for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy in Australia: outcomes of 12,000 tests [published online October 26, 2017; published correction appears in Genet Med. 2018. doi:10.1038/gim.2017.266]. Genet Med. doi:10.1038/gim.2017.134.
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125(3):653–662.
- Committee on Genetics. Committee opinion no. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129(3):e35–e40.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15(6):482–483.
- Lazarin GA, Haque IS, Nazareth S, et al. An empirical estimate of carrier frequencies for 400+ causal Mendelian variants: results from an ethnically diverse clinical sample of 23,453 individuals. Genet Med. 2013;15(3):178–186.
- Chen LS, Goodson P. Factors affecting decisions to accept or decline cystic fibrosis carrier testing/screening: a theory-guided systematic review. Genet Med. 2007;9(7):442–450.
- Ioannou L, McClaren BJ, Massie J, et al. Population-based carrier screening for cystic fibrosis: a systematic review of 23 years of research. Genet Med. 2014;16(3):207-216.
- Archibald AD, Smith MJ, Burgess T, et al. Reproductive genetic carrier screening for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy in Australia: outcomes of 12,000 tests [published online October 26, 2017; published correction appears in Genet Med. 2018. doi:10.1038/gim.2017.266]. Genet Med. doi:10.1038/gim.2017.134.
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125(3):653–662.
- Committee on Genetics. Committee opinion no. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129(3):e35–e40.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15(6):482–483.
- Lazarin GA, Haque IS, Nazareth S, et al. An empirical estimate of carrier frequencies for 400+ causal Mendelian variants: results from an ethnically diverse clinical sample of 23,453 individuals. Genet Med. 2013;15(3):178–186.
- Chen LS, Goodson P. Factors affecting decisions to accept or decline cystic fibrosis carrier testing/screening: a theory-guided systematic review. Genet Med. 2007;9(7):442–450.
- Ioannou L, McClaren BJ, Massie J, et al. Population-based carrier screening for cystic fibrosis: a systematic review of 23 years of research. Genet Med. 2014;16(3):207-216.
Metformin reduces preterm births, late miscarriages in PCOS
CHICAGO – , preterm births and late miscarriages were reduced, but researchers saw no effect on blood glucose levels or other diabetes-related maternal outcomes.
A Nordic study of pregnant women, dubbed PregMet 2, followed the course of 487 women with PCOS who were randomized to take 2,000 mg of metformin or placebo daily during pregnancy.
Among the entire study population, those with PCOS who took placebo had a 9.6% incidence of late miscarriage or preterm birth. For those taking metformin, the figure was 5%, similar to the 5.2% risk seen in the population-wide Norwegian Birth Registry, said Dr. Løvvik, of the Norwegian University of Science and Technology, Trondheim.
This analysis yielded a number needed to treat (NNT) for benefit of metformin of 22, she said.
However, Dr. Løvvik said that “the elephant in the room” is the lack of benefit of metformin for rates of gestational diabetes, hypertension in pregnancy, or preeclampsia in PregMet 2.
Of the various endpoint analyses, “the most surprising and striking one is the absolute lack of effect on gestational diabetes in this high-risk population,” said Dr. Løvvik. “There’s not even a tendency towards effect. … Metformin had no effect on prevention, treatment, or the need for additional insulin, indicating it was actually as effective as placebo.”
Considering that “metformin is now a part of the standard treatment for gestational diabetes, according to many national guidelines, we think that it’s questionable that it’s never, ever been tested against placebo for this diagnosis,” said Dr. Løvvik.
Pregnant women with PCOS have an increased risk of complications, and metformin is often prescribed off label to attempt to address some of these complications. Some previous work had shown metformin to be helpful, but previous studies have been underpowered, said Dr. Løvvik.
PregMet 2’s protocol attempted to address the literature gap; over 3 years, it called for enrollment of 1,000 women in the first trimester of pregnancy, randomized 1:1 to receive metformin or placebo in pregnancy.
At that enrollment level, the study would aim to show a 50% reduction in late miscarriages and preterm birth, with a power of 85%. Two predetermined analysis were planned at the end of the 3-year study, one for the intent-to-treat population and one for the per-protocol group.
In the end, however, just 487 patients were enrolled and randomized over a period of 5 years, despite recruitment at 14 centers in Iceland, Norway, and Sweden.
Richard Legro, MD, an ob.gyn. from Pennsylvania State University, Hershey, was in attendance. While praising the overall high quality of the study, he commented, “The primary concern is that you stopped the study when you hadn’t even achieved half your sample size.”
Dr. Løvvik said that a variety of factors contributed to the low study enrollment. One primary issue was that participation required additional visits on the part of participants; the study was not part of routine prenatal care, she said. Also, the placebo used in the study expired before enrollment was completed, complicating execution of the study.
She also noted that nearly a quarter of patients had a history of depression, and one in five had a history of migraine. More than half of patients overall had at least one previous or ongoing chronic medical condition.
A small number of patients in each group were excluded for protocol violations, pregnancy termination, intrauterine fetal demise, or loss to follow-up. The final intent-to-treat population included 238 in the metformin group and 240 who received placebo.
The per-protocol analysis was conducted two ways: In the first analysis, only those who dropped out were excluded, leaving 209 in the metformin group and 223 in the placebo group. The second analysis included those whose adherence to taking the study medication was assessed at 90% or better. For this second per-protocol analysis, just 142 metformin takers and 156 in the placebo group remained.
In the intent-to-treat population, the primary endpoint – a composite outcome of late miscarriage and preterm delivery – yielded an odds ratio of 1.99 favoring metformin over placebo, a figure that fell short of statistical significance (95% confidence interval, 0.93-4.51; P = .08).
However, there was significantly less weight gain in those taking metformin in this population (8.7 vs. 11.48 kg; P less than .0001). And newborns had significantly larger head circumferences if their mothers with PCOS took metformin (35.4 vs. 35.0 cm; P = .006).
Dr. Løvvik and her colleagues subsequently performed the same analysis on the first per-protocol group, one that excluded the 46 patients who were early dropouts after randomization. Here, the odds ratio favoring metformin for the composite primary outcome measure was a significant 2.55 (n = 432; 95% CI, 1.10-6.42; P = .03). Results for the final per-protocol analysis that excluded dropouts and those with less than 90% adherence to study medication were similar, with an odds ratio of 2.76 in favor of metformin use during pregnancy (n = 298; 95% CI, 1.0-8.82; P = .05).
In response to an audience question, Dr. Løvvik said that, though a per-protocol analysis of some sort had been predetermined, the decision to perform the narrower analysis on the highly adherent group was made later. However, compared to thresholds for adherence in other studies, “ours was way higher – so it’s too strict,” she acknowledged.
At baseline, the median age overall was about 30 years, and the median body mass index was about 28 kg/m2. Patients were at a median 74 days gestational age of pregnancy at randomization, and 53%-61% of patients were on metformin at the time of conception, with no significant difference between the metformin and placebo group.
A little less than half (40%-46%) of participants had a spontaneous conception, and most participants (56%-59%) had no prior term deliveries. Participants were overwhelmingly (91%-97%) white and Nordic.
There were no serious maternal, fetal, or neonatal safety signals in either study group.
The study followed the earlier PregMet study, which also tracked use of metformin in pregnancy for women with PCOS.
CHICAGO – , preterm births and late miscarriages were reduced, but researchers saw no effect on blood glucose levels or other diabetes-related maternal outcomes.
A Nordic study of pregnant women, dubbed PregMet 2, followed the course of 487 women with PCOS who were randomized to take 2,000 mg of metformin or placebo daily during pregnancy.
Among the entire study population, those with PCOS who took placebo had a 9.6% incidence of late miscarriage or preterm birth. For those taking metformin, the figure was 5%, similar to the 5.2% risk seen in the population-wide Norwegian Birth Registry, said Dr. Løvvik, of the Norwegian University of Science and Technology, Trondheim.
This analysis yielded a number needed to treat (NNT) for benefit of metformin of 22, she said.
However, Dr. Løvvik said that “the elephant in the room” is the lack of benefit of metformin for rates of gestational diabetes, hypertension in pregnancy, or preeclampsia in PregMet 2.
Of the various endpoint analyses, “the most surprising and striking one is the absolute lack of effect on gestational diabetes in this high-risk population,” said Dr. Løvvik. “There’s not even a tendency towards effect. … Metformin had no effect on prevention, treatment, or the need for additional insulin, indicating it was actually as effective as placebo.”
Considering that “metformin is now a part of the standard treatment for gestational diabetes, according to many national guidelines, we think that it’s questionable that it’s never, ever been tested against placebo for this diagnosis,” said Dr. Løvvik.
Pregnant women with PCOS have an increased risk of complications, and metformin is often prescribed off label to attempt to address some of these complications. Some previous work had shown metformin to be helpful, but previous studies have been underpowered, said Dr. Løvvik.
PregMet 2’s protocol attempted to address the literature gap; over 3 years, it called for enrollment of 1,000 women in the first trimester of pregnancy, randomized 1:1 to receive metformin or placebo in pregnancy.
At that enrollment level, the study would aim to show a 50% reduction in late miscarriages and preterm birth, with a power of 85%. Two predetermined analysis were planned at the end of the 3-year study, one for the intent-to-treat population and one for the per-protocol group.
In the end, however, just 487 patients were enrolled and randomized over a period of 5 years, despite recruitment at 14 centers in Iceland, Norway, and Sweden.
Richard Legro, MD, an ob.gyn. from Pennsylvania State University, Hershey, was in attendance. While praising the overall high quality of the study, he commented, “The primary concern is that you stopped the study when you hadn’t even achieved half your sample size.”
Dr. Løvvik said that a variety of factors contributed to the low study enrollment. One primary issue was that participation required additional visits on the part of participants; the study was not part of routine prenatal care, she said. Also, the placebo used in the study expired before enrollment was completed, complicating execution of the study.
She also noted that nearly a quarter of patients had a history of depression, and one in five had a history of migraine. More than half of patients overall had at least one previous or ongoing chronic medical condition.
A small number of patients in each group were excluded for protocol violations, pregnancy termination, intrauterine fetal demise, or loss to follow-up. The final intent-to-treat population included 238 in the metformin group and 240 who received placebo.
The per-protocol analysis was conducted two ways: In the first analysis, only those who dropped out were excluded, leaving 209 in the metformin group and 223 in the placebo group. The second analysis included those whose adherence to taking the study medication was assessed at 90% or better. For this second per-protocol analysis, just 142 metformin takers and 156 in the placebo group remained.
In the intent-to-treat population, the primary endpoint – a composite outcome of late miscarriage and preterm delivery – yielded an odds ratio of 1.99 favoring metformin over placebo, a figure that fell short of statistical significance (95% confidence interval, 0.93-4.51; P = .08).
However, there was significantly less weight gain in those taking metformin in this population (8.7 vs. 11.48 kg; P less than .0001). And newborns had significantly larger head circumferences if their mothers with PCOS took metformin (35.4 vs. 35.0 cm; P = .006).
Dr. Løvvik and her colleagues subsequently performed the same analysis on the first per-protocol group, one that excluded the 46 patients who were early dropouts after randomization. Here, the odds ratio favoring metformin for the composite primary outcome measure was a significant 2.55 (n = 432; 95% CI, 1.10-6.42; P = .03). Results for the final per-protocol analysis that excluded dropouts and those with less than 90% adherence to study medication were similar, with an odds ratio of 2.76 in favor of metformin use during pregnancy (n = 298; 95% CI, 1.0-8.82; P = .05).
In response to an audience question, Dr. Løvvik said that, though a per-protocol analysis of some sort had been predetermined, the decision to perform the narrower analysis on the highly adherent group was made later. However, compared to thresholds for adherence in other studies, “ours was way higher – so it’s too strict,” she acknowledged.
At baseline, the median age overall was about 30 years, and the median body mass index was about 28 kg/m2. Patients were at a median 74 days gestational age of pregnancy at randomization, and 53%-61% of patients were on metformin at the time of conception, with no significant difference between the metformin and placebo group.
A little less than half (40%-46%) of participants had a spontaneous conception, and most participants (56%-59%) had no prior term deliveries. Participants were overwhelmingly (91%-97%) white and Nordic.
There were no serious maternal, fetal, or neonatal safety signals in either study group.
The study followed the earlier PregMet study, which also tracked use of metformin in pregnancy for women with PCOS.
CHICAGO – , preterm births and late miscarriages were reduced, but researchers saw no effect on blood glucose levels or other diabetes-related maternal outcomes.
A Nordic study of pregnant women, dubbed PregMet 2, followed the course of 487 women with PCOS who were randomized to take 2,000 mg of metformin or placebo daily during pregnancy.
Among the entire study population, those with PCOS who took placebo had a 9.6% incidence of late miscarriage or preterm birth. For those taking metformin, the figure was 5%, similar to the 5.2% risk seen in the population-wide Norwegian Birth Registry, said Dr. Løvvik, of the Norwegian University of Science and Technology, Trondheim.
This analysis yielded a number needed to treat (NNT) for benefit of metformin of 22, she said.
However, Dr. Løvvik said that “the elephant in the room” is the lack of benefit of metformin for rates of gestational diabetes, hypertension in pregnancy, or preeclampsia in PregMet 2.
Of the various endpoint analyses, “the most surprising and striking one is the absolute lack of effect on gestational diabetes in this high-risk population,” said Dr. Løvvik. “There’s not even a tendency towards effect. … Metformin had no effect on prevention, treatment, or the need for additional insulin, indicating it was actually as effective as placebo.”
Considering that “metformin is now a part of the standard treatment for gestational diabetes, according to many national guidelines, we think that it’s questionable that it’s never, ever been tested against placebo for this diagnosis,” said Dr. Løvvik.
Pregnant women with PCOS have an increased risk of complications, and metformin is often prescribed off label to attempt to address some of these complications. Some previous work had shown metformin to be helpful, but previous studies have been underpowered, said Dr. Løvvik.
PregMet 2’s protocol attempted to address the literature gap; over 3 years, it called for enrollment of 1,000 women in the first trimester of pregnancy, randomized 1:1 to receive metformin or placebo in pregnancy.
At that enrollment level, the study would aim to show a 50% reduction in late miscarriages and preterm birth, with a power of 85%. Two predetermined analysis were planned at the end of the 3-year study, one for the intent-to-treat population and one for the per-protocol group.
In the end, however, just 487 patients were enrolled and randomized over a period of 5 years, despite recruitment at 14 centers in Iceland, Norway, and Sweden.
Richard Legro, MD, an ob.gyn. from Pennsylvania State University, Hershey, was in attendance. While praising the overall high quality of the study, he commented, “The primary concern is that you stopped the study when you hadn’t even achieved half your sample size.”
Dr. Løvvik said that a variety of factors contributed to the low study enrollment. One primary issue was that participation required additional visits on the part of participants; the study was not part of routine prenatal care, she said. Also, the placebo used in the study expired before enrollment was completed, complicating execution of the study.
She also noted that nearly a quarter of patients had a history of depression, and one in five had a history of migraine. More than half of patients overall had at least one previous or ongoing chronic medical condition.
A small number of patients in each group were excluded for protocol violations, pregnancy termination, intrauterine fetal demise, or loss to follow-up. The final intent-to-treat population included 238 in the metformin group and 240 who received placebo.
The per-protocol analysis was conducted two ways: In the first analysis, only those who dropped out were excluded, leaving 209 in the metformin group and 223 in the placebo group. The second analysis included those whose adherence to taking the study medication was assessed at 90% or better. For this second per-protocol analysis, just 142 metformin takers and 156 in the placebo group remained.
In the intent-to-treat population, the primary endpoint – a composite outcome of late miscarriage and preterm delivery – yielded an odds ratio of 1.99 favoring metformin over placebo, a figure that fell short of statistical significance (95% confidence interval, 0.93-4.51; P = .08).
However, there was significantly less weight gain in those taking metformin in this population (8.7 vs. 11.48 kg; P less than .0001). And newborns had significantly larger head circumferences if their mothers with PCOS took metformin (35.4 vs. 35.0 cm; P = .006).
Dr. Løvvik and her colleagues subsequently performed the same analysis on the first per-protocol group, one that excluded the 46 patients who were early dropouts after randomization. Here, the odds ratio favoring metformin for the composite primary outcome measure was a significant 2.55 (n = 432; 95% CI, 1.10-6.42; P = .03). Results for the final per-protocol analysis that excluded dropouts and those with less than 90% adherence to study medication were similar, with an odds ratio of 2.76 in favor of metformin use during pregnancy (n = 298; 95% CI, 1.0-8.82; P = .05).
In response to an audience question, Dr. Løvvik said that, though a per-protocol analysis of some sort had been predetermined, the decision to perform the narrower analysis on the highly adherent group was made later. However, compared to thresholds for adherence in other studies, “ours was way higher – so it’s too strict,” she acknowledged.
At baseline, the median age overall was about 30 years, and the median body mass index was about 28 kg/m2. Patients were at a median 74 days gestational age of pregnancy at randomization, and 53%-61% of patients were on metformin at the time of conception, with no significant difference between the metformin and placebo group.
A little less than half (40%-46%) of participants had a spontaneous conception, and most participants (56%-59%) had no prior term deliveries. Participants were overwhelmingly (91%-97%) white and Nordic.
There were no serious maternal, fetal, or neonatal safety signals in either study group.
The study followed the earlier PregMet study, which also tracked use of metformin in pregnancy for women with PCOS.
REPORTING FROM ENDO 2018
Key clinical point: Metformin reduced the rate of late preterm births and miscarriages in pregnant women with PCOS.
Major finding: The rate of the composite outcome was 5% for those on metformin, with a NNT of 22.
Study details: Randomized placebo-controlled trial of 487 pregnant women.
Disclosures: None of the study authors reported conflicts of interest.
Source: Løvvik T et al. ENDO 2018, Abstract 33-4.
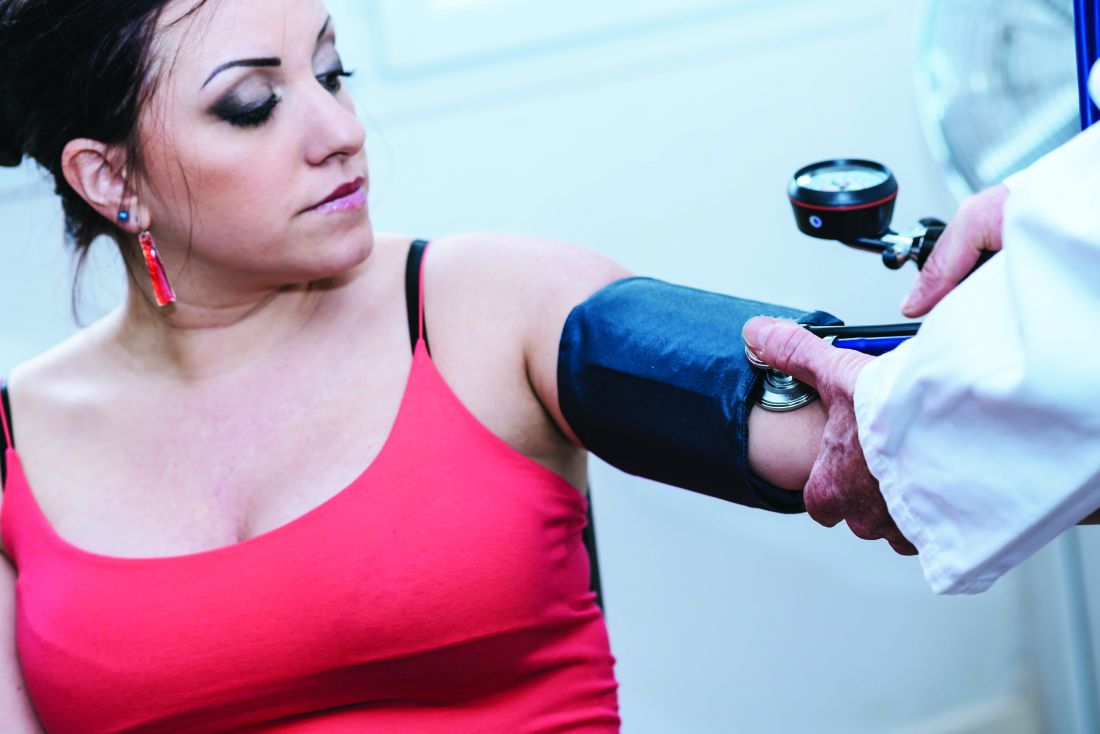
Higher preconception blood pressure linked to pregnancy loss
High preconception blood pressure is associated with a greater risk of pregnancy loss, according to analysis of data from a randomized clinical trial of aspirin and pregnancy outcomes.
Researchers in the EAGeR (Effects of Aspirin on Gestational and Reproduction) trial analyzed data from 1,228 women attempting pregnancy with a history of pregnancy loss. After researchers adjusted for treatment assignment, body mass index (BMI), race, marital status, smoking, parity, and time from last pregnancy loss, an increase in all blood pressure measures was associated with a 17% increase in the risk of pregnancy loss (Hypertension. doi: 10.1161/hypertensionaha.117.10705).
although the authors noted that group sizes were small.
Overall, one-quarter of the women enrolled in the study met the criteria for hypertension stage I, and 4.3% met the criteria for hypertension stage II.
“Screening and lifestyle interventions targeting maintenance of healthy blood pressure levels among reproductive-aged women may have additional important short-term benefits on reproductive health,” wrote Carrie J. Nobles, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and coauthors.
The authors also saw an impact of early pregnancy blood pressure on pregnancy loss, with an 18% greater risk of loss with each 10–mm Hg increase in mean arterial pressure.
Higher blood pressure during preconception was also associated with a decrease in the chance of live birth, but this association disappeared after adjusting for other confounders.
The study also examined the relationship between preconception blood pressure and the probability of conception. While the unadjusted models suggested 10% lower odds of fecundability, adjusting for all covariates except for BMI found similar effect estimates.
“We observed no clear associations of preconception blood pressure with fecundability after adjustment for BMI, suggesting that pathways related to BMI, which is strongly related to fecundability, may explain the marginal association of blood pressure with fecundability,” the authors wrote.
There was also some evidence that aspirin may influence the association between higher preconception blood pressure and pregnancy loss, as this association was marginally stronger in the placebo group than in the group randomized to low-dose aspirin.
“Pregnancy loss and other adverse reproductive outcomes may serve as sensitive markers of early-stage progression toward cardiometabolic disease in young adults,” Dr. Noble and coauthors wrote. “Further elucidating the cardiometabolic risk factors for pregnancy loss may help identify early intervention strategies, such as regular physical activity and following a DASH-type (Dietary Approaches to Stop Hypertension) diet.”
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. No conflicts of interest were declared.
SOURCE: Nobles CJ et al. Hypertension. 2018 Apr 2;71. doi: 10.1161/hypertensionaha.117.10705.
High preconception blood pressure is associated with a greater risk of pregnancy loss, according to analysis of data from a randomized clinical trial of aspirin and pregnancy outcomes.
Researchers in the EAGeR (Effects of Aspirin on Gestational and Reproduction) trial analyzed data from 1,228 women attempting pregnancy with a history of pregnancy loss. After researchers adjusted for treatment assignment, body mass index (BMI), race, marital status, smoking, parity, and time from last pregnancy loss, an increase in all blood pressure measures was associated with a 17% increase in the risk of pregnancy loss (Hypertension. doi: 10.1161/hypertensionaha.117.10705).
although the authors noted that group sizes were small.
Overall, one-quarter of the women enrolled in the study met the criteria for hypertension stage I, and 4.3% met the criteria for hypertension stage II.
“Screening and lifestyle interventions targeting maintenance of healthy blood pressure levels among reproductive-aged women may have additional important short-term benefits on reproductive health,” wrote Carrie J. Nobles, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and coauthors.
The authors also saw an impact of early pregnancy blood pressure on pregnancy loss, with an 18% greater risk of loss with each 10–mm Hg increase in mean arterial pressure.
Higher blood pressure during preconception was also associated with a decrease in the chance of live birth, but this association disappeared after adjusting for other confounders.
The study also examined the relationship between preconception blood pressure and the probability of conception. While the unadjusted models suggested 10% lower odds of fecundability, adjusting for all covariates except for BMI found similar effect estimates.
“We observed no clear associations of preconception blood pressure with fecundability after adjustment for BMI, suggesting that pathways related to BMI, which is strongly related to fecundability, may explain the marginal association of blood pressure with fecundability,” the authors wrote.
There was also some evidence that aspirin may influence the association between higher preconception blood pressure and pregnancy loss, as this association was marginally stronger in the placebo group than in the group randomized to low-dose aspirin.
“Pregnancy loss and other adverse reproductive outcomes may serve as sensitive markers of early-stage progression toward cardiometabolic disease in young adults,” Dr. Noble and coauthors wrote. “Further elucidating the cardiometabolic risk factors for pregnancy loss may help identify early intervention strategies, such as regular physical activity and following a DASH-type (Dietary Approaches to Stop Hypertension) diet.”
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. No conflicts of interest were declared.
SOURCE: Nobles CJ et al. Hypertension. 2018 Apr 2;71. doi: 10.1161/hypertensionaha.117.10705.
High preconception blood pressure is associated with a greater risk of pregnancy loss, according to analysis of data from a randomized clinical trial of aspirin and pregnancy outcomes.
Researchers in the EAGeR (Effects of Aspirin on Gestational and Reproduction) trial analyzed data from 1,228 women attempting pregnancy with a history of pregnancy loss. After researchers adjusted for treatment assignment, body mass index (BMI), race, marital status, smoking, parity, and time from last pregnancy loss, an increase in all blood pressure measures was associated with a 17% increase in the risk of pregnancy loss (Hypertension. doi: 10.1161/hypertensionaha.117.10705).
although the authors noted that group sizes were small.
Overall, one-quarter of the women enrolled in the study met the criteria for hypertension stage I, and 4.3% met the criteria for hypertension stage II.
“Screening and lifestyle interventions targeting maintenance of healthy blood pressure levels among reproductive-aged women may have additional important short-term benefits on reproductive health,” wrote Carrie J. Nobles, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and coauthors.
The authors also saw an impact of early pregnancy blood pressure on pregnancy loss, with an 18% greater risk of loss with each 10–mm Hg increase in mean arterial pressure.
Higher blood pressure during preconception was also associated with a decrease in the chance of live birth, but this association disappeared after adjusting for other confounders.
The study also examined the relationship between preconception blood pressure and the probability of conception. While the unadjusted models suggested 10% lower odds of fecundability, adjusting for all covariates except for BMI found similar effect estimates.
“We observed no clear associations of preconception blood pressure with fecundability after adjustment for BMI, suggesting that pathways related to BMI, which is strongly related to fecundability, may explain the marginal association of blood pressure with fecundability,” the authors wrote.
There was also some evidence that aspirin may influence the association between higher preconception blood pressure and pregnancy loss, as this association was marginally stronger in the placebo group than in the group randomized to low-dose aspirin.
“Pregnancy loss and other adverse reproductive outcomes may serve as sensitive markers of early-stage progression toward cardiometabolic disease in young adults,” Dr. Noble and coauthors wrote. “Further elucidating the cardiometabolic risk factors for pregnancy loss may help identify early intervention strategies, such as regular physical activity and following a DASH-type (Dietary Approaches to Stop Hypertension) diet.”
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. No conflicts of interest were declared.
SOURCE: Nobles CJ et al. Hypertension. 2018 Apr 2;71. doi: 10.1161/hypertensionaha.117.10705.
FROM HYPERTENSION
Key clinical point: Maintaining normal blood pressure is even more important for women who previously have miscarried.
Major finding: Higher preconception blood pressure was associated with a 17% increase in the risk of pregnancy loss.
Study details: Analysis of data from a randomized clinical trial in 1,228 women.
Disclosures: The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. No conflicts of interest were declared.
Source: Nobles CJ et al. Hypertension. 2018 Apr 2;71. doi: 10.1161/hypertensionaha.117.10705.
Fetal pathology exam performed without consent: $500,000 verdict
Fetal pathology exam performed without consent: $500,000 verdict
At 12 to 15 weeks' gestation, a fetus was found to have died. An ObGyn stimulated uterine contractions with misoprostol and the fetus was delivered.
The parents requested chromosomal testing to determine the cause of death. The ObGyn took samples of the baby's skin near the umbilical cord and from the placenta. He told the parents that the tests might come back as inconclusive, and that it may be necessary to take further samples. The couple was also told that, under hospital policy, the fetus must go to the pathology department before being taken to a funeral home. The parents told the ObGyn that they did not want further samples taken for any reason, and that nothing else should be done to their child before funeral preparations. The placenta, umbilical cord, and fetus were placed in 2 separate containers. The container with the placenta and umbilical cord had an anatomic pathology requisition form, indicating that a surgical pathology exam was to be performed. The container with the fetus did not have a requisition form. When the father accompanied the nurse who carried the containers to the pathology department, he told a general technologist that no additional tissue samples were to be taken from the fetus.
The next day, the pathologist made an incision on the fetus' left side and took a sample to create a slide for microscopic analysis. The parents discovered the incision at the funeral home.
PARENTS' CLAIM: The parents sued the hospital and pathologist. The pathologist was negligent in performing a surgical pathology examination without consent. The hospital had a duty to communicate the parents' desires to the pathologist.
According to hospital protocol, the nurse who attended the delivery was to fill out the proper forms and ensure that they were available to the pathologist. The container with the fetus should have had its own requisition form that instructed the pathologist to not perform any further testing. Every specimen that goes to pathology should have a requisition form, per guidelines from the College of American Pathologists (CAP). Without a form, the pathologist should not do anything with the sample.
The parents' verbal instructions not to take additional samples from the fetus were never relayed to the pathologist.
DEFENDANTS' DEFENSE: The hospital and pathologist denied negligence. A single requisition form was appropriate; the containers' labeling and the form made it clear that the placenta, umbilical cord, and fetus were from the same patient. CAP guidelines do not require separate forms. The remains were in the gross room at pathology, which is where specimens are taken for review and analysis. Therefore, the pathologist assumed he was to perform a standard pathology exam on both containers. The technologist with whom the father had spoken met the accepted standard of care.
VERDICT: A $500,000 Maine verdict was returned. The pathologist (through insurance) and the hospital equally shared payment of the award.
When should delivery have occurred? $4M verdict
Concerned that her fetus had stopped moving, a mother presented to the ED. Results of fetal heart-rate (FHR) monitoring ordered by the attending ObGyn (Dr. A) were nonreassuring. A second ObGyn (Dr. B) ordered a fetal biophysical profile (BPP); the score was 2 points. Although a low score usually results in immediate delivery, Dr. B consulted a maternal-fetal medicine (MFM) specialist. After another fetal BPP scored 8 points, the mother was discharged.
The next day, the mother called her ObGyn (Dr. C), who told her to immediately come to his office. A fetal BPP scored 4 points, with nonreassuring fetal heart sounds.
The mother was transported to the hospital for emergency cesarean delivery. At birth, the baby was blue, not breathing, and had meconium in his lungs. After 6 minutes' resuscitation, he began breathing. The child has an hypoxic brain injury.
PARENTS' CLAIM: Based on the nonreassuring FHR readings when the mother first reported lack of fetal movement, and a BPP of 2 points, an immediate cesarean delivery should have been performed. If the child had been delivered in a timely manner, he would have escaped a brain injury. At the very least, the mother should have been kept in the hospital for monitoring.
DEFENDANTS' DEFENSE: Drs. A and B and the hospital claimed that the child did not have a hypoxic injury; he had gastroschisis.
VERDICT: A $4,098,266 New York verdict was returned.
Second twin's birth delayed; brain damage: $1.5M settlement
A 35-year-old woman was 30 weeks' pregnant with twins when she was admitted to a hospital at high risk. At 36 weeks' gestation, she went into labor. A resident called the ObGyn to report that the patient was ready to deliver and waiting to push. The ObGyn advised that he was tied up in another procedure and for the mother to wait until he could get there.
Forty minutes later, the ObGyn arrived and the mother was allowed to push. A first-year resident delivered the first twin without incident. The second twin shifted from a cephalic presentation to a double foot- ling breech presentation and his FHR reflected severe bradycardia. Under the supervision of the ObGyn, a fourth-year resident managed the delivery, which took 28 minutes. The second twin's Apgar scores were low. He was intubated and transferred to a children's hospital for brain cooling.
PARENTS' CLAIM: Although excellent care following the birth reduced the degree of brain damage, the delay caused by the ObGyn's late arrival was responsible for the child's injuries.
PHYSICIAN'S DEFENSE: In pretrial findings, a panel of physicians reported that the child did not have a qualifying injury. However, the case settled before the trial began.
VERDICT: A $1.5 million Virginia settlement was reached.
Pregnant mother prepares for child's congenital heart disorder
A mother was told that her fetus had tricuspid atresia, a congenital defect. The mother and pediatric cardiologist developed a treatment plan to perform a balloon heart procedure at birth then transfer the baby to a specialized hospital.
After birth at a local hospital, the child was also found to have a lung disorder. He was promptly placed on a respirator. The hospital's pediatric cardiologist decided that the baby was too fragile to undergo the balloon procedure, and that the procedure could wait until the child was more stable. The baby died on his third day of life.
The neonatologists involved in treating the baby determined that the cause of death was tricuspid atresia. A pathologist reported that the death was due to a combination of congenital heart and lung disorders.
ESTATE'S CLAIM: The hospital team was sued for not following the prenatal plan. The baby's condition was serious but survivable if treated properly.
DEFENDANTS' DEFENSE: The hospital team made the correct decision to stabilize the baby's lung conditions before performing the balloon repair. The baby died because both his heart and lungs were compromised. The child's chance of survival was very small.
VERDICT: A Mississippi defense verdict was returned.
Severe uterine atony and PPH: hysterectomy
A 35-year-old woman at 41 weeks' gestation was admitted to the hospital in active labor at 5:45 am on July 26. At 2:45 pm, an ObGyn noted that she was 8+ cm dilated, 90% effaced, and that the baby's head was at -1 station. At 6:00 pm, the membranes were artificially ruptured. At 7:45 pm, examination revealed no change in her cervix. The ObGyn reviewed the options with the mother, including offering an epidural and cesarean delivery. At 8:30 pm, an intrauterine pressure catheter was placed. The ObGyn noted an inadequate labor pattern and ordered augmentation with oxytocin. On July 27 at 12:50 am, examination revealed that the cervix was completely dilated and the baby's head was at +1 station. At 2:30 am, oxytocin was infusing at 12 mU/m. At 2:30 am, the patient began to push. By 4:30 am, oxytocin was running at 14 mU/m and the patient continued to push. At 5:00 am, the ObGyn noted no further progression of labor and recommended a cesarean delivery, but the mother wanted to push a little longer. At 6:00 am, with no further progress, the decision was made to perform cesarean delivery. At 6:40 am, the patient was transferred to the operating room and delivery occurred at 7:08 am. The baby weighed 9.5 lb.
The mother's uterus was closed and hemostasis was reached. However, uterine atony was persistent and did not respond to multiple doses of medication or uterine massage. Persistent atony led to postpartum hemorrhaging (PPH). The ObGyn used conservative methods to control the hemorrhage, including medications and sutures. He then called for assistance from another ObGyn, and performed ligation of the utero-ovarian ligaments and additional suturing, which had no effect on the bleeding. They attempted an ovarian artery ligation and placed additional sutures, but the PPH continued. The ObGyn recommended a hysterectomy to the patient and her husband, who agreed. A supracervical hysterectomy was performed. During the procedure, the left ovary tore and was removed.
PARENTS' CLAIM: The patient and her husband spoke at trial of their intention to have more than one child. They sued the ObGyn, her practice, and the hospital. The ObGyn was negligent in allowing labor to continue for too long, for using oxytocin to stimulate contractions, and for allowing the patient to push for several hours, given that the baby was large. Oxytocin stimulation for so many hours without labor progression created an unacceptably high risk of uterine atony. This led to a nonreversible atonic state after delivery, which caused the patient to lose her uterus, left ovary, and her ability to reproduce.
DEFENDANTS' DEFENSE: The ObGyn was not negligent. The ObGyn met the standard of care in the management of labor and delivery. She was attentive, present at appropriate intervals, and there was no point at which a different decision should have been made. The ObGyn allowed the patient to have input in the decision-making process, as long as the ObGyn was comfortable that the decision was not compromising the safety of mother and baby. The length of labor and size of the fetus increased the risk of atony. However, in most cases, this does not occur; it can occur without those risk factors. PPH is a serious but frequently unavoidable complication of childbirth. The ObGyn's management was appropriate and prevented the patient's death.
VERDICT: A Pennsylvania defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Fetal pathology exam performed without consent: $500,000 verdict
At 12 to 15 weeks' gestation, a fetus was found to have died. An ObGyn stimulated uterine contractions with misoprostol and the fetus was delivered.
The parents requested chromosomal testing to determine the cause of death. The ObGyn took samples of the baby's skin near the umbilical cord and from the placenta. He told the parents that the tests might come back as inconclusive, and that it may be necessary to take further samples. The couple was also told that, under hospital policy, the fetus must go to the pathology department before being taken to a funeral home. The parents told the ObGyn that they did not want further samples taken for any reason, and that nothing else should be done to their child before funeral preparations. The placenta, umbilical cord, and fetus were placed in 2 separate containers. The container with the placenta and umbilical cord had an anatomic pathology requisition form, indicating that a surgical pathology exam was to be performed. The container with the fetus did not have a requisition form. When the father accompanied the nurse who carried the containers to the pathology department, he told a general technologist that no additional tissue samples were to be taken from the fetus.
The next day, the pathologist made an incision on the fetus' left side and took a sample to create a slide for microscopic analysis. The parents discovered the incision at the funeral home.
PARENTS' CLAIM: The parents sued the hospital and pathologist. The pathologist was negligent in performing a surgical pathology examination without consent. The hospital had a duty to communicate the parents' desires to the pathologist.
According to hospital protocol, the nurse who attended the delivery was to fill out the proper forms and ensure that they were available to the pathologist. The container with the fetus should have had its own requisition form that instructed the pathologist to not perform any further testing. Every specimen that goes to pathology should have a requisition form, per guidelines from the College of American Pathologists (CAP). Without a form, the pathologist should not do anything with the sample.
The parents' verbal instructions not to take additional samples from the fetus were never relayed to the pathologist.
DEFENDANTS' DEFENSE: The hospital and pathologist denied negligence. A single requisition form was appropriate; the containers' labeling and the form made it clear that the placenta, umbilical cord, and fetus were from the same patient. CAP guidelines do not require separate forms. The remains were in the gross room at pathology, which is where specimens are taken for review and analysis. Therefore, the pathologist assumed he was to perform a standard pathology exam on both containers. The technologist with whom the father had spoken met the accepted standard of care.
VERDICT: A $500,000 Maine verdict was returned. The pathologist (through insurance) and the hospital equally shared payment of the award.
When should delivery have occurred? $4M verdict
Concerned that her fetus had stopped moving, a mother presented to the ED. Results of fetal heart-rate (FHR) monitoring ordered by the attending ObGyn (Dr. A) were nonreassuring. A second ObGyn (Dr. B) ordered a fetal biophysical profile (BPP); the score was 2 points. Although a low score usually results in immediate delivery, Dr. B consulted a maternal-fetal medicine (MFM) specialist. After another fetal BPP scored 8 points, the mother was discharged.
The next day, the mother called her ObGyn (Dr. C), who told her to immediately come to his office. A fetal BPP scored 4 points, with nonreassuring fetal heart sounds.
The mother was transported to the hospital for emergency cesarean delivery. At birth, the baby was blue, not breathing, and had meconium in his lungs. After 6 minutes' resuscitation, he began breathing. The child has an hypoxic brain injury.
PARENTS' CLAIM: Based on the nonreassuring FHR readings when the mother first reported lack of fetal movement, and a BPP of 2 points, an immediate cesarean delivery should have been performed. If the child had been delivered in a timely manner, he would have escaped a brain injury. At the very least, the mother should have been kept in the hospital for monitoring.
DEFENDANTS' DEFENSE: Drs. A and B and the hospital claimed that the child did not have a hypoxic injury; he had gastroschisis.
VERDICT: A $4,098,266 New York verdict was returned.
Second twin's birth delayed; brain damage: $1.5M settlement
A 35-year-old woman was 30 weeks' pregnant with twins when she was admitted to a hospital at high risk. At 36 weeks' gestation, she went into labor. A resident called the ObGyn to report that the patient was ready to deliver and waiting to push. The ObGyn advised that he was tied up in another procedure and for the mother to wait until he could get there.
Forty minutes later, the ObGyn arrived and the mother was allowed to push. A first-year resident delivered the first twin without incident. The second twin shifted from a cephalic presentation to a double foot- ling breech presentation and his FHR reflected severe bradycardia. Under the supervision of the ObGyn, a fourth-year resident managed the delivery, which took 28 minutes. The second twin's Apgar scores were low. He was intubated and transferred to a children's hospital for brain cooling.
PARENTS' CLAIM: Although excellent care following the birth reduced the degree of brain damage, the delay caused by the ObGyn's late arrival was responsible for the child's injuries.
PHYSICIAN'S DEFENSE: In pretrial findings, a panel of physicians reported that the child did not have a qualifying injury. However, the case settled before the trial began.
VERDICT: A $1.5 million Virginia settlement was reached.
Pregnant mother prepares for child's congenital heart disorder
A mother was told that her fetus had tricuspid atresia, a congenital defect. The mother and pediatric cardiologist developed a treatment plan to perform a balloon heart procedure at birth then transfer the baby to a specialized hospital.
After birth at a local hospital, the child was also found to have a lung disorder. He was promptly placed on a respirator. The hospital's pediatric cardiologist decided that the baby was too fragile to undergo the balloon procedure, and that the procedure could wait until the child was more stable. The baby died on his third day of life.
The neonatologists involved in treating the baby determined that the cause of death was tricuspid atresia. A pathologist reported that the death was due to a combination of congenital heart and lung disorders.
ESTATE'S CLAIM: The hospital team was sued for not following the prenatal plan. The baby's condition was serious but survivable if treated properly.
DEFENDANTS' DEFENSE: The hospital team made the correct decision to stabilize the baby's lung conditions before performing the balloon repair. The baby died because both his heart and lungs were compromised. The child's chance of survival was very small.
VERDICT: A Mississippi defense verdict was returned.
Severe uterine atony and PPH: hysterectomy
A 35-year-old woman at 41 weeks' gestation was admitted to the hospital in active labor at 5:45 am on July 26. At 2:45 pm, an ObGyn noted that she was 8+ cm dilated, 90% effaced, and that the baby's head was at -1 station. At 6:00 pm, the membranes were artificially ruptured. At 7:45 pm, examination revealed no change in her cervix. The ObGyn reviewed the options with the mother, including offering an epidural and cesarean delivery. At 8:30 pm, an intrauterine pressure catheter was placed. The ObGyn noted an inadequate labor pattern and ordered augmentation with oxytocin. On July 27 at 12:50 am, examination revealed that the cervix was completely dilated and the baby's head was at +1 station. At 2:30 am, oxytocin was infusing at 12 mU/m. At 2:30 am, the patient began to push. By 4:30 am, oxytocin was running at 14 mU/m and the patient continued to push. At 5:00 am, the ObGyn noted no further progression of labor and recommended a cesarean delivery, but the mother wanted to push a little longer. At 6:00 am, with no further progress, the decision was made to perform cesarean delivery. At 6:40 am, the patient was transferred to the operating room and delivery occurred at 7:08 am. The baby weighed 9.5 lb.
The mother's uterus was closed and hemostasis was reached. However, uterine atony was persistent and did not respond to multiple doses of medication or uterine massage. Persistent atony led to postpartum hemorrhaging (PPH). The ObGyn used conservative methods to control the hemorrhage, including medications and sutures. He then called for assistance from another ObGyn, and performed ligation of the utero-ovarian ligaments and additional suturing, which had no effect on the bleeding. They attempted an ovarian artery ligation and placed additional sutures, but the PPH continued. The ObGyn recommended a hysterectomy to the patient and her husband, who agreed. A supracervical hysterectomy was performed. During the procedure, the left ovary tore and was removed.
PARENTS' CLAIM: The patient and her husband spoke at trial of their intention to have more than one child. They sued the ObGyn, her practice, and the hospital. The ObGyn was negligent in allowing labor to continue for too long, for using oxytocin to stimulate contractions, and for allowing the patient to push for several hours, given that the baby was large. Oxytocin stimulation for so many hours without labor progression created an unacceptably high risk of uterine atony. This led to a nonreversible atonic state after delivery, which caused the patient to lose her uterus, left ovary, and her ability to reproduce.
DEFENDANTS' DEFENSE: The ObGyn was not negligent. The ObGyn met the standard of care in the management of labor and delivery. She was attentive, present at appropriate intervals, and there was no point at which a different decision should have been made. The ObGyn allowed the patient to have input in the decision-making process, as long as the ObGyn was comfortable that the decision was not compromising the safety of mother and baby. The length of labor and size of the fetus increased the risk of atony. However, in most cases, this does not occur; it can occur without those risk factors. PPH is a serious but frequently unavoidable complication of childbirth. The ObGyn's management was appropriate and prevented the patient's death.
VERDICT: A Pennsylvania defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Fetal pathology exam performed without consent: $500,000 verdict
At 12 to 15 weeks' gestation, a fetus was found to have died. An ObGyn stimulated uterine contractions with misoprostol and the fetus was delivered.
The parents requested chromosomal testing to determine the cause of death. The ObGyn took samples of the baby's skin near the umbilical cord and from the placenta. He told the parents that the tests might come back as inconclusive, and that it may be necessary to take further samples. The couple was also told that, under hospital policy, the fetus must go to the pathology department before being taken to a funeral home. The parents told the ObGyn that they did not want further samples taken for any reason, and that nothing else should be done to their child before funeral preparations. The placenta, umbilical cord, and fetus were placed in 2 separate containers. The container with the placenta and umbilical cord had an anatomic pathology requisition form, indicating that a surgical pathology exam was to be performed. The container with the fetus did not have a requisition form. When the father accompanied the nurse who carried the containers to the pathology department, he told a general technologist that no additional tissue samples were to be taken from the fetus.
The next day, the pathologist made an incision on the fetus' left side and took a sample to create a slide for microscopic analysis. The parents discovered the incision at the funeral home.
PARENTS' CLAIM: The parents sued the hospital and pathologist. The pathologist was negligent in performing a surgical pathology examination without consent. The hospital had a duty to communicate the parents' desires to the pathologist.
According to hospital protocol, the nurse who attended the delivery was to fill out the proper forms and ensure that they were available to the pathologist. The container with the fetus should have had its own requisition form that instructed the pathologist to not perform any further testing. Every specimen that goes to pathology should have a requisition form, per guidelines from the College of American Pathologists (CAP). Without a form, the pathologist should not do anything with the sample.
The parents' verbal instructions not to take additional samples from the fetus were never relayed to the pathologist.
DEFENDANTS' DEFENSE: The hospital and pathologist denied negligence. A single requisition form was appropriate; the containers' labeling and the form made it clear that the placenta, umbilical cord, and fetus were from the same patient. CAP guidelines do not require separate forms. The remains were in the gross room at pathology, which is where specimens are taken for review and analysis. Therefore, the pathologist assumed he was to perform a standard pathology exam on both containers. The technologist with whom the father had spoken met the accepted standard of care.
VERDICT: A $500,000 Maine verdict was returned. The pathologist (through insurance) and the hospital equally shared payment of the award.
When should delivery have occurred? $4M verdict
Concerned that her fetus had stopped moving, a mother presented to the ED. Results of fetal heart-rate (FHR) monitoring ordered by the attending ObGyn (Dr. A) were nonreassuring. A second ObGyn (Dr. B) ordered a fetal biophysical profile (BPP); the score was 2 points. Although a low score usually results in immediate delivery, Dr. B consulted a maternal-fetal medicine (MFM) specialist. After another fetal BPP scored 8 points, the mother was discharged.
The next day, the mother called her ObGyn (Dr. C), who told her to immediately come to his office. A fetal BPP scored 4 points, with nonreassuring fetal heart sounds.
The mother was transported to the hospital for emergency cesarean delivery. At birth, the baby was blue, not breathing, and had meconium in his lungs. After 6 minutes' resuscitation, he began breathing. The child has an hypoxic brain injury.
PARENTS' CLAIM: Based on the nonreassuring FHR readings when the mother first reported lack of fetal movement, and a BPP of 2 points, an immediate cesarean delivery should have been performed. If the child had been delivered in a timely manner, he would have escaped a brain injury. At the very least, the mother should have been kept in the hospital for monitoring.
DEFENDANTS' DEFENSE: Drs. A and B and the hospital claimed that the child did not have a hypoxic injury; he had gastroschisis.
VERDICT: A $4,098,266 New York verdict was returned.
Second twin's birth delayed; brain damage: $1.5M settlement
A 35-year-old woman was 30 weeks' pregnant with twins when she was admitted to a hospital at high risk. At 36 weeks' gestation, she went into labor. A resident called the ObGyn to report that the patient was ready to deliver and waiting to push. The ObGyn advised that he was tied up in another procedure and for the mother to wait until he could get there.
Forty minutes later, the ObGyn arrived and the mother was allowed to push. A first-year resident delivered the first twin without incident. The second twin shifted from a cephalic presentation to a double foot- ling breech presentation and his FHR reflected severe bradycardia. Under the supervision of the ObGyn, a fourth-year resident managed the delivery, which took 28 minutes. The second twin's Apgar scores were low. He was intubated and transferred to a children's hospital for brain cooling.
PARENTS' CLAIM: Although excellent care following the birth reduced the degree of brain damage, the delay caused by the ObGyn's late arrival was responsible for the child's injuries.
PHYSICIAN'S DEFENSE: In pretrial findings, a panel of physicians reported that the child did not have a qualifying injury. However, the case settled before the trial began.
VERDICT: A $1.5 million Virginia settlement was reached.
Pregnant mother prepares for child's congenital heart disorder
A mother was told that her fetus had tricuspid atresia, a congenital defect. The mother and pediatric cardiologist developed a treatment plan to perform a balloon heart procedure at birth then transfer the baby to a specialized hospital.
After birth at a local hospital, the child was also found to have a lung disorder. He was promptly placed on a respirator. The hospital's pediatric cardiologist decided that the baby was too fragile to undergo the balloon procedure, and that the procedure could wait until the child was more stable. The baby died on his third day of life.
The neonatologists involved in treating the baby determined that the cause of death was tricuspid atresia. A pathologist reported that the death was due to a combination of congenital heart and lung disorders.
ESTATE'S CLAIM: The hospital team was sued for not following the prenatal plan. The baby's condition was serious but survivable if treated properly.
DEFENDANTS' DEFENSE: The hospital team made the correct decision to stabilize the baby's lung conditions before performing the balloon repair. The baby died because both his heart and lungs were compromised. The child's chance of survival was very small.
VERDICT: A Mississippi defense verdict was returned.
Severe uterine atony and PPH: hysterectomy
A 35-year-old woman at 41 weeks' gestation was admitted to the hospital in active labor at 5:45 am on July 26. At 2:45 pm, an ObGyn noted that she was 8+ cm dilated, 90% effaced, and that the baby's head was at -1 station. At 6:00 pm, the membranes were artificially ruptured. At 7:45 pm, examination revealed no change in her cervix. The ObGyn reviewed the options with the mother, including offering an epidural and cesarean delivery. At 8:30 pm, an intrauterine pressure catheter was placed. The ObGyn noted an inadequate labor pattern and ordered augmentation with oxytocin. On July 27 at 12:50 am, examination revealed that the cervix was completely dilated and the baby's head was at +1 station. At 2:30 am, oxytocin was infusing at 12 mU/m. At 2:30 am, the patient began to push. By 4:30 am, oxytocin was running at 14 mU/m and the patient continued to push. At 5:00 am, the ObGyn noted no further progression of labor and recommended a cesarean delivery, but the mother wanted to push a little longer. At 6:00 am, with no further progress, the decision was made to perform cesarean delivery. At 6:40 am, the patient was transferred to the operating room and delivery occurred at 7:08 am. The baby weighed 9.5 lb.
The mother's uterus was closed and hemostasis was reached. However, uterine atony was persistent and did not respond to multiple doses of medication or uterine massage. Persistent atony led to postpartum hemorrhaging (PPH). The ObGyn used conservative methods to control the hemorrhage, including medications and sutures. He then called for assistance from another ObGyn, and performed ligation of the utero-ovarian ligaments and additional suturing, which had no effect on the bleeding. They attempted an ovarian artery ligation and placed additional sutures, but the PPH continued. The ObGyn recommended a hysterectomy to the patient and her husband, who agreed. A supracervical hysterectomy was performed. During the procedure, the left ovary tore and was removed.
PARENTS' CLAIM: The patient and her husband spoke at trial of their intention to have more than one child. They sued the ObGyn, her practice, and the hospital. The ObGyn was negligent in allowing labor to continue for too long, for using oxytocin to stimulate contractions, and for allowing the patient to push for several hours, given that the baby was large. Oxytocin stimulation for so many hours without labor progression created an unacceptably high risk of uterine atony. This led to a nonreversible atonic state after delivery, which caused the patient to lose her uterus, left ovary, and her ability to reproduce.
DEFENDANTS' DEFENSE: The ObGyn was not negligent. The ObGyn met the standard of care in the management of labor and delivery. She was attentive, present at appropriate intervals, and there was no point at which a different decision should have been made. The ObGyn allowed the patient to have input in the decision-making process, as long as the ObGyn was comfortable that the decision was not compromising the safety of mother and baby. The length of labor and size of the fetus increased the risk of atony. However, in most cases, this does not occur; it can occur without those risk factors. PPH is a serious but frequently unavoidable complication of childbirth. The ObGyn's management was appropriate and prevented the patient's death.
VERDICT: A Pennsylvania defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
It costs what?! How we can educate residents and students on how much things cost
“Why are you ordering a CBC on the patient when her white blood cell count, hemoglobin, and platelets have been stable for the past 3 days?” sternly inquired the attending gynecologic oncologist. “Don’t order tests without any clinical indication. If she is infected or bleeding, there will be signs and thus an indication to order a CBC. The physical exam is your test.” There was an authoritative pause before he invoked the “value-based care” maxim.
For many residents who graduated in the past decade, education in value-based care and alternative payment models (APMs) was cobbled together from experience, demonstrated by attendings who labeled it as such, and from rare didactic education classroom sessions and inpatient environments.
In today’s health care environment, professional survival requires the ability to successfully deliver high-value care to patients. Attendings often illustrate and champion how to do this by using patient care to highlight the definition: Value = Quality ÷ Cost.
For residency education programs to create the ObGyns of the future, they must teach trainees what they will be evaluated on and held accountable for.1 Today’s clinicians will have to take responsibility for reigning in health care costs from the fee-for-service era, which in the United States have snowballed into one of the unhealthiest cost-to-outcomes ratios worldwide. Residents will be required to understand not only value but also areas in which they can influence the cost of care and how their outcome metrics are valued.
Modifiable factors in value-based care
As mentioned, value is defined by the equation, Value = Quality ÷ Cost. The granularity of these terms helps clarify the depth and the multitude of levels that clinicians can modify and influence to achieve the highest value.
Quality, as defined by the National Academy of Medicine, includes2:
- effectiveness: providing care processes and achieving outcomes as supported by scientific evidence
- efficiency: maximizing the quality of a comparable unit of health care delivered or unit of health benefit achieved for a given unit of health care resources used
- equity: providing health care of equal quality to those who may differ in personal characteristics other than their clinical condition or preferences for care
- patient-centeredness: meeting patient needs and preferences and providing education and support
- safety: actual or potential bodily harm
- timeliness: obtaining needed care while minimizing delays.
From electronic health records, which were mandated in the Patient Protection and Affordable Care Act of 2010, offices, hospitals, and medical systems have gained robust databases of mineable information. Even data abstraction from paper records has been made easier, allowing better reflection of practitioner-based delivery of care.
Understanding cost breakdown in the overall value equation
With regard to value-based care, cost is generally related to money. When broadly explored, however, cost can be broken down into cost to the patient, the health care system, and society this way:
- patient: time spent receiving evaluation and management from a clinician; money spent for family care needs while undergoing management; money spent for procedures and tests; wages lost due to appointments
- health system: preventive services versus costly emergency room visit; community-based interventions to improve population health
- society: cost to tax payers; equitable distribution of vital resources (for example, vaccines); prevention of iatrogenic antibiotic resistance.
To understand how physicians are paid, it is important to see how payers value our services. The Centers for Medicare and Medicaid Services states that it is “promoting value-based care as part of its larger quality strategy to reform how health care is delivered and paid for.” In 2018, the US Department of Health and Human Services is striving to have half of Medicare payments in APMs.3
It is the physician’s responsibility to recognize that costs to the patient, payer, health system, and society can compete with and directly influence the outcome of each other. For example, because the patient pays an insurance premium to participate in a risk pool where cost-sharing is the primary cost-containment strategy, poor-value interventions can directly translate into increased premiums, copayments, or deductibles for the entire pool.4
By clearly identifying the different variables involved in the value-based care equation, residents can better understand their responsibility in their day-to-day work in medicine to address value, not just quality or cost. Clarifying the tenets of value-based care will help guide educators in identifying “teaching moments” and organizing didactic sessions focused on practical implementation of value.
Less is more
In our opening anecdote, the attending shows how curbing overuse of resources can increase the value of care delivered. But that example illustrates only one of the many levels on which educators can help residents understand their impact on value. A multidisciplinary education that incorporates outpatient and inpatient pharmacists, social workers, occupational therapists, pelvic floor physiotherapists, office staff, billing specialists, operating room (OR) technologists, and others can be beneficial in learning how to deliver high-value care.
Read about selecting value-based interventions at work.
Value-based interventions at work
In the discussion that follows, we illustrate how residents can identify, evaluate, and put into practice value-based interventions that can occur at multiple levels.
Antibiotic selection. Resident choices for outpatient antibiotics can severely affect patient adherence. Subtle differences in the formulation of certain antibiotics affect the price and thus pose a significant potential obstacle. Judicious use of inexpensive drug formulations with fewer dosing frequencies can help patients engage in their own care.
Knowing the pharmacologic difference between doxycycline hyclate and doxycycline monohydrate, for example, is to know the difference between esoteric salts—undeniably worthless information with regard to successfully treating a patient’s infection. Knowing that one formula is on the bargain formulary at the patient’s local pharmacy, or that one drug requires twice-daily dosing versus 4-times-daily dosing, however, can mean the difference between the patient’s adherence or nonadherence to your expert recommendation.
Contraception options. Contraceptives pose a challenge with respect to value because of the myriad delivery systems, doses, and generic formulations available. There are dozens of oral contraceptive pills (OCPs) on the market that vary in their dosing, phasic nature (monophasic, multiphasic), iron content in the hormone-free week, and different progestogens for different conditions (such as drospirenone for androgen excess).
When weighing contraceptive options, the clinician must look at value not only from a cost perspective but also from an effectiveness perspective. The desired outcome in this scenario is preventing unwanted pregnancy with ideal or typical contraceptive use at the most inexpensive price point. When working within the value equation, the clinician must individualize the prescribed contraceptive to one that is most acceptable to the patient and that optimizes the various costs and quality measures. “Cost” can mean the cost of OCPs, menstrual control products, backup contraception, failed or unwanted pregnancy management, or suffering lost wages from missed days of work from, for example, dysmenorrhea. “Quality” can mean a low contraceptive failure rate, predictable cyclicality, the need for patient administration and the risk of forgetting, and the need for backup contraceptives.
In comparing the subdermal contraceptive implant (which can cost up to $1,300 every 3 years, equivalent to $36.11 per month) with OCPs (which can cost as low as $324 for 3 years for an ethinyl estradiol and norgestimate combination, or $9 per month), the OCPs significantly outweigh the implant in terms of cost. When comparing failure rates, the degree of patient intervention, and decreased use of menstrual control products due to amenorrhea, the subdermal contraceptive wins. As we know, long-acting reversible contraception (LARC), including the intrauterine device (IUD) and subdermal implant, is the most effective but often the most expensive contraceptive option.5 When cost is evaluated from a global perspective, as highlighted by the adage “an IUD is cheaper than a baby,” the LARC’s value is derived from its overall high effectiveness and low cost.
If the patient elects to choose OCPs, the clinician should direct the prescription to a pharmacy that has discounted generic pills on its formulary. Generic OCPs have a low- cost burden without loss of efficacy, thus providing maximal value.6 This requires an intimate knowledge of the local pharmacies and what their formularies provide. Sometimes the patient will need to drive out of her way to access cost-effective, quality medications, or the high-value option.
Surgery considerations. Judicious instrument selection in the OR can decrease overall operative costs. While most advanced sealing and cutting instrumentation is for single use, for example, it also can be reprocessed for reuse. Although the cost of reprocessed, single-use instruments is lower, studies evaluating the quality of these instruments “found a significant rate of physical defects, performance issues, or improper decontamination.”7
Marketing largely has driven physician choice in the use of certain vessel sealing and cutting devices, but there has yet to be evidence that using any one device actually improves performance or outcomes, such as length of surgery, blood loss, or postoperative complications. Technology companies that create these instruments likely will have to start designing studies to test performance and outcomes as they relate to their devices to persuade hospital systems that using their products improves outcomes and reduces costs.
While learning laparoscopic hysterectomy, residents may see that some attending surgeons can complete the entire procedure with monopolar scissors, bipolar forceps, and laparoscopic needle drivers, while other surgeons use those instruments plus others, such as a LigaSure instrument or a Harmonic scalpel. With outcomes being the same between these surgeons, it is reasonable for hospitals to audit each surgeon using the Value = Quality ÷ Cost equation and to seek data to describe why the latter surgeon requires additional instrumentation.
Residency training poses a unique opportunity for physicians to learn numerous ways to perform the same procedure so they can fill their armamentarium with various effective techniques. Residency also should be a time in which proficiency with basic surgical instrumentation is emphasized. Attending physicians can help residents improve their skills, for example, by having them use only one advanced sealing and cutting device, or no device at all. This practice will make the trainee better able to adapt to situations in which an advanced device may fail or be unavailable. Future performance metrics may evaluate the physician’s cost effectiveness with regard to single-use instruments during routine surgical procedures.
Standardized order sets. Evidence-based order sets help in the management of pneumonia, sepsis, deep vein thrombosis prophylaxis, and numerous other conditions. In the era of computerized physician order entry systems (CPOEs), a resident needs to enter just a few clicks to order all necessary tests, interventions, and imaging studies for a condition. In one fell swoop, orders are placed not only for admission but also for the patient’s entire hospitalization. The paradox of the order set is that it uses a template to deliver individualized patient-centered care.
In the age of enhanced recovery pathways after surgery, we see patients who undergo a hysterectomy being discharged home directly from the postoperative anesthesia care unit (PACU). Generally, follow-up laboratory testing is not ordered on an outpatient basis. If, however, the patient needs to remain in the hospital for social reasons (such as delayed PACU transfer, transportation, weather), she receives the standardized orders from the post hysterectomy order set: a morning complete blood count ($55) with a basic metabolic panel ($45). As an academic exercise, the order set may help residents learn which orders they must consider when admitting a postoperative hysterectomy patient, but overuse of order sets can be a setback for a value-based care system.
Read about evaluating competence and individualizing care.
Evaluating competence in value-based care
Research is an integral component of all residency programs accredited by the Accreditation Council for Graduate Medical Education (ACGME). The implementation of value-based care—with all its nuances, quality metrics, and cost parameters—creates a space for resident-led studies to contribute to peer education. The ACGME’s Obstetrics and Gynecology Milestones project was developed to assess the development of ObGyn residents’ competence as they progress through training. Despite national laws tying reimbursements to value-based care, there is no mention of value as it relates to the basic formula, Value = Quality ÷ Cost, in the project.
With the nuances that value-based care offers, it would behoove the Council on Resident Education in Obstetrics and Gynecology of the American College of Obstetricians and Gynecologists to incorporate a method of evaluation to determine competence in this evolving field.
Care also must be individualized
Academic ObGyns and instructors should focus their pedagogy not only on value-based care but also on individualized care that will maximize desired outcomes for each patient. Incorporating multidisciplinary didactics, focused research, and a 360-degree evaluation in the residency curriculum will create new ObGyns who are known for successfully delivering high-value care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Wieand E, Lagrew DC Jr. Value-based payment: what does it mean, and how can ObGyns get out ahead? OBG Manag. 2018;30(1):17–19, 25–26.
- Agency for Healthcare Research and Quality. The six domains of health care quality. https://www.ahrq.gov/professionals/quality-patient-safety/talkingquality/create/sixdomains.html. Reviewed March 2016. Accessed March 22, 2018.
- Centers for Medicare and Medicaid Services. Better care. Smarter spending. Healthier people: paying providers for value, not volume. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-01-26-3.html. Published January 26, 2015. Accessed March 22, 2018.
- Society for Human Resource Management. Managing health care costs. https://www.shrm.org/resourcesandtools/tools-and-samples/toolkits/pages/managinghealthcarecosts.aspx. Published January 11, 2017. Accessed March 18, 2018.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice, Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 642: Increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126(4):e44–e48.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 375: Brand versus generic oral contraceptives. Obstet Gynecol. 2007;110(2 pt 1):447–448.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 537: R
eprocessed single-use devices. Obstet Gynecol. 2012;120(4):974–976.
“Why are you ordering a CBC on the patient when her white blood cell count, hemoglobin, and platelets have been stable for the past 3 days?” sternly inquired the attending gynecologic oncologist. “Don’t order tests without any clinical indication. If she is infected or bleeding, there will be signs and thus an indication to order a CBC. The physical exam is your test.” There was an authoritative pause before he invoked the “value-based care” maxim.
For many residents who graduated in the past decade, education in value-based care and alternative payment models (APMs) was cobbled together from experience, demonstrated by attendings who labeled it as such, and from rare didactic education classroom sessions and inpatient environments.
In today’s health care environment, professional survival requires the ability to successfully deliver high-value care to patients. Attendings often illustrate and champion how to do this by using patient care to highlight the definition: Value = Quality ÷ Cost.
For residency education programs to create the ObGyns of the future, they must teach trainees what they will be evaluated on and held accountable for.1 Today’s clinicians will have to take responsibility for reigning in health care costs from the fee-for-service era, which in the United States have snowballed into one of the unhealthiest cost-to-outcomes ratios worldwide. Residents will be required to understand not only value but also areas in which they can influence the cost of care and how their outcome metrics are valued.
Modifiable factors in value-based care
As mentioned, value is defined by the equation, Value = Quality ÷ Cost. The granularity of these terms helps clarify the depth and the multitude of levels that clinicians can modify and influence to achieve the highest value.
Quality, as defined by the National Academy of Medicine, includes2:
- effectiveness: providing care processes and achieving outcomes as supported by scientific evidence
- efficiency: maximizing the quality of a comparable unit of health care delivered or unit of health benefit achieved for a given unit of health care resources used
- equity: providing health care of equal quality to those who may differ in personal characteristics other than their clinical condition or preferences for care
- patient-centeredness: meeting patient needs and preferences and providing education and support
- safety: actual or potential bodily harm
- timeliness: obtaining needed care while minimizing delays.
From electronic health records, which were mandated in the Patient Protection and Affordable Care Act of 2010, offices, hospitals, and medical systems have gained robust databases of mineable information. Even data abstraction from paper records has been made easier, allowing better reflection of practitioner-based delivery of care.
Understanding cost breakdown in the overall value equation
With regard to value-based care, cost is generally related to money. When broadly explored, however, cost can be broken down into cost to the patient, the health care system, and society this way:
- patient: time spent receiving evaluation and management from a clinician; money spent for family care needs while undergoing management; money spent for procedures and tests; wages lost due to appointments
- health system: preventive services versus costly emergency room visit; community-based interventions to improve population health
- society: cost to tax payers; equitable distribution of vital resources (for example, vaccines); prevention of iatrogenic antibiotic resistance.
To understand how physicians are paid, it is important to see how payers value our services. The Centers for Medicare and Medicaid Services states that it is “promoting value-based care as part of its larger quality strategy to reform how health care is delivered and paid for.” In 2018, the US Department of Health and Human Services is striving to have half of Medicare payments in APMs.3
It is the physician’s responsibility to recognize that costs to the patient, payer, health system, and society can compete with and directly influence the outcome of each other. For example, because the patient pays an insurance premium to participate in a risk pool where cost-sharing is the primary cost-containment strategy, poor-value interventions can directly translate into increased premiums, copayments, or deductibles for the entire pool.4
By clearly identifying the different variables involved in the value-based care equation, residents can better understand their responsibility in their day-to-day work in medicine to address value, not just quality or cost. Clarifying the tenets of value-based care will help guide educators in identifying “teaching moments” and organizing didactic sessions focused on practical implementation of value.
Less is more
In our opening anecdote, the attending shows how curbing overuse of resources can increase the value of care delivered. But that example illustrates only one of the many levels on which educators can help residents understand their impact on value. A multidisciplinary education that incorporates outpatient and inpatient pharmacists, social workers, occupational therapists, pelvic floor physiotherapists, office staff, billing specialists, operating room (OR) technologists, and others can be beneficial in learning how to deliver high-value care.
Read about selecting value-based interventions at work.
Value-based interventions at work
In the discussion that follows, we illustrate how residents can identify, evaluate, and put into practice value-based interventions that can occur at multiple levels.
Antibiotic selection. Resident choices for outpatient antibiotics can severely affect patient adherence. Subtle differences in the formulation of certain antibiotics affect the price and thus pose a significant potential obstacle. Judicious use of inexpensive drug formulations with fewer dosing frequencies can help patients engage in their own care.
Knowing the pharmacologic difference between doxycycline hyclate and doxycycline monohydrate, for example, is to know the difference between esoteric salts—undeniably worthless information with regard to successfully treating a patient’s infection. Knowing that one formula is on the bargain formulary at the patient’s local pharmacy, or that one drug requires twice-daily dosing versus 4-times-daily dosing, however, can mean the difference between the patient’s adherence or nonadherence to your expert recommendation.
Contraception options. Contraceptives pose a challenge with respect to value because of the myriad delivery systems, doses, and generic formulations available. There are dozens of oral contraceptive pills (OCPs) on the market that vary in their dosing, phasic nature (monophasic, multiphasic), iron content in the hormone-free week, and different progestogens for different conditions (such as drospirenone for androgen excess).
When weighing contraceptive options, the clinician must look at value not only from a cost perspective but also from an effectiveness perspective. The desired outcome in this scenario is preventing unwanted pregnancy with ideal or typical contraceptive use at the most inexpensive price point. When working within the value equation, the clinician must individualize the prescribed contraceptive to one that is most acceptable to the patient and that optimizes the various costs and quality measures. “Cost” can mean the cost of OCPs, menstrual control products, backup contraception, failed or unwanted pregnancy management, or suffering lost wages from missed days of work from, for example, dysmenorrhea. “Quality” can mean a low contraceptive failure rate, predictable cyclicality, the need for patient administration and the risk of forgetting, and the need for backup contraceptives.
In comparing the subdermal contraceptive implant (which can cost up to $1,300 every 3 years, equivalent to $36.11 per month) with OCPs (which can cost as low as $324 for 3 years for an ethinyl estradiol and norgestimate combination, or $9 per month), the OCPs significantly outweigh the implant in terms of cost. When comparing failure rates, the degree of patient intervention, and decreased use of menstrual control products due to amenorrhea, the subdermal contraceptive wins. As we know, long-acting reversible contraception (LARC), including the intrauterine device (IUD) and subdermal implant, is the most effective but often the most expensive contraceptive option.5 When cost is evaluated from a global perspective, as highlighted by the adage “an IUD is cheaper than a baby,” the LARC’s value is derived from its overall high effectiveness and low cost.
If the patient elects to choose OCPs, the clinician should direct the prescription to a pharmacy that has discounted generic pills on its formulary. Generic OCPs have a low- cost burden without loss of efficacy, thus providing maximal value.6 This requires an intimate knowledge of the local pharmacies and what their formularies provide. Sometimes the patient will need to drive out of her way to access cost-effective, quality medications, or the high-value option.
Surgery considerations. Judicious instrument selection in the OR can decrease overall operative costs. While most advanced sealing and cutting instrumentation is for single use, for example, it also can be reprocessed for reuse. Although the cost of reprocessed, single-use instruments is lower, studies evaluating the quality of these instruments “found a significant rate of physical defects, performance issues, or improper decontamination.”7
Marketing largely has driven physician choice in the use of certain vessel sealing and cutting devices, but there has yet to be evidence that using any one device actually improves performance or outcomes, such as length of surgery, blood loss, or postoperative complications. Technology companies that create these instruments likely will have to start designing studies to test performance and outcomes as they relate to their devices to persuade hospital systems that using their products improves outcomes and reduces costs.
While learning laparoscopic hysterectomy, residents may see that some attending surgeons can complete the entire procedure with monopolar scissors, bipolar forceps, and laparoscopic needle drivers, while other surgeons use those instruments plus others, such as a LigaSure instrument or a Harmonic scalpel. With outcomes being the same between these surgeons, it is reasonable for hospitals to audit each surgeon using the Value = Quality ÷ Cost equation and to seek data to describe why the latter surgeon requires additional instrumentation.
Residency training poses a unique opportunity for physicians to learn numerous ways to perform the same procedure so they can fill their armamentarium with various effective techniques. Residency also should be a time in which proficiency with basic surgical instrumentation is emphasized. Attending physicians can help residents improve their skills, for example, by having them use only one advanced sealing and cutting device, or no device at all. This practice will make the trainee better able to adapt to situations in which an advanced device may fail or be unavailable. Future performance metrics may evaluate the physician’s cost effectiveness with regard to single-use instruments during routine surgical procedures.
Standardized order sets. Evidence-based order sets help in the management of pneumonia, sepsis, deep vein thrombosis prophylaxis, and numerous other conditions. In the era of computerized physician order entry systems (CPOEs), a resident needs to enter just a few clicks to order all necessary tests, interventions, and imaging studies for a condition. In one fell swoop, orders are placed not only for admission but also for the patient’s entire hospitalization. The paradox of the order set is that it uses a template to deliver individualized patient-centered care.
In the age of enhanced recovery pathways after surgery, we see patients who undergo a hysterectomy being discharged home directly from the postoperative anesthesia care unit (PACU). Generally, follow-up laboratory testing is not ordered on an outpatient basis. If, however, the patient needs to remain in the hospital for social reasons (such as delayed PACU transfer, transportation, weather), she receives the standardized orders from the post hysterectomy order set: a morning complete blood count ($55) with a basic metabolic panel ($45). As an academic exercise, the order set may help residents learn which orders they must consider when admitting a postoperative hysterectomy patient, but overuse of order sets can be a setback for a value-based care system.
Read about evaluating competence and individualizing care.
Evaluating competence in value-based care
Research is an integral component of all residency programs accredited by the Accreditation Council for Graduate Medical Education (ACGME). The implementation of value-based care—with all its nuances, quality metrics, and cost parameters—creates a space for resident-led studies to contribute to peer education. The ACGME’s Obstetrics and Gynecology Milestones project was developed to assess the development of ObGyn residents’ competence as they progress through training. Despite national laws tying reimbursements to value-based care, there is no mention of value as it relates to the basic formula, Value = Quality ÷ Cost, in the project.
With the nuances that value-based care offers, it would behoove the Council on Resident Education in Obstetrics and Gynecology of the American College of Obstetricians and Gynecologists to incorporate a method of evaluation to determine competence in this evolving field.
Care also must be individualized
Academic ObGyns and instructors should focus their pedagogy not only on value-based care but also on individualized care that will maximize desired outcomes for each patient. Incorporating multidisciplinary didactics, focused research, and a 360-degree evaluation in the residency curriculum will create new ObGyns who are known for successfully delivering high-value care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“Why are you ordering a CBC on the patient when her white blood cell count, hemoglobin, and platelets have been stable for the past 3 days?” sternly inquired the attending gynecologic oncologist. “Don’t order tests without any clinical indication. If she is infected or bleeding, there will be signs and thus an indication to order a CBC. The physical exam is your test.” There was an authoritative pause before he invoked the “value-based care” maxim.
For many residents who graduated in the past decade, education in value-based care and alternative payment models (APMs) was cobbled together from experience, demonstrated by attendings who labeled it as such, and from rare didactic education classroom sessions and inpatient environments.
In today’s health care environment, professional survival requires the ability to successfully deliver high-value care to patients. Attendings often illustrate and champion how to do this by using patient care to highlight the definition: Value = Quality ÷ Cost.
For residency education programs to create the ObGyns of the future, they must teach trainees what they will be evaluated on and held accountable for.1 Today’s clinicians will have to take responsibility for reigning in health care costs from the fee-for-service era, which in the United States have snowballed into one of the unhealthiest cost-to-outcomes ratios worldwide. Residents will be required to understand not only value but also areas in which they can influence the cost of care and how their outcome metrics are valued.
Modifiable factors in value-based care
As mentioned, value is defined by the equation, Value = Quality ÷ Cost. The granularity of these terms helps clarify the depth and the multitude of levels that clinicians can modify and influence to achieve the highest value.
Quality, as defined by the National Academy of Medicine, includes2:
- effectiveness: providing care processes and achieving outcomes as supported by scientific evidence
- efficiency: maximizing the quality of a comparable unit of health care delivered or unit of health benefit achieved for a given unit of health care resources used
- equity: providing health care of equal quality to those who may differ in personal characteristics other than their clinical condition or preferences for care
- patient-centeredness: meeting patient needs and preferences and providing education and support
- safety: actual or potential bodily harm
- timeliness: obtaining needed care while minimizing delays.
From electronic health records, which were mandated in the Patient Protection and Affordable Care Act of 2010, offices, hospitals, and medical systems have gained robust databases of mineable information. Even data abstraction from paper records has been made easier, allowing better reflection of practitioner-based delivery of care.
Understanding cost breakdown in the overall value equation
With regard to value-based care, cost is generally related to money. When broadly explored, however, cost can be broken down into cost to the patient, the health care system, and society this way:
- patient: time spent receiving evaluation and management from a clinician; money spent for family care needs while undergoing management; money spent for procedures and tests; wages lost due to appointments
- health system: preventive services versus costly emergency room visit; community-based interventions to improve population health
- society: cost to tax payers; equitable distribution of vital resources (for example, vaccines); prevention of iatrogenic antibiotic resistance.
To understand how physicians are paid, it is important to see how payers value our services. The Centers for Medicare and Medicaid Services states that it is “promoting value-based care as part of its larger quality strategy to reform how health care is delivered and paid for.” In 2018, the US Department of Health and Human Services is striving to have half of Medicare payments in APMs.3
It is the physician’s responsibility to recognize that costs to the patient, payer, health system, and society can compete with and directly influence the outcome of each other. For example, because the patient pays an insurance premium to participate in a risk pool where cost-sharing is the primary cost-containment strategy, poor-value interventions can directly translate into increased premiums, copayments, or deductibles for the entire pool.4
By clearly identifying the different variables involved in the value-based care equation, residents can better understand their responsibility in their day-to-day work in medicine to address value, not just quality or cost. Clarifying the tenets of value-based care will help guide educators in identifying “teaching moments” and organizing didactic sessions focused on practical implementation of value.
Less is more
In our opening anecdote, the attending shows how curbing overuse of resources can increase the value of care delivered. But that example illustrates only one of the many levels on which educators can help residents understand their impact on value. A multidisciplinary education that incorporates outpatient and inpatient pharmacists, social workers, occupational therapists, pelvic floor physiotherapists, office staff, billing specialists, operating room (OR) technologists, and others can be beneficial in learning how to deliver high-value care.
Read about selecting value-based interventions at work.
Value-based interventions at work
In the discussion that follows, we illustrate how residents can identify, evaluate, and put into practice value-based interventions that can occur at multiple levels.
Antibiotic selection. Resident choices for outpatient antibiotics can severely affect patient adherence. Subtle differences in the formulation of certain antibiotics affect the price and thus pose a significant potential obstacle. Judicious use of inexpensive drug formulations with fewer dosing frequencies can help patients engage in their own care.
Knowing the pharmacologic difference between doxycycline hyclate and doxycycline monohydrate, for example, is to know the difference between esoteric salts—undeniably worthless information with regard to successfully treating a patient’s infection. Knowing that one formula is on the bargain formulary at the patient’s local pharmacy, or that one drug requires twice-daily dosing versus 4-times-daily dosing, however, can mean the difference between the patient’s adherence or nonadherence to your expert recommendation.
Contraception options. Contraceptives pose a challenge with respect to value because of the myriad delivery systems, doses, and generic formulations available. There are dozens of oral contraceptive pills (OCPs) on the market that vary in their dosing, phasic nature (monophasic, multiphasic), iron content in the hormone-free week, and different progestogens for different conditions (such as drospirenone for androgen excess).
When weighing contraceptive options, the clinician must look at value not only from a cost perspective but also from an effectiveness perspective. The desired outcome in this scenario is preventing unwanted pregnancy with ideal or typical contraceptive use at the most inexpensive price point. When working within the value equation, the clinician must individualize the prescribed contraceptive to one that is most acceptable to the patient and that optimizes the various costs and quality measures. “Cost” can mean the cost of OCPs, menstrual control products, backup contraception, failed or unwanted pregnancy management, or suffering lost wages from missed days of work from, for example, dysmenorrhea. “Quality” can mean a low contraceptive failure rate, predictable cyclicality, the need for patient administration and the risk of forgetting, and the need for backup contraceptives.
In comparing the subdermal contraceptive implant (which can cost up to $1,300 every 3 years, equivalent to $36.11 per month) with OCPs (which can cost as low as $324 for 3 years for an ethinyl estradiol and norgestimate combination, or $9 per month), the OCPs significantly outweigh the implant in terms of cost. When comparing failure rates, the degree of patient intervention, and decreased use of menstrual control products due to amenorrhea, the subdermal contraceptive wins. As we know, long-acting reversible contraception (LARC), including the intrauterine device (IUD) and subdermal implant, is the most effective but often the most expensive contraceptive option.5 When cost is evaluated from a global perspective, as highlighted by the adage “an IUD is cheaper than a baby,” the LARC’s value is derived from its overall high effectiveness and low cost.
If the patient elects to choose OCPs, the clinician should direct the prescription to a pharmacy that has discounted generic pills on its formulary. Generic OCPs have a low- cost burden without loss of efficacy, thus providing maximal value.6 This requires an intimate knowledge of the local pharmacies and what their formularies provide. Sometimes the patient will need to drive out of her way to access cost-effective, quality medications, or the high-value option.
Surgery considerations. Judicious instrument selection in the OR can decrease overall operative costs. While most advanced sealing and cutting instrumentation is for single use, for example, it also can be reprocessed for reuse. Although the cost of reprocessed, single-use instruments is lower, studies evaluating the quality of these instruments “found a significant rate of physical defects, performance issues, or improper decontamination.”7
Marketing largely has driven physician choice in the use of certain vessel sealing and cutting devices, but there has yet to be evidence that using any one device actually improves performance or outcomes, such as length of surgery, blood loss, or postoperative complications. Technology companies that create these instruments likely will have to start designing studies to test performance and outcomes as they relate to their devices to persuade hospital systems that using their products improves outcomes and reduces costs.
While learning laparoscopic hysterectomy, residents may see that some attending surgeons can complete the entire procedure with monopolar scissors, bipolar forceps, and laparoscopic needle drivers, while other surgeons use those instruments plus others, such as a LigaSure instrument or a Harmonic scalpel. With outcomes being the same between these surgeons, it is reasonable for hospitals to audit each surgeon using the Value = Quality ÷ Cost equation and to seek data to describe why the latter surgeon requires additional instrumentation.
Residency training poses a unique opportunity for physicians to learn numerous ways to perform the same procedure so they can fill their armamentarium with various effective techniques. Residency also should be a time in which proficiency with basic surgical instrumentation is emphasized. Attending physicians can help residents improve their skills, for example, by having them use only one advanced sealing and cutting device, or no device at all. This practice will make the trainee better able to adapt to situations in which an advanced device may fail or be unavailable. Future performance metrics may evaluate the physician’s cost effectiveness with regard to single-use instruments during routine surgical procedures.
Standardized order sets. Evidence-based order sets help in the management of pneumonia, sepsis, deep vein thrombosis prophylaxis, and numerous other conditions. In the era of computerized physician order entry systems (CPOEs), a resident needs to enter just a few clicks to order all necessary tests, interventions, and imaging studies for a condition. In one fell swoop, orders are placed not only for admission but also for the patient’s entire hospitalization. The paradox of the order set is that it uses a template to deliver individualized patient-centered care.
In the age of enhanced recovery pathways after surgery, we see patients who undergo a hysterectomy being discharged home directly from the postoperative anesthesia care unit (PACU). Generally, follow-up laboratory testing is not ordered on an outpatient basis. If, however, the patient needs to remain in the hospital for social reasons (such as delayed PACU transfer, transportation, weather), she receives the standardized orders from the post hysterectomy order set: a morning complete blood count ($55) with a basic metabolic panel ($45). As an academic exercise, the order set may help residents learn which orders they must consider when admitting a postoperative hysterectomy patient, but overuse of order sets can be a setback for a value-based care system.
Read about evaluating competence and individualizing care.
Evaluating competence in value-based care
Research is an integral component of all residency programs accredited by the Accreditation Council for Graduate Medical Education (ACGME). The implementation of value-based care—with all its nuances, quality metrics, and cost parameters—creates a space for resident-led studies to contribute to peer education. The ACGME’s Obstetrics and Gynecology Milestones project was developed to assess the development of ObGyn residents’ competence as they progress through training. Despite national laws tying reimbursements to value-based care, there is no mention of value as it relates to the basic formula, Value = Quality ÷ Cost, in the project.
With the nuances that value-based care offers, it would behoove the Council on Resident Education in Obstetrics and Gynecology of the American College of Obstetricians and Gynecologists to incorporate a method of evaluation to determine competence in this evolving field.
Care also must be individualized
Academic ObGyns and instructors should focus their pedagogy not only on value-based care but also on individualized care that will maximize desired outcomes for each patient. Incorporating multidisciplinary didactics, focused research, and a 360-degree evaluation in the residency curriculum will create new ObGyns who are known for successfully delivering high-value care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Wieand E, Lagrew DC Jr. Value-based payment: what does it mean, and how can ObGyns get out ahead? OBG Manag. 2018;30(1):17–19, 25–26.
- Agency for Healthcare Research and Quality. The six domains of health care quality. https://www.ahrq.gov/professionals/quality-patient-safety/talkingquality/create/sixdomains.html. Reviewed March 2016. Accessed March 22, 2018.
- Centers for Medicare and Medicaid Services. Better care. Smarter spending. Healthier people: paying providers for value, not volume. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-01-26-3.html. Published January 26, 2015. Accessed March 22, 2018.
- Society for Human Resource Management. Managing health care costs. https://www.shrm.org/resourcesandtools/tools-and-samples/toolkits/pages/managinghealthcarecosts.aspx. Published January 11, 2017. Accessed March 18, 2018.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice, Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 642: Increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126(4):e44–e48.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 375: Brand versus generic oral contraceptives. Obstet Gynecol. 2007;110(2 pt 1):447–448.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 537: R
eprocessed single-use devices. Obstet Gynecol. 2012;120(4):974–976.
- Wieand E, Lagrew DC Jr. Value-based payment: what does it mean, and how can ObGyns get out ahead? OBG Manag. 2018;30(1):17–19, 25–26.
- Agency for Healthcare Research and Quality. The six domains of health care quality. https://www.ahrq.gov/professionals/quality-patient-safety/talkingquality/create/sixdomains.html. Reviewed March 2016. Accessed March 22, 2018.
- Centers for Medicare and Medicaid Services. Better care. Smarter spending. Healthier people: paying providers for value, not volume. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-01-26-3.html. Published January 26, 2015. Accessed March 22, 2018.
- Society for Human Resource Management. Managing health care costs. https://www.shrm.org/resourcesandtools/tools-and-samples/toolkits/pages/managinghealthcarecosts.aspx. Published January 11, 2017. Accessed March 18, 2018.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice, Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 642: Increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126(4):e44–e48.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 375: Brand versus generic oral contraceptives. Obstet Gynecol. 2007;110(2 pt 1):447–448.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 537: R
eprocessed single-use devices. Obstet Gynecol. 2012;120(4):974–976.
Read all parts of this series
PART 1 Value-based payment: What does it mean and how can ObGyns get out ahead
PART 2 What makes a “quality” quality measure?
PART 3 The role of patient-reported outcomes in women’s health
PART 4 It costs what?! How we can educate residents and students on how much things cost
Tactics for reducing the rate of surgical site infection following cesarean delivery
The 25-year-old patient (G1P0) is at 41 weeks’ gestation. She has been fully dilated and pushing for 3.5 hours, at station 0, with regular strong contractions, no descent and a Category II fetal heart-rate tracing. The estimated fetal weight is 8 lb. Membranes have been ruptured for 10 hours. Maternal temperature is 99° F and her prepregnancy body mass index (BMI) was 32 kg/m2. After examining the patient and reviewing the labor progress, you recommend a cesarean delivery. As you prepare for the delivery, you identify the patient as high risk for surgical site infection and begin to recall all the interventions that might reduce postoperative infection for a patient at high risk for infection.
Halsted’s surgical principles
Dr. William Steward Halsted, the first chief of surgery at Johns Hopkins Hospital, articulated a set of surgical principles that included strict aseptic technique, gentle tissue handling, meticulous hemostasis, minimum tension on tissue, accurate tissue apposition, preservation of blood supply, and obliteration of dead space where appropriate. These principles of “safe surgery” are believed to improve surgical outcomes and reduce the risk of surgical site infection.1
Preoperative antibiotics
All obstetricians who perform cesarean delivery know the importance of administering a narrow-spectrum antibiotic, such as cefazolin or ampicillin, prior to the skin incision, but not more than 60 minutes before the incision, to help reduce the risk of wound infection and endometritis. In a meta-analysis of 82 studies involving more than 13,000 women the administration of a preoperative antibiotic compared with placebo reduced the risk of wound infection (relative risk [RR], 0.40; 95% confidence interval [CI], 0.35–0.46) and endometritis (RR, 0.38; 95% CI, 0.34–0.42).2
Cefazolin 3 g versus 2 g for obese patients
There are no data from randomized trials of cesarean delivery that directly compare the efficacy of preoperative cefazolin at doses of 2 g and 3 g to reduce the risk of infection. However, based on the observation that, for any given dose of cefazolin, circulating levels are reduced in obese patients, many authorities recommend that if the patient weighs ≥120 kg that 3 g of cefazolin should be administered.3
Extended-spectrum preoperative antibiotics
Some experts recommend that, for women in labor and for women with more than 4 hours of ruptured membranes, IV azithromycin 500 mg be added to the standard narrow-spectrum cefazolin regimen to reduce the rate of postoperative infection. In one trial, 2,013 women who were in labor or had more than 4 hours of ruptured membranes were randomly assigned to IV cefazolin alone or IV cefazolin plus azithromycin 500 mg prior to cesarean delivery.4 The cefazolin dose was reported to be weight-based utilizing the BMI at the time of delivery. The rates of endometritis (3.8% vs 6.1%) and wound infection (2.4% vs 6.6%) were lower in the women receiving extended-spectrum antibiotics versus cefazolin monotherapy.
Concerns have been raised about the impact of extended-spectrum antibiotics on the newborn microbiome and risk of accelerating the emergence of bacteria resistant to available antibiotics. Limiting the use of azithromycin to those cesarean delivery cases in which the patient is immunosuppressed, diabetic, obese, in labor and/or with prolonged ruptured membranes would reduce the number of women and newborns exposed to the drug and achieve the immediate health goal of reducing surgical infection.
Preoperative vaginal preparation
Many authorities recommend the use of a preoperative povidone- iodine vaginal scrub for 30 seconds prior to cesarean delivery for women in labor and women with ruptured membranes. In a meta-analysis of 16 trials involving 4,837 women, the women who received vaginal cleansing before cesarean delivery had a significantly lower incidence of endometritis (4.5% vs 8.8%) and postoperative fever (9.4% vs 14.9%) compared with those who did not have vaginal cleansing.5 Most of the benefit in reducing the risk of endometritis was confined to women in labor before the cesarean delivery (8.1% vs 13.8%) and women with ruptured membranes (4.3% vs 20.1%).5
Metronidazole gel 5 g also has been reported to be effective in reducing the rate of endometritis associated with cesarean delivery. In one study, 224 women having a cesarean delivery for various indications were randomly assigned to preoperative treatment with vaginally administered metronidazole gel 5 g or placebo gel. All women also received one dose of preoperative intravenous antibiotics. The rates of endometritis were 7% and 17% in the metronidazole and placebo groups, respectively.6
Povidone-iodine is approved for vaginal surgical site cleansing. For women with allergies to iodine or povidone-iodine, the options for vaginal cleansing are limited. The American College of Obstetricians and Gynecologists has noted the chlorhexidine gluconate solutions with a high concentration of alcohol should not be used for vaginal cleansing because the alcohol can irritate the mucosal epithelium. However, although not US Food and Drug Administration–approved for vaginal cleansing, solutions of chlorhexidine with a low alcohol content (Hibiclens, chlorhexidine with 4% alcohol concentration) are thought to be safe and may be considered for off-label use in vaginal cleansing.7
Preoperative abdominal preparation with chlorhexidine
Some authorities recommend skin preparation with chlorhexidine rather than povidone-iodine prior to cesarean delivery. Two recent randomized trials in women undergoing cesarean delivery8,9 and one trial in patients undergoing general surgery operations10 reported a reduction in surgical site infection with chlorhexidine. However, other trials have reported no difference in the rate of surgical site infection with these two skin preparation methods.11,12
Changing gloves and equipment after delivery of the newborn
Currently there is no high-quality evidence that changing gloves after delivery of the newborn or using new surgical instruments for closure reduces the risk of postcesarean infection. Two small clinical trials reported that changing gloves after delivery of the newborn did not reduce the rate of postcesarean infection.13,14
Postoperative antibiotics (a heretical challenge to the central dogma of antibiotic prophylaxis in surgery)
The central dogma of antibiotic prevention of postoperative infection is that antibiotics administered just before skin incision are effective, and postoperative antibiotics to prevent surgical infection generally are not useful. For the case of cesarean delivery, where the rate of postcesarean infection is very high, that dogma is being questioned. In a recent clinical trial, 403 women with a prepregnancy BMI ≥30 kg/m2 were randomly assigned to postcesarean treatment with oral cephalexin plus metronidazole (500 mg of each medication every 8 hours for 6 doses) or placebo pills.15 All women received preoperative IV cefazolin 2 g, indicating that the dosing was probably not weight-based. The surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 6.4% and 15.4%, respectively (RR, 0.41; 95% CI, 0.22–0.77; P = .01). In a subgroup analysis based on the presence or absence of ruptured membranes, postoperative oral cephalexin plus metronidazole was most beneficial for the women with ruptured membranes. Among women with ruptured membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 9.5% and 30.2%, respectively. Among women with intact membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 5% and 8.7%, respectively.
Given that these findings are not consistent with current dogma, clinicians should be cautious about using postcesarean antibiotics and await confirmation in additional trials. Of relevance, a randomized study of women with chorioamnionitis who were treated precesarean delivery with ampicillin, gentamicin, and clindamycin did not benefit from the administration of additional postoperative antibiotics (one additional dose of gentamicin and clindamycin) compared with no postdelivery antibiotics.16
Does suture selection matter?
In one randomized trial comparing two suture types, 550 women undergoing nonemergent cesarean delivery were randomly assigned to subcuticular skin closure with polyglactin 910 (Vicryl) or poliglecaprone 25 (Monocryl) suture. The poliglecaprone 25 suture was associated with a lower rate of wound complications (8.8% vs 14.4%; 95% CI, 0.37–99; P = .04).17 However, a post-hoc analysis of a randomized trial of skin preparation did not observe a difference in wound complications between the use of polyglactinor poliglecaprone suture for skin closure.18
Prophylactic negative-pressure wound therapy: An evolving best practice?
A meta-analysis of 6 randomized trials and 3 cohort studies reported that in high-risk obese women the use of prophylactic negative-pressure wound therapy compared with standard wound dressing resulted in a decrease in surgical site infection (RR, 0.45; 95% CI, 0.31–0.66).19 The number needed to treat was 17. In one recent study, the wound outcomes following cesarean delivery among women with a BMI ≥40 kg/m2 were compared in 234 women who received and 233 women who did not receive negative-pressure wound therapy.20 Wound infection was observed in 5.6% and 9.9% of the treated and untreated women, respectively.20 However, another meta-analysis of prophylactic negative-pressure wound therapy for obese women undergoing cesarean delivery did not report any benefit.21
Let’s work on continuous improvement
Cesarean delivery is a common major operation and is associated with wound infections and endometritis at rates much greater than those observed after vaginal delivery or other major intra-abdominal operations. As obstetricians, we can do more to guide practice toward continuous improvement in surgical outcomes. Systematically using a bundle of evidence-based interventions, including proper antibiotic selection, timing, and dosing; use of hair removal with clippers; use of chlorhexidine abdominal prep; removal of the placenta with gentle traction; and closure of the subcutaneous layer if tissue depth is ≥2 cm, will reduce the rate of postcesarean infection.22 Although aspirational, we may, someday, achieve a post‑cesarean infection rate less than 1%!
CASE Conclusion
The patient was noted to be at high risk for postcesarean infection because she had both an elevated BMI and ruptured membranes. The surgeon astutely decided to administer cefazolin 3 g and azithromycin 500 mg, cleanse the vagina with povidone-iodine, use chlorhexidine for the abdominal prep, use poliglecaprone 25 subcuticular skin closure, and did not use postoperative antibiotics or prophylactic wound vacuum. Following an uneventful cesarean delivery, the patient was discharged without an infection on postoperative day 4.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Cameron JL. William Steward Halsted: our surgical heritage. Ann Surg. 1997;225(5):445–458.
- Smaill FM, Grivell RM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2014;(10):CD007482.
- Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283.
- Tita AT, Szychowski JM, Boggess K, et al; C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231–1241.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systemic review and meta-analysis. Obstet Gynecol. 2017;130(3):527–538.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- American College of Obstetricians and Gynecologists; Committee on Gynecologic Practice. Committee Opinion No. 571: solutions for surgicalpreparation of the vagina. Obstet Gynecol. 2013;122(3):718–720.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Kunkle CM, Marchan J, Safadi S, Whitman S, Chmait RH. Chlorhexidine gluconate versus povidone iodine at cesarean delivery: a randomized controlled trial. J Matern Fetal Neonatal Med. 2015;28(5):573–577.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Springel EH, Wang XY, Sarfoh VM, Stetzer BP, Weight SA, Mercer BM. A randomized open-label controlled trial of chlorhexidine-alcohol vs povidone-iodine for cesarean antisepsis: the CAPICA trial. Am J Obstet Gynecol. 2017;217(4):463.e1–e8.
- Turrentine MA, Banks TA. Effect of changing gloves before placental extraction on incidence of postcesarean endometritis. Infect Dis Obstet Gynecol. 1996;4(1):16–19.
- Cernadas M, Smulian JC, Giannina G, Ananth CV. Effects of placental delivery method and intraoperative glove changing on postcesareanfebrile morbidity. J Matern Fetal Med. 1998;7(2):100–104.
- Valent AM, DeArmond C, Houston JM, et al. Effect of post-cesarean delivery oral cephalexin and metronidazole on surgical site infection among obese women: a randomized clinical trial. JAMA. 2017;318(11):1026–1034.
- Shanks AL, Mehra S, Gross G, Colvin R, Harper LM, Tuuli MG. Treatment utility of postpartum antibiotics in chorioamnionitis study. Am J Perinatol. 2016;33(8):732–737.
- Buresch AM, Van Arsdale A, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2017;130(3): 521–526.
- Tuuli MG, Stout MJ, Martin S, Rampersad RM, Cahill AG, Macones GA. Comparison of suture materials for subcuticular skin closure at cesarean delivery. Am J Obstet Gynecol. 2016;215(4): 490.e1–e5.
- Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200–210.e1.
- Looby MA, Vogel RI, Bangdiwala A, Hyer B, Das K. Prophylactic negative pressure wound therapy in obese patients following cesarean delivery. Surg Innov. 2018;25(1):43–49.
- Smid MD, Dotters-Katz SK, Grace M, et al. Prophylactic negative pressure wound therapy for obese women after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(5):969–978.
- Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(4):735–746.
The 25-year-old patient (G1P0) is at 41 weeks’ gestation. She has been fully dilated and pushing for 3.5 hours, at station 0, with regular strong contractions, no descent and a Category II fetal heart-rate tracing. The estimated fetal weight is 8 lb. Membranes have been ruptured for 10 hours. Maternal temperature is 99° F and her prepregnancy body mass index (BMI) was 32 kg/m2. After examining the patient and reviewing the labor progress, you recommend a cesarean delivery. As you prepare for the delivery, you identify the patient as high risk for surgical site infection and begin to recall all the interventions that might reduce postoperative infection for a patient at high risk for infection.
Halsted’s surgical principles
Dr. William Steward Halsted, the first chief of surgery at Johns Hopkins Hospital, articulated a set of surgical principles that included strict aseptic technique, gentle tissue handling, meticulous hemostasis, minimum tension on tissue, accurate tissue apposition, preservation of blood supply, and obliteration of dead space where appropriate. These principles of “safe surgery” are believed to improve surgical outcomes and reduce the risk of surgical site infection.1
Preoperative antibiotics
All obstetricians who perform cesarean delivery know the importance of administering a narrow-spectrum antibiotic, such as cefazolin or ampicillin, prior to the skin incision, but not more than 60 minutes before the incision, to help reduce the risk of wound infection and endometritis. In a meta-analysis of 82 studies involving more than 13,000 women the administration of a preoperative antibiotic compared with placebo reduced the risk of wound infection (relative risk [RR], 0.40; 95% confidence interval [CI], 0.35–0.46) and endometritis (RR, 0.38; 95% CI, 0.34–0.42).2
Cefazolin 3 g versus 2 g for obese patients
There are no data from randomized trials of cesarean delivery that directly compare the efficacy of preoperative cefazolin at doses of 2 g and 3 g to reduce the risk of infection. However, based on the observation that, for any given dose of cefazolin, circulating levels are reduced in obese patients, many authorities recommend that if the patient weighs ≥120 kg that 3 g of cefazolin should be administered.3
Extended-spectrum preoperative antibiotics
Some experts recommend that, for women in labor and for women with more than 4 hours of ruptured membranes, IV azithromycin 500 mg be added to the standard narrow-spectrum cefazolin regimen to reduce the rate of postoperative infection. In one trial, 2,013 women who were in labor or had more than 4 hours of ruptured membranes were randomly assigned to IV cefazolin alone or IV cefazolin plus azithromycin 500 mg prior to cesarean delivery.4 The cefazolin dose was reported to be weight-based utilizing the BMI at the time of delivery. The rates of endometritis (3.8% vs 6.1%) and wound infection (2.4% vs 6.6%) were lower in the women receiving extended-spectrum antibiotics versus cefazolin monotherapy.
Concerns have been raised about the impact of extended-spectrum antibiotics on the newborn microbiome and risk of accelerating the emergence of bacteria resistant to available antibiotics. Limiting the use of azithromycin to those cesarean delivery cases in which the patient is immunosuppressed, diabetic, obese, in labor and/or with prolonged ruptured membranes would reduce the number of women and newborns exposed to the drug and achieve the immediate health goal of reducing surgical infection.
Preoperative vaginal preparation
Many authorities recommend the use of a preoperative povidone- iodine vaginal scrub for 30 seconds prior to cesarean delivery for women in labor and women with ruptured membranes. In a meta-analysis of 16 trials involving 4,837 women, the women who received vaginal cleansing before cesarean delivery had a significantly lower incidence of endometritis (4.5% vs 8.8%) and postoperative fever (9.4% vs 14.9%) compared with those who did not have vaginal cleansing.5 Most of the benefit in reducing the risk of endometritis was confined to women in labor before the cesarean delivery (8.1% vs 13.8%) and women with ruptured membranes (4.3% vs 20.1%).5
Metronidazole gel 5 g also has been reported to be effective in reducing the rate of endometritis associated with cesarean delivery. In one study, 224 women having a cesarean delivery for various indications were randomly assigned to preoperative treatment with vaginally administered metronidazole gel 5 g or placebo gel. All women also received one dose of preoperative intravenous antibiotics. The rates of endometritis were 7% and 17% in the metronidazole and placebo groups, respectively.6
Povidone-iodine is approved for vaginal surgical site cleansing. For women with allergies to iodine or povidone-iodine, the options for vaginal cleansing are limited. The American College of Obstetricians and Gynecologists has noted the chlorhexidine gluconate solutions with a high concentration of alcohol should not be used for vaginal cleansing because the alcohol can irritate the mucosal epithelium. However, although not US Food and Drug Administration–approved for vaginal cleansing, solutions of chlorhexidine with a low alcohol content (Hibiclens, chlorhexidine with 4% alcohol concentration) are thought to be safe and may be considered for off-label use in vaginal cleansing.7
Preoperative abdominal preparation with chlorhexidine
Some authorities recommend skin preparation with chlorhexidine rather than povidone-iodine prior to cesarean delivery. Two recent randomized trials in women undergoing cesarean delivery8,9 and one trial in patients undergoing general surgery operations10 reported a reduction in surgical site infection with chlorhexidine. However, other trials have reported no difference in the rate of surgical site infection with these two skin preparation methods.11,12
Changing gloves and equipment after delivery of the newborn
Currently there is no high-quality evidence that changing gloves after delivery of the newborn or using new surgical instruments for closure reduces the risk of postcesarean infection. Two small clinical trials reported that changing gloves after delivery of the newborn did not reduce the rate of postcesarean infection.13,14
Postoperative antibiotics (a heretical challenge to the central dogma of antibiotic prophylaxis in surgery)
The central dogma of antibiotic prevention of postoperative infection is that antibiotics administered just before skin incision are effective, and postoperative antibiotics to prevent surgical infection generally are not useful. For the case of cesarean delivery, where the rate of postcesarean infection is very high, that dogma is being questioned. In a recent clinical trial, 403 women with a prepregnancy BMI ≥30 kg/m2 were randomly assigned to postcesarean treatment with oral cephalexin plus metronidazole (500 mg of each medication every 8 hours for 6 doses) or placebo pills.15 All women received preoperative IV cefazolin 2 g, indicating that the dosing was probably not weight-based. The surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 6.4% and 15.4%, respectively (RR, 0.41; 95% CI, 0.22–0.77; P = .01). In a subgroup analysis based on the presence or absence of ruptured membranes, postoperative oral cephalexin plus metronidazole was most beneficial for the women with ruptured membranes. Among women with ruptured membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 9.5% and 30.2%, respectively. Among women with intact membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 5% and 8.7%, respectively.
Given that these findings are not consistent with current dogma, clinicians should be cautious about using postcesarean antibiotics and await confirmation in additional trials. Of relevance, a randomized study of women with chorioamnionitis who were treated precesarean delivery with ampicillin, gentamicin, and clindamycin did not benefit from the administration of additional postoperative antibiotics (one additional dose of gentamicin and clindamycin) compared with no postdelivery antibiotics.16
Does suture selection matter?
In one randomized trial comparing two suture types, 550 women undergoing nonemergent cesarean delivery were randomly assigned to subcuticular skin closure with polyglactin 910 (Vicryl) or poliglecaprone 25 (Monocryl) suture. The poliglecaprone 25 suture was associated with a lower rate of wound complications (8.8% vs 14.4%; 95% CI, 0.37–99; P = .04).17 However, a post-hoc analysis of a randomized trial of skin preparation did not observe a difference in wound complications between the use of polyglactinor poliglecaprone suture for skin closure.18
Prophylactic negative-pressure wound therapy: An evolving best practice?
A meta-analysis of 6 randomized trials and 3 cohort studies reported that in high-risk obese women the use of prophylactic negative-pressure wound therapy compared with standard wound dressing resulted in a decrease in surgical site infection (RR, 0.45; 95% CI, 0.31–0.66).19 The number needed to treat was 17. In one recent study, the wound outcomes following cesarean delivery among women with a BMI ≥40 kg/m2 were compared in 234 women who received and 233 women who did not receive negative-pressure wound therapy.20 Wound infection was observed in 5.6% and 9.9% of the treated and untreated women, respectively.20 However, another meta-analysis of prophylactic negative-pressure wound therapy for obese women undergoing cesarean delivery did not report any benefit.21
Let’s work on continuous improvement
Cesarean delivery is a common major operation and is associated with wound infections and endometritis at rates much greater than those observed after vaginal delivery or other major intra-abdominal operations. As obstetricians, we can do more to guide practice toward continuous improvement in surgical outcomes. Systematically using a bundle of evidence-based interventions, including proper antibiotic selection, timing, and dosing; use of hair removal with clippers; use of chlorhexidine abdominal prep; removal of the placenta with gentle traction; and closure of the subcutaneous layer if tissue depth is ≥2 cm, will reduce the rate of postcesarean infection.22 Although aspirational, we may, someday, achieve a post‑cesarean infection rate less than 1%!
CASE Conclusion
The patient was noted to be at high risk for postcesarean infection because she had both an elevated BMI and ruptured membranes. The surgeon astutely decided to administer cefazolin 3 g and azithromycin 500 mg, cleanse the vagina with povidone-iodine, use chlorhexidine for the abdominal prep, use poliglecaprone 25 subcuticular skin closure, and did not use postoperative antibiotics or prophylactic wound vacuum. Following an uneventful cesarean delivery, the patient was discharged without an infection on postoperative day 4.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The 25-year-old patient (G1P0) is at 41 weeks’ gestation. She has been fully dilated and pushing for 3.5 hours, at station 0, with regular strong contractions, no descent and a Category II fetal heart-rate tracing. The estimated fetal weight is 8 lb. Membranes have been ruptured for 10 hours. Maternal temperature is 99° F and her prepregnancy body mass index (BMI) was 32 kg/m2. After examining the patient and reviewing the labor progress, you recommend a cesarean delivery. As you prepare for the delivery, you identify the patient as high risk for surgical site infection and begin to recall all the interventions that might reduce postoperative infection for a patient at high risk for infection.
Halsted’s surgical principles
Dr. William Steward Halsted, the first chief of surgery at Johns Hopkins Hospital, articulated a set of surgical principles that included strict aseptic technique, gentle tissue handling, meticulous hemostasis, minimum tension on tissue, accurate tissue apposition, preservation of blood supply, and obliteration of dead space where appropriate. These principles of “safe surgery” are believed to improve surgical outcomes and reduce the risk of surgical site infection.1
Preoperative antibiotics
All obstetricians who perform cesarean delivery know the importance of administering a narrow-spectrum antibiotic, such as cefazolin or ampicillin, prior to the skin incision, but not more than 60 minutes before the incision, to help reduce the risk of wound infection and endometritis. In a meta-analysis of 82 studies involving more than 13,000 women the administration of a preoperative antibiotic compared with placebo reduced the risk of wound infection (relative risk [RR], 0.40; 95% confidence interval [CI], 0.35–0.46) and endometritis (RR, 0.38; 95% CI, 0.34–0.42).2
Cefazolin 3 g versus 2 g for obese patients
There are no data from randomized trials of cesarean delivery that directly compare the efficacy of preoperative cefazolin at doses of 2 g and 3 g to reduce the risk of infection. However, based on the observation that, for any given dose of cefazolin, circulating levels are reduced in obese patients, many authorities recommend that if the patient weighs ≥120 kg that 3 g of cefazolin should be administered.3
Extended-spectrum preoperative antibiotics
Some experts recommend that, for women in labor and for women with more than 4 hours of ruptured membranes, IV azithromycin 500 mg be added to the standard narrow-spectrum cefazolin regimen to reduce the rate of postoperative infection. In one trial, 2,013 women who were in labor or had more than 4 hours of ruptured membranes were randomly assigned to IV cefazolin alone or IV cefazolin plus azithromycin 500 mg prior to cesarean delivery.4 The cefazolin dose was reported to be weight-based utilizing the BMI at the time of delivery. The rates of endometritis (3.8% vs 6.1%) and wound infection (2.4% vs 6.6%) were lower in the women receiving extended-spectrum antibiotics versus cefazolin monotherapy.
Concerns have been raised about the impact of extended-spectrum antibiotics on the newborn microbiome and risk of accelerating the emergence of bacteria resistant to available antibiotics. Limiting the use of azithromycin to those cesarean delivery cases in which the patient is immunosuppressed, diabetic, obese, in labor and/or with prolonged ruptured membranes would reduce the number of women and newborns exposed to the drug and achieve the immediate health goal of reducing surgical infection.
Preoperative vaginal preparation
Many authorities recommend the use of a preoperative povidone- iodine vaginal scrub for 30 seconds prior to cesarean delivery for women in labor and women with ruptured membranes. In a meta-analysis of 16 trials involving 4,837 women, the women who received vaginal cleansing before cesarean delivery had a significantly lower incidence of endometritis (4.5% vs 8.8%) and postoperative fever (9.4% vs 14.9%) compared with those who did not have vaginal cleansing.5 Most of the benefit in reducing the risk of endometritis was confined to women in labor before the cesarean delivery (8.1% vs 13.8%) and women with ruptured membranes (4.3% vs 20.1%).5
Metronidazole gel 5 g also has been reported to be effective in reducing the rate of endometritis associated with cesarean delivery. In one study, 224 women having a cesarean delivery for various indications were randomly assigned to preoperative treatment with vaginally administered metronidazole gel 5 g or placebo gel. All women also received one dose of preoperative intravenous antibiotics. The rates of endometritis were 7% and 17% in the metronidazole and placebo groups, respectively.6
Povidone-iodine is approved for vaginal surgical site cleansing. For women with allergies to iodine or povidone-iodine, the options for vaginal cleansing are limited. The American College of Obstetricians and Gynecologists has noted the chlorhexidine gluconate solutions with a high concentration of alcohol should not be used for vaginal cleansing because the alcohol can irritate the mucosal epithelium. However, although not US Food and Drug Administration–approved for vaginal cleansing, solutions of chlorhexidine with a low alcohol content (Hibiclens, chlorhexidine with 4% alcohol concentration) are thought to be safe and may be considered for off-label use in vaginal cleansing.7
Preoperative abdominal preparation with chlorhexidine
Some authorities recommend skin preparation with chlorhexidine rather than povidone-iodine prior to cesarean delivery. Two recent randomized trials in women undergoing cesarean delivery8,9 and one trial in patients undergoing general surgery operations10 reported a reduction in surgical site infection with chlorhexidine. However, other trials have reported no difference in the rate of surgical site infection with these two skin preparation methods.11,12
Changing gloves and equipment after delivery of the newborn
Currently there is no high-quality evidence that changing gloves after delivery of the newborn or using new surgical instruments for closure reduces the risk of postcesarean infection. Two small clinical trials reported that changing gloves after delivery of the newborn did not reduce the rate of postcesarean infection.13,14
Postoperative antibiotics (a heretical challenge to the central dogma of antibiotic prophylaxis in surgery)
The central dogma of antibiotic prevention of postoperative infection is that antibiotics administered just before skin incision are effective, and postoperative antibiotics to prevent surgical infection generally are not useful. For the case of cesarean delivery, where the rate of postcesarean infection is very high, that dogma is being questioned. In a recent clinical trial, 403 women with a prepregnancy BMI ≥30 kg/m2 were randomly assigned to postcesarean treatment with oral cephalexin plus metronidazole (500 mg of each medication every 8 hours for 6 doses) or placebo pills.15 All women received preoperative IV cefazolin 2 g, indicating that the dosing was probably not weight-based. The surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 6.4% and 15.4%, respectively (RR, 0.41; 95% CI, 0.22–0.77; P = .01). In a subgroup analysis based on the presence or absence of ruptured membranes, postoperative oral cephalexin plus metronidazole was most beneficial for the women with ruptured membranes. Among women with ruptured membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 9.5% and 30.2%, respectively. Among women with intact membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 5% and 8.7%, respectively.
Given that these findings are not consistent with current dogma, clinicians should be cautious about using postcesarean antibiotics and await confirmation in additional trials. Of relevance, a randomized study of women with chorioamnionitis who were treated precesarean delivery with ampicillin, gentamicin, and clindamycin did not benefit from the administration of additional postoperative antibiotics (one additional dose of gentamicin and clindamycin) compared with no postdelivery antibiotics.16
Does suture selection matter?
In one randomized trial comparing two suture types, 550 women undergoing nonemergent cesarean delivery were randomly assigned to subcuticular skin closure with polyglactin 910 (Vicryl) or poliglecaprone 25 (Monocryl) suture. The poliglecaprone 25 suture was associated with a lower rate of wound complications (8.8% vs 14.4%; 95% CI, 0.37–99; P = .04).17 However, a post-hoc analysis of a randomized trial of skin preparation did not observe a difference in wound complications between the use of polyglactinor poliglecaprone suture for skin closure.18
Prophylactic negative-pressure wound therapy: An evolving best practice?
A meta-analysis of 6 randomized trials and 3 cohort studies reported that in high-risk obese women the use of prophylactic negative-pressure wound therapy compared with standard wound dressing resulted in a decrease in surgical site infection (RR, 0.45; 95% CI, 0.31–0.66).19 The number needed to treat was 17. In one recent study, the wound outcomes following cesarean delivery among women with a BMI ≥40 kg/m2 were compared in 234 women who received and 233 women who did not receive negative-pressure wound therapy.20 Wound infection was observed in 5.6% and 9.9% of the treated and untreated women, respectively.20 However, another meta-analysis of prophylactic negative-pressure wound therapy for obese women undergoing cesarean delivery did not report any benefit.21
Let’s work on continuous improvement
Cesarean delivery is a common major operation and is associated with wound infections and endometritis at rates much greater than those observed after vaginal delivery or other major intra-abdominal operations. As obstetricians, we can do more to guide practice toward continuous improvement in surgical outcomes. Systematically using a bundle of evidence-based interventions, including proper antibiotic selection, timing, and dosing; use of hair removal with clippers; use of chlorhexidine abdominal prep; removal of the placenta with gentle traction; and closure of the subcutaneous layer if tissue depth is ≥2 cm, will reduce the rate of postcesarean infection.22 Although aspirational, we may, someday, achieve a post‑cesarean infection rate less than 1%!
CASE Conclusion
The patient was noted to be at high risk for postcesarean infection because she had both an elevated BMI and ruptured membranes. The surgeon astutely decided to administer cefazolin 3 g and azithromycin 500 mg, cleanse the vagina with povidone-iodine, use chlorhexidine for the abdominal prep, use poliglecaprone 25 subcuticular skin closure, and did not use postoperative antibiotics or prophylactic wound vacuum. Following an uneventful cesarean delivery, the patient was discharged without an infection on postoperative day 4.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Cameron JL. William Steward Halsted: our surgical heritage. Ann Surg. 1997;225(5):445–458.
- Smaill FM, Grivell RM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2014;(10):CD007482.
- Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283.
- Tita AT, Szychowski JM, Boggess K, et al; C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231–1241.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systemic review and meta-analysis. Obstet Gynecol. 2017;130(3):527–538.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- American College of Obstetricians and Gynecologists; Committee on Gynecologic Practice. Committee Opinion No. 571: solutions for surgicalpreparation of the vagina. Obstet Gynecol. 2013;122(3):718–720.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Kunkle CM, Marchan J, Safadi S, Whitman S, Chmait RH. Chlorhexidine gluconate versus povidone iodine at cesarean delivery: a randomized controlled trial. J Matern Fetal Neonatal Med. 2015;28(5):573–577.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Springel EH, Wang XY, Sarfoh VM, Stetzer BP, Weight SA, Mercer BM. A randomized open-label controlled trial of chlorhexidine-alcohol vs povidone-iodine for cesarean antisepsis: the CAPICA trial. Am J Obstet Gynecol. 2017;217(4):463.e1–e8.
- Turrentine MA, Banks TA. Effect of changing gloves before placental extraction on incidence of postcesarean endometritis. Infect Dis Obstet Gynecol. 1996;4(1):16–19.
- Cernadas M, Smulian JC, Giannina G, Ananth CV. Effects of placental delivery method and intraoperative glove changing on postcesareanfebrile morbidity. J Matern Fetal Med. 1998;7(2):100–104.
- Valent AM, DeArmond C, Houston JM, et al. Effect of post-cesarean delivery oral cephalexin and metronidazole on surgical site infection among obese women: a randomized clinical trial. JAMA. 2017;318(11):1026–1034.
- Shanks AL, Mehra S, Gross G, Colvin R, Harper LM, Tuuli MG. Treatment utility of postpartum antibiotics in chorioamnionitis study. Am J Perinatol. 2016;33(8):732–737.
- Buresch AM, Van Arsdale A, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2017;130(3): 521–526.
- Tuuli MG, Stout MJ, Martin S, Rampersad RM, Cahill AG, Macones GA. Comparison of suture materials for subcuticular skin closure at cesarean delivery. Am J Obstet Gynecol. 2016;215(4): 490.e1–e5.
- Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200–210.e1.
- Looby MA, Vogel RI, Bangdiwala A, Hyer B, Das K. Prophylactic negative pressure wound therapy in obese patients following cesarean delivery. Surg Innov. 2018;25(1):43–49.
- Smid MD, Dotters-Katz SK, Grace M, et al. Prophylactic negative pressure wound therapy for obese women after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(5):969–978.
- Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(4):735–746.
- Cameron JL. William Steward Halsted: our surgical heritage. Ann Surg. 1997;225(5):445–458.
- Smaill FM, Grivell RM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2014;(10):CD007482.
- Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283.
- Tita AT, Szychowski JM, Boggess K, et al; C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231–1241.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systemic review and meta-analysis. Obstet Gynecol. 2017;130(3):527–538.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- American College of Obstetricians and Gynecologists; Committee on Gynecologic Practice. Committee Opinion No. 571: solutions for surgicalpreparation of the vagina. Obstet Gynecol. 2013;122(3):718–720.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Kunkle CM, Marchan J, Safadi S, Whitman S, Chmait RH. Chlorhexidine gluconate versus povidone iodine at cesarean delivery: a randomized controlled trial. J Matern Fetal Neonatal Med. 2015;28(5):573–577.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Springel EH, Wang XY, Sarfoh VM, Stetzer BP, Weight SA, Mercer BM. A randomized open-label controlled trial of chlorhexidine-alcohol vs povidone-iodine for cesarean antisepsis: the CAPICA trial. Am J Obstet Gynecol. 2017;217(4):463.e1–e8.
- Turrentine MA, Banks TA. Effect of changing gloves before placental extraction on incidence of postcesarean endometritis. Infect Dis Obstet Gynecol. 1996;4(1):16–19.
- Cernadas M, Smulian JC, Giannina G, Ananth CV. Effects of placental delivery method and intraoperative glove changing on postcesareanfebrile morbidity. J Matern Fetal Med. 1998;7(2):100–104.
- Valent AM, DeArmond C, Houston JM, et al. Effect of post-cesarean delivery oral cephalexin and metronidazole on surgical site infection among obese women: a randomized clinical trial. JAMA. 2017;318(11):1026–1034.
- Shanks AL, Mehra S, Gross G, Colvin R, Harper LM, Tuuli MG. Treatment utility of postpartum antibiotics in chorioamnionitis study. Am J Perinatol. 2016;33(8):732–737.
- Buresch AM, Van Arsdale A, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2017;130(3): 521–526.
- Tuuli MG, Stout MJ, Martin S, Rampersad RM, Cahill AG, Macones GA. Comparison of suture materials for subcuticular skin closure at cesarean delivery. Am J Obstet Gynecol. 2016;215(4): 490.e1–e5.
- Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200–210.e1.
- Looby MA, Vogel RI, Bangdiwala A, Hyer B, Das K. Prophylactic negative pressure wound therapy in obese patients following cesarean delivery. Surg Innov. 2018;25(1):43–49.
- Smid MD, Dotters-Katz SK, Grace M, et al. Prophylactic negative pressure wound therapy for obese women after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(5):969–978.
- Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(4):735–746.
MDedge Daily News: Why most heart failure may be preventable
Why most heart failure may be preventable. Statins miss the mark in familial high cholesterol. Why mumps outbreaks are on the rise. And how using epileptic drugs in pregnancy affects school test scores.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Why most heart failure may be preventable. Statins miss the mark in familial high cholesterol. Why mumps outbreaks are on the rise. And how using epileptic drugs in pregnancy affects school test scores.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Why most heart failure may be preventable. Statins miss the mark in familial high cholesterol. Why mumps outbreaks are on the rise. And how using epileptic drugs in pregnancy affects school test scores.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Here’s one issue blue and red states agree on: Preventing deaths of expectant and new mothers
Alarmed that the U.S. is the most dangerous affluent country in which to give birth, state and local lawmakers around the country are adopting a flurry of bipartisan bills aimed at reforming how maternal deaths are identified and investigated.
In Indiana earlier this month, Republican Gov. Eric Holcomb signed a bill creating a maternal mortality review committee to scrutinize deaths and near-deaths among expectant and new mothers and make policy recommendations to improve maternal health.
Oregon’s governor and Washington, D.C.’s mayor, both Democrats, are expected to sign similar legislation in the coming days. Proposals are pending in Pennsylvania, Connecticut, Maryland and New Jersey.
Legislators from several of these states credited the ProPublica/NPR “Lost Mothers” series with raising their awareness and concern about the issue. Maryland Delegate Jheanelle Wilkins, who introduced a bill there, said that the series, especially articles looking at why black mothers are at greatest risk of dying and nearly dying, inspired her and her fellow lawmakers.
“A friend of mine posted one of the stories on Facebook and she challenged her elected officials — Who’s going to do something about it?” Wilkins said.
About 35 states have now established review committees or are in the process of doing so, as well as four cities: New York, Philadelphia, Baltimore and Washington. Two federal bills introduced last year, which would create a grant program to help states introduce or improve review committees, remain stalled in committee.
Between 700 and 900 women die each year in the U.S. from causes related to pregnancy or childbirth, and the rate has risen even as it has declined in other wealthy countries. The rate of life-threatening complications has also soared since the 1990s, endangering more than 50,000 U.S. women a year. A new report by the CDC Foundation — a nonprofit created by Congress to support the Centers for Disease Control and Prevention — suggests that more than 60 percent of pregnancy- and childbirth-related deaths in the U.S. are preventable.
The “Lost Mothers” project highlighted a number of reasons the U.S. has fallen behind other countries, including a greater focus on the health of the baby than of the mother, treatment guidelines that vary from one doctor or hospital to the next, and government failures to collect accurate data and to study maternal deaths and near-deaths to understand how they might be prevented.
Maternal mortality review committees can play a key role in this process, public health experts say, by identifying pregnancy-related deaths that might otherwise be overlooked, analyzing the factors contributing to those deaths, and translating the lessons into policy changes. That’s what happens in Great Britain, where a national committee investigates every maternal death and the findings help set women’s health policy across the country.
As recently as 2016, only about half the states had such panels. The number has been growing quickly, said Andria Cornell, senior program manager for women’s health and maternal health lead at the Association of Maternal & Child Health Programs, a nonprofit advocacy group.
“This is a time of unprecedented political and social will for establishing maternal mortality review committees,” she said. “We’ve definitely come to a tipping point.”
Cornell credited two forces for driving the change: journalism focused on maternal deaths and a national project led by AMCHP, the Centers for Disease Control and the CDC Foundation.
With money from Merck for Mothers, a charitable initiative created by the pharmaceutical giant, the project has funded a web portal that provides information on starting and improving review committees and a tool, the Maternal Mortality Review Information Application, that shows jurisdictions how to standardize data collection from review panels so that it’s comparable from one state to the next.
Review committees do have limitations. Many are understaffed and poorly funded, with limited authority to dig deeply into systemic problems or implement meaningful reforms. It generally takes several years for them to produce their reports, in part because committee members — including doctors, public health experts, medical examiners, and the like — have other demands on their time and aren’t compensated.
Also, committee records and reports are de-identified — stripped of any information that might point to a particular woman, caregiver or hospital. Thus the review is of little use in assigning responsibility for individual deaths, or evaluating whether some hospitals, doctors or nurses are especially prone to error. Still, recommendations and findings from reviews have proven helpful in states such as California in shaping preventive efforts that have reduced maternal mortality rates.
Indiana epitomizes the national movement to use the review committee process to scrutinize maternal deaths. There, the focus had long been on reducing infant mortality: The state has the highest rate of neonatal deaths outside the South. Maternal deaths weren’t on the radar, even though the state’s maternal mortality rate is around 41 women per 100,000 births, according to a new analysis of federal data by United Health Foundation — or double the rate of maternal deaths in neighboring Illinois and Ohio.
“I’ll be honest,” said state Sen. Jean Leising, a Republican from rural southeastern Indiana. “I’m on the Health Committee ... and I had no idea our maternal statistics were so lousy.”
The bill she sponsored — creating a committee for the next five years to study not just maternal mortality but also life-threatening complications, or severe maternal morbidity — sailed through the legislature, in part because of a change in governors. Holcomb, who replaced Mike Pence and is seen as more of a pragmatist, appointed a female obstetrician-gynecologist to be his new health commissioner.
The bill’s supporters drew a connection between maternal and infant mortality, said Dr. Brownsyne Tucker Edmonds, legislative chair for the Indiana chapter of the American Congress of Obstetricians and Gynecologists: “We could bring forth the idea that healthy moms have healthy babies.”
Oregon’s bill, which also passed easily this month, creates a review committee that will start by focusing on maternal deaths; by 2021, it will also begin looking at severe maternal morbidity. Over that period, it will cost the state more than $450,000 — a significant public commitment to a women’s health initiative.
“I did think, wow, that’s more money than I thought it was going to be, but no one blinked an eye,” said Rep. Alissa Keny-Guyer of Portland, the bill’s chief sponsor. “That just shows how much support this idea has.”
In the District of Columbia, concerns about the high maternal mortality rate — in 2014, it stood at about 40.7 deaths per 100,000 births, according to the analysis by United Health Foundation, substantially exceeding the U.S. rate and those of neighboring Virginia and Maryland — have periodically sparked talk of a review committee, but not enough to push a measure through.
Last year, after two hospitals in Northeast and predominantly black Southeast Washington closed maternity units, concerns grew over access to quality care, particularly for low-income and minority women. Nationally, black women have a maternal mortality rate three to four times higher than white women, and the District suspects its gap is even wider.
“Those disparities were the more acute driver of why we felt we needed to take this action,” said Councilmember Charles Allen, who introduced the measure to establish the panel. “You have to know what is driving this wide disparity before you can really have the strategies for how to fix it.”
The D.C. bill still must be signed into law and, like all District legislation, reviewed by Congress before it becomes effective. It calls for one full-time employee to assist the panel’s work, a position that Allen said he expects to be funded in the budget that will be passed later in the year. In addition to health care professionals, a social worker and representatives of community groups that specialize in women’s health, the D.C. committee will also include “one person who has been directly impacted by a maternal mortality or severe maternal morbidity.” Maternal health advocates say listening to such voices is a critical step in addressing how disparities in race, income and education affect outcomes.
That’s what prompted Wilkins, the Maryland delegate, to introduce her bill, which passed the House this month and will be taken up in the Senate in April. Maryland established its review committee in 2000, but in the panel’s most recent report, the participants consisted almost exclusively of medical professionals, mostly doctors and nurse-midwives. Wilkins’s bill would require the committee to meet at least twice a year with a group that includes representatives from the Maryland Office of Minority Health and Health Disparities, the Maryland Patient Safety Center, women’s health advocacy organizations, and a relative of a mother who died, and to incorporate their recommendations into its final report.
“The women who are impacted and the organizations that work with the communities they live in — we need to make sure they are at the table,” Wilkins said.
Other pending proposals would revamp New Jersey’s 80-plus-year-old review process and establish a new review committee in Connecticut.
Pennsylvania, which ranks sixth in the number of births in the U.S., is currently the largest state without a maternal mortality review committee, but lawmakers are advancing a measure to change that. It passed the House in December and recently cleared a Senate committee; it’s now headed for consideration by the full Senate.
State Rep. Ryan Mackenzie, R-Lehigh, introduced the bill last October after doctors from his district showed him grim data on rising maternal mortality rates in the nation and the state. Pennsylvania’s rate has more than doubled since 1994, according to a December 2017 report in the Pittsburgh Post-Gazette. When Mackenzie ran the idea of creating a statewide review process past House colleagues, several responded that media reports about maternal mortality, including our “Lost Mothers” series, had spurred them to consider similar measures.
Mackenzie’s bill calls for a committee of at least 14 members, most of them health care professionals, with a special emphasis on members working in communities most affected by maternal deaths and a lack of access to care. The measure does not include funding, and specifies that committee members would be unpaid, but Mackenzie said the state Department of Health would redirect existing staff to support it.
Another important aim in creating a statewide review process is making sure maternal deaths are being defined and tracked consistently, Mackenzie said. The state health department has tabulated annual totals for years, but counts only deaths that occur up to 42 days after pregnancy and not those that happen within a year, the standard used by the CDC and most review committees. When Philadelphia’s maternal mortality review panel compared the state’s numbers with its own for the city from 2010 to 2012, the state’s count was about 30 percent lower. Mackenzie’s bill would align Pennsylvania’s committee with the one-year standard.
“We’re hoping to save lives,” Mackenzie said in proposing the review committee. “Based on the results in other states, we think this is realistic.”
ProPublica is a Pulitzer Prize-winning investigative newsroom.
Alarmed that the U.S. is the most dangerous affluent country in which to give birth, state and local lawmakers around the country are adopting a flurry of bipartisan bills aimed at reforming how maternal deaths are identified and investigated.
In Indiana earlier this month, Republican Gov. Eric Holcomb signed a bill creating a maternal mortality review committee to scrutinize deaths and near-deaths among expectant and new mothers and make policy recommendations to improve maternal health.
Oregon’s governor and Washington, D.C.’s mayor, both Democrats, are expected to sign similar legislation in the coming days. Proposals are pending in Pennsylvania, Connecticut, Maryland and New Jersey.
Legislators from several of these states credited the ProPublica/NPR “Lost Mothers” series with raising their awareness and concern about the issue. Maryland Delegate Jheanelle Wilkins, who introduced a bill there, said that the series, especially articles looking at why black mothers are at greatest risk of dying and nearly dying, inspired her and her fellow lawmakers.
“A friend of mine posted one of the stories on Facebook and she challenged her elected officials — Who’s going to do something about it?” Wilkins said.
About 35 states have now established review committees or are in the process of doing so, as well as four cities: New York, Philadelphia, Baltimore and Washington. Two federal bills introduced last year, which would create a grant program to help states introduce or improve review committees, remain stalled in committee.
Between 700 and 900 women die each year in the U.S. from causes related to pregnancy or childbirth, and the rate has risen even as it has declined in other wealthy countries. The rate of life-threatening complications has also soared since the 1990s, endangering more than 50,000 U.S. women a year. A new report by the CDC Foundation — a nonprofit created by Congress to support the Centers for Disease Control and Prevention — suggests that more than 60 percent of pregnancy- and childbirth-related deaths in the U.S. are preventable.
The “Lost Mothers” project highlighted a number of reasons the U.S. has fallen behind other countries, including a greater focus on the health of the baby than of the mother, treatment guidelines that vary from one doctor or hospital to the next, and government failures to collect accurate data and to study maternal deaths and near-deaths to understand how they might be prevented.
Maternal mortality review committees can play a key role in this process, public health experts say, by identifying pregnancy-related deaths that might otherwise be overlooked, analyzing the factors contributing to those deaths, and translating the lessons into policy changes. That’s what happens in Great Britain, where a national committee investigates every maternal death and the findings help set women’s health policy across the country.
As recently as 2016, only about half the states had such panels. The number has been growing quickly, said Andria Cornell, senior program manager for women’s health and maternal health lead at the Association of Maternal & Child Health Programs, a nonprofit advocacy group.
“This is a time of unprecedented political and social will for establishing maternal mortality review committees,” she said. “We’ve definitely come to a tipping point.”
Cornell credited two forces for driving the change: journalism focused on maternal deaths and a national project led by AMCHP, the Centers for Disease Control and the CDC Foundation.
With money from Merck for Mothers, a charitable initiative created by the pharmaceutical giant, the project has funded a web portal that provides information on starting and improving review committees and a tool, the Maternal Mortality Review Information Application, that shows jurisdictions how to standardize data collection from review panels so that it’s comparable from one state to the next.
Review committees do have limitations. Many are understaffed and poorly funded, with limited authority to dig deeply into systemic problems or implement meaningful reforms. It generally takes several years for them to produce their reports, in part because committee members — including doctors, public health experts, medical examiners, and the like — have other demands on their time and aren’t compensated.
Also, committee records and reports are de-identified — stripped of any information that might point to a particular woman, caregiver or hospital. Thus the review is of little use in assigning responsibility for individual deaths, or evaluating whether some hospitals, doctors or nurses are especially prone to error. Still, recommendations and findings from reviews have proven helpful in states such as California in shaping preventive efforts that have reduced maternal mortality rates.
Indiana epitomizes the national movement to use the review committee process to scrutinize maternal deaths. There, the focus had long been on reducing infant mortality: The state has the highest rate of neonatal deaths outside the South. Maternal deaths weren’t on the radar, even though the state’s maternal mortality rate is around 41 women per 100,000 births, according to a new analysis of federal data by United Health Foundation — or double the rate of maternal deaths in neighboring Illinois and Ohio.
“I’ll be honest,” said state Sen. Jean Leising, a Republican from rural southeastern Indiana. “I’m on the Health Committee ... and I had no idea our maternal statistics were so lousy.”
The bill she sponsored — creating a committee for the next five years to study not just maternal mortality but also life-threatening complications, or severe maternal morbidity — sailed through the legislature, in part because of a change in governors. Holcomb, who replaced Mike Pence and is seen as more of a pragmatist, appointed a female obstetrician-gynecologist to be his new health commissioner.
The bill’s supporters drew a connection between maternal and infant mortality, said Dr. Brownsyne Tucker Edmonds, legislative chair for the Indiana chapter of the American Congress of Obstetricians and Gynecologists: “We could bring forth the idea that healthy moms have healthy babies.”
Oregon’s bill, which also passed easily this month, creates a review committee that will start by focusing on maternal deaths; by 2021, it will also begin looking at severe maternal morbidity. Over that period, it will cost the state more than $450,000 — a significant public commitment to a women’s health initiative.
“I did think, wow, that’s more money than I thought it was going to be, but no one blinked an eye,” said Rep. Alissa Keny-Guyer of Portland, the bill’s chief sponsor. “That just shows how much support this idea has.”
In the District of Columbia, concerns about the high maternal mortality rate — in 2014, it stood at about 40.7 deaths per 100,000 births, according to the analysis by United Health Foundation, substantially exceeding the U.S. rate and those of neighboring Virginia and Maryland — have periodically sparked talk of a review committee, but not enough to push a measure through.
Last year, after two hospitals in Northeast and predominantly black Southeast Washington closed maternity units, concerns grew over access to quality care, particularly for low-income and minority women. Nationally, black women have a maternal mortality rate three to four times higher than white women, and the District suspects its gap is even wider.
“Those disparities were the more acute driver of why we felt we needed to take this action,” said Councilmember Charles Allen, who introduced the measure to establish the panel. “You have to know what is driving this wide disparity before you can really have the strategies for how to fix it.”
The D.C. bill still must be signed into law and, like all District legislation, reviewed by Congress before it becomes effective. It calls for one full-time employee to assist the panel’s work, a position that Allen said he expects to be funded in the budget that will be passed later in the year. In addition to health care professionals, a social worker and representatives of community groups that specialize in women’s health, the D.C. committee will also include “one person who has been directly impacted by a maternal mortality or severe maternal morbidity.” Maternal health advocates say listening to such voices is a critical step in addressing how disparities in race, income and education affect outcomes.
That’s what prompted Wilkins, the Maryland delegate, to introduce her bill, which passed the House this month and will be taken up in the Senate in April. Maryland established its review committee in 2000, but in the panel’s most recent report, the participants consisted almost exclusively of medical professionals, mostly doctors and nurse-midwives. Wilkins’s bill would require the committee to meet at least twice a year with a group that includes representatives from the Maryland Office of Minority Health and Health Disparities, the Maryland Patient Safety Center, women’s health advocacy organizations, and a relative of a mother who died, and to incorporate their recommendations into its final report.
“The women who are impacted and the organizations that work with the communities they live in — we need to make sure they are at the table,” Wilkins said.
Other pending proposals would revamp New Jersey’s 80-plus-year-old review process and establish a new review committee in Connecticut.
Pennsylvania, which ranks sixth in the number of births in the U.S., is currently the largest state without a maternal mortality review committee, but lawmakers are advancing a measure to change that. It passed the House in December and recently cleared a Senate committee; it’s now headed for consideration by the full Senate.
State Rep. Ryan Mackenzie, R-Lehigh, introduced the bill last October after doctors from his district showed him grim data on rising maternal mortality rates in the nation and the state. Pennsylvania’s rate has more than doubled since 1994, according to a December 2017 report in the Pittsburgh Post-Gazette. When Mackenzie ran the idea of creating a statewide review process past House colleagues, several responded that media reports about maternal mortality, including our “Lost Mothers” series, had spurred them to consider similar measures.
Mackenzie’s bill calls for a committee of at least 14 members, most of them health care professionals, with a special emphasis on members working in communities most affected by maternal deaths and a lack of access to care. The measure does not include funding, and specifies that committee members would be unpaid, but Mackenzie said the state Department of Health would redirect existing staff to support it.
Another important aim in creating a statewide review process is making sure maternal deaths are being defined and tracked consistently, Mackenzie said. The state health department has tabulated annual totals for years, but counts only deaths that occur up to 42 days after pregnancy and not those that happen within a year, the standard used by the CDC and most review committees. When Philadelphia’s maternal mortality review panel compared the state’s numbers with its own for the city from 2010 to 2012, the state’s count was about 30 percent lower. Mackenzie’s bill would align Pennsylvania’s committee with the one-year standard.
“We’re hoping to save lives,” Mackenzie said in proposing the review committee. “Based on the results in other states, we think this is realistic.”
ProPublica is a Pulitzer Prize-winning investigative newsroom.
Alarmed that the U.S. is the most dangerous affluent country in which to give birth, state and local lawmakers around the country are adopting a flurry of bipartisan bills aimed at reforming how maternal deaths are identified and investigated.
In Indiana earlier this month, Republican Gov. Eric Holcomb signed a bill creating a maternal mortality review committee to scrutinize deaths and near-deaths among expectant and new mothers and make policy recommendations to improve maternal health.
Oregon’s governor and Washington, D.C.’s mayor, both Democrats, are expected to sign similar legislation in the coming days. Proposals are pending in Pennsylvania, Connecticut, Maryland and New Jersey.
Legislators from several of these states credited the ProPublica/NPR “Lost Mothers” series with raising their awareness and concern about the issue. Maryland Delegate Jheanelle Wilkins, who introduced a bill there, said that the series, especially articles looking at why black mothers are at greatest risk of dying and nearly dying, inspired her and her fellow lawmakers.
“A friend of mine posted one of the stories on Facebook and she challenged her elected officials — Who’s going to do something about it?” Wilkins said.
About 35 states have now established review committees or are in the process of doing so, as well as four cities: New York, Philadelphia, Baltimore and Washington. Two federal bills introduced last year, which would create a grant program to help states introduce or improve review committees, remain stalled in committee.
Between 700 and 900 women die each year in the U.S. from causes related to pregnancy or childbirth, and the rate has risen even as it has declined in other wealthy countries. The rate of life-threatening complications has also soared since the 1990s, endangering more than 50,000 U.S. women a year. A new report by the CDC Foundation — a nonprofit created by Congress to support the Centers for Disease Control and Prevention — suggests that more than 60 percent of pregnancy- and childbirth-related deaths in the U.S. are preventable.
The “Lost Mothers” project highlighted a number of reasons the U.S. has fallen behind other countries, including a greater focus on the health of the baby than of the mother, treatment guidelines that vary from one doctor or hospital to the next, and government failures to collect accurate data and to study maternal deaths and near-deaths to understand how they might be prevented.
Maternal mortality review committees can play a key role in this process, public health experts say, by identifying pregnancy-related deaths that might otherwise be overlooked, analyzing the factors contributing to those deaths, and translating the lessons into policy changes. That’s what happens in Great Britain, where a national committee investigates every maternal death and the findings help set women’s health policy across the country.
As recently as 2016, only about half the states had such panels. The number has been growing quickly, said Andria Cornell, senior program manager for women’s health and maternal health lead at the Association of Maternal & Child Health Programs, a nonprofit advocacy group.
“This is a time of unprecedented political and social will for establishing maternal mortality review committees,” she said. “We’ve definitely come to a tipping point.”
Cornell credited two forces for driving the change: journalism focused on maternal deaths and a national project led by AMCHP, the Centers for Disease Control and the CDC Foundation.
With money from Merck for Mothers, a charitable initiative created by the pharmaceutical giant, the project has funded a web portal that provides information on starting and improving review committees and a tool, the Maternal Mortality Review Information Application, that shows jurisdictions how to standardize data collection from review panels so that it’s comparable from one state to the next.
Review committees do have limitations. Many are understaffed and poorly funded, with limited authority to dig deeply into systemic problems or implement meaningful reforms. It generally takes several years for them to produce their reports, in part because committee members — including doctors, public health experts, medical examiners, and the like — have other demands on their time and aren’t compensated.
Also, committee records and reports are de-identified — stripped of any information that might point to a particular woman, caregiver or hospital. Thus the review is of little use in assigning responsibility for individual deaths, or evaluating whether some hospitals, doctors or nurses are especially prone to error. Still, recommendations and findings from reviews have proven helpful in states such as California in shaping preventive efforts that have reduced maternal mortality rates.
Indiana epitomizes the national movement to use the review committee process to scrutinize maternal deaths. There, the focus had long been on reducing infant mortality: The state has the highest rate of neonatal deaths outside the South. Maternal deaths weren’t on the radar, even though the state’s maternal mortality rate is around 41 women per 100,000 births, according to a new analysis of federal data by United Health Foundation — or double the rate of maternal deaths in neighboring Illinois and Ohio.
“I’ll be honest,” said state Sen. Jean Leising, a Republican from rural southeastern Indiana. “I’m on the Health Committee ... and I had no idea our maternal statistics were so lousy.”
The bill she sponsored — creating a committee for the next five years to study not just maternal mortality but also life-threatening complications, or severe maternal morbidity — sailed through the legislature, in part because of a change in governors. Holcomb, who replaced Mike Pence and is seen as more of a pragmatist, appointed a female obstetrician-gynecologist to be his new health commissioner.
The bill’s supporters drew a connection between maternal and infant mortality, said Dr. Brownsyne Tucker Edmonds, legislative chair for the Indiana chapter of the American Congress of Obstetricians and Gynecologists: “We could bring forth the idea that healthy moms have healthy babies.”
Oregon’s bill, which also passed easily this month, creates a review committee that will start by focusing on maternal deaths; by 2021, it will also begin looking at severe maternal morbidity. Over that period, it will cost the state more than $450,000 — a significant public commitment to a women’s health initiative.
“I did think, wow, that’s more money than I thought it was going to be, but no one blinked an eye,” said Rep. Alissa Keny-Guyer of Portland, the bill’s chief sponsor. “That just shows how much support this idea has.”
In the District of Columbia, concerns about the high maternal mortality rate — in 2014, it stood at about 40.7 deaths per 100,000 births, according to the analysis by United Health Foundation, substantially exceeding the U.S. rate and those of neighboring Virginia and Maryland — have periodically sparked talk of a review committee, but not enough to push a measure through.
Last year, after two hospitals in Northeast and predominantly black Southeast Washington closed maternity units, concerns grew over access to quality care, particularly for low-income and minority women. Nationally, black women have a maternal mortality rate three to four times higher than white women, and the District suspects its gap is even wider.
“Those disparities were the more acute driver of why we felt we needed to take this action,” said Councilmember Charles Allen, who introduced the measure to establish the panel. “You have to know what is driving this wide disparity before you can really have the strategies for how to fix it.”
The D.C. bill still must be signed into law and, like all District legislation, reviewed by Congress before it becomes effective. It calls for one full-time employee to assist the panel’s work, a position that Allen said he expects to be funded in the budget that will be passed later in the year. In addition to health care professionals, a social worker and representatives of community groups that specialize in women’s health, the D.C. committee will also include “one person who has been directly impacted by a maternal mortality or severe maternal morbidity.” Maternal health advocates say listening to such voices is a critical step in addressing how disparities in race, income and education affect outcomes.
That’s what prompted Wilkins, the Maryland delegate, to introduce her bill, which passed the House this month and will be taken up in the Senate in April. Maryland established its review committee in 2000, but in the panel’s most recent report, the participants consisted almost exclusively of medical professionals, mostly doctors and nurse-midwives. Wilkins’s bill would require the committee to meet at least twice a year with a group that includes representatives from the Maryland Office of Minority Health and Health Disparities, the Maryland Patient Safety Center, women’s health advocacy organizations, and a relative of a mother who died, and to incorporate their recommendations into its final report.
“The women who are impacted and the organizations that work with the communities they live in — we need to make sure they are at the table,” Wilkins said.
Other pending proposals would revamp New Jersey’s 80-plus-year-old review process and establish a new review committee in Connecticut.
Pennsylvania, which ranks sixth in the number of births in the U.S., is currently the largest state without a maternal mortality review committee, but lawmakers are advancing a measure to change that. It passed the House in December and recently cleared a Senate committee; it’s now headed for consideration by the full Senate.
State Rep. Ryan Mackenzie, R-Lehigh, introduced the bill last October after doctors from his district showed him grim data on rising maternal mortality rates in the nation and the state. Pennsylvania’s rate has more than doubled since 1994, according to a December 2017 report in the Pittsburgh Post-Gazette. When Mackenzie ran the idea of creating a statewide review process past House colleagues, several responded that media reports about maternal mortality, including our “Lost Mothers” series, had spurred them to consider similar measures.
Mackenzie’s bill calls for a committee of at least 14 members, most of them health care professionals, with a special emphasis on members working in communities most affected by maternal deaths and a lack of access to care. The measure does not include funding, and specifies that committee members would be unpaid, but Mackenzie said the state Department of Health would redirect existing staff to support it.
Another important aim in creating a statewide review process is making sure maternal deaths are being defined and tracked consistently, Mackenzie said. The state health department has tabulated annual totals for years, but counts only deaths that occur up to 42 days after pregnancy and not those that happen within a year, the standard used by the CDC and most review committees. When Philadelphia’s maternal mortality review panel compared the state’s numbers with its own for the city from 2010 to 2012, the state’s count was about 30 percent lower. Mackenzie’s bill would align Pennsylvania’s committee with the one-year standard.
“We’re hoping to save lives,” Mackenzie said in proposing the review committee. “Based on the results in other states, we think this is realistic.”
ProPublica is a Pulitzer Prize-winning investigative newsroom.
Axial SpA disease activity remains mostly stable throughout pregnancy
, according to results of a prospective study.
“In the largest prospective study to date exploring disease activity during pregnancy in women with axSpA, we found that the majority experienced stable, low disease activity,” Kristin Ursin, MD, of the Trondheim (Norway) University Hospital and her associates wrote in Rheumatology. “In accordance with two previous studies, we found a small increase in disease activity in the second trimester.”
All women in the study fulfilled the Assessment of SpondyloArthritis International Society criteria for axSpA and had seven clinic visits throughout their pregnancy: one before conception, one at each trimester, another at 6 weeks, and two visits 6 and 12 months after delivery. No differentiation was made between women with radiographic or nonradiographic axSpA. At each visit, patients’ disease activity was determined using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), which is calculated based on six patient factors scored from 1 to 10, including: fatigue, back pain, peripheral joint pain and swelling, localized tenderness, duration of morning stiffness, and severity of morning stiffness. A disease score of 4 is commonly used to define active disease. Disease activity was also assessed by measuring C-reactive protein (CRP). Women’s function and health was also assessed using the Bath Ankylosing Spondylitis Functional Index (BASFI), scored similarly to the BASDAI, and the RAND-36 questionnaire to assess general health.
The research team found that, across the span of pregnancy, axSpA disease activity was relatively low and consistent throughout the study. But there was a significant relationship between disease activity and time (P = .029) in which disease activity was highest during the second trimester and proved to be significantly higher than 6 weeks after giving birth (mean BASDAI 3.97 vs. 3.46, P = .005). In fact, 45% of women had active disease in their second trimester (BASDAI of 4 or greater). But among women with data from the second trimester and 6 weeks post partum, 42% had a decrease in BASDAI score of 1 or more, and 22% had an equivalent increase. CRP values remained low and stable throughout the study.
Like the BASDAI scores, physical function and well being seemed to correlate with later time points during pregnancy. The lowest level of physical functioning for most women was during the second and third trimesters, and those periods were significantly worse than 6 weeks after giving birth (mean BASFI of 3.2 during the second trimester and 3.6 during the third vs. 2.6 at 6 weeks post partum). Similarly, patients reported overall worse general health during the second and third trimesters, compared with 6 weeks after giving birth, based on RAND-36 scores (mean physical functioning score of 63.1 in the second trimester and 54.5 in the third vs. 71.0 at 6 weeks post partum).
Use of nonbiologic medications remained relatively stable during pregnancy, while biologic disease-modifying antirheumatic drug (DMARD) use (all tumor necrosis factor [TNF] inhibitors) dropped significantly when pregnancy was confirmed. About 20% used NSAIDs during pregnancy, except for during the third trimester when use decreased to 8%. Prednisolone use remained stable at 5%-8%. A total of 11% had used synthetic DMARDs prior to pregnancy, and this rate stayed at 10%-16% during the three trimesters. Overall, 44% had used a biologic DMARD (TNF inhibitor) prior to pregnancy, but this declined sharply to 6% during the first trimester and 1% in the third.
The main limitations of the study included not having data from all of the women for all the time points. In fact, only 22% were included prior to conceiving, which could suggest that women with low disease activity were less likely to visit their rheumatologist, thereby biasing the sample toward an overestimated disease activity at preconception that could have potentially hidden a “more evident deterioration between the preconception visit and the second trimester,” the authors said.
“Future research on pregnancy in women with axSpA should differentiate between subgroups of the disease and aim to include objective assessment of inflammation.”
The study was funded by the Research Fund of the Norwegian Organization for People With Rheumatic Diseases. None of the authors had any conflicts of interest to declare.
SOURCE: Ursin K et al. Rheumatology. 2018 Mar 14. doi: 10.1093/rheumatology/key047.
, according to results of a prospective study.
“In the largest prospective study to date exploring disease activity during pregnancy in women with axSpA, we found that the majority experienced stable, low disease activity,” Kristin Ursin, MD, of the Trondheim (Norway) University Hospital and her associates wrote in Rheumatology. “In accordance with two previous studies, we found a small increase in disease activity in the second trimester.”
All women in the study fulfilled the Assessment of SpondyloArthritis International Society criteria for axSpA and had seven clinic visits throughout their pregnancy: one before conception, one at each trimester, another at 6 weeks, and two visits 6 and 12 months after delivery. No differentiation was made between women with radiographic or nonradiographic axSpA. At each visit, patients’ disease activity was determined using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), which is calculated based on six patient factors scored from 1 to 10, including: fatigue, back pain, peripheral joint pain and swelling, localized tenderness, duration of morning stiffness, and severity of morning stiffness. A disease score of 4 is commonly used to define active disease. Disease activity was also assessed by measuring C-reactive protein (CRP). Women’s function and health was also assessed using the Bath Ankylosing Spondylitis Functional Index (BASFI), scored similarly to the BASDAI, and the RAND-36 questionnaire to assess general health.
The research team found that, across the span of pregnancy, axSpA disease activity was relatively low and consistent throughout the study. But there was a significant relationship between disease activity and time (P = .029) in which disease activity was highest during the second trimester and proved to be significantly higher than 6 weeks after giving birth (mean BASDAI 3.97 vs. 3.46, P = .005). In fact, 45% of women had active disease in their second trimester (BASDAI of 4 or greater). But among women with data from the second trimester and 6 weeks post partum, 42% had a decrease in BASDAI score of 1 or more, and 22% had an equivalent increase. CRP values remained low and stable throughout the study.
Like the BASDAI scores, physical function and well being seemed to correlate with later time points during pregnancy. The lowest level of physical functioning for most women was during the second and third trimesters, and those periods were significantly worse than 6 weeks after giving birth (mean BASFI of 3.2 during the second trimester and 3.6 during the third vs. 2.6 at 6 weeks post partum). Similarly, patients reported overall worse general health during the second and third trimesters, compared with 6 weeks after giving birth, based on RAND-36 scores (mean physical functioning score of 63.1 in the second trimester and 54.5 in the third vs. 71.0 at 6 weeks post partum).
Use of nonbiologic medications remained relatively stable during pregnancy, while biologic disease-modifying antirheumatic drug (DMARD) use (all tumor necrosis factor [TNF] inhibitors) dropped significantly when pregnancy was confirmed. About 20% used NSAIDs during pregnancy, except for during the third trimester when use decreased to 8%. Prednisolone use remained stable at 5%-8%. A total of 11% had used synthetic DMARDs prior to pregnancy, and this rate stayed at 10%-16% during the three trimesters. Overall, 44% had used a biologic DMARD (TNF inhibitor) prior to pregnancy, but this declined sharply to 6% during the first trimester and 1% in the third.
The main limitations of the study included not having data from all of the women for all the time points. In fact, only 22% were included prior to conceiving, which could suggest that women with low disease activity were less likely to visit their rheumatologist, thereby biasing the sample toward an overestimated disease activity at preconception that could have potentially hidden a “more evident deterioration between the preconception visit and the second trimester,” the authors said.
“Future research on pregnancy in women with axSpA should differentiate between subgroups of the disease and aim to include objective assessment of inflammation.”
The study was funded by the Research Fund of the Norwegian Organization for People With Rheumatic Diseases. None of the authors had any conflicts of interest to declare.
SOURCE: Ursin K et al. Rheumatology. 2018 Mar 14. doi: 10.1093/rheumatology/key047.
, according to results of a prospective study.
“In the largest prospective study to date exploring disease activity during pregnancy in women with axSpA, we found that the majority experienced stable, low disease activity,” Kristin Ursin, MD, of the Trondheim (Norway) University Hospital and her associates wrote in Rheumatology. “In accordance with two previous studies, we found a small increase in disease activity in the second trimester.”
All women in the study fulfilled the Assessment of SpondyloArthritis International Society criteria for axSpA and had seven clinic visits throughout their pregnancy: one before conception, one at each trimester, another at 6 weeks, and two visits 6 and 12 months after delivery. No differentiation was made between women with radiographic or nonradiographic axSpA. At each visit, patients’ disease activity was determined using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), which is calculated based on six patient factors scored from 1 to 10, including: fatigue, back pain, peripheral joint pain and swelling, localized tenderness, duration of morning stiffness, and severity of morning stiffness. A disease score of 4 is commonly used to define active disease. Disease activity was also assessed by measuring C-reactive protein (CRP). Women’s function and health was also assessed using the Bath Ankylosing Spondylitis Functional Index (BASFI), scored similarly to the BASDAI, and the RAND-36 questionnaire to assess general health.
The research team found that, across the span of pregnancy, axSpA disease activity was relatively low and consistent throughout the study. But there was a significant relationship between disease activity and time (P = .029) in which disease activity was highest during the second trimester and proved to be significantly higher than 6 weeks after giving birth (mean BASDAI 3.97 vs. 3.46, P = .005). In fact, 45% of women had active disease in their second trimester (BASDAI of 4 or greater). But among women with data from the second trimester and 6 weeks post partum, 42% had a decrease in BASDAI score of 1 or more, and 22% had an equivalent increase. CRP values remained low and stable throughout the study.
Like the BASDAI scores, physical function and well being seemed to correlate with later time points during pregnancy. The lowest level of physical functioning for most women was during the second and third trimesters, and those periods were significantly worse than 6 weeks after giving birth (mean BASFI of 3.2 during the second trimester and 3.6 during the third vs. 2.6 at 6 weeks post partum). Similarly, patients reported overall worse general health during the second and third trimesters, compared with 6 weeks after giving birth, based on RAND-36 scores (mean physical functioning score of 63.1 in the second trimester and 54.5 in the third vs. 71.0 at 6 weeks post partum).
Use of nonbiologic medications remained relatively stable during pregnancy, while biologic disease-modifying antirheumatic drug (DMARD) use (all tumor necrosis factor [TNF] inhibitors) dropped significantly when pregnancy was confirmed. About 20% used NSAIDs during pregnancy, except for during the third trimester when use decreased to 8%. Prednisolone use remained stable at 5%-8%. A total of 11% had used synthetic DMARDs prior to pregnancy, and this rate stayed at 10%-16% during the three trimesters. Overall, 44% had used a biologic DMARD (TNF inhibitor) prior to pregnancy, but this declined sharply to 6% during the first trimester and 1% in the third.
The main limitations of the study included not having data from all of the women for all the time points. In fact, only 22% were included prior to conceiving, which could suggest that women with low disease activity were less likely to visit their rheumatologist, thereby biasing the sample toward an overestimated disease activity at preconception that could have potentially hidden a “more evident deterioration between the preconception visit and the second trimester,” the authors said.
“Future research on pregnancy in women with axSpA should differentiate between subgroups of the disease and aim to include objective assessment of inflammation.”
The study was funded by the Research Fund of the Norwegian Organization for People With Rheumatic Diseases. None of the authors had any conflicts of interest to declare.
SOURCE: Ursin K et al. Rheumatology. 2018 Mar 14. doi: 10.1093/rheumatology/key047.
FROM RHEUMATOLOGY
Key clinical point: Higher disease activity and lower physical well being correspond with late stage pregnancy.
Major finding: Disease activity appears to be highest in the second trimester, compared with 6 weeks post partum (mean BASDAI 3.97 vs. 3.46, P = .005).
Study details: A prospective study of 179 pregnancies in 166 Norwegian women with axSpA from a Norwegian health registry between 2006 and 2016.
Disclosures: The study was funded by the Research Fund of the Norwegian Organization for People With Rheumatic Diseases. None of the authors had any conflicts of interest to declare.
Source: Ursin K et al. Rheumatology. 2018 Mar 14. doi: 10.1093/rheumatology/key047.
Interventions urged to stop rising NAS, stem Medicaid costs
The incidence of neonatal abstinence syndrome (NAS) and the corresponding costs to Medicaid are unlikely to decline unless interventions focus on stopping opioid use by low-income mothers, said Tyler N.A. Winkelman, MD, of Hennepin County Medical Center, Minneapolis, and his associates.
Dr. Winkelman and his associates conducted a serial cross-sectional analysis that used 9,115,457 birth discharge records from the 2004-2014 National Inpatient Sample (NIS), which were representative of 43.6 million weighted births. Overall, 3,991,336 infants were covered by Medicaid, which were representative of 19.1 million weighted births. There were 35,629 (0.89%) infants with a diagnosis of NAS, which were representative of 173,384 weighted births. Medicaid was the primary payer for 74% (95% confidence interval, 68.9%-77.9%) of NAS-related births in 2004 and 82% (95% CI, 80.5%-83.5%) of NAS-related births in 2014.
Infants with NAS who were enrolled in Medicaid were significantly more likely to be male, live in a rural county, and have comorbidities reflective of the syndrome than were infants without NAS who were enrolled in Medicaid, the researchers wrote in Pediatrics.
from 1.5 per 1,000 hospital births (95% CI, 1.2-1.9) to 8 per 1,000 hospital births (95% CI, 7.2-8.7), as the opioid epidemic worsened in the country.
Infants with NAS who were covered by Medicaid had a greater chance of being transferred to another hospital for care (9% vs. 7%; P = .02) and stay in the hospital longer (17 days vs. 15 days; P less than .001), compared with infants with NAS who were covered by private insurance.
NAS is costly. In the 2011-2014 era, mean hospital costs for a NAS infant covered by Medicaid were more than fivefold higher than for an infant without NAS ($19,340/birth vs. $3,700/birth; P less than .001). After adjustment for inflation, mean hospital costs for infants with NAS who were covered by Medicaid increased 26% between 2004-2006 and 2011-2014 ($15,350 vs. $19,340; P less than .001), the researchers reported. Annual hospital costs, which were adjusted for inflation to 2014 U.S. dollars, for all infants with NAS who were covered by Medicaid rose from $65.4 million in 2004 to $462 million in 2014.
“With the disproportionate impact of NAS on the Medicaid population, we suggest that NAS incidence rates are unlikely to improve without interventions targeted at low-income mothers and infants,” Dr. Winkelman and his associates concluded.
Nonpharmacologic interventions are available to help NAS infants. “Systematic implementation of policies that support rooming-in, breastfeeding, swaddling, on-demand feeding schedules, and minimization of sleep disruption may reduce symptoms of NAS and reduce the duration of, or even eliminate the need for, pharmacologic treatment of NAS,” the researchers said. “Pharmacologic treatment with buprenorphine, for example, has been shown to reduce hospital length of stay by 35%.”
Another intervention is medication-assisted treatment during pregnancy, which studies have shown “improves outcomes and reduces costs associated with NAS, compared with attempted abstinence,” Dr. Winkelman and his associates said. It does not necessarily reduce NAS incidence, but “it may prevent prolonged hospital stays due to preterm birth, reduce NICU [neonatal intensive care unit] admissions, decrease the severity of NAS symptoms, and improve birth outcomes for some infants.”
The researchers also endorsed screening, referral, and treatment for substance abuse and mental health disorders among reproductive age women, including adolescents, because these are risk factors for opioid abuse.
One author was supported by an award from the National Institute on Drug Abuse. None of the other authors had relevant financial disclosures.
SOURCE: Winkelman TNA et al. Pediatrics. 2018;141(4):e20173520.
The incidence of neonatal abstinence syndrome (NAS) and the corresponding costs to Medicaid are unlikely to decline unless interventions focus on stopping opioid use by low-income mothers, said Tyler N.A. Winkelman, MD, of Hennepin County Medical Center, Minneapolis, and his associates.
Dr. Winkelman and his associates conducted a serial cross-sectional analysis that used 9,115,457 birth discharge records from the 2004-2014 National Inpatient Sample (NIS), which were representative of 43.6 million weighted births. Overall, 3,991,336 infants were covered by Medicaid, which were representative of 19.1 million weighted births. There were 35,629 (0.89%) infants with a diagnosis of NAS, which were representative of 173,384 weighted births. Medicaid was the primary payer for 74% (95% confidence interval, 68.9%-77.9%) of NAS-related births in 2004 and 82% (95% CI, 80.5%-83.5%) of NAS-related births in 2014.
Infants with NAS who were enrolled in Medicaid were significantly more likely to be male, live in a rural county, and have comorbidities reflective of the syndrome than were infants without NAS who were enrolled in Medicaid, the researchers wrote in Pediatrics.
from 1.5 per 1,000 hospital births (95% CI, 1.2-1.9) to 8 per 1,000 hospital births (95% CI, 7.2-8.7), as the opioid epidemic worsened in the country.
Infants with NAS who were covered by Medicaid had a greater chance of being transferred to another hospital for care (9% vs. 7%; P = .02) and stay in the hospital longer (17 days vs. 15 days; P less than .001), compared with infants with NAS who were covered by private insurance.
NAS is costly. In the 2011-2014 era, mean hospital costs for a NAS infant covered by Medicaid were more than fivefold higher than for an infant without NAS ($19,340/birth vs. $3,700/birth; P less than .001). After adjustment for inflation, mean hospital costs for infants with NAS who were covered by Medicaid increased 26% between 2004-2006 and 2011-2014 ($15,350 vs. $19,340; P less than .001), the researchers reported. Annual hospital costs, which were adjusted for inflation to 2014 U.S. dollars, for all infants with NAS who were covered by Medicaid rose from $65.4 million in 2004 to $462 million in 2014.
“With the disproportionate impact of NAS on the Medicaid population, we suggest that NAS incidence rates are unlikely to improve without interventions targeted at low-income mothers and infants,” Dr. Winkelman and his associates concluded.
Nonpharmacologic interventions are available to help NAS infants. “Systematic implementation of policies that support rooming-in, breastfeeding, swaddling, on-demand feeding schedules, and minimization of sleep disruption may reduce symptoms of NAS and reduce the duration of, or even eliminate the need for, pharmacologic treatment of NAS,” the researchers said. “Pharmacologic treatment with buprenorphine, for example, has been shown to reduce hospital length of stay by 35%.”
Another intervention is medication-assisted treatment during pregnancy, which studies have shown “improves outcomes and reduces costs associated with NAS, compared with attempted abstinence,” Dr. Winkelman and his associates said. It does not necessarily reduce NAS incidence, but “it may prevent prolonged hospital stays due to preterm birth, reduce NICU [neonatal intensive care unit] admissions, decrease the severity of NAS symptoms, and improve birth outcomes for some infants.”
The researchers also endorsed screening, referral, and treatment for substance abuse and mental health disorders among reproductive age women, including adolescents, because these are risk factors for opioid abuse.
One author was supported by an award from the National Institute on Drug Abuse. None of the other authors had relevant financial disclosures.
SOURCE: Winkelman TNA et al. Pediatrics. 2018;141(4):e20173520.
The incidence of neonatal abstinence syndrome (NAS) and the corresponding costs to Medicaid are unlikely to decline unless interventions focus on stopping opioid use by low-income mothers, said Tyler N.A. Winkelman, MD, of Hennepin County Medical Center, Minneapolis, and his associates.
Dr. Winkelman and his associates conducted a serial cross-sectional analysis that used 9,115,457 birth discharge records from the 2004-2014 National Inpatient Sample (NIS), which were representative of 43.6 million weighted births. Overall, 3,991,336 infants were covered by Medicaid, which were representative of 19.1 million weighted births. There were 35,629 (0.89%) infants with a diagnosis of NAS, which were representative of 173,384 weighted births. Medicaid was the primary payer for 74% (95% confidence interval, 68.9%-77.9%) of NAS-related births in 2004 and 82% (95% CI, 80.5%-83.5%) of NAS-related births in 2014.
Infants with NAS who were enrolled in Medicaid were significantly more likely to be male, live in a rural county, and have comorbidities reflective of the syndrome than were infants without NAS who were enrolled in Medicaid, the researchers wrote in Pediatrics.
from 1.5 per 1,000 hospital births (95% CI, 1.2-1.9) to 8 per 1,000 hospital births (95% CI, 7.2-8.7), as the opioid epidemic worsened in the country.
Infants with NAS who were covered by Medicaid had a greater chance of being transferred to another hospital for care (9% vs. 7%; P = .02) and stay in the hospital longer (17 days vs. 15 days; P less than .001), compared with infants with NAS who were covered by private insurance.
NAS is costly. In the 2011-2014 era, mean hospital costs for a NAS infant covered by Medicaid were more than fivefold higher than for an infant without NAS ($19,340/birth vs. $3,700/birth; P less than .001). After adjustment for inflation, mean hospital costs for infants with NAS who were covered by Medicaid increased 26% between 2004-2006 and 2011-2014 ($15,350 vs. $19,340; P less than .001), the researchers reported. Annual hospital costs, which were adjusted for inflation to 2014 U.S. dollars, for all infants with NAS who were covered by Medicaid rose from $65.4 million in 2004 to $462 million in 2014.
“With the disproportionate impact of NAS on the Medicaid population, we suggest that NAS incidence rates are unlikely to improve without interventions targeted at low-income mothers and infants,” Dr. Winkelman and his associates concluded.
Nonpharmacologic interventions are available to help NAS infants. “Systematic implementation of policies that support rooming-in, breastfeeding, swaddling, on-demand feeding schedules, and minimization of sleep disruption may reduce symptoms of NAS and reduce the duration of, or even eliminate the need for, pharmacologic treatment of NAS,” the researchers said. “Pharmacologic treatment with buprenorphine, for example, has been shown to reduce hospital length of stay by 35%.”
Another intervention is medication-assisted treatment during pregnancy, which studies have shown “improves outcomes and reduces costs associated with NAS, compared with attempted abstinence,” Dr. Winkelman and his associates said. It does not necessarily reduce NAS incidence, but “it may prevent prolonged hospital stays due to preterm birth, reduce NICU [neonatal intensive care unit] admissions, decrease the severity of NAS symptoms, and improve birth outcomes for some infants.”
The researchers also endorsed screening, referral, and treatment for substance abuse and mental health disorders among reproductive age women, including adolescents, because these are risk factors for opioid abuse.
One author was supported by an award from the National Institute on Drug Abuse. None of the other authors had relevant financial disclosures.
SOURCE: Winkelman TNA et al. Pediatrics. 2018;141(4):e20173520.
FROM PEDIATRICS
Key clinical point:
Major finding: In 2011-2014, mean hospital costs for NAS infants covered by Medicaid were more than fivefold higher than for infants without NAS ($19,340/birth vs. $3,700/birth).
Study details: A serial cross-sectional analysis that usd data from the 2004-2014 National Inpatient Sample (NIS) of 9,115,457 birth discharge records.
Disclosures: Stephen W. Patrick, MD, was supported by an award from the National Institute on Drug Abuse. None of the other authors had relevant financial disclosures.
Source: Winkelman TNA et al. Pediatrics. 2018;141(4):e20173520.