User login
Postpartum depression screening in well-child care appears promising
, according to results of a study from the Netherlands.
“This promising finding warrants wider implementation of screening for postpartum depression,” said Dr. Angarath I. Van der Zee-van den Berg of the University of Twente, Enschede, the Netherlands, and associates.
Results showed significantly fewer mothers in the intervention group were depressed at 9 months post partum, compared with the CAU group (0.6% of 1,843 vs. 2.5% 1,246 for major depression), with an adjusted odds ratio of 0.28 (95% confidence interval, 0.12-0.63). The difference also was significant for minor and major depression, with 3.0% of the intervention group affected vs. 8.4% of the CAU group, and the adjusted odds ratio was 0.40 (95% confidence interval, 0.27-0.58). For parenting, anxiety symptoms, and mental health functioning, the intervention resulted in effect sizes ranging from 0.23 to 0.27.
“We found screening for postpartum depression to have a negligible effect on socioemotional development of the child with no former evidence to compare with,” Dr. Van der Zee-van den Berg and his associates said. “Attention for the mother-child interaction in the trajectory after screening may improve child outcomes; this evidently requires further study.”
To find out more information see Pediatrics (2017;140[4]:e20170110).
, according to results of a study from the Netherlands.
“This promising finding warrants wider implementation of screening for postpartum depression,” said Dr. Angarath I. Van der Zee-van den Berg of the University of Twente, Enschede, the Netherlands, and associates.
Results showed significantly fewer mothers in the intervention group were depressed at 9 months post partum, compared with the CAU group (0.6% of 1,843 vs. 2.5% 1,246 for major depression), with an adjusted odds ratio of 0.28 (95% confidence interval, 0.12-0.63). The difference also was significant for minor and major depression, with 3.0% of the intervention group affected vs. 8.4% of the CAU group, and the adjusted odds ratio was 0.40 (95% confidence interval, 0.27-0.58). For parenting, anxiety symptoms, and mental health functioning, the intervention resulted in effect sizes ranging from 0.23 to 0.27.
“We found screening for postpartum depression to have a negligible effect on socioemotional development of the child with no former evidence to compare with,” Dr. Van der Zee-van den Berg and his associates said. “Attention for the mother-child interaction in the trajectory after screening may improve child outcomes; this evidently requires further study.”
To find out more information see Pediatrics (2017;140[4]:e20170110).
, according to results of a study from the Netherlands.
“This promising finding warrants wider implementation of screening for postpartum depression,” said Dr. Angarath I. Van der Zee-van den Berg of the University of Twente, Enschede, the Netherlands, and associates.
Results showed significantly fewer mothers in the intervention group were depressed at 9 months post partum, compared with the CAU group (0.6% of 1,843 vs. 2.5% 1,246 for major depression), with an adjusted odds ratio of 0.28 (95% confidence interval, 0.12-0.63). The difference also was significant for minor and major depression, with 3.0% of the intervention group affected vs. 8.4% of the CAU group, and the adjusted odds ratio was 0.40 (95% confidence interval, 0.27-0.58). For parenting, anxiety symptoms, and mental health functioning, the intervention resulted in effect sizes ranging from 0.23 to 0.27.
“We found screening for postpartum depression to have a negligible effect on socioemotional development of the child with no former evidence to compare with,” Dr. Van der Zee-van den Berg and his associates said. “Attention for the mother-child interaction in the trajectory after screening may improve child outcomes; this evidently requires further study.”
To find out more information see Pediatrics (2017;140[4]:e20170110).
FROM PEDIATRICS
Prenatal antidepressant use linked to psychiatric illness in offspring
Antidepressant use before and during pregnancy may be associated with an increased risk of psychiatric disorders in offspring, according to the results of a population-based cohort study published Sept. 7 in the BMJ.
There have been contradictory findings in the literature about whether in utero exposure to SSRIs is associated with autism spectrum disorder and ADHD. “However, these studies did not investigate the overall risk of psychiatric disorders, which is important because differentiating between overlapping symptoms and diagnosing specific disorders are challenging in children and adolescents,” Xiaoqin Liu, MD, PhD, of Aarhus University in Denmark, and her coauthors wrote (BMJ 2017;358:j3668. doi: 10.1136/bmj.j3668).
Participants were categorized into four groups based on maternal use of antidepressants; unexposed, antidepressant discontinuation (if the mother had used in the 2 years before but not during pregnancy), antidepressant continuation (if the use happened in the 2 years before pregnancy and during it), and new users (if antidepressant use happened only during pregnancy).
The study found that children whose mothers used antidepressants both in the 2 years before pregnancy and during pregnancy had a 27% higher incidence of any psychiatric disorder, compared with children whose mothers had used antidepressants but discontinued them before becoming pregnant (95% confidence interval, 1.17-1.38).
This figure was adjusted for factors such as maternal age and psychiatric history at delivery, psychiatric treatment in the 2 years before pregnancy, other psychotropic medications used during pregnancy, and paternal psychiatric history at the time of delivery.
Any maternal antidepressant use was associated with an increased risk of psychiatric disorders in the offspring, compared with the unexposed group. The 15-year cumulative incidence of psychiatric disorders in offspring was 8% in the unexposed group, 11.5% in the discontinuation group, 13.6% in the continuation group, and 14.5% in the new user group.
There were no differences in risk between children exposed to SSRI monotherapy and those exposed to non-SSRI monotherapy, although the statistical precision for the latter was low, the researchers noted. However, they did see a lower risk of psychiatric disorder in children who were exposed only during the first trimester, compared with those exposed in the second or third trimesters.
The researchers suggested that the association between in utero exposure and the risk of psychiatric disorders in offspring may be the result of a combination of underlying maternal disorders and in utero antidepressant exposure. “We speculated that this increased risk could be due to the severity of underlying maternal psychiatric disorders because mothers with severe symptoms are more likely to continue treatment during pregnancy,” they wrote.
The researchers cautioned that discontinuation of treatment could lead to psychiatric episodes that could have long-lasting effects on both mother and child.
The investigators reported support from several research foundations, as well as institutional grants from Sage Therapeutics and Janssen. No conflicts of interest were declared.
Only the most severely sick women have drugs prescribed during pregnancy. Consequently, confounding by indication is a major challenge in pharmacoepidemiological studies. Including a disease comparison group with women discontinuing antidepressants before pregnancy, as in Liu and her colleagues’ study, offers an important advantage over studies that use only healthy comparison groups because it allows researchers to disentangle the effect of antidepressants from the underlying maternal psychiatric disease.
It is important that researchers report absolute risks to facilitate communication between clinicians and pregnant women. For example, if prenatal exposure to antidepressants is associated with a 23% increased risk of autism in children, and if we assume a baseline prevalence of autism of 1%, then for every 10,000 women who continue treatment during pregnancy, 23 additional cases of autism would occur. This number may be alarming to some patients and reassuring to others.
Hedvig Nordeng, PhD, Angela Lupattelli, PhD, and Mollie Wood, PhD, are from the University of Oslo. These comments are adapted from an accompanying editorial (BMJ 2017;358:j3950 doi: 10.1136/bmj.j3950). No conflicts of interest were declared.
Only the most severely sick women have drugs prescribed during pregnancy. Consequently, confounding by indication is a major challenge in pharmacoepidemiological studies. Including a disease comparison group with women discontinuing antidepressants before pregnancy, as in Liu and her colleagues’ study, offers an important advantage over studies that use only healthy comparison groups because it allows researchers to disentangle the effect of antidepressants from the underlying maternal psychiatric disease.
It is important that researchers report absolute risks to facilitate communication between clinicians and pregnant women. For example, if prenatal exposure to antidepressants is associated with a 23% increased risk of autism in children, and if we assume a baseline prevalence of autism of 1%, then for every 10,000 women who continue treatment during pregnancy, 23 additional cases of autism would occur. This number may be alarming to some patients and reassuring to others.
Hedvig Nordeng, PhD, Angela Lupattelli, PhD, and Mollie Wood, PhD, are from the University of Oslo. These comments are adapted from an accompanying editorial (BMJ 2017;358:j3950 doi: 10.1136/bmj.j3950). No conflicts of interest were declared.
Only the most severely sick women have drugs prescribed during pregnancy. Consequently, confounding by indication is a major challenge in pharmacoepidemiological studies. Including a disease comparison group with women discontinuing antidepressants before pregnancy, as in Liu and her colleagues’ study, offers an important advantage over studies that use only healthy comparison groups because it allows researchers to disentangle the effect of antidepressants from the underlying maternal psychiatric disease.
It is important that researchers report absolute risks to facilitate communication between clinicians and pregnant women. For example, if prenatal exposure to antidepressants is associated with a 23% increased risk of autism in children, and if we assume a baseline prevalence of autism of 1%, then for every 10,000 women who continue treatment during pregnancy, 23 additional cases of autism would occur. This number may be alarming to some patients and reassuring to others.
Hedvig Nordeng, PhD, Angela Lupattelli, PhD, and Mollie Wood, PhD, are from the University of Oslo. These comments are adapted from an accompanying editorial (BMJ 2017;358:j3950 doi: 10.1136/bmj.j3950). No conflicts of interest were declared.
Antidepressant use before and during pregnancy may be associated with an increased risk of psychiatric disorders in offspring, according to the results of a population-based cohort study published Sept. 7 in the BMJ.
There have been contradictory findings in the literature about whether in utero exposure to SSRIs is associated with autism spectrum disorder and ADHD. “However, these studies did not investigate the overall risk of psychiatric disorders, which is important because differentiating between overlapping symptoms and diagnosing specific disorders are challenging in children and adolescents,” Xiaoqin Liu, MD, PhD, of Aarhus University in Denmark, and her coauthors wrote (BMJ 2017;358:j3668. doi: 10.1136/bmj.j3668).
Participants were categorized into four groups based on maternal use of antidepressants; unexposed, antidepressant discontinuation (if the mother had used in the 2 years before but not during pregnancy), antidepressant continuation (if the use happened in the 2 years before pregnancy and during it), and new users (if antidepressant use happened only during pregnancy).
The study found that children whose mothers used antidepressants both in the 2 years before pregnancy and during pregnancy had a 27% higher incidence of any psychiatric disorder, compared with children whose mothers had used antidepressants but discontinued them before becoming pregnant (95% confidence interval, 1.17-1.38).
This figure was adjusted for factors such as maternal age and psychiatric history at delivery, psychiatric treatment in the 2 years before pregnancy, other psychotropic medications used during pregnancy, and paternal psychiatric history at the time of delivery.
Any maternal antidepressant use was associated with an increased risk of psychiatric disorders in the offspring, compared with the unexposed group. The 15-year cumulative incidence of psychiatric disorders in offspring was 8% in the unexposed group, 11.5% in the discontinuation group, 13.6% in the continuation group, and 14.5% in the new user group.
There were no differences in risk between children exposed to SSRI monotherapy and those exposed to non-SSRI monotherapy, although the statistical precision for the latter was low, the researchers noted. However, they did see a lower risk of psychiatric disorder in children who were exposed only during the first trimester, compared with those exposed in the second or third trimesters.
The researchers suggested that the association between in utero exposure and the risk of psychiatric disorders in offspring may be the result of a combination of underlying maternal disorders and in utero antidepressant exposure. “We speculated that this increased risk could be due to the severity of underlying maternal psychiatric disorders because mothers with severe symptoms are more likely to continue treatment during pregnancy,” they wrote.
The researchers cautioned that discontinuation of treatment could lead to psychiatric episodes that could have long-lasting effects on both mother and child.
The investigators reported support from several research foundations, as well as institutional grants from Sage Therapeutics and Janssen. No conflicts of interest were declared.
Antidepressant use before and during pregnancy may be associated with an increased risk of psychiatric disorders in offspring, according to the results of a population-based cohort study published Sept. 7 in the BMJ.
There have been contradictory findings in the literature about whether in utero exposure to SSRIs is associated with autism spectrum disorder and ADHD. “However, these studies did not investigate the overall risk of psychiatric disorders, which is important because differentiating between overlapping symptoms and diagnosing specific disorders are challenging in children and adolescents,” Xiaoqin Liu, MD, PhD, of Aarhus University in Denmark, and her coauthors wrote (BMJ 2017;358:j3668. doi: 10.1136/bmj.j3668).
Participants were categorized into four groups based on maternal use of antidepressants; unexposed, antidepressant discontinuation (if the mother had used in the 2 years before but not during pregnancy), antidepressant continuation (if the use happened in the 2 years before pregnancy and during it), and new users (if antidepressant use happened only during pregnancy).
The study found that children whose mothers used antidepressants both in the 2 years before pregnancy and during pregnancy had a 27% higher incidence of any psychiatric disorder, compared with children whose mothers had used antidepressants but discontinued them before becoming pregnant (95% confidence interval, 1.17-1.38).
This figure was adjusted for factors such as maternal age and psychiatric history at delivery, psychiatric treatment in the 2 years before pregnancy, other psychotropic medications used during pregnancy, and paternal psychiatric history at the time of delivery.
Any maternal antidepressant use was associated with an increased risk of psychiatric disorders in the offspring, compared with the unexposed group. The 15-year cumulative incidence of psychiatric disorders in offspring was 8% in the unexposed group, 11.5% in the discontinuation group, 13.6% in the continuation group, and 14.5% in the new user group.
There were no differences in risk between children exposed to SSRI monotherapy and those exposed to non-SSRI monotherapy, although the statistical precision for the latter was low, the researchers noted. However, they did see a lower risk of psychiatric disorder in children who were exposed only during the first trimester, compared with those exposed in the second or third trimesters.
The researchers suggested that the association between in utero exposure and the risk of psychiatric disorders in offspring may be the result of a combination of underlying maternal disorders and in utero antidepressant exposure. “We speculated that this increased risk could be due to the severity of underlying maternal psychiatric disorders because mothers with severe symptoms are more likely to continue treatment during pregnancy,” they wrote.
The researchers cautioned that discontinuation of treatment could lead to psychiatric episodes that could have long-lasting effects on both mother and child.
The investigators reported support from several research foundations, as well as institutional grants from Sage Therapeutics and Janssen. No conflicts of interest were declared.
FROM THE BMJ
Key clinical point:
Major finding: Children whose mothers took antidepressants both before and during pregnancy are 27% more likely to develop psychiatric illness than are those whose mothers stopped taking antidepressants before pregnancy.
Data source: A population-based cohort study in 905,383 liveborn singletons.
Disclosures: The investigators reported support from several research foundations, as well as institutional grants from Sage Therapeutics and Janssen. No conflicts of interest were declared.
Zika’s 2017 summer less active than 2016
Zika may not have gone away this summer, but it didn’t make a comeback, either.
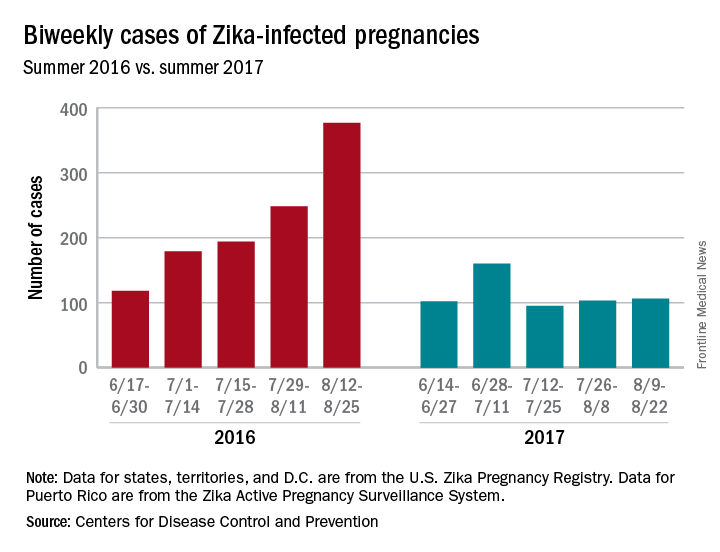
New cases in pregnant women are still being reported, but the numbers are much lower than a year ago, when the infection was kicking into high gear. For the 2 weeks ending Aug. 22, 106 pregnant women with laboratory evidence of Zika virus infection were reported: 43 in the U.S. states and the District of Columbia, and 63 in the U.S. territories, according to the Centers for Disease Control and Prevention.
The total cases reported for the previous 2-week periods, going back to mid-June, look like this: 102 (June 14-27), 160 (June 28–July 11), 95 (July 12-25), and 103 (July 26–Aug. 8). In the summer of 2016, the 2-week period of Aug. 12-25 produced 375 new reports of Zika-infected pregnant women, the CDC data show.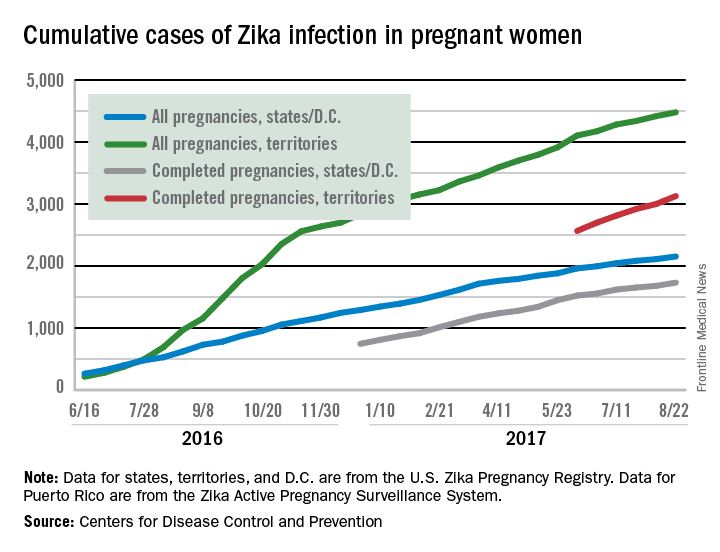
Zika may not have gone away this summer, but it didn’t make a comeback, either.
New cases in pregnant women are still being reported, but the numbers are much lower than a year ago, when the infection was kicking into high gear. For the 2 weeks ending Aug. 22, 106 pregnant women with laboratory evidence of Zika virus infection were reported: 43 in the U.S. states and the District of Columbia, and 63 in the U.S. territories, according to the Centers for Disease Control and Prevention.
The total cases reported for the previous 2-week periods, going back to mid-June, look like this: 102 (June 14-27), 160 (June 28–July 11), 95 (July 12-25), and 103 (July 26–Aug. 8). In the summer of 2016, the 2-week period of Aug. 12-25 produced 375 new reports of Zika-infected pregnant women, the CDC data show.

Zika may not have gone away this summer, but it didn’t make a comeback, either.
New cases in pregnant women are still being reported, but the numbers are much lower than a year ago, when the infection was kicking into high gear. For the 2 weeks ending Aug. 22, 106 pregnant women with laboratory evidence of Zika virus infection were reported: 43 in the U.S. states and the District of Columbia, and 63 in the U.S. territories, according to the Centers for Disease Control and Prevention.
The total cases reported for the previous 2-week periods, going back to mid-June, look like this: 102 (June 14-27), 160 (June 28–July 11), 95 (July 12-25), and 103 (July 26–Aug. 8). In the summer of 2016, the 2-week period of Aug. 12-25 produced 375 new reports of Zika-infected pregnant women, the CDC data show.

External cephalic version: How to increase the chances for success
About 3% to 4% of all fetuses at term are in breech presentation. Since 2000, when Hannah and colleagues reported finding that vaginal delivery of breech-presenting babies was riskier than cesarean delivery,1 most breech-presenting neonates in the United States have been delivered abdominally2—despite subsequent questioning of some of that study’s conclusions.
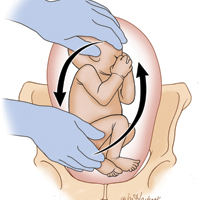
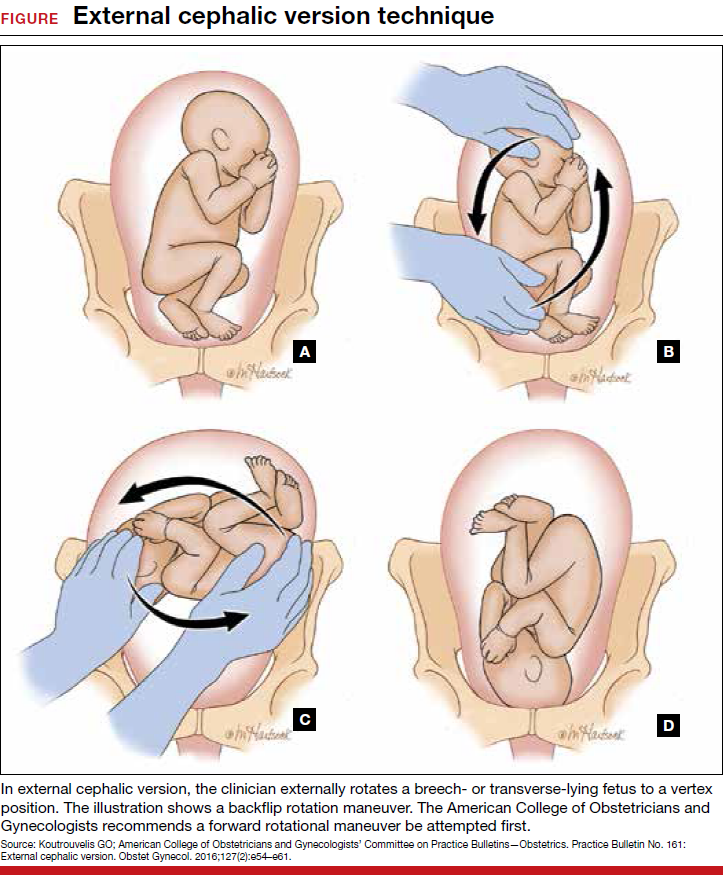
Each year in the United States, approximately 4 million babies are born, and fetal malpresentation accounts for 110,000 to 150,000 cesarean deliveries. In fact, about 15% of all cesarean deliveries in the United States are for breech presentation or transverse lie; in England the percentage is 10%.3 Fortunately, the repopularized technique of external cephalic version (ECV), in which the clinician externally rotates a breech- or transverse-lying fetus to a vertex position (FIGURE), along with the facilitating tools of tocolysis and neuraxial analgesia/anesthesia, is helping to reduce the number of breech presentations in fetuses at term and thus the number of cesarean deliveries and their sequelae—placenta accreta, prolonged recovery, and cesarean deliveries in subsequent pregnancies.
Reluctance to perform ECV is unfounded
In the United States, the practice of offering ECV to women who present with their fetus in breech presentation at term varies tremendously. It is routine at some institutions but not even offered at others.
Many ObGyns are reluctant to perform ECV. Cited reasons include the potential for injury to the fetus and mother (and related liability concerns), the ease of elective cesarean delivery, the variable success rate of ECV (35% to 86%),4 and the pain that women often have with the procedure. According to the literature, however, these concerns either are unfounded or can be mitigated with use of current techniques. Multiple studies have found that the risk of ECV to the fetus and mother is minimal, and that tocolysis and neuraxial anesthesia can facilitate the success of ECV and relieve the pain associated with the procedure.
Related article:
2017 Update on obstetrics
Indications for ECV
The indications for ECV include breech, oblique, or transverse lie presentation after 36 weeks’ gestation and the mother’s desire to avoid cesarean delivery. A clinician skilled in ECV and a facility where emergency cesarean delivery is possible are essential.
There are several instances in which ECV should not be attempted.
Contraindications include:
- concerns about fetal status, including nonreactive nonstress test, biophysical profile score <6/8, severe intrauterine growth restriction, decreased end-diastolic umbilical blood flow
- placenta previa
- multifetal gestation before delivery of first twin
- severe oligohydramnios
- severe preeclampsia
- significant fetal anomaly
- known malformation of uterus
- breech with hyperextended head or arms above shoulders, as seen on ultrasonography.
More controversial contraindications include prior uterine incision, maternal obesity (body mass index >40 kg/m2), ruptured membranes, and fetal macrosomia.
Read about timing, success rates, risk factors, alternate approaches for ECV
Optimal timing for the ECV procedure
Current practice is to wait until 36 to 37 weeks to perform ECV, as most fetuses spontaneously move into vertex presentation by 36 weeks’ gestation. This time frame has several advantages: Many unnecessary attempts at ECV are avoided; only 8% of fetuses in breech presentation after 36 weeks spontaneously change to vertex5; many fetuses revert to breech if ECV is performed too early; and prematurity generally is not an issue in the rare case that immediate delivery is required during or just after attempted ECV.
ECV during labor. Performing ECV during labor appears to pose no increased risk to mother or fetus if membranes are intact and there are no other contraindications to the procedure. Some clinicians perform ECV only during labor. The advantages are that the fetus has had every chance to move into vertex presentation on its own, the equipment used to continuously monitor the fetus during ECV is in place, and cesarean delivery and anesthesia are immediately available in the event ECV is unsuccessful.
The major disadvantage of waiting until labor is that the increased size of the fetus makes ECV more difficult. In addition, the membranes may have already ruptured, and the breech may have descended deeply into the pelvis.
Related article:
For the management of labor, patience is a virtue
Success rates in breech-to-vertex conversions
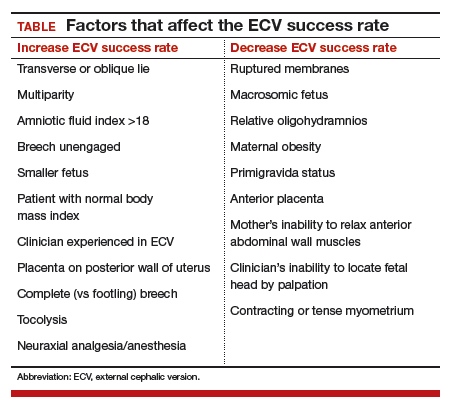
In 2016, the American College of Obstetricians and Gynecologists (ACOG) reported an average ECV success rate of 58% (range, 16% to 100%).6 ACOG noted that, with transverse lie, the success rate was significantly higher. Other studies have found a wide range of rates: 58% in 1,308 patients in a Cochrane review by Hofmeyr and colleagues7; 47% in a study by Beuckens and colleagues8; and 63.1% for primiparas and 82.7% for multiparas in a study by Tong Leung and colleagues.9 These rates were affected by whether ECV was performed with or without tocolysis, with or without intravenous analgesia, and with or without neuraxial analgesia/anesthesia (TABLE).
Likelihood of vaginal delivery after successful ECV
The rate of vaginal delivery after successful ECV is roughly half that of fetuses that were never in breech presentation.10 In successful ECV cases, dystocia and nonreassuring fetal heart rate patterns are the major indications for cesarean delivery. Some experts have speculated that the factors leading to near-term breech presentation—such as an unengaged presenting part or a mother’s smaller pelvis—also may be risk factors for dystocia in labor. Despite this, the rate of vaginal delivery of successfully verted babies has been reported to be as high as 80%.10
As might be expected, post-ECV vaginal deliveries are more common in multiparous than in primiparous women.
Although multiple problems may occur with ECV, generally they are rare and reversible. For instance, Grootscholten and colleagues found a stillbirth and placental abruption rate of only 0.25% in a large group of patients who underwent ECV.11 Similarly, the rate of emergency cesarean delivery was 0.35%. In addition, Hofmeyr and Kulier, in their Cochrane Data Review of 2015, found no significant differences in the Apgar scores and pH’s of babies in the ECV group compared with babies in breech presentation whose mothers did not undergo ECV.7 Results of other studies have confirmed the safety of ECV.12,13
One significant risk of ECV attempts is fetal-to-maternal blood transfer. Boucher and colleagues found that 2.4% of 1,244 women who underwent ECV had a positive Kleihauer-Betke test result, and, in one-third of the positive cases, more than 1 mL of fetal blood was found in maternal circulation.14 This risk can be minimized by administering Rho (D) immune globulin to all Rh-negative mothers after the procedure.
Even these small risks, however, should not be considered in isolation. The infrequent complications of ECV must be compared with what can occur with breech-presenting fetuses during labor or cesarean delivery: complications of breech vaginal delivery, cord prolapse, difficulties with cesarean delivery, and maternal operative complications related to present and future cesarean deliveries.
Alternative approaches to converting breech presentation of unproven efficacy
Over the years, attempts have been made to address breech presentations with measures short of ECV. There is little evidence that these measures work, or work consistently.
- Observation. After 36 weeks’ gestation, only 8% of fetuses in breech presentationspontaneously move into vertex presentation.5
- Maternal positioning. There is no good evidence that such maneuvers are effective in changing fetal presentation.15
- Moxibustion and acupuncture. Moxibustion is inhalation of smoke from burning herbal compounds. In formal studies using controls, these techniques did not consistently increase the rate of movement from breech to vertex presentation.16–18 Likewise, studies with the use of acupuncture have not shown consistent success in changing fetal presentation.19
Read about various methods to facilitate ECV success
Methods to facilitate ECV success
Two techniques that can facilitate ECV success are tocolysis, which relaxes the uterus, and neuraxial analgesia/anesthesia, which relaxes anterior abdominal wall muscles and reduces or relieves ECV-associated pain.
Tocolysis
In tocolysis, a medication is administered to reduce myometrial activity and to relax the uterine muscle so that it stretches more easily around the fetus during repositioning. Tocolytic medications originally were studied for their use in decreasing myometrial tone during preterm labor.
Tocolysis clearly is effective in increasing ECV success rates. Reviewing the results of 4 randomized trials, Cluver showed a 1.38 risk ratio for successful ECV when terbutaline was used versus when there was no tocolysis. The risk ratio for cesarean delivery was 0.82.20 Fernandez, in a study of 103 women divided into terbutaline versus placebo groups, had a 52% success rate for ECV with the terbutaline group versus only a 27% success rate with the placebo group.21
Tocolytic medications include terbutaline, nifedipine, and nitroglycerin.
Tocolysis most often involves the use of β2-adrenergic receptor agonists, particularly terbutaline (despite the boxed safety warning in its prescribing information). A 0.25-mg dose of terbutaline is given subcutaneously 15 to 30 minutes before ECV. Clinicians have successfully used β2-adrenergic receptor agonists in the treatment of patients in preterm labor, and there are more data on this class of medications than on other agents used to facilitate ECV.
Although nifedipine is as effective as terbutaline in the temporary treatment of preterm uterine contractions, several studies have found this calcium channel blocker less effective than terbutaline in facilitating ECV.22,23
The uterus-relaxing effect of nitroglycerin was once thought to make this medication appropriate for facilitating ECV, but multiple studies have found success rates unimproved. In some cases, the drug performed more poorly than placebo.24 Moreover, nitroglycerin is associated with a fairly high rate of adverse effects, such as headaches and blood pressure changes.
Neuraxial analgesia/anesthesia
Over the past 2 decades, there has been a resurgence in the use of neuraxial analgesia/anesthesia in ECV. This technique is more effective than others in improving ECV success rates, it reduces maternal discomfort, and it is very safe. Specifically, it relaxes the maternal abdominal wall muscles and thereby facilitates ECV. Another benefit is that the anesthesia is in place and available for use should emergency cesarean delivery be needed during or after attempted ECV. Neuraxial anesthesia, which includes spinal, epidural, and combined spinal-epidural techniques, is almost always used with tocolysis.
The major complications of neuraxial analgesia/anesthesia are maternal hypotension and fetal bradycardia. Each is dose related and usually transient.
In the past, there was concern that using regional anesthesia to control pain would reduce a patient’s natural warning symptoms and result in a clinician applying excessive force, thus increasing the chances of fetal and maternal injury and even fetal death. However, multiple studies have found that ECV complication rates are not increased with use of neuraxial methods.
Higher doses of neuraxial anesthesia produce higher ECV success rates. This dose-dependent relationship is almost surely attributable to the fact that, although lower dose neuraxial analgesia can relieve the pain associated with ECV, an anesthetic dose is needed to relax the abdominal wall muscles and facilitate fetus repositioning.
The literature is clear: ECV success rates are significantly increased with the use of neuraxial techniques, with anesthesia having higher success rates than analgesia. Reviewing the results of 6 controlled trials in which a total of 508 patients underwent ECV with tocolysis, Goetzinger and colleagues found that the chance of ECV success was almost 60% higher in the 253 patients who received regional anesthesia than in the 255 patients who received intravenous or no analgesia.25 Moreover, only 48.4% of the regional anesthesia patients as compared with 59.3% of patients who did not have regional anesthesia underwent cesarean delivery, roughly a 20% decrease. Pain scores were consistently lower in the regional anesthesia group. Multiple other studies have reported similar results.
Although the use of neuraxial anesthesia increases the ECV success rate, and decreases the cesarean delivery rate for breech presentation by 5% to 15%,25 some groups of obstetrics professionals, noting that the decreased cesarean delivery rate does not meet the formal criterion for statistical significance, have expressed reservations about recommending regional anesthesia for ECV. Thus, despite the positive results obtained with neuraxial anesthesia, neither the literature nor authoritative professional organizations definitively recommend the use of neuraxial anesthesia in facilitating ECV.
This lack of official recommendation, however, overlooks an important point: While the cesarean delivery percentage decrease that occurs with the use of neuraxial anesthesia may not be statistically significant, the promise of a pain-free procedure will encourage more women to undergo ECV. If the procedure population increases, then the average ECV success rate of roughly 60%6 applies to a larger base of patients, reducing the total number of cesarean deliveries for breech presentation. As only a small percentage of the 110,000 to 150,000 women with breech presentation at 36 weeks currently elects to undergo ECV, any increase in the number of women who proceed with attempts at fetal repositioning once procedural pain is no longer an issue will accordingly reduce the number of cesarean deliveries for the indication of malpresentation.
Related article:
Nitrous oxide for labor pain
Overarching goal: Reduce cesarean delivery rate and associated risks
In the United States, increasing the use of ECV in cases of breech-presenting fetuses would reduce the cesarean delivery rate by about 10%, thereby reducing recovery time for cesarean deliveries, minimizing the risks associated with these deliveries (current and future), and providing the health care system with a major cost savings.
Tocolysis and the use of neuraxial anesthesia each increases the ECV success rate and each is remarkably safe within the context of a well-defined protocol. Reducing the pain associated with ECV by administering neuraxial anesthesia will increase the number of women electing to undergo the procedure and ultimately will reduce the number of cesarean deliveries performed for the indication of breech presentation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR. Planned cesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Term Breech Trial Collaborative Group. Lancet. 2000;356(9239):1375–1383.
- Weiniger CF, Lyell DJ, Tsen LC, et al. Maternal outcomes of term breech presentation delivery: impact of successful external cephalic version in a nationwide sample of delivery admissions in the United States. BMC Pregnancy Childbirth. 2016;16(1):150.
- Eller DP, Van Dorsten JP. Breech presentation. Curr Opin Obstet Gynecol.1993;5(5)664–668.
- Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics. 24th ed. New York, NY: McGraw Hill; 2014:570.
- Westgren M, Edvall H, Nordstrom L, Svalenius E, Ranstam J. Spontaneous cephalic version of breech presentation in the last trimester. Br J Obstet Gynaecol. 1985;92(1):19–22.
- External cephalic version. ACOG Practice Bulletin No. 161. American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2016.
- Hofmeyr GJ, Kulier R, West HM. External cephalic version for breech presentation at term. Cochrane Database Syst Rev. 2015;(4):CD000083.
- Beuckens A, Rijnders M, Verburgt-Doeleman GH, Rijninks-van Driel GC, Thorpe J, Hutton EK. An observational study of the success and complications of 2546 external cephalic versions in low-risk pregnant women performed by trained midwives. BJOG. 2016;123(3):415–423.
- Tong Leung VK, Suen SS, Singh Sahota D, Lau TK, Yeung Leung T. External cephalic version does not increase the risk of intra-uterine death: a 17-year experience and literature review. J Matern Fetal Neonatal Med. 2012;25(9):1774–1778.
- de Hundt M, Velzel J, de Groot CJ, Mol BW, Kok M. Mode of delivery after successful external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(6):1327–1334.
- Grootscholten K, Kok M, Oei SG, Mol BW, van der Post JA. External cephalic version–related risks: a meta-analysis. Obstet Gynecol. 2008;112(5):1143–1151.
- Collaris RJ, Oei SG. External cephalic version: a safe procedure? A systematic review of version-related risk. Acta Obstet Gynecol Scand. 2004;83(6):511–518.
- Khaw KS, Lee SW, Ngan Kee WD, et al. Randomized trial of anesthetic interventions in external cephalic version for breech presentation. Br J Anaesth. 2015;114(6):944–950.
- Boucher M, Marquette GP, Varin J, Champagne J, Bujold E. Fetomaternal hemorrhage during external cephalic version. Obstet Gynecol. 2008;112(1):79–84.
- Hofmeyr GJ, Kulier R. Cephalic version by postural management for breech presentation. Cochrane Database Syst Rev. 2012;(10):CD00051.
- Coulon C, Poleszczuk M, Paty-Montaigne MH, et al. Version of breech fetuses by moxibustion with acupuncture: a randomized controlled trial. Obstet Gynecol. 2014;124(1):32–39.
- Bue L, Lauszus FF. Moxibustion did not have an effect in a randomised clinical trial for version of breech position. Dan Med J. 2016;63(2):pii:A5199.
- Coyle ME, Smith CA, Peat B. Cephalic version by moxibustion for breech presentation. Cochrane Database Syst Rev. 2012;(5):CD003928.
- Sananes N, Roth GE, Aissi GA, et al. Acupuncture version of breech presentation: a randomized sham-controlled single-blinded trial. Eur J Obstet Gynecol Reprod Biol. 2016;204:24–30.
- Cluver C, Gyte GM, Sinclair M, Dowswell T, Hofmeyr G. Interventions for helping to turn breech babies to head first presentation when using external cephalic version. Cochrane Database Syst Rev. 2015;(2):CD000184.
- Fernandez CO, Bloom SL, Smulian JC, Ananth CV, Wendel GD Jr. A randomized placebo-controlled evaluation of terbutaline for external cephalic version. Obstet Gynecol. 1997;90(5):775–779.
- Mohamed Ismail NA, Ibrahim M, Mohd Naim N, Mahdy ZA, Jamil MA, Mohd Razi ZR. Nifedipine versus terbutaline for tocolysis in external cephalic version. Int J Gynaecol Obstet. 2008;102(3):263–266.
- Kok M, Bais J, van Lith J, et al. Nifedipine as a uterine relaxant for external cephalic version: a meta-analysis. Am J Obstet Gynecol. 2008;112(2 pt 1):271–276.
- Bujold E, Boucher M, Rinfred D, Berman S, Ferreira E, Marquette GP. Sublingual nitroglycerin versus placebo as a tocolytic for external cephalic version: a randomized controlled trial in parous women. Am J Obstet Gynecol. 2003;189(4):1070–1073.
- Goetzinger KR, Harper LM, Tuuli MG, Macones GA, Colditz GA. Effect of regional anesthesia on the success of external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1137–1144.
About 3% to 4% of all fetuses at term are in breech presentation. Since 2000, when Hannah and colleagues reported finding that vaginal delivery of breech-presenting babies was riskier than cesarean delivery,1 most breech-presenting neonates in the United States have been delivered abdominally2—despite subsequent questioning of some of that study’s conclusions.
Each year in the United States, approximately 4 million babies are born, and fetal malpresentation accounts for 110,000 to 150,000 cesarean deliveries. In fact, about 15% of all cesarean deliveries in the United States are for breech presentation or transverse lie; in England the percentage is 10%.3 Fortunately, the repopularized technique of external cephalic version (ECV), in which the clinician externally rotates a breech- or transverse-lying fetus to a vertex position (FIGURE), along with the facilitating tools of tocolysis and neuraxial analgesia/anesthesia, is helping to reduce the number of breech presentations in fetuses at term and thus the number of cesarean deliveries and their sequelae—placenta accreta, prolonged recovery, and cesarean deliveries in subsequent pregnancies.

Reluctance to perform ECV is unfounded
In the United States, the practice of offering ECV to women who present with their fetus in breech presentation at term varies tremendously. It is routine at some institutions but not even offered at others.
Many ObGyns are reluctant to perform ECV. Cited reasons include the potential for injury to the fetus and mother (and related liability concerns), the ease of elective cesarean delivery, the variable success rate of ECV (35% to 86%),4 and the pain that women often have with the procedure. According to the literature, however, these concerns either are unfounded or can be mitigated with use of current techniques. Multiple studies have found that the risk of ECV to the fetus and mother is minimal, and that tocolysis and neuraxial anesthesia can facilitate the success of ECV and relieve the pain associated with the procedure.
Related article:
2017 Update on obstetrics
Indications for ECV
The indications for ECV include breech, oblique, or transverse lie presentation after 36 weeks’ gestation and the mother’s desire to avoid cesarean delivery. A clinician skilled in ECV and a facility where emergency cesarean delivery is possible are essential.
There are several instances in which ECV should not be attempted.
Contraindications include:
- concerns about fetal status, including nonreactive nonstress test, biophysical profile score <6/8, severe intrauterine growth restriction, decreased end-diastolic umbilical blood flow
- placenta previa
- multifetal gestation before delivery of first twin
- severe oligohydramnios
- severe preeclampsia
- significant fetal anomaly
- known malformation of uterus
- breech with hyperextended head or arms above shoulders, as seen on ultrasonography.
More controversial contraindications include prior uterine incision, maternal obesity (body mass index >40 kg/m2), ruptured membranes, and fetal macrosomia.
Read about timing, success rates, risk factors, alternate approaches for ECV
Optimal timing for the ECV procedure
Current practice is to wait until 36 to 37 weeks to perform ECV, as most fetuses spontaneously move into vertex presentation by 36 weeks’ gestation. This time frame has several advantages: Many unnecessary attempts at ECV are avoided; only 8% of fetuses in breech presentation after 36 weeks spontaneously change to vertex5; many fetuses revert to breech if ECV is performed too early; and prematurity generally is not an issue in the rare case that immediate delivery is required during or just after attempted ECV.
ECV during labor. Performing ECV during labor appears to pose no increased risk to mother or fetus if membranes are intact and there are no other contraindications to the procedure. Some clinicians perform ECV only during labor. The advantages are that the fetus has had every chance to move into vertex presentation on its own, the equipment used to continuously monitor the fetus during ECV is in place, and cesarean delivery and anesthesia are immediately available in the event ECV is unsuccessful.
The major disadvantage of waiting until labor is that the increased size of the fetus makes ECV more difficult. In addition, the membranes may have already ruptured, and the breech may have descended deeply into the pelvis.
Related article:
For the management of labor, patience is a virtue
Success rates in breech-to-vertex conversions
In 2016, the American College of Obstetricians and Gynecologists (ACOG) reported an average ECV success rate of 58% (range, 16% to 100%).6 ACOG noted that, with transverse lie, the success rate was significantly higher. Other studies have found a wide range of rates: 58% in 1,308 patients in a Cochrane review by Hofmeyr and colleagues7; 47% in a study by Beuckens and colleagues8; and 63.1% for primiparas and 82.7% for multiparas in a study by Tong Leung and colleagues.9 These rates were affected by whether ECV was performed with or without tocolysis, with or without intravenous analgesia, and with or without neuraxial analgesia/anesthesia (TABLE).

Likelihood of vaginal delivery after successful ECV
The rate of vaginal delivery after successful ECV is roughly half that of fetuses that were never in breech presentation.10 In successful ECV cases, dystocia and nonreassuring fetal heart rate patterns are the major indications for cesarean delivery. Some experts have speculated that the factors leading to near-term breech presentation—such as an unengaged presenting part or a mother’s smaller pelvis—also may be risk factors for dystocia in labor. Despite this, the rate of vaginal delivery of successfully verted babies has been reported to be as high as 80%.10
As might be expected, post-ECV vaginal deliveries are more common in multiparous than in primiparous women.
Although multiple problems may occur with ECV, generally they are rare and reversible. For instance, Grootscholten and colleagues found a stillbirth and placental abruption rate of only 0.25% in a large group of patients who underwent ECV.11 Similarly, the rate of emergency cesarean delivery was 0.35%. In addition, Hofmeyr and Kulier, in their Cochrane Data Review of 2015, found no significant differences in the Apgar scores and pH’s of babies in the ECV group compared with babies in breech presentation whose mothers did not undergo ECV.7 Results of other studies have confirmed the safety of ECV.12,13
One significant risk of ECV attempts is fetal-to-maternal blood transfer. Boucher and colleagues found that 2.4% of 1,244 women who underwent ECV had a positive Kleihauer-Betke test result, and, in one-third of the positive cases, more than 1 mL of fetal blood was found in maternal circulation.14 This risk can be minimized by administering Rho (D) immune globulin to all Rh-negative mothers after the procedure.
Even these small risks, however, should not be considered in isolation. The infrequent complications of ECV must be compared with what can occur with breech-presenting fetuses during labor or cesarean delivery: complications of breech vaginal delivery, cord prolapse, difficulties with cesarean delivery, and maternal operative complications related to present and future cesarean deliveries.
Alternative approaches to converting breech presentation of unproven efficacy
Over the years, attempts have been made to address breech presentations with measures short of ECV. There is little evidence that these measures work, or work consistently.
- Observation. After 36 weeks’ gestation, only 8% of fetuses in breech presentationspontaneously move into vertex presentation.5
- Maternal positioning. There is no good evidence that such maneuvers are effective in changing fetal presentation.15
- Moxibustion and acupuncture. Moxibustion is inhalation of smoke from burning herbal compounds. In formal studies using controls, these techniques did not consistently increase the rate of movement from breech to vertex presentation.16–18 Likewise, studies with the use of acupuncture have not shown consistent success in changing fetal presentation.19
Read about various methods to facilitate ECV success
Methods to facilitate ECV success
Two techniques that can facilitate ECV success are tocolysis, which relaxes the uterus, and neuraxial analgesia/anesthesia, which relaxes anterior abdominal wall muscles and reduces or relieves ECV-associated pain.
Tocolysis
In tocolysis, a medication is administered to reduce myometrial activity and to relax the uterine muscle so that it stretches more easily around the fetus during repositioning. Tocolytic medications originally were studied for their use in decreasing myometrial tone during preterm labor.
Tocolysis clearly is effective in increasing ECV success rates. Reviewing the results of 4 randomized trials, Cluver showed a 1.38 risk ratio for successful ECV when terbutaline was used versus when there was no tocolysis. The risk ratio for cesarean delivery was 0.82.20 Fernandez, in a study of 103 women divided into terbutaline versus placebo groups, had a 52% success rate for ECV with the terbutaline group versus only a 27% success rate with the placebo group.21
Tocolytic medications include terbutaline, nifedipine, and nitroglycerin.
Tocolysis most often involves the use of β2-adrenergic receptor agonists, particularly terbutaline (despite the boxed safety warning in its prescribing information). A 0.25-mg dose of terbutaline is given subcutaneously 15 to 30 minutes before ECV. Clinicians have successfully used β2-adrenergic receptor agonists in the treatment of patients in preterm labor, and there are more data on this class of medications than on other agents used to facilitate ECV.
Although nifedipine is as effective as terbutaline in the temporary treatment of preterm uterine contractions, several studies have found this calcium channel blocker less effective than terbutaline in facilitating ECV.22,23
The uterus-relaxing effect of nitroglycerin was once thought to make this medication appropriate for facilitating ECV, but multiple studies have found success rates unimproved. In some cases, the drug performed more poorly than placebo.24 Moreover, nitroglycerin is associated with a fairly high rate of adverse effects, such as headaches and blood pressure changes.
Neuraxial analgesia/anesthesia
Over the past 2 decades, there has been a resurgence in the use of neuraxial analgesia/anesthesia in ECV. This technique is more effective than others in improving ECV success rates, it reduces maternal discomfort, and it is very safe. Specifically, it relaxes the maternal abdominal wall muscles and thereby facilitates ECV. Another benefit is that the anesthesia is in place and available for use should emergency cesarean delivery be needed during or after attempted ECV. Neuraxial anesthesia, which includes spinal, epidural, and combined spinal-epidural techniques, is almost always used with tocolysis.
The major complications of neuraxial analgesia/anesthesia are maternal hypotension and fetal bradycardia. Each is dose related and usually transient.
In the past, there was concern that using regional anesthesia to control pain would reduce a patient’s natural warning symptoms and result in a clinician applying excessive force, thus increasing the chances of fetal and maternal injury and even fetal death. However, multiple studies have found that ECV complication rates are not increased with use of neuraxial methods.
Higher doses of neuraxial anesthesia produce higher ECV success rates. This dose-dependent relationship is almost surely attributable to the fact that, although lower dose neuraxial analgesia can relieve the pain associated with ECV, an anesthetic dose is needed to relax the abdominal wall muscles and facilitate fetus repositioning.
The literature is clear: ECV success rates are significantly increased with the use of neuraxial techniques, with anesthesia having higher success rates than analgesia. Reviewing the results of 6 controlled trials in which a total of 508 patients underwent ECV with tocolysis, Goetzinger and colleagues found that the chance of ECV success was almost 60% higher in the 253 patients who received regional anesthesia than in the 255 patients who received intravenous or no analgesia.25 Moreover, only 48.4% of the regional anesthesia patients as compared with 59.3% of patients who did not have regional anesthesia underwent cesarean delivery, roughly a 20% decrease. Pain scores were consistently lower in the regional anesthesia group. Multiple other studies have reported similar results.
Although the use of neuraxial anesthesia increases the ECV success rate, and decreases the cesarean delivery rate for breech presentation by 5% to 15%,25 some groups of obstetrics professionals, noting that the decreased cesarean delivery rate does not meet the formal criterion for statistical significance, have expressed reservations about recommending regional anesthesia for ECV. Thus, despite the positive results obtained with neuraxial anesthesia, neither the literature nor authoritative professional organizations definitively recommend the use of neuraxial anesthesia in facilitating ECV.
This lack of official recommendation, however, overlooks an important point: While the cesarean delivery percentage decrease that occurs with the use of neuraxial anesthesia may not be statistically significant, the promise of a pain-free procedure will encourage more women to undergo ECV. If the procedure population increases, then the average ECV success rate of roughly 60%6 applies to a larger base of patients, reducing the total number of cesarean deliveries for breech presentation. As only a small percentage of the 110,000 to 150,000 women with breech presentation at 36 weeks currently elects to undergo ECV, any increase in the number of women who proceed with attempts at fetal repositioning once procedural pain is no longer an issue will accordingly reduce the number of cesarean deliveries for the indication of malpresentation.
Related article:
Nitrous oxide for labor pain
Overarching goal: Reduce cesarean delivery rate and associated risks
In the United States, increasing the use of ECV in cases of breech-presenting fetuses would reduce the cesarean delivery rate by about 10%, thereby reducing recovery time for cesarean deliveries, minimizing the risks associated with these deliveries (current and future), and providing the health care system with a major cost savings.
Tocolysis and the use of neuraxial anesthesia each increases the ECV success rate and each is remarkably safe within the context of a well-defined protocol. Reducing the pain associated with ECV by administering neuraxial anesthesia will increase the number of women electing to undergo the procedure and ultimately will reduce the number of cesarean deliveries performed for the indication of breech presentation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
About 3% to 4% of all fetuses at term are in breech presentation. Since 2000, when Hannah and colleagues reported finding that vaginal delivery of breech-presenting babies was riskier than cesarean delivery,1 most breech-presenting neonates in the United States have been delivered abdominally2—despite subsequent questioning of some of that study’s conclusions.
Each year in the United States, approximately 4 million babies are born, and fetal malpresentation accounts for 110,000 to 150,000 cesarean deliveries. In fact, about 15% of all cesarean deliveries in the United States are for breech presentation or transverse lie; in England the percentage is 10%.3 Fortunately, the repopularized technique of external cephalic version (ECV), in which the clinician externally rotates a breech- or transverse-lying fetus to a vertex position (FIGURE), along with the facilitating tools of tocolysis and neuraxial analgesia/anesthesia, is helping to reduce the number of breech presentations in fetuses at term and thus the number of cesarean deliveries and their sequelae—placenta accreta, prolonged recovery, and cesarean deliveries in subsequent pregnancies.

Reluctance to perform ECV is unfounded
In the United States, the practice of offering ECV to women who present with their fetus in breech presentation at term varies tremendously. It is routine at some institutions but not even offered at others.
Many ObGyns are reluctant to perform ECV. Cited reasons include the potential for injury to the fetus and mother (and related liability concerns), the ease of elective cesarean delivery, the variable success rate of ECV (35% to 86%),4 and the pain that women often have with the procedure. According to the literature, however, these concerns either are unfounded or can be mitigated with use of current techniques. Multiple studies have found that the risk of ECV to the fetus and mother is minimal, and that tocolysis and neuraxial anesthesia can facilitate the success of ECV and relieve the pain associated with the procedure.
Related article:
2017 Update on obstetrics
Indications for ECV
The indications for ECV include breech, oblique, or transverse lie presentation after 36 weeks’ gestation and the mother’s desire to avoid cesarean delivery. A clinician skilled in ECV and a facility where emergency cesarean delivery is possible are essential.
There are several instances in which ECV should not be attempted.
Contraindications include:
- concerns about fetal status, including nonreactive nonstress test, biophysical profile score <6/8, severe intrauterine growth restriction, decreased end-diastolic umbilical blood flow
- placenta previa
- multifetal gestation before delivery of first twin
- severe oligohydramnios
- severe preeclampsia
- significant fetal anomaly
- known malformation of uterus
- breech with hyperextended head or arms above shoulders, as seen on ultrasonography.
More controversial contraindications include prior uterine incision, maternal obesity (body mass index >40 kg/m2), ruptured membranes, and fetal macrosomia.
Read about timing, success rates, risk factors, alternate approaches for ECV
Optimal timing for the ECV procedure
Current practice is to wait until 36 to 37 weeks to perform ECV, as most fetuses spontaneously move into vertex presentation by 36 weeks’ gestation. This time frame has several advantages: Many unnecessary attempts at ECV are avoided; only 8% of fetuses in breech presentation after 36 weeks spontaneously change to vertex5; many fetuses revert to breech if ECV is performed too early; and prematurity generally is not an issue in the rare case that immediate delivery is required during or just after attempted ECV.
ECV during labor. Performing ECV during labor appears to pose no increased risk to mother or fetus if membranes are intact and there are no other contraindications to the procedure. Some clinicians perform ECV only during labor. The advantages are that the fetus has had every chance to move into vertex presentation on its own, the equipment used to continuously monitor the fetus during ECV is in place, and cesarean delivery and anesthesia are immediately available in the event ECV is unsuccessful.
The major disadvantage of waiting until labor is that the increased size of the fetus makes ECV more difficult. In addition, the membranes may have already ruptured, and the breech may have descended deeply into the pelvis.
Related article:
For the management of labor, patience is a virtue
Success rates in breech-to-vertex conversions
In 2016, the American College of Obstetricians and Gynecologists (ACOG) reported an average ECV success rate of 58% (range, 16% to 100%).6 ACOG noted that, with transverse lie, the success rate was significantly higher. Other studies have found a wide range of rates: 58% in 1,308 patients in a Cochrane review by Hofmeyr and colleagues7; 47% in a study by Beuckens and colleagues8; and 63.1% for primiparas and 82.7% for multiparas in a study by Tong Leung and colleagues.9 These rates were affected by whether ECV was performed with or without tocolysis, with or without intravenous analgesia, and with or without neuraxial analgesia/anesthesia (TABLE).

Likelihood of vaginal delivery after successful ECV
The rate of vaginal delivery after successful ECV is roughly half that of fetuses that were never in breech presentation.10 In successful ECV cases, dystocia and nonreassuring fetal heart rate patterns are the major indications for cesarean delivery. Some experts have speculated that the factors leading to near-term breech presentation—such as an unengaged presenting part or a mother’s smaller pelvis—also may be risk factors for dystocia in labor. Despite this, the rate of vaginal delivery of successfully verted babies has been reported to be as high as 80%.10
As might be expected, post-ECV vaginal deliveries are more common in multiparous than in primiparous women.
Although multiple problems may occur with ECV, generally they are rare and reversible. For instance, Grootscholten and colleagues found a stillbirth and placental abruption rate of only 0.25% in a large group of patients who underwent ECV.11 Similarly, the rate of emergency cesarean delivery was 0.35%. In addition, Hofmeyr and Kulier, in their Cochrane Data Review of 2015, found no significant differences in the Apgar scores and pH’s of babies in the ECV group compared with babies in breech presentation whose mothers did not undergo ECV.7 Results of other studies have confirmed the safety of ECV.12,13
One significant risk of ECV attempts is fetal-to-maternal blood transfer. Boucher and colleagues found that 2.4% of 1,244 women who underwent ECV had a positive Kleihauer-Betke test result, and, in one-third of the positive cases, more than 1 mL of fetal blood was found in maternal circulation.14 This risk can be minimized by administering Rho (D) immune globulin to all Rh-negative mothers after the procedure.
Even these small risks, however, should not be considered in isolation. The infrequent complications of ECV must be compared with what can occur with breech-presenting fetuses during labor or cesarean delivery: complications of breech vaginal delivery, cord prolapse, difficulties with cesarean delivery, and maternal operative complications related to present and future cesarean deliveries.
Alternative approaches to converting breech presentation of unproven efficacy
Over the years, attempts have been made to address breech presentations with measures short of ECV. There is little evidence that these measures work, or work consistently.
- Observation. After 36 weeks’ gestation, only 8% of fetuses in breech presentationspontaneously move into vertex presentation.5
- Maternal positioning. There is no good evidence that such maneuvers are effective in changing fetal presentation.15
- Moxibustion and acupuncture. Moxibustion is inhalation of smoke from burning herbal compounds. In formal studies using controls, these techniques did not consistently increase the rate of movement from breech to vertex presentation.16–18 Likewise, studies with the use of acupuncture have not shown consistent success in changing fetal presentation.19
Read about various methods to facilitate ECV success
Methods to facilitate ECV success
Two techniques that can facilitate ECV success are tocolysis, which relaxes the uterus, and neuraxial analgesia/anesthesia, which relaxes anterior abdominal wall muscles and reduces or relieves ECV-associated pain.
Tocolysis
In tocolysis, a medication is administered to reduce myometrial activity and to relax the uterine muscle so that it stretches more easily around the fetus during repositioning. Tocolytic medications originally were studied for their use in decreasing myometrial tone during preterm labor.
Tocolysis clearly is effective in increasing ECV success rates. Reviewing the results of 4 randomized trials, Cluver showed a 1.38 risk ratio for successful ECV when terbutaline was used versus when there was no tocolysis. The risk ratio for cesarean delivery was 0.82.20 Fernandez, in a study of 103 women divided into terbutaline versus placebo groups, had a 52% success rate for ECV with the terbutaline group versus only a 27% success rate with the placebo group.21
Tocolytic medications include terbutaline, nifedipine, and nitroglycerin.
Tocolysis most often involves the use of β2-adrenergic receptor agonists, particularly terbutaline (despite the boxed safety warning in its prescribing information). A 0.25-mg dose of terbutaline is given subcutaneously 15 to 30 minutes before ECV. Clinicians have successfully used β2-adrenergic receptor agonists in the treatment of patients in preterm labor, and there are more data on this class of medications than on other agents used to facilitate ECV.
Although nifedipine is as effective as terbutaline in the temporary treatment of preterm uterine contractions, several studies have found this calcium channel blocker less effective than terbutaline in facilitating ECV.22,23
The uterus-relaxing effect of nitroglycerin was once thought to make this medication appropriate for facilitating ECV, but multiple studies have found success rates unimproved. In some cases, the drug performed more poorly than placebo.24 Moreover, nitroglycerin is associated with a fairly high rate of adverse effects, such as headaches and blood pressure changes.
Neuraxial analgesia/anesthesia
Over the past 2 decades, there has been a resurgence in the use of neuraxial analgesia/anesthesia in ECV. This technique is more effective than others in improving ECV success rates, it reduces maternal discomfort, and it is very safe. Specifically, it relaxes the maternal abdominal wall muscles and thereby facilitates ECV. Another benefit is that the anesthesia is in place and available for use should emergency cesarean delivery be needed during or after attempted ECV. Neuraxial anesthesia, which includes spinal, epidural, and combined spinal-epidural techniques, is almost always used with tocolysis.
The major complications of neuraxial analgesia/anesthesia are maternal hypotension and fetal bradycardia. Each is dose related and usually transient.
In the past, there was concern that using regional anesthesia to control pain would reduce a patient’s natural warning symptoms and result in a clinician applying excessive force, thus increasing the chances of fetal and maternal injury and even fetal death. However, multiple studies have found that ECV complication rates are not increased with use of neuraxial methods.
Higher doses of neuraxial anesthesia produce higher ECV success rates. This dose-dependent relationship is almost surely attributable to the fact that, although lower dose neuraxial analgesia can relieve the pain associated with ECV, an anesthetic dose is needed to relax the abdominal wall muscles and facilitate fetus repositioning.
The literature is clear: ECV success rates are significantly increased with the use of neuraxial techniques, with anesthesia having higher success rates than analgesia. Reviewing the results of 6 controlled trials in which a total of 508 patients underwent ECV with tocolysis, Goetzinger and colleagues found that the chance of ECV success was almost 60% higher in the 253 patients who received regional anesthesia than in the 255 patients who received intravenous or no analgesia.25 Moreover, only 48.4% of the regional anesthesia patients as compared with 59.3% of patients who did not have regional anesthesia underwent cesarean delivery, roughly a 20% decrease. Pain scores were consistently lower in the regional anesthesia group. Multiple other studies have reported similar results.
Although the use of neuraxial anesthesia increases the ECV success rate, and decreases the cesarean delivery rate for breech presentation by 5% to 15%,25 some groups of obstetrics professionals, noting that the decreased cesarean delivery rate does not meet the formal criterion for statistical significance, have expressed reservations about recommending regional anesthesia for ECV. Thus, despite the positive results obtained with neuraxial anesthesia, neither the literature nor authoritative professional organizations definitively recommend the use of neuraxial anesthesia in facilitating ECV.
This lack of official recommendation, however, overlooks an important point: While the cesarean delivery percentage decrease that occurs with the use of neuraxial anesthesia may not be statistically significant, the promise of a pain-free procedure will encourage more women to undergo ECV. If the procedure population increases, then the average ECV success rate of roughly 60%6 applies to a larger base of patients, reducing the total number of cesarean deliveries for breech presentation. As only a small percentage of the 110,000 to 150,000 women with breech presentation at 36 weeks currently elects to undergo ECV, any increase in the number of women who proceed with attempts at fetal repositioning once procedural pain is no longer an issue will accordingly reduce the number of cesarean deliveries for the indication of malpresentation.
Related article:
Nitrous oxide for labor pain
Overarching goal: Reduce cesarean delivery rate and associated risks
In the United States, increasing the use of ECV in cases of breech-presenting fetuses would reduce the cesarean delivery rate by about 10%, thereby reducing recovery time for cesarean deliveries, minimizing the risks associated with these deliveries (current and future), and providing the health care system with a major cost savings.
Tocolysis and the use of neuraxial anesthesia each increases the ECV success rate and each is remarkably safe within the context of a well-defined protocol. Reducing the pain associated with ECV by administering neuraxial anesthesia will increase the number of women electing to undergo the procedure and ultimately will reduce the number of cesarean deliveries performed for the indication of breech presentation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR. Planned cesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Term Breech Trial Collaborative Group. Lancet. 2000;356(9239):1375–1383.
- Weiniger CF, Lyell DJ, Tsen LC, et al. Maternal outcomes of term breech presentation delivery: impact of successful external cephalic version in a nationwide sample of delivery admissions in the United States. BMC Pregnancy Childbirth. 2016;16(1):150.
- Eller DP, Van Dorsten JP. Breech presentation. Curr Opin Obstet Gynecol.1993;5(5)664–668.
- Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics. 24th ed. New York, NY: McGraw Hill; 2014:570.
- Westgren M, Edvall H, Nordstrom L, Svalenius E, Ranstam J. Spontaneous cephalic version of breech presentation in the last trimester. Br J Obstet Gynaecol. 1985;92(1):19–22.
- External cephalic version. ACOG Practice Bulletin No. 161. American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2016.
- Hofmeyr GJ, Kulier R, West HM. External cephalic version for breech presentation at term. Cochrane Database Syst Rev. 2015;(4):CD000083.
- Beuckens A, Rijnders M, Verburgt-Doeleman GH, Rijninks-van Driel GC, Thorpe J, Hutton EK. An observational study of the success and complications of 2546 external cephalic versions in low-risk pregnant women performed by trained midwives. BJOG. 2016;123(3):415–423.
- Tong Leung VK, Suen SS, Singh Sahota D, Lau TK, Yeung Leung T. External cephalic version does not increase the risk of intra-uterine death: a 17-year experience and literature review. J Matern Fetal Neonatal Med. 2012;25(9):1774–1778.
- de Hundt M, Velzel J, de Groot CJ, Mol BW, Kok M. Mode of delivery after successful external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(6):1327–1334.
- Grootscholten K, Kok M, Oei SG, Mol BW, van der Post JA. External cephalic version–related risks: a meta-analysis. Obstet Gynecol. 2008;112(5):1143–1151.
- Collaris RJ, Oei SG. External cephalic version: a safe procedure? A systematic review of version-related risk. Acta Obstet Gynecol Scand. 2004;83(6):511–518.
- Khaw KS, Lee SW, Ngan Kee WD, et al. Randomized trial of anesthetic interventions in external cephalic version for breech presentation. Br J Anaesth. 2015;114(6):944–950.
- Boucher M, Marquette GP, Varin J, Champagne J, Bujold E. Fetomaternal hemorrhage during external cephalic version. Obstet Gynecol. 2008;112(1):79–84.
- Hofmeyr GJ, Kulier R. Cephalic version by postural management for breech presentation. Cochrane Database Syst Rev. 2012;(10):CD00051.
- Coulon C, Poleszczuk M, Paty-Montaigne MH, et al. Version of breech fetuses by moxibustion with acupuncture: a randomized controlled trial. Obstet Gynecol. 2014;124(1):32–39.
- Bue L, Lauszus FF. Moxibustion did not have an effect in a randomised clinical trial for version of breech position. Dan Med J. 2016;63(2):pii:A5199.
- Coyle ME, Smith CA, Peat B. Cephalic version by moxibustion for breech presentation. Cochrane Database Syst Rev. 2012;(5):CD003928.
- Sananes N, Roth GE, Aissi GA, et al. Acupuncture version of breech presentation: a randomized sham-controlled single-blinded trial. Eur J Obstet Gynecol Reprod Biol. 2016;204:24–30.
- Cluver C, Gyte GM, Sinclair M, Dowswell T, Hofmeyr G. Interventions for helping to turn breech babies to head first presentation when using external cephalic version. Cochrane Database Syst Rev. 2015;(2):CD000184.
- Fernandez CO, Bloom SL, Smulian JC, Ananth CV, Wendel GD Jr. A randomized placebo-controlled evaluation of terbutaline for external cephalic version. Obstet Gynecol. 1997;90(5):775–779.
- Mohamed Ismail NA, Ibrahim M, Mohd Naim N, Mahdy ZA, Jamil MA, Mohd Razi ZR. Nifedipine versus terbutaline for tocolysis in external cephalic version. Int J Gynaecol Obstet. 2008;102(3):263–266.
- Kok M, Bais J, van Lith J, et al. Nifedipine as a uterine relaxant for external cephalic version: a meta-analysis. Am J Obstet Gynecol. 2008;112(2 pt 1):271–276.
- Bujold E, Boucher M, Rinfred D, Berman S, Ferreira E, Marquette GP. Sublingual nitroglycerin versus placebo as a tocolytic for external cephalic version: a randomized controlled trial in parous women. Am J Obstet Gynecol. 2003;189(4):1070–1073.
- Goetzinger KR, Harper LM, Tuuli MG, Macones GA, Colditz GA. Effect of regional anesthesia on the success of external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1137–1144.
- Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR. Planned cesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Term Breech Trial Collaborative Group. Lancet. 2000;356(9239):1375–1383.
- Weiniger CF, Lyell DJ, Tsen LC, et al. Maternal outcomes of term breech presentation delivery: impact of successful external cephalic version in a nationwide sample of delivery admissions in the United States. BMC Pregnancy Childbirth. 2016;16(1):150.
- Eller DP, Van Dorsten JP. Breech presentation. Curr Opin Obstet Gynecol.1993;5(5)664–668.
- Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics. 24th ed. New York, NY: McGraw Hill; 2014:570.
- Westgren M, Edvall H, Nordstrom L, Svalenius E, Ranstam J. Spontaneous cephalic version of breech presentation in the last trimester. Br J Obstet Gynaecol. 1985;92(1):19–22.
- External cephalic version. ACOG Practice Bulletin No. 161. American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2016.
- Hofmeyr GJ, Kulier R, West HM. External cephalic version for breech presentation at term. Cochrane Database Syst Rev. 2015;(4):CD000083.
- Beuckens A, Rijnders M, Verburgt-Doeleman GH, Rijninks-van Driel GC, Thorpe J, Hutton EK. An observational study of the success and complications of 2546 external cephalic versions in low-risk pregnant women performed by trained midwives. BJOG. 2016;123(3):415–423.
- Tong Leung VK, Suen SS, Singh Sahota D, Lau TK, Yeung Leung T. External cephalic version does not increase the risk of intra-uterine death: a 17-year experience and literature review. J Matern Fetal Neonatal Med. 2012;25(9):1774–1778.
- de Hundt M, Velzel J, de Groot CJ, Mol BW, Kok M. Mode of delivery after successful external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(6):1327–1334.
- Grootscholten K, Kok M, Oei SG, Mol BW, van der Post JA. External cephalic version–related risks: a meta-analysis. Obstet Gynecol. 2008;112(5):1143–1151.
- Collaris RJ, Oei SG. External cephalic version: a safe procedure? A systematic review of version-related risk. Acta Obstet Gynecol Scand. 2004;83(6):511–518.
- Khaw KS, Lee SW, Ngan Kee WD, et al. Randomized trial of anesthetic interventions in external cephalic version for breech presentation. Br J Anaesth. 2015;114(6):944–950.
- Boucher M, Marquette GP, Varin J, Champagne J, Bujold E. Fetomaternal hemorrhage during external cephalic version. Obstet Gynecol. 2008;112(1):79–84.
- Hofmeyr GJ, Kulier R. Cephalic version by postural management for breech presentation. Cochrane Database Syst Rev. 2012;(10):CD00051.
- Coulon C, Poleszczuk M, Paty-Montaigne MH, et al. Version of breech fetuses by moxibustion with acupuncture: a randomized controlled trial. Obstet Gynecol. 2014;124(1):32–39.
- Bue L, Lauszus FF. Moxibustion did not have an effect in a randomised clinical trial for version of breech position. Dan Med J. 2016;63(2):pii:A5199.
- Coyle ME, Smith CA, Peat B. Cephalic version by moxibustion for breech presentation. Cochrane Database Syst Rev. 2012;(5):CD003928.
- Sananes N, Roth GE, Aissi GA, et al. Acupuncture version of breech presentation: a randomized sham-controlled single-blinded trial. Eur J Obstet Gynecol Reprod Biol. 2016;204:24–30.
- Cluver C, Gyte GM, Sinclair M, Dowswell T, Hofmeyr G. Interventions for helping to turn breech babies to head first presentation when using external cephalic version. Cochrane Database Syst Rev. 2015;(2):CD000184.
- Fernandez CO, Bloom SL, Smulian JC, Ananth CV, Wendel GD Jr. A randomized placebo-controlled evaluation of terbutaline for external cephalic version. Obstet Gynecol. 1997;90(5):775–779.
- Mohamed Ismail NA, Ibrahim M, Mohd Naim N, Mahdy ZA, Jamil MA, Mohd Razi ZR. Nifedipine versus terbutaline for tocolysis in external cephalic version. Int J Gynaecol Obstet. 2008;102(3):263–266.
- Kok M, Bais J, van Lith J, et al. Nifedipine as a uterine relaxant for external cephalic version: a meta-analysis. Am J Obstet Gynecol. 2008;112(2 pt 1):271–276.
- Bujold E, Boucher M, Rinfred D, Berman S, Ferreira E, Marquette GP. Sublingual nitroglycerin versus placebo as a tocolytic for external cephalic version: a randomized controlled trial in parous women. Am J Obstet Gynecol. 2003;189(4):1070–1073.
- Goetzinger KR, Harper LM, Tuuli MG, Macones GA, Colditz GA. Effect of regional anesthesia on the success of external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1137–1144.
Fast Tracks
- Current practice is to wait until 36 to 37 weeks of gestation to perform ECV, since most fetuses spontaneously move into vertex presentation by 36 weeks
- Tocolysis, which relaxes the uterus, and neuraxial analgesia/anesthesia, which relaxes anterior abdominal wall muscles and reduces ECV-associated pain, can facilitate ECV success
- Several studies have found that nifedipine is less effective than terbutaline in facilitating ECV
- Higher doses of neuraxial anesthesia produce higher ECV success rates, possibly because the higher anesthetic dose relaxes the abdominal wall muscles and facilitates fetus repositioning
Should oxytocin and a Foley catheter be used concurrently for cervical ripening in induction of labor?
EXPERT COMMENTARY
The concurrent use of mechanical and pharmacologic cervical ripening is an area of active interest. Combination methods typically involve placing a Foley catheter and simultaneously administering either prostaglandins or oxytocin. Despite the long-standing belief that using 2 cervical ripening agents simultaneously has no benefit compared with using only 1 cervical ripening agent, several recent large randomized trials are challenging this paradigm by suggesting that using 2 cervical ripening agents together may in fact be superior.
Related Article:
Q: Following cesarean delivery, what is the optimal oxytocin infusion duration to prevent postpartum bleeding?
Details of the study
Schoen and colleagues conducted a randomized controlled trial that included 184 nulliparous and 139 multiparous women with an unfavorable cervix undergoing induction of labor after 24 weeks of gestation. All participants had a Foley catheter placed intracervically and then were randomly assigned to receive either concurrent oxytocin infusion within 60 minutes or no oxytocin until after Foley catheter expulsion or removal. Nulliparous and multiparous women were randomly assigned separately. Women with premature rupture of membranes and with 1 prior cesarean delivery were included in the trial, but women were excluded if they were in active labor, had suspected abruption, or had a nonreassuring fetal tracing.
The study was powered to detect a 20% increase in total delivery rate within 24 hours of Foley placement, which was the primary study outcome. Secondary induction outcomes of note included time to Foley expulsion, time to second stage, delivery within 12 hours, total time to delivery, duration of oxytocin use, and mode of delivery. Several maternal and neonatal outcomes also were examined, including tachysystole, chorioamnionitis, meconium, postpartum hemorrhage, birth weight, maternal intensive care unit (ICU) admission, and neonatal ICU admission.
Related Article:
Start offering antenatal corticosteroids to women delivering between 34 0/7 and 36 6/7 weeks of gestation to improve newborn outcomes
Women receiving concurrent Foley and oxytocin delivered sooner. Among nulliparous women, the overall rate of delivery within 24 hours of Foley catheter placement was 64% in the Foley with concurrent oxytocin group compared with 43% in those who received a Foley catheter alone followed by oxytocin (P = .003). The overall time to delivery was 5 hours less in nulliparous women who received combination cervical ripening compared with those who had a Foley catheter alone.
Similarly, multiparous women had an overall rate of delivery within 24 hours of 87% in the concurrent Foley and oxytocin group compared with 72% in women who received Foley catheter followed by oxytocin (P = .022).
Meanwhile, there were no statistically significant differences in mode of delivery between groups for either multiparous or nulliparous patients, and there were no differences in adverse maternal or neonatal outcomes between groups.
Related Article:
How and when umbilical cord gas analysis can justify your obstetric management
Study strengths and weaknesses
This well-designed, randomized control trial clearly demonstrated that the combination of Foley catheter and oxytocin for cervical ripening increases the rate of delivery within 24 hours compared with use of Foley catheter alone. This finding is consistent with those of 2 other large randomized trials in the past 2 years that similarly demonstrated reduced time to delivery when oxytocin infusion was used in combination with Foley catheter compared with Foley alone.1,2
Despite these findings, important questions remain regarding concurrent use of cervical ripening agents. The study by Schoen and colleagues does not address the other option for dual cervical ripening, namely, concurrent use of Foley catheter and misoprostol. Several large randomized trials using Foley catheter with vaginal or oral misoprostol demonstrated reduced time to delivery compared with using either method alone.1,3,4 Only 1 randomized study has compared these 2 dual cervical ripening regimens head-to-head; that study demonstrated that the misoprostol and Foley combination significantly reduced time to delivery compared with combining Foley catheter and oxytocin together.1
Additionally, it is important to note that the study by Schoen and colleagues was not large enough to adequately evaluate potential safety risks with dual combination cervical ripening. More safety data are needed before combination cervical ripening methods can be recommended universally.
-- Christina A. Penfield, MD, MPH, and Deborah A. Wing, MD, MBA
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Levine LD, Downes KL, Elovitz MA, Parry S, Sammel MD, Srinivas SK. Mechanical and pharmacologic methods of labor induction: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1357–1364.
- Connolly KA, Kohari KS, Rekawek P, et al. A randomized trial of Foley balloon induction of labor trial in nulliparas (FIAT-N). Am J Obstet Gynecol. 2016;215(3):392.e1–e6.
- Carbone JF, Tuuli MG, Fogertey PJ, Roehl KA, Macones GA. Combination of Foley bulb and vaginal misoprostol compared with vaginal misoprostol alone for cervical ripening and labor induction: a randomized controlled trial. Obstet Gynecol. 2013;121(2 pt 1):247–252.
- Hill JB, Thigpen BD, Bofill JA, Magann E, Moore LE, Martin JN Jr. A randomized clinical trial comparing vaginal misoprostol versus cervical Foley plus oral misoprostol for cervical ripening and labor induction. Am J Perinatol. 2009;26(1):33–38.
EXPERT COMMENTARY
The concurrent use of mechanical and pharmacologic cervical ripening is an area of active interest. Combination methods typically involve placing a Foley catheter and simultaneously administering either prostaglandins or oxytocin. Despite the long-standing belief that using 2 cervical ripening agents simultaneously has no benefit compared with using only 1 cervical ripening agent, several recent large randomized trials are challenging this paradigm by suggesting that using 2 cervical ripening agents together may in fact be superior.
Related Article:
Q: Following cesarean delivery, what is the optimal oxytocin infusion duration to prevent postpartum bleeding?
Details of the study
Schoen and colleagues conducted a randomized controlled trial that included 184 nulliparous and 139 multiparous women with an unfavorable cervix undergoing induction of labor after 24 weeks of gestation. All participants had a Foley catheter placed intracervically and then were randomly assigned to receive either concurrent oxytocin infusion within 60 minutes or no oxytocin until after Foley catheter expulsion or removal. Nulliparous and multiparous women were randomly assigned separately. Women with premature rupture of membranes and with 1 prior cesarean delivery were included in the trial, but women were excluded if they were in active labor, had suspected abruption, or had a nonreassuring fetal tracing.
The study was powered to detect a 20% increase in total delivery rate within 24 hours of Foley placement, which was the primary study outcome. Secondary induction outcomes of note included time to Foley expulsion, time to second stage, delivery within 12 hours, total time to delivery, duration of oxytocin use, and mode of delivery. Several maternal and neonatal outcomes also were examined, including tachysystole, chorioamnionitis, meconium, postpartum hemorrhage, birth weight, maternal intensive care unit (ICU) admission, and neonatal ICU admission.
Related Article:
Start offering antenatal corticosteroids to women delivering between 34 0/7 and 36 6/7 weeks of gestation to improve newborn outcomes
Women receiving concurrent Foley and oxytocin delivered sooner. Among nulliparous women, the overall rate of delivery within 24 hours of Foley catheter placement was 64% in the Foley with concurrent oxytocin group compared with 43% in those who received a Foley catheter alone followed by oxytocin (P = .003). The overall time to delivery was 5 hours less in nulliparous women who received combination cervical ripening compared with those who had a Foley catheter alone.
Similarly, multiparous women had an overall rate of delivery within 24 hours of 87% in the concurrent Foley and oxytocin group compared with 72% in women who received Foley catheter followed by oxytocin (P = .022).
Meanwhile, there were no statistically significant differences in mode of delivery between groups for either multiparous or nulliparous patients, and there were no differences in adverse maternal or neonatal outcomes between groups.
Related Article:
How and when umbilical cord gas analysis can justify your obstetric management
Study strengths and weaknesses
This well-designed, randomized control trial clearly demonstrated that the combination of Foley catheter and oxytocin for cervical ripening increases the rate of delivery within 24 hours compared with use of Foley catheter alone. This finding is consistent with those of 2 other large randomized trials in the past 2 years that similarly demonstrated reduced time to delivery when oxytocin infusion was used in combination with Foley catheter compared with Foley alone.1,2
Despite these findings, important questions remain regarding concurrent use of cervical ripening agents. The study by Schoen and colleagues does not address the other option for dual cervical ripening, namely, concurrent use of Foley catheter and misoprostol. Several large randomized trials using Foley catheter with vaginal or oral misoprostol demonstrated reduced time to delivery compared with using either method alone.1,3,4 Only 1 randomized study has compared these 2 dual cervical ripening regimens head-to-head; that study demonstrated that the misoprostol and Foley combination significantly reduced time to delivery compared with combining Foley catheter and oxytocin together.1
Additionally, it is important to note that the study by Schoen and colleagues was not large enough to adequately evaluate potential safety risks with dual combination cervical ripening. More safety data are needed before combination cervical ripening methods can be recommended universally.
-- Christina A. Penfield, MD, MPH, and Deborah A. Wing, MD, MBA
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
The concurrent use of mechanical and pharmacologic cervical ripening is an area of active interest. Combination methods typically involve placing a Foley catheter and simultaneously administering either prostaglandins or oxytocin. Despite the long-standing belief that using 2 cervical ripening agents simultaneously has no benefit compared with using only 1 cervical ripening agent, several recent large randomized trials are challenging this paradigm by suggesting that using 2 cervical ripening agents together may in fact be superior.
Related Article:
Q: Following cesarean delivery, what is the optimal oxytocin infusion duration to prevent postpartum bleeding?
Details of the study
Schoen and colleagues conducted a randomized controlled trial that included 184 nulliparous and 139 multiparous women with an unfavorable cervix undergoing induction of labor after 24 weeks of gestation. All participants had a Foley catheter placed intracervically and then were randomly assigned to receive either concurrent oxytocin infusion within 60 minutes or no oxytocin until after Foley catheter expulsion or removal. Nulliparous and multiparous women were randomly assigned separately. Women with premature rupture of membranes and with 1 prior cesarean delivery were included in the trial, but women were excluded if they were in active labor, had suspected abruption, or had a nonreassuring fetal tracing.
The study was powered to detect a 20% increase in total delivery rate within 24 hours of Foley placement, which was the primary study outcome. Secondary induction outcomes of note included time to Foley expulsion, time to second stage, delivery within 12 hours, total time to delivery, duration of oxytocin use, and mode of delivery. Several maternal and neonatal outcomes also were examined, including tachysystole, chorioamnionitis, meconium, postpartum hemorrhage, birth weight, maternal intensive care unit (ICU) admission, and neonatal ICU admission.
Related Article:
Start offering antenatal corticosteroids to women delivering between 34 0/7 and 36 6/7 weeks of gestation to improve newborn outcomes
Women receiving concurrent Foley and oxytocin delivered sooner. Among nulliparous women, the overall rate of delivery within 24 hours of Foley catheter placement was 64% in the Foley with concurrent oxytocin group compared with 43% in those who received a Foley catheter alone followed by oxytocin (P = .003). The overall time to delivery was 5 hours less in nulliparous women who received combination cervical ripening compared with those who had a Foley catheter alone.
Similarly, multiparous women had an overall rate of delivery within 24 hours of 87% in the concurrent Foley and oxytocin group compared with 72% in women who received Foley catheter followed by oxytocin (P = .022).
Meanwhile, there were no statistically significant differences in mode of delivery between groups for either multiparous or nulliparous patients, and there were no differences in adverse maternal or neonatal outcomes between groups.
Related Article:
How and when umbilical cord gas analysis can justify your obstetric management
Study strengths and weaknesses
This well-designed, randomized control trial clearly demonstrated that the combination of Foley catheter and oxytocin for cervical ripening increases the rate of delivery within 24 hours compared with use of Foley catheter alone. This finding is consistent with those of 2 other large randomized trials in the past 2 years that similarly demonstrated reduced time to delivery when oxytocin infusion was used in combination with Foley catheter compared with Foley alone.1,2
Despite these findings, important questions remain regarding concurrent use of cervical ripening agents. The study by Schoen and colleagues does not address the other option for dual cervical ripening, namely, concurrent use of Foley catheter and misoprostol. Several large randomized trials using Foley catheter with vaginal or oral misoprostol demonstrated reduced time to delivery compared with using either method alone.1,3,4 Only 1 randomized study has compared these 2 dual cervical ripening regimens head-to-head; that study demonstrated that the misoprostol and Foley combination significantly reduced time to delivery compared with combining Foley catheter and oxytocin together.1
Additionally, it is important to note that the study by Schoen and colleagues was not large enough to adequately evaluate potential safety risks with dual combination cervical ripening. More safety data are needed before combination cervical ripening methods can be recommended universally.
-- Christina A. Penfield, MD, MPH, and Deborah A. Wing, MD, MBA
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Levine LD, Downes KL, Elovitz MA, Parry S, Sammel MD, Srinivas SK. Mechanical and pharmacologic methods of labor induction: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1357–1364.
- Connolly KA, Kohari KS, Rekawek P, et al. A randomized trial of Foley balloon induction of labor trial in nulliparas (FIAT-N). Am J Obstet Gynecol. 2016;215(3):392.e1–e6.
- Carbone JF, Tuuli MG, Fogertey PJ, Roehl KA, Macones GA. Combination of Foley bulb and vaginal misoprostol compared with vaginal misoprostol alone for cervical ripening and labor induction: a randomized controlled trial. Obstet Gynecol. 2013;121(2 pt 1):247–252.
- Hill JB, Thigpen BD, Bofill JA, Magann E, Moore LE, Martin JN Jr. A randomized clinical trial comparing vaginal misoprostol versus cervical Foley plus oral misoprostol for cervical ripening and labor induction. Am J Perinatol. 2009;26(1):33–38.
- Levine LD, Downes KL, Elovitz MA, Parry S, Sammel MD, Srinivas SK. Mechanical and pharmacologic methods of labor induction: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1357–1364.
- Connolly KA, Kohari KS, Rekawek P, et al. A randomized trial of Foley balloon induction of labor trial in nulliparas (FIAT-N). Am J Obstet Gynecol. 2016;215(3):392.e1–e6.
- Carbone JF, Tuuli MG, Fogertey PJ, Roehl KA, Macones GA. Combination of Foley bulb and vaginal misoprostol compared with vaginal misoprostol alone for cervical ripening and labor induction: a randomized controlled trial. Obstet Gynecol. 2013;121(2 pt 1):247–252.
- Hill JB, Thigpen BD, Bofill JA, Magann E, Moore LE, Martin JN Jr. A randomized clinical trial comparing vaginal misoprostol versus cervical Foley plus oral misoprostol for cervical ripening and labor induction. Am J Perinatol. 2009;26(1):33–38.
How to Increase HPV Vaccination Rates
CE/CME No: CR-1709
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand and identify the low- and high-risk human papillomavirus (HPV) types that can lead to benign and malignant manifestations.
• Know the recommended age range and dosing schedule for individuals who can and should receive the vaccination.
• Recognize important barriers to HPV vaccination in the health care setting.
• Understand how to promote HPV vaccination to parents/caregivers and patients.
• Find resources and educational material from national organizations that recommend and support HPV vaccination.
FACULTY
Tyler Cole practices at Coastal Community Health Services in Brunswick, Georgia, and is a clinical instructor in the DNP-APRN program at the Medical University of South Carolina (MUSC). Marie C. Thomas is a registered nurse on a surgical oncology unit at MUSC and will receive her DNP-FNP from MUSC in December 2017. Katlyn Straup practices at Roper St. Francis Healthcare and Southern Care Hospice in Charleston, South Carolina; she is also a clinical associate faculty member in the MUSC College of Nursing. Ashlyn Savage is an Associate Professor of Obstetrics and Gynecology at MUSC College of Nursing and is certified by the American Board of Obstetrics and Gynecology.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through August 31, 2018.
Article begins on next page >>
Although human papillomavirus (HPV) vaccine is a safe and effective means of preventing most HPV-related cancers, HPV vaccination rates lag well behind those of other vaccines recommended for children and adolescents. Understanding the barriers to HPV vaccine acceptance and effective strategies for overcoming them will improve vaccine uptake and completion in adolescents.
Human papillomavirus (HPV) infection is the most common sexually transmitted infection in the United States.1,2 HPV causes approximately 30,700 new cancer cases in the US annually.3 It is the primary cause of cervical cancer, which resulted in more than 4,000 deaths in the US in 2016.4 HPV is also associated with some vaginal, vulvar, penile, anal, and oropharyngeal cancers and causes anogenital warts.3
Although HPV vaccines are available to protect against infection with the HPV types that lead to these sequelae, HPV vaccination rates remain low compared with other routinely administered vaccines.5 Reasons for these lower rates include vaccine cost, lack of patient and provider education, providers’ failure to recommend, stigmas related to sexual behavior, and misconceptions about the vaccine, such as concerns about harm.5 This article discusses these barriers to better educate providers about the HPV vaccine and encourage them to assist in increasing vaccination rates.
EPIDEMIOLOGY
Approximately 79 million Americans are currently infected with HPV, and 14 million new cases are reported each year.2 In the US, the prevalence of HPV is highest among sexually active adolescents and young adults, especially those ages 20 to 24.2 Of the more than 150 types of HPV that have been identified, 40 infect the genital area. HPV genital infections are mainly spread through sexual intercourse but can also be spread through oral-to-genital contact.2
The genital HPV types are categorized as low-risk and high-risk based on their association with cervical cancer.2 High-risk types 16 and 18 are the most troublesome, accounting for 63% of all HPV-associated cancers, with HPV 16 posing the highest risk for cancer.3 High-risk types HPV 31, 33, 45, 52, and 58 account for another 10% of these cancers.3 Low-risk types, such as HPV 6 and 11, can cause low-grade cervical intraepithelial lesions, and HPV 6 and 11 account for more than 90% of genital warts.2
Most HPV infections, whether with high- or low-risk types, do not cause symptoms and resolve spontaneously in about two years.2 Persistent high-risk HPV infection is necessary for the development of cervical cancer precursor lesions—and therefore, once the infection has cleared, the risk for cancer declines.2
HPV VACCINES
Three HPV vaccines are licensed for use in the US: bivalent (Cervarix), quadrivalent (Gardasil), and 9-valent (Gardasil 9) vaccines (see Table 1).2,6,7 The bivalent, Cervarix, has recently been removed from the US market due to a decrease in product demand.6,8
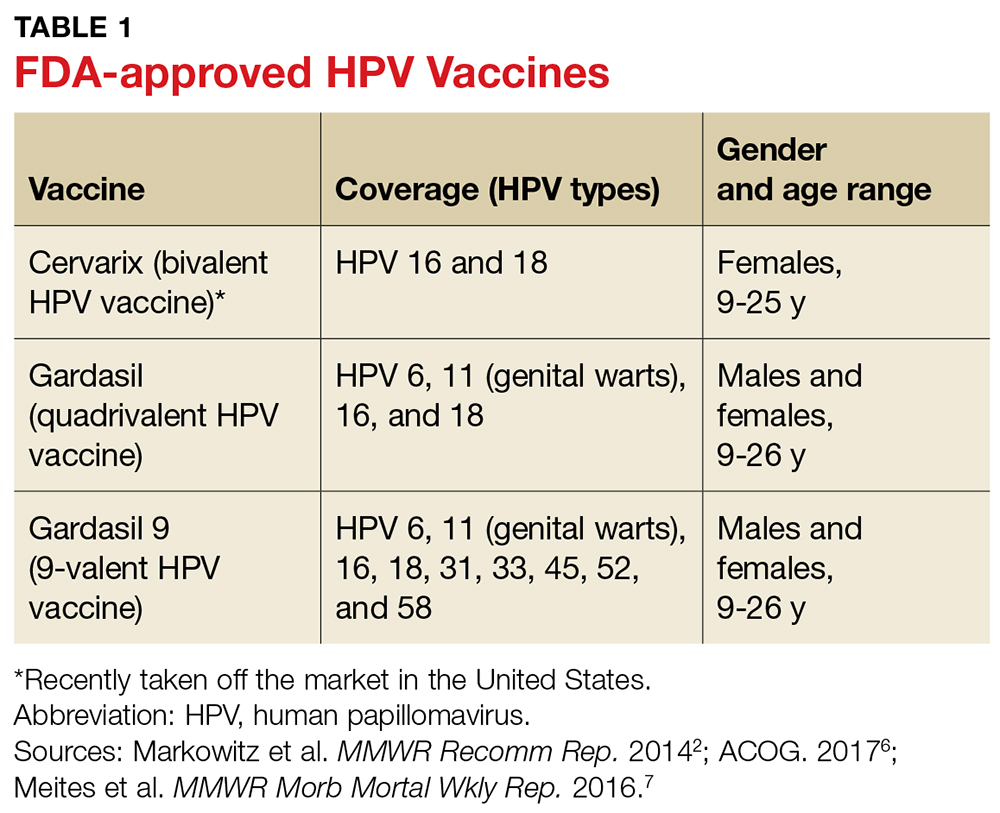
To ensure optimal protection, the vaccines must be administered in a series of scheduled doses over six to 12 months. The Advisory Committee on Immunization Practices (ACIP) recently updated their recommendations to include a two- or three-dose series based on age (see Table 2).7
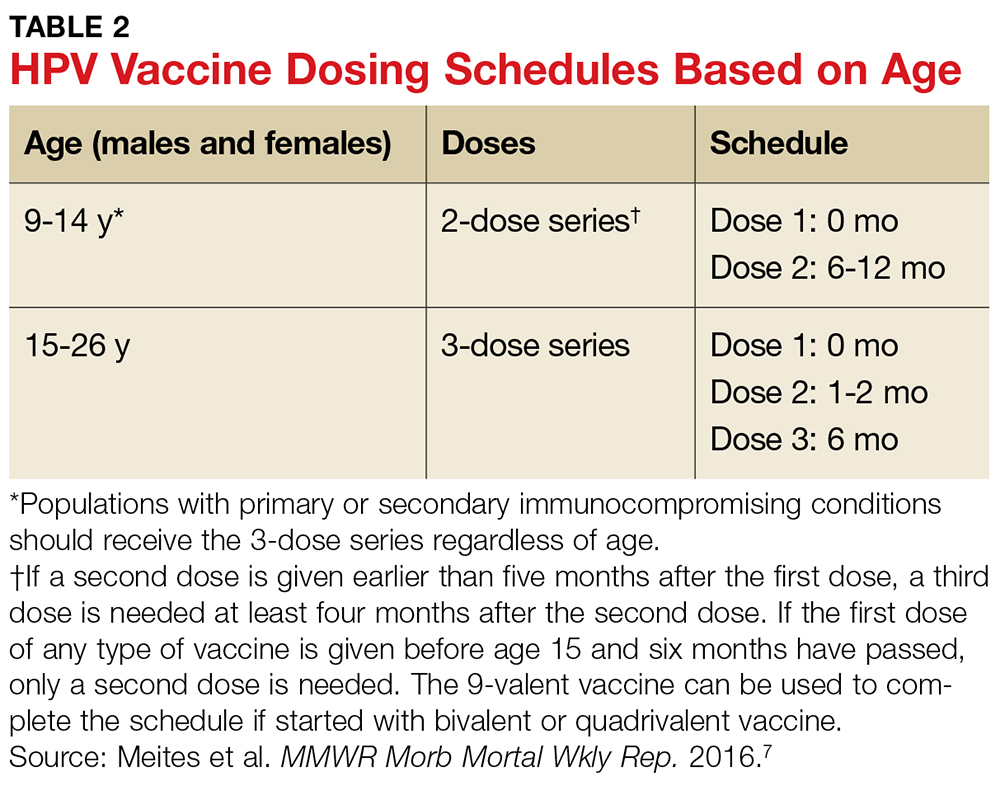
HPV vaccines are recommended for males and females between the ages of 9 and 26 years, but the ACIP and the American College of Obstetricians and Gynecologists (ACOG) strongly promote a targeted age range for vaccination between 11 and 12 years for both genders.6,7 Earlier vaccination is preferred because clinical data show a more rapid antibody response at a younger age, and because the vaccines are more effective if administered before an individual is exposed to or infected with HPV (ie, before the start of sexual activity).6,7
LOW VACCINATION RATES
HPV vaccination rates in the US are significantly lower than rates for other regularly administered vaccines; furthermore, they do not meet the Healthy People 2020 national goal of 80% for all vaccines.9 Immunization rates for most childhood vaccines range from 80% to 90%, but in 2015 only 28.1% of males and 41.9% of females ages 13 to 17 had completed the entire HPV vaccine series.9-11
The total HPV vaccination rates for male and female adolescents combined were 56.1% for one dose or more, 45.4% for two or more doses, and 34.9% for all three doses.9 In comparison, coverage rates for the meningococcal and Tdap (tetanus, diphtheria, and pertussis) immunizations, also recommended at the same age range as the HPV vaccine, were 81.3% and 86.4%, respectively.9
In addition to variation by gender and age, factors such as race, insurance coverage, and socioeconomic status influence vaccination rates.11 For the HPV vaccine specifically, Hispanic, non-Hispanic black, and American Indian/Alaska Native adolescents have higher rates of receiving each of the vaccine doses and higher rates of completing the vaccine series, compared to non-Hispanic white adolescents.9 Adolescents with Medicaid insurance and those living below the federal poverty level have better HPV vaccination coverage compared with adolescents with commercial insurance plans or those living at or above the poverty level.9,11
The HPV vaccine series completion rates in 2015 for males and females ages 13 to 17 living below the poverty level were 31.0% and 44.4%, respectively, compared to 27.4% and 41.3% for those living at or above the poverty level.9 One reason for increased rates among those living in lower-income households may be their eligibility for vaccinations at no cost through the Vaccines for Children (VFC) program, a federal program that provides vaccines to children who might otherwise forgo vaccination because of inability to pay.9
BARRIERS TO VACCINATION
Impediments that prevent adolescents and young adults from receiving the HPV vaccine exist throughout the vaccination process, with providers, parents, and the medical system itself contributing to low rates. Barriers to vaccination include fear and misconceptions, costs and socioeconomic status, lack of understanding and education, and logistic obstacles to completing the full series.5 Understanding these barriers, as well as discussing methods to overcome them, is key to increasing HPV vaccination rates and preventing the spread of this cancer-causing infection.
Health care provider barriers
Even though accredited national institutions and committees such as the CDC, ACIP, and ACOG strongly recommend vaccination based on current evidence, some health care providers still do not recommend the HPV vaccine to parents and patients.2,6,7,11 Lack of provider recommendation and the resulting lack of parental awareness of the vaccine account for many adolescents not receiving the vaccination.10,12
Providers do not recommend the vaccine for a number of reasons. Some have limited knowledge or conflicting ideas about the specific disease protection of the HPV vaccine, while others are hesitant to administer the vaccine before the onset of sexual activity, because they feel the suggested age for vaccination (11 to 12 years) is too young.10,11 Still other providers report difficulty approaching parents who they perceive as having concerns about the vaccine’s association with a sexually transmitted infection or believing that it might promote sexual activity.10
Some professionals simply claim that they forget to address the HPV vaccine at health visits, or that they propose it as optional and up to the parent’s discretion.5,10 Many providers do recommend and administer the initial dose of the vaccine, but have difficulties ensuring that patients complete the full multidose series.13 Evidence has shown that a strong provider recommendation is one of the most important incentives for parents and patients to accept vaccination.14
Parental and caregiver barriers
Lack of knowledge about the HPV vaccine and lack of recommendation from providers are two top reasons parents and caregivers cite for not vaccinating their children.5,10,14,15 In a national survey, almost all parents whose daughters completed the full vaccination series reported being counseled by their provider on the appropriate age for vaccination and the timeline of the series.14
Fears and apprehensions about side effects, especially with newer vaccines, can prevent some parents from having their children vaccinated.15 Although there is some stigma related to the vaccine’s association with the sexually transmitted HPV, this is a much less significant barrier than lack of provider recommendation or knowledge about the vaccine.5,11
Health care system barriers
Both providers and parents agree that system-level issues such as access, follow-up, and cost are barriers to initiating or completing the vaccination series.11,13 Many adolescents have few opportunities to receive the vaccine because they do not have a primary care provider.11 For those with access to primary care, visits are often problem-focused and frequently do not include a review of immunization history.13 Health care professionals also report challenges with scheduling follow-up visits for the second and third doses to complete the series within the recommended timeframe.13
Cost, insurance coverage, and reimbursement pose additional hurdles for both providers and patients, with some providers citing concerns about the cost of stocking the vaccine.16 Providers, both family practice providers and gynecologists, agree that reimbursement for administering the HPV vaccine in office poses a barrier when recommending the vaccine to patients.17 Lack of insurance coverage and type of insurance also pose barriers, with Medicaid patients more often completing the full series compared to those with private or no insurance, because Medicaid covers the cost of vaccination for men up to age 19.9,18 A national survey of males ages 9 to 17 found that the percentage of HPV vaccine initiation was double for those with public insurance compared to those with private insurance.19 Changes at the system-level, such as participation in the VFC program, in coordination with better provider recommendation should help increase HPV vaccination rates.9,11
STRATEGIES TO IMPROVE VACCINATION RATES
Many strategies for increasing HPV vaccination acceptance, decreasing barriers to access, and improving compliance with vaccine completion have been reported in the literature, with some strategies achieving more success than others. This section discusses interventions and strategies designed to help overcome provider-, parent-, and system-related barriers that have been shown to be effective (see Table 3).
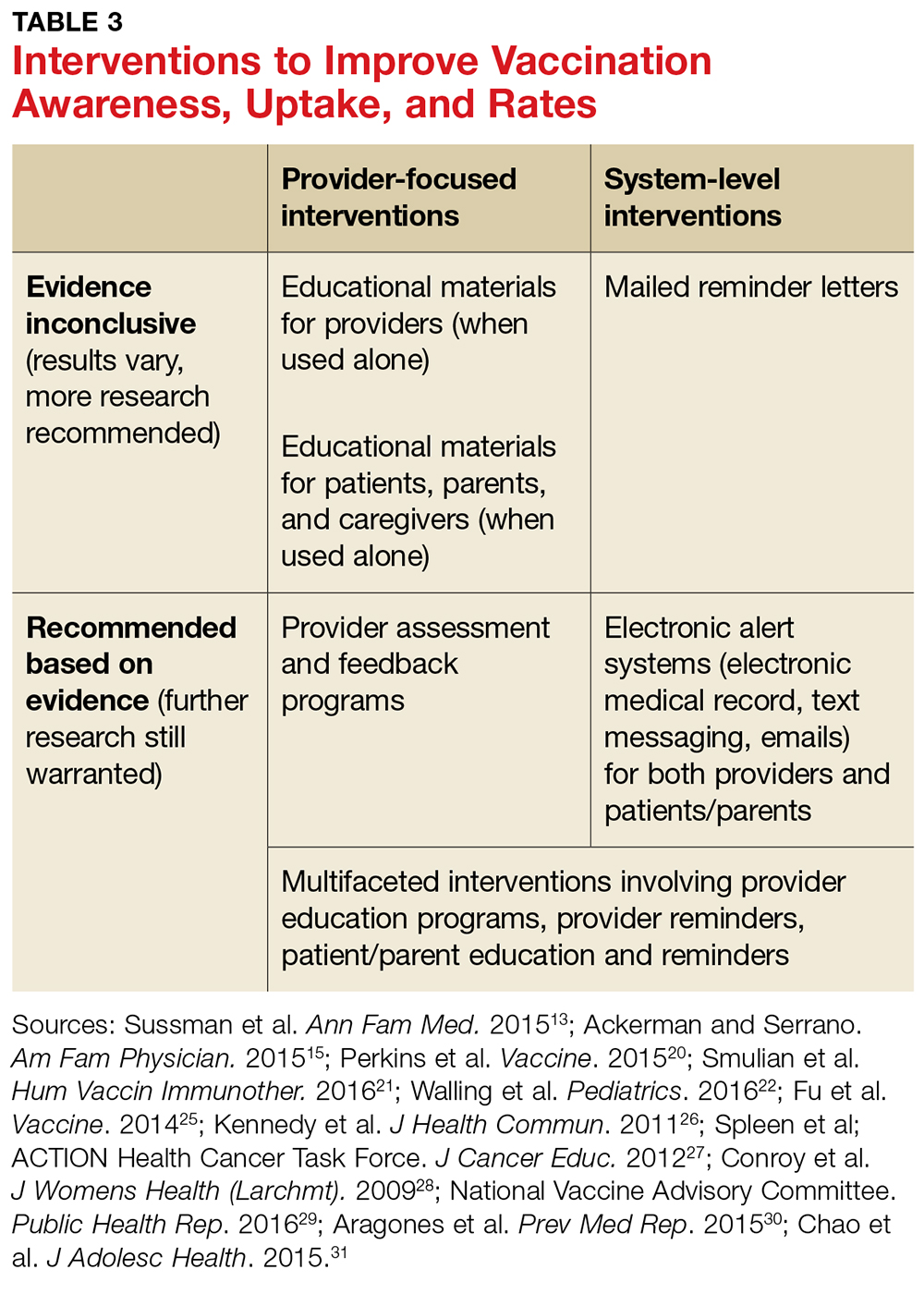
Health care provider interventions
Evidence supports a number of provider-level strategies to increase HPV vaccination rates (see Table 3). An improvement in vaccination acceptance was observed when providers promoted the vaccine as a safe, effective way to prevent cancer, rather than as a means to prevent a sexually transmitted infection.10,11,20
Some primary care providers found that encouraging the HPV vaccine at the same time as the meningococcal and Tdap vaccinations, which are also recommended at age 11 to 12 years, increased vaccination rates as well.13,20 Another successful strategy is reviewing vaccination history at every visit, whether the visit is for an acute event or an annual well exam.10,13,20 These tactics are most useful when providers practice them consistently, which may require them to change or adapt their way of practice.
Provider-based trainings that educate and prepare them to consistently recommend the vaccine have demonstrated success in increasing HPV vaccination uptake.21,22 The CDC’s Assessment/Feedback/Incentive/eXchange (AFIX) quality improvement program to increase vaccination rates, which includes Web-based or in-person consults, has been shown to increase HPV vaccination rates.20-23 The Assessment phase of the AFIX program determines a practice’s current immunization practices and rates, while the Feedback portion provides strategies for increasing vaccination rates.23 A study by Perkins and colleagues utilized AFIX strategies, specifically for the HPV vaccine, such as focusing provider education on HPV-related cancers and vaccine efficacy, as well as preparing providers to discuss and answer questions through basic motivational interviewing tactics.20
The CDC also offers PowerPoint presentations, flyers, posters, videos, and other informational resources to guide and educate providers, parents, and patients about the HPV vaccine.24 Educational resources, such as pamphlets, flyers, or fact sheets given to parents and patients, have been shown to improve intent to vaccinate as well as awareness of the vaccine.25-27
Although Fu and colleagues in a systematic review concluded that there was insufficient data to support a specific educational intervention for widespread use, the authors did recommend utilizing educational pieces and adapting them to specific populations.25 These simple interventions help increase awareness and can be implemented with other interventions in health care offices by providers and other staff.
System-level interventions
The use of systems that track patients for necessary vaccines and remind providers, parents, and patients about vaccine appointments have increased vaccination rates.13,28 Facility-based interventions, such as electronic medical records (EMR) that track patients for scheduled vaccines and remind providers when patients are due for vaccinations, will help increase provider recommendations and completion of the entire vaccination series.13
The National Vaccine Advisory Committee (NVAC) suggests that provider offices implement reminder-recall systems and provide educational material for parents and patients to increase vaccination rates.29 One specific study using both educational material and text-message reminders for parents found that these interventions significantly increased vaccination rates.30 Health care facilities could also incorporate reminder letters mailed to patients and parents to promote vaccine initiation and completion.31 The evidence supports the use of reminder alerts and EMR tracking systems to increase rates, but more research is warranted to determine the most cost-effective approach.
National programs, committees, and organizations have provided recommendations for overcoming system-related barriers to HPV vaccination, such as access and cost.29,32 The NVAC recommends incorporating alternative venues for vaccination delivery, such as pharmacies, schools, and health clinics, to increase availability to the adolescent population, especially to those who do not have primary care providers.29 One study that addressed parental opinions of vaccination administration in schools found that the majority of parents were in favor of this type of program.33 Although these recommendations seem promising and are accepted by parents, logistical barriers such as reimbursement to the pharmacies, schools, and clinics and accurate documentation of the doses received need to be addressed.29 The NVAC recommends continued evaluation and efforts to develop these programs in the future.29
In addition to school-based interventions, providing home visits for vaccination and implementing standing orders are other suggestions to overcome access and cost barriers for vaccinations, including HPV.32 Standing orders allow for individuals to receive a vaccine by a health care professional in an approved institution, where allowed by state law.32 This provides easier access to vaccinations, especially for those who do not see a primary care provider.
Although some of the system-level interventions mentioned in this article are outside the realm of what providers can do in the office, understanding and advocating for these advancements will promote vaccine uptake.
CONCLUSION
Lack of provider recommendation, coupled with poor or no parental knowledge about the HPV vaccine, are significant factors affecting vaccination uptake. Evidence supports the use of multifaceted interventions that promote and support provider recommendation and parent/patient education. Studies of interventions that incorporated educational resources and alert systems for both providers and patients or their caregivers have shown these strategies to be effective in increasing vaccination uptake and completion.
In addition to recommending the HPV vaccine, providers must educate parents/caregivers and patients about it, particularly by presenting the vaccine as a means of cancer prevention. Primary care facilities should implement reminder plans and provide educational literature to promote vaccine uptake. Although the interventions highlighted here have increased HPV vaccination rates, further research is warranted to evaluate more effective strategies for overcoming barriers and to determine which strategies are most cost-effective.
1. Juckett G, Hartsman-Adams H. Human papillomavirus: clinical manifestations and prevention. Am Fam Physician. 2010;82(10):1209-1213.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1-30.
3. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661-666.
4. American Cancer Society. Cancer facts and figures 2016. Atlanta: American Cancer Society; 2016.
5. Holman DM, Benard V, Roland KB, et al. Barriers to the human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76-82.
6. American College of Obstetricians and Gynecologists. Human papillomavirus vaccination. Committee Opinion. Number 704. June 2017. www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Adolescent-Health-Care/Human-Papillomavirus-Vaccination. Accessed August 17, 2017.
7. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendation of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405-1408.
8. Mulcahy N. GSK’s HPV vaccine, Cervarix, no longer available in the US. Medscape Medical News. October 26, 2016. www.medscape.com/viewarticle/870853. Accessed June 11, 2017.
9. Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784-792.
10. Muncie HL, Lebato AL. HPV vaccination: overcoming parental and physician impediments. Am Fam Physician. 2015;92(6):449-454.
11. Bratic JS, Seyferth ER, Bocchini JA Jr. Update on barriers to human papillomavirus vaccination and effective strategies to promote vaccine acceptance. Curr Opin Pediatr. 2016;28(3):407-412.
12. Rahman M, Laz TH, McGrath CJ, Berenson AB. Provider recommendation mediates the relationship between parental human papillomavirus (HPV) vaccine awareness and HPV vaccine initiation and completion among 13- to 17-year-old US adolescent children. Clin Pediatr. 2015;54(4):371-375.
13. Sussman AL, Helitzer D, Bennett A, et al. Catching up with the HPV vaccine: challenges and opportunities in primary care. Ann Fam Med. 2015;13(4):354-360.
14. Clark SJ, Cowan AE, Filipp SL, et al. Parent perception of provider interactions influences HPV vaccination status of adolescent females. Clin Pediatr. 2016;55(8):701-706.
15. Ackerman LK, Serrano JL. Update on routine childhood and adolescent immunizations. Am Fam Physician. 2015;92(6):460-468.
16. Tom A, Robinett H, Buenconsejo-Lum L, et al. Promoting and providing the HPV vaccination in Hawaii: barriers faced by health providers. J Community Health. 2016;41(5):1069-1077.
17. Young JL, Bernheim RG, Korte JE, et al. Human papillomavirus vaccination recommendation may be linked to reimbursement: A survey of Virginia family practitioners and gynecologists. J Pediat Adolesc Gynecol. 2011;24(6):380-385.
18. Thomas R, Higgins L, Ding L, et al. Factors associated with HPV vaccine initiation, vaccine completion, and accuracy of self-reported vaccination status among 13- to 26-year-old men. Am J Mens Health. 2016;1-9.
19. Laz TH, Rahman M, Berenson AB. Human papillomavirus vaccine uptake among 9-17 year old males in the United States: the National Health Interview Survey, 2010. Hum Vaccin Immunother. 2013;9(4):874-878.
20. Perkins RB, Zisblatt L, Legler A, et al. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine. 2015;33:1223-1229.
21. Smulian EA, Mitchell KR, Stokley S. Interventions to increase HPV vaccination coverage: a systematic review. Hum Vaccin Immunother. 2016;12:1566-1588.
22. Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics. 2016;138(1).
23. CDC. Overview of AFIX. www.cdc.gov/vaccines/programs/afix/index.html. Accessed August 17, 2017.
24. CDC. Human papillomavirus (HPV). www.cdc.gov/hpv/. Accessed August 17, 2017.
25. Fu LY, Bonhomme LA, Cooper SC, et al. Educational interventions to increase HPV vaccination acceptance: a systematic review. Vaccine. 2014;32(17):1901-1920.
26. Kennedy A, Sapsis KF, Stokley S, et al. Parental attitudes toward human papillomavirus vaccination: evaluation of an educational intervention, 2008. J Health Commun. 2011;16(3):300-313.
27. Spleen AM, Kluhsman BC, Clark AD, et al; ACTION Health Cancer Task Force. An increase in HPV-related knowledge and vaccination intent among parental and non-parental caregivers of adolescent girls, ages 9-17 years, in Appalachian Pennsylvania. J Cancer Educ. 2012;27(2):312-319.
28. Conroy K, Rosenthal SL, Zimet GD, et al. Human papillomavirus vaccine uptake, predictors of vaccination, and self-reported barriers to vaccination. J Womens Health (Larchmt). 2009;18(10):1679-1686.
29. National Vaccine Advisory Committee. Overcoming barriers to low HPV vaccine uptake in the United States: recommendations from the National Vaccine Advisory Committee. Public Health Rep. 2016;131(1):17-25.
30. Aragones A, Bruno DM, Ehrenberg M, et al. Parental education and text messaging reminders as effective community based tools to increase HPV vaccination rates among Mexican American children. Prev Med Rep. 2015;2:554-558.
31. Chao C, Preciado M, Slezak J, Xu L. A randomized intervention of reminder letter for human papillomavirus vaccine series completion. J Adolesc Health. 2015;56(1):85-90.
32. Community Preventive Services Task Force. The community guide—guide to community preventive services: increasing appropriate vaccinations. Atlanta, GA: Community Preventive Services Task Force; 2016
33. Kelminson K, Saville A, Seewald L, et al. Parental views of school-located delivery of adolescent vaccines. J Adolesc Health. 2012;51(2):190-196.
CE/CME No: CR-1709
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand and identify the low- and high-risk human papillomavirus (HPV) types that can lead to benign and malignant manifestations.
• Know the recommended age range and dosing schedule for individuals who can and should receive the vaccination.
• Recognize important barriers to HPV vaccination in the health care setting.
• Understand how to promote HPV vaccination to parents/caregivers and patients.
• Find resources and educational material from national organizations that recommend and support HPV vaccination.
FACULTY
Tyler Cole practices at Coastal Community Health Services in Brunswick, Georgia, and is a clinical instructor in the DNP-APRN program at the Medical University of South Carolina (MUSC). Marie C. Thomas is a registered nurse on a surgical oncology unit at MUSC and will receive her DNP-FNP from MUSC in December 2017. Katlyn Straup practices at Roper St. Francis Healthcare and Southern Care Hospice in Charleston, South Carolina; she is also a clinical associate faculty member in the MUSC College of Nursing. Ashlyn Savage is an Associate Professor of Obstetrics and Gynecology at MUSC College of Nursing and is certified by the American Board of Obstetrics and Gynecology.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through August 31, 2018.
Article begins on next page >>
Although human papillomavirus (HPV) vaccine is a safe and effective means of preventing most HPV-related cancers, HPV vaccination rates lag well behind those of other vaccines recommended for children and adolescents. Understanding the barriers to HPV vaccine acceptance and effective strategies for overcoming them will improve vaccine uptake and completion in adolescents.
Human papillomavirus (HPV) infection is the most common sexually transmitted infection in the United States.1,2 HPV causes approximately 30,700 new cancer cases in the US annually.3 It is the primary cause of cervical cancer, which resulted in more than 4,000 deaths in the US in 2016.4 HPV is also associated with some vaginal, vulvar, penile, anal, and oropharyngeal cancers and causes anogenital warts.3
Although HPV vaccines are available to protect against infection with the HPV types that lead to these sequelae, HPV vaccination rates remain low compared with other routinely administered vaccines.5 Reasons for these lower rates include vaccine cost, lack of patient and provider education, providers’ failure to recommend, stigmas related to sexual behavior, and misconceptions about the vaccine, such as concerns about harm.5 This article discusses these barriers to better educate providers about the HPV vaccine and encourage them to assist in increasing vaccination rates.
EPIDEMIOLOGY
Approximately 79 million Americans are currently infected with HPV, and 14 million new cases are reported each year.2 In the US, the prevalence of HPV is highest among sexually active adolescents and young adults, especially those ages 20 to 24.2 Of the more than 150 types of HPV that have been identified, 40 infect the genital area. HPV genital infections are mainly spread through sexual intercourse but can also be spread through oral-to-genital contact.2
The genital HPV types are categorized as low-risk and high-risk based on their association with cervical cancer.2 High-risk types 16 and 18 are the most troublesome, accounting for 63% of all HPV-associated cancers, with HPV 16 posing the highest risk for cancer.3 High-risk types HPV 31, 33, 45, 52, and 58 account for another 10% of these cancers.3 Low-risk types, such as HPV 6 and 11, can cause low-grade cervical intraepithelial lesions, and HPV 6 and 11 account for more than 90% of genital warts.2
Most HPV infections, whether with high- or low-risk types, do not cause symptoms and resolve spontaneously in about two years.2 Persistent high-risk HPV infection is necessary for the development of cervical cancer precursor lesions—and therefore, once the infection has cleared, the risk for cancer declines.2
HPV VACCINES
Three HPV vaccines are licensed for use in the US: bivalent (Cervarix), quadrivalent (Gardasil), and 9-valent (Gardasil 9) vaccines (see Table 1).2,6,7 The bivalent, Cervarix, has recently been removed from the US market due to a decrease in product demand.6,8

To ensure optimal protection, the vaccines must be administered in a series of scheduled doses over six to 12 months. The Advisory Committee on Immunization Practices (ACIP) recently updated their recommendations to include a two- or three-dose series based on age (see Table 2).7

HPV vaccines are recommended for males and females between the ages of 9 and 26 years, but the ACIP and the American College of Obstetricians and Gynecologists (ACOG) strongly promote a targeted age range for vaccination between 11 and 12 years for both genders.6,7 Earlier vaccination is preferred because clinical data show a more rapid antibody response at a younger age, and because the vaccines are more effective if administered before an individual is exposed to or infected with HPV (ie, before the start of sexual activity).6,7
LOW VACCINATION RATES
HPV vaccination rates in the US are significantly lower than rates for other regularly administered vaccines; furthermore, they do not meet the Healthy People 2020 national goal of 80% for all vaccines.9 Immunization rates for most childhood vaccines range from 80% to 90%, but in 2015 only 28.1% of males and 41.9% of females ages 13 to 17 had completed the entire HPV vaccine series.9-11
The total HPV vaccination rates for male and female adolescents combined were 56.1% for one dose or more, 45.4% for two or more doses, and 34.9% for all three doses.9 In comparison, coverage rates for the meningococcal and Tdap (tetanus, diphtheria, and pertussis) immunizations, also recommended at the same age range as the HPV vaccine, were 81.3% and 86.4%, respectively.9
In addition to variation by gender and age, factors such as race, insurance coverage, and socioeconomic status influence vaccination rates.11 For the HPV vaccine specifically, Hispanic, non-Hispanic black, and American Indian/Alaska Native adolescents have higher rates of receiving each of the vaccine doses and higher rates of completing the vaccine series, compared to non-Hispanic white adolescents.9 Adolescents with Medicaid insurance and those living below the federal poverty level have better HPV vaccination coverage compared with adolescents with commercial insurance plans or those living at or above the poverty level.9,11
The HPV vaccine series completion rates in 2015 for males and females ages 13 to 17 living below the poverty level were 31.0% and 44.4%, respectively, compared to 27.4% and 41.3% for those living at or above the poverty level.9 One reason for increased rates among those living in lower-income households may be their eligibility for vaccinations at no cost through the Vaccines for Children (VFC) program, a federal program that provides vaccines to children who might otherwise forgo vaccination because of inability to pay.9
BARRIERS TO VACCINATION
Impediments that prevent adolescents and young adults from receiving the HPV vaccine exist throughout the vaccination process, with providers, parents, and the medical system itself contributing to low rates. Barriers to vaccination include fear and misconceptions, costs and socioeconomic status, lack of understanding and education, and logistic obstacles to completing the full series.5 Understanding these barriers, as well as discussing methods to overcome them, is key to increasing HPV vaccination rates and preventing the spread of this cancer-causing infection.
Health care provider barriers
Even though accredited national institutions and committees such as the CDC, ACIP, and ACOG strongly recommend vaccination based on current evidence, some health care providers still do not recommend the HPV vaccine to parents and patients.2,6,7,11 Lack of provider recommendation and the resulting lack of parental awareness of the vaccine account for many adolescents not receiving the vaccination.10,12
Providers do not recommend the vaccine for a number of reasons. Some have limited knowledge or conflicting ideas about the specific disease protection of the HPV vaccine, while others are hesitant to administer the vaccine before the onset of sexual activity, because they feel the suggested age for vaccination (11 to 12 years) is too young.10,11 Still other providers report difficulty approaching parents who they perceive as having concerns about the vaccine’s association with a sexually transmitted infection or believing that it might promote sexual activity.10
Some professionals simply claim that they forget to address the HPV vaccine at health visits, or that they propose it as optional and up to the parent’s discretion.5,10 Many providers do recommend and administer the initial dose of the vaccine, but have difficulties ensuring that patients complete the full multidose series.13 Evidence has shown that a strong provider recommendation is one of the most important incentives for parents and patients to accept vaccination.14
Parental and caregiver barriers
Lack of knowledge about the HPV vaccine and lack of recommendation from providers are two top reasons parents and caregivers cite for not vaccinating their children.5,10,14,15 In a national survey, almost all parents whose daughters completed the full vaccination series reported being counseled by their provider on the appropriate age for vaccination and the timeline of the series.14
Fears and apprehensions about side effects, especially with newer vaccines, can prevent some parents from having their children vaccinated.15 Although there is some stigma related to the vaccine’s association with the sexually transmitted HPV, this is a much less significant barrier than lack of provider recommendation or knowledge about the vaccine.5,11
Health care system barriers
Both providers and parents agree that system-level issues such as access, follow-up, and cost are barriers to initiating or completing the vaccination series.11,13 Many adolescents have few opportunities to receive the vaccine because they do not have a primary care provider.11 For those with access to primary care, visits are often problem-focused and frequently do not include a review of immunization history.13 Health care professionals also report challenges with scheduling follow-up visits for the second and third doses to complete the series within the recommended timeframe.13
Cost, insurance coverage, and reimbursement pose additional hurdles for both providers and patients, with some providers citing concerns about the cost of stocking the vaccine.16 Providers, both family practice providers and gynecologists, agree that reimbursement for administering the HPV vaccine in office poses a barrier when recommending the vaccine to patients.17 Lack of insurance coverage and type of insurance also pose barriers, with Medicaid patients more often completing the full series compared to those with private or no insurance, because Medicaid covers the cost of vaccination for men up to age 19.9,18 A national survey of males ages 9 to 17 found that the percentage of HPV vaccine initiation was double for those with public insurance compared to those with private insurance.19 Changes at the system-level, such as participation in the VFC program, in coordination with better provider recommendation should help increase HPV vaccination rates.9,11
STRATEGIES TO IMPROVE VACCINATION RATES
Many strategies for increasing HPV vaccination acceptance, decreasing barriers to access, and improving compliance with vaccine completion have been reported in the literature, with some strategies achieving more success than others. This section discusses interventions and strategies designed to help overcome provider-, parent-, and system-related barriers that have been shown to be effective (see Table 3).

Health care provider interventions
Evidence supports a number of provider-level strategies to increase HPV vaccination rates (see Table 3). An improvement in vaccination acceptance was observed when providers promoted the vaccine as a safe, effective way to prevent cancer, rather than as a means to prevent a sexually transmitted infection.10,11,20
Some primary care providers found that encouraging the HPV vaccine at the same time as the meningococcal and Tdap vaccinations, which are also recommended at age 11 to 12 years, increased vaccination rates as well.13,20 Another successful strategy is reviewing vaccination history at every visit, whether the visit is for an acute event or an annual well exam.10,13,20 These tactics are most useful when providers practice them consistently, which may require them to change or adapt their way of practice.
Provider-based trainings that educate and prepare them to consistently recommend the vaccine have demonstrated success in increasing HPV vaccination uptake.21,22 The CDC’s Assessment/Feedback/Incentive/eXchange (AFIX) quality improvement program to increase vaccination rates, which includes Web-based or in-person consults, has been shown to increase HPV vaccination rates.20-23 The Assessment phase of the AFIX program determines a practice’s current immunization practices and rates, while the Feedback portion provides strategies for increasing vaccination rates.23 A study by Perkins and colleagues utilized AFIX strategies, specifically for the HPV vaccine, such as focusing provider education on HPV-related cancers and vaccine efficacy, as well as preparing providers to discuss and answer questions through basic motivational interviewing tactics.20
The CDC also offers PowerPoint presentations, flyers, posters, videos, and other informational resources to guide and educate providers, parents, and patients about the HPV vaccine.24 Educational resources, such as pamphlets, flyers, or fact sheets given to parents and patients, have been shown to improve intent to vaccinate as well as awareness of the vaccine.25-27
Although Fu and colleagues in a systematic review concluded that there was insufficient data to support a specific educational intervention for widespread use, the authors did recommend utilizing educational pieces and adapting them to specific populations.25 These simple interventions help increase awareness and can be implemented with other interventions in health care offices by providers and other staff.

System-level interventions
The use of systems that track patients for necessary vaccines and remind providers, parents, and patients about vaccine appointments have increased vaccination rates.13,28 Facility-based interventions, such as electronic medical records (EMR) that track patients for scheduled vaccines and remind providers when patients are due for vaccinations, will help increase provider recommendations and completion of the entire vaccination series.13
The National Vaccine Advisory Committee (NVAC) suggests that provider offices implement reminder-recall systems and provide educational material for parents and patients to increase vaccination rates.29 One specific study using both educational material and text-message reminders for parents found that these interventions significantly increased vaccination rates.30 Health care facilities could also incorporate reminder letters mailed to patients and parents to promote vaccine initiation and completion.31 The evidence supports the use of reminder alerts and EMR tracking systems to increase rates, but more research is warranted to determine the most cost-effective approach.
National programs, committees, and organizations have provided recommendations for overcoming system-related barriers to HPV vaccination, such as access and cost.29,32 The NVAC recommends incorporating alternative venues for vaccination delivery, such as pharmacies, schools, and health clinics, to increase availability to the adolescent population, especially to those who do not have primary care providers.29 One study that addressed parental opinions of vaccination administration in schools found that the majority of parents were in favor of this type of program.33 Although these recommendations seem promising and are accepted by parents, logistical barriers such as reimbursement to the pharmacies, schools, and clinics and accurate documentation of the doses received need to be addressed.29 The NVAC recommends continued evaluation and efforts to develop these programs in the future.29
In addition to school-based interventions, providing home visits for vaccination and implementing standing orders are other suggestions to overcome access and cost barriers for vaccinations, including HPV.32 Standing orders allow for individuals to receive a vaccine by a health care professional in an approved institution, where allowed by state law.32 This provides easier access to vaccinations, especially for those who do not see a primary care provider.
Although some of the system-level interventions mentioned in this article are outside the realm of what providers can do in the office, understanding and advocating for these advancements will promote vaccine uptake.
CONCLUSION
Lack of provider recommendation, coupled with poor or no parental knowledge about the HPV vaccine, are significant factors affecting vaccination uptake. Evidence supports the use of multifaceted interventions that promote and support provider recommendation and parent/patient education. Studies of interventions that incorporated educational resources and alert systems for both providers and patients or their caregivers have shown these strategies to be effective in increasing vaccination uptake and completion.
In addition to recommending the HPV vaccine, providers must educate parents/caregivers and patients about it, particularly by presenting the vaccine as a means of cancer prevention. Primary care facilities should implement reminder plans and provide educational literature to promote vaccine uptake. Although the interventions highlighted here have increased HPV vaccination rates, further research is warranted to evaluate more effective strategies for overcoming barriers and to determine which strategies are most cost-effective.
CE/CME No: CR-1709
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand and identify the low- and high-risk human papillomavirus (HPV) types that can lead to benign and malignant manifestations.
• Know the recommended age range and dosing schedule for individuals who can and should receive the vaccination.
• Recognize important barriers to HPV vaccination in the health care setting.
• Understand how to promote HPV vaccination to parents/caregivers and patients.
• Find resources and educational material from national organizations that recommend and support HPV vaccination.
FACULTY
Tyler Cole practices at Coastal Community Health Services in Brunswick, Georgia, and is a clinical instructor in the DNP-APRN program at the Medical University of South Carolina (MUSC). Marie C. Thomas is a registered nurse on a surgical oncology unit at MUSC and will receive her DNP-FNP from MUSC in December 2017. Katlyn Straup practices at Roper St. Francis Healthcare and Southern Care Hospice in Charleston, South Carolina; she is also a clinical associate faculty member in the MUSC College of Nursing. Ashlyn Savage is an Associate Professor of Obstetrics and Gynecology at MUSC College of Nursing and is certified by the American Board of Obstetrics and Gynecology.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through August 31, 2018.
Article begins on next page >>
Although human papillomavirus (HPV) vaccine is a safe and effective means of preventing most HPV-related cancers, HPV vaccination rates lag well behind those of other vaccines recommended for children and adolescents. Understanding the barriers to HPV vaccine acceptance and effective strategies for overcoming them will improve vaccine uptake and completion in adolescents.
Human papillomavirus (HPV) infection is the most common sexually transmitted infection in the United States.1,2 HPV causes approximately 30,700 new cancer cases in the US annually.3 It is the primary cause of cervical cancer, which resulted in more than 4,000 deaths in the US in 2016.4 HPV is also associated with some vaginal, vulvar, penile, anal, and oropharyngeal cancers and causes anogenital warts.3
Although HPV vaccines are available to protect against infection with the HPV types that lead to these sequelae, HPV vaccination rates remain low compared with other routinely administered vaccines.5 Reasons for these lower rates include vaccine cost, lack of patient and provider education, providers’ failure to recommend, stigmas related to sexual behavior, and misconceptions about the vaccine, such as concerns about harm.5 This article discusses these barriers to better educate providers about the HPV vaccine and encourage them to assist in increasing vaccination rates.
EPIDEMIOLOGY
Approximately 79 million Americans are currently infected with HPV, and 14 million new cases are reported each year.2 In the US, the prevalence of HPV is highest among sexually active adolescents and young adults, especially those ages 20 to 24.2 Of the more than 150 types of HPV that have been identified, 40 infect the genital area. HPV genital infections are mainly spread through sexual intercourse but can also be spread through oral-to-genital contact.2
The genital HPV types are categorized as low-risk and high-risk based on their association with cervical cancer.2 High-risk types 16 and 18 are the most troublesome, accounting for 63% of all HPV-associated cancers, with HPV 16 posing the highest risk for cancer.3 High-risk types HPV 31, 33, 45, 52, and 58 account for another 10% of these cancers.3 Low-risk types, such as HPV 6 and 11, can cause low-grade cervical intraepithelial lesions, and HPV 6 and 11 account for more than 90% of genital warts.2
Most HPV infections, whether with high- or low-risk types, do not cause symptoms and resolve spontaneously in about two years.2 Persistent high-risk HPV infection is necessary for the development of cervical cancer precursor lesions—and therefore, once the infection has cleared, the risk for cancer declines.2
HPV VACCINES
Three HPV vaccines are licensed for use in the US: bivalent (Cervarix), quadrivalent (Gardasil), and 9-valent (Gardasil 9) vaccines (see Table 1).2,6,7 The bivalent, Cervarix, has recently been removed from the US market due to a decrease in product demand.6,8

To ensure optimal protection, the vaccines must be administered in a series of scheduled doses over six to 12 months. The Advisory Committee on Immunization Practices (ACIP) recently updated their recommendations to include a two- or three-dose series based on age (see Table 2).7

HPV vaccines are recommended for males and females between the ages of 9 and 26 years, but the ACIP and the American College of Obstetricians and Gynecologists (ACOG) strongly promote a targeted age range for vaccination between 11 and 12 years for both genders.6,7 Earlier vaccination is preferred because clinical data show a more rapid antibody response at a younger age, and because the vaccines are more effective if administered before an individual is exposed to or infected with HPV (ie, before the start of sexual activity).6,7
LOW VACCINATION RATES
HPV vaccination rates in the US are significantly lower than rates for other regularly administered vaccines; furthermore, they do not meet the Healthy People 2020 national goal of 80% for all vaccines.9 Immunization rates for most childhood vaccines range from 80% to 90%, but in 2015 only 28.1% of males and 41.9% of females ages 13 to 17 had completed the entire HPV vaccine series.9-11
The total HPV vaccination rates for male and female adolescents combined were 56.1% for one dose or more, 45.4% for two or more doses, and 34.9% for all three doses.9 In comparison, coverage rates for the meningococcal and Tdap (tetanus, diphtheria, and pertussis) immunizations, also recommended at the same age range as the HPV vaccine, were 81.3% and 86.4%, respectively.9
In addition to variation by gender and age, factors such as race, insurance coverage, and socioeconomic status influence vaccination rates.11 For the HPV vaccine specifically, Hispanic, non-Hispanic black, and American Indian/Alaska Native adolescents have higher rates of receiving each of the vaccine doses and higher rates of completing the vaccine series, compared to non-Hispanic white adolescents.9 Adolescents with Medicaid insurance and those living below the federal poverty level have better HPV vaccination coverage compared with adolescents with commercial insurance plans or those living at or above the poverty level.9,11
The HPV vaccine series completion rates in 2015 for males and females ages 13 to 17 living below the poverty level were 31.0% and 44.4%, respectively, compared to 27.4% and 41.3% for those living at or above the poverty level.9 One reason for increased rates among those living in lower-income households may be their eligibility for vaccinations at no cost through the Vaccines for Children (VFC) program, a federal program that provides vaccines to children who might otherwise forgo vaccination because of inability to pay.9
BARRIERS TO VACCINATION
Impediments that prevent adolescents and young adults from receiving the HPV vaccine exist throughout the vaccination process, with providers, parents, and the medical system itself contributing to low rates. Barriers to vaccination include fear and misconceptions, costs and socioeconomic status, lack of understanding and education, and logistic obstacles to completing the full series.5 Understanding these barriers, as well as discussing methods to overcome them, is key to increasing HPV vaccination rates and preventing the spread of this cancer-causing infection.
Health care provider barriers
Even though accredited national institutions and committees such as the CDC, ACIP, and ACOG strongly recommend vaccination based on current evidence, some health care providers still do not recommend the HPV vaccine to parents and patients.2,6,7,11 Lack of provider recommendation and the resulting lack of parental awareness of the vaccine account for many adolescents not receiving the vaccination.10,12
Providers do not recommend the vaccine for a number of reasons. Some have limited knowledge or conflicting ideas about the specific disease protection of the HPV vaccine, while others are hesitant to administer the vaccine before the onset of sexual activity, because they feel the suggested age for vaccination (11 to 12 years) is too young.10,11 Still other providers report difficulty approaching parents who they perceive as having concerns about the vaccine’s association with a sexually transmitted infection or believing that it might promote sexual activity.10
Some professionals simply claim that they forget to address the HPV vaccine at health visits, or that they propose it as optional and up to the parent’s discretion.5,10 Many providers do recommend and administer the initial dose of the vaccine, but have difficulties ensuring that patients complete the full multidose series.13 Evidence has shown that a strong provider recommendation is one of the most important incentives for parents and patients to accept vaccination.14
Parental and caregiver barriers
Lack of knowledge about the HPV vaccine and lack of recommendation from providers are two top reasons parents and caregivers cite for not vaccinating their children.5,10,14,15 In a national survey, almost all parents whose daughters completed the full vaccination series reported being counseled by their provider on the appropriate age for vaccination and the timeline of the series.14
Fears and apprehensions about side effects, especially with newer vaccines, can prevent some parents from having their children vaccinated.15 Although there is some stigma related to the vaccine’s association with the sexually transmitted HPV, this is a much less significant barrier than lack of provider recommendation or knowledge about the vaccine.5,11
Health care system barriers
Both providers and parents agree that system-level issues such as access, follow-up, and cost are barriers to initiating or completing the vaccination series.11,13 Many adolescents have few opportunities to receive the vaccine because they do not have a primary care provider.11 For those with access to primary care, visits are often problem-focused and frequently do not include a review of immunization history.13 Health care professionals also report challenges with scheduling follow-up visits for the second and third doses to complete the series within the recommended timeframe.13
Cost, insurance coverage, and reimbursement pose additional hurdles for both providers and patients, with some providers citing concerns about the cost of stocking the vaccine.16 Providers, both family practice providers and gynecologists, agree that reimbursement for administering the HPV vaccine in office poses a barrier when recommending the vaccine to patients.17 Lack of insurance coverage and type of insurance also pose barriers, with Medicaid patients more often completing the full series compared to those with private or no insurance, because Medicaid covers the cost of vaccination for men up to age 19.9,18 A national survey of males ages 9 to 17 found that the percentage of HPV vaccine initiation was double for those with public insurance compared to those with private insurance.19 Changes at the system-level, such as participation in the VFC program, in coordination with better provider recommendation should help increase HPV vaccination rates.9,11
STRATEGIES TO IMPROVE VACCINATION RATES
Many strategies for increasing HPV vaccination acceptance, decreasing barriers to access, and improving compliance with vaccine completion have been reported in the literature, with some strategies achieving more success than others. This section discusses interventions and strategies designed to help overcome provider-, parent-, and system-related barriers that have been shown to be effective (see Table 3).

Health care provider interventions
Evidence supports a number of provider-level strategies to increase HPV vaccination rates (see Table 3). An improvement in vaccination acceptance was observed when providers promoted the vaccine as a safe, effective way to prevent cancer, rather than as a means to prevent a sexually transmitted infection.10,11,20
Some primary care providers found that encouraging the HPV vaccine at the same time as the meningococcal and Tdap vaccinations, which are also recommended at age 11 to 12 years, increased vaccination rates as well.13,20 Another successful strategy is reviewing vaccination history at every visit, whether the visit is for an acute event or an annual well exam.10,13,20 These tactics are most useful when providers practice them consistently, which may require them to change or adapt their way of practice.
Provider-based trainings that educate and prepare them to consistently recommend the vaccine have demonstrated success in increasing HPV vaccination uptake.21,22 The CDC’s Assessment/Feedback/Incentive/eXchange (AFIX) quality improvement program to increase vaccination rates, which includes Web-based or in-person consults, has been shown to increase HPV vaccination rates.20-23 The Assessment phase of the AFIX program determines a practice’s current immunization practices and rates, while the Feedback portion provides strategies for increasing vaccination rates.23 A study by Perkins and colleagues utilized AFIX strategies, specifically for the HPV vaccine, such as focusing provider education on HPV-related cancers and vaccine efficacy, as well as preparing providers to discuss and answer questions through basic motivational interviewing tactics.20
The CDC also offers PowerPoint presentations, flyers, posters, videos, and other informational resources to guide and educate providers, parents, and patients about the HPV vaccine.24 Educational resources, such as pamphlets, flyers, or fact sheets given to parents and patients, have been shown to improve intent to vaccinate as well as awareness of the vaccine.25-27
Although Fu and colleagues in a systematic review concluded that there was insufficient data to support a specific educational intervention for widespread use, the authors did recommend utilizing educational pieces and adapting them to specific populations.25 These simple interventions help increase awareness and can be implemented with other interventions in health care offices by providers and other staff.

System-level interventions
The use of systems that track patients for necessary vaccines and remind providers, parents, and patients about vaccine appointments have increased vaccination rates.13,28 Facility-based interventions, such as electronic medical records (EMR) that track patients for scheduled vaccines and remind providers when patients are due for vaccinations, will help increase provider recommendations and completion of the entire vaccination series.13
The National Vaccine Advisory Committee (NVAC) suggests that provider offices implement reminder-recall systems and provide educational material for parents and patients to increase vaccination rates.29 One specific study using both educational material and text-message reminders for parents found that these interventions significantly increased vaccination rates.30 Health care facilities could also incorporate reminder letters mailed to patients and parents to promote vaccine initiation and completion.31 The evidence supports the use of reminder alerts and EMR tracking systems to increase rates, but more research is warranted to determine the most cost-effective approach.
National programs, committees, and organizations have provided recommendations for overcoming system-related barriers to HPV vaccination, such as access and cost.29,32 The NVAC recommends incorporating alternative venues for vaccination delivery, such as pharmacies, schools, and health clinics, to increase availability to the adolescent population, especially to those who do not have primary care providers.29 One study that addressed parental opinions of vaccination administration in schools found that the majority of parents were in favor of this type of program.33 Although these recommendations seem promising and are accepted by parents, logistical barriers such as reimbursement to the pharmacies, schools, and clinics and accurate documentation of the doses received need to be addressed.29 The NVAC recommends continued evaluation and efforts to develop these programs in the future.29
In addition to school-based interventions, providing home visits for vaccination and implementing standing orders are other suggestions to overcome access and cost barriers for vaccinations, including HPV.32 Standing orders allow for individuals to receive a vaccine by a health care professional in an approved institution, where allowed by state law.32 This provides easier access to vaccinations, especially for those who do not see a primary care provider.
Although some of the system-level interventions mentioned in this article are outside the realm of what providers can do in the office, understanding and advocating for these advancements will promote vaccine uptake.
CONCLUSION
Lack of provider recommendation, coupled with poor or no parental knowledge about the HPV vaccine, are significant factors affecting vaccination uptake. Evidence supports the use of multifaceted interventions that promote and support provider recommendation and parent/patient education. Studies of interventions that incorporated educational resources and alert systems for both providers and patients or their caregivers have shown these strategies to be effective in increasing vaccination uptake and completion.
In addition to recommending the HPV vaccine, providers must educate parents/caregivers and patients about it, particularly by presenting the vaccine as a means of cancer prevention. Primary care facilities should implement reminder plans and provide educational literature to promote vaccine uptake. Although the interventions highlighted here have increased HPV vaccination rates, further research is warranted to evaluate more effective strategies for overcoming barriers and to determine which strategies are most cost-effective.
1. Juckett G, Hartsman-Adams H. Human papillomavirus: clinical manifestations and prevention. Am Fam Physician. 2010;82(10):1209-1213.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1-30.
3. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661-666.
4. American Cancer Society. Cancer facts and figures 2016. Atlanta: American Cancer Society; 2016.
5. Holman DM, Benard V, Roland KB, et al. Barriers to the human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76-82.
6. American College of Obstetricians and Gynecologists. Human papillomavirus vaccination. Committee Opinion. Number 704. June 2017. www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Adolescent-Health-Care/Human-Papillomavirus-Vaccination. Accessed August 17, 2017.
7. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendation of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405-1408.
8. Mulcahy N. GSK’s HPV vaccine, Cervarix, no longer available in the US. Medscape Medical News. October 26, 2016. www.medscape.com/viewarticle/870853. Accessed June 11, 2017.
9. Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784-792.
10. Muncie HL, Lebato AL. HPV vaccination: overcoming parental and physician impediments. Am Fam Physician. 2015;92(6):449-454.
11. Bratic JS, Seyferth ER, Bocchini JA Jr. Update on barriers to human papillomavirus vaccination and effective strategies to promote vaccine acceptance. Curr Opin Pediatr. 2016;28(3):407-412.
12. Rahman M, Laz TH, McGrath CJ, Berenson AB. Provider recommendation mediates the relationship between parental human papillomavirus (HPV) vaccine awareness and HPV vaccine initiation and completion among 13- to 17-year-old US adolescent children. Clin Pediatr. 2015;54(4):371-375.
13. Sussman AL, Helitzer D, Bennett A, et al. Catching up with the HPV vaccine: challenges and opportunities in primary care. Ann Fam Med. 2015;13(4):354-360.
14. Clark SJ, Cowan AE, Filipp SL, et al. Parent perception of provider interactions influences HPV vaccination status of adolescent females. Clin Pediatr. 2016;55(8):701-706.
15. Ackerman LK, Serrano JL. Update on routine childhood and adolescent immunizations. Am Fam Physician. 2015;92(6):460-468.
16. Tom A, Robinett H, Buenconsejo-Lum L, et al. Promoting and providing the HPV vaccination in Hawaii: barriers faced by health providers. J Community Health. 2016;41(5):1069-1077.
17. Young JL, Bernheim RG, Korte JE, et al. Human papillomavirus vaccination recommendation may be linked to reimbursement: A survey of Virginia family practitioners and gynecologists. J Pediat Adolesc Gynecol. 2011;24(6):380-385.
18. Thomas R, Higgins L, Ding L, et al. Factors associated with HPV vaccine initiation, vaccine completion, and accuracy of self-reported vaccination status among 13- to 26-year-old men. Am J Mens Health. 2016;1-9.
19. Laz TH, Rahman M, Berenson AB. Human papillomavirus vaccine uptake among 9-17 year old males in the United States: the National Health Interview Survey, 2010. Hum Vaccin Immunother. 2013;9(4):874-878.
20. Perkins RB, Zisblatt L, Legler A, et al. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine. 2015;33:1223-1229.
21. Smulian EA, Mitchell KR, Stokley S. Interventions to increase HPV vaccination coverage: a systematic review. Hum Vaccin Immunother. 2016;12:1566-1588.
22. Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics. 2016;138(1).
23. CDC. Overview of AFIX. www.cdc.gov/vaccines/programs/afix/index.html. Accessed August 17, 2017.
24. CDC. Human papillomavirus (HPV). www.cdc.gov/hpv/. Accessed August 17, 2017.
25. Fu LY, Bonhomme LA, Cooper SC, et al. Educational interventions to increase HPV vaccination acceptance: a systematic review. Vaccine. 2014;32(17):1901-1920.
26. Kennedy A, Sapsis KF, Stokley S, et al. Parental attitudes toward human papillomavirus vaccination: evaluation of an educational intervention, 2008. J Health Commun. 2011;16(3):300-313.
27. Spleen AM, Kluhsman BC, Clark AD, et al; ACTION Health Cancer Task Force. An increase in HPV-related knowledge and vaccination intent among parental and non-parental caregivers of adolescent girls, ages 9-17 years, in Appalachian Pennsylvania. J Cancer Educ. 2012;27(2):312-319.
28. Conroy K, Rosenthal SL, Zimet GD, et al. Human papillomavirus vaccine uptake, predictors of vaccination, and self-reported barriers to vaccination. J Womens Health (Larchmt). 2009;18(10):1679-1686.
29. National Vaccine Advisory Committee. Overcoming barriers to low HPV vaccine uptake in the United States: recommendations from the National Vaccine Advisory Committee. Public Health Rep. 2016;131(1):17-25.
30. Aragones A, Bruno DM, Ehrenberg M, et al. Parental education and text messaging reminders as effective community based tools to increase HPV vaccination rates among Mexican American children. Prev Med Rep. 2015;2:554-558.
31. Chao C, Preciado M, Slezak J, Xu L. A randomized intervention of reminder letter for human papillomavirus vaccine series completion. J Adolesc Health. 2015;56(1):85-90.
32. Community Preventive Services Task Force. The community guide—guide to community preventive services: increasing appropriate vaccinations. Atlanta, GA: Community Preventive Services Task Force; 2016
33. Kelminson K, Saville A, Seewald L, et al. Parental views of school-located delivery of adolescent vaccines. J Adolesc Health. 2012;51(2):190-196.
1. Juckett G, Hartsman-Adams H. Human papillomavirus: clinical manifestations and prevention. Am Fam Physician. 2010;82(10):1209-1213.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1-30.
3. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661-666.
4. American Cancer Society. Cancer facts and figures 2016. Atlanta: American Cancer Society; 2016.
5. Holman DM, Benard V, Roland KB, et al. Barriers to the human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76-82.
6. American College of Obstetricians and Gynecologists. Human papillomavirus vaccination. Committee Opinion. Number 704. June 2017. www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Adolescent-Health-Care/Human-Papillomavirus-Vaccination. Accessed August 17, 2017.
7. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendation of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405-1408.
8. Mulcahy N. GSK’s HPV vaccine, Cervarix, no longer available in the US. Medscape Medical News. October 26, 2016. www.medscape.com/viewarticle/870853. Accessed June 11, 2017.
9. Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784-792.
10. Muncie HL, Lebato AL. HPV vaccination: overcoming parental and physician impediments. Am Fam Physician. 2015;92(6):449-454.
11. Bratic JS, Seyferth ER, Bocchini JA Jr. Update on barriers to human papillomavirus vaccination and effective strategies to promote vaccine acceptance. Curr Opin Pediatr. 2016;28(3):407-412.
12. Rahman M, Laz TH, McGrath CJ, Berenson AB. Provider recommendation mediates the relationship between parental human papillomavirus (HPV) vaccine awareness and HPV vaccine initiation and completion among 13- to 17-year-old US adolescent children. Clin Pediatr. 2015;54(4):371-375.
13. Sussman AL, Helitzer D, Bennett A, et al. Catching up with the HPV vaccine: challenges and opportunities in primary care. Ann Fam Med. 2015;13(4):354-360.
14. Clark SJ, Cowan AE, Filipp SL, et al. Parent perception of provider interactions influences HPV vaccination status of adolescent females. Clin Pediatr. 2016;55(8):701-706.
15. Ackerman LK, Serrano JL. Update on routine childhood and adolescent immunizations. Am Fam Physician. 2015;92(6):460-468.
16. Tom A, Robinett H, Buenconsejo-Lum L, et al. Promoting and providing the HPV vaccination in Hawaii: barriers faced by health providers. J Community Health. 2016;41(5):1069-1077.
17. Young JL, Bernheim RG, Korte JE, et al. Human papillomavirus vaccination recommendation may be linked to reimbursement: A survey of Virginia family practitioners and gynecologists. J Pediat Adolesc Gynecol. 2011;24(6):380-385.
18. Thomas R, Higgins L, Ding L, et al. Factors associated with HPV vaccine initiation, vaccine completion, and accuracy of self-reported vaccination status among 13- to 26-year-old men. Am J Mens Health. 2016;1-9.
19. Laz TH, Rahman M, Berenson AB. Human papillomavirus vaccine uptake among 9-17 year old males in the United States: the National Health Interview Survey, 2010. Hum Vaccin Immunother. 2013;9(4):874-878.
20. Perkins RB, Zisblatt L, Legler A, et al. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine. 2015;33:1223-1229.
21. Smulian EA, Mitchell KR, Stokley S. Interventions to increase HPV vaccination coverage: a systematic review. Hum Vaccin Immunother. 2016;12:1566-1588.
22. Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics. 2016;138(1).
23. CDC. Overview of AFIX. www.cdc.gov/vaccines/programs/afix/index.html. Accessed August 17, 2017.
24. CDC. Human papillomavirus (HPV). www.cdc.gov/hpv/. Accessed August 17, 2017.
25. Fu LY, Bonhomme LA, Cooper SC, et al. Educational interventions to increase HPV vaccination acceptance: a systematic review. Vaccine. 2014;32(17):1901-1920.
26. Kennedy A, Sapsis KF, Stokley S, et al. Parental attitudes toward human papillomavirus vaccination: evaluation of an educational intervention, 2008. J Health Commun. 2011;16(3):300-313.
27. Spleen AM, Kluhsman BC, Clark AD, et al; ACTION Health Cancer Task Force. An increase in HPV-related knowledge and vaccination intent among parental and non-parental caregivers of adolescent girls, ages 9-17 years, in Appalachian Pennsylvania. J Cancer Educ. 2012;27(2):312-319.
28. Conroy K, Rosenthal SL, Zimet GD, et al. Human papillomavirus vaccine uptake, predictors of vaccination, and self-reported barriers to vaccination. J Womens Health (Larchmt). 2009;18(10):1679-1686.
29. National Vaccine Advisory Committee. Overcoming barriers to low HPV vaccine uptake in the United States: recommendations from the National Vaccine Advisory Committee. Public Health Rep. 2016;131(1):17-25.
30. Aragones A, Bruno DM, Ehrenberg M, et al. Parental education and text messaging reminders as effective community based tools to increase HPV vaccination rates among Mexican American children. Prev Med Rep. 2015;2:554-558.
31. Chao C, Preciado M, Slezak J, Xu L. A randomized intervention of reminder letter for human papillomavirus vaccine series completion. J Adolesc Health. 2015;56(1):85-90.
32. Community Preventive Services Task Force. The community guide—guide to community preventive services: increasing appropriate vaccinations. Atlanta, GA: Community Preventive Services Task Force; 2016
33. Kelminson K, Saville A, Seewald L, et al. Parental views of school-located delivery of adolescent vaccines. J Adolesc Health. 2012;51(2):190-196.
Opioid antagonists in pregnancy: Naltrexone or not?
With the increasing concern about rising rates of opioid abuse in the general population, including women of reproductive age and pregnant and breastfeeding women, clear guidelines regarding treatment in pregnancy and lactation are needed.
The Committee on Obstetric Practice of the American College of Obstetricians and Gynecologists and the American Society of Addiction Medicine addressed this issue comprehensively in the ACOG Committee Opinion issued in August 2017.1 In this document, universal screening and medication-assisted treatment for opioid use disorder were recommended. Opioid agonists including methadone and buprenorphine were considered the treatments with the most evidence of benefit, and limited concern about adverse fetal effects, other than predictable and treatable neonatal abstinence syndrome.
Two types of scenarios make this topic relevant. In the first, a woman who has been successful in avoiding relapse by naltrexone treatment, although advised not to become pregnant, could inadvertently conceive. She would then be at risk of relapse if treatment were discontinued. In the second, a woman who overdoses with an opioid in pregnancy might require rapid detoxification with naltrexone in order to survive. In either case, there are quite limited data on potential fetal consequences.
In a 2001 report, Hulse et al. described a series of fetal outcomes following prenatal naltrexone exposure. In one set of cases accumulated from three countries, rapid opiate detoxification with naltrexone was performed for 18 pregnant women. One woman received two detoxification treatments. Two treatments occurred in the first trimester, 11 in the second, and 6 in the third. Maternal and fetal outcomes were said to be unremarkable, except for two cases of low birth weight infants (less than 2,500 g). In another set of cases, seven opioid-dependent women in Australia who had been maintained on 50 mg naltrexone per day became pregnant. In six of the seven cases, naltrexone was discontinued at 7 weeks’ gestation because of the unknown risks of teratogenicity. Of these, three restarted naltrexone maintenance therapy in the second trimester. One mother continued naltrexone throughout pregnancy. One of the seven women delivered at 36 weeks by induction for high blood pressure, and the infant was less than 2,500 g. One other term infant was small at 2,625 g. Otherwise, outcomes were considered normal.3
In two subsequent reports by some of the same authors, pregnancy outcomes in 9 and 17 heroin users with naltrexone implants were unremarkable and comparable to those of women on methadone maintenance therapy.4,5
While the very limited data on naltrexone safety in pregnancy have not suggested substantial increased risks, the numbers are too small to provide strong reassurance, and the animal data remain concerning. Long-term behavioral outcome studies are also lacking. More research in this area is needed to weigh the safety of naltrexone for the fetus against the risk of relapse with discontinuation of this drug.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures.
References
1. Obstet Gynecol. 2017 Aug;130(2):e81-e94.
2. Curr Neuropharmacol. 2008 Jun;6(2):125–50.
3. Aust N Z J Obstet Gynaecol. 2001 Nov;41(4):424-8.
4. Aust N Z J Obstet Gynaecol. 2002 Feb;42(1):104-5.
5. Int J Gynaecol Obstet. 2004 May;85(2):170-1.
6. Drugs. 2017 Jul;77(11):1211-9.
With the increasing concern about rising rates of opioid abuse in the general population, including women of reproductive age and pregnant and breastfeeding women, clear guidelines regarding treatment in pregnancy and lactation are needed.
The Committee on Obstetric Practice of the American College of Obstetricians and Gynecologists and the American Society of Addiction Medicine addressed this issue comprehensively in the ACOG Committee Opinion issued in August 2017.1 In this document, universal screening and medication-assisted treatment for opioid use disorder were recommended. Opioid agonists including methadone and buprenorphine were considered the treatments with the most evidence of benefit, and limited concern about adverse fetal effects, other than predictable and treatable neonatal abstinence syndrome.
Two types of scenarios make this topic relevant. In the first, a woman who has been successful in avoiding relapse by naltrexone treatment, although advised not to become pregnant, could inadvertently conceive. She would then be at risk of relapse if treatment were discontinued. In the second, a woman who overdoses with an opioid in pregnancy might require rapid detoxification with naltrexone in order to survive. In either case, there are quite limited data on potential fetal consequences.
In a 2001 report, Hulse et al. described a series of fetal outcomes following prenatal naltrexone exposure. In one set of cases accumulated from three countries, rapid opiate detoxification with naltrexone was performed for 18 pregnant women. One woman received two detoxification treatments. Two treatments occurred in the first trimester, 11 in the second, and 6 in the third. Maternal and fetal outcomes were said to be unremarkable, except for two cases of low birth weight infants (less than 2,500 g). In another set of cases, seven opioid-dependent women in Australia who had been maintained on 50 mg naltrexone per day became pregnant. In six of the seven cases, naltrexone was discontinued at 7 weeks’ gestation because of the unknown risks of teratogenicity. Of these, three restarted naltrexone maintenance therapy in the second trimester. One mother continued naltrexone throughout pregnancy. One of the seven women delivered at 36 weeks by induction for high blood pressure, and the infant was less than 2,500 g. One other term infant was small at 2,625 g. Otherwise, outcomes were considered normal.3
In two subsequent reports by some of the same authors, pregnancy outcomes in 9 and 17 heroin users with naltrexone implants were unremarkable and comparable to those of women on methadone maintenance therapy.4,5
While the very limited data on naltrexone safety in pregnancy have not suggested substantial increased risks, the numbers are too small to provide strong reassurance, and the animal data remain concerning. Long-term behavioral outcome studies are also lacking. More research in this area is needed to weigh the safety of naltrexone for the fetus against the risk of relapse with discontinuation of this drug.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures.
References
1. Obstet Gynecol. 2017 Aug;130(2):e81-e94.
2. Curr Neuropharmacol. 2008 Jun;6(2):125–50.
3. Aust N Z J Obstet Gynaecol. 2001 Nov;41(4):424-8.
4. Aust N Z J Obstet Gynaecol. 2002 Feb;42(1):104-5.
5. Int J Gynaecol Obstet. 2004 May;85(2):170-1.
6. Drugs. 2017 Jul;77(11):1211-9.
With the increasing concern about rising rates of opioid abuse in the general population, including women of reproductive age and pregnant and breastfeeding women, clear guidelines regarding treatment in pregnancy and lactation are needed.
The Committee on Obstetric Practice of the American College of Obstetricians and Gynecologists and the American Society of Addiction Medicine addressed this issue comprehensively in the ACOG Committee Opinion issued in August 2017.1 In this document, universal screening and medication-assisted treatment for opioid use disorder were recommended. Opioid agonists including methadone and buprenorphine were considered the treatments with the most evidence of benefit, and limited concern about adverse fetal effects, other than predictable and treatable neonatal abstinence syndrome.
Two types of scenarios make this topic relevant. In the first, a woman who has been successful in avoiding relapse by naltrexone treatment, although advised not to become pregnant, could inadvertently conceive. She would then be at risk of relapse if treatment were discontinued. In the second, a woman who overdoses with an opioid in pregnancy might require rapid detoxification with naltrexone in order to survive. In either case, there are quite limited data on potential fetal consequences.
In a 2001 report, Hulse et al. described a series of fetal outcomes following prenatal naltrexone exposure. In one set of cases accumulated from three countries, rapid opiate detoxification with naltrexone was performed for 18 pregnant women. One woman received two detoxification treatments. Two treatments occurred in the first trimester, 11 in the second, and 6 in the third. Maternal and fetal outcomes were said to be unremarkable, except for two cases of low birth weight infants (less than 2,500 g). In another set of cases, seven opioid-dependent women in Australia who had been maintained on 50 mg naltrexone per day became pregnant. In six of the seven cases, naltrexone was discontinued at 7 weeks’ gestation because of the unknown risks of teratogenicity. Of these, three restarted naltrexone maintenance therapy in the second trimester. One mother continued naltrexone throughout pregnancy. One of the seven women delivered at 36 weeks by induction for high blood pressure, and the infant was less than 2,500 g. One other term infant was small at 2,625 g. Otherwise, outcomes were considered normal.3
In two subsequent reports by some of the same authors, pregnancy outcomes in 9 and 17 heroin users with naltrexone implants were unremarkable and comparable to those of women on methadone maintenance therapy.4,5
While the very limited data on naltrexone safety in pregnancy have not suggested substantial increased risks, the numbers are too small to provide strong reassurance, and the animal data remain concerning. Long-term behavioral outcome studies are also lacking. More research in this area is needed to weigh the safety of naltrexone for the fetus against the risk of relapse with discontinuation of this drug.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures.
References
1. Obstet Gynecol. 2017 Aug;130(2):e81-e94.
2. Curr Neuropharmacol. 2008 Jun;6(2):125–50.
3. Aust N Z J Obstet Gynaecol. 2001 Nov;41(4):424-8.
4. Aust N Z J Obstet Gynaecol. 2002 Feb;42(1):104-5.
5. Int J Gynaecol Obstet. 2004 May;85(2):170-1.
6. Drugs. 2017 Jul;77(11):1211-9.
Premature birth after preeclampsia: $23.1M verdict
Premature birth after preeclampsia: $23.1M verdict
When a woman saw her ObGyn on August 16 at 24 weeks’ gestation, test results showed proteinuria and high blood pressure (BP). The following day, she was hospitalized for a 24-hour urine test and BP evaluation supervised by an on-call ObGyn and her ObGyn. Test results confirmed preeclampsia. She was released from the hospital. A few days later, she was found to have continued high BP and increased proteinuria, and restricted fetal growth was detected. On August 29 at 26 weeks’ gestation, the baby girl was born with severe cystic periventricular leukomalacia by emergency cesarean delivery. She cannot perform basic tasks and will need 24-hour care for the rest of her life.
PARENTS' CLAIM:
The hospital staff and 2 ObGyns failed to timely diagnose and treat preeclampsia. The treating ObGyn neither prescribed medication to treat preeclampsia nor administered antenatal corticosteroids to enhance fetal lung and brain development, both of which should have been started on August 17. Hospital health care providers failed to transfer her to a Level III facility equipped to handle a premature birth of less than 33 weeks’ gestation.
DEFENDANTS' DEFENSE:
The hospital and ObGyn denied negligence.
VERDICT:
Prior to trial, the mother settled with the on-call ObGyn for an undisclosed amount. A $23.15 million Florida verdict was returned, apportioning 70% liability to the treating ObGyn and 30% to the hospital.
Related article:
For the management of labor, patience is a virtue
Shoulder dystocia, paralysis: $950,000 settlement
During delivery, shoulder dystocia was encountered. The ObGyn used maneuvers to release the shoulder and completed the delivery. The child has a brachial plexus injury. Despite nerve graft surgery, her right arm, shoulder, and hand are paralyzed.
PARENTS' CLAIM:
The ObGyn failed to properly manage the delivery. Shoulder dystocia had been encountered during the delivery of a sibling, but the ObGyn failed to communicate the need for cesarean delivery in future pregnancies.
DEFENDANTS' DEFENSE:
There was no negligence. The case settled during trial.
VERDICT:
A $950,000 California settlement was reached with the hospital and ObGyn.
Related article:
Shoulder dystocia: Taking the fear out of management
Child has brachial plexus injury
A mother was admitted to the hospital shortly after her membranes broke. Meconium was detected but the fetal heart-rate (FHR) monitor results were normal. About 15 minutes after admission, she was seen by an attending ObGyn, who started oxytocin to induce labor. FHR monitoring results were acceptable throughout the day, and by midafternoon, the mother was ready to deliver. A fetal baseline heart rate of less than 110 bpm was detected as staff prepared for the delivery. Less than an hour later, the baby’s head crowned and the ObGyn quickly identified shoulder dystocia. Nurses repositioned the mother, the baby rotated, and was delivered. Apgar scores were normal despite a shoulder injury.
PARENTS' CLAIM:
The ObGyn caused the injury by using excessive force during delivery. After attempting gentle traction, the ObGyn should have changed strategies.
DEFENDANTS' DEFENSE:
The ObGyn asserted that she used gentle traction that prevented twisting or stretching the baby’s nerves. The birth was normal and she followed all protocols, resulting in the birth of a cognitively intact baby, as evidenced by the child’s Apgar scores. The baby was large and labor and delivery went very quickly, both factors that could have led to the baby’s injuries. The ObGyn’s actions did not cause the injuries.
VERDICT:
A Pennsylvania defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Premature birth after preeclampsia: $23.1M verdict
When a woman saw her ObGyn on August 16 at 24 weeks’ gestation, test results showed proteinuria and high blood pressure (BP). The following day, she was hospitalized for a 24-hour urine test and BP evaluation supervised by an on-call ObGyn and her ObGyn. Test results confirmed preeclampsia. She was released from the hospital. A few days later, she was found to have continued high BP and increased proteinuria, and restricted fetal growth was detected. On August 29 at 26 weeks’ gestation, the baby girl was born with severe cystic periventricular leukomalacia by emergency cesarean delivery. She cannot perform basic tasks and will need 24-hour care for the rest of her life.
PARENTS' CLAIM:
The hospital staff and 2 ObGyns failed to timely diagnose and treat preeclampsia. The treating ObGyn neither prescribed medication to treat preeclampsia nor administered antenatal corticosteroids to enhance fetal lung and brain development, both of which should have been started on August 17. Hospital health care providers failed to transfer her to a Level III facility equipped to handle a premature birth of less than 33 weeks’ gestation.
DEFENDANTS' DEFENSE:
The hospital and ObGyn denied negligence.
VERDICT:
Prior to trial, the mother settled with the on-call ObGyn for an undisclosed amount. A $23.15 million Florida verdict was returned, apportioning 70% liability to the treating ObGyn and 30% to the hospital.
Related article:
For the management of labor, patience is a virtue
Shoulder dystocia, paralysis: $950,000 settlement
During delivery, shoulder dystocia was encountered. The ObGyn used maneuvers to release the shoulder and completed the delivery. The child has a brachial plexus injury. Despite nerve graft surgery, her right arm, shoulder, and hand are paralyzed.
PARENTS' CLAIM:
The ObGyn failed to properly manage the delivery. Shoulder dystocia had been encountered during the delivery of a sibling, but the ObGyn failed to communicate the need for cesarean delivery in future pregnancies.
DEFENDANTS' DEFENSE:
There was no negligence. The case settled during trial.
VERDICT:
A $950,000 California settlement was reached with the hospital and ObGyn.
Related article:
Shoulder dystocia: Taking the fear out of management
Child has brachial plexus injury
A mother was admitted to the hospital shortly after her membranes broke. Meconium was detected but the fetal heart-rate (FHR) monitor results were normal. About 15 minutes after admission, she was seen by an attending ObGyn, who started oxytocin to induce labor. FHR monitoring results were acceptable throughout the day, and by midafternoon, the mother was ready to deliver. A fetal baseline heart rate of less than 110 bpm was detected as staff prepared for the delivery. Less than an hour later, the baby’s head crowned and the ObGyn quickly identified shoulder dystocia. Nurses repositioned the mother, the baby rotated, and was delivered. Apgar scores were normal despite a shoulder injury.
PARENTS' CLAIM:
The ObGyn caused the injury by using excessive force during delivery. After attempting gentle traction, the ObGyn should have changed strategies.
DEFENDANTS' DEFENSE:
The ObGyn asserted that she used gentle traction that prevented twisting or stretching the baby’s nerves. The birth was normal and she followed all protocols, resulting in the birth of a cognitively intact baby, as evidenced by the child’s Apgar scores. The baby was large and labor and delivery went very quickly, both factors that could have led to the baby’s injuries. The ObGyn’s actions did not cause the injuries.
VERDICT:
A Pennsylvania defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Premature birth after preeclampsia: $23.1M verdict
When a woman saw her ObGyn on August 16 at 24 weeks’ gestation, test results showed proteinuria and high blood pressure (BP). The following day, she was hospitalized for a 24-hour urine test and BP evaluation supervised by an on-call ObGyn and her ObGyn. Test results confirmed preeclampsia. She was released from the hospital. A few days later, she was found to have continued high BP and increased proteinuria, and restricted fetal growth was detected. On August 29 at 26 weeks’ gestation, the baby girl was born with severe cystic periventricular leukomalacia by emergency cesarean delivery. She cannot perform basic tasks and will need 24-hour care for the rest of her life.
PARENTS' CLAIM:
The hospital staff and 2 ObGyns failed to timely diagnose and treat preeclampsia. The treating ObGyn neither prescribed medication to treat preeclampsia nor administered antenatal corticosteroids to enhance fetal lung and brain development, both of which should have been started on August 17. Hospital health care providers failed to transfer her to a Level III facility equipped to handle a premature birth of less than 33 weeks’ gestation.
DEFENDANTS' DEFENSE:
The hospital and ObGyn denied negligence.
VERDICT:
Prior to trial, the mother settled with the on-call ObGyn for an undisclosed amount. A $23.15 million Florida verdict was returned, apportioning 70% liability to the treating ObGyn and 30% to the hospital.
Related article:
For the management of labor, patience is a virtue
Shoulder dystocia, paralysis: $950,000 settlement
During delivery, shoulder dystocia was encountered. The ObGyn used maneuvers to release the shoulder and completed the delivery. The child has a brachial plexus injury. Despite nerve graft surgery, her right arm, shoulder, and hand are paralyzed.
PARENTS' CLAIM:
The ObGyn failed to properly manage the delivery. Shoulder dystocia had been encountered during the delivery of a sibling, but the ObGyn failed to communicate the need for cesarean delivery in future pregnancies.
DEFENDANTS' DEFENSE:
There was no negligence. The case settled during trial.
VERDICT:
A $950,000 California settlement was reached with the hospital and ObGyn.
Related article:
Shoulder dystocia: Taking the fear out of management
Child has brachial plexus injury
A mother was admitted to the hospital shortly after her membranes broke. Meconium was detected but the fetal heart-rate (FHR) monitor results were normal. About 15 minutes after admission, she was seen by an attending ObGyn, who started oxytocin to induce labor. FHR monitoring results were acceptable throughout the day, and by midafternoon, the mother was ready to deliver. A fetal baseline heart rate of less than 110 bpm was detected as staff prepared for the delivery. Less than an hour later, the baby’s head crowned and the ObGyn quickly identified shoulder dystocia. Nurses repositioned the mother, the baby rotated, and was delivered. Apgar scores were normal despite a shoulder injury.
PARENTS' CLAIM:
The ObGyn caused the injury by using excessive force during delivery. After attempting gentle traction, the ObGyn should have changed strategies.
DEFENDANTS' DEFENSE:
The ObGyn asserted that she used gentle traction that prevented twisting or stretching the baby’s nerves. The birth was normal and she followed all protocols, resulting in the birth of a cognitively intact baby, as evidenced by the child’s Apgar scores. The baby was large and labor and delivery went very quickly, both factors that could have led to the baby’s injuries. The ObGyn’s actions did not cause the injuries.
VERDICT:
A Pennsylvania defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CDC: Flu vaccine recommendations broaden for pregnant women and children
, according to new recommendations from the Centers for Disease Control and Prevention.
This change from the CDC’s previous guidance that pregnant women receive a seasonal inactivated influenza vaccine (IIV) was recommended by the Advisory Committee on Immunization Practices after some heated debate among committee members over evidence presented to support the change in wording (MMWR. 2017 Aug 25;66[(RR-2]:1-20).
The new update gives women the ability to choose between receiving an IIV and FluBlok, a recombinant influenza vaccine (RIV) that is not egg based and can be manufactured more quickly, making it ideal in cases of pandemic or supply shortages, according to the CDC.
Although pregnant women may choose to receive a vaccination during the first trimester, the CDC warns there may be some risk involved.
“Although experience with the use of IIVs is substantial, and data from observational studies are available to support the safety of these vaccines in pregnancy, data are more limited for vaccination during the first trimester,” according to the CDC. “Moreover, there is substantially less experience with more recently licensed IIV products (e.g., quadrivalent, cell culture-based, and adjuvanted vaccines) during pregnancy in general.”
Data also are limited regarding RIVs, the CDC said, with the data used to determine safety among pregnant women “limited to reports of pregnancies occurring incidentally during clinical trials, Vaccine Adverse Event Reporting System (VAERS) reports, and pregnancy registry reports.”
Changes for children
The CDC chose to accept ACIP recommendations regarding Afluria (IIV3), expanding the age of children who can receive the vaccine from 9 years and older to 5 years and older.
Similar labeling changes were accepted for FluLaval Quadrivalent (IIV4), which had previously been given to children 3 years and older but now but will be available for children starting at 6 months of age.
New products
Recent product licensures included in the MMWR report are Afluria Quadrivalent (IIV4) and Flublok Quadrivalent (RIV4), both for persons over 18 years.
According to the CDC, Flublok Quadrivalent (an RIV) met noninferiority measures, compared with a similar IIV quadrivalent vaccine, for the A(H3H2) and B/Yamagata viruses but not for A(H1N1) or B/Victoria viruses.
Vaccine composition for 2017-2018
Approved viruses for the 2017-2018 season trivalent vaccines are an A/Michigan/45/2015 (H1N1) pdm09–like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008–like virus (Victoria lineage), according to the MMWR. Quadrivalent vaccines will include those viruses, with the addition of an B/Phuket/3073/2013–like virus (Yamagata lineage).
The CDC continues to recommend that the quadrivalent live attenuated influenza vaccine FluMist not be used by anyone for the 2017-2018 season, a decision that was made after evidence showed poor effectiveness against influenza A(H1N1)pdm09 viruses in the 2013-2014 and 2015-2016 seasons.
Vaccine updates published in this report were recommended by ACIP during meetings held in October 2016 and February and June 2017.
[email protected]
On Twitter @eaztweets
, according to new recommendations from the Centers for Disease Control and Prevention.
This change from the CDC’s previous guidance that pregnant women receive a seasonal inactivated influenza vaccine (IIV) was recommended by the Advisory Committee on Immunization Practices after some heated debate among committee members over evidence presented to support the change in wording (MMWR. 2017 Aug 25;66[(RR-2]:1-20).
The new update gives women the ability to choose between receiving an IIV and FluBlok, a recombinant influenza vaccine (RIV) that is not egg based and can be manufactured more quickly, making it ideal in cases of pandemic or supply shortages, according to the CDC.
Although pregnant women may choose to receive a vaccination during the first trimester, the CDC warns there may be some risk involved.
“Although experience with the use of IIVs is substantial, and data from observational studies are available to support the safety of these vaccines in pregnancy, data are more limited for vaccination during the first trimester,” according to the CDC. “Moreover, there is substantially less experience with more recently licensed IIV products (e.g., quadrivalent, cell culture-based, and adjuvanted vaccines) during pregnancy in general.”
Data also are limited regarding RIVs, the CDC said, with the data used to determine safety among pregnant women “limited to reports of pregnancies occurring incidentally during clinical trials, Vaccine Adverse Event Reporting System (VAERS) reports, and pregnancy registry reports.”
Changes for children
The CDC chose to accept ACIP recommendations regarding Afluria (IIV3), expanding the age of children who can receive the vaccine from 9 years and older to 5 years and older.
Similar labeling changes were accepted for FluLaval Quadrivalent (IIV4), which had previously been given to children 3 years and older but now but will be available for children starting at 6 months of age.
New products
Recent product licensures included in the MMWR report are Afluria Quadrivalent (IIV4) and Flublok Quadrivalent (RIV4), both for persons over 18 years.
According to the CDC, Flublok Quadrivalent (an RIV) met noninferiority measures, compared with a similar IIV quadrivalent vaccine, for the A(H3H2) and B/Yamagata viruses but not for A(H1N1) or B/Victoria viruses.
Vaccine composition for 2017-2018
Approved viruses for the 2017-2018 season trivalent vaccines are an A/Michigan/45/2015 (H1N1) pdm09–like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008–like virus (Victoria lineage), according to the MMWR. Quadrivalent vaccines will include those viruses, with the addition of an B/Phuket/3073/2013–like virus (Yamagata lineage).
The CDC continues to recommend that the quadrivalent live attenuated influenza vaccine FluMist not be used by anyone for the 2017-2018 season, a decision that was made after evidence showed poor effectiveness against influenza A(H1N1)pdm09 viruses in the 2013-2014 and 2015-2016 seasons.
Vaccine updates published in this report were recommended by ACIP during meetings held in October 2016 and February and June 2017.
[email protected]
On Twitter @eaztweets
, according to new recommendations from the Centers for Disease Control and Prevention.
This change from the CDC’s previous guidance that pregnant women receive a seasonal inactivated influenza vaccine (IIV) was recommended by the Advisory Committee on Immunization Practices after some heated debate among committee members over evidence presented to support the change in wording (MMWR. 2017 Aug 25;66[(RR-2]:1-20).
The new update gives women the ability to choose between receiving an IIV and FluBlok, a recombinant influenza vaccine (RIV) that is not egg based and can be manufactured more quickly, making it ideal in cases of pandemic or supply shortages, according to the CDC.
Although pregnant women may choose to receive a vaccination during the first trimester, the CDC warns there may be some risk involved.
“Although experience with the use of IIVs is substantial, and data from observational studies are available to support the safety of these vaccines in pregnancy, data are more limited for vaccination during the first trimester,” according to the CDC. “Moreover, there is substantially less experience with more recently licensed IIV products (e.g., quadrivalent, cell culture-based, and adjuvanted vaccines) during pregnancy in general.”
Data also are limited regarding RIVs, the CDC said, with the data used to determine safety among pregnant women “limited to reports of pregnancies occurring incidentally during clinical trials, Vaccine Adverse Event Reporting System (VAERS) reports, and pregnancy registry reports.”
Changes for children
The CDC chose to accept ACIP recommendations regarding Afluria (IIV3), expanding the age of children who can receive the vaccine from 9 years and older to 5 years and older.
Similar labeling changes were accepted for FluLaval Quadrivalent (IIV4), which had previously been given to children 3 years and older but now but will be available for children starting at 6 months of age.
New products
Recent product licensures included in the MMWR report are Afluria Quadrivalent (IIV4) and Flublok Quadrivalent (RIV4), both for persons over 18 years.
According to the CDC, Flublok Quadrivalent (an RIV) met noninferiority measures, compared with a similar IIV quadrivalent vaccine, for the A(H3H2) and B/Yamagata viruses but not for A(H1N1) or B/Victoria viruses.
Vaccine composition for 2017-2018
Approved viruses for the 2017-2018 season trivalent vaccines are an A/Michigan/45/2015 (H1N1) pdm09–like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008–like virus (Victoria lineage), according to the MMWR. Quadrivalent vaccines will include those viruses, with the addition of an B/Phuket/3073/2013–like virus (Yamagata lineage).
The CDC continues to recommend that the quadrivalent live attenuated influenza vaccine FluMist not be used by anyone for the 2017-2018 season, a decision that was made after evidence showed poor effectiveness against influenza A(H1N1)pdm09 viruses in the 2013-2014 and 2015-2016 seasons.
Vaccine updates published in this report were recommended by ACIP during meetings held in October 2016 and February and June 2017.
[email protected]
On Twitter @eaztweets
FROM MMWR
Consider routine penicillin allergy testing in obstetrics
PARK CITY, UTAH – When attendees at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology were asked if their institutions test to confirm alleged penicillin allergies, the only hands that went up were from clinicians at Duke University.
That’s a problem, according to Robert Heine, MD, a maternal-fetal medicine specialist at Duke, in Durham, N.C. “We, as a group, need to be doing [penicillin] allergy testing,” he said.
It’s become clear in recent years that patients who say they have a penicillin allergy often don’t have one, or remember a mild reaction from childhood that doesn’t preclude the use of beta-lactam antibiotics as adults. For decades, however, clinicians have taken those claims at face value, and duly noted them in charts and switched patients to non–beta-lactam antibiotics that don’t work as well.
That’s what happened at Duke in 2014. A total of 81 women with documented penicillin allergies were put on gentamicin and clindamycin to protect against cesarean wound infections and 16% ended up with infections anyway. Among the 864 women who received cefazolin – the first-line cesarean prophylaxis choice at Duke – the infection rate was 7%.
“Beta-lactam antibiotic prophylaxis reduced the risk of surgical site infections after cesareans by 60%,” said Benjamin Harris, MD, the lead investigator and an ob.gyn. resident at Duke, who presented the findings at the meeting.
When the investigators took a closer look at the 81 women who reported penicillin allergies, most of them had rashes and other mild reactions noted in their charts.
Findings such as those led Dr. Heine to push for routine testing. “I brought Duke into it kicking and screaming,” he said. The biggest obstacle was concern over liability, specifically that pregnant women would go into anaphylaxis and deliver prematurely, he said.
After a lot of lobbying, Dr. Heine and his colleagues started routine penicillin allergy testing in March 2016. There hasn’t been a single reaction among the 80-plus pregnant women tested so far, he reported.
Duke administrators were also concerned about reimbursement, but it hasn’t turned out to be a problem. Reimbursements from public and private payers “cover our costs,” a little over $100 per test, Dr. Heine said.
Dr. Heine said he can imagine outpatient testing at some point, but for now women are checked into triage. They get a fetal heart tone before 24 weeks, and a fetal heart rate monitor afterward. “We try to do it before 20 weeks so we don’t have to worry about the fetus,” he said.
When penicillin allergies are in the chart, or women say they are allergic, ask what type of reaction they had in the past. Type 1 reactions should be confirmed with testing. It’s okay to skip testing and give beta-lactams for non–type 1 reactions, but “if a woman has a non–type 1, and they’re already set up for testing, I’m going to do it anyway because getting the penicillin allergy off her chart is good for her and her life,” Dr. Heine said.
Dr. Heine and Dr. Harris reported having no financial disclosures.
PARK CITY, UTAH – When attendees at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology were asked if their institutions test to confirm alleged penicillin allergies, the only hands that went up were from clinicians at Duke University.
That’s a problem, according to Robert Heine, MD, a maternal-fetal medicine specialist at Duke, in Durham, N.C. “We, as a group, need to be doing [penicillin] allergy testing,” he said.
It’s become clear in recent years that patients who say they have a penicillin allergy often don’t have one, or remember a mild reaction from childhood that doesn’t preclude the use of beta-lactam antibiotics as adults. For decades, however, clinicians have taken those claims at face value, and duly noted them in charts and switched patients to non–beta-lactam antibiotics that don’t work as well.
That’s what happened at Duke in 2014. A total of 81 women with documented penicillin allergies were put on gentamicin and clindamycin to protect against cesarean wound infections and 16% ended up with infections anyway. Among the 864 women who received cefazolin – the first-line cesarean prophylaxis choice at Duke – the infection rate was 7%.
“Beta-lactam antibiotic prophylaxis reduced the risk of surgical site infections after cesareans by 60%,” said Benjamin Harris, MD, the lead investigator and an ob.gyn. resident at Duke, who presented the findings at the meeting.
When the investigators took a closer look at the 81 women who reported penicillin allergies, most of them had rashes and other mild reactions noted in their charts.
Findings such as those led Dr. Heine to push for routine testing. “I brought Duke into it kicking and screaming,” he said. The biggest obstacle was concern over liability, specifically that pregnant women would go into anaphylaxis and deliver prematurely, he said.
After a lot of lobbying, Dr. Heine and his colleagues started routine penicillin allergy testing in March 2016. There hasn’t been a single reaction among the 80-plus pregnant women tested so far, he reported.
Duke administrators were also concerned about reimbursement, but it hasn’t turned out to be a problem. Reimbursements from public and private payers “cover our costs,” a little over $100 per test, Dr. Heine said.
Dr. Heine said he can imagine outpatient testing at some point, but for now women are checked into triage. They get a fetal heart tone before 24 weeks, and a fetal heart rate monitor afterward. “We try to do it before 20 weeks so we don’t have to worry about the fetus,” he said.
When penicillin allergies are in the chart, or women say they are allergic, ask what type of reaction they had in the past. Type 1 reactions should be confirmed with testing. It’s okay to skip testing and give beta-lactams for non–type 1 reactions, but “if a woman has a non–type 1, and they’re already set up for testing, I’m going to do it anyway because getting the penicillin allergy off her chart is good for her and her life,” Dr. Heine said.
Dr. Heine and Dr. Harris reported having no financial disclosures.
PARK CITY, UTAH – When attendees at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology were asked if their institutions test to confirm alleged penicillin allergies, the only hands that went up were from clinicians at Duke University.
That’s a problem, according to Robert Heine, MD, a maternal-fetal medicine specialist at Duke, in Durham, N.C. “We, as a group, need to be doing [penicillin] allergy testing,” he said.
It’s become clear in recent years that patients who say they have a penicillin allergy often don’t have one, or remember a mild reaction from childhood that doesn’t preclude the use of beta-lactam antibiotics as adults. For decades, however, clinicians have taken those claims at face value, and duly noted them in charts and switched patients to non–beta-lactam antibiotics that don’t work as well.
That’s what happened at Duke in 2014. A total of 81 women with documented penicillin allergies were put on gentamicin and clindamycin to protect against cesarean wound infections and 16% ended up with infections anyway. Among the 864 women who received cefazolin – the first-line cesarean prophylaxis choice at Duke – the infection rate was 7%.
“Beta-lactam antibiotic prophylaxis reduced the risk of surgical site infections after cesareans by 60%,” said Benjamin Harris, MD, the lead investigator and an ob.gyn. resident at Duke, who presented the findings at the meeting.
When the investigators took a closer look at the 81 women who reported penicillin allergies, most of them had rashes and other mild reactions noted in their charts.
Findings such as those led Dr. Heine to push for routine testing. “I brought Duke into it kicking and screaming,” he said. The biggest obstacle was concern over liability, specifically that pregnant women would go into anaphylaxis and deliver prematurely, he said.
After a lot of lobbying, Dr. Heine and his colleagues started routine penicillin allergy testing in March 2016. There hasn’t been a single reaction among the 80-plus pregnant women tested so far, he reported.
Duke administrators were also concerned about reimbursement, but it hasn’t turned out to be a problem. Reimbursements from public and private payers “cover our costs,” a little over $100 per test, Dr. Heine said.
Dr. Heine said he can imagine outpatient testing at some point, but for now women are checked into triage. They get a fetal heart tone before 24 weeks, and a fetal heart rate monitor afterward. “We try to do it before 20 weeks so we don’t have to worry about the fetus,” he said.
When penicillin allergies are in the chart, or women say they are allergic, ask what type of reaction they had in the past. Type 1 reactions should be confirmed with testing. It’s okay to skip testing and give beta-lactams for non–type 1 reactions, but “if a woman has a non–type 1, and they’re already set up for testing, I’m going to do it anyway because getting the penicillin allergy off her chart is good for her and her life,” Dr. Heine said.
Dr. Heine and Dr. Harris reported having no financial disclosures.
AT IDSOG
Key clinical point:
Major finding: Among 81 women with documented penicillin allergies who received gentamicin and clindamycin, 16% developed surgical site infections. In contrast, among the 864 women who received cefazolin, the infection rate was 7%.
Data source: A single-center review at Duke University.
Disclosures: The investigators reported having no relevant financial disclosures.