User login
LMWH doesn’t reduce late pregnancy loss in women with thrombophilias
Prophylactic-dose low molecular weight heparin (LMWH), with or without aspirin, did not reduce the risk of pregnancy loss in women with inherited thrombophilia and a history of prior late or recurrent early pregnancy loss, based on a meta-analysis of randomized, controlled trials.
“To our knowledge, this is the largest study published to date that evaluates LMWH in women with inherited thrombophilia and previous pregnancy loss,” Dr. Leslie Skeith, of the University of Ottawa, and her colleagues wrote in Blood (2016 Mar 31;127[13]:1650-55). A recent Cochrane Review (2014 Jul 4;7:CD004734) similarly found no difference in live birth rates in women with or without inherited thrombophilia treated with LMWH and aspirin, compared with women given no treatment. Additionally, the Effects of Aspirin in Gestation and Reproduction [EAGeR] trial found no difference in live birth rates in women with previous pregnancy loss given aspirin or placebo (Lancet. 2014;384[9937]:29-36).
Based on a literature search, 8 publications and 483 participants met eligibility criteria as randomized, controlled trials for the meta-analysis. Four trials included an LMWH-plus-aspirin arm, and five trials included an LMWH-only arm. The control groups included four trials with an aspirin arm, and five trials with a placebo or no-treatment arm. The data indicated no difference in the treated groups and controls (relative risk of 0.81; 95% confidence interval, 0.55-1.19; P = .28).
As there is the potential for adverse side effects and significant cost with LMWH, the researchers advise against the use of LMWH to prevent recurrent and prior late pregnancy loss (greater than 10 weeks gestation) in women with inherited thrombophilia (Grade 1B, strong recommendation with moderate-quality evidence) and suggest against LMWH to prevent recurrent pregnancy loss in women with inherited thrombophilia and prior recurrent early (less than 10 weeks) pregnancy loss. (Grade 2B, weak recommendation with moderate-quality evidence.)
Given that the analysis included just 66 women with thrombophilia and prior recurrent early pregnancy loss, the researchers could not exclude a beneficial effect of LMWH in this subgroup. An ongoing randomized controlled trial, ALIFE2 (Netherlands Trial Registration Identifier: NTR3361), “is evaluating LMWH in women with inherited thrombophilia and a history of two or more miscarriages and/or intrauterine fetal death, which we hope will provide definitive answers to this question,” the researchers wrote.
They also suggest not testing for inherited thrombophilia in women with prior late or recurrent early pregnancy loss (Grade 2B, weak recommendation with moderate-quality evidence).
The study was supported by a series of university and institutional investigator research awards. Dr. Skeith received a Thrombosis Canada CanVECTOR Research Fellowship award.
On Twitter @maryjodales
Prophylactic-dose low molecular weight heparin (LMWH), with or without aspirin, did not reduce the risk of pregnancy loss in women with inherited thrombophilia and a history of prior late or recurrent early pregnancy loss, based on a meta-analysis of randomized, controlled trials.
“To our knowledge, this is the largest study published to date that evaluates LMWH in women with inherited thrombophilia and previous pregnancy loss,” Dr. Leslie Skeith, of the University of Ottawa, and her colleagues wrote in Blood (2016 Mar 31;127[13]:1650-55). A recent Cochrane Review (2014 Jul 4;7:CD004734) similarly found no difference in live birth rates in women with or without inherited thrombophilia treated with LMWH and aspirin, compared with women given no treatment. Additionally, the Effects of Aspirin in Gestation and Reproduction [EAGeR] trial found no difference in live birth rates in women with previous pregnancy loss given aspirin or placebo (Lancet. 2014;384[9937]:29-36).
Based on a literature search, 8 publications and 483 participants met eligibility criteria as randomized, controlled trials for the meta-analysis. Four trials included an LMWH-plus-aspirin arm, and five trials included an LMWH-only arm. The control groups included four trials with an aspirin arm, and five trials with a placebo or no-treatment arm. The data indicated no difference in the treated groups and controls (relative risk of 0.81; 95% confidence interval, 0.55-1.19; P = .28).
As there is the potential for adverse side effects and significant cost with LMWH, the researchers advise against the use of LMWH to prevent recurrent and prior late pregnancy loss (greater than 10 weeks gestation) in women with inherited thrombophilia (Grade 1B, strong recommendation with moderate-quality evidence) and suggest against LMWH to prevent recurrent pregnancy loss in women with inherited thrombophilia and prior recurrent early (less than 10 weeks) pregnancy loss. (Grade 2B, weak recommendation with moderate-quality evidence.)
Given that the analysis included just 66 women with thrombophilia and prior recurrent early pregnancy loss, the researchers could not exclude a beneficial effect of LMWH in this subgroup. An ongoing randomized controlled trial, ALIFE2 (Netherlands Trial Registration Identifier: NTR3361), “is evaluating LMWH in women with inherited thrombophilia and a history of two or more miscarriages and/or intrauterine fetal death, which we hope will provide definitive answers to this question,” the researchers wrote.
They also suggest not testing for inherited thrombophilia in women with prior late or recurrent early pregnancy loss (Grade 2B, weak recommendation with moderate-quality evidence).
The study was supported by a series of university and institutional investigator research awards. Dr. Skeith received a Thrombosis Canada CanVECTOR Research Fellowship award.
On Twitter @maryjodales
Prophylactic-dose low molecular weight heparin (LMWH), with or without aspirin, did not reduce the risk of pregnancy loss in women with inherited thrombophilia and a history of prior late or recurrent early pregnancy loss, based on a meta-analysis of randomized, controlled trials.
“To our knowledge, this is the largest study published to date that evaluates LMWH in women with inherited thrombophilia and previous pregnancy loss,” Dr. Leslie Skeith, of the University of Ottawa, and her colleagues wrote in Blood (2016 Mar 31;127[13]:1650-55). A recent Cochrane Review (2014 Jul 4;7:CD004734) similarly found no difference in live birth rates in women with or without inherited thrombophilia treated with LMWH and aspirin, compared with women given no treatment. Additionally, the Effects of Aspirin in Gestation and Reproduction [EAGeR] trial found no difference in live birth rates in women with previous pregnancy loss given aspirin or placebo (Lancet. 2014;384[9937]:29-36).
Based on a literature search, 8 publications and 483 participants met eligibility criteria as randomized, controlled trials for the meta-analysis. Four trials included an LMWH-plus-aspirin arm, and five trials included an LMWH-only arm. The control groups included four trials with an aspirin arm, and five trials with a placebo or no-treatment arm. The data indicated no difference in the treated groups and controls (relative risk of 0.81; 95% confidence interval, 0.55-1.19; P = .28).
As there is the potential for adverse side effects and significant cost with LMWH, the researchers advise against the use of LMWH to prevent recurrent and prior late pregnancy loss (greater than 10 weeks gestation) in women with inherited thrombophilia (Grade 1B, strong recommendation with moderate-quality evidence) and suggest against LMWH to prevent recurrent pregnancy loss in women with inherited thrombophilia and prior recurrent early (less than 10 weeks) pregnancy loss. (Grade 2B, weak recommendation with moderate-quality evidence.)
Given that the analysis included just 66 women with thrombophilia and prior recurrent early pregnancy loss, the researchers could not exclude a beneficial effect of LMWH in this subgroup. An ongoing randomized controlled trial, ALIFE2 (Netherlands Trial Registration Identifier: NTR3361), “is evaluating LMWH in women with inherited thrombophilia and a history of two or more miscarriages and/or intrauterine fetal death, which we hope will provide definitive answers to this question,” the researchers wrote.
They also suggest not testing for inherited thrombophilia in women with prior late or recurrent early pregnancy loss (Grade 2B, weak recommendation with moderate-quality evidence).
The study was supported by a series of university and institutional investigator research awards. Dr. Skeith received a Thrombosis Canada CanVECTOR Research Fellowship award.
On Twitter @maryjodales
FROM BLOOD
Key clinical point: Given the potential for adverse side effects and significant cost, the researchers advise against the use of LMWH to prevent recurrent and prior late pregnancy loss (greater than 10 weeks gestation) in women with inherited thrombophilia.
Major finding: The data indicated no difference in the treated groups and controls (relative risk of 0.81; 95% confidence interval, 0.55-1.19; P = .28).
Data source: Based on a literature search, 8 publications and 483 participants met eligibility criteria as randomized, controlled trials for the meta-analysis.
Disclosures: The study was supported by a series of university and institutional investigator research awards. Dr. Skeith received a Thrombosis Canada CanVECTOR Research Fellowship award.
Intractable shoulder dystocia: A posterior axilla maneuver may save the day
Shoulder dystocia is an unpredictable obstetric emergency that challenges all obstetricians and midwives. In response to a shoulder dystocia emergency, most clinicians implement a sequence of well-practiced steps that begin with early recognition of the problem, clear communication of the emergency with delivery room staff, and a call for help to available clinicians. Management steps may include:
- instructing the mother to stop pushing and moving the mother's buttocks to the edge of the bed
- ensuring there is not a tight nuchal cord
- committing to avoiding the use of excessive force on the fetal head and neck
- considering performing an episiotomy
- performing the McRoberts maneuver combined with suprapubic pressure
- using a rotational maneuver, such as the Woods maneuver or the Rubin maneuver
- delivering the posterior arm
- considering the Gaskin all-four maneuver.
When initial management steps are not enoughIf this sequence of steps does not result in successful vaginal delivery, additional options include: clavicle fracture, cephalic replacement followed by cesarean delivery (Zavanelli maneuver), symphysiotomy, or fundal pressure combined with a rotational maneuver. Another simple intervention that is not discussed widely in medical textbooks or taught during training is the posterior axilla maneuver.
Posterior axilla maneuversVarying posterior axilla maneuvers have been described by many expert obstetricians, including Willughby (17th Century),1 Holman (1963),2 Schramm (1983),3 Menticoglou (2006),4 and Hofmeyr and Cluver (2009, 2015).5−7
Willughby maneuverPercival Willughby’s (1596−1685) description of a posterior axilla maneuver was brief1:
After the head is born, if the child through the greatness of the shoulders, should stick at the neck, let the midwife put her fingers under the child's armpit and give it a nudge, thrusting it to the other side with her finger, drawing the child or she may quickly bring forth the shoulders, without offering to put it forth by her hands clasped about the neck, which might endanger the breaking of the neck.
Holman maneuverHolman described a maneuver with the following steps2:
- perform an episiotomy
- place a finger in the posterior axilla and draw the posterior shoulder down along the pelvic axis
- simultaneously have an assistant perform suprapubic pressure and
- if necessary, insert two supinated fingers under the pubic arch and press and rock the anterior shoulder, tilting the anterior shoulder toward the hollow of the sacrum while simultaneously gently pulling the posterior axilla along the pelvic axis.
Schramm maneuverSchramm, working with a population enriched with women with diabetes, frequently encountered shoulder dystocia and recommended3:
If the posterior axilla can be reached—in other words, if the posterior shoulder is engaged—in my experience it can always be delivered by rotating it to the anterior position while at the same time applying traction....I normally place 1 or 2 fingers of my right hand in the posterior axilla and “scruff” the neck with my left hand, applying both rotation and traction. Because this grip is somewhat insecure, the resultant tractive force is limited and I consider this manoeuvre to be the most effective and least traumatic method of relieving moderate to severe obstruction.
Practice your shoulder dystocia maneuvers using simulation
Obstetric emergencies trigger a rush of adrenaline and great stress for the obstetrician and delivery room team. This may adversely impact motor performance, decision making, and communication skills.1 Low- and high-fidelity simulation exercises create an environment in which the obstetrics team can practice the sequence of maneuvers and seamless teamwork needed to successfully resolve a shoulder dystocia.2,3 Implementing a shoulder dystocia protocol and practicing the protocol using team-based simulation may help to reduce the adverse outcomes of shoulder dystocia.3,4
Reference
1. Wetzel CM, Kneebone RL, Woloshynowych M, et al. The effects of stress on surgical performance. Am J Surg. 2006;191(1):5−10.
2. Crofts JF, Fox R, Ellis D, Winter C, Hinshaw K, Draycott TJ. Observations from 450 shoulder dystocia simulations. Obstet Gynecol. 2008;112(4):906−912.
3. Draycott TJ, Crofts JF, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14−20.
4. Grobman WA, Miller D, Burke C, Hornbogen A, Tam K, Costello R. Outcomes associated with introduction of a shoulder dystocia protocol. Am J Obstet Gynecol. 2011;205(6):513−517.
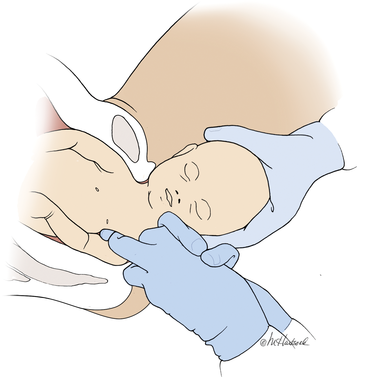
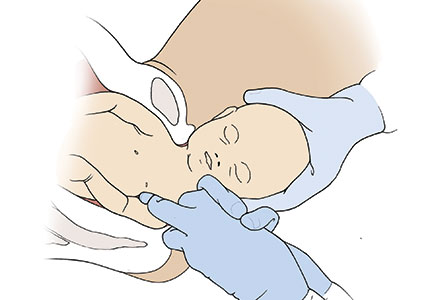
Manipulation of the posterior axilla |
|
The right and left third fingers are locked into the posterior axilla, one finger from the front and one from the back of the fetus. Gentle downward guidance is provided by the fingers to draw the posterior shoulder down and out along the curve of the sacrum, thus releasing the anterior shoulder.4 In this drawing, an assistant gently holds the head up. |
Menticoglou maneuverMenticoglou noted that delivery of the posterior arm generally resolves almost all cases of shoulder dystocia. However, if the posterior arm is extended and trapped between the fetus and maternal pelvic side-wall, it may be difficult to deliver the posterior arm. In these cases he recommended having an assistant gently hold, not pull, the fetal head upward and, at the same time, having the obstetrician get on one knee, placing the middle fingers of both hands into the posterior axilla of the fetus.4
The right middle finger is placed into the axilla from the left side of the maternal pelvis, and the left middle finger is placed into the axilla from the right side of the maternal pelvis, resulting in the two middle fingers overlapping in the fetal axilla (FIGURE).4 Gentle force is then used to pull the posterior shoulder and arm downward and outward along the curve of the sacrum. Once the shoulder has emerged from the pelvis, the posterior arm is delivered. Alternatively, if the posterior shoulder is brought well down into the pelvis, another attempt can be made at delivering the posterior arm.4
My preferred approach. The Menticoglou maneuver is my preferred posterior axilla maneuver because it can be accomplished rapidly; requires no equipment, such as a sling catheter; and the obstetrician has good tactile feedback throughout the application of gentle force.
Hofmeyr-Cluver maneuverIn cases of difficult shoulder dystocia, Dr. William Smellie (1762)8 recommended placing one or two fingers in the anterior or posterior fetal axilla and gentling pulling on the axilla to deliver the body. If the axillae were too high to reach, he recommended using a blunt hook in the axilla to draw forth the impacted child. He advised caution when using a blunt hook because the fetus might be injured or lacerated.
Instead of using a hook, Hofmeyr and Cluver5−7 have recommended using a catheter sling to deliver the posterior shoulder. In this maneuver, a loop of a suction catheter or firm urinary catheter is placed over the obstetrician’s index finger and the loop is pushed through the posterior axilla, back to front, with guidance from the index finger. The index finger of the opposite hand is used to catch the loop and pull the catheter through, creating a single-stranded sling that is positioned in the axilla. Gentle force is then applied to the sling in the axis of the pelvis to deliver the posterior shoulder.
“If the posterior arm does not follow it is then swept out easily because room has been created by delivering the posterior shoulder. If the aforementioned procedure fails, the sling can be used to rotate the shoulder. To perform a rotational maneuver, sling traction is directed laterally towards the side of the baby’s back then anteriorly while digital pressure is applied behind the anterior shoulder to assist rotation.”7
Use ACOG’s checklist for documenting a shoulder dystocia
Following the resolution of a shoulder dystocia, it is important to gather all the necessary facts to complete a detailed medical record entry describing the situation and interventions used. The checklist from the American College of Obstetricians and Gynecologists (ACOG) helps you to prepare a standardized medical record entry that is comprehensive.
My experience is that “free form” medical record entries describing the events at a shoulder dystocia event are generally not optimally organized, creating future problems when the case is reviewed.
ACOG obstetric checklists are available for download at http://www.acog.org/Resources-And-Publications, or use your web browser to search for “ACOG Shoulder Dystocia checklist.”
With scant literature, know the benefits and risksThe world’s literature on posterior axilla maneuvers to resolve shoulder dystocia consists of case series and individual case reports.2−7 Hence, the quality of the data supporting this intervention is not optimal, and risks associated with the maneuver are not well characterized. Application of a controlled and gentle force to the posterior axilla may cause fracture of the fetal humerus5 or dislocation of the fetal shoulder. The posterior axilla maneuver also may increase the risk of a maternal third- or fourth-degree perineal laceration.
As a general rule, as the number of maneuvers used to resolve a difficult shoulder dystocia increase, the risk of neonatal injury increases.9 Since the posterior axilla maneuver typically is only attempted after multiple previous maneuvers have failed, the risk of fetal injury is increased. However, as time passes and a shoulder dystocia remains unresolved for 4 or 5 minutes, the risk of neurologic injury and fetal death increases.10
In resolving a shoulder dystocia, speed and skill are essential. A posterior axilla maneuver can be performed more rapidly than a Zavanelli maneuver or a symphysiotomy. Although manipulation of the posterior axilla and arm may cause a fracture of the humerus, this complication is a modest price to pay for preventing permanent fetal brain injury or fetal death.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Willughby P. Observations in midwifery. New York, NY: MW Books; 1972:312−313.
- Holman MS. A new manoeuvre for delivery of an impacted shoulder based on a mechanical analysis. S Afr Med J. 1963;37:247−249.
- Schramm M. Impacted shoulders—a personal experience. Aust N Z J Obstet Gynaecol. 1983;23(1):28−31.
- Menticoglou SM. A modified technique to deliver the posterior arm in severe shoulder dystocia. Obstet Gynecol. 2006;108(3 pt 2):755−757.
- Cluver CA, Hofmeyr GJ. Posterior axilla sling traction: a technique for intractable shoulder dystocia. Obstet Gynecol. 2009;113(2 pt 2):486–488.
- Hofmeyr GJ, Cluver CA. Posterior axilla sling traction for intractable shoulder dystocia. BJOG. 2009;116(13):1818−1820.
- Cluver CA, Hofmeyr GJ. Posterior axilla sling traction for shoulder dystocia: case review and a new method for shoulder rotation with the sling. Am J Obstet Gynecol. 2015;212(6):784.e1−e7.
- Smellie W. A treatise on the theory and practice of midwifery. 4th ed. London, England; 1762:226−227.
- Hoffman MK, Bailit JL, Branch DW, et al; Consortium on Safe Labor. A comparison of obstetric maneuvers for the acute management of shoulder dystocia. Obstet Gynecol. 2011;117(6):1272−1278.
- Lerner H, Durlacher K, Smith S, Hamilton E. Relationship between head-to-body delivery interval in shoulder dystocia and neonatal depression. Obstet Gynecol. 2011;118(2 pt 1):318−322.
Shoulder dystocia is an unpredictable obstetric emergency that challenges all obstetricians and midwives. In response to a shoulder dystocia emergency, most clinicians implement a sequence of well-practiced steps that begin with early recognition of the problem, clear communication of the emergency with delivery room staff, and a call for help to available clinicians. Management steps may include:
- instructing the mother to stop pushing and moving the mother's buttocks to the edge of the bed
- ensuring there is not a tight nuchal cord
- committing to avoiding the use of excessive force on the fetal head and neck
- considering performing an episiotomy
- performing the McRoberts maneuver combined with suprapubic pressure
- using a rotational maneuver, such as the Woods maneuver or the Rubin maneuver
- delivering the posterior arm
- considering the Gaskin all-four maneuver.
When initial management steps are not enoughIf this sequence of steps does not result in successful vaginal delivery, additional options include: clavicle fracture, cephalic replacement followed by cesarean delivery (Zavanelli maneuver), symphysiotomy, or fundal pressure combined with a rotational maneuver. Another simple intervention that is not discussed widely in medical textbooks or taught during training is the posterior axilla maneuver.
Posterior axilla maneuversVarying posterior axilla maneuvers have been described by many expert obstetricians, including Willughby (17th Century),1 Holman (1963),2 Schramm (1983),3 Menticoglou (2006),4 and Hofmeyr and Cluver (2009, 2015).5−7
Willughby maneuverPercival Willughby’s (1596−1685) description of a posterior axilla maneuver was brief1:
After the head is born, if the child through the greatness of the shoulders, should stick at the neck, let the midwife put her fingers under the child's armpit and give it a nudge, thrusting it to the other side with her finger, drawing the child or she may quickly bring forth the shoulders, without offering to put it forth by her hands clasped about the neck, which might endanger the breaking of the neck.
Holman maneuverHolman described a maneuver with the following steps2:
- perform an episiotomy
- place a finger in the posterior axilla and draw the posterior shoulder down along the pelvic axis
- simultaneously have an assistant perform suprapubic pressure and
- if necessary, insert two supinated fingers under the pubic arch and press and rock the anterior shoulder, tilting the anterior shoulder toward the hollow of the sacrum while simultaneously gently pulling the posterior axilla along the pelvic axis.
Schramm maneuverSchramm, working with a population enriched with women with diabetes, frequently encountered shoulder dystocia and recommended3:
If the posterior axilla can be reached—in other words, if the posterior shoulder is engaged—in my experience it can always be delivered by rotating it to the anterior position while at the same time applying traction....I normally place 1 or 2 fingers of my right hand in the posterior axilla and “scruff” the neck with my left hand, applying both rotation and traction. Because this grip is somewhat insecure, the resultant tractive force is limited and I consider this manoeuvre to be the most effective and least traumatic method of relieving moderate to severe obstruction.
Practice your shoulder dystocia maneuvers using simulation
Obstetric emergencies trigger a rush of adrenaline and great stress for the obstetrician and delivery room team. This may adversely impact motor performance, decision making, and communication skills.1 Low- and high-fidelity simulation exercises create an environment in which the obstetrics team can practice the sequence of maneuvers and seamless teamwork needed to successfully resolve a shoulder dystocia.2,3 Implementing a shoulder dystocia protocol and practicing the protocol using team-based simulation may help to reduce the adverse outcomes of shoulder dystocia.3,4
Reference
1. Wetzel CM, Kneebone RL, Woloshynowych M, et al. The effects of stress on surgical performance. Am J Surg. 2006;191(1):5−10.
2. Crofts JF, Fox R, Ellis D, Winter C, Hinshaw K, Draycott TJ. Observations from 450 shoulder dystocia simulations. Obstet Gynecol. 2008;112(4):906−912.
3. Draycott TJ, Crofts JF, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14−20.
4. Grobman WA, Miller D, Burke C, Hornbogen A, Tam K, Costello R. Outcomes associated with introduction of a shoulder dystocia protocol. Am J Obstet Gynecol. 2011;205(6):513−517.
Manipulation of the posterior axilla |
|
The right and left third fingers are locked into the posterior axilla, one finger from the front and one from the back of the fetus. Gentle downward guidance is provided by the fingers to draw the posterior shoulder down and out along the curve of the sacrum, thus releasing the anterior shoulder.4 In this drawing, an assistant gently holds the head up. |
Menticoglou maneuverMenticoglou noted that delivery of the posterior arm generally resolves almost all cases of shoulder dystocia. However, if the posterior arm is extended and trapped between the fetus and maternal pelvic side-wall, it may be difficult to deliver the posterior arm. In these cases he recommended having an assistant gently hold, not pull, the fetal head upward and, at the same time, having the obstetrician get on one knee, placing the middle fingers of both hands into the posterior axilla of the fetus.4
The right middle finger is placed into the axilla from the left side of the maternal pelvis, and the left middle finger is placed into the axilla from the right side of the maternal pelvis, resulting in the two middle fingers overlapping in the fetal axilla (FIGURE).4 Gentle force is then used to pull the posterior shoulder and arm downward and outward along the curve of the sacrum. Once the shoulder has emerged from the pelvis, the posterior arm is delivered. Alternatively, if the posterior shoulder is brought well down into the pelvis, another attempt can be made at delivering the posterior arm.4
My preferred approach. The Menticoglou maneuver is my preferred posterior axilla maneuver because it can be accomplished rapidly; requires no equipment, such as a sling catheter; and the obstetrician has good tactile feedback throughout the application of gentle force.
Hofmeyr-Cluver maneuverIn cases of difficult shoulder dystocia, Dr. William Smellie (1762)8 recommended placing one or two fingers in the anterior or posterior fetal axilla and gentling pulling on the axilla to deliver the body. If the axillae were too high to reach, he recommended using a blunt hook in the axilla to draw forth the impacted child. He advised caution when using a blunt hook because the fetus might be injured or lacerated.
Instead of using a hook, Hofmeyr and Cluver5−7 have recommended using a catheter sling to deliver the posterior shoulder. In this maneuver, a loop of a suction catheter or firm urinary catheter is placed over the obstetrician’s index finger and the loop is pushed through the posterior axilla, back to front, with guidance from the index finger. The index finger of the opposite hand is used to catch the loop and pull the catheter through, creating a single-stranded sling that is positioned in the axilla. Gentle force is then applied to the sling in the axis of the pelvis to deliver the posterior shoulder.
“If the posterior arm does not follow it is then swept out easily because room has been created by delivering the posterior shoulder. If the aforementioned procedure fails, the sling can be used to rotate the shoulder. To perform a rotational maneuver, sling traction is directed laterally towards the side of the baby’s back then anteriorly while digital pressure is applied behind the anterior shoulder to assist rotation.”7
Use ACOG’s checklist for documenting a shoulder dystocia
Following the resolution of a shoulder dystocia, it is important to gather all the necessary facts to complete a detailed medical record entry describing the situation and interventions used. The checklist from the American College of Obstetricians and Gynecologists (ACOG) helps you to prepare a standardized medical record entry that is comprehensive.
My experience is that “free form” medical record entries describing the events at a shoulder dystocia event are generally not optimally organized, creating future problems when the case is reviewed.
ACOG obstetric checklists are available for download at http://www.acog.org/Resources-And-Publications, or use your web browser to search for “ACOG Shoulder Dystocia checklist.”
With scant literature, know the benefits and risksThe world’s literature on posterior axilla maneuvers to resolve shoulder dystocia consists of case series and individual case reports.2−7 Hence, the quality of the data supporting this intervention is not optimal, and risks associated with the maneuver are not well characterized. Application of a controlled and gentle force to the posterior axilla may cause fracture of the fetal humerus5 or dislocation of the fetal shoulder. The posterior axilla maneuver also may increase the risk of a maternal third- or fourth-degree perineal laceration.
As a general rule, as the number of maneuvers used to resolve a difficult shoulder dystocia increase, the risk of neonatal injury increases.9 Since the posterior axilla maneuver typically is only attempted after multiple previous maneuvers have failed, the risk of fetal injury is increased. However, as time passes and a shoulder dystocia remains unresolved for 4 or 5 minutes, the risk of neurologic injury and fetal death increases.10
In resolving a shoulder dystocia, speed and skill are essential. A posterior axilla maneuver can be performed more rapidly than a Zavanelli maneuver or a symphysiotomy. Although manipulation of the posterior axilla and arm may cause a fracture of the humerus, this complication is a modest price to pay for preventing permanent fetal brain injury or fetal death.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Shoulder dystocia is an unpredictable obstetric emergency that challenges all obstetricians and midwives. In response to a shoulder dystocia emergency, most clinicians implement a sequence of well-practiced steps that begin with early recognition of the problem, clear communication of the emergency with delivery room staff, and a call for help to available clinicians. Management steps may include:
- instructing the mother to stop pushing and moving the mother's buttocks to the edge of the bed
- ensuring there is not a tight nuchal cord
- committing to avoiding the use of excessive force on the fetal head and neck
- considering performing an episiotomy
- performing the McRoberts maneuver combined with suprapubic pressure
- using a rotational maneuver, such as the Woods maneuver or the Rubin maneuver
- delivering the posterior arm
- considering the Gaskin all-four maneuver.
When initial management steps are not enoughIf this sequence of steps does not result in successful vaginal delivery, additional options include: clavicle fracture, cephalic replacement followed by cesarean delivery (Zavanelli maneuver), symphysiotomy, or fundal pressure combined with a rotational maneuver. Another simple intervention that is not discussed widely in medical textbooks or taught during training is the posterior axilla maneuver.
Posterior axilla maneuversVarying posterior axilla maneuvers have been described by many expert obstetricians, including Willughby (17th Century),1 Holman (1963),2 Schramm (1983),3 Menticoglou (2006),4 and Hofmeyr and Cluver (2009, 2015).5−7
Willughby maneuverPercival Willughby’s (1596−1685) description of a posterior axilla maneuver was brief1:
After the head is born, if the child through the greatness of the shoulders, should stick at the neck, let the midwife put her fingers under the child's armpit and give it a nudge, thrusting it to the other side with her finger, drawing the child or she may quickly bring forth the shoulders, without offering to put it forth by her hands clasped about the neck, which might endanger the breaking of the neck.
Holman maneuverHolman described a maneuver with the following steps2:
- perform an episiotomy
- place a finger in the posterior axilla and draw the posterior shoulder down along the pelvic axis
- simultaneously have an assistant perform suprapubic pressure and
- if necessary, insert two supinated fingers under the pubic arch and press and rock the anterior shoulder, tilting the anterior shoulder toward the hollow of the sacrum while simultaneously gently pulling the posterior axilla along the pelvic axis.
Schramm maneuverSchramm, working with a population enriched with women with diabetes, frequently encountered shoulder dystocia and recommended3:
If the posterior axilla can be reached—in other words, if the posterior shoulder is engaged—in my experience it can always be delivered by rotating it to the anterior position while at the same time applying traction....I normally place 1 or 2 fingers of my right hand in the posterior axilla and “scruff” the neck with my left hand, applying both rotation and traction. Because this grip is somewhat insecure, the resultant tractive force is limited and I consider this manoeuvre to be the most effective and least traumatic method of relieving moderate to severe obstruction.
Practice your shoulder dystocia maneuvers using simulation
Obstetric emergencies trigger a rush of adrenaline and great stress for the obstetrician and delivery room team. This may adversely impact motor performance, decision making, and communication skills.1 Low- and high-fidelity simulation exercises create an environment in which the obstetrics team can practice the sequence of maneuvers and seamless teamwork needed to successfully resolve a shoulder dystocia.2,3 Implementing a shoulder dystocia protocol and practicing the protocol using team-based simulation may help to reduce the adverse outcomes of shoulder dystocia.3,4
Reference
1. Wetzel CM, Kneebone RL, Woloshynowych M, et al. The effects of stress on surgical performance. Am J Surg. 2006;191(1):5−10.
2. Crofts JF, Fox R, Ellis D, Winter C, Hinshaw K, Draycott TJ. Observations from 450 shoulder dystocia simulations. Obstet Gynecol. 2008;112(4):906−912.
3. Draycott TJ, Crofts JF, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14−20.
4. Grobman WA, Miller D, Burke C, Hornbogen A, Tam K, Costello R. Outcomes associated with introduction of a shoulder dystocia protocol. Am J Obstet Gynecol. 2011;205(6):513−517.
Manipulation of the posterior axilla |
|
The right and left third fingers are locked into the posterior axilla, one finger from the front and one from the back of the fetus. Gentle downward guidance is provided by the fingers to draw the posterior shoulder down and out along the curve of the sacrum, thus releasing the anterior shoulder.4 In this drawing, an assistant gently holds the head up. |
Menticoglou maneuverMenticoglou noted that delivery of the posterior arm generally resolves almost all cases of shoulder dystocia. However, if the posterior arm is extended and trapped between the fetus and maternal pelvic side-wall, it may be difficult to deliver the posterior arm. In these cases he recommended having an assistant gently hold, not pull, the fetal head upward and, at the same time, having the obstetrician get on one knee, placing the middle fingers of both hands into the posterior axilla of the fetus.4
The right middle finger is placed into the axilla from the left side of the maternal pelvis, and the left middle finger is placed into the axilla from the right side of the maternal pelvis, resulting in the two middle fingers overlapping in the fetal axilla (FIGURE).4 Gentle force is then used to pull the posterior shoulder and arm downward and outward along the curve of the sacrum. Once the shoulder has emerged from the pelvis, the posterior arm is delivered. Alternatively, if the posterior shoulder is brought well down into the pelvis, another attempt can be made at delivering the posterior arm.4
My preferred approach. The Menticoglou maneuver is my preferred posterior axilla maneuver because it can be accomplished rapidly; requires no equipment, such as a sling catheter; and the obstetrician has good tactile feedback throughout the application of gentle force.
Hofmeyr-Cluver maneuverIn cases of difficult shoulder dystocia, Dr. William Smellie (1762)8 recommended placing one or two fingers in the anterior or posterior fetal axilla and gentling pulling on the axilla to deliver the body. If the axillae were too high to reach, he recommended using a blunt hook in the axilla to draw forth the impacted child. He advised caution when using a blunt hook because the fetus might be injured or lacerated.
Instead of using a hook, Hofmeyr and Cluver5−7 have recommended using a catheter sling to deliver the posterior shoulder. In this maneuver, a loop of a suction catheter or firm urinary catheter is placed over the obstetrician’s index finger and the loop is pushed through the posterior axilla, back to front, with guidance from the index finger. The index finger of the opposite hand is used to catch the loop and pull the catheter through, creating a single-stranded sling that is positioned in the axilla. Gentle force is then applied to the sling in the axis of the pelvis to deliver the posterior shoulder.
“If the posterior arm does not follow it is then swept out easily because room has been created by delivering the posterior shoulder. If the aforementioned procedure fails, the sling can be used to rotate the shoulder. To perform a rotational maneuver, sling traction is directed laterally towards the side of the baby’s back then anteriorly while digital pressure is applied behind the anterior shoulder to assist rotation.”7
Use ACOG’s checklist for documenting a shoulder dystocia
Following the resolution of a shoulder dystocia, it is important to gather all the necessary facts to complete a detailed medical record entry describing the situation and interventions used. The checklist from the American College of Obstetricians and Gynecologists (ACOG) helps you to prepare a standardized medical record entry that is comprehensive.
My experience is that “free form” medical record entries describing the events at a shoulder dystocia event are generally not optimally organized, creating future problems when the case is reviewed.
ACOG obstetric checklists are available for download at http://www.acog.org/Resources-And-Publications, or use your web browser to search for “ACOG Shoulder Dystocia checklist.”
With scant literature, know the benefits and risksThe world’s literature on posterior axilla maneuvers to resolve shoulder dystocia consists of case series and individual case reports.2−7 Hence, the quality of the data supporting this intervention is not optimal, and risks associated with the maneuver are not well characterized. Application of a controlled and gentle force to the posterior axilla may cause fracture of the fetal humerus5 or dislocation of the fetal shoulder. The posterior axilla maneuver also may increase the risk of a maternal third- or fourth-degree perineal laceration.
As a general rule, as the number of maneuvers used to resolve a difficult shoulder dystocia increase, the risk of neonatal injury increases.9 Since the posterior axilla maneuver typically is only attempted after multiple previous maneuvers have failed, the risk of fetal injury is increased. However, as time passes and a shoulder dystocia remains unresolved for 4 or 5 minutes, the risk of neurologic injury and fetal death increases.10
In resolving a shoulder dystocia, speed and skill are essential. A posterior axilla maneuver can be performed more rapidly than a Zavanelli maneuver or a symphysiotomy. Although manipulation of the posterior axilla and arm may cause a fracture of the humerus, this complication is a modest price to pay for preventing permanent fetal brain injury or fetal death.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Willughby P. Observations in midwifery. New York, NY: MW Books; 1972:312−313.
- Holman MS. A new manoeuvre for delivery of an impacted shoulder based on a mechanical analysis. S Afr Med J. 1963;37:247−249.
- Schramm M. Impacted shoulders—a personal experience. Aust N Z J Obstet Gynaecol. 1983;23(1):28−31.
- Menticoglou SM. A modified technique to deliver the posterior arm in severe shoulder dystocia. Obstet Gynecol. 2006;108(3 pt 2):755−757.
- Cluver CA, Hofmeyr GJ. Posterior axilla sling traction: a technique for intractable shoulder dystocia. Obstet Gynecol. 2009;113(2 pt 2):486–488.
- Hofmeyr GJ, Cluver CA. Posterior axilla sling traction for intractable shoulder dystocia. BJOG. 2009;116(13):1818−1820.
- Cluver CA, Hofmeyr GJ. Posterior axilla sling traction for shoulder dystocia: case review and a new method for shoulder rotation with the sling. Am J Obstet Gynecol. 2015;212(6):784.e1−e7.
- Smellie W. A treatise on the theory and practice of midwifery. 4th ed. London, England; 1762:226−227.
- Hoffman MK, Bailit JL, Branch DW, et al; Consortium on Safe Labor. A comparison of obstetric maneuvers for the acute management of shoulder dystocia. Obstet Gynecol. 2011;117(6):1272−1278.
- Lerner H, Durlacher K, Smith S, Hamilton E. Relationship between head-to-body delivery interval in shoulder dystocia and neonatal depression. Obstet Gynecol. 2011;118(2 pt 1):318−322.
- Willughby P. Observations in midwifery. New York, NY: MW Books; 1972:312−313.
- Holman MS. A new manoeuvre for delivery of an impacted shoulder based on a mechanical analysis. S Afr Med J. 1963;37:247−249.
- Schramm M. Impacted shoulders—a personal experience. Aust N Z J Obstet Gynaecol. 1983;23(1):28−31.
- Menticoglou SM. A modified technique to deliver the posterior arm in severe shoulder dystocia. Obstet Gynecol. 2006;108(3 pt 2):755−757.
- Cluver CA, Hofmeyr GJ. Posterior axilla sling traction: a technique for intractable shoulder dystocia. Obstet Gynecol. 2009;113(2 pt 2):486–488.
- Hofmeyr GJ, Cluver CA. Posterior axilla sling traction for intractable shoulder dystocia. BJOG. 2009;116(13):1818−1820.
- Cluver CA, Hofmeyr GJ. Posterior axilla sling traction for shoulder dystocia: case review and a new method for shoulder rotation with the sling. Am J Obstet Gynecol. 2015;212(6):784.e1−e7.
- Smellie W. A treatise on the theory and practice of midwifery. 4th ed. London, England; 1762:226−227.
- Hoffman MK, Bailit JL, Branch DW, et al; Consortium on Safe Labor. A comparison of obstetric maneuvers for the acute management of shoulder dystocia. Obstet Gynecol. 2011;117(6):1272−1278.
- Lerner H, Durlacher K, Smith S, Hamilton E. Relationship between head-to-body delivery interval in shoulder dystocia and neonatal depression. Obstet Gynecol. 2011;118(2 pt 1):318−322.
In this article
- Menticoglou maneuver
- Importance of simulation
Reader reactions to the problem of inadequate contraception for high-risk women
“Contraception as a vital sign”
In his recent Editorial Dr. Barbieri asked for ideas to improve contraception counseling for women with medical problems that put them at risk for adverse pregnancy outcomes. His idea of “contraception status as a vital sign” is applied in our very large group practice in Northern California using the electronic health record (EHR).
Over 10 years ago, I attempted to put a hard stop in the EHR to require documentation that women of reproductive age be evaluated for contraception. This scheme seemed to be too cumbersome and was rejected at the time.
The idea was not abandoned, however. Medical assistants must now document a means of contraception for each woman of reproductive age. This does not guarantee that a physician will look at the information, but it is a step in the right direction.
My hope is that someday we will have automatic contraception as a vital sign documentation for all reproductive-age women, including “children” who are documented as menstruating. In the meantime, thank you for highlighting this critical issue.
Tia Will, MD
Sacramento, California
Reduce reimbursement when standard of care is not met
When I read Dr. Barbieri’s Editorial, I was surprised that he avoided the elephant in the room: the current political climate of denying contraception to women, including the defunding of Planned Parenthood and the Supreme Court decision to allow corporations to deny contraceptive coverage for religious issues.
Although I am not currently involved in women’s health, I do work under the auspices of a large Catholic health care system in the United States. Here, all employees are prohibited from providing contraceptive procedures, prescriptions, or even counseling unless it is a Natural Family Planning/ Fertility Awareness Method. These employees also are not provided individual contraceptive health coverage by their employer; this coverage is provided by the federal government thanks to the Affordable Care Act.
Contraception is part of the standard of care for women. However, many women are denied this standard of care due to “religious” reasons, which I suspect may be partially financial and/or political in nature.
This issue must therefore be addressed by political and financial means. My recommendation is for legislation that mandates lower reimbursement rates for health care systems and providers that refuse to offer full contraceptive options to women. If they do not provide full care, they do not get full payment for services. The money saved by reduced reimbursements could then fund federal women’s health clinics in areas dominated by “religious” health care systems that would guarantee full reproductive health options to all.
Name and practice location withheld
Remove Medicaid barriers to postpartum sterilization
An issue not addressed in Dr. Barbieri’s Editorial is that of women who, after appropriate and extensive counseling by a physician and with a full understanding of the reproductive implications and the possible adverse effect of additional pregnancies on their health and life, decide for permanent contraception. A woman’s opportunity to obtain postpartum or interval contraceptive procedure varies by her insurance coverage, which is indirectly associated with her ethnicity or race.
In 1979, Medicaid Title XIX imposed a 30-day interval between the signing of the sterilization informed consent by the patient and the performance of the procedure. These regulations are still in effect today. What was instituted to protect vulnerable populations from coerced methods in the 1970s represents an anachronistic and archaic approach in the 21st Century. This regulation discriminates against low-income and minority women whose health care is covered by public insurance yet who are frequently at highest risk for unintended and possible risky pregnancy or abortion. In simple words, this imposition violates the standards of justice, beneficence, and nonmaleficence as it treats publicly insured women differently from privately insured women.
The American Medical Association and the American College of Obstetricians and Gynecologists1 state that this regulation must be revised and charged practitioners to develop policies and procedures to ensure all women who desire postpartum sterilization can receive it. It is incumbent upon all women’s health care physicians to see that this barrier is removed.
Federico G. Mariona, MD, MHSA
Dearborn, Michigan
Reference
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. ACOG Committee Opinion No. 530: access to postpartum sterilization. Obstet Gynecol. 2012;120(1):212–215.
Educate the sexual partners of at-risk women
It always strikes me how little emphasis is placed on including the sexual partners of women with serious medical problems in the dialogue about responsibility for at-risk pregnancy. As advocates for women’s health, we should educate the couple about vasectomy and liberally provide referrals. Community outreach to help men understand how they can protect their partner from potentially dangerous unwanted pregnancy is extremely important and not stressed enough. Vasectomy is a quick, safe procedure performed in a physician’s office under local anesthesia. Why should any woman who has already risked her life carrying and delivering a baby be required to bear the contraceptive burden when there is a safe and convenient alternative?
Emily Gubert, MD
East Islip, New York
Dr. Barbieri responds
I thank Drs. Will, Mariona, and Gubert and the anonymous author for their wonderful recommendations on approaches to help improve contraceptive care for women. I agree with Dr. Will that the EHR is a valuable tool to advance contraceptive care. The anonymous author and Dr. Mariona make the critically important point that all women should have access to desired contraception without any barriers based on institutional beliefs or government regulations. The patient’s needs should be prioritized in all medical decision making. I agree with Dr. Gubert that including the male partner in the care process is an important part of effective contraception for women. I enthusiastically agree with her that the best permanent contraceptive for a stable couple is vasectomy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“Contraception as a vital sign”
In his recent Editorial Dr. Barbieri asked for ideas to improve contraception counseling for women with medical problems that put them at risk for adverse pregnancy outcomes. His idea of “contraception status as a vital sign” is applied in our very large group practice in Northern California using the electronic health record (EHR).
Over 10 years ago, I attempted to put a hard stop in the EHR to require documentation that women of reproductive age be evaluated for contraception. This scheme seemed to be too cumbersome and was rejected at the time.
The idea was not abandoned, however. Medical assistants must now document a means of contraception for each woman of reproductive age. This does not guarantee that a physician will look at the information, but it is a step in the right direction.
My hope is that someday we will have automatic contraception as a vital sign documentation for all reproductive-age women, including “children” who are documented as menstruating. In the meantime, thank you for highlighting this critical issue.
Tia Will, MD
Sacramento, California
Reduce reimbursement when standard of care is not met
When I read Dr. Barbieri’s Editorial, I was surprised that he avoided the elephant in the room: the current political climate of denying contraception to women, including the defunding of Planned Parenthood and the Supreme Court decision to allow corporations to deny contraceptive coverage for religious issues.
Although I am not currently involved in women’s health, I do work under the auspices of a large Catholic health care system in the United States. Here, all employees are prohibited from providing contraceptive procedures, prescriptions, or even counseling unless it is a Natural Family Planning/ Fertility Awareness Method. These employees also are not provided individual contraceptive health coverage by their employer; this coverage is provided by the federal government thanks to the Affordable Care Act.
Contraception is part of the standard of care for women. However, many women are denied this standard of care due to “religious” reasons, which I suspect may be partially financial and/or political in nature.
This issue must therefore be addressed by political and financial means. My recommendation is for legislation that mandates lower reimbursement rates for health care systems and providers that refuse to offer full contraceptive options to women. If they do not provide full care, they do not get full payment for services. The money saved by reduced reimbursements could then fund federal women’s health clinics in areas dominated by “religious” health care systems that would guarantee full reproductive health options to all.
Name and practice location withheld
Remove Medicaid barriers to postpartum sterilization
An issue not addressed in Dr. Barbieri’s Editorial is that of women who, after appropriate and extensive counseling by a physician and with a full understanding of the reproductive implications and the possible adverse effect of additional pregnancies on their health and life, decide for permanent contraception. A woman’s opportunity to obtain postpartum or interval contraceptive procedure varies by her insurance coverage, which is indirectly associated with her ethnicity or race.
In 1979, Medicaid Title XIX imposed a 30-day interval between the signing of the sterilization informed consent by the patient and the performance of the procedure. These regulations are still in effect today. What was instituted to protect vulnerable populations from coerced methods in the 1970s represents an anachronistic and archaic approach in the 21st Century. This regulation discriminates against low-income and minority women whose health care is covered by public insurance yet who are frequently at highest risk for unintended and possible risky pregnancy or abortion. In simple words, this imposition violates the standards of justice, beneficence, and nonmaleficence as it treats publicly insured women differently from privately insured women.
The American Medical Association and the American College of Obstetricians and Gynecologists1 state that this regulation must be revised and charged practitioners to develop policies and procedures to ensure all women who desire postpartum sterilization can receive it. It is incumbent upon all women’s health care physicians to see that this barrier is removed.
Federico G. Mariona, MD, MHSA
Dearborn, Michigan
Reference
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. ACOG Committee Opinion No. 530: access to postpartum sterilization. Obstet Gynecol. 2012;120(1):212–215.
Educate the sexual partners of at-risk women
It always strikes me how little emphasis is placed on including the sexual partners of women with serious medical problems in the dialogue about responsibility for at-risk pregnancy. As advocates for women’s health, we should educate the couple about vasectomy and liberally provide referrals. Community outreach to help men understand how they can protect their partner from potentially dangerous unwanted pregnancy is extremely important and not stressed enough. Vasectomy is a quick, safe procedure performed in a physician’s office under local anesthesia. Why should any woman who has already risked her life carrying and delivering a baby be required to bear the contraceptive burden when there is a safe and convenient alternative?
Emily Gubert, MD
East Islip, New York
Dr. Barbieri responds
I thank Drs. Will, Mariona, and Gubert and the anonymous author for their wonderful recommendations on approaches to help improve contraceptive care for women. I agree with Dr. Will that the EHR is a valuable tool to advance contraceptive care. The anonymous author and Dr. Mariona make the critically important point that all women should have access to desired contraception without any barriers based on institutional beliefs or government regulations. The patient’s needs should be prioritized in all medical decision making. I agree with Dr. Gubert that including the male partner in the care process is an important part of effective contraception for women. I enthusiastically agree with her that the best permanent contraceptive for a stable couple is vasectomy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“Contraception as a vital sign”
In his recent Editorial Dr. Barbieri asked for ideas to improve contraception counseling for women with medical problems that put them at risk for adverse pregnancy outcomes. His idea of “contraception status as a vital sign” is applied in our very large group practice in Northern California using the electronic health record (EHR).
Over 10 years ago, I attempted to put a hard stop in the EHR to require documentation that women of reproductive age be evaluated for contraception. This scheme seemed to be too cumbersome and was rejected at the time.
The idea was not abandoned, however. Medical assistants must now document a means of contraception for each woman of reproductive age. This does not guarantee that a physician will look at the information, but it is a step in the right direction.
My hope is that someday we will have automatic contraception as a vital sign documentation for all reproductive-age women, including “children” who are documented as menstruating. In the meantime, thank you for highlighting this critical issue.
Tia Will, MD
Sacramento, California
Reduce reimbursement when standard of care is not met
When I read Dr. Barbieri’s Editorial, I was surprised that he avoided the elephant in the room: the current political climate of denying contraception to women, including the defunding of Planned Parenthood and the Supreme Court decision to allow corporations to deny contraceptive coverage for religious issues.
Although I am not currently involved in women’s health, I do work under the auspices of a large Catholic health care system in the United States. Here, all employees are prohibited from providing contraceptive procedures, prescriptions, or even counseling unless it is a Natural Family Planning/ Fertility Awareness Method. These employees also are not provided individual contraceptive health coverage by their employer; this coverage is provided by the federal government thanks to the Affordable Care Act.
Contraception is part of the standard of care for women. However, many women are denied this standard of care due to “religious” reasons, which I suspect may be partially financial and/or political in nature.
This issue must therefore be addressed by political and financial means. My recommendation is for legislation that mandates lower reimbursement rates for health care systems and providers that refuse to offer full contraceptive options to women. If they do not provide full care, they do not get full payment for services. The money saved by reduced reimbursements could then fund federal women’s health clinics in areas dominated by “religious” health care systems that would guarantee full reproductive health options to all.
Name and practice location withheld
Remove Medicaid barriers to postpartum sterilization
An issue not addressed in Dr. Barbieri’s Editorial is that of women who, after appropriate and extensive counseling by a physician and with a full understanding of the reproductive implications and the possible adverse effect of additional pregnancies on their health and life, decide for permanent contraception. A woman’s opportunity to obtain postpartum or interval contraceptive procedure varies by her insurance coverage, which is indirectly associated with her ethnicity or race.
In 1979, Medicaid Title XIX imposed a 30-day interval between the signing of the sterilization informed consent by the patient and the performance of the procedure. These regulations are still in effect today. What was instituted to protect vulnerable populations from coerced methods in the 1970s represents an anachronistic and archaic approach in the 21st Century. This regulation discriminates against low-income and minority women whose health care is covered by public insurance yet who are frequently at highest risk for unintended and possible risky pregnancy or abortion. In simple words, this imposition violates the standards of justice, beneficence, and nonmaleficence as it treats publicly insured women differently from privately insured women.
The American Medical Association and the American College of Obstetricians and Gynecologists1 state that this regulation must be revised and charged practitioners to develop policies and procedures to ensure all women who desire postpartum sterilization can receive it. It is incumbent upon all women’s health care physicians to see that this barrier is removed.
Federico G. Mariona, MD, MHSA
Dearborn, Michigan
Reference
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. ACOG Committee Opinion No. 530: access to postpartum sterilization. Obstet Gynecol. 2012;120(1):212–215.
Educate the sexual partners of at-risk women
It always strikes me how little emphasis is placed on including the sexual partners of women with serious medical problems in the dialogue about responsibility for at-risk pregnancy. As advocates for women’s health, we should educate the couple about vasectomy and liberally provide referrals. Community outreach to help men understand how they can protect their partner from potentially dangerous unwanted pregnancy is extremely important and not stressed enough. Vasectomy is a quick, safe procedure performed in a physician’s office under local anesthesia. Why should any woman who has already risked her life carrying and delivering a baby be required to bear the contraceptive burden when there is a safe and convenient alternative?
Emily Gubert, MD
East Islip, New York
Dr. Barbieri responds
I thank Drs. Will, Mariona, and Gubert and the anonymous author for their wonderful recommendations on approaches to help improve contraceptive care for women. I agree with Dr. Will that the EHR is a valuable tool to advance contraceptive care. The anonymous author and Dr. Mariona make the critically important point that all women should have access to desired contraception without any barriers based on institutional beliefs or government regulations. The patient’s needs should be prioritized in all medical decision making. I agree with Dr. Gubert that including the male partner in the care process is an important part of effective contraception for women. I enthusiastically agree with her that the best permanent contraceptive for a stable couple is vasectomy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Do antidepressants really cause autism?
Presently it seems that anything a pregnant woman ingests can be correlated with a teratology or an unfortunate neurobehavioral outcome. In an era when up to 15% of pregnant women are taking antidepressant therapy, antidepressants are obvious drugs to be correlated with an untoward fetal outcome, despite the fact that untreated maternal depression itself is significantly worse.1
A recent retrospective secondary end point study by Boukhris and colleagues on antidepressant use in pregnancy and the risk of autism spectrum disorder (ASD) in children is an example of correlation without substantive evidence of causation. Although this study received media attention,2 it is a “data-dredge” study. While the authors correctly note that the database is derived from a prospective registry-based population-based cohort study (the Quebec Pregnancy/Children Cohort), their study’s design more closely resembles a post hoc nested case-control study.
Details of the study
Researchers evaluated data from 145,456 singleton full-term infants born alive between January 1, 1998, and December 31, 2009, with antidepressant exposure during pregnancy defined according to trimester and specific antidepressant classes. Children were considered as having autism if they had received at least 1 autism diagnosis between their date of birth and the last date of follow-up.
We perceive several problems in the study’s design and the authors’ conclusions.
Shortcomings of study design
The study results are based on a post hoc analysis. Autism spectrum disorder was not the primary end point of interest in this database. Accordingly, in a secondary end point study, the risk for bias and confounding is substantial. This study design cannot prove causation.3–5
Exposure is defined by number of antidepressant prescriptions filled. No data regarding adherence (true exposure) are provided. Many women will not take antidepressant drugs as prescribed during pregnancy. It has been reported that antidepressants dispensed to pregnant women during the last 2 trimesters of pregnancy were taken by only 55% of the women.6
The specific antidepressant agents and dosages used were not identified, and the study provided no good sense of duration of use. Is it biologically plausible, therefore, to suggest that all antidepressants—with their disparate structures and mechanisms, in all doses, and for various durations of use—have a uniform effect on fetal neurodevelopment?
Notably, in another prescription drug study of 668,468 pregnancies in 2013, investigators found no significant association between prenatal exposure to antidepressants and ASD.7
Some data suggest that ASD and depression may share preexisting risk factors.8 The increased risk for ASD proposed by Boukhris and colleagues’ study cannot likely be separated from the well-described genetic risk of ASD that might be shared with that of depression.9,10
The stated hazard ratios (HRs) are all <2.2. Given this study’s design, it is plausible that various biases and confounders account for these findings. True significance of these HRs are suspect unless they exceed 3.0, and there is a greater probability of avoiding a type I error when the risk ratios are greater than 4 to 5.3,4
What this evidence means for practice
In this registry-based study of an ongoing population-based cohort, the authors suggest a sensational 87% increased risk of ASD with use of antidepressants during pregnancy. While technically correct, the absolute risk (if real) is really less than 1%. Using sound epidemiologic principles, we would advise against speculating on a number needed to harm based on this study design. Such a projection would require a prospective randomized trial.
—Robert P. Kauffman, MD; Teresa Baker, MD; and Thomas W. Hale, PhD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dawson AL, Ailes EC, Gilboa SM, et al. Antidepressant prescription claims among reproductive-aged women with private employer-sponsored insurance--United States 2008 -2013. MMWR Morb Mortal Wkly Rep. 2016;65(3):41–46.
- Cha AE. Maternal exposure to anti-depressant SSRIs linked to autism in children. https://www.washingtonpost.com/news/to-your-health/wp/2015/12/14/maternal-exposure-to-anti-depressant-ssris-linked-to-autism-in-children/. Published December 17, 2015. Accessed March 13, 2017.
- Taubes G. Epidemiology faces its limits. Science. 1995;269(5221):164–169.
- Grimes DA, Schulz KF. False alarms and pseudo-epidemics: the limitations of observational epidemiology. Obstet Gynecol. 2012;120(4):920–927.
- Smith GD, Ebrahim S. Data dredging, bias, and confounding: they can all get you into the BMJ and the Friday papers. BMJ. 2002;325(7378):1437–1438.
- Källén B, Nilsson E, Olausson PO. Antidepressant use during pregnancy: comparison of data obtained from a prescription register and from antenatal care records. Eur J Clin Pharmacol. 2011;67(8):839–845.
- Sørensen MJ, Grønborg TK, Christensen J, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol. 2013;5:449–459.
- King BH. Assessing risk of autism spectrum disorder in children after antidepressant use during pregnancy. JAMA Pediatr. 2016;170(2):111–112.
- Daniels JL, Forssen U, Hultman CM, et al. Parental psychiatric disorders associated with autism spectrum disorders in the offspring. Pediatrics. 2008;121(5):e1357–e1362.
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil. 2011;32(5):1910–1917.
Presently it seems that anything a pregnant woman ingests can be correlated with a teratology or an unfortunate neurobehavioral outcome. In an era when up to 15% of pregnant women are taking antidepressant therapy, antidepressants are obvious drugs to be correlated with an untoward fetal outcome, despite the fact that untreated maternal depression itself is significantly worse.1
A recent retrospective secondary end point study by Boukhris and colleagues on antidepressant use in pregnancy and the risk of autism spectrum disorder (ASD) in children is an example of correlation without substantive evidence of causation. Although this study received media attention,2 it is a “data-dredge” study. While the authors correctly note that the database is derived from a prospective registry-based population-based cohort study (the Quebec Pregnancy/Children Cohort), their study’s design more closely resembles a post hoc nested case-control study.
Details of the study
Researchers evaluated data from 145,456 singleton full-term infants born alive between January 1, 1998, and December 31, 2009, with antidepressant exposure during pregnancy defined according to trimester and specific antidepressant classes. Children were considered as having autism if they had received at least 1 autism diagnosis between their date of birth and the last date of follow-up.
We perceive several problems in the study’s design and the authors’ conclusions.
Shortcomings of study design
The study results are based on a post hoc analysis. Autism spectrum disorder was not the primary end point of interest in this database. Accordingly, in a secondary end point study, the risk for bias and confounding is substantial. This study design cannot prove causation.3–5
Exposure is defined by number of antidepressant prescriptions filled. No data regarding adherence (true exposure) are provided. Many women will not take antidepressant drugs as prescribed during pregnancy. It has been reported that antidepressants dispensed to pregnant women during the last 2 trimesters of pregnancy were taken by only 55% of the women.6
The specific antidepressant agents and dosages used were not identified, and the study provided no good sense of duration of use. Is it biologically plausible, therefore, to suggest that all antidepressants—with their disparate structures and mechanisms, in all doses, and for various durations of use—have a uniform effect on fetal neurodevelopment?
Notably, in another prescription drug study of 668,468 pregnancies in 2013, investigators found no significant association between prenatal exposure to antidepressants and ASD.7
Some data suggest that ASD and depression may share preexisting risk factors.8 The increased risk for ASD proposed by Boukhris and colleagues’ study cannot likely be separated from the well-described genetic risk of ASD that might be shared with that of depression.9,10
The stated hazard ratios (HRs) are all <2.2. Given this study’s design, it is plausible that various biases and confounders account for these findings. True significance of these HRs are suspect unless they exceed 3.0, and there is a greater probability of avoiding a type I error when the risk ratios are greater than 4 to 5.3,4
What this evidence means for practice
In this registry-based study of an ongoing population-based cohort, the authors suggest a sensational 87% increased risk of ASD with use of antidepressants during pregnancy. While technically correct, the absolute risk (if real) is really less than 1%. Using sound epidemiologic principles, we would advise against speculating on a number needed to harm based on this study design. Such a projection would require a prospective randomized trial.
—Robert P. Kauffman, MD; Teresa Baker, MD; and Thomas W. Hale, PhD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Presently it seems that anything a pregnant woman ingests can be correlated with a teratology or an unfortunate neurobehavioral outcome. In an era when up to 15% of pregnant women are taking antidepressant therapy, antidepressants are obvious drugs to be correlated with an untoward fetal outcome, despite the fact that untreated maternal depression itself is significantly worse.1
A recent retrospective secondary end point study by Boukhris and colleagues on antidepressant use in pregnancy and the risk of autism spectrum disorder (ASD) in children is an example of correlation without substantive evidence of causation. Although this study received media attention,2 it is a “data-dredge” study. While the authors correctly note that the database is derived from a prospective registry-based population-based cohort study (the Quebec Pregnancy/Children Cohort), their study’s design more closely resembles a post hoc nested case-control study.
Details of the study
Researchers evaluated data from 145,456 singleton full-term infants born alive between January 1, 1998, and December 31, 2009, with antidepressant exposure during pregnancy defined according to trimester and specific antidepressant classes. Children were considered as having autism if they had received at least 1 autism diagnosis between their date of birth and the last date of follow-up.
We perceive several problems in the study’s design and the authors’ conclusions.
Shortcomings of study design
The study results are based on a post hoc analysis. Autism spectrum disorder was not the primary end point of interest in this database. Accordingly, in a secondary end point study, the risk for bias and confounding is substantial. This study design cannot prove causation.3–5
Exposure is defined by number of antidepressant prescriptions filled. No data regarding adherence (true exposure) are provided. Many women will not take antidepressant drugs as prescribed during pregnancy. It has been reported that antidepressants dispensed to pregnant women during the last 2 trimesters of pregnancy were taken by only 55% of the women.6
The specific antidepressant agents and dosages used were not identified, and the study provided no good sense of duration of use. Is it biologically plausible, therefore, to suggest that all antidepressants—with their disparate structures and mechanisms, in all doses, and for various durations of use—have a uniform effect on fetal neurodevelopment?
Notably, in another prescription drug study of 668,468 pregnancies in 2013, investigators found no significant association between prenatal exposure to antidepressants and ASD.7
Some data suggest that ASD and depression may share preexisting risk factors.8 The increased risk for ASD proposed by Boukhris and colleagues’ study cannot likely be separated from the well-described genetic risk of ASD that might be shared with that of depression.9,10
The stated hazard ratios (HRs) are all <2.2. Given this study’s design, it is plausible that various biases and confounders account for these findings. True significance of these HRs are suspect unless they exceed 3.0, and there is a greater probability of avoiding a type I error when the risk ratios are greater than 4 to 5.3,4
What this evidence means for practice
In this registry-based study of an ongoing population-based cohort, the authors suggest a sensational 87% increased risk of ASD with use of antidepressants during pregnancy. While technically correct, the absolute risk (if real) is really less than 1%. Using sound epidemiologic principles, we would advise against speculating on a number needed to harm based on this study design. Such a projection would require a prospective randomized trial.
—Robert P. Kauffman, MD; Teresa Baker, MD; and Thomas W. Hale, PhD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dawson AL, Ailes EC, Gilboa SM, et al. Antidepressant prescription claims among reproductive-aged women with private employer-sponsored insurance--United States 2008 -2013. MMWR Morb Mortal Wkly Rep. 2016;65(3):41–46.
- Cha AE. Maternal exposure to anti-depressant SSRIs linked to autism in children. https://www.washingtonpost.com/news/to-your-health/wp/2015/12/14/maternal-exposure-to-anti-depressant-ssris-linked-to-autism-in-children/. Published December 17, 2015. Accessed March 13, 2017.
- Taubes G. Epidemiology faces its limits. Science. 1995;269(5221):164–169.
- Grimes DA, Schulz KF. False alarms and pseudo-epidemics: the limitations of observational epidemiology. Obstet Gynecol. 2012;120(4):920–927.
- Smith GD, Ebrahim S. Data dredging, bias, and confounding: they can all get you into the BMJ and the Friday papers. BMJ. 2002;325(7378):1437–1438.
- Källén B, Nilsson E, Olausson PO. Antidepressant use during pregnancy: comparison of data obtained from a prescription register and from antenatal care records. Eur J Clin Pharmacol. 2011;67(8):839–845.
- Sørensen MJ, Grønborg TK, Christensen J, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol. 2013;5:449–459.
- King BH. Assessing risk of autism spectrum disorder in children after antidepressant use during pregnancy. JAMA Pediatr. 2016;170(2):111–112.
- Daniels JL, Forssen U, Hultman CM, et al. Parental psychiatric disorders associated with autism spectrum disorders in the offspring. Pediatrics. 2008;121(5):e1357–e1362.
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil. 2011;32(5):1910–1917.
- Dawson AL, Ailes EC, Gilboa SM, et al. Antidepressant prescription claims among reproductive-aged women with private employer-sponsored insurance--United States 2008 -2013. MMWR Morb Mortal Wkly Rep. 2016;65(3):41–46.
- Cha AE. Maternal exposure to anti-depressant SSRIs linked to autism in children. https://www.washingtonpost.com/news/to-your-health/wp/2015/12/14/maternal-exposure-to-anti-depressant-ssris-linked-to-autism-in-children/. Published December 17, 2015. Accessed March 13, 2017.
- Taubes G. Epidemiology faces its limits. Science. 1995;269(5221):164–169.
- Grimes DA, Schulz KF. False alarms and pseudo-epidemics: the limitations of observational epidemiology. Obstet Gynecol. 2012;120(4):920–927.
- Smith GD, Ebrahim S. Data dredging, bias, and confounding: they can all get you into the BMJ and the Friday papers. BMJ. 2002;325(7378):1437–1438.
- Källén B, Nilsson E, Olausson PO. Antidepressant use during pregnancy: comparison of data obtained from a prescription register and from antenatal care records. Eur J Clin Pharmacol. 2011;67(8):839–845.
- Sørensen MJ, Grønborg TK, Christensen J, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol. 2013;5:449–459.
- King BH. Assessing risk of autism spectrum disorder in children after antidepressant use during pregnancy. JAMA Pediatr. 2016;170(2):111–112.
- Daniels JL, Forssen U, Hultman CM, et al. Parental psychiatric disorders associated with autism spectrum disorders in the offspring. Pediatrics. 2008;121(5):e1357–e1362.
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil. 2011;32(5):1910–1917.
CDC urges states to prepare for Zika
As warmer weather raises the possibility of local Zika virus transmission in the continental United States, federal health officials are pushing state and local governments to devise plans aimed at protecting pregnant women from infection, to increase access to contraception, and to ensure better coordination by mosquito control districts.
On April 1, the U.S. Centers for Disease Prevention and Control hosted a day-long seminar on Zika attended by some 300 state and local public health professionals. Zika virus, which is increasingly linked to adverse fetal outcomes including microcephaly, is now spreading in Puerto Rico, the U.S. Virgin Islands, and American Samoa.
Though most of the U.S. response effort is currently concentrated in Puerto Rico, and no local transmission has yet been reported in the continental U.S., a CDC report issued concurrently with the conference highlighted the potential for Zika transmission within the U.S. The entire southern half of the continental U.S., and much of its east coast, is home to the Aedes aegypti mosquitoes that can carry Zika virus.
In the same report, CDC also stressed that pregnant women should avoid travel to areas where Zika is being rapidly transmitted, and avoid sexual contact or consistently use condoms with partners who “reside in or have traveled to areas with active Zika virus transmission.”
“The key here is to reduce the risk to pregnant women,” Dr. Tom Frieden, CDC director, said at a news conference during the meeting. “There is an urgent need for all of us to learn more and do more,” he noted, acknowledging that officials had concerns about securing sufficient federal funding, getting rapid screening tools commercially developed in the absence of such funding, and improving the capabilities of U.S. mosquito control districts in the states most likely to be affected.
As local clusters of dengue virus disease have occurred in Florida, Texas, and Hawaii, Dr. Frieden said these states need to be particularly responsive to the Zika threat. However, Zika transmission could follow a different pattern, he said.
While Dr. Frieden described some local mosquito control districts and their capabilities as robust, others are considerably less so. Many districts in vulnerable states are not contiguous, leading to potential gaps in vector control. Dr. Frieden noted that even where control is most intensive, such as in Puerto Rico, vector resistance to common pesticides is a problem.
Dr. Frieden also stressed the importance of widening local access to contraception. He clarified that the CDC was not advising couples to avoid or delay pregnancy, even in Puerto Rico. However, he said, “if a woman and her partner choose not to become pregnant [there should be] ready access to effective contraception,” particularly the long-term reversible methods likely to be most effective.
Amy Pope, deputy homeland security advisor in the Obama administration, underscored the concern about Zika-specific funding. In February, the administration requested some $1.9 billion from Congress to combat Zika, including by improving access to contraception, developing vaccines and diagnostics, and other efforts. This funding has yet to be approved, Ms. Pope noted, adding that some members of Congress proposed redirecting funding that had been earmarked to combat Ebola.
“Congress is asking the American people to choose which disease it wants the most protection from,” Ms. Pope said, and Dr. Frieden reminded the conference that the Ebola crisis was not over. A new case from Liberia was announced April 1, he noted, and an ongoing cluster of transmission is occurring in Guinea.
As warmer weather raises the possibility of local Zika virus transmission in the continental United States, federal health officials are pushing state and local governments to devise plans aimed at protecting pregnant women from infection, to increase access to contraception, and to ensure better coordination by mosquito control districts.
On April 1, the U.S. Centers for Disease Prevention and Control hosted a day-long seminar on Zika attended by some 300 state and local public health professionals. Zika virus, which is increasingly linked to adverse fetal outcomes including microcephaly, is now spreading in Puerto Rico, the U.S. Virgin Islands, and American Samoa.
Though most of the U.S. response effort is currently concentrated in Puerto Rico, and no local transmission has yet been reported in the continental U.S., a CDC report issued concurrently with the conference highlighted the potential for Zika transmission within the U.S. The entire southern half of the continental U.S., and much of its east coast, is home to the Aedes aegypti mosquitoes that can carry Zika virus.
In the same report, CDC also stressed that pregnant women should avoid travel to areas where Zika is being rapidly transmitted, and avoid sexual contact or consistently use condoms with partners who “reside in or have traveled to areas with active Zika virus transmission.”
“The key here is to reduce the risk to pregnant women,” Dr. Tom Frieden, CDC director, said at a news conference during the meeting. “There is an urgent need for all of us to learn more and do more,” he noted, acknowledging that officials had concerns about securing sufficient federal funding, getting rapid screening tools commercially developed in the absence of such funding, and improving the capabilities of U.S. mosquito control districts in the states most likely to be affected.
As local clusters of dengue virus disease have occurred in Florida, Texas, and Hawaii, Dr. Frieden said these states need to be particularly responsive to the Zika threat. However, Zika transmission could follow a different pattern, he said.
While Dr. Frieden described some local mosquito control districts and their capabilities as robust, others are considerably less so. Many districts in vulnerable states are not contiguous, leading to potential gaps in vector control. Dr. Frieden noted that even where control is most intensive, such as in Puerto Rico, vector resistance to common pesticides is a problem.
Dr. Frieden also stressed the importance of widening local access to contraception. He clarified that the CDC was not advising couples to avoid or delay pregnancy, even in Puerto Rico. However, he said, “if a woman and her partner choose not to become pregnant [there should be] ready access to effective contraception,” particularly the long-term reversible methods likely to be most effective.
Amy Pope, deputy homeland security advisor in the Obama administration, underscored the concern about Zika-specific funding. In February, the administration requested some $1.9 billion from Congress to combat Zika, including by improving access to contraception, developing vaccines and diagnostics, and other efforts. This funding has yet to be approved, Ms. Pope noted, adding that some members of Congress proposed redirecting funding that had been earmarked to combat Ebola.
“Congress is asking the American people to choose which disease it wants the most protection from,” Ms. Pope said, and Dr. Frieden reminded the conference that the Ebola crisis was not over. A new case from Liberia was announced April 1, he noted, and an ongoing cluster of transmission is occurring in Guinea.
As warmer weather raises the possibility of local Zika virus transmission in the continental United States, federal health officials are pushing state and local governments to devise plans aimed at protecting pregnant women from infection, to increase access to contraception, and to ensure better coordination by mosquito control districts.
On April 1, the U.S. Centers for Disease Prevention and Control hosted a day-long seminar on Zika attended by some 300 state and local public health professionals. Zika virus, which is increasingly linked to adverse fetal outcomes including microcephaly, is now spreading in Puerto Rico, the U.S. Virgin Islands, and American Samoa.
Though most of the U.S. response effort is currently concentrated in Puerto Rico, and no local transmission has yet been reported in the continental U.S., a CDC report issued concurrently with the conference highlighted the potential for Zika transmission within the U.S. The entire southern half of the continental U.S., and much of its east coast, is home to the Aedes aegypti mosquitoes that can carry Zika virus.
In the same report, CDC also stressed that pregnant women should avoid travel to areas where Zika is being rapidly transmitted, and avoid sexual contact or consistently use condoms with partners who “reside in or have traveled to areas with active Zika virus transmission.”
“The key here is to reduce the risk to pregnant women,” Dr. Tom Frieden, CDC director, said at a news conference during the meeting. “There is an urgent need for all of us to learn more and do more,” he noted, acknowledging that officials had concerns about securing sufficient federal funding, getting rapid screening tools commercially developed in the absence of such funding, and improving the capabilities of U.S. mosquito control districts in the states most likely to be affected.
As local clusters of dengue virus disease have occurred in Florida, Texas, and Hawaii, Dr. Frieden said these states need to be particularly responsive to the Zika threat. However, Zika transmission could follow a different pattern, he said.
While Dr. Frieden described some local mosquito control districts and their capabilities as robust, others are considerably less so. Many districts in vulnerable states are not contiguous, leading to potential gaps in vector control. Dr. Frieden noted that even where control is most intensive, such as in Puerto Rico, vector resistance to common pesticides is a problem.
Dr. Frieden also stressed the importance of widening local access to contraception. He clarified that the CDC was not advising couples to avoid or delay pregnancy, even in Puerto Rico. However, he said, “if a woman and her partner choose not to become pregnant [there should be] ready access to effective contraception,” particularly the long-term reversible methods likely to be most effective.
Amy Pope, deputy homeland security advisor in the Obama administration, underscored the concern about Zika-specific funding. In February, the administration requested some $1.9 billion from Congress to combat Zika, including by improving access to contraception, developing vaccines and diagnostics, and other efforts. This funding has yet to be approved, Ms. Pope noted, adding that some members of Congress proposed redirecting funding that had been earmarked to combat Ebola.
“Congress is asking the American people to choose which disease it wants the most protection from,” Ms. Pope said, and Dr. Frieden reminded the conference that the Ebola crisis was not over. A new case from Liberia was announced April 1, he noted, and an ongoing cluster of transmission is occurring in Guinea.
High-grade cervical dysplasia in pregnancy
Cervical intraepithelial neoplasia (CIN) describes a precancerous lesion of the squamous epithelium of the ectocervix. The cervical cancer screening paradigm in the United States begins with collection of cervical cytology with a Pap smear, frequently in conjunction with human papillomavirus testing. Abnormalities will frequently lead to colposcopy with directed biopsy, which can result in a diagnosis of CIN. There are different grades of severity within CIN, which aids in making treatment recommendations.
Pregnancy is a convenient time to capture women for cervical cancer screening, given the increased contact with health care providers. Routine guidelines should be followed for screening women who are pregnant, as collection of cervical cytology and human papillomavirus (HPV) cotesting is safe.
In women who have been found to have abnormal cytology, CIN or malignancy has been identified in up to 19% of cases (Am J Obstet Gynecol. 2004 Jul;191[1]:105-13). High-grade lesions identified in pregnant women create a unique management dilemma.
Terminology
The Bethesda system describes colposcopic abnormalities as CIN and divides premalignant lesions into grades from 1 to 3 with the highest grade representing more worrisome lesions. CIN2 has been found to have poor reproducibility and likely represents a mix of low- and high-grade lesions. In addition, there is concern that HPV-associated lesions of the lower anogenital tract have incongruent terminology among different specialties that may not accurately represent the current understanding of HPV pathogenesis.
In 2012, the Lower Anogenital Squamous Terminology (LAST) project of the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology (ASCCP) advocated for consistent terminology across all lower anogenital tract lesions with HPV, including CIN (Int J Gynecol Pathol. 2013 Jan;32[1]:76-115).
With this new terminology, CIN1 is referred to as low-grade squamous intraepithelial lesion (LSIL). CIN2 is characterized by its p16 immunostaining; lesions that are p16 negative are considered LSIL, while those that are positive are considered HSIL (high-grade squamous intraepithelial lesion). While this staining is not universally performed, physicians will start seeing p16 staining results with increasing frequency on their cervical biopsies. CIN3 lesions are referred to as HSIL.
Given the current understanding of HPV-mediated disease, and a commitment to represent the most up-to-date information, the LAST project terminology of HSIL to represent previously identified CIN2 and CIN3 lesions will be used for the remainder of this text.
Diagnosis
There is little data on the natural history of HSIL diagnosed after colposcopy, as most women get some form of therapy. The information that is available suggests that in patients with untreated HSIL, the cumulative incidence of malignancy is as high as 30% at 30 years (Lancet Oncol. 2008 May;9[5]:425-34). Treatment recommendations for excision are aimed at addressing this alarming number; however, care must be individualized, especially in the setting of pregnancy.
If abnormal cervical cytology is obtained on routine screening, appropriate patients should be referred for colposcopic exam. Physicians performing colposcopy should be familiar with the physiologic effects of pregnancy that can obscure the exam, including the increased cervical mucus production, prominence of endocervical glands, and increased vascularity.
Colposcopic-directed ectocervical biopsies have been found to be safe in pregnancy, and these women should be provided the same care as those who are not pregnant (Obstet Gynecol. 1993 Jun;81[6]:915-8). Endocervical sampling and endometrial sampling should not be performed, however, and physicians should remain dedicated to checking pregnancy tests prior to colposcopy.
HSIL cytology should prompt a biopsy in pregnancy; a decision to skip the biopsy and perform an excisional procedure in this setting is not recommended regardless of patient or gestational age. If LSIL (CIN1) is noted on biopsy, reevaluation post partum should be strongly considered, unless a suspicious lesion was felt to be inadequately biopsied.
Management
Managing HSIL in pregnancy focuses on diagnosis and excluding malignancy, while treatment can be reserved for the postpartum period. When choosing a management option, consider individual patient factors such as colposcopic appearance of the lesion, gestational age, and access to health care.
If HSIL is noted on colposcopic-directed biopsy, consider one of several options. The most conservative approach is reevaluation with cytology and colposcopy 6 weeks post partum. This is an option for patients who do not have a colposcopic exam that was concerning for an invasive lesion, were able to be adequately biopsied, and will reliably return for follow-up. Many physicians feel more comfortable with repeat cytology and colposcopy in 3 months from the original biopsy. The most aggressive management would include an excisional procedure during pregnancy.
There are varying rates of regression of biopsy-proven HSIL in pregnancy ranging from 34% to 70% (Obstet Gynecol. 1999 Mar;93[3]:359-62; Acta Obstet Gynecol Scand. 2006;85[9]:1134-7; Reprod Sci. 2009 Nov;16[11]:1034-9). Out of more than 200 patients across these three studies, just two patients were diagnosed with an invasive lesion post partum. Given the low likelihood of progression during pregnancy and the high rate of regression, an excisional procedure should be considered only in cases where there is concern about invasive carcinoma.
In cases where an invasive lesion is suspected, consider an an excisional procedure. While there is some evidence that performing a laser excisional procedure early in pregnancy (18 weeks and earlier) can be safely done, that is not the most common management strategy in the United States (Tumori. 1998 Sep-Oct;84[5]:567-70; Int J Gynecol Cancer. 2007 Jan-Feb;17[1]:127-31). In this circumstance, referral to a gynecologic oncologist is warranted where consideration can be made for performing a cold knife conization. Physicians should be aware of the increased risk of bleeding with this procedure in pregnancy and the potential for preterm birth. There is little literature to guide counseling regarding these risks, and the decision to perform an excisional procedure should be made with a multidisciplinary team (Arch Gynecol Obstet. 2016 Jan 4. doi: 10.1007/s00404-015-3980-y).
The see-and-treat paradigm is not recommended in pregnancy. Those patients with poor follow-up should still undergo colposcopic-directed biopsies prior to any excisional procedure.
Treatment recommendations in pregnancy should be made on the basis of careful consideration of individual patient factors, with strong consideration of repeat testing with cytology and colposcopy prior to an excision procedure.
Dr. Sullivan is a fellow in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. Dr. Sullivan and Dr. Gehrig reported having no relevant financial disclosures. Email them at [email protected].
Cervical intraepithelial neoplasia (CIN) describes a precancerous lesion of the squamous epithelium of the ectocervix. The cervical cancer screening paradigm in the United States begins with collection of cervical cytology with a Pap smear, frequently in conjunction with human papillomavirus testing. Abnormalities will frequently lead to colposcopy with directed biopsy, which can result in a diagnosis of CIN. There are different grades of severity within CIN, which aids in making treatment recommendations.
Pregnancy is a convenient time to capture women for cervical cancer screening, given the increased contact with health care providers. Routine guidelines should be followed for screening women who are pregnant, as collection of cervical cytology and human papillomavirus (HPV) cotesting is safe.
In women who have been found to have abnormal cytology, CIN or malignancy has been identified in up to 19% of cases (Am J Obstet Gynecol. 2004 Jul;191[1]:105-13). High-grade lesions identified in pregnant women create a unique management dilemma.
Terminology
The Bethesda system describes colposcopic abnormalities as CIN and divides premalignant lesions into grades from 1 to 3 with the highest grade representing more worrisome lesions. CIN2 has been found to have poor reproducibility and likely represents a mix of low- and high-grade lesions. In addition, there is concern that HPV-associated lesions of the lower anogenital tract have incongruent terminology among different specialties that may not accurately represent the current understanding of HPV pathogenesis.
In 2012, the Lower Anogenital Squamous Terminology (LAST) project of the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology (ASCCP) advocated for consistent terminology across all lower anogenital tract lesions with HPV, including CIN (Int J Gynecol Pathol. 2013 Jan;32[1]:76-115).
With this new terminology, CIN1 is referred to as low-grade squamous intraepithelial lesion (LSIL). CIN2 is characterized by its p16 immunostaining; lesions that are p16 negative are considered LSIL, while those that are positive are considered HSIL (high-grade squamous intraepithelial lesion). While this staining is not universally performed, physicians will start seeing p16 staining results with increasing frequency on their cervical biopsies. CIN3 lesions are referred to as HSIL.
Given the current understanding of HPV-mediated disease, and a commitment to represent the most up-to-date information, the LAST project terminology of HSIL to represent previously identified CIN2 and CIN3 lesions will be used for the remainder of this text.
Diagnosis
There is little data on the natural history of HSIL diagnosed after colposcopy, as most women get some form of therapy. The information that is available suggests that in patients with untreated HSIL, the cumulative incidence of malignancy is as high as 30% at 30 years (Lancet Oncol. 2008 May;9[5]:425-34). Treatment recommendations for excision are aimed at addressing this alarming number; however, care must be individualized, especially in the setting of pregnancy.
If abnormal cervical cytology is obtained on routine screening, appropriate patients should be referred for colposcopic exam. Physicians performing colposcopy should be familiar with the physiologic effects of pregnancy that can obscure the exam, including the increased cervical mucus production, prominence of endocervical glands, and increased vascularity.
Colposcopic-directed ectocervical biopsies have been found to be safe in pregnancy, and these women should be provided the same care as those who are not pregnant (Obstet Gynecol. 1993 Jun;81[6]:915-8). Endocervical sampling and endometrial sampling should not be performed, however, and physicians should remain dedicated to checking pregnancy tests prior to colposcopy.
HSIL cytology should prompt a biopsy in pregnancy; a decision to skip the biopsy and perform an excisional procedure in this setting is not recommended regardless of patient or gestational age. If LSIL (CIN1) is noted on biopsy, reevaluation post partum should be strongly considered, unless a suspicious lesion was felt to be inadequately biopsied.
Management
Managing HSIL in pregnancy focuses on diagnosis and excluding malignancy, while treatment can be reserved for the postpartum period. When choosing a management option, consider individual patient factors such as colposcopic appearance of the lesion, gestational age, and access to health care.
If HSIL is noted on colposcopic-directed biopsy, consider one of several options. The most conservative approach is reevaluation with cytology and colposcopy 6 weeks post partum. This is an option for patients who do not have a colposcopic exam that was concerning for an invasive lesion, were able to be adequately biopsied, and will reliably return for follow-up. Many physicians feel more comfortable with repeat cytology and colposcopy in 3 months from the original biopsy. The most aggressive management would include an excisional procedure during pregnancy.
There are varying rates of regression of biopsy-proven HSIL in pregnancy ranging from 34% to 70% (Obstet Gynecol. 1999 Mar;93[3]:359-62; Acta Obstet Gynecol Scand. 2006;85[9]:1134-7; Reprod Sci. 2009 Nov;16[11]:1034-9). Out of more than 200 patients across these three studies, just two patients were diagnosed with an invasive lesion post partum. Given the low likelihood of progression during pregnancy and the high rate of regression, an excisional procedure should be considered only in cases where there is concern about invasive carcinoma.
In cases where an invasive lesion is suspected, consider an an excisional procedure. While there is some evidence that performing a laser excisional procedure early in pregnancy (18 weeks and earlier) can be safely done, that is not the most common management strategy in the United States (Tumori. 1998 Sep-Oct;84[5]:567-70; Int J Gynecol Cancer. 2007 Jan-Feb;17[1]:127-31). In this circumstance, referral to a gynecologic oncologist is warranted where consideration can be made for performing a cold knife conization. Physicians should be aware of the increased risk of bleeding with this procedure in pregnancy and the potential for preterm birth. There is little literature to guide counseling regarding these risks, and the decision to perform an excisional procedure should be made with a multidisciplinary team (Arch Gynecol Obstet. 2016 Jan 4. doi: 10.1007/s00404-015-3980-y).
The see-and-treat paradigm is not recommended in pregnancy. Those patients with poor follow-up should still undergo colposcopic-directed biopsies prior to any excisional procedure.
Treatment recommendations in pregnancy should be made on the basis of careful consideration of individual patient factors, with strong consideration of repeat testing with cytology and colposcopy prior to an excision procedure.
Dr. Sullivan is a fellow in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. Dr. Sullivan and Dr. Gehrig reported having no relevant financial disclosures. Email them at [email protected].
Cervical intraepithelial neoplasia (CIN) describes a precancerous lesion of the squamous epithelium of the ectocervix. The cervical cancer screening paradigm in the United States begins with collection of cervical cytology with a Pap smear, frequently in conjunction with human papillomavirus testing. Abnormalities will frequently lead to colposcopy with directed biopsy, which can result in a diagnosis of CIN. There are different grades of severity within CIN, which aids in making treatment recommendations.
Pregnancy is a convenient time to capture women for cervical cancer screening, given the increased contact with health care providers. Routine guidelines should be followed for screening women who are pregnant, as collection of cervical cytology and human papillomavirus (HPV) cotesting is safe.
In women who have been found to have abnormal cytology, CIN or malignancy has been identified in up to 19% of cases (Am J Obstet Gynecol. 2004 Jul;191[1]:105-13). High-grade lesions identified in pregnant women create a unique management dilemma.
Terminology
The Bethesda system describes colposcopic abnormalities as CIN and divides premalignant lesions into grades from 1 to 3 with the highest grade representing more worrisome lesions. CIN2 has been found to have poor reproducibility and likely represents a mix of low- and high-grade lesions. In addition, there is concern that HPV-associated lesions of the lower anogenital tract have incongruent terminology among different specialties that may not accurately represent the current understanding of HPV pathogenesis.
In 2012, the Lower Anogenital Squamous Terminology (LAST) project of the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology (ASCCP) advocated for consistent terminology across all lower anogenital tract lesions with HPV, including CIN (Int J Gynecol Pathol. 2013 Jan;32[1]:76-115).
With this new terminology, CIN1 is referred to as low-grade squamous intraepithelial lesion (LSIL). CIN2 is characterized by its p16 immunostaining; lesions that are p16 negative are considered LSIL, while those that are positive are considered HSIL (high-grade squamous intraepithelial lesion). While this staining is not universally performed, physicians will start seeing p16 staining results with increasing frequency on their cervical biopsies. CIN3 lesions are referred to as HSIL.
Given the current understanding of HPV-mediated disease, and a commitment to represent the most up-to-date information, the LAST project terminology of HSIL to represent previously identified CIN2 and CIN3 lesions will be used for the remainder of this text.
Diagnosis
There is little data on the natural history of HSIL diagnosed after colposcopy, as most women get some form of therapy. The information that is available suggests that in patients with untreated HSIL, the cumulative incidence of malignancy is as high as 30% at 30 years (Lancet Oncol. 2008 May;9[5]:425-34). Treatment recommendations for excision are aimed at addressing this alarming number; however, care must be individualized, especially in the setting of pregnancy.
If abnormal cervical cytology is obtained on routine screening, appropriate patients should be referred for colposcopic exam. Physicians performing colposcopy should be familiar with the physiologic effects of pregnancy that can obscure the exam, including the increased cervical mucus production, prominence of endocervical glands, and increased vascularity.
Colposcopic-directed ectocervical biopsies have been found to be safe in pregnancy, and these women should be provided the same care as those who are not pregnant (Obstet Gynecol. 1993 Jun;81[6]:915-8). Endocervical sampling and endometrial sampling should not be performed, however, and physicians should remain dedicated to checking pregnancy tests prior to colposcopy.
HSIL cytology should prompt a biopsy in pregnancy; a decision to skip the biopsy and perform an excisional procedure in this setting is not recommended regardless of patient or gestational age. If LSIL (CIN1) is noted on biopsy, reevaluation post partum should be strongly considered, unless a suspicious lesion was felt to be inadequately biopsied.
Management
Managing HSIL in pregnancy focuses on diagnosis and excluding malignancy, while treatment can be reserved for the postpartum period. When choosing a management option, consider individual patient factors such as colposcopic appearance of the lesion, gestational age, and access to health care.
If HSIL is noted on colposcopic-directed biopsy, consider one of several options. The most conservative approach is reevaluation with cytology and colposcopy 6 weeks post partum. This is an option for patients who do not have a colposcopic exam that was concerning for an invasive lesion, were able to be adequately biopsied, and will reliably return for follow-up. Many physicians feel more comfortable with repeat cytology and colposcopy in 3 months from the original biopsy. The most aggressive management would include an excisional procedure during pregnancy.
There are varying rates of regression of biopsy-proven HSIL in pregnancy ranging from 34% to 70% (Obstet Gynecol. 1999 Mar;93[3]:359-62; Acta Obstet Gynecol Scand. 2006;85[9]:1134-7; Reprod Sci. 2009 Nov;16[11]:1034-9). Out of more than 200 patients across these three studies, just two patients were diagnosed with an invasive lesion post partum. Given the low likelihood of progression during pregnancy and the high rate of regression, an excisional procedure should be considered only in cases where there is concern about invasive carcinoma.
In cases where an invasive lesion is suspected, consider an an excisional procedure. While there is some evidence that performing a laser excisional procedure early in pregnancy (18 weeks and earlier) can be safely done, that is not the most common management strategy in the United States (Tumori. 1998 Sep-Oct;84[5]:567-70; Int J Gynecol Cancer. 2007 Jan-Feb;17[1]:127-31). In this circumstance, referral to a gynecologic oncologist is warranted where consideration can be made for performing a cold knife conization. Physicians should be aware of the increased risk of bleeding with this procedure in pregnancy and the potential for preterm birth. There is little literature to guide counseling regarding these risks, and the decision to perform an excisional procedure should be made with a multidisciplinary team (Arch Gynecol Obstet. 2016 Jan 4. doi: 10.1007/s00404-015-3980-y).
The see-and-treat paradigm is not recommended in pregnancy. Those patients with poor follow-up should still undergo colposcopic-directed biopsies prior to any excisional procedure.
Treatment recommendations in pregnancy should be made on the basis of careful consideration of individual patient factors, with strong consideration of repeat testing with cytology and colposcopy prior to an excision procedure.
Dr. Sullivan is a fellow in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. Dr. Sullivan and Dr. Gehrig reported having no relevant financial disclosures. Email them at [email protected].
New Zika case study fills some research gaps
A new case study adds yet more evidence to the link between congenital Zika virus infection and fetal brain damage while offering insights into how the virus affects brain development at different stages.
“Our study highlights the possible importance of [Zika virus] RNA testing of serum obtained from pregnant women beyond the first week after symptom onset, as well as a more detailed evaluation of the fetal intracranial anatomy by means of serial fetal ultrasonography or fetal brain MRI,” wrote Dr. Rita W. Driggers of Johns Hopkins University, Baltimore, and her associates (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMoa1601824).
The study also continues to help fill in the gaps in research highlighted by Dr. Lyle R. Petersen and his associates at the Centers for Disease Control and Prevention, in an overview of the virus published in the same issue (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMra1602113).
“These include a complete understanding of the frequency and full spectrum of clinical outcomes resulting from fetal Zika virus infection and of the environmental factors that influence emergence, as well as the development of discriminating diagnostic tools for flaviruses, animal models for fetal developmental effects due to viral infection, new vector control products and strategies, effective therapeutics, and vaccines to protect humans against the disease,” the CDC authors wrote.
In the case study, a 33-year-old Finnish woman developed an infection from Zika virus in her 11th week of pregnancy while on vacation in Mexico, Guatemala, and Belize. She experienced the common Zika virus symptoms of a mild fever, eye pain, rash, and muscle pain for 5 days and had evidence of Zika virus RNA in her blood between 16 and 21 weeks’ gestation.
Although the fetal head size remained within the normal range during the 16th and 17th weeks of pregnancy, fetal head circumference dropped from the 47th percentile at 16 weeks gestation to the 24th percentile at 20 weeks’ gestation. Ultrasound and MRI imagery found fetal brain abnormalities, including a thin cerebral mantle and potential agenesis of the corpus callosum at 19 and 20 weeks’ gestation, but neither microcephaly nor calcifications in the brain were seen.
“We suspect these reductions in brain growth would have eventually met the criteria for microcephaly,” the researchers wrote. “As this case shows, the latency period between Zika virus infection of the fetal brain and the detection of microcephaly and intracranial calcifications on ultrasonography is likely to be prolonged.”
Negative findings during this time could be “falsely reassuring and might delay critical time-sensitive decision making,” they added.
The woman chose to terminate the pregnancy at 21 weeks, and high viral loads of Zika were found in the fetal brain during a postmortem exam. The fetus also had lower amounts of Zika RNA in the muscle, liver, lung, and spleen, as did the mother’s amniotic fluid.
“Although the evidence of the association between the presence of Zika virus in pregnant women and fetal brain abnormalities continues to grow, the timing of infection during fetal development and other factors that may have an effect on viral pathogenesis and their effects on the appearance of the brain abnormalities are poorly understood,” the case study researchers wrote.
The CDC authors also note the challenges of differentiating Zika virus infections from dengue or other flavivirus infections. “Reliable testing regimens for the diagnosis of prenatal and antenatal Zika virus infection have not been established,” they wrote.
The CDC authors also predict millions more Zika cases in the Americas, given the incidence of dengue and chikungunya cases previously, but the burden of long-term effects is harder to predict. “The long-term outlook with regard to the current Zika outbreak in the Americas is uncertain,” they wrote. “Herd immunity sufficient to slow further transmission will undoubtedly occur, although this will not obviate the need for immediate and long-term prevention and control strategies.”
Researchers from the CDC and those who reported the case study reported having no financial disclosures.
We need research to clarify the best way to provide protection and to prevent serious consequences of Zika virus and other flaviviruses that were previously unknown. Until recently, Zika virus was believed to cause only mild disease, which it still does in the majority of cases. The main concern today is the growing body of evidence that Zika virus infection results in severe neurologic complications – Guillain-Barré syndrome in infected patients and microcephaly in unborn babies – combined with the very rapid spread of the virus.
Although vaccines may come too late for countries currently affected by the Zika virus epidemic, the development of a vaccine that can, above all, protect pregnant women and their babies remains an imperative for countries where the epidemic is expected to arrive in the foreseeable future. The goal would be to allow for medium- to long-term control of Zika virus analogous in some ways to the control of rubella. It is critical that we collaborate rather than compete to find answers to the questions that worry millions of women of child-bearing age in areas where Zika virus is spreading rapidly and may become endemic.
Dr. Charlotte J. Haug is an international correspondent for the New England Journal of Medicine; Marie-Paule Kieny, Ph.D., is assistant director-general for health systems and innovation at the World Health Organization; and Dr. Bernadette Murgue is the project manager of the WHO’s R&D Blueprint. They reported having no financial disclosures. These comments are adapted from an editorial (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMp1603734).
We need research to clarify the best way to provide protection and to prevent serious consequences of Zika virus and other flaviviruses that were previously unknown. Until recently, Zika virus was believed to cause only mild disease, which it still does in the majority of cases. The main concern today is the growing body of evidence that Zika virus infection results in severe neurologic complications – Guillain-Barré syndrome in infected patients and microcephaly in unborn babies – combined with the very rapid spread of the virus.
Although vaccines may come too late for countries currently affected by the Zika virus epidemic, the development of a vaccine that can, above all, protect pregnant women and their babies remains an imperative for countries where the epidemic is expected to arrive in the foreseeable future. The goal would be to allow for medium- to long-term control of Zika virus analogous in some ways to the control of rubella. It is critical that we collaborate rather than compete to find answers to the questions that worry millions of women of child-bearing age in areas where Zika virus is spreading rapidly and may become endemic.
Dr. Charlotte J. Haug is an international correspondent for the New England Journal of Medicine; Marie-Paule Kieny, Ph.D., is assistant director-general for health systems and innovation at the World Health Organization; and Dr. Bernadette Murgue is the project manager of the WHO’s R&D Blueprint. They reported having no financial disclosures. These comments are adapted from an editorial (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMp1603734).
We need research to clarify the best way to provide protection and to prevent serious consequences of Zika virus and other flaviviruses that were previously unknown. Until recently, Zika virus was believed to cause only mild disease, which it still does in the majority of cases. The main concern today is the growing body of evidence that Zika virus infection results in severe neurologic complications – Guillain-Barré syndrome in infected patients and microcephaly in unborn babies – combined with the very rapid spread of the virus.
Although vaccines may come too late for countries currently affected by the Zika virus epidemic, the development of a vaccine that can, above all, protect pregnant women and their babies remains an imperative for countries where the epidemic is expected to arrive in the foreseeable future. The goal would be to allow for medium- to long-term control of Zika virus analogous in some ways to the control of rubella. It is critical that we collaborate rather than compete to find answers to the questions that worry millions of women of child-bearing age in areas where Zika virus is spreading rapidly and may become endemic.
Dr. Charlotte J. Haug is an international correspondent for the New England Journal of Medicine; Marie-Paule Kieny, Ph.D., is assistant director-general for health systems and innovation at the World Health Organization; and Dr. Bernadette Murgue is the project manager of the WHO’s R&D Blueprint. They reported having no financial disclosures. These comments are adapted from an editorial (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMp1603734).
A new case study adds yet more evidence to the link between congenital Zika virus infection and fetal brain damage while offering insights into how the virus affects brain development at different stages.
“Our study highlights the possible importance of [Zika virus] RNA testing of serum obtained from pregnant women beyond the first week after symptom onset, as well as a more detailed evaluation of the fetal intracranial anatomy by means of serial fetal ultrasonography or fetal brain MRI,” wrote Dr. Rita W. Driggers of Johns Hopkins University, Baltimore, and her associates (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMoa1601824).
The study also continues to help fill in the gaps in research highlighted by Dr. Lyle R. Petersen and his associates at the Centers for Disease Control and Prevention, in an overview of the virus published in the same issue (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMra1602113).
“These include a complete understanding of the frequency and full spectrum of clinical outcomes resulting from fetal Zika virus infection and of the environmental factors that influence emergence, as well as the development of discriminating diagnostic tools for flaviruses, animal models for fetal developmental effects due to viral infection, new vector control products and strategies, effective therapeutics, and vaccines to protect humans against the disease,” the CDC authors wrote.
In the case study, a 33-year-old Finnish woman developed an infection from Zika virus in her 11th week of pregnancy while on vacation in Mexico, Guatemala, and Belize. She experienced the common Zika virus symptoms of a mild fever, eye pain, rash, and muscle pain for 5 days and had evidence of Zika virus RNA in her blood between 16 and 21 weeks’ gestation.
Although the fetal head size remained within the normal range during the 16th and 17th weeks of pregnancy, fetal head circumference dropped from the 47th percentile at 16 weeks gestation to the 24th percentile at 20 weeks’ gestation. Ultrasound and MRI imagery found fetal brain abnormalities, including a thin cerebral mantle and potential agenesis of the corpus callosum at 19 and 20 weeks’ gestation, but neither microcephaly nor calcifications in the brain were seen.
“We suspect these reductions in brain growth would have eventually met the criteria for microcephaly,” the researchers wrote. “As this case shows, the latency period between Zika virus infection of the fetal brain and the detection of microcephaly and intracranial calcifications on ultrasonography is likely to be prolonged.”
Negative findings during this time could be “falsely reassuring and might delay critical time-sensitive decision making,” they added.
The woman chose to terminate the pregnancy at 21 weeks, and high viral loads of Zika were found in the fetal brain during a postmortem exam. The fetus also had lower amounts of Zika RNA in the muscle, liver, lung, and spleen, as did the mother’s amniotic fluid.
“Although the evidence of the association between the presence of Zika virus in pregnant women and fetal brain abnormalities continues to grow, the timing of infection during fetal development and other factors that may have an effect on viral pathogenesis and their effects on the appearance of the brain abnormalities are poorly understood,” the case study researchers wrote.
The CDC authors also note the challenges of differentiating Zika virus infections from dengue or other flavivirus infections. “Reliable testing regimens for the diagnosis of prenatal and antenatal Zika virus infection have not been established,” they wrote.
The CDC authors also predict millions more Zika cases in the Americas, given the incidence of dengue and chikungunya cases previously, but the burden of long-term effects is harder to predict. “The long-term outlook with regard to the current Zika outbreak in the Americas is uncertain,” they wrote. “Herd immunity sufficient to slow further transmission will undoubtedly occur, although this will not obviate the need for immediate and long-term prevention and control strategies.”
Researchers from the CDC and those who reported the case study reported having no financial disclosures.
A new case study adds yet more evidence to the link between congenital Zika virus infection and fetal brain damage while offering insights into how the virus affects brain development at different stages.
“Our study highlights the possible importance of [Zika virus] RNA testing of serum obtained from pregnant women beyond the first week after symptom onset, as well as a more detailed evaluation of the fetal intracranial anatomy by means of serial fetal ultrasonography or fetal brain MRI,” wrote Dr. Rita W. Driggers of Johns Hopkins University, Baltimore, and her associates (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMoa1601824).
The study also continues to help fill in the gaps in research highlighted by Dr. Lyle R. Petersen and his associates at the Centers for Disease Control and Prevention, in an overview of the virus published in the same issue (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMra1602113).
“These include a complete understanding of the frequency and full spectrum of clinical outcomes resulting from fetal Zika virus infection and of the environmental factors that influence emergence, as well as the development of discriminating diagnostic tools for flaviruses, animal models for fetal developmental effects due to viral infection, new vector control products and strategies, effective therapeutics, and vaccines to protect humans against the disease,” the CDC authors wrote.
In the case study, a 33-year-old Finnish woman developed an infection from Zika virus in her 11th week of pregnancy while on vacation in Mexico, Guatemala, and Belize. She experienced the common Zika virus symptoms of a mild fever, eye pain, rash, and muscle pain for 5 days and had evidence of Zika virus RNA in her blood between 16 and 21 weeks’ gestation.
Although the fetal head size remained within the normal range during the 16th and 17th weeks of pregnancy, fetal head circumference dropped from the 47th percentile at 16 weeks gestation to the 24th percentile at 20 weeks’ gestation. Ultrasound and MRI imagery found fetal brain abnormalities, including a thin cerebral mantle and potential agenesis of the corpus callosum at 19 and 20 weeks’ gestation, but neither microcephaly nor calcifications in the brain were seen.
“We suspect these reductions in brain growth would have eventually met the criteria for microcephaly,” the researchers wrote. “As this case shows, the latency period between Zika virus infection of the fetal brain and the detection of microcephaly and intracranial calcifications on ultrasonography is likely to be prolonged.”
Negative findings during this time could be “falsely reassuring and might delay critical time-sensitive decision making,” they added.
The woman chose to terminate the pregnancy at 21 weeks, and high viral loads of Zika were found in the fetal brain during a postmortem exam. The fetus also had lower amounts of Zika RNA in the muscle, liver, lung, and spleen, as did the mother’s amniotic fluid.
“Although the evidence of the association between the presence of Zika virus in pregnant women and fetal brain abnormalities continues to grow, the timing of infection during fetal development and other factors that may have an effect on viral pathogenesis and their effects on the appearance of the brain abnormalities are poorly understood,” the case study researchers wrote.
The CDC authors also note the challenges of differentiating Zika virus infections from dengue or other flavivirus infections. “Reliable testing regimens for the diagnosis of prenatal and antenatal Zika virus infection have not been established,” they wrote.
The CDC authors also predict millions more Zika cases in the Americas, given the incidence of dengue and chikungunya cases previously, but the burden of long-term effects is harder to predict. “The long-term outlook with regard to the current Zika outbreak in the Americas is uncertain,” they wrote. “Herd immunity sufficient to slow further transmission will undoubtedly occur, although this will not obviate the need for immediate and long-term prevention and control strategies.”
Researchers from the CDC and those who reported the case study reported having no financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
CDC updates advice on preventing sexual transmission of Zika virus
Men potentially exposed to Zika virus should use a condom during all sex or abstain from sex for at least 8 weeks, according to new recommendations from the Centers for Disease Control and Prevention on reducing the risk of sexual transmission of the virus.
Men with confirmed infections or clinical symptoms of Zika should similarly abstain or use a condom for at least 6 months, the CDC recommends in the Morbidity and Mortality Weekly Report released on March 25 (MMWR 2016. Mar 25. doi: http://dx.doi.org/10.15585/mmwr.mm6512e3er).
These recommendations update and replace those issued by the CDC on Feb. 5 and include new guidance for men who live in, or have traveled to, an area with active Zika virus transmission. The recommendations apply to all types of sexual activity involving the penis, including vaginal intercourse, anal intercourse, or fellatio.
“The previous recommendations focused on women who were already pregnant,” Dr. Denise J. Jamieson, co-lead of the Pregnancy and Birth Defects Team of the CDC Zika Virus Response Team, said during a press briefing. “What’s new is that we are now concerned about the periconceptional period, around the time the woman conceives.”
For men with pregnant sex partners, the agency recommends consistent and accurate use of condoms during any type of sex, or abstinence during the length of the pregnancy.
“This course is the best way to avoid even a minimal risk of sexual transmission of Zika virus, which could have adverse fetal effects when contracted during pregnancy,” the CDC report states, adding that pregnant women should ask their male sex partners about recent travel to areas with currently circulating Zika virus.
For couples not expecting a child, but concerned about sexual transmission of Zika, men with a confirmed Zika infection or clinical symptoms of Zika infection should consider using condoms or abstaining from sex for at least 6 months after their symptoms appear. This recommendation is based on tripling 62 days – the longest time interval after infection during which the virus was successfully isolated from semen.
If men have traveled to areas with active Zika transmission but have not developed symptoms, the CDC recommends condom use or abstinence for at least 8 weeks after leaving the area. Those living in areas with active transmission should also consider condom use during sex or abstaining from sex until active transmission has ceased.
These recommendations come as more evidence points to a link between Zika infection and fetal abnormalities, including microcephaly and fetal mortality.
“I think we’re learning more every day, and I think the evidence of a link between Zika and a range of poor pregnancy outcomes is becoming stronger and stronger,” Dr. Jamieson said. “At this point, we’re not using causal language, but the evidence is mounting.”
The CDC also released two other reports focusing on the need to increase access to contraception for residents of Puerto Rico and interim guidance for health care providers of women of childbearing age who have been potentially exposed to Zika virus.
As of March 25, the CDC has reported 273 U.S. cases of Zika virus infections from 35 states and Washington, D.C. All of these – except six sexually transmitted cases – are travel related.
Additionally, Puerto Rico’s most recent case total is 261, all locally transmitted by mosquitoes, except for three travel-associated cases. American Samoa has 14 cases, and the U.S. Virgin Islands have 11 cases, all thought to be locally transmitted.
“Long-acting contraception methods are not readily available in Puerto Rico, and from our health care provider colleagues in Puerto Rico, there is a desire to provide a more broad range of contraception options to women in Puerto Rico,” Dr. Jamieson said.
She said the CDC is developing a plan to make long-acting contraceptive methods more available in Puerto Rico.
When advising couples who wish to become pregnant after the man has had confirmed or suspected Zika infection, the CDC recommends waiting at least 6 months after the man’s onset of Zika symptoms or confirmed infection before attempting to conceive.
Although no evidence suggests that Zika virus will cause congenital infections in pregnancies conceived after a woman’s infection has resolved, data on the virus’s incubation period is limited, according to the CDC.
“Women with Zika virus disease should wait until at least 8 weeks after symptom onset before attempting conception,” wrote Dr. Emily E. Petersen and her colleagues in the guidance on caring for women of reproductive age with possible Zika virus exposure. “No data are available regarding the risk for congenital infection among pregnant women with asymptomatic infection.”
Similarly, asymptomatic women potentially exposed to Zika virus should also wait at least 8 weeks after the possible exposure date before trying to conceive.
Men potentially exposed to Zika virus should use a condom during all sex or abstain from sex for at least 8 weeks, according to new recommendations from the Centers for Disease Control and Prevention on reducing the risk of sexual transmission of the virus.
Men with confirmed infections or clinical symptoms of Zika should similarly abstain or use a condom for at least 6 months, the CDC recommends in the Morbidity and Mortality Weekly Report released on March 25 (MMWR 2016. Mar 25. doi: http://dx.doi.org/10.15585/mmwr.mm6512e3er).
These recommendations update and replace those issued by the CDC on Feb. 5 and include new guidance for men who live in, or have traveled to, an area with active Zika virus transmission. The recommendations apply to all types of sexual activity involving the penis, including vaginal intercourse, anal intercourse, or fellatio.
“The previous recommendations focused on women who were already pregnant,” Dr. Denise J. Jamieson, co-lead of the Pregnancy and Birth Defects Team of the CDC Zika Virus Response Team, said during a press briefing. “What’s new is that we are now concerned about the periconceptional period, around the time the woman conceives.”
For men with pregnant sex partners, the agency recommends consistent and accurate use of condoms during any type of sex, or abstinence during the length of the pregnancy.
“This course is the best way to avoid even a minimal risk of sexual transmission of Zika virus, which could have adverse fetal effects when contracted during pregnancy,” the CDC report states, adding that pregnant women should ask their male sex partners about recent travel to areas with currently circulating Zika virus.
For couples not expecting a child, but concerned about sexual transmission of Zika, men with a confirmed Zika infection or clinical symptoms of Zika infection should consider using condoms or abstaining from sex for at least 6 months after their symptoms appear. This recommendation is based on tripling 62 days – the longest time interval after infection during which the virus was successfully isolated from semen.
If men have traveled to areas with active Zika transmission but have not developed symptoms, the CDC recommends condom use or abstinence for at least 8 weeks after leaving the area. Those living in areas with active transmission should also consider condom use during sex or abstaining from sex until active transmission has ceased.
These recommendations come as more evidence points to a link between Zika infection and fetal abnormalities, including microcephaly and fetal mortality.
“I think we’re learning more every day, and I think the evidence of a link between Zika and a range of poor pregnancy outcomes is becoming stronger and stronger,” Dr. Jamieson said. “At this point, we’re not using causal language, but the evidence is mounting.”
The CDC also released two other reports focusing on the need to increase access to contraception for residents of Puerto Rico and interim guidance for health care providers of women of childbearing age who have been potentially exposed to Zika virus.
As of March 25, the CDC has reported 273 U.S. cases of Zika virus infections from 35 states and Washington, D.C. All of these – except six sexually transmitted cases – are travel related.
Additionally, Puerto Rico’s most recent case total is 261, all locally transmitted by mosquitoes, except for three travel-associated cases. American Samoa has 14 cases, and the U.S. Virgin Islands have 11 cases, all thought to be locally transmitted.
“Long-acting contraception methods are not readily available in Puerto Rico, and from our health care provider colleagues in Puerto Rico, there is a desire to provide a more broad range of contraception options to women in Puerto Rico,” Dr. Jamieson said.
She said the CDC is developing a plan to make long-acting contraceptive methods more available in Puerto Rico.
When advising couples who wish to become pregnant after the man has had confirmed or suspected Zika infection, the CDC recommends waiting at least 6 months after the man’s onset of Zika symptoms or confirmed infection before attempting to conceive.
Although no evidence suggests that Zika virus will cause congenital infections in pregnancies conceived after a woman’s infection has resolved, data on the virus’s incubation period is limited, according to the CDC.
“Women with Zika virus disease should wait until at least 8 weeks after symptom onset before attempting conception,” wrote Dr. Emily E. Petersen and her colleagues in the guidance on caring for women of reproductive age with possible Zika virus exposure. “No data are available regarding the risk for congenital infection among pregnant women with asymptomatic infection.”
Similarly, asymptomatic women potentially exposed to Zika virus should also wait at least 8 weeks after the possible exposure date before trying to conceive.
Men potentially exposed to Zika virus should use a condom during all sex or abstain from sex for at least 8 weeks, according to new recommendations from the Centers for Disease Control and Prevention on reducing the risk of sexual transmission of the virus.
Men with confirmed infections or clinical symptoms of Zika should similarly abstain or use a condom for at least 6 months, the CDC recommends in the Morbidity and Mortality Weekly Report released on March 25 (MMWR 2016. Mar 25. doi: http://dx.doi.org/10.15585/mmwr.mm6512e3er).
These recommendations update and replace those issued by the CDC on Feb. 5 and include new guidance for men who live in, or have traveled to, an area with active Zika virus transmission. The recommendations apply to all types of sexual activity involving the penis, including vaginal intercourse, anal intercourse, or fellatio.
“The previous recommendations focused on women who were already pregnant,” Dr. Denise J. Jamieson, co-lead of the Pregnancy and Birth Defects Team of the CDC Zika Virus Response Team, said during a press briefing. “What’s new is that we are now concerned about the periconceptional period, around the time the woman conceives.”
For men with pregnant sex partners, the agency recommends consistent and accurate use of condoms during any type of sex, or abstinence during the length of the pregnancy.
“This course is the best way to avoid even a minimal risk of sexual transmission of Zika virus, which could have adverse fetal effects when contracted during pregnancy,” the CDC report states, adding that pregnant women should ask their male sex partners about recent travel to areas with currently circulating Zika virus.
For couples not expecting a child, but concerned about sexual transmission of Zika, men with a confirmed Zika infection or clinical symptoms of Zika infection should consider using condoms or abstaining from sex for at least 6 months after their symptoms appear. This recommendation is based on tripling 62 days – the longest time interval after infection during which the virus was successfully isolated from semen.
If men have traveled to areas with active Zika transmission but have not developed symptoms, the CDC recommends condom use or abstinence for at least 8 weeks after leaving the area. Those living in areas with active transmission should also consider condom use during sex or abstaining from sex until active transmission has ceased.
These recommendations come as more evidence points to a link between Zika infection and fetal abnormalities, including microcephaly and fetal mortality.
“I think we’re learning more every day, and I think the evidence of a link between Zika and a range of poor pregnancy outcomes is becoming stronger and stronger,” Dr. Jamieson said. “At this point, we’re not using causal language, but the evidence is mounting.”
The CDC also released two other reports focusing on the need to increase access to contraception for residents of Puerto Rico and interim guidance for health care providers of women of childbearing age who have been potentially exposed to Zika virus.
As of March 25, the CDC has reported 273 U.S. cases of Zika virus infections from 35 states and Washington, D.C. All of these – except six sexually transmitted cases – are travel related.
Additionally, Puerto Rico’s most recent case total is 261, all locally transmitted by mosquitoes, except for three travel-associated cases. American Samoa has 14 cases, and the U.S. Virgin Islands have 11 cases, all thought to be locally transmitted.
“Long-acting contraception methods are not readily available in Puerto Rico, and from our health care provider colleagues in Puerto Rico, there is a desire to provide a more broad range of contraception options to women in Puerto Rico,” Dr. Jamieson said.
She said the CDC is developing a plan to make long-acting contraceptive methods more available in Puerto Rico.
When advising couples who wish to become pregnant after the man has had confirmed or suspected Zika infection, the CDC recommends waiting at least 6 months after the man’s onset of Zika symptoms or confirmed infection before attempting to conceive.
Although no evidence suggests that Zika virus will cause congenital infections in pregnancies conceived after a woman’s infection has resolved, data on the virus’s incubation period is limited, according to the CDC.
“Women with Zika virus disease should wait until at least 8 weeks after symptom onset before attempting conception,” wrote Dr. Emily E. Petersen and her colleagues in the guidance on caring for women of reproductive age with possible Zika virus exposure. “No data are available regarding the risk for congenital infection among pregnant women with asymptomatic infection.”
Similarly, asymptomatic women potentially exposed to Zika virus should also wait at least 8 weeks after the possible exposure date before trying to conceive.
Perinatal depression screening: New recommendations and challenges
It was almost a year ago that the American College of Obstetricians and Gynecologists came out unequivocally in favor of universal screening for perinatal depression.
In the revised policy statement from ACOG’s Committee on Obstetric Practice, the college recommended that physicians screen women for depression and anxiety symptoms at least once during the perinatal period using a standard, validated tool. ACOG also noted that screening must be coupled with appropriate follow-up and that clinical staff must be prepared to start therapy or refer patients to treatment (Obstet. Gynecol. 2015;125:1268-71).
This move toward routine screening was intuitive given the prevalence of perinatal mood and anxiety disorders.
Fast forward to January 2016 and the U.S. Preventive Services Task Force final recommendation calling for screening all adults for depression, including the at-risk populations of pregnant and postpartum women. Much like the ACOG guidelines, the USPSTF recommendations call for adequate systems to ensure treatment and follow-up (JAMA. 2016 Jan. 26;315[4]:380-7).
These recommendations, although timely, derive from relatively sparse data on the actual effectiveness of perinatal screening. Although the move toward screening is welcome and simply commonsense, it is concerning that there has been very little systematic study of the effectiveness of screening for such a prevalent and impactful illness. At the end of the day, the question remains: Will screening for perinatal depression in obstetric and possibly pediatric settings lead to improved outcomes for patients and families?
We’re screening, but will it make a difference?
As more U.S. states, along with other countries around the world, have begun routine screening of women in the perinatal period, it’s become clear that screening itself is easy to do. What has yet to be adequately demonstrated is how screening moves us toward getting women into treatment and ultimately toward getting women well.
New Jersey and Illinois are good examples of states that should be applauded for recognizing early on how important it is to identify women with perinatal depression. But even in these early-adopter states, the actual implementation of referral systems has been lacking.
Here in Massachusetts, we have a state-funded program designed to teach local women’s health providers – including ob.gyns. – about diagnosing perinatal depression. The MCPAP (Massachusetts Child Psychiatry Access Project) for Moms program also offers resources for consultation and referral. The program is fairly new, so it’s still unclear whether ob.gyns. and primary care physicians will accept the role of de facto mental health treaters, as well as whether the women who are identified through screening will go on to recover acutely and, more importantly, over the long term.
These experiences among the states highlight how great a challenge it is to go from screening to positive health outcomes for women.
Downstream difficulties
A lack of evidence isn’t the only problem. A recent editorial in the Lancet raised the concern that the currently available screening tests are not suitable for clinical practice. The suggestion read to some like heresy.
The Edinburgh Postnatal Depression Scale, which is the most commonly used screening instrument, has a positive predictive value of detecting major depressive disorder of 47%-64%, according to the editorial, making it prone to delivering false positives (doi: 10.1016/S0140-6736[16]00265-8).
“This situation is potentially dangerous,” the Lancet editorial noted, since results of qualitative studies “suggest that women are extremely concerned about depression screening, about the stigma associated with a diagnosis of depression, and that a positive result might lead to an automatic social service referral, and potentially removal of their baby.”
A recent article, published in the New York Times, raises an additional concern about what a depression diagnosis could mean for insurability. The article highlights the experience of a woman whose diagnosis of postpartum depression is creating difficulties for her in getting life insurance. The point is underscored that it is perfectly legal for life and disability insurers to charge more to patients with a diagnosis of mental illness or to deny coverage outright.
No going back
The whole issue of perinatal depression screening has opened a Pandora’s box, and that is a good thing. The conversation is long overdue in America. It is time for greater national awareness and focus on a disease that is as prevalent as perinatal depression and as disabling for women and their families.
The focus up to this point has been on perinatal depression screening, but we’re about to see a shift toward building the community infrastructure that will be critical for managing patients, including those women who have previously been marginalized and have had very poor access to care.
Widespread screening and treatment will also require a level of cooperation between advocacy groups and providers who are multidisciplinary in their approach, taking advantage of both pharmacologic and nonpharmacologic approaches. A model of crossdisciplinary collaboration will include, for example, providers from psychiatrists to therapists to doulas to social workers to clinicians who focus on mother-infant interaction. It is a long list and models for such collaboration are somewhat lacking.
One good example of a pilot effort for such a collaboration is the Massachusetts Postpartum Depression Commission, which includes a full spectrum of participants from doulas, social workers, and perinatal psychiatrists to lay people. The partnerships and the networking that’s going on across disciplines is absolutely new and is going to be essential if we’re going to manage an issue as large as the treatment of perinatal depression.
The enhanced awareness of the need to screen for, identify, and treat postpartum depression will also lead to better tools with greater specificity, perhaps using new technologies for better identification and treatment, everything from telemedicine to smartphone applications.
There will certainly be growing pains as we gather evidence, refine our screening instruments, and build referral systems, but I don’t see this as a reason not to identify illness in this vulnerable population. Rather, it is a charge to the field that there is work to be done.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
It was almost a year ago that the American College of Obstetricians and Gynecologists came out unequivocally in favor of universal screening for perinatal depression.
In the revised policy statement from ACOG’s Committee on Obstetric Practice, the college recommended that physicians screen women for depression and anxiety symptoms at least once during the perinatal period using a standard, validated tool. ACOG also noted that screening must be coupled with appropriate follow-up and that clinical staff must be prepared to start therapy or refer patients to treatment (Obstet. Gynecol. 2015;125:1268-71).
This move toward routine screening was intuitive given the prevalence of perinatal mood and anxiety disorders.
Fast forward to January 2016 and the U.S. Preventive Services Task Force final recommendation calling for screening all adults for depression, including the at-risk populations of pregnant and postpartum women. Much like the ACOG guidelines, the USPSTF recommendations call for adequate systems to ensure treatment and follow-up (JAMA. 2016 Jan. 26;315[4]:380-7).
These recommendations, although timely, derive from relatively sparse data on the actual effectiveness of perinatal screening. Although the move toward screening is welcome and simply commonsense, it is concerning that there has been very little systematic study of the effectiveness of screening for such a prevalent and impactful illness. At the end of the day, the question remains: Will screening for perinatal depression in obstetric and possibly pediatric settings lead to improved outcomes for patients and families?
We’re screening, but will it make a difference?
As more U.S. states, along with other countries around the world, have begun routine screening of women in the perinatal period, it’s become clear that screening itself is easy to do. What has yet to be adequately demonstrated is how screening moves us toward getting women into treatment and ultimately toward getting women well.
New Jersey and Illinois are good examples of states that should be applauded for recognizing early on how important it is to identify women with perinatal depression. But even in these early-adopter states, the actual implementation of referral systems has been lacking.
Here in Massachusetts, we have a state-funded program designed to teach local women’s health providers – including ob.gyns. – about diagnosing perinatal depression. The MCPAP (Massachusetts Child Psychiatry Access Project) for Moms program also offers resources for consultation and referral. The program is fairly new, so it’s still unclear whether ob.gyns. and primary care physicians will accept the role of de facto mental health treaters, as well as whether the women who are identified through screening will go on to recover acutely and, more importantly, over the long term.
These experiences among the states highlight how great a challenge it is to go from screening to positive health outcomes for women.
Downstream difficulties
A lack of evidence isn’t the only problem. A recent editorial in the Lancet raised the concern that the currently available screening tests are not suitable for clinical practice. The suggestion read to some like heresy.
The Edinburgh Postnatal Depression Scale, which is the most commonly used screening instrument, has a positive predictive value of detecting major depressive disorder of 47%-64%, according to the editorial, making it prone to delivering false positives (doi: 10.1016/S0140-6736[16]00265-8).
“This situation is potentially dangerous,” the Lancet editorial noted, since results of qualitative studies “suggest that women are extremely concerned about depression screening, about the stigma associated with a diagnosis of depression, and that a positive result might lead to an automatic social service referral, and potentially removal of their baby.”
A recent article, published in the New York Times, raises an additional concern about what a depression diagnosis could mean for insurability. The article highlights the experience of a woman whose diagnosis of postpartum depression is creating difficulties for her in getting life insurance. The point is underscored that it is perfectly legal for life and disability insurers to charge more to patients with a diagnosis of mental illness or to deny coverage outright.
No going back
The whole issue of perinatal depression screening has opened a Pandora’s box, and that is a good thing. The conversation is long overdue in America. It is time for greater national awareness and focus on a disease that is as prevalent as perinatal depression and as disabling for women and their families.
The focus up to this point has been on perinatal depression screening, but we’re about to see a shift toward building the community infrastructure that will be critical for managing patients, including those women who have previously been marginalized and have had very poor access to care.
Widespread screening and treatment will also require a level of cooperation between advocacy groups and providers who are multidisciplinary in their approach, taking advantage of both pharmacologic and nonpharmacologic approaches. A model of crossdisciplinary collaboration will include, for example, providers from psychiatrists to therapists to doulas to social workers to clinicians who focus on mother-infant interaction. It is a long list and models for such collaboration are somewhat lacking.
One good example of a pilot effort for such a collaboration is the Massachusetts Postpartum Depression Commission, which includes a full spectrum of participants from doulas, social workers, and perinatal psychiatrists to lay people. The partnerships and the networking that’s going on across disciplines is absolutely new and is going to be essential if we’re going to manage an issue as large as the treatment of perinatal depression.
The enhanced awareness of the need to screen for, identify, and treat postpartum depression will also lead to better tools with greater specificity, perhaps using new technologies for better identification and treatment, everything from telemedicine to smartphone applications.
There will certainly be growing pains as we gather evidence, refine our screening instruments, and build referral systems, but I don’t see this as a reason not to identify illness in this vulnerable population. Rather, it is a charge to the field that there is work to be done.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
It was almost a year ago that the American College of Obstetricians and Gynecologists came out unequivocally in favor of universal screening for perinatal depression.
In the revised policy statement from ACOG’s Committee on Obstetric Practice, the college recommended that physicians screen women for depression and anxiety symptoms at least once during the perinatal period using a standard, validated tool. ACOG also noted that screening must be coupled with appropriate follow-up and that clinical staff must be prepared to start therapy or refer patients to treatment (Obstet. Gynecol. 2015;125:1268-71).
This move toward routine screening was intuitive given the prevalence of perinatal mood and anxiety disorders.
Fast forward to January 2016 and the U.S. Preventive Services Task Force final recommendation calling for screening all adults for depression, including the at-risk populations of pregnant and postpartum women. Much like the ACOG guidelines, the USPSTF recommendations call for adequate systems to ensure treatment and follow-up (JAMA. 2016 Jan. 26;315[4]:380-7).
These recommendations, although timely, derive from relatively sparse data on the actual effectiveness of perinatal screening. Although the move toward screening is welcome and simply commonsense, it is concerning that there has been very little systematic study of the effectiveness of screening for such a prevalent and impactful illness. At the end of the day, the question remains: Will screening for perinatal depression in obstetric and possibly pediatric settings lead to improved outcomes for patients and families?
We’re screening, but will it make a difference?
As more U.S. states, along with other countries around the world, have begun routine screening of women in the perinatal period, it’s become clear that screening itself is easy to do. What has yet to be adequately demonstrated is how screening moves us toward getting women into treatment and ultimately toward getting women well.
New Jersey and Illinois are good examples of states that should be applauded for recognizing early on how important it is to identify women with perinatal depression. But even in these early-adopter states, the actual implementation of referral systems has been lacking.
Here in Massachusetts, we have a state-funded program designed to teach local women’s health providers – including ob.gyns. – about diagnosing perinatal depression. The MCPAP (Massachusetts Child Psychiatry Access Project) for Moms program also offers resources for consultation and referral. The program is fairly new, so it’s still unclear whether ob.gyns. and primary care physicians will accept the role of de facto mental health treaters, as well as whether the women who are identified through screening will go on to recover acutely and, more importantly, over the long term.
These experiences among the states highlight how great a challenge it is to go from screening to positive health outcomes for women.
Downstream difficulties
A lack of evidence isn’t the only problem. A recent editorial in the Lancet raised the concern that the currently available screening tests are not suitable for clinical practice. The suggestion read to some like heresy.
The Edinburgh Postnatal Depression Scale, which is the most commonly used screening instrument, has a positive predictive value of detecting major depressive disorder of 47%-64%, according to the editorial, making it prone to delivering false positives (doi: 10.1016/S0140-6736[16]00265-8).
“This situation is potentially dangerous,” the Lancet editorial noted, since results of qualitative studies “suggest that women are extremely concerned about depression screening, about the stigma associated with a diagnosis of depression, and that a positive result might lead to an automatic social service referral, and potentially removal of their baby.”
A recent article, published in the New York Times, raises an additional concern about what a depression diagnosis could mean for insurability. The article highlights the experience of a woman whose diagnosis of postpartum depression is creating difficulties for her in getting life insurance. The point is underscored that it is perfectly legal for life and disability insurers to charge more to patients with a diagnosis of mental illness or to deny coverage outright.
No going back
The whole issue of perinatal depression screening has opened a Pandora’s box, and that is a good thing. The conversation is long overdue in America. It is time for greater national awareness and focus on a disease that is as prevalent as perinatal depression and as disabling for women and their families.
The focus up to this point has been on perinatal depression screening, but we’re about to see a shift toward building the community infrastructure that will be critical for managing patients, including those women who have previously been marginalized and have had very poor access to care.
Widespread screening and treatment will also require a level of cooperation between advocacy groups and providers who are multidisciplinary in their approach, taking advantage of both pharmacologic and nonpharmacologic approaches. A model of crossdisciplinary collaboration will include, for example, providers from psychiatrists to therapists to doulas to social workers to clinicians who focus on mother-infant interaction. It is a long list and models for such collaboration are somewhat lacking.
One good example of a pilot effort for such a collaboration is the Massachusetts Postpartum Depression Commission, which includes a full spectrum of participants from doulas, social workers, and perinatal psychiatrists to lay people. The partnerships and the networking that’s going on across disciplines is absolutely new and is going to be essential if we’re going to manage an issue as large as the treatment of perinatal depression.
The enhanced awareness of the need to screen for, identify, and treat postpartum depression will also lead to better tools with greater specificity, perhaps using new technologies for better identification and treatment, everything from telemedicine to smartphone applications.
There will certainly be growing pains as we gather evidence, refine our screening instruments, and build referral systems, but I don’t see this as a reason not to identify illness in this vulnerable population. Rather, it is a charge to the field that there is work to be done.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
CDC urges precautions during L&D to prevent Zika transmission
Health care providers working in labor and delivery rooms should employ the standard precautions for infection control to reduce the theoretical risk of Zika transmission, according to recommendations from the Centers for Disease Control and Prevention.
“Because of the potential for exposure to large volumes of body fluids during the labor and delivery process and the sometimes unpredictable and fast-paced nature of obstetrical care, the use of Standard Precautions in these settings is essential to prevent possible transmission of Zika virus from patients to health care personnel,” Dr. Christine K. Olson and her colleagues at the CDC wrote in the Morbidity and Mortality Weekly Report.
No cases of occupational transmission of Zika via bodily fluids have been reported so far, but sexual transmission has occurred and the virus’s RNA has been found in blood, urine, saliva, and amniotic fluid. The risk of occupational exposure therefore theoretically exists, the authors wrote (MMWR. 2016 Mar 22. doi: 10.15585/mmwr.mm6511e3er).
The standard precautions are aimed at preventing transmission of any infectious agent present in blood, body fluids, secretions, nonperspiration excretions, nonintact skin, and mucous membranes. They include five main elements: hand hygiene, use of personal protection equipment (PPE), respiratory hygiene and cough etiquette, safe injection practices, and safe handling of potentially contaminated equipment or surfaces in the patient environment.
Standard PPE recommendations in labor and delivery rooms include eye protection during deliveries to prevent contamination from blood and bodily fluids and the use of double-gloving since the outer layer often contains perforations.
Health care personnel should already be following these standard precautions in all health care settings, but the CDC report will likely remind providers of the importance of these infection control procedures and improve compliance, said Dr. Aaron Caughey, chair of the department of obstetrics and gynecology and associate dean for Women’s Health Research and Policy at Oregon Health and Science University, Portland.
“Those are the standard recommendations on every labor floor for delivery currently, so absolutely it’s feasible,” he said in an interview. However, “for a low-risk woman doing a vaginal delivery without complications, I know that some people skimp on eyewear, and I know there are occasionally people who will wear just one pair of gloves,” he said.
The CDC report also noted varying levels of adherence to the standard precautions.
“Numerous barriers to the appropriate use of PPE have been cited, including the perception that PPE is uncomfortable and limits dexterity, fogging of goggles or face masks, the misperception that prescription eyeglasses provide adequate eye protection, lack of available PPE, forgetting to use PPE, lack of time in urgent clinical situations to don appropriate PPE, the perception that the patient poses minimal risk, and concerns about interference with patient care,” Dr. Olson and her colleagues wrote.
Dr. Caughey drew parallels to the late 1980s and early 1990s, when compliance with eye safety and glove safety precautions increased dramatically alongside the HIV epidemic.
“With these recommendations, it will probably get a little more heightened, particularly if there are parts of the country where Zika becomes endemic,” he said. “The big difference with Zika is if a man contracts Zika or a woman who’s not pregnant contracts Zika, the risks to them are very, very low. It’s really about pregnant women and the risk to the fetus.”
The CDC authors specifically noted that the theoretical risk for Zika transmission is most relevant for female health care personnel who may be pregnant, or for male or female health care personnel attempting to conceive. They recommended that personnel determine the most appropriate PPE based on the likelihood of body fluid exposure for each type of procedure or activity.
An amniotomy or placement of an intrauterine pressure catheter may require mask, eye protection, gloves and an impermeable gown, for example, but vaginal exams of pregnant women with minimal cervical dilation and intact membranes likely only call for the use of gloves. Anesthesia providers should wear sterile gloves and a surgical mask when they place catheters or administer intrathecal injections, and all providers should wear double gloves while handling sharps.
“When performing procedures including vaginal deliveries, manual placenta removal, bimanual uterine massage, and repair of vaginal lacerations, PPE should include (in addition to mucous membrane and skin protection) impermeable gowns and knee-high impermeable shoe covers,” they wrote.
Health care providers working in labor and delivery rooms should employ the standard precautions for infection control to reduce the theoretical risk of Zika transmission, according to recommendations from the Centers for Disease Control and Prevention.
“Because of the potential for exposure to large volumes of body fluids during the labor and delivery process and the sometimes unpredictable and fast-paced nature of obstetrical care, the use of Standard Precautions in these settings is essential to prevent possible transmission of Zika virus from patients to health care personnel,” Dr. Christine K. Olson and her colleagues at the CDC wrote in the Morbidity and Mortality Weekly Report.
No cases of occupational transmission of Zika via bodily fluids have been reported so far, but sexual transmission has occurred and the virus’s RNA has been found in blood, urine, saliva, and amniotic fluid. The risk of occupational exposure therefore theoretically exists, the authors wrote (MMWR. 2016 Mar 22. doi: 10.15585/mmwr.mm6511e3er).
The standard precautions are aimed at preventing transmission of any infectious agent present in blood, body fluids, secretions, nonperspiration excretions, nonintact skin, and mucous membranes. They include five main elements: hand hygiene, use of personal protection equipment (PPE), respiratory hygiene and cough etiquette, safe injection practices, and safe handling of potentially contaminated equipment or surfaces in the patient environment.
Standard PPE recommendations in labor and delivery rooms include eye protection during deliveries to prevent contamination from blood and bodily fluids and the use of double-gloving since the outer layer often contains perforations.
Health care personnel should already be following these standard precautions in all health care settings, but the CDC report will likely remind providers of the importance of these infection control procedures and improve compliance, said Dr. Aaron Caughey, chair of the department of obstetrics and gynecology and associate dean for Women’s Health Research and Policy at Oregon Health and Science University, Portland.
“Those are the standard recommendations on every labor floor for delivery currently, so absolutely it’s feasible,” he said in an interview. However, “for a low-risk woman doing a vaginal delivery without complications, I know that some people skimp on eyewear, and I know there are occasionally people who will wear just one pair of gloves,” he said.
The CDC report also noted varying levels of adherence to the standard precautions.
“Numerous barriers to the appropriate use of PPE have been cited, including the perception that PPE is uncomfortable and limits dexterity, fogging of goggles or face masks, the misperception that prescription eyeglasses provide adequate eye protection, lack of available PPE, forgetting to use PPE, lack of time in urgent clinical situations to don appropriate PPE, the perception that the patient poses minimal risk, and concerns about interference with patient care,” Dr. Olson and her colleagues wrote.
Dr. Caughey drew parallels to the late 1980s and early 1990s, when compliance with eye safety and glove safety precautions increased dramatically alongside the HIV epidemic.
“With these recommendations, it will probably get a little more heightened, particularly if there are parts of the country where Zika becomes endemic,” he said. “The big difference with Zika is if a man contracts Zika or a woman who’s not pregnant contracts Zika, the risks to them are very, very low. It’s really about pregnant women and the risk to the fetus.”
The CDC authors specifically noted that the theoretical risk for Zika transmission is most relevant for female health care personnel who may be pregnant, or for male or female health care personnel attempting to conceive. They recommended that personnel determine the most appropriate PPE based on the likelihood of body fluid exposure for each type of procedure or activity.
An amniotomy or placement of an intrauterine pressure catheter may require mask, eye protection, gloves and an impermeable gown, for example, but vaginal exams of pregnant women with minimal cervical dilation and intact membranes likely only call for the use of gloves. Anesthesia providers should wear sterile gloves and a surgical mask when they place catheters or administer intrathecal injections, and all providers should wear double gloves while handling sharps.
“When performing procedures including vaginal deliveries, manual placenta removal, bimanual uterine massage, and repair of vaginal lacerations, PPE should include (in addition to mucous membrane and skin protection) impermeable gowns and knee-high impermeable shoe covers,” they wrote.
Health care providers working in labor and delivery rooms should employ the standard precautions for infection control to reduce the theoretical risk of Zika transmission, according to recommendations from the Centers for Disease Control and Prevention.
“Because of the potential for exposure to large volumes of body fluids during the labor and delivery process and the sometimes unpredictable and fast-paced nature of obstetrical care, the use of Standard Precautions in these settings is essential to prevent possible transmission of Zika virus from patients to health care personnel,” Dr. Christine K. Olson and her colleagues at the CDC wrote in the Morbidity and Mortality Weekly Report.
No cases of occupational transmission of Zika via bodily fluids have been reported so far, but sexual transmission has occurred and the virus’s RNA has been found in blood, urine, saliva, and amniotic fluid. The risk of occupational exposure therefore theoretically exists, the authors wrote (MMWR. 2016 Mar 22. doi: 10.15585/mmwr.mm6511e3er).
The standard precautions are aimed at preventing transmission of any infectious agent present in blood, body fluids, secretions, nonperspiration excretions, nonintact skin, and mucous membranes. They include five main elements: hand hygiene, use of personal protection equipment (PPE), respiratory hygiene and cough etiquette, safe injection practices, and safe handling of potentially contaminated equipment or surfaces in the patient environment.
Standard PPE recommendations in labor and delivery rooms include eye protection during deliveries to prevent contamination from blood and bodily fluids and the use of double-gloving since the outer layer often contains perforations.
Health care personnel should already be following these standard precautions in all health care settings, but the CDC report will likely remind providers of the importance of these infection control procedures and improve compliance, said Dr. Aaron Caughey, chair of the department of obstetrics and gynecology and associate dean for Women’s Health Research and Policy at Oregon Health and Science University, Portland.
“Those are the standard recommendations on every labor floor for delivery currently, so absolutely it’s feasible,” he said in an interview. However, “for a low-risk woman doing a vaginal delivery without complications, I know that some people skimp on eyewear, and I know there are occasionally people who will wear just one pair of gloves,” he said.
The CDC report also noted varying levels of adherence to the standard precautions.
“Numerous barriers to the appropriate use of PPE have been cited, including the perception that PPE is uncomfortable and limits dexterity, fogging of goggles or face masks, the misperception that prescription eyeglasses provide adequate eye protection, lack of available PPE, forgetting to use PPE, lack of time in urgent clinical situations to don appropriate PPE, the perception that the patient poses minimal risk, and concerns about interference with patient care,” Dr. Olson and her colleagues wrote.
Dr. Caughey drew parallels to the late 1980s and early 1990s, when compliance with eye safety and glove safety precautions increased dramatically alongside the HIV epidemic.
“With these recommendations, it will probably get a little more heightened, particularly if there are parts of the country where Zika becomes endemic,” he said. “The big difference with Zika is if a man contracts Zika or a woman who’s not pregnant contracts Zika, the risks to them are very, very low. It’s really about pregnant women and the risk to the fetus.”
The CDC authors specifically noted that the theoretical risk for Zika transmission is most relevant for female health care personnel who may be pregnant, or for male or female health care personnel attempting to conceive. They recommended that personnel determine the most appropriate PPE based on the likelihood of body fluid exposure for each type of procedure or activity.
An amniotomy or placement of an intrauterine pressure catheter may require mask, eye protection, gloves and an impermeable gown, for example, but vaginal exams of pregnant women with minimal cervical dilation and intact membranes likely only call for the use of gloves. Anesthesia providers should wear sterile gloves and a surgical mask when they place catheters or administer intrathecal injections, and all providers should wear double gloves while handling sharps.
“When performing procedures including vaginal deliveries, manual placenta removal, bimanual uterine massage, and repair of vaginal lacerations, PPE should include (in addition to mucous membrane and skin protection) impermeable gowns and knee-high impermeable shoe covers,” they wrote.
FROM MMWR