User login
Colorectal cancer screening, 2021: An update
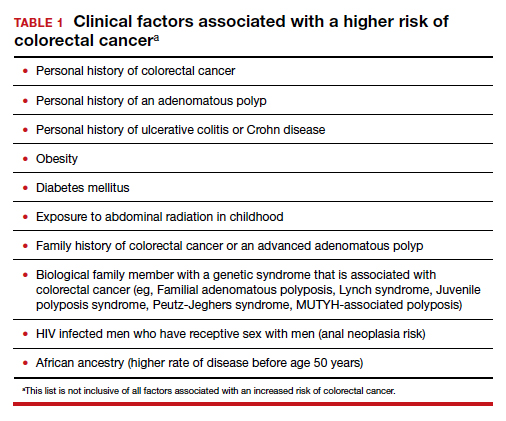
Colorectal cancer is a common disease that has a very lengthy natural history of progression from small (<8 mm) to large (≥8 mm) polyps, then to dysplasia, and eventually to invasive cancer. It is estimated that this progression takes 10 years.1 The long natural history from preneoplasia to cancer makes colorectal cancer an ideal target for screening. Screening for colorectal cancer is divided into two clinical pathways, screening for people at average risk and for those at high risk. Clinical factors that increase the risk of colorectal cancer are listed in TABLE 1. This editorial is focused on the clinical approach to screening for people at average risk for colorectal cancer.
Colorectal cancer is the second most common cause of cancer death
The top 6 causes of cancer death in the United States are2:
- lung cancer (23% of cancer deaths)
- colon and rectum (9%)
- pancreas (8%)
- female breast (7%)
- prostate (5%)
- liver/bile ducts (5%).
In 2020 it is estimated that 147,950 people were diagnosed with colorectal cancer, including 17,930 people less than 50 years of age.3 In 2020, it is also estimated that 53,200 people in the United States died of colorectal cancer, including 3,640 people younger than age 50.3 By contrast, the American Cancer Society estimates that, in 2021, cervical cancer will be diagnosed in 14,480 women and 4,290 women with the disease will die.4
According to a Centers for Disease Control and Prevention (CDC) study, among people 50 to 64 years of age, 63% report being up to date with colorectal cancer screening—leaving a full one-third not up to date with their screening.5 Among people aged 65 to 75, 79% report being up to date with colorectal cancer screening. Among those aged 50 to 64, those with health insurance were more likely to be up to date with screening than people without insurance—67% versus 33%, respectively. People with a household income greater than $75,000 and less than $35,000 reported up-to-date screening rates of 71% and 55%, respectively. Among people aged 50 to 64, non-Hispanic White and Black people reported similar rates of being up to date with colorectal screening (66% and 65%, respectively). Hispanic people, however, reported a significantly lower rate of being up to date with colorectal cancer screening (51%).5
A weakness of this CDC study is that the response rate from the surveyed population was less than 50%, raising questions about validity and generalizability of the reported results. Of note, other studies report that Black men may have lower rates of colorectal cancer screening than non-Black men.6 These data show that focused interventions to improve colorectal cancer screening are required for people 50 to 64 years of age, particularly among underinsured and some minority populations.
Continue to: Inequitable health outcomes for colorectal cancer...
Inequitable health outcomes for colorectal cancer
The purpose of screening for cancer is to reduce the morbidity and mortality associated with the disease. Based on the Surveillance, Epidemiology and End Results (SEER) national reporting system, from 2014 to 2018 colorectal death rates per 100,000 adults were 18 for Black adults; 15.1 for American Indian/Alaska native adults; 13.6 for White non-Hispanic adults; 10.9 for White, Hispanic adults; and 9.4 for Asian/Pacific Islander adults.7 Lack of access to and a lower utilization rate of high-quality colon cancer screening modalities, for example colonoscopy, and a lower rate of optimal colon cancer treatment may account for the higher colorectal death rate among Black adults.8,9
Colorectal cancer screening should begin at age 45
In 2015 the Agency for Health Research and Quality (AHRQ) published data showing that the benefit of initiating screening for colorectal cancer at 45 years of age outweighed the additional cost.10 In 2018, the American Cancer Society recommended that screening for colorectal cancer should begin at age 45.11 In 2021, after resisting the change for many years, the US Preventive Services Task Force (USPSTF) also recommended that screening for colorectal cancer should begin at 45.7 The new recommendation is based on statistical models that showed a significant increase in life-years gained at a small incremental cost. The USPSTF also recommended that clinicians and patients could consider discontinuing colorectal cancer screening at 75 years of age because the net benefit of continuing screening after age 75 is minimal.
Prior to 2021 the USPSTF recommended that screening be initiated at age 50. However, from 2010 to 2020 there was a significant increase in the percentage of new cases of colorectal cancer detected in people younger than 50. In 2010, colon and rectal cancer among people under 50 years of age accounted for 5% and 9% of all cases, respectively.12 In 2020, colon and rectal cancer in people younger than age 50 accounted for 11% and 15% of all cases, respectively.3
Options for colon cancer screening
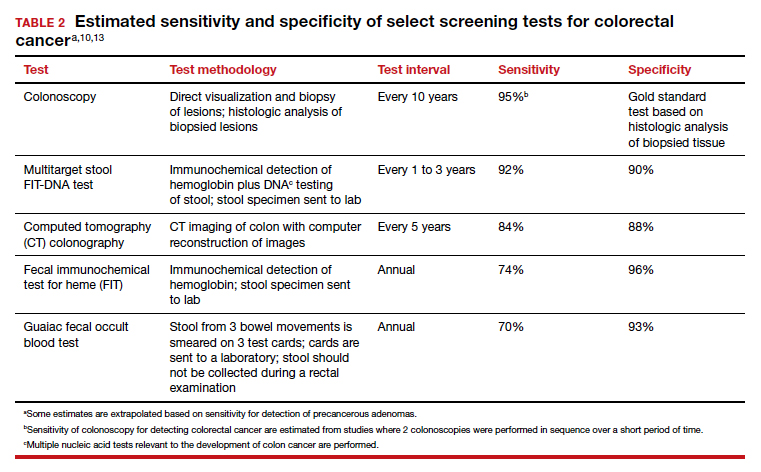
There are many options for colorectal cancer screening (TABLE 2).10,13 Experts conclude that the best colorectal cancer screening test is the test that the patient will complete. Among options for screening, colonoscopy and the multitarget stool FIT-DNA test (Cologuard) have greater sensitivity for detecting colorectal precancer and cancer lesions compared with fecal immunochemical testing (FIT), computed tomography colonography imaging (CTC), and stool guaiac testing (see TABLE 1).
In my practice, I suggest patients use either colonoscopy (every 10 years) or the multitarget stool FIT-DNA test (every 1 to 3 years) for screening. Most of my patients select colonoscopy, but some prefer the multitarget stool FIT-DNA test because they fear the pre-colonoscopy bowel preparation and the risk of bowel perforation with colonoscopy. Most colonoscopy procedures are performed with sedation, requiring an adult to take responsibility for transporting the patient to their residence, adding complexity to the performance of colonoscopy. These two tests are discussed in more detail below.
Colonoscopy
Colonoscopy occupies a unique position among the options for colorectal cancer screening because it is both a screening test and the gold standard for diagnosis, based on histologic analysis of the polypoid tissue biopsied at the time of colonoscopy. For all other screening tests, if the test yields an abnormal result, it is necessary to perform a colonoscopy. Colonoscopy screening offers the advantage of “one and done for 10 years.” In my practice it is much easier to manage a test that is performed every 10 years than a test that should be performed annually.
Colonoscopy also accounts for most of the harms of colorectal screening because of serious procedure complications, including bowel perforation (1 in 2,000 cases) and major bleeding (1 in 500 cases).7
Continue to: Multitarget stool FIT-DNA test (Cologuard)...
Multitarget stool FIT-DNA test (Cologuard)
The multitarget stool FIT-DNA test is a remarkable innovation in cancer screening combining 3 independent biomarkers associated with precancerous lesions and colorectal cancer.14 The 3 test components include14:
- a fecal immunochemical test (FIT) for hemoglobin (which uses antibodies to detect hemoglobin)
- a test for epigenetic changes in the methylation pattern of promoter DNA, including the promoter regions on the N-Myc Downstream-Regulated Gene 4 (NDRG4) and Bone Morphogenetic Protein 3 (BMP3) genes
- a test for 7 gene mutations in the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).
In addition, the amount of the beta-actin DNA present in the stool specimen is assessed and used as a quantitative control for the total amount of DNA in the specimen.
In one large clinical study, 9,989 people at average risk for colorectal cancer were screened with both a multitarget stool FIT-DNA test and a stool FIT test.15 Positive test results triggered a referral for colonoscopy. Among this cohort, 1% of participants were diagnosed with colorectal cancer and 7.6% with a precancerous lesion. The sensitivity of the multitarget stool FIT-DNA test and the FIT test for detecting colorectal cancer was 92.3% and 73.8%, respectively. The sensitivities of the multitarget stool FIT-DNA test and the FIT test for detecting precancerous lesions were 42.4% and 23.8%, respectively. The specificity of the FIT-DNA and FIT tests for detecting any cancer or precancerous lesion was 90% and 96.4%, respectively.15 The FIT test is less expensive than the multitarget stool FIT-DNA test. Eligible patients can order the FIT test through a Quest website.16 In June 2021 the published cost was $89 for the test plus a $6 physician fee. Most insurers will reimburse the expense of the test for eligible patients.
The multitarget stool FIT-DNA test should be performed every 1 to 3 years. Unlike colonoscopy or CT colonography, the stool is collected at home and sent to a testing laboratory, saving the patient time and travel costs. A disadvantage of the test is that it is more expensive than FIT or guaiac testing. Eligible patients can request a test kit by completing a telemedicine visit through the Cologuard website.17 One website lists the cost of a Cologuard test at $599.18 This test is eligible for reimbursement by most insurers.
Ensure patients are informed of needed screening
Most obstetrician-gynecologists have many women in their practice who are aged 45 to 64, a key target group for colorectal cancer screening. The American Cancer Society and the USPSTF strongly recommend that people in this age range be screened for colorectal cancer. Given that one-third of people these ages have not been screened, obstetrician-gynecologists can play an important role in reducing the health burden of the second most common cause of cancer death by ensuring that their patients are up to date with colorectal screening. ●
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening, clinical guidelines and rationale. Gastroenterology. 1997;112:594. doi: 10.1053/gast.1997.v112.agast970594.
- Centers for Disease Control and Prevention website. An update on cancer deaths in the United States. Accessed July 14, 2021.
- Siegel RL, Miller KD, Goding SA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. doi: 10.3322/caac.21601.
- American Cancer Society website. Key statistics for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed July 14, 2021.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use, United States. Morb Mortal Wkly Rep. 2020;69:253-259.
- Rogers CR, Matthews P, Xu L, et al. Interventions for increasing colorectal cancer screening uptake among African-American men: a systematic review and meta-analysis. PLoS One. 2020;15:e0238354. doi: 10.1371/journal.pone.0238354.
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi: 10.1001/jama.2021.6238.
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354-367. doi: 10.1053/j.gastro.2019.10.029.
- Rutter CM, Knudsen AB, Lin JS, et al. Black and White differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30:3-12. doi: 10.1158/1055-9965.EPI-19-1537.
- Zauber A, Knudsen A, Rutter CM, et al; Writing Committee of the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Working Group. Evaluating the benefits and harms of colorectal cancer screening strategies: a collaborative modeling approach. AHRQ Publication No. 14-05203-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; October 2015. file:///C:/Users/loconnor/Downloads/cisnet-draft-modeling-report.pdf. Accessed July 15, 2021.
- American Cancer Society website. Cancer screening guidelines by age. . Accessed July 15, 2021.
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17-22. doi: 10.1001/jamasurg.2014.1756.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595. doi: 10.1001/jama.2016.6828.
- FDA summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130017B.pdf. Accessed July 15, 2021.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Mulitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. doi: 10.1056/NEJMoa1311194.
- FIT colorectal cancer screening. Quest Diagnostics website. https://questdirect.questdiagnostics.com/products/fit-colorectal-cancer-screening/d41c67cb-a16d-4ad6-82b9-1a77d32daf41?utm_source=google&utm_medium=cpc&utm_campaign=71700000081635378&utm_content=58700006943838348&utm_term=p62498361603&gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYAiAAEgKHqfD_BwE. Accessed July 15, 2021.
- Request Cologuard without leaving your home. Cologuard website. https://www.cologuard.com/how-to-get-cologuard?gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYASAAEgKHIfD_BwE. Accessed July 15, 2021.
- Cologuard. Colonoscopy Assist website. https: //colonoscopyassist.com/Cologuard.html. Accessed July 15, 2021.
Colorectal cancer is a common disease that has a very lengthy natural history of progression from small (<8 mm) to large (≥8 mm) polyps, then to dysplasia, and eventually to invasive cancer. It is estimated that this progression takes 10 years.1 The long natural history from preneoplasia to cancer makes colorectal cancer an ideal target for screening. Screening for colorectal cancer is divided into two clinical pathways, screening for people at average risk and for those at high risk. Clinical factors that increase the risk of colorectal cancer are listed in TABLE 1. This editorial is focused on the clinical approach to screening for people at average risk for colorectal cancer.

Colorectal cancer is the second most common cause of cancer death
The top 6 causes of cancer death in the United States are2:
- lung cancer (23% of cancer deaths)
- colon and rectum (9%)
- pancreas (8%)
- female breast (7%)
- prostate (5%)
- liver/bile ducts (5%).
In 2020 it is estimated that 147,950 people were diagnosed with colorectal cancer, including 17,930 people less than 50 years of age.3 In 2020, it is also estimated that 53,200 people in the United States died of colorectal cancer, including 3,640 people younger than age 50.3 By contrast, the American Cancer Society estimates that, in 2021, cervical cancer will be diagnosed in 14,480 women and 4,290 women with the disease will die.4
According to a Centers for Disease Control and Prevention (CDC) study, among people 50 to 64 years of age, 63% report being up to date with colorectal cancer screening—leaving a full one-third not up to date with their screening.5 Among people aged 65 to 75, 79% report being up to date with colorectal cancer screening. Among those aged 50 to 64, those with health insurance were more likely to be up to date with screening than people without insurance—67% versus 33%, respectively. People with a household income greater than $75,000 and less than $35,000 reported up-to-date screening rates of 71% and 55%, respectively. Among people aged 50 to 64, non-Hispanic White and Black people reported similar rates of being up to date with colorectal screening (66% and 65%, respectively). Hispanic people, however, reported a significantly lower rate of being up to date with colorectal cancer screening (51%).5
A weakness of this CDC study is that the response rate from the surveyed population was less than 50%, raising questions about validity and generalizability of the reported results. Of note, other studies report that Black men may have lower rates of colorectal cancer screening than non-Black men.6 These data show that focused interventions to improve colorectal cancer screening are required for people 50 to 64 years of age, particularly among underinsured and some minority populations.
Continue to: Inequitable health outcomes for colorectal cancer...
Inequitable health outcomes for colorectal cancer
The purpose of screening for cancer is to reduce the morbidity and mortality associated with the disease. Based on the Surveillance, Epidemiology and End Results (SEER) national reporting system, from 2014 to 2018 colorectal death rates per 100,000 adults were 18 for Black adults; 15.1 for American Indian/Alaska native adults; 13.6 for White non-Hispanic adults; 10.9 for White, Hispanic adults; and 9.4 for Asian/Pacific Islander adults.7 Lack of access to and a lower utilization rate of high-quality colon cancer screening modalities, for example colonoscopy, and a lower rate of optimal colon cancer treatment may account for the higher colorectal death rate among Black adults.8,9
Colorectal cancer screening should begin at age 45
In 2015 the Agency for Health Research and Quality (AHRQ) published data showing that the benefit of initiating screening for colorectal cancer at 45 years of age outweighed the additional cost.10 In 2018, the American Cancer Society recommended that screening for colorectal cancer should begin at age 45.11 In 2021, after resisting the change for many years, the US Preventive Services Task Force (USPSTF) also recommended that screening for colorectal cancer should begin at 45.7 The new recommendation is based on statistical models that showed a significant increase in life-years gained at a small incremental cost. The USPSTF also recommended that clinicians and patients could consider discontinuing colorectal cancer screening at 75 years of age because the net benefit of continuing screening after age 75 is minimal.
Prior to 2021 the USPSTF recommended that screening be initiated at age 50. However, from 2010 to 2020 there was a significant increase in the percentage of new cases of colorectal cancer detected in people younger than 50. In 2010, colon and rectal cancer among people under 50 years of age accounted for 5% and 9% of all cases, respectively.12 In 2020, colon and rectal cancer in people younger than age 50 accounted for 11% and 15% of all cases, respectively.3
Options for colon cancer screening
There are many options for colorectal cancer screening (TABLE 2).10,13 Experts conclude that the best colorectal cancer screening test is the test that the patient will complete. Among options for screening, colonoscopy and the multitarget stool FIT-DNA test (Cologuard) have greater sensitivity for detecting colorectal precancer and cancer lesions compared with fecal immunochemical testing (FIT), computed tomography colonography imaging (CTC), and stool guaiac testing (see TABLE 1).

In my practice, I suggest patients use either colonoscopy (every 10 years) or the multitarget stool FIT-DNA test (every 1 to 3 years) for screening. Most of my patients select colonoscopy, but some prefer the multitarget stool FIT-DNA test because they fear the pre-colonoscopy bowel preparation and the risk of bowel perforation with colonoscopy. Most colonoscopy procedures are performed with sedation, requiring an adult to take responsibility for transporting the patient to their residence, adding complexity to the performance of colonoscopy. These two tests are discussed in more detail below.
Colonoscopy
Colonoscopy occupies a unique position among the options for colorectal cancer screening because it is both a screening test and the gold standard for diagnosis, based on histologic analysis of the polypoid tissue biopsied at the time of colonoscopy. For all other screening tests, if the test yields an abnormal result, it is necessary to perform a colonoscopy. Colonoscopy screening offers the advantage of “one and done for 10 years.” In my practice it is much easier to manage a test that is performed every 10 years than a test that should be performed annually.
Colonoscopy also accounts for most of the harms of colorectal screening because of serious procedure complications, including bowel perforation (1 in 2,000 cases) and major bleeding (1 in 500 cases).7
Continue to: Multitarget stool FIT-DNA test (Cologuard)...
Multitarget stool FIT-DNA test (Cologuard)
The multitarget stool FIT-DNA test is a remarkable innovation in cancer screening combining 3 independent biomarkers associated with precancerous lesions and colorectal cancer.14 The 3 test components include14:
- a fecal immunochemical test (FIT) for hemoglobin (which uses antibodies to detect hemoglobin)
- a test for epigenetic changes in the methylation pattern of promoter DNA, including the promoter regions on the N-Myc Downstream-Regulated Gene 4 (NDRG4) and Bone Morphogenetic Protein 3 (BMP3) genes
- a test for 7 gene mutations in the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).
In addition, the amount of the beta-actin DNA present in the stool specimen is assessed and used as a quantitative control for the total amount of DNA in the specimen.
In one large clinical study, 9,989 people at average risk for colorectal cancer were screened with both a multitarget stool FIT-DNA test and a stool FIT test.15 Positive test results triggered a referral for colonoscopy. Among this cohort, 1% of participants were diagnosed with colorectal cancer and 7.6% with a precancerous lesion. The sensitivity of the multitarget stool FIT-DNA test and the FIT test for detecting colorectal cancer was 92.3% and 73.8%, respectively. The sensitivities of the multitarget stool FIT-DNA test and the FIT test for detecting precancerous lesions were 42.4% and 23.8%, respectively. The specificity of the FIT-DNA and FIT tests for detecting any cancer or precancerous lesion was 90% and 96.4%, respectively.15 The FIT test is less expensive than the multitarget stool FIT-DNA test. Eligible patients can order the FIT test through a Quest website.16 In June 2021 the published cost was $89 for the test plus a $6 physician fee. Most insurers will reimburse the expense of the test for eligible patients.
The multitarget stool FIT-DNA test should be performed every 1 to 3 years. Unlike colonoscopy or CT colonography, the stool is collected at home and sent to a testing laboratory, saving the patient time and travel costs. A disadvantage of the test is that it is more expensive than FIT or guaiac testing. Eligible patients can request a test kit by completing a telemedicine visit through the Cologuard website.17 One website lists the cost of a Cologuard test at $599.18 This test is eligible for reimbursement by most insurers.
Ensure patients are informed of needed screening
Most obstetrician-gynecologists have many women in their practice who are aged 45 to 64, a key target group for colorectal cancer screening. The American Cancer Society and the USPSTF strongly recommend that people in this age range be screened for colorectal cancer. Given that one-third of people these ages have not been screened, obstetrician-gynecologists can play an important role in reducing the health burden of the second most common cause of cancer death by ensuring that their patients are up to date with colorectal screening. ●
Colorectal cancer is a common disease that has a very lengthy natural history of progression from small (<8 mm) to large (≥8 mm) polyps, then to dysplasia, and eventually to invasive cancer. It is estimated that this progression takes 10 years.1 The long natural history from preneoplasia to cancer makes colorectal cancer an ideal target for screening. Screening for colorectal cancer is divided into two clinical pathways, screening for people at average risk and for those at high risk. Clinical factors that increase the risk of colorectal cancer are listed in TABLE 1. This editorial is focused on the clinical approach to screening for people at average risk for colorectal cancer.

Colorectal cancer is the second most common cause of cancer death
The top 6 causes of cancer death in the United States are2:
- lung cancer (23% of cancer deaths)
- colon and rectum (9%)
- pancreas (8%)
- female breast (7%)
- prostate (5%)
- liver/bile ducts (5%).
In 2020 it is estimated that 147,950 people were diagnosed with colorectal cancer, including 17,930 people less than 50 years of age.3 In 2020, it is also estimated that 53,200 people in the United States died of colorectal cancer, including 3,640 people younger than age 50.3 By contrast, the American Cancer Society estimates that, in 2021, cervical cancer will be diagnosed in 14,480 women and 4,290 women with the disease will die.4
According to a Centers for Disease Control and Prevention (CDC) study, among people 50 to 64 years of age, 63% report being up to date with colorectal cancer screening—leaving a full one-third not up to date with their screening.5 Among people aged 65 to 75, 79% report being up to date with colorectal cancer screening. Among those aged 50 to 64, those with health insurance were more likely to be up to date with screening than people without insurance—67% versus 33%, respectively. People with a household income greater than $75,000 and less than $35,000 reported up-to-date screening rates of 71% and 55%, respectively. Among people aged 50 to 64, non-Hispanic White and Black people reported similar rates of being up to date with colorectal screening (66% and 65%, respectively). Hispanic people, however, reported a significantly lower rate of being up to date with colorectal cancer screening (51%).5
A weakness of this CDC study is that the response rate from the surveyed population was less than 50%, raising questions about validity and generalizability of the reported results. Of note, other studies report that Black men may have lower rates of colorectal cancer screening than non-Black men.6 These data show that focused interventions to improve colorectal cancer screening are required for people 50 to 64 years of age, particularly among underinsured and some minority populations.
Continue to: Inequitable health outcomes for colorectal cancer...
Inequitable health outcomes for colorectal cancer
The purpose of screening for cancer is to reduce the morbidity and mortality associated with the disease. Based on the Surveillance, Epidemiology and End Results (SEER) national reporting system, from 2014 to 2018 colorectal death rates per 100,000 adults were 18 for Black adults; 15.1 for American Indian/Alaska native adults; 13.6 for White non-Hispanic adults; 10.9 for White, Hispanic adults; and 9.4 for Asian/Pacific Islander adults.7 Lack of access to and a lower utilization rate of high-quality colon cancer screening modalities, for example colonoscopy, and a lower rate of optimal colon cancer treatment may account for the higher colorectal death rate among Black adults.8,9
Colorectal cancer screening should begin at age 45
In 2015 the Agency for Health Research and Quality (AHRQ) published data showing that the benefit of initiating screening for colorectal cancer at 45 years of age outweighed the additional cost.10 In 2018, the American Cancer Society recommended that screening for colorectal cancer should begin at age 45.11 In 2021, after resisting the change for many years, the US Preventive Services Task Force (USPSTF) also recommended that screening for colorectal cancer should begin at 45.7 The new recommendation is based on statistical models that showed a significant increase in life-years gained at a small incremental cost. The USPSTF also recommended that clinicians and patients could consider discontinuing colorectal cancer screening at 75 years of age because the net benefit of continuing screening after age 75 is minimal.
Prior to 2021 the USPSTF recommended that screening be initiated at age 50. However, from 2010 to 2020 there was a significant increase in the percentage of new cases of colorectal cancer detected in people younger than 50. In 2010, colon and rectal cancer among people under 50 years of age accounted for 5% and 9% of all cases, respectively.12 In 2020, colon and rectal cancer in people younger than age 50 accounted for 11% and 15% of all cases, respectively.3
Options for colon cancer screening
There are many options for colorectal cancer screening (TABLE 2).10,13 Experts conclude that the best colorectal cancer screening test is the test that the patient will complete. Among options for screening, colonoscopy and the multitarget stool FIT-DNA test (Cologuard) have greater sensitivity for detecting colorectal precancer and cancer lesions compared with fecal immunochemical testing (FIT), computed tomography colonography imaging (CTC), and stool guaiac testing (see TABLE 1).

In my practice, I suggest patients use either colonoscopy (every 10 years) or the multitarget stool FIT-DNA test (every 1 to 3 years) for screening. Most of my patients select colonoscopy, but some prefer the multitarget stool FIT-DNA test because they fear the pre-colonoscopy bowel preparation and the risk of bowel perforation with colonoscopy. Most colonoscopy procedures are performed with sedation, requiring an adult to take responsibility for transporting the patient to their residence, adding complexity to the performance of colonoscopy. These two tests are discussed in more detail below.
Colonoscopy
Colonoscopy occupies a unique position among the options for colorectal cancer screening because it is both a screening test and the gold standard for diagnosis, based on histologic analysis of the polypoid tissue biopsied at the time of colonoscopy. For all other screening tests, if the test yields an abnormal result, it is necessary to perform a colonoscopy. Colonoscopy screening offers the advantage of “one and done for 10 years.” In my practice it is much easier to manage a test that is performed every 10 years than a test that should be performed annually.
Colonoscopy also accounts for most of the harms of colorectal screening because of serious procedure complications, including bowel perforation (1 in 2,000 cases) and major bleeding (1 in 500 cases).7
Continue to: Multitarget stool FIT-DNA test (Cologuard)...
Multitarget stool FIT-DNA test (Cologuard)
The multitarget stool FIT-DNA test is a remarkable innovation in cancer screening combining 3 independent biomarkers associated with precancerous lesions and colorectal cancer.14 The 3 test components include14:
- a fecal immunochemical test (FIT) for hemoglobin (which uses antibodies to detect hemoglobin)
- a test for epigenetic changes in the methylation pattern of promoter DNA, including the promoter regions on the N-Myc Downstream-Regulated Gene 4 (NDRG4) and Bone Morphogenetic Protein 3 (BMP3) genes
- a test for 7 gene mutations in the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).
In addition, the amount of the beta-actin DNA present in the stool specimen is assessed and used as a quantitative control for the total amount of DNA in the specimen.
In one large clinical study, 9,989 people at average risk for colorectal cancer were screened with both a multitarget stool FIT-DNA test and a stool FIT test.15 Positive test results triggered a referral for colonoscopy. Among this cohort, 1% of participants were diagnosed with colorectal cancer and 7.6% with a precancerous lesion. The sensitivity of the multitarget stool FIT-DNA test and the FIT test for detecting colorectal cancer was 92.3% and 73.8%, respectively. The sensitivities of the multitarget stool FIT-DNA test and the FIT test for detecting precancerous lesions were 42.4% and 23.8%, respectively. The specificity of the FIT-DNA and FIT tests for detecting any cancer or precancerous lesion was 90% and 96.4%, respectively.15 The FIT test is less expensive than the multitarget stool FIT-DNA test. Eligible patients can order the FIT test through a Quest website.16 In June 2021 the published cost was $89 for the test plus a $6 physician fee. Most insurers will reimburse the expense of the test for eligible patients.
The multitarget stool FIT-DNA test should be performed every 1 to 3 years. Unlike colonoscopy or CT colonography, the stool is collected at home and sent to a testing laboratory, saving the patient time and travel costs. A disadvantage of the test is that it is more expensive than FIT or guaiac testing. Eligible patients can request a test kit by completing a telemedicine visit through the Cologuard website.17 One website lists the cost of a Cologuard test at $599.18 This test is eligible for reimbursement by most insurers.
Ensure patients are informed of needed screening
Most obstetrician-gynecologists have many women in their practice who are aged 45 to 64, a key target group for colorectal cancer screening. The American Cancer Society and the USPSTF strongly recommend that people in this age range be screened for colorectal cancer. Given that one-third of people these ages have not been screened, obstetrician-gynecologists can play an important role in reducing the health burden of the second most common cause of cancer death by ensuring that their patients are up to date with colorectal screening. ●
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening, clinical guidelines and rationale. Gastroenterology. 1997;112:594. doi: 10.1053/gast.1997.v112.agast970594.
- Centers for Disease Control and Prevention website. An update on cancer deaths in the United States. Accessed July 14, 2021.
- Siegel RL, Miller KD, Goding SA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. doi: 10.3322/caac.21601.
- American Cancer Society website. Key statistics for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed July 14, 2021.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use, United States. Morb Mortal Wkly Rep. 2020;69:253-259.
- Rogers CR, Matthews P, Xu L, et al. Interventions for increasing colorectal cancer screening uptake among African-American men: a systematic review and meta-analysis. PLoS One. 2020;15:e0238354. doi: 10.1371/journal.pone.0238354.
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi: 10.1001/jama.2021.6238.
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354-367. doi: 10.1053/j.gastro.2019.10.029.
- Rutter CM, Knudsen AB, Lin JS, et al. Black and White differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30:3-12. doi: 10.1158/1055-9965.EPI-19-1537.
- Zauber A, Knudsen A, Rutter CM, et al; Writing Committee of the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Working Group. Evaluating the benefits and harms of colorectal cancer screening strategies: a collaborative modeling approach. AHRQ Publication No. 14-05203-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; October 2015. file:///C:/Users/loconnor/Downloads/cisnet-draft-modeling-report.pdf. Accessed July 15, 2021.
- American Cancer Society website. Cancer screening guidelines by age. . Accessed July 15, 2021.
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17-22. doi: 10.1001/jamasurg.2014.1756.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595. doi: 10.1001/jama.2016.6828.
- FDA summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130017B.pdf. Accessed July 15, 2021.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Mulitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. doi: 10.1056/NEJMoa1311194.
- FIT colorectal cancer screening. Quest Diagnostics website. https://questdirect.questdiagnostics.com/products/fit-colorectal-cancer-screening/d41c67cb-a16d-4ad6-82b9-1a77d32daf41?utm_source=google&utm_medium=cpc&utm_campaign=71700000081635378&utm_content=58700006943838348&utm_term=p62498361603&gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYAiAAEgKHqfD_BwE. Accessed July 15, 2021.
- Request Cologuard without leaving your home. Cologuard website. https://www.cologuard.com/how-to-get-cologuard?gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYASAAEgKHIfD_BwE. Accessed July 15, 2021.
- Cologuard. Colonoscopy Assist website. https: //colonoscopyassist.com/Cologuard.html. Accessed July 15, 2021.
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening, clinical guidelines and rationale. Gastroenterology. 1997;112:594. doi: 10.1053/gast.1997.v112.agast970594.
- Centers for Disease Control and Prevention website. An update on cancer deaths in the United States. Accessed July 14, 2021.
- Siegel RL, Miller KD, Goding SA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. doi: 10.3322/caac.21601.
- American Cancer Society website. Key statistics for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed July 14, 2021.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use, United States. Morb Mortal Wkly Rep. 2020;69:253-259.
- Rogers CR, Matthews P, Xu L, et al. Interventions for increasing colorectal cancer screening uptake among African-American men: a systematic review and meta-analysis. PLoS One. 2020;15:e0238354. doi: 10.1371/journal.pone.0238354.
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi: 10.1001/jama.2021.6238.
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354-367. doi: 10.1053/j.gastro.2019.10.029.
- Rutter CM, Knudsen AB, Lin JS, et al. Black and White differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30:3-12. doi: 10.1158/1055-9965.EPI-19-1537.
- Zauber A, Knudsen A, Rutter CM, et al; Writing Committee of the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Working Group. Evaluating the benefits and harms of colorectal cancer screening strategies: a collaborative modeling approach. AHRQ Publication No. 14-05203-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; October 2015. file:///C:/Users/loconnor/Downloads/cisnet-draft-modeling-report.pdf. Accessed July 15, 2021.
- American Cancer Society website. Cancer screening guidelines by age. . Accessed July 15, 2021.
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17-22. doi: 10.1001/jamasurg.2014.1756.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595. doi: 10.1001/jama.2016.6828.
- FDA summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130017B.pdf. Accessed July 15, 2021.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Mulitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. doi: 10.1056/NEJMoa1311194.
- FIT colorectal cancer screening. Quest Diagnostics website. https://questdirect.questdiagnostics.com/products/fit-colorectal-cancer-screening/d41c67cb-a16d-4ad6-82b9-1a77d32daf41?utm_source=google&utm_medium=cpc&utm_campaign=71700000081635378&utm_content=58700006943838348&utm_term=p62498361603&gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYAiAAEgKHqfD_BwE. Accessed July 15, 2021.
- Request Cologuard without leaving your home. Cologuard website. https://www.cologuard.com/how-to-get-cologuard?gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYASAAEgKHIfD_BwE. Accessed July 15, 2021.
- Cologuard. Colonoscopy Assist website. https: //colonoscopyassist.com/Cologuard.html. Accessed July 15, 2021.
One center’s experience delivering monochorionic twins
Between 2005 and 2021, mode of delivery of diamniotic twins at this practice did not significantly differ by chorionicity, researchers affiliated with Maternal Fetal Medicine Associates and the department of obstetrics, gynecology, and reproductive science at Icahn School of Medicine at Mount Sinai, New York reported in Obstetrics & Gynecology.
The study supports a recommendation from the American College of Obstetricians and Gynecologists that vaginal delivery “is a reasonable option in well selected diamniotic twin pregnancies, irrespective of chorionicity, and should be considered, provided that an experienced obstetrician is available,” said Iris Krishna, MD, assistant professor of maternal-fetal medicine at Emory University, Atlanta.
The experience at this practice, however, may not apply to many practices in the United States, said Dr. Krishna, who was not involved in the study.
Of 1,121 diamniotic twin pregnancies included in the analysis, 202 (18%) were monochorionic. The cesarean delivery rate was not significantly different between groups: 61% for monochorionic and 63% for dichorionic pregnancies.
Among women with planned vaginal delivery (101 monochorionic pregnancies and 422 dichorionic pregnancies), the cesarean delivery rate likewise did not significantly differ by chorionicity. Twenty-two percent of the monochorionic pregnancies and 21% of the dichorionic pregnancies in this subgroup had a cesarean delivery.
Among patients with a vaginal delivery of twin A, chorionicity was not associated with mode of delivery for twin B. Combined vaginal-cesarean deliveries occurred less than 1% of the time, and breech extraction of twin B occurred approximately 75% of the time, regardless of chorionicity.
The researchers also compared neonatal outcomes for monochorionic-diamniotic twin pregnancies at or after 34 weeks of gestation, based on the intended mode of delivery (95 women with planned vaginal delivery and 68 with planned cesarean delivery). Neonatal outcomes generally were similar, although the incidence of mechanical ventilation was less common in cases with planned vaginal delivery (7% vs. 21%).
“Our data affirm that an attempt at a vaginal birth for twin pregnancies, without contraindications to vaginal delivery and regardless of chorionicity, is reasonable and achievable,” wrote study author Henry N. Lesser, MD, with the department of obstetrics and gynecology at Sinai Hospital in Baltimore, and colleagues.
The patients with planned cesarean delivery had a contraindication to vaginal delivery or otherwise chose to have a cesarean delivery. The researchers excluded from their analysis pregnancies with intrauterine fetal demise of either twin before labor or planned cesarean delivery.
The study’s reliance on data from a single practice decreases its external validity, the researchers noted. Induction of labor at this center typically occurs at 37 weeks’ gestation for monochorionic twins and at 38 weeks for dichorionic twins, and “senior personnel experienced in intrauterine twin manipulation are always present at delivery,” the study authors said.
The study describes “the experience of a single site with skilled obstetricians following a standardized approach to management of diamniotic twin deliveries,” Dr. Krishna said. “Findings may not be generalizable to many U.S. practices as obstetrics and gynecology residents often lack training in breech extraction or internal podalic version of the second twin. This underscores the importance of a concerted effort by skilled senior physicians to train junior physicians in vaginal delivery of the second twin to improve overall outcomes amongst women with diamniotic twin gestations.”
Michael F. Greene, MD, professor emeritus of obstetrics, gynecology, and reproductive biology at Massachusetts General Hospital, Boston, agreed that the findings are not generalizable to the national population. Approximately 10% of the patients in the study had prepregnancy obesity, whereas doctors practicing in other areas likely encounter higher rates, Dr. Greene said in an interview.
He also wondered about other data points that could be of interest but were not reported, such as the racial or ethnic distribution of the patients, rates of birth defects, the use of instruments to aid delivery, and neonatal outcomes for the dichorionic twins.
Monochorionic pregnancies entail a risk of twin-twin transfusion syndrome and other complications, including an increased likelihood of birth defects.
Dr. Greene is an associate editor with the New England Journal of Medicine, which in 2013 published results from the Twin Birth Study, an international trial where women with dichorionic or monochorionic twins were randomly assigned to planned vaginal delivery or planned cesarean delivery. Outcomes did not significantly differ between groups. In the trial, the rate of cesarean delivery in the group with planned vaginal delivery was 43.8%, and Dr. Greene discussed the implications of the study in an accompanying editorial.
Since then, the obstetrics and gynecology community “has been focusing in recent years on trying to avoid the first cesarean section” when it is safe to do so, Dr. Greene said. “That has become almost a bumper sticker in modern obstetrics.”
And patients should know that it is an option, Dr. Krishna added.
“Women with monochorionic-diamniotic twins should be counseled that with an experienced obstetrician that an attempt at vaginal delivery is not associated with adverse neonatal outcomes when compared with planned cesarean delivery,” Dr. Krishna said.
A study coauthor disclosed serving on the speakers bureau for Natera and Hologic. Dr. Krishna is a member of the editorial advisory board for Ob.Gyn. News.
Between 2005 and 2021, mode of delivery of diamniotic twins at this practice did not significantly differ by chorionicity, researchers affiliated with Maternal Fetal Medicine Associates and the department of obstetrics, gynecology, and reproductive science at Icahn School of Medicine at Mount Sinai, New York reported in Obstetrics & Gynecology.
The study supports a recommendation from the American College of Obstetricians and Gynecologists that vaginal delivery “is a reasonable option in well selected diamniotic twin pregnancies, irrespective of chorionicity, and should be considered, provided that an experienced obstetrician is available,” said Iris Krishna, MD, assistant professor of maternal-fetal medicine at Emory University, Atlanta.
The experience at this practice, however, may not apply to many practices in the United States, said Dr. Krishna, who was not involved in the study.
Of 1,121 diamniotic twin pregnancies included in the analysis, 202 (18%) were monochorionic. The cesarean delivery rate was not significantly different between groups: 61% for monochorionic and 63% for dichorionic pregnancies.
Among women with planned vaginal delivery (101 monochorionic pregnancies and 422 dichorionic pregnancies), the cesarean delivery rate likewise did not significantly differ by chorionicity. Twenty-two percent of the monochorionic pregnancies and 21% of the dichorionic pregnancies in this subgroup had a cesarean delivery.
Among patients with a vaginal delivery of twin A, chorionicity was not associated with mode of delivery for twin B. Combined vaginal-cesarean deliveries occurred less than 1% of the time, and breech extraction of twin B occurred approximately 75% of the time, regardless of chorionicity.
The researchers also compared neonatal outcomes for monochorionic-diamniotic twin pregnancies at or after 34 weeks of gestation, based on the intended mode of delivery (95 women with planned vaginal delivery and 68 with planned cesarean delivery). Neonatal outcomes generally were similar, although the incidence of mechanical ventilation was less common in cases with planned vaginal delivery (7% vs. 21%).
“Our data affirm that an attempt at a vaginal birth for twin pregnancies, without contraindications to vaginal delivery and regardless of chorionicity, is reasonable and achievable,” wrote study author Henry N. Lesser, MD, with the department of obstetrics and gynecology at Sinai Hospital in Baltimore, and colleagues.
The patients with planned cesarean delivery had a contraindication to vaginal delivery or otherwise chose to have a cesarean delivery. The researchers excluded from their analysis pregnancies with intrauterine fetal demise of either twin before labor or planned cesarean delivery.
The study’s reliance on data from a single practice decreases its external validity, the researchers noted. Induction of labor at this center typically occurs at 37 weeks’ gestation for monochorionic twins and at 38 weeks for dichorionic twins, and “senior personnel experienced in intrauterine twin manipulation are always present at delivery,” the study authors said.
The study describes “the experience of a single site with skilled obstetricians following a standardized approach to management of diamniotic twin deliveries,” Dr. Krishna said. “Findings may not be generalizable to many U.S. practices as obstetrics and gynecology residents often lack training in breech extraction or internal podalic version of the second twin. This underscores the importance of a concerted effort by skilled senior physicians to train junior physicians in vaginal delivery of the second twin to improve overall outcomes amongst women with diamniotic twin gestations.”
Michael F. Greene, MD, professor emeritus of obstetrics, gynecology, and reproductive biology at Massachusetts General Hospital, Boston, agreed that the findings are not generalizable to the national population. Approximately 10% of the patients in the study had prepregnancy obesity, whereas doctors practicing in other areas likely encounter higher rates, Dr. Greene said in an interview.
He also wondered about other data points that could be of interest but were not reported, such as the racial or ethnic distribution of the patients, rates of birth defects, the use of instruments to aid delivery, and neonatal outcomes for the dichorionic twins.
Monochorionic pregnancies entail a risk of twin-twin transfusion syndrome and other complications, including an increased likelihood of birth defects.
Dr. Greene is an associate editor with the New England Journal of Medicine, which in 2013 published results from the Twin Birth Study, an international trial where women with dichorionic or monochorionic twins were randomly assigned to planned vaginal delivery or planned cesarean delivery. Outcomes did not significantly differ between groups. In the trial, the rate of cesarean delivery in the group with planned vaginal delivery was 43.8%, and Dr. Greene discussed the implications of the study in an accompanying editorial.
Since then, the obstetrics and gynecology community “has been focusing in recent years on trying to avoid the first cesarean section” when it is safe to do so, Dr. Greene said. “That has become almost a bumper sticker in modern obstetrics.”
And patients should know that it is an option, Dr. Krishna added.
“Women with monochorionic-diamniotic twins should be counseled that with an experienced obstetrician that an attempt at vaginal delivery is not associated with adverse neonatal outcomes when compared with planned cesarean delivery,” Dr. Krishna said.
A study coauthor disclosed serving on the speakers bureau for Natera and Hologic. Dr. Krishna is a member of the editorial advisory board for Ob.Gyn. News.
Between 2005 and 2021, mode of delivery of diamniotic twins at this practice did not significantly differ by chorionicity, researchers affiliated with Maternal Fetal Medicine Associates and the department of obstetrics, gynecology, and reproductive science at Icahn School of Medicine at Mount Sinai, New York reported in Obstetrics & Gynecology.
The study supports a recommendation from the American College of Obstetricians and Gynecologists that vaginal delivery “is a reasonable option in well selected diamniotic twin pregnancies, irrespective of chorionicity, and should be considered, provided that an experienced obstetrician is available,” said Iris Krishna, MD, assistant professor of maternal-fetal medicine at Emory University, Atlanta.
The experience at this practice, however, may not apply to many practices in the United States, said Dr. Krishna, who was not involved in the study.
Of 1,121 diamniotic twin pregnancies included in the analysis, 202 (18%) were monochorionic. The cesarean delivery rate was not significantly different between groups: 61% for monochorionic and 63% for dichorionic pregnancies.
Among women with planned vaginal delivery (101 monochorionic pregnancies and 422 dichorionic pregnancies), the cesarean delivery rate likewise did not significantly differ by chorionicity. Twenty-two percent of the monochorionic pregnancies and 21% of the dichorionic pregnancies in this subgroup had a cesarean delivery.
Among patients with a vaginal delivery of twin A, chorionicity was not associated with mode of delivery for twin B. Combined vaginal-cesarean deliveries occurred less than 1% of the time, and breech extraction of twin B occurred approximately 75% of the time, regardless of chorionicity.
The researchers also compared neonatal outcomes for monochorionic-diamniotic twin pregnancies at or after 34 weeks of gestation, based on the intended mode of delivery (95 women with planned vaginal delivery and 68 with planned cesarean delivery). Neonatal outcomes generally were similar, although the incidence of mechanical ventilation was less common in cases with planned vaginal delivery (7% vs. 21%).
“Our data affirm that an attempt at a vaginal birth for twin pregnancies, without contraindications to vaginal delivery and regardless of chorionicity, is reasonable and achievable,” wrote study author Henry N. Lesser, MD, with the department of obstetrics and gynecology at Sinai Hospital in Baltimore, and colleagues.
The patients with planned cesarean delivery had a contraindication to vaginal delivery or otherwise chose to have a cesarean delivery. The researchers excluded from their analysis pregnancies with intrauterine fetal demise of either twin before labor or planned cesarean delivery.
The study’s reliance on data from a single practice decreases its external validity, the researchers noted. Induction of labor at this center typically occurs at 37 weeks’ gestation for monochorionic twins and at 38 weeks for dichorionic twins, and “senior personnel experienced in intrauterine twin manipulation are always present at delivery,” the study authors said.
The study describes “the experience of a single site with skilled obstetricians following a standardized approach to management of diamniotic twin deliveries,” Dr. Krishna said. “Findings may not be generalizable to many U.S. practices as obstetrics and gynecology residents often lack training in breech extraction or internal podalic version of the second twin. This underscores the importance of a concerted effort by skilled senior physicians to train junior physicians in vaginal delivery of the second twin to improve overall outcomes amongst women with diamniotic twin gestations.”
Michael F. Greene, MD, professor emeritus of obstetrics, gynecology, and reproductive biology at Massachusetts General Hospital, Boston, agreed that the findings are not generalizable to the national population. Approximately 10% of the patients in the study had prepregnancy obesity, whereas doctors practicing in other areas likely encounter higher rates, Dr. Greene said in an interview.
He also wondered about other data points that could be of interest but were not reported, such as the racial or ethnic distribution of the patients, rates of birth defects, the use of instruments to aid delivery, and neonatal outcomes for the dichorionic twins.
Monochorionic pregnancies entail a risk of twin-twin transfusion syndrome and other complications, including an increased likelihood of birth defects.
Dr. Greene is an associate editor with the New England Journal of Medicine, which in 2013 published results from the Twin Birth Study, an international trial where women with dichorionic or monochorionic twins were randomly assigned to planned vaginal delivery or planned cesarean delivery. Outcomes did not significantly differ between groups. In the trial, the rate of cesarean delivery in the group with planned vaginal delivery was 43.8%, and Dr. Greene discussed the implications of the study in an accompanying editorial.
Since then, the obstetrics and gynecology community “has been focusing in recent years on trying to avoid the first cesarean section” when it is safe to do so, Dr. Greene said. “That has become almost a bumper sticker in modern obstetrics.”
And patients should know that it is an option, Dr. Krishna added.
“Women with monochorionic-diamniotic twins should be counseled that with an experienced obstetrician that an attempt at vaginal delivery is not associated with adverse neonatal outcomes when compared with planned cesarean delivery,” Dr. Krishna said.
A study coauthor disclosed serving on the speakers bureau for Natera and Hologic. Dr. Krishna is a member of the editorial advisory board for Ob.Gyn. News.
FROM OBSTETRICS & GYNECOLOGY
Specific COVID-19 antibodies found in breast milk of vaccinated women
The breast milk of women who had received Pfizer’s COVID-19 vaccine contained specific antibodies against the infectious disease, new research found.
“The COVID-19 pandemic has raised questions among individuals who are breastfeeding, both because of the possibility of viral transmission to infants during breastfeeding and, more recently, of the potential risks and benefits of vaccination in this specific population,” researchers wrote.
In August, the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, and most recently, the Centers for Disease Control and Prevention, recommended that pregnant people receive the COVID-19 vaccine.
The study, published Aug. 11 in JAMA Network Open, adds to a growing collection of research that has found COVID-19 antibodies in the breast milk of women who were vaccinated against or have been infected with the illness.
Study author Erika Esteve-Palau, MD, PhD, and her colleagues collected blood and milk samples from 33 people who were on average 37 years old and who were on average 17.5 months post partum to examine the correlation of the levels of immunoglobulin G antibodies against the spike protein (S1 subunit) and against the nucleocapsid (NC) of SARS-CoV-2.
Blood and milk samples were taken from each study participant at three time points – 2 weeks after receiving the first dose of the vaccine, 2 weeks after receiving the second dose, and 4 weeks after the second dose. No participants had confirmed SARS-CoV-2 infection prior to vaccination or during the study period.
Researchers found that, after the second dose of the vaccine, IgG(S1) levels in breast milk increased and were positively associated with corresponding levels in the blood samples. The median range of IgG(S1) levels for serum-milk pairs at each time point were 519 to 1 arbitrary units (AU) per mL 2 weeks after receiving the first dose of the vaccine, 8,644 to 78 AU/mL 2 weeks after receiving the second dose, and 12,478 to 50.4 AU/mL 4 weeks after receiving the second dose.
Lisette D. Tanner, MD, MPH, FACOG, who was not involved in the study, said she was not surprised by the findings as previous studies have shown the passage of antibodies in breast milk in vaccinated women. One 2021 study published in JAMA found SARS-CoV-2–specific IgA and IgG antibodies in breast milk for 6 weeks after vaccination. IgA secretion was evident as early as 2 weeks after vaccination followed by a spike in IgG after 4 weeks (a week after the second vaccine). Meanwhile, another 2021 study published in mBio found that breast milk produced by parents with COVID-19 is a source of SARS-CoV-2 IgA and IgG antibodies and can neutralize COVID-19 activity.
“While the data from this and other studies is promising in regards to the passage of antibodies, it is currently unclear what the long-term effects for children will be,” said Dr. Tanner of the department of gynecology and obstetrics at Emory University, Atlanta. “It is not yet known what level of antibodies is necessary to convey protection to either neonates or children. This is an active area of investigation at multiple institutions.”
Dr. Tanner said she wished the study “evaluated neonatal cord blood or serum levels to better understand the immune response mounted by the children of women who received vaccination.”
Researchers of the current study said larger prospective studies are needed to confirm the safety of SARS-CoV-2 vaccination in individuals who are breastfeeding and further assess the association of vaccination with infants’ health and SARS-CoV-2–specific immunity.
Dr. Palau and Dr. Tanner had no relevant financial disclosures.
The breast milk of women who had received Pfizer’s COVID-19 vaccine contained specific antibodies against the infectious disease, new research found.
“The COVID-19 pandemic has raised questions among individuals who are breastfeeding, both because of the possibility of viral transmission to infants during breastfeeding and, more recently, of the potential risks and benefits of vaccination in this specific population,” researchers wrote.
In August, the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, and most recently, the Centers for Disease Control and Prevention, recommended that pregnant people receive the COVID-19 vaccine.
The study, published Aug. 11 in JAMA Network Open, adds to a growing collection of research that has found COVID-19 antibodies in the breast milk of women who were vaccinated against or have been infected with the illness.
Study author Erika Esteve-Palau, MD, PhD, and her colleagues collected blood and milk samples from 33 people who were on average 37 years old and who were on average 17.5 months post partum to examine the correlation of the levels of immunoglobulin G antibodies against the spike protein (S1 subunit) and against the nucleocapsid (NC) of SARS-CoV-2.
Blood and milk samples were taken from each study participant at three time points – 2 weeks after receiving the first dose of the vaccine, 2 weeks after receiving the second dose, and 4 weeks after the second dose. No participants had confirmed SARS-CoV-2 infection prior to vaccination or during the study period.
Researchers found that, after the second dose of the vaccine, IgG(S1) levels in breast milk increased and were positively associated with corresponding levels in the blood samples. The median range of IgG(S1) levels for serum-milk pairs at each time point were 519 to 1 arbitrary units (AU) per mL 2 weeks after receiving the first dose of the vaccine, 8,644 to 78 AU/mL 2 weeks after receiving the second dose, and 12,478 to 50.4 AU/mL 4 weeks after receiving the second dose.
Lisette D. Tanner, MD, MPH, FACOG, who was not involved in the study, said she was not surprised by the findings as previous studies have shown the passage of antibodies in breast milk in vaccinated women. One 2021 study published in JAMA found SARS-CoV-2–specific IgA and IgG antibodies in breast milk for 6 weeks after vaccination. IgA secretion was evident as early as 2 weeks after vaccination followed by a spike in IgG after 4 weeks (a week after the second vaccine). Meanwhile, another 2021 study published in mBio found that breast milk produced by parents with COVID-19 is a source of SARS-CoV-2 IgA and IgG antibodies and can neutralize COVID-19 activity.
“While the data from this and other studies is promising in regards to the passage of antibodies, it is currently unclear what the long-term effects for children will be,” said Dr. Tanner of the department of gynecology and obstetrics at Emory University, Atlanta. “It is not yet known what level of antibodies is necessary to convey protection to either neonates or children. This is an active area of investigation at multiple institutions.”
Dr. Tanner said she wished the study “evaluated neonatal cord blood or serum levels to better understand the immune response mounted by the children of women who received vaccination.”
Researchers of the current study said larger prospective studies are needed to confirm the safety of SARS-CoV-2 vaccination in individuals who are breastfeeding and further assess the association of vaccination with infants’ health and SARS-CoV-2–specific immunity.
Dr. Palau and Dr. Tanner had no relevant financial disclosures.
The breast milk of women who had received Pfizer’s COVID-19 vaccine contained specific antibodies against the infectious disease, new research found.
“The COVID-19 pandemic has raised questions among individuals who are breastfeeding, both because of the possibility of viral transmission to infants during breastfeeding and, more recently, of the potential risks and benefits of vaccination in this specific population,” researchers wrote.
In August, the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, and most recently, the Centers for Disease Control and Prevention, recommended that pregnant people receive the COVID-19 vaccine.
The study, published Aug. 11 in JAMA Network Open, adds to a growing collection of research that has found COVID-19 antibodies in the breast milk of women who were vaccinated against or have been infected with the illness.
Study author Erika Esteve-Palau, MD, PhD, and her colleagues collected blood and milk samples from 33 people who were on average 37 years old and who were on average 17.5 months post partum to examine the correlation of the levels of immunoglobulin G antibodies against the spike protein (S1 subunit) and against the nucleocapsid (NC) of SARS-CoV-2.
Blood and milk samples were taken from each study participant at three time points – 2 weeks after receiving the first dose of the vaccine, 2 weeks after receiving the second dose, and 4 weeks after the second dose. No participants had confirmed SARS-CoV-2 infection prior to vaccination or during the study period.
Researchers found that, after the second dose of the vaccine, IgG(S1) levels in breast milk increased and were positively associated with corresponding levels in the blood samples. The median range of IgG(S1) levels for serum-milk pairs at each time point were 519 to 1 arbitrary units (AU) per mL 2 weeks after receiving the first dose of the vaccine, 8,644 to 78 AU/mL 2 weeks after receiving the second dose, and 12,478 to 50.4 AU/mL 4 weeks after receiving the second dose.
Lisette D. Tanner, MD, MPH, FACOG, who was not involved in the study, said she was not surprised by the findings as previous studies have shown the passage of antibodies in breast milk in vaccinated women. One 2021 study published in JAMA found SARS-CoV-2–specific IgA and IgG antibodies in breast milk for 6 weeks after vaccination. IgA secretion was evident as early as 2 weeks after vaccination followed by a spike in IgG after 4 weeks (a week after the second vaccine). Meanwhile, another 2021 study published in mBio found that breast milk produced by parents with COVID-19 is a source of SARS-CoV-2 IgA and IgG antibodies and can neutralize COVID-19 activity.
“While the data from this and other studies is promising in regards to the passage of antibodies, it is currently unclear what the long-term effects for children will be,” said Dr. Tanner of the department of gynecology and obstetrics at Emory University, Atlanta. “It is not yet known what level of antibodies is necessary to convey protection to either neonates or children. This is an active area of investigation at multiple institutions.”
Dr. Tanner said she wished the study “evaluated neonatal cord blood or serum levels to better understand the immune response mounted by the children of women who received vaccination.”
Researchers of the current study said larger prospective studies are needed to confirm the safety of SARS-CoV-2 vaccination in individuals who are breastfeeding and further assess the association of vaccination with infants’ health and SARS-CoV-2–specific immunity.
Dr. Palau and Dr. Tanner had no relevant financial disclosures.
JAMA NETWORK OPEN
Task force affirms routine gestational diabetes testing
Asymptomatic pregnant women with no previous diagnosis of type 1 or 2 diabetes should be screened for gestational diabetes at 24 weeks’ gestation or later, according to an updated recommendation from the U.S. Preventive Services Task Force.
Pregnant individuals who develop gestational diabetes are at increased risk for complications including preeclampsia, fetal macrosomia, and neonatal hypoglycemia, as well as negative long-term outcomes for themselves and their children, wrote lead author Karina W. Davidson, PhD, of Feinstein Institute for Medical Research, Manhasset, N.Y., and colleagues. The statement was published online in JAMA.
The B recommendation and I statement reflect “moderate certainty” that current evidence supports the recommendation in terms of harms versus benefits, and is consistent with the 2014 USPSTF recommendation.
The statement calls for a one-time screening using a glucose tolerance test at or after 24 weeks’ gestation. Although most screening in the United States takes place prior to 28 weeks’ gestation, it can be performed later in patients who begin prenatal care after 28 weeks’ gestation, according to the statement. Data on the harms and benefits of gestational diabetes screening prior to 24 weeks’ gestation are limited, the authors noted. Gestational diabetes was defined as diabetes that develops during pregnancy that is not clearly overt diabetes.
To update the 2014 recommendation, the USPSTF commissioned a systematic review. In 45 prospective studies on the accuracy of gestational diabetes screening, several tests, included oral glucose challenge test, oral glucose tolerance test, and fasting plasma glucose using either a one- or two-step approach were accurate detectors of gestational diabetes; therefore, the USPSTF does not recommend a specific test.
In 13 trials on the impact of treating gestational diabetes on intermediate and health outcomes, treatment was associated with a reduced risk of outcomes, including primary cesarean delivery (but not total cesarean delivery) and preterm delivery, but not with a reduced risk of outcomes including preeclampsia, emergency cesarean delivery, induction of labor, or maternal birth trauma.
The task force also reviewed seven studies of harms associated with screening for gestational diabetes, including three on psychosocial harms, three on hospital experiences, and one of the odds of cesarean delivery after a diagnosis of gestational diabetes. No increase in anxiety or depression occurred following a positive diagnosis or false-positive test result, but data suggested that a gestational diabetes diagnosis may be associated with higher rates of cesarean delivery.
A total of 13 trials evaluated the harms associated with treatment of gestational diabetes, and found no association between treatment and increased risk of several outcomes including severe maternal hypoglycemia, low birth weight, and small for gestational age, and no effect was noted on the number of cesarean deliveries.
Evidence gaps that require additional research include randomized, controlled trials on the effects of gestational diabetes screening on health outcomes, as well as benefits versus harms of screening for pregnant individuals prior to 24 weeks, and studies on the effects of screening in subpopulations of race/ethnicity, age, and socioeconomic factors, according to the task force. Additional research also is needed in areas of maternal health outcomes, long-term outcomes, and the effect on outcomes of one-step versus two-step screening, the USPSTF said.
However, “screening for and detecting gestational diabetes provides a potential opportunity to control blood glucose levels (through lifestyle changes, pharmacological interventions, or both) and reduce the risk of macrosomia and LGA [large for gestational age] infants,” the task force wrote. “In turn, this can prevent associated complications such as primary cesarean delivery, shoulder dystocia, and [neonatal] ICU admissions.”
Support screening with counseling on risk reduction
The USPSTF recommendation is important at this time because “the prevalence of gestational diabetes is increasing secondary to rising rates of obesity,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview.
“In 2014, based on a systematic review of literature, the USPSTF recommended screening all asymptomatic pregnant women for gestational diabetes mellitus [GDM] starting at 24 weeks’ gestation. The recommended gestational age for screening coincides with increasing insulin resistance during pregnancy with advancing gestational age,” Dr. Krishna said.
“An updated systematic review by the USPSTF concluded that existing literature continues to affirm current recommendations of universal screening for GDM at 24 weeks gestation or later. There continues, however, to be no consensus on the optimal approach to screening,” she noted.
“Screening can be performed as a two-step or one-step approach,” said Dr. Krishna. “The two-step approach is commonly used in the United States, and all pregnant women are first screened with a 50-gram oral glucose solution followed by a diagnostic test if they have a positive initial screening.
“Women with risk factors for diabetes, such as prior GDM, obesity, strong family history of diabetes, or history of fetal macrosomia, should be screened early in pregnancy for GDM and have the GDM screen repeated at 24 weeks’ gestation or later if normal in early pregnancy,” Dr. Krishna said. “Pregnant women should be counseled on the importance of diet and exercise and appropriate weight gain in pregnancy to reduce the risk of GDM. Overall, timely diagnosis of gestational diabetes is crucial to improving maternal and fetal pregnancy outcomes.”
The full recommendation statement is also available on the USPSTF website. The research was supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose. Dr. Krishna had no disclosures, but serves on the editorial advisory board of Ob.Gyn News.
Asymptomatic pregnant women with no previous diagnosis of type 1 or 2 diabetes should be screened for gestational diabetes at 24 weeks’ gestation or later, according to an updated recommendation from the U.S. Preventive Services Task Force.
Pregnant individuals who develop gestational diabetes are at increased risk for complications including preeclampsia, fetal macrosomia, and neonatal hypoglycemia, as well as negative long-term outcomes for themselves and their children, wrote lead author Karina W. Davidson, PhD, of Feinstein Institute for Medical Research, Manhasset, N.Y., and colleagues. The statement was published online in JAMA.
The B recommendation and I statement reflect “moderate certainty” that current evidence supports the recommendation in terms of harms versus benefits, and is consistent with the 2014 USPSTF recommendation.
The statement calls for a one-time screening using a glucose tolerance test at or after 24 weeks’ gestation. Although most screening in the United States takes place prior to 28 weeks’ gestation, it can be performed later in patients who begin prenatal care after 28 weeks’ gestation, according to the statement. Data on the harms and benefits of gestational diabetes screening prior to 24 weeks’ gestation are limited, the authors noted. Gestational diabetes was defined as diabetes that develops during pregnancy that is not clearly overt diabetes.
To update the 2014 recommendation, the USPSTF commissioned a systematic review. In 45 prospective studies on the accuracy of gestational diabetes screening, several tests, included oral glucose challenge test, oral glucose tolerance test, and fasting plasma glucose using either a one- or two-step approach were accurate detectors of gestational diabetes; therefore, the USPSTF does not recommend a specific test.
In 13 trials on the impact of treating gestational diabetes on intermediate and health outcomes, treatment was associated with a reduced risk of outcomes, including primary cesarean delivery (but not total cesarean delivery) and preterm delivery, but not with a reduced risk of outcomes including preeclampsia, emergency cesarean delivery, induction of labor, or maternal birth trauma.
The task force also reviewed seven studies of harms associated with screening for gestational diabetes, including three on psychosocial harms, three on hospital experiences, and one of the odds of cesarean delivery after a diagnosis of gestational diabetes. No increase in anxiety or depression occurred following a positive diagnosis or false-positive test result, but data suggested that a gestational diabetes diagnosis may be associated with higher rates of cesarean delivery.
A total of 13 trials evaluated the harms associated with treatment of gestational diabetes, and found no association between treatment and increased risk of several outcomes including severe maternal hypoglycemia, low birth weight, and small for gestational age, and no effect was noted on the number of cesarean deliveries.
Evidence gaps that require additional research include randomized, controlled trials on the effects of gestational diabetes screening on health outcomes, as well as benefits versus harms of screening for pregnant individuals prior to 24 weeks, and studies on the effects of screening in subpopulations of race/ethnicity, age, and socioeconomic factors, according to the task force. Additional research also is needed in areas of maternal health outcomes, long-term outcomes, and the effect on outcomes of one-step versus two-step screening, the USPSTF said.
However, “screening for and detecting gestational diabetes provides a potential opportunity to control blood glucose levels (through lifestyle changes, pharmacological interventions, or both) and reduce the risk of macrosomia and LGA [large for gestational age] infants,” the task force wrote. “In turn, this can prevent associated complications such as primary cesarean delivery, shoulder dystocia, and [neonatal] ICU admissions.”
Support screening with counseling on risk reduction
The USPSTF recommendation is important at this time because “the prevalence of gestational diabetes is increasing secondary to rising rates of obesity,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview.
“In 2014, based on a systematic review of literature, the USPSTF recommended screening all asymptomatic pregnant women for gestational diabetes mellitus [GDM] starting at 24 weeks’ gestation. The recommended gestational age for screening coincides with increasing insulin resistance during pregnancy with advancing gestational age,” Dr. Krishna said.
“An updated systematic review by the USPSTF concluded that existing literature continues to affirm current recommendations of universal screening for GDM at 24 weeks gestation or later. There continues, however, to be no consensus on the optimal approach to screening,” she noted.
“Screening can be performed as a two-step or one-step approach,” said Dr. Krishna. “The two-step approach is commonly used in the United States, and all pregnant women are first screened with a 50-gram oral glucose solution followed by a diagnostic test if they have a positive initial screening.
“Women with risk factors for diabetes, such as prior GDM, obesity, strong family history of diabetes, or history of fetal macrosomia, should be screened early in pregnancy for GDM and have the GDM screen repeated at 24 weeks’ gestation or later if normal in early pregnancy,” Dr. Krishna said. “Pregnant women should be counseled on the importance of diet and exercise and appropriate weight gain in pregnancy to reduce the risk of GDM. Overall, timely diagnosis of gestational diabetes is crucial to improving maternal and fetal pregnancy outcomes.”
The full recommendation statement is also available on the USPSTF website. The research was supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose. Dr. Krishna had no disclosures, but serves on the editorial advisory board of Ob.Gyn News.
Asymptomatic pregnant women with no previous diagnosis of type 1 or 2 diabetes should be screened for gestational diabetes at 24 weeks’ gestation or later, according to an updated recommendation from the U.S. Preventive Services Task Force.
Pregnant individuals who develop gestational diabetes are at increased risk for complications including preeclampsia, fetal macrosomia, and neonatal hypoglycemia, as well as negative long-term outcomes for themselves and their children, wrote lead author Karina W. Davidson, PhD, of Feinstein Institute for Medical Research, Manhasset, N.Y., and colleagues. The statement was published online in JAMA.
The B recommendation and I statement reflect “moderate certainty” that current evidence supports the recommendation in terms of harms versus benefits, and is consistent with the 2014 USPSTF recommendation.
The statement calls for a one-time screening using a glucose tolerance test at or after 24 weeks’ gestation. Although most screening in the United States takes place prior to 28 weeks’ gestation, it can be performed later in patients who begin prenatal care after 28 weeks’ gestation, according to the statement. Data on the harms and benefits of gestational diabetes screening prior to 24 weeks’ gestation are limited, the authors noted. Gestational diabetes was defined as diabetes that develops during pregnancy that is not clearly overt diabetes.
To update the 2014 recommendation, the USPSTF commissioned a systematic review. In 45 prospective studies on the accuracy of gestational diabetes screening, several tests, included oral glucose challenge test, oral glucose tolerance test, and fasting plasma glucose using either a one- or two-step approach were accurate detectors of gestational diabetes; therefore, the USPSTF does not recommend a specific test.
In 13 trials on the impact of treating gestational diabetes on intermediate and health outcomes, treatment was associated with a reduced risk of outcomes, including primary cesarean delivery (but not total cesarean delivery) and preterm delivery, but not with a reduced risk of outcomes including preeclampsia, emergency cesarean delivery, induction of labor, or maternal birth trauma.
The task force also reviewed seven studies of harms associated with screening for gestational diabetes, including three on psychosocial harms, three on hospital experiences, and one of the odds of cesarean delivery after a diagnosis of gestational diabetes. No increase in anxiety or depression occurred following a positive diagnosis or false-positive test result, but data suggested that a gestational diabetes diagnosis may be associated with higher rates of cesarean delivery.
A total of 13 trials evaluated the harms associated with treatment of gestational diabetes, and found no association between treatment and increased risk of several outcomes including severe maternal hypoglycemia, low birth weight, and small for gestational age, and no effect was noted on the number of cesarean deliveries.
Evidence gaps that require additional research include randomized, controlled trials on the effects of gestational diabetes screening on health outcomes, as well as benefits versus harms of screening for pregnant individuals prior to 24 weeks, and studies on the effects of screening in subpopulations of race/ethnicity, age, and socioeconomic factors, according to the task force. Additional research also is needed in areas of maternal health outcomes, long-term outcomes, and the effect on outcomes of one-step versus two-step screening, the USPSTF said.
However, “screening for and detecting gestational diabetes provides a potential opportunity to control blood glucose levels (through lifestyle changes, pharmacological interventions, or both) and reduce the risk of macrosomia and LGA [large for gestational age] infants,” the task force wrote. “In turn, this can prevent associated complications such as primary cesarean delivery, shoulder dystocia, and [neonatal] ICU admissions.”
Support screening with counseling on risk reduction
The USPSTF recommendation is important at this time because “the prevalence of gestational diabetes is increasing secondary to rising rates of obesity,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview.
“In 2014, based on a systematic review of literature, the USPSTF recommended screening all asymptomatic pregnant women for gestational diabetes mellitus [GDM] starting at 24 weeks’ gestation. The recommended gestational age for screening coincides with increasing insulin resistance during pregnancy with advancing gestational age,” Dr. Krishna said.
“An updated systematic review by the USPSTF concluded that existing literature continues to affirm current recommendations of universal screening for GDM at 24 weeks gestation or later. There continues, however, to be no consensus on the optimal approach to screening,” she noted.
“Screening can be performed as a two-step or one-step approach,” said Dr. Krishna. “The two-step approach is commonly used in the United States, and all pregnant women are first screened with a 50-gram oral glucose solution followed by a diagnostic test if they have a positive initial screening.
“Women with risk factors for diabetes, such as prior GDM, obesity, strong family history of diabetes, or history of fetal macrosomia, should be screened early in pregnancy for GDM and have the GDM screen repeated at 24 weeks’ gestation or later if normal in early pregnancy,” Dr. Krishna said. “Pregnant women should be counseled on the importance of diet and exercise and appropriate weight gain in pregnancy to reduce the risk of GDM. Overall, timely diagnosis of gestational diabetes is crucial to improving maternal and fetal pregnancy outcomes.”
The full recommendation statement is also available on the USPSTF website. The research was supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose. Dr. Krishna had no disclosures, but serves on the editorial advisory board of Ob.Gyn News.
FROM JAMA
New moms latched on to remote breastfeeding help. Will demand wane as pandemic fades?
Madison Cano knew she wanted to breastfeed her son, Theo. But breastfeeding was painful for her. The skin on her breasts was chafed and blistered last July when she returned home from the hospital. And Theo sometimes screamed during feedings.
Ms. Cano, 30, realized she needed help to get the short- and long-term health benefits of breastfeeding for moms and babies. New studies (Am J Obstet Gynecol. 2021 Mar 26. doi: 10.1016/j.ajog.2021.03.023) also have shown that COVID-vaccinated mothers pass protective antibodies on to their newborns. However, Ms. Cano lives in Montrose in western Colorado, 60 miles away from her lactation counselor, Ali Reynolds, in Grand Junction – and it was during the thick of the pandemic.
She messaged Ms. Reynolds on Facebook and took photos and recorded videos of herself breastfeeding so Reynolds could offer advice and encouragement from afar. It worked. She no longer had pain. Ms. Cano is still breastfeeding Theo, who just turned 1.
“I don’t think I would have understood what was happening and been able to work through it without that resource,” said Ms. Cano.
Support for breastfeeding was upended last year, when it no longer seemed safe to take a baby class at the hospital or invite a nurse into one’s home. Hospitals, lactation counselors, and support groups turned to virtual platforms like Zoom or phone calls. That made lactation support accessible to struggling families during the pandemic, said Danielle Harmon, executive director of the United States Lactation Consultant Association.
Today, although lactation specialists have more options to safely meet in person with families after their COVID-19 vaccinations, many are choosing to continue virtual classes, keeping alive the online communities they created and relying on the technology that worked for many families. Virtual options especially help those in remote areas or those with limited transportation access, breastfeeding experts say.
Right before the pandemic, for example, Sandrine Druon typically had one or two moms attend in-person meetings she held for La Leche League of Longmont at the First Evangelical Lutheran Church or at a Ziggi’s Coffee shop. But because they could no longer meet in person, last June she launched two monthly virtual meetings. Now, an online meeting will typically include 9 or 10 moms. She started an online Spanish-speaking meeting in May and parents joined from their homes in several states and even from other countries. She hopes eventually to have a mix of online and in-person meetings.
The virtual switch hasn’t worked for everyone. Ms. Harmon said the logistics of video support remain difficult, along with privacy concerns on platforms that could be hacked. Other lactation experts noted Black and Hispanic mothers are sometimes still left behind. So lactation specialists are trying to learn from the pandemic on what worked – and what didn’t – to reach all kinds of new parents.
Before the pandemic, 84% of U.S. mothers breastfed at least initially, according to 2019 data from the Centers for Disease Control and Prevention, while Colorado had a 93% rate.
The pandemic hasn’t seemed to change the picture, said Stacy Miller, Colorado’s breastfeeding coordinator for the Special Supplemental Nutrition Program for Women, Infants, and Children, shorthanded as WIC. Citing state birth certificate data, Ms. Miller said preliminary breastfeeding rates among families discharged from Colorado hospitals remained similar in the first quarter of 2021 to rates from 2020 or 2019.
Throughout the pandemic, lactation specialists have tried to offer convenient options for parents. St. Joseph Hospital in Denver launched virtual breastfeeding support groups that still occur today, in addition to breastfeeding help during families’ hospital stays, said Katie Halverstadt, the hospital’s clinical nurse manager of lactation and family education.
Last year in North Carolina, experts adapted an in-person prenatal breastfeeding program to an interactive video platform in English and Spanish. A separate effort on New York’s Long Island successfully converted in-person breastfeeding support to phone and video calls in 2020.
To help support parents in Grand Junction, Colo., Ms. Reynolds expanded her private practice, Valley Lactation, by offering virtual appointments while continuing to see some clients in their homes. That hybrid model continues today, although Ms. Reynolds said the demand for virtual or phone appointments has decreased lately as the country reopens.
Paying out-of-pocket for appointments is a hurdle her clients face, said Ms. Reynolds, but she encourages them to submit claims for telehealth or in-person visits to their health insurance companies for reimbursement. Early in the pandemic, telehealth rules were relaxed to encourage more telephone and virtual appointments – many of which have been covered by insurance.
But insurance coverage for lactation support will likely continue to be an issue independent of whether pandemic telehealth rules expire, Ms. Harmon said. While the Affordable Care Act mandates that insurance companies cover lactation support and supplies, such as breast pumps, Ms. Harmon said reimbursement is often spotty. Mirroring Medicaid, insurance providers often cover services only from licensed providers, she said, but just four states – Georgia, New Mexico, Oregon, and Rhode Island – license lactation consultants.
Experts such as Jennifer Schindler-Ruwisch, an assistant professor at Fairfield (Conn.) University, found the pandemic may have exacerbated breastfeeding barriers for those without access to online technology or translation services, among other things. She published one of the first studies in the United States to examine COVID’s effect on lactation services by collecting experiences from lactation support providers in Connecticut, including many working in WIC programs. For income-eligible WIC families, all breastfeeding classes, peer groups and one-on-one consultations are free.
Birdie Johnson, a doula who provides breastfeeding and other postpartum support to Black families as part of Sacred Seeds Black Doula Collective of Colorado, said virtual support groups during the pandemic also did not meet her clients’ needs for connection and interaction. Social media built communities online, particularly by normalizing breastfeeding struggles among Black parents, she said, but obstacles remained.
“COVID brought our community together and at the same time destroyed it,” Ms. Johnson said.
Black parents in the United States already had lower rates of breastfeeding than Asian or White parents, according to 2017 CDC data, and both Black and Hispanic parents have had lower rates of exclusively breastfeeding their babies at 6 months, which is what the American Academy of Pediatrics recommends. Socioeconomics and lack of workplace support have been found to contribute to the gap. Research also has found Black mothers are more likely than White moms to be introduced to infant formula at hospitals.
A scarcity of Black health care providers in lactation, women’s health and pediatrics is a continuing concern, Ms. Johnson said. In Colorado last year, the Colorado Breastfeeding Coalition, the Center for African American Health, Elephant Circle and Families Forward Resource Center held three training sessions for people of color to become lactation specialists, said Ms. Halverstadt, who chairs the coalition.
Jefferson County, which encompasses much of Denver’s western suburbs, is now training at least a dozen Spanish-speaking community members for lactation certification. In addition to classes, the trainees log clinical hours in breastfeeding support, sometimes during virtual meetings of a Spanish-speaking support group called Cuenta Conmigo Lactancia.
“You are more confident and more at ease with someone who knows your language, your culture and who is part of the community,” said Brenda Rodriguez, a dietitian and certified lactation consultant for Jefferson County Public Health, which reaches roughly 400 breastfeeding families each month through its WIC programs.
Angelica Pereda, a maternal and child health registered nurse, is part of that training program. Ms. Pereda, who is Hispanic and bilingual, gave birth to son Ahmias in April 2020 and struggled with breastfeeding because he could not latch on to her breasts. A lactation consultant could not come into her home during the pandemic, and she was skeptical of virtual consultations because of privacy concerns. So she pumped her breast milk and bottle-fed it to her son.
Her experience gave her newfound empathy for families, and she wants to help other Spanish-speaking parents find solutions – whether in person or virtually.
“There is just not enough breastfeeding support in general, but especially when that support is in a different language,” said Ms. Pereda.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Madison Cano knew she wanted to breastfeed her son, Theo. But breastfeeding was painful for her. The skin on her breasts was chafed and blistered last July when she returned home from the hospital. And Theo sometimes screamed during feedings.
Ms. Cano, 30, realized she needed help to get the short- and long-term health benefits of breastfeeding for moms and babies. New studies (Am J Obstet Gynecol. 2021 Mar 26. doi: 10.1016/j.ajog.2021.03.023) also have shown that COVID-vaccinated mothers pass protective antibodies on to their newborns. However, Ms. Cano lives in Montrose in western Colorado, 60 miles away from her lactation counselor, Ali Reynolds, in Grand Junction – and it was during the thick of the pandemic.
She messaged Ms. Reynolds on Facebook and took photos and recorded videos of herself breastfeeding so Reynolds could offer advice and encouragement from afar. It worked. She no longer had pain. Ms. Cano is still breastfeeding Theo, who just turned 1.
“I don’t think I would have understood what was happening and been able to work through it without that resource,” said Ms. Cano.
Support for breastfeeding was upended last year, when it no longer seemed safe to take a baby class at the hospital or invite a nurse into one’s home. Hospitals, lactation counselors, and support groups turned to virtual platforms like Zoom or phone calls. That made lactation support accessible to struggling families during the pandemic, said Danielle Harmon, executive director of the United States Lactation Consultant Association.
Today, although lactation specialists have more options to safely meet in person with families after their COVID-19 vaccinations, many are choosing to continue virtual classes, keeping alive the online communities they created and relying on the technology that worked for many families. Virtual options especially help those in remote areas or those with limited transportation access, breastfeeding experts say.
Right before the pandemic, for example, Sandrine Druon typically had one or two moms attend in-person meetings she held for La Leche League of Longmont at the First Evangelical Lutheran Church or at a Ziggi’s Coffee shop. But because they could no longer meet in person, last June she launched two monthly virtual meetings. Now, an online meeting will typically include 9 or 10 moms. She started an online Spanish-speaking meeting in May and parents joined from their homes in several states and even from other countries. She hopes eventually to have a mix of online and in-person meetings.
The virtual switch hasn’t worked for everyone. Ms. Harmon said the logistics of video support remain difficult, along with privacy concerns on platforms that could be hacked. Other lactation experts noted Black and Hispanic mothers are sometimes still left behind. So lactation specialists are trying to learn from the pandemic on what worked – and what didn’t – to reach all kinds of new parents.
Before the pandemic, 84% of U.S. mothers breastfed at least initially, according to 2019 data from the Centers for Disease Control and Prevention, while Colorado had a 93% rate.
The pandemic hasn’t seemed to change the picture, said Stacy Miller, Colorado’s breastfeeding coordinator for the Special Supplemental Nutrition Program for Women, Infants, and Children, shorthanded as WIC. Citing state birth certificate data, Ms. Miller said preliminary breastfeeding rates among families discharged from Colorado hospitals remained similar in the first quarter of 2021 to rates from 2020 or 2019.
Throughout the pandemic, lactation specialists have tried to offer convenient options for parents. St. Joseph Hospital in Denver launched virtual breastfeeding support groups that still occur today, in addition to breastfeeding help during families’ hospital stays, said Katie Halverstadt, the hospital’s clinical nurse manager of lactation and family education.
Last year in North Carolina, experts adapted an in-person prenatal breastfeeding program to an interactive video platform in English and Spanish. A separate effort on New York’s Long Island successfully converted in-person breastfeeding support to phone and video calls in 2020.
To help support parents in Grand Junction, Colo., Ms. Reynolds expanded her private practice, Valley Lactation, by offering virtual appointments while continuing to see some clients in their homes. That hybrid model continues today, although Ms. Reynolds said the demand for virtual or phone appointments has decreased lately as the country reopens.
Paying out-of-pocket for appointments is a hurdle her clients face, said Ms. Reynolds, but she encourages them to submit claims for telehealth or in-person visits to their health insurance companies for reimbursement. Early in the pandemic, telehealth rules were relaxed to encourage more telephone and virtual appointments – many of which have been covered by insurance.
But insurance coverage for lactation support will likely continue to be an issue independent of whether pandemic telehealth rules expire, Ms. Harmon said. While the Affordable Care Act mandates that insurance companies cover lactation support and supplies, such as breast pumps, Ms. Harmon said reimbursement is often spotty. Mirroring Medicaid, insurance providers often cover services only from licensed providers, she said, but just four states – Georgia, New Mexico, Oregon, and Rhode Island – license lactation consultants.
Experts such as Jennifer Schindler-Ruwisch, an assistant professor at Fairfield (Conn.) University, found the pandemic may have exacerbated breastfeeding barriers for those without access to online technology or translation services, among other things. She published one of the first studies in the United States to examine COVID’s effect on lactation services by collecting experiences from lactation support providers in Connecticut, including many working in WIC programs. For income-eligible WIC families, all breastfeeding classes, peer groups and one-on-one consultations are free.
Birdie Johnson, a doula who provides breastfeeding and other postpartum support to Black families as part of Sacred Seeds Black Doula Collective of Colorado, said virtual support groups during the pandemic also did not meet her clients’ needs for connection and interaction. Social media built communities online, particularly by normalizing breastfeeding struggles among Black parents, she said, but obstacles remained.
“COVID brought our community together and at the same time destroyed it,” Ms. Johnson said.
Black parents in the United States already had lower rates of breastfeeding than Asian or White parents, according to 2017 CDC data, and both Black and Hispanic parents have had lower rates of exclusively breastfeeding their babies at 6 months, which is what the American Academy of Pediatrics recommends. Socioeconomics and lack of workplace support have been found to contribute to the gap. Research also has found Black mothers are more likely than White moms to be introduced to infant formula at hospitals.
A scarcity of Black health care providers in lactation, women’s health and pediatrics is a continuing concern, Ms. Johnson said. In Colorado last year, the Colorado Breastfeeding Coalition, the Center for African American Health, Elephant Circle and Families Forward Resource Center held three training sessions for people of color to become lactation specialists, said Ms. Halverstadt, who chairs the coalition.
Jefferson County, which encompasses much of Denver’s western suburbs, is now training at least a dozen Spanish-speaking community members for lactation certification. In addition to classes, the trainees log clinical hours in breastfeeding support, sometimes during virtual meetings of a Spanish-speaking support group called Cuenta Conmigo Lactancia.
“You are more confident and more at ease with someone who knows your language, your culture and who is part of the community,” said Brenda Rodriguez, a dietitian and certified lactation consultant for Jefferson County Public Health, which reaches roughly 400 breastfeeding families each month through its WIC programs.
Angelica Pereda, a maternal and child health registered nurse, is part of that training program. Ms. Pereda, who is Hispanic and bilingual, gave birth to son Ahmias in April 2020 and struggled with breastfeeding because he could not latch on to her breasts. A lactation consultant could not come into her home during the pandemic, and she was skeptical of virtual consultations because of privacy concerns. So she pumped her breast milk and bottle-fed it to her son.
Her experience gave her newfound empathy for families, and she wants to help other Spanish-speaking parents find solutions – whether in person or virtually.
“There is just not enough breastfeeding support in general, but especially when that support is in a different language,” said Ms. Pereda.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Madison Cano knew she wanted to breastfeed her son, Theo. But breastfeeding was painful for her. The skin on her breasts was chafed and blistered last July when she returned home from the hospital. And Theo sometimes screamed during feedings.
Ms. Cano, 30, realized she needed help to get the short- and long-term health benefits of breastfeeding for moms and babies. New studies (Am J Obstet Gynecol. 2021 Mar 26. doi: 10.1016/j.ajog.2021.03.023) also have shown that COVID-vaccinated mothers pass protective antibodies on to their newborns. However, Ms. Cano lives in Montrose in western Colorado, 60 miles away from her lactation counselor, Ali Reynolds, in Grand Junction – and it was during the thick of the pandemic.
She messaged Ms. Reynolds on Facebook and took photos and recorded videos of herself breastfeeding so Reynolds could offer advice and encouragement from afar. It worked. She no longer had pain. Ms. Cano is still breastfeeding Theo, who just turned 1.
“I don’t think I would have understood what was happening and been able to work through it without that resource,” said Ms. Cano.
Support for breastfeeding was upended last year, when it no longer seemed safe to take a baby class at the hospital or invite a nurse into one’s home. Hospitals, lactation counselors, and support groups turned to virtual platforms like Zoom or phone calls. That made lactation support accessible to struggling families during the pandemic, said Danielle Harmon, executive director of the United States Lactation Consultant Association.
Today, although lactation specialists have more options to safely meet in person with families after their COVID-19 vaccinations, many are choosing to continue virtual classes, keeping alive the online communities they created and relying on the technology that worked for many families. Virtual options especially help those in remote areas or those with limited transportation access, breastfeeding experts say.
Right before the pandemic, for example, Sandrine Druon typically had one or two moms attend in-person meetings she held for La Leche League of Longmont at the First Evangelical Lutheran Church or at a Ziggi’s Coffee shop. But because they could no longer meet in person, last June she launched two monthly virtual meetings. Now, an online meeting will typically include 9 or 10 moms. She started an online Spanish-speaking meeting in May and parents joined from their homes in several states and even from other countries. She hopes eventually to have a mix of online and in-person meetings.
The virtual switch hasn’t worked for everyone. Ms. Harmon said the logistics of video support remain difficult, along with privacy concerns on platforms that could be hacked. Other lactation experts noted Black and Hispanic mothers are sometimes still left behind. So lactation specialists are trying to learn from the pandemic on what worked – and what didn’t – to reach all kinds of new parents.
Before the pandemic, 84% of U.S. mothers breastfed at least initially, according to 2019 data from the Centers for Disease Control and Prevention, while Colorado had a 93% rate.
The pandemic hasn’t seemed to change the picture, said Stacy Miller, Colorado’s breastfeeding coordinator for the Special Supplemental Nutrition Program for Women, Infants, and Children, shorthanded as WIC. Citing state birth certificate data, Ms. Miller said preliminary breastfeeding rates among families discharged from Colorado hospitals remained similar in the first quarter of 2021 to rates from 2020 or 2019.
Throughout the pandemic, lactation specialists have tried to offer convenient options for parents. St. Joseph Hospital in Denver launched virtual breastfeeding support groups that still occur today, in addition to breastfeeding help during families’ hospital stays, said Katie Halverstadt, the hospital’s clinical nurse manager of lactation and family education.
Last year in North Carolina, experts adapted an in-person prenatal breastfeeding program to an interactive video platform in English and Spanish. A separate effort on New York’s Long Island successfully converted in-person breastfeeding support to phone and video calls in 2020.
To help support parents in Grand Junction, Colo., Ms. Reynolds expanded her private practice, Valley Lactation, by offering virtual appointments while continuing to see some clients in their homes. That hybrid model continues today, although Ms. Reynolds said the demand for virtual or phone appointments has decreased lately as the country reopens.
Paying out-of-pocket for appointments is a hurdle her clients face, said Ms. Reynolds, but she encourages them to submit claims for telehealth or in-person visits to their health insurance companies for reimbursement. Early in the pandemic, telehealth rules were relaxed to encourage more telephone and virtual appointments – many of which have been covered by insurance.
But insurance coverage for lactation support will likely continue to be an issue independent of whether pandemic telehealth rules expire, Ms. Harmon said. While the Affordable Care Act mandates that insurance companies cover lactation support and supplies, such as breast pumps, Ms. Harmon said reimbursement is often spotty. Mirroring Medicaid, insurance providers often cover services only from licensed providers, she said, but just four states – Georgia, New Mexico, Oregon, and Rhode Island – license lactation consultants.
Experts such as Jennifer Schindler-Ruwisch, an assistant professor at Fairfield (Conn.) University, found the pandemic may have exacerbated breastfeeding barriers for those without access to online technology or translation services, among other things. She published one of the first studies in the United States to examine COVID’s effect on lactation services by collecting experiences from lactation support providers in Connecticut, including many working in WIC programs. For income-eligible WIC families, all breastfeeding classes, peer groups and one-on-one consultations are free.
Birdie Johnson, a doula who provides breastfeeding and other postpartum support to Black families as part of Sacred Seeds Black Doula Collective of Colorado, said virtual support groups during the pandemic also did not meet her clients’ needs for connection and interaction. Social media built communities online, particularly by normalizing breastfeeding struggles among Black parents, she said, but obstacles remained.
“COVID brought our community together and at the same time destroyed it,” Ms. Johnson said.
Black parents in the United States already had lower rates of breastfeeding than Asian or White parents, according to 2017 CDC data, and both Black and Hispanic parents have had lower rates of exclusively breastfeeding their babies at 6 months, which is what the American Academy of Pediatrics recommends. Socioeconomics and lack of workplace support have been found to contribute to the gap. Research also has found Black mothers are more likely than White moms to be introduced to infant formula at hospitals.
A scarcity of Black health care providers in lactation, women’s health and pediatrics is a continuing concern, Ms. Johnson said. In Colorado last year, the Colorado Breastfeeding Coalition, the Center for African American Health, Elephant Circle and Families Forward Resource Center held three training sessions for people of color to become lactation specialists, said Ms. Halverstadt, who chairs the coalition.
Jefferson County, which encompasses much of Denver’s western suburbs, is now training at least a dozen Spanish-speaking community members for lactation certification. In addition to classes, the trainees log clinical hours in breastfeeding support, sometimes during virtual meetings of a Spanish-speaking support group called Cuenta Conmigo Lactancia.
“You are more confident and more at ease with someone who knows your language, your culture and who is part of the community,” said Brenda Rodriguez, a dietitian and certified lactation consultant for Jefferson County Public Health, which reaches roughly 400 breastfeeding families each month through its WIC programs.
Angelica Pereda, a maternal and child health registered nurse, is part of that training program. Ms. Pereda, who is Hispanic and bilingual, gave birth to son Ahmias in April 2020 and struggled with breastfeeding because he could not latch on to her breasts. A lactation consultant could not come into her home during the pandemic, and she was skeptical of virtual consultations because of privacy concerns. So she pumped her breast milk and bottle-fed it to her son.
Her experience gave her newfound empathy for families, and she wants to help other Spanish-speaking parents find solutions – whether in person or virtually.
“There is just not enough breastfeeding support in general, but especially when that support is in a different language,” said Ms. Pereda.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
ACOG, SMFM urge all pregnant women to get COVID-19 vaccine
The American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) strongly recommend that all pregnant women be vaccinated against COVID-19.
Only about 16% of pregnant people have received one or more doses of a COVID-19 vaccine, according to the Centers for Disease Control and Prevention, despite evidence that COVID-19 infection puts pregnant people at an increased risk of severe complications and death.
That CDC report in June also found that vaccination during pregnancy was lowest among Hispanic (11.9%) and non-Hispanic Black women (6%) and women aged 18-24 years (5.5%) and highest among non-Hispanic Asian women (24.7%) and women aged 35-49 years (22.7%).
Linda Eckert, MD, professor of obstetrics and gynecology at University of Washington, Seattle, and a member of ACOG’s immunization expert work group, said in an interview that previously, ACOG has said that pregnant women should have the opportunity to be vaccinated, should they choose it.
Now the urgency has increased, she said: “This is a strong recommendation.”
The recommendation comes after mounting evidence demonstrating that COVID-19 vaccines are safe during pregnancy “from tens of thousands of reporting individuals over the last several months, as well as the current low vaccination rates and concerning increase in cases,” ACOG and SMFM said in the statement.
Both organizations said the timing of the advisory comes amid growing concern about the Delta variant.
CDC Director Rochelle Walensky, MD, has called the variant “one of the most infectious respiratory viruses we know of.”
No evidence of maternal/fetal harm
There is no evidence that COVID-19 vaccines could cause maternal or fetal harm, ACOG stated.
“ACOG encourages its members to enthusiastically recommend vaccination to their patients. This means emphasizing the known safety of the vaccines and the increased risk of severe complications associated with COVID-19 infection, including death, during pregnancy,” said J. Martin Tucker, MD, FACOG, president of ACOG. “It is clear that pregnant people need to feel confident in the decision to choose vaccination, and a strong recommendation from their obstetrician-gynecologist could make a meaningful difference for many pregnant people.”
Pregnant women are considered high risk because of concerns about the effect of COVID-19 during and after pregnancy, and on their offspring.
As this news organization has reported, research published in The BMJ found that pregnant women with COVID-19 may be at higher risk of admission to a hospital intensive care unit.
Preterm birth rates also were found to be higher among pregnant women with COVID-19 than among pregnant women without the disease.
Dr. Eckert said several of her patients have declined the vaccine. Among the reasons are that they don’t want to take any medications while pregnant or that they have heard that effects of the vaccines were not studied in pregnant women.
“Sometimes as I review with them the ongoing data coming in from pregnant individuals and newborns, [these patients] may change their minds and get the vaccine,” Dr. Eckert said.
In some cases, a pregnant patient’s family has pressured the patient not to get the vaccine.
The ACOG/SMFM advice notes that pregnant women who have decided to wait until after delivery to be vaccinated “may be inadvertently exposing themselves to an increased risk of severe illness or death.”
The recommendation extends to those who have already given birth.
“Those who have recently delivered and were not vaccinated during pregnancy are also strongly encouraged to get vaccinated as soon as possible,” the statement reads.
ACOG has developed talking points about the safety and efficacy of COVID-19 vaccines for pregnant patients.
Dr. Eckert disclosed no relevant financial relationships.
The American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) strongly recommend that all pregnant women be vaccinated against COVID-19.
Only about 16% of pregnant people have received one or more doses of a COVID-19 vaccine, according to the Centers for Disease Control and Prevention, despite evidence that COVID-19 infection puts pregnant people at an increased risk of severe complications and death.
That CDC report in June also found that vaccination during pregnancy was lowest among Hispanic (11.9%) and non-Hispanic Black women (6%) and women aged 18-24 years (5.5%) and highest among non-Hispanic Asian women (24.7%) and women aged 35-49 years (22.7%).
Linda Eckert, MD, professor of obstetrics and gynecology at University of Washington, Seattle, and a member of ACOG’s immunization expert work group, said in an interview that previously, ACOG has said that pregnant women should have the opportunity to be vaccinated, should they choose it.
Now the urgency has increased, she said: “This is a strong recommendation.”
The recommendation comes after mounting evidence demonstrating that COVID-19 vaccines are safe during pregnancy “from tens of thousands of reporting individuals over the last several months, as well as the current low vaccination rates and concerning increase in cases,” ACOG and SMFM said in the statement.
Both organizations said the timing of the advisory comes amid growing concern about the Delta variant.
CDC Director Rochelle Walensky, MD, has called the variant “one of the most infectious respiratory viruses we know of.”
No evidence of maternal/fetal harm
There is no evidence that COVID-19 vaccines could cause maternal or fetal harm, ACOG stated.
“ACOG encourages its members to enthusiastically recommend vaccination to their patients. This means emphasizing the known safety of the vaccines and the increased risk of severe complications associated with COVID-19 infection, including death, during pregnancy,” said J. Martin Tucker, MD, FACOG, president of ACOG. “It is clear that pregnant people need to feel confident in the decision to choose vaccination, and a strong recommendation from their obstetrician-gynecologist could make a meaningful difference for many pregnant people.”
Pregnant women are considered high risk because of concerns about the effect of COVID-19 during and after pregnancy, and on their offspring.
As this news organization has reported, research published in The BMJ found that pregnant women with COVID-19 may be at higher risk of admission to a hospital intensive care unit.
Preterm birth rates also were found to be higher among pregnant women with COVID-19 than among pregnant women without the disease.
Dr. Eckert said several of her patients have declined the vaccine. Among the reasons are that they don’t want to take any medications while pregnant or that they have heard that effects of the vaccines were not studied in pregnant women.
“Sometimes as I review with them the ongoing data coming in from pregnant individuals and newborns, [these patients] may change their minds and get the vaccine,” Dr. Eckert said.
In some cases, a pregnant patient’s family has pressured the patient not to get the vaccine.
The ACOG/SMFM advice notes that pregnant women who have decided to wait until after delivery to be vaccinated “may be inadvertently exposing themselves to an increased risk of severe illness or death.”
The recommendation extends to those who have already given birth.
“Those who have recently delivered and were not vaccinated during pregnancy are also strongly encouraged to get vaccinated as soon as possible,” the statement reads.
ACOG has developed talking points about the safety and efficacy of COVID-19 vaccines for pregnant patients.
Dr. Eckert disclosed no relevant financial relationships.
The American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) strongly recommend that all pregnant women be vaccinated against COVID-19.
Only about 16% of pregnant people have received one or more doses of a COVID-19 vaccine, according to the Centers for Disease Control and Prevention, despite evidence that COVID-19 infection puts pregnant people at an increased risk of severe complications and death.
That CDC report in June also found that vaccination during pregnancy was lowest among Hispanic (11.9%) and non-Hispanic Black women (6%) and women aged 18-24 years (5.5%) and highest among non-Hispanic Asian women (24.7%) and women aged 35-49 years (22.7%).
Linda Eckert, MD, professor of obstetrics and gynecology at University of Washington, Seattle, and a member of ACOG’s immunization expert work group, said in an interview that previously, ACOG has said that pregnant women should have the opportunity to be vaccinated, should they choose it.
Now the urgency has increased, she said: “This is a strong recommendation.”
The recommendation comes after mounting evidence demonstrating that COVID-19 vaccines are safe during pregnancy “from tens of thousands of reporting individuals over the last several months, as well as the current low vaccination rates and concerning increase in cases,” ACOG and SMFM said in the statement.
Both organizations said the timing of the advisory comes amid growing concern about the Delta variant.
CDC Director Rochelle Walensky, MD, has called the variant “one of the most infectious respiratory viruses we know of.”
No evidence of maternal/fetal harm
There is no evidence that COVID-19 vaccines could cause maternal or fetal harm, ACOG stated.
“ACOG encourages its members to enthusiastically recommend vaccination to their patients. This means emphasizing the known safety of the vaccines and the increased risk of severe complications associated with COVID-19 infection, including death, during pregnancy,” said J. Martin Tucker, MD, FACOG, president of ACOG. “It is clear that pregnant people need to feel confident in the decision to choose vaccination, and a strong recommendation from their obstetrician-gynecologist could make a meaningful difference for many pregnant people.”
Pregnant women are considered high risk because of concerns about the effect of COVID-19 during and after pregnancy, and on their offspring.
As this news organization has reported, research published in The BMJ found that pregnant women with COVID-19 may be at higher risk of admission to a hospital intensive care unit.
Preterm birth rates also were found to be higher among pregnant women with COVID-19 than among pregnant women without the disease.
Dr. Eckert said several of her patients have declined the vaccine. Among the reasons are that they don’t want to take any medications while pregnant or that they have heard that effects of the vaccines were not studied in pregnant women.
“Sometimes as I review with them the ongoing data coming in from pregnant individuals and newborns, [these patients] may change their minds and get the vaccine,” Dr. Eckert said.
In some cases, a pregnant patient’s family has pressured the patient not to get the vaccine.
The ACOG/SMFM advice notes that pregnant women who have decided to wait until after delivery to be vaccinated “may be inadvertently exposing themselves to an increased risk of severe illness or death.”
The recommendation extends to those who have already given birth.
“Those who have recently delivered and were not vaccinated during pregnancy are also strongly encouraged to get vaccinated as soon as possible,” the statement reads.
ACOG has developed talking points about the safety and efficacy of COVID-19 vaccines for pregnant patients.
Dr. Eckert disclosed no relevant financial relationships.
Nearly half of female surgeons surveyed lost a pregnancy
– according to an article published online July 28 in JAMA Surgery.
The authors, led by Erika L. Rangel, MD, division of general and gastrointestinal surgery, department of surgery, Brigham and Women’s Hospital, Boston, found that after the losses, the women took little or no time off.
Of 692 surgeons surveyed, 347 female surgeons had experienced a pregnancy loss. Of those, 244 had had a miscarriage at less than 10 weeks’ gestation, 92 had had a miscarriage between 10 and 20 weeks’ gestation, and 11 had had a stillbirth (loss at 20 weeks or later).
Most took no time off after miscarriage
After a miscarriage, 225 of 336 women (75%) took no time off work, and after a stillbirth, 5 of 11 (45%) took off 1 week or less, the authors found.
The study addressed an issue that people have talked about anecdotally or on social media, Dr. Rangel told this news organization.
“This was finally an opportunity to do a study of enough magnitude to show that there is a very quantifiable difference in complication rate, use of IVF [in vitro fertilization], and the age at which we have children. These are not just anecdotal stories,” she said.
For the study, a self-administered questionnaire was distributed electronically. Answers were collected from November 2020 to January 2021 through multiple U.S. surgical societies and social media among attending and resident surgeons with children. The control group for the study comprised 158 male surgeons who answered questions regarding their partners’ pregnancies.
Female surgeons had fewer children compared with male surgeons and their female partners (mean [SD],1.8 [0.8], versus 2.3 [1.1]; P < .001) and were more likely to delay having children because of surgical training (450 of 692 [65.0%] versus 69 of 158 [43.7%]; P < .001).
In addition, Dr. Rangel and colleagues found that 57% of female surgeons worked more than 60 hours a week during pregnancy and that 37% took more than six overnight calls.
The data show that female surgeons who operated 12 or more hours per week during the last trimester of pregnancy were at higher risk compared with those who operated fewer hours (odds ratio, 1.57; 95% confidence interval, 1.08-2.26).
“Pregnant surgeons should not be operating more than 12 hours a week when they are in the third trimester,” Dr. Rangel said.
“That is a modifiable risk factor,” she told this news organization. “It’s a very brief period of support – a couple of months of support for a woman who may do 25-30 more years of serving the public with surgical skills.”
She said that training programs should be organized so as to have colleagues cover operating room (OR) shifts to reduce the operating hours for pregnant colleagues. In addition, advanced practice health care professionals should be paid to take up the paperwork and perform non-OR care to reduce the stigma associated with pregnant trainees overburdening other surgical trainees.
‘It’s too big an ask’
Obstetrician-gynecologist Maryam Siddiqui, MD, said in an interview that she was particularly struck by the number of female surgeons who experience involuntary childlessness.
“That’s a big ask for people who want childbearing to be a part of the fulfillment of their life. It’s too big,” said Dr. Siddiqui, a gynecologic surgeon at UChicago Medicine.
She said the amount of detail in the article and the large number of participants were persuasive factors that can support establishing a more humane system than one in which one person at a time has to ask for change.
Pointing to the finding that three-fourths of the women in the study who had had miscarriages didn’t take time off, she said, “That’s not really humane. But they’re afraid to ask or they don’t want to reveal they’re trying [to get pregnant]. Why should you be afraid of building your family?”
The authors also found other adverse outcomes. Female surgeons were more likely to have musculoskeletal disorders compared with female nonsurgeon partners (36.9% versus 18.4%; P < .001), and they were more likely to undergo nonelective cesarean delivery (25.5% versus 15.3%; P = .01) and to experience postpartum depression (11.1% versus 5.7%; P = .04).
Dr. Siddiqui said the conditions that surgeons encounter on their return to work after childbirth are “a perfect storm” for postpartum depression among women who are not accustomed to being reliant on others.
Women often feel coerced into returning to work before they are physically or emotionally ready, then toggle back and forth from night shift to day shift, losing sleep, she said. “We can do better.”
One of the solutions, she said, is to provide better work coverage for the surgeon while she is pregnant and when she returns to work. That includes properly compensating the person covering for the surgeon by giving that person extra pay or additional time off.
“You have to value both people,” she said. “If both people are valued, there’s still collegiality.”
She acknowledged that that kind of compensation may be more readily available at large academic centers.
At UChicago, she said, they are creative with scheduling in training. For women at the height of pregnancy, rotations are less intensive, and trauma rotations are avoided.
Dr. Siddiqui said one of the most important aspects of the article is the authors’ list of two dozen ways, both big and small, to improve conditions.
Adopting such changes will become increasingly important for hiring and retaining female surgeons. “You want to work someplace where you’re respected as a whole person,” she said.
Sarah Blair, MD, a surgical oncologist at University of California, San Diego, stated that the number of miscarriages in particular provides disturbing proof of a problem women in surgery frequently discuss.
For nearly a decade, she led a women-in-surgery committee at UCSD in which they discussed such issues regarding pregnancy and medicine.
She said she hopes these data can help push for change in flexibility in residency so that women can graduate on time and have the families they want.
“There’s a movement away from time-based training to competency-based training, so maybe that will help women,” she said.
‘We have to figure this out’
“We will have to figure this out, because more than half of the people in medical school are women, and there are a lot more women in surgery than when I trained more than 20 years ago. It’s not a problem that’s going away,” she said.
One sign of improvement happened recently, Dr. Rangel said.
As previously reported, according to the American Board of Medical Specialties, as of July 1, 2021, residents and fellows are allowed a minimum 6 weeks away for medical leave or caregiving once during training, without having to use vacation time or sick leave and without having to extend their training.
“That’s huge,” she said. “But we still have a long way to go, because the residency programs still don’t have to have policy that abides that. It merely says you can take 6 weeks off and take your boards. It doesn’t say that the residency program has to allow you to take 6 weeks off.”
The authors noted that the United States and Papua New Guinea are the only countries in the world without federally mandated paid parental leave.
“Most U.S. female surgeons rely on their employer for this benefit, but only half of top-ranked medical schools offer paid leave, and 33%-65% of U.S. surgical training programs lack clear maternity leave policies,” she said.
Funding for the study was provided by the department of surgery at Brigham and Women’s Hospital. The study authors, Dr. Blair, and Dr. Siddiqui have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– according to an article published online July 28 in JAMA Surgery.
The authors, led by Erika L. Rangel, MD, division of general and gastrointestinal surgery, department of surgery, Brigham and Women’s Hospital, Boston, found that after the losses, the women took little or no time off.
Of 692 surgeons surveyed, 347 female surgeons had experienced a pregnancy loss. Of those, 244 had had a miscarriage at less than 10 weeks’ gestation, 92 had had a miscarriage between 10 and 20 weeks’ gestation, and 11 had had a stillbirth (loss at 20 weeks or later).
Most took no time off after miscarriage
After a miscarriage, 225 of 336 women (75%) took no time off work, and after a stillbirth, 5 of 11 (45%) took off 1 week or less, the authors found.
The study addressed an issue that people have talked about anecdotally or on social media, Dr. Rangel told this news organization.
“This was finally an opportunity to do a study of enough magnitude to show that there is a very quantifiable difference in complication rate, use of IVF [in vitro fertilization], and the age at which we have children. These are not just anecdotal stories,” she said.
For the study, a self-administered questionnaire was distributed electronically. Answers were collected from November 2020 to January 2021 through multiple U.S. surgical societies and social media among attending and resident surgeons with children. The control group for the study comprised 158 male surgeons who answered questions regarding their partners’ pregnancies.
Female surgeons had fewer children compared with male surgeons and their female partners (mean [SD],1.8 [0.8], versus 2.3 [1.1]; P < .001) and were more likely to delay having children because of surgical training (450 of 692 [65.0%] versus 69 of 158 [43.7%]; P < .001).
In addition, Dr. Rangel and colleagues found that 57% of female surgeons worked more than 60 hours a week during pregnancy and that 37% took more than six overnight calls.
The data show that female surgeons who operated 12 or more hours per week during the last trimester of pregnancy were at higher risk compared with those who operated fewer hours (odds ratio, 1.57; 95% confidence interval, 1.08-2.26).
“Pregnant surgeons should not be operating more than 12 hours a week when they are in the third trimester,” Dr. Rangel said.
“That is a modifiable risk factor,” she told this news organization. “It’s a very brief period of support – a couple of months of support for a woman who may do 25-30 more years of serving the public with surgical skills.”
She said that training programs should be organized so as to have colleagues cover operating room (OR) shifts to reduce the operating hours for pregnant colleagues. In addition, advanced practice health care professionals should be paid to take up the paperwork and perform non-OR care to reduce the stigma associated with pregnant trainees overburdening other surgical trainees.
‘It’s too big an ask’
Obstetrician-gynecologist Maryam Siddiqui, MD, said in an interview that she was particularly struck by the number of female surgeons who experience involuntary childlessness.
“That’s a big ask for people who want childbearing to be a part of the fulfillment of their life. It’s too big,” said Dr. Siddiqui, a gynecologic surgeon at UChicago Medicine.
She said the amount of detail in the article and the large number of participants were persuasive factors that can support establishing a more humane system than one in which one person at a time has to ask for change.
Pointing to the finding that three-fourths of the women in the study who had had miscarriages didn’t take time off, she said, “That’s not really humane. But they’re afraid to ask or they don’t want to reveal they’re trying [to get pregnant]. Why should you be afraid of building your family?”
The authors also found other adverse outcomes. Female surgeons were more likely to have musculoskeletal disorders compared with female nonsurgeon partners (36.9% versus 18.4%; P < .001), and they were more likely to undergo nonelective cesarean delivery (25.5% versus 15.3%; P = .01) and to experience postpartum depression (11.1% versus 5.7%; P = .04).
Dr. Siddiqui said the conditions that surgeons encounter on their return to work after childbirth are “a perfect storm” for postpartum depression among women who are not accustomed to being reliant on others.
Women often feel coerced into returning to work before they are physically or emotionally ready, then toggle back and forth from night shift to day shift, losing sleep, she said. “We can do better.”
One of the solutions, she said, is to provide better work coverage for the surgeon while she is pregnant and when she returns to work. That includes properly compensating the person covering for the surgeon by giving that person extra pay or additional time off.
“You have to value both people,” she said. “If both people are valued, there’s still collegiality.”
She acknowledged that that kind of compensation may be more readily available at large academic centers.
At UChicago, she said, they are creative with scheduling in training. For women at the height of pregnancy, rotations are less intensive, and trauma rotations are avoided.
Dr. Siddiqui said one of the most important aspects of the article is the authors’ list of two dozen ways, both big and small, to improve conditions.
Adopting such changes will become increasingly important for hiring and retaining female surgeons. “You want to work someplace where you’re respected as a whole person,” she said.
Sarah Blair, MD, a surgical oncologist at University of California, San Diego, stated that the number of miscarriages in particular provides disturbing proof of a problem women in surgery frequently discuss.
For nearly a decade, she led a women-in-surgery committee at UCSD in which they discussed such issues regarding pregnancy and medicine.
She said she hopes these data can help push for change in flexibility in residency so that women can graduate on time and have the families they want.
“There’s a movement away from time-based training to competency-based training, so maybe that will help women,” she said.
‘We have to figure this out’
“We will have to figure this out, because more than half of the people in medical school are women, and there are a lot more women in surgery than when I trained more than 20 years ago. It’s not a problem that’s going away,” she said.
One sign of improvement happened recently, Dr. Rangel said.
As previously reported, according to the American Board of Medical Specialties, as of July 1, 2021, residents and fellows are allowed a minimum 6 weeks away for medical leave or caregiving once during training, without having to use vacation time or sick leave and without having to extend their training.
“That’s huge,” she said. “But we still have a long way to go, because the residency programs still don’t have to have policy that abides that. It merely says you can take 6 weeks off and take your boards. It doesn’t say that the residency program has to allow you to take 6 weeks off.”
The authors noted that the United States and Papua New Guinea are the only countries in the world without federally mandated paid parental leave.
“Most U.S. female surgeons rely on their employer for this benefit, but only half of top-ranked medical schools offer paid leave, and 33%-65% of U.S. surgical training programs lack clear maternity leave policies,” she said.
Funding for the study was provided by the department of surgery at Brigham and Women’s Hospital. The study authors, Dr. Blair, and Dr. Siddiqui have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– according to an article published online July 28 in JAMA Surgery.
The authors, led by Erika L. Rangel, MD, division of general and gastrointestinal surgery, department of surgery, Brigham and Women’s Hospital, Boston, found that after the losses, the women took little or no time off.
Of 692 surgeons surveyed, 347 female surgeons had experienced a pregnancy loss. Of those, 244 had had a miscarriage at less than 10 weeks’ gestation, 92 had had a miscarriage between 10 and 20 weeks’ gestation, and 11 had had a stillbirth (loss at 20 weeks or later).
Most took no time off after miscarriage
After a miscarriage, 225 of 336 women (75%) took no time off work, and after a stillbirth, 5 of 11 (45%) took off 1 week or less, the authors found.
The study addressed an issue that people have talked about anecdotally or on social media, Dr. Rangel told this news organization.
“This was finally an opportunity to do a study of enough magnitude to show that there is a very quantifiable difference in complication rate, use of IVF [in vitro fertilization], and the age at which we have children. These are not just anecdotal stories,” she said.
For the study, a self-administered questionnaire was distributed electronically. Answers were collected from November 2020 to January 2021 through multiple U.S. surgical societies and social media among attending and resident surgeons with children. The control group for the study comprised 158 male surgeons who answered questions regarding their partners’ pregnancies.
Female surgeons had fewer children compared with male surgeons and their female partners (mean [SD],1.8 [0.8], versus 2.3 [1.1]; P < .001) and were more likely to delay having children because of surgical training (450 of 692 [65.0%] versus 69 of 158 [43.7%]; P < .001).
In addition, Dr. Rangel and colleagues found that 57% of female surgeons worked more than 60 hours a week during pregnancy and that 37% took more than six overnight calls.
The data show that female surgeons who operated 12 or more hours per week during the last trimester of pregnancy were at higher risk compared with those who operated fewer hours (odds ratio, 1.57; 95% confidence interval, 1.08-2.26).
“Pregnant surgeons should not be operating more than 12 hours a week when they are in the third trimester,” Dr. Rangel said.
“That is a modifiable risk factor,” she told this news organization. “It’s a very brief period of support – a couple of months of support for a woman who may do 25-30 more years of serving the public with surgical skills.”
She said that training programs should be organized so as to have colleagues cover operating room (OR) shifts to reduce the operating hours for pregnant colleagues. In addition, advanced practice health care professionals should be paid to take up the paperwork and perform non-OR care to reduce the stigma associated with pregnant trainees overburdening other surgical trainees.
‘It’s too big an ask’
Obstetrician-gynecologist Maryam Siddiqui, MD, said in an interview that she was particularly struck by the number of female surgeons who experience involuntary childlessness.
“That’s a big ask for people who want childbearing to be a part of the fulfillment of their life. It’s too big,” said Dr. Siddiqui, a gynecologic surgeon at UChicago Medicine.
She said the amount of detail in the article and the large number of participants were persuasive factors that can support establishing a more humane system than one in which one person at a time has to ask for change.
Pointing to the finding that three-fourths of the women in the study who had had miscarriages didn’t take time off, she said, “That’s not really humane. But they’re afraid to ask or they don’t want to reveal they’re trying [to get pregnant]. Why should you be afraid of building your family?”
The authors also found other adverse outcomes. Female surgeons were more likely to have musculoskeletal disorders compared with female nonsurgeon partners (36.9% versus 18.4%; P < .001), and they were more likely to undergo nonelective cesarean delivery (25.5% versus 15.3%; P = .01) and to experience postpartum depression (11.1% versus 5.7%; P = .04).
Dr. Siddiqui said the conditions that surgeons encounter on their return to work after childbirth are “a perfect storm” for postpartum depression among women who are not accustomed to being reliant on others.
Women often feel coerced into returning to work before they are physically or emotionally ready, then toggle back and forth from night shift to day shift, losing sleep, she said. “We can do better.”
One of the solutions, she said, is to provide better work coverage for the surgeon while she is pregnant and when she returns to work. That includes properly compensating the person covering for the surgeon by giving that person extra pay or additional time off.
“You have to value both people,” she said. “If both people are valued, there’s still collegiality.”
She acknowledged that that kind of compensation may be more readily available at large academic centers.
At UChicago, she said, they are creative with scheduling in training. For women at the height of pregnancy, rotations are less intensive, and trauma rotations are avoided.
Dr. Siddiqui said one of the most important aspects of the article is the authors’ list of two dozen ways, both big and small, to improve conditions.
Adopting such changes will become increasingly important for hiring and retaining female surgeons. “You want to work someplace where you’re respected as a whole person,” she said.
Sarah Blair, MD, a surgical oncologist at University of California, San Diego, stated that the number of miscarriages in particular provides disturbing proof of a problem women in surgery frequently discuss.
For nearly a decade, she led a women-in-surgery committee at UCSD in which they discussed such issues regarding pregnancy and medicine.
She said she hopes these data can help push for change in flexibility in residency so that women can graduate on time and have the families they want.
“There’s a movement away from time-based training to competency-based training, so maybe that will help women,” she said.
‘We have to figure this out’
“We will have to figure this out, because more than half of the people in medical school are women, and there are a lot more women in surgery than when I trained more than 20 years ago. It’s not a problem that’s going away,” she said.
One sign of improvement happened recently, Dr. Rangel said.
As previously reported, according to the American Board of Medical Specialties, as of July 1, 2021, residents and fellows are allowed a minimum 6 weeks away for medical leave or caregiving once during training, without having to use vacation time or sick leave and without having to extend their training.
“That’s huge,” she said. “But we still have a long way to go, because the residency programs still don’t have to have policy that abides that. It merely says you can take 6 weeks off and take your boards. It doesn’t say that the residency program has to allow you to take 6 weeks off.”
The authors noted that the United States and Papua New Guinea are the only countries in the world without federally mandated paid parental leave.
“Most U.S. female surgeons rely on their employer for this benefit, but only half of top-ranked medical schools offer paid leave, and 33%-65% of U.S. surgical training programs lack clear maternity leave policies,” she said.
Funding for the study was provided by the department of surgery at Brigham and Women’s Hospital. The study authors, Dr. Blair, and Dr. Siddiqui have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hyperimmune globulin fails to prevent congenital CMV infection
Administering hyperimmune globulin to pregnant women who tested positive for cytomegalovirus did not reduce CMV infections or deaths among their fetuses or newborns, according to a randomized controlled trial published online July 28 in the New England Journal of Medicine.
Up to 40,000 infants a year have congenital CMV infections, which can lead to stillbirth, neonatal death, deafness, and cognitive and motor delay. An estimated 35%-40% of fetuses of women with a primary CMV infection will develop an infection, write Brenna Hughes, MD, an associate professor of ob/gyn and chief of the division of maternal fetal medicine at Duke University, Durham, N.C., and colleagues.
Previous trials and observational studies have shown mixed results with hyperimmune globulin for the prevention of congenital CMV infection.
“It was surprising to us that none of the outcomes in this trial were in the direction of potential benefit,” Dr. Hughes told this news organization. “However, this is why it is important to do large trials in a diverse population.”
The study cohort comprised 206,082 pregnant women who were screened for CMV infection before 23 weeks’ gestation. Of those women, 712 (0.35%) tested positive for CMV. The researchers enrolled 399 women who had tested positive and randomly assigned them to receive either a monthly infusion of CMV hyperimmune globulin (100 mg/kg) or placebo until delivery. The researchers used a composite of CMV infection or, if no testing occurred, fetal/neonatal death as the primary endpoint.
The trial was stopped early for futility when data from 394 participants revealed that 22.7% of offspring in the hyperimmune globulin group and 19.4% of those in the placebo group had had a CMV infection or had died (relative risk = 1.17; P = .42).
When individual endpoints were examined, trends were detected in favor of the placebo, but they did not reach statistical significance. The incidence of death was higher in the hyperimmune globulin group (4.9%) than in the placebo group (2.6%). The rate of preterm birth was also higher in the intervention group (12.2%) than in the group that received placebo (8.3%). The incidence of birth weight below the fifth percentile was 10.3% in the intervention group and 5.4% in the placebo group.
One woman who received hyperimmune globulin experienced a severe allergic reaction to the first infusion. Additionally, more women in the hyperimmune globulin group experienced headaches and shaking chills during infusions than did those who received placebo. There were no differences in maternal outcomes between the groups. There were no thromboembolic or ischemic events in either group.
“These findings suggest CMV hyperimmune globulin should not be used for the prevention of congenital CMV in pregnant patients with primary CMV during pregnancy,” Dr. Hughes said in an interview.
“A CMV vaccine is likely to be the most effective public health measure that we can offer, and that should be at the forefront of research investments,” she said. “But some of the other medications that work against CMV should be tested on a large scale as well,” she said. For example, a small trial in Israel showed that high-dose valacyclovir in early pregnancy decreased congenital CMV, and thus the drug merits study in a larger trial, she said.
Other experts agree that developing a vaccine should be the priority.
“The ultimate goal for preventing the brain damage and birth defects caused by congenital CMV infection is a vaccine that is as effective as the rubella vaccine has been for eliminating congenital rubella syndrome and that can be given well before pregnancy,” said Sallie Permar, MD, PhD, chair of pediatrics at Weill Cornell Medicine and pediatrician-in-chief at New York–Presbyterian/Weill Cornell Medical Center and the New York–Presbyterian Komansky Children’s Hospital in New York.
“While trials of vaccines are ongoing, there is a need to have a therapeutic option, especially for the high-risk setting of a mother acquiring the virus for the first time during pregnancy,” Dr. Permar said in an interview.
Dr. Permar was not involved in this study but is involved in follow-up studies of this cohort and is conducting research on CMV maternal vaccines. She noted the need for safe, effective antiviral treatments and for research into newer immunoglobulin products, such as monoclonal antibodies.
Both Dr. Permar and Dr. Hughes highlighted the challenge of raising awareness about the danger of CMV infections during pregnancy.
“Pregnant women, and especially those who have or work with young children, who are frequently carriers of the infection, should be informed of this risk,” Dr. Permar said. She hopes universal testing of newborns will be implemented and that it enables people to recognize the frequency and burden of these infections. She remains optimistic about a vaccine.
“After 60 years of research into a CMV vaccine, I believe we are currently in a ‘golden age’ of CMV vaccine development,” she said. She noted that Moderna is about to launch a phase 3 mRNA vaccine trial for CMV. “Moreover, immune correlates of protection against CMV have been identified from previous partially effective vaccines, and animal models have improved for preclinical studies. Therefore, I believe we will have an effective and safe vaccine against this most common congenital infection in the coming years.”
The research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Advancing Translational Sciences. Dr. Hughes has served on Merck’s scientific advisory board. Various coauthors have received personal fees from Medela and nonfinancial support from Hologic; personal fees from Moderna and VBI vaccines, and grants from Novavax. Dr. Permar consults for Pfizer, Moderna, Merck, Sanofi, and Dynavax on their CMV vaccine programs, and she has a sponsored research program with Merck and Moderna on CMV vaccines.
A version of this article first appeared on Medscape.com.
Administering hyperimmune globulin to pregnant women who tested positive for cytomegalovirus did not reduce CMV infections or deaths among their fetuses or newborns, according to a randomized controlled trial published online July 28 in the New England Journal of Medicine.
Up to 40,000 infants a year have congenital CMV infections, which can lead to stillbirth, neonatal death, deafness, and cognitive and motor delay. An estimated 35%-40% of fetuses of women with a primary CMV infection will develop an infection, write Brenna Hughes, MD, an associate professor of ob/gyn and chief of the division of maternal fetal medicine at Duke University, Durham, N.C., and colleagues.
Previous trials and observational studies have shown mixed results with hyperimmune globulin for the prevention of congenital CMV infection.
“It was surprising to us that none of the outcomes in this trial were in the direction of potential benefit,” Dr. Hughes told this news organization. “However, this is why it is important to do large trials in a diverse population.”
The study cohort comprised 206,082 pregnant women who were screened for CMV infection before 23 weeks’ gestation. Of those women, 712 (0.35%) tested positive for CMV. The researchers enrolled 399 women who had tested positive and randomly assigned them to receive either a monthly infusion of CMV hyperimmune globulin (100 mg/kg) or placebo until delivery. The researchers used a composite of CMV infection or, if no testing occurred, fetal/neonatal death as the primary endpoint.
The trial was stopped early for futility when data from 394 participants revealed that 22.7% of offspring in the hyperimmune globulin group and 19.4% of those in the placebo group had had a CMV infection or had died (relative risk = 1.17; P = .42).
When individual endpoints were examined, trends were detected in favor of the placebo, but they did not reach statistical significance. The incidence of death was higher in the hyperimmune globulin group (4.9%) than in the placebo group (2.6%). The rate of preterm birth was also higher in the intervention group (12.2%) than in the group that received placebo (8.3%). The incidence of birth weight below the fifth percentile was 10.3% in the intervention group and 5.4% in the placebo group.
One woman who received hyperimmune globulin experienced a severe allergic reaction to the first infusion. Additionally, more women in the hyperimmune globulin group experienced headaches and shaking chills during infusions than did those who received placebo. There were no differences in maternal outcomes between the groups. There were no thromboembolic or ischemic events in either group.
“These findings suggest CMV hyperimmune globulin should not be used for the prevention of congenital CMV in pregnant patients with primary CMV during pregnancy,” Dr. Hughes said in an interview.
“A CMV vaccine is likely to be the most effective public health measure that we can offer, and that should be at the forefront of research investments,” she said. “But some of the other medications that work against CMV should be tested on a large scale as well,” she said. For example, a small trial in Israel showed that high-dose valacyclovir in early pregnancy decreased congenital CMV, and thus the drug merits study in a larger trial, she said.
Other experts agree that developing a vaccine should be the priority.
“The ultimate goal for preventing the brain damage and birth defects caused by congenital CMV infection is a vaccine that is as effective as the rubella vaccine has been for eliminating congenital rubella syndrome and that can be given well before pregnancy,” said Sallie Permar, MD, PhD, chair of pediatrics at Weill Cornell Medicine and pediatrician-in-chief at New York–Presbyterian/Weill Cornell Medical Center and the New York–Presbyterian Komansky Children’s Hospital in New York.
“While trials of vaccines are ongoing, there is a need to have a therapeutic option, especially for the high-risk setting of a mother acquiring the virus for the first time during pregnancy,” Dr. Permar said in an interview.
Dr. Permar was not involved in this study but is involved in follow-up studies of this cohort and is conducting research on CMV maternal vaccines. She noted the need for safe, effective antiviral treatments and for research into newer immunoglobulin products, such as monoclonal antibodies.
Both Dr. Permar and Dr. Hughes highlighted the challenge of raising awareness about the danger of CMV infections during pregnancy.
“Pregnant women, and especially those who have or work with young children, who are frequently carriers of the infection, should be informed of this risk,” Dr. Permar said. She hopes universal testing of newborns will be implemented and that it enables people to recognize the frequency and burden of these infections. She remains optimistic about a vaccine.
“After 60 years of research into a CMV vaccine, I believe we are currently in a ‘golden age’ of CMV vaccine development,” she said. She noted that Moderna is about to launch a phase 3 mRNA vaccine trial for CMV. “Moreover, immune correlates of protection against CMV have been identified from previous partially effective vaccines, and animal models have improved for preclinical studies. Therefore, I believe we will have an effective and safe vaccine against this most common congenital infection in the coming years.”
The research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Advancing Translational Sciences. Dr. Hughes has served on Merck’s scientific advisory board. Various coauthors have received personal fees from Medela and nonfinancial support from Hologic; personal fees from Moderna and VBI vaccines, and grants from Novavax. Dr. Permar consults for Pfizer, Moderna, Merck, Sanofi, and Dynavax on their CMV vaccine programs, and she has a sponsored research program with Merck and Moderna on CMV vaccines.
A version of this article first appeared on Medscape.com.
Administering hyperimmune globulin to pregnant women who tested positive for cytomegalovirus did not reduce CMV infections or deaths among their fetuses or newborns, according to a randomized controlled trial published online July 28 in the New England Journal of Medicine.
Up to 40,000 infants a year have congenital CMV infections, which can lead to stillbirth, neonatal death, deafness, and cognitive and motor delay. An estimated 35%-40% of fetuses of women with a primary CMV infection will develop an infection, write Brenna Hughes, MD, an associate professor of ob/gyn and chief of the division of maternal fetal medicine at Duke University, Durham, N.C., and colleagues.
Previous trials and observational studies have shown mixed results with hyperimmune globulin for the prevention of congenital CMV infection.
“It was surprising to us that none of the outcomes in this trial were in the direction of potential benefit,” Dr. Hughes told this news organization. “However, this is why it is important to do large trials in a diverse population.”
The study cohort comprised 206,082 pregnant women who were screened for CMV infection before 23 weeks’ gestation. Of those women, 712 (0.35%) tested positive for CMV. The researchers enrolled 399 women who had tested positive and randomly assigned them to receive either a monthly infusion of CMV hyperimmune globulin (100 mg/kg) or placebo until delivery. The researchers used a composite of CMV infection or, if no testing occurred, fetal/neonatal death as the primary endpoint.
The trial was stopped early for futility when data from 394 participants revealed that 22.7% of offspring in the hyperimmune globulin group and 19.4% of those in the placebo group had had a CMV infection or had died (relative risk = 1.17; P = .42).
When individual endpoints were examined, trends were detected in favor of the placebo, but they did not reach statistical significance. The incidence of death was higher in the hyperimmune globulin group (4.9%) than in the placebo group (2.6%). The rate of preterm birth was also higher in the intervention group (12.2%) than in the group that received placebo (8.3%). The incidence of birth weight below the fifth percentile was 10.3% in the intervention group and 5.4% in the placebo group.
One woman who received hyperimmune globulin experienced a severe allergic reaction to the first infusion. Additionally, more women in the hyperimmune globulin group experienced headaches and shaking chills during infusions than did those who received placebo. There were no differences in maternal outcomes between the groups. There were no thromboembolic or ischemic events in either group.
“These findings suggest CMV hyperimmune globulin should not be used for the prevention of congenital CMV in pregnant patients with primary CMV during pregnancy,” Dr. Hughes said in an interview.
“A CMV vaccine is likely to be the most effective public health measure that we can offer, and that should be at the forefront of research investments,” she said. “But some of the other medications that work against CMV should be tested on a large scale as well,” she said. For example, a small trial in Israel showed that high-dose valacyclovir in early pregnancy decreased congenital CMV, and thus the drug merits study in a larger trial, she said.
Other experts agree that developing a vaccine should be the priority.
“The ultimate goal for preventing the brain damage and birth defects caused by congenital CMV infection is a vaccine that is as effective as the rubella vaccine has been for eliminating congenital rubella syndrome and that can be given well before pregnancy,” said Sallie Permar, MD, PhD, chair of pediatrics at Weill Cornell Medicine and pediatrician-in-chief at New York–Presbyterian/Weill Cornell Medical Center and the New York–Presbyterian Komansky Children’s Hospital in New York.
“While trials of vaccines are ongoing, there is a need to have a therapeutic option, especially for the high-risk setting of a mother acquiring the virus for the first time during pregnancy,” Dr. Permar said in an interview.
Dr. Permar was not involved in this study but is involved in follow-up studies of this cohort and is conducting research on CMV maternal vaccines. She noted the need for safe, effective antiviral treatments and for research into newer immunoglobulin products, such as monoclonal antibodies.
Both Dr. Permar and Dr. Hughes highlighted the challenge of raising awareness about the danger of CMV infections during pregnancy.
“Pregnant women, and especially those who have or work with young children, who are frequently carriers of the infection, should be informed of this risk,” Dr. Permar said. She hopes universal testing of newborns will be implemented and that it enables people to recognize the frequency and burden of these infections. She remains optimistic about a vaccine.
“After 60 years of research into a CMV vaccine, I believe we are currently in a ‘golden age’ of CMV vaccine development,” she said. She noted that Moderna is about to launch a phase 3 mRNA vaccine trial for CMV. “Moreover, immune correlates of protection against CMV have been identified from previous partially effective vaccines, and animal models have improved for preclinical studies. Therefore, I believe we will have an effective and safe vaccine against this most common congenital infection in the coming years.”
The research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Advancing Translational Sciences. Dr. Hughes has served on Merck’s scientific advisory board. Various coauthors have received personal fees from Medela and nonfinancial support from Hologic; personal fees from Moderna and VBI vaccines, and grants from Novavax. Dr. Permar consults for Pfizer, Moderna, Merck, Sanofi, and Dynavax on their CMV vaccine programs, and she has a sponsored research program with Merck and Moderna on CMV vaccines.
A version of this article first appeared on Medscape.com.
Hematologic cancer increases risk of delivery complications
The risk of in-hospital complications and poor birth outcomes were greater in pregnant women with current or historical cancer diagnoses, new research suggests.
The study, published in Mayo Clinic Proceedings, found that women with current and historical cancer diagnoses had an increased risk of death, kidney injury, and stroke during delivery hospitalizations, compared with those with no cancer. When it came to delivery outcomes, this group also had a higher risk for preterm birth and postpartum hemorrhage. Those with a current cancer diagnoses had a 1.7-fold increase in odds for a preterm birth, compared with women without cancer.
“Our study found that metastases increased the odds of mortality, cesarean delivery, preterm birth, and stillbirth,” the researchers noted. “Coupled with previous research reporting that pregnant women are more likely to be diagnosed with advanced disease, this implies that pregnant women with newly diagnosed cancer have poor prognoses.”
However, although women with prior cancer had increased odds of mortality, the researchers said it was not statistically significant.
“The study really did not show an increase of mortality [for women with prior cancer diagnosis],” said Justin Chura, MD, a specialist in gynecologic oncology who was not involved in the study. “And the reason might be because there is not or the reason might be because it’s such a rare event. You would need 100 million births to assess that. So I would actually use caution in that interpretation.”
Researchers analyzed more than 43 million delivery hospitalizations of women with or without current or historical cancer diagnoses between January 2004 and December 2014. They found that the most common cancer diagnoses were hematologic, thyroid, cervical, skin, and breast.
Of the five most common cancers, the prevalence of all maternal complications and negative delivery outcomes was the highest among women with hematologic cancers. They were more likely to experience peripartum cardiomyopathy, acute kidney injury, and arrhythmia, compared with other cancers. Postpartum hemorrhage, maternal mortality, and placental abruption was also more likely to occur in those with this type of cancer.
“I was surprised that it was the hematologic cancers that were worse when they did it by cancer type,” said Dr. Chura, who is the chief of surgery and the director of gynecologic oncology and robotic surgery at the Cancer Treatment Centers of America’s Eastern Regional Medical Center in Philadelphia. “I think this is a useful bit of information for counseling our patients and also to identify the cohort with the highest risk.”
The findings also suggested that those with skin cancer had the highest odds for stroke, while women with cervical and breast cancers were more likely to experience acute kidney injury and preterm birth.
Dr. Chura said cancer treatments can have an impact on a woman’s health when she’s giving birth. For example, if a woman is diagnosed with cervical cancer, doctors may perform a cone biopsy on her where they remove a large portion of the cervix and still leave them with the ability to conceive and become pregnant. However, those patients are left with a higher risk of a preterm delivery.
For women with a hematologic cancer like non-Hodgkin’s lymphoma, chest radiation may cause some subsequent damage to their heart muscles “and now the stress of pregnancy puts more demand on the heart that can lead to cardiac complications for that patient,” Dr. Chura said.
“There are potential long-term effects from radiation and chemotherapy,” Dr. Chura said.
Previous studies have shown that chemotherapy may affect pregnancy and delivery. A 2019 study published in the Journal of Cancer also found that 59 pregnant women with cancer had increased mortality compared with those without the long-term illness. Meanwhile, another 2018 study published in Cancer found that women who conceived less than a year after starting chemotherapy had higher risks of preterm birth in comparison with those who conceived more than a year after starting chemotherapy. The study also found that cancer survivors who conceived more than a year after finishing chemotherapy with or without radiation had no higher risk of a preterm birth than those without cancer.
Dr. Chura said the new study could force doctors to think about the long-term effects of their cancer therapies and make them more apt to think about how to make cancer therapy less toxic with less long-term health consequences, while still curing patients.
“Most oncologists, when dealing with younger patients, are very focused on curing the cancer at hand, but not necessarily thinking 5 or 10 years down the road,” Dr. Chura said. “[This study] could help inform or at least make us aware of the long-term consequences of our cancer therapies.”
Dr. Chura had no relevant financial disclosures.
The risk of in-hospital complications and poor birth outcomes were greater in pregnant women with current or historical cancer diagnoses, new research suggests.
The study, published in Mayo Clinic Proceedings, found that women with current and historical cancer diagnoses had an increased risk of death, kidney injury, and stroke during delivery hospitalizations, compared with those with no cancer. When it came to delivery outcomes, this group also had a higher risk for preterm birth and postpartum hemorrhage. Those with a current cancer diagnoses had a 1.7-fold increase in odds for a preterm birth, compared with women without cancer.
“Our study found that metastases increased the odds of mortality, cesarean delivery, preterm birth, and stillbirth,” the researchers noted. “Coupled with previous research reporting that pregnant women are more likely to be diagnosed with advanced disease, this implies that pregnant women with newly diagnosed cancer have poor prognoses.”
However, although women with prior cancer had increased odds of mortality, the researchers said it was not statistically significant.
“The study really did not show an increase of mortality [for women with prior cancer diagnosis],” said Justin Chura, MD, a specialist in gynecologic oncology who was not involved in the study. “And the reason might be because there is not or the reason might be because it’s such a rare event. You would need 100 million births to assess that. So I would actually use caution in that interpretation.”
Researchers analyzed more than 43 million delivery hospitalizations of women with or without current or historical cancer diagnoses between January 2004 and December 2014. They found that the most common cancer diagnoses were hematologic, thyroid, cervical, skin, and breast.
Of the five most common cancers, the prevalence of all maternal complications and negative delivery outcomes was the highest among women with hematologic cancers. They were more likely to experience peripartum cardiomyopathy, acute kidney injury, and arrhythmia, compared with other cancers. Postpartum hemorrhage, maternal mortality, and placental abruption was also more likely to occur in those with this type of cancer.
“I was surprised that it was the hematologic cancers that were worse when they did it by cancer type,” said Dr. Chura, who is the chief of surgery and the director of gynecologic oncology and robotic surgery at the Cancer Treatment Centers of America’s Eastern Regional Medical Center in Philadelphia. “I think this is a useful bit of information for counseling our patients and also to identify the cohort with the highest risk.”
The findings also suggested that those with skin cancer had the highest odds for stroke, while women with cervical and breast cancers were more likely to experience acute kidney injury and preterm birth.
Dr. Chura said cancer treatments can have an impact on a woman’s health when she’s giving birth. For example, if a woman is diagnosed with cervical cancer, doctors may perform a cone biopsy on her where they remove a large portion of the cervix and still leave them with the ability to conceive and become pregnant. However, those patients are left with a higher risk of a preterm delivery.
For women with a hematologic cancer like non-Hodgkin’s lymphoma, chest radiation may cause some subsequent damage to their heart muscles “and now the stress of pregnancy puts more demand on the heart that can lead to cardiac complications for that patient,” Dr. Chura said.
“There are potential long-term effects from radiation and chemotherapy,” Dr. Chura said.
Previous studies have shown that chemotherapy may affect pregnancy and delivery. A 2019 study published in the Journal of Cancer also found that 59 pregnant women with cancer had increased mortality compared with those without the long-term illness. Meanwhile, another 2018 study published in Cancer found that women who conceived less than a year after starting chemotherapy had higher risks of preterm birth in comparison with those who conceived more than a year after starting chemotherapy. The study also found that cancer survivors who conceived more than a year after finishing chemotherapy with or without radiation had no higher risk of a preterm birth than those without cancer.
Dr. Chura said the new study could force doctors to think about the long-term effects of their cancer therapies and make them more apt to think about how to make cancer therapy less toxic with less long-term health consequences, while still curing patients.
“Most oncologists, when dealing with younger patients, are very focused on curing the cancer at hand, but not necessarily thinking 5 or 10 years down the road,” Dr. Chura said. “[This study] could help inform or at least make us aware of the long-term consequences of our cancer therapies.”
Dr. Chura had no relevant financial disclosures.
The risk of in-hospital complications and poor birth outcomes were greater in pregnant women with current or historical cancer diagnoses, new research suggests.
The study, published in Mayo Clinic Proceedings, found that women with current and historical cancer diagnoses had an increased risk of death, kidney injury, and stroke during delivery hospitalizations, compared with those with no cancer. When it came to delivery outcomes, this group also had a higher risk for preterm birth and postpartum hemorrhage. Those with a current cancer diagnoses had a 1.7-fold increase in odds for a preterm birth, compared with women without cancer.
“Our study found that metastases increased the odds of mortality, cesarean delivery, preterm birth, and stillbirth,” the researchers noted. “Coupled with previous research reporting that pregnant women are more likely to be diagnosed with advanced disease, this implies that pregnant women with newly diagnosed cancer have poor prognoses.”
However, although women with prior cancer had increased odds of mortality, the researchers said it was not statistically significant.
“The study really did not show an increase of mortality [for women with prior cancer diagnosis],” said Justin Chura, MD, a specialist in gynecologic oncology who was not involved in the study. “And the reason might be because there is not or the reason might be because it’s such a rare event. You would need 100 million births to assess that. So I would actually use caution in that interpretation.”
Researchers analyzed more than 43 million delivery hospitalizations of women with or without current or historical cancer diagnoses between January 2004 and December 2014. They found that the most common cancer diagnoses were hematologic, thyroid, cervical, skin, and breast.
Of the five most common cancers, the prevalence of all maternal complications and negative delivery outcomes was the highest among women with hematologic cancers. They were more likely to experience peripartum cardiomyopathy, acute kidney injury, and arrhythmia, compared with other cancers. Postpartum hemorrhage, maternal mortality, and placental abruption was also more likely to occur in those with this type of cancer.
“I was surprised that it was the hematologic cancers that were worse when they did it by cancer type,” said Dr. Chura, who is the chief of surgery and the director of gynecologic oncology and robotic surgery at the Cancer Treatment Centers of America’s Eastern Regional Medical Center in Philadelphia. “I think this is a useful bit of information for counseling our patients and also to identify the cohort with the highest risk.”
The findings also suggested that those with skin cancer had the highest odds for stroke, while women with cervical and breast cancers were more likely to experience acute kidney injury and preterm birth.
Dr. Chura said cancer treatments can have an impact on a woman’s health when she’s giving birth. For example, if a woman is diagnosed with cervical cancer, doctors may perform a cone biopsy on her where they remove a large portion of the cervix and still leave them with the ability to conceive and become pregnant. However, those patients are left with a higher risk of a preterm delivery.
For women with a hematologic cancer like non-Hodgkin’s lymphoma, chest radiation may cause some subsequent damage to their heart muscles “and now the stress of pregnancy puts more demand on the heart that can lead to cardiac complications for that patient,” Dr. Chura said.
“There are potential long-term effects from radiation and chemotherapy,” Dr. Chura said.
Previous studies have shown that chemotherapy may affect pregnancy and delivery. A 2019 study published in the Journal of Cancer also found that 59 pregnant women with cancer had increased mortality compared with those without the long-term illness. Meanwhile, another 2018 study published in Cancer found that women who conceived less than a year after starting chemotherapy had higher risks of preterm birth in comparison with those who conceived more than a year after starting chemotherapy. The study also found that cancer survivors who conceived more than a year after finishing chemotherapy with or without radiation had no higher risk of a preterm birth than those without cancer.
Dr. Chura said the new study could force doctors to think about the long-term effects of their cancer therapies and make them more apt to think about how to make cancer therapy less toxic with less long-term health consequences, while still curing patients.
“Most oncologists, when dealing with younger patients, are very focused on curing the cancer at hand, but not necessarily thinking 5 or 10 years down the road,” Dr. Chura said. “[This study] could help inform or at least make us aware of the long-term consequences of our cancer therapies.”
Dr. Chura had no relevant financial disclosures.
FROM MAYO CLINIC PROCEEDINGS
FDA to revise statin pregnancy contraindication
The U.S. Food and Drug Administration (FDA) aims to update the labeling on all statins to remove the drugs’ blanket contraindication in all pregnant patients, the agency has announced. The change should reinforce for both physicians and patients that statin use in women with unrecognized pregnancy is unlikely to be harmful, it said.
“Because the benefits of statins may include prevention of serious or potentially fatal events in a small group of very high-risk pregnant patients, contraindicating these drugs in all pregnant women is not appropriate.”
The revision should emphasize for clinicians “that statins are safe to prescribe in patients who can become pregnant and help them reassure patients with unintended statin exposure in early pregnancy,” the FDA explained.
Removal of the broadly worded contraindication should “enable health care professionals and patients to make individual decisions about benefit and risk, especially for those at very high risk of heart attack or stroke." That includes women with homozygous familial hypercholesterolemia and those who are prescribed statins for secondary prevention, the agency said.
Clinicians “should discontinue statin therapy in most pregnant patients, or they can consider the ongoing therapeutic needs of the individual patient, particularly those at very high risk for cardiovascular events during pregnancy. Because of the chronic nature of cardiovascular disease, treatment of hyperlipidemia is not generally necessary during pregnancy.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration (FDA) aims to update the labeling on all statins to remove the drugs’ blanket contraindication in all pregnant patients, the agency has announced. The change should reinforce for both physicians and patients that statin use in women with unrecognized pregnancy is unlikely to be harmful, it said.
“Because the benefits of statins may include prevention of serious or potentially fatal events in a small group of very high-risk pregnant patients, contraindicating these drugs in all pregnant women is not appropriate.”
The revision should emphasize for clinicians “that statins are safe to prescribe in patients who can become pregnant and help them reassure patients with unintended statin exposure in early pregnancy,” the FDA explained.
Removal of the broadly worded contraindication should “enable health care professionals and patients to make individual decisions about benefit and risk, especially for those at very high risk of heart attack or stroke." That includes women with homozygous familial hypercholesterolemia and those who are prescribed statins for secondary prevention, the agency said.
Clinicians “should discontinue statin therapy in most pregnant patients, or they can consider the ongoing therapeutic needs of the individual patient, particularly those at very high risk for cardiovascular events during pregnancy. Because of the chronic nature of cardiovascular disease, treatment of hyperlipidemia is not generally necessary during pregnancy.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration (FDA) aims to update the labeling on all statins to remove the drugs’ blanket contraindication in all pregnant patients, the agency has announced. The change should reinforce for both physicians and patients that statin use in women with unrecognized pregnancy is unlikely to be harmful, it said.
“Because the benefits of statins may include prevention of serious or potentially fatal events in a small group of very high-risk pregnant patients, contraindicating these drugs in all pregnant women is not appropriate.”
The revision should emphasize for clinicians “that statins are safe to prescribe in patients who can become pregnant and help them reassure patients with unintended statin exposure in early pregnancy,” the FDA explained.
Removal of the broadly worded contraindication should “enable health care professionals and patients to make individual decisions about benefit and risk, especially for those at very high risk of heart attack or stroke." That includes women with homozygous familial hypercholesterolemia and those who are prescribed statins for secondary prevention, the agency said.
Clinicians “should discontinue statin therapy in most pregnant patients, or they can consider the ongoing therapeutic needs of the individual patient, particularly those at very high risk for cardiovascular events during pregnancy. Because of the chronic nature of cardiovascular disease, treatment of hyperlipidemia is not generally necessary during pregnancy.”
A version of this article first appeared on Medscape.com.




