User login
COVID-19 may increase risk of preterm birth and cesarean delivery
Among 57 hospitalized patients with SARS-CoV-2 infection who underwent vaginal or cesarean delivery, 7 had spontaneous preterm or respiratory-indicated preterm delivery, a rate of 12%, according to a study published in Obstetrics & Gynecology. For comparison, 7% of patients had preterm delivery in 2019, researchers reported “We also noted a high cesarean delivery rate in the study population (39% vs. 27% in the same area in 2019), mainly as a result of maternal respiratory-indicated urgent delivery,” wrote Valeria M. Savasi, MD, PhD, of the University of Milan and Luigi Sacco Hospital, also in Milan, and colleagues.
Data do not indicate that pregnant women are more susceptible to severe COVID-19 infection, nor have studies suggested an increased risk of miscarriage, congenital anomalies, or early pregnancy loss in pregnant patients with COVID-19, the authors wrote. Studies have described an increased risk of preterm birth, however.
To study clinical features of maternal SARS-CoV-2 infection and potential factors associated with severe disease and iatrogenic delivery, Dr. Savasi and colleagues conducted a prospective study of 77 women with laboratory-confirmed SARS-CoV-2 infection who were admitted during pregnancy or the immediate postpartum period in 12 maternity hospitals in northern Italy between Feb. 23 and March 28, 2020.
The investigators classified patients as having severe disease if they underwent urgent delivery based on maternal respiratory function or if they were admitted to an ICU or subintensive care department. In all, 14 patients (18%) were classified as having severe disease.
“Three patients were intubated after emergency cesarean delivery performed for maternal deterioration, and one patient underwent extracorporeal membrane oxygenation,” Dr. Savasi and colleagues reported. The results are consistent with epidemiologic data in the nonpregnant population with COVID-19 disease.
Of 11 patients with severe disease who underwent urgent delivery for respiratory compromise, 6 had significant postpartum improvement in clinical conditions. No maternal deaths occurred.
“Increased BMI [body mass index] was a significant risk factor for severe disease,” Dr. Savasi and colleagues wrote. “Fever and dyspnea on admission were symptoms significantly associated with subsequent severe maternal respiratory deterioration.”
Most patients (65%) were admitted during the third trimester, and 20 patients were still pregnant at discharge.
“Nine newborns were admitted to the neonatal intensive care unit,” the authors wrote. “Interestingly, besides prematurity, fetal oxygenation and well-being at delivery were not apparently affected by the maternal acute conditions.” Three newborns with vaginal delivery and one with cesarean delivery tested positive for SARS-CoV-2. The newborns may have been infected after delivery, Dr. Savasi and colleagues added. For all newborns, rooming-in and breastfeeding were performed, and none developed respiratory symptoms.
Criteria for hospital admission and therapeutic protocols may have varied between hospitals, the authors noted. In addition, the study included 12 patients who were asymptomatic and admitted for obstetric indications. These patients were tested for SARS-CoV-2 because of contact with an infected individual. Most patients were symptomatic, however, which explains the high rate of maternal severe outcomes. Hospitals have since adopted a universal SARS-CoV-2 screening policy for hospitalized pregnant patients.
Kristina Adams Waldorf, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, commented in an interview that Savasi et al. describe one of the larger COVID-19 in pregnancy cohorts to date with rates of severe disease and delivery for respiratory compromise, which is remarkably similar to Washington state (severe disease, 18% vs. nearly 15%; delivery for respiratory compromise, 16% vs. 20%). As in Washington state, Italian women with a higher prepregnancy BMI were overrepresented in the severe disease group.
“Data are beginning to emerge that identify women who were overweight or obese prior to pregnancy as a high risk group for developing severe COVID-19. These data are similar to known associations between obesity and critical illness in pregnancy during the 2009 ‘swine flu’ (influenza A virus, H1N1) pandemic,” she said.
“This study and others indicate that the late second and third trimesters may be a time when women are more likely to be symptomatic from COVID-19. It remains unclear if women in the first trimester are protected from severe COVID-19 outcomes or have outcomes similar to nonpregnant women,” concluded Dr. Waldorf.
One study author disclosed receiving funds from Lo Li Pharma and Zambongroup. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Savasi VM et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003979.
Among 57 hospitalized patients with SARS-CoV-2 infection who underwent vaginal or cesarean delivery, 7 had spontaneous preterm or respiratory-indicated preterm delivery, a rate of 12%, according to a study published in Obstetrics & Gynecology. For comparison, 7% of patients had preterm delivery in 2019, researchers reported “We also noted a high cesarean delivery rate in the study population (39% vs. 27% in the same area in 2019), mainly as a result of maternal respiratory-indicated urgent delivery,” wrote Valeria M. Savasi, MD, PhD, of the University of Milan and Luigi Sacco Hospital, also in Milan, and colleagues.
Data do not indicate that pregnant women are more susceptible to severe COVID-19 infection, nor have studies suggested an increased risk of miscarriage, congenital anomalies, or early pregnancy loss in pregnant patients with COVID-19, the authors wrote. Studies have described an increased risk of preterm birth, however.
To study clinical features of maternal SARS-CoV-2 infection and potential factors associated with severe disease and iatrogenic delivery, Dr. Savasi and colleagues conducted a prospective study of 77 women with laboratory-confirmed SARS-CoV-2 infection who were admitted during pregnancy or the immediate postpartum period in 12 maternity hospitals in northern Italy between Feb. 23 and March 28, 2020.
The investigators classified patients as having severe disease if they underwent urgent delivery based on maternal respiratory function or if they were admitted to an ICU or subintensive care department. In all, 14 patients (18%) were classified as having severe disease.
“Three patients were intubated after emergency cesarean delivery performed for maternal deterioration, and one patient underwent extracorporeal membrane oxygenation,” Dr. Savasi and colleagues reported. The results are consistent with epidemiologic data in the nonpregnant population with COVID-19 disease.
Of 11 patients with severe disease who underwent urgent delivery for respiratory compromise, 6 had significant postpartum improvement in clinical conditions. No maternal deaths occurred.
“Increased BMI [body mass index] was a significant risk factor for severe disease,” Dr. Savasi and colleagues wrote. “Fever and dyspnea on admission were symptoms significantly associated with subsequent severe maternal respiratory deterioration.”
Most patients (65%) were admitted during the third trimester, and 20 patients were still pregnant at discharge.
“Nine newborns were admitted to the neonatal intensive care unit,” the authors wrote. “Interestingly, besides prematurity, fetal oxygenation and well-being at delivery were not apparently affected by the maternal acute conditions.” Three newborns with vaginal delivery and one with cesarean delivery tested positive for SARS-CoV-2. The newborns may have been infected after delivery, Dr. Savasi and colleagues added. For all newborns, rooming-in and breastfeeding were performed, and none developed respiratory symptoms.
Criteria for hospital admission and therapeutic protocols may have varied between hospitals, the authors noted. In addition, the study included 12 patients who were asymptomatic and admitted for obstetric indications. These patients were tested for SARS-CoV-2 because of contact with an infected individual. Most patients were symptomatic, however, which explains the high rate of maternal severe outcomes. Hospitals have since adopted a universal SARS-CoV-2 screening policy for hospitalized pregnant patients.
Kristina Adams Waldorf, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, commented in an interview that Savasi et al. describe one of the larger COVID-19 in pregnancy cohorts to date with rates of severe disease and delivery for respiratory compromise, which is remarkably similar to Washington state (severe disease, 18% vs. nearly 15%; delivery for respiratory compromise, 16% vs. 20%). As in Washington state, Italian women with a higher prepregnancy BMI were overrepresented in the severe disease group.
“Data are beginning to emerge that identify women who were overweight or obese prior to pregnancy as a high risk group for developing severe COVID-19. These data are similar to known associations between obesity and critical illness in pregnancy during the 2009 ‘swine flu’ (influenza A virus, H1N1) pandemic,” she said.
“This study and others indicate that the late second and third trimesters may be a time when women are more likely to be symptomatic from COVID-19. It remains unclear if women in the first trimester are protected from severe COVID-19 outcomes or have outcomes similar to nonpregnant women,” concluded Dr. Waldorf.
One study author disclosed receiving funds from Lo Li Pharma and Zambongroup. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Savasi VM et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003979.
Among 57 hospitalized patients with SARS-CoV-2 infection who underwent vaginal or cesarean delivery, 7 had spontaneous preterm or respiratory-indicated preterm delivery, a rate of 12%, according to a study published in Obstetrics & Gynecology. For comparison, 7% of patients had preterm delivery in 2019, researchers reported “We also noted a high cesarean delivery rate in the study population (39% vs. 27% in the same area in 2019), mainly as a result of maternal respiratory-indicated urgent delivery,” wrote Valeria M. Savasi, MD, PhD, of the University of Milan and Luigi Sacco Hospital, also in Milan, and colleagues.
Data do not indicate that pregnant women are more susceptible to severe COVID-19 infection, nor have studies suggested an increased risk of miscarriage, congenital anomalies, or early pregnancy loss in pregnant patients with COVID-19, the authors wrote. Studies have described an increased risk of preterm birth, however.
To study clinical features of maternal SARS-CoV-2 infection and potential factors associated with severe disease and iatrogenic delivery, Dr. Savasi and colleagues conducted a prospective study of 77 women with laboratory-confirmed SARS-CoV-2 infection who were admitted during pregnancy or the immediate postpartum period in 12 maternity hospitals in northern Italy between Feb. 23 and March 28, 2020.
The investigators classified patients as having severe disease if they underwent urgent delivery based on maternal respiratory function or if they were admitted to an ICU or subintensive care department. In all, 14 patients (18%) were classified as having severe disease.
“Three patients were intubated after emergency cesarean delivery performed for maternal deterioration, and one patient underwent extracorporeal membrane oxygenation,” Dr. Savasi and colleagues reported. The results are consistent with epidemiologic data in the nonpregnant population with COVID-19 disease.
Of 11 patients with severe disease who underwent urgent delivery for respiratory compromise, 6 had significant postpartum improvement in clinical conditions. No maternal deaths occurred.
“Increased BMI [body mass index] was a significant risk factor for severe disease,” Dr. Savasi and colleagues wrote. “Fever and dyspnea on admission were symptoms significantly associated with subsequent severe maternal respiratory deterioration.”
Most patients (65%) were admitted during the third trimester, and 20 patients were still pregnant at discharge.
“Nine newborns were admitted to the neonatal intensive care unit,” the authors wrote. “Interestingly, besides prematurity, fetal oxygenation and well-being at delivery were not apparently affected by the maternal acute conditions.” Three newborns with vaginal delivery and one with cesarean delivery tested positive for SARS-CoV-2. The newborns may have been infected after delivery, Dr. Savasi and colleagues added. For all newborns, rooming-in and breastfeeding were performed, and none developed respiratory symptoms.
Criteria for hospital admission and therapeutic protocols may have varied between hospitals, the authors noted. In addition, the study included 12 patients who were asymptomatic and admitted for obstetric indications. These patients were tested for SARS-CoV-2 because of contact with an infected individual. Most patients were symptomatic, however, which explains the high rate of maternal severe outcomes. Hospitals have since adopted a universal SARS-CoV-2 screening policy for hospitalized pregnant patients.
Kristina Adams Waldorf, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, commented in an interview that Savasi et al. describe one of the larger COVID-19 in pregnancy cohorts to date with rates of severe disease and delivery for respiratory compromise, which is remarkably similar to Washington state (severe disease, 18% vs. nearly 15%; delivery for respiratory compromise, 16% vs. 20%). As in Washington state, Italian women with a higher prepregnancy BMI were overrepresented in the severe disease group.
“Data are beginning to emerge that identify women who were overweight or obese prior to pregnancy as a high risk group for developing severe COVID-19. These data are similar to known associations between obesity and critical illness in pregnancy during the 2009 ‘swine flu’ (influenza A virus, H1N1) pandemic,” she said.
“This study and others indicate that the late second and third trimesters may be a time when women are more likely to be symptomatic from COVID-19. It remains unclear if women in the first trimester are protected from severe COVID-19 outcomes or have outcomes similar to nonpregnant women,” concluded Dr. Waldorf.
One study author disclosed receiving funds from Lo Li Pharma and Zambongroup. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Savasi VM et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003979.
FROM OBSTETRICS & GYNECOLOGY
Improving care for women who have experienced stillbirth
Think of the current standard of care and do the opposite
One of hardest parts of being an obstetrician is taking care of patients who experience a stillbirth. I am very comfortable with the care of a grieving patient and I always have been, although I am not sure why. I have a model of care that I have evolved in my 16 years since medical school graduation. This model is not based on formal instruction because I received none, but on my natural instincts of what a grieving mom and her family need to hear and receive in the worst moments of their lives. All obstetrics providers grieve the loss of the baby, but often not with the patient but on our own. We may do this because we want to respect the patient’s privacy or because we are not sure of the words to say. I hope I can provide some guidance for those who struggle with what to do.
I delivered my first stillborn baby as a third-year medical student. My mentor, a chief resident, saw something in me and encouraged me to care for this mother. She had twins and one baby was still living, but the prognosis was poor since this was the surviving twin from a monochorionic diamniotic pregnancy. On the day of the mom’s induction, I just pulled up a chair and talked with her. We talked about her life, the loss of the first baby several weeks before, and her hope that her surviving son would be okay. She felt so bonded to me that she refused to push until I was there. Her delivery is still firm in my mind. I still remember 17 years later the room she delivered in.
My first loss (stillbirth) as a resident was my intern year – a beautiful baby named Jude, who was stillborn at 39 weeks. After delivering Jude, I asked the family about the funeral arrangements. Three days later, I attended his funeral. I looked all around for the mother’s attending doctors but none of them were there. I remember thinking then that it was a given that they would be there, but now I know that it is rare. I also learned a lot about the grief a stillborn baby brings while listening to Jude’s father’s eulogy. He talked about how Jude would never bake cookies with Aunt Jane, ride the slide with cousin Chris, or put on a yellow backpack and ride the bus on the first day of school. Because of this eulogy, I understood this unique kind of grief that these losses bring early in my training.
I delivered many losses in my residency. The attendings left soon after the birth and I stayed behind with the family. I sat and counseled the families, and I helped them make memories. I realized that to care for these patients, I would need to trust my instincts because there was no formal and little informal training on how to care for families who lost their babies.
Once I completed residency, I was really able to do my “thing.” At loss deliveries, I was able to model for residents my method of care. I showed them that families want and need attention, support, and guidance. I modeled for them how to deliver and greet the baby. That it is not necessary to leave the room right after the birth, and it is okay to grieve and help families meet their babies. I modeled commenting on the baby’s features, on who they looked like. I showed how laughing about how the baby has grandma’s nose is okay. I showed them that it is okay to ask to hold and get a picture taken with the baby. I showed them that these are the only moments these families will get with their babies, and it is our job to help them do this. The family will have many moments alone in the days and weeks to come. They need our support and guidance. It is a part of being an ob.gyn. to care for families after stillbirths, and we do not want our patients to feel abandoned during this time by those they entrust to care for them.
I also was able to create a model for aftercare. I call my families often after they go home. Sometimes I catch them in the anger stage of the grief process and I let them vent. I work through this with them, and I answer their hardest and sometimes accusatory questions regarding care leading up to the diagnosis. I am not saying this is easy for me or for them. I think fear of these tough conversations is a barrier to giving the emotional support that these families need. I work through this with them in an honest and open manner. I also call to check on the patients as much as possible, especially on anniversaries. I am not saying that all providers must follow this model. This is my passion and is natural for me, but data clearly show that the standard of emotional care we provide is not what patients need at this time. Thankfully, there is an amazing resource of grief counselors, social workers, online resources, and support groups for these families to help them get through the tragedy. These resources, however, are not the provider who spent this precious time with them and their beloved baby, and our emotional support is invaluable.
This past year has been very eventful for me. One of my patients delivered a new baby, after a prior loss, and asked if we could teach together. I had mentioned that everything I do with stillbirths is not based on my residency education, but on my experience and instinctual feeling of what families need. She knew from friends in bereavement circles that they felt that their care was different. We started teaching last summer and have done 10 training sessions to date; hopefully we will continue to teach new groups of nurses, residents, medical students, doulas, and physician assistant students each year.
This year also was eventful because I discovered the Star Legacy Foundation, a national not-for-profit organization with the goal of spreading awareness, education, and prevention regarding stillbirth. I attended their 2019 Summit in Minnesota. I thought I would meet many more doctors and midwives like myself, and I would learn even more about care for bereaved patients. However, that summer I learned preventing stillbirth may be possible from the then chief medical officer of Scotland, Catherine Calderwood, MB ChB. She talked about the preventive protocol she had created that had reduced the stillbirth rate by 23%. Because I was one of only five ob.gyn. nonspeaker attendees in a room of 400, I realized I had a real opportunity to try to bring some model for prevention to the United States. I brought the U.K. protocol to my practice and we have been doing it now for 9 months. (See “Decreased fetal movement: Time to educate patients and ourselves” at mdedge.com/pediatrics.)
I have had a year to think about why the U.S. stillbirth rate is higher than that of many high-income nations and why we have the lowest annual rate of reduction in the 2016 Lancet series among high resource nations.1 I think it is due to lack of education and training for providers in stillbirth prevention and care, which has led to further marginalization and stigmatization of bereaved moms. This has pushed them further into the shadows and makes it taboo to share their stories. It is providers being fearful to even mention to patients that stillbirth still happens. It is the lack of any protocol on how to educate patients and providers about fetal movement, and what to do if pregnant women complain about a decrease or change in fetal movement. I think a lot of this stems from an innate discomfort that obstetric providers have in the care of these patients. That if women felt cared for and empowered to tell their stories, there would be more efforts at stillbirth education and prevention.
I often think of an experience that the founder of Star Legacy, Lindsey Wimmer, experienced when she lost her son, Garrett, 16 years ago. She told a story in the documentary, “Don’t talk about the baby.” She tells that on the first night of the induction, the nurse came in and told her that the attending wanted to turn off the oxytocin so “she could get her rest.” I heard this and immediately knew the attending’s true reason for turning off the oxytocin. Lindsey then said she knew it was because the attending did not want to wake up to deliver a dead baby. I wrote Lindsey that day and told her I completely agreed and apologized on behalf of my profession for that care. She wrote me back that she had waited 16 years to have a provider validate her feelings about this. I told her I think her doctor was fearful and uncomfortable with this birth and was avoiding it, but I believe with better education and training this can change. I want to deliver babies like Garrett during my shift, because it is giving this vital care that reminds me why I became a doctor in the first place.
I know there are many providers out there who follow a similar model, but I want more providers to do so, and so does the bereavement community. In one study of 20 parents, all but 2 were frustrated about how the ob.gyn. and staff handled their deliveries.2 I truly believe that every person who delivers babies does it because they love it. Part of doing this job we love is realizing there will be times of great sadness. I also believe if this model of care is attempted by wary providers, they will quickly realize that this is what patients and their families need. With this care, stillbirth may become less of a taboo subject, and our stillbirth rate may fall.
Dr. Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, N.Y. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures. Email her a [email protected].
References
1. “Stillbirths 2016: ending preventable stillbirths.” Series from The Lancet journals. Published: Jan. 20, 2016.
2. BMC Pregnancy Childbirth. 2012 Nov 27. doi: 10.1186/1471-2393-12-137.
Think of the current standard of care and do the opposite
Think of the current standard of care and do the opposite
One of hardest parts of being an obstetrician is taking care of patients who experience a stillbirth. I am very comfortable with the care of a grieving patient and I always have been, although I am not sure why. I have a model of care that I have evolved in my 16 years since medical school graduation. This model is not based on formal instruction because I received none, but on my natural instincts of what a grieving mom and her family need to hear and receive in the worst moments of their lives. All obstetrics providers grieve the loss of the baby, but often not with the patient but on our own. We may do this because we want to respect the patient’s privacy or because we are not sure of the words to say. I hope I can provide some guidance for those who struggle with what to do.
I delivered my first stillborn baby as a third-year medical student. My mentor, a chief resident, saw something in me and encouraged me to care for this mother. She had twins and one baby was still living, but the prognosis was poor since this was the surviving twin from a monochorionic diamniotic pregnancy. On the day of the mom’s induction, I just pulled up a chair and talked with her. We talked about her life, the loss of the first baby several weeks before, and her hope that her surviving son would be okay. She felt so bonded to me that she refused to push until I was there. Her delivery is still firm in my mind. I still remember 17 years later the room she delivered in.
My first loss (stillbirth) as a resident was my intern year – a beautiful baby named Jude, who was stillborn at 39 weeks. After delivering Jude, I asked the family about the funeral arrangements. Three days later, I attended his funeral. I looked all around for the mother’s attending doctors but none of them were there. I remember thinking then that it was a given that they would be there, but now I know that it is rare. I also learned a lot about the grief a stillborn baby brings while listening to Jude’s father’s eulogy. He talked about how Jude would never bake cookies with Aunt Jane, ride the slide with cousin Chris, or put on a yellow backpack and ride the bus on the first day of school. Because of this eulogy, I understood this unique kind of grief that these losses bring early in my training.
I delivered many losses in my residency. The attendings left soon after the birth and I stayed behind with the family. I sat and counseled the families, and I helped them make memories. I realized that to care for these patients, I would need to trust my instincts because there was no formal and little informal training on how to care for families who lost their babies.
Once I completed residency, I was really able to do my “thing.” At loss deliveries, I was able to model for residents my method of care. I showed them that families want and need attention, support, and guidance. I modeled for them how to deliver and greet the baby. That it is not necessary to leave the room right after the birth, and it is okay to grieve and help families meet their babies. I modeled commenting on the baby’s features, on who they looked like. I showed how laughing about how the baby has grandma’s nose is okay. I showed them that it is okay to ask to hold and get a picture taken with the baby. I showed them that these are the only moments these families will get with their babies, and it is our job to help them do this. The family will have many moments alone in the days and weeks to come. They need our support and guidance. It is a part of being an ob.gyn. to care for families after stillbirths, and we do not want our patients to feel abandoned during this time by those they entrust to care for them.
I also was able to create a model for aftercare. I call my families often after they go home. Sometimes I catch them in the anger stage of the grief process and I let them vent. I work through this with them, and I answer their hardest and sometimes accusatory questions regarding care leading up to the diagnosis. I am not saying this is easy for me or for them. I think fear of these tough conversations is a barrier to giving the emotional support that these families need. I work through this with them in an honest and open manner. I also call to check on the patients as much as possible, especially on anniversaries. I am not saying that all providers must follow this model. This is my passion and is natural for me, but data clearly show that the standard of emotional care we provide is not what patients need at this time. Thankfully, there is an amazing resource of grief counselors, social workers, online resources, and support groups for these families to help them get through the tragedy. These resources, however, are not the provider who spent this precious time with them and their beloved baby, and our emotional support is invaluable.
This past year has been very eventful for me. One of my patients delivered a new baby, after a prior loss, and asked if we could teach together. I had mentioned that everything I do with stillbirths is not based on my residency education, but on my experience and instinctual feeling of what families need. She knew from friends in bereavement circles that they felt that their care was different. We started teaching last summer and have done 10 training sessions to date; hopefully we will continue to teach new groups of nurses, residents, medical students, doulas, and physician assistant students each year.
This year also was eventful because I discovered the Star Legacy Foundation, a national not-for-profit organization with the goal of spreading awareness, education, and prevention regarding stillbirth. I attended their 2019 Summit in Minnesota. I thought I would meet many more doctors and midwives like myself, and I would learn even more about care for bereaved patients. However, that summer I learned preventing stillbirth may be possible from the then chief medical officer of Scotland, Catherine Calderwood, MB ChB. She talked about the preventive protocol she had created that had reduced the stillbirth rate by 23%. Because I was one of only five ob.gyn. nonspeaker attendees in a room of 400, I realized I had a real opportunity to try to bring some model for prevention to the United States. I brought the U.K. protocol to my practice and we have been doing it now for 9 months. (See “Decreased fetal movement: Time to educate patients and ourselves” at mdedge.com/pediatrics.)
I have had a year to think about why the U.S. stillbirth rate is higher than that of many high-income nations and why we have the lowest annual rate of reduction in the 2016 Lancet series among high resource nations.1 I think it is due to lack of education and training for providers in stillbirth prevention and care, which has led to further marginalization and stigmatization of bereaved moms. This has pushed them further into the shadows and makes it taboo to share their stories. It is providers being fearful to even mention to patients that stillbirth still happens. It is the lack of any protocol on how to educate patients and providers about fetal movement, and what to do if pregnant women complain about a decrease or change in fetal movement. I think a lot of this stems from an innate discomfort that obstetric providers have in the care of these patients. That if women felt cared for and empowered to tell their stories, there would be more efforts at stillbirth education and prevention.
I often think of an experience that the founder of Star Legacy, Lindsey Wimmer, experienced when she lost her son, Garrett, 16 years ago. She told a story in the documentary, “Don’t talk about the baby.” She tells that on the first night of the induction, the nurse came in and told her that the attending wanted to turn off the oxytocin so “she could get her rest.” I heard this and immediately knew the attending’s true reason for turning off the oxytocin. Lindsey then said she knew it was because the attending did not want to wake up to deliver a dead baby. I wrote Lindsey that day and told her I completely agreed and apologized on behalf of my profession for that care. She wrote me back that she had waited 16 years to have a provider validate her feelings about this. I told her I think her doctor was fearful and uncomfortable with this birth and was avoiding it, but I believe with better education and training this can change. I want to deliver babies like Garrett during my shift, because it is giving this vital care that reminds me why I became a doctor in the first place.
I know there are many providers out there who follow a similar model, but I want more providers to do so, and so does the bereavement community. In one study of 20 parents, all but 2 were frustrated about how the ob.gyn. and staff handled their deliveries.2 I truly believe that every person who delivers babies does it because they love it. Part of doing this job we love is realizing there will be times of great sadness. I also believe if this model of care is attempted by wary providers, they will quickly realize that this is what patients and their families need. With this care, stillbirth may become less of a taboo subject, and our stillbirth rate may fall.
Dr. Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, N.Y. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures. Email her a [email protected].
References
1. “Stillbirths 2016: ending preventable stillbirths.” Series from The Lancet journals. Published: Jan. 20, 2016.
2. BMC Pregnancy Childbirth. 2012 Nov 27. doi: 10.1186/1471-2393-12-137.
One of hardest parts of being an obstetrician is taking care of patients who experience a stillbirth. I am very comfortable with the care of a grieving patient and I always have been, although I am not sure why. I have a model of care that I have evolved in my 16 years since medical school graduation. This model is not based on formal instruction because I received none, but on my natural instincts of what a grieving mom and her family need to hear and receive in the worst moments of their lives. All obstetrics providers grieve the loss of the baby, but often not with the patient but on our own. We may do this because we want to respect the patient’s privacy or because we are not sure of the words to say. I hope I can provide some guidance for those who struggle with what to do.
I delivered my first stillborn baby as a third-year medical student. My mentor, a chief resident, saw something in me and encouraged me to care for this mother. She had twins and one baby was still living, but the prognosis was poor since this was the surviving twin from a monochorionic diamniotic pregnancy. On the day of the mom’s induction, I just pulled up a chair and talked with her. We talked about her life, the loss of the first baby several weeks before, and her hope that her surviving son would be okay. She felt so bonded to me that she refused to push until I was there. Her delivery is still firm in my mind. I still remember 17 years later the room she delivered in.
My first loss (stillbirth) as a resident was my intern year – a beautiful baby named Jude, who was stillborn at 39 weeks. After delivering Jude, I asked the family about the funeral arrangements. Three days later, I attended his funeral. I looked all around for the mother’s attending doctors but none of them were there. I remember thinking then that it was a given that they would be there, but now I know that it is rare. I also learned a lot about the grief a stillborn baby brings while listening to Jude’s father’s eulogy. He talked about how Jude would never bake cookies with Aunt Jane, ride the slide with cousin Chris, or put on a yellow backpack and ride the bus on the first day of school. Because of this eulogy, I understood this unique kind of grief that these losses bring early in my training.
I delivered many losses in my residency. The attendings left soon after the birth and I stayed behind with the family. I sat and counseled the families, and I helped them make memories. I realized that to care for these patients, I would need to trust my instincts because there was no formal and little informal training on how to care for families who lost their babies.
Once I completed residency, I was really able to do my “thing.” At loss deliveries, I was able to model for residents my method of care. I showed them that families want and need attention, support, and guidance. I modeled for them how to deliver and greet the baby. That it is not necessary to leave the room right after the birth, and it is okay to grieve and help families meet their babies. I modeled commenting on the baby’s features, on who they looked like. I showed how laughing about how the baby has grandma’s nose is okay. I showed them that it is okay to ask to hold and get a picture taken with the baby. I showed them that these are the only moments these families will get with their babies, and it is our job to help them do this. The family will have many moments alone in the days and weeks to come. They need our support and guidance. It is a part of being an ob.gyn. to care for families after stillbirths, and we do not want our patients to feel abandoned during this time by those they entrust to care for them.
I also was able to create a model for aftercare. I call my families often after they go home. Sometimes I catch them in the anger stage of the grief process and I let them vent. I work through this with them, and I answer their hardest and sometimes accusatory questions regarding care leading up to the diagnosis. I am not saying this is easy for me or for them. I think fear of these tough conversations is a barrier to giving the emotional support that these families need. I work through this with them in an honest and open manner. I also call to check on the patients as much as possible, especially on anniversaries. I am not saying that all providers must follow this model. This is my passion and is natural for me, but data clearly show that the standard of emotional care we provide is not what patients need at this time. Thankfully, there is an amazing resource of grief counselors, social workers, online resources, and support groups for these families to help them get through the tragedy. These resources, however, are not the provider who spent this precious time with them and their beloved baby, and our emotional support is invaluable.
This past year has been very eventful for me. One of my patients delivered a new baby, after a prior loss, and asked if we could teach together. I had mentioned that everything I do with stillbirths is not based on my residency education, but on my experience and instinctual feeling of what families need. She knew from friends in bereavement circles that they felt that their care was different. We started teaching last summer and have done 10 training sessions to date; hopefully we will continue to teach new groups of nurses, residents, medical students, doulas, and physician assistant students each year.
This year also was eventful because I discovered the Star Legacy Foundation, a national not-for-profit organization with the goal of spreading awareness, education, and prevention regarding stillbirth. I attended their 2019 Summit in Minnesota. I thought I would meet many more doctors and midwives like myself, and I would learn even more about care for bereaved patients. However, that summer I learned preventing stillbirth may be possible from the then chief medical officer of Scotland, Catherine Calderwood, MB ChB. She talked about the preventive protocol she had created that had reduced the stillbirth rate by 23%. Because I was one of only five ob.gyn. nonspeaker attendees in a room of 400, I realized I had a real opportunity to try to bring some model for prevention to the United States. I brought the U.K. protocol to my practice and we have been doing it now for 9 months. (See “Decreased fetal movement: Time to educate patients and ourselves” at mdedge.com/pediatrics.)
I have had a year to think about why the U.S. stillbirth rate is higher than that of many high-income nations and why we have the lowest annual rate of reduction in the 2016 Lancet series among high resource nations.1 I think it is due to lack of education and training for providers in stillbirth prevention and care, which has led to further marginalization and stigmatization of bereaved moms. This has pushed them further into the shadows and makes it taboo to share their stories. It is providers being fearful to even mention to patients that stillbirth still happens. It is the lack of any protocol on how to educate patients and providers about fetal movement, and what to do if pregnant women complain about a decrease or change in fetal movement. I think a lot of this stems from an innate discomfort that obstetric providers have in the care of these patients. That if women felt cared for and empowered to tell their stories, there would be more efforts at stillbirth education and prevention.
I often think of an experience that the founder of Star Legacy, Lindsey Wimmer, experienced when she lost her son, Garrett, 16 years ago. She told a story in the documentary, “Don’t talk about the baby.” She tells that on the first night of the induction, the nurse came in and told her that the attending wanted to turn off the oxytocin so “she could get her rest.” I heard this and immediately knew the attending’s true reason for turning off the oxytocin. Lindsey then said she knew it was because the attending did not want to wake up to deliver a dead baby. I wrote Lindsey that day and told her I completely agreed and apologized on behalf of my profession for that care. She wrote me back that she had waited 16 years to have a provider validate her feelings about this. I told her I think her doctor was fearful and uncomfortable with this birth and was avoiding it, but I believe with better education and training this can change. I want to deliver babies like Garrett during my shift, because it is giving this vital care that reminds me why I became a doctor in the first place.
I know there are many providers out there who follow a similar model, but I want more providers to do so, and so does the bereavement community. In one study of 20 parents, all but 2 were frustrated about how the ob.gyn. and staff handled their deliveries.2 I truly believe that every person who delivers babies does it because they love it. Part of doing this job we love is realizing there will be times of great sadness. I also believe if this model of care is attempted by wary providers, they will quickly realize that this is what patients and their families need. With this care, stillbirth may become less of a taboo subject, and our stillbirth rate may fall.
Dr. Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, N.Y. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures. Email her a [email protected].
References
1. “Stillbirths 2016: ending preventable stillbirths.” Series from The Lancet journals. Published: Jan. 20, 2016.
2. BMC Pregnancy Childbirth. 2012 Nov 27. doi: 10.1186/1471-2393-12-137.
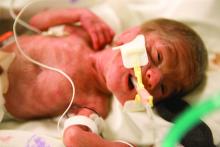
Placental injury reported in women with COVID-19
Neonates appear healthy so far
Maternal vascular malperfusion and intervillous thrombi were more common in the placentas of women infected with SARS-CoV-2, compared with historic controls, report researchers who conducted the first-of-its-kind case series in the English literature. Nevertheless, the neonates in the report appear to be healthy so far and all tested negative for the virus.
Although the series examining placentas from 16 women is small, it carries a larger implication – that increased antenatal surveillance for pregnant women infected with SARS-CoV-2 may be indicated, the researchers noted.
Furthermore, the results could align with other reports of coagulation and vascular abnormalities among people with COVID-19. “I would say that our findings fit into that larger picture of vascular injury. This is developing, and there are some significant ways that these feeder vessels to the placenta are different, but if this is the emerging paradigm, our findings can fit into it,” Jeffrey A. Goldstein, MD, PhD, assistant professor of pathology at Northwestern University, Chicago, said in an interview.
The research was published in the American Journal of Clinical Pathology.
Prior case series reported in Wuhan, China, do not currently suggest that pregnant women are more likely to experience severe COVID-19, in contrast to observations during severe acute respiratory syndrome and Middle East respiratory syndrome outbreaks. “However,” the researchers noted, “adverse perinatal outcomes have been reported, including increased risks of miscarriage, preeclampsia, preterm birth, and stillbirth.”
To learn more, Dr. Goldstein, lead author Elisheva D. Shanes, MD, and colleagues examined the histology of placentas from women with COVID-19 giving birth between March 18 and May 5, 2020. They compared these placentas with over 17,000 historic controls and 215 women who had their placentas evaluated as part of a melanoma history study.
A total of 10 women were diagnosed with COVID-19 upon presentation to labor and delivery, 4 others were diagnosed approximately 1 month before delivery and the remaining 2 within 1 week of delivery. Ten of the patients were symptomatic and two required oxygen. None of the patients received intubation or died. A total of 14 patients delivered at term, 1 delivered at 34 weeks, and the remaining case experienced a 16-week intrauterine fetal demise (IUFD). The IUFD was excluded from subsequent statistical analysis.
The neonates each had a 5-minute Apgar score of 9. Most infants were discharged on the first or second day of life, and there were no neonatal deaths.
Key findings
Of the 15 placentas, 12 featured maternal vascular malperfusion. This rate was significantly higher than historic controls (P = .046) and melanoma study controls (P = .001).
Specific features varied between groups, with decidual arteriopathy, atherosis and fibrinoid necrosis of maternal vessels, and mural hypertrophy of membrane arterioles observed more often in COVID-19 cases than in all historical controls. In addition, peripheral infarctions, decidual arteriopathy, atherosis, and fibrinoid necrosis, and mural hypertrophy being more common in COVID-19 cases than in placentas of women with a history of melanoma.
In contrast, features of fetal vascular malperfusion were observed in 12 of 15 cases, but not at rates significantly different from the control groups. Chorangiosis, villous edema, and intervillous thrombi also were more common in the COVID-19 cohort.
Dr. Goldstein was surprised they did not observe much acute or chronic inflammation. “We see chronic inflammation in the placenta in response to many viruses, such as cytomegalovirus, so you might expect similar findings, but we didn’t see any increase above the controls.”
There are a couple of case reports of histiocytic intervillositis – a particularly severe form of chronic inflammation – associated with COVID-19, “but we didn’t see that in our study,” he added.
Clinical implications
The healthy neonatal outcomes reported in the study occurred despite the placental injury, which may be caused by the redundancy built into placentas for delivering oxygen and nutrients and for removing waste.
The negative COVID-19 test results in all infants also supports existing evidence that vertical transmission of the virus is uncommon. The finding also suggests that any damage to the placenta is likely related to maternal infection.
Only one mother in the COVID-19 cohort was hypertensive, which surprised the researchers because intervillous thrombi have been associated with maternal high blood pressure. “In the context of research suggesting an increase of thrombotic and thromboembolic disorders in COVID-19,” the researchers noted, “these may represent placental formation or deposition of thrombi in response to the virus.”
One of the priorities for the researchers going forward is to monitor the longer-term outcomes of the infants, Dr. Goldstein said. “We know the people in utero during the 1918-1919 flu pandemic had higher rates of heart disease and other long-term problems, so we want to be on the lookout for something similar.”
Valuable insight
“This is a comprehensive case series of this topic, with findings worth noting and sharing in a timely fashion,” Karen Mestan, MD, associate professor of pediatrics within the division of neonatology at Northwestern University, said when asked to comment on the study.
“The information is valuable to neonatologists as the short- and long-term effects of COVID-19 exposure on newborn infants are still largely unknown,” she added. “Details of placental pathology provide emerging insight and may help us understand mother-baby vertical transmission during the current pandemic.”
Dr. Goldstein and Dr. Mestan had no relevant financial disclosures.
SOURCE: Shanes ED et al. Am J Clin Pathol. 2020 May 22. doi: 10.1093/ajcp/aqaa089.
Neonates appear healthy so far
Neonates appear healthy so far
Maternal vascular malperfusion and intervillous thrombi were more common in the placentas of women infected with SARS-CoV-2, compared with historic controls, report researchers who conducted the first-of-its-kind case series in the English literature. Nevertheless, the neonates in the report appear to be healthy so far and all tested negative for the virus.
Although the series examining placentas from 16 women is small, it carries a larger implication – that increased antenatal surveillance for pregnant women infected with SARS-CoV-2 may be indicated, the researchers noted.
Furthermore, the results could align with other reports of coagulation and vascular abnormalities among people with COVID-19. “I would say that our findings fit into that larger picture of vascular injury. This is developing, and there are some significant ways that these feeder vessels to the placenta are different, but if this is the emerging paradigm, our findings can fit into it,” Jeffrey A. Goldstein, MD, PhD, assistant professor of pathology at Northwestern University, Chicago, said in an interview.
The research was published in the American Journal of Clinical Pathology.
Prior case series reported in Wuhan, China, do not currently suggest that pregnant women are more likely to experience severe COVID-19, in contrast to observations during severe acute respiratory syndrome and Middle East respiratory syndrome outbreaks. “However,” the researchers noted, “adverse perinatal outcomes have been reported, including increased risks of miscarriage, preeclampsia, preterm birth, and stillbirth.”
To learn more, Dr. Goldstein, lead author Elisheva D. Shanes, MD, and colleagues examined the histology of placentas from women with COVID-19 giving birth between March 18 and May 5, 2020. They compared these placentas with over 17,000 historic controls and 215 women who had their placentas evaluated as part of a melanoma history study.
A total of 10 women were diagnosed with COVID-19 upon presentation to labor and delivery, 4 others were diagnosed approximately 1 month before delivery and the remaining 2 within 1 week of delivery. Ten of the patients were symptomatic and two required oxygen. None of the patients received intubation or died. A total of 14 patients delivered at term, 1 delivered at 34 weeks, and the remaining case experienced a 16-week intrauterine fetal demise (IUFD). The IUFD was excluded from subsequent statistical analysis.
The neonates each had a 5-minute Apgar score of 9. Most infants were discharged on the first or second day of life, and there were no neonatal deaths.
Key findings
Of the 15 placentas, 12 featured maternal vascular malperfusion. This rate was significantly higher than historic controls (P = .046) and melanoma study controls (P = .001).
Specific features varied between groups, with decidual arteriopathy, atherosis and fibrinoid necrosis of maternal vessels, and mural hypertrophy of membrane arterioles observed more often in COVID-19 cases than in all historical controls. In addition, peripheral infarctions, decidual arteriopathy, atherosis, and fibrinoid necrosis, and mural hypertrophy being more common in COVID-19 cases than in placentas of women with a history of melanoma.
In contrast, features of fetal vascular malperfusion were observed in 12 of 15 cases, but not at rates significantly different from the control groups. Chorangiosis, villous edema, and intervillous thrombi also were more common in the COVID-19 cohort.
Dr. Goldstein was surprised they did not observe much acute or chronic inflammation. “We see chronic inflammation in the placenta in response to many viruses, such as cytomegalovirus, so you might expect similar findings, but we didn’t see any increase above the controls.”
There are a couple of case reports of histiocytic intervillositis – a particularly severe form of chronic inflammation – associated with COVID-19, “but we didn’t see that in our study,” he added.
Clinical implications
The healthy neonatal outcomes reported in the study occurred despite the placental injury, which may be caused by the redundancy built into placentas for delivering oxygen and nutrients and for removing waste.
The negative COVID-19 test results in all infants also supports existing evidence that vertical transmission of the virus is uncommon. The finding also suggests that any damage to the placenta is likely related to maternal infection.
Only one mother in the COVID-19 cohort was hypertensive, which surprised the researchers because intervillous thrombi have been associated with maternal high blood pressure. “In the context of research suggesting an increase of thrombotic and thromboembolic disorders in COVID-19,” the researchers noted, “these may represent placental formation or deposition of thrombi in response to the virus.”
One of the priorities for the researchers going forward is to monitor the longer-term outcomes of the infants, Dr. Goldstein said. “We know the people in utero during the 1918-1919 flu pandemic had higher rates of heart disease and other long-term problems, so we want to be on the lookout for something similar.”
Valuable insight
“This is a comprehensive case series of this topic, with findings worth noting and sharing in a timely fashion,” Karen Mestan, MD, associate professor of pediatrics within the division of neonatology at Northwestern University, said when asked to comment on the study.
“The information is valuable to neonatologists as the short- and long-term effects of COVID-19 exposure on newborn infants are still largely unknown,” she added. “Details of placental pathology provide emerging insight and may help us understand mother-baby vertical transmission during the current pandemic.”
Dr. Goldstein and Dr. Mestan had no relevant financial disclosures.
SOURCE: Shanes ED et al. Am J Clin Pathol. 2020 May 22. doi: 10.1093/ajcp/aqaa089.
Maternal vascular malperfusion and intervillous thrombi were more common in the placentas of women infected with SARS-CoV-2, compared with historic controls, report researchers who conducted the first-of-its-kind case series in the English literature. Nevertheless, the neonates in the report appear to be healthy so far and all tested negative for the virus.
Although the series examining placentas from 16 women is small, it carries a larger implication – that increased antenatal surveillance for pregnant women infected with SARS-CoV-2 may be indicated, the researchers noted.
Furthermore, the results could align with other reports of coagulation and vascular abnormalities among people with COVID-19. “I would say that our findings fit into that larger picture of vascular injury. This is developing, and there are some significant ways that these feeder vessels to the placenta are different, but if this is the emerging paradigm, our findings can fit into it,” Jeffrey A. Goldstein, MD, PhD, assistant professor of pathology at Northwestern University, Chicago, said in an interview.
The research was published in the American Journal of Clinical Pathology.
Prior case series reported in Wuhan, China, do not currently suggest that pregnant women are more likely to experience severe COVID-19, in contrast to observations during severe acute respiratory syndrome and Middle East respiratory syndrome outbreaks. “However,” the researchers noted, “adverse perinatal outcomes have been reported, including increased risks of miscarriage, preeclampsia, preterm birth, and stillbirth.”
To learn more, Dr. Goldstein, lead author Elisheva D. Shanes, MD, and colleagues examined the histology of placentas from women with COVID-19 giving birth between March 18 and May 5, 2020. They compared these placentas with over 17,000 historic controls and 215 women who had their placentas evaluated as part of a melanoma history study.
A total of 10 women were diagnosed with COVID-19 upon presentation to labor and delivery, 4 others were diagnosed approximately 1 month before delivery and the remaining 2 within 1 week of delivery. Ten of the patients were symptomatic and two required oxygen. None of the patients received intubation or died. A total of 14 patients delivered at term, 1 delivered at 34 weeks, and the remaining case experienced a 16-week intrauterine fetal demise (IUFD). The IUFD was excluded from subsequent statistical analysis.
The neonates each had a 5-minute Apgar score of 9. Most infants were discharged on the first or second day of life, and there were no neonatal deaths.
Key findings
Of the 15 placentas, 12 featured maternal vascular malperfusion. This rate was significantly higher than historic controls (P = .046) and melanoma study controls (P = .001).
Specific features varied between groups, with decidual arteriopathy, atherosis and fibrinoid necrosis of maternal vessels, and mural hypertrophy of membrane arterioles observed more often in COVID-19 cases than in all historical controls. In addition, peripheral infarctions, decidual arteriopathy, atherosis, and fibrinoid necrosis, and mural hypertrophy being more common in COVID-19 cases than in placentas of women with a history of melanoma.
In contrast, features of fetal vascular malperfusion were observed in 12 of 15 cases, but not at rates significantly different from the control groups. Chorangiosis, villous edema, and intervillous thrombi also were more common in the COVID-19 cohort.
Dr. Goldstein was surprised they did not observe much acute or chronic inflammation. “We see chronic inflammation in the placenta in response to many viruses, such as cytomegalovirus, so you might expect similar findings, but we didn’t see any increase above the controls.”
There are a couple of case reports of histiocytic intervillositis – a particularly severe form of chronic inflammation – associated with COVID-19, “but we didn’t see that in our study,” he added.
Clinical implications
The healthy neonatal outcomes reported in the study occurred despite the placental injury, which may be caused by the redundancy built into placentas for delivering oxygen and nutrients and for removing waste.
The negative COVID-19 test results in all infants also supports existing evidence that vertical transmission of the virus is uncommon. The finding also suggests that any damage to the placenta is likely related to maternal infection.
Only one mother in the COVID-19 cohort was hypertensive, which surprised the researchers because intervillous thrombi have been associated with maternal high blood pressure. “In the context of research suggesting an increase of thrombotic and thromboembolic disorders in COVID-19,” the researchers noted, “these may represent placental formation or deposition of thrombi in response to the virus.”
One of the priorities for the researchers going forward is to monitor the longer-term outcomes of the infants, Dr. Goldstein said. “We know the people in utero during the 1918-1919 flu pandemic had higher rates of heart disease and other long-term problems, so we want to be on the lookout for something similar.”
Valuable insight
“This is a comprehensive case series of this topic, with findings worth noting and sharing in a timely fashion,” Karen Mestan, MD, associate professor of pediatrics within the division of neonatology at Northwestern University, said when asked to comment on the study.
“The information is valuable to neonatologists as the short- and long-term effects of COVID-19 exposure on newborn infants are still largely unknown,” she added. “Details of placental pathology provide emerging insight and may help us understand mother-baby vertical transmission during the current pandemic.”
Dr. Goldstein and Dr. Mestan had no relevant financial disclosures.
SOURCE: Shanes ED et al. Am J Clin Pathol. 2020 May 22. doi: 10.1093/ajcp/aqaa089.
FROM THE AMERICAN JOURNAL OF CLINICAL PATHOLOGY
SARS-CoV-2 infection rate 16% in asymptomatic pregnant women at delivery
Among women with a planned delivery in a New York City health system during the first half of April, the rate of asymptomatic SARS-CoV-2 infection was 16%, according to a study published in Obstetrics & Gynecology. Among the patients’ designated support persons, the asymptomatic carrier rate was 10%.
“If universal testing of pregnant patients in a high prevalence area is not performed, health care workers will be inadvertently exposed to COVID-19, unless universal precautions with personal protective equipment are taken,” wrote the researchers affiliated with the department of obstetrics, gynecology, and reproductive medicine at Icahn School of Medicine at Mount Sinai, New York.
Angela Bianco, MD, and colleagues conducted an observational study of women who were scheduled for a planned delivery within the Mount Sinai Health System between April 4 and April 15, 2020. Patients and their designated support person completed a telephone screen and underwent COVID-19 testing the day before a scheduled delivery. If support persons screened positive during the telephone interview about COVID-19 symptoms, they could not attend the birth, and patients could contact a different support person to be screened and tested. “All patients and their support persons were informed of their SARS-CoV-2 test results before admission,” the investigators wrote. “Those who tested positive were counseled regarding symptomatology that should prompt medical attention.”
In all, researchers screened 158 patients with a planned delivery, and 155 agreed to undergo COVID-19 testing. Of the 155 women tested, 24 (16%) tested positive for SARS CoV-2 infection. Among 146 support persons who had a negative interview screen and underwent SARS-CoV-2 testing, 14 (10%) tested positive for SARS-CoV-2 infection.
Test results were substantially concordant among patient and support person pairs. “Among patients who tested positive for COVID-19 infection and had a support person present, 11 of 19 (58%) support persons also tested positive for COVID-19 infection,” the authors reported. “Among patients who tested negative for COVID-19 infection and had a support person present, only 3 of 127 (2.4%) support persons tested positive for COVID-19 infection.”
Telephone screening did not identify any of the COVID-19–positive cases. Of the 24 patients with SARS-CoV-2 infection, none of their newborns tested positive at birth.
“Universal testing ... provides a mechanism for more accurate counseling of patients regarding issues such as newborn skin-to-skin contact and breastfeeding,” noted Dr. Bianco and colleagues. At their institution, parents with COVID-19 are instructed to wear a mask and practice proper hand hygiene when caring for their newborns.
Kristina Adams Waldorf, MD, said in an interview that the study by Bianco et al. underscores the high rate of asymptomatic or mildly symptomatic COVID-19 infections detected with universal screening in a hospital at the U.S. epicenter of the pandemic. “Each state and hospital will need to evaluate their own data to determine the value of universal screening for their patient population. In rural parts of America that have yet to see cases, universal screening may not make sense, but these areas are likely to be few and far between. The rest of America will need to quickly get on board with universal screening to protect their labor and delivery staff.”
Testing the partner was a strength of the study. “It is reassuring that when a pregnant woman tested negative for SARS-CoV-2, the rate was very, very low (2.4%) that her partner would test positive. However, it was disconcerting that telephone screening for common symptoms associated with COVID-19 was not very helpful in identifying cases,” said Dr. Waldorf, a professor of obstetrics and gynecology at the University of Washington, Seattle. She was not involved in the study by Bianco et al.
One study author receives payment from the American Board of Obstetrics and Gynecology for serving as a board examiner, receives payment from UpToDate, and serves as an expert witness in malpractice and products liability cases. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Bianco A et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003985.
Among women with a planned delivery in a New York City health system during the first half of April, the rate of asymptomatic SARS-CoV-2 infection was 16%, according to a study published in Obstetrics & Gynecology. Among the patients’ designated support persons, the asymptomatic carrier rate was 10%.
“If universal testing of pregnant patients in a high prevalence area is not performed, health care workers will be inadvertently exposed to COVID-19, unless universal precautions with personal protective equipment are taken,” wrote the researchers affiliated with the department of obstetrics, gynecology, and reproductive medicine at Icahn School of Medicine at Mount Sinai, New York.
Angela Bianco, MD, and colleagues conducted an observational study of women who were scheduled for a planned delivery within the Mount Sinai Health System between April 4 and April 15, 2020. Patients and their designated support person completed a telephone screen and underwent COVID-19 testing the day before a scheduled delivery. If support persons screened positive during the telephone interview about COVID-19 symptoms, they could not attend the birth, and patients could contact a different support person to be screened and tested. “All patients and their support persons were informed of their SARS-CoV-2 test results before admission,” the investigators wrote. “Those who tested positive were counseled regarding symptomatology that should prompt medical attention.”
In all, researchers screened 158 patients with a planned delivery, and 155 agreed to undergo COVID-19 testing. Of the 155 women tested, 24 (16%) tested positive for SARS CoV-2 infection. Among 146 support persons who had a negative interview screen and underwent SARS-CoV-2 testing, 14 (10%) tested positive for SARS-CoV-2 infection.
Test results were substantially concordant among patient and support person pairs. “Among patients who tested positive for COVID-19 infection and had a support person present, 11 of 19 (58%) support persons also tested positive for COVID-19 infection,” the authors reported. “Among patients who tested negative for COVID-19 infection and had a support person present, only 3 of 127 (2.4%) support persons tested positive for COVID-19 infection.”
Telephone screening did not identify any of the COVID-19–positive cases. Of the 24 patients with SARS-CoV-2 infection, none of their newborns tested positive at birth.
“Universal testing ... provides a mechanism for more accurate counseling of patients regarding issues such as newborn skin-to-skin contact and breastfeeding,” noted Dr. Bianco and colleagues. At their institution, parents with COVID-19 are instructed to wear a mask and practice proper hand hygiene when caring for their newborns.
Kristina Adams Waldorf, MD, said in an interview that the study by Bianco et al. underscores the high rate of asymptomatic or mildly symptomatic COVID-19 infections detected with universal screening in a hospital at the U.S. epicenter of the pandemic. “Each state and hospital will need to evaluate their own data to determine the value of universal screening for their patient population. In rural parts of America that have yet to see cases, universal screening may not make sense, but these areas are likely to be few and far between. The rest of America will need to quickly get on board with universal screening to protect their labor and delivery staff.”
Testing the partner was a strength of the study. “It is reassuring that when a pregnant woman tested negative for SARS-CoV-2, the rate was very, very low (2.4%) that her partner would test positive. However, it was disconcerting that telephone screening for common symptoms associated with COVID-19 was not very helpful in identifying cases,” said Dr. Waldorf, a professor of obstetrics and gynecology at the University of Washington, Seattle. She was not involved in the study by Bianco et al.
One study author receives payment from the American Board of Obstetrics and Gynecology for serving as a board examiner, receives payment from UpToDate, and serves as an expert witness in malpractice and products liability cases. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Bianco A et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003985.
Among women with a planned delivery in a New York City health system during the first half of April, the rate of asymptomatic SARS-CoV-2 infection was 16%, according to a study published in Obstetrics & Gynecology. Among the patients’ designated support persons, the asymptomatic carrier rate was 10%.
“If universal testing of pregnant patients in a high prevalence area is not performed, health care workers will be inadvertently exposed to COVID-19, unless universal precautions with personal protective equipment are taken,” wrote the researchers affiliated with the department of obstetrics, gynecology, and reproductive medicine at Icahn School of Medicine at Mount Sinai, New York.
Angela Bianco, MD, and colleagues conducted an observational study of women who were scheduled for a planned delivery within the Mount Sinai Health System between April 4 and April 15, 2020. Patients and their designated support person completed a telephone screen and underwent COVID-19 testing the day before a scheduled delivery. If support persons screened positive during the telephone interview about COVID-19 symptoms, they could not attend the birth, and patients could contact a different support person to be screened and tested. “All patients and their support persons were informed of their SARS-CoV-2 test results before admission,” the investigators wrote. “Those who tested positive were counseled regarding symptomatology that should prompt medical attention.”
In all, researchers screened 158 patients with a planned delivery, and 155 agreed to undergo COVID-19 testing. Of the 155 women tested, 24 (16%) tested positive for SARS CoV-2 infection. Among 146 support persons who had a negative interview screen and underwent SARS-CoV-2 testing, 14 (10%) tested positive for SARS-CoV-2 infection.
Test results were substantially concordant among patient and support person pairs. “Among patients who tested positive for COVID-19 infection and had a support person present, 11 of 19 (58%) support persons also tested positive for COVID-19 infection,” the authors reported. “Among patients who tested negative for COVID-19 infection and had a support person present, only 3 of 127 (2.4%) support persons tested positive for COVID-19 infection.”
Telephone screening did not identify any of the COVID-19–positive cases. Of the 24 patients with SARS-CoV-2 infection, none of their newborns tested positive at birth.
“Universal testing ... provides a mechanism for more accurate counseling of patients regarding issues such as newborn skin-to-skin contact and breastfeeding,” noted Dr. Bianco and colleagues. At their institution, parents with COVID-19 are instructed to wear a mask and practice proper hand hygiene when caring for their newborns.
Kristina Adams Waldorf, MD, said in an interview that the study by Bianco et al. underscores the high rate of asymptomatic or mildly symptomatic COVID-19 infections detected with universal screening in a hospital at the U.S. epicenter of the pandemic. “Each state and hospital will need to evaluate their own data to determine the value of universal screening for their patient population. In rural parts of America that have yet to see cases, universal screening may not make sense, but these areas are likely to be few and far between. The rest of America will need to quickly get on board with universal screening to protect their labor and delivery staff.”
Testing the partner was a strength of the study. “It is reassuring that when a pregnant woman tested negative for SARS-CoV-2, the rate was very, very low (2.4%) that her partner would test positive. However, it was disconcerting that telephone screening for common symptoms associated with COVID-19 was not very helpful in identifying cases,” said Dr. Waldorf, a professor of obstetrics and gynecology at the University of Washington, Seattle. She was not involved in the study by Bianco et al.
One study author receives payment from the American Board of Obstetrics and Gynecology for serving as a board examiner, receives payment from UpToDate, and serves as an expert witness in malpractice and products liability cases. The other authors did not report any potential conflicts of interest. Dr. Waldorf said she had no relevant financial disclosures.
SOURCE: Bianco A et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003985.
FROM OBSTETRICS & GYNECOLOGY
Whether to test laboring women for SARS-CoV-2 may hinge on regional prevalence
at the time of admission, research published online in Obstetrics & Gynecology suggests.
In Los Angeles, researchers stopped universal testing after none of the first 80 asymptomatic women had positive results. Researchers in Chicago, on the other hand, found a positive rate of approximately 1.6% among 614 asymptomatic patients and continue to test all patients.
“Decisions regarding universal testing need to be made in the context of regional prevalence of COVID-19 infection, with recognition that a ‘one-size-fits-all’ approach is unlikely to be justifiable,” Torri D. Metz, MD,of University of Utah Health in Salt Lake City said in an editorial accompanying research letters that described the experience in Los Angeles and Chicago. “In the setting of low population prevalence of COVID-19 infection or in locations with limited testing availability, deferring universal testing may represent the better part of valor when weighing risks, benefits, economic burden, and unintended consequences of testing for SARS-CoV-2 infection. In high-prevalence regions, universal testing may be a valuable addition to obstetric care that will prevent infections in health care workers and neonates.”
Testing all patients also may provide valuable population-level surveillance, added Dr. Metz, who is an associate professor of obstetrics and gynecology, a maternal-fetal medicine subspecialist, and vice-chair of research in obstetrics and gynecology.
One week of data
After New York hospitals reported an approximately 13% prevalence of SARS-CoV-2 infection among asymptomatic laboring women, Cedars-Sinai Medical Center in Los Angeles changed its policy from testing only women with COVID-19 symptoms to testing all women beginning April 4, 2020. “Data from New York made us very concerned about the possibility of asymptomatic infections among our own pregnant patients,” Mariam Naqvi, MD, a maternal-fetal medicine specialist at Cedars-Sinai Medical Center, said in a news release. “This would have implications for them, their babies, their households, and for the health of our staff caring for them.”
In 1 week, 82 pregnant women admitted to the obstetric unit were tested for SARS-CoV-2 infection. Of two women who reported COVID-19 symptoms, one tested positive for SARS-CoV-2. “Of the remaining 80 asymptomatic women, none tested positive for SARS-CoV-2 infection, and all remained symptom free throughout their hospitalizations,” Dr. Naqvi and colleagues reported. “One asymptomatic patient had an inadequate nasopharyngeal specimen and declined repeat testing.”
Precautions taken during universal testing meant that all members of the treatment team used valuable personal protective equipment. In some cases, mothers and newborns were separated until test results were available.
“We discontinued universal testing after a 7-day period, because we could not justify continued testing of asymptomatic women in the absence of positive test results for SARS-CoV-2 infection,” they noted. “Though universal testing did not yield enough positive results on our obstetric unit to warrant continued testing at this time, our approach may change if local rates of infection increase.”
20 days of testing
In a prospective case series of pregnant women admitted to Northwestern Memorial Hospital in Chicago from April 8 to April 27, 2020, universal testing did detect asymptomatic infections. Women with scheduled admissions were tested 12-36 hours before admission in a drive-through testing center, and women with unscheduled admissions received a test that has a 2- to 3-hour turnaround time. In addition, patients were screened for symptoms such as fever, shortness of breath, cough, sore throat, body aches, chills, new-onset vomiting, diarrhea, loss of taste or smell, and red or painful eyes.
“Asymptomatic women with pending tests were managed on the routine labor floor, but health care workers used personal protective equipment that included a respirator during the second stage of labor and delivery until the test result became available,” wrote Emily S. Miller, MD, MPH, of Northwestern University, Chicago, and colleagues.
During the first 20 days of universal testing, 635 pregnant women were admitted, and 23 (3.6%) tested positive for SARS-CoV-2 infection. Of 21 women with COVID-19 symptoms, 13 (62%) tested positive for SARS-CoV-2 infection. Of 614 women who were asymptomatic, 10 (1.6%) tested positive for SARS-CoV-2. “Our data corroborate the observation that pregnant women with SARS-CoV-2 infection on admission do not seem to be reliably identified using symptom screening alone,” the researchers wrote.
Unintended consequences
Despite a lack of effective treatments for mild to moderate COVID-19, “knowledge of the disease state allows ... health care workers to wear appropriate personal protective equipment to avoid exposure,” Dr. Metz wrote. It also allows “women to be counseled about ways to decrease transmission to neonates” and enables close monitoring of patients with infection.
At the same time, universal testing may have unintended consequences for infected patients, such as stigmatization, separation from the newborn, and delays in care related to health care providers spending more time donning personal protective equipment or changes in medical decision-making regarding cesarean delivery, she emphasized.
“Obstetricians should remain aware of disease prevalence in their communities and consider universal screening of asymptomatic women on an ongoing basis as new ‘hot spots’ for COVID-19 infection are identified,” Dr. Metz concluded.
One of Dr. Naqvi’s coauthors disclosed receiving funds from Contemporary OB/GYN, Keneka, and the American College of Obstetricians and Gynecologists and serving as a board examiner for the American Board of Obstetrics and Gynecology; her coauthors did not report any relevant financial disclosures. Dr. Metz disclosed that money was paid to her institution from Pfizer and GestVision for work related to an RSV vaccination trial and a preeclampsia test, respectively. Dr. Miller and colleagues did not report any potential conflicts of interest.
SOURCES: Naqvi M et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003987; Miller ES et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003983; Metz TD. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003972.
at the time of admission, research published online in Obstetrics & Gynecology suggests.
In Los Angeles, researchers stopped universal testing after none of the first 80 asymptomatic women had positive results. Researchers in Chicago, on the other hand, found a positive rate of approximately 1.6% among 614 asymptomatic patients and continue to test all patients.
“Decisions regarding universal testing need to be made in the context of regional prevalence of COVID-19 infection, with recognition that a ‘one-size-fits-all’ approach is unlikely to be justifiable,” Torri D. Metz, MD,of University of Utah Health in Salt Lake City said in an editorial accompanying research letters that described the experience in Los Angeles and Chicago. “In the setting of low population prevalence of COVID-19 infection or in locations with limited testing availability, deferring universal testing may represent the better part of valor when weighing risks, benefits, economic burden, and unintended consequences of testing for SARS-CoV-2 infection. In high-prevalence regions, universal testing may be a valuable addition to obstetric care that will prevent infections in health care workers and neonates.”
Testing all patients also may provide valuable population-level surveillance, added Dr. Metz, who is an associate professor of obstetrics and gynecology, a maternal-fetal medicine subspecialist, and vice-chair of research in obstetrics and gynecology.
One week of data
After New York hospitals reported an approximately 13% prevalence of SARS-CoV-2 infection among asymptomatic laboring women, Cedars-Sinai Medical Center in Los Angeles changed its policy from testing only women with COVID-19 symptoms to testing all women beginning April 4, 2020. “Data from New York made us very concerned about the possibility of asymptomatic infections among our own pregnant patients,” Mariam Naqvi, MD, a maternal-fetal medicine specialist at Cedars-Sinai Medical Center, said in a news release. “This would have implications for them, their babies, their households, and for the health of our staff caring for them.”
In 1 week, 82 pregnant women admitted to the obstetric unit were tested for SARS-CoV-2 infection. Of two women who reported COVID-19 symptoms, one tested positive for SARS-CoV-2. “Of the remaining 80 asymptomatic women, none tested positive for SARS-CoV-2 infection, and all remained symptom free throughout their hospitalizations,” Dr. Naqvi and colleagues reported. “One asymptomatic patient had an inadequate nasopharyngeal specimen and declined repeat testing.”
Precautions taken during universal testing meant that all members of the treatment team used valuable personal protective equipment. In some cases, mothers and newborns were separated until test results were available.
“We discontinued universal testing after a 7-day period, because we could not justify continued testing of asymptomatic women in the absence of positive test results for SARS-CoV-2 infection,” they noted. “Though universal testing did not yield enough positive results on our obstetric unit to warrant continued testing at this time, our approach may change if local rates of infection increase.”
20 days of testing
In a prospective case series of pregnant women admitted to Northwestern Memorial Hospital in Chicago from April 8 to April 27, 2020, universal testing did detect asymptomatic infections. Women with scheduled admissions were tested 12-36 hours before admission in a drive-through testing center, and women with unscheduled admissions received a test that has a 2- to 3-hour turnaround time. In addition, patients were screened for symptoms such as fever, shortness of breath, cough, sore throat, body aches, chills, new-onset vomiting, diarrhea, loss of taste or smell, and red or painful eyes.
“Asymptomatic women with pending tests were managed on the routine labor floor, but health care workers used personal protective equipment that included a respirator during the second stage of labor and delivery until the test result became available,” wrote Emily S. Miller, MD, MPH, of Northwestern University, Chicago, and colleagues.
During the first 20 days of universal testing, 635 pregnant women were admitted, and 23 (3.6%) tested positive for SARS-CoV-2 infection. Of 21 women with COVID-19 symptoms, 13 (62%) tested positive for SARS-CoV-2 infection. Of 614 women who were asymptomatic, 10 (1.6%) tested positive for SARS-CoV-2. “Our data corroborate the observation that pregnant women with SARS-CoV-2 infection on admission do not seem to be reliably identified using symptom screening alone,” the researchers wrote.
Unintended consequences
Despite a lack of effective treatments for mild to moderate COVID-19, “knowledge of the disease state allows ... health care workers to wear appropriate personal protective equipment to avoid exposure,” Dr. Metz wrote. It also allows “women to be counseled about ways to decrease transmission to neonates” and enables close monitoring of patients with infection.
At the same time, universal testing may have unintended consequences for infected patients, such as stigmatization, separation from the newborn, and delays in care related to health care providers spending more time donning personal protective equipment or changes in medical decision-making regarding cesarean delivery, she emphasized.
“Obstetricians should remain aware of disease prevalence in their communities and consider universal screening of asymptomatic women on an ongoing basis as new ‘hot spots’ for COVID-19 infection are identified,” Dr. Metz concluded.
One of Dr. Naqvi’s coauthors disclosed receiving funds from Contemporary OB/GYN, Keneka, and the American College of Obstetricians and Gynecologists and serving as a board examiner for the American Board of Obstetrics and Gynecology; her coauthors did not report any relevant financial disclosures. Dr. Metz disclosed that money was paid to her institution from Pfizer and GestVision for work related to an RSV vaccination trial and a preeclampsia test, respectively. Dr. Miller and colleagues did not report any potential conflicts of interest.
SOURCES: Naqvi M et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003987; Miller ES et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003983; Metz TD. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003972.
at the time of admission, research published online in Obstetrics & Gynecology suggests.
In Los Angeles, researchers stopped universal testing after none of the first 80 asymptomatic women had positive results. Researchers in Chicago, on the other hand, found a positive rate of approximately 1.6% among 614 asymptomatic patients and continue to test all patients.
“Decisions regarding universal testing need to be made in the context of regional prevalence of COVID-19 infection, with recognition that a ‘one-size-fits-all’ approach is unlikely to be justifiable,” Torri D. Metz, MD,of University of Utah Health in Salt Lake City said in an editorial accompanying research letters that described the experience in Los Angeles and Chicago. “In the setting of low population prevalence of COVID-19 infection or in locations with limited testing availability, deferring universal testing may represent the better part of valor when weighing risks, benefits, economic burden, and unintended consequences of testing for SARS-CoV-2 infection. In high-prevalence regions, universal testing may be a valuable addition to obstetric care that will prevent infections in health care workers and neonates.”
Testing all patients also may provide valuable population-level surveillance, added Dr. Metz, who is an associate professor of obstetrics and gynecology, a maternal-fetal medicine subspecialist, and vice-chair of research in obstetrics and gynecology.
One week of data
After New York hospitals reported an approximately 13% prevalence of SARS-CoV-2 infection among asymptomatic laboring women, Cedars-Sinai Medical Center in Los Angeles changed its policy from testing only women with COVID-19 symptoms to testing all women beginning April 4, 2020. “Data from New York made us very concerned about the possibility of asymptomatic infections among our own pregnant patients,” Mariam Naqvi, MD, a maternal-fetal medicine specialist at Cedars-Sinai Medical Center, said in a news release. “This would have implications for them, their babies, their households, and for the health of our staff caring for them.”
In 1 week, 82 pregnant women admitted to the obstetric unit were tested for SARS-CoV-2 infection. Of two women who reported COVID-19 symptoms, one tested positive for SARS-CoV-2. “Of the remaining 80 asymptomatic women, none tested positive for SARS-CoV-2 infection, and all remained symptom free throughout their hospitalizations,” Dr. Naqvi and colleagues reported. “One asymptomatic patient had an inadequate nasopharyngeal specimen and declined repeat testing.”
Precautions taken during universal testing meant that all members of the treatment team used valuable personal protective equipment. In some cases, mothers and newborns were separated until test results were available.
“We discontinued universal testing after a 7-day period, because we could not justify continued testing of asymptomatic women in the absence of positive test results for SARS-CoV-2 infection,” they noted. “Though universal testing did not yield enough positive results on our obstetric unit to warrant continued testing at this time, our approach may change if local rates of infection increase.”
20 days of testing
In a prospective case series of pregnant women admitted to Northwestern Memorial Hospital in Chicago from April 8 to April 27, 2020, universal testing did detect asymptomatic infections. Women with scheduled admissions were tested 12-36 hours before admission in a drive-through testing center, and women with unscheduled admissions received a test that has a 2- to 3-hour turnaround time. In addition, patients were screened for symptoms such as fever, shortness of breath, cough, sore throat, body aches, chills, new-onset vomiting, diarrhea, loss of taste or smell, and red or painful eyes.
“Asymptomatic women with pending tests were managed on the routine labor floor, but health care workers used personal protective equipment that included a respirator during the second stage of labor and delivery until the test result became available,” wrote Emily S. Miller, MD, MPH, of Northwestern University, Chicago, and colleagues.
During the first 20 days of universal testing, 635 pregnant women were admitted, and 23 (3.6%) tested positive for SARS-CoV-2 infection. Of 21 women with COVID-19 symptoms, 13 (62%) tested positive for SARS-CoV-2 infection. Of 614 women who were asymptomatic, 10 (1.6%) tested positive for SARS-CoV-2. “Our data corroborate the observation that pregnant women with SARS-CoV-2 infection on admission do not seem to be reliably identified using symptom screening alone,” the researchers wrote.
Unintended consequences
Despite a lack of effective treatments for mild to moderate COVID-19, “knowledge of the disease state allows ... health care workers to wear appropriate personal protective equipment to avoid exposure,” Dr. Metz wrote. It also allows “women to be counseled about ways to decrease transmission to neonates” and enables close monitoring of patients with infection.
At the same time, universal testing may have unintended consequences for infected patients, such as stigmatization, separation from the newborn, and delays in care related to health care providers spending more time donning personal protective equipment or changes in medical decision-making regarding cesarean delivery, she emphasized.
“Obstetricians should remain aware of disease prevalence in their communities and consider universal screening of asymptomatic women on an ongoing basis as new ‘hot spots’ for COVID-19 infection are identified,” Dr. Metz concluded.
One of Dr. Naqvi’s coauthors disclosed receiving funds from Contemporary OB/GYN, Keneka, and the American College of Obstetricians and Gynecologists and serving as a board examiner for the American Board of Obstetrics and Gynecology; her coauthors did not report any relevant financial disclosures. Dr. Metz disclosed that money was paid to her institution from Pfizer and GestVision for work related to an RSV vaccination trial and a preeclampsia test, respectively. Dr. Miller and colleagues did not report any potential conflicts of interest.
SOURCES: Naqvi M et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003987; Miller ES et al. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003983; Metz TD. Obstet Gynecol. 2020 May 19. doi: 10.1097/AOG.0000000000003972.
FROM OBSTETRICS & GYNECOLOGY
Seek safe strategies to diagnose gestational diabetes during pandemic
Clinicians and pregnant women are less likely to prescribe and undergo the oral glucose tolerance test (OGTT) to diagnose gestational diabetes in the context of the COVID-19 pandemic, according to a review by H. David McIntyre, MD, of the University of Queensland, Brisbane, Australia, and Robert G. Moses, MD, of Wollongong (Australia) Hospital.
National and international discussions of whether a one- or two-step test for gestational diabetes mellitus (GDM) is optimal, and which women should be tested are ongoing, but the potential for exposure risks to COVID-19 are impacting the test process, they wrote in a commentary published in Diabetes Care.
“Any national or local guidelines should be developed with the primary aim of being protective for pregnant women and workable in the current health crisis,” they wrote.
Key concerns expressed by women and health care providers include the need for travel to be tested, the possible need for two visits, and the several hours spent in a potentially high-risk specimen collection center.
“Further, a GDM diagnosis generally involves additional health service visits for diabetes education, glucose monitoring review, and fetal ultrasonography, all of which carry exposure risks during a pandemic,” Dr. McIntyre and Dr. Moses noted.
Professional societies in the United Kingdom, Canada, and Australia have issued guidance to clinicians for modifying GDM diagnoses criteria during the pandemic that aim to reduce the need for the oral glucose tolerance test both during and after pregnancy.
Pandemic guidelines for all three of these countries support the identification of GDM using early pregnancy hemoglobin A1c (HbA1c) of at least 41 mmol/mol (5.9%).
Then, professionals in the United Kingdom recommend testing based on risk factors and diagnosing GDM based on any of these criteria: HbA1c of at least 39 mmol/mol (5.7%), fasting venous plasma glucose of at least 5.6 mmol/L (preferred), or random VPG of at least 9.0 mmol/L.
The revised testing pathway for Canada accepts an HbA1c of at least 39 mmol/mol (5.7%) and/or random VPG of at least 11.1 mmol/L.
“The revised Australian pathway does not include HbA1c but recommends a fasting VPG with progression to OGTT only if this result is 4.7-5.0 mmol/L,” Dr. McIntyre and Dr. Moses explained.
Overall, the revised guidelines for GDM testing will likely miss some women and only identify those with higher levels of hyperglycemia, the authors wrote. In addition, “the evidence base for these revised pathways is limited and that each alternative strategy should be evaluated over the course of the current pandemic.”
Validation of new testing strategies are needed, and the pandemic may provide and opportunity to adopt an alternative to the OGTT. The World Health Organization has not issued revised guidance for other methods of testing, but fasting VPG alone may be the simplest and most cost effective, at least for the short term, they noted.
“In this ‘new COVID world,’ GDM should not be ignored but pragmatically merits a lower priority than the avoidance of exposure to the COVID-19 virus,” although no single alternative strategy applies in all countries and situations, the authors concluded. Pragmatic measures and documentation of outcomes at the local level will offer the “least worst” solution while the pandemic continues.
The authors had no relevant financial disclosures.
SOURCE: McIntyre HD, Moses RG. Diabetes Care. 2020 May. doi: 10.2337/dci20-0026.
A major concern against the backdrop of COVID-19 is ensuring long-term health while urgent care is – understandably so – being prioritized over preventive care. We can already see the impact that the decrease in primary care has had: Rates of childhood vaccination appear to have dropped; the cancellation or indefinite delay of elective medical procedures has meant a reduction in preventive cancer screenings, such as colonoscopies and mammograms; and concerns about COVID-19 may be keeping those experiencing cardiac events from seeking emergency care.
However, an outcropping of the coronavirus pandemic is an ingenuity to adapt to our new “normal.” Medical licenses have been recognized across state lines to allow much-needed professionals to practice in the hardest-hit areas. Doctors retrofitted a sleep apnea machine to be used as a makeshift ventilator. Those in the wearable device market now have a greater onus to deliver on quality, utility, security, and accuracy.
Obstetricians have had to dramatically change delivery of ante-, intra- and postpartum care. The recent commentary by Dr. McIntyre and Dr. Moses focuses on one particular area of concern: screening, diagnosis, and management of gestational diabetes mellitus (GDM).
Screening and diagnosis are mainstays to reduce the adverse maternal and neonatal outcomes of diabetes in pregnancy. Although there is no universally accepted approach to evaluating GDM, all current methods utilize an oral glucose tolerance test (OGTT), which requires significant time spent in a clinical office setting, thus increasing risk for COVID-19 exposure.
Several countries have adopted modified GDM criteria within the last months. At the time of this writing, the United States has not. Although not testing women for GDM, which is what Dr. McIntyre and Dr. Moses point out may be happening in countries with modified guidelines, seems questionable, perhaps we should think differently about our approach.
More than 20 years ago, it was reported that jelly beans could be used as an alternative to the 50-g GDM screening test (Am J Obstet Gynecol. 1999 Nov;181[5 Pt 1]:1154‐7; Am J Obstet Gynecol. 1995 Dec;173[6]:1889‐92); more recently, candy twists were used with similar results (Am J Obstet Gynecol. 2015 Apr;212[4]:522.e1-5). In addition, a number of articles have reported on the utility of capillary whole blood glucose measurements to screen for GDM in developing and resource-limited countries (Diabetes Technol Ther. 2011;13[5]:586‐91; Acta Diabetol. 2016 Feb;53[1]:91‐7; Diabetes Technol Ther. 2012 Feb;14[2]:131-4). Therefore, rather than forgo GDM screening, women could self-administer a jelly bean test at home, measure blood sugar with a glucometer, and depending on the results, have an OGTT. Importantly, this would allow ob.gyns. to maintain medical standards while managing patients via telemedicine.
We have evidence that GDM can establish poor health for generations. We know that people with underlying conditions have greater morbidity and mortality from infectious diseases. We recognize that accurate screening and diagnosis is the key to prevention and management. Rather than accept a “least worst” scenario, as Dr. McIntyre and Dr. Moses state, we must find ways to provide the best possible care under the current circumstances.
E. Albert Reece, MD, PhD, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the University of Maryland School of Medicine. He said he had no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
A major concern against the backdrop of COVID-19 is ensuring long-term health while urgent care is – understandably so – being prioritized over preventive care. We can already see the impact that the decrease in primary care has had: Rates of childhood vaccination appear to have dropped; the cancellation or indefinite delay of elective medical procedures has meant a reduction in preventive cancer screenings, such as colonoscopies and mammograms; and concerns about COVID-19 may be keeping those experiencing cardiac events from seeking emergency care.
However, an outcropping of the coronavirus pandemic is an ingenuity to adapt to our new “normal.” Medical licenses have been recognized across state lines to allow much-needed professionals to practice in the hardest-hit areas. Doctors retrofitted a sleep apnea machine to be used as a makeshift ventilator. Those in the wearable device market now have a greater onus to deliver on quality, utility, security, and accuracy.
Obstetricians have had to dramatically change delivery of ante-, intra- and postpartum care. The recent commentary by Dr. McIntyre and Dr. Moses focuses on one particular area of concern: screening, diagnosis, and management of gestational diabetes mellitus (GDM).
Screening and diagnosis are mainstays to reduce the adverse maternal and neonatal outcomes of diabetes in pregnancy. Although there is no universally accepted approach to evaluating GDM, all current methods utilize an oral glucose tolerance test (OGTT), which requires significant time spent in a clinical office setting, thus increasing risk for COVID-19 exposure.
Several countries have adopted modified GDM criteria within the last months. At the time of this writing, the United States has not. Although not testing women for GDM, which is what Dr. McIntyre and Dr. Moses point out may be happening in countries with modified guidelines, seems questionable, perhaps we should think differently about our approach.
More than 20 years ago, it was reported that jelly beans could be used as an alternative to the 50-g GDM screening test (Am J Obstet Gynecol. 1999 Nov;181[5 Pt 1]:1154‐7; Am J Obstet Gynecol. 1995 Dec;173[6]:1889‐92); more recently, candy twists were used with similar results (Am J Obstet Gynecol. 2015 Apr;212[4]:522.e1-5). In addition, a number of articles have reported on the utility of capillary whole blood glucose measurements to screen for GDM in developing and resource-limited countries (Diabetes Technol Ther. 2011;13[5]:586‐91; Acta Diabetol. 2016 Feb;53[1]:91‐7; Diabetes Technol Ther. 2012 Feb;14[2]:131-4). Therefore, rather than forgo GDM screening, women could self-administer a jelly bean test at home, measure blood sugar with a glucometer, and depending on the results, have an OGTT. Importantly, this would allow ob.gyns. to maintain medical standards while managing patients via telemedicine.
We have evidence that GDM can establish poor health for generations. We know that people with underlying conditions have greater morbidity and mortality from infectious diseases. We recognize that accurate screening and diagnosis is the key to prevention and management. Rather than accept a “least worst” scenario, as Dr. McIntyre and Dr. Moses state, we must find ways to provide the best possible care under the current circumstances.
E. Albert Reece, MD, PhD, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the University of Maryland School of Medicine. He said he had no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
A major concern against the backdrop of COVID-19 is ensuring long-term health while urgent care is – understandably so – being prioritized over preventive care. We can already see the impact that the decrease in primary care has had: Rates of childhood vaccination appear to have dropped; the cancellation or indefinite delay of elective medical procedures has meant a reduction in preventive cancer screenings, such as colonoscopies and mammograms; and concerns about COVID-19 may be keeping those experiencing cardiac events from seeking emergency care.
However, an outcropping of the coronavirus pandemic is an ingenuity to adapt to our new “normal.” Medical licenses have been recognized across state lines to allow much-needed professionals to practice in the hardest-hit areas. Doctors retrofitted a sleep apnea machine to be used as a makeshift ventilator. Those in the wearable device market now have a greater onus to deliver on quality, utility, security, and accuracy.
Obstetricians have had to dramatically change delivery of ante-, intra- and postpartum care. The recent commentary by Dr. McIntyre and Dr. Moses focuses on one particular area of concern: screening, diagnosis, and management of gestational diabetes mellitus (GDM).
Screening and diagnosis are mainstays to reduce the adverse maternal and neonatal outcomes of diabetes in pregnancy. Although there is no universally accepted approach to evaluating GDM, all current methods utilize an oral glucose tolerance test (OGTT), which requires significant time spent in a clinical office setting, thus increasing risk for COVID-19 exposure.
Several countries have adopted modified GDM criteria within the last months. At the time of this writing, the United States has not. Although not testing women for GDM, which is what Dr. McIntyre and Dr. Moses point out may be happening in countries with modified guidelines, seems questionable, perhaps we should think differently about our approach.
More than 20 years ago, it was reported that jelly beans could be used as an alternative to the 50-g GDM screening test (Am J Obstet Gynecol. 1999 Nov;181[5 Pt 1]:1154‐7; Am J Obstet Gynecol. 1995 Dec;173[6]:1889‐92); more recently, candy twists were used with similar results (Am J Obstet Gynecol. 2015 Apr;212[4]:522.e1-5). In addition, a number of articles have reported on the utility of capillary whole blood glucose measurements to screen for GDM in developing and resource-limited countries (Diabetes Technol Ther. 2011;13[5]:586‐91; Acta Diabetol. 2016 Feb;53[1]:91‐7; Diabetes Technol Ther. 2012 Feb;14[2]:131-4). Therefore, rather than forgo GDM screening, women could self-administer a jelly bean test at home, measure blood sugar with a glucometer, and depending on the results, have an OGTT. Importantly, this would allow ob.gyns. to maintain medical standards while managing patients via telemedicine.
We have evidence that GDM can establish poor health for generations. We know that people with underlying conditions have greater morbidity and mortality from infectious diseases. We recognize that accurate screening and diagnosis is the key to prevention and management. Rather than accept a “least worst” scenario, as Dr. McIntyre and Dr. Moses state, we must find ways to provide the best possible care under the current circumstances.
E. Albert Reece, MD, PhD, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the University of Maryland School of Medicine. He said he had no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
Clinicians and pregnant women are less likely to prescribe and undergo the oral glucose tolerance test (OGTT) to diagnose gestational diabetes in the context of the COVID-19 pandemic, according to a review by H. David McIntyre, MD, of the University of Queensland, Brisbane, Australia, and Robert G. Moses, MD, of Wollongong (Australia) Hospital.
National and international discussions of whether a one- or two-step test for gestational diabetes mellitus (GDM) is optimal, and which women should be tested are ongoing, but the potential for exposure risks to COVID-19 are impacting the test process, they wrote in a commentary published in Diabetes Care.
“Any national or local guidelines should be developed with the primary aim of being protective for pregnant women and workable in the current health crisis,” they wrote.
Key concerns expressed by women and health care providers include the need for travel to be tested, the possible need for two visits, and the several hours spent in a potentially high-risk specimen collection center.
“Further, a GDM diagnosis generally involves additional health service visits for diabetes education, glucose monitoring review, and fetal ultrasonography, all of which carry exposure risks during a pandemic,” Dr. McIntyre and Dr. Moses noted.
Professional societies in the United Kingdom, Canada, and Australia have issued guidance to clinicians for modifying GDM diagnoses criteria during the pandemic that aim to reduce the need for the oral glucose tolerance test both during and after pregnancy.
Pandemic guidelines for all three of these countries support the identification of GDM using early pregnancy hemoglobin A1c (HbA1c) of at least 41 mmol/mol (5.9%).
Then, professionals in the United Kingdom recommend testing based on risk factors and diagnosing GDM based on any of these criteria: HbA1c of at least 39 mmol/mol (5.7%), fasting venous plasma glucose of at least 5.6 mmol/L (preferred), or random VPG of at least 9.0 mmol/L.
The revised testing pathway for Canada accepts an HbA1c of at least 39 mmol/mol (5.7%) and/or random VPG of at least 11.1 mmol/L.
“The revised Australian pathway does not include HbA1c but recommends a fasting VPG with progression to OGTT only if this result is 4.7-5.0 mmol/L,” Dr. McIntyre and Dr. Moses explained.
Overall, the revised guidelines for GDM testing will likely miss some women and only identify those with higher levels of hyperglycemia, the authors wrote. In addition, “the evidence base for these revised pathways is limited and that each alternative strategy should be evaluated over the course of the current pandemic.”
Validation of new testing strategies are needed, and the pandemic may provide and opportunity to adopt an alternative to the OGTT. The World Health Organization has not issued revised guidance for other methods of testing, but fasting VPG alone may be the simplest and most cost effective, at least for the short term, they noted.
“In this ‘new COVID world,’ GDM should not be ignored but pragmatically merits a lower priority than the avoidance of exposure to the COVID-19 virus,” although no single alternative strategy applies in all countries and situations, the authors concluded. Pragmatic measures and documentation of outcomes at the local level will offer the “least worst” solution while the pandemic continues.
The authors had no relevant financial disclosures.
SOURCE: McIntyre HD, Moses RG. Diabetes Care. 2020 May. doi: 10.2337/dci20-0026.
Clinicians and pregnant women are less likely to prescribe and undergo the oral glucose tolerance test (OGTT) to diagnose gestational diabetes in the context of the COVID-19 pandemic, according to a review by H. David McIntyre, MD, of the University of Queensland, Brisbane, Australia, and Robert G. Moses, MD, of Wollongong (Australia) Hospital.
National and international discussions of whether a one- or two-step test for gestational diabetes mellitus (GDM) is optimal, and which women should be tested are ongoing, but the potential for exposure risks to COVID-19 are impacting the test process, they wrote in a commentary published in Diabetes Care.
“Any national or local guidelines should be developed with the primary aim of being protective for pregnant women and workable in the current health crisis,” they wrote.
Key concerns expressed by women and health care providers include the need for travel to be tested, the possible need for two visits, and the several hours spent in a potentially high-risk specimen collection center.
“Further, a GDM diagnosis generally involves additional health service visits for diabetes education, glucose monitoring review, and fetal ultrasonography, all of which carry exposure risks during a pandemic,” Dr. McIntyre and Dr. Moses noted.
Professional societies in the United Kingdom, Canada, and Australia have issued guidance to clinicians for modifying GDM diagnoses criteria during the pandemic that aim to reduce the need for the oral glucose tolerance test both during and after pregnancy.
Pandemic guidelines for all three of these countries support the identification of GDM using early pregnancy hemoglobin A1c (HbA1c) of at least 41 mmol/mol (5.9%).
Then, professionals in the United Kingdom recommend testing based on risk factors and diagnosing GDM based on any of these criteria: HbA1c of at least 39 mmol/mol (5.7%), fasting venous plasma glucose of at least 5.6 mmol/L (preferred), or random VPG of at least 9.0 mmol/L.
The revised testing pathway for Canada accepts an HbA1c of at least 39 mmol/mol (5.7%) and/or random VPG of at least 11.1 mmol/L.
“The revised Australian pathway does not include HbA1c but recommends a fasting VPG with progression to OGTT only if this result is 4.7-5.0 mmol/L,” Dr. McIntyre and Dr. Moses explained.
Overall, the revised guidelines for GDM testing will likely miss some women and only identify those with higher levels of hyperglycemia, the authors wrote. In addition, “the evidence base for these revised pathways is limited and that each alternative strategy should be evaluated over the course of the current pandemic.”
Validation of new testing strategies are needed, and the pandemic may provide and opportunity to adopt an alternative to the OGTT. The World Health Organization has not issued revised guidance for other methods of testing, but fasting VPG alone may be the simplest and most cost effective, at least for the short term, they noted.
“In this ‘new COVID world,’ GDM should not be ignored but pragmatically merits a lower priority than the avoidance of exposure to the COVID-19 virus,” although no single alternative strategy applies in all countries and situations, the authors concluded. Pragmatic measures and documentation of outcomes at the local level will offer the “least worst” solution while the pandemic continues.
The authors had no relevant financial disclosures.
SOURCE: McIntyre HD, Moses RG. Diabetes Care. 2020 May. doi: 10.2337/dci20-0026.
Today’s top news highlights: ACE inhibitors in COVID patients, fewer AMI admissions, and more
Here are the stories our MDedge editors across specialties think you need to know about today:
Are ACE inhibitors protective in COVID-19?
Older patients with COVID-19 had a lower risk of developing severe illness if they were taking ACE inhibitors, according to a large observational U.S. study. ACE inhibitor use was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. Senior investigator Harlan M. Krumholz, MD, said that while he and his associates think this finding is worthy of further study, “We don’t believe this is enough info to change practice.” The study was published on the MedRxiv preprint server and has not yet been peer reviewed.
READ MORE.
AMI: Admissions drop, deaths rise
In Italy, sharp nationwide decreases in hospitalizations for acute myocardial infarctions (AMIs) during the height of COVID-19 were offset by higher mortality for patients who did present. The study counted AMIs at 54 hospitals nationwide for the week of March 12-19, 2020, and compared that with an equivalent week in 2019 – 319 vs. 618 AMIs, respectively, representing a 48% reduction in hospitalizations. Mortality for ST-segment elevation MI cases more than tripled to 14% during the outbreak, compared with 4% in 2019. “The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” commented Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, who was not involved with the study.
READ MORE.
Prenatal, postpartum screening for depression falls short
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Researchers analyzed self-reported data on postpartum depressive symptoms collected in 2018 by the Pregnancy Risk Assessment Monitoring System. Mental health conditions play a role in approximately 9% of pregnancy-related deaths and not asking about depression represents “missed opportunities to potentially identify and treat women with depression,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Are ACE inhibitors protective in COVID-19?
Older patients with COVID-19 had a lower risk of developing severe illness if they were taking ACE inhibitors, according to a large observational U.S. study. ACE inhibitor use was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. Senior investigator Harlan M. Krumholz, MD, said that while he and his associates think this finding is worthy of further study, “We don’t believe this is enough info to change practice.” The study was published on the MedRxiv preprint server and has not yet been peer reviewed.
READ MORE.
AMI: Admissions drop, deaths rise
In Italy, sharp nationwide decreases in hospitalizations for acute myocardial infarctions (AMIs) during the height of COVID-19 were offset by higher mortality for patients who did present. The study counted AMIs at 54 hospitals nationwide for the week of March 12-19, 2020, and compared that with an equivalent week in 2019 – 319 vs. 618 AMIs, respectively, representing a 48% reduction in hospitalizations. Mortality for ST-segment elevation MI cases more than tripled to 14% during the outbreak, compared with 4% in 2019. “The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” commented Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, who was not involved with the study.
READ MORE.
Prenatal, postpartum screening for depression falls short
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Researchers analyzed self-reported data on postpartum depressive symptoms collected in 2018 by the Pregnancy Risk Assessment Monitoring System. Mental health conditions play a role in approximately 9% of pregnancy-related deaths and not asking about depression represents “missed opportunities to potentially identify and treat women with depression,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Are ACE inhibitors protective in COVID-19?
Older patients with COVID-19 had a lower risk of developing severe illness if they were taking ACE inhibitors, according to a large observational U.S. study. ACE inhibitor use was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. Senior investigator Harlan M. Krumholz, MD, said that while he and his associates think this finding is worthy of further study, “We don’t believe this is enough info to change practice.” The study was published on the MedRxiv preprint server and has not yet been peer reviewed.
READ MORE.
AMI: Admissions drop, deaths rise
In Italy, sharp nationwide decreases in hospitalizations for acute myocardial infarctions (AMIs) during the height of COVID-19 were offset by higher mortality for patients who did present. The study counted AMIs at 54 hospitals nationwide for the week of March 12-19, 2020, and compared that with an equivalent week in 2019 – 319 vs. 618 AMIs, respectively, representing a 48% reduction in hospitalizations. Mortality for ST-segment elevation MI cases more than tripled to 14% during the outbreak, compared with 4% in 2019. “The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” commented Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, who was not involved with the study.
READ MORE.
Prenatal, postpartum screening for depression falls short
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Researchers analyzed self-reported data on postpartum depressive symptoms collected in 2018 by the Pregnancy Risk Assessment Monitoring System. Mental health conditions play a role in approximately 9% of pregnancy-related deaths and not asking about depression represents “missed opportunities to potentially identify and treat women with depression,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Out of the pipeline: Remdesivir
Although the US Food and Drug Administration (FDA) has granted emergency use authorization of remdesivir (Gilead Sciences, Inc., Foster City, California) to treat COVID-19, the disease caused by SARS-CoV-2, the drug is considered an investigational agent, not yet formally approved by the FDA and whose efficacy and safety has not yet been fully characterized. Remdesivir has been shown to be effective in reducing the time to recovery of people with COVID-19 disease. It has not been tested in a large controlled clinical trial of pregnant women with COVID-19; however, remdesivir has been given to pregnant women infected with COVID-19 in a compassionate use protocol. For pregnant women, the drug should only be used if the potential benefit justifies the potential risk to the mother and fetus.1
Pharmacology. Remdesivir is a nucleoside RNA polymerase inhibitor. It has a molecular formula of
C27H35N6O8P and a molecular weight of 602.6 g/mol.1
Mechanism of action. From FDA’s fact sheet: “Remdesivir is an adenosine nucleotide prodrug that distributes into cells where it is metabolized to form the pharmacologically active nucleoside triphosphate metabolite. Metabolism of remdesivir to remdesivir triphosphate has been demonstrated in multiple cell types. Remdesivir triphosphate acts as an analog of adenosine triphosphate (ATP) and competes with the natural ATP substrate for incorporation into nascent RNA chains by the SARS-CoV-2 RNA-dependent RNA polymerase, which results in chain termination during replication of the viral RNA. Remdesivir triphosphate is a weak inhibitor of mammalian DNA and RNA polymerases with low potential for mitochondrial toxicity.”1
Treatment protocols
Remdesivir is authorized for treatment of hospitalized patients with severe COVID-19 disease, defined as patients with an oxygen saturation ≤ 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). The optimal dose and duration of treatment of COVID-19 with remdesivir is unknown.1
Prior to initiating treatment, the estimated glomerular filtration rate should be documented to be ≥ 30 mL/min. An excipient used in the remdesivir formulation—sulfobutylether-β-cylcodextrin sodium salt—is renally cleared and accumulates in patients with decreased renal function.
Baseline liver function tests should be performed prior to treatment and daily during the course of treatment. Remdesivir should not be initiated in patients with an alanine aminotransferase (ALT) level ≥ 5 times the upper limit of normal at baseline. Remdesivir should be discontinued in patients who develop an ALT level ≥ 5 times the upper limit of normal or in patients who develop elevated ALT levels and have increased bilirubin, alkaline phosphatase, or international normalized ratio.1
In one open-label study (GS-US-540-5773), remdesivir treatment was discontinued due to an adverse event in 5% of patients on a 5-day regimen and in 10% of patients on a 10-day regimen.1
Under the emergency use authorization, two treatment protocols have been proposed depending on the clinical severity of the COVID-19 infection1:
- Protocol 1: For people with COVID-19 requiring mechanical ventilation and/or ECMO, the duration of therapy is 10 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 9 days.
- Protocol 2: For people with COVID-19 disease not requiring mechanical ventilation and/or ECMO, the duration of therapy is 5 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 4 days. If the patient does not show clinical improvement, treatment may be extended for an additional 5 days.
Continue to: Randomized placebo-controlled trial results...
Randomized placebo-controlled trial results
The Adaptive COVID-19 Treatment Trial (ACTT), sponsored by the National Institute of Allergy and Infectious Diseases, is a randomized, double-blind, placebo-controlled trial conducted by Gilead Sciences. The study began in February and evaluated up to 10 days of remdesivir treatment—200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days in hospitalized adult patients with COVID-19. Patients were enrolled in a 1:1 manner to remdesivir or placebo, and time to recovery within 28 days after randomization was the trial’s endpoint. According to preliminary analysis of 606 recovered patients, recovery took a median of 11 days in the remdesivir group and 15 days in the placebo group (hazard ratio, 1.31; 95% confidence interval (CI), 1.12‒1.54; P<.001). Mortality rates were 8.0% and 11.6% in the remdesivir and placebo groups, respectively (P=.059).1
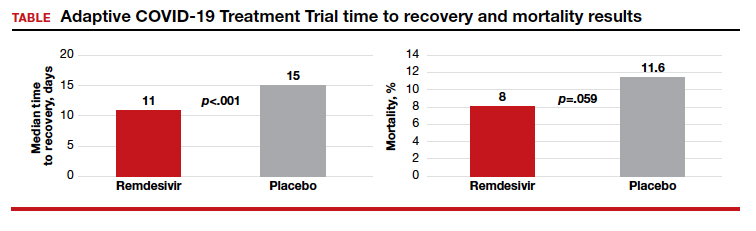
5 vs 10 days of remdesivir treatment
The Gilead Sciences‒sponsored study GS-US-540-5773 was a randomized, open-label multicenter trial of patients with severe COVID-19. A total of 197 adult patients received 10-day remdesivir treatment (200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days). An additional 200 adult patients received 5-day remdesivir treatment (200 mg IV once daily followed by 100 mg IV for 4 days). Both groups also received standard of care. Results suggested that patients receiving 10 days of remdesivir had similar improvement in clinical status compared with those receiving a 5-day treatment course (10-to-5 day odds ratio, 0.76; 95% CI, 0.51‒1.13] on day 14).1 Improvement in clinical status was defined as an improvement of 2 or more points from baseline on a predefined 7-point scale that ranged from hospital discharge to increasing levels of oxygen support to death. Clinical recovery was achieved if patients ceased the need for oxygen support or were discharged.1
The time to clinical improvement for 50% of patients was similar in each treatment group (10 days in the 5-day group versus 11 days in the 10-day group). By day 14, observed clinical improvement rates were 65% and 54% in the 5- and 10-day treatment groups, respectively. Clinical recovery rates were 70% and 59% in the 5- and 10-day treatment groups and mortality rates were 8% and 11%.1
Adverse events
The use of remdesivir is contraindicated in patients who are hypersensitive to the drug. Its infusion may cause hypotension, nausea, vomiting, diaphoresis, and shivering. If signs of a clinically significant infusion reaction are observed the infusion should be discontinued. As noted above, elevation in ALT levels occurs with remdesivir treatment.1
Reporting serious adverse events. If a serious and unexpected adverse event occurs and appears to be associated with the use of remdesivir, the prescribing health care provider and/or the provider’s designee should complete and submit a MedWatch form to the FDA using one of the following methods1:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Return form FDA 3500 (available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf) to the FDA by mail (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787) or fax (1-800-FDA-0178)
- Gilead requests that all FDA MedWatch forms also be returned to Gilead Pharmacovigilance and Epidemiology: fax: 1-650-522-5477 726; e-mail: [email protected]
Continue to: Drug interactions...
Drug interactions
Remdesivir has not been evaluated for drug-drug interactions in humans. The clinical relevance of in vitro drug interactions also has not been established. According to the FDA, remdesivir is a substrate for the drug metabolizing enzymes CYP2C8, CYP2D6, and CYP3A4, and is a substrate for organic anion transporting polypeptides 1B1 (OAPT1B1) and P-glycoprotein (P-gp) transporters. In vitro, remdesivir inhibits CYP3A4, OATP1B1, OATP1B3, BSEP, MRP4, and NTCP.1
Pregnancy risk summary
Remdesivir has not been studied adequately in pregnant women and only should be used during pregnancy if the potential benefit of the drug justifies the potential risk to both mother and fetus.
Nonclinical animal studies that included systemic exposure of the predominant circulating metabolite of remdesivir in pregnant rats and rabbits (at 4 times the recommended dose of human exposure) demonstrated no adverse effect on embryofetal development.1
Breastfeeding
The only information regarding breastfeeding and remdesivir comes from animal studies. The drug and its metabolites were detected in the plasma of nursing rat pups whose mothers given intravenous remdesivir daily from gestation day 6 to lactation day 20. Measured on lactation day 10, remdesivir exposure in the pups was about 1% that of maternal exposure.1
“Because of the potential for viral transmission to SARS-CoV-2-negative infants and adverse reactions from the drug in breastfeeding infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for remdesivir and any potential adverse effects on the breastfed child from remdesivir or from the underlying maternal condition.”1
- US Food and Drug Administration. Fact Sheet for Health Care Providers Emergency Use Authorization (UA) of Remdesivir (GS-5734)TM. https://www.fda.gov/media/137566/download. Accessed May 19, 2020.
Although the US Food and Drug Administration (FDA) has granted emergency use authorization of remdesivir (Gilead Sciences, Inc., Foster City, California) to treat COVID-19, the disease caused by SARS-CoV-2, the drug is considered an investigational agent, not yet formally approved by the FDA and whose efficacy and safety has not yet been fully characterized. Remdesivir has been shown to be effective in reducing the time to recovery of people with COVID-19 disease. It has not been tested in a large controlled clinical trial of pregnant women with COVID-19; however, remdesivir has been given to pregnant women infected with COVID-19 in a compassionate use protocol. For pregnant women, the drug should only be used if the potential benefit justifies the potential risk to the mother and fetus.1
Pharmacology. Remdesivir is a nucleoside RNA polymerase inhibitor. It has a molecular formula of
C27H35N6O8P and a molecular weight of 602.6 g/mol.1
Mechanism of action. From FDA’s fact sheet: “Remdesivir is an adenosine nucleotide prodrug that distributes into cells where it is metabolized to form the pharmacologically active nucleoside triphosphate metabolite. Metabolism of remdesivir to remdesivir triphosphate has been demonstrated in multiple cell types. Remdesivir triphosphate acts as an analog of adenosine triphosphate (ATP) and competes with the natural ATP substrate for incorporation into nascent RNA chains by the SARS-CoV-2 RNA-dependent RNA polymerase, which results in chain termination during replication of the viral RNA. Remdesivir triphosphate is a weak inhibitor of mammalian DNA and RNA polymerases with low potential for mitochondrial toxicity.”1
Treatment protocols
Remdesivir is authorized for treatment of hospitalized patients with severe COVID-19 disease, defined as patients with an oxygen saturation ≤ 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). The optimal dose and duration of treatment of COVID-19 with remdesivir is unknown.1
Prior to initiating treatment, the estimated glomerular filtration rate should be documented to be ≥ 30 mL/min. An excipient used in the remdesivir formulation—sulfobutylether-β-cylcodextrin sodium salt—is renally cleared and accumulates in patients with decreased renal function.
Baseline liver function tests should be performed prior to treatment and daily during the course of treatment. Remdesivir should not be initiated in patients with an alanine aminotransferase (ALT) level ≥ 5 times the upper limit of normal at baseline. Remdesivir should be discontinued in patients who develop an ALT level ≥ 5 times the upper limit of normal or in patients who develop elevated ALT levels and have increased bilirubin, alkaline phosphatase, or international normalized ratio.1
In one open-label study (GS-US-540-5773), remdesivir treatment was discontinued due to an adverse event in 5% of patients on a 5-day regimen and in 10% of patients on a 10-day regimen.1
Under the emergency use authorization, two treatment protocols have been proposed depending on the clinical severity of the COVID-19 infection1:
- Protocol 1: For people with COVID-19 requiring mechanical ventilation and/or ECMO, the duration of therapy is 10 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 9 days.
- Protocol 2: For people with COVID-19 disease not requiring mechanical ventilation and/or ECMO, the duration of therapy is 5 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 4 days. If the patient does not show clinical improvement, treatment may be extended for an additional 5 days.
Continue to: Randomized placebo-controlled trial results...
Randomized placebo-controlled trial results
The Adaptive COVID-19 Treatment Trial (ACTT), sponsored by the National Institute of Allergy and Infectious Diseases, is a randomized, double-blind, placebo-controlled trial conducted by Gilead Sciences. The study began in February and evaluated up to 10 days of remdesivir treatment—200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days in hospitalized adult patients with COVID-19. Patients were enrolled in a 1:1 manner to remdesivir or placebo, and time to recovery within 28 days after randomization was the trial’s endpoint. According to preliminary analysis of 606 recovered patients, recovery took a median of 11 days in the remdesivir group and 15 days in the placebo group (hazard ratio, 1.31; 95% confidence interval (CI), 1.12‒1.54; P<.001). Mortality rates were 8.0% and 11.6% in the remdesivir and placebo groups, respectively (P=.059).1

5 vs 10 days of remdesivir treatment
The Gilead Sciences‒sponsored study GS-US-540-5773 was a randomized, open-label multicenter trial of patients with severe COVID-19. A total of 197 adult patients received 10-day remdesivir treatment (200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days). An additional 200 adult patients received 5-day remdesivir treatment (200 mg IV once daily followed by 100 mg IV for 4 days). Both groups also received standard of care. Results suggested that patients receiving 10 days of remdesivir had similar improvement in clinical status compared with those receiving a 5-day treatment course (10-to-5 day odds ratio, 0.76; 95% CI, 0.51‒1.13] on day 14).1 Improvement in clinical status was defined as an improvement of 2 or more points from baseline on a predefined 7-point scale that ranged from hospital discharge to increasing levels of oxygen support to death. Clinical recovery was achieved if patients ceased the need for oxygen support or were discharged.1
The time to clinical improvement for 50% of patients was similar in each treatment group (10 days in the 5-day group versus 11 days in the 10-day group). By day 14, observed clinical improvement rates were 65% and 54% in the 5- and 10-day treatment groups, respectively. Clinical recovery rates were 70% and 59% in the 5- and 10-day treatment groups and mortality rates were 8% and 11%.1
Adverse events
The use of remdesivir is contraindicated in patients who are hypersensitive to the drug. Its infusion may cause hypotension, nausea, vomiting, diaphoresis, and shivering. If signs of a clinically significant infusion reaction are observed the infusion should be discontinued. As noted above, elevation in ALT levels occurs with remdesivir treatment.1
Reporting serious adverse events. If a serious and unexpected adverse event occurs and appears to be associated with the use of remdesivir, the prescribing health care provider and/or the provider’s designee should complete and submit a MedWatch form to the FDA using one of the following methods1:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Return form FDA 3500 (available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf) to the FDA by mail (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787) or fax (1-800-FDA-0178)
- Gilead requests that all FDA MedWatch forms also be returned to Gilead Pharmacovigilance and Epidemiology: fax: 1-650-522-5477 726; e-mail: [email protected]
Continue to: Drug interactions...
Drug interactions
Remdesivir has not been evaluated for drug-drug interactions in humans. The clinical relevance of in vitro drug interactions also has not been established. According to the FDA, remdesivir is a substrate for the drug metabolizing enzymes CYP2C8, CYP2D6, and CYP3A4, and is a substrate for organic anion transporting polypeptides 1B1 (OAPT1B1) and P-glycoprotein (P-gp) transporters. In vitro, remdesivir inhibits CYP3A4, OATP1B1, OATP1B3, BSEP, MRP4, and NTCP.1
Pregnancy risk summary
Remdesivir has not been studied adequately in pregnant women and only should be used during pregnancy if the potential benefit of the drug justifies the potential risk to both mother and fetus.
Nonclinical animal studies that included systemic exposure of the predominant circulating metabolite of remdesivir in pregnant rats and rabbits (at 4 times the recommended dose of human exposure) demonstrated no adverse effect on embryofetal development.1
Breastfeeding
The only information regarding breastfeeding and remdesivir comes from animal studies. The drug and its metabolites were detected in the plasma of nursing rat pups whose mothers given intravenous remdesivir daily from gestation day 6 to lactation day 20. Measured on lactation day 10, remdesivir exposure in the pups was about 1% that of maternal exposure.1
“Because of the potential for viral transmission to SARS-CoV-2-negative infants and adverse reactions from the drug in breastfeeding infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for remdesivir and any potential adverse effects on the breastfed child from remdesivir or from the underlying maternal condition.”1
Although the US Food and Drug Administration (FDA) has granted emergency use authorization of remdesivir (Gilead Sciences, Inc., Foster City, California) to treat COVID-19, the disease caused by SARS-CoV-2, the drug is considered an investigational agent, not yet formally approved by the FDA and whose efficacy and safety has not yet been fully characterized. Remdesivir has been shown to be effective in reducing the time to recovery of people with COVID-19 disease. It has not been tested in a large controlled clinical trial of pregnant women with COVID-19; however, remdesivir has been given to pregnant women infected with COVID-19 in a compassionate use protocol. For pregnant women, the drug should only be used if the potential benefit justifies the potential risk to the mother and fetus.1
Pharmacology. Remdesivir is a nucleoside RNA polymerase inhibitor. It has a molecular formula of
C27H35N6O8P and a molecular weight of 602.6 g/mol.1
Mechanism of action. From FDA’s fact sheet: “Remdesivir is an adenosine nucleotide prodrug that distributes into cells where it is metabolized to form the pharmacologically active nucleoside triphosphate metabolite. Metabolism of remdesivir to remdesivir triphosphate has been demonstrated in multiple cell types. Remdesivir triphosphate acts as an analog of adenosine triphosphate (ATP) and competes with the natural ATP substrate for incorporation into nascent RNA chains by the SARS-CoV-2 RNA-dependent RNA polymerase, which results in chain termination during replication of the viral RNA. Remdesivir triphosphate is a weak inhibitor of mammalian DNA and RNA polymerases with low potential for mitochondrial toxicity.”1
Treatment protocols
Remdesivir is authorized for treatment of hospitalized patients with severe COVID-19 disease, defined as patients with an oxygen saturation ≤ 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). The optimal dose and duration of treatment of COVID-19 with remdesivir is unknown.1
Prior to initiating treatment, the estimated glomerular filtration rate should be documented to be ≥ 30 mL/min. An excipient used in the remdesivir formulation—sulfobutylether-β-cylcodextrin sodium salt—is renally cleared and accumulates in patients with decreased renal function.
Baseline liver function tests should be performed prior to treatment and daily during the course of treatment. Remdesivir should not be initiated in patients with an alanine aminotransferase (ALT) level ≥ 5 times the upper limit of normal at baseline. Remdesivir should be discontinued in patients who develop an ALT level ≥ 5 times the upper limit of normal or in patients who develop elevated ALT levels and have increased bilirubin, alkaline phosphatase, or international normalized ratio.1
In one open-label study (GS-US-540-5773), remdesivir treatment was discontinued due to an adverse event in 5% of patients on a 5-day regimen and in 10% of patients on a 10-day regimen.1
Under the emergency use authorization, two treatment protocols have been proposed depending on the clinical severity of the COVID-19 infection1:
- Protocol 1: For people with COVID-19 requiring mechanical ventilation and/or ECMO, the duration of therapy is 10 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 9 days.
- Protocol 2: For people with COVID-19 disease not requiring mechanical ventilation and/or ECMO, the duration of therapy is 5 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 4 days. If the patient does not show clinical improvement, treatment may be extended for an additional 5 days.
Continue to: Randomized placebo-controlled trial results...
Randomized placebo-controlled trial results
The Adaptive COVID-19 Treatment Trial (ACTT), sponsored by the National Institute of Allergy and Infectious Diseases, is a randomized, double-blind, placebo-controlled trial conducted by Gilead Sciences. The study began in February and evaluated up to 10 days of remdesivir treatment—200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days in hospitalized adult patients with COVID-19. Patients were enrolled in a 1:1 manner to remdesivir or placebo, and time to recovery within 28 days after randomization was the trial’s endpoint. According to preliminary analysis of 606 recovered patients, recovery took a median of 11 days in the remdesivir group and 15 days in the placebo group (hazard ratio, 1.31; 95% confidence interval (CI), 1.12‒1.54; P<.001). Mortality rates were 8.0% and 11.6% in the remdesivir and placebo groups, respectively (P=.059).1

5 vs 10 days of remdesivir treatment
The Gilead Sciences‒sponsored study GS-US-540-5773 was a randomized, open-label multicenter trial of patients with severe COVID-19. A total of 197 adult patients received 10-day remdesivir treatment (200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days). An additional 200 adult patients received 5-day remdesivir treatment (200 mg IV once daily followed by 100 mg IV for 4 days). Both groups also received standard of care. Results suggested that patients receiving 10 days of remdesivir had similar improvement in clinical status compared with those receiving a 5-day treatment course (10-to-5 day odds ratio, 0.76; 95% CI, 0.51‒1.13] on day 14).1 Improvement in clinical status was defined as an improvement of 2 or more points from baseline on a predefined 7-point scale that ranged from hospital discharge to increasing levels of oxygen support to death. Clinical recovery was achieved if patients ceased the need for oxygen support or were discharged.1
The time to clinical improvement for 50% of patients was similar in each treatment group (10 days in the 5-day group versus 11 days in the 10-day group). By day 14, observed clinical improvement rates were 65% and 54% in the 5- and 10-day treatment groups, respectively. Clinical recovery rates were 70% and 59% in the 5- and 10-day treatment groups and mortality rates were 8% and 11%.1
Adverse events
The use of remdesivir is contraindicated in patients who are hypersensitive to the drug. Its infusion may cause hypotension, nausea, vomiting, diaphoresis, and shivering. If signs of a clinically significant infusion reaction are observed the infusion should be discontinued. As noted above, elevation in ALT levels occurs with remdesivir treatment.1
Reporting serious adverse events. If a serious and unexpected adverse event occurs and appears to be associated with the use of remdesivir, the prescribing health care provider and/or the provider’s designee should complete and submit a MedWatch form to the FDA using one of the following methods1:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Return form FDA 3500 (available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf) to the FDA by mail (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787) or fax (1-800-FDA-0178)
- Gilead requests that all FDA MedWatch forms also be returned to Gilead Pharmacovigilance and Epidemiology: fax: 1-650-522-5477 726; e-mail: [email protected]
Continue to: Drug interactions...
Drug interactions
Remdesivir has not been evaluated for drug-drug interactions in humans. The clinical relevance of in vitro drug interactions also has not been established. According to the FDA, remdesivir is a substrate for the drug metabolizing enzymes CYP2C8, CYP2D6, and CYP3A4, and is a substrate for organic anion transporting polypeptides 1B1 (OAPT1B1) and P-glycoprotein (P-gp) transporters. In vitro, remdesivir inhibits CYP3A4, OATP1B1, OATP1B3, BSEP, MRP4, and NTCP.1
Pregnancy risk summary
Remdesivir has not been studied adequately in pregnant women and only should be used during pregnancy if the potential benefit of the drug justifies the potential risk to both mother and fetus.
Nonclinical animal studies that included systemic exposure of the predominant circulating metabolite of remdesivir in pregnant rats and rabbits (at 4 times the recommended dose of human exposure) demonstrated no adverse effect on embryofetal development.1
Breastfeeding
The only information regarding breastfeeding and remdesivir comes from animal studies. The drug and its metabolites were detected in the plasma of nursing rat pups whose mothers given intravenous remdesivir daily from gestation day 6 to lactation day 20. Measured on lactation day 10, remdesivir exposure in the pups was about 1% that of maternal exposure.1
“Because of the potential for viral transmission to SARS-CoV-2-negative infants and adverse reactions from the drug in breastfeeding infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for remdesivir and any potential adverse effects on the breastfed child from remdesivir or from the underlying maternal condition.”1
- US Food and Drug Administration. Fact Sheet for Health Care Providers Emergency Use Authorization (UA) of Remdesivir (GS-5734)TM. https://www.fda.gov/media/137566/download. Accessed May 19, 2020.
- US Food and Drug Administration. Fact Sheet for Health Care Providers Emergency Use Authorization (UA) of Remdesivir (GS-5734)TM. https://www.fda.gov/media/137566/download. Accessed May 19, 2020.
Many clinicians still not asking about postpartum depression
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Although the prevalence of screening has risen in recent years, many women could be suffering in silence.
“[U]ndetected and untreated perinatal depression can have negative health consequences for the mothers and their babies,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
Dr. Ko and colleagues reported their findings in an article published in Morbidity and Mortality Weekly Report.
The researchers analyzed self-reported data on postpartum depressive symptoms (PDS) collected in 2018 by the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were stratified on the basis of location and maternal and infant characteristics, including age, race/ethnicity, and education level. Women who had recently given birth to one or more live infants answered questions about whether they had been screened by health care providers for depression during perinatal visits.
The prevalence of PDS among women from 31 PRAMS sites was 13.2%. States with lower prevalences included Illinois (9.7%), Massachusetts (10.3%), and Wisconsin (10.5%); states with higher prevalences included Mississippi (23.5%), West Virginia (19.4%), and Michigan (16.4%).
Some groups were at higher risk for PDS than others. The prevalence was greater than 20% among women who were aged 19 years or younger, were of American Indian or Alaska Native ethnicity, smoked during the perinatal period, experienced perinatal depression, or whose infant died after birth.
Depressive symptoms were also more common among women who received assistance from the Women, Infants, and Children program; were Medicaid beneficiaries at the time of delivery; smoked cigarettes during the last trimester of pregnancy; breastfed their infants for fewer than 8 weeks; or had experienced intimate partner violence while pregnant or before.
Small rise in screening
Overall, 79.1% of women said a health care provider had inquired about depression during the prenatal period. Prenatal screening for depression was lowest in Puerto Rico (50.7%), Mississippi (69.4%), Utah (69.5%), and Kentucky (69.5%) and was highest in Alaska (90.7%), Minnesota (90.6%), and Maine (90.5%).
Among 22 continuously reporting sites, the prevalence of prenatal depression screening rose significantly from 76.2% in 2016 to 79.3% in 2018 (P < .05) .
“It is unclear what might account for this small increase,” Dr. Ko said. “There may be additional factors, such as women may be becoming more comfortable reporting symptoms of depression. With continued awareness about the need to screen every pregnant and postpartum woman for depression, we can expect things to continue to improve.”
Overall, 90.1% of respondents reported a postpartum visit; of those, 87.4% said a health care provider had asked about depression during that visit.
Screening during the postpartum period was highest in Vermont (96.2%), Minnesota (95.9%), and Maine (95.5%) and was lowest in Puerto Rico (50.7%), New York City (73.1%), and Louisiana (75.0%).
Among the 22 sites that reported continuously, the prevalence of screening for postpartum depression rose significantly from 84.1% to 88.0% (P < .05), “with an average annual percentage point increase of 1.8%,” the authors wrote.
‘Missed opportunities’
“PRAMS responses are reported an average of 4 months postpartum, which suggests persistence of [depressive] symptoms,” the authors wrote.
Dr. Ko said that mental health conditions play a role in approximately 9% of pregnancy-related deaths and that not asking about depression represents “missed opportunities to potentially identify and treat women with depression.” The United States Preventive Services Task Force recommends screening all adults for depression, including women during pregnancy and the postpartum period, she added.
When asked what can be done to improve screening that has not already been tried, Dr. Ko said the CDC is currently evaluating a study called the Program in Support of Moms (PRISM), which “is designed to help obstetrics and gynecology practices address the significant public health issue of depression during and after pregnancy. PRISM aims to close gaps in health care delivery to ensure that women with depression during and after pregnancy receive the best treatment, which can result in improvement in their symptoms.”
Dr. Ko added that the Health Resources and Services Administration has funded seven states to begin “programs to support providers to screen, assess, refer, and treat pregnant and postpartum women for depression and other behavioral health conditions. States can use initiatives like Healthy Start, home visiting, and Title V Maternal and Child Health Services Block Grant programs as levers to improve screening and address maternal depression.
“Screening is just one part of addressing perinatal depression. Health care providers need to refer women to appropriate resources in order to get the proper diagnosis, treatment, and follow-up care for management of depression,” Dr. Ko concluded.
The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Although the prevalence of screening has risen in recent years, many women could be suffering in silence.
“[U]ndetected and untreated perinatal depression can have negative health consequences for the mothers and their babies,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
Dr. Ko and colleagues reported their findings in an article published in Morbidity and Mortality Weekly Report.
The researchers analyzed self-reported data on postpartum depressive symptoms (PDS) collected in 2018 by the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were stratified on the basis of location and maternal and infant characteristics, including age, race/ethnicity, and education level. Women who had recently given birth to one or more live infants answered questions about whether they had been screened by health care providers for depression during perinatal visits.
The prevalence of PDS among women from 31 PRAMS sites was 13.2%. States with lower prevalences included Illinois (9.7%), Massachusetts (10.3%), and Wisconsin (10.5%); states with higher prevalences included Mississippi (23.5%), West Virginia (19.4%), and Michigan (16.4%).
Some groups were at higher risk for PDS than others. The prevalence was greater than 20% among women who were aged 19 years or younger, were of American Indian or Alaska Native ethnicity, smoked during the perinatal period, experienced perinatal depression, or whose infant died after birth.
Depressive symptoms were also more common among women who received assistance from the Women, Infants, and Children program; were Medicaid beneficiaries at the time of delivery; smoked cigarettes during the last trimester of pregnancy; breastfed their infants for fewer than 8 weeks; or had experienced intimate partner violence while pregnant or before.
Small rise in screening
Overall, 79.1% of women said a health care provider had inquired about depression during the prenatal period. Prenatal screening for depression was lowest in Puerto Rico (50.7%), Mississippi (69.4%), Utah (69.5%), and Kentucky (69.5%) and was highest in Alaska (90.7%), Minnesota (90.6%), and Maine (90.5%).
Among 22 continuously reporting sites, the prevalence of prenatal depression screening rose significantly from 76.2% in 2016 to 79.3% in 2018 (P < .05) .
“It is unclear what might account for this small increase,” Dr. Ko said. “There may be additional factors, such as women may be becoming more comfortable reporting symptoms of depression. With continued awareness about the need to screen every pregnant and postpartum woman for depression, we can expect things to continue to improve.”
Overall, 90.1% of respondents reported a postpartum visit; of those, 87.4% said a health care provider had asked about depression during that visit.
Screening during the postpartum period was highest in Vermont (96.2%), Minnesota (95.9%), and Maine (95.5%) and was lowest in Puerto Rico (50.7%), New York City (73.1%), and Louisiana (75.0%).
Among the 22 sites that reported continuously, the prevalence of screening for postpartum depression rose significantly from 84.1% to 88.0% (P < .05), “with an average annual percentage point increase of 1.8%,” the authors wrote.
‘Missed opportunities’
“PRAMS responses are reported an average of 4 months postpartum, which suggests persistence of [depressive] symptoms,” the authors wrote.
Dr. Ko said that mental health conditions play a role in approximately 9% of pregnancy-related deaths and that not asking about depression represents “missed opportunities to potentially identify and treat women with depression.” The United States Preventive Services Task Force recommends screening all adults for depression, including women during pregnancy and the postpartum period, she added.
When asked what can be done to improve screening that has not already been tried, Dr. Ko said the CDC is currently evaluating a study called the Program in Support of Moms (PRISM), which “is designed to help obstetrics and gynecology practices address the significant public health issue of depression during and after pregnancy. PRISM aims to close gaps in health care delivery to ensure that women with depression during and after pregnancy receive the best treatment, which can result in improvement in their symptoms.”
Dr. Ko added that the Health Resources and Services Administration has funded seven states to begin “programs to support providers to screen, assess, refer, and treat pregnant and postpartum women for depression and other behavioral health conditions. States can use initiatives like Healthy Start, home visiting, and Title V Maternal and Child Health Services Block Grant programs as levers to improve screening and address maternal depression.
“Screening is just one part of addressing perinatal depression. Health care providers need to refer women to appropriate resources in order to get the proper diagnosis, treatment, and follow-up care for management of depression,” Dr. Ko concluded.
The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Although the prevalence of screening has risen in recent years, many women could be suffering in silence.
“[U]ndetected and untreated perinatal depression can have negative health consequences for the mothers and their babies,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
Dr. Ko and colleagues reported their findings in an article published in Morbidity and Mortality Weekly Report.
The researchers analyzed self-reported data on postpartum depressive symptoms (PDS) collected in 2018 by the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were stratified on the basis of location and maternal and infant characteristics, including age, race/ethnicity, and education level. Women who had recently given birth to one or more live infants answered questions about whether they had been screened by health care providers for depression during perinatal visits.
The prevalence of PDS among women from 31 PRAMS sites was 13.2%. States with lower prevalences included Illinois (9.7%), Massachusetts (10.3%), and Wisconsin (10.5%); states with higher prevalences included Mississippi (23.5%), West Virginia (19.4%), and Michigan (16.4%).
Some groups were at higher risk for PDS than others. The prevalence was greater than 20% among women who were aged 19 years or younger, were of American Indian or Alaska Native ethnicity, smoked during the perinatal period, experienced perinatal depression, or whose infant died after birth.
Depressive symptoms were also more common among women who received assistance from the Women, Infants, and Children program; were Medicaid beneficiaries at the time of delivery; smoked cigarettes during the last trimester of pregnancy; breastfed their infants for fewer than 8 weeks; or had experienced intimate partner violence while pregnant or before.
Small rise in screening
Overall, 79.1% of women said a health care provider had inquired about depression during the prenatal period. Prenatal screening for depression was lowest in Puerto Rico (50.7%), Mississippi (69.4%), Utah (69.5%), and Kentucky (69.5%) and was highest in Alaska (90.7%), Minnesota (90.6%), and Maine (90.5%).
Among 22 continuously reporting sites, the prevalence of prenatal depression screening rose significantly from 76.2% in 2016 to 79.3% in 2018 (P < .05) .
“It is unclear what might account for this small increase,” Dr. Ko said. “There may be additional factors, such as women may be becoming more comfortable reporting symptoms of depression. With continued awareness about the need to screen every pregnant and postpartum woman for depression, we can expect things to continue to improve.”
Overall, 90.1% of respondents reported a postpartum visit; of those, 87.4% said a health care provider had asked about depression during that visit.
Screening during the postpartum period was highest in Vermont (96.2%), Minnesota (95.9%), and Maine (95.5%) and was lowest in Puerto Rico (50.7%), New York City (73.1%), and Louisiana (75.0%).
Among the 22 sites that reported continuously, the prevalence of screening for postpartum depression rose significantly from 84.1% to 88.0% (P < .05), “with an average annual percentage point increase of 1.8%,” the authors wrote.
‘Missed opportunities’
“PRAMS responses are reported an average of 4 months postpartum, which suggests persistence of [depressive] symptoms,” the authors wrote.
Dr. Ko said that mental health conditions play a role in approximately 9% of pregnancy-related deaths and that not asking about depression represents “missed opportunities to potentially identify and treat women with depression.” The United States Preventive Services Task Force recommends screening all adults for depression, including women during pregnancy and the postpartum period, she added.
When asked what can be done to improve screening that has not already been tried, Dr. Ko said the CDC is currently evaluating a study called the Program in Support of Moms (PRISM), which “is designed to help obstetrics and gynecology practices address the significant public health issue of depression during and after pregnancy. PRISM aims to close gaps in health care delivery to ensure that women with depression during and after pregnancy receive the best treatment, which can result in improvement in their symptoms.”
Dr. Ko added that the Health Resources and Services Administration has funded seven states to begin “programs to support providers to screen, assess, refer, and treat pregnant and postpartum women for depression and other behavioral health conditions. States can use initiatives like Healthy Start, home visiting, and Title V Maternal and Child Health Services Block Grant programs as levers to improve screening and address maternal depression.
“Screening is just one part of addressing perinatal depression. Health care providers need to refer women to appropriate resources in order to get the proper diagnosis, treatment, and follow-up care for management of depression,” Dr. Ko concluded.
The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Extremely preterm infants fare better with corticosteroid and magnesium combo
Children born before 27 weeks’ gestation had lower combined risk of death or severe neurodevelopmental impairment when exposed to antenatal corticosteroids and magnesium sulfate together, compared with exposure of either or neither therapy, according to a prospective observational study published in Obstetrics & Gynecology.
“If there is sufficient time to administer antenatal corticosteroids, there should similarly be sufficient time to administer magnesium sulfate,” wrote Samuel J. Gentle, MD, of the University of Alabama at Birmingham, and colleagues. “Given the lower rate of severe neurodevelopmental impairment or death in children exposed to both antenatal corticosteroids and magnesium sulfate in the present study, compared with those exposed to antenatal corticosteroids alone, increasing the rates of magnesium sulfate exposure through quality improvement or other interventions may improve infant outcomes.”
Although previous randomized controlled trials had shown neurologic benefits of each therapy independently in preterm children, few data exist on extremely preterm children, the authors noted. They also pointed out differences in the findings when they analyzed neurodevelopmental outcomes and death separately.
“Whereas exposure to both therapies was associated with a lower rate of death, exposure to magnesium sulfate in addition to antenatal corticosteroids was not associated with a lower rate of severe neurodevelopmental impairment or components of severe neurodevelopmental impairment including Bayley scores, bilateral hearing impairment, and cerebral palsy,” Dr Gentle and his coauthors wrote.
The researchers used prospectively collected data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Generic Database to track 3,093 children born extremely preterm – from 22 weeks 0 days to 26 weeks 6 days – during 2011-2014.
The researchers compared outcomes of death or severe neurodevelopmental impairment when the children were 18-26 months of corrected age based on whether they had been exposed to antenatal corticosteroids alone (betamethasone or dexamethasone) or antenatal corticosteroids in addition to magnesium sulfate. Severe neurodevelopmental impairment included “severe cerebral palsy, motor or cognitive composite score less than 70 on the Bayley-III exam, bilateral blindness, or bilateral severe functional hearing impairment with or without amplification.”
The researchers also looked at severe neurodevelopmental impairment and death among children with only magnesium sulfate exposure or with no exposure to steroids or magnesium.
In the study population, 73% of infants had been exposed to both therapies, 16% had been exposed to only corticosteroids, 3% to only magnesium sulfate, and 8% to neither therapy.
“Importantly, a larger proportion of mothers unexposed to either therapy, compared with both therapies, received high school or less education or had no maternal private health insurance which may suggest health inequity as a driver for antenatal therapy exposure rates,” Dr. Gentle and associates noted.
Children whose mothers received corticosteroids and magnesium had a 27% lower risk of severe neurodevelopmental impairment or death, compared with those whose mothers only received corticosteroids (adjusted odds ratio, 0.73). Just over a third of children exposed to both interventions (36%) had severe neurodevelopmental impairment or died, compared with 44% of those exposed only to steroids.
Similarly, corticosteroids and magnesium together were associated with approximately half the risk of death or severe neurodevelopmental impairment, compared with magnesium alone (aOR, 0.49) and 34% lower risk, compared with neither therapy (aOR 0.66).
When the researchers uncoupled the outcomes, severe neurodevelopmental impairment rates were similar among all exposure groups, but rates of death were lower among those who received both therapies than among those who received just one or neither therapy.
“The therapeutic mechanism for neuroprotection in children exposed to magnesium sulfate is unclear but may result from neuronal stabilization or anti-inflammatory properties,” Dr. Gentle and colleagues said.
They also compared rates in the exposure groups of grade 3-4 intracranial hemorrhage, which has been linked to poor neurodevelopmental outcomes in extremely preterm children.
“The rate of grade 3-4 intracranial hemorrhage did not differ between children exposed to both antenatal corticosteroids and magnesium sulfate and those exposed to antenatal corticosteroids alone,” they said. “These findings further support data from randomized controlled trials showing benefit for antenatal corticosteroids but not for magnesium sulfate.”
They further noted a Cochrane Review that found significantly reduced risk of severe or any intracranial hemorrhage among children exposed to antenatal corticosteroids. No similar reduction in intracranial hemorrhage occurred in a separate Cochrane Review of antenatal magnesium sulfate trials.
The research was funded by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Center for Advancing Translational Sciences. One author is a consultant for Mednax who has received travel funds. Another author disclosed Catholic Health Professionals of Houston paid honorarium for an ethics talk he gave.
SOURCE: Gentle SJ et al. Obstet. Gynecol. 2020. doi: 10.1097/AOG.0000000000003882.
Children born before 27 weeks’ gestation had lower combined risk of death or severe neurodevelopmental impairment when exposed to antenatal corticosteroids and magnesium sulfate together, compared with exposure of either or neither therapy, according to a prospective observational study published in Obstetrics & Gynecology.
“If there is sufficient time to administer antenatal corticosteroids, there should similarly be sufficient time to administer magnesium sulfate,” wrote Samuel J. Gentle, MD, of the University of Alabama at Birmingham, and colleagues. “Given the lower rate of severe neurodevelopmental impairment or death in children exposed to both antenatal corticosteroids and magnesium sulfate in the present study, compared with those exposed to antenatal corticosteroids alone, increasing the rates of magnesium sulfate exposure through quality improvement or other interventions may improve infant outcomes.”
Although previous randomized controlled trials had shown neurologic benefits of each therapy independently in preterm children, few data exist on extremely preterm children, the authors noted. They also pointed out differences in the findings when they analyzed neurodevelopmental outcomes and death separately.
“Whereas exposure to both therapies was associated with a lower rate of death, exposure to magnesium sulfate in addition to antenatal corticosteroids was not associated with a lower rate of severe neurodevelopmental impairment or components of severe neurodevelopmental impairment including Bayley scores, bilateral hearing impairment, and cerebral palsy,” Dr Gentle and his coauthors wrote.
The researchers used prospectively collected data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Generic Database to track 3,093 children born extremely preterm – from 22 weeks 0 days to 26 weeks 6 days – during 2011-2014.
The researchers compared outcomes of death or severe neurodevelopmental impairment when the children were 18-26 months of corrected age based on whether they had been exposed to antenatal corticosteroids alone (betamethasone or dexamethasone) or antenatal corticosteroids in addition to magnesium sulfate. Severe neurodevelopmental impairment included “severe cerebral palsy, motor or cognitive composite score less than 70 on the Bayley-III exam, bilateral blindness, or bilateral severe functional hearing impairment with or without amplification.”
The researchers also looked at severe neurodevelopmental impairment and death among children with only magnesium sulfate exposure or with no exposure to steroids or magnesium.
In the study population, 73% of infants had been exposed to both therapies, 16% had been exposed to only corticosteroids, 3% to only magnesium sulfate, and 8% to neither therapy.
“Importantly, a larger proportion of mothers unexposed to either therapy, compared with both therapies, received high school or less education or had no maternal private health insurance which may suggest health inequity as a driver for antenatal therapy exposure rates,” Dr. Gentle and associates noted.
Children whose mothers received corticosteroids and magnesium had a 27% lower risk of severe neurodevelopmental impairment or death, compared with those whose mothers only received corticosteroids (adjusted odds ratio, 0.73). Just over a third of children exposed to both interventions (36%) had severe neurodevelopmental impairment or died, compared with 44% of those exposed only to steroids.
Similarly, corticosteroids and magnesium together were associated with approximately half the risk of death or severe neurodevelopmental impairment, compared with magnesium alone (aOR, 0.49) and 34% lower risk, compared with neither therapy (aOR 0.66).
When the researchers uncoupled the outcomes, severe neurodevelopmental impairment rates were similar among all exposure groups, but rates of death were lower among those who received both therapies than among those who received just one or neither therapy.
“The therapeutic mechanism for neuroprotection in children exposed to magnesium sulfate is unclear but may result from neuronal stabilization or anti-inflammatory properties,” Dr. Gentle and colleagues said.
They also compared rates in the exposure groups of grade 3-4 intracranial hemorrhage, which has been linked to poor neurodevelopmental outcomes in extremely preterm children.
“The rate of grade 3-4 intracranial hemorrhage did not differ between children exposed to both antenatal corticosteroids and magnesium sulfate and those exposed to antenatal corticosteroids alone,” they said. “These findings further support data from randomized controlled trials showing benefit for antenatal corticosteroids but not for magnesium sulfate.”
They further noted a Cochrane Review that found significantly reduced risk of severe or any intracranial hemorrhage among children exposed to antenatal corticosteroids. No similar reduction in intracranial hemorrhage occurred in a separate Cochrane Review of antenatal magnesium sulfate trials.
The research was funded by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Center for Advancing Translational Sciences. One author is a consultant for Mednax who has received travel funds. Another author disclosed Catholic Health Professionals of Houston paid honorarium for an ethics talk he gave.
SOURCE: Gentle SJ et al. Obstet. Gynecol. 2020. doi: 10.1097/AOG.0000000000003882.
Children born before 27 weeks’ gestation had lower combined risk of death or severe neurodevelopmental impairment when exposed to antenatal corticosteroids and magnesium sulfate together, compared with exposure of either or neither therapy, according to a prospective observational study published in Obstetrics & Gynecology.
“If there is sufficient time to administer antenatal corticosteroids, there should similarly be sufficient time to administer magnesium sulfate,” wrote Samuel J. Gentle, MD, of the University of Alabama at Birmingham, and colleagues. “Given the lower rate of severe neurodevelopmental impairment or death in children exposed to both antenatal corticosteroids and magnesium sulfate in the present study, compared with those exposed to antenatal corticosteroids alone, increasing the rates of magnesium sulfate exposure through quality improvement or other interventions may improve infant outcomes.”
Although previous randomized controlled trials had shown neurologic benefits of each therapy independently in preterm children, few data exist on extremely preterm children, the authors noted. They also pointed out differences in the findings when they analyzed neurodevelopmental outcomes and death separately.
“Whereas exposure to both therapies was associated with a lower rate of death, exposure to magnesium sulfate in addition to antenatal corticosteroids was not associated with a lower rate of severe neurodevelopmental impairment or components of severe neurodevelopmental impairment including Bayley scores, bilateral hearing impairment, and cerebral palsy,” Dr Gentle and his coauthors wrote.
The researchers used prospectively collected data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Generic Database to track 3,093 children born extremely preterm – from 22 weeks 0 days to 26 weeks 6 days – during 2011-2014.
The researchers compared outcomes of death or severe neurodevelopmental impairment when the children were 18-26 months of corrected age based on whether they had been exposed to antenatal corticosteroids alone (betamethasone or dexamethasone) or antenatal corticosteroids in addition to magnesium sulfate. Severe neurodevelopmental impairment included “severe cerebral palsy, motor or cognitive composite score less than 70 on the Bayley-III exam, bilateral blindness, or bilateral severe functional hearing impairment with or without amplification.”
The researchers also looked at severe neurodevelopmental impairment and death among children with only magnesium sulfate exposure or with no exposure to steroids or magnesium.
In the study population, 73% of infants had been exposed to both therapies, 16% had been exposed to only corticosteroids, 3% to only magnesium sulfate, and 8% to neither therapy.
“Importantly, a larger proportion of mothers unexposed to either therapy, compared with both therapies, received high school or less education or had no maternal private health insurance which may suggest health inequity as a driver for antenatal therapy exposure rates,” Dr. Gentle and associates noted.
Children whose mothers received corticosteroids and magnesium had a 27% lower risk of severe neurodevelopmental impairment or death, compared with those whose mothers only received corticosteroids (adjusted odds ratio, 0.73). Just over a third of children exposed to both interventions (36%) had severe neurodevelopmental impairment or died, compared with 44% of those exposed only to steroids.
Similarly, corticosteroids and magnesium together were associated with approximately half the risk of death or severe neurodevelopmental impairment, compared with magnesium alone (aOR, 0.49) and 34% lower risk, compared with neither therapy (aOR 0.66).
When the researchers uncoupled the outcomes, severe neurodevelopmental impairment rates were similar among all exposure groups, but rates of death were lower among those who received both therapies than among those who received just one or neither therapy.
“The therapeutic mechanism for neuroprotection in children exposed to magnesium sulfate is unclear but may result from neuronal stabilization or anti-inflammatory properties,” Dr. Gentle and colleagues said.
They also compared rates in the exposure groups of grade 3-4 intracranial hemorrhage, which has been linked to poor neurodevelopmental outcomes in extremely preterm children.
“The rate of grade 3-4 intracranial hemorrhage did not differ between children exposed to both antenatal corticosteroids and magnesium sulfate and those exposed to antenatal corticosteroids alone,” they said. “These findings further support data from randomized controlled trials showing benefit for antenatal corticosteroids but not for magnesium sulfate.”
They further noted a Cochrane Review that found significantly reduced risk of severe or any intracranial hemorrhage among children exposed to antenatal corticosteroids. No similar reduction in intracranial hemorrhage occurred in a separate Cochrane Review of antenatal magnesium sulfate trials.
The research was funded by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Center for Advancing Translational Sciences. One author is a consultant for Mednax who has received travel funds. Another author disclosed Catholic Health Professionals of Houston paid honorarium for an ethics talk he gave.
SOURCE: Gentle SJ et al. Obstet. Gynecol. 2020. doi: 10.1097/AOG.0000000000003882.
FROM OBSTETRICS & GYNECOLOGY