User login
U.S. fertility rates fall to record lows
and birth rates for women under age 30 fell to record lows, according to the National Center for Health Statistics.
To be exact – at least as exact as is possible from these provisional data – there were 3,745,540 births in the United States last year. That’s down about 1% from 2018 and is the lowest number of births since 1985, Brady E. Hamilton, PhD, and associates at the NCHS said in a rapid release report.
As births go, so goes the general fertility rate. A 2% decrease from 2018 to 2019 left the fertility rate at its lowest point ever: 58.2 births per 1,000 women aged 15-44 years, compared with 59.1 per 1,000 in 2018, the investigators said, based on data from the National Vital Statistics System.
The total fertility rate – defined as “the number of births that a hypothetical group of 1,000 women would have over their lifetimes, based on the age-specific birth rate in a given year” – also reached a record low of 1,705 births per 1,000 women last year after falling 1% from 2018, they reported.
The falling birth rates did not include women over age 35. The birth rate among women aged 40-44 increased by 2% from 2018, as it reached 12.0 births per 1,000 in 2019. “The rate for this age group has risen almost continuously since 1985 by an average of 3% per year,” Dr. Hamilton and associates wrote.
The birth rate for women aged 30-34 years, 98.3 per 1,000, was down 1% from 2018 but was still the highest for any age category. Among younger women, rates all dropped to record lows: 16.6 (ages 15-19), 66.6 (ages 20-24), and 93.7 (ages 25-29), they said.
Preterm birth rates, on the other hand, rose for the fifth year in a row. The rate for 2019, 10.23% of all births, represents an increase of 2% over 2018 and is “the highest level reported in more than a decade,” the investigators noted.
and birth rates for women under age 30 fell to record lows, according to the National Center for Health Statistics.
To be exact – at least as exact as is possible from these provisional data – there were 3,745,540 births in the United States last year. That’s down about 1% from 2018 and is the lowest number of births since 1985, Brady E. Hamilton, PhD, and associates at the NCHS said in a rapid release report.
As births go, so goes the general fertility rate. A 2% decrease from 2018 to 2019 left the fertility rate at its lowest point ever: 58.2 births per 1,000 women aged 15-44 years, compared with 59.1 per 1,000 in 2018, the investigators said, based on data from the National Vital Statistics System.
The total fertility rate – defined as “the number of births that a hypothetical group of 1,000 women would have over their lifetimes, based on the age-specific birth rate in a given year” – also reached a record low of 1,705 births per 1,000 women last year after falling 1% from 2018, they reported.
The falling birth rates did not include women over age 35. The birth rate among women aged 40-44 increased by 2% from 2018, as it reached 12.0 births per 1,000 in 2019. “The rate for this age group has risen almost continuously since 1985 by an average of 3% per year,” Dr. Hamilton and associates wrote.
The birth rate for women aged 30-34 years, 98.3 per 1,000, was down 1% from 2018 but was still the highest for any age category. Among younger women, rates all dropped to record lows: 16.6 (ages 15-19), 66.6 (ages 20-24), and 93.7 (ages 25-29), they said.
Preterm birth rates, on the other hand, rose for the fifth year in a row. The rate for 2019, 10.23% of all births, represents an increase of 2% over 2018 and is “the highest level reported in more than a decade,” the investigators noted.
and birth rates for women under age 30 fell to record lows, according to the National Center for Health Statistics.
To be exact – at least as exact as is possible from these provisional data – there were 3,745,540 births in the United States last year. That’s down about 1% from 2018 and is the lowest number of births since 1985, Brady E. Hamilton, PhD, and associates at the NCHS said in a rapid release report.
As births go, so goes the general fertility rate. A 2% decrease from 2018 to 2019 left the fertility rate at its lowest point ever: 58.2 births per 1,000 women aged 15-44 years, compared with 59.1 per 1,000 in 2018, the investigators said, based on data from the National Vital Statistics System.
The total fertility rate – defined as “the number of births that a hypothetical group of 1,000 women would have over their lifetimes, based on the age-specific birth rate in a given year” – also reached a record low of 1,705 births per 1,000 women last year after falling 1% from 2018, they reported.
The falling birth rates did not include women over age 35. The birth rate among women aged 40-44 increased by 2% from 2018, as it reached 12.0 births per 1,000 in 2019. “The rate for this age group has risen almost continuously since 1985 by an average of 3% per year,” Dr. Hamilton and associates wrote.
The birth rate for women aged 30-34 years, 98.3 per 1,000, was down 1% from 2018 but was still the highest for any age category. Among younger women, rates all dropped to record lows: 16.6 (ages 15-19), 66.6 (ages 20-24), and 93.7 (ages 25-29), they said.
Preterm birth rates, on the other hand, rose for the fifth year in a row. The rate for 2019, 10.23% of all births, represents an increase of 2% over 2018 and is “the highest level reported in more than a decade,” the investigators noted.
Pedometer use improves postcesarean mobility for high-risk patients
based on data from a randomized trial of 215 patients.
“Patient immobility after surgery is associated with an increased risk of VTE [venous thromboembolism], whereas adequate mobility offers the benefits of enhanced bowel movement resumption and decreasing hospitalization length,” wrote Hadas Ganer Herman, MD, of Tel Aviv University, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers randomized 108 women to a personalized feedback program using pedometers to promote mobility after cesarean delivery; 107 served as controls. Patient demographics and intrapartum experiences, including age, body mass index, and gestation week at delivery, were similar between the groups, as were postpartum complications and the use of analgesics.
Patients who used the pedometers took significantly more steps, compared with controls (5,918 vs. 4,161, P < .001). In addition, women in the pedometer group reported improved physical and mental postpartum recovery and higher levels of satisfaction with their delivery experience, the researchers noted.
The study findings were limited by several factors including potential selection bias among patients who completed the full follow-up, as well as the effect of preset visits from the research team during the study and lack of blinding of the participants. In addition, data on thromboembolic events after hospital discharge were available only through patient phone calls, the researchers noted.
“Our trial is notable for its novelty in exploring an intervention to improve postcesarean delivery mobility, using an objective means of digital step counters,” and for focusing on high-risk patients of clinical interest, Dr. Herman and associates wrote.
Larger studies are needed to explore interventions to improve mobility after cesarean deliveries, they emphasized. However, “because the integration between technology and medicine has continued to evolve and has successfully been proven for additional patient care issues in obstetrics, the current trial offers a basis for interpretation, with the possible use of low-cost interventions such as smart phone applications in maternity wards and simple digital feedback.”
“VTEs are still among the leading causes of maternal morbidity and mortality with peak incidence in the immediate postpartum period,” Martina L. Badell, MD, of Emory University, Atlanta, said in an interview. “As the age and body mass index of our pregnant patients continues to increase, focused attention to prevent VTEs in high-risk populations is very important.”
Dr. Badell said that pedometers are a feasible strategy “provided there is funding available to pay for and provide them.” Pedometers “don’t cause pain/discomfort and can be easily worn and reused. If the hospital isn’t able to provide them, however, then cost could be a barrier to high-risk women using pedometers in the immediate postpartum period.”
“The take-home message is that wearing a pedometer is a simple, low-risk strategy to encourage increased ambulation in a high-risk postpartum population with good patient satisfaction,” Dr. Badell said. The next step for research in this area “is to determine how many steps during the immediate postpartum period is optimal to reduce not only VTE risk, but potentially other postoperative markers such as pain and infection,” she added. Another research question is whether “focused feedback-based pedometers during the prolonged postpartum period result in improved weight loss.”
The researchers had no relevant financial disclosures. Dr. Badell said she had no relevant financial disclosures.
SOURCE: Herman HG et al. Obstet Gynecol. 2020 May 7. doi: 10.1097/AOG.0000000000003879.
based on data from a randomized trial of 215 patients.
“Patient immobility after surgery is associated with an increased risk of VTE [venous thromboembolism], whereas adequate mobility offers the benefits of enhanced bowel movement resumption and decreasing hospitalization length,” wrote Hadas Ganer Herman, MD, of Tel Aviv University, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers randomized 108 women to a personalized feedback program using pedometers to promote mobility after cesarean delivery; 107 served as controls. Patient demographics and intrapartum experiences, including age, body mass index, and gestation week at delivery, were similar between the groups, as were postpartum complications and the use of analgesics.
Patients who used the pedometers took significantly more steps, compared with controls (5,918 vs. 4,161, P < .001). In addition, women in the pedometer group reported improved physical and mental postpartum recovery and higher levels of satisfaction with their delivery experience, the researchers noted.
The study findings were limited by several factors including potential selection bias among patients who completed the full follow-up, as well as the effect of preset visits from the research team during the study and lack of blinding of the participants. In addition, data on thromboembolic events after hospital discharge were available only through patient phone calls, the researchers noted.
“Our trial is notable for its novelty in exploring an intervention to improve postcesarean delivery mobility, using an objective means of digital step counters,” and for focusing on high-risk patients of clinical interest, Dr. Herman and associates wrote.
Larger studies are needed to explore interventions to improve mobility after cesarean deliveries, they emphasized. However, “because the integration between technology and medicine has continued to evolve and has successfully been proven for additional patient care issues in obstetrics, the current trial offers a basis for interpretation, with the possible use of low-cost interventions such as smart phone applications in maternity wards and simple digital feedback.”
“VTEs are still among the leading causes of maternal morbidity and mortality with peak incidence in the immediate postpartum period,” Martina L. Badell, MD, of Emory University, Atlanta, said in an interview. “As the age and body mass index of our pregnant patients continues to increase, focused attention to prevent VTEs in high-risk populations is very important.”
Dr. Badell said that pedometers are a feasible strategy “provided there is funding available to pay for and provide them.” Pedometers “don’t cause pain/discomfort and can be easily worn and reused. If the hospital isn’t able to provide them, however, then cost could be a barrier to high-risk women using pedometers in the immediate postpartum period.”
“The take-home message is that wearing a pedometer is a simple, low-risk strategy to encourage increased ambulation in a high-risk postpartum population with good patient satisfaction,” Dr. Badell said. The next step for research in this area “is to determine how many steps during the immediate postpartum period is optimal to reduce not only VTE risk, but potentially other postoperative markers such as pain and infection,” she added. Another research question is whether “focused feedback-based pedometers during the prolonged postpartum period result in improved weight loss.”
The researchers had no relevant financial disclosures. Dr. Badell said she had no relevant financial disclosures.
SOURCE: Herman HG et al. Obstet Gynecol. 2020 May 7. doi: 10.1097/AOG.0000000000003879.
based on data from a randomized trial of 215 patients.
“Patient immobility after surgery is associated with an increased risk of VTE [venous thromboembolism], whereas adequate mobility offers the benefits of enhanced bowel movement resumption and decreasing hospitalization length,” wrote Hadas Ganer Herman, MD, of Tel Aviv University, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers randomized 108 women to a personalized feedback program using pedometers to promote mobility after cesarean delivery; 107 served as controls. Patient demographics and intrapartum experiences, including age, body mass index, and gestation week at delivery, were similar between the groups, as were postpartum complications and the use of analgesics.
Patients who used the pedometers took significantly more steps, compared with controls (5,918 vs. 4,161, P < .001). In addition, women in the pedometer group reported improved physical and mental postpartum recovery and higher levels of satisfaction with their delivery experience, the researchers noted.
The study findings were limited by several factors including potential selection bias among patients who completed the full follow-up, as well as the effect of preset visits from the research team during the study and lack of blinding of the participants. In addition, data on thromboembolic events after hospital discharge were available only through patient phone calls, the researchers noted.
“Our trial is notable for its novelty in exploring an intervention to improve postcesarean delivery mobility, using an objective means of digital step counters,” and for focusing on high-risk patients of clinical interest, Dr. Herman and associates wrote.
Larger studies are needed to explore interventions to improve mobility after cesarean deliveries, they emphasized. However, “because the integration between technology and medicine has continued to evolve and has successfully been proven for additional patient care issues in obstetrics, the current trial offers a basis for interpretation, with the possible use of low-cost interventions such as smart phone applications in maternity wards and simple digital feedback.”
“VTEs are still among the leading causes of maternal morbidity and mortality with peak incidence in the immediate postpartum period,” Martina L. Badell, MD, of Emory University, Atlanta, said in an interview. “As the age and body mass index of our pregnant patients continues to increase, focused attention to prevent VTEs in high-risk populations is very important.”
Dr. Badell said that pedometers are a feasible strategy “provided there is funding available to pay for and provide them.” Pedometers “don’t cause pain/discomfort and can be easily worn and reused. If the hospital isn’t able to provide them, however, then cost could be a barrier to high-risk women using pedometers in the immediate postpartum period.”
“The take-home message is that wearing a pedometer is a simple, low-risk strategy to encourage increased ambulation in a high-risk postpartum population with good patient satisfaction,” Dr. Badell said. The next step for research in this area “is to determine how many steps during the immediate postpartum period is optimal to reduce not only VTE risk, but potentially other postoperative markers such as pain and infection,” she added. Another research question is whether “focused feedback-based pedometers during the prolonged postpartum period result in improved weight loss.”
The researchers had no relevant financial disclosures. Dr. Badell said she had no relevant financial disclosures.
SOURCE: Herman HG et al. Obstet Gynecol. 2020 May 7. doi: 10.1097/AOG.0000000000003879.
FROM OBSTETRICS & GYNECOLOGY
Managing Trichomonas vaginalis infections
CASE Woman with malodorous vaginal discharge
A 26-year-old nulligravid woman with 2 current sexual partners requests evaluation because she has a yellow-green frothy vaginal discharge that is slightly malodorous. One of her sexual partners has noted a similar discharge from his urethra. On physical examination, the clinician notes that the patient’s discharge emanates from the vaginal mucosa, and the exocervix has multiple punctate hemorrhages. Considerations in this case include:
- What is the most likely diagnosis?
- How should this patient be evaluated and treated?
- Should the patient’s sexual partners be treated?
This clinical scenario is most consistent with a trichomonas infection, although other conditions, including bacterial vaginosis, gonorrhea, and chlamydia infection, must be considered in the differential diagnosis.
In this article, we examine the microbiology, epidemiology, clinical manifestations, and diagnosis and treatment of this common sexually transmitted infection (STI).
The causative microbe
Trichomonas vaginalis is a free-living flagellated protozoan that accounts for almost half of all nonviral STIs globally. It has a predilection for the mucosal epithelium of the genitourinary tract, including the vagina and urethra. Humans are the only known host for T vaginalis. The infection is transmitted through sexual intercourse, and the organism reproduces through binary fission in the lower genital tract of women and in the urethra and prostate of men.
This anaerobic trophozoite has 4 flagella anteriorly and 1 flagellum that projects posteriorly, with an undulating membrane that gives its characteristic motile appearance on saline microscopy.1
T vaginalis infection causes major mechanical stress on epithelial cells, which results in disruption of the plasma cell membrane and, ultimately, cell death. The necrotic cell fragments are then phagocytosed by trichomonads, thus accelerating the infection.2
Groups at risk
Trichomonal infections are not reportable to public health authorities, which makes assessing the true prevalence of infection difficult.
The World Health Organization estimated the incidence of infection to be more than 156 million cases globally in 2016, with a prevalence of 110.4 million people at any one time.3
The 2013-2014 National Health and Nutrition Examination Survey tested 4,057 men and women aged 18 to 59 years for T vaginalis and found a prevalence of 0.5% among men and 1.8% among women.4 The prevalence increased with age. There was a disproportionate burden of trichomonas infections in the non-Hispanic black population, with 4.2% of black men and 8.9% of black women affected.4
Targeted screening of urogenital samples for T vaginalis in a population of US women undergoing Chlamydia trachomatis/Neisseria gonorrhoeae screening demonstrated prevalence rates of 8.7%, 6.7%, and 1.7% for T vaginalis, C trachomatis, and N gonorrhoeae, respectively.5
Differences in prevalence estimates may be due to differences in the varying sensitivity of each testing modality and patient populations. In one study, nucleic acid amplification testing (NAAT) for T vaginalis detected rates as high as 11.3% in women and 6.1% in men undergoing evaluations at STI clinics.6
Continue to: Clinical manifestations of infection...
Clinical manifestations of infection
Most cases of T vaginalis remain in an asymptomatic carrier state, with up to 85% of women and 77% of men reporting no clinical symptoms.1 However, approximately one-third of asymptomatic carriers will experience symptoms within 6 months of infection acquisition. This latency in appearance of clinical symptoms certainly contributes to the high transmission rate of T vaginalis.
Infected men may experience purulent urethritis, dysuria, and postcoital pruritus. Common clinical symptoms in women include abnormal vaginal discharge that may be malodorous, purulent, thin, frothy, and yellow-green, as well as symptoms of dyspareunia and vulvar irritation. Punctate hemorrhages in the cervix (colpitis macularis) and vaginal walls (macular vaginitis) give the characteristic “strawberry appearance,” but these findings are seen in only 2% of affected women.7
Complications in ObGyn patients
Although T vaginalis once was regarded as more of an annoyance than a public health issue, awareness of the infection’s ramifications has increased in recent years. Because of these complications, treatment of both symptomatic and asymptomatic patients is clearly indicated.
Complications of trichomonal infection in men include balanoposthitis, epididymitis, prostatitis, urethritis, and infertility.7 In women, complications include infections of the adnexa, endometrium, and vestibular glands, as well as cervical neoplasia and increased co-infection rates with other STIs, such as bacterial vaginosis, chlamydia infection, gonorrhea, syphilis, and herpes simplex virus type 2.1
Infection in pregnancy. Adverse outcomes in pregnant women with T vaginalis infections at mid-gestation include low birth weight, preterm premature rupture of membranes, preterm delivery, and postpartum endometritis.8 A disproportionately larger share of the low birth weight rate associated with T vaginalis infections occurs in black women compared with white and Hispanic women.8 Perinatal transmission to newborns can cause fever; respiratory difficulties; urinary tract infections; nasal discharge; and, in female infants, vaginal discharge.9,10
Co-infection concerns. The increased rate of co-infection with human immunodeficiency virus type 1 (HIV-1) and T vaginalis is a major concern.11 One study found a higher concentration of HIV-1 in semen samples from men with T vaginalis and symptomatic urethritis.12 Further, T vaginalis was found in 17.4% of women with HIV screened at a public clinic in California, with almost 38% of black women affected.13 Trichomoniasis can increase the risk of HIV-1 acquisition by 1.52-fold (95% confidence interval, 1.04- to 2.24-fold), pointing toward a potential amplifying effect of T vaginalis on HIV transmission rates.14 This association may be based at least in part on the organism’s ability to cause microulcerations in the genital and urinary tract epithelium, thus creating pathways for other microorganisms to enter the vascular system.
Making the diagnosis
The nonspecific symptoms of T vaginalis create a wide differential to consider. Vaginal discharge may be due to bacterial vaginosis, vulvovaginal candidiasis, physiologic discharge, atrophy, and nonspecific inflammation. The presence of malodorous and discolored discharge increases the likelihood of bacterial vaginosis or T vaginalis infection. Pruritus often is associated with candidiasis co-infection.
The diagnosis of trichomoniasis can be confirmed in the outpatient office with the use of saline microscopy, an inexpensive test that is based on observation of motile trichomonads in a wet mount of vaginal fluid. The sensitivity of the wet mount ranges from 44% to 68% compared with culture. Culture, traditionally using Diamond’s medium, has a sensitivity of 81% to 94% and was long the gold standard; however, culture has been replaced largely by molecular and antigen testing.
Three US Food and Drug Administration (FDA)-approved NAATs for T vaginalis currently are on the market; all can detect co-infection with gonorrhea and chlamydia from the same specimen. These tests include the Aptima T vaginalis rRNA target assay (Hologic, Bedford, Massachusetts) and the BD ProbTec T vaginalis Qx (TVQ) amplified DNA assay (BD Diagnostics, Baltimore, Maryland), both of which require up to 8 hours to yield results. The Xpert T vaginalis (TV) assay (Cepheid, Sunnyvale, California) is the first NAAT that is FDA approved for use with male urine (in addition to female urine), and it yields results in 60 to 90 minutes. Sensitivity for these NAAT assays ranges from 88% to 100%.15
Point-of-care testing is preferred for rapid diagnosis and for helping the clinician provide same-visit treatment for STIs. The Solana trichomonas assay (Quidel, San Diego, California) detects T vaginalis DNA and can yield results within 40 minutes, but it requires specialized equipment for running the samples. The AmpliVue trichomonas assay (Quidel, San Diego, California) is similar to the Solana assay but it is contained within a small handheld cartridge that does not require additional equipment. Sensitivities are 92% to 98% for Solona and 90.7% to 100% for AmpliVue. The OSOM trichomonas rapid test (Sekisui, Framingham, Massachusetts) uses antigen-detection immunochromatography to provide results in 10 to 15 minutes, with 83% to 92% sensitivity and 99% specificity for vaginal specimens.15,16
Continue to: The TABLE provides a summary...
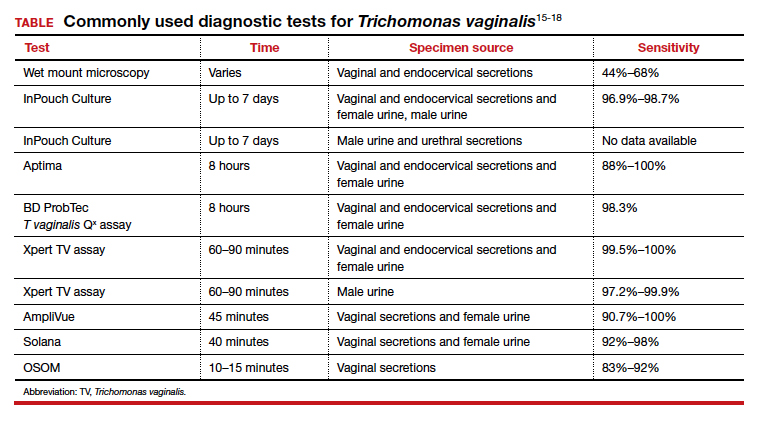
The TABLE provides a summary of the clinical performance of the various tests for T vaginalis. 15-18
Treatment options
The 5-nitroimidazole agents, which include metronidazole and tinidazole, are the preferred agents for the treatment of trichomoniasis.
Dosing regimen. While a single oral dose of metronidazole 2 g has long been the mainstay of treatment for T vaginalis, this regimen recently has been questioned, at least in women, due to the high posttreatment positive rate of T vaginalis, which ranges from 5% to 37%.19,20 These cases may be due to reinfection by untreated sexual partners. They also may result from treatment failure, however, specifically inadequate treatment time.21 Overall, patients treated with single-dose metronidazole are 1.87 times more likely to experience treatment failure compared with those treated with a multidose regimen.19 Since many cases of T vaginalis infection are associated with bacterial vaginosis co-infection, recommending metronidazole 500 mg twice daily for 7 days is beneficial because this course provides optimal treatment for both infections.
Treatment during pregnancy. In the minds of some investigators, treatment of T vaginalis in asymptomatic pregnant women is problematic. One study demonstrated a similar to slightly increased risk of preterm delivery for metronidazole-treated patients compared with a placebo-treated group.22 Limitations of the study included atypical treatment dosing (2 doses of metronidazole 2 g given 48 hours apart at 16 to 23 weeks’ gestation and repeated at 24 to 29 weeks’ gestation) and a latency between the last dose of metronidazole and preterm delivery.22
We believe that all pregnant women, symptomatic or asymptomatic, should be treated because of the sexually transmitted nature of the infection and the probability that most asymptomatic carriers ultimately become symptomatic.
Cost of treatment. Generic oral metronidazole is very inexpensive. The approximate retail price for 14 metronidazole 500-mg tablets is $15.69 (www.goodrx.com). By contrast, a single-dose course of tinidazole (four 500-mg tablets) costs approximately $45. Accordingly, we reserve tinidazole for patients who have experienced a treatment failure with metronidazole or who cannot tolerate metronidazole.
Drug‒alcohol interaction. With both metronidazole and tinidazole, patients must abstain from alcohol during treatment and for 72 hours after completing therapy because these drugs have a disulfiram-like reaction with ethanol.
- Kissinger P. Epidemiology and treatment of trichomoniasis. Curr Infect Dis Rep. 2015;17:484.
- Midlej V, Benchimol M. Trichomonas vaginalis kills and eats—evidence for phagocytic activity as a cytopathic effect. Parasitology. 2010;137:65-76.
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P.
- Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601-2608.
- Schwebke J, Merriweather A, Massingale S, et al. Screening for Trichomonas vaginalis in a large high-risk population: prevalence among men and women determined by nucleic acid amplification testing. Sex Transm Dis. 2018;45:e23-e24.
- Petrin D, Delgaty K, Bhatt R, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300-317.
- Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353-360.
- Smith LM, Wang M, Zangwill K, et al. Trichomonas vaginalis infection in a premature newborn. J Perinatol. 2002;22:502-503.
- Temesvári P, Kerekes A, Tege A, et al. Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiratory failure. J Matern Fetal Neonatal Med. 2002;11:347-349.
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426-433.
- Cohen MS, Hoffman IF, Royce RA, et al; AIDSCAP Malawi Research Group. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868-1873.
- Sorvillo F, Kovacs A, Kerndt P, et al. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495-500.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698-702.
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;8:F1000 Faculty Rev-1666.
- Gaydos CA, Klausner JD, Pai NP, et al. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect. 2017;93(S4):S31-S35.
- Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol. 2013;51:3875-3876.
- Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper System. J Clin Microbiol. 2014;52:885-889.
- Howe K, Kissinger P. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44:29-34.
- Duff P. Should the length of treatment for trichomoniasis in women be reconsidered? OBG Manag. 2017;29(3):48-49.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37:440-444.
- Klebanoff MA, Carey JC, Hauth JC, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487-493.
CASE Woman with malodorous vaginal discharge
A 26-year-old nulligravid woman with 2 current sexual partners requests evaluation because she has a yellow-green frothy vaginal discharge that is slightly malodorous. One of her sexual partners has noted a similar discharge from his urethra. On physical examination, the clinician notes that the patient’s discharge emanates from the vaginal mucosa, and the exocervix has multiple punctate hemorrhages. Considerations in this case include:
- What is the most likely diagnosis?
- How should this patient be evaluated and treated?
- Should the patient’s sexual partners be treated?
This clinical scenario is most consistent with a trichomonas infection, although other conditions, including bacterial vaginosis, gonorrhea, and chlamydia infection, must be considered in the differential diagnosis.
In this article, we examine the microbiology, epidemiology, clinical manifestations, and diagnosis and treatment of this common sexually transmitted infection (STI).
The causative microbe
Trichomonas vaginalis is a free-living flagellated protozoan that accounts for almost half of all nonviral STIs globally. It has a predilection for the mucosal epithelium of the genitourinary tract, including the vagina and urethra. Humans are the only known host for T vaginalis. The infection is transmitted through sexual intercourse, and the organism reproduces through binary fission in the lower genital tract of women and in the urethra and prostate of men.
This anaerobic trophozoite has 4 flagella anteriorly and 1 flagellum that projects posteriorly, with an undulating membrane that gives its characteristic motile appearance on saline microscopy.1
T vaginalis infection causes major mechanical stress on epithelial cells, which results in disruption of the plasma cell membrane and, ultimately, cell death. The necrotic cell fragments are then phagocytosed by trichomonads, thus accelerating the infection.2
Groups at risk
Trichomonal infections are not reportable to public health authorities, which makes assessing the true prevalence of infection difficult.
The World Health Organization estimated the incidence of infection to be more than 156 million cases globally in 2016, with a prevalence of 110.4 million people at any one time.3
The 2013-2014 National Health and Nutrition Examination Survey tested 4,057 men and women aged 18 to 59 years for T vaginalis and found a prevalence of 0.5% among men and 1.8% among women.4 The prevalence increased with age. There was a disproportionate burden of trichomonas infections in the non-Hispanic black population, with 4.2% of black men and 8.9% of black women affected.4
Targeted screening of urogenital samples for T vaginalis in a population of US women undergoing Chlamydia trachomatis/Neisseria gonorrhoeae screening demonstrated prevalence rates of 8.7%, 6.7%, and 1.7% for T vaginalis, C trachomatis, and N gonorrhoeae, respectively.5
Differences in prevalence estimates may be due to differences in the varying sensitivity of each testing modality and patient populations. In one study, nucleic acid amplification testing (NAAT) for T vaginalis detected rates as high as 11.3% in women and 6.1% in men undergoing evaluations at STI clinics.6
Continue to: Clinical manifestations of infection...
Clinical manifestations of infection
Most cases of T vaginalis remain in an asymptomatic carrier state, with up to 85% of women and 77% of men reporting no clinical symptoms.1 However, approximately one-third of asymptomatic carriers will experience symptoms within 6 months of infection acquisition. This latency in appearance of clinical symptoms certainly contributes to the high transmission rate of T vaginalis.
Infected men may experience purulent urethritis, dysuria, and postcoital pruritus. Common clinical symptoms in women include abnormal vaginal discharge that may be malodorous, purulent, thin, frothy, and yellow-green, as well as symptoms of dyspareunia and vulvar irritation. Punctate hemorrhages in the cervix (colpitis macularis) and vaginal walls (macular vaginitis) give the characteristic “strawberry appearance,” but these findings are seen in only 2% of affected women.7
Complications in ObGyn patients
Although T vaginalis once was regarded as more of an annoyance than a public health issue, awareness of the infection’s ramifications has increased in recent years. Because of these complications, treatment of both symptomatic and asymptomatic patients is clearly indicated.
Complications of trichomonal infection in men include balanoposthitis, epididymitis, prostatitis, urethritis, and infertility.7 In women, complications include infections of the adnexa, endometrium, and vestibular glands, as well as cervical neoplasia and increased co-infection rates with other STIs, such as bacterial vaginosis, chlamydia infection, gonorrhea, syphilis, and herpes simplex virus type 2.1
Infection in pregnancy. Adverse outcomes in pregnant women with T vaginalis infections at mid-gestation include low birth weight, preterm premature rupture of membranes, preterm delivery, and postpartum endometritis.8 A disproportionately larger share of the low birth weight rate associated with T vaginalis infections occurs in black women compared with white and Hispanic women.8 Perinatal transmission to newborns can cause fever; respiratory difficulties; urinary tract infections; nasal discharge; and, in female infants, vaginal discharge.9,10
Co-infection concerns. The increased rate of co-infection with human immunodeficiency virus type 1 (HIV-1) and T vaginalis is a major concern.11 One study found a higher concentration of HIV-1 in semen samples from men with T vaginalis and symptomatic urethritis.12 Further, T vaginalis was found in 17.4% of women with HIV screened at a public clinic in California, with almost 38% of black women affected.13 Trichomoniasis can increase the risk of HIV-1 acquisition by 1.52-fold (95% confidence interval, 1.04- to 2.24-fold), pointing toward a potential amplifying effect of T vaginalis on HIV transmission rates.14 This association may be based at least in part on the organism’s ability to cause microulcerations in the genital and urinary tract epithelium, thus creating pathways for other microorganisms to enter the vascular system.
Making the diagnosis
The nonspecific symptoms of T vaginalis create a wide differential to consider. Vaginal discharge may be due to bacterial vaginosis, vulvovaginal candidiasis, physiologic discharge, atrophy, and nonspecific inflammation. The presence of malodorous and discolored discharge increases the likelihood of bacterial vaginosis or T vaginalis infection. Pruritus often is associated with candidiasis co-infection.
The diagnosis of trichomoniasis can be confirmed in the outpatient office with the use of saline microscopy, an inexpensive test that is based on observation of motile trichomonads in a wet mount of vaginal fluid. The sensitivity of the wet mount ranges from 44% to 68% compared with culture. Culture, traditionally using Diamond’s medium, has a sensitivity of 81% to 94% and was long the gold standard; however, culture has been replaced largely by molecular and antigen testing.
Three US Food and Drug Administration (FDA)-approved NAATs for T vaginalis currently are on the market; all can detect co-infection with gonorrhea and chlamydia from the same specimen. These tests include the Aptima T vaginalis rRNA target assay (Hologic, Bedford, Massachusetts) and the BD ProbTec T vaginalis Qx (TVQ) amplified DNA assay (BD Diagnostics, Baltimore, Maryland), both of which require up to 8 hours to yield results. The Xpert T vaginalis (TV) assay (Cepheid, Sunnyvale, California) is the first NAAT that is FDA approved for use with male urine (in addition to female urine), and it yields results in 60 to 90 minutes. Sensitivity for these NAAT assays ranges from 88% to 100%.15
Point-of-care testing is preferred for rapid diagnosis and for helping the clinician provide same-visit treatment for STIs. The Solana trichomonas assay (Quidel, San Diego, California) detects T vaginalis DNA and can yield results within 40 minutes, but it requires specialized equipment for running the samples. The AmpliVue trichomonas assay (Quidel, San Diego, California) is similar to the Solana assay but it is contained within a small handheld cartridge that does not require additional equipment. Sensitivities are 92% to 98% for Solona and 90.7% to 100% for AmpliVue. The OSOM trichomonas rapid test (Sekisui, Framingham, Massachusetts) uses antigen-detection immunochromatography to provide results in 10 to 15 minutes, with 83% to 92% sensitivity and 99% specificity for vaginal specimens.15,16
Continue to: The TABLE provides a summary...
The TABLE provides a summary of the clinical performance of the various tests for T vaginalis. 15-18

Treatment options
The 5-nitroimidazole agents, which include metronidazole and tinidazole, are the preferred agents for the treatment of trichomoniasis.
Dosing regimen. While a single oral dose of metronidazole 2 g has long been the mainstay of treatment for T vaginalis, this regimen recently has been questioned, at least in women, due to the high posttreatment positive rate of T vaginalis, which ranges from 5% to 37%.19,20 These cases may be due to reinfection by untreated sexual partners. They also may result from treatment failure, however, specifically inadequate treatment time.21 Overall, patients treated with single-dose metronidazole are 1.87 times more likely to experience treatment failure compared with those treated with a multidose regimen.19 Since many cases of T vaginalis infection are associated with bacterial vaginosis co-infection, recommending metronidazole 500 mg twice daily for 7 days is beneficial because this course provides optimal treatment for both infections.
Treatment during pregnancy. In the minds of some investigators, treatment of T vaginalis in asymptomatic pregnant women is problematic. One study demonstrated a similar to slightly increased risk of preterm delivery for metronidazole-treated patients compared with a placebo-treated group.22 Limitations of the study included atypical treatment dosing (2 doses of metronidazole 2 g given 48 hours apart at 16 to 23 weeks’ gestation and repeated at 24 to 29 weeks’ gestation) and a latency between the last dose of metronidazole and preterm delivery.22
We believe that all pregnant women, symptomatic or asymptomatic, should be treated because of the sexually transmitted nature of the infection and the probability that most asymptomatic carriers ultimately become symptomatic.
Cost of treatment. Generic oral metronidazole is very inexpensive. The approximate retail price for 14 metronidazole 500-mg tablets is $15.69 (www.goodrx.com). By contrast, a single-dose course of tinidazole (four 500-mg tablets) costs approximately $45. Accordingly, we reserve tinidazole for patients who have experienced a treatment failure with metronidazole or who cannot tolerate metronidazole.
Drug‒alcohol interaction. With both metronidazole and tinidazole, patients must abstain from alcohol during treatment and for 72 hours after completing therapy because these drugs have a disulfiram-like reaction with ethanol.
CASE Woman with malodorous vaginal discharge
A 26-year-old nulligravid woman with 2 current sexual partners requests evaluation because she has a yellow-green frothy vaginal discharge that is slightly malodorous. One of her sexual partners has noted a similar discharge from his urethra. On physical examination, the clinician notes that the patient’s discharge emanates from the vaginal mucosa, and the exocervix has multiple punctate hemorrhages. Considerations in this case include:
- What is the most likely diagnosis?
- How should this patient be evaluated and treated?
- Should the patient’s sexual partners be treated?
This clinical scenario is most consistent with a trichomonas infection, although other conditions, including bacterial vaginosis, gonorrhea, and chlamydia infection, must be considered in the differential diagnosis.
In this article, we examine the microbiology, epidemiology, clinical manifestations, and diagnosis and treatment of this common sexually transmitted infection (STI).
The causative microbe
Trichomonas vaginalis is a free-living flagellated protozoan that accounts for almost half of all nonviral STIs globally. It has a predilection for the mucosal epithelium of the genitourinary tract, including the vagina and urethra. Humans are the only known host for T vaginalis. The infection is transmitted through sexual intercourse, and the organism reproduces through binary fission in the lower genital tract of women and in the urethra and prostate of men.
This anaerobic trophozoite has 4 flagella anteriorly and 1 flagellum that projects posteriorly, with an undulating membrane that gives its characteristic motile appearance on saline microscopy.1
T vaginalis infection causes major mechanical stress on epithelial cells, which results in disruption of the plasma cell membrane and, ultimately, cell death. The necrotic cell fragments are then phagocytosed by trichomonads, thus accelerating the infection.2
Groups at risk
Trichomonal infections are not reportable to public health authorities, which makes assessing the true prevalence of infection difficult.
The World Health Organization estimated the incidence of infection to be more than 156 million cases globally in 2016, with a prevalence of 110.4 million people at any one time.3
The 2013-2014 National Health and Nutrition Examination Survey tested 4,057 men and women aged 18 to 59 years for T vaginalis and found a prevalence of 0.5% among men and 1.8% among women.4 The prevalence increased with age. There was a disproportionate burden of trichomonas infections in the non-Hispanic black population, with 4.2% of black men and 8.9% of black women affected.4
Targeted screening of urogenital samples for T vaginalis in a population of US women undergoing Chlamydia trachomatis/Neisseria gonorrhoeae screening demonstrated prevalence rates of 8.7%, 6.7%, and 1.7% for T vaginalis, C trachomatis, and N gonorrhoeae, respectively.5
Differences in prevalence estimates may be due to differences in the varying sensitivity of each testing modality and patient populations. In one study, nucleic acid amplification testing (NAAT) for T vaginalis detected rates as high as 11.3% in women and 6.1% in men undergoing evaluations at STI clinics.6
Continue to: Clinical manifestations of infection...
Clinical manifestations of infection
Most cases of T vaginalis remain in an asymptomatic carrier state, with up to 85% of women and 77% of men reporting no clinical symptoms.1 However, approximately one-third of asymptomatic carriers will experience symptoms within 6 months of infection acquisition. This latency in appearance of clinical symptoms certainly contributes to the high transmission rate of T vaginalis.
Infected men may experience purulent urethritis, dysuria, and postcoital pruritus. Common clinical symptoms in women include abnormal vaginal discharge that may be malodorous, purulent, thin, frothy, and yellow-green, as well as symptoms of dyspareunia and vulvar irritation. Punctate hemorrhages in the cervix (colpitis macularis) and vaginal walls (macular vaginitis) give the characteristic “strawberry appearance,” but these findings are seen in only 2% of affected women.7
Complications in ObGyn patients
Although T vaginalis once was regarded as more of an annoyance than a public health issue, awareness of the infection’s ramifications has increased in recent years. Because of these complications, treatment of both symptomatic and asymptomatic patients is clearly indicated.
Complications of trichomonal infection in men include balanoposthitis, epididymitis, prostatitis, urethritis, and infertility.7 In women, complications include infections of the adnexa, endometrium, and vestibular glands, as well as cervical neoplasia and increased co-infection rates with other STIs, such as bacterial vaginosis, chlamydia infection, gonorrhea, syphilis, and herpes simplex virus type 2.1
Infection in pregnancy. Adverse outcomes in pregnant women with T vaginalis infections at mid-gestation include low birth weight, preterm premature rupture of membranes, preterm delivery, and postpartum endometritis.8 A disproportionately larger share of the low birth weight rate associated with T vaginalis infections occurs in black women compared with white and Hispanic women.8 Perinatal transmission to newborns can cause fever; respiratory difficulties; urinary tract infections; nasal discharge; and, in female infants, vaginal discharge.9,10
Co-infection concerns. The increased rate of co-infection with human immunodeficiency virus type 1 (HIV-1) and T vaginalis is a major concern.11 One study found a higher concentration of HIV-1 in semen samples from men with T vaginalis and symptomatic urethritis.12 Further, T vaginalis was found in 17.4% of women with HIV screened at a public clinic in California, with almost 38% of black women affected.13 Trichomoniasis can increase the risk of HIV-1 acquisition by 1.52-fold (95% confidence interval, 1.04- to 2.24-fold), pointing toward a potential amplifying effect of T vaginalis on HIV transmission rates.14 This association may be based at least in part on the organism’s ability to cause microulcerations in the genital and urinary tract epithelium, thus creating pathways for other microorganisms to enter the vascular system.
Making the diagnosis
The nonspecific symptoms of T vaginalis create a wide differential to consider. Vaginal discharge may be due to bacterial vaginosis, vulvovaginal candidiasis, physiologic discharge, atrophy, and nonspecific inflammation. The presence of malodorous and discolored discharge increases the likelihood of bacterial vaginosis or T vaginalis infection. Pruritus often is associated with candidiasis co-infection.
The diagnosis of trichomoniasis can be confirmed in the outpatient office with the use of saline microscopy, an inexpensive test that is based on observation of motile trichomonads in a wet mount of vaginal fluid. The sensitivity of the wet mount ranges from 44% to 68% compared with culture. Culture, traditionally using Diamond’s medium, has a sensitivity of 81% to 94% and was long the gold standard; however, culture has been replaced largely by molecular and antigen testing.
Three US Food and Drug Administration (FDA)-approved NAATs for T vaginalis currently are on the market; all can detect co-infection with gonorrhea and chlamydia from the same specimen. These tests include the Aptima T vaginalis rRNA target assay (Hologic, Bedford, Massachusetts) and the BD ProbTec T vaginalis Qx (TVQ) amplified DNA assay (BD Diagnostics, Baltimore, Maryland), both of which require up to 8 hours to yield results. The Xpert T vaginalis (TV) assay (Cepheid, Sunnyvale, California) is the first NAAT that is FDA approved for use with male urine (in addition to female urine), and it yields results in 60 to 90 minutes. Sensitivity for these NAAT assays ranges from 88% to 100%.15
Point-of-care testing is preferred for rapid diagnosis and for helping the clinician provide same-visit treatment for STIs. The Solana trichomonas assay (Quidel, San Diego, California) detects T vaginalis DNA and can yield results within 40 minutes, but it requires specialized equipment for running the samples. The AmpliVue trichomonas assay (Quidel, San Diego, California) is similar to the Solana assay but it is contained within a small handheld cartridge that does not require additional equipment. Sensitivities are 92% to 98% for Solona and 90.7% to 100% for AmpliVue. The OSOM trichomonas rapid test (Sekisui, Framingham, Massachusetts) uses antigen-detection immunochromatography to provide results in 10 to 15 minutes, with 83% to 92% sensitivity and 99% specificity for vaginal specimens.15,16
Continue to: The TABLE provides a summary...
The TABLE provides a summary of the clinical performance of the various tests for T vaginalis. 15-18

Treatment options
The 5-nitroimidazole agents, which include metronidazole and tinidazole, are the preferred agents for the treatment of trichomoniasis.
Dosing regimen. While a single oral dose of metronidazole 2 g has long been the mainstay of treatment for T vaginalis, this regimen recently has been questioned, at least in women, due to the high posttreatment positive rate of T vaginalis, which ranges from 5% to 37%.19,20 These cases may be due to reinfection by untreated sexual partners. They also may result from treatment failure, however, specifically inadequate treatment time.21 Overall, patients treated with single-dose metronidazole are 1.87 times more likely to experience treatment failure compared with those treated with a multidose regimen.19 Since many cases of T vaginalis infection are associated with bacterial vaginosis co-infection, recommending metronidazole 500 mg twice daily for 7 days is beneficial because this course provides optimal treatment for both infections.
Treatment during pregnancy. In the minds of some investigators, treatment of T vaginalis in asymptomatic pregnant women is problematic. One study demonstrated a similar to slightly increased risk of preterm delivery for metronidazole-treated patients compared with a placebo-treated group.22 Limitations of the study included atypical treatment dosing (2 doses of metronidazole 2 g given 48 hours apart at 16 to 23 weeks’ gestation and repeated at 24 to 29 weeks’ gestation) and a latency between the last dose of metronidazole and preterm delivery.22
We believe that all pregnant women, symptomatic or asymptomatic, should be treated because of the sexually transmitted nature of the infection and the probability that most asymptomatic carriers ultimately become symptomatic.
Cost of treatment. Generic oral metronidazole is very inexpensive. The approximate retail price for 14 metronidazole 500-mg tablets is $15.69 (www.goodrx.com). By contrast, a single-dose course of tinidazole (four 500-mg tablets) costs approximately $45. Accordingly, we reserve tinidazole for patients who have experienced a treatment failure with metronidazole or who cannot tolerate metronidazole.
Drug‒alcohol interaction. With both metronidazole and tinidazole, patients must abstain from alcohol during treatment and for 72 hours after completing therapy because these drugs have a disulfiram-like reaction with ethanol.
- Kissinger P. Epidemiology and treatment of trichomoniasis. Curr Infect Dis Rep. 2015;17:484.
- Midlej V, Benchimol M. Trichomonas vaginalis kills and eats—evidence for phagocytic activity as a cytopathic effect. Parasitology. 2010;137:65-76.
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P.
- Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601-2608.
- Schwebke J, Merriweather A, Massingale S, et al. Screening for Trichomonas vaginalis in a large high-risk population: prevalence among men and women determined by nucleic acid amplification testing. Sex Transm Dis. 2018;45:e23-e24.
- Petrin D, Delgaty K, Bhatt R, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300-317.
- Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353-360.
- Smith LM, Wang M, Zangwill K, et al. Trichomonas vaginalis infection in a premature newborn. J Perinatol. 2002;22:502-503.
- Temesvári P, Kerekes A, Tege A, et al. Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiratory failure. J Matern Fetal Neonatal Med. 2002;11:347-349.
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426-433.
- Cohen MS, Hoffman IF, Royce RA, et al; AIDSCAP Malawi Research Group. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868-1873.
- Sorvillo F, Kovacs A, Kerndt P, et al. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495-500.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698-702.
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;8:F1000 Faculty Rev-1666.
- Gaydos CA, Klausner JD, Pai NP, et al. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect. 2017;93(S4):S31-S35.
- Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol. 2013;51:3875-3876.
- Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper System. J Clin Microbiol. 2014;52:885-889.
- Howe K, Kissinger P. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44:29-34.
- Duff P. Should the length of treatment for trichomoniasis in women be reconsidered? OBG Manag. 2017;29(3):48-49.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37:440-444.
- Klebanoff MA, Carey JC, Hauth JC, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487-493.
- Kissinger P. Epidemiology and treatment of trichomoniasis. Curr Infect Dis Rep. 2015;17:484.
- Midlej V, Benchimol M. Trichomonas vaginalis kills and eats—evidence for phagocytic activity as a cytopathic effect. Parasitology. 2010;137:65-76.
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P.
- Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601-2608.
- Schwebke J, Merriweather A, Massingale S, et al. Screening for Trichomonas vaginalis in a large high-risk population: prevalence among men and women determined by nucleic acid amplification testing. Sex Transm Dis. 2018;45:e23-e24.
- Petrin D, Delgaty K, Bhatt R, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300-317.
- Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353-360.
- Smith LM, Wang M, Zangwill K, et al. Trichomonas vaginalis infection in a premature newborn. J Perinatol. 2002;22:502-503.
- Temesvári P, Kerekes A, Tege A, et al. Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiratory failure. J Matern Fetal Neonatal Med. 2002;11:347-349.
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426-433.
- Cohen MS, Hoffman IF, Royce RA, et al; AIDSCAP Malawi Research Group. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868-1873.
- Sorvillo F, Kovacs A, Kerndt P, et al. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495-500.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698-702.
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;8:F1000 Faculty Rev-1666.
- Gaydos CA, Klausner JD, Pai NP, et al. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect. 2017;93(S4):S31-S35.
- Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol. 2013;51:3875-3876.
- Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper System. J Clin Microbiol. 2014;52:885-889.
- Howe K, Kissinger P. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44:29-34.
- Duff P. Should the length of treatment for trichomoniasis in women be reconsidered? OBG Manag. 2017;29(3):48-49.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37:440-444.
- Klebanoff MA, Carey JC, Hauth JC, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487-493.
COVID-19 in pregnancy: Supplement oxygen if saturation dips below 94%
Oxygen supplementation for pregnant women with COVID-19 should begin when saturations fall below 94%, according to physicians in the divisions of maternal-fetal medicine and surgical critical care at the University of Texas Medical Branch at Galveston.
That’s a bit higher than the 92% cut point for nonpregnant women, but necessary due to the increased oxygen demand and oxygen partial pressure in pregnancy. The goal is a saturation of 94%-96%, said Luis Pacheco, MD, a maternal-fetal medicine and critical care specialist at the university, and associates.
so Dr. Pacheco and associates addressed the issue in a commentary in Obstetrics & Gynecology.
Women on respiratory support should lie prone if under 20 weeks’ gestation to help with posterior lung recruitment and oxygenation.
If conventional oxygen therapy isn’t enough, high-flow nasal cannula (HFNC) at 60 L/min and 100% oxygen should be the next step, not positive-pressure ventilation. Positive pressure, another option, kicks off aerosols that increase the risk of viral transmission to medical staff. “This makes high-flow nasal cannula the first-line option for patients not responding to conventional oxygen therapy but who are not yet candidates for endotracheal intubation,” the team said. If women do well, the fraction of inspired oxygen should be weaned before the nasal cannula flow is decreased.
However, if they continue to struggle with dyspnea, tachypnea, and oxygen saturation after 30-60 minutes on HFNC, it’s time for mechanical ventilation, and fast. “Delays in recognizing early failure of high-flow nasal cannula ... may result in life-threatening hypoxemia at the time of induction and intubation (especially in pregnant patients with difficult airway anatomy),” the authors said.
For birth, Dr. Pacheco and associates recommended controlled delivery, likely cesarean, if respiration continues to deteriorate despite intubation, especially after 28 weeks’ gestation, instead of waiting for fetal distress and an ICU delivery. A single course of steroids is reasonable to help fetal lung development beforehand, if indicated.
As for fluid strategy during respiratory support, pregnant women are at higher risk for pulmonary edema with lung inflammation, so the authors cautioned against giving maintenance fluids, and said “if daily positive fluid balances are present, combined with worsening respiratory status, the use of furosemide (10-20 mg intravenously every 12 hours) may be indicated.”
For women stable on conventional oxygen therapy or HFNC, they suggested daily nonstress tests starting at 25 weeks’ gestation instead of continuous monitoring, to minimize the COVID-19 transmission risk for staff.
The team cautioned against nebulized treatments and sputum-inducing agents when possible as this may aerosolize the virus.
There was no external funding for the report, and the authors didn’t have any relevant financial disclosures.
SOURCE: Pacheco LD et al. Obstet Gynecol. 2020 Apr 29. doi: 10.1097/AOG.0000000000003929.
Oxygen supplementation for pregnant women with COVID-19 should begin when saturations fall below 94%, according to physicians in the divisions of maternal-fetal medicine and surgical critical care at the University of Texas Medical Branch at Galveston.
That’s a bit higher than the 92% cut point for nonpregnant women, but necessary due to the increased oxygen demand and oxygen partial pressure in pregnancy. The goal is a saturation of 94%-96%, said Luis Pacheco, MD, a maternal-fetal medicine and critical care specialist at the university, and associates.
so Dr. Pacheco and associates addressed the issue in a commentary in Obstetrics & Gynecology.
Women on respiratory support should lie prone if under 20 weeks’ gestation to help with posterior lung recruitment and oxygenation.
If conventional oxygen therapy isn’t enough, high-flow nasal cannula (HFNC) at 60 L/min and 100% oxygen should be the next step, not positive-pressure ventilation. Positive pressure, another option, kicks off aerosols that increase the risk of viral transmission to medical staff. “This makes high-flow nasal cannula the first-line option for patients not responding to conventional oxygen therapy but who are not yet candidates for endotracheal intubation,” the team said. If women do well, the fraction of inspired oxygen should be weaned before the nasal cannula flow is decreased.
However, if they continue to struggle with dyspnea, tachypnea, and oxygen saturation after 30-60 minutes on HFNC, it’s time for mechanical ventilation, and fast. “Delays in recognizing early failure of high-flow nasal cannula ... may result in life-threatening hypoxemia at the time of induction and intubation (especially in pregnant patients with difficult airway anatomy),” the authors said.
For birth, Dr. Pacheco and associates recommended controlled delivery, likely cesarean, if respiration continues to deteriorate despite intubation, especially after 28 weeks’ gestation, instead of waiting for fetal distress and an ICU delivery. A single course of steroids is reasonable to help fetal lung development beforehand, if indicated.
As for fluid strategy during respiratory support, pregnant women are at higher risk for pulmonary edema with lung inflammation, so the authors cautioned against giving maintenance fluids, and said “if daily positive fluid balances are present, combined with worsening respiratory status, the use of furosemide (10-20 mg intravenously every 12 hours) may be indicated.”
For women stable on conventional oxygen therapy or HFNC, they suggested daily nonstress tests starting at 25 weeks’ gestation instead of continuous monitoring, to minimize the COVID-19 transmission risk for staff.
The team cautioned against nebulized treatments and sputum-inducing agents when possible as this may aerosolize the virus.
There was no external funding for the report, and the authors didn’t have any relevant financial disclosures.
SOURCE: Pacheco LD et al. Obstet Gynecol. 2020 Apr 29. doi: 10.1097/AOG.0000000000003929.
Oxygen supplementation for pregnant women with COVID-19 should begin when saturations fall below 94%, according to physicians in the divisions of maternal-fetal medicine and surgical critical care at the University of Texas Medical Branch at Galveston.
That’s a bit higher than the 92% cut point for nonpregnant women, but necessary due to the increased oxygen demand and oxygen partial pressure in pregnancy. The goal is a saturation of 94%-96%, said Luis Pacheco, MD, a maternal-fetal medicine and critical care specialist at the university, and associates.
so Dr. Pacheco and associates addressed the issue in a commentary in Obstetrics & Gynecology.
Women on respiratory support should lie prone if under 20 weeks’ gestation to help with posterior lung recruitment and oxygenation.
If conventional oxygen therapy isn’t enough, high-flow nasal cannula (HFNC) at 60 L/min and 100% oxygen should be the next step, not positive-pressure ventilation. Positive pressure, another option, kicks off aerosols that increase the risk of viral transmission to medical staff. “This makes high-flow nasal cannula the first-line option for patients not responding to conventional oxygen therapy but who are not yet candidates for endotracheal intubation,” the team said. If women do well, the fraction of inspired oxygen should be weaned before the nasal cannula flow is decreased.
However, if they continue to struggle with dyspnea, tachypnea, and oxygen saturation after 30-60 minutes on HFNC, it’s time for mechanical ventilation, and fast. “Delays in recognizing early failure of high-flow nasal cannula ... may result in life-threatening hypoxemia at the time of induction and intubation (especially in pregnant patients with difficult airway anatomy),” the authors said.
For birth, Dr. Pacheco and associates recommended controlled delivery, likely cesarean, if respiration continues to deteriorate despite intubation, especially after 28 weeks’ gestation, instead of waiting for fetal distress and an ICU delivery. A single course of steroids is reasonable to help fetal lung development beforehand, if indicated.
As for fluid strategy during respiratory support, pregnant women are at higher risk for pulmonary edema with lung inflammation, so the authors cautioned against giving maintenance fluids, and said “if daily positive fluid balances are present, combined with worsening respiratory status, the use of furosemide (10-20 mg intravenously every 12 hours) may be indicated.”
For women stable on conventional oxygen therapy or HFNC, they suggested daily nonstress tests starting at 25 weeks’ gestation instead of continuous monitoring, to minimize the COVID-19 transmission risk for staff.
The team cautioned against nebulized treatments and sputum-inducing agents when possible as this may aerosolize the virus.
There was no external funding for the report, and the authors didn’t have any relevant financial disclosures.
SOURCE: Pacheco LD et al. Obstet Gynecol. 2020 Apr 29. doi: 10.1097/AOG.0000000000003929.
OBSTETRICS & GYNECOLOGY
Cardiovascular health among US pregnant women



A multicenter RCT makes a case for transabdominal cerclage
Since the 1950s, when Shirodkar (1955) and McDonald (1957) published their seminal works detailing a transvaginal method to suture a “weak” cervix, clinicians and researchers have debated the indications for and utility of cerclage for preventing pregnancy loss and preterm birth.1,2
Originally based on a history of recurrent mid-trimester loss (that is, a clinical diagnosis of cervical insufficiency), cerclage has been expanded to capture both ultrasonography and physical-exam indications. While cerclage has proven useful in select patient populations, an infrequent but vexing problem is what to do when a woman has experienced 1 or more (transvaginal) cerclage “failures.”
With a dearth of well-controlled, randomized data to support the use of cerclage for either history- or physical-exam indications, it is not surprising that we still debate whether the Shirodkar method is superior to the McDonald technique as well as how to best manage a patient when either or both methods previously resulted in an unsatisfactory outcome.
First randomized study to directly compare cerclage techniques
Fortunately, Shennan and colleagues in the United Kingdom have greatly enlarged our knowledge in this area by performing the first well-powered, 3-arm, randomized trial of transabdominal cerclage (TAC) compared with both high and low vaginal cerclage (HVC, LVC).3 They analyzed data for 111 women who were randomly assigned to TAC
(n = 39), HVC (n = 39), or LVC (n = 33).
Interestingly, the investigators chose to not attach conventional eponymous labels to their transvaginal methods, and they do not even provide a reference or detailed description of the surgical methods, telling us instead that, “Techniques used were left to the local clinician’s discretion.” Writing also that HVC cases, like the transabdominal surgeries, were carried out in specialty centers, they implied that additional training was required for the HVC. I inferred that indeed they actually were performing the McDonald and Shirodkar transvaginal methods and with possible by-physician, local modifications.
I am certain that the authors’ results did not surprise proponents of transabdominal cerclage for transvaginal cerclage failures, defined in this trial as prior birth from 14 to 28 weeks’ gestation. Since some clinicians use a more generous definition of cerclage failure (such as birth at less than 34 weeks), this study population was clearly at high risk for poor outcomes; in fact, more than 90% of each group had experienced at least 2 prior mid-trimester losses. As anticipated with randomization, other characteristics were well distributed across the 3 groups.
Continue to: Transabdominal cerclage significantly reduced preterm birth rates...
Transabdominal cerclage significantly reduced preterm birth rates
Using a primary outcome of preterm birth less than 32 weeks, which concentrates neonatal morbidities, the investigators observed an overall 4.5-fold higher rate of preterm birth in the transvaginal cohorts compared with the transabdominal patients (33% and 38% versus 8%, respectively). Comparing the TAC group individually with both LVC and HVC groups, the relative risk of preterm birth was 0.20 compared with the HVC group and 0.23 compared with the LVC group, reflecting an approximate 80% reduction.
Not surprising to me, the investigators observed nearly identical outcomes between the HVC and LVC cohorts, substantiating my bias that the 2 transvaginal methods are similarly effective. Opponents will quickly remind me that the study was not well-powered to detect a clinically significant difference between these 2 groups; touché!
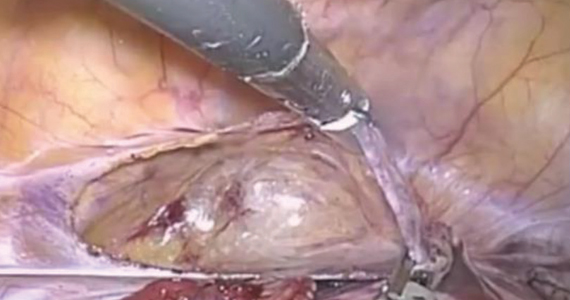
Risks of TAC. We all know that, despite its now-proven benefits, the transabdominal approach is associated with a risk of special complications, including the surgical risks of placement (and removal) of the cerclage, the management of fetal death beyond approximately 14 weeks, and the absolute requisite for hysterotomy/cesarean birth. While serious complications are rare, in the trial by Shennan and colleagues none were recorded in the 39 TAC cases. Nevertheless, for women with no children or only prior early births, the risks seem to be justified; the number needed to treat was less than 4 to prevent 1 birth at less than 32 weeks and was 5.3 to prevent a fetal loss.
TAC is an option for select patients
Given that TAC now can be successfully placed using minimally invasive surgery, either prior to or following conception, this study provides unique level I evidence that should not be discounted and should further be considered in the context of confirming prior cohort studies that suggested a significant benefit. Although specialized training is required and the procedure may involve travel to a specialty center, the weight of clinical data clearly supports the use of TAC.
In summary, based largely on the trial by Shennan and colleagues, women with prior failed vaginal cerclage can and should be counseled regarding the availability of TAC and given the opportunity to weigh the reported risks and benefits. ●
1. Shirodkar VN. A new method of operative treatment for habitual abortion in the second trimester of pregnancy. Antiseptic. 1955;52:299-303.
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynecol Br Emp. 1957;64:346-350.
3. Shennan A, Chandiramani M, Bennett P, et al. MAVRIC: a multicenter randomized trial of transabdominal vs transvaginal cervical cerclage. Am J Obstet Gynecol. 2020;222:261.e1-261.e9.
Since the 1950s, when Shirodkar (1955) and McDonald (1957) published their seminal works detailing a transvaginal method to suture a “weak” cervix, clinicians and researchers have debated the indications for and utility of cerclage for preventing pregnancy loss and preterm birth.1,2
Originally based on a history of recurrent mid-trimester loss (that is, a clinical diagnosis of cervical insufficiency), cerclage has been expanded to capture both ultrasonography and physical-exam indications. While cerclage has proven useful in select patient populations, an infrequent but vexing problem is what to do when a woman has experienced 1 or more (transvaginal) cerclage “failures.”
With a dearth of well-controlled, randomized data to support the use of cerclage for either history- or physical-exam indications, it is not surprising that we still debate whether the Shirodkar method is superior to the McDonald technique as well as how to best manage a patient when either or both methods previously resulted in an unsatisfactory outcome.
First randomized study to directly compare cerclage techniques
Fortunately, Shennan and colleagues in the United Kingdom have greatly enlarged our knowledge in this area by performing the first well-powered, 3-arm, randomized trial of transabdominal cerclage (TAC) compared with both high and low vaginal cerclage (HVC, LVC).3 They analyzed data for 111 women who were randomly assigned to TAC
(n = 39), HVC (n = 39), or LVC (n = 33).
Interestingly, the investigators chose to not attach conventional eponymous labels to their transvaginal methods, and they do not even provide a reference or detailed description of the surgical methods, telling us instead that, “Techniques used were left to the local clinician’s discretion.” Writing also that HVC cases, like the transabdominal surgeries, were carried out in specialty centers, they implied that additional training was required for the HVC. I inferred that indeed they actually were performing the McDonald and Shirodkar transvaginal methods and with possible by-physician, local modifications.
I am certain that the authors’ results did not surprise proponents of transabdominal cerclage for transvaginal cerclage failures, defined in this trial as prior birth from 14 to 28 weeks’ gestation. Since some clinicians use a more generous definition of cerclage failure (such as birth at less than 34 weeks), this study population was clearly at high risk for poor outcomes; in fact, more than 90% of each group had experienced at least 2 prior mid-trimester losses. As anticipated with randomization, other characteristics were well distributed across the 3 groups.
Continue to: Transabdominal cerclage significantly reduced preterm birth rates...
Transabdominal cerclage significantly reduced preterm birth rates
Using a primary outcome of preterm birth less than 32 weeks, which concentrates neonatal morbidities, the investigators observed an overall 4.5-fold higher rate of preterm birth in the transvaginal cohorts compared with the transabdominal patients (33% and 38% versus 8%, respectively). Comparing the TAC group individually with both LVC and HVC groups, the relative risk of preterm birth was 0.20 compared with the HVC group and 0.23 compared with the LVC group, reflecting an approximate 80% reduction.
Not surprising to me, the investigators observed nearly identical outcomes between the HVC and LVC cohorts, substantiating my bias that the 2 transvaginal methods are similarly effective. Opponents will quickly remind me that the study was not well-powered to detect a clinically significant difference between these 2 groups; touché!
Risks of TAC. We all know that, despite its now-proven benefits, the transabdominal approach is associated with a risk of special complications, including the surgical risks of placement (and removal) of the cerclage, the management of fetal death beyond approximately 14 weeks, and the absolute requisite for hysterotomy/cesarean birth. While serious complications are rare, in the trial by Shennan and colleagues none were recorded in the 39 TAC cases. Nevertheless, for women with no children or only prior early births, the risks seem to be justified; the number needed to treat was less than 4 to prevent 1 birth at less than 32 weeks and was 5.3 to prevent a fetal loss.
TAC is an option for select patients
Given that TAC now can be successfully placed using minimally invasive surgery, either prior to or following conception, this study provides unique level I evidence that should not be discounted and should further be considered in the context of confirming prior cohort studies that suggested a significant benefit. Although specialized training is required and the procedure may involve travel to a specialty center, the weight of clinical data clearly supports the use of TAC.
In summary, based largely on the trial by Shennan and colleagues, women with prior failed vaginal cerclage can and should be counseled regarding the availability of TAC and given the opportunity to weigh the reported risks and benefits. ●
Since the 1950s, when Shirodkar (1955) and McDonald (1957) published their seminal works detailing a transvaginal method to suture a “weak” cervix, clinicians and researchers have debated the indications for and utility of cerclage for preventing pregnancy loss and preterm birth.1,2
Originally based on a history of recurrent mid-trimester loss (that is, a clinical diagnosis of cervical insufficiency), cerclage has been expanded to capture both ultrasonography and physical-exam indications. While cerclage has proven useful in select patient populations, an infrequent but vexing problem is what to do when a woman has experienced 1 or more (transvaginal) cerclage “failures.”
With a dearth of well-controlled, randomized data to support the use of cerclage for either history- or physical-exam indications, it is not surprising that we still debate whether the Shirodkar method is superior to the McDonald technique as well as how to best manage a patient when either or both methods previously resulted in an unsatisfactory outcome.
First randomized study to directly compare cerclage techniques
Fortunately, Shennan and colleagues in the United Kingdom have greatly enlarged our knowledge in this area by performing the first well-powered, 3-arm, randomized trial of transabdominal cerclage (TAC) compared with both high and low vaginal cerclage (HVC, LVC).3 They analyzed data for 111 women who were randomly assigned to TAC
(n = 39), HVC (n = 39), or LVC (n = 33).
Interestingly, the investigators chose to not attach conventional eponymous labels to their transvaginal methods, and they do not even provide a reference or detailed description of the surgical methods, telling us instead that, “Techniques used were left to the local clinician’s discretion.” Writing also that HVC cases, like the transabdominal surgeries, were carried out in specialty centers, they implied that additional training was required for the HVC. I inferred that indeed they actually were performing the McDonald and Shirodkar transvaginal methods and with possible by-physician, local modifications.
I am certain that the authors’ results did not surprise proponents of transabdominal cerclage for transvaginal cerclage failures, defined in this trial as prior birth from 14 to 28 weeks’ gestation. Since some clinicians use a more generous definition of cerclage failure (such as birth at less than 34 weeks), this study population was clearly at high risk for poor outcomes; in fact, more than 90% of each group had experienced at least 2 prior mid-trimester losses. As anticipated with randomization, other characteristics were well distributed across the 3 groups.
Continue to: Transabdominal cerclage significantly reduced preterm birth rates...
Transabdominal cerclage significantly reduced preterm birth rates
Using a primary outcome of preterm birth less than 32 weeks, which concentrates neonatal morbidities, the investigators observed an overall 4.5-fold higher rate of preterm birth in the transvaginal cohorts compared with the transabdominal patients (33% and 38% versus 8%, respectively). Comparing the TAC group individually with both LVC and HVC groups, the relative risk of preterm birth was 0.20 compared with the HVC group and 0.23 compared with the LVC group, reflecting an approximate 80% reduction.
Not surprising to me, the investigators observed nearly identical outcomes between the HVC and LVC cohorts, substantiating my bias that the 2 transvaginal methods are similarly effective. Opponents will quickly remind me that the study was not well-powered to detect a clinically significant difference between these 2 groups; touché!
Risks of TAC. We all know that, despite its now-proven benefits, the transabdominal approach is associated with a risk of special complications, including the surgical risks of placement (and removal) of the cerclage, the management of fetal death beyond approximately 14 weeks, and the absolute requisite for hysterotomy/cesarean birth. While serious complications are rare, in the trial by Shennan and colleagues none were recorded in the 39 TAC cases. Nevertheless, for women with no children or only prior early births, the risks seem to be justified; the number needed to treat was less than 4 to prevent 1 birth at less than 32 weeks and was 5.3 to prevent a fetal loss.
TAC is an option for select patients
Given that TAC now can be successfully placed using minimally invasive surgery, either prior to or following conception, this study provides unique level I evidence that should not be discounted and should further be considered in the context of confirming prior cohort studies that suggested a significant benefit. Although specialized training is required and the procedure may involve travel to a specialty center, the weight of clinical data clearly supports the use of TAC.
In summary, based largely on the trial by Shennan and colleagues, women with prior failed vaginal cerclage can and should be counseled regarding the availability of TAC and given the opportunity to weigh the reported risks and benefits. ●
1. Shirodkar VN. A new method of operative treatment for habitual abortion in the second trimester of pregnancy. Antiseptic. 1955;52:299-303.
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynecol Br Emp. 1957;64:346-350.
3. Shennan A, Chandiramani M, Bennett P, et al. MAVRIC: a multicenter randomized trial of transabdominal vs transvaginal cervical cerclage. Am J Obstet Gynecol. 2020;222:261.e1-261.e9.
1. Shirodkar VN. A new method of operative treatment for habitual abortion in the second trimester of pregnancy. Antiseptic. 1955;52:299-303.
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynecol Br Emp. 1957;64:346-350.
3. Shennan A, Chandiramani M, Bennett P, et al. MAVRIC: a multicenter randomized trial of transabdominal vs transvaginal cervical cerclage. Am J Obstet Gynecol. 2020;222:261.e1-261.e9.
Transabdominal cerclage for managing recurrent pregnancy loss
CASE A woman with recurrent pregnancy loss
A 38-year-old woman (G4P0221) presents to your office for preconception counseling. Her history is significant for the following: a spontaneous pregnancy loss at 15 weeks’ gestation; a pregnancy loss at 17 weeks secondary to preterm premature rupture of membranes (PPROM); a cesarean delivery at 30 weeks and 6 days’ gestation after placement of a transvaginal cerclage at 20 weeks for cervical dilation noted on physical exam (the child now has developmental delays); and most recently a delivery at 24 weeks and 4 days due to preterm labor with subsequent neonatal demise (this followed a transvaginal cerclage placed at 13 weeks and 6 days).
How would you counsel this patient?
Cervical insufficiency describes the inability of the cervix to retain a pregnancy in the absence of the signs and symptoms of clinical contractions, labor, or both in the second trimester.1 This condition affects an estimated 1% of obstetric patients and 8% of women with recurrent losses who have experienced a second-trimester loss.2
Diagnosis of cervical insufficiency is based on a history of painless cervical dilation after the first trimester with expulsion of the pregnancy in the second trimester before 24 weeks of gestation without contractions and in the absence of other pathology, such as bleeding, infection, or ruptured membranes.1 Diagnosis also can be made by noting cervical dilation on physical exam during the second trimester; more recently, short cervical length on transvaginal ultrasonography in the second trimester has been used to try to predict when a cervical cerclage may be indicated, although sonographic cervical length is more a marker for risk of preterm birth than for cervical insufficiency specifically.1,3
Given the considerable emotional and physical distress that patients experience with recurrent second-trimester losses and the significant neonatal morbidity and mortality that can occur with preterm delivery, substantial efforts are made to prevent these outcomes by treating patients with cervical insufficiency and those at risk for preterm delivery.
Transvaginal cerclage: A treatment mainstay
Standard treatment options for cervical insufficiency depend on the patient’s history. One of the treatment mainstays for women with prior second-trimester losses or preterm deliveries is transvaginal cervical cerclage. A transvaginal cerclage can be placed using either a Shirodkar technique, in which the vesicocervical mucosa is dissected and a suture is placed as close to the internal cervical os as possible, or a McDonald technique, in which a purse-string suture is placed around the cervicovaginal junction. No randomized trials have compared the effectiveness of these 2 methods, but most observational studies show no difference, and one suggests that the Shirodkar technique may be more effective in obese women specifically.4-6
Indications for transvaginal cerclage. The indication for transvaginal cerclage is based on history, physical exam, or ultrasonography.
A physical-exam indication is the most straightforward of the 3. Transvaginal cerclage placement is indicated if on physical exam in the second trimester a patient has cervical dilation without contractions or infection.1,7
A history-indicated cerclage (typically placed between 12 and 14 weeks’ gestation) is based on a cerclage having been placed in a prior pregnancy due to painless cervical dilation in the second trimester (either ultrasonography- or physical-exam indicated), and it also can be considered in the case of a history of 1 or more second-trimester pregnancy losses related to painless cervical dilation.1
More recent evidence suggests that in patients with 1 prior second-trimester loss or preterm delivery, serial sonographic cervical length can be measured safely from 16 to 24 weeks, with a cerclage being placed only if cervical length decreases to less than 25 mm. By using the ultrasonography-based indication, unnecessary history-indicated cerclages for 1 prior second-trimester or preterm birth can be avoided in more than one-half of patients (FIGURE 1).1,7
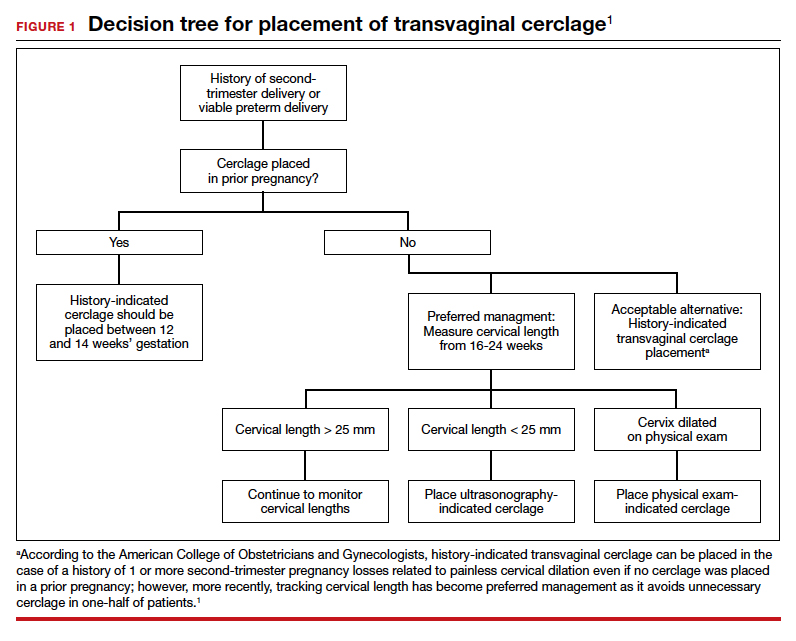
Efficacy. The effectiveness of transvaginal cerclage varies by the indication. Authors of a 2017 Cochrane review found an overall reduced risk of giving birth before 34 weeks’ gestation for any indication, with an average relative risk of 0.77.2 Other recent studies showed the following8-10:
- a 63% delivery rate after 28 weeks’ gestation for physical-exam indicated cerclages in the presence of bulging amniotic membranes
- an 86.2% delivery rate after 32 weeks’ gestation for ultrasonography-indicated cerclages
- an 86% delivery rate after 32 weeks’ gestation for a history-indicated cerclage in patients with 2 or more prior second-trimester losses.
Success rates, especially for ultrasonography- and history-indicated cerclage, are thus high. For the 14% who still fail these methods, however, a different management strategy is needed, which is where transabdominal cerclage comes into play.
Continue to: Transabdominal cerclage is an option for certain patients...
Transabdominal cerclage is an option for certain patients
In transabdominal cerclage, an abdominal approach is used to place a stitch at the cervicouterine junction. With this approach, the cerclage can reach a closer proximity to the internal os compared with the vaginal approach, providing better support of the cervical tissue (FIGURE 2).11 Whether performed via laparotomy or laparoscopy, the transabdominal cerclage procedure likely carries higher morbidity than a transvaginal approach, and cesarean delivery is required after placement.

Since transvaginal cerclage often is successful, in most cases the transabdominal approach should not be viewed as the first-line treatment for cervical insufficiency if a history-indicated transvaginal cerclage has not been attempted. For women who fail a history-indicated transvaginal cerclage, however, a transabdominal cerclage has been proven to decrease the rate of preterm delivery and PPROM compared with attempting another history-indicated transvaginal cerclage.11,12
A recent systematic review of pregnancy outcomes after transabdominal cerclage placement reported neonatal survival of 96.5% and an 83% delivery rate after 34 weeks’ gestation.13 Thus, even among a population that failed transvaginal cerclage, a transabdominal cerclage has a high success rate in providing a good pregnancy outcome (TABLE). Transabdominal cerclage also can be considered as first-line treatment in patients who had prior cervical surgery or cervical deformities that might preclude the ability to place a cerclage transvaginally.

CASE Continued: A candidate for transabdominal cerclage
Given the patient’s poor obstetric history, which includes a preterm delivery and neonatal loss despite a history-indicated cerclage, you recommend that the patient have a transabdominal cerclage placed as the procedure has been proven to increase the chances of neonatal survival and delivery after 34 weeks in women with a similar obstetric history. The patient is interested in this option and asks about how this cerclage is placed and when it would need to be placed during her next pregnancy.
Surgical technique for transabdominal cerclage placement
A transabdominal cerclage can be placed via laparotomy, laparoscopy, or robot-assisted laparoscopy. No differences in obstetric outcomes have been shown between the laparotomy and laparoscopic approaches.14,15 Given the benefits of minimally invasive surgery, a laparoscopic or robot-assisted approach is preferred when feasible.
Additionally, for ease of placement, transabdominal cerclage can be placed prior to conception—known as interval placement—or during pregnancy between 10 and 14 weeks (preferably closer to 10 weeks). Because of the increased difficulty in placing a cerclage in the gravid uterus, interval transabdominal cerclage placement is recommended when possible.13,16 Authors of one observational study noted that improved obstetric outcomes occurred with interval placement compared with cerclage placement between 9 and 10 weeks’ gestation, with a delivery rate at more than 34 weeks’ gestation in 90% versus 74% of patients, respectively.16
Continue to: Steps for interval cerclage and during pregnancy...
Steps for interval cerclage and during pregnancy
Our practice is to place transabdominal cerclage via conventional laparoscopy as an interval procedure when possible. We find no benefit in using robotic assistance.
For an interval procedure, the patient is placed in a dorsal lithotomy position, and we place a 10-mm umbilical port, 2 lateral 5-mm ports, 1 suprapubic 5-mm port, and a uterine manipulator. We use a flexible laparoscope to provide optimal visualization of the pelvis from any angle.
The first step of the surgery involves dissecting the vesicouterine peritoneum in order to move the bladder inferiorly (FIGURE 3A). Uterine arteries are then identified lateral to the cervix as part of this dissection, and a window is created in the inferior aspect of the broad ligament just anterior and lateral to the insertion of the uterosacral ligaments onto the uterus, with care taken to avoid the uterine vessels superiorly (FIGURE 3B). Two 5-mm Mersilene tape sutures are then tied together to create 1 suture with a needle at each end. This is then passed into the abdomen, and 1 needle is passed through the parametrial space at the level of the internal os inferior to the uterine vessels on 1 side of the uterus while the other needle is passed through the parametrial space on the opposite side.
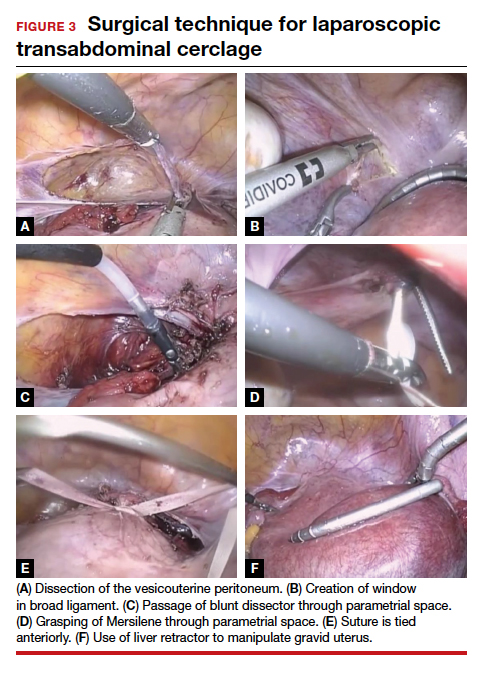
Alternatively, rather than using the suture needles, a blunt dissector can be passed through this same space bilaterally (FIGURE 3C) via the suprapubic port and can pull the Mersilene tape through the parametrial space (FIGURE 3D). The suture is then tied anterior at the level of the internal os intracorporally (FIGURE 3E), and the needles are cut off the suture and removed from the abdomen.
To perform transabdominal cerclage when the patient is pregnant, a few modifications are needed to help with placement. First, the patient may be placed in supine position since a uterine manipulator cannot be used. Second, use of a flexible laparoscope becomes even more imperative in order to properly see around the gravid uterus. Lastly, a 5-mm laparoscopic liver retractor can be used to aid in blunt manipulation of the gravid uterus (FIGURE 3F). (The surgical video below highlights the steps to transabdominal cerclage placement in a pregnant patient.) All other port placements and steps to dissection and suture placement are the same as in interval placement.

CASE Continued: Patient pursues transabdominal cerclage
You explain to your patient that ideally the cerclage should be placed now in a laparoscopic fashion before she becomes pregnant. You then refer her to a local gynecologic surgeon who places many laparoscopic transabdominal cerclages. She undergoes the procedure, becomes pregnant, and after presenting in labor at 35 weeks’ gestation has a cesarean delivery. Her baby is born without any neonatal complications, and the patient is overjoyed with the outcome.
Management during and after pregnancy
Pregnant patients with a transabdominal cerclage are precluded from having a vaginal delivery and must deliver via cesarean. During the antepartum period, patients are managed in the same manner as those who have a transvaginal cerclage. Delivery via cesarean at the onset of regular contractions is recommended to reduce the risk of uterine rupture. In the absence of labor, scheduled cesarean is performed at term.
Our practice is to schedule cesarean delivery at 38 weeks’ gestation, although there are no data or consensus to support a specific gestational age between 37 and 39 weeks. Unlike a transvaginal cerclage, a transabdominal cerclage can be left in place for use in subsequent pregnancies. Data are limited on whether the transabdominal cerclage should be removed in women who no longer desire childbearing and whether there are long-term sequelae if the suture is left in situ.17
Continue to: Complications and risks of abdominal cerclage...
Complications and risks of abdominal cerclage
As the data suggest and our experience confirms, transabdominal cerclage is highly successful in patients who have failed a history-indicated transvaginal cerclage; however, the transabdominal approach carries a higher surgical risk. Risks include intraoperative hemorrhage, conversion to laparotomy, and a range of rare surgical and obstetric complications, such as bladder injury and PPROM.13,18
If a patient experiences a fetal loss in the first trimester, a dilation and curettage (D&C) can be performed, with good obstetric outcomes in subsequent pregnancies.19 If the patient experiences an early-to-mid second-trimester loss, some studies suggest that a dilation and evacuation (D&E) of the uterus can be done with sufficient dilation of the cervix to accommodate up to a 15-mm cannula and Sopher forceps.19 Laminaria also may be used in this process. However, no data exist regarding success of future pregnancies and transabdominal cerclage integrity after a D&E.20 If the cerclage prevents successful dilation of the cervix, the cerclage must be removed laparoscopically prior to performing the D&E.
In late second-trimester and third-trimester loss, the cerclage must be removed to allow passage of the fetus and placenta prior to a D&E or an induction of labor.20
For patients with PPROM or preterm labor, data are limited regarding management recommendations. However, in these complex cases, we strongly recommend an individualized approach and co-management with maternal-fetal medicine specialists.
CASE Resolved
The cerclage is left in place during the patient’s cesarean delivery, and her postpartum course is uneventful. She continued without complications for the next year, at which time she sees you in the office with plans to have another pregnancy later in the year. You counsel her that her abdominal cerclage will still be effective and that she can get pregnant with expectations of similar outcomes as her previous pregnancy. She thanks you for everything and reports that she hopes to return later in the year for her first prenatal visit. ●
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 142: Cerclage for the management of cervical insufficiency. Obstet Gynecol. 2014;123(2 pt 1): 372-379.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6(6):CD008991.
- Brown R, Gagnon R, Delisle M-F. No. 373—cervical insufficiency and cervical cerclage. J Obstet Gynaecol Can. 2019;41:233-247.
- Odibo AO, Berghella V, To MS, et al. Shirodkar versus McDonald cerclage for the prevention of preterm birth in women with short cervical length. Am J Perinatol. 2007;24: 55-60.
- Basbug A, Bayrak M, Dogan O, et al. McDonald versus modified Shirodkar rescue cerclage in women with prolapsed fetal membranes. J Matern Fetal Neonatal Med. 2020;33: 1075-1097.
- Figueroa R, Crowell R, Martinez A, et al. McDonald versus Shirodkar cervical cerclage for the prevention of preterm birth: impact of body mass index. J Matern Fetal Neonatal Med. 2019;32:3408-3414.
- Suhag A, Berghella V. Cervical cerclage. Clin Obstet Gynecol. 2014;57:557-567.
- Bayrak M, Gul A, Goynumer G. Rescue cerclage when foetal membranes prolapse into the vagina. J Obstet Gynaecol. 2017;37:471-475.
- Drassinower D, Coviello E, Landy HJ, et al. Outcomes after periviable ultrasound-indicated cerclage. J Matern Fetal Neonatal Med. 2019;32:932-938.
- Lee KN, Whang EJ, Chang KH, et al. History-indicated cerclage: the association between previous preterm history and cerclage outcome. Obstet Gynecol Sci. 2018;61:23-29. doi:10.5468/ogs.2018.61.1.23.
- Sneider K, Christiansen OB, Sundtoft IB, et al. Recurrence rates after abdominal and vaginal cerclages in women with cervical insufficiency: a validated cohort study. Arch Gynecol Obstet. 2017;295:859-866.
- Davis G, Berghella V, Talucci M, et al. Patients with a prior failed transvaginal cerclage: a comparison of obstetric outcomes with either transabdominal or transvaginal cerclage. Am J Obstet Gynecol. 2000;183:836-839.
- Moawad GN, Tyan P, Bracke T, et al. Systematic review of transabdominal cerclage placed via laparoscopy for the prevention of preterm birth. J Mimim Invasive Gynecol. 2018;25:277-286.
- Burger NB, Brölmann HAM, Einarsson JI, et al. Effectiveness of abdominal cerclage placed via laparotomy or laparoscopy: systematic review. J Minim Invasive Gynecol. 2011;18:696-704.
- Kim S, Hill A, Menderes G, et al. Minimally invasive abdominal cerclage compared to laparotomy: a comparison of surgical and obstetric outcomes. J Robot Surg. 2018;12:295-301.
- Dawood F, Farquharson RG. Transabdominal cerclage: preconceptual versus first trimester insertion. Eur J Obstet Gynecol Reprod Biol. 2016;199:27-31.
- Hawkins E, Nimaroff M. Vaginal erosion of an abdominal cerclage 7 years after laparoscopic placement. Obstet Gynecol. 2014;123(2 pt 2 suppl 2):420-423.
- Foster TL, Moore ES, Sumners JE. Operative complications and fetal morbidity encountered in 300 prophylactic transabdominal cervical cerclage procedures by one obstetric surgeon. J Obstet Gynaecol. 2011;31:713-717.
- Dethier D, Lassey SC, Pilliod R, et al. Uterine evacuation in the setting of transabdominal cerclage. Contraception. 2020;101:174-177.
- Martin A, Lathrop E. Controversies in family planning: management of second-trimester losses in the setting of an abdominal cerclage. Contraception. 2013;87:728-731.
CASE A woman with recurrent pregnancy loss
A 38-year-old woman (G4P0221) presents to your office for preconception counseling. Her history is significant for the following: a spontaneous pregnancy loss at 15 weeks’ gestation; a pregnancy loss at 17 weeks secondary to preterm premature rupture of membranes (PPROM); a cesarean delivery at 30 weeks and 6 days’ gestation after placement of a transvaginal cerclage at 20 weeks for cervical dilation noted on physical exam (the child now has developmental delays); and most recently a delivery at 24 weeks and 4 days due to preterm labor with subsequent neonatal demise (this followed a transvaginal cerclage placed at 13 weeks and 6 days).
How would you counsel this patient?
Cervical insufficiency describes the inability of the cervix to retain a pregnancy in the absence of the signs and symptoms of clinical contractions, labor, or both in the second trimester.1 This condition affects an estimated 1% of obstetric patients and 8% of women with recurrent losses who have experienced a second-trimester loss.2
Diagnosis of cervical insufficiency is based on a history of painless cervical dilation after the first trimester with expulsion of the pregnancy in the second trimester before 24 weeks of gestation without contractions and in the absence of other pathology, such as bleeding, infection, or ruptured membranes.1 Diagnosis also can be made by noting cervical dilation on physical exam during the second trimester; more recently, short cervical length on transvaginal ultrasonography in the second trimester has been used to try to predict when a cervical cerclage may be indicated, although sonographic cervical length is more a marker for risk of preterm birth than for cervical insufficiency specifically.1,3
Given the considerable emotional and physical distress that patients experience with recurrent second-trimester losses and the significant neonatal morbidity and mortality that can occur with preterm delivery, substantial efforts are made to prevent these outcomes by treating patients with cervical insufficiency and those at risk for preterm delivery.
Transvaginal cerclage: A treatment mainstay
Standard treatment options for cervical insufficiency depend on the patient’s history. One of the treatment mainstays for women with prior second-trimester losses or preterm deliveries is transvaginal cervical cerclage. A transvaginal cerclage can be placed using either a Shirodkar technique, in which the vesicocervical mucosa is dissected and a suture is placed as close to the internal cervical os as possible, or a McDonald technique, in which a purse-string suture is placed around the cervicovaginal junction. No randomized trials have compared the effectiveness of these 2 methods, but most observational studies show no difference, and one suggests that the Shirodkar technique may be more effective in obese women specifically.4-6
Indications for transvaginal cerclage. The indication for transvaginal cerclage is based on history, physical exam, or ultrasonography.
A physical-exam indication is the most straightforward of the 3. Transvaginal cerclage placement is indicated if on physical exam in the second trimester a patient has cervical dilation without contractions or infection.1,7
A history-indicated cerclage (typically placed between 12 and 14 weeks’ gestation) is based on a cerclage having been placed in a prior pregnancy due to painless cervical dilation in the second trimester (either ultrasonography- or physical-exam indicated), and it also can be considered in the case of a history of 1 or more second-trimester pregnancy losses related to painless cervical dilation.1
More recent evidence suggests that in patients with 1 prior second-trimester loss or preterm delivery, serial sonographic cervical length can be measured safely from 16 to 24 weeks, with a cerclage being placed only if cervical length decreases to less than 25 mm. By using the ultrasonography-based indication, unnecessary history-indicated cerclages for 1 prior second-trimester or preterm birth can be avoided in more than one-half of patients (FIGURE 1).1,7

Efficacy. The effectiveness of transvaginal cerclage varies by the indication. Authors of a 2017 Cochrane review found an overall reduced risk of giving birth before 34 weeks’ gestation for any indication, with an average relative risk of 0.77.2 Other recent studies showed the following8-10:
- a 63% delivery rate after 28 weeks’ gestation for physical-exam indicated cerclages in the presence of bulging amniotic membranes
- an 86.2% delivery rate after 32 weeks’ gestation for ultrasonography-indicated cerclages
- an 86% delivery rate after 32 weeks’ gestation for a history-indicated cerclage in patients with 2 or more prior second-trimester losses.
Success rates, especially for ultrasonography- and history-indicated cerclage, are thus high. For the 14% who still fail these methods, however, a different management strategy is needed, which is where transabdominal cerclage comes into play.
Continue to: Transabdominal cerclage is an option for certain patients...
Transabdominal cerclage is an option for certain patients
In transabdominal cerclage, an abdominal approach is used to place a stitch at the cervicouterine junction. With this approach, the cerclage can reach a closer proximity to the internal os compared with the vaginal approach, providing better support of the cervical tissue (FIGURE 2).11 Whether performed via laparotomy or laparoscopy, the transabdominal cerclage procedure likely carries higher morbidity than a transvaginal approach, and cesarean delivery is required after placement.

Since transvaginal cerclage often is successful, in most cases the transabdominal approach should not be viewed as the first-line treatment for cervical insufficiency if a history-indicated transvaginal cerclage has not been attempted. For women who fail a history-indicated transvaginal cerclage, however, a transabdominal cerclage has been proven to decrease the rate of preterm delivery and PPROM compared with attempting another history-indicated transvaginal cerclage.11,12
A recent systematic review of pregnancy outcomes after transabdominal cerclage placement reported neonatal survival of 96.5% and an 83% delivery rate after 34 weeks’ gestation.13 Thus, even among a population that failed transvaginal cerclage, a transabdominal cerclage has a high success rate in providing a good pregnancy outcome (TABLE). Transabdominal cerclage also can be considered as first-line treatment in patients who had prior cervical surgery or cervical deformities that might preclude the ability to place a cerclage transvaginally.

CASE Continued: A candidate for transabdominal cerclage
Given the patient’s poor obstetric history, which includes a preterm delivery and neonatal loss despite a history-indicated cerclage, you recommend that the patient have a transabdominal cerclage placed as the procedure has been proven to increase the chances of neonatal survival and delivery after 34 weeks in women with a similar obstetric history. The patient is interested in this option and asks about how this cerclage is placed and when it would need to be placed during her next pregnancy.
Surgical technique for transabdominal cerclage placement
A transabdominal cerclage can be placed via laparotomy, laparoscopy, or robot-assisted laparoscopy. No differences in obstetric outcomes have been shown between the laparotomy and laparoscopic approaches.14,15 Given the benefits of minimally invasive surgery, a laparoscopic or robot-assisted approach is preferred when feasible.
Additionally, for ease of placement, transabdominal cerclage can be placed prior to conception—known as interval placement—or during pregnancy between 10 and 14 weeks (preferably closer to 10 weeks). Because of the increased difficulty in placing a cerclage in the gravid uterus, interval transabdominal cerclage placement is recommended when possible.13,16 Authors of one observational study noted that improved obstetric outcomes occurred with interval placement compared with cerclage placement between 9 and 10 weeks’ gestation, with a delivery rate at more than 34 weeks’ gestation in 90% versus 74% of patients, respectively.16
Continue to: Steps for interval cerclage and during pregnancy...
Steps for interval cerclage and during pregnancy
Our practice is to place transabdominal cerclage via conventional laparoscopy as an interval procedure when possible. We find no benefit in using robotic assistance.
For an interval procedure, the patient is placed in a dorsal lithotomy position, and we place a 10-mm umbilical port, 2 lateral 5-mm ports, 1 suprapubic 5-mm port, and a uterine manipulator. We use a flexible laparoscope to provide optimal visualization of the pelvis from any angle.
The first step of the surgery involves dissecting the vesicouterine peritoneum in order to move the bladder inferiorly (FIGURE 3A). Uterine arteries are then identified lateral to the cervix as part of this dissection, and a window is created in the inferior aspect of the broad ligament just anterior and lateral to the insertion of the uterosacral ligaments onto the uterus, with care taken to avoid the uterine vessels superiorly (FIGURE 3B). Two 5-mm Mersilene tape sutures are then tied together to create 1 suture with a needle at each end. This is then passed into the abdomen, and 1 needle is passed through the parametrial space at the level of the internal os inferior to the uterine vessels on 1 side of the uterus while the other needle is passed through the parametrial space on the opposite side.

Alternatively, rather than using the suture needles, a blunt dissector can be passed through this same space bilaterally (FIGURE 3C) via the suprapubic port and can pull the Mersilene tape through the parametrial space (FIGURE 3D). The suture is then tied anterior at the level of the internal os intracorporally (FIGURE 3E), and the needles are cut off the suture and removed from the abdomen.
To perform transabdominal cerclage when the patient is pregnant, a few modifications are needed to help with placement. First, the patient may be placed in supine position since a uterine manipulator cannot be used. Second, use of a flexible laparoscope becomes even more imperative in order to properly see around the gravid uterus. Lastly, a 5-mm laparoscopic liver retractor can be used to aid in blunt manipulation of the gravid uterus (FIGURE 3F). (The surgical video below highlights the steps to transabdominal cerclage placement in a pregnant patient.) All other port placements and steps to dissection and suture placement are the same as in interval placement.

CASE Continued: Patient pursues transabdominal cerclage
You explain to your patient that ideally the cerclage should be placed now in a laparoscopic fashion before she becomes pregnant. You then refer her to a local gynecologic surgeon who places many laparoscopic transabdominal cerclages. She undergoes the procedure, becomes pregnant, and after presenting in labor at 35 weeks’ gestation has a cesarean delivery. Her baby is born without any neonatal complications, and the patient is overjoyed with the outcome.
Management during and after pregnancy
Pregnant patients with a transabdominal cerclage are precluded from having a vaginal delivery and must deliver via cesarean. During the antepartum period, patients are managed in the same manner as those who have a transvaginal cerclage. Delivery via cesarean at the onset of regular contractions is recommended to reduce the risk of uterine rupture. In the absence of labor, scheduled cesarean is performed at term.
Our practice is to schedule cesarean delivery at 38 weeks’ gestation, although there are no data or consensus to support a specific gestational age between 37 and 39 weeks. Unlike a transvaginal cerclage, a transabdominal cerclage can be left in place for use in subsequent pregnancies. Data are limited on whether the transabdominal cerclage should be removed in women who no longer desire childbearing and whether there are long-term sequelae if the suture is left in situ.17
Continue to: Complications and risks of abdominal cerclage...
Complications and risks of abdominal cerclage
As the data suggest and our experience confirms, transabdominal cerclage is highly successful in patients who have failed a history-indicated transvaginal cerclage; however, the transabdominal approach carries a higher surgical risk. Risks include intraoperative hemorrhage, conversion to laparotomy, and a range of rare surgical and obstetric complications, such as bladder injury and PPROM.13,18
If a patient experiences a fetal loss in the first trimester, a dilation and curettage (D&C) can be performed, with good obstetric outcomes in subsequent pregnancies.19 If the patient experiences an early-to-mid second-trimester loss, some studies suggest that a dilation and evacuation (D&E) of the uterus can be done with sufficient dilation of the cervix to accommodate up to a 15-mm cannula and Sopher forceps.19 Laminaria also may be used in this process. However, no data exist regarding success of future pregnancies and transabdominal cerclage integrity after a D&E.20 If the cerclage prevents successful dilation of the cervix, the cerclage must be removed laparoscopically prior to performing the D&E.
In late second-trimester and third-trimester loss, the cerclage must be removed to allow passage of the fetus and placenta prior to a D&E or an induction of labor.20
For patients with PPROM or preterm labor, data are limited regarding management recommendations. However, in these complex cases, we strongly recommend an individualized approach and co-management with maternal-fetal medicine specialists.
CASE Resolved
The cerclage is left in place during the patient’s cesarean delivery, and her postpartum course is uneventful. She continued without complications for the next year, at which time she sees you in the office with plans to have another pregnancy later in the year. You counsel her that her abdominal cerclage will still be effective and that she can get pregnant with expectations of similar outcomes as her previous pregnancy. She thanks you for everything and reports that she hopes to return later in the year for her first prenatal visit. ●
CASE A woman with recurrent pregnancy loss
A 38-year-old woman (G4P0221) presents to your office for preconception counseling. Her history is significant for the following: a spontaneous pregnancy loss at 15 weeks’ gestation; a pregnancy loss at 17 weeks secondary to preterm premature rupture of membranes (PPROM); a cesarean delivery at 30 weeks and 6 days’ gestation after placement of a transvaginal cerclage at 20 weeks for cervical dilation noted on physical exam (the child now has developmental delays); and most recently a delivery at 24 weeks and 4 days due to preterm labor with subsequent neonatal demise (this followed a transvaginal cerclage placed at 13 weeks and 6 days).
How would you counsel this patient?
Cervical insufficiency describes the inability of the cervix to retain a pregnancy in the absence of the signs and symptoms of clinical contractions, labor, or both in the second trimester.1 This condition affects an estimated 1% of obstetric patients and 8% of women with recurrent losses who have experienced a second-trimester loss.2
Diagnosis of cervical insufficiency is based on a history of painless cervical dilation after the first trimester with expulsion of the pregnancy in the second trimester before 24 weeks of gestation without contractions and in the absence of other pathology, such as bleeding, infection, or ruptured membranes.1 Diagnosis also can be made by noting cervical dilation on physical exam during the second trimester; more recently, short cervical length on transvaginal ultrasonography in the second trimester has been used to try to predict when a cervical cerclage may be indicated, although sonographic cervical length is more a marker for risk of preterm birth than for cervical insufficiency specifically.1,3
Given the considerable emotional and physical distress that patients experience with recurrent second-trimester losses and the significant neonatal morbidity and mortality that can occur with preterm delivery, substantial efforts are made to prevent these outcomes by treating patients with cervical insufficiency and those at risk for preterm delivery.
Transvaginal cerclage: A treatment mainstay
Standard treatment options for cervical insufficiency depend on the patient’s history. One of the treatment mainstays for women with prior second-trimester losses or preterm deliveries is transvaginal cervical cerclage. A transvaginal cerclage can be placed using either a Shirodkar technique, in which the vesicocervical mucosa is dissected and a suture is placed as close to the internal cervical os as possible, or a McDonald technique, in which a purse-string suture is placed around the cervicovaginal junction. No randomized trials have compared the effectiveness of these 2 methods, but most observational studies show no difference, and one suggests that the Shirodkar technique may be more effective in obese women specifically.4-6
Indications for transvaginal cerclage. The indication for transvaginal cerclage is based on history, physical exam, or ultrasonography.
A physical-exam indication is the most straightforward of the 3. Transvaginal cerclage placement is indicated if on physical exam in the second trimester a patient has cervical dilation without contractions or infection.1,7
A history-indicated cerclage (typically placed between 12 and 14 weeks’ gestation) is based on a cerclage having been placed in a prior pregnancy due to painless cervical dilation in the second trimester (either ultrasonography- or physical-exam indicated), and it also can be considered in the case of a history of 1 or more second-trimester pregnancy losses related to painless cervical dilation.1
More recent evidence suggests that in patients with 1 prior second-trimester loss or preterm delivery, serial sonographic cervical length can be measured safely from 16 to 24 weeks, with a cerclage being placed only if cervical length decreases to less than 25 mm. By using the ultrasonography-based indication, unnecessary history-indicated cerclages for 1 prior second-trimester or preterm birth can be avoided in more than one-half of patients (FIGURE 1).1,7

Efficacy. The effectiveness of transvaginal cerclage varies by the indication. Authors of a 2017 Cochrane review found an overall reduced risk of giving birth before 34 weeks’ gestation for any indication, with an average relative risk of 0.77.2 Other recent studies showed the following8-10:
- a 63% delivery rate after 28 weeks’ gestation for physical-exam indicated cerclages in the presence of bulging amniotic membranes
- an 86.2% delivery rate after 32 weeks’ gestation for ultrasonography-indicated cerclages
- an 86% delivery rate after 32 weeks’ gestation for a history-indicated cerclage in patients with 2 or more prior second-trimester losses.
Success rates, especially for ultrasonography- and history-indicated cerclage, are thus high. For the 14% who still fail these methods, however, a different management strategy is needed, which is where transabdominal cerclage comes into play.
Continue to: Transabdominal cerclage is an option for certain patients...
Transabdominal cerclage is an option for certain patients
In transabdominal cerclage, an abdominal approach is used to place a stitch at the cervicouterine junction. With this approach, the cerclage can reach a closer proximity to the internal os compared with the vaginal approach, providing better support of the cervical tissue (FIGURE 2).11 Whether performed via laparotomy or laparoscopy, the transabdominal cerclage procedure likely carries higher morbidity than a transvaginal approach, and cesarean delivery is required after placement.

Since transvaginal cerclage often is successful, in most cases the transabdominal approach should not be viewed as the first-line treatment for cervical insufficiency if a history-indicated transvaginal cerclage has not been attempted. For women who fail a history-indicated transvaginal cerclage, however, a transabdominal cerclage has been proven to decrease the rate of preterm delivery and PPROM compared with attempting another history-indicated transvaginal cerclage.11,12
A recent systematic review of pregnancy outcomes after transabdominal cerclage placement reported neonatal survival of 96.5% and an 83% delivery rate after 34 weeks’ gestation.13 Thus, even among a population that failed transvaginal cerclage, a transabdominal cerclage has a high success rate in providing a good pregnancy outcome (TABLE). Transabdominal cerclage also can be considered as first-line treatment in patients who had prior cervical surgery or cervical deformities that might preclude the ability to place a cerclage transvaginally.

CASE Continued: A candidate for transabdominal cerclage
Given the patient’s poor obstetric history, which includes a preterm delivery and neonatal loss despite a history-indicated cerclage, you recommend that the patient have a transabdominal cerclage placed as the procedure has been proven to increase the chances of neonatal survival and delivery after 34 weeks in women with a similar obstetric history. The patient is interested in this option and asks about how this cerclage is placed and when it would need to be placed during her next pregnancy.
Surgical technique for transabdominal cerclage placement
A transabdominal cerclage can be placed via laparotomy, laparoscopy, or robot-assisted laparoscopy. No differences in obstetric outcomes have been shown between the laparotomy and laparoscopic approaches.14,15 Given the benefits of minimally invasive surgery, a laparoscopic or robot-assisted approach is preferred when feasible.
Additionally, for ease of placement, transabdominal cerclage can be placed prior to conception—known as interval placement—or during pregnancy between 10 and 14 weeks (preferably closer to 10 weeks). Because of the increased difficulty in placing a cerclage in the gravid uterus, interval transabdominal cerclage placement is recommended when possible.13,16 Authors of one observational study noted that improved obstetric outcomes occurred with interval placement compared with cerclage placement between 9 and 10 weeks’ gestation, with a delivery rate at more than 34 weeks’ gestation in 90% versus 74% of patients, respectively.16
Continue to: Steps for interval cerclage and during pregnancy...
Steps for interval cerclage and during pregnancy
Our practice is to place transabdominal cerclage via conventional laparoscopy as an interval procedure when possible. We find no benefit in using robotic assistance.
For an interval procedure, the patient is placed in a dorsal lithotomy position, and we place a 10-mm umbilical port, 2 lateral 5-mm ports, 1 suprapubic 5-mm port, and a uterine manipulator. We use a flexible laparoscope to provide optimal visualization of the pelvis from any angle.
The first step of the surgery involves dissecting the vesicouterine peritoneum in order to move the bladder inferiorly (FIGURE 3A). Uterine arteries are then identified lateral to the cervix as part of this dissection, and a window is created in the inferior aspect of the broad ligament just anterior and lateral to the insertion of the uterosacral ligaments onto the uterus, with care taken to avoid the uterine vessels superiorly (FIGURE 3B). Two 5-mm Mersilene tape sutures are then tied together to create 1 suture with a needle at each end. This is then passed into the abdomen, and 1 needle is passed through the parametrial space at the level of the internal os inferior to the uterine vessels on 1 side of the uterus while the other needle is passed through the parametrial space on the opposite side.

Alternatively, rather than using the suture needles, a blunt dissector can be passed through this same space bilaterally (FIGURE 3C) via the suprapubic port and can pull the Mersilene tape through the parametrial space (FIGURE 3D). The suture is then tied anterior at the level of the internal os intracorporally (FIGURE 3E), and the needles are cut off the suture and removed from the abdomen.
To perform transabdominal cerclage when the patient is pregnant, a few modifications are needed to help with placement. First, the patient may be placed in supine position since a uterine manipulator cannot be used. Second, use of a flexible laparoscope becomes even more imperative in order to properly see around the gravid uterus. Lastly, a 5-mm laparoscopic liver retractor can be used to aid in blunt manipulation of the gravid uterus (FIGURE 3F). (The surgical video below highlights the steps to transabdominal cerclage placement in a pregnant patient.) All other port placements and steps to dissection and suture placement are the same as in interval placement.

CASE Continued: Patient pursues transabdominal cerclage
You explain to your patient that ideally the cerclage should be placed now in a laparoscopic fashion before she becomes pregnant. You then refer her to a local gynecologic surgeon who places many laparoscopic transabdominal cerclages. She undergoes the procedure, becomes pregnant, and after presenting in labor at 35 weeks’ gestation has a cesarean delivery. Her baby is born without any neonatal complications, and the patient is overjoyed with the outcome.
Management during and after pregnancy
Pregnant patients with a transabdominal cerclage are precluded from having a vaginal delivery and must deliver via cesarean. During the antepartum period, patients are managed in the same manner as those who have a transvaginal cerclage. Delivery via cesarean at the onset of regular contractions is recommended to reduce the risk of uterine rupture. In the absence of labor, scheduled cesarean is performed at term.
Our practice is to schedule cesarean delivery at 38 weeks’ gestation, although there are no data or consensus to support a specific gestational age between 37 and 39 weeks. Unlike a transvaginal cerclage, a transabdominal cerclage can be left in place for use in subsequent pregnancies. Data are limited on whether the transabdominal cerclage should be removed in women who no longer desire childbearing and whether there are long-term sequelae if the suture is left in situ.17
Continue to: Complications and risks of abdominal cerclage...
Complications and risks of abdominal cerclage
As the data suggest and our experience confirms, transabdominal cerclage is highly successful in patients who have failed a history-indicated transvaginal cerclage; however, the transabdominal approach carries a higher surgical risk. Risks include intraoperative hemorrhage, conversion to laparotomy, and a range of rare surgical and obstetric complications, such as bladder injury and PPROM.13,18
If a patient experiences a fetal loss in the first trimester, a dilation and curettage (D&C) can be performed, with good obstetric outcomes in subsequent pregnancies.19 If the patient experiences an early-to-mid second-trimester loss, some studies suggest that a dilation and evacuation (D&E) of the uterus can be done with sufficient dilation of the cervix to accommodate up to a 15-mm cannula and Sopher forceps.19 Laminaria also may be used in this process. However, no data exist regarding success of future pregnancies and transabdominal cerclage integrity after a D&E.20 If the cerclage prevents successful dilation of the cervix, the cerclage must be removed laparoscopically prior to performing the D&E.
In late second-trimester and third-trimester loss, the cerclage must be removed to allow passage of the fetus and placenta prior to a D&E or an induction of labor.20
For patients with PPROM or preterm labor, data are limited regarding management recommendations. However, in these complex cases, we strongly recommend an individualized approach and co-management with maternal-fetal medicine specialists.
CASE Resolved
The cerclage is left in place during the patient’s cesarean delivery, and her postpartum course is uneventful. She continued without complications for the next year, at which time she sees you in the office with plans to have another pregnancy later in the year. You counsel her that her abdominal cerclage will still be effective and that she can get pregnant with expectations of similar outcomes as her previous pregnancy. She thanks you for everything and reports that she hopes to return later in the year for her first prenatal visit. ●
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 142: Cerclage for the management of cervical insufficiency. Obstet Gynecol. 2014;123(2 pt 1): 372-379.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6(6):CD008991.
- Brown R, Gagnon R, Delisle M-F. No. 373—cervical insufficiency and cervical cerclage. J Obstet Gynaecol Can. 2019;41:233-247.
- Odibo AO, Berghella V, To MS, et al. Shirodkar versus McDonald cerclage for the prevention of preterm birth in women with short cervical length. Am J Perinatol. 2007;24: 55-60.
- Basbug A, Bayrak M, Dogan O, et al. McDonald versus modified Shirodkar rescue cerclage in women with prolapsed fetal membranes. J Matern Fetal Neonatal Med. 2020;33: 1075-1097.
- Figueroa R, Crowell R, Martinez A, et al. McDonald versus Shirodkar cervical cerclage for the prevention of preterm birth: impact of body mass index. J Matern Fetal Neonatal Med. 2019;32:3408-3414.
- Suhag A, Berghella V. Cervical cerclage. Clin Obstet Gynecol. 2014;57:557-567.
- Bayrak M, Gul A, Goynumer G. Rescue cerclage when foetal membranes prolapse into the vagina. J Obstet Gynaecol. 2017;37:471-475.
- Drassinower D, Coviello E, Landy HJ, et al. Outcomes after periviable ultrasound-indicated cerclage. J Matern Fetal Neonatal Med. 2019;32:932-938.
- Lee KN, Whang EJ, Chang KH, et al. History-indicated cerclage: the association between previous preterm history and cerclage outcome. Obstet Gynecol Sci. 2018;61:23-29. doi:10.5468/ogs.2018.61.1.23.
- Sneider K, Christiansen OB, Sundtoft IB, et al. Recurrence rates after abdominal and vaginal cerclages in women with cervical insufficiency: a validated cohort study. Arch Gynecol Obstet. 2017;295:859-866.
- Davis G, Berghella V, Talucci M, et al. Patients with a prior failed transvaginal cerclage: a comparison of obstetric outcomes with either transabdominal or transvaginal cerclage. Am J Obstet Gynecol. 2000;183:836-839.
- Moawad GN, Tyan P, Bracke T, et al. Systematic review of transabdominal cerclage placed via laparoscopy for the prevention of preterm birth. J Mimim Invasive Gynecol. 2018;25:277-286.
- Burger NB, Brölmann HAM, Einarsson JI, et al. Effectiveness of abdominal cerclage placed via laparotomy or laparoscopy: systematic review. J Minim Invasive Gynecol. 2011;18:696-704.
- Kim S, Hill A, Menderes G, et al. Minimally invasive abdominal cerclage compared to laparotomy: a comparison of surgical and obstetric outcomes. J Robot Surg. 2018;12:295-301.
- Dawood F, Farquharson RG. Transabdominal cerclage: preconceptual versus first trimester insertion. Eur J Obstet Gynecol Reprod Biol. 2016;199:27-31.
- Hawkins E, Nimaroff M. Vaginal erosion of an abdominal cerclage 7 years after laparoscopic placement. Obstet Gynecol. 2014;123(2 pt 2 suppl 2):420-423.
- Foster TL, Moore ES, Sumners JE. Operative complications and fetal morbidity encountered in 300 prophylactic transabdominal cervical cerclage procedures by one obstetric surgeon. J Obstet Gynaecol. 2011;31:713-717.
- Dethier D, Lassey SC, Pilliod R, et al. Uterine evacuation in the setting of transabdominal cerclage. Contraception. 2020;101:174-177.
- Martin A, Lathrop E. Controversies in family planning: management of second-trimester losses in the setting of an abdominal cerclage. Contraception. 2013;87:728-731.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 142: Cerclage for the management of cervical insufficiency. Obstet Gynecol. 2014;123(2 pt 1): 372-379.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6(6):CD008991.
- Brown R, Gagnon R, Delisle M-F. No. 373—cervical insufficiency and cervical cerclage. J Obstet Gynaecol Can. 2019;41:233-247.
- Odibo AO, Berghella V, To MS, et al. Shirodkar versus McDonald cerclage for the prevention of preterm birth in women with short cervical length. Am J Perinatol. 2007;24: 55-60.
- Basbug A, Bayrak M, Dogan O, et al. McDonald versus modified Shirodkar rescue cerclage in women with prolapsed fetal membranes. J Matern Fetal Neonatal Med. 2020;33: 1075-1097.
- Figueroa R, Crowell R, Martinez A, et al. McDonald versus Shirodkar cervical cerclage for the prevention of preterm birth: impact of body mass index. J Matern Fetal Neonatal Med. 2019;32:3408-3414.
- Suhag A, Berghella V. Cervical cerclage. Clin Obstet Gynecol. 2014;57:557-567.
- Bayrak M, Gul A, Goynumer G. Rescue cerclage when foetal membranes prolapse into the vagina. J Obstet Gynaecol. 2017;37:471-475.
- Drassinower D, Coviello E, Landy HJ, et al. Outcomes after periviable ultrasound-indicated cerclage. J Matern Fetal Neonatal Med. 2019;32:932-938.
- Lee KN, Whang EJ, Chang KH, et al. History-indicated cerclage: the association between previous preterm history and cerclage outcome. Obstet Gynecol Sci. 2018;61:23-29. doi:10.5468/ogs.2018.61.1.23.
- Sneider K, Christiansen OB, Sundtoft IB, et al. Recurrence rates after abdominal and vaginal cerclages in women with cervical insufficiency: a validated cohort study. Arch Gynecol Obstet. 2017;295:859-866.
- Davis G, Berghella V, Talucci M, et al. Patients with a prior failed transvaginal cerclage: a comparison of obstetric outcomes with either transabdominal or transvaginal cerclage. Am J Obstet Gynecol. 2000;183:836-839.
- Moawad GN, Tyan P, Bracke T, et al. Systematic review of transabdominal cerclage placed via laparoscopy for the prevention of preterm birth. J Mimim Invasive Gynecol. 2018;25:277-286.
- Burger NB, Brölmann HAM, Einarsson JI, et al. Effectiveness of abdominal cerclage placed via laparotomy or laparoscopy: systematic review. J Minim Invasive Gynecol. 2011;18:696-704.
- Kim S, Hill A, Menderes G, et al. Minimally invasive abdominal cerclage compared to laparotomy: a comparison of surgical and obstetric outcomes. J Robot Surg. 2018;12:295-301.
- Dawood F, Farquharson RG. Transabdominal cerclage: preconceptual versus first trimester insertion. Eur J Obstet Gynecol Reprod Biol. 2016;199:27-31.
- Hawkins E, Nimaroff M. Vaginal erosion of an abdominal cerclage 7 years after laparoscopic placement. Obstet Gynecol. 2014;123(2 pt 2 suppl 2):420-423.
- Foster TL, Moore ES, Sumners JE. Operative complications and fetal morbidity encountered in 300 prophylactic transabdominal cervical cerclage procedures by one obstetric surgeon. J Obstet Gynaecol. 2011;31:713-717.
- Dethier D, Lassey SC, Pilliod R, et al. Uterine evacuation in the setting of transabdominal cerclage. Contraception. 2020;101:174-177.
- Martin A, Lathrop E. Controversies in family planning: management of second-trimester losses in the setting of an abdominal cerclage. Contraception. 2013;87:728-731.
AHA emphasizes the need for cardio-obstetrics teams
according to a statement from the American Heart Association.
Cardiovascular disease (CVD) remains the leading cause of pregnancy-related mortality in the United States, and accounted for approximately 17 deaths per 100,000 live births in 2015, wrote Laxmi S. Mehta, MD, of The Ohio State University, Columbus, and colleagues.
Ideally, a woman with CVD at the time of pregnancy should be managed by a multidisciplinary cardio-obstetrics team that can assess cardiovascular risk, obstetric risk, and fetal risk throughout pregnancy, delivery, and up to a year post partum. The team should develop a shared strategy to promote best outcomes, according to the statement. The cardio-obstetrics team may include obstetricians, cardiologists, anesthesiologists, maternal-fetal medicine specialists, geneticists, neurologists, nurses, and pharmacists, according to the statement.
Women with preexisting CVD should receive counseling about maternal and fetal risks before conception, if possible, to involve the women in shared decision-making and to develop strategies for each stage of pregnancy and delivery, Dr. Mehta and associates said. Such counseling should include a review of all medications and assessment of risk factors.
However, some women present already in the early stages of pregnancy even with severe conditions such as pulmonary arterial hypertension, severe ventricular dysfunction, severe left-sided heart obstruction, and significant aortic dilatation with underlying connective tissue disease. Women with these conditions often are counseled to avoid pregnancy, but if they already are pregnant, a high-risk cardio-obstetrics team will need to work together to discover the best strategies going forward to mitigate risk, Dr. Mehta and associates said.
Common CVD conditions that affect pregnancy include hypertensive disorders, notably preeclampsia, defined as systolic blood pressure greater than 140 mm Hg or diastolic blood pressure greater than 90 mm Hg in women after 20 weeks of gestation whose blood pressure was normal prior to pregnancy. A management strategy to reduce the risk of pregnancy-related complications from hypertension includes healthy lifestyle behaviors such as exercise, nutrition, and smoking cessation, according to the statement. However, patients with severe hypertension may require intravenous labetalol or hydralazine. The statement gives more information about handling preeclampsia with pulmonary edema, and prevention of eclampsia and treatment of seizures.
It is important to recognize that severe hypertension or superimposed preeclampsia may occur for the first time post partum. Early ambulatory visits in the first 1-2 weeks are sensible. Medications may be needed to keep a systolic blood pressure not higher than 150 mm Hg and a diastolic blood pressure not higher than 100 mm Hg, Dr. Mehta and associates said.
According to the statement, severe hypertriglyceridemia and familial hypercholesterolemia are the two most common conditions in which lipids should be addressed during pregnancy, with consideration of the fetal risks associated with certain medications.
“Statins are contraindicated during pregnancy, and all women who are on any lipid-lowering agents should review with their physician the safety of treatment during pregnancy and whether to discontinue treatment before pregnancy,” according to the statement. A heart-healthy lifestyle can help improve lipid profiles in all pregnant patients, Dr. Mehta and associates said. Patients with extremely high triglycerides above 500 mg/dL are at risk of pancreatitis and “may benefit from pharmacological agents (omega-3 fatty acids with or without fenofibrate or gemfibrozil) during the second trimester,” they noted. Pregnant women with familial hypercholesterolemia might take bile acid sequestrants, or as a last resort, low-density lipoprotein apheresis.
Other conditions calling for a multidisciplinary cardio-obstetric approach include preexisting coronary artery disease, cardiomyopathies, arrhythmias, valvular heart disease, cerebrovascular disease, and deep venous thrombosis, according to the statement, which provides information about the risks, diagnosis, and management.
When it is time for delivery, spontaneous labor and vaginal birth are preferable for most women with heart disease, as cesarean delivery is associated with increased risk of infection, thrombotic complications, and blood loss, according to the statement.
Women with CVD and associated complications will require “specialized long-term cardiovascular follow-up,” Dr. Mehta and associates said. “In women with a high-risk pregnancy, a cardio-obstetrics team is essential to prevent maternal morbidity and mortality during the length of the pregnancy and post partum.”
“The release of this document demonstrates the AHA’s recognition of the importance of CVD in pregnancy-related death and their commitment to education and ensuring best practices in this field,” said Lisa M. Hollier, MD, past president of the American College of Obstetricians and Gynecologists and chief medical officer at Texas Children’s Health Plan, Bellaire.
“I think one of the most important outcomes from the release of this scientific statement from AHA will be increased implementation of cardio-obstetrics teams,” she said in an interview.
“In the United States, cardiovascular disease and cardiomyopathy together are now the leading cause of death in pregnancy and the postpartum period, and constitute 26.5% of pregnancy-related deaths, with higher rates of mortality among women of color and women with lower incomes,” she said. “The rising trend in cardiovascular-related maternal deaths appears to be due to acquired, not congenital, heart disease.”
During her tenure as president of ACOG, Dr. Hollier convened a task force on cardiovascular disease in pregnancy that developed guidance that outlines screening, diagnosis, and management of CVD for women from prepregnancy through post partum.
Dr. Hollier noted that COVID-19 emphasizes racial disparities for maternal mortality.
“Pregnant patients with comorbidities, like heart conditions, may be at increased risk for severe illness from COVID-19 – consistent with the general population with similar comorbidities,” she said. “And as we know, black women’s risk of dying from CVD-related pregnancy complications is 3.4 times higher than that of white women. During the COVID-19 pandemic, we are seeing these racial health disparities exacerbated.”
However, any pregnant patients should not hesitate to communicate with their health care providers despite the pandemic situation, Dr. Hollier emphasized. “Communication between a patient and her ob.gyn., cardiologist, or other clinician is even more critical now during the COVID-19 pandemic. We’re hearing reports that patients who are experiencing symptoms or those with known cardiac conditions are avoiding the hospital and delaying or not seeking necessary treatment. This has the very real possibility of worsening the devastating maternal mortality crisis that we’re already experiencing in this country.”
To help overcome barriers to treatment, “collaboration between ob.gyns. and cardiologists, such as the cardio-obstetrics team or pregnancy heart team, is critical,” said Dr. Hollier. “These collaborative teams with a multidisciplinary approach can prospectively reduce the communication gaps across specialties when patients are seen separately. They can also improve the communication during care transitions such as between outpatient and inpatient care.
“In reviews of maternal deaths, we have found that there are often delays in diagnosis of heart conditions during and after pregnancy,” Dr. Hollier added. “Most maternal deaths from CVD are due to either undiagnosed cardiovascular disease or new-onset cardiomyopathy. ACOG recommends that all women be assessed for cardiovascular disease in the antepartum and postpartum periods using a recently developed algorithm,” she said. “Women who have known CVD and women who have concerning symptoms should have a consultation with this team. With increased awareness and screening, women can receive the additional care that they need.
“Because management of cardiac conditions in pregnancy is so complex, it is important to ensure that women receive care with teams and in facilities that have appropriate resources,” explained Dr. Hollier. “Women with known heart disease should see a cardiologist prior to pregnancy and receive prepregnancy counseling,” as noted in the AHA statement. “Patients determined to have moderate and high-risk CVD should be managed during pregnancy, delivery, and post partum in a medical center that is able to provide a higher level of care, including a cardio-obstetrics team.”
Early recognition of cardiovascular conditions is essential to help manage care and reduce risks to mother and baby, said Dr. Hollier. “Identification before a woman becomes pregnant means the patient’s care can be properly managed throughout the pregnancy and a detailed delivery plan can be developed through shared decision making between the patient and provider. We must think of heart disease as a possibility in every pregnant or postpartum patient we see to detect and treat at-risk mothers,” she said.
Additional research should focus on identifying risk factors prior to pregnancy, said Dr. Hollier. “There are often delays in recognizing symptoms during pregnancy and post partum, particularly for black women. We need data to understand which protocols are best to identify heart disease,”
Dr. Hollier had no financial conflicts to disclose. The authors of the AHA statement had no financial conflicts to disclose. The scientific statement was produced on behalf of the American Heart Association Council on Clinical Cardiology; Council on Atherosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
SOURCE: Mehta LS et al. Circulation. 2020 May 4. doi: 10.1161/CIR.0000000000000772.
according to a statement from the American Heart Association.
Cardiovascular disease (CVD) remains the leading cause of pregnancy-related mortality in the United States, and accounted for approximately 17 deaths per 100,000 live births in 2015, wrote Laxmi S. Mehta, MD, of The Ohio State University, Columbus, and colleagues.
Ideally, a woman with CVD at the time of pregnancy should be managed by a multidisciplinary cardio-obstetrics team that can assess cardiovascular risk, obstetric risk, and fetal risk throughout pregnancy, delivery, and up to a year post partum. The team should develop a shared strategy to promote best outcomes, according to the statement. The cardio-obstetrics team may include obstetricians, cardiologists, anesthesiologists, maternal-fetal medicine specialists, geneticists, neurologists, nurses, and pharmacists, according to the statement.
Women with preexisting CVD should receive counseling about maternal and fetal risks before conception, if possible, to involve the women in shared decision-making and to develop strategies for each stage of pregnancy and delivery, Dr. Mehta and associates said. Such counseling should include a review of all medications and assessment of risk factors.
However, some women present already in the early stages of pregnancy even with severe conditions such as pulmonary arterial hypertension, severe ventricular dysfunction, severe left-sided heart obstruction, and significant aortic dilatation with underlying connective tissue disease. Women with these conditions often are counseled to avoid pregnancy, but if they already are pregnant, a high-risk cardio-obstetrics team will need to work together to discover the best strategies going forward to mitigate risk, Dr. Mehta and associates said.
Common CVD conditions that affect pregnancy include hypertensive disorders, notably preeclampsia, defined as systolic blood pressure greater than 140 mm Hg or diastolic blood pressure greater than 90 mm Hg in women after 20 weeks of gestation whose blood pressure was normal prior to pregnancy. A management strategy to reduce the risk of pregnancy-related complications from hypertension includes healthy lifestyle behaviors such as exercise, nutrition, and smoking cessation, according to the statement. However, patients with severe hypertension may require intravenous labetalol or hydralazine. The statement gives more information about handling preeclampsia with pulmonary edema, and prevention of eclampsia and treatment of seizures.
It is important to recognize that severe hypertension or superimposed preeclampsia may occur for the first time post partum. Early ambulatory visits in the first 1-2 weeks are sensible. Medications may be needed to keep a systolic blood pressure not higher than 150 mm Hg and a diastolic blood pressure not higher than 100 mm Hg, Dr. Mehta and associates said.
According to the statement, severe hypertriglyceridemia and familial hypercholesterolemia are the two most common conditions in which lipids should be addressed during pregnancy, with consideration of the fetal risks associated with certain medications.
“Statins are contraindicated during pregnancy, and all women who are on any lipid-lowering agents should review with their physician the safety of treatment during pregnancy and whether to discontinue treatment before pregnancy,” according to the statement. A heart-healthy lifestyle can help improve lipid profiles in all pregnant patients, Dr. Mehta and associates said. Patients with extremely high triglycerides above 500 mg/dL are at risk of pancreatitis and “may benefit from pharmacological agents (omega-3 fatty acids with or without fenofibrate or gemfibrozil) during the second trimester,” they noted. Pregnant women with familial hypercholesterolemia might take bile acid sequestrants, or as a last resort, low-density lipoprotein apheresis.
Other conditions calling for a multidisciplinary cardio-obstetric approach include preexisting coronary artery disease, cardiomyopathies, arrhythmias, valvular heart disease, cerebrovascular disease, and deep venous thrombosis, according to the statement, which provides information about the risks, diagnosis, and management.
When it is time for delivery, spontaneous labor and vaginal birth are preferable for most women with heart disease, as cesarean delivery is associated with increased risk of infection, thrombotic complications, and blood loss, according to the statement.
Women with CVD and associated complications will require “specialized long-term cardiovascular follow-up,” Dr. Mehta and associates said. “In women with a high-risk pregnancy, a cardio-obstetrics team is essential to prevent maternal morbidity and mortality during the length of the pregnancy and post partum.”
“The release of this document demonstrates the AHA’s recognition of the importance of CVD in pregnancy-related death and their commitment to education and ensuring best practices in this field,” said Lisa M. Hollier, MD, past president of the American College of Obstetricians and Gynecologists and chief medical officer at Texas Children’s Health Plan, Bellaire.
“I think one of the most important outcomes from the release of this scientific statement from AHA will be increased implementation of cardio-obstetrics teams,” she said in an interview.
“In the United States, cardiovascular disease and cardiomyopathy together are now the leading cause of death in pregnancy and the postpartum period, and constitute 26.5% of pregnancy-related deaths, with higher rates of mortality among women of color and women with lower incomes,” she said. “The rising trend in cardiovascular-related maternal deaths appears to be due to acquired, not congenital, heart disease.”
During her tenure as president of ACOG, Dr. Hollier convened a task force on cardiovascular disease in pregnancy that developed guidance that outlines screening, diagnosis, and management of CVD for women from prepregnancy through post partum.
Dr. Hollier noted that COVID-19 emphasizes racial disparities for maternal mortality.
“Pregnant patients with comorbidities, like heart conditions, may be at increased risk for severe illness from COVID-19 – consistent with the general population with similar comorbidities,” she said. “And as we know, black women’s risk of dying from CVD-related pregnancy complications is 3.4 times higher than that of white women. During the COVID-19 pandemic, we are seeing these racial health disparities exacerbated.”
However, any pregnant patients should not hesitate to communicate with their health care providers despite the pandemic situation, Dr. Hollier emphasized. “Communication between a patient and her ob.gyn., cardiologist, or other clinician is even more critical now during the COVID-19 pandemic. We’re hearing reports that patients who are experiencing symptoms or those with known cardiac conditions are avoiding the hospital and delaying or not seeking necessary treatment. This has the very real possibility of worsening the devastating maternal mortality crisis that we’re already experiencing in this country.”
To help overcome barriers to treatment, “collaboration between ob.gyns. and cardiologists, such as the cardio-obstetrics team or pregnancy heart team, is critical,” said Dr. Hollier. “These collaborative teams with a multidisciplinary approach can prospectively reduce the communication gaps across specialties when patients are seen separately. They can also improve the communication during care transitions such as between outpatient and inpatient care.
“In reviews of maternal deaths, we have found that there are often delays in diagnosis of heart conditions during and after pregnancy,” Dr. Hollier added. “Most maternal deaths from CVD are due to either undiagnosed cardiovascular disease or new-onset cardiomyopathy. ACOG recommends that all women be assessed for cardiovascular disease in the antepartum and postpartum periods using a recently developed algorithm,” she said. “Women who have known CVD and women who have concerning symptoms should have a consultation with this team. With increased awareness and screening, women can receive the additional care that they need.
“Because management of cardiac conditions in pregnancy is so complex, it is important to ensure that women receive care with teams and in facilities that have appropriate resources,” explained Dr. Hollier. “Women with known heart disease should see a cardiologist prior to pregnancy and receive prepregnancy counseling,” as noted in the AHA statement. “Patients determined to have moderate and high-risk CVD should be managed during pregnancy, delivery, and post partum in a medical center that is able to provide a higher level of care, including a cardio-obstetrics team.”
Early recognition of cardiovascular conditions is essential to help manage care and reduce risks to mother and baby, said Dr. Hollier. “Identification before a woman becomes pregnant means the patient’s care can be properly managed throughout the pregnancy and a detailed delivery plan can be developed through shared decision making between the patient and provider. We must think of heart disease as a possibility in every pregnant or postpartum patient we see to detect and treat at-risk mothers,” she said.
Additional research should focus on identifying risk factors prior to pregnancy, said Dr. Hollier. “There are often delays in recognizing symptoms during pregnancy and post partum, particularly for black women. We need data to understand which protocols are best to identify heart disease,”
Dr. Hollier had no financial conflicts to disclose. The authors of the AHA statement had no financial conflicts to disclose. The scientific statement was produced on behalf of the American Heart Association Council on Clinical Cardiology; Council on Atherosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
SOURCE: Mehta LS et al. Circulation. 2020 May 4. doi: 10.1161/CIR.0000000000000772.
according to a statement from the American Heart Association.
Cardiovascular disease (CVD) remains the leading cause of pregnancy-related mortality in the United States, and accounted for approximately 17 deaths per 100,000 live births in 2015, wrote Laxmi S. Mehta, MD, of The Ohio State University, Columbus, and colleagues.
Ideally, a woman with CVD at the time of pregnancy should be managed by a multidisciplinary cardio-obstetrics team that can assess cardiovascular risk, obstetric risk, and fetal risk throughout pregnancy, delivery, and up to a year post partum. The team should develop a shared strategy to promote best outcomes, according to the statement. The cardio-obstetrics team may include obstetricians, cardiologists, anesthesiologists, maternal-fetal medicine specialists, geneticists, neurologists, nurses, and pharmacists, according to the statement.
Women with preexisting CVD should receive counseling about maternal and fetal risks before conception, if possible, to involve the women in shared decision-making and to develop strategies for each stage of pregnancy and delivery, Dr. Mehta and associates said. Such counseling should include a review of all medications and assessment of risk factors.
However, some women present already in the early stages of pregnancy even with severe conditions such as pulmonary arterial hypertension, severe ventricular dysfunction, severe left-sided heart obstruction, and significant aortic dilatation with underlying connective tissue disease. Women with these conditions often are counseled to avoid pregnancy, but if they already are pregnant, a high-risk cardio-obstetrics team will need to work together to discover the best strategies going forward to mitigate risk, Dr. Mehta and associates said.
Common CVD conditions that affect pregnancy include hypertensive disorders, notably preeclampsia, defined as systolic blood pressure greater than 140 mm Hg or diastolic blood pressure greater than 90 mm Hg in women after 20 weeks of gestation whose blood pressure was normal prior to pregnancy. A management strategy to reduce the risk of pregnancy-related complications from hypertension includes healthy lifestyle behaviors such as exercise, nutrition, and smoking cessation, according to the statement. However, patients with severe hypertension may require intravenous labetalol or hydralazine. The statement gives more information about handling preeclampsia with pulmonary edema, and prevention of eclampsia and treatment of seizures.
It is important to recognize that severe hypertension or superimposed preeclampsia may occur for the first time post partum. Early ambulatory visits in the first 1-2 weeks are sensible. Medications may be needed to keep a systolic blood pressure not higher than 150 mm Hg and a diastolic blood pressure not higher than 100 mm Hg, Dr. Mehta and associates said.
According to the statement, severe hypertriglyceridemia and familial hypercholesterolemia are the two most common conditions in which lipids should be addressed during pregnancy, with consideration of the fetal risks associated with certain medications.
“Statins are contraindicated during pregnancy, and all women who are on any lipid-lowering agents should review with their physician the safety of treatment during pregnancy and whether to discontinue treatment before pregnancy,” according to the statement. A heart-healthy lifestyle can help improve lipid profiles in all pregnant patients, Dr. Mehta and associates said. Patients with extremely high triglycerides above 500 mg/dL are at risk of pancreatitis and “may benefit from pharmacological agents (omega-3 fatty acids with or without fenofibrate or gemfibrozil) during the second trimester,” they noted. Pregnant women with familial hypercholesterolemia might take bile acid sequestrants, or as a last resort, low-density lipoprotein apheresis.
Other conditions calling for a multidisciplinary cardio-obstetric approach include preexisting coronary artery disease, cardiomyopathies, arrhythmias, valvular heart disease, cerebrovascular disease, and deep venous thrombosis, according to the statement, which provides information about the risks, diagnosis, and management.
When it is time for delivery, spontaneous labor and vaginal birth are preferable for most women with heart disease, as cesarean delivery is associated with increased risk of infection, thrombotic complications, and blood loss, according to the statement.
Women with CVD and associated complications will require “specialized long-term cardiovascular follow-up,” Dr. Mehta and associates said. “In women with a high-risk pregnancy, a cardio-obstetrics team is essential to prevent maternal morbidity and mortality during the length of the pregnancy and post partum.”
“The release of this document demonstrates the AHA’s recognition of the importance of CVD in pregnancy-related death and their commitment to education and ensuring best practices in this field,” said Lisa M. Hollier, MD, past president of the American College of Obstetricians and Gynecologists and chief medical officer at Texas Children’s Health Plan, Bellaire.
“I think one of the most important outcomes from the release of this scientific statement from AHA will be increased implementation of cardio-obstetrics teams,” she said in an interview.
“In the United States, cardiovascular disease and cardiomyopathy together are now the leading cause of death in pregnancy and the postpartum period, and constitute 26.5% of pregnancy-related deaths, with higher rates of mortality among women of color and women with lower incomes,” she said. “The rising trend in cardiovascular-related maternal deaths appears to be due to acquired, not congenital, heart disease.”
During her tenure as president of ACOG, Dr. Hollier convened a task force on cardiovascular disease in pregnancy that developed guidance that outlines screening, diagnosis, and management of CVD for women from prepregnancy through post partum.
Dr. Hollier noted that COVID-19 emphasizes racial disparities for maternal mortality.
“Pregnant patients with comorbidities, like heart conditions, may be at increased risk for severe illness from COVID-19 – consistent with the general population with similar comorbidities,” she said. “And as we know, black women’s risk of dying from CVD-related pregnancy complications is 3.4 times higher than that of white women. During the COVID-19 pandemic, we are seeing these racial health disparities exacerbated.”
However, any pregnant patients should not hesitate to communicate with their health care providers despite the pandemic situation, Dr. Hollier emphasized. “Communication between a patient and her ob.gyn., cardiologist, or other clinician is even more critical now during the COVID-19 pandemic. We’re hearing reports that patients who are experiencing symptoms or those with known cardiac conditions are avoiding the hospital and delaying or not seeking necessary treatment. This has the very real possibility of worsening the devastating maternal mortality crisis that we’re already experiencing in this country.”
To help overcome barriers to treatment, “collaboration between ob.gyns. and cardiologists, such as the cardio-obstetrics team or pregnancy heart team, is critical,” said Dr. Hollier. “These collaborative teams with a multidisciplinary approach can prospectively reduce the communication gaps across specialties when patients are seen separately. They can also improve the communication during care transitions such as between outpatient and inpatient care.
“In reviews of maternal deaths, we have found that there are often delays in diagnosis of heart conditions during and after pregnancy,” Dr. Hollier added. “Most maternal deaths from CVD are due to either undiagnosed cardiovascular disease or new-onset cardiomyopathy. ACOG recommends that all women be assessed for cardiovascular disease in the antepartum and postpartum periods using a recently developed algorithm,” she said. “Women who have known CVD and women who have concerning symptoms should have a consultation with this team. With increased awareness and screening, women can receive the additional care that they need.
“Because management of cardiac conditions in pregnancy is so complex, it is important to ensure that women receive care with teams and in facilities that have appropriate resources,” explained Dr. Hollier. “Women with known heart disease should see a cardiologist prior to pregnancy and receive prepregnancy counseling,” as noted in the AHA statement. “Patients determined to have moderate and high-risk CVD should be managed during pregnancy, delivery, and post partum in a medical center that is able to provide a higher level of care, including a cardio-obstetrics team.”
Early recognition of cardiovascular conditions is essential to help manage care and reduce risks to mother and baby, said Dr. Hollier. “Identification before a woman becomes pregnant means the patient’s care can be properly managed throughout the pregnancy and a detailed delivery plan can be developed through shared decision making between the patient and provider. We must think of heart disease as a possibility in every pregnant or postpartum patient we see to detect and treat at-risk mothers,” she said.
Additional research should focus on identifying risk factors prior to pregnancy, said Dr. Hollier. “There are often delays in recognizing symptoms during pregnancy and post partum, particularly for black women. We need data to understand which protocols are best to identify heart disease,”
Dr. Hollier had no financial conflicts to disclose. The authors of the AHA statement had no financial conflicts to disclose. The scientific statement was produced on behalf of the American Heart Association Council on Clinical Cardiology; Council on Atherosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
SOURCE: Mehta LS et al. Circulation. 2020 May 4. doi: 10.1161/CIR.0000000000000772.
FROM CIRCULATION
Triage, L&D, postpartum care during the COVID-19 pandemic
The meteoric rise in the number of test-positive and clinical cases of COVID-19 because of infection with the SARS coronavirus (SARS-CoV-2) in states and cities across the United States has added urgency to the efforts to develop protocols for hospital triage, admission, labor and delivery management, and other aspects of obstetrical care.
Emerging data suggest that, while SARS-CoV-2 is less lethal overall than the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) proved to be, it is significantly more contagious. Although a severe disease, the limited worldwide data so far available (as of early May) do not indicate that pregnant women are at greater risk of severe disease, compared with the general population. However, there remains a critical need for data on maternal and perinatal outcomes in women infected with SARS-CoV-2.
Multiple physiological changes in pregnancy, from reduced cell-based immune competence to changes in respiratory tract and pulmonary function – e.g., edema of the respiratory tract, increases in secretions and oxygen consumption, elevation of the diaphragm, and decrease in functional residual capacity – have historically contributed to worse obstetric outcomes in pregnant women who have had viral pneumonias. Furthermore, limited published experience with COVID-19 in China suggests worse perinatal outcomes in some affected pregnancies, including prematurity and perinatal death.
With evolution of the pandemic and accumulation of experience, it is expected that data-driven guidelines on assessment and management of infected pregnant women will contribute to improved maternal and perinatal outcomes. What is clear now, however, is that,
Here are my recommendations, based on a currently limited body of literature on COVID-19 and other communicable viral respiratory disorders, as well my experience in the greater Detroit area, a COVID-19 hot spot.
Preparing for hospital evaluation and admission
The obstetric triage or labor and delivery (L&D) unit should be notified prior to the arrival of a patient suspected of or known to be infected with the virus. This will minimize staff exposure and allow sufficient time to prepare appropriate accommodations, equipment, and supplies for the patient’s care. Hospital infection control should be promptly notified by L&D of the expected arrival of such a patient. Placement ideally should be in a negative-pressure room, which allows outside air to flow into the room but prevents contaminated air from escaping. In the absence of a negative-pressure room, an infection isolation area should be utilized.
The patient and one accompanying support individual should wear either medical-grade masks brought from home or supplied upon entry to the hospital or homemade masks or bandanas. This will reduce the risk of viral transmission to hospital workers and other individuals encountered in the hospital prior to arriving in L&D. An ideal setup is to have separate entry areas, access corridors, and elevators for patients known or suspected to have COVID-19 infection. The patient and visitor should be expeditiously escorted to the prepared area for evaluation. Patients who are not known or suspected to be infected ideally should be tested.
Screening of patients & support individuals
Proper screening of patients and support individuals is critical to protecting both patients and staff in the L&D unit. This should include an expanded questionnaire that asks about disturbances of smell and taste and GI symptoms like loss of appetite – not only the more commonly queried symptoms of fever, shortness of breath, coughing, and exposure to someone who may have been ill.
Recent studies regarding presenting symptoms cast significant doubt, in fact, on the validity of patients with “asymptomatic COVID-19.” Over 15% of patients with confirmed infection in one published case series had solely GI symptoms and almost all had some digestive symptoms, for example, and almost 90% in another study had absent or reduced sense of smell and/or taste.1,2 In fact, the use of the term “paucisymptomatic” rather than “asymptomatic” may be most appropriate.
Support individuals also should undergo temperature screening, ideally with laser noncontact thermometers on entry to the hospital or triage.
Visitor policy
The number of visitors/support individuals should be kept to a minimum to reduce transmission risk. The actual number will be determined by hospital or state policy, but up to one visitor in the labor room appears reasonable. Very strong individual justification should be required to exceed this threshold! The visitor should not only be screened for an expanded list of symptoms, but they also should be queried for underlying illnesses (e.g., diabetes, cardiovascular disease, significant lung disease, undergoing cancer therapy) as well as for age over 65 years, each of which increase the chances of severe COVID-19 disease should infection occur. The visitor should be informed of such risks and, especially when accompanying a patient with known or suspected COVID-19, provided the option of voluntarily revoking their visitor status. A visitor with known or suspected COVID-19 infection based on testing or screening should not be allowed into the L&D unit.
In addition, institutions may be considered to have obligations to the visitor/support person beyond screening. These include instructions in proper mask usage, hand washing, and limiting the touching of surfaces to lower infection risk.
“Visitor relays” where one visitor replaces another should be strongly discouraged. Visitors should similarly not be allowed to wander around the hospital (to use phones, for instance); transiting back and forth to obtain food and coffee should be kept to a strict minimum. For visitors accompanying COVID-19–-infected women, “visitor’s plates” provided by the hospital at reasonable cost is a much-preferred arrangement for obtaining meals during the course of the hospital stay. In addition, visitors should be sent out of the room during the performance of aerosolizing procedures.
Labor and delivery management
The successful management of patients with COVID-19 requires a rigorous infection control protocol informed by guidelines from national entities, such as the Centers for Disease Control and Prevention, the Society for Maternal-Fetal Medicine, and the American College of Obstetricians and Gynecologists, and by state health departments when available.
Strict limits on the number of obstetricians and other health care workers (HCWs) entering the patient’s room should be enforced and documented to minimize risk to the HCWs attending to patients who have a positive diagnosis or who are under investigation. Only in cases of demonstrable clinical benefit should repeat visits by the same or additional HCWs be permitted. Conventional and electronic tablets present an excellent opportunity for patient follow-up visits without room entry. In our institution, this has been successfully piloted in nonpregnant patients. Obstetricians and others caring for obstetrical patients – especially those who are infected or under investigation for infection – should always wear a properly fitted N95 mask.
Because patients with COVID-19 may have or go on to develop a constellation of organ abnormalities (e.g., cardiovascular, renal, pulmonary), it is vital that a standardized panel of baseline laboratory studies be developed for pregnant patients. This will minimize the need for repeated blood draws and other testing which may increase HCW exposure.
A negative screen based on nonreport of symptoms, lack of temperature elevation, and reported nonexposure to individuals with COVID-19 symptoms still has limitations in terms of disease detection. A recent report from a tertiary care hospital in New York City found that close to one-third of pregnant patients with confirmed COVID-19 admitted over a 2-week period had no viral symptoms or instructive history on initial admission.3 This is consistent with our clinical experience. Most importantly, therefore, routine quantitative reverse transcription polymerase chain reaction testing should be performed on all patients admitted to the L&D unit.
Given the reported variability in the accuracy of polymerase chain reaction testing induced by variable effectiveness of sampling techniques, stage of infection, and inherent test accuracy issues, symptomatic patients with a negative test should first obtain clearance from infectious disease specialists before isolation precautions are discontinued. Repeat testing in 24 hours, including testing of multiple sites, may subsequently yield a positive result in persistently symptomatic patients.
Intrapartum management
As much as possible, standard obstetric indications should guide the timing and route of delivery. In the case of a COVID-19–positive patient or a patient under investigation, nonobstetric factors may bear heavily on decision making, and management flexibility is of great value. For example, in cases of severe or critical disease status, evidence suggests that early delivery regardless of gestational age can improve maternal oxygenation; this supports the liberal use of C-sections in these circumstances. In addition, shortening labor length as well as duration of hospitalization may be expected to reduce the risk of transmission to HCWs, other staff, and other patients.
High rates of cesarean delivery unsurprisingly have been reported thus far: One review of 108 case reports and series of test-positive COVID-19 pregnancies found a 92% C-section rate, and another review and meta-analysis of studies of SARS, MERS, and COVID-19 during pregnancy similarly found that the majority of patients – 84% across all coronavirus infections and 91% in COVID-19 pregnancies – were delivered by C-section.4,5 Given these high rates of cesarean deliveries, the early placement of neuraxial anesthesia while the patient is stable appears to be prudent and obviates the need for intubation, the latter of which is associated with increased aerosol generation and increased virus transmission risk.
Strict protocols for the optimal protection of staff should be observed, including proper personal protective equipment (PPE) protection. Protocols have been detailed in various guidelines and publications; they include the wearing of shoe covers, gowns, N95 masks, goggles, face shields, and two layers of gloves.
For institutions that currently do not offer routine COVID-19 testing to pregnant patients – especially those in areas of outbreaks – N95 masks and eye protection should still be provided to all HCWs involved in the intrapartum management of untested asymptomatic patients, particularly those in the active phase of labor. This protection is justified given the limitations of symptom- and history-based screening and the not-uncommon experience of the patient with a negative screen who subsequently develops the clinical syndrome.
Obstetric management of labor requires close patient contact that potentially elevates the risk of contamination and infection. During the active stage of labor, patient shouting, rapid mouth breathing, and other behaviors inherent to labor all increase the risk of aerosolization of oronasal secretions. In addition, nasal-prong oxygen administration is believed to independently increase the risk of aerosolization of secretions. The casual practice of nasal oxygen application should thus be discontinued and, where felt to be absolutely necessary, a mask should be worn on top of the prongs.
Regarding operative delivery, each participating obstetric surgeon should observe guidelines and recommendations of governing national organizations and professional groups – including the American College of Surgeons – regarding the safe conduct of operations on patients with COVID-19. Written guidelines should be tailored as needed to the performance of C-sections and readily available in L&D. Drills and simulations are generally valuable, and expertise and support should always be available in the labor room to assist with donning and doffing of PPE.
Postpartum care
Expeditious separation of the COVID-19–positive mother from her infant is recommended, including avoidance of delayed cord clamping because of insufficient evidence of benefit to the infant. Insufficient evidence exists to support vertical transmission, but the possibility of maternal-infant transmission is clinically accepted based on small case reports of infection in a neonate at 30 hours of life and in infants of mothers with suspected or confirmed COVID-19.6,7 Accordingly, it is recommended that the benefit of early infant separation should be discussed with the mother. If approved, the infant should be kept in a separate isolation area and observed.
There is no evidence of breast milk transmission of the virus. For those electing to breastfeed, the patient should be provided with a breast pump to express and store the milk for subsequent bottle feeding. For mothers who elect to room in with the infant, a separation distance of 6 feet is recommended with an intervening barrier curtain. For COVID-19–positive mothers who elect breastfeeding, meticulous hand and face washing, continuous wearing of a mask, and cleansing of the breast prior to feeding needs to be maintained.
Restrictive visiting policies of no more than one visitor should be maintained. For severely or critically ill patients with COVID-19, it has been suggested that no visitors be allowed. As with other hospitalizations of COVID-19 patients, the HCW contact should be kept at a justifiable minimum to reduce the risk of transmission.
Protecting the obstetrician and other HCWs
Protecting the health of obstetricians and other HCWs is central to any successful strategy to fight the COVID-19 epidemic. For the individual obstetrician, careful attention to national and local hospital guidelines is required as these are rapidly evolving.
Physicians and their leadership must maintain an ongoing dialogue with hospital leadership to continually upgrade and optimize infection prevention and control measures, and to uphold best practices. The experience in Wuhan, China, illustrates the effectiveness of the proper use of PPE along with population control measures to reduce infections in HCWs. Prior to understanding the mechanism of virus transmission and using protective equipment, infection rates of 3%-29% were reported among HCWs. With the meticulous utilization of mitigation strategies and population control measures – including consistent use of PPE – the rate of infection of HCWs reportedly fell to zero.
In outpatient offices, all staff and HCWs should wear masks at all times and engage in social distancing and in frequent hand sanitization. Patients should be strongly encouraged to wear masks during office visits and on all other occasions when they will be in physical proximity to other individuals outside of the home.
Reports from epidemic areas describe transmission from household sources as a significant cause of HCW infection. The information emphasizes the need for ongoing vigilance and attention to sanitization measures even when at home with one’s family. An additional benefit is reduced risk of transmission from HCWs to family members.
Dr. Bahado-Singh is professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System.
References
1. Luo S et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.043.
2. Lechien JR et al. Eur Arch Otorhinolaryngol. 2020 Apr 6. doi: 10.1007/s00405-020-05965-1.
3. Breslin N et al. Am J Obstet Gynecol MFM. 2020 Apr 9. doi: 10.1016/j.ajogmf.2020.100118.
4. Zaigham M, Andersson O. Acta Obstet Gynecol Scand. 2020 Apr 7. doi: 10.1111/aogs.13867.
5. Di Mascio D et al. Am J Obstet Gynecol MFM. 2020 Mar 25. doi: 10.1016/j.ajogmf.2020.100107.
6. Ital J. Pediatr 2020;46(1) doi: 10.1186/s13052-020-0820-x.
7. Int J Gynaecol Obstet. 2020;149(2):130-6.
*This article was updated 5/6/2020.
The meteoric rise in the number of test-positive and clinical cases of COVID-19 because of infection with the SARS coronavirus (SARS-CoV-2) in states and cities across the United States has added urgency to the efforts to develop protocols for hospital triage, admission, labor and delivery management, and other aspects of obstetrical care.
Emerging data suggest that, while SARS-CoV-2 is less lethal overall than the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) proved to be, it is significantly more contagious. Although a severe disease, the limited worldwide data so far available (as of early May) do not indicate that pregnant women are at greater risk of severe disease, compared with the general population. However, there remains a critical need for data on maternal and perinatal outcomes in women infected with SARS-CoV-2.
Multiple physiological changes in pregnancy, from reduced cell-based immune competence to changes in respiratory tract and pulmonary function – e.g., edema of the respiratory tract, increases in secretions and oxygen consumption, elevation of the diaphragm, and decrease in functional residual capacity – have historically contributed to worse obstetric outcomes in pregnant women who have had viral pneumonias. Furthermore, limited published experience with COVID-19 in China suggests worse perinatal outcomes in some affected pregnancies, including prematurity and perinatal death.
With evolution of the pandemic and accumulation of experience, it is expected that data-driven guidelines on assessment and management of infected pregnant women will contribute to improved maternal and perinatal outcomes. What is clear now, however, is that,
Here are my recommendations, based on a currently limited body of literature on COVID-19 and other communicable viral respiratory disorders, as well my experience in the greater Detroit area, a COVID-19 hot spot.
Preparing for hospital evaluation and admission
The obstetric triage or labor and delivery (L&D) unit should be notified prior to the arrival of a patient suspected of or known to be infected with the virus. This will minimize staff exposure and allow sufficient time to prepare appropriate accommodations, equipment, and supplies for the patient’s care. Hospital infection control should be promptly notified by L&D of the expected arrival of such a patient. Placement ideally should be in a negative-pressure room, which allows outside air to flow into the room but prevents contaminated air from escaping. In the absence of a negative-pressure room, an infection isolation area should be utilized.
The patient and one accompanying support individual should wear either medical-grade masks brought from home or supplied upon entry to the hospital or homemade masks or bandanas. This will reduce the risk of viral transmission to hospital workers and other individuals encountered in the hospital prior to arriving in L&D. An ideal setup is to have separate entry areas, access corridors, and elevators for patients known or suspected to have COVID-19 infection. The patient and visitor should be expeditiously escorted to the prepared area for evaluation. Patients who are not known or suspected to be infected ideally should be tested.
Screening of patients & support individuals
Proper screening of patients and support individuals is critical to protecting both patients and staff in the L&D unit. This should include an expanded questionnaire that asks about disturbances of smell and taste and GI symptoms like loss of appetite – not only the more commonly queried symptoms of fever, shortness of breath, coughing, and exposure to someone who may have been ill.
Recent studies regarding presenting symptoms cast significant doubt, in fact, on the validity of patients with “asymptomatic COVID-19.” Over 15% of patients with confirmed infection in one published case series had solely GI symptoms and almost all had some digestive symptoms, for example, and almost 90% in another study had absent or reduced sense of smell and/or taste.1,2 In fact, the use of the term “paucisymptomatic” rather than “asymptomatic” may be most appropriate.
Support individuals also should undergo temperature screening, ideally with laser noncontact thermometers on entry to the hospital or triage.
Visitor policy
The number of visitors/support individuals should be kept to a minimum to reduce transmission risk. The actual number will be determined by hospital or state policy, but up to one visitor in the labor room appears reasonable. Very strong individual justification should be required to exceed this threshold! The visitor should not only be screened for an expanded list of symptoms, but they also should be queried for underlying illnesses (e.g., diabetes, cardiovascular disease, significant lung disease, undergoing cancer therapy) as well as for age over 65 years, each of which increase the chances of severe COVID-19 disease should infection occur. The visitor should be informed of such risks and, especially when accompanying a patient with known or suspected COVID-19, provided the option of voluntarily revoking their visitor status. A visitor with known or suspected COVID-19 infection based on testing or screening should not be allowed into the L&D unit.
In addition, institutions may be considered to have obligations to the visitor/support person beyond screening. These include instructions in proper mask usage, hand washing, and limiting the touching of surfaces to lower infection risk.
“Visitor relays” where one visitor replaces another should be strongly discouraged. Visitors should similarly not be allowed to wander around the hospital (to use phones, for instance); transiting back and forth to obtain food and coffee should be kept to a strict minimum. For visitors accompanying COVID-19–-infected women, “visitor’s plates” provided by the hospital at reasonable cost is a much-preferred arrangement for obtaining meals during the course of the hospital stay. In addition, visitors should be sent out of the room during the performance of aerosolizing procedures.
Labor and delivery management
The successful management of patients with COVID-19 requires a rigorous infection control protocol informed by guidelines from national entities, such as the Centers for Disease Control and Prevention, the Society for Maternal-Fetal Medicine, and the American College of Obstetricians and Gynecologists, and by state health departments when available.
Strict limits on the number of obstetricians and other health care workers (HCWs) entering the patient’s room should be enforced and documented to minimize risk to the HCWs attending to patients who have a positive diagnosis or who are under investigation. Only in cases of demonstrable clinical benefit should repeat visits by the same or additional HCWs be permitted. Conventional and electronic tablets present an excellent opportunity for patient follow-up visits without room entry. In our institution, this has been successfully piloted in nonpregnant patients. Obstetricians and others caring for obstetrical patients – especially those who are infected or under investigation for infection – should always wear a properly fitted N95 mask.
Because patients with COVID-19 may have or go on to develop a constellation of organ abnormalities (e.g., cardiovascular, renal, pulmonary), it is vital that a standardized panel of baseline laboratory studies be developed for pregnant patients. This will minimize the need for repeated blood draws and other testing which may increase HCW exposure.
A negative screen based on nonreport of symptoms, lack of temperature elevation, and reported nonexposure to individuals with COVID-19 symptoms still has limitations in terms of disease detection. A recent report from a tertiary care hospital in New York City found that close to one-third of pregnant patients with confirmed COVID-19 admitted over a 2-week period had no viral symptoms or instructive history on initial admission.3 This is consistent with our clinical experience. Most importantly, therefore, routine quantitative reverse transcription polymerase chain reaction testing should be performed on all patients admitted to the L&D unit.
Given the reported variability in the accuracy of polymerase chain reaction testing induced by variable effectiveness of sampling techniques, stage of infection, and inherent test accuracy issues, symptomatic patients with a negative test should first obtain clearance from infectious disease specialists before isolation precautions are discontinued. Repeat testing in 24 hours, including testing of multiple sites, may subsequently yield a positive result in persistently symptomatic patients.
Intrapartum management
As much as possible, standard obstetric indications should guide the timing and route of delivery. In the case of a COVID-19–positive patient or a patient under investigation, nonobstetric factors may bear heavily on decision making, and management flexibility is of great value. For example, in cases of severe or critical disease status, evidence suggests that early delivery regardless of gestational age can improve maternal oxygenation; this supports the liberal use of C-sections in these circumstances. In addition, shortening labor length as well as duration of hospitalization may be expected to reduce the risk of transmission to HCWs, other staff, and other patients.
High rates of cesarean delivery unsurprisingly have been reported thus far: One review of 108 case reports and series of test-positive COVID-19 pregnancies found a 92% C-section rate, and another review and meta-analysis of studies of SARS, MERS, and COVID-19 during pregnancy similarly found that the majority of patients – 84% across all coronavirus infections and 91% in COVID-19 pregnancies – were delivered by C-section.4,5 Given these high rates of cesarean deliveries, the early placement of neuraxial anesthesia while the patient is stable appears to be prudent and obviates the need for intubation, the latter of which is associated with increased aerosol generation and increased virus transmission risk.
Strict protocols for the optimal protection of staff should be observed, including proper personal protective equipment (PPE) protection. Protocols have been detailed in various guidelines and publications; they include the wearing of shoe covers, gowns, N95 masks, goggles, face shields, and two layers of gloves.
For institutions that currently do not offer routine COVID-19 testing to pregnant patients – especially those in areas of outbreaks – N95 masks and eye protection should still be provided to all HCWs involved in the intrapartum management of untested asymptomatic patients, particularly those in the active phase of labor. This protection is justified given the limitations of symptom- and history-based screening and the not-uncommon experience of the patient with a negative screen who subsequently develops the clinical syndrome.
Obstetric management of labor requires close patient contact that potentially elevates the risk of contamination and infection. During the active stage of labor, patient shouting, rapid mouth breathing, and other behaviors inherent to labor all increase the risk of aerosolization of oronasal secretions. In addition, nasal-prong oxygen administration is believed to independently increase the risk of aerosolization of secretions. The casual practice of nasal oxygen application should thus be discontinued and, where felt to be absolutely necessary, a mask should be worn on top of the prongs.
Regarding operative delivery, each participating obstetric surgeon should observe guidelines and recommendations of governing national organizations and professional groups – including the American College of Surgeons – regarding the safe conduct of operations on patients with COVID-19. Written guidelines should be tailored as needed to the performance of C-sections and readily available in L&D. Drills and simulations are generally valuable, and expertise and support should always be available in the labor room to assist with donning and doffing of PPE.
Postpartum care
Expeditious separation of the COVID-19–positive mother from her infant is recommended, including avoidance of delayed cord clamping because of insufficient evidence of benefit to the infant. Insufficient evidence exists to support vertical transmission, but the possibility of maternal-infant transmission is clinically accepted based on small case reports of infection in a neonate at 30 hours of life and in infants of mothers with suspected or confirmed COVID-19.6,7 Accordingly, it is recommended that the benefit of early infant separation should be discussed with the mother. If approved, the infant should be kept in a separate isolation area and observed.
There is no evidence of breast milk transmission of the virus. For those electing to breastfeed, the patient should be provided with a breast pump to express and store the milk for subsequent bottle feeding. For mothers who elect to room in with the infant, a separation distance of 6 feet is recommended with an intervening barrier curtain. For COVID-19–positive mothers who elect breastfeeding, meticulous hand and face washing, continuous wearing of a mask, and cleansing of the breast prior to feeding needs to be maintained.
Restrictive visiting policies of no more than one visitor should be maintained. For severely or critically ill patients with COVID-19, it has been suggested that no visitors be allowed. As with other hospitalizations of COVID-19 patients, the HCW contact should be kept at a justifiable minimum to reduce the risk of transmission.
Protecting the obstetrician and other HCWs
Protecting the health of obstetricians and other HCWs is central to any successful strategy to fight the COVID-19 epidemic. For the individual obstetrician, careful attention to national and local hospital guidelines is required as these are rapidly evolving.
Physicians and their leadership must maintain an ongoing dialogue with hospital leadership to continually upgrade and optimize infection prevention and control measures, and to uphold best practices. The experience in Wuhan, China, illustrates the effectiveness of the proper use of PPE along with population control measures to reduce infections in HCWs. Prior to understanding the mechanism of virus transmission and using protective equipment, infection rates of 3%-29% were reported among HCWs. With the meticulous utilization of mitigation strategies and population control measures – including consistent use of PPE – the rate of infection of HCWs reportedly fell to zero.
In outpatient offices, all staff and HCWs should wear masks at all times and engage in social distancing and in frequent hand sanitization. Patients should be strongly encouraged to wear masks during office visits and on all other occasions when they will be in physical proximity to other individuals outside of the home.
Reports from epidemic areas describe transmission from household sources as a significant cause of HCW infection. The information emphasizes the need for ongoing vigilance and attention to sanitization measures even when at home with one’s family. An additional benefit is reduced risk of transmission from HCWs to family members.
Dr. Bahado-Singh is professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System.
References
1. Luo S et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.043.
2. Lechien JR et al. Eur Arch Otorhinolaryngol. 2020 Apr 6. doi: 10.1007/s00405-020-05965-1.
3. Breslin N et al. Am J Obstet Gynecol MFM. 2020 Apr 9. doi: 10.1016/j.ajogmf.2020.100118.
4. Zaigham M, Andersson O. Acta Obstet Gynecol Scand. 2020 Apr 7. doi: 10.1111/aogs.13867.
5. Di Mascio D et al. Am J Obstet Gynecol MFM. 2020 Mar 25. doi: 10.1016/j.ajogmf.2020.100107.
6. Ital J. Pediatr 2020;46(1) doi: 10.1186/s13052-020-0820-x.
7. Int J Gynaecol Obstet. 2020;149(2):130-6.
*This article was updated 5/6/2020.
The meteoric rise in the number of test-positive and clinical cases of COVID-19 because of infection with the SARS coronavirus (SARS-CoV-2) in states and cities across the United States has added urgency to the efforts to develop protocols for hospital triage, admission, labor and delivery management, and other aspects of obstetrical care.
Emerging data suggest that, while SARS-CoV-2 is less lethal overall than the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) proved to be, it is significantly more contagious. Although a severe disease, the limited worldwide data so far available (as of early May) do not indicate that pregnant women are at greater risk of severe disease, compared with the general population. However, there remains a critical need for data on maternal and perinatal outcomes in women infected with SARS-CoV-2.
Multiple physiological changes in pregnancy, from reduced cell-based immune competence to changes in respiratory tract and pulmonary function – e.g., edema of the respiratory tract, increases in secretions and oxygen consumption, elevation of the diaphragm, and decrease in functional residual capacity – have historically contributed to worse obstetric outcomes in pregnant women who have had viral pneumonias. Furthermore, limited published experience with COVID-19 in China suggests worse perinatal outcomes in some affected pregnancies, including prematurity and perinatal death.
With evolution of the pandemic and accumulation of experience, it is expected that data-driven guidelines on assessment and management of infected pregnant women will contribute to improved maternal and perinatal outcomes. What is clear now, however, is that,
Here are my recommendations, based on a currently limited body of literature on COVID-19 and other communicable viral respiratory disorders, as well my experience in the greater Detroit area, a COVID-19 hot spot.
Preparing for hospital evaluation and admission
The obstetric triage or labor and delivery (L&D) unit should be notified prior to the arrival of a patient suspected of or known to be infected with the virus. This will minimize staff exposure and allow sufficient time to prepare appropriate accommodations, equipment, and supplies for the patient’s care. Hospital infection control should be promptly notified by L&D of the expected arrival of such a patient. Placement ideally should be in a negative-pressure room, which allows outside air to flow into the room but prevents contaminated air from escaping. In the absence of a negative-pressure room, an infection isolation area should be utilized.
The patient and one accompanying support individual should wear either medical-grade masks brought from home or supplied upon entry to the hospital or homemade masks or bandanas. This will reduce the risk of viral transmission to hospital workers and other individuals encountered in the hospital prior to arriving in L&D. An ideal setup is to have separate entry areas, access corridors, and elevators for patients known or suspected to have COVID-19 infection. The patient and visitor should be expeditiously escorted to the prepared area for evaluation. Patients who are not known or suspected to be infected ideally should be tested.
Screening of patients & support individuals
Proper screening of patients and support individuals is critical to protecting both patients and staff in the L&D unit. This should include an expanded questionnaire that asks about disturbances of smell and taste and GI symptoms like loss of appetite – not only the more commonly queried symptoms of fever, shortness of breath, coughing, and exposure to someone who may have been ill.
Recent studies regarding presenting symptoms cast significant doubt, in fact, on the validity of patients with “asymptomatic COVID-19.” Over 15% of patients with confirmed infection in one published case series had solely GI symptoms and almost all had some digestive symptoms, for example, and almost 90% in another study had absent or reduced sense of smell and/or taste.1,2 In fact, the use of the term “paucisymptomatic” rather than “asymptomatic” may be most appropriate.
Support individuals also should undergo temperature screening, ideally with laser noncontact thermometers on entry to the hospital or triage.
Visitor policy
The number of visitors/support individuals should be kept to a minimum to reduce transmission risk. The actual number will be determined by hospital or state policy, but up to one visitor in the labor room appears reasonable. Very strong individual justification should be required to exceed this threshold! The visitor should not only be screened for an expanded list of symptoms, but they also should be queried for underlying illnesses (e.g., diabetes, cardiovascular disease, significant lung disease, undergoing cancer therapy) as well as for age over 65 years, each of which increase the chances of severe COVID-19 disease should infection occur. The visitor should be informed of such risks and, especially when accompanying a patient with known or suspected COVID-19, provided the option of voluntarily revoking their visitor status. A visitor with known or suspected COVID-19 infection based on testing or screening should not be allowed into the L&D unit.
In addition, institutions may be considered to have obligations to the visitor/support person beyond screening. These include instructions in proper mask usage, hand washing, and limiting the touching of surfaces to lower infection risk.
“Visitor relays” where one visitor replaces another should be strongly discouraged. Visitors should similarly not be allowed to wander around the hospital (to use phones, for instance); transiting back and forth to obtain food and coffee should be kept to a strict minimum. For visitors accompanying COVID-19–-infected women, “visitor’s plates” provided by the hospital at reasonable cost is a much-preferred arrangement for obtaining meals during the course of the hospital stay. In addition, visitors should be sent out of the room during the performance of aerosolizing procedures.
Labor and delivery management
The successful management of patients with COVID-19 requires a rigorous infection control protocol informed by guidelines from national entities, such as the Centers for Disease Control and Prevention, the Society for Maternal-Fetal Medicine, and the American College of Obstetricians and Gynecologists, and by state health departments when available.
Strict limits on the number of obstetricians and other health care workers (HCWs) entering the patient’s room should be enforced and documented to minimize risk to the HCWs attending to patients who have a positive diagnosis or who are under investigation. Only in cases of demonstrable clinical benefit should repeat visits by the same or additional HCWs be permitted. Conventional and electronic tablets present an excellent opportunity for patient follow-up visits without room entry. In our institution, this has been successfully piloted in nonpregnant patients. Obstetricians and others caring for obstetrical patients – especially those who are infected or under investigation for infection – should always wear a properly fitted N95 mask.
Because patients with COVID-19 may have or go on to develop a constellation of organ abnormalities (e.g., cardiovascular, renal, pulmonary), it is vital that a standardized panel of baseline laboratory studies be developed for pregnant patients. This will minimize the need for repeated blood draws and other testing which may increase HCW exposure.
A negative screen based on nonreport of symptoms, lack of temperature elevation, and reported nonexposure to individuals with COVID-19 symptoms still has limitations in terms of disease detection. A recent report from a tertiary care hospital in New York City found that close to one-third of pregnant patients with confirmed COVID-19 admitted over a 2-week period had no viral symptoms or instructive history on initial admission.3 This is consistent with our clinical experience. Most importantly, therefore, routine quantitative reverse transcription polymerase chain reaction testing should be performed on all patients admitted to the L&D unit.
Given the reported variability in the accuracy of polymerase chain reaction testing induced by variable effectiveness of sampling techniques, stage of infection, and inherent test accuracy issues, symptomatic patients with a negative test should first obtain clearance from infectious disease specialists before isolation precautions are discontinued. Repeat testing in 24 hours, including testing of multiple sites, may subsequently yield a positive result in persistently symptomatic patients.
Intrapartum management
As much as possible, standard obstetric indications should guide the timing and route of delivery. In the case of a COVID-19–positive patient or a patient under investigation, nonobstetric factors may bear heavily on decision making, and management flexibility is of great value. For example, in cases of severe or critical disease status, evidence suggests that early delivery regardless of gestational age can improve maternal oxygenation; this supports the liberal use of C-sections in these circumstances. In addition, shortening labor length as well as duration of hospitalization may be expected to reduce the risk of transmission to HCWs, other staff, and other patients.
High rates of cesarean delivery unsurprisingly have been reported thus far: One review of 108 case reports and series of test-positive COVID-19 pregnancies found a 92% C-section rate, and another review and meta-analysis of studies of SARS, MERS, and COVID-19 during pregnancy similarly found that the majority of patients – 84% across all coronavirus infections and 91% in COVID-19 pregnancies – were delivered by C-section.4,5 Given these high rates of cesarean deliveries, the early placement of neuraxial anesthesia while the patient is stable appears to be prudent and obviates the need for intubation, the latter of which is associated with increased aerosol generation and increased virus transmission risk.
Strict protocols for the optimal protection of staff should be observed, including proper personal protective equipment (PPE) protection. Protocols have been detailed in various guidelines and publications; they include the wearing of shoe covers, gowns, N95 masks, goggles, face shields, and two layers of gloves.
For institutions that currently do not offer routine COVID-19 testing to pregnant patients – especially those in areas of outbreaks – N95 masks and eye protection should still be provided to all HCWs involved in the intrapartum management of untested asymptomatic patients, particularly those in the active phase of labor. This protection is justified given the limitations of symptom- and history-based screening and the not-uncommon experience of the patient with a negative screen who subsequently develops the clinical syndrome.
Obstetric management of labor requires close patient contact that potentially elevates the risk of contamination and infection. During the active stage of labor, patient shouting, rapid mouth breathing, and other behaviors inherent to labor all increase the risk of aerosolization of oronasal secretions. In addition, nasal-prong oxygen administration is believed to independently increase the risk of aerosolization of secretions. The casual practice of nasal oxygen application should thus be discontinued and, where felt to be absolutely necessary, a mask should be worn on top of the prongs.
Regarding operative delivery, each participating obstetric surgeon should observe guidelines and recommendations of governing national organizations and professional groups – including the American College of Surgeons – regarding the safe conduct of operations on patients with COVID-19. Written guidelines should be tailored as needed to the performance of C-sections and readily available in L&D. Drills and simulations are generally valuable, and expertise and support should always be available in the labor room to assist with donning and doffing of PPE.
Postpartum care
Expeditious separation of the COVID-19–positive mother from her infant is recommended, including avoidance of delayed cord clamping because of insufficient evidence of benefit to the infant. Insufficient evidence exists to support vertical transmission, but the possibility of maternal-infant transmission is clinically accepted based on small case reports of infection in a neonate at 30 hours of life and in infants of mothers with suspected or confirmed COVID-19.6,7 Accordingly, it is recommended that the benefit of early infant separation should be discussed with the mother. If approved, the infant should be kept in a separate isolation area and observed.
There is no evidence of breast milk transmission of the virus. For those electing to breastfeed, the patient should be provided with a breast pump to express and store the milk for subsequent bottle feeding. For mothers who elect to room in with the infant, a separation distance of 6 feet is recommended with an intervening barrier curtain. For COVID-19–positive mothers who elect breastfeeding, meticulous hand and face washing, continuous wearing of a mask, and cleansing of the breast prior to feeding needs to be maintained.
Restrictive visiting policies of no more than one visitor should be maintained. For severely or critically ill patients with COVID-19, it has been suggested that no visitors be allowed. As with other hospitalizations of COVID-19 patients, the HCW contact should be kept at a justifiable minimum to reduce the risk of transmission.
Protecting the obstetrician and other HCWs
Protecting the health of obstetricians and other HCWs is central to any successful strategy to fight the COVID-19 epidemic. For the individual obstetrician, careful attention to national and local hospital guidelines is required as these are rapidly evolving.
Physicians and their leadership must maintain an ongoing dialogue with hospital leadership to continually upgrade and optimize infection prevention and control measures, and to uphold best practices. The experience in Wuhan, China, illustrates the effectiveness of the proper use of PPE along with population control measures to reduce infections in HCWs. Prior to understanding the mechanism of virus transmission and using protective equipment, infection rates of 3%-29% were reported among HCWs. With the meticulous utilization of mitigation strategies and population control measures – including consistent use of PPE – the rate of infection of HCWs reportedly fell to zero.
In outpatient offices, all staff and HCWs should wear masks at all times and engage in social distancing and in frequent hand sanitization. Patients should be strongly encouraged to wear masks during office visits and on all other occasions when they will be in physical proximity to other individuals outside of the home.
Reports from epidemic areas describe transmission from household sources as a significant cause of HCW infection. The information emphasizes the need for ongoing vigilance and attention to sanitization measures even when at home with one’s family. An additional benefit is reduced risk of transmission from HCWs to family members.
Dr. Bahado-Singh is professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System.
References
1. Luo S et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.043.
2. Lechien JR et al. Eur Arch Otorhinolaryngol. 2020 Apr 6. doi: 10.1007/s00405-020-05965-1.
3. Breslin N et al. Am J Obstet Gynecol MFM. 2020 Apr 9. doi: 10.1016/j.ajogmf.2020.100118.
4. Zaigham M, Andersson O. Acta Obstet Gynecol Scand. 2020 Apr 7. doi: 10.1111/aogs.13867.
5. Di Mascio D et al. Am J Obstet Gynecol MFM. 2020 Mar 25. doi: 10.1016/j.ajogmf.2020.100107.
6. Ital J. Pediatr 2020;46(1) doi: 10.1186/s13052-020-0820-x.
7. Int J Gynaecol Obstet. 2020;149(2):130-6.
*This article was updated 5/6/2020.
Obstetrics during the COVID-19 pandemic
The identification of the SARS coronavirus (SARS-CoV-2) and emergence of the associated infectious respiratory disease, COVID-19, in late 2019 catapulted the citizens of the world, especially those in the health care professions, into an era of considerable uncertainty. At this moment in human history, calm reassurance – founded in fact and evidence – seems its greatest need. Much of the focus within the biomedical community has been on containment, prevention, and treatment of this highly contagious and, for some, extremely virulent disease.
However, for ob.gyns on the front lines of the COVID-19 fight, there is the additional challenge of caring for at least two patients simultaneously: the mother and her unborn baby. Studies in mother-baby dyads, while being published at an incredible pace, are still quite scarce. In addition, published reports are limited by the small sample size of the patient population (many are single-case reports), lack of uniformity in the timing and types of clinical samples collected, testing delays, and varying isolation protocols in cases where the mother has confirmed SARS-CoV-2.
Five months into a pandemic that has swept the world, we still know very little about COVID-19 infection in the general population, let alone the obstetric one. We do not know if having and resolving COVID-19 infection provides any long-term protection against future disease. We do not know if vertical transmission of SARS-CoV-2 occurs. We do not know if maternal infection confers any immunologic benefit to the neonate. The list goes on.
What we do know is that taking extra precautions works. Use of personal protective equipment saves health care practitioner and patient lives. Prohibiting or restricting visitors to only one person in hospitals reduces risk of transmission to vulnerable patients.
Additionally, we know that leading with compassion is vital to easing patient – and practitioner – anxiety and stress. Most importantly, we know that people are extraordinarily resilient, especially when it comes to safeguarding the health of their families.
To address some of the major concerns that many ob.gyns. have regarding their risk of coronavirus exposure when caring for patients, we have invited Ray Bahado-Singh, MD, professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System, who works in a suburb of Detroit, one of our nation’s COVID-19 hot spots.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
The identification of the SARS coronavirus (SARS-CoV-2) and emergence of the associated infectious respiratory disease, COVID-19, in late 2019 catapulted the citizens of the world, especially those in the health care professions, into an era of considerable uncertainty. At this moment in human history, calm reassurance – founded in fact and evidence – seems its greatest need. Much of the focus within the biomedical community has been on containment, prevention, and treatment of this highly contagious and, for some, extremely virulent disease.
However, for ob.gyns on the front lines of the COVID-19 fight, there is the additional challenge of caring for at least two patients simultaneously: the mother and her unborn baby. Studies in mother-baby dyads, while being published at an incredible pace, are still quite scarce. In addition, published reports are limited by the small sample size of the patient population (many are single-case reports), lack of uniformity in the timing and types of clinical samples collected, testing delays, and varying isolation protocols in cases where the mother has confirmed SARS-CoV-2.
Five months into a pandemic that has swept the world, we still know very little about COVID-19 infection in the general population, let alone the obstetric one. We do not know if having and resolving COVID-19 infection provides any long-term protection against future disease. We do not know if vertical transmission of SARS-CoV-2 occurs. We do not know if maternal infection confers any immunologic benefit to the neonate. The list goes on.
What we do know is that taking extra precautions works. Use of personal protective equipment saves health care practitioner and patient lives. Prohibiting or restricting visitors to only one person in hospitals reduces risk of transmission to vulnerable patients.
Additionally, we know that leading with compassion is vital to easing patient – and practitioner – anxiety and stress. Most importantly, we know that people are extraordinarily resilient, especially when it comes to safeguarding the health of their families.
To address some of the major concerns that many ob.gyns. have regarding their risk of coronavirus exposure when caring for patients, we have invited Ray Bahado-Singh, MD, professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System, who works in a suburb of Detroit, one of our nation’s COVID-19 hot spots.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
The identification of the SARS coronavirus (SARS-CoV-2) and emergence of the associated infectious respiratory disease, COVID-19, in late 2019 catapulted the citizens of the world, especially those in the health care professions, into an era of considerable uncertainty. At this moment in human history, calm reassurance – founded in fact and evidence – seems its greatest need. Much of the focus within the biomedical community has been on containment, prevention, and treatment of this highly contagious and, for some, extremely virulent disease.
However, for ob.gyns on the front lines of the COVID-19 fight, there is the additional challenge of caring for at least two patients simultaneously: the mother and her unborn baby. Studies in mother-baby dyads, while being published at an incredible pace, are still quite scarce. In addition, published reports are limited by the small sample size of the patient population (many are single-case reports), lack of uniformity in the timing and types of clinical samples collected, testing delays, and varying isolation protocols in cases where the mother has confirmed SARS-CoV-2.
Five months into a pandemic that has swept the world, we still know very little about COVID-19 infection in the general population, let alone the obstetric one. We do not know if having and resolving COVID-19 infection provides any long-term protection against future disease. We do not know if vertical transmission of SARS-CoV-2 occurs. We do not know if maternal infection confers any immunologic benefit to the neonate. The list goes on.
What we do know is that taking extra precautions works. Use of personal protective equipment saves health care practitioner and patient lives. Prohibiting or restricting visitors to only one person in hospitals reduces risk of transmission to vulnerable patients.
Additionally, we know that leading with compassion is vital to easing patient – and practitioner – anxiety and stress. Most importantly, we know that people are extraordinarily resilient, especially when it comes to safeguarding the health of their families.
To address some of the major concerns that many ob.gyns. have regarding their risk of coronavirus exposure when caring for patients, we have invited Ray Bahado-Singh, MD, professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System, who works in a suburb of Detroit, one of our nation’s COVID-19 hot spots.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].