User login
The X-waiver is dead
In 2016, when Erin Schanning lost her brother Ethan to an overdose, she wanted to know what could have been done to have helped him. Ethan, who had struggled with opioids since getting a prescription for the drugs after a dental procedure in middle school, had tried dozens of treatments. But at the age of 30, he was gone.
“After my brother died, I started researching and was surprised to learn that there were many evidence-based ways to treat substance use disorder that he hadn’t had access to, even though he had doggedly pursued treatment,” Ms. Schanning told me in an interview. One of those treatments, buprenorphine, is one of the most effective tools that health care providers have to treat opioid use disorder. A partial opioid agonist, it reduces cravings and prevents overdose, decreasing mortality more effectively than almost any medication for any disease. Yet most providers have never prescribed it.
That may be about to change. The special license to prescribe the medication, commonly known as the “X-waiver,” was officially eliminated as part of the passage of the Mainstreaming Addiction Treatment (MAT) Act. Immediately, following the passage of the Act, any provider with a DEA license became eligible to prescribe buprenorphine to treat opioid use disorder, and limits on the number of patients they could treat were eliminated.
Previously, buprenorphine, which has a better safety profile than almost any other prescription opioid because of its ceiling effect on respiratory depression, nonetheless required providers to obtain a special license to prescribe it, and – prior to an executive order from the Biden administration – 8 to 24 hours of training to do so. This led to a misconception that buprenorphine was dangerous, and created barriers for treatment during the worst overdose crisis in our country’s history. More than 110,00 overdose deaths occurred in 2021, representing a 468% increase in the last 2 decades.
Along with the MAT Act, the Medication Access and Training Expansion Act was passed in the same spending bill, requiring all prescribers who obtain a DEA license to do 8 hours of training on the treatment of substance use disorders. According to the Act, addiction specialty societies will have a role in creating trainings. Medical schools and residencies will also be able to fulfill this requirement with a “comprehensive” curriculum that covers all approved medications for the treatment of substance use disorders.
The DEA has not yet confirmed what training will be accepted, according to the Chief Medical Officer of the Substance Abuse and Mental Health Services Administration, Neeraj Gandotra, MD, who spoke to me in an interview. However, it is required to do so by April 5, 2023. Dr. Gandotra also emphasized that state and local laws, as well as insurance requirements, remain in place, and may place other constraints on prescribing. According to the Act, this new rule will be in effect by June 2023.
As an addiction medicine specialist and longtime buprenorphine prescriber, I am excited about these changes but wary of lingering resistance among health care providers. Will providers who have chosen not to get an X-waiver now look for another reason to not treat patients with substance use disorders?
Ms. Schanning remains hopeful. “I’m incredibly optimistic that health care providers are going to learn about buprenorphine and prescribe it to patients, and that patients are going to start asking about this medication,” she told me. “Seven in 10 providers say that they do feel an obligation to treat their patients with [opioid use disorder], but the federal government has made it very difficult to do so.”
Now with the X-waiver gone, providers and patients may be able to push for a long overdue shift in how we treat and conceptualize substance use disorders, she noted.
“Health care providers need to recognize substance use disorder as a medical condition that deserves treatment, and to speak about it like a medical condition,” Ms. Schanning said, by, for instance, moving away from using words such as “abuse” and “clean” and, instead, talking about treatable substance use disorders that can improve with evidence-based care, such as buprenorphine and methadone. “We also need to share stories of success and hope with people,” she added. “Once you’ve seen how someone can be transformed by treatment, it’s really difficult to say that substance use disorder is a character flaw, or their fault.”
A patient-centered approach
Over the past decade of practicing medicine, I have experienced this transformation personally. In residency, I believed that people had to be ready for help, to stop using, to change. I failed to recognize that many of those same people were asking me for help, and I wasn’t offering what they needed. The person who had to change was me.
As I moved toward a patient-centered approach, lowering barriers to starting and remaining in treatment, and collaborating with teams that could meet people wherever they might be, addictions became the most rewarding part of my practice.
I have never had more people thank me spontaneously and deeply for the care I provide. Plus, I have never seen a more profound change in the students I work with than when they witness someone with a substance use disorder offered treatment that works.
The X-waiver was not the only barrier to care, and the overdose crisis is not slowing down. But maybe with a new tool widely accessible, more of us will be ready to help.
Dr. Poorman is board certified in internal medicine and addiction medicine, assistant professor of medicine, University of Illinois at Chicago, and provides primary care and addiction services in Chicago. Her views do not necessarily reflect the views of her employer. She has reported no relevant disclosures, and she serves on the editorial advisory board of Internal Medicine News.
In 2016, when Erin Schanning lost her brother Ethan to an overdose, she wanted to know what could have been done to have helped him. Ethan, who had struggled with opioids since getting a prescription for the drugs after a dental procedure in middle school, had tried dozens of treatments. But at the age of 30, he was gone.
“After my brother died, I started researching and was surprised to learn that there were many evidence-based ways to treat substance use disorder that he hadn’t had access to, even though he had doggedly pursued treatment,” Ms. Schanning told me in an interview. One of those treatments, buprenorphine, is one of the most effective tools that health care providers have to treat opioid use disorder. A partial opioid agonist, it reduces cravings and prevents overdose, decreasing mortality more effectively than almost any medication for any disease. Yet most providers have never prescribed it.
That may be about to change. The special license to prescribe the medication, commonly known as the “X-waiver,” was officially eliminated as part of the passage of the Mainstreaming Addiction Treatment (MAT) Act. Immediately, following the passage of the Act, any provider with a DEA license became eligible to prescribe buprenorphine to treat opioid use disorder, and limits on the number of patients they could treat were eliminated.
Previously, buprenorphine, which has a better safety profile than almost any other prescription opioid because of its ceiling effect on respiratory depression, nonetheless required providers to obtain a special license to prescribe it, and – prior to an executive order from the Biden administration – 8 to 24 hours of training to do so. This led to a misconception that buprenorphine was dangerous, and created barriers for treatment during the worst overdose crisis in our country’s history. More than 110,00 overdose deaths occurred in 2021, representing a 468% increase in the last 2 decades.
Along with the MAT Act, the Medication Access and Training Expansion Act was passed in the same spending bill, requiring all prescribers who obtain a DEA license to do 8 hours of training on the treatment of substance use disorders. According to the Act, addiction specialty societies will have a role in creating trainings. Medical schools and residencies will also be able to fulfill this requirement with a “comprehensive” curriculum that covers all approved medications for the treatment of substance use disorders.
The DEA has not yet confirmed what training will be accepted, according to the Chief Medical Officer of the Substance Abuse and Mental Health Services Administration, Neeraj Gandotra, MD, who spoke to me in an interview. However, it is required to do so by April 5, 2023. Dr. Gandotra also emphasized that state and local laws, as well as insurance requirements, remain in place, and may place other constraints on prescribing. According to the Act, this new rule will be in effect by June 2023.
As an addiction medicine specialist and longtime buprenorphine prescriber, I am excited about these changes but wary of lingering resistance among health care providers. Will providers who have chosen not to get an X-waiver now look for another reason to not treat patients with substance use disorders?
Ms. Schanning remains hopeful. “I’m incredibly optimistic that health care providers are going to learn about buprenorphine and prescribe it to patients, and that patients are going to start asking about this medication,” she told me. “Seven in 10 providers say that they do feel an obligation to treat their patients with [opioid use disorder], but the federal government has made it very difficult to do so.”
Now with the X-waiver gone, providers and patients may be able to push for a long overdue shift in how we treat and conceptualize substance use disorders, she noted.
“Health care providers need to recognize substance use disorder as a medical condition that deserves treatment, and to speak about it like a medical condition,” Ms. Schanning said, by, for instance, moving away from using words such as “abuse” and “clean” and, instead, talking about treatable substance use disorders that can improve with evidence-based care, such as buprenorphine and methadone. “We also need to share stories of success and hope with people,” she added. “Once you’ve seen how someone can be transformed by treatment, it’s really difficult to say that substance use disorder is a character flaw, or their fault.”
A patient-centered approach
Over the past decade of practicing medicine, I have experienced this transformation personally. In residency, I believed that people had to be ready for help, to stop using, to change. I failed to recognize that many of those same people were asking me for help, and I wasn’t offering what they needed. The person who had to change was me.
As I moved toward a patient-centered approach, lowering barriers to starting and remaining in treatment, and collaborating with teams that could meet people wherever they might be, addictions became the most rewarding part of my practice.
I have never had more people thank me spontaneously and deeply for the care I provide. Plus, I have never seen a more profound change in the students I work with than when they witness someone with a substance use disorder offered treatment that works.
The X-waiver was not the only barrier to care, and the overdose crisis is not slowing down. But maybe with a new tool widely accessible, more of us will be ready to help.
Dr. Poorman is board certified in internal medicine and addiction medicine, assistant professor of medicine, University of Illinois at Chicago, and provides primary care and addiction services in Chicago. Her views do not necessarily reflect the views of her employer. She has reported no relevant disclosures, and she serves on the editorial advisory board of Internal Medicine News.
In 2016, when Erin Schanning lost her brother Ethan to an overdose, she wanted to know what could have been done to have helped him. Ethan, who had struggled with opioids since getting a prescription for the drugs after a dental procedure in middle school, had tried dozens of treatments. But at the age of 30, he was gone.
“After my brother died, I started researching and was surprised to learn that there were many evidence-based ways to treat substance use disorder that he hadn’t had access to, even though he had doggedly pursued treatment,” Ms. Schanning told me in an interview. One of those treatments, buprenorphine, is one of the most effective tools that health care providers have to treat opioid use disorder. A partial opioid agonist, it reduces cravings and prevents overdose, decreasing mortality more effectively than almost any medication for any disease. Yet most providers have never prescribed it.
That may be about to change. The special license to prescribe the medication, commonly known as the “X-waiver,” was officially eliminated as part of the passage of the Mainstreaming Addiction Treatment (MAT) Act. Immediately, following the passage of the Act, any provider with a DEA license became eligible to prescribe buprenorphine to treat opioid use disorder, and limits on the number of patients they could treat were eliminated.
Previously, buprenorphine, which has a better safety profile than almost any other prescription opioid because of its ceiling effect on respiratory depression, nonetheless required providers to obtain a special license to prescribe it, and – prior to an executive order from the Biden administration – 8 to 24 hours of training to do so. This led to a misconception that buprenorphine was dangerous, and created barriers for treatment during the worst overdose crisis in our country’s history. More than 110,00 overdose deaths occurred in 2021, representing a 468% increase in the last 2 decades.
Along with the MAT Act, the Medication Access and Training Expansion Act was passed in the same spending bill, requiring all prescribers who obtain a DEA license to do 8 hours of training on the treatment of substance use disorders. According to the Act, addiction specialty societies will have a role in creating trainings. Medical schools and residencies will also be able to fulfill this requirement with a “comprehensive” curriculum that covers all approved medications for the treatment of substance use disorders.
The DEA has not yet confirmed what training will be accepted, according to the Chief Medical Officer of the Substance Abuse and Mental Health Services Administration, Neeraj Gandotra, MD, who spoke to me in an interview. However, it is required to do so by April 5, 2023. Dr. Gandotra also emphasized that state and local laws, as well as insurance requirements, remain in place, and may place other constraints on prescribing. According to the Act, this new rule will be in effect by June 2023.
As an addiction medicine specialist and longtime buprenorphine prescriber, I am excited about these changes but wary of lingering resistance among health care providers. Will providers who have chosen not to get an X-waiver now look for another reason to not treat patients with substance use disorders?
Ms. Schanning remains hopeful. “I’m incredibly optimistic that health care providers are going to learn about buprenorphine and prescribe it to patients, and that patients are going to start asking about this medication,” she told me. “Seven in 10 providers say that they do feel an obligation to treat their patients with [opioid use disorder], but the federal government has made it very difficult to do so.”
Now with the X-waiver gone, providers and patients may be able to push for a long overdue shift in how we treat and conceptualize substance use disorders, she noted.
“Health care providers need to recognize substance use disorder as a medical condition that deserves treatment, and to speak about it like a medical condition,” Ms. Schanning said, by, for instance, moving away from using words such as “abuse” and “clean” and, instead, talking about treatable substance use disorders that can improve with evidence-based care, such as buprenorphine and methadone. “We also need to share stories of success and hope with people,” she added. “Once you’ve seen how someone can be transformed by treatment, it’s really difficult to say that substance use disorder is a character flaw, or their fault.”
A patient-centered approach
Over the past decade of practicing medicine, I have experienced this transformation personally. In residency, I believed that people had to be ready for help, to stop using, to change. I failed to recognize that many of those same people were asking me for help, and I wasn’t offering what they needed. The person who had to change was me.
As I moved toward a patient-centered approach, lowering barriers to starting and remaining in treatment, and collaborating with teams that could meet people wherever they might be, addictions became the most rewarding part of my practice.
I have never had more people thank me spontaneously and deeply for the care I provide. Plus, I have never seen a more profound change in the students I work with than when they witness someone with a substance use disorder offered treatment that works.
The X-waiver was not the only barrier to care, and the overdose crisis is not slowing down. But maybe with a new tool widely accessible, more of us will be ready to help.
Dr. Poorman is board certified in internal medicine and addiction medicine, assistant professor of medicine, University of Illinois at Chicago, and provides primary care and addiction services in Chicago. Her views do not necessarily reflect the views of her employer. She has reported no relevant disclosures, and she serves on the editorial advisory board of Internal Medicine News.
Incorporating medication abortion into your ObGyn practice: Why and how
The Supreme Court’s Dobbs decision on June 24, 2022, which nullified the federal protections of Roe v Wade, resulted in the swift and devastating dissolution of access to abortion care for hundreds of thousands of patients in the United States.1 Within days of the decision, 11 states in the South and Midwest implemented complete or 6-week abortion bans that, in part, led to the closure of over half the abortion clinics in these states.2 Abortion bans, severe restrictions, and clinic closures affect all patients and magnify existing health care inequities.
Medication abortion is becoming increasingly popular; as of 2020, approximately 50% of US abortions were performed using this method.3 Through a combination of mifepristone and misoprostol, medication abortion induces the physiologic process and symptoms similar to those of a miscarriage. Notably, this regimen is also the most effective medical management method for a missed abortion in the first trimester, and therefore, should already be incorporated into any general ObGyn practice.4
Although a recent study found that 97% of ObGyn physicians report encountering patients who seek an abortion, only 15% to 25% of them reported providing abortion services.5,6 Given our expertise, ObGyns are well-positioned to incorporate medication abortion into our practices. For those ObGyn providers who practice in states without extreme abortion bans, this article provides guidance on how to incorporate medication abortion into your practice (FIGURE). Several states now have early gestational limits on abortion, and the abortion-dedicated clinics that remain open are over capacity. Therefore, by incorporating medication abortion into your practice you can contribute to timely abortion access for your patients.
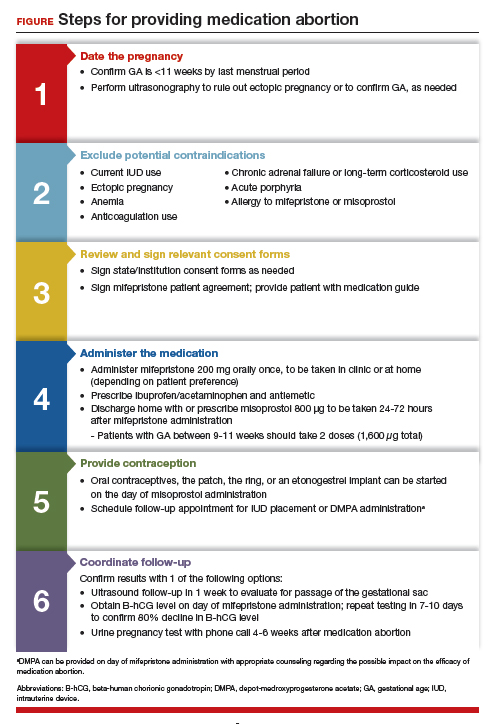
Medication abortion: The process
Determine your ability and patient’s eligibility
Abortion-specific laws for your state have now become the first determinant of your ability to provide medication abortion to your patients. The Guttmacher Institute is one reliable source of specific state laws that your practice can reference and is updated regularly.7
From a practice perspective, most ObGyn physicians already have the technical capabilities in place to provide medication abortion. First, you must be able to accurately determine the patient’s gestational age by their last menstrual period, which is often confirmed through ultrasonography.
Medication abortion is safe and routinely used in many practices up to 77 days, or 11 weeks, of gestation. Authors of a recent retrospective cohort study found that medication abortion also may be initiated for a pregnancy of unknown location in patients who are asymptomatic and determined to have low risk for an ectopic pregnancy. In this study, initiation of medication abortion on the day of presentation, with concurrent evaluation for ectopic pregnancy, was associated with a shorter time to a completed abortion, but a lower rate of successful medication abortion when compared with patients who delayed the initiation of medication abortion until a clear intrauterine pregnancy was diagnosed.8
Few medical contraindications exist for patients who seek a medication abortion. These contraindications include allergy to or medication interaction with mifepristone or misoprostol, chronic adrenal failure or long-term corticosteroid therapy, acute porphyria, anemia or the use of anticoagulation therapy, or current intrauterine device (IUD) use.
Continue to: Gather consents and administer treatment...
Gather consents and administer treatment
Historically, mifepristone has been dispensed directly at an ObGyn physician’s office. However, the US Food and Drug Administration (FDA) regulations requiring this were lifted during the COVID-19 pandemic, and as of December 2021, the inperson dispensing requirement was permanently removed.9 To provide mifepristone in a medical practice under current guidelines, a confidential prescriber agreement must be completed once by one person on behalf of the practice. Then each patient must read the manufacturer’s medication guide and sign the patient agreement form as part of the consent process (available on the FDA’s website).10 These agreement forms must be filled out by a physician and each patient if your practice uses mifepristone for any pregnancy indication, including induction of labor or medical management of miscarriage. Given the multiple evidence-based indications for mifepristone in pregnancy, it is hoped that these agreement forms will become a routine part of most ObGyn practices. Other consent requirements vary by state.
After signing consent forms, patients receive and often immediately take mifepristone 200 mg orally. Mifepristone is a progesterone receptor antagonist that sensitizes the uterine myometrium to the effects of prostaglandin.11 Rarely, patients may experience symptoms of bleeding or cramping after mifepristone administration alone.
Patients are discharged home with ibuprofen and an antiemetic for symptom relief to be taken around the time of administration of misoprostol. Misoprostol is a synthetic prostaglandin that causes uterine cramping and expulsion of the pregnancy typically within 4 hours of administration. Patients leave with the pills of misoprostol 800 μg (4 tablets, 200 µg each), which they self-administer buccally 24-48 hours after mifepristone administration. A prescription for misoprostol can be given instead of the actual pills, but geographic distance to the pharmacy and other potential barriers should be considered when evaluating the feasibility and convenience of providing pharmacy-dispensed misoprostol.
We instruct patients to place 2 tablets buccally between each gum and cheek, dosing all 4 tablets at the same time. Patients are instructed to let the tablets dissolve buccally and, after 30 minutes, to swallow the tablets with water. Administration of an automatic second dose of misoprostol 3-6 hours after the first dose for pregnancies between 9-11 weeks of gestation is recommended to increase success rate at these later gestational ages.12,13 Several different routes of administration, including buccal, vaginal, and sublingual, have been used for first trimester medication abortion with misoprostol.
Follow up and confirm the results
Patients can safely follow up after their medication abortion in several ways. In our practice, patients are offered 3 possible options.
- The first is ultrasound follow-up, whereby the patient returns to the clinic 1 week after their medication abortion for a pelvic ultrasound to confirm the gestational sac has passed.
- The second method is to test beta-human chorionic gonadotropin (B-hCG) levels. Patients interested in this option have a baseline B-hCG drawn on the day of presentation and follow up 7-10 days later for a repeat B-hCG test. An 80% drop in B-hCG level is consistent with a successful medication abortion.
- The third option, a phone checklist that is usually combined with a urine pregnancy test 4-6 weeks after a medication abortion, is an effective patient-centered approach. The COVID-19 pandemic and the subsequent compulsory shift to providing medical care via telemedicine highlighted the safety, acceptability, and patient preference for the provision of medication abortion using telehealth platforms.14
Outcomes and complications
Medication abortion using a combined regimen of mifepristone followed by misoprostol is approximately 95% effective at complete expulsion of the pregnancy.15,16 Complications after a first trimester medication abortion are rare. In a retrospective cohort study of 54,911 abortions, the most common complication was incomplete abortion.17 Symptoms concerning for incomplete abortion included persistent heavy vaginal bleeding and pelvic cramping. An incomplete or failed abortion should be managed with an additional dose of misoprostol or dilation and evacuation. Other possible complications such as infection are also rare, and prophylactic antibiotics are not encouraged.18
Future fertility and pregnancy implications
Patients should be counseled that a medication abortion is not associated with infertility or increased risk for adverse outcomes in future pregnancies.19 Contraceptive counseling should be provided to all interested patients at the time of a medication abortion and ideally provided to the patient on the day of their visit. Oral contraceptives, the patch, and the ring can be started on the day of misoprostol administration.20 The optimal timing of IUD insertion has been examined in 2 randomized control trials. Results indicated a higher uptake in the group of patients who received their IUD approximately 1 week after medication abortion versus delaying placement for several weeks, with no difference in IUD expulsion rates.21,22 Patients interested in depot-medroxyprogesterone acetate (DMPA) injection should be counseled on the theoretical decreased efficacy of medication abortion in the setting of concurrent DMPA administration. If possible, a follow-up plan should be made so that the patient can receive DMPA, if desired, at a later date.23 The etonogestrel implant (Nexplanon), however, can be placed on the day of mifepristone administration and does not affect the efficacy of a medication abortion.24,25
Summary
During this critical time for reproductive health care, it is essential that ObGyns consider how their professional position and expertise can assist with the provision of medication abortions. Most ObGyn practices already have the resources in place to effectively care for patients before, during, and after a medication abortion. Integrating abortion health care into your practice promotes patient-centered care, continuity, and patient satisfaction. Furthermore, by improving abortion referrals or offering information on safe, self-procured abortion, you can contribute to destigmatizing abortion care, while playing an integral role in connecting your patients with the care they need and desire. ●
- Jones RK, Philbin J, Kirstein M, et al. Long-term decline in US abortions reverses, showing rising need for abortion as Supreme Court is poised to overturn Roe v. Wade. Guttmacher Institute. August 30, 2022. https://www.gut. Accessed November 2, 2022. tmacher.org/article/2022/06 /long-term-decline-us-abortions-reverses-showing-rising -need-abortion-supreme-court.
- Kirstein M, Jones RK, Philbin J. One month post-roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. September 5, 2022. https://www.guttmacher.org/article/2022/07/one-month -post-roe-least-43-abortion-clinics-across-11-states-have -stopped-offering. Accessed November 2, 2022.
- Jones RK, Nash E, Cross L, et al. Medication abortion now accounts for more than half of all US abortions. Guttmacher Institute. September 12, 2022. https://www.guttmacher.org /article/2022/02/medication-abortion-now-accounts-more-half-all-us-abortions. Accessed November 2, 2022.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi:10.1056/ nejmoa1715726.
- Stulberg DB, Dude AM, Dahlquist I, Curlin, FA. Abortion provision among practicing obstetrician-gynecologists. Obstet Gynecol. 2011;118:609-614. doi:10.1097/aog.0b013e31822ad973.
- Daniel S, Schulkin J, Grossman D. Obstetrician-gynecologist willingness to provide medication abortion with removal of the in-person dispensing requirement for mifepristone. Contraception. 2021;104:73-76. doi:10.1016/j. contraception.2021.03.026.
- Guttmacher Institute. State legislation tracker. Updated October 31, 2022. https://www.guttmacher.org/state-policy. Accessed November 2, 2022.
- Goldberg AB, Fulcher IR, Fortin J, et al. Mifepristone and misoprostol for undesired pregnancy of unknown location. Obstet Gynecol. 2022;139:771-780. doi:10.1097/ aog.0000000000004756.
- The American College of Obstetricians and Gynecologists. Understanding the practical implications of the FDA’s December 2021 mifepristone REMS decision: a Q&A with Dr. Nisha Verma and Vanessa Wellbery. March 28, 2022. https:// www.acog.org/news/news-articles/2022/03/understanding -the-practical-implications-of-the-fdas-december-2021 -mifepristone-rems-decision. Accessed November 2, 2022.
- US Food and Drug Administration. Mifeprex (mifepristone) information. December 16, 2021. https://www.fda.gov/ drugs/postmarket-drug-safety-information-patients-and-providers/ifeprex-mifepristone-information. Accessed November 2, 2022.
- Cadepond F, Ulmann A, Baulieu EE. Ru486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129-156. doi:10.1146/annurev.med.48.1.129.
- Ashok PW, Templeton A, Wagaarachchi PT, Flett GMM. Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG. 2002;109:1281-1289. doi:10.1046/j.1471-0528.2002.02156.x.
- Coyaji K, Krishna U, Ambardekar S, et al. Are two doses of misoprostol after mifepristone for early abortion better than one? BJOG. 2007;114:271-278. doi:10.1111/j.14710528.2006.01208.x.
- Aiken A, Lohr PA, Lord J, et al. Effectiveness, safety and acceptability of no‐test medical abortion (termination of pregnancy) provided via telemedicine: a national cohort study. BJOG. 2021;128:1464-1474. doi:10.1111/14710528.16668.
- Schaff EA, Eisinger SH, Stadalius LS, et al. Low-dose mifepristone 200 mg and vaginal misoprostol for abortion. Contraception. 1999;59:1-6. doi:10.1016/s00107824(98)00150-4.
- Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception. 2001;64:81-85. doi:10.1016/s0010-7824(01)00229-3.
- Upadhyay UD, Desai S, Zlidar V, et al. Incidence of emergency department visits and complications after abortion. Obstet Gynecol. 2015;125:175-183. doi:10.1097/ aog.0000000000000603.
- Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: a review of the literature. Contraception. 2004;70:183-190. doi:10.1016/j.contraception.2004.04.009.
- Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653. doi:10.1056/nejmoa070445.
- Mittal S. Contraception after medical abortion. Contraception. 2006;74:56-60. doi:10.1016/j.contraception.2006.03.006.
- Shimoni N, Davis A, Ramos ME, et al. Timing of copper intrauterine device insertion after medical abortion. Obstet Gynecol. 2011;118:623-628. doi:10.1097/aog.0b013e31822ade67.
- Sääv I, Stephansson O, Gemzell-Danielsson K. Early versus delayed insertion of intrauterine contraception after medical abortion—a randomized controlled trial. PloS ONE. 2012;7:e48948. doi:10.1371/journal.pone.0048948.
- Raymond EG, Weaver MA, Louie KS, et al. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;128:739-745. doi:10.1097/aog.0000000000001627.
- Hognert H, Kopp Kallner H, Cameron S, et al. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion—a randomized controlled equivalence trial. Hum Reprod. 2016;31:2484-2490. doi:10.1093/humrep/ dew238.
- Raymond EG, Weaver MA, Tan Y-L, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy. Obstet Gynecol. 2016;127:306-312. doi:10.1097/ aog.0000000000001274.
The Supreme Court’s Dobbs decision on June 24, 2022, which nullified the federal protections of Roe v Wade, resulted in the swift and devastating dissolution of access to abortion care for hundreds of thousands of patients in the United States.1 Within days of the decision, 11 states in the South and Midwest implemented complete or 6-week abortion bans that, in part, led to the closure of over half the abortion clinics in these states.2 Abortion bans, severe restrictions, and clinic closures affect all patients and magnify existing health care inequities.
Medication abortion is becoming increasingly popular; as of 2020, approximately 50% of US abortions were performed using this method.3 Through a combination of mifepristone and misoprostol, medication abortion induces the physiologic process and symptoms similar to those of a miscarriage. Notably, this regimen is also the most effective medical management method for a missed abortion in the first trimester, and therefore, should already be incorporated into any general ObGyn practice.4
Although a recent study found that 97% of ObGyn physicians report encountering patients who seek an abortion, only 15% to 25% of them reported providing abortion services.5,6 Given our expertise, ObGyns are well-positioned to incorporate medication abortion into our practices. For those ObGyn providers who practice in states without extreme abortion bans, this article provides guidance on how to incorporate medication abortion into your practice (FIGURE). Several states now have early gestational limits on abortion, and the abortion-dedicated clinics that remain open are over capacity. Therefore, by incorporating medication abortion into your practice you can contribute to timely abortion access for your patients.

Medication abortion: The process
Determine your ability and patient’s eligibility
Abortion-specific laws for your state have now become the first determinant of your ability to provide medication abortion to your patients. The Guttmacher Institute is one reliable source of specific state laws that your practice can reference and is updated regularly.7
From a practice perspective, most ObGyn physicians already have the technical capabilities in place to provide medication abortion. First, you must be able to accurately determine the patient’s gestational age by their last menstrual period, which is often confirmed through ultrasonography.
Medication abortion is safe and routinely used in many practices up to 77 days, or 11 weeks, of gestation. Authors of a recent retrospective cohort study found that medication abortion also may be initiated for a pregnancy of unknown location in patients who are asymptomatic and determined to have low risk for an ectopic pregnancy. In this study, initiation of medication abortion on the day of presentation, with concurrent evaluation for ectopic pregnancy, was associated with a shorter time to a completed abortion, but a lower rate of successful medication abortion when compared with patients who delayed the initiation of medication abortion until a clear intrauterine pregnancy was diagnosed.8
Few medical contraindications exist for patients who seek a medication abortion. These contraindications include allergy to or medication interaction with mifepristone or misoprostol, chronic adrenal failure or long-term corticosteroid therapy, acute porphyria, anemia or the use of anticoagulation therapy, or current intrauterine device (IUD) use.
Continue to: Gather consents and administer treatment...
Gather consents and administer treatment
Historically, mifepristone has been dispensed directly at an ObGyn physician’s office. However, the US Food and Drug Administration (FDA) regulations requiring this were lifted during the COVID-19 pandemic, and as of December 2021, the inperson dispensing requirement was permanently removed.9 To provide mifepristone in a medical practice under current guidelines, a confidential prescriber agreement must be completed once by one person on behalf of the practice. Then each patient must read the manufacturer’s medication guide and sign the patient agreement form as part of the consent process (available on the FDA’s website).10 These agreement forms must be filled out by a physician and each patient if your practice uses mifepristone for any pregnancy indication, including induction of labor or medical management of miscarriage. Given the multiple evidence-based indications for mifepristone in pregnancy, it is hoped that these agreement forms will become a routine part of most ObGyn practices. Other consent requirements vary by state.
After signing consent forms, patients receive and often immediately take mifepristone 200 mg orally. Mifepristone is a progesterone receptor antagonist that sensitizes the uterine myometrium to the effects of prostaglandin.11 Rarely, patients may experience symptoms of bleeding or cramping after mifepristone administration alone.
Patients are discharged home with ibuprofen and an antiemetic for symptom relief to be taken around the time of administration of misoprostol. Misoprostol is a synthetic prostaglandin that causes uterine cramping and expulsion of the pregnancy typically within 4 hours of administration. Patients leave with the pills of misoprostol 800 μg (4 tablets, 200 µg each), which they self-administer buccally 24-48 hours after mifepristone administration. A prescription for misoprostol can be given instead of the actual pills, but geographic distance to the pharmacy and other potential barriers should be considered when evaluating the feasibility and convenience of providing pharmacy-dispensed misoprostol.
We instruct patients to place 2 tablets buccally between each gum and cheek, dosing all 4 tablets at the same time. Patients are instructed to let the tablets dissolve buccally and, after 30 minutes, to swallow the tablets with water. Administration of an automatic second dose of misoprostol 3-6 hours after the first dose for pregnancies between 9-11 weeks of gestation is recommended to increase success rate at these later gestational ages.12,13 Several different routes of administration, including buccal, vaginal, and sublingual, have been used for first trimester medication abortion with misoprostol.
Follow up and confirm the results
Patients can safely follow up after their medication abortion in several ways. In our practice, patients are offered 3 possible options.
- The first is ultrasound follow-up, whereby the patient returns to the clinic 1 week after their medication abortion for a pelvic ultrasound to confirm the gestational sac has passed.
- The second method is to test beta-human chorionic gonadotropin (B-hCG) levels. Patients interested in this option have a baseline B-hCG drawn on the day of presentation and follow up 7-10 days later for a repeat B-hCG test. An 80% drop in B-hCG level is consistent with a successful medication abortion.
- The third option, a phone checklist that is usually combined with a urine pregnancy test 4-6 weeks after a medication abortion, is an effective patient-centered approach. The COVID-19 pandemic and the subsequent compulsory shift to providing medical care via telemedicine highlighted the safety, acceptability, and patient preference for the provision of medication abortion using telehealth platforms.14
Outcomes and complications
Medication abortion using a combined regimen of mifepristone followed by misoprostol is approximately 95% effective at complete expulsion of the pregnancy.15,16 Complications after a first trimester medication abortion are rare. In a retrospective cohort study of 54,911 abortions, the most common complication was incomplete abortion.17 Symptoms concerning for incomplete abortion included persistent heavy vaginal bleeding and pelvic cramping. An incomplete or failed abortion should be managed with an additional dose of misoprostol or dilation and evacuation. Other possible complications such as infection are also rare, and prophylactic antibiotics are not encouraged.18
Future fertility and pregnancy implications
Patients should be counseled that a medication abortion is not associated with infertility or increased risk for adverse outcomes in future pregnancies.19 Contraceptive counseling should be provided to all interested patients at the time of a medication abortion and ideally provided to the patient on the day of their visit. Oral contraceptives, the patch, and the ring can be started on the day of misoprostol administration.20 The optimal timing of IUD insertion has been examined in 2 randomized control trials. Results indicated a higher uptake in the group of patients who received their IUD approximately 1 week after medication abortion versus delaying placement for several weeks, with no difference in IUD expulsion rates.21,22 Patients interested in depot-medroxyprogesterone acetate (DMPA) injection should be counseled on the theoretical decreased efficacy of medication abortion in the setting of concurrent DMPA administration. If possible, a follow-up plan should be made so that the patient can receive DMPA, if desired, at a later date.23 The etonogestrel implant (Nexplanon), however, can be placed on the day of mifepristone administration and does not affect the efficacy of a medication abortion.24,25
Summary
During this critical time for reproductive health care, it is essential that ObGyns consider how their professional position and expertise can assist with the provision of medication abortions. Most ObGyn practices already have the resources in place to effectively care for patients before, during, and after a medication abortion. Integrating abortion health care into your practice promotes patient-centered care, continuity, and patient satisfaction. Furthermore, by improving abortion referrals or offering information on safe, self-procured abortion, you can contribute to destigmatizing abortion care, while playing an integral role in connecting your patients with the care they need and desire. ●
The Supreme Court’s Dobbs decision on June 24, 2022, which nullified the federal protections of Roe v Wade, resulted in the swift and devastating dissolution of access to abortion care for hundreds of thousands of patients in the United States.1 Within days of the decision, 11 states in the South and Midwest implemented complete or 6-week abortion bans that, in part, led to the closure of over half the abortion clinics in these states.2 Abortion bans, severe restrictions, and clinic closures affect all patients and magnify existing health care inequities.
Medication abortion is becoming increasingly popular; as of 2020, approximately 50% of US abortions were performed using this method.3 Through a combination of mifepristone and misoprostol, medication abortion induces the physiologic process and symptoms similar to those of a miscarriage. Notably, this regimen is also the most effective medical management method for a missed abortion in the first trimester, and therefore, should already be incorporated into any general ObGyn practice.4
Although a recent study found that 97% of ObGyn physicians report encountering patients who seek an abortion, only 15% to 25% of them reported providing abortion services.5,6 Given our expertise, ObGyns are well-positioned to incorporate medication abortion into our practices. For those ObGyn providers who practice in states without extreme abortion bans, this article provides guidance on how to incorporate medication abortion into your practice (FIGURE). Several states now have early gestational limits on abortion, and the abortion-dedicated clinics that remain open are over capacity. Therefore, by incorporating medication abortion into your practice you can contribute to timely abortion access for your patients.

Medication abortion: The process
Determine your ability and patient’s eligibility
Abortion-specific laws for your state have now become the first determinant of your ability to provide medication abortion to your patients. The Guttmacher Institute is one reliable source of specific state laws that your practice can reference and is updated regularly.7
From a practice perspective, most ObGyn physicians already have the technical capabilities in place to provide medication abortion. First, you must be able to accurately determine the patient’s gestational age by their last menstrual period, which is often confirmed through ultrasonography.
Medication abortion is safe and routinely used in many practices up to 77 days, or 11 weeks, of gestation. Authors of a recent retrospective cohort study found that medication abortion also may be initiated for a pregnancy of unknown location in patients who are asymptomatic and determined to have low risk for an ectopic pregnancy. In this study, initiation of medication abortion on the day of presentation, with concurrent evaluation for ectopic pregnancy, was associated with a shorter time to a completed abortion, but a lower rate of successful medication abortion when compared with patients who delayed the initiation of medication abortion until a clear intrauterine pregnancy was diagnosed.8
Few medical contraindications exist for patients who seek a medication abortion. These contraindications include allergy to or medication interaction with mifepristone or misoprostol, chronic adrenal failure or long-term corticosteroid therapy, acute porphyria, anemia or the use of anticoagulation therapy, or current intrauterine device (IUD) use.
Continue to: Gather consents and administer treatment...
Gather consents and administer treatment
Historically, mifepristone has been dispensed directly at an ObGyn physician’s office. However, the US Food and Drug Administration (FDA) regulations requiring this were lifted during the COVID-19 pandemic, and as of December 2021, the inperson dispensing requirement was permanently removed.9 To provide mifepristone in a medical practice under current guidelines, a confidential prescriber agreement must be completed once by one person on behalf of the practice. Then each patient must read the manufacturer’s medication guide and sign the patient agreement form as part of the consent process (available on the FDA’s website).10 These agreement forms must be filled out by a physician and each patient if your practice uses mifepristone for any pregnancy indication, including induction of labor or medical management of miscarriage. Given the multiple evidence-based indications for mifepristone in pregnancy, it is hoped that these agreement forms will become a routine part of most ObGyn practices. Other consent requirements vary by state.
After signing consent forms, patients receive and often immediately take mifepristone 200 mg orally. Mifepristone is a progesterone receptor antagonist that sensitizes the uterine myometrium to the effects of prostaglandin.11 Rarely, patients may experience symptoms of bleeding or cramping after mifepristone administration alone.
Patients are discharged home with ibuprofen and an antiemetic for symptom relief to be taken around the time of administration of misoprostol. Misoprostol is a synthetic prostaglandin that causes uterine cramping and expulsion of the pregnancy typically within 4 hours of administration. Patients leave with the pills of misoprostol 800 μg (4 tablets, 200 µg each), which they self-administer buccally 24-48 hours after mifepristone administration. A prescription for misoprostol can be given instead of the actual pills, but geographic distance to the pharmacy and other potential barriers should be considered when evaluating the feasibility and convenience of providing pharmacy-dispensed misoprostol.
We instruct patients to place 2 tablets buccally between each gum and cheek, dosing all 4 tablets at the same time. Patients are instructed to let the tablets dissolve buccally and, after 30 minutes, to swallow the tablets with water. Administration of an automatic second dose of misoprostol 3-6 hours after the first dose for pregnancies between 9-11 weeks of gestation is recommended to increase success rate at these later gestational ages.12,13 Several different routes of administration, including buccal, vaginal, and sublingual, have been used for first trimester medication abortion with misoprostol.
Follow up and confirm the results
Patients can safely follow up after their medication abortion in several ways. In our practice, patients are offered 3 possible options.
- The first is ultrasound follow-up, whereby the patient returns to the clinic 1 week after their medication abortion for a pelvic ultrasound to confirm the gestational sac has passed.
- The second method is to test beta-human chorionic gonadotropin (B-hCG) levels. Patients interested in this option have a baseline B-hCG drawn on the day of presentation and follow up 7-10 days later for a repeat B-hCG test. An 80% drop in B-hCG level is consistent with a successful medication abortion.
- The third option, a phone checklist that is usually combined with a urine pregnancy test 4-6 weeks after a medication abortion, is an effective patient-centered approach. The COVID-19 pandemic and the subsequent compulsory shift to providing medical care via telemedicine highlighted the safety, acceptability, and patient preference for the provision of medication abortion using telehealth platforms.14
Outcomes and complications
Medication abortion using a combined regimen of mifepristone followed by misoprostol is approximately 95% effective at complete expulsion of the pregnancy.15,16 Complications after a first trimester medication abortion are rare. In a retrospective cohort study of 54,911 abortions, the most common complication was incomplete abortion.17 Symptoms concerning for incomplete abortion included persistent heavy vaginal bleeding and pelvic cramping. An incomplete or failed abortion should be managed with an additional dose of misoprostol or dilation and evacuation. Other possible complications such as infection are also rare, and prophylactic antibiotics are not encouraged.18
Future fertility and pregnancy implications
Patients should be counseled that a medication abortion is not associated with infertility or increased risk for adverse outcomes in future pregnancies.19 Contraceptive counseling should be provided to all interested patients at the time of a medication abortion and ideally provided to the patient on the day of their visit. Oral contraceptives, the patch, and the ring can be started on the day of misoprostol administration.20 The optimal timing of IUD insertion has been examined in 2 randomized control trials. Results indicated a higher uptake in the group of patients who received their IUD approximately 1 week after medication abortion versus delaying placement for several weeks, with no difference in IUD expulsion rates.21,22 Patients interested in depot-medroxyprogesterone acetate (DMPA) injection should be counseled on the theoretical decreased efficacy of medication abortion in the setting of concurrent DMPA administration. If possible, a follow-up plan should be made so that the patient can receive DMPA, if desired, at a later date.23 The etonogestrel implant (Nexplanon), however, can be placed on the day of mifepristone administration and does not affect the efficacy of a medication abortion.24,25
Summary
During this critical time for reproductive health care, it is essential that ObGyns consider how their professional position and expertise can assist with the provision of medication abortions. Most ObGyn practices already have the resources in place to effectively care for patients before, during, and after a medication abortion. Integrating abortion health care into your practice promotes patient-centered care, continuity, and patient satisfaction. Furthermore, by improving abortion referrals or offering information on safe, self-procured abortion, you can contribute to destigmatizing abortion care, while playing an integral role in connecting your patients with the care they need and desire. ●
- Jones RK, Philbin J, Kirstein M, et al. Long-term decline in US abortions reverses, showing rising need for abortion as Supreme Court is poised to overturn Roe v. Wade. Guttmacher Institute. August 30, 2022. https://www.gut. Accessed November 2, 2022. tmacher.org/article/2022/06 /long-term-decline-us-abortions-reverses-showing-rising -need-abortion-supreme-court.
- Kirstein M, Jones RK, Philbin J. One month post-roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. September 5, 2022. https://www.guttmacher.org/article/2022/07/one-month -post-roe-least-43-abortion-clinics-across-11-states-have -stopped-offering. Accessed November 2, 2022.
- Jones RK, Nash E, Cross L, et al. Medication abortion now accounts for more than half of all US abortions. Guttmacher Institute. September 12, 2022. https://www.guttmacher.org /article/2022/02/medication-abortion-now-accounts-more-half-all-us-abortions. Accessed November 2, 2022.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi:10.1056/ nejmoa1715726.
- Stulberg DB, Dude AM, Dahlquist I, Curlin, FA. Abortion provision among practicing obstetrician-gynecologists. Obstet Gynecol. 2011;118:609-614. doi:10.1097/aog.0b013e31822ad973.
- Daniel S, Schulkin J, Grossman D. Obstetrician-gynecologist willingness to provide medication abortion with removal of the in-person dispensing requirement for mifepristone. Contraception. 2021;104:73-76. doi:10.1016/j. contraception.2021.03.026.
- Guttmacher Institute. State legislation tracker. Updated October 31, 2022. https://www.guttmacher.org/state-policy. Accessed November 2, 2022.
- Goldberg AB, Fulcher IR, Fortin J, et al. Mifepristone and misoprostol for undesired pregnancy of unknown location. Obstet Gynecol. 2022;139:771-780. doi:10.1097/ aog.0000000000004756.
- The American College of Obstetricians and Gynecologists. Understanding the practical implications of the FDA’s December 2021 mifepristone REMS decision: a Q&A with Dr. Nisha Verma and Vanessa Wellbery. March 28, 2022. https:// www.acog.org/news/news-articles/2022/03/understanding -the-practical-implications-of-the-fdas-december-2021 -mifepristone-rems-decision. Accessed November 2, 2022.
- US Food and Drug Administration. Mifeprex (mifepristone) information. December 16, 2021. https://www.fda.gov/ drugs/postmarket-drug-safety-information-patients-and-providers/ifeprex-mifepristone-information. Accessed November 2, 2022.
- Cadepond F, Ulmann A, Baulieu EE. Ru486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129-156. doi:10.1146/annurev.med.48.1.129.
- Ashok PW, Templeton A, Wagaarachchi PT, Flett GMM. Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG. 2002;109:1281-1289. doi:10.1046/j.1471-0528.2002.02156.x.
- Coyaji K, Krishna U, Ambardekar S, et al. Are two doses of misoprostol after mifepristone for early abortion better than one? BJOG. 2007;114:271-278. doi:10.1111/j.14710528.2006.01208.x.
- Aiken A, Lohr PA, Lord J, et al. Effectiveness, safety and acceptability of no‐test medical abortion (termination of pregnancy) provided via telemedicine: a national cohort study. BJOG. 2021;128:1464-1474. doi:10.1111/14710528.16668.
- Schaff EA, Eisinger SH, Stadalius LS, et al. Low-dose mifepristone 200 mg and vaginal misoprostol for abortion. Contraception. 1999;59:1-6. doi:10.1016/s00107824(98)00150-4.
- Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception. 2001;64:81-85. doi:10.1016/s0010-7824(01)00229-3.
- Upadhyay UD, Desai S, Zlidar V, et al. Incidence of emergency department visits and complications after abortion. Obstet Gynecol. 2015;125:175-183. doi:10.1097/ aog.0000000000000603.
- Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: a review of the literature. Contraception. 2004;70:183-190. doi:10.1016/j.contraception.2004.04.009.
- Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653. doi:10.1056/nejmoa070445.
- Mittal S. Contraception after medical abortion. Contraception. 2006;74:56-60. doi:10.1016/j.contraception.2006.03.006.
- Shimoni N, Davis A, Ramos ME, et al. Timing of copper intrauterine device insertion after medical abortion. Obstet Gynecol. 2011;118:623-628. doi:10.1097/aog.0b013e31822ade67.
- Sääv I, Stephansson O, Gemzell-Danielsson K. Early versus delayed insertion of intrauterine contraception after medical abortion—a randomized controlled trial. PloS ONE. 2012;7:e48948. doi:10.1371/journal.pone.0048948.
- Raymond EG, Weaver MA, Louie KS, et al. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;128:739-745. doi:10.1097/aog.0000000000001627.
- Hognert H, Kopp Kallner H, Cameron S, et al. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion—a randomized controlled equivalence trial. Hum Reprod. 2016;31:2484-2490. doi:10.1093/humrep/ dew238.
- Raymond EG, Weaver MA, Tan Y-L, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy. Obstet Gynecol. 2016;127:306-312. doi:10.1097/ aog.0000000000001274.
- Jones RK, Philbin J, Kirstein M, et al. Long-term decline in US abortions reverses, showing rising need for abortion as Supreme Court is poised to overturn Roe v. Wade. Guttmacher Institute. August 30, 2022. https://www.gut. Accessed November 2, 2022. tmacher.org/article/2022/06 /long-term-decline-us-abortions-reverses-showing-rising -need-abortion-supreme-court.
- Kirstein M, Jones RK, Philbin J. One month post-roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. September 5, 2022. https://www.guttmacher.org/article/2022/07/one-month -post-roe-least-43-abortion-clinics-across-11-states-have -stopped-offering. Accessed November 2, 2022.
- Jones RK, Nash E, Cross L, et al. Medication abortion now accounts for more than half of all US abortions. Guttmacher Institute. September 12, 2022. https://www.guttmacher.org /article/2022/02/medication-abortion-now-accounts-more-half-all-us-abortions. Accessed November 2, 2022.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi:10.1056/ nejmoa1715726.
- Stulberg DB, Dude AM, Dahlquist I, Curlin, FA. Abortion provision among practicing obstetrician-gynecologists. Obstet Gynecol. 2011;118:609-614. doi:10.1097/aog.0b013e31822ad973.
- Daniel S, Schulkin J, Grossman D. Obstetrician-gynecologist willingness to provide medication abortion with removal of the in-person dispensing requirement for mifepristone. Contraception. 2021;104:73-76. doi:10.1016/j. contraception.2021.03.026.
- Guttmacher Institute. State legislation tracker. Updated October 31, 2022. https://www.guttmacher.org/state-policy. Accessed November 2, 2022.
- Goldberg AB, Fulcher IR, Fortin J, et al. Mifepristone and misoprostol for undesired pregnancy of unknown location. Obstet Gynecol. 2022;139:771-780. doi:10.1097/ aog.0000000000004756.
- The American College of Obstetricians and Gynecologists. Understanding the practical implications of the FDA’s December 2021 mifepristone REMS decision: a Q&A with Dr. Nisha Verma and Vanessa Wellbery. March 28, 2022. https:// www.acog.org/news/news-articles/2022/03/understanding -the-practical-implications-of-the-fdas-december-2021 -mifepristone-rems-decision. Accessed November 2, 2022.
- US Food and Drug Administration. Mifeprex (mifepristone) information. December 16, 2021. https://www.fda.gov/ drugs/postmarket-drug-safety-information-patients-and-providers/ifeprex-mifepristone-information. Accessed November 2, 2022.
- Cadepond F, Ulmann A, Baulieu EE. Ru486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129-156. doi:10.1146/annurev.med.48.1.129.
- Ashok PW, Templeton A, Wagaarachchi PT, Flett GMM. Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG. 2002;109:1281-1289. doi:10.1046/j.1471-0528.2002.02156.x.
- Coyaji K, Krishna U, Ambardekar S, et al. Are two doses of misoprostol after mifepristone for early abortion better than one? BJOG. 2007;114:271-278. doi:10.1111/j.14710528.2006.01208.x.
- Aiken A, Lohr PA, Lord J, et al. Effectiveness, safety and acceptability of no‐test medical abortion (termination of pregnancy) provided via telemedicine: a national cohort study. BJOG. 2021;128:1464-1474. doi:10.1111/14710528.16668.
- Schaff EA, Eisinger SH, Stadalius LS, et al. Low-dose mifepristone 200 mg and vaginal misoprostol for abortion. Contraception. 1999;59:1-6. doi:10.1016/s00107824(98)00150-4.
- Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception. 2001;64:81-85. doi:10.1016/s0010-7824(01)00229-3.
- Upadhyay UD, Desai S, Zlidar V, et al. Incidence of emergency department visits and complications after abortion. Obstet Gynecol. 2015;125:175-183. doi:10.1097/ aog.0000000000000603.
- Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: a review of the literature. Contraception. 2004;70:183-190. doi:10.1016/j.contraception.2004.04.009.
- Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653. doi:10.1056/nejmoa070445.
- Mittal S. Contraception after medical abortion. Contraception. 2006;74:56-60. doi:10.1016/j.contraception.2006.03.006.
- Shimoni N, Davis A, Ramos ME, et al. Timing of copper intrauterine device insertion after medical abortion. Obstet Gynecol. 2011;118:623-628. doi:10.1097/aog.0b013e31822ade67.
- Sääv I, Stephansson O, Gemzell-Danielsson K. Early versus delayed insertion of intrauterine contraception after medical abortion—a randomized controlled trial. PloS ONE. 2012;7:e48948. doi:10.1371/journal.pone.0048948.
- Raymond EG, Weaver MA, Louie KS, et al. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;128:739-745. doi:10.1097/aog.0000000000001627.
- Hognert H, Kopp Kallner H, Cameron S, et al. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion—a randomized controlled equivalence trial. Hum Reprod. 2016;31:2484-2490. doi:10.1093/humrep/ dew238.
- Raymond EG, Weaver MA, Tan Y-L, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy. Obstet Gynecol. 2016;127:306-312. doi:10.1097/ aog.0000000000001274.
Buprenorphine linked with lower risk for neonatal harms than methadone
Using buprenorphine for opioid use disorder in pregnancy was linked with a lower risk of neonatal side effects than using methadone, but the risk of adverse maternal outcomes was similar between the two treatments, according to new research.
Elizabeth A. Suarez, PhD, MPH, with Brigham and Women’s Hospital in Boston, led the study published online in the New England Journal of Medicine.
Opioid use disorder in pregnant women has increased steadily in the United States since 2000, the authors write. As of 2017, about 8.2 per 1,000 deliveries were estimated to be affected by the disorder. The numbers were particularly high in people insured by Medicaid. In that group, an estimated 14.6 per 1,000 deliveries were affected.
Researchers studied pregnant women enrolled in public insurance programs in the United States from 2000 through 2018 in a dataset of 2,548,372 pregnancies that ended in live births. They analyzed outcomes in those who received buprenorphine as compared with those who received methadone.
They looked at different periods of exposure to the two medications: early pregnancy (through gestational week 19); late pregnancy (week 20 through the day before delivery); and the 30 days before delivery.
Highlighted differences in infants included:
- Neonatal abstinence syndrome in 52% of the infants who were exposed to buprenorphine in the 30 days before delivery as compared with 69.2% of those exposed to methadone (adjusted relative risk, 0.73).
- Preterm birth in 14.4% of infants exposed to buprenorphine in early pregnancy and in 24.9% of those exposed to methadone (ARR, 0.58).
- Small size for gestational age in 12.1% (buprenorphine) and 15.3% (methadone) (ARR, 0.72).
- Low birth weight in 8.3% (buprenorphine) and 14.9% (methadone) (ARR, 0.56).
- Delivery by cesarean section occurred in 33.6% of pregnant women exposed to buprenorphine in early pregnancy and 33.1% of those exposed to methadone (ARR, 1.02.).
Severe maternal complications developed in 3.3% of the women exposed to buprenorphine and 3.5% of those on methadone (ARR, 0.91.) Exposures in late pregnancy and early pregnancy yielded similar results, the authors say.
Michael Caucci, MD, of the department of psychiatry at Vanderbilt University Medical Center in Nashville, Tenn. who also runs the Women’s Mental Health Clinic at the university, said this paper supports preliminary findings from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study that suggested infants exposed to buprenorphine (compared with methadone) appeared to have lower rates of neonatal complications.
“It also supports buprenorphine as a relatively safe option for treatment of opioid use disorder during pregnancy,” said Dr. Caucci, who was not part of the study by Dr. Suarez and associates. “Reducing the fear of harming the fetus or neonate will help eliminate this barrier to perinatal substance use disorder treatment.”
But he cautions against concluding that, because buprenorphine has lower risks of fetal/neonatal complications, it is safer and therefore better than methadone in pregnancy.
“Some women do not tolerate buprenorphine and do much better on methadone, Dr. Caucci said. “Current recommendations are that both buprenorphine and methadone are relatively safe options for treatment of OUD [opioid use disorder] in pregnancy.”
Among the differences between the treatments is that while methadone is administered daily during in-person visits to federally regulated opioid treatment programs, buprenorphine can be prescribed by approved providers, which allows patients to administer buprenorphine themselves.
Dr. Caucci said he was intrigued by the finding that there was no difference in pregnancy, neonatal, and maternal outcomes depending on the time of exposure to the agents.
“I would have expected higher rates of neonatal abstinence syndrome (NAS) or poor fetal growth in those exposed later in pregnancy vs. those with early exposure,” he said.
The work was supported by the National Institute on Drug Abuse. Dr. Caucci reports no relevant financial relationships. The authors’ disclosures are available with the full text.
Using buprenorphine for opioid use disorder in pregnancy was linked with a lower risk of neonatal side effects than using methadone, but the risk of adverse maternal outcomes was similar between the two treatments, according to new research.
Elizabeth A. Suarez, PhD, MPH, with Brigham and Women’s Hospital in Boston, led the study published online in the New England Journal of Medicine.
Opioid use disorder in pregnant women has increased steadily in the United States since 2000, the authors write. As of 2017, about 8.2 per 1,000 deliveries were estimated to be affected by the disorder. The numbers were particularly high in people insured by Medicaid. In that group, an estimated 14.6 per 1,000 deliveries were affected.
Researchers studied pregnant women enrolled in public insurance programs in the United States from 2000 through 2018 in a dataset of 2,548,372 pregnancies that ended in live births. They analyzed outcomes in those who received buprenorphine as compared with those who received methadone.
They looked at different periods of exposure to the two medications: early pregnancy (through gestational week 19); late pregnancy (week 20 through the day before delivery); and the 30 days before delivery.
Highlighted differences in infants included:
- Neonatal abstinence syndrome in 52% of the infants who were exposed to buprenorphine in the 30 days before delivery as compared with 69.2% of those exposed to methadone (adjusted relative risk, 0.73).
- Preterm birth in 14.4% of infants exposed to buprenorphine in early pregnancy and in 24.9% of those exposed to methadone (ARR, 0.58).
- Small size for gestational age in 12.1% (buprenorphine) and 15.3% (methadone) (ARR, 0.72).
- Low birth weight in 8.3% (buprenorphine) and 14.9% (methadone) (ARR, 0.56).
- Delivery by cesarean section occurred in 33.6% of pregnant women exposed to buprenorphine in early pregnancy and 33.1% of those exposed to methadone (ARR, 1.02.).
Severe maternal complications developed in 3.3% of the women exposed to buprenorphine and 3.5% of those on methadone (ARR, 0.91.) Exposures in late pregnancy and early pregnancy yielded similar results, the authors say.
Michael Caucci, MD, of the department of psychiatry at Vanderbilt University Medical Center in Nashville, Tenn. who also runs the Women’s Mental Health Clinic at the university, said this paper supports preliminary findings from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study that suggested infants exposed to buprenorphine (compared with methadone) appeared to have lower rates of neonatal complications.
“It also supports buprenorphine as a relatively safe option for treatment of opioid use disorder during pregnancy,” said Dr. Caucci, who was not part of the study by Dr. Suarez and associates. “Reducing the fear of harming the fetus or neonate will help eliminate this barrier to perinatal substance use disorder treatment.”
But he cautions against concluding that, because buprenorphine has lower risks of fetal/neonatal complications, it is safer and therefore better than methadone in pregnancy.
“Some women do not tolerate buprenorphine and do much better on methadone, Dr. Caucci said. “Current recommendations are that both buprenorphine and methadone are relatively safe options for treatment of OUD [opioid use disorder] in pregnancy.”
Among the differences between the treatments is that while methadone is administered daily during in-person visits to federally regulated opioid treatment programs, buprenorphine can be prescribed by approved providers, which allows patients to administer buprenorphine themselves.
Dr. Caucci said he was intrigued by the finding that there was no difference in pregnancy, neonatal, and maternal outcomes depending on the time of exposure to the agents.
“I would have expected higher rates of neonatal abstinence syndrome (NAS) or poor fetal growth in those exposed later in pregnancy vs. those with early exposure,” he said.
The work was supported by the National Institute on Drug Abuse. Dr. Caucci reports no relevant financial relationships. The authors’ disclosures are available with the full text.
Using buprenorphine for opioid use disorder in pregnancy was linked with a lower risk of neonatal side effects than using methadone, but the risk of adverse maternal outcomes was similar between the two treatments, according to new research.
Elizabeth A. Suarez, PhD, MPH, with Brigham and Women’s Hospital in Boston, led the study published online in the New England Journal of Medicine.
Opioid use disorder in pregnant women has increased steadily in the United States since 2000, the authors write. As of 2017, about 8.2 per 1,000 deliveries were estimated to be affected by the disorder. The numbers were particularly high in people insured by Medicaid. In that group, an estimated 14.6 per 1,000 deliveries were affected.
Researchers studied pregnant women enrolled in public insurance programs in the United States from 2000 through 2018 in a dataset of 2,548,372 pregnancies that ended in live births. They analyzed outcomes in those who received buprenorphine as compared with those who received methadone.
They looked at different periods of exposure to the two medications: early pregnancy (through gestational week 19); late pregnancy (week 20 through the day before delivery); and the 30 days before delivery.
Highlighted differences in infants included:
- Neonatal abstinence syndrome in 52% of the infants who were exposed to buprenorphine in the 30 days before delivery as compared with 69.2% of those exposed to methadone (adjusted relative risk, 0.73).
- Preterm birth in 14.4% of infants exposed to buprenorphine in early pregnancy and in 24.9% of those exposed to methadone (ARR, 0.58).
- Small size for gestational age in 12.1% (buprenorphine) and 15.3% (methadone) (ARR, 0.72).
- Low birth weight in 8.3% (buprenorphine) and 14.9% (methadone) (ARR, 0.56).
- Delivery by cesarean section occurred in 33.6% of pregnant women exposed to buprenorphine in early pregnancy and 33.1% of those exposed to methadone (ARR, 1.02.).
Severe maternal complications developed in 3.3% of the women exposed to buprenorphine and 3.5% of those on methadone (ARR, 0.91.) Exposures in late pregnancy and early pregnancy yielded similar results, the authors say.
Michael Caucci, MD, of the department of psychiatry at Vanderbilt University Medical Center in Nashville, Tenn. who also runs the Women’s Mental Health Clinic at the university, said this paper supports preliminary findings from the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study that suggested infants exposed to buprenorphine (compared with methadone) appeared to have lower rates of neonatal complications.
“It also supports buprenorphine as a relatively safe option for treatment of opioid use disorder during pregnancy,” said Dr. Caucci, who was not part of the study by Dr. Suarez and associates. “Reducing the fear of harming the fetus or neonate will help eliminate this barrier to perinatal substance use disorder treatment.”
But he cautions against concluding that, because buprenorphine has lower risks of fetal/neonatal complications, it is safer and therefore better than methadone in pregnancy.
“Some women do not tolerate buprenorphine and do much better on methadone, Dr. Caucci said. “Current recommendations are that both buprenorphine and methadone are relatively safe options for treatment of OUD [opioid use disorder] in pregnancy.”
Among the differences between the treatments is that while methadone is administered daily during in-person visits to federally regulated opioid treatment programs, buprenorphine can be prescribed by approved providers, which allows patients to administer buprenorphine themselves.
Dr. Caucci said he was intrigued by the finding that there was no difference in pregnancy, neonatal, and maternal outcomes depending on the time of exposure to the agents.
“I would have expected higher rates of neonatal abstinence syndrome (NAS) or poor fetal growth in those exposed later in pregnancy vs. those with early exposure,” he said.
The work was supported by the National Institute on Drug Abuse. Dr. Caucci reports no relevant financial relationships. The authors’ disclosures are available with the full text.
FROM NEW ENGLAND JOURNAL OF MEDICINE
HCV reinfection uncommon among people who inject drugs
The findings, which are based on prospective data from 13 countries, including the United States, and were published in Annals of Internal Medicine (2022 Aug 8. doi: 10.7326/M21-4119), should encourage physicians to treat HCV in people with a history of injection drug use, said lead author Jason Grebely, PhD. They should also pressure payers to lift reimbursement restrictions on the same population.
“Direct-acting antiviral medications for HCV infection are safe and effective among people receiving OAT and people with recent injecting-drug use,” the investigators wrote. “Concerns remain, however, that HCV reinfection may reduce the benefits of cure among people who inject drugs and compromise HCV elimination efforts.”
They explored these concerns through a 3-year extension of the phase 3 CO-STAR trial that evaluated elbasvir and grazoprevir in people consistently taking OAT. Participants in the CO-STAR trial, which had a 96% sustained virologic response rate among those who completed therapy, could elect to participate in the present study, offering a prospective look at long-term reinfection.
Out of 296 participants in the CO-STAR trial, 286 were evaluable for reinfection and 199 enrolled in the present extension. The majority were White (79.4%) and male (75.9%), with most taking methadone (79%), followed by buprenorphine (20%). At 6 months, 40 out of 191 respondents (21%) reported injection-drug use in the previous month. At the 3-year mark, 26 out of 142 respondents (18%) disclosed injection-drug use in the previous month.
For all participants in the CO-STAR trial, the overall rate of reinfection at 3 years was 1.7 per 100 person-years (95% confidence interval, 0.8-3.0), which is lower than the rate reported in systematic reviews (3.8 per 100 person-years), according to the investigators.
In the extension analysis, the 3-year reinfection rate was lower still, at 1.2 per 100 person-years. The rate was slightly higher among people who reported injection-drug use in the previous month (1.9 per 100 person-years), and slightly lower among those who did not report injection-drug use in the prior month (0.5 per 100 person-years). More pronounced differences in reinfection were observed among participants who shared needles (6.4 per 100 person-years), versus those who didn’t share needles (1.5 per 100 person years).
Low reinfection rate may help facilitate removal of reimbursement restrictions
“Most of the reinfections in this study occurred within 24 weeks of completing treatment, suggesting that this is a key period for optimizing treatment of opioid use disorder and for providing access to needle and syringe programs that have documented benefits in preventing HCV transmission,” the investigators wrote.
This is one of the largest observational studies of its kind to date, bolstered by “excellent study retention” and a “well-characterized cohort,” with findings that should prompt real-world action, said Dr. Grebely, who is head of the hepatitis C and drug use group in the viral hepatitis clinical research program at the Kirby Institute, University of New South Wales, Sydney.
“Given that reinfection has often been cited ... by some providers as a reason for not offering treatment to people receiving OAT, the low reinfection rate in this study will be incredibly important for guiding practice and ensuring therapy is not withheld from this group,” Dr. Grebely said in an interview. “In terms of policy implications, these data may also help to facilitate the removal of reimbursement restrictions based on recent drug/alcohol use criteria that are in place among many payers in the United States.”
More research needed to determine optimal intervention strategies
Carl Latkin, PhD, professor and vice chair of the department of health, behavior, and society at Johns Hopkins University, Baltimore, called the present publication a “great article and well-done study with long-term follow-up.”
Dr. Latkin, who investigates biobehavioral interventions for disadvantaged communities, said the reported rate of reinfection is “very low among a group of current and former injectors.”
Affirming Dr. Grebely’s call for supportive practices by physicians and payers, Dr. Latkin said: “The study highlights the importance of improving access to medication for opioid use disorder. This level of treatment adherence in this group is much higher than for many other medications. Given these data, it would be difficult for payers to have a rational reason for blanket restrictions for HCV treatment among people who use drugs.”
Dr. Latkin explained that “it isn’t simply injection drug use per se” that drives HCV reinfection; instead, he cited social factors, such as lack of housing, as well as withdrawal symptoms, especially among those without access to medications for opioid use disorder (MOUD).
Dr. Latkin and Grebely also agreed that more research is needed to determine optimal intervention strategies.
Dr. Grebely called for one to enhance HCV testing and linkage to care, a topic he covered in a recent review article (Lancet Gastroenterol Hepatol. 2022 May;7[5]:426-45.).
Dr. Latkin said that, while it’s clear that “syringe services programs, accessible HCV treatment, and MOUD are needed,” it is unclear how much coverage is necessary for a given population.
Findings support critical nature of needle and syringe exchange programs
Sarah M. Kattakuzhy, MD, an associate professor in the division of clinical care & research at the Institute of Human Virology, University of Maryland, Baltimore, agreed that the findings “support the critical nature of needle and syringe exchange programs.”
“As most cities in the United States fall well below the high coverage needle and syringe program threshold required to maximally prevent disease transmission, the study serves as a push toward an evidence-based harm reduction policy,” she said.
Dr. Kattakuzhy he added that the study “supports the need to longitudinally engage individuals after HCV treatment to monitor reinfection risk behaviors and test for reinfection,” she continued.
When it came to translating all the data to populations in the United States, she offered a more guarded view.
“Critically, the study population included only individuals who were engaged with OAT and adherent for 3 or more months, selecting to a population of individuals with high adherence and engagement in care,” Dr. Kattakuzhy said in an interview. “As such, the study findings are not applicable to other cross sections of the drug-using community, including individuals not engaged in OAT, and cohorts with higher rates of ongoing injection drug use. Furthermore, there are known genetic impacts on spontaneous clearance, and emerging data on the immunology of reinfection.
“Studies with a focus on less engaged, higher-risk, and minority populations with active drug use are required to answer the remaining questions in HCV reinfection,” she said.
The study was supported by Merck, the Australian Government Department of Health, and the Australian National Health and Medical Research Council. Dr. Grebely disclosed receiving funding from Cepheid, the manufacturer of the Xpert HCV assay. The other investigators disclosed additional relationships with Gilead, AbbVie, Cepheid, and others. Dr. Latkin and Dr. Kattakuzhy disclosed no relevant conflicts of interest.
The findings, which are based on prospective data from 13 countries, including the United States, and were published in Annals of Internal Medicine (2022 Aug 8. doi: 10.7326/M21-4119), should encourage physicians to treat HCV in people with a history of injection drug use, said lead author Jason Grebely, PhD. They should also pressure payers to lift reimbursement restrictions on the same population.
“Direct-acting antiviral medications for HCV infection are safe and effective among people receiving OAT and people with recent injecting-drug use,” the investigators wrote. “Concerns remain, however, that HCV reinfection may reduce the benefits of cure among people who inject drugs and compromise HCV elimination efforts.”
They explored these concerns through a 3-year extension of the phase 3 CO-STAR trial that evaluated elbasvir and grazoprevir in people consistently taking OAT. Participants in the CO-STAR trial, which had a 96% sustained virologic response rate among those who completed therapy, could elect to participate in the present study, offering a prospective look at long-term reinfection.
Out of 296 participants in the CO-STAR trial, 286 were evaluable for reinfection and 199 enrolled in the present extension. The majority were White (79.4%) and male (75.9%), with most taking methadone (79%), followed by buprenorphine (20%). At 6 months, 40 out of 191 respondents (21%) reported injection-drug use in the previous month. At the 3-year mark, 26 out of 142 respondents (18%) disclosed injection-drug use in the previous month.
For all participants in the CO-STAR trial, the overall rate of reinfection at 3 years was 1.7 per 100 person-years (95% confidence interval, 0.8-3.0), which is lower than the rate reported in systematic reviews (3.8 per 100 person-years), according to the investigators.
In the extension analysis, the 3-year reinfection rate was lower still, at 1.2 per 100 person-years. The rate was slightly higher among people who reported injection-drug use in the previous month (1.9 per 100 person-years), and slightly lower among those who did not report injection-drug use in the prior month (0.5 per 100 person-years). More pronounced differences in reinfection were observed among participants who shared needles (6.4 per 100 person-years), versus those who didn’t share needles (1.5 per 100 person years).
Low reinfection rate may help facilitate removal of reimbursement restrictions
“Most of the reinfections in this study occurred within 24 weeks of completing treatment, suggesting that this is a key period for optimizing treatment of opioid use disorder and for providing access to needle and syringe programs that have documented benefits in preventing HCV transmission,” the investigators wrote.
This is one of the largest observational studies of its kind to date, bolstered by “excellent study retention” and a “well-characterized cohort,” with findings that should prompt real-world action, said Dr. Grebely, who is head of the hepatitis C and drug use group in the viral hepatitis clinical research program at the Kirby Institute, University of New South Wales, Sydney.
“Given that reinfection has often been cited ... by some providers as a reason for not offering treatment to people receiving OAT, the low reinfection rate in this study will be incredibly important for guiding practice and ensuring therapy is not withheld from this group,” Dr. Grebely said in an interview. “In terms of policy implications, these data may also help to facilitate the removal of reimbursement restrictions based on recent drug/alcohol use criteria that are in place among many payers in the United States.”
More research needed to determine optimal intervention strategies
Carl Latkin, PhD, professor and vice chair of the department of health, behavior, and society at Johns Hopkins University, Baltimore, called the present publication a “great article and well-done study with long-term follow-up.”
Dr. Latkin, who investigates biobehavioral interventions for disadvantaged communities, said the reported rate of reinfection is “very low among a group of current and former injectors.”
Affirming Dr. Grebely’s call for supportive practices by physicians and payers, Dr. Latkin said: “The study highlights the importance of improving access to medication for opioid use disorder. This level of treatment adherence in this group is much higher than for many other medications. Given these data, it would be difficult for payers to have a rational reason for blanket restrictions for HCV treatment among people who use drugs.”
Dr. Latkin explained that “it isn’t simply injection drug use per se” that drives HCV reinfection; instead, he cited social factors, such as lack of housing, as well as withdrawal symptoms, especially among those without access to medications for opioid use disorder (MOUD).
Dr. Latkin and Grebely also agreed that more research is needed to determine optimal intervention strategies.
Dr. Grebely called for one to enhance HCV testing and linkage to care, a topic he covered in a recent review article (Lancet Gastroenterol Hepatol. 2022 May;7[5]:426-45.).
Dr. Latkin said that, while it’s clear that “syringe services programs, accessible HCV treatment, and MOUD are needed,” it is unclear how much coverage is necessary for a given population.
Findings support critical nature of needle and syringe exchange programs
Sarah M. Kattakuzhy, MD, an associate professor in the division of clinical care & research at the Institute of Human Virology, University of Maryland, Baltimore, agreed that the findings “support the critical nature of needle and syringe exchange programs.”
“As most cities in the United States fall well below the high coverage needle and syringe program threshold required to maximally prevent disease transmission, the study serves as a push toward an evidence-based harm reduction policy,” she said.
Dr. Kattakuzhy he added that the study “supports the need to longitudinally engage individuals after HCV treatment to monitor reinfection risk behaviors and test for reinfection,” she continued.
When it came to translating all the data to populations in the United States, she offered a more guarded view.
“Critically, the study population included only individuals who were engaged with OAT and adherent for 3 or more months, selecting to a population of individuals with high adherence and engagement in care,” Dr. Kattakuzhy said in an interview. “As such, the study findings are not applicable to other cross sections of the drug-using community, including individuals not engaged in OAT, and cohorts with higher rates of ongoing injection drug use. Furthermore, there are known genetic impacts on spontaneous clearance, and emerging data on the immunology of reinfection.
“Studies with a focus on less engaged, higher-risk, and minority populations with active drug use are required to answer the remaining questions in HCV reinfection,” she said.
The study was supported by Merck, the Australian Government Department of Health, and the Australian National Health and Medical Research Council. Dr. Grebely disclosed receiving funding from Cepheid, the manufacturer of the Xpert HCV assay. The other investigators disclosed additional relationships with Gilead, AbbVie, Cepheid, and others. Dr. Latkin and Dr. Kattakuzhy disclosed no relevant conflicts of interest.
The findings, which are based on prospective data from 13 countries, including the United States, and were published in Annals of Internal Medicine (2022 Aug 8. doi: 10.7326/M21-4119), should encourage physicians to treat HCV in people with a history of injection drug use, said lead author Jason Grebely, PhD. They should also pressure payers to lift reimbursement restrictions on the same population.
“Direct-acting antiviral medications for HCV infection are safe and effective among people receiving OAT and people with recent injecting-drug use,” the investigators wrote. “Concerns remain, however, that HCV reinfection may reduce the benefits of cure among people who inject drugs and compromise HCV elimination efforts.”
They explored these concerns through a 3-year extension of the phase 3 CO-STAR trial that evaluated elbasvir and grazoprevir in people consistently taking OAT. Participants in the CO-STAR trial, which had a 96% sustained virologic response rate among those who completed therapy, could elect to participate in the present study, offering a prospective look at long-term reinfection.
Out of 296 participants in the CO-STAR trial, 286 were evaluable for reinfection and 199 enrolled in the present extension. The majority were White (79.4%) and male (75.9%), with most taking methadone (79%), followed by buprenorphine (20%). At 6 months, 40 out of 191 respondents (21%) reported injection-drug use in the previous month. At the 3-year mark, 26 out of 142 respondents (18%) disclosed injection-drug use in the previous month.
For all participants in the CO-STAR trial, the overall rate of reinfection at 3 years was 1.7 per 100 person-years (95% confidence interval, 0.8-3.0), which is lower than the rate reported in systematic reviews (3.8 per 100 person-years), according to the investigators.
In the extension analysis, the 3-year reinfection rate was lower still, at 1.2 per 100 person-years. The rate was slightly higher among people who reported injection-drug use in the previous month (1.9 per 100 person-years), and slightly lower among those who did not report injection-drug use in the prior month (0.5 per 100 person-years). More pronounced differences in reinfection were observed among participants who shared needles (6.4 per 100 person-years), versus those who didn’t share needles (1.5 per 100 person years).
Low reinfection rate may help facilitate removal of reimbursement restrictions
“Most of the reinfections in this study occurred within 24 weeks of completing treatment, suggesting that this is a key period for optimizing treatment of opioid use disorder and for providing access to needle and syringe programs that have documented benefits in preventing HCV transmission,” the investigators wrote.
This is one of the largest observational studies of its kind to date, bolstered by “excellent study retention” and a “well-characterized cohort,” with findings that should prompt real-world action, said Dr. Grebely, who is head of the hepatitis C and drug use group in the viral hepatitis clinical research program at the Kirby Institute, University of New South Wales, Sydney.
“Given that reinfection has often been cited ... by some providers as a reason for not offering treatment to people receiving OAT, the low reinfection rate in this study will be incredibly important for guiding practice and ensuring therapy is not withheld from this group,” Dr. Grebely said in an interview. “In terms of policy implications, these data may also help to facilitate the removal of reimbursement restrictions based on recent drug/alcohol use criteria that are in place among many payers in the United States.”
More research needed to determine optimal intervention strategies
Carl Latkin, PhD, professor and vice chair of the department of health, behavior, and society at Johns Hopkins University, Baltimore, called the present publication a “great article and well-done study with long-term follow-up.”
Dr. Latkin, who investigates biobehavioral interventions for disadvantaged communities, said the reported rate of reinfection is “very low among a group of current and former injectors.”
Affirming Dr. Grebely’s call for supportive practices by physicians and payers, Dr. Latkin said: “The study highlights the importance of improving access to medication for opioid use disorder. This level of treatment adherence in this group is much higher than for many other medications. Given these data, it would be difficult for payers to have a rational reason for blanket restrictions for HCV treatment among people who use drugs.”
Dr. Latkin explained that “it isn’t simply injection drug use per se” that drives HCV reinfection; instead, he cited social factors, such as lack of housing, as well as withdrawal symptoms, especially among those without access to medications for opioid use disorder (MOUD).
Dr. Latkin and Grebely also agreed that more research is needed to determine optimal intervention strategies.
Dr. Grebely called for one to enhance HCV testing and linkage to care, a topic he covered in a recent review article (Lancet Gastroenterol Hepatol. 2022 May;7[5]:426-45.).
Dr. Latkin said that, while it’s clear that “syringe services programs, accessible HCV treatment, and MOUD are needed,” it is unclear how much coverage is necessary for a given population.
Findings support critical nature of needle and syringe exchange programs
Sarah M. Kattakuzhy, MD, an associate professor in the division of clinical care & research at the Institute of Human Virology, University of Maryland, Baltimore, agreed that the findings “support the critical nature of needle and syringe exchange programs.”
“As most cities in the United States fall well below the high coverage needle and syringe program threshold required to maximally prevent disease transmission, the study serves as a push toward an evidence-based harm reduction policy,” she said.
Dr. Kattakuzhy he added that the study “supports the need to longitudinally engage individuals after HCV treatment to monitor reinfection risk behaviors and test for reinfection,” she continued.
When it came to translating all the data to populations in the United States, she offered a more guarded view.
“Critically, the study population included only individuals who were engaged with OAT and adherent for 3 or more months, selecting to a population of individuals with high adherence and engagement in care,” Dr. Kattakuzhy said in an interview. “As such, the study findings are not applicable to other cross sections of the drug-using community, including individuals not engaged in OAT, and cohorts with higher rates of ongoing injection drug use. Furthermore, there are known genetic impacts on spontaneous clearance, and emerging data on the immunology of reinfection.
“Studies with a focus on less engaged, higher-risk, and minority populations with active drug use are required to answer the remaining questions in HCV reinfection,” she said.
The study was supported by Merck, the Australian Government Department of Health, and the Australian National Health and Medical Research Council. Dr. Grebely disclosed receiving funding from Cepheid, the manufacturer of the Xpert HCV assay. The other investigators disclosed additional relationships with Gilead, AbbVie, Cepheid, and others. Dr. Latkin and Dr. Kattakuzhy disclosed no relevant conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Moms’ cooing swapped with morphine for newborns in withdrawal
Four years ago, Atrium Health, in Charlotte, N.C., embarked on a dramatic change in how it cares for newborns exposed to opioids in the womb.
Until then, most of the 700 or so babies who underwent opioid withdrawal each year in the hospital system spent their first weeks in a neonatal intensive care unit (NICU), isolated from their parents and treated with regular doses of morphine to ease their symptoms.
Now, most babies stay in the hospital for just a few days under a new approach called Eat, Sleep, Console. These young patients stay in private rooms where they can bond with their parents and volunteer caregivers. The usual course of treatment is no longer extended therapy with opioid replacements. Instead, mothers are encouraged to stay overnight and are taught how to sooth their babies with swaddling, rocking, and cooing.
As a result, the average length of stay for newborns with neonatal abstinence syndrome (NAS) has dropped from 12 days to 6. Use of morphine has fallen by 79%, from 2.25 to 0.45 mg/kg per stay, according to results of a quality improvement pilot project at one of Atrium’s community hospitals.
Similar outcomes from other hospitals around the country have led to widespread uptake of Eat, Sleep, Console since its advent in 2017. That year, according to federal data, seven newborns were diagnosed with NAS for every 1,000 births.
Advocates say the family-centric model helps parents feel less stigmatized and more confident in their ability to care for their babies, who can have symptoms such as irritability and difficulty feeding for months.
The approach “really empowers families to do what they do best, which is take care of each other,” Douglas Dodds, MD, a pediatrician who led the effort at Atrium, told this news organization.
Questioning the old protocols
Numerous state perinatal collaboratives, hospital associations, and health systems say the program is the new standard of care for infants with NAS and neonatal opioid withdrawal syndrome (NOWS).
Twenty-six hospitals have adopted Eat, Sleep, Console as part of a clinical trial sponsored by the National Institutes of Health and a program called Advancing Clinical Trials in Neonatal Opioid Withdrawal Syndrome (ACT NOW). Researchers are comparing the approach to previous care protocols in regard to 12 outcomes, including time to medical readiness for discharge, frequency of opioid replacement therapy, and safety problems, such as seizures during treatment.
The transition has been swift. Less than a decade ago, most hospitals used the Finnegan Neonatal Abstinence Scoring System, which was developed in the 1970s to assess babies whose mothers had used heroin during pregnancy.
The Finnegan score entails monitoring babies every 3 hours for 21 symptoms, including high-pitched crying, sneezing, gastrointestinal problems, and yawning. If a baby scores an 8 or more three times in a row, most protocols using the traditional Finnegan approach recommend that providers move infants to an NICU, where they receive morphine or methadone. Once opioid replacement therapy is started, the protocols require a gradual weaning that lasts 3-4 weeks.
As the opioid epidemic grew and NICUs around the country began to fill with babies experiencing NAS or NOW, some clinicians began to question the Finnegan-driven approach.
“You have these miserable babies who are going through this really tough experience, and our first move is to separate them from their moms,” said Matthew Grossman, MD, a pediatric hospitalist at Yale New Haven Children’s Hospital, New Haven, Conn., who created Eat, Sleep, Console.
Dr. Grossman, associate professor and vice chair for quality in the department of pediatrics at Yale University, said he noticed that when mothers stayed overnight with their babies, the infants tended to have fewer withdrawal symptoms. Indeed, previous studies had demonstrated the benefits of breastfeeding and allowing mothers and babies to share a room.
“If you think of mom as a medicine, then you can’t put the baby in a unit where the mom can’t be there,” Dr. Grossman told this news organization. “It would be like taking a kid with pneumonia and putting him in a unit that doesn’t have antibiotics.”
Despite its prominence, the Finnegan score has never been validated for guiding the treatment of NAS. In addition, Finnegan scores can be inconsistent, and the assessment requires disturbing an infant to check signs such as its startle reflex, which, as Dr. Grossman and his fellow researchers pointed out, flies in the face of American Academy of Pediatrics’ recommendations to prioritize swaddling and minimize stimulation for infants with NAS.
By contrast, Eat, Sleep, Console offers a simplified assessment. Interventions are called for if a baby eats less than an ounce of food at a time/does not breastfeed, sleeps less than an hour at a stretch, or takes more than 10 minutes to be consoled. After nonpharmacologic interventions have been tried, doses of medication are used as needed. Babies who are doing well can be discharged in as few as 4 days.
Quashing bias against parents with substance abuse disorder
Even with the promise of shorter stays and better care, switching to nonpharmacologic care presents hurdles for hospitals. Among these is a lack of physical space for mothers to room with their babies in a quiet environment.
“In many community hospitals, the only place for infants to go is a neonatal intensive care unit, outside of the newborn nursery,” said Stephen Patrick, MD, MPH, associate professor and director of the Center for Child Health Policy at Vanderbilt University, Nashville, Tenn., who researches stigma associated with opioid use during pregnancy.
Administrators at SSM St. Mary’s Hospital in St. Louis initially balked at providing private rooms for mothers and their babies with NAS and NOWS, according to Kimberly Spence, MD, a neonatologist at SSM Health. She said the initial plan was to put the babies in a busy, brightly lit nursery.
But resistance waned as the hospital convinced health plans to pay for private rooms for the 5-7 days it typically takes a baby to go through withdrawal, said Dr. Spence, associate professor of pediatrics at Saint Louis University.
“We were able to provide enough data that this is evidence-based medicine and babies do better with their moms, and that ethically, this is the right thing to do, to reduce transfers to an NICU,” she said.
In addition, news stories about the family-centric approach and shorter stays for infants, along with SSM’s launch of an outpatient clinic to treat pregnant women with opioid use disorder, helped the system to attract more patients and increase its market share, said Dr. Spence.
Another challenge was getting physicians and nurses to set aside any judgments of parents with substance abuse disorder, according to Dr. Grossman and others.
“A lot of faculty and staff on the medical team didn’t feel like we should trust moms with their babies’ medical care” at SSM, Dr. Spence said.
Some hospitals conduct anti-bias training to teach providers that substance abuse is a disease that deserves proper medical treatment and not the moral failing of a patient. Such education may involve explaining that babies’ outcomes are improved when women undergo treatment with methadone or buprenorphine during pregnancy, even though use of those medications does pose a risk of NAS.
Creating a system that supports parents with substance abuse disorders may help to change perceptions. At Atrium Health, some staff members now enjoy working with these families because they can make a profound impact, Dr. Dodds said. He said they’ve learned that families suffering from substance abuse disorder “are not that different than any other family.”
Dr. Dodds, Dr. Patrick, Dr. Spence, and Dr. Grossman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Four years ago, Atrium Health, in Charlotte, N.C., embarked on a dramatic change in how it cares for newborns exposed to opioids in the womb.
Until then, most of the 700 or so babies who underwent opioid withdrawal each year in the hospital system spent their first weeks in a neonatal intensive care unit (NICU), isolated from their parents and treated with regular doses of morphine to ease their symptoms.
Now, most babies stay in the hospital for just a few days under a new approach called Eat, Sleep, Console. These young patients stay in private rooms where they can bond with their parents and volunteer caregivers. The usual course of treatment is no longer extended therapy with opioid replacements. Instead, mothers are encouraged to stay overnight and are taught how to sooth their babies with swaddling, rocking, and cooing.
As a result, the average length of stay for newborns with neonatal abstinence syndrome (NAS) has dropped from 12 days to 6. Use of morphine has fallen by 79%, from 2.25 to 0.45 mg/kg per stay, according to results of a quality improvement pilot project at one of Atrium’s community hospitals.
Similar outcomes from other hospitals around the country have led to widespread uptake of Eat, Sleep, Console since its advent in 2017. That year, according to federal data, seven newborns were diagnosed with NAS for every 1,000 births.
Advocates say the family-centric model helps parents feel less stigmatized and more confident in their ability to care for their babies, who can have symptoms such as irritability and difficulty feeding for months.
The approach “really empowers families to do what they do best, which is take care of each other,” Douglas Dodds, MD, a pediatrician who led the effort at Atrium, told this news organization.
Questioning the old protocols
Numerous state perinatal collaboratives, hospital associations, and health systems say the program is the new standard of care for infants with NAS and neonatal opioid withdrawal syndrome (NOWS).
Twenty-six hospitals have adopted Eat, Sleep, Console as part of a clinical trial sponsored by the National Institutes of Health and a program called Advancing Clinical Trials in Neonatal Opioid Withdrawal Syndrome (ACT NOW). Researchers are comparing the approach to previous care protocols in regard to 12 outcomes, including time to medical readiness for discharge, frequency of opioid replacement therapy, and safety problems, such as seizures during treatment.
The transition has been swift. Less than a decade ago, most hospitals used the Finnegan Neonatal Abstinence Scoring System, which was developed in the 1970s to assess babies whose mothers had used heroin during pregnancy.
The Finnegan score entails monitoring babies every 3 hours for 21 symptoms, including high-pitched crying, sneezing, gastrointestinal problems, and yawning. If a baby scores an 8 or more three times in a row, most protocols using the traditional Finnegan approach recommend that providers move infants to an NICU, where they receive morphine or methadone. Once opioid replacement therapy is started, the protocols require a gradual weaning that lasts 3-4 weeks.
As the opioid epidemic grew and NICUs around the country began to fill with babies experiencing NAS or NOW, some clinicians began to question the Finnegan-driven approach.
“You have these miserable babies who are going through this really tough experience, and our first move is to separate them from their moms,” said Matthew Grossman, MD, a pediatric hospitalist at Yale New Haven Children’s Hospital, New Haven, Conn., who created Eat, Sleep, Console.
Dr. Grossman, associate professor and vice chair for quality in the department of pediatrics at Yale University, said he noticed that when mothers stayed overnight with their babies, the infants tended to have fewer withdrawal symptoms. Indeed, previous studies had demonstrated the benefits of breastfeeding and allowing mothers and babies to share a room.
“If you think of mom as a medicine, then you can’t put the baby in a unit where the mom can’t be there,” Dr. Grossman told this news organization. “It would be like taking a kid with pneumonia and putting him in a unit that doesn’t have antibiotics.”
Despite its prominence, the Finnegan score has never been validated for guiding the treatment of NAS. In addition, Finnegan scores can be inconsistent, and the assessment requires disturbing an infant to check signs such as its startle reflex, which, as Dr. Grossman and his fellow researchers pointed out, flies in the face of American Academy of Pediatrics’ recommendations to prioritize swaddling and minimize stimulation for infants with NAS.
By contrast, Eat, Sleep, Console offers a simplified assessment. Interventions are called for if a baby eats less than an ounce of food at a time/does not breastfeed, sleeps less than an hour at a stretch, or takes more than 10 minutes to be consoled. After nonpharmacologic interventions have been tried, doses of medication are used as needed. Babies who are doing well can be discharged in as few as 4 days.
Quashing bias against parents with substance abuse disorder
Even with the promise of shorter stays and better care, switching to nonpharmacologic care presents hurdles for hospitals. Among these is a lack of physical space for mothers to room with their babies in a quiet environment.
“In many community hospitals, the only place for infants to go is a neonatal intensive care unit, outside of the newborn nursery,” said Stephen Patrick, MD, MPH, associate professor and director of the Center for Child Health Policy at Vanderbilt University, Nashville, Tenn., who researches stigma associated with opioid use during pregnancy.
Administrators at SSM St. Mary’s Hospital in St. Louis initially balked at providing private rooms for mothers and their babies with NAS and NOWS, according to Kimberly Spence, MD, a neonatologist at SSM Health. She said the initial plan was to put the babies in a busy, brightly lit nursery.
But resistance waned as the hospital convinced health plans to pay for private rooms for the 5-7 days it typically takes a baby to go through withdrawal, said Dr. Spence, associate professor of pediatrics at Saint Louis University.
“We were able to provide enough data that this is evidence-based medicine and babies do better with their moms, and that ethically, this is the right thing to do, to reduce transfers to an NICU,” she said.
In addition, news stories about the family-centric approach and shorter stays for infants, along with SSM’s launch of an outpatient clinic to treat pregnant women with opioid use disorder, helped the system to attract more patients and increase its market share, said Dr. Spence.
Another challenge was getting physicians and nurses to set aside any judgments of parents with substance abuse disorder, according to Dr. Grossman and others.
“A lot of faculty and staff on the medical team didn’t feel like we should trust moms with their babies’ medical care” at SSM, Dr. Spence said.
Some hospitals conduct anti-bias training to teach providers that substance abuse is a disease that deserves proper medical treatment and not the moral failing of a patient. Such education may involve explaining that babies’ outcomes are improved when women undergo treatment with methadone or buprenorphine during pregnancy, even though use of those medications does pose a risk of NAS.
Creating a system that supports parents with substance abuse disorders may help to change perceptions. At Atrium Health, some staff members now enjoy working with these families because they can make a profound impact, Dr. Dodds said. He said they’ve learned that families suffering from substance abuse disorder “are not that different than any other family.”
Dr. Dodds, Dr. Patrick, Dr. Spence, and Dr. Grossman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Four years ago, Atrium Health, in Charlotte, N.C., embarked on a dramatic change in how it cares for newborns exposed to opioids in the womb.
Until then, most of the 700 or so babies who underwent opioid withdrawal each year in the hospital system spent their first weeks in a neonatal intensive care unit (NICU), isolated from their parents and treated with regular doses of morphine to ease their symptoms.
Now, most babies stay in the hospital for just a few days under a new approach called Eat, Sleep, Console. These young patients stay in private rooms where they can bond with their parents and volunteer caregivers. The usual course of treatment is no longer extended therapy with opioid replacements. Instead, mothers are encouraged to stay overnight and are taught how to sooth their babies with swaddling, rocking, and cooing.
As a result, the average length of stay for newborns with neonatal abstinence syndrome (NAS) has dropped from 12 days to 6. Use of morphine has fallen by 79%, from 2.25 to 0.45 mg/kg per stay, according to results of a quality improvement pilot project at one of Atrium’s community hospitals.
Similar outcomes from other hospitals around the country have led to widespread uptake of Eat, Sleep, Console since its advent in 2017. That year, according to federal data, seven newborns were diagnosed with NAS for every 1,000 births.
Advocates say the family-centric model helps parents feel less stigmatized and more confident in their ability to care for their babies, who can have symptoms such as irritability and difficulty feeding for months.
The approach “really empowers families to do what they do best, which is take care of each other,” Douglas Dodds, MD, a pediatrician who led the effort at Atrium, told this news organization.
Questioning the old protocols
Numerous state perinatal collaboratives, hospital associations, and health systems say the program is the new standard of care for infants with NAS and neonatal opioid withdrawal syndrome (NOWS).
Twenty-six hospitals have adopted Eat, Sleep, Console as part of a clinical trial sponsored by the National Institutes of Health and a program called Advancing Clinical Trials in Neonatal Opioid Withdrawal Syndrome (ACT NOW). Researchers are comparing the approach to previous care protocols in regard to 12 outcomes, including time to medical readiness for discharge, frequency of opioid replacement therapy, and safety problems, such as seizures during treatment.
The transition has been swift. Less than a decade ago, most hospitals used the Finnegan Neonatal Abstinence Scoring System, which was developed in the 1970s to assess babies whose mothers had used heroin during pregnancy.
The Finnegan score entails monitoring babies every 3 hours for 21 symptoms, including high-pitched crying, sneezing, gastrointestinal problems, and yawning. If a baby scores an 8 or more three times in a row, most protocols using the traditional Finnegan approach recommend that providers move infants to an NICU, where they receive morphine or methadone. Once opioid replacement therapy is started, the protocols require a gradual weaning that lasts 3-4 weeks.
As the opioid epidemic grew and NICUs around the country began to fill with babies experiencing NAS or NOW, some clinicians began to question the Finnegan-driven approach.
“You have these miserable babies who are going through this really tough experience, and our first move is to separate them from their moms,” said Matthew Grossman, MD, a pediatric hospitalist at Yale New Haven Children’s Hospital, New Haven, Conn., who created Eat, Sleep, Console.
Dr. Grossman, associate professor and vice chair for quality in the department of pediatrics at Yale University, said he noticed that when mothers stayed overnight with their babies, the infants tended to have fewer withdrawal symptoms. Indeed, previous studies had demonstrated the benefits of breastfeeding and allowing mothers and babies to share a room.
“If you think of mom as a medicine, then you can’t put the baby in a unit where the mom can’t be there,” Dr. Grossman told this news organization. “It would be like taking a kid with pneumonia and putting him in a unit that doesn’t have antibiotics.”
Despite its prominence, the Finnegan score has never been validated for guiding the treatment of NAS. In addition, Finnegan scores can be inconsistent, and the assessment requires disturbing an infant to check signs such as its startle reflex, which, as Dr. Grossman and his fellow researchers pointed out, flies in the face of American Academy of Pediatrics’ recommendations to prioritize swaddling and minimize stimulation for infants with NAS.
By contrast, Eat, Sleep, Console offers a simplified assessment. Interventions are called for if a baby eats less than an ounce of food at a time/does not breastfeed, sleeps less than an hour at a stretch, or takes more than 10 minutes to be consoled. After nonpharmacologic interventions have been tried, doses of medication are used as needed. Babies who are doing well can be discharged in as few as 4 days.
Quashing bias against parents with substance abuse disorder
Even with the promise of shorter stays and better care, switching to nonpharmacologic care presents hurdles for hospitals. Among these is a lack of physical space for mothers to room with their babies in a quiet environment.
“In many community hospitals, the only place for infants to go is a neonatal intensive care unit, outside of the newborn nursery,” said Stephen Patrick, MD, MPH, associate professor and director of the Center for Child Health Policy at Vanderbilt University, Nashville, Tenn., who researches stigma associated with opioid use during pregnancy.
Administrators at SSM St. Mary’s Hospital in St. Louis initially balked at providing private rooms for mothers and their babies with NAS and NOWS, according to Kimberly Spence, MD, a neonatologist at SSM Health. She said the initial plan was to put the babies in a busy, brightly lit nursery.
But resistance waned as the hospital convinced health plans to pay for private rooms for the 5-7 days it typically takes a baby to go through withdrawal, said Dr. Spence, associate professor of pediatrics at Saint Louis University.
“We were able to provide enough data that this is evidence-based medicine and babies do better with their moms, and that ethically, this is the right thing to do, to reduce transfers to an NICU,” she said.
In addition, news stories about the family-centric approach and shorter stays for infants, along with SSM’s launch of an outpatient clinic to treat pregnant women with opioid use disorder, helped the system to attract more patients and increase its market share, said Dr. Spence.
Another challenge was getting physicians and nurses to set aside any judgments of parents with substance abuse disorder, according to Dr. Grossman and others.
“A lot of faculty and staff on the medical team didn’t feel like we should trust moms with their babies’ medical care” at SSM, Dr. Spence said.
Some hospitals conduct anti-bias training to teach providers that substance abuse is a disease that deserves proper medical treatment and not the moral failing of a patient. Such education may involve explaining that babies’ outcomes are improved when women undergo treatment with methadone or buprenorphine during pregnancy, even though use of those medications does pose a risk of NAS.
Creating a system that supports parents with substance abuse disorders may help to change perceptions. At Atrium Health, some staff members now enjoy working with these families because they can make a profound impact, Dr. Dodds said. He said they’ve learned that families suffering from substance abuse disorder “are not that different than any other family.”
Dr. Dodds, Dr. Patrick, Dr. Spence, and Dr. Grossman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Opioid use in the elderly a dementia risk factor?
in new findings that suggest exposure to these drugs may be another modifiable risk factor for dementia.
“Clinicians and others may want to consider that opioid exposure in those aged 75-80 increases dementia risk, and to balance the potential benefits of opioid use in old age with adverse side effects,” said Stephen Z. Levine, PhD, professor, department of community mental health, University of Haifa (Israel).
The study was published online in the American Journal of Geriatric Psychiatry.
Widespread use
Evidence points to a relatively high rate of opioid prescriptions among older adults. A Morbidity and Mortality Weekly Report noted 19.2% of the U.S. adult population filled an opioid prescription in 2018, with the rate in those over 65 double that of adults aged 20-24 years (25% vs. 11.2%).
Disorders and illnesses for which opioids might be prescribed, including cancer and some pain conditions, “are far more prevalent in old age than at a younger age,” said Dr. Levine.
This high rate of opioid use underscores the need to consider the risks of opioid use in old age, said Dr. Levine. “Unfortunately, studies of the association between opioid use and dementia risk in old age are few, and their results are inconsistent.”
The study included 91,307 Israeli citizens aged 60 and over without dementia who were enrolled in the Meuhedet Healthcare Services, a nonprofit health maintenance organization (HMO) serving 14% of the country’s population. Meuhedet has maintained an up-to-date dementia registry since 2002.
The average age of the study sample was 68.29 years at the start of the study (in 2012).
In Israel, opioids are prescribed for a 30-day period. In this study, opioid exposure was defined as opioid medication fills covering 60 days (or two prescriptions) within a 120-day interval.
The primary outcome was incident dementia during follow-up from Jan. 1, 2013 to Oct. 30, 2017. The analysis controlled for a number of factors, including age, sex, smoking status, health conditions such as arthritis, depression, diabetes, osteoporosis, cognitive decline, vitamin deficiencies, cancer, cardiovascular conditions, and hospitalizations for falls.
Researchers also accounted for the competing risk of mortality.
During the study, 3.1% of subjects were exposed to opioids at a mean age of 73.94 years, and 5.8% of subjects developed dementia at an average age of 78.07 years.
Increased dementia risk
The risk of incident dementia was significantly increased in those exposed to opioids versus unexposed individuals in the 75- to 80-year age group (adjusted hazard ratio, 1.39; 95% confidence interval, 1.01-1.92; z statistic = 2.02; P < .05).
The authors noted the effect size for opioid exposure in this elderly age group is like other potentially modifiable risk factors for dementia, including body mass index and smoking.
The current study could not determine the biological explanation for the increased dementia risk among older opioid users. “Causal notions are challenging in observational studies and should be viewed with caution,” Dr. Levine noted.
However, a plausible mechanism highlighted in the literature is that opioids promote apoptosis of microglia and neurons that contribute to neurodegenerative diseases, he said.
The study included 14 sensitivity analyses, including those that looked at females, subjects older than 70, smokers, and groups with and without comorbid health conditions. The only sensitivity analysis that didn’t have similar findings to the primary analysis looked at dementia risk restricted to subjects without a vitamin deficiency.
“It’s reassuring that 13 or 14 sensitivity analyses found a significant association between opioid exposure and dementia risk,” said Dr. Levine.
Some prior studies did not show an association between opioid exposure and dementia risk. One possible reason for the discrepancy with the current findings is that the previous research didn’t account for age-specific opioid use effects, or the competing risk of mortality, said Dr. Levine.
Clinicians have a number of potential alternatives to opioids to treat various conditions including acetaminophen, non-steroidal anti-inflammatory drugs, amine reuptake inhibitors (ARIs), membrane stabilizers, muscle relaxants, topical capsaicin, botulinum toxin, cannabinoids, and steroids.
A limitation of the study was that it didn’t adjust for all possible comorbid health conditions, including vascular conditions, or for use of benzodiazepines, and surgical procedures.
In addition, since up to 50% of dementia cases are undetected, it’s possible some in the unexposed opioid group may actually have undiagnosed dementia, thereby reducing the effect sizes in the results.
Reverse causality is also a possibility as the neuropathological process associated with dementia could have started prior to opioid exposure. In addition, the results are limited to prolonged opioid exposure.
Interpret with caution
Commenting on the study, David Knopman, MD, a neurologist at Mayo Clinic in Rochester, Minn., whose research involves late-life cognitive disorders, was skeptical.
“On the face of it, the fact that an association was seen only in one narrow age range – 75+ to 80 years – ought to raise serious suspicion about the reliability and validity of the claim that opioid use is a risk factor for dementia, he said.
Although the researchers performed several sensitivity analyses, including accounting for mortality, “pharmacoepidemiological studies are terribly sensitive to residual biases” related to physician and patient choices related to medication use, added Dr. Knopman.
The claim that opioids are a dementia risk “should be viewed with great caution” and should not influence use of opioids where they’re truly indicated, he said.
“It would be a great pity if patients with pain requiring opioids avoid them because of fears about dementia based on the dubious relationship between age and opioid use.”
Dr. Levine and Dr. Knopman report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
in new findings that suggest exposure to these drugs may be another modifiable risk factor for dementia.
“Clinicians and others may want to consider that opioid exposure in those aged 75-80 increases dementia risk, and to balance the potential benefits of opioid use in old age with adverse side effects,” said Stephen Z. Levine, PhD, professor, department of community mental health, University of Haifa (Israel).
The study was published online in the American Journal of Geriatric Psychiatry.
Widespread use
Evidence points to a relatively high rate of opioid prescriptions among older adults. A Morbidity and Mortality Weekly Report noted 19.2% of the U.S. adult population filled an opioid prescription in 2018, with the rate in those over 65 double that of adults aged 20-24 years (25% vs. 11.2%).
Disorders and illnesses for which opioids might be prescribed, including cancer and some pain conditions, “are far more prevalent in old age than at a younger age,” said Dr. Levine.
This high rate of opioid use underscores the need to consider the risks of opioid use in old age, said Dr. Levine. “Unfortunately, studies of the association between opioid use and dementia risk in old age are few, and their results are inconsistent.”
The study included 91,307 Israeli citizens aged 60 and over without dementia who were enrolled in the Meuhedet Healthcare Services, a nonprofit health maintenance organization (HMO) serving 14% of the country’s population. Meuhedet has maintained an up-to-date dementia registry since 2002.
The average age of the study sample was 68.29 years at the start of the study (in 2012).
In Israel, opioids are prescribed for a 30-day period. In this study, opioid exposure was defined as opioid medication fills covering 60 days (or two prescriptions) within a 120-day interval.
The primary outcome was incident dementia during follow-up from Jan. 1, 2013 to Oct. 30, 2017. The analysis controlled for a number of factors, including age, sex, smoking status, health conditions such as arthritis, depression, diabetes, osteoporosis, cognitive decline, vitamin deficiencies, cancer, cardiovascular conditions, and hospitalizations for falls.
Researchers also accounted for the competing risk of mortality.
During the study, 3.1% of subjects were exposed to opioids at a mean age of 73.94 years, and 5.8% of subjects developed dementia at an average age of 78.07 years.
Increased dementia risk
The risk of incident dementia was significantly increased in those exposed to opioids versus unexposed individuals in the 75- to 80-year age group (adjusted hazard ratio, 1.39; 95% confidence interval, 1.01-1.92; z statistic = 2.02; P < .05).
The authors noted the effect size for opioid exposure in this elderly age group is like other potentially modifiable risk factors for dementia, including body mass index and smoking.
The current study could not determine the biological explanation for the increased dementia risk among older opioid users. “Causal notions are challenging in observational studies and should be viewed with caution,” Dr. Levine noted.
However, a plausible mechanism highlighted in the literature is that opioids promote apoptosis of microglia and neurons that contribute to neurodegenerative diseases, he said.
The study included 14 sensitivity analyses, including those that looked at females, subjects older than 70, smokers, and groups with and without comorbid health conditions. The only sensitivity analysis that didn’t have similar findings to the primary analysis looked at dementia risk restricted to subjects without a vitamin deficiency.
“It’s reassuring that 13 or 14 sensitivity analyses found a significant association between opioid exposure and dementia risk,” said Dr. Levine.
Some prior studies did not show an association between opioid exposure and dementia risk. One possible reason for the discrepancy with the current findings is that the previous research didn’t account for age-specific opioid use effects, or the competing risk of mortality, said Dr. Levine.
Clinicians have a number of potential alternatives to opioids to treat various conditions including acetaminophen, non-steroidal anti-inflammatory drugs, amine reuptake inhibitors (ARIs), membrane stabilizers, muscle relaxants, topical capsaicin, botulinum toxin, cannabinoids, and steroids.
A limitation of the study was that it didn’t adjust for all possible comorbid health conditions, including vascular conditions, or for use of benzodiazepines, and surgical procedures.
In addition, since up to 50% of dementia cases are undetected, it’s possible some in the unexposed opioid group may actually have undiagnosed dementia, thereby reducing the effect sizes in the results.
Reverse causality is also a possibility as the neuropathological process associated with dementia could have started prior to opioid exposure. In addition, the results are limited to prolonged opioid exposure.
Interpret with caution
Commenting on the study, David Knopman, MD, a neurologist at Mayo Clinic in Rochester, Minn., whose research involves late-life cognitive disorders, was skeptical.
“On the face of it, the fact that an association was seen only in one narrow age range – 75+ to 80 years – ought to raise serious suspicion about the reliability and validity of the claim that opioid use is a risk factor for dementia, he said.
Although the researchers performed several sensitivity analyses, including accounting for mortality, “pharmacoepidemiological studies are terribly sensitive to residual biases” related to physician and patient choices related to medication use, added Dr. Knopman.
The claim that opioids are a dementia risk “should be viewed with great caution” and should not influence use of opioids where they’re truly indicated, he said.
“It would be a great pity if patients with pain requiring opioids avoid them because of fears about dementia based on the dubious relationship between age and opioid use.”
Dr. Levine and Dr. Knopman report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
in new findings that suggest exposure to these drugs may be another modifiable risk factor for dementia.
“Clinicians and others may want to consider that opioid exposure in those aged 75-80 increases dementia risk, and to balance the potential benefits of opioid use in old age with adverse side effects,” said Stephen Z. Levine, PhD, professor, department of community mental health, University of Haifa (Israel).
The study was published online in the American Journal of Geriatric Psychiatry.
Widespread use
Evidence points to a relatively high rate of opioid prescriptions among older adults. A Morbidity and Mortality Weekly Report noted 19.2% of the U.S. adult population filled an opioid prescription in 2018, with the rate in those over 65 double that of adults aged 20-24 years (25% vs. 11.2%).
Disorders and illnesses for which opioids might be prescribed, including cancer and some pain conditions, “are far more prevalent in old age than at a younger age,” said Dr. Levine.
This high rate of opioid use underscores the need to consider the risks of opioid use in old age, said Dr. Levine. “Unfortunately, studies of the association between opioid use and dementia risk in old age are few, and their results are inconsistent.”
The study included 91,307 Israeli citizens aged 60 and over without dementia who were enrolled in the Meuhedet Healthcare Services, a nonprofit health maintenance organization (HMO) serving 14% of the country’s population. Meuhedet has maintained an up-to-date dementia registry since 2002.
The average age of the study sample was 68.29 years at the start of the study (in 2012).
In Israel, opioids are prescribed for a 30-day period. In this study, opioid exposure was defined as opioid medication fills covering 60 days (or two prescriptions) within a 120-day interval.
The primary outcome was incident dementia during follow-up from Jan. 1, 2013 to Oct. 30, 2017. The analysis controlled for a number of factors, including age, sex, smoking status, health conditions such as arthritis, depression, diabetes, osteoporosis, cognitive decline, vitamin deficiencies, cancer, cardiovascular conditions, and hospitalizations for falls.
Researchers also accounted for the competing risk of mortality.
During the study, 3.1% of subjects were exposed to opioids at a mean age of 73.94 years, and 5.8% of subjects developed dementia at an average age of 78.07 years.
Increased dementia risk
The risk of incident dementia was significantly increased in those exposed to opioids versus unexposed individuals in the 75- to 80-year age group (adjusted hazard ratio, 1.39; 95% confidence interval, 1.01-1.92; z statistic = 2.02; P < .05).
The authors noted the effect size for opioid exposure in this elderly age group is like other potentially modifiable risk factors for dementia, including body mass index and smoking.
The current study could not determine the biological explanation for the increased dementia risk among older opioid users. “Causal notions are challenging in observational studies and should be viewed with caution,” Dr. Levine noted.
However, a plausible mechanism highlighted in the literature is that opioids promote apoptosis of microglia and neurons that contribute to neurodegenerative diseases, he said.
The study included 14 sensitivity analyses, including those that looked at females, subjects older than 70, smokers, and groups with and without comorbid health conditions. The only sensitivity analysis that didn’t have similar findings to the primary analysis looked at dementia risk restricted to subjects without a vitamin deficiency.
“It’s reassuring that 13 or 14 sensitivity analyses found a significant association between opioid exposure and dementia risk,” said Dr. Levine.
Some prior studies did not show an association between opioid exposure and dementia risk. One possible reason for the discrepancy with the current findings is that the previous research didn’t account for age-specific opioid use effects, or the competing risk of mortality, said Dr. Levine.
Clinicians have a number of potential alternatives to opioids to treat various conditions including acetaminophen, non-steroidal anti-inflammatory drugs, amine reuptake inhibitors (ARIs), membrane stabilizers, muscle relaxants, topical capsaicin, botulinum toxin, cannabinoids, and steroids.
A limitation of the study was that it didn’t adjust for all possible comorbid health conditions, including vascular conditions, or for use of benzodiazepines, and surgical procedures.
In addition, since up to 50% of dementia cases are undetected, it’s possible some in the unexposed opioid group may actually have undiagnosed dementia, thereby reducing the effect sizes in the results.
Reverse causality is also a possibility as the neuropathological process associated with dementia could have started prior to opioid exposure. In addition, the results are limited to prolonged opioid exposure.
Interpret with caution
Commenting on the study, David Knopman, MD, a neurologist at Mayo Clinic in Rochester, Minn., whose research involves late-life cognitive disorders, was skeptical.
“On the face of it, the fact that an association was seen only in one narrow age range – 75+ to 80 years – ought to raise serious suspicion about the reliability and validity of the claim that opioid use is a risk factor for dementia, he said.
Although the researchers performed several sensitivity analyses, including accounting for mortality, “pharmacoepidemiological studies are terribly sensitive to residual biases” related to physician and patient choices related to medication use, added Dr. Knopman.
The claim that opioids are a dementia risk “should be viewed with great caution” and should not influence use of opioids where they’re truly indicated, he said.
“It would be a great pity if patients with pain requiring opioids avoid them because of fears about dementia based on the dubious relationship between age and opioid use.”
Dr. Levine and Dr. Knopman report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM AMERICAN JOURNAL OF GERIATRIC PSYCHIATRY
Study: No more autopilot opioids after cesarean delivery
A group of clinicians is hoping to prompt a rethink of how opioids are prescribed to new mothers after cesarean deliveries after finding that sending patients home with smaller prescriptions led to dramatic drops in use of the drugs.
In research scheduled to be presented at the annual meeting of the American College of Obstetricians and Gynecologists (ACOG) in San Diego, women undergoing cesarean delivery were randomly assigned to receive prescriptions for either 10 or 20 oxycodone tablets at discharge. Interim data revealed that not only did 10-tablet prescriptions correlate with significantly less opioid consumption, but 35% of all women left their opioid scripts unfilled.
The results suggest physicians should stop “automatically prescribing opioids for caesarean section patients at discharge and, instead, work on individualized prescribing,” said Amanda Selk, MD, associate professor of obstetrics and gynecology at the University of Toronto, who was not involved with the study.
“We need to move away from a culture that says a patient can’t be discharged without an opioid prescription,” Dr. Selk said in an interview.
For the study, researchers at Virginia Tech Carilion School of Medicine, Roanoke, Virginia, set out to understand how discharge opioid prescription sizes impact opioid consumption in women who undergo caesarean delivery. Other specialties have found a correlation between the two.
With “no standard dose recommendation for physicians to follow when it came to prescribing opioids for post-cesarean pain management,” they also wanted to help their own clinicians optimize their prescribing, co-investigator Jaclyn Nunziato, MD, assistant professor of obstetrics and gynecology at Virginia Tech Carilion, told this news organization.
They randomly assigned 134 women undergoing a scheduled cesarean delivery to receive a prescription for 10 or 20 tablets of 5 mg oxycodone at discharge. Most women had one or more previous cesarean deliveries, and none had used opioids in the previous 30 days.
Data from 97 patients presented at the ACOG meeting showed that 35% of women had not filled their opioid prescriptions 6 weeks after surgery. For those who did fill the orders, the average consumption at week 6 of the study was 6.6 tablets in the 10-tablet group and 13.3 tablets in the 20-tablet group (P = .0005), according to the researchers. No patients in either group had requested a refill of their opioid medication.
Despite differences in opioid use, both groups reported similar pain scores throughout the study period, and almost all patients in both groups said they were satisfied with their pain management, the investigators reported.
With an average of 9.2 tablets consumed in the two groups combined, the findings indicate “a standard prescription of 10 tablets may be adequate to manage most patients’ postoperative pain,” Dr. Nunziato said.
She said she hoped the results help change how physicians view the treatment of post-cesarean pain and also help “break down the stigma that opioids are the best treatment option for patients.”
Potential for ‘huge’ benefit
Given the number of cesarean deliveries performed every year in the United States – more than 1.1 million in 2020, according to the U.S. Centers for Disease Control and Prevention – providing smaller post-cesarean opioid prescriptions would be “hugely beneficial to individual patients and our larger community,” said Robyn Goodrich, a medical student at Virginia Tech and the first author of the study.
“The patient would receive an amount that is adequate for controlling their pain and keeping them comfortable but does not put them at risk of developing tolerance and addiction,” she added. “For the community, it reduces the number of prescription opioids that are left over and may be diverted or misused if not properly disposed of.”
Dr. Selk pointed to her own group’s randomized controlled trial, showing that post-cesarean patients who do not receive opioids for pain while in the hospital also do not ask for opioid prescriptions after discharge and that their pain can be well-managed with acetaminophen and nonsteroidal anti-inflammatory drugs.
Dr. Selk, Dr. Nunziato, and Ms. Goodrich have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A group of clinicians is hoping to prompt a rethink of how opioids are prescribed to new mothers after cesarean deliveries after finding that sending patients home with smaller prescriptions led to dramatic drops in use of the drugs.
In research scheduled to be presented at the annual meeting of the American College of Obstetricians and Gynecologists (ACOG) in San Diego, women undergoing cesarean delivery were randomly assigned to receive prescriptions for either 10 or 20 oxycodone tablets at discharge. Interim data revealed that not only did 10-tablet prescriptions correlate with significantly less opioid consumption, but 35% of all women left their opioid scripts unfilled.
The results suggest physicians should stop “automatically prescribing opioids for caesarean section patients at discharge and, instead, work on individualized prescribing,” said Amanda Selk, MD, associate professor of obstetrics and gynecology at the University of Toronto, who was not involved with the study.
“We need to move away from a culture that says a patient can’t be discharged without an opioid prescription,” Dr. Selk said in an interview.
For the study, researchers at Virginia Tech Carilion School of Medicine, Roanoke, Virginia, set out to understand how discharge opioid prescription sizes impact opioid consumption in women who undergo caesarean delivery. Other specialties have found a correlation between the two.
With “no standard dose recommendation for physicians to follow when it came to prescribing opioids for post-cesarean pain management,” they also wanted to help their own clinicians optimize their prescribing, co-investigator Jaclyn Nunziato, MD, assistant professor of obstetrics and gynecology at Virginia Tech Carilion, told this news organization.
They randomly assigned 134 women undergoing a scheduled cesarean delivery to receive a prescription for 10 or 20 tablets of 5 mg oxycodone at discharge. Most women had one or more previous cesarean deliveries, and none had used opioids in the previous 30 days.
Data from 97 patients presented at the ACOG meeting showed that 35% of women had not filled their opioid prescriptions 6 weeks after surgery. For those who did fill the orders, the average consumption at week 6 of the study was 6.6 tablets in the 10-tablet group and 13.3 tablets in the 20-tablet group (P = .0005), according to the researchers. No patients in either group had requested a refill of their opioid medication.
Despite differences in opioid use, both groups reported similar pain scores throughout the study period, and almost all patients in both groups said they were satisfied with their pain management, the investigators reported.
With an average of 9.2 tablets consumed in the two groups combined, the findings indicate “a standard prescription of 10 tablets may be adequate to manage most patients’ postoperative pain,” Dr. Nunziato said.
She said she hoped the results help change how physicians view the treatment of post-cesarean pain and also help “break down the stigma that opioids are the best treatment option for patients.”
Potential for ‘huge’ benefit
Given the number of cesarean deliveries performed every year in the United States – more than 1.1 million in 2020, according to the U.S. Centers for Disease Control and Prevention – providing smaller post-cesarean opioid prescriptions would be “hugely beneficial to individual patients and our larger community,” said Robyn Goodrich, a medical student at Virginia Tech and the first author of the study.
“The patient would receive an amount that is adequate for controlling their pain and keeping them comfortable but does not put them at risk of developing tolerance and addiction,” she added. “For the community, it reduces the number of prescription opioids that are left over and may be diverted or misused if not properly disposed of.”
Dr. Selk pointed to her own group’s randomized controlled trial, showing that post-cesarean patients who do not receive opioids for pain while in the hospital also do not ask for opioid prescriptions after discharge and that their pain can be well-managed with acetaminophen and nonsteroidal anti-inflammatory drugs.
Dr. Selk, Dr. Nunziato, and Ms. Goodrich have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A group of clinicians is hoping to prompt a rethink of how opioids are prescribed to new mothers after cesarean deliveries after finding that sending patients home with smaller prescriptions led to dramatic drops in use of the drugs.
In research scheduled to be presented at the annual meeting of the American College of Obstetricians and Gynecologists (ACOG) in San Diego, women undergoing cesarean delivery were randomly assigned to receive prescriptions for either 10 or 20 oxycodone tablets at discharge. Interim data revealed that not only did 10-tablet prescriptions correlate with significantly less opioid consumption, but 35% of all women left their opioid scripts unfilled.
The results suggest physicians should stop “automatically prescribing opioids for caesarean section patients at discharge and, instead, work on individualized prescribing,” said Amanda Selk, MD, associate professor of obstetrics and gynecology at the University of Toronto, who was not involved with the study.
“We need to move away from a culture that says a patient can’t be discharged without an opioid prescription,” Dr. Selk said in an interview.
For the study, researchers at Virginia Tech Carilion School of Medicine, Roanoke, Virginia, set out to understand how discharge opioid prescription sizes impact opioid consumption in women who undergo caesarean delivery. Other specialties have found a correlation between the two.
With “no standard dose recommendation for physicians to follow when it came to prescribing opioids for post-cesarean pain management,” they also wanted to help their own clinicians optimize their prescribing, co-investigator Jaclyn Nunziato, MD, assistant professor of obstetrics and gynecology at Virginia Tech Carilion, told this news organization.
They randomly assigned 134 women undergoing a scheduled cesarean delivery to receive a prescription for 10 or 20 tablets of 5 mg oxycodone at discharge. Most women had one or more previous cesarean deliveries, and none had used opioids in the previous 30 days.
Data from 97 patients presented at the ACOG meeting showed that 35% of women had not filled their opioid prescriptions 6 weeks after surgery. For those who did fill the orders, the average consumption at week 6 of the study was 6.6 tablets in the 10-tablet group and 13.3 tablets in the 20-tablet group (P = .0005), according to the researchers. No patients in either group had requested a refill of their opioid medication.
Despite differences in opioid use, both groups reported similar pain scores throughout the study period, and almost all patients in both groups said they were satisfied with their pain management, the investigators reported.
With an average of 9.2 tablets consumed in the two groups combined, the findings indicate “a standard prescription of 10 tablets may be adequate to manage most patients’ postoperative pain,” Dr. Nunziato said.
She said she hoped the results help change how physicians view the treatment of post-cesarean pain and also help “break down the stigma that opioids are the best treatment option for patients.”
Potential for ‘huge’ benefit
Given the number of cesarean deliveries performed every year in the United States – more than 1.1 million in 2020, according to the U.S. Centers for Disease Control and Prevention – providing smaller post-cesarean opioid prescriptions would be “hugely beneficial to individual patients and our larger community,” said Robyn Goodrich, a medical student at Virginia Tech and the first author of the study.
“The patient would receive an amount that is adequate for controlling their pain and keeping them comfortable but does not put them at risk of developing tolerance and addiction,” she added. “For the community, it reduces the number of prescription opioids that are left over and may be diverted or misused if not properly disposed of.”
Dr. Selk pointed to her own group’s randomized controlled trial, showing that post-cesarean patients who do not receive opioids for pain while in the hospital also do not ask for opioid prescriptions after discharge and that their pain can be well-managed with acetaminophen and nonsteroidal anti-inflammatory drugs.
Dr. Selk, Dr. Nunziato, and Ms. Goodrich have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACOG 2022
Adolescent overdose deaths nearly doubled in 2020 and spiked again in 2021
The number of overdose deaths in adolescents nearly doubled in 2020 from the year before and increased substantially again in 2021 after nearly a decade of fairly stable rates, according to data published in a JAMA research letter.
Most of the deaths involved fentanyl, the researchers found.
Joseph Friedman, MPH, of the Center for Social Medicine and Humanities at the University of California, Los Angeles, led the study, which analyzed adolescent (14-18 years old) overdose deaths in the United States from 2010 to June 2021 in light of increasing contamination in the supply of illicit drugs.
The researchers found there were 518 deaths among adolescents (2.40 per 100,000 population) in 2010, and the rates remained stable through 2019 with 492 deaths (2.36 per 100,000).
In 2020, however, deaths spiked to 954 (4.57 per 100 000), increasing by 94.3%, compared with 2019. In 2021, they increased another 20%.
The rise in fentanyl-involved deaths was particularly striking. Fentanyl-involved deaths increased from 253 (1.21 per 100,000) in 2019 to 680 (3.26 per 100,000) in 2020. The numbers through June 2021 were annualized for 2021 and calculations predicted 884 deaths (4.23 per 100,000) for the year.
Numbers point to fentanyl potency
In 2021, more than three-fourths (77.14%) of adolescent overdose deaths involved fentanyl, compared with 13.26% for benzodiazepines, 9.77% for methamphetamine, 7.33% for cocaine, 5.76% for prescription opioids, and 2.27% for heroin.
American Indian and Alaska Native adolescents had the highest overdose rate in 2021 (n = 24; 11.79 per 100,000), followed by Latinx adolescents (n = 354; 6.98 per 100,000).
“These adolescent trends fit a wider pattern of increasing racial and ethnic inequalities in overdose that deserve further investigation and intervention efforts,” the authors wrote.
Pandemic’s role unclear
The spikes in adolescent overdoses overlap the COVID-19 pandemic, but Dr. Friedman said in an interview the pandemic “may or may not have been a big factor. “
The authors wrote that drug use had generally been stable among adolescents between 2010 and 2020. The number of 10th graders reporting any illicit drug use was 30.2% in 2010 and 30.4% in 2020.
“So it’s not that more teens are using drugs. It’s just that drug use is becoming more dangerous due to the spread of counterfeit pills containing fentanyls,” Dr. Friedman said.
The authors noted that “the illicit drug supply has increasingly become contaminated with illicitly manufactured fentanyls and other synthetic opioid and benzodiazepine analogues.”
Mr. Friedman said the pandemic may have accelerated the spread of more dangerous forms of drugs as supply chains were disrupted.
Benjamin Brady, DrPH, an assistant professor at the University of Arizona, Tucson, who also has an appointment in the university’s Comprehensive Pain and Addiction Center, said in an interview the numbers that Dr. Friedman and colleagues present represent “worst fears coming true.”
He said he and his colleagues in the field “were anticipating a rise in overdose deaths for the next 5-10 years because of the way the supply-and-demand environment exists in the U.S.”
Dr. Brady explained that restricting access to prescription opioids has had an unfortunate side effect in decreasing access to a safer supply of drugs.
“Without having solutions that would reduce demand at the same rate, supply of the safer form of the drug has been reduced; that has pushed people toward heroin and street drugs and from 2016 on those have been adulterated with fentanyl,” he said.
He said the United States, compared with other developed nations, has been slower to embrace longer-term harm-reduction strategies and to improve access to treatment and care.
COVID likely also has exacerbated the problem in terms of isolation and reduction in quality of life that has adolescents seeking to fill that void with drugs, Dr. Brady said. They may be completely unaware that the drugs they are seeking are commonly cut with counterfeit fentanyl.
“Fentanyl can be up to 50 times stronger than heroin,” he noted. “Even just a little bit of fentanyl dramatically changes the risk profile on an overdose.”
Increasing rates of mental health concerns among adolescents over decades also contribute to drug-seeking trends, Dr. Brady noted.
Overdose increases in the overall population were smaller
In the overall population, the percentage increases were not nearly as large in 2020 and 2021 as they were for adolescents.
Rates of overdose deaths in the overall population increased steadily from 2010 and reached 70,630 in 2019. In 2020, the deaths increased to 91,799 (an increase of 29.48% from 2019) and increased 11.48% in 2021.
The researchers analyzed numbers from the Centers for Disease Control and Prevention WONDER (Wide-Ranging Online Data for Epidemiologic Research) database, which has records of all U.S. deaths for which drug overdose was listed as the underlying cause.
The authors and Dr. Brady report no relevant financial relationships.
The number of overdose deaths in adolescents nearly doubled in 2020 from the year before and increased substantially again in 2021 after nearly a decade of fairly stable rates, according to data published in a JAMA research letter.
Most of the deaths involved fentanyl, the researchers found.
Joseph Friedman, MPH, of the Center for Social Medicine and Humanities at the University of California, Los Angeles, led the study, which analyzed adolescent (14-18 years old) overdose deaths in the United States from 2010 to June 2021 in light of increasing contamination in the supply of illicit drugs.
The researchers found there were 518 deaths among adolescents (2.40 per 100,000 population) in 2010, and the rates remained stable through 2019 with 492 deaths (2.36 per 100,000).
In 2020, however, deaths spiked to 954 (4.57 per 100 000), increasing by 94.3%, compared with 2019. In 2021, they increased another 20%.
The rise in fentanyl-involved deaths was particularly striking. Fentanyl-involved deaths increased from 253 (1.21 per 100,000) in 2019 to 680 (3.26 per 100,000) in 2020. The numbers through June 2021 were annualized for 2021 and calculations predicted 884 deaths (4.23 per 100,000) for the year.
Numbers point to fentanyl potency
In 2021, more than three-fourths (77.14%) of adolescent overdose deaths involved fentanyl, compared with 13.26% for benzodiazepines, 9.77% for methamphetamine, 7.33% for cocaine, 5.76% for prescription opioids, and 2.27% for heroin.
American Indian and Alaska Native adolescents had the highest overdose rate in 2021 (n = 24; 11.79 per 100,000), followed by Latinx adolescents (n = 354; 6.98 per 100,000).
“These adolescent trends fit a wider pattern of increasing racial and ethnic inequalities in overdose that deserve further investigation and intervention efforts,” the authors wrote.
Pandemic’s role unclear
The spikes in adolescent overdoses overlap the COVID-19 pandemic, but Dr. Friedman said in an interview the pandemic “may or may not have been a big factor. “
The authors wrote that drug use had generally been stable among adolescents between 2010 and 2020. The number of 10th graders reporting any illicit drug use was 30.2% in 2010 and 30.4% in 2020.
“So it’s not that more teens are using drugs. It’s just that drug use is becoming more dangerous due to the spread of counterfeit pills containing fentanyls,” Dr. Friedman said.
The authors noted that “the illicit drug supply has increasingly become contaminated with illicitly manufactured fentanyls and other synthetic opioid and benzodiazepine analogues.”
Mr. Friedman said the pandemic may have accelerated the spread of more dangerous forms of drugs as supply chains were disrupted.
Benjamin Brady, DrPH, an assistant professor at the University of Arizona, Tucson, who also has an appointment in the university’s Comprehensive Pain and Addiction Center, said in an interview the numbers that Dr. Friedman and colleagues present represent “worst fears coming true.”
He said he and his colleagues in the field “were anticipating a rise in overdose deaths for the next 5-10 years because of the way the supply-and-demand environment exists in the U.S.”
Dr. Brady explained that restricting access to prescription opioids has had an unfortunate side effect in decreasing access to a safer supply of drugs.
“Without having solutions that would reduce demand at the same rate, supply of the safer form of the drug has been reduced; that has pushed people toward heroin and street drugs and from 2016 on those have been adulterated with fentanyl,” he said.
He said the United States, compared with other developed nations, has been slower to embrace longer-term harm-reduction strategies and to improve access to treatment and care.
COVID likely also has exacerbated the problem in terms of isolation and reduction in quality of life that has adolescents seeking to fill that void with drugs, Dr. Brady said. They may be completely unaware that the drugs they are seeking are commonly cut with counterfeit fentanyl.
“Fentanyl can be up to 50 times stronger than heroin,” he noted. “Even just a little bit of fentanyl dramatically changes the risk profile on an overdose.”
Increasing rates of mental health concerns among adolescents over decades also contribute to drug-seeking trends, Dr. Brady noted.
Overdose increases in the overall population were smaller
In the overall population, the percentage increases were not nearly as large in 2020 and 2021 as they were for adolescents.
Rates of overdose deaths in the overall population increased steadily from 2010 and reached 70,630 in 2019. In 2020, the deaths increased to 91,799 (an increase of 29.48% from 2019) and increased 11.48% in 2021.
The researchers analyzed numbers from the Centers for Disease Control and Prevention WONDER (Wide-Ranging Online Data for Epidemiologic Research) database, which has records of all U.S. deaths for which drug overdose was listed as the underlying cause.
The authors and Dr. Brady report no relevant financial relationships.
The number of overdose deaths in adolescents nearly doubled in 2020 from the year before and increased substantially again in 2021 after nearly a decade of fairly stable rates, according to data published in a JAMA research letter.
Most of the deaths involved fentanyl, the researchers found.
Joseph Friedman, MPH, of the Center for Social Medicine and Humanities at the University of California, Los Angeles, led the study, which analyzed adolescent (14-18 years old) overdose deaths in the United States from 2010 to June 2021 in light of increasing contamination in the supply of illicit drugs.
The researchers found there were 518 deaths among adolescents (2.40 per 100,000 population) in 2010, and the rates remained stable through 2019 with 492 deaths (2.36 per 100,000).
In 2020, however, deaths spiked to 954 (4.57 per 100 000), increasing by 94.3%, compared with 2019. In 2021, they increased another 20%.
The rise in fentanyl-involved deaths was particularly striking. Fentanyl-involved deaths increased from 253 (1.21 per 100,000) in 2019 to 680 (3.26 per 100,000) in 2020. The numbers through June 2021 were annualized for 2021 and calculations predicted 884 deaths (4.23 per 100,000) for the year.
Numbers point to fentanyl potency
In 2021, more than three-fourths (77.14%) of adolescent overdose deaths involved fentanyl, compared with 13.26% for benzodiazepines, 9.77% for methamphetamine, 7.33% for cocaine, 5.76% for prescription opioids, and 2.27% for heroin.
American Indian and Alaska Native adolescents had the highest overdose rate in 2021 (n = 24; 11.79 per 100,000), followed by Latinx adolescents (n = 354; 6.98 per 100,000).
“These adolescent trends fit a wider pattern of increasing racial and ethnic inequalities in overdose that deserve further investigation and intervention efforts,” the authors wrote.
Pandemic’s role unclear
The spikes in adolescent overdoses overlap the COVID-19 pandemic, but Dr. Friedman said in an interview the pandemic “may or may not have been a big factor. “
The authors wrote that drug use had generally been stable among adolescents between 2010 and 2020. The number of 10th graders reporting any illicit drug use was 30.2% in 2010 and 30.4% in 2020.
“So it’s not that more teens are using drugs. It’s just that drug use is becoming more dangerous due to the spread of counterfeit pills containing fentanyls,” Dr. Friedman said.
The authors noted that “the illicit drug supply has increasingly become contaminated with illicitly manufactured fentanyls and other synthetic opioid and benzodiazepine analogues.”
Mr. Friedman said the pandemic may have accelerated the spread of more dangerous forms of drugs as supply chains were disrupted.
Benjamin Brady, DrPH, an assistant professor at the University of Arizona, Tucson, who also has an appointment in the university’s Comprehensive Pain and Addiction Center, said in an interview the numbers that Dr. Friedman and colleagues present represent “worst fears coming true.”
He said he and his colleagues in the field “were anticipating a rise in overdose deaths for the next 5-10 years because of the way the supply-and-demand environment exists in the U.S.”
Dr. Brady explained that restricting access to prescription opioids has had an unfortunate side effect in decreasing access to a safer supply of drugs.
“Without having solutions that would reduce demand at the same rate, supply of the safer form of the drug has been reduced; that has pushed people toward heroin and street drugs and from 2016 on those have been adulterated with fentanyl,” he said.
He said the United States, compared with other developed nations, has been slower to embrace longer-term harm-reduction strategies and to improve access to treatment and care.
COVID likely also has exacerbated the problem in terms of isolation and reduction in quality of life that has adolescents seeking to fill that void with drugs, Dr. Brady said. They may be completely unaware that the drugs they are seeking are commonly cut with counterfeit fentanyl.
“Fentanyl can be up to 50 times stronger than heroin,” he noted. “Even just a little bit of fentanyl dramatically changes the risk profile on an overdose.”
Increasing rates of mental health concerns among adolescents over decades also contribute to drug-seeking trends, Dr. Brady noted.
Overdose increases in the overall population were smaller
In the overall population, the percentage increases were not nearly as large in 2020 and 2021 as they were for adolescents.
Rates of overdose deaths in the overall population increased steadily from 2010 and reached 70,630 in 2019. In 2020, the deaths increased to 91,799 (an increase of 29.48% from 2019) and increased 11.48% in 2021.
The researchers analyzed numbers from the Centers for Disease Control and Prevention WONDER (Wide-Ranging Online Data for Epidemiologic Research) database, which has records of all U.S. deaths for which drug overdose was listed as the underlying cause.
The authors and Dr. Brady report no relevant financial relationships.
FROM JAMA
Postpartum HCV treatment rare in infected mothers with opioid use disorder
Despite the availability of effective direct-acting antivirals, very few a mothers with opioid use disorder (OUD) and hepatitis C virus (HCV) during pregnancy received follow-up care or treatment for the infection within 6 months of giving birth, a retrospective study of Medicaid maternity patients found.
The study pooled data on 23,780 Medicaid-enrolled pregnant women with OUD who had a live or stillbirth during 2016-2019 and were followed for 6 months after delivery. Among these women – drawn from six states in the Medicaid Outcomes Distributed Research Network – the pooled average probability of HCV testing during pregnancy was 70.3% (95% confidence interval, 61.5%-79.1%). Of these, 30.9% (95% CI, 23.8%-38%) tested positive. At 60 days postpartum, just 3.2% (95% CI, 2.6%-3.8%) had a follow-up visit or treatment for HCV. In a subset of patients followed for 6 months, only 5.9% (95% CI, 4.9%-6.9%) had any HCV follow-up visit or medication within 6 months of delivery.
While HCV screening and diagnosis rates varied across states, postpartum follow-up rates were universally low. The results suggest a need to improve the cascade of postpartum care for HCV and, ultimately perhaps, introduce antenatal HCV treatment, as is currently given safely for HIV, if current clinical research establishes safety, according to Marian P. Jarlenski, PhD, MPH, an associate professor of public health policy and management at the University of Pittsburgh. The study was published in Obstetrics & Gynecology.
HCV infection has risen substantially in people of reproductive age in tandem with an increase in OUDs. HCV is transmitted from an infected mother to her baby in about 6% of cases, according to the Centers for Disease Control and Prevention, which in 2020 expanded its HCV screening recommendations to include all pregnant women. Currently no treatment for HCV during pregnancy has been approved.
In light of those recent recommendations, Dr. Jarlenski said in an interview that her group was “interested in looking at high-risk screened people and estimating what proportion received follow-up care and treatment for HCV. What is the promise of screening? The promise is that you can treat. Otherwise why screen?”
She acknowledged, however, that the postpartum period is a challenging time for a mother to seek health information or care for herself, whether she’s a new parent or has other children in the home. Nevertheless, the low rate of follow-up and treatment was unexpected. “Even the 70% rate of screening was low – we felt it should have been closer to 100% – but the follow-up rate was surprisingly low,” Dr. Jarlenski said.
Mishka Terplan, MD, MPH, medical director of Friends Research Institute in Baltimore, was not surprised at the low follow-up rate. “The cascade of care for hep C is demoralizing,” said Dr. Terplan, who was not involved in the study. “We know that hep C is syndemic with OUD and other opioid crises and we know that screening is effective for identifying hep C and that antiviral medications are now more effective and less toxic than ever before. But despite this, we’re failing pregnant women and their kids at every step along the cascade. We do a better job with initial testing than with the follow-up testing. We do a horrible job with postpartum medication initiation.”
He pointed to the systemic challenges mothers face in getting postpartum HCV care. “They may be transferred to a subspecialist for treatment, and this transfer is compounded by issues of insurance coverage and eligibility.” With the onus on new mothers to submit the paperwork, “the idea that mothers would be able to initiate much less continue postpartum treatment is absurd,” Dr. Terplan said.
He added that the children born to HCV-positive mothers need surveillance as well, but data suggest that the rates of newborn testing are also low. “There’s a preventable public health burden in all of this.”
The obvious way to increase eradicative therapy would be to treat women while they are getting antenatal care. A small phase 1 trial found that all pregnant participants who were HCV positive and given antivirals in their second trimester were safely treated and gave birth to healthy babies.
“If larger trials prove this treatment is safe and effective, then these results should be communicated to care providers and pregnant patients,” Dr. Jarlenski said. Otherwise, the public health potential of universal screening in pregnancy will not be realized.
This research was supported by the National Institute of Drug Abuse and by the Delaware Division of Medicaid and Medical Assistance and the University of Delaware, Center for Community Research & Service. Dr. Jarlenski disclosed no competing interests. One coauthor disclosed grant funding through her institution from Gilead Sciences and Organon unrelated to this work. Dr. Terplan reported no relevant competing interests.
Despite the availability of effective direct-acting antivirals, very few a mothers with opioid use disorder (OUD) and hepatitis C virus (HCV) during pregnancy received follow-up care or treatment for the infection within 6 months of giving birth, a retrospective study of Medicaid maternity patients found.
The study pooled data on 23,780 Medicaid-enrolled pregnant women with OUD who had a live or stillbirth during 2016-2019 and were followed for 6 months after delivery. Among these women – drawn from six states in the Medicaid Outcomes Distributed Research Network – the pooled average probability of HCV testing during pregnancy was 70.3% (95% confidence interval, 61.5%-79.1%). Of these, 30.9% (95% CI, 23.8%-38%) tested positive. At 60 days postpartum, just 3.2% (95% CI, 2.6%-3.8%) had a follow-up visit or treatment for HCV. In a subset of patients followed for 6 months, only 5.9% (95% CI, 4.9%-6.9%) had any HCV follow-up visit or medication within 6 months of delivery.
While HCV screening and diagnosis rates varied across states, postpartum follow-up rates were universally low. The results suggest a need to improve the cascade of postpartum care for HCV and, ultimately perhaps, introduce antenatal HCV treatment, as is currently given safely for HIV, if current clinical research establishes safety, according to Marian P. Jarlenski, PhD, MPH, an associate professor of public health policy and management at the University of Pittsburgh. The study was published in Obstetrics & Gynecology.
HCV infection has risen substantially in people of reproductive age in tandem with an increase in OUDs. HCV is transmitted from an infected mother to her baby in about 6% of cases, according to the Centers for Disease Control and Prevention, which in 2020 expanded its HCV screening recommendations to include all pregnant women. Currently no treatment for HCV during pregnancy has been approved.
In light of those recent recommendations, Dr. Jarlenski said in an interview that her group was “interested in looking at high-risk screened people and estimating what proportion received follow-up care and treatment for HCV. What is the promise of screening? The promise is that you can treat. Otherwise why screen?”
She acknowledged, however, that the postpartum period is a challenging time for a mother to seek health information or care for herself, whether she’s a new parent or has other children in the home. Nevertheless, the low rate of follow-up and treatment was unexpected. “Even the 70% rate of screening was low – we felt it should have been closer to 100% – but the follow-up rate was surprisingly low,” Dr. Jarlenski said.
Mishka Terplan, MD, MPH, medical director of Friends Research Institute in Baltimore, was not surprised at the low follow-up rate. “The cascade of care for hep C is demoralizing,” said Dr. Terplan, who was not involved in the study. “We know that hep C is syndemic with OUD and other opioid crises and we know that screening is effective for identifying hep C and that antiviral medications are now more effective and less toxic than ever before. But despite this, we’re failing pregnant women and their kids at every step along the cascade. We do a better job with initial testing than with the follow-up testing. We do a horrible job with postpartum medication initiation.”
He pointed to the systemic challenges mothers face in getting postpartum HCV care. “They may be transferred to a subspecialist for treatment, and this transfer is compounded by issues of insurance coverage and eligibility.” With the onus on new mothers to submit the paperwork, “the idea that mothers would be able to initiate much less continue postpartum treatment is absurd,” Dr. Terplan said.
He added that the children born to HCV-positive mothers need surveillance as well, but data suggest that the rates of newborn testing are also low. “There’s a preventable public health burden in all of this.”
The obvious way to increase eradicative therapy would be to treat women while they are getting antenatal care. A small phase 1 trial found that all pregnant participants who were HCV positive and given antivirals in their second trimester were safely treated and gave birth to healthy babies.
“If larger trials prove this treatment is safe and effective, then these results should be communicated to care providers and pregnant patients,” Dr. Jarlenski said. Otherwise, the public health potential of universal screening in pregnancy will not be realized.
This research was supported by the National Institute of Drug Abuse and by the Delaware Division of Medicaid and Medical Assistance and the University of Delaware, Center for Community Research & Service. Dr. Jarlenski disclosed no competing interests. One coauthor disclosed grant funding through her institution from Gilead Sciences and Organon unrelated to this work. Dr. Terplan reported no relevant competing interests.
Despite the availability of effective direct-acting antivirals, very few a mothers with opioid use disorder (OUD) and hepatitis C virus (HCV) during pregnancy received follow-up care or treatment for the infection within 6 months of giving birth, a retrospective study of Medicaid maternity patients found.
The study pooled data on 23,780 Medicaid-enrolled pregnant women with OUD who had a live or stillbirth during 2016-2019 and were followed for 6 months after delivery. Among these women – drawn from six states in the Medicaid Outcomes Distributed Research Network – the pooled average probability of HCV testing during pregnancy was 70.3% (95% confidence interval, 61.5%-79.1%). Of these, 30.9% (95% CI, 23.8%-38%) tested positive. At 60 days postpartum, just 3.2% (95% CI, 2.6%-3.8%) had a follow-up visit or treatment for HCV. In a subset of patients followed for 6 months, only 5.9% (95% CI, 4.9%-6.9%) had any HCV follow-up visit or medication within 6 months of delivery.
While HCV screening and diagnosis rates varied across states, postpartum follow-up rates were universally low. The results suggest a need to improve the cascade of postpartum care for HCV and, ultimately perhaps, introduce antenatal HCV treatment, as is currently given safely for HIV, if current clinical research establishes safety, according to Marian P. Jarlenski, PhD, MPH, an associate professor of public health policy and management at the University of Pittsburgh. The study was published in Obstetrics & Gynecology.
HCV infection has risen substantially in people of reproductive age in tandem with an increase in OUDs. HCV is transmitted from an infected mother to her baby in about 6% of cases, according to the Centers for Disease Control and Prevention, which in 2020 expanded its HCV screening recommendations to include all pregnant women. Currently no treatment for HCV during pregnancy has been approved.
In light of those recent recommendations, Dr. Jarlenski said in an interview that her group was “interested in looking at high-risk screened people and estimating what proportion received follow-up care and treatment for HCV. What is the promise of screening? The promise is that you can treat. Otherwise why screen?”
She acknowledged, however, that the postpartum period is a challenging time for a mother to seek health information or care for herself, whether she’s a new parent or has other children in the home. Nevertheless, the low rate of follow-up and treatment was unexpected. “Even the 70% rate of screening was low – we felt it should have been closer to 100% – but the follow-up rate was surprisingly low,” Dr. Jarlenski said.
Mishka Terplan, MD, MPH, medical director of Friends Research Institute in Baltimore, was not surprised at the low follow-up rate. “The cascade of care for hep C is demoralizing,” said Dr. Terplan, who was not involved in the study. “We know that hep C is syndemic with OUD and other opioid crises and we know that screening is effective for identifying hep C and that antiviral medications are now more effective and less toxic than ever before. But despite this, we’re failing pregnant women and their kids at every step along the cascade. We do a better job with initial testing than with the follow-up testing. We do a horrible job with postpartum medication initiation.”
He pointed to the systemic challenges mothers face in getting postpartum HCV care. “They may be transferred to a subspecialist for treatment, and this transfer is compounded by issues of insurance coverage and eligibility.” With the onus on new mothers to submit the paperwork, “the idea that mothers would be able to initiate much less continue postpartum treatment is absurd,” Dr. Terplan said.
He added that the children born to HCV-positive mothers need surveillance as well, but data suggest that the rates of newborn testing are also low. “There’s a preventable public health burden in all of this.”
The obvious way to increase eradicative therapy would be to treat women while they are getting antenatal care. A small phase 1 trial found that all pregnant participants who were HCV positive and given antivirals in their second trimester were safely treated and gave birth to healthy babies.
“If larger trials prove this treatment is safe and effective, then these results should be communicated to care providers and pregnant patients,” Dr. Jarlenski said. Otherwise, the public health potential of universal screening in pregnancy will not be realized.
This research was supported by the National Institute of Drug Abuse and by the Delaware Division of Medicaid and Medical Assistance and the University of Delaware, Center for Community Research & Service. Dr. Jarlenski disclosed no competing interests. One coauthor disclosed grant funding through her institution from Gilead Sciences and Organon unrelated to this work. Dr. Terplan reported no relevant competing interests.
FROM OBSTETRICS & GYNECOLOGY
ADHD link to prenatal opioid exposure shifts with other substances
Children prenatally exposed to opioids alone have an increased risk of attention-deficit/hyperactivity disorder (ADHD), but interactions between opioids and both cannabis use and alcohol use were linked to varying levels of ADHD risk as well, according to findings published March 11 in JAMA Network Open.
While many prenatal exposure studies examine associations with one substance, the results of this case-control study “suggest that it is important to consider prenatal exposure to multiple substances and the interactions between these substances when counseling women regarding substance use during pregnancy,” wrote Henri M. Garrison-Desany of the Johns Hopkins University, Baltimore, and colleagues.
Using data from children in the prospective Boston Birth Cohort between 1998 and 2019, the researchers did a secondary analysis on the 3,138 children (50.4% of whom were male) with at least 2 years of follow-up, excluding children from multiple-gestation pregnancies, in vitro fertilization pregnancies, and deliveries involving major maternal trauma or major chromosomal anomalies. Mothers answered a questionnaire within 24-72 hours of delivery regarding their demographics, substance use, pregnancy history, and health status. Among the mothers, 58.6% were Black, 22.3% were Hispanic, 7.2% were White, 1.5% were Asian, and 10.4% were other races/ethnicities.
The children’s electronic medical records were used to identify those with ADHD diagnoses. The researchers did not assess prescription opioid exposure during pregnancy, but they based opioid exposure on mothers’ reports of recreationally using heroin or oxycodone, mothers’ reports of receiving methadone treatment, or a newborn diagnosis of neonatal abstinence syndrome or neonatal opioid withdrawal syndrome.
Just under a quarter of the women (24.2%) reported using at least one substance during pregnancy. After tobacco smoking (18.5%), the next most reported substances were alcohol (8.1%), cannabis (3.9%), and opioids (1.9%). With a median 12 years of follow-up, 15.5% of the children had been diagnosed with ADHD, most of whom (71.6%) were male.
Before considering interaction of different substances, children exposed to opioids had a little over twice the risk of ADHD (hazard ratio [HR], 2.19) compared to those with no prenatal substance exposure. Although neither cannabis nor alcohol was independently associated with ADHD, smoking had a 40% increased risk, and researchers found a 21% increase in risk of ADHD with each additional substance mothers used during pregnancy. The researchers had adjusted these findings for maternal age, race/ethnicity, marital status, educational level, annual household income, parity, number of perinatal visits, and general stress during pregnancy, based on a structured interview.
When the researchers considered all the substances together, opioid exposure increased risk of ADHD by 60% (HR, 1.6), opioids with cannabis increased risk by 42%, opioids with alcohol increased risk by 15%, and opioids with smoking increased risk by 17%.
”Our findings suggest opioids may interact with other substances (including cannabis), which may be particularly deleterious,” the researchers reported. “It is not clear whether this interaction is owing to biological or environmental factors, such as whether individuals with illicit polysubstance use are more likely to use more substances or whether they have other characteristics that may impact child development.”
The authors noted that cannabis exposure has been linked to other neurodevelopmental outcomes, including reduced executive and motor function in infants. ”Notably, although we did not find a significant independent association between cannabis exposure and ADHD, children exposed to both cannabis and opioids had a 23% greater risk than expected from either exposure individually,” they reported.
The researchers suggest that their findings provide data for considering harm reduction approaches that reduce use of any single substance during pregnancy. “Focusing on the most obviously harmful exposures may be a useful way to reduce the risk of ADHD,” they wrote. “Further work is needed to directly investigate this hypothesis and examine whether reduction in the use of any substance among those with polysubstance use could be acceptable compared with abstinence.”
In an invited commentary, Angela Lupattelli, PhD, and Nhung T. H. Trinh, PhD, both of the department of pharmacy at the University of Oslo, noted the methodological challenges of assessing exposures and associations from multiple different substances during pregnancy.
“First, how can we disentangle the consequences of individual and/or combined substance exposures during pregnancy from the underlying risks?” they asked. In addition to differences in baseline characteristic between those who use opioids or cannabis, Dr. Lupattelli and Dr. Trinh noted that other important unmeasured factors, such as genetics and family environment, may confound the effect size estimates for ADHD.
They also noted the need to consider intensity, dose, duration, and timing of substance use during pregnancy.
“Understanding the longer-term safety of substance use during pregnancy is paramount to inform prevention policy and shape counseling strategies. Observational studies, despite their limitations, are a necessary piece of the puzzle,” they wrote. “However, the study findings should be interpreted with caution, as the use of advanced analytical methods cannot overcome the unavailability of some important confounding factors and exposure information.”
The research was funded by the U.S. Department of Health and Human Services and the National Institutes of Health. The authors had no industry-related disclosures.
Children prenatally exposed to opioids alone have an increased risk of attention-deficit/hyperactivity disorder (ADHD), but interactions between opioids and both cannabis use and alcohol use were linked to varying levels of ADHD risk as well, according to findings published March 11 in JAMA Network Open.
While many prenatal exposure studies examine associations with one substance, the results of this case-control study “suggest that it is important to consider prenatal exposure to multiple substances and the interactions between these substances when counseling women regarding substance use during pregnancy,” wrote Henri M. Garrison-Desany of the Johns Hopkins University, Baltimore, and colleagues.
Using data from children in the prospective Boston Birth Cohort between 1998 and 2019, the researchers did a secondary analysis on the 3,138 children (50.4% of whom were male) with at least 2 years of follow-up, excluding children from multiple-gestation pregnancies, in vitro fertilization pregnancies, and deliveries involving major maternal trauma or major chromosomal anomalies. Mothers answered a questionnaire within 24-72 hours of delivery regarding their demographics, substance use, pregnancy history, and health status. Among the mothers, 58.6% were Black, 22.3% were Hispanic, 7.2% were White, 1.5% were Asian, and 10.4% were other races/ethnicities.
The children’s electronic medical records were used to identify those with ADHD diagnoses. The researchers did not assess prescription opioid exposure during pregnancy, but they based opioid exposure on mothers’ reports of recreationally using heroin or oxycodone, mothers’ reports of receiving methadone treatment, or a newborn diagnosis of neonatal abstinence syndrome or neonatal opioid withdrawal syndrome.
Just under a quarter of the women (24.2%) reported using at least one substance during pregnancy. After tobacco smoking (18.5%), the next most reported substances were alcohol (8.1%), cannabis (3.9%), and opioids (1.9%). With a median 12 years of follow-up, 15.5% of the children had been diagnosed with ADHD, most of whom (71.6%) were male.
Before considering interaction of different substances, children exposed to opioids had a little over twice the risk of ADHD (hazard ratio [HR], 2.19) compared to those with no prenatal substance exposure. Although neither cannabis nor alcohol was independently associated with ADHD, smoking had a 40% increased risk, and researchers found a 21% increase in risk of ADHD with each additional substance mothers used during pregnancy. The researchers had adjusted these findings for maternal age, race/ethnicity, marital status, educational level, annual household income, parity, number of perinatal visits, and general stress during pregnancy, based on a structured interview.
When the researchers considered all the substances together, opioid exposure increased risk of ADHD by 60% (HR, 1.6), opioids with cannabis increased risk by 42%, opioids with alcohol increased risk by 15%, and opioids with smoking increased risk by 17%.
”Our findings suggest opioids may interact with other substances (including cannabis), which may be particularly deleterious,” the researchers reported. “It is not clear whether this interaction is owing to biological or environmental factors, such as whether individuals with illicit polysubstance use are more likely to use more substances or whether they have other characteristics that may impact child development.”
The authors noted that cannabis exposure has been linked to other neurodevelopmental outcomes, including reduced executive and motor function in infants. ”Notably, although we did not find a significant independent association between cannabis exposure and ADHD, children exposed to both cannabis and opioids had a 23% greater risk than expected from either exposure individually,” they reported.
The researchers suggest that their findings provide data for considering harm reduction approaches that reduce use of any single substance during pregnancy. “Focusing on the most obviously harmful exposures may be a useful way to reduce the risk of ADHD,” they wrote. “Further work is needed to directly investigate this hypothesis and examine whether reduction in the use of any substance among those with polysubstance use could be acceptable compared with abstinence.”
In an invited commentary, Angela Lupattelli, PhD, and Nhung T. H. Trinh, PhD, both of the department of pharmacy at the University of Oslo, noted the methodological challenges of assessing exposures and associations from multiple different substances during pregnancy.
“First, how can we disentangle the consequences of individual and/or combined substance exposures during pregnancy from the underlying risks?” they asked. In addition to differences in baseline characteristic between those who use opioids or cannabis, Dr. Lupattelli and Dr. Trinh noted that other important unmeasured factors, such as genetics and family environment, may confound the effect size estimates for ADHD.
They also noted the need to consider intensity, dose, duration, and timing of substance use during pregnancy.
“Understanding the longer-term safety of substance use during pregnancy is paramount to inform prevention policy and shape counseling strategies. Observational studies, despite their limitations, are a necessary piece of the puzzle,” they wrote. “However, the study findings should be interpreted with caution, as the use of advanced analytical methods cannot overcome the unavailability of some important confounding factors and exposure information.”
The research was funded by the U.S. Department of Health and Human Services and the National Institutes of Health. The authors had no industry-related disclosures.
Children prenatally exposed to opioids alone have an increased risk of attention-deficit/hyperactivity disorder (ADHD), but interactions between opioids and both cannabis use and alcohol use were linked to varying levels of ADHD risk as well, according to findings published March 11 in JAMA Network Open.
While many prenatal exposure studies examine associations with one substance, the results of this case-control study “suggest that it is important to consider prenatal exposure to multiple substances and the interactions between these substances when counseling women regarding substance use during pregnancy,” wrote Henri M. Garrison-Desany of the Johns Hopkins University, Baltimore, and colleagues.
Using data from children in the prospective Boston Birth Cohort between 1998 and 2019, the researchers did a secondary analysis on the 3,138 children (50.4% of whom were male) with at least 2 years of follow-up, excluding children from multiple-gestation pregnancies, in vitro fertilization pregnancies, and deliveries involving major maternal trauma or major chromosomal anomalies. Mothers answered a questionnaire within 24-72 hours of delivery regarding their demographics, substance use, pregnancy history, and health status. Among the mothers, 58.6% were Black, 22.3% were Hispanic, 7.2% were White, 1.5% were Asian, and 10.4% were other races/ethnicities.
The children’s electronic medical records were used to identify those with ADHD diagnoses. The researchers did not assess prescription opioid exposure during pregnancy, but they based opioid exposure on mothers’ reports of recreationally using heroin or oxycodone, mothers’ reports of receiving methadone treatment, or a newborn diagnosis of neonatal abstinence syndrome or neonatal opioid withdrawal syndrome.
Just under a quarter of the women (24.2%) reported using at least one substance during pregnancy. After tobacco smoking (18.5%), the next most reported substances were alcohol (8.1%), cannabis (3.9%), and opioids (1.9%). With a median 12 years of follow-up, 15.5% of the children had been diagnosed with ADHD, most of whom (71.6%) were male.
Before considering interaction of different substances, children exposed to opioids had a little over twice the risk of ADHD (hazard ratio [HR], 2.19) compared to those with no prenatal substance exposure. Although neither cannabis nor alcohol was independently associated with ADHD, smoking had a 40% increased risk, and researchers found a 21% increase in risk of ADHD with each additional substance mothers used during pregnancy. The researchers had adjusted these findings for maternal age, race/ethnicity, marital status, educational level, annual household income, parity, number of perinatal visits, and general stress during pregnancy, based on a structured interview.
When the researchers considered all the substances together, opioid exposure increased risk of ADHD by 60% (HR, 1.6), opioids with cannabis increased risk by 42%, opioids with alcohol increased risk by 15%, and opioids with smoking increased risk by 17%.
”Our findings suggest opioids may interact with other substances (including cannabis), which may be particularly deleterious,” the researchers reported. “It is not clear whether this interaction is owing to biological or environmental factors, such as whether individuals with illicit polysubstance use are more likely to use more substances or whether they have other characteristics that may impact child development.”
The authors noted that cannabis exposure has been linked to other neurodevelopmental outcomes, including reduced executive and motor function in infants. ”Notably, although we did not find a significant independent association between cannabis exposure and ADHD, children exposed to both cannabis and opioids had a 23% greater risk than expected from either exposure individually,” they reported.
The researchers suggest that their findings provide data for considering harm reduction approaches that reduce use of any single substance during pregnancy. “Focusing on the most obviously harmful exposures may be a useful way to reduce the risk of ADHD,” they wrote. “Further work is needed to directly investigate this hypothesis and examine whether reduction in the use of any substance among those with polysubstance use could be acceptable compared with abstinence.”
In an invited commentary, Angela Lupattelli, PhD, and Nhung T. H. Trinh, PhD, both of the department of pharmacy at the University of Oslo, noted the methodological challenges of assessing exposures and associations from multiple different substances during pregnancy.
“First, how can we disentangle the consequences of individual and/or combined substance exposures during pregnancy from the underlying risks?” they asked. In addition to differences in baseline characteristic between those who use opioids or cannabis, Dr. Lupattelli and Dr. Trinh noted that other important unmeasured factors, such as genetics and family environment, may confound the effect size estimates for ADHD.
They also noted the need to consider intensity, dose, duration, and timing of substance use during pregnancy.
“Understanding the longer-term safety of substance use during pregnancy is paramount to inform prevention policy and shape counseling strategies. Observational studies, despite their limitations, are a necessary piece of the puzzle,” they wrote. “However, the study findings should be interpreted with caution, as the use of advanced analytical methods cannot overcome the unavailability of some important confounding factors and exposure information.”
The research was funded by the U.S. Department of Health and Human Services and the National Institutes of Health. The authors had no industry-related disclosures.
FROM JAMA NETWORK OPEN












