User login
Volar Plate Capsulodesis for Metacarpophalangeal Hyperextension With Basal Joint Arthritis
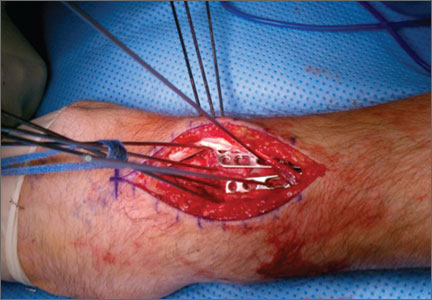
En Bloc Joystick Reduction of a Comminuted Intra-articular Distal Radius Fracture: A Technical Trick
A Perspective on the Evolution of Distal Radius Fracture Treatment
The treatment for distal radius fractures has changed significantly over time. Initially, distal radius fractures were treated as relatively innocuous injuries that befell the elderly and the comparatively inactive, and casts were the mainstay of treatment. However, closer scrutiny of the clinical results revealed a myriad of problems with these treatments, including “cast disease,” stiffness, inability to hold skeletal position, and soft-tissue compromise that affected the overall function of the wrist and hand.
Additional techniques to improve results included the “pins and plaster” technique, with the introduction of 2 pins in the radius and metacarpals to retard collapse of the fracture while in the cast. This was in some sense an early version of external fixation, with pins giving support to the unstable wrist and the body of the cast serving as the external support. There was further evolution of the adaptation of early versions of external fixation used for the lower extremity towards the treatment of the distal radius. For example, when I was a resident at Massachusetts General Hospital, we routinely applied femoral distractors as external fixation devices for selected distal radius fractures. This was a time when more specific anatomic devices and implants were not yet available.
External fixation evolved,1 and distal radius–specific systems, with enhanced ability to adjust and achieve reduction, became available in the late 1980s. At the same time, distal radius fracture plating evolved from simple “stamped metal” plates with screws that merely fit in the screw holes, to more highly engineered implants with screws that engaged the plate at a fixed angle, much like the blade plate
technology used for lower extremity fractures.2 Over time, the volar fixed-angle plating system supplanted the other treatments and emerged as a popular treatment method.
Use of Kirschner wires or simple pins has been promoted in the past for treatment of distal radius fractures. In France, Kapandji3 described the use of “intra-focal
pinning.” In this technique, smooth Kirschner wires are introduced in the fracture site itself, and then using leverage so that the pins act like “crowbars,” the distal fragment that is malpositioned becomes adjusted into a more anatomic position.3 Kapandji’s treatment can be very effective in achieving reduction; however, as there is no fixation into the distal fragment, this technique has limitations in maintaining the reduction until healing has occurred. Interfragmentary pinning from the dorsal radial and dorsal ulnar aspects were nicely described by Clancey.4 I have found great utility in combining the Kapandji intra-focal techniques to achieve reduction with Clancey pin fixation or distal radius plating to maintain reduction.
I was intrigued with the article by Drs. Siegall and Ziran, “En Bloc Joystick Reduction of a Comminuted Intraarticular Distal Radius Fracture: A Technical Trick,” in this month’s issue of The American Journal of Orthopedics. In their technique, the authors introduced a series of parallel pins or screws below the articular surface from radius to ulna in parallel fashion to provide provisional fixation for the intra-articular components of their complex fracture. Once having done so, they felt more secure in manipulating the distal radius component en bloc; in fact, they used strapping to provide distal traction on the external protruding portion of the pins to help achieve and maintain reduction for their definitive fixation. Drs. Siegall and Ziran describe the use of either Kirschner wires or plating to provide definitive fixation. In the example cited, they performed (via an open method) both the scaffolding and plating without the need of an assistant to hold or maintain the reduction during the osteosynthesis. I can envision adapting the technique they describe to percutaneous treatments for placement of the scaffolding pins, and even the Kapandji/Clancey pins under fluoroscopic guidance or arthroscopeassisted placement.
Despite the popularity and utility of volar fixed-angle plating techniques to treat distal radius fractures, there remain certain situations in which these techniques are faced with challenges. Certainly one of them is the more complex intra-articular fracture with multiple components, or in the very distal fracture patterns in which there is limited bone for the surgeon to use in providing distal screw fixation in the plating systems. Additionally, the nascent malunion presents some challenges as well in terms of performing a “takedown” of the partially healed fracture without destroying the soft, partially healed distal bone that contains the all-important articular component. These are the instances where supplemental techniques such as the one described by Drs. Siegall and Ziran, as well as the
Kapandji and Clancey techniques, have their greatest utility and appeal. Despite one’s wishes and best efforts, some distal radius fractures are not easily reconstructable. In these cases, use of external fixation or temporary arthrodesis
dorsal plating with subsequent plate removal5,6 can be the best reconstructive option and a great “bailout.” The prepared surgeon should have these supplemental techniques in their armamentarium to be able to adapt to the conditions that present themselves in the operating room and to do the best job they can for the patient.
References
1. Agee JM. External fixation. Technical advances based upon multiplanar
ligamentotaxis. Orthop Clin North Am. 1993;24(2):265-274.
2. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable
distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
3. Kapandji A. Internal fixation by double intrafocal plate. Functional treatment
of non articular fractures of the lower end of the radius (author’s transl) [in French]. Ann Chir. 1976;30(11-12):903-908.
4. Clancey GJ. Percutaneous Kirschner-wire fixation of Colles fractures. A prospective study of thirty cases. J Bone Joint Surg Am. 1984;66(7):1008-1014.
5. Burke EF, Singer RM. Treatment of comminuted distal radius with the use of an internal distraction plate. Tech Hand Up Extrem Surg. 1998;2(4):248-252.
6. Ruch DS, Ginn TA, Yang CC, Smith BP, Rushing J, Hanel DP. Use of a distraction plate for distal radial fractures with metaphyseal and diaphyseal comminution. J Bone Joint Surg Am. 2005;87(5):945-954.
The treatment for distal radius fractures has changed significantly over time. Initially, distal radius fractures were treated as relatively innocuous injuries that befell the elderly and the comparatively inactive, and casts were the mainstay of treatment. However, closer scrutiny of the clinical results revealed a myriad of problems with these treatments, including “cast disease,” stiffness, inability to hold skeletal position, and soft-tissue compromise that affected the overall function of the wrist and hand.
Additional techniques to improve results included the “pins and plaster” technique, with the introduction of 2 pins in the radius and metacarpals to retard collapse of the fracture while in the cast. This was in some sense an early version of external fixation, with pins giving support to the unstable wrist and the body of the cast serving as the external support. There was further evolution of the adaptation of early versions of external fixation used for the lower extremity towards the treatment of the distal radius. For example, when I was a resident at Massachusetts General Hospital, we routinely applied femoral distractors as external fixation devices for selected distal radius fractures. This was a time when more specific anatomic devices and implants were not yet available.
External fixation evolved,1 and distal radius–specific systems, with enhanced ability to adjust and achieve reduction, became available in the late 1980s. At the same time, distal radius fracture plating evolved from simple “stamped metal” plates with screws that merely fit in the screw holes, to more highly engineered implants with screws that engaged the plate at a fixed angle, much like the blade plate
technology used for lower extremity fractures.2 Over time, the volar fixed-angle plating system supplanted the other treatments and emerged as a popular treatment method.
Use of Kirschner wires or simple pins has been promoted in the past for treatment of distal radius fractures. In France, Kapandji3 described the use of “intra-focal
pinning.” In this technique, smooth Kirschner wires are introduced in the fracture site itself, and then using leverage so that the pins act like “crowbars,” the distal fragment that is malpositioned becomes adjusted into a more anatomic position.3 Kapandji’s treatment can be very effective in achieving reduction; however, as there is no fixation into the distal fragment, this technique has limitations in maintaining the reduction until healing has occurred. Interfragmentary pinning from the dorsal radial and dorsal ulnar aspects were nicely described by Clancey.4 I have found great utility in combining the Kapandji intra-focal techniques to achieve reduction with Clancey pin fixation or distal radius plating to maintain reduction.
I was intrigued with the article by Drs. Siegall and Ziran, “En Bloc Joystick Reduction of a Comminuted Intraarticular Distal Radius Fracture: A Technical Trick,” in this month’s issue of The American Journal of Orthopedics. In their technique, the authors introduced a series of parallel pins or screws below the articular surface from radius to ulna in parallel fashion to provide provisional fixation for the intra-articular components of their complex fracture. Once having done so, they felt more secure in manipulating the distal radius component en bloc; in fact, they used strapping to provide distal traction on the external protruding portion of the pins to help achieve and maintain reduction for their definitive fixation. Drs. Siegall and Ziran describe the use of either Kirschner wires or plating to provide definitive fixation. In the example cited, they performed (via an open method) both the scaffolding and plating without the need of an assistant to hold or maintain the reduction during the osteosynthesis. I can envision adapting the technique they describe to percutaneous treatments for placement of the scaffolding pins, and even the Kapandji/Clancey pins under fluoroscopic guidance or arthroscopeassisted placement.
Despite the popularity and utility of volar fixed-angle plating techniques to treat distal radius fractures, there remain certain situations in which these techniques are faced with challenges. Certainly one of them is the more complex intra-articular fracture with multiple components, or in the very distal fracture patterns in which there is limited bone for the surgeon to use in providing distal screw fixation in the plating systems. Additionally, the nascent malunion presents some challenges as well in terms of performing a “takedown” of the partially healed fracture without destroying the soft, partially healed distal bone that contains the all-important articular component. These are the instances where supplemental techniques such as the one described by Drs. Siegall and Ziran, as well as the
Kapandji and Clancey techniques, have their greatest utility and appeal. Despite one’s wishes and best efforts, some distal radius fractures are not easily reconstructable. In these cases, use of external fixation or temporary arthrodesis
dorsal plating with subsequent plate removal5,6 can be the best reconstructive option and a great “bailout.” The prepared surgeon should have these supplemental techniques in their armamentarium to be able to adapt to the conditions that present themselves in the operating room and to do the best job they can for the patient.
References
1. Agee JM. External fixation. Technical advances based upon multiplanar
ligamentotaxis. Orthop Clin North Am. 1993;24(2):265-274.
2. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable
distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
3. Kapandji A. Internal fixation by double intrafocal plate. Functional treatment
of non articular fractures of the lower end of the radius (author’s transl) [in French]. Ann Chir. 1976;30(11-12):903-908.
4. Clancey GJ. Percutaneous Kirschner-wire fixation of Colles fractures. A prospective study of thirty cases. J Bone Joint Surg Am. 1984;66(7):1008-1014.
5. Burke EF, Singer RM. Treatment of comminuted distal radius with the use of an internal distraction plate. Tech Hand Up Extrem Surg. 1998;2(4):248-252.
6. Ruch DS, Ginn TA, Yang CC, Smith BP, Rushing J, Hanel DP. Use of a distraction plate for distal radial fractures with metaphyseal and diaphyseal comminution. J Bone Joint Surg Am. 2005;87(5):945-954.
The treatment for distal radius fractures has changed significantly over time. Initially, distal radius fractures were treated as relatively innocuous injuries that befell the elderly and the comparatively inactive, and casts were the mainstay of treatment. However, closer scrutiny of the clinical results revealed a myriad of problems with these treatments, including “cast disease,” stiffness, inability to hold skeletal position, and soft-tissue compromise that affected the overall function of the wrist and hand.
Additional techniques to improve results included the “pins and plaster” technique, with the introduction of 2 pins in the radius and metacarpals to retard collapse of the fracture while in the cast. This was in some sense an early version of external fixation, with pins giving support to the unstable wrist and the body of the cast serving as the external support. There was further evolution of the adaptation of early versions of external fixation used for the lower extremity towards the treatment of the distal radius. For example, when I was a resident at Massachusetts General Hospital, we routinely applied femoral distractors as external fixation devices for selected distal radius fractures. This was a time when more specific anatomic devices and implants were not yet available.
External fixation evolved,1 and distal radius–specific systems, with enhanced ability to adjust and achieve reduction, became available in the late 1980s. At the same time, distal radius fracture plating evolved from simple “stamped metal” plates with screws that merely fit in the screw holes, to more highly engineered implants with screws that engaged the plate at a fixed angle, much like the blade plate
technology used for lower extremity fractures.2 Over time, the volar fixed-angle plating system supplanted the other treatments and emerged as a popular treatment method.
Use of Kirschner wires or simple pins has been promoted in the past for treatment of distal radius fractures. In France, Kapandji3 described the use of “intra-focal
pinning.” In this technique, smooth Kirschner wires are introduced in the fracture site itself, and then using leverage so that the pins act like “crowbars,” the distal fragment that is malpositioned becomes adjusted into a more anatomic position.3 Kapandji’s treatment can be very effective in achieving reduction; however, as there is no fixation into the distal fragment, this technique has limitations in maintaining the reduction until healing has occurred. Interfragmentary pinning from the dorsal radial and dorsal ulnar aspects were nicely described by Clancey.4 I have found great utility in combining the Kapandji intra-focal techniques to achieve reduction with Clancey pin fixation or distal radius plating to maintain reduction.
I was intrigued with the article by Drs. Siegall and Ziran, “En Bloc Joystick Reduction of a Comminuted Intraarticular Distal Radius Fracture: A Technical Trick,” in this month’s issue of The American Journal of Orthopedics. In their technique, the authors introduced a series of parallel pins or screws below the articular surface from radius to ulna in parallel fashion to provide provisional fixation for the intra-articular components of their complex fracture. Once having done so, they felt more secure in manipulating the distal radius component en bloc; in fact, they used strapping to provide distal traction on the external protruding portion of the pins to help achieve and maintain reduction for their definitive fixation. Drs. Siegall and Ziran describe the use of either Kirschner wires or plating to provide definitive fixation. In the example cited, they performed (via an open method) both the scaffolding and plating without the need of an assistant to hold or maintain the reduction during the osteosynthesis. I can envision adapting the technique they describe to percutaneous treatments for placement of the scaffolding pins, and even the Kapandji/Clancey pins under fluoroscopic guidance or arthroscopeassisted placement.
Despite the popularity and utility of volar fixed-angle plating techniques to treat distal radius fractures, there remain certain situations in which these techniques are faced with challenges. Certainly one of them is the more complex intra-articular fracture with multiple components, or in the very distal fracture patterns in which there is limited bone for the surgeon to use in providing distal screw fixation in the plating systems. Additionally, the nascent malunion presents some challenges as well in terms of performing a “takedown” of the partially healed fracture without destroying the soft, partially healed distal bone that contains the all-important articular component. These are the instances where supplemental techniques such as the one described by Drs. Siegall and Ziran, as well as the
Kapandji and Clancey techniques, have their greatest utility and appeal. Despite one’s wishes and best efforts, some distal radius fractures are not easily reconstructable. In these cases, use of external fixation or temporary arthrodesis
dorsal plating with subsequent plate removal5,6 can be the best reconstructive option and a great “bailout.” The prepared surgeon should have these supplemental techniques in their armamentarium to be able to adapt to the conditions that present themselves in the operating room and to do the best job they can for the patient.
References
1. Agee JM. External fixation. Technical advances based upon multiplanar
ligamentotaxis. Orthop Clin North Am. 1993;24(2):265-274.
2. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable
distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
3. Kapandji A. Internal fixation by double intrafocal plate. Functional treatment
of non articular fractures of the lower end of the radius (author’s transl) [in French]. Ann Chir. 1976;30(11-12):903-908.
4. Clancey GJ. Percutaneous Kirschner-wire fixation of Colles fractures. A prospective study of thirty cases. J Bone Joint Surg Am. 1984;66(7):1008-1014.
5. Burke EF, Singer RM. Treatment of comminuted distal radius with the use of an internal distraction plate. Tech Hand Up Extrem Surg. 1998;2(4):248-252.
6. Ruch DS, Ginn TA, Yang CC, Smith BP, Rushing J, Hanel DP. Use of a distraction plate for distal radial fractures with metaphyseal and diaphyseal comminution. J Bone Joint Surg Am. 2005;87(5):945-954.
Most kneecap pain can be managed conservatively
SEATTLE – In about 80% of patients, patellofemoral knee pain is due to muscular dysfunction and often responds to strengthening and other conservative measures, according to Dr. Christian Lattermann of the department of orthopedic surgery at the University of Kentucky, Lexington.
Surgery should be the last option, and "only if you have identified a structural or functional pathology that you can correct." When surgery is indicated, lateral releases should be avoided; lateral retinacular lengthening is the better option because it preserves the anatomical bridle that keeps the knee cap tracking properly. "Surgically, you can lengthen it by about 2 cm, and that’s usually enough," Dr. Lattermann said at the annual meeting of the American Orthopaedic Society for Sports Medicine.
Patellofemoral pain is common but also frustrating to treat. Finding the cause is difficult; whether it’s subluxation, instability, plica, tendinitis, or simply a noisy knee, "all of it will be described to you as pain. You need to figure it out," he said.
Injections might help. "If you inject them, and they have absolutely no pain," it suggests extraarticular issues. In those cases, "rehab is probably your first bet." Meanwhile, effusions hint at intraarticular pathology, he said.
"McConnell taping is an excellent tool, and will help make the surgical decision. If it’s truly effective," soft tissue realignment might be enough, he said.
"If you have a patient who tells you they have pain all the time everywhere, then think about deconditioning." After perhaps years of compensating for chronic knee problems, the musculature is weak – perhaps in both knees – and gait alterations are also likely. Patients "are basically slamming into their knee cap with every step. You have to start the process of getting them out of that," and it doesn’t involve surgery, at least at first, he said.
"Many of these patients are difficult to manage because as soon as you want to look at their knee, they start becoming apprehensive. It’s not because they are crazy, but because they’ve had experiences that were hard to deal with. You can often help them by talking and identifying those for them, and putting them into perspective," he said.
If they only have pain, "we essentially start with physical therapy," including strengthening and stretching the core, hip, and leg muscles. Even in patients with apprehension or instability, "I’d be very careful to try to identify muscular dysfunction. You always need to treat that," Dr. Lattermann said.
Runners and other athletes might get into problems by overdoing it, which can lead to chronic muscle fatigue and patella overload. For some, the root of the problem might be an odd gait or an odd way or running. "You need to basically teach these people how to move properly," he said.
In addition to MRI and CT to assess anatomy and biomechanics, bone scans might demonstrate a "hot spot that will help guide you." Also, when it’s an issue, weight is a "discussion that happens almost every time with every patient," he said.
Dr. Lattermann is a consultant for Ceterix, Icartilage, and Sanofi/Genzyme.
SEATTLE – In about 80% of patients, patellofemoral knee pain is due to muscular dysfunction and often responds to strengthening and other conservative measures, according to Dr. Christian Lattermann of the department of orthopedic surgery at the University of Kentucky, Lexington.
Surgery should be the last option, and "only if you have identified a structural or functional pathology that you can correct." When surgery is indicated, lateral releases should be avoided; lateral retinacular lengthening is the better option because it preserves the anatomical bridle that keeps the knee cap tracking properly. "Surgically, you can lengthen it by about 2 cm, and that’s usually enough," Dr. Lattermann said at the annual meeting of the American Orthopaedic Society for Sports Medicine.
Patellofemoral pain is common but also frustrating to treat. Finding the cause is difficult; whether it’s subluxation, instability, plica, tendinitis, or simply a noisy knee, "all of it will be described to you as pain. You need to figure it out," he said.
Injections might help. "If you inject them, and they have absolutely no pain," it suggests extraarticular issues. In those cases, "rehab is probably your first bet." Meanwhile, effusions hint at intraarticular pathology, he said.
"McConnell taping is an excellent tool, and will help make the surgical decision. If it’s truly effective," soft tissue realignment might be enough, he said.
"If you have a patient who tells you they have pain all the time everywhere, then think about deconditioning." After perhaps years of compensating for chronic knee problems, the musculature is weak – perhaps in both knees – and gait alterations are also likely. Patients "are basically slamming into their knee cap with every step. You have to start the process of getting them out of that," and it doesn’t involve surgery, at least at first, he said.
"Many of these patients are difficult to manage because as soon as you want to look at their knee, they start becoming apprehensive. It’s not because they are crazy, but because they’ve had experiences that were hard to deal with. You can often help them by talking and identifying those for them, and putting them into perspective," he said.
If they only have pain, "we essentially start with physical therapy," including strengthening and stretching the core, hip, and leg muscles. Even in patients with apprehension or instability, "I’d be very careful to try to identify muscular dysfunction. You always need to treat that," Dr. Lattermann said.
Runners and other athletes might get into problems by overdoing it, which can lead to chronic muscle fatigue and patella overload. For some, the root of the problem might be an odd gait or an odd way or running. "You need to basically teach these people how to move properly," he said.
In addition to MRI and CT to assess anatomy and biomechanics, bone scans might demonstrate a "hot spot that will help guide you." Also, when it’s an issue, weight is a "discussion that happens almost every time with every patient," he said.
Dr. Lattermann is a consultant for Ceterix, Icartilage, and Sanofi/Genzyme.
SEATTLE – In about 80% of patients, patellofemoral knee pain is due to muscular dysfunction and often responds to strengthening and other conservative measures, according to Dr. Christian Lattermann of the department of orthopedic surgery at the University of Kentucky, Lexington.
Surgery should be the last option, and "only if you have identified a structural or functional pathology that you can correct." When surgery is indicated, lateral releases should be avoided; lateral retinacular lengthening is the better option because it preserves the anatomical bridle that keeps the knee cap tracking properly. "Surgically, you can lengthen it by about 2 cm, and that’s usually enough," Dr. Lattermann said at the annual meeting of the American Orthopaedic Society for Sports Medicine.
Patellofemoral pain is common but also frustrating to treat. Finding the cause is difficult; whether it’s subluxation, instability, plica, tendinitis, or simply a noisy knee, "all of it will be described to you as pain. You need to figure it out," he said.
Injections might help. "If you inject them, and they have absolutely no pain," it suggests extraarticular issues. In those cases, "rehab is probably your first bet." Meanwhile, effusions hint at intraarticular pathology, he said.
"McConnell taping is an excellent tool, and will help make the surgical decision. If it’s truly effective," soft tissue realignment might be enough, he said.
"If you have a patient who tells you they have pain all the time everywhere, then think about deconditioning." After perhaps years of compensating for chronic knee problems, the musculature is weak – perhaps in both knees – and gait alterations are also likely. Patients "are basically slamming into their knee cap with every step. You have to start the process of getting them out of that," and it doesn’t involve surgery, at least at first, he said.
"Many of these patients are difficult to manage because as soon as you want to look at their knee, they start becoming apprehensive. It’s not because they are crazy, but because they’ve had experiences that were hard to deal with. You can often help them by talking and identifying those for them, and putting them into perspective," he said.
If they only have pain, "we essentially start with physical therapy," including strengthening and stretching the core, hip, and leg muscles. Even in patients with apprehension or instability, "I’d be very careful to try to identify muscular dysfunction. You always need to treat that," Dr. Lattermann said.
Runners and other athletes might get into problems by overdoing it, which can lead to chronic muscle fatigue and patella overload. For some, the root of the problem might be an odd gait or an odd way or running. "You need to basically teach these people how to move properly," he said.
In addition to MRI and CT to assess anatomy and biomechanics, bone scans might demonstrate a "hot spot that will help guide you." Also, when it’s an issue, weight is a "discussion that happens almost every time with every patient," he said.
Dr. Lattermann is a consultant for Ceterix, Icartilage, and Sanofi/Genzyme.
AT THE AOSSM 2014 ANNUAL MEETING
VIDEO: Consider cognitive function in elderly before surgery
COPENHAGEN – Almost half of patients with mild cognitive impairment progressed to dementia within 1 year of undergoing either arthroplasty or coronary angiography, according to a small Australian study.
Baseline cognitive impairment appeared to be the main driver of progression, increasing the risk more than sevenfold – significantly more than age or heart attack, Lisbeth Evered, Ph.D., said at the annual Alzheimer’s Association International Conference.
"After 12 months, 42% met the criteria for dementia," said Dr. Evered, a researcher at the University of Melbourne. "The expected annual progression from mild cognitive impairment [MCI] to dementia would be about 10%-12% per year."
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Her study included 67 patients with a mean age of 70 years. All had MCI at baseline, with a mean score of 23 on the Mini-Mental State Exam (MMSE). They underwent either arthroplasty (26 patients) or coronary angiography (41 patients).
A year after surgery, about 34% of the arthroplasty patients and 46% of the angiography patients had progressed to dementia. Baseline cognitive impairment was the only factor significantly associated with the change.
Postsurgical cognitive decline, both transient and long lasting, is a well-documented phenomenon, with studies going back to the late 1800s. Although the causative link isn’t entirely clear, anesthetics have long been implicated, said Dr. Evered. In animal models, some anesthesia drugs do seem to precipitate an Alzheimer’s-like amyloidosis and tau hyperphosphorylation.
More recent animal data suggest that inflammation might be a powerful influence.
"When a patient has surgery with a general anesthetic, they experience peripheral inflammation," Dr. Evered explained. "In a healthy normal brain, there’s plenty of cognitive reserve, and although there might be some cognitive decline afterward, the person won’t really notice and will certainly recover."
In a vulnerable brain, however, the inflammation may be amplified and may cause significant collateral damage that accelerates cognitive decline. "The problem is, we don’t know why they are vulnerable or what we might do about it," she noted.
The best approach now is to routinely assess cognition before surgery and monitor it afterward, Dr. Evered explained. "Then, the perioperative period is not something occurring in isolation," she noted. "We will be better able to identify those at risk and implement strategies to improve their outcomes."
In a video interview, Dr. Evered and Dr. Brendan Silbert of St. Vincent’s Hospital, Melbourne, discuss the study and its implications.
Dr. Evered had no financial disclosures
On Twitter @alz_gal
COPENHAGEN – Almost half of patients with mild cognitive impairment progressed to dementia within 1 year of undergoing either arthroplasty or coronary angiography, according to a small Australian study.
Baseline cognitive impairment appeared to be the main driver of progression, increasing the risk more than sevenfold – significantly more than age or heart attack, Lisbeth Evered, Ph.D., said at the annual Alzheimer’s Association International Conference.
"After 12 months, 42% met the criteria for dementia," said Dr. Evered, a researcher at the University of Melbourne. "The expected annual progression from mild cognitive impairment [MCI] to dementia would be about 10%-12% per year."
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Her study included 67 patients with a mean age of 70 years. All had MCI at baseline, with a mean score of 23 on the Mini-Mental State Exam (MMSE). They underwent either arthroplasty (26 patients) or coronary angiography (41 patients).
A year after surgery, about 34% of the arthroplasty patients and 46% of the angiography patients had progressed to dementia. Baseline cognitive impairment was the only factor significantly associated with the change.
Postsurgical cognitive decline, both transient and long lasting, is a well-documented phenomenon, with studies going back to the late 1800s. Although the causative link isn’t entirely clear, anesthetics have long been implicated, said Dr. Evered. In animal models, some anesthesia drugs do seem to precipitate an Alzheimer’s-like amyloidosis and tau hyperphosphorylation.
More recent animal data suggest that inflammation might be a powerful influence.
"When a patient has surgery with a general anesthetic, they experience peripheral inflammation," Dr. Evered explained. "In a healthy normal brain, there’s plenty of cognitive reserve, and although there might be some cognitive decline afterward, the person won’t really notice and will certainly recover."
In a vulnerable brain, however, the inflammation may be amplified and may cause significant collateral damage that accelerates cognitive decline. "The problem is, we don’t know why they are vulnerable or what we might do about it," she noted.
The best approach now is to routinely assess cognition before surgery and monitor it afterward, Dr. Evered explained. "Then, the perioperative period is not something occurring in isolation," she noted. "We will be better able to identify those at risk and implement strategies to improve their outcomes."
In a video interview, Dr. Evered and Dr. Brendan Silbert of St. Vincent’s Hospital, Melbourne, discuss the study and its implications.
Dr. Evered had no financial disclosures
On Twitter @alz_gal
COPENHAGEN – Almost half of patients with mild cognitive impairment progressed to dementia within 1 year of undergoing either arthroplasty or coronary angiography, according to a small Australian study.
Baseline cognitive impairment appeared to be the main driver of progression, increasing the risk more than sevenfold – significantly more than age or heart attack, Lisbeth Evered, Ph.D., said at the annual Alzheimer’s Association International Conference.
"After 12 months, 42% met the criteria for dementia," said Dr. Evered, a researcher at the University of Melbourne. "The expected annual progression from mild cognitive impairment [MCI] to dementia would be about 10%-12% per year."
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Her study included 67 patients with a mean age of 70 years. All had MCI at baseline, with a mean score of 23 on the Mini-Mental State Exam (MMSE). They underwent either arthroplasty (26 patients) or coronary angiography (41 patients).
A year after surgery, about 34% of the arthroplasty patients and 46% of the angiography patients had progressed to dementia. Baseline cognitive impairment was the only factor significantly associated with the change.
Postsurgical cognitive decline, both transient and long lasting, is a well-documented phenomenon, with studies going back to the late 1800s. Although the causative link isn’t entirely clear, anesthetics have long been implicated, said Dr. Evered. In animal models, some anesthesia drugs do seem to precipitate an Alzheimer’s-like amyloidosis and tau hyperphosphorylation.
More recent animal data suggest that inflammation might be a powerful influence.
"When a patient has surgery with a general anesthetic, they experience peripheral inflammation," Dr. Evered explained. "In a healthy normal brain, there’s plenty of cognitive reserve, and although there might be some cognitive decline afterward, the person won’t really notice and will certainly recover."
In a vulnerable brain, however, the inflammation may be amplified and may cause significant collateral damage that accelerates cognitive decline. "The problem is, we don’t know why they are vulnerable or what we might do about it," she noted.
The best approach now is to routinely assess cognition before surgery and monitor it afterward, Dr. Evered explained. "Then, the perioperative period is not something occurring in isolation," she noted. "We will be better able to identify those at risk and implement strategies to improve their outcomes."
In a video interview, Dr. Evered and Dr. Brendan Silbert of St. Vincent’s Hospital, Melbourne, discuss the study and its implications.
Dr. Evered had no financial disclosures
On Twitter @alz_gal
AT AAIC 2014
Obesity Behind Rise in Knee Replacement Surgeries
There is a direct link between the number of total knee replacement surgeries and obesity, according to a study published online ahead of print June 4 in the Journal of Bone and Joint Surgery. Lead study author Peter B. Derman, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery in New York, and colleagues reported that the number of total knee replacement surgeries more than tripled between 1993 and 2009, while the number of total hip replacements doubled in the same time period.
“We observed that growth of knee replacement volumes was far outpacing that of hip replacements and were curious as to the origins of this trend,” said Dr. Derman. He and his research colleagues found that an increase in the prevalence of overweight and obesity in the US accounted for 95% of the higher demand for knee replacements, with younger patients affected to a greater degree.
The researchers compiled longitudinal data on total hip arthroplasty and total knee arthroplasty volume, length of hospital stay, and in-hospital mortality from the Nationwide Inpatient Sample. They then calculated reimbursement using information available in the Federal Register and Centers for Medicare and Medicaid databases. Trends in body mass index (BMI) were determined from Behavioral Risk Factor Surveillance System findings and the size of the surgical workforce was based on membership data from the American Academy of Orthopaedic Surgeons.
Among the study’s findings:
• Growth in total knee arthroplasty volume far outpaced that of total hip arthroplasty among those patients with a BMI of 25 kg/m2 or above, but not for those with a BMI of less than 25 kg/m2.
• In 1993, surgeons performed 1.16 total knee replacements for every total hip replacement, but this ratio grew to 1.60 by the year 2009.
• Patients ages 18 to 64 years experienced a more rapid rise in obesity, compared to patients older than 65 years.
• From 1997 to 2009, the share of patient’s ages 18 to 64 years undergoing total knee replacement rose 56% compared to 35% for total hip replacement.
• Surgeon per-case reimbursement for total knee replacement fell from approximately $3000 in 1995 to $1560 in 2009. Surgeon fees for total hip replacement dropped from $2840 to $1460 in the same time period.
• As the data represent an approximate 48% drop in fees for both procedures, surgeons do not appear to be performing more total knee replacements over total hip replacements due to higher reimbursement.
• Hospital reimbursement, length of hospital stay, and in-hospital mortality pertaining to total knee replacement and total hip replacement also declined between 1995 and 2009.
Commenting on the study findings, Dr. Derman said, “Because excess body weight appears to be more damaging to the knee than to the hip, the increasing prevalence of overweight and obesity may explain the growing demand for knee replacements over hip replacements. If rates of overweight and obesity continue to climb, we should expect further acceleration in the number of knee replacements performed annually in the United States with a more modest increase in hip replacement volumes.”
Suggested Reading
Derman PB, Fabricant PD, David G. The role of overweight and obesity in relation to the more rapid growth of total knee arthroplasty volume compared with total hip arthroplasty volume. J Bone Joint Surg Am. 2014;96(11):922-928 [Epub ahead of print].
There is a direct link between the number of total knee replacement surgeries and obesity, according to a study published online ahead of print June 4 in the Journal of Bone and Joint Surgery. Lead study author Peter B. Derman, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery in New York, and colleagues reported that the number of total knee replacement surgeries more than tripled between 1993 and 2009, while the number of total hip replacements doubled in the same time period.
“We observed that growth of knee replacement volumes was far outpacing that of hip replacements and were curious as to the origins of this trend,” said Dr. Derman. He and his research colleagues found that an increase in the prevalence of overweight and obesity in the US accounted for 95% of the higher demand for knee replacements, with younger patients affected to a greater degree.
The researchers compiled longitudinal data on total hip arthroplasty and total knee arthroplasty volume, length of hospital stay, and in-hospital mortality from the Nationwide Inpatient Sample. They then calculated reimbursement using information available in the Federal Register and Centers for Medicare and Medicaid databases. Trends in body mass index (BMI) were determined from Behavioral Risk Factor Surveillance System findings and the size of the surgical workforce was based on membership data from the American Academy of Orthopaedic Surgeons.
Among the study’s findings:
• Growth in total knee arthroplasty volume far outpaced that of total hip arthroplasty among those patients with a BMI of 25 kg/m2 or above, but not for those with a BMI of less than 25 kg/m2.
• In 1993, surgeons performed 1.16 total knee replacements for every total hip replacement, but this ratio grew to 1.60 by the year 2009.
• Patients ages 18 to 64 years experienced a more rapid rise in obesity, compared to patients older than 65 years.
• From 1997 to 2009, the share of patient’s ages 18 to 64 years undergoing total knee replacement rose 56% compared to 35% for total hip replacement.
• Surgeon per-case reimbursement for total knee replacement fell from approximately $3000 in 1995 to $1560 in 2009. Surgeon fees for total hip replacement dropped from $2840 to $1460 in the same time period.
• As the data represent an approximate 48% drop in fees for both procedures, surgeons do not appear to be performing more total knee replacements over total hip replacements due to higher reimbursement.
• Hospital reimbursement, length of hospital stay, and in-hospital mortality pertaining to total knee replacement and total hip replacement also declined between 1995 and 2009.
Commenting on the study findings, Dr. Derman said, “Because excess body weight appears to be more damaging to the knee than to the hip, the increasing prevalence of overweight and obesity may explain the growing demand for knee replacements over hip replacements. If rates of overweight and obesity continue to climb, we should expect further acceleration in the number of knee replacements performed annually in the United States with a more modest increase in hip replacement volumes.”
There is a direct link between the number of total knee replacement surgeries and obesity, according to a study published online ahead of print June 4 in the Journal of Bone and Joint Surgery. Lead study author Peter B. Derman, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery in New York, and colleagues reported that the number of total knee replacement surgeries more than tripled between 1993 and 2009, while the number of total hip replacements doubled in the same time period.
“We observed that growth of knee replacement volumes was far outpacing that of hip replacements and were curious as to the origins of this trend,” said Dr. Derman. He and his research colleagues found that an increase in the prevalence of overweight and obesity in the US accounted for 95% of the higher demand for knee replacements, with younger patients affected to a greater degree.
The researchers compiled longitudinal data on total hip arthroplasty and total knee arthroplasty volume, length of hospital stay, and in-hospital mortality from the Nationwide Inpatient Sample. They then calculated reimbursement using information available in the Federal Register and Centers for Medicare and Medicaid databases. Trends in body mass index (BMI) were determined from Behavioral Risk Factor Surveillance System findings and the size of the surgical workforce was based on membership data from the American Academy of Orthopaedic Surgeons.
Among the study’s findings:
• Growth in total knee arthroplasty volume far outpaced that of total hip arthroplasty among those patients with a BMI of 25 kg/m2 or above, but not for those with a BMI of less than 25 kg/m2.
• In 1993, surgeons performed 1.16 total knee replacements for every total hip replacement, but this ratio grew to 1.60 by the year 2009.
• Patients ages 18 to 64 years experienced a more rapid rise in obesity, compared to patients older than 65 years.
• From 1997 to 2009, the share of patient’s ages 18 to 64 years undergoing total knee replacement rose 56% compared to 35% for total hip replacement.
• Surgeon per-case reimbursement for total knee replacement fell from approximately $3000 in 1995 to $1560 in 2009. Surgeon fees for total hip replacement dropped from $2840 to $1460 in the same time period.
• As the data represent an approximate 48% drop in fees for both procedures, surgeons do not appear to be performing more total knee replacements over total hip replacements due to higher reimbursement.
• Hospital reimbursement, length of hospital stay, and in-hospital mortality pertaining to total knee replacement and total hip replacement also declined between 1995 and 2009.
Commenting on the study findings, Dr. Derman said, “Because excess body weight appears to be more damaging to the knee than to the hip, the increasing prevalence of overweight and obesity may explain the growing demand for knee replacements over hip replacements. If rates of overweight and obesity continue to climb, we should expect further acceleration in the number of knee replacements performed annually in the United States with a more modest increase in hip replacement volumes.”
Suggested Reading
Derman PB, Fabricant PD, David G. The role of overweight and obesity in relation to the more rapid growth of total knee arthroplasty volume compared with total hip arthroplasty volume. J Bone Joint Surg Am. 2014;96(11):922-928 [Epub ahead of print].
Suggested Reading
Derman PB, Fabricant PD, David G. The role of overweight and obesity in relation to the more rapid growth of total knee arthroplasty volume compared with total hip arthroplasty volume. J Bone Joint Surg Am. 2014;96(11):922-928 [Epub ahead of print].
NIH Tackles Chronic Low Back Pain
Standardized research methods are needed to advance efforts toward reducing the costs and high burden of chronic low back pain, according to a multidisciplinary NIH Task Force report published online ahead of print May 30 in Spine.
The article introduces a set of proposed research standards to help in comparing the results of chronic low back pain studies. The Task Force co-chairs were Drs. Richard A. Deyo of Oregon Health and Science University, and Samuel F. Dworkin of the University of Washington in Seattle.
“Researchers use varied inclusion criteria, definitions, baseline assessments, and outcome measures, which impede comparisons and consensus,” the Task Force wrote.
To address this issue, the Task Force followed a structured approach to developing a set of standards for chronic low back pain research. Overriding issues included defining the problem of chronic low back pain, identifying the minimum dataset that should be collected in chronic low back pain research, assessing its impact on patients’ lives, and defining the best outcomes to evaluate treatment effectiveness.
The Task Force recommends that chronic low back pain be defined as back pain that lasts at least 3 months and causing pain on at least half of days over the past 6 months. Their recommended definition does not include ratings of pain severity.
In terms of impact, the Task Force recommends focusing on how back pain affects patients’ lives. They recommend a 9-item chronic low back pain “Impact Score” that incorporates ratings of pain intensity, interference with normal activities, and functional ability.
A key point for the Task Force was to define a minimum set of data to be gathered in any study of chronic low back pain. The recommended dataset included legal or workers compensation issues, previous treatments, and important contributing factors (eg, smoking, obesity, substance abuse, and widespread pain).
For outcomes, the Task Force concluded there was no set definition of what degree of improvement should be considered “clinically important.” Likewise, there was no consensus on the use of combined outcome measures, time frame for improvement, or adverse effects.
Finally, testing and developing new combined outcome measures was identified as an important aspect of future research, more specifically, other included approaches to predicting treatment results and studies to evaluate and improve the minimum dataset.
Overall, the recommendations stress the importance of getting a fuller picture of the patient’s medical history, even more so than the physical examination. On the other hand, the Task Force specified no standard laboratory or imaging tests, citing the lack of association with patient functioning or symptoms. Evaluations of depression, sleep disturbance, physical functioning, and catastrophic thinking were rated as important for all groups of patients with chronic low back pain.
The Task Force hopes that their recommended standards reflect the “complex, intertwined factors” affecting the development and clinical course of chronic low back pain. The NIH Pain Consortium has approved the recommendations and advises that investigators should incorporate them into NIH grant proposals. “As adopted by the NIH, these recommendations have the potential to standardize methods for identifying chronic low back pain research cases, describe research subjects, and compare published reports.” The Task Force added that recommendations should be subject to periodic validation and refinement in years ahead.
Suggested Reading
Deyo RA, Dworkin SF, Amtmann D, et al. NIH task force proposes standards for research on chronic low back pain. Spine. 2014 May 30 [Epub ahead of print].
Standardized research methods are needed to advance efforts toward reducing the costs and high burden of chronic low back pain, according to a multidisciplinary NIH Task Force report published online ahead of print May 30 in Spine.
The article introduces a set of proposed research standards to help in comparing the results of chronic low back pain studies. The Task Force co-chairs were Drs. Richard A. Deyo of Oregon Health and Science University, and Samuel F. Dworkin of the University of Washington in Seattle.
“Researchers use varied inclusion criteria, definitions, baseline assessments, and outcome measures, which impede comparisons and consensus,” the Task Force wrote.
To address this issue, the Task Force followed a structured approach to developing a set of standards for chronic low back pain research. Overriding issues included defining the problem of chronic low back pain, identifying the minimum dataset that should be collected in chronic low back pain research, assessing its impact on patients’ lives, and defining the best outcomes to evaluate treatment effectiveness.
The Task Force recommends that chronic low back pain be defined as back pain that lasts at least 3 months and causing pain on at least half of days over the past 6 months. Their recommended definition does not include ratings of pain severity.
In terms of impact, the Task Force recommends focusing on how back pain affects patients’ lives. They recommend a 9-item chronic low back pain “Impact Score” that incorporates ratings of pain intensity, interference with normal activities, and functional ability.
A key point for the Task Force was to define a minimum set of data to be gathered in any study of chronic low back pain. The recommended dataset included legal or workers compensation issues, previous treatments, and important contributing factors (eg, smoking, obesity, substance abuse, and widespread pain).
For outcomes, the Task Force concluded there was no set definition of what degree of improvement should be considered “clinically important.” Likewise, there was no consensus on the use of combined outcome measures, time frame for improvement, or adverse effects.
Finally, testing and developing new combined outcome measures was identified as an important aspect of future research, more specifically, other included approaches to predicting treatment results and studies to evaluate and improve the minimum dataset.
Overall, the recommendations stress the importance of getting a fuller picture of the patient’s medical history, even more so than the physical examination. On the other hand, the Task Force specified no standard laboratory or imaging tests, citing the lack of association with patient functioning or symptoms. Evaluations of depression, sleep disturbance, physical functioning, and catastrophic thinking were rated as important for all groups of patients with chronic low back pain.
The Task Force hopes that their recommended standards reflect the “complex, intertwined factors” affecting the development and clinical course of chronic low back pain. The NIH Pain Consortium has approved the recommendations and advises that investigators should incorporate them into NIH grant proposals. “As adopted by the NIH, these recommendations have the potential to standardize methods for identifying chronic low back pain research cases, describe research subjects, and compare published reports.” The Task Force added that recommendations should be subject to periodic validation and refinement in years ahead.
Standardized research methods are needed to advance efforts toward reducing the costs and high burden of chronic low back pain, according to a multidisciplinary NIH Task Force report published online ahead of print May 30 in Spine.
The article introduces a set of proposed research standards to help in comparing the results of chronic low back pain studies. The Task Force co-chairs were Drs. Richard A. Deyo of Oregon Health and Science University, and Samuel F. Dworkin of the University of Washington in Seattle.
“Researchers use varied inclusion criteria, definitions, baseline assessments, and outcome measures, which impede comparisons and consensus,” the Task Force wrote.
To address this issue, the Task Force followed a structured approach to developing a set of standards for chronic low back pain research. Overriding issues included defining the problem of chronic low back pain, identifying the minimum dataset that should be collected in chronic low back pain research, assessing its impact on patients’ lives, and defining the best outcomes to evaluate treatment effectiveness.
The Task Force recommends that chronic low back pain be defined as back pain that lasts at least 3 months and causing pain on at least half of days over the past 6 months. Their recommended definition does not include ratings of pain severity.
In terms of impact, the Task Force recommends focusing on how back pain affects patients’ lives. They recommend a 9-item chronic low back pain “Impact Score” that incorporates ratings of pain intensity, interference with normal activities, and functional ability.
A key point for the Task Force was to define a minimum set of data to be gathered in any study of chronic low back pain. The recommended dataset included legal or workers compensation issues, previous treatments, and important contributing factors (eg, smoking, obesity, substance abuse, and widespread pain).
For outcomes, the Task Force concluded there was no set definition of what degree of improvement should be considered “clinically important.” Likewise, there was no consensus on the use of combined outcome measures, time frame for improvement, or adverse effects.
Finally, testing and developing new combined outcome measures was identified as an important aspect of future research, more specifically, other included approaches to predicting treatment results and studies to evaluate and improve the minimum dataset.
Overall, the recommendations stress the importance of getting a fuller picture of the patient’s medical history, even more so than the physical examination. On the other hand, the Task Force specified no standard laboratory or imaging tests, citing the lack of association with patient functioning or symptoms. Evaluations of depression, sleep disturbance, physical functioning, and catastrophic thinking were rated as important for all groups of patients with chronic low back pain.
The Task Force hopes that their recommended standards reflect the “complex, intertwined factors” affecting the development and clinical course of chronic low back pain. The NIH Pain Consortium has approved the recommendations and advises that investigators should incorporate them into NIH grant proposals. “As adopted by the NIH, these recommendations have the potential to standardize methods for identifying chronic low back pain research cases, describe research subjects, and compare published reports.” The Task Force added that recommendations should be subject to periodic validation and refinement in years ahead.
Suggested Reading
Deyo RA, Dworkin SF, Amtmann D, et al. NIH task force proposes standards for research on chronic low back pain. Spine. 2014 May 30 [Epub ahead of print].
Suggested Reading
Deyo RA, Dworkin SF, Amtmann D, et al. NIH task force proposes standards for research on chronic low back pain. Spine. 2014 May 30 [Epub ahead of print].
Bionic Arm Still in Development Stage
Within the past 13 years, roughly 2000 veterans who have returned from Afghanistan and Iraq have sustained injuries that required amputations. Of these injured veterans, 14% required upper extremity amputations. An article published in the June issue of the Journal of the American Academy of Orthopaedic Surgeons reviewed recent advancements in upper extremity bionics. Also reviewed were the challenges that linger in creating a prosthesis that meets or surpasses the abilities of the human hand and arm.
During the next 50 years, “I truly believe we will be able to make artificial arms that function better than many injured arms that doctors are saving today,” said article author Dr. Douglas T. Hutchinson, Associate Professor of Orthopedics at the University of Utah Medical School and Chief of Hand Surgery at Primary Children’s Medical Center, the Veterans Affairs Medical Center, and Shriners Intermountain Hospital.
Created more than 50 years ago, the myoelectric prosthesis continues to be the most commonly used upper extremity prostheses. This prosthesis allows residual muscles to act as natural batteries to create transcutaneous signals, to control the movements of the prosthetic hand and arm. However, the muscles used most often are the triceps and biceps, which do not inherently translate to the opening and closing of the hand. Another drawback is that sometimes the socket interface used to attach the prosthesis may interfere with the function of the residual joint, such as the elbow. Myoelectric prosthetics also do not look natural and are heavy, hot, and uncomfortable, and are not waterproof.
The current federal budget for prostheses research is $2.5 billion. The US Department of Defense Advanced Research Project (DARPA) already has invested more than $150 million for their Revolutionizing Prosthetics Program. The later program, which seeks to create an upper extremity prosthesis that can function as a normal hand and arm does, but with full sensory and motor functions.
In order for these prosthetic devices to be used effectively in a broad range for patients, adjustments still need to be made. For example, many have short-life batteries, along with being weighty and uncomfortable. Particularly challenging is the problem of accurately and efficiently sending brain signals through the peripheral nerves and muscles of the hands and arm, a feat that may warrant the creation and use of a reliable wireless device or direct wiring through an osseous-integrated implant. Current infection rates (nearly 45%) with osseous-integrated devices at the prosthesis-skin interface also pose an issue.
Suggested Reading
Hutchinson DT. The quest for the bionic arm. J Am Acad Orthop Surg. 2014;22(6):346-351.
Within the past 13 years, roughly 2000 veterans who have returned from Afghanistan and Iraq have sustained injuries that required amputations. Of these injured veterans, 14% required upper extremity amputations. An article published in the June issue of the Journal of the American Academy of Orthopaedic Surgeons reviewed recent advancements in upper extremity bionics. Also reviewed were the challenges that linger in creating a prosthesis that meets or surpasses the abilities of the human hand and arm.
During the next 50 years, “I truly believe we will be able to make artificial arms that function better than many injured arms that doctors are saving today,” said article author Dr. Douglas T. Hutchinson, Associate Professor of Orthopedics at the University of Utah Medical School and Chief of Hand Surgery at Primary Children’s Medical Center, the Veterans Affairs Medical Center, and Shriners Intermountain Hospital.
Created more than 50 years ago, the myoelectric prosthesis continues to be the most commonly used upper extremity prostheses. This prosthesis allows residual muscles to act as natural batteries to create transcutaneous signals, to control the movements of the prosthetic hand and arm. However, the muscles used most often are the triceps and biceps, which do not inherently translate to the opening and closing of the hand. Another drawback is that sometimes the socket interface used to attach the prosthesis may interfere with the function of the residual joint, such as the elbow. Myoelectric prosthetics also do not look natural and are heavy, hot, and uncomfortable, and are not waterproof.
The current federal budget for prostheses research is $2.5 billion. The US Department of Defense Advanced Research Project (DARPA) already has invested more than $150 million for their Revolutionizing Prosthetics Program. The later program, which seeks to create an upper extremity prosthesis that can function as a normal hand and arm does, but with full sensory and motor functions.
In order for these prosthetic devices to be used effectively in a broad range for patients, adjustments still need to be made. For example, many have short-life batteries, along with being weighty and uncomfortable. Particularly challenging is the problem of accurately and efficiently sending brain signals through the peripheral nerves and muscles of the hands and arm, a feat that may warrant the creation and use of a reliable wireless device or direct wiring through an osseous-integrated implant. Current infection rates (nearly 45%) with osseous-integrated devices at the prosthesis-skin interface also pose an issue.
Within the past 13 years, roughly 2000 veterans who have returned from Afghanistan and Iraq have sustained injuries that required amputations. Of these injured veterans, 14% required upper extremity amputations. An article published in the June issue of the Journal of the American Academy of Orthopaedic Surgeons reviewed recent advancements in upper extremity bionics. Also reviewed were the challenges that linger in creating a prosthesis that meets or surpasses the abilities of the human hand and arm.
During the next 50 years, “I truly believe we will be able to make artificial arms that function better than many injured arms that doctors are saving today,” said article author Dr. Douglas T. Hutchinson, Associate Professor of Orthopedics at the University of Utah Medical School and Chief of Hand Surgery at Primary Children’s Medical Center, the Veterans Affairs Medical Center, and Shriners Intermountain Hospital.
Created more than 50 years ago, the myoelectric prosthesis continues to be the most commonly used upper extremity prostheses. This prosthesis allows residual muscles to act as natural batteries to create transcutaneous signals, to control the movements of the prosthetic hand and arm. However, the muscles used most often are the triceps and biceps, which do not inherently translate to the opening and closing of the hand. Another drawback is that sometimes the socket interface used to attach the prosthesis may interfere with the function of the residual joint, such as the elbow. Myoelectric prosthetics also do not look natural and are heavy, hot, and uncomfortable, and are not waterproof.
The current federal budget for prostheses research is $2.5 billion. The US Department of Defense Advanced Research Project (DARPA) already has invested more than $150 million for their Revolutionizing Prosthetics Program. The later program, which seeks to create an upper extremity prosthesis that can function as a normal hand and arm does, but with full sensory and motor functions.
In order for these prosthetic devices to be used effectively in a broad range for patients, adjustments still need to be made. For example, many have short-life batteries, along with being weighty and uncomfortable. Particularly challenging is the problem of accurately and efficiently sending brain signals through the peripheral nerves and muscles of the hands and arm, a feat that may warrant the creation and use of a reliable wireless device or direct wiring through an osseous-integrated implant. Current infection rates (nearly 45%) with osseous-integrated devices at the prosthesis-skin interface also pose an issue.
Suggested Reading
Hutchinson DT. The quest for the bionic arm. J Am Acad Orthop Surg. 2014;22(6):346-351.
Suggested Reading
Hutchinson DT. The quest for the bionic arm. J Am Acad Orthop Surg. 2014;22(6):346-351.
Glucocorticoid shots ineffective for spinal stenosis
Epidural injections of glucocorticoids plus lidocaine proved ineffective at improving pain or function in older adults with central spinal stenosis in a double-blind, randomized, controlled trial performed at 16 medical centers across the United States.
In the LESS (Lumbar Epidural Steroid Injections for Spinal Stenosis) trial, fluoroscopically guided transforaminal or interlaminar injections of lidocaine plus triamcinolone, betamethasone, dexamethasone, or methylprednisolone failed to reduce back, buttock, and leg pain or to enhance pain-related function any better than injections of lidocaine alone, reported Dr. Janna L. Friedly of the rehabilitation medicine department at the University of Washington, Seattle, and her associates. (Lidocaine alone has never been demonstrated to offer long-term benefit, they noted.)
The use of steroid injections for spinal stenosis has risen nearly 300% over the past 20 years even though "there is little evidence of effectiveness from clinical trials." The few studies that have examined this treatment have been "small, uncontrolled, or conducted without the use of fluoroscopy," the investigators said.
In their study, patients aged 50 years and older (mean age, 68 years) who had MRI- or CT-proven central spinal stenosis were enrolled over a 2-year period and randomly assigned to receive either a lidocaine plus glucocorticoid injection (200 intervention subjects) or a matching lidocaine injection (200 control subjects) under fluoroscopic guidance. All the study participants reported back, buttock, and leg pain scores of 5 or more on a scale of 0-10 (with 10 representing the worst pain imaginable) and disability scores of 7 or higher on the Roland-Morris Disability Questionnaire (RMDQ) scale of 0-24 (with higher scores indicating greater disability).
The patients were allowed to receive a repeat injection after 3 weeks, if they wished, at the discretion of their treating physicians. The physicians also chose whether to use the transforaminal or interlaminar approach, based on a variety of clinical factors.
At 6 weeks of follow-up, patients in both the glucocorticoid-lidocaine and lidocaine-only study groups reported some improvement in pain and function, but there was no significant difference in improvement between the two groups (–4.2 points and –3.1 points, respectively, on the RMDQ). There also were no significant differences across any of the subgroups of patients studied; in particular, steroid injections were no more effective than lidocaine alone according to the duration of spinal stenosis or the type of injection approach (transforaminal or interlaminar), Dr. Friedly and her associates said (N. Engl. J. Med. 2014 July 3 [doi: 10.1056/NEJMoa1313265]).
There were no differences in the percentage of patients in either group who achieved 30% or 50% improvement RMDQ scores at either 3 or 6 weeks, nor were there any differences in "any improvement" on pain scores or in 50% improvement on pain scores at 6 weeks.
There also were no differences between the groups in scores on secondary outcome measures, including the Brief Pain Inventory, which measures pain-related interference with activities; the Swiss Spinal Stenosis Questionnaire (SSSQ), which measures pain, function, and satisfaction with treatment; the EQ-5D, which measures quality of life; or the Generalized Anxiety Disorder-7 scale.
On the Patient Health Questionnaire-8, the glucocorticoid-lidocaine group reported more improvement in depression symptoms, and on one portion of the SSSQ, more patients in the glucocorticoid-lidocaine group (67%) than in the control group (54%) reported satisfaction with treatment. This is consistent with the systemic effects of cortisol suppression and is likely to be transient, the investigators said.
The glucocorticoid plus lidocaine injections were associated with more adverse effects (21.5% of patients) than were the lidocaine-only injections (15.5%), although the difference was not significant. However, the number of adverse events reported per person was significantly higher among patients who received glucocorticoid plus lidocaine injections (0.29 vs. 0.17), they added.
This study was funded by the Agency for Healthcare Research and Quality and received no commercial support. Dr. Friedly reported no potential financial conflicts of interest; her associates reported ties to Johnson & Johnson, ArthroCare, and other companies.
These findings raise serious questions about the benefits of epidural glucocorticoid injections for spinal stenosis. Patients seeking them should be informed that the best available data do not support a clinically significant long-term benefit, and that complications, though rare, are possible, said Dr. Gunnar B.J. Andersson.
Currently, many insurance companies require that epidural injections be performed as part of nonsurgical treatment before surgery will be approved. The study by Dr. Friedly and her colleagues, together with the Food and Drug Administration safety announcement in April of this year regarding possible catastrophic complications, "suggests that this requirement should be reconsidered," he said.
Dr. Gunnar B.J. Andersson is in the department of orthopedic surgery at Rush University Medical Center, Chicago. These remarks were taken from his editorial accompanying Dr. Friedly’s report (N. Engl. J. Med. 2014 July 3 [doi: 10.1056/NEJMe1405475]).
These findings raise serious questions about the benefits of epidural glucocorticoid injections for spinal stenosis. Patients seeking them should be informed that the best available data do not support a clinically significant long-term benefit, and that complications, though rare, are possible, said Dr. Gunnar B.J. Andersson.
Currently, many insurance companies require that epidural injections be performed as part of nonsurgical treatment before surgery will be approved. The study by Dr. Friedly and her colleagues, together with the Food and Drug Administration safety announcement in April of this year regarding possible catastrophic complications, "suggests that this requirement should be reconsidered," he said.
Dr. Gunnar B.J. Andersson is in the department of orthopedic surgery at Rush University Medical Center, Chicago. These remarks were taken from his editorial accompanying Dr. Friedly’s report (N. Engl. J. Med. 2014 July 3 [doi: 10.1056/NEJMe1405475]).
These findings raise serious questions about the benefits of epidural glucocorticoid injections for spinal stenosis. Patients seeking them should be informed that the best available data do not support a clinically significant long-term benefit, and that complications, though rare, are possible, said Dr. Gunnar B.J. Andersson.
Currently, many insurance companies require that epidural injections be performed as part of nonsurgical treatment before surgery will be approved. The study by Dr. Friedly and her colleagues, together with the Food and Drug Administration safety announcement in April of this year regarding possible catastrophic complications, "suggests that this requirement should be reconsidered," he said.
Dr. Gunnar B.J. Andersson is in the department of orthopedic surgery at Rush University Medical Center, Chicago. These remarks were taken from his editorial accompanying Dr. Friedly’s report (N. Engl. J. Med. 2014 July 3 [doi: 10.1056/NEJMe1405475]).
Epidural injections of glucocorticoids plus lidocaine proved ineffective at improving pain or function in older adults with central spinal stenosis in a double-blind, randomized, controlled trial performed at 16 medical centers across the United States.
In the LESS (Lumbar Epidural Steroid Injections for Spinal Stenosis) trial, fluoroscopically guided transforaminal or interlaminar injections of lidocaine plus triamcinolone, betamethasone, dexamethasone, or methylprednisolone failed to reduce back, buttock, and leg pain or to enhance pain-related function any better than injections of lidocaine alone, reported Dr. Janna L. Friedly of the rehabilitation medicine department at the University of Washington, Seattle, and her associates. (Lidocaine alone has never been demonstrated to offer long-term benefit, they noted.)
The use of steroid injections for spinal stenosis has risen nearly 300% over the past 20 years even though "there is little evidence of effectiveness from clinical trials." The few studies that have examined this treatment have been "small, uncontrolled, or conducted without the use of fluoroscopy," the investigators said.
In their study, patients aged 50 years and older (mean age, 68 years) who had MRI- or CT-proven central spinal stenosis were enrolled over a 2-year period and randomly assigned to receive either a lidocaine plus glucocorticoid injection (200 intervention subjects) or a matching lidocaine injection (200 control subjects) under fluoroscopic guidance. All the study participants reported back, buttock, and leg pain scores of 5 or more on a scale of 0-10 (with 10 representing the worst pain imaginable) and disability scores of 7 or higher on the Roland-Morris Disability Questionnaire (RMDQ) scale of 0-24 (with higher scores indicating greater disability).
The patients were allowed to receive a repeat injection after 3 weeks, if they wished, at the discretion of their treating physicians. The physicians also chose whether to use the transforaminal or interlaminar approach, based on a variety of clinical factors.
At 6 weeks of follow-up, patients in both the glucocorticoid-lidocaine and lidocaine-only study groups reported some improvement in pain and function, but there was no significant difference in improvement between the two groups (–4.2 points and –3.1 points, respectively, on the RMDQ). There also were no significant differences across any of the subgroups of patients studied; in particular, steroid injections were no more effective than lidocaine alone according to the duration of spinal stenosis or the type of injection approach (transforaminal or interlaminar), Dr. Friedly and her associates said (N. Engl. J. Med. 2014 July 3 [doi: 10.1056/NEJMoa1313265]).
There were no differences in the percentage of patients in either group who achieved 30% or 50% improvement RMDQ scores at either 3 or 6 weeks, nor were there any differences in "any improvement" on pain scores or in 50% improvement on pain scores at 6 weeks.
There also were no differences between the groups in scores on secondary outcome measures, including the Brief Pain Inventory, which measures pain-related interference with activities; the Swiss Spinal Stenosis Questionnaire (SSSQ), which measures pain, function, and satisfaction with treatment; the EQ-5D, which measures quality of life; or the Generalized Anxiety Disorder-7 scale.
On the Patient Health Questionnaire-8, the glucocorticoid-lidocaine group reported more improvement in depression symptoms, and on one portion of the SSSQ, more patients in the glucocorticoid-lidocaine group (67%) than in the control group (54%) reported satisfaction with treatment. This is consistent with the systemic effects of cortisol suppression and is likely to be transient, the investigators said.
The glucocorticoid plus lidocaine injections were associated with more adverse effects (21.5% of patients) than were the lidocaine-only injections (15.5%), although the difference was not significant. However, the number of adverse events reported per person was significantly higher among patients who received glucocorticoid plus lidocaine injections (0.29 vs. 0.17), they added.
This study was funded by the Agency for Healthcare Research and Quality and received no commercial support. Dr. Friedly reported no potential financial conflicts of interest; her associates reported ties to Johnson & Johnson, ArthroCare, and other companies.
Epidural injections of glucocorticoids plus lidocaine proved ineffective at improving pain or function in older adults with central spinal stenosis in a double-blind, randomized, controlled trial performed at 16 medical centers across the United States.
In the LESS (Lumbar Epidural Steroid Injections for Spinal Stenosis) trial, fluoroscopically guided transforaminal or interlaminar injections of lidocaine plus triamcinolone, betamethasone, dexamethasone, or methylprednisolone failed to reduce back, buttock, and leg pain or to enhance pain-related function any better than injections of lidocaine alone, reported Dr. Janna L. Friedly of the rehabilitation medicine department at the University of Washington, Seattle, and her associates. (Lidocaine alone has never been demonstrated to offer long-term benefit, they noted.)
The use of steroid injections for spinal stenosis has risen nearly 300% over the past 20 years even though "there is little evidence of effectiveness from clinical trials." The few studies that have examined this treatment have been "small, uncontrolled, or conducted without the use of fluoroscopy," the investigators said.
In their study, patients aged 50 years and older (mean age, 68 years) who had MRI- or CT-proven central spinal stenosis were enrolled over a 2-year period and randomly assigned to receive either a lidocaine plus glucocorticoid injection (200 intervention subjects) or a matching lidocaine injection (200 control subjects) under fluoroscopic guidance. All the study participants reported back, buttock, and leg pain scores of 5 or more on a scale of 0-10 (with 10 representing the worst pain imaginable) and disability scores of 7 or higher on the Roland-Morris Disability Questionnaire (RMDQ) scale of 0-24 (with higher scores indicating greater disability).
The patients were allowed to receive a repeat injection after 3 weeks, if they wished, at the discretion of their treating physicians. The physicians also chose whether to use the transforaminal or interlaminar approach, based on a variety of clinical factors.
At 6 weeks of follow-up, patients in both the glucocorticoid-lidocaine and lidocaine-only study groups reported some improvement in pain and function, but there was no significant difference in improvement between the two groups (–4.2 points and –3.1 points, respectively, on the RMDQ). There also were no significant differences across any of the subgroups of patients studied; in particular, steroid injections were no more effective than lidocaine alone according to the duration of spinal stenosis or the type of injection approach (transforaminal or interlaminar), Dr. Friedly and her associates said (N. Engl. J. Med. 2014 July 3 [doi: 10.1056/NEJMoa1313265]).
There were no differences in the percentage of patients in either group who achieved 30% or 50% improvement RMDQ scores at either 3 or 6 weeks, nor were there any differences in "any improvement" on pain scores or in 50% improvement on pain scores at 6 weeks.
There also were no differences between the groups in scores on secondary outcome measures, including the Brief Pain Inventory, which measures pain-related interference with activities; the Swiss Spinal Stenosis Questionnaire (SSSQ), which measures pain, function, and satisfaction with treatment; the EQ-5D, which measures quality of life; or the Generalized Anxiety Disorder-7 scale.
On the Patient Health Questionnaire-8, the glucocorticoid-lidocaine group reported more improvement in depression symptoms, and on one portion of the SSSQ, more patients in the glucocorticoid-lidocaine group (67%) than in the control group (54%) reported satisfaction with treatment. This is consistent with the systemic effects of cortisol suppression and is likely to be transient, the investigators said.
The glucocorticoid plus lidocaine injections were associated with more adverse effects (21.5% of patients) than were the lidocaine-only injections (15.5%), although the difference was not significant. However, the number of adverse events reported per person was significantly higher among patients who received glucocorticoid plus lidocaine injections (0.29 vs. 0.17), they added.
This study was funded by the Agency for Healthcare Research and Quality and received no commercial support. Dr. Friedly reported no potential financial conflicts of interest; her associates reported ties to Johnson & Johnson, ArthroCare, and other companies.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: There is no evidence of a treatment effect at 6 weeks with fluoroscopically guided epidural injections of glucocorticoids plus lidocaine.
Major finding: At 6 weeks of follow-up, patients in both the glucocorticoid-lidocaine and lidocaine-only study groups reported some improvement in pain and function, but there was no significant difference in improvement between the two groups (–4.2 points and –3.1 points, respectively, on the RMDQ).
Data source: A multicenter, double-blind, randomized, controlled trial involving 400 patients aged 50 years and older with central spinal stenosis who received epidural injections of either glucocorticoids plus lidocaine or lidocaine alone and were followed for 6 weeks.
Disclosures: This study was funded by the Agency for Healthcare Research and Quality and received no commercial support. Dr. Friedly reported no potential financial conflicts of interest; her associates reported ties to Johnson & Johnson, ArthroCare, and other companies.