User login
Humeral Bone Loss in Revision Shoulder Arthroplasty
ABSTRACT
Revision shoulder arthroplasty is becoming more prevalent as the rate of primary shoulder arthroplasty in the US continues to increase. The management of proximal humeral bone loss in the revision setting presents a difficult problem without a clear solution. Different preoperative diagnoses often lead to distinctly different patterns of bone loss. Successful management of these cases requires a clear understanding of the normal anatomy of the proximal humerus, as well as structural limitations imposed by significant bone loss and the effect this loss has on component fixation. Our preferred technique differs depending on the pattern of bone loss encountered. The use of allograft-prosthetic composites, the cement-within-cement technique, and combinations of these strategies comprise the mainstay of our treatment algorithm. This article focuses on indications, surgical techniques, and some of the published outcomes using these strategies in the management of proximal humeral bone loss.
Continue to: The demand for shoulder arthroplasty...
The demand for shoulder arthroplasty (SA) has increased significantly over the past decade, with a 200% increase witnessed from 2011 to 2015.1 SA performed in patients younger than 55 years is expected to increase 333% between 2011 to 2030.2 With increasing rates of SA being performed in younger patient populations, rates of revision SA also can be expected to climb. Revision to reverse shoulder arthroplasty (RSA) has arisen as a viable option in these patients, and multiple studies demonstrate excellent outcomes that can be obtained with RSA.3-11
Despite significant improvements obtained in revision SA since the mainstream acceptance of RSA, bone loss remains a problematic issue. Loss of humeral bone stock, in particular, can be a challenging problem to solve with multiple clinical implications. Biomechanical studies have demonstrated that if bone loss is left unaddressed, increased bending and torsional forces on the prosthesis result, which ultimately contribute to increased micromotion and eventual component failure.12 In addition, existing challenges are associated with the lack of attachment sites for both multiple muscles and tendons. Also, there is a loss of the normal lateralized pull of the deltoid, which results in a decreased amount of force generated by this muscle.13,14 Ultimately, the increased loss of bone can lead to a devastating situation where there is not enough bone to provide adequate fixation while maintaining the appropriate humeral length necessary to achieve stability of the articulation, which will inevitably lead to instability.4,15 Therefore, techniques are needed to address proximal humeral bone loss while maintaining as much native humeral bone as possible.
PROXIMAL HUMERUS: ANATOMICAL CONSIDERATIONS
The anatomy of the proximal humerus has been studied in great detail and reported in a number of different studies.16-23 The average humeral head thickness (24 mm in men and 19 mm in women) and offset relative to the humeral shaft (2.1 mm posterior and 6.6 mm medial) act to tension the rotator cuff musculature appropriately and contribute to a wrapping effect that allows the deltoid to function more effectively.13,14 Knowledge regarding the rotator cuff footprint has advanced over the past 10 years, specifically with regard to the supraspinatus and infraspinatus.24 The current belief is that the supraspinatus has a triangular insertion onto the most anterior aspect of the greater tuberosity, with a maximum medial-to-lateral length of 6.9 mm and a maximum anterior-to-posterior width of 12.6 mm. The infraspinatus insertion has a trapezoidal insertion, with a maximum medial-to-lateral length of 10.2 mm and anterior-to-posterior width of 32.7 mm. The subscapularis, by far the largest of all the rotator cuff muscles, has a complex geometry with regard to its insertion on the lesser tuberosity, with 4 different insertion points and an overall lateral footprint measuring 37.6 mm and a medial footprint measuring 40.7 mm.25 Finally, the teres minor, with the smallest volume of all the rotator cuff muscles, inserts immediately inferior to the infraspinatus along the inferior facet of the greater tuberosity.26
Aside from the rotator cuff, there are various other muscles and tendons that insert about the proximal humerus and are essential for normal function. The deltoid, which inserts at a point approximately 6 cm from the greater tuberosity along the length of the humerus, with an insertion length between 5 cm to 7 cm,13,27 is the primary mover of the shoulder and essential for proper function after RSA.28,29 The pectoralis major tendon, which begins inserting at a point approximately 5.6 cm from the humeral head and spans a distance of 7.7 cm along the length of the humerus,30-32 is important not only for function but as an anatomical landmark in reconstruction. Lastly, the latissimus dorsi and teres major, which share a role in extension, adduction, and internal rotation of the glenohumeral joint, insert along the floor and medial lip of the intertubercular groove of the humerus, respectively.33,34 In addition to their role in tendon transfer procedures because of treating irreparable posterosuperior cuff and subscapularis tears,35,36 it has been suggested that these tendons may play some role in glenohumeral joint stability.37
In addition to the loss of muscular attachments, the absence of proximal humeral bone stock, in and of itself, can have deleterious effects on fixation of the humeral component. RSA is a semiconstrained device, which results in increased transmission of forces to the interface between the humeral implant and its surrounding structures, including cement (when present) and the bone itself. When there is the absence of significant amounts of bone, the remaining bone must now account for an even higher proportion of these forces. A previous biomechanical study showed that cemented humeral stems demonstrated significantly increased micromotion in the presence of proximal humeral bone loss, particularly when a modular humeral component was used.12
Continue to: TYPES OF BONE LOSS
TYPES OF BONE LOSS
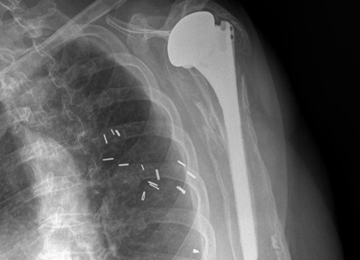
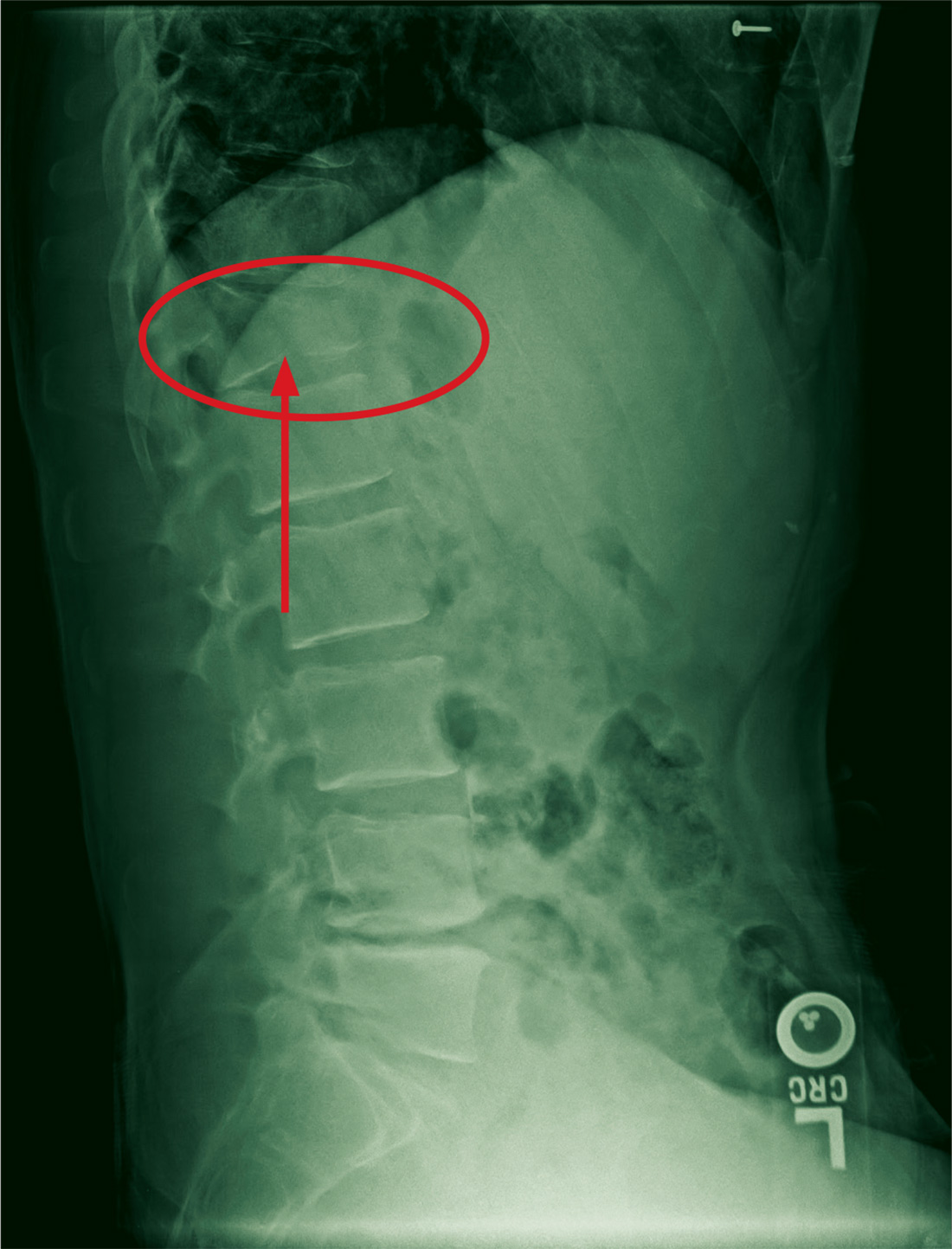
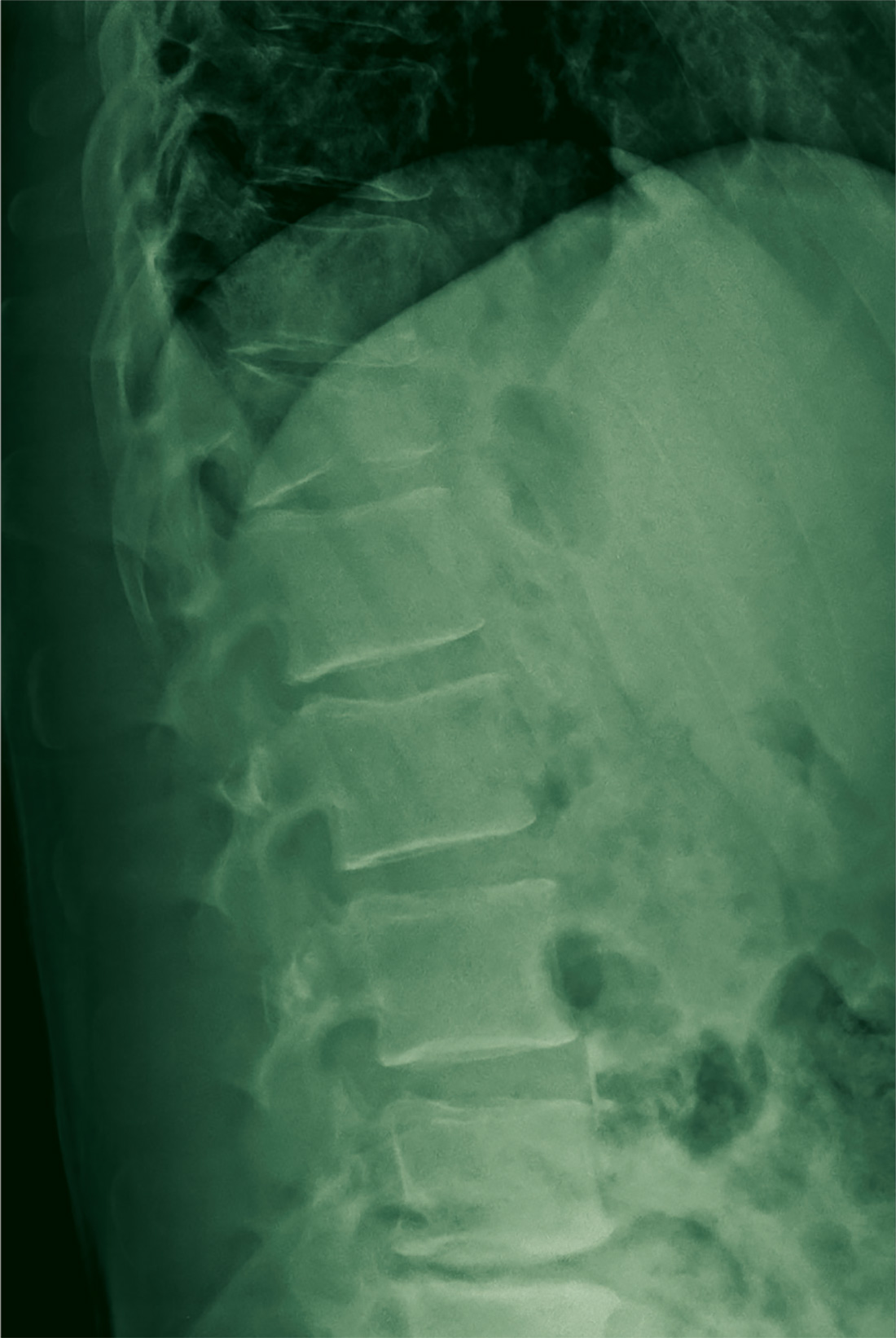
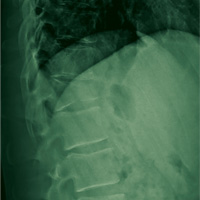
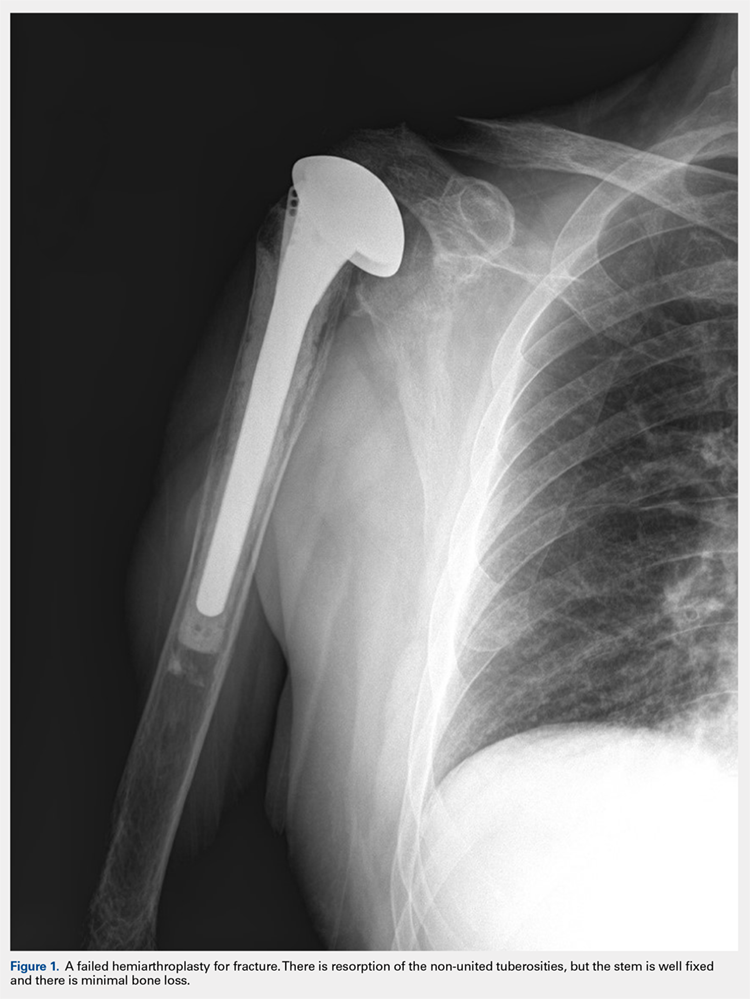
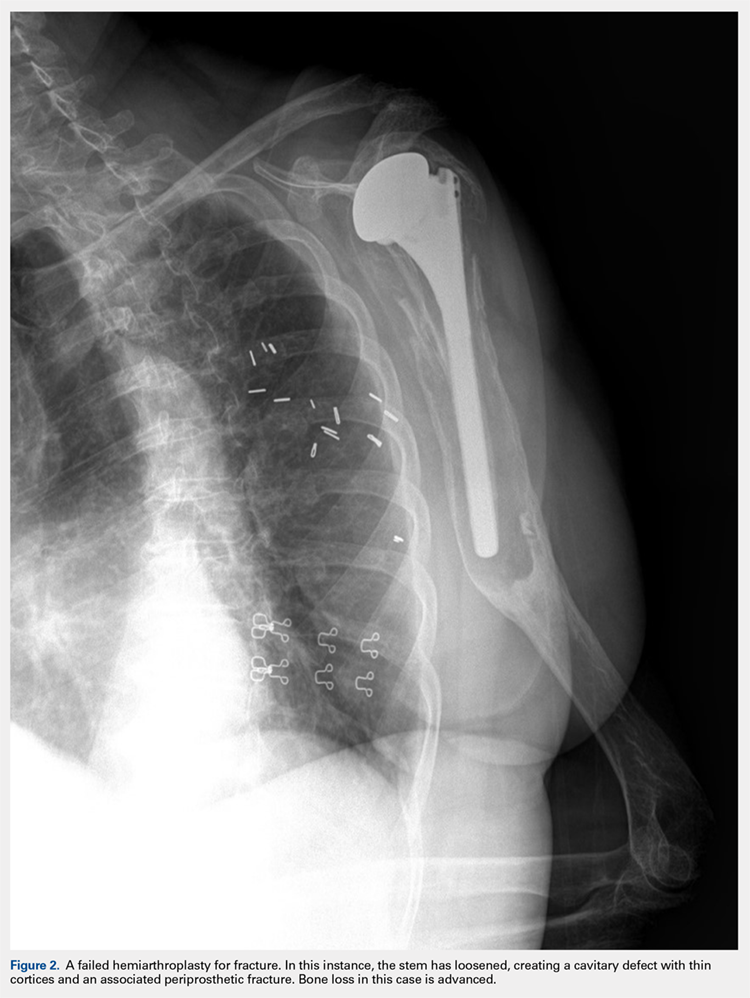
There are a variety of different etiologies of proximal humeral bone loss that result in distinctly different clinical presentations. These can be fairly mild, as is the case of isolated resorption of the greater tuberosity in a non-united proximal humerus fracture (Figure 1). Alternatively, they can be severe, as seen in a grossly loose cemented long-stemmed component that is freely mobile, creating a windshield-wiper effect throughout the length of the humerus (Figure 2). This can be somewhat deceiving, however, as the amount of bone loss, as well as the pathophysiologic process that led to the bone loss, are important factors to determine ideal reconstructive methods. In the case of a failed open reduction internal fixation, where the tuberosity has failed to unite or has been resected, there is much less of a biologic response in comparison with implant loosening associated with osteolysis. This latter condition will be associated with a much more destructive inflammatory response resulting in poor tissue quality and often dramatic thinning of the cortex. If one simply measured the distance from the most proximal remaining bone stock to the area where the greater tuberosity should be, a loose stem with subsidence and ballooning of the cortices may appear to have a similar amount of bone loss as a failed hemiarthroplasty for fracture with a well-fixed stem. However, intraoperatively, one will find that the bone that appeared to be present radiographically in the case of the loose stem is of such poor quality that it cannot reasonably provide any beneficial fixation. In light of this, different treatment modalities are recommended for different types of bone loss, and the revision surgeon must be able to anticipate this and possess a full armamentarium of options to treat these challenging cases successfully.
INDICATIONS
Our technique to manage proximal humeral bone loss is dependent on both the quantity of bone loss, which can be measured radiographically, as well as the anticipated inflammatory response described above. As both the destructive process and the amount of bone loss increase, the importance of more advanced reconstructive procedures that will sustain implant security and soft-tissue management becomes apparent. In the least destructive cases with <5 cm of bone loss, successful revision can typically be accomplished with stem removal and placement of a new monoblock humeral stem. In cases where more advanced destructive pathology is present, and bone loss is >5 cm, an allograft-prosthetic composite (APC) is typically used. In both scenarios, if the stem being revised is cemented and the cement mantle remains intact, and of reasonable length, consideration is given to the cement-within-cement technique. Finally, in the most destructive cases where bone loss exceeds 10 cm and a large biological response is anticipated (eg, periprosthetic fractures with humeral loosening), the use of a longer diaphyseal-incorporating APC is often necessary. This prosthetic composite can be combined with a cement-within-cement technique as well.
It is important to comment on the implications of using modular stems in this setting. With advanced bone loss, a situation is often encountered where the newly implanted stem geometry and working length may be insufficient to acquire adequate rotational stability. In this setting, if a modular junction is positioned close to the stem and cement/bone interface, it will be exposed to very high stress concentrations which can lead to component fracture38 as well as taper corrosion, also referred to as trunnionosis. This latter phenomenon, which has been well studied in the total hip arthroplasty literature with the use of modular components,39 is especially relevant given the high torsional loads imparted at the modular junction. Ultimately, high torsional loads lead to micromotion and electrochemical ion release via degradation of the passivation layer, initiating the process of mechanically assisted crevice corrosion.40 For these reasons, when a modular stem must be used in the presence of mild to moderate bone loss, using a proximal humeral allograft to protect the junction or to provide additional fixation may be implemented with a lower threshold than when using a monoblock stem.
SURGICAL TECHNIQUE: ALLOGRAFT-PROSTHETIC COMPOSITES
A standard deltopectoral approach is used, taking care to preserve all viable muscular attachments to the proximal humerus. After removal of the prosthetic humeral head, the decision to proceed with removal of the stem at this juncture is based on several factors. If the remaining proximal humeral bone is so compromised that it might not be able to withstand the forces exerted upon it during retraction for glenoid exposure, the component is left in place. Additionally, if there is consideration that the glenoid-sided bone loss may be so severe that a glenoid baseplate cannot be implanted, and the stem remains well fixed, it is retained so that it can be converted to a hemiarthroplasty.
If neither of the above issues is present, the humeral stem is removed. If a well-fixed press-fit stem is in place, it is typically removed using a combination of burrs and osteotomes to disrupt the bone-implant interface, and the stem is then carefully removed using an impactor and mallet. If a cemented stem is present, the stem is removed in a similar manner, and the cement mantle is left in place if stable, in anticipation of a cement-within-cement technique. If the mantle is disrupted, standard cement removal instruments are used to remove all cement from the canal meticulously.
Continue to: Management of the glenoid...
Management of the glenoid can have significant implications with regard to the humerus. Most notably, the size of the glenosphere has direct implications on the fixation of the humeral component. Use of larger diameter glenospheres result in increased contact area between the glenosphere and humerosocket, adding constraint to the articulation and further increasing the stresses at the implant-bone interface. As such, the use of larger glenospheres to prevent instability must be balanced with the resulting implications on humeral component fixation, especially in cases of severe bone loss.
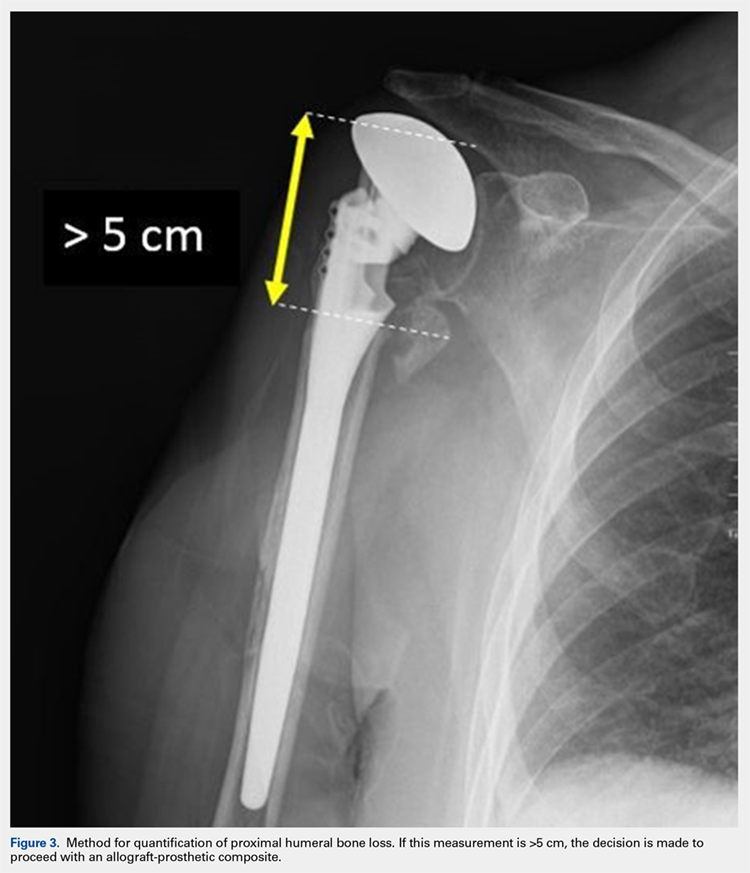
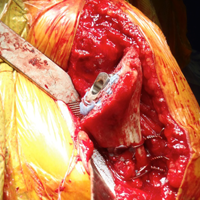
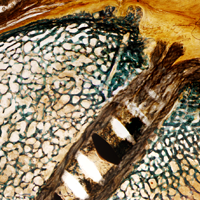
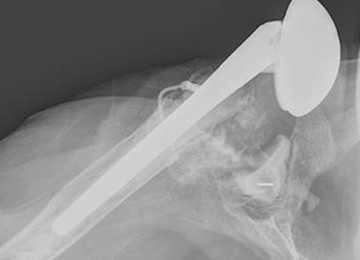
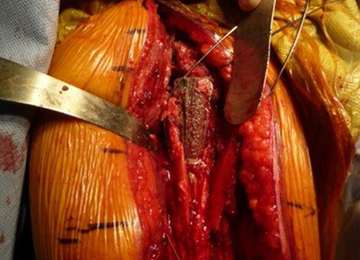
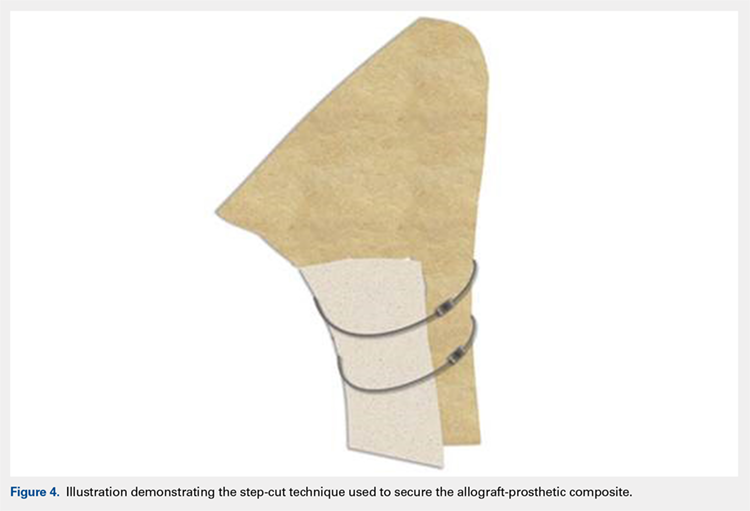
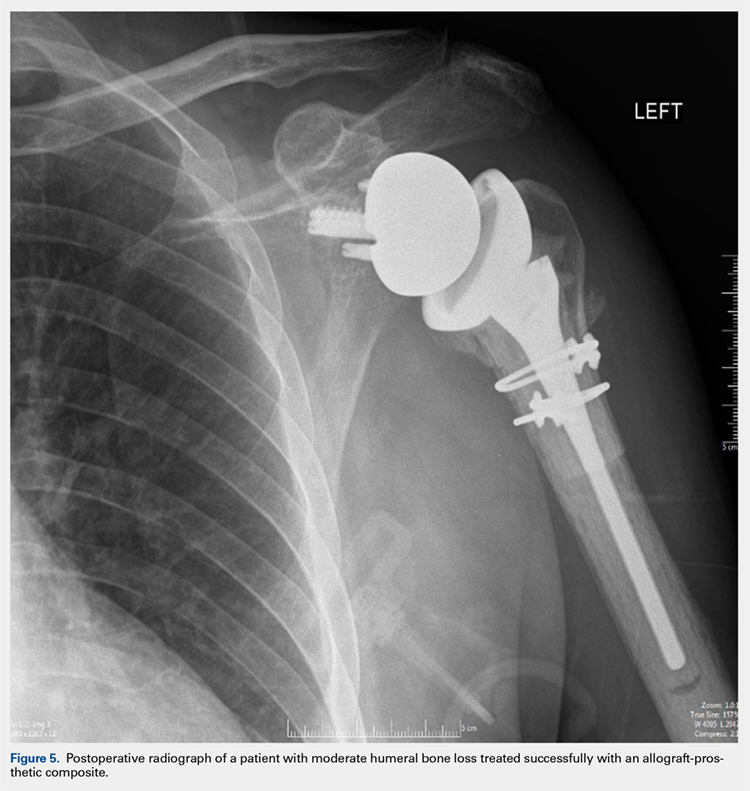
After implanting the appropriate glenosphere, attention is then turned back to the humerus. Trial implants are sequentially used to obtain adequate humeral length and stability. Once this is accomplished, the amount of humeral bone loss is quantified by measuring the distance from the superior aspect of the medial humeral tray to the medial humeral shaft. If this number is >5 cm (Figure 3), the decision is made to proceed with an APC. The allograft humeral head is cut, cancellous bone is removed, and a step-cut is performed, with the medial portion of the allograft measuring the same length as that of bone loss and the lateral plate extending an additional several centimeters distally (Figure 4). Additional soft tissue is removed from the allograft, leaving the subscapularis stump intact for later repair with the patient’s native tissue. The allograft is secured to the patient’s proximal humerus using multiple cerclage wires, and the humeral stem is cemented into place. The final construct is shown in Figure 5.
ADDITIONAL CONSIDERATIONS: CASES OF ADVANCED BONE LOSS
In cases of advanced humeral bone loss, as is often seen when revising loose humeral stems, larger allografts that span a significant length of the diaphysis are often required. This type of bone loss has implications with regard to how the deltoid insertion is managed. Interestingly, even in situations when the vast majority of the remaining diaphysis consists of ectatic egg-shell bone, the deltoid tuberosity remains of fairly substantial quality due to the continued pull of the muscular insertion on this area. This fragment is isolated, carefully mobilized, and subsequently repaired back on top of the allograft using cables.
POSTOPERATIVE CARE
Patients are kept in a shoulder immobilizer for 6 weeks after surgery to facilitate allograft incorporation and subscapularis tendon healing. During this time, pendulum exercises are initiated. Active assisted range of motion (ROM) exercises begin after 6 weeks, consisting of supine forward elevation. A sling is given to be used in public. Light strengthening exercises begin at 3 months postoperatively.
DISCUSSION
In cases of mild to moderate proximal humeral bone loss, RSA using a long-stem humeral component without allograft augmentation is a viable option. Budge and colleagues38 demonstrated excellent results in a population of 15 patients with an average of 38 mm of proximal humeral bone loss without use of allografts. Interestingly, they noted 1 case of component fracture in a modular prosthesis and therefore concluded that monoblock humeral stems should be used in the absence of allograft augmentation.
Continue to: In more advanced cases of bone loss...
In more advanced cases of bone loss, our data shows that use of APCs can result in equally satisfactory results. In a series of 25 patients with an average bone loss of 54 mm, patients were able to achieve statistically significant improvements in pain, ROM, and function with high rates of allograft incorporation.9 Overall, a low rate of complications was noted, including 1 infection. This finding is consistent with an additional study looking specifically at factors associated with infection in revision SA, which found that the use of allografts was not associated with increased risk of infection.41
As stated previously, the size of allograft needed for the APC construct is related to the distinct pathology encountered. In our experience, we have noted that well-fixed stems can be treated with short metaphyseal APCs in 85% of cases. On the other hand, loose stems require long allografts measuring >10 cm in 90% of cases. As such, these cases typically require mobilization of the deltoid insertion as described above, and therefore it is important that the surgeon is prepared for this aspect of the procedure preoperatively.
Finally, the cement-within-cement technique, originally popularized for use in revision total hip arthroplasty, has demonstrated reliable results when utilized in revision SA.42 To date, there are no recommendations regarding the minimal length of existing cement mantle that is needed to perform this technique. In situations in which the length of the cement mantle is questionable, our preference is to combine the cement-within-cement technique with an APC when possible.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi:10.1016/j.jse.2010.02.009.
2. Padegimas EM, Maltenfort M, Lazarus MD, Ramsey ML, Williams GR, Namdari S. Future patient demand for shoulder arthroplasty by younger patients: national projections. Clin Orthop Relat Res. 2015;473(6):1860-1867. doi:10.1007/s11999-015-4231-z.
3. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522. doi:10.1016/j.jse.2011.03.006.
4. Levy JC, Virani N, Pupello D, et al. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195. doi:10.1302/0301-620X.89B2.
5. Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21(3):342-349. doi:10.1016/j.jse.2011.05.021.
6. Deutsch A, Abboud JA, Kelly J, et al. Clinical results of revision shoulder arthroplasty for glenoid component loosening. J Shoulder Elbow Surg. 2007;16(6):706-716. doi:10.1016/j.jse.2007.01.007.
7. Kelly JD, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525. doi:10.1016/j.jse.2011.11.021.
8. Black EM, Roberts SM, Siegel E, Yannopoulos P, Higgins LD, Warner JJP. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg. 2014;23(7):1036-1042. doi:10.1016/j.jse.2014.02.019.
9. Composite P, Chacon BA, Virani N, et al. Revision arthroplasty with use of a reverse shoulder. J Bone Joint Surg. 2009;1:119-127. doi:10.2106/JBJS.H.00094.
10. Klein SM, Dunning P, Mulieri P, Pupello D, Downes K, Frankle MA. Effects of acquired glenoid bone defects on surgical technique and clinical outcomes in reverse shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1144-1154. doi:10.2106/JBJS.I.00778.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
12. Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651. doi:10.1016/j.jse.2010.10.026.
13. Morgan SJ, Furry K, Parekh A, Agudelo JF, Smith WR. The deltoid muscle: an anatomic description of the deltoid insertion to the proximal humerus. J Orthop Trauma. 2006;20(1):19-21. doi:10.1097/01.bot.0000187063.43267.18.
14. Gagey O, Hue E. Mechanics of the deltoid muscle. A new approach. Clin Orthop Relat Res. 2000;375:250-257. doi:10.1097/00003086-200006000-00030.
15. De Wilde L, Plasschaert F. Prosthetic treatment and functional recovery of the shoulder after tumor resection 10 years ago: a case report. J Shoulder Elbow Surg. 2005;14(6):645-649. doi:10.1016/j.jse.2004.11.001.
16. Wataru S, Kazuomi S, Yoshikazu N, Hiroaki I, Takaharu Y, Hideki Y. Three-dimensional morphological analysis of humeral heads: a study in cadavers. Acta Orthop. 2005;76(3):392-396. doi:10.1080/00016470510030878.
17. Tillett E, Smith M, Fulcher M, Shanklin J. Anatomic determination of humeral head retroversion: the relationship of the central axis of the humeral head to the bicipital groove. J Shoulder Elbow Surg. 1993;2(5):255-256. doi:10.1016/S1058-2746(09)80085-2.
18. Doyle AJ, Burks RT. Comparison of humeral head retroversion with the humeral axis/biceps groove relationship: a study in live subjects and cadavers. J Shoulder Elbow Surg. 1998;7(5):453-457. doi:10.1016/S1058-2746(98)90193-8.
19. Johnson JW, Thostenson JD, Suva LJ, Hasan SA. Relationship of bicipital groove rotation with humeral head retroversion: a three-dimensional computed tomographic analysis. J Bone Joint Surg Am. 2013;95(8):719-724. doi:10.2106/JBJS.J.00085.
20. Hromádka R, Kuběna AA, Pokorný D, Popelka S, Jahoda D, Sosna A. Lesser tuberosity is more reliable than bicipital groove when determining orientation of humeral head in primary shoulder arthroplasty. Surg Radiol Anat. 2010;32(1):31-37. doi:10.1007/s00276-009-0543-6.
21. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338. doi:10.1067/mse.2002.124429.
22. Pearl ML. Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. J Shoulder Elbow Surg. 2005;14(suppl 1):99-104. doi:10.1016/j.jse.2004.09.025.
23. Robertson DD, Yuan J, Bigliani LU, Flatow EL, Yamaguchi K. Three-dimensional analysis of the proximal part of the humerus: relevance to arthroplasty. J Bone Joint Surg Am. 2000;82-A(11):1594-1602.
24. Mochizuki T, Sugaya H, Uomizu M, et al. Humeral insertion of the supraspinatus and infraspinatus. J Bone Joint Surg Am. 2008;90(5):962-969. doi:10.2106/JBJS.G.00427.
25. Arai R, Sugaya H, Mochizuki T, Nimura A, Moriishi J, Akita K. Subscapularis tendon tear: an anatomic and clinical investigation. Arthroscopy. 2008;24(9):997-1004. doi:10.1016/j.arthro.2008.04.076.
26. Nimura A, Kato A, Yamaguchi K, et al. The superior capsule of the shoulder joint complements the insertion of the rotator cuff. J Shoulder Elbow Surg. 2012;21(7):867-872. doi:10.1016/j.jse.2011.04.034.
27. Rispoli DM, Athwal GS, Sperling JW, Cofield RH. The anatomy of the deltoid insertion. J Shoulder Elbow Surg. 2009;18(3):386-390. doi:10.1016/j.jse.2008.10.012.
28. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364. doi:10.1016/j.jse.2012.02.002.
29. Walker M, Brooks J, Willis M, Frankle M. How reverse shoulder arthroplasty works. Clinical Orthop Relat Res. 2011;469(9):2440-2451. doi:10.1007/s11999-011-1892-0.
30. Torrens C, Corrales M, Melendo E, Solano A, Rodríguez-Baeza A, Cáceres E. The pectoralis major tendon as a reference for restoring humeral length and retroversion with hemiarthroplasty for fracture. J Shoulder Elbow Surg. 2008;17(6):947-950. doi:10.1016/j.jse.2008.05.041.
31. Ponce BA, Thompson KJ, Rosenzweig SD, et al. Re-evaluation of pectoralis major height as an anatomic reference for humeral height in fracture hemiarthroplasty. J Shoulder Elbow Surg. 2013;22(11):1567-1572. doi:10.1016/j.jse.2013.01.039.
32. LaFrance R, Madsen W, Yaseen Z, Giordano B, Maloney M, Voloshin I. Relevant anatomic landmarks and measurements for biceps tenodesis. Am J Sports Med. 2013;41(6):1395-1399. doi:10.1177/0363546513482297.
33. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
34. Bhatt CR, Prajapati B, Patil DS, Patel VD, Singh BGP, Mehta CD. Variation in the insertion of the latissimus dorsi & its clinical importance. J Orthop. 2013;10(1):25-28. doi:10.1016/j.jor.2013.01.002.
35. Gerber C, Maquieira G, Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg. 2006;88(1):113-120. doi:10.2106/JBJS.E.00282.
36. Elhassan B, Christensen TJ, Wagner ER. Feasibility of latissimus and teres major transfer to reconstruct irreparable subscapularis tendon tear: an anatomic study. J Shoulder Elbow Surg. 2014;23(4):492-499. doi:10.1016/j.jse.2013.07.046.
37. Pouliart N, Gagey O. Significance of the latissimus dorsi for shoulder instability. II. Its influence on dislocation behavior in a sequential cutting protocol of the glenohumeral capsule. Clin Anat. 2005;18(7):500-509. doi:10.1002/ca.20181.
38. Budge MD, Moravek JE, Zimel MN, Nolan EM, Wiater JM. Reverse total shoulder arthroplasty for the management of failed shoulder arthroplasty with proximal humeral bone loss: is allograft augmentation necessary? J Shoulder Elbow Surg. 2013;22(6):739-744. doi:10.1016/j.jse.2012.08.008.
39. Weiser MC, Lavernia CJ. Trunnionosis in total hip arthroplasty. J Bone Joint Surg Am. 2017;99(17):27-29. doi:10.2106/JBJS.17.00345.
40. Cohen J. Current concepts review. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80(10):1554.
41. Meijer ST, Paulino Pereira NR, Nota SPFT, Ferrone ML, Schwab JH, Lozano Calderón SA. Factors associated with infection after reconstructive shoulder surgery for proximal humerus tumors. J Shoulder Elbow Surg. 2017;26(6):931-938. doi:10.1016/j.jse.2016.10.014.
42. Wagner ER, Houdek MT, Hernandez NM, Cofield RH, Sánchez-Sotelo J, Sperling JW. Cement-within-cement technique in revision reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(8):1448-1453. doi:10.1016/j.jse.2017.01.013.
ABSTRACT
Revision shoulder arthroplasty is becoming more prevalent as the rate of primary shoulder arthroplasty in the US continues to increase. The management of proximal humeral bone loss in the revision setting presents a difficult problem without a clear solution. Different preoperative diagnoses often lead to distinctly different patterns of bone loss. Successful management of these cases requires a clear understanding of the normal anatomy of the proximal humerus, as well as structural limitations imposed by significant bone loss and the effect this loss has on component fixation. Our preferred technique differs depending on the pattern of bone loss encountered. The use of allograft-prosthetic composites, the cement-within-cement technique, and combinations of these strategies comprise the mainstay of our treatment algorithm. This article focuses on indications, surgical techniques, and some of the published outcomes using these strategies in the management of proximal humeral bone loss.
Continue to: The demand for shoulder arthroplasty...
The demand for shoulder arthroplasty (SA) has increased significantly over the past decade, with a 200% increase witnessed from 2011 to 2015.1 SA performed in patients younger than 55 years is expected to increase 333% between 2011 to 2030.2 With increasing rates of SA being performed in younger patient populations, rates of revision SA also can be expected to climb. Revision to reverse shoulder arthroplasty (RSA) has arisen as a viable option in these patients, and multiple studies demonstrate excellent outcomes that can be obtained with RSA.3-11
Despite significant improvements obtained in revision SA since the mainstream acceptance of RSA, bone loss remains a problematic issue. Loss of humeral bone stock, in particular, can be a challenging problem to solve with multiple clinical implications. Biomechanical studies have demonstrated that if bone loss is left unaddressed, increased bending and torsional forces on the prosthesis result, which ultimately contribute to increased micromotion and eventual component failure.12 In addition, existing challenges are associated with the lack of attachment sites for both multiple muscles and tendons. Also, there is a loss of the normal lateralized pull of the deltoid, which results in a decreased amount of force generated by this muscle.13,14 Ultimately, the increased loss of bone can lead to a devastating situation where there is not enough bone to provide adequate fixation while maintaining the appropriate humeral length necessary to achieve stability of the articulation, which will inevitably lead to instability.4,15 Therefore, techniques are needed to address proximal humeral bone loss while maintaining as much native humeral bone as possible.
PROXIMAL HUMERUS: ANATOMICAL CONSIDERATIONS
The anatomy of the proximal humerus has been studied in great detail and reported in a number of different studies.16-23 The average humeral head thickness (24 mm in men and 19 mm in women) and offset relative to the humeral shaft (2.1 mm posterior and 6.6 mm medial) act to tension the rotator cuff musculature appropriately and contribute to a wrapping effect that allows the deltoid to function more effectively.13,14 Knowledge regarding the rotator cuff footprint has advanced over the past 10 years, specifically with regard to the supraspinatus and infraspinatus.24 The current belief is that the supraspinatus has a triangular insertion onto the most anterior aspect of the greater tuberosity, with a maximum medial-to-lateral length of 6.9 mm and a maximum anterior-to-posterior width of 12.6 mm. The infraspinatus insertion has a trapezoidal insertion, with a maximum medial-to-lateral length of 10.2 mm and anterior-to-posterior width of 32.7 mm. The subscapularis, by far the largest of all the rotator cuff muscles, has a complex geometry with regard to its insertion on the lesser tuberosity, with 4 different insertion points and an overall lateral footprint measuring 37.6 mm and a medial footprint measuring 40.7 mm.25 Finally, the teres minor, with the smallest volume of all the rotator cuff muscles, inserts immediately inferior to the infraspinatus along the inferior facet of the greater tuberosity.26
Aside from the rotator cuff, there are various other muscles and tendons that insert about the proximal humerus and are essential for normal function. The deltoid, which inserts at a point approximately 6 cm from the greater tuberosity along the length of the humerus, with an insertion length between 5 cm to 7 cm,13,27 is the primary mover of the shoulder and essential for proper function after RSA.28,29 The pectoralis major tendon, which begins inserting at a point approximately 5.6 cm from the humeral head and spans a distance of 7.7 cm along the length of the humerus,30-32 is important not only for function but as an anatomical landmark in reconstruction. Lastly, the latissimus dorsi and teres major, which share a role in extension, adduction, and internal rotation of the glenohumeral joint, insert along the floor and medial lip of the intertubercular groove of the humerus, respectively.33,34 In addition to their role in tendon transfer procedures because of treating irreparable posterosuperior cuff and subscapularis tears,35,36 it has been suggested that these tendons may play some role in glenohumeral joint stability.37
In addition to the loss of muscular attachments, the absence of proximal humeral bone stock, in and of itself, can have deleterious effects on fixation of the humeral component. RSA is a semiconstrained device, which results in increased transmission of forces to the interface between the humeral implant and its surrounding structures, including cement (when present) and the bone itself. When there is the absence of significant amounts of bone, the remaining bone must now account for an even higher proportion of these forces. A previous biomechanical study showed that cemented humeral stems demonstrated significantly increased micromotion in the presence of proximal humeral bone loss, particularly when a modular humeral component was used.12
Continue to: TYPES OF BONE LOSS
TYPES OF BONE LOSS
There are a variety of different etiologies of proximal humeral bone loss that result in distinctly different clinical presentations. These can be fairly mild, as is the case of isolated resorption of the greater tuberosity in a non-united proximal humerus fracture (Figure 1). Alternatively, they can be severe, as seen in a grossly loose cemented long-stemmed component that is freely mobile, creating a windshield-wiper effect throughout the length of the humerus (Figure 2). This can be somewhat deceiving, however, as the amount of bone loss, as well as the pathophysiologic process that led to the bone loss, are important factors to determine ideal reconstructive methods. In the case of a failed open reduction internal fixation, where the tuberosity has failed to unite or has been resected, there is much less of a biologic response in comparison with implant loosening associated with osteolysis. This latter condition will be associated with a much more destructive inflammatory response resulting in poor tissue quality and often dramatic thinning of the cortex. If one simply measured the distance from the most proximal remaining bone stock to the area where the greater tuberosity should be, a loose stem with subsidence and ballooning of the cortices may appear to have a similar amount of bone loss as a failed hemiarthroplasty for fracture with a well-fixed stem. However, intraoperatively, one will find that the bone that appeared to be present radiographically in the case of the loose stem is of such poor quality that it cannot reasonably provide any beneficial fixation. In light of this, different treatment modalities are recommended for different types of bone loss, and the revision surgeon must be able to anticipate this and possess a full armamentarium of options to treat these challenging cases successfully.

INDICATIONS
Our technique to manage proximal humeral bone loss is dependent on both the quantity of bone loss, which can be measured radiographically, as well as the anticipated inflammatory response described above. As both the destructive process and the amount of bone loss increase, the importance of more advanced reconstructive procedures that will sustain implant security and soft-tissue management becomes apparent. In the least destructive cases with <5 cm of bone loss, successful revision can typically be accomplished with stem removal and placement of a new monoblock humeral stem. In cases where more advanced destructive pathology is present, and bone loss is >5 cm, an allograft-prosthetic composite (APC) is typically used. In both scenarios, if the stem being revised is cemented and the cement mantle remains intact, and of reasonable length, consideration is given to the cement-within-cement technique. Finally, in the most destructive cases where bone loss exceeds 10 cm and a large biological response is anticipated (eg, periprosthetic fractures with humeral loosening), the use of a longer diaphyseal-incorporating APC is often necessary. This prosthetic composite can be combined with a cement-within-cement technique as well.

It is important to comment on the implications of using modular stems in this setting. With advanced bone loss, a situation is often encountered where the newly implanted stem geometry and working length may be insufficient to acquire adequate rotational stability. In this setting, if a modular junction is positioned close to the stem and cement/bone interface, it will be exposed to very high stress concentrations which can lead to component fracture38 as well as taper corrosion, also referred to as trunnionosis. This latter phenomenon, which has been well studied in the total hip arthroplasty literature with the use of modular components,39 is especially relevant given the high torsional loads imparted at the modular junction. Ultimately, high torsional loads lead to micromotion and electrochemical ion release via degradation of the passivation layer, initiating the process of mechanically assisted crevice corrosion.40 For these reasons, when a modular stem must be used in the presence of mild to moderate bone loss, using a proximal humeral allograft to protect the junction or to provide additional fixation may be implemented with a lower threshold than when using a monoblock stem.
SURGICAL TECHNIQUE: ALLOGRAFT-PROSTHETIC COMPOSITES
A standard deltopectoral approach is used, taking care to preserve all viable muscular attachments to the proximal humerus. After removal of the prosthetic humeral head, the decision to proceed with removal of the stem at this juncture is based on several factors. If the remaining proximal humeral bone is so compromised that it might not be able to withstand the forces exerted upon it during retraction for glenoid exposure, the component is left in place. Additionally, if there is consideration that the glenoid-sided bone loss may be so severe that a glenoid baseplate cannot be implanted, and the stem remains well fixed, it is retained so that it can be converted to a hemiarthroplasty.
If neither of the above issues is present, the humeral stem is removed. If a well-fixed press-fit stem is in place, it is typically removed using a combination of burrs and osteotomes to disrupt the bone-implant interface, and the stem is then carefully removed using an impactor and mallet. If a cemented stem is present, the stem is removed in a similar manner, and the cement mantle is left in place if stable, in anticipation of a cement-within-cement technique. If the mantle is disrupted, standard cement removal instruments are used to remove all cement from the canal meticulously.
Continue to: Management of the glenoid...
Management of the glenoid can have significant implications with regard to the humerus. Most notably, the size of the glenosphere has direct implications on the fixation of the humeral component. Use of larger diameter glenospheres result in increased contact area between the glenosphere and humerosocket, adding constraint to the articulation and further increasing the stresses at the implant-bone interface. As such, the use of larger glenospheres to prevent instability must be balanced with the resulting implications on humeral component fixation, especially in cases of severe bone loss.

After implanting the appropriate glenosphere, attention is then turned back to the humerus. Trial implants are sequentially used to obtain adequate humeral length and stability. Once this is accomplished, the amount of humeral bone loss is quantified by measuring the distance from the superior aspect of the medial humeral tray to the medial humeral shaft. If this number is >5 cm (Figure 3), the decision is made to proceed with an APC. The allograft humeral head is cut, cancellous bone is removed, and a step-cut is performed, with the medial portion of the allograft measuring the same length as that of bone loss and the lateral plate extending an additional several centimeters distally (Figure 4). Additional soft tissue is removed from the allograft, leaving the subscapularis stump intact for later repair with the patient’s native tissue. The allograft is secured to the patient’s proximal humerus using multiple cerclage wires, and the humeral stem is cemented into place. The final construct is shown in Figure 5.

ADDITIONAL CONSIDERATIONS: CASES OF ADVANCED BONE LOSS
In cases of advanced humeral bone loss, as is often seen when revising loose humeral stems, larger allografts that span a significant length of the diaphysis are often required. This type of bone loss has implications with regard to how the deltoid insertion is managed. Interestingly, even in situations when the vast majority of the remaining diaphysis consists of ectatic egg-shell bone, the deltoid tuberosity remains of fairly substantial quality due to the continued pull of the muscular insertion on this area. This fragment is isolated, carefully mobilized, and subsequently repaired back on top of the allograft using cables.

POSTOPERATIVE CARE
Patients are kept in a shoulder immobilizer for 6 weeks after surgery to facilitate allograft incorporation and subscapularis tendon healing. During this time, pendulum exercises are initiated. Active assisted range of motion (ROM) exercises begin after 6 weeks, consisting of supine forward elevation. A sling is given to be used in public. Light strengthening exercises begin at 3 months postoperatively.
DISCUSSION
In cases of mild to moderate proximal humeral bone loss, RSA using a long-stem humeral component without allograft augmentation is a viable option. Budge and colleagues38 demonstrated excellent results in a population of 15 patients with an average of 38 mm of proximal humeral bone loss without use of allografts. Interestingly, they noted 1 case of component fracture in a modular prosthesis and therefore concluded that monoblock humeral stems should be used in the absence of allograft augmentation.
Continue to: In more advanced cases of bone loss...
In more advanced cases of bone loss, our data shows that use of APCs can result in equally satisfactory results. In a series of 25 patients with an average bone loss of 54 mm, patients were able to achieve statistically significant improvements in pain, ROM, and function with high rates of allograft incorporation.9 Overall, a low rate of complications was noted, including 1 infection. This finding is consistent with an additional study looking specifically at factors associated with infection in revision SA, which found that the use of allografts was not associated with increased risk of infection.41
As stated previously, the size of allograft needed for the APC construct is related to the distinct pathology encountered. In our experience, we have noted that well-fixed stems can be treated with short metaphyseal APCs in 85% of cases. On the other hand, loose stems require long allografts measuring >10 cm in 90% of cases. As such, these cases typically require mobilization of the deltoid insertion as described above, and therefore it is important that the surgeon is prepared for this aspect of the procedure preoperatively.
Finally, the cement-within-cement technique, originally popularized for use in revision total hip arthroplasty, has demonstrated reliable results when utilized in revision SA.42 To date, there are no recommendations regarding the minimal length of existing cement mantle that is needed to perform this technique. In situations in which the length of the cement mantle is questionable, our preference is to combine the cement-within-cement technique with an APC when possible.
ABSTRACT
Revision shoulder arthroplasty is becoming more prevalent as the rate of primary shoulder arthroplasty in the US continues to increase. The management of proximal humeral bone loss in the revision setting presents a difficult problem without a clear solution. Different preoperative diagnoses often lead to distinctly different patterns of bone loss. Successful management of these cases requires a clear understanding of the normal anatomy of the proximal humerus, as well as structural limitations imposed by significant bone loss and the effect this loss has on component fixation. Our preferred technique differs depending on the pattern of bone loss encountered. The use of allograft-prosthetic composites, the cement-within-cement technique, and combinations of these strategies comprise the mainstay of our treatment algorithm. This article focuses on indications, surgical techniques, and some of the published outcomes using these strategies in the management of proximal humeral bone loss.
Continue to: The demand for shoulder arthroplasty...
The demand for shoulder arthroplasty (SA) has increased significantly over the past decade, with a 200% increase witnessed from 2011 to 2015.1 SA performed in patients younger than 55 years is expected to increase 333% between 2011 to 2030.2 With increasing rates of SA being performed in younger patient populations, rates of revision SA also can be expected to climb. Revision to reverse shoulder arthroplasty (RSA) has arisen as a viable option in these patients, and multiple studies demonstrate excellent outcomes that can be obtained with RSA.3-11
Despite significant improvements obtained in revision SA since the mainstream acceptance of RSA, bone loss remains a problematic issue. Loss of humeral bone stock, in particular, can be a challenging problem to solve with multiple clinical implications. Biomechanical studies have demonstrated that if bone loss is left unaddressed, increased bending and torsional forces on the prosthesis result, which ultimately contribute to increased micromotion and eventual component failure.12 In addition, existing challenges are associated with the lack of attachment sites for both multiple muscles and tendons. Also, there is a loss of the normal lateralized pull of the deltoid, which results in a decreased amount of force generated by this muscle.13,14 Ultimately, the increased loss of bone can lead to a devastating situation where there is not enough bone to provide adequate fixation while maintaining the appropriate humeral length necessary to achieve stability of the articulation, which will inevitably lead to instability.4,15 Therefore, techniques are needed to address proximal humeral bone loss while maintaining as much native humeral bone as possible.
PROXIMAL HUMERUS: ANATOMICAL CONSIDERATIONS
The anatomy of the proximal humerus has been studied in great detail and reported in a number of different studies.16-23 The average humeral head thickness (24 mm in men and 19 mm in women) and offset relative to the humeral shaft (2.1 mm posterior and 6.6 mm medial) act to tension the rotator cuff musculature appropriately and contribute to a wrapping effect that allows the deltoid to function more effectively.13,14 Knowledge regarding the rotator cuff footprint has advanced over the past 10 years, specifically with regard to the supraspinatus and infraspinatus.24 The current belief is that the supraspinatus has a triangular insertion onto the most anterior aspect of the greater tuberosity, with a maximum medial-to-lateral length of 6.9 mm and a maximum anterior-to-posterior width of 12.6 mm. The infraspinatus insertion has a trapezoidal insertion, with a maximum medial-to-lateral length of 10.2 mm and anterior-to-posterior width of 32.7 mm. The subscapularis, by far the largest of all the rotator cuff muscles, has a complex geometry with regard to its insertion on the lesser tuberosity, with 4 different insertion points and an overall lateral footprint measuring 37.6 mm and a medial footprint measuring 40.7 mm.25 Finally, the teres minor, with the smallest volume of all the rotator cuff muscles, inserts immediately inferior to the infraspinatus along the inferior facet of the greater tuberosity.26
Aside from the rotator cuff, there are various other muscles and tendons that insert about the proximal humerus and are essential for normal function. The deltoid, which inserts at a point approximately 6 cm from the greater tuberosity along the length of the humerus, with an insertion length between 5 cm to 7 cm,13,27 is the primary mover of the shoulder and essential for proper function after RSA.28,29 The pectoralis major tendon, which begins inserting at a point approximately 5.6 cm from the humeral head and spans a distance of 7.7 cm along the length of the humerus,30-32 is important not only for function but as an anatomical landmark in reconstruction. Lastly, the latissimus dorsi and teres major, which share a role in extension, adduction, and internal rotation of the glenohumeral joint, insert along the floor and medial lip of the intertubercular groove of the humerus, respectively.33,34 In addition to their role in tendon transfer procedures because of treating irreparable posterosuperior cuff and subscapularis tears,35,36 it has been suggested that these tendons may play some role in glenohumeral joint stability.37
In addition to the loss of muscular attachments, the absence of proximal humeral bone stock, in and of itself, can have deleterious effects on fixation of the humeral component. RSA is a semiconstrained device, which results in increased transmission of forces to the interface between the humeral implant and its surrounding structures, including cement (when present) and the bone itself. When there is the absence of significant amounts of bone, the remaining bone must now account for an even higher proportion of these forces. A previous biomechanical study showed that cemented humeral stems demonstrated significantly increased micromotion in the presence of proximal humeral bone loss, particularly when a modular humeral component was used.12
Continue to: TYPES OF BONE LOSS
TYPES OF BONE LOSS
There are a variety of different etiologies of proximal humeral bone loss that result in distinctly different clinical presentations. These can be fairly mild, as is the case of isolated resorption of the greater tuberosity in a non-united proximal humerus fracture (Figure 1). Alternatively, they can be severe, as seen in a grossly loose cemented long-stemmed component that is freely mobile, creating a windshield-wiper effect throughout the length of the humerus (Figure 2). This can be somewhat deceiving, however, as the amount of bone loss, as well as the pathophysiologic process that led to the bone loss, are important factors to determine ideal reconstructive methods. In the case of a failed open reduction internal fixation, where the tuberosity has failed to unite or has been resected, there is much less of a biologic response in comparison with implant loosening associated with osteolysis. This latter condition will be associated with a much more destructive inflammatory response resulting in poor tissue quality and often dramatic thinning of the cortex. If one simply measured the distance from the most proximal remaining bone stock to the area where the greater tuberosity should be, a loose stem with subsidence and ballooning of the cortices may appear to have a similar amount of bone loss as a failed hemiarthroplasty for fracture with a well-fixed stem. However, intraoperatively, one will find that the bone that appeared to be present radiographically in the case of the loose stem is of such poor quality that it cannot reasonably provide any beneficial fixation. In light of this, different treatment modalities are recommended for different types of bone loss, and the revision surgeon must be able to anticipate this and possess a full armamentarium of options to treat these challenging cases successfully.

INDICATIONS
Our technique to manage proximal humeral bone loss is dependent on both the quantity of bone loss, which can be measured radiographically, as well as the anticipated inflammatory response described above. As both the destructive process and the amount of bone loss increase, the importance of more advanced reconstructive procedures that will sustain implant security and soft-tissue management becomes apparent. In the least destructive cases with <5 cm of bone loss, successful revision can typically be accomplished with stem removal and placement of a new monoblock humeral stem. In cases where more advanced destructive pathology is present, and bone loss is >5 cm, an allograft-prosthetic composite (APC) is typically used. In both scenarios, if the stem being revised is cemented and the cement mantle remains intact, and of reasonable length, consideration is given to the cement-within-cement technique. Finally, in the most destructive cases where bone loss exceeds 10 cm and a large biological response is anticipated (eg, periprosthetic fractures with humeral loosening), the use of a longer diaphyseal-incorporating APC is often necessary. This prosthetic composite can be combined with a cement-within-cement technique as well.

It is important to comment on the implications of using modular stems in this setting. With advanced bone loss, a situation is often encountered where the newly implanted stem geometry and working length may be insufficient to acquire adequate rotational stability. In this setting, if a modular junction is positioned close to the stem and cement/bone interface, it will be exposed to very high stress concentrations which can lead to component fracture38 as well as taper corrosion, also referred to as trunnionosis. This latter phenomenon, which has been well studied in the total hip arthroplasty literature with the use of modular components,39 is especially relevant given the high torsional loads imparted at the modular junction. Ultimately, high torsional loads lead to micromotion and electrochemical ion release via degradation of the passivation layer, initiating the process of mechanically assisted crevice corrosion.40 For these reasons, when a modular stem must be used in the presence of mild to moderate bone loss, using a proximal humeral allograft to protect the junction or to provide additional fixation may be implemented with a lower threshold than when using a monoblock stem.
SURGICAL TECHNIQUE: ALLOGRAFT-PROSTHETIC COMPOSITES
A standard deltopectoral approach is used, taking care to preserve all viable muscular attachments to the proximal humerus. After removal of the prosthetic humeral head, the decision to proceed with removal of the stem at this juncture is based on several factors. If the remaining proximal humeral bone is so compromised that it might not be able to withstand the forces exerted upon it during retraction for glenoid exposure, the component is left in place. Additionally, if there is consideration that the glenoid-sided bone loss may be so severe that a glenoid baseplate cannot be implanted, and the stem remains well fixed, it is retained so that it can be converted to a hemiarthroplasty.
If neither of the above issues is present, the humeral stem is removed. If a well-fixed press-fit stem is in place, it is typically removed using a combination of burrs and osteotomes to disrupt the bone-implant interface, and the stem is then carefully removed using an impactor and mallet. If a cemented stem is present, the stem is removed in a similar manner, and the cement mantle is left in place if stable, in anticipation of a cement-within-cement technique. If the mantle is disrupted, standard cement removal instruments are used to remove all cement from the canal meticulously.
Continue to: Management of the glenoid...
Management of the glenoid can have significant implications with regard to the humerus. Most notably, the size of the glenosphere has direct implications on the fixation of the humeral component. Use of larger diameter glenospheres result in increased contact area between the glenosphere and humerosocket, adding constraint to the articulation and further increasing the stresses at the implant-bone interface. As such, the use of larger glenospheres to prevent instability must be balanced with the resulting implications on humeral component fixation, especially in cases of severe bone loss.

After implanting the appropriate glenosphere, attention is then turned back to the humerus. Trial implants are sequentially used to obtain adequate humeral length and stability. Once this is accomplished, the amount of humeral bone loss is quantified by measuring the distance from the superior aspect of the medial humeral tray to the medial humeral shaft. If this number is >5 cm (Figure 3), the decision is made to proceed with an APC. The allograft humeral head is cut, cancellous bone is removed, and a step-cut is performed, with the medial portion of the allograft measuring the same length as that of bone loss and the lateral plate extending an additional several centimeters distally (Figure 4). Additional soft tissue is removed from the allograft, leaving the subscapularis stump intact for later repair with the patient’s native tissue. The allograft is secured to the patient’s proximal humerus using multiple cerclage wires, and the humeral stem is cemented into place. The final construct is shown in Figure 5.

ADDITIONAL CONSIDERATIONS: CASES OF ADVANCED BONE LOSS
In cases of advanced humeral bone loss, as is often seen when revising loose humeral stems, larger allografts that span a significant length of the diaphysis are often required. This type of bone loss has implications with regard to how the deltoid insertion is managed. Interestingly, even in situations when the vast majority of the remaining diaphysis consists of ectatic egg-shell bone, the deltoid tuberosity remains of fairly substantial quality due to the continued pull of the muscular insertion on this area. This fragment is isolated, carefully mobilized, and subsequently repaired back on top of the allograft using cables.

POSTOPERATIVE CARE
Patients are kept in a shoulder immobilizer for 6 weeks after surgery to facilitate allograft incorporation and subscapularis tendon healing. During this time, pendulum exercises are initiated. Active assisted range of motion (ROM) exercises begin after 6 weeks, consisting of supine forward elevation. A sling is given to be used in public. Light strengthening exercises begin at 3 months postoperatively.
DISCUSSION
In cases of mild to moderate proximal humeral bone loss, RSA using a long-stem humeral component without allograft augmentation is a viable option. Budge and colleagues38 demonstrated excellent results in a population of 15 patients with an average of 38 mm of proximal humeral bone loss without use of allografts. Interestingly, they noted 1 case of component fracture in a modular prosthesis and therefore concluded that monoblock humeral stems should be used in the absence of allograft augmentation.
Continue to: In more advanced cases of bone loss...
In more advanced cases of bone loss, our data shows that use of APCs can result in equally satisfactory results. In a series of 25 patients with an average bone loss of 54 mm, patients were able to achieve statistically significant improvements in pain, ROM, and function with high rates of allograft incorporation.9 Overall, a low rate of complications was noted, including 1 infection. This finding is consistent with an additional study looking specifically at factors associated with infection in revision SA, which found that the use of allografts was not associated with increased risk of infection.41
As stated previously, the size of allograft needed for the APC construct is related to the distinct pathology encountered. In our experience, we have noted that well-fixed stems can be treated with short metaphyseal APCs in 85% of cases. On the other hand, loose stems require long allografts measuring >10 cm in 90% of cases. As such, these cases typically require mobilization of the deltoid insertion as described above, and therefore it is important that the surgeon is prepared for this aspect of the procedure preoperatively.
Finally, the cement-within-cement technique, originally popularized for use in revision total hip arthroplasty, has demonstrated reliable results when utilized in revision SA.42 To date, there are no recommendations regarding the minimal length of existing cement mantle that is needed to perform this technique. In situations in which the length of the cement mantle is questionable, our preference is to combine the cement-within-cement technique with an APC when possible.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi:10.1016/j.jse.2010.02.009.
2. Padegimas EM, Maltenfort M, Lazarus MD, Ramsey ML, Williams GR, Namdari S. Future patient demand for shoulder arthroplasty by younger patients: national projections. Clin Orthop Relat Res. 2015;473(6):1860-1867. doi:10.1007/s11999-015-4231-z.
3. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522. doi:10.1016/j.jse.2011.03.006.
4. Levy JC, Virani N, Pupello D, et al. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195. doi:10.1302/0301-620X.89B2.
5. Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21(3):342-349. doi:10.1016/j.jse.2011.05.021.
6. Deutsch A, Abboud JA, Kelly J, et al. Clinical results of revision shoulder arthroplasty for glenoid component loosening. J Shoulder Elbow Surg. 2007;16(6):706-716. doi:10.1016/j.jse.2007.01.007.
7. Kelly JD, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525. doi:10.1016/j.jse.2011.11.021.
8. Black EM, Roberts SM, Siegel E, Yannopoulos P, Higgins LD, Warner JJP. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg. 2014;23(7):1036-1042. doi:10.1016/j.jse.2014.02.019.
9. Composite P, Chacon BA, Virani N, et al. Revision arthroplasty with use of a reverse shoulder. J Bone Joint Surg. 2009;1:119-127. doi:10.2106/JBJS.H.00094.
10. Klein SM, Dunning P, Mulieri P, Pupello D, Downes K, Frankle MA. Effects of acquired glenoid bone defects on surgical technique and clinical outcomes in reverse shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1144-1154. doi:10.2106/JBJS.I.00778.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
12. Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651. doi:10.1016/j.jse.2010.10.026.
13. Morgan SJ, Furry K, Parekh A, Agudelo JF, Smith WR. The deltoid muscle: an anatomic description of the deltoid insertion to the proximal humerus. J Orthop Trauma. 2006;20(1):19-21. doi:10.1097/01.bot.0000187063.43267.18.
14. Gagey O, Hue E. Mechanics of the deltoid muscle. A new approach. Clin Orthop Relat Res. 2000;375:250-257. doi:10.1097/00003086-200006000-00030.
15. De Wilde L, Plasschaert F. Prosthetic treatment and functional recovery of the shoulder after tumor resection 10 years ago: a case report. J Shoulder Elbow Surg. 2005;14(6):645-649. doi:10.1016/j.jse.2004.11.001.
16. Wataru S, Kazuomi S, Yoshikazu N, Hiroaki I, Takaharu Y, Hideki Y. Three-dimensional morphological analysis of humeral heads: a study in cadavers. Acta Orthop. 2005;76(3):392-396. doi:10.1080/00016470510030878.
17. Tillett E, Smith M, Fulcher M, Shanklin J. Anatomic determination of humeral head retroversion: the relationship of the central axis of the humeral head to the bicipital groove. J Shoulder Elbow Surg. 1993;2(5):255-256. doi:10.1016/S1058-2746(09)80085-2.
18. Doyle AJ, Burks RT. Comparison of humeral head retroversion with the humeral axis/biceps groove relationship: a study in live subjects and cadavers. J Shoulder Elbow Surg. 1998;7(5):453-457. doi:10.1016/S1058-2746(98)90193-8.
19. Johnson JW, Thostenson JD, Suva LJ, Hasan SA. Relationship of bicipital groove rotation with humeral head retroversion: a three-dimensional computed tomographic analysis. J Bone Joint Surg Am. 2013;95(8):719-724. doi:10.2106/JBJS.J.00085.
20. Hromádka R, Kuběna AA, Pokorný D, Popelka S, Jahoda D, Sosna A. Lesser tuberosity is more reliable than bicipital groove when determining orientation of humeral head in primary shoulder arthroplasty. Surg Radiol Anat. 2010;32(1):31-37. doi:10.1007/s00276-009-0543-6.
21. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338. doi:10.1067/mse.2002.124429.
22. Pearl ML. Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. J Shoulder Elbow Surg. 2005;14(suppl 1):99-104. doi:10.1016/j.jse.2004.09.025.
23. Robertson DD, Yuan J, Bigliani LU, Flatow EL, Yamaguchi K. Three-dimensional analysis of the proximal part of the humerus: relevance to arthroplasty. J Bone Joint Surg Am. 2000;82-A(11):1594-1602.
24. Mochizuki T, Sugaya H, Uomizu M, et al. Humeral insertion of the supraspinatus and infraspinatus. J Bone Joint Surg Am. 2008;90(5):962-969. doi:10.2106/JBJS.G.00427.
25. Arai R, Sugaya H, Mochizuki T, Nimura A, Moriishi J, Akita K. Subscapularis tendon tear: an anatomic and clinical investigation. Arthroscopy. 2008;24(9):997-1004. doi:10.1016/j.arthro.2008.04.076.
26. Nimura A, Kato A, Yamaguchi K, et al. The superior capsule of the shoulder joint complements the insertion of the rotator cuff. J Shoulder Elbow Surg. 2012;21(7):867-872. doi:10.1016/j.jse.2011.04.034.
27. Rispoli DM, Athwal GS, Sperling JW, Cofield RH. The anatomy of the deltoid insertion. J Shoulder Elbow Surg. 2009;18(3):386-390. doi:10.1016/j.jse.2008.10.012.
28. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364. doi:10.1016/j.jse.2012.02.002.
29. Walker M, Brooks J, Willis M, Frankle M. How reverse shoulder arthroplasty works. Clinical Orthop Relat Res. 2011;469(9):2440-2451. doi:10.1007/s11999-011-1892-0.
30. Torrens C, Corrales M, Melendo E, Solano A, Rodríguez-Baeza A, Cáceres E. The pectoralis major tendon as a reference for restoring humeral length and retroversion with hemiarthroplasty for fracture. J Shoulder Elbow Surg. 2008;17(6):947-950. doi:10.1016/j.jse.2008.05.041.
31. Ponce BA, Thompson KJ, Rosenzweig SD, et al. Re-evaluation of pectoralis major height as an anatomic reference for humeral height in fracture hemiarthroplasty. J Shoulder Elbow Surg. 2013;22(11):1567-1572. doi:10.1016/j.jse.2013.01.039.
32. LaFrance R, Madsen W, Yaseen Z, Giordano B, Maloney M, Voloshin I. Relevant anatomic landmarks and measurements for biceps tenodesis. Am J Sports Med. 2013;41(6):1395-1399. doi:10.1177/0363546513482297.
33. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
34. Bhatt CR, Prajapati B, Patil DS, Patel VD, Singh BGP, Mehta CD. Variation in the insertion of the latissimus dorsi & its clinical importance. J Orthop. 2013;10(1):25-28. doi:10.1016/j.jor.2013.01.002.
35. Gerber C, Maquieira G, Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg. 2006;88(1):113-120. doi:10.2106/JBJS.E.00282.
36. Elhassan B, Christensen TJ, Wagner ER. Feasibility of latissimus and teres major transfer to reconstruct irreparable subscapularis tendon tear: an anatomic study. J Shoulder Elbow Surg. 2014;23(4):492-499. doi:10.1016/j.jse.2013.07.046.
37. Pouliart N, Gagey O. Significance of the latissimus dorsi for shoulder instability. II. Its influence on dislocation behavior in a sequential cutting protocol of the glenohumeral capsule. Clin Anat. 2005;18(7):500-509. doi:10.1002/ca.20181.
38. Budge MD, Moravek JE, Zimel MN, Nolan EM, Wiater JM. Reverse total shoulder arthroplasty for the management of failed shoulder arthroplasty with proximal humeral bone loss: is allograft augmentation necessary? J Shoulder Elbow Surg. 2013;22(6):739-744. doi:10.1016/j.jse.2012.08.008.
39. Weiser MC, Lavernia CJ. Trunnionosis in total hip arthroplasty. J Bone Joint Surg Am. 2017;99(17):27-29. doi:10.2106/JBJS.17.00345.
40. Cohen J. Current concepts review. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80(10):1554.
41. Meijer ST, Paulino Pereira NR, Nota SPFT, Ferrone ML, Schwab JH, Lozano Calderón SA. Factors associated with infection after reconstructive shoulder surgery for proximal humerus tumors. J Shoulder Elbow Surg. 2017;26(6):931-938. doi:10.1016/j.jse.2016.10.014.
42. Wagner ER, Houdek MT, Hernandez NM, Cofield RH, Sánchez-Sotelo J, Sperling JW. Cement-within-cement technique in revision reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(8):1448-1453. doi:10.1016/j.jse.2017.01.013.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi:10.1016/j.jse.2010.02.009.
2. Padegimas EM, Maltenfort M, Lazarus MD, Ramsey ML, Williams GR, Namdari S. Future patient demand for shoulder arthroplasty by younger patients: national projections. Clin Orthop Relat Res. 2015;473(6):1860-1867. doi:10.1007/s11999-015-4231-z.
3. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522. doi:10.1016/j.jse.2011.03.006.
4. Levy JC, Virani N, Pupello D, et al. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195. doi:10.1302/0301-620X.89B2.
5. Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21(3):342-349. doi:10.1016/j.jse.2011.05.021.
6. Deutsch A, Abboud JA, Kelly J, et al. Clinical results of revision shoulder arthroplasty for glenoid component loosening. J Shoulder Elbow Surg. 2007;16(6):706-716. doi:10.1016/j.jse.2007.01.007.
7. Kelly JD, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525. doi:10.1016/j.jse.2011.11.021.
8. Black EM, Roberts SM, Siegel E, Yannopoulos P, Higgins LD, Warner JJP. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg. 2014;23(7):1036-1042. doi:10.1016/j.jse.2014.02.019.
9. Composite P, Chacon BA, Virani N, et al. Revision arthroplasty with use of a reverse shoulder. J Bone Joint Surg. 2009;1:119-127. doi:10.2106/JBJS.H.00094.
10. Klein SM, Dunning P, Mulieri P, Pupello D, Downes K, Frankle MA. Effects of acquired glenoid bone defects on surgical technique and clinical outcomes in reverse shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1144-1154. doi:10.2106/JBJS.I.00778.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
12. Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651. doi:10.1016/j.jse.2010.10.026.
13. Morgan SJ, Furry K, Parekh A, Agudelo JF, Smith WR. The deltoid muscle: an anatomic description of the deltoid insertion to the proximal humerus. J Orthop Trauma. 2006;20(1):19-21. doi:10.1097/01.bot.0000187063.43267.18.
14. Gagey O, Hue E. Mechanics of the deltoid muscle. A new approach. Clin Orthop Relat Res. 2000;375:250-257. doi:10.1097/00003086-200006000-00030.
15. De Wilde L, Plasschaert F. Prosthetic treatment and functional recovery of the shoulder after tumor resection 10 years ago: a case report. J Shoulder Elbow Surg. 2005;14(6):645-649. doi:10.1016/j.jse.2004.11.001.
16. Wataru S, Kazuomi S, Yoshikazu N, Hiroaki I, Takaharu Y, Hideki Y. Three-dimensional morphological analysis of humeral heads: a study in cadavers. Acta Orthop. 2005;76(3):392-396. doi:10.1080/00016470510030878.
17. Tillett E, Smith M, Fulcher M, Shanklin J. Anatomic determination of humeral head retroversion: the relationship of the central axis of the humeral head to the bicipital groove. J Shoulder Elbow Surg. 1993;2(5):255-256. doi:10.1016/S1058-2746(09)80085-2.
18. Doyle AJ, Burks RT. Comparison of humeral head retroversion with the humeral axis/biceps groove relationship: a study in live subjects and cadavers. J Shoulder Elbow Surg. 1998;7(5):453-457. doi:10.1016/S1058-2746(98)90193-8.
19. Johnson JW, Thostenson JD, Suva LJ, Hasan SA. Relationship of bicipital groove rotation with humeral head retroversion: a three-dimensional computed tomographic analysis. J Bone Joint Surg Am. 2013;95(8):719-724. doi:10.2106/JBJS.J.00085.
20. Hromádka R, Kuběna AA, Pokorný D, Popelka S, Jahoda D, Sosna A. Lesser tuberosity is more reliable than bicipital groove when determining orientation of humeral head in primary shoulder arthroplasty. Surg Radiol Anat. 2010;32(1):31-37. doi:10.1007/s00276-009-0543-6.
21. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338. doi:10.1067/mse.2002.124429.
22. Pearl ML. Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. J Shoulder Elbow Surg. 2005;14(suppl 1):99-104. doi:10.1016/j.jse.2004.09.025.
23. Robertson DD, Yuan J, Bigliani LU, Flatow EL, Yamaguchi K. Three-dimensional analysis of the proximal part of the humerus: relevance to arthroplasty. J Bone Joint Surg Am. 2000;82-A(11):1594-1602.
24. Mochizuki T, Sugaya H, Uomizu M, et al. Humeral insertion of the supraspinatus and infraspinatus. J Bone Joint Surg Am. 2008;90(5):962-969. doi:10.2106/JBJS.G.00427.
25. Arai R, Sugaya H, Mochizuki T, Nimura A, Moriishi J, Akita K. Subscapularis tendon tear: an anatomic and clinical investigation. Arthroscopy. 2008;24(9):997-1004. doi:10.1016/j.arthro.2008.04.076.
26. Nimura A, Kato A, Yamaguchi K, et al. The superior capsule of the shoulder joint complements the insertion of the rotator cuff. J Shoulder Elbow Surg. 2012;21(7):867-872. doi:10.1016/j.jse.2011.04.034.
27. Rispoli DM, Athwal GS, Sperling JW, Cofield RH. The anatomy of the deltoid insertion. J Shoulder Elbow Surg. 2009;18(3):386-390. doi:10.1016/j.jse.2008.10.012.
28. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364. doi:10.1016/j.jse.2012.02.002.
29. Walker M, Brooks J, Willis M, Frankle M. How reverse shoulder arthroplasty works. Clinical Orthop Relat Res. 2011;469(9):2440-2451. doi:10.1007/s11999-011-1892-0.
30. Torrens C, Corrales M, Melendo E, Solano A, Rodríguez-Baeza A, Cáceres E. The pectoralis major tendon as a reference for restoring humeral length and retroversion with hemiarthroplasty for fracture. J Shoulder Elbow Surg. 2008;17(6):947-950. doi:10.1016/j.jse.2008.05.041.
31. Ponce BA, Thompson KJ, Rosenzweig SD, et al. Re-evaluation of pectoralis major height as an anatomic reference for humeral height in fracture hemiarthroplasty. J Shoulder Elbow Surg. 2013;22(11):1567-1572. doi:10.1016/j.jse.2013.01.039.
32. LaFrance R, Madsen W, Yaseen Z, Giordano B, Maloney M, Voloshin I. Relevant anatomic landmarks and measurements for biceps tenodesis. Am J Sports Med. 2013;41(6):1395-1399. doi:10.1177/0363546513482297.
33. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
34. Bhatt CR, Prajapati B, Patil DS, Patel VD, Singh BGP, Mehta CD. Variation in the insertion of the latissimus dorsi & its clinical importance. J Orthop. 2013;10(1):25-28. doi:10.1016/j.jor.2013.01.002.
35. Gerber C, Maquieira G, Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg. 2006;88(1):113-120. doi:10.2106/JBJS.E.00282.
36. Elhassan B, Christensen TJ, Wagner ER. Feasibility of latissimus and teres major transfer to reconstruct irreparable subscapularis tendon tear: an anatomic study. J Shoulder Elbow Surg. 2014;23(4):492-499. doi:10.1016/j.jse.2013.07.046.
37. Pouliart N, Gagey O. Significance of the latissimus dorsi for shoulder instability. II. Its influence on dislocation behavior in a sequential cutting protocol of the glenohumeral capsule. Clin Anat. 2005;18(7):500-509. doi:10.1002/ca.20181.
38. Budge MD, Moravek JE, Zimel MN, Nolan EM, Wiater JM. Reverse total shoulder arthroplasty for the management of failed shoulder arthroplasty with proximal humeral bone loss: is allograft augmentation necessary? J Shoulder Elbow Surg. 2013;22(6):739-744. doi:10.1016/j.jse.2012.08.008.
39. Weiser MC, Lavernia CJ. Trunnionosis in total hip arthroplasty. J Bone Joint Surg Am. 2017;99(17):27-29. doi:10.2106/JBJS.17.00345.
40. Cohen J. Current concepts review. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80(10):1554.
41. Meijer ST, Paulino Pereira NR, Nota SPFT, Ferrone ML, Schwab JH, Lozano Calderón SA. Factors associated with infection after reconstructive shoulder surgery for proximal humerus tumors. J Shoulder Elbow Surg. 2017;26(6):931-938. doi:10.1016/j.jse.2016.10.014.
42. Wagner ER, Houdek MT, Hernandez NM, Cofield RH, Sánchez-Sotelo J, Sperling JW. Cement-within-cement technique in revision reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(8):1448-1453. doi:10.1016/j.jse.2017.01.013.
TAKE-HOME POINTS
- Different preoperative diagnoses lead to distinct patterns of bone loss in revision shoulder arthroplasty.
- A variety of techniques should be utilized to address the specific pathologies encountered.
- Advanced proximal humeral bone loss results in limited substrate available for humeral component fixation.
- Monoblock humeral stems can be used without allografts in cases with mild humeral bone loss.
- The revision of loose humeral stems dictates the use of large diaphyseal allografts in the majority of cases.
Treating Humeral Bone Loss in Shoulder Arthroplasty: Modular Humeral Components or Allografts
ABSTRACT
Reconstructing proximal humeral bone loss in the setting of shoulder arthroplasty can be a daunting task. Proposed techniques include long-stemmed humeral components, allograft-prosthesis composites (APCs), and modular endoprosthetic reconstruction. While unsupported long-stemmed components are at high risk for component loosening, APC reconstruction techniques have been reported with success. However, graft resorption and eventual failure are significant concerns. Modular endoprosthetic systems allow bone deficiencies to be reconstructed with metal, which may allow for a more durable reconstruction.
Continue to: Shoulder arthroplasty is an established procedure...
Shoulder arthroplasty is an established procedure with good results for restoring motion and decreasing pain for a variety of indications, including arthritis, fracture, posttraumatic sequelae, and tumor resection.1-4 As the population ages, the incidence of these shoulder disorders increases, with the incidence of total shoulder arthroplasty (TSA) and reverse total shoulder arthroplasty (RTSA) increasing at faster rates than that of hemiarthroplasty.5,6 These expanding indications will, in turn, result in more revisions that would present challenges for surgeons.1,7,8
The glenoid component is much more commonly revised than the humeral component; however, the humeral component may also require revision or removal to allow exposure of the glenoid component.9 Revision of the humeral stem might be required in cases of infection, periprosthetic fracture, dislocation, or aseptic loosening.10 The survival rate of humeral stems is generally >90% at 10 years and >80% at 20-year follow-up.7 Despite these good survival rates, a revision setting the humeral component requires exchange in about half of all cases.11
Humeral bone loss or deficiency is one of the challenges encountered in both primary and revision TSA. The amount of proximal bone loss can be determined by measuring the distance from the top of the prosthesis laterally to the intact lateral cortex.12 Methods for treating bone loss may involve monoblock revision stems to bypass the deficiency, allografts to rebuild the bone stock, or modular components or endoprostheses to restore the length and stability of the extremity.
Proximal humeral bone loss may make component positioning difficult and may create problems with fixation of the humeral stem. Proper sizing and placement of components are important for improving postoperative function, decreasing component wear and instability, and restoring humeral height and offset. Determining the appropriate center of rotation is important for the function and avoidance of impingement on the acromion, as well as for the restoration of the lever arm of the deltoid without overtensioning. The selection of components with the correct size and the accurate intraoperative placement are important to restore humeral height and offset.13,14 Components must be positioned <4 mm from the height of the greater tuberosity and <8 mm of offset to avoid compromising motion.15 De Wilde and Walch16 described about 3 patients who underwent revision reverse shoulder arthroplasty after failure of the humeral implant because of inadequate proximal humeral bone stock. They concluded that treatment of the bone loss was critical to achieve a successful outcome.
LONG-STEMMED HUMERAL COMPONENTS WITHOUT GRAFTING
There is some evidence indicating that humeral bone loss can be managed without allograft or augmentation. Owens and colleagues17 evaluated the use of intermediate- or long-stemmed humeral components for primary shoulder arthroplasty in 17 patients with severe proximal humeral bone loss and in 18 patients with large humeral canals. The stems were fully cemented, cemented distally only with proximal allografting, and uncemented. Indications for fully cemented stems were loss of proximal bone that could be filled with a proximal cement mantle to ensure a secure fit. Distal cement fixation was applied when there was significant proximal bone loss and was often supplemented with cancellous or structural allograft and/or cancellous autograft. Intraoperative complications included cortical perforation or cement extrusion in 16% of patients. Excellent or satisfactory results were obtained in 21 (60%) of the 35 shoulders, 14 (78%) of the 18 shoulders with large humeral canals, and 7 (41%) of the 17 shoulders with bone loss. All the 17 components implanted in patients with proximal humeral bone loss were stable with no gross loosening at an average 6-year follow-up.
Continue to: Budge and colleagues...
Budge and colleagues12 prospectively enrolled 15 patients with substantial proximal humeral bone loss (38.4 mm) who had conversion to RTSA without allografting after a failed TSA. All patients showed improvements in terms of the American Shoulder and Elbow Surgeons (ASES) score, subjective shoulder value, Constant score, and Visual Analog Scale (VAS) pain score, as well as an improved active range of motion (ROM) and good radiographic outcomes at 2-year follow-up. Although the complication rate was high (7 of 15), most of the complications were minor, with only 2 requiring operative intervention. The only component fracture occurred in a patient with a modular prosthesis that was unsupported by bone proximally. Budge and colleagues12 suggested that concerns about prosthetic fracture can be alleviated using a nonmodular monoblock design. No prosthesis-related complications occurred in their series, leading them to recommend monoblock humeral stems in patients with severe proximal humeral bone loss.
Stephens and colleagues18 reported revision to RTSA in 32 patients with hemiarthroplasties, half of whom had proximal humeral bone loss (average 36.3 mm). Of these 16 patients, 10 were treated with monoblock stems and 6 with modular components, with cement fixation of all implants. At an average 4-year follow-up, patients with proximal bone loss had improved motion and outcomes, and decreased pain compared to their preoperative condition; however, they had lower functional and pain ratings, as well as less ROM compared to those of patients with intact proximal bone stock. Complications occurred in 5 (31%) of those with bone loss and in 1 (0.6%) of those without bone loss. Three of the 16 patients with bone loss had humeral stem loosening, with 2 of the 3 having subsidence. Only 1 patient required return to the operating room for the treatment of a periprosthetic fracture sustained in a fall. Of the 16 patients with bone loss, 14 patients demonstrated scapular notching, which was severe in 5 of them. Because patients with altered humeral length and/or standard length stems had worse outcomes, the authors recommended longer stems to improve fixation and advocated the use of a long-stemmed monoblock prosthesis over an allograft-prosthesis composite (APC).18
However, Werner and colleagues19 reported high rates of loosening and distal migration with the use of long-stemmed humeral implants in 50 patients with revision RTSA. They noted periprosthetic lucency on radiographs in 48% of patients, with more than half of them requiring revision. In 6 patients with subsidence of the humeral shaft, revision was done using custom, modular implants, with the anatomic curve being further stabilized using distal screw and cable fixation to provide rotational stability.
Using a biomechanical model, Cuff and colleagues20 compared 3 RTSA humeral designs, 2 modular designs, and 1 monoblock design in 12 intact models and in 12 models simulating 5 cm of proximal humeral bone loss. They observed that proximal humeral bone loss led to increased humeral component micromotion and rotational instability. The bone loss group had 5 failures compared to 2 in the control group. All failures occurred in those with modular components, whereas those with monoblock implants had no failures.
ALLOGRAFT-PROSTHESIS COMPOSITE
Composite treatment with a humeral stem and a metaphyseal allograft was described by Kassab and colleagues21 in 2005 and Levy and colleagues22 in 2007 (Figures 1A-1C) in patients with tumor resections21 or failed hemiarthroplasties.22 Allograft was used when there was insufficient metaphyseal bone to support the implant, and a graft was fashioned and fixed with cerclage wire before the component was cemented in place. In the 29 patients reported by Levy and colleagues,22 subjective and objective measurements trended toward better results in those with an APC than in those with RTSA alone, but this difference did not reach statistical significance. Several authors have identified a lack of proximal humeral bone support as 1 of the 4 possible causes of failure, and suggested that the allograft provides structural support, serves as an attachment for subscapularis repair, and maximizes deltoid function by increasing lateral offset and setting the moment arm of the deltoid.21-23
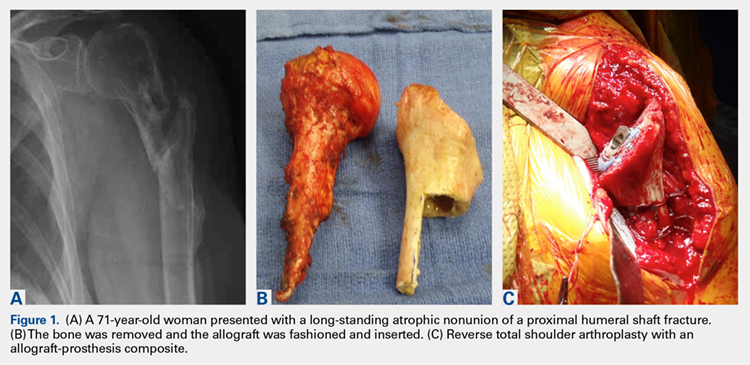
Continue to: In a prospective study of RTSA...
In a prospective study of RTSA using structural allografts for failed hemiarthroplasty in 25 patients with an average bone loss of 5 cm, 19 patients (76%) reported good or excellent results, 5 reported satisfactory results, and 1 patient reported an unsatisfactory result.1 Patients had significantly improved forward flexion, abduction, and external rotation and improved outcome scores (ASES and SST). Graft incorporation was good, with 88% and 79% incorporation in the metaphysis and diaphysis, respectively. This technique used a fresh-frozen proximal humeral allograft to fashion a custom proximal block with a lateral step-cut, which was fixed around the stem with cables. A long stem and cement were used. If there was no cement mantle remaining or if the medial portion of the graft was longer than 120 mm, the cement mantle was completely excised. The allograft stump of the subscapularis was used to repair the subscapularis tendon either end-to-end or pants-over-vest. The authors noted that the subscapularis repair provided increased stability; the only dislocation not caused by trauma did not have an identifiable tendon to repair. In this manner, APC reconstruction provided structural and rotational support to the humeral stem as well as bone stock for future revision.1,20
One significant concern with APC reconstruction is the potential for graft resorption, which can lead to humeral stem loosening, loss of contour of the tuberosity, or weakening to the point of fracture.24,25 This may be worsened by stress shielding of the allograft by distal stem cement fixation.26 Other concerns include the cost of the allograft, increased risk of de novo infection, donor-to-host infection, increased operative time and complexity, and failure of allograft incorporation.
The use of a proximal femoral allograft has been described when there is a lack of a proximal humeral allograft.1,27 Kelly and colleagues27 described good results in 2 patients in whom proximal femoral allograft was used along with bone morphogenetic protein, cemented long-stemmed revision implants, and locking plate augmentation.
ENDOPROSTHETIC RECONSTRUCTION
Various forms of prosthetic augmentation have been described to compensate for proximal humeral bone loss, with the majority of reports involving the use of endoprosthetic replacement for tumor procedures.28-31 Use of endoprostheses has also been described for revision procedures in patients with rheumatoid arthritis with massive bone loss, demonstrating modest improvements compared to severe preoperative functional limitations.32
Tumor patients, as well as revision arthroplasty patients, may present difficulties with prosthetic fixation due to massive bone loss. Chao and colleagues29 reported about the long-term outcomes after the use of implants with a porous ongrowth surface and extracortical bridging bone graft in multiple anatomic locations, including the proximal humerus, the proximal and distal femur, and the femoral diaphysis. In 3 patients with proximal humeral reconstruction, the measured ongrowth was only 30%. Given the small number of patients with a proximal humerus, no statistical significance was observed in the prosthesis location and the amount of bony ongrowth, but it was far less than that in the lower extremity.
Continue to: Endoprosthetic reconstruction...
Endoprosthetic reconstruction of the proximal humerus is commonly used for tumor resection that resulted in bone loss. Cannon and colleagues28 reported a 97.6% survival rate at a mean follow-up of 30 months in 83 patients with modular and custom reconstruction with a unipolar head. The ROM was limited, but the prosthesis provided adequate stability to allow elbow and hand function. Proximal migration of the prosthetic head was noticed with increasing frequency as the length of follow-up increased.
Use of an endoprosthesis with compressive osteointegration (Zimmer Biomet) has been described in lower extremities and more recently with follow-up on several cases, including 2 proximal humeral replacements for oncology patients to treat severe bone loss. One case was for a primary sarcoma resection, and the other was for the revision of aseptic loosening of a previous endoprosthesis. Follow-up periods for these 2 patients were 54 and 141 months, respectively. Both these patients had complications, but both retained the endoprosthesis. The authors concluded that this is a salvage operation with high risk.30 In another study, Guven and colleagues31 reported about reverse endoprosthetic reconstruction for tumor resection with bone loss. The ROM was improved, with a mean active forward elevation of 96° (range, 30°-160°), an abduction of 88° (range, 30-160°), and an external rotation of 13° (range, 0°-20°).
Modular endoprostheses have been evaluated as a method for improving bone fixation and restoring soft-tissue tension, while avoiding the complications associated with traditional endoprostheses or allografts (Figures 2A-2D). These systems allow precise adjustments of length using different trial lengths intraoperatively to obtain proper stability and deltoid tension. Of the 12 patients in a 2 center study, 11 had cementless components inserted using a press-fit technique (unpublished data, J. Feldman). At a minimum 2-year follow-up, the patients had an average improvement in forward elevation from 78° to 97°. Excluding 2 patients with loss of the deltoid tuberosity, the forward elevation averaged 109°. There were significant improvements in internal rotation (from 18° to 38°), as well as in the scores of Quick Disabilities of the Arm, Shoulder and Hand (DASH), forward elevation strength, ASES, and VAS pain. However, the overall complication rate was 41%. Therefore, despite these promising early results, longer-term studies are needed.
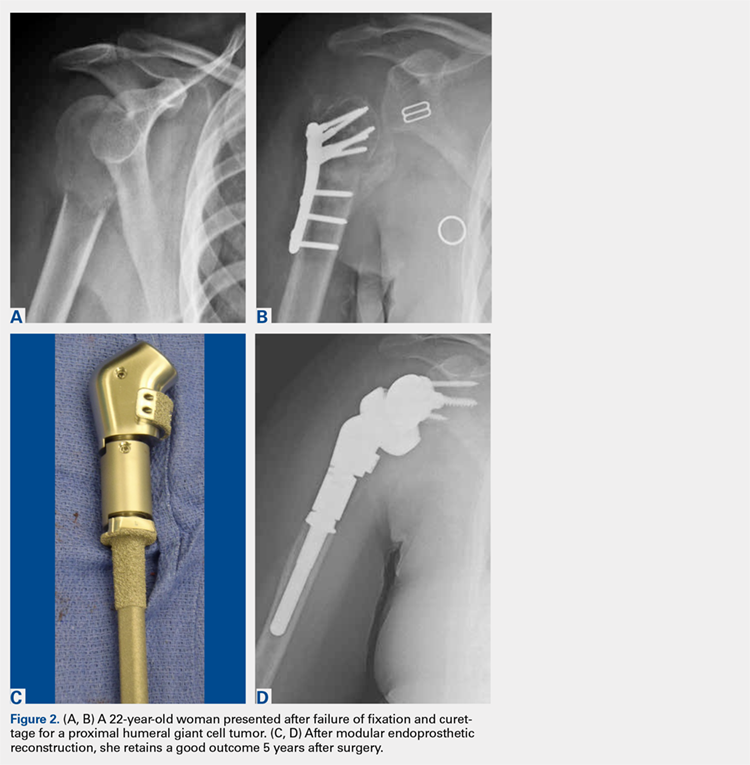
CONCLUSION
Proximal humeral bone loss remains a significant challenge for the shoulder arthroplasty surgeon. In the setting of a primary or a revision arthroplasty, the bone stock must be thoroughly evaluated during preoperative planning, and a surgical plan for addressing the deficits should be developed. Because proximal humeral bone loss may contribute to prosthetic failure, every effort should be made to reconstitute the bone stock.16 If the bone loss is less extensive, impaction grafting may be considered. Options to address massive proximal humeral bone loss include APCs and endoprosthetic reconstruction. The use of an allograft allows subscapularis repair, which may help stabilize the shoulder and restore the natural lever arm, as well as the tension of the deltoid.1,21-23 In addition, it helps avoid rotational instability and micromotion and provides bone stock for future revisions. However, concern persists regarding allograft resorption over time. More recently, modular endoprosthetic reconstruction systems have been developed to address bone deficiency with metal augmentation. Early clinical results demonstrate a high complication rate in this complex cohort of patients, not unlike those in the series of APCs, but clinical outcomes were improved compared to those in historical series. Nevertheless, longer-term clinical studies are necessary to determine the role of these modular endoprosthetic implant systems.
1. Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91(1):119-127. doi:10.2106/JBJS.H.00094.
2. Hattrup SJ, Waldrop R, Sanchez-Sotelo J. Reverse total shoulder arthroplasty for posttraumatic sequelae. J Orthop Trauma. 2016;30(2):e41-e47. doi:10.1097/BOT.0000000000000416.
3. Sewell MD, Kang SN, Al-Hadithy N, et al. Management of peri-prosthetic fracture of the humerus with severe bone loss and loosening of the humeral component after total shoulder replacement. J Bone Joint Surg Br. 2012;94(10):1382-1389. doi:10.1302/0301-620X.94B10.29248.
4. Trompeter AJ, Gupta RR. The management of complex periprosthetic humeral fractures: a case series of strut allograft augmentation, and a review of the literature. Strategies Trauma Limb Reconstr. 2013;8(1):43-51. doi:10.1007/s11751-013-0155-x.
5. Khatib O, Onyekwelu I, Yu S, Zuckerman JD. Shoulder arthroplasty in New York State, 1991 to 2010: changing patterns of utilization. J Shoulder Elbow Surg. 2015;24(10):e286-e291. doi:10.1016/j.jse.2015.05.038.
6. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
7. Cil A, Veillette CJ, Sanchez-Sotelo J, Sperling JW, Schleck CD, Cofield RH. Survivorship of the humeral component in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):143-150. doi:10.1016/j.jse.2009.04.011.
8. Wright TW. Revision of humeral components in shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(2 suppl):S77-S81.
9. Duquin TR, Sperling JW. Revision shoulder arthroplasty—how to manage the humerus. Oper Tech Orthop. 2011;21(1):44-51. doi:10.1053/j.oto.2010.09.008.
10. Cil A, Veillette CJ, Sanchez-Sotelo J, Sperling JW, Schleck C, Cofield RH. Revision of the humeral component for aseptic loosening in arthroplasty of the shoulder. J Bone Joint Surg Br. 2009;91(1):75-81. doi:10.1302/0301-620X.91B1.21094.
11. Cofield RH. Revision of hemiarthroplasty to total shoulder arthroplasty. In: Zuckerman JD, ed. Advanced Reconstruction: Shoulder. 1st edition. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2007;613-622.
12. Budge MD, Moravek JE, Zimel MN, Nolan EM, Wiater JM. Reverse total shoulder arthroplasty for the management of failed shoulder arthroplasty with proximal humeral bone loss: is allograft augmentation necessary? J Shoulder Elbow Surg. 2013;22(6):739-744. doi:10.1016/j.jse.2012.08.008.
13. Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997;79(5):857-865.
14. Throckmorton TW. Reconstructive procedures of the shoulder and elbow. In: Azar FM, Beaty JH, Canale ST, eds. Campbell’s Operative Orthopaedics. 13th edition. Philadelphia, PA: Elsevier; 2017;570-622.
15. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409. doi:10.1067/mse.2001.116871.
16. De Wilde L, Walch G. Humeral prosthetic failure of reversed total shoulder arthroplasty: a report of three cases. J Shoulder Elbow Surg. 2006;15(2):260-264. doi:10.1016/j.jse.2005.07.014.
17. Owens CJ, Sperling JW, Cofield RH. Utility and complications of long-stem humeral components in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(7):e7-e12. doi:10.1016/j.jse.2012.10.034.
18. Stephens SP, Paisley KC, Giveans MR, Wirth MA. The effect of proximal humeral bone loss on revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):1519-1526. doi:10.1016/j.jse.2015.02.020.
19. Werner BS, Abdelkawi AF, Boehm D, et al. Long-term analysis of revision reverse shoulder arthroplasty using cemented long stems. J Shoulder Elbow Surg. 2017;26(2):273-278. doi:10.1016/j.jse.2016.05.015.
20. Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651. doi:10.1016/j.jse.2010.10.026.
21. Kassab M, Dumaine V, Babinet A, Ouaknine M, Tomeno B, Anract P. Twenty nine shoulder reconstructions after resection of the proximal humerus for neoplasm with mean 7-year follow-up. Rev Chir Orthop Reparatrice Appar Mot. 2005;91(1):15-23.
22. Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg. 2007;98(2):292-300. doi:10.2106/JBJS.E.01310.
23. Gagey O, Pourjamasb B, Court C. Revision arthroplasty of the shoulder for painful glenoid loosening: a series of 14 cases with acromial prostheses reviewed at four year follow up. Rev Chir Reparatrice Appar Mot. 2001;87(3):221-228.
24. Abdeen A, Hoang BH, Althanasina EA, Morris CD, Boland PJ, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am. 2009;91(10):2406-2415. doi:10.2106/JBJS.H.00815.
25. Getty PJ, Peabody TD. Complications and functional outcomes of reconstruction with an osteoarticular allograft after intra-articular resection of the proximal aspect of the humerus. J Bone Joint Surg Am. 1999;81(8):1138-1146.
26. Chen CF, Chen WM, Cheng YC, Chiang CC, Huang CK, Chen TH. Extracorporeally irradiated autograft-prosthetic composite arthroplasty using AML® extensively porous-coated stem for proximal femur reconstruction: a clinical analysis of 14 patients. J Surg Oncol. 2009;100(5):418-422. doi:10.1002/jso.21351.
27. Kelly JD 2nd, Purchase RJ, Kam G, Norris TR. Alloprosthetic composite reconstruction using the reverse shoulder arthroplasty. Tech Shoulder Elbow Surg. 2009;10(1):5-10.
28. Cannon CP, Paraliticci GU, Lin PP, Lewis VO, Yasko AW. Functional outcome following endoprosthetic reconstruction of the proximal humerus. J Shoulder Elbow Surg. 2009;18(5):705-710. doi:10.1016/j.jse.2008.10.011.
29. Chao EY, Fuchs B, Rowland CM, Ilstrup DM, Pritchard DJ, Sim FH. Long-term results of segmental prosthesis fixation by extracortical bone-bridging and ingrowth. J Bone Joint Surg Am. 2004;86-A(5):948-955.
30. Goulding KA, Schwartz A, Hattrup SJ, et al. Use of compressive osseointegration endoprostheses for massive bone loss from tumor and failed arthroplasty: a viable option in the upper extremity. Clin Orthop Relat Res. 2017;475(6):1702-1711. doi:10.1007/s11999-017-5258-0.
31. Guven MF, Aslan L, Botanlioglu H, Kaynak G, Kesmezacar H, Babacan M. Functional outcome of reverse shoulder tumor prosthesis in the treatment of proximal humeral tumors. J Shoulder Elbow Surg. 2016;25(1):e1-e6. doi:10.1016/j.jse.2015.06.012.
32. Wang ML, Ballard BL, Kulidjian AA, Abrams RA. Upper extremity reconstruction with a humeral tumor endoprosthesis: a novel salvage procedure after multiple revisions of total shoulder and elbow replacement. J Shoulder Elbow Surg. 2011;20(1):e1-e8. doi:10.1016/j.jse.2010.07.018.
ABSTRACT
Reconstructing proximal humeral bone loss in the setting of shoulder arthroplasty can be a daunting task. Proposed techniques include long-stemmed humeral components, allograft-prosthesis composites (APCs), and modular endoprosthetic reconstruction. While unsupported long-stemmed components are at high risk for component loosening, APC reconstruction techniques have been reported with success. However, graft resorption and eventual failure are significant concerns. Modular endoprosthetic systems allow bone deficiencies to be reconstructed with metal, which may allow for a more durable reconstruction.
Continue to: Shoulder arthroplasty is an established procedure...
Shoulder arthroplasty is an established procedure with good results for restoring motion and decreasing pain for a variety of indications, including arthritis, fracture, posttraumatic sequelae, and tumor resection.1-4 As the population ages, the incidence of these shoulder disorders increases, with the incidence of total shoulder arthroplasty (TSA) and reverse total shoulder arthroplasty (RTSA) increasing at faster rates than that of hemiarthroplasty.5,6 These expanding indications will, in turn, result in more revisions that would present challenges for surgeons.1,7,8
The glenoid component is much more commonly revised than the humeral component; however, the humeral component may also require revision or removal to allow exposure of the glenoid component.9 Revision of the humeral stem might be required in cases of infection, periprosthetic fracture, dislocation, or aseptic loosening.10 The survival rate of humeral stems is generally >90% at 10 years and >80% at 20-year follow-up.7 Despite these good survival rates, a revision setting the humeral component requires exchange in about half of all cases.11
Humeral bone loss or deficiency is one of the challenges encountered in both primary and revision TSA. The amount of proximal bone loss can be determined by measuring the distance from the top of the prosthesis laterally to the intact lateral cortex.12 Methods for treating bone loss may involve monoblock revision stems to bypass the deficiency, allografts to rebuild the bone stock, or modular components or endoprostheses to restore the length and stability of the extremity.
Proximal humeral bone loss may make component positioning difficult and may create problems with fixation of the humeral stem. Proper sizing and placement of components are important for improving postoperative function, decreasing component wear and instability, and restoring humeral height and offset. Determining the appropriate center of rotation is important for the function and avoidance of impingement on the acromion, as well as for the restoration of the lever arm of the deltoid without overtensioning. The selection of components with the correct size and the accurate intraoperative placement are important to restore humeral height and offset.13,14 Components must be positioned <4 mm from the height of the greater tuberosity and <8 mm of offset to avoid compromising motion.15 De Wilde and Walch16 described about 3 patients who underwent revision reverse shoulder arthroplasty after failure of the humeral implant because of inadequate proximal humeral bone stock. They concluded that treatment of the bone loss was critical to achieve a successful outcome.
LONG-STEMMED HUMERAL COMPONENTS WITHOUT GRAFTING
There is some evidence indicating that humeral bone loss can be managed without allograft or augmentation. Owens and colleagues17 evaluated the use of intermediate- or long-stemmed humeral components for primary shoulder arthroplasty in 17 patients with severe proximal humeral bone loss and in 18 patients with large humeral canals. The stems were fully cemented, cemented distally only with proximal allografting, and uncemented. Indications for fully cemented stems were loss of proximal bone that could be filled with a proximal cement mantle to ensure a secure fit. Distal cement fixation was applied when there was significant proximal bone loss and was often supplemented with cancellous or structural allograft and/or cancellous autograft. Intraoperative complications included cortical perforation or cement extrusion in 16% of patients. Excellent or satisfactory results were obtained in 21 (60%) of the 35 shoulders, 14 (78%) of the 18 shoulders with large humeral canals, and 7 (41%) of the 17 shoulders with bone loss. All the 17 components implanted in patients with proximal humeral bone loss were stable with no gross loosening at an average 6-year follow-up.
Continue to: Budge and colleagues...
Budge and colleagues12 prospectively enrolled 15 patients with substantial proximal humeral bone loss (38.4 mm) who had conversion to RTSA without allografting after a failed TSA. All patients showed improvements in terms of the American Shoulder and Elbow Surgeons (ASES) score, subjective shoulder value, Constant score, and Visual Analog Scale (VAS) pain score, as well as an improved active range of motion (ROM) and good radiographic outcomes at 2-year follow-up. Although the complication rate was high (7 of 15), most of the complications were minor, with only 2 requiring operative intervention. The only component fracture occurred in a patient with a modular prosthesis that was unsupported by bone proximally. Budge and colleagues12 suggested that concerns about prosthetic fracture can be alleviated using a nonmodular monoblock design. No prosthesis-related complications occurred in their series, leading them to recommend monoblock humeral stems in patients with severe proximal humeral bone loss.
Stephens and colleagues18 reported revision to RTSA in 32 patients with hemiarthroplasties, half of whom had proximal humeral bone loss (average 36.3 mm). Of these 16 patients, 10 were treated with monoblock stems and 6 with modular components, with cement fixation of all implants. At an average 4-year follow-up, patients with proximal bone loss had improved motion and outcomes, and decreased pain compared to their preoperative condition; however, they had lower functional and pain ratings, as well as less ROM compared to those of patients with intact proximal bone stock. Complications occurred in 5 (31%) of those with bone loss and in 1 (0.6%) of those without bone loss. Three of the 16 patients with bone loss had humeral stem loosening, with 2 of the 3 having subsidence. Only 1 patient required return to the operating room for the treatment of a periprosthetic fracture sustained in a fall. Of the 16 patients with bone loss, 14 patients demonstrated scapular notching, which was severe in 5 of them. Because patients with altered humeral length and/or standard length stems had worse outcomes, the authors recommended longer stems to improve fixation and advocated the use of a long-stemmed monoblock prosthesis over an allograft-prosthesis composite (APC).18
However, Werner and colleagues19 reported high rates of loosening and distal migration with the use of long-stemmed humeral implants in 50 patients with revision RTSA. They noted periprosthetic lucency on radiographs in 48% of patients, with more than half of them requiring revision. In 6 patients with subsidence of the humeral shaft, revision was done using custom, modular implants, with the anatomic curve being further stabilized using distal screw and cable fixation to provide rotational stability.
Using a biomechanical model, Cuff and colleagues20 compared 3 RTSA humeral designs, 2 modular designs, and 1 monoblock design in 12 intact models and in 12 models simulating 5 cm of proximal humeral bone loss. They observed that proximal humeral bone loss led to increased humeral component micromotion and rotational instability. The bone loss group had 5 failures compared to 2 in the control group. All failures occurred in those with modular components, whereas those with monoblock implants had no failures.
ALLOGRAFT-PROSTHESIS COMPOSITE
Composite treatment with a humeral stem and a metaphyseal allograft was described by Kassab and colleagues21 in 2005 and Levy and colleagues22 in 2007 (Figures 1A-1C) in patients with tumor resections21 or failed hemiarthroplasties.22 Allograft was used when there was insufficient metaphyseal bone to support the implant, and a graft was fashioned and fixed with cerclage wire before the component was cemented in place. In the 29 patients reported by Levy and colleagues,22 subjective and objective measurements trended toward better results in those with an APC than in those with RTSA alone, but this difference did not reach statistical significance. Several authors have identified a lack of proximal humeral bone support as 1 of the 4 possible causes of failure, and suggested that the allograft provides structural support, serves as an attachment for subscapularis repair, and maximizes deltoid function by increasing lateral offset and setting the moment arm of the deltoid.21-23

Continue to: In a prospective study of RTSA...
In a prospective study of RTSA using structural allografts for failed hemiarthroplasty in 25 patients with an average bone loss of 5 cm, 19 patients (76%) reported good or excellent results, 5 reported satisfactory results, and 1 patient reported an unsatisfactory result.1 Patients had significantly improved forward flexion, abduction, and external rotation and improved outcome scores (ASES and SST). Graft incorporation was good, with 88% and 79% incorporation in the metaphysis and diaphysis, respectively. This technique used a fresh-frozen proximal humeral allograft to fashion a custom proximal block with a lateral step-cut, which was fixed around the stem with cables. A long stem and cement were used. If there was no cement mantle remaining or if the medial portion of the graft was longer than 120 mm, the cement mantle was completely excised. The allograft stump of the subscapularis was used to repair the subscapularis tendon either end-to-end or pants-over-vest. The authors noted that the subscapularis repair provided increased stability; the only dislocation not caused by trauma did not have an identifiable tendon to repair. In this manner, APC reconstruction provided structural and rotational support to the humeral stem as well as bone stock for future revision.1,20
One significant concern with APC reconstruction is the potential for graft resorption, which can lead to humeral stem loosening, loss of contour of the tuberosity, or weakening to the point of fracture.24,25 This may be worsened by stress shielding of the allograft by distal stem cement fixation.26 Other concerns include the cost of the allograft, increased risk of de novo infection, donor-to-host infection, increased operative time and complexity, and failure of allograft incorporation.
The use of a proximal femoral allograft has been described when there is a lack of a proximal humeral allograft.1,27 Kelly and colleagues27 described good results in 2 patients in whom proximal femoral allograft was used along with bone morphogenetic protein, cemented long-stemmed revision implants, and locking plate augmentation.
ENDOPROSTHETIC RECONSTRUCTION
Various forms of prosthetic augmentation have been described to compensate for proximal humeral bone loss, with the majority of reports involving the use of endoprosthetic replacement for tumor procedures.28-31 Use of endoprostheses has also been described for revision procedures in patients with rheumatoid arthritis with massive bone loss, demonstrating modest improvements compared to severe preoperative functional limitations.32
Tumor patients, as well as revision arthroplasty patients, may present difficulties with prosthetic fixation due to massive bone loss. Chao and colleagues29 reported about the long-term outcomes after the use of implants with a porous ongrowth surface and extracortical bridging bone graft in multiple anatomic locations, including the proximal humerus, the proximal and distal femur, and the femoral diaphysis. In 3 patients with proximal humeral reconstruction, the measured ongrowth was only 30%. Given the small number of patients with a proximal humerus, no statistical significance was observed in the prosthesis location and the amount of bony ongrowth, but it was far less than that in the lower extremity.
Continue to: Endoprosthetic reconstruction...
Endoprosthetic reconstruction of the proximal humerus is commonly used for tumor resection that resulted in bone loss. Cannon and colleagues28 reported a 97.6% survival rate at a mean follow-up of 30 months in 83 patients with modular and custom reconstruction with a unipolar head. The ROM was limited, but the prosthesis provided adequate stability to allow elbow and hand function. Proximal migration of the prosthetic head was noticed with increasing frequency as the length of follow-up increased.
Use of an endoprosthesis with compressive osteointegration (Zimmer Biomet) has been described in lower extremities and more recently with follow-up on several cases, including 2 proximal humeral replacements for oncology patients to treat severe bone loss. One case was for a primary sarcoma resection, and the other was for the revision of aseptic loosening of a previous endoprosthesis. Follow-up periods for these 2 patients were 54 and 141 months, respectively. Both these patients had complications, but both retained the endoprosthesis. The authors concluded that this is a salvage operation with high risk.30 In another study, Guven and colleagues31 reported about reverse endoprosthetic reconstruction for tumor resection with bone loss. The ROM was improved, with a mean active forward elevation of 96° (range, 30°-160°), an abduction of 88° (range, 30-160°), and an external rotation of 13° (range, 0°-20°).
Modular endoprostheses have been evaluated as a method for improving bone fixation and restoring soft-tissue tension, while avoiding the complications associated with traditional endoprostheses or allografts (Figures 2A-2D). These systems allow precise adjustments of length using different trial lengths intraoperatively to obtain proper stability and deltoid tension. Of the 12 patients in a 2 center study, 11 had cementless components inserted using a press-fit technique (unpublished data, J. Feldman). At a minimum 2-year follow-up, the patients had an average improvement in forward elevation from 78° to 97°. Excluding 2 patients with loss of the deltoid tuberosity, the forward elevation averaged 109°. There were significant improvements in internal rotation (from 18° to 38°), as well as in the scores of Quick Disabilities of the Arm, Shoulder and Hand (DASH), forward elevation strength, ASES, and VAS pain. However, the overall complication rate was 41%. Therefore, despite these promising early results, longer-term studies are needed.

CONCLUSION
Proximal humeral bone loss remains a significant challenge for the shoulder arthroplasty surgeon. In the setting of a primary or a revision arthroplasty, the bone stock must be thoroughly evaluated during preoperative planning, and a surgical plan for addressing the deficits should be developed. Because proximal humeral bone loss may contribute to prosthetic failure, every effort should be made to reconstitute the bone stock.16 If the bone loss is less extensive, impaction grafting may be considered. Options to address massive proximal humeral bone loss include APCs and endoprosthetic reconstruction. The use of an allograft allows subscapularis repair, which may help stabilize the shoulder and restore the natural lever arm, as well as the tension of the deltoid.1,21-23 In addition, it helps avoid rotational instability and micromotion and provides bone stock for future revisions. However, concern persists regarding allograft resorption over time. More recently, modular endoprosthetic reconstruction systems have been developed to address bone deficiency with metal augmentation. Early clinical results demonstrate a high complication rate in this complex cohort of patients, not unlike those in the series of APCs, but clinical outcomes were improved compared to those in historical series. Nevertheless, longer-term clinical studies are necessary to determine the role of these modular endoprosthetic implant systems.
ABSTRACT
Reconstructing proximal humeral bone loss in the setting of shoulder arthroplasty can be a daunting task. Proposed techniques include long-stemmed humeral components, allograft-prosthesis composites (APCs), and modular endoprosthetic reconstruction. While unsupported long-stemmed components are at high risk for component loosening, APC reconstruction techniques have been reported with success. However, graft resorption and eventual failure are significant concerns. Modular endoprosthetic systems allow bone deficiencies to be reconstructed with metal, which may allow for a more durable reconstruction.
Continue to: Shoulder arthroplasty is an established procedure...
Shoulder arthroplasty is an established procedure with good results for restoring motion and decreasing pain for a variety of indications, including arthritis, fracture, posttraumatic sequelae, and tumor resection.1-4 As the population ages, the incidence of these shoulder disorders increases, with the incidence of total shoulder arthroplasty (TSA) and reverse total shoulder arthroplasty (RTSA) increasing at faster rates than that of hemiarthroplasty.5,6 These expanding indications will, in turn, result in more revisions that would present challenges for surgeons.1,7,8
The glenoid component is much more commonly revised than the humeral component; however, the humeral component may also require revision or removal to allow exposure of the glenoid component.9 Revision of the humeral stem might be required in cases of infection, periprosthetic fracture, dislocation, or aseptic loosening.10 The survival rate of humeral stems is generally >90% at 10 years and >80% at 20-year follow-up.7 Despite these good survival rates, a revision setting the humeral component requires exchange in about half of all cases.11
Humeral bone loss or deficiency is one of the challenges encountered in both primary and revision TSA. The amount of proximal bone loss can be determined by measuring the distance from the top of the prosthesis laterally to the intact lateral cortex.12 Methods for treating bone loss may involve monoblock revision stems to bypass the deficiency, allografts to rebuild the bone stock, or modular components or endoprostheses to restore the length and stability of the extremity.
Proximal humeral bone loss may make component positioning difficult and may create problems with fixation of the humeral stem. Proper sizing and placement of components are important for improving postoperative function, decreasing component wear and instability, and restoring humeral height and offset. Determining the appropriate center of rotation is important for the function and avoidance of impingement on the acromion, as well as for the restoration of the lever arm of the deltoid without overtensioning. The selection of components with the correct size and the accurate intraoperative placement are important to restore humeral height and offset.13,14 Components must be positioned <4 mm from the height of the greater tuberosity and <8 mm of offset to avoid compromising motion.15 De Wilde and Walch16 described about 3 patients who underwent revision reverse shoulder arthroplasty after failure of the humeral implant because of inadequate proximal humeral bone stock. They concluded that treatment of the bone loss was critical to achieve a successful outcome.
LONG-STEMMED HUMERAL COMPONENTS WITHOUT GRAFTING
There is some evidence indicating that humeral bone loss can be managed without allograft or augmentation. Owens and colleagues17 evaluated the use of intermediate- or long-stemmed humeral components for primary shoulder arthroplasty in 17 patients with severe proximal humeral bone loss and in 18 patients with large humeral canals. The stems were fully cemented, cemented distally only with proximal allografting, and uncemented. Indications for fully cemented stems were loss of proximal bone that could be filled with a proximal cement mantle to ensure a secure fit. Distal cement fixation was applied when there was significant proximal bone loss and was often supplemented with cancellous or structural allograft and/or cancellous autograft. Intraoperative complications included cortical perforation or cement extrusion in 16% of patients. Excellent or satisfactory results were obtained in 21 (60%) of the 35 shoulders, 14 (78%) of the 18 shoulders with large humeral canals, and 7 (41%) of the 17 shoulders with bone loss. All the 17 components implanted in patients with proximal humeral bone loss were stable with no gross loosening at an average 6-year follow-up.
Continue to: Budge and colleagues...
Budge and colleagues12 prospectively enrolled 15 patients with substantial proximal humeral bone loss (38.4 mm) who had conversion to RTSA without allografting after a failed TSA. All patients showed improvements in terms of the American Shoulder and Elbow Surgeons (ASES) score, subjective shoulder value, Constant score, and Visual Analog Scale (VAS) pain score, as well as an improved active range of motion (ROM) and good radiographic outcomes at 2-year follow-up. Although the complication rate was high (7 of 15), most of the complications were minor, with only 2 requiring operative intervention. The only component fracture occurred in a patient with a modular prosthesis that was unsupported by bone proximally. Budge and colleagues12 suggested that concerns about prosthetic fracture can be alleviated using a nonmodular monoblock design. No prosthesis-related complications occurred in their series, leading them to recommend monoblock humeral stems in patients with severe proximal humeral bone loss.
Stephens and colleagues18 reported revision to RTSA in 32 patients with hemiarthroplasties, half of whom had proximal humeral bone loss (average 36.3 mm). Of these 16 patients, 10 were treated with monoblock stems and 6 with modular components, with cement fixation of all implants. At an average 4-year follow-up, patients with proximal bone loss had improved motion and outcomes, and decreased pain compared to their preoperative condition; however, they had lower functional and pain ratings, as well as less ROM compared to those of patients with intact proximal bone stock. Complications occurred in 5 (31%) of those with bone loss and in 1 (0.6%) of those without bone loss. Three of the 16 patients with bone loss had humeral stem loosening, with 2 of the 3 having subsidence. Only 1 patient required return to the operating room for the treatment of a periprosthetic fracture sustained in a fall. Of the 16 patients with bone loss, 14 patients demonstrated scapular notching, which was severe in 5 of them. Because patients with altered humeral length and/or standard length stems had worse outcomes, the authors recommended longer stems to improve fixation and advocated the use of a long-stemmed monoblock prosthesis over an allograft-prosthesis composite (APC).18
However, Werner and colleagues19 reported high rates of loosening and distal migration with the use of long-stemmed humeral implants in 50 patients with revision RTSA. They noted periprosthetic lucency on radiographs in 48% of patients, with more than half of them requiring revision. In 6 patients with subsidence of the humeral shaft, revision was done using custom, modular implants, with the anatomic curve being further stabilized using distal screw and cable fixation to provide rotational stability.
Using a biomechanical model, Cuff and colleagues20 compared 3 RTSA humeral designs, 2 modular designs, and 1 monoblock design in 12 intact models and in 12 models simulating 5 cm of proximal humeral bone loss. They observed that proximal humeral bone loss led to increased humeral component micromotion and rotational instability. The bone loss group had 5 failures compared to 2 in the control group. All failures occurred in those with modular components, whereas those with monoblock implants had no failures.
ALLOGRAFT-PROSTHESIS COMPOSITE
Composite treatment with a humeral stem and a metaphyseal allograft was described by Kassab and colleagues21 in 2005 and Levy and colleagues22 in 2007 (Figures 1A-1C) in patients with tumor resections21 or failed hemiarthroplasties.22 Allograft was used when there was insufficient metaphyseal bone to support the implant, and a graft was fashioned and fixed with cerclage wire before the component was cemented in place. In the 29 patients reported by Levy and colleagues,22 subjective and objective measurements trended toward better results in those with an APC than in those with RTSA alone, but this difference did not reach statistical significance. Several authors have identified a lack of proximal humeral bone support as 1 of the 4 possible causes of failure, and suggested that the allograft provides structural support, serves as an attachment for subscapularis repair, and maximizes deltoid function by increasing lateral offset and setting the moment arm of the deltoid.21-23

Continue to: In a prospective study of RTSA...
In a prospective study of RTSA using structural allografts for failed hemiarthroplasty in 25 patients with an average bone loss of 5 cm, 19 patients (76%) reported good or excellent results, 5 reported satisfactory results, and 1 patient reported an unsatisfactory result.1 Patients had significantly improved forward flexion, abduction, and external rotation and improved outcome scores (ASES and SST). Graft incorporation was good, with 88% and 79% incorporation in the metaphysis and diaphysis, respectively. This technique used a fresh-frozen proximal humeral allograft to fashion a custom proximal block with a lateral step-cut, which was fixed around the stem with cables. A long stem and cement were used. If there was no cement mantle remaining or if the medial portion of the graft was longer than 120 mm, the cement mantle was completely excised. The allograft stump of the subscapularis was used to repair the subscapularis tendon either end-to-end or pants-over-vest. The authors noted that the subscapularis repair provided increased stability; the only dislocation not caused by trauma did not have an identifiable tendon to repair. In this manner, APC reconstruction provided structural and rotational support to the humeral stem as well as bone stock for future revision.1,20
One significant concern with APC reconstruction is the potential for graft resorption, which can lead to humeral stem loosening, loss of contour of the tuberosity, or weakening to the point of fracture.24,25 This may be worsened by stress shielding of the allograft by distal stem cement fixation.26 Other concerns include the cost of the allograft, increased risk of de novo infection, donor-to-host infection, increased operative time and complexity, and failure of allograft incorporation.
The use of a proximal femoral allograft has been described when there is a lack of a proximal humeral allograft.1,27 Kelly and colleagues27 described good results in 2 patients in whom proximal femoral allograft was used along with bone morphogenetic protein, cemented long-stemmed revision implants, and locking plate augmentation.
ENDOPROSTHETIC RECONSTRUCTION
Various forms of prosthetic augmentation have been described to compensate for proximal humeral bone loss, with the majority of reports involving the use of endoprosthetic replacement for tumor procedures.28-31 Use of endoprostheses has also been described for revision procedures in patients with rheumatoid arthritis with massive bone loss, demonstrating modest improvements compared to severe preoperative functional limitations.32
Tumor patients, as well as revision arthroplasty patients, may present difficulties with prosthetic fixation due to massive bone loss. Chao and colleagues29 reported about the long-term outcomes after the use of implants with a porous ongrowth surface and extracortical bridging bone graft in multiple anatomic locations, including the proximal humerus, the proximal and distal femur, and the femoral diaphysis. In 3 patients with proximal humeral reconstruction, the measured ongrowth was only 30%. Given the small number of patients with a proximal humerus, no statistical significance was observed in the prosthesis location and the amount of bony ongrowth, but it was far less than that in the lower extremity.
Continue to: Endoprosthetic reconstruction...
Endoprosthetic reconstruction of the proximal humerus is commonly used for tumor resection that resulted in bone loss. Cannon and colleagues28 reported a 97.6% survival rate at a mean follow-up of 30 months in 83 patients with modular and custom reconstruction with a unipolar head. The ROM was limited, but the prosthesis provided adequate stability to allow elbow and hand function. Proximal migration of the prosthetic head was noticed with increasing frequency as the length of follow-up increased.
Use of an endoprosthesis with compressive osteointegration (Zimmer Biomet) has been described in lower extremities and more recently with follow-up on several cases, including 2 proximal humeral replacements for oncology patients to treat severe bone loss. One case was for a primary sarcoma resection, and the other was for the revision of aseptic loosening of a previous endoprosthesis. Follow-up periods for these 2 patients were 54 and 141 months, respectively. Both these patients had complications, but both retained the endoprosthesis. The authors concluded that this is a salvage operation with high risk.30 In another study, Guven and colleagues31 reported about reverse endoprosthetic reconstruction for tumor resection with bone loss. The ROM was improved, with a mean active forward elevation of 96° (range, 30°-160°), an abduction of 88° (range, 30-160°), and an external rotation of 13° (range, 0°-20°).
Modular endoprostheses have been evaluated as a method for improving bone fixation and restoring soft-tissue tension, while avoiding the complications associated with traditional endoprostheses or allografts (Figures 2A-2D). These systems allow precise adjustments of length using different trial lengths intraoperatively to obtain proper stability and deltoid tension. Of the 12 patients in a 2 center study, 11 had cementless components inserted using a press-fit technique (unpublished data, J. Feldman). At a minimum 2-year follow-up, the patients had an average improvement in forward elevation from 78° to 97°. Excluding 2 patients with loss of the deltoid tuberosity, the forward elevation averaged 109°. There were significant improvements in internal rotation (from 18° to 38°), as well as in the scores of Quick Disabilities of the Arm, Shoulder and Hand (DASH), forward elevation strength, ASES, and VAS pain. However, the overall complication rate was 41%. Therefore, despite these promising early results, longer-term studies are needed.

CONCLUSION
Proximal humeral bone loss remains a significant challenge for the shoulder arthroplasty surgeon. In the setting of a primary or a revision arthroplasty, the bone stock must be thoroughly evaluated during preoperative planning, and a surgical plan for addressing the deficits should be developed. Because proximal humeral bone loss may contribute to prosthetic failure, every effort should be made to reconstitute the bone stock.16 If the bone loss is less extensive, impaction grafting may be considered. Options to address massive proximal humeral bone loss include APCs and endoprosthetic reconstruction. The use of an allograft allows subscapularis repair, which may help stabilize the shoulder and restore the natural lever arm, as well as the tension of the deltoid.1,21-23 In addition, it helps avoid rotational instability and micromotion and provides bone stock for future revisions. However, concern persists regarding allograft resorption over time. More recently, modular endoprosthetic reconstruction systems have been developed to address bone deficiency with metal augmentation. Early clinical results demonstrate a high complication rate in this complex cohort of patients, not unlike those in the series of APCs, but clinical outcomes were improved compared to those in historical series. Nevertheless, longer-term clinical studies are necessary to determine the role of these modular endoprosthetic implant systems.
1. Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91(1):119-127. doi:10.2106/JBJS.H.00094.
2. Hattrup SJ, Waldrop R, Sanchez-Sotelo J. Reverse total shoulder arthroplasty for posttraumatic sequelae. J Orthop Trauma. 2016;30(2):e41-e47. doi:10.1097/BOT.0000000000000416.
3. Sewell MD, Kang SN, Al-Hadithy N, et al. Management of peri-prosthetic fracture of the humerus with severe bone loss and loosening of the humeral component after total shoulder replacement. J Bone Joint Surg Br. 2012;94(10):1382-1389. doi:10.1302/0301-620X.94B10.29248.
4. Trompeter AJ, Gupta RR. The management of complex periprosthetic humeral fractures: a case series of strut allograft augmentation, and a review of the literature. Strategies Trauma Limb Reconstr. 2013;8(1):43-51. doi:10.1007/s11751-013-0155-x.
5. Khatib O, Onyekwelu I, Yu S, Zuckerman JD. Shoulder arthroplasty in New York State, 1991 to 2010: changing patterns of utilization. J Shoulder Elbow Surg. 2015;24(10):e286-e291. doi:10.1016/j.jse.2015.05.038.
6. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
7. Cil A, Veillette CJ, Sanchez-Sotelo J, Sperling JW, Schleck CD, Cofield RH. Survivorship of the humeral component in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):143-150. doi:10.1016/j.jse.2009.04.011.
8. Wright TW. Revision of humeral components in shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(2 suppl):S77-S81.
9. Duquin TR, Sperling JW. Revision shoulder arthroplasty—how to manage the humerus. Oper Tech Orthop. 2011;21(1):44-51. doi:10.1053/j.oto.2010.09.008.
10. Cil A, Veillette CJ, Sanchez-Sotelo J, Sperling JW, Schleck C, Cofield RH. Revision of the humeral component for aseptic loosening in arthroplasty of the shoulder. J Bone Joint Surg Br. 2009;91(1):75-81. doi:10.1302/0301-620X.91B1.21094.
11. Cofield RH. Revision of hemiarthroplasty to total shoulder arthroplasty. In: Zuckerman JD, ed. Advanced Reconstruction: Shoulder. 1st edition. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2007;613-622.
12. Budge MD, Moravek JE, Zimel MN, Nolan EM, Wiater JM. Reverse total shoulder arthroplasty for the management of failed shoulder arthroplasty with proximal humeral bone loss: is allograft augmentation necessary? J Shoulder Elbow Surg. 2013;22(6):739-744. doi:10.1016/j.jse.2012.08.008.
13. Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997;79(5):857-865.
14. Throckmorton TW. Reconstructive procedures of the shoulder and elbow. In: Azar FM, Beaty JH, Canale ST, eds. Campbell’s Operative Orthopaedics. 13th edition. Philadelphia, PA: Elsevier; 2017;570-622.
15. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409. doi:10.1067/mse.2001.116871.
16. De Wilde L, Walch G. Humeral prosthetic failure of reversed total shoulder arthroplasty: a report of three cases. J Shoulder Elbow Surg. 2006;15(2):260-264. doi:10.1016/j.jse.2005.07.014.
17. Owens CJ, Sperling JW, Cofield RH. Utility and complications of long-stem humeral components in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(7):e7-e12. doi:10.1016/j.jse.2012.10.034.
18. Stephens SP, Paisley KC, Giveans MR, Wirth MA. The effect of proximal humeral bone loss on revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):1519-1526. doi:10.1016/j.jse.2015.02.020.
19. Werner BS, Abdelkawi AF, Boehm D, et al. Long-term analysis of revision reverse shoulder arthroplasty using cemented long stems. J Shoulder Elbow Surg. 2017;26(2):273-278. doi:10.1016/j.jse.2016.05.015.
20. Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651. doi:10.1016/j.jse.2010.10.026.
21. Kassab M, Dumaine V, Babinet A, Ouaknine M, Tomeno B, Anract P. Twenty nine shoulder reconstructions after resection of the proximal humerus for neoplasm with mean 7-year follow-up. Rev Chir Orthop Reparatrice Appar Mot. 2005;91(1):15-23.
22. Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg. 2007;98(2):292-300. doi:10.2106/JBJS.E.01310.
23. Gagey O, Pourjamasb B, Court C. Revision arthroplasty of the shoulder for painful glenoid loosening: a series of 14 cases with acromial prostheses reviewed at four year follow up. Rev Chir Reparatrice Appar Mot. 2001;87(3):221-228.
24. Abdeen A, Hoang BH, Althanasina EA, Morris CD, Boland PJ, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am. 2009;91(10):2406-2415. doi:10.2106/JBJS.H.00815.
25. Getty PJ, Peabody TD. Complications and functional outcomes of reconstruction with an osteoarticular allograft after intra-articular resection of the proximal aspect of the humerus. J Bone Joint Surg Am. 1999;81(8):1138-1146.
26. Chen CF, Chen WM, Cheng YC, Chiang CC, Huang CK, Chen TH. Extracorporeally irradiated autograft-prosthetic composite arthroplasty using AML® extensively porous-coated stem for proximal femur reconstruction: a clinical analysis of 14 patients. J Surg Oncol. 2009;100(5):418-422. doi:10.1002/jso.21351.
27. Kelly JD 2nd, Purchase RJ, Kam G, Norris TR. Alloprosthetic composite reconstruction using the reverse shoulder arthroplasty. Tech Shoulder Elbow Surg. 2009;10(1):5-10.
28. Cannon CP, Paraliticci GU, Lin PP, Lewis VO, Yasko AW. Functional outcome following endoprosthetic reconstruction of the proximal humerus. J Shoulder Elbow Surg. 2009;18(5):705-710. doi:10.1016/j.jse.2008.10.011.
29. Chao EY, Fuchs B, Rowland CM, Ilstrup DM, Pritchard DJ, Sim FH. Long-term results of segmental prosthesis fixation by extracortical bone-bridging and ingrowth. J Bone Joint Surg Am. 2004;86-A(5):948-955.
30. Goulding KA, Schwartz A, Hattrup SJ, et al. Use of compressive osseointegration endoprostheses for massive bone loss from tumor and failed arthroplasty: a viable option in the upper extremity. Clin Orthop Relat Res. 2017;475(6):1702-1711. doi:10.1007/s11999-017-5258-0.
31. Guven MF, Aslan L, Botanlioglu H, Kaynak G, Kesmezacar H, Babacan M. Functional outcome of reverse shoulder tumor prosthesis in the treatment of proximal humeral tumors. J Shoulder Elbow Surg. 2016;25(1):e1-e6. doi:10.1016/j.jse.2015.06.012.
32. Wang ML, Ballard BL, Kulidjian AA, Abrams RA. Upper extremity reconstruction with a humeral tumor endoprosthesis: a novel salvage procedure after multiple revisions of total shoulder and elbow replacement. J Shoulder Elbow Surg. 2011;20(1):e1-e8. doi:10.1016/j.jse.2010.07.018.
1. Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91(1):119-127. doi:10.2106/JBJS.H.00094.
2. Hattrup SJ, Waldrop R, Sanchez-Sotelo J. Reverse total shoulder arthroplasty for posttraumatic sequelae. J Orthop Trauma. 2016;30(2):e41-e47. doi:10.1097/BOT.0000000000000416.
3. Sewell MD, Kang SN, Al-Hadithy N, et al. Management of peri-prosthetic fracture of the humerus with severe bone loss and loosening of the humeral component after total shoulder replacement. J Bone Joint Surg Br. 2012;94(10):1382-1389. doi:10.1302/0301-620X.94B10.29248.
4. Trompeter AJ, Gupta RR. The management of complex periprosthetic humeral fractures: a case series of strut allograft augmentation, and a review of the literature. Strategies Trauma Limb Reconstr. 2013;8(1):43-51. doi:10.1007/s11751-013-0155-x.
5. Khatib O, Onyekwelu I, Yu S, Zuckerman JD. Shoulder arthroplasty in New York State, 1991 to 2010: changing patterns of utilization. J Shoulder Elbow Surg. 2015;24(10):e286-e291. doi:10.1016/j.jse.2015.05.038.
6. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
7. Cil A, Veillette CJ, Sanchez-Sotelo J, Sperling JW, Schleck CD, Cofield RH. Survivorship of the humeral component in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):143-150. doi:10.1016/j.jse.2009.04.011.
8. Wright TW. Revision of humeral components in shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(2 suppl):S77-S81.
9. Duquin TR, Sperling JW. Revision shoulder arthroplasty—how to manage the humerus. Oper Tech Orthop. 2011;21(1):44-51. doi:10.1053/j.oto.2010.09.008.
10. Cil A, Veillette CJ, Sanchez-Sotelo J, Sperling JW, Schleck C, Cofield RH. Revision of the humeral component for aseptic loosening in arthroplasty of the shoulder. J Bone Joint Surg Br. 2009;91(1):75-81. doi:10.1302/0301-620X.91B1.21094.
11. Cofield RH. Revision of hemiarthroplasty to total shoulder arthroplasty. In: Zuckerman JD, ed. Advanced Reconstruction: Shoulder. 1st edition. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2007;613-622.
12. Budge MD, Moravek JE, Zimel MN, Nolan EM, Wiater JM. Reverse total shoulder arthroplasty for the management of failed shoulder arthroplasty with proximal humeral bone loss: is allograft augmentation necessary? J Shoulder Elbow Surg. 2013;22(6):739-744. doi:10.1016/j.jse.2012.08.008.
13. Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997;79(5):857-865.
14. Throckmorton TW. Reconstructive procedures of the shoulder and elbow. In: Azar FM, Beaty JH, Canale ST, eds. Campbell’s Operative Orthopaedics. 13th edition. Philadelphia, PA: Elsevier; 2017;570-622.
15. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409. doi:10.1067/mse.2001.116871.
16. De Wilde L, Walch G. Humeral prosthetic failure of reversed total shoulder arthroplasty: a report of three cases. J Shoulder Elbow Surg. 2006;15(2):260-264. doi:10.1016/j.jse.2005.07.014.
17. Owens CJ, Sperling JW, Cofield RH. Utility and complications of long-stem humeral components in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(7):e7-e12. doi:10.1016/j.jse.2012.10.034.
18. Stephens SP, Paisley KC, Giveans MR, Wirth MA. The effect of proximal humeral bone loss on revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):1519-1526. doi:10.1016/j.jse.2015.02.020.
19. Werner BS, Abdelkawi AF, Boehm D, et al. Long-term analysis of revision reverse shoulder arthroplasty using cemented long stems. J Shoulder Elbow Surg. 2017;26(2):273-278. doi:10.1016/j.jse.2016.05.015.
20. Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651. doi:10.1016/j.jse.2010.10.026.
21. Kassab M, Dumaine V, Babinet A, Ouaknine M, Tomeno B, Anract P. Twenty nine shoulder reconstructions after resection of the proximal humerus for neoplasm with mean 7-year follow-up. Rev Chir Orthop Reparatrice Appar Mot. 2005;91(1):15-23.
22. Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg. 2007;98(2):292-300. doi:10.2106/JBJS.E.01310.
23. Gagey O, Pourjamasb B, Court C. Revision arthroplasty of the shoulder for painful glenoid loosening: a series of 14 cases with acromial prostheses reviewed at four year follow up. Rev Chir Reparatrice Appar Mot. 2001;87(3):221-228.
24. Abdeen A, Hoang BH, Althanasina EA, Morris CD, Boland PJ, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am. 2009;91(10):2406-2415. doi:10.2106/JBJS.H.00815.
25. Getty PJ, Peabody TD. Complications and functional outcomes of reconstruction with an osteoarticular allograft after intra-articular resection of the proximal aspect of the humerus. J Bone Joint Surg Am. 1999;81(8):1138-1146.
26. Chen CF, Chen WM, Cheng YC, Chiang CC, Huang CK, Chen TH. Extracorporeally irradiated autograft-prosthetic composite arthroplasty using AML® extensively porous-coated stem for proximal femur reconstruction: a clinical analysis of 14 patients. J Surg Oncol. 2009;100(5):418-422. doi:10.1002/jso.21351.
27. Kelly JD 2nd, Purchase RJ, Kam G, Norris TR. Alloprosthetic composite reconstruction using the reverse shoulder arthroplasty. Tech Shoulder Elbow Surg. 2009;10(1):5-10.
28. Cannon CP, Paraliticci GU, Lin PP, Lewis VO, Yasko AW. Functional outcome following endoprosthetic reconstruction of the proximal humerus. J Shoulder Elbow Surg. 2009;18(5):705-710. doi:10.1016/j.jse.2008.10.011.
29. Chao EY, Fuchs B, Rowland CM, Ilstrup DM, Pritchard DJ, Sim FH. Long-term results of segmental prosthesis fixation by extracortical bone-bridging and ingrowth. J Bone Joint Surg Am. 2004;86-A(5):948-955.
30. Goulding KA, Schwartz A, Hattrup SJ, et al. Use of compressive osseointegration endoprostheses for massive bone loss from tumor and failed arthroplasty: a viable option in the upper extremity. Clin Orthop Relat Res. 2017;475(6):1702-1711. doi:10.1007/s11999-017-5258-0.
31. Guven MF, Aslan L, Botanlioglu H, Kaynak G, Kesmezacar H, Babacan M. Functional outcome of reverse shoulder tumor prosthesis in the treatment of proximal humeral tumors. J Shoulder Elbow Surg. 2016;25(1):e1-e6. doi:10.1016/j.jse.2015.06.012.
32. Wang ML, Ballard BL, Kulidjian AA, Abrams RA. Upper extremity reconstruction with a humeral tumor endoprosthesis: a novel salvage procedure after multiple revisions of total shoulder and elbow replacement. J Shoulder Elbow Surg. 2011;20(1):e1-e8. doi:10.1016/j.jse.2010.07.018.
TAKE-HOME POINTS
- Proximal humeral bone loss presents a significant challenge for the shoulder arthroplasty surgeon.
- Unsupported long-stemmed humeral components in this setting are prone to early loosening.
- APCs can rebuild proximal humeral bone stock, but have concerns with graft resorption and long-term failure.
- Modular endoprosthetic reconstruction of proximal humeral bone loss potentially allows those deficiencies to be addressed in a more durable fashion.
- Longer-term and larger studies are needed to determine the optimal reconstruction technique for proximal humeral bone loss.
Use of a Novel Magnesium-Based Resorbable Bone Cement for Augmenting Anchor and Tendon Fixation
ABSTRACT
The aim of this study was to assess the efficacy and safety of a novel magnesium-based resorbable bone cement (OsteoCrete, Bone Solutions Incorporated) for anchor and tendon fixation.
Cadaveric humeral testing involved straight pull-to-failure of rotator cuff suture anchors; OsteoCrete was injected through one anchor, and a second anchor served as the uninjected control. Testing was conducted 15 minutes post-injection. A canine preclinical model was used to evaluate the safety of the following parameters: Rotator cuff repair: A double-row technique was used to repair transected infraspinatus tendons; OsteoCrete was injected through both anchors in one limb, and the contralateral limb served as the uninjected control. Biceps tenodesis: The transected biceps tendon was implanted into a proximal humeral socket with a transcortical button; OsteoCrete was injected into the socket of one limb, and a screw was used for final fixation in the contralateral control limb. Nondestructive biomechanical testing and histologic assessment were performed after 12 weeks.
OsteoCrete-augmented anchors showed significantly higher load-to-failure compared to that with uninjected controls. In cadaveric humeri with reduced bone quality, OsteoCrete increased the mean load-to-failure by 99%. Within the preclinical model, there were no complications or statistically significant biomechanical/histologic differences between the techniques.
OsteoCrete has the potential for safely providing improved suture anchor and tissue fixation in patients with poor bone or tissue quality.
Continue to: Calcium phosphate bone void fillers...
Calcium phosphate bone void fillers have been commonly used in orthopedic surgery for several applications, including, but not limited to, a variety of fracture fixation or augmentation procedures.1-8 Continuing research on calcium phosphates has evidenced that the addition of magnesium phosphate to the formulation results in improved reactivity of the bone void filler. An in vitro study demonstrated enhanced attachment and proliferation of MG63 osteoblast-like cells on calcium magnesium phosphate cement (CMPC), in comparison with calcium phosphate cement (CPC), along with increased cellular alkaline phosphatase activity.9 The authors further explored the proliferation rates of MG63 cells by comparing CMPC with CPC and magnesium phosphate cement (MPC), and observed significantly increased proliferation of cells on CMPC. They also compared CMPC and CPC using a rabbit bone void model and observed substantial CMPC resorption with new bone formation at the 3-month time point and further reported that the majority of the defect had filled with new bone at 6 months, whereas CPC resulted in <10% new bone formation after 6 months.10 The authors continued to study the differences between CPC, MPC, and CMPC and identified increased proliferation of bone marrow stromal cells (bMSCs), when the cells were associated with CMPC and MPC, and when compared to that with CPC. The osteogenic differentiation of bMSCs was highest in the CMPC and CPC groups, when compared to that in the MPC group, with no significant difference between the CMPC and CPC groups. The authors also compared these 3 different formulations using a rabbit maxillary sinus floor elevation model, in which CMPC resulted in increased new bone formation and mineralization compared to that with CPC and MPC, which was further enhanced with the addition of bMSCs.11
These studies highlight the importance of having both a magnesium phosphate and a calcium phosphate component for a resorbable cement intended for use as a bone void filler. The rationale behind this strategy is related to the release of magnesium ions from the magnesium phosphate component. Magnesium has been shown to increase the proliferation of bMSCs, improve the attachment and growth of osteoblasts, stimulate the proteins involved in bone regeneration, enhance new bone formation, and boost bone mineralization.12,13
OsteoCrete (Bone Solutions Incorporation) is a novel CMPC composed of magnesium oxide, monopotassium phosphate, monosodium phosphate, hydroxyapatite, and sucrose. OsteoCrete has been demonstrated to significantly increase peak torque-to-failure of stainless-steel cortical bone screw fixation, when compared with screw fixation without augmentation and screw fixation with calcium phosphate augmentation using an in vivo equine model. In the same study, the authors showed that OsteoCrete resulted in an interface toughness that was significantly increased compared to that with no treatment, CPC augmentation, and polymethylmethacrylate (PMMA) augmentation. At 6 months after implantation, woven bone had replaced 69% of the OsteoCrete at the screw interface, compared to 44% of that with CPC.14 An equine study examined the effects of OsteoCrete on bone stability and healing using a metatarsal osteotomy model; the study reported significantly improved radiographic callus formation and a greater amount of new bone formation within the fracture gap when compared to that with CPC augmentation or no augmentation. OsteoCrete also secured the fragment significantly better than the CPC and control groups based on a decreased fracture gap over time.15 Another study using a preclinical anterior cruciate ligament (ACL) reconstruction model reported that OsteoCrete resulted in significantly better new bone formation in the tibial tunnel, a smaller amount of fibrous tissue, more cartilage formation at the tendon-bone interface, and a higher ultimate load-to-failure compared to that with standard ACL reconstruction in the contralateral limb after 6 weeks.16 OsteoCrete and PMMA were evaluated in terms of biomechanical fixation of a stemless humeral prosthesis, with data showing that both groups have higher failure loads, failure displacements, and failure cycles when compared to those with the control, nonaugmented group.17 Another preclinical model evaluated cranial bone flap augmentation with 2 resorbable cements and highlighted faster cement resorption and replacement with bone, along with superior stability within the OsteoCrete group compared to that with CPC.18 In a preclinical bone void study conducted for obtaining US Food and Drug Administration 510(k) clearance, OsteoCrete resulted in 83% greater resorption than that with CPC after 12 weeks and 35% greater resorption at 26 weeks, with 84% of OsteoCrete being resorbed and replaced with woven or lamellar mineralized bone of normal morphology at the 26-week time point (unpublished data provided by Bone Solutions Incorporated [BSI]).
These data indicate that CMPCs such as OsteoCrete appear to have potential benefits for augmenting the healing of bone implants and bone soft tissue. Therefore, the objective of this study was to assess the safety and efficacy of OsteoCrete in applications for the augmentation of anchor and tendon fixation in rotator cuff repair and biceps tenodesis procedures, respectively. Improving healing for these 2 commonly performed procedures would be of great benefit in improving the functional outcomes and mitigating the complications and morbidity.
MATERIALS AND METHODS
IN VITRO STUDY METHODS
Cadaveric humeri (N = 12, six matched pairs) of females (age, 70-75 years) were warmed to 37°C prior to testing. Two 4.75-mm vented anchors (SwiveLock, Arthrex) with FiberTape were implanted into a lateral row position (anterior and posterior anchor positioning) of a double-row rotator cuff repair within the greater tuberosity. One anchor was injected with 1 ml of OsteoCrete–after preparation according to the manufacturer’s instructions–through the cannulation channel after placement, and the other anchor served as the uninjected control for each humerus. For the six matched pairs, the OsteoCrete group and the control group were rotated with respect to anterior vs posterior location within the lateral row position. After 15 minutes of the injection, straight pull-to-failure (12 in/min) was performed. Data were compared between the groups for significant (P < .05) differences using t-tests and Pearson correlation.
Continue to: IN VIVO STUDY METHODS
IN VIVO STUDY METHODS
With Institutional Animal Care and Use Committee approval, adult (age, 2-4 years) purpose-bred dogs (N = 8) underwent aseptic surgery of both forelimbs for rotator cuff (infraspinatus) tendon repair (Figure 1) and biceps tenodesis (Figure 2). For the rotator cuff repair, two 4.75-mm vented anchors (1 medial and 1 lateral) with FiberTape were used in a modified double-row technique to repair the acutely transected infraspinatus tendon. In one limb, 1 ml of OsteoCrete was injected through both anchors; the other limb served as the uninjected control. For the biceps tenodesis procedure, the long head of the biceps tendon was transected at its origin and whip-stitched. The tendon was transposed and inserted into a 7-mm diameter socket drilled into the proximal humerus using a tension-slide technique with a transcortical button for fixation. In one limb, 1 ml of OsteoCrete was injected into the socket prior to final tensioning and tying. In the contralateral limb, a 7-mm interference screw (Bio-Tenodesis™ Screw, Arthrex) was inserted into the socket after tensioning and tying. The dogs were allowed to perform out-of-kennel monitored exercise daily for a period of 12 weeks after surgery and were then sacrificed.
The infraspinatus and biceps bone-tendon-muscle units were excised en bloc. Custom-designed jigs were used for biomechanical testing of the bone-tendon-muscle units along the anatomical vector of muscle contraction. Optical markers were mounted at standardized anatomical locations. Elongation of the repair site was defined as the change in distance between markers and was measured to 0.01-mm resolution using an optical tracking system (Optotrak Certus, NDI), synchronized with measurement of the applied tension load. The bone-tendon-muscle units were loaded in tension to 3-mm elongation at a displacement controlled rate of 0.01 mm/s. Load at 1-mm, 2-mm, and 3-mm displacement of the tendon-bone junction was extracted from the load vs the displacement curve of each sample. Stiffness was calculated as the slope of the linear portion of the load vs the displacement curve.19,20
For histologic assessments, sections of each treatment site were obtained using a microsaw and alternated between decalcified and non-decalcified processing. For decalcified bone processing, formalin-fixed tissues were placed in 10% ethylenediaminetetraacetic acid with phosphate-buffered saline for 39 days and then processed routinely for the assessment of sections stained with hematoxylin and eosin (H&E), toluidine blue, and picrosirius red. For non-decalcified bone processing, the tissues were dehydrated through a series of graded ethyl alcohol solutions, embedded in PMMA, sectioned, and stained with toluidine blue and Goldner’s trichrome. Two pathologists who were blinded to the clinical application and the differences between techniques assessed the histologic sections and scored each section using the modified Bonar score that assesses cell morphology, collagen arrangement, cellularity, vascularity, and extracellular matrix using a 15-point scale, where a higher score indicates more pathology.21
Categorical data were compared for detecting statistically significant differences using the rank sum test. Continuous data were compared for identifying statistically significant differences using the t-test or one-way ANOVA. Significance was set at P < .05.
RESULTS
IN VITRO RESULTS
OsteoCrete-augmented anchors (mean = 225 N; range, 158-287 N) had significantly (P < .001) higher pull-out load-to-failure compared to that in the uninjected controls (mean = 161 N; range, 68-202 N), which translated to a 50% mean increase (range, 3%-134%) in load-to-failure (Table 1). For humeri with reduced bone quality (control anchors that failed at <160 N, 4 humeri), the mean increase in load-to-failure for OsteoCrete-augmented anchors was 99% (range, 58%-135%), with the difference between mean values being again significantly different (OsteoCrete mean = 205 N; control mean = 110 N, P < .001). When the control and OsteoCrete load-to-failure values were compared using Pearson correlation, a significantly strong positive correlation (r = 0.66, P = 0.02) was detected. When the control load-to-failure values were compared with its percent increase value when OsteoCrete was used, there was a significantly very strong negative correlation (r = −0.90, P < .001).
Table 1. Cadaveric Lateral Row Rotator Cuff Anchor Pull-To-Failure; Testing Occurred 15 Minutes Post-Injection
| Humerus No. | Control (N) | OsteoCrete (N)a | Percent Increase |
| 1-Right (PA) | 197.28 | 278.73 | 41% |
| 1-Left (AP) | 152.62 | 241.72 | 58% |
| 2-Right (PA) | 178.60 | 196.03 | 10% |
| 2-Left (AP) | 170.10 | 175.57 | 3% |
| 3-Right (PA) | 67.70 | 158.31 | 134% |
| 3-Left (AP) | 74.24 | 173.08 | 133% |
| 4-Right (PA) | 195.81 | 248.12 | 27% |
| 4-Left (AP) | 201.95 | 209.42 | 4% |
| 5-Right (PA) | 173.30 | 220.59 | 27% |
| 5-Left (AP) | 146.61 | 247.37 | 69% |
| 6-Right (PA) | 171.03 | 266.14 | 56% |
| 6-Left (AP) | 199.99 | 286.91 | 43% |
| Average | 160.77 + 45.60 | 225.17 + 43.08 | 50% + 44 |
aOsteoCrete (Bone Solutions Incorporated) resulted in significantly increased (P < 0.001) pull-to-failure. Abbreviations: AP, control anchor located in anterior position, OsteoCrete anchor located in posterior position; PA, control anchor located in posterior position, OsteoCrete anchor located in anterior position.
Continue to: IN VIVO RESULTS
IN VIVO RESULT
No intraoperative or postoperative complications were noted. All repairs were found to be intact based on the gross assessment and the completed biomechanical testing without failure. No statistically significant (P > 0.3) biomechanical differences were found between the techniques (Table 2). Histologic assessments showed low-to-mild pathology scores for all sites with no statistically significant (P > 0.3) differences between the techniques (Table 2). Both control and OsteoCrete rotator cuff repairs demonstrated tendon-to-bone integration via fibrous connective tissue attachment to bone. All anchors were in place with no evidence for loosening, tunnel expansion, or cyst formation. OsteoCrete-augmented anchor repairs were associated with cement remaining within their lumens along with a thin layer of cement interposed between the anchor and the bone interface around their entire periphery. The cement-bone interface was discrete with typical inflammatory cell infiltrate without evidence for infection, membrane or cyst formation, or other untoward pathologic responses. In the OsteoCrete biceps tenodesis group, the tendons filled the tunnels with a thin layer of cement remaining interposed between the tendon and the bone interface around the entire periphery. The tendon-cement-bone interface was discrete with typical inflammatory cell infiltrates and without evidence for infection, membrane or cyst formation, or other untoward pathologic responses. Tendon-to-bone integration was not observed in the control or OsteoCrete biceps tenodesis groups at the 12-week study endpoint. Representative histologic images of the rotator cuff tendon repairs and biceps tenodesis procedures are shown in Figures 3A, 3B and Figures 4A, 4B, respectively.
Table 2. Biomechanical Testing And Histologic Scoring Of Rotator Cuff And Biceps Tendon Repairs In A Preclinical Model
| Procedure | Force (N) at 1 mm | Force (N) at 2 mm | Force (N) at 3 mm | Stiffness (N/mm) | Histologic Score |
| Rotator Cuff - Control | 14.0 + 3.3 | 19.3 + 5.5 | 25.0 + 7.0 | 5.4 + 2.0 | 4.6 + 1.1 |
| Rotator Cuff - OsteoCrete (Bone Solutions Incorporated) | 14.8 + 3.7 | 20.4 + 6.0 | 26.4 + 8.5 | 6.3 + 2.5 | 3.9 + 1.7 |
| Biceps - Control | 23.1 + 6.2 | 35.5 + 8.5 | 52.6 + 15.0 | 17.8 + 6.4 | 3.4 + 1.2 |
| Biceps - OsteoCrete | 22.4 + 7.3 | 36.8 + 10.1 | 57.8 + 16.0 | 21.1 + 8.5 | 3.4 + 0.7 |
There were no significant differences (P < 0.05) between groups. Histologic scoring based on a 15-point scale with higher scores indicating more pathology.
DISCUSSION
The results of this study highlight the safety and efficacy of OsteoCrete in applications for the augmentation of anchor and tendon fixation in rotator cuff repair and biceps tenodesis procedures, respectively. Anchors augmented with OsteoCrete resulted in significantly increased load-to-failure pull-out strength 15 minutes after insertion. In addition, a very strong negative correlation was found between the percentage of improved load-to-failure after OsteoCrete injection and the bone quality of the humerus, which was based on the control load-to-failure values. In the validated preclinical model used in this study, OsteoCrete-based fixation was found to be noninferior to current standard-of-care techniques and was not associated with any untoward pathologic responses of humeral bone, rotator cuff tendon, or biceps tendon based on the biomechanical and histologic analyses. These data highlight the functional efficacy and biocompatibility of OsteoCrete when used for these common indications.

More than 270,000 rotator cuff procedures have been reported to be performed in the US annually (average patient age: 61 years for women, 56 years for men).22 Rotator cuff repair procedures have been associated with a 20% failure rate, with one of the causes being related to an inability for the tendon to heal, even with strong initial fixation.23 Rotator cuff repair techniques are being continuously optimized with the goal of improving patient outcomes. This goal is being realized, primarily with respect to re-tear rates.24,25 However, even with advanced techniques, there are still relatively high rates of failure reported, with increasing patient age serving as one of the primary negative prognostic factors.26 An older patient population is associated with decreased bone mass and strength, and postmenopausal females have decreased bone quality; these factors are associated with higher rotator cuff failure rates due to poor tendon healing, with anchor fixation failure also playing a role.27-29 Therefore, it is critically important to develop methods for augmenting implant and tendon fixation to bone to achieve functional healing. The results of this study suggest that OsteoCrete provides a valid method for accomplishing this goal based on the observation that proximal humeral anchor fixation was improved by 50% in load-to-failure 15 minutes post-injection with an even more profound impact on the anchors placed in poor-quality bone (99% increased load-to-failure 15 minutes post-injection). It is probable that the degree of improvement in fixation strength would be even greater 1 day after fixation, since the strength of OsteoCrete continues to increase over the first 30 hours of curing.

Based on the preclinical animal model data of this study, OsteoCrete augmentation of rotator cuff anchor fixation had no untoward effects on tendon healing or function and can be considered as safe for use. Previously published data also suggest that OsteoCrete may improve osseous replacement of anchors as a result of magnesium ion release, which can drive adjacent attachment and growth of osteoblasts, leading to enhanced new bone formation.9-16,18 As such, surgeons may consider this means of anchor augmentation in situations of questionable or poor-quality bone and/or when accelerated postoperative rehabilitation protocols are desired.
A very low early incidence failure rate (1.2%) has been reported when a distal biceps tendon rupture is repaired using cortical suspensory fixation in conjunction with an interference screw.30 When an early re-rupture does occur, the most common explanation for failure tends to be a lack of patient compliance, with excessive force being placed on the repair.31 This study was not meant to investigate the methods to increase the strength of a biceps tendon repair using OsteoCrete but instead to replace the interference screw with OsteoCrete in a safe and noninferior manner. Primary fixation was still dependent on cortical suspensory fixation; however, OsteoCrete was used to help aid in stabilization of the tendon without the need for interference screw fixation. Although rare, osteolysis and perianchor cyst formation have been reported adjacent to nonbiodegradable anchors (PEEK), along with several types of biodegradable anchors (PLLA, hydroxyapatite plus PLLA, β-tricalcium phosphate plus PLLA, and polyglycolic acid; the latter of the 3 resulted in the lowest incidence of perianchor cyst formation) in the shoulder and elbow.32-34 Whenever osteolysis or cyst formation occurs around an anchor, it leads to decreased bone volume and potential adjacent bone weakness, which may act as a stress riser, thus increasing the risk for fracture. This potential is probably more of a concern within the proximal radius where there is a decreased amount of bone stock around the anchor.34
Continue to: In this study...
In this study, a short-term 12-week analysis revealed no significant differences in the nondestructive biomechanical testing and histologic analysis results between the use of OsteoCrete and the use of a tenodesis anchor. These results indicate the potential for using OsteoCrete as an anchor replacement. The biceps tendon did not react negatively to the OsteoCrete material, which indicated that OsteoCrete can be used adjacent to tendons without the concern of weakening the tendon due to an inflammatory reaction. This being said, tendon-to-bone integration was not evident at this early time point. It would be helpful to further explore the potential of this technique with a longer-term study investigating tendon-to-bone integration in more detail. Ideally, a long-term study would reveal an increased amount of new bone formation within the socket when compared to that with the anchor comparison, similar to the results reported by Gulotta and colleagues16 when using a tendon for ACL reconstruction with OsteoCrete.
We do note several limitations in this study. The dogs used in this study were healthy with normal bone and tendon morphology, the tendons were transected and repaired during the course of the same surgery, and only 1 early time point was evaluated. Additional investigations continuing the characterization of these clinical applications using an osteopenic or osteoporotic preclinical model with chronic tendon pathology and longer-term evaluation are now warranted based on the positive findings of this initial work.
CONCLUSION
OsteoCrete augmentation significantly improved initial rotator cuff anchor fixation (human in vitro) and was safe and effective for anchor and tendon fixation in rotator cuff tendon repair and biceps tenodesis procedures (canine in vivo), respectively, when compared with the current standard-of-care. Of note, the significant improvements associated with OsteoCrete were the greatest in poor-quality bone. Based on these results and considering the previously discussed limitations, it can be concluded that OsteoCrete has the potential for safely providing improved suture anchor and tissue fixation in patients with poor bone or tissue quality. Further in vivo study toward potential clinical applications is warranted.
1. Russell TA, Leighton RK, Group A-BTPFS. Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J Bone Joint Surg Am. 2008; 90(10):2057-2061. doi:10.2106/JBJS.G.01191.
2. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction-internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012; 21(6):741-748. doi:10.1016/j.jse.2011.09.017.
3. Cassidy C, Jupiter JB, Cohen M, et al. Norian SRS cement compared with conventional fixation in distal radial fractures. A randomized study. J Bone Joint Surg Am. 2003;85-A(11):2127-2137.
4. Mattsson P, Alberts A, Dahlberg G, Sohlman M, Hyldahl HC, Larsson S. Resorbable cement for the augmentation of internally-fixed unstable trochanteric fractures. A prospective, randomised multicentre study. J Bone Joint Surg Br. 2005;87(9):1203-1209.
5. Cohen SB, Sharkey PF. Subchondroplasty for treating bone marrow lesions. J Knee Surg. 2016;29(07):555-563. doi:10.1302/0301-620X.87B9.15792.
6. Guida P, Ragozzino R, Sorrentino B, et al. Three-in-One minimally invasive approach to surgical treatment of pediatric pathological fractures with wide bone loss through bone cysts: ESIN, curettage and packing with injectable HA bone substitute. A retrospective series of 116 cases. Injury. 2016;47(6):1222-1228. doi:10.1016/j.injury.2016.01.006.
7. Maestretti G, Sutter P, Monnard E, et al. A prospective study of percutaneous balloon kyphoplasty with calcium phosphate cement in traumatic vertebral fractures: 10-year results. Eur Spine J. 2014;23(6):1354-1360. doi:10.1007/s00586-014-3206-1.
8. Nakano M, Hirano N, Zukawa M, et al. Vertebroplasty using calcium phosphate cement for osteoporotic vertebral fractures: study of outcomes at a minimum follow-up of two years. Asian Spine J. 2012;6(1):34-42. doi:10.4184/asj.2012.6.1.34.
9. Jia J, Zhou H, Wei J, et al. Development of magnesium calcium phosphate biocement for bone regeneration. J R Soc Interface. 2010;7(49):1171-1180. doi:10.1098/rsif.2009.0559.
10. Wu F, Wei J, Guo H, Chen F, Hong H, Liu C. Self-setting bioactive calcium-magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008;4(6):1873-1884. doi:10.1016/j.actbio.2008.06.020.
11. Zeng D, Xia L, Zhang W, et al. Maxillary sinus floor elevation using a tissue-engineered bone with calcium-magnesium phosphate cement and bone marrow stromal cells in rabbits. Tissue Eng Part A. 2012;18(7-8):870-881. doi:10.1089/ten.TEA.2011.0379.
12. Yoshizawa S, Brown A, Barchowsky A, Sfeir C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014;10(6):2834-2842. doi:10.1016/j.actbio.2014.02.002.
13. Liao J, Qu Y, Chu B, Zhang X, Qian Z. Biodegradable CSMA/PECA/Graphene porous hybrid scaffold for cartilage tissue engineering. Sci Rep. 2015;5:9879. doi:10.1038/srep09879.
14. Hirvinen LJ, Litsky AS, Samii VF, Weisbrode SE, Bertone AL. Influence of bone cements on bone-screw interfaces in the third metacarpal and third metatarsal bones of horses. Am J Vet Res. 2009;70(8):964-972. doi:10.2460/ajvr.70.8.964.
15. Waselau M, Samii VF, Weisbrode SE, Litsky AS, Bertone AL. Effects of a magnesium adhesive cement on bone stability and healing following a metatarsal osteotomy in horses. Am J Vet Res. 2007;68(4):370-378. doi:10.2460/ajvr.68.4.370.
16. Gulotta LV, Kovacevic D, Ying L, Ehteshami JR, Montgomery S, Rodeo SA. Augmentation of tendon-to-bone healing with a magnesium-based bone adhesive. Am J Sports Med. 2008;36(7):1290-1297. doi:10.1177/0363546508314396.
17. Kim MS, Kovacevic D, Milks RA, et al. Bone graft substitute provides metaphyseal fixation for a stemless humeral implant. Orthopedics. 2015;38(7):e597-e603. doi:10.3928/01477447-20150701-58.
18. Schendel SA, Peauroi J. Magnesium-based bone cement and bone void filler: preliminary experimental studies. J Craniofac Surg. 2009;20(2):461-464. doi:10.1097/SCS.0b013e31819b9819.
19. Pfeiffer FM, Smith MJ, Cook JL, Kuroki K. The histologic and biomechanical response of two commercially available small glenoid anchors for use in labral repairs. J Shoulder Elbow Surg. 2014;23(8):1156-1161. doi:10.1016/j.jse.2013.12.036.
20. Smith MJ, Cook JL, Kuroki K, et al. Comparison of a novel bone-tendon allograft with a human dermis-derived patch for repair of chronic large rotator cuff tears using a canine model. Arthroscopy. 2012;28(2):169-177. doi:10.1016/j.arthro.2011.08.296.
21. Fearon A, Dahlstrom JE, Twin J, Cook J, Scott A. The Bonar score revisited: region of evaluation significantly influences the standardized assessment of tendon degeneration. J Sci Med Sport. 2014;17(4):346-350. doi:10.1016/j.jsams.2013.07.008.
22. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233. doi:10.2106/JBJS.J.00739.
23. Lädermann A, Denard PJ, Burkhart SS. Management of failed rotator cuff repair: a systematic review. J ISAKOS. 2016;1(1):32-37. doi:10.1136/jisakos-2015-000027.
24. Franceschi F, Papalia R, Franceschetti E, et al. Double-Row repair lowers the retear risk after accelerated rehabilitation. Am J Sports Med. 2016;44(4):948-956. doi:10.1177/0363546515623031.
25. Wang E, Wang L, Gao P, Li Z, Zhou X, Wang S. Single-versus double-row arthroscopic rotator cuff repair in massive tears. Med Sci Monit. 2015;21:1556-1561. doi:10.12659/MSM.893058.
26. Abtahi AM, Granger EK, Tashjian RZ. Factors affecting healing after arthroscopic rotator cuff repair. World J Orthop. 2015;6(2):211-220. doi:10.5312/wjo.v6.i2.211.
27. Chung SW, Oh JH, Gong HS, Kim JY, Kim SH. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med. 2011;39(10):2099-2107. doi:10.1177/0363546511415659.
28. Tsiouri C, Mok DH. Early pullout of lateral row knotless anchor in rotator cuff repair. Int J Shoulder Surg. 2009;3(3):63-65. doi:10.4103/0973-6042.59972.
29. Boskey AL, Coleman R. Aging and bone. J Dent Res. 2010;89(12):1333-1348. doi:10.1177/0022034510377791.
30. Cusick MC, Cottrell BJ, Cain RA, Mighell MA. Low incidence of tendon rerupture after distal biceps repair by cortical button and interference screw. J Shoulder Elbow Surg. 2014;23(10):1532-1536. doi:10.1016/j.jse.2014.04.013.
31. Hinchey JW, Aronowitz JG, Sanchez-Sotelo J, Morrey BF. Re-rupture rate of primarily repaired distal biceps tendon injuries. J Shoulder Elbow Surg. 2014;23(6):850-854. doi:10.1016/j.jse.2014.02.006.
32. Shahrulazua A, Duckworth D, Bokor DJ. Perianchor radiolucency following PEEK suture anchor application associated with recurrent shoulder dislocation: a case report. Clin Ter. 2014;165(1):31-34. doi:10.7471/CT.2014.1658.
33. Kim SH, Kim dY, Kwon JE, Park JS, Oh JH. Perianchor cyst formation around biocomposite biodegradable suture anchors after rotator cuff repair. Am J Sports Med. 2015;43(12):2907-2912. doi:10.1177/0363546515608484
34. Potapov A, Laflamme YG, Gagnon S, Canet F, Rouleau DM. Progressive osteolysis of the radius after distal biceps tendon repair with the bioabsorbable screw. J Shoulder Elbow Surg. 2011;20(5):819-826. doi:10.1016/j.jse.2011.02.021.
ABSTRACT
The aim of this study was to assess the efficacy and safety of a novel magnesium-based resorbable bone cement (OsteoCrete, Bone Solutions Incorporated) for anchor and tendon fixation.
Cadaveric humeral testing involved straight pull-to-failure of rotator cuff suture anchors; OsteoCrete was injected through one anchor, and a second anchor served as the uninjected control. Testing was conducted 15 minutes post-injection. A canine preclinical model was used to evaluate the safety of the following parameters: Rotator cuff repair: A double-row technique was used to repair transected infraspinatus tendons; OsteoCrete was injected through both anchors in one limb, and the contralateral limb served as the uninjected control. Biceps tenodesis: The transected biceps tendon was implanted into a proximal humeral socket with a transcortical button; OsteoCrete was injected into the socket of one limb, and a screw was used for final fixation in the contralateral control limb. Nondestructive biomechanical testing and histologic assessment were performed after 12 weeks.
OsteoCrete-augmented anchors showed significantly higher load-to-failure compared to that with uninjected controls. In cadaveric humeri with reduced bone quality, OsteoCrete increased the mean load-to-failure by 99%. Within the preclinical model, there were no complications or statistically significant biomechanical/histologic differences between the techniques.
OsteoCrete has the potential for safely providing improved suture anchor and tissue fixation in patients with poor bone or tissue quality.
Continue to: Calcium phosphate bone void fillers...
Calcium phosphate bone void fillers have been commonly used in orthopedic surgery for several applications, including, but not limited to, a variety of fracture fixation or augmentation procedures.1-8 Continuing research on calcium phosphates has evidenced that the addition of magnesium phosphate to the formulation results in improved reactivity of the bone void filler. An in vitro study demonstrated enhanced attachment and proliferation of MG63 osteoblast-like cells on calcium magnesium phosphate cement (CMPC), in comparison with calcium phosphate cement (CPC), along with increased cellular alkaline phosphatase activity.9 The authors further explored the proliferation rates of MG63 cells by comparing CMPC with CPC and magnesium phosphate cement (MPC), and observed significantly increased proliferation of cells on CMPC. They also compared CMPC and CPC using a rabbit bone void model and observed substantial CMPC resorption with new bone formation at the 3-month time point and further reported that the majority of the defect had filled with new bone at 6 months, whereas CPC resulted in <10% new bone formation after 6 months.10 The authors continued to study the differences between CPC, MPC, and CMPC and identified increased proliferation of bone marrow stromal cells (bMSCs), when the cells were associated with CMPC and MPC, and when compared to that with CPC. The osteogenic differentiation of bMSCs was highest in the CMPC and CPC groups, when compared to that in the MPC group, with no significant difference between the CMPC and CPC groups. The authors also compared these 3 different formulations using a rabbit maxillary sinus floor elevation model, in which CMPC resulted in increased new bone formation and mineralization compared to that with CPC and MPC, which was further enhanced with the addition of bMSCs.11
These studies highlight the importance of having both a magnesium phosphate and a calcium phosphate component for a resorbable cement intended for use as a bone void filler. The rationale behind this strategy is related to the release of magnesium ions from the magnesium phosphate component. Magnesium has been shown to increase the proliferation of bMSCs, improve the attachment and growth of osteoblasts, stimulate the proteins involved in bone regeneration, enhance new bone formation, and boost bone mineralization.12,13
OsteoCrete (Bone Solutions Incorporation) is a novel CMPC composed of magnesium oxide, monopotassium phosphate, monosodium phosphate, hydroxyapatite, and sucrose. OsteoCrete has been demonstrated to significantly increase peak torque-to-failure of stainless-steel cortical bone screw fixation, when compared with screw fixation without augmentation and screw fixation with calcium phosphate augmentation using an in vivo equine model. In the same study, the authors showed that OsteoCrete resulted in an interface toughness that was significantly increased compared to that with no treatment, CPC augmentation, and polymethylmethacrylate (PMMA) augmentation. At 6 months after implantation, woven bone had replaced 69% of the OsteoCrete at the screw interface, compared to 44% of that with CPC.14 An equine study examined the effects of OsteoCrete on bone stability and healing using a metatarsal osteotomy model; the study reported significantly improved radiographic callus formation and a greater amount of new bone formation within the fracture gap when compared to that with CPC augmentation or no augmentation. OsteoCrete also secured the fragment significantly better than the CPC and control groups based on a decreased fracture gap over time.15 Another study using a preclinical anterior cruciate ligament (ACL) reconstruction model reported that OsteoCrete resulted in significantly better new bone formation in the tibial tunnel, a smaller amount of fibrous tissue, more cartilage formation at the tendon-bone interface, and a higher ultimate load-to-failure compared to that with standard ACL reconstruction in the contralateral limb after 6 weeks.16 OsteoCrete and PMMA were evaluated in terms of biomechanical fixation of a stemless humeral prosthesis, with data showing that both groups have higher failure loads, failure displacements, and failure cycles when compared to those with the control, nonaugmented group.17 Another preclinical model evaluated cranial bone flap augmentation with 2 resorbable cements and highlighted faster cement resorption and replacement with bone, along with superior stability within the OsteoCrete group compared to that with CPC.18 In a preclinical bone void study conducted for obtaining US Food and Drug Administration 510(k) clearance, OsteoCrete resulted in 83% greater resorption than that with CPC after 12 weeks and 35% greater resorption at 26 weeks, with 84% of OsteoCrete being resorbed and replaced with woven or lamellar mineralized bone of normal morphology at the 26-week time point (unpublished data provided by Bone Solutions Incorporated [BSI]).
These data indicate that CMPCs such as OsteoCrete appear to have potential benefits for augmenting the healing of bone implants and bone soft tissue. Therefore, the objective of this study was to assess the safety and efficacy of OsteoCrete in applications for the augmentation of anchor and tendon fixation in rotator cuff repair and biceps tenodesis procedures, respectively. Improving healing for these 2 commonly performed procedures would be of great benefit in improving the functional outcomes and mitigating the complications and morbidity.
MATERIALS AND METHODS
IN VITRO STUDY METHODS
Cadaveric humeri (N = 12, six matched pairs) of females (age, 70-75 years) were warmed to 37°C prior to testing. Two 4.75-mm vented anchors (SwiveLock, Arthrex) with FiberTape were implanted into a lateral row position (anterior and posterior anchor positioning) of a double-row rotator cuff repair within the greater tuberosity. One anchor was injected with 1 ml of OsteoCrete–after preparation according to the manufacturer’s instructions–through the cannulation channel after placement, and the other anchor served as the uninjected control for each humerus. For the six matched pairs, the OsteoCrete group and the control group were rotated with respect to anterior vs posterior location within the lateral row position. After 15 minutes of the injection, straight pull-to-failure (12 in/min) was performed. Data were compared between the groups for significant (P < .05) differences using t-tests and Pearson correlation.
Continue to: IN VIVO STUDY METHODS
IN VIVO STUDY METHODS
With Institutional Animal Care and Use Committee approval, adult (age, 2-4 years) purpose-bred dogs (N = 8) underwent aseptic surgery of both forelimbs for rotator cuff (infraspinatus) tendon repair (Figure 1) and biceps tenodesis (Figure 2). For the rotator cuff repair, two 4.75-mm vented anchors (1 medial and 1 lateral) with FiberTape were used in a modified double-row technique to repair the acutely transected infraspinatus tendon. In one limb, 1 ml of OsteoCrete was injected through both anchors; the other limb served as the uninjected control. For the biceps tenodesis procedure, the long head of the biceps tendon was transected at its origin and whip-stitched. The tendon was transposed and inserted into a 7-mm diameter socket drilled into the proximal humerus using a tension-slide technique with a transcortical button for fixation. In one limb, 1 ml of OsteoCrete was injected into the socket prior to final tensioning and tying. In the contralateral limb, a 7-mm interference screw (Bio-Tenodesis™ Screw, Arthrex) was inserted into the socket after tensioning and tying. The dogs were allowed to perform out-of-kennel monitored exercise daily for a period of 12 weeks after surgery and were then sacrificed.

The infraspinatus and biceps bone-tendon-muscle units were excised en bloc. Custom-designed jigs were used for biomechanical testing of the bone-tendon-muscle units along the anatomical vector of muscle contraction. Optical markers were mounted at standardized anatomical locations. Elongation of the repair site was defined as the change in distance between markers and was measured to 0.01-mm resolution using an optical tracking system (Optotrak Certus, NDI), synchronized with measurement of the applied tension load. The bone-tendon-muscle units were loaded in tension to 3-mm elongation at a displacement controlled rate of 0.01 mm/s. Load at 1-mm, 2-mm, and 3-mm displacement of the tendon-bone junction was extracted from the load vs the displacement curve of each sample. Stiffness was calculated as the slope of the linear portion of the load vs the displacement curve.19,20

For histologic assessments, sections of each treatment site were obtained using a microsaw and alternated between decalcified and non-decalcified processing. For decalcified bone processing, formalin-fixed tissues were placed in 10% ethylenediaminetetraacetic acid with phosphate-buffered saline for 39 days and then processed routinely for the assessment of sections stained with hematoxylin and eosin (H&E), toluidine blue, and picrosirius red. For non-decalcified bone processing, the tissues were dehydrated through a series of graded ethyl alcohol solutions, embedded in PMMA, sectioned, and stained with toluidine blue and Goldner’s trichrome. Two pathologists who were blinded to the clinical application and the differences between techniques assessed the histologic sections and scored each section using the modified Bonar score that assesses cell morphology, collagen arrangement, cellularity, vascularity, and extracellular matrix using a 15-point scale, where a higher score indicates more pathology.21
Categorical data were compared for detecting statistically significant differences using the rank sum test. Continuous data were compared for identifying statistically significant differences using the t-test or one-way ANOVA. Significance was set at P < .05.
RESULTS
IN VITRO RESULTS
OsteoCrete-augmented anchors (mean = 225 N; range, 158-287 N) had significantly (P < .001) higher pull-out load-to-failure compared to that in the uninjected controls (mean = 161 N; range, 68-202 N), which translated to a 50% mean increase (range, 3%-134%) in load-to-failure (Table 1). For humeri with reduced bone quality (control anchors that failed at <160 N, 4 humeri), the mean increase in load-to-failure for OsteoCrete-augmented anchors was 99% (range, 58%-135%), with the difference between mean values being again significantly different (OsteoCrete mean = 205 N; control mean = 110 N, P < .001). When the control and OsteoCrete load-to-failure values were compared using Pearson correlation, a significantly strong positive correlation (r = 0.66, P = 0.02) was detected. When the control load-to-failure values were compared with its percent increase value when OsteoCrete was used, there was a significantly very strong negative correlation (r = −0.90, P < .001).
Table 1. Cadaveric Lateral Row Rotator Cuff Anchor Pull-To-Failure; Testing Occurred 15 Minutes Post-Injection
| Humerus No. | Control (N) | OsteoCrete (N)a | Percent Increase |
| 1-Right (PA) | 197.28 | 278.73 | 41% |
| 1-Left (AP) | 152.62 | 241.72 | 58% |
| 2-Right (PA) | 178.60 | 196.03 | 10% |
| 2-Left (AP) | 170.10 | 175.57 | 3% |
| 3-Right (PA) | 67.70 | 158.31 | 134% |
| 3-Left (AP) | 74.24 | 173.08 | 133% |
| 4-Right (PA) | 195.81 | 248.12 | 27% |
| 4-Left (AP) | 201.95 | 209.42 | 4% |
| 5-Right (PA) | 173.30 | 220.59 | 27% |
| 5-Left (AP) | 146.61 | 247.37 | 69% |
| 6-Right (PA) | 171.03 | 266.14 | 56% |
| 6-Left (AP) | 199.99 | 286.91 | 43% |
| Average | 160.77 + 45.60 | 225.17 + 43.08 | 50% + 44 |
aOsteoCrete (Bone Solutions Incorporated) resulted in significantly increased (P < 0.001) pull-to-failure. Abbreviations: AP, control anchor located in anterior position, OsteoCrete anchor located in posterior position; PA, control anchor located in posterior position, OsteoCrete anchor located in anterior position.
Continue to: IN VIVO RESULTS
IN VIVO RESULT
No intraoperative or postoperative complications were noted. All repairs were found to be intact based on the gross assessment and the completed biomechanical testing without failure. No statistically significant (P > 0.3) biomechanical differences were found between the techniques (Table 2). Histologic assessments showed low-to-mild pathology scores for all sites with no statistically significant (P > 0.3) differences between the techniques (Table 2). Both control and OsteoCrete rotator cuff repairs demonstrated tendon-to-bone integration via fibrous connective tissue attachment to bone. All anchors were in place with no evidence for loosening, tunnel expansion, or cyst formation. OsteoCrete-augmented anchor repairs were associated with cement remaining within their lumens along with a thin layer of cement interposed between the anchor and the bone interface around their entire periphery. The cement-bone interface was discrete with typical inflammatory cell infiltrate without evidence for infection, membrane or cyst formation, or other untoward pathologic responses. In the OsteoCrete biceps tenodesis group, the tendons filled the tunnels with a thin layer of cement remaining interposed between the tendon and the bone interface around the entire periphery. The tendon-cement-bone interface was discrete with typical inflammatory cell infiltrates and without evidence for infection, membrane or cyst formation, or other untoward pathologic responses. Tendon-to-bone integration was not observed in the control or OsteoCrete biceps tenodesis groups at the 12-week study endpoint. Representative histologic images of the rotator cuff tendon repairs and biceps tenodesis procedures are shown in Figures 3A, 3B and Figures 4A, 4B, respectively.
Table 2. Biomechanical Testing And Histologic Scoring Of Rotator Cuff And Biceps Tendon Repairs In A Preclinical Model
| Procedure | Force (N) at 1 mm | Force (N) at 2 mm | Force (N) at 3 mm | Stiffness (N/mm) | Histologic Score |
| Rotator Cuff - Control | 14.0 + 3.3 | 19.3 + 5.5 | 25.0 + 7.0 | 5.4 + 2.0 | 4.6 + 1.1 |
| Rotator Cuff - OsteoCrete (Bone Solutions Incorporated) | 14.8 + 3.7 | 20.4 + 6.0 | 26.4 + 8.5 | 6.3 + 2.5 | 3.9 + 1.7 |
| Biceps - Control | 23.1 + 6.2 | 35.5 + 8.5 | 52.6 + 15.0 | 17.8 + 6.4 | 3.4 + 1.2 |
| Biceps - OsteoCrete | 22.4 + 7.3 | 36.8 + 10.1 | 57.8 + 16.0 | 21.1 + 8.5 | 3.4 + 0.7 |
There were no significant differences (P < 0.05) between groups. Histologic scoring based on a 15-point scale with higher scores indicating more pathology.
DISCUSSION
The results of this study highlight the safety and efficacy of OsteoCrete in applications for the augmentation of anchor and tendon fixation in rotator cuff repair and biceps tenodesis procedures, respectively. Anchors augmented with OsteoCrete resulted in significantly increased load-to-failure pull-out strength 15 minutes after insertion. In addition, a very strong negative correlation was found between the percentage of improved load-to-failure after OsteoCrete injection and the bone quality of the humerus, which was based on the control load-to-failure values. In the validated preclinical model used in this study, OsteoCrete-based fixation was found to be noninferior to current standard-of-care techniques and was not associated with any untoward pathologic responses of humeral bone, rotator cuff tendon, or biceps tendon based on the biomechanical and histologic analyses. These data highlight the functional efficacy and biocompatibility of OsteoCrete when used for these common indications.

More than 270,000 rotator cuff procedures have been reported to be performed in the US annually (average patient age: 61 years for women, 56 years for men).22 Rotator cuff repair procedures have been associated with a 20% failure rate, with one of the causes being related to an inability for the tendon to heal, even with strong initial fixation.23 Rotator cuff repair techniques are being continuously optimized with the goal of improving patient outcomes. This goal is being realized, primarily with respect to re-tear rates.24,25 However, even with advanced techniques, there are still relatively high rates of failure reported, with increasing patient age serving as one of the primary negative prognostic factors.26 An older patient population is associated with decreased bone mass and strength, and postmenopausal females have decreased bone quality; these factors are associated with higher rotator cuff failure rates due to poor tendon healing, with anchor fixation failure also playing a role.27-29 Therefore, it is critically important to develop methods for augmenting implant and tendon fixation to bone to achieve functional healing. The results of this study suggest that OsteoCrete provides a valid method for accomplishing this goal based on the observation that proximal humeral anchor fixation was improved by 50% in load-to-failure 15 minutes post-injection with an even more profound impact on the anchors placed in poor-quality bone (99% increased load-to-failure 15 minutes post-injection). It is probable that the degree of improvement in fixation strength would be even greater 1 day after fixation, since the strength of OsteoCrete continues to increase over the first 30 hours of curing.

Based on the preclinical animal model data of this study, OsteoCrete augmentation of rotator cuff anchor fixation had no untoward effects on tendon healing or function and can be considered as safe for use. Previously published data also suggest that OsteoCrete may improve osseous replacement of anchors as a result of magnesium ion release, which can drive adjacent attachment and growth of osteoblasts, leading to enhanced new bone formation.9-16,18 As such, surgeons may consider this means of anchor augmentation in situations of questionable or poor-quality bone and/or when accelerated postoperative rehabilitation protocols are desired.
A very low early incidence failure rate (1.2%) has been reported when a distal biceps tendon rupture is repaired using cortical suspensory fixation in conjunction with an interference screw.30 When an early re-rupture does occur, the most common explanation for failure tends to be a lack of patient compliance, with excessive force being placed on the repair.31 This study was not meant to investigate the methods to increase the strength of a biceps tendon repair using OsteoCrete but instead to replace the interference screw with OsteoCrete in a safe and noninferior manner. Primary fixation was still dependent on cortical suspensory fixation; however, OsteoCrete was used to help aid in stabilization of the tendon without the need for interference screw fixation. Although rare, osteolysis and perianchor cyst formation have been reported adjacent to nonbiodegradable anchors (PEEK), along with several types of biodegradable anchors (PLLA, hydroxyapatite plus PLLA, β-tricalcium phosphate plus PLLA, and polyglycolic acid; the latter of the 3 resulted in the lowest incidence of perianchor cyst formation) in the shoulder and elbow.32-34 Whenever osteolysis or cyst formation occurs around an anchor, it leads to decreased bone volume and potential adjacent bone weakness, which may act as a stress riser, thus increasing the risk for fracture. This potential is probably more of a concern within the proximal radius where there is a decreased amount of bone stock around the anchor.34
Continue to: In this study...
In this study, a short-term 12-week analysis revealed no significant differences in the nondestructive biomechanical testing and histologic analysis results between the use of OsteoCrete and the use of a tenodesis anchor. These results indicate the potential for using OsteoCrete as an anchor replacement. The biceps tendon did not react negatively to the OsteoCrete material, which indicated that OsteoCrete can be used adjacent to tendons without the concern of weakening the tendon due to an inflammatory reaction. This being said, tendon-to-bone integration was not evident at this early time point. It would be helpful to further explore the potential of this technique with a longer-term study investigating tendon-to-bone integration in more detail. Ideally, a long-term study would reveal an increased amount of new bone formation within the socket when compared to that with the anchor comparison, similar to the results reported by Gulotta and colleagues16 when using a tendon for ACL reconstruction with OsteoCrete.
We do note several limitations in this study. The dogs used in this study were healthy with normal bone and tendon morphology, the tendons were transected and repaired during the course of the same surgery, and only 1 early time point was evaluated. Additional investigations continuing the characterization of these clinical applications using an osteopenic or osteoporotic preclinical model with chronic tendon pathology and longer-term evaluation are now warranted based on the positive findings of this initial work.
CONCLUSION
OsteoCrete augmentation significantly improved initial rotator cuff anchor fixation (human in vitro) and was safe and effective for anchor and tendon fixation in rotator cuff tendon repair and biceps tenodesis procedures (canine in vivo), respectively, when compared with the current standard-of-care. Of note, the significant improvements associated with OsteoCrete were the greatest in poor-quality bone. Based on these results and considering the previously discussed limitations, it can be concluded that OsteoCrete has the potential for safely providing improved suture anchor and tissue fixation in patients with poor bone or tissue quality. Further in vivo study toward potential clinical applications is warranted.
ABSTRACT
The aim of this study was to assess the efficacy and safety of a novel magnesium-based resorbable bone cement (OsteoCrete, Bone Solutions Incorporated) for anchor and tendon fixation.
Cadaveric humeral testing involved straight pull-to-failure of rotator cuff suture anchors; OsteoCrete was injected through one anchor, and a second anchor served as the uninjected control. Testing was conducted 15 minutes post-injection. A canine preclinical model was used to evaluate the safety of the following parameters: Rotator cuff repair: A double-row technique was used to repair transected infraspinatus tendons; OsteoCrete was injected through both anchors in one limb, and the contralateral limb served as the uninjected control. Biceps tenodesis: The transected biceps tendon was implanted into a proximal humeral socket with a transcortical button; OsteoCrete was injected into the socket of one limb, and a screw was used for final fixation in the contralateral control limb. Nondestructive biomechanical testing and histologic assessment were performed after 12 weeks.
OsteoCrete-augmented anchors showed significantly higher load-to-failure compared to that with uninjected controls. In cadaveric humeri with reduced bone quality, OsteoCrete increased the mean load-to-failure by 99%. Within the preclinical model, there were no complications or statistically significant biomechanical/histologic differences between the techniques.
OsteoCrete has the potential for safely providing improved suture anchor and tissue fixation in patients with poor bone or tissue quality.
Continue to: Calcium phosphate bone void fillers...
Calcium phosphate bone void fillers have been commonly used in orthopedic surgery for several applications, including, but not limited to, a variety of fracture fixation or augmentation procedures.1-8 Continuing research on calcium phosphates has evidenced that the addition of magnesium phosphate to the formulation results in improved reactivity of the bone void filler. An in vitro study demonstrated enhanced attachment and proliferation of MG63 osteoblast-like cells on calcium magnesium phosphate cement (CMPC), in comparison with calcium phosphate cement (CPC), along with increased cellular alkaline phosphatase activity.9 The authors further explored the proliferation rates of MG63 cells by comparing CMPC with CPC and magnesium phosphate cement (MPC), and observed significantly increased proliferation of cells on CMPC. They also compared CMPC and CPC using a rabbit bone void model and observed substantial CMPC resorption with new bone formation at the 3-month time point and further reported that the majority of the defect had filled with new bone at 6 months, whereas CPC resulted in <10% new bone formation after 6 months.10 The authors continued to study the differences between CPC, MPC, and CMPC and identified increased proliferation of bone marrow stromal cells (bMSCs), when the cells were associated with CMPC and MPC, and when compared to that with CPC. The osteogenic differentiation of bMSCs was highest in the CMPC and CPC groups, when compared to that in the MPC group, with no significant difference between the CMPC and CPC groups. The authors also compared these 3 different formulations using a rabbit maxillary sinus floor elevation model, in which CMPC resulted in increased new bone formation and mineralization compared to that with CPC and MPC, which was further enhanced with the addition of bMSCs.11
These studies highlight the importance of having both a magnesium phosphate and a calcium phosphate component for a resorbable cement intended for use as a bone void filler. The rationale behind this strategy is related to the release of magnesium ions from the magnesium phosphate component. Magnesium has been shown to increase the proliferation of bMSCs, improve the attachment and growth of osteoblasts, stimulate the proteins involved in bone regeneration, enhance new bone formation, and boost bone mineralization.12,13
OsteoCrete (Bone Solutions Incorporation) is a novel CMPC composed of magnesium oxide, monopotassium phosphate, monosodium phosphate, hydroxyapatite, and sucrose. OsteoCrete has been demonstrated to significantly increase peak torque-to-failure of stainless-steel cortical bone screw fixation, when compared with screw fixation without augmentation and screw fixation with calcium phosphate augmentation using an in vivo equine model. In the same study, the authors showed that OsteoCrete resulted in an interface toughness that was significantly increased compared to that with no treatment, CPC augmentation, and polymethylmethacrylate (PMMA) augmentation. At 6 months after implantation, woven bone had replaced 69% of the OsteoCrete at the screw interface, compared to 44% of that with CPC.14 An equine study examined the effects of OsteoCrete on bone stability and healing using a metatarsal osteotomy model; the study reported significantly improved radiographic callus formation and a greater amount of new bone formation within the fracture gap when compared to that with CPC augmentation or no augmentation. OsteoCrete also secured the fragment significantly better than the CPC and control groups based on a decreased fracture gap over time.15 Another study using a preclinical anterior cruciate ligament (ACL) reconstruction model reported that OsteoCrete resulted in significantly better new bone formation in the tibial tunnel, a smaller amount of fibrous tissue, more cartilage formation at the tendon-bone interface, and a higher ultimate load-to-failure compared to that with standard ACL reconstruction in the contralateral limb after 6 weeks.16 OsteoCrete and PMMA were evaluated in terms of biomechanical fixation of a stemless humeral prosthesis, with data showing that both groups have higher failure loads, failure displacements, and failure cycles when compared to those with the control, nonaugmented group.17 Another preclinical model evaluated cranial bone flap augmentation with 2 resorbable cements and highlighted faster cement resorption and replacement with bone, along with superior stability within the OsteoCrete group compared to that with CPC.18 In a preclinical bone void study conducted for obtaining US Food and Drug Administration 510(k) clearance, OsteoCrete resulted in 83% greater resorption than that with CPC after 12 weeks and 35% greater resorption at 26 weeks, with 84% of OsteoCrete being resorbed and replaced with woven or lamellar mineralized bone of normal morphology at the 26-week time point (unpublished data provided by Bone Solutions Incorporated [BSI]).
These data indicate that CMPCs such as OsteoCrete appear to have potential benefits for augmenting the healing of bone implants and bone soft tissue. Therefore, the objective of this study was to assess the safety and efficacy of OsteoCrete in applications for the augmentation of anchor and tendon fixation in rotator cuff repair and biceps tenodesis procedures, respectively. Improving healing for these 2 commonly performed procedures would be of great benefit in improving the functional outcomes and mitigating the complications and morbidity.
MATERIALS AND METHODS
IN VITRO STUDY METHODS
Cadaveric humeri (N = 12, six matched pairs) of females (age, 70-75 years) were warmed to 37°C prior to testing. Two 4.75-mm vented anchors (SwiveLock, Arthrex) with FiberTape were implanted into a lateral row position (anterior and posterior anchor positioning) of a double-row rotator cuff repair within the greater tuberosity. One anchor was injected with 1 ml of OsteoCrete–after preparation according to the manufacturer’s instructions–through the cannulation channel after placement, and the other anchor served as the uninjected control for each humerus. For the six matched pairs, the OsteoCrete group and the control group were rotated with respect to anterior vs posterior location within the lateral row position. After 15 minutes of the injection, straight pull-to-failure (12 in/min) was performed. Data were compared between the groups for significant (P < .05) differences using t-tests and Pearson correlation.
Continue to: IN VIVO STUDY METHODS
IN VIVO STUDY METHODS
With Institutional Animal Care and Use Committee approval, adult (age, 2-4 years) purpose-bred dogs (N = 8) underwent aseptic surgery of both forelimbs for rotator cuff (infraspinatus) tendon repair (Figure 1) and biceps tenodesis (Figure 2). For the rotator cuff repair, two 4.75-mm vented anchors (1 medial and 1 lateral) with FiberTape were used in a modified double-row technique to repair the acutely transected infraspinatus tendon. In one limb, 1 ml of OsteoCrete was injected through both anchors; the other limb served as the uninjected control. For the biceps tenodesis procedure, the long head of the biceps tendon was transected at its origin and whip-stitched. The tendon was transposed and inserted into a 7-mm diameter socket drilled into the proximal humerus using a tension-slide technique with a transcortical button for fixation. In one limb, 1 ml of OsteoCrete was injected into the socket prior to final tensioning and tying. In the contralateral limb, a 7-mm interference screw (Bio-Tenodesis™ Screw, Arthrex) was inserted into the socket after tensioning and tying. The dogs were allowed to perform out-of-kennel monitored exercise daily for a period of 12 weeks after surgery and were then sacrificed.

The infraspinatus and biceps bone-tendon-muscle units were excised en bloc. Custom-designed jigs were used for biomechanical testing of the bone-tendon-muscle units along the anatomical vector of muscle contraction. Optical markers were mounted at standardized anatomical locations. Elongation of the repair site was defined as the change in distance between markers and was measured to 0.01-mm resolution using an optical tracking system (Optotrak Certus, NDI), synchronized with measurement of the applied tension load. The bone-tendon-muscle units were loaded in tension to 3-mm elongation at a displacement controlled rate of 0.01 mm/s. Load at 1-mm, 2-mm, and 3-mm displacement of the tendon-bone junction was extracted from the load vs the displacement curve of each sample. Stiffness was calculated as the slope of the linear portion of the load vs the displacement curve.19,20

For histologic assessments, sections of each treatment site were obtained using a microsaw and alternated between decalcified and non-decalcified processing. For decalcified bone processing, formalin-fixed tissues were placed in 10% ethylenediaminetetraacetic acid with phosphate-buffered saline for 39 days and then processed routinely for the assessment of sections stained with hematoxylin and eosin (H&E), toluidine blue, and picrosirius red. For non-decalcified bone processing, the tissues were dehydrated through a series of graded ethyl alcohol solutions, embedded in PMMA, sectioned, and stained with toluidine blue and Goldner’s trichrome. Two pathologists who were blinded to the clinical application and the differences between techniques assessed the histologic sections and scored each section using the modified Bonar score that assesses cell morphology, collagen arrangement, cellularity, vascularity, and extracellular matrix using a 15-point scale, where a higher score indicates more pathology.21
Categorical data were compared for detecting statistically significant differences using the rank sum test. Continuous data were compared for identifying statistically significant differences using the t-test or one-way ANOVA. Significance was set at P < .05.
RESULTS
IN VITRO RESULTS
OsteoCrete-augmented anchors (mean = 225 N; range, 158-287 N) had significantly (P < .001) higher pull-out load-to-failure compared to that in the uninjected controls (mean = 161 N; range, 68-202 N), which translated to a 50% mean increase (range, 3%-134%) in load-to-failure (Table 1). For humeri with reduced bone quality (control anchors that failed at <160 N, 4 humeri), the mean increase in load-to-failure for OsteoCrete-augmented anchors was 99% (range, 58%-135%), with the difference between mean values being again significantly different (OsteoCrete mean = 205 N; control mean = 110 N, P < .001). When the control and OsteoCrete load-to-failure values were compared using Pearson correlation, a significantly strong positive correlation (r = 0.66, P = 0.02) was detected. When the control load-to-failure values were compared with its percent increase value when OsteoCrete was used, there was a significantly very strong negative correlation (r = −0.90, P < .001).
Table 1. Cadaveric Lateral Row Rotator Cuff Anchor Pull-To-Failure; Testing Occurred 15 Minutes Post-Injection
| Humerus No. | Control (N) | OsteoCrete (N)a | Percent Increase |
| 1-Right (PA) | 197.28 | 278.73 | 41% |
| 1-Left (AP) | 152.62 | 241.72 | 58% |
| 2-Right (PA) | 178.60 | 196.03 | 10% |
| 2-Left (AP) | 170.10 | 175.57 | 3% |
| 3-Right (PA) | 67.70 | 158.31 | 134% |
| 3-Left (AP) | 74.24 | 173.08 | 133% |
| 4-Right (PA) | 195.81 | 248.12 | 27% |
| 4-Left (AP) | 201.95 | 209.42 | 4% |
| 5-Right (PA) | 173.30 | 220.59 | 27% |
| 5-Left (AP) | 146.61 | 247.37 | 69% |
| 6-Right (PA) | 171.03 | 266.14 | 56% |
| 6-Left (AP) | 199.99 | 286.91 | 43% |
| Average | 160.77 + 45.60 | 225.17 + 43.08 | 50% + 44 |
aOsteoCrete (Bone Solutions Incorporated) resulted in significantly increased (P < 0.001) pull-to-failure. Abbreviations: AP, control anchor located in anterior position, OsteoCrete anchor located in posterior position; PA, control anchor located in posterior position, OsteoCrete anchor located in anterior position.
Continue to: IN VIVO RESULTS
IN VIVO RESULT
No intraoperative or postoperative complications were noted. All repairs were found to be intact based on the gross assessment and the completed biomechanical testing without failure. No statistically significant (P > 0.3) biomechanical differences were found between the techniques (Table 2). Histologic assessments showed low-to-mild pathology scores for all sites with no statistically significant (P > 0.3) differences between the techniques (Table 2). Both control and OsteoCrete rotator cuff repairs demonstrated tendon-to-bone integration via fibrous connective tissue attachment to bone. All anchors were in place with no evidence for loosening, tunnel expansion, or cyst formation. OsteoCrete-augmented anchor repairs were associated with cement remaining within their lumens along with a thin layer of cement interposed between the anchor and the bone interface around their entire periphery. The cement-bone interface was discrete with typical inflammatory cell infiltrate without evidence for infection, membrane or cyst formation, or other untoward pathologic responses. In the OsteoCrete biceps tenodesis group, the tendons filled the tunnels with a thin layer of cement remaining interposed between the tendon and the bone interface around the entire periphery. The tendon-cement-bone interface was discrete with typical inflammatory cell infiltrates and without evidence for infection, membrane or cyst formation, or other untoward pathologic responses. Tendon-to-bone integration was not observed in the control or OsteoCrete biceps tenodesis groups at the 12-week study endpoint. Representative histologic images of the rotator cuff tendon repairs and biceps tenodesis procedures are shown in Figures 3A, 3B and Figures 4A, 4B, respectively.
Table 2. Biomechanical Testing And Histologic Scoring Of Rotator Cuff And Biceps Tendon Repairs In A Preclinical Model
| Procedure | Force (N) at 1 mm | Force (N) at 2 mm | Force (N) at 3 mm | Stiffness (N/mm) | Histologic Score |
| Rotator Cuff - Control | 14.0 + 3.3 | 19.3 + 5.5 | 25.0 + 7.0 | 5.4 + 2.0 | 4.6 + 1.1 |
| Rotator Cuff - OsteoCrete (Bone Solutions Incorporated) | 14.8 + 3.7 | 20.4 + 6.0 | 26.4 + 8.5 | 6.3 + 2.5 | 3.9 + 1.7 |
| Biceps - Control | 23.1 + 6.2 | 35.5 + 8.5 | 52.6 + 15.0 | 17.8 + 6.4 | 3.4 + 1.2 |
| Biceps - OsteoCrete | 22.4 + 7.3 | 36.8 + 10.1 | 57.8 + 16.0 | 21.1 + 8.5 | 3.4 + 0.7 |
There were no significant differences (P < 0.05) between groups. Histologic scoring based on a 15-point scale with higher scores indicating more pathology.
DISCUSSION
The results of this study highlight the safety and efficacy of OsteoCrete in applications for the augmentation of anchor and tendon fixation in rotator cuff repair and biceps tenodesis procedures, respectively. Anchors augmented with OsteoCrete resulted in significantly increased load-to-failure pull-out strength 15 minutes after insertion. In addition, a very strong negative correlation was found between the percentage of improved load-to-failure after OsteoCrete injection and the bone quality of the humerus, which was based on the control load-to-failure values. In the validated preclinical model used in this study, OsteoCrete-based fixation was found to be noninferior to current standard-of-care techniques and was not associated with any untoward pathologic responses of humeral bone, rotator cuff tendon, or biceps tendon based on the biomechanical and histologic analyses. These data highlight the functional efficacy and biocompatibility of OsteoCrete when used for these common indications.

More than 270,000 rotator cuff procedures have been reported to be performed in the US annually (average patient age: 61 years for women, 56 years for men).22 Rotator cuff repair procedures have been associated with a 20% failure rate, with one of the causes being related to an inability for the tendon to heal, even with strong initial fixation.23 Rotator cuff repair techniques are being continuously optimized with the goal of improving patient outcomes. This goal is being realized, primarily with respect to re-tear rates.24,25 However, even with advanced techniques, there are still relatively high rates of failure reported, with increasing patient age serving as one of the primary negative prognostic factors.26 An older patient population is associated with decreased bone mass and strength, and postmenopausal females have decreased bone quality; these factors are associated with higher rotator cuff failure rates due to poor tendon healing, with anchor fixation failure also playing a role.27-29 Therefore, it is critically important to develop methods for augmenting implant and tendon fixation to bone to achieve functional healing. The results of this study suggest that OsteoCrete provides a valid method for accomplishing this goal based on the observation that proximal humeral anchor fixation was improved by 50% in load-to-failure 15 minutes post-injection with an even more profound impact on the anchors placed in poor-quality bone (99% increased load-to-failure 15 minutes post-injection). It is probable that the degree of improvement in fixation strength would be even greater 1 day after fixation, since the strength of OsteoCrete continues to increase over the first 30 hours of curing.

Based on the preclinical animal model data of this study, OsteoCrete augmentation of rotator cuff anchor fixation had no untoward effects on tendon healing or function and can be considered as safe for use. Previously published data also suggest that OsteoCrete may improve osseous replacement of anchors as a result of magnesium ion release, which can drive adjacent attachment and growth of osteoblasts, leading to enhanced new bone formation.9-16,18 As such, surgeons may consider this means of anchor augmentation in situations of questionable or poor-quality bone and/or when accelerated postoperative rehabilitation protocols are desired.
A very low early incidence failure rate (1.2%) has been reported when a distal biceps tendon rupture is repaired using cortical suspensory fixation in conjunction with an interference screw.30 When an early re-rupture does occur, the most common explanation for failure tends to be a lack of patient compliance, with excessive force being placed on the repair.31 This study was not meant to investigate the methods to increase the strength of a biceps tendon repair using OsteoCrete but instead to replace the interference screw with OsteoCrete in a safe and noninferior manner. Primary fixation was still dependent on cortical suspensory fixation; however, OsteoCrete was used to help aid in stabilization of the tendon without the need for interference screw fixation. Although rare, osteolysis and perianchor cyst formation have been reported adjacent to nonbiodegradable anchors (PEEK), along with several types of biodegradable anchors (PLLA, hydroxyapatite plus PLLA, β-tricalcium phosphate plus PLLA, and polyglycolic acid; the latter of the 3 resulted in the lowest incidence of perianchor cyst formation) in the shoulder and elbow.32-34 Whenever osteolysis or cyst formation occurs around an anchor, it leads to decreased bone volume and potential adjacent bone weakness, which may act as a stress riser, thus increasing the risk for fracture. This potential is probably more of a concern within the proximal radius where there is a decreased amount of bone stock around the anchor.34
Continue to: In this study...
In this study, a short-term 12-week analysis revealed no significant differences in the nondestructive biomechanical testing and histologic analysis results between the use of OsteoCrete and the use of a tenodesis anchor. These results indicate the potential for using OsteoCrete as an anchor replacement. The biceps tendon did not react negatively to the OsteoCrete material, which indicated that OsteoCrete can be used adjacent to tendons without the concern of weakening the tendon due to an inflammatory reaction. This being said, tendon-to-bone integration was not evident at this early time point. It would be helpful to further explore the potential of this technique with a longer-term study investigating tendon-to-bone integration in more detail. Ideally, a long-term study would reveal an increased amount of new bone formation within the socket when compared to that with the anchor comparison, similar to the results reported by Gulotta and colleagues16 when using a tendon for ACL reconstruction with OsteoCrete.
We do note several limitations in this study. The dogs used in this study were healthy with normal bone and tendon morphology, the tendons were transected and repaired during the course of the same surgery, and only 1 early time point was evaluated. Additional investigations continuing the characterization of these clinical applications using an osteopenic or osteoporotic preclinical model with chronic tendon pathology and longer-term evaluation are now warranted based on the positive findings of this initial work.
CONCLUSION
OsteoCrete augmentation significantly improved initial rotator cuff anchor fixation (human in vitro) and was safe and effective for anchor and tendon fixation in rotator cuff tendon repair and biceps tenodesis procedures (canine in vivo), respectively, when compared with the current standard-of-care. Of note, the significant improvements associated with OsteoCrete were the greatest in poor-quality bone. Based on these results and considering the previously discussed limitations, it can be concluded that OsteoCrete has the potential for safely providing improved suture anchor and tissue fixation in patients with poor bone or tissue quality. Further in vivo study toward potential clinical applications is warranted.
1. Russell TA, Leighton RK, Group A-BTPFS. Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J Bone Joint Surg Am. 2008; 90(10):2057-2061. doi:10.2106/JBJS.G.01191.
2. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction-internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012; 21(6):741-748. doi:10.1016/j.jse.2011.09.017.
3. Cassidy C, Jupiter JB, Cohen M, et al. Norian SRS cement compared with conventional fixation in distal radial fractures. A randomized study. J Bone Joint Surg Am. 2003;85-A(11):2127-2137.
4. Mattsson P, Alberts A, Dahlberg G, Sohlman M, Hyldahl HC, Larsson S. Resorbable cement for the augmentation of internally-fixed unstable trochanteric fractures. A prospective, randomised multicentre study. J Bone Joint Surg Br. 2005;87(9):1203-1209.
5. Cohen SB, Sharkey PF. Subchondroplasty for treating bone marrow lesions. J Knee Surg. 2016;29(07):555-563. doi:10.1302/0301-620X.87B9.15792.
6. Guida P, Ragozzino R, Sorrentino B, et al. Three-in-One minimally invasive approach to surgical treatment of pediatric pathological fractures with wide bone loss through bone cysts: ESIN, curettage and packing with injectable HA bone substitute. A retrospective series of 116 cases. Injury. 2016;47(6):1222-1228. doi:10.1016/j.injury.2016.01.006.
7. Maestretti G, Sutter P, Monnard E, et al. A prospective study of percutaneous balloon kyphoplasty with calcium phosphate cement in traumatic vertebral fractures: 10-year results. Eur Spine J. 2014;23(6):1354-1360. doi:10.1007/s00586-014-3206-1.
8. Nakano M, Hirano N, Zukawa M, et al. Vertebroplasty using calcium phosphate cement for osteoporotic vertebral fractures: study of outcomes at a minimum follow-up of two years. Asian Spine J. 2012;6(1):34-42. doi:10.4184/asj.2012.6.1.34.
9. Jia J, Zhou H, Wei J, et al. Development of magnesium calcium phosphate biocement for bone regeneration. J R Soc Interface. 2010;7(49):1171-1180. doi:10.1098/rsif.2009.0559.
10. Wu F, Wei J, Guo H, Chen F, Hong H, Liu C. Self-setting bioactive calcium-magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008;4(6):1873-1884. doi:10.1016/j.actbio.2008.06.020.
11. Zeng D, Xia L, Zhang W, et al. Maxillary sinus floor elevation using a tissue-engineered bone with calcium-magnesium phosphate cement and bone marrow stromal cells in rabbits. Tissue Eng Part A. 2012;18(7-8):870-881. doi:10.1089/ten.TEA.2011.0379.
12. Yoshizawa S, Brown A, Barchowsky A, Sfeir C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014;10(6):2834-2842. doi:10.1016/j.actbio.2014.02.002.
13. Liao J, Qu Y, Chu B, Zhang X, Qian Z. Biodegradable CSMA/PECA/Graphene porous hybrid scaffold for cartilage tissue engineering. Sci Rep. 2015;5:9879. doi:10.1038/srep09879.
14. Hirvinen LJ, Litsky AS, Samii VF, Weisbrode SE, Bertone AL. Influence of bone cements on bone-screw interfaces in the third metacarpal and third metatarsal bones of horses. Am J Vet Res. 2009;70(8):964-972. doi:10.2460/ajvr.70.8.964.
15. Waselau M, Samii VF, Weisbrode SE, Litsky AS, Bertone AL. Effects of a magnesium adhesive cement on bone stability and healing following a metatarsal osteotomy in horses. Am J Vet Res. 2007;68(4):370-378. doi:10.2460/ajvr.68.4.370.
16. Gulotta LV, Kovacevic D, Ying L, Ehteshami JR, Montgomery S, Rodeo SA. Augmentation of tendon-to-bone healing with a magnesium-based bone adhesive. Am J Sports Med. 2008;36(7):1290-1297. doi:10.1177/0363546508314396.
17. Kim MS, Kovacevic D, Milks RA, et al. Bone graft substitute provides metaphyseal fixation for a stemless humeral implant. Orthopedics. 2015;38(7):e597-e603. doi:10.3928/01477447-20150701-58.
18. Schendel SA, Peauroi J. Magnesium-based bone cement and bone void filler: preliminary experimental studies. J Craniofac Surg. 2009;20(2):461-464. doi:10.1097/SCS.0b013e31819b9819.
19. Pfeiffer FM, Smith MJ, Cook JL, Kuroki K. The histologic and biomechanical response of two commercially available small glenoid anchors for use in labral repairs. J Shoulder Elbow Surg. 2014;23(8):1156-1161. doi:10.1016/j.jse.2013.12.036.
20. Smith MJ, Cook JL, Kuroki K, et al. Comparison of a novel bone-tendon allograft with a human dermis-derived patch for repair of chronic large rotator cuff tears using a canine model. Arthroscopy. 2012;28(2):169-177. doi:10.1016/j.arthro.2011.08.296.
21. Fearon A, Dahlstrom JE, Twin J, Cook J, Scott A. The Bonar score revisited: region of evaluation significantly influences the standardized assessment of tendon degeneration. J Sci Med Sport. 2014;17(4):346-350. doi:10.1016/j.jsams.2013.07.008.
22. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233. doi:10.2106/JBJS.J.00739.
23. Lädermann A, Denard PJ, Burkhart SS. Management of failed rotator cuff repair: a systematic review. J ISAKOS. 2016;1(1):32-37. doi:10.1136/jisakos-2015-000027.
24. Franceschi F, Papalia R, Franceschetti E, et al. Double-Row repair lowers the retear risk after accelerated rehabilitation. Am J Sports Med. 2016;44(4):948-956. doi:10.1177/0363546515623031.
25. Wang E, Wang L, Gao P, Li Z, Zhou X, Wang S. Single-versus double-row arthroscopic rotator cuff repair in massive tears. Med Sci Monit. 2015;21:1556-1561. doi:10.12659/MSM.893058.
26. Abtahi AM, Granger EK, Tashjian RZ. Factors affecting healing after arthroscopic rotator cuff repair. World J Orthop. 2015;6(2):211-220. doi:10.5312/wjo.v6.i2.211.
27. Chung SW, Oh JH, Gong HS, Kim JY, Kim SH. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med. 2011;39(10):2099-2107. doi:10.1177/0363546511415659.
28. Tsiouri C, Mok DH. Early pullout of lateral row knotless anchor in rotator cuff repair. Int J Shoulder Surg. 2009;3(3):63-65. doi:10.4103/0973-6042.59972.
29. Boskey AL, Coleman R. Aging and bone. J Dent Res. 2010;89(12):1333-1348. doi:10.1177/0022034510377791.
30. Cusick MC, Cottrell BJ, Cain RA, Mighell MA. Low incidence of tendon rerupture after distal biceps repair by cortical button and interference screw. J Shoulder Elbow Surg. 2014;23(10):1532-1536. doi:10.1016/j.jse.2014.04.013.
31. Hinchey JW, Aronowitz JG, Sanchez-Sotelo J, Morrey BF. Re-rupture rate of primarily repaired distal biceps tendon injuries. J Shoulder Elbow Surg. 2014;23(6):850-854. doi:10.1016/j.jse.2014.02.006.
32. Shahrulazua A, Duckworth D, Bokor DJ. Perianchor radiolucency following PEEK suture anchor application associated with recurrent shoulder dislocation: a case report. Clin Ter. 2014;165(1):31-34. doi:10.7471/CT.2014.1658.
33. Kim SH, Kim dY, Kwon JE, Park JS, Oh JH. Perianchor cyst formation around biocomposite biodegradable suture anchors after rotator cuff repair. Am J Sports Med. 2015;43(12):2907-2912. doi:10.1177/0363546515608484
34. Potapov A, Laflamme YG, Gagnon S, Canet F, Rouleau DM. Progressive osteolysis of the radius after distal biceps tendon repair with the bioabsorbable screw. J Shoulder Elbow Surg. 2011;20(5):819-826. doi:10.1016/j.jse.2011.02.021.
1. Russell TA, Leighton RK, Group A-BTPFS. Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J Bone Joint Surg Am. 2008; 90(10):2057-2061. doi:10.2106/JBJS.G.01191.
2. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction-internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012; 21(6):741-748. doi:10.1016/j.jse.2011.09.017.
3. Cassidy C, Jupiter JB, Cohen M, et al. Norian SRS cement compared with conventional fixation in distal radial fractures. A randomized study. J Bone Joint Surg Am. 2003;85-A(11):2127-2137.
4. Mattsson P, Alberts A, Dahlberg G, Sohlman M, Hyldahl HC, Larsson S. Resorbable cement for the augmentation of internally-fixed unstable trochanteric fractures. A prospective, randomised multicentre study. J Bone Joint Surg Br. 2005;87(9):1203-1209.
5. Cohen SB, Sharkey PF. Subchondroplasty for treating bone marrow lesions. J Knee Surg. 2016;29(07):555-563. doi:10.1302/0301-620X.87B9.15792.
6. Guida P, Ragozzino R, Sorrentino B, et al. Three-in-One minimally invasive approach to surgical treatment of pediatric pathological fractures with wide bone loss through bone cysts: ESIN, curettage and packing with injectable HA bone substitute. A retrospective series of 116 cases. Injury. 2016;47(6):1222-1228. doi:10.1016/j.injury.2016.01.006.
7. Maestretti G, Sutter P, Monnard E, et al. A prospective study of percutaneous balloon kyphoplasty with calcium phosphate cement in traumatic vertebral fractures: 10-year results. Eur Spine J. 2014;23(6):1354-1360. doi:10.1007/s00586-014-3206-1.
8. Nakano M, Hirano N, Zukawa M, et al. Vertebroplasty using calcium phosphate cement for osteoporotic vertebral fractures: study of outcomes at a minimum follow-up of two years. Asian Spine J. 2012;6(1):34-42. doi:10.4184/asj.2012.6.1.34.
9. Jia J, Zhou H, Wei J, et al. Development of magnesium calcium phosphate biocement for bone regeneration. J R Soc Interface. 2010;7(49):1171-1180. doi:10.1098/rsif.2009.0559.
10. Wu F, Wei J, Guo H, Chen F, Hong H, Liu C. Self-setting bioactive calcium-magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008;4(6):1873-1884. doi:10.1016/j.actbio.2008.06.020.
11. Zeng D, Xia L, Zhang W, et al. Maxillary sinus floor elevation using a tissue-engineered bone with calcium-magnesium phosphate cement and bone marrow stromal cells in rabbits. Tissue Eng Part A. 2012;18(7-8):870-881. doi:10.1089/ten.TEA.2011.0379.
12. Yoshizawa S, Brown A, Barchowsky A, Sfeir C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014;10(6):2834-2842. doi:10.1016/j.actbio.2014.02.002.
13. Liao J, Qu Y, Chu B, Zhang X, Qian Z. Biodegradable CSMA/PECA/Graphene porous hybrid scaffold for cartilage tissue engineering. Sci Rep. 2015;5:9879. doi:10.1038/srep09879.
14. Hirvinen LJ, Litsky AS, Samii VF, Weisbrode SE, Bertone AL. Influence of bone cements on bone-screw interfaces in the third metacarpal and third metatarsal bones of horses. Am J Vet Res. 2009;70(8):964-972. doi:10.2460/ajvr.70.8.964.
15. Waselau M, Samii VF, Weisbrode SE, Litsky AS, Bertone AL. Effects of a magnesium adhesive cement on bone stability and healing following a metatarsal osteotomy in horses. Am J Vet Res. 2007;68(4):370-378. doi:10.2460/ajvr.68.4.370.
16. Gulotta LV, Kovacevic D, Ying L, Ehteshami JR, Montgomery S, Rodeo SA. Augmentation of tendon-to-bone healing with a magnesium-based bone adhesive. Am J Sports Med. 2008;36(7):1290-1297. doi:10.1177/0363546508314396.
17. Kim MS, Kovacevic D, Milks RA, et al. Bone graft substitute provides metaphyseal fixation for a stemless humeral implant. Orthopedics. 2015;38(7):e597-e603. doi:10.3928/01477447-20150701-58.
18. Schendel SA, Peauroi J. Magnesium-based bone cement and bone void filler: preliminary experimental studies. J Craniofac Surg. 2009;20(2):461-464. doi:10.1097/SCS.0b013e31819b9819.
19. Pfeiffer FM, Smith MJ, Cook JL, Kuroki K. The histologic and biomechanical response of two commercially available small glenoid anchors for use in labral repairs. J Shoulder Elbow Surg. 2014;23(8):1156-1161. doi:10.1016/j.jse.2013.12.036.
20. Smith MJ, Cook JL, Kuroki K, et al. Comparison of a novel bone-tendon allograft with a human dermis-derived patch for repair of chronic large rotator cuff tears using a canine model. Arthroscopy. 2012;28(2):169-177. doi:10.1016/j.arthro.2011.08.296.
21. Fearon A, Dahlstrom JE, Twin J, Cook J, Scott A. The Bonar score revisited: region of evaluation significantly influences the standardized assessment of tendon degeneration. J Sci Med Sport. 2014;17(4):346-350. doi:10.1016/j.jsams.2013.07.008.
22. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233. doi:10.2106/JBJS.J.00739.
23. Lädermann A, Denard PJ, Burkhart SS. Management of failed rotator cuff repair: a systematic review. J ISAKOS. 2016;1(1):32-37. doi:10.1136/jisakos-2015-000027.
24. Franceschi F, Papalia R, Franceschetti E, et al. Double-Row repair lowers the retear risk after accelerated rehabilitation. Am J Sports Med. 2016;44(4):948-956. doi:10.1177/0363546515623031.
25. Wang E, Wang L, Gao P, Li Z, Zhou X, Wang S. Single-versus double-row arthroscopic rotator cuff repair in massive tears. Med Sci Monit. 2015;21:1556-1561. doi:10.12659/MSM.893058.
26. Abtahi AM, Granger EK, Tashjian RZ. Factors affecting healing after arthroscopic rotator cuff repair. World J Orthop. 2015;6(2):211-220. doi:10.5312/wjo.v6.i2.211.
27. Chung SW, Oh JH, Gong HS, Kim JY, Kim SH. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med. 2011;39(10):2099-2107. doi:10.1177/0363546511415659.
28. Tsiouri C, Mok DH. Early pullout of lateral row knotless anchor in rotator cuff repair. Int J Shoulder Surg. 2009;3(3):63-65. doi:10.4103/0973-6042.59972.
29. Boskey AL, Coleman R. Aging and bone. J Dent Res. 2010;89(12):1333-1348. doi:10.1177/0022034510377791.
30. Cusick MC, Cottrell BJ, Cain RA, Mighell MA. Low incidence of tendon rerupture after distal biceps repair by cortical button and interference screw. J Shoulder Elbow Surg. 2014;23(10):1532-1536. doi:10.1016/j.jse.2014.04.013.
31. Hinchey JW, Aronowitz JG, Sanchez-Sotelo J, Morrey BF. Re-rupture rate of primarily repaired distal biceps tendon injuries. J Shoulder Elbow Surg. 2014;23(6):850-854. doi:10.1016/j.jse.2014.02.006.
32. Shahrulazua A, Duckworth D, Bokor DJ. Perianchor radiolucency following PEEK suture anchor application associated with recurrent shoulder dislocation: a case report. Clin Ter. 2014;165(1):31-34. doi:10.7471/CT.2014.1658.
33. Kim SH, Kim dY, Kwon JE, Park JS, Oh JH. Perianchor cyst formation around biocomposite biodegradable suture anchors after rotator cuff repair. Am J Sports Med. 2015;43(12):2907-2912. doi:10.1177/0363546515608484
34. Potapov A, Laflamme YG, Gagnon S, Canet F, Rouleau DM. Progressive osteolysis of the radius after distal biceps tendon repair with the bioabsorbable screw. J Shoulder Elbow Surg. 2011;20(5):819-826. doi:10.1016/j.jse.2011.02.021.
TAKE-HOME POINTS
- OsteoCrete, a magnesium-based resorbable bone cement, has potential to safely and effectively augment suture anchor fixation.
- OsteoCrete increases anchor pull-out strength within 15 minutes of injection.
- OsteoCrete has a more profound impact on anchors when used within bone of decreased density and quality.
- OsteoCrete does not result in any untoward effect when placed near, or in contact with, rotator cuff or biceps tendons during fixation procedures.
- OsteoCrete can potentially be used to replace the anchor within tenodesis procedures that utilize transcortical button fixation in addition to anchor fixation.
The Deer Stand Strikes Back
ANSWER
The radiograph demonstrates a compression fracture of T12 with moderate loss of height approaching 50% anteriorly. In addition, there is a vertically oriented fracture within the middle of the vertebral body, causing the back portion to be posteriorly displaced.
This type of burst fracture is potentially unstable and should be treated as such. The patient was placed on strict spine precautions and transferred to a facility where trauma and neurosurgical services were available.
ANSWER
The radiograph demonstrates a compression fracture of T12 with moderate loss of height approaching 50% anteriorly. In addition, there is a vertically oriented fracture within the middle of the vertebral body, causing the back portion to be posteriorly displaced.
This type of burst fracture is potentially unstable and should be treated as such. The patient was placed on strict spine precautions and transferred to a facility where trauma and neurosurgical services were available.
ANSWER
The radiograph demonstrates a compression fracture of T12 with moderate loss of height approaching 50% anteriorly. In addition, there is a vertically oriented fracture within the middle of the vertebral body, causing the back portion to be posteriorly displaced.
This type of burst fracture is potentially unstable and should be treated as such. The patient was placed on strict spine precautions and transferred to a facility where trauma and neurosurgical services were available.
A 45-year-old man presents to your facility for evaluation of ongoing back pain. He reports that he fell out of a deer stand from an approximate height of 15 to 20 ft. He landed on his back but was able to get up, walk a short distance to his car, and drive home. Persistent pain is what brings him to the emergency department to seek treatment.
He denies any weakness or numbness in his lower extremities. There are no bowel or bladder issues. His medical history is unremarkable.
On physical examination, you note a moderately uncomfortable male whose vital signs are normal. He is able to move all four extremities well, and his strength is intact throughout. He does have moderate tenderness within the thoracolumbar region, with no step-off appreciated. The paraspinous muscles are tender as well.
You order lumbosacral radiographs (lateral view shown). What is your impression?
Patient-Specific Implants in Severe Glenoid Bone Loss
ABSTRACT
Complex glenoid bone deformities present the treating surgeon with a complex reconstructive challenge. Although glenoid bone loss can be encountered in the primary setting (degenerative, congenital, post-traumatic), severe glenoid bone loss is encountered in most revision total shoulder arthroplasties. Severe glenoid bone loss is treated with various techniques including hemiarthroplasty, eccentric reaming, and glenoid reconstruction with bone autografts and allografts. Despite encouraging short- to mid-term results reported with these reconstruction techniques, the clinical and radiographic outcomes remain inconsistent and the high number of complications is a concern. To overcome this problem, more recently augmented components and patient specific implants were introduced. Using the computer-aided design and computer-aided manufacturing technology patient-specific implants have been created to reconstruct the glenoid vault in cases of severe glenoid bone loss.
In this article we describe a patient specific glenoid implant, its indication, technical aspects and surgical technique, based on the author's experience as well as a review of the current literature on custom glenoid implants.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) is an effective operation for providing pain relief and improving function in patients with end-stage degenerative shoulder disease that is nonresponsive to nonoperative treatments.1-4 With the increasing number of arthroplasties performed, and the expanding indication for shoulder arthroplasty, the number of revision shoulder arthroplasties is also increasing.5-14 Complex glenoid bone deformities present the treating surgeon with a complex reconstructive challenge. Although glenoid bone loss can be seen in the primary setting (degenerative, congenital, and post-traumatic), severe glenoid bone loss is encountered mostly in revision TSAs.
Historically, patients with severe glenoid bone loss were treated with a hemiarthroplasty.15-17 However, due to inferior outcomes associated with the use of shoulder hemiarthroplasties compared with TSA in these cases,18-20 various techniques were developed with the aim of realigning the glenoid axis and securing the implants into the deficient glenoid vault.21-25 Options have included eccentric reaming, glenoid reconstruction with bone autografts and allografts, and more recently augmented components and patient-specific implants. Studies with eccentric reaming and reconstruction with bone graft during complex shoulder arthroplasty have reported encouraging short- to mid-term results, but the clinical and radiographic outcomes remain inconsistent, and the high number of complications is a concern.25-28
Complications with these techniques include component loosening, graft resorption, nonunion, failure of graft incorporation, infection, and instability.25-28
Computer-aided design and computer-aided manufacturing (CAD/CAM) of patient-specific implants have been used successfully by hip arthroplasty surgeons to deal with complex acetabular reconstructions in the setting of severe bone loss. More recently, the same technology has been used to reconstruct the glenoid vault in cases of severe glenoid bone loss.
In this article, we describe a patient-specific glenoid implant, its indication, and both technical aspects and the surgical technique, based on the authors’ experience as well as a review of the current literature on custom glenoid implants.
Continue to: PATIENT-SPECIFIC GLENOID COMPONENT
PATIENT-SPECIFIC GLENOID COMPONENT
The Vault Reconstruction System ([VRS], Zimmer Biomet) is a patient-specific glenoid vault reconstruction system developed with the use of CAD/CAM to address severe glenoid bone loss encountered during shoulder arthroplasty. For several years, the VRS was available only as a custom implant according to the US Food and Drug Administration rules, and therefore its use was limited to a few cases per year. Recently, a 510(k) envelope clearance was granted to use the VRS in reverse TSA to address significant glenoid bone defects.
The VRS is made of porous plasma spray titanium to provide high strength and flexibility, and allows for biologic fixation. This system can accommodate a restricted bone loss envelope of about 50 mm × 50 mm × 35 mm according to the previous experience of the manufacturer in the custom scenario, covering 96% of defects previously addressed. One 6.5-mm nonlocking central screw and a minimum of four 4.75-mm nonlocking or locking peripheral screws are required for optimal fixation of the implant in the native scapula. A custom boss can be added in to enhance fixation in the native scapula when the bone is sufficient. To facilitate the surgical procedure, a trial implant, a bone model of the scapula, and a custom boss reaming guide are 3-dimensional (3-D) and printed in sterilizable material. These are all provided as single-use disposable instruments and can be available for surgeons during both the initial plan review and surgery.
PREOPERATIVE PLANNING
Patients undergo a preoperative fine-cut 2-dimensional computed tomography scan of the scapula and adjacent humerus following a predefined protocol with a slice thickness of 2 mm to 3 mm. An accurate 3-D bone model of the scapula is obtained using a 3-D image processing software system (Figure 1). The 3-D scapular model is used to create a patient-specific glenoid implant proposal that is approved by the surgeon (Figure 2). Implant position, orientation, size, screw trajectory, and recommended bone removal, if necessary, are determined to create a more normal glenohumeral center of rotation and to secure a glenoid implant in severely deficient glenoid bone (Figure 3). Once the implant design is approved by the surgeon, the final patient-specific implant is manufactured.
SURGICAL TECHNIQUE
The exposure of the glenoid is a critical step for the successful implantation of the patient-specific glenoid implant. Soft tissue and scar tissue around the glenoid must be removed to allow for optimal fit of the custom-made reaming guide. Also, removal of the entire capsulolabral complex on the anteroinferior rim of the glenoid is essential to both enhance glenoid exposure and to allow a perfect fit of the guide to the pathologic bone stock. Attention should be paid during débridement and/or implant removal in case of revision, to make sure that no excessive bone is removed because the patient-specific guide is referenced to this anatomy. Excessive bone removal can change the orientation of the patient-specific guide and ultimately the fixation of the implant. Once the custom-made patient-specific guide is positioned, a 3.2-mm Steinmann pin is placed through the inserter for temporary fixation. The pin should engage or perforate the medial cortical wall to ensure that the subsequent reamer has a stable cannula over which to ream. After the glenoid is reamed, the final implant can be placed in the ideal position according to the preoperative planning. A central 6.5-mm nonlocking central screw and 4.75-mm nonlocking or locking peripheral screws are required to complete the fixation of the implant in the native scapula. Once the patient-specific glenoid component is positioned and strongly fixed to the bone, the glenosphere can be positioned according to the preoperative planning, and the reverse shoulder arthroplasty can be completed in the usual fashion.
CASE EXAMPLES
A 68-year-old woman underwent a TSA for end-stage osteoarthritis in 2000. The implant failed due to a cuff failure. The patient underwent several surgeries, including an open cuff repair, with no success. She had no active elevation preoperatively. Because of the significant glenoid bone loss, a patient-specific glenoid reconstruction was planned. Within 24 months after this surgery, the patient was able to get her hand to her head and elevate to 90º (Figures 4A-4F).
Continue to: In October 2013...
In October 2013, a 68-year-old man underwent a TSA for end-stage osteoarthritis. After 18 months, the implant failed due to active Propionibacterium acnes infection, which required excisional arthroplasty with insertion of an antibiotic spacer. Significant glenoid bone loss (Figure 5) and global soft-tissue deficiency caused substantial disability and led to an indication for a reverse TSA with a patient-specific glenoid vault reconstruction (Figures 6A-6D) after infection eradication. Within 20 months after this surgery, the patient had resumed a satisfactory range of motion (130º forward elevation, 20º external rotation) and outcome.
DISCUSSION
Although glenoid bone loss is often seen in the primary setting (degenerative, congenital, and post-traumatic), severe glenoid bone loss is encountered in most revision TSAs. The best treatment method for massive glenoid bone defects during complex shoulder arthroplasty remains uncertain. Options have included eccentric reaming, glenoid reconstruction with bone allograft and autograft, and more recently augmented components and patient-specific implants.21-25 The advent and availability of CAD/CAM technology have enabled shoulder surgeons to create patient-specific metal solutions to these challenging cases. Currently, only a few reports exist in the literature on patient-specific glenoid components in the setting of severe bone loss.29-32
Chammaa and colleagues29 reported the outcomes of 37 patients with a hip-inspired glenoid component (Total Shoulder Replacement, Stanmore Implants Worldwide). The 5-year results with this implant were promising, with a 16% revision rate and only 1 case of glenoid loosening.
Stoffelen and colleagues30 recently described the successful use of a patient-specific anatomic metal-backed glenoid component for the management of severe glenoid bone loss with excellent results at 2.5 years of follow-up. A different approach was pursued by Gunther and Lynch,31 who reported on 7 patients with a custom inset glenoid implant for deficient glenoid vaults. These circular anatomic, custom-made glenoid components were created with the intention of placing the implants partially inside the glenoid vault and relying partially on sclerotic cortical bone. Despite excellent results at 3 years of follow-up, their use is limited to specific defect geometries and cannot be used in cases of extreme bone loss.
CONCLUSION
We have described the use of a patient-specific glenoid component in 2 patients with severe glenoid bone loss. Despite the satisfactory clinical and short-term radiographic results, we acknowledge that longer-term follow-up is needed to confirm the efficacy of this type of reconstruction. We believe that patient-specific glenoid components represent a valuable addition to the armamentarium of shoulder surgeons who address complex glenoid bone deformities.
1. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860. doi:10.1016/j.arth.2013.07.002.
2. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479. doi:10.1016/j.jse.2005.02.009.
3. Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635. doi:10.1016/j.jse.2012.07.005.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224. doi:10.1067/mse.2001.113961.
6. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):737-744. doi:10.1016/j.jse.2013.08.015.
7. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563. doi:10.1016/j.jse.2010.11.005.
8. Farshad M, Grogli M, Catanzaro S, Gerber C. Revision of reversed total shoulder arthroplasty. Indications and outcome. BMC Musculoskelet Disord. 2012;13(1):160. doi:10.1186/1471-2474-13-160.
9. Fevang BT, Lie SA, Havelin LI, Skredderstuen A, Furnes O. Risk factors for revision after shoulder arthroplasty: 1,825 shoulder arthroplasties from the Norwegian Arthroplasty Register. Acta Orthop. 2009;80(1):83-91.
10. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863. doi:10.1016/j.jse.2008.11.020.
11. Rasmussen JV. Outcome and risk of revision following shoulder replacement in patients with glenohumeral osteoarthritis. Acta Orthop Suppl. 2014;85(355 suppl):1-23. doi:10.3109/17453674.2014.922007.
12. Rasmussen JV, Polk A, Brorson S, Sorensen AK, Olsen BS. Patient-reported outcome and risk of revision after shoulder replacement for osteoarthritis. 1,209 cases from the Danish Shoulder Arthroplasty Registry, 2006-2010. Acta Orthop. 2014;85(2):117-122. doi:10.3109/17453674.2014.893497.
13. Sajadi KR, Kwon YW, Zuckerman JD. Revision shoulder arthroplasty: an analysis of indications and outcomes. J Shoulder Elbow Surg. 2010;19(2):308-313. doi:10.1016/j.jse.2009.05.016.
14. Singh JA, Sperling JW, Cofield RH. Revision surgery following total shoulder arthroplasty: analysis of 2588 shoulders over three decades (1976 to 2008). J Bone Joint Surg Br. 2011;93(11):1513-1517. doi:10.1302/0301-620X.93B11.26938.
15. Levine WN, Djurasovic M, Glasson JM, Pollock RG, Flatow EL, Bigliani LU. Hemiarthroplasty for glenohumeral osteoarthritis: results correlated to degree of glenoid wear. J Shoulder Elbow Surg. 1997;6(5):449-454.
16. Levine WN, Fischer CR, Nguyen D, Flatow EL, Ahmad CS, Bigliani LU. Long-term follow-up of shoulder hemiarthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2012;94(22):e164. doi:10.2106/JBJS.K.00603.
17. Lynch JR, Franta AK, Montgomery WH, Lenters TR, Mounce D, Matsen FA. Self-assessed outcome at two to four years after shoulder hemiarthroplasty with concentric glenoid reaming. J Bone Joint Surg Am. 2007;89(6):1284-1292. doi:10.2106/JBJS.E.00942.
18. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85-A(2):251-258.
19. Sperling JW, Cofield RH, Rowland CM. Neer hemiarthroplasty and Neer total shoulder arthroplasty in patients fifty years old or less. Long-term results. J Bone Joint Surg Am. 1998;80(4):464-473.
20. Strauss EJ, Roche C, Flurin PH, Wright T, Zuckerman JD. The glenoid in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(5):819-833. doi:10.1016/j.jse.2009.05.008.
21. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157. doi:10.1016/j.jse.2013.09.023.
22. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598. doi:10.1016/j.jse.2013.06.017.
23. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684. doi:10.1016/j.jse.2011.03.023.
24. Neer CS, Morrison DS. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70(8):1154-1162.
25. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367. doi:10.1067/mse.2000.106921.
26. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012:21(6):765-771. doi:10.1016/j.jse.2011.08.069.
27. Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83-A(6):877-883.
28. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymptomatic posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308.
29. Chammaa R, Uri O, Lambert S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J Shoulder Elbow Surg. 2017;26(1):101-107. doi:10.1016/j.jse.2016.05.027.
30. Stoffelen DV, Eraly K, Debeer P. The use of 3D printing technology in reconstruction of a severe glenoid defect: a case report with 2.5 years of follow-up. J Shoulder Elbow Surg. 2015;24(8):e218-e222. doi:10.1016/j.jse.2015.04.006.
31. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684. doi:10.1016/j.jse.2011.03.023.
32. Dines DM, Gulotta L, Craig EV, Dines JS. Novel solution for massive glenoid defects in shoulder arthroplasty: a patient-specific glenoid vault reconstruction system. Am J Orthop. 2017;46(2):104-108.
ABSTRACT
Complex glenoid bone deformities present the treating surgeon with a complex reconstructive challenge. Although glenoid bone loss can be encountered in the primary setting (degenerative, congenital, post-traumatic), severe glenoid bone loss is encountered in most revision total shoulder arthroplasties. Severe glenoid bone loss is treated with various techniques including hemiarthroplasty, eccentric reaming, and glenoid reconstruction with bone autografts and allografts. Despite encouraging short- to mid-term results reported with these reconstruction techniques, the clinical and radiographic outcomes remain inconsistent and the high number of complications is a concern. To overcome this problem, more recently augmented components and patient specific implants were introduced. Using the computer-aided design and computer-aided manufacturing technology patient-specific implants have been created to reconstruct the glenoid vault in cases of severe glenoid bone loss.
In this article we describe a patient specific glenoid implant, its indication, technical aspects and surgical technique, based on the author's experience as well as a review of the current literature on custom glenoid implants.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) is an effective operation for providing pain relief and improving function in patients with end-stage degenerative shoulder disease that is nonresponsive to nonoperative treatments.1-4 With the increasing number of arthroplasties performed, and the expanding indication for shoulder arthroplasty, the number of revision shoulder arthroplasties is also increasing.5-14 Complex glenoid bone deformities present the treating surgeon with a complex reconstructive challenge. Although glenoid bone loss can be seen in the primary setting (degenerative, congenital, and post-traumatic), severe glenoid bone loss is encountered mostly in revision TSAs.
Historically, patients with severe glenoid bone loss were treated with a hemiarthroplasty.15-17 However, due to inferior outcomes associated with the use of shoulder hemiarthroplasties compared with TSA in these cases,18-20 various techniques were developed with the aim of realigning the glenoid axis and securing the implants into the deficient glenoid vault.21-25 Options have included eccentric reaming, glenoid reconstruction with bone autografts and allografts, and more recently augmented components and patient-specific implants. Studies with eccentric reaming and reconstruction with bone graft during complex shoulder arthroplasty have reported encouraging short- to mid-term results, but the clinical and radiographic outcomes remain inconsistent, and the high number of complications is a concern.25-28
Complications with these techniques include component loosening, graft resorption, nonunion, failure of graft incorporation, infection, and instability.25-28
Computer-aided design and computer-aided manufacturing (CAD/CAM) of patient-specific implants have been used successfully by hip arthroplasty surgeons to deal with complex acetabular reconstructions in the setting of severe bone loss. More recently, the same technology has been used to reconstruct the glenoid vault in cases of severe glenoid bone loss.
In this article, we describe a patient-specific glenoid implant, its indication, and both technical aspects and the surgical technique, based on the authors’ experience as well as a review of the current literature on custom glenoid implants.
Continue to: PATIENT-SPECIFIC GLENOID COMPONENT
PATIENT-SPECIFIC GLENOID COMPONENT
The Vault Reconstruction System ([VRS], Zimmer Biomet) is a patient-specific glenoid vault reconstruction system developed with the use of CAD/CAM to address severe glenoid bone loss encountered during shoulder arthroplasty. For several years, the VRS was available only as a custom implant according to the US Food and Drug Administration rules, and therefore its use was limited to a few cases per year. Recently, a 510(k) envelope clearance was granted to use the VRS in reverse TSA to address significant glenoid bone defects.
The VRS is made of porous plasma spray titanium to provide high strength and flexibility, and allows for biologic fixation. This system can accommodate a restricted bone loss envelope of about 50 mm × 50 mm × 35 mm according to the previous experience of the manufacturer in the custom scenario, covering 96% of defects previously addressed. One 6.5-mm nonlocking central screw and a minimum of four 4.75-mm nonlocking or locking peripheral screws are required for optimal fixation of the implant in the native scapula. A custom boss can be added in to enhance fixation in the native scapula when the bone is sufficient. To facilitate the surgical procedure, a trial implant, a bone model of the scapula, and a custom boss reaming guide are 3-dimensional (3-D) and printed in sterilizable material. These are all provided as single-use disposable instruments and can be available for surgeons during both the initial plan review and surgery.

PREOPERATIVE PLANNING
Patients undergo a preoperative fine-cut 2-dimensional computed tomography scan of the scapula and adjacent humerus following a predefined protocol with a slice thickness of 2 mm to 3 mm. An accurate 3-D bone model of the scapula is obtained using a 3-D image processing software system (Figure 1). The 3-D scapular model is used to create a patient-specific glenoid implant proposal that is approved by the surgeon (Figure 2). Implant position, orientation, size, screw trajectory, and recommended bone removal, if necessary, are determined to create a more normal glenohumeral center of rotation and to secure a glenoid implant in severely deficient glenoid bone (Figure 3). Once the implant design is approved by the surgeon, the final patient-specific implant is manufactured.

SURGICAL TECHNIQUE
The exposure of the glenoid is a critical step for the successful implantation of the patient-specific glenoid implant. Soft tissue and scar tissue around the glenoid must be removed to allow for optimal fit of the custom-made reaming guide. Also, removal of the entire capsulolabral complex on the anteroinferior rim of the glenoid is essential to both enhance glenoid exposure and to allow a perfect fit of the guide to the pathologic bone stock. Attention should be paid during débridement and/or implant removal in case of revision, to make sure that no excessive bone is removed because the patient-specific guide is referenced to this anatomy. Excessive bone removal can change the orientation of the patient-specific guide and ultimately the fixation of the implant. Once the custom-made patient-specific guide is positioned, a 3.2-mm Steinmann pin is placed through the inserter for temporary fixation. The pin should engage or perforate the medial cortical wall to ensure that the subsequent reamer has a stable cannula over which to ream. After the glenoid is reamed, the final implant can be placed in the ideal position according to the preoperative planning. A central 6.5-mm nonlocking central screw and 4.75-mm nonlocking or locking peripheral screws are required to complete the fixation of the implant in the native scapula. Once the patient-specific glenoid component is positioned and strongly fixed to the bone, the glenosphere can be positioned according to the preoperative planning, and the reverse shoulder arthroplasty can be completed in the usual fashion.

CASE EXAMPLES
A 68-year-old woman underwent a TSA for end-stage osteoarthritis in 2000. The implant failed due to a cuff failure. The patient underwent several surgeries, including an open cuff repair, with no success. She had no active elevation preoperatively. Because of the significant glenoid bone loss, a patient-specific glenoid reconstruction was planned. Within 24 months after this surgery, the patient was able to get her hand to her head and elevate to 90º (Figures 4A-4F).

Continue to: In October 2013...
In October 2013, a 68-year-old man underwent a TSA for end-stage osteoarthritis. After 18 months, the implant failed due to active Propionibacterium acnes infection, which required excisional arthroplasty with insertion of an antibiotic spacer. Significant glenoid bone loss (Figure 5) and global soft-tissue deficiency caused substantial disability and led to an indication for a reverse TSA with a patient-specific glenoid vault reconstruction (Figures 6A-6D) after infection eradication. Within 20 months after this surgery, the patient had resumed a satisfactory range of motion (130º forward elevation, 20º external rotation) and outcome.

DISCUSSION
Although glenoid bone loss is often seen in the primary setting (degenerative, congenital, and post-traumatic), severe glenoid bone loss is encountered in most revision TSAs. The best treatment method for massive glenoid bone defects during complex shoulder arthroplasty remains uncertain. Options have included eccentric reaming, glenoid reconstruction with bone allograft and autograft, and more recently augmented components and patient-specific implants.21-25 The advent and availability of CAD/CAM technology have enabled shoulder surgeons to create patient-specific metal solutions to these challenging cases. Currently, only a few reports exist in the literature on patient-specific glenoid components in the setting of severe bone loss.29-32

Chammaa and colleagues29 reported the outcomes of 37 patients with a hip-inspired glenoid component (Total Shoulder Replacement, Stanmore Implants Worldwide). The 5-year results with this implant were promising, with a 16% revision rate and only 1 case of glenoid loosening.
Stoffelen and colleagues30 recently described the successful use of a patient-specific anatomic metal-backed glenoid component for the management of severe glenoid bone loss with excellent results at 2.5 years of follow-up. A different approach was pursued by Gunther and Lynch,31 who reported on 7 patients with a custom inset glenoid implant for deficient glenoid vaults. These circular anatomic, custom-made glenoid components were created with the intention of placing the implants partially inside the glenoid vault and relying partially on sclerotic cortical bone. Despite excellent results at 3 years of follow-up, their use is limited to specific defect geometries and cannot be used in cases of extreme bone loss.
CONCLUSION
We have described the use of a patient-specific glenoid component in 2 patients with severe glenoid bone loss. Despite the satisfactory clinical and short-term radiographic results, we acknowledge that longer-term follow-up is needed to confirm the efficacy of this type of reconstruction. We believe that patient-specific glenoid components represent a valuable addition to the armamentarium of shoulder surgeons who address complex glenoid bone deformities.
ABSTRACT
Complex glenoid bone deformities present the treating surgeon with a complex reconstructive challenge. Although glenoid bone loss can be encountered in the primary setting (degenerative, congenital, post-traumatic), severe glenoid bone loss is encountered in most revision total shoulder arthroplasties. Severe glenoid bone loss is treated with various techniques including hemiarthroplasty, eccentric reaming, and glenoid reconstruction with bone autografts and allografts. Despite encouraging short- to mid-term results reported with these reconstruction techniques, the clinical and radiographic outcomes remain inconsistent and the high number of complications is a concern. To overcome this problem, more recently augmented components and patient specific implants were introduced. Using the computer-aided design and computer-aided manufacturing technology patient-specific implants have been created to reconstruct the glenoid vault in cases of severe glenoid bone loss.
In this article we describe a patient specific glenoid implant, its indication, technical aspects and surgical technique, based on the author's experience as well as a review of the current literature on custom glenoid implants.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) is an effective operation for providing pain relief and improving function in patients with end-stage degenerative shoulder disease that is nonresponsive to nonoperative treatments.1-4 With the increasing number of arthroplasties performed, and the expanding indication for shoulder arthroplasty, the number of revision shoulder arthroplasties is also increasing.5-14 Complex glenoid bone deformities present the treating surgeon with a complex reconstructive challenge. Although glenoid bone loss can be seen in the primary setting (degenerative, congenital, and post-traumatic), severe glenoid bone loss is encountered mostly in revision TSAs.
Historically, patients with severe glenoid bone loss were treated with a hemiarthroplasty.15-17 However, due to inferior outcomes associated with the use of shoulder hemiarthroplasties compared with TSA in these cases,18-20 various techniques were developed with the aim of realigning the glenoid axis and securing the implants into the deficient glenoid vault.21-25 Options have included eccentric reaming, glenoid reconstruction with bone autografts and allografts, and more recently augmented components and patient-specific implants. Studies with eccentric reaming and reconstruction with bone graft during complex shoulder arthroplasty have reported encouraging short- to mid-term results, but the clinical and radiographic outcomes remain inconsistent, and the high number of complications is a concern.25-28
Complications with these techniques include component loosening, graft resorption, nonunion, failure of graft incorporation, infection, and instability.25-28
Computer-aided design and computer-aided manufacturing (CAD/CAM) of patient-specific implants have been used successfully by hip arthroplasty surgeons to deal with complex acetabular reconstructions in the setting of severe bone loss. More recently, the same technology has been used to reconstruct the glenoid vault in cases of severe glenoid bone loss.
In this article, we describe a patient-specific glenoid implant, its indication, and both technical aspects and the surgical technique, based on the authors’ experience as well as a review of the current literature on custom glenoid implants.
Continue to: PATIENT-SPECIFIC GLENOID COMPONENT
PATIENT-SPECIFIC GLENOID COMPONENT
The Vault Reconstruction System ([VRS], Zimmer Biomet) is a patient-specific glenoid vault reconstruction system developed with the use of CAD/CAM to address severe glenoid bone loss encountered during shoulder arthroplasty. For several years, the VRS was available only as a custom implant according to the US Food and Drug Administration rules, and therefore its use was limited to a few cases per year. Recently, a 510(k) envelope clearance was granted to use the VRS in reverse TSA to address significant glenoid bone defects.
The VRS is made of porous plasma spray titanium to provide high strength and flexibility, and allows for biologic fixation. This system can accommodate a restricted bone loss envelope of about 50 mm × 50 mm × 35 mm according to the previous experience of the manufacturer in the custom scenario, covering 96% of defects previously addressed. One 6.5-mm nonlocking central screw and a minimum of four 4.75-mm nonlocking or locking peripheral screws are required for optimal fixation of the implant in the native scapula. A custom boss can be added in to enhance fixation in the native scapula when the bone is sufficient. To facilitate the surgical procedure, a trial implant, a bone model of the scapula, and a custom boss reaming guide are 3-dimensional (3-D) and printed in sterilizable material. These are all provided as single-use disposable instruments and can be available for surgeons during both the initial plan review and surgery.

PREOPERATIVE PLANNING
Patients undergo a preoperative fine-cut 2-dimensional computed tomography scan of the scapula and adjacent humerus following a predefined protocol with a slice thickness of 2 mm to 3 mm. An accurate 3-D bone model of the scapula is obtained using a 3-D image processing software system (Figure 1). The 3-D scapular model is used to create a patient-specific glenoid implant proposal that is approved by the surgeon (Figure 2). Implant position, orientation, size, screw trajectory, and recommended bone removal, if necessary, are determined to create a more normal glenohumeral center of rotation and to secure a glenoid implant in severely deficient glenoid bone (Figure 3). Once the implant design is approved by the surgeon, the final patient-specific implant is manufactured.

SURGICAL TECHNIQUE
The exposure of the glenoid is a critical step for the successful implantation of the patient-specific glenoid implant. Soft tissue and scar tissue around the glenoid must be removed to allow for optimal fit of the custom-made reaming guide. Also, removal of the entire capsulolabral complex on the anteroinferior rim of the glenoid is essential to both enhance glenoid exposure and to allow a perfect fit of the guide to the pathologic bone stock. Attention should be paid during débridement and/or implant removal in case of revision, to make sure that no excessive bone is removed because the patient-specific guide is referenced to this anatomy. Excessive bone removal can change the orientation of the patient-specific guide and ultimately the fixation of the implant. Once the custom-made patient-specific guide is positioned, a 3.2-mm Steinmann pin is placed through the inserter for temporary fixation. The pin should engage or perforate the medial cortical wall to ensure that the subsequent reamer has a stable cannula over which to ream. After the glenoid is reamed, the final implant can be placed in the ideal position according to the preoperative planning. A central 6.5-mm nonlocking central screw and 4.75-mm nonlocking or locking peripheral screws are required to complete the fixation of the implant in the native scapula. Once the patient-specific glenoid component is positioned and strongly fixed to the bone, the glenosphere can be positioned according to the preoperative planning, and the reverse shoulder arthroplasty can be completed in the usual fashion.

CASE EXAMPLES
A 68-year-old woman underwent a TSA for end-stage osteoarthritis in 2000. The implant failed due to a cuff failure. The patient underwent several surgeries, including an open cuff repair, with no success. She had no active elevation preoperatively. Because of the significant glenoid bone loss, a patient-specific glenoid reconstruction was planned. Within 24 months after this surgery, the patient was able to get her hand to her head and elevate to 90º (Figures 4A-4F).

Continue to: In October 2013...
In October 2013, a 68-year-old man underwent a TSA for end-stage osteoarthritis. After 18 months, the implant failed due to active Propionibacterium acnes infection, which required excisional arthroplasty with insertion of an antibiotic spacer. Significant glenoid bone loss (Figure 5) and global soft-tissue deficiency caused substantial disability and led to an indication for a reverse TSA with a patient-specific glenoid vault reconstruction (Figures 6A-6D) after infection eradication. Within 20 months after this surgery, the patient had resumed a satisfactory range of motion (130º forward elevation, 20º external rotation) and outcome.

DISCUSSION
Although glenoid bone loss is often seen in the primary setting (degenerative, congenital, and post-traumatic), severe glenoid bone loss is encountered in most revision TSAs. The best treatment method for massive glenoid bone defects during complex shoulder arthroplasty remains uncertain. Options have included eccentric reaming, glenoid reconstruction with bone allograft and autograft, and more recently augmented components and patient-specific implants.21-25 The advent and availability of CAD/CAM technology have enabled shoulder surgeons to create patient-specific metal solutions to these challenging cases. Currently, only a few reports exist in the literature on patient-specific glenoid components in the setting of severe bone loss.29-32

Chammaa and colleagues29 reported the outcomes of 37 patients with a hip-inspired glenoid component (Total Shoulder Replacement, Stanmore Implants Worldwide). The 5-year results with this implant were promising, with a 16% revision rate and only 1 case of glenoid loosening.
Stoffelen and colleagues30 recently described the successful use of a patient-specific anatomic metal-backed glenoid component for the management of severe glenoid bone loss with excellent results at 2.5 years of follow-up. A different approach was pursued by Gunther and Lynch,31 who reported on 7 patients with a custom inset glenoid implant for deficient glenoid vaults. These circular anatomic, custom-made glenoid components were created with the intention of placing the implants partially inside the glenoid vault and relying partially on sclerotic cortical bone. Despite excellent results at 3 years of follow-up, their use is limited to specific defect geometries and cannot be used in cases of extreme bone loss.
CONCLUSION
We have described the use of a patient-specific glenoid component in 2 patients with severe glenoid bone loss. Despite the satisfactory clinical and short-term radiographic results, we acknowledge that longer-term follow-up is needed to confirm the efficacy of this type of reconstruction. We believe that patient-specific glenoid components represent a valuable addition to the armamentarium of shoulder surgeons who address complex glenoid bone deformities.
1. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860. doi:10.1016/j.arth.2013.07.002.
2. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479. doi:10.1016/j.jse.2005.02.009.
3. Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635. doi:10.1016/j.jse.2012.07.005.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224. doi:10.1067/mse.2001.113961.
6. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):737-744. doi:10.1016/j.jse.2013.08.015.
7. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563. doi:10.1016/j.jse.2010.11.005.
8. Farshad M, Grogli M, Catanzaro S, Gerber C. Revision of reversed total shoulder arthroplasty. Indications and outcome. BMC Musculoskelet Disord. 2012;13(1):160. doi:10.1186/1471-2474-13-160.
9. Fevang BT, Lie SA, Havelin LI, Skredderstuen A, Furnes O. Risk factors for revision after shoulder arthroplasty: 1,825 shoulder arthroplasties from the Norwegian Arthroplasty Register. Acta Orthop. 2009;80(1):83-91.
10. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863. doi:10.1016/j.jse.2008.11.020.
11. Rasmussen JV. Outcome and risk of revision following shoulder replacement in patients with glenohumeral osteoarthritis. Acta Orthop Suppl. 2014;85(355 suppl):1-23. doi:10.3109/17453674.2014.922007.
12. Rasmussen JV, Polk A, Brorson S, Sorensen AK, Olsen BS. Patient-reported outcome and risk of revision after shoulder replacement for osteoarthritis. 1,209 cases from the Danish Shoulder Arthroplasty Registry, 2006-2010. Acta Orthop. 2014;85(2):117-122. doi:10.3109/17453674.2014.893497.
13. Sajadi KR, Kwon YW, Zuckerman JD. Revision shoulder arthroplasty: an analysis of indications and outcomes. J Shoulder Elbow Surg. 2010;19(2):308-313. doi:10.1016/j.jse.2009.05.016.
14. Singh JA, Sperling JW, Cofield RH. Revision surgery following total shoulder arthroplasty: analysis of 2588 shoulders over three decades (1976 to 2008). J Bone Joint Surg Br. 2011;93(11):1513-1517. doi:10.1302/0301-620X.93B11.26938.
15. Levine WN, Djurasovic M, Glasson JM, Pollock RG, Flatow EL, Bigliani LU. Hemiarthroplasty for glenohumeral osteoarthritis: results correlated to degree of glenoid wear. J Shoulder Elbow Surg. 1997;6(5):449-454.
16. Levine WN, Fischer CR, Nguyen D, Flatow EL, Ahmad CS, Bigliani LU. Long-term follow-up of shoulder hemiarthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2012;94(22):e164. doi:10.2106/JBJS.K.00603.
17. Lynch JR, Franta AK, Montgomery WH, Lenters TR, Mounce D, Matsen FA. Self-assessed outcome at two to four years after shoulder hemiarthroplasty with concentric glenoid reaming. J Bone Joint Surg Am. 2007;89(6):1284-1292. doi:10.2106/JBJS.E.00942.
18. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85-A(2):251-258.
19. Sperling JW, Cofield RH, Rowland CM. Neer hemiarthroplasty and Neer total shoulder arthroplasty in patients fifty years old or less. Long-term results. J Bone Joint Surg Am. 1998;80(4):464-473.
20. Strauss EJ, Roche C, Flurin PH, Wright T, Zuckerman JD. The glenoid in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(5):819-833. doi:10.1016/j.jse.2009.05.008.
21. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157. doi:10.1016/j.jse.2013.09.023.
22. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598. doi:10.1016/j.jse.2013.06.017.
23. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684. doi:10.1016/j.jse.2011.03.023.
24. Neer CS, Morrison DS. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70(8):1154-1162.
25. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367. doi:10.1067/mse.2000.106921.
26. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012:21(6):765-771. doi:10.1016/j.jse.2011.08.069.
27. Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83-A(6):877-883.
28. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymptomatic posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308.
29. Chammaa R, Uri O, Lambert S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J Shoulder Elbow Surg. 2017;26(1):101-107. doi:10.1016/j.jse.2016.05.027.
30. Stoffelen DV, Eraly K, Debeer P. The use of 3D printing technology in reconstruction of a severe glenoid defect: a case report with 2.5 years of follow-up. J Shoulder Elbow Surg. 2015;24(8):e218-e222. doi:10.1016/j.jse.2015.04.006.
31. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684. doi:10.1016/j.jse.2011.03.023.
32. Dines DM, Gulotta L, Craig EV, Dines JS. Novel solution for massive glenoid defects in shoulder arthroplasty: a patient-specific glenoid vault reconstruction system. Am J Orthop. 2017;46(2):104-108.
1. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860. doi:10.1016/j.arth.2013.07.002.
2. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479. doi:10.1016/j.jse.2005.02.009.
3. Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635. doi:10.1016/j.jse.2012.07.005.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224. doi:10.1067/mse.2001.113961.
6. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):737-744. doi:10.1016/j.jse.2013.08.015.
7. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563. doi:10.1016/j.jse.2010.11.005.
8. Farshad M, Grogli M, Catanzaro S, Gerber C. Revision of reversed total shoulder arthroplasty. Indications and outcome. BMC Musculoskelet Disord. 2012;13(1):160. doi:10.1186/1471-2474-13-160.
9. Fevang BT, Lie SA, Havelin LI, Skredderstuen A, Furnes O. Risk factors for revision after shoulder arthroplasty: 1,825 shoulder arthroplasties from the Norwegian Arthroplasty Register. Acta Orthop. 2009;80(1):83-91.
10. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863. doi:10.1016/j.jse.2008.11.020.
11. Rasmussen JV. Outcome and risk of revision following shoulder replacement in patients with glenohumeral osteoarthritis. Acta Orthop Suppl. 2014;85(355 suppl):1-23. doi:10.3109/17453674.2014.922007.
12. Rasmussen JV, Polk A, Brorson S, Sorensen AK, Olsen BS. Patient-reported outcome and risk of revision after shoulder replacement for osteoarthritis. 1,209 cases from the Danish Shoulder Arthroplasty Registry, 2006-2010. Acta Orthop. 2014;85(2):117-122. doi:10.3109/17453674.2014.893497.
13. Sajadi KR, Kwon YW, Zuckerman JD. Revision shoulder arthroplasty: an analysis of indications and outcomes. J Shoulder Elbow Surg. 2010;19(2):308-313. doi:10.1016/j.jse.2009.05.016.
14. Singh JA, Sperling JW, Cofield RH. Revision surgery following total shoulder arthroplasty: analysis of 2588 shoulders over three decades (1976 to 2008). J Bone Joint Surg Br. 2011;93(11):1513-1517. doi:10.1302/0301-620X.93B11.26938.
15. Levine WN, Djurasovic M, Glasson JM, Pollock RG, Flatow EL, Bigliani LU. Hemiarthroplasty for glenohumeral osteoarthritis: results correlated to degree of glenoid wear. J Shoulder Elbow Surg. 1997;6(5):449-454.
16. Levine WN, Fischer CR, Nguyen D, Flatow EL, Ahmad CS, Bigliani LU. Long-term follow-up of shoulder hemiarthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2012;94(22):e164. doi:10.2106/JBJS.K.00603.
17. Lynch JR, Franta AK, Montgomery WH, Lenters TR, Mounce D, Matsen FA. Self-assessed outcome at two to four years after shoulder hemiarthroplasty with concentric glenoid reaming. J Bone Joint Surg Am. 2007;89(6):1284-1292. doi:10.2106/JBJS.E.00942.
18. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85-A(2):251-258.
19. Sperling JW, Cofield RH, Rowland CM. Neer hemiarthroplasty and Neer total shoulder arthroplasty in patients fifty years old or less. Long-term results. J Bone Joint Surg Am. 1998;80(4):464-473.
20. Strauss EJ, Roche C, Flurin PH, Wright T, Zuckerman JD. The glenoid in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(5):819-833. doi:10.1016/j.jse.2009.05.008.
21. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157. doi:10.1016/j.jse.2013.09.023.
22. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598. doi:10.1016/j.jse.2013.06.017.
23. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684. doi:10.1016/j.jse.2011.03.023.
24. Neer CS, Morrison DS. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70(8):1154-1162.
25. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367. doi:10.1067/mse.2000.106921.
26. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012:21(6):765-771. doi:10.1016/j.jse.2011.08.069.
27. Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83-A(6):877-883.
28. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymptomatic posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308.
29. Chammaa R, Uri O, Lambert S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J Shoulder Elbow Surg. 2017;26(1):101-107. doi:10.1016/j.jse.2016.05.027.
30. Stoffelen DV, Eraly K, Debeer P. The use of 3D printing technology in reconstruction of a severe glenoid defect: a case report with 2.5 years of follow-up. J Shoulder Elbow Surg. 2015;24(8):e218-e222. doi:10.1016/j.jse.2015.04.006.
31. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684. doi:10.1016/j.jse.2011.03.023.
32. Dines DM, Gulotta L, Craig EV, Dines JS. Novel solution for massive glenoid defects in shoulder arthroplasty: a patient-specific glenoid vault reconstruction system. Am J Orthop. 2017;46(2):104-108.
TAKE-HOME POINTS
- With the increasing number of arthroplasties performed, and the expanding indication for shoulder arthroplasty, the number of revision shoulder arthroplasties is also increasing.
- Complex glenoid bone defects are sometimes encountered in revision shoulder arthroplasties.
- Glenoid reconstructions with bone graft have reported encouraging short- to mid-term results, but the high number of complications is a concern.
- Using the CAD/CAM technology patient-specific glenoid components have been created to reconstruct the glenoid vault in cases of severe glenoid bone loss.
- Short-term clinical and radiographic results of patient-specific glenoid components are encouraging, however longer-term follow-up are needed to confirm the efficacy of this type of reconstruction.
Convertible Glenoid Components Facilitate Revisions to Reverse Shoulder Arthroplasty Easier: Retrospective Review of 13 Cases
ABSTRACT
Removal of a cemented glenoid component often leads to massive glenoid bone loss, which makes it difficult to implant a new glenoid baseplate. The purpose of this study was to demonstrate the feasibility of revisions with a completely convertible system and to report clinical and radiographic results of a retrospective review of 13 cases.
Between 2003 and 2011, 104 primary total shoulder arthroplasties (TSAs) were performed with an uncemented glenoid component in our group. Of these patients, 13 (average age, 64 years) were revised to reverse shoulder arthroplasty (RSA) using a modular convertible platform system and were included in this study. Average follow-up after revision was 22 months. Outcome measures included pain, range of motion, Constant-Murley scores, Simple Shoulder Tests, and subjective shoulder values. Active flexion increased significantly from a mean of 93° (range, 30°-120°) to 138° (range, 95°-170°) (P = 0.021), and active external rotation increased significantly from 8° (range, −20°-15°) to 25° (range, −10°-60°). Mean pain scores significantly improved from 4.2 to 13.3 points. The mean Constant Scores improved from 21 (range, 18-32) to 63 (range, 43-90). Subjectively, 12 patients rated their shoulder as better or much better than preoperatively. This retrospective study shows that a complete convertible system facilitates conversion of TSAs to RSAs with excellent pain relief and a significant improvement in shoulder function.
Continue to: Polyethylene glenoid components...
Polyethylene glenoid components are the gold standard in anatomic total shoulder arthroplasty (TSA). However, even though TSA survivorship exceeds 95% at 10-year follow-up,1 glenoid component loosening remains the main complication and the weak link in these implants. This complication accounts for 25% of all complications related to TSA in the literature.2 In most cases, glenoid component loosening is not isolated but combined with a rotator cuff tear, glenohumeral instability, or component malposition.
We hypothesized that a completely convertible platform system on both the humeral and the glenoid side could facilitate the revision of a failed TSA to a RSA. This would enable the surgeon to leave the humeral stem and the glenoid baseplate in place, avoiding the difficulty of stem removal and the reimplantation of a glenoid component, especially in osteoporotic glenoid bone and elderly patients. The revision procedure would then only consist of replacing the humeral head by a metallic tray and polyethylene bearing on the humeral side and by impacting a glenosphere on the glenoid baseplate (Figures 2A, 2B).
The purpose of this study was to demonstrate the feasibility of revisions with this completely convertible system and to report clinical and radiographic results of a retrospective review of 13 cases.
MATERIALS AND METHODS
PATIENT SELECTION
Between 2003 and 2011, 104 primary TSAs were performed with an uncemented glenoid component in our group. Of these patients, 18 underwent revision (17.3%). Among these 18 patients, 13 were revised to RSA using a modular convertible platform system and were included in this study, while 5 patients were revised to another TSA (2 dissociations of the polyethylene glenoid implant, 2 excessively low implantations of the glenoid baseplate, and 1 glenoid loosening). The mean age of the 13 patients (9 women, 4 men) included in this retrospective study at the time of revision was 64 years (range, 50-75 years). The reasons for revision surgery were rotator cuff tear (5, among which 2 were posterosuperior tears, and 3 were tears of the subscapularis), dislocations (5 posterior and 1 anterior, among which 4 had a B2 or C glenoid), suprascapular nerve paralysis (1), and dissociation of the polyethylene (1). The initial TSA was indicated for primary osteoarthritis with a normal cuff (9), primary osteoarthritis with a reparable cuff tear (2), posttraumatic osteoarthritis (1), and chronic dislocation (1). The right dominant shoulder was involved in 10 cases. The mean time interval between the primary TSA and the revision was 15 months (range, 1-61 months).
OPERATIVE TECHNIQUE
PREOPERATIVE PLANNING
Revision of a failed TSA is always a difficult challenge, and evaluation of bone loss on both the humeral and the glenoid sides, as well as the status of the cuff, is mandatory, even with a completely convertible arthroplasty system. The surgeon must be prepared to remove the humeral stem in case reduction of the joint is impossible. We systematically performed standard radiographs (anteroposterior, axillary, and outlet views) and computed tomography (CT) scans in order to assess both the version and positioning, as well as potential signs of loosening of the implants and the status of the cuff (continuity, degree of muscle trophicity, and fatty infiltration). A preoperative leucocyte count, sedimentation rate, and C-reactive protein rates were requested in every revision case, even if a mechanical etiology was strongly suspected.
Continue to: REVISION PROCEDURE
REVISION PROCEDURE
All the implants that had been used in the primary TSAs were Arrow Universal Shoulder Prostheses (FH Orthopedics). All revisions were performed through the previous deltopectoral approach in the beach chair position under general anesthesia with an interscalene block. Adhesions of the deep part of the deltoid were carefully released. The conjoint tendon was released, and the location of the musculocutaneous and axillary nerves was identified before any retractor was placed. In the 10 cases where the subscapularis was intact, it was peeled off the medial border of the bicipital groove to obtain sufficient length for a tension-free reinsertion.
The anatomical head of the humeral implant was disconnected from the stem and removed. All stems were found to be well fixed; there were no cases of loosening or evidence of infection. A circumferential capsular release was systematically carried out. The polyethylene glenoid onlay was then unlocked from the baseplate.
The quality of the fixation of the glenoid baseplate was systematically evaluated; no screw was found to be loose, and the fixation of all baseplates was stable. Therefore, there was no need to revise the glenoid baseplate, even when its position was considered excessively retroverted (Glenoid B2) or high. A glenosphere was impacted on the baseplate, and a polyethylene humeral bearing was then implanted on the humeral stem. The thinnest polyethylene bearing available (number 0) was chosen in all cases, and a size 36 glenosphere was chosen in 12 out of 13 cases. Intraoperative stability of the implant was satisfactory, and no impingement was found posteriorly, anteriorly, or inferiorly.
In one case, the humeral stem was a first-generation humeral implant which was not compatible with the new-generation humeral bearing, and the humeral stem had to be replaced.
In 2 cases, reduction of the RSA was either impossible or felt to be too tight, even after extensive soft-tissue release and resection of the remaining supraspinatus. The main reason for this was an excessively proud humeral stem because of an onlay polyethylene humeral bearing instead of an inlay design. However, removal of the uncemented humeral stem was always possible with no osteotomy or cortical window of the humeral shaft as the humeral stem has been designed with a bone ingrowth surface only on the metaphyseal part with a smooth surface on diaphyseal part. After removal of the stem, a small amount of humeral metaphysis was cut, and a new humeral stem was press-fit in a lower position. This allowed restoration of an appropriate tension of the soft tissue and, therefore, an easier reduction. The subscapularis was medialized and reinserted transosseously when possible with a double-row repair. In 3 cases, the subscapularis was torn and retracted at the level of the glenoid, or impossible to identify to allow its reinsertion.
Continue to: According to our infectious disease department...
According to our infectious disease department, we made a minimum of 5 cultures for each revision case looking for a possible low-grade infection. All cultures in our group are held for 14 days to assess for Propionibacterium acnes.
POSTOPERATIVE MANAGEMENT
A shoulder splint in neutral rotation was used for the first 4 weeks. Passive range of motion (ROM) was started immediately with pendulum exercises and passive anterior elevation. Active assisted and active ROM were allowed after 4 weeks, and physiotherapy was continued for 6 months. Elderly patients were referred to a center of rehabilitation. We found only 1 or 2 positive cultures (Propionibacterium acnes) for 4 patients, and we decided to consider them as a contamination. None of the patients were treated with antibiotics.
CLINICAL AND RADIOLOGICAL ASSESSMENT
Clinical evaluation included pre- and postoperative pain scores (visual analog scale [VAS]), ROM, the Constant-Murley13 score, the Simple Shoulder Test (SST),14 and the subjective shoulder value.15 Subjective satisfaction was assessed by asking the patients at follow-up how they felt compared with before surgery and was graded using a 4-point scale: 1, much better; 2, better; 3, the same; and 4, worse. Radiographic evaluation was performed on pre- and postoperative standard anteroposterior, outlet, and axillary views. Radiographs were reviewed to determine the presence of glenohumeral subluxation, periprosthetic lucency, component shift in position, and scapular notching.
STATISTICAL ANALYSIS
Descriptive statistics are reported as mean (range) for continuous measures and number (percentage) for discrete variables. The Wilcoxon signed-rank test was used for preoperative vs postoperative changes. The alpha level for all tests was set at 0.05 for statistical significance.
RESULTS
CLINICAL OUTCOME
At a mean of 22 months (range, 7-38 months) follow-up after revision, active ROM was significantly improved. Active flexion increased significantly from a mean of 93° (range, 30°-120°) to 138° (range, 95°-170°) (P = 0.021). Active external rotation with the elbow on the side increased significantly from 8° (range, −20°-15°) to 25° (range, −10°-60°) (P = 0.034), and increased with the arm held at 90° abduction from 13° (range, 0°-20°) to 49° (range, 0°-80°) (P = 0.025). Mean pain scores improved from 4.2 to 13.3 points (P < 0.001). VAS improved significantly from 9 to 1 (P < 0.0001). The mean Constant Scores improved from 21 (range, 18-32) to 63 (range, 43-90) (P = 0.006). The final SST was 7 per 12. Subjectively, 4 patients rated their shoulder as much better, 8 as better, and 1 as the same as preoperatively. No intra- or postoperative complications, including infections, were observed. The mean duration of the procedure was 60 minutes (range, 30-75 minutes).
Continue to: RADIOLOGICAL OUTCOME
RADIOLOGICAL OUTCOME
No periprosthetic lucency or shift in component was observed at the last follow-up. There was no scapular notching. No resorption of the tuberosities, and no fractures of the acromion or the scapular spine were observed.
DISCUSSION
In this retrospective study, failure of TSA with a metal-backed glenoid implant was successfully revised to RSA. In 10 patients, the use of a universal platform system allowed an easier conversion without removal of the humeral stem or the glenoid component (Figures 3A-3D). Twelve of the 13 patients were satisfied or very satisfied at the last follow-up. None of the patients were in pain, and the mean Constant score was 63. In all the cases, the glenoid baseplate was not changed. In 3 cases the humeral stem was changed without any fracture of the tuberosities of need for an osteotomy. This greatly simplified the revision procedure, as glenoid revisions can be very challenging. Indeed, it is often difficult to assess precisely preoperatively the remaining glenoid bone stock after removal of the glenoid component and the cement. Many therapeutic options to deal with glenoid loosening have been reported in the literature: glenoid bone reconstruction after glenoid component removal and revision to a hemiarthroplasty (HA),10,16-18 glenoid bone reconstruction after glenoid component removal and revision to a new TSA with a cemented glenoid implant,16,17,19,20 and glenoid reconstruction after glenoid component removal and revision to a RSA.12,21 These authors reported that glenoid reconstruction frequently necessitates an iliac bone graft associated with a special design of the baseplate with a long post fixed into the native glenoid bone. However, sometimes implantation of an uncemented glenoid component can be unstable with a high risk of early mobilization of the implant, and 2 steps may be necessary. Conversion to a HA,10,16-18 or a TSA16,17,19,20 with a new cemented implant have both been associated with poor clinical outcome, with a high rate of recurrent glenoid loosening for the TSAs.
In our retrospective study, we reported no intra- or postoperative complications. Flury and colleagues22 reported a complication rate of 38% in 21 patients after conversion from a TSA to a RSA with a mean follow-up of 46 months. They removed all the components of the prosthesis with a crack or fracture of the humerus and/or the glenoid. Ortmaier and colleagues23 reported a rate of complication of 22.7% during the conversion of TSA to RSA. They did an osteotomy of the humeral diaphysis to extract the stem in 40% of cases and had to remove the glenoid cement in 86% of cases with severe damage of the glenoid bone in 10% of cases. Fewer complications were found in our study, as we did not need any procedure such as humeral osteotomy, cerclage, bone grafting, and/or reconstruction of the glenoid. The short operative time and the absence of extensive soft-tissue dissection, thanks to a standard deltopectoral approach, could explain the absence of infection in our series.
Other authors shared our strategy of a universal convertible system and reported their results in the literature. Castagna and colleagues24 in 2013 reported the clinical and radiological results of conversions of HA or TSA to RSA using a modular, convertible system (SMR Shoulder System, Lima Corporate). In their series, only 8 cases of TSAs were converted to RSA. They preserved, in each case, the humeral stem and the glenoid baseplate. There were no intra- or postoperative complications. The mean VAS score decreased from 8 to 2. Weber-Spickschen and colleague25 reported recently in 2015 the same experience with the same system (SMR Shoulder System). They reviewed 15 conversions of TSAs to RSAs without any removal of the implants at a mean 43-month follow-up. They reported excellent pain relief (VAS decreased from 8 to 1) and improvement in shoulder function with a low rate of complications.
Kany and colleagues26 in 2015 had already reported the advantages of a shoulder platform system for revisions. In their series, the authors included cases of failure of HAs and TSAs with loose cemented glenoids and metal-backed glenoids. The clinical and radiological results were similar, with a final Constant score of 60 (range, 42-85) and a similar rate of humeral stems which had to be changed (24%). These stems were replaced either because they were too proud or because there was not enough space to add an onlay polyethylene socket.
Continue to: Despite the encouraging results...
Despite the encouraging results reported in this study, there are some limitations. Firstly, no control group was used. Attempting to address this issue, we compared our results with the literature. Secondly, the number of patients in our study was small. Finally, the follow-up duration (mean 22 months) did not provide long-term outcomes.
CONCLUSION
This retrospective study shows that a complete convertible system facilitates conversion of TSAs to RSAs with excellent pain relief and a significant improvement in shoulder function. A platform system on both the humeral and the glenoid side reduces the operative time of the conversion with a low risk of complications.
1. Brenner BC, Ferlic DC, Clayton ML, Dennis DA. Survivorship of unconstrained total shoulder arthroplasty. J Bone Joint Surg Am. 1989;71(9):1289-1296.
2. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541. doi:10.1016/j.jse.2012.06.001.
3. Chin PY, Sperling JW, Cofield RH, Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15(1):19-22. doi:10.1016/j.jse.2005.05.005.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement surgery. J Bone Joint Surg Am. 1996;78(4):603-616.
6. Gohlke F, Rolf O. Revision of failed fracture hemiarthroplasties to reverse total shoulder prosthesis through the transhumeral approach: method incorporating a pectoralis-major-pedicled bone window. Oper Orthop Traumatol. 2007;19(2):185-208. doi:10.1007/s00064-007-1202-x.
7. Goldberg SH, Cohen MS, Young M, Bradnock B. Thermal tissue damage caused by ultrasonic cement removal from the humerus. J Bone Joint Surg Am. 2005;87(3):583-591. doi:10.2106/JBJS.D.01966.
8. Sperling JW, Cofield RH. Humeral windows in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(3):258-263. doi:10.1016/j.jse.2004.09.004.
9. Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91(1):119-127. doi:10.2106/JBJS.H.00094.
10. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012;21(6):765-771. doi:10.1016/j.jse.2011.08.069.
11. Kelly JD 2nd, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525. doi:10.1016/j.jse.2011.11.021.
12. Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21(3):342-349. doi:10.1016/j.jse.2011.05.021.
13. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
14. Matsen FA 3rd, Ziegler DW, DeBartolo SE. Patient self-assessment of health status and function in glenohumeral degenerative joint disease. J Shoulder Elbow Surg. 1995;4(5):345-351.
15. Gilbart MK, Gerber C. Comparison of the subjective shoulder value and the Constant score. J Shoulder Elbow Surg. 2007;16(6):717-721. doi:10.1016/j.jse.2007.02.123.
16. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224. doi:10.1067/mse.2001.113961.
17. Cofield RH, Edgerton BC. Total shoulder arthroplasty: complications and revision surgery. Instr Course Lect. 1990;39:449-462.
18. Neyton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid corticocancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg. 2006;15(2):173-179. doi:10.1016/j.jse.2005.07.010.
19. Bonnevialle N, Melis B, Neyton L, et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22(6):745-751. doi:10.1016/j.jse.2012.08.009.
20. Rodosky MW, Bigliani LU. Indications for glenoid resurfacing in shoulder arthroplasty. J Shoulder Elbow Surg. 1996;5(3):231-248.
21. Bateman E, Donald SM. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg. 2012;21(7):925-934. doi:10.1016/j.jse.2011.07.009.
22. Flury MP, Frey P, Goldhahn J, Schwyzer HK, Simmen BR. Reverse shoulder arthroplasty as a salvage procedure for failed conventional shoulder replacement due to cuff failure--midterm results. Int Orthop. 2011;35(1):53-60. doi:10.1007/s00264-010-0990-z.
23. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2013;38(3):1-7. doi:10.1007/s00264-013-2139-3.
24. Castagna A, Delcogliano M, de Caro F, et al. Conversion of shoulder arthroplasty to reverse implants: clinical and radiological results using a modular system. Int Orthop. 2013;37(7):1297-1305. doi:10.1007/s00264-013-1907-4.
25. Weber-Spickschen TS, Alfke D, Agneskirchner JD. The use of a modular system to convert an anatomical total shoulder arthroplasty to a reverse shoulder arthroplasty: Clinical and radiological results. Bone Joint J. 2015;97-B(12):1662-1667. doi:10.1302/0301-620X.97B12.35176.
26. Kany J, Amouyel T, Flamand O, Katz D, Valenti P. A convertible shoulder system: is it useful in total shoulder arthroplasty revisions? Int Orthop. 2015;39(2):299-304. doi:10.1007/s00264-014-2563-z.
ABSTRACT
Removal of a cemented glenoid component often leads to massive glenoid bone loss, which makes it difficult to implant a new glenoid baseplate. The purpose of this study was to demonstrate the feasibility of revisions with a completely convertible system and to report clinical and radiographic results of a retrospective review of 13 cases.
Between 2003 and 2011, 104 primary total shoulder arthroplasties (TSAs) were performed with an uncemented glenoid component in our group. Of these patients, 13 (average age, 64 years) were revised to reverse shoulder arthroplasty (RSA) using a modular convertible platform system and were included in this study. Average follow-up after revision was 22 months. Outcome measures included pain, range of motion, Constant-Murley scores, Simple Shoulder Tests, and subjective shoulder values. Active flexion increased significantly from a mean of 93° (range, 30°-120°) to 138° (range, 95°-170°) (P = 0.021), and active external rotation increased significantly from 8° (range, −20°-15°) to 25° (range, −10°-60°). Mean pain scores significantly improved from 4.2 to 13.3 points. The mean Constant Scores improved from 21 (range, 18-32) to 63 (range, 43-90). Subjectively, 12 patients rated their shoulder as better or much better than preoperatively. This retrospective study shows that a complete convertible system facilitates conversion of TSAs to RSAs with excellent pain relief and a significant improvement in shoulder function.
Continue to: Polyethylene glenoid components...
Polyethylene glenoid components are the gold standard in anatomic total shoulder arthroplasty (TSA). However, even though TSA survivorship exceeds 95% at 10-year follow-up,1 glenoid component loosening remains the main complication and the weak link in these implants. This complication accounts for 25% of all complications related to TSA in the literature.2 In most cases, glenoid component loosening is not isolated but combined with a rotator cuff tear, glenohumeral instability, or component malposition.

We hypothesized that a completely convertible platform system on both the humeral and the glenoid side could facilitate the revision of a failed TSA to a RSA. This would enable the surgeon to leave the humeral stem and the glenoid baseplate in place, avoiding the difficulty of stem removal and the reimplantation of a glenoid component, especially in osteoporotic glenoid bone and elderly patients. The revision procedure would then only consist of replacing the humeral head by a metallic tray and polyethylene bearing on the humeral side and by impacting a glenosphere on the glenoid baseplate (Figures 2A, 2B).

The purpose of this study was to demonstrate the feasibility of revisions with this completely convertible system and to report clinical and radiographic results of a retrospective review of 13 cases.
MATERIALS AND METHODS
PATIENT SELECTION
Between 2003 and 2011, 104 primary TSAs were performed with an uncemented glenoid component in our group. Of these patients, 18 underwent revision (17.3%). Among these 18 patients, 13 were revised to RSA using a modular convertible platform system and were included in this study, while 5 patients were revised to another TSA (2 dissociations of the polyethylene glenoid implant, 2 excessively low implantations of the glenoid baseplate, and 1 glenoid loosening). The mean age of the 13 patients (9 women, 4 men) included in this retrospective study at the time of revision was 64 years (range, 50-75 years). The reasons for revision surgery were rotator cuff tear (5, among which 2 were posterosuperior tears, and 3 were tears of the subscapularis), dislocations (5 posterior and 1 anterior, among which 4 had a B2 or C glenoid), suprascapular nerve paralysis (1), and dissociation of the polyethylene (1). The initial TSA was indicated for primary osteoarthritis with a normal cuff (9), primary osteoarthritis with a reparable cuff tear (2), posttraumatic osteoarthritis (1), and chronic dislocation (1). The right dominant shoulder was involved in 10 cases. The mean time interval between the primary TSA and the revision was 15 months (range, 1-61 months).
OPERATIVE TECHNIQUE
PREOPERATIVE PLANNING
Revision of a failed TSA is always a difficult challenge, and evaluation of bone loss on both the humeral and the glenoid sides, as well as the status of the cuff, is mandatory, even with a completely convertible arthroplasty system. The surgeon must be prepared to remove the humeral stem in case reduction of the joint is impossible. We systematically performed standard radiographs (anteroposterior, axillary, and outlet views) and computed tomography (CT) scans in order to assess both the version and positioning, as well as potential signs of loosening of the implants and the status of the cuff (continuity, degree of muscle trophicity, and fatty infiltration). A preoperative leucocyte count, sedimentation rate, and C-reactive protein rates were requested in every revision case, even if a mechanical etiology was strongly suspected.
Continue to: REVISION PROCEDURE
REVISION PROCEDURE
All the implants that had been used in the primary TSAs were Arrow Universal Shoulder Prostheses (FH Orthopedics). All revisions were performed through the previous deltopectoral approach in the beach chair position under general anesthesia with an interscalene block. Adhesions of the deep part of the deltoid were carefully released. The conjoint tendon was released, and the location of the musculocutaneous and axillary nerves was identified before any retractor was placed. In the 10 cases where the subscapularis was intact, it was peeled off the medial border of the bicipital groove to obtain sufficient length for a tension-free reinsertion.
The anatomical head of the humeral implant was disconnected from the stem and removed. All stems were found to be well fixed; there were no cases of loosening or evidence of infection. A circumferential capsular release was systematically carried out. The polyethylene glenoid onlay was then unlocked from the baseplate.
The quality of the fixation of the glenoid baseplate was systematically evaluated; no screw was found to be loose, and the fixation of all baseplates was stable. Therefore, there was no need to revise the glenoid baseplate, even when its position was considered excessively retroverted (Glenoid B2) or high. A glenosphere was impacted on the baseplate, and a polyethylene humeral bearing was then implanted on the humeral stem. The thinnest polyethylene bearing available (number 0) was chosen in all cases, and a size 36 glenosphere was chosen in 12 out of 13 cases. Intraoperative stability of the implant was satisfactory, and no impingement was found posteriorly, anteriorly, or inferiorly.
In one case, the humeral stem was a first-generation humeral implant which was not compatible with the new-generation humeral bearing, and the humeral stem had to be replaced.
In 2 cases, reduction of the RSA was either impossible or felt to be too tight, even after extensive soft-tissue release and resection of the remaining supraspinatus. The main reason for this was an excessively proud humeral stem because of an onlay polyethylene humeral bearing instead of an inlay design. However, removal of the uncemented humeral stem was always possible with no osteotomy or cortical window of the humeral shaft as the humeral stem has been designed with a bone ingrowth surface only on the metaphyseal part with a smooth surface on diaphyseal part. After removal of the stem, a small amount of humeral metaphysis was cut, and a new humeral stem was press-fit in a lower position. This allowed restoration of an appropriate tension of the soft tissue and, therefore, an easier reduction. The subscapularis was medialized and reinserted transosseously when possible with a double-row repair. In 3 cases, the subscapularis was torn and retracted at the level of the glenoid, or impossible to identify to allow its reinsertion.
Continue to: According to our infectious disease department...
According to our infectious disease department, we made a minimum of 5 cultures for each revision case looking for a possible low-grade infection. All cultures in our group are held for 14 days to assess for Propionibacterium acnes.
POSTOPERATIVE MANAGEMENT
A shoulder splint in neutral rotation was used for the first 4 weeks. Passive range of motion (ROM) was started immediately with pendulum exercises and passive anterior elevation. Active assisted and active ROM were allowed after 4 weeks, and physiotherapy was continued for 6 months. Elderly patients were referred to a center of rehabilitation. We found only 1 or 2 positive cultures (Propionibacterium acnes) for 4 patients, and we decided to consider them as a contamination. None of the patients were treated with antibiotics.
CLINICAL AND RADIOLOGICAL ASSESSMENT
Clinical evaluation included pre- and postoperative pain scores (visual analog scale [VAS]), ROM, the Constant-Murley13 score, the Simple Shoulder Test (SST),14 and the subjective shoulder value.15 Subjective satisfaction was assessed by asking the patients at follow-up how they felt compared with before surgery and was graded using a 4-point scale: 1, much better; 2, better; 3, the same; and 4, worse. Radiographic evaluation was performed on pre- and postoperative standard anteroposterior, outlet, and axillary views. Radiographs were reviewed to determine the presence of glenohumeral subluxation, periprosthetic lucency, component shift in position, and scapular notching.
STATISTICAL ANALYSIS
Descriptive statistics are reported as mean (range) for continuous measures and number (percentage) for discrete variables. The Wilcoxon signed-rank test was used for preoperative vs postoperative changes. The alpha level for all tests was set at 0.05 for statistical significance.
RESULTS
CLINICAL OUTCOME
At a mean of 22 months (range, 7-38 months) follow-up after revision, active ROM was significantly improved. Active flexion increased significantly from a mean of 93° (range, 30°-120°) to 138° (range, 95°-170°) (P = 0.021). Active external rotation with the elbow on the side increased significantly from 8° (range, −20°-15°) to 25° (range, −10°-60°) (P = 0.034), and increased with the arm held at 90° abduction from 13° (range, 0°-20°) to 49° (range, 0°-80°) (P = 0.025). Mean pain scores improved from 4.2 to 13.3 points (P < 0.001). VAS improved significantly from 9 to 1 (P < 0.0001). The mean Constant Scores improved from 21 (range, 18-32) to 63 (range, 43-90) (P = 0.006). The final SST was 7 per 12. Subjectively, 4 patients rated their shoulder as much better, 8 as better, and 1 as the same as preoperatively. No intra- or postoperative complications, including infections, were observed. The mean duration of the procedure was 60 minutes (range, 30-75 minutes).
Continue to: RADIOLOGICAL OUTCOME
RADIOLOGICAL OUTCOME
No periprosthetic lucency or shift in component was observed at the last follow-up. There was no scapular notching. No resorption of the tuberosities, and no fractures of the acromion or the scapular spine were observed.
DISCUSSION
In this retrospective study, failure of TSA with a metal-backed glenoid implant was successfully revised to RSA. In 10 patients, the use of a universal platform system allowed an easier conversion without removal of the humeral stem or the glenoid component (Figures 3A-3D). Twelve of the 13 patients were satisfied or very satisfied at the last follow-up. None of the patients were in pain, and the mean Constant score was 63. In all the cases, the glenoid baseplate was not changed. In 3 cases the humeral stem was changed without any fracture of the tuberosities of need for an osteotomy. This greatly simplified the revision procedure, as glenoid revisions can be very challenging. Indeed, it is often difficult to assess precisely preoperatively the remaining glenoid bone stock after removal of the glenoid component and the cement. Many therapeutic options to deal with glenoid loosening have been reported in the literature: glenoid bone reconstruction after glenoid component removal and revision to a hemiarthroplasty (HA),10,16-18 glenoid bone reconstruction after glenoid component removal and revision to a new TSA with a cemented glenoid implant,16,17,19,20 and glenoid reconstruction after glenoid component removal and revision to a RSA.12,21 These authors reported that glenoid reconstruction frequently necessitates an iliac bone graft associated with a special design of the baseplate with a long post fixed into the native glenoid bone. However, sometimes implantation of an uncemented glenoid component can be unstable with a high risk of early mobilization of the implant, and 2 steps may be necessary. Conversion to a HA,10,16-18 or a TSA16,17,19,20 with a new cemented implant have both been associated with poor clinical outcome, with a high rate of recurrent glenoid loosening for the TSAs.

In our retrospective study, we reported no intra- or postoperative complications. Flury and colleagues22 reported a complication rate of 38% in 21 patients after conversion from a TSA to a RSA with a mean follow-up of 46 months. They removed all the components of the prosthesis with a crack or fracture of the humerus and/or the glenoid. Ortmaier and colleagues23 reported a rate of complication of 22.7% during the conversion of TSA to RSA. They did an osteotomy of the humeral diaphysis to extract the stem in 40% of cases and had to remove the glenoid cement in 86% of cases with severe damage of the glenoid bone in 10% of cases. Fewer complications were found in our study, as we did not need any procedure such as humeral osteotomy, cerclage, bone grafting, and/or reconstruction of the glenoid. The short operative time and the absence of extensive soft-tissue dissection, thanks to a standard deltopectoral approach, could explain the absence of infection in our series.
Other authors shared our strategy of a universal convertible system and reported their results in the literature. Castagna and colleagues24 in 2013 reported the clinical and radiological results of conversions of HA or TSA to RSA using a modular, convertible system (SMR Shoulder System, Lima Corporate). In their series, only 8 cases of TSAs were converted to RSA. They preserved, in each case, the humeral stem and the glenoid baseplate. There were no intra- or postoperative complications. The mean VAS score decreased from 8 to 2. Weber-Spickschen and colleague25 reported recently in 2015 the same experience with the same system (SMR Shoulder System). They reviewed 15 conversions of TSAs to RSAs without any removal of the implants at a mean 43-month follow-up. They reported excellent pain relief (VAS decreased from 8 to 1) and improvement in shoulder function with a low rate of complications.
Kany and colleagues26 in 2015 had already reported the advantages of a shoulder platform system for revisions. In their series, the authors included cases of failure of HAs and TSAs with loose cemented glenoids and metal-backed glenoids. The clinical and radiological results were similar, with a final Constant score of 60 (range, 42-85) and a similar rate of humeral stems which had to be changed (24%). These stems were replaced either because they were too proud or because there was not enough space to add an onlay polyethylene socket.
Continue to: Despite the encouraging results...
Despite the encouraging results reported in this study, there are some limitations. Firstly, no control group was used. Attempting to address this issue, we compared our results with the literature. Secondly, the number of patients in our study was small. Finally, the follow-up duration (mean 22 months) did not provide long-term outcomes.
CONCLUSION
This retrospective study shows that a complete convertible system facilitates conversion of TSAs to RSAs with excellent pain relief and a significant improvement in shoulder function. A platform system on both the humeral and the glenoid side reduces the operative time of the conversion with a low risk of complications.
ABSTRACT
Removal of a cemented glenoid component often leads to massive glenoid bone loss, which makes it difficult to implant a new glenoid baseplate. The purpose of this study was to demonstrate the feasibility of revisions with a completely convertible system and to report clinical and radiographic results of a retrospective review of 13 cases.
Between 2003 and 2011, 104 primary total shoulder arthroplasties (TSAs) were performed with an uncemented glenoid component in our group. Of these patients, 13 (average age, 64 years) were revised to reverse shoulder arthroplasty (RSA) using a modular convertible platform system and were included in this study. Average follow-up after revision was 22 months. Outcome measures included pain, range of motion, Constant-Murley scores, Simple Shoulder Tests, and subjective shoulder values. Active flexion increased significantly from a mean of 93° (range, 30°-120°) to 138° (range, 95°-170°) (P = 0.021), and active external rotation increased significantly from 8° (range, −20°-15°) to 25° (range, −10°-60°). Mean pain scores significantly improved from 4.2 to 13.3 points. The mean Constant Scores improved from 21 (range, 18-32) to 63 (range, 43-90). Subjectively, 12 patients rated their shoulder as better or much better than preoperatively. This retrospective study shows that a complete convertible system facilitates conversion of TSAs to RSAs with excellent pain relief and a significant improvement in shoulder function.
Continue to: Polyethylene glenoid components...
Polyethylene glenoid components are the gold standard in anatomic total shoulder arthroplasty (TSA). However, even though TSA survivorship exceeds 95% at 10-year follow-up,1 glenoid component loosening remains the main complication and the weak link in these implants. This complication accounts for 25% of all complications related to TSA in the literature.2 In most cases, glenoid component loosening is not isolated but combined with a rotator cuff tear, glenohumeral instability, or component malposition.

We hypothesized that a completely convertible platform system on both the humeral and the glenoid side could facilitate the revision of a failed TSA to a RSA. This would enable the surgeon to leave the humeral stem and the glenoid baseplate in place, avoiding the difficulty of stem removal and the reimplantation of a glenoid component, especially in osteoporotic glenoid bone and elderly patients. The revision procedure would then only consist of replacing the humeral head by a metallic tray and polyethylene bearing on the humeral side and by impacting a glenosphere on the glenoid baseplate (Figures 2A, 2B).

The purpose of this study was to demonstrate the feasibility of revisions with this completely convertible system and to report clinical and radiographic results of a retrospective review of 13 cases.
MATERIALS AND METHODS
PATIENT SELECTION
Between 2003 and 2011, 104 primary TSAs were performed with an uncemented glenoid component in our group. Of these patients, 18 underwent revision (17.3%). Among these 18 patients, 13 were revised to RSA using a modular convertible platform system and were included in this study, while 5 patients were revised to another TSA (2 dissociations of the polyethylene glenoid implant, 2 excessively low implantations of the glenoid baseplate, and 1 glenoid loosening). The mean age of the 13 patients (9 women, 4 men) included in this retrospective study at the time of revision was 64 years (range, 50-75 years). The reasons for revision surgery were rotator cuff tear (5, among which 2 were posterosuperior tears, and 3 were tears of the subscapularis), dislocations (5 posterior and 1 anterior, among which 4 had a B2 or C glenoid), suprascapular nerve paralysis (1), and dissociation of the polyethylene (1). The initial TSA was indicated for primary osteoarthritis with a normal cuff (9), primary osteoarthritis with a reparable cuff tear (2), posttraumatic osteoarthritis (1), and chronic dislocation (1). The right dominant shoulder was involved in 10 cases. The mean time interval between the primary TSA and the revision was 15 months (range, 1-61 months).
OPERATIVE TECHNIQUE
PREOPERATIVE PLANNING
Revision of a failed TSA is always a difficult challenge, and evaluation of bone loss on both the humeral and the glenoid sides, as well as the status of the cuff, is mandatory, even with a completely convertible arthroplasty system. The surgeon must be prepared to remove the humeral stem in case reduction of the joint is impossible. We systematically performed standard radiographs (anteroposterior, axillary, and outlet views) and computed tomography (CT) scans in order to assess both the version and positioning, as well as potential signs of loosening of the implants and the status of the cuff (continuity, degree of muscle trophicity, and fatty infiltration). A preoperative leucocyte count, sedimentation rate, and C-reactive protein rates were requested in every revision case, even if a mechanical etiology was strongly suspected.
Continue to: REVISION PROCEDURE
REVISION PROCEDURE
All the implants that had been used in the primary TSAs were Arrow Universal Shoulder Prostheses (FH Orthopedics). All revisions were performed through the previous deltopectoral approach in the beach chair position under general anesthesia with an interscalene block. Adhesions of the deep part of the deltoid were carefully released. The conjoint tendon was released, and the location of the musculocutaneous and axillary nerves was identified before any retractor was placed. In the 10 cases where the subscapularis was intact, it was peeled off the medial border of the bicipital groove to obtain sufficient length for a tension-free reinsertion.
The anatomical head of the humeral implant was disconnected from the stem and removed. All stems were found to be well fixed; there were no cases of loosening or evidence of infection. A circumferential capsular release was systematically carried out. The polyethylene glenoid onlay was then unlocked from the baseplate.
The quality of the fixation of the glenoid baseplate was systematically evaluated; no screw was found to be loose, and the fixation of all baseplates was stable. Therefore, there was no need to revise the glenoid baseplate, even when its position was considered excessively retroverted (Glenoid B2) or high. A glenosphere was impacted on the baseplate, and a polyethylene humeral bearing was then implanted on the humeral stem. The thinnest polyethylene bearing available (number 0) was chosen in all cases, and a size 36 glenosphere was chosen in 12 out of 13 cases. Intraoperative stability of the implant was satisfactory, and no impingement was found posteriorly, anteriorly, or inferiorly.
In one case, the humeral stem was a first-generation humeral implant which was not compatible with the new-generation humeral bearing, and the humeral stem had to be replaced.
In 2 cases, reduction of the RSA was either impossible or felt to be too tight, even after extensive soft-tissue release and resection of the remaining supraspinatus. The main reason for this was an excessively proud humeral stem because of an onlay polyethylene humeral bearing instead of an inlay design. However, removal of the uncemented humeral stem was always possible with no osteotomy or cortical window of the humeral shaft as the humeral stem has been designed with a bone ingrowth surface only on the metaphyseal part with a smooth surface on diaphyseal part. After removal of the stem, a small amount of humeral metaphysis was cut, and a new humeral stem was press-fit in a lower position. This allowed restoration of an appropriate tension of the soft tissue and, therefore, an easier reduction. The subscapularis was medialized and reinserted transosseously when possible with a double-row repair. In 3 cases, the subscapularis was torn and retracted at the level of the glenoid, or impossible to identify to allow its reinsertion.
Continue to: According to our infectious disease department...
According to our infectious disease department, we made a minimum of 5 cultures for each revision case looking for a possible low-grade infection. All cultures in our group are held for 14 days to assess for Propionibacterium acnes.
POSTOPERATIVE MANAGEMENT
A shoulder splint in neutral rotation was used for the first 4 weeks. Passive range of motion (ROM) was started immediately with pendulum exercises and passive anterior elevation. Active assisted and active ROM were allowed after 4 weeks, and physiotherapy was continued for 6 months. Elderly patients were referred to a center of rehabilitation. We found only 1 or 2 positive cultures (Propionibacterium acnes) for 4 patients, and we decided to consider them as a contamination. None of the patients were treated with antibiotics.
CLINICAL AND RADIOLOGICAL ASSESSMENT
Clinical evaluation included pre- and postoperative pain scores (visual analog scale [VAS]), ROM, the Constant-Murley13 score, the Simple Shoulder Test (SST),14 and the subjective shoulder value.15 Subjective satisfaction was assessed by asking the patients at follow-up how they felt compared with before surgery and was graded using a 4-point scale: 1, much better; 2, better; 3, the same; and 4, worse. Radiographic evaluation was performed on pre- and postoperative standard anteroposterior, outlet, and axillary views. Radiographs were reviewed to determine the presence of glenohumeral subluxation, periprosthetic lucency, component shift in position, and scapular notching.
STATISTICAL ANALYSIS
Descriptive statistics are reported as mean (range) for continuous measures and number (percentage) for discrete variables. The Wilcoxon signed-rank test was used for preoperative vs postoperative changes. The alpha level for all tests was set at 0.05 for statistical significance.
RESULTS
CLINICAL OUTCOME
At a mean of 22 months (range, 7-38 months) follow-up after revision, active ROM was significantly improved. Active flexion increased significantly from a mean of 93° (range, 30°-120°) to 138° (range, 95°-170°) (P = 0.021). Active external rotation with the elbow on the side increased significantly from 8° (range, −20°-15°) to 25° (range, −10°-60°) (P = 0.034), and increased with the arm held at 90° abduction from 13° (range, 0°-20°) to 49° (range, 0°-80°) (P = 0.025). Mean pain scores improved from 4.2 to 13.3 points (P < 0.001). VAS improved significantly from 9 to 1 (P < 0.0001). The mean Constant Scores improved from 21 (range, 18-32) to 63 (range, 43-90) (P = 0.006). The final SST was 7 per 12. Subjectively, 4 patients rated their shoulder as much better, 8 as better, and 1 as the same as preoperatively. No intra- or postoperative complications, including infections, were observed. The mean duration of the procedure was 60 minutes (range, 30-75 minutes).
Continue to: RADIOLOGICAL OUTCOME
RADIOLOGICAL OUTCOME
No periprosthetic lucency or shift in component was observed at the last follow-up. There was no scapular notching. No resorption of the tuberosities, and no fractures of the acromion or the scapular spine were observed.
DISCUSSION
In this retrospective study, failure of TSA with a metal-backed glenoid implant was successfully revised to RSA. In 10 patients, the use of a universal platform system allowed an easier conversion without removal of the humeral stem or the glenoid component (Figures 3A-3D). Twelve of the 13 patients were satisfied or very satisfied at the last follow-up. None of the patients were in pain, and the mean Constant score was 63. In all the cases, the glenoid baseplate was not changed. In 3 cases the humeral stem was changed without any fracture of the tuberosities of need for an osteotomy. This greatly simplified the revision procedure, as glenoid revisions can be very challenging. Indeed, it is often difficult to assess precisely preoperatively the remaining glenoid bone stock after removal of the glenoid component and the cement. Many therapeutic options to deal with glenoid loosening have been reported in the literature: glenoid bone reconstruction after glenoid component removal and revision to a hemiarthroplasty (HA),10,16-18 glenoid bone reconstruction after glenoid component removal and revision to a new TSA with a cemented glenoid implant,16,17,19,20 and glenoid reconstruction after glenoid component removal and revision to a RSA.12,21 These authors reported that glenoid reconstruction frequently necessitates an iliac bone graft associated with a special design of the baseplate with a long post fixed into the native glenoid bone. However, sometimes implantation of an uncemented glenoid component can be unstable with a high risk of early mobilization of the implant, and 2 steps may be necessary. Conversion to a HA,10,16-18 or a TSA16,17,19,20 with a new cemented implant have both been associated with poor clinical outcome, with a high rate of recurrent glenoid loosening for the TSAs.

In our retrospective study, we reported no intra- or postoperative complications. Flury and colleagues22 reported a complication rate of 38% in 21 patients after conversion from a TSA to a RSA with a mean follow-up of 46 months. They removed all the components of the prosthesis with a crack or fracture of the humerus and/or the glenoid. Ortmaier and colleagues23 reported a rate of complication of 22.7% during the conversion of TSA to RSA. They did an osteotomy of the humeral diaphysis to extract the stem in 40% of cases and had to remove the glenoid cement in 86% of cases with severe damage of the glenoid bone in 10% of cases. Fewer complications were found in our study, as we did not need any procedure such as humeral osteotomy, cerclage, bone grafting, and/or reconstruction of the glenoid. The short operative time and the absence of extensive soft-tissue dissection, thanks to a standard deltopectoral approach, could explain the absence of infection in our series.
Other authors shared our strategy of a universal convertible system and reported their results in the literature. Castagna and colleagues24 in 2013 reported the clinical and radiological results of conversions of HA or TSA to RSA using a modular, convertible system (SMR Shoulder System, Lima Corporate). In their series, only 8 cases of TSAs were converted to RSA. They preserved, in each case, the humeral stem and the glenoid baseplate. There were no intra- or postoperative complications. The mean VAS score decreased from 8 to 2. Weber-Spickschen and colleague25 reported recently in 2015 the same experience with the same system (SMR Shoulder System). They reviewed 15 conversions of TSAs to RSAs without any removal of the implants at a mean 43-month follow-up. They reported excellent pain relief (VAS decreased from 8 to 1) and improvement in shoulder function with a low rate of complications.
Kany and colleagues26 in 2015 had already reported the advantages of a shoulder platform system for revisions. In their series, the authors included cases of failure of HAs and TSAs with loose cemented glenoids and metal-backed glenoids. The clinical and radiological results were similar, with a final Constant score of 60 (range, 42-85) and a similar rate of humeral stems which had to be changed (24%). These stems were replaced either because they were too proud or because there was not enough space to add an onlay polyethylene socket.
Continue to: Despite the encouraging results...
Despite the encouraging results reported in this study, there are some limitations. Firstly, no control group was used. Attempting to address this issue, we compared our results with the literature. Secondly, the number of patients in our study was small. Finally, the follow-up duration (mean 22 months) did not provide long-term outcomes.
CONCLUSION
This retrospective study shows that a complete convertible system facilitates conversion of TSAs to RSAs with excellent pain relief and a significant improvement in shoulder function. A platform system on both the humeral and the glenoid side reduces the operative time of the conversion with a low risk of complications.
1. Brenner BC, Ferlic DC, Clayton ML, Dennis DA. Survivorship of unconstrained total shoulder arthroplasty. J Bone Joint Surg Am. 1989;71(9):1289-1296.
2. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541. doi:10.1016/j.jse.2012.06.001.
3. Chin PY, Sperling JW, Cofield RH, Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15(1):19-22. doi:10.1016/j.jse.2005.05.005.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement surgery. J Bone Joint Surg Am. 1996;78(4):603-616.
6. Gohlke F, Rolf O. Revision of failed fracture hemiarthroplasties to reverse total shoulder prosthesis through the transhumeral approach: method incorporating a pectoralis-major-pedicled bone window. Oper Orthop Traumatol. 2007;19(2):185-208. doi:10.1007/s00064-007-1202-x.
7. Goldberg SH, Cohen MS, Young M, Bradnock B. Thermal tissue damage caused by ultrasonic cement removal from the humerus. J Bone Joint Surg Am. 2005;87(3):583-591. doi:10.2106/JBJS.D.01966.
8. Sperling JW, Cofield RH. Humeral windows in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(3):258-263. doi:10.1016/j.jse.2004.09.004.
9. Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91(1):119-127. doi:10.2106/JBJS.H.00094.
10. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012;21(6):765-771. doi:10.1016/j.jse.2011.08.069.
11. Kelly JD 2nd, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525. doi:10.1016/j.jse.2011.11.021.
12. Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21(3):342-349. doi:10.1016/j.jse.2011.05.021.
13. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
14. Matsen FA 3rd, Ziegler DW, DeBartolo SE. Patient self-assessment of health status and function in glenohumeral degenerative joint disease. J Shoulder Elbow Surg. 1995;4(5):345-351.
15. Gilbart MK, Gerber C. Comparison of the subjective shoulder value and the Constant score. J Shoulder Elbow Surg. 2007;16(6):717-721. doi:10.1016/j.jse.2007.02.123.
16. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224. doi:10.1067/mse.2001.113961.
17. Cofield RH, Edgerton BC. Total shoulder arthroplasty: complications and revision surgery. Instr Course Lect. 1990;39:449-462.
18. Neyton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid corticocancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg. 2006;15(2):173-179. doi:10.1016/j.jse.2005.07.010.
19. Bonnevialle N, Melis B, Neyton L, et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22(6):745-751. doi:10.1016/j.jse.2012.08.009.
20. Rodosky MW, Bigliani LU. Indications for glenoid resurfacing in shoulder arthroplasty. J Shoulder Elbow Surg. 1996;5(3):231-248.
21. Bateman E, Donald SM. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg. 2012;21(7):925-934. doi:10.1016/j.jse.2011.07.009.
22. Flury MP, Frey P, Goldhahn J, Schwyzer HK, Simmen BR. Reverse shoulder arthroplasty as a salvage procedure for failed conventional shoulder replacement due to cuff failure--midterm results. Int Orthop. 2011;35(1):53-60. doi:10.1007/s00264-010-0990-z.
23. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2013;38(3):1-7. doi:10.1007/s00264-013-2139-3.
24. Castagna A, Delcogliano M, de Caro F, et al. Conversion of shoulder arthroplasty to reverse implants: clinical and radiological results using a modular system. Int Orthop. 2013;37(7):1297-1305. doi:10.1007/s00264-013-1907-4.
25. Weber-Spickschen TS, Alfke D, Agneskirchner JD. The use of a modular system to convert an anatomical total shoulder arthroplasty to a reverse shoulder arthroplasty: Clinical and radiological results. Bone Joint J. 2015;97-B(12):1662-1667. doi:10.1302/0301-620X.97B12.35176.
26. Kany J, Amouyel T, Flamand O, Katz D, Valenti P. A convertible shoulder system: is it useful in total shoulder arthroplasty revisions? Int Orthop. 2015;39(2):299-304. doi:10.1007/s00264-014-2563-z.
1. Brenner BC, Ferlic DC, Clayton ML, Dennis DA. Survivorship of unconstrained total shoulder arthroplasty. J Bone Joint Surg Am. 1989;71(9):1289-1296.
2. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541. doi:10.1016/j.jse.2012.06.001.
3. Chin PY, Sperling JW, Cofield RH, Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15(1):19-22. doi:10.1016/j.jse.2005.05.005.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement surgery. J Bone Joint Surg Am. 1996;78(4):603-616.
6. Gohlke F, Rolf O. Revision of failed fracture hemiarthroplasties to reverse total shoulder prosthesis through the transhumeral approach: method incorporating a pectoralis-major-pedicled bone window. Oper Orthop Traumatol. 2007;19(2):185-208. doi:10.1007/s00064-007-1202-x.
7. Goldberg SH, Cohen MS, Young M, Bradnock B. Thermal tissue damage caused by ultrasonic cement removal from the humerus. J Bone Joint Surg Am. 2005;87(3):583-591. doi:10.2106/JBJS.D.01966.
8. Sperling JW, Cofield RH. Humeral windows in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(3):258-263. doi:10.1016/j.jse.2004.09.004.
9. Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91(1):119-127. doi:10.2106/JBJS.H.00094.
10. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012;21(6):765-771. doi:10.1016/j.jse.2011.08.069.
11. Kelly JD 2nd, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525. doi:10.1016/j.jse.2011.11.021.
12. Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21(3):342-349. doi:10.1016/j.jse.2011.05.021.
13. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
14. Matsen FA 3rd, Ziegler DW, DeBartolo SE. Patient self-assessment of health status and function in glenohumeral degenerative joint disease. J Shoulder Elbow Surg. 1995;4(5):345-351.
15. Gilbart MK, Gerber C. Comparison of the subjective shoulder value and the Constant score. J Shoulder Elbow Surg. 2007;16(6):717-721. doi:10.1016/j.jse.2007.02.123.
16. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224. doi:10.1067/mse.2001.113961.
17. Cofield RH, Edgerton BC. Total shoulder arthroplasty: complications and revision surgery. Instr Course Lect. 1990;39:449-462.
18. Neyton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid corticocancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg. 2006;15(2):173-179. doi:10.1016/j.jse.2005.07.010.
19. Bonnevialle N, Melis B, Neyton L, et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22(6):745-751. doi:10.1016/j.jse.2012.08.009.
20. Rodosky MW, Bigliani LU. Indications for glenoid resurfacing in shoulder arthroplasty. J Shoulder Elbow Surg. 1996;5(3):231-248.
21. Bateman E, Donald SM. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg. 2012;21(7):925-934. doi:10.1016/j.jse.2011.07.009.
22. Flury MP, Frey P, Goldhahn J, Schwyzer HK, Simmen BR. Reverse shoulder arthroplasty as a salvage procedure for failed conventional shoulder replacement due to cuff failure--midterm results. Int Orthop. 2011;35(1):53-60. doi:10.1007/s00264-010-0990-z.
23. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2013;38(3):1-7. doi:10.1007/s00264-013-2139-3.
24. Castagna A, Delcogliano M, de Caro F, et al. Conversion of shoulder arthroplasty to reverse implants: clinical and radiological results using a modular system. Int Orthop. 2013;37(7):1297-1305. doi:10.1007/s00264-013-1907-4.
25. Weber-Spickschen TS, Alfke D, Agneskirchner JD. The use of a modular system to convert an anatomical total shoulder arthroplasty to a reverse shoulder arthroplasty: Clinical and radiological results. Bone Joint J. 2015;97-B(12):1662-1667. doi:10.1302/0301-620X.97B12.35176.
26. Kany J, Amouyel T, Flamand O, Katz D, Valenti P. A convertible shoulder system: is it useful in total shoulder arthroplasty revisions? Int Orthop. 2015;39(2):299-304. doi:10.1007/s00264-014-2563-z.
TAKE-HOME POINTS
- Full polyethylene is the gold standard, but the revision of glenoid loosening leads a difficult reconstruction of a glenoid bone.
- A complete convertible system facilitates the revision and decreases the rate of complications.
- The functional and subjective results of the revision are good.
- During the revision, the metalback was well fixed without any sign of loosening.
- In 3 cases the humeral stem was changed; in 2 cases there was no space to reduce (onlay system) and in 1 case it was an older design, nonadapted.
Use of a Small-Bore Needle Arthroscope to Diagnose Intra-Articular Knee Pathology: Comparison With Magnetic Resonance Imaging
ABSTRACT
The use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). The mi-eye+TM (Trice Medical) technology is a small-bore needle unit for in-office arthroscopy. We conducted a pilot study comparing the mi-eye+TM unit with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to MRI for the diagnosis of intra-articular pathology of the knee.
This prospective, multicenter, observational study was approved by the Institutional Review Board. There were 106 patients (53 males, 53 females) in the study. MRIs were interpreted by musculoskeletally trained radiologists. The study was conducted in the operating room using the mi-eye+TM device. The mi-eye+ TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings.
The mi-eye+TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 41.7%; P < .0001).
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI, but our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology.
Continue to: Surgical arthroscopy is the gold standard...
Surgical arthroscopy is the gold standard for the diagnosis of intra-articular knee pathologies. Nevertheless, the use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). Although MRI is considered the standard diagnostic tool for acute and chronic soft-tissue injuries of the knee, its use is not without contraindication and some potential inconveniences. Contraindications to MRI are well documented. In terms of inconvenience, MRI usually requires a separate visit followed by another visit to the prescribing physician. In addition, required interpretation by a radiologist may lead to a delay in care and increase in cost.
In the early 1990s, in-office needle arthroscopy was described as a viable means of diagnosing pathologies and obtaining synovial biopsies from the knee.1-3 Initial results were good, and the procedures had very low complication rates. Nevertheless, in-office arthroscopy of the knee is not yet widely performed, likely given concerns about the technical difficulties of in-office arthroscopy, the potential for patient discomfort, and the cumbersomeness of in-office arthroscopy units. However, significant advances have been made in the resolution capability of small-bore needle arthroscopy, resulting in much less painful procedures. Additionally, the early hardware designs, which mimicked operating room setups using towers, fluid irrigation systems, and larger arthroscopes, have been replaced with small-needle arthroscopes that use syringes for irrigation and tablet computers for visualization (Figures 1A, 1B).
The mi-eye+TM technology (Trice Medical) is a small-bore needle unit for in-office arthroscopy with digital optics that does not need an irrigation tower. We conducted a pilot study of the sensitivity and specificity of the mi-eye+TM unit in comparison with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to the standard of care (MRI) for the diagnosis of intra-articular pathology of the knee.
METHODS
Central regulatory approval for this prospective, multicenter, observational study was obtained from the Western Institutional Review Board for 3 of the sites, and 1 institution required and was granted internal Institutional Review Board approval.
The study was performed by 4 sports medicine orthopedic surgeons experienced in using the mi-eye+TM in-office arthroscope. Patients were enrolled from December 2015 through June 2016. Inclusion criteria were an indication for an arthroscopic procedure of the knee based on history, physical examination, and MRI findings. Patients were excluded from the study if there were any contraindications to completing an MRI. Acute hemarthroses of the knee or active systemic infections were also excluded. Once a patient was identified as meeting the criteria for participation, informed consent was obtained. Of the 113 patients who enrolled, 7 did not have a complete study dataset available, leaving 106 patients (53 males, 53 females) in the study. Mean age was 47 years (range, 18-82 years).
Continue to: A test result form was used...
A test result form was used to record mi-eye+TM, surgical arthroscopy, and MRI results. This form required a “positive” or “negative” result for all of several diagnoses: medial and lateral meniscal tears, intra-articular loose body, osteoarthritis (OA), osteochondritis dissecans (OCD), and tears of the anterior and posterior cruciate ligaments (ACL, PCL). MRI was performed at a variety of imaging facilities, but the images were interpreted by musculoskeletally trained radiologists.
The study was conducted in the operating room. After the patient was appropriately anesthetized, and the extremity prepared and draped, the mi-eye+TM procedure was performed immediately prior to surgical arthroscopy. A tourniquet was not used. At surgeon discretion, medial, lateral, or both approaches were used with the mi-eye+TM, and diagnostic arthroscopy was performed. During the procedure, the mi-eye+TM was advanced into the knee. Once in the synovial compartment, the external 14-gauge needle was retracted, exposing the unit’s optics. Visualization was improved by injecting normal saline through the lure lock in the mi-eye+TM needle arthroscope. An average of 20 mL of saline was used, though the amount varied with surgeon discretion. Subsequently, the surgeon visualized structures in the knee and documented all findings.
At the end of the mi-eye+TM procedure, the scheduled surgical arthroscopy was performed. After the surgical procedure, if there were no issues or complications, the patient was discharged from the study. No follow-up was required for the study, as arthroscopic findings served as the conclusive diagnosis for each patient, and no interventions were being studied. There were no complications related to use of the mi-eye+TM.
The mi-eye+TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings. When a test had no false-positive or false-negative findings in comparison with surgical arthroscopy, it was identified as having complete accuracy for all intra-articular knee pathologies. For these methods, the 95% confidence interval was determined based on binomial distribution.
RESULTS
The mi-eye+ TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and surgical arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. On the other hand, MRI demonstrated both false-negative and false-positive results, failing to reveal some aspect of the knee’s pathology for 31 patients, and potentially overcalling some aspect of the knee’s pathology among 18 patients.
Continue to: The pathology most frequently...
The pathology most frequently identified in the study was a meniscal tear. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 87.5%; P < .0002). The difference in specificity resulted from the false MRI diagnosis of a meniscal tear among 24 patients, who were found to have no tear by both mi-eye+TM and surgical arthroscopy.
Table 1. Raw Data of mi-eye+TM and Magnetic Resonance Imaging Findings
| Data | True-Positive | False-Negative | False-Negative | True-Negative |
| mi-eye+TM | ||||
| Medial meniscal tear | 68 | 3 | 0 | 35 |
| Lateral meniscal tear | 32 | 5 | 0 | 69 |
| Any meniscal tear | 100 | 8 | 0 | 104 |
| Intra-articular loose body | 13 | 2 | 0 | 87 |
| Osteoarthritis | 31 | 2 | 00 | 73 |
| Osteochondritis dissecans | 8 | 2 | 0 | 97 |
| Anterior cruciate ligament tear | 16 | 0 | 0 | 90 |
| Posterior cruciate ligament tear | 0 | 0 | 0 | 106 |
| All pathologies | 168 | 14 | 0 | 557 |
| Magnetic resonance imaging | ||||
| Medial meniscal tear | 62 | 9 | 6 | 29 |
| Lateral meniscal tear | 22 | 15 | 7 | 62 |
| Any meniscal tear | 84 | 24 | 13 | 91 |
| Intra-articular loose body | 3 | 12 | 0 | 87 |
| Osteoarthritis | 26 | 7 | 8 | 65 |
| Osteochondritis dissecans | 5 | 5 | 4 | 93 |
| Anterior cruciate ligament tear | 14 | 2 | 3 | 87 |
| Posterior cruciate ligament tear | 0 | 0 | 2 | 104 |
| All pathologies | 132 | 500 | 30 | 527 |
The second most frequent pathology was an intra-articular loose body. The mi-eye+TM was more sensitive than MRI in identifying loose bodies (86.7% vs 20%; P = .0007). The specificity of the mi-eye+TM and the specificity of MRI were equivalent in diagnosing loose bodies (100%). Table 1 and Table 2 show the complete set of diagnoses and associated diagnostic profiles.
Table 2. Diagnostic Profiles: Sensitivity and Specificity of mi-eye+TM and Magnetic Resonance Imaging
| Patient Group | mi-eye+TM | MRI | |||
| Estimate, % | CI, % | Estimate, % | CI, % | Pa | |
| Sensitivity | |||||
| Medial meniscal tear | 95.77 | 88.1-99.1 | 87.32 | 77.3-94.0 | .0129 |
| Lateral meniscal tear | 86.49 | 71.2-95.5 | 59.46 | 42.1-75.3 | .0172 |
| Any meniscal tear | 92.59 | 85.9-96.8 | 77.78 | 68.8-85.2 | .0035 |
| Intra-articular loose body | 86.70 | 59.5-98.3 | 20 | 4.3-48.1 | .0006789 |
| Osteoarthritis | 93.90 | 79.8-99.3 | 78.80 | 61.1-91.0 | .1487 |
| Osteochondritis dissecans | 80.00 | 44.4-97.5 | 50 | 18.7-81.3 | .3498 |
| Anterior crucitate ligament tear | 100.00 | 79.4-100.0 | 87.50 | 61.7-98.4 | .4839 |
| Posterior cruciate ligament tear | N/A | N/A | N/A | N/A | N/A |
| Specificity | |||||
| Medial meniscal tear | 100.00 | 90.0-100.0 | 82.86 | 66.4-93.4 | .0246 |
| Lateral meniscal tear | 100.00 | 94.8-100.0 | 89.86 | 80.2-95.8 | .0133 |
| Any meniscal tear | 100.00 | 96.5-100.0 | 87.50 | 79.6-93.2 | .0002 |
| Intra-articular loose body | 100.00 | 95.9-100.0 | 100.00 | 95.9-100.0 | 1 |
| Osteoarthritis | 100.00 | 95.1-100.0 | 89.00 | 79.5-95.1 | .006382 |
| Osteochondritis dissecans | 100.00 | 96.3-100.0 | 95.90 | 89.8-98.9 | .1211 |
| Anterior cruciate ligament tear | 100.00 | 96.0-100.0 | 96.70 | 90.6-99.3 | .2458 |
| Posterior crttuciate ligament tear | 100.00 | 96.6-100.0 | 98.10 | 93.4-99.8 | .4976 |
aBold P values are significant. Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging; N/A, not applicable.
DISCUSSION
The overall accuracy of the mi-eye+TM was superior to that of MRI relative to the arthroscopic gold standard in this pilot study. Other studies have demonstrated the accuracy, feasibility, and cost-efficacy of in-office arthroscopy. However, likely because of the cumbersomeness of in-office arthroscopy equipment and the potential for patient discomfort, the technique is not yet standard in the field. Recent advances in small-bore technology, digital optics, and ergonomics have addressed the difficulties associated with in-office arthroscopy, facilitating a faster and more efficient procedure. Our goal in this study was to evaluate the diagnostic capability of the mi-eye+TM in-office arthroscopy unit, which features a small bore, digital optics, and functionality without an irrigation tower.
This study of 106 patients demonstrated equivalent or better accuracy of the mi-eye+TM relative to MRI when compared with the gold standard of surgical arthroscopy. This was not surprising given that both the mi-eye+TM and surgical arthroscopy are based on direct visualization of intra-articular pathology. The mi-eye+TM unit identified more meniscal tears, intra-articular loose bodies, ACL tears, and OCD lesions than MRI did, and with enough power to demonstrate statistically significant improved sensitivity for meniscal tears and loose bodies. Furthermore, MRI demonstrated false-positive meniscal tears, ACL tears, OCD lesions, and OA, whereas the mi-eye+TM did not demonstrate any false-positive results in comparison with surgical arthroscopy. This study demonstrated statistically significant improved specificity of the mi-eye+ compared with MRI in the diagnosis of meniscal tears and OA.
There are several limitations to our study. We refer to it as a pilot study because it was performed in a standard operating room. Before taking the technology to an outpatient setting, we wanted to confirm efficacy and safety in an operating room. However, the techniques used in this study are readily transferable to the outpatient clinic setting and to date have been used in more than 2000 cases.
Continue to: The specificity of MRI...
The specificity of MRI for meniscal tears was unexpectedly low compared with previous studies, which may reflect the multi-institution, multi-surgeon, multi-radiologist involvement in MRI interpretation.4-10 MRI was performed at a variety of institutions without a standardized protocol. This lack of standardization of image capture and interpretation may have contributed to the suboptimal performance of MRI, falsely decreasing the potential ideal specificity for meniscal tears. Although this study may have underestimated the specificity of MRI for meniscal tears, we think the mi-eye+TM and MRI results reported here reflect the findings of standard practice, without the standardization usually applied in studies. For example, a study of 139 knee MRI reports at 14 different institutions confirmed arthroscopic findings and concluded that 37% of the operations supported by a significant MRI finding were unjustified.11 The authors attributed the rate of false-positive MRI findings to the wide variety of places where patients had their MRIs performed, and the subsequent variation in quality of imaging and MRI reader skill level.11
Before inserting the mi-eye+TM needle arthroscope, the surgeons had a working diagnosis of the pathology based on their clinical examination and MRI results. Clearly, this introduced a bias. Further studies will be conducted in a prospective, blinded manner to address this limitation.
Although studies of in-office arthroscopy technology date to the 1990s, there is an overall lack of data comparing in-office arthroscopy with MRI. Halbrecht and Jackson2 conducted a study of 20 knee patients with both MRI and in-office needle arthroscopy. Overall, MRI was poor in detecting cartilage defects, with sensitivity of 34.6%, using the in-office arthroscopy as the confirmatory diagnosis. Although the authors did not compare in-office diagnoses with surgical arthroscopic findings, they concluded that office arthroscopy is an accurate and cost-efficient alternative to MRI in diagnostic evaluation of knee patients. Xerogeanes and colleagues12 studied 110 patients in a prospective, blinded, multicenter trial comparing a minimally invasive office-based arthroscopy with MRI, using surgical arthroscopy as the confirmatory diagnosis. They concluded that the office-based arthroscope was statistically equivalent to diagnostic surgical arthroscopy and that it outperformed MRI in helping make accurate diagnoses. The authors applied a cost analysis to their findings and determined that office-based arthroscopy could result in an annual potential savings of $177 million for the healthcare system.12
Modern imaging sequences on high-Tesla MRI machines provide excellent visualization. Nevertheless, a significant number of patients do not undergo MRI, owing to time constraints, contraindications, body habitus, or anxiety/claustrophobia. Our study results confirmed that doctors treating such patients now have a viable alternative to help diagnose pathology.
CONCLUSION
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at the time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI; our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology. More studies of the mi-eye+TM device in a clinical setting are warranted.
1. Baeten D, Van den Bosch F, Elewaut D, Stuer A, Veys EM, De Keyser F. Needle arthroscopy of the knee with synovial biopsy sampling: technical experience in 150 patients. Clin Rheumatol. 1999;18(6):434-441.
2. Halbrecht J, Jackson D. Office arthroscopy: a diagnostic alternative. Arthroscopy. 1992;8(3):320-326.
3. Batcheleor R, Henshaw K, Astin P, Emery P, Reece R, Leeds DM. Rheumatological needle arthroscopy: a 5-year follow up of safety and efficacy. Arthritis Rheum Ann Sci Meet Abstr. 2001;(9 suppl).
4. Barronian AD, Zoltan JD, Bucon KA. Magnetic resonance imaging of the knee: correlation with arthroscopy. Arthroscopy. 1989;5(3):187-191.
5. Crues JV 3rd, Ryu R, Morgan FW. Meniscal pathology. The expanding role of magnetic resonance imaging. Clin Orthop Relat Res. 1990;(252):80-87.
6. Raunest J, Oberle K, Leohnert J, Hoetzinger H. The clinical value of magnetic resonance imaging in the evaluation of meniscal disorders. J Bone Joint Surg Am. 1991;73(1):11-16.
7. Spiers AS, Meagher T, Ostlere SJ, Wilson DJ, Dodd CA. Can MRI of the knee affect arthroscopic practice? A prospective study of 58 patients. J Bone Joint Surg Br. 1993;75(1):49-52.
8. O’Shea KJ, Murphy KP, Heekin RD, Herzwurm PJ. The diagnostic accuracy of history, physical examination, and radiographs in the evaluation of traumatic knee disorders. Am J Sports Med. 1996;24(2):164-167.
9. Ben-Galim P, Steinberg EL, Amir H, Ash N, Dekel S, Arbel R. Accuracy of magnetic resonance imaging of the knee and unjustified surgery. Clin Orthop Relat Res. 2006;(447):100-104.
10. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
11. Voigt JD, Mosier M, Huber B. In-office diagnostic arthroscopy for knee and shoulder intra-articular injuries: its potential impact on cost savings in the United States. BMC Health Serv Res. 2014;14:203.
12. Xerogeanes JW, Safran MR, Huber B, Mandelbaum BR, Robertson W, Gambardella RA. A prospective multi-center clinical trial to compare efficiency, accuracy and safety of the VisionScope imaging system compared to MRI and diagnostic arthroscopy. Orthop J Sports Med. 2014;2(2 suppl):1.
ABSTRACT
The use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). The mi-eye+TM (Trice Medical) technology is a small-bore needle unit for in-office arthroscopy. We conducted a pilot study comparing the mi-eye+TM unit with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to MRI for the diagnosis of intra-articular pathology of the knee.
This prospective, multicenter, observational study was approved by the Institutional Review Board. There were 106 patients (53 males, 53 females) in the study. MRIs were interpreted by musculoskeletally trained radiologists. The study was conducted in the operating room using the mi-eye+TM device. The mi-eye+ TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings.
The mi-eye+TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 41.7%; P < .0001).
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI, but our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology.
Continue to: Surgical arthroscopy is the gold standard...
Surgical arthroscopy is the gold standard for the diagnosis of intra-articular knee pathologies. Nevertheless, the use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). Although MRI is considered the standard diagnostic tool for acute and chronic soft-tissue injuries of the knee, its use is not without contraindication and some potential inconveniences. Contraindications to MRI are well documented. In terms of inconvenience, MRI usually requires a separate visit followed by another visit to the prescribing physician. In addition, required interpretation by a radiologist may lead to a delay in care and increase in cost.
In the early 1990s, in-office needle arthroscopy was described as a viable means of diagnosing pathologies and obtaining synovial biopsies from the knee.1-3 Initial results were good, and the procedures had very low complication rates. Nevertheless, in-office arthroscopy of the knee is not yet widely performed, likely given concerns about the technical difficulties of in-office arthroscopy, the potential for patient discomfort, and the cumbersomeness of in-office arthroscopy units. However, significant advances have been made in the resolution capability of small-bore needle arthroscopy, resulting in much less painful procedures. Additionally, the early hardware designs, which mimicked operating room setups using towers, fluid irrigation systems, and larger arthroscopes, have been replaced with small-needle arthroscopes that use syringes for irrigation and tablet computers for visualization (Figures 1A, 1B).

The mi-eye+TM technology (Trice Medical) is a small-bore needle unit for in-office arthroscopy with digital optics that does not need an irrigation tower. We conducted a pilot study of the sensitivity and specificity of the mi-eye+TM unit in comparison with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to the standard of care (MRI) for the diagnosis of intra-articular pathology of the knee.
METHODS
Central regulatory approval for this prospective, multicenter, observational study was obtained from the Western Institutional Review Board for 3 of the sites, and 1 institution required and was granted internal Institutional Review Board approval.
The study was performed by 4 sports medicine orthopedic surgeons experienced in using the mi-eye+TM in-office arthroscope. Patients were enrolled from December 2015 through June 2016. Inclusion criteria were an indication for an arthroscopic procedure of the knee based on history, physical examination, and MRI findings. Patients were excluded from the study if there were any contraindications to completing an MRI. Acute hemarthroses of the knee or active systemic infections were also excluded. Once a patient was identified as meeting the criteria for participation, informed consent was obtained. Of the 113 patients who enrolled, 7 did not have a complete study dataset available, leaving 106 patients (53 males, 53 females) in the study. Mean age was 47 years (range, 18-82 years).
Continue to: A test result form was used...
A test result form was used to record mi-eye+TM, surgical arthroscopy, and MRI results. This form required a “positive” or “negative” result for all of several diagnoses: medial and lateral meniscal tears, intra-articular loose body, osteoarthritis (OA), osteochondritis dissecans (OCD), and tears of the anterior and posterior cruciate ligaments (ACL, PCL). MRI was performed at a variety of imaging facilities, but the images were interpreted by musculoskeletally trained radiologists.
The study was conducted in the operating room. After the patient was appropriately anesthetized, and the extremity prepared and draped, the mi-eye+TM procedure was performed immediately prior to surgical arthroscopy. A tourniquet was not used. At surgeon discretion, medial, lateral, or both approaches were used with the mi-eye+TM, and diagnostic arthroscopy was performed. During the procedure, the mi-eye+TM was advanced into the knee. Once in the synovial compartment, the external 14-gauge needle was retracted, exposing the unit’s optics. Visualization was improved by injecting normal saline through the lure lock in the mi-eye+TM needle arthroscope. An average of 20 mL of saline was used, though the amount varied with surgeon discretion. Subsequently, the surgeon visualized structures in the knee and documented all findings.
At the end of the mi-eye+TM procedure, the scheduled surgical arthroscopy was performed. After the surgical procedure, if there were no issues or complications, the patient was discharged from the study. No follow-up was required for the study, as arthroscopic findings served as the conclusive diagnosis for each patient, and no interventions were being studied. There were no complications related to use of the mi-eye+TM.
The mi-eye+TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings. When a test had no false-positive or false-negative findings in comparison with surgical arthroscopy, it was identified as having complete accuracy for all intra-articular knee pathologies. For these methods, the 95% confidence interval was determined based on binomial distribution.
RESULTS
The mi-eye+ TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and surgical arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. On the other hand, MRI demonstrated both false-negative and false-positive results, failing to reveal some aspect of the knee’s pathology for 31 patients, and potentially overcalling some aspect of the knee’s pathology among 18 patients.
Continue to: The pathology most frequently...
The pathology most frequently identified in the study was a meniscal tear. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 87.5%; P < .0002). The difference in specificity resulted from the false MRI diagnosis of a meniscal tear among 24 patients, who were found to have no tear by both mi-eye+TM and surgical arthroscopy.
Table 1. Raw Data of mi-eye+TM and Magnetic Resonance Imaging Findings
| Data | True-Positive | False-Negative | False-Negative | True-Negative |
| mi-eye+TM | ||||
| Medial meniscal tear | 68 | 3 | 0 | 35 |
| Lateral meniscal tear | 32 | 5 | 0 | 69 |
| Any meniscal tear | 100 | 8 | 0 | 104 |
| Intra-articular loose body | 13 | 2 | 0 | 87 |
| Osteoarthritis | 31 | 2 | 00 | 73 |
| Osteochondritis dissecans | 8 | 2 | 0 | 97 |
| Anterior cruciate ligament tear | 16 | 0 | 0 | 90 |
| Posterior cruciate ligament tear | 0 | 0 | 0 | 106 |
| All pathologies | 168 | 14 | 0 | 557 |
| Magnetic resonance imaging | ||||
| Medial meniscal tear | 62 | 9 | 6 | 29 |
| Lateral meniscal tear | 22 | 15 | 7 | 62 |
| Any meniscal tear | 84 | 24 | 13 | 91 |
| Intra-articular loose body | 3 | 12 | 0 | 87 |
| Osteoarthritis | 26 | 7 | 8 | 65 |
| Osteochondritis dissecans | 5 | 5 | 4 | 93 |
| Anterior cruciate ligament tear | 14 | 2 | 3 | 87 |
| Posterior cruciate ligament tear | 0 | 0 | 2 | 104 |
| All pathologies | 132 | 500 | 30 | 527 |
The second most frequent pathology was an intra-articular loose body. The mi-eye+TM was more sensitive than MRI in identifying loose bodies (86.7% vs 20%; P = .0007). The specificity of the mi-eye+TM and the specificity of MRI were equivalent in diagnosing loose bodies (100%). Table 1 and Table 2 show the complete set of diagnoses and associated diagnostic profiles.
Table 2. Diagnostic Profiles: Sensitivity and Specificity of mi-eye+TM and Magnetic Resonance Imaging
| Patient Group | mi-eye+TM | MRI | |||
| Estimate, % | CI, % | Estimate, % | CI, % | Pa | |
| Sensitivity | |||||
| Medial meniscal tear | 95.77 | 88.1-99.1 | 87.32 | 77.3-94.0 | .0129 |
| Lateral meniscal tear | 86.49 | 71.2-95.5 | 59.46 | 42.1-75.3 | .0172 |
| Any meniscal tear | 92.59 | 85.9-96.8 | 77.78 | 68.8-85.2 | .0035 |
| Intra-articular loose body | 86.70 | 59.5-98.3 | 20 | 4.3-48.1 | .0006789 |
| Osteoarthritis | 93.90 | 79.8-99.3 | 78.80 | 61.1-91.0 | .1487 |
| Osteochondritis dissecans | 80.00 | 44.4-97.5 | 50 | 18.7-81.3 | .3498 |
| Anterior crucitate ligament tear | 100.00 | 79.4-100.0 | 87.50 | 61.7-98.4 | .4839 |
| Posterior cruciate ligament tear | N/A | N/A | N/A | N/A | N/A |
| Specificity | |||||
| Medial meniscal tear | 100.00 | 90.0-100.0 | 82.86 | 66.4-93.4 | .0246 |
| Lateral meniscal tear | 100.00 | 94.8-100.0 | 89.86 | 80.2-95.8 | .0133 |
| Any meniscal tear | 100.00 | 96.5-100.0 | 87.50 | 79.6-93.2 | .0002 |
| Intra-articular loose body | 100.00 | 95.9-100.0 | 100.00 | 95.9-100.0 | 1 |
| Osteoarthritis | 100.00 | 95.1-100.0 | 89.00 | 79.5-95.1 | .006382 |
| Osteochondritis dissecans | 100.00 | 96.3-100.0 | 95.90 | 89.8-98.9 | .1211 |
| Anterior cruciate ligament tear | 100.00 | 96.0-100.0 | 96.70 | 90.6-99.3 | .2458 |
| Posterior crttuciate ligament tear | 100.00 | 96.6-100.0 | 98.10 | 93.4-99.8 | .4976 |
aBold P values are significant. Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging; N/A, not applicable.
DISCUSSION
The overall accuracy of the mi-eye+TM was superior to that of MRI relative to the arthroscopic gold standard in this pilot study. Other studies have demonstrated the accuracy, feasibility, and cost-efficacy of in-office arthroscopy. However, likely because of the cumbersomeness of in-office arthroscopy equipment and the potential for patient discomfort, the technique is not yet standard in the field. Recent advances in small-bore technology, digital optics, and ergonomics have addressed the difficulties associated with in-office arthroscopy, facilitating a faster and more efficient procedure. Our goal in this study was to evaluate the diagnostic capability of the mi-eye+TM in-office arthroscopy unit, which features a small bore, digital optics, and functionality without an irrigation tower.
This study of 106 patients demonstrated equivalent or better accuracy of the mi-eye+TM relative to MRI when compared with the gold standard of surgical arthroscopy. This was not surprising given that both the mi-eye+TM and surgical arthroscopy are based on direct visualization of intra-articular pathology. The mi-eye+TM unit identified more meniscal tears, intra-articular loose bodies, ACL tears, and OCD lesions than MRI did, and with enough power to demonstrate statistically significant improved sensitivity for meniscal tears and loose bodies. Furthermore, MRI demonstrated false-positive meniscal tears, ACL tears, OCD lesions, and OA, whereas the mi-eye+TM did not demonstrate any false-positive results in comparison with surgical arthroscopy. This study demonstrated statistically significant improved specificity of the mi-eye+ compared with MRI in the diagnosis of meniscal tears and OA.
There are several limitations to our study. We refer to it as a pilot study because it was performed in a standard operating room. Before taking the technology to an outpatient setting, we wanted to confirm efficacy and safety in an operating room. However, the techniques used in this study are readily transferable to the outpatient clinic setting and to date have been used in more than 2000 cases.
Continue to: The specificity of MRI...
The specificity of MRI for meniscal tears was unexpectedly low compared with previous studies, which may reflect the multi-institution, multi-surgeon, multi-radiologist involvement in MRI interpretation.4-10 MRI was performed at a variety of institutions without a standardized protocol. This lack of standardization of image capture and interpretation may have contributed to the suboptimal performance of MRI, falsely decreasing the potential ideal specificity for meniscal tears. Although this study may have underestimated the specificity of MRI for meniscal tears, we think the mi-eye+TM and MRI results reported here reflect the findings of standard practice, without the standardization usually applied in studies. For example, a study of 139 knee MRI reports at 14 different institutions confirmed arthroscopic findings and concluded that 37% of the operations supported by a significant MRI finding were unjustified.11 The authors attributed the rate of false-positive MRI findings to the wide variety of places where patients had their MRIs performed, and the subsequent variation in quality of imaging and MRI reader skill level.11
Before inserting the mi-eye+TM needle arthroscope, the surgeons had a working diagnosis of the pathology based on their clinical examination and MRI results. Clearly, this introduced a bias. Further studies will be conducted in a prospective, blinded manner to address this limitation.
Although studies of in-office arthroscopy technology date to the 1990s, there is an overall lack of data comparing in-office arthroscopy with MRI. Halbrecht and Jackson2 conducted a study of 20 knee patients with both MRI and in-office needle arthroscopy. Overall, MRI was poor in detecting cartilage defects, with sensitivity of 34.6%, using the in-office arthroscopy as the confirmatory diagnosis. Although the authors did not compare in-office diagnoses with surgical arthroscopic findings, they concluded that office arthroscopy is an accurate and cost-efficient alternative to MRI in diagnostic evaluation of knee patients. Xerogeanes and colleagues12 studied 110 patients in a prospective, blinded, multicenter trial comparing a minimally invasive office-based arthroscopy with MRI, using surgical arthroscopy as the confirmatory diagnosis. They concluded that the office-based arthroscope was statistically equivalent to diagnostic surgical arthroscopy and that it outperformed MRI in helping make accurate diagnoses. The authors applied a cost analysis to their findings and determined that office-based arthroscopy could result in an annual potential savings of $177 million for the healthcare system.12
Modern imaging sequences on high-Tesla MRI machines provide excellent visualization. Nevertheless, a significant number of patients do not undergo MRI, owing to time constraints, contraindications, body habitus, or anxiety/claustrophobia. Our study results confirmed that doctors treating such patients now have a viable alternative to help diagnose pathology.
CONCLUSION
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at the time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI; our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology. More studies of the mi-eye+TM device in a clinical setting are warranted.
ABSTRACT
The use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). The mi-eye+TM (Trice Medical) technology is a small-bore needle unit for in-office arthroscopy. We conducted a pilot study comparing the mi-eye+TM unit with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to MRI for the diagnosis of intra-articular pathology of the knee.
This prospective, multicenter, observational study was approved by the Institutional Review Board. There were 106 patients (53 males, 53 females) in the study. MRIs were interpreted by musculoskeletally trained radiologists. The study was conducted in the operating room using the mi-eye+TM device. The mi-eye+ TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings.
The mi-eye+TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 41.7%; P < .0001).
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI, but our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology.
Continue to: Surgical arthroscopy is the gold standard...
Surgical arthroscopy is the gold standard for the diagnosis of intra-articular knee pathologies. Nevertheless, the use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). Although MRI is considered the standard diagnostic tool for acute and chronic soft-tissue injuries of the knee, its use is not without contraindication and some potential inconveniences. Contraindications to MRI are well documented. In terms of inconvenience, MRI usually requires a separate visit followed by another visit to the prescribing physician. In addition, required interpretation by a radiologist may lead to a delay in care and increase in cost.
In the early 1990s, in-office needle arthroscopy was described as a viable means of diagnosing pathologies and obtaining synovial biopsies from the knee.1-3 Initial results were good, and the procedures had very low complication rates. Nevertheless, in-office arthroscopy of the knee is not yet widely performed, likely given concerns about the technical difficulties of in-office arthroscopy, the potential for patient discomfort, and the cumbersomeness of in-office arthroscopy units. However, significant advances have been made in the resolution capability of small-bore needle arthroscopy, resulting in much less painful procedures. Additionally, the early hardware designs, which mimicked operating room setups using towers, fluid irrigation systems, and larger arthroscopes, have been replaced with small-needle arthroscopes that use syringes for irrigation and tablet computers for visualization (Figures 1A, 1B).

The mi-eye+TM technology (Trice Medical) is a small-bore needle unit for in-office arthroscopy with digital optics that does not need an irrigation tower. We conducted a pilot study of the sensitivity and specificity of the mi-eye+TM unit in comparison with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to the standard of care (MRI) for the diagnosis of intra-articular pathology of the knee.
METHODS
Central regulatory approval for this prospective, multicenter, observational study was obtained from the Western Institutional Review Board for 3 of the sites, and 1 institution required and was granted internal Institutional Review Board approval.
The study was performed by 4 sports medicine orthopedic surgeons experienced in using the mi-eye+TM in-office arthroscope. Patients were enrolled from December 2015 through June 2016. Inclusion criteria were an indication for an arthroscopic procedure of the knee based on history, physical examination, and MRI findings. Patients were excluded from the study if there were any contraindications to completing an MRI. Acute hemarthroses of the knee or active systemic infections were also excluded. Once a patient was identified as meeting the criteria for participation, informed consent was obtained. Of the 113 patients who enrolled, 7 did not have a complete study dataset available, leaving 106 patients (53 males, 53 females) in the study. Mean age was 47 years (range, 18-82 years).
Continue to: A test result form was used...
A test result form was used to record mi-eye+TM, surgical arthroscopy, and MRI results. This form required a “positive” or “negative” result for all of several diagnoses: medial and lateral meniscal tears, intra-articular loose body, osteoarthritis (OA), osteochondritis dissecans (OCD), and tears of the anterior and posterior cruciate ligaments (ACL, PCL). MRI was performed at a variety of imaging facilities, but the images were interpreted by musculoskeletally trained radiologists.
The study was conducted in the operating room. After the patient was appropriately anesthetized, and the extremity prepared and draped, the mi-eye+TM procedure was performed immediately prior to surgical arthroscopy. A tourniquet was not used. At surgeon discretion, medial, lateral, or both approaches were used with the mi-eye+TM, and diagnostic arthroscopy was performed. During the procedure, the mi-eye+TM was advanced into the knee. Once in the synovial compartment, the external 14-gauge needle was retracted, exposing the unit’s optics. Visualization was improved by injecting normal saline through the lure lock in the mi-eye+TM needle arthroscope. An average of 20 mL of saline was used, though the amount varied with surgeon discretion. Subsequently, the surgeon visualized structures in the knee and documented all findings.
At the end of the mi-eye+TM procedure, the scheduled surgical arthroscopy was performed. After the surgical procedure, if there were no issues or complications, the patient was discharged from the study. No follow-up was required for the study, as arthroscopic findings served as the conclusive diagnosis for each patient, and no interventions were being studied. There were no complications related to use of the mi-eye+TM.
The mi-eye+TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings. When a test had no false-positive or false-negative findings in comparison with surgical arthroscopy, it was identified as having complete accuracy for all intra-articular knee pathologies. For these methods, the 95% confidence interval was determined based on binomial distribution.
RESULTS
The mi-eye+ TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and surgical arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. On the other hand, MRI demonstrated both false-negative and false-positive results, failing to reveal some aspect of the knee’s pathology for 31 patients, and potentially overcalling some aspect of the knee’s pathology among 18 patients.
Continue to: The pathology most frequently...
The pathology most frequently identified in the study was a meniscal tear. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 87.5%; P < .0002). The difference in specificity resulted from the false MRI diagnosis of a meniscal tear among 24 patients, who were found to have no tear by both mi-eye+TM and surgical arthroscopy.
Table 1. Raw Data of mi-eye+TM and Magnetic Resonance Imaging Findings
| Data | True-Positive | False-Negative | False-Negative | True-Negative |
| mi-eye+TM | ||||
| Medial meniscal tear | 68 | 3 | 0 | 35 |
| Lateral meniscal tear | 32 | 5 | 0 | 69 |
| Any meniscal tear | 100 | 8 | 0 | 104 |
| Intra-articular loose body | 13 | 2 | 0 | 87 |
| Osteoarthritis | 31 | 2 | 00 | 73 |
| Osteochondritis dissecans | 8 | 2 | 0 | 97 |
| Anterior cruciate ligament tear | 16 | 0 | 0 | 90 |
| Posterior cruciate ligament tear | 0 | 0 | 0 | 106 |
| All pathologies | 168 | 14 | 0 | 557 |
| Magnetic resonance imaging | ||||
| Medial meniscal tear | 62 | 9 | 6 | 29 |
| Lateral meniscal tear | 22 | 15 | 7 | 62 |
| Any meniscal tear | 84 | 24 | 13 | 91 |
| Intra-articular loose body | 3 | 12 | 0 | 87 |
| Osteoarthritis | 26 | 7 | 8 | 65 |
| Osteochondritis dissecans | 5 | 5 | 4 | 93 |
| Anterior cruciate ligament tear | 14 | 2 | 3 | 87 |
| Posterior cruciate ligament tear | 0 | 0 | 2 | 104 |
| All pathologies | 132 | 500 | 30 | 527 |
The second most frequent pathology was an intra-articular loose body. The mi-eye+TM was more sensitive than MRI in identifying loose bodies (86.7% vs 20%; P = .0007). The specificity of the mi-eye+TM and the specificity of MRI were equivalent in diagnosing loose bodies (100%). Table 1 and Table 2 show the complete set of diagnoses and associated diagnostic profiles.
Table 2. Diagnostic Profiles: Sensitivity and Specificity of mi-eye+TM and Magnetic Resonance Imaging
| Patient Group | mi-eye+TM | MRI | |||
| Estimate, % | CI, % | Estimate, % | CI, % | Pa | |
| Sensitivity | |||||
| Medial meniscal tear | 95.77 | 88.1-99.1 | 87.32 | 77.3-94.0 | .0129 |
| Lateral meniscal tear | 86.49 | 71.2-95.5 | 59.46 | 42.1-75.3 | .0172 |
| Any meniscal tear | 92.59 | 85.9-96.8 | 77.78 | 68.8-85.2 | .0035 |
| Intra-articular loose body | 86.70 | 59.5-98.3 | 20 | 4.3-48.1 | .0006789 |
| Osteoarthritis | 93.90 | 79.8-99.3 | 78.80 | 61.1-91.0 | .1487 |
| Osteochondritis dissecans | 80.00 | 44.4-97.5 | 50 | 18.7-81.3 | .3498 |
| Anterior crucitate ligament tear | 100.00 | 79.4-100.0 | 87.50 | 61.7-98.4 | .4839 |
| Posterior cruciate ligament tear | N/A | N/A | N/A | N/A | N/A |
| Specificity | |||||
| Medial meniscal tear | 100.00 | 90.0-100.0 | 82.86 | 66.4-93.4 | .0246 |
| Lateral meniscal tear | 100.00 | 94.8-100.0 | 89.86 | 80.2-95.8 | .0133 |
| Any meniscal tear | 100.00 | 96.5-100.0 | 87.50 | 79.6-93.2 | .0002 |
| Intra-articular loose body | 100.00 | 95.9-100.0 | 100.00 | 95.9-100.0 | 1 |
| Osteoarthritis | 100.00 | 95.1-100.0 | 89.00 | 79.5-95.1 | .006382 |
| Osteochondritis dissecans | 100.00 | 96.3-100.0 | 95.90 | 89.8-98.9 | .1211 |
| Anterior cruciate ligament tear | 100.00 | 96.0-100.0 | 96.70 | 90.6-99.3 | .2458 |
| Posterior crttuciate ligament tear | 100.00 | 96.6-100.0 | 98.10 | 93.4-99.8 | .4976 |
aBold P values are significant. Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging; N/A, not applicable.
DISCUSSION
The overall accuracy of the mi-eye+TM was superior to that of MRI relative to the arthroscopic gold standard in this pilot study. Other studies have demonstrated the accuracy, feasibility, and cost-efficacy of in-office arthroscopy. However, likely because of the cumbersomeness of in-office arthroscopy equipment and the potential for patient discomfort, the technique is not yet standard in the field. Recent advances in small-bore technology, digital optics, and ergonomics have addressed the difficulties associated with in-office arthroscopy, facilitating a faster and more efficient procedure. Our goal in this study was to evaluate the diagnostic capability of the mi-eye+TM in-office arthroscopy unit, which features a small bore, digital optics, and functionality without an irrigation tower.
This study of 106 patients demonstrated equivalent or better accuracy of the mi-eye+TM relative to MRI when compared with the gold standard of surgical arthroscopy. This was not surprising given that both the mi-eye+TM and surgical arthroscopy are based on direct visualization of intra-articular pathology. The mi-eye+TM unit identified more meniscal tears, intra-articular loose bodies, ACL tears, and OCD lesions than MRI did, and with enough power to demonstrate statistically significant improved sensitivity for meniscal tears and loose bodies. Furthermore, MRI demonstrated false-positive meniscal tears, ACL tears, OCD lesions, and OA, whereas the mi-eye+TM did not demonstrate any false-positive results in comparison with surgical arthroscopy. This study demonstrated statistically significant improved specificity of the mi-eye+ compared with MRI in the diagnosis of meniscal tears and OA.
There are several limitations to our study. We refer to it as a pilot study because it was performed in a standard operating room. Before taking the technology to an outpatient setting, we wanted to confirm efficacy and safety in an operating room. However, the techniques used in this study are readily transferable to the outpatient clinic setting and to date have been used in more than 2000 cases.
Continue to: The specificity of MRI...
The specificity of MRI for meniscal tears was unexpectedly low compared with previous studies, which may reflect the multi-institution, multi-surgeon, multi-radiologist involvement in MRI interpretation.4-10 MRI was performed at a variety of institutions without a standardized protocol. This lack of standardization of image capture and interpretation may have contributed to the suboptimal performance of MRI, falsely decreasing the potential ideal specificity for meniscal tears. Although this study may have underestimated the specificity of MRI for meniscal tears, we think the mi-eye+TM and MRI results reported here reflect the findings of standard practice, without the standardization usually applied in studies. For example, a study of 139 knee MRI reports at 14 different institutions confirmed arthroscopic findings and concluded that 37% of the operations supported by a significant MRI finding were unjustified.11 The authors attributed the rate of false-positive MRI findings to the wide variety of places where patients had their MRIs performed, and the subsequent variation in quality of imaging and MRI reader skill level.11
Before inserting the mi-eye+TM needle arthroscope, the surgeons had a working diagnosis of the pathology based on their clinical examination and MRI results. Clearly, this introduced a bias. Further studies will be conducted in a prospective, blinded manner to address this limitation.
Although studies of in-office arthroscopy technology date to the 1990s, there is an overall lack of data comparing in-office arthroscopy with MRI. Halbrecht and Jackson2 conducted a study of 20 knee patients with both MRI and in-office needle arthroscopy. Overall, MRI was poor in detecting cartilage defects, with sensitivity of 34.6%, using the in-office arthroscopy as the confirmatory diagnosis. Although the authors did not compare in-office diagnoses with surgical arthroscopic findings, they concluded that office arthroscopy is an accurate and cost-efficient alternative to MRI in diagnostic evaluation of knee patients. Xerogeanes and colleagues12 studied 110 patients in a prospective, blinded, multicenter trial comparing a minimally invasive office-based arthroscopy with MRI, using surgical arthroscopy as the confirmatory diagnosis. They concluded that the office-based arthroscope was statistically equivalent to diagnostic surgical arthroscopy and that it outperformed MRI in helping make accurate diagnoses. The authors applied a cost analysis to their findings and determined that office-based arthroscopy could result in an annual potential savings of $177 million for the healthcare system.12
Modern imaging sequences on high-Tesla MRI machines provide excellent visualization. Nevertheless, a significant number of patients do not undergo MRI, owing to time constraints, contraindications, body habitus, or anxiety/claustrophobia. Our study results confirmed that doctors treating such patients now have a viable alternative to help diagnose pathology.
CONCLUSION
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at the time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI; our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology. More studies of the mi-eye+TM device in a clinical setting are warranted.
1. Baeten D, Van den Bosch F, Elewaut D, Stuer A, Veys EM, De Keyser F. Needle arthroscopy of the knee with synovial biopsy sampling: technical experience in 150 patients. Clin Rheumatol. 1999;18(6):434-441.
2. Halbrecht J, Jackson D. Office arthroscopy: a diagnostic alternative. Arthroscopy. 1992;8(3):320-326.
3. Batcheleor R, Henshaw K, Astin P, Emery P, Reece R, Leeds DM. Rheumatological needle arthroscopy: a 5-year follow up of safety and efficacy. Arthritis Rheum Ann Sci Meet Abstr. 2001;(9 suppl).
4. Barronian AD, Zoltan JD, Bucon KA. Magnetic resonance imaging of the knee: correlation with arthroscopy. Arthroscopy. 1989;5(3):187-191.
5. Crues JV 3rd, Ryu R, Morgan FW. Meniscal pathology. The expanding role of magnetic resonance imaging. Clin Orthop Relat Res. 1990;(252):80-87.
6. Raunest J, Oberle K, Leohnert J, Hoetzinger H. The clinical value of magnetic resonance imaging in the evaluation of meniscal disorders. J Bone Joint Surg Am. 1991;73(1):11-16.
7. Spiers AS, Meagher T, Ostlere SJ, Wilson DJ, Dodd CA. Can MRI of the knee affect arthroscopic practice? A prospective study of 58 patients. J Bone Joint Surg Br. 1993;75(1):49-52.
8. O’Shea KJ, Murphy KP, Heekin RD, Herzwurm PJ. The diagnostic accuracy of history, physical examination, and radiographs in the evaluation of traumatic knee disorders. Am J Sports Med. 1996;24(2):164-167.
9. Ben-Galim P, Steinberg EL, Amir H, Ash N, Dekel S, Arbel R. Accuracy of magnetic resonance imaging of the knee and unjustified surgery. Clin Orthop Relat Res. 2006;(447):100-104.
10. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
11. Voigt JD, Mosier M, Huber B. In-office diagnostic arthroscopy for knee and shoulder intra-articular injuries: its potential impact on cost savings in the United States. BMC Health Serv Res. 2014;14:203.
12. Xerogeanes JW, Safran MR, Huber B, Mandelbaum BR, Robertson W, Gambardella RA. A prospective multi-center clinical trial to compare efficiency, accuracy and safety of the VisionScope imaging system compared to MRI and diagnostic arthroscopy. Orthop J Sports Med. 2014;2(2 suppl):1.
1. Baeten D, Van den Bosch F, Elewaut D, Stuer A, Veys EM, De Keyser F. Needle arthroscopy of the knee with synovial biopsy sampling: technical experience in 150 patients. Clin Rheumatol. 1999;18(6):434-441.
2. Halbrecht J, Jackson D. Office arthroscopy: a diagnostic alternative. Arthroscopy. 1992;8(3):320-326.
3. Batcheleor R, Henshaw K, Astin P, Emery P, Reece R, Leeds DM. Rheumatological needle arthroscopy: a 5-year follow up of safety and efficacy. Arthritis Rheum Ann Sci Meet Abstr. 2001;(9 suppl).
4. Barronian AD, Zoltan JD, Bucon KA. Magnetic resonance imaging of the knee: correlation with arthroscopy. Arthroscopy. 1989;5(3):187-191.
5. Crues JV 3rd, Ryu R, Morgan FW. Meniscal pathology. The expanding role of magnetic resonance imaging. Clin Orthop Relat Res. 1990;(252):80-87.
6. Raunest J, Oberle K, Leohnert J, Hoetzinger H. The clinical value of magnetic resonance imaging in the evaluation of meniscal disorders. J Bone Joint Surg Am. 1991;73(1):11-16.
7. Spiers AS, Meagher T, Ostlere SJ, Wilson DJ, Dodd CA. Can MRI of the knee affect arthroscopic practice? A prospective study of 58 patients. J Bone Joint Surg Br. 1993;75(1):49-52.
8. O’Shea KJ, Murphy KP, Heekin RD, Herzwurm PJ. The diagnostic accuracy of history, physical examination, and radiographs in the evaluation of traumatic knee disorders. Am J Sports Med. 1996;24(2):164-167.
9. Ben-Galim P, Steinberg EL, Amir H, Ash N, Dekel S, Arbel R. Accuracy of magnetic resonance imaging of the knee and unjustified surgery. Clin Orthop Relat Res. 2006;(447):100-104.
10. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
11. Voigt JD, Mosier M, Huber B. In-office diagnostic arthroscopy for knee and shoulder intra-articular injuries: its potential impact on cost savings in the United States. BMC Health Serv Res. 2014;14:203.
12. Xerogeanes JW, Safran MR, Huber B, Mandelbaum BR, Robertson W, Gambardella RA. A prospective multi-center clinical trial to compare efficiency, accuracy and safety of the VisionScope imaging system compared to MRI and diagnostic arthroscopy. Orthop J Sports Med. 2014;2(2 suppl):1.
TAKE-HOME POINTS
- Small-bore needle arthroscopy is an effective way to diagnose intra-articular knee pathology.
- Small-bore needle arthroscopy is safe and easy to use with no complications reported in this series.
- Small-bore needle arthroscopy is a useful diagnostic tool in office settings.
- In this series, small-bore needle arthroscopy was more accurate than MRI to diagnose knee meniscal tears.
- In-office diagnostic arthroscopy can be used for other joints such as shoulder, elbow, and ankle.
Total Shoulder Arthroplasty Using a Bone-Sparing, Precision Multiplanar Humeral Prosthesis
ABSTRACT
Proper reconstruction of proximal humeral anatomy is of primary importance to maximize patient outcomes after total shoulder arthroplasty. This article describes a new arthroplasty technique, where a fixed multiplanar bone resection is made and a novel implant, which is designed to precisely match the bone resection, is inserted.
Continue to: The success of total shoulder arthroplasty...
The success of total shoulder arthroplasty (TSA) is largely dependent on how accurate the proximal humeral anatomy is reconstructed and the glenohumeral relationships are restored.1-4 Numerous studies have demonstrated a relationship of worse clinical outcomes and implant failure with nonanatomic implant placement.5-8 The majority of arthroplasty systems rely on surgeon-dependent decision-making to determine the location of the border of the articular surface and, ultimately, the amount and location of bone to be resected. Even in experienced hands, the ability to reproducibly restore the joint line is inconsistent.3
In contrast, the majority of total knee arthroplasty (TKA) systems have been designed with instrumentation that guides the surgeon precisely regarding where and how much femoral bone must be resected, and the corresponding implant is designed with the same thickness to preserve the location of the joint line. Cutting block instrumentation rather than freehand cuts enables reproducibility of TKA while being performed for an estimated 700,000 times annually in the US.9
To achieve similar high levels of reproducibility in shoulder arthroplasty, a new technique was developed based on the principle of providing instrumentation to assist the surgeon in accurately restoring the proximal humeral joint line. This technical article describes the technique of using a multiplanar instrumented cutting system and matching implants to perform TSA. The technique shown was previously studied and was found to allow surgeons to recreate the original anatomy of the humerus with very high precision.10

The humeral prosthesis described in this article has an articular surface that is slightly elliptical to more closely match the actual shape of the humerus bone.11 Biomechanical studies have demonstrated that implants designed with a nonspherical shape have more similar motion and kinematics to those of the native humeral head.

This provides rotation stability, and the implant rests on the strong subchondral bone of the proximal humerus proximal to the anatomic neck rather than relying on metaphyseal bone or canal fixation, as recommended by Aldoiusti.13 It also allows optimal implant placement with complete freedom with respect to inclination, version, and medial/posterior offset from the humeral canal.
Continue to: The implant respects the relationship...
The implant respects the relationship of the rotator cuff insertion and has a recessed superior margin to keep both the implant and the saw blade 3 mm to 5 mm away from the supraspinatus fibers to protect the rotator cuff from iatrogenic injury.
TECHNIQUE
The technique described in this article uses the Catalyst CSR Total Shoulder System (Catalyst OrthoScience), which was cleared to treat arthritis of the shoulder by the US Food and Drug Administration in May 2016.
A standard deltopectoral incision is made, and the surgeon dissects the interval between the pectoralis major medially and the deltoid laterally. The subscapularis can be incised by tenotomy; alternatively, the surgeon can perform a subscapularis peel or a lesser tuberosity osteotomy using this technique.
Once the glenohumeral joint is exposed, the surgeon delivers the humeral head anteriorly. A preferred method is to place a Darrach retractor between the humeral head and the glenoid, and a cobra or a second Darrach retractor behind the superolateral humeral head superficial to the supraspinatus tendon. By simultaneously pressing on both retractors and externally rotating the patient’s arm, the humeral head is delivered anteriorly. Osteophytes on the anterior and inferior edge of the humeral head are generously removed at this time using a rongeur.
Using a pin guide, the long 3.2-mm guidewire pin is drilled under power into the center of the articular surface. The pin guide is then removed, leaving the pin in the center of the humerus (Figure 3).
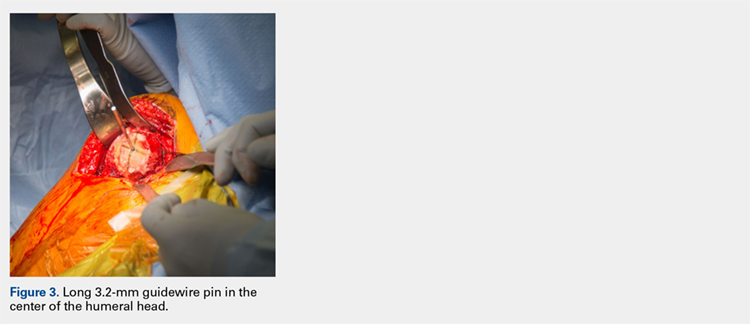
Continue to: Next, the surgeon...
Next, the surgeon slides the cannulated reamer over the long guidewire pin and under power removes a small portion of the humeral head subchondral bone until the surgeon feels and observes that the reamer is no longer removing bone (Figure 4). The patent-pending reamer design prevents the surgeon from removing more than a few millimeters of bone, after which point the reamer spins on the surface of the bone without resecting further.

The surgeon is aware that the reamer has achieved its desired depth when it is no longer creating new bone shavings, and the surgeon can hear and feel that the reamer is spinning and no longer cutting. Then the surgeon removes the reamer.

The surgeon places the first humeral cut guide over the long guidewire pin, oriented superiorly-inferiorly and secures the guide using 4 short pins, and the long pin is removed. The surgeon uses an oscillating saw to cut the anterior and posterior plane cuts through the saw captures in the cut guide (Figure 5). The humeral cut guide and short pins are removed (Figure 6).
The surgeon then applies the second humeral cut guide to the proximal humerus and secures it using 2 short pins. The surgeon then uses the 6-mm drill to drill the 4 holes for the pegs of the implant. The top portion of the guide is removed, and the surgeon makes the superior and inferior cuts along the top and bottom surfaces of the guide using an oscillating saw (Figure 7).
The surgeon then uses a rongeur to slightly round the edges of the 4 corners at the periphery of the humerus. The second humeral cut guide and short pins are removed (Figure 8).

Continue to: Next, the surgeon trials...
Next, the surgeon trials humeral implants to determine the correct implant size (Figure 9). Once the proper humeral size is chosen, the trial is removed and the humeral cover is placed over the prepared humeral head. The surgeon then proceeds to glenoid preparation (Figure 10), which is easily accessible and facilitated by angled planar cuts on the humeral head. Glenoid technique will be discussed in a subsequent article.

After glenoid preparation and insertion, the humerus is delivered anteriorly. The proximal humerus is washed and dried, and cement is applied to the peg holes in the humerus bone and the underside of the humeral implant. The implant is then inserted using the humeral impactor to apply pressure and assure that the implant is fully seated. Once the humeral cement is hardened, the glenohumeral joint is irrigated and closure begins. Postoperative radiograph is shown in Figure 11.
DISCUSSION
Numerous authors have demonstrated that accurate implant placement is crucial for restoring normal glenoid kinematics and motion,1-4 while some authors have reported worsening clinical outcomes and higher rates of pain and implant loosening when the implants were not placed anatomically.5-8 This is such an important concept that it essentially was the primary inspiration for creating this TSA system. In addition, the system utilizes a nonspherical, elliptical humeral head that more closely matches the anatomy of the proximal humerus,14,15 and this type of shape has shown improved biomechanics in laboratory testing.12
Good results have been demonstrated in restoring the normal anatomy using stemmed devices on the radiographic analysis of cadavers.16 The creation of stemmed implants with variable inclination and offset has improved computer models17 compared with previous studies,18 with the exception of scenarios with extreme offset.
In theory, resurfacing implants and implants without a canal stem should have a better implant placement than that with stemmed implants; however, the ability to restore the center of rotation was even worse for resurfacing prostheses, with 65% of all implants being measured as outliers postoperatively in one study.19 Most of the resurfacing implants and their instrumentation techniques offer little to help the surgeon control for implant height. The depth of the reaming is variable, not calibrated, and not correlated with the implant size, frequently leading to overstuffing after surgery. Second, the use of spherical prostheses forces the surgeon to choose between matching the superior-inferior humeral size, leading to overhang of the implant, or matching the anteroposterior, leading to frequent undersizing in the coronal plane. The nonspherical, elliptical head shape can potentially simplify implant selection.
In summary, new techniques have been developed in an attempt to achieve increased consistency and precision in TSA. By more accurately reproducing the proximal humeral anatomy, it is proposed that clinical outcomes in terms of the range of motion and patient satisfaction may also be improved through newer techniques. Cadaver studies have validated the anatomic precision of this technique.10 Clinical data comprising of patient-reported outcome measures and radiographic outcome studies are currently underway for this arthroplasty system.
1. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409.
2. Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86-A(3):575-580.
3. Iannotti JP, Spencer EE, Winter U, Deffenbaugh D, Williams G. Prosthetic positioning in total shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1 Supple S):111S-121S.
4. Terrier A, Ramondetti S, Merlini F, Pioletti DD, Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(8):1184-1190.
5. Denard PJ, Raiss P, Sowa B, Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22(7):894-900.
6. Figgie HE 3rd, Inglis AE, Goldberg VM, Ranawat CS, Figgie MP, Wile JM. An analysis of factors affecting the long-term results of total shoulder arthroplasty in inflammatory arthritis. J Arthroplasty. 1988;3(2):123-130.
7. Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA 3rd. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16(5):555-562.
8. Flurin PH, Roche CP, Wright TW, Zuckerman JD. Correlation between clinical outcomes and anatomic reconstruction with anatomic total shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73 Suppl 1:S92-S98.
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Goldberg SS, Akyuz E, Murthi AM, Blaine T. Accuracy of humeral articular surface restoration in a novel anatomic shoulder arthroplasty technique and design: a cadaveric study. Journal of Shoulder and Elbow Arthroplasty. 2018;2:2471549217750791.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Jun BJ, Lee TQ, McGarry MH, Quigley RJ, Shin SJ, Iannotti JP. The effects of prosthetic humeral head shape on glenohumeral joint kinematics during humeral axial rotation in total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25(7):1084-1093.
13. Alidousti H, Giles JW, Emery RJH, Jeffers J. Spatial mapping of humeral head bone density. J Shoulder Elbow Surg. 2017;26(9):1653-1661.
14. Harrold F, Wigderowitz C. Humeral head arthroplasty and its ability to restore original humeral head geometry. J Shoulder Elbow Surg. 2013;22(1):115-121.
15. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338.
16. Wirth MA, Ondrla J, Southworth C, Kaar K, Anderson BC, Rockwood CA 3rd. Replicating proximal humeral articular geometry with a third-generation implant: a radiographic study in cadaveric shoulders. J Shoulder Elbow Surg. 2007;16(3 Suppl):S111-S116.
17. Pearl ML, Kurutz S, Postacchini R. Geometric variables in anatomic replacement of the proximal humerus: How much prosthetic geometry is necessary? J Shoulder Elbow Surg. 2009;18(3):366-370.
18. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326.
19. Alolabi B, Youderian AR, Napolitano L, et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1740-1746.
ABSTRACT
Proper reconstruction of proximal humeral anatomy is of primary importance to maximize patient outcomes after total shoulder arthroplasty. This article describes a new arthroplasty technique, where a fixed multiplanar bone resection is made and a novel implant, which is designed to precisely match the bone resection, is inserted.
Continue to: The success of total shoulder arthroplasty...
The success of total shoulder arthroplasty (TSA) is largely dependent on how accurate the proximal humeral anatomy is reconstructed and the glenohumeral relationships are restored.1-4 Numerous studies have demonstrated a relationship of worse clinical outcomes and implant failure with nonanatomic implant placement.5-8 The majority of arthroplasty systems rely on surgeon-dependent decision-making to determine the location of the border of the articular surface and, ultimately, the amount and location of bone to be resected. Even in experienced hands, the ability to reproducibly restore the joint line is inconsistent.3
In contrast, the majority of total knee arthroplasty (TKA) systems have been designed with instrumentation that guides the surgeon precisely regarding where and how much femoral bone must be resected, and the corresponding implant is designed with the same thickness to preserve the location of the joint line. Cutting block instrumentation rather than freehand cuts enables reproducibility of TKA while being performed for an estimated 700,000 times annually in the US.9
To achieve similar high levels of reproducibility in shoulder arthroplasty, a new technique was developed based on the principle of providing instrumentation to assist the surgeon in accurately restoring the proximal humeral joint line. This technical article describes the technique of using a multiplanar instrumented cutting system and matching implants to perform TSA. The technique shown was previously studied and was found to allow surgeons to recreate the original anatomy of the humerus with very high precision.10

The humeral prosthesis described in this article has an articular surface that is slightly elliptical to more closely match the actual shape of the humerus bone.11 Biomechanical studies have demonstrated that implants designed with a nonspherical shape have more similar motion and kinematics to those of the native humeral head.

This provides rotation stability, and the implant rests on the strong subchondral bone of the proximal humerus proximal to the anatomic neck rather than relying on metaphyseal bone or canal fixation, as recommended by Aldoiusti.13 It also allows optimal implant placement with complete freedom with respect to inclination, version, and medial/posterior offset from the humeral canal.
Continue to: The implant respects the relationship...
The implant respects the relationship of the rotator cuff insertion and has a recessed superior margin to keep both the implant and the saw blade 3 mm to 5 mm away from the supraspinatus fibers to protect the rotator cuff from iatrogenic injury.
TECHNIQUE
The technique described in this article uses the Catalyst CSR Total Shoulder System (Catalyst OrthoScience), which was cleared to treat arthritis of the shoulder by the US Food and Drug Administration in May 2016.
A standard deltopectoral incision is made, and the surgeon dissects the interval between the pectoralis major medially and the deltoid laterally. The subscapularis can be incised by tenotomy; alternatively, the surgeon can perform a subscapularis peel or a lesser tuberosity osteotomy using this technique.
Once the glenohumeral joint is exposed, the surgeon delivers the humeral head anteriorly. A preferred method is to place a Darrach retractor between the humeral head and the glenoid, and a cobra or a second Darrach retractor behind the superolateral humeral head superficial to the supraspinatus tendon. By simultaneously pressing on both retractors and externally rotating the patient’s arm, the humeral head is delivered anteriorly. Osteophytes on the anterior and inferior edge of the humeral head are generously removed at this time using a rongeur.
Using a pin guide, the long 3.2-mm guidewire pin is drilled under power into the center of the articular surface. The pin guide is then removed, leaving the pin in the center of the humerus (Figure 3).

Continue to: Next, the surgeon...
Next, the surgeon slides the cannulated reamer over the long guidewire pin and under power removes a small portion of the humeral head subchondral bone until the surgeon feels and observes that the reamer is no longer removing bone (Figure 4). The patent-pending reamer design prevents the surgeon from removing more than a few millimeters of bone, after which point the reamer spins on the surface of the bone without resecting further.

The surgeon is aware that the reamer has achieved its desired depth when it is no longer creating new bone shavings, and the surgeon can hear and feel that the reamer is spinning and no longer cutting. Then the surgeon removes the reamer.

The surgeon places the first humeral cut guide over the long guidewire pin, oriented superiorly-inferiorly and secures the guide using 4 short pins, and the long pin is removed. The surgeon uses an oscillating saw to cut the anterior and posterior plane cuts through the saw captures in the cut guide (Figure 5). The humeral cut guide and short pins are removed (Figure 6).

The surgeon then applies the second humeral cut guide to the proximal humerus and secures it using 2 short pins. The surgeon then uses the 6-mm drill to drill the 4 holes for the pegs of the implant. The top portion of the guide is removed, and the surgeon makes the superior and inferior cuts along the top and bottom surfaces of the guide using an oscillating saw (Figure 7).

The surgeon then uses a rongeur to slightly round the edges of the 4 corners at the periphery of the humerus. The second humeral cut guide and short pins are removed (Figure 8).

Continue to: Next, the surgeon trials...
Next, the surgeon trials humeral implants to determine the correct implant size (Figure 9). Once the proper humeral size is chosen, the trial is removed and the humeral cover is placed over the prepared humeral head. The surgeon then proceeds to glenoid preparation (Figure 10), which is easily accessible and facilitated by angled planar cuts on the humeral head. Glenoid technique will be discussed in a subsequent article.

After glenoid preparation and insertion, the humerus is delivered anteriorly. The proximal humerus is washed and dried, and cement is applied to the peg holes in the humerus bone and the underside of the humeral implant. The implant is then inserted using the humeral impactor to apply pressure and assure that the implant is fully seated. Once the humeral cement is hardened, the glenohumeral joint is irrigated and closure begins. Postoperative radiograph is shown in Figure 11.

DISCUSSION
Numerous authors have demonstrated that accurate implant placement is crucial for restoring normal glenoid kinematics and motion,1-4 while some authors have reported worsening clinical outcomes and higher rates of pain and implant loosening when the implants were not placed anatomically.5-8 This is such an important concept that it essentially was the primary inspiration for creating this TSA system. In addition, the system utilizes a nonspherical, elliptical humeral head that more closely matches the anatomy of the proximal humerus,14,15 and this type of shape has shown improved biomechanics in laboratory testing.12

Good results have been demonstrated in restoring the normal anatomy using stemmed devices on the radiographic analysis of cadavers.16 The creation of stemmed implants with variable inclination and offset has improved computer models17 compared with previous studies,18 with the exception of scenarios with extreme offset.
In theory, resurfacing implants and implants without a canal stem should have a better implant placement than that with stemmed implants; however, the ability to restore the center of rotation was even worse for resurfacing prostheses, with 65% of all implants being measured as outliers postoperatively in one study.19 Most of the resurfacing implants and their instrumentation techniques offer little to help the surgeon control for implant height. The depth of the reaming is variable, not calibrated, and not correlated with the implant size, frequently leading to overstuffing after surgery. Second, the use of spherical prostheses forces the surgeon to choose between matching the superior-inferior humeral size, leading to overhang of the implant, or matching the anteroposterior, leading to frequent undersizing in the coronal plane. The nonspherical, elliptical head shape can potentially simplify implant selection.
In summary, new techniques have been developed in an attempt to achieve increased consistency and precision in TSA. By more accurately reproducing the proximal humeral anatomy, it is proposed that clinical outcomes in terms of the range of motion and patient satisfaction may also be improved through newer techniques. Cadaver studies have validated the anatomic precision of this technique.10 Clinical data comprising of patient-reported outcome measures and radiographic outcome studies are currently underway for this arthroplasty system.
ABSTRACT
Proper reconstruction of proximal humeral anatomy is of primary importance to maximize patient outcomes after total shoulder arthroplasty. This article describes a new arthroplasty technique, where a fixed multiplanar bone resection is made and a novel implant, which is designed to precisely match the bone resection, is inserted.
Continue to: The success of total shoulder arthroplasty...
The success of total shoulder arthroplasty (TSA) is largely dependent on how accurate the proximal humeral anatomy is reconstructed and the glenohumeral relationships are restored.1-4 Numerous studies have demonstrated a relationship of worse clinical outcomes and implant failure with nonanatomic implant placement.5-8 The majority of arthroplasty systems rely on surgeon-dependent decision-making to determine the location of the border of the articular surface and, ultimately, the amount and location of bone to be resected. Even in experienced hands, the ability to reproducibly restore the joint line is inconsistent.3
In contrast, the majority of total knee arthroplasty (TKA) systems have been designed with instrumentation that guides the surgeon precisely regarding where and how much femoral bone must be resected, and the corresponding implant is designed with the same thickness to preserve the location of the joint line. Cutting block instrumentation rather than freehand cuts enables reproducibility of TKA while being performed for an estimated 700,000 times annually in the US.9
To achieve similar high levels of reproducibility in shoulder arthroplasty, a new technique was developed based on the principle of providing instrumentation to assist the surgeon in accurately restoring the proximal humeral joint line. This technical article describes the technique of using a multiplanar instrumented cutting system and matching implants to perform TSA. The technique shown was previously studied and was found to allow surgeons to recreate the original anatomy of the humerus with very high precision.10

The humeral prosthesis described in this article has an articular surface that is slightly elliptical to more closely match the actual shape of the humerus bone.11 Biomechanical studies have demonstrated that implants designed with a nonspherical shape have more similar motion and kinematics to those of the native humeral head.

This provides rotation stability, and the implant rests on the strong subchondral bone of the proximal humerus proximal to the anatomic neck rather than relying on metaphyseal bone or canal fixation, as recommended by Aldoiusti.13 It also allows optimal implant placement with complete freedom with respect to inclination, version, and medial/posterior offset from the humeral canal.
Continue to: The implant respects the relationship...
The implant respects the relationship of the rotator cuff insertion and has a recessed superior margin to keep both the implant and the saw blade 3 mm to 5 mm away from the supraspinatus fibers to protect the rotator cuff from iatrogenic injury.
TECHNIQUE
The technique described in this article uses the Catalyst CSR Total Shoulder System (Catalyst OrthoScience), which was cleared to treat arthritis of the shoulder by the US Food and Drug Administration in May 2016.
A standard deltopectoral incision is made, and the surgeon dissects the interval between the pectoralis major medially and the deltoid laterally. The subscapularis can be incised by tenotomy; alternatively, the surgeon can perform a subscapularis peel or a lesser tuberosity osteotomy using this technique.
Once the glenohumeral joint is exposed, the surgeon delivers the humeral head anteriorly. A preferred method is to place a Darrach retractor between the humeral head and the glenoid, and a cobra or a second Darrach retractor behind the superolateral humeral head superficial to the supraspinatus tendon. By simultaneously pressing on both retractors and externally rotating the patient’s arm, the humeral head is delivered anteriorly. Osteophytes on the anterior and inferior edge of the humeral head are generously removed at this time using a rongeur.
Using a pin guide, the long 3.2-mm guidewire pin is drilled under power into the center of the articular surface. The pin guide is then removed, leaving the pin in the center of the humerus (Figure 3).

Continue to: Next, the surgeon...
Next, the surgeon slides the cannulated reamer over the long guidewire pin and under power removes a small portion of the humeral head subchondral bone until the surgeon feels and observes that the reamer is no longer removing bone (Figure 4). The patent-pending reamer design prevents the surgeon from removing more than a few millimeters of bone, after which point the reamer spins on the surface of the bone without resecting further.

The surgeon is aware that the reamer has achieved its desired depth when it is no longer creating new bone shavings, and the surgeon can hear and feel that the reamer is spinning and no longer cutting. Then the surgeon removes the reamer.

The surgeon places the first humeral cut guide over the long guidewire pin, oriented superiorly-inferiorly and secures the guide using 4 short pins, and the long pin is removed. The surgeon uses an oscillating saw to cut the anterior and posterior plane cuts through the saw captures in the cut guide (Figure 5). The humeral cut guide and short pins are removed (Figure 6).

The surgeon then applies the second humeral cut guide to the proximal humerus and secures it using 2 short pins. The surgeon then uses the 6-mm drill to drill the 4 holes for the pegs of the implant. The top portion of the guide is removed, and the surgeon makes the superior and inferior cuts along the top and bottom surfaces of the guide using an oscillating saw (Figure 7).

The surgeon then uses a rongeur to slightly round the edges of the 4 corners at the periphery of the humerus. The second humeral cut guide and short pins are removed (Figure 8).

Continue to: Next, the surgeon trials...
Next, the surgeon trials humeral implants to determine the correct implant size (Figure 9). Once the proper humeral size is chosen, the trial is removed and the humeral cover is placed over the prepared humeral head. The surgeon then proceeds to glenoid preparation (Figure 10), which is easily accessible and facilitated by angled planar cuts on the humeral head. Glenoid technique will be discussed in a subsequent article.

After glenoid preparation and insertion, the humerus is delivered anteriorly. The proximal humerus is washed and dried, and cement is applied to the peg holes in the humerus bone and the underside of the humeral implant. The implant is then inserted using the humeral impactor to apply pressure and assure that the implant is fully seated. Once the humeral cement is hardened, the glenohumeral joint is irrigated and closure begins. Postoperative radiograph is shown in Figure 11.

DISCUSSION
Numerous authors have demonstrated that accurate implant placement is crucial for restoring normal glenoid kinematics and motion,1-4 while some authors have reported worsening clinical outcomes and higher rates of pain and implant loosening when the implants were not placed anatomically.5-8 This is such an important concept that it essentially was the primary inspiration for creating this TSA system. In addition, the system utilizes a nonspherical, elliptical humeral head that more closely matches the anatomy of the proximal humerus,14,15 and this type of shape has shown improved biomechanics in laboratory testing.12

Good results have been demonstrated in restoring the normal anatomy using stemmed devices on the radiographic analysis of cadavers.16 The creation of stemmed implants with variable inclination and offset has improved computer models17 compared with previous studies,18 with the exception of scenarios with extreme offset.
In theory, resurfacing implants and implants without a canal stem should have a better implant placement than that with stemmed implants; however, the ability to restore the center of rotation was even worse for resurfacing prostheses, with 65% of all implants being measured as outliers postoperatively in one study.19 Most of the resurfacing implants and their instrumentation techniques offer little to help the surgeon control for implant height. The depth of the reaming is variable, not calibrated, and not correlated with the implant size, frequently leading to overstuffing after surgery. Second, the use of spherical prostheses forces the surgeon to choose between matching the superior-inferior humeral size, leading to overhang of the implant, or matching the anteroposterior, leading to frequent undersizing in the coronal plane. The nonspherical, elliptical head shape can potentially simplify implant selection.
In summary, new techniques have been developed in an attempt to achieve increased consistency and precision in TSA. By more accurately reproducing the proximal humeral anatomy, it is proposed that clinical outcomes in terms of the range of motion and patient satisfaction may also be improved through newer techniques. Cadaver studies have validated the anatomic precision of this technique.10 Clinical data comprising of patient-reported outcome measures and radiographic outcome studies are currently underway for this arthroplasty system.
1. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409.
2. Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86-A(3):575-580.
3. Iannotti JP, Spencer EE, Winter U, Deffenbaugh D, Williams G. Prosthetic positioning in total shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1 Supple S):111S-121S.
4. Terrier A, Ramondetti S, Merlini F, Pioletti DD, Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(8):1184-1190.
5. Denard PJ, Raiss P, Sowa B, Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22(7):894-900.
6. Figgie HE 3rd, Inglis AE, Goldberg VM, Ranawat CS, Figgie MP, Wile JM. An analysis of factors affecting the long-term results of total shoulder arthroplasty in inflammatory arthritis. J Arthroplasty. 1988;3(2):123-130.
7. Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA 3rd. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16(5):555-562.
8. Flurin PH, Roche CP, Wright TW, Zuckerman JD. Correlation between clinical outcomes and anatomic reconstruction with anatomic total shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73 Suppl 1:S92-S98.
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Goldberg SS, Akyuz E, Murthi AM, Blaine T. Accuracy of humeral articular surface restoration in a novel anatomic shoulder arthroplasty technique and design: a cadaveric study. Journal of Shoulder and Elbow Arthroplasty. 2018;2:2471549217750791.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Jun BJ, Lee TQ, McGarry MH, Quigley RJ, Shin SJ, Iannotti JP. The effects of prosthetic humeral head shape on glenohumeral joint kinematics during humeral axial rotation in total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25(7):1084-1093.
13. Alidousti H, Giles JW, Emery RJH, Jeffers J. Spatial mapping of humeral head bone density. J Shoulder Elbow Surg. 2017;26(9):1653-1661.
14. Harrold F, Wigderowitz C. Humeral head arthroplasty and its ability to restore original humeral head geometry. J Shoulder Elbow Surg. 2013;22(1):115-121.
15. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338.
16. Wirth MA, Ondrla J, Southworth C, Kaar K, Anderson BC, Rockwood CA 3rd. Replicating proximal humeral articular geometry with a third-generation implant: a radiographic study in cadaveric shoulders. J Shoulder Elbow Surg. 2007;16(3 Suppl):S111-S116.
17. Pearl ML, Kurutz S, Postacchini R. Geometric variables in anatomic replacement of the proximal humerus: How much prosthetic geometry is necessary? J Shoulder Elbow Surg. 2009;18(3):366-370.
18. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326.
19. Alolabi B, Youderian AR, Napolitano L, et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1740-1746.
1. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409.
2. Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86-A(3):575-580.
3. Iannotti JP, Spencer EE, Winter U, Deffenbaugh D, Williams G. Prosthetic positioning in total shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1 Supple S):111S-121S.
4. Terrier A, Ramondetti S, Merlini F, Pioletti DD, Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(8):1184-1190.
5. Denard PJ, Raiss P, Sowa B, Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22(7):894-900.
6. Figgie HE 3rd, Inglis AE, Goldberg VM, Ranawat CS, Figgie MP, Wile JM. An analysis of factors affecting the long-term results of total shoulder arthroplasty in inflammatory arthritis. J Arthroplasty. 1988;3(2):123-130.
7. Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA 3rd. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16(5):555-562.
8. Flurin PH, Roche CP, Wright TW, Zuckerman JD. Correlation between clinical outcomes and anatomic reconstruction with anatomic total shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73 Suppl 1:S92-S98.
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Goldberg SS, Akyuz E, Murthi AM, Blaine T. Accuracy of humeral articular surface restoration in a novel anatomic shoulder arthroplasty technique and design: a cadaveric study. Journal of Shoulder and Elbow Arthroplasty. 2018;2:2471549217750791.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Jun BJ, Lee TQ, McGarry MH, Quigley RJ, Shin SJ, Iannotti JP. The effects of prosthetic humeral head shape on glenohumeral joint kinematics during humeral axial rotation in total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25(7):1084-1093.
13. Alidousti H, Giles JW, Emery RJH, Jeffers J. Spatial mapping of humeral head bone density. J Shoulder Elbow Surg. 2017;26(9):1653-1661.
14. Harrold F, Wigderowitz C. Humeral head arthroplasty and its ability to restore original humeral head geometry. J Shoulder Elbow Surg. 2013;22(1):115-121.
15. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338.
16. Wirth MA, Ondrla J, Southworth C, Kaar K, Anderson BC, Rockwood CA 3rd. Replicating proximal humeral articular geometry with a third-generation implant: a radiographic study in cadaveric shoulders. J Shoulder Elbow Surg. 2007;16(3 Suppl):S111-S116.
17. Pearl ML, Kurutz S, Postacchini R. Geometric variables in anatomic replacement of the proximal humerus: How much prosthetic geometry is necessary? J Shoulder Elbow Surg. 2009;18(3):366-370.
18. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326.
19. Alolabi B, Youderian AR, Napolitano L, et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1740-1746.
TAKE-HOME POINTS
- Bone-preserving shoulder arthroplasty is now available and rapidly growing in the US.
- The calibrated, multiplanar instruments and prosthesis shown here allow surgeons to recreate the normal humerus shape with high precision.
- The elliptical, non-spherical design of the humerus prosthesis has shown improved shoulder kinematics compared to standard spherical prostheses.
- The implant rests on dense bone proximal to the anatomic neck where bone support is strong.
- Glenoid implant insertion is routinely performed using this technique and access is facilitated by the angled bone resections.
In Throwers With Posterior Instability, Rotator Cuff Tears Are Common but Do Not Affect Surgical Outcomes
ABSTRACT
In a previous study, compared with throwing athletes with superior labral anterior posterior (SLAP) tears, those with concomitant SLAP tears and rotator cuff tears (RCTs) had significantly poorer outcome scores and return to play. Posterior shoulder instability also occurs in throwing athletes, but no studies currently exist regarding outcomes of these patients with concomitant RCTs.
The authors hypothesized that throwing athletes treated with arthroscopic capsulolabral repair for posterior shoulder instability with coexistent rotator cuff pathology would have poorer outcome scores and return to play.
Fifty-six consecutive throwing athletes with unidirectional posterior shoulder instability underwent arthroscopic capsulolabral repair. Preoperative and postoperative patient-centered outcomes of pain, stability, function, range of motion, strength, and American Shoulder and Elbow Surgeons Shoulder (ASES) scores, as well as return to play, were evaluated. Patients with and without rotator cuff pathology were compared.
Forty-three percent (24/56) of throwing athletes had rotator cuff pathology in addition to posterior capsulolabral pathology. All RCTs were débrided. At a mean of 3 years, there were no differences in preoperative and postoperative patient-centered outcomes between those with and without RCTs. Return-to-play rates showed no between-group differences; 92% (22/24) of athletes with concomitant RCTs returned to sport (P = .414) and 67% (16/24) returned to the same level (P = .430).
Arthroscopic capsulolabral reconstruction is successful in throwing athletes with RCTs treated with arthroscopic débridement. Unlike the previous study evaluating throwers outcomes after surgical treatment for concomitant SLAP tears and RCTs, the authors found no difference in patient-reported outcome measures or return to play for throwing athletes with concomitant posterior shoulder instability and RCTs. In throwing athletes with concomitant posterior instability and RCTs, arthroscopic posterior capsulolabral repair with rotator cuff débridement is successful.
Continue to: Posterior shoulder instability...
Posterior shoulder instability is an important and increasingly recognized pathology among throwers. Like the superior labrum, the posterior capsulolabral complex is also susceptible to injury during the throwing motion; the posterior labrum being most at risk during the late cocking and follow-through phases. Recent studies have found that arthroscopic capsulolabral reconstruction in posterior shoulder instability is successful in allowing athletes to return to their preinjury sports activities, with 2 studies detailing outcomes in throwing athletes.1-4 However, superior labral anterior posterior (SLAP) tears are common in throwing athletes and have been treated with varying and limited success. Further, in a study of outcomes of arthroscopic repair of SLAP lesions, Neri and colleagues5 found that, compared with throwing athletes with SLAP tears, throwing athletes with concomitant SLAP tears and partial-thickness rotator cuff tears (RCTs) had significantly poorer outcomes and return-to-play rates after surgical repair.
The purpose of this study was to determine outcome scores and return to play of throwing athletes treated with arthroscopic capsulolabral repair for posterior shoulder instability with coexistent RCTs and to compare them with outcome scores as well as return to play of throwing athletes with isolated posterior shoulder instability. It was hypothesized that throwing athletes with a combination of posterior shoulder instability and RCT would have poorer outcomes and poorer return to play after surgery.5
METHODS
PATIENT SELECTION
After Institutional Review Board approval, informed consent was obtained, and consecutive throwing athletes who underwent arthroscopic posterior capsulolabral reconstruction for posterior shoulder instability were followed in the perioperative period. Inclusion criteria were throwing athletes participating in competitive sports at the high school, collegiate, or professional level, minimum 1-year follow-up, presence of unidirectional posterior instability, and absence of symptoms of instability in any direction other than posterior. Patients with inferior instability, SLAP pathology on examination and on magnetic resonance imaging, multidirectional instability, or habitual or psychogenic voluntary shoulder subluxations were excluded. Patients with diagnoses of both posterior shoulder instability and impingement treated with subacromial decompression and distal clavicle resection were also excluded.
After this cohort was identified, patient records were reviewed for pertinent operative data, such as procedure, complications, and evidence of RCT by operative report and arthroscopic photographs. A partial RCT was defined as a tear of 10% to 50%; those with rotator cuff fraying were determined not to be significant.
PATIENT EVALUATION
Surgeries were performed between January 1998 and December 2009 by the senior author (JPB). All patients were followed with clinical examinations, radiographs, and subjective grading scales. Recorded patient demographic data included age, sex, sport, position, competition level, and follow-up duration.
Continue to: All patients had...
All patients had symptomatic posterior shoulder instability, including posterior shoulder pain, clicking, a sensation of subluxation, or instability/apprehension with motion. Each athlete’s shoulder was palpated for tenderness and tested for impingement. Specific posterior glenohumeral instability tests, including the Kim test,6 the circumduction test, the jerk test,7 the posterior load-and-shift test,8 and the posterior stress test,9 were performed on all patients. Patients with multidirectional instability on the sulcus test, as well as provocative tests indicating SLAP pathology, such as the Crank test and the active compression test, were not included. Standard radiography and magnetic resonance arthrography (MRA) were performed to further narrow inclusion and exclusion criteria.
Both before surgery and at latest follow-up, patient outcomes were evaluated using the American Shoulder and Elbow Surgeons (ASES) score (range, 0-100) which combines a subjective functional scale measuring activities of daily living (0-3 for each of 10 tasks, with a total of 0-30) and a subjective pain scale (0-10, with 10 being worst pain). Values >80 were described as excellent, and failures were defined as scores <60 after surgery.10 A subjective stability scale (0-10, with 0 indicating completely stable and 10 completely unstable), strength scale (0-3, with 0 indicating none, 1 markedly decreased, 2 slightly decreased, and 3 normal), and ROM scale (0-3, with 0 indicating poor, 1 limited, 2 satisfactory, and 3 full) were evaluated both before surgery and at the latest follow-up. A stability score >5 after surgery was defined as a failure.1,2,11 Patients were also asked if, based on their current state, they would undergo surgery again. Intraoperative findings and specific surgical procedures performed were correlated with the aforementioned subjective and objective outcome scores.
OPERATIVE TREATMENT
Throwing athletes who met inclusion criteria and failed nonoperative management underwent surgery by the senior author (JPB). Each patient was examined under anesthesia and, with the patient in the lateral decubitus position, a diagnostic arthroscopy was performed to identify posterior capsulolabral complex pathology, including a patulous capsule, capsular tears, labral fraying, and labral tears. A careful examination for rotator cuff pathology was also performed. Based on preoperative clinical examination, MRA, examination under anesthesia, pathologic findings at diagnostic arthroscopic surgery, and surgeon experience, capsulolabral plication was performed with or without suture anchors.2,5 After capsulolabral repair, the capsule was evaluated for residual laxity, and additional plication sutures were placed, as indicated, with care to avoid overconstraint in these throwing athletes.1 Posterior glenohumeral stability restoration was judged by removing traction and performing posterior load-and-shift and posterior stress tests. Any RCT with <50% thickness was débrided. Postoperative care and rehabilitation were carried out as previously described and were not altered by the presence or absence of a RCT.3
STATISTICAL ANALYSIS
Preoperative and latest follow-up ASES scores, stability scores, functional scores, and pain-level findings were compared using paired-samples Comparisons between groups, including throwing athletes with and without rotator cuff pathology, were done using the Student t test. Outcome comparisons between multiple groups, which included intraoperative findings and surgical fixation methods, were analyzed with c2 modeling for nonparametric data. Statistical significance was set at P < .05. A power analysis found that this study was able to detect a meaningful difference of 10 ASES points.
RESULTS
PATIENT DEMOGRAPHIC CHARACTERISTICS
Of the 56 throwing athletes who met the inclusion criteria, 24 were found to have rotator cuff pathology in addition to posterior capsulolabral pathology, while 32 were found to have capsulolabral pathology alone. Demographic data are listed in Table 1. Mean age was 20.1 years for patients with rotator cuff pathology and 17.8 years for patients without RCTs. All 24 athletes with rotator cuff pathology were treated with arthroscopic débridement. Mean follow-up was 38.6 months (range, 16.5-63.6 months) for patients with RCTs and 39.1 months (range, 12-98.8 months) for patients without RCTs. No significant difference was found in age, sports level, or follow-up between groups.
Table 1. Demographic Data for Athletes With Posterior Instability With and Without Rotator Cuff Tears (N = 56 Shoulders)a
Characteristic | Rotator Cuff Tears | |
Yes | No | |
| Total | 24 | 32 |
| Sex | ||
| Male | 16 | 27 |
| Female | 8 | 5 |
| Mean age, y | 20.1 | 17.8 |
| Mean follow up, mo | 38.6 | 39.1 |
| Participation level | ||
| Professional | 1 | 0 |
| College | 4 | 4 |
| High school | 17 | 26 |
| Recreational | 2 | 2 |
aThe majority of athletes were males in high school and their mean follow-up was 3 years.
Continue to: Outcomes
OUTCOMES
Table 2 lists the preoperative and postoperative scores for shoulder performance in throwing athletes with posterior shoulder instability, with and without RCTs.
Table 2. Preoperative and Postoperative Scores for Shoulder Performance in Throwing Athletes With Posterior Shoulder Instability With and Without Rotator Cuff Tearsa
| With Rotator Cuff Tears (n=24 shoulders) | Without Rotator Cuff Tears (n=32 shoulders) | |||||||||
| Preoperative | Latest Follow-Up | Preoperative | Latest Follow-Up | |||||||
| Outcome Measure | Mean Score | Range | Mean Score | Range | P | Mean Score | Range | Mean Score | Range | P |
ASES 0-100 0 = worst | 41.8 | 20-70 | 85.4 | 67-100 | <.05 | 49.7 | 20-85 | 83.1 | 25-100 | <.05 |
Stability 0-10 0 = most stable | 6.7 | 2-10 | 2.4 | 0-6 | <.05 | 7.8 | 0-10 | 2.4 | 0-8 | <.05 |
Pain 0-10 10 = worst | 7.6 | 5-10 | 1.9 | 0-5 | <.05 | 6.3 | 0-10 | 2.2 | 0-7 | <.05 |
Function 0-30 0 = worst | 18.5 | 6-27 | 27 | 16-30 | <.05 | 19.0 | 8-26 | 26.4 | 6-30 | <.05 |
aThere was no difference in ASES, stability, pain, or functional scores between athletes with posterior instability alone compared with patients with concomitant rotator cuff tears.
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
ASES Scores. Mean preoperative ASES scores for patients with RCTs improved significantly (t = –13.8, P < .001), as did those for patients without rotator cuff pathology (t = –8.9, P < .001). No significant differences in ASES score were found between patients with and without rotator cuff pathology before or after surgery (t = 1.9, P = .07; t = .58, P = .06). In addition, 70.8% (17/24) of throwing athletes with rotator cuff pathology had an excellent postoperative outcome (ASES score >80), and 29.2% (7/24) had a satisfactory outcome (ASES score, 60-80). Thus, 100% of those with concomitant posterior shoulder instability and RCTs had a good or excellent outcome after surgical intervention. In those without rotator cuff pathology, 78.1% (25/32) had an excellent outcome, 12.5% (4/32) had a satisfactory outcome, and 9.4% (3/32) had a poor outcome. Thus, 91% of those without rotator cuff pathology had a good or excellent outcome after surgery.
Stability. Preoperative stability scores improved significantly after surgery in both groups (t = 7.2, P < .001; t = 10.5, P < .001). There were no statistical differences between preoperative or postoperative stability scores in those with or without rotator cuff pathology (t = 1.7, P = .095; t = .03, P = .975). Of throwing athletes with RCTs, 54.2% (13/24) had an excellent outcome, 33.3% (8/24) a good outcome, and 12.5% (3/24) a satisfactory outcome. Thus, 87.5% (21/24) of those with RCTs had a good or excellent outcome in terms of stability. In those without rotator cuff pathology, 46.9% (15/32) had excellent stability, 46.9% (15/32) had good stability, and 3.1% (1/32) had satisfactory stability after surgery. Thus, 93.8% (30/32) of throwing athletes without rotator cuff pathology had good or excellent stability after surgery.
Pain. Mean preoperative pain scores for those with and without rotator cuff pathology improved significantly (t = 13.4, P < .001; t = 7.1, P < .001). There was no statistical difference in preoperative or postoperative pain scores between those with and without rotator cuff pathology (t = 1.99, P = .051; t = .49, P = .627).
Function. Mean preoperative function scores for both groups improved significantly (t = 7.7, P < .001; t = 8.0, P < .001). There was no difference in improvement in functional scores between the two groups before or after surgery (t = .36, P = .721; t = .5, P = .622).
Continue to: ROM
ROM. Of those with rotator cuff pathology, 54% (13/24) had normal ROM, 42% (10/24) had satisfactory ROM, and 4% (1/24) had limited ROM. In throwing athletes without rotator cuff pathology, 34% (11/32) had normal ROM, 53.1% (17/32) had satisfactory ROM, and 9% (3/32) had limited ROM after surgery. There was no significant difference in ROM between the groups (c2 = 2.7, P = .260).
Strength. Of those with RCTs, 67% (16/24) reported normal strength, 29% (7/24) slightly decreased strength, and 4% (1/24) markedly decreased strength. Of those throwing athletes without rotator cuff pathology, 50% (16/32) had normal strength, 41% (13/32) had slightly decreased strength, and 9% (3/32) had markedly decreased strength. No statistical difference was noted between the two groups (c2 = 1.7, P = .429).
Return to Sport. Of those with RCTs, 92% (22/24) returned to sport while 84% (27/32) of throwing athletes without RCTs returned to sport. There was no difference between the two groups (c2 = .667, P = .414). Sixty-seven percent (16/24) of those with RCTs and 56% (18/32) of those without RCTs returned to the same level of sport. No statistical difference was found in return to play between throwing athletes with and without rotator cuff pathology (c2 = .624, P = .430).
Failures. According to ASES scores, no throwers with RCTs failed, while 9.4% (3/32) with posterior instability alone failed. Regarding stability, 8.3% (2/24) of athletes with RCTs failed, while 6.3% (2/32) with posterior instability alone failed.
SURGICAL FINDINGS AND PROCEDURES
Of the 24 throwing athletes with rotator cuff pathology, 92% (22/24) had labral tears, while 78% (25/32) of those without RCTs had labral tears. The majority of RCTs were in the posterior supraspinatus and anterior infraspinatus regions. This was not significantly different between groups (c2 = 1.86, P = .172). All labral pathology was posterior-inferior, and all RCTs were <50% thickness, and therefore were débrided. Fifty-four percent (13/24) of those with RCTs had a patulous capsule and 63% (20/32) of throwing athletes without rotator cuff pathology had a patulous capsule. There was no significant difference between groups (c2 = .393, P = .530). Of those with RCTs, 92% (22/24) had surgical fixation with anchors, while 78% (25/32) of those without rotator cuff pathology underwent repair with anchor fixation. There was no statistically significant difference in anchor use between groups (c2 = 1.86, P = .172).
Continue to: Discussion
DISCUSSION
Throwing athletes with and without RCTs had similar rates of recovery and return to play after arthroscopic capsular labral repair, with rotator cuff débridement if a tear was present. The mean follow-up was 3.2 years. Further, there was no difference in return to play (92% vs 84%), ASES score, stability, pain, function, ROM, or strength between the 2 groups before or after surgery. In this cohort of 56 patients, 24 throwing athletes (43%) were found to have RCTs.
Return-to-play rates showed no between-group differences; 92% (22/24) of athletes with concomitant RCTs returned to sport, and 67% (16/24) returned to the same level. Eight percent of throwing athletes with RCTs were unable to return to sport after surgery. These return-to-play rates are an improvement over most previously reported rates in throwing athletes and in posterior shoulder instability in general.1-4,11 When these athletes are compared with their counterparts with combined SLAP tears and RCTs, return-to-play rates are notably higher. There may be discrepancies in interpreting return-to-play between the two studies, but in the current study, 67% of those with concomitant RCTs achieved return to preinjury level of play. This is 10% higher than the rate reported in athletes with SLAP tears alone (57%) and even higher than in those with concomitant SLAP and RCTs. It is also essential to note that a number of this cohort’s athletes who did not return to play did so for factors (eg, graduation) unrelated to the shoulder. However, the study by Neri and colleagues5 included professional athletes who likely all attempted to return to play and, if unable to perform at the same level, likely were unable to continue their professional career.5
All patients with RCTs had a good or excellent outcome (ASES score), and 70.8% had an excellent outcome. Similarly, 97% of those without rotator cuff pathology had a good or excellent outcome, and 81.3% had an excellent outcome. There was no significant difference between the two groups. These results parallel those of Neri and colleagues’5 study of SLAP tears with RCTs, where 96% (22/23) of throwing athletes had a good or excellent outcome. Despite these high outcome scores in patients with SLAP tears, only 57% were able to return to elite pitching.5 In the current study, pain was slightly higher for those with rotator cuff pathology before surgery—a finding consistent with pain frequently being found in patients with isolated partial-thickness RCTs. Their postoperative pain scores were actually lower on average than those of patients without RCTs, which suggests simple débridement of undersurface tears adequately addressed the pathology. The authors theorize that the main pain generator in this population may be posterior instability, and that the rotator cuff has less of an influence. In the SLAP population, the main pain generator likely is the RCT.
Failures by ASES score or strength were fairly rare in this cohort. Many patients opted to have revision surgery because of continued instability, pain, decreased function, or reinjury. One potential cause of failure in this cohort is inadequate capsular shift. However, capsular plication in throwing athletes is difficult to address, as overtensioning the repair can lead to the inability to adequately perform overhead activites.3,4 This cannot be overemphasized, particularly with pitchers.
Partial-thickness RCTs, particularly those on the articular side, are common in throwing athletes because of high tensile and compressive loads.12 Despite the known risk of RCTs with posterior shoulder instability in throwing athletes, the authors are unaware of reports of the incidence or treatment of this pathology. RCTs in this posterior instability group likely represent a pathology other than internal impingement. The high proportion of throwing athletes with RCTs in this study (43%) indicates a need for close evaluation of rotator cuff pathology in young throwing athletes. Ide et al found that 75% of patients with SLAP tears had partial articular-sided RCTs.13 In the current study, all RCTs were small partial tears, and arthroscopic débridement was performed. It is unknown whether repair of these RCTs would impact return to play. However, rotator cuff repair in this population has been shown to have poor outcomes. Tear thickness typically is used to determine treatment, with débridement performed if <50% tendon thickness is affected. More recently, many have advocated having greater tendon involvement in throwers before repair, because of poor outcomes. Although studies are limited, tear size does seem to correlate with outcomes.14
Continue to: Study Limitations
STUDY LIMITATIONS
Limitations of this study include its small number of professional throwing athletes, with the majority being high school athletes. Further, although ASES scores are consistently used in posterior shoulder instability studies, these scores are influenced highly by pain scores, and some argue that other scoring systems may provide more useful information. However, none of the more modern scoring systems have been studied extensively in posterior glenohumeral instability. Further, because the authors used the present scoring systems previously,1-4 they were continued to be used for comparison and consistency. Outcomes such as ROM and strength may carry more weight if measured and documented by clinical examination. Further testing, such as clinical evaluation of the jerk test or the posterior load-and-shift test, and their comparison before and after surgery may provide more objective data.
CONCLUSION
Arthroscopic capsulolabral reconstruction is successful in throwing athletes with RCTs treated with arthroscopic débridement. Unlike a previous study of throwing athletes’ outcomes after surgery for concomitant SLAP tears and RCTs,5 this study of throwing athletes with concomitant posterior shoulder instability and RCTs found no difference in patient-reported outcome measures or return to play. In throwing athletes with posterior instability and RCTs, arthroscopic posterior capsulolabral repair with rotator cuff débridement is successful.
1. Bradley JP, Baker CL 3rd, Kline AJ, Armfield DR, Chhabra A. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 100 shoulders. Am J Sports Med. 2006;34(7):1061-1071.
2. Bradley JP, McClincy MP, Arner JW, Tejwani SG. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 200 shoulders. Am J Sports Med. 2013;41(9):2005-2014.
3. McClincy MP, Arner JW, Bradley JP. Posterior shoulder instability in throwing athletes: a case-matched comparison of throwers and non-throwers. Arthroscopy. 2015;31(6):1041-1051.
4. Radkowski CA, Chhabra A, Baker CL 3rd, Tejwani SG, Bradley JP. Arthroscopic capsulolabral repair for posterior shoulder instability in throwing athletes compared with nonthrowing athletes. Am J Sports Med. 2008;36(4):693-699.
5. Neri BR, ElAttrache NS, Owsley KC, Mohr K, Yocum LA. Outcome of type II superior labral anterior posterior repairs in elite overhead athletes: effect of concomitant partial-thickness rotator cuff tears. Am J Sports Med. 2011;39(1):114-120.
6. Kim SH, Park JS, Jeong WK, Shin SK. The Kim test: a novel test for posteroinferior labral lesion of the shoulder—a comparison to the jerk test. Am J Sports Med. 2005;33(8):1188-1192.
7. Antoniou J, Duckworth DT, Harryman DT 2nd. Capsulolabral augmentation for the management of posteroinferior instability of the shoulder. J Bone Joint Surg Am. 2000;82(9):1220-1230.
8. Altchek DW, Hobbs WR. Evaluation and management of shoulder instability in the elite overhead thrower. Orthop Clin North Am. 2001;32(3):423-430, viii.
9. Fuchs B, Jost B, Gerber C. Posterior-inferior capsular shift for the treatment of recurrent, voluntary posterior subluxation of the shoulder. J Bone Joint Surg Am. 2000;82(1):16-25.
10. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
11. Arner JW, McClincy MP, Bradley JP. Arthroscopic stabilization of posterior shoulder instability is successful in American football players. Arthroscopy. 2015;31(8):1466-1471.
12. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
13. Ide J, Maeda S, Takagi K. Sports activity after arthroscopic superior labral repair using suture anchors in overhead-throwing athletes. Am J Sports Med. 2005;33(4):507-514.
14. Economopoulos KJ, Brockmeier SF. Rotator cuff tears in overhead athletes. Clin Sports Med. 2012;31(4):675-692.
ABSTRACT
In a previous study, compared with throwing athletes with superior labral anterior posterior (SLAP) tears, those with concomitant SLAP tears and rotator cuff tears (RCTs) had significantly poorer outcome scores and return to play. Posterior shoulder instability also occurs in throwing athletes, but no studies currently exist regarding outcomes of these patients with concomitant RCTs.
The authors hypothesized that throwing athletes treated with arthroscopic capsulolabral repair for posterior shoulder instability with coexistent rotator cuff pathology would have poorer outcome scores and return to play.
Fifty-six consecutive throwing athletes with unidirectional posterior shoulder instability underwent arthroscopic capsulolabral repair. Preoperative and postoperative patient-centered outcomes of pain, stability, function, range of motion, strength, and American Shoulder and Elbow Surgeons Shoulder (ASES) scores, as well as return to play, were evaluated. Patients with and without rotator cuff pathology were compared.
Forty-three percent (24/56) of throwing athletes had rotator cuff pathology in addition to posterior capsulolabral pathology. All RCTs were débrided. At a mean of 3 years, there were no differences in preoperative and postoperative patient-centered outcomes between those with and without RCTs. Return-to-play rates showed no between-group differences; 92% (22/24) of athletes with concomitant RCTs returned to sport (P = .414) and 67% (16/24) returned to the same level (P = .430).
Arthroscopic capsulolabral reconstruction is successful in throwing athletes with RCTs treated with arthroscopic débridement. Unlike the previous study evaluating throwers outcomes after surgical treatment for concomitant SLAP tears and RCTs, the authors found no difference in patient-reported outcome measures or return to play for throwing athletes with concomitant posterior shoulder instability and RCTs. In throwing athletes with concomitant posterior instability and RCTs, arthroscopic posterior capsulolabral repair with rotator cuff débridement is successful.
Continue to: Posterior shoulder instability...
Posterior shoulder instability is an important and increasingly recognized pathology among throwers. Like the superior labrum, the posterior capsulolabral complex is also susceptible to injury during the throwing motion; the posterior labrum being most at risk during the late cocking and follow-through phases. Recent studies have found that arthroscopic capsulolabral reconstruction in posterior shoulder instability is successful in allowing athletes to return to their preinjury sports activities, with 2 studies detailing outcomes in throwing athletes.1-4 However, superior labral anterior posterior (SLAP) tears are common in throwing athletes and have been treated with varying and limited success. Further, in a study of outcomes of arthroscopic repair of SLAP lesions, Neri and colleagues5 found that, compared with throwing athletes with SLAP tears, throwing athletes with concomitant SLAP tears and partial-thickness rotator cuff tears (RCTs) had significantly poorer outcomes and return-to-play rates after surgical repair.
The purpose of this study was to determine outcome scores and return to play of throwing athletes treated with arthroscopic capsulolabral repair for posterior shoulder instability with coexistent RCTs and to compare them with outcome scores as well as return to play of throwing athletes with isolated posterior shoulder instability. It was hypothesized that throwing athletes with a combination of posterior shoulder instability and RCT would have poorer outcomes and poorer return to play after surgery.5
METHODS
PATIENT SELECTION
After Institutional Review Board approval, informed consent was obtained, and consecutive throwing athletes who underwent arthroscopic posterior capsulolabral reconstruction for posterior shoulder instability were followed in the perioperative period. Inclusion criteria were throwing athletes participating in competitive sports at the high school, collegiate, or professional level, minimum 1-year follow-up, presence of unidirectional posterior instability, and absence of symptoms of instability in any direction other than posterior. Patients with inferior instability, SLAP pathology on examination and on magnetic resonance imaging, multidirectional instability, or habitual or psychogenic voluntary shoulder subluxations were excluded. Patients with diagnoses of both posterior shoulder instability and impingement treated with subacromial decompression and distal clavicle resection were also excluded.
After this cohort was identified, patient records were reviewed for pertinent operative data, such as procedure, complications, and evidence of RCT by operative report and arthroscopic photographs. A partial RCT was defined as a tear of 10% to 50%; those with rotator cuff fraying were determined not to be significant.
PATIENT EVALUATION
Surgeries were performed between January 1998 and December 2009 by the senior author (JPB). All patients were followed with clinical examinations, radiographs, and subjective grading scales. Recorded patient demographic data included age, sex, sport, position, competition level, and follow-up duration.
Continue to: All patients had...
All patients had symptomatic posterior shoulder instability, including posterior shoulder pain, clicking, a sensation of subluxation, or instability/apprehension with motion. Each athlete’s shoulder was palpated for tenderness and tested for impingement. Specific posterior glenohumeral instability tests, including the Kim test,6 the circumduction test, the jerk test,7 the posterior load-and-shift test,8 and the posterior stress test,9 were performed on all patients. Patients with multidirectional instability on the sulcus test, as well as provocative tests indicating SLAP pathology, such as the Crank test and the active compression test, were not included. Standard radiography and magnetic resonance arthrography (MRA) were performed to further narrow inclusion and exclusion criteria.
Both before surgery and at latest follow-up, patient outcomes were evaluated using the American Shoulder and Elbow Surgeons (ASES) score (range, 0-100) which combines a subjective functional scale measuring activities of daily living (0-3 for each of 10 tasks, with a total of 0-30) and a subjective pain scale (0-10, with 10 being worst pain). Values >80 were described as excellent, and failures were defined as scores <60 after surgery.10 A subjective stability scale (0-10, with 0 indicating completely stable and 10 completely unstable), strength scale (0-3, with 0 indicating none, 1 markedly decreased, 2 slightly decreased, and 3 normal), and ROM scale (0-3, with 0 indicating poor, 1 limited, 2 satisfactory, and 3 full) were evaluated both before surgery and at the latest follow-up. A stability score >5 after surgery was defined as a failure.1,2,11 Patients were also asked if, based on their current state, they would undergo surgery again. Intraoperative findings and specific surgical procedures performed were correlated with the aforementioned subjective and objective outcome scores.
OPERATIVE TREATMENT
Throwing athletes who met inclusion criteria and failed nonoperative management underwent surgery by the senior author (JPB). Each patient was examined under anesthesia and, with the patient in the lateral decubitus position, a diagnostic arthroscopy was performed to identify posterior capsulolabral complex pathology, including a patulous capsule, capsular tears, labral fraying, and labral tears. A careful examination for rotator cuff pathology was also performed. Based on preoperative clinical examination, MRA, examination under anesthesia, pathologic findings at diagnostic arthroscopic surgery, and surgeon experience, capsulolabral plication was performed with or without suture anchors.2,5 After capsulolabral repair, the capsule was evaluated for residual laxity, and additional plication sutures were placed, as indicated, with care to avoid overconstraint in these throwing athletes.1 Posterior glenohumeral stability restoration was judged by removing traction and performing posterior load-and-shift and posterior stress tests. Any RCT with <50% thickness was débrided. Postoperative care and rehabilitation were carried out as previously described and were not altered by the presence or absence of a RCT.3
STATISTICAL ANALYSIS
Preoperative and latest follow-up ASES scores, stability scores, functional scores, and pain-level findings were compared using paired-samples Comparisons between groups, including throwing athletes with and without rotator cuff pathology, were done using the Student t test. Outcome comparisons between multiple groups, which included intraoperative findings and surgical fixation methods, were analyzed with c2 modeling for nonparametric data. Statistical significance was set at P < .05. A power analysis found that this study was able to detect a meaningful difference of 10 ASES points.
RESULTS
PATIENT DEMOGRAPHIC CHARACTERISTICS
Of the 56 throwing athletes who met the inclusion criteria, 24 were found to have rotator cuff pathology in addition to posterior capsulolabral pathology, while 32 were found to have capsulolabral pathology alone. Demographic data are listed in Table 1. Mean age was 20.1 years for patients with rotator cuff pathology and 17.8 years for patients without RCTs. All 24 athletes with rotator cuff pathology were treated with arthroscopic débridement. Mean follow-up was 38.6 months (range, 16.5-63.6 months) for patients with RCTs and 39.1 months (range, 12-98.8 months) for patients without RCTs. No significant difference was found in age, sports level, or follow-up between groups.
Table 1. Demographic Data for Athletes With Posterior Instability With and Without Rotator Cuff Tears (N = 56 Shoulders)a
Characteristic | Rotator Cuff Tears | |
Yes | No | |
| Total | 24 | 32 |
| Sex | ||
| Male | 16 | 27 |
| Female | 8 | 5 |
| Mean age, y | 20.1 | 17.8 |
| Mean follow up, mo | 38.6 | 39.1 |
| Participation level | ||
| Professional | 1 | 0 |
| College | 4 | 4 |
| High school | 17 | 26 |
| Recreational | 2 | 2 |
aThe majority of athletes were males in high school and their mean follow-up was 3 years.
Continue to: Outcomes
OUTCOMES
Table 2 lists the preoperative and postoperative scores for shoulder performance in throwing athletes with posterior shoulder instability, with and without RCTs.
Table 2. Preoperative and Postoperative Scores for Shoulder Performance in Throwing Athletes With Posterior Shoulder Instability With and Without Rotator Cuff Tearsa
| With Rotator Cuff Tears (n=24 shoulders) | Without Rotator Cuff Tears (n=32 shoulders) | |||||||||
| Preoperative | Latest Follow-Up | Preoperative | Latest Follow-Up | |||||||
| Outcome Measure | Mean Score | Range | Mean Score | Range | P | Mean Score | Range | Mean Score | Range | P |
ASES 0-100 0 = worst | 41.8 | 20-70 | 85.4 | 67-100 | <.05 | 49.7 | 20-85 | 83.1 | 25-100 | <.05 |
Stability 0-10 0 = most stable | 6.7 | 2-10 | 2.4 | 0-6 | <.05 | 7.8 | 0-10 | 2.4 | 0-8 | <.05 |
Pain 0-10 10 = worst | 7.6 | 5-10 | 1.9 | 0-5 | <.05 | 6.3 | 0-10 | 2.2 | 0-7 | <.05 |
Function 0-30 0 = worst | 18.5 | 6-27 | 27 | 16-30 | <.05 | 19.0 | 8-26 | 26.4 | 6-30 | <.05 |
aThere was no difference in ASES, stability, pain, or functional scores between athletes with posterior instability alone compared with patients with concomitant rotator cuff tears.
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
ASES Scores. Mean preoperative ASES scores for patients with RCTs improved significantly (t = –13.8, P < .001), as did those for patients without rotator cuff pathology (t = –8.9, P < .001). No significant differences in ASES score were found between patients with and without rotator cuff pathology before or after surgery (t = 1.9, P = .07; t = .58, P = .06). In addition, 70.8% (17/24) of throwing athletes with rotator cuff pathology had an excellent postoperative outcome (ASES score >80), and 29.2% (7/24) had a satisfactory outcome (ASES score, 60-80). Thus, 100% of those with concomitant posterior shoulder instability and RCTs had a good or excellent outcome after surgical intervention. In those without rotator cuff pathology, 78.1% (25/32) had an excellent outcome, 12.5% (4/32) had a satisfactory outcome, and 9.4% (3/32) had a poor outcome. Thus, 91% of those without rotator cuff pathology had a good or excellent outcome after surgery.
Stability. Preoperative stability scores improved significantly after surgery in both groups (t = 7.2, P < .001; t = 10.5, P < .001). There were no statistical differences between preoperative or postoperative stability scores in those with or without rotator cuff pathology (t = 1.7, P = .095; t = .03, P = .975). Of throwing athletes with RCTs, 54.2% (13/24) had an excellent outcome, 33.3% (8/24) a good outcome, and 12.5% (3/24) a satisfactory outcome. Thus, 87.5% (21/24) of those with RCTs had a good or excellent outcome in terms of stability. In those without rotator cuff pathology, 46.9% (15/32) had excellent stability, 46.9% (15/32) had good stability, and 3.1% (1/32) had satisfactory stability after surgery. Thus, 93.8% (30/32) of throwing athletes without rotator cuff pathology had good or excellent stability after surgery.
Pain. Mean preoperative pain scores for those with and without rotator cuff pathology improved significantly (t = 13.4, P < .001; t = 7.1, P < .001). There was no statistical difference in preoperative or postoperative pain scores between those with and without rotator cuff pathology (t = 1.99, P = .051; t = .49, P = .627).
Function. Mean preoperative function scores for both groups improved significantly (t = 7.7, P < .001; t = 8.0, P < .001). There was no difference in improvement in functional scores between the two groups before or after surgery (t = .36, P = .721; t = .5, P = .622).
Continue to: ROM
ROM. Of those with rotator cuff pathology, 54% (13/24) had normal ROM, 42% (10/24) had satisfactory ROM, and 4% (1/24) had limited ROM. In throwing athletes without rotator cuff pathology, 34% (11/32) had normal ROM, 53.1% (17/32) had satisfactory ROM, and 9% (3/32) had limited ROM after surgery. There was no significant difference in ROM between the groups (c2 = 2.7, P = .260).
Strength. Of those with RCTs, 67% (16/24) reported normal strength, 29% (7/24) slightly decreased strength, and 4% (1/24) markedly decreased strength. Of those throwing athletes without rotator cuff pathology, 50% (16/32) had normal strength, 41% (13/32) had slightly decreased strength, and 9% (3/32) had markedly decreased strength. No statistical difference was noted between the two groups (c2 = 1.7, P = .429).
Return to Sport. Of those with RCTs, 92% (22/24) returned to sport while 84% (27/32) of throwing athletes without RCTs returned to sport. There was no difference between the two groups (c2 = .667, P = .414). Sixty-seven percent (16/24) of those with RCTs and 56% (18/32) of those without RCTs returned to the same level of sport. No statistical difference was found in return to play between throwing athletes with and without rotator cuff pathology (c2 = .624, P = .430).
Failures. According to ASES scores, no throwers with RCTs failed, while 9.4% (3/32) with posterior instability alone failed. Regarding stability, 8.3% (2/24) of athletes with RCTs failed, while 6.3% (2/32) with posterior instability alone failed.
SURGICAL FINDINGS AND PROCEDURES
Of the 24 throwing athletes with rotator cuff pathology, 92% (22/24) had labral tears, while 78% (25/32) of those without RCTs had labral tears. The majority of RCTs were in the posterior supraspinatus and anterior infraspinatus regions. This was not significantly different between groups (c2 = 1.86, P = .172). All labral pathology was posterior-inferior, and all RCTs were <50% thickness, and therefore were débrided. Fifty-four percent (13/24) of those with RCTs had a patulous capsule and 63% (20/32) of throwing athletes without rotator cuff pathology had a patulous capsule. There was no significant difference between groups (c2 = .393, P = .530). Of those with RCTs, 92% (22/24) had surgical fixation with anchors, while 78% (25/32) of those without rotator cuff pathology underwent repair with anchor fixation. There was no statistically significant difference in anchor use between groups (c2 = 1.86, P = .172).
Continue to: Discussion
DISCUSSION
Throwing athletes with and without RCTs had similar rates of recovery and return to play after arthroscopic capsular labral repair, with rotator cuff débridement if a tear was present. The mean follow-up was 3.2 years. Further, there was no difference in return to play (92% vs 84%), ASES score, stability, pain, function, ROM, or strength between the 2 groups before or after surgery. In this cohort of 56 patients, 24 throwing athletes (43%) were found to have RCTs.
Return-to-play rates showed no between-group differences; 92% (22/24) of athletes with concomitant RCTs returned to sport, and 67% (16/24) returned to the same level. Eight percent of throwing athletes with RCTs were unable to return to sport after surgery. These return-to-play rates are an improvement over most previously reported rates in throwing athletes and in posterior shoulder instability in general.1-4,11 When these athletes are compared with their counterparts with combined SLAP tears and RCTs, return-to-play rates are notably higher. There may be discrepancies in interpreting return-to-play between the two studies, but in the current study, 67% of those with concomitant RCTs achieved return to preinjury level of play. This is 10% higher than the rate reported in athletes with SLAP tears alone (57%) and even higher than in those with concomitant SLAP and RCTs. It is also essential to note that a number of this cohort’s athletes who did not return to play did so for factors (eg, graduation) unrelated to the shoulder. However, the study by Neri and colleagues5 included professional athletes who likely all attempted to return to play and, if unable to perform at the same level, likely were unable to continue their professional career.5
All patients with RCTs had a good or excellent outcome (ASES score), and 70.8% had an excellent outcome. Similarly, 97% of those without rotator cuff pathology had a good or excellent outcome, and 81.3% had an excellent outcome. There was no significant difference between the two groups. These results parallel those of Neri and colleagues’5 study of SLAP tears with RCTs, where 96% (22/23) of throwing athletes had a good or excellent outcome. Despite these high outcome scores in patients with SLAP tears, only 57% were able to return to elite pitching.5 In the current study, pain was slightly higher for those with rotator cuff pathology before surgery—a finding consistent with pain frequently being found in patients with isolated partial-thickness RCTs. Their postoperative pain scores were actually lower on average than those of patients without RCTs, which suggests simple débridement of undersurface tears adequately addressed the pathology. The authors theorize that the main pain generator in this population may be posterior instability, and that the rotator cuff has less of an influence. In the SLAP population, the main pain generator likely is the RCT.
Failures by ASES score or strength were fairly rare in this cohort. Many patients opted to have revision surgery because of continued instability, pain, decreased function, or reinjury. One potential cause of failure in this cohort is inadequate capsular shift. However, capsular plication in throwing athletes is difficult to address, as overtensioning the repair can lead to the inability to adequately perform overhead activites.3,4 This cannot be overemphasized, particularly with pitchers.
Partial-thickness RCTs, particularly those on the articular side, are common in throwing athletes because of high tensile and compressive loads.12 Despite the known risk of RCTs with posterior shoulder instability in throwing athletes, the authors are unaware of reports of the incidence or treatment of this pathology. RCTs in this posterior instability group likely represent a pathology other than internal impingement. The high proportion of throwing athletes with RCTs in this study (43%) indicates a need for close evaluation of rotator cuff pathology in young throwing athletes. Ide et al found that 75% of patients with SLAP tears had partial articular-sided RCTs.13 In the current study, all RCTs were small partial tears, and arthroscopic débridement was performed. It is unknown whether repair of these RCTs would impact return to play. However, rotator cuff repair in this population has been shown to have poor outcomes. Tear thickness typically is used to determine treatment, with débridement performed if <50% tendon thickness is affected. More recently, many have advocated having greater tendon involvement in throwers before repair, because of poor outcomes. Although studies are limited, tear size does seem to correlate with outcomes.14
Continue to: Study Limitations
STUDY LIMITATIONS
Limitations of this study include its small number of professional throwing athletes, with the majority being high school athletes. Further, although ASES scores are consistently used in posterior shoulder instability studies, these scores are influenced highly by pain scores, and some argue that other scoring systems may provide more useful information. However, none of the more modern scoring systems have been studied extensively in posterior glenohumeral instability. Further, because the authors used the present scoring systems previously,1-4 they were continued to be used for comparison and consistency. Outcomes such as ROM and strength may carry more weight if measured and documented by clinical examination. Further testing, such as clinical evaluation of the jerk test or the posterior load-and-shift test, and their comparison before and after surgery may provide more objective data.
CONCLUSION
Arthroscopic capsulolabral reconstruction is successful in throwing athletes with RCTs treated with arthroscopic débridement. Unlike a previous study of throwing athletes’ outcomes after surgery for concomitant SLAP tears and RCTs,5 this study of throwing athletes with concomitant posterior shoulder instability and RCTs found no difference in patient-reported outcome measures or return to play. In throwing athletes with posterior instability and RCTs, arthroscopic posterior capsulolabral repair with rotator cuff débridement is successful.
ABSTRACT
In a previous study, compared with throwing athletes with superior labral anterior posterior (SLAP) tears, those with concomitant SLAP tears and rotator cuff tears (RCTs) had significantly poorer outcome scores and return to play. Posterior shoulder instability also occurs in throwing athletes, but no studies currently exist regarding outcomes of these patients with concomitant RCTs.
The authors hypothesized that throwing athletes treated with arthroscopic capsulolabral repair for posterior shoulder instability with coexistent rotator cuff pathology would have poorer outcome scores and return to play.
Fifty-six consecutive throwing athletes with unidirectional posterior shoulder instability underwent arthroscopic capsulolabral repair. Preoperative and postoperative patient-centered outcomes of pain, stability, function, range of motion, strength, and American Shoulder and Elbow Surgeons Shoulder (ASES) scores, as well as return to play, were evaluated. Patients with and without rotator cuff pathology were compared.
Forty-three percent (24/56) of throwing athletes had rotator cuff pathology in addition to posterior capsulolabral pathology. All RCTs were débrided. At a mean of 3 years, there were no differences in preoperative and postoperative patient-centered outcomes between those with and without RCTs. Return-to-play rates showed no between-group differences; 92% (22/24) of athletes with concomitant RCTs returned to sport (P = .414) and 67% (16/24) returned to the same level (P = .430).
Arthroscopic capsulolabral reconstruction is successful in throwing athletes with RCTs treated with arthroscopic débridement. Unlike the previous study evaluating throwers outcomes after surgical treatment for concomitant SLAP tears and RCTs, the authors found no difference in patient-reported outcome measures or return to play for throwing athletes with concomitant posterior shoulder instability and RCTs. In throwing athletes with concomitant posterior instability and RCTs, arthroscopic posterior capsulolabral repair with rotator cuff débridement is successful.
Continue to: Posterior shoulder instability...
Posterior shoulder instability is an important and increasingly recognized pathology among throwers. Like the superior labrum, the posterior capsulolabral complex is also susceptible to injury during the throwing motion; the posterior labrum being most at risk during the late cocking and follow-through phases. Recent studies have found that arthroscopic capsulolabral reconstruction in posterior shoulder instability is successful in allowing athletes to return to their preinjury sports activities, with 2 studies detailing outcomes in throwing athletes.1-4 However, superior labral anterior posterior (SLAP) tears are common in throwing athletes and have been treated with varying and limited success. Further, in a study of outcomes of arthroscopic repair of SLAP lesions, Neri and colleagues5 found that, compared with throwing athletes with SLAP tears, throwing athletes with concomitant SLAP tears and partial-thickness rotator cuff tears (RCTs) had significantly poorer outcomes and return-to-play rates after surgical repair.
The purpose of this study was to determine outcome scores and return to play of throwing athletes treated with arthroscopic capsulolabral repair for posterior shoulder instability with coexistent RCTs and to compare them with outcome scores as well as return to play of throwing athletes with isolated posterior shoulder instability. It was hypothesized that throwing athletes with a combination of posterior shoulder instability and RCT would have poorer outcomes and poorer return to play after surgery.5
METHODS
PATIENT SELECTION
After Institutional Review Board approval, informed consent was obtained, and consecutive throwing athletes who underwent arthroscopic posterior capsulolabral reconstruction for posterior shoulder instability were followed in the perioperative period. Inclusion criteria were throwing athletes participating in competitive sports at the high school, collegiate, or professional level, minimum 1-year follow-up, presence of unidirectional posterior instability, and absence of symptoms of instability in any direction other than posterior. Patients with inferior instability, SLAP pathology on examination and on magnetic resonance imaging, multidirectional instability, or habitual or psychogenic voluntary shoulder subluxations were excluded. Patients with diagnoses of both posterior shoulder instability and impingement treated with subacromial decompression and distal clavicle resection were also excluded.
After this cohort was identified, patient records were reviewed for pertinent operative data, such as procedure, complications, and evidence of RCT by operative report and arthroscopic photographs. A partial RCT was defined as a tear of 10% to 50%; those with rotator cuff fraying were determined not to be significant.
PATIENT EVALUATION
Surgeries were performed between January 1998 and December 2009 by the senior author (JPB). All patients were followed with clinical examinations, radiographs, and subjective grading scales. Recorded patient demographic data included age, sex, sport, position, competition level, and follow-up duration.
Continue to: All patients had...
All patients had symptomatic posterior shoulder instability, including posterior shoulder pain, clicking, a sensation of subluxation, or instability/apprehension with motion. Each athlete’s shoulder was palpated for tenderness and tested for impingement. Specific posterior glenohumeral instability tests, including the Kim test,6 the circumduction test, the jerk test,7 the posterior load-and-shift test,8 and the posterior stress test,9 were performed on all patients. Patients with multidirectional instability on the sulcus test, as well as provocative tests indicating SLAP pathology, such as the Crank test and the active compression test, were not included. Standard radiography and magnetic resonance arthrography (MRA) were performed to further narrow inclusion and exclusion criteria.
Both before surgery and at latest follow-up, patient outcomes were evaluated using the American Shoulder and Elbow Surgeons (ASES) score (range, 0-100) which combines a subjective functional scale measuring activities of daily living (0-3 for each of 10 tasks, with a total of 0-30) and a subjective pain scale (0-10, with 10 being worst pain). Values >80 were described as excellent, and failures were defined as scores <60 after surgery.10 A subjective stability scale (0-10, with 0 indicating completely stable and 10 completely unstable), strength scale (0-3, with 0 indicating none, 1 markedly decreased, 2 slightly decreased, and 3 normal), and ROM scale (0-3, with 0 indicating poor, 1 limited, 2 satisfactory, and 3 full) were evaluated both before surgery and at the latest follow-up. A stability score >5 after surgery was defined as a failure.1,2,11 Patients were also asked if, based on their current state, they would undergo surgery again. Intraoperative findings and specific surgical procedures performed were correlated with the aforementioned subjective and objective outcome scores.
OPERATIVE TREATMENT
Throwing athletes who met inclusion criteria and failed nonoperative management underwent surgery by the senior author (JPB). Each patient was examined under anesthesia and, with the patient in the lateral decubitus position, a diagnostic arthroscopy was performed to identify posterior capsulolabral complex pathology, including a patulous capsule, capsular tears, labral fraying, and labral tears. A careful examination for rotator cuff pathology was also performed. Based on preoperative clinical examination, MRA, examination under anesthesia, pathologic findings at diagnostic arthroscopic surgery, and surgeon experience, capsulolabral plication was performed with or without suture anchors.2,5 After capsulolabral repair, the capsule was evaluated for residual laxity, and additional plication sutures were placed, as indicated, with care to avoid overconstraint in these throwing athletes.1 Posterior glenohumeral stability restoration was judged by removing traction and performing posterior load-and-shift and posterior stress tests. Any RCT with <50% thickness was débrided. Postoperative care and rehabilitation were carried out as previously described and were not altered by the presence or absence of a RCT.3
STATISTICAL ANALYSIS
Preoperative and latest follow-up ASES scores, stability scores, functional scores, and pain-level findings were compared using paired-samples Comparisons between groups, including throwing athletes with and without rotator cuff pathology, were done using the Student t test. Outcome comparisons between multiple groups, which included intraoperative findings and surgical fixation methods, were analyzed with c2 modeling for nonparametric data. Statistical significance was set at P < .05. A power analysis found that this study was able to detect a meaningful difference of 10 ASES points.
RESULTS
PATIENT DEMOGRAPHIC CHARACTERISTICS
Of the 56 throwing athletes who met the inclusion criteria, 24 were found to have rotator cuff pathology in addition to posterior capsulolabral pathology, while 32 were found to have capsulolabral pathology alone. Demographic data are listed in Table 1. Mean age was 20.1 years for patients with rotator cuff pathology and 17.8 years for patients without RCTs. All 24 athletes with rotator cuff pathology were treated with arthroscopic débridement. Mean follow-up was 38.6 months (range, 16.5-63.6 months) for patients with RCTs and 39.1 months (range, 12-98.8 months) for patients without RCTs. No significant difference was found in age, sports level, or follow-up between groups.
Table 1. Demographic Data for Athletes With Posterior Instability With and Without Rotator Cuff Tears (N = 56 Shoulders)a
Characteristic | Rotator Cuff Tears | |
Yes | No | |
| Total | 24 | 32 |
| Sex | ||
| Male | 16 | 27 |
| Female | 8 | 5 |
| Mean age, y | 20.1 | 17.8 |
| Mean follow up, mo | 38.6 | 39.1 |
| Participation level | ||
| Professional | 1 | 0 |
| College | 4 | 4 |
| High school | 17 | 26 |
| Recreational | 2 | 2 |
aThe majority of athletes were males in high school and their mean follow-up was 3 years.
Continue to: Outcomes
OUTCOMES
Table 2 lists the preoperative and postoperative scores for shoulder performance in throwing athletes with posterior shoulder instability, with and without RCTs.
Table 2. Preoperative and Postoperative Scores for Shoulder Performance in Throwing Athletes With Posterior Shoulder Instability With and Without Rotator Cuff Tearsa
| With Rotator Cuff Tears (n=24 shoulders) | Without Rotator Cuff Tears (n=32 shoulders) | |||||||||
| Preoperative | Latest Follow-Up | Preoperative | Latest Follow-Up | |||||||
| Outcome Measure | Mean Score | Range | Mean Score | Range | P | Mean Score | Range | Mean Score | Range | P |
ASES 0-100 0 = worst | 41.8 | 20-70 | 85.4 | 67-100 | <.05 | 49.7 | 20-85 | 83.1 | 25-100 | <.05 |
Stability 0-10 0 = most stable | 6.7 | 2-10 | 2.4 | 0-6 | <.05 | 7.8 | 0-10 | 2.4 | 0-8 | <.05 |
Pain 0-10 10 = worst | 7.6 | 5-10 | 1.9 | 0-5 | <.05 | 6.3 | 0-10 | 2.2 | 0-7 | <.05 |
Function 0-30 0 = worst | 18.5 | 6-27 | 27 | 16-30 | <.05 | 19.0 | 8-26 | 26.4 | 6-30 | <.05 |
aThere was no difference in ASES, stability, pain, or functional scores between athletes with posterior instability alone compared with patients with concomitant rotator cuff tears.
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
ASES Scores. Mean preoperative ASES scores for patients with RCTs improved significantly (t = –13.8, P < .001), as did those for patients without rotator cuff pathology (t = –8.9, P < .001). No significant differences in ASES score were found between patients with and without rotator cuff pathology before or after surgery (t = 1.9, P = .07; t = .58, P = .06). In addition, 70.8% (17/24) of throwing athletes with rotator cuff pathology had an excellent postoperative outcome (ASES score >80), and 29.2% (7/24) had a satisfactory outcome (ASES score, 60-80). Thus, 100% of those with concomitant posterior shoulder instability and RCTs had a good or excellent outcome after surgical intervention. In those without rotator cuff pathology, 78.1% (25/32) had an excellent outcome, 12.5% (4/32) had a satisfactory outcome, and 9.4% (3/32) had a poor outcome. Thus, 91% of those without rotator cuff pathology had a good or excellent outcome after surgery.
Stability. Preoperative stability scores improved significantly after surgery in both groups (t = 7.2, P < .001; t = 10.5, P < .001). There were no statistical differences between preoperative or postoperative stability scores in those with or without rotator cuff pathology (t = 1.7, P = .095; t = .03, P = .975). Of throwing athletes with RCTs, 54.2% (13/24) had an excellent outcome, 33.3% (8/24) a good outcome, and 12.5% (3/24) a satisfactory outcome. Thus, 87.5% (21/24) of those with RCTs had a good or excellent outcome in terms of stability. In those without rotator cuff pathology, 46.9% (15/32) had excellent stability, 46.9% (15/32) had good stability, and 3.1% (1/32) had satisfactory stability after surgery. Thus, 93.8% (30/32) of throwing athletes without rotator cuff pathology had good or excellent stability after surgery.
Pain. Mean preoperative pain scores for those with and without rotator cuff pathology improved significantly (t = 13.4, P < .001; t = 7.1, P < .001). There was no statistical difference in preoperative or postoperative pain scores between those with and without rotator cuff pathology (t = 1.99, P = .051; t = .49, P = .627).
Function. Mean preoperative function scores for both groups improved significantly (t = 7.7, P < .001; t = 8.0, P < .001). There was no difference in improvement in functional scores between the two groups before or after surgery (t = .36, P = .721; t = .5, P = .622).
Continue to: ROM
ROM. Of those with rotator cuff pathology, 54% (13/24) had normal ROM, 42% (10/24) had satisfactory ROM, and 4% (1/24) had limited ROM. In throwing athletes without rotator cuff pathology, 34% (11/32) had normal ROM, 53.1% (17/32) had satisfactory ROM, and 9% (3/32) had limited ROM after surgery. There was no significant difference in ROM between the groups (c2 = 2.7, P = .260).
Strength. Of those with RCTs, 67% (16/24) reported normal strength, 29% (7/24) slightly decreased strength, and 4% (1/24) markedly decreased strength. Of those throwing athletes without rotator cuff pathology, 50% (16/32) had normal strength, 41% (13/32) had slightly decreased strength, and 9% (3/32) had markedly decreased strength. No statistical difference was noted between the two groups (c2 = 1.7, P = .429).
Return to Sport. Of those with RCTs, 92% (22/24) returned to sport while 84% (27/32) of throwing athletes without RCTs returned to sport. There was no difference between the two groups (c2 = .667, P = .414). Sixty-seven percent (16/24) of those with RCTs and 56% (18/32) of those without RCTs returned to the same level of sport. No statistical difference was found in return to play between throwing athletes with and without rotator cuff pathology (c2 = .624, P = .430).
Failures. According to ASES scores, no throwers with RCTs failed, while 9.4% (3/32) with posterior instability alone failed. Regarding stability, 8.3% (2/24) of athletes with RCTs failed, while 6.3% (2/32) with posterior instability alone failed.
SURGICAL FINDINGS AND PROCEDURES
Of the 24 throwing athletes with rotator cuff pathology, 92% (22/24) had labral tears, while 78% (25/32) of those without RCTs had labral tears. The majority of RCTs were in the posterior supraspinatus and anterior infraspinatus regions. This was not significantly different between groups (c2 = 1.86, P = .172). All labral pathology was posterior-inferior, and all RCTs were <50% thickness, and therefore were débrided. Fifty-four percent (13/24) of those with RCTs had a patulous capsule and 63% (20/32) of throwing athletes without rotator cuff pathology had a patulous capsule. There was no significant difference between groups (c2 = .393, P = .530). Of those with RCTs, 92% (22/24) had surgical fixation with anchors, while 78% (25/32) of those without rotator cuff pathology underwent repair with anchor fixation. There was no statistically significant difference in anchor use between groups (c2 = 1.86, P = .172).
Continue to: Discussion
DISCUSSION
Throwing athletes with and without RCTs had similar rates of recovery and return to play after arthroscopic capsular labral repair, with rotator cuff débridement if a tear was present. The mean follow-up was 3.2 years. Further, there was no difference in return to play (92% vs 84%), ASES score, stability, pain, function, ROM, or strength between the 2 groups before or after surgery. In this cohort of 56 patients, 24 throwing athletes (43%) were found to have RCTs.
Return-to-play rates showed no between-group differences; 92% (22/24) of athletes with concomitant RCTs returned to sport, and 67% (16/24) returned to the same level. Eight percent of throwing athletes with RCTs were unable to return to sport after surgery. These return-to-play rates are an improvement over most previously reported rates in throwing athletes and in posterior shoulder instability in general.1-4,11 When these athletes are compared with their counterparts with combined SLAP tears and RCTs, return-to-play rates are notably higher. There may be discrepancies in interpreting return-to-play between the two studies, but in the current study, 67% of those with concomitant RCTs achieved return to preinjury level of play. This is 10% higher than the rate reported in athletes with SLAP tears alone (57%) and even higher than in those with concomitant SLAP and RCTs. It is also essential to note that a number of this cohort’s athletes who did not return to play did so for factors (eg, graduation) unrelated to the shoulder. However, the study by Neri and colleagues5 included professional athletes who likely all attempted to return to play and, if unable to perform at the same level, likely were unable to continue their professional career.5
All patients with RCTs had a good or excellent outcome (ASES score), and 70.8% had an excellent outcome. Similarly, 97% of those without rotator cuff pathology had a good or excellent outcome, and 81.3% had an excellent outcome. There was no significant difference between the two groups. These results parallel those of Neri and colleagues’5 study of SLAP tears with RCTs, where 96% (22/23) of throwing athletes had a good or excellent outcome. Despite these high outcome scores in patients with SLAP tears, only 57% were able to return to elite pitching.5 In the current study, pain was slightly higher for those with rotator cuff pathology before surgery—a finding consistent with pain frequently being found in patients with isolated partial-thickness RCTs. Their postoperative pain scores were actually lower on average than those of patients without RCTs, which suggests simple débridement of undersurface tears adequately addressed the pathology. The authors theorize that the main pain generator in this population may be posterior instability, and that the rotator cuff has less of an influence. In the SLAP population, the main pain generator likely is the RCT.
Failures by ASES score or strength were fairly rare in this cohort. Many patients opted to have revision surgery because of continued instability, pain, decreased function, or reinjury. One potential cause of failure in this cohort is inadequate capsular shift. However, capsular plication in throwing athletes is difficult to address, as overtensioning the repair can lead to the inability to adequately perform overhead activites.3,4 This cannot be overemphasized, particularly with pitchers.
Partial-thickness RCTs, particularly those on the articular side, are common in throwing athletes because of high tensile and compressive loads.12 Despite the known risk of RCTs with posterior shoulder instability in throwing athletes, the authors are unaware of reports of the incidence or treatment of this pathology. RCTs in this posterior instability group likely represent a pathology other than internal impingement. The high proportion of throwing athletes with RCTs in this study (43%) indicates a need for close evaluation of rotator cuff pathology in young throwing athletes. Ide et al found that 75% of patients with SLAP tears had partial articular-sided RCTs.13 In the current study, all RCTs were small partial tears, and arthroscopic débridement was performed. It is unknown whether repair of these RCTs would impact return to play. However, rotator cuff repair in this population has been shown to have poor outcomes. Tear thickness typically is used to determine treatment, with débridement performed if <50% tendon thickness is affected. More recently, many have advocated having greater tendon involvement in throwers before repair, because of poor outcomes. Although studies are limited, tear size does seem to correlate with outcomes.14
Continue to: Study Limitations
STUDY LIMITATIONS
Limitations of this study include its small number of professional throwing athletes, with the majority being high school athletes. Further, although ASES scores are consistently used in posterior shoulder instability studies, these scores are influenced highly by pain scores, and some argue that other scoring systems may provide more useful information. However, none of the more modern scoring systems have been studied extensively in posterior glenohumeral instability. Further, because the authors used the present scoring systems previously,1-4 they were continued to be used for comparison and consistency. Outcomes such as ROM and strength may carry more weight if measured and documented by clinical examination. Further testing, such as clinical evaluation of the jerk test or the posterior load-and-shift test, and their comparison before and after surgery may provide more objective data.
CONCLUSION
Arthroscopic capsulolabral reconstruction is successful in throwing athletes with RCTs treated with arthroscopic débridement. Unlike a previous study of throwing athletes’ outcomes after surgery for concomitant SLAP tears and RCTs,5 this study of throwing athletes with concomitant posterior shoulder instability and RCTs found no difference in patient-reported outcome measures or return to play. In throwing athletes with posterior instability and RCTs, arthroscopic posterior capsulolabral repair with rotator cuff débridement is successful.
1. Bradley JP, Baker CL 3rd, Kline AJ, Armfield DR, Chhabra A. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 100 shoulders. Am J Sports Med. 2006;34(7):1061-1071.
2. Bradley JP, McClincy MP, Arner JW, Tejwani SG. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 200 shoulders. Am J Sports Med. 2013;41(9):2005-2014.
3. McClincy MP, Arner JW, Bradley JP. Posterior shoulder instability in throwing athletes: a case-matched comparison of throwers and non-throwers. Arthroscopy. 2015;31(6):1041-1051.
4. Radkowski CA, Chhabra A, Baker CL 3rd, Tejwani SG, Bradley JP. Arthroscopic capsulolabral repair for posterior shoulder instability in throwing athletes compared with nonthrowing athletes. Am J Sports Med. 2008;36(4):693-699.
5. Neri BR, ElAttrache NS, Owsley KC, Mohr K, Yocum LA. Outcome of type II superior labral anterior posterior repairs in elite overhead athletes: effect of concomitant partial-thickness rotator cuff tears. Am J Sports Med. 2011;39(1):114-120.
6. Kim SH, Park JS, Jeong WK, Shin SK. The Kim test: a novel test for posteroinferior labral lesion of the shoulder—a comparison to the jerk test. Am J Sports Med. 2005;33(8):1188-1192.
7. Antoniou J, Duckworth DT, Harryman DT 2nd. Capsulolabral augmentation for the management of posteroinferior instability of the shoulder. J Bone Joint Surg Am. 2000;82(9):1220-1230.
8. Altchek DW, Hobbs WR. Evaluation and management of shoulder instability in the elite overhead thrower. Orthop Clin North Am. 2001;32(3):423-430, viii.
9. Fuchs B, Jost B, Gerber C. Posterior-inferior capsular shift for the treatment of recurrent, voluntary posterior subluxation of the shoulder. J Bone Joint Surg Am. 2000;82(1):16-25.
10. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
11. Arner JW, McClincy MP, Bradley JP. Arthroscopic stabilization of posterior shoulder instability is successful in American football players. Arthroscopy. 2015;31(8):1466-1471.
12. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
13. Ide J, Maeda S, Takagi K. Sports activity after arthroscopic superior labral repair using suture anchors in overhead-throwing athletes. Am J Sports Med. 2005;33(4):507-514.
14. Economopoulos KJ, Brockmeier SF. Rotator cuff tears in overhead athletes. Clin Sports Med. 2012;31(4):675-692.
1. Bradley JP, Baker CL 3rd, Kline AJ, Armfield DR, Chhabra A. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 100 shoulders. Am J Sports Med. 2006;34(7):1061-1071.
2. Bradley JP, McClincy MP, Arner JW, Tejwani SG. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 200 shoulders. Am J Sports Med. 2013;41(9):2005-2014.
3. McClincy MP, Arner JW, Bradley JP. Posterior shoulder instability in throwing athletes: a case-matched comparison of throwers and non-throwers. Arthroscopy. 2015;31(6):1041-1051.
4. Radkowski CA, Chhabra A, Baker CL 3rd, Tejwani SG, Bradley JP. Arthroscopic capsulolabral repair for posterior shoulder instability in throwing athletes compared with nonthrowing athletes. Am J Sports Med. 2008;36(4):693-699.
5. Neri BR, ElAttrache NS, Owsley KC, Mohr K, Yocum LA. Outcome of type II superior labral anterior posterior repairs in elite overhead athletes: effect of concomitant partial-thickness rotator cuff tears. Am J Sports Med. 2011;39(1):114-120.
6. Kim SH, Park JS, Jeong WK, Shin SK. The Kim test: a novel test for posteroinferior labral lesion of the shoulder—a comparison to the jerk test. Am J Sports Med. 2005;33(8):1188-1192.
7. Antoniou J, Duckworth DT, Harryman DT 2nd. Capsulolabral augmentation for the management of posteroinferior instability of the shoulder. J Bone Joint Surg Am. 2000;82(9):1220-1230.
8. Altchek DW, Hobbs WR. Evaluation and management of shoulder instability in the elite overhead thrower. Orthop Clin North Am. 2001;32(3):423-430, viii.
9. Fuchs B, Jost B, Gerber C. Posterior-inferior capsular shift for the treatment of recurrent, voluntary posterior subluxation of the shoulder. J Bone Joint Surg Am. 2000;82(1):16-25.
10. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
11. Arner JW, McClincy MP, Bradley JP. Arthroscopic stabilization of posterior shoulder instability is successful in American football players. Arthroscopy. 2015;31(8):1466-1471.
12. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
13. Ide J, Maeda S, Takagi K. Sports activity after arthroscopic superior labral repair using suture anchors in overhead-throwing athletes. Am J Sports Med. 2005;33(4):507-514.
14. Economopoulos KJ, Brockmeier SF. Rotator cuff tears in overhead athletes. Clin Sports Med. 2012;31(4):675-692.
TAKE-HOME POINTS
- Arthroscopic capsulolabral reconstruction is successful in throwing athletes with concomitant RCTs treated with arthroscopic débridement.
- A previous study of throwing athletes found poor outcomes after surgery for concomitant SLAP tears and RCTs.
- Throwing athletes with concomitant posterior shoulder instability and RCTs were no different in patient-reported outcomes or return to play.
- The high proportion of throwing athletes with partial thickness RCTs in this study (43%) indicates a need for close evaluation of rotator cuff pathology in young throwing athletes.
- The authors theorize the main pain generator in this population may be posterior instability and that the rotator cuff has less of an influence.
Shoulder Arthroplasty in Cases of Significant Bone Loss: An Overview
Over the past few decades, there has been a dramatic increase in the number of shoulder arthroplasties performed around the world. This increase is the result of an aging and increasingly more active population, better implant technology, and the advent of reverse shoulder arthroplasty (RSA) for rotator cuff arthropathy. Additionally, as the indications for RSA have expanded to include pathologies such as rotator cuff insufficiency, chronic instabilities, trauma, and tumors, the number of arthroplasties will continue to increase. Although the results of most arthroplasties are good and predictable, any glenoid and/or humeral bone deficiencies can have detrimental effects on the clinical outcomes of these procedures. Bone loss becomes more of a problem in revision cases, and, as the number of primary arthroplasties increases, it follows that the number of revision procedures will also increase.
Many of the disease- or procedure-specific processes indicated for shoulder arthroplasty have predictable patterns of bone loss, especially on the glenoid side. Walch and colleagues1 and Bercik and colleagues2 made us aware that many patients with primary osteoarthritis have significant glenoid bone deformity. Similarly, there have been a number of first- and second-generation classification systems for delineating glenoid deformity in rotator cuff tear arthropathy and in revision settings. In revision settings, both glenoid and humeral bone deficiencies can occur as a result of implant removal, iatrogenic fracture, and even infection. Each of these bone loss patterns must be recognized and treated appropriately for the best surgical outcome.
The articles in this month of The American Journal of Orthopedics address the most up-to-date concepts and solutions regarding both humeral and glenoid bone loss in shoulder arthroplasty of all types.
HUMERAL BONE LOSS
Humeral bone loss is typically encountered in proximal humerus fractures, in revision surgery necessitating humeral component removal, and, less commonly, in tumors and infection.
In many displaced proximal humeral fractures indicated for shoulder arthroplasty, the bone is comminuted with displacement of the lesser and greater tuberosities. In these situations, failure of tuberosity healing may result in loss of rotator cuff function with loss of elevation, rotation, and even instability. Humeral shortening can also occur as a result of bone loss and can compromise deltoid function by loss of proper muscle tension, leading to instability, dysfunction, or both. In addition to possible instability, humeral shortening with metaphyseal bone loss can adversely affect long-term fixation of the humeral component, leading to stem loosening or failure. Cuff and colleagues3 showed significantly more rotational micromotion in cases lacking metaphyseal support, leading to aseptic loosening of the humeral stem.
Humeral bone loss can also result from humeral stem component removal in revision shoulder arthroplasty for infection, component failure or loosening, and even periprosthetic fracture resulting from surgery or trauma.
For the surgeon, humeral bone loss can create a complex set of circumstances related to rotator cuff attachment failure, soft-tissue balancing effects, and component fixation issues. Any such issue must be recognized and addressed for best outcomes. Best results can be obtained with preoperative imaging, planning, use of bone graft techniques, proximal humeral allografts, and, more recently, modular and patient-specific implants. All of these issues are discussed comprehensively in the articles this month.
Continue to: GLENOID BONE LOSS
GLENOID BONE LOSS
Proper glenoid component placement with durable fixation is crucial for success in anatomical total shoulder arthroplasty and RSA. Glenoid bone deformity and loss can result from intrinsic deformity characteristics seen in primary osteoarthritis, cuff tear arthropathy, or glenoid component removal in revision situations and infection. These bone deformity complications can be extremely difficult to treat and in some cases lead to catastrophic failure of the index arthroplasty.
We are now aware that one key to success in the face of moderate to severe deformity is proper recognition. Newer imaging techniques, including 2-dimensional (2-D) computed tomography (CT) and 3-dimensional (3-D) modeling and surgical planning software tools, which are outlined in an upcoming article, have given surgeons important new instruments that can help in treating these difficult cases.
Glenoid bone deformity in primary osteoarthritis was well delineated in the 1999 seminal study of CT changes by Walch and colleagues.1 The Walch classification system, which characterized glenoid morphology based on 2-D CT findings, was recently upgraded, based on 3-D imaging technology, to include Walch B3 and D patterns (Figure 1).2 Recognition of certain primary deformities in osteoarthritis has led to increased use of RSA in some cases of Walch B2, B3, and C deformities with substantial glenoid retroversion and/or humeral head subluxation.4
In cases of rotator cuff tear arthropathy, glenoid bone deformities are well described with several classification systems based on degree and dimension of bone insufficiency. The Hamada classification system defines the degree of medial glenoid erosion and superior bone loss, as well as acetabularization of the acromion in 5 grades; 5 Rispoli and colleagues6 defined and graded the degree of medicalization of the glenohumeral joint based on degree of subchondral plate erosion; and Visotsky and colleagues7 based their classification system on wear patterns of bone loss, alignment, and concomitant soft-tissue insufficiencies leading to instability and rotation loss.
In severe glenoid bone deficiency after glenoid component removal, Antuna and colleagues8 described the classic findings related to medial bone loss, anterior and posterior wall failure, and combinations thereof.
Continue to: All these classification systems...
All these classification systems are based on the 2-D appearance of the glenoid and should be considered cautiously. The glenoid is a complex 3-D structure that can be affected by any number of disease processes, trauma, and surgical intervention. Using more modern CT techniques and 3-D imaging, we now know that many deformities previously classified as unidirectional are, instead, complex and multidirectional.
Frankle and colleagues9 developed a classification based more 3-D CT models which has further classified severe glenoid vault deformities in relation to direction and degree of bone loss (Figures 2A-2E). Using this system, they were better able to determine degree and direction of deformity than in previous 2-D evaluations, and they were able to determine the amount of glenoid vault bone available for baseplate fixation. Scalise and colleagues10 further defined the influence of such 3-D planning in total shoulder arthroplasty.
With knowledge of these classification systems and use of contemporary imaging systems, shoulder arthroplasty in cases of severe glenoid deficiency can be more successful. Potentially, we can improve outcomes even more in the more severe cases of bone loss with use of patient-specific planning tools, including the guides and patient-specific implants that are now readily available with many implant systems.11
Preoperative planning tools, bone-grafting techniques, augmented and specialized glenoid and humeral implants, and patient-specific implants are discussed this month to give our readers a comprehensive review of the latest concepts in shoulder arthroplasty in cases of significant bone loss or deformity.
This month of The American Journal of Orthopedics presents the most current and cutting-edge solutions for humeral and glenoid bone deformities and deficiencies in contemporary shoulder arthroplasties.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
3. Cuff D, Levy JC, Gutiérrez S, Frankle M. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651.
4. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598.
5. Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;(254):92-96.
6. Rispoli D, Sperling JW, Athwal GS, Schleck CD, Cofield RH. Humeral head replacement for the treatment of osteoarthritis. J Bone Joint Surg Am. 2006;88(12):2637-2644.
7. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
8. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
9. Frankle MA, Teramoto A, Luo ZP, Levy JC, Pupello D. Glenoid morphology in reverse shoulder arthroplasty: classification and surgical implications. J Shoulder Elbow Surg. 2009;18(6):874-885.
10. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
11. Dines DM, Gulotta L, Craig EV, Dines JS. Novel solution for massive glenoid defects in shoulder arthroplasty: a patient-specific glenoid vault reconstruction system. Am J Orthop. 2017;46(2):104-108.
Over the past few decades, there has been a dramatic increase in the number of shoulder arthroplasties performed around the world. This increase is the result of an aging and increasingly more active population, better implant technology, and the advent of reverse shoulder arthroplasty (RSA) for rotator cuff arthropathy. Additionally, as the indications for RSA have expanded to include pathologies such as rotator cuff insufficiency, chronic instabilities, trauma, and tumors, the number of arthroplasties will continue to increase. Although the results of most arthroplasties are good and predictable, any glenoid and/or humeral bone deficiencies can have detrimental effects on the clinical outcomes of these procedures. Bone loss becomes more of a problem in revision cases, and, as the number of primary arthroplasties increases, it follows that the number of revision procedures will also increase.
Many of the disease- or procedure-specific processes indicated for shoulder arthroplasty have predictable patterns of bone loss, especially on the glenoid side. Walch and colleagues1 and Bercik and colleagues2 made us aware that many patients with primary osteoarthritis have significant glenoid bone deformity. Similarly, there have been a number of first- and second-generation classification systems for delineating glenoid deformity in rotator cuff tear arthropathy and in revision settings. In revision settings, both glenoid and humeral bone deficiencies can occur as a result of implant removal, iatrogenic fracture, and even infection. Each of these bone loss patterns must be recognized and treated appropriately for the best surgical outcome.
The articles in this month of The American Journal of Orthopedics address the most up-to-date concepts and solutions regarding both humeral and glenoid bone loss in shoulder arthroplasty of all types.
HUMERAL BONE LOSS
Humeral bone loss is typically encountered in proximal humerus fractures, in revision surgery necessitating humeral component removal, and, less commonly, in tumors and infection.
In many displaced proximal humeral fractures indicated for shoulder arthroplasty, the bone is comminuted with displacement of the lesser and greater tuberosities. In these situations, failure of tuberosity healing may result in loss of rotator cuff function with loss of elevation, rotation, and even instability. Humeral shortening can also occur as a result of bone loss and can compromise deltoid function by loss of proper muscle tension, leading to instability, dysfunction, or both. In addition to possible instability, humeral shortening with metaphyseal bone loss can adversely affect long-term fixation of the humeral component, leading to stem loosening or failure. Cuff and colleagues3 showed significantly more rotational micromotion in cases lacking metaphyseal support, leading to aseptic loosening of the humeral stem.
Humeral bone loss can also result from humeral stem component removal in revision shoulder arthroplasty for infection, component failure or loosening, and even periprosthetic fracture resulting from surgery or trauma.
For the surgeon, humeral bone loss can create a complex set of circumstances related to rotator cuff attachment failure, soft-tissue balancing effects, and component fixation issues. Any such issue must be recognized and addressed for best outcomes. Best results can be obtained with preoperative imaging, planning, use of bone graft techniques, proximal humeral allografts, and, more recently, modular and patient-specific implants. All of these issues are discussed comprehensively in the articles this month.
Continue to: GLENOID BONE LOSS
GLENOID BONE LOSS
Proper glenoid component placement with durable fixation is crucial for success in anatomical total shoulder arthroplasty and RSA. Glenoid bone deformity and loss can result from intrinsic deformity characteristics seen in primary osteoarthritis, cuff tear arthropathy, or glenoid component removal in revision situations and infection. These bone deformity complications can be extremely difficult to treat and in some cases lead to catastrophic failure of the index arthroplasty.
We are now aware that one key to success in the face of moderate to severe deformity is proper recognition. Newer imaging techniques, including 2-dimensional (2-D) computed tomography (CT) and 3-dimensional (3-D) modeling and surgical planning software tools, which are outlined in an upcoming article, have given surgeons important new instruments that can help in treating these difficult cases.
Glenoid bone deformity in primary osteoarthritis was well delineated in the 1999 seminal study of CT changes by Walch and colleagues.1 The Walch classification system, which characterized glenoid morphology based on 2-D CT findings, was recently upgraded, based on 3-D imaging technology, to include Walch B3 and D patterns (Figure 1).2 Recognition of certain primary deformities in osteoarthritis has led to increased use of RSA in some cases of Walch B2, B3, and C deformities with substantial glenoid retroversion and/or humeral head subluxation.4

In cases of rotator cuff tear arthropathy, glenoid bone deformities are well described with several classification systems based on degree and dimension of bone insufficiency. The Hamada classification system defines the degree of medial glenoid erosion and superior bone loss, as well as acetabularization of the acromion in 5 grades; 5 Rispoli and colleagues6 defined and graded the degree of medicalization of the glenohumeral joint based on degree of subchondral plate erosion; and Visotsky and colleagues7 based their classification system on wear patterns of bone loss, alignment, and concomitant soft-tissue insufficiencies leading to instability and rotation loss.
In severe glenoid bone deficiency after glenoid component removal, Antuna and colleagues8 described the classic findings related to medial bone loss, anterior and posterior wall failure, and combinations thereof.
Continue to: All these classification systems...
All these classification systems are based on the 2-D appearance of the glenoid and should be considered cautiously. The glenoid is a complex 3-D structure that can be affected by any number of disease processes, trauma, and surgical intervention. Using more modern CT techniques and 3-D imaging, we now know that many deformities previously classified as unidirectional are, instead, complex and multidirectional.
Frankle and colleagues9 developed a classification based more 3-D CT models which has further classified severe glenoid vault deformities in relation to direction and degree of bone loss (Figures 2A-2E). Using this system, they were better able to determine degree and direction of deformity than in previous 2-D evaluations, and they were able to determine the amount of glenoid vault bone available for baseplate fixation. Scalise and colleagues10 further defined the influence of such 3-D planning in total shoulder arthroplasty.

With knowledge of these classification systems and use of contemporary imaging systems, shoulder arthroplasty in cases of severe glenoid deficiency can be more successful. Potentially, we can improve outcomes even more in the more severe cases of bone loss with use of patient-specific planning tools, including the guides and patient-specific implants that are now readily available with many implant systems.11
Preoperative planning tools, bone-grafting techniques, augmented and specialized glenoid and humeral implants, and patient-specific implants are discussed this month to give our readers a comprehensive review of the latest concepts in shoulder arthroplasty in cases of significant bone loss or deformity.
This month of The American Journal of Orthopedics presents the most current and cutting-edge solutions for humeral and glenoid bone deformities and deficiencies in contemporary shoulder arthroplasties.
Over the past few decades, there has been a dramatic increase in the number of shoulder arthroplasties performed around the world. This increase is the result of an aging and increasingly more active population, better implant technology, and the advent of reverse shoulder arthroplasty (RSA) for rotator cuff arthropathy. Additionally, as the indications for RSA have expanded to include pathologies such as rotator cuff insufficiency, chronic instabilities, trauma, and tumors, the number of arthroplasties will continue to increase. Although the results of most arthroplasties are good and predictable, any glenoid and/or humeral bone deficiencies can have detrimental effects on the clinical outcomes of these procedures. Bone loss becomes more of a problem in revision cases, and, as the number of primary arthroplasties increases, it follows that the number of revision procedures will also increase.
Many of the disease- or procedure-specific processes indicated for shoulder arthroplasty have predictable patterns of bone loss, especially on the glenoid side. Walch and colleagues1 and Bercik and colleagues2 made us aware that many patients with primary osteoarthritis have significant glenoid bone deformity. Similarly, there have been a number of first- and second-generation classification systems for delineating glenoid deformity in rotator cuff tear arthropathy and in revision settings. In revision settings, both glenoid and humeral bone deficiencies can occur as a result of implant removal, iatrogenic fracture, and even infection. Each of these bone loss patterns must be recognized and treated appropriately for the best surgical outcome.
The articles in this month of The American Journal of Orthopedics address the most up-to-date concepts and solutions regarding both humeral and glenoid bone loss in shoulder arthroplasty of all types.
HUMERAL BONE LOSS
Humeral bone loss is typically encountered in proximal humerus fractures, in revision surgery necessitating humeral component removal, and, less commonly, in tumors and infection.
In many displaced proximal humeral fractures indicated for shoulder arthroplasty, the bone is comminuted with displacement of the lesser and greater tuberosities. In these situations, failure of tuberosity healing may result in loss of rotator cuff function with loss of elevation, rotation, and even instability. Humeral shortening can also occur as a result of bone loss and can compromise deltoid function by loss of proper muscle tension, leading to instability, dysfunction, or both. In addition to possible instability, humeral shortening with metaphyseal bone loss can adversely affect long-term fixation of the humeral component, leading to stem loosening or failure. Cuff and colleagues3 showed significantly more rotational micromotion in cases lacking metaphyseal support, leading to aseptic loosening of the humeral stem.
Humeral bone loss can also result from humeral stem component removal in revision shoulder arthroplasty for infection, component failure or loosening, and even periprosthetic fracture resulting from surgery or trauma.
For the surgeon, humeral bone loss can create a complex set of circumstances related to rotator cuff attachment failure, soft-tissue balancing effects, and component fixation issues. Any such issue must be recognized and addressed for best outcomes. Best results can be obtained with preoperative imaging, planning, use of bone graft techniques, proximal humeral allografts, and, more recently, modular and patient-specific implants. All of these issues are discussed comprehensively in the articles this month.
Continue to: GLENOID BONE LOSS
GLENOID BONE LOSS
Proper glenoid component placement with durable fixation is crucial for success in anatomical total shoulder arthroplasty and RSA. Glenoid bone deformity and loss can result from intrinsic deformity characteristics seen in primary osteoarthritis, cuff tear arthropathy, or glenoid component removal in revision situations and infection. These bone deformity complications can be extremely difficult to treat and in some cases lead to catastrophic failure of the index arthroplasty.
We are now aware that one key to success in the face of moderate to severe deformity is proper recognition. Newer imaging techniques, including 2-dimensional (2-D) computed tomography (CT) and 3-dimensional (3-D) modeling and surgical planning software tools, which are outlined in an upcoming article, have given surgeons important new instruments that can help in treating these difficult cases.
Glenoid bone deformity in primary osteoarthritis was well delineated in the 1999 seminal study of CT changes by Walch and colleagues.1 The Walch classification system, which characterized glenoid morphology based on 2-D CT findings, was recently upgraded, based on 3-D imaging technology, to include Walch B3 and D patterns (Figure 1).2 Recognition of certain primary deformities in osteoarthritis has led to increased use of RSA in some cases of Walch B2, B3, and C deformities with substantial glenoid retroversion and/or humeral head subluxation.4

In cases of rotator cuff tear arthropathy, glenoid bone deformities are well described with several classification systems based on degree and dimension of bone insufficiency. The Hamada classification system defines the degree of medial glenoid erosion and superior bone loss, as well as acetabularization of the acromion in 5 grades; 5 Rispoli and colleagues6 defined and graded the degree of medicalization of the glenohumeral joint based on degree of subchondral plate erosion; and Visotsky and colleagues7 based their classification system on wear patterns of bone loss, alignment, and concomitant soft-tissue insufficiencies leading to instability and rotation loss.
In severe glenoid bone deficiency after glenoid component removal, Antuna and colleagues8 described the classic findings related to medial bone loss, anterior and posterior wall failure, and combinations thereof.
Continue to: All these classification systems...
All these classification systems are based on the 2-D appearance of the glenoid and should be considered cautiously. The glenoid is a complex 3-D structure that can be affected by any number of disease processes, trauma, and surgical intervention. Using more modern CT techniques and 3-D imaging, we now know that many deformities previously classified as unidirectional are, instead, complex and multidirectional.
Frankle and colleagues9 developed a classification based more 3-D CT models which has further classified severe glenoid vault deformities in relation to direction and degree of bone loss (Figures 2A-2E). Using this system, they were better able to determine degree and direction of deformity than in previous 2-D evaluations, and they were able to determine the amount of glenoid vault bone available for baseplate fixation. Scalise and colleagues10 further defined the influence of such 3-D planning in total shoulder arthroplasty.

With knowledge of these classification systems and use of contemporary imaging systems, shoulder arthroplasty in cases of severe glenoid deficiency can be more successful. Potentially, we can improve outcomes even more in the more severe cases of bone loss with use of patient-specific planning tools, including the guides and patient-specific implants that are now readily available with many implant systems.11
Preoperative planning tools, bone-grafting techniques, augmented and specialized glenoid and humeral implants, and patient-specific implants are discussed this month to give our readers a comprehensive review of the latest concepts in shoulder arthroplasty in cases of significant bone loss or deformity.
This month of The American Journal of Orthopedics presents the most current and cutting-edge solutions for humeral and glenoid bone deformities and deficiencies in contemporary shoulder arthroplasties.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
3. Cuff D, Levy JC, Gutiérrez S, Frankle M. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651.
4. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598.
5. Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;(254):92-96.
6. Rispoli D, Sperling JW, Athwal GS, Schleck CD, Cofield RH. Humeral head replacement for the treatment of osteoarthritis. J Bone Joint Surg Am. 2006;88(12):2637-2644.
7. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
8. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
9. Frankle MA, Teramoto A, Luo ZP, Levy JC, Pupello D. Glenoid morphology in reverse shoulder arthroplasty: classification and surgical implications. J Shoulder Elbow Surg. 2009;18(6):874-885.
10. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
11. Dines DM, Gulotta L, Craig EV, Dines JS. Novel solution for massive glenoid defects in shoulder arthroplasty: a patient-specific glenoid vault reconstruction system. Am J Orthop. 2017;46(2):104-108.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
3. Cuff D, Levy JC, Gutiérrez S, Frankle M. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651.
4. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598.
5. Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;(254):92-96.
6. Rispoli D, Sperling JW, Athwal GS, Schleck CD, Cofield RH. Humeral head replacement for the treatment of osteoarthritis. J Bone Joint Surg Am. 2006;88(12):2637-2644.
7. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
8. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
9. Frankle MA, Teramoto A, Luo ZP, Levy JC, Pupello D. Glenoid morphology in reverse shoulder arthroplasty: classification and surgical implications. J Shoulder Elbow Surg. 2009;18(6):874-885.
10. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
11. Dines DM, Gulotta L, Craig EV, Dines JS. Novel solution for massive glenoid defects in shoulder arthroplasty: a patient-specific glenoid vault reconstruction system. Am J Orthop. 2017;46(2):104-108.