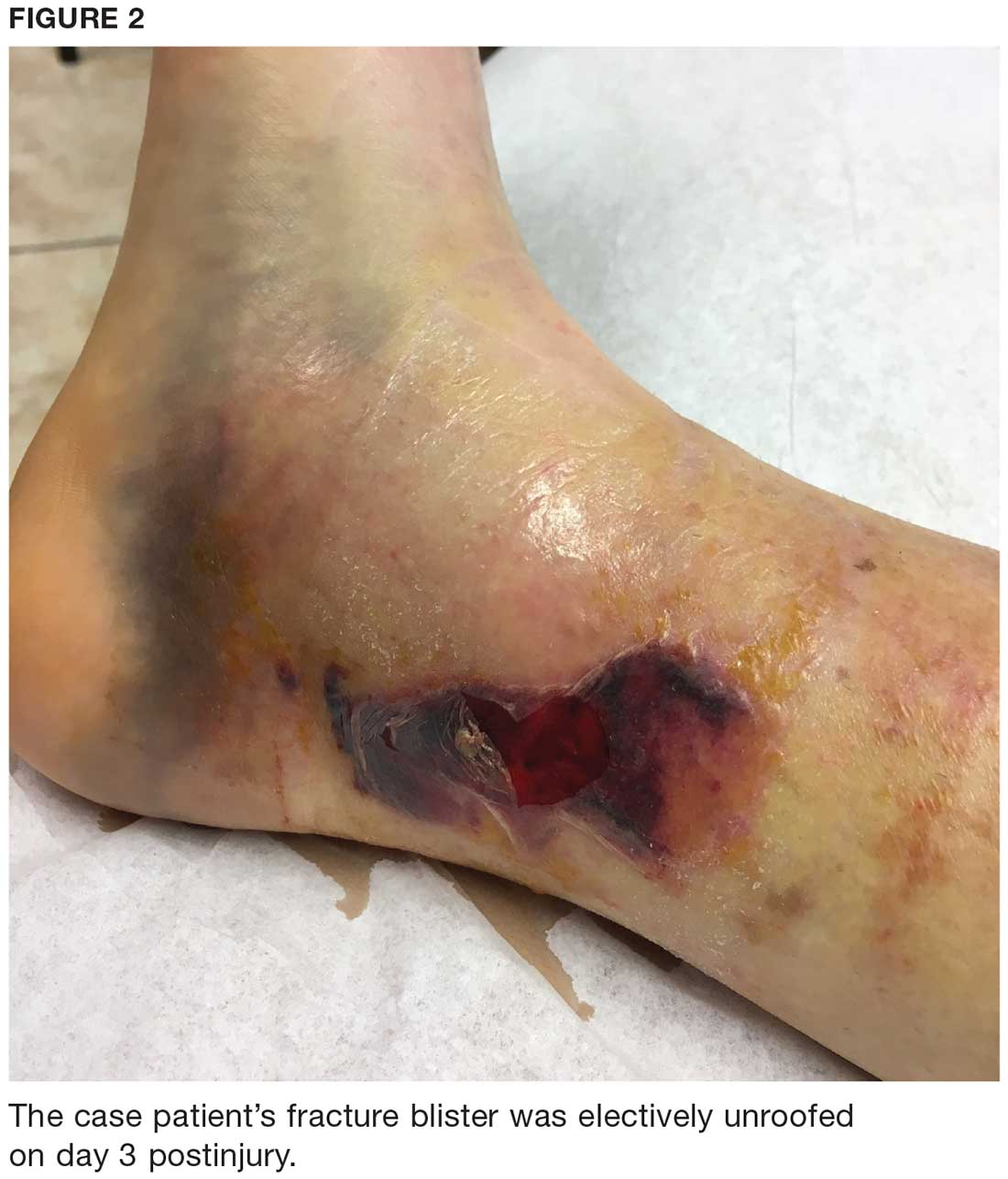
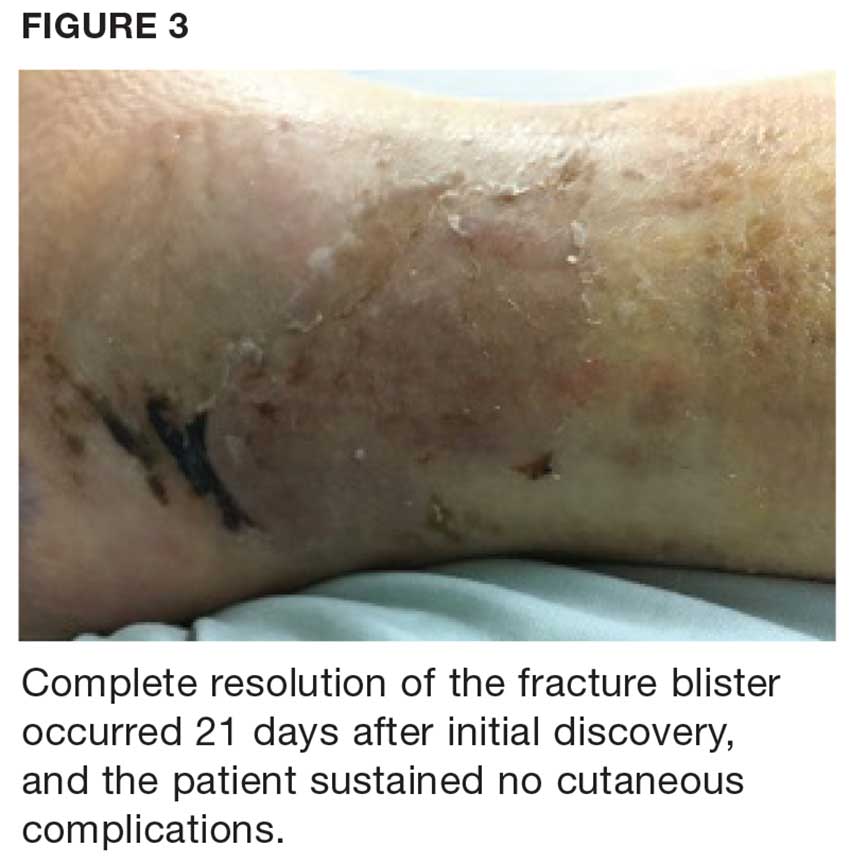
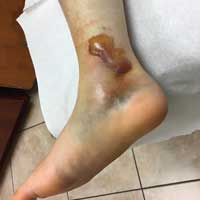
User login
The Prevention and Treatment of Femoral Trial Head Loss in Total Hip Arthroplasty
ABSTRACT
This article aims to provide the information necessary to prevent femoral trial head loss and to offer information regarding retrieval of the trial head if it is lost within the surgical field. These techniques can be used to help guide practice in the future. A review of the literature was conducted using a computerized search of PubMed in regard to this issue to investigate how such an occurrence can be prevented and what steps can be taken if preventative measures fail.
Continue to: Total hip arthroplasty...
Total hip arthroplasty (THA) is becoming an increasingly common procedure. Although this procedure is frequently performed, intraoperative complications still arise; therefore, methods of preventing and ameliorating these complications must be devised. One such complication is the loss of the femoral trial head component within the patient.
Loss of the trial head has been documented in THA cases that have used a number of different surgical approaches.1 Although it is uncommon to lose the trial within the pelvis, it is not an entirely unlikely phenomenon. The possibility of such an event makes prevention important, especially given the associated morbidity that loss of the component could cause. Fortunately, there are preventative measures that can be taken to minimize the probability of losing the femoral trial head, in addition to techniques that can be utilized if prevention fails.
SURGICAL TECHNIQUE
PREVENTION
Firstly, it is important to avoid the use of worn-out femoral trial components. It is thought that the incidence of femoral trial head loss is increased when the trunnion is older and has been used repeatedly.2,3 Therefore, it is advised that the use of worn femoral trial stems and other older trial components be avoided.
When the femoral trial head disengages anteriorly, it has the potential to enter the pelvis/retroperitoneal space.2,4 The femoral trial head may move more freely in the absence of resistance offered by the anterior capsule.4 Therefore, when extensive anterior capsular dissection has taken place, such as during extensive capsulectomy, caution should be exercised when manipulating the hip. This emphasizes the necessity to closely monitor the head during any manipulation, particularly in the presence of significant anterior capsule disruption.
Modular hip arthroplasty prosthetics allow for various intraoperative changes to be made to the femoral component, providing greater specificity to the prosthesis.5 However, the modularity of the femoral component has been described as a factor contributing to loss of the femoral trial head.4 This also has been discussed with respect to the implantable prosthetic femoral head itself because of disengagement from the femoral stem during reduction and dislocation.4
Continue to: Case reports have cited...
Case reports have cited the tension of the soft tissues as a definitive factor in trial head loss.1,4,6 These reports discuss the notion that more tension within the soft tissue can increase the likelihood that the trial head will dislodge during reduction or dislocation. Surgeons should therefore consider taking special care when manipulating the trial joint when the soft tissues are particularly tight and offer significant resistance. It has been suggested that the incision be packed with gauze during reductions when the soft tissue is under significant tension in order to keep the femoral trial head from entering the pelvis.6
A simple technique that can be utilized in the prevention of femoral trial head loss is the placement of a suture through the apical hole in the trial head to aid in the retrieval of the implant if it is lost.1 Madsen and colleagues1 suggest the placement of a No.1 (or thicker) suture through this hole. Although this takes some time to perform, it could prove useful in the prevention of complicated implant loss.
Lastly, and perhaps most importantly, it is essential that there is communication and understanding between the surgeon and any assistants. This has been noted to be particularly important during posterior or lateral surgical approaches when the trial head can be lost during attempts at reduction with traction and internal rotation.2 Given the possibility of losing the trial head during this reduction maneuver, communication between the team during the reduction is instrumental.
RETRIEVAL
If the femoral trial head dissociates from the trunnion of the femoral trial manipulation, there are some techniques that can be used to aid in retrieval. It has been described that when the trial head is lost within the surgical wound, it can travel underneath the rectus femoris muscle and cross the pelvic brim, subsequently entering the pelvis along the psoas tendon, as the psoas bursa offers little resistance to the smooth femoral trial head.1 The trial head has been found to follow this path along the psoas tendon until it is located in the posterior pelvis within the retroperitoneal space.1,7 What follows is a compilation of techniques for approaching loss of the femoral trial head when it occurs.
The femoral trial head is round and smooth, which complicates its retrieval. If the surgeon tries to simply grab the component with fingers, it may slip away into the pelvis. When trialing the hip to assess for anterior stability, if the femoral trial head is lost, the leg should not be moved.7 At this point, a manual attempt to recover the trial head before it moves into the pelvis along the psoas tendon should be made.7 It is possible that the femoral trial head may spin when trying to retrieve it, however this should still be attempted before a formal additional surgical approach is employed.7 It has also been noted that one can manually simultaneously press down on the hypogastrium toward the iliac fossa in order to inhibit the movement of the disarticulated trial head from advancing proximally.3 After performing this maneuver, the femoral trial head can be retrieved through the inguinal canal.3
Continue to: Additional surgical approaches...
Additional surgical approaches can also be utilized for retrieval of the femoral trial head if other measures fail. Callaghan and colleagues7 describe a separate surgical approach that can be used to retrieve the trial component after losing the trial head during a posterolateral approach for THA. This technique is commenced by making a 6-cm to 7-cm incision along the iliac crest to the anteromedial aspect of the anterior superior iliac spine.7 The interval between the iliacus and the inner table of the iliac wing is developed, and an attempt is made to locate the femoral trial head and guide it distally along the pelvis toward the hip. Fingers or napkin forceps can be used to accomplish this advancement of the trial head distally toward the hip, and once reaching surgical site, the trial can then be retrieved.7 Further extension of the incision can be made distally if this limited approach is unsuccessful.7 In the event the femoral trial head is still unable to be retrieved, the authors suggest considering a dedicated retroperitoneal approach for trial retrieval after the arthroplasty procedure has been completed.7
Another method for retrieval of the femoral trial head has been described specifically in the setting of a direct lateral approach.8 Kalra and colleagues8 describe a case in which the trial femoral head dislocated anteriorly, and although it was unable to be visualized, the component was able to be palpated posterior to the superior pubic ramus. With the trial head still disassociated within the pelvis, the final implants were implanted. Although the trial was unable to be viewed, using the same incision for the direct lateral approach, the trial femoral head was guided posteriorly toward the sciatic notch. A posterior approach to the hip was then performed using the same initial direct lateral incision used. Subsequent exposure and release of the external rotators and posterior capsule was performed, as was release of the insertion of the gluteus maximus in order to facilitate better visualization and to prevent excessive tension on the sciatic nerve. Blunt finger dissection of the soft tissues was then performed, and the trial head was retrieved from the sciatic notch with a Kocher clamp.8
Madsen and colleagues1 highlight two different cases in which the trial head was lost into the pelvis when using an anterolateral (modified Watson-Jones) approach to the hip to perform THA. As previously alluded to, the trial heads traveled along the patients’ psoas muscle and stopped directly anterior to their sacroiliac joint. In both cases, the trial head was retrieved using a large Satinsky aortic clamp, which enabled the surgeons to drag the trial head to the pelvic brim where it could be removed with a hemostat.1
Multiple authors have discussed the decision to leave the component within the pelvis if the femoral trial head cannot be retrieved.2,4,7 Batouk and colleagues4 noted that in a case of loss of the femoral trial head, the component would be unlikely to disrupt any of the structures within the pelvis, and in the absence of compression of any vital structures, leaving the implant in the patient could be considered. Although the short-term follow-up of 3 months noted in this particular case did not yield any obvious detriment to the patient in regard to symptomatology, the authors note that the long-term effects of such a practice is unclear.4 In another case, in which the decision was made to leave the femoral trial head, the patient at postoperative week 6 began to hear clicking in the hip with an associated loss of range of motion.7 This subsequently prompted removal of the trial component.
DISCUSSION
Although not a particularly common complication, loss of the femoral trial head can occur; therefore, a plan of action should be in place to prevent its loss or to retrieve it if prevention is ineffective. Given the modularity of various arthroplasty systems in regard to the different trial components or even the final implantable prosthetic devices, component loss is a possibility. An understanding of this complication and the appropriate steps to approaching it could aid in preventing patient morbidity. Because of this, it is imperative that surgeons who perform THA be aware of the potential complications and the measures that can be taken to address them.
Continue to: CONCLUSION
CONCLUSION
The femoral trial head often can be quickly and easily recovered; however, trial component recovery can sometimes be more complicated. Loss of the trial femoral head could potentially occur during dislocation, reduction, or any of the trial positions. An example of a more complicated recovery is when the femoral trial head is lost into the retroperitoneal space, which could occur when trialing the hip in extension to assess the anterior stability of the hip. Loss of the femoral trial head is an avoidable occurrence, and it has the potential to cause a number of complications as well as the need for additional incisions/surgery to retrieve the femoral trial head. The subsequent issues that could arise after loss occurs can not only lead to extensive surgical complications, but can also foster patient dissatisfaction regarding surgical outcomes. Therefore, consistent attempts to utilize preventative techniques are essential. As discussed, simple measures such as placement of a suture through the apical hole of the trial component and adequate communication between those involved in reduction and trialing maneuvers, can serve to avert femoral trial head loss.
1. Madsen WY, Mitchell BS, Kates SL. Successful intraoperative retrieval of dislocated femoral trial head during total hip arthroplasty. J Arthroplasty. 2012;27(5):820.e9-e11. doi:10.1016/j.arth.2011.08.006.
2. Ozkan K, Ugutmen E, Altintas F, Eren A, Mahirogullari M. Intraoperative dislocation of the prosthetic femoral head into the pelvis during total hip arthroplasty. Acta Orthop Belg. 2008;74(4):553-555.
3. Rachbauer F, Nogler M, Krismer M, Moritz M. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):881-882.
4. Batouk O, Gilbart M, Jain R. Intraoperative dislocation of the trial femoral head into the pelvis during total hip arthroplasty: a case report. J Bone Joint Surg Am. 2001;83-A(10):1549-1551.
5. Srinivasan A, Jung E, Levine BR. Modularity of the femoral component in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(4):214-222. doi:10.5435/JAAOS-20-04-214.
6. Princep A. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):880-881.
7. Callaghan JJ, McAndrew C, Boese CK, Forest E. Intrapelvic migration of the trial femoral head during total hip arthroplasty: is retrieval necessary? A report of four cases. Iowa Orthop J. 2006;26:60-62.
8. Kalra K, Ries MD, Bozic KJ. Intrapelvic displacement of a trial femoral head during total hip arthroplasty and a method to retrieve it. J Arthroplasty 2011;26(2):338.e21-e23. doi:10.1016/j.arth.2009.12.005.
ABSTRACT
This article aims to provide the information necessary to prevent femoral trial head loss and to offer information regarding retrieval of the trial head if it is lost within the surgical field. These techniques can be used to help guide practice in the future. A review of the literature was conducted using a computerized search of PubMed in regard to this issue to investigate how such an occurrence can be prevented and what steps can be taken if preventative measures fail.
Continue to: Total hip arthroplasty...
Total hip arthroplasty (THA) is becoming an increasingly common procedure. Although this procedure is frequently performed, intraoperative complications still arise; therefore, methods of preventing and ameliorating these complications must be devised. One such complication is the loss of the femoral trial head component within the patient.
Loss of the trial head has been documented in THA cases that have used a number of different surgical approaches.1 Although it is uncommon to lose the trial within the pelvis, it is not an entirely unlikely phenomenon. The possibility of such an event makes prevention important, especially given the associated morbidity that loss of the component could cause. Fortunately, there are preventative measures that can be taken to minimize the probability of losing the femoral trial head, in addition to techniques that can be utilized if prevention fails.
SURGICAL TECHNIQUE
PREVENTION
Firstly, it is important to avoid the use of worn-out femoral trial components. It is thought that the incidence of femoral trial head loss is increased when the trunnion is older and has been used repeatedly.2,3 Therefore, it is advised that the use of worn femoral trial stems and other older trial components be avoided.
When the femoral trial head disengages anteriorly, it has the potential to enter the pelvis/retroperitoneal space.2,4 The femoral trial head may move more freely in the absence of resistance offered by the anterior capsule.4 Therefore, when extensive anterior capsular dissection has taken place, such as during extensive capsulectomy, caution should be exercised when manipulating the hip. This emphasizes the necessity to closely monitor the head during any manipulation, particularly in the presence of significant anterior capsule disruption.
Modular hip arthroplasty prosthetics allow for various intraoperative changes to be made to the femoral component, providing greater specificity to the prosthesis.5 However, the modularity of the femoral component has been described as a factor contributing to loss of the femoral trial head.4 This also has been discussed with respect to the implantable prosthetic femoral head itself because of disengagement from the femoral stem during reduction and dislocation.4
Continue to: Case reports have cited...
Case reports have cited the tension of the soft tissues as a definitive factor in trial head loss.1,4,6 These reports discuss the notion that more tension within the soft tissue can increase the likelihood that the trial head will dislodge during reduction or dislocation. Surgeons should therefore consider taking special care when manipulating the trial joint when the soft tissues are particularly tight and offer significant resistance. It has been suggested that the incision be packed with gauze during reductions when the soft tissue is under significant tension in order to keep the femoral trial head from entering the pelvis.6
A simple technique that can be utilized in the prevention of femoral trial head loss is the placement of a suture through the apical hole in the trial head to aid in the retrieval of the implant if it is lost.1 Madsen and colleagues1 suggest the placement of a No.1 (or thicker) suture through this hole. Although this takes some time to perform, it could prove useful in the prevention of complicated implant loss.
Lastly, and perhaps most importantly, it is essential that there is communication and understanding between the surgeon and any assistants. This has been noted to be particularly important during posterior or lateral surgical approaches when the trial head can be lost during attempts at reduction with traction and internal rotation.2 Given the possibility of losing the trial head during this reduction maneuver, communication between the team during the reduction is instrumental.
RETRIEVAL
If the femoral trial head dissociates from the trunnion of the femoral trial manipulation, there are some techniques that can be used to aid in retrieval. It has been described that when the trial head is lost within the surgical wound, it can travel underneath the rectus femoris muscle and cross the pelvic brim, subsequently entering the pelvis along the psoas tendon, as the psoas bursa offers little resistance to the smooth femoral trial head.1 The trial head has been found to follow this path along the psoas tendon until it is located in the posterior pelvis within the retroperitoneal space.1,7 What follows is a compilation of techniques for approaching loss of the femoral trial head when it occurs.
The femoral trial head is round and smooth, which complicates its retrieval. If the surgeon tries to simply grab the component with fingers, it may slip away into the pelvis. When trialing the hip to assess for anterior stability, if the femoral trial head is lost, the leg should not be moved.7 At this point, a manual attempt to recover the trial head before it moves into the pelvis along the psoas tendon should be made.7 It is possible that the femoral trial head may spin when trying to retrieve it, however this should still be attempted before a formal additional surgical approach is employed.7 It has also been noted that one can manually simultaneously press down on the hypogastrium toward the iliac fossa in order to inhibit the movement of the disarticulated trial head from advancing proximally.3 After performing this maneuver, the femoral trial head can be retrieved through the inguinal canal.3
Continue to: Additional surgical approaches...
Additional surgical approaches can also be utilized for retrieval of the femoral trial head if other measures fail. Callaghan and colleagues7 describe a separate surgical approach that can be used to retrieve the trial component after losing the trial head during a posterolateral approach for THA. This technique is commenced by making a 6-cm to 7-cm incision along the iliac crest to the anteromedial aspect of the anterior superior iliac spine.7 The interval between the iliacus and the inner table of the iliac wing is developed, and an attempt is made to locate the femoral trial head and guide it distally along the pelvis toward the hip. Fingers or napkin forceps can be used to accomplish this advancement of the trial head distally toward the hip, and once reaching surgical site, the trial can then be retrieved.7 Further extension of the incision can be made distally if this limited approach is unsuccessful.7 In the event the femoral trial head is still unable to be retrieved, the authors suggest considering a dedicated retroperitoneal approach for trial retrieval after the arthroplasty procedure has been completed.7
Another method for retrieval of the femoral trial head has been described specifically in the setting of a direct lateral approach.8 Kalra and colleagues8 describe a case in which the trial femoral head dislocated anteriorly, and although it was unable to be visualized, the component was able to be palpated posterior to the superior pubic ramus. With the trial head still disassociated within the pelvis, the final implants were implanted. Although the trial was unable to be viewed, using the same incision for the direct lateral approach, the trial femoral head was guided posteriorly toward the sciatic notch. A posterior approach to the hip was then performed using the same initial direct lateral incision used. Subsequent exposure and release of the external rotators and posterior capsule was performed, as was release of the insertion of the gluteus maximus in order to facilitate better visualization and to prevent excessive tension on the sciatic nerve. Blunt finger dissection of the soft tissues was then performed, and the trial head was retrieved from the sciatic notch with a Kocher clamp.8
Madsen and colleagues1 highlight two different cases in which the trial head was lost into the pelvis when using an anterolateral (modified Watson-Jones) approach to the hip to perform THA. As previously alluded to, the trial heads traveled along the patients’ psoas muscle and stopped directly anterior to their sacroiliac joint. In both cases, the trial head was retrieved using a large Satinsky aortic clamp, which enabled the surgeons to drag the trial head to the pelvic brim where it could be removed with a hemostat.1
Multiple authors have discussed the decision to leave the component within the pelvis if the femoral trial head cannot be retrieved.2,4,7 Batouk and colleagues4 noted that in a case of loss of the femoral trial head, the component would be unlikely to disrupt any of the structures within the pelvis, and in the absence of compression of any vital structures, leaving the implant in the patient could be considered. Although the short-term follow-up of 3 months noted in this particular case did not yield any obvious detriment to the patient in regard to symptomatology, the authors note that the long-term effects of such a practice is unclear.4 In another case, in which the decision was made to leave the femoral trial head, the patient at postoperative week 6 began to hear clicking in the hip with an associated loss of range of motion.7 This subsequently prompted removal of the trial component.
DISCUSSION
Although not a particularly common complication, loss of the femoral trial head can occur; therefore, a plan of action should be in place to prevent its loss or to retrieve it if prevention is ineffective. Given the modularity of various arthroplasty systems in regard to the different trial components or even the final implantable prosthetic devices, component loss is a possibility. An understanding of this complication and the appropriate steps to approaching it could aid in preventing patient morbidity. Because of this, it is imperative that surgeons who perform THA be aware of the potential complications and the measures that can be taken to address them.
Continue to: CONCLUSION
CONCLUSION
The femoral trial head often can be quickly and easily recovered; however, trial component recovery can sometimes be more complicated. Loss of the trial femoral head could potentially occur during dislocation, reduction, or any of the trial positions. An example of a more complicated recovery is when the femoral trial head is lost into the retroperitoneal space, which could occur when trialing the hip in extension to assess the anterior stability of the hip. Loss of the femoral trial head is an avoidable occurrence, and it has the potential to cause a number of complications as well as the need for additional incisions/surgery to retrieve the femoral trial head. The subsequent issues that could arise after loss occurs can not only lead to extensive surgical complications, but can also foster patient dissatisfaction regarding surgical outcomes. Therefore, consistent attempts to utilize preventative techniques are essential. As discussed, simple measures such as placement of a suture through the apical hole of the trial component and adequate communication between those involved in reduction and trialing maneuvers, can serve to avert femoral trial head loss.
ABSTRACT
This article aims to provide the information necessary to prevent femoral trial head loss and to offer information regarding retrieval of the trial head if it is lost within the surgical field. These techniques can be used to help guide practice in the future. A review of the literature was conducted using a computerized search of PubMed in regard to this issue to investigate how such an occurrence can be prevented and what steps can be taken if preventative measures fail.
Continue to: Total hip arthroplasty...
Total hip arthroplasty (THA) is becoming an increasingly common procedure. Although this procedure is frequently performed, intraoperative complications still arise; therefore, methods of preventing and ameliorating these complications must be devised. One such complication is the loss of the femoral trial head component within the patient.
Loss of the trial head has been documented in THA cases that have used a number of different surgical approaches.1 Although it is uncommon to lose the trial within the pelvis, it is not an entirely unlikely phenomenon. The possibility of such an event makes prevention important, especially given the associated morbidity that loss of the component could cause. Fortunately, there are preventative measures that can be taken to minimize the probability of losing the femoral trial head, in addition to techniques that can be utilized if prevention fails.
SURGICAL TECHNIQUE
PREVENTION
Firstly, it is important to avoid the use of worn-out femoral trial components. It is thought that the incidence of femoral trial head loss is increased when the trunnion is older and has been used repeatedly.2,3 Therefore, it is advised that the use of worn femoral trial stems and other older trial components be avoided.
When the femoral trial head disengages anteriorly, it has the potential to enter the pelvis/retroperitoneal space.2,4 The femoral trial head may move more freely in the absence of resistance offered by the anterior capsule.4 Therefore, when extensive anterior capsular dissection has taken place, such as during extensive capsulectomy, caution should be exercised when manipulating the hip. This emphasizes the necessity to closely monitor the head during any manipulation, particularly in the presence of significant anterior capsule disruption.
Modular hip arthroplasty prosthetics allow for various intraoperative changes to be made to the femoral component, providing greater specificity to the prosthesis.5 However, the modularity of the femoral component has been described as a factor contributing to loss of the femoral trial head.4 This also has been discussed with respect to the implantable prosthetic femoral head itself because of disengagement from the femoral stem during reduction and dislocation.4
Continue to: Case reports have cited...
Case reports have cited the tension of the soft tissues as a definitive factor in trial head loss.1,4,6 These reports discuss the notion that more tension within the soft tissue can increase the likelihood that the trial head will dislodge during reduction or dislocation. Surgeons should therefore consider taking special care when manipulating the trial joint when the soft tissues are particularly tight and offer significant resistance. It has been suggested that the incision be packed with gauze during reductions when the soft tissue is under significant tension in order to keep the femoral trial head from entering the pelvis.6
A simple technique that can be utilized in the prevention of femoral trial head loss is the placement of a suture through the apical hole in the trial head to aid in the retrieval of the implant if it is lost.1 Madsen and colleagues1 suggest the placement of a No.1 (or thicker) suture through this hole. Although this takes some time to perform, it could prove useful in the prevention of complicated implant loss.
Lastly, and perhaps most importantly, it is essential that there is communication and understanding between the surgeon and any assistants. This has been noted to be particularly important during posterior or lateral surgical approaches when the trial head can be lost during attempts at reduction with traction and internal rotation.2 Given the possibility of losing the trial head during this reduction maneuver, communication between the team during the reduction is instrumental.
RETRIEVAL
If the femoral trial head dissociates from the trunnion of the femoral trial manipulation, there are some techniques that can be used to aid in retrieval. It has been described that when the trial head is lost within the surgical wound, it can travel underneath the rectus femoris muscle and cross the pelvic brim, subsequently entering the pelvis along the psoas tendon, as the psoas bursa offers little resistance to the smooth femoral trial head.1 The trial head has been found to follow this path along the psoas tendon until it is located in the posterior pelvis within the retroperitoneal space.1,7 What follows is a compilation of techniques for approaching loss of the femoral trial head when it occurs.
The femoral trial head is round and smooth, which complicates its retrieval. If the surgeon tries to simply grab the component with fingers, it may slip away into the pelvis. When trialing the hip to assess for anterior stability, if the femoral trial head is lost, the leg should not be moved.7 At this point, a manual attempt to recover the trial head before it moves into the pelvis along the psoas tendon should be made.7 It is possible that the femoral trial head may spin when trying to retrieve it, however this should still be attempted before a formal additional surgical approach is employed.7 It has also been noted that one can manually simultaneously press down on the hypogastrium toward the iliac fossa in order to inhibit the movement of the disarticulated trial head from advancing proximally.3 After performing this maneuver, the femoral trial head can be retrieved through the inguinal canal.3
Continue to: Additional surgical approaches...
Additional surgical approaches can also be utilized for retrieval of the femoral trial head if other measures fail. Callaghan and colleagues7 describe a separate surgical approach that can be used to retrieve the trial component after losing the trial head during a posterolateral approach for THA. This technique is commenced by making a 6-cm to 7-cm incision along the iliac crest to the anteromedial aspect of the anterior superior iliac spine.7 The interval between the iliacus and the inner table of the iliac wing is developed, and an attempt is made to locate the femoral trial head and guide it distally along the pelvis toward the hip. Fingers or napkin forceps can be used to accomplish this advancement of the trial head distally toward the hip, and once reaching surgical site, the trial can then be retrieved.7 Further extension of the incision can be made distally if this limited approach is unsuccessful.7 In the event the femoral trial head is still unable to be retrieved, the authors suggest considering a dedicated retroperitoneal approach for trial retrieval after the arthroplasty procedure has been completed.7
Another method for retrieval of the femoral trial head has been described specifically in the setting of a direct lateral approach.8 Kalra and colleagues8 describe a case in which the trial femoral head dislocated anteriorly, and although it was unable to be visualized, the component was able to be palpated posterior to the superior pubic ramus. With the trial head still disassociated within the pelvis, the final implants were implanted. Although the trial was unable to be viewed, using the same incision for the direct lateral approach, the trial femoral head was guided posteriorly toward the sciatic notch. A posterior approach to the hip was then performed using the same initial direct lateral incision used. Subsequent exposure and release of the external rotators and posterior capsule was performed, as was release of the insertion of the gluteus maximus in order to facilitate better visualization and to prevent excessive tension on the sciatic nerve. Blunt finger dissection of the soft tissues was then performed, and the trial head was retrieved from the sciatic notch with a Kocher clamp.8
Madsen and colleagues1 highlight two different cases in which the trial head was lost into the pelvis when using an anterolateral (modified Watson-Jones) approach to the hip to perform THA. As previously alluded to, the trial heads traveled along the patients’ psoas muscle and stopped directly anterior to their sacroiliac joint. In both cases, the trial head was retrieved using a large Satinsky aortic clamp, which enabled the surgeons to drag the trial head to the pelvic brim where it could be removed with a hemostat.1
Multiple authors have discussed the decision to leave the component within the pelvis if the femoral trial head cannot be retrieved.2,4,7 Batouk and colleagues4 noted that in a case of loss of the femoral trial head, the component would be unlikely to disrupt any of the structures within the pelvis, and in the absence of compression of any vital structures, leaving the implant in the patient could be considered. Although the short-term follow-up of 3 months noted in this particular case did not yield any obvious detriment to the patient in regard to symptomatology, the authors note that the long-term effects of such a practice is unclear.4 In another case, in which the decision was made to leave the femoral trial head, the patient at postoperative week 6 began to hear clicking in the hip with an associated loss of range of motion.7 This subsequently prompted removal of the trial component.
DISCUSSION
Although not a particularly common complication, loss of the femoral trial head can occur; therefore, a plan of action should be in place to prevent its loss or to retrieve it if prevention is ineffective. Given the modularity of various arthroplasty systems in regard to the different trial components or even the final implantable prosthetic devices, component loss is a possibility. An understanding of this complication and the appropriate steps to approaching it could aid in preventing patient morbidity. Because of this, it is imperative that surgeons who perform THA be aware of the potential complications and the measures that can be taken to address them.
Continue to: CONCLUSION
CONCLUSION
The femoral trial head often can be quickly and easily recovered; however, trial component recovery can sometimes be more complicated. Loss of the trial femoral head could potentially occur during dislocation, reduction, or any of the trial positions. An example of a more complicated recovery is when the femoral trial head is lost into the retroperitoneal space, which could occur when trialing the hip in extension to assess the anterior stability of the hip. Loss of the femoral trial head is an avoidable occurrence, and it has the potential to cause a number of complications as well as the need for additional incisions/surgery to retrieve the femoral trial head. The subsequent issues that could arise after loss occurs can not only lead to extensive surgical complications, but can also foster patient dissatisfaction regarding surgical outcomes. Therefore, consistent attempts to utilize preventative techniques are essential. As discussed, simple measures such as placement of a suture through the apical hole of the trial component and adequate communication between those involved in reduction and trialing maneuvers, can serve to avert femoral trial head loss.
1. Madsen WY, Mitchell BS, Kates SL. Successful intraoperative retrieval of dislocated femoral trial head during total hip arthroplasty. J Arthroplasty. 2012;27(5):820.e9-e11. doi:10.1016/j.arth.2011.08.006.
2. Ozkan K, Ugutmen E, Altintas F, Eren A, Mahirogullari M. Intraoperative dislocation of the prosthetic femoral head into the pelvis during total hip arthroplasty. Acta Orthop Belg. 2008;74(4):553-555.
3. Rachbauer F, Nogler M, Krismer M, Moritz M. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):881-882.
4. Batouk O, Gilbart M, Jain R. Intraoperative dislocation of the trial femoral head into the pelvis during total hip arthroplasty: a case report. J Bone Joint Surg Am. 2001;83-A(10):1549-1551.
5. Srinivasan A, Jung E, Levine BR. Modularity of the femoral component in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(4):214-222. doi:10.5435/JAAOS-20-04-214.
6. Princep A. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):880-881.
7. Callaghan JJ, McAndrew C, Boese CK, Forest E. Intrapelvic migration of the trial femoral head during total hip arthroplasty: is retrieval necessary? A report of four cases. Iowa Orthop J. 2006;26:60-62.
8. Kalra K, Ries MD, Bozic KJ. Intrapelvic displacement of a trial femoral head during total hip arthroplasty and a method to retrieve it. J Arthroplasty 2011;26(2):338.e21-e23. doi:10.1016/j.arth.2009.12.005.
1. Madsen WY, Mitchell BS, Kates SL. Successful intraoperative retrieval of dislocated femoral trial head during total hip arthroplasty. J Arthroplasty. 2012;27(5):820.e9-e11. doi:10.1016/j.arth.2011.08.006.
2. Ozkan K, Ugutmen E, Altintas F, Eren A, Mahirogullari M. Intraoperative dislocation of the prosthetic femoral head into the pelvis during total hip arthroplasty. Acta Orthop Belg. 2008;74(4):553-555.
3. Rachbauer F, Nogler M, Krismer M, Moritz M. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):881-882.
4. Batouk O, Gilbart M, Jain R. Intraoperative dislocation of the trial femoral head into the pelvis during total hip arthroplasty: a case report. J Bone Joint Surg Am. 2001;83-A(10):1549-1551.
5. Srinivasan A, Jung E, Levine BR. Modularity of the femoral component in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(4):214-222. doi:10.5435/JAAOS-20-04-214.
6. Princep A. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):880-881.
7. Callaghan JJ, McAndrew C, Boese CK, Forest E. Intrapelvic migration of the trial femoral head during total hip arthroplasty: is retrieval necessary? A report of four cases. Iowa Orthop J. 2006;26:60-62.
8. Kalra K, Ries MD, Bozic KJ. Intrapelvic displacement of a trial femoral head during total hip arthroplasty and a method to retrieve it. J Arthroplasty 2011;26(2):338.e21-e23. doi:10.1016/j.arth.2009.12.005.
TAKE-HOME POINTS
- Femoral head trial loss is a complication that can occur during THA.
- This event can be a source of avoidable morbidity.
- Preventative measures can be taken to avoid this complication.
- If preventative measures fail, retrieval of the femoral trial head can be performed.
- A thorough understanding of preventative and retrieval methods is essential for surgeons that perform THA.
Special Considerations for Pediatric Patellar Instability
ABSTRACT
Patellar instability in children and adolescents is a challenging subset to treat. Varied forms of instability, ranging from episodic dislocation to fixed dislocation, have been recognized. It is of utmost importance for the treating physician to recognize these different patterns of instability and their associated risk factors, as more complex patterns of instability would require more extensive surgical procedures. Medial patellofemoral ligament (MPFL) reconstruction, by itself, may not suffice or may not be appropriate for the more complex instability patterns. Appropriate and early treatment of such instability in children would allow for functional progression and possible remodeling of the trochlea. However, early treatment has the associated risk of growth disturbances when surgical procedures are performed around open physis or if adult-type bony procedures are performed in children. Recent knowledge about the relationship between trochlea, MPFL femoral attachment, and distal femoral physis could help to advance safe surgical care for these patients. This article reviews the pathophysiology, risk factors, and the existing classification systems for patellar instability in children and adolescents. It focuses on varied surgical techniques, which are unique to the pediatric population, and summarizes the outcomes of these surgical techniques.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
In a prospective 2-year study of Finnish children, the annual incidence rate of patellar instability was 43/100,000 pediatric population.1 In patients 9 to 15 years of age, the incidence was approximately 1/1000.1 In another study, patients at highest risk for a first-time patellar dislocation were females aged 10 to 17 years.2 In a study in patients with traumatic hemarthrosis, 36% in the younger age group (10-14 years) and 28% in the older age group (15-18 years) had sustained patellar dislocation. In contrast, 22% in the younger age group and 40% in the older age group had sustained an anterior cruciate ligament tear.3
Approximately one-half of patients who dislocate their patella suffer from long-term complications.4,5 These complications include recurrent instability, patellofemoral pain, osteochondral lesions, and eventual arthritis.1,4,5 Young, active individuals are more prone to these issues.6 Also, 39% or more of patellar dislocation patients have an associated osteochondral fracture that might influence the management.1 Thus, patellar instability in young patients is an area of concern.
DEVELOPMENTAL ANATOMY
At 4-week gestation, the patellofemoral joint is an ectodermal sac filled with mesenchyme of the somatic mesoderm.7 Mesenchymal condensations then appear at 4 to 5 weeks gestation, followed by chondrification of both the femur and patella.7 The joint space is present by 6 weeks, and the patellar and distal femoral condyles are present at 7 weeks gestation.7 By 8 weeks gestation, the basic knee anatomy resembles that of an adult with the chondroepiphysis forming the articular surfaces of the femur, tibia, and patella.7 By this time, the extensor mechanism is formed, and active joint motion has begun, facilitating the development of the trochlear sulcus.7 The secondary ossification center in the distal femoral epiphysis forms around 36 weeks gestation.8 Postnatally, both the patella and distal femur grow through endochondral ossification.9,10
The patella is the largest sesamoid bone in the human body.11 The patella begins as a dense consolidation of cells that differentiate as the quadriceps mechanisms develop.12,13 The patellar anlage becomes distinguishable within the quadriceps tendon around 7.5-week gestation.12 The morphology of the patella conforms to the distal femur.12 The patella molds or re-models as the knee begins to move in response to mechanical stresses.7 The patella increases in relative size during the first 6 months of gestation, then enlarges proportionately to the rest of the bones.7 Ossification begins around 3 years of age for females and 4 to 5 years of age for males.8,14 The ossification center may appear irregular as it rapidly expands.14 Ossification proceeds in a proximal to distal direction, thus giving a spurious estimation of patellar height on radiographs in children. The overall morphology of the cartilaginous patella during development is comparable to the final mature shape.14 Abnormal contact stresses on the articular surface of the patella during skeletal immaturity can lead to deformation.7
Ultrasonographic measurements in normal patients show that trochlear groove (TG) morphology is present early and becomes more radiographically apparent as distal femoral ossification is completed.15 Anatomic dissections of aborted fetuses have verified the morphology of the TG as it remains constant during growth and the groove morphology is the same for both fetuses and adults.16 An ultrasound study performed on patients aged 12 to 18 years showed the cartilaginous sulcus angle (CSA) remained constant throughout all age groups (146°).17 The CSA however, differed in patients who suffered a patellar dislocation (average, 164°; range, 154°-195°) compared with normal knees (average CSA, 145°; range, 131°-158°).15,17,18 The osseous sulcus angle, on the other hand, appears flat at birth and the TG deepens with age. This increase in depth is more of a reflection of progressive ossification of a well-formed cartilaginous trochlea, rather than a true deepening of the sulcus.17 Thus, the axial radiographic view of the patella provides misleading information about the sulcus angle in children and should not be used to define trochlear morphology.
Continue to: MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
The medial patellofemoral ligament (MPFL) functions to limit the lateral translation of the patella.19 The attachment sites on the femur and patella for the MPFL have been studied in children.20-23 Cadaveric dissections in specimens aged 2 to 11 years have noted the patellar attachment to be an average of 12 mm in length with the midpoint approximately 5 mm superior to the mid-pole of the patella.22 The patellar footprint of the MPFL insertion was a mean 41% of the entire patellar length.22
It is important to be aware of the characteristic anatomy of the MPFL, as fixation points should mimic the anatomic insertion as best as possible while also avoiding violation of the nearby physis. The MPFL originates between the adductor tubercle and the medial femoral epicondyle just distal to the distal femoral physis and attaches to the superomedial aspect of the patella.20-25 In relation to the physis in pediatric patients, the midpoint of MPFL insertion has been measured to be 4 mm to 9 mm distal to the femoral physis.21,24,25 These measurements represent averages as cadaveric studies have reported that some part of MPFL femoral insertion extends proximal to the distal femoral physis.21 A recent report of physeal injury to the posterior distal femoral physis during MPFL reconstruction leading to femoral flexion deformity highlights the importance of physeal-respecting surgery.26
TROCHLEA AND ANTERIOR DISTAL FEMORAL PHYSIS
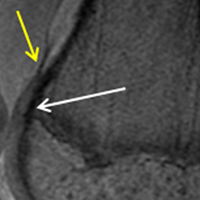
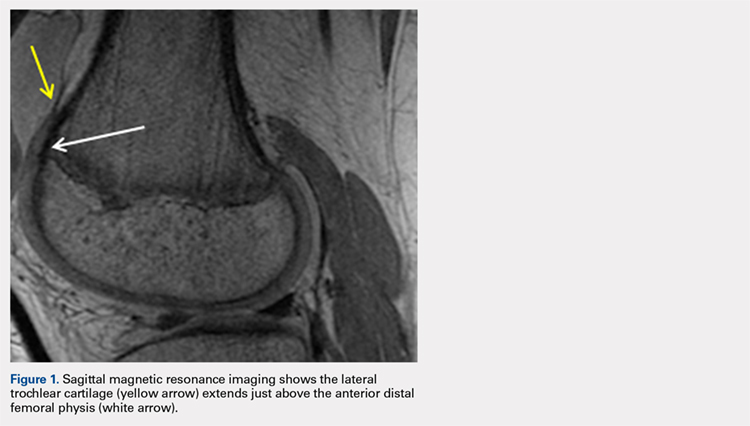
The relationship between the proximal aspect of the trochlea and the anterior distal femoral physis has been recently studied in 175 knees with dysplastic trochlea.27 Based on magnetic resonance imaging evaluation, the lateral aspect of the trochlea extended proximal to the anterior distal femoral physis in 13% of patients and was at the level of the anterior physis in another 13% of patients (Figure 1).27 Hence, a cautious approach is recommended for any surgery to address trochlear dysplasia or trochlear bump in younger patients to prevent iatrogenic injury to anterior distal femoral physis and resultant genu recurvatum. The distance between the trochlea and the physis increased with increasing age.
LIMB ALIGNMENT
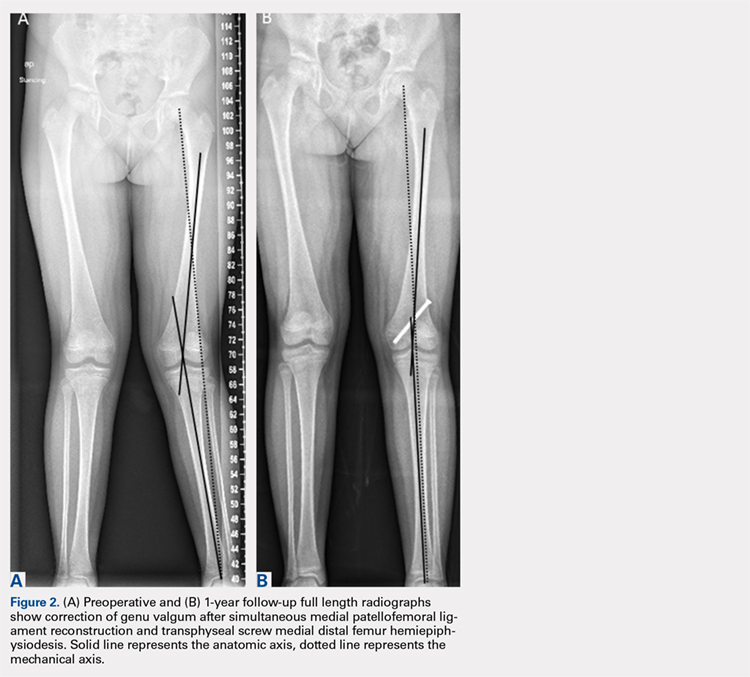
Physiologically, the quadriceps angle (Q angle) changes through the course of growth. As children begin standing and walking, they stand with their feet wider apart and in genu varum.28 Physiologic genu varum can reach 15°.28 This degree lessens during the first 1.5 to 2 years of life, transitioning to physiologic valgus of nearly 12° by 3 years of age.28 Genu valgum, thereafter, gradually decreases to reach the adult value of around 7° to 8° by age 7 years.28 Increased genu valgum is a risk factor for patellar instability. In skeletally immature patients, correction of genu valgum through guided growth may be desirable in patients undergoing patellar stabilization surgery (Figures 2A, 2B).29
PATHOPHYSIOLOGY OF PEDIATRIC PATELLAR DISLOCATION
TROCHLEAR DYSPLASIA
Trochlear dysplasia is an abnormal shape and depth of the TG.30 Up to 96% of patients with patellar dislocation have trochlear dysplasia.30-33 In a study of patellar instability in children, at least 1 of the 3 signs of trochlear dysplasia (the crossing sign, supratrochlear bump, and double contour sign) was present on lateral radiographs.34 In another study on the growth of trochlear dysplasia in children and adolescents, all grades of trochlear dysplasia were present at all ages (ie, the dysplasia was most likely present at birth and did not necessarily worsen with age and growth).35 The linear dimensions of lateral and medial condylar height as well as trochlear bump increased with age but both the sulcus angle and shape of the trochlea did not change significantly.35 Remodeling of a dysplastic trochlea can happen if the patella is stabilized and appropriately located at a younger age, preferably before 10 years of age.36,37
Continue to: PATELLAR HEIGHT
PATELLAR HEIGHT
The role of patellar height in patellar instability has been well established.38 In patients with patella alta, the patella remains proximal to the TG during the greater arc of knee motion, which predisposes it to patellar instability. Calculation of patellar height in children could be challenging due to incomplete ossification, as well as asymmetric ossification of the patella and the tibial tubercle (TT). Since the patella ossifies from proximal to distal, most radiographic methods that measure the patellar height from the distal aspect of the patella provide a spurious elevation of the measurement.
The Caton-Deschamps (CD) method measures the length of the patellar articular surface and the distance from the inferior edge of the articular surface to the anterosuperior corner of the tibial plateau.39 A ratio >1.3 signifies patella alta. The CD ratio has been verified as a simple and reliable index for measuring patellar height in children.40 Two other methods have been described for determining patellar height in children.41,42 Based on anteroposterior (AP) radiographs of the knee in full extension, Micheli and colleagues41 calculated the difference between the distance from the superior pole of the patella to the tibial plateau and the length of the patella. A positive difference signified patella alta. The Koshino method involves the ratio between a reference line from the midpoint of the patella to the midpoint of the proximal tibial physis and a second distance from the midpoint of the distal femoral physis to the midpoint of the proximal tibial physis on lateral knee radiographs.42 Normal values range from 0.99 to 1.20 with the knee in >30° flexion, in children 3 to 18 years of age.
HYPERLAXITY
In contrast to adults, children have increased levels of collagen III compared with collagen I, which is responsible for tissue elasticity.43 Tissue elasticity leads to increased joint mobility, which is more common in children. Joint hypermobility or hyperlaxity has to be differentiated from symptomatic instability. The traditional Beighton score identifies individuals as having joint hypermobility with a score of 5/9 or higher in school-aged children.44-46 Smits-Engelsman and colleagues44 suggested using stricter criteria with scores of 7/9 or higher being indicative of hyperlaxity in school-aged children. A study of 1845 Swedish school children noted that females have a higher degree of joint laxity.45 Maximal laxity was noted in females at 15 years of age.45 Hyperlaxity has been demonstrated to be greater on the left side of the body44 and can be part of generalized syndromes including Down’s syndrome, Marfan’s syndrome, or Ehlers-Danlos syndrome.
LIMB TORSION
Staheli and colleagues47 described the normative values of a lower extremity rotational profile, including femoral anteversion and tibial torsion. Children normally have increased femoral anteversion, which decreases with growth. Miserable malalignment is a term used to denote increased femoral anteversion and increased external tibial torsion.48,49 These rotational abnormalities can increase the Q angle and the lateral forces on the patella. Femoral anteversion or internal rotation of the femur of 30° significantly increases strain in all areas of the MPFL.48 This increased strain may lead to MPFL failure and patellar instability.48 Increased internal rotation of the femur also increases contact pressure on the lateral aspect of the patellofemoral joint.48 Miserable malalignment frequently manifests following a pubertal growth spurt and may require femoral and tibial osteotomy.50
SYNDROMIC ASSOCIATIONS
Several syndromes have patellar instability as a part of their manifestation. The more common syndromes include nail-patella syndrome, Kabuki syndrome, Down’s syndrome, and Rubinstein-Taybi syndrome.51-54 Other syndromes less commonly associated with patellar instability include Turner syndrome, patella aplasia, or absent patella syndrome. Since many patients with syndromic patellar instability are functionally limited, they may not require an aggressive approach to treatment. When treating these patients, it is important to recognize the unique features of a specific syndrome, which may affect the anesthesia risk profile, management decisions, rehabilitation, and prognosis.
Continue to: MPFL TEAR PATTERN
MPFL TEAR PATTERN
The MPFL serves as an important constraint to the patella to prevent lateral dislocation, primarily during the first 20° to 30° of knee flexion.55,56 Injury to the MPFL is noted in over 90% of patients who suffer a patellar dislocation.57 The location of MPFL tears in pediatric patients is variably reported at the patellar attachment (10%-61%), femoral attachment (12%-73%), both (12%-35%) or mid-substance (2.5%-15%).25,57 The most common tear patterns in pediatric patients are tears at the patellar attachment.25,57 This tear pattern may be accompanied by an avulsion fracture of the medial rim of the patella, though this fracture, being extra-articular, seldom needs treatment.
CLASSIFICATION
While several authors have established extensive classification systems of patellar dislocation based on both clinical and radiographic presentation and reviews of the literature, a single classification system has not been recognized as the gold standard. In this section, in addition to presenting our preferred methods of classification, we will review some of the more recent and extensive classification systems for patellar dislocation and patellar instability.
Dejour and colleagues31 initially used both the presence of patellofemoral anatomic abnormalities and pain to define 3 types of patellar instability: major, objective, and potential patellar instability. Major patellar instability indicates that the patient has experienced more than 1 documented dislocation, objective instability involves one dislocation in addition to an associated anatomic abnormality, and potential patellar instability refers to cases in which the patient has radiographic abnormalities and patellar pain.31 Garin and colleagues58 more simplistically divided patellar dislocation patients into 2 groups: major (permanent or habitual) dislocation of the patella and recurrent dislocation. Sillanpaa59 stressed the distinction between first-time dislocation and recurrent dislocation specifically in the context of acute injuries. These classification systems were formulated with adults as the most relevant population; however, classifications targeted specifically to pediatric patients have recently been presented in the literature.
Historically, pediatric patella dislocations were simply categorized as traumatic or congenital.60 In 2014, Chotel and colleagues61 focused on classifying patellar dislocation by extensively reviewing anatomic, biomechanical, pathophysiological, and clinical patterns seen most commonly in children. They included 5 categories: congenital dislocation, permanent dislocation, habitual dislocation during knee flexion, habitual dislocation during knee extension, and recurrent dislocation; however, they did not address traumatic dislocations.61 Congenital dislocation is a rare condition, typically presenting at birth, which produces a pattern of functional genu valgum.62 Permanent dislocation typically presents after the child has started walking, but before the age of 5 years.61 The 2 variations of habitual dislocation typically present between ages 5 and 8 years.61 The final category is the most common and typically occurs during pre-adolescence or adolescence as a result of an atraumatic or trivial traumatic event or sports injury.1 Using more specific terminology, Hiemstra and colleagues63 modeled a classification system based on the traumatic, unilateral, bankart lesion, surgery (TUBS)/atraumatic, multidirectional, bilateral, rehabilitation, inferior shift (AMBRI) for shoulder dislocation classifications. The patellar dislocation system is used to identify 2 distinct subsets of patients in the patellofemoral instability population. One subset is defined by the acronym WARPS (weak, atraumatic, risky anatomy, pain, and subluxation), the other is STAID (strong, traumatic, anatomy normal, instability, and dislocation).64 Patients categorized by the WARPS acronym tend to experience atraumatic onsets of patellofemoral instability and demonstrate anatomic issues that increase this instability. These underlying anatomic issues include valgus alignment, ligamentous laxity, rotational abnormalities, shallow and short TG, and patella alta. On the other hand, STAID patients describe a traumatic dislocation event and do not have underlying anatomic abnormalities that predispose them to instability.64
Taking into account these previous classifications, Frosch and colleagues65 added specific pathologies including “instability,” “maltracking,” and “loss of patellar tracking,” in addition to both clinical and radiographic factors to define 5 types of patellar dislocation and their specific treatment recommendations.65 Type 1 involves simple dislocation with neither maltracking nor instability and a low risk of redislocation.65 Type 2 is defined as primary dislocation followed by subsequent high risk of dislocation and no maltracking.65 Type 3 is divided into 5 subcategories of instability and maltracking issues involving soft tissue contracture, patella alta, pathological tibial tuberosity, and TG distance.65 Type 4 is defined as the highly unstable “floating patella,” and type 5 involves patellar maltracking without instability 65. In terms of treatment, conservative rehabilitation is recommended for type 1 whereas MPFL reconstruction tends to show positive outcomes for both types 2 and 3.66-70
Continue to: Parikh and Lykissas recently published...
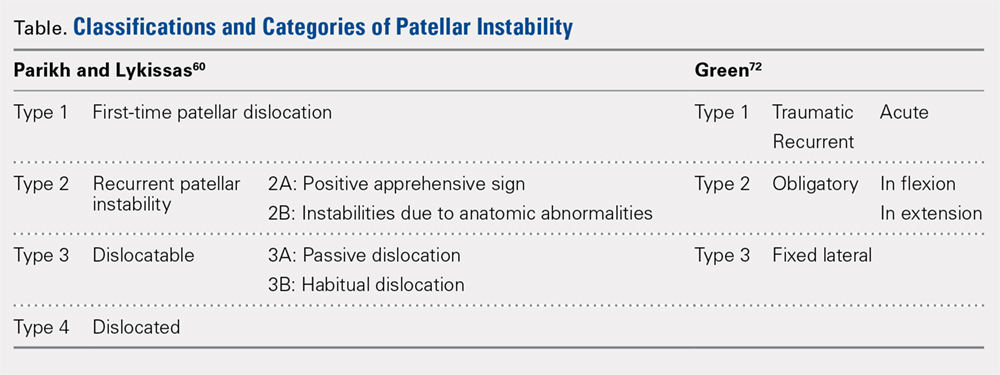
Parikh and Lykissas recently published a comprehensive classification system of 4 defined types of patellar dislocation in addition to voluntary patellar instability and syndromic patellar instability (Table).60 The 4 types are Type 1, first-time patellar dislocation; Type 2, recurrent patellar instability; Type 3, dislocatable; and Type 4, dislocated. Type 2 is further subdivided into Type 2A, which presents with positive apprehension signs, and Type 2B, which involves instabilities related to anatomic abnormalities.60 A distinction is also made between Type 3A or passive patellar dislocation and Type 3B habitual patellar dislocation.60
The classification system proposed by Green and colleagues is more simplified with 3 main categories (Table) of pediatric patellar dislocation: traumatic (acute or recurrent), obligatory (either in flexion or extension), and fixed laterally.71,72 The acute traumatic categorization refers to patients who experienced an initial dislocation event due to trauma whereas recurrent traumatic involves repeated patella dislocations following an initial incident. Studies report that between 60% to 70% of these acute traumatic dislocations occur as a result of a sports-related incident.2,33,73 Obligatory dislocations occur with every episode of either knee flexion or extension, depending on the subtype. Obligatory patella dislocation in flexion typically cannot be manipulated or relocated into the trochlea while the knee is fixed but does reduce into the trochlea in full extension. Fixed lateral dislocations are rare, irreducible dislocations in which the patella stays dislocated laterally in flexion and extension. These dislocations often present with other congenital abnormalities. Each of these categories can be further specified as syndromic if the dislocation is associated with genetic or congenital conditions including skeletal dysplasia, Ehlers-Danlos syndrome, cerebral palsy, Marfan disease, nail-patella syndrome, Down syndrome, Rubenstein-Taybi syndrome, and Kabuki syndrome.51-54,61,74-76
SURGICAL TECHNIQUES IN SKELETALLY IMMATURE PATIENTS
While nonsurgical, conservative treatment involving physical therapy and activity modification is recommended for most patients who experience first-time traumatic patellar dislocations, many patients experience complicating factors that indicate them for surgery. These factors include recurrent dislocation, risk factors for patellofemoral instability, underlying malalignment issues, and congenital deformities. When evaluating these factors, particularly patellofemoral instability, the authors recommend assessing osteochondral lesions, age, skeletal maturity, number of previous dislocations, family history, and anatomic risk factors.2,5,77-79 Extra care should be taken when considering surgical treatment for skeletally immature patients at elevated risk for recurrent instability as the risk of cartilage damage in these cases is high.80-82
Recently, there has been a reported increase in surgical treatment for patellar instability in the skeletally immature.83 This finding may be attributed to heightened awareness of factors that indicate patients for surgical treatment and increased familiarity of surgeons with newer techniques.83 Many surgical techniques have been described to address patellar instability involving both soft-tissue procedures and bony corrections.84 In this article, we discuss the various surgical techniques for MPFL reconstruction, quadricepsplasty, and distal realignment. These procedures can be paired with any number of additional procedures including, but not limited to, lateral retinacular release or lengthening, chondroplasty, TT osteotomy (in skeletally mature patients), and removal of loose bodies.83
There is a need for more comprehensive studies, particularly randomized controlled trials, to evaluate the outcomes for both surgical and nonsurgical treatments for first-time dislocations. In the current literature, only very recently have surgical treatments shown outcomes that are more positive. In 2009, Nietosvaara and colleagues85 conducted a randomized controlled trial of nonoperative and operative treatment of primary acute patellar dislocation in both children and adolescents. After a long-term mean follow-up of 14 years, there was not a significant difference between the groups in recurrent dislocation and instability, subjective outcome, or activity scores.85 In a subsequent review of 5 studies including 339 knees, Hing and colleagues86 also found similar results in both the operative and nonoperative cohorts at risk of recurrent dislocations, Kujala scores, and reoperations. However, a recent systematic review comparing redislocation rates and clinical outcomes between surgical and conservative management of acute patellar dislocation reported more positive outcomes for the surgical cohort.87 This review included 627 knees, 470 of which received conservative management, 157 of which received operative treatment. The conservative cohort was followed for an average of 3.9 years and had a 31% rate of recurrent dislocation while the surgical group was followed for a mean 4.7 years and experienced a 22% redislocation rate.87 This study indicates that operative management for acute first-time dislocations may be the preferred treatment option.
Continue to: A potential reason some of these studies...
A potential reason some of these studies did not show any significant difference between the operative and nonoperative cohort could be that the surgical cohorts included a wide range of procedures including lateral releases and MPFL repairs. Recent publications have indicated that these techniques do not produce overall positive outcomes. While each surgical treatment plan is unique depending on the patient; recently, MPFL reconstruction has been shown to have better outcomes than both nonoperative management and simple medial repair and/or lateral
release.67,88-90
MPFL RECONSTRUCTION
INDICATIONS/OVERVIEW
The MPFL is an important stabilizer for the knee that primarily resists lateral translation of the patella. Damage to the MPFL is very common in acute patellar dislocations with up to 90% of first-time dislocations resulting in injury to the MPFL.91,92 Historically, simple medial and/or lateral MPFL repairs have not been shown to improve patellofemoral kinematics significantly and often result in recurrence.90,93 To address this issue, during the past few decades, numerous MPFL reconstruction techniques have been developed to reconstruct a stronger ligament with the same kinematics as the anatomic MPFL.2,19,69,81,94-106 The ultimate goal of MPFL reconstruction is to reestablish the anatomic “checkrein” to guide the patella into the trochlea between 0° and 30° of knee flexion.107,108 An essential secondary surgical goal in skeletally immature patients is to avoid damaging the distal femoral physis.
There are many variations in both the grafts used to replace the MPFL and the means by which to secure them. The ones discussed below include free semitendinosus or gracilis autografts or grafts constructed from a pedicled adductor, patellar, or quadriceps tendon.69,105,109 While not used as frequently, allografts have also been used.110 Methods to secure these grafts in osseous tunnels include suture anchors or tenodesis screws. Incomplete osseous sockets or medial-sided bone tunnels have also been used as a method to decrease patellar fractures as they preserve the lateral patellar cortex.111-114
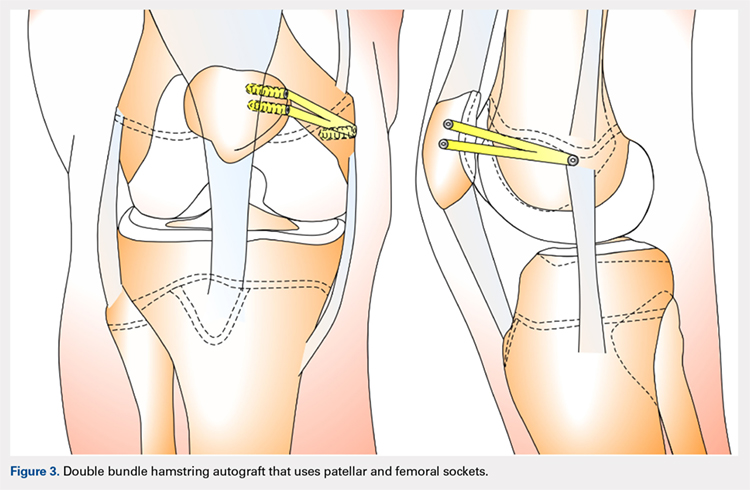
DOUBLE-BUNDLE HAMSTRING AUTOGRAFT
The technique most often used by the author is a double-bundle hamstring autograft harvested from either the semitendinosus or the gracilis secured by short patellar and femoral sockets (Figure 3). After harvesting the hamstring graft from a posteromedial incision, an approximately 90-mm graft is prepared with Krackow stitches to secure 15 mm of the tendon in each socket.115 Lateral radiographs are used intraoperatively to ensure the guidewire for the femoral drill hole falls along the posterior cortex of the diaphysis of the femur while AP radiographs confirm placement distal to the physis. It is important to take both AP and lateral radiographs intraoperatively due to the concave curvature of the distal femoral physis. This unique anatomy can make a point that is located distally to the physis on the AP view appear on or proximal to it on the lateral cross reference view.24,116 For the patellar socket, 2 short sockets are made in the superior half of the patella. Once the sockets have been drilled, the graft is adjusted so that the patella stays seated in the center of the trochlea between 20° and 30° of flexion. This anchoring is accomplished by securing the graft while the knee is kept at 30° of flexion. Proper tension is confirmed by ensuring that the graft does not allow lateral patella movement over one-fourth the width of the patella in extension while crepitation must not appear throughout the ROM.92
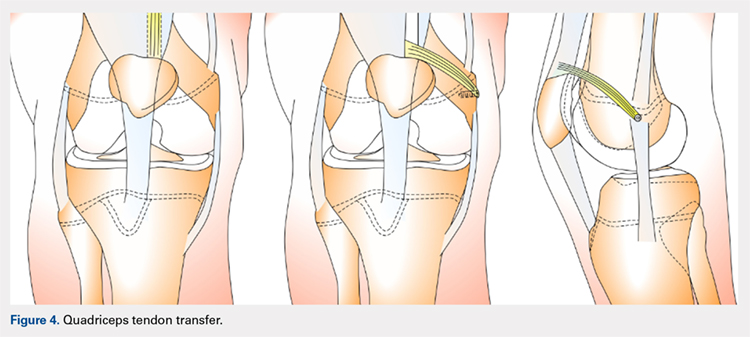
QUADRICPETS TENDON TRANSFER
A combination of techniques by Steensen and colleagues,105 Goyal,109 Noyes and Albright,117 and Pinkowsky and Hennrikus118 describe an MPFL reconstruction in which the proximal end of a small medial portion of the quadriceps tendon is released and then attached to the medial epicondyle through a subcutaneous tunnel (Figure 4). This technique is particularly useful for cases in which the extra strength provided by the bone-quadriceps tendon is necessary to correct more severe dysplasia. Leaving the distal end of the quadriceps tendon intact at its patellar insertion, a graft of about 8 mm x 70 mm thickness is harvested from the tendon. The free distal end of the tendon is then run anatomically through the synovium and retinaculum to be either sutured to the medial intermuscular septum at the medial femoral epicondyle or fixed in femoral tunnel using interference screw.105,109,118 The placement of the femoral fixation point is essential to ensure positive surgical outcomes. If the graft is secured too anteriorly, it may be too loose in extension and too tight in flexion, both of which can lead to postoperative pain, loss of normal kinematics, and overload of the medial patellofemoral cartilage.119-121 Once the ideal placement of the femoral fixation point has been confirmed by intraoperative radiographs, the graft is secured with a small absorbable suture.122,123 While this technique has good clinical results, the longitudinal scar that results from graft harvesting is cosmetically unappealing, and it is technically challenging to harvest a consistent strip of the quadriceps tendon. To address some of these concerns, Fink and colleagues124 described a new harvesting technique that produces more consistent grafts and requires a smaller incision.
Continue to: ADDUCTOR MAGNUS TENDON TRANSFER
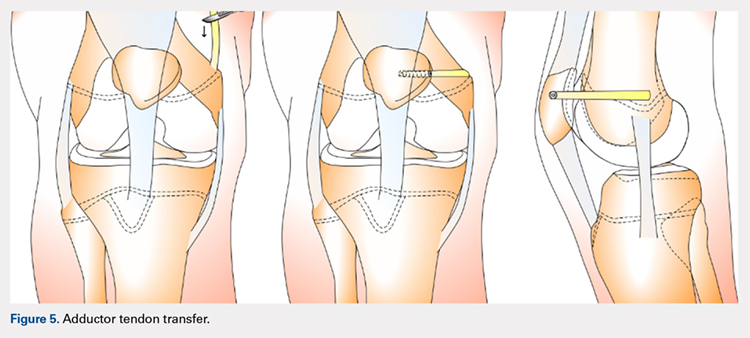
ADDUCTOR MAGNUS TENDON TRANSFER
This technique is a double-bundle MPFL reconstruction that uses a pedicled graft of the distal adductor magnus tendon and suture anchors or incomplete osseous sockets to recreate the MPFL anatomically (Figure 5). Avikainen and colleagues96 and Sillanpää and colleagues125 described this procedure as a progression from the original single-strand adductor magnus transfer technique. First, maintaining the distal insertion, a graft of approximately 14 cm to 18 cm is harvested from the adductor tendon and then passed through a subcutaneous tunnel between the distal vastus medialis obliquus and the superficial joint capsule. The graft is then looped at the medial patella so that the distal bundle runs back to the adductor tubercle.125 With the knee at 30° of flexion to assure proper tension, the graft is secured at both the patella and near the adductor tubercle with suture anchors.125 Hambridge and colleagues126 compared a similar adductor magnus transfer with other pedicled techniques including bone-quadriceps tendon autograft and bone-patellar tendon allograft and found positive results for all 3 methods of reconstruction.
HEMI-PATELLA TENDON TRANSFER
In a similar technique to the adductor tendon transfer, the medial section of the patellar tendon is harvested from the TT and run from its proximal insertion at the medial patella to the medial femoral attachment via a subcutaneous tunnel. The free end of the graft is then secured with suture anchors or incomplete osseous sockets with the knee at 30° of flexion.127
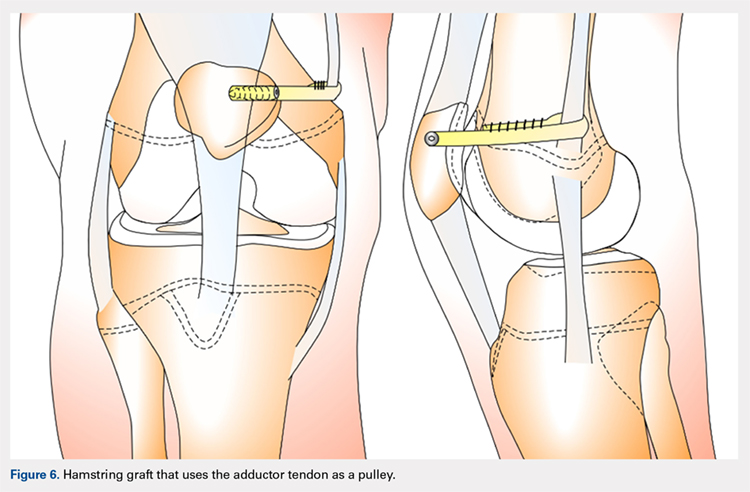
HAMSTRING GRAFT WITH ADDUCTOR TENDON AS A PULLEY
Several techniques opt to use a more dynamic model of MPFL reconstruction in which the adductor tendon or medial collateral ligament (MCL) is used as a pulley for the hamstring graft (Figure 6).128,129 The site of the pulley approximates the normal attachment of the MPFL to the femur and so acts as an effective anatomic replica of the MPFL origin. A semitendinosus graft is harvested and is prepared with continuous sutures, and 2 tunnels to secure the graft are drilled into the patella. The graft is then run subcutaneously from the medial side of the patella to the adductor magnus tubercle into which an osteoperiosteal tunnel is drilled at its distal femoral insertion. The graft is looped through the adductor tunnel and secured with sutures. Proper knee kinematics was ensured by placing the knee at 30° of flexion as the ends of the tendon are secured to the patella.114,130
HAMSTRING GRAFT WITH MCL AS A PULLEY
The MCL can also be used as a pulley rather than the adductor tendon. The semitendinosus graft is harvested and prepared and the patella drilled as it is in the previous technique. The MCL was fashioned into a pulley by making a slit in its posterior one-third. The semitendinosus graft is looped through this slit, and both ends of the graft are held in place with suture anchors on the surface of the patella.129
ADDITIONAL PROCEDURAL COMBINATIONS
Depending on the needs of the individual patient, MPFL reconstruction, and other patellar stabilization techniques can also be combined with additional procedures. Arshi and colleagues83 conducted a review of 6190 adolescents surgically treated for patellar instability and reported the most common additional procedures performed at the time of the stabilization. They found 43.7% of the population underwent lateral retinacular release, which while not effective as an isolated technique to treat patellar instability, has often been used in combination with MPFL reconstruction.131-133 There is currently a lack of consensus regarding the success of adding a lateral release to the reconstruction. Some studies report no difference while others report a decrease in stability after lateral release.90,134-136 While lateral retinacular release has been shown to decrease the force required to displace the patella, it can be surgically indicated in certain patients undergoing MPFL reconstruction.131 The authors advocate that if the lateral retinaculum is tight such that centralized patellar tracking is inhibited following the reconstruction, or if the patella cannot be pushed passively from a laterally tilted position to the neutral horizontal position, lateral retinacular lengthening should be performed to improve kinematics.132
Continue to: Arshi and colleagues...
Arshi and colleagues83 also reported a high rate of cartilage procedures, with chondroplasty performed in 31.1% and chondral fragment/loose body removal in 10.2%. These statistics suggest that a significant level of cartilage damage has occurred by the time of surgery.83
COMPLICATIONS
As MPFL reconstruction techniques have only recently been popularized and developed, there are not many comprehensive studies evaluating the outcomes and complications associated with these procedures. However, in the current literature, there is a general consensus that patients usually experience positive short-term clinical outcomes and relatively low complication rates.68,77 In one of the largest retrospective cohort studies of pediatric patients undergoing MPFL reconstruction, Parikh and colleagues114 reported both the type and rate of complications. They found complications occurred in 16.2% of patients, and the most common complications were recurrent patellar instability, patellar fractures, patellofemoral arthrosis, motion deficits, and stiffness with over half classified as avoidable. Most of these complications were due to technical errors with episodes of recurrent instability only reported in 4.5% of patients.114 In a comprehensive meta-analysis of MPFL reconstruction studies, Shah and colleagues137 reported a complication rate of 26% in both pediatric and adult patients. The cohort was not stratified by age, yet complications were similar to those reported by Parikh and colleagues,114 including pain, loss of knee flexion, wound complications, and patellar fracture.137
As indicated by the frequency of technical complications reported by Parikh and colleagues,114 extra caution should be taken in the operating room to minimize potential errors. In techniques that require drilling of femoral sockets, proper length for and placement of the graft is essential to reestablish proper kinematics. Studies have reported that placing the femoral socket too proximally can result in loss of ROM during flexion and increased compressive forces across the patella.138 A graft that is too short can have similar negative outcomes, and a graft that is too long can result in recurrent instability. Positioning the graft while the knee is in 30° of flexion can help ensure the proper length and tension is achieved. Once the graft is in place, it is important to ensure the ROM and isometry before completing the fixation.72 It is also essential to be vigilant about potential violation of the physes and subsequent growth disturbances. To establish the safest angles for drilling the distal femoral epiphysis for graft placement, Nguyen and colleagues139 conducted a study using high-resolution 3-dimensional images of cadaveric distal femoral epiphyses. By recording which tunnels disrupted the physis before reaching 20 mm of depth, the authors concluded that it is safest to drill distally and anteriorly at an angle between 15° and 20°.139 This technique should minimize damage to the physis, notch, and distal femoral cartilage and decrease potential complications.139
OUTCOMES
In general, the literature reports positive outcomes for MPFL reconstruction—in both studies that address a specific technique and all-encompassing studies. Outcomes are typically reported as Kujala and Tegner scores, results from clinical examinations, and rates of subsequence recurrences. Several recent studies have also evaluated the ability of MPFL reconstruction to restore proper kinematics. Edmonds and colleagues140 evaluated the difference in patellofemoral joint reaction forces and load experienced by 3 groups of adolescents: a cohort treated with MPFL reconstruction, a cohort treated with soft-tissue realignment of the extensor mechanism (the Insall method), and controls. While both surgical techniques were able to restore medial constraints to the patella, the study showed that only the MPFL reconstruction cohort experienced joint reaction forces that were analogous to the control group. In comparison, the cohort that was treated with soft-tissue realignment alone experienced higher patellofemoral joint reaction forces and did not regain normal joint mechanics.140 These results can be used to advocate for the further use of MPFL reconstruction as an effective anatomic replacement of the native ligament. Radiographic studies have similarly reported MPFL reconstruction as an effective means to restore anatomic normality. Fabricant and colleagues141 conducted a radiographic study in which patella alta was corrected to normal childhood ranges in patients who underwent MPFL reconstruction technique using a hamstring autograft. Lykissas and colleagues142 corroborated these results with another radiographic study that reported small but significant decreases in the Blackburne-Peel index and CD index following MPFL reconstruction in 25 adolescents. As correction of patella alta allows the patella to rest in a deeper, more secure position in the TG, these results indicate that effective early MPFL reconstruction can correct for patellar anatomic abnormalities that could be future risk factors.143,144 Several studies have also reported outcomes addressing specific MPFL techniques; these are reported and discussed in this article.
OUTCOMES BY TECHNIQUE
HAMSTRING AUTOGRAFT
Reports on outcomes following MPFL reconstructions using hamstring autografts have been particularly promising. A cohort of 21 skeletally immature patients who underwent MPFL reconstruction was evaluated pre- and postoperatively with an average of a 2.8-year follow-up. The authors of the study reported no redislocation events and significant improvement in the Kujala scores, and patients were able to return to athletic activities safely.145 Previous studies report similar positive increases in Kujala scores, subjective patient reports, and lack of subsequent redislocation for patients who underwent either semitendinosus or gracilis autograft MPFL reconstructions. One such study further documented an average patellar inclination angle decrease from 34.3° to 18.6° following MPFL reconstruction.146 However, while the literature typically reports positive Kujala scores and subjective outcomes for the hamstring autograft procedure, a study arthroscopically evaluating patellar tracking immediately following surgery and then at 6 to 26 months follow-up found that patellar tracking correction was not maintained for all patients who underwent this type of MPFL reconstruction.147
Continue to: QUADRICEPS TENDON TRANSFER OUTCOMES
QUADRICEPS TENDON TRANSFER OUTCOMES
Studies specifically evaluating the quadriceps tendon transfer technique for MPFL reconstruction in children are sparse, but authors have reported positive clinical outcomes and low complication rates in adults. After following 32 young adults who underwent this MPFL reconstruction technique for 3 years, Goyal109 reported a significant increase in mean Kujala scores from 49.31 to 91.25 and no complications or redislocation. He argues this type of quadriceps graft has a high success rate because it is anatomically more similar to the MPFL than other grafts and does not require additional patellar fixation.101,109 Similar positive Kujala scores and minimal complications have been reported in adult patient populations.148 Abouelsoud and colleagues149 conducted one of the few studies in skeletally immature patients and reported similarly positive results with no redislocations and significantly improved Kujala scores at a mean follow-up of 29.25 months in their 16-patient cohorts.
ADDUCTOR MAGNUS TENDON TRANSFER
After initially describing this technique in 14 adult patients, Avikainen and colleagues96 followed this cohort and reported positive subjective results and only 1 redislocation. In a more recent study in which the adductor tendon transfer technique was compared with the quadriceps tendon transfer described above and the bone-patellar tendon allograft, Steiner and colleagues69 reported similarly significant improvement in all cohorts in Lysholm, Kujala, and Tegner scores with no redislocations. Additionally, Malecki and colleagues150 followed a cohort of 33 children with 39 knees diagnosed with recurrent patellar dislocation, who underwent MPFL reconstruction using the adductor magnus tendon. After evaluating this cohort functionally and radiographically, the authors reported improvements in Lysholm and Kujala scores, patellar tilt and congruence angles, and peak torque of the quadriceps muscle and flexor.150 However, this cohort did report postoperative redislocations in 36.4% of patients (4 of 11).150
HEMI-PATELLA TENDON TRANSFER
In 2012, in the first randomized controlled trial, Bitar and colleagues67 compared the outcomes of patients who underwent MPFL reconstruction via the hemi-patellar tendon technique with those who were managed nonoperatively with immobilization and physiotherapy after first-time patellar dislocation. At 2-year follow-up, the surgical cohort presented positive results with a significantly higher mean Kujala score (88.9 to 70.8) and no redislocations or subluxations. In contrast, 35% of nonoperative cases presented with recurrences and subluxations over the 2-year period.67
MCL OR ADDUCTOR TENDON AS A PULLEY
Studies have reported good postoperative results and low complication rates for these dynamic techniques.128,129 In terms of kinematics, while hypermobility and patellar height were not fully corrected, improvements in patellar tilt and lateral shift were reported in a cohort of 6 patients with a minimum 4-year follow-up.129 To further evaluate whether the more dynamic pulley reconstruction technique resulted in better outcomes, Gomes and colleagues128 compared the subjective reports, clinical evaluations, and complication rates of patients who underwent MPFL reconstruction with a rigid adductor magnus fixation vs a semitendinosus tendon dynamic femoral fixation. One case in the rigid cohort experienced a subsequent subluxation, while patients in the semitendinosus group had better subjective reports and a higher rate of return to sport.128 More recently, Kumahashi and colleagues151 specifically studied the outcomes of the MCL tendon as a pulley in 5 patients aged 14 to 15 years. They reported similar successful results as no patients experienced recurrence, and all patients exhibited improvement in radiographic measures of patellar tilt and congruence angle, lateral shift ratio, and both Kujala and Lysholm scores.151
While there has yet to be a randomized controlled trial comparing all of these different techniques, there is a general consensus in the literature that patients tend to perform better following MPFL reconstruction vs MPFL repair.
OTHER STABILIZATION PROCEDURES, INCLUDING DISTAL REALIGNMENT
Patients with additional underlying deficits and malalignment issues such as significant trochlear dysplasia, increased TT-TG distance, patella alta, increased Q angle, and/or positive J sign may require stabilization procedures beyond MPFL reconstruction.152,153 TT osteotomies are often used to correct alignment issues in the adult patient population; however, these procedures are typically contraindicated in skeletally immature patients. Alternative realignment procedures for the pediatric population include both proximal and distal realignment, with proximal realignment performed primarily in children under the age of 12 years.153 Many variations on these procedures exist, some of which are no longer regularly performed due to poor reported outcomes. In this article, we discuss several of the techniques, focusing primarily on those that have demonstrated higher success rates.
Continue to: GALEAZZI TECHNIQUE
GALEAZZI TECHNIQUE
One of the first and most famous soft-tissue techniques to address patellar instability was the semitendinosus tenodesis, published by Galeazzi154 in 1922 (Figure 7). This technique stabilizes the patella without altering the TT. In the original technique, a portion of the semitendinosus tendon is harvested with its tibial insertion left intact. The free end of the tendon is then secured with sutures at the periosteal groove of the medial patella.154,155 Fiume156 modified this technique by adding a lateral release and medial retinacular reefing. The most recent addition to this procedure was introduced by Baker and colleagues,157 in which a tunnel is drilled from the medial to the lateral border of the patella. Tension placed on the grafted tendon is used to reposition the patella medially and draw it downward. Preliminary literature on this modified procedure reported fair clinical results with success rates of approximately 75%.155,158-160 A recent study evaluating both the clinical and radiographic outcomes of this technique also indicated that while clinical results were excellent in 62.5% of patients, this technique alone was unsuccessful in fully addressing patellar instability in patients with underlying anatomic abnormalities such as patellar alta.161 In light of these less than ideal reports, the authors no longer recommend this technique for patellofemoral instability cases.
ROUX-GOLDTHWAIT PROCEDURE
The Roux-Goldthwait procedure, first described by both Roux162 and Goldthwait163 in 1888 and 1895 respectively, was later modified in 1985 to involve a lateral release, plication of the medial retinaculum, medial transfer of the lateral patellar tendon without advancement, and advancement of the vastus medialis (Figure 8).164 More recently, Marsh and colleagues152 introduced an addition to aligning the extensor mechanism with the femoral shaft better. In this technique modification, the patellar tendon is split longitudinally, and its lateral half is detached and transferred distally beneath its medial half. The free end is then sutured to the periosteum on the medial side of the tibia.152 With a mean long-term follow-up of 6.2 years, Marsh and colleagues152 reported excellent results in 65%, good in 11%, and fair in 3% of the knees operated on with this modified technique. Of the patients in this cohort whose strength was evaluated, 80% had their strength returned to 90% of preoperative levels in the operated leg.152 While this study and others report improved outcomes, an increasing body of literature has found high rates of recurrence, patella infera, and other complications following the modified Roux-Goldthwait procedure.36,165-171 Also, a study comparing MPFL reconstruction using adductus magnus transfer with the Roux-Goldthwait procedure reported that patients in the MPFL cohort reported less pain postoperatively.150 In addition, whereas the Kujala and Lysholm scores, recurrence rates, patellofemoral angles, and apprehension test results did not demonstrate significant differences between these 2 groups, the MPFL group had significantly fewer abnormal congruence angles, better patellar medialization, and higher peak torque of the hamstring.150
COMBINED MPFL AND MEDIAL PATELLOTIBIAL LIGAMENT RECONSTRUCTION
While the medial patellotibial ligament (MPTL) has not received much attention with regard to patellar stability, recent studies have indicated its role during higher degrees of both flexion and extension.172 The MPTL acts as a secondary restrictor ligament which helps release stress on the MPFL by decreasing the Q angle and further normalizing patellar kinematics.173 Patients who present with hyperlaxity or knee hyperextension combined with extension subluxation and flexion instability could be indicated for this additional stabilizing procedure. Both Nietosvaara and colleagues85 and Brown and Ahmad174 have described a dual MPTL and MPFL reconstruction technique using a semitendinosus hamstring graft. More recently Hinckel and colleagues172 described a combined MPFL and MPTL reconstruction, using a graft from the quadriceps tendon to reconstruct the MPFL and one from the patellar tendon to reconstruct the MPTL. In this technique, once the respective grafts have been harvested, a femoral insertion for the graft recreating the MPFL is fluoroscopically established so that an anchor can be inserted distal to the femoral physeal growth plate. For the MPTL insertion, attachment to the tibia below the joint line and 2 cm medial to the patellar tendon is established fluoroscopically just above the physeal growth plate on the proximal epiphysis.19,175 The MPTL graft is sutured first with the knee at 90° of flexion to establish tension similar to that of the patellar tendon.176 Then, the knee is placed in 30° of flexion to fix the MPFL graft to the medial patella to prevent excessive lateral translation of the patella.
PATELLAR TENDON TRANSFER
Patellar tendon transfer with proximal realignment is a technique used in particularly young patients to address cases of patellofemoral instability involving concomitant bony or anatomic abnormalities. This procedure is effective for young children with substantial amounts of remaining growth as it better mimics native anatomy than other realignment procedures and does not require bony remodeling.152-154 It is important to familiarize with surgical techniques to address malalignment issues in young patients as neglected alignment issues can lead to worsening of trochlear dysplasia and instability, which are very difficult to treat later on when patients are older.153
The patellar tendon transfer technique (Figure 9), as described by Gordon and Schoenecker,177 starts with an extensive lateral retinacular release. The patellar tendon is then released from its distal insertion at the TT so that it can be moved medially without moving it inferiorly. After confirming patellar tracking and alignment by flexing the knee from 0° to 90° with the graft in place, the patellar tendon graft is secured with multiple nonabsorbable horizontal sutures.177 Of note, in skeletally mature patients, a TT osteotomy is used to accomplish the same goal. This osteotomy has been shown to improve both patellar height and TT-TG distance in skeletally mature patients, but is contraindicated in skeletally immature patients.92,178
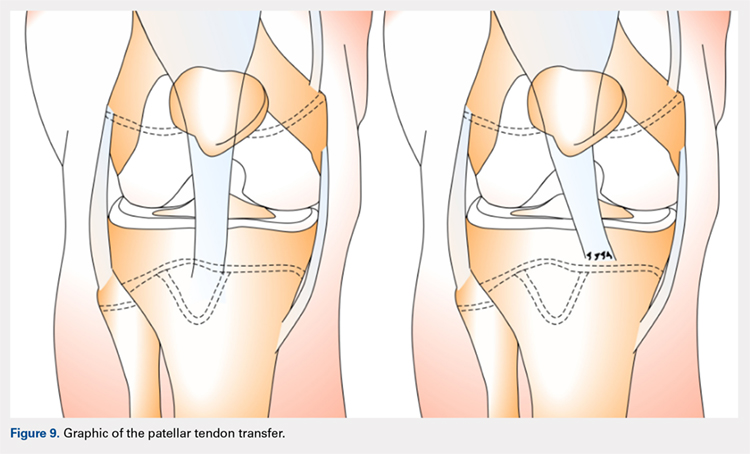
Continue to: Initial studies conducted on patellar tendon...
Initial studies conducted on patellar tendon transfer have positive outcomes.179 At a mean follow-up of 5.1 years, patients reported a decrease in pain and increased the ROM and activity, and only 1 reported a postoperative redislocation.179 In more recent studies, both Benoit and colleagues36 and Garin and colleagues58 reviewed cases of patellar instability treated with patellar tendon transfer to address concomitant patellar alignment and anatomic abnormalities. They reported good functional, clinical, and radiographic outcomes with 12.5% and 16% recurrence rates, respectively.36,58 They also noted radiographic improvements in femoral sulcus angle, particularly in younger patients, which indicate this procedure is effective in addressing bony abnormalities that can result from neglected malalignment issues.36,58,154
QUADRICEPSPLASTY
Quadricepsplasty is a lengthening and remodeling technique not frequently used in the pediatric population. The goal of this procedure in patients with significant amounts of growth remaining is to reposition the patella to ameliorate trochlear remodeling and prevent worsening symptoms and anatomic abnormalities.36 A quadricepsplasty accomplishes this by de-rotating and/or lengthening the extensor mechanism and may or may not involve a concomitant MPFL reconstruction. This procedure is particularly effective in young patients who experience obligatory dislocation.60,72 Several quadricepsplasty techniques have been described including Thompson, Curtis and Fisher, Judet, Stanisavljevic, and V-Y technique.180-186 Most techniques initially involve sharp dissection of the vastus medialis and lateralis from the rectus femoral tendon. A tongue is then fashioned out of the rectus femoral tendon. Once the vastus medialis and lateralis are detached from the margins of the patella, the knee is extended, and the distal ends of the vasti are sutured to the tongue of the rectus tendon. Effective extension facilitates flexion to 90°.184 The authors recommend a modification of this technique in which a Z lengthening of the quadriceps tendon is performed after the vastus lateralis is removed distally from the patella and the quadriceps tendon.
Several series and case reports evaluating quadricepsplasty in adult patients report positive outcomes with most patients achieving good or excellent flexion with minimal complications.183,185,187-189 Reports on quadricepsplasty used to treat conditions other than patellofemoral instability in children have reported similar positive outcomes.190-192 As quadricepsplasty for patellar instability is relatively rare in pediatric patients, there is not much relevant literature. However, Kocon and colleagues193 reported results of quadricepsplasty and quadricepsplasty combined with the modified Galeazzi procedure in 8 children (10 knees) with a mean follow-up of 3.25 years. Seventy percent of cases resulted in stabilization and correction of patellar position, and only 2 postoperative redislocations were noted.193 Additionally, in a study evaluating 6 patients suffering from patellar instability, 2 of whom were obligate dislocators, quadricepsplasty resulted in patellar stability, satisfaction, and near normal gait patterns.194
Figure 10 shows the surgical algorithm used for patellar instability characteristics.
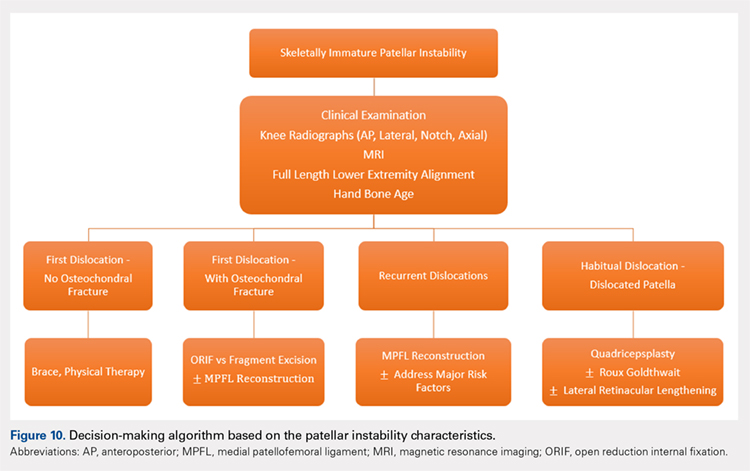
CONCLUSION
Patellofemoral joint stability relies on a complex interplay of musculotendinous units, ligaments and the osteocartilaginous morphology of the patellofemoral joint. Patellar instability in pediatric patients is different from adults. Having an in-depth understanding of the remodeling potential, the insertion sites for the MPFL and its relationship to the physis are of utmost importance when planning surgery. Reducing and maintaining the patella within the patellofemoral joint early enough can allow for remodeling of the patella and/or the trochlea to provide for lasting stability. Appropriate surgical principles, such as tensioning, can help both prevent continued pain and minimize future complications.
1. Nietosvaara Y, Aalto K, Kallio PE. Acute patellar dislocation in children: incidence and associated osteochondral fractures. J Pediatr Orthop. 1994;14(4):513-515.
2. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121. doi:10.1177/0363546503260788.
3. Abbasi D, May MM, Wall EJ, Chan G, Parikh SN. MRI findings in adolescent patients with acute traumatic knee hemarthrosis. J Pediatr Orthop. 2012;32(8):760-764. doi:10.1097/BPO.0b013e3182648d45.
4. Mäenpää H, Lehto MU. Patellar dislocation. The long-term results of nonoperative management in 100 patients. Am J Sports Med. 1997;25(2):213-217. doi:10.1177/036354659702500213.
5. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120. doi:10.1177/036354658601400204.
6. Cofield RH, Bryan RS. Acute dislocation of the patella: results of conservative treatment. J Trauma. 1977;17(7):526-531.
7. Wasserlauf BL, Paletta GA. Developmental anatomy of the pediatric and adolescent knee. In: Micheli LJ, Kocher MS, eds. The Pediatric and Adolescent Knee. 1st ed. Elsevier; 2006:27-32.
8. Birch JG. Growth and development. In: Herring J, ed. Tachdjian’s Pediatric Orthopedics. 4th ed. Saunders/Elsevier; 2007:3-22.
9. Sarin VK, Carter DR. Mechanobiology and joint conformity regulate endochondral ossification of sesamoids. J Orthop Res. 2000;18(5):706-712. doi:10.1002/jor.1100180505.
10. Maes C, Kronenberg HM. Postnatal bone growth: growth plate biology, bone formation, and remodeling. In: Pediatric Bone. Elsevier; 2012:55-82.
11. Tecklenburg K, Dejour D, Hoser C, Fink C. Bony and cartilaginous anatomy of the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):235-240. doi:10.1007/s00167-005-0683-0.
12. Walmsley R. The development of the patella. J Anat. 1940;74(Pt 3):360-368.3.
13. Fulkerson J, Hungerford D. Disorders of the patellofemoral Joint. In: Normal Anatomy. 2nd ed. Baltimore: Williams & Wilkins; 1990:1-24.
14. Ogden JA. Radiology of postnatal skeletal development. X. Patella and tibial tuberosity. Skeletal Radiol. 1984;11(4):246-257.
15. Nietosvaara Y, Aalto K. The cartilaginous femoral sulcus in children with patellar dislocation: an ultrasonographic study. J Pediatr Orthop. 1997;17(1):50-53.
16. Glard Y, Jouve JL, Garron E, Adalian P, Tardieu C, Bollini G. Anatomic study of femoral patellar groove in fetus. J Pediatr Orthop. 2005;25(3):305-308.
17. Nietosvaara Y. The femoral sulcus in children. An ultrasonographic study. J Bone Joint Surg Br. 1994;76(5):807-809.
18. Nietosvaara AY, Aalto KA. Ultrasonographic evaluation of patellar tracking in children. Clin Orthop Relat Res. 1993;(297):62-64.
19. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 26(1):59-65. doi:10.1177/03635465980260012701.
20. Shea KG, Styhl AC, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: a cadaveric study. Am J Sports Med. 2016;44(11):2833-2837. doi:10.1177/0363546516656366.
21. Shea KG, Polousky JD, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children. J Pediatr Orthop. 2014;34(8):808-813. doi:10.1097/BPO.0000000000000165.
22. Shea KG, Polousky JD, Jacobs JC, et al. The patellar insertion of the medial patellofemoral ligament in children. J Pediatr Orthop. 2015;35(4):e31-e35. doi:10.1097/BPO.0000000000000399.
23. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi:10.1016/j.arthro.2009.12.020.
24. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sport Traumatol Arthrosc. 2011;19(12):2067-2071. doi:10.1007/s00167-011-1548-3.
25. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi:10.1177/0363546510397174.
26. Seitlinger G, Moroder P, Fink C, Wierer G. Acquired femoral flexion deformity due to physeal injury during medial patellofemoral ligament reconstruction. Knee. 2017;24(3):680-685. doi:10.1016/j.knee.2017.02.003.
27. Parikh SN, Rajdev N. Relationship between trochlear dysplasia and the anterior distal femoral physis in pediatric and adolescent patients with patellar instability. In: 36th Annual Meething AANA. Denver; 2017.
28. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57(2):259-261.
29. Parikh SN. Medial patellofemoral ligament reconstruction and simultaneous guided correction of genu valgum for patellar instability in skeletally immature patients. In: EPOSNA. 2017.
30. Brattstroem H. Shape of the intercondylar groove normally and in recurrent dislocation of patella. A clinical and X-ray-Anatomical investigation. Acta Orthop Scand Suppl. 1964;68(suppl 68):1-148.
31. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
32. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi:10.1177/0363546512472873.
33. Jaquith BP, Parikh SN. Predictors of recurrent patellar instability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi:10.1097/BPO.0000000000000674.
34. Lippacher S, Reichel H, Nelitz M. Radiological criteria for trochlear dysplasia in children and adolescents. J Pediatr Orthop B. 2011;20(5):341-344. doi:10.1097/BPB.0b013e3283474c8b.
35. Parikh SN, Rajdev N, Sun Q. The growth of trochlear dysplasia during adolescence. J Pediatr Orthop. 2018. doi:10.1097/BPO.0000000000001168. [Epub ahead of print]
36. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177. doi:10.1302/0301-620X.89B9.19065.
37. Sugimoto D, Christino MA, Micheli LJ. Effects of surgical intervention on trochlear remodeling in pediatric patients with recurrent patella dislocation cases. J Pediatr Orthop B. 2016;25(4):349-353. doi:10.1097/BPB.0000000000000341.
38. Insall J, Goldberg V, Salvati E. Recurrent dislocation and the high-riding patella. Clin Orthop Relat Res. 1972;88:67-69.
39. Caton J. [Method of measuring the height of the patella]. Acta Orthop Belg. 1989;55(3):385-386.
40. Thévenin-Lemoine C, Ferrand M, Courvoisier A, Damsin JP, Ducou le Pointe H, Vialle R. Is the Caton-Deschamps index a valuable ratio to investigate patellar height in children? J Bone Joint Surg Am. 2011;93(8):e35. doi:10.2106/JBJS.J.00759.
41. Micheli LJ, Slater JA, Woods E, Gerbino PG. Patella alta and the adolescent growth spurt. Clin Orthop Relat Res. 1986;213:159-162.
42. Koshino T, Sugimoto K. New measurement of patellar height in the knees of children using the epiphyseal line midpoint. J Pediatr Orthop. 1989;9(2):216-218.
43. Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45(3):203-212.
44. Smits-Engelsman B, Klerks M, Kirby A. Beighton score: a valid measure for generalized hypermobility in children. J Pediatr. 2011;158(1):119-123. doi:10.1016/j.jpeds.2010.07.021.
45. Jansson A, Saartok T, Werner S, Renström P. General joint laxity in 1845 Swedish school children of different ages: age- and gender-specific distributions. Acta Paediatr. 2004;93(9):1202-1206.
46. Beighton P, Solomon L, Soskolnet CL. Articular mobility in an African population. Ann rheum Dis. 1973;32(5):413-418.
47. Staheli LT, Corbett M, Wyss C, King H. Lower-extremity rotational problems in children. Normal values to guide management. J Bone Joint Surg Am. 1985;67(1):39-47.
48. Kijowski R, Plagens D, Shaeh S, Teitge R. The effects of rotational deformities of the femur on contact pressure and contact area in the patellofemoral joint and on strain in the medial patellofemoral ligament. Annu Meet Int Patellofemoral Study Group, Napa Val San Fr CA. 1999.
49. Post W, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
50. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
51. Kurosawa K, Kawame H, Ochiai Y, Nakashima M, Tohma T, Ohashi H. Patellar dislocation in Kabuki syndrome. Am J Med Genet. 2002;108(2):160-163.
52. Mik G, Gholve PA, Scher DM, Widmann RF, Green DW. Down syndrome: orthopedic issues. Curr Opin Pediatr. 2008;20(1):30-36. doi:10.1097/MOP.0b013e3282f35f19.
53. Sweeney E, Fryer A, Mountford R, Green A, McIntosh I. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40(3):153-162.
54. Stevens CA. Patellar dislocation in Rubenstein-Taybi syndrome. Am J Med Genet. 1997;72(2):188-190.
55. Andrish J. The biomechanics of patellofemoral stability. J Knee Surg. 2004;17(1):35-39.
56. Conlan T, Garth WP, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682-693.
57. Balcarek P, Ammon J, Frosch S, et al. Magnetic resonance imaging characteristics of the medial patellofemoral ligament lesion in acute lateral patellar dislocations considering trochlear dysplasia, patella alta, and tibial tuberosity-TG distance. Arthroscopy. 2010;26(7):926-935. doi:10.1016/j.arthro.2009.11.004.
58. Garin C, Chaker M, Dohin B, Kohler R. Permanent, habitual dislocation and recurrent dislocation of the patella in children: surgical management by patellar ligamentous transfer in 50 knees. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(7):690-700.
59. Sillanpaa P. Terminology of patellar dislocation. In: Sillanpaa P, ed. Trauma. Saarbrucken, Germany: Lambert Academic Publishing; 2010:16-18.
60. Parikh SN, Lykissas MG. Classification of lateral patellar instability in children and adolescents. Orthop Clin North Am. 2016;47(1):145-152. doi:10.1016/j.ocl.2015.08.016.
61. Chotel F, Bérard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(suppl 1):125-137. doi:10.1016/j.otsr.2013.06.014.
62. Wada A, Fujii T, Takamura K, Yanagida H, Surijamorn P. Congenital dislocation of the patella. J Child Orthop. 2008;2(2):119-123. doi:10.1007/s11832-008-0090-4.
63. Südkamp NP, Granrath M, Hoffmann R, Haas NP. Instability of the shoulder joint in the athlete. Chirurg. 1994;65(11):901-909.
64. Hiemstra LA, Kerslake S, Lafave M, Heard SM, Buchko GML. Introduction of a classification system for patients with patellofemoral instability (WARPS and STAID). Knee Surg Sport Traumatol Arthrosc. 2014;22(11):2776-2782. doi:10.1007/s00167-013-2477-0.
65. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497. doi:10.1007/s00402-015-2381-9.
66. Balcarek P, Oberthür S, Hopfensitz S, et al. Which patellae are likely to redislocate? Knee Surg Sport Traumatol Arthrosc. 2014;22(10):2308-2314. doi:10.1007/s00167-013-2650-5.
67. Bitar AC, Demange MK, D’Elia CO, Camanho GL. Traumatic patellar dislocation: nonoperative treatment compared with mpfl reconstruction using patellar tendon. Am J Sports Med. 2012;40(1):114-122. doi:10.1177/0363546511423742.
68. Smith TO, Walker J, Russell N. Outcomes of medial patellofemoral ligament reconstruction for patellar instability: a systematic review. Knee Surg Sport Traumatol Arthrosc. 2007;15(11):1301-1314. doi:10.1007/s00167-007-0390-0.
69. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261. doi:10.1177/0363546505285584.
70. Ma LF, Wang F, Chen BC, Wang CH, Zhou JW, Wang HY. Medial retinaculum plasty versus medial patellofemoral ligament reconstruction for recurrent patellar instability in adults: a randomized controlled trial. Arthrosc J Arthrosc Relat Surg. 2013;29(5):891-897. doi:10.1016/j.arthro.2013.01.030.
71. Weeks KD, Fabricant PD, Ladenhauf HN, Green DW. Surgical options for patellar stabilization in the skeletally immature patient. Sports Med Arthrosc Rev. 2012;20(3):194-202.
72. Green D. Surgical treatment of pediatric patella instability. Die Ther der Instabilen patella. 2016:80-89.
73. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309. doi:10.1055/s-0034-1398373.
74. Ghanem I, Wattincourt L, Seringe R. Congenital dislocation of the patella. Part I: pathologic anatomy. J Pediatr Orthop. 2000;20(6):812-816.
75. Bongers E, Van Kampen A, Van Bokhoven H, Knoers N. Human syndromes with congenital patellar anomalies and the underlying gene defects. Clin Genet. 2005;68(4):302-319. doi:10.1111/j.1399-0004.2005.00508.x.
76. Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51(3):444-453.
77. Gausden EB, Fabricant PD, Taylor SA, et al. Medial patellofemoral reconstruction in children and adolescents. JBJS Rev. 2015;3(10):1-11. doi:10.2106/JBJS.RVW.N.00091.
78. Palmu S, Kallio PE, Donell ST, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90(3):463-470. doi:10.2106/JBJS.G.00072.
79. Webb JE, Lewallen LW, Christophersen C, Krych AJ, McIntosh AL. Clinical outcome of internal fixation of unstable juvenile osteochondritis dissecans lesions of the knee. Orthopedics. 2013;36(11):e1444-e1449. doi:10.3928/01477447-20131021-30.
80. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
81. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):e47. doi:10.1053/jars.2003.50167.
82. Nomura E, Inoue M, Kobayashi S. Long-term follow-up and knee osteoarthritis change after medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2007;35(11):1851-1858. doi:10.1177/0363546507306161.
83. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States. Orthop J Sport Med. 2016;4(8):2325967116662873. doi:10.1177/2325967116662873.
84. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc. 2007;15(2):61-67. doi:10.1097/JSA.0b013e3180479464.
85. Nietosvaara Y, Paukku R, Palmu S, Donell ST. Acute patellar dislocation in children and adolescents. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2):139-145. doi:10.2106/JBJS.H.01289.
86. Hing CBC, Smith TO, Donell S, Song F. Surgical versus non-surgical interventions for treating patellar dislocation. In Hing CB, ed. Cochrane Database Syst Rev. 2011;(11):CD008106. doi:10.1002/14651858.CD008106.pub2.
87. Nwachukwu BU, So C, Schairer WW, Green DW, Dodwell ER. Surgical versus conservative management of acute patellar dislocation in children and adolescents: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2016;24(3):760-767. doi:10.1007/s00167-015-3948-2.
88. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi:10.1177/0363546510376230.
89. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. Operative treatment of primary patellar dislocation does not improve medium-term outcome: a 7-year follow-up report and risk analysis of 127 randomized patients. Acta Orthop. 2005;76(5):699-704. doi:10.1080/17453670510041790.
90. Ostermeier S, Holst M, Hurschler C, Windhagen H, Stukenborg-Colsman C. Dynamic measurement of patellofemoral kinematics and contact pressure after lateral retinacular release: an in vitro study. Knee Surg Sport Traumatol Arthrosc. 2007;15(5):547-554. doi:10.1007/s00167-006-0261-0.
91. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60. doi: 10.1177/036354659602400110.
92. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
93. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914. doi:10.1007/s00167-011-1516-y.
94. Ellera Gomes JL. Medial patellofemoral ligament reconstruction for recurrent dislocation of the patella: a preliminary report. Arthroscopy. 1992;8(3):335-340.
95. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
96. Avikainen VJ, Nikku RK, Seppänen-Lehmonen TK. Adductor magnus tenodesis for patellar dislocation. Technique and preliminary results. Clin Orthop Relat Res. 1993;(297):12-16.
97. Davis DK, Fithian DC. Techniques of medial retinacular repair and reconstruction. Clin Orthop Relat Res. 2002;(402):38-52.
98. Dhillon MS, Mohan P, Nagi ON. Does harvesting the medial third of the patellar tendon cause lateral shift of the patella after ACL reconstruction? Acta Orthop Belg. 2003;69(4):334-340.
99. Drez D, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthrosc J Arthrosc Relat Surg. 2001;17(3):298-306. doi:10.1053/jars.2001.21490.
100. Ellera Gomes JL, Stigler Marczyk LR, César de César P, Jungblut CF. Medial patellofemoral ligament reconstruction with semitendinosus autograft for chronic patellar instability: a follow-up study. Arthrosc J Arthrosc Relat Surg. 2004;20(2):147-151. doi:10.1016/j.arthro.2003.11.006.
101. Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. Arthrosc J Arthrosc Relat Surg. 2007;23(5):542-553. doi:10.1016/j.arthro.2007.03.006.
102. Nomura E, Horiuchi Y, Kihara M. Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee. 2000;7(2):121-127.
103. Ostermeier S, Stukenborg-Colsman C, Hurschler C, Wirth CJ. In vitro investigation of the effect of medial patellofemoral ligament reconstruction and medial tibial tuberosity transfer on lateral patellar stability. Arthrosc J Arthrosc Relat Surg. 2006;22(3):308-319. doi:10.1016/j.arthro.2005.09.024.
104. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
105. Steensen RN, Dopirak RM, Maurus PB. A simple technique for reconstruction of the medial patellofemoral ligament using a quadriceps tendon graft. Arthroscopy. 2005;21(3):365-370. doi:10.1016/j.arthro.2004.10.007.
106. Steensen RN, Dopirak RM, McDonald WG. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513. doi:10.1177/0363546503261505.
107. Farahmand F, Tahmasbi MN, Amis AA. Lateral force–displacement behaviour of the human patella and its variation with knee flexion—a biomechanical study in vitro. J Biomech. 1998;31(12):1147-1152.
108. Heegaard J, Leyvraz PF, Van Kampen A, Rakotomanana L, Rubin PJ, Blankevoort L. Influence of soft structures on patellar 3-dimensional tracking. Clin Orthop Relat Res. 1994;(299):235-243.
109. Goyal D. Medial patellofemoral ligament reconstruction: the superficial quad technique. Am J Sports Med. 2013;41(5):1022-1029. doi:10.1177/0363546513477828.
110. Hohn E, Pandya NK. Does the utilization of allograft tissue in medial patellofemoral ligament reconstruction in pediatric and adolescent patients restore patellar stability? Clin Orthop Relat Res. 2017;475(6):1563-1569. doi:10.1007/s11999-016-5060-4.
111. Csintalan R, Latt L, Fornalski S, Raiszadeh K, Inacio M, Fithian D. Medial patellofemoral ligament (MPFL) reconstruction for the treatment of patellofemoral instability. J Knee Surg. 2013;27(2):139-146. doi:10.1055/s-0033-1360652.
112. Ahmad CS, Brown GD, Stein BS. The docking technique for medial patellofemoral ligament reconstruction: surgical technique and clinical outcome. Am J Sports Med. 2009;37(10):2021-2027. doi:10.1177/0363546509336261.
113. Fernandez E, Sala D, Castejon M. Reconstruction of the medial patellofemoral ligament for patellar instability using a semitendinosus autograft. Acta Orthop Belg. 2005;71(3):303-308.
114. Parikh SN, Nathan ST, Wall EJ, Eismann EA. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41(5):1030-1038. doi:10.1177/0363546513482085.
115. Green D, Gausden E. Medial patellofemoral ligament reconstruction: hamstring technique. In: Green D, Cordasco F, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:150-157.
116. Craig JG, Cody DD, van Holsbeeck M. The distal femoral and proximal tibial growth plates: MR imaging, 3-dimensional modeling and estimation of area and volume. Skeletal Radiol. 2004;33(6):337-344.
117. Noyes FR, Albright JC. Reconstruction of the medial patellofemoral ligament with autologous quadriceps tendon. Arthroscopy. 2006;22(8):904.e1-e7. doi:10.1016/j.arthro.2005.12.058.
118. Pinkowsky G, Hennrikus. Technique: quad tendon medial patellofemoral ligament reconstruction. In: Green DW, Cordasco FA, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:133-139.
119. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159. doi:10.1016/j.arthro.2011.02.014.
120. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485. doi:10.1177/0363546506287486.
121. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483. doi:10.1007/s00167-008-0702-z.
122. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
123. Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293-297. doi:10.1177/0363546509347602.
124. Fink C, Veselko M, Herbort M, Hoser C. Minimally invasive reconstruction of the medial patellofemoral ligament using quadriceps tendon. Arthrosc Tech. 2014;3(3):e325-e329. doi:10.1016/j.eats.2014.01.012.
125. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. A mini-invasive adductor magnus tendon transfer technique for medial patellofemoral ligament reconstruction: a technical note. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):508-512. doi:10.1007/s00167-008-0713-9.
126. Hambidge SJ, Davidson AJ, Gonzales R, Steiner JF. Epidemiology of pediatric injury-related primary care office visits in the United States. Pediatrics. 2002;109(4):559-565.
127. Camanho GL, Bitar AC, Hernandez AJ, Olivi R. Medial patellofemoral ligament reconstruction: a novel technique using the patellar ligament. Arthroscopy. 2007;23(1):108.e1-e4. doi:10.1016/j.arthro.2006.07.008.
128. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435. doi:10.1016/j.arthro.2007.11.005.
129. Deie M, Ochi M, Sumen Y, Yasumoto M, Kobayashi K, Kimura H. Reconstruction of the medial patellofemoral ligament for the treatment of habitual or recurrent dislocation of the patella in children. J Bone Joint Surg Br. 2003;85(6):887-890.
130. Ladenhauf HN, Berkes MB, Green DW. Medial patellofemoral ligament reconstruction using hamstring autograft in children and adolescents. Arthrosc Tech. 2013;2(2):e151-e154. doi:10.1016/j.eats.2013.01.006.
131. Bedi H, Marzo J. The biomechanics of medial patellofemoral ligament repair followed by lateral retinacular release. Am J Sports Med. 2010;38(7):1462-1467. doi:10.1177/0363546510373581.
132. Lattermann C, Toth J, Bach BR. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc Rev. 2007;15(2):57-60. doi:10.1097/JSA.0b013e318042af30
133. Ricchetti ET, Mehta S, Sennett BJ, Huffman GR. Comparison of lateral release versus lateral release with medial soft-tissue realignment for the treatment of recurrent patellar instability: a systematic review. Arthroscopy. 2007;23(5):463-468. doi:10.1016/j.arthro.2007.01.007.
134. Dainer RD, Barrack RL, Buckley SL, Alexander AH. Arthroscopic treatment of acute patellar dislocations. Arthroscopy. 1988;4(4):267-271.
135. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629. doi:10.1177/0363546506296041.
136. Vainionpää S, Laasonen E, Silvennoinen T, Vasenius J, Rokkanen P. Acute dislocation of the patella. A prospective review of operative treatment. J Bone Joint Surg Br. 1990;72(3):366-369.
137. Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40(8):1916-1923. doi:10.1177/0363546512442330.
138. Tanaka MJ, Bollier MJ, Andrish JT, Fulkerson JP, Cosgarea AJ. Complications of medial patellofemoral ligament reconstruction: common technical errors and factors for success: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(12):e87. doi:10.2106/JBJS.K.01449.
139. Nguyen CV, Farrow LD, Liu RW, Gilmore A. Safe drilling paths in the distal femoral epiphysis for pediatric medial patellofemoral ligament reconstruction. Am J Sports Med. 2017;45(5):1085-1089. doi:10.1177/0363546516677795.
140. Edmonds EW, Glaser DA. Adolescent patella instability extensor mechanics. J Pediatr Orthop. 2016;36(3):262-267. doi:10.1097/BPO.0000000000000430.
141. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184. doi:10.1016/j.knee.2014.07.023.
142. Lykissas MG, Li T, Eismann EA, Parikh SN. Does medial patellofemoral ligament reconstruction decrease patellar height? A preliminary report. J Pediatr Orthop. 2014;34(1):78-85. doi:10.1097/BPO.0b013e3182a12102.
143. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2014;22(10):2545-2550. doi:10.1007/s00167-013-2445-8.
144. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521. doi:10.1007/s00167-005-0659-0.
145. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi:10.1177/0363546512463683.
146. Raghuveer RK, Mishra CB. Reconstruction of medial patellofemoral ligament for chronic patellar instability. Indian J Orthop. 2012;46(4):447-454. doi:10.4103/0019-5413.97259.
147. Kita K, Horibe S, Toritsuka Y, et al. Effects of medial patellofemoral ligament reconstruction on patellar tracking. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):829-837. doi:10.1007/s00167-011-1609-7.
148. Lenschow S, Herbort M, Fink C. Medial patellofemoral ligament reconstruction using quadriceps tendon. Oper Orthop Traumatol. 2015;27(6):474-483. doi:10.1007/s00064-015-0416-6.
149. Abouelsoud MM, Abdelhady A, Elshazly O. Anatomic physeal-sparing technique for medial patellofemoral ligament reconstruction in skeletally immature patients with ligamentous laxity. Eur J Orthop Surg Traumatol. 2015;25(5):921-926. doi:10.1007/s00590-015-1618-1.
150. Malecki K, Fabis J, Flont P, Niedzielski KR. The results of adductor magnus tenodesis in adolescents with recurrent patellar dislocation. Biomed Res Int. 2015;2015:1-7. doi:10.1155/2015/456858.
151. Kumahashi N, Kuwata S, Tadenuma T, Kadowaki M, Uchio Y. A “sandwich” method of reconstruction of the medial patellofemoral ligament using a titanium interference screw for patellar instability in skeletally immature patients. Arch Orthop Trauma Surg. 2012;132(8):1077-1083. doi:10.1007/s00402-012-1516-5.
152. Marsh JS, Daigneault JP, Sethi P, Polzhofer GK. Treatment of recurrent patellar instability with a modification of the Roux-Goldthwait technique. J Pediatr Orthop. 2006;26(4):461-465. doi:10.1097/01.bpo.0000217711.34492.48.
153. Nepple J, Luhmann S. Medial patellar tendon transfer with proximal realignment. In: Green D, Cordasco F, eds. Pediatric and Adolescent Knee Surgery. New York, NY: Wolters Kluwer; 2015:148-153.
154. Galeazzi R. Nuove applicazioni del trapianto musculare e tendineo. Ard Di Orthop Milano. 1922;38:315-323.
155. Grannatt K, Heyworth BE, Ogunwole O, Micheli LJ, Kocher MS. Galeazzi semitendinosus tenodesis for patellofemoral instability in skeletally immature patients. J Pediatr Orthop. 2012;32(6):621-625. doi:10.1097/BPO.0b013e318263a230.
156. Fiume M. La rotulopessi secondi Galeazzi nella lussazione recidivante di rotula [in Italian]. Minerva Ortop. 1954;5:171-174.
157. Baker RH, Carroll N, Dewar FP, Hall JE. The semitendinosus tenodesis for recurrent dislocation of the patella. J Bone Joint Surg Br. 1972;54(1):103-109.
158. Hall JE, Micheli LJ, McManama GB. Semitendinosus tenodesis for recurrent subluxation or dislocation of the patella. Clin Orthop Relat Res. 1979;(144):31-35.
159. Moyad TF, Blakemore L. Modified Galeazzi technique for recurrent patellar dislocation in children. Orthopedics. 2006;29(4):302-304.
160. Letts RM, Davidson D, Beaule P. Semitendinosus tenodesis for repair of recurrent dislocation of the patella in children. J Pediatr Orthop. 1999;19(6):742-747.
161. Aulisa AG, Falciglia F, Giordano M, Savignoni P, Guzzanti V. Galeazzi’s modified technique for recurrent patella dislocation in skeletally immature patients. J Orthop Sci. 2012;17(2):148-155. doi:10.1007/s00776-011-0189-1.
162. Roux C. Recurrent dislocation of the patella: operative treatment. 1888. Clin Orthop Relat Res. 2006;452:17-20.
163. Goldthwait J. Dislocation of the Patella. Trans Am Orthop Assn. 1895.
164. Fondren FB, Goldner JL, Bassett FH. Recurrent dislocation of the patella treated by the modified Roux-Goldthwait procedure. A prospective study of forty-seven knees. J Bone Joint Surg Am. 1985;67(7):993-1005.
165. Aärimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446. doi:10.1111/j.1600-0838.2007.00730.x.
166. Nelitz M, Reichel H, Dornacher D, Lippacher S. Anatomical reconstruction of the medial patellofemoral ligament in children with open growth-plates. Arch Orthop Trauma Surg. 2012;132(11):1647-1651. doi:10.1007/s00402-012-1593-5.
167. Vähäsarja V, Kinnunen P, Lanning P, Serlo W. Operative realignment of patellar malalignment in children. J Pediatr Orthop. 1995;15(3):281-285.
168. Abraham E, Washington E, Huang TL. Insall proximal realignment for disorders of the patella. Clin Orthop Relat Res. 1989;248:61-65.
169. Insall JN, Aglietti P, Tria AJ. Patellar pain and incongruence. II: Clinical application. Clin Orthop Relat Res. 1983;176:225-232.
170. Chrisman OD, Snook GA, Wilson TC. A long-term prospective study of the Hauser and Roux-Goldthwait procedures for recurrent patellar dislocation. Clin Orthop Relat Res. 1979;144:27-30.
171. Niedzielski KR, Malecki K, Flont P, Fabis J. The results of an extensive soft-tissue procedure in the treatment of obligatory patellar dislocation in children with ligamentous laxity: a post-operative isokinetic study. Bone Joint J. 2015;97-B(1):129-133. doi:10.1302/0301-620X.97B1.33941.
172. Hinckel BB, Gobbi RG, Demange MK, Bonadio MB, Pécora JR, Camanho GL. Combined reconstruction of the medial patellofemoral ligament with quadricipital tendon and the medial patellotibial ligament with patellar tendon. Arthrosc Tech. 2016;5(1):e79-e84. doi:10.1016/j.eats.2015.10.004.
173. Mani S, Kirkpatrick MS, Saranathan A, Smith LG, Cosgarea AJ, Elias JJ. Tibial tuberosity osteotomy for patellofemoral realignment alters tibiofemoral kinematics. Am J Sports Med. 2011;39(5):1024-1031. doi:10.1177/0363546510390188.
174. Brown GD, Ahmad CS. Combined medial patellofemoral ligament and medial patellotibial ligament reconstruction in skeletally immature patients. J Knee Surg. 2008;21(4):328-332.
175. Panagiotopoulos E, Strzelczyk P, Herrmann M, Scuderi G. Cadaveric study on static medial patellar stabilizers: the dynamizing role of the vastus medialis obliquus on medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):7-12. doi:10.1007/s00167-005-0631-z.
176. Philippot R, Boyer B, Testa R, Farizon F, Moyen B. The role of the medial ligamentous structures on patellar tracking during knee flexion. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):331-336. doi:10.1007/s00167-011-1598-6.
177. Gordon JE, Schoenecker PL. Surgical treatment of congenital dislocation of the patella. J Pediatr Orthop. 1999;19(2):260-264.
178. Koh JL, Stewart C. Patellar instability. Orthop Clin North Am. 2015;46(1):147-157. doi:10.1016/j.ocl.2014.09.011.
179. Pal S, Besier TF, Draper CE, et al. Patellar tilt correlates with vastus lateralis: vastus medialis activation ratio in maltracking patellofemoral pain patients. J Orthop Res. 2012;30(6):927-933. doi:10.1002/jor.22008.
180. Thompson TC. Quadricepsplasty. Ann Surg. 1945;121(5):751-754.
181. Daoud H, O’Farrell T, Cruess RL. Quadricepsplasty. The Judet technique and results of six cases. J Bone Joint Surg Br. 1982;64(2):194-197.
182. Stanisavljevic S, Zemenick G, Miller D. Congenital, irreducible, permanent lateral dislocation of the patella. Clin Orthop Relat Res. 1976;(116):190-199.
183. Kundu Z, Sangwan S, Guliani G, Siwach R, Kamboj P, Singh R. Thompson’s quadricepsplasty for stiff knee. Indian J Orthop. 2007;41(4):390-394. doi:10.4103/0019-5413.37004.
184. Tercier S, Shah H, Joseph B. Quadricepsplasty for congenital dislocation of the knee and congenital quadriceps contracture. J Child Orthop. 2012;6(5):397-410. doi:10.1007/s11832-012-0437-8.
185. Rose RE. Judet quadricepsplasty for extension contracture of the knee. West Indian Med J. 2005;54(4):238-241.
186. Tsukamoto N, Miura H, Matsuda S, Mawatari T, Kato H, Iwamoto Y. Functional evaluation of four patients treated with V-Y quadricepsplasty in total knee arthroplasty. J Orthop Sci. 2006;11(4):394-400. doi:10.1007/s00776-006-1023-z.
187. Dao Q, Chen DB, Scott RD. Proximal patellar quadricepsplasty realignment during total knee arthroplasty for irreducible congenital dislocation of the patella. J Bone Joint Surg Am. 2010;92(14):2457-2461. doi:10.2106/JBJS.H.00812.
188. Judet R, Judet J, Lord G. Results of treatment of stiffness of the knee caused by arthrolysis and disinsertion of the quadriceps femoris. Mem Acad Chir. 1959;85:645-654.
189. Oliveira VG, D’Elia LF, Tirico LEP, et al. Judet quadricepsplasty in the treatment of posttraumatic knee rigidity: long-term outcomes of 45 cases. J Trauma Acute Care Surg. 2012;72(2):e77-e80.
190. Hahn SB, Choi YR, Kang HJ, Lee SH. Prognostic factors and long-term outcomes following a modified Thompson’s quadricepsplasty for severely stiff knees. J Bone Joint Surg Br. 2010;92(2):217-221. doi:10.1302/0301-620X.92B2.22936.
191. Hosalkar HS, Jones S, Chowdhury M, Hartley J, Hill RA. Quadricepsplasty for knee stiffness after femoral lengthening in congenital short femur. J Bone Joint Surg Br. 2003;85(2):261-264.
192. Massè A, Biasibetti A, Demangos J, Dutto E, Pazzano S, Gallinaro P. The judet quadricepsplasty: long-term outcome of 21 cases. J Trauma. 2006;61(2):358-362. doi:10.1097/01.ta.0000230281.31144.1d.
193. Kocon H, Kabacyj M, Zgoda M. The results of the operative treatment of patellar instability in children with Down’s syndrome. J Pediatr Orthop B. 2012;21(5):407-410. doi:10.1097/BPB.0b013e328354f684.
194. Martin BD, Cherkashin AM, Tulchin K, Samchukov M, Birch JG. Treatment of femoral lengthening-related knee stiffness with a novel quadricepsplasty. J Pediatr Orthop. 2013;33(4):446-452. doi:10.1097/BPO.0b013e3182784e5d.
ABSTRACT
Patellar instability in children and adolescents is a challenging subset to treat. Varied forms of instability, ranging from episodic dislocation to fixed dislocation, have been recognized. It is of utmost importance for the treating physician to recognize these different patterns of instability and their associated risk factors, as more complex patterns of instability would require more extensive surgical procedures. Medial patellofemoral ligament (MPFL) reconstruction, by itself, may not suffice or may not be appropriate for the more complex instability patterns. Appropriate and early treatment of such instability in children would allow for functional progression and possible remodeling of the trochlea. However, early treatment has the associated risk of growth disturbances when surgical procedures are performed around open physis or if adult-type bony procedures are performed in children. Recent knowledge about the relationship between trochlea, MPFL femoral attachment, and distal femoral physis could help to advance safe surgical care for these patients. This article reviews the pathophysiology, risk factors, and the existing classification systems for patellar instability in children and adolescents. It focuses on varied surgical techniques, which are unique to the pediatric population, and summarizes the outcomes of these surgical techniques.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
In a prospective 2-year study of Finnish children, the annual incidence rate of patellar instability was 43/100,000 pediatric population.1 In patients 9 to 15 years of age, the incidence was approximately 1/1000.1 In another study, patients at highest risk for a first-time patellar dislocation were females aged 10 to 17 years.2 In a study in patients with traumatic hemarthrosis, 36% in the younger age group (10-14 years) and 28% in the older age group (15-18 years) had sustained patellar dislocation. In contrast, 22% in the younger age group and 40% in the older age group had sustained an anterior cruciate ligament tear.3
Approximately one-half of patients who dislocate their patella suffer from long-term complications.4,5 These complications include recurrent instability, patellofemoral pain, osteochondral lesions, and eventual arthritis.1,4,5 Young, active individuals are more prone to these issues.6 Also, 39% or more of patellar dislocation patients have an associated osteochondral fracture that might influence the management.1 Thus, patellar instability in young patients is an area of concern.
DEVELOPMENTAL ANATOMY
At 4-week gestation, the patellofemoral joint is an ectodermal sac filled with mesenchyme of the somatic mesoderm.7 Mesenchymal condensations then appear at 4 to 5 weeks gestation, followed by chondrification of both the femur and patella.7 The joint space is present by 6 weeks, and the patellar and distal femoral condyles are present at 7 weeks gestation.7 By 8 weeks gestation, the basic knee anatomy resembles that of an adult with the chondroepiphysis forming the articular surfaces of the femur, tibia, and patella.7 By this time, the extensor mechanism is formed, and active joint motion has begun, facilitating the development of the trochlear sulcus.7 The secondary ossification center in the distal femoral epiphysis forms around 36 weeks gestation.8 Postnatally, both the patella and distal femur grow through endochondral ossification.9,10
The patella is the largest sesamoid bone in the human body.11 The patella begins as a dense consolidation of cells that differentiate as the quadriceps mechanisms develop.12,13 The patellar anlage becomes distinguishable within the quadriceps tendon around 7.5-week gestation.12 The morphology of the patella conforms to the distal femur.12 The patella molds or re-models as the knee begins to move in response to mechanical stresses.7 The patella increases in relative size during the first 6 months of gestation, then enlarges proportionately to the rest of the bones.7 Ossification begins around 3 years of age for females and 4 to 5 years of age for males.8,14 The ossification center may appear irregular as it rapidly expands.14 Ossification proceeds in a proximal to distal direction, thus giving a spurious estimation of patellar height on radiographs in children. The overall morphology of the cartilaginous patella during development is comparable to the final mature shape.14 Abnormal contact stresses on the articular surface of the patella during skeletal immaturity can lead to deformation.7
Ultrasonographic measurements in normal patients show that trochlear groove (TG) morphology is present early and becomes more radiographically apparent as distal femoral ossification is completed.15 Anatomic dissections of aborted fetuses have verified the morphology of the TG as it remains constant during growth and the groove morphology is the same for both fetuses and adults.16 An ultrasound study performed on patients aged 12 to 18 years showed the cartilaginous sulcus angle (CSA) remained constant throughout all age groups (146°).17 The CSA however, differed in patients who suffered a patellar dislocation (average, 164°; range, 154°-195°) compared with normal knees (average CSA, 145°; range, 131°-158°).15,17,18 The osseous sulcus angle, on the other hand, appears flat at birth and the TG deepens with age. This increase in depth is more of a reflection of progressive ossification of a well-formed cartilaginous trochlea, rather than a true deepening of the sulcus.17 Thus, the axial radiographic view of the patella provides misleading information about the sulcus angle in children and should not be used to define trochlear morphology.
Continue to: MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
The medial patellofemoral ligament (MPFL) functions to limit the lateral translation of the patella.19 The attachment sites on the femur and patella for the MPFL have been studied in children.20-23 Cadaveric dissections in specimens aged 2 to 11 years have noted the patellar attachment to be an average of 12 mm in length with the midpoint approximately 5 mm superior to the mid-pole of the patella.22 The patellar footprint of the MPFL insertion was a mean 41% of the entire patellar length.22
It is important to be aware of the characteristic anatomy of the MPFL, as fixation points should mimic the anatomic insertion as best as possible while also avoiding violation of the nearby physis. The MPFL originates between the adductor tubercle and the medial femoral epicondyle just distal to the distal femoral physis and attaches to the superomedial aspect of the patella.20-25 In relation to the physis in pediatric patients, the midpoint of MPFL insertion has been measured to be 4 mm to 9 mm distal to the femoral physis.21,24,25 These measurements represent averages as cadaveric studies have reported that some part of MPFL femoral insertion extends proximal to the distal femoral physis.21 A recent report of physeal injury to the posterior distal femoral physis during MPFL reconstruction leading to femoral flexion deformity highlights the importance of physeal-respecting surgery.26
TROCHLEA AND ANTERIOR DISTAL FEMORAL PHYSIS
The relationship between the proximal aspect of the trochlea and the anterior distal femoral physis has been recently studied in 175 knees with dysplastic trochlea.27 Based on magnetic resonance imaging evaluation, the lateral aspect of the trochlea extended proximal to the anterior distal femoral physis in 13% of patients and was at the level of the anterior physis in another 13% of patients (Figure 1).27 Hence, a cautious approach is recommended for any surgery to address trochlear dysplasia or trochlear bump in younger patients to prevent iatrogenic injury to anterior distal femoral physis and resultant genu recurvatum. The distance between the trochlea and the physis increased with increasing age.

LIMB ALIGNMENT
Physiologically, the quadriceps angle (Q angle) changes through the course of growth. As children begin standing and walking, they stand with their feet wider apart and in genu varum.28 Physiologic genu varum can reach 15°.28 This degree lessens during the first 1.5 to 2 years of life, transitioning to physiologic valgus of nearly 12° by 3 years of age.28 Genu valgum, thereafter, gradually decreases to reach the adult value of around 7° to 8° by age 7 years.28 Increased genu valgum is a risk factor for patellar instability. In skeletally immature patients, correction of genu valgum through guided growth may be desirable in patients undergoing patellar stabilization surgery (Figures 2A, 2B).29

PATHOPHYSIOLOGY OF PEDIATRIC PATELLAR DISLOCATION
TROCHLEAR DYSPLASIA
Trochlear dysplasia is an abnormal shape and depth of the TG.30 Up to 96% of patients with patellar dislocation have trochlear dysplasia.30-33 In a study of patellar instability in children, at least 1 of the 3 signs of trochlear dysplasia (the crossing sign, supratrochlear bump, and double contour sign) was present on lateral radiographs.34 In another study on the growth of trochlear dysplasia in children and adolescents, all grades of trochlear dysplasia were present at all ages (ie, the dysplasia was most likely present at birth and did not necessarily worsen with age and growth).35 The linear dimensions of lateral and medial condylar height as well as trochlear bump increased with age but both the sulcus angle and shape of the trochlea did not change significantly.35 Remodeling of a dysplastic trochlea can happen if the patella is stabilized and appropriately located at a younger age, preferably before 10 years of age.36,37
Continue to: PATELLAR HEIGHT
PATELLAR HEIGHT
The role of patellar height in patellar instability has been well established.38 In patients with patella alta, the patella remains proximal to the TG during the greater arc of knee motion, which predisposes it to patellar instability. Calculation of patellar height in children could be challenging due to incomplete ossification, as well as asymmetric ossification of the patella and the tibial tubercle (TT). Since the patella ossifies from proximal to distal, most radiographic methods that measure the patellar height from the distal aspect of the patella provide a spurious elevation of the measurement.
The Caton-Deschamps (CD) method measures the length of the patellar articular surface and the distance from the inferior edge of the articular surface to the anterosuperior corner of the tibial plateau.39 A ratio >1.3 signifies patella alta. The CD ratio has been verified as a simple and reliable index for measuring patellar height in children.40 Two other methods have been described for determining patellar height in children.41,42 Based on anteroposterior (AP) radiographs of the knee in full extension, Micheli and colleagues41 calculated the difference between the distance from the superior pole of the patella to the tibial plateau and the length of the patella. A positive difference signified patella alta. The Koshino method involves the ratio between a reference line from the midpoint of the patella to the midpoint of the proximal tibial physis and a second distance from the midpoint of the distal femoral physis to the midpoint of the proximal tibial physis on lateral knee radiographs.42 Normal values range from 0.99 to 1.20 with the knee in >30° flexion, in children 3 to 18 years of age.
HYPERLAXITY
In contrast to adults, children have increased levels of collagen III compared with collagen I, which is responsible for tissue elasticity.43 Tissue elasticity leads to increased joint mobility, which is more common in children. Joint hypermobility or hyperlaxity has to be differentiated from symptomatic instability. The traditional Beighton score identifies individuals as having joint hypermobility with a score of 5/9 or higher in school-aged children.44-46 Smits-Engelsman and colleagues44 suggested using stricter criteria with scores of 7/9 or higher being indicative of hyperlaxity in school-aged children. A study of 1845 Swedish school children noted that females have a higher degree of joint laxity.45 Maximal laxity was noted in females at 15 years of age.45 Hyperlaxity has been demonstrated to be greater on the left side of the body44 and can be part of generalized syndromes including Down’s syndrome, Marfan’s syndrome, or Ehlers-Danlos syndrome.
LIMB TORSION
Staheli and colleagues47 described the normative values of a lower extremity rotational profile, including femoral anteversion and tibial torsion. Children normally have increased femoral anteversion, which decreases with growth. Miserable malalignment is a term used to denote increased femoral anteversion and increased external tibial torsion.48,49 These rotational abnormalities can increase the Q angle and the lateral forces on the patella. Femoral anteversion or internal rotation of the femur of 30° significantly increases strain in all areas of the MPFL.48 This increased strain may lead to MPFL failure and patellar instability.48 Increased internal rotation of the femur also increases contact pressure on the lateral aspect of the patellofemoral joint.48 Miserable malalignment frequently manifests following a pubertal growth spurt and may require femoral and tibial osteotomy.50
SYNDROMIC ASSOCIATIONS
Several syndromes have patellar instability as a part of their manifestation. The more common syndromes include nail-patella syndrome, Kabuki syndrome, Down’s syndrome, and Rubinstein-Taybi syndrome.51-54 Other syndromes less commonly associated with patellar instability include Turner syndrome, patella aplasia, or absent patella syndrome. Since many patients with syndromic patellar instability are functionally limited, they may not require an aggressive approach to treatment. When treating these patients, it is important to recognize the unique features of a specific syndrome, which may affect the anesthesia risk profile, management decisions, rehabilitation, and prognosis.
Continue to: MPFL TEAR PATTERN
MPFL TEAR PATTERN
The MPFL serves as an important constraint to the patella to prevent lateral dislocation, primarily during the first 20° to 30° of knee flexion.55,56 Injury to the MPFL is noted in over 90% of patients who suffer a patellar dislocation.57 The location of MPFL tears in pediatric patients is variably reported at the patellar attachment (10%-61%), femoral attachment (12%-73%), both (12%-35%) or mid-substance (2.5%-15%).25,57 The most common tear patterns in pediatric patients are tears at the patellar attachment.25,57 This tear pattern may be accompanied by an avulsion fracture of the medial rim of the patella, though this fracture, being extra-articular, seldom needs treatment.
CLASSIFICATION
While several authors have established extensive classification systems of patellar dislocation based on both clinical and radiographic presentation and reviews of the literature, a single classification system has not been recognized as the gold standard. In this section, in addition to presenting our preferred methods of classification, we will review some of the more recent and extensive classification systems for patellar dislocation and patellar instability.
Dejour and colleagues31 initially used both the presence of patellofemoral anatomic abnormalities and pain to define 3 types of patellar instability: major, objective, and potential patellar instability. Major patellar instability indicates that the patient has experienced more than 1 documented dislocation, objective instability involves one dislocation in addition to an associated anatomic abnormality, and potential patellar instability refers to cases in which the patient has radiographic abnormalities and patellar pain.31 Garin and colleagues58 more simplistically divided patellar dislocation patients into 2 groups: major (permanent or habitual) dislocation of the patella and recurrent dislocation. Sillanpaa59 stressed the distinction between first-time dislocation and recurrent dislocation specifically in the context of acute injuries. These classification systems were formulated with adults as the most relevant population; however, classifications targeted specifically to pediatric patients have recently been presented in the literature.
Historically, pediatric patella dislocations were simply categorized as traumatic or congenital.60 In 2014, Chotel and colleagues61 focused on classifying patellar dislocation by extensively reviewing anatomic, biomechanical, pathophysiological, and clinical patterns seen most commonly in children. They included 5 categories: congenital dislocation, permanent dislocation, habitual dislocation during knee flexion, habitual dislocation during knee extension, and recurrent dislocation; however, they did not address traumatic dislocations.61 Congenital dislocation is a rare condition, typically presenting at birth, which produces a pattern of functional genu valgum.62 Permanent dislocation typically presents after the child has started walking, but before the age of 5 years.61 The 2 variations of habitual dislocation typically present between ages 5 and 8 years.61 The final category is the most common and typically occurs during pre-adolescence or adolescence as a result of an atraumatic or trivial traumatic event or sports injury.1 Using more specific terminology, Hiemstra and colleagues63 modeled a classification system based on the traumatic, unilateral, bankart lesion, surgery (TUBS)/atraumatic, multidirectional, bilateral, rehabilitation, inferior shift (AMBRI) for shoulder dislocation classifications. The patellar dislocation system is used to identify 2 distinct subsets of patients in the patellofemoral instability population. One subset is defined by the acronym WARPS (weak, atraumatic, risky anatomy, pain, and subluxation), the other is STAID (strong, traumatic, anatomy normal, instability, and dislocation).64 Patients categorized by the WARPS acronym tend to experience atraumatic onsets of patellofemoral instability and demonstrate anatomic issues that increase this instability. These underlying anatomic issues include valgus alignment, ligamentous laxity, rotational abnormalities, shallow and short TG, and patella alta. On the other hand, STAID patients describe a traumatic dislocation event and do not have underlying anatomic abnormalities that predispose them to instability.64
Taking into account these previous classifications, Frosch and colleagues65 added specific pathologies including “instability,” “maltracking,” and “loss of patellar tracking,” in addition to both clinical and radiographic factors to define 5 types of patellar dislocation and their specific treatment recommendations.65 Type 1 involves simple dislocation with neither maltracking nor instability and a low risk of redislocation.65 Type 2 is defined as primary dislocation followed by subsequent high risk of dislocation and no maltracking.65 Type 3 is divided into 5 subcategories of instability and maltracking issues involving soft tissue contracture, patella alta, pathological tibial tuberosity, and TG distance.65 Type 4 is defined as the highly unstable “floating patella,” and type 5 involves patellar maltracking without instability 65. In terms of treatment, conservative rehabilitation is recommended for type 1 whereas MPFL reconstruction tends to show positive outcomes for both types 2 and 3.66-70
Continue to: Parikh and Lykissas recently published...
Parikh and Lykissas recently published a comprehensive classification system of 4 defined types of patellar dislocation in addition to voluntary patellar instability and syndromic patellar instability (Table).60 The 4 types are Type 1, first-time patellar dislocation; Type 2, recurrent patellar instability; Type 3, dislocatable; and Type 4, dislocated. Type 2 is further subdivided into Type 2A, which presents with positive apprehension signs, and Type 2B, which involves instabilities related to anatomic abnormalities.60 A distinction is also made between Type 3A or passive patellar dislocation and Type 3B habitual patellar dislocation.60

The classification system proposed by Green and colleagues is more simplified with 3 main categories (Table) of pediatric patellar dislocation: traumatic (acute or recurrent), obligatory (either in flexion or extension), and fixed laterally.71,72 The acute traumatic categorization refers to patients who experienced an initial dislocation event due to trauma whereas recurrent traumatic involves repeated patella dislocations following an initial incident. Studies report that between 60% to 70% of these acute traumatic dislocations occur as a result of a sports-related incident.2,33,73 Obligatory dislocations occur with every episode of either knee flexion or extension, depending on the subtype. Obligatory patella dislocation in flexion typically cannot be manipulated or relocated into the trochlea while the knee is fixed but does reduce into the trochlea in full extension. Fixed lateral dislocations are rare, irreducible dislocations in which the patella stays dislocated laterally in flexion and extension. These dislocations often present with other congenital abnormalities. Each of these categories can be further specified as syndromic if the dislocation is associated with genetic or congenital conditions including skeletal dysplasia, Ehlers-Danlos syndrome, cerebral palsy, Marfan disease, nail-patella syndrome, Down syndrome, Rubenstein-Taybi syndrome, and Kabuki syndrome.51-54,61,74-76
SURGICAL TECHNIQUES IN SKELETALLY IMMATURE PATIENTS
While nonsurgical, conservative treatment involving physical therapy and activity modification is recommended for most patients who experience first-time traumatic patellar dislocations, many patients experience complicating factors that indicate them for surgery. These factors include recurrent dislocation, risk factors for patellofemoral instability, underlying malalignment issues, and congenital deformities. When evaluating these factors, particularly patellofemoral instability, the authors recommend assessing osteochondral lesions, age, skeletal maturity, number of previous dislocations, family history, and anatomic risk factors.2,5,77-79 Extra care should be taken when considering surgical treatment for skeletally immature patients at elevated risk for recurrent instability as the risk of cartilage damage in these cases is high.80-82
Recently, there has been a reported increase in surgical treatment for patellar instability in the skeletally immature.83 This finding may be attributed to heightened awareness of factors that indicate patients for surgical treatment and increased familiarity of surgeons with newer techniques.83 Many surgical techniques have been described to address patellar instability involving both soft-tissue procedures and bony corrections.84 In this article, we discuss the various surgical techniques for MPFL reconstruction, quadricepsplasty, and distal realignment. These procedures can be paired with any number of additional procedures including, but not limited to, lateral retinacular release or lengthening, chondroplasty, TT osteotomy (in skeletally mature patients), and removal of loose bodies.83
There is a need for more comprehensive studies, particularly randomized controlled trials, to evaluate the outcomes for both surgical and nonsurgical treatments for first-time dislocations. In the current literature, only very recently have surgical treatments shown outcomes that are more positive. In 2009, Nietosvaara and colleagues85 conducted a randomized controlled trial of nonoperative and operative treatment of primary acute patellar dislocation in both children and adolescents. After a long-term mean follow-up of 14 years, there was not a significant difference between the groups in recurrent dislocation and instability, subjective outcome, or activity scores.85 In a subsequent review of 5 studies including 339 knees, Hing and colleagues86 also found similar results in both the operative and nonoperative cohorts at risk of recurrent dislocations, Kujala scores, and reoperations. However, a recent systematic review comparing redislocation rates and clinical outcomes between surgical and conservative management of acute patellar dislocation reported more positive outcomes for the surgical cohort.87 This review included 627 knees, 470 of which received conservative management, 157 of which received operative treatment. The conservative cohort was followed for an average of 3.9 years and had a 31% rate of recurrent dislocation while the surgical group was followed for a mean 4.7 years and experienced a 22% redislocation rate.87 This study indicates that operative management for acute first-time dislocations may be the preferred treatment option.
Continue to: A potential reason some of these studies...
A potential reason some of these studies did not show any significant difference between the operative and nonoperative cohort could be that the surgical cohorts included a wide range of procedures including lateral releases and MPFL repairs. Recent publications have indicated that these techniques do not produce overall positive outcomes. While each surgical treatment plan is unique depending on the patient; recently, MPFL reconstruction has been shown to have better outcomes than both nonoperative management and simple medial repair and/or lateral
release.67,88-90
MPFL RECONSTRUCTION
INDICATIONS/OVERVIEW
The MPFL is an important stabilizer for the knee that primarily resists lateral translation of the patella. Damage to the MPFL is very common in acute patellar dislocations with up to 90% of first-time dislocations resulting in injury to the MPFL.91,92 Historically, simple medial and/or lateral MPFL repairs have not been shown to improve patellofemoral kinematics significantly and often result in recurrence.90,93 To address this issue, during the past few decades, numerous MPFL reconstruction techniques have been developed to reconstruct a stronger ligament with the same kinematics as the anatomic MPFL.2,19,69,81,94-106 The ultimate goal of MPFL reconstruction is to reestablish the anatomic “checkrein” to guide the patella into the trochlea between 0° and 30° of knee flexion.107,108 An essential secondary surgical goal in skeletally immature patients is to avoid damaging the distal femoral physis.
There are many variations in both the grafts used to replace the MPFL and the means by which to secure them. The ones discussed below include free semitendinosus or gracilis autografts or grafts constructed from a pedicled adductor, patellar, or quadriceps tendon.69,105,109 While not used as frequently, allografts have also been used.110 Methods to secure these grafts in osseous tunnels include suture anchors or tenodesis screws. Incomplete osseous sockets or medial-sided bone tunnels have also been used as a method to decrease patellar fractures as they preserve the lateral patellar cortex.111-114
DOUBLE-BUNDLE HAMSTRING AUTOGRAFT
The technique most often used by the author is a double-bundle hamstring autograft harvested from either the semitendinosus or the gracilis secured by short patellar and femoral sockets (Figure 3). After harvesting the hamstring graft from a posteromedial incision, an approximately 90-mm graft is prepared with Krackow stitches to secure 15 mm of the tendon in each socket.115 Lateral radiographs are used intraoperatively to ensure the guidewire for the femoral drill hole falls along the posterior cortex of the diaphysis of the femur while AP radiographs confirm placement distal to the physis. It is important to take both AP and lateral radiographs intraoperatively due to the concave curvature of the distal femoral physis. This unique anatomy can make a point that is located distally to the physis on the AP view appear on or proximal to it on the lateral cross reference view.24,116 For the patellar socket, 2 short sockets are made in the superior half of the patella. Once the sockets have been drilled, the graft is adjusted so that the patella stays seated in the center of the trochlea between 20° and 30° of flexion. This anchoring is accomplished by securing the graft while the knee is kept at 30° of flexion. Proper tension is confirmed by ensuring that the graft does not allow lateral patella movement over one-fourth the width of the patella in extension while crepitation must not appear throughout the ROM.92

QUADRICPETS TENDON TRANSFER
A combination of techniques by Steensen and colleagues,105 Goyal,109 Noyes and Albright,117 and Pinkowsky and Hennrikus118 describe an MPFL reconstruction in which the proximal end of a small medial portion of the quadriceps tendon is released and then attached to the medial epicondyle through a subcutaneous tunnel (Figure 4). This technique is particularly useful for cases in which the extra strength provided by the bone-quadriceps tendon is necessary to correct more severe dysplasia. Leaving the distal end of the quadriceps tendon intact at its patellar insertion, a graft of about 8 mm x 70 mm thickness is harvested from the tendon. The free distal end of the tendon is then run anatomically through the synovium and retinaculum to be either sutured to the medial intermuscular septum at the medial femoral epicondyle or fixed in femoral tunnel using interference screw.105,109,118 The placement of the femoral fixation point is essential to ensure positive surgical outcomes. If the graft is secured too anteriorly, it may be too loose in extension and too tight in flexion, both of which can lead to postoperative pain, loss of normal kinematics, and overload of the medial patellofemoral cartilage.119-121 Once the ideal placement of the femoral fixation point has been confirmed by intraoperative radiographs, the graft is secured with a small absorbable suture.122,123 While this technique has good clinical results, the longitudinal scar that results from graft harvesting is cosmetically unappealing, and it is technically challenging to harvest a consistent strip of the quadriceps tendon. To address some of these concerns, Fink and colleagues124 described a new harvesting technique that produces more consistent grafts and requires a smaller incision.

Continue to: ADDUCTOR MAGNUS TENDON TRANSFER
ADDUCTOR MAGNUS TENDON TRANSFER
This technique is a double-bundle MPFL reconstruction that uses a pedicled graft of the distal adductor magnus tendon and suture anchors or incomplete osseous sockets to recreate the MPFL anatomically (Figure 5). Avikainen and colleagues96 and Sillanpää and colleagues125 described this procedure as a progression from the original single-strand adductor magnus transfer technique. First, maintaining the distal insertion, a graft of approximately 14 cm to 18 cm is harvested from the adductor tendon and then passed through a subcutaneous tunnel between the distal vastus medialis obliquus and the superficial joint capsule. The graft is then looped at the medial patella so that the distal bundle runs back to the adductor tubercle.125 With the knee at 30° of flexion to assure proper tension, the graft is secured at both the patella and near the adductor tubercle with suture anchors.125 Hambridge and colleagues126 compared a similar adductor magnus transfer with other pedicled techniques including bone-quadriceps tendon autograft and bone-patellar tendon allograft and found positive results for all 3 methods of reconstruction.

HEMI-PATELLA TENDON TRANSFER
In a similar technique to the adductor tendon transfer, the medial section of the patellar tendon is harvested from the TT and run from its proximal insertion at the medial patella to the medial femoral attachment via a subcutaneous tunnel. The free end of the graft is then secured with suture anchors or incomplete osseous sockets with the knee at 30° of flexion.127
HAMSTRING GRAFT WITH ADDUCTOR TENDON AS A PULLEY
Several techniques opt to use a more dynamic model of MPFL reconstruction in which the adductor tendon or medial collateral ligament (MCL) is used as a pulley for the hamstring graft (Figure 6).128,129 The site of the pulley approximates the normal attachment of the MPFL to the femur and so acts as an effective anatomic replica of the MPFL origin. A semitendinosus graft is harvested and is prepared with continuous sutures, and 2 tunnels to secure the graft are drilled into the patella. The graft is then run subcutaneously from the medial side of the patella to the adductor magnus tubercle into which an osteoperiosteal tunnel is drilled at its distal femoral insertion. The graft is looped through the adductor tunnel and secured with sutures. Proper knee kinematics was ensured by placing the knee at 30° of flexion as the ends of the tendon are secured to the patella.114,130

HAMSTRING GRAFT WITH MCL AS A PULLEY
The MCL can also be used as a pulley rather than the adductor tendon. The semitendinosus graft is harvested and prepared and the patella drilled as it is in the previous technique. The MCL was fashioned into a pulley by making a slit in its posterior one-third. The semitendinosus graft is looped through this slit, and both ends of the graft are held in place with suture anchors on the surface of the patella.129
ADDITIONAL PROCEDURAL COMBINATIONS
Depending on the needs of the individual patient, MPFL reconstruction, and other patellar stabilization techniques can also be combined with additional procedures. Arshi and colleagues83 conducted a review of 6190 adolescents surgically treated for patellar instability and reported the most common additional procedures performed at the time of the stabilization. They found 43.7% of the population underwent lateral retinacular release, which while not effective as an isolated technique to treat patellar instability, has often been used in combination with MPFL reconstruction.131-133 There is currently a lack of consensus regarding the success of adding a lateral release to the reconstruction. Some studies report no difference while others report a decrease in stability after lateral release.90,134-136 While lateral retinacular release has been shown to decrease the force required to displace the patella, it can be surgically indicated in certain patients undergoing MPFL reconstruction.131 The authors advocate that if the lateral retinaculum is tight such that centralized patellar tracking is inhibited following the reconstruction, or if the patella cannot be pushed passively from a laterally tilted position to the neutral horizontal position, lateral retinacular lengthening should be performed to improve kinematics.132
Continue to: Arshi and colleagues...
Arshi and colleagues83 also reported a high rate of cartilage procedures, with chondroplasty performed in 31.1% and chondral fragment/loose body removal in 10.2%. These statistics suggest that a significant level of cartilage damage has occurred by the time of surgery.83
COMPLICATIONS
As MPFL reconstruction techniques have only recently been popularized and developed, there are not many comprehensive studies evaluating the outcomes and complications associated with these procedures. However, in the current literature, there is a general consensus that patients usually experience positive short-term clinical outcomes and relatively low complication rates.68,77 In one of the largest retrospective cohort studies of pediatric patients undergoing MPFL reconstruction, Parikh and colleagues114 reported both the type and rate of complications. They found complications occurred in 16.2% of patients, and the most common complications were recurrent patellar instability, patellar fractures, patellofemoral arthrosis, motion deficits, and stiffness with over half classified as avoidable. Most of these complications were due to technical errors with episodes of recurrent instability only reported in 4.5% of patients.114 In a comprehensive meta-analysis of MPFL reconstruction studies, Shah and colleagues137 reported a complication rate of 26% in both pediatric and adult patients. The cohort was not stratified by age, yet complications were similar to those reported by Parikh and colleagues,114 including pain, loss of knee flexion, wound complications, and patellar fracture.137
As indicated by the frequency of technical complications reported by Parikh and colleagues,114 extra caution should be taken in the operating room to minimize potential errors. In techniques that require drilling of femoral sockets, proper length for and placement of the graft is essential to reestablish proper kinematics. Studies have reported that placing the femoral socket too proximally can result in loss of ROM during flexion and increased compressive forces across the patella.138 A graft that is too short can have similar negative outcomes, and a graft that is too long can result in recurrent instability. Positioning the graft while the knee is in 30° of flexion can help ensure the proper length and tension is achieved. Once the graft is in place, it is important to ensure the ROM and isometry before completing the fixation.72 It is also essential to be vigilant about potential violation of the physes and subsequent growth disturbances. To establish the safest angles for drilling the distal femoral epiphysis for graft placement, Nguyen and colleagues139 conducted a study using high-resolution 3-dimensional images of cadaveric distal femoral epiphyses. By recording which tunnels disrupted the physis before reaching 20 mm of depth, the authors concluded that it is safest to drill distally and anteriorly at an angle between 15° and 20°.139 This technique should minimize damage to the physis, notch, and distal femoral cartilage and decrease potential complications.139
OUTCOMES
In general, the literature reports positive outcomes for MPFL reconstruction—in both studies that address a specific technique and all-encompassing studies. Outcomes are typically reported as Kujala and Tegner scores, results from clinical examinations, and rates of subsequence recurrences. Several recent studies have also evaluated the ability of MPFL reconstruction to restore proper kinematics. Edmonds and colleagues140 evaluated the difference in patellofemoral joint reaction forces and load experienced by 3 groups of adolescents: a cohort treated with MPFL reconstruction, a cohort treated with soft-tissue realignment of the extensor mechanism (the Insall method), and controls. While both surgical techniques were able to restore medial constraints to the patella, the study showed that only the MPFL reconstruction cohort experienced joint reaction forces that were analogous to the control group. In comparison, the cohort that was treated with soft-tissue realignment alone experienced higher patellofemoral joint reaction forces and did not regain normal joint mechanics.140 These results can be used to advocate for the further use of MPFL reconstruction as an effective anatomic replacement of the native ligament. Radiographic studies have similarly reported MPFL reconstruction as an effective means to restore anatomic normality. Fabricant and colleagues141 conducted a radiographic study in which patella alta was corrected to normal childhood ranges in patients who underwent MPFL reconstruction technique using a hamstring autograft. Lykissas and colleagues142 corroborated these results with another radiographic study that reported small but significant decreases in the Blackburne-Peel index and CD index following MPFL reconstruction in 25 adolescents. As correction of patella alta allows the patella to rest in a deeper, more secure position in the TG, these results indicate that effective early MPFL reconstruction can correct for patellar anatomic abnormalities that could be future risk factors.143,144 Several studies have also reported outcomes addressing specific MPFL techniques; these are reported and discussed in this article.
OUTCOMES BY TECHNIQUE
HAMSTRING AUTOGRAFT
Reports on outcomes following MPFL reconstructions using hamstring autografts have been particularly promising. A cohort of 21 skeletally immature patients who underwent MPFL reconstruction was evaluated pre- and postoperatively with an average of a 2.8-year follow-up. The authors of the study reported no redislocation events and significant improvement in the Kujala scores, and patients were able to return to athletic activities safely.145 Previous studies report similar positive increases in Kujala scores, subjective patient reports, and lack of subsequent redislocation for patients who underwent either semitendinosus or gracilis autograft MPFL reconstructions. One such study further documented an average patellar inclination angle decrease from 34.3° to 18.6° following MPFL reconstruction.146 However, while the literature typically reports positive Kujala scores and subjective outcomes for the hamstring autograft procedure, a study arthroscopically evaluating patellar tracking immediately following surgery and then at 6 to 26 months follow-up found that patellar tracking correction was not maintained for all patients who underwent this type of MPFL reconstruction.147
Continue to: QUADRICEPS TENDON TRANSFER OUTCOMES
QUADRICEPS TENDON TRANSFER OUTCOMES
Studies specifically evaluating the quadriceps tendon transfer technique for MPFL reconstruction in children are sparse, but authors have reported positive clinical outcomes and low complication rates in adults. After following 32 young adults who underwent this MPFL reconstruction technique for 3 years, Goyal109 reported a significant increase in mean Kujala scores from 49.31 to 91.25 and no complications or redislocation. He argues this type of quadriceps graft has a high success rate because it is anatomically more similar to the MPFL than other grafts and does not require additional patellar fixation.101,109 Similar positive Kujala scores and minimal complications have been reported in adult patient populations.148 Abouelsoud and colleagues149 conducted one of the few studies in skeletally immature patients and reported similarly positive results with no redislocations and significantly improved Kujala scores at a mean follow-up of 29.25 months in their 16-patient cohorts.
ADDUCTOR MAGNUS TENDON TRANSFER
After initially describing this technique in 14 adult patients, Avikainen and colleagues96 followed this cohort and reported positive subjective results and only 1 redislocation. In a more recent study in which the adductor tendon transfer technique was compared with the quadriceps tendon transfer described above and the bone-patellar tendon allograft, Steiner and colleagues69 reported similarly significant improvement in all cohorts in Lysholm, Kujala, and Tegner scores with no redislocations. Additionally, Malecki and colleagues150 followed a cohort of 33 children with 39 knees diagnosed with recurrent patellar dislocation, who underwent MPFL reconstruction using the adductor magnus tendon. After evaluating this cohort functionally and radiographically, the authors reported improvements in Lysholm and Kujala scores, patellar tilt and congruence angles, and peak torque of the quadriceps muscle and flexor.150 However, this cohort did report postoperative redislocations in 36.4% of patients (4 of 11).150
HEMI-PATELLA TENDON TRANSFER
In 2012, in the first randomized controlled trial, Bitar and colleagues67 compared the outcomes of patients who underwent MPFL reconstruction via the hemi-patellar tendon technique with those who were managed nonoperatively with immobilization and physiotherapy after first-time patellar dislocation. At 2-year follow-up, the surgical cohort presented positive results with a significantly higher mean Kujala score (88.9 to 70.8) and no redislocations or subluxations. In contrast, 35% of nonoperative cases presented with recurrences and subluxations over the 2-year period.67
MCL OR ADDUCTOR TENDON AS A PULLEY
Studies have reported good postoperative results and low complication rates for these dynamic techniques.128,129 In terms of kinematics, while hypermobility and patellar height were not fully corrected, improvements in patellar tilt and lateral shift were reported in a cohort of 6 patients with a minimum 4-year follow-up.129 To further evaluate whether the more dynamic pulley reconstruction technique resulted in better outcomes, Gomes and colleagues128 compared the subjective reports, clinical evaluations, and complication rates of patients who underwent MPFL reconstruction with a rigid adductor magnus fixation vs a semitendinosus tendon dynamic femoral fixation. One case in the rigid cohort experienced a subsequent subluxation, while patients in the semitendinosus group had better subjective reports and a higher rate of return to sport.128 More recently, Kumahashi and colleagues151 specifically studied the outcomes of the MCL tendon as a pulley in 5 patients aged 14 to 15 years. They reported similar successful results as no patients experienced recurrence, and all patients exhibited improvement in radiographic measures of patellar tilt and congruence angle, lateral shift ratio, and both Kujala and Lysholm scores.151
While there has yet to be a randomized controlled trial comparing all of these different techniques, there is a general consensus in the literature that patients tend to perform better following MPFL reconstruction vs MPFL repair.
OTHER STABILIZATION PROCEDURES, INCLUDING DISTAL REALIGNMENT
Patients with additional underlying deficits and malalignment issues such as significant trochlear dysplasia, increased TT-TG distance, patella alta, increased Q angle, and/or positive J sign may require stabilization procedures beyond MPFL reconstruction.152,153 TT osteotomies are often used to correct alignment issues in the adult patient population; however, these procedures are typically contraindicated in skeletally immature patients. Alternative realignment procedures for the pediatric population include both proximal and distal realignment, with proximal realignment performed primarily in children under the age of 12 years.153 Many variations on these procedures exist, some of which are no longer regularly performed due to poor reported outcomes. In this article, we discuss several of the techniques, focusing primarily on those that have demonstrated higher success rates.
Continue to: GALEAZZI TECHNIQUE
GALEAZZI TECHNIQUE
One of the first and most famous soft-tissue techniques to address patellar instability was the semitendinosus tenodesis, published by Galeazzi154 in 1922 (Figure 7). This technique stabilizes the patella without altering the TT. In the original technique, a portion of the semitendinosus tendon is harvested with its tibial insertion left intact. The free end of the tendon is then secured with sutures at the periosteal groove of the medial patella.154,155 Fiume156 modified this technique by adding a lateral release and medial retinacular reefing. The most recent addition to this procedure was introduced by Baker and colleagues,157 in which a tunnel is drilled from the medial to the lateral border of the patella. Tension placed on the grafted tendon is used to reposition the patella medially and draw it downward. Preliminary literature on this modified procedure reported fair clinical results with success rates of approximately 75%.155,158-160 A recent study evaluating both the clinical and radiographic outcomes of this technique also indicated that while clinical results were excellent in 62.5% of patients, this technique alone was unsuccessful in fully addressing patellar instability in patients with underlying anatomic abnormalities such as patellar alta.161 In light of these less than ideal reports, the authors no longer recommend this technique for patellofemoral instability cases.

ROUX-GOLDTHWAIT PROCEDURE
The Roux-Goldthwait procedure, first described by both Roux162 and Goldthwait163 in 1888 and 1895 respectively, was later modified in 1985 to involve a lateral release, plication of the medial retinaculum, medial transfer of the lateral patellar tendon without advancement, and advancement of the vastus medialis (Figure 8).164 More recently, Marsh and colleagues152 introduced an addition to aligning the extensor mechanism with the femoral shaft better. In this technique modification, the patellar tendon is split longitudinally, and its lateral half is detached and transferred distally beneath its medial half. The free end is then sutured to the periosteum on the medial side of the tibia.152 With a mean long-term follow-up of 6.2 years, Marsh and colleagues152 reported excellent results in 65%, good in 11%, and fair in 3% of the knees operated on with this modified technique. Of the patients in this cohort whose strength was evaluated, 80% had their strength returned to 90% of preoperative levels in the operated leg.152 While this study and others report improved outcomes, an increasing body of literature has found high rates of recurrence, patella infera, and other complications following the modified Roux-Goldthwait procedure.36,165-171 Also, a study comparing MPFL reconstruction using adductus magnus transfer with the Roux-Goldthwait procedure reported that patients in the MPFL cohort reported less pain postoperatively.150 In addition, whereas the Kujala and Lysholm scores, recurrence rates, patellofemoral angles, and apprehension test results did not demonstrate significant differences between these 2 groups, the MPFL group had significantly fewer abnormal congruence angles, better patellar medialization, and higher peak torque of the hamstring.150

COMBINED MPFL AND MEDIAL PATELLOTIBIAL LIGAMENT RECONSTRUCTION
While the medial patellotibial ligament (MPTL) has not received much attention with regard to patellar stability, recent studies have indicated its role during higher degrees of both flexion and extension.172 The MPTL acts as a secondary restrictor ligament which helps release stress on the MPFL by decreasing the Q angle and further normalizing patellar kinematics.173 Patients who present with hyperlaxity or knee hyperextension combined with extension subluxation and flexion instability could be indicated for this additional stabilizing procedure. Both Nietosvaara and colleagues85 and Brown and Ahmad174 have described a dual MPTL and MPFL reconstruction technique using a semitendinosus hamstring graft. More recently Hinckel and colleagues172 described a combined MPFL and MPTL reconstruction, using a graft from the quadriceps tendon to reconstruct the MPFL and one from the patellar tendon to reconstruct the MPTL. In this technique, once the respective grafts have been harvested, a femoral insertion for the graft recreating the MPFL is fluoroscopically established so that an anchor can be inserted distal to the femoral physeal growth plate. For the MPTL insertion, attachment to the tibia below the joint line and 2 cm medial to the patellar tendon is established fluoroscopically just above the physeal growth plate on the proximal epiphysis.19,175 The MPTL graft is sutured first with the knee at 90° of flexion to establish tension similar to that of the patellar tendon.176 Then, the knee is placed in 30° of flexion to fix the MPFL graft to the medial patella to prevent excessive lateral translation of the patella.
PATELLAR TENDON TRANSFER
Patellar tendon transfer with proximal realignment is a technique used in particularly young patients to address cases of patellofemoral instability involving concomitant bony or anatomic abnormalities. This procedure is effective for young children with substantial amounts of remaining growth as it better mimics native anatomy than other realignment procedures and does not require bony remodeling.152-154 It is important to familiarize with surgical techniques to address malalignment issues in young patients as neglected alignment issues can lead to worsening of trochlear dysplasia and instability, which are very difficult to treat later on when patients are older.153
The patellar tendon transfer technique (Figure 9), as described by Gordon and Schoenecker,177 starts with an extensive lateral retinacular release. The patellar tendon is then released from its distal insertion at the TT so that it can be moved medially without moving it inferiorly. After confirming patellar tracking and alignment by flexing the knee from 0° to 90° with the graft in place, the patellar tendon graft is secured with multiple nonabsorbable horizontal sutures.177 Of note, in skeletally mature patients, a TT osteotomy is used to accomplish the same goal. This osteotomy has been shown to improve both patellar height and TT-TG distance in skeletally mature patients, but is contraindicated in skeletally immature patients.92,178

Continue to: Initial studies conducted on patellar tendon...
Initial studies conducted on patellar tendon transfer have positive outcomes.179 At a mean follow-up of 5.1 years, patients reported a decrease in pain and increased the ROM and activity, and only 1 reported a postoperative redislocation.179 In more recent studies, both Benoit and colleagues36 and Garin and colleagues58 reviewed cases of patellar instability treated with patellar tendon transfer to address concomitant patellar alignment and anatomic abnormalities. They reported good functional, clinical, and radiographic outcomes with 12.5% and 16% recurrence rates, respectively.36,58 They also noted radiographic improvements in femoral sulcus angle, particularly in younger patients, which indicate this procedure is effective in addressing bony abnormalities that can result from neglected malalignment issues.36,58,154
QUADRICEPSPLASTY
Quadricepsplasty is a lengthening and remodeling technique not frequently used in the pediatric population. The goal of this procedure in patients with significant amounts of growth remaining is to reposition the patella to ameliorate trochlear remodeling and prevent worsening symptoms and anatomic abnormalities.36 A quadricepsplasty accomplishes this by de-rotating and/or lengthening the extensor mechanism and may or may not involve a concomitant MPFL reconstruction. This procedure is particularly effective in young patients who experience obligatory dislocation.60,72 Several quadricepsplasty techniques have been described including Thompson, Curtis and Fisher, Judet, Stanisavljevic, and V-Y technique.180-186 Most techniques initially involve sharp dissection of the vastus medialis and lateralis from the rectus femoral tendon. A tongue is then fashioned out of the rectus femoral tendon. Once the vastus medialis and lateralis are detached from the margins of the patella, the knee is extended, and the distal ends of the vasti are sutured to the tongue of the rectus tendon. Effective extension facilitates flexion to 90°.184 The authors recommend a modification of this technique in which a Z lengthening of the quadriceps tendon is performed after the vastus lateralis is removed distally from the patella and the quadriceps tendon.
Several series and case reports evaluating quadricepsplasty in adult patients report positive outcomes with most patients achieving good or excellent flexion with minimal complications.183,185,187-189 Reports on quadricepsplasty used to treat conditions other than patellofemoral instability in children have reported similar positive outcomes.190-192 As quadricepsplasty for patellar instability is relatively rare in pediatric patients, there is not much relevant literature. However, Kocon and colleagues193 reported results of quadricepsplasty and quadricepsplasty combined with the modified Galeazzi procedure in 8 children (10 knees) with a mean follow-up of 3.25 years. Seventy percent of cases resulted in stabilization and correction of patellar position, and only 2 postoperative redislocations were noted.193 Additionally, in a study evaluating 6 patients suffering from patellar instability, 2 of whom were obligate dislocators, quadricepsplasty resulted in patellar stability, satisfaction, and near normal gait patterns.194
Figure 10 shows the surgical algorithm used for patellar instability characteristics.

CONCLUSION
Patellofemoral joint stability relies on a complex interplay of musculotendinous units, ligaments and the osteocartilaginous morphology of the patellofemoral joint. Patellar instability in pediatric patients is different from adults. Having an in-depth understanding of the remodeling potential, the insertion sites for the MPFL and its relationship to the physis are of utmost importance when planning surgery. Reducing and maintaining the patella within the patellofemoral joint early enough can allow for remodeling of the patella and/or the trochlea to provide for lasting stability. Appropriate surgical principles, such as tensioning, can help both prevent continued pain and minimize future complications.
ABSTRACT
Patellar instability in children and adolescents is a challenging subset to treat. Varied forms of instability, ranging from episodic dislocation to fixed dislocation, have been recognized. It is of utmost importance for the treating physician to recognize these different patterns of instability and their associated risk factors, as more complex patterns of instability would require more extensive surgical procedures. Medial patellofemoral ligament (MPFL) reconstruction, by itself, may not suffice or may not be appropriate for the more complex instability patterns. Appropriate and early treatment of such instability in children would allow for functional progression and possible remodeling of the trochlea. However, early treatment has the associated risk of growth disturbances when surgical procedures are performed around open physis or if adult-type bony procedures are performed in children. Recent knowledge about the relationship between trochlea, MPFL femoral attachment, and distal femoral physis could help to advance safe surgical care for these patients. This article reviews the pathophysiology, risk factors, and the existing classification systems for patellar instability in children and adolescents. It focuses on varied surgical techniques, which are unique to the pediatric population, and summarizes the outcomes of these surgical techniques.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
In a prospective 2-year study of Finnish children, the annual incidence rate of patellar instability was 43/100,000 pediatric population.1 In patients 9 to 15 years of age, the incidence was approximately 1/1000.1 In another study, patients at highest risk for a first-time patellar dislocation were females aged 10 to 17 years.2 In a study in patients with traumatic hemarthrosis, 36% in the younger age group (10-14 years) and 28% in the older age group (15-18 years) had sustained patellar dislocation. In contrast, 22% in the younger age group and 40% in the older age group had sustained an anterior cruciate ligament tear.3
Approximately one-half of patients who dislocate their patella suffer from long-term complications.4,5 These complications include recurrent instability, patellofemoral pain, osteochondral lesions, and eventual arthritis.1,4,5 Young, active individuals are more prone to these issues.6 Also, 39% or more of patellar dislocation patients have an associated osteochondral fracture that might influence the management.1 Thus, patellar instability in young patients is an area of concern.
DEVELOPMENTAL ANATOMY
At 4-week gestation, the patellofemoral joint is an ectodermal sac filled with mesenchyme of the somatic mesoderm.7 Mesenchymal condensations then appear at 4 to 5 weeks gestation, followed by chondrification of both the femur and patella.7 The joint space is present by 6 weeks, and the patellar and distal femoral condyles are present at 7 weeks gestation.7 By 8 weeks gestation, the basic knee anatomy resembles that of an adult with the chondroepiphysis forming the articular surfaces of the femur, tibia, and patella.7 By this time, the extensor mechanism is formed, and active joint motion has begun, facilitating the development of the trochlear sulcus.7 The secondary ossification center in the distal femoral epiphysis forms around 36 weeks gestation.8 Postnatally, both the patella and distal femur grow through endochondral ossification.9,10
The patella is the largest sesamoid bone in the human body.11 The patella begins as a dense consolidation of cells that differentiate as the quadriceps mechanisms develop.12,13 The patellar anlage becomes distinguishable within the quadriceps tendon around 7.5-week gestation.12 The morphology of the patella conforms to the distal femur.12 The patella molds or re-models as the knee begins to move in response to mechanical stresses.7 The patella increases in relative size during the first 6 months of gestation, then enlarges proportionately to the rest of the bones.7 Ossification begins around 3 years of age for females and 4 to 5 years of age for males.8,14 The ossification center may appear irregular as it rapidly expands.14 Ossification proceeds in a proximal to distal direction, thus giving a spurious estimation of patellar height on radiographs in children. The overall morphology of the cartilaginous patella during development is comparable to the final mature shape.14 Abnormal contact stresses on the articular surface of the patella during skeletal immaturity can lead to deformation.7
Ultrasonographic measurements in normal patients show that trochlear groove (TG) morphology is present early and becomes more radiographically apparent as distal femoral ossification is completed.15 Anatomic dissections of aborted fetuses have verified the morphology of the TG as it remains constant during growth and the groove morphology is the same for both fetuses and adults.16 An ultrasound study performed on patients aged 12 to 18 years showed the cartilaginous sulcus angle (CSA) remained constant throughout all age groups (146°).17 The CSA however, differed in patients who suffered a patellar dislocation (average, 164°; range, 154°-195°) compared with normal knees (average CSA, 145°; range, 131°-158°).15,17,18 The osseous sulcus angle, on the other hand, appears flat at birth and the TG deepens with age. This increase in depth is more of a reflection of progressive ossification of a well-formed cartilaginous trochlea, rather than a true deepening of the sulcus.17 Thus, the axial radiographic view of the patella provides misleading information about the sulcus angle in children and should not be used to define trochlear morphology.
Continue to: MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
The medial patellofemoral ligament (MPFL) functions to limit the lateral translation of the patella.19 The attachment sites on the femur and patella for the MPFL have been studied in children.20-23 Cadaveric dissections in specimens aged 2 to 11 years have noted the patellar attachment to be an average of 12 mm in length with the midpoint approximately 5 mm superior to the mid-pole of the patella.22 The patellar footprint of the MPFL insertion was a mean 41% of the entire patellar length.22
It is important to be aware of the characteristic anatomy of the MPFL, as fixation points should mimic the anatomic insertion as best as possible while also avoiding violation of the nearby physis. The MPFL originates between the adductor tubercle and the medial femoral epicondyle just distal to the distal femoral physis and attaches to the superomedial aspect of the patella.20-25 In relation to the physis in pediatric patients, the midpoint of MPFL insertion has been measured to be 4 mm to 9 mm distal to the femoral physis.21,24,25 These measurements represent averages as cadaveric studies have reported that some part of MPFL femoral insertion extends proximal to the distal femoral physis.21 A recent report of physeal injury to the posterior distal femoral physis during MPFL reconstruction leading to femoral flexion deformity highlights the importance of physeal-respecting surgery.26
TROCHLEA AND ANTERIOR DISTAL FEMORAL PHYSIS
The relationship between the proximal aspect of the trochlea and the anterior distal femoral physis has been recently studied in 175 knees with dysplastic trochlea.27 Based on magnetic resonance imaging evaluation, the lateral aspect of the trochlea extended proximal to the anterior distal femoral physis in 13% of patients and was at the level of the anterior physis in another 13% of patients (Figure 1).27 Hence, a cautious approach is recommended for any surgery to address trochlear dysplasia or trochlear bump in younger patients to prevent iatrogenic injury to anterior distal femoral physis and resultant genu recurvatum. The distance between the trochlea and the physis increased with increasing age.

LIMB ALIGNMENT
Physiologically, the quadriceps angle (Q angle) changes through the course of growth. As children begin standing and walking, they stand with their feet wider apart and in genu varum.28 Physiologic genu varum can reach 15°.28 This degree lessens during the first 1.5 to 2 years of life, transitioning to physiologic valgus of nearly 12° by 3 years of age.28 Genu valgum, thereafter, gradually decreases to reach the adult value of around 7° to 8° by age 7 years.28 Increased genu valgum is a risk factor for patellar instability. In skeletally immature patients, correction of genu valgum through guided growth may be desirable in patients undergoing patellar stabilization surgery (Figures 2A, 2B).29

PATHOPHYSIOLOGY OF PEDIATRIC PATELLAR DISLOCATION
TROCHLEAR DYSPLASIA
Trochlear dysplasia is an abnormal shape and depth of the TG.30 Up to 96% of patients with patellar dislocation have trochlear dysplasia.30-33 In a study of patellar instability in children, at least 1 of the 3 signs of trochlear dysplasia (the crossing sign, supratrochlear bump, and double contour sign) was present on lateral radiographs.34 In another study on the growth of trochlear dysplasia in children and adolescents, all grades of trochlear dysplasia were present at all ages (ie, the dysplasia was most likely present at birth and did not necessarily worsen with age and growth).35 The linear dimensions of lateral and medial condylar height as well as trochlear bump increased with age but both the sulcus angle and shape of the trochlea did not change significantly.35 Remodeling of a dysplastic trochlea can happen if the patella is stabilized and appropriately located at a younger age, preferably before 10 years of age.36,37
Continue to: PATELLAR HEIGHT
PATELLAR HEIGHT
The role of patellar height in patellar instability has been well established.38 In patients with patella alta, the patella remains proximal to the TG during the greater arc of knee motion, which predisposes it to patellar instability. Calculation of patellar height in children could be challenging due to incomplete ossification, as well as asymmetric ossification of the patella and the tibial tubercle (TT). Since the patella ossifies from proximal to distal, most radiographic methods that measure the patellar height from the distal aspect of the patella provide a spurious elevation of the measurement.
The Caton-Deschamps (CD) method measures the length of the patellar articular surface and the distance from the inferior edge of the articular surface to the anterosuperior corner of the tibial plateau.39 A ratio >1.3 signifies patella alta. The CD ratio has been verified as a simple and reliable index for measuring patellar height in children.40 Two other methods have been described for determining patellar height in children.41,42 Based on anteroposterior (AP) radiographs of the knee in full extension, Micheli and colleagues41 calculated the difference between the distance from the superior pole of the patella to the tibial plateau and the length of the patella. A positive difference signified patella alta. The Koshino method involves the ratio between a reference line from the midpoint of the patella to the midpoint of the proximal tibial physis and a second distance from the midpoint of the distal femoral physis to the midpoint of the proximal tibial physis on lateral knee radiographs.42 Normal values range from 0.99 to 1.20 with the knee in >30° flexion, in children 3 to 18 years of age.
HYPERLAXITY
In contrast to adults, children have increased levels of collagen III compared with collagen I, which is responsible for tissue elasticity.43 Tissue elasticity leads to increased joint mobility, which is more common in children. Joint hypermobility or hyperlaxity has to be differentiated from symptomatic instability. The traditional Beighton score identifies individuals as having joint hypermobility with a score of 5/9 or higher in school-aged children.44-46 Smits-Engelsman and colleagues44 suggested using stricter criteria with scores of 7/9 or higher being indicative of hyperlaxity in school-aged children. A study of 1845 Swedish school children noted that females have a higher degree of joint laxity.45 Maximal laxity was noted in females at 15 years of age.45 Hyperlaxity has been demonstrated to be greater on the left side of the body44 and can be part of generalized syndromes including Down’s syndrome, Marfan’s syndrome, or Ehlers-Danlos syndrome.
LIMB TORSION
Staheli and colleagues47 described the normative values of a lower extremity rotational profile, including femoral anteversion and tibial torsion. Children normally have increased femoral anteversion, which decreases with growth. Miserable malalignment is a term used to denote increased femoral anteversion and increased external tibial torsion.48,49 These rotational abnormalities can increase the Q angle and the lateral forces on the patella. Femoral anteversion or internal rotation of the femur of 30° significantly increases strain in all areas of the MPFL.48 This increased strain may lead to MPFL failure and patellar instability.48 Increased internal rotation of the femur also increases contact pressure on the lateral aspect of the patellofemoral joint.48 Miserable malalignment frequently manifests following a pubertal growth spurt and may require femoral and tibial osteotomy.50
SYNDROMIC ASSOCIATIONS
Several syndromes have patellar instability as a part of their manifestation. The more common syndromes include nail-patella syndrome, Kabuki syndrome, Down’s syndrome, and Rubinstein-Taybi syndrome.51-54 Other syndromes less commonly associated with patellar instability include Turner syndrome, patella aplasia, or absent patella syndrome. Since many patients with syndromic patellar instability are functionally limited, they may not require an aggressive approach to treatment. When treating these patients, it is important to recognize the unique features of a specific syndrome, which may affect the anesthesia risk profile, management decisions, rehabilitation, and prognosis.
Continue to: MPFL TEAR PATTERN
MPFL TEAR PATTERN
The MPFL serves as an important constraint to the patella to prevent lateral dislocation, primarily during the first 20° to 30° of knee flexion.55,56 Injury to the MPFL is noted in over 90% of patients who suffer a patellar dislocation.57 The location of MPFL tears in pediatric patients is variably reported at the patellar attachment (10%-61%), femoral attachment (12%-73%), both (12%-35%) or mid-substance (2.5%-15%).25,57 The most common tear patterns in pediatric patients are tears at the patellar attachment.25,57 This tear pattern may be accompanied by an avulsion fracture of the medial rim of the patella, though this fracture, being extra-articular, seldom needs treatment.
CLASSIFICATION
While several authors have established extensive classification systems of patellar dislocation based on both clinical and radiographic presentation and reviews of the literature, a single classification system has not been recognized as the gold standard. In this section, in addition to presenting our preferred methods of classification, we will review some of the more recent and extensive classification systems for patellar dislocation and patellar instability.
Dejour and colleagues31 initially used both the presence of patellofemoral anatomic abnormalities and pain to define 3 types of patellar instability: major, objective, and potential patellar instability. Major patellar instability indicates that the patient has experienced more than 1 documented dislocation, objective instability involves one dislocation in addition to an associated anatomic abnormality, and potential patellar instability refers to cases in which the patient has radiographic abnormalities and patellar pain.31 Garin and colleagues58 more simplistically divided patellar dislocation patients into 2 groups: major (permanent or habitual) dislocation of the patella and recurrent dislocation. Sillanpaa59 stressed the distinction between first-time dislocation and recurrent dislocation specifically in the context of acute injuries. These classification systems were formulated with adults as the most relevant population; however, classifications targeted specifically to pediatric patients have recently been presented in the literature.
Historically, pediatric patella dislocations were simply categorized as traumatic or congenital.60 In 2014, Chotel and colleagues61 focused on classifying patellar dislocation by extensively reviewing anatomic, biomechanical, pathophysiological, and clinical patterns seen most commonly in children. They included 5 categories: congenital dislocation, permanent dislocation, habitual dislocation during knee flexion, habitual dislocation during knee extension, and recurrent dislocation; however, they did not address traumatic dislocations.61 Congenital dislocation is a rare condition, typically presenting at birth, which produces a pattern of functional genu valgum.62 Permanent dislocation typically presents after the child has started walking, but before the age of 5 years.61 The 2 variations of habitual dislocation typically present between ages 5 and 8 years.61 The final category is the most common and typically occurs during pre-adolescence or adolescence as a result of an atraumatic or trivial traumatic event or sports injury.1 Using more specific terminology, Hiemstra and colleagues63 modeled a classification system based on the traumatic, unilateral, bankart lesion, surgery (TUBS)/atraumatic, multidirectional, bilateral, rehabilitation, inferior shift (AMBRI) for shoulder dislocation classifications. The patellar dislocation system is used to identify 2 distinct subsets of patients in the patellofemoral instability population. One subset is defined by the acronym WARPS (weak, atraumatic, risky anatomy, pain, and subluxation), the other is STAID (strong, traumatic, anatomy normal, instability, and dislocation).64 Patients categorized by the WARPS acronym tend to experience atraumatic onsets of patellofemoral instability and demonstrate anatomic issues that increase this instability. These underlying anatomic issues include valgus alignment, ligamentous laxity, rotational abnormalities, shallow and short TG, and patella alta. On the other hand, STAID patients describe a traumatic dislocation event and do not have underlying anatomic abnormalities that predispose them to instability.64
Taking into account these previous classifications, Frosch and colleagues65 added specific pathologies including “instability,” “maltracking,” and “loss of patellar tracking,” in addition to both clinical and radiographic factors to define 5 types of patellar dislocation and their specific treatment recommendations.65 Type 1 involves simple dislocation with neither maltracking nor instability and a low risk of redislocation.65 Type 2 is defined as primary dislocation followed by subsequent high risk of dislocation and no maltracking.65 Type 3 is divided into 5 subcategories of instability and maltracking issues involving soft tissue contracture, patella alta, pathological tibial tuberosity, and TG distance.65 Type 4 is defined as the highly unstable “floating patella,” and type 5 involves patellar maltracking without instability 65. In terms of treatment, conservative rehabilitation is recommended for type 1 whereas MPFL reconstruction tends to show positive outcomes for both types 2 and 3.66-70
Continue to: Parikh and Lykissas recently published...
Parikh and Lykissas recently published a comprehensive classification system of 4 defined types of patellar dislocation in addition to voluntary patellar instability and syndromic patellar instability (Table).60 The 4 types are Type 1, first-time patellar dislocation; Type 2, recurrent patellar instability; Type 3, dislocatable; and Type 4, dislocated. Type 2 is further subdivided into Type 2A, which presents with positive apprehension signs, and Type 2B, which involves instabilities related to anatomic abnormalities.60 A distinction is also made between Type 3A or passive patellar dislocation and Type 3B habitual patellar dislocation.60

The classification system proposed by Green and colleagues is more simplified with 3 main categories (Table) of pediatric patellar dislocation: traumatic (acute or recurrent), obligatory (either in flexion or extension), and fixed laterally.71,72 The acute traumatic categorization refers to patients who experienced an initial dislocation event due to trauma whereas recurrent traumatic involves repeated patella dislocations following an initial incident. Studies report that between 60% to 70% of these acute traumatic dislocations occur as a result of a sports-related incident.2,33,73 Obligatory dislocations occur with every episode of either knee flexion or extension, depending on the subtype. Obligatory patella dislocation in flexion typically cannot be manipulated or relocated into the trochlea while the knee is fixed but does reduce into the trochlea in full extension. Fixed lateral dislocations are rare, irreducible dislocations in which the patella stays dislocated laterally in flexion and extension. These dislocations often present with other congenital abnormalities. Each of these categories can be further specified as syndromic if the dislocation is associated with genetic or congenital conditions including skeletal dysplasia, Ehlers-Danlos syndrome, cerebral palsy, Marfan disease, nail-patella syndrome, Down syndrome, Rubenstein-Taybi syndrome, and Kabuki syndrome.51-54,61,74-76
SURGICAL TECHNIQUES IN SKELETALLY IMMATURE PATIENTS
While nonsurgical, conservative treatment involving physical therapy and activity modification is recommended for most patients who experience first-time traumatic patellar dislocations, many patients experience complicating factors that indicate them for surgery. These factors include recurrent dislocation, risk factors for patellofemoral instability, underlying malalignment issues, and congenital deformities. When evaluating these factors, particularly patellofemoral instability, the authors recommend assessing osteochondral lesions, age, skeletal maturity, number of previous dislocations, family history, and anatomic risk factors.2,5,77-79 Extra care should be taken when considering surgical treatment for skeletally immature patients at elevated risk for recurrent instability as the risk of cartilage damage in these cases is high.80-82
Recently, there has been a reported increase in surgical treatment for patellar instability in the skeletally immature.83 This finding may be attributed to heightened awareness of factors that indicate patients for surgical treatment and increased familiarity of surgeons with newer techniques.83 Many surgical techniques have been described to address patellar instability involving both soft-tissue procedures and bony corrections.84 In this article, we discuss the various surgical techniques for MPFL reconstruction, quadricepsplasty, and distal realignment. These procedures can be paired with any number of additional procedures including, but not limited to, lateral retinacular release or lengthening, chondroplasty, TT osteotomy (in skeletally mature patients), and removal of loose bodies.83
There is a need for more comprehensive studies, particularly randomized controlled trials, to evaluate the outcomes for both surgical and nonsurgical treatments for first-time dislocations. In the current literature, only very recently have surgical treatments shown outcomes that are more positive. In 2009, Nietosvaara and colleagues85 conducted a randomized controlled trial of nonoperative and operative treatment of primary acute patellar dislocation in both children and adolescents. After a long-term mean follow-up of 14 years, there was not a significant difference between the groups in recurrent dislocation and instability, subjective outcome, or activity scores.85 In a subsequent review of 5 studies including 339 knees, Hing and colleagues86 also found similar results in both the operative and nonoperative cohorts at risk of recurrent dislocations, Kujala scores, and reoperations. However, a recent systematic review comparing redislocation rates and clinical outcomes between surgical and conservative management of acute patellar dislocation reported more positive outcomes for the surgical cohort.87 This review included 627 knees, 470 of which received conservative management, 157 of which received operative treatment. The conservative cohort was followed for an average of 3.9 years and had a 31% rate of recurrent dislocation while the surgical group was followed for a mean 4.7 years and experienced a 22% redislocation rate.87 This study indicates that operative management for acute first-time dislocations may be the preferred treatment option.
Continue to: A potential reason some of these studies...
A potential reason some of these studies did not show any significant difference between the operative and nonoperative cohort could be that the surgical cohorts included a wide range of procedures including lateral releases and MPFL repairs. Recent publications have indicated that these techniques do not produce overall positive outcomes. While each surgical treatment plan is unique depending on the patient; recently, MPFL reconstruction has been shown to have better outcomes than both nonoperative management and simple medial repair and/or lateral
release.67,88-90
MPFL RECONSTRUCTION
INDICATIONS/OVERVIEW
The MPFL is an important stabilizer for the knee that primarily resists lateral translation of the patella. Damage to the MPFL is very common in acute patellar dislocations with up to 90% of first-time dislocations resulting in injury to the MPFL.91,92 Historically, simple medial and/or lateral MPFL repairs have not been shown to improve patellofemoral kinematics significantly and often result in recurrence.90,93 To address this issue, during the past few decades, numerous MPFL reconstruction techniques have been developed to reconstruct a stronger ligament with the same kinematics as the anatomic MPFL.2,19,69,81,94-106 The ultimate goal of MPFL reconstruction is to reestablish the anatomic “checkrein” to guide the patella into the trochlea between 0° and 30° of knee flexion.107,108 An essential secondary surgical goal in skeletally immature patients is to avoid damaging the distal femoral physis.
There are many variations in both the grafts used to replace the MPFL and the means by which to secure them. The ones discussed below include free semitendinosus or gracilis autografts or grafts constructed from a pedicled adductor, patellar, or quadriceps tendon.69,105,109 While not used as frequently, allografts have also been used.110 Methods to secure these grafts in osseous tunnels include suture anchors or tenodesis screws. Incomplete osseous sockets or medial-sided bone tunnels have also been used as a method to decrease patellar fractures as they preserve the lateral patellar cortex.111-114
DOUBLE-BUNDLE HAMSTRING AUTOGRAFT
The technique most often used by the author is a double-bundle hamstring autograft harvested from either the semitendinosus or the gracilis secured by short patellar and femoral sockets (Figure 3). After harvesting the hamstring graft from a posteromedial incision, an approximately 90-mm graft is prepared with Krackow stitches to secure 15 mm of the tendon in each socket.115 Lateral radiographs are used intraoperatively to ensure the guidewire for the femoral drill hole falls along the posterior cortex of the diaphysis of the femur while AP radiographs confirm placement distal to the physis. It is important to take both AP and lateral radiographs intraoperatively due to the concave curvature of the distal femoral physis. This unique anatomy can make a point that is located distally to the physis on the AP view appear on or proximal to it on the lateral cross reference view.24,116 For the patellar socket, 2 short sockets are made in the superior half of the patella. Once the sockets have been drilled, the graft is adjusted so that the patella stays seated in the center of the trochlea between 20° and 30° of flexion. This anchoring is accomplished by securing the graft while the knee is kept at 30° of flexion. Proper tension is confirmed by ensuring that the graft does not allow lateral patella movement over one-fourth the width of the patella in extension while crepitation must not appear throughout the ROM.92

QUADRICPETS TENDON TRANSFER
A combination of techniques by Steensen and colleagues,105 Goyal,109 Noyes and Albright,117 and Pinkowsky and Hennrikus118 describe an MPFL reconstruction in which the proximal end of a small medial portion of the quadriceps tendon is released and then attached to the medial epicondyle through a subcutaneous tunnel (Figure 4). This technique is particularly useful for cases in which the extra strength provided by the bone-quadriceps tendon is necessary to correct more severe dysplasia. Leaving the distal end of the quadriceps tendon intact at its patellar insertion, a graft of about 8 mm x 70 mm thickness is harvested from the tendon. The free distal end of the tendon is then run anatomically through the synovium and retinaculum to be either sutured to the medial intermuscular septum at the medial femoral epicondyle or fixed in femoral tunnel using interference screw.105,109,118 The placement of the femoral fixation point is essential to ensure positive surgical outcomes. If the graft is secured too anteriorly, it may be too loose in extension and too tight in flexion, both of which can lead to postoperative pain, loss of normal kinematics, and overload of the medial patellofemoral cartilage.119-121 Once the ideal placement of the femoral fixation point has been confirmed by intraoperative radiographs, the graft is secured with a small absorbable suture.122,123 While this technique has good clinical results, the longitudinal scar that results from graft harvesting is cosmetically unappealing, and it is technically challenging to harvest a consistent strip of the quadriceps tendon. To address some of these concerns, Fink and colleagues124 described a new harvesting technique that produces more consistent grafts and requires a smaller incision.

Continue to: ADDUCTOR MAGNUS TENDON TRANSFER
ADDUCTOR MAGNUS TENDON TRANSFER
This technique is a double-bundle MPFL reconstruction that uses a pedicled graft of the distal adductor magnus tendon and suture anchors or incomplete osseous sockets to recreate the MPFL anatomically (Figure 5). Avikainen and colleagues96 and Sillanpää and colleagues125 described this procedure as a progression from the original single-strand adductor magnus transfer technique. First, maintaining the distal insertion, a graft of approximately 14 cm to 18 cm is harvested from the adductor tendon and then passed through a subcutaneous tunnel between the distal vastus medialis obliquus and the superficial joint capsule. The graft is then looped at the medial patella so that the distal bundle runs back to the adductor tubercle.125 With the knee at 30° of flexion to assure proper tension, the graft is secured at both the patella and near the adductor tubercle with suture anchors.125 Hambridge and colleagues126 compared a similar adductor magnus transfer with other pedicled techniques including bone-quadriceps tendon autograft and bone-patellar tendon allograft and found positive results for all 3 methods of reconstruction.

HEMI-PATELLA TENDON TRANSFER
In a similar technique to the adductor tendon transfer, the medial section of the patellar tendon is harvested from the TT and run from its proximal insertion at the medial patella to the medial femoral attachment via a subcutaneous tunnel. The free end of the graft is then secured with suture anchors or incomplete osseous sockets with the knee at 30° of flexion.127
HAMSTRING GRAFT WITH ADDUCTOR TENDON AS A PULLEY
Several techniques opt to use a more dynamic model of MPFL reconstruction in which the adductor tendon or medial collateral ligament (MCL) is used as a pulley for the hamstring graft (Figure 6).128,129 The site of the pulley approximates the normal attachment of the MPFL to the femur and so acts as an effective anatomic replica of the MPFL origin. A semitendinosus graft is harvested and is prepared with continuous sutures, and 2 tunnels to secure the graft are drilled into the patella. The graft is then run subcutaneously from the medial side of the patella to the adductor magnus tubercle into which an osteoperiosteal tunnel is drilled at its distal femoral insertion. The graft is looped through the adductor tunnel and secured with sutures. Proper knee kinematics was ensured by placing the knee at 30° of flexion as the ends of the tendon are secured to the patella.114,130

HAMSTRING GRAFT WITH MCL AS A PULLEY
The MCL can also be used as a pulley rather than the adductor tendon. The semitendinosus graft is harvested and prepared and the patella drilled as it is in the previous technique. The MCL was fashioned into a pulley by making a slit in its posterior one-third. The semitendinosus graft is looped through this slit, and both ends of the graft are held in place with suture anchors on the surface of the patella.129
ADDITIONAL PROCEDURAL COMBINATIONS
Depending on the needs of the individual patient, MPFL reconstruction, and other patellar stabilization techniques can also be combined with additional procedures. Arshi and colleagues83 conducted a review of 6190 adolescents surgically treated for patellar instability and reported the most common additional procedures performed at the time of the stabilization. They found 43.7% of the population underwent lateral retinacular release, which while not effective as an isolated technique to treat patellar instability, has often been used in combination with MPFL reconstruction.131-133 There is currently a lack of consensus regarding the success of adding a lateral release to the reconstruction. Some studies report no difference while others report a decrease in stability after lateral release.90,134-136 While lateral retinacular release has been shown to decrease the force required to displace the patella, it can be surgically indicated in certain patients undergoing MPFL reconstruction.131 The authors advocate that if the lateral retinaculum is tight such that centralized patellar tracking is inhibited following the reconstruction, or if the patella cannot be pushed passively from a laterally tilted position to the neutral horizontal position, lateral retinacular lengthening should be performed to improve kinematics.132
Continue to: Arshi and colleagues...
Arshi and colleagues83 also reported a high rate of cartilage procedures, with chondroplasty performed in 31.1% and chondral fragment/loose body removal in 10.2%. These statistics suggest that a significant level of cartilage damage has occurred by the time of surgery.83
COMPLICATIONS
As MPFL reconstruction techniques have only recently been popularized and developed, there are not many comprehensive studies evaluating the outcomes and complications associated with these procedures. However, in the current literature, there is a general consensus that patients usually experience positive short-term clinical outcomes and relatively low complication rates.68,77 In one of the largest retrospective cohort studies of pediatric patients undergoing MPFL reconstruction, Parikh and colleagues114 reported both the type and rate of complications. They found complications occurred in 16.2% of patients, and the most common complications were recurrent patellar instability, patellar fractures, patellofemoral arthrosis, motion deficits, and stiffness with over half classified as avoidable. Most of these complications were due to technical errors with episodes of recurrent instability only reported in 4.5% of patients.114 In a comprehensive meta-analysis of MPFL reconstruction studies, Shah and colleagues137 reported a complication rate of 26% in both pediatric and adult patients. The cohort was not stratified by age, yet complications were similar to those reported by Parikh and colleagues,114 including pain, loss of knee flexion, wound complications, and patellar fracture.137
As indicated by the frequency of technical complications reported by Parikh and colleagues,114 extra caution should be taken in the operating room to minimize potential errors. In techniques that require drilling of femoral sockets, proper length for and placement of the graft is essential to reestablish proper kinematics. Studies have reported that placing the femoral socket too proximally can result in loss of ROM during flexion and increased compressive forces across the patella.138 A graft that is too short can have similar negative outcomes, and a graft that is too long can result in recurrent instability. Positioning the graft while the knee is in 30° of flexion can help ensure the proper length and tension is achieved. Once the graft is in place, it is important to ensure the ROM and isometry before completing the fixation.72 It is also essential to be vigilant about potential violation of the physes and subsequent growth disturbances. To establish the safest angles for drilling the distal femoral epiphysis for graft placement, Nguyen and colleagues139 conducted a study using high-resolution 3-dimensional images of cadaveric distal femoral epiphyses. By recording which tunnels disrupted the physis before reaching 20 mm of depth, the authors concluded that it is safest to drill distally and anteriorly at an angle between 15° and 20°.139 This technique should minimize damage to the physis, notch, and distal femoral cartilage and decrease potential complications.139
OUTCOMES
In general, the literature reports positive outcomes for MPFL reconstruction—in both studies that address a specific technique and all-encompassing studies. Outcomes are typically reported as Kujala and Tegner scores, results from clinical examinations, and rates of subsequence recurrences. Several recent studies have also evaluated the ability of MPFL reconstruction to restore proper kinematics. Edmonds and colleagues140 evaluated the difference in patellofemoral joint reaction forces and load experienced by 3 groups of adolescents: a cohort treated with MPFL reconstruction, a cohort treated with soft-tissue realignment of the extensor mechanism (the Insall method), and controls. While both surgical techniques were able to restore medial constraints to the patella, the study showed that only the MPFL reconstruction cohort experienced joint reaction forces that were analogous to the control group. In comparison, the cohort that was treated with soft-tissue realignment alone experienced higher patellofemoral joint reaction forces and did not regain normal joint mechanics.140 These results can be used to advocate for the further use of MPFL reconstruction as an effective anatomic replacement of the native ligament. Radiographic studies have similarly reported MPFL reconstruction as an effective means to restore anatomic normality. Fabricant and colleagues141 conducted a radiographic study in which patella alta was corrected to normal childhood ranges in patients who underwent MPFL reconstruction technique using a hamstring autograft. Lykissas and colleagues142 corroborated these results with another radiographic study that reported small but significant decreases in the Blackburne-Peel index and CD index following MPFL reconstruction in 25 adolescents. As correction of patella alta allows the patella to rest in a deeper, more secure position in the TG, these results indicate that effective early MPFL reconstruction can correct for patellar anatomic abnormalities that could be future risk factors.143,144 Several studies have also reported outcomes addressing specific MPFL techniques; these are reported and discussed in this article.
OUTCOMES BY TECHNIQUE
HAMSTRING AUTOGRAFT
Reports on outcomes following MPFL reconstructions using hamstring autografts have been particularly promising. A cohort of 21 skeletally immature patients who underwent MPFL reconstruction was evaluated pre- and postoperatively with an average of a 2.8-year follow-up. The authors of the study reported no redislocation events and significant improvement in the Kujala scores, and patients were able to return to athletic activities safely.145 Previous studies report similar positive increases in Kujala scores, subjective patient reports, and lack of subsequent redislocation for patients who underwent either semitendinosus or gracilis autograft MPFL reconstructions. One such study further documented an average patellar inclination angle decrease from 34.3° to 18.6° following MPFL reconstruction.146 However, while the literature typically reports positive Kujala scores and subjective outcomes for the hamstring autograft procedure, a study arthroscopically evaluating patellar tracking immediately following surgery and then at 6 to 26 months follow-up found that patellar tracking correction was not maintained for all patients who underwent this type of MPFL reconstruction.147
Continue to: QUADRICEPS TENDON TRANSFER OUTCOMES
QUADRICEPS TENDON TRANSFER OUTCOMES
Studies specifically evaluating the quadriceps tendon transfer technique for MPFL reconstruction in children are sparse, but authors have reported positive clinical outcomes and low complication rates in adults. After following 32 young adults who underwent this MPFL reconstruction technique for 3 years, Goyal109 reported a significant increase in mean Kujala scores from 49.31 to 91.25 and no complications or redislocation. He argues this type of quadriceps graft has a high success rate because it is anatomically more similar to the MPFL than other grafts and does not require additional patellar fixation.101,109 Similar positive Kujala scores and minimal complications have been reported in adult patient populations.148 Abouelsoud and colleagues149 conducted one of the few studies in skeletally immature patients and reported similarly positive results with no redislocations and significantly improved Kujala scores at a mean follow-up of 29.25 months in their 16-patient cohorts.
ADDUCTOR MAGNUS TENDON TRANSFER
After initially describing this technique in 14 adult patients, Avikainen and colleagues96 followed this cohort and reported positive subjective results and only 1 redislocation. In a more recent study in which the adductor tendon transfer technique was compared with the quadriceps tendon transfer described above and the bone-patellar tendon allograft, Steiner and colleagues69 reported similarly significant improvement in all cohorts in Lysholm, Kujala, and Tegner scores with no redislocations. Additionally, Malecki and colleagues150 followed a cohort of 33 children with 39 knees diagnosed with recurrent patellar dislocation, who underwent MPFL reconstruction using the adductor magnus tendon. After evaluating this cohort functionally and radiographically, the authors reported improvements in Lysholm and Kujala scores, patellar tilt and congruence angles, and peak torque of the quadriceps muscle and flexor.150 However, this cohort did report postoperative redislocations in 36.4% of patients (4 of 11).150
HEMI-PATELLA TENDON TRANSFER
In 2012, in the first randomized controlled trial, Bitar and colleagues67 compared the outcomes of patients who underwent MPFL reconstruction via the hemi-patellar tendon technique with those who were managed nonoperatively with immobilization and physiotherapy after first-time patellar dislocation. At 2-year follow-up, the surgical cohort presented positive results with a significantly higher mean Kujala score (88.9 to 70.8) and no redislocations or subluxations. In contrast, 35% of nonoperative cases presented with recurrences and subluxations over the 2-year period.67
MCL OR ADDUCTOR TENDON AS A PULLEY
Studies have reported good postoperative results and low complication rates for these dynamic techniques.128,129 In terms of kinematics, while hypermobility and patellar height were not fully corrected, improvements in patellar tilt and lateral shift were reported in a cohort of 6 patients with a minimum 4-year follow-up.129 To further evaluate whether the more dynamic pulley reconstruction technique resulted in better outcomes, Gomes and colleagues128 compared the subjective reports, clinical evaluations, and complication rates of patients who underwent MPFL reconstruction with a rigid adductor magnus fixation vs a semitendinosus tendon dynamic femoral fixation. One case in the rigid cohort experienced a subsequent subluxation, while patients in the semitendinosus group had better subjective reports and a higher rate of return to sport.128 More recently, Kumahashi and colleagues151 specifically studied the outcomes of the MCL tendon as a pulley in 5 patients aged 14 to 15 years. They reported similar successful results as no patients experienced recurrence, and all patients exhibited improvement in radiographic measures of patellar tilt and congruence angle, lateral shift ratio, and both Kujala and Lysholm scores.151
While there has yet to be a randomized controlled trial comparing all of these different techniques, there is a general consensus in the literature that patients tend to perform better following MPFL reconstruction vs MPFL repair.
OTHER STABILIZATION PROCEDURES, INCLUDING DISTAL REALIGNMENT
Patients with additional underlying deficits and malalignment issues such as significant trochlear dysplasia, increased TT-TG distance, patella alta, increased Q angle, and/or positive J sign may require stabilization procedures beyond MPFL reconstruction.152,153 TT osteotomies are often used to correct alignment issues in the adult patient population; however, these procedures are typically contraindicated in skeletally immature patients. Alternative realignment procedures for the pediatric population include both proximal and distal realignment, with proximal realignment performed primarily in children under the age of 12 years.153 Many variations on these procedures exist, some of which are no longer regularly performed due to poor reported outcomes. In this article, we discuss several of the techniques, focusing primarily on those that have demonstrated higher success rates.
Continue to: GALEAZZI TECHNIQUE
GALEAZZI TECHNIQUE
One of the first and most famous soft-tissue techniques to address patellar instability was the semitendinosus tenodesis, published by Galeazzi154 in 1922 (Figure 7). This technique stabilizes the patella without altering the TT. In the original technique, a portion of the semitendinosus tendon is harvested with its tibial insertion left intact. The free end of the tendon is then secured with sutures at the periosteal groove of the medial patella.154,155 Fiume156 modified this technique by adding a lateral release and medial retinacular reefing. The most recent addition to this procedure was introduced by Baker and colleagues,157 in which a tunnel is drilled from the medial to the lateral border of the patella. Tension placed on the grafted tendon is used to reposition the patella medially and draw it downward. Preliminary literature on this modified procedure reported fair clinical results with success rates of approximately 75%.155,158-160 A recent study evaluating both the clinical and radiographic outcomes of this technique also indicated that while clinical results were excellent in 62.5% of patients, this technique alone was unsuccessful in fully addressing patellar instability in patients with underlying anatomic abnormalities such as patellar alta.161 In light of these less than ideal reports, the authors no longer recommend this technique for patellofemoral instability cases.

ROUX-GOLDTHWAIT PROCEDURE
The Roux-Goldthwait procedure, first described by both Roux162 and Goldthwait163 in 1888 and 1895 respectively, was later modified in 1985 to involve a lateral release, plication of the medial retinaculum, medial transfer of the lateral patellar tendon without advancement, and advancement of the vastus medialis (Figure 8).164 More recently, Marsh and colleagues152 introduced an addition to aligning the extensor mechanism with the femoral shaft better. In this technique modification, the patellar tendon is split longitudinally, and its lateral half is detached and transferred distally beneath its medial half. The free end is then sutured to the periosteum on the medial side of the tibia.152 With a mean long-term follow-up of 6.2 years, Marsh and colleagues152 reported excellent results in 65%, good in 11%, and fair in 3% of the knees operated on with this modified technique. Of the patients in this cohort whose strength was evaluated, 80% had their strength returned to 90% of preoperative levels in the operated leg.152 While this study and others report improved outcomes, an increasing body of literature has found high rates of recurrence, patella infera, and other complications following the modified Roux-Goldthwait procedure.36,165-171 Also, a study comparing MPFL reconstruction using adductus magnus transfer with the Roux-Goldthwait procedure reported that patients in the MPFL cohort reported less pain postoperatively.150 In addition, whereas the Kujala and Lysholm scores, recurrence rates, patellofemoral angles, and apprehension test results did not demonstrate significant differences between these 2 groups, the MPFL group had significantly fewer abnormal congruence angles, better patellar medialization, and higher peak torque of the hamstring.150

COMBINED MPFL AND MEDIAL PATELLOTIBIAL LIGAMENT RECONSTRUCTION
While the medial patellotibial ligament (MPTL) has not received much attention with regard to patellar stability, recent studies have indicated its role during higher degrees of both flexion and extension.172 The MPTL acts as a secondary restrictor ligament which helps release stress on the MPFL by decreasing the Q angle and further normalizing patellar kinematics.173 Patients who present with hyperlaxity or knee hyperextension combined with extension subluxation and flexion instability could be indicated for this additional stabilizing procedure. Both Nietosvaara and colleagues85 and Brown and Ahmad174 have described a dual MPTL and MPFL reconstruction technique using a semitendinosus hamstring graft. More recently Hinckel and colleagues172 described a combined MPFL and MPTL reconstruction, using a graft from the quadriceps tendon to reconstruct the MPFL and one from the patellar tendon to reconstruct the MPTL. In this technique, once the respective grafts have been harvested, a femoral insertion for the graft recreating the MPFL is fluoroscopically established so that an anchor can be inserted distal to the femoral physeal growth plate. For the MPTL insertion, attachment to the tibia below the joint line and 2 cm medial to the patellar tendon is established fluoroscopically just above the physeal growth plate on the proximal epiphysis.19,175 The MPTL graft is sutured first with the knee at 90° of flexion to establish tension similar to that of the patellar tendon.176 Then, the knee is placed in 30° of flexion to fix the MPFL graft to the medial patella to prevent excessive lateral translation of the patella.
PATELLAR TENDON TRANSFER
Patellar tendon transfer with proximal realignment is a technique used in particularly young patients to address cases of patellofemoral instability involving concomitant bony or anatomic abnormalities. This procedure is effective for young children with substantial amounts of remaining growth as it better mimics native anatomy than other realignment procedures and does not require bony remodeling.152-154 It is important to familiarize with surgical techniques to address malalignment issues in young patients as neglected alignment issues can lead to worsening of trochlear dysplasia and instability, which are very difficult to treat later on when patients are older.153
The patellar tendon transfer technique (Figure 9), as described by Gordon and Schoenecker,177 starts with an extensive lateral retinacular release. The patellar tendon is then released from its distal insertion at the TT so that it can be moved medially without moving it inferiorly. After confirming patellar tracking and alignment by flexing the knee from 0° to 90° with the graft in place, the patellar tendon graft is secured with multiple nonabsorbable horizontal sutures.177 Of note, in skeletally mature patients, a TT osteotomy is used to accomplish the same goal. This osteotomy has been shown to improve both patellar height and TT-TG distance in skeletally mature patients, but is contraindicated in skeletally immature patients.92,178

Continue to: Initial studies conducted on patellar tendon...
Initial studies conducted on patellar tendon transfer have positive outcomes.179 At a mean follow-up of 5.1 years, patients reported a decrease in pain and increased the ROM and activity, and only 1 reported a postoperative redislocation.179 In more recent studies, both Benoit and colleagues36 and Garin and colleagues58 reviewed cases of patellar instability treated with patellar tendon transfer to address concomitant patellar alignment and anatomic abnormalities. They reported good functional, clinical, and radiographic outcomes with 12.5% and 16% recurrence rates, respectively.36,58 They also noted radiographic improvements in femoral sulcus angle, particularly in younger patients, which indicate this procedure is effective in addressing bony abnormalities that can result from neglected malalignment issues.36,58,154
QUADRICEPSPLASTY
Quadricepsplasty is a lengthening and remodeling technique not frequently used in the pediatric population. The goal of this procedure in patients with significant amounts of growth remaining is to reposition the patella to ameliorate trochlear remodeling and prevent worsening symptoms and anatomic abnormalities.36 A quadricepsplasty accomplishes this by de-rotating and/or lengthening the extensor mechanism and may or may not involve a concomitant MPFL reconstruction. This procedure is particularly effective in young patients who experience obligatory dislocation.60,72 Several quadricepsplasty techniques have been described including Thompson, Curtis and Fisher, Judet, Stanisavljevic, and V-Y technique.180-186 Most techniques initially involve sharp dissection of the vastus medialis and lateralis from the rectus femoral tendon. A tongue is then fashioned out of the rectus femoral tendon. Once the vastus medialis and lateralis are detached from the margins of the patella, the knee is extended, and the distal ends of the vasti are sutured to the tongue of the rectus tendon. Effective extension facilitates flexion to 90°.184 The authors recommend a modification of this technique in which a Z lengthening of the quadriceps tendon is performed after the vastus lateralis is removed distally from the patella and the quadriceps tendon.
Several series and case reports evaluating quadricepsplasty in adult patients report positive outcomes with most patients achieving good or excellent flexion with minimal complications.183,185,187-189 Reports on quadricepsplasty used to treat conditions other than patellofemoral instability in children have reported similar positive outcomes.190-192 As quadricepsplasty for patellar instability is relatively rare in pediatric patients, there is not much relevant literature. However, Kocon and colleagues193 reported results of quadricepsplasty and quadricepsplasty combined with the modified Galeazzi procedure in 8 children (10 knees) with a mean follow-up of 3.25 years. Seventy percent of cases resulted in stabilization and correction of patellar position, and only 2 postoperative redislocations were noted.193 Additionally, in a study evaluating 6 patients suffering from patellar instability, 2 of whom were obligate dislocators, quadricepsplasty resulted in patellar stability, satisfaction, and near normal gait patterns.194
Figure 10 shows the surgical algorithm used for patellar instability characteristics.

CONCLUSION
Patellofemoral joint stability relies on a complex interplay of musculotendinous units, ligaments and the osteocartilaginous morphology of the patellofemoral joint. Patellar instability in pediatric patients is different from adults. Having an in-depth understanding of the remodeling potential, the insertion sites for the MPFL and its relationship to the physis are of utmost importance when planning surgery. Reducing and maintaining the patella within the patellofemoral joint early enough can allow for remodeling of the patella and/or the trochlea to provide for lasting stability. Appropriate surgical principles, such as tensioning, can help both prevent continued pain and minimize future complications.
1. Nietosvaara Y, Aalto K, Kallio PE. Acute patellar dislocation in children: incidence and associated osteochondral fractures. J Pediatr Orthop. 1994;14(4):513-515.
2. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121. doi:10.1177/0363546503260788.
3. Abbasi D, May MM, Wall EJ, Chan G, Parikh SN. MRI findings in adolescent patients with acute traumatic knee hemarthrosis. J Pediatr Orthop. 2012;32(8):760-764. doi:10.1097/BPO.0b013e3182648d45.
4. Mäenpää H, Lehto MU. Patellar dislocation. The long-term results of nonoperative management in 100 patients. Am J Sports Med. 1997;25(2):213-217. doi:10.1177/036354659702500213.
5. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120. doi:10.1177/036354658601400204.
6. Cofield RH, Bryan RS. Acute dislocation of the patella: results of conservative treatment. J Trauma. 1977;17(7):526-531.
7. Wasserlauf BL, Paletta GA. Developmental anatomy of the pediatric and adolescent knee. In: Micheli LJ, Kocher MS, eds. The Pediatric and Adolescent Knee. 1st ed. Elsevier; 2006:27-32.
8. Birch JG. Growth and development. In: Herring J, ed. Tachdjian’s Pediatric Orthopedics. 4th ed. Saunders/Elsevier; 2007:3-22.
9. Sarin VK, Carter DR. Mechanobiology and joint conformity regulate endochondral ossification of sesamoids. J Orthop Res. 2000;18(5):706-712. doi:10.1002/jor.1100180505.
10. Maes C, Kronenberg HM. Postnatal bone growth: growth plate biology, bone formation, and remodeling. In: Pediatric Bone. Elsevier; 2012:55-82.
11. Tecklenburg K, Dejour D, Hoser C, Fink C. Bony and cartilaginous anatomy of the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):235-240. doi:10.1007/s00167-005-0683-0.
12. Walmsley R. The development of the patella. J Anat. 1940;74(Pt 3):360-368.3.
13. Fulkerson J, Hungerford D. Disorders of the patellofemoral Joint. In: Normal Anatomy. 2nd ed. Baltimore: Williams & Wilkins; 1990:1-24.
14. Ogden JA. Radiology of postnatal skeletal development. X. Patella and tibial tuberosity. Skeletal Radiol. 1984;11(4):246-257.
15. Nietosvaara Y, Aalto K. The cartilaginous femoral sulcus in children with patellar dislocation: an ultrasonographic study. J Pediatr Orthop. 1997;17(1):50-53.
16. Glard Y, Jouve JL, Garron E, Adalian P, Tardieu C, Bollini G. Anatomic study of femoral patellar groove in fetus. J Pediatr Orthop. 2005;25(3):305-308.
17. Nietosvaara Y. The femoral sulcus in children. An ultrasonographic study. J Bone Joint Surg Br. 1994;76(5):807-809.
18. Nietosvaara AY, Aalto KA. Ultrasonographic evaluation of patellar tracking in children. Clin Orthop Relat Res. 1993;(297):62-64.
19. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 26(1):59-65. doi:10.1177/03635465980260012701.
20. Shea KG, Styhl AC, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: a cadaveric study. Am J Sports Med. 2016;44(11):2833-2837. doi:10.1177/0363546516656366.
21. Shea KG, Polousky JD, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children. J Pediatr Orthop. 2014;34(8):808-813. doi:10.1097/BPO.0000000000000165.
22. Shea KG, Polousky JD, Jacobs JC, et al. The patellar insertion of the medial patellofemoral ligament in children. J Pediatr Orthop. 2015;35(4):e31-e35. doi:10.1097/BPO.0000000000000399.
23. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi:10.1016/j.arthro.2009.12.020.
24. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sport Traumatol Arthrosc. 2011;19(12):2067-2071. doi:10.1007/s00167-011-1548-3.
25. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi:10.1177/0363546510397174.
26. Seitlinger G, Moroder P, Fink C, Wierer G. Acquired femoral flexion deformity due to physeal injury during medial patellofemoral ligament reconstruction. Knee. 2017;24(3):680-685. doi:10.1016/j.knee.2017.02.003.
27. Parikh SN, Rajdev N. Relationship between trochlear dysplasia and the anterior distal femoral physis in pediatric and adolescent patients with patellar instability. In: 36th Annual Meething AANA. Denver; 2017.
28. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57(2):259-261.
29. Parikh SN. Medial patellofemoral ligament reconstruction and simultaneous guided correction of genu valgum for patellar instability in skeletally immature patients. In: EPOSNA. 2017.
30. Brattstroem H. Shape of the intercondylar groove normally and in recurrent dislocation of patella. A clinical and X-ray-Anatomical investigation. Acta Orthop Scand Suppl. 1964;68(suppl 68):1-148.
31. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
32. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi:10.1177/0363546512472873.
33. Jaquith BP, Parikh SN. Predictors of recurrent patellar instability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi:10.1097/BPO.0000000000000674.
34. Lippacher S, Reichel H, Nelitz M. Radiological criteria for trochlear dysplasia in children and adolescents. J Pediatr Orthop B. 2011;20(5):341-344. doi:10.1097/BPB.0b013e3283474c8b.
35. Parikh SN, Rajdev N, Sun Q. The growth of trochlear dysplasia during adolescence. J Pediatr Orthop. 2018. doi:10.1097/BPO.0000000000001168. [Epub ahead of print]
36. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177. doi:10.1302/0301-620X.89B9.19065.
37. Sugimoto D, Christino MA, Micheli LJ. Effects of surgical intervention on trochlear remodeling in pediatric patients with recurrent patella dislocation cases. J Pediatr Orthop B. 2016;25(4):349-353. doi:10.1097/BPB.0000000000000341.
38. Insall J, Goldberg V, Salvati E. Recurrent dislocation and the high-riding patella. Clin Orthop Relat Res. 1972;88:67-69.
39. Caton J. [Method of measuring the height of the patella]. Acta Orthop Belg. 1989;55(3):385-386.
40. Thévenin-Lemoine C, Ferrand M, Courvoisier A, Damsin JP, Ducou le Pointe H, Vialle R. Is the Caton-Deschamps index a valuable ratio to investigate patellar height in children? J Bone Joint Surg Am. 2011;93(8):e35. doi:10.2106/JBJS.J.00759.
41. Micheli LJ, Slater JA, Woods E, Gerbino PG. Patella alta and the adolescent growth spurt. Clin Orthop Relat Res. 1986;213:159-162.
42. Koshino T, Sugimoto K. New measurement of patellar height in the knees of children using the epiphyseal line midpoint. J Pediatr Orthop. 1989;9(2):216-218.
43. Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45(3):203-212.
44. Smits-Engelsman B, Klerks M, Kirby A. Beighton score: a valid measure for generalized hypermobility in children. J Pediatr. 2011;158(1):119-123. doi:10.1016/j.jpeds.2010.07.021.
45. Jansson A, Saartok T, Werner S, Renström P. General joint laxity in 1845 Swedish school children of different ages: age- and gender-specific distributions. Acta Paediatr. 2004;93(9):1202-1206.
46. Beighton P, Solomon L, Soskolnet CL. Articular mobility in an African population. Ann rheum Dis. 1973;32(5):413-418.
47. Staheli LT, Corbett M, Wyss C, King H. Lower-extremity rotational problems in children. Normal values to guide management. J Bone Joint Surg Am. 1985;67(1):39-47.
48. Kijowski R, Plagens D, Shaeh S, Teitge R. The effects of rotational deformities of the femur on contact pressure and contact area in the patellofemoral joint and on strain in the medial patellofemoral ligament. Annu Meet Int Patellofemoral Study Group, Napa Val San Fr CA. 1999.
49. Post W, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
50. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
51. Kurosawa K, Kawame H, Ochiai Y, Nakashima M, Tohma T, Ohashi H. Patellar dislocation in Kabuki syndrome. Am J Med Genet. 2002;108(2):160-163.
52. Mik G, Gholve PA, Scher DM, Widmann RF, Green DW. Down syndrome: orthopedic issues. Curr Opin Pediatr. 2008;20(1):30-36. doi:10.1097/MOP.0b013e3282f35f19.
53. Sweeney E, Fryer A, Mountford R, Green A, McIntosh I. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40(3):153-162.
54. Stevens CA. Patellar dislocation in Rubenstein-Taybi syndrome. Am J Med Genet. 1997;72(2):188-190.
55. Andrish J. The biomechanics of patellofemoral stability. J Knee Surg. 2004;17(1):35-39.
56. Conlan T, Garth WP, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682-693.
57. Balcarek P, Ammon J, Frosch S, et al. Magnetic resonance imaging characteristics of the medial patellofemoral ligament lesion in acute lateral patellar dislocations considering trochlear dysplasia, patella alta, and tibial tuberosity-TG distance. Arthroscopy. 2010;26(7):926-935. doi:10.1016/j.arthro.2009.11.004.
58. Garin C, Chaker M, Dohin B, Kohler R. Permanent, habitual dislocation and recurrent dislocation of the patella in children: surgical management by patellar ligamentous transfer in 50 knees. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(7):690-700.
59. Sillanpaa P. Terminology of patellar dislocation. In: Sillanpaa P, ed. Trauma. Saarbrucken, Germany: Lambert Academic Publishing; 2010:16-18.
60. Parikh SN, Lykissas MG. Classification of lateral patellar instability in children and adolescents. Orthop Clin North Am. 2016;47(1):145-152. doi:10.1016/j.ocl.2015.08.016.
61. Chotel F, Bérard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(suppl 1):125-137. doi:10.1016/j.otsr.2013.06.014.
62. Wada A, Fujii T, Takamura K, Yanagida H, Surijamorn P. Congenital dislocation of the patella. J Child Orthop. 2008;2(2):119-123. doi:10.1007/s11832-008-0090-4.
63. Südkamp NP, Granrath M, Hoffmann R, Haas NP. Instability of the shoulder joint in the athlete. Chirurg. 1994;65(11):901-909.
64. Hiemstra LA, Kerslake S, Lafave M, Heard SM, Buchko GML. Introduction of a classification system for patients with patellofemoral instability (WARPS and STAID). Knee Surg Sport Traumatol Arthrosc. 2014;22(11):2776-2782. doi:10.1007/s00167-013-2477-0.
65. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497. doi:10.1007/s00402-015-2381-9.
66. Balcarek P, Oberthür S, Hopfensitz S, et al. Which patellae are likely to redislocate? Knee Surg Sport Traumatol Arthrosc. 2014;22(10):2308-2314. doi:10.1007/s00167-013-2650-5.
67. Bitar AC, Demange MK, D’Elia CO, Camanho GL. Traumatic patellar dislocation: nonoperative treatment compared with mpfl reconstruction using patellar tendon. Am J Sports Med. 2012;40(1):114-122. doi:10.1177/0363546511423742.
68. Smith TO, Walker J, Russell N. Outcomes of medial patellofemoral ligament reconstruction for patellar instability: a systematic review. Knee Surg Sport Traumatol Arthrosc. 2007;15(11):1301-1314. doi:10.1007/s00167-007-0390-0.
69. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261. doi:10.1177/0363546505285584.
70. Ma LF, Wang F, Chen BC, Wang CH, Zhou JW, Wang HY. Medial retinaculum plasty versus medial patellofemoral ligament reconstruction for recurrent patellar instability in adults: a randomized controlled trial. Arthrosc J Arthrosc Relat Surg. 2013;29(5):891-897. doi:10.1016/j.arthro.2013.01.030.
71. Weeks KD, Fabricant PD, Ladenhauf HN, Green DW. Surgical options for patellar stabilization in the skeletally immature patient. Sports Med Arthrosc Rev. 2012;20(3):194-202.
72. Green D. Surgical treatment of pediatric patella instability. Die Ther der Instabilen patella. 2016:80-89.
73. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309. doi:10.1055/s-0034-1398373.
74. Ghanem I, Wattincourt L, Seringe R. Congenital dislocation of the patella. Part I: pathologic anatomy. J Pediatr Orthop. 2000;20(6):812-816.
75. Bongers E, Van Kampen A, Van Bokhoven H, Knoers N. Human syndromes with congenital patellar anomalies and the underlying gene defects. Clin Genet. 2005;68(4):302-319. doi:10.1111/j.1399-0004.2005.00508.x.
76. Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51(3):444-453.
77. Gausden EB, Fabricant PD, Taylor SA, et al. Medial patellofemoral reconstruction in children and adolescents. JBJS Rev. 2015;3(10):1-11. doi:10.2106/JBJS.RVW.N.00091.
78. Palmu S, Kallio PE, Donell ST, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90(3):463-470. doi:10.2106/JBJS.G.00072.
79. Webb JE, Lewallen LW, Christophersen C, Krych AJ, McIntosh AL. Clinical outcome of internal fixation of unstable juvenile osteochondritis dissecans lesions of the knee. Orthopedics. 2013;36(11):e1444-e1449. doi:10.3928/01477447-20131021-30.
80. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
81. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):e47. doi:10.1053/jars.2003.50167.
82. Nomura E, Inoue M, Kobayashi S. Long-term follow-up and knee osteoarthritis change after medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2007;35(11):1851-1858. doi:10.1177/0363546507306161.
83. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States. Orthop J Sport Med. 2016;4(8):2325967116662873. doi:10.1177/2325967116662873.
84. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc. 2007;15(2):61-67. doi:10.1097/JSA.0b013e3180479464.
85. Nietosvaara Y, Paukku R, Palmu S, Donell ST. Acute patellar dislocation in children and adolescents. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2):139-145. doi:10.2106/JBJS.H.01289.
86. Hing CBC, Smith TO, Donell S, Song F. Surgical versus non-surgical interventions for treating patellar dislocation. In Hing CB, ed. Cochrane Database Syst Rev. 2011;(11):CD008106. doi:10.1002/14651858.CD008106.pub2.
87. Nwachukwu BU, So C, Schairer WW, Green DW, Dodwell ER. Surgical versus conservative management of acute patellar dislocation in children and adolescents: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2016;24(3):760-767. doi:10.1007/s00167-015-3948-2.
88. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi:10.1177/0363546510376230.
89. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. Operative treatment of primary patellar dislocation does not improve medium-term outcome: a 7-year follow-up report and risk analysis of 127 randomized patients. Acta Orthop. 2005;76(5):699-704. doi:10.1080/17453670510041790.
90. Ostermeier S, Holst M, Hurschler C, Windhagen H, Stukenborg-Colsman C. Dynamic measurement of patellofemoral kinematics and contact pressure after lateral retinacular release: an in vitro study. Knee Surg Sport Traumatol Arthrosc. 2007;15(5):547-554. doi:10.1007/s00167-006-0261-0.
91. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60. doi: 10.1177/036354659602400110.
92. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
93. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914. doi:10.1007/s00167-011-1516-y.
94. Ellera Gomes JL. Medial patellofemoral ligament reconstruction for recurrent dislocation of the patella: a preliminary report. Arthroscopy. 1992;8(3):335-340.
95. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
96. Avikainen VJ, Nikku RK, Seppänen-Lehmonen TK. Adductor magnus tenodesis for patellar dislocation. Technique and preliminary results. Clin Orthop Relat Res. 1993;(297):12-16.
97. Davis DK, Fithian DC. Techniques of medial retinacular repair and reconstruction. Clin Orthop Relat Res. 2002;(402):38-52.
98. Dhillon MS, Mohan P, Nagi ON. Does harvesting the medial third of the patellar tendon cause lateral shift of the patella after ACL reconstruction? Acta Orthop Belg. 2003;69(4):334-340.
99. Drez D, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthrosc J Arthrosc Relat Surg. 2001;17(3):298-306. doi:10.1053/jars.2001.21490.
100. Ellera Gomes JL, Stigler Marczyk LR, César de César P, Jungblut CF. Medial patellofemoral ligament reconstruction with semitendinosus autograft for chronic patellar instability: a follow-up study. Arthrosc J Arthrosc Relat Surg. 2004;20(2):147-151. doi:10.1016/j.arthro.2003.11.006.
101. Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. Arthrosc J Arthrosc Relat Surg. 2007;23(5):542-553. doi:10.1016/j.arthro.2007.03.006.
102. Nomura E, Horiuchi Y, Kihara M. Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee. 2000;7(2):121-127.
103. Ostermeier S, Stukenborg-Colsman C, Hurschler C, Wirth CJ. In vitro investigation of the effect of medial patellofemoral ligament reconstruction and medial tibial tuberosity transfer on lateral patellar stability. Arthrosc J Arthrosc Relat Surg. 2006;22(3):308-319. doi:10.1016/j.arthro.2005.09.024.
104. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
105. Steensen RN, Dopirak RM, Maurus PB. A simple technique for reconstruction of the medial patellofemoral ligament using a quadriceps tendon graft. Arthroscopy. 2005;21(3):365-370. doi:10.1016/j.arthro.2004.10.007.
106. Steensen RN, Dopirak RM, McDonald WG. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513. doi:10.1177/0363546503261505.
107. Farahmand F, Tahmasbi MN, Amis AA. Lateral force–displacement behaviour of the human patella and its variation with knee flexion—a biomechanical study in vitro. J Biomech. 1998;31(12):1147-1152.
108. Heegaard J, Leyvraz PF, Van Kampen A, Rakotomanana L, Rubin PJ, Blankevoort L. Influence of soft structures on patellar 3-dimensional tracking. Clin Orthop Relat Res. 1994;(299):235-243.
109. Goyal D. Medial patellofemoral ligament reconstruction: the superficial quad technique. Am J Sports Med. 2013;41(5):1022-1029. doi:10.1177/0363546513477828.
110. Hohn E, Pandya NK. Does the utilization of allograft tissue in medial patellofemoral ligament reconstruction in pediatric and adolescent patients restore patellar stability? Clin Orthop Relat Res. 2017;475(6):1563-1569. doi:10.1007/s11999-016-5060-4.
111. Csintalan R, Latt L, Fornalski S, Raiszadeh K, Inacio M, Fithian D. Medial patellofemoral ligament (MPFL) reconstruction for the treatment of patellofemoral instability. J Knee Surg. 2013;27(2):139-146. doi:10.1055/s-0033-1360652.
112. Ahmad CS, Brown GD, Stein BS. The docking technique for medial patellofemoral ligament reconstruction: surgical technique and clinical outcome. Am J Sports Med. 2009;37(10):2021-2027. doi:10.1177/0363546509336261.
113. Fernandez E, Sala D, Castejon M. Reconstruction of the medial patellofemoral ligament for patellar instability using a semitendinosus autograft. Acta Orthop Belg. 2005;71(3):303-308.
114. Parikh SN, Nathan ST, Wall EJ, Eismann EA. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41(5):1030-1038. doi:10.1177/0363546513482085.
115. Green D, Gausden E. Medial patellofemoral ligament reconstruction: hamstring technique. In: Green D, Cordasco F, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:150-157.
116. Craig JG, Cody DD, van Holsbeeck M. The distal femoral and proximal tibial growth plates: MR imaging, 3-dimensional modeling and estimation of area and volume. Skeletal Radiol. 2004;33(6):337-344.
117. Noyes FR, Albright JC. Reconstruction of the medial patellofemoral ligament with autologous quadriceps tendon. Arthroscopy. 2006;22(8):904.e1-e7. doi:10.1016/j.arthro.2005.12.058.
118. Pinkowsky G, Hennrikus. Technique: quad tendon medial patellofemoral ligament reconstruction. In: Green DW, Cordasco FA, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:133-139.
119. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159. doi:10.1016/j.arthro.2011.02.014.
120. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485. doi:10.1177/0363546506287486.
121. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483. doi:10.1007/s00167-008-0702-z.
122. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
123. Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293-297. doi:10.1177/0363546509347602.
124. Fink C, Veselko M, Herbort M, Hoser C. Minimally invasive reconstruction of the medial patellofemoral ligament using quadriceps tendon. Arthrosc Tech. 2014;3(3):e325-e329. doi:10.1016/j.eats.2014.01.012.
125. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. A mini-invasive adductor magnus tendon transfer technique for medial patellofemoral ligament reconstruction: a technical note. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):508-512. doi:10.1007/s00167-008-0713-9.
126. Hambidge SJ, Davidson AJ, Gonzales R, Steiner JF. Epidemiology of pediatric injury-related primary care office visits in the United States. Pediatrics. 2002;109(4):559-565.
127. Camanho GL, Bitar AC, Hernandez AJ, Olivi R. Medial patellofemoral ligament reconstruction: a novel technique using the patellar ligament. Arthroscopy. 2007;23(1):108.e1-e4. doi:10.1016/j.arthro.2006.07.008.
128. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435. doi:10.1016/j.arthro.2007.11.005.
129. Deie M, Ochi M, Sumen Y, Yasumoto M, Kobayashi K, Kimura H. Reconstruction of the medial patellofemoral ligament for the treatment of habitual or recurrent dislocation of the patella in children. J Bone Joint Surg Br. 2003;85(6):887-890.
130. Ladenhauf HN, Berkes MB, Green DW. Medial patellofemoral ligament reconstruction using hamstring autograft in children and adolescents. Arthrosc Tech. 2013;2(2):e151-e154. doi:10.1016/j.eats.2013.01.006.
131. Bedi H, Marzo J. The biomechanics of medial patellofemoral ligament repair followed by lateral retinacular release. Am J Sports Med. 2010;38(7):1462-1467. doi:10.1177/0363546510373581.
132. Lattermann C, Toth J, Bach BR. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc Rev. 2007;15(2):57-60. doi:10.1097/JSA.0b013e318042af30
133. Ricchetti ET, Mehta S, Sennett BJ, Huffman GR. Comparison of lateral release versus lateral release with medial soft-tissue realignment for the treatment of recurrent patellar instability: a systematic review. Arthroscopy. 2007;23(5):463-468. doi:10.1016/j.arthro.2007.01.007.
134. Dainer RD, Barrack RL, Buckley SL, Alexander AH. Arthroscopic treatment of acute patellar dislocations. Arthroscopy. 1988;4(4):267-271.
135. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629. doi:10.1177/0363546506296041.
136. Vainionpää S, Laasonen E, Silvennoinen T, Vasenius J, Rokkanen P. Acute dislocation of the patella. A prospective review of operative treatment. J Bone Joint Surg Br. 1990;72(3):366-369.
137. Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40(8):1916-1923. doi:10.1177/0363546512442330.
138. Tanaka MJ, Bollier MJ, Andrish JT, Fulkerson JP, Cosgarea AJ. Complications of medial patellofemoral ligament reconstruction: common technical errors and factors for success: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(12):e87. doi:10.2106/JBJS.K.01449.
139. Nguyen CV, Farrow LD, Liu RW, Gilmore A. Safe drilling paths in the distal femoral epiphysis for pediatric medial patellofemoral ligament reconstruction. Am J Sports Med. 2017;45(5):1085-1089. doi:10.1177/0363546516677795.
140. Edmonds EW, Glaser DA. Adolescent patella instability extensor mechanics. J Pediatr Orthop. 2016;36(3):262-267. doi:10.1097/BPO.0000000000000430.
141. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184. doi:10.1016/j.knee.2014.07.023.
142. Lykissas MG, Li T, Eismann EA, Parikh SN. Does medial patellofemoral ligament reconstruction decrease patellar height? A preliminary report. J Pediatr Orthop. 2014;34(1):78-85. doi:10.1097/BPO.0b013e3182a12102.
143. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2014;22(10):2545-2550. doi:10.1007/s00167-013-2445-8.
144. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521. doi:10.1007/s00167-005-0659-0.
145. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi:10.1177/0363546512463683.
146. Raghuveer RK, Mishra CB. Reconstruction of medial patellofemoral ligament for chronic patellar instability. Indian J Orthop. 2012;46(4):447-454. doi:10.4103/0019-5413.97259.
147. Kita K, Horibe S, Toritsuka Y, et al. Effects of medial patellofemoral ligament reconstruction on patellar tracking. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):829-837. doi:10.1007/s00167-011-1609-7.
148. Lenschow S, Herbort M, Fink C. Medial patellofemoral ligament reconstruction using quadriceps tendon. Oper Orthop Traumatol. 2015;27(6):474-483. doi:10.1007/s00064-015-0416-6.
149. Abouelsoud MM, Abdelhady A, Elshazly O. Anatomic physeal-sparing technique for medial patellofemoral ligament reconstruction in skeletally immature patients with ligamentous laxity. Eur J Orthop Surg Traumatol. 2015;25(5):921-926. doi:10.1007/s00590-015-1618-1.
150. Malecki K, Fabis J, Flont P, Niedzielski KR. The results of adductor magnus tenodesis in adolescents with recurrent patellar dislocation. Biomed Res Int. 2015;2015:1-7. doi:10.1155/2015/456858.
151. Kumahashi N, Kuwata S, Tadenuma T, Kadowaki M, Uchio Y. A “sandwich” method of reconstruction of the medial patellofemoral ligament using a titanium interference screw for patellar instability in skeletally immature patients. Arch Orthop Trauma Surg. 2012;132(8):1077-1083. doi:10.1007/s00402-012-1516-5.
152. Marsh JS, Daigneault JP, Sethi P, Polzhofer GK. Treatment of recurrent patellar instability with a modification of the Roux-Goldthwait technique. J Pediatr Orthop. 2006;26(4):461-465. doi:10.1097/01.bpo.0000217711.34492.48.
153. Nepple J, Luhmann S. Medial patellar tendon transfer with proximal realignment. In: Green D, Cordasco F, eds. Pediatric and Adolescent Knee Surgery. New York, NY: Wolters Kluwer; 2015:148-153.
154. Galeazzi R. Nuove applicazioni del trapianto musculare e tendineo. Ard Di Orthop Milano. 1922;38:315-323.
155. Grannatt K, Heyworth BE, Ogunwole O, Micheli LJ, Kocher MS. Galeazzi semitendinosus tenodesis for patellofemoral instability in skeletally immature patients. J Pediatr Orthop. 2012;32(6):621-625. doi:10.1097/BPO.0b013e318263a230.
156. Fiume M. La rotulopessi secondi Galeazzi nella lussazione recidivante di rotula [in Italian]. Minerva Ortop. 1954;5:171-174.
157. Baker RH, Carroll N, Dewar FP, Hall JE. The semitendinosus tenodesis for recurrent dislocation of the patella. J Bone Joint Surg Br. 1972;54(1):103-109.
158. Hall JE, Micheli LJ, McManama GB. Semitendinosus tenodesis for recurrent subluxation or dislocation of the patella. Clin Orthop Relat Res. 1979;(144):31-35.
159. Moyad TF, Blakemore L. Modified Galeazzi technique for recurrent patellar dislocation in children. Orthopedics. 2006;29(4):302-304.
160. Letts RM, Davidson D, Beaule P. Semitendinosus tenodesis for repair of recurrent dislocation of the patella in children. J Pediatr Orthop. 1999;19(6):742-747.
161. Aulisa AG, Falciglia F, Giordano M, Savignoni P, Guzzanti V. Galeazzi’s modified technique for recurrent patella dislocation in skeletally immature patients. J Orthop Sci. 2012;17(2):148-155. doi:10.1007/s00776-011-0189-1.
162. Roux C. Recurrent dislocation of the patella: operative treatment. 1888. Clin Orthop Relat Res. 2006;452:17-20.
163. Goldthwait J. Dislocation of the Patella. Trans Am Orthop Assn. 1895.
164. Fondren FB, Goldner JL, Bassett FH. Recurrent dislocation of the patella treated by the modified Roux-Goldthwait procedure. A prospective study of forty-seven knees. J Bone Joint Surg Am. 1985;67(7):993-1005.
165. Aärimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446. doi:10.1111/j.1600-0838.2007.00730.x.
166. Nelitz M, Reichel H, Dornacher D, Lippacher S. Anatomical reconstruction of the medial patellofemoral ligament in children with open growth-plates. Arch Orthop Trauma Surg. 2012;132(11):1647-1651. doi:10.1007/s00402-012-1593-5.
167. Vähäsarja V, Kinnunen P, Lanning P, Serlo W. Operative realignment of patellar malalignment in children. J Pediatr Orthop. 1995;15(3):281-285.
168. Abraham E, Washington E, Huang TL. Insall proximal realignment for disorders of the patella. Clin Orthop Relat Res. 1989;248:61-65.
169. Insall JN, Aglietti P, Tria AJ. Patellar pain and incongruence. II: Clinical application. Clin Orthop Relat Res. 1983;176:225-232.
170. Chrisman OD, Snook GA, Wilson TC. A long-term prospective study of the Hauser and Roux-Goldthwait procedures for recurrent patellar dislocation. Clin Orthop Relat Res. 1979;144:27-30.
171. Niedzielski KR, Malecki K, Flont P, Fabis J. The results of an extensive soft-tissue procedure in the treatment of obligatory patellar dislocation in children with ligamentous laxity: a post-operative isokinetic study. Bone Joint J. 2015;97-B(1):129-133. doi:10.1302/0301-620X.97B1.33941.
172. Hinckel BB, Gobbi RG, Demange MK, Bonadio MB, Pécora JR, Camanho GL. Combined reconstruction of the medial patellofemoral ligament with quadricipital tendon and the medial patellotibial ligament with patellar tendon. Arthrosc Tech. 2016;5(1):e79-e84. doi:10.1016/j.eats.2015.10.004.
173. Mani S, Kirkpatrick MS, Saranathan A, Smith LG, Cosgarea AJ, Elias JJ. Tibial tuberosity osteotomy for patellofemoral realignment alters tibiofemoral kinematics. Am J Sports Med. 2011;39(5):1024-1031. doi:10.1177/0363546510390188.
174. Brown GD, Ahmad CS. Combined medial patellofemoral ligament and medial patellotibial ligament reconstruction in skeletally immature patients. J Knee Surg. 2008;21(4):328-332.
175. Panagiotopoulos E, Strzelczyk P, Herrmann M, Scuderi G. Cadaveric study on static medial patellar stabilizers: the dynamizing role of the vastus medialis obliquus on medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):7-12. doi:10.1007/s00167-005-0631-z.
176. Philippot R, Boyer B, Testa R, Farizon F, Moyen B. The role of the medial ligamentous structures on patellar tracking during knee flexion. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):331-336. doi:10.1007/s00167-011-1598-6.
177. Gordon JE, Schoenecker PL. Surgical treatment of congenital dislocation of the patella. J Pediatr Orthop. 1999;19(2):260-264.
178. Koh JL, Stewart C. Patellar instability. Orthop Clin North Am. 2015;46(1):147-157. doi:10.1016/j.ocl.2014.09.011.
179. Pal S, Besier TF, Draper CE, et al. Patellar tilt correlates with vastus lateralis: vastus medialis activation ratio in maltracking patellofemoral pain patients. J Orthop Res. 2012;30(6):927-933. doi:10.1002/jor.22008.
180. Thompson TC. Quadricepsplasty. Ann Surg. 1945;121(5):751-754.
181. Daoud H, O’Farrell T, Cruess RL. Quadricepsplasty. The Judet technique and results of six cases. J Bone Joint Surg Br. 1982;64(2):194-197.
182. Stanisavljevic S, Zemenick G, Miller D. Congenital, irreducible, permanent lateral dislocation of the patella. Clin Orthop Relat Res. 1976;(116):190-199.
183. Kundu Z, Sangwan S, Guliani G, Siwach R, Kamboj P, Singh R. Thompson’s quadricepsplasty for stiff knee. Indian J Orthop. 2007;41(4):390-394. doi:10.4103/0019-5413.37004.
184. Tercier S, Shah H, Joseph B. Quadricepsplasty for congenital dislocation of the knee and congenital quadriceps contracture. J Child Orthop. 2012;6(5):397-410. doi:10.1007/s11832-012-0437-8.
185. Rose RE. Judet quadricepsplasty for extension contracture of the knee. West Indian Med J. 2005;54(4):238-241.
186. Tsukamoto N, Miura H, Matsuda S, Mawatari T, Kato H, Iwamoto Y. Functional evaluation of four patients treated with V-Y quadricepsplasty in total knee arthroplasty. J Orthop Sci. 2006;11(4):394-400. doi:10.1007/s00776-006-1023-z.
187. Dao Q, Chen DB, Scott RD. Proximal patellar quadricepsplasty realignment during total knee arthroplasty for irreducible congenital dislocation of the patella. J Bone Joint Surg Am. 2010;92(14):2457-2461. doi:10.2106/JBJS.H.00812.
188. Judet R, Judet J, Lord G. Results of treatment of stiffness of the knee caused by arthrolysis and disinsertion of the quadriceps femoris. Mem Acad Chir. 1959;85:645-654.
189. Oliveira VG, D’Elia LF, Tirico LEP, et al. Judet quadricepsplasty in the treatment of posttraumatic knee rigidity: long-term outcomes of 45 cases. J Trauma Acute Care Surg. 2012;72(2):e77-e80.
190. Hahn SB, Choi YR, Kang HJ, Lee SH. Prognostic factors and long-term outcomes following a modified Thompson’s quadricepsplasty for severely stiff knees. J Bone Joint Surg Br. 2010;92(2):217-221. doi:10.1302/0301-620X.92B2.22936.
191. Hosalkar HS, Jones S, Chowdhury M, Hartley J, Hill RA. Quadricepsplasty for knee stiffness after femoral lengthening in congenital short femur. J Bone Joint Surg Br. 2003;85(2):261-264.
192. Massè A, Biasibetti A, Demangos J, Dutto E, Pazzano S, Gallinaro P. The judet quadricepsplasty: long-term outcome of 21 cases. J Trauma. 2006;61(2):358-362. doi:10.1097/01.ta.0000230281.31144.1d.
193. Kocon H, Kabacyj M, Zgoda M. The results of the operative treatment of patellar instability in children with Down’s syndrome. J Pediatr Orthop B. 2012;21(5):407-410. doi:10.1097/BPB.0b013e328354f684.
194. Martin BD, Cherkashin AM, Tulchin K, Samchukov M, Birch JG. Treatment of femoral lengthening-related knee stiffness with a novel quadricepsplasty. J Pediatr Orthop. 2013;33(4):446-452. doi:10.1097/BPO.0b013e3182784e5d.
1. Nietosvaara Y, Aalto K, Kallio PE. Acute patellar dislocation in children: incidence and associated osteochondral fractures. J Pediatr Orthop. 1994;14(4):513-515.
2. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121. doi:10.1177/0363546503260788.
3. Abbasi D, May MM, Wall EJ, Chan G, Parikh SN. MRI findings in adolescent patients with acute traumatic knee hemarthrosis. J Pediatr Orthop. 2012;32(8):760-764. doi:10.1097/BPO.0b013e3182648d45.
4. Mäenpää H, Lehto MU. Patellar dislocation. The long-term results of nonoperative management in 100 patients. Am J Sports Med. 1997;25(2):213-217. doi:10.1177/036354659702500213.
5. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120. doi:10.1177/036354658601400204.
6. Cofield RH, Bryan RS. Acute dislocation of the patella: results of conservative treatment. J Trauma. 1977;17(7):526-531.
7. Wasserlauf BL, Paletta GA. Developmental anatomy of the pediatric and adolescent knee. In: Micheli LJ, Kocher MS, eds. The Pediatric and Adolescent Knee. 1st ed. Elsevier; 2006:27-32.
8. Birch JG. Growth and development. In: Herring J, ed. Tachdjian’s Pediatric Orthopedics. 4th ed. Saunders/Elsevier; 2007:3-22.
9. Sarin VK, Carter DR. Mechanobiology and joint conformity regulate endochondral ossification of sesamoids. J Orthop Res. 2000;18(5):706-712. doi:10.1002/jor.1100180505.
10. Maes C, Kronenberg HM. Postnatal bone growth: growth plate biology, bone formation, and remodeling. In: Pediatric Bone. Elsevier; 2012:55-82.
11. Tecklenburg K, Dejour D, Hoser C, Fink C. Bony and cartilaginous anatomy of the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):235-240. doi:10.1007/s00167-005-0683-0.
12. Walmsley R. The development of the patella. J Anat. 1940;74(Pt 3):360-368.3.
13. Fulkerson J, Hungerford D. Disorders of the patellofemoral Joint. In: Normal Anatomy. 2nd ed. Baltimore: Williams & Wilkins; 1990:1-24.
14. Ogden JA. Radiology of postnatal skeletal development. X. Patella and tibial tuberosity. Skeletal Radiol. 1984;11(4):246-257.
15. Nietosvaara Y, Aalto K. The cartilaginous femoral sulcus in children with patellar dislocation: an ultrasonographic study. J Pediatr Orthop. 1997;17(1):50-53.
16. Glard Y, Jouve JL, Garron E, Adalian P, Tardieu C, Bollini G. Anatomic study of femoral patellar groove in fetus. J Pediatr Orthop. 2005;25(3):305-308.
17. Nietosvaara Y. The femoral sulcus in children. An ultrasonographic study. J Bone Joint Surg Br. 1994;76(5):807-809.
18. Nietosvaara AY, Aalto KA. Ultrasonographic evaluation of patellar tracking in children. Clin Orthop Relat Res. 1993;(297):62-64.
19. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 26(1):59-65. doi:10.1177/03635465980260012701.
20. Shea KG, Styhl AC, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: a cadaveric study. Am J Sports Med. 2016;44(11):2833-2837. doi:10.1177/0363546516656366.
21. Shea KG, Polousky JD, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children. J Pediatr Orthop. 2014;34(8):808-813. doi:10.1097/BPO.0000000000000165.
22. Shea KG, Polousky JD, Jacobs JC, et al. The patellar insertion of the medial patellofemoral ligament in children. J Pediatr Orthop. 2015;35(4):e31-e35. doi:10.1097/BPO.0000000000000399.
23. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi:10.1016/j.arthro.2009.12.020.
24. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sport Traumatol Arthrosc. 2011;19(12):2067-2071. doi:10.1007/s00167-011-1548-3.
25. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi:10.1177/0363546510397174.
26. Seitlinger G, Moroder P, Fink C, Wierer G. Acquired femoral flexion deformity due to physeal injury during medial patellofemoral ligament reconstruction. Knee. 2017;24(3):680-685. doi:10.1016/j.knee.2017.02.003.
27. Parikh SN, Rajdev N. Relationship between trochlear dysplasia and the anterior distal femoral physis in pediatric and adolescent patients with patellar instability. In: 36th Annual Meething AANA. Denver; 2017.
28. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57(2):259-261.
29. Parikh SN. Medial patellofemoral ligament reconstruction and simultaneous guided correction of genu valgum for patellar instability in skeletally immature patients. In: EPOSNA. 2017.
30. Brattstroem H. Shape of the intercondylar groove normally and in recurrent dislocation of patella. A clinical and X-ray-Anatomical investigation. Acta Orthop Scand Suppl. 1964;68(suppl 68):1-148.
31. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
32. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi:10.1177/0363546512472873.
33. Jaquith BP, Parikh SN. Predictors of recurrent patellar instability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi:10.1097/BPO.0000000000000674.
34. Lippacher S, Reichel H, Nelitz M. Radiological criteria for trochlear dysplasia in children and adolescents. J Pediatr Orthop B. 2011;20(5):341-344. doi:10.1097/BPB.0b013e3283474c8b.
35. Parikh SN, Rajdev N, Sun Q. The growth of trochlear dysplasia during adolescence. J Pediatr Orthop. 2018. doi:10.1097/BPO.0000000000001168. [Epub ahead of print]
36. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177. doi:10.1302/0301-620X.89B9.19065.
37. Sugimoto D, Christino MA, Micheli LJ. Effects of surgical intervention on trochlear remodeling in pediatric patients with recurrent patella dislocation cases. J Pediatr Orthop B. 2016;25(4):349-353. doi:10.1097/BPB.0000000000000341.
38. Insall J, Goldberg V, Salvati E. Recurrent dislocation and the high-riding patella. Clin Orthop Relat Res. 1972;88:67-69.
39. Caton J. [Method of measuring the height of the patella]. Acta Orthop Belg. 1989;55(3):385-386.
40. Thévenin-Lemoine C, Ferrand M, Courvoisier A, Damsin JP, Ducou le Pointe H, Vialle R. Is the Caton-Deschamps index a valuable ratio to investigate patellar height in children? J Bone Joint Surg Am. 2011;93(8):e35. doi:10.2106/JBJS.J.00759.
41. Micheli LJ, Slater JA, Woods E, Gerbino PG. Patella alta and the adolescent growth spurt. Clin Orthop Relat Res. 1986;213:159-162.
42. Koshino T, Sugimoto K. New measurement of patellar height in the knees of children using the epiphyseal line midpoint. J Pediatr Orthop. 1989;9(2):216-218.
43. Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45(3):203-212.
44. Smits-Engelsman B, Klerks M, Kirby A. Beighton score: a valid measure for generalized hypermobility in children. J Pediatr. 2011;158(1):119-123. doi:10.1016/j.jpeds.2010.07.021.
45. Jansson A, Saartok T, Werner S, Renström P. General joint laxity in 1845 Swedish school children of different ages: age- and gender-specific distributions. Acta Paediatr. 2004;93(9):1202-1206.
46. Beighton P, Solomon L, Soskolnet CL. Articular mobility in an African population. Ann rheum Dis. 1973;32(5):413-418.
47. Staheli LT, Corbett M, Wyss C, King H. Lower-extremity rotational problems in children. Normal values to guide management. J Bone Joint Surg Am. 1985;67(1):39-47.
48. Kijowski R, Plagens D, Shaeh S, Teitge R. The effects of rotational deformities of the femur on contact pressure and contact area in the patellofemoral joint and on strain in the medial patellofemoral ligament. Annu Meet Int Patellofemoral Study Group, Napa Val San Fr CA. 1999.
49. Post W, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
50. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
51. Kurosawa K, Kawame H, Ochiai Y, Nakashima M, Tohma T, Ohashi H. Patellar dislocation in Kabuki syndrome. Am J Med Genet. 2002;108(2):160-163.
52. Mik G, Gholve PA, Scher DM, Widmann RF, Green DW. Down syndrome: orthopedic issues. Curr Opin Pediatr. 2008;20(1):30-36. doi:10.1097/MOP.0b013e3282f35f19.
53. Sweeney E, Fryer A, Mountford R, Green A, McIntosh I. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40(3):153-162.
54. Stevens CA. Patellar dislocation in Rubenstein-Taybi syndrome. Am J Med Genet. 1997;72(2):188-190.
55. Andrish J. The biomechanics of patellofemoral stability. J Knee Surg. 2004;17(1):35-39.
56. Conlan T, Garth WP, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682-693.
57. Balcarek P, Ammon J, Frosch S, et al. Magnetic resonance imaging characteristics of the medial patellofemoral ligament lesion in acute lateral patellar dislocations considering trochlear dysplasia, patella alta, and tibial tuberosity-TG distance. Arthroscopy. 2010;26(7):926-935. doi:10.1016/j.arthro.2009.11.004.
58. Garin C, Chaker M, Dohin B, Kohler R. Permanent, habitual dislocation and recurrent dislocation of the patella in children: surgical management by patellar ligamentous transfer in 50 knees. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(7):690-700.
59. Sillanpaa P. Terminology of patellar dislocation. In: Sillanpaa P, ed. Trauma. Saarbrucken, Germany: Lambert Academic Publishing; 2010:16-18.
60. Parikh SN, Lykissas MG. Classification of lateral patellar instability in children and adolescents. Orthop Clin North Am. 2016;47(1):145-152. doi:10.1016/j.ocl.2015.08.016.
61. Chotel F, Bérard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(suppl 1):125-137. doi:10.1016/j.otsr.2013.06.014.
62. Wada A, Fujii T, Takamura K, Yanagida H, Surijamorn P. Congenital dislocation of the patella. J Child Orthop. 2008;2(2):119-123. doi:10.1007/s11832-008-0090-4.
63. Südkamp NP, Granrath M, Hoffmann R, Haas NP. Instability of the shoulder joint in the athlete. Chirurg. 1994;65(11):901-909.
64. Hiemstra LA, Kerslake S, Lafave M, Heard SM, Buchko GML. Introduction of a classification system for patients with patellofemoral instability (WARPS and STAID). Knee Surg Sport Traumatol Arthrosc. 2014;22(11):2776-2782. doi:10.1007/s00167-013-2477-0.
65. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497. doi:10.1007/s00402-015-2381-9.
66. Balcarek P, Oberthür S, Hopfensitz S, et al. Which patellae are likely to redislocate? Knee Surg Sport Traumatol Arthrosc. 2014;22(10):2308-2314. doi:10.1007/s00167-013-2650-5.
67. Bitar AC, Demange MK, D’Elia CO, Camanho GL. Traumatic patellar dislocation: nonoperative treatment compared with mpfl reconstruction using patellar tendon. Am J Sports Med. 2012;40(1):114-122. doi:10.1177/0363546511423742.
68. Smith TO, Walker J, Russell N. Outcomes of medial patellofemoral ligament reconstruction for patellar instability: a systematic review. Knee Surg Sport Traumatol Arthrosc. 2007;15(11):1301-1314. doi:10.1007/s00167-007-0390-0.
69. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261. doi:10.1177/0363546505285584.
70. Ma LF, Wang F, Chen BC, Wang CH, Zhou JW, Wang HY. Medial retinaculum plasty versus medial patellofemoral ligament reconstruction for recurrent patellar instability in adults: a randomized controlled trial. Arthrosc J Arthrosc Relat Surg. 2013;29(5):891-897. doi:10.1016/j.arthro.2013.01.030.
71. Weeks KD, Fabricant PD, Ladenhauf HN, Green DW. Surgical options for patellar stabilization in the skeletally immature patient. Sports Med Arthrosc Rev. 2012;20(3):194-202.
72. Green D. Surgical treatment of pediatric patella instability. Die Ther der Instabilen patella. 2016:80-89.
73. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309. doi:10.1055/s-0034-1398373.
74. Ghanem I, Wattincourt L, Seringe R. Congenital dislocation of the patella. Part I: pathologic anatomy. J Pediatr Orthop. 2000;20(6):812-816.
75. Bongers E, Van Kampen A, Van Bokhoven H, Knoers N. Human syndromes with congenital patellar anomalies and the underlying gene defects. Clin Genet. 2005;68(4):302-319. doi:10.1111/j.1399-0004.2005.00508.x.
76. Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51(3):444-453.
77. Gausden EB, Fabricant PD, Taylor SA, et al. Medial patellofemoral reconstruction in children and adolescents. JBJS Rev. 2015;3(10):1-11. doi:10.2106/JBJS.RVW.N.00091.
78. Palmu S, Kallio PE, Donell ST, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90(3):463-470. doi:10.2106/JBJS.G.00072.
79. Webb JE, Lewallen LW, Christophersen C, Krych AJ, McIntosh AL. Clinical outcome of internal fixation of unstable juvenile osteochondritis dissecans lesions of the knee. Orthopedics. 2013;36(11):e1444-e1449. doi:10.3928/01477447-20131021-30.
80. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
81. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):e47. doi:10.1053/jars.2003.50167.
82. Nomura E, Inoue M, Kobayashi S. Long-term follow-up and knee osteoarthritis change after medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2007;35(11):1851-1858. doi:10.1177/0363546507306161.
83. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States. Orthop J Sport Med. 2016;4(8):2325967116662873. doi:10.1177/2325967116662873.
84. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc. 2007;15(2):61-67. doi:10.1097/JSA.0b013e3180479464.
85. Nietosvaara Y, Paukku R, Palmu S, Donell ST. Acute patellar dislocation in children and adolescents. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2):139-145. doi:10.2106/JBJS.H.01289.
86. Hing CBC, Smith TO, Donell S, Song F. Surgical versus non-surgical interventions for treating patellar dislocation. In Hing CB, ed. Cochrane Database Syst Rev. 2011;(11):CD008106. doi:10.1002/14651858.CD008106.pub2.
87. Nwachukwu BU, So C, Schairer WW, Green DW, Dodwell ER. Surgical versus conservative management of acute patellar dislocation in children and adolescents: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2016;24(3):760-767. doi:10.1007/s00167-015-3948-2.
88. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi:10.1177/0363546510376230.
89. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. Operative treatment of primary patellar dislocation does not improve medium-term outcome: a 7-year follow-up report and risk analysis of 127 randomized patients. Acta Orthop. 2005;76(5):699-704. doi:10.1080/17453670510041790.
90. Ostermeier S, Holst M, Hurschler C, Windhagen H, Stukenborg-Colsman C. Dynamic measurement of patellofemoral kinematics and contact pressure after lateral retinacular release: an in vitro study. Knee Surg Sport Traumatol Arthrosc. 2007;15(5):547-554. doi:10.1007/s00167-006-0261-0.
91. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60. doi: 10.1177/036354659602400110.
92. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
93. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914. doi:10.1007/s00167-011-1516-y.
94. Ellera Gomes JL. Medial patellofemoral ligament reconstruction for recurrent dislocation of the patella: a preliminary report. Arthroscopy. 1992;8(3):335-340.
95. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
96. Avikainen VJ, Nikku RK, Seppänen-Lehmonen TK. Adductor magnus tenodesis for patellar dislocation. Technique and preliminary results. Clin Orthop Relat Res. 1993;(297):12-16.
97. Davis DK, Fithian DC. Techniques of medial retinacular repair and reconstruction. Clin Orthop Relat Res. 2002;(402):38-52.
98. Dhillon MS, Mohan P, Nagi ON. Does harvesting the medial third of the patellar tendon cause lateral shift of the patella after ACL reconstruction? Acta Orthop Belg. 2003;69(4):334-340.
99. Drez D, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthrosc J Arthrosc Relat Surg. 2001;17(3):298-306. doi:10.1053/jars.2001.21490.
100. Ellera Gomes JL, Stigler Marczyk LR, César de César P, Jungblut CF. Medial patellofemoral ligament reconstruction with semitendinosus autograft for chronic patellar instability: a follow-up study. Arthrosc J Arthrosc Relat Surg. 2004;20(2):147-151. doi:10.1016/j.arthro.2003.11.006.
101. Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. Arthrosc J Arthrosc Relat Surg. 2007;23(5):542-553. doi:10.1016/j.arthro.2007.03.006.
102. Nomura E, Horiuchi Y, Kihara M. Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee. 2000;7(2):121-127.
103. Ostermeier S, Stukenborg-Colsman C, Hurschler C, Wirth CJ. In vitro investigation of the effect of medial patellofemoral ligament reconstruction and medial tibial tuberosity transfer on lateral patellar stability. Arthrosc J Arthrosc Relat Surg. 2006;22(3):308-319. doi:10.1016/j.arthro.2005.09.024.
104. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
105. Steensen RN, Dopirak RM, Maurus PB. A simple technique for reconstruction of the medial patellofemoral ligament using a quadriceps tendon graft. Arthroscopy. 2005;21(3):365-370. doi:10.1016/j.arthro.2004.10.007.
106. Steensen RN, Dopirak RM, McDonald WG. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513. doi:10.1177/0363546503261505.
107. Farahmand F, Tahmasbi MN, Amis AA. Lateral force–displacement behaviour of the human patella and its variation with knee flexion—a biomechanical study in vitro. J Biomech. 1998;31(12):1147-1152.
108. Heegaard J, Leyvraz PF, Van Kampen A, Rakotomanana L, Rubin PJ, Blankevoort L. Influence of soft structures on patellar 3-dimensional tracking. Clin Orthop Relat Res. 1994;(299):235-243.
109. Goyal D. Medial patellofemoral ligament reconstruction: the superficial quad technique. Am J Sports Med. 2013;41(5):1022-1029. doi:10.1177/0363546513477828.
110. Hohn E, Pandya NK. Does the utilization of allograft tissue in medial patellofemoral ligament reconstruction in pediatric and adolescent patients restore patellar stability? Clin Orthop Relat Res. 2017;475(6):1563-1569. doi:10.1007/s11999-016-5060-4.
111. Csintalan R, Latt L, Fornalski S, Raiszadeh K, Inacio M, Fithian D. Medial patellofemoral ligament (MPFL) reconstruction for the treatment of patellofemoral instability. J Knee Surg. 2013;27(2):139-146. doi:10.1055/s-0033-1360652.
112. Ahmad CS, Brown GD, Stein BS. The docking technique for medial patellofemoral ligament reconstruction: surgical technique and clinical outcome. Am J Sports Med. 2009;37(10):2021-2027. doi:10.1177/0363546509336261.
113. Fernandez E, Sala D, Castejon M. Reconstruction of the medial patellofemoral ligament for patellar instability using a semitendinosus autograft. Acta Orthop Belg. 2005;71(3):303-308.
114. Parikh SN, Nathan ST, Wall EJ, Eismann EA. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41(5):1030-1038. doi:10.1177/0363546513482085.
115. Green D, Gausden E. Medial patellofemoral ligament reconstruction: hamstring technique. In: Green D, Cordasco F, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:150-157.
116. Craig JG, Cody DD, van Holsbeeck M. The distal femoral and proximal tibial growth plates: MR imaging, 3-dimensional modeling and estimation of area and volume. Skeletal Radiol. 2004;33(6):337-344.
117. Noyes FR, Albright JC. Reconstruction of the medial patellofemoral ligament with autologous quadriceps tendon. Arthroscopy. 2006;22(8):904.e1-e7. doi:10.1016/j.arthro.2005.12.058.
118. Pinkowsky G, Hennrikus. Technique: quad tendon medial patellofemoral ligament reconstruction. In: Green DW, Cordasco FA, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:133-139.
119. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159. doi:10.1016/j.arthro.2011.02.014.
120. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485. doi:10.1177/0363546506287486.
121. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483. doi:10.1007/s00167-008-0702-z.
122. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
123. Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293-297. doi:10.1177/0363546509347602.
124. Fink C, Veselko M, Herbort M, Hoser C. Minimally invasive reconstruction of the medial patellofemoral ligament using quadriceps tendon. Arthrosc Tech. 2014;3(3):e325-e329. doi:10.1016/j.eats.2014.01.012.
125. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. A mini-invasive adductor magnus tendon transfer technique for medial patellofemoral ligament reconstruction: a technical note. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):508-512. doi:10.1007/s00167-008-0713-9.
126. Hambidge SJ, Davidson AJ, Gonzales R, Steiner JF. Epidemiology of pediatric injury-related primary care office visits in the United States. Pediatrics. 2002;109(4):559-565.
127. Camanho GL, Bitar AC, Hernandez AJ, Olivi R. Medial patellofemoral ligament reconstruction: a novel technique using the patellar ligament. Arthroscopy. 2007;23(1):108.e1-e4. doi:10.1016/j.arthro.2006.07.008.
128. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435. doi:10.1016/j.arthro.2007.11.005.
129. Deie M, Ochi M, Sumen Y, Yasumoto M, Kobayashi K, Kimura H. Reconstruction of the medial patellofemoral ligament for the treatment of habitual or recurrent dislocation of the patella in children. J Bone Joint Surg Br. 2003;85(6):887-890.
130. Ladenhauf HN, Berkes MB, Green DW. Medial patellofemoral ligament reconstruction using hamstring autograft in children and adolescents. Arthrosc Tech. 2013;2(2):e151-e154. doi:10.1016/j.eats.2013.01.006.
131. Bedi H, Marzo J. The biomechanics of medial patellofemoral ligament repair followed by lateral retinacular release. Am J Sports Med. 2010;38(7):1462-1467. doi:10.1177/0363546510373581.
132. Lattermann C, Toth J, Bach BR. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc Rev. 2007;15(2):57-60. doi:10.1097/JSA.0b013e318042af30
133. Ricchetti ET, Mehta S, Sennett BJ, Huffman GR. Comparison of lateral release versus lateral release with medial soft-tissue realignment for the treatment of recurrent patellar instability: a systematic review. Arthroscopy. 2007;23(5):463-468. doi:10.1016/j.arthro.2007.01.007.
134. Dainer RD, Barrack RL, Buckley SL, Alexander AH. Arthroscopic treatment of acute patellar dislocations. Arthroscopy. 1988;4(4):267-271.
135. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629. doi:10.1177/0363546506296041.
136. Vainionpää S, Laasonen E, Silvennoinen T, Vasenius J, Rokkanen P. Acute dislocation of the patella. A prospective review of operative treatment. J Bone Joint Surg Br. 1990;72(3):366-369.
137. Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40(8):1916-1923. doi:10.1177/0363546512442330.
138. Tanaka MJ, Bollier MJ, Andrish JT, Fulkerson JP, Cosgarea AJ. Complications of medial patellofemoral ligament reconstruction: common technical errors and factors for success: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(12):e87. doi:10.2106/JBJS.K.01449.
139. Nguyen CV, Farrow LD, Liu RW, Gilmore A. Safe drilling paths in the distal femoral epiphysis for pediatric medial patellofemoral ligament reconstruction. Am J Sports Med. 2017;45(5):1085-1089. doi:10.1177/0363546516677795.
140. Edmonds EW, Glaser DA. Adolescent patella instability extensor mechanics. J Pediatr Orthop. 2016;36(3):262-267. doi:10.1097/BPO.0000000000000430.
141. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184. doi:10.1016/j.knee.2014.07.023.
142. Lykissas MG, Li T, Eismann EA, Parikh SN. Does medial patellofemoral ligament reconstruction decrease patellar height? A preliminary report. J Pediatr Orthop. 2014;34(1):78-85. doi:10.1097/BPO.0b013e3182a12102.
143. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2014;22(10):2545-2550. doi:10.1007/s00167-013-2445-8.
144. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521. doi:10.1007/s00167-005-0659-0.
145. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi:10.1177/0363546512463683.
146. Raghuveer RK, Mishra CB. Reconstruction of medial patellofemoral ligament for chronic patellar instability. Indian J Orthop. 2012;46(4):447-454. doi:10.4103/0019-5413.97259.
147. Kita K, Horibe S, Toritsuka Y, et al. Effects of medial patellofemoral ligament reconstruction on patellar tracking. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):829-837. doi:10.1007/s00167-011-1609-7.
148. Lenschow S, Herbort M, Fink C. Medial patellofemoral ligament reconstruction using quadriceps tendon. Oper Orthop Traumatol. 2015;27(6):474-483. doi:10.1007/s00064-015-0416-6.
149. Abouelsoud MM, Abdelhady A, Elshazly O. Anatomic physeal-sparing technique for medial patellofemoral ligament reconstruction in skeletally immature patients with ligamentous laxity. Eur J Orthop Surg Traumatol. 2015;25(5):921-926. doi:10.1007/s00590-015-1618-1.
150. Malecki K, Fabis J, Flont P, Niedzielski KR. The results of adductor magnus tenodesis in adolescents with recurrent patellar dislocation. Biomed Res Int. 2015;2015:1-7. doi:10.1155/2015/456858.
151. Kumahashi N, Kuwata S, Tadenuma T, Kadowaki M, Uchio Y. A “sandwich” method of reconstruction of the medial patellofemoral ligament using a titanium interference screw for patellar instability in skeletally immature patients. Arch Orthop Trauma Surg. 2012;132(8):1077-1083. doi:10.1007/s00402-012-1516-5.
152. Marsh JS, Daigneault JP, Sethi P, Polzhofer GK. Treatment of recurrent patellar instability with a modification of the Roux-Goldthwait technique. J Pediatr Orthop. 2006;26(4):461-465. doi:10.1097/01.bpo.0000217711.34492.48.
153. Nepple J, Luhmann S. Medial patellar tendon transfer with proximal realignment. In: Green D, Cordasco F, eds. Pediatric and Adolescent Knee Surgery. New York, NY: Wolters Kluwer; 2015:148-153.
154. Galeazzi R. Nuove applicazioni del trapianto musculare e tendineo. Ard Di Orthop Milano. 1922;38:315-323.
155. Grannatt K, Heyworth BE, Ogunwole O, Micheli LJ, Kocher MS. Galeazzi semitendinosus tenodesis for patellofemoral instability in skeletally immature patients. J Pediatr Orthop. 2012;32(6):621-625. doi:10.1097/BPO.0b013e318263a230.
156. Fiume M. La rotulopessi secondi Galeazzi nella lussazione recidivante di rotula [in Italian]. Minerva Ortop. 1954;5:171-174.
157. Baker RH, Carroll N, Dewar FP, Hall JE. The semitendinosus tenodesis for recurrent dislocation of the patella. J Bone Joint Surg Br. 1972;54(1):103-109.
158. Hall JE, Micheli LJ, McManama GB. Semitendinosus tenodesis for recurrent subluxation or dislocation of the patella. Clin Orthop Relat Res. 1979;(144):31-35.
159. Moyad TF, Blakemore L. Modified Galeazzi technique for recurrent patellar dislocation in children. Orthopedics. 2006;29(4):302-304.
160. Letts RM, Davidson D, Beaule P. Semitendinosus tenodesis for repair of recurrent dislocation of the patella in children. J Pediatr Orthop. 1999;19(6):742-747.
161. Aulisa AG, Falciglia F, Giordano M, Savignoni P, Guzzanti V. Galeazzi’s modified technique for recurrent patella dislocation in skeletally immature patients. J Orthop Sci. 2012;17(2):148-155. doi:10.1007/s00776-011-0189-1.
162. Roux C. Recurrent dislocation of the patella: operative treatment. 1888. Clin Orthop Relat Res. 2006;452:17-20.
163. Goldthwait J. Dislocation of the Patella. Trans Am Orthop Assn. 1895.
164. Fondren FB, Goldner JL, Bassett FH. Recurrent dislocation of the patella treated by the modified Roux-Goldthwait procedure. A prospective study of forty-seven knees. J Bone Joint Surg Am. 1985;67(7):993-1005.
165. Aärimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446. doi:10.1111/j.1600-0838.2007.00730.x.
166. Nelitz M, Reichel H, Dornacher D, Lippacher S. Anatomical reconstruction of the medial patellofemoral ligament in children with open growth-plates. Arch Orthop Trauma Surg. 2012;132(11):1647-1651. doi:10.1007/s00402-012-1593-5.
167. Vähäsarja V, Kinnunen P, Lanning P, Serlo W. Operative realignment of patellar malalignment in children. J Pediatr Orthop. 1995;15(3):281-285.
168. Abraham E, Washington E, Huang TL. Insall proximal realignment for disorders of the patella. Clin Orthop Relat Res. 1989;248:61-65.
169. Insall JN, Aglietti P, Tria AJ. Patellar pain and incongruence. II: Clinical application. Clin Orthop Relat Res. 1983;176:225-232.
170. Chrisman OD, Snook GA, Wilson TC. A long-term prospective study of the Hauser and Roux-Goldthwait procedures for recurrent patellar dislocation. Clin Orthop Relat Res. 1979;144:27-30.
171. Niedzielski KR, Malecki K, Flont P, Fabis J. The results of an extensive soft-tissue procedure in the treatment of obligatory patellar dislocation in children with ligamentous laxity: a post-operative isokinetic study. Bone Joint J. 2015;97-B(1):129-133. doi:10.1302/0301-620X.97B1.33941.
172. Hinckel BB, Gobbi RG, Demange MK, Bonadio MB, Pécora JR, Camanho GL. Combined reconstruction of the medial patellofemoral ligament with quadricipital tendon and the medial patellotibial ligament with patellar tendon. Arthrosc Tech. 2016;5(1):e79-e84. doi:10.1016/j.eats.2015.10.004.
173. Mani S, Kirkpatrick MS, Saranathan A, Smith LG, Cosgarea AJ, Elias JJ. Tibial tuberosity osteotomy for patellofemoral realignment alters tibiofemoral kinematics. Am J Sports Med. 2011;39(5):1024-1031. doi:10.1177/0363546510390188.
174. Brown GD, Ahmad CS. Combined medial patellofemoral ligament and medial patellotibial ligament reconstruction in skeletally immature patients. J Knee Surg. 2008;21(4):328-332.
175. Panagiotopoulos E, Strzelczyk P, Herrmann M, Scuderi G. Cadaveric study on static medial patellar stabilizers: the dynamizing role of the vastus medialis obliquus on medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):7-12. doi:10.1007/s00167-005-0631-z.
176. Philippot R, Boyer B, Testa R, Farizon F, Moyen B. The role of the medial ligamentous structures on patellar tracking during knee flexion. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):331-336. doi:10.1007/s00167-011-1598-6.
177. Gordon JE, Schoenecker PL. Surgical treatment of congenital dislocation of the patella. J Pediatr Orthop. 1999;19(2):260-264.
178. Koh JL, Stewart C. Patellar instability. Orthop Clin North Am. 2015;46(1):147-157. doi:10.1016/j.ocl.2014.09.011.
179. Pal S, Besier TF, Draper CE, et al. Patellar tilt correlates with vastus lateralis: vastus medialis activation ratio in maltracking patellofemoral pain patients. J Orthop Res. 2012;30(6):927-933. doi:10.1002/jor.22008.
180. Thompson TC. Quadricepsplasty. Ann Surg. 1945;121(5):751-754.
181. Daoud H, O’Farrell T, Cruess RL. Quadricepsplasty. The Judet technique and results of six cases. J Bone Joint Surg Br. 1982;64(2):194-197.
182. Stanisavljevic S, Zemenick G, Miller D. Congenital, irreducible, permanent lateral dislocation of the patella. Clin Orthop Relat Res. 1976;(116):190-199.
183. Kundu Z, Sangwan S, Guliani G, Siwach R, Kamboj P, Singh R. Thompson’s quadricepsplasty for stiff knee. Indian J Orthop. 2007;41(4):390-394. doi:10.4103/0019-5413.37004.
184. Tercier S, Shah H, Joseph B. Quadricepsplasty for congenital dislocation of the knee and congenital quadriceps contracture. J Child Orthop. 2012;6(5):397-410. doi:10.1007/s11832-012-0437-8.
185. Rose RE. Judet quadricepsplasty for extension contracture of the knee. West Indian Med J. 2005;54(4):238-241.
186. Tsukamoto N, Miura H, Matsuda S, Mawatari T, Kato H, Iwamoto Y. Functional evaluation of four patients treated with V-Y quadricepsplasty in total knee arthroplasty. J Orthop Sci. 2006;11(4):394-400. doi:10.1007/s00776-006-1023-z.
187. Dao Q, Chen DB, Scott RD. Proximal patellar quadricepsplasty realignment during total knee arthroplasty for irreducible congenital dislocation of the patella. J Bone Joint Surg Am. 2010;92(14):2457-2461. doi:10.2106/JBJS.H.00812.
188. Judet R, Judet J, Lord G. Results of treatment of stiffness of the knee caused by arthrolysis and disinsertion of the quadriceps femoris. Mem Acad Chir. 1959;85:645-654.
189. Oliveira VG, D’Elia LF, Tirico LEP, et al. Judet quadricepsplasty in the treatment of posttraumatic knee rigidity: long-term outcomes of 45 cases. J Trauma Acute Care Surg. 2012;72(2):e77-e80.
190. Hahn SB, Choi YR, Kang HJ, Lee SH. Prognostic factors and long-term outcomes following a modified Thompson’s quadricepsplasty for severely stiff knees. J Bone Joint Surg Br. 2010;92(2):217-221. doi:10.1302/0301-620X.92B2.22936.
191. Hosalkar HS, Jones S, Chowdhury M, Hartley J, Hill RA. Quadricepsplasty for knee stiffness after femoral lengthening in congenital short femur. J Bone Joint Surg Br. 2003;85(2):261-264.
192. Massè A, Biasibetti A, Demangos J, Dutto E, Pazzano S, Gallinaro P. The judet quadricepsplasty: long-term outcome of 21 cases. J Trauma. 2006;61(2):358-362. doi:10.1097/01.ta.0000230281.31144.1d.
193. Kocon H, Kabacyj M, Zgoda M. The results of the operative treatment of patellar instability in children with Down’s syndrome. J Pediatr Orthop B. 2012;21(5):407-410. doi:10.1097/BPB.0b013e328354f684.
194. Martin BD, Cherkashin AM, Tulchin K, Samchukov M, Birch JG. Treatment of femoral lengthening-related knee stiffness with a novel quadricepsplasty. J Pediatr Orthop. 2013;33(4):446-452. doi:10.1097/BPO.0b013e3182784e5d.
TAKE-HOME POINTS
- Patellofemoral joint stability is dependent on a complex interplay of musculotendinous units, ligaments, and the osteocartilaginous morphology of the patellofemoral joint.
- Varied patterns of patellar instability in the pediatric population should be recognized. Habitual dislocation in flexion and permanent dislocation are the more severe types.
- Assessment of major risk factors and, if required, their correction would influence management decisions and would have prognostic value related to outcomes.
- Physeal-sparing MPFL reconstruction can suffice for most children and adolescents with recurrent patellar dislocation.
- Distal stabilization techniques and quadricepsplasty are an important part of surgical armamentarium, especially for the more complex patellar instability patterns.
Glenoid Bone Loss in Reverse Shoulder Arthroplasty Treated with Bone Graft Techniques
ABSTRACT
The reverse shoulder arthroplasty facilitates surgical treatment of primary and revision shoulder with rotator cuff and bone deficiencies. Wear pattern classifications and a logical treatment approach for glenoid bone loss enable the surgeon to address a difficult series of problems in the reconstructions where the glenoid might not otherwise be able to support the implants. Bone grafting using the native humeral head in primary cases, and in revision cases, iliac crest are the most reliable sources for structural grafts for the worn or deficient glenoid vault.
Continue to: The reverse shoulder arthroplasty...
The reverse shoulder arthroplasty (RSA) technique was approved by the US Food and Drug Administration and introduced to the US market in 2004. It has been a successful addition to the treatment of shoulder pathologies with bone and rotator cuff loss. Its indications have expanded from treatment of very elderly patients with rotator cuff deficiencies to now include younger patients with humeral and glenoid bone loss, arthritis, soft-tissue losses, fractures, instability, and revision arthroplasty. Many of these conditions, when not adequately addressed with anatomic arthroplasty, now have viable treatment options for newer complex and successful reconstructions.
Glenoid bone deficiencies offer unique challenges for successful arthroplasty management. Basing treatment on bone loss classifications permits meaningful evaluation of these surgical options and whether they might be carried out in 1- or 2-stage reconstructions. An underlying premise is that restoration of the glenoid joint line and version assist in final stability, power, and functional results. For this purpose, bone graft options, or augmented implants are beneficial. This review covers the bone grafting options for autografts and allografts for deficient glenoids in reverse shoulder arthroplasty reconstructions.
OPERATIVE TECHNIQUES
For patients without prior arthroplasty, the humeral head is available for bone grafting the glenoid bone deficits. Favard and Hamada have described vertical glenoid classifications for uneven glenoid bone loss applicable to cuff tear arthropathy and inflammatory arthritis patients.1,2 The more severe E3 superior and medial bone loss is ideally addressed with the humeral head. An early example in 2004 confirmed that this was a good indication for glenoid bone grafting and using the reverse shoulder in these advanced cases (Figures 1A-1E).
In this case, it was noted that with bone grafts the base plate post did not engage the native scapula glenoid vault. Given that the on-growth central post was the strongest part of the fixation, it was fortunate that this healed. The need for a longer post with bone grafts was recognized. Laurent Comtat with the Wright Medical company accommodated the author’s request to develop the first 25- and 30-mm-long posts to allow better fixation and on-growth potential when used with bone grafts.
Gilles Walch’s classification addresses arthritic central and horizontal bone loss.3,4 Considerations relevant in RSA include the severe A2 central bone loss found in inflammatory arthritis and the B2, B3, and C patterns with posterior bone loss seen in osteoarthritis, rheumatoid arthritis, and congenital dysplasia5,6 as seen in Figures 2A, 2B. The 3-dimensional (3-D) computed tomography (CT) scan is considered the most accurate method of assessment when compared with axial radiographs.7 The glenoid vault model as a measurement of glenoid bone loss has great promise in designing prosthetic replacements and bone graft techniques.8
Continue to: Modern methods for determining glenoid version...
Modern methods for determining glenoid version, medialization, and eccentric bone wear include 3-D reconstruction and patient-specific instruments. For many years, version determination has been confirmed at surgery with subscapularis elevation, palpating the glenoid center point along Friedman’s line, and then inserting a Steinmann pin as a guide to restore version and the lateral joint line at the time of bone grafting. An example of this is demonstrated in Figures 3A-3E.9
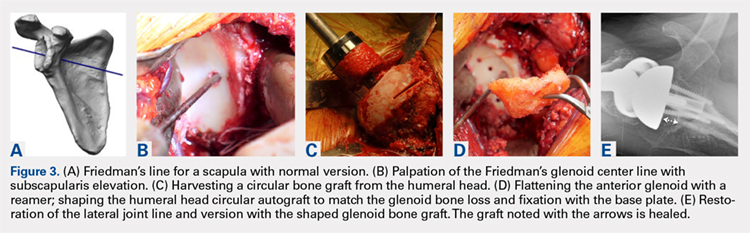
All grafts are harvested with a hole saw from the humeral head. The inner diameter is 29 mm, the same as that of the base plate. Originally, the hole saw and mandrel were obtained from the hardware store, but Pascal Boileau upgraded the hole saw quality when he had industry develop a stainless-steel hole saw and published his results with the BIO-RSA (Wright Medical).10 In an unpublished study, Harmsen reviewed our 220 consecutive humeral head bone grafts for use of this technique with successful and reproducible results. In a separate evaluation, 29 shaped humeral head bone grafts for B2, B3, and C glenoid bone deficits showed 100% healing.11 This technique has good reproducibility when performed with an autogenous bone graft from a local donor source.
The more challenging cases involve glenoid bone loss from polyethylene osteolysis and, in some revision cases, concomitant sepsis.12 The humeral head is no longer available, and the distal clavicle or humeral metaphysis are often insufficient to restore the glenoid vault and joint line. Gunther and associates at the UC Berkeley biomaterials laboratory have made many contributions to our understanding of polyethylene wear and the factors leading to its loosening that result in massive glenoid bone loss.13
Antuna and colleagues14 classified these cases as having a central vault cavitary defect, or one combined with a peripheral glenoid wall bone loss of either the anterior or posterior glenoid. Newton and colleagues15 described the structural tricortical iliac crest bone graft as a 2-stage reconstruction. The second stage could be performed 4 to 6 months later after graft incorporation. With the excellent Association for Osteosynthesis (AO) type fixation using the base plate with compression and locking screws, it was reasonable to perform this in 1 stage, assuming that adequate fixation could be obtained with the iliac bone graft to the glenoid.16 This worked well with the cavitary glenoid defects and those in which either the anterior or posterior wall was absent.17-19
EXCEPTIONS TO THE 1-STAGE FIXATION TECHNIQUE
Fixation could still be obtained medially, but more severe cases were encountered with loss of both the anterior and posterior walls. In these more advanced cases, the vault was no longer present after removal of the polyethylene, cement, and rubbery osteolytic tissue that replaced the bone. To account for this, a simplified 3-stage classification was proposed.20 The cavitary vault defect is designated as type 1 bone loss. Type 2A includes the cavitary central defect plus loss of the anterior glenoid wall, and 2B is similar with loss of the posterior wall (Figures 4A-4F). Type 3 involves loss of the glenoid vault and both anterior and posterior walls with erosion down to the medial juncture of the base of the scapular spine, coracoid, and pillar of the scapula.
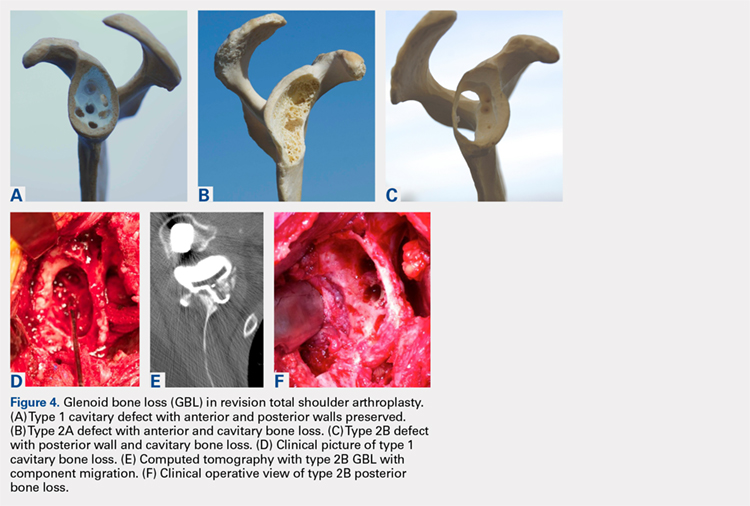
Continue to: The tricortical iliac crest bone graft...
The tricortical iliac crest bone graft (TICBG) offered a structural graft that worked well for these cases of bone loss. When the graft is performed in 1 stage, the glenoid is exposed, and the defect measured after removing the osteolytic, polyethylene-laden tissue from the glenoid. The iliac graft is harvested and placed with the long post base plate engaging the native scapula medially (Figures 5A-5C).
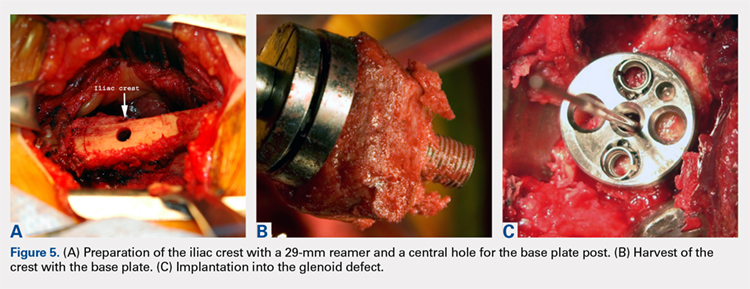
This technique worked well with the type 1 and 2 defects, but when attempted with the type 3 glenoid defect with global glenoid bone loss, adequate fixation for a single-stage reconstruction could not be predictably obtained with type 3 loss of the vault and both walls. In this situation, the base plate post is wider than the remaining medialized glenoid vault (Figures 6A-6C). The iliac crest provides better bone for this global loss when harvested sideways, fixed with screws, and after secure healing, the second-stage base plate is placed (Figures 7A-7F).
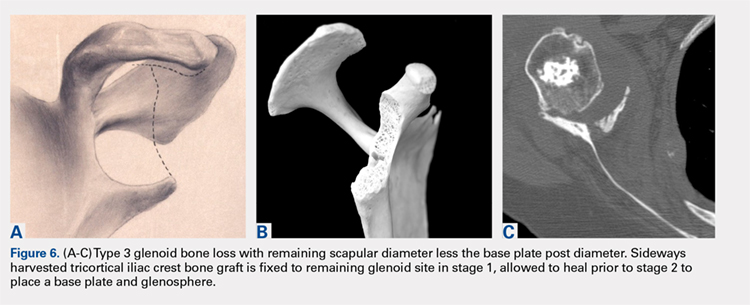
An alternative to the iliac crest as a bone graft donor site is the femoral neck allograft.21 It avoids the additional surgery and pain at the donor site, but healing is less assured. Scalise and Iannotti22 have had good clinical results but noted substantial graft resorption when revising a total shoulder to a humeral head arthroplasty. In a recent report by Ozgur and colleagues,23 64% of femoral neck allografts were still intact at 1-year follow-up. The technique involved harvesting the graft with a hole saw, shaping and affixing it to the deficient glenoid, and gaining central fixation with a threaded or solid post base plate and peripheral screws. Poor results were obtained with the use of the femoral shaft, as it is brittle. Angled peripheral screws caused the allograft shaft to fracture. Low-grade sepsis remained an unanswered problem in the patient group, which averaged 6 prior procedures, and often led to another revision. Less favorable results were found using the 1-piece threaded post base plate with grafts.24 It is assumed that the allograft has less healing potential, and micro motion plays a role when the long central screw has no on-growth healing potential in the native scapula. This graft choice is the author’s least favorite, but is available in desperate situations. Jones and colleagues25 report promising results with bulk allografts and autografts for large glenoid defects with good clinical results. The results in the graft cohort were inferior to those in a matched group not requiring grafts. Their complications were consistent with the revision setting for shoulders having multiple operations. It is well known that preoperative factors are strong predictors of postoperative outcomes.26
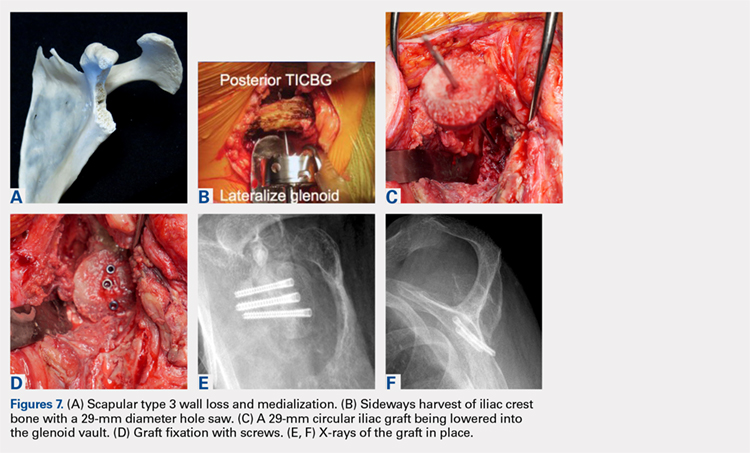
CONCLUSION
The author’s current technique is to use the native humeral head when available, or iliac crest for structural support to the base plate and glenosphere. Secure fixation to the native scapula is necessary if the operation is to be done in 1-stage. Incorporation with calcium orthophosphate bone substitution does not replace the need for structural support as shown in Figures 8A-8D.
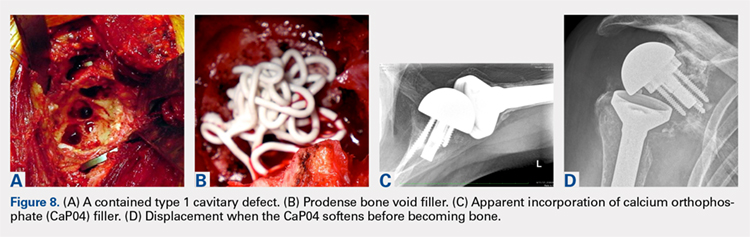
For the type 2 vault and 1 wall glenoid bone loss defects, the TICBG is still the most useful option. For the type 3 global bone loss defects, a 2-stage approach is the safer option. Additional options that may replace some of these grafting techniques are the introduction of the metallic augmented ingrowth base plates to correct for superior, anterior, and posterior glenoid bone losses. The early unpublished experiences by Wright and colleagues are very promising. All of the above options should be available in the operating room for a busy arthroplasty surgeon.
1. Hamada K, Fukuda H, Mikasa M, Kobay Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;254:92-96.
2. Favard L, Alami G. The glenoid in the frontal plane: The Favard and Hamada radiographic classifications of cuff tear osteoarthritis. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:53-58.
3. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Walch G. The glenoid in the horizontal plane: Walch classification revisited humeral subluxation and glenoid retroversion. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:45-51.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphological study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. Iannotti JP, Ricchetti E. Walch classification: adding two new glenoid types. Orthopaedic Insights Cleveland Clinic. 2017:6-7.
6. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse shoulder arthroplasty for primary glenohumeral osteoarthritis with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.
7. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: conventional radiographs verses computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
8. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional compute tomography scans. [published online ahead of print January 22, 2008]. J Shoulder Elbow Surg. 2008;17(2):328-335.
9. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
10. Boileau P, Moineau G, Roussanne Y, O’Shea K. Bony increased-offset reverse shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567.
11. Harmsen S, Casagrande D, Norris T: “Shaped” humeral head autograft reverse shoulder arthroplasty: Treatment for primary glenohumeral osteoarthritis with significant posterior glenoid bone loss (B2, B3, and C-type). Orthopade. 2017;46(12):1045-1054.
12. Norris TR, Phipatanakul WP. Treatment of glenoid loosening and bone loss due to osteolysis with glenoid bone grafting. J Shoulder Elbow Surg. 2006;15(1):84-87.
13. Farzana F, Lee T, Malito L, et al. Analysis of severely fractured glenoid components: clinical consequences of biomechanics, design, and materials selection on implant performance. J Shoulder Elbow Surg. 2016;25(7):1041-1050.
14. Antuña SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
15. Newton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid cortical cancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg. 2006;15(2):173-179.
16. Norris TR, Kelly JD, Humphrey CS. Management of glenoid bone defects in revision shoulder arthroplasty: a new application of the reverse total shoulder prosthesis. Techniques Shoulder Elbow Surgery. 2007;8(1):37-46.
17. Kelly JD II, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525.
18. Norris TR. Reconstruction of glenoid bone loss in total shoulder arthroplasty. In: Boileau P, ed. Shoulder Concepts 2008-Arthroscopy and Arthroplasty. Paris, France: Sauramps Medical; 2008:397-404.
19. Humphrey CS, Kelly JD, Norris TR. Management of glenoid deficiency in reverse shoulder arthroplasty. In: Fealy S, Warren RF, Craig EV, Sperling JW, eds. Shoulder Arthroplasty. New York, NY: Thieme; 2006.
20. Norris TR, Abdus-Salaam S. Lessons learned from the Hylamer experience and technical salvage for glenoid reconstruction. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:265-278.
21. Bateman E, Donald SM. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg. 2012;21(7):925-934.
22. Scalise JJ, Iannotti JP. Bone grafting severe glenoid defects in revision shoulder arthroplasty. Clin Orthop. 2008;466(1):139-145.
23. Ozgur S, Sadeghpour R, Norris TR. Revision shoulder arthroplasty with a reverse shoulder prosthesis. Use of structural allograft for glenoid bone loss. Orthopade. 2017;46(12):1055-1062.
24. Sadeghpour R, Ozgur S, Norris TR. Threaded post baseplate failures in RSA. In: Hardy PH, Valenti PH, Scheibel M, eds. Shoulder Arthroplasty, Current Concepts. Paris International Shoulder Course 2017. 2017:148-157.
25. Jones RB, Wright TW, Zuckerman JD. Reverse total shoulder arthroplasty with structural bone grafting of large glenoid defects. J Shoulder Elbow Surg. 2016;25(9):1425-1432.
26. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg. 2003;85(2):251-258.
ABSTRACT
The reverse shoulder arthroplasty facilitates surgical treatment of primary and revision shoulder with rotator cuff and bone deficiencies. Wear pattern classifications and a logical treatment approach for glenoid bone loss enable the surgeon to address a difficult series of problems in the reconstructions where the glenoid might not otherwise be able to support the implants. Bone grafting using the native humeral head in primary cases, and in revision cases, iliac crest are the most reliable sources for structural grafts for the worn or deficient glenoid vault.
Continue to: The reverse shoulder arthroplasty...
The reverse shoulder arthroplasty (RSA) technique was approved by the US Food and Drug Administration and introduced to the US market in 2004. It has been a successful addition to the treatment of shoulder pathologies with bone and rotator cuff loss. Its indications have expanded from treatment of very elderly patients with rotator cuff deficiencies to now include younger patients with humeral and glenoid bone loss, arthritis, soft-tissue losses, fractures, instability, and revision arthroplasty. Many of these conditions, when not adequately addressed with anatomic arthroplasty, now have viable treatment options for newer complex and successful reconstructions.
Glenoid bone deficiencies offer unique challenges for successful arthroplasty management. Basing treatment on bone loss classifications permits meaningful evaluation of these surgical options and whether they might be carried out in 1- or 2-stage reconstructions. An underlying premise is that restoration of the glenoid joint line and version assist in final stability, power, and functional results. For this purpose, bone graft options, or augmented implants are beneficial. This review covers the bone grafting options for autografts and allografts for deficient glenoids in reverse shoulder arthroplasty reconstructions.
OPERATIVE TECHNIQUES
For patients without prior arthroplasty, the humeral head is available for bone grafting the glenoid bone deficits. Favard and Hamada have described vertical glenoid classifications for uneven glenoid bone loss applicable to cuff tear arthropathy and inflammatory arthritis patients.1,2 The more severe E3 superior and medial bone loss is ideally addressed with the humeral head. An early example in 2004 confirmed that this was a good indication for glenoid bone grafting and using the reverse shoulder in these advanced cases (Figures 1A-1E).

In this case, it was noted that with bone grafts the base plate post did not engage the native scapula glenoid vault. Given that the on-growth central post was the strongest part of the fixation, it was fortunate that this healed. The need for a longer post with bone grafts was recognized. Laurent Comtat with the Wright Medical company accommodated the author’s request to develop the first 25- and 30-mm-long posts to allow better fixation and on-growth potential when used with bone grafts.
Gilles Walch’s classification addresses arthritic central and horizontal bone loss.3,4 Considerations relevant in RSA include the severe A2 central bone loss found in inflammatory arthritis and the B2, B3, and C patterns with posterior bone loss seen in osteoarthritis, rheumatoid arthritis, and congenital dysplasia5,6 as seen in Figures 2A, 2B. The 3-dimensional (3-D) computed tomography (CT) scan is considered the most accurate method of assessment when compared with axial radiographs.7 The glenoid vault model as a measurement of glenoid bone loss has great promise in designing prosthetic replacements and bone graft techniques.8

Continue to: Modern methods for determining glenoid version...
Modern methods for determining glenoid version, medialization, and eccentric bone wear include 3-D reconstruction and patient-specific instruments. For many years, version determination has been confirmed at surgery with subscapularis elevation, palpating the glenoid center point along Friedman’s line, and then inserting a Steinmann pin as a guide to restore version and the lateral joint line at the time of bone grafting. An example of this is demonstrated in Figures 3A-3E.9

All grafts are harvested with a hole saw from the humeral head. The inner diameter is 29 mm, the same as that of the base plate. Originally, the hole saw and mandrel were obtained from the hardware store, but Pascal Boileau upgraded the hole saw quality when he had industry develop a stainless-steel hole saw and published his results with the BIO-RSA (Wright Medical).10 In an unpublished study, Harmsen reviewed our 220 consecutive humeral head bone grafts for use of this technique with successful and reproducible results. In a separate evaluation, 29 shaped humeral head bone grafts for B2, B3, and C glenoid bone deficits showed 100% healing.11 This technique has good reproducibility when performed with an autogenous bone graft from a local donor source.
The more challenging cases involve glenoid bone loss from polyethylene osteolysis and, in some revision cases, concomitant sepsis.12 The humeral head is no longer available, and the distal clavicle or humeral metaphysis are often insufficient to restore the glenoid vault and joint line. Gunther and associates at the UC Berkeley biomaterials laboratory have made many contributions to our understanding of polyethylene wear and the factors leading to its loosening that result in massive glenoid bone loss.13
Antuna and colleagues14 classified these cases as having a central vault cavitary defect, or one combined with a peripheral glenoid wall bone loss of either the anterior or posterior glenoid. Newton and colleagues15 described the structural tricortical iliac crest bone graft as a 2-stage reconstruction. The second stage could be performed 4 to 6 months later after graft incorporation. With the excellent Association for Osteosynthesis (AO) type fixation using the base plate with compression and locking screws, it was reasonable to perform this in 1 stage, assuming that adequate fixation could be obtained with the iliac bone graft to the glenoid.16 This worked well with the cavitary glenoid defects and those in which either the anterior or posterior wall was absent.17-19
EXCEPTIONS TO THE 1-STAGE FIXATION TECHNIQUE
Fixation could still be obtained medially, but more severe cases were encountered with loss of both the anterior and posterior walls. In these more advanced cases, the vault was no longer present after removal of the polyethylene, cement, and rubbery osteolytic tissue that replaced the bone. To account for this, a simplified 3-stage classification was proposed.20 The cavitary vault defect is designated as type 1 bone loss. Type 2A includes the cavitary central defect plus loss of the anterior glenoid wall, and 2B is similar with loss of the posterior wall (Figures 4A-4F). Type 3 involves loss of the glenoid vault and both anterior and posterior walls with erosion down to the medial juncture of the base of the scapular spine, coracoid, and pillar of the scapula.

Continue to: The tricortical iliac crest bone graft...
The tricortical iliac crest bone graft (TICBG) offered a structural graft that worked well for these cases of bone loss. When the graft is performed in 1 stage, the glenoid is exposed, and the defect measured after removing the osteolytic, polyethylene-laden tissue from the glenoid. The iliac graft is harvested and placed with the long post base plate engaging the native scapula medially (Figures 5A-5C).

This technique worked well with the type 1 and 2 defects, but when attempted with the type 3 glenoid defect with global glenoid bone loss, adequate fixation for a single-stage reconstruction could not be predictably obtained with type 3 loss of the vault and both walls. In this situation, the base plate post is wider than the remaining medialized glenoid vault (Figures 6A-6C). The iliac crest provides better bone for this global loss when harvested sideways, fixed with screws, and after secure healing, the second-stage base plate is placed (Figures 7A-7F).

An alternative to the iliac crest as a bone graft donor site is the femoral neck allograft.21 It avoids the additional surgery and pain at the donor site, but healing is less assured. Scalise and Iannotti22 have had good clinical results but noted substantial graft resorption when revising a total shoulder to a humeral head arthroplasty. In a recent report by Ozgur and colleagues,23 64% of femoral neck allografts were still intact at 1-year follow-up. The technique involved harvesting the graft with a hole saw, shaping and affixing it to the deficient glenoid, and gaining central fixation with a threaded or solid post base plate and peripheral screws. Poor results were obtained with the use of the femoral shaft, as it is brittle. Angled peripheral screws caused the allograft shaft to fracture. Low-grade sepsis remained an unanswered problem in the patient group, which averaged 6 prior procedures, and often led to another revision. Less favorable results were found using the 1-piece threaded post base plate with grafts.24 It is assumed that the allograft has less healing potential, and micro motion plays a role when the long central screw has no on-growth healing potential in the native scapula. This graft choice is the author’s least favorite, but is available in desperate situations. Jones and colleagues25 report promising results with bulk allografts and autografts for large glenoid defects with good clinical results. The results in the graft cohort were inferior to those in a matched group not requiring grafts. Their complications were consistent with the revision setting for shoulders having multiple operations. It is well known that preoperative factors are strong predictors of postoperative outcomes.26

CONCLUSION
The author’s current technique is to use the native humeral head when available, or iliac crest for structural support to the base plate and glenosphere. Secure fixation to the native scapula is necessary if the operation is to be done in 1-stage. Incorporation with calcium orthophosphate bone substitution does not replace the need for structural support as shown in Figures 8A-8D.

For the type 2 vault and 1 wall glenoid bone loss defects, the TICBG is still the most useful option. For the type 3 global bone loss defects, a 2-stage approach is the safer option. Additional options that may replace some of these grafting techniques are the introduction of the metallic augmented ingrowth base plates to correct for superior, anterior, and posterior glenoid bone losses. The early unpublished experiences by Wright and colleagues are very promising. All of the above options should be available in the operating room for a busy arthroplasty surgeon.
ABSTRACT
The reverse shoulder arthroplasty facilitates surgical treatment of primary and revision shoulder with rotator cuff and bone deficiencies. Wear pattern classifications and a logical treatment approach for glenoid bone loss enable the surgeon to address a difficult series of problems in the reconstructions where the glenoid might not otherwise be able to support the implants. Bone grafting using the native humeral head in primary cases, and in revision cases, iliac crest are the most reliable sources for structural grafts for the worn or deficient glenoid vault.
Continue to: The reverse shoulder arthroplasty...
The reverse shoulder arthroplasty (RSA) technique was approved by the US Food and Drug Administration and introduced to the US market in 2004. It has been a successful addition to the treatment of shoulder pathologies with bone and rotator cuff loss. Its indications have expanded from treatment of very elderly patients with rotator cuff deficiencies to now include younger patients with humeral and glenoid bone loss, arthritis, soft-tissue losses, fractures, instability, and revision arthroplasty. Many of these conditions, when not adequately addressed with anatomic arthroplasty, now have viable treatment options for newer complex and successful reconstructions.
Glenoid bone deficiencies offer unique challenges for successful arthroplasty management. Basing treatment on bone loss classifications permits meaningful evaluation of these surgical options and whether they might be carried out in 1- or 2-stage reconstructions. An underlying premise is that restoration of the glenoid joint line and version assist in final stability, power, and functional results. For this purpose, bone graft options, or augmented implants are beneficial. This review covers the bone grafting options for autografts and allografts for deficient glenoids in reverse shoulder arthroplasty reconstructions.
OPERATIVE TECHNIQUES
For patients without prior arthroplasty, the humeral head is available for bone grafting the glenoid bone deficits. Favard and Hamada have described vertical glenoid classifications for uneven glenoid bone loss applicable to cuff tear arthropathy and inflammatory arthritis patients.1,2 The more severe E3 superior and medial bone loss is ideally addressed with the humeral head. An early example in 2004 confirmed that this was a good indication for glenoid bone grafting and using the reverse shoulder in these advanced cases (Figures 1A-1E).

In this case, it was noted that with bone grafts the base plate post did not engage the native scapula glenoid vault. Given that the on-growth central post was the strongest part of the fixation, it was fortunate that this healed. The need for a longer post with bone grafts was recognized. Laurent Comtat with the Wright Medical company accommodated the author’s request to develop the first 25- and 30-mm-long posts to allow better fixation and on-growth potential when used with bone grafts.
Gilles Walch’s classification addresses arthritic central and horizontal bone loss.3,4 Considerations relevant in RSA include the severe A2 central bone loss found in inflammatory arthritis and the B2, B3, and C patterns with posterior bone loss seen in osteoarthritis, rheumatoid arthritis, and congenital dysplasia5,6 as seen in Figures 2A, 2B. The 3-dimensional (3-D) computed tomography (CT) scan is considered the most accurate method of assessment when compared with axial radiographs.7 The glenoid vault model as a measurement of glenoid bone loss has great promise in designing prosthetic replacements and bone graft techniques.8

Continue to: Modern methods for determining glenoid version...
Modern methods for determining glenoid version, medialization, and eccentric bone wear include 3-D reconstruction and patient-specific instruments. For many years, version determination has been confirmed at surgery with subscapularis elevation, palpating the glenoid center point along Friedman’s line, and then inserting a Steinmann pin as a guide to restore version and the lateral joint line at the time of bone grafting. An example of this is demonstrated in Figures 3A-3E.9

All grafts are harvested with a hole saw from the humeral head. The inner diameter is 29 mm, the same as that of the base plate. Originally, the hole saw and mandrel were obtained from the hardware store, but Pascal Boileau upgraded the hole saw quality when he had industry develop a stainless-steel hole saw and published his results with the BIO-RSA (Wright Medical).10 In an unpublished study, Harmsen reviewed our 220 consecutive humeral head bone grafts for use of this technique with successful and reproducible results. In a separate evaluation, 29 shaped humeral head bone grafts for B2, B3, and C glenoid bone deficits showed 100% healing.11 This technique has good reproducibility when performed with an autogenous bone graft from a local donor source.
The more challenging cases involve glenoid bone loss from polyethylene osteolysis and, in some revision cases, concomitant sepsis.12 The humeral head is no longer available, and the distal clavicle or humeral metaphysis are often insufficient to restore the glenoid vault and joint line. Gunther and associates at the UC Berkeley biomaterials laboratory have made many contributions to our understanding of polyethylene wear and the factors leading to its loosening that result in massive glenoid bone loss.13
Antuna and colleagues14 classified these cases as having a central vault cavitary defect, or one combined with a peripheral glenoid wall bone loss of either the anterior or posterior glenoid. Newton and colleagues15 described the structural tricortical iliac crest bone graft as a 2-stage reconstruction. The second stage could be performed 4 to 6 months later after graft incorporation. With the excellent Association for Osteosynthesis (AO) type fixation using the base plate with compression and locking screws, it was reasonable to perform this in 1 stage, assuming that adequate fixation could be obtained with the iliac bone graft to the glenoid.16 This worked well with the cavitary glenoid defects and those in which either the anterior or posterior wall was absent.17-19
EXCEPTIONS TO THE 1-STAGE FIXATION TECHNIQUE
Fixation could still be obtained medially, but more severe cases were encountered with loss of both the anterior and posterior walls. In these more advanced cases, the vault was no longer present after removal of the polyethylene, cement, and rubbery osteolytic tissue that replaced the bone. To account for this, a simplified 3-stage classification was proposed.20 The cavitary vault defect is designated as type 1 bone loss. Type 2A includes the cavitary central defect plus loss of the anterior glenoid wall, and 2B is similar with loss of the posterior wall (Figures 4A-4F). Type 3 involves loss of the glenoid vault and both anterior and posterior walls with erosion down to the medial juncture of the base of the scapular spine, coracoid, and pillar of the scapula.

Continue to: The tricortical iliac crest bone graft...
The tricortical iliac crest bone graft (TICBG) offered a structural graft that worked well for these cases of bone loss. When the graft is performed in 1 stage, the glenoid is exposed, and the defect measured after removing the osteolytic, polyethylene-laden tissue from the glenoid. The iliac graft is harvested and placed with the long post base plate engaging the native scapula medially (Figures 5A-5C).

This technique worked well with the type 1 and 2 defects, but when attempted with the type 3 glenoid defect with global glenoid bone loss, adequate fixation for a single-stage reconstruction could not be predictably obtained with type 3 loss of the vault and both walls. In this situation, the base plate post is wider than the remaining medialized glenoid vault (Figures 6A-6C). The iliac crest provides better bone for this global loss when harvested sideways, fixed with screws, and after secure healing, the second-stage base plate is placed (Figures 7A-7F).

An alternative to the iliac crest as a bone graft donor site is the femoral neck allograft.21 It avoids the additional surgery and pain at the donor site, but healing is less assured. Scalise and Iannotti22 have had good clinical results but noted substantial graft resorption when revising a total shoulder to a humeral head arthroplasty. In a recent report by Ozgur and colleagues,23 64% of femoral neck allografts were still intact at 1-year follow-up. The technique involved harvesting the graft with a hole saw, shaping and affixing it to the deficient glenoid, and gaining central fixation with a threaded or solid post base plate and peripheral screws. Poor results were obtained with the use of the femoral shaft, as it is brittle. Angled peripheral screws caused the allograft shaft to fracture. Low-grade sepsis remained an unanswered problem in the patient group, which averaged 6 prior procedures, and often led to another revision. Less favorable results were found using the 1-piece threaded post base plate with grafts.24 It is assumed that the allograft has less healing potential, and micro motion plays a role when the long central screw has no on-growth healing potential in the native scapula. This graft choice is the author’s least favorite, but is available in desperate situations. Jones and colleagues25 report promising results with bulk allografts and autografts for large glenoid defects with good clinical results. The results in the graft cohort were inferior to those in a matched group not requiring grafts. Their complications were consistent with the revision setting for shoulders having multiple operations. It is well known that preoperative factors are strong predictors of postoperative outcomes.26

CONCLUSION
The author’s current technique is to use the native humeral head when available, or iliac crest for structural support to the base plate and glenosphere. Secure fixation to the native scapula is necessary if the operation is to be done in 1-stage. Incorporation with calcium orthophosphate bone substitution does not replace the need for structural support as shown in Figures 8A-8D.

For the type 2 vault and 1 wall glenoid bone loss defects, the TICBG is still the most useful option. For the type 3 global bone loss defects, a 2-stage approach is the safer option. Additional options that may replace some of these grafting techniques are the introduction of the metallic augmented ingrowth base plates to correct for superior, anterior, and posterior glenoid bone losses. The early unpublished experiences by Wright and colleagues are very promising. All of the above options should be available in the operating room for a busy arthroplasty surgeon.
1. Hamada K, Fukuda H, Mikasa M, Kobay Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;254:92-96.
2. Favard L, Alami G. The glenoid in the frontal plane: The Favard and Hamada radiographic classifications of cuff tear osteoarthritis. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:53-58.
3. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Walch G. The glenoid in the horizontal plane: Walch classification revisited humeral subluxation and glenoid retroversion. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:45-51.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphological study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. Iannotti JP, Ricchetti E. Walch classification: adding two new glenoid types. Orthopaedic Insights Cleveland Clinic. 2017:6-7.
6. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse shoulder arthroplasty for primary glenohumeral osteoarthritis with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.
7. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: conventional radiographs verses computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
8. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional compute tomography scans. [published online ahead of print January 22, 2008]. J Shoulder Elbow Surg. 2008;17(2):328-335.
9. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
10. Boileau P, Moineau G, Roussanne Y, O’Shea K. Bony increased-offset reverse shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567.
11. Harmsen S, Casagrande D, Norris T: “Shaped” humeral head autograft reverse shoulder arthroplasty: Treatment for primary glenohumeral osteoarthritis with significant posterior glenoid bone loss (B2, B3, and C-type). Orthopade. 2017;46(12):1045-1054.
12. Norris TR, Phipatanakul WP. Treatment of glenoid loosening and bone loss due to osteolysis with glenoid bone grafting. J Shoulder Elbow Surg. 2006;15(1):84-87.
13. Farzana F, Lee T, Malito L, et al. Analysis of severely fractured glenoid components: clinical consequences of biomechanics, design, and materials selection on implant performance. J Shoulder Elbow Surg. 2016;25(7):1041-1050.
14. Antuña SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
15. Newton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid cortical cancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg. 2006;15(2):173-179.
16. Norris TR, Kelly JD, Humphrey CS. Management of glenoid bone defects in revision shoulder arthroplasty: a new application of the reverse total shoulder prosthesis. Techniques Shoulder Elbow Surgery. 2007;8(1):37-46.
17. Kelly JD II, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525.
18. Norris TR. Reconstruction of glenoid bone loss in total shoulder arthroplasty. In: Boileau P, ed. Shoulder Concepts 2008-Arthroscopy and Arthroplasty. Paris, France: Sauramps Medical; 2008:397-404.
19. Humphrey CS, Kelly JD, Norris TR. Management of glenoid deficiency in reverse shoulder arthroplasty. In: Fealy S, Warren RF, Craig EV, Sperling JW, eds. Shoulder Arthroplasty. New York, NY: Thieme; 2006.
20. Norris TR, Abdus-Salaam S. Lessons learned from the Hylamer experience and technical salvage for glenoid reconstruction. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:265-278.
21. Bateman E, Donald SM. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg. 2012;21(7):925-934.
22. Scalise JJ, Iannotti JP. Bone grafting severe glenoid defects in revision shoulder arthroplasty. Clin Orthop. 2008;466(1):139-145.
23. Ozgur S, Sadeghpour R, Norris TR. Revision shoulder arthroplasty with a reverse shoulder prosthesis. Use of structural allograft for glenoid bone loss. Orthopade. 2017;46(12):1055-1062.
24. Sadeghpour R, Ozgur S, Norris TR. Threaded post baseplate failures in RSA. In: Hardy PH, Valenti PH, Scheibel M, eds. Shoulder Arthroplasty, Current Concepts. Paris International Shoulder Course 2017. 2017:148-157.
25. Jones RB, Wright TW, Zuckerman JD. Reverse total shoulder arthroplasty with structural bone grafting of large glenoid defects. J Shoulder Elbow Surg. 2016;25(9):1425-1432.
26. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg. 2003;85(2):251-258.
1. Hamada K, Fukuda H, Mikasa M, Kobay Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;254:92-96.
2. Favard L, Alami G. The glenoid in the frontal plane: The Favard and Hamada radiographic classifications of cuff tear osteoarthritis. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:53-58.
3. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Walch G. The glenoid in the horizontal plane: Walch classification revisited humeral subluxation and glenoid retroversion. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:45-51.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphological study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. Iannotti JP, Ricchetti E. Walch classification: adding two new glenoid types. Orthopaedic Insights Cleveland Clinic. 2017:6-7.
6. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse shoulder arthroplasty for primary glenohumeral osteoarthritis with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.
7. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: conventional radiographs verses computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
8. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional compute tomography scans. [published online ahead of print January 22, 2008]. J Shoulder Elbow Surg. 2008;17(2):328-335.
9. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
10. Boileau P, Moineau G, Roussanne Y, O’Shea K. Bony increased-offset reverse shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567.
11. Harmsen S, Casagrande D, Norris T: “Shaped” humeral head autograft reverse shoulder arthroplasty: Treatment for primary glenohumeral osteoarthritis with significant posterior glenoid bone loss (B2, B3, and C-type). Orthopade. 2017;46(12):1045-1054.
12. Norris TR, Phipatanakul WP. Treatment of glenoid loosening and bone loss due to osteolysis with glenoid bone grafting. J Shoulder Elbow Surg. 2006;15(1):84-87.
13. Farzana F, Lee T, Malito L, et al. Analysis of severely fractured glenoid components: clinical consequences of biomechanics, design, and materials selection on implant performance. J Shoulder Elbow Surg. 2016;25(7):1041-1050.
14. Antuña SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
15. Newton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid cortical cancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg. 2006;15(2):173-179.
16. Norris TR, Kelly JD, Humphrey CS. Management of glenoid bone defects in revision shoulder arthroplasty: a new application of the reverse total shoulder prosthesis. Techniques Shoulder Elbow Surgery. 2007;8(1):37-46.
17. Kelly JD II, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525.
18. Norris TR. Reconstruction of glenoid bone loss in total shoulder arthroplasty. In: Boileau P, ed. Shoulder Concepts 2008-Arthroscopy and Arthroplasty. Paris, France: Sauramps Medical; 2008:397-404.
19. Humphrey CS, Kelly JD, Norris TR. Management of glenoid deficiency in reverse shoulder arthroplasty. In: Fealy S, Warren RF, Craig EV, Sperling JW, eds. Shoulder Arthroplasty. New York, NY: Thieme; 2006.
20. Norris TR, Abdus-Salaam S. Lessons learned from the Hylamer experience and technical salvage for glenoid reconstruction. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:265-278.
21. Bateman E, Donald SM. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg. 2012;21(7):925-934.
22. Scalise JJ, Iannotti JP. Bone grafting severe glenoid defects in revision shoulder arthroplasty. Clin Orthop. 2008;466(1):139-145.
23. Ozgur S, Sadeghpour R, Norris TR. Revision shoulder arthroplasty with a reverse shoulder prosthesis. Use of structural allograft for glenoid bone loss. Orthopade. 2017;46(12):1055-1062.
24. Sadeghpour R, Ozgur S, Norris TR. Threaded post baseplate failures in RSA. In: Hardy PH, Valenti PH, Scheibel M, eds. Shoulder Arthroplasty, Current Concepts. Paris International Shoulder Course 2017. 2017:148-157.
25. Jones RB, Wright TW, Zuckerman JD. Reverse total shoulder arthroplasty with structural bone grafting of large glenoid defects. J Shoulder Elbow Surg. 2016;25(9):1425-1432.
26. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg. 2003;85(2):251-258.
TAKE-HOME POINTS
- Glenoid deficiencies that occur from dysplasia, arthritis, or polyethylene osteolysis may be successfully addressed with bone grafting techniques and reverse shoulder arthroplasty.
- The intact humeral head in a primary case is ideal graft to be shaped to fit the glenoid deficits.
- The reverse shoulder with a long post base plate that is fixed securely to the native scapula is the author’s preferred technique.
- As the native humeral head is not available in revision cases, the tricortical iliac crest bone graft may be fixed as a structural graft in 1-stage.
- When the scapular walls are deficient and medial fixation is not secure, 2 stages 4 months to 6 months apart will be necessary before loading the construct.
Study links RA flares after joint replacement to disease activity, not medications
Patients with the most severe cases of rheumatoid arthritis are more likely to suffer flares after knee or hip replacement surgery, a new study finds, and it doesn’t seem to matter whether they stop taking biologics before their operation.
“We found that the majority of patients had active disease at the time of surgery, contrary to prior statements that RA patients have inactive disease at the time they go for hip or knee replacement. In fact, the majority – 65% of the patients – reported a flare of RA within 6 weeks of surgery,” lead author Susan M. Goodman, MD, of Cornell University and the Hospital for Special Surgery, New York, said in an interview. “Surprisingly, although more of the flaring patients were taking potent biologics that had been withheld preoperatively, the major risk factor for flares was their baseline disease activity.”
According to Dr. Goodman, the researchers launched the study to better understand how medical decisions prior to joint replacement surgery affect the progress of RA afterward.
In terms of continuing RA drug treatment, she said, “the decision really hinges on the risk of infection versus the risk of flare, and we didn’t know the usual course of events for these patients.”
In addition, she said, “many doctors incorrectly think that the majority of patients with RA have ‘burnt-out’ or inactive disease at the time of hip or knee replacement surgery.”
For the study, the researchers prospectively followed 120 patients who were to undergo joint replacement surgery. (The researchers initially approached 354 patients, of whom 169 declined to participate. Another 65 were dropped from the study for various reasons, including 42 who did not sufficiently fill out questionnaires and were deleted from the final analysis.)
The researchers tracked the patients before surgery and for 6 weeks after surgery. A majority of the patients were female (83%) and white (81%), with a mean age of 62 and a median RA symptom duration of 15 years. A total of 44% underwent hip replacement surgery while the rest underwent knee replacement surgery. Just over half of the patients were taking biologics, which were stopped prior to surgery, while glucocorticoids and methotrexate were usually continued.
Just under two-thirds of the patients flared within the first 6 weeks after surgery. The researchers didn’t find any connection between the flares and stopping biologics or using methotrexate. They did, however, link higher baseline RA activity to postsurgery flaring (odds ratio, 2.11; P = .015).
Dr. Goodman said that she and her colleagues continue to collect data to better understand flares and the link to disease severity. “The long-term implications of this are not yet known. We would like to know the effect on long-term functional outcome and complication rate.”
The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. Dr. Goodman disclosed receiving research funding from Novartis and Roche.
SOURCE: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366
Patients with the most severe cases of rheumatoid arthritis are more likely to suffer flares after knee or hip replacement surgery, a new study finds, and it doesn’t seem to matter whether they stop taking biologics before their operation.
“We found that the majority of patients had active disease at the time of surgery, contrary to prior statements that RA patients have inactive disease at the time they go for hip or knee replacement. In fact, the majority – 65% of the patients – reported a flare of RA within 6 weeks of surgery,” lead author Susan M. Goodman, MD, of Cornell University and the Hospital for Special Surgery, New York, said in an interview. “Surprisingly, although more of the flaring patients were taking potent biologics that had been withheld preoperatively, the major risk factor for flares was their baseline disease activity.”
According to Dr. Goodman, the researchers launched the study to better understand how medical decisions prior to joint replacement surgery affect the progress of RA afterward.
In terms of continuing RA drug treatment, she said, “the decision really hinges on the risk of infection versus the risk of flare, and we didn’t know the usual course of events for these patients.”
In addition, she said, “many doctors incorrectly think that the majority of patients with RA have ‘burnt-out’ or inactive disease at the time of hip or knee replacement surgery.”
For the study, the researchers prospectively followed 120 patients who were to undergo joint replacement surgery. (The researchers initially approached 354 patients, of whom 169 declined to participate. Another 65 were dropped from the study for various reasons, including 42 who did not sufficiently fill out questionnaires and were deleted from the final analysis.)
The researchers tracked the patients before surgery and for 6 weeks after surgery. A majority of the patients were female (83%) and white (81%), with a mean age of 62 and a median RA symptom duration of 15 years. A total of 44% underwent hip replacement surgery while the rest underwent knee replacement surgery. Just over half of the patients were taking biologics, which were stopped prior to surgery, while glucocorticoids and methotrexate were usually continued.
Just under two-thirds of the patients flared within the first 6 weeks after surgery. The researchers didn’t find any connection between the flares and stopping biologics or using methotrexate. They did, however, link higher baseline RA activity to postsurgery flaring (odds ratio, 2.11; P = .015).
Dr. Goodman said that she and her colleagues continue to collect data to better understand flares and the link to disease severity. “The long-term implications of this are not yet known. We would like to know the effect on long-term functional outcome and complication rate.”
The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. Dr. Goodman disclosed receiving research funding from Novartis and Roche.
SOURCE: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366
Patients with the most severe cases of rheumatoid arthritis are more likely to suffer flares after knee or hip replacement surgery, a new study finds, and it doesn’t seem to matter whether they stop taking biologics before their operation.
“We found that the majority of patients had active disease at the time of surgery, contrary to prior statements that RA patients have inactive disease at the time they go for hip or knee replacement. In fact, the majority – 65% of the patients – reported a flare of RA within 6 weeks of surgery,” lead author Susan M. Goodman, MD, of Cornell University and the Hospital for Special Surgery, New York, said in an interview. “Surprisingly, although more of the flaring patients were taking potent biologics that had been withheld preoperatively, the major risk factor for flares was their baseline disease activity.”
According to Dr. Goodman, the researchers launched the study to better understand how medical decisions prior to joint replacement surgery affect the progress of RA afterward.
In terms of continuing RA drug treatment, she said, “the decision really hinges on the risk of infection versus the risk of flare, and we didn’t know the usual course of events for these patients.”
In addition, she said, “many doctors incorrectly think that the majority of patients with RA have ‘burnt-out’ or inactive disease at the time of hip or knee replacement surgery.”
For the study, the researchers prospectively followed 120 patients who were to undergo joint replacement surgery. (The researchers initially approached 354 patients, of whom 169 declined to participate. Another 65 were dropped from the study for various reasons, including 42 who did not sufficiently fill out questionnaires and were deleted from the final analysis.)
The researchers tracked the patients before surgery and for 6 weeks after surgery. A majority of the patients were female (83%) and white (81%), with a mean age of 62 and a median RA symptom duration of 15 years. A total of 44% underwent hip replacement surgery while the rest underwent knee replacement surgery. Just over half of the patients were taking biologics, which were stopped prior to surgery, while glucocorticoids and methotrexate were usually continued.
Just under two-thirds of the patients flared within the first 6 weeks after surgery. The researchers didn’t find any connection between the flares and stopping biologics or using methotrexate. They did, however, link higher baseline RA activity to postsurgery flaring (odds ratio, 2.11; P = .015).
Dr. Goodman said that she and her colleagues continue to collect data to better understand flares and the link to disease severity. “The long-term implications of this are not yet known. We would like to know the effect on long-term functional outcome and complication rate.”
The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. Dr. Goodman disclosed receiving research funding from Novartis and Roche.
SOURCE: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point:
Major finding: Sixty-five percent of RA patients developed flares after joint replacement surgery, and it was more common in those with higher baseline RA activity (odds ratio, 2.11; P = .015).
Study details: Prospective study of 120 patients with RA who underwent hip replacement (44%) or knee replacement (56%).
Disclosures: The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. The lead author disclosed receiving research funding from Novartis and Roche.
Source: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366.
Prevalence and Impact of Self-Citation in Academic Orthopedic Surgery
ABSTRACT
The h-index is a commonly utilized metric for academic productivity. Previous studies have proposed that self-citation may limit the utility of the h-index. The purpose of this study is to evaluate the impact of self-citation on the h-index among orthopedic investigators. The study cohort consisted of program directors, chairpersons, and faculty at orthopedic surgery residency programs in the United States. The Scopus database was used to determine the h-index and number of citations ± self-citations. The total number of publications was correlated with the change in the h-index via self-citation. A total of 463 researchers were included (198 National Institutes of Health-funded faculty, 147 chairpersons, 118 program directors). Of these researchers, 83.8% cited previous work at least once (mean, 123.9 ± 277.6). Self-citations accounted for 5.8% of all citations. Including these citations increased the author h-index from 18.5 ± 14.9 to 19.2 ± 15.6 (P < .001). A minority of researchers (36.3%, P < .001) had increased their h-index via self-citation (range, 0-11). The proportional increase in the h-index via self-citation was positively related to the number of publications (P < .001). While the practice of self-citation is prevalent in orthopedics, its impact on the h-index is minimal for most researchers. With more publications, researchers can increase their h-index to a greater degree via self-citation.
Continue to: The competitive nature of academic research...
The competitive nature of academic research requires objective metrics to define career end points, such as promotion and funding procurement. While various criteria are used to assess performance in academia, publications and research funding are particularly regarded.1 Quantifying research dollars is relatively straightforward, but measuring research productivity is more complex. Not all articles are created equal, and disparities exist regarding effort and the ultimate impact of articles. In 2005, a physicist created the h-index to measure both research impact and productivity.2 As a bibliometric, the h-index equals the number of publications h that have been cited at least h times. Given its simplicity, the h-index has gained wide popularity in diverse medical specialties, including orthopedic surgery.3 Other recent studies have applied the h-index to hand surgery and spine surgery.4,5
Importantly, some authors have raised concerns regarding potential limitations of the h-index. One potentially significant limitation is the ability of authors to artificially inflate their h-index via self-citation. The impact of this practice is of particular interest as the h-index becomes widely adopted as a metric for promotion at many academic institutions.6-7 Furthermore, scholarly productivity has remained a critical component of successful grant funding procurement, and future grant funding applications may evaluate the h-index.8-10
The purpose of this study is to determine the prevalence and impact of self-citation on the h-index in a large cohort of orthopedic investigators. Given their high level of investment in academic orthopedic surgery, we focused on program directors, chairpersons, and National Institutes of Health (NIH)-funded research faculty at orthopedic surgery residency programs.
METHODS
INCLUSION CRITERIA
This study qualified as non-human and non-animal research and received exemption per the standing policy of the Institutional Review Board. The Fellowship and Residency Electronic Interactive Database (FREIDA) was accessed to generate a list of orthopedic residency program directors.11 This database was also used to generate a list of allopathic orthopedic surgery residency programs. Official program websites were accessed to generate a list of orthopedic chairpersons. Lastly, the NIH RePORTER was used to generate a list of basic science orthopedic investigators who received funding anytime during 2011 to 2014.12 This methodology was used due to the lack of reporting of basic science investigators on program websites. A list of NIH-funded orthopedic investigators was cross-referenced via an online search to isolate a cohort of PhD investigators.
Orthopedic faculty were defined as chairpersons, program directors, or NIH-funded investigators. In cases of overlap, preference was given in that order. Orthopedic investigators who had not published an article after 1995 were excluded (6 chairpersons, 1 program director).
BIBLIOMETRIC ANALYSIS
While several resources exist to calculate the h-index, the Scopus database (Elsevier) is one of the easiest programs to use.13 Author entries are created via institutional affiliations, thereby alleviating the need for manual reconciliations. Investigators were identified on Scopus via “author last name” and “first name, middle initial.” For each author, publications were screened for relevance to the field of orthopedics. Affiliated institutions were cross-referenced with information obtained from individual program websites. The “view h-graph” feature was used to calculate the number of publications, h-index, and number of citations. Then, the “Exclude self-citations” feature was used to calculate the number of corrected citations and the h-index excluding self-citations. Metrics were calculated over a 2-day period.
Continue to: STATISTICAL ANALYSIS
STATISTICAL ANALYSIS
Bibliometric analyses were presented descriptively with means ± standard deviation. After testing for normality, differences in the h-index between groups were assessed via analysis of variance tests. The proportional increase in the number of citations and the h-index were calculated by dividing the difference between the before and after exclusion of self-citations by the total number of citations before exclusion. The relationship between the number of publications and the proportional change in the h-index was assessed via calculation of the Spearman correlation coefficient. The independent variable was the number of publications, and the proportional increase in the h-index via self-citation was the dependent variable. Statistical tests were performed on STATA 13 (StataCorp) and the results considered significant if P < .05. Figures were created using GraphPad Prism 6.02 Software.
RESULTS
A total of 463 orthopedic investigators were included (147 chairpersons, 118 program directors, and 198 NIH-funded faculty) (Table 1). On average, these researchers produced 72.3 ± 83.0 articles and referenced 2139 ± 3222 articles (mean, 29.6 references per article). The cumulative h-index was 19.2 ± 15.6, and was the highest among NIH-funded researchers (24.3 ± 17.0) (P < .001). In all, 83.8% of orthopedic investigators self-cited their previous work at least once, and the total number of self-citations was highest among NIH-funded investigators (221 ± 355) (P < .001). After these self-citations were excluded, the h-index changed by 0.6 ± 1.1 for all investigators, and this change was greatest among NIH-funded researchers (1.1 ± 1.3) (P < .001).
Table 1. Effect of Self-Citation on NIH-funded Investigators, Chairpersons, and Program Directors in Orthopedics
| Investigator | N (%) | Articles, n (mean ± SD) |
Total Citations (mean ± SD)
| h-index | Self-Citations (mean ± SD) | Corrected h-index | ∆ h-index |
| NIH-funded | 198 (42.8) | 87.6 ± 84.9 | 3086 ± 3799 | 24.3 ± 17.0 | 221 ± 355 | 23.2 ± 16.3 | 1.1 ± 1.3 |
| Chairperson | 147 (31.7) | 85.3 ± 95.5 | 2151 ± 3098 | 19.9 ± 15.0 | 85.2 ± 221 | 19.5 ± 14.5 | 0.4 ± 0.8 |
| Program Director | 118 (25.5) | 30.5 ± 35.9 | 536.8 ± 785 | 9.6 ± 7.2 | 8.8 ± 19.9 | 9.5 ± 7.1 | 0.1 ± 0.3 |
| Total | 463 (100) | 72.3 ± 83.0 | 2139 ± 3222 | 19.2 ± 15.6 | 123.9 ± 277.6 | 18.5 ± 14.9 | 0.6 ± 1.1 |
Abbreviation: NIH, National Institutes of Health.
Most orthopedic investigators did not increase their h-index via self-citation (63.7%, P < .001). Table 2 categorizes investigators by changes in their h-index after excluding self-citations (range, 0-11). The maximal change in the h-index was seen in the most prolific group of investigators, who produced 261.0 ± 149.3 articles. In this group, the h-index increased by 11.1% ± 5.2%. The Figure investigates the relationship between the number of articles and the proportional increase in the h-index. The number of publications was positively correlated with the change in h-index after self-citations were excluded (r = 0.448, P < .001).
Table 2. Stratification of Orthopedic Researcher Investigators by Change in h-index After Self-Citation
∆ h-index
| N (%) |
Articles (mean ± SD)
| Self-Citations (mean ± SD) |
h-index (mean ± SD) | % Increase in h-index |
| 0 | 295 (63.7) | 43.8 ± 51.3 | 27.6 ± 58.4 | 13.1 ± 10.7 | 0 |
| 1 | 101 (21.8) | 87.9 ± 68.3 | 126.0 ± 130.6 | 24.0 ± 13.3 | 5.9 ± 4.1 |
| 2 | 42 (9.1) | 141.9 ± 111.1 | 331.6 ± 318.0 | 32.4 ± 16.6 | 8.4 ± 5.5 |
| 3 | 14 (3.0) | 203.1 ± 92.6 | 611.6 ± 332.9 | 45.4 ± 14.9 | 7.6 ± 3.6 |
| 4+ | 11 (2.4) | 261.0 ± 149.3 | 1277.1 ± 692.4 | 53.1 ± 18.9 | 11.1 ± 5.2 |
DISCUSSION
The practice of self-citation is widely prevalent among experienced orthopedic investigators. However, this practice seems to have minimal impact on the h-index for most investigators. Self-citation had a measurable impact on the h-index only after an investigator had many publications. At a mean of 87.9 ± 68.3 articles, investigators had a ∆h-index of 1. This represented a mean 5.9% increase. Overall, these findings underscore the utility of the h-index in assessing scholarly impact and ameliorate concerns over bibliometric manipulation.
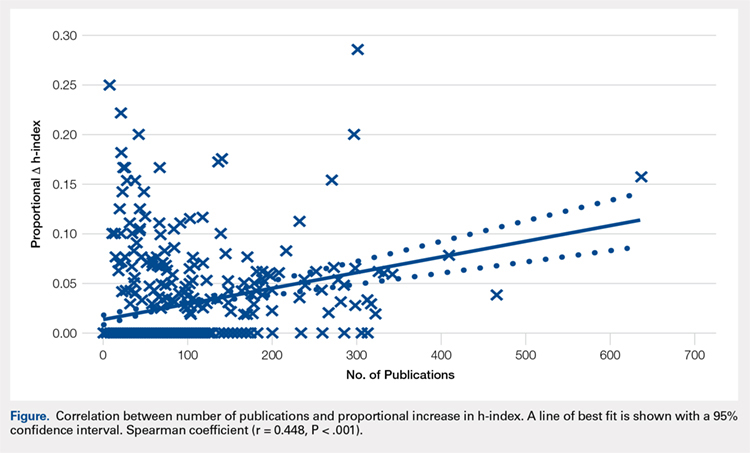
Among a large group of experienced orthopedic investigators, self-citation has minimal effect on the h-index. Importantly, most investigators (63.7%) did not experience a full integer increase in their h-index. At a threshold of ∆ h-index increase of 1, investigators had impressive h-indices (24.0 ± 13.3), which eclipsed those of recent studies of hand surgeons (10.2 ± 9.9) and spine surgeons (13.6 ± 8.7).4,5 This finding suggests that committees for academic promotion in orthopedic surgery may disregard the impact of self-citation on the h-index. While the thresholds for promotion have not been defined in the orthopedic literature, a study in plastic surgery established an h-index threshold of 14.5 for promotion from associate to full professor.14 It may be, however, that h-indices are higher among orthopedic surgeons, as a previous study reported an h-index of 20 among full professors.15 Future research is needed to determine thresholds for promotion within orthopedic surgery, as the h-index varies by specialty according to unique citation patterns.
Continue to: It is worth highlighting...
It is worth highlighting the academic performance of NIH-funded PhD researchers in orthopedics. Even including training grant awardees in this group, this cohort exceeded the academic productivity of their orthopedic chairpersons, as measured by the h-index. Previous studies in urology, neurosurgery, and otolaryngology have demonstrated the impact of NIH-funding on academic productivity.8-10 Ultimately, orthopedic departments could increase academic productivity by recruiting more PhD investigators with NIH funding.
In contrast to academic radiology,16 this study demonstrated a correlation between the number of publications and the increase in h-index via self-citation. Several reasons may help explain this disparity. The first reason is a technical one, as at the time of this study, the Scopus database had been updated to include citations before 1996. Considering that the h-index increases over time as older publications are cited, the exclusion of older articles is a significant limitation of previous h-index studies. Applying the same logic, the mean h-index for chairpersons of 19.9 quoted in this study contradicts a recent study, which quoted a mean h-index of 15.3.3 This previous study utilized citations that were limited to articles published after 1996.
Previous work on self-citation in the field of orthopedics has been limited to its influence on journal impact factors. Our results build on this literature in several important ways. Firstly, the calculation of a journal’s impact factor is a highly scrutinized process, and authors have criticized the mechanisms employed by editors to inflate impact factors.17 One study reported that 20% of authors have been encouraged to cite a journal during the revision process.18 Self-citation practices have been demonstrated in journals of cardiology,19 diabetes,20 anesthesia,21 and medicine.22 A study using a hypothetical model to assess the maximum potential for h-index increase by self-citation demonstrated an h-index inflation of 5 points over 20 years (5/14, 35.7%) by publishing 3 papers per year with 3 self-citations each.23 This study highlights a potential limitation of the h-index, but our study observed an h-index inflation of ≥4 in only 11 researchers (2.4%). Thus, results from our study ameliorate self-citation concerns in academic orthopedic surgery.
There are several limitations to this study that offer future areas of research. First, the validity of the h-index academic promotion in orthopedic surgery has not been evaluated. This was a motivation for the present study, and the authors have ongoing efforts to characterize the h-index in a larger cohort of orthopedic investigators. Importantly, an appropriate amount of self-citation was not established. It may be necessary for orthopedic researchers to cite their works as they become experts on a specific topic. Lastly, our analyses are prone to limitations inherent in the h-index, which does not account for author contribution or journal impact factors. Despite these limitations, we show that for most orthopedic researchers, the practice of self-citation does not impact the h-index.
In summary, self-citation is a widely prevalent practice among orthopedic investigators, but this practice has minimal impact on an author’s h-index. Approximately one third of orthopedic faculty in our study had a higher h-index due to self-citation. Greater h-index inflation through self-citation correlated with more publications. For the majority of orthopedic faculty, however, self-citation did not inflate the h-index, suggesting that promotional committees may disregard this concern when using the h-index as an adjunct measure for career advancement.
1. Atasoylu AA, Wright SM, Beasley BW, et al. Promotion criteria for clinician-educators. J Gen Intern Med. 2003;18(9):711-716.
2. Hirsch JE. An index to quantify an individual's scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572.
3. Stavrakis AI, Patel AD, Burke ZD, et al. The role of chairman and research director in influencing scholarly productivity and research funding in academic orthopaedic surgery. J Orthop Res. 2015;33(10)1407-1411. doi:10.1002/jor.22919.
4. Lopez J, Susarla SM, Swanson EW, Calotta N, Lifchez SD. The association of the H-index and academic rank among full-time academic hand surgeons affiliated with fellowship programs. J Hand Surg Am. 2015;40(7):1434-1441. doi:10.1016/j.jhsa.2015.03.026.
5. Schoenfeld AJ, Bhalla A, George J, Harris MB, Bono CM. Academic productivity and contributions to the literature among spine surgery fellowship faculty. Spine J. 2015;15(10)2126-2131. doi:10.1016/j.spinee.2015.03.026.
6. Jackson JB. Promotion at the Johns Hopkins School of Medicine. Johns Hopkins Medicine. https://www.hopkinsmedicine.org/gim/useful_links/PPC%20Promotion.pdf. Accessed February 1, 2015.
7. Appointments, promotion, and tenure. The Ohio State University School of Medicine. https://oaa.osu.edu/sites/default/files/uploads/governance-documents/college-of-medicine/surgery/Surgery_APT_5-20-14.pdf. Accessed February 1, 2015.
8. Colaco M, Svider PF, Mauro KM, Eloy JA, Jackson-Rosario I. Is there a relationship between National Institutes of Health funding and research impact on academic urology? J Urol .2013;190(3):999-1003. doi:10.1016/j.juro.2013.02.3186.
9. Svider PF, Husain Q, Folbe AJ, Couldwell WT, Liu JK, Eloy JA. Assessing National Institutes of Health funding and scholarly impact in neurological surgery. J Neurosurg. 2014;120(1):191-196. doi:10.3171/2013.8.JNS13938.
10. Svider PF, Mauro KM, Sanghvi S, Setzen M, Baredes S, Eloy JA. Is NIH funding predictive of greater research productivity and impact among academic otolaryngologists? Laryngoscope. 2013;123(1):118-122. doi:10.1002/lary.23659.
11. American Medical Association. FREIDA Online. http://www.ama-assn.org/ama/pub/education-careers/graduate-medical-education/freida-online.page? Accessed February 1, 2015.
12. NIH. Research Portfolio Online Reporting Tools. https://projectreporter.nih.gov/reporter.cfm. Accessed February 1, 2015.
13. Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB Journal. 2008;22(2):338-342. doi:10.1096/fj.07-9492LSF.
14. Gast KM, Kuzon WM Jr, Waljee JF. Bibliometric indices and academic promotion within plastic surgery. Plast Reconstr Surg. 2014;134(5):838e-844e. doi:10.1097/PRS.0000000000000594.
15. Svider PF, Pashkova AA, Choudhry Z, et al. Comparison of scholarly impact among surgical specialties: an examination of 2429 academic surgeons. Laryngoscope. 2013;123(4):884-889. doi:10.1002/lary.23951.
16. Rad AE, Shahgholi L, Kallmes D. Impact of self-citation on the H index in the field of academic radiology. Acad Radiol. 2012;19(4):455-457. doi:10.1016/j.acra.2011.11.013.
17. Hakkalamani S, Rawal A, Hennessy MS, Parkinson RW. The impact factor of seven orthopaedic journals: factors influencing it. J Bone Joint Surg Br. 2006;88(2):159-162. doi:10.1302/0301-620X.88B2.16983.
18. Foley JA, Della Sala S. The impact of self-citation. Cortex. 2010;46(6):802-810. doi:10.1016/j.cortex.2010.01.004.
19. Opthof T. Inflation of impact factors by journal self-citation in cardiovascular science. Neth Heart J. 2013;21(4):163-165. doi:10.1007/s12471-013-0384-0.
20. Gami AS, Montori VM, Wilczynski NL, Haynes RB. Author self-citation in the diabetes literature. CMAJ. 2004;170(13):1925-1927.
21. Fassoulaki A, Paraskeva A, Papilas K, Karabinis G. Self-citations in six anaesthesia journals and their significance in determining the impact factor. Br J Anaesth. 2000;84(2):266-269.
22. Kulkarni AV, Aziz B, Shams I, Busse JW. Author self-citation in the general medicine literature. PloS One. 2011;6(6): e20885. doi:10.1371/journal.pone.0020885.
23. Bartneck C, Kokkelmans S. Detecting h-index manipulation through self-citation analysis. Scientometrics. 2011;87(1):85-98. doi:10.1007/s11192-010-0306-5.
ABSTRACT
The h-index is a commonly utilized metric for academic productivity. Previous studies have proposed that self-citation may limit the utility of the h-index. The purpose of this study is to evaluate the impact of self-citation on the h-index among orthopedic investigators. The study cohort consisted of program directors, chairpersons, and faculty at orthopedic surgery residency programs in the United States. The Scopus database was used to determine the h-index and number of citations ± self-citations. The total number of publications was correlated with the change in the h-index via self-citation. A total of 463 researchers were included (198 National Institutes of Health-funded faculty, 147 chairpersons, 118 program directors). Of these researchers, 83.8% cited previous work at least once (mean, 123.9 ± 277.6). Self-citations accounted for 5.8% of all citations. Including these citations increased the author h-index from 18.5 ± 14.9 to 19.2 ± 15.6 (P < .001). A minority of researchers (36.3%, P < .001) had increased their h-index via self-citation (range, 0-11). The proportional increase in the h-index via self-citation was positively related to the number of publications (P < .001). While the practice of self-citation is prevalent in orthopedics, its impact on the h-index is minimal for most researchers. With more publications, researchers can increase their h-index to a greater degree via self-citation.
Continue to: The competitive nature of academic research...
The competitive nature of academic research requires objective metrics to define career end points, such as promotion and funding procurement. While various criteria are used to assess performance in academia, publications and research funding are particularly regarded.1 Quantifying research dollars is relatively straightforward, but measuring research productivity is more complex. Not all articles are created equal, and disparities exist regarding effort and the ultimate impact of articles. In 2005, a physicist created the h-index to measure both research impact and productivity.2 As a bibliometric, the h-index equals the number of publications h that have been cited at least h times. Given its simplicity, the h-index has gained wide popularity in diverse medical specialties, including orthopedic surgery.3 Other recent studies have applied the h-index to hand surgery and spine surgery.4,5
Importantly, some authors have raised concerns regarding potential limitations of the h-index. One potentially significant limitation is the ability of authors to artificially inflate their h-index via self-citation. The impact of this practice is of particular interest as the h-index becomes widely adopted as a metric for promotion at many academic institutions.6-7 Furthermore, scholarly productivity has remained a critical component of successful grant funding procurement, and future grant funding applications may evaluate the h-index.8-10
The purpose of this study is to determine the prevalence and impact of self-citation on the h-index in a large cohort of orthopedic investigators. Given their high level of investment in academic orthopedic surgery, we focused on program directors, chairpersons, and National Institutes of Health (NIH)-funded research faculty at orthopedic surgery residency programs.
METHODS
INCLUSION CRITERIA
This study qualified as non-human and non-animal research and received exemption per the standing policy of the Institutional Review Board. The Fellowship and Residency Electronic Interactive Database (FREIDA) was accessed to generate a list of orthopedic residency program directors.11 This database was also used to generate a list of allopathic orthopedic surgery residency programs. Official program websites were accessed to generate a list of orthopedic chairpersons. Lastly, the NIH RePORTER was used to generate a list of basic science orthopedic investigators who received funding anytime during 2011 to 2014.12 This methodology was used due to the lack of reporting of basic science investigators on program websites. A list of NIH-funded orthopedic investigators was cross-referenced via an online search to isolate a cohort of PhD investigators.
Orthopedic faculty were defined as chairpersons, program directors, or NIH-funded investigators. In cases of overlap, preference was given in that order. Orthopedic investigators who had not published an article after 1995 were excluded (6 chairpersons, 1 program director).
BIBLIOMETRIC ANALYSIS
While several resources exist to calculate the h-index, the Scopus database (Elsevier) is one of the easiest programs to use.13 Author entries are created via institutional affiliations, thereby alleviating the need for manual reconciliations. Investigators were identified on Scopus via “author last name” and “first name, middle initial.” For each author, publications were screened for relevance to the field of orthopedics. Affiliated institutions were cross-referenced with information obtained from individual program websites. The “view h-graph” feature was used to calculate the number of publications, h-index, and number of citations. Then, the “Exclude self-citations” feature was used to calculate the number of corrected citations and the h-index excluding self-citations. Metrics were calculated over a 2-day period.
Continue to: STATISTICAL ANALYSIS
STATISTICAL ANALYSIS
Bibliometric analyses were presented descriptively with means ± standard deviation. After testing for normality, differences in the h-index between groups were assessed via analysis of variance tests. The proportional increase in the number of citations and the h-index were calculated by dividing the difference between the before and after exclusion of self-citations by the total number of citations before exclusion. The relationship between the number of publications and the proportional change in the h-index was assessed via calculation of the Spearman correlation coefficient. The independent variable was the number of publications, and the proportional increase in the h-index via self-citation was the dependent variable. Statistical tests were performed on STATA 13 (StataCorp) and the results considered significant if P < .05. Figures were created using GraphPad Prism 6.02 Software.
RESULTS
A total of 463 orthopedic investigators were included (147 chairpersons, 118 program directors, and 198 NIH-funded faculty) (Table 1). On average, these researchers produced 72.3 ± 83.0 articles and referenced 2139 ± 3222 articles (mean, 29.6 references per article). The cumulative h-index was 19.2 ± 15.6, and was the highest among NIH-funded researchers (24.3 ± 17.0) (P < .001). In all, 83.8% of orthopedic investigators self-cited their previous work at least once, and the total number of self-citations was highest among NIH-funded investigators (221 ± 355) (P < .001). After these self-citations were excluded, the h-index changed by 0.6 ± 1.1 for all investigators, and this change was greatest among NIH-funded researchers (1.1 ± 1.3) (P < .001).
Table 1. Effect of Self-Citation on NIH-funded Investigators, Chairpersons, and Program Directors in Orthopedics
| Investigator | N (%) | Articles, n (mean ± SD) |
Total Citations (mean ± SD)
| h-index | Self-Citations (mean ± SD) | Corrected h-index | ∆ h-index |
| NIH-funded | 198 (42.8) | 87.6 ± 84.9 | 3086 ± 3799 | 24.3 ± 17.0 | 221 ± 355 | 23.2 ± 16.3 | 1.1 ± 1.3 |
| Chairperson | 147 (31.7) | 85.3 ± 95.5 | 2151 ± 3098 | 19.9 ± 15.0 | 85.2 ± 221 | 19.5 ± 14.5 | 0.4 ± 0.8 |
| Program Director | 118 (25.5) | 30.5 ± 35.9 | 536.8 ± 785 | 9.6 ± 7.2 | 8.8 ± 19.9 | 9.5 ± 7.1 | 0.1 ± 0.3 |
| Total | 463 (100) | 72.3 ± 83.0 | 2139 ± 3222 | 19.2 ± 15.6 | 123.9 ± 277.6 | 18.5 ± 14.9 | 0.6 ± 1.1 |
Abbreviation: NIH, National Institutes of Health.
Most orthopedic investigators did not increase their h-index via self-citation (63.7%, P < .001). Table 2 categorizes investigators by changes in their h-index after excluding self-citations (range, 0-11). The maximal change in the h-index was seen in the most prolific group of investigators, who produced 261.0 ± 149.3 articles. In this group, the h-index increased by 11.1% ± 5.2%. The Figure investigates the relationship between the number of articles and the proportional increase in the h-index. The number of publications was positively correlated with the change in h-index after self-citations were excluded (r = 0.448, P < .001).
Table 2. Stratification of Orthopedic Researcher Investigators by Change in h-index After Self-Citation
∆ h-index
| N (%) |
Articles (mean ± SD)
| Self-Citations (mean ± SD) |
h-index (mean ± SD) | % Increase in h-index |
| 0 | 295 (63.7) | 43.8 ± 51.3 | 27.6 ± 58.4 | 13.1 ± 10.7 | 0 |
| 1 | 101 (21.8) | 87.9 ± 68.3 | 126.0 ± 130.6 | 24.0 ± 13.3 | 5.9 ± 4.1 |
| 2 | 42 (9.1) | 141.9 ± 111.1 | 331.6 ± 318.0 | 32.4 ± 16.6 | 8.4 ± 5.5 |
| 3 | 14 (3.0) | 203.1 ± 92.6 | 611.6 ± 332.9 | 45.4 ± 14.9 | 7.6 ± 3.6 |
| 4+ | 11 (2.4) | 261.0 ± 149.3 | 1277.1 ± 692.4 | 53.1 ± 18.9 | 11.1 ± 5.2 |
DISCUSSION
The practice of self-citation is widely prevalent among experienced orthopedic investigators. However, this practice seems to have minimal impact on the h-index for most investigators. Self-citation had a measurable impact on the h-index only after an investigator had many publications. At a mean of 87.9 ± 68.3 articles, investigators had a ∆h-index of 1. This represented a mean 5.9% increase. Overall, these findings underscore the utility of the h-index in assessing scholarly impact and ameliorate concerns over bibliometric manipulation.

Among a large group of experienced orthopedic investigators, self-citation has minimal effect on the h-index. Importantly, most investigators (63.7%) did not experience a full integer increase in their h-index. At a threshold of ∆ h-index increase of 1, investigators had impressive h-indices (24.0 ± 13.3), which eclipsed those of recent studies of hand surgeons (10.2 ± 9.9) and spine surgeons (13.6 ± 8.7).4,5 This finding suggests that committees for academic promotion in orthopedic surgery may disregard the impact of self-citation on the h-index. While the thresholds for promotion have not been defined in the orthopedic literature, a study in plastic surgery established an h-index threshold of 14.5 for promotion from associate to full professor.14 It may be, however, that h-indices are higher among orthopedic surgeons, as a previous study reported an h-index of 20 among full professors.15 Future research is needed to determine thresholds for promotion within orthopedic surgery, as the h-index varies by specialty according to unique citation patterns.
Continue to: It is worth highlighting...
It is worth highlighting the academic performance of NIH-funded PhD researchers in orthopedics. Even including training grant awardees in this group, this cohort exceeded the academic productivity of their orthopedic chairpersons, as measured by the h-index. Previous studies in urology, neurosurgery, and otolaryngology have demonstrated the impact of NIH-funding on academic productivity.8-10 Ultimately, orthopedic departments could increase academic productivity by recruiting more PhD investigators with NIH funding.
In contrast to academic radiology,16 this study demonstrated a correlation between the number of publications and the increase in h-index via self-citation. Several reasons may help explain this disparity. The first reason is a technical one, as at the time of this study, the Scopus database had been updated to include citations before 1996. Considering that the h-index increases over time as older publications are cited, the exclusion of older articles is a significant limitation of previous h-index studies. Applying the same logic, the mean h-index for chairpersons of 19.9 quoted in this study contradicts a recent study, which quoted a mean h-index of 15.3.3 This previous study utilized citations that were limited to articles published after 1996.
Previous work on self-citation in the field of orthopedics has been limited to its influence on journal impact factors. Our results build on this literature in several important ways. Firstly, the calculation of a journal’s impact factor is a highly scrutinized process, and authors have criticized the mechanisms employed by editors to inflate impact factors.17 One study reported that 20% of authors have been encouraged to cite a journal during the revision process.18 Self-citation practices have been demonstrated in journals of cardiology,19 diabetes,20 anesthesia,21 and medicine.22 A study using a hypothetical model to assess the maximum potential for h-index increase by self-citation demonstrated an h-index inflation of 5 points over 20 years (5/14, 35.7%) by publishing 3 papers per year with 3 self-citations each.23 This study highlights a potential limitation of the h-index, but our study observed an h-index inflation of ≥4 in only 11 researchers (2.4%). Thus, results from our study ameliorate self-citation concerns in academic orthopedic surgery.
There are several limitations to this study that offer future areas of research. First, the validity of the h-index academic promotion in orthopedic surgery has not been evaluated. This was a motivation for the present study, and the authors have ongoing efforts to characterize the h-index in a larger cohort of orthopedic investigators. Importantly, an appropriate amount of self-citation was not established. It may be necessary for orthopedic researchers to cite their works as they become experts on a specific topic. Lastly, our analyses are prone to limitations inherent in the h-index, which does not account for author contribution or journal impact factors. Despite these limitations, we show that for most orthopedic researchers, the practice of self-citation does not impact the h-index.
In summary, self-citation is a widely prevalent practice among orthopedic investigators, but this practice has minimal impact on an author’s h-index. Approximately one third of orthopedic faculty in our study had a higher h-index due to self-citation. Greater h-index inflation through self-citation correlated with more publications. For the majority of orthopedic faculty, however, self-citation did not inflate the h-index, suggesting that promotional committees may disregard this concern when using the h-index as an adjunct measure for career advancement.
ABSTRACT
The h-index is a commonly utilized metric for academic productivity. Previous studies have proposed that self-citation may limit the utility of the h-index. The purpose of this study is to evaluate the impact of self-citation on the h-index among orthopedic investigators. The study cohort consisted of program directors, chairpersons, and faculty at orthopedic surgery residency programs in the United States. The Scopus database was used to determine the h-index and number of citations ± self-citations. The total number of publications was correlated with the change in the h-index via self-citation. A total of 463 researchers were included (198 National Institutes of Health-funded faculty, 147 chairpersons, 118 program directors). Of these researchers, 83.8% cited previous work at least once (mean, 123.9 ± 277.6). Self-citations accounted for 5.8% of all citations. Including these citations increased the author h-index from 18.5 ± 14.9 to 19.2 ± 15.6 (P < .001). A minority of researchers (36.3%, P < .001) had increased their h-index via self-citation (range, 0-11). The proportional increase in the h-index via self-citation was positively related to the number of publications (P < .001). While the practice of self-citation is prevalent in orthopedics, its impact on the h-index is minimal for most researchers. With more publications, researchers can increase their h-index to a greater degree via self-citation.
Continue to: The competitive nature of academic research...
The competitive nature of academic research requires objective metrics to define career end points, such as promotion and funding procurement. While various criteria are used to assess performance in academia, publications and research funding are particularly regarded.1 Quantifying research dollars is relatively straightforward, but measuring research productivity is more complex. Not all articles are created equal, and disparities exist regarding effort and the ultimate impact of articles. In 2005, a physicist created the h-index to measure both research impact and productivity.2 As a bibliometric, the h-index equals the number of publications h that have been cited at least h times. Given its simplicity, the h-index has gained wide popularity in diverse medical specialties, including orthopedic surgery.3 Other recent studies have applied the h-index to hand surgery and spine surgery.4,5
Importantly, some authors have raised concerns regarding potential limitations of the h-index. One potentially significant limitation is the ability of authors to artificially inflate their h-index via self-citation. The impact of this practice is of particular interest as the h-index becomes widely adopted as a metric for promotion at many academic institutions.6-7 Furthermore, scholarly productivity has remained a critical component of successful grant funding procurement, and future grant funding applications may evaluate the h-index.8-10
The purpose of this study is to determine the prevalence and impact of self-citation on the h-index in a large cohort of orthopedic investigators. Given their high level of investment in academic orthopedic surgery, we focused on program directors, chairpersons, and National Institutes of Health (NIH)-funded research faculty at orthopedic surgery residency programs.
METHODS
INCLUSION CRITERIA
This study qualified as non-human and non-animal research and received exemption per the standing policy of the Institutional Review Board. The Fellowship and Residency Electronic Interactive Database (FREIDA) was accessed to generate a list of orthopedic residency program directors.11 This database was also used to generate a list of allopathic orthopedic surgery residency programs. Official program websites were accessed to generate a list of orthopedic chairpersons. Lastly, the NIH RePORTER was used to generate a list of basic science orthopedic investigators who received funding anytime during 2011 to 2014.12 This methodology was used due to the lack of reporting of basic science investigators on program websites. A list of NIH-funded orthopedic investigators was cross-referenced via an online search to isolate a cohort of PhD investigators.
Orthopedic faculty were defined as chairpersons, program directors, or NIH-funded investigators. In cases of overlap, preference was given in that order. Orthopedic investigators who had not published an article after 1995 were excluded (6 chairpersons, 1 program director).
BIBLIOMETRIC ANALYSIS
While several resources exist to calculate the h-index, the Scopus database (Elsevier) is one of the easiest programs to use.13 Author entries are created via institutional affiliations, thereby alleviating the need for manual reconciliations. Investigators were identified on Scopus via “author last name” and “first name, middle initial.” For each author, publications were screened for relevance to the field of orthopedics. Affiliated institutions were cross-referenced with information obtained from individual program websites. The “view h-graph” feature was used to calculate the number of publications, h-index, and number of citations. Then, the “Exclude self-citations” feature was used to calculate the number of corrected citations and the h-index excluding self-citations. Metrics were calculated over a 2-day period.
Continue to: STATISTICAL ANALYSIS
STATISTICAL ANALYSIS
Bibliometric analyses were presented descriptively with means ± standard deviation. After testing for normality, differences in the h-index between groups were assessed via analysis of variance tests. The proportional increase in the number of citations and the h-index were calculated by dividing the difference between the before and after exclusion of self-citations by the total number of citations before exclusion. The relationship between the number of publications and the proportional change in the h-index was assessed via calculation of the Spearman correlation coefficient. The independent variable was the number of publications, and the proportional increase in the h-index via self-citation was the dependent variable. Statistical tests were performed on STATA 13 (StataCorp) and the results considered significant if P < .05. Figures were created using GraphPad Prism 6.02 Software.
RESULTS
A total of 463 orthopedic investigators were included (147 chairpersons, 118 program directors, and 198 NIH-funded faculty) (Table 1). On average, these researchers produced 72.3 ± 83.0 articles and referenced 2139 ± 3222 articles (mean, 29.6 references per article). The cumulative h-index was 19.2 ± 15.6, and was the highest among NIH-funded researchers (24.3 ± 17.0) (P < .001). In all, 83.8% of orthopedic investigators self-cited their previous work at least once, and the total number of self-citations was highest among NIH-funded investigators (221 ± 355) (P < .001). After these self-citations were excluded, the h-index changed by 0.6 ± 1.1 for all investigators, and this change was greatest among NIH-funded researchers (1.1 ± 1.3) (P < .001).
Table 1. Effect of Self-Citation on NIH-funded Investigators, Chairpersons, and Program Directors in Orthopedics
| Investigator | N (%) | Articles, n (mean ± SD) |
Total Citations (mean ± SD)
| h-index | Self-Citations (mean ± SD) | Corrected h-index | ∆ h-index |
| NIH-funded | 198 (42.8) | 87.6 ± 84.9 | 3086 ± 3799 | 24.3 ± 17.0 | 221 ± 355 | 23.2 ± 16.3 | 1.1 ± 1.3 |
| Chairperson | 147 (31.7) | 85.3 ± 95.5 | 2151 ± 3098 | 19.9 ± 15.0 | 85.2 ± 221 | 19.5 ± 14.5 | 0.4 ± 0.8 |
| Program Director | 118 (25.5) | 30.5 ± 35.9 | 536.8 ± 785 | 9.6 ± 7.2 | 8.8 ± 19.9 | 9.5 ± 7.1 | 0.1 ± 0.3 |
| Total | 463 (100) | 72.3 ± 83.0 | 2139 ± 3222 | 19.2 ± 15.6 | 123.9 ± 277.6 | 18.5 ± 14.9 | 0.6 ± 1.1 |
Abbreviation: NIH, National Institutes of Health.
Most orthopedic investigators did not increase their h-index via self-citation (63.7%, P < .001). Table 2 categorizes investigators by changes in their h-index after excluding self-citations (range, 0-11). The maximal change in the h-index was seen in the most prolific group of investigators, who produced 261.0 ± 149.3 articles. In this group, the h-index increased by 11.1% ± 5.2%. The Figure investigates the relationship between the number of articles and the proportional increase in the h-index. The number of publications was positively correlated with the change in h-index after self-citations were excluded (r = 0.448, P < .001).
Table 2. Stratification of Orthopedic Researcher Investigators by Change in h-index After Self-Citation
∆ h-index
| N (%) |
Articles (mean ± SD)
| Self-Citations (mean ± SD) |
h-index (mean ± SD) | % Increase in h-index |
| 0 | 295 (63.7) | 43.8 ± 51.3 | 27.6 ± 58.4 | 13.1 ± 10.7 | 0 |
| 1 | 101 (21.8) | 87.9 ± 68.3 | 126.0 ± 130.6 | 24.0 ± 13.3 | 5.9 ± 4.1 |
| 2 | 42 (9.1) | 141.9 ± 111.1 | 331.6 ± 318.0 | 32.4 ± 16.6 | 8.4 ± 5.5 |
| 3 | 14 (3.0) | 203.1 ± 92.6 | 611.6 ± 332.9 | 45.4 ± 14.9 | 7.6 ± 3.6 |
| 4+ | 11 (2.4) | 261.0 ± 149.3 | 1277.1 ± 692.4 | 53.1 ± 18.9 | 11.1 ± 5.2 |
DISCUSSION
The practice of self-citation is widely prevalent among experienced orthopedic investigators. However, this practice seems to have minimal impact on the h-index for most investigators. Self-citation had a measurable impact on the h-index only after an investigator had many publications. At a mean of 87.9 ± 68.3 articles, investigators had a ∆h-index of 1. This represented a mean 5.9% increase. Overall, these findings underscore the utility of the h-index in assessing scholarly impact and ameliorate concerns over bibliometric manipulation.

Among a large group of experienced orthopedic investigators, self-citation has minimal effect on the h-index. Importantly, most investigators (63.7%) did not experience a full integer increase in their h-index. At a threshold of ∆ h-index increase of 1, investigators had impressive h-indices (24.0 ± 13.3), which eclipsed those of recent studies of hand surgeons (10.2 ± 9.9) and spine surgeons (13.6 ± 8.7).4,5 This finding suggests that committees for academic promotion in orthopedic surgery may disregard the impact of self-citation on the h-index. While the thresholds for promotion have not been defined in the orthopedic literature, a study in plastic surgery established an h-index threshold of 14.5 for promotion from associate to full professor.14 It may be, however, that h-indices are higher among orthopedic surgeons, as a previous study reported an h-index of 20 among full professors.15 Future research is needed to determine thresholds for promotion within orthopedic surgery, as the h-index varies by specialty according to unique citation patterns.
Continue to: It is worth highlighting...
It is worth highlighting the academic performance of NIH-funded PhD researchers in orthopedics. Even including training grant awardees in this group, this cohort exceeded the academic productivity of their orthopedic chairpersons, as measured by the h-index. Previous studies in urology, neurosurgery, and otolaryngology have demonstrated the impact of NIH-funding on academic productivity.8-10 Ultimately, orthopedic departments could increase academic productivity by recruiting more PhD investigators with NIH funding.
In contrast to academic radiology,16 this study demonstrated a correlation between the number of publications and the increase in h-index via self-citation. Several reasons may help explain this disparity. The first reason is a technical one, as at the time of this study, the Scopus database had been updated to include citations before 1996. Considering that the h-index increases over time as older publications are cited, the exclusion of older articles is a significant limitation of previous h-index studies. Applying the same logic, the mean h-index for chairpersons of 19.9 quoted in this study contradicts a recent study, which quoted a mean h-index of 15.3.3 This previous study utilized citations that were limited to articles published after 1996.
Previous work on self-citation in the field of orthopedics has been limited to its influence on journal impact factors. Our results build on this literature in several important ways. Firstly, the calculation of a journal’s impact factor is a highly scrutinized process, and authors have criticized the mechanisms employed by editors to inflate impact factors.17 One study reported that 20% of authors have been encouraged to cite a journal during the revision process.18 Self-citation practices have been demonstrated in journals of cardiology,19 diabetes,20 anesthesia,21 and medicine.22 A study using a hypothetical model to assess the maximum potential for h-index increase by self-citation demonstrated an h-index inflation of 5 points over 20 years (5/14, 35.7%) by publishing 3 papers per year with 3 self-citations each.23 This study highlights a potential limitation of the h-index, but our study observed an h-index inflation of ≥4 in only 11 researchers (2.4%). Thus, results from our study ameliorate self-citation concerns in academic orthopedic surgery.
There are several limitations to this study that offer future areas of research. First, the validity of the h-index academic promotion in orthopedic surgery has not been evaluated. This was a motivation for the present study, and the authors have ongoing efforts to characterize the h-index in a larger cohort of orthopedic investigators. Importantly, an appropriate amount of self-citation was not established. It may be necessary for orthopedic researchers to cite their works as they become experts on a specific topic. Lastly, our analyses are prone to limitations inherent in the h-index, which does not account for author contribution or journal impact factors. Despite these limitations, we show that for most orthopedic researchers, the practice of self-citation does not impact the h-index.
In summary, self-citation is a widely prevalent practice among orthopedic investigators, but this practice has minimal impact on an author’s h-index. Approximately one third of orthopedic faculty in our study had a higher h-index due to self-citation. Greater h-index inflation through self-citation correlated with more publications. For the majority of orthopedic faculty, however, self-citation did not inflate the h-index, suggesting that promotional committees may disregard this concern when using the h-index as an adjunct measure for career advancement.
1. Atasoylu AA, Wright SM, Beasley BW, et al. Promotion criteria for clinician-educators. J Gen Intern Med. 2003;18(9):711-716.
2. Hirsch JE. An index to quantify an individual's scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572.
3. Stavrakis AI, Patel AD, Burke ZD, et al. The role of chairman and research director in influencing scholarly productivity and research funding in academic orthopaedic surgery. J Orthop Res. 2015;33(10)1407-1411. doi:10.1002/jor.22919.
4. Lopez J, Susarla SM, Swanson EW, Calotta N, Lifchez SD. The association of the H-index and academic rank among full-time academic hand surgeons affiliated with fellowship programs. J Hand Surg Am. 2015;40(7):1434-1441. doi:10.1016/j.jhsa.2015.03.026.
5. Schoenfeld AJ, Bhalla A, George J, Harris MB, Bono CM. Academic productivity and contributions to the literature among spine surgery fellowship faculty. Spine J. 2015;15(10)2126-2131. doi:10.1016/j.spinee.2015.03.026.
6. Jackson JB. Promotion at the Johns Hopkins School of Medicine. Johns Hopkins Medicine. https://www.hopkinsmedicine.org/gim/useful_links/PPC%20Promotion.pdf. Accessed February 1, 2015.
7. Appointments, promotion, and tenure. The Ohio State University School of Medicine. https://oaa.osu.edu/sites/default/files/uploads/governance-documents/college-of-medicine/surgery/Surgery_APT_5-20-14.pdf. Accessed February 1, 2015.
8. Colaco M, Svider PF, Mauro KM, Eloy JA, Jackson-Rosario I. Is there a relationship between National Institutes of Health funding and research impact on academic urology? J Urol .2013;190(3):999-1003. doi:10.1016/j.juro.2013.02.3186.
9. Svider PF, Husain Q, Folbe AJ, Couldwell WT, Liu JK, Eloy JA. Assessing National Institutes of Health funding and scholarly impact in neurological surgery. J Neurosurg. 2014;120(1):191-196. doi:10.3171/2013.8.JNS13938.
10. Svider PF, Mauro KM, Sanghvi S, Setzen M, Baredes S, Eloy JA. Is NIH funding predictive of greater research productivity and impact among academic otolaryngologists? Laryngoscope. 2013;123(1):118-122. doi:10.1002/lary.23659.
11. American Medical Association. FREIDA Online. http://www.ama-assn.org/ama/pub/education-careers/graduate-medical-education/freida-online.page? Accessed February 1, 2015.
12. NIH. Research Portfolio Online Reporting Tools. https://projectreporter.nih.gov/reporter.cfm. Accessed February 1, 2015.
13. Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB Journal. 2008;22(2):338-342. doi:10.1096/fj.07-9492LSF.
14. Gast KM, Kuzon WM Jr, Waljee JF. Bibliometric indices and academic promotion within plastic surgery. Plast Reconstr Surg. 2014;134(5):838e-844e. doi:10.1097/PRS.0000000000000594.
15. Svider PF, Pashkova AA, Choudhry Z, et al. Comparison of scholarly impact among surgical specialties: an examination of 2429 academic surgeons. Laryngoscope. 2013;123(4):884-889. doi:10.1002/lary.23951.
16. Rad AE, Shahgholi L, Kallmes D. Impact of self-citation on the H index in the field of academic radiology. Acad Radiol. 2012;19(4):455-457. doi:10.1016/j.acra.2011.11.013.
17. Hakkalamani S, Rawal A, Hennessy MS, Parkinson RW. The impact factor of seven orthopaedic journals: factors influencing it. J Bone Joint Surg Br. 2006;88(2):159-162. doi:10.1302/0301-620X.88B2.16983.
18. Foley JA, Della Sala S. The impact of self-citation. Cortex. 2010;46(6):802-810. doi:10.1016/j.cortex.2010.01.004.
19. Opthof T. Inflation of impact factors by journal self-citation in cardiovascular science. Neth Heart J. 2013;21(4):163-165. doi:10.1007/s12471-013-0384-0.
20. Gami AS, Montori VM, Wilczynski NL, Haynes RB. Author self-citation in the diabetes literature. CMAJ. 2004;170(13):1925-1927.
21. Fassoulaki A, Paraskeva A, Papilas K, Karabinis G. Self-citations in six anaesthesia journals and their significance in determining the impact factor. Br J Anaesth. 2000;84(2):266-269.
22. Kulkarni AV, Aziz B, Shams I, Busse JW. Author self-citation in the general medicine literature. PloS One. 2011;6(6): e20885. doi:10.1371/journal.pone.0020885.
23. Bartneck C, Kokkelmans S. Detecting h-index manipulation through self-citation analysis. Scientometrics. 2011;87(1):85-98. doi:10.1007/s11192-010-0306-5.
1. Atasoylu AA, Wright SM, Beasley BW, et al. Promotion criteria for clinician-educators. J Gen Intern Med. 2003;18(9):711-716.
2. Hirsch JE. An index to quantify an individual's scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572.
3. Stavrakis AI, Patel AD, Burke ZD, et al. The role of chairman and research director in influencing scholarly productivity and research funding in academic orthopaedic surgery. J Orthop Res. 2015;33(10)1407-1411. doi:10.1002/jor.22919.
4. Lopez J, Susarla SM, Swanson EW, Calotta N, Lifchez SD. The association of the H-index and academic rank among full-time academic hand surgeons affiliated with fellowship programs. J Hand Surg Am. 2015;40(7):1434-1441. doi:10.1016/j.jhsa.2015.03.026.
5. Schoenfeld AJ, Bhalla A, George J, Harris MB, Bono CM. Academic productivity and contributions to the literature among spine surgery fellowship faculty. Spine J. 2015;15(10)2126-2131. doi:10.1016/j.spinee.2015.03.026.
6. Jackson JB. Promotion at the Johns Hopkins School of Medicine. Johns Hopkins Medicine. https://www.hopkinsmedicine.org/gim/useful_links/PPC%20Promotion.pdf. Accessed February 1, 2015.
7. Appointments, promotion, and tenure. The Ohio State University School of Medicine. https://oaa.osu.edu/sites/default/files/uploads/governance-documents/college-of-medicine/surgery/Surgery_APT_5-20-14.pdf. Accessed February 1, 2015.
8. Colaco M, Svider PF, Mauro KM, Eloy JA, Jackson-Rosario I. Is there a relationship between National Institutes of Health funding and research impact on academic urology? J Urol .2013;190(3):999-1003. doi:10.1016/j.juro.2013.02.3186.
9. Svider PF, Husain Q, Folbe AJ, Couldwell WT, Liu JK, Eloy JA. Assessing National Institutes of Health funding and scholarly impact in neurological surgery. J Neurosurg. 2014;120(1):191-196. doi:10.3171/2013.8.JNS13938.
10. Svider PF, Mauro KM, Sanghvi S, Setzen M, Baredes S, Eloy JA. Is NIH funding predictive of greater research productivity and impact among academic otolaryngologists? Laryngoscope. 2013;123(1):118-122. doi:10.1002/lary.23659.
11. American Medical Association. FREIDA Online. http://www.ama-assn.org/ama/pub/education-careers/graduate-medical-education/freida-online.page? Accessed February 1, 2015.
12. NIH. Research Portfolio Online Reporting Tools. https://projectreporter.nih.gov/reporter.cfm. Accessed February 1, 2015.
13. Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB Journal. 2008;22(2):338-342. doi:10.1096/fj.07-9492LSF.
14. Gast KM, Kuzon WM Jr, Waljee JF. Bibliometric indices and academic promotion within plastic surgery. Plast Reconstr Surg. 2014;134(5):838e-844e. doi:10.1097/PRS.0000000000000594.
15. Svider PF, Pashkova AA, Choudhry Z, et al. Comparison of scholarly impact among surgical specialties: an examination of 2429 academic surgeons. Laryngoscope. 2013;123(4):884-889. doi:10.1002/lary.23951.
16. Rad AE, Shahgholi L, Kallmes D. Impact of self-citation on the H index in the field of academic radiology. Acad Radiol. 2012;19(4):455-457. doi:10.1016/j.acra.2011.11.013.
17. Hakkalamani S, Rawal A, Hennessy MS, Parkinson RW. The impact factor of seven orthopaedic journals: factors influencing it. J Bone Joint Surg Br. 2006;88(2):159-162. doi:10.1302/0301-620X.88B2.16983.
18. Foley JA, Della Sala S. The impact of self-citation. Cortex. 2010;46(6):802-810. doi:10.1016/j.cortex.2010.01.004.
19. Opthof T. Inflation of impact factors by journal self-citation in cardiovascular science. Neth Heart J. 2013;21(4):163-165. doi:10.1007/s12471-013-0384-0.
20. Gami AS, Montori VM, Wilczynski NL, Haynes RB. Author self-citation in the diabetes literature. CMAJ. 2004;170(13):1925-1927.
21. Fassoulaki A, Paraskeva A, Papilas K, Karabinis G. Self-citations in six anaesthesia journals and their significance in determining the impact factor. Br J Anaesth. 2000;84(2):266-269.
22. Kulkarni AV, Aziz B, Shams I, Busse JW. Author self-citation in the general medicine literature. PloS One. 2011;6(6): e20885. doi:10.1371/journal.pone.0020885.
23. Bartneck C, Kokkelmans S. Detecting h-index manipulation through self-citation analysis. Scientometrics. 2011;87(1):85-98. doi:10.1007/s11192-010-0306-5.
TAKE-HOME POINTS
- In all, 83.8% of orthopedic surgeons cite previous work at least once.
- Self-citations account for only 5.8% of all citations.
- Including self-citations increases the mean h-index from 18.5 ± 14.9 to 19.2 ± 15.6 (P < .001).
- The magnitude of increase in h-index via self-citation is proportional to the career number of publications.
- Overall, while prevalent, the practice of self-citation has minimal impact on an academic orthopedic surgeon’s h-index.
His Old Pain Is Back
ANSWER
The radiograph demonstrates a fracture within the femoral neck. These types of fractures, which are slightly angulated and located at the base of the neck, are typically referred to as basicervical femur fractures.
Prompt orthopedic surgery consultation was obtained, and the patient underwent an open reduction and internal fixation of his fracture.
ANSWER
The radiograph demonstrates a fracture within the femoral neck. These types of fractures, which are slightly angulated and located at the base of the neck, are typically referred to as basicervical femur fractures.
Prompt orthopedic surgery consultation was obtained, and the patient underwent an open reduction and internal fixation of his fracture.
ANSWER
The radiograph demonstrates a fracture within the femoral neck. These types of fractures, which are slightly angulated and located at the base of the neck, are typically referred to as basicervical femur fractures.
Prompt orthopedic surgery consultation was obtained, and the patient underwent an open reduction and internal fixation of his fracture.
You are called in for a neurosurgical consult of a 60-year-old man who underwent a lumbar decompression approximately 18 months ago. For the past several weeks, he has been experiencing worsening back pain that radiates down his left leg; at times, he has to use his cane and walker. The patient denies any acute injury or trauma preceding the pain; however, last night he slipped and fell in his bedroom, which made it much worse.
Medical history is significant for hypertension and hyperlipidemia. The patient responded well to his previous back surgery. The emergency department clinician is concerned that his symptoms may be caused by a new lumbar stenosis at an adjacent level.
On exam, you note a mildly obese male who appears uncomfortable but is in no obvious distress. His vital signs are stable. He has some tenderness within his left hip. Distally, his strength is good, and there is no evidence of neurovascular compromise. Internal and external rotation causes some discomfort.
A portable pelvic radiograph is obtained (shown). What is your impression?
Osteoporosis: Overview, Workup, Diagnosis
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This video was filmed at Metabolic & Endocrine Disease Summit (MEDS). Click here to learn more.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This video was filmed at Metabolic & Endocrine Disease Summit (MEDS). Click here to learn more.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This video was filmed at Metabolic & Endocrine Disease Summit (MEDS). Click here to learn more.
Managing Glenoid Bone Deficiency—The Augment Experience in Anatomic and Reverse Shoulder Arthroplasty
ABSTRACT
Glenoid bone deficiency in the setting of shoulder replacement surgery is far more common than originally reported. The frequency and severity of the glenoid defects are noted to be more common and severe with the advent of computer-assisted surgery. The results of an anatomic total shoulder arthroplasty (aTSA) with glenoid deficiency have been reported to be inferior to aTSA patients without a glenoid deficiency. Options for treating the glenoid deficiency include eccentric reaming, bone grafting, and the use of augmented glenoid components. The purpose of this article is to present the indications, technique, and results of augmented glenoids for both aTSA and reverse TSA (RTSA).
Augments for both aTSA and RTSA are viable options. They preserve subchondral bone at the same time as optimizing the joint line without the need for bone grafts. Complications, revisions and results are as good as compared to shoulder arthroplasties without glenoid wear.
Continue to: Glenoid bone deficiency...
Glenoid bone deficiency in arthritic or cuff-deficient shoulder has been reported in up to 50% of shoulder defect cases.1,2 The type and severity of glenoid deformities vary depending on the underlying pathology and time of manifestation. Osteoarthritis with bone loss typically results in posterior or posterior inferior glenoid wear and is commonly classified as Walch types B1 or B2 (biconcave). In cases of severe erosion, B3 classification has been proposed; in this classification, bone loss becomes extremely severe, progressing to resemble a type C glenoid. Unlike primary osteoarthritis, inflammatory arthropathy more commonly causes central loss of glenoid bone (Walch A2). With the rotator cuff insufficiency, superior migration of the humeral head occurs. As these conditions progress, cuff tear arthropathy (CTA) changes result in superior or posterior-superior bone loss.1 Anterior bone loss (type D) will be rarely encountered due to recurrent anterior instability.3
Classically, with anatomic total shoulder arthroplasty (aTSA), the surgeon considers several options for managing glenoid deficiencies. The most commonly employed technique involves eccentrically reaming the glenoid and correcting the deformity. This procedure is relatively easy but features significant drawbacks, such as sacrificing the subchondral bone, medializing the glenohumeral joint line, and secondarily shrinking the glenoid surface area. Other options include structural bone grafting behind the glenoid component. Most anatomic prosthetic glenoids prove to be unsuitable for fixation of structural bone graft. Therefore, the graft is first internally fixed, followed by placement of the glenoid component. Cement, which is commonly used for glenoid fixation, may potentially inhibit bone-graft healing. Reports using this technique documented high radiographic failure rate of up to 40% at midterm follow-up.4 Although leaving the glenoid component retroverted may be considered, surgeons should develop awareness of the possibility of peg penetration of the anterior glenoid neck. Additionally, retroversion in excess of 5°may increase the risk of recurrent posterior subluxation, resulting in early glenoid loosening.5-7 Results of aTSA under significant glenoid deficiency are inferior to those of aTSA patients without glenoid deficiency.8 Such findings have been extremely inferior in patients with significant glenoid wear, prompting numerous surgeons to abandon aTSA in this population in favor of reverse TSA (RTSA) due to improved bony fixation.
In 2010, augmented anatomic glenoids were first introduced as a wedge (Exactech) and as a step shortly thereafter (DePuy Synthes; Figures 1A-1C). More recently, hemi-wedges have been introduced (Wright Medical Group). Augments have gained popularity due to improved range of motion vs reverse shoulder arthroplasty (RSA). However, debates remain regarding the use of posteriorly augmented components in the setting of posterior glenoid bone loss.8 Augments serve as another viable option for handling glenoid bone deficiency in aTSA.
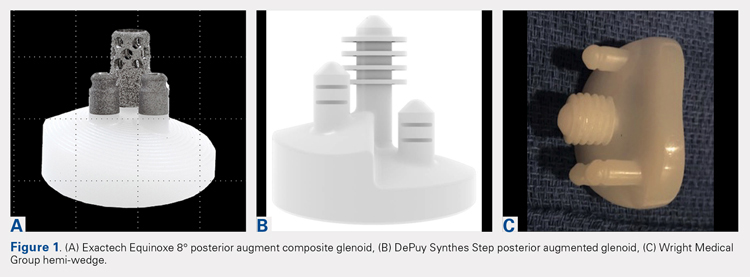
Glenoid bone loss in RTSA presents similar options to aTSA. However, screw fixation of the glenoid component offers several distinct advantages. Baseplate fixation can readily be used with bone grafting and with a highly anticipated success rate. With multiple screw options, 100% support of the baseplate is not mandatory. Although bony increase offset RSAs (BIO-RSAs) have shown success, augmentation with allograft or autograft increases operative time and relies on osseous integration for long-term implant success.9 Metal augmented baseplates were first introduced in 2011 (Exactech) as a means of managing glenoid bone loss without structural grafting. Although initial results have been encouraging, additional studies are needed to assess the longevity of these implants (Figures 1A-1C).
aTSA AUGMENTS
aTSA augments were introduced as a means of correcting acquired glenoid bone deficiency, restoring native glenoid version, correcting humeral subluxation, and preserving the native subchondral bone. Compared with glenoid bone grafting, augmented glenoid components decrease operative time, allow for a technically easier operation, and require no bone healing for clinical success. Early and midterm results are encouraging, showing similar findings comparable to those of aTSA in non-glenoid deficient shoulders.10-12
Continue to: INDICATIONS
INDICATIONS
Indications and limitations for augmented aTSA glenoids remain incompletely defined. The most common indication for an augmented aTSA is osteoarthritis with a B2 glenoid. We recommend augments in the occurrence of any indication of significant eccentric glenoid wear. With the expertise of surgeons, deformities of up to 20° to 25° of deformity can be readily handled with good predictability. More severe deformities can be managed with augmented aTSA components, but early failure rates may be high. The most severe acquired deformities remain best managed with RTSA. Currently, we prefer RTSA when glenoid bone loss exceeds 25°. With the widespread availability of computed tomography (CT) scans with 3-dimensional (3-D) reconstruction, glenoid bone defects are increasingly recognized. When correcting deformity, surgeons should strive to limit residual retroversion to a maximum of 5°.13 Preoperative planning software and computer-assisted surgery (ExactechGPS) may allow surgeons to better define the limits of augmented glenoid fixation prior to the date of surgery. We routinely utilize computer-guided glenoid preparation to control glenoid version to within 5° of neutral position.
The differences between B3 and a true type C glenoid must be recognized. Although B3 glenoids may still be a candidate for an augmented anatomic glenoid component, type C glenoids are not. Developmental abnormalities of type C glenoid occur simultaneously with humeral deformities, including medialized posterior rotator cuff musculature. Correction of the joint line to neutral version may not replicate the non-diseased state of a dysplastic type shoulder. Davis and colleagues14 have proposed treating these patients by leaving both the humerus and glenoid in their native version without correction.
TECHNIQUE
The implant that we have the most experience with is an 8° full-wedge augmented glenoid component. Such an implant is typically utilized for B2 glenoids. We recommend that a high-quality CT scan be performed for preoperative planning. As a general rule, the starting point often lies close to the ridge of B2 glenoid and more anterior than the apparent glenoid center, which is viewed intraoperatively due to asymmetric posterior wear. Full-wedge component is utilized to ream the ridge separating the neo and paleoglenoids to create a flat surface. This condition is best achieved by drilling a pilot hole at the planned glenoid central peg position to prevent the reamer from sliding anteriorly during reaming. Glenoid preparation begins with the smallest reamer until the ridge has been flattened, and the reamer makes full contact with the glenoid. The reamer diameter is then increased based on glenoid size. Slightly downsizing the glenoid implant will require less reaming to achieve full backside support. Once the glenoid is properly reamed, the central and peripheral peg holes are drilled using the appropriate guides. Holes are then dried, and all-polyethylene or composite glenoid component (either partially or completely cemented) is installed using favored cementing techniques. The advantage of composite glenoid component is that the central cage allows for bone ingrowth and may potentially improve long-term implant survival. Press fit of the central cage requires no waiting time for glenoid cement hardening before proceeding to the humerus. When placing an augmented component, adequate glenoid exposure is imperative to allow in-line placement and appropriate seating of the component without impingement on adjacent retractors.
When using the step-augmented glenoid, the paleoglenoid is prepared in a similar fashion to a standard aTSA. Once the paleoglenoid has been reamed to a neutral position, a protector plate is placed onto the paleoglenoid. and a step-cut saw is used to prepare the posterior stepped bone cut. Peripheral pegs are then drilled, and the component is installed in routine fashion. When using hemi-wedge augments, the paleoglenoid is again prepared in a similar fashion as a standard glenoid component over a cannulated guidewire. The neoglenoid is subsequently prepared using a specialized angled reamer with a positive stop to prevent over-reaming. These glenoid implants improve rotational force neutralization given the absence of flat back against the glenoid. All 3 designs preserve bone when compared with eccentric reaming alone,15 with the half-augmented wedge preserving the most bone.
Table 1. Results of Various Augmented Glenoid Components in Anatomic Total Shoulder
Arthroplasty
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
8° cage (N = 21) |
|
|
|
|
| |||||||||||||||
All-polyethylene 8° (N = 45) |
|
|
|
|
| |||||||||||||||
All-polyethylene 16° (N = 7) |
|
|
|
|
|
RESULTS
In our institution, we first used all-polyethylene posteriorly augmented glenoid components in 2010. Between 2010 and 2015, 45 patients received an 8° all-polyethylene posterior augment, and 7 patients received a 16° augment. In 2015, we transitioned to the composite caged posterior augment. All patients in our database who received an augmented glenoid component experienced improvement in active forward elevation, external rotation, American Shoulder and Elbow Surgeons (ASES), and Constant scores (Table 1). Minimum follow-up was 1 year for patients receiving both an 8° cage (mean, 1.48 years) and an 8° all-polyethylene augment (mean, 3.18 years). Figures 2A-2C show a patient with significant posterior glenoid wear and humeral head subluxation treated with an 8° wedge composite posterior augment glenoid 3 years postoperative.
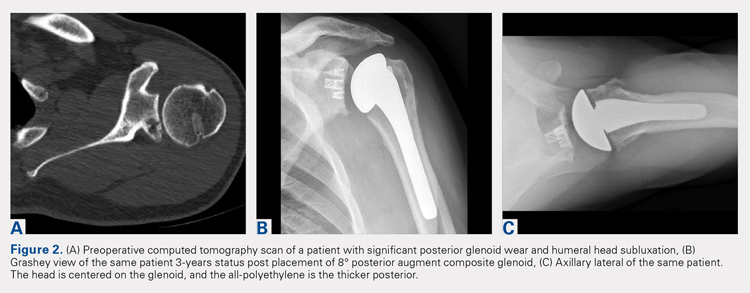
Continue to: COMPLICATIONS
COMPLICATIONS
Two complications developed in the group undergoing composite cage augment. One patient experienced glenoid loosening after a motor vehicle accident. Another patient sustained significant intraoperative tuberosity avulsion during implantation of the humeral component, requiring a change of implant and tuberosity fixation. Although no complications were noted in the 8° all-polyethylene group, 3 patients in the 16° augment group sustained complications. One of these patients suffered a cardiac event that was unrelated to the implant. Two complications in this group were both related to loosening of the glenoid component, requiring subsequent revision.
DISCUSSION
The first report on augmented aTSA was published in 2008, and it involved a 5° augmented, anatomic glenoid.12 One study was based on a small series of augments; the poor results led the reporting surgeons to subsequently abandon the implant.12 This early design produced a correction on the articular side of the implant rather than the pathologic bony side. By performing such correction, the component pegs remained anteriorly oriented, placing the component at risk of perforation through the anterior glenoid neck. All current augment designs feature pegs that are oriented down the glenoid vault, with corrections occurring on the bony surface. This condition requires 2 different axes for reaming the glenoid and drilling the pegs. This approach allows the pegs to be directed down the glenoid neck, and is a far superior solution to neutralizing shear forces when compared with the implants used in the 1990s.
Early to midterm results of modern aTSA augments have been extremely encouraging with low revision rates. The main concern of recurrent posterior subluxation has been rarely reported. The concerns over glenoid loosening due to high shear forces, similarly, have not been described to date. However, surgeons should remain cautious, as longer-term follow-up remains unavailable.
The main advantage of aTSA augments is their capacity to preserve bone compared with eccentric reaming and better long-term stability. Each of the augment designs requires varying amounts of bone removal. Through biomechanics and using finite element analysis, the 3 augment types act differently, with no design demonstrating remarkable biomechanical superiority.6 Favorito and colleagues16 performed a retrospective review of 22 patients who underwent aTSA using an all-polyethylene, posteriorly augmented, and stepped glenoid component for posterior bone loss. At an average follow-up of 36 months, all patients experienced improvements in active forward elevation, external rotation, visual analog scale, Short Form-36 Physical Component Summary, and Western Ontario Osteoarthritis of the Shoulder scores. The authors noted that 2 patients (9%) experienced complications: 1 with an anterior dislocation and the other with recurrent posterior instability requiring revision. Sandow and Schutz17 reported the preliminary results of 10 patients who underwent aTSA using trabecular metal augment with a minimum of 2-year follow-up. All patients received either a 15° or 30° posterior, metal-backed augment for severe glenoid bone loss (Walch grade B2 or C). At a minimum of 2-year follow-up, all patients received correction to within 10° of neutral glenoid version, without any complications nor implant failures.
Regardless of augment design, all current components restore the native glenoid version, improving the length and subsequent tension of rotator cuff musculature. Similarly, re-centering the humeral head decreases the forces on the glenoid and allows for optimal function with decreasing loss of vital subchondral bone.
Continue to: RTSA AUGMENTS
RTSA AUGMENTS
Similar to anatomic augments, metal augments were introduced for use with RTSA in 2011. Unlike anatomic augments, those for RTSA were manufactured with metal. Given the difference in bony wear patterns in patients requiring RTSA, augments were available in a number of configurations. With CTA, wear is most commonly superior. Leaving a superiorly inclined baseplate must be avoided due to risks of notching, loosening, and early failure. However, correcting this tilt will require significant reaming of the inferior glenoid. A superior augment is ideally suited for this bone-loss pattern. If the glenoid is retroverted significantly, difficulty can also arise during glenoid preparation and baseplate placement. Posterior augments may ease this aspect of the procedure. Posterior augments feature the additional benefits of tensioning any remaining posterior rotator cuff, minimizing posterior inferior impingement, and technically easing the operation.18 As we improve our awareness of glenoid orientation using computer navigation, a posterior-superior augmented implant is commonly needed to simultaneously optimize the baseplate position and to minimize reaming (Figure 3). The posterior-superior augmented baseplate has become the most commonly used baseplate augment of choice in 90% of our RTSA cases that require an augment.

INDICATIONS
Augmented RTSA baseplates are indicated when adequate backside contact cannot be achieved with eccentric reaming, thus compromising potential fixation. In our practice, we preferably use augments at <50% contact with the backside of the baseplate. Excessive superior inclination is observed in a CTA setting, commonly indicating the use of superior augments. Similarly, severe primary osteoarthritis may contain elements of posterior bone loss, leading to increased retroversion, which is where we use posterior augments. When patients exhibit combined deformities, or when the surgeon wishes to tension the posterior rotator cuff, a posterior-superior augmented glenoid baseplate is used. For extremely severe defects, we have combined bone grafting and augments. In patients with a highly deficient glenoid but good quality of the remaining bone stock, an augment allows for better contact with less reaming although it is not fully supported when compared with a non-augmented baseplate. Bone grafts can function similarly, but the autograft humeral head is not constantly present in revision situations and requires increased operative time to allow for precision carpentry. Additionally, the success of BIO-RSA requires healing of bone graft on the native glenoid to support the baseplate.19 Jones and colleagues9 compared metal augmented RTSA with BIO-RSA and presented equivalent results.
To minimize reaming and to obtain appropriately inferior inclination, we have discovered preoperative templating and intraoperative, computer-guided glenoid preparation to be extremely valuable (ExactechGPS). These tools allow appropriate assessment of augments and for minimal bone removal when preparing the glenoid.
TECHNIQUE
When using an augment, a fine-cut CT scan is highly recommended to aid in surgery planning. We also find 3-D reconstructions to be helpful. Preoperative planning software also allows surgeons to maximize fixation of implant within the glenoid vault. The starting point for reaming is planned based on CT. Some surgeons using augments perform minimal or no reaming at all, electing to remove the remaining cartilage with a Cobb elevator. Different reaming and drilling axes are used when using augments. In cases of severe glenoid deformity and unavailability of computer assistance, a guide wire with inferior inclination can be installed based on CT scan. Penetration of this wire down the glenoid neck can be palpated and compared with the preoperative plan. We generally prefer at least 24 mm of bone containment for the central cage. Once the surgeon is satisfied with the placement of the wire, the appropriate augment guide is placed, followed by a second guide wire. This second wire acts as the reaming axis. The first wire is removed, and the glenoid is reamed with a cannulated reamer. Once reaming is completed, the original wire is replaced in the same hole and trajectory, and the reaming wire is removed. The first wire is then drilled with a cannulated drill for the central cage. The augmented baseplate is then impacted into place, and screw fixation is performed. Again, intraoperative computer guidance allows for precision screw placement with maximal bone attachment.
Table 2. Results of Reverse Total Shoulder Arthroplasty Augmented Baseplates
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
Superior (N = 22) |
|
|
|
|
| |||||||||||||||
Posterior (N = 50) |
|
|
|
|
| |||||||||||||||
Posterosuperior (N = 67) |
|
|
|
|
|
RESULTS
Based on our experience, glenoid augments for RTSA have performed well at short- and mid-term follow-up. From October 2011 to July 2016, 139 patients undergoing RTSA received a posterior, superior, or posterior-superior augmented glenoid baseplate. All groups demonstrated improvements in functional outcome measures, including Constant, ASES, Shoulder Pain and Disability Index, and Simple Shoulder Test scores compared with baseline values (Table 2). The posterior-superior augment group experienced the most significant improvement in active forward flexion and external rotation, whereas the posterior augment group experienced the most significant improvement in ASES and Constant scores. Figures 4A-4C displays the radiographs of a patient with significant glenoid wear treated with a posterior-superior augment RTSA.
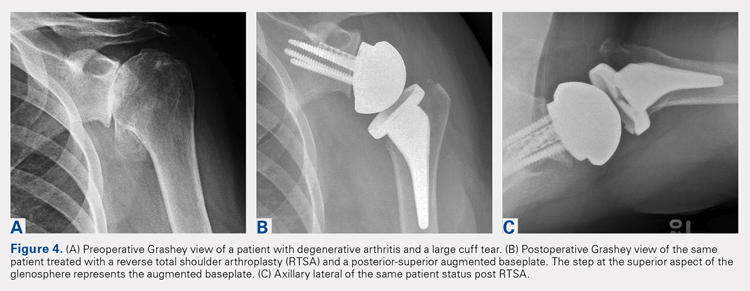
Continue to: COMPLICATIONS
COMPLICATIONS
In the superior augment group, 3 patients (13%) sustained 5 complications. One patient sustained 3 separate episodes of instability, eventually requiring revision of prosthesis. In the posterior augment group, 4 patients (8%) sustained complications. Two of the 4 patients presented postoperative humeral fractures related to traumatic events, whereas another patient sustained an intraoperative tuberosity fracture. The last complication in this group involved a postoperative draining wound that was treated with oral antibiotics.
Nine complications developed in the posterior-superior augment group (13%); these complications included aseptic baseplate loosening (5), glenoid fracture (1), humeral fracture (1), acromial stress fracture (1), and cerebrovascular accident (1).
DISCUSSION
As the use of augments in RTSA is relatively new, significantly scarce data exist regarding their outcomes and longevity. A few studies have focused on the short-term outcomes of these augments. Jones and colleagues9 performed a retrospective review of 80 patients who underwent RTSA and required either a structural bone graft or an augmented glenoid baseplate.9 They observed that although all patients showed improvements in pain, range of motion, and functional scores, the structural bone graft group incurred a 14.6% complication rate compared with none observed in the augment group. Additionally, Jones and colleagues9 noted that the augmented baseplate group exhibited a significantly lower rate of scapular notching compared with the bone-graft group (10% vs 18.5%) at similar follow-up intervals. A separate study by Wright and colleagues18 compared posterior vs superior augmented baseplates in RTSA. The posterior augment group demonstrated lower rates of scapular notching (6.3% vs 14.3%) and showed more significant improvements in Constant, ASES, and active forward elevation measures, compared with the superior augment group.
As more manufacturers develop augments for RTSA, and as ExactechGPS uses become more widespread, the use of RTSA baseplate augments will continually grow. Custom implants using massive metal augments are now also being introduced. Although currently too expensive for most cases, as technology drives the cost down, every patient may receive customized augmented implants in the future.
The advantages of augmented baseplate designs include minimized reaming and notching, improved tension of the remaining rotator cuff, and decreased operating room time. The disadvantages include increased cost and lack of mid- or long-term clinical data. The concerns with baseplate loosening with augments in RTSA are much less than those with augments for aTSA due to the outstanding baseplate fixation that can be achieved in RTSA.
Continue to: CONLCLUSION
CONCLUSION
Augments offer an excellent tool for surgeons performing both aTSA and RTSA with glenoid bone loss. Use of augments will become more common as more manufacturers develop them. Although clinical results fall short in full midterm, they have been positive for both augmented RTSA and aTSA. Concerns arise when performing augmented aTSA, as an upper limit of correction has not been defined with regard to component failure. Currently, no data support the maximum amount of correction that can be achieved. In our current practice, we face difficulty in correcting more than 25° of version in young active patients with aTSA augment. Beyond this point, we perform a RTSA with an augment. In older patients or low-demand patients, we only correct minor deformities (<20°) with an aTSA augment, opting instead for an augmented RTSA due to the lower midterm failure rates observed with this implant.
1. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. J Bone Joint Surg Br. 2004;86(3):388-395. doi:10.1302/0301-620X.86B3.
2. Churchill RS, Spencer Jr EE, Fehringer EV. Quantification of B2 glenoid morphology in total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(8):1212-1217. doi:10.1016/j.jse.2015.01.007.
3. Bercik MJ, Kruse K, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606. doi:10.1016/j.jse.2016.03.010.
4. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072. doi:10.1016/j.jse.2013.09.017.
5. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
6. Hermida JC, Flores-Hernandez C, Hoenecke HR, D’Lima DD. Augmented wedge-shaped glenoid component for the correction of glenoid retroversion: a finite element analysis. J Shoulder Elbow Surg. 2014;23(3):347-354. doi:10.1016/j.jse.2013.06.008.
7. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82. doi:10.2106/JBJS.L.00336.
8. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598. doi:10.1016/j.jse.2013.06.017.
9. Jones RB, Wright TW, Roche CP. Bone grafting the glenoid versus use of augmented glenoid baseplates with reverse shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73(suppl 1):S129-S135.
10. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymmetric posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308. doi:10.1016/j.jse.2013.04.014.
11. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157. doi:10.1016/j.jse.2013.09.023.
12. Rice RS, Sperling JW, Miletti J, Schleck C, Cofield RH. Augmented glenoid component for bone deficiency in shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):579-583. doi:10.1007/s11999-007-0104-4.
13. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973. doi:10.1016/j.jse.2013.09.019.
14. Davis DE, Acevedo D, Williams A, Williams G. Total shoulder arthroplasty using an inlay mini-glenoid component for glenoid deficiency: a 2-year follow-up of 9 shoulders in 7 patients. J Shoulder Elbow Surg. 2016;25(8):1354-1361. doi:10.1016/j.jse.2015.12.010.
15. Kersten AD, Flores-Hernandez C, Hoenecke HR, D'Lima DD. Posterior augmented glenoid designs preserve more bone in biconcave glenoids. J Shoulder Elbow Surg. 2015;24(7):1135-1141. doi:10.1016/j.jse.2014.12.007.
16. Favorito PJ, Freed RJ, Passanise AM, Brown MJ. Total shoulder arthroplasty for glenohumeral arthritis associated with posterior glenoid bone loss: results of an all-polyethylene, posteriorly augmented glenoid component. J Shoulder Elbow Surg. 2016;25(10):1681-1689. doi:10.1016/j.jse.2016.02.020.
17. Sandow M, Schutz C. Total shoulder arthroplasty using trabecular metal augments to address glenoid retroversion: the preliminary result of 10 patients with minimum 2-year follow-up. J Shoulder Elbow Surg. 2016;25(4):598-607. doi:10.1016/j.jse.2016.01.001.
18. Wright TW, Roche CP, Wright L, Flurin PH, Crosby LA, Zuckerman JD. Reverse shoulder arthroplasty augments for glenoid wear: A comparison of posterior augments to superior augments. Bull Hosp Jt Dis. 2015;73(suppl 1):S124-S128.
19. Boileau P, Morin-Salvo N, Gauci MO, et al. Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty): a solution for the management glenoid bone loss and erosion. J Shoulder Elbow Surg. 2017;26(12):2133-2142. doi:10.1016/j.jse.2017.05.024.
ABSTRACT
Glenoid bone deficiency in the setting of shoulder replacement surgery is far more common than originally reported. The frequency and severity of the glenoid defects are noted to be more common and severe with the advent of computer-assisted surgery. The results of an anatomic total shoulder arthroplasty (aTSA) with glenoid deficiency have been reported to be inferior to aTSA patients without a glenoid deficiency. Options for treating the glenoid deficiency include eccentric reaming, bone grafting, and the use of augmented glenoid components. The purpose of this article is to present the indications, technique, and results of augmented glenoids for both aTSA and reverse TSA (RTSA).
Augments for both aTSA and RTSA are viable options. They preserve subchondral bone at the same time as optimizing the joint line without the need for bone grafts. Complications, revisions and results are as good as compared to shoulder arthroplasties without glenoid wear.
Continue to: Glenoid bone deficiency...
Glenoid bone deficiency in arthritic or cuff-deficient shoulder has been reported in up to 50% of shoulder defect cases.1,2 The type and severity of glenoid deformities vary depending on the underlying pathology and time of manifestation. Osteoarthritis with bone loss typically results in posterior or posterior inferior glenoid wear and is commonly classified as Walch types B1 or B2 (biconcave). In cases of severe erosion, B3 classification has been proposed; in this classification, bone loss becomes extremely severe, progressing to resemble a type C glenoid. Unlike primary osteoarthritis, inflammatory arthropathy more commonly causes central loss of glenoid bone (Walch A2). With the rotator cuff insufficiency, superior migration of the humeral head occurs. As these conditions progress, cuff tear arthropathy (CTA) changes result in superior or posterior-superior bone loss.1 Anterior bone loss (type D) will be rarely encountered due to recurrent anterior instability.3
Classically, with anatomic total shoulder arthroplasty (aTSA), the surgeon considers several options for managing glenoid deficiencies. The most commonly employed technique involves eccentrically reaming the glenoid and correcting the deformity. This procedure is relatively easy but features significant drawbacks, such as sacrificing the subchondral bone, medializing the glenohumeral joint line, and secondarily shrinking the glenoid surface area. Other options include structural bone grafting behind the glenoid component. Most anatomic prosthetic glenoids prove to be unsuitable for fixation of structural bone graft. Therefore, the graft is first internally fixed, followed by placement of the glenoid component. Cement, which is commonly used for glenoid fixation, may potentially inhibit bone-graft healing. Reports using this technique documented high radiographic failure rate of up to 40% at midterm follow-up.4 Although leaving the glenoid component retroverted may be considered, surgeons should develop awareness of the possibility of peg penetration of the anterior glenoid neck. Additionally, retroversion in excess of 5°may increase the risk of recurrent posterior subluxation, resulting in early glenoid loosening.5-7 Results of aTSA under significant glenoid deficiency are inferior to those of aTSA patients without glenoid deficiency.8 Such findings have been extremely inferior in patients with significant glenoid wear, prompting numerous surgeons to abandon aTSA in this population in favor of reverse TSA (RTSA) due to improved bony fixation.
In 2010, augmented anatomic glenoids were first introduced as a wedge (Exactech) and as a step shortly thereafter (DePuy Synthes; Figures 1A-1C). More recently, hemi-wedges have been introduced (Wright Medical Group). Augments have gained popularity due to improved range of motion vs reverse shoulder arthroplasty (RSA). However, debates remain regarding the use of posteriorly augmented components in the setting of posterior glenoid bone loss.8 Augments serve as another viable option for handling glenoid bone deficiency in aTSA.

Glenoid bone loss in RTSA presents similar options to aTSA. However, screw fixation of the glenoid component offers several distinct advantages. Baseplate fixation can readily be used with bone grafting and with a highly anticipated success rate. With multiple screw options, 100% support of the baseplate is not mandatory. Although bony increase offset RSAs (BIO-RSAs) have shown success, augmentation with allograft or autograft increases operative time and relies on osseous integration for long-term implant success.9 Metal augmented baseplates were first introduced in 2011 (Exactech) as a means of managing glenoid bone loss without structural grafting. Although initial results have been encouraging, additional studies are needed to assess the longevity of these implants (Figures 1A-1C).
aTSA AUGMENTS
aTSA augments were introduced as a means of correcting acquired glenoid bone deficiency, restoring native glenoid version, correcting humeral subluxation, and preserving the native subchondral bone. Compared with glenoid bone grafting, augmented glenoid components decrease operative time, allow for a technically easier operation, and require no bone healing for clinical success. Early and midterm results are encouraging, showing similar findings comparable to those of aTSA in non-glenoid deficient shoulders.10-12
Continue to: INDICATIONS
INDICATIONS
Indications and limitations for augmented aTSA glenoids remain incompletely defined. The most common indication for an augmented aTSA is osteoarthritis with a B2 glenoid. We recommend augments in the occurrence of any indication of significant eccentric glenoid wear. With the expertise of surgeons, deformities of up to 20° to 25° of deformity can be readily handled with good predictability. More severe deformities can be managed with augmented aTSA components, but early failure rates may be high. The most severe acquired deformities remain best managed with RTSA. Currently, we prefer RTSA when glenoid bone loss exceeds 25°. With the widespread availability of computed tomography (CT) scans with 3-dimensional (3-D) reconstruction, glenoid bone defects are increasingly recognized. When correcting deformity, surgeons should strive to limit residual retroversion to a maximum of 5°.13 Preoperative planning software and computer-assisted surgery (ExactechGPS) may allow surgeons to better define the limits of augmented glenoid fixation prior to the date of surgery. We routinely utilize computer-guided glenoid preparation to control glenoid version to within 5° of neutral position.
The differences between B3 and a true type C glenoid must be recognized. Although B3 glenoids may still be a candidate for an augmented anatomic glenoid component, type C glenoids are not. Developmental abnormalities of type C glenoid occur simultaneously with humeral deformities, including medialized posterior rotator cuff musculature. Correction of the joint line to neutral version may not replicate the non-diseased state of a dysplastic type shoulder. Davis and colleagues14 have proposed treating these patients by leaving both the humerus and glenoid in their native version without correction.
TECHNIQUE
The implant that we have the most experience with is an 8° full-wedge augmented glenoid component. Such an implant is typically utilized for B2 glenoids. We recommend that a high-quality CT scan be performed for preoperative planning. As a general rule, the starting point often lies close to the ridge of B2 glenoid and more anterior than the apparent glenoid center, which is viewed intraoperatively due to asymmetric posterior wear. Full-wedge component is utilized to ream the ridge separating the neo and paleoglenoids to create a flat surface. This condition is best achieved by drilling a pilot hole at the planned glenoid central peg position to prevent the reamer from sliding anteriorly during reaming. Glenoid preparation begins with the smallest reamer until the ridge has been flattened, and the reamer makes full contact with the glenoid. The reamer diameter is then increased based on glenoid size. Slightly downsizing the glenoid implant will require less reaming to achieve full backside support. Once the glenoid is properly reamed, the central and peripheral peg holes are drilled using the appropriate guides. Holes are then dried, and all-polyethylene or composite glenoid component (either partially or completely cemented) is installed using favored cementing techniques. The advantage of composite glenoid component is that the central cage allows for bone ingrowth and may potentially improve long-term implant survival. Press fit of the central cage requires no waiting time for glenoid cement hardening before proceeding to the humerus. When placing an augmented component, adequate glenoid exposure is imperative to allow in-line placement and appropriate seating of the component without impingement on adjacent retractors.
When using the step-augmented glenoid, the paleoglenoid is prepared in a similar fashion to a standard aTSA. Once the paleoglenoid has been reamed to a neutral position, a protector plate is placed onto the paleoglenoid. and a step-cut saw is used to prepare the posterior stepped bone cut. Peripheral pegs are then drilled, and the component is installed in routine fashion. When using hemi-wedge augments, the paleoglenoid is again prepared in a similar fashion as a standard glenoid component over a cannulated guidewire. The neoglenoid is subsequently prepared using a specialized angled reamer with a positive stop to prevent over-reaming. These glenoid implants improve rotational force neutralization given the absence of flat back against the glenoid. All 3 designs preserve bone when compared with eccentric reaming alone,15 with the half-augmented wedge preserving the most bone.
Table 1. Results of Various Augmented Glenoid Components in Anatomic Total Shoulder
Arthroplasty
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
8° cage (N = 21) |
|
|
|
|
| |||||||||||||||
All-polyethylene 8° (N = 45) |
|
|
|
|
| |||||||||||||||
All-polyethylene 16° (N = 7) |
|
|
|
|
|
RESULTS
In our institution, we first used all-polyethylene posteriorly augmented glenoid components in 2010. Between 2010 and 2015, 45 patients received an 8° all-polyethylene posterior augment, and 7 patients received a 16° augment. In 2015, we transitioned to the composite caged posterior augment. All patients in our database who received an augmented glenoid component experienced improvement in active forward elevation, external rotation, American Shoulder and Elbow Surgeons (ASES), and Constant scores (Table 1). Minimum follow-up was 1 year for patients receiving both an 8° cage (mean, 1.48 years) and an 8° all-polyethylene augment (mean, 3.18 years). Figures 2A-2C show a patient with significant posterior glenoid wear and humeral head subluxation treated with an 8° wedge composite posterior augment glenoid 3 years postoperative.

Continue to: COMPLICATIONS
COMPLICATIONS
Two complications developed in the group undergoing composite cage augment. One patient experienced glenoid loosening after a motor vehicle accident. Another patient sustained significant intraoperative tuberosity avulsion during implantation of the humeral component, requiring a change of implant and tuberosity fixation. Although no complications were noted in the 8° all-polyethylene group, 3 patients in the 16° augment group sustained complications. One of these patients suffered a cardiac event that was unrelated to the implant. Two complications in this group were both related to loosening of the glenoid component, requiring subsequent revision.
DISCUSSION
The first report on augmented aTSA was published in 2008, and it involved a 5° augmented, anatomic glenoid.12 One study was based on a small series of augments; the poor results led the reporting surgeons to subsequently abandon the implant.12 This early design produced a correction on the articular side of the implant rather than the pathologic bony side. By performing such correction, the component pegs remained anteriorly oriented, placing the component at risk of perforation through the anterior glenoid neck. All current augment designs feature pegs that are oriented down the glenoid vault, with corrections occurring on the bony surface. This condition requires 2 different axes for reaming the glenoid and drilling the pegs. This approach allows the pegs to be directed down the glenoid neck, and is a far superior solution to neutralizing shear forces when compared with the implants used in the 1990s.
Early to midterm results of modern aTSA augments have been extremely encouraging with low revision rates. The main concern of recurrent posterior subluxation has been rarely reported. The concerns over glenoid loosening due to high shear forces, similarly, have not been described to date. However, surgeons should remain cautious, as longer-term follow-up remains unavailable.
The main advantage of aTSA augments is their capacity to preserve bone compared with eccentric reaming and better long-term stability. Each of the augment designs requires varying amounts of bone removal. Through biomechanics and using finite element analysis, the 3 augment types act differently, with no design demonstrating remarkable biomechanical superiority.6 Favorito and colleagues16 performed a retrospective review of 22 patients who underwent aTSA using an all-polyethylene, posteriorly augmented, and stepped glenoid component for posterior bone loss. At an average follow-up of 36 months, all patients experienced improvements in active forward elevation, external rotation, visual analog scale, Short Form-36 Physical Component Summary, and Western Ontario Osteoarthritis of the Shoulder scores. The authors noted that 2 patients (9%) experienced complications: 1 with an anterior dislocation and the other with recurrent posterior instability requiring revision. Sandow and Schutz17 reported the preliminary results of 10 patients who underwent aTSA using trabecular metal augment with a minimum of 2-year follow-up. All patients received either a 15° or 30° posterior, metal-backed augment for severe glenoid bone loss (Walch grade B2 or C). At a minimum of 2-year follow-up, all patients received correction to within 10° of neutral glenoid version, without any complications nor implant failures.
Regardless of augment design, all current components restore the native glenoid version, improving the length and subsequent tension of rotator cuff musculature. Similarly, re-centering the humeral head decreases the forces on the glenoid and allows for optimal function with decreasing loss of vital subchondral bone.
Continue to: RTSA AUGMENTS
RTSA AUGMENTS
Similar to anatomic augments, metal augments were introduced for use with RTSA in 2011. Unlike anatomic augments, those for RTSA were manufactured with metal. Given the difference in bony wear patterns in patients requiring RTSA, augments were available in a number of configurations. With CTA, wear is most commonly superior. Leaving a superiorly inclined baseplate must be avoided due to risks of notching, loosening, and early failure. However, correcting this tilt will require significant reaming of the inferior glenoid. A superior augment is ideally suited for this bone-loss pattern. If the glenoid is retroverted significantly, difficulty can also arise during glenoid preparation and baseplate placement. Posterior augments may ease this aspect of the procedure. Posterior augments feature the additional benefits of tensioning any remaining posterior rotator cuff, minimizing posterior inferior impingement, and technically easing the operation.18 As we improve our awareness of glenoid orientation using computer navigation, a posterior-superior augmented implant is commonly needed to simultaneously optimize the baseplate position and to minimize reaming (Figure 3). The posterior-superior augmented baseplate has become the most commonly used baseplate augment of choice in 90% of our RTSA cases that require an augment.

INDICATIONS
Augmented RTSA baseplates are indicated when adequate backside contact cannot be achieved with eccentric reaming, thus compromising potential fixation. In our practice, we preferably use augments at <50% contact with the backside of the baseplate. Excessive superior inclination is observed in a CTA setting, commonly indicating the use of superior augments. Similarly, severe primary osteoarthritis may contain elements of posterior bone loss, leading to increased retroversion, which is where we use posterior augments. When patients exhibit combined deformities, or when the surgeon wishes to tension the posterior rotator cuff, a posterior-superior augmented glenoid baseplate is used. For extremely severe defects, we have combined bone grafting and augments. In patients with a highly deficient glenoid but good quality of the remaining bone stock, an augment allows for better contact with less reaming although it is not fully supported when compared with a non-augmented baseplate. Bone grafts can function similarly, but the autograft humeral head is not constantly present in revision situations and requires increased operative time to allow for precision carpentry. Additionally, the success of BIO-RSA requires healing of bone graft on the native glenoid to support the baseplate.19 Jones and colleagues9 compared metal augmented RTSA with BIO-RSA and presented equivalent results.
To minimize reaming and to obtain appropriately inferior inclination, we have discovered preoperative templating and intraoperative, computer-guided glenoid preparation to be extremely valuable (ExactechGPS). These tools allow appropriate assessment of augments and for minimal bone removal when preparing the glenoid.
TECHNIQUE
When using an augment, a fine-cut CT scan is highly recommended to aid in surgery planning. We also find 3-D reconstructions to be helpful. Preoperative planning software also allows surgeons to maximize fixation of implant within the glenoid vault. The starting point for reaming is planned based on CT. Some surgeons using augments perform minimal or no reaming at all, electing to remove the remaining cartilage with a Cobb elevator. Different reaming and drilling axes are used when using augments. In cases of severe glenoid deformity and unavailability of computer assistance, a guide wire with inferior inclination can be installed based on CT scan. Penetration of this wire down the glenoid neck can be palpated and compared with the preoperative plan. We generally prefer at least 24 mm of bone containment for the central cage. Once the surgeon is satisfied with the placement of the wire, the appropriate augment guide is placed, followed by a second guide wire. This second wire acts as the reaming axis. The first wire is removed, and the glenoid is reamed with a cannulated reamer. Once reaming is completed, the original wire is replaced in the same hole and trajectory, and the reaming wire is removed. The first wire is then drilled with a cannulated drill for the central cage. The augmented baseplate is then impacted into place, and screw fixation is performed. Again, intraoperative computer guidance allows for precision screw placement with maximal bone attachment.
Table 2. Results of Reverse Total Shoulder Arthroplasty Augmented Baseplates
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
Superior (N = 22) |
|
|
|
|
| |||||||||||||||
Posterior (N = 50) |
|
|
|
|
| |||||||||||||||
Posterosuperior (N = 67) |
|
|
|
|
|
RESULTS
Based on our experience, glenoid augments for RTSA have performed well at short- and mid-term follow-up. From October 2011 to July 2016, 139 patients undergoing RTSA received a posterior, superior, or posterior-superior augmented glenoid baseplate. All groups demonstrated improvements in functional outcome measures, including Constant, ASES, Shoulder Pain and Disability Index, and Simple Shoulder Test scores compared with baseline values (Table 2). The posterior-superior augment group experienced the most significant improvement in active forward flexion and external rotation, whereas the posterior augment group experienced the most significant improvement in ASES and Constant scores. Figures 4A-4C displays the radiographs of a patient with significant glenoid wear treated with a posterior-superior augment RTSA.

Continue to: COMPLICATIONS
COMPLICATIONS
In the superior augment group, 3 patients (13%) sustained 5 complications. One patient sustained 3 separate episodes of instability, eventually requiring revision of prosthesis. In the posterior augment group, 4 patients (8%) sustained complications. Two of the 4 patients presented postoperative humeral fractures related to traumatic events, whereas another patient sustained an intraoperative tuberosity fracture. The last complication in this group involved a postoperative draining wound that was treated with oral antibiotics.
Nine complications developed in the posterior-superior augment group (13%); these complications included aseptic baseplate loosening (5), glenoid fracture (1), humeral fracture (1), acromial stress fracture (1), and cerebrovascular accident (1).
DISCUSSION
As the use of augments in RTSA is relatively new, significantly scarce data exist regarding their outcomes and longevity. A few studies have focused on the short-term outcomes of these augments. Jones and colleagues9 performed a retrospective review of 80 patients who underwent RTSA and required either a structural bone graft or an augmented glenoid baseplate.9 They observed that although all patients showed improvements in pain, range of motion, and functional scores, the structural bone graft group incurred a 14.6% complication rate compared with none observed in the augment group. Additionally, Jones and colleagues9 noted that the augmented baseplate group exhibited a significantly lower rate of scapular notching compared with the bone-graft group (10% vs 18.5%) at similar follow-up intervals. A separate study by Wright and colleagues18 compared posterior vs superior augmented baseplates in RTSA. The posterior augment group demonstrated lower rates of scapular notching (6.3% vs 14.3%) and showed more significant improvements in Constant, ASES, and active forward elevation measures, compared with the superior augment group.
As more manufacturers develop augments for RTSA, and as ExactechGPS uses become more widespread, the use of RTSA baseplate augments will continually grow. Custom implants using massive metal augments are now also being introduced. Although currently too expensive for most cases, as technology drives the cost down, every patient may receive customized augmented implants in the future.
The advantages of augmented baseplate designs include minimized reaming and notching, improved tension of the remaining rotator cuff, and decreased operating room time. The disadvantages include increased cost and lack of mid- or long-term clinical data. The concerns with baseplate loosening with augments in RTSA are much less than those with augments for aTSA due to the outstanding baseplate fixation that can be achieved in RTSA.
Continue to: CONLCLUSION
CONCLUSION
Augments offer an excellent tool for surgeons performing both aTSA and RTSA with glenoid bone loss. Use of augments will become more common as more manufacturers develop them. Although clinical results fall short in full midterm, they have been positive for both augmented RTSA and aTSA. Concerns arise when performing augmented aTSA, as an upper limit of correction has not been defined with regard to component failure. Currently, no data support the maximum amount of correction that can be achieved. In our current practice, we face difficulty in correcting more than 25° of version in young active patients with aTSA augment. Beyond this point, we perform a RTSA with an augment. In older patients or low-demand patients, we only correct minor deformities (<20°) with an aTSA augment, opting instead for an augmented RTSA due to the lower midterm failure rates observed with this implant.
ABSTRACT
Glenoid bone deficiency in the setting of shoulder replacement surgery is far more common than originally reported. The frequency and severity of the glenoid defects are noted to be more common and severe with the advent of computer-assisted surgery. The results of an anatomic total shoulder arthroplasty (aTSA) with glenoid deficiency have been reported to be inferior to aTSA patients without a glenoid deficiency. Options for treating the glenoid deficiency include eccentric reaming, bone grafting, and the use of augmented glenoid components. The purpose of this article is to present the indications, technique, and results of augmented glenoids for both aTSA and reverse TSA (RTSA).
Augments for both aTSA and RTSA are viable options. They preserve subchondral bone at the same time as optimizing the joint line without the need for bone grafts. Complications, revisions and results are as good as compared to shoulder arthroplasties without glenoid wear.
Continue to: Glenoid bone deficiency...
Glenoid bone deficiency in arthritic or cuff-deficient shoulder has been reported in up to 50% of shoulder defect cases.1,2 The type and severity of glenoid deformities vary depending on the underlying pathology and time of manifestation. Osteoarthritis with bone loss typically results in posterior or posterior inferior glenoid wear and is commonly classified as Walch types B1 or B2 (biconcave). In cases of severe erosion, B3 classification has been proposed; in this classification, bone loss becomes extremely severe, progressing to resemble a type C glenoid. Unlike primary osteoarthritis, inflammatory arthropathy more commonly causes central loss of glenoid bone (Walch A2). With the rotator cuff insufficiency, superior migration of the humeral head occurs. As these conditions progress, cuff tear arthropathy (CTA) changes result in superior or posterior-superior bone loss.1 Anterior bone loss (type D) will be rarely encountered due to recurrent anterior instability.3
Classically, with anatomic total shoulder arthroplasty (aTSA), the surgeon considers several options for managing glenoid deficiencies. The most commonly employed technique involves eccentrically reaming the glenoid and correcting the deformity. This procedure is relatively easy but features significant drawbacks, such as sacrificing the subchondral bone, medializing the glenohumeral joint line, and secondarily shrinking the glenoid surface area. Other options include structural bone grafting behind the glenoid component. Most anatomic prosthetic glenoids prove to be unsuitable for fixation of structural bone graft. Therefore, the graft is first internally fixed, followed by placement of the glenoid component. Cement, which is commonly used for glenoid fixation, may potentially inhibit bone-graft healing. Reports using this technique documented high radiographic failure rate of up to 40% at midterm follow-up.4 Although leaving the glenoid component retroverted may be considered, surgeons should develop awareness of the possibility of peg penetration of the anterior glenoid neck. Additionally, retroversion in excess of 5°may increase the risk of recurrent posterior subluxation, resulting in early glenoid loosening.5-7 Results of aTSA under significant glenoid deficiency are inferior to those of aTSA patients without glenoid deficiency.8 Such findings have been extremely inferior in patients with significant glenoid wear, prompting numerous surgeons to abandon aTSA in this population in favor of reverse TSA (RTSA) due to improved bony fixation.
In 2010, augmented anatomic glenoids were first introduced as a wedge (Exactech) and as a step shortly thereafter (DePuy Synthes; Figures 1A-1C). More recently, hemi-wedges have been introduced (Wright Medical Group). Augments have gained popularity due to improved range of motion vs reverse shoulder arthroplasty (RSA). However, debates remain regarding the use of posteriorly augmented components in the setting of posterior glenoid bone loss.8 Augments serve as another viable option for handling glenoid bone deficiency in aTSA.

Glenoid bone loss in RTSA presents similar options to aTSA. However, screw fixation of the glenoid component offers several distinct advantages. Baseplate fixation can readily be used with bone grafting and with a highly anticipated success rate. With multiple screw options, 100% support of the baseplate is not mandatory. Although bony increase offset RSAs (BIO-RSAs) have shown success, augmentation with allograft or autograft increases operative time and relies on osseous integration for long-term implant success.9 Metal augmented baseplates were first introduced in 2011 (Exactech) as a means of managing glenoid bone loss without structural grafting. Although initial results have been encouraging, additional studies are needed to assess the longevity of these implants (Figures 1A-1C).
aTSA AUGMENTS
aTSA augments were introduced as a means of correcting acquired glenoid bone deficiency, restoring native glenoid version, correcting humeral subluxation, and preserving the native subchondral bone. Compared with glenoid bone grafting, augmented glenoid components decrease operative time, allow for a technically easier operation, and require no bone healing for clinical success. Early and midterm results are encouraging, showing similar findings comparable to those of aTSA in non-glenoid deficient shoulders.10-12
Continue to: INDICATIONS
INDICATIONS
Indications and limitations for augmented aTSA glenoids remain incompletely defined. The most common indication for an augmented aTSA is osteoarthritis with a B2 glenoid. We recommend augments in the occurrence of any indication of significant eccentric glenoid wear. With the expertise of surgeons, deformities of up to 20° to 25° of deformity can be readily handled with good predictability. More severe deformities can be managed with augmented aTSA components, but early failure rates may be high. The most severe acquired deformities remain best managed with RTSA. Currently, we prefer RTSA when glenoid bone loss exceeds 25°. With the widespread availability of computed tomography (CT) scans with 3-dimensional (3-D) reconstruction, glenoid bone defects are increasingly recognized. When correcting deformity, surgeons should strive to limit residual retroversion to a maximum of 5°.13 Preoperative planning software and computer-assisted surgery (ExactechGPS) may allow surgeons to better define the limits of augmented glenoid fixation prior to the date of surgery. We routinely utilize computer-guided glenoid preparation to control glenoid version to within 5° of neutral position.
The differences between B3 and a true type C glenoid must be recognized. Although B3 glenoids may still be a candidate for an augmented anatomic glenoid component, type C glenoids are not. Developmental abnormalities of type C glenoid occur simultaneously with humeral deformities, including medialized posterior rotator cuff musculature. Correction of the joint line to neutral version may not replicate the non-diseased state of a dysplastic type shoulder. Davis and colleagues14 have proposed treating these patients by leaving both the humerus and glenoid in their native version without correction.
TECHNIQUE
The implant that we have the most experience with is an 8° full-wedge augmented glenoid component. Such an implant is typically utilized for B2 glenoids. We recommend that a high-quality CT scan be performed for preoperative planning. As a general rule, the starting point often lies close to the ridge of B2 glenoid and more anterior than the apparent glenoid center, which is viewed intraoperatively due to asymmetric posterior wear. Full-wedge component is utilized to ream the ridge separating the neo and paleoglenoids to create a flat surface. This condition is best achieved by drilling a pilot hole at the planned glenoid central peg position to prevent the reamer from sliding anteriorly during reaming. Glenoid preparation begins with the smallest reamer until the ridge has been flattened, and the reamer makes full contact with the glenoid. The reamer diameter is then increased based on glenoid size. Slightly downsizing the glenoid implant will require less reaming to achieve full backside support. Once the glenoid is properly reamed, the central and peripheral peg holes are drilled using the appropriate guides. Holes are then dried, and all-polyethylene or composite glenoid component (either partially or completely cemented) is installed using favored cementing techniques. The advantage of composite glenoid component is that the central cage allows for bone ingrowth and may potentially improve long-term implant survival. Press fit of the central cage requires no waiting time for glenoid cement hardening before proceeding to the humerus. When placing an augmented component, adequate glenoid exposure is imperative to allow in-line placement and appropriate seating of the component without impingement on adjacent retractors.
When using the step-augmented glenoid, the paleoglenoid is prepared in a similar fashion to a standard aTSA. Once the paleoglenoid has been reamed to a neutral position, a protector plate is placed onto the paleoglenoid. and a step-cut saw is used to prepare the posterior stepped bone cut. Peripheral pegs are then drilled, and the component is installed in routine fashion. When using hemi-wedge augments, the paleoglenoid is again prepared in a similar fashion as a standard glenoid component over a cannulated guidewire. The neoglenoid is subsequently prepared using a specialized angled reamer with a positive stop to prevent over-reaming. These glenoid implants improve rotational force neutralization given the absence of flat back against the glenoid. All 3 designs preserve bone when compared with eccentric reaming alone,15 with the half-augmented wedge preserving the most bone.
Table 1. Results of Various Augmented Glenoid Components in Anatomic Total Shoulder
Arthroplasty
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
8° cage (N = 21) |
|
|
|
|
| |||||||||||||||
All-polyethylene 8° (N = 45) |
|
|
|
|
| |||||||||||||||
All-polyethylene 16° (N = 7) |
|
|
|
|
|
RESULTS
In our institution, we first used all-polyethylene posteriorly augmented glenoid components in 2010. Between 2010 and 2015, 45 patients received an 8° all-polyethylene posterior augment, and 7 patients received a 16° augment. In 2015, we transitioned to the composite caged posterior augment. All patients in our database who received an augmented glenoid component experienced improvement in active forward elevation, external rotation, American Shoulder and Elbow Surgeons (ASES), and Constant scores (Table 1). Minimum follow-up was 1 year for patients receiving both an 8° cage (mean, 1.48 years) and an 8° all-polyethylene augment (mean, 3.18 years). Figures 2A-2C show a patient with significant posterior glenoid wear and humeral head subluxation treated with an 8° wedge composite posterior augment glenoid 3 years postoperative.

Continue to: COMPLICATIONS
COMPLICATIONS
Two complications developed in the group undergoing composite cage augment. One patient experienced glenoid loosening after a motor vehicle accident. Another patient sustained significant intraoperative tuberosity avulsion during implantation of the humeral component, requiring a change of implant and tuberosity fixation. Although no complications were noted in the 8° all-polyethylene group, 3 patients in the 16° augment group sustained complications. One of these patients suffered a cardiac event that was unrelated to the implant. Two complications in this group were both related to loosening of the glenoid component, requiring subsequent revision.
DISCUSSION
The first report on augmented aTSA was published in 2008, and it involved a 5° augmented, anatomic glenoid.12 One study was based on a small series of augments; the poor results led the reporting surgeons to subsequently abandon the implant.12 This early design produced a correction on the articular side of the implant rather than the pathologic bony side. By performing such correction, the component pegs remained anteriorly oriented, placing the component at risk of perforation through the anterior glenoid neck. All current augment designs feature pegs that are oriented down the glenoid vault, with corrections occurring on the bony surface. This condition requires 2 different axes for reaming the glenoid and drilling the pegs. This approach allows the pegs to be directed down the glenoid neck, and is a far superior solution to neutralizing shear forces when compared with the implants used in the 1990s.
Early to midterm results of modern aTSA augments have been extremely encouraging with low revision rates. The main concern of recurrent posterior subluxation has been rarely reported. The concerns over glenoid loosening due to high shear forces, similarly, have not been described to date. However, surgeons should remain cautious, as longer-term follow-up remains unavailable.
The main advantage of aTSA augments is their capacity to preserve bone compared with eccentric reaming and better long-term stability. Each of the augment designs requires varying amounts of bone removal. Through biomechanics and using finite element analysis, the 3 augment types act differently, with no design demonstrating remarkable biomechanical superiority.6 Favorito and colleagues16 performed a retrospective review of 22 patients who underwent aTSA using an all-polyethylene, posteriorly augmented, and stepped glenoid component for posterior bone loss. At an average follow-up of 36 months, all patients experienced improvements in active forward elevation, external rotation, visual analog scale, Short Form-36 Physical Component Summary, and Western Ontario Osteoarthritis of the Shoulder scores. The authors noted that 2 patients (9%) experienced complications: 1 with an anterior dislocation and the other with recurrent posterior instability requiring revision. Sandow and Schutz17 reported the preliminary results of 10 patients who underwent aTSA using trabecular metal augment with a minimum of 2-year follow-up. All patients received either a 15° or 30° posterior, metal-backed augment for severe glenoid bone loss (Walch grade B2 or C). At a minimum of 2-year follow-up, all patients received correction to within 10° of neutral glenoid version, without any complications nor implant failures.
Regardless of augment design, all current components restore the native glenoid version, improving the length and subsequent tension of rotator cuff musculature. Similarly, re-centering the humeral head decreases the forces on the glenoid and allows for optimal function with decreasing loss of vital subchondral bone.
Continue to: RTSA AUGMENTS
RTSA AUGMENTS
Similar to anatomic augments, metal augments were introduced for use with RTSA in 2011. Unlike anatomic augments, those for RTSA were manufactured with metal. Given the difference in bony wear patterns in patients requiring RTSA, augments were available in a number of configurations. With CTA, wear is most commonly superior. Leaving a superiorly inclined baseplate must be avoided due to risks of notching, loosening, and early failure. However, correcting this tilt will require significant reaming of the inferior glenoid. A superior augment is ideally suited for this bone-loss pattern. If the glenoid is retroverted significantly, difficulty can also arise during glenoid preparation and baseplate placement. Posterior augments may ease this aspect of the procedure. Posterior augments feature the additional benefits of tensioning any remaining posterior rotator cuff, minimizing posterior inferior impingement, and technically easing the operation.18 As we improve our awareness of glenoid orientation using computer navigation, a posterior-superior augmented implant is commonly needed to simultaneously optimize the baseplate position and to minimize reaming (Figure 3). The posterior-superior augmented baseplate has become the most commonly used baseplate augment of choice in 90% of our RTSA cases that require an augment.

INDICATIONS
Augmented RTSA baseplates are indicated when adequate backside contact cannot be achieved with eccentric reaming, thus compromising potential fixation. In our practice, we preferably use augments at <50% contact with the backside of the baseplate. Excessive superior inclination is observed in a CTA setting, commonly indicating the use of superior augments. Similarly, severe primary osteoarthritis may contain elements of posterior bone loss, leading to increased retroversion, which is where we use posterior augments. When patients exhibit combined deformities, or when the surgeon wishes to tension the posterior rotator cuff, a posterior-superior augmented glenoid baseplate is used. For extremely severe defects, we have combined bone grafting and augments. In patients with a highly deficient glenoid but good quality of the remaining bone stock, an augment allows for better contact with less reaming although it is not fully supported when compared with a non-augmented baseplate. Bone grafts can function similarly, but the autograft humeral head is not constantly present in revision situations and requires increased operative time to allow for precision carpentry. Additionally, the success of BIO-RSA requires healing of bone graft on the native glenoid to support the baseplate.19 Jones and colleagues9 compared metal augmented RTSA with BIO-RSA and presented equivalent results.
To minimize reaming and to obtain appropriately inferior inclination, we have discovered preoperative templating and intraoperative, computer-guided glenoid preparation to be extremely valuable (ExactechGPS). These tools allow appropriate assessment of augments and for minimal bone removal when preparing the glenoid.
TECHNIQUE
When using an augment, a fine-cut CT scan is highly recommended to aid in surgery planning. We also find 3-D reconstructions to be helpful. Preoperative planning software also allows surgeons to maximize fixation of implant within the glenoid vault. The starting point for reaming is planned based on CT. Some surgeons using augments perform minimal or no reaming at all, electing to remove the remaining cartilage with a Cobb elevator. Different reaming and drilling axes are used when using augments. In cases of severe glenoid deformity and unavailability of computer assistance, a guide wire with inferior inclination can be installed based on CT scan. Penetration of this wire down the glenoid neck can be palpated and compared with the preoperative plan. We generally prefer at least 24 mm of bone containment for the central cage. Once the surgeon is satisfied with the placement of the wire, the appropriate augment guide is placed, followed by a second guide wire. This second wire acts as the reaming axis. The first wire is removed, and the glenoid is reamed with a cannulated reamer. Once reaming is completed, the original wire is replaced in the same hole and trajectory, and the reaming wire is removed. The first wire is then drilled with a cannulated drill for the central cage. The augmented baseplate is then impacted into place, and screw fixation is performed. Again, intraoperative computer guidance allows for precision screw placement with maximal bone attachment.
Table 2. Results of Reverse Total Shoulder Arthroplasty Augmented Baseplates
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
Superior (N = 22) |
|
|
|
|
| |||||||||||||||
Posterior (N = 50) |
|
|
|
|
| |||||||||||||||
Posterosuperior (N = 67) |
|
|
|
|
|
RESULTS
Based on our experience, glenoid augments for RTSA have performed well at short- and mid-term follow-up. From October 2011 to July 2016, 139 patients undergoing RTSA received a posterior, superior, or posterior-superior augmented glenoid baseplate. All groups demonstrated improvements in functional outcome measures, including Constant, ASES, Shoulder Pain and Disability Index, and Simple Shoulder Test scores compared with baseline values (Table 2). The posterior-superior augment group experienced the most significant improvement in active forward flexion and external rotation, whereas the posterior augment group experienced the most significant improvement in ASES and Constant scores. Figures 4A-4C displays the radiographs of a patient with significant glenoid wear treated with a posterior-superior augment RTSA.

Continue to: COMPLICATIONS
COMPLICATIONS
In the superior augment group, 3 patients (13%) sustained 5 complications. One patient sustained 3 separate episodes of instability, eventually requiring revision of prosthesis. In the posterior augment group, 4 patients (8%) sustained complications. Two of the 4 patients presented postoperative humeral fractures related to traumatic events, whereas another patient sustained an intraoperative tuberosity fracture. The last complication in this group involved a postoperative draining wound that was treated with oral antibiotics.
Nine complications developed in the posterior-superior augment group (13%); these complications included aseptic baseplate loosening (5), glenoid fracture (1), humeral fracture (1), acromial stress fracture (1), and cerebrovascular accident (1).
DISCUSSION
As the use of augments in RTSA is relatively new, significantly scarce data exist regarding their outcomes and longevity. A few studies have focused on the short-term outcomes of these augments. Jones and colleagues9 performed a retrospective review of 80 patients who underwent RTSA and required either a structural bone graft or an augmented glenoid baseplate.9 They observed that although all patients showed improvements in pain, range of motion, and functional scores, the structural bone graft group incurred a 14.6% complication rate compared with none observed in the augment group. Additionally, Jones and colleagues9 noted that the augmented baseplate group exhibited a significantly lower rate of scapular notching compared with the bone-graft group (10% vs 18.5%) at similar follow-up intervals. A separate study by Wright and colleagues18 compared posterior vs superior augmented baseplates in RTSA. The posterior augment group demonstrated lower rates of scapular notching (6.3% vs 14.3%) and showed more significant improvements in Constant, ASES, and active forward elevation measures, compared with the superior augment group.
As more manufacturers develop augments for RTSA, and as ExactechGPS uses become more widespread, the use of RTSA baseplate augments will continually grow. Custom implants using massive metal augments are now also being introduced. Although currently too expensive for most cases, as technology drives the cost down, every patient may receive customized augmented implants in the future.
The advantages of augmented baseplate designs include minimized reaming and notching, improved tension of the remaining rotator cuff, and decreased operating room time. The disadvantages include increased cost and lack of mid- or long-term clinical data. The concerns with baseplate loosening with augments in RTSA are much less than those with augments for aTSA due to the outstanding baseplate fixation that can be achieved in RTSA.
Continue to: CONLCLUSION
CONCLUSION
Augments offer an excellent tool for surgeons performing both aTSA and RTSA with glenoid bone loss. Use of augments will become more common as more manufacturers develop them. Although clinical results fall short in full midterm, they have been positive for both augmented RTSA and aTSA. Concerns arise when performing augmented aTSA, as an upper limit of correction has not been defined with regard to component failure. Currently, no data support the maximum amount of correction that can be achieved. In our current practice, we face difficulty in correcting more than 25° of version in young active patients with aTSA augment. Beyond this point, we perform a RTSA with an augment. In older patients or low-demand patients, we only correct minor deformities (<20°) with an aTSA augment, opting instead for an augmented RTSA due to the lower midterm failure rates observed with this implant.
1. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. J Bone Joint Surg Br. 2004;86(3):388-395. doi:10.1302/0301-620X.86B3.
2. Churchill RS, Spencer Jr EE, Fehringer EV. Quantification of B2 glenoid morphology in total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(8):1212-1217. doi:10.1016/j.jse.2015.01.007.
3. Bercik MJ, Kruse K, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606. doi:10.1016/j.jse.2016.03.010.
4. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072. doi:10.1016/j.jse.2013.09.017.
5. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
6. Hermida JC, Flores-Hernandez C, Hoenecke HR, D’Lima DD. Augmented wedge-shaped glenoid component for the correction of glenoid retroversion: a finite element analysis. J Shoulder Elbow Surg. 2014;23(3):347-354. doi:10.1016/j.jse.2013.06.008.
7. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82. doi:10.2106/JBJS.L.00336.
8. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598. doi:10.1016/j.jse.2013.06.017.
9. Jones RB, Wright TW, Roche CP. Bone grafting the glenoid versus use of augmented glenoid baseplates with reverse shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73(suppl 1):S129-S135.
10. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymmetric posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308. doi:10.1016/j.jse.2013.04.014.
11. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157. doi:10.1016/j.jse.2013.09.023.
12. Rice RS, Sperling JW, Miletti J, Schleck C, Cofield RH. Augmented glenoid component for bone deficiency in shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):579-583. doi:10.1007/s11999-007-0104-4.
13. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973. doi:10.1016/j.jse.2013.09.019.
14. Davis DE, Acevedo D, Williams A, Williams G. Total shoulder arthroplasty using an inlay mini-glenoid component for glenoid deficiency: a 2-year follow-up of 9 shoulders in 7 patients. J Shoulder Elbow Surg. 2016;25(8):1354-1361. doi:10.1016/j.jse.2015.12.010.
15. Kersten AD, Flores-Hernandez C, Hoenecke HR, D'Lima DD. Posterior augmented glenoid designs preserve more bone in biconcave glenoids. J Shoulder Elbow Surg. 2015;24(7):1135-1141. doi:10.1016/j.jse.2014.12.007.
16. Favorito PJ, Freed RJ, Passanise AM, Brown MJ. Total shoulder arthroplasty for glenohumeral arthritis associated with posterior glenoid bone loss: results of an all-polyethylene, posteriorly augmented glenoid component. J Shoulder Elbow Surg. 2016;25(10):1681-1689. doi:10.1016/j.jse.2016.02.020.
17. Sandow M, Schutz C. Total shoulder arthroplasty using trabecular metal augments to address glenoid retroversion: the preliminary result of 10 patients with minimum 2-year follow-up. J Shoulder Elbow Surg. 2016;25(4):598-607. doi:10.1016/j.jse.2016.01.001.
18. Wright TW, Roche CP, Wright L, Flurin PH, Crosby LA, Zuckerman JD. Reverse shoulder arthroplasty augments for glenoid wear: A comparison of posterior augments to superior augments. Bull Hosp Jt Dis. 2015;73(suppl 1):S124-S128.
19. Boileau P, Morin-Salvo N, Gauci MO, et al. Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty): a solution for the management glenoid bone loss and erosion. J Shoulder Elbow Surg. 2017;26(12):2133-2142. doi:10.1016/j.jse.2017.05.024.
1. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. J Bone Joint Surg Br. 2004;86(3):388-395. doi:10.1302/0301-620X.86B3.
2. Churchill RS, Spencer Jr EE, Fehringer EV. Quantification of B2 glenoid morphology in total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(8):1212-1217. doi:10.1016/j.jse.2015.01.007.
3. Bercik MJ, Kruse K, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606. doi:10.1016/j.jse.2016.03.010.
4. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072. doi:10.1016/j.jse.2013.09.017.
5. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
6. Hermida JC, Flores-Hernandez C, Hoenecke HR, D’Lima DD. Augmented wedge-shaped glenoid component for the correction of glenoid retroversion: a finite element analysis. J Shoulder Elbow Surg. 2014;23(3):347-354. doi:10.1016/j.jse.2013.06.008.
7. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82. doi:10.2106/JBJS.L.00336.
8. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598. doi:10.1016/j.jse.2013.06.017.
9. Jones RB, Wright TW, Roche CP. Bone grafting the glenoid versus use of augmented glenoid baseplates with reverse shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73(suppl 1):S129-S135.
10. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymmetric posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308. doi:10.1016/j.jse.2013.04.014.
11. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157. doi:10.1016/j.jse.2013.09.023.
12. Rice RS, Sperling JW, Miletti J, Schleck C, Cofield RH. Augmented glenoid component for bone deficiency in shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):579-583. doi:10.1007/s11999-007-0104-4.
13. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973. doi:10.1016/j.jse.2013.09.019.
14. Davis DE, Acevedo D, Williams A, Williams G. Total shoulder arthroplasty using an inlay mini-glenoid component for glenoid deficiency: a 2-year follow-up of 9 shoulders in 7 patients. J Shoulder Elbow Surg. 2016;25(8):1354-1361. doi:10.1016/j.jse.2015.12.010.
15. Kersten AD, Flores-Hernandez C, Hoenecke HR, D'Lima DD. Posterior augmented glenoid designs preserve more bone in biconcave glenoids. J Shoulder Elbow Surg. 2015;24(7):1135-1141. doi:10.1016/j.jse.2014.12.007.
16. Favorito PJ, Freed RJ, Passanise AM, Brown MJ. Total shoulder arthroplasty for glenohumeral arthritis associated with posterior glenoid bone loss: results of an all-polyethylene, posteriorly augmented glenoid component. J Shoulder Elbow Surg. 2016;25(10):1681-1689. doi:10.1016/j.jse.2016.02.020.
17. Sandow M, Schutz C. Total shoulder arthroplasty using trabecular metal augments to address glenoid retroversion: the preliminary result of 10 patients with minimum 2-year follow-up. J Shoulder Elbow Surg. 2016;25(4):598-607. doi:10.1016/j.jse.2016.01.001.
18. Wright TW, Roche CP, Wright L, Flurin PH, Crosby LA, Zuckerman JD. Reverse shoulder arthroplasty augments for glenoid wear: A comparison of posterior augments to superior augments. Bull Hosp Jt Dis. 2015;73(suppl 1):S124-S128.
19. Boileau P, Morin-Salvo N, Gauci MO, et al. Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty): a solution for the management glenoid bone loss and erosion. J Shoulder Elbow Surg. 2017;26(12):2133-2142. doi:10.1016/j.jse.2017.05.024.
TAKE-HOME POINTS
- Glenoid defects are very common.
- Options for treating glenoid defects include eccentric reaming, bone grafting, and augmented glenoids.
- As computer-assisted surgery use becomes more widespread the use of augments in both TSA and RTSA will become very common.
- Subchondral bone is precious and cannot be replaced once reamed away. Eccentric glenoids introduce a mechanism to minimize reaming and preserve this precious bone.
- On short-term to midterm follow-up augments perform at least as well if not better than non-augmented glenoid components with complication rate and revisions likewise similar.
Woman, 57, With Painful, Swollen Ankle
IN THIS ARTICLE
- Diagnosis
- Treatment
- Care outcome
A 57-year-old horticulturist is working on a ladder leaned up against a tree trunk when the ladder slips, causing her to fall six feet onto concrete. Her right foot and ankle sustain the force of the fall; she is in excruciating pain and unable to bear weight on the foot. She is immediately transported to a local emergency department for evaluation.
Physical exam reveals a tearful middle-aged female in moderate distress and acute pain. There is moderate swelling of the right medial and lateral malleolus, as well as the midfoot, with blue and purple discoloration on the medial and lateral malleolus. Radiographs of the right ankle identify nondisplaced fractures of the distal fibula and tibia. Foot x-rays are unremarkable. A splint is ordered. The patient is given crutches (non-weight-bearing status), pain medication, and a referral to orthopedics.
On day 3, the patient presents to orthopedics, where the splint is removed. An irregular, 4 × 3–in (at largest diameter), serohemorrhagic blister is discovered on the medial aspect of the lower leg, above the right malleolus (see Figure 1). Multiple 1- to 3-mm vesicles surround much of the anterior border. Moderate edema is noted from the top of the lesion to the midfoot, concentrated around the lateral and medial malleolus. Extensive blue, purple, and black discoloration is seen below the malleolus. The patient is diagnosed with a fracture blister.
DISCUSSION
Fracture blisters are taut, bullous, subepidermal vesicles that can accompany fractures or severe twisting injuries. They overlie markedly edematous soft tissue and histologically resemble a second-degree burn.1,2
Physiologically, blisters are caused by increased interstitial pressure due to swelling, with subsequent increased filtration pressure and colloid osmotic pressure in the epidermal gap.3 This causes a disruption that allows fluid to move into the weakened area.3 Areas most at risk for fracture blister formation are those with tight, closely adhered skin without muscle or enveloping fascia, where there is less soft tissue between the skin and bone prominences (eg, ankle, elbow, foot, distal tibia).2-4
Approximately 3% of all patients with acute fractures requiring hospitalization develop a fracture blister.4 Any condition that predisposes a patient to poor wound healing (eg, peripheral vascular disease, diabetes, hypertension) increases risk for a fracture blister.2 Recognizing which patients are at greatest risk is vital, as implementing prevention strategies and intervening when fracture blisters do form can help decrease complications—including infection and delayed surgery—and improve fracture resolution. In this patient’s case, the extent of the injury and force of the fall caused the fracture blister to form.
Diagnosis
Diagnosis of a fracture blister is based on clinical presentation. There are two types: hemorrhagic blisters and clear fluid-filled blisters. Hemorrhagic blisters indicate more severe injury and longer healing time (approximately 16 d), while clear fluid-filled blisters demonstrate minimal injury and therefore are quicker to heal.2,4
The differential diagnosis for fracture blisters includes friction blisters and disorders such as epidermolysis bullosa and bullous pemphigoid. Friction blisters form when the epidermis is subjected to repeated friction or shear forces (eg, from a cast or splint).5,6 These forces mechanically separate epidermal cells at the stratum spinosum layer.7 The pressure that moves across the skin forces fluid into the deeper open spaces, filling them but leaving the surface layer intact.1
Epidermolysis bullosa (EB) is a group of rare inherited cutaneous and mucus membrane disorders. EB involves fragility and detachment of subepithelial tissues, which results in blistering and erosions.8,9 The blisters tend to develop in areas subject to minor trauma, such as the extensor aspects of the elbows and the dorsal aspects of the hands and feet.9 They can also be triggered by exposure to heat, friction, scratching, and adhesive tape.10
Bullous pemphigoid, a chronic autoimmune skin disorder, is characterized by pruritic, bullous lesions. When IgG autoantibodies bind to certain hemidesmosomal antigens, complement activation causes a subepidermal blister.11While bullous pemphigoid most commonly affects those older than 60, it can also occur in children. Diagnosis is confirmed by skin biopsy and immunofluorescence testing.11
Treatment and management
Although several recommendations have been published, there is no gold standard and treatment of fracture blisters remains controversial. Early surgical intervention for fractures could decrease the incidence of fracture blisters.1,3
The goal of treatment is to achieve re-epithelialization of the dermis.3,12,13 Once a blister forms, management techniques vary. Some recommend keeping closed blisters covered with a dry dressing to protect them from damage.3 Strauss et al recommend unroofing to avoid traumatic rupture; however, this does increase risk for infection.12 Recommendations differ depending on provider preference and each patient’s individual situation.
Elective unroofing of a blister is typically followed with one of several treatment options. These include covering the open blister with a topical antibiotic cream (eg, silver sulfadiazine 2%); applying a nonadherent, occlusive bismuth-tribromophenate-petroleum gauze dressing; or elevating and immobilizing the affected extremity.12,13
Treatment of spontaneously ruptured fracture blisters entails
- Unroofing the blister completely and applying a topical antimicrobial (eg, silver sulfadiazine, polymyxin B, neomycin, bacitracin).
- Applying a hydrocolloid dressing to keep the environment moist.
- Using a first-aid gel containing melaleuca (tea tree) oil.
- Initiating prophylactic oral antibiotics.
- Using whirlpool treatments.
- Elevating and immobilizing the affected extremity.3,12,14
OUTCOME FOR THE CASE PATIENT
The fracture blister was electively unroofed (see Figure 2) based on provider preference. The patient was instructed to clean the wound daily and apply topical cream (silver sulfadiazine 2% bid) to the wound and cover it with gauze. The patient was made non-weight-bearing to the right lower extremity. Continuous elevation was highly encouraged except for bathing and restroom use, and an NSAID was recommended as needed for pain. She was reassessed the following day and, due to partial refilling, the blister required additional unroofing. The patient was instructed to resume previous wound care orders.
No surgical intervention was required. CT of the right foot and ankle without contrast (performed on day 4 postinjury) confirmed a nondisplaced transverse fracture of the medial malleolus and a sagittal avulsion fracture of the anterior-inferior lateral malleolus. Multiple smaller fracture fragments were noted posterior and medial to the medial malleolus as well as inferiorly along the course of the deltoid ligament. There was a small, nondisplaced avulsion fracture of the medial malleolus at the anterolateral and posterolateral tibial plafond.
Due to the extent of the swelling, multiple fractures, and blister formation, the patient was essentially bed bound for the first three weeks; complete resolution of the fracture blister occurred 21 days after initial discovery (see Figure 3). The patient did not experience cutaneous complications. Her lower extremity was then casted in a short-leg removable cast for 10 weeks. She underwent physical therapy, and after 12 weeks, the patient was weight-bearing and was discharged from orthopedics. The patient reported refractory pain and swelling for an additional eight weeks following injury, warranting daily ibuprofen.
CONCLUSION
Fracture blisters are rare, and experience and knowledge about them in primary care is lacking. But clinicians need to be able to identify, diagnose, and refer at-risk patients to orthopedics in a timely manner.
Current management and treatment recommendations are inconsistent. Treatment varies depending on the site, severity, type, and status of the blister and the overall health of the patient. Fracture blisters may be left intact, electively unroofed, or treated after spontaneous rupture. More research is needed to clarify management recommendations, specifically regarding the decision to unroof a blister or leave it intact. Early surgical intervention may prevent the development of a fracture blister.
1. Wallace GF, Sullivan J. Fracture blisters. Clin Podiatr Med Surg. 1995;12(4):801-811.
2. Halawi MJ. Fracture blisters after primary total knee arthroplasty. Am J Orthop. 2015; 44(8):E291-E293.
3. McCann S, Gruen G. Fracture blisters: a review of the literature. Orthop Nurs. 1997; 16(2):17-24.
4. Uebbing CM, Walsh M, Miller JB, et al. Fracture blister. West J Emerg Med. 2011; 12(1):131-133.
5. Kirkham S, Lam S, Nester C, Hashmi F. The effect of hydration on the risk of friction blister formation on the heel of the foot. Skin Res Tech. 2014;20:246-253.
6. Boyd A, Benjamin H, Asplund C. Principles of casting and splinting. Am Fam Physician. 2009;79(1):16-24.
7. Knapik J, Reynolds K, Duplantis K, Jones B. Friction blisters. Pathophysiology, prevention and treatment. Sports Med. 1995; 20(3):136-147.
8. Iranzo P, Herrero-González JE, Mascaró-Galy JM, et al. Epidermolysis bullosa acquisita: a retrospective analysis of 12 patients evaluated in four tertiary hospitals in Spain. Br J Dermatol. 2014;171(5):1022-1030.
9. Peraza DM. Epidermolysis bullosa acquisita. Merck Manual Professional Version. August 2016. www.merckmanuals.com/professional/dermatologic-disorders/bullous-diseases/epidermolysis-bullosa-acquisita. Accessed January 26, 2018.
10. Lyons F, Ousley L. Dermatology for the Advanced Practice Nurse. New York, NY: Springer; 2015.
11. Peraza D. Bullous pemphigoid. Merck Manual Professional Version. August 2016. www.merckmanuals.com/professional/dermatologic-disorders/bullous-diseases/bullous-pemphigoid. Accessed January 26, 2018.
12. Strauss EJ, Petrucelli G, Bong M, et al. Blisters associated with lower-extremity fracture: Results of a prospective treatment protocol. J Orthop Trauma. 2006;20(9): 618-622.
13. Tolpinrud WL, Rebolledo BJ, Lorich DG, Grossman ME. A case of extensive fracture bullae: a multidisciplinary approach for acute management. JAAD Case Rep. 2015;1(3):132-135.
14. Cox H, Nealon L. Case report: the use of Burnaid Gel on fracture blisters. Wound Practice and Research. 2008;16(1):32-36.
IN THIS ARTICLE
- Diagnosis
- Treatment
- Care outcome
A 57-year-old horticulturist is working on a ladder leaned up against a tree trunk when the ladder slips, causing her to fall six feet onto concrete. Her right foot and ankle sustain the force of the fall; she is in excruciating pain and unable to bear weight on the foot. She is immediately transported to a local emergency department for evaluation.
Physical exam reveals a tearful middle-aged female in moderate distress and acute pain. There is moderate swelling of the right medial and lateral malleolus, as well as the midfoot, with blue and purple discoloration on the medial and lateral malleolus. Radiographs of the right ankle identify nondisplaced fractures of the distal fibula and tibia. Foot x-rays are unremarkable. A splint is ordered. The patient is given crutches (non-weight-bearing status), pain medication, and a referral to orthopedics.
On day 3, the patient presents to orthopedics, where the splint is removed. An irregular, 4 × 3–in (at largest diameter), serohemorrhagic blister is discovered on the medial aspect of the lower leg, above the right malleolus (see Figure 1). Multiple 1- to 3-mm vesicles surround much of the anterior border. Moderate edema is noted from the top of the lesion to the midfoot, concentrated around the lateral and medial malleolus. Extensive blue, purple, and black discoloration is seen below the malleolus. The patient is diagnosed with a fracture blister.
DISCUSSION
Fracture blisters are taut, bullous, subepidermal vesicles that can accompany fractures or severe twisting injuries. They overlie markedly edematous soft tissue and histologically resemble a second-degree burn.1,2
Physiologically, blisters are caused by increased interstitial pressure due to swelling, with subsequent increased filtration pressure and colloid osmotic pressure in the epidermal gap.3 This causes a disruption that allows fluid to move into the weakened area.3 Areas most at risk for fracture blister formation are those with tight, closely adhered skin without muscle or enveloping fascia, where there is less soft tissue between the skin and bone prominences (eg, ankle, elbow, foot, distal tibia).2-4
Approximately 3% of all patients with acute fractures requiring hospitalization develop a fracture blister.4 Any condition that predisposes a patient to poor wound healing (eg, peripheral vascular disease, diabetes, hypertension) increases risk for a fracture blister.2 Recognizing which patients are at greatest risk is vital, as implementing prevention strategies and intervening when fracture blisters do form can help decrease complications—including infection and delayed surgery—and improve fracture resolution. In this patient’s case, the extent of the injury and force of the fall caused the fracture blister to form.
Diagnosis
Diagnosis of a fracture blister is based on clinical presentation. There are two types: hemorrhagic blisters and clear fluid-filled blisters. Hemorrhagic blisters indicate more severe injury and longer healing time (approximately 16 d), while clear fluid-filled blisters demonstrate minimal injury and therefore are quicker to heal.2,4
The differential diagnosis for fracture blisters includes friction blisters and disorders such as epidermolysis bullosa and bullous pemphigoid. Friction blisters form when the epidermis is subjected to repeated friction or shear forces (eg, from a cast or splint).5,6 These forces mechanically separate epidermal cells at the stratum spinosum layer.7 The pressure that moves across the skin forces fluid into the deeper open spaces, filling them but leaving the surface layer intact.1
Epidermolysis bullosa (EB) is a group of rare inherited cutaneous and mucus membrane disorders. EB involves fragility and detachment of subepithelial tissues, which results in blistering and erosions.8,9 The blisters tend to develop in areas subject to minor trauma, such as the extensor aspects of the elbows and the dorsal aspects of the hands and feet.9 They can also be triggered by exposure to heat, friction, scratching, and adhesive tape.10
Bullous pemphigoid, a chronic autoimmune skin disorder, is characterized by pruritic, bullous lesions. When IgG autoantibodies bind to certain hemidesmosomal antigens, complement activation causes a subepidermal blister.11While bullous pemphigoid most commonly affects those older than 60, it can also occur in children. Diagnosis is confirmed by skin biopsy and immunofluorescence testing.11
Treatment and management
Although several recommendations have been published, there is no gold standard and treatment of fracture blisters remains controversial. Early surgical intervention for fractures could decrease the incidence of fracture blisters.1,3
The goal of treatment is to achieve re-epithelialization of the dermis.3,12,13 Once a blister forms, management techniques vary. Some recommend keeping closed blisters covered with a dry dressing to protect them from damage.3 Strauss et al recommend unroofing to avoid traumatic rupture; however, this does increase risk for infection.12 Recommendations differ depending on provider preference and each patient’s individual situation.
Elective unroofing of a blister is typically followed with one of several treatment options. These include covering the open blister with a topical antibiotic cream (eg, silver sulfadiazine 2%); applying a nonadherent, occlusive bismuth-tribromophenate-petroleum gauze dressing; or elevating and immobilizing the affected extremity.12,13
Treatment of spontaneously ruptured fracture blisters entails
- Unroofing the blister completely and applying a topical antimicrobial (eg, silver sulfadiazine, polymyxin B, neomycin, bacitracin).
- Applying a hydrocolloid dressing to keep the environment moist.
- Using a first-aid gel containing melaleuca (tea tree) oil.
- Initiating prophylactic oral antibiotics.
- Using whirlpool treatments.
- Elevating and immobilizing the affected extremity.3,12,14
OUTCOME FOR THE CASE PATIENT
The fracture blister was electively unroofed (see Figure 2) based on provider preference. The patient was instructed to clean the wound daily and apply topical cream (silver sulfadiazine 2% bid) to the wound and cover it with gauze. The patient was made non-weight-bearing to the right lower extremity. Continuous elevation was highly encouraged except for bathing and restroom use, and an NSAID was recommended as needed for pain. She was reassessed the following day and, due to partial refilling, the blister required additional unroofing. The patient was instructed to resume previous wound care orders.
No surgical intervention was required. CT of the right foot and ankle without contrast (performed on day 4 postinjury) confirmed a nondisplaced transverse fracture of the medial malleolus and a sagittal avulsion fracture of the anterior-inferior lateral malleolus. Multiple smaller fracture fragments were noted posterior and medial to the medial malleolus as well as inferiorly along the course of the deltoid ligament. There was a small, nondisplaced avulsion fracture of the medial malleolus at the anterolateral and posterolateral tibial plafond.
Due to the extent of the swelling, multiple fractures, and blister formation, the patient was essentially bed bound for the first three weeks; complete resolution of the fracture blister occurred 21 days after initial discovery (see Figure 3). The patient did not experience cutaneous complications. Her lower extremity was then casted in a short-leg removable cast for 10 weeks. She underwent physical therapy, and after 12 weeks, the patient was weight-bearing and was discharged from orthopedics. The patient reported refractory pain and swelling for an additional eight weeks following injury, warranting daily ibuprofen.
CONCLUSION
Fracture blisters are rare, and experience and knowledge about them in primary care is lacking. But clinicians need to be able to identify, diagnose, and refer at-risk patients to orthopedics in a timely manner.
Current management and treatment recommendations are inconsistent. Treatment varies depending on the site, severity, type, and status of the blister and the overall health of the patient. Fracture blisters may be left intact, electively unroofed, or treated after spontaneous rupture. More research is needed to clarify management recommendations, specifically regarding the decision to unroof a blister or leave it intact. Early surgical intervention may prevent the development of a fracture blister.
IN THIS ARTICLE
- Diagnosis
- Treatment
- Care outcome
A 57-year-old horticulturist is working on a ladder leaned up against a tree trunk when the ladder slips, causing her to fall six feet onto concrete. Her right foot and ankle sustain the force of the fall; she is in excruciating pain and unable to bear weight on the foot. She is immediately transported to a local emergency department for evaluation.
Physical exam reveals a tearful middle-aged female in moderate distress and acute pain. There is moderate swelling of the right medial and lateral malleolus, as well as the midfoot, with blue and purple discoloration on the medial and lateral malleolus. Radiographs of the right ankle identify nondisplaced fractures of the distal fibula and tibia. Foot x-rays are unremarkable. A splint is ordered. The patient is given crutches (non-weight-bearing status), pain medication, and a referral to orthopedics.
On day 3, the patient presents to orthopedics, where the splint is removed. An irregular, 4 × 3–in (at largest diameter), serohemorrhagic blister is discovered on the medial aspect of the lower leg, above the right malleolus (see Figure 1). Multiple 1- to 3-mm vesicles surround much of the anterior border. Moderate edema is noted from the top of the lesion to the midfoot, concentrated around the lateral and medial malleolus. Extensive blue, purple, and black discoloration is seen below the malleolus. The patient is diagnosed with a fracture blister.
DISCUSSION
Fracture blisters are taut, bullous, subepidermal vesicles that can accompany fractures or severe twisting injuries. They overlie markedly edematous soft tissue and histologically resemble a second-degree burn.1,2
Physiologically, blisters are caused by increased interstitial pressure due to swelling, with subsequent increased filtration pressure and colloid osmotic pressure in the epidermal gap.3 This causes a disruption that allows fluid to move into the weakened area.3 Areas most at risk for fracture blister formation are those with tight, closely adhered skin without muscle or enveloping fascia, where there is less soft tissue between the skin and bone prominences (eg, ankle, elbow, foot, distal tibia).2-4
Approximately 3% of all patients with acute fractures requiring hospitalization develop a fracture blister.4 Any condition that predisposes a patient to poor wound healing (eg, peripheral vascular disease, diabetes, hypertension) increases risk for a fracture blister.2 Recognizing which patients are at greatest risk is vital, as implementing prevention strategies and intervening when fracture blisters do form can help decrease complications—including infection and delayed surgery—and improve fracture resolution. In this patient’s case, the extent of the injury and force of the fall caused the fracture blister to form.
Diagnosis
Diagnosis of a fracture blister is based on clinical presentation. There are two types: hemorrhagic blisters and clear fluid-filled blisters. Hemorrhagic blisters indicate more severe injury and longer healing time (approximately 16 d), while clear fluid-filled blisters demonstrate minimal injury and therefore are quicker to heal.2,4
The differential diagnosis for fracture blisters includes friction blisters and disorders such as epidermolysis bullosa and bullous pemphigoid. Friction blisters form when the epidermis is subjected to repeated friction or shear forces (eg, from a cast or splint).5,6 These forces mechanically separate epidermal cells at the stratum spinosum layer.7 The pressure that moves across the skin forces fluid into the deeper open spaces, filling them but leaving the surface layer intact.1
Epidermolysis bullosa (EB) is a group of rare inherited cutaneous and mucus membrane disorders. EB involves fragility and detachment of subepithelial tissues, which results in blistering and erosions.8,9 The blisters tend to develop in areas subject to minor trauma, such as the extensor aspects of the elbows and the dorsal aspects of the hands and feet.9 They can also be triggered by exposure to heat, friction, scratching, and adhesive tape.10
Bullous pemphigoid, a chronic autoimmune skin disorder, is characterized by pruritic, bullous lesions. When IgG autoantibodies bind to certain hemidesmosomal antigens, complement activation causes a subepidermal blister.11While bullous pemphigoid most commonly affects those older than 60, it can also occur in children. Diagnosis is confirmed by skin biopsy and immunofluorescence testing.11
Treatment and management
Although several recommendations have been published, there is no gold standard and treatment of fracture blisters remains controversial. Early surgical intervention for fractures could decrease the incidence of fracture blisters.1,3
The goal of treatment is to achieve re-epithelialization of the dermis.3,12,13 Once a blister forms, management techniques vary. Some recommend keeping closed blisters covered with a dry dressing to protect them from damage.3 Strauss et al recommend unroofing to avoid traumatic rupture; however, this does increase risk for infection.12 Recommendations differ depending on provider preference and each patient’s individual situation.
Elective unroofing of a blister is typically followed with one of several treatment options. These include covering the open blister with a topical antibiotic cream (eg, silver sulfadiazine 2%); applying a nonadherent, occlusive bismuth-tribromophenate-petroleum gauze dressing; or elevating and immobilizing the affected extremity.12,13
Treatment of spontaneously ruptured fracture blisters entails
- Unroofing the blister completely and applying a topical antimicrobial (eg, silver sulfadiazine, polymyxin B, neomycin, bacitracin).
- Applying a hydrocolloid dressing to keep the environment moist.
- Using a first-aid gel containing melaleuca (tea tree) oil.
- Initiating prophylactic oral antibiotics.
- Using whirlpool treatments.
- Elevating and immobilizing the affected extremity.3,12,14
OUTCOME FOR THE CASE PATIENT
The fracture blister was electively unroofed (see Figure 2) based on provider preference. The patient was instructed to clean the wound daily and apply topical cream (silver sulfadiazine 2% bid) to the wound and cover it with gauze. The patient was made non-weight-bearing to the right lower extremity. Continuous elevation was highly encouraged except for bathing and restroom use, and an NSAID was recommended as needed for pain. She was reassessed the following day and, due to partial refilling, the blister required additional unroofing. The patient was instructed to resume previous wound care orders.
No surgical intervention was required. CT of the right foot and ankle without contrast (performed on day 4 postinjury) confirmed a nondisplaced transverse fracture of the medial malleolus and a sagittal avulsion fracture of the anterior-inferior lateral malleolus. Multiple smaller fracture fragments were noted posterior and medial to the medial malleolus as well as inferiorly along the course of the deltoid ligament. There was a small, nondisplaced avulsion fracture of the medial malleolus at the anterolateral and posterolateral tibial plafond.
Due to the extent of the swelling, multiple fractures, and blister formation, the patient was essentially bed bound for the first three weeks; complete resolution of the fracture blister occurred 21 days after initial discovery (see Figure 3). The patient did not experience cutaneous complications. Her lower extremity was then casted in a short-leg removable cast for 10 weeks. She underwent physical therapy, and after 12 weeks, the patient was weight-bearing and was discharged from orthopedics. The patient reported refractory pain and swelling for an additional eight weeks following injury, warranting daily ibuprofen.
CONCLUSION
Fracture blisters are rare, and experience and knowledge about them in primary care is lacking. But clinicians need to be able to identify, diagnose, and refer at-risk patients to orthopedics in a timely manner.
Current management and treatment recommendations are inconsistent. Treatment varies depending on the site, severity, type, and status of the blister and the overall health of the patient. Fracture blisters may be left intact, electively unroofed, or treated after spontaneous rupture. More research is needed to clarify management recommendations, specifically regarding the decision to unroof a blister or leave it intact. Early surgical intervention may prevent the development of a fracture blister.
1. Wallace GF, Sullivan J. Fracture blisters. Clin Podiatr Med Surg. 1995;12(4):801-811.
2. Halawi MJ. Fracture blisters after primary total knee arthroplasty. Am J Orthop. 2015; 44(8):E291-E293.
3. McCann S, Gruen G. Fracture blisters: a review of the literature. Orthop Nurs. 1997; 16(2):17-24.
4. Uebbing CM, Walsh M, Miller JB, et al. Fracture blister. West J Emerg Med. 2011; 12(1):131-133.
5. Kirkham S, Lam S, Nester C, Hashmi F. The effect of hydration on the risk of friction blister formation on the heel of the foot. Skin Res Tech. 2014;20:246-253.
6. Boyd A, Benjamin H, Asplund C. Principles of casting and splinting. Am Fam Physician. 2009;79(1):16-24.
7. Knapik J, Reynolds K, Duplantis K, Jones B. Friction blisters. Pathophysiology, prevention and treatment. Sports Med. 1995; 20(3):136-147.
8. Iranzo P, Herrero-González JE, Mascaró-Galy JM, et al. Epidermolysis bullosa acquisita: a retrospective analysis of 12 patients evaluated in four tertiary hospitals in Spain. Br J Dermatol. 2014;171(5):1022-1030.
9. Peraza DM. Epidermolysis bullosa acquisita. Merck Manual Professional Version. August 2016. www.merckmanuals.com/professional/dermatologic-disorders/bullous-diseases/epidermolysis-bullosa-acquisita. Accessed January 26, 2018.
10. Lyons F, Ousley L. Dermatology for the Advanced Practice Nurse. New York, NY: Springer; 2015.
11. Peraza D. Bullous pemphigoid. Merck Manual Professional Version. August 2016. www.merckmanuals.com/professional/dermatologic-disorders/bullous-diseases/bullous-pemphigoid. Accessed January 26, 2018.
12. Strauss EJ, Petrucelli G, Bong M, et al. Blisters associated with lower-extremity fracture: Results of a prospective treatment protocol. J Orthop Trauma. 2006;20(9): 618-622.
13. Tolpinrud WL, Rebolledo BJ, Lorich DG, Grossman ME. A case of extensive fracture bullae: a multidisciplinary approach for acute management. JAAD Case Rep. 2015;1(3):132-135.
14. Cox H, Nealon L. Case report: the use of Burnaid Gel on fracture blisters. Wound Practice and Research. 2008;16(1):32-36.
1. Wallace GF, Sullivan J. Fracture blisters. Clin Podiatr Med Surg. 1995;12(4):801-811.
2. Halawi MJ. Fracture blisters after primary total knee arthroplasty. Am J Orthop. 2015; 44(8):E291-E293.
3. McCann S, Gruen G. Fracture blisters: a review of the literature. Orthop Nurs. 1997; 16(2):17-24.
4. Uebbing CM, Walsh M, Miller JB, et al. Fracture blister. West J Emerg Med. 2011; 12(1):131-133.
5. Kirkham S, Lam S, Nester C, Hashmi F. The effect of hydration on the risk of friction blister formation on the heel of the foot. Skin Res Tech. 2014;20:246-253.
6. Boyd A, Benjamin H, Asplund C. Principles of casting and splinting. Am Fam Physician. 2009;79(1):16-24.
7. Knapik J, Reynolds K, Duplantis K, Jones B. Friction blisters. Pathophysiology, prevention and treatment. Sports Med. 1995; 20(3):136-147.
8. Iranzo P, Herrero-González JE, Mascaró-Galy JM, et al. Epidermolysis bullosa acquisita: a retrospective analysis of 12 patients evaluated in four tertiary hospitals in Spain. Br J Dermatol. 2014;171(5):1022-1030.
9. Peraza DM. Epidermolysis bullosa acquisita. Merck Manual Professional Version. August 2016. www.merckmanuals.com/professional/dermatologic-disorders/bullous-diseases/epidermolysis-bullosa-acquisita. Accessed January 26, 2018.
10. Lyons F, Ousley L. Dermatology for the Advanced Practice Nurse. New York, NY: Springer; 2015.
11. Peraza D. Bullous pemphigoid. Merck Manual Professional Version. August 2016. www.merckmanuals.com/professional/dermatologic-disorders/bullous-diseases/bullous-pemphigoid. Accessed January 26, 2018.
12. Strauss EJ, Petrucelli G, Bong M, et al. Blisters associated with lower-extremity fracture: Results of a prospective treatment protocol. J Orthop Trauma. 2006;20(9): 618-622.
13. Tolpinrud WL, Rebolledo BJ, Lorich DG, Grossman ME. A case of extensive fracture bullae: a multidisciplinary approach for acute management. JAAD Case Rep. 2015;1(3):132-135.
14. Cox H, Nealon L. Case report: the use of Burnaid Gel on fracture blisters. Wound Practice and Research. 2008;16(1):32-36.
Patient-Specific Guides/Instrumentation in Shoulder Arthroplasty
ABSTRACT
Optimal outcomes following total shoulder arthroplasty TSA and reverse shoulder arthroplasty RSA are dependent on proper implant position. Multiple cadaver studies have demonstrated improved accuracy of implant positioning with use of patient-specific guides/instrumentation compared to traditional methods. At this time, there are 3 commercially available single use patient-specific instrumentation systems and 1 commercially available reusable patient-specific instrumentation system. Currently though, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research has been done comparing the accuracy of each system’s 3-dimensional planning software. Future work is necessary to elucidate the ideal indications for the use of patient-specific guides and instrumentation, but it is likely, particularly in the setting of advanced glenoid deformity, that these systems will improve a surgeon's ability to put the implant in the best position possible.
Continue to: Optimal functional recovery...
Optimal functional recovery and implant longevity following both total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) depend, in large part, on proper placement of the glenoid component. Glenoid component malpositioning has an adverse effect on shoulder stability, range of motion (ROM), impingement, and glenoid implant longevity.
Traditionally, glenoid component positioning has been done manually by the surgeons based on their review of preoperative films and knowledge of glenoid anatomy. Anatomic studies have demonstrated high individual variability in the version of the native glenoid, thus making ideal placement of the initial glenoid guide pin difficult using standard guide pin guides.1
The following 2 methods have been described for improving the accuracy of glenoid guide pin insertion and subsequent glenoid implant placement: (1) computerized navigation and (2) patient-specific guides/instrumentation. Although navigated shoulder systems have demonstrated improved accuracy in glenoid placement compared with traditional methods, navigated systems require often large and expensive systems for implementation. The majority of them also require placement of guide pins or arrays on scapular bony landmarks, likely leading to an increase in operative time and possible iatrogenic complications, including fracture and pin site infections.
This review focuses on the use of patient-specific guides/instrumentation in shoulder arthroplasty. This includes the topic of proper glenoid and glenosphere placement as well as patient-specific guides/instrumentation and their accuracy.
GLENOID PLACEMENT
Glenohumeral osteoarthritis is the most common indication for TSA2 and commonly results in glenoid deformity. Using computed tomography (CT) scans of 45 arthritic shoulders and 19 normal shoulders, Mullaji and colleagues3 reported that the anteroposterior dimensions of the glenoid were increased by an average of 5 mm to 8 mm in osteoarthritic shoulders and by an average of 6 mm in rheumatoid arthritic shoulders compared to those in normal shoulders. A retrospective review of serial CT scans performed preoperatively on 113 osteoarthritic shoulders by Walch and colleagues4 demonstrated an average retroversion of 16°, and it has been the basis for the commonly used Walch classification of glenoid wear in osteoarthritis. Increased glenoid wear and increased glenoid retroversion make the proper restoration of glenoid version, inclination, and offset during shoulder arthroplasty more difficult and lead to increased glenoid component malpositioning.
Continue to: The ideal placement of the glenoid...
The ideal placement of the glenoid to maximize function, ROM, and implant longevity is in a mechanically neutral alignment with no superoinferior inclination1 and neutral version with respect to the transverse axis of the scapula.5
Improper glenoid positioning has an adverse effect on the functional results of shoulder arthroplasty. Yian and colleagues6 evaluated 47 cemented, pegged glenoids using standard radiography and CT scans at a mean follow-up of 40 months. They observed a significant correlation between increased glenoid component retroversion and lower Constant scores. Hasan and colleagues7 evaluated 139 consecutive patients who were dissatisfied with the result of their primary arthroplasty and found that 28% of them had at least 1 substantially malpositioned component identified either on radiography or during a revision surgery. They also found a significant correlation between stiffness, instability, and component malposition in their cohort.
Glenoid longevity is also dependent on proper component positioning, with the worst outcomes coming if the glenoid is malaligned with either superior or inferior inclination. Hasan and colleagues7 found that of their 74 patients with failed TSAs, 44 patients (59%) demonstrated mechanical loosening of their glenoid components either radiographically or during revision surgery, and 10 of their 44 patients with loose glenoids (23%) also had a malpositioned component. Using finite element analysis, Hopkins and colleagues8 analyzed the stresses through the cement mantle in glenoid prostheses that were centrally aligned, superiorly inclined, inferiorly inclined, anteverted, and retroverted. They found that malalignment of the glenoid increases the stresses through the cement mantle, leading to increased likelihood of mantle failure compared to that of centrally aligned glenoids, especially if there is malalignment with superior or inferior inclination or retroversion.
The accuracy of traditional methods of glenoid placement using an initial guide pin is limited and decreases with increasing amounts of glenoid deformity and retroversion. Iannotti and colleagues 9 investigated 13 patients undergoing TSA with an average preoperative retroversion of 13° and evaluated them using a 3-dimensional (3-D) surgical simulator. They found that the postoperative glenoid version was within 5° of ideal version in only 7 of their 13 patients (54%) and within 10° of ideal version in only 10 of their 13 patients (77%). In their study, the ideal version was considered to be the version as close to perpendicular to the plane of the scapula as possible with complete contact of the back side of the component on glenoid bone and maintenance of the center peg of the component within bone. In addition, they found that of their 7 patients with preoperative retroversion >10°, only 1 patient (14%) had a postoperative glenoid with <10° of retroversion with regard to the plane of the scapula and that all 6 of their patients with preoperative glenoid retroversion of <10° had a postoperative glenoid version of <10°.
Preoperative CT scans are much more accurate at determining glenoid version and thus how much glenoid correction is required to reestablish neutral version than plain radiography. Nyffeler and colleagues10 compared CT scans with axillary views for comparing glenoid version in 25 patients with no shoulder prosthesis present and 25 patients with a TSA in place. They found that glenoid retroversion was overestimated on plain radiographs in 86% of their patients with an average difference between CT and plain radiography of 6.4° and a maximum difference of 21°. They also found poor interobserver reliability in the plain radiography group and good interobserver reliability in the CT group, with coefficients of correlation of 0.77 for the plain radiography group and 0.93 for the CT group. Thus, they concluded that glenoid version cannot be accurately measured by plain radiography and that CT should be used. Hoenecke and colleagues11 subsequently evaluated 33 patients scheduled for TSA and found that CT version measurements made on 2-dimensional (2-D) CT slices compared with 3-D-reconstructed models of the same CT slices differed by an average of 5.1° because the axial CT slices were most often made perpendicular to the axis of the patient’s torso and not perpendicular to the body of the scapula. Accurate version assessment is critically important in planning for the degree of correction required to restore neutral glenoid version, and differences of 6.4° between CT assessment and plain radiography, and 5.1° between 2-D and 3-D CT scan assessments may lead to inadequate version correction intraoperatively and inferior postoperative results.
Continue to: GLENOSPHERE PLACEMENT
GLENOSPHERE PLACEMENT
The most common indication for reverse TSA is rotator cuff arthropathy characterized by rotator cuff dysfunction and end-stage glenohumeral arthritis.12 These patients require accurate and reproducible glenoid placement to optimize their postoperative range of motion and stability and minimize scapular notching.
Ideal glenosphere placement is the location and orientation that maximizes impingement-free ROM and stability while avoiding notching. Individual patient anatomy determines ideal placement; however, several guidelines for placement include inferior translation on the glenoid with neutral to inferior inclination. Gutiérrez and colleagues13 developed a computer model to assess the hierarchy of surgical factors affecting the ROM after a reverse TSA. They found that lateralizing the center of rotation gave the largest increase in impingement-free abduction, followed closely by inferior translation of the glenosphere on the glenoid.
Avoiding scapular notching is also a very important factor in ideal glenosphere placement. Scapular notching can be described as impingement of the humeral cup against the scapular neck during arm adduction and/or humeral rotation. Gutiérrez and colleagues13 also found that decreasing the neck shaft angle to create a more varus proximal humerus was the most important factor in increasing the impingement-free adduction. Roche and colleagues14 reviewed the radiographs of 151 patients who underwent primary reverse TSA at a mean follow-up of 28.3 months postoperatively; they found that 13.2% of their patients had a notch and that, on average, their patients who had no scapular notch had significantly more inferior glenosphere overhang than those who had a scapular notch. Poon and colleagues15 found that a glenosphere overhang of >3.5 mm prevented notching in their randomized control trial comparing concentrically and eccentrically placed glenospheres. Multiple other studies have demonstrated similar results and recommended inferior glenoid translation and inferior glenoid inclination to avoid scapular notching.16,17 Lévigne and colleagues18 retrospectively reviewed 337 reverse TSAs and observed a correlation between scapular notching and radiolucencies around the glenosphere component, with 14% of patients with scapular notching displaying radiolucencies vs 4% of patients without scapula notching displaying radiolucencies.
Several studies have also focused on the ideal amount of inferior glenoid inclination to maximize impingement-free ROM. Li and colleagues17 performed a computer simulation study on the Comprehensive Reverse Shoulder System (Zimmer Biomet) to determine impingement-free internal and external ROM with varying amounts of glenosphere offset, translation, and inclination. They found that progressive glenosphere inferior inclination up to 30° improved impingement-free rotational ROM at all degrees of scaption. Gutiérrez and colleagues19 used computer modeling to compare concentrically placed glenospheres in neutral inclination with eccentrically placed glenospheres in varying degrees of inclination. They found that the lowest forces across the baseplate occurred in the lateralized and inferiorly inclined glenospheres, and the highest forces occurred in the lateralized and superiorly inclined glenospheres. Together, these studies show that inferior glenoid inclination increases impingement-free ROM and, combined with lateralization, may result in improved glenosphere longevity due to significantly decreased forces at the RSA glenoid baseplate when compared to that at superiorly inclined glenoids.
The ideal amount of mediolateral glenosphere offset has not been well defined. Grammont design systems place the center of rotation of the glenosphere medial to the glenoid baseplate together with valgus humeral component neck shaft angles of around 155°. These design elements are believed to decrease shear stresses through the glenoid baseplate to the glenoid interface and improve shoulder stability, but they are also associated with reduced impingement-free ROM and increased rates of scapular notching.13 This effect is accentuated in patients with preexisting glenoid bone loss and/or congenitally short scapular necks that further medialize the glenosphere. Medialization of the glenosphere may also shorten the remaining rotator cuff muscles and result in decreased implant stability and external rotation strength. Several implant systems have options to vary the amount of lateral offset. The correct amount of lateral offset for each patient requires the understanding that improving patients’ impingement-free ROM by increasing the amount of lateral offset comes at the price of increasing the shear forces experienced by the interface between the glenoid baseplate and the glenoid. As glenoid fixation technology improves increased lateralization of glenospheres without increased rates of glenoid baseplate, loosening should improve the ROM after reverse TSA.
Continue to: Regardless of the intraoperative goals...
Regardless of the intraoperative goals for placement and orientation of the glenosphere components, it is vitally important to accurately and consistently meet those goals for achieving optimal patient outcomes. Verborgt and colleagues20 implanted 7 glenospheres in cadaveric specimens without any glenohumeral arthritis using standard techniques to evaluate the accuracy of glenosphere version and inclination. Their goal was to place components in neutral version and with 10° of inferior inclination. Their average glenoid version postoperatively was 8.7° of anteversion, and their average inclination was 0.9° of superior inclination. Throckmorton and colleagues21 randomized 35 cadaveric shoulders to receive either an anatomic or a reverse total shoulder prosthesis from high-, mid-, and low-volume surgeons. They found that components placed using traditional guides averaged 6° of deviation in version and 5° of deviation in inclination from their target values, with no significant differences between surgeons of different volumes.
PATIENT-SPECIFIC GUIDES/INSTRUMENTATION
Patient-specific guides/instrumentation and intraoperative navigation are the 2 techniques that have been used to improve the accuracy of glenoid and glenosphere placement. Both techniques require the use of high-resolution CT scans and computer software to determine the proper position for glenoid or glenosphere placement based on the patient’s individual anatomy. Patient-specific guides and instrumentation use the data acquired from a CT scan to generate a preoperative plan for the location and orientation of the glenoid baseplate. Once the surgeon approves the preoperative plan, a patient-specific guide is created using the patient’s glenoid as a reference for the location and orientation of the central guide pin. The location of the central guide pin on the glenoid determines the center of the glenoid baseplate, and the guide pin’s orientation determines the version and inclination of the glenoid or the glenosphere. Once the guide pin is placed in the glenoid, the remainder of the glenoid implantation uses the guide pin as a reference, and, in that way, patient-specific guides control the orientation of the glenoid at the time of surgery.
Intraoperative navigation uses an optical tracking system to determine the location and orientation of the central guide pin. Navigation systems require intraoperative calibration of the optical tracking system before they can track the location of implantation relative to bony landmarks on the patient’s scapula. Their advantage over patient-specific instrumentation (PSI) is that they do not require the manufacture of a custom guide; however, they may add significantly increased cost and surgical time due to the need for calibration prior to use and the cost of the navigation system along with any disposable components associated with it. Kircher and colleagues22 performed a prospective randomized clinical study of navigation-aided TSA compared with conventional TSA and found that operating time was significantly increased for the navigated group with an average operating room time of 169.5 minutes compared to 138 minutes for the conventional group. They also found that navigation had to be abandoned in 37.5% of their navigated patients due to technical errors during glenoid referencing.
COMMERCIAL PATIENT-SPECIFIC INSTRUMENTATION SYSTEMS
The 2 types of PSI that are currently available are single-use PSI and reusable PSI. The single-use PSI involves the fabrication of unique guides based on surgeon-approved preoperative plans generated by computer-software-processed preoperative CT scans. The guides are fabricated to rest on the glenoid articular surface and direct the guide pin to the correct location and in the correct direction to place the glenoid baseplate in the desired position with the desired version and inclination. Most of these systems also provide a 3-D model of the patient’s glenoid so that surgeons can visualize glenoid deformities and the correct guide placement on the glenoid. Single-use PSI systems are available from DJO Global, Wright Medical Group, and Zimmer Biomet. The second category of PSI is reusable and is available from Arthrex. The guide pin for this system is adjusted to fit individual patient anatomy and guide the guide pin into the glenoid in a location and orientation preplanned on the CT-scan-based computer software or using a 3-D model of the patient’s glenoid (Table).
Table. Details of Available Patient-Specific Instrumentation Systems
| System | Manufacturer | Single-Use/Reusable | Guides |
| MatchPoint System | DJO Global | Single-use | Central guide pin |
| Blueprint 3D Planning + PSI | Wright Medical Group | Single-use | Central guide pin |
| Zimmer Patient Specific Instruments Shoulder | Zimmer Biomet | Single-use | Central guide pin, reaming guide, roll guide, screw drill guide |
| Virtual Implant Positioning System | Arthrex | Reusable | Central guide pin |
The DJO Global patient-specific guide is termed as the MatchPoint System. This system creates 3-D renderings of the scapula and allows the surgeon to manipulate the glenoid baseplate on the scapula. The surgeon chooses the glenoid baseplate, location, version, and inclination on the computerized 3-D model. The system then fabricates a guide pin matching the computerized template that references the patient’s glenoid surface with a hook to orient it against the coracoid. A 3-D model of the glenoid is also provided along with the customized guide pin.
Continue to: Blueprint 3D Planning + PSI...
Blueprint 3D Planning + PSI (Wright Medical Group) allows custom placement of the glenoid version, inclination, and position on computerized 3-D models of the patient’s scapula. This PSI references the glenoid with 4 feet that captures the edge of the patient’s glenoid at specific locations and is unique because it allows the surgeon to control where on the glenoid edge to 4 feet contact as long as 1 foot is placed on the posterior edge of the glenoid and the remaining 3 feet are placed on the anterior edge of the glenoid. A 3-D model of the glenoid is also provided with this guide.
The Zimmer Biomet patient-specific guide is termed as the Zimmer Patient Specific Instruments Shoulder. Its computer software allows custom placement of the glenoid as well, but it also includes computerized customization of the reaming depth, screw angles, and screw lengths to optimize fixation. Their system includes a central guide pin to set the glenoid baseplate’s location and orientation, a reaming guide to control reaming depth and direction, a roll guide to control the glenoid baseplate’s rotation, and a drill guide to control the screw direction. They also provide a 3-D model of the glenoid.
The Arthrex Virtual Implant Positioning (VIP) System is similar to other systems in that its 3-D planning software is based on CT images uploaded by the surgeon. The unique aspect of this system is that the guide pin is adjusted by the surgeon for each individual patient based on instructions generated by the planning software; however, after use, the instruments are resterilized and reused on subsequent patients (Figures 1A, 1B). In this manner, their instruments are reusable and allow custom adjustment for each patient with the ability to set the pin location and glenoid version in a patient-specific manner. This has the potential benefit of keeping costs down. For more complex deformity cases, the Arthrex VIP System can also 3-D-print a sterile model of the glenoid to help surgeons appreciate the deformity better (Figure 2).

DATA ON PATIENT-SPECIFIC INSTRUMENTS
Several studies have measured the accuracy of patient-specific guides and have compared the accuracy of patient-specific guides to that of traditional methods. Levy and colleagues23 investigated the accuracy of single-use patient-specific guides compared to that of preoperative plans. They used patient-specific guides on 14 cadaveric shoulders based on plans developed by virtual preoperative 3-D planning system using CT images. Once the guide pin was drilled using the patient-specific guide, they obtained a second CT scan to compare the accuracy of the patient-specific guide to the surgical plan generated preoperatively. They found that the translational accuracy of the starting point for the guide pin averaged 1.2 mm ± 0.7 mm, the accuracy of the inferior inclination was 1.2° ± 1.2°, and the accuracy of the glenoid version was 2.6° ± 1.7°. They concluded that patient-specific guides were highly accurate in reproducing the starting point, inclination, and version set on preoperative guides.
Walch and colleagues24 subsequently performed a similar study using 15 cadaveric scapulae without any other shoulder soft tissue or bone attached. They also used CT-scan-based 3-D planning software to plan their glenoid placement with a subsequently fabricated single-use patient-specific guide used to place a guide pin. They obtained a second CT scan after guide pin implantation and compared the preoperative plan with the subsequent guide pin. They found a mean entry point position error of 1.05 mm ± 0.31 mm, a mean inclination error of 1.42° ± 1.37°, and a mean version error of 1.64° ± 1.01°.
Continue to: Throckmorton and colleagues...
Throckmorton and colleagues21 used 70 cadaveric shoulders with radiographically confirmed arthritis and randomized them to undergo either anatomic or reverse TSA using either a patient-specific guide or standard instrumentation. Postoperative CT scans were used to evaluate the glenoid inclination, version, and starting point. They found that glenoid components implanted using patient-specific guides were more accurate than those placed using traditional instrumentation. The average deviation from intended inclination was 3° for patient-specific guides and 7° for traditional instrumentation, the average deviation from intended version was 5° for patient-specific guides and 8° for traditional instrumentation, and the average deviation in intended starting point was 2 mm for patient-specific guides and 3 mm for traditional instrumentation. They also analyzed significantly malpositioned components as defined by a variation in version or inclination of >10° or >4 mm in starting point. They found that 6 of their 35 glenoids using patient-specific guides were significantly malpositioned compared to 23 of 35 glenoids using traditional instrumentation. They concluded that patient-specific guides were more accurate and reduced the number of significantly malpositioned implants when compared with traditional instrumentation.
Early and colleagues25 analyzed the effect of severe glenoid bone defects on the accuracy of patient-specific guides compared with traditional guides. Using 10 cadaveric shoulders, they created anterior, central, or posterior glenoid defects using a reamer and chisel to erode the bone past the coracoid base. Subsequent CT scans were performed on the specimens, and patient-specific guides were fabricated and used for reverse TSA in 5 of the 10 specimens. A reverse TSA was performed using traditional instrumentation in the remaining 5 specimens. They found that the average deviation in inclination and version from preoperative plan was more accurate in the patient-specific guide cohort than that in the traditional instrument cohort, with an average deviation in inclination and version of 1.2° ± 1.2° and 1.8° ± 1.2° respectively for the cohort using patient-specific instruments vs 2.8° ± 1.8° and 3.5° ± 3° for the cohort using traditional instruments. They also found that their total bone screw lengths were longer in the patient-specific guide group than those in the traditional group, with screws averaging 52% of preoperatively planned length in the traditional instrument cohort vs 89% of preoperatively planned length in the patient-specific instrument cohort.
Gauci and colleagues26 measured the accuracy of patient-specific guides in vivo in 17 patients receiving TSA. Preoperative CT scans were used to fabricate patient-specific guides, and postoperative CT scans were used to measure version, inclination, and error of entry in comparison with the templated goals used to create patient-specific guides. They found a mean error in version and inclination of 3.4° and 1.8°, respectively, and a mean error in entry of 0.9 mm of translation on the glenoid. Dallalana and colleagues27 performed a very similar study on 20 patients and found a mean deviation in glenoid version of 1.8° ± 1.9°, a mean deviation in glenoid inclination of 1.3° ± 1.0°, a mean translation in anterior-posterior plane of 0.5 mm ± 0.3 mm, and a mean translation in the superior-inferior plane of 0.8 mm ± 0.5 mm.
Hendel and colleagues28 performed a randomized prospective clinical trial comparing patient-specific guides with traditional methods for glenoid insertion. They randomized 31 patients to receive a glenoid implant using either a patient-specific guide or traditional methods and compared glenoid retroversion and inclination with their preoperative plan. They found an average version deviation of 6.9° in the traditional method cohort and 4.3° in the patient-specific guide cohort. Their average deviation in inclination was 11.6° in the traditional method cohort and 2.9° in the patient-specific guide cohort. For patients with preoperative retroversion >16°, the average deviation was 10° in the standard surgical cohort and 1.2° in the patient-specific instrument cohort. Their data suggest that increasing preoperative retroversion leads to an increased version variation from preoperative plan.
Iannotti and colleagues29 randomly assigned 46 patients to preoperatively undergo either CT scan with 3-D templating of glenoid component without patient-specific guide fabrication or CT scan with 3-D templating and patient-specific guide fabrication prior to receiving a TSA. They recorded the postoperative inclination and version for each patient and compared them to those of a nonrandomized control group of 17 patients who underwent TSA using standard instrumentation. They found no difference between the cohorts with or without patient-specific guide use with regard to implant location, inclination, or version; however, they did find a difference between the combined 3-D templating cohort compared with their standard instrumentation cohort. They concluded that 3-D templating significantly improved the surgeons’ ability to correctly position the glenoid component with or without the fabrication and the use of a patient-specific guide.
Continue to: Denard and colleagues...
Denard and colleagues30 compared the preoperative glenoid version and inclination measurements obtained using the Blueprint 3D Planning + PSI software and the VIP System 3D planning software. They analyzed the preoperative CT scans of 63 consecutive patients undergoing either TSA or reverse TSA using both the Blueprint and the VIP System 3D planning software and compared the resulting native glenoid version and inclination measured by the software. They found a statistically significant difference (P = 0.04) in the version measurements provided by the different planning software; however, the differences found in inclination did not reach statistical significance (P = 0.463). In 19 of the 63 patients (30%), the version measurements between the systems were >5°, and in 29 of the 63 patients (46%), the inclination measurements between the systems were 5° or greater. In addition, 12 of the 63 patients (19%) had both version and inclination measurement differences of >5° between the systems. In total, they found that 35 of the 63 patients had at least 1 measurement that varied by >5° between the systems, and that in 15 patients (24%), 1 measurement varied by >10°. Their data demonstrate considerable variability in the preoperative measurements provided by different 3-D planning software systems, and that further study of each commercially available 3-D planning software system is needed to evaluate their accuracy.
CONCLUSION
Optimal outcomes following TSA and reverse TSA are dependent on proper implant position. Multiple studies have demonstrated improved accuracy in implant positioning with the use of patient-specific guides compared to that with traditional methods. Currently, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research had been done comparing the accuracy of each system’s 3-D planning software with each other and with standardized measurements of glenoid version and inclination. Further research is required to determine the accuracy of each commercially available 3-D planning software system as well as the clinical benefit of patient-specific guides in shoulder arthroplasty.
1. Churchill RS, Brems JJ, Kotschi H. Glenoid size, inclination, and version: an anatomic study. J Shoulder Elbow Surg. 2001;10(4):327-332. doi:10.1067/mse.2001.115269.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135. doi:10.1067/mse.2002.121146.
3. Mullaji AB, Beddow FH, Lamb GH. CT measurement of glenoid erosion in arthritis. J Bone Joint Surg Br. 1994;76(3):384-388.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. RJ Friedman, KB Hawthorne, BM Genez. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg. 1992;74(7):1032-1037. doi:10.2106/00004623-199274070-00009.
6. Yian EH, Werner CM, Nyffeler RW, et al. Radiographic and computed tomography analysis of cemented pegged polyethylene glenoid components in total shoulder replacement. J Bone Joint Surg. 2005;87(9):1928-1936. doi:10.2106/00004623-200509000-00004.
7. Hasan SS, Leith JM, Campbell B, Kapil R, Smith KL, Matsen FA. Characteristics of unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2002;11(5):431-441.
8. Hopkins AR, Hansen UN, Amis AA, Emery R. The effects of glenoid component alignment variations on cement mantle stresses in total shoulder arthroplasty. J Shoulder Elbow Surg. 2004;13(6):668-675. doi:10.1016/S1058274604001399.
9. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55. doi:10.1016/j.jse.2011.02.011.
10. Nyffeler RW, Jost B, Pfirrmann CWA, Gerber C. Measurement of glenoid version: conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496. doi:10.1016/S1058274603001812.
11. Hoenecke HR, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(2):166-171. doi:10.1016/j.jse.2009.08.009.
12. Wall B, Nové-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485. doi:10.2106/JBJS.F.00666.
13. Gutiérrez S, Comiskey 4, Charles A, Luo Z, Pupello DR, Frankle MA. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am. 2008;90(12):2606-2615. doi:10.2106/JBJS.H.00012.
14. Roche CP, Marczuk Y, Wright TW, et al. Scapular notching and osteophyte formation after reverse shoulder replacement: Radiological analysis of implant position in male and female patients. Bone Joint J. 2013;95-B(4):530-535. doi:10.1302/0301-620X.95B4.30442.
15. Poon PC, Chou J, Young SW, Astley T. A comparison of concentric and eccentric glenospheres in reverse shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(16):e138. doi:10.2106/JBJS.M.00941.
16. Nyffeler RW, Werner CML, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528. doi:10.1016/j.jse.2004.09.010.
17. Li X, Knutson Z, Choi D, et al. Effects of glenosphere positioning on impingement-free internal and external rotation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(6):807-813. doi:10.1016/j.jse.2012.07.013.
18. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935. doi:10.1016/j.jse.2008.02.010.
19. Gutiérrez S, Walker M, Willis M, Pupello DR, Frankle MA. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(5):732-739. doi:10.1016/j.jse.2010.10.035.
20. Verborgt O, De Smedt T, Vanhees M, Clockaerts S, Parizel PM, Van Glabbeek F. Accuracy of placement of the glenoid component in reversed shoulder arthroplasty with and without navigation. J Shoulder Elbow Surg. 2011;20(1):21-26. doi:10.1016/j.jse.2010.07.014.
21. Throckmorton TW, Gulotta LV, Bonnarens FO, et al. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: A multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg. 2015;24(6):965-971. doi:10.1016/j.jse.2014.10.013.
22. Kircher J, Wiedemann M, Magosch P, Lichtenberg S, Habermeyer P. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: a prospective-randomized clinical study. J Shoulder Elbow Surg. 2009;18(4):515-520. doi:10.1016/j.jse.2009.03.014.
23. Levy JC, Everding NG, Frankle MA, Keppler LJ. Accuracy of patient-specific guided glenoid baseplate positioning for reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(10):1563-1567. doi:10.1016/j.jse.2014.01.051.
24. Walch G, Vezeridis PS, Boileau P, Deransart P, Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg. 2015;24(2):302-309. doi:10.1016/j.jse.2014.05.029.
25. Eraly K, Stoffelen D, Vander Sloten J, Jonkers I, Debeer P. A patient-specific guide for optimizing custom-made glenoid implantation in cases of severe glenoid defects: an in vitro study. J Shoulder Elbow Surg. 2016;25(5):837-845. doi:10.1016/j.jse.2015.09.034.
26. Gauci MO, Boileau P, Baba M, Chaoui J, Walch G. Patient-specific glenoid guides provide accuracy and reproducibility in total shoulder arthroplasty. Bone Joint J. 2016;98-B(8):1080-1085. doi:10.1302/0301-620X.98B8.37257.
27. Dallalana RJ, McMahon RA, East B, Geraghty L. Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty. Int J Shoulder Surg. 2016;10(2):59-66. doi:10.4103/09736042.180717.
28. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg. 2012;94(23):2167-2175. doi:10.2106/JBJS.K.01209.
29. Iannotti JP, Weiner S, Rodriguez E, et al. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Joint Surg. 2015;97(8):651-658. doi:10.2106/JBJS.N.00493.
30. Denard PJ, Provencher MT, Lädermann A, Romeo AA, Dines JS. Version and inclination obtained with 3D planning in total shoulder arthroplasty: do different programs produce the same results? SECEC-ESSSE Congress, Berlin 2017. 2017.
ABSTRACT
Optimal outcomes following total shoulder arthroplasty TSA and reverse shoulder arthroplasty RSA are dependent on proper implant position. Multiple cadaver studies have demonstrated improved accuracy of implant positioning with use of patient-specific guides/instrumentation compared to traditional methods. At this time, there are 3 commercially available single use patient-specific instrumentation systems and 1 commercially available reusable patient-specific instrumentation system. Currently though, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research has been done comparing the accuracy of each system’s 3-dimensional planning software. Future work is necessary to elucidate the ideal indications for the use of patient-specific guides and instrumentation, but it is likely, particularly in the setting of advanced glenoid deformity, that these systems will improve a surgeon's ability to put the implant in the best position possible.
Continue to: Optimal functional recovery...
Optimal functional recovery and implant longevity following both total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) depend, in large part, on proper placement of the glenoid component. Glenoid component malpositioning has an adverse effect on shoulder stability, range of motion (ROM), impingement, and glenoid implant longevity.
Traditionally, glenoid component positioning has been done manually by the surgeons based on their review of preoperative films and knowledge of glenoid anatomy. Anatomic studies have demonstrated high individual variability in the version of the native glenoid, thus making ideal placement of the initial glenoid guide pin difficult using standard guide pin guides.1
The following 2 methods have been described for improving the accuracy of glenoid guide pin insertion and subsequent glenoid implant placement: (1) computerized navigation and (2) patient-specific guides/instrumentation. Although navigated shoulder systems have demonstrated improved accuracy in glenoid placement compared with traditional methods, navigated systems require often large and expensive systems for implementation. The majority of them also require placement of guide pins or arrays on scapular bony landmarks, likely leading to an increase in operative time and possible iatrogenic complications, including fracture and pin site infections.
This review focuses on the use of patient-specific guides/instrumentation in shoulder arthroplasty. This includes the topic of proper glenoid and glenosphere placement as well as patient-specific guides/instrumentation and their accuracy.
GLENOID PLACEMENT
Glenohumeral osteoarthritis is the most common indication for TSA2 and commonly results in glenoid deformity. Using computed tomography (CT) scans of 45 arthritic shoulders and 19 normal shoulders, Mullaji and colleagues3 reported that the anteroposterior dimensions of the glenoid were increased by an average of 5 mm to 8 mm in osteoarthritic shoulders and by an average of 6 mm in rheumatoid arthritic shoulders compared to those in normal shoulders. A retrospective review of serial CT scans performed preoperatively on 113 osteoarthritic shoulders by Walch and colleagues4 demonstrated an average retroversion of 16°, and it has been the basis for the commonly used Walch classification of glenoid wear in osteoarthritis. Increased glenoid wear and increased glenoid retroversion make the proper restoration of glenoid version, inclination, and offset during shoulder arthroplasty more difficult and lead to increased glenoid component malpositioning.
Continue to: The ideal placement of the glenoid...
The ideal placement of the glenoid to maximize function, ROM, and implant longevity is in a mechanically neutral alignment with no superoinferior inclination1 and neutral version with respect to the transverse axis of the scapula.5
Improper glenoid positioning has an adverse effect on the functional results of shoulder arthroplasty. Yian and colleagues6 evaluated 47 cemented, pegged glenoids using standard radiography and CT scans at a mean follow-up of 40 months. They observed a significant correlation between increased glenoid component retroversion and lower Constant scores. Hasan and colleagues7 evaluated 139 consecutive patients who were dissatisfied with the result of their primary arthroplasty and found that 28% of them had at least 1 substantially malpositioned component identified either on radiography or during a revision surgery. They also found a significant correlation between stiffness, instability, and component malposition in their cohort.
Glenoid longevity is also dependent on proper component positioning, with the worst outcomes coming if the glenoid is malaligned with either superior or inferior inclination. Hasan and colleagues7 found that of their 74 patients with failed TSAs, 44 patients (59%) demonstrated mechanical loosening of their glenoid components either radiographically or during revision surgery, and 10 of their 44 patients with loose glenoids (23%) also had a malpositioned component. Using finite element analysis, Hopkins and colleagues8 analyzed the stresses through the cement mantle in glenoid prostheses that were centrally aligned, superiorly inclined, inferiorly inclined, anteverted, and retroverted. They found that malalignment of the glenoid increases the stresses through the cement mantle, leading to increased likelihood of mantle failure compared to that of centrally aligned glenoids, especially if there is malalignment with superior or inferior inclination or retroversion.
The accuracy of traditional methods of glenoid placement using an initial guide pin is limited and decreases with increasing amounts of glenoid deformity and retroversion. Iannotti and colleagues 9 investigated 13 patients undergoing TSA with an average preoperative retroversion of 13° and evaluated them using a 3-dimensional (3-D) surgical simulator. They found that the postoperative glenoid version was within 5° of ideal version in only 7 of their 13 patients (54%) and within 10° of ideal version in only 10 of their 13 patients (77%). In their study, the ideal version was considered to be the version as close to perpendicular to the plane of the scapula as possible with complete contact of the back side of the component on glenoid bone and maintenance of the center peg of the component within bone. In addition, they found that of their 7 patients with preoperative retroversion >10°, only 1 patient (14%) had a postoperative glenoid with <10° of retroversion with regard to the plane of the scapula and that all 6 of their patients with preoperative glenoid retroversion of <10° had a postoperative glenoid version of <10°.
Preoperative CT scans are much more accurate at determining glenoid version and thus how much glenoid correction is required to reestablish neutral version than plain radiography. Nyffeler and colleagues10 compared CT scans with axillary views for comparing glenoid version in 25 patients with no shoulder prosthesis present and 25 patients with a TSA in place. They found that glenoid retroversion was overestimated on plain radiographs in 86% of their patients with an average difference between CT and plain radiography of 6.4° and a maximum difference of 21°. They also found poor interobserver reliability in the plain radiography group and good interobserver reliability in the CT group, with coefficients of correlation of 0.77 for the plain radiography group and 0.93 for the CT group. Thus, they concluded that glenoid version cannot be accurately measured by plain radiography and that CT should be used. Hoenecke and colleagues11 subsequently evaluated 33 patients scheduled for TSA and found that CT version measurements made on 2-dimensional (2-D) CT slices compared with 3-D-reconstructed models of the same CT slices differed by an average of 5.1° because the axial CT slices were most often made perpendicular to the axis of the patient’s torso and not perpendicular to the body of the scapula. Accurate version assessment is critically important in planning for the degree of correction required to restore neutral glenoid version, and differences of 6.4° between CT assessment and plain radiography, and 5.1° between 2-D and 3-D CT scan assessments may lead to inadequate version correction intraoperatively and inferior postoperative results.
Continue to: GLENOSPHERE PLACEMENT
GLENOSPHERE PLACEMENT
The most common indication for reverse TSA is rotator cuff arthropathy characterized by rotator cuff dysfunction and end-stage glenohumeral arthritis.12 These patients require accurate and reproducible glenoid placement to optimize their postoperative range of motion and stability and minimize scapular notching.
Ideal glenosphere placement is the location and orientation that maximizes impingement-free ROM and stability while avoiding notching. Individual patient anatomy determines ideal placement; however, several guidelines for placement include inferior translation on the glenoid with neutral to inferior inclination. Gutiérrez and colleagues13 developed a computer model to assess the hierarchy of surgical factors affecting the ROM after a reverse TSA. They found that lateralizing the center of rotation gave the largest increase in impingement-free abduction, followed closely by inferior translation of the glenosphere on the glenoid.
Avoiding scapular notching is also a very important factor in ideal glenosphere placement. Scapular notching can be described as impingement of the humeral cup against the scapular neck during arm adduction and/or humeral rotation. Gutiérrez and colleagues13 also found that decreasing the neck shaft angle to create a more varus proximal humerus was the most important factor in increasing the impingement-free adduction. Roche and colleagues14 reviewed the radiographs of 151 patients who underwent primary reverse TSA at a mean follow-up of 28.3 months postoperatively; they found that 13.2% of their patients had a notch and that, on average, their patients who had no scapular notch had significantly more inferior glenosphere overhang than those who had a scapular notch. Poon and colleagues15 found that a glenosphere overhang of >3.5 mm prevented notching in their randomized control trial comparing concentrically and eccentrically placed glenospheres. Multiple other studies have demonstrated similar results and recommended inferior glenoid translation and inferior glenoid inclination to avoid scapular notching.16,17 Lévigne and colleagues18 retrospectively reviewed 337 reverse TSAs and observed a correlation between scapular notching and radiolucencies around the glenosphere component, with 14% of patients with scapular notching displaying radiolucencies vs 4% of patients without scapula notching displaying radiolucencies.
Several studies have also focused on the ideal amount of inferior glenoid inclination to maximize impingement-free ROM. Li and colleagues17 performed a computer simulation study on the Comprehensive Reverse Shoulder System (Zimmer Biomet) to determine impingement-free internal and external ROM with varying amounts of glenosphere offset, translation, and inclination. They found that progressive glenosphere inferior inclination up to 30° improved impingement-free rotational ROM at all degrees of scaption. Gutiérrez and colleagues19 used computer modeling to compare concentrically placed glenospheres in neutral inclination with eccentrically placed glenospheres in varying degrees of inclination. They found that the lowest forces across the baseplate occurred in the lateralized and inferiorly inclined glenospheres, and the highest forces occurred in the lateralized and superiorly inclined glenospheres. Together, these studies show that inferior glenoid inclination increases impingement-free ROM and, combined with lateralization, may result in improved glenosphere longevity due to significantly decreased forces at the RSA glenoid baseplate when compared to that at superiorly inclined glenoids.
The ideal amount of mediolateral glenosphere offset has not been well defined. Grammont design systems place the center of rotation of the glenosphere medial to the glenoid baseplate together with valgus humeral component neck shaft angles of around 155°. These design elements are believed to decrease shear stresses through the glenoid baseplate to the glenoid interface and improve shoulder stability, but they are also associated with reduced impingement-free ROM and increased rates of scapular notching.13 This effect is accentuated in patients with preexisting glenoid bone loss and/or congenitally short scapular necks that further medialize the glenosphere. Medialization of the glenosphere may also shorten the remaining rotator cuff muscles and result in decreased implant stability and external rotation strength. Several implant systems have options to vary the amount of lateral offset. The correct amount of lateral offset for each patient requires the understanding that improving patients’ impingement-free ROM by increasing the amount of lateral offset comes at the price of increasing the shear forces experienced by the interface between the glenoid baseplate and the glenoid. As glenoid fixation technology improves increased lateralization of glenospheres without increased rates of glenoid baseplate, loosening should improve the ROM after reverse TSA.
Continue to: Regardless of the intraoperative goals...
Regardless of the intraoperative goals for placement and orientation of the glenosphere components, it is vitally important to accurately and consistently meet those goals for achieving optimal patient outcomes. Verborgt and colleagues20 implanted 7 glenospheres in cadaveric specimens without any glenohumeral arthritis using standard techniques to evaluate the accuracy of glenosphere version and inclination. Their goal was to place components in neutral version and with 10° of inferior inclination. Their average glenoid version postoperatively was 8.7° of anteversion, and their average inclination was 0.9° of superior inclination. Throckmorton and colleagues21 randomized 35 cadaveric shoulders to receive either an anatomic or a reverse total shoulder prosthesis from high-, mid-, and low-volume surgeons. They found that components placed using traditional guides averaged 6° of deviation in version and 5° of deviation in inclination from their target values, with no significant differences between surgeons of different volumes.
PATIENT-SPECIFIC GUIDES/INSTRUMENTATION
Patient-specific guides/instrumentation and intraoperative navigation are the 2 techniques that have been used to improve the accuracy of glenoid and glenosphere placement. Both techniques require the use of high-resolution CT scans and computer software to determine the proper position for glenoid or glenosphere placement based on the patient’s individual anatomy. Patient-specific guides and instrumentation use the data acquired from a CT scan to generate a preoperative plan for the location and orientation of the glenoid baseplate. Once the surgeon approves the preoperative plan, a patient-specific guide is created using the patient’s glenoid as a reference for the location and orientation of the central guide pin. The location of the central guide pin on the glenoid determines the center of the glenoid baseplate, and the guide pin’s orientation determines the version and inclination of the glenoid or the glenosphere. Once the guide pin is placed in the glenoid, the remainder of the glenoid implantation uses the guide pin as a reference, and, in that way, patient-specific guides control the orientation of the glenoid at the time of surgery.
Intraoperative navigation uses an optical tracking system to determine the location and orientation of the central guide pin. Navigation systems require intraoperative calibration of the optical tracking system before they can track the location of implantation relative to bony landmarks on the patient’s scapula. Their advantage over patient-specific instrumentation (PSI) is that they do not require the manufacture of a custom guide; however, they may add significantly increased cost and surgical time due to the need for calibration prior to use and the cost of the navigation system along with any disposable components associated with it. Kircher and colleagues22 performed a prospective randomized clinical study of navigation-aided TSA compared with conventional TSA and found that operating time was significantly increased for the navigated group with an average operating room time of 169.5 minutes compared to 138 minutes for the conventional group. They also found that navigation had to be abandoned in 37.5% of their navigated patients due to technical errors during glenoid referencing.
COMMERCIAL PATIENT-SPECIFIC INSTRUMENTATION SYSTEMS
The 2 types of PSI that are currently available are single-use PSI and reusable PSI. The single-use PSI involves the fabrication of unique guides based on surgeon-approved preoperative plans generated by computer-software-processed preoperative CT scans. The guides are fabricated to rest on the glenoid articular surface and direct the guide pin to the correct location and in the correct direction to place the glenoid baseplate in the desired position with the desired version and inclination. Most of these systems also provide a 3-D model of the patient’s glenoid so that surgeons can visualize glenoid deformities and the correct guide placement on the glenoid. Single-use PSI systems are available from DJO Global, Wright Medical Group, and Zimmer Biomet. The second category of PSI is reusable and is available from Arthrex. The guide pin for this system is adjusted to fit individual patient anatomy and guide the guide pin into the glenoid in a location and orientation preplanned on the CT-scan-based computer software or using a 3-D model of the patient’s glenoid (Table).
Table. Details of Available Patient-Specific Instrumentation Systems
| System | Manufacturer | Single-Use/Reusable | Guides |
| MatchPoint System | DJO Global | Single-use | Central guide pin |
| Blueprint 3D Planning + PSI | Wright Medical Group | Single-use | Central guide pin |
| Zimmer Patient Specific Instruments Shoulder | Zimmer Biomet | Single-use | Central guide pin, reaming guide, roll guide, screw drill guide |
| Virtual Implant Positioning System | Arthrex | Reusable | Central guide pin |
The DJO Global patient-specific guide is termed as the MatchPoint System. This system creates 3-D renderings of the scapula and allows the surgeon to manipulate the glenoid baseplate on the scapula. The surgeon chooses the glenoid baseplate, location, version, and inclination on the computerized 3-D model. The system then fabricates a guide pin matching the computerized template that references the patient’s glenoid surface with a hook to orient it against the coracoid. A 3-D model of the glenoid is also provided along with the customized guide pin.
Continue to: Blueprint 3D Planning + PSI...
Blueprint 3D Planning + PSI (Wright Medical Group) allows custom placement of the glenoid version, inclination, and position on computerized 3-D models of the patient’s scapula. This PSI references the glenoid with 4 feet that captures the edge of the patient’s glenoid at specific locations and is unique because it allows the surgeon to control where on the glenoid edge to 4 feet contact as long as 1 foot is placed on the posterior edge of the glenoid and the remaining 3 feet are placed on the anterior edge of the glenoid. A 3-D model of the glenoid is also provided with this guide.
The Zimmer Biomet patient-specific guide is termed as the Zimmer Patient Specific Instruments Shoulder. Its computer software allows custom placement of the glenoid as well, but it also includes computerized customization of the reaming depth, screw angles, and screw lengths to optimize fixation. Their system includes a central guide pin to set the glenoid baseplate’s location and orientation, a reaming guide to control reaming depth and direction, a roll guide to control the glenoid baseplate’s rotation, and a drill guide to control the screw direction. They also provide a 3-D model of the glenoid.

The Arthrex Virtual Implant Positioning (VIP) System is similar to other systems in that its 3-D planning software is based on CT images uploaded by the surgeon. The unique aspect of this system is that the guide pin is adjusted by the surgeon for each individual patient based on instructions generated by the planning software; however, after use, the instruments are resterilized and reused on subsequent patients (Figures 1A, 1B). In this manner, their instruments are reusable and allow custom adjustment for each patient with the ability to set the pin location and glenoid version in a patient-specific manner. This has the potential benefit of keeping costs down. For more complex deformity cases, the Arthrex VIP System can also 3-D-print a sterile model of the glenoid to help surgeons appreciate the deformity better (Figure 2).

DATA ON PATIENT-SPECIFIC INSTRUMENTS
Several studies have measured the accuracy of patient-specific guides and have compared the accuracy of patient-specific guides to that of traditional methods. Levy and colleagues23 investigated the accuracy of single-use patient-specific guides compared to that of preoperative plans. They used patient-specific guides on 14 cadaveric shoulders based on plans developed by virtual preoperative 3-D planning system using CT images. Once the guide pin was drilled using the patient-specific guide, they obtained a second CT scan to compare the accuracy of the patient-specific guide to the surgical plan generated preoperatively. They found that the translational accuracy of the starting point for the guide pin averaged 1.2 mm ± 0.7 mm, the accuracy of the inferior inclination was 1.2° ± 1.2°, and the accuracy of the glenoid version was 2.6° ± 1.7°. They concluded that patient-specific guides were highly accurate in reproducing the starting point, inclination, and version set on preoperative guides.
Walch and colleagues24 subsequently performed a similar study using 15 cadaveric scapulae without any other shoulder soft tissue or bone attached. They also used CT-scan-based 3-D planning software to plan their glenoid placement with a subsequently fabricated single-use patient-specific guide used to place a guide pin. They obtained a second CT scan after guide pin implantation and compared the preoperative plan with the subsequent guide pin. They found a mean entry point position error of 1.05 mm ± 0.31 mm, a mean inclination error of 1.42° ± 1.37°, and a mean version error of 1.64° ± 1.01°.
Continue to: Throckmorton and colleagues...
Throckmorton and colleagues21 used 70 cadaveric shoulders with radiographically confirmed arthritis and randomized them to undergo either anatomic or reverse TSA using either a patient-specific guide or standard instrumentation. Postoperative CT scans were used to evaluate the glenoid inclination, version, and starting point. They found that glenoid components implanted using patient-specific guides were more accurate than those placed using traditional instrumentation. The average deviation from intended inclination was 3° for patient-specific guides and 7° for traditional instrumentation, the average deviation from intended version was 5° for patient-specific guides and 8° for traditional instrumentation, and the average deviation in intended starting point was 2 mm for patient-specific guides and 3 mm for traditional instrumentation. They also analyzed significantly malpositioned components as defined by a variation in version or inclination of >10° or >4 mm in starting point. They found that 6 of their 35 glenoids using patient-specific guides were significantly malpositioned compared to 23 of 35 glenoids using traditional instrumentation. They concluded that patient-specific guides were more accurate and reduced the number of significantly malpositioned implants when compared with traditional instrumentation.
Early and colleagues25 analyzed the effect of severe glenoid bone defects on the accuracy of patient-specific guides compared with traditional guides. Using 10 cadaveric shoulders, they created anterior, central, or posterior glenoid defects using a reamer and chisel to erode the bone past the coracoid base. Subsequent CT scans were performed on the specimens, and patient-specific guides were fabricated and used for reverse TSA in 5 of the 10 specimens. A reverse TSA was performed using traditional instrumentation in the remaining 5 specimens. They found that the average deviation in inclination and version from preoperative plan was more accurate in the patient-specific guide cohort than that in the traditional instrument cohort, with an average deviation in inclination and version of 1.2° ± 1.2° and 1.8° ± 1.2° respectively for the cohort using patient-specific instruments vs 2.8° ± 1.8° and 3.5° ± 3° for the cohort using traditional instruments. They also found that their total bone screw lengths were longer in the patient-specific guide group than those in the traditional group, with screws averaging 52% of preoperatively planned length in the traditional instrument cohort vs 89% of preoperatively planned length in the patient-specific instrument cohort.
Gauci and colleagues26 measured the accuracy of patient-specific guides in vivo in 17 patients receiving TSA. Preoperative CT scans were used to fabricate patient-specific guides, and postoperative CT scans were used to measure version, inclination, and error of entry in comparison with the templated goals used to create patient-specific guides. They found a mean error in version and inclination of 3.4° and 1.8°, respectively, and a mean error in entry of 0.9 mm of translation on the glenoid. Dallalana and colleagues27 performed a very similar study on 20 patients and found a mean deviation in glenoid version of 1.8° ± 1.9°, a mean deviation in glenoid inclination of 1.3° ± 1.0°, a mean translation in anterior-posterior plane of 0.5 mm ± 0.3 mm, and a mean translation in the superior-inferior plane of 0.8 mm ± 0.5 mm.
Hendel and colleagues28 performed a randomized prospective clinical trial comparing patient-specific guides with traditional methods for glenoid insertion. They randomized 31 patients to receive a glenoid implant using either a patient-specific guide or traditional methods and compared glenoid retroversion and inclination with their preoperative plan. They found an average version deviation of 6.9° in the traditional method cohort and 4.3° in the patient-specific guide cohort. Their average deviation in inclination was 11.6° in the traditional method cohort and 2.9° in the patient-specific guide cohort. For patients with preoperative retroversion >16°, the average deviation was 10° in the standard surgical cohort and 1.2° in the patient-specific instrument cohort. Their data suggest that increasing preoperative retroversion leads to an increased version variation from preoperative plan.
Iannotti and colleagues29 randomly assigned 46 patients to preoperatively undergo either CT scan with 3-D templating of glenoid component without patient-specific guide fabrication or CT scan with 3-D templating and patient-specific guide fabrication prior to receiving a TSA. They recorded the postoperative inclination and version for each patient and compared them to those of a nonrandomized control group of 17 patients who underwent TSA using standard instrumentation. They found no difference between the cohorts with or without patient-specific guide use with regard to implant location, inclination, or version; however, they did find a difference between the combined 3-D templating cohort compared with their standard instrumentation cohort. They concluded that 3-D templating significantly improved the surgeons’ ability to correctly position the glenoid component with or without the fabrication and the use of a patient-specific guide.
Continue to: Denard and colleagues...
Denard and colleagues30 compared the preoperative glenoid version and inclination measurements obtained using the Blueprint 3D Planning + PSI software and the VIP System 3D planning software. They analyzed the preoperative CT scans of 63 consecutive patients undergoing either TSA or reverse TSA using both the Blueprint and the VIP System 3D planning software and compared the resulting native glenoid version and inclination measured by the software. They found a statistically significant difference (P = 0.04) in the version measurements provided by the different planning software; however, the differences found in inclination did not reach statistical significance (P = 0.463). In 19 of the 63 patients (30%), the version measurements between the systems were >5°, and in 29 of the 63 patients (46%), the inclination measurements between the systems were 5° or greater. In addition, 12 of the 63 patients (19%) had both version and inclination measurement differences of >5° between the systems. In total, they found that 35 of the 63 patients had at least 1 measurement that varied by >5° between the systems, and that in 15 patients (24%), 1 measurement varied by >10°. Their data demonstrate considerable variability in the preoperative measurements provided by different 3-D planning software systems, and that further study of each commercially available 3-D planning software system is needed to evaluate their accuracy.
CONCLUSION
Optimal outcomes following TSA and reverse TSA are dependent on proper implant position. Multiple studies have demonstrated improved accuracy in implant positioning with the use of patient-specific guides compared to that with traditional methods. Currently, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research had been done comparing the accuracy of each system’s 3-D planning software with each other and with standardized measurements of glenoid version and inclination. Further research is required to determine the accuracy of each commercially available 3-D planning software system as well as the clinical benefit of patient-specific guides in shoulder arthroplasty.
ABSTRACT
Optimal outcomes following total shoulder arthroplasty TSA and reverse shoulder arthroplasty RSA are dependent on proper implant position. Multiple cadaver studies have demonstrated improved accuracy of implant positioning with use of patient-specific guides/instrumentation compared to traditional methods. At this time, there are 3 commercially available single use patient-specific instrumentation systems and 1 commercially available reusable patient-specific instrumentation system. Currently though, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research has been done comparing the accuracy of each system’s 3-dimensional planning software. Future work is necessary to elucidate the ideal indications for the use of patient-specific guides and instrumentation, but it is likely, particularly in the setting of advanced glenoid deformity, that these systems will improve a surgeon's ability to put the implant in the best position possible.
Continue to: Optimal functional recovery...
Optimal functional recovery and implant longevity following both total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) depend, in large part, on proper placement of the glenoid component. Glenoid component malpositioning has an adverse effect on shoulder stability, range of motion (ROM), impingement, and glenoid implant longevity.
Traditionally, glenoid component positioning has been done manually by the surgeons based on their review of preoperative films and knowledge of glenoid anatomy. Anatomic studies have demonstrated high individual variability in the version of the native glenoid, thus making ideal placement of the initial glenoid guide pin difficult using standard guide pin guides.1
The following 2 methods have been described for improving the accuracy of glenoid guide pin insertion and subsequent glenoid implant placement: (1) computerized navigation and (2) patient-specific guides/instrumentation. Although navigated shoulder systems have demonstrated improved accuracy in glenoid placement compared with traditional methods, navigated systems require often large and expensive systems for implementation. The majority of them also require placement of guide pins or arrays on scapular bony landmarks, likely leading to an increase in operative time and possible iatrogenic complications, including fracture and pin site infections.
This review focuses on the use of patient-specific guides/instrumentation in shoulder arthroplasty. This includes the topic of proper glenoid and glenosphere placement as well as patient-specific guides/instrumentation and their accuracy.
GLENOID PLACEMENT
Glenohumeral osteoarthritis is the most common indication for TSA2 and commonly results in glenoid deformity. Using computed tomography (CT) scans of 45 arthritic shoulders and 19 normal shoulders, Mullaji and colleagues3 reported that the anteroposterior dimensions of the glenoid were increased by an average of 5 mm to 8 mm in osteoarthritic shoulders and by an average of 6 mm in rheumatoid arthritic shoulders compared to those in normal shoulders. A retrospective review of serial CT scans performed preoperatively on 113 osteoarthritic shoulders by Walch and colleagues4 demonstrated an average retroversion of 16°, and it has been the basis for the commonly used Walch classification of glenoid wear in osteoarthritis. Increased glenoid wear and increased glenoid retroversion make the proper restoration of glenoid version, inclination, and offset during shoulder arthroplasty more difficult and lead to increased glenoid component malpositioning.
Continue to: The ideal placement of the glenoid...
The ideal placement of the glenoid to maximize function, ROM, and implant longevity is in a mechanically neutral alignment with no superoinferior inclination1 and neutral version with respect to the transverse axis of the scapula.5
Improper glenoid positioning has an adverse effect on the functional results of shoulder arthroplasty. Yian and colleagues6 evaluated 47 cemented, pegged glenoids using standard radiography and CT scans at a mean follow-up of 40 months. They observed a significant correlation between increased glenoid component retroversion and lower Constant scores. Hasan and colleagues7 evaluated 139 consecutive patients who were dissatisfied with the result of their primary arthroplasty and found that 28% of them had at least 1 substantially malpositioned component identified either on radiography or during a revision surgery. They also found a significant correlation between stiffness, instability, and component malposition in their cohort.
Glenoid longevity is also dependent on proper component positioning, with the worst outcomes coming if the glenoid is malaligned with either superior or inferior inclination. Hasan and colleagues7 found that of their 74 patients with failed TSAs, 44 patients (59%) demonstrated mechanical loosening of their glenoid components either radiographically or during revision surgery, and 10 of their 44 patients with loose glenoids (23%) also had a malpositioned component. Using finite element analysis, Hopkins and colleagues8 analyzed the stresses through the cement mantle in glenoid prostheses that were centrally aligned, superiorly inclined, inferiorly inclined, anteverted, and retroverted. They found that malalignment of the glenoid increases the stresses through the cement mantle, leading to increased likelihood of mantle failure compared to that of centrally aligned glenoids, especially if there is malalignment with superior or inferior inclination or retroversion.
The accuracy of traditional methods of glenoid placement using an initial guide pin is limited and decreases with increasing amounts of glenoid deformity and retroversion. Iannotti and colleagues 9 investigated 13 patients undergoing TSA with an average preoperative retroversion of 13° and evaluated them using a 3-dimensional (3-D) surgical simulator. They found that the postoperative glenoid version was within 5° of ideal version in only 7 of their 13 patients (54%) and within 10° of ideal version in only 10 of their 13 patients (77%). In their study, the ideal version was considered to be the version as close to perpendicular to the plane of the scapula as possible with complete contact of the back side of the component on glenoid bone and maintenance of the center peg of the component within bone. In addition, they found that of their 7 patients with preoperative retroversion >10°, only 1 patient (14%) had a postoperative glenoid with <10° of retroversion with regard to the plane of the scapula and that all 6 of their patients with preoperative glenoid retroversion of <10° had a postoperative glenoid version of <10°.
Preoperative CT scans are much more accurate at determining glenoid version and thus how much glenoid correction is required to reestablish neutral version than plain radiography. Nyffeler and colleagues10 compared CT scans with axillary views for comparing glenoid version in 25 patients with no shoulder prosthesis present and 25 patients with a TSA in place. They found that glenoid retroversion was overestimated on plain radiographs in 86% of their patients with an average difference between CT and plain radiography of 6.4° and a maximum difference of 21°. They also found poor interobserver reliability in the plain radiography group and good interobserver reliability in the CT group, with coefficients of correlation of 0.77 for the plain radiography group and 0.93 for the CT group. Thus, they concluded that glenoid version cannot be accurately measured by plain radiography and that CT should be used. Hoenecke and colleagues11 subsequently evaluated 33 patients scheduled for TSA and found that CT version measurements made on 2-dimensional (2-D) CT slices compared with 3-D-reconstructed models of the same CT slices differed by an average of 5.1° because the axial CT slices were most often made perpendicular to the axis of the patient’s torso and not perpendicular to the body of the scapula. Accurate version assessment is critically important in planning for the degree of correction required to restore neutral glenoid version, and differences of 6.4° between CT assessment and plain radiography, and 5.1° between 2-D and 3-D CT scan assessments may lead to inadequate version correction intraoperatively and inferior postoperative results.
Continue to: GLENOSPHERE PLACEMENT
GLENOSPHERE PLACEMENT
The most common indication for reverse TSA is rotator cuff arthropathy characterized by rotator cuff dysfunction and end-stage glenohumeral arthritis.12 These patients require accurate and reproducible glenoid placement to optimize their postoperative range of motion and stability and minimize scapular notching.
Ideal glenosphere placement is the location and orientation that maximizes impingement-free ROM and stability while avoiding notching. Individual patient anatomy determines ideal placement; however, several guidelines for placement include inferior translation on the glenoid with neutral to inferior inclination. Gutiérrez and colleagues13 developed a computer model to assess the hierarchy of surgical factors affecting the ROM after a reverse TSA. They found that lateralizing the center of rotation gave the largest increase in impingement-free abduction, followed closely by inferior translation of the glenosphere on the glenoid.
Avoiding scapular notching is also a very important factor in ideal glenosphere placement. Scapular notching can be described as impingement of the humeral cup against the scapular neck during arm adduction and/or humeral rotation. Gutiérrez and colleagues13 also found that decreasing the neck shaft angle to create a more varus proximal humerus was the most important factor in increasing the impingement-free adduction. Roche and colleagues14 reviewed the radiographs of 151 patients who underwent primary reverse TSA at a mean follow-up of 28.3 months postoperatively; they found that 13.2% of their patients had a notch and that, on average, their patients who had no scapular notch had significantly more inferior glenosphere overhang than those who had a scapular notch. Poon and colleagues15 found that a glenosphere overhang of >3.5 mm prevented notching in their randomized control trial comparing concentrically and eccentrically placed glenospheres. Multiple other studies have demonstrated similar results and recommended inferior glenoid translation and inferior glenoid inclination to avoid scapular notching.16,17 Lévigne and colleagues18 retrospectively reviewed 337 reverse TSAs and observed a correlation between scapular notching and radiolucencies around the glenosphere component, with 14% of patients with scapular notching displaying radiolucencies vs 4% of patients without scapula notching displaying radiolucencies.
Several studies have also focused on the ideal amount of inferior glenoid inclination to maximize impingement-free ROM. Li and colleagues17 performed a computer simulation study on the Comprehensive Reverse Shoulder System (Zimmer Biomet) to determine impingement-free internal and external ROM with varying amounts of glenosphere offset, translation, and inclination. They found that progressive glenosphere inferior inclination up to 30° improved impingement-free rotational ROM at all degrees of scaption. Gutiérrez and colleagues19 used computer modeling to compare concentrically placed glenospheres in neutral inclination with eccentrically placed glenospheres in varying degrees of inclination. They found that the lowest forces across the baseplate occurred in the lateralized and inferiorly inclined glenospheres, and the highest forces occurred in the lateralized and superiorly inclined glenospheres. Together, these studies show that inferior glenoid inclination increases impingement-free ROM and, combined with lateralization, may result in improved glenosphere longevity due to significantly decreased forces at the RSA glenoid baseplate when compared to that at superiorly inclined glenoids.
The ideal amount of mediolateral glenosphere offset has not been well defined. Grammont design systems place the center of rotation of the glenosphere medial to the glenoid baseplate together with valgus humeral component neck shaft angles of around 155°. These design elements are believed to decrease shear stresses through the glenoid baseplate to the glenoid interface and improve shoulder stability, but they are also associated with reduced impingement-free ROM and increased rates of scapular notching.13 This effect is accentuated in patients with preexisting glenoid bone loss and/or congenitally short scapular necks that further medialize the glenosphere. Medialization of the glenosphere may also shorten the remaining rotator cuff muscles and result in decreased implant stability and external rotation strength. Several implant systems have options to vary the amount of lateral offset. The correct amount of lateral offset for each patient requires the understanding that improving patients’ impingement-free ROM by increasing the amount of lateral offset comes at the price of increasing the shear forces experienced by the interface between the glenoid baseplate and the glenoid. As glenoid fixation technology improves increased lateralization of glenospheres without increased rates of glenoid baseplate, loosening should improve the ROM after reverse TSA.
Continue to: Regardless of the intraoperative goals...
Regardless of the intraoperative goals for placement and orientation of the glenosphere components, it is vitally important to accurately and consistently meet those goals for achieving optimal patient outcomes. Verborgt and colleagues20 implanted 7 glenospheres in cadaveric specimens without any glenohumeral arthritis using standard techniques to evaluate the accuracy of glenosphere version and inclination. Their goal was to place components in neutral version and with 10° of inferior inclination. Their average glenoid version postoperatively was 8.7° of anteversion, and their average inclination was 0.9° of superior inclination. Throckmorton and colleagues21 randomized 35 cadaveric shoulders to receive either an anatomic or a reverse total shoulder prosthesis from high-, mid-, and low-volume surgeons. They found that components placed using traditional guides averaged 6° of deviation in version and 5° of deviation in inclination from their target values, with no significant differences between surgeons of different volumes.
PATIENT-SPECIFIC GUIDES/INSTRUMENTATION
Patient-specific guides/instrumentation and intraoperative navigation are the 2 techniques that have been used to improve the accuracy of glenoid and glenosphere placement. Both techniques require the use of high-resolution CT scans and computer software to determine the proper position for glenoid or glenosphere placement based on the patient’s individual anatomy. Patient-specific guides and instrumentation use the data acquired from a CT scan to generate a preoperative plan for the location and orientation of the glenoid baseplate. Once the surgeon approves the preoperative plan, a patient-specific guide is created using the patient’s glenoid as a reference for the location and orientation of the central guide pin. The location of the central guide pin on the glenoid determines the center of the glenoid baseplate, and the guide pin’s orientation determines the version and inclination of the glenoid or the glenosphere. Once the guide pin is placed in the glenoid, the remainder of the glenoid implantation uses the guide pin as a reference, and, in that way, patient-specific guides control the orientation of the glenoid at the time of surgery.
Intraoperative navigation uses an optical tracking system to determine the location and orientation of the central guide pin. Navigation systems require intraoperative calibration of the optical tracking system before they can track the location of implantation relative to bony landmarks on the patient’s scapula. Their advantage over patient-specific instrumentation (PSI) is that they do not require the manufacture of a custom guide; however, they may add significantly increased cost and surgical time due to the need for calibration prior to use and the cost of the navigation system along with any disposable components associated with it. Kircher and colleagues22 performed a prospective randomized clinical study of navigation-aided TSA compared with conventional TSA and found that operating time was significantly increased for the navigated group with an average operating room time of 169.5 minutes compared to 138 minutes for the conventional group. They also found that navigation had to be abandoned in 37.5% of their navigated patients due to technical errors during glenoid referencing.
COMMERCIAL PATIENT-SPECIFIC INSTRUMENTATION SYSTEMS
The 2 types of PSI that are currently available are single-use PSI and reusable PSI. The single-use PSI involves the fabrication of unique guides based on surgeon-approved preoperative plans generated by computer-software-processed preoperative CT scans. The guides are fabricated to rest on the glenoid articular surface and direct the guide pin to the correct location and in the correct direction to place the glenoid baseplate in the desired position with the desired version and inclination. Most of these systems also provide a 3-D model of the patient’s glenoid so that surgeons can visualize glenoid deformities and the correct guide placement on the glenoid. Single-use PSI systems are available from DJO Global, Wright Medical Group, and Zimmer Biomet. The second category of PSI is reusable and is available from Arthrex. The guide pin for this system is adjusted to fit individual patient anatomy and guide the guide pin into the glenoid in a location and orientation preplanned on the CT-scan-based computer software or using a 3-D model of the patient’s glenoid (Table).
Table. Details of Available Patient-Specific Instrumentation Systems
| System | Manufacturer | Single-Use/Reusable | Guides |
| MatchPoint System | DJO Global | Single-use | Central guide pin |
| Blueprint 3D Planning + PSI | Wright Medical Group | Single-use | Central guide pin |
| Zimmer Patient Specific Instruments Shoulder | Zimmer Biomet | Single-use | Central guide pin, reaming guide, roll guide, screw drill guide |
| Virtual Implant Positioning System | Arthrex | Reusable | Central guide pin |
The DJO Global patient-specific guide is termed as the MatchPoint System. This system creates 3-D renderings of the scapula and allows the surgeon to manipulate the glenoid baseplate on the scapula. The surgeon chooses the glenoid baseplate, location, version, and inclination on the computerized 3-D model. The system then fabricates a guide pin matching the computerized template that references the patient’s glenoid surface with a hook to orient it against the coracoid. A 3-D model of the glenoid is also provided along with the customized guide pin.
Continue to: Blueprint 3D Planning + PSI...
Blueprint 3D Planning + PSI (Wright Medical Group) allows custom placement of the glenoid version, inclination, and position on computerized 3-D models of the patient’s scapula. This PSI references the glenoid with 4 feet that captures the edge of the patient’s glenoid at specific locations and is unique because it allows the surgeon to control where on the glenoid edge to 4 feet contact as long as 1 foot is placed on the posterior edge of the glenoid and the remaining 3 feet are placed on the anterior edge of the glenoid. A 3-D model of the glenoid is also provided with this guide.
The Zimmer Biomet patient-specific guide is termed as the Zimmer Patient Specific Instruments Shoulder. Its computer software allows custom placement of the glenoid as well, but it also includes computerized customization of the reaming depth, screw angles, and screw lengths to optimize fixation. Their system includes a central guide pin to set the glenoid baseplate’s location and orientation, a reaming guide to control reaming depth and direction, a roll guide to control the glenoid baseplate’s rotation, and a drill guide to control the screw direction. They also provide a 3-D model of the glenoid.

The Arthrex Virtual Implant Positioning (VIP) System is similar to other systems in that its 3-D planning software is based on CT images uploaded by the surgeon. The unique aspect of this system is that the guide pin is adjusted by the surgeon for each individual patient based on instructions generated by the planning software; however, after use, the instruments are resterilized and reused on subsequent patients (Figures 1A, 1B). In this manner, their instruments are reusable and allow custom adjustment for each patient with the ability to set the pin location and glenoid version in a patient-specific manner. This has the potential benefit of keeping costs down. For more complex deformity cases, the Arthrex VIP System can also 3-D-print a sterile model of the glenoid to help surgeons appreciate the deformity better (Figure 2).

DATA ON PATIENT-SPECIFIC INSTRUMENTS
Several studies have measured the accuracy of patient-specific guides and have compared the accuracy of patient-specific guides to that of traditional methods. Levy and colleagues23 investigated the accuracy of single-use patient-specific guides compared to that of preoperative plans. They used patient-specific guides on 14 cadaveric shoulders based on plans developed by virtual preoperative 3-D planning system using CT images. Once the guide pin was drilled using the patient-specific guide, they obtained a second CT scan to compare the accuracy of the patient-specific guide to the surgical plan generated preoperatively. They found that the translational accuracy of the starting point for the guide pin averaged 1.2 mm ± 0.7 mm, the accuracy of the inferior inclination was 1.2° ± 1.2°, and the accuracy of the glenoid version was 2.6° ± 1.7°. They concluded that patient-specific guides were highly accurate in reproducing the starting point, inclination, and version set on preoperative guides.
Walch and colleagues24 subsequently performed a similar study using 15 cadaveric scapulae without any other shoulder soft tissue or bone attached. They also used CT-scan-based 3-D planning software to plan their glenoid placement with a subsequently fabricated single-use patient-specific guide used to place a guide pin. They obtained a second CT scan after guide pin implantation and compared the preoperative plan with the subsequent guide pin. They found a mean entry point position error of 1.05 mm ± 0.31 mm, a mean inclination error of 1.42° ± 1.37°, and a mean version error of 1.64° ± 1.01°.
Continue to: Throckmorton and colleagues...
Throckmorton and colleagues21 used 70 cadaveric shoulders with radiographically confirmed arthritis and randomized them to undergo either anatomic or reverse TSA using either a patient-specific guide or standard instrumentation. Postoperative CT scans were used to evaluate the glenoid inclination, version, and starting point. They found that glenoid components implanted using patient-specific guides were more accurate than those placed using traditional instrumentation. The average deviation from intended inclination was 3° for patient-specific guides and 7° for traditional instrumentation, the average deviation from intended version was 5° for patient-specific guides and 8° for traditional instrumentation, and the average deviation in intended starting point was 2 mm for patient-specific guides and 3 mm for traditional instrumentation. They also analyzed significantly malpositioned components as defined by a variation in version or inclination of >10° or >4 mm in starting point. They found that 6 of their 35 glenoids using patient-specific guides were significantly malpositioned compared to 23 of 35 glenoids using traditional instrumentation. They concluded that patient-specific guides were more accurate and reduced the number of significantly malpositioned implants when compared with traditional instrumentation.
Early and colleagues25 analyzed the effect of severe glenoid bone defects on the accuracy of patient-specific guides compared with traditional guides. Using 10 cadaveric shoulders, they created anterior, central, or posterior glenoid defects using a reamer and chisel to erode the bone past the coracoid base. Subsequent CT scans were performed on the specimens, and patient-specific guides were fabricated and used for reverse TSA in 5 of the 10 specimens. A reverse TSA was performed using traditional instrumentation in the remaining 5 specimens. They found that the average deviation in inclination and version from preoperative plan was more accurate in the patient-specific guide cohort than that in the traditional instrument cohort, with an average deviation in inclination and version of 1.2° ± 1.2° and 1.8° ± 1.2° respectively for the cohort using patient-specific instruments vs 2.8° ± 1.8° and 3.5° ± 3° for the cohort using traditional instruments. They also found that their total bone screw lengths were longer in the patient-specific guide group than those in the traditional group, with screws averaging 52% of preoperatively planned length in the traditional instrument cohort vs 89% of preoperatively planned length in the patient-specific instrument cohort.
Gauci and colleagues26 measured the accuracy of patient-specific guides in vivo in 17 patients receiving TSA. Preoperative CT scans were used to fabricate patient-specific guides, and postoperative CT scans were used to measure version, inclination, and error of entry in comparison with the templated goals used to create patient-specific guides. They found a mean error in version and inclination of 3.4° and 1.8°, respectively, and a mean error in entry of 0.9 mm of translation on the glenoid. Dallalana and colleagues27 performed a very similar study on 20 patients and found a mean deviation in glenoid version of 1.8° ± 1.9°, a mean deviation in glenoid inclination of 1.3° ± 1.0°, a mean translation in anterior-posterior plane of 0.5 mm ± 0.3 mm, and a mean translation in the superior-inferior plane of 0.8 mm ± 0.5 mm.
Hendel and colleagues28 performed a randomized prospective clinical trial comparing patient-specific guides with traditional methods for glenoid insertion. They randomized 31 patients to receive a glenoid implant using either a patient-specific guide or traditional methods and compared glenoid retroversion and inclination with their preoperative plan. They found an average version deviation of 6.9° in the traditional method cohort and 4.3° in the patient-specific guide cohort. Their average deviation in inclination was 11.6° in the traditional method cohort and 2.9° in the patient-specific guide cohort. For patients with preoperative retroversion >16°, the average deviation was 10° in the standard surgical cohort and 1.2° in the patient-specific instrument cohort. Their data suggest that increasing preoperative retroversion leads to an increased version variation from preoperative plan.
Iannotti and colleagues29 randomly assigned 46 patients to preoperatively undergo either CT scan with 3-D templating of glenoid component without patient-specific guide fabrication or CT scan with 3-D templating and patient-specific guide fabrication prior to receiving a TSA. They recorded the postoperative inclination and version for each patient and compared them to those of a nonrandomized control group of 17 patients who underwent TSA using standard instrumentation. They found no difference between the cohorts with or without patient-specific guide use with regard to implant location, inclination, or version; however, they did find a difference between the combined 3-D templating cohort compared with their standard instrumentation cohort. They concluded that 3-D templating significantly improved the surgeons’ ability to correctly position the glenoid component with or without the fabrication and the use of a patient-specific guide.
Continue to: Denard and colleagues...
Denard and colleagues30 compared the preoperative glenoid version and inclination measurements obtained using the Blueprint 3D Planning + PSI software and the VIP System 3D planning software. They analyzed the preoperative CT scans of 63 consecutive patients undergoing either TSA or reverse TSA using both the Blueprint and the VIP System 3D planning software and compared the resulting native glenoid version and inclination measured by the software. They found a statistically significant difference (P = 0.04) in the version measurements provided by the different planning software; however, the differences found in inclination did not reach statistical significance (P = 0.463). In 19 of the 63 patients (30%), the version measurements between the systems were >5°, and in 29 of the 63 patients (46%), the inclination measurements between the systems were 5° or greater. In addition, 12 of the 63 patients (19%) had both version and inclination measurement differences of >5° between the systems. In total, they found that 35 of the 63 patients had at least 1 measurement that varied by >5° between the systems, and that in 15 patients (24%), 1 measurement varied by >10°. Their data demonstrate considerable variability in the preoperative measurements provided by different 3-D planning software systems, and that further study of each commercially available 3-D planning software system is needed to evaluate their accuracy.
CONCLUSION
Optimal outcomes following TSA and reverse TSA are dependent on proper implant position. Multiple studies have demonstrated improved accuracy in implant positioning with the use of patient-specific guides compared to that with traditional methods. Currently, there are no studies comparing the clinical outcomes of patient-specific guides to those of traditional methods of glenoid placement, and limited research had been done comparing the accuracy of each system’s 3-D planning software with each other and with standardized measurements of glenoid version and inclination. Further research is required to determine the accuracy of each commercially available 3-D planning software system as well as the clinical benefit of patient-specific guides in shoulder arthroplasty.
1. Churchill RS, Brems JJ, Kotschi H. Glenoid size, inclination, and version: an anatomic study. J Shoulder Elbow Surg. 2001;10(4):327-332. doi:10.1067/mse.2001.115269.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135. doi:10.1067/mse.2002.121146.
3. Mullaji AB, Beddow FH, Lamb GH. CT measurement of glenoid erosion in arthritis. J Bone Joint Surg Br. 1994;76(3):384-388.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. RJ Friedman, KB Hawthorne, BM Genez. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg. 1992;74(7):1032-1037. doi:10.2106/00004623-199274070-00009.
6. Yian EH, Werner CM, Nyffeler RW, et al. Radiographic and computed tomography analysis of cemented pegged polyethylene glenoid components in total shoulder replacement. J Bone Joint Surg. 2005;87(9):1928-1936. doi:10.2106/00004623-200509000-00004.
7. Hasan SS, Leith JM, Campbell B, Kapil R, Smith KL, Matsen FA. Characteristics of unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2002;11(5):431-441.
8. Hopkins AR, Hansen UN, Amis AA, Emery R. The effects of glenoid component alignment variations on cement mantle stresses in total shoulder arthroplasty. J Shoulder Elbow Surg. 2004;13(6):668-675. doi:10.1016/S1058274604001399.
9. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55. doi:10.1016/j.jse.2011.02.011.
10. Nyffeler RW, Jost B, Pfirrmann CWA, Gerber C. Measurement of glenoid version: conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496. doi:10.1016/S1058274603001812.
11. Hoenecke HR, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(2):166-171. doi:10.1016/j.jse.2009.08.009.
12. Wall B, Nové-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485. doi:10.2106/JBJS.F.00666.
13. Gutiérrez S, Comiskey 4, Charles A, Luo Z, Pupello DR, Frankle MA. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am. 2008;90(12):2606-2615. doi:10.2106/JBJS.H.00012.
14. Roche CP, Marczuk Y, Wright TW, et al. Scapular notching and osteophyte formation after reverse shoulder replacement: Radiological analysis of implant position in male and female patients. Bone Joint J. 2013;95-B(4):530-535. doi:10.1302/0301-620X.95B4.30442.
15. Poon PC, Chou J, Young SW, Astley T. A comparison of concentric and eccentric glenospheres in reverse shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(16):e138. doi:10.2106/JBJS.M.00941.
16. Nyffeler RW, Werner CML, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528. doi:10.1016/j.jse.2004.09.010.
17. Li X, Knutson Z, Choi D, et al. Effects of glenosphere positioning on impingement-free internal and external rotation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(6):807-813. doi:10.1016/j.jse.2012.07.013.
18. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935. doi:10.1016/j.jse.2008.02.010.
19. Gutiérrez S, Walker M, Willis M, Pupello DR, Frankle MA. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(5):732-739. doi:10.1016/j.jse.2010.10.035.
20. Verborgt O, De Smedt T, Vanhees M, Clockaerts S, Parizel PM, Van Glabbeek F. Accuracy of placement of the glenoid component in reversed shoulder arthroplasty with and without navigation. J Shoulder Elbow Surg. 2011;20(1):21-26. doi:10.1016/j.jse.2010.07.014.
21. Throckmorton TW, Gulotta LV, Bonnarens FO, et al. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: A multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg. 2015;24(6):965-971. doi:10.1016/j.jse.2014.10.013.
22. Kircher J, Wiedemann M, Magosch P, Lichtenberg S, Habermeyer P. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: a prospective-randomized clinical study. J Shoulder Elbow Surg. 2009;18(4):515-520. doi:10.1016/j.jse.2009.03.014.
23. Levy JC, Everding NG, Frankle MA, Keppler LJ. Accuracy of patient-specific guided glenoid baseplate positioning for reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(10):1563-1567. doi:10.1016/j.jse.2014.01.051.
24. Walch G, Vezeridis PS, Boileau P, Deransart P, Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg. 2015;24(2):302-309. doi:10.1016/j.jse.2014.05.029.
25. Eraly K, Stoffelen D, Vander Sloten J, Jonkers I, Debeer P. A patient-specific guide for optimizing custom-made glenoid implantation in cases of severe glenoid defects: an in vitro study. J Shoulder Elbow Surg. 2016;25(5):837-845. doi:10.1016/j.jse.2015.09.034.
26. Gauci MO, Boileau P, Baba M, Chaoui J, Walch G. Patient-specific glenoid guides provide accuracy and reproducibility in total shoulder arthroplasty. Bone Joint J. 2016;98-B(8):1080-1085. doi:10.1302/0301-620X.98B8.37257.
27. Dallalana RJ, McMahon RA, East B, Geraghty L. Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty. Int J Shoulder Surg. 2016;10(2):59-66. doi:10.4103/09736042.180717.
28. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg. 2012;94(23):2167-2175. doi:10.2106/JBJS.K.01209.
29. Iannotti JP, Weiner S, Rodriguez E, et al. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Joint Surg. 2015;97(8):651-658. doi:10.2106/JBJS.N.00493.
30. Denard PJ, Provencher MT, Lädermann A, Romeo AA, Dines JS. Version and inclination obtained with 3D planning in total shoulder arthroplasty: do different programs produce the same results? SECEC-ESSSE Congress, Berlin 2017. 2017.
1. Churchill RS, Brems JJ, Kotschi H. Glenoid size, inclination, and version: an anatomic study. J Shoulder Elbow Surg. 2001;10(4):327-332. doi:10.1067/mse.2001.115269.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135. doi:10.1067/mse.2002.121146.
3. Mullaji AB, Beddow FH, Lamb GH. CT measurement of glenoid erosion in arthritis. J Bone Joint Surg Br. 1994;76(3):384-388.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. RJ Friedman, KB Hawthorne, BM Genez. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg. 1992;74(7):1032-1037. doi:10.2106/00004623-199274070-00009.
6. Yian EH, Werner CM, Nyffeler RW, et al. Radiographic and computed tomography analysis of cemented pegged polyethylene glenoid components in total shoulder replacement. J Bone Joint Surg. 2005;87(9):1928-1936. doi:10.2106/00004623-200509000-00004.
7. Hasan SS, Leith JM, Campbell B, Kapil R, Smith KL, Matsen FA. Characteristics of unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2002;11(5):431-441.
8. Hopkins AR, Hansen UN, Amis AA, Emery R. The effects of glenoid component alignment variations on cement mantle stresses in total shoulder arthroplasty. J Shoulder Elbow Surg. 2004;13(6):668-675. doi:10.1016/S1058274604001399.
9. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55. doi:10.1016/j.jse.2011.02.011.
10. Nyffeler RW, Jost B, Pfirrmann CWA, Gerber C. Measurement of glenoid version: conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496. doi:10.1016/S1058274603001812.
11. Hoenecke HR, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(2):166-171. doi:10.1016/j.jse.2009.08.009.
12. Wall B, Nové-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485. doi:10.2106/JBJS.F.00666.
13. Gutiérrez S, Comiskey 4, Charles A, Luo Z, Pupello DR, Frankle MA. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am. 2008;90(12):2606-2615. doi:10.2106/JBJS.H.00012.
14. Roche CP, Marczuk Y, Wright TW, et al. Scapular notching and osteophyte formation after reverse shoulder replacement: Radiological analysis of implant position in male and female patients. Bone Joint J. 2013;95-B(4):530-535. doi:10.1302/0301-620X.95B4.30442.
15. Poon PC, Chou J, Young SW, Astley T. A comparison of concentric and eccentric glenospheres in reverse shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(16):e138. doi:10.2106/JBJS.M.00941.
16. Nyffeler RW, Werner CML, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528. doi:10.1016/j.jse.2004.09.010.
17. Li X, Knutson Z, Choi D, et al. Effects of glenosphere positioning on impingement-free internal and external rotation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(6):807-813. doi:10.1016/j.jse.2012.07.013.
18. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935. doi:10.1016/j.jse.2008.02.010.
19. Gutiérrez S, Walker M, Willis M, Pupello DR, Frankle MA. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(5):732-739. doi:10.1016/j.jse.2010.10.035.
20. Verborgt O, De Smedt T, Vanhees M, Clockaerts S, Parizel PM, Van Glabbeek F. Accuracy of placement of the glenoid component in reversed shoulder arthroplasty with and without navigation. J Shoulder Elbow Surg. 2011;20(1):21-26. doi:10.1016/j.jse.2010.07.014.
21. Throckmorton TW, Gulotta LV, Bonnarens FO, et al. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: A multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg. 2015;24(6):965-971. doi:10.1016/j.jse.2014.10.013.
22. Kircher J, Wiedemann M, Magosch P, Lichtenberg S, Habermeyer P. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: a prospective-randomized clinical study. J Shoulder Elbow Surg. 2009;18(4):515-520. doi:10.1016/j.jse.2009.03.014.
23. Levy JC, Everding NG, Frankle MA, Keppler LJ. Accuracy of patient-specific guided glenoid baseplate positioning for reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(10):1563-1567. doi:10.1016/j.jse.2014.01.051.
24. Walch G, Vezeridis PS, Boileau P, Deransart P, Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg. 2015;24(2):302-309. doi:10.1016/j.jse.2014.05.029.
25. Eraly K, Stoffelen D, Vander Sloten J, Jonkers I, Debeer P. A patient-specific guide for optimizing custom-made glenoid implantation in cases of severe glenoid defects: an in vitro study. J Shoulder Elbow Surg. 2016;25(5):837-845. doi:10.1016/j.jse.2015.09.034.
26. Gauci MO, Boileau P, Baba M, Chaoui J, Walch G. Patient-specific glenoid guides provide accuracy and reproducibility in total shoulder arthroplasty. Bone Joint J. 2016;98-B(8):1080-1085. doi:10.1302/0301-620X.98B8.37257.
27. Dallalana RJ, McMahon RA, East B, Geraghty L. Accuracy of patient-specific instrumentation in anatomic and reverse total shoulder arthroplasty. Int J Shoulder Surg. 2016;10(2):59-66. doi:10.4103/09736042.180717.
28. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg. 2012;94(23):2167-2175. doi:10.2106/JBJS.K.01209.
29. Iannotti JP, Weiner S, Rodriguez E, et al. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Joint Surg. 2015;97(8):651-658. doi:10.2106/JBJS.N.00493.
30. Denard PJ, Provencher MT, Lädermann A, Romeo AA, Dines JS. Version and inclination obtained with 3D planning in total shoulder arthroplasty: do different programs produce the same results? SECEC-ESSSE Congress, Berlin 2017. 2017.
TAKE-HOME POINTS
- Optimal outcomes following TSA and RSA are dependent on proper implant position.
- Patient-specific guides/instrumentation result in improved accuracy of implant positioning compared to traditional methods.
- Currently, there are no clinical studies demonstrating superiority of patient-specific guide/instrumentation use on patient outcomes.
- At this time there are 3 commercially available single use patient-specific instrumentation systems (DJO Global, Wright Medical Group, and Zimmer Biomet) and 1 commercially available reusable patient-specific instrumentation system (Arthrex).
- Limited research is available comparing the accuracy of different commercially available 3-D planning systems.