User login
Poor Sleep, Negative Attitude, Amplify Pain in Knee Osteoarthritis
Patients with knee osteoarthritis (OA) who have poor sleep habits display greater central sensitization of pain, according to a study published online ahead of print June 4 in Arthritis Care & Research. Study findings also showed that OA patients who catastrophize had increased central sensitization that was associated with greater pain.
“Our study is the largest and most comprehensive examination of the relationship between sleep disturbance, catastrophizing, and central sensitization in knee OA,” stated lead author Claudia Campbell, PhD, an Associate Professor of Psychiatry and Behavioral Sciences at Johns Hopkins University School of Medicine in Baltimore.
The case-controlled study included 208 participants who were categorized according to 4 groups: patients who have OA and insomnia, patients who have OA and normal sleep habits, healthy controls with insomnia, and healthy controls without a pain syndrome and normal sleep. In all, 72% of the study’s participants were female.
Participants completed multimodal sleep assessments (eg, questionnaire, diary, actigraphy, and polysmnography) and extensive evaluation of pain using clinical measures and quantitative sensory testing to evaluate associations between central sensitization, catastrophizing, and insomnia.
Results showed that the participants with knee OA and insomnia had the greatest amount of central sensitization compared with controls. The team found patients with poor sleep and high catastrophizing scores reported increased levels of central sensitization. In turn, central sensitization was significantly associated with increased clinical pain.
“While no causal processes may be determined from this study, our data suggest that those with low sleep efficiency and higher catastrophizing have the greatest central sensitization. Understanding the intricate relationship between sleep, central sensitization, and catastrophizing has important clinical implications for treating those with chronic pain conditions such as knee OA,” Dr. Campbell stated.
Suggested Reading
Campbell CM, Buenaver LF, Finan P, et al. Sleep, pain catastrophizing and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res. 2015 June 4. [Epub ahead of print]
Patients with knee osteoarthritis (OA) who have poor sleep habits display greater central sensitization of pain, according to a study published online ahead of print June 4 in Arthritis Care & Research. Study findings also showed that OA patients who catastrophize had increased central sensitization that was associated with greater pain.
“Our study is the largest and most comprehensive examination of the relationship between sleep disturbance, catastrophizing, and central sensitization in knee OA,” stated lead author Claudia Campbell, PhD, an Associate Professor of Psychiatry and Behavioral Sciences at Johns Hopkins University School of Medicine in Baltimore.
The case-controlled study included 208 participants who were categorized according to 4 groups: patients who have OA and insomnia, patients who have OA and normal sleep habits, healthy controls with insomnia, and healthy controls without a pain syndrome and normal sleep. In all, 72% of the study’s participants were female.
Participants completed multimodal sleep assessments (eg, questionnaire, diary, actigraphy, and polysmnography) and extensive evaluation of pain using clinical measures and quantitative sensory testing to evaluate associations between central sensitization, catastrophizing, and insomnia.
Results showed that the participants with knee OA and insomnia had the greatest amount of central sensitization compared with controls. The team found patients with poor sleep and high catastrophizing scores reported increased levels of central sensitization. In turn, central sensitization was significantly associated with increased clinical pain.
“While no causal processes may be determined from this study, our data suggest that those with low sleep efficiency and higher catastrophizing have the greatest central sensitization. Understanding the intricate relationship between sleep, central sensitization, and catastrophizing has important clinical implications for treating those with chronic pain conditions such as knee OA,” Dr. Campbell stated.
Patients with knee osteoarthritis (OA) who have poor sleep habits display greater central sensitization of pain, according to a study published online ahead of print June 4 in Arthritis Care & Research. Study findings also showed that OA patients who catastrophize had increased central sensitization that was associated with greater pain.
“Our study is the largest and most comprehensive examination of the relationship between sleep disturbance, catastrophizing, and central sensitization in knee OA,” stated lead author Claudia Campbell, PhD, an Associate Professor of Psychiatry and Behavioral Sciences at Johns Hopkins University School of Medicine in Baltimore.
The case-controlled study included 208 participants who were categorized according to 4 groups: patients who have OA and insomnia, patients who have OA and normal sleep habits, healthy controls with insomnia, and healthy controls without a pain syndrome and normal sleep. In all, 72% of the study’s participants were female.
Participants completed multimodal sleep assessments (eg, questionnaire, diary, actigraphy, and polysmnography) and extensive evaluation of pain using clinical measures and quantitative sensory testing to evaluate associations between central sensitization, catastrophizing, and insomnia.
Results showed that the participants with knee OA and insomnia had the greatest amount of central sensitization compared with controls. The team found patients with poor sleep and high catastrophizing scores reported increased levels of central sensitization. In turn, central sensitization was significantly associated with increased clinical pain.
“While no causal processes may be determined from this study, our data suggest that those with low sleep efficiency and higher catastrophizing have the greatest central sensitization. Understanding the intricate relationship between sleep, central sensitization, and catastrophizing has important clinical implications for treating those with chronic pain conditions such as knee OA,” Dr. Campbell stated.
Suggested Reading
Campbell CM, Buenaver LF, Finan P, et al. Sleep, pain catastrophizing and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res. 2015 June 4. [Epub ahead of print]
Suggested Reading
Campbell CM, Buenaver LF, Finan P, et al. Sleep, pain catastrophizing and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res. 2015 June 4. [Epub ahead of print]
FORCE-TJR Now Certified as CMS Qualified Clinical Data Registry
Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement and Quality Improvement (FORCE-TJR), the most comprehensive national registry for total hip and knee joint replacement patients and their outcomes, is now certified as a Qualified Clinical Data Registry (QCDR).
In meeting QCDR requirements, FORCE-TJR has successfully collected and tracked more than 30,000 patients with total joint replacements across the US, in more than 150 provider institutions. The FORCE-TJR registry continues to expand, providing patient and disease tracking, implant performance, patient-reported outcomes, and quality monitoring of total joint replacements.
With QCDR certification, FORCE-TJR is able to complete the collection and submission of Physician Quality Reporting System (PQRS) quality measures on behalf of member hospitals and physicians, allowing FORCE-TJR members to avoid the 2016 payment adjustment of 2.0%.
“The value of being involved in a registry such as FORCE-TJR is that I can concentrate on my patient and my practice,” said Courtland Lewis, MD, Orthopedic Surgery Chief, Department of Orthopedics, at Hartford Hospital and core member of FORCE-TJR. “FORCE-TJR makes it easy to capture and report this data to QCDR and PQRS for incentive payments, internal quality monitoring, and improving the value of the care we provide to patients and insurance plans.”
As part of this certification, FORCE-TJR has developed new non-PQRS measures, which include:
• Pain and functional status assessment for hip and knee replacements
• Improvement in pain and function after hip and knee replacements
• Assessment and improvement on patients with osteoarthritis in the hip or knee
• Mental health assessment for patients who undergo hip and knee replacements
“The new QCDR designation allows FORCE-TJR to define new quality measures, including patient-reported outcomes, and to submit these data to Centers for Medicare and Medicaid Services (CMS) on behalf of our members—without any additional data collection. The data serve both their internal quality monitoring and meet the CMS mandate,” said Patricia Franklin, MD, FORCE-TJR’s registry director.
FORCE-TJR, originally a 4-year, national research project funded by the Agency for Healthcare Research and Quality (AHRQ), is the first registry for total joint replacement to identify risk-adjusted national benchmarks, including patient risk factors, and other clinical measures, to guide surgeon and patient decisions regarding timing of surgery and optimal patient selection.
FORCE-TJR is now serving as a comprehensive orthopedic registry, expanding to enroll surgeons and hospitals beyond the original Agency for Healthcare Research and Quality-funded cohort.
In addition to assisting with reporting requirements and securing quality incentive payments, the FORCE-TJR registry provides access to national TJR benchmarks, real-time patient-reported outcome scoring, comprehensive, comparative arthroplasty practice feedback and data to improve patient care, and compare performance to peer surgeons and institutions.
Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement and Quality Improvement (FORCE-TJR), the most comprehensive national registry for total hip and knee joint replacement patients and their outcomes, is now certified as a Qualified Clinical Data Registry (QCDR).
In meeting QCDR requirements, FORCE-TJR has successfully collected and tracked more than 30,000 patients with total joint replacements across the US, in more than 150 provider institutions. The FORCE-TJR registry continues to expand, providing patient and disease tracking, implant performance, patient-reported outcomes, and quality monitoring of total joint replacements.
With QCDR certification, FORCE-TJR is able to complete the collection and submission of Physician Quality Reporting System (PQRS) quality measures on behalf of member hospitals and physicians, allowing FORCE-TJR members to avoid the 2016 payment adjustment of 2.0%.
“The value of being involved in a registry such as FORCE-TJR is that I can concentrate on my patient and my practice,” said Courtland Lewis, MD, Orthopedic Surgery Chief, Department of Orthopedics, at Hartford Hospital and core member of FORCE-TJR. “FORCE-TJR makes it easy to capture and report this data to QCDR and PQRS for incentive payments, internal quality monitoring, and improving the value of the care we provide to patients and insurance plans.”
As part of this certification, FORCE-TJR has developed new non-PQRS measures, which include:
• Pain and functional status assessment for hip and knee replacements
• Improvement in pain and function after hip and knee replacements
• Assessment and improvement on patients with osteoarthritis in the hip or knee
• Mental health assessment for patients who undergo hip and knee replacements
“The new QCDR designation allows FORCE-TJR to define new quality measures, including patient-reported outcomes, and to submit these data to Centers for Medicare and Medicaid Services (CMS) on behalf of our members—without any additional data collection. The data serve both their internal quality monitoring and meet the CMS mandate,” said Patricia Franklin, MD, FORCE-TJR’s registry director.
FORCE-TJR, originally a 4-year, national research project funded by the Agency for Healthcare Research and Quality (AHRQ), is the first registry for total joint replacement to identify risk-adjusted national benchmarks, including patient risk factors, and other clinical measures, to guide surgeon and patient decisions regarding timing of surgery and optimal patient selection.
FORCE-TJR is now serving as a comprehensive orthopedic registry, expanding to enroll surgeons and hospitals beyond the original Agency for Healthcare Research and Quality-funded cohort.
In addition to assisting with reporting requirements and securing quality incentive payments, the FORCE-TJR registry provides access to national TJR benchmarks, real-time patient-reported outcome scoring, comprehensive, comparative arthroplasty practice feedback and data to improve patient care, and compare performance to peer surgeons and institutions.
Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement and Quality Improvement (FORCE-TJR), the most comprehensive national registry for total hip and knee joint replacement patients and their outcomes, is now certified as a Qualified Clinical Data Registry (QCDR).
In meeting QCDR requirements, FORCE-TJR has successfully collected and tracked more than 30,000 patients with total joint replacements across the US, in more than 150 provider institutions. The FORCE-TJR registry continues to expand, providing patient and disease tracking, implant performance, patient-reported outcomes, and quality monitoring of total joint replacements.
With QCDR certification, FORCE-TJR is able to complete the collection and submission of Physician Quality Reporting System (PQRS) quality measures on behalf of member hospitals and physicians, allowing FORCE-TJR members to avoid the 2016 payment adjustment of 2.0%.
“The value of being involved in a registry such as FORCE-TJR is that I can concentrate on my patient and my practice,” said Courtland Lewis, MD, Orthopedic Surgery Chief, Department of Orthopedics, at Hartford Hospital and core member of FORCE-TJR. “FORCE-TJR makes it easy to capture and report this data to QCDR and PQRS for incentive payments, internal quality monitoring, and improving the value of the care we provide to patients and insurance plans.”
As part of this certification, FORCE-TJR has developed new non-PQRS measures, which include:
• Pain and functional status assessment for hip and knee replacements
• Improvement in pain and function after hip and knee replacements
• Assessment and improvement on patients with osteoarthritis in the hip or knee
• Mental health assessment for patients who undergo hip and knee replacements
“The new QCDR designation allows FORCE-TJR to define new quality measures, including patient-reported outcomes, and to submit these data to Centers for Medicare and Medicaid Services (CMS) on behalf of our members—without any additional data collection. The data serve both their internal quality monitoring and meet the CMS mandate,” said Patricia Franklin, MD, FORCE-TJR’s registry director.
FORCE-TJR, originally a 4-year, national research project funded by the Agency for Healthcare Research and Quality (AHRQ), is the first registry for total joint replacement to identify risk-adjusted national benchmarks, including patient risk factors, and other clinical measures, to guide surgeon and patient decisions regarding timing of surgery and optimal patient selection.
FORCE-TJR is now serving as a comprehensive orthopedic registry, expanding to enroll surgeons and hospitals beyond the original Agency for Healthcare Research and Quality-funded cohort.
In addition to assisting with reporting requirements and securing quality incentive payments, the FORCE-TJR registry provides access to national TJR benchmarks, real-time patient-reported outcome scoring, comprehensive, comparative arthroplasty practice feedback and data to improve patient care, and compare performance to peer surgeons and institutions.
ICD-10 Race to the Finish: 8 High Priorities in the 11th Hour
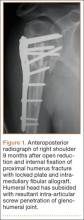
As late as mid-April 2015, a survey of 121 orthopedic practices indicated that 30% had done nothing to start preparing for ICD-10 (International Classification of Diseases, Tenth Revision).1 That’s scary. And even the practices that had begun to prepare had not completed a number of key tasks (Figure 1).
Certainly, the will-they-or-won’t-they possibility of another congressional delay had many practices sitting on their hands this year. But now that the October 1, 2015, implementation is set in stone, this lack of inertia has many practices woefully behind. If your practice is one of many that hasn’t mapped your common ICD-9 (International Classification of Diseases, Ninth Revision) codes to ICD-10 codes, completed payer testing, or attended training, it’s time for a “full-court press.”
Being unprepared for ICD-10 will cause more than just an increase in claim denials. If your surgery schedule is booked a few months out, your staff will need to pre-authorize cases using ICD-10 as early as August 1—and they won’t be able to do that if you haven’t dictated the clinical terms required to choose an ICD-10 code. Without an understanding of ICD-10, severity of illness coding will suffer, and that will affect your bundled and value-based payments. And, if you don’t provide an adequate diagnosis when sending patients off-site for physical therapy, you’ll soon be getting phone calls from their billing staff demanding more specifics.
The clock is ticking and time is short. Here’s a prioritized list of what needs to get done.
1. Generate an ICD-9 frequency report
Identifying which diagnosis codes are the most frequently used, and therefore drive a significant portion of practice revenue, is an absolute must. The data will help prioritize training and code-mapping activities.
Most practices generate Current Procedural Terminology (CPT) code-frequency reports regularly, but few have ever run an ICD-9 code-frequency report. Call your vendor and ask for assistance, as there are multiple ways to run this report and they vary by practice management system. Sort the data elements and generate the ICD-9 frequency report by:
- Primary diagnosis.
- Unique patient.
- Revenue. (If your practice management system can’t give you diagnosis data by revenue, which enables you to focus on the codes that generate the most revenue, generate it by charges.)
The result should be a report that identifies the 20 to 25 diagnosis codes (or charges, depending on the reports generated) that drive the most revenue for the practice. Use the data to focus and prioritize your training and code-mapping activities.
2. Schedule training
Forget about “general” ICD-10 training courses. You need orthopedic-specific guidance. That’s because ICD-10 for orthopedics is more complex than for other specialties. Dictating fractures under ICD-10 is not so simple. Selecting an injury code requires confidence in correctly using the seventh character.
“Everyone who uses diagnosis codes must have baseline knowledge: surgeons, billing staff, surgical coordinators, and clinical team,” according to Sarah Wiskerchen, MBA, CPC, consultant and ICD-10 educator with KarenZupko & Associates (KZA). Training must include the practical details of ICD-10, such as assigning laterality, understanding the system architecture, and limiting the use of unspecified codes.
The American Academy of Orthopaedic Surgeons (AAOS) offers a self-paced, online training series that provides details for the top 3 diagnosis codes for each subspecialty. The 10-program course, ICD-10-CM: By the Numbers, is available at www.aaos.org ($299 for members, $399 for nonmembers). If you prefer live instruction, there is one more AAOS-sponsored, regional ICD-10 workshop left before the October 1 deadline, and more may be added. (Details at www.karenzupko.com)
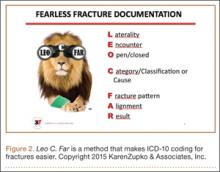
These courses provide highly specific and granular ICD-10 knowledge and incorporate the use of Code-X, an AAOS-developed software tool. They also feature tools for handling the complexities of fractures and injury codes, such as Leo C. Far, an acronym developed by KZA consultant and coding educator Margie Maley, BSN, MS, to make ICD-10 diagnosis coding for fractures easier (Figure 2).
Some subspecialty societies also offer ICD-10 training. The American Society for Surgery of the Hand (www.assh.org), for example, offers a series of webinars and member-developed ICD-9-to-ICD-10 code maps.
3. Crosswalk your common codes from ICD-9 to ICD-10
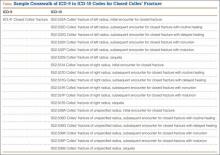
Crosswalking is the process of mapping your most commonly used ICD-9 codes to their equivalent ICD-10 codes. This exercise familiarizes your team with ICD-10 language and terms, and gives a sense of which ICD-9 codes expand to just 1 or 2 ICD-10 codes and which codes expand into 10 or more codes—as some injury codes do (Table).
“Attempting to map the codes before completing ICD-10 training is like trying to write a letter in Greek when you only speak English,” Wiskerchen warns. “So start this process after at least some of your team have grasped the fundamentals of ICD-10.” This is where the data from your ICD-9 frequency report comes in. Use it to prioritize which codes to map first with a goal of mapping your top 25 ICD-9 codes to their ICD-10 equivalents by August 31.
Invest in good tools to support your mapping efforts. Avoid general mapping equivalent (GEM) coding tools, which are free for a good reason—they are incomplete and don’t always lead you to the correct ICD-10 code. Instead, purchase resources from credible sources, such as the American Medical Association (AMA; www.ama-assn.org). The AMA publishes ICD-10-CM 2016: The Complete Official Codebook as well as ICD-10-CM Mappings 2016, which links ICD-9 codes to all valid ICD-10 alternatives. The AMA also offers electronic ICD-10-CM Express Reference Mapping Cards for multiple specialties.
Practice makes perfect and crosswalking from ICD-9 to ICD-10 is one of the best ways for your team to become aware of the nuances in the new coding system. Like learning a new language, “speaking” ICD-10 does not become automatic just because you’ve attended training or completed the coding maps. Training teaches the architecture of the new coding system. Mapping provides a structured way to become familiar with the codes the practice will use most often. Once these 2 primary pieces are understood and assimilated, most physicians find that dictating the necessary new terms becomes quite easy.
4. Conduct a gap analysis to identify the ICD-10 terms missing from each provider’s current documentation
Conduct the gap analysis after your team has completed training, and once you’ve at least begun the process of mapping codes from ICD-9 to ICD-10. Here’s how:
- Generate a CPT frequency report.
- Select the top 5 procedures for each physician.
- Pull 2 patients’ notes for each of the top procedures.
- Review the notes and try to select ICD-10 code(s).
If key ICD-10 terms are not included in current documentation, physicians should modify the templates or macros they rely on for dictation.
“This simple exercise makes it obvious which clinical information physicians must add for ICD-10,” Wiskerchen says. For example, if the patient had an arthroscopy, but the note doesn’t specify on which leg, that’s a clear indication that the physician must dictate laterality. “The gap analysis is a great way to coach physicians about the clinical details to document, so staff can bill under ICD-10,” Wiskerchen says.
5. Contact technology vendors
Given the number of new ICD-10 codes in orthopedics, paper cheat sheets will be obsolete. Instead, you’ll need to rely on pull-down menus and/or search fields in the electronic health record (EHR) and practice management systems.
“Get clarity about how the new features and workflow processes will work in your systems,” suggests Wiskerchen. “Ask questions such as: Which features will be added or changed to accommodate the new codes? Will there be new screens or pick lists for ICD-10, or search fields? How will new screens and features change our current workflow? And schedule any necessary training as soon as possible.”
In addition to software upgrades and training, vendors and clearinghouses offer an array of services to help practices make the transition. Some vendors even provide help coordinating your internal plan with their new product features and training. Contact vendors to find out what they offer.
6. Use completed code maps to build diagnosis code databases, EHR templates, charge tickets, pick lists, prompters, and other coding tools
“Provide the code crosswalks and results of your documentation gap analysis to the IT [information technology] team so they can get started,” Wiskerchen advises. “And assign a physician or midlevel provider to work with IT so that the tools are clinically accurate.”
7. Schedule testing with clearinghouses and payers
“Successful testing indicates that your hard work has paid off, and that claims will be processed with few, if any, ICD-10–related hiccups,” Wiskerchen says. Essentially, the testing confirms that your ICD-10 code database, pick lists, vendor features, and other coding fields are working properly. “Testing with a clearinghouse is good. Testing directly with the payer is even better, if you are a direct submitter and it is allowed,” Wiskerchen suggests. Contact your clearinghouse and/or payers for testing opportunities prior to October 1.
8. Develop a plan for a potential cash flow crunch
So what happens if your best efforts in the 11th hour still aren’t enough to get your practice to the ICD-10 finish line? Prepare for the possibility of increased claim denials and temporary cash flow stalls, and put a plan in place to deal with them.
Start now by cleaning up as much of the accounts receivable as possible, and moving patient collections up front. Ask the billing team for a weekly status update of the largest unpaid balances in the 60-day aging column, and what has been done to appeal or otherwise address them. Analyze denial patterns and trends and fix their causes at the source—some may be ICD-10–related, others may simply be a gap in the reimbursement process that needs improvement.
Use payer cost estimators to calculate patient out-of-pocket cost and to collect unmet deductibles, coinsurance, and noncovered services prior to surgery. The surgeon-developed iPhone app Health Insurance Arithmetic2 ($1.99 in the iTunes Store) can help staff do this math on one, simple screen.
Finally, secure a line of credit to guard against a claim denial pile up this fall. A line of credit mitigates financial risk by making cash available quickly, should you need it to cover temporary revenue shortfalls, meet payroll, or pay operational expenses. It’s not too late to meet with your banker and apply for this protection, and the peace of mind may even help you sleep better.
1. KarenZupko & Associates, Inc. Pre-course survey of Q1 2015 coding and reimbursement workshop attendees. [Workshops are cosponsored by the American Academy of Orthopaedic Surgeons.] Unpublished data, April 2015.
2. Health Insurance Arithmetic. iTunes Store website. https://itunes.apple.com/us/app/healthinsurancearithmetic/id953262818. Accessed May 12, 2015.
As late as mid-April 2015, a survey of 121 orthopedic practices indicated that 30% had done nothing to start preparing for ICD-10 (International Classification of Diseases, Tenth Revision).1 That’s scary. And even the practices that had begun to prepare had not completed a number of key tasks (Figure 1).
Certainly, the will-they-or-won’t-they possibility of another congressional delay had many practices sitting on their hands this year. But now that the October 1, 2015, implementation is set in stone, this lack of inertia has many practices woefully behind. If your practice is one of many that hasn’t mapped your common ICD-9 (International Classification of Diseases, Ninth Revision) codes to ICD-10 codes, completed payer testing, or attended training, it’s time for a “full-court press.”
Being unprepared for ICD-10 will cause more than just an increase in claim denials. If your surgery schedule is booked a few months out, your staff will need to pre-authorize cases using ICD-10 as early as August 1—and they won’t be able to do that if you haven’t dictated the clinical terms required to choose an ICD-10 code. Without an understanding of ICD-10, severity of illness coding will suffer, and that will affect your bundled and value-based payments. And, if you don’t provide an adequate diagnosis when sending patients off-site for physical therapy, you’ll soon be getting phone calls from their billing staff demanding more specifics.
The clock is ticking and time is short. Here’s a prioritized list of what needs to get done.
1. Generate an ICD-9 frequency report
Identifying which diagnosis codes are the most frequently used, and therefore drive a significant portion of practice revenue, is an absolute must. The data will help prioritize training and code-mapping activities.
Most practices generate Current Procedural Terminology (CPT) code-frequency reports regularly, but few have ever run an ICD-9 code-frequency report. Call your vendor and ask for assistance, as there are multiple ways to run this report and they vary by practice management system. Sort the data elements and generate the ICD-9 frequency report by:
- Primary diagnosis.
- Unique patient.
- Revenue. (If your practice management system can’t give you diagnosis data by revenue, which enables you to focus on the codes that generate the most revenue, generate it by charges.)
The result should be a report that identifies the 20 to 25 diagnosis codes (or charges, depending on the reports generated) that drive the most revenue for the practice. Use the data to focus and prioritize your training and code-mapping activities.
2. Schedule training
Forget about “general” ICD-10 training courses. You need orthopedic-specific guidance. That’s because ICD-10 for orthopedics is more complex than for other specialties. Dictating fractures under ICD-10 is not so simple. Selecting an injury code requires confidence in correctly using the seventh character.
“Everyone who uses diagnosis codes must have baseline knowledge: surgeons, billing staff, surgical coordinators, and clinical team,” according to Sarah Wiskerchen, MBA, CPC, consultant and ICD-10 educator with KarenZupko & Associates (KZA). Training must include the practical details of ICD-10, such as assigning laterality, understanding the system architecture, and limiting the use of unspecified codes.
The American Academy of Orthopaedic Surgeons (AAOS) offers a self-paced, online training series that provides details for the top 3 diagnosis codes for each subspecialty. The 10-program course, ICD-10-CM: By the Numbers, is available at www.aaos.org ($299 for members, $399 for nonmembers). If you prefer live instruction, there is one more AAOS-sponsored, regional ICD-10 workshop left before the October 1 deadline, and more may be added. (Details at www.karenzupko.com)
These courses provide highly specific and granular ICD-10 knowledge and incorporate the use of Code-X, an AAOS-developed software tool. They also feature tools for handling the complexities of fractures and injury codes, such as Leo C. Far, an acronym developed by KZA consultant and coding educator Margie Maley, BSN, MS, to make ICD-10 diagnosis coding for fractures easier (Figure 2).
Some subspecialty societies also offer ICD-10 training. The American Society for Surgery of the Hand (www.assh.org), for example, offers a series of webinars and member-developed ICD-9-to-ICD-10 code maps.
3. Crosswalk your common codes from ICD-9 to ICD-10
Crosswalking is the process of mapping your most commonly used ICD-9 codes to their equivalent ICD-10 codes. This exercise familiarizes your team with ICD-10 language and terms, and gives a sense of which ICD-9 codes expand to just 1 or 2 ICD-10 codes and which codes expand into 10 or more codes—as some injury codes do (Table).
“Attempting to map the codes before completing ICD-10 training is like trying to write a letter in Greek when you only speak English,” Wiskerchen warns. “So start this process after at least some of your team have grasped the fundamentals of ICD-10.” This is where the data from your ICD-9 frequency report comes in. Use it to prioritize which codes to map first with a goal of mapping your top 25 ICD-9 codes to their ICD-10 equivalents by August 31.
Invest in good tools to support your mapping efforts. Avoid general mapping equivalent (GEM) coding tools, which are free for a good reason—they are incomplete and don’t always lead you to the correct ICD-10 code. Instead, purchase resources from credible sources, such as the American Medical Association (AMA; www.ama-assn.org). The AMA publishes ICD-10-CM 2016: The Complete Official Codebook as well as ICD-10-CM Mappings 2016, which links ICD-9 codes to all valid ICD-10 alternatives. The AMA also offers electronic ICD-10-CM Express Reference Mapping Cards for multiple specialties.
Practice makes perfect and crosswalking from ICD-9 to ICD-10 is one of the best ways for your team to become aware of the nuances in the new coding system. Like learning a new language, “speaking” ICD-10 does not become automatic just because you’ve attended training or completed the coding maps. Training teaches the architecture of the new coding system. Mapping provides a structured way to become familiar with the codes the practice will use most often. Once these 2 primary pieces are understood and assimilated, most physicians find that dictating the necessary new terms becomes quite easy.
4. Conduct a gap analysis to identify the ICD-10 terms missing from each provider’s current documentation
Conduct the gap analysis after your team has completed training, and once you’ve at least begun the process of mapping codes from ICD-9 to ICD-10. Here’s how:
- Generate a CPT frequency report.
- Select the top 5 procedures for each physician.
- Pull 2 patients’ notes for each of the top procedures.
- Review the notes and try to select ICD-10 code(s).
If key ICD-10 terms are not included in current documentation, physicians should modify the templates or macros they rely on for dictation.
“This simple exercise makes it obvious which clinical information physicians must add for ICD-10,” Wiskerchen says. For example, if the patient had an arthroscopy, but the note doesn’t specify on which leg, that’s a clear indication that the physician must dictate laterality. “The gap analysis is a great way to coach physicians about the clinical details to document, so staff can bill under ICD-10,” Wiskerchen says.
5. Contact technology vendors
Given the number of new ICD-10 codes in orthopedics, paper cheat sheets will be obsolete. Instead, you’ll need to rely on pull-down menus and/or search fields in the electronic health record (EHR) and practice management systems.
“Get clarity about how the new features and workflow processes will work in your systems,” suggests Wiskerchen. “Ask questions such as: Which features will be added or changed to accommodate the new codes? Will there be new screens or pick lists for ICD-10, or search fields? How will new screens and features change our current workflow? And schedule any necessary training as soon as possible.”
In addition to software upgrades and training, vendors and clearinghouses offer an array of services to help practices make the transition. Some vendors even provide help coordinating your internal plan with their new product features and training. Contact vendors to find out what they offer.
6. Use completed code maps to build diagnosis code databases, EHR templates, charge tickets, pick lists, prompters, and other coding tools
“Provide the code crosswalks and results of your documentation gap analysis to the IT [information technology] team so they can get started,” Wiskerchen advises. “And assign a physician or midlevel provider to work with IT so that the tools are clinically accurate.”
7. Schedule testing with clearinghouses and payers
“Successful testing indicates that your hard work has paid off, and that claims will be processed with few, if any, ICD-10–related hiccups,” Wiskerchen says. Essentially, the testing confirms that your ICD-10 code database, pick lists, vendor features, and other coding fields are working properly. “Testing with a clearinghouse is good. Testing directly with the payer is even better, if you are a direct submitter and it is allowed,” Wiskerchen suggests. Contact your clearinghouse and/or payers for testing opportunities prior to October 1.
8. Develop a plan for a potential cash flow crunch
So what happens if your best efforts in the 11th hour still aren’t enough to get your practice to the ICD-10 finish line? Prepare for the possibility of increased claim denials and temporary cash flow stalls, and put a plan in place to deal with them.
Start now by cleaning up as much of the accounts receivable as possible, and moving patient collections up front. Ask the billing team for a weekly status update of the largest unpaid balances in the 60-day aging column, and what has been done to appeal or otherwise address them. Analyze denial patterns and trends and fix their causes at the source—some may be ICD-10–related, others may simply be a gap in the reimbursement process that needs improvement.
Use payer cost estimators to calculate patient out-of-pocket cost and to collect unmet deductibles, coinsurance, and noncovered services prior to surgery. The surgeon-developed iPhone app Health Insurance Arithmetic2 ($1.99 in the iTunes Store) can help staff do this math on one, simple screen.
Finally, secure a line of credit to guard against a claim denial pile up this fall. A line of credit mitigates financial risk by making cash available quickly, should you need it to cover temporary revenue shortfalls, meet payroll, or pay operational expenses. It’s not too late to meet with your banker and apply for this protection, and the peace of mind may even help you sleep better.
As late as mid-April 2015, a survey of 121 orthopedic practices indicated that 30% had done nothing to start preparing for ICD-10 (International Classification of Diseases, Tenth Revision).1 That’s scary. And even the practices that had begun to prepare had not completed a number of key tasks (Figure 1).
Certainly, the will-they-or-won’t-they possibility of another congressional delay had many practices sitting on their hands this year. But now that the October 1, 2015, implementation is set in stone, this lack of inertia has many practices woefully behind. If your practice is one of many that hasn’t mapped your common ICD-9 (International Classification of Diseases, Ninth Revision) codes to ICD-10 codes, completed payer testing, or attended training, it’s time for a “full-court press.”
Being unprepared for ICD-10 will cause more than just an increase in claim denials. If your surgery schedule is booked a few months out, your staff will need to pre-authorize cases using ICD-10 as early as August 1—and they won’t be able to do that if you haven’t dictated the clinical terms required to choose an ICD-10 code. Without an understanding of ICD-10, severity of illness coding will suffer, and that will affect your bundled and value-based payments. And, if you don’t provide an adequate diagnosis when sending patients off-site for physical therapy, you’ll soon be getting phone calls from their billing staff demanding more specifics.
The clock is ticking and time is short. Here’s a prioritized list of what needs to get done.
1. Generate an ICD-9 frequency report
Identifying which diagnosis codes are the most frequently used, and therefore drive a significant portion of practice revenue, is an absolute must. The data will help prioritize training and code-mapping activities.
Most practices generate Current Procedural Terminology (CPT) code-frequency reports regularly, but few have ever run an ICD-9 code-frequency report. Call your vendor and ask for assistance, as there are multiple ways to run this report and they vary by practice management system. Sort the data elements and generate the ICD-9 frequency report by:
- Primary diagnosis.
- Unique patient.
- Revenue. (If your practice management system can’t give you diagnosis data by revenue, which enables you to focus on the codes that generate the most revenue, generate it by charges.)
The result should be a report that identifies the 20 to 25 diagnosis codes (or charges, depending on the reports generated) that drive the most revenue for the practice. Use the data to focus and prioritize your training and code-mapping activities.
2. Schedule training
Forget about “general” ICD-10 training courses. You need orthopedic-specific guidance. That’s because ICD-10 for orthopedics is more complex than for other specialties. Dictating fractures under ICD-10 is not so simple. Selecting an injury code requires confidence in correctly using the seventh character.
“Everyone who uses diagnosis codes must have baseline knowledge: surgeons, billing staff, surgical coordinators, and clinical team,” according to Sarah Wiskerchen, MBA, CPC, consultant and ICD-10 educator with KarenZupko & Associates (KZA). Training must include the practical details of ICD-10, such as assigning laterality, understanding the system architecture, and limiting the use of unspecified codes.
The American Academy of Orthopaedic Surgeons (AAOS) offers a self-paced, online training series that provides details for the top 3 diagnosis codes for each subspecialty. The 10-program course, ICD-10-CM: By the Numbers, is available at www.aaos.org ($299 for members, $399 for nonmembers). If you prefer live instruction, there is one more AAOS-sponsored, regional ICD-10 workshop left before the October 1 deadline, and more may be added. (Details at www.karenzupko.com)
These courses provide highly specific and granular ICD-10 knowledge and incorporate the use of Code-X, an AAOS-developed software tool. They also feature tools for handling the complexities of fractures and injury codes, such as Leo C. Far, an acronym developed by KZA consultant and coding educator Margie Maley, BSN, MS, to make ICD-10 diagnosis coding for fractures easier (Figure 2).
Some subspecialty societies also offer ICD-10 training. The American Society for Surgery of the Hand (www.assh.org), for example, offers a series of webinars and member-developed ICD-9-to-ICD-10 code maps.
3. Crosswalk your common codes from ICD-9 to ICD-10
Crosswalking is the process of mapping your most commonly used ICD-9 codes to their equivalent ICD-10 codes. This exercise familiarizes your team with ICD-10 language and terms, and gives a sense of which ICD-9 codes expand to just 1 or 2 ICD-10 codes and which codes expand into 10 or more codes—as some injury codes do (Table).
“Attempting to map the codes before completing ICD-10 training is like trying to write a letter in Greek when you only speak English,” Wiskerchen warns. “So start this process after at least some of your team have grasped the fundamentals of ICD-10.” This is where the data from your ICD-9 frequency report comes in. Use it to prioritize which codes to map first with a goal of mapping your top 25 ICD-9 codes to their ICD-10 equivalents by August 31.
Invest in good tools to support your mapping efforts. Avoid general mapping equivalent (GEM) coding tools, which are free for a good reason—they are incomplete and don’t always lead you to the correct ICD-10 code. Instead, purchase resources from credible sources, such as the American Medical Association (AMA; www.ama-assn.org). The AMA publishes ICD-10-CM 2016: The Complete Official Codebook as well as ICD-10-CM Mappings 2016, which links ICD-9 codes to all valid ICD-10 alternatives. The AMA also offers electronic ICD-10-CM Express Reference Mapping Cards for multiple specialties.
Practice makes perfect and crosswalking from ICD-9 to ICD-10 is one of the best ways for your team to become aware of the nuances in the new coding system. Like learning a new language, “speaking” ICD-10 does not become automatic just because you’ve attended training or completed the coding maps. Training teaches the architecture of the new coding system. Mapping provides a structured way to become familiar with the codes the practice will use most often. Once these 2 primary pieces are understood and assimilated, most physicians find that dictating the necessary new terms becomes quite easy.
4. Conduct a gap analysis to identify the ICD-10 terms missing from each provider’s current documentation
Conduct the gap analysis after your team has completed training, and once you’ve at least begun the process of mapping codes from ICD-9 to ICD-10. Here’s how:
- Generate a CPT frequency report.
- Select the top 5 procedures for each physician.
- Pull 2 patients’ notes for each of the top procedures.
- Review the notes and try to select ICD-10 code(s).
If key ICD-10 terms are not included in current documentation, physicians should modify the templates or macros they rely on for dictation.
“This simple exercise makes it obvious which clinical information physicians must add for ICD-10,” Wiskerchen says. For example, if the patient had an arthroscopy, but the note doesn’t specify on which leg, that’s a clear indication that the physician must dictate laterality. “The gap analysis is a great way to coach physicians about the clinical details to document, so staff can bill under ICD-10,” Wiskerchen says.
5. Contact technology vendors
Given the number of new ICD-10 codes in orthopedics, paper cheat sheets will be obsolete. Instead, you’ll need to rely on pull-down menus and/or search fields in the electronic health record (EHR) and practice management systems.
“Get clarity about how the new features and workflow processes will work in your systems,” suggests Wiskerchen. “Ask questions such as: Which features will be added or changed to accommodate the new codes? Will there be new screens or pick lists for ICD-10, or search fields? How will new screens and features change our current workflow? And schedule any necessary training as soon as possible.”
In addition to software upgrades and training, vendors and clearinghouses offer an array of services to help practices make the transition. Some vendors even provide help coordinating your internal plan with their new product features and training. Contact vendors to find out what they offer.
6. Use completed code maps to build diagnosis code databases, EHR templates, charge tickets, pick lists, prompters, and other coding tools
“Provide the code crosswalks and results of your documentation gap analysis to the IT [information technology] team so they can get started,” Wiskerchen advises. “And assign a physician or midlevel provider to work with IT so that the tools are clinically accurate.”
7. Schedule testing with clearinghouses and payers
“Successful testing indicates that your hard work has paid off, and that claims will be processed with few, if any, ICD-10–related hiccups,” Wiskerchen says. Essentially, the testing confirms that your ICD-10 code database, pick lists, vendor features, and other coding fields are working properly. “Testing with a clearinghouse is good. Testing directly with the payer is even better, if you are a direct submitter and it is allowed,” Wiskerchen suggests. Contact your clearinghouse and/or payers for testing opportunities prior to October 1.
8. Develop a plan for a potential cash flow crunch
So what happens if your best efforts in the 11th hour still aren’t enough to get your practice to the ICD-10 finish line? Prepare for the possibility of increased claim denials and temporary cash flow stalls, and put a plan in place to deal with them.
Start now by cleaning up as much of the accounts receivable as possible, and moving patient collections up front. Ask the billing team for a weekly status update of the largest unpaid balances in the 60-day aging column, and what has been done to appeal or otherwise address them. Analyze denial patterns and trends and fix their causes at the source—some may be ICD-10–related, others may simply be a gap in the reimbursement process that needs improvement.
Use payer cost estimators to calculate patient out-of-pocket cost and to collect unmet deductibles, coinsurance, and noncovered services prior to surgery. The surgeon-developed iPhone app Health Insurance Arithmetic2 ($1.99 in the iTunes Store) can help staff do this math on one, simple screen.
Finally, secure a line of credit to guard against a claim denial pile up this fall. A line of credit mitigates financial risk by making cash available quickly, should you need it to cover temporary revenue shortfalls, meet payroll, or pay operational expenses. It’s not too late to meet with your banker and apply for this protection, and the peace of mind may even help you sleep better.
1. KarenZupko & Associates, Inc. Pre-course survey of Q1 2015 coding and reimbursement workshop attendees. [Workshops are cosponsored by the American Academy of Orthopaedic Surgeons.] Unpublished data, April 2015.
2. Health Insurance Arithmetic. iTunes Store website. https://itunes.apple.com/us/app/healthinsurancearithmetic/id953262818. Accessed May 12, 2015.
1. KarenZupko & Associates, Inc. Pre-course survey of Q1 2015 coding and reimbursement workshop attendees. [Workshops are cosponsored by the American Academy of Orthopaedic Surgeons.] Unpublished data, April 2015.
2. Health Insurance Arithmetic. iTunes Store website. https://itunes.apple.com/us/app/healthinsurancearithmetic/id953262818. Accessed May 12, 2015.
Revision Rotator Cuff Reconstruction for Large Tears With Retraction: A Novel Technique Using Autogenous Tendon and Autologous Marrow
Primary rotator cuff repair is a common procedure that consistently yields favorable clinical results.1 Revision rotator cuff repair and reconstruction yield less consistent clinical results and are associated with a significant incidence of recurrent cuff tearing.2 Possible factors contributing to the loss of tissue continuity have included poor quality or frank loss of rotator cuff tissue, diminished biological potential of the rotator cuff tendon, and excessive mechanical stress on or strain of the reconstructive surgical construct.3
I conducted a pilot study involving a technique that addresses these potential factors, amalgamating several contemporary surgical methods with the addition of a novel step: an autogenous tendon graft incubated in autologous bone marrow concentrate.
Materials and Methods
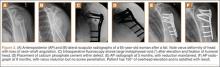
Ten consecutive patients (7 men, 3 women) enrolled in this retrospective case series. Mean age at time of surgery was 58 years (range, 47-65 years). Mean follow-up was 24 months (range, 12-44 months), and no patients were lost to follow-up. Mean time between original primary repair and current reconstruction was 36 months (range, 6-120 months). Criteria for enrollment included unremitting shoulder pain, radiographs showing no significant degenerative joint disease, magnetic resonance imaging confirming a large (3-5 cm) full-thickness rotator cuff tear with retraction, and history of prior rotator cuff repair on the affected shoulder without associated biceps tenodesis. The intraoperative inclusion criterion was direct visualization of a 3- to 5-cm full-thickness rotator cuff tear with retraction of at least 3 cm. Validated Constant, American Shoulder and Elbow Surgeons (ASES), and University of California Los Angeles (UCLA) shoulder scoring systems were used to collect range-of-motion, pain, strength, daily function, and patient satisfaction data before and after surgery. Standard error was calculated. Two-sample t test was used for preoperative–postoperative comparisons. Postoperative integrity of the rotator cuff reconstruction was evaluated by an independent full-time academic musculoskeletal radiologist using dynamic diagnostic ultrasound (iU22 xMatrix Ultrasound System [Philips Healthcare] at L 9-3 MHz). Informed consent was obtained from each patient. The study was approved by institutional review board.
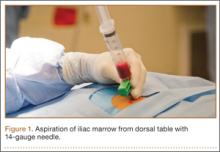
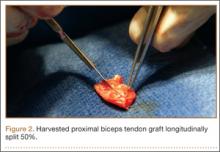
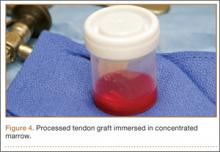
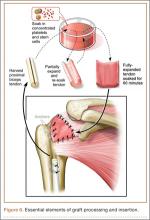
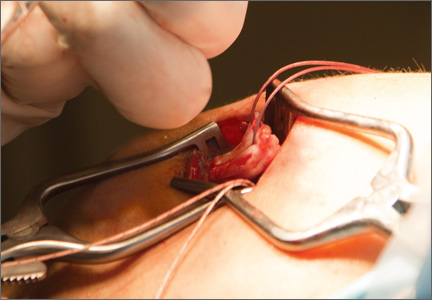
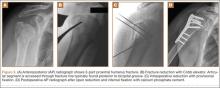
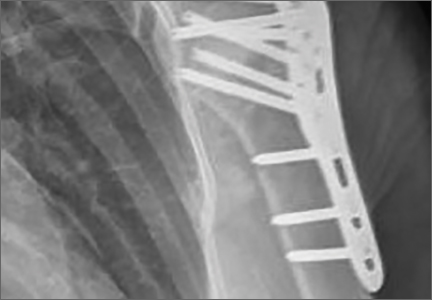
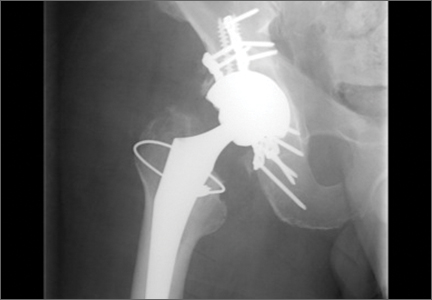
After induction of general anesthesia, each patient was placed in the lateral decubitus position. Bone marrow (60 mL) was aspirated through a 14-gauge needle from a dorsal iliac table, just inferior to the iliac crest (Figure 1). The patient was then placed into the beach-chair position on a surgical shoulder table. The aspirated marrow was centrifuged at 2800 and 3800 rpm for 14 to 17 minutes (Magellan Autologous Platelet Separator; Arteriocyte Medical Systems) to yield 10 mL of a concentrated (4- to 5-fold) mixture of platelet-rich plasma (PRP) and mesenchymal stem cells. Surgery was performed through a 3-cm oblique anterior mini-open incision between the anterior corner of the acromion and the coracoid process, as I previously described.4 The deltoid muscle was split, not detached. Acromioplasty and release of the coracoacromial ligament were performed. The rotator cuff was inspected under ×4.5 optical magnification. The cuff tissue was mobilized and débrided back to a healthy-appearing margin. The size and shape of the rotator cuff defect were then estimated. The long head of the biceps was harvested from its origin just distal to the superior glenoid labrum unto the intertubercular sulcus on the proximal humerus. The remainder of the biceps tendon was tenodesed to the surgical neck of the humerus. The biceps tendon graft was then manipulated and fashioned (by longitudinal partial-thickness incision and expansion) to fit the cuff defect (Figures 2, 3). The expanded graft was incubated in the concentrated marrow (10 mL) for 60 minutes (Figure 4). Débridement at the base of the greater tuberosity down to bleeding cancellous bone was followed by insertion of multiple bone anchors bearing several strands of No. 2 synthetic suture. These strands were then passed through the biceps tendon graft for secure fixation (Figure 5). The débrided end of the rotator cuff was then sewn to the biceps tendon graft using locking stitches under zero tissue tension with the arm in full adduction. The free end of the graft was sewn to the subscapularis tendon (Figure 6). The remaining marrow concentrate was injected both deep and superficial to the rotator cuff construct. No additional wound irrigation fluid was injected or suction drain inserted. After surgery, the patient was placed into an abduction pillow for 1 month and then engaged in passive motion for 1 month. Active-assisted motion began 3 months after surgery.
Results
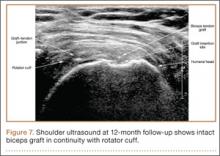
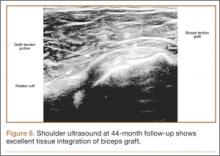
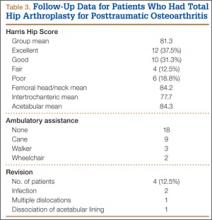
Clinically, all patients improved with respect to pain, motion, strength, function, and satisfaction by virtue of the reconstructive surgery. After surgery, mean Constant score was increased, from 13 to 71 (P < .001). Mean ASES score increased from 18 to 75 (P < .001). Mean UCLA score increased from 4 to 28 (P < .001) (Table). Ultrasound showed 0% incidence of full-thickness retearing. Dynamic scanning during abduction showed maintained reduction of the humeral head within the glenoid socket; superior subluxation of the humeral head was not detected. The biceps tendon graft was continuous with the rotator cuff tendon, indicative of graft integration: tissue healing at the graft–bone and graft–tendon interfaces (Figures 7, 8). There were no intraoperative or postoperative patient-related complications.
Discussion
Primary rotator cuff surgery is beneficial.5 Irrespective of technique, open versus arthroscopic,6 single- versus double-row repair,7 the clinical results have been satisfactory.8 Nevertheless, the “tissue failure” rate of rotator cuff surgery (full-thickness discontinuity of rotator cuff) has been as high as 31% in primary repairs.9 In revision rotator cuff repair and reconstruction, the radiographic tissue failure rate has been even higher, particularly in the setting of chronic large tears with retraction, with tissue failure rates up to 91%.10 Although small to medium full-thickness tears and retears are well tolerated by patients with reduced activity levels,11 and pain symptoms do not necessarily correlate with rotator cuff tear size,12 large retracted full-thickness tears in active patients seldom result in optimal clinical outcomes or patient satisfaction.13,14 In addition, although recurrent tearing does not preclude a satisfactory clinical result, maintenance of cuff tissue integrity tends to produce a better objective clinical score and a more desirable clinical outcome.2
Few evidence-based restorative solutions exist for large recurrent rotator cuff tears with retraction in active nongeriatric patients.15 The no-treatment option in this context may result in gradual enlargement of the tear, chronic pain, weakness, and progressive degeneration of the glenohumeral joint and acromiohumeral confluence—so-called rotator cuff arthropathy, for which reverse total shoulder arthroplasty is required.16,17 Partial repair of a large rotator cuff tear by margin convergence, interval slide, split deltoid flap, or nonanatomical reinsertion may improve clinical outcome scores but may not alter or prevent the progressive degenerative changes associated with rotator cuff arthropathy.18,19 Synthetic scaffolds with and without biological enhancement have been used with varying degrees of success, particularly pain improvement and tissue integration.20 Nevertheless, the failure rate has been reported to be 17% to 51%,21 and no evidence exists that allograft augmentation improves functional outcomes.22 Tendon transfer using the latissimus dorsi has also proved to be a surgical alternative in younger, active patients.23 However, dissection in this procedure is a major undertaking for both surgeon and patient—compared with the minimally invasive technique used in the present study.24
I selected a cohort of active, symptomatic patients for application of a synthesis of accepted surgical techniques through a mini-open incision in order to improve the reliability of the surgical construct while minimizing surgical morbidity. Débridement of marginal tissue, safe mobilization of remaining cuff, and tension-free suture line using locking sutures maximized the mechanical strength of the construct.25,26 Biological enhancement with autogenous tissue (the patient’s own biceps tendon) as graft material (scaffolding), as well as autologous concentrated marrow delivering viable responding cells and chemokine/cytokine biofactors, increased the probability of reparative activity at the graft site.27 The net effect was consistent tissue healing at a biologically challenging locus. Nonenhanced biceps tendon grafting in the setting of “irreparable” primary rotator cuff repair has had a 40-year history of orthopedic utility and an excellent record of clinical success.28 Nevertheless, the retear rate has been 7% to 30%.29 There are no previous reports of biologically enhanced autogenous biceps tendon grafting for reconstruction of a torn rotator cuff, either primary or in the setting of chronic revision surgery.
Previous well-designed PRP enhancement studies in the context of primary rotator cuff repair failed to demonstrate a consistent benefit with concentrated platelet-only augmentation.30,31 The shared experimental design of these published studies used intra-articular injection as the sole delivery method without guarantee that the injected platelets would migrate, adhere to, and persist at the intended destination, the healing edge of the rotator cuff. In the present study, extended exposure of the splayed tendon graft by incubation in concentrated marrow was specifically designed to increase the probability that biologically active components would settle at the desired location by cellular seeding and plasmatic imbibition.32 Furthermore, use of PRP for growth factor (platelet-derived, PDGF; basic fibroblast, bFGF; transforming, TGF-β; epidermal, EGF; vascular endothelial, VEGF; connective tissue, CTGF) therapy, in addition to pluripotential mesenchymal cells for marrow-derived stem cell therapy, is in theory biologically superior to use of PRP alone.33,34
The recent expansion of information about biologics has generated much interest in augmentation of soft-tissue healing. Unfortunately, the optimal technique of using cellular processing to upregulate stem-cell capacity at the graft interface is yet to be defined.35 Clinical studies using PRP and related products to promote tendon healing have been both inconsistent and contradictory with respect to benefit of outcome. As we have been unable to harness the biological potential of this medium, application of biologics in contemporary clinical orthopedics remains narrow, random, and infrequent. The technique presented in this clinical series appears to be a small advancement in a positive direction. The described construct provides a starting point for study, combining mechanical as well as biological steps to promote rotator cuff healing. The consistency of the outcome in a clinical model in which retearing is an expectation rather than an exception is noteworthy. The zero tissue failure rate at 1 to 4 years, compared with the literature values in similar patient cohorts, is very promising.36 The clinical outcome as measured by validated shoulder scores is also comparable to literature outcome values.19 Also noteworthy is the dynamic stability the construct gives to the glenohumeral joint. Ideally, the reconstructed rotator cuff provides active force coupling with the deltoid, simulating normal shoulder biomechanics. At a minimum, the reconstructed cuff provides a viable passive barrier to superior migration of the humeral head—thus supporting the mechanical efficiency of the deltoid and preventing rotator cuff arthropathy.
This study’s small sample (10 patients) puts its conclusions at risk for type I statistical error, in that too few patients were examined over a long enough period to demonstrate failure. Nevertheless, retears typically occur within 6 months of repair.37,38 Therefore, minimum follow-up of 1 year was deemed sufficient. None of the 10 patients had diabetes or another chronic comorbidity. Nine of the 10 had either no or only mild preoperative fatty atrophy of the rotator cuff muscles. Eight of the 10 were nonsmokers. These factors, which suggest optimal surgical candidates, may prove to be significant as the clinical series expands over time. Incubation of the autogenous biceps graft in concentrated marrow for 60 minutes was arbitrarily chosen. In future in vitro examination, marrow cell viability as a function of incubation time will be assessed.
Conclusion
In active, middle-aged patients with chronic recurrent large retracted rotator cuff tears, the technique presented here, using autogenous biceps tendon and autologous concentrated marrow containing PRP and mesenchymal cells, consistently yielded satisfactory clinical results and promoted rotator cuff tissue healing without full-thickness retearing.
1. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
2. Kim HM, Caldwell JM, Buza JA, et al. Factors affecting satisfaction and shoulder function in patients with a recurrent rotator cuff tear. J Bone Joint Surg Am. 2014;96(2):106-112.
3. George MS, Khazzam M. Current concepts review: revision rotator cuff repair. J Shoulder Elbow Surg. 2012;21(4):431-440.
4. Skoff HD. Conservative open acromioplasty. J Bone Joint Surg Br. 1995;77(6):933-936.
5. Mather RC 3rd, Koenig L, Acevedo D, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993-2000.
6. Sauerbrey AM, Getz CL, Piancastelli M, Iannotti JP, Ramsey ML, Williams GR. Arthroscopic versus mini-open rotator cuff repair: a comparison of clinical outcome. Arthroscopy. 2005;21(12):1415-1420.
7. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
8. Galatz LM, Griggs S, Cameron BD, Iannotti JP. Prospective longitudinal analysis of post-operative shoulder function: a ten-year follow-up study of full thickness rotator cuff tears. J Bone Joint Surg Am. 2001;83(7):1052-1056.
9. Oh JH, Kim SH, Kang JY, Oh CH, Gong HS. Effect of age on functional and structural outcome after rotator cuff repair. Am J Sports Med. 2010;38(4):672-678.
10. Kim JH, Kim SH, Lee SK, Seo JW, Chun YMC. Arthroscopic repair of massive contracted rotator cuff tears: aggressive release with anterior and posterior interval slides do not improve cuff healing and integrity. J Bone Joint Surg Am. 2014;95(16):1482-1488.
11. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears. J Bone Joint Surg Am. 2014;96(18):1504-1514.
12. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity. J Bone Joint Surg Am. 2014;96(10):793-800.
13. Lubiatowski P, Kaczmarek P, Dzianach M, et al. Clinical and biomechanical performance of patients with failed rotator cuff repair. Int Orthop. 2013;37(12):2395-2401.
14. Holtby R, Razmjou H. Relationship between clinical and surgical findings and reparability of large and massive rotator cuff tears: a longitudinal study. BMC Musculoskelet Disord. 2014;15:180.
15. Nho SJ, Delos D, Yadav H, et al. Biomechanical and biological augmentation for the treatment of massive rotator cuff tears. Am J Sports Med. 2010;38(3):619-629.
16. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
17. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
18. Bartl C, Louloumentas P, Konstantin H, et al. Long-term outcome and structural integrity following open repair of massive rotator cuff tears. Int J Shoulder Surg. 2012;6(1):1-8.
19. Paxton ES, Teefey SA, Dahiya N, Keener JD, Yamaguchi K, Galatz LM. Clinical and radiographic outcomes of failed repairs of large or massive rotator cuff tears: minimum ten-year follow-up. J Bone Joint Surg Am. 2013;95(7):627-632.
20. Longo UG, Lamberti A, Maffulli N, Denaro V. Tendon augmentation grafts: a systematic review. Br Med Bull. 2010;94:165-188.
21. Ciampi P, Scotti C, Nonis A, et al. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: a 3-year follow-up study. Am J Sports Med. 2014;42(5):1169-1175.
22. Murhi AM. Rotator cuff tears and cuff tear arthropathy. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:921-929.
23. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
24. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
25. Wagner JP, Krushall RJ, Masqueloet A, Gerber C. Anatomy and relationships of the suprascapular nerve: anatomical constraints to mobilization of the supraspinatus and infraspinatus muscles in the management of massive rotator cuff tears. J Bone Joint Surg Am. 1992;74(1):36-45.
26. Ponce BA, Hosemann CD, Reghava P, Tate JP, Sheppard ED, Ebenhardt AW. A biomechanical analysis of controllable intraoperative variables affecting the strength of rotator cuff repairs at the suture–tendon interface. Am J Sports Med. 2013;41(10):2256-2261.
27. Thomopoulos S. Tendon and ligaments. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:105-111.
28. Sano H, Mineta M, Kitz A, Itoi E. Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci. 2010;15(3):310-316.
29. Rhee YG, Cho NS, Lim CT, Yi JW, Vishvanathan T. Bridging the gap in immobile massive rotator cuff tears: augmentation using the tenotomized biceps. Am J Sports Med. 2008;36(8):1511-1518.
30. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
31. Rodeo SA, Delos, D, Williams, RJ, Adler RS, Pearle A, Warren RF. The effects of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
32. Beitzel K, McCarthy MB, Cote MP, et al. Properties of biologic scaffolds and their response to mesenchymal stem cells. Arthroscopy. 2014;30(3):289-298.
33. Anz AW, Hackel JG, Nilssen ED, Andrews JR. Application of biologics in the treatment of rotator cuff, meniscus, cartilage and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
34. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
35. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
36. Kowalsky MS, Keener JD. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome: surgical technique. J Bone Joint Surg Am. 2011;93(suppl 1):62-74.
37. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
38. Le BT, Wu XL, Lam PH, Murrell GA. Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 2014;42(5):1134-1142.
Primary rotator cuff repair is a common procedure that consistently yields favorable clinical results.1 Revision rotator cuff repair and reconstruction yield less consistent clinical results and are associated with a significant incidence of recurrent cuff tearing.2 Possible factors contributing to the loss of tissue continuity have included poor quality or frank loss of rotator cuff tissue, diminished biological potential of the rotator cuff tendon, and excessive mechanical stress on or strain of the reconstructive surgical construct.3
I conducted a pilot study involving a technique that addresses these potential factors, amalgamating several contemporary surgical methods with the addition of a novel step: an autogenous tendon graft incubated in autologous bone marrow concentrate.
Materials and Methods
Ten consecutive patients (7 men, 3 women) enrolled in this retrospective case series. Mean age at time of surgery was 58 years (range, 47-65 years). Mean follow-up was 24 months (range, 12-44 months), and no patients were lost to follow-up. Mean time between original primary repair and current reconstruction was 36 months (range, 6-120 months). Criteria for enrollment included unremitting shoulder pain, radiographs showing no significant degenerative joint disease, magnetic resonance imaging confirming a large (3-5 cm) full-thickness rotator cuff tear with retraction, and history of prior rotator cuff repair on the affected shoulder without associated biceps tenodesis. The intraoperative inclusion criterion was direct visualization of a 3- to 5-cm full-thickness rotator cuff tear with retraction of at least 3 cm. Validated Constant, American Shoulder and Elbow Surgeons (ASES), and University of California Los Angeles (UCLA) shoulder scoring systems were used to collect range-of-motion, pain, strength, daily function, and patient satisfaction data before and after surgery. Standard error was calculated. Two-sample t test was used for preoperative–postoperative comparisons. Postoperative integrity of the rotator cuff reconstruction was evaluated by an independent full-time academic musculoskeletal radiologist using dynamic diagnostic ultrasound (iU22 xMatrix Ultrasound System [Philips Healthcare] at L 9-3 MHz). Informed consent was obtained from each patient. The study was approved by institutional review board.
After induction of general anesthesia, each patient was placed in the lateral decubitus position. Bone marrow (60 mL) was aspirated through a 14-gauge needle from a dorsal iliac table, just inferior to the iliac crest (Figure 1). The patient was then placed into the beach-chair position on a surgical shoulder table. The aspirated marrow was centrifuged at 2800 and 3800 rpm for 14 to 17 minutes (Magellan Autologous Platelet Separator; Arteriocyte Medical Systems) to yield 10 mL of a concentrated (4- to 5-fold) mixture of platelet-rich plasma (PRP) and mesenchymal stem cells. Surgery was performed through a 3-cm oblique anterior mini-open incision between the anterior corner of the acromion and the coracoid process, as I previously described.4 The deltoid muscle was split, not detached. Acromioplasty and release of the coracoacromial ligament were performed. The rotator cuff was inspected under ×4.5 optical magnification. The cuff tissue was mobilized and débrided back to a healthy-appearing margin. The size and shape of the rotator cuff defect were then estimated. The long head of the biceps was harvested from its origin just distal to the superior glenoid labrum unto the intertubercular sulcus on the proximal humerus. The remainder of the biceps tendon was tenodesed to the surgical neck of the humerus. The biceps tendon graft was then manipulated and fashioned (by longitudinal partial-thickness incision and expansion) to fit the cuff defect (Figures 2, 3). The expanded graft was incubated in the concentrated marrow (10 mL) for 60 minutes (Figure 4). Débridement at the base of the greater tuberosity down to bleeding cancellous bone was followed by insertion of multiple bone anchors bearing several strands of No. 2 synthetic suture. These strands were then passed through the biceps tendon graft for secure fixation (Figure 5). The débrided end of the rotator cuff was then sewn to the biceps tendon graft using locking stitches under zero tissue tension with the arm in full adduction. The free end of the graft was sewn to the subscapularis tendon (Figure 6). The remaining marrow concentrate was injected both deep and superficial to the rotator cuff construct. No additional wound irrigation fluid was injected or suction drain inserted. After surgery, the patient was placed into an abduction pillow for 1 month and then engaged in passive motion for 1 month. Active-assisted motion began 3 months after surgery.
Results
Clinically, all patients improved with respect to pain, motion, strength, function, and satisfaction by virtue of the reconstructive surgery. After surgery, mean Constant score was increased, from 13 to 71 (P < .001). Mean ASES score increased from 18 to 75 (P < .001). Mean UCLA score increased from 4 to 28 (P < .001) (Table). Ultrasound showed 0% incidence of full-thickness retearing. Dynamic scanning during abduction showed maintained reduction of the humeral head within the glenoid socket; superior subluxation of the humeral head was not detected. The biceps tendon graft was continuous with the rotator cuff tendon, indicative of graft integration: tissue healing at the graft–bone and graft–tendon interfaces (Figures 7, 8). There were no intraoperative or postoperative patient-related complications.
Discussion
Primary rotator cuff surgery is beneficial.5 Irrespective of technique, open versus arthroscopic,6 single- versus double-row repair,7 the clinical results have been satisfactory.8 Nevertheless, the “tissue failure” rate of rotator cuff surgery (full-thickness discontinuity of rotator cuff) has been as high as 31% in primary repairs.9 In revision rotator cuff repair and reconstruction, the radiographic tissue failure rate has been even higher, particularly in the setting of chronic large tears with retraction, with tissue failure rates up to 91%.10 Although small to medium full-thickness tears and retears are well tolerated by patients with reduced activity levels,11 and pain symptoms do not necessarily correlate with rotator cuff tear size,12 large retracted full-thickness tears in active patients seldom result in optimal clinical outcomes or patient satisfaction.13,14 In addition, although recurrent tearing does not preclude a satisfactory clinical result, maintenance of cuff tissue integrity tends to produce a better objective clinical score and a more desirable clinical outcome.2
Few evidence-based restorative solutions exist for large recurrent rotator cuff tears with retraction in active nongeriatric patients.15 The no-treatment option in this context may result in gradual enlargement of the tear, chronic pain, weakness, and progressive degeneration of the glenohumeral joint and acromiohumeral confluence—so-called rotator cuff arthropathy, for which reverse total shoulder arthroplasty is required.16,17 Partial repair of a large rotator cuff tear by margin convergence, interval slide, split deltoid flap, or nonanatomical reinsertion may improve clinical outcome scores but may not alter or prevent the progressive degenerative changes associated with rotator cuff arthropathy.18,19 Synthetic scaffolds with and without biological enhancement have been used with varying degrees of success, particularly pain improvement and tissue integration.20 Nevertheless, the failure rate has been reported to be 17% to 51%,21 and no evidence exists that allograft augmentation improves functional outcomes.22 Tendon transfer using the latissimus dorsi has also proved to be a surgical alternative in younger, active patients.23 However, dissection in this procedure is a major undertaking for both surgeon and patient—compared with the minimally invasive technique used in the present study.24
I selected a cohort of active, symptomatic patients for application of a synthesis of accepted surgical techniques through a mini-open incision in order to improve the reliability of the surgical construct while minimizing surgical morbidity. Débridement of marginal tissue, safe mobilization of remaining cuff, and tension-free suture line using locking sutures maximized the mechanical strength of the construct.25,26 Biological enhancement with autogenous tissue (the patient’s own biceps tendon) as graft material (scaffolding), as well as autologous concentrated marrow delivering viable responding cells and chemokine/cytokine biofactors, increased the probability of reparative activity at the graft site.27 The net effect was consistent tissue healing at a biologically challenging locus. Nonenhanced biceps tendon grafting in the setting of “irreparable” primary rotator cuff repair has had a 40-year history of orthopedic utility and an excellent record of clinical success.28 Nevertheless, the retear rate has been 7% to 30%.29 There are no previous reports of biologically enhanced autogenous biceps tendon grafting for reconstruction of a torn rotator cuff, either primary or in the setting of chronic revision surgery.
Previous well-designed PRP enhancement studies in the context of primary rotator cuff repair failed to demonstrate a consistent benefit with concentrated platelet-only augmentation.30,31 The shared experimental design of these published studies used intra-articular injection as the sole delivery method without guarantee that the injected platelets would migrate, adhere to, and persist at the intended destination, the healing edge of the rotator cuff. In the present study, extended exposure of the splayed tendon graft by incubation in concentrated marrow was specifically designed to increase the probability that biologically active components would settle at the desired location by cellular seeding and plasmatic imbibition.32 Furthermore, use of PRP for growth factor (platelet-derived, PDGF; basic fibroblast, bFGF; transforming, TGF-β; epidermal, EGF; vascular endothelial, VEGF; connective tissue, CTGF) therapy, in addition to pluripotential mesenchymal cells for marrow-derived stem cell therapy, is in theory biologically superior to use of PRP alone.33,34
The recent expansion of information about biologics has generated much interest in augmentation of soft-tissue healing. Unfortunately, the optimal technique of using cellular processing to upregulate stem-cell capacity at the graft interface is yet to be defined.35 Clinical studies using PRP and related products to promote tendon healing have been both inconsistent and contradictory with respect to benefit of outcome. As we have been unable to harness the biological potential of this medium, application of biologics in contemporary clinical orthopedics remains narrow, random, and infrequent. The technique presented in this clinical series appears to be a small advancement in a positive direction. The described construct provides a starting point for study, combining mechanical as well as biological steps to promote rotator cuff healing. The consistency of the outcome in a clinical model in which retearing is an expectation rather than an exception is noteworthy. The zero tissue failure rate at 1 to 4 years, compared with the literature values in similar patient cohorts, is very promising.36 The clinical outcome as measured by validated shoulder scores is also comparable to literature outcome values.19 Also noteworthy is the dynamic stability the construct gives to the glenohumeral joint. Ideally, the reconstructed rotator cuff provides active force coupling with the deltoid, simulating normal shoulder biomechanics. At a minimum, the reconstructed cuff provides a viable passive barrier to superior migration of the humeral head—thus supporting the mechanical efficiency of the deltoid and preventing rotator cuff arthropathy.
This study’s small sample (10 patients) puts its conclusions at risk for type I statistical error, in that too few patients were examined over a long enough period to demonstrate failure. Nevertheless, retears typically occur within 6 months of repair.37,38 Therefore, minimum follow-up of 1 year was deemed sufficient. None of the 10 patients had diabetes or another chronic comorbidity. Nine of the 10 had either no or only mild preoperative fatty atrophy of the rotator cuff muscles. Eight of the 10 were nonsmokers. These factors, which suggest optimal surgical candidates, may prove to be significant as the clinical series expands over time. Incubation of the autogenous biceps graft in concentrated marrow for 60 minutes was arbitrarily chosen. In future in vitro examination, marrow cell viability as a function of incubation time will be assessed.
Conclusion
In active, middle-aged patients with chronic recurrent large retracted rotator cuff tears, the technique presented here, using autogenous biceps tendon and autologous concentrated marrow containing PRP and mesenchymal cells, consistently yielded satisfactory clinical results and promoted rotator cuff tissue healing without full-thickness retearing.
Primary rotator cuff repair is a common procedure that consistently yields favorable clinical results.1 Revision rotator cuff repair and reconstruction yield less consistent clinical results and are associated with a significant incidence of recurrent cuff tearing.2 Possible factors contributing to the loss of tissue continuity have included poor quality or frank loss of rotator cuff tissue, diminished biological potential of the rotator cuff tendon, and excessive mechanical stress on or strain of the reconstructive surgical construct.3
I conducted a pilot study involving a technique that addresses these potential factors, amalgamating several contemporary surgical methods with the addition of a novel step: an autogenous tendon graft incubated in autologous bone marrow concentrate.
Materials and Methods
Ten consecutive patients (7 men, 3 women) enrolled in this retrospective case series. Mean age at time of surgery was 58 years (range, 47-65 years). Mean follow-up was 24 months (range, 12-44 months), and no patients were lost to follow-up. Mean time between original primary repair and current reconstruction was 36 months (range, 6-120 months). Criteria for enrollment included unremitting shoulder pain, radiographs showing no significant degenerative joint disease, magnetic resonance imaging confirming a large (3-5 cm) full-thickness rotator cuff tear with retraction, and history of prior rotator cuff repair on the affected shoulder without associated biceps tenodesis. The intraoperative inclusion criterion was direct visualization of a 3- to 5-cm full-thickness rotator cuff tear with retraction of at least 3 cm. Validated Constant, American Shoulder and Elbow Surgeons (ASES), and University of California Los Angeles (UCLA) shoulder scoring systems were used to collect range-of-motion, pain, strength, daily function, and patient satisfaction data before and after surgery. Standard error was calculated. Two-sample t test was used for preoperative–postoperative comparisons. Postoperative integrity of the rotator cuff reconstruction was evaluated by an independent full-time academic musculoskeletal radiologist using dynamic diagnostic ultrasound (iU22 xMatrix Ultrasound System [Philips Healthcare] at L 9-3 MHz). Informed consent was obtained from each patient. The study was approved by institutional review board.
After induction of general anesthesia, each patient was placed in the lateral decubitus position. Bone marrow (60 mL) was aspirated through a 14-gauge needle from a dorsal iliac table, just inferior to the iliac crest (Figure 1). The patient was then placed into the beach-chair position on a surgical shoulder table. The aspirated marrow was centrifuged at 2800 and 3800 rpm for 14 to 17 minutes (Magellan Autologous Platelet Separator; Arteriocyte Medical Systems) to yield 10 mL of a concentrated (4- to 5-fold) mixture of platelet-rich plasma (PRP) and mesenchymal stem cells. Surgery was performed through a 3-cm oblique anterior mini-open incision between the anterior corner of the acromion and the coracoid process, as I previously described.4 The deltoid muscle was split, not detached. Acromioplasty and release of the coracoacromial ligament were performed. The rotator cuff was inspected under ×4.5 optical magnification. The cuff tissue was mobilized and débrided back to a healthy-appearing margin. The size and shape of the rotator cuff defect were then estimated. The long head of the biceps was harvested from its origin just distal to the superior glenoid labrum unto the intertubercular sulcus on the proximal humerus. The remainder of the biceps tendon was tenodesed to the surgical neck of the humerus. The biceps tendon graft was then manipulated and fashioned (by longitudinal partial-thickness incision and expansion) to fit the cuff defect (Figures 2, 3). The expanded graft was incubated in the concentrated marrow (10 mL) for 60 minutes (Figure 4). Débridement at the base of the greater tuberosity down to bleeding cancellous bone was followed by insertion of multiple bone anchors bearing several strands of No. 2 synthetic suture. These strands were then passed through the biceps tendon graft for secure fixation (Figure 5). The débrided end of the rotator cuff was then sewn to the biceps tendon graft using locking stitches under zero tissue tension with the arm in full adduction. The free end of the graft was sewn to the subscapularis tendon (Figure 6). The remaining marrow concentrate was injected both deep and superficial to the rotator cuff construct. No additional wound irrigation fluid was injected or suction drain inserted. After surgery, the patient was placed into an abduction pillow for 1 month and then engaged in passive motion for 1 month. Active-assisted motion began 3 months after surgery.
Results
Clinically, all patients improved with respect to pain, motion, strength, function, and satisfaction by virtue of the reconstructive surgery. After surgery, mean Constant score was increased, from 13 to 71 (P < .001). Mean ASES score increased from 18 to 75 (P < .001). Mean UCLA score increased from 4 to 28 (P < .001) (Table). Ultrasound showed 0% incidence of full-thickness retearing. Dynamic scanning during abduction showed maintained reduction of the humeral head within the glenoid socket; superior subluxation of the humeral head was not detected. The biceps tendon graft was continuous with the rotator cuff tendon, indicative of graft integration: tissue healing at the graft–bone and graft–tendon interfaces (Figures 7, 8). There were no intraoperative or postoperative patient-related complications.
Discussion
Primary rotator cuff surgery is beneficial.5 Irrespective of technique, open versus arthroscopic,6 single- versus double-row repair,7 the clinical results have been satisfactory.8 Nevertheless, the “tissue failure” rate of rotator cuff surgery (full-thickness discontinuity of rotator cuff) has been as high as 31% in primary repairs.9 In revision rotator cuff repair and reconstruction, the radiographic tissue failure rate has been even higher, particularly in the setting of chronic large tears with retraction, with tissue failure rates up to 91%.10 Although small to medium full-thickness tears and retears are well tolerated by patients with reduced activity levels,11 and pain symptoms do not necessarily correlate with rotator cuff tear size,12 large retracted full-thickness tears in active patients seldom result in optimal clinical outcomes or patient satisfaction.13,14 In addition, although recurrent tearing does not preclude a satisfactory clinical result, maintenance of cuff tissue integrity tends to produce a better objective clinical score and a more desirable clinical outcome.2
Few evidence-based restorative solutions exist for large recurrent rotator cuff tears with retraction in active nongeriatric patients.15 The no-treatment option in this context may result in gradual enlargement of the tear, chronic pain, weakness, and progressive degeneration of the glenohumeral joint and acromiohumeral confluence—so-called rotator cuff arthropathy, for which reverse total shoulder arthroplasty is required.16,17 Partial repair of a large rotator cuff tear by margin convergence, interval slide, split deltoid flap, or nonanatomical reinsertion may improve clinical outcome scores but may not alter or prevent the progressive degenerative changes associated with rotator cuff arthropathy.18,19 Synthetic scaffolds with and without biological enhancement have been used with varying degrees of success, particularly pain improvement and tissue integration.20 Nevertheless, the failure rate has been reported to be 17% to 51%,21 and no evidence exists that allograft augmentation improves functional outcomes.22 Tendon transfer using the latissimus dorsi has also proved to be a surgical alternative in younger, active patients.23 However, dissection in this procedure is a major undertaking for both surgeon and patient—compared with the minimally invasive technique used in the present study.24
I selected a cohort of active, symptomatic patients for application of a synthesis of accepted surgical techniques through a mini-open incision in order to improve the reliability of the surgical construct while minimizing surgical morbidity. Débridement of marginal tissue, safe mobilization of remaining cuff, and tension-free suture line using locking sutures maximized the mechanical strength of the construct.25,26 Biological enhancement with autogenous tissue (the patient’s own biceps tendon) as graft material (scaffolding), as well as autologous concentrated marrow delivering viable responding cells and chemokine/cytokine biofactors, increased the probability of reparative activity at the graft site.27 The net effect was consistent tissue healing at a biologically challenging locus. Nonenhanced biceps tendon grafting in the setting of “irreparable” primary rotator cuff repair has had a 40-year history of orthopedic utility and an excellent record of clinical success.28 Nevertheless, the retear rate has been 7% to 30%.29 There are no previous reports of biologically enhanced autogenous biceps tendon grafting for reconstruction of a torn rotator cuff, either primary or in the setting of chronic revision surgery.
Previous well-designed PRP enhancement studies in the context of primary rotator cuff repair failed to demonstrate a consistent benefit with concentrated platelet-only augmentation.30,31 The shared experimental design of these published studies used intra-articular injection as the sole delivery method without guarantee that the injected platelets would migrate, adhere to, and persist at the intended destination, the healing edge of the rotator cuff. In the present study, extended exposure of the splayed tendon graft by incubation in concentrated marrow was specifically designed to increase the probability that biologically active components would settle at the desired location by cellular seeding and plasmatic imbibition.32 Furthermore, use of PRP for growth factor (platelet-derived, PDGF; basic fibroblast, bFGF; transforming, TGF-β; epidermal, EGF; vascular endothelial, VEGF; connective tissue, CTGF) therapy, in addition to pluripotential mesenchymal cells for marrow-derived stem cell therapy, is in theory biologically superior to use of PRP alone.33,34
The recent expansion of information about biologics has generated much interest in augmentation of soft-tissue healing. Unfortunately, the optimal technique of using cellular processing to upregulate stem-cell capacity at the graft interface is yet to be defined.35 Clinical studies using PRP and related products to promote tendon healing have been both inconsistent and contradictory with respect to benefit of outcome. As we have been unable to harness the biological potential of this medium, application of biologics in contemporary clinical orthopedics remains narrow, random, and infrequent. The technique presented in this clinical series appears to be a small advancement in a positive direction. The described construct provides a starting point for study, combining mechanical as well as biological steps to promote rotator cuff healing. The consistency of the outcome in a clinical model in which retearing is an expectation rather than an exception is noteworthy. The zero tissue failure rate at 1 to 4 years, compared with the literature values in similar patient cohorts, is very promising.36 The clinical outcome as measured by validated shoulder scores is also comparable to literature outcome values.19 Also noteworthy is the dynamic stability the construct gives to the glenohumeral joint. Ideally, the reconstructed rotator cuff provides active force coupling with the deltoid, simulating normal shoulder biomechanics. At a minimum, the reconstructed cuff provides a viable passive barrier to superior migration of the humeral head—thus supporting the mechanical efficiency of the deltoid and preventing rotator cuff arthropathy.
This study’s small sample (10 patients) puts its conclusions at risk for type I statistical error, in that too few patients were examined over a long enough period to demonstrate failure. Nevertheless, retears typically occur within 6 months of repair.37,38 Therefore, minimum follow-up of 1 year was deemed sufficient. None of the 10 patients had diabetes or another chronic comorbidity. Nine of the 10 had either no or only mild preoperative fatty atrophy of the rotator cuff muscles. Eight of the 10 were nonsmokers. These factors, which suggest optimal surgical candidates, may prove to be significant as the clinical series expands over time. Incubation of the autogenous biceps graft in concentrated marrow for 60 minutes was arbitrarily chosen. In future in vitro examination, marrow cell viability as a function of incubation time will be assessed.
Conclusion
In active, middle-aged patients with chronic recurrent large retracted rotator cuff tears, the technique presented here, using autogenous biceps tendon and autologous concentrated marrow containing PRP and mesenchymal cells, consistently yielded satisfactory clinical results and promoted rotator cuff tissue healing without full-thickness retearing.
1. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
2. Kim HM, Caldwell JM, Buza JA, et al. Factors affecting satisfaction and shoulder function in patients with a recurrent rotator cuff tear. J Bone Joint Surg Am. 2014;96(2):106-112.
3. George MS, Khazzam M. Current concepts review: revision rotator cuff repair. J Shoulder Elbow Surg. 2012;21(4):431-440.
4. Skoff HD. Conservative open acromioplasty. J Bone Joint Surg Br. 1995;77(6):933-936.
5. Mather RC 3rd, Koenig L, Acevedo D, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993-2000.
6. Sauerbrey AM, Getz CL, Piancastelli M, Iannotti JP, Ramsey ML, Williams GR. Arthroscopic versus mini-open rotator cuff repair: a comparison of clinical outcome. Arthroscopy. 2005;21(12):1415-1420.
7. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
8. Galatz LM, Griggs S, Cameron BD, Iannotti JP. Prospective longitudinal analysis of post-operative shoulder function: a ten-year follow-up study of full thickness rotator cuff tears. J Bone Joint Surg Am. 2001;83(7):1052-1056.
9. Oh JH, Kim SH, Kang JY, Oh CH, Gong HS. Effect of age on functional and structural outcome after rotator cuff repair. Am J Sports Med. 2010;38(4):672-678.
10. Kim JH, Kim SH, Lee SK, Seo JW, Chun YMC. Arthroscopic repair of massive contracted rotator cuff tears: aggressive release with anterior and posterior interval slides do not improve cuff healing and integrity. J Bone Joint Surg Am. 2014;95(16):1482-1488.
11. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears. J Bone Joint Surg Am. 2014;96(18):1504-1514.
12. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity. J Bone Joint Surg Am. 2014;96(10):793-800.
13. Lubiatowski P, Kaczmarek P, Dzianach M, et al. Clinical and biomechanical performance of patients with failed rotator cuff repair. Int Orthop. 2013;37(12):2395-2401.
14. Holtby R, Razmjou H. Relationship between clinical and surgical findings and reparability of large and massive rotator cuff tears: a longitudinal study. BMC Musculoskelet Disord. 2014;15:180.
15. Nho SJ, Delos D, Yadav H, et al. Biomechanical and biological augmentation for the treatment of massive rotator cuff tears. Am J Sports Med. 2010;38(3):619-629.
16. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
17. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
18. Bartl C, Louloumentas P, Konstantin H, et al. Long-term outcome and structural integrity following open repair of massive rotator cuff tears. Int J Shoulder Surg. 2012;6(1):1-8.
19. Paxton ES, Teefey SA, Dahiya N, Keener JD, Yamaguchi K, Galatz LM. Clinical and radiographic outcomes of failed repairs of large or massive rotator cuff tears: minimum ten-year follow-up. J Bone Joint Surg Am. 2013;95(7):627-632.
20. Longo UG, Lamberti A, Maffulli N, Denaro V. Tendon augmentation grafts: a systematic review. Br Med Bull. 2010;94:165-188.
21. Ciampi P, Scotti C, Nonis A, et al. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: a 3-year follow-up study. Am J Sports Med. 2014;42(5):1169-1175.
22. Murhi AM. Rotator cuff tears and cuff tear arthropathy. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:921-929.
23. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
24. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
25. Wagner JP, Krushall RJ, Masqueloet A, Gerber C. Anatomy and relationships of the suprascapular nerve: anatomical constraints to mobilization of the supraspinatus and infraspinatus muscles in the management of massive rotator cuff tears. J Bone Joint Surg Am. 1992;74(1):36-45.
26. Ponce BA, Hosemann CD, Reghava P, Tate JP, Sheppard ED, Ebenhardt AW. A biomechanical analysis of controllable intraoperative variables affecting the strength of rotator cuff repairs at the suture–tendon interface. Am J Sports Med. 2013;41(10):2256-2261.
27. Thomopoulos S. Tendon and ligaments. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:105-111.
28. Sano H, Mineta M, Kitz A, Itoi E. Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci. 2010;15(3):310-316.
29. Rhee YG, Cho NS, Lim CT, Yi JW, Vishvanathan T. Bridging the gap in immobile massive rotator cuff tears: augmentation using the tenotomized biceps. Am J Sports Med. 2008;36(8):1511-1518.
30. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
31. Rodeo SA, Delos, D, Williams, RJ, Adler RS, Pearle A, Warren RF. The effects of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
32. Beitzel K, McCarthy MB, Cote MP, et al. Properties of biologic scaffolds and their response to mesenchymal stem cells. Arthroscopy. 2014;30(3):289-298.
33. Anz AW, Hackel JG, Nilssen ED, Andrews JR. Application of biologics in the treatment of rotator cuff, meniscus, cartilage and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
34. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
35. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
36. Kowalsky MS, Keener JD. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome: surgical technique. J Bone Joint Surg Am. 2011;93(suppl 1):62-74.
37. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
38. Le BT, Wu XL, Lam PH, Murrell GA. Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 2014;42(5):1134-1142.
1. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
2. Kim HM, Caldwell JM, Buza JA, et al. Factors affecting satisfaction and shoulder function in patients with a recurrent rotator cuff tear. J Bone Joint Surg Am. 2014;96(2):106-112.
3. George MS, Khazzam M. Current concepts review: revision rotator cuff repair. J Shoulder Elbow Surg. 2012;21(4):431-440.
4. Skoff HD. Conservative open acromioplasty. J Bone Joint Surg Br. 1995;77(6):933-936.
5. Mather RC 3rd, Koenig L, Acevedo D, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993-2000.
6. Sauerbrey AM, Getz CL, Piancastelli M, Iannotti JP, Ramsey ML, Williams GR. Arthroscopic versus mini-open rotator cuff repair: a comparison of clinical outcome. Arthroscopy. 2005;21(12):1415-1420.
7. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
8. Galatz LM, Griggs S, Cameron BD, Iannotti JP. Prospective longitudinal analysis of post-operative shoulder function: a ten-year follow-up study of full thickness rotator cuff tears. J Bone Joint Surg Am. 2001;83(7):1052-1056.
9. Oh JH, Kim SH, Kang JY, Oh CH, Gong HS. Effect of age on functional and structural outcome after rotator cuff repair. Am J Sports Med. 2010;38(4):672-678.
10. Kim JH, Kim SH, Lee SK, Seo JW, Chun YMC. Arthroscopic repair of massive contracted rotator cuff tears: aggressive release with anterior and posterior interval slides do not improve cuff healing and integrity. J Bone Joint Surg Am. 2014;95(16):1482-1488.
11. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears. J Bone Joint Surg Am. 2014;96(18):1504-1514.
12. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity. J Bone Joint Surg Am. 2014;96(10):793-800.
13. Lubiatowski P, Kaczmarek P, Dzianach M, et al. Clinical and biomechanical performance of patients with failed rotator cuff repair. Int Orthop. 2013;37(12):2395-2401.
14. Holtby R, Razmjou H. Relationship between clinical and surgical findings and reparability of large and massive rotator cuff tears: a longitudinal study. BMC Musculoskelet Disord. 2014;15:180.
15. Nho SJ, Delos D, Yadav H, et al. Biomechanical and biological augmentation for the treatment of massive rotator cuff tears. Am J Sports Med. 2010;38(3):619-629.
16. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
17. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
18. Bartl C, Louloumentas P, Konstantin H, et al. Long-term outcome and structural integrity following open repair of massive rotator cuff tears. Int J Shoulder Surg. 2012;6(1):1-8.
19. Paxton ES, Teefey SA, Dahiya N, Keener JD, Yamaguchi K, Galatz LM. Clinical and radiographic outcomes of failed repairs of large or massive rotator cuff tears: minimum ten-year follow-up. J Bone Joint Surg Am. 2013;95(7):627-632.
20. Longo UG, Lamberti A, Maffulli N, Denaro V. Tendon augmentation grafts: a systematic review. Br Med Bull. 2010;94:165-188.
21. Ciampi P, Scotti C, Nonis A, et al. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: a 3-year follow-up study. Am J Sports Med. 2014;42(5):1169-1175.
22. Murhi AM. Rotator cuff tears and cuff tear arthropathy. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:921-929.
23. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
24. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
25. Wagner JP, Krushall RJ, Masqueloet A, Gerber C. Anatomy and relationships of the suprascapular nerve: anatomical constraints to mobilization of the supraspinatus and infraspinatus muscles in the management of massive rotator cuff tears. J Bone Joint Surg Am. 1992;74(1):36-45.
26. Ponce BA, Hosemann CD, Reghava P, Tate JP, Sheppard ED, Ebenhardt AW. A biomechanical analysis of controllable intraoperative variables affecting the strength of rotator cuff repairs at the suture–tendon interface. Am J Sports Med. 2013;41(10):2256-2261.
27. Thomopoulos S. Tendon and ligaments. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:105-111.
28. Sano H, Mineta M, Kitz A, Itoi E. Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci. 2010;15(3):310-316.
29. Rhee YG, Cho NS, Lim CT, Yi JW, Vishvanathan T. Bridging the gap in immobile massive rotator cuff tears: augmentation using the tenotomized biceps. Am J Sports Med. 2008;36(8):1511-1518.
30. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
31. Rodeo SA, Delos, D, Williams, RJ, Adler RS, Pearle A, Warren RF. The effects of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
32. Beitzel K, McCarthy MB, Cote MP, et al. Properties of biologic scaffolds and their response to mesenchymal stem cells. Arthroscopy. 2014;30(3):289-298.
33. Anz AW, Hackel JG, Nilssen ED, Andrews JR. Application of biologics in the treatment of rotator cuff, meniscus, cartilage and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
34. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
35. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
36. Kowalsky MS, Keener JD. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome: surgical technique. J Bone Joint Surg Am. 2011;93(suppl 1):62-74.
37. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
38. Le BT, Wu XL, Lam PH, Murrell GA. Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 2014;42(5):1134-1142.
Reducing Postoperative Fracture Displacement After Locked Plating of Proximal Humerus Fractures: Current Concepts
Proximal humerus fractures account for 4% to 5% of all fractures.1 These fractures occur most frequently in the elderly—patients older than 60 years sustain 71% of these injuries2—and in females.1,3 Given an aging population, this incidence is predicted to increase 3-fold over the next 30 years.4 There is much debate regarding management of acute, displaced proximal humerus fractures. A recent Cochrane Review of published outcomes of operative and nonoperative treatment of displaced proximal humerus fractures found insufficient evidence supporting either modality, though surgery was associated with additional procedures.5 A review of 1000 proximal humerus fractures found that 49% had less than 1 cm of displacement of the major fragments or angulation of less than 45°.3 Other authors have reported similar findings.6,7 Although the incidence of proximal humerus fractures has remained stable over the past decade, from 1999 to 2005 there was a 25% relative increase in surgical management, including a relative increase of 29% in open reduction and internal fixation (ORIF) versus a 20% increase in arthroplasty.1
Locking plates have consistently demonstrated biomechanical superiority over other forms of fixation in osteoporotic bone.8-11 Egol and colleagues8 found that osteoporotic bone limited the torque of fixation to values less than what is required for adequate frictional force between the plate and the bone. This problem can be overcome with fixed-angle devices, such as locked plates.9 Compared with locked nail constructs, proximal humerus locking plates have demonstrated superiority in torsion, loading, and varus bending.10,11 Compared with blade plates, proximal humerus locking plates exhibited increased stiffness and torsional fatigue resistance.12 In a randomized clinical trial, Olerud and colleagues13 reported superior functional results with locking plate fixation compared with nonoperative treatment of displaced 3-part fractures in elderly patients with 2-year follow-up, though these clinical results were not supported by others.14 Two recent case–control studies comparing functional outcomes for 3- and 4-part fractures with follow-up of more than 2 years revealed higher Constant scores after locked plating compared with hemiarthroplasty, though complications were higher with locked plates.15,16 Adoption of locked proximal humerus plating has been correlated with good clinical outcomes and union rates, though this has been accompanied by a higher rate of reoperation.7 Reoperation rates from 1999 to 2005 increased both in the immediate postoperative period (odds ratio, 3.36) and at 1 year (odds ratio, 3.90).1
Complications of Locked Plating
Regardless of fixation type, reduced humeral head bone mass and quality may lead to implant loosening, fracture redisplacement, and, ultimately, poor outcomes. Baseline osteoporosis may predict likelihood of fixation failure.17 Multiple studies have reported on the implant-related complications associated with locking plate fixation—most commonly, intra-articular screw penetration, postoperative fracture displacement, and avascular necrosis (AVN)18-24 (Figure 1). A meta-analysis of 12 studies with a total of 514 proximal humerus fractures treated with locking plate fixation showed an overall complication rate of 49% and a 13.8% reoperation rate.25 The most common indication for reoperation involved intra-articular screw perforation. The most common complications were varus malunion (16%), osteonecrosis (10%), intra-articular screw penetration (8%), subacromial impingement (6%), and infection (4%).
Suboptimal intraoperative fracture reduction, specifically with residual varus, has been correlated with loss of fracture fixation. In a series of 153 fractures, loss of fixation occurred in 13.7% of cases, with the leading risk factor being varus malreduction.19 Failure rates were 30.4% and 11% when the head shaft angle was less than 120° and when it was 120° or more, respectively. Solberg and colleagues16 found that initial postoperative varus angulation of more than 20° resulted in universal loss of fixation. Conversion of these cases to hemiarthroplasty resulted in poor outcomes. Preoperative fracture alignment may also predict fixation failure.22 In one series, initial varus angulation healed with a mean 16° varus and a Constant score of 63, whereas initial valgus alignment healed with 6° varus and a Constant score of 71.22 Complications occurred in fractures that were initially in varus 79% of the time and initially in valgus 19% of the time. Screw perforation has been associated with loss of reduction 44% of the time.20
In an analysis of locking plate constructs revised after early (<4 weeks) failure in 8 patients with osteoporosis, Micic and colleagues21 found implant pullout leading to varus malalignment. All cases lacked medial support and subchondral screw purchase; 3 were initially malreduced. Owsley and Gorczyca23 retrospectively reviewed 53 cases of displaced proximal humerus fractures treated with locked plating. Despite the high rate of radiographic union, 36% developed complications, including screw cutout (23%), varus displacement of more than 10° (25%), and AVN (4%); 13% required revision. These complications disproportionately affected patients older than 60 years (57%) and negatively affected functional outcomes.
Augmentation Techniques
Despite its reported complications, proximal humerus locked plating remains the most widely used type of fixation.1 Advancements in locking plate design, improved understanding of fixation principles, and adoption of techniques augmenting proximal humerus locking plate fixation, particularly in osteoporotic bone, have reduced postoperative complications (Table 1).
Rotator Cuff Sutures
A widely adopted technique for neutralizing rotator cuff–deforming forces, which theoretically can cause fracture displacement, is incorporation of heavy nonabsorbable sutures. These sutures are placed through the rotator cuff–tuberosity junction and tied down after being passed through the plate. Obtaining and maintaining tuberosity reduction are essential in achieving good functional outcomes after fixation. In addition, tension band sutures may be particularly useful in the setting of initial varus deformity.26
Although clinical use of these sutures is common, biomechanical studies of their adjunctive contribution to fracture stability are lacking.27 The rotator cuff musculature has a maximal contractile force of 3.5 kg/cm2.28 Ricchetti and colleagues29 described a technique that involves using a locked plate and tagging the rotator cuff with heavy nonabsorbable sutures. Selective traction on the sutures can help obtain and maintain fracture reduction. Multiple studies have reported on suture use with locked plating for proximal humerus fractures.29-34 Badman and colleagues30 retrospectively reviewed 81 cases of metaphyseal defects or medial comminution treated with locked plating, rotator cuff sutures, and structural allograft. All cases healed within 6 months after surgery. The incidence of screw cutout was 3.7%, the incidence of AVN was 6.2%, and the incidence of varus collapse was 6%. A cadaveric study that used specimens (mean age, 77 years) with a simulated 3-part proximal humerus fracture treated with a locked plate both with and without cerclage sutures found no difference in interfragmentary motion between the groups.27 The authors concluded that additive sutures are not required for anatomically reduced fractures. Multiple sutures may counteract the deforming forces that act on bony segments that cannot be adequately maintained with screws, such as an osteoporotic greater tuberosity.
Medial Column Restoration
The importance of reducing and maintaining the medial calcar to provide biomechanical support for a laterally placed plate has been recognized.26,34-37 Gardner and colleagues26 suggested that medial support was achieved if the medial cortex was anatomically reduced, if the proximal fragment was impacted laterally onto the shaft, or if 1 or more inferomedial screws were placed. Cases that did not achieve medial support developed significantly more humeral head subsidence (5.8 mm vs 1.2 mm) and screw penetration. Krappinger and colleagues36 found that factors leading to fixation failure included age, local bone mineral density, anatomical reduction, and restoration of the medial cortical support. The authors concluded that anatomical reduction and restoration of the medial cortex were important in minimizing mechanical loads at the bone–implant interface. Biomechanically, Lescheid and colleagues37 found that the most stable construct was anatomical reduction with medial cortical contact. In the setting of comminution, however, it may be preferable to intentionally perform varus malreduction to achieve medial contact than to achieve anatomical reduction with a fracture gap. Badman and colleagues30 found that the incidence of screw penetration was 6% in patients with an intact medial calcar versus 29% in patients without medial support. In a retrospective analysis of patients treated with a locking plate and suture augmentation, Jung and colleagues35 concluded that restoring medial support was the most reliable factor in the prevention of loss of reduction with or without screw perforation. Last, Solberg and colleagues16 reported better clinical outcomes when the length of the metaphyseal segment attached to the articular fragment was more than 2 mm. A length of less than 2 mm was predictive of developing AVN.
Use of Bone Void Fillers
Allograft. Allograft is cancellous or corticocancellous chips or tricortical graft used as osteoconductive filler for metaphyseal defects.38 An increasingly popular technique involves using an endosteal fibular allograft strut to indirectly reduce the fracture and help support the medial calcar.39-42 Hettrich and colleagues40 reported on radiographic outcomes of displaced proximal humerus fractures with medial comminution treated with a locked plate and an endosteal fibular allograft or semitubular plate. The reduction was maintained in 96% of cases; there was 1 varus collapse. There were no cases of implant failure, screw perforation, or AVN. Other authors have also reported on successful use of fibular allograft in conjunction with a locked plate; the rate of reduction loss was low, and there were no cases of screw cutout or intra-articular screw penetration.30,41,42 These clinical outcomes are supported by results of biomechanical studies of the added benefit of intramedullary fibular allograft.43-46 Mathison and colleagues43 reported that a construct with fibular allograft and a locking plate increased the failure load by 1.72 times and the stiffness by 3.84 times compared with a control group of locking plate only. Bae and colleagues46 found significantly higher maximum failure load and construct stiffness with no varus collapse in specimens prepared with locked plate and fibular strut augmentation compared with a control group.
Others have successfully used cancellous allograft to fill humeral head bone defects.29,32,47-49 Duralde and Leddy47 reported 100% radiographic union and 81% good to excellent results in cases treated with a locking plate and morselized cancellous allograft to fill bone voids. Varus collapse and screw cutout did not occur, but there were 2 cases of AVN. Ricchetti and colleagues29 reviewed 54 cases treated with a locking plate and rotator cuff suture construct. Allograft cancellous chips and demineralized bone matrix were used in 3- and 4-part fractures (70% of cases) along with shorter screws in the humeral head. Major complications included AVN (1), fixation failure (3), and varus malunion (5). Others investigators have had less favorable results with use of cancellous bone graft. Schliemann and colleagues19 reported on 27 patients who were older than 65 years when they underwent ORIF with rotator cuff sutures to stabilize the tuberosities and either cancellous graft or a synthetic bone substitute in patients with massive metaphyseal defects. Patient-reported outcomes were superior to Constant scores. Complications included screw penetration (22.2%), reduction loss (44.4%), implant failure (3.7%), and AVN (29.6%).
Autograft. Autograft has both osteoconductive and osteoinductive properties and has been successfully used for metaphyseal defects.32,50 Kim and colleagues50 reported on patients with 4-part proximal humerus fractures treated with a locking plate and autologous iliac graft. All cases achieved union and had good or excellent outcomes. There were no cases of AVN, varus collapse, or hardware-related complications.
Bone Cement. Calcium phosphate cement has osteoconductive properties and enhances screw purchase in cancellous bone (Figures 2A–2F). It can be injected or molded into bone voids to provide improved compressive strength. It is resorbed through cell-mediated processes resembling bone remodeling and does not disappear until new bone forms. (Calcium sulfate cement, on the other hand, resorbs through a chemical process independent of new bone formation.51) Egol and colleagues52 reviewed the cases of 92 patients (mean age, 61 years) with 2-, 3-, and 4-part proximal humerus fractures treated with locked plate fixation. Metaphyseal defects were treated with no augmentation, augmentation with cancellous chips, or augmentation with calcium phosphate cement. Adding calcium phosphate cement was associated with lower incidence of intra-articular screw penetration and humeral head settling. In a recent cadaveric biomechanical study using 2-part proximal humerus fractures with metaphyseal comminution, the group augmented with calcium phosphate cement had enhanced axial stiffness and load to failure with reduced screw penetration.53 Other biomechanical studies have found increased screw pullout strength54 and decreased interfragmentary motion when specimens were augmented with calcium phosphate cement.55
Similar good clinical and radiographic outcomes have been observed with use of calcium sulfate cement.56,57 Somasundaram and colleagues56 reported good clinical outcomes in 82% of patients treated with locking plates and calcium sulfate cement used to fill metaphyseal voids. All fractures united without infection, fixation failure, subsequent malunion, tuberosity failure, or AVN. Lee and Shin57 compared outcomes of 14 patients who received calcium sulfate augmentation with outcomes of patients who did not receive this augmentation. Overall, 89% of patients had good or excellent results. Calcium sulfate cement did not affect the reduction failure rate or clinical outcomes in cases in which medial cortical reduction was achieved. However, postoperative displacement caused by lack of medial support was associated with poor outcomes.
Screw Placement
Screws optimally should be placed in the posterior-medial-inferior aspect of the humeral head to provide medial support for the fracture and mechanical stability.58 Cadaveric studies have shown the highest cancellous bone density in the proximal, posterior, and medial portions of the humeral head.59-63 Similarly, in a cadaveric study, Liew and colleagues61 found greater screw purchase and higher pullout strength when the screw was placed in the center of the humeral head, within subchondral bone; fixation was poorest when the screw was placed in the anterosuperior region of the humeral head. Tingart and colleagues62 reported that humeral head trabecular density significantly affected pullout strength of cancellous screws. In addition, the most pullout strength was at the center of the head, and the least within the anterosuperior head. Trabecular density was higher in the inferior and posterior regions than in the superior and anterior regions.
Most locking plate designs allow screws to be placed at the level of the medial calcar—the goal being to provide medial column support (Table 2). Zhang and colleagues58 treated 2-, 3-, and 4-part fractures with a locking plate and randomized them into receiving the plate with or without medial support screws. For 3- and 4-part fractures, the group with these screws had a significantly greater final neck-shaft angle and smaller angulation loss compared with the group without screws. No additional benefit was found for 2-part fractures. Erhardt and colleagues63 simulated unstable proximal humerus fractures using cadavers and testing different fixation methods using a polyaxial locking plate. They found that 5 screws in the head fragment and an inferomedial support screw significantly reduced the risk of screw perforation. Other authors have concluded that placing 1 or more inferomedial screws is important in cases of medial comminution or medial column malreduction.26 Interestingly, compared with use of a polyaxial implant, which allows for adjustment of screw direction, use of a monoaxial locking plate did not lead to a clinically different outcome or complication profile.64
Techniques have been used to achieve subchondral purchase of locking screws while reducing iatrogenic articular perforation.65 However, given the incidence of fracture settling and subsequent postoperative screw penetration, many authors currently recommend using shorter divergent screws combined with other augmentation techniques, described previously.17,29,32
Physical Therapy
There is no standardized physiotherapy regimen for postoperative management of proximal humerus fractures treated with locking plates.25 In older patients, immediate active range of motion (ROM) exercises should be delayed until early callus is noted, though there is a risk for stiffness. Lee and Shin57 found that a delay in rehabilitation after ORIF was an independent risk for poor clinical outcome. Namdari and colleagues17 recommended sling use only for comfort and initiated non-load-bearing activities and pendulum exercises immediately after surgery. Patients with adequate reduction at 4 to 6 weeks were advanced to full weight-bearing. Badman and colleagues30 initiated passive-assisted ROM exercises when the wound was healed at 2 weeks in 2-part fractures, whereas patients with 3- and 4-part fractures were immobilized until radiographic healing. Formal therapy was started after 6 weeks. Stiffness was reported in 5% of patients. For patients with stable fixation, Ricchetti and colleagues29 recommended passive shoulder ROM exercises on postoperative day 1; at 4 to 6 weeks, patients should start active shoulder ROM exercises, and then resistance exercises at 10 to 12 weeks. Other authors are more conservative—only sling immobilization and pendulum exercises the first month.66 Barlow and colleagues32 immobilized their patients (age, >75 years) for 6 weeks. No patient developed disabling stiffness. The authors suggested that patients older than 75 years may not be prone to stiffness.
Our Preferred Treatment Method
All proximal humerus fractures are approached anteriorly through the deltopectoral interval (Figure 3A). The long head biceps is identified and truncated for later tenodesis. Multiple No. 5 Ethibond sutures (Ethicon) are placed at the bone–tendon interface. The fracture is reduced with a Cobb elevator (Figure 3B), and provisional Kirschner wires are placed within the head (Figure 3C). The plate is affixed to the humeral head with its anterior border paralleling the posterior aspect of the bicipital groove. Multiple locking screws are placed within the superior and posterior humeral head. Nonlocking screws are then used to fix the plate to the shaft to reduce the specific deformity. Under fluoroscopy, any metaphyseal void is filled with calcium phosphate cement (Figure 3D). The remaining inferior screws are placed within the humeral head. Dr. Gruson uses screws 4 to 6 mm short of subchondral bone to reduce the risk for joint penetration. The rotator cuff sutures are tied down through the plate. Patients are started on progressive supine passive ROM exercises at 7 days, followed by supine active-assisted ROM exercises 6 weeks after fracture healing is confirmed radiographically.
Conclusion
Use of locked plating for proximal humerus fractures has increased, particularly in the elderly. Resulting complications include intra-articular screw penetration, postoperative fracture displacement, and AVN. Recognition of the importance of reducing and supporting the medial calcar, filling any metaphyseal defects, and selectively placing screws within the humeral head has lowered the incidence of these complications. Further comparative studies evaluating the efficacy of individual augmentation techniques are needed to determine their contribution to successful fracture healing and their cost-effectiveness. Results of such studies may help in the development of protocols for more standardized implementation of these techniques and in understanding which specific fracture patterns and patients would benefit from their use.
1. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
2. Aaron D, Shatsky J, Paredes JC, Jiang C, Parsons BO, Flatow EL. Proximal humeral fractures: internal fixation. J Bone Joint Surg Am. 2012;94(24):2280-2288.
3. Court-Brown CM, Garg A, McQueen MM. The epidemiology of proximal humeral fractures. Acta Orthop Scand. 2001;72(4):365-371.
4. Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M, Vuori I. Increasing number and incidence of osteoporotic fractures of the proximal humerus in elderly people. BMJ. 1996;313(7064):1051-1052.
5. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
6. Tamai K, Ishige N, Kuroda S, et al. Four-segment classification of proximal humeral fractures revisited: a multicenter study on 509 cases. J Shoulder Elbow Surg. 2009;18(6):845-850.
7. Rothberg D, Higgins T. Fractures of the proximal humerus. Orthop Clin North Am. 2013;44(1):9-19.
8. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
9. Miranda MA. Locking plate technology and its role in osteoporotic fractures. Injury. 2007;38(suppl 3):35-39.
10. Foruria AM, Carrascal MT, Revilla C, Munuera L, Sanchez-Sotelo J. Proximal humerus fracture rotational stability after fixation using a locking plate or a fixed-angle locked nail: the role of implant stiffness. Clin Biomech. 2010;25(4):307-311.
11. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
12. Siffri PC, Peindl RD, Coley ER, Norton J, Connor PM, Kellam JF. Biomechanical analysis of blade plate versus locking plate fixation for a proximal humerus fracture: comparison using cadaveric and synthetic humeri. J Orthop Trauma. 2006;20(8):547-554.
13. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
14. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
15. Wild JR, DeMers A, French R, et al. Functional outcomes for surgically treated 3- and 4-part proximal humerus fractures. Orthopedics. 2011;34(10):e629-e633.
16. Solberg BD, Moon CN, Franco DP, Paiement GD. Surgical treatment of three and four-part proximal humeral fractures. J Bone Joint Surg Am. 2009;91(7):1689-1697.
17. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
18. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
19. Schliemann B, Siemoneit J, Theisen C, Kosters C, Weimann A, Raschke MJ. Complex fractures of the proximal humerus in the elderly—outcome and complications after locking plate fixation. Musculoskelet Surg. 2012;96(suppl 1):S3-S11.
20. Thanasas C, Kontakis G, Angoules A, Limb D, Giannoudis P. Treatment of proximal humerus fractures with locking plates: a systematic review. J Shoulder Elbow Surg. 2009;18(6):837-844.
21. Micic ID, Kim KC, Shin DJ, et al. Analysis of early failure of the locking compression plate in osteoporotic proximal humerus fractures. J Orthop Sci. 2009;14(5):596-601.
22. Solberg BD, Moon CN, Franco DP, Paiement GD. Locked plating of 3- and 4-part proximal humerus fractures in older patients: the effect of initial fracture pattern on outcome. J Orthop Trauma. 2009;23(2):113-119.
23. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures [corrected]. J Bone Joint Surg Am. 2008;90(2):233-240.
24. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
25. Sproul RC, Iyengar JJ, Devcic Z, Feeley BT. A systematic review of locking plate fixation of proximal humerus fractures. Injury. 2011;42(4):408-413.
26. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
27. Voigt C, Hurschler C, Rech L, Vossenrich R, Lill H. Additive fiber-cerclages in proximal humeral fractures stabilized by locking plates. No effect on fracture stabilization and rotator cuff function in human shoulder specimens. Acta Orthop. 2009;80(4):465-471.
28. Lo IK, Burkhart SS. Biomechanical principles of arthroscopic repair of the rotator cuff. Oper Tech Orthop. 2002;12(3):140-155.
29. Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2010;19(2 suppl):66-75.
30. Badman B, Frankle M, Keating C, Henderson L, Brooks J, Mighell M. Results of proximal humeral locked plating with supplemental suture fixation of rotator cuff. J Shoulder Elbow Surg. 2011;20(4):616-624.
31. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Management of proximal humeral fractures based on current literature. J Bone Joint Surg Am. 2007;89(suppl 3):44-58.
32. Barlow JD, Sanchez-Sotelo J, Torchia M. Proximal humerus fractures in the elderly can be reliably fixed with a “hybrid” locked-plating technique. Clin Orthop. 2011;469(12):3281-3291.
33. Cho CH, Jung GH, Song KS. Tension suture fixation using 2 washers for proximal humeral fractures. Orthopedics. 2012;35(3):202-205.
34. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
35. Jung WB, Moon ES, Kim SK, Kovacevic D, Kim MS. Does medial support decrease major complications of unstable proximal humerus fractures treated with locking plate? BMC Musculoskelet Disord. 2013;14:102.
36. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury. 2011;42(11):1283-1288.
37. Lescheid J, Zdero R, Shah S, Kuzyk PR, Schemitsch EH. The biomechanics of locked plating for repairing proximal humerus fractures with or without medial cortical support. J Trauma. 2010;69(5):1235-1242.
38. De Long WG Jr, Einhorn TA, Koval K, et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89(3):649-658.
39. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
40. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
41. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
42. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop. 2011;469(12):3300-3306.
43. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
44. Osterhoff G, Baumgartner D, Favre P, et al. Medial support by fibula bone graft in angular stable plate fixation of proximal humeral fractures: an in vitro study with synthetic bone. J Shoulder Elbow Surg. 2011;20(5):740-746.
45. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
46. Bae JH, Oh JK, Chon CS, Oh CW, Hwang JH, Yoon YC. The biomechanical performance of locking plate fixation with intramedullary fibular strut graft augmentation in the treatment of unstable fractures of the proximal humerus. J Bone Joint Surg Br. 2011;93(7):937-941.
47. Duralde XA, Leddy LR. The results of ORIF of displaced unstable proximal humeral fractures using a locking plate. J Shoulder Elbow Surg. 2010;19(4):480-488.
48. Robinson CM, Wylie JR, Ray AG, et al. Proximal humeral fractures with a severe varus deformity treated by fixation with a locking plate. J Bone Joint Surg Br. 2010;92(5):672-678.
49. Ong C, Bechtel C, Walsh M, Zuckerman JD, Egol KA. Three- and four-part fractures have poorer function than one-part proximal humerus fractures. Clin Orthop. 2011;469(12):3292-3299.
50. Kim SH, Lee YH, Chung SW, et al. Outcomes for four-part proximal humerus fractures treated with a locking compression plate and an autologous iliac bone impaction graft. Injury. 2012;43(10):1724-1731.
51. Larsson S. Calcium phosphates: what is the evidence? J Orthop Trauma. 2010;24(suppl 1):S41-S45.
52. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction–internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
53. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
54. Collinge C, Merk B, Lautenschlager EP. Mechanical evaluation of fracture fixation augmented with tricalcium phosphate bone cement in a porous osteoporotic cancellous bone model. J Orthop Trauma. 2007;21(2):124-128.
55. Kwon BK, Goertzen DJ, O’Brien PJ, Broekhuyse HM, Oxland TR. Biomechanical evaluation of proximal humeral fracture fixation supplemented with calcium phosphate cement. J Bone Joint Surg Am. 2002;84(6):951-961.
56. Somasundaram K, Huber CP, Babu V, Zadeh H. Proximal humeral fractures: the role of calcium sulphate augmentation and extended deltoid splitting approach in internal fixation using locking plates. Injury. 2013;44(4):481-487.
57. Lee CW, Shin SJ. Prognostic factors for unstable proximal humeral fractures treated with locking-plate fixation. J Shoulder Elbow Surg. 2009;18(1):83-88.
58. Zhang L, Zheng J, Wang W, et al. The clinical benefit of medial support screws in locking plating of proximal humerus fractures: a prospective randomized study. Int Orthop. 2011;35(11):1655-1661.
59. Brianza S, Roderer G, Schiuma D, et al. Where do locking screws purchase in the humeral head? Injury. 2012;43(6):850-855.
60. Hepp P, Lill H, Bail H, et al. Where should implants be anchored in the humeral head? Clin Orthop. 2003;(415):139-147.
61. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
62. Tingart MJ, Lehtinen J, Zurakowski D, Warner JJ, Apreleva M. Proximal humeral fractures: regional differences in bone mineral density of the humeral head affect the fixation strength of cancellous screws. J Shoulder Elbow Surg. 2006;15(5):620-624.
63. Erhardt JB, Stoffel K, Kampshoff J, Badur N, Yates P, Kuster MS. The position and number of screws influence screw perforation of the humeral head in modern locking plates: a cadaver study. J Orthop Trauma. 2012;26(10):e188-e192.
64. Konigshausen M, Kubler L, Godry H, Citak M, Schildhauer TA, Seybold D. Clinical outcome and complications using a polyaxial locking plate in the treatment of displaced proximal humerus fractures. A reliable system? Injury. 2012;43(2):223-231.
65. Bengard MJ, Gardner MJ. Screw depth sounding in proximal humerus fractures to avoid iatrogenic intra-articular penetration. J Orthop Trauma. 2011;25(10):630-633.
66. Ring D. Current concepts in plate and screw fixation of osteoporotic proximal humerus fractures. Injury. 2007;38(3):S59-S68.
Proximal humerus fractures account for 4% to 5% of all fractures.1 These fractures occur most frequently in the elderly—patients older than 60 years sustain 71% of these injuries2—and in females.1,3 Given an aging population, this incidence is predicted to increase 3-fold over the next 30 years.4 There is much debate regarding management of acute, displaced proximal humerus fractures. A recent Cochrane Review of published outcomes of operative and nonoperative treatment of displaced proximal humerus fractures found insufficient evidence supporting either modality, though surgery was associated with additional procedures.5 A review of 1000 proximal humerus fractures found that 49% had less than 1 cm of displacement of the major fragments or angulation of less than 45°.3 Other authors have reported similar findings.6,7 Although the incidence of proximal humerus fractures has remained stable over the past decade, from 1999 to 2005 there was a 25% relative increase in surgical management, including a relative increase of 29% in open reduction and internal fixation (ORIF) versus a 20% increase in arthroplasty.1
Locking plates have consistently demonstrated biomechanical superiority over other forms of fixation in osteoporotic bone.8-11 Egol and colleagues8 found that osteoporotic bone limited the torque of fixation to values less than what is required for adequate frictional force between the plate and the bone. This problem can be overcome with fixed-angle devices, such as locked plates.9 Compared with locked nail constructs, proximal humerus locking plates have demonstrated superiority in torsion, loading, and varus bending.10,11 Compared with blade plates, proximal humerus locking plates exhibited increased stiffness and torsional fatigue resistance.12 In a randomized clinical trial, Olerud and colleagues13 reported superior functional results with locking plate fixation compared with nonoperative treatment of displaced 3-part fractures in elderly patients with 2-year follow-up, though these clinical results were not supported by others.14 Two recent case–control studies comparing functional outcomes for 3- and 4-part fractures with follow-up of more than 2 years revealed higher Constant scores after locked plating compared with hemiarthroplasty, though complications were higher with locked plates.15,16 Adoption of locked proximal humerus plating has been correlated with good clinical outcomes and union rates, though this has been accompanied by a higher rate of reoperation.7 Reoperation rates from 1999 to 2005 increased both in the immediate postoperative period (odds ratio, 3.36) and at 1 year (odds ratio, 3.90).1
Complications of Locked Plating
Regardless of fixation type, reduced humeral head bone mass and quality may lead to implant loosening, fracture redisplacement, and, ultimately, poor outcomes. Baseline osteoporosis may predict likelihood of fixation failure.17 Multiple studies have reported on the implant-related complications associated with locking plate fixation—most commonly, intra-articular screw penetration, postoperative fracture displacement, and avascular necrosis (AVN)18-24 (Figure 1). A meta-analysis of 12 studies with a total of 514 proximal humerus fractures treated with locking plate fixation showed an overall complication rate of 49% and a 13.8% reoperation rate.25 The most common indication for reoperation involved intra-articular screw perforation. The most common complications were varus malunion (16%), osteonecrosis (10%), intra-articular screw penetration (8%), subacromial impingement (6%), and infection (4%).
Suboptimal intraoperative fracture reduction, specifically with residual varus, has been correlated with loss of fracture fixation. In a series of 153 fractures, loss of fixation occurred in 13.7% of cases, with the leading risk factor being varus malreduction.19 Failure rates were 30.4% and 11% when the head shaft angle was less than 120° and when it was 120° or more, respectively. Solberg and colleagues16 found that initial postoperative varus angulation of more than 20° resulted in universal loss of fixation. Conversion of these cases to hemiarthroplasty resulted in poor outcomes. Preoperative fracture alignment may also predict fixation failure.22 In one series, initial varus angulation healed with a mean 16° varus and a Constant score of 63, whereas initial valgus alignment healed with 6° varus and a Constant score of 71.22 Complications occurred in fractures that were initially in varus 79% of the time and initially in valgus 19% of the time. Screw perforation has been associated with loss of reduction 44% of the time.20
In an analysis of locking plate constructs revised after early (<4 weeks) failure in 8 patients with osteoporosis, Micic and colleagues21 found implant pullout leading to varus malalignment. All cases lacked medial support and subchondral screw purchase; 3 were initially malreduced. Owsley and Gorczyca23 retrospectively reviewed 53 cases of displaced proximal humerus fractures treated with locked plating. Despite the high rate of radiographic union, 36% developed complications, including screw cutout (23%), varus displacement of more than 10° (25%), and AVN (4%); 13% required revision. These complications disproportionately affected patients older than 60 years (57%) and negatively affected functional outcomes.
Augmentation Techniques
Despite its reported complications, proximal humerus locked plating remains the most widely used type of fixation.1 Advancements in locking plate design, improved understanding of fixation principles, and adoption of techniques augmenting proximal humerus locking plate fixation, particularly in osteoporotic bone, have reduced postoperative complications (Table 1).
Rotator Cuff Sutures
A widely adopted technique for neutralizing rotator cuff–deforming forces, which theoretically can cause fracture displacement, is incorporation of heavy nonabsorbable sutures. These sutures are placed through the rotator cuff–tuberosity junction and tied down after being passed through the plate. Obtaining and maintaining tuberosity reduction are essential in achieving good functional outcomes after fixation. In addition, tension band sutures may be particularly useful in the setting of initial varus deformity.26
Although clinical use of these sutures is common, biomechanical studies of their adjunctive contribution to fracture stability are lacking.27 The rotator cuff musculature has a maximal contractile force of 3.5 kg/cm2.28 Ricchetti and colleagues29 described a technique that involves using a locked plate and tagging the rotator cuff with heavy nonabsorbable sutures. Selective traction on the sutures can help obtain and maintain fracture reduction. Multiple studies have reported on suture use with locked plating for proximal humerus fractures.29-34 Badman and colleagues30 retrospectively reviewed 81 cases of metaphyseal defects or medial comminution treated with locked plating, rotator cuff sutures, and structural allograft. All cases healed within 6 months after surgery. The incidence of screw cutout was 3.7%, the incidence of AVN was 6.2%, and the incidence of varus collapse was 6%. A cadaveric study that used specimens (mean age, 77 years) with a simulated 3-part proximal humerus fracture treated with a locked plate both with and without cerclage sutures found no difference in interfragmentary motion between the groups.27 The authors concluded that additive sutures are not required for anatomically reduced fractures. Multiple sutures may counteract the deforming forces that act on bony segments that cannot be adequately maintained with screws, such as an osteoporotic greater tuberosity.
Medial Column Restoration
The importance of reducing and maintaining the medial calcar to provide biomechanical support for a laterally placed plate has been recognized.26,34-37 Gardner and colleagues26 suggested that medial support was achieved if the medial cortex was anatomically reduced, if the proximal fragment was impacted laterally onto the shaft, or if 1 or more inferomedial screws were placed. Cases that did not achieve medial support developed significantly more humeral head subsidence (5.8 mm vs 1.2 mm) and screw penetration. Krappinger and colleagues36 found that factors leading to fixation failure included age, local bone mineral density, anatomical reduction, and restoration of the medial cortical support. The authors concluded that anatomical reduction and restoration of the medial cortex were important in minimizing mechanical loads at the bone–implant interface. Biomechanically, Lescheid and colleagues37 found that the most stable construct was anatomical reduction with medial cortical contact. In the setting of comminution, however, it may be preferable to intentionally perform varus malreduction to achieve medial contact than to achieve anatomical reduction with a fracture gap. Badman and colleagues30 found that the incidence of screw penetration was 6% in patients with an intact medial calcar versus 29% in patients without medial support. In a retrospective analysis of patients treated with a locking plate and suture augmentation, Jung and colleagues35 concluded that restoring medial support was the most reliable factor in the prevention of loss of reduction with or without screw perforation. Last, Solberg and colleagues16 reported better clinical outcomes when the length of the metaphyseal segment attached to the articular fragment was more than 2 mm. A length of less than 2 mm was predictive of developing AVN.
Use of Bone Void Fillers
Allograft. Allograft is cancellous or corticocancellous chips or tricortical graft used as osteoconductive filler for metaphyseal defects.38 An increasingly popular technique involves using an endosteal fibular allograft strut to indirectly reduce the fracture and help support the medial calcar.39-42 Hettrich and colleagues40 reported on radiographic outcomes of displaced proximal humerus fractures with medial comminution treated with a locked plate and an endosteal fibular allograft or semitubular plate. The reduction was maintained in 96% of cases; there was 1 varus collapse. There were no cases of implant failure, screw perforation, or AVN. Other authors have also reported on successful use of fibular allograft in conjunction with a locked plate; the rate of reduction loss was low, and there were no cases of screw cutout or intra-articular screw penetration.30,41,42 These clinical outcomes are supported by results of biomechanical studies of the added benefit of intramedullary fibular allograft.43-46 Mathison and colleagues43 reported that a construct with fibular allograft and a locking plate increased the failure load by 1.72 times and the stiffness by 3.84 times compared with a control group of locking plate only. Bae and colleagues46 found significantly higher maximum failure load and construct stiffness with no varus collapse in specimens prepared with locked plate and fibular strut augmentation compared with a control group.
Others have successfully used cancellous allograft to fill humeral head bone defects.29,32,47-49 Duralde and Leddy47 reported 100% radiographic union and 81% good to excellent results in cases treated with a locking plate and morselized cancellous allograft to fill bone voids. Varus collapse and screw cutout did not occur, but there were 2 cases of AVN. Ricchetti and colleagues29 reviewed 54 cases treated with a locking plate and rotator cuff suture construct. Allograft cancellous chips and demineralized bone matrix were used in 3- and 4-part fractures (70% of cases) along with shorter screws in the humeral head. Major complications included AVN (1), fixation failure (3), and varus malunion (5). Others investigators have had less favorable results with use of cancellous bone graft. Schliemann and colleagues19 reported on 27 patients who were older than 65 years when they underwent ORIF with rotator cuff sutures to stabilize the tuberosities and either cancellous graft or a synthetic bone substitute in patients with massive metaphyseal defects. Patient-reported outcomes were superior to Constant scores. Complications included screw penetration (22.2%), reduction loss (44.4%), implant failure (3.7%), and AVN (29.6%).
Autograft. Autograft has both osteoconductive and osteoinductive properties and has been successfully used for metaphyseal defects.32,50 Kim and colleagues50 reported on patients with 4-part proximal humerus fractures treated with a locking plate and autologous iliac graft. All cases achieved union and had good or excellent outcomes. There were no cases of AVN, varus collapse, or hardware-related complications.
Bone Cement. Calcium phosphate cement has osteoconductive properties and enhances screw purchase in cancellous bone (Figures 2A–2F). It can be injected or molded into bone voids to provide improved compressive strength. It is resorbed through cell-mediated processes resembling bone remodeling and does not disappear until new bone forms. (Calcium sulfate cement, on the other hand, resorbs through a chemical process independent of new bone formation.51) Egol and colleagues52 reviewed the cases of 92 patients (mean age, 61 years) with 2-, 3-, and 4-part proximal humerus fractures treated with locked plate fixation. Metaphyseal defects were treated with no augmentation, augmentation with cancellous chips, or augmentation with calcium phosphate cement. Adding calcium phosphate cement was associated with lower incidence of intra-articular screw penetration and humeral head settling. In a recent cadaveric biomechanical study using 2-part proximal humerus fractures with metaphyseal comminution, the group augmented with calcium phosphate cement had enhanced axial stiffness and load to failure with reduced screw penetration.53 Other biomechanical studies have found increased screw pullout strength54 and decreased interfragmentary motion when specimens were augmented with calcium phosphate cement.55
Similar good clinical and radiographic outcomes have been observed with use of calcium sulfate cement.56,57 Somasundaram and colleagues56 reported good clinical outcomes in 82% of patients treated with locking plates and calcium sulfate cement used to fill metaphyseal voids. All fractures united without infection, fixation failure, subsequent malunion, tuberosity failure, or AVN. Lee and Shin57 compared outcomes of 14 patients who received calcium sulfate augmentation with outcomes of patients who did not receive this augmentation. Overall, 89% of patients had good or excellent results. Calcium sulfate cement did not affect the reduction failure rate or clinical outcomes in cases in which medial cortical reduction was achieved. However, postoperative displacement caused by lack of medial support was associated with poor outcomes.
Screw Placement
Screws optimally should be placed in the posterior-medial-inferior aspect of the humeral head to provide medial support for the fracture and mechanical stability.58 Cadaveric studies have shown the highest cancellous bone density in the proximal, posterior, and medial portions of the humeral head.59-63 Similarly, in a cadaveric study, Liew and colleagues61 found greater screw purchase and higher pullout strength when the screw was placed in the center of the humeral head, within subchondral bone; fixation was poorest when the screw was placed in the anterosuperior region of the humeral head. Tingart and colleagues62 reported that humeral head trabecular density significantly affected pullout strength of cancellous screws. In addition, the most pullout strength was at the center of the head, and the least within the anterosuperior head. Trabecular density was higher in the inferior and posterior regions than in the superior and anterior regions.
Most locking plate designs allow screws to be placed at the level of the medial calcar—the goal being to provide medial column support (Table 2). Zhang and colleagues58 treated 2-, 3-, and 4-part fractures with a locking plate and randomized them into receiving the plate with or without medial support screws. For 3- and 4-part fractures, the group with these screws had a significantly greater final neck-shaft angle and smaller angulation loss compared with the group without screws. No additional benefit was found for 2-part fractures. Erhardt and colleagues63 simulated unstable proximal humerus fractures using cadavers and testing different fixation methods using a polyaxial locking plate. They found that 5 screws in the head fragment and an inferomedial support screw significantly reduced the risk of screw perforation. Other authors have concluded that placing 1 or more inferomedial screws is important in cases of medial comminution or medial column malreduction.26 Interestingly, compared with use of a polyaxial implant, which allows for adjustment of screw direction, use of a monoaxial locking plate did not lead to a clinically different outcome or complication profile.64
Techniques have been used to achieve subchondral purchase of locking screws while reducing iatrogenic articular perforation.65 However, given the incidence of fracture settling and subsequent postoperative screw penetration, many authors currently recommend using shorter divergent screws combined with other augmentation techniques, described previously.17,29,32
Physical Therapy
There is no standardized physiotherapy regimen for postoperative management of proximal humerus fractures treated with locking plates.25 In older patients, immediate active range of motion (ROM) exercises should be delayed until early callus is noted, though there is a risk for stiffness. Lee and Shin57 found that a delay in rehabilitation after ORIF was an independent risk for poor clinical outcome. Namdari and colleagues17 recommended sling use only for comfort and initiated non-load-bearing activities and pendulum exercises immediately after surgery. Patients with adequate reduction at 4 to 6 weeks were advanced to full weight-bearing. Badman and colleagues30 initiated passive-assisted ROM exercises when the wound was healed at 2 weeks in 2-part fractures, whereas patients with 3- and 4-part fractures were immobilized until radiographic healing. Formal therapy was started after 6 weeks. Stiffness was reported in 5% of patients. For patients with stable fixation, Ricchetti and colleagues29 recommended passive shoulder ROM exercises on postoperative day 1; at 4 to 6 weeks, patients should start active shoulder ROM exercises, and then resistance exercises at 10 to 12 weeks. Other authors are more conservative—only sling immobilization and pendulum exercises the first month.66 Barlow and colleagues32 immobilized their patients (age, >75 years) for 6 weeks. No patient developed disabling stiffness. The authors suggested that patients older than 75 years may not be prone to stiffness.
Our Preferred Treatment Method
All proximal humerus fractures are approached anteriorly through the deltopectoral interval (Figure 3A). The long head biceps is identified and truncated for later tenodesis. Multiple No. 5 Ethibond sutures (Ethicon) are placed at the bone–tendon interface. The fracture is reduced with a Cobb elevator (Figure 3B), and provisional Kirschner wires are placed within the head (Figure 3C). The plate is affixed to the humeral head with its anterior border paralleling the posterior aspect of the bicipital groove. Multiple locking screws are placed within the superior and posterior humeral head. Nonlocking screws are then used to fix the plate to the shaft to reduce the specific deformity. Under fluoroscopy, any metaphyseal void is filled with calcium phosphate cement (Figure 3D). The remaining inferior screws are placed within the humeral head. Dr. Gruson uses screws 4 to 6 mm short of subchondral bone to reduce the risk for joint penetration. The rotator cuff sutures are tied down through the plate. Patients are started on progressive supine passive ROM exercises at 7 days, followed by supine active-assisted ROM exercises 6 weeks after fracture healing is confirmed radiographically.
Conclusion
Use of locked plating for proximal humerus fractures has increased, particularly in the elderly. Resulting complications include intra-articular screw penetration, postoperative fracture displacement, and AVN. Recognition of the importance of reducing and supporting the medial calcar, filling any metaphyseal defects, and selectively placing screws within the humeral head has lowered the incidence of these complications. Further comparative studies evaluating the efficacy of individual augmentation techniques are needed to determine their contribution to successful fracture healing and their cost-effectiveness. Results of such studies may help in the development of protocols for more standardized implementation of these techniques and in understanding which specific fracture patterns and patients would benefit from their use.
Proximal humerus fractures account for 4% to 5% of all fractures.1 These fractures occur most frequently in the elderly—patients older than 60 years sustain 71% of these injuries2—and in females.1,3 Given an aging population, this incidence is predicted to increase 3-fold over the next 30 years.4 There is much debate regarding management of acute, displaced proximal humerus fractures. A recent Cochrane Review of published outcomes of operative and nonoperative treatment of displaced proximal humerus fractures found insufficient evidence supporting either modality, though surgery was associated with additional procedures.5 A review of 1000 proximal humerus fractures found that 49% had less than 1 cm of displacement of the major fragments or angulation of less than 45°.3 Other authors have reported similar findings.6,7 Although the incidence of proximal humerus fractures has remained stable over the past decade, from 1999 to 2005 there was a 25% relative increase in surgical management, including a relative increase of 29% in open reduction and internal fixation (ORIF) versus a 20% increase in arthroplasty.1
Locking plates have consistently demonstrated biomechanical superiority over other forms of fixation in osteoporotic bone.8-11 Egol and colleagues8 found that osteoporotic bone limited the torque of fixation to values less than what is required for adequate frictional force between the plate and the bone. This problem can be overcome with fixed-angle devices, such as locked plates.9 Compared with locked nail constructs, proximal humerus locking plates have demonstrated superiority in torsion, loading, and varus bending.10,11 Compared with blade plates, proximal humerus locking plates exhibited increased stiffness and torsional fatigue resistance.12 In a randomized clinical trial, Olerud and colleagues13 reported superior functional results with locking plate fixation compared with nonoperative treatment of displaced 3-part fractures in elderly patients with 2-year follow-up, though these clinical results were not supported by others.14 Two recent case–control studies comparing functional outcomes for 3- and 4-part fractures with follow-up of more than 2 years revealed higher Constant scores after locked plating compared with hemiarthroplasty, though complications were higher with locked plates.15,16 Adoption of locked proximal humerus plating has been correlated with good clinical outcomes and union rates, though this has been accompanied by a higher rate of reoperation.7 Reoperation rates from 1999 to 2005 increased both in the immediate postoperative period (odds ratio, 3.36) and at 1 year (odds ratio, 3.90).1
Complications of Locked Plating
Regardless of fixation type, reduced humeral head bone mass and quality may lead to implant loosening, fracture redisplacement, and, ultimately, poor outcomes. Baseline osteoporosis may predict likelihood of fixation failure.17 Multiple studies have reported on the implant-related complications associated with locking plate fixation—most commonly, intra-articular screw penetration, postoperative fracture displacement, and avascular necrosis (AVN)18-24 (Figure 1). A meta-analysis of 12 studies with a total of 514 proximal humerus fractures treated with locking plate fixation showed an overall complication rate of 49% and a 13.8% reoperation rate.25 The most common indication for reoperation involved intra-articular screw perforation. The most common complications were varus malunion (16%), osteonecrosis (10%), intra-articular screw penetration (8%), subacromial impingement (6%), and infection (4%).
Suboptimal intraoperative fracture reduction, specifically with residual varus, has been correlated with loss of fracture fixation. In a series of 153 fractures, loss of fixation occurred in 13.7% of cases, with the leading risk factor being varus malreduction.19 Failure rates were 30.4% and 11% when the head shaft angle was less than 120° and when it was 120° or more, respectively. Solberg and colleagues16 found that initial postoperative varus angulation of more than 20° resulted in universal loss of fixation. Conversion of these cases to hemiarthroplasty resulted in poor outcomes. Preoperative fracture alignment may also predict fixation failure.22 In one series, initial varus angulation healed with a mean 16° varus and a Constant score of 63, whereas initial valgus alignment healed with 6° varus and a Constant score of 71.22 Complications occurred in fractures that were initially in varus 79% of the time and initially in valgus 19% of the time. Screw perforation has been associated with loss of reduction 44% of the time.20
In an analysis of locking plate constructs revised after early (<4 weeks) failure in 8 patients with osteoporosis, Micic and colleagues21 found implant pullout leading to varus malalignment. All cases lacked medial support and subchondral screw purchase; 3 were initially malreduced. Owsley and Gorczyca23 retrospectively reviewed 53 cases of displaced proximal humerus fractures treated with locked plating. Despite the high rate of radiographic union, 36% developed complications, including screw cutout (23%), varus displacement of more than 10° (25%), and AVN (4%); 13% required revision. These complications disproportionately affected patients older than 60 years (57%) and negatively affected functional outcomes.
Augmentation Techniques
Despite its reported complications, proximal humerus locked plating remains the most widely used type of fixation.1 Advancements in locking plate design, improved understanding of fixation principles, and adoption of techniques augmenting proximal humerus locking plate fixation, particularly in osteoporotic bone, have reduced postoperative complications (Table 1).
Rotator Cuff Sutures
A widely adopted technique for neutralizing rotator cuff–deforming forces, which theoretically can cause fracture displacement, is incorporation of heavy nonabsorbable sutures. These sutures are placed through the rotator cuff–tuberosity junction and tied down after being passed through the plate. Obtaining and maintaining tuberosity reduction are essential in achieving good functional outcomes after fixation. In addition, tension band sutures may be particularly useful in the setting of initial varus deformity.26
Although clinical use of these sutures is common, biomechanical studies of their adjunctive contribution to fracture stability are lacking.27 The rotator cuff musculature has a maximal contractile force of 3.5 kg/cm2.28 Ricchetti and colleagues29 described a technique that involves using a locked plate and tagging the rotator cuff with heavy nonabsorbable sutures. Selective traction on the sutures can help obtain and maintain fracture reduction. Multiple studies have reported on suture use with locked plating for proximal humerus fractures.29-34 Badman and colleagues30 retrospectively reviewed 81 cases of metaphyseal defects or medial comminution treated with locked plating, rotator cuff sutures, and structural allograft. All cases healed within 6 months after surgery. The incidence of screw cutout was 3.7%, the incidence of AVN was 6.2%, and the incidence of varus collapse was 6%. A cadaveric study that used specimens (mean age, 77 years) with a simulated 3-part proximal humerus fracture treated with a locked plate both with and without cerclage sutures found no difference in interfragmentary motion between the groups.27 The authors concluded that additive sutures are not required for anatomically reduced fractures. Multiple sutures may counteract the deforming forces that act on bony segments that cannot be adequately maintained with screws, such as an osteoporotic greater tuberosity.
Medial Column Restoration
The importance of reducing and maintaining the medial calcar to provide biomechanical support for a laterally placed plate has been recognized.26,34-37 Gardner and colleagues26 suggested that medial support was achieved if the medial cortex was anatomically reduced, if the proximal fragment was impacted laterally onto the shaft, or if 1 or more inferomedial screws were placed. Cases that did not achieve medial support developed significantly more humeral head subsidence (5.8 mm vs 1.2 mm) and screw penetration. Krappinger and colleagues36 found that factors leading to fixation failure included age, local bone mineral density, anatomical reduction, and restoration of the medial cortical support. The authors concluded that anatomical reduction and restoration of the medial cortex were important in minimizing mechanical loads at the bone–implant interface. Biomechanically, Lescheid and colleagues37 found that the most stable construct was anatomical reduction with medial cortical contact. In the setting of comminution, however, it may be preferable to intentionally perform varus malreduction to achieve medial contact than to achieve anatomical reduction with a fracture gap. Badman and colleagues30 found that the incidence of screw penetration was 6% in patients with an intact medial calcar versus 29% in patients without medial support. In a retrospective analysis of patients treated with a locking plate and suture augmentation, Jung and colleagues35 concluded that restoring medial support was the most reliable factor in the prevention of loss of reduction with or without screw perforation. Last, Solberg and colleagues16 reported better clinical outcomes when the length of the metaphyseal segment attached to the articular fragment was more than 2 mm. A length of less than 2 mm was predictive of developing AVN.
Use of Bone Void Fillers
Allograft. Allograft is cancellous or corticocancellous chips or tricortical graft used as osteoconductive filler for metaphyseal defects.38 An increasingly popular technique involves using an endosteal fibular allograft strut to indirectly reduce the fracture and help support the medial calcar.39-42 Hettrich and colleagues40 reported on radiographic outcomes of displaced proximal humerus fractures with medial comminution treated with a locked plate and an endosteal fibular allograft or semitubular plate. The reduction was maintained in 96% of cases; there was 1 varus collapse. There were no cases of implant failure, screw perforation, or AVN. Other authors have also reported on successful use of fibular allograft in conjunction with a locked plate; the rate of reduction loss was low, and there were no cases of screw cutout or intra-articular screw penetration.30,41,42 These clinical outcomes are supported by results of biomechanical studies of the added benefit of intramedullary fibular allograft.43-46 Mathison and colleagues43 reported that a construct with fibular allograft and a locking plate increased the failure load by 1.72 times and the stiffness by 3.84 times compared with a control group of locking plate only. Bae and colleagues46 found significantly higher maximum failure load and construct stiffness with no varus collapse in specimens prepared with locked plate and fibular strut augmentation compared with a control group.
Others have successfully used cancellous allograft to fill humeral head bone defects.29,32,47-49 Duralde and Leddy47 reported 100% radiographic union and 81% good to excellent results in cases treated with a locking plate and morselized cancellous allograft to fill bone voids. Varus collapse and screw cutout did not occur, but there were 2 cases of AVN. Ricchetti and colleagues29 reviewed 54 cases treated with a locking plate and rotator cuff suture construct. Allograft cancellous chips and demineralized bone matrix were used in 3- and 4-part fractures (70% of cases) along with shorter screws in the humeral head. Major complications included AVN (1), fixation failure (3), and varus malunion (5). Others investigators have had less favorable results with use of cancellous bone graft. Schliemann and colleagues19 reported on 27 patients who were older than 65 years when they underwent ORIF with rotator cuff sutures to stabilize the tuberosities and either cancellous graft or a synthetic bone substitute in patients with massive metaphyseal defects. Patient-reported outcomes were superior to Constant scores. Complications included screw penetration (22.2%), reduction loss (44.4%), implant failure (3.7%), and AVN (29.6%).
Autograft. Autograft has both osteoconductive and osteoinductive properties and has been successfully used for metaphyseal defects.32,50 Kim and colleagues50 reported on patients with 4-part proximal humerus fractures treated with a locking plate and autologous iliac graft. All cases achieved union and had good or excellent outcomes. There were no cases of AVN, varus collapse, or hardware-related complications.
Bone Cement. Calcium phosphate cement has osteoconductive properties and enhances screw purchase in cancellous bone (Figures 2A–2F). It can be injected or molded into bone voids to provide improved compressive strength. It is resorbed through cell-mediated processes resembling bone remodeling and does not disappear until new bone forms. (Calcium sulfate cement, on the other hand, resorbs through a chemical process independent of new bone formation.51) Egol and colleagues52 reviewed the cases of 92 patients (mean age, 61 years) with 2-, 3-, and 4-part proximal humerus fractures treated with locked plate fixation. Metaphyseal defects were treated with no augmentation, augmentation with cancellous chips, or augmentation with calcium phosphate cement. Adding calcium phosphate cement was associated with lower incidence of intra-articular screw penetration and humeral head settling. In a recent cadaveric biomechanical study using 2-part proximal humerus fractures with metaphyseal comminution, the group augmented with calcium phosphate cement had enhanced axial stiffness and load to failure with reduced screw penetration.53 Other biomechanical studies have found increased screw pullout strength54 and decreased interfragmentary motion when specimens were augmented with calcium phosphate cement.55
Similar good clinical and radiographic outcomes have been observed with use of calcium sulfate cement.56,57 Somasundaram and colleagues56 reported good clinical outcomes in 82% of patients treated with locking plates and calcium sulfate cement used to fill metaphyseal voids. All fractures united without infection, fixation failure, subsequent malunion, tuberosity failure, or AVN. Lee and Shin57 compared outcomes of 14 patients who received calcium sulfate augmentation with outcomes of patients who did not receive this augmentation. Overall, 89% of patients had good or excellent results. Calcium sulfate cement did not affect the reduction failure rate or clinical outcomes in cases in which medial cortical reduction was achieved. However, postoperative displacement caused by lack of medial support was associated with poor outcomes.
Screw Placement
Screws optimally should be placed in the posterior-medial-inferior aspect of the humeral head to provide medial support for the fracture and mechanical stability.58 Cadaveric studies have shown the highest cancellous bone density in the proximal, posterior, and medial portions of the humeral head.59-63 Similarly, in a cadaveric study, Liew and colleagues61 found greater screw purchase and higher pullout strength when the screw was placed in the center of the humeral head, within subchondral bone; fixation was poorest when the screw was placed in the anterosuperior region of the humeral head. Tingart and colleagues62 reported that humeral head trabecular density significantly affected pullout strength of cancellous screws. In addition, the most pullout strength was at the center of the head, and the least within the anterosuperior head. Trabecular density was higher in the inferior and posterior regions than in the superior and anterior regions.
Most locking plate designs allow screws to be placed at the level of the medial calcar—the goal being to provide medial column support (Table 2). Zhang and colleagues58 treated 2-, 3-, and 4-part fractures with a locking plate and randomized them into receiving the plate with or without medial support screws. For 3- and 4-part fractures, the group with these screws had a significantly greater final neck-shaft angle and smaller angulation loss compared with the group without screws. No additional benefit was found for 2-part fractures. Erhardt and colleagues63 simulated unstable proximal humerus fractures using cadavers and testing different fixation methods using a polyaxial locking plate. They found that 5 screws in the head fragment and an inferomedial support screw significantly reduced the risk of screw perforation. Other authors have concluded that placing 1 or more inferomedial screws is important in cases of medial comminution or medial column malreduction.26 Interestingly, compared with use of a polyaxial implant, which allows for adjustment of screw direction, use of a monoaxial locking plate did not lead to a clinically different outcome or complication profile.64
Techniques have been used to achieve subchondral purchase of locking screws while reducing iatrogenic articular perforation.65 However, given the incidence of fracture settling and subsequent postoperative screw penetration, many authors currently recommend using shorter divergent screws combined with other augmentation techniques, described previously.17,29,32
Physical Therapy
There is no standardized physiotherapy regimen for postoperative management of proximal humerus fractures treated with locking plates.25 In older patients, immediate active range of motion (ROM) exercises should be delayed until early callus is noted, though there is a risk for stiffness. Lee and Shin57 found that a delay in rehabilitation after ORIF was an independent risk for poor clinical outcome. Namdari and colleagues17 recommended sling use only for comfort and initiated non-load-bearing activities and pendulum exercises immediately after surgery. Patients with adequate reduction at 4 to 6 weeks were advanced to full weight-bearing. Badman and colleagues30 initiated passive-assisted ROM exercises when the wound was healed at 2 weeks in 2-part fractures, whereas patients with 3- and 4-part fractures were immobilized until radiographic healing. Formal therapy was started after 6 weeks. Stiffness was reported in 5% of patients. For patients with stable fixation, Ricchetti and colleagues29 recommended passive shoulder ROM exercises on postoperative day 1; at 4 to 6 weeks, patients should start active shoulder ROM exercises, and then resistance exercises at 10 to 12 weeks. Other authors are more conservative—only sling immobilization and pendulum exercises the first month.66 Barlow and colleagues32 immobilized their patients (age, >75 years) for 6 weeks. No patient developed disabling stiffness. The authors suggested that patients older than 75 years may not be prone to stiffness.
Our Preferred Treatment Method
All proximal humerus fractures are approached anteriorly through the deltopectoral interval (Figure 3A). The long head biceps is identified and truncated for later tenodesis. Multiple No. 5 Ethibond sutures (Ethicon) are placed at the bone–tendon interface. The fracture is reduced with a Cobb elevator (Figure 3B), and provisional Kirschner wires are placed within the head (Figure 3C). The plate is affixed to the humeral head with its anterior border paralleling the posterior aspect of the bicipital groove. Multiple locking screws are placed within the superior and posterior humeral head. Nonlocking screws are then used to fix the plate to the shaft to reduce the specific deformity. Under fluoroscopy, any metaphyseal void is filled with calcium phosphate cement (Figure 3D). The remaining inferior screws are placed within the humeral head. Dr. Gruson uses screws 4 to 6 mm short of subchondral bone to reduce the risk for joint penetration. The rotator cuff sutures are tied down through the plate. Patients are started on progressive supine passive ROM exercises at 7 days, followed by supine active-assisted ROM exercises 6 weeks after fracture healing is confirmed radiographically.
Conclusion
Use of locked plating for proximal humerus fractures has increased, particularly in the elderly. Resulting complications include intra-articular screw penetration, postoperative fracture displacement, and AVN. Recognition of the importance of reducing and supporting the medial calcar, filling any metaphyseal defects, and selectively placing screws within the humeral head has lowered the incidence of these complications. Further comparative studies evaluating the efficacy of individual augmentation techniques are needed to determine their contribution to successful fracture healing and their cost-effectiveness. Results of such studies may help in the development of protocols for more standardized implementation of these techniques and in understanding which specific fracture patterns and patients would benefit from their use.
1. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
2. Aaron D, Shatsky J, Paredes JC, Jiang C, Parsons BO, Flatow EL. Proximal humeral fractures: internal fixation. J Bone Joint Surg Am. 2012;94(24):2280-2288.
3. Court-Brown CM, Garg A, McQueen MM. The epidemiology of proximal humeral fractures. Acta Orthop Scand. 2001;72(4):365-371.
4. Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M, Vuori I. Increasing number and incidence of osteoporotic fractures of the proximal humerus in elderly people. BMJ. 1996;313(7064):1051-1052.
5. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
6. Tamai K, Ishige N, Kuroda S, et al. Four-segment classification of proximal humeral fractures revisited: a multicenter study on 509 cases. J Shoulder Elbow Surg. 2009;18(6):845-850.
7. Rothberg D, Higgins T. Fractures of the proximal humerus. Orthop Clin North Am. 2013;44(1):9-19.
8. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
9. Miranda MA. Locking plate technology and its role in osteoporotic fractures. Injury. 2007;38(suppl 3):35-39.
10. Foruria AM, Carrascal MT, Revilla C, Munuera L, Sanchez-Sotelo J. Proximal humerus fracture rotational stability after fixation using a locking plate or a fixed-angle locked nail: the role of implant stiffness. Clin Biomech. 2010;25(4):307-311.
11. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
12. Siffri PC, Peindl RD, Coley ER, Norton J, Connor PM, Kellam JF. Biomechanical analysis of blade plate versus locking plate fixation for a proximal humerus fracture: comparison using cadaveric and synthetic humeri. J Orthop Trauma. 2006;20(8):547-554.
13. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
14. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
15. Wild JR, DeMers A, French R, et al. Functional outcomes for surgically treated 3- and 4-part proximal humerus fractures. Orthopedics. 2011;34(10):e629-e633.
16. Solberg BD, Moon CN, Franco DP, Paiement GD. Surgical treatment of three and four-part proximal humeral fractures. J Bone Joint Surg Am. 2009;91(7):1689-1697.
17. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
18. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
19. Schliemann B, Siemoneit J, Theisen C, Kosters C, Weimann A, Raschke MJ. Complex fractures of the proximal humerus in the elderly—outcome and complications after locking plate fixation. Musculoskelet Surg. 2012;96(suppl 1):S3-S11.
20. Thanasas C, Kontakis G, Angoules A, Limb D, Giannoudis P. Treatment of proximal humerus fractures with locking plates: a systematic review. J Shoulder Elbow Surg. 2009;18(6):837-844.
21. Micic ID, Kim KC, Shin DJ, et al. Analysis of early failure of the locking compression plate in osteoporotic proximal humerus fractures. J Orthop Sci. 2009;14(5):596-601.
22. Solberg BD, Moon CN, Franco DP, Paiement GD. Locked plating of 3- and 4-part proximal humerus fractures in older patients: the effect of initial fracture pattern on outcome. J Orthop Trauma. 2009;23(2):113-119.
23. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures [corrected]. J Bone Joint Surg Am. 2008;90(2):233-240.
24. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
25. Sproul RC, Iyengar JJ, Devcic Z, Feeley BT. A systematic review of locking plate fixation of proximal humerus fractures. Injury. 2011;42(4):408-413.
26. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
27. Voigt C, Hurschler C, Rech L, Vossenrich R, Lill H. Additive fiber-cerclages in proximal humeral fractures stabilized by locking plates. No effect on fracture stabilization and rotator cuff function in human shoulder specimens. Acta Orthop. 2009;80(4):465-471.
28. Lo IK, Burkhart SS. Biomechanical principles of arthroscopic repair of the rotator cuff. Oper Tech Orthop. 2002;12(3):140-155.
29. Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2010;19(2 suppl):66-75.
30. Badman B, Frankle M, Keating C, Henderson L, Brooks J, Mighell M. Results of proximal humeral locked plating with supplemental suture fixation of rotator cuff. J Shoulder Elbow Surg. 2011;20(4):616-624.
31. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Management of proximal humeral fractures based on current literature. J Bone Joint Surg Am. 2007;89(suppl 3):44-58.
32. Barlow JD, Sanchez-Sotelo J, Torchia M. Proximal humerus fractures in the elderly can be reliably fixed with a “hybrid” locked-plating technique. Clin Orthop. 2011;469(12):3281-3291.
33. Cho CH, Jung GH, Song KS. Tension suture fixation using 2 washers for proximal humeral fractures. Orthopedics. 2012;35(3):202-205.
34. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
35. Jung WB, Moon ES, Kim SK, Kovacevic D, Kim MS. Does medial support decrease major complications of unstable proximal humerus fractures treated with locking plate? BMC Musculoskelet Disord. 2013;14:102.
36. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury. 2011;42(11):1283-1288.
37. Lescheid J, Zdero R, Shah S, Kuzyk PR, Schemitsch EH. The biomechanics of locked plating for repairing proximal humerus fractures with or without medial cortical support. J Trauma. 2010;69(5):1235-1242.
38. De Long WG Jr, Einhorn TA, Koval K, et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89(3):649-658.
39. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
40. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
41. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
42. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop. 2011;469(12):3300-3306.
43. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
44. Osterhoff G, Baumgartner D, Favre P, et al. Medial support by fibula bone graft in angular stable plate fixation of proximal humeral fractures: an in vitro study with synthetic bone. J Shoulder Elbow Surg. 2011;20(5):740-746.
45. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
46. Bae JH, Oh JK, Chon CS, Oh CW, Hwang JH, Yoon YC. The biomechanical performance of locking plate fixation with intramedullary fibular strut graft augmentation in the treatment of unstable fractures of the proximal humerus. J Bone Joint Surg Br. 2011;93(7):937-941.
47. Duralde XA, Leddy LR. The results of ORIF of displaced unstable proximal humeral fractures using a locking plate. J Shoulder Elbow Surg. 2010;19(4):480-488.
48. Robinson CM, Wylie JR, Ray AG, et al. Proximal humeral fractures with a severe varus deformity treated by fixation with a locking plate. J Bone Joint Surg Br. 2010;92(5):672-678.
49. Ong C, Bechtel C, Walsh M, Zuckerman JD, Egol KA. Three- and four-part fractures have poorer function than one-part proximal humerus fractures. Clin Orthop. 2011;469(12):3292-3299.
50. Kim SH, Lee YH, Chung SW, et al. Outcomes for four-part proximal humerus fractures treated with a locking compression plate and an autologous iliac bone impaction graft. Injury. 2012;43(10):1724-1731.
51. Larsson S. Calcium phosphates: what is the evidence? J Orthop Trauma. 2010;24(suppl 1):S41-S45.
52. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction–internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
53. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
54. Collinge C, Merk B, Lautenschlager EP. Mechanical evaluation of fracture fixation augmented with tricalcium phosphate bone cement in a porous osteoporotic cancellous bone model. J Orthop Trauma. 2007;21(2):124-128.
55. Kwon BK, Goertzen DJ, O’Brien PJ, Broekhuyse HM, Oxland TR. Biomechanical evaluation of proximal humeral fracture fixation supplemented with calcium phosphate cement. J Bone Joint Surg Am. 2002;84(6):951-961.
56. Somasundaram K, Huber CP, Babu V, Zadeh H. Proximal humeral fractures: the role of calcium sulphate augmentation and extended deltoid splitting approach in internal fixation using locking plates. Injury. 2013;44(4):481-487.
57. Lee CW, Shin SJ. Prognostic factors for unstable proximal humeral fractures treated with locking-plate fixation. J Shoulder Elbow Surg. 2009;18(1):83-88.
58. Zhang L, Zheng J, Wang W, et al. The clinical benefit of medial support screws in locking plating of proximal humerus fractures: a prospective randomized study. Int Orthop. 2011;35(11):1655-1661.
59. Brianza S, Roderer G, Schiuma D, et al. Where do locking screws purchase in the humeral head? Injury. 2012;43(6):850-855.
60. Hepp P, Lill H, Bail H, et al. Where should implants be anchored in the humeral head? Clin Orthop. 2003;(415):139-147.
61. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
62. Tingart MJ, Lehtinen J, Zurakowski D, Warner JJ, Apreleva M. Proximal humeral fractures: regional differences in bone mineral density of the humeral head affect the fixation strength of cancellous screws. J Shoulder Elbow Surg. 2006;15(5):620-624.
63. Erhardt JB, Stoffel K, Kampshoff J, Badur N, Yates P, Kuster MS. The position and number of screws influence screw perforation of the humeral head in modern locking plates: a cadaver study. J Orthop Trauma. 2012;26(10):e188-e192.
64. Konigshausen M, Kubler L, Godry H, Citak M, Schildhauer TA, Seybold D. Clinical outcome and complications using a polyaxial locking plate in the treatment of displaced proximal humerus fractures. A reliable system? Injury. 2012;43(2):223-231.
65. Bengard MJ, Gardner MJ. Screw depth sounding in proximal humerus fractures to avoid iatrogenic intra-articular penetration. J Orthop Trauma. 2011;25(10):630-633.
66. Ring D. Current concepts in plate and screw fixation of osteoporotic proximal humerus fractures. Injury. 2007;38(3):S59-S68.
1. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
2. Aaron D, Shatsky J, Paredes JC, Jiang C, Parsons BO, Flatow EL. Proximal humeral fractures: internal fixation. J Bone Joint Surg Am. 2012;94(24):2280-2288.
3. Court-Brown CM, Garg A, McQueen MM. The epidemiology of proximal humeral fractures. Acta Orthop Scand. 2001;72(4):365-371.
4. Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M, Vuori I. Increasing number and incidence of osteoporotic fractures of the proximal humerus in elderly people. BMJ. 1996;313(7064):1051-1052.
5. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
6. Tamai K, Ishige N, Kuroda S, et al. Four-segment classification of proximal humeral fractures revisited: a multicenter study on 509 cases. J Shoulder Elbow Surg. 2009;18(6):845-850.
7. Rothberg D, Higgins T. Fractures of the proximal humerus. Orthop Clin North Am. 2013;44(1):9-19.
8. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
9. Miranda MA. Locking plate technology and its role in osteoporotic fractures. Injury. 2007;38(suppl 3):35-39.
10. Foruria AM, Carrascal MT, Revilla C, Munuera L, Sanchez-Sotelo J. Proximal humerus fracture rotational stability after fixation using a locking plate or a fixed-angle locked nail: the role of implant stiffness. Clin Biomech. 2010;25(4):307-311.
11. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
12. Siffri PC, Peindl RD, Coley ER, Norton J, Connor PM, Kellam JF. Biomechanical analysis of blade plate versus locking plate fixation for a proximal humerus fracture: comparison using cadaveric and synthetic humeri. J Orthop Trauma. 2006;20(8):547-554.
13. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
14. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
15. Wild JR, DeMers A, French R, et al. Functional outcomes for surgically treated 3- and 4-part proximal humerus fractures. Orthopedics. 2011;34(10):e629-e633.
16. Solberg BD, Moon CN, Franco DP, Paiement GD. Surgical treatment of three and four-part proximal humeral fractures. J Bone Joint Surg Am. 2009;91(7):1689-1697.
17. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
18. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
19. Schliemann B, Siemoneit J, Theisen C, Kosters C, Weimann A, Raschke MJ. Complex fractures of the proximal humerus in the elderly—outcome and complications after locking plate fixation. Musculoskelet Surg. 2012;96(suppl 1):S3-S11.
20. Thanasas C, Kontakis G, Angoules A, Limb D, Giannoudis P. Treatment of proximal humerus fractures with locking plates: a systematic review. J Shoulder Elbow Surg. 2009;18(6):837-844.
21. Micic ID, Kim KC, Shin DJ, et al. Analysis of early failure of the locking compression plate in osteoporotic proximal humerus fractures. J Orthop Sci. 2009;14(5):596-601.
22. Solberg BD, Moon CN, Franco DP, Paiement GD. Locked plating of 3- and 4-part proximal humerus fractures in older patients: the effect of initial fracture pattern on outcome. J Orthop Trauma. 2009;23(2):113-119.
23. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures [corrected]. J Bone Joint Surg Am. 2008;90(2):233-240.
24. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
25. Sproul RC, Iyengar JJ, Devcic Z, Feeley BT. A systematic review of locking plate fixation of proximal humerus fractures. Injury. 2011;42(4):408-413.
26. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
27. Voigt C, Hurschler C, Rech L, Vossenrich R, Lill H. Additive fiber-cerclages in proximal humeral fractures stabilized by locking plates. No effect on fracture stabilization and rotator cuff function in human shoulder specimens. Acta Orthop. 2009;80(4):465-471.
28. Lo IK, Burkhart SS. Biomechanical principles of arthroscopic repair of the rotator cuff. Oper Tech Orthop. 2002;12(3):140-155.
29. Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2010;19(2 suppl):66-75.
30. Badman B, Frankle M, Keating C, Henderson L, Brooks J, Mighell M. Results of proximal humeral locked plating with supplemental suture fixation of rotator cuff. J Shoulder Elbow Surg. 2011;20(4):616-624.
31. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Management of proximal humeral fractures based on current literature. J Bone Joint Surg Am. 2007;89(suppl 3):44-58.
32. Barlow JD, Sanchez-Sotelo J, Torchia M. Proximal humerus fractures in the elderly can be reliably fixed with a “hybrid” locked-plating technique. Clin Orthop. 2011;469(12):3281-3291.
33. Cho CH, Jung GH, Song KS. Tension suture fixation using 2 washers for proximal humeral fractures. Orthopedics. 2012;35(3):202-205.
34. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
35. Jung WB, Moon ES, Kim SK, Kovacevic D, Kim MS. Does medial support decrease major complications of unstable proximal humerus fractures treated with locking plate? BMC Musculoskelet Disord. 2013;14:102.
36. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury. 2011;42(11):1283-1288.
37. Lescheid J, Zdero R, Shah S, Kuzyk PR, Schemitsch EH. The biomechanics of locked plating for repairing proximal humerus fractures with or without medial cortical support. J Trauma. 2010;69(5):1235-1242.
38. De Long WG Jr, Einhorn TA, Koval K, et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89(3):649-658.
39. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
40. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
41. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
42. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop. 2011;469(12):3300-3306.
43. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
44. Osterhoff G, Baumgartner D, Favre P, et al. Medial support by fibula bone graft in angular stable plate fixation of proximal humeral fractures: an in vitro study with synthetic bone. J Shoulder Elbow Surg. 2011;20(5):740-746.
45. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
46. Bae JH, Oh JK, Chon CS, Oh CW, Hwang JH, Yoon YC. The biomechanical performance of locking plate fixation with intramedullary fibular strut graft augmentation in the treatment of unstable fractures of the proximal humerus. J Bone Joint Surg Br. 2011;93(7):937-941.
47. Duralde XA, Leddy LR. The results of ORIF of displaced unstable proximal humeral fractures using a locking plate. J Shoulder Elbow Surg. 2010;19(4):480-488.
48. Robinson CM, Wylie JR, Ray AG, et al. Proximal humeral fractures with a severe varus deformity treated by fixation with a locking plate. J Bone Joint Surg Br. 2010;92(5):672-678.
49. Ong C, Bechtel C, Walsh M, Zuckerman JD, Egol KA. Three- and four-part fractures have poorer function than one-part proximal humerus fractures. Clin Orthop. 2011;469(12):3292-3299.
50. Kim SH, Lee YH, Chung SW, et al. Outcomes for four-part proximal humerus fractures treated with a locking compression plate and an autologous iliac bone impaction graft. Injury. 2012;43(10):1724-1731.
51. Larsson S. Calcium phosphates: what is the evidence? J Orthop Trauma. 2010;24(suppl 1):S41-S45.
52. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction–internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
53. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
54. Collinge C, Merk B, Lautenschlager EP. Mechanical evaluation of fracture fixation augmented with tricalcium phosphate bone cement in a porous osteoporotic cancellous bone model. J Orthop Trauma. 2007;21(2):124-128.
55. Kwon BK, Goertzen DJ, O’Brien PJ, Broekhuyse HM, Oxland TR. Biomechanical evaluation of proximal humeral fracture fixation supplemented with calcium phosphate cement. J Bone Joint Surg Am. 2002;84(6):951-961.
56. Somasundaram K, Huber CP, Babu V, Zadeh H. Proximal humeral fractures: the role of calcium sulphate augmentation and extended deltoid splitting approach in internal fixation using locking plates. Injury. 2013;44(4):481-487.
57. Lee CW, Shin SJ. Prognostic factors for unstable proximal humeral fractures treated with locking-plate fixation. J Shoulder Elbow Surg. 2009;18(1):83-88.
58. Zhang L, Zheng J, Wang W, et al. The clinical benefit of medial support screws in locking plating of proximal humerus fractures: a prospective randomized study. Int Orthop. 2011;35(11):1655-1661.
59. Brianza S, Roderer G, Schiuma D, et al. Where do locking screws purchase in the humeral head? Injury. 2012;43(6):850-855.
60. Hepp P, Lill H, Bail H, et al. Where should implants be anchored in the humeral head? Clin Orthop. 2003;(415):139-147.
61. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
62. Tingart MJ, Lehtinen J, Zurakowski D, Warner JJ, Apreleva M. Proximal humeral fractures: regional differences in bone mineral density of the humeral head affect the fixation strength of cancellous screws. J Shoulder Elbow Surg. 2006;15(5):620-624.
63. Erhardt JB, Stoffel K, Kampshoff J, Badur N, Yates P, Kuster MS. The position and number of screws influence screw perforation of the humeral head in modern locking plates: a cadaver study. J Orthop Trauma. 2012;26(10):e188-e192.
64. Konigshausen M, Kubler L, Godry H, Citak M, Schildhauer TA, Seybold D. Clinical outcome and complications using a polyaxial locking plate in the treatment of displaced proximal humerus fractures. A reliable system? Injury. 2012;43(2):223-231.
65. Bengard MJ, Gardner MJ. Screw depth sounding in proximal humerus fractures to avoid iatrogenic intra-articular penetration. J Orthop Trauma. 2011;25(10):630-633.
66. Ring D. Current concepts in plate and screw fixation of osteoporotic proximal humerus fractures. Injury. 2007;38(3):S59-S68.
Closed Reduction of Subacute Patellar Dislocation Using Saline Joint Insufflation: A Technical Trick
As the largest sesamoid bone in the human body, the patella acts as a fulcrum to enhance the biomechanical advantage of the quadriceps in extension.1 It is subject to a variety of forces while improving distribution of forces along the extensor mechanism.2 With sufficient force, the patella can be dislocated. Acute patellar dislocations are the most common knee injury, encompassing 2% to 3% of all knee injuries3 and occurring in 5.8 per 100,000 individuals.4-5 These injuries are associated with acute trauma, frequently from sports and physical activities, occurring while in terminal extension with an axial-valgus stress on the knee during rotation.6
With acute patellar dislocations, patients are usually in significant discomfort. Often, the patella may spontaneously reduce; if not, closed reduction is usually successful with pressure applied anteromedially on the lateral patellar margin, while simultaneously attempting gentle extension of the leg.7 Closed reduction is almost universally successful, and there have only been case reports of irreducible, mainly fixed vertical axis patellar dislocations.8-11 No reports in the literature have described subacute patellar dislocations because of their rarity. Patients present immediately after dislocation, spontaneously reduce, or have a painless, chronically dislocated patella.
We present a case of an elderly man with dementia and a subacute fixed irreducible patellar dislocation, which was reduced using a technique not described in the literature. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 68-year-old nonambulatory man with a history of dementia and stroke presented to the emergency department with complaints of left knee pain and his knee locked in flexion. The patient’s knee had been in that fixed hyperflexed position for at least 10 days after he sustained a twisting injury to his knee while attempting to get out of bed. At baseline, the patient was mostly bedbound and could walk minimally with maximum support, but, given his dementia, he would often attempt to ambulate by himself. After the injury, the patient did not complain of much pain at rest, but attempts at his group home to straighten his leg had caused severe pain. As a result, the patient was brought to the emergency department to be evaluated for fractures.
Physical examination in the emergency department revealed atrophy of the lower extremity musculature and a left knee fixed at 120º in flexion. The skin was intact, and there was minimal effusion of the knee joint. The patella was noted to be laterally subluxated and tender to palpation over the lateral and medial facets. He was neurovascularly intact distally and had painless range of motion of his hips. His contralateral right knee had full range of motion with good patellar tracking.
Radiographs of the patient’s knee confirmed a lateral dislocation of the patella (Figures 1A-1C). After oral and intravenous administration of pain medication, a reduction was attempted without success. Next, an intra-articular knee injection of 10 mL of 1% lidocaine was given. After waiting 15 minutes, another reduction was tried. While the pain control was sufficient, the reduction was again unsuccessful. The knee was insufflated with 120 mL of sterile saline and reduction attempted again. By extending the knee and applying a medially directed force to the patella, reduction was successful. The patient was placed into a knee immobilizer and postreduction radiographs were taken (Figures 2A, 2B). Saline was extracted from the knee. The patient was admitted to the hospital where repeat examination of his knees during the next week revealed markedly less pain. The patient was lost to follow-up.
Discussion
Our patient presumably had a low-energy mechanism of injury, resulting in an undiagnosed patellar dislocation with delayed treatment. This subacute patellar dislocation was irreducible using the standard techniques. Alternatively, insufflation of the joint with saline provided the necessary impetus to allow for successful patellar reduction. The history of the patient reveals clues about the mechanism of injury. It is likely that the patient’s nonambulatory status resulted in a weak vastus medialis muscle that placed the patella at risk for dislocation. While the exact mechanism of dislocation is unknown, the patella was unable to be reduced spontaneously because our patient’s knee was maintained in a state of flexion secondary to pain and muscle contraction. The combination of weak quadriceps musculature, increased Q angle, and forced hyperflexion of the knee prevented closed reduction of the patella.
Fixed, irreducible patellar dislocations are rare and discussed infrequently in the literature.9,11-12 Reported mechanisms are mostly high energy, including blows during athletics and impacts from motor vehicle collisions.9,13 Vertical axis rotation, as first described by Cooper,14 is commonly implicated in irreducible patellar dislocations. This occurs when the patella internally rotates 180º on its vertical axis, associated with a large tear of the medial retinaculum but intact quadriceps tendon. The patella is fixed over the lateral femoral condyle with the articular surface pointing anterolaterally. Despite adequate sedation and analgesia, these are notoriously difficult to close-reduce and may necessitate open reduction.3 Our patient, while having a fixed dislocation, did not have a vertical axis component and, therefore, was amenable to our closed reduction attempt.
Our first reduction attempts were unsuccessful, likely because the patient continued to be tense, firing his quadriceps. Even after injecting the knee with lidocaine and eliminating the pain component, the patella was still impinging on the lateral femoral condyle (Figure 3A). By insufflating the knee with saline, we were able to increase the distance from the patella to the trochlea (Figure 3B). This is comparable to a knee arthroscopy, in which joint fluid pressure allows passage of arthroscopic instruments into the patellofemoral joint. We postulate that the farther the patella is anterior to the trochlea, the higher the likelihood that the patella can be reduced to its anatomic position.
Insufflation of the knee with sterile saline is a novel technique that involves minimal risk compared with the alternatives. Sometimes, for closed reduction to be successful, individuals need to be consciously sedated to relax their muscles and eliminate pain. While conscious sedation is generally considered low risk, complications have been noted, including hypotension, apnea, and retrograde amnesia.15 Manual closed reduction may also cause additional chondral damage when the medial patellar facet contacts the lateral femoral trochlea. When closed reduction of the patella fails, open reduction is required; this inherently includes all the risks of surgery, such as bleeding, infection, neurovascular injury, and wound complications.
Our insufflation technique does not require sedation and is minimally invasive. The saline creates space and provides lubrication to allow for easier manipulation of the patella. This theoretically protects the cartilage as the patella passes over the lateral trochlea. In addition to the intended effect of providing more space and lubrication for the reduction of the patella, insufflation of the joint may also relax the vastus musculature.16 In their study, Torry and colleagues16 injected 13 knees with 20 mL sterile saline and noted reduction in electromyography readings in the vastus medialis and lateralis muscles. This inhibition of vastus musculature may provide enough relaxation to aid in the successful reduction of the patella.
Our study is limited by our sample size of 1. Because acute patellar dislocations are often easily reduced, our technical trick is not frequently used. Additionally, while we were able to monitor his progress during his inpatient stay, our patient was lost to follow-up after his discharge from the hospital.
If successful, the insufflation technique eliminates the need for urgent open reduction in the operating room. As a result, we recommend attempting closed reduction using insufflation of the knee with sterile saline for irreducible patellar dislocations before proceeding with open reduction.
Conclusion
Saline insufflation of the knee can be safely and easily performed to aid in the reduction of subacute, difficult patellar dislocations.
1. Fu FH, Seel M, Berger RA. Patellofemoral biomechanics. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:49.
2. Dye SF. Patellofemoral anatomy. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:2-3.
3. Li X, Nielsen NM, Zhou H, Stein BS, Shelton YA, Busconi BD. Surgical treatment of a chronically fixed lateral patella dislocation in an adolescent patient. Orthop Rev (Pavia). 2013;5(2):45-47.
4. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
5. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90(12):2751-2762.
6. Panni AS, Vasso M, Cerciello S. Acute patellar dislocation. What to do? Knee Surg Sports Traumatol Arthrosc. 2013;21(2):275-278.
7. Lu DW, Wang EE, Self WH, Kharasch M. Patellar dislocation reduction. Acad Emerg Med. 2010;17(2):226.
8. Michels F, Pouliart N, Oosterlinck D. Locked patellar dislocation: a case report. J Med Case Rep. 2008;2:371.
9. ElMaraghy AW, Berry GK, Kreder HJ. Irreducible lateral patellar dislocation with vertical axis rotation: case report and review of the literature. J Trauma. 2002;53(1):131-132.
10. Wajid MA, Cheema MQ, Siddique MS. Vertical axis patellar dislocation with ipsilateral femoral fracture: use of a closed percutaneous technique for reduction of the dislocation. J Orthop Trauma. 2006;20(2):143-146.
11. Shetty S, Ramesh B, Gul A, Madhusudan TR, Altayeb T. Vertical dislocation of the patella: report of 2 cases. Orthopedics. 2009;32(10). doi: 10.3928/01477447-20090818-25.
12. Hackl W, Benedetto KP, Fink C, Sailer R, Rieger M. Locked lateral patellar dislocation: a rare case of irreducible patellar dislocation requiring open reduction. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):352-355.
13. Gidden DJ, Bell KM. An unusual case of irreducible intra-articular patellar dislocation with vertical axis rotation. Injury. 1995;26(9):643-644.
14. Cooper A. Dislocation of the patella. In: Cooper A, ed. A Treatise on the Dislocations and Fractures of the Joints. Philadelphia, PA: Lea & Febiger; 1844:195.
15. Swanson ER, Seaberg DC, Mathias S. The use of propofol for sedation in the emergency department. Acad Emerg Med. 2008;3(3):234-238.
16. Torry MR, Decker MJ, Millett PJ, Steadman JR, Sterett WI. The effects of knee joint effusion on quadriceps electromyography during jogging. J Sports Sci Med. 2005;4(1):1-8.
As the largest sesamoid bone in the human body, the patella acts as a fulcrum to enhance the biomechanical advantage of the quadriceps in extension.1 It is subject to a variety of forces while improving distribution of forces along the extensor mechanism.2 With sufficient force, the patella can be dislocated. Acute patellar dislocations are the most common knee injury, encompassing 2% to 3% of all knee injuries3 and occurring in 5.8 per 100,000 individuals.4-5 These injuries are associated with acute trauma, frequently from sports and physical activities, occurring while in terminal extension with an axial-valgus stress on the knee during rotation.6
With acute patellar dislocations, patients are usually in significant discomfort. Often, the patella may spontaneously reduce; if not, closed reduction is usually successful with pressure applied anteromedially on the lateral patellar margin, while simultaneously attempting gentle extension of the leg.7 Closed reduction is almost universally successful, and there have only been case reports of irreducible, mainly fixed vertical axis patellar dislocations.8-11 No reports in the literature have described subacute patellar dislocations because of their rarity. Patients present immediately after dislocation, spontaneously reduce, or have a painless, chronically dislocated patella.
We present a case of an elderly man with dementia and a subacute fixed irreducible patellar dislocation, which was reduced using a technique not described in the literature. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 68-year-old nonambulatory man with a history of dementia and stroke presented to the emergency department with complaints of left knee pain and his knee locked in flexion. The patient’s knee had been in that fixed hyperflexed position for at least 10 days after he sustained a twisting injury to his knee while attempting to get out of bed. At baseline, the patient was mostly bedbound and could walk minimally with maximum support, but, given his dementia, he would often attempt to ambulate by himself. After the injury, the patient did not complain of much pain at rest, but attempts at his group home to straighten his leg had caused severe pain. As a result, the patient was brought to the emergency department to be evaluated for fractures.
Physical examination in the emergency department revealed atrophy of the lower extremity musculature and a left knee fixed at 120º in flexion. The skin was intact, and there was minimal effusion of the knee joint. The patella was noted to be laterally subluxated and tender to palpation over the lateral and medial facets. He was neurovascularly intact distally and had painless range of motion of his hips. His contralateral right knee had full range of motion with good patellar tracking.
Radiographs of the patient’s knee confirmed a lateral dislocation of the patella (Figures 1A-1C). After oral and intravenous administration of pain medication, a reduction was attempted without success. Next, an intra-articular knee injection of 10 mL of 1% lidocaine was given. After waiting 15 minutes, another reduction was tried. While the pain control was sufficient, the reduction was again unsuccessful. The knee was insufflated with 120 mL of sterile saline and reduction attempted again. By extending the knee and applying a medially directed force to the patella, reduction was successful. The patient was placed into a knee immobilizer and postreduction radiographs were taken (Figures 2A, 2B). Saline was extracted from the knee. The patient was admitted to the hospital where repeat examination of his knees during the next week revealed markedly less pain. The patient was lost to follow-up.
Discussion
Our patient presumably had a low-energy mechanism of injury, resulting in an undiagnosed patellar dislocation with delayed treatment. This subacute patellar dislocation was irreducible using the standard techniques. Alternatively, insufflation of the joint with saline provided the necessary impetus to allow for successful patellar reduction. The history of the patient reveals clues about the mechanism of injury. It is likely that the patient’s nonambulatory status resulted in a weak vastus medialis muscle that placed the patella at risk for dislocation. While the exact mechanism of dislocation is unknown, the patella was unable to be reduced spontaneously because our patient’s knee was maintained in a state of flexion secondary to pain and muscle contraction. The combination of weak quadriceps musculature, increased Q angle, and forced hyperflexion of the knee prevented closed reduction of the patella.
Fixed, irreducible patellar dislocations are rare and discussed infrequently in the literature.9,11-12 Reported mechanisms are mostly high energy, including blows during athletics and impacts from motor vehicle collisions.9,13 Vertical axis rotation, as first described by Cooper,14 is commonly implicated in irreducible patellar dislocations. This occurs when the patella internally rotates 180º on its vertical axis, associated with a large tear of the medial retinaculum but intact quadriceps tendon. The patella is fixed over the lateral femoral condyle with the articular surface pointing anterolaterally. Despite adequate sedation and analgesia, these are notoriously difficult to close-reduce and may necessitate open reduction.3 Our patient, while having a fixed dislocation, did not have a vertical axis component and, therefore, was amenable to our closed reduction attempt.
Our first reduction attempts were unsuccessful, likely because the patient continued to be tense, firing his quadriceps. Even after injecting the knee with lidocaine and eliminating the pain component, the patella was still impinging on the lateral femoral condyle (Figure 3A). By insufflating the knee with saline, we were able to increase the distance from the patella to the trochlea (Figure 3B). This is comparable to a knee arthroscopy, in which joint fluid pressure allows passage of arthroscopic instruments into the patellofemoral joint. We postulate that the farther the patella is anterior to the trochlea, the higher the likelihood that the patella can be reduced to its anatomic position.
Insufflation of the knee with sterile saline is a novel technique that involves minimal risk compared with the alternatives. Sometimes, for closed reduction to be successful, individuals need to be consciously sedated to relax their muscles and eliminate pain. While conscious sedation is generally considered low risk, complications have been noted, including hypotension, apnea, and retrograde amnesia.15 Manual closed reduction may also cause additional chondral damage when the medial patellar facet contacts the lateral femoral trochlea. When closed reduction of the patella fails, open reduction is required; this inherently includes all the risks of surgery, such as bleeding, infection, neurovascular injury, and wound complications.
Our insufflation technique does not require sedation and is minimally invasive. The saline creates space and provides lubrication to allow for easier manipulation of the patella. This theoretically protects the cartilage as the patella passes over the lateral trochlea. In addition to the intended effect of providing more space and lubrication for the reduction of the patella, insufflation of the joint may also relax the vastus musculature.16 In their study, Torry and colleagues16 injected 13 knees with 20 mL sterile saline and noted reduction in electromyography readings in the vastus medialis and lateralis muscles. This inhibition of vastus musculature may provide enough relaxation to aid in the successful reduction of the patella.
Our study is limited by our sample size of 1. Because acute patellar dislocations are often easily reduced, our technical trick is not frequently used. Additionally, while we were able to monitor his progress during his inpatient stay, our patient was lost to follow-up after his discharge from the hospital.
If successful, the insufflation technique eliminates the need for urgent open reduction in the operating room. As a result, we recommend attempting closed reduction using insufflation of the knee with sterile saline for irreducible patellar dislocations before proceeding with open reduction.
Conclusion
Saline insufflation of the knee can be safely and easily performed to aid in the reduction of subacute, difficult patellar dislocations.
As the largest sesamoid bone in the human body, the patella acts as a fulcrum to enhance the biomechanical advantage of the quadriceps in extension.1 It is subject to a variety of forces while improving distribution of forces along the extensor mechanism.2 With sufficient force, the patella can be dislocated. Acute patellar dislocations are the most common knee injury, encompassing 2% to 3% of all knee injuries3 and occurring in 5.8 per 100,000 individuals.4-5 These injuries are associated with acute trauma, frequently from sports and physical activities, occurring while in terminal extension with an axial-valgus stress on the knee during rotation.6
With acute patellar dislocations, patients are usually in significant discomfort. Often, the patella may spontaneously reduce; if not, closed reduction is usually successful with pressure applied anteromedially on the lateral patellar margin, while simultaneously attempting gentle extension of the leg.7 Closed reduction is almost universally successful, and there have only been case reports of irreducible, mainly fixed vertical axis patellar dislocations.8-11 No reports in the literature have described subacute patellar dislocations because of their rarity. Patients present immediately after dislocation, spontaneously reduce, or have a painless, chronically dislocated patella.
We present a case of an elderly man with dementia and a subacute fixed irreducible patellar dislocation, which was reduced using a technique not described in the literature. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 68-year-old nonambulatory man with a history of dementia and stroke presented to the emergency department with complaints of left knee pain and his knee locked in flexion. The patient’s knee had been in that fixed hyperflexed position for at least 10 days after he sustained a twisting injury to his knee while attempting to get out of bed. At baseline, the patient was mostly bedbound and could walk minimally with maximum support, but, given his dementia, he would often attempt to ambulate by himself. After the injury, the patient did not complain of much pain at rest, but attempts at his group home to straighten his leg had caused severe pain. As a result, the patient was brought to the emergency department to be evaluated for fractures.
Physical examination in the emergency department revealed atrophy of the lower extremity musculature and a left knee fixed at 120º in flexion. The skin was intact, and there was minimal effusion of the knee joint. The patella was noted to be laterally subluxated and tender to palpation over the lateral and medial facets. He was neurovascularly intact distally and had painless range of motion of his hips. His contralateral right knee had full range of motion with good patellar tracking.
Radiographs of the patient’s knee confirmed a lateral dislocation of the patella (Figures 1A-1C). After oral and intravenous administration of pain medication, a reduction was attempted without success. Next, an intra-articular knee injection of 10 mL of 1% lidocaine was given. After waiting 15 minutes, another reduction was tried. While the pain control was sufficient, the reduction was again unsuccessful. The knee was insufflated with 120 mL of sterile saline and reduction attempted again. By extending the knee and applying a medially directed force to the patella, reduction was successful. The patient was placed into a knee immobilizer and postreduction radiographs were taken (Figures 2A, 2B). Saline was extracted from the knee. The patient was admitted to the hospital where repeat examination of his knees during the next week revealed markedly less pain. The patient was lost to follow-up.
Discussion
Our patient presumably had a low-energy mechanism of injury, resulting in an undiagnosed patellar dislocation with delayed treatment. This subacute patellar dislocation was irreducible using the standard techniques. Alternatively, insufflation of the joint with saline provided the necessary impetus to allow for successful patellar reduction. The history of the patient reveals clues about the mechanism of injury. It is likely that the patient’s nonambulatory status resulted in a weak vastus medialis muscle that placed the patella at risk for dislocation. While the exact mechanism of dislocation is unknown, the patella was unable to be reduced spontaneously because our patient’s knee was maintained in a state of flexion secondary to pain and muscle contraction. The combination of weak quadriceps musculature, increased Q angle, and forced hyperflexion of the knee prevented closed reduction of the patella.
Fixed, irreducible patellar dislocations are rare and discussed infrequently in the literature.9,11-12 Reported mechanisms are mostly high energy, including blows during athletics and impacts from motor vehicle collisions.9,13 Vertical axis rotation, as first described by Cooper,14 is commonly implicated in irreducible patellar dislocations. This occurs when the patella internally rotates 180º on its vertical axis, associated with a large tear of the medial retinaculum but intact quadriceps tendon. The patella is fixed over the lateral femoral condyle with the articular surface pointing anterolaterally. Despite adequate sedation and analgesia, these are notoriously difficult to close-reduce and may necessitate open reduction.3 Our patient, while having a fixed dislocation, did not have a vertical axis component and, therefore, was amenable to our closed reduction attempt.
Our first reduction attempts were unsuccessful, likely because the patient continued to be tense, firing his quadriceps. Even after injecting the knee with lidocaine and eliminating the pain component, the patella was still impinging on the lateral femoral condyle (Figure 3A). By insufflating the knee with saline, we were able to increase the distance from the patella to the trochlea (Figure 3B). This is comparable to a knee arthroscopy, in which joint fluid pressure allows passage of arthroscopic instruments into the patellofemoral joint. We postulate that the farther the patella is anterior to the trochlea, the higher the likelihood that the patella can be reduced to its anatomic position.
Insufflation of the knee with sterile saline is a novel technique that involves minimal risk compared with the alternatives. Sometimes, for closed reduction to be successful, individuals need to be consciously sedated to relax their muscles and eliminate pain. While conscious sedation is generally considered low risk, complications have been noted, including hypotension, apnea, and retrograde amnesia.15 Manual closed reduction may also cause additional chondral damage when the medial patellar facet contacts the lateral femoral trochlea. When closed reduction of the patella fails, open reduction is required; this inherently includes all the risks of surgery, such as bleeding, infection, neurovascular injury, and wound complications.
Our insufflation technique does not require sedation and is minimally invasive. The saline creates space and provides lubrication to allow for easier manipulation of the patella. This theoretically protects the cartilage as the patella passes over the lateral trochlea. In addition to the intended effect of providing more space and lubrication for the reduction of the patella, insufflation of the joint may also relax the vastus musculature.16 In their study, Torry and colleagues16 injected 13 knees with 20 mL sterile saline and noted reduction in electromyography readings in the vastus medialis and lateralis muscles. This inhibition of vastus musculature may provide enough relaxation to aid in the successful reduction of the patella.
Our study is limited by our sample size of 1. Because acute patellar dislocations are often easily reduced, our technical trick is not frequently used. Additionally, while we were able to monitor his progress during his inpatient stay, our patient was lost to follow-up after his discharge from the hospital.
If successful, the insufflation technique eliminates the need for urgent open reduction in the operating room. As a result, we recommend attempting closed reduction using insufflation of the knee with sterile saline for irreducible patellar dislocations before proceeding with open reduction.
Conclusion
Saline insufflation of the knee can be safely and easily performed to aid in the reduction of subacute, difficult patellar dislocations.
1. Fu FH, Seel M, Berger RA. Patellofemoral biomechanics. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:49.
2. Dye SF. Patellofemoral anatomy. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:2-3.
3. Li X, Nielsen NM, Zhou H, Stein BS, Shelton YA, Busconi BD. Surgical treatment of a chronically fixed lateral patella dislocation in an adolescent patient. Orthop Rev (Pavia). 2013;5(2):45-47.
4. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
5. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90(12):2751-2762.
6. Panni AS, Vasso M, Cerciello S. Acute patellar dislocation. What to do? Knee Surg Sports Traumatol Arthrosc. 2013;21(2):275-278.
7. Lu DW, Wang EE, Self WH, Kharasch M. Patellar dislocation reduction. Acad Emerg Med. 2010;17(2):226.
8. Michels F, Pouliart N, Oosterlinck D. Locked patellar dislocation: a case report. J Med Case Rep. 2008;2:371.
9. ElMaraghy AW, Berry GK, Kreder HJ. Irreducible lateral patellar dislocation with vertical axis rotation: case report and review of the literature. J Trauma. 2002;53(1):131-132.
10. Wajid MA, Cheema MQ, Siddique MS. Vertical axis patellar dislocation with ipsilateral femoral fracture: use of a closed percutaneous technique for reduction of the dislocation. J Orthop Trauma. 2006;20(2):143-146.
11. Shetty S, Ramesh B, Gul A, Madhusudan TR, Altayeb T. Vertical dislocation of the patella: report of 2 cases. Orthopedics. 2009;32(10). doi: 10.3928/01477447-20090818-25.
12. Hackl W, Benedetto KP, Fink C, Sailer R, Rieger M. Locked lateral patellar dislocation: a rare case of irreducible patellar dislocation requiring open reduction. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):352-355.
13. Gidden DJ, Bell KM. An unusual case of irreducible intra-articular patellar dislocation with vertical axis rotation. Injury. 1995;26(9):643-644.
14. Cooper A. Dislocation of the patella. In: Cooper A, ed. A Treatise on the Dislocations and Fractures of the Joints. Philadelphia, PA: Lea & Febiger; 1844:195.
15. Swanson ER, Seaberg DC, Mathias S. The use of propofol for sedation in the emergency department. Acad Emerg Med. 2008;3(3):234-238.
16. Torry MR, Decker MJ, Millett PJ, Steadman JR, Sterett WI. The effects of knee joint effusion on quadriceps electromyography during jogging. J Sports Sci Med. 2005;4(1):1-8.
1. Fu FH, Seel M, Berger RA. Patellofemoral biomechanics. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:49.
2. Dye SF. Patellofemoral anatomy. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:2-3.
3. Li X, Nielsen NM, Zhou H, Stein BS, Shelton YA, Busconi BD. Surgical treatment of a chronically fixed lateral patella dislocation in an adolescent patient. Orthop Rev (Pavia). 2013;5(2):45-47.
4. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
5. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90(12):2751-2762.
6. Panni AS, Vasso M, Cerciello S. Acute patellar dislocation. What to do? Knee Surg Sports Traumatol Arthrosc. 2013;21(2):275-278.
7. Lu DW, Wang EE, Self WH, Kharasch M. Patellar dislocation reduction. Acad Emerg Med. 2010;17(2):226.
8. Michels F, Pouliart N, Oosterlinck D. Locked patellar dislocation: a case report. J Med Case Rep. 2008;2:371.
9. ElMaraghy AW, Berry GK, Kreder HJ. Irreducible lateral patellar dislocation with vertical axis rotation: case report and review of the literature. J Trauma. 2002;53(1):131-132.
10. Wajid MA, Cheema MQ, Siddique MS. Vertical axis patellar dislocation with ipsilateral femoral fracture: use of a closed percutaneous technique for reduction of the dislocation. J Orthop Trauma. 2006;20(2):143-146.
11. Shetty S, Ramesh B, Gul A, Madhusudan TR, Altayeb T. Vertical dislocation of the patella: report of 2 cases. Orthopedics. 2009;32(10). doi: 10.3928/01477447-20090818-25.
12. Hackl W, Benedetto KP, Fink C, Sailer R, Rieger M. Locked lateral patellar dislocation: a rare case of irreducible patellar dislocation requiring open reduction. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):352-355.
13. Gidden DJ, Bell KM. An unusual case of irreducible intra-articular patellar dislocation with vertical axis rotation. Injury. 1995;26(9):643-644.
14. Cooper A. Dislocation of the patella. In: Cooper A, ed. A Treatise on the Dislocations and Fractures of the Joints. Philadelphia, PA: Lea & Febiger; 1844:195.
15. Swanson ER, Seaberg DC, Mathias S. The use of propofol for sedation in the emergency department. Acad Emerg Med. 2008;3(3):234-238.
16. Torry MR, Decker MJ, Millett PJ, Steadman JR, Sterett WI. The effects of knee joint effusion on quadriceps electromyography during jogging. J Sports Sci Med. 2005;4(1):1-8.
A Conversation With AAOS President David D. Teuscher, MD
For the past 9 years, I have interviewed the president of the American Academy of Orthopaedic Surgeons (AAOS) to better understand the roles the AAOS and its president play in our professional lives.
At the 2015 AAOS Annual Meeting in Las Vegas this past March, David D. Teuscher, MD, assumed leadership of the AAOS as its 83rd president. Dr. Teuscher is a partner and past president of the Beaumont Bone & Joint Institute in Beaumont, Texas, and has had a broad experience in leadership positions in both Texas medical professional societies and the AAOS. Dr. Teuscher obtained his undergraduate degree from the University of Illinois at Champaign/Urbana and his medical degree from the University of Texas Medical School at San Antonio. He completed his orthopedic residency at the Brooke Army Medical Center, in Fort Sam Houston, and, following 13 years of military service, he entered private practice in 1993.
He has led numerous AAOS committees over the years, most notably the team that in 2014 completed a revision of the AAOS Strategic Plan, “Vision 20/20,” which outlines the Academy’s goals over the next 6 years, including the following elements:
- AAOS Mission: Serving our profession to provide the highest-quality musculoskeletal care.
- AAOS Vision: Keeping the world in motion through the prevention and treatment of musculoskeletal conditions.
- Core Values: Excellence, Professionalism, Leadership, Collegiality, Lifelong Learning.
- Strategic Domains: Advocacy, Education, Membership, Organizational Excellence, Quality and Patient Value.
Read more at: http://www.aaos.org/about/strategicplan.asp.
Dr. Teuscher explained that his role as president for the coming year is really that of spokesperson for a leadership group that has developed a 4-year presidential line and governance structure to ensure a solid platform for continuity and to achieve the goals of the AAOS Strategic Plan year after year. While the Academy president does not set his or her own agenda for the year, David has several priority goals during his tenure, which include ensuring that the rules governing the repeal and replacement of the Medicare Sustainable Growth Rate (SGR) formula treat our patients fairly, opening of the new digital and modular Orthopaedic Learning Center (OLC), preventing the harmful effects of unnecessary and premature ICD-10 (International Classification of Diseases, Tenth Revision) implementation, leading a cultural change in surgical patient safety, and advances in AAOS technology offerings in education and online lifelong learning.
Dr. Teuscher stated that the repeal of the SGR formula this year was a major step forward for orthopedic surgeons. Averting a 21% reduction in physician reimbursement in 2015, the new legislation will increase physician payments by 0.5% annually through 2019, at which time the Centers for Medicare and Medicaid Services (CMS) will begin a new payment system, based not on the traditional fee-for-service model, but on a new incentive: the quality and value of care.1 David firmly believes that the AAOS has a major role to assist the practicing orthopedic surgeon manage this new payment system by:
- establishing standards of performance and quality that will drive payment for medical services.
- helping the practicing orthopedic surgeon report useful quality outcomes in a simple and accessible format.
- linking these new reporting measures to satisfy Maintenance of Certification (MOC) requirements.
David is especially proud of the recently opened OLC. This cutting-edge facility, sponsored by the AAOS and its equity partners (Arthroscopy Association of North America, American Orthopaedic Society for Sports Medicine, American Association of Hip and Knee Surgeons, OLC), is clear evidence of the Academy’s commitment to the highest quality of musculoskeletal care and lifelong learning for its members.
Dr. Teuscher is concerned that CMS may not be fully prepared for implementation of the new ICD-10 codes on October 1, 2015. In the spirit of advocacy for its members, the AAOS is actively engaged to recommend delay of ICD-10 implementation until reliable operating systems to process this new system can be ensured.
David and orthopedic patient safety experts are working with national perioperative stakeholders to plan and implement a National Surgical Patient Safety Summit in 2016. This will cause a cultural change in how we lead treatment teams to deliver a highly reliable and safe surgical experience for all our patients.
Finally, Dr. Teuscher is extremely excited about improvements in technology offered to Academy members. Many of us enjoyed the new AAOS My Academy app available this year at the Las Vegas meeting that enabled review of the 2015 program on your smartphone. Dr. Teuscher anticipates that upgrades to the AAOS Access app will provide the most comprehensive mobile platform for continuing medical education and educational videos available to all Academy members. The AAOS website is undergoing a complete update and expansion of offerings by the end of this year.
Over the years of interviewing current presidents of the AAOS, I have been impressed by consistent characteristics of our leaders: enormously energetic, engaging, articulate, and tirelessly committed to the Academy and its members. David Teuscher processes all these qualities. We are very fortunate to have someone of David’s organizational and leadership skills navigate our course through the turbulent health care waters that lie ahead of us in the coming years.◾
Reference
1. Lowes R. Congress repeals Medicare SGR formula. Medscape website. http://www.medscape.com/viewarticle/843078. Published April 14, 2015. Accessed June 8, 2015.
For the past 9 years, I have interviewed the president of the American Academy of Orthopaedic Surgeons (AAOS) to better understand the roles the AAOS and its president play in our professional lives.
At the 2015 AAOS Annual Meeting in Las Vegas this past March, David D. Teuscher, MD, assumed leadership of the AAOS as its 83rd president. Dr. Teuscher is a partner and past president of the Beaumont Bone & Joint Institute in Beaumont, Texas, and has had a broad experience in leadership positions in both Texas medical professional societies and the AAOS. Dr. Teuscher obtained his undergraduate degree from the University of Illinois at Champaign/Urbana and his medical degree from the University of Texas Medical School at San Antonio. He completed his orthopedic residency at the Brooke Army Medical Center, in Fort Sam Houston, and, following 13 years of military service, he entered private practice in 1993.
He has led numerous AAOS committees over the years, most notably the team that in 2014 completed a revision of the AAOS Strategic Plan, “Vision 20/20,” which outlines the Academy’s goals over the next 6 years, including the following elements:
- AAOS Mission: Serving our profession to provide the highest-quality musculoskeletal care.
- AAOS Vision: Keeping the world in motion through the prevention and treatment of musculoskeletal conditions.
- Core Values: Excellence, Professionalism, Leadership, Collegiality, Lifelong Learning.
- Strategic Domains: Advocacy, Education, Membership, Organizational Excellence, Quality and Patient Value.
Read more at: http://www.aaos.org/about/strategicplan.asp.
Dr. Teuscher explained that his role as president for the coming year is really that of spokesperson for a leadership group that has developed a 4-year presidential line and governance structure to ensure a solid platform for continuity and to achieve the goals of the AAOS Strategic Plan year after year. While the Academy president does not set his or her own agenda for the year, David has several priority goals during his tenure, which include ensuring that the rules governing the repeal and replacement of the Medicare Sustainable Growth Rate (SGR) formula treat our patients fairly, opening of the new digital and modular Orthopaedic Learning Center (OLC), preventing the harmful effects of unnecessary and premature ICD-10 (International Classification of Diseases, Tenth Revision) implementation, leading a cultural change in surgical patient safety, and advances in AAOS technology offerings in education and online lifelong learning.
Dr. Teuscher stated that the repeal of the SGR formula this year was a major step forward for orthopedic surgeons. Averting a 21% reduction in physician reimbursement in 2015, the new legislation will increase physician payments by 0.5% annually through 2019, at which time the Centers for Medicare and Medicaid Services (CMS) will begin a new payment system, based not on the traditional fee-for-service model, but on a new incentive: the quality and value of care.1 David firmly believes that the AAOS has a major role to assist the practicing orthopedic surgeon manage this new payment system by:
- establishing standards of performance and quality that will drive payment for medical services.
- helping the practicing orthopedic surgeon report useful quality outcomes in a simple and accessible format.
- linking these new reporting measures to satisfy Maintenance of Certification (MOC) requirements.
David is especially proud of the recently opened OLC. This cutting-edge facility, sponsored by the AAOS and its equity partners (Arthroscopy Association of North America, American Orthopaedic Society for Sports Medicine, American Association of Hip and Knee Surgeons, OLC), is clear evidence of the Academy’s commitment to the highest quality of musculoskeletal care and lifelong learning for its members.
Dr. Teuscher is concerned that CMS may not be fully prepared for implementation of the new ICD-10 codes on October 1, 2015. In the spirit of advocacy for its members, the AAOS is actively engaged to recommend delay of ICD-10 implementation until reliable operating systems to process this new system can be ensured.
David and orthopedic patient safety experts are working with national perioperative stakeholders to plan and implement a National Surgical Patient Safety Summit in 2016. This will cause a cultural change in how we lead treatment teams to deliver a highly reliable and safe surgical experience for all our patients.
Finally, Dr. Teuscher is extremely excited about improvements in technology offered to Academy members. Many of us enjoyed the new AAOS My Academy app available this year at the Las Vegas meeting that enabled review of the 2015 program on your smartphone. Dr. Teuscher anticipates that upgrades to the AAOS Access app will provide the most comprehensive mobile platform for continuing medical education and educational videos available to all Academy members. The AAOS website is undergoing a complete update and expansion of offerings by the end of this year.
Over the years of interviewing current presidents of the AAOS, I have been impressed by consistent characteristics of our leaders: enormously energetic, engaging, articulate, and tirelessly committed to the Academy and its members. David Teuscher processes all these qualities. We are very fortunate to have someone of David’s organizational and leadership skills navigate our course through the turbulent health care waters that lie ahead of us in the coming years.◾
For the past 9 years, I have interviewed the president of the American Academy of Orthopaedic Surgeons (AAOS) to better understand the roles the AAOS and its president play in our professional lives.
At the 2015 AAOS Annual Meeting in Las Vegas this past March, David D. Teuscher, MD, assumed leadership of the AAOS as its 83rd president. Dr. Teuscher is a partner and past president of the Beaumont Bone & Joint Institute in Beaumont, Texas, and has had a broad experience in leadership positions in both Texas medical professional societies and the AAOS. Dr. Teuscher obtained his undergraduate degree from the University of Illinois at Champaign/Urbana and his medical degree from the University of Texas Medical School at San Antonio. He completed his orthopedic residency at the Brooke Army Medical Center, in Fort Sam Houston, and, following 13 years of military service, he entered private practice in 1993.
He has led numerous AAOS committees over the years, most notably the team that in 2014 completed a revision of the AAOS Strategic Plan, “Vision 20/20,” which outlines the Academy’s goals over the next 6 years, including the following elements:
- AAOS Mission: Serving our profession to provide the highest-quality musculoskeletal care.
- AAOS Vision: Keeping the world in motion through the prevention and treatment of musculoskeletal conditions.
- Core Values: Excellence, Professionalism, Leadership, Collegiality, Lifelong Learning.
- Strategic Domains: Advocacy, Education, Membership, Organizational Excellence, Quality and Patient Value.
Read more at: http://www.aaos.org/about/strategicplan.asp.
Dr. Teuscher explained that his role as president for the coming year is really that of spokesperson for a leadership group that has developed a 4-year presidential line and governance structure to ensure a solid platform for continuity and to achieve the goals of the AAOS Strategic Plan year after year. While the Academy president does not set his or her own agenda for the year, David has several priority goals during his tenure, which include ensuring that the rules governing the repeal and replacement of the Medicare Sustainable Growth Rate (SGR) formula treat our patients fairly, opening of the new digital and modular Orthopaedic Learning Center (OLC), preventing the harmful effects of unnecessary and premature ICD-10 (International Classification of Diseases, Tenth Revision) implementation, leading a cultural change in surgical patient safety, and advances in AAOS technology offerings in education and online lifelong learning.
Dr. Teuscher stated that the repeal of the SGR formula this year was a major step forward for orthopedic surgeons. Averting a 21% reduction in physician reimbursement in 2015, the new legislation will increase physician payments by 0.5% annually through 2019, at which time the Centers for Medicare and Medicaid Services (CMS) will begin a new payment system, based not on the traditional fee-for-service model, but on a new incentive: the quality and value of care.1 David firmly believes that the AAOS has a major role to assist the practicing orthopedic surgeon manage this new payment system by:
- establishing standards of performance and quality that will drive payment for medical services.
- helping the practicing orthopedic surgeon report useful quality outcomes in a simple and accessible format.
- linking these new reporting measures to satisfy Maintenance of Certification (MOC) requirements.
David is especially proud of the recently opened OLC. This cutting-edge facility, sponsored by the AAOS and its equity partners (Arthroscopy Association of North America, American Orthopaedic Society for Sports Medicine, American Association of Hip and Knee Surgeons, OLC), is clear evidence of the Academy’s commitment to the highest quality of musculoskeletal care and lifelong learning for its members.
Dr. Teuscher is concerned that CMS may not be fully prepared for implementation of the new ICD-10 codes on October 1, 2015. In the spirit of advocacy for its members, the AAOS is actively engaged to recommend delay of ICD-10 implementation until reliable operating systems to process this new system can be ensured.
David and orthopedic patient safety experts are working with national perioperative stakeholders to plan and implement a National Surgical Patient Safety Summit in 2016. This will cause a cultural change in how we lead treatment teams to deliver a highly reliable and safe surgical experience for all our patients.
Finally, Dr. Teuscher is extremely excited about improvements in technology offered to Academy members. Many of us enjoyed the new AAOS My Academy app available this year at the Las Vegas meeting that enabled review of the 2015 program on your smartphone. Dr. Teuscher anticipates that upgrades to the AAOS Access app will provide the most comprehensive mobile platform for continuing medical education and educational videos available to all Academy members. The AAOS website is undergoing a complete update and expansion of offerings by the end of this year.
Over the years of interviewing current presidents of the AAOS, I have been impressed by consistent characteristics of our leaders: enormously energetic, engaging, articulate, and tirelessly committed to the Academy and its members. David Teuscher processes all these qualities. We are very fortunate to have someone of David’s organizational and leadership skills navigate our course through the turbulent health care waters that lie ahead of us in the coming years.◾
Reference
1. Lowes R. Congress repeals Medicare SGR formula. Medscape website. http://www.medscape.com/viewarticle/843078. Published April 14, 2015. Accessed June 8, 2015.
Reference
1. Lowes R. Congress repeals Medicare SGR formula. Medscape website. http://www.medscape.com/viewarticle/843078. Published April 14, 2015. Accessed June 8, 2015.
Total Hip Arthroplasty for Posttraumatic Osteoarthritis of the Hip Fares Worse Than THA for Primary Osteoarthritis
The incidence of hip fractures decreased between 1995 and 2005, but these injuries continue to occur in large numbers. Between 1986 and 2005, the mean annual number of hip fractures was 957.3/100,000, and the majority of these occurred in patients 75 to 84 years old.1 Investigators have described total hip arthroplasty (THA) performed after initial surgical treatment in patients who developed osteoarthritis (OA) of the hip secondary to a fracture.2-7 Only 1 of these studies compared these patients with a control group of patients who had THA for primary hip OA.2 No study included both previous proximal femur and acetabular fractures.
Postfracture OA may occur when there is residual articular incongruity after fracture or osteonecrosis of the femoral head. THA is commonly used to treat OA when more conservative treatments have failed.6 Other indications for conversion to THA include femoral neck nonunion, significant leg-length discrepancy, and femoral head damage caused by previous internal fixation.4
Given these conditions and previous study findings, THA performed in patients with previous hip fracture fixation is potentially more complicated than THA for primary OA. We therefore conducted a study to evaluate differences in sociodemographic factors, surgical details, and outcomes between patients who had THA for posttraumatic OA and patients who had THA for primary OA.
Materials and Methods
After obtaining institutional review board approval and patient consent, we used a prospective database to follow 3844 patients who had THA performed for OA by 1 of 17 different surgeons at a single center over an 8-year period. Patients who had THA for secondary causes of hip OA, developmental hip dysplasia, or inflammatory processes were excluded. Of the remaining 1199 patients, 62 (5.2%) had THA for posttraumatic OA after previous acetabular or proximal femur fracture fixation (Figures 1, 2) (no THA was performed at time of initial fracture treatment), and 1137 had THA for primary OA and served as the control group.
We collected data on age, sex, fracture location, reason for THA, time between open reduction and internal fixation (ORIF) and THA, type of components, cement use, leg-length discrepancy, intraoperative complications, blood loss, operating room time, and postoperative complications. All patients were aseptic at time of THA. All posttraumatic OA patients had previous hardware removed; the extent of hardware removal was dictated by the exposure required for prosthesis implantation. These patients were contacted, and clinical follow-up was assessed with modified Harris Hip Score (HHS).8 HHS was determined by Dr. Khurana. Statistical analysis was performed with Student t test and Pearson χ2 test using PASW Statistics 18 (SPSS, Chicago, Illinois).
The 62 posttraumatic OA patients had 63 fractures, 41 of the proximal femur (femoral neck and intertrochanteric; 65%) and 22 acetabular (35%). This group consisted of 33 females and 29 males. Their mean age at time of THA surgery was 58 years (range, 31-90 years). Mean age of the control patients was 59.4 years (range, 18-95 years). There were 35 right hips and 27 left hips in the posttrauma group. Mean body mass index (BMI) was 28.4 for the posttrauma group and 28.9 for the control group. There were no differences in age (P = .451), sex (P = .674), or BMI (P = .592) between the 2 groups (Table 1).
All 62 posttraumatic OA patients had complete hospital data, and 32 (52%) of the 62 underwent long-term follow-up (mean, 4.3 years; range, 4 months–10.5 years). At time of attempted contact (mean, 6.79 years after THA), 7 patients were deceased; cause of death was an unrelated medical condition (1) or unknown (6). The rest of the patients did not respond to multiple telephone and mail summons. Primary reasons for conversion to THA included OA (34 patients, 54%), development of osteonecrosis (12 patients, 19%), and nonunion (12 patients, 19%). The rest of the patients had fixation failure. The mechanisms of injury were motor vehicle accidents (30 patients), falls (20), and other causes (15).
Results
Thirty-two (52%) of the posttraumatic OA patients had a preoperative leg-length discrepancy. For these patients, mean time between initial fracture fixation and conversion to THA was 74 months (range, 1-480 months). Four patients required grafting with cancellous autogenous bone graft or allograft chips to fill a bony defect. Mean acetabular component diameter was 54 mm. Nineteen patients had acetabular fixation supplemented with screws. (Screw supplementation data were not recorded for control patients.) Three patients (4.7%) with an acetabular fracture had heterotopic bone removed at time of THA. Two patients underwent neurolysis of the sciatic nerve at time of surgery for preexisting nerve palsy.
Mean postoperative hemoglobin was 109 g/L in the posttraumatic OA group and 121 g/L in the control group (P <. 001). Mean postoperative hematocrit was 0.327 and 0.367, respectively (P < .001). Mean amount of Cell Saver (Haemonetics) used by patients was 176.2 and 72.9 mL, respectively (P < .001). Posttrauma patients lost a mean of 360 mL of blood more than control patients did (P < .001) and were transfused a mean of 1.59 units of blood, compared with 0.85 unit in the controls (P < .001). Patients with acetabular fractures required a mean of only 0.65 unit of transfused blood. Mean operating room time was 240.5 minutes for posttrauma patients and 135.6 minutes for control patients (P < .001). In the posttrauma group, mean size of the head of the femoral component was 29 mm (head size was not recorded for the control group). Posttrauma patients had 18 (29%) hybrid cemented hip replacements (femoral component only) and 44 uncemented hip replacements. Data on femoral stem size and type were not reported for either group.
Twenty-four posttrauma patients (39%) had a total of 63 perioperative complications, and 131 control patients (11.5%) had a total of 160 complications (P < .001). Complications in posttrauma patients with proximal femur fractures included excess bleeding (5 patients), in-hospital dislocations (2), and postoperative infections (4: 2 superficial wound infections, 1 implant infection requiring explant, 1 Clostridium difficile infection); in patients with acetabular fractures, there was only 1 dislocation (no infections). The posttraumatic OA group did not develop any symptomatic venous thromboembolic complications. One patient developed a sciatic nerve palsy after surgery. Of the 3 patients who sustained dislocations, 2 were treated with closed reduction and maintenance of implants, and 1 with revision THA. Complications in the control group included 3 infections, 4 dislocations, and 12 cases of extensive blood loss (Table 2).
In patients with long-term follow-up, mean postoperative modified HHS was 81.33 (range, 34.1-100.1). Twelve patients had an excellent score (>90), 10 a good score (80-89), 4 a fair score (70-79), and 6 a poor score (<70). Mean HHS was 84.2 for the 16 patients with a femoral head or neck fracture, 77.7 for the 6 patients with an intertrochanteric fracture, and 84.3 for the 9 patients with an acetabular fracture. Nine patients reported using a cane, 3 required walkers, 2 required wheelchairs, and 18 did not require any walking support. Four (12.5%) of the 32 patients required THA revision a mean of 3.5 years (range, 2 months–8 years) after initial arthroplasty. Reasons for revision were infections (2 patients), multiple dislocations (1), and dissociation of acetabular lining (1) (Table 3). Two of the patients who underwent THA revision had a cemented femoral stem, and 2 did not have any cemented implants. Additional details of the femoral stem components were not available for either group.
Discussion
Patients who develop posttraumatic OA of the hip have limited options. THA has emerged as an excellent option in cases of failed repair of fractures about the hip joint. The results of the present study are consistent with earlier findings of the effectiveness of THA in salvaging posttraumatic hips.2-7 THA for patients with posttraumatic arthritis of the hip after acetabular or proximal femur fracture is longer and more complicated than THA for primary OA, and there is significantly more blood loss. In addition, the rate of early failure appears to be higher.9
In this study, mean amount of blood transfused for patients with previous acetabular fracture was 0.65 unit, much less than the mean of 3.5 units noted by Weber and colleagues.6 In their study, complications associated with THA were increased in patients with posttraumatic OA from acetabular fractures. The authors attributed these complications to scarring from previous surgery, retained hardware, heterotopic bone, and residual osseous deformity and deficiency. Our results support their conclusion. Operating times were longer, as well as blood loss and the need for blood transfusions and other blood products were increased in the patients with posttraumatic OA, as compared with patients with primary OA. Fifteen percent of patients with an acetabular fracture had undergone removal of heterotopic bone at time of surgery—similar to the rate of 18% noted in the Weber study.6
Our results showed that the rate of revision THA was also higher than in patients with primary THA within the general population—reported to be about 4%.9 The higher rate may be the result of the additional surgeries performed on patients with fractures, or hardware retention increasing the infection risk over the years. Our revision rate of 12.5% was similar to the 19% found by Ranawat and colleagues7 in their study.
A majority of the patients in our study had favorable long-term HHS. Mean overall HHS was 83, slightly better than the 79 reported by Srivastav and colleagues.4 We found that patients with intertrochanteric fractures ultimately had worse outcome scores than patients with acetabular or femoral neck fractures. These results are consistent with findings reported by Mehlhoff and colleagues5 in a study comparing patients with femoral neck and intertrochanteric fractures. Mean HHS for the intertrochanteric fracture patients in our study was 77.7, comparable to the mean of 78 reported by Mehlhoff and colleagues.5 Mean HHS for the femoral neck or head fractures in our study was 84.2, similar to the mean of 81 they noted. Patients with a previous acetabular fracture in our study had a mean HHS of 84.3, consistent with the 84 reported by Ranawat and colleagues7 for patients who had initially undergone ORIF for acetabular fracture. Mean HHS in our study (83) was slightly less than the 88.5 reported by Shi and colleagues10 in their study of primary THAs.
Few studies have been conducted exclusively on one type of hip fracture (acetabular) or another (proximal femur), and all except 1 did not perform a comparison. Tabsh and colleagues2 compared similar cohorts but focused solely on patients with previous proximal femur fractures. The present study included a control group and both acetabular and proximal femur fractures, which allowed us to compare patients with and without previous fracture fixation and to consider the 2 different fracture types and see if they affected outcomes.
The strengths of this study include the large control group and the relatively short data-collection period. The shorter period decreased the influence of improvements in implants on patient outcomes. In addition, the control group was our own population, as we did not compare our cohort of patients with previous internal fixation and patients who had primary THAs in other studies, aside from comparisons for revision rates and HHS.
Although the ultimate long-term follow-up rate for patients with previous internal fixation was 50%, our sample size was still larger than that in most reported studies. Another weakness of our study was the large number of surgeons (17), representing an array of techniques, approaches, and surgical experience. All these factors could have influenced patient outcomes and operative data. In addition, data on revision rates and HHS were not available for our control group, so we could not directly compare these outcomes with those of the posttraumatic group. However, we used previously reported data on revision rates and HHS in primary THAs for comparison with the posttraumatic group.9,10
Conclusion
In this study, THA was a viable option for patients with posttraumatic arthritis from a previous acetabular or proximal femur fracture. The outcomes, however, were less reliable than the outcomes of primary THA for degenerative arthritis, and the complication rates were higher. Surgeons should counsel patients about the complexity of the procedure as well as its ultimately favorable outcomes. Surgeons should expect additional technical difficulties in the operating room when treating this patient population.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573-1579.
2. Tabsh I, Waddell JP, Morton J. Total hip arthroplasty for complications of proximal femoral fractures. J Orthop Trauma. 1997;11(3):166-169.
3. Haidukewych GJ, Berry DJ. Hip arthroplasty for salvage of failed treatment of intertrochanteric hip fractures. J Bone Joint Surg Am. 2003;85(5):899-904.
4. Srivastav S, Mittal V, Agarwal S. Total hip arthroplasty following failed fixation of proximal hip fractures. Indian J Orthop. 2008;42(3):279-286.
5. Mehlhoff T, Landon GC, Tullos HS. Total hip arthroplasty following failed internal fixation of hip fractures. Clin Orthop Relat Res. 1991;(269):32-37.
6. Weber M, Berry DJ, Harmsen WS. Total hip arthroplasty after operative treatment of an acetabular fracture. J Bone Joint Surg Am. 1998;80(9):1295-1305.
7. Ranawat A, Zelken J, Helfet D, Buly R. Total hip arthroplasty for posttraumatic arthritis after acetabular fracture. J Arthroplasty. 2009;24(5):759-767.
8. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
9. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
10. Shi HY, Mau LW, Chang JK, Wang JW, Chiu HC. Responsiveness of the Harris Hip Score and the SF-36: five years after total hip arthroplasty. Qual Life Res. 2009;18(8):1053-1060.
The incidence of hip fractures decreased between 1995 and 2005, but these injuries continue to occur in large numbers. Between 1986 and 2005, the mean annual number of hip fractures was 957.3/100,000, and the majority of these occurred in patients 75 to 84 years old.1 Investigators have described total hip arthroplasty (THA) performed after initial surgical treatment in patients who developed osteoarthritis (OA) of the hip secondary to a fracture.2-7 Only 1 of these studies compared these patients with a control group of patients who had THA for primary hip OA.2 No study included both previous proximal femur and acetabular fractures.
Postfracture OA may occur when there is residual articular incongruity after fracture or osteonecrosis of the femoral head. THA is commonly used to treat OA when more conservative treatments have failed.6 Other indications for conversion to THA include femoral neck nonunion, significant leg-length discrepancy, and femoral head damage caused by previous internal fixation.4
Given these conditions and previous study findings, THA performed in patients with previous hip fracture fixation is potentially more complicated than THA for primary OA. We therefore conducted a study to evaluate differences in sociodemographic factors, surgical details, and outcomes between patients who had THA for posttraumatic OA and patients who had THA for primary OA.
Materials and Methods
After obtaining institutional review board approval and patient consent, we used a prospective database to follow 3844 patients who had THA performed for OA by 1 of 17 different surgeons at a single center over an 8-year period. Patients who had THA for secondary causes of hip OA, developmental hip dysplasia, or inflammatory processes were excluded. Of the remaining 1199 patients, 62 (5.2%) had THA for posttraumatic OA after previous acetabular or proximal femur fracture fixation (Figures 1, 2) (no THA was performed at time of initial fracture treatment), and 1137 had THA for primary OA and served as the control group.
We collected data on age, sex, fracture location, reason for THA, time between open reduction and internal fixation (ORIF) and THA, type of components, cement use, leg-length discrepancy, intraoperative complications, blood loss, operating room time, and postoperative complications. All patients were aseptic at time of THA. All posttraumatic OA patients had previous hardware removed; the extent of hardware removal was dictated by the exposure required for prosthesis implantation. These patients were contacted, and clinical follow-up was assessed with modified Harris Hip Score (HHS).8 HHS was determined by Dr. Khurana. Statistical analysis was performed with Student t test and Pearson χ2 test using PASW Statistics 18 (SPSS, Chicago, Illinois).
The 62 posttraumatic OA patients had 63 fractures, 41 of the proximal femur (femoral neck and intertrochanteric; 65%) and 22 acetabular (35%). This group consisted of 33 females and 29 males. Their mean age at time of THA surgery was 58 years (range, 31-90 years). Mean age of the control patients was 59.4 years (range, 18-95 years). There were 35 right hips and 27 left hips in the posttrauma group. Mean body mass index (BMI) was 28.4 for the posttrauma group and 28.9 for the control group. There were no differences in age (P = .451), sex (P = .674), or BMI (P = .592) between the 2 groups (Table 1).
All 62 posttraumatic OA patients had complete hospital data, and 32 (52%) of the 62 underwent long-term follow-up (mean, 4.3 years; range, 4 months–10.5 years). At time of attempted contact (mean, 6.79 years after THA), 7 patients were deceased; cause of death was an unrelated medical condition (1) or unknown (6). The rest of the patients did not respond to multiple telephone and mail summons. Primary reasons for conversion to THA included OA (34 patients, 54%), development of osteonecrosis (12 patients, 19%), and nonunion (12 patients, 19%). The rest of the patients had fixation failure. The mechanisms of injury were motor vehicle accidents (30 patients), falls (20), and other causes (15).
Results
Thirty-two (52%) of the posttraumatic OA patients had a preoperative leg-length discrepancy. For these patients, mean time between initial fracture fixation and conversion to THA was 74 months (range, 1-480 months). Four patients required grafting with cancellous autogenous bone graft or allograft chips to fill a bony defect. Mean acetabular component diameter was 54 mm. Nineteen patients had acetabular fixation supplemented with screws. (Screw supplementation data were not recorded for control patients.) Three patients (4.7%) with an acetabular fracture had heterotopic bone removed at time of THA. Two patients underwent neurolysis of the sciatic nerve at time of surgery for preexisting nerve palsy.
Mean postoperative hemoglobin was 109 g/L in the posttraumatic OA group and 121 g/L in the control group (P <. 001). Mean postoperative hematocrit was 0.327 and 0.367, respectively (P < .001). Mean amount of Cell Saver (Haemonetics) used by patients was 176.2 and 72.9 mL, respectively (P < .001). Posttrauma patients lost a mean of 360 mL of blood more than control patients did (P < .001) and were transfused a mean of 1.59 units of blood, compared with 0.85 unit in the controls (P < .001). Patients with acetabular fractures required a mean of only 0.65 unit of transfused blood. Mean operating room time was 240.5 minutes for posttrauma patients and 135.6 minutes for control patients (P < .001). In the posttrauma group, mean size of the head of the femoral component was 29 mm (head size was not recorded for the control group). Posttrauma patients had 18 (29%) hybrid cemented hip replacements (femoral component only) and 44 uncemented hip replacements. Data on femoral stem size and type were not reported for either group.
Twenty-four posttrauma patients (39%) had a total of 63 perioperative complications, and 131 control patients (11.5%) had a total of 160 complications (P < .001). Complications in posttrauma patients with proximal femur fractures included excess bleeding (5 patients), in-hospital dislocations (2), and postoperative infections (4: 2 superficial wound infections, 1 implant infection requiring explant, 1 Clostridium difficile infection); in patients with acetabular fractures, there was only 1 dislocation (no infections). The posttraumatic OA group did not develop any symptomatic venous thromboembolic complications. One patient developed a sciatic nerve palsy after surgery. Of the 3 patients who sustained dislocations, 2 were treated with closed reduction and maintenance of implants, and 1 with revision THA. Complications in the control group included 3 infections, 4 dislocations, and 12 cases of extensive blood loss (Table 2).
In patients with long-term follow-up, mean postoperative modified HHS was 81.33 (range, 34.1-100.1). Twelve patients had an excellent score (>90), 10 a good score (80-89), 4 a fair score (70-79), and 6 a poor score (<70). Mean HHS was 84.2 for the 16 patients with a femoral head or neck fracture, 77.7 for the 6 patients with an intertrochanteric fracture, and 84.3 for the 9 patients with an acetabular fracture. Nine patients reported using a cane, 3 required walkers, 2 required wheelchairs, and 18 did not require any walking support. Four (12.5%) of the 32 patients required THA revision a mean of 3.5 years (range, 2 months–8 years) after initial arthroplasty. Reasons for revision were infections (2 patients), multiple dislocations (1), and dissociation of acetabular lining (1) (Table 3). Two of the patients who underwent THA revision had a cemented femoral stem, and 2 did not have any cemented implants. Additional details of the femoral stem components were not available for either group.
Discussion
Patients who develop posttraumatic OA of the hip have limited options. THA has emerged as an excellent option in cases of failed repair of fractures about the hip joint. The results of the present study are consistent with earlier findings of the effectiveness of THA in salvaging posttraumatic hips.2-7 THA for patients with posttraumatic arthritis of the hip after acetabular or proximal femur fracture is longer and more complicated than THA for primary OA, and there is significantly more blood loss. In addition, the rate of early failure appears to be higher.9
In this study, mean amount of blood transfused for patients with previous acetabular fracture was 0.65 unit, much less than the mean of 3.5 units noted by Weber and colleagues.6 In their study, complications associated with THA were increased in patients with posttraumatic OA from acetabular fractures. The authors attributed these complications to scarring from previous surgery, retained hardware, heterotopic bone, and residual osseous deformity and deficiency. Our results support their conclusion. Operating times were longer, as well as blood loss and the need for blood transfusions and other blood products were increased in the patients with posttraumatic OA, as compared with patients with primary OA. Fifteen percent of patients with an acetabular fracture had undergone removal of heterotopic bone at time of surgery—similar to the rate of 18% noted in the Weber study.6
Our results showed that the rate of revision THA was also higher than in patients with primary THA within the general population—reported to be about 4%.9 The higher rate may be the result of the additional surgeries performed on patients with fractures, or hardware retention increasing the infection risk over the years. Our revision rate of 12.5% was similar to the 19% found by Ranawat and colleagues7 in their study.
A majority of the patients in our study had favorable long-term HHS. Mean overall HHS was 83, slightly better than the 79 reported by Srivastav and colleagues.4 We found that patients with intertrochanteric fractures ultimately had worse outcome scores than patients with acetabular or femoral neck fractures. These results are consistent with findings reported by Mehlhoff and colleagues5 in a study comparing patients with femoral neck and intertrochanteric fractures. Mean HHS for the intertrochanteric fracture patients in our study was 77.7, comparable to the mean of 78 reported by Mehlhoff and colleagues.5 Mean HHS for the femoral neck or head fractures in our study was 84.2, similar to the mean of 81 they noted. Patients with a previous acetabular fracture in our study had a mean HHS of 84.3, consistent with the 84 reported by Ranawat and colleagues7 for patients who had initially undergone ORIF for acetabular fracture. Mean HHS in our study (83) was slightly less than the 88.5 reported by Shi and colleagues10 in their study of primary THAs.
Few studies have been conducted exclusively on one type of hip fracture (acetabular) or another (proximal femur), and all except 1 did not perform a comparison. Tabsh and colleagues2 compared similar cohorts but focused solely on patients with previous proximal femur fractures. The present study included a control group and both acetabular and proximal femur fractures, which allowed us to compare patients with and without previous fracture fixation and to consider the 2 different fracture types and see if they affected outcomes.
The strengths of this study include the large control group and the relatively short data-collection period. The shorter period decreased the influence of improvements in implants on patient outcomes. In addition, the control group was our own population, as we did not compare our cohort of patients with previous internal fixation and patients who had primary THAs in other studies, aside from comparisons for revision rates and HHS.
Although the ultimate long-term follow-up rate for patients with previous internal fixation was 50%, our sample size was still larger than that in most reported studies. Another weakness of our study was the large number of surgeons (17), representing an array of techniques, approaches, and surgical experience. All these factors could have influenced patient outcomes and operative data. In addition, data on revision rates and HHS were not available for our control group, so we could not directly compare these outcomes with those of the posttraumatic group. However, we used previously reported data on revision rates and HHS in primary THAs for comparison with the posttraumatic group.9,10
Conclusion
In this study, THA was a viable option for patients with posttraumatic arthritis from a previous acetabular or proximal femur fracture. The outcomes, however, were less reliable than the outcomes of primary THA for degenerative arthritis, and the complication rates were higher. Surgeons should counsel patients about the complexity of the procedure as well as its ultimately favorable outcomes. Surgeons should expect additional technical difficulties in the operating room when treating this patient population.
The incidence of hip fractures decreased between 1995 and 2005, but these injuries continue to occur in large numbers. Between 1986 and 2005, the mean annual number of hip fractures was 957.3/100,000, and the majority of these occurred in patients 75 to 84 years old.1 Investigators have described total hip arthroplasty (THA) performed after initial surgical treatment in patients who developed osteoarthritis (OA) of the hip secondary to a fracture.2-7 Only 1 of these studies compared these patients with a control group of patients who had THA for primary hip OA.2 No study included both previous proximal femur and acetabular fractures.
Postfracture OA may occur when there is residual articular incongruity after fracture or osteonecrosis of the femoral head. THA is commonly used to treat OA when more conservative treatments have failed.6 Other indications for conversion to THA include femoral neck nonunion, significant leg-length discrepancy, and femoral head damage caused by previous internal fixation.4
Given these conditions and previous study findings, THA performed in patients with previous hip fracture fixation is potentially more complicated than THA for primary OA. We therefore conducted a study to evaluate differences in sociodemographic factors, surgical details, and outcomes between patients who had THA for posttraumatic OA and patients who had THA for primary OA.
Materials and Methods
After obtaining institutional review board approval and patient consent, we used a prospective database to follow 3844 patients who had THA performed for OA by 1 of 17 different surgeons at a single center over an 8-year period. Patients who had THA for secondary causes of hip OA, developmental hip dysplasia, or inflammatory processes were excluded. Of the remaining 1199 patients, 62 (5.2%) had THA for posttraumatic OA after previous acetabular or proximal femur fracture fixation (Figures 1, 2) (no THA was performed at time of initial fracture treatment), and 1137 had THA for primary OA and served as the control group.
We collected data on age, sex, fracture location, reason for THA, time between open reduction and internal fixation (ORIF) and THA, type of components, cement use, leg-length discrepancy, intraoperative complications, blood loss, operating room time, and postoperative complications. All patients were aseptic at time of THA. All posttraumatic OA patients had previous hardware removed; the extent of hardware removal was dictated by the exposure required for prosthesis implantation. These patients were contacted, and clinical follow-up was assessed with modified Harris Hip Score (HHS).8 HHS was determined by Dr. Khurana. Statistical analysis was performed with Student t test and Pearson χ2 test using PASW Statistics 18 (SPSS, Chicago, Illinois).
The 62 posttraumatic OA patients had 63 fractures, 41 of the proximal femur (femoral neck and intertrochanteric; 65%) and 22 acetabular (35%). This group consisted of 33 females and 29 males. Their mean age at time of THA surgery was 58 years (range, 31-90 years). Mean age of the control patients was 59.4 years (range, 18-95 years). There were 35 right hips and 27 left hips in the posttrauma group. Mean body mass index (BMI) was 28.4 for the posttrauma group and 28.9 for the control group. There were no differences in age (P = .451), sex (P = .674), or BMI (P = .592) between the 2 groups (Table 1).
All 62 posttraumatic OA patients had complete hospital data, and 32 (52%) of the 62 underwent long-term follow-up (mean, 4.3 years; range, 4 months–10.5 years). At time of attempted contact (mean, 6.79 years after THA), 7 patients were deceased; cause of death was an unrelated medical condition (1) or unknown (6). The rest of the patients did not respond to multiple telephone and mail summons. Primary reasons for conversion to THA included OA (34 patients, 54%), development of osteonecrosis (12 patients, 19%), and nonunion (12 patients, 19%). The rest of the patients had fixation failure. The mechanisms of injury were motor vehicle accidents (30 patients), falls (20), and other causes (15).
Results
Thirty-two (52%) of the posttraumatic OA patients had a preoperative leg-length discrepancy. For these patients, mean time between initial fracture fixation and conversion to THA was 74 months (range, 1-480 months). Four patients required grafting with cancellous autogenous bone graft or allograft chips to fill a bony defect. Mean acetabular component diameter was 54 mm. Nineteen patients had acetabular fixation supplemented with screws. (Screw supplementation data were not recorded for control patients.) Three patients (4.7%) with an acetabular fracture had heterotopic bone removed at time of THA. Two patients underwent neurolysis of the sciatic nerve at time of surgery for preexisting nerve palsy.
Mean postoperative hemoglobin was 109 g/L in the posttraumatic OA group and 121 g/L in the control group (P <. 001). Mean postoperative hematocrit was 0.327 and 0.367, respectively (P < .001). Mean amount of Cell Saver (Haemonetics) used by patients was 176.2 and 72.9 mL, respectively (P < .001). Posttrauma patients lost a mean of 360 mL of blood more than control patients did (P < .001) and were transfused a mean of 1.59 units of blood, compared with 0.85 unit in the controls (P < .001). Patients with acetabular fractures required a mean of only 0.65 unit of transfused blood. Mean operating room time was 240.5 minutes for posttrauma patients and 135.6 minutes for control patients (P < .001). In the posttrauma group, mean size of the head of the femoral component was 29 mm (head size was not recorded for the control group). Posttrauma patients had 18 (29%) hybrid cemented hip replacements (femoral component only) and 44 uncemented hip replacements. Data on femoral stem size and type were not reported for either group.
Twenty-four posttrauma patients (39%) had a total of 63 perioperative complications, and 131 control patients (11.5%) had a total of 160 complications (P < .001). Complications in posttrauma patients with proximal femur fractures included excess bleeding (5 patients), in-hospital dislocations (2), and postoperative infections (4: 2 superficial wound infections, 1 implant infection requiring explant, 1 Clostridium difficile infection); in patients with acetabular fractures, there was only 1 dislocation (no infections). The posttraumatic OA group did not develop any symptomatic venous thromboembolic complications. One patient developed a sciatic nerve palsy after surgery. Of the 3 patients who sustained dislocations, 2 were treated with closed reduction and maintenance of implants, and 1 with revision THA. Complications in the control group included 3 infections, 4 dislocations, and 12 cases of extensive blood loss (Table 2).
In patients with long-term follow-up, mean postoperative modified HHS was 81.33 (range, 34.1-100.1). Twelve patients had an excellent score (>90), 10 a good score (80-89), 4 a fair score (70-79), and 6 a poor score (<70). Mean HHS was 84.2 for the 16 patients with a femoral head or neck fracture, 77.7 for the 6 patients with an intertrochanteric fracture, and 84.3 for the 9 patients with an acetabular fracture. Nine patients reported using a cane, 3 required walkers, 2 required wheelchairs, and 18 did not require any walking support. Four (12.5%) of the 32 patients required THA revision a mean of 3.5 years (range, 2 months–8 years) after initial arthroplasty. Reasons for revision were infections (2 patients), multiple dislocations (1), and dissociation of acetabular lining (1) (Table 3). Two of the patients who underwent THA revision had a cemented femoral stem, and 2 did not have any cemented implants. Additional details of the femoral stem components were not available for either group.
Discussion
Patients who develop posttraumatic OA of the hip have limited options. THA has emerged as an excellent option in cases of failed repair of fractures about the hip joint. The results of the present study are consistent with earlier findings of the effectiveness of THA in salvaging posttraumatic hips.2-7 THA for patients with posttraumatic arthritis of the hip after acetabular or proximal femur fracture is longer and more complicated than THA for primary OA, and there is significantly more blood loss. In addition, the rate of early failure appears to be higher.9
In this study, mean amount of blood transfused for patients with previous acetabular fracture was 0.65 unit, much less than the mean of 3.5 units noted by Weber and colleagues.6 In their study, complications associated with THA were increased in patients with posttraumatic OA from acetabular fractures. The authors attributed these complications to scarring from previous surgery, retained hardware, heterotopic bone, and residual osseous deformity and deficiency. Our results support their conclusion. Operating times were longer, as well as blood loss and the need for blood transfusions and other blood products were increased in the patients with posttraumatic OA, as compared with patients with primary OA. Fifteen percent of patients with an acetabular fracture had undergone removal of heterotopic bone at time of surgery—similar to the rate of 18% noted in the Weber study.6
Our results showed that the rate of revision THA was also higher than in patients with primary THA within the general population—reported to be about 4%.9 The higher rate may be the result of the additional surgeries performed on patients with fractures, or hardware retention increasing the infection risk over the years. Our revision rate of 12.5% was similar to the 19% found by Ranawat and colleagues7 in their study.
A majority of the patients in our study had favorable long-term HHS. Mean overall HHS was 83, slightly better than the 79 reported by Srivastav and colleagues.4 We found that patients with intertrochanteric fractures ultimately had worse outcome scores than patients with acetabular or femoral neck fractures. These results are consistent with findings reported by Mehlhoff and colleagues5 in a study comparing patients with femoral neck and intertrochanteric fractures. Mean HHS for the intertrochanteric fracture patients in our study was 77.7, comparable to the mean of 78 reported by Mehlhoff and colleagues.5 Mean HHS for the femoral neck or head fractures in our study was 84.2, similar to the mean of 81 they noted. Patients with a previous acetabular fracture in our study had a mean HHS of 84.3, consistent with the 84 reported by Ranawat and colleagues7 for patients who had initially undergone ORIF for acetabular fracture. Mean HHS in our study (83) was slightly less than the 88.5 reported by Shi and colleagues10 in their study of primary THAs.
Few studies have been conducted exclusively on one type of hip fracture (acetabular) or another (proximal femur), and all except 1 did not perform a comparison. Tabsh and colleagues2 compared similar cohorts but focused solely on patients with previous proximal femur fractures. The present study included a control group and both acetabular and proximal femur fractures, which allowed us to compare patients with and without previous fracture fixation and to consider the 2 different fracture types and see if they affected outcomes.
The strengths of this study include the large control group and the relatively short data-collection period. The shorter period decreased the influence of improvements in implants on patient outcomes. In addition, the control group was our own population, as we did not compare our cohort of patients with previous internal fixation and patients who had primary THAs in other studies, aside from comparisons for revision rates and HHS.
Although the ultimate long-term follow-up rate for patients with previous internal fixation was 50%, our sample size was still larger than that in most reported studies. Another weakness of our study was the large number of surgeons (17), representing an array of techniques, approaches, and surgical experience. All these factors could have influenced patient outcomes and operative data. In addition, data on revision rates and HHS were not available for our control group, so we could not directly compare these outcomes with those of the posttraumatic group. However, we used previously reported data on revision rates and HHS in primary THAs for comparison with the posttraumatic group.9,10
Conclusion
In this study, THA was a viable option for patients with posttraumatic arthritis from a previous acetabular or proximal femur fracture. The outcomes, however, were less reliable than the outcomes of primary THA for degenerative arthritis, and the complication rates were higher. Surgeons should counsel patients about the complexity of the procedure as well as its ultimately favorable outcomes. Surgeons should expect additional technical difficulties in the operating room when treating this patient population.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573-1579.
2. Tabsh I, Waddell JP, Morton J. Total hip arthroplasty for complications of proximal femoral fractures. J Orthop Trauma. 1997;11(3):166-169.
3. Haidukewych GJ, Berry DJ. Hip arthroplasty for salvage of failed treatment of intertrochanteric hip fractures. J Bone Joint Surg Am. 2003;85(5):899-904.
4. Srivastav S, Mittal V, Agarwal S. Total hip arthroplasty following failed fixation of proximal hip fractures. Indian J Orthop. 2008;42(3):279-286.
5. Mehlhoff T, Landon GC, Tullos HS. Total hip arthroplasty following failed internal fixation of hip fractures. Clin Orthop Relat Res. 1991;(269):32-37.
6. Weber M, Berry DJ, Harmsen WS. Total hip arthroplasty after operative treatment of an acetabular fracture. J Bone Joint Surg Am. 1998;80(9):1295-1305.
7. Ranawat A, Zelken J, Helfet D, Buly R. Total hip arthroplasty for posttraumatic arthritis after acetabular fracture. J Arthroplasty. 2009;24(5):759-767.
8. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
9. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
10. Shi HY, Mau LW, Chang JK, Wang JW, Chiu HC. Responsiveness of the Harris Hip Score and the SF-36: five years after total hip arthroplasty. Qual Life Res. 2009;18(8):1053-1060.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573-1579.
2. Tabsh I, Waddell JP, Morton J. Total hip arthroplasty for complications of proximal femoral fractures. J Orthop Trauma. 1997;11(3):166-169.
3. Haidukewych GJ, Berry DJ. Hip arthroplasty for salvage of failed treatment of intertrochanteric hip fractures. J Bone Joint Surg Am. 2003;85(5):899-904.
4. Srivastav S, Mittal V, Agarwal S. Total hip arthroplasty following failed fixation of proximal hip fractures. Indian J Orthop. 2008;42(3):279-286.
5. Mehlhoff T, Landon GC, Tullos HS. Total hip arthroplasty following failed internal fixation of hip fractures. Clin Orthop Relat Res. 1991;(269):32-37.
6. Weber M, Berry DJ, Harmsen WS. Total hip arthroplasty after operative treatment of an acetabular fracture. J Bone Joint Surg Am. 1998;80(9):1295-1305.
7. Ranawat A, Zelken J, Helfet D, Buly R. Total hip arthroplasty for posttraumatic arthritis after acetabular fracture. J Arthroplasty. 2009;24(5):759-767.
8. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
9. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
10. Shi HY, Mau LW, Chang JK, Wang JW, Chiu HC. Responsiveness of the Harris Hip Score and the SF-36: five years after total hip arthroplasty. Qual Life Res. 2009;18(8):1053-1060.
The Role of Vitamin C in Orthopedic Trauma and Bone Health
L-ascorbic acid, more commonly know as vitamin C, is an essential micronutrient used in numerous metabolic pathways. It functions physiologically as a water-soluble antioxidant by virtue of its high reducing power, playing a key role in the function of leukocytes, protein metabolism, and production of neurotransmitters.1-3 Vitamin C also contributes to musculoskeletal health through biosynthesis of carnitine and collagen4 and enhancement of intestinal absorption of dietary iron5 from plants and vegetables. Unlike most animals, humans are unable to synthesize this essential vitamin and therefore require intake from natural dietary sources or supplements.6 The ability of vitamin C to prevent or treat disease has been an area of research interest since the vitamin was identified and isolated by Szent-Györgyi in the 1930s.7-16 Research in orthopedic surgery has focused on the effects of vitamin C on fracture healing, its potential use in preventing complex regional pain syndrome (CRPS), and its role in the pathophysiology of osteoarthritis. In this article, we review the basics of vitamin C metabolism and summarize the evidence surrounding the role of vitamin C supplementation in orthopedics.
Sources and Metabolism
Vitamin C is found naturally in many fruits and vegetables (Table 1) and is a common fortification in cereals, juices, and multivitamins. Daily recommended intake (Table 2) depends on age and smoking status. Absorption occurs in the distal small intestine, with blood plasma vitamin C concentrations reflecting dietary intake. Pharmacokinetic studies have shown that vitamin C concentrations are tightly regulated through absorption, tissue accumulation, and renal resorption, with plasma concentrations rarely exceeding 100 μmol/L without additional supplementation.17 Although the usual dietary doses of 100 mg/d (adult) are almost completely absorbed, producing a plasma concentration of 60 μmol/L, higher intake results in an increasingly smaller fraction absorbed.1,18 Intake of more than 1000 mg/d results in less than 50% absorption19 (unmetabolized vitamin C is excreted in stool and urine1). Even at higher doses, vitamin C has low toxicity3; the most common complaints are diarrhea, nausea, and abdominal cramps caused by the osmotic effect of unabsorbed vitamin C in the gastrointestinal tract.1
Vitamin C Deficiency
The relationship between vitamin C deficiency and the development of scurvy has been documented for centuries. Symptoms are described in the ancient Egyptian, Greek, and Roman literature.20 Ascorbic acid is essential for normal collagen function, as it is a required cofactor for enzymatic transfer of hydroxyl groups to select proline and lysine residues during procollagen formation. Hydroxylysine contributes to the intermolecular cross-links in collagen, and hydroxyproline stabilizes the triple-helix structure of collagen.21 Insufficient vitamin C during this process results in collagen that is non-cross-linked, nonhelical, structurally unstable, and weak.21 Clinical manifestations of scurvy stem from an underlying impairment of collagen production causing a systemic decrease in connective tissue integrity, capillary fragility, poor wound healing, fatigue, myalgias, arthritis, and even death.22 Vitamin C deficiency has also been implicated as a cause of diffuse bleeding in surgical patients with normal coagulation parameters secondary to capillary fragility.23 In the United States, the 2003–2004 National Health and Nutrition Examination Survey (NHANES) measured serum vitamin C concentrations in 7277 noninstitutionalized patients 6 years old or older.24 Age-adjusted incidence of subnormal serum vitamin C levels (<28 μmol/L) was 19.6%, and incidence of frank vitamin C deficiency (<11.4 μmol/L) was 7.1%. Reported rates of vitamin C deficiency in hospitalized patients are much higher, with 47% to 60% having subnormal values (<28 μmol/L) and 17% to 19% being vitamin C–deficient (<11.4 μmol/L).22,25 Identified risk factors for hypovitaminosis C include advanced age, obesity, low socioeconomic status, unemployment, male sex, and concomitant alcohol and tobacco consumption.22,24,25
Fracture Healing and Prevention
The effects of vitamin C deficiency on bone healing have been studied with animal models as early as the 1940s.26,27 Early experiments using guinea pigs demonstrated failure of bone graft incorporation, delayed collagen maturation, and decreased collagen and callus formation in scorbutic animals compared with controls that received vitamin C supplementation.26,27 Based on his work with guinea pigs, Bourne26 reported in 1942 that vitamin C deficiency significantly inhibited the reparative process in damaged bone and that patients with fractures should receive vitamin C supplementation. Building on this early research, Yilmaz and colleagues28 found faster histologic healing for tibia fractures in a rat model for animals that received a single injection of vitamin C 0.5 mg/kg compared with a nonscorbutic control group, and Sarisözen and colleagues29 showed significantly accelerated histologic bone formation and mineralization at the fracture site for rats that received vitamin C supplementation. Moreover, Kipp and colleagues30 found that scorbutic guinea pigs had lower bone mineral density (BMD), decreased bone mineral content, and impaired collagen synthesis of articular cartilage and tendons compared with nondeficient controls.
Besides promoting bone formation, vitamin C improves the mechanical strength of callus formation. Alcantara-Martos and colleagues31 used an osteogenic disorder Shionogi (ODS) rat model to examine the effects of vitamin C intake on femoral fracture healing. This particular animal model is unable to produce its own vitamin C. The groups with lower serum vitamin C levels demonstrated lower mechanical resistance of the fracture callus to torsional loads 5 weeks after fracture. Moreover, the group that received vitamin C supplementation showed higher histologic grade of callus formation and demonstrated faster healing rates. The authors suggested that subclinical vitamin C deficiency can delay fracture healing and that vitamin C supplementation in nondeficient patients would improve bone healing.
Other research has demonstrated a link between vitamin C and mesenchymal cell differentiation. Mohan and colleagues32 used an sfx mouse model to show that vitamin C deficiency results in decreased bone formation secondary to impaired osteoblast differentiation, diminished bone density, and development of spontaneous fractures. The authors indicated that not only is vitamin C essential for maintenance of differentiated functions of osteoblasts, but deficiency during early active growth may affect peak BMD levels in humans. Additional studies have demonstrated the role of vitamin C in endochondral bone formation through both induction of osteoblast differentiation and modulation of gene expression in hypertrophic chondrocytes.33-36 Chronic vitamin C deficiency has been found to depress osteoblast function and differentiation of chondrocytes.37 More recently, Kim and colleagues38 examined the effect of vitamin C insufficiency in Gulo-deficient mice, which are unable to synthesize ascorbic acid. Ascorbic acid insufficiency over 4 weeks led to decreased plasma levels of osteocalcin and bone formation in vivo as well as significantly diminished metaphyseal trabecular bone. Despite all the evidence demonstrating the importance of vitamin C in bone formation and maintenance, many of the underlying processes in this relationship have yet to be determined.
Bone Mineral Density
Several observational studies have found a positive association between vitamin C intake and BMD in postmenopausal women. In a retrospective, cross-sectional study by Hall and Greendale,39 a positive association was found between vitamin C intake and BMD of the femoral neck in 775 participants in the Postmenopausal Estrogen/Progestin Interventions trial. After calcium intake, physical activity level, smoking, estrogen use, age, and body mass index were adjusted for, each 100-mg increase in dietary vitamin C was associated with a 0.017 g/cm2 increase in BMD. Wang and colleagues40 found a positive association between dietary vitamin C intake and femoral neck BMD in a retrospective analysis of 125 postmenopausal Mexican American women. Other observational studies have reported that decreased intake of vitamin C is associated with osteoporosis41 and increased rates of BMD loss42 and that supplementation with vitamin C may suppress bone resorption in postmenopausal women.43
The results of these studies contrast with the findings of Leveille and colleagues,44 who examined the relationship between dietary vitamin C and hip BMD in 1892 postmenopausal women. Although the authors found that women (age, 55-64 years) using vitamin C supplements for more than 10 years had an average BMD 6.7% higher than that of nonusers, they did not find any association between dietary vitamin C intake and BMD. Moreover, NHANES III also found inconsistent associations between vitamin C and BMD among 13,080 adults surveyed in the United States.45 Although for premenopausal women dietary ascorbic acid was associated with increased BMD, for postmenopausal women with a history of smoking and estrogen replacement, it was actually associated with lower BMD values. For other subgroups in the study, the relationship was also inconsistent or nonlinear.
The exact mechanism by which ascorbic acid contributes to BMD is not fully delineated. However, it likely is related to the known role of vitamin C in collagen formation, bone matrix development, osteoblast differentiation, and its antioxidant effects limiting bone resorption.44,46
Hip Fractures
Besides demonstrating positive effects of vitamin C on bone healing and BMD, epidemiologic studies have found evidence of a protective effect of vitamin C on hip fracture risk. In a study of the Swedish Mammography cohort, 66,651 women (age, 40-76 years) were prospectively followed.47 The authors found that the odds ratio (OR) for hip fractures among smokers with a low intake of vitamin E (median intake, ≤6.2 mg/d) was 3.0 (95% CI, 1.6-5.4) and for vitamin C (median intake, ≤67 mg/d) was 3.0 (95% CI, 1.6-5.6). Moreover, in smokers with a low intake of both vitamins E and C, OR increased to 4.9 (95% CI, 2.2-11.0). In addition, the Utah Study of Nutrition and Bone Health matched 1215 cases of hip fractures in patients who had ever smoked (age, >50 years) with 1349 controls and found that vitamin C intake above 159 mg/d had a significant protective effect on the incidence of hip fracture; however, a graded relationship was not observed.48 Despite the inconsistencies in the NHANES III study regarding the relationship between vitamin C and BMD, Simon and Hudes45 found that serum vitamin C was associated with lower risk for self-reported fracture in postmenopausal women who had ever smoked and had a history of estrogen therapy (OR, 0.51; 95% CI, 0.36-0.70). Finally, Sahni and colleagues49 followed 958 Framingham cohort men and women (mean age, 75 years) over 17 years and found that those in the highest tertile of total vitamin C intake (median, 313 mg/d) had significantly fewer hip fractures and nonvertebral fractures compared with those in the lowest tertile of intake (median, 94 mg/d). Dietary vitamin C intake was not associated with fracture risk in this study.
Complex Regional Pain Syndrome
Type 1 CRPS is a debilitating condition characterized by severe pain, swelling, and vasomotor instability. It is commonly precipitated by an injury or surgery to an extremity and is a dreaded sequelae in orthopedics,50 with incidence rates of 10% to 22% in wrist fractures51-53 and 10% after foot and ankle surgery.54 Although the pathophysiology of CRPS remains unknown, dysregulation and increased permeability of the vasculature caused by free radicals are thought to play an important role.55 In dermal burns, high doses of vitamin C therapy slowed progression of vascular permeability and therefore reduced extravascular leakage of fluids and protein.56,57 The ability of vitamin C to prevent CRPS has been studied in only a handful of trials.
In a double-blind trial, Zollinger and colleagues51 randomized 127 conservatively treated distal radius fractures to receive either vitamin C 500 mg or placebo daily for 50 days starting on day of injury. Incidence of CRPS (using the diagnostic criteria proposed by Veldman and colleagues58) at 1-year follow-up was 22% in the placebo group and 7% in the vitamin C group (95% CI for difference, 2%-26%). Complaints while wearing the cast and fracture type increased the risk for developing CRPS. This initial study was followed up by a prospective, randomized, double-blind multicenter trial by the same authors,52 who had 416 patients with 427 wrist fractures receive either placebo or vitamin C 200 mg/d, 500 mg/d, or 1500 mg/d for 50 days. This follow-up study included both operative (11%) and nonoperative (89%) distal radius fractures. Incidence of CRPS was 10.1% in the placebo group and 2.4% in the vitamin C group (P < .002). Although there was an appreciable drop in the relative risk (RR) of developing CRPS between the vitamin C 200-mg/d and 500-mg/d groups (0.41-0.17), there was no additional benefit in the 1500-mg/d group. Pooling the data for these 2 randomized trials showed that the overall RR for developing CRPS was lower with vitamin C supplementation (RR, 0.28; 95% CI, 0.14-0.56; P = .0003).59
Results of the 2 trials by Zollinger and colleagues51,52 have been met with several concerns.60-62 As a corollary to the unclear etiology of CRPS, several different sets of diagnostic criteria exist, and the criteria are somewhat subjective and imprecise. Although both trials used the Veldman criteria,58 the incidence of CRPS in the placebo group dropped unexpectedly between trials, from 22% to 10.1%, and the results may have been different had other criteria been used. Moreover, the idea that toxic oxygen radicals have a role in CRPS and that vitamin C can scavenge these radicals is based on limited data.61 In the absence of a clear pathophysiologic explanation, some surgeons have been reluctant to treat patients with vitamin C supplementation.
Cazeneuve and colleagues53 also studied the effect of vitamin C supplementation on CRPS in patients with distal radius fractures treated with reduction and intrafocal pinning. Group 1 consisted of 100 patients (treated from 1995 to 1998) who did not receive vitamin C supplementation, and group 2 consisted of 95 patients (treated from 1998 to 2002) who received vitamin C 1000 mg/d for 45 days starting on day of fracture. Patients were followed for up to 90 days after surgery. Incidence of CRPS type 1 was 10% in the untreated group and 2.1% in the group that received vitamin C supplementation.
Vitamin C prophylaxis for CRPS has also been studied in foot and ankle surgery. Besse and colleagues54 prospectively compared 2 chronologically successive groups that received (235 feet) or did not receive (185 feet) vitamin C 1000-mg/d supplementation for 45 days. Incidence of CRPS type 1 as diagnosed with International Association for the Study of Pain (IASP) criteria dropped from 9.6% to 1.7% with vitamin C supplementation. In a case series, Zollinger and colleagues63 examined CRPS type 1 rates after performing cementless total trapeziometacarpal semiconstrained joint prosthesis implantations for trapeziometacarpal arthritis. Forty implantations were performed in 34 patients. All patients received vitamin C 500 mg/d for CRPS prevention starting 2 days before surgery for 50 days. There were no cases of CRPS in the postoperative period, according to Veldman or IASP criteria. Although the results of the studies by Cazeneuve and colleagues53 and Besse and colleagues54 agree with those of the distal radius fracture trials by Zollinger and colleagues,51,52 the quasi-experimental design and the lack of blinding and randomization temper the conclusions that can be drawn because of the risk for significant bias.
In a recent systematic review examining the effectiveness of vitamin C supplementation in preventing CRPS in trauma and surgery in the extremities, Shibuya and colleagues64 concluded that taking at least 500 mg of vitamin C daily for 45 to 50 days after injury or surgery may help decrease the incidence of CRPS after a traumatic event.
Osteoarthritis
Damage caused by free radicals has long been thought to play an important role in osteoarthritis (OA).65-67 A cross-sectional study in knee OA found that amounts of joint fluid antioxidants were lower in patients with severe arthritis than in those with intact cartilage, further implicating free radicals in the pathophysiology of OA.68 Use of vitamin C for prophylaxis against development or progression of OA is therefore a hot research topic. Thus far, animal studies have had mixed results—several showing a chondroprotective effect of vitamin C69,70 and others finding either no effect or even a positive association with the development of arthritis.71
The literature on human subjects, chiefly observational studies, is just as controversial. Wang and colleagues40 found vitamin C intake associated with both a 50% risk reduction of bone marrow lesions on magnetic resonance imaging over a 10-year interval (OR, 0.5; 95% CI, 0.29-0.87) and inversely associated with the tibial plateau bone area. Similarly, the Clearwater Osteoarthritis Study, which followed 1023 patients (age, >40 years), showed that participants who took vitamin C supplements were 11% less likely to develop radiographic evidence of OA (RR, 0.89; 95% CI, 0.85-0.93).72 Nonetheless, other studies have failed to show such associations73 or have demonstrated the opposite effect. Chaganti and colleagues74 analyzed levels of vitamins C and E in the Multicenter Osteoarthritis Study (MOST) cohort of 3026 men and women (age, 50-79 years) and found higher vitamin levels were not protective against incidence of radiographic whole-knee OA and may even have been associated with increased risk.
Conclusion
Vitamin C is an essential micronutrient and a powerful water-soluble antioxidant in numerous biochemical pathways that influence bone health. It has been implicated in the biology of fracture healing, and vitamin C supplementation has been proposed as prophylaxis against hip fractures based on observational data. Results of 2 high-quality double-blind randomized trials support use of vitamin C as prophylaxis against CRPS in wrist fractures treated conservatively and operatively; the evidence for foot and ankle surgery is weaker. Use of vitamin C in OA prevention has tremendous potential, though animal and human study results are controversial. Heterogeneous results and lack of prospective trials preclude any recommendation at this time.
1. Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002;5(2):66-74.
2. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86(16):6377-6381.
3. Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100(6):637-640.
4. Padh H. Vitamin C: newer insights into its biochemical functions. Nutr Rev. 1991;49(3):65-70.
5. Gershoff SN. Vitamin C (ascorbic acid): new roles, new requirements? Nutr Rev. 1993;51(11):313-326.
6. Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137(10):2171-2184.
7. Szent-Györgyi A. On the function of hexuronic acid in the respiration of the cabbage leaf. J Biol Chem. 1931;90(1):385-393.
8. Svirbely JL, Szent-Györgyi A. The chemical nature of vitamin C. Biochem J. 1933;27(1):279-285.
9. Pauling L. Vitamin C and the Common Cold. San Francisco, CA: Freeman; 1970.
10. Spittle CR. Atherosclerosis and vitamin C. Lancet. 1971;2(7737):1280-1281.
11. Chappell LC, Seed PT, Briley AL, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354(9181):810-816.
12. Block G. Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr. 1991;53(1 suppl):270S-282S.
13. Creagan ET, Moertel CG, O’Fallon JR, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979;301(13):687-690.
14. Hemila H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;1:CD000980.
15. Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH; Vitamins in Pre-eclampsia (VIP) Trial Consortium. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367(9517):1145-1154.
16. Roberts JM, Myatt L, Spong CY, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362(14):1282-1291.
17. Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78-88.
18. Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281(15):1415-1423.
19. Glatthaar BE, Hornig DH, Moser U. The role of ascorbic acid in carcinogenesis. Adv Exp Med Biol. 1986;206:357-377.
20. Carpenter KJ. The History of Scurvy and Vitamin C. New York, NY: Cambridge University Press; 1986.
21. Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78(5):2879-2882.
22. Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14(7):419-425.
23. Blee TH, Cogbill TH, Lambert PJ. Hemorrhage associated with vitamin C deficiency in surgical patients. Surgery. 2002;131(4):408-412.
24. Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90(5):1252-1263.
25. Gan R, Eintracht S, Hoffer LJ. Vitamin C deficiency in a university teaching hospital. J Am Coll Nutr. 2008;27(3):428-433.
26. Bourne G. The effect of graded doses of vitamin C upon the regeneration of bone in guinea-pigs on a scorbutic diet. J Physiol. 1942;101(3):327-336.
27. Bourne GH. The relative importance of periosteum and endosteum in bone healing and the relationship of vitamin C to their activities. Proc R Soc Med. 1944;37(6):275-279.
28. Yilmaz C, Erdemli E, Selek H, Kinik H, Arikan M, Erdemli B. The contribution of vitamin C to healing of experimental fractures. Arch Orthop Trauma Surg. 2001;121(7):426-428.
29. Sarisözen B, Durak K, Dinçer G, Bilgen OF. The effects of vitamins E and C on fracture healing in rats. J Int Med Res. 2002;30(3):309-313.
30. Kipp DE, McElvain M, Kimmel DB, Akhter MP, Robinson RG, Lukert BP. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone. 1996;18(3):281-288.
31. Alcantara-Martos T, Delgado-Martinez AD, Vega MV, Carrascal MT, Munuera-Martinez L. Effect of vitamin C on fracture healing in elderly osteogenic disorder Shionogi rats. J Bone Joint Surg Br. 2007;89(3):402-407.
32. Mohan S, Kapoor A, Singgih A, et al. Spontaneous fractures in the mouse mutant sfx are caused by deletion of the gulonolactone oxidase gene, causing vitamin C deficiency. J Bone Miner Res. 2005;20(9):1597-1610.
33. Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol. 1990;143(2):213-221.
34. Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7(2):235-246.
35. Leboy PS, Vaias L, Uschmann B, Golub E, Adams SL, Pacifici M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J Biol Chem. 1989;264(29):17281-17286.
36. Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid–dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol Endocrinol. 1997;11(8):1103-1113.
37. Sakamoto Y, Takano Y. Morphological influence of ascorbic acid deficiency on endochondral ossification in osteogenic disorder Shionogi rat. Anat Rec. 2002;268(2):93-104.
38. Kim W, Bae S, Kim H, et al. Ascorbic acid insufficiency induces the severe defect on bone formation via the down-regulation of osteocalcin production. Anat Cell Biol. 2013;46(4):254-261.
39. Hall SL, Greendale GA. The relation of dietary vitamin C intake to bone mineral density: results from the PEPI study. Calcif Tissue Int. 1998;63(3):183-189.
40. Wang Y, Hodge AM, Wluka AE, et al. Effect of antioxidants on knee cartilage and bone in healthy, middle-aged subjects: a cross-sectional study. Arthritis Res Ther. 2007;9(4):R66.
41. Maggio D, Barabani M, Pierandrei M, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88(4):1523-1527.
42. Kaptoge S, Welch A, McTaggart A, et al. Effects of dietary nutrients and food groups on bone loss from the proximal femur in men and women in the 7th and 8th decades of age. Osteoporosis Int. 2003;14(5):418-428.
43. Pasco JA, Henry MJ, Wilkinson LK, Nicholson GC, Schneider HG, Kotowicz MA. Antioxidant vitamin supplements and markers of bone turnover in a community sample of nonsmoking women. J Womens Health. 2006;15(3):295-300.
44. Leveille SG, LaCroix AZ, Koepsell TD, Beresford SA, Van Belle G, Buchner DM. Dietary vitamin C and bone mineral density in postmenopausal women in Washington state, USA. J Epidemiol Community Health. 1997;51(5):479-485.
45. Simon JA, Hudes ES. Relation of ascorbic acid to bone mineral density and self-reported fractures among US adults. Am J Epidemiol. 2001;154(5):427-433.
46. Wolf RL, Cauley JA, Pettinger M, et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: results from the Women’s Health Initiative. Am J Clin Nutr. 2005;82(3):581-588.
47. Melhus H, Michaelsson K, Holmberg L, Wolk A, Ljunghall S. Smoking, antioxidant vitamins, and the risk of hip fracture. J Bone Miner Res. 1999;14(1):129-135.
48. Zhang J, Munger RG, West NA, Cutler DR, Wengreen HJ, Corcoran CD. Antioxidant intake and risk of osteoporotic hip fracture in Utah: an effect modified by smoking status. Am J Epidemiol. 2006;163(1):9-17.
49. Sahni S, Hannan MT, Blumberg J, Cupples LA, Kiel DP, Tucker KL. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: a 17-year follow-up from the Framingham Osteoporosis Study. J Bone Miner Res. 2009;24(6):1086-1094.
50. Rho RH, Brewer RP, Lamer TJ, Wilson PR. Complex regional pain syndrome. Mayo Clin Proc. 2002;77(2):174-180.
51. Zollinger PE, Tuinebreijer WE, Kreis RW, Breederveld RS. Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: a randomised trial. Lancet. 1999;354(9195):2025-2028.
52. Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose–response study. J Bone Joint Surg Am. 2007;89(7):1424-1431.
53. Cazeneuve JF, Leborgne JM, Kermad K, Hassan Y. Vitamin C and prevention of reflex sympathetic dystrophy following surgical management of distal radius fractures [in French]. Acta Orthop Belg. 2002;68(5):481-484.
54. Besse JL, Gadeyne S, Galand-Desme S, Lerat JL, Moyen B. Effect of vitamin C on prevention of complex regional pain syndrome type I in foot and ankle surgery. Foot Ankle Surg. 2009;15(4):179-182.
55. Goris RJ, Dongen LM, Winters HA. Are toxic oxygen radicals involved in the pathogenesis of reflex sympathetic dystrophy? Free Radic Res Commun. 1987;3(1-5):13-18.
56. Matsuda T, Tanaka H, Shimazaki S, et al. High-dose vitamin C therapy for extensive deep dermal burns. Burns. 1992;18(2):127-131.
57. Matsuda T, Tanaka H, Hanumadass M, et al. Effects of high-dose vitamin C administration on postburn microvascular fluid and protein flux. J Burn Care Rehabil. 1992;13(5):560-566.
58. Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342(8878):1012-1016.
59. Zollinger PE. The administration of vitamin C in prevention of CRPS-I after distal radial fractures and hand surgery—a review of two RCTs and one observational prospective study. Open Conference Proc J. 2011;2:1-4.
60. Rogers BA, Ricketts DM. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2008;90(2):447-448.
61. Amadio PC. Vitamin C reduced the incidence of reflex sympathetic dystrophy after wrist fracture. J Bone Joint Surg Am. 2000;82(6):873.
62. Frolke JP. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2007;89(11):2550-2551.
63. Zollinger PE, Unal H, Ellis ML, Tuinebreijer WE. Clinical results of 40 consecutive basal thumb prostheses and no CRPS type I after vitamin C prophylaxis. Open Orthop J. 2010;4:62-66.
64. Shibuya N, Humphers JM, Agarwal MR, Jupiter DC. Efficacy and safety of high-dose vitamin C on complex regional pain syndrome in extremity trauma and surgery—systematic review and meta-analysis. J Foot Ankle Surg. 2013;52(1):62-66.
65. Henrotin Y, Deby-Dupont G, Deby C, De Bruyn M, Lamy M, Franchimont P. Production of active oxygen species by isolated human chondrocytes. Br J Rheumatol. 1993;32(7):562-567.
66. McAlindon TE, Jacques P, Zhang Y, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39(4):648-656.
67. Kaiki G, Tsuji H, Yonezawa T, et al. Osteoarthrosis induced by intra-articular hydrogen peroxide injection and running load. J Orthop Res. 1990;8(5):731-740.
68. Regan EA, Bowler RP, Crapo JD. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthritis Cartilage. 2008;16(4):515-521.
69. Meacock SC, Bodmer JL, Billingham ME. Experimental osteoarthritis in guinea-pigs. J Exp Pathol. 1990;71(2):279-293.
70. Kurz B, Jost B, Schunke M. Dietary vitamins and selenium diminish the development of mechanically induced osteoarthritis and increase the expression of antioxidative enzymes in the knee joint of STR/1N mice. Osteoarthritis Cartilage. 2002;10(2):119-126.
71. Kraus VB, Huebner JL, Stabler T, et al. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum. 2004;50(6):1822-1831.
72. Peregoy J, Wilder FV. The effects of vitamin C supplementation on incident and progressive knee osteoarthritis: a longitudinal study. Public Health Nutr. 2011;14(4):709-715.
73. Hill J, Bird HA. Failure of selenium-ace to improve osteoarthritis. Br J Rheumatol. 1990;29(3):211-213.
74. Chaganti RK, Tolstykh I, Javaid MK, et al; Multicenter Osteoarthritis Study Group (MOST). High plasma levels of vitamin C and E are associated with incident radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):190-196.
75. US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference. Release 26. http://www.ars.usda.gov/Services/docs.htm?docid=24936. Published August 2013. Revised November 2013. Accessed May 14, 2015.
76. National Institutes of Health, Office of Dietary Supplements. Vitamin C: fact sheet for health professionals. National Institutes of Health website. http://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/. Reviewed June 5, 2013. Accessed May 14, 2015.
77. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000.
L-ascorbic acid, more commonly know as vitamin C, is an essential micronutrient used in numerous metabolic pathways. It functions physiologically as a water-soluble antioxidant by virtue of its high reducing power, playing a key role in the function of leukocytes, protein metabolism, and production of neurotransmitters.1-3 Vitamin C also contributes to musculoskeletal health through biosynthesis of carnitine and collagen4 and enhancement of intestinal absorption of dietary iron5 from plants and vegetables. Unlike most animals, humans are unable to synthesize this essential vitamin and therefore require intake from natural dietary sources or supplements.6 The ability of vitamin C to prevent or treat disease has been an area of research interest since the vitamin was identified and isolated by Szent-Györgyi in the 1930s.7-16 Research in orthopedic surgery has focused on the effects of vitamin C on fracture healing, its potential use in preventing complex regional pain syndrome (CRPS), and its role in the pathophysiology of osteoarthritis. In this article, we review the basics of vitamin C metabolism and summarize the evidence surrounding the role of vitamin C supplementation in orthopedics.
Sources and Metabolism
Vitamin C is found naturally in many fruits and vegetables (Table 1) and is a common fortification in cereals, juices, and multivitamins. Daily recommended intake (Table 2) depends on age and smoking status. Absorption occurs in the distal small intestine, with blood plasma vitamin C concentrations reflecting dietary intake. Pharmacokinetic studies have shown that vitamin C concentrations are tightly regulated through absorption, tissue accumulation, and renal resorption, with plasma concentrations rarely exceeding 100 μmol/L without additional supplementation.17 Although the usual dietary doses of 100 mg/d (adult) are almost completely absorbed, producing a plasma concentration of 60 μmol/L, higher intake results in an increasingly smaller fraction absorbed.1,18 Intake of more than 1000 mg/d results in less than 50% absorption19 (unmetabolized vitamin C is excreted in stool and urine1). Even at higher doses, vitamin C has low toxicity3; the most common complaints are diarrhea, nausea, and abdominal cramps caused by the osmotic effect of unabsorbed vitamin C in the gastrointestinal tract.1
Vitamin C Deficiency
The relationship between vitamin C deficiency and the development of scurvy has been documented for centuries. Symptoms are described in the ancient Egyptian, Greek, and Roman literature.20 Ascorbic acid is essential for normal collagen function, as it is a required cofactor for enzymatic transfer of hydroxyl groups to select proline and lysine residues during procollagen formation. Hydroxylysine contributes to the intermolecular cross-links in collagen, and hydroxyproline stabilizes the triple-helix structure of collagen.21 Insufficient vitamin C during this process results in collagen that is non-cross-linked, nonhelical, structurally unstable, and weak.21 Clinical manifestations of scurvy stem from an underlying impairment of collagen production causing a systemic decrease in connective tissue integrity, capillary fragility, poor wound healing, fatigue, myalgias, arthritis, and even death.22 Vitamin C deficiency has also been implicated as a cause of diffuse bleeding in surgical patients with normal coagulation parameters secondary to capillary fragility.23 In the United States, the 2003–2004 National Health and Nutrition Examination Survey (NHANES) measured serum vitamin C concentrations in 7277 noninstitutionalized patients 6 years old or older.24 Age-adjusted incidence of subnormal serum vitamin C levels (<28 μmol/L) was 19.6%, and incidence of frank vitamin C deficiency (<11.4 μmol/L) was 7.1%. Reported rates of vitamin C deficiency in hospitalized patients are much higher, with 47% to 60% having subnormal values (<28 μmol/L) and 17% to 19% being vitamin C–deficient (<11.4 μmol/L).22,25 Identified risk factors for hypovitaminosis C include advanced age, obesity, low socioeconomic status, unemployment, male sex, and concomitant alcohol and tobacco consumption.22,24,25
Fracture Healing and Prevention
The effects of vitamin C deficiency on bone healing have been studied with animal models as early as the 1940s.26,27 Early experiments using guinea pigs demonstrated failure of bone graft incorporation, delayed collagen maturation, and decreased collagen and callus formation in scorbutic animals compared with controls that received vitamin C supplementation.26,27 Based on his work with guinea pigs, Bourne26 reported in 1942 that vitamin C deficiency significantly inhibited the reparative process in damaged bone and that patients with fractures should receive vitamin C supplementation. Building on this early research, Yilmaz and colleagues28 found faster histologic healing for tibia fractures in a rat model for animals that received a single injection of vitamin C 0.5 mg/kg compared with a nonscorbutic control group, and Sarisözen and colleagues29 showed significantly accelerated histologic bone formation and mineralization at the fracture site for rats that received vitamin C supplementation. Moreover, Kipp and colleagues30 found that scorbutic guinea pigs had lower bone mineral density (BMD), decreased bone mineral content, and impaired collagen synthesis of articular cartilage and tendons compared with nondeficient controls.
Besides promoting bone formation, vitamin C improves the mechanical strength of callus formation. Alcantara-Martos and colleagues31 used an osteogenic disorder Shionogi (ODS) rat model to examine the effects of vitamin C intake on femoral fracture healing. This particular animal model is unable to produce its own vitamin C. The groups with lower serum vitamin C levels demonstrated lower mechanical resistance of the fracture callus to torsional loads 5 weeks after fracture. Moreover, the group that received vitamin C supplementation showed higher histologic grade of callus formation and demonstrated faster healing rates. The authors suggested that subclinical vitamin C deficiency can delay fracture healing and that vitamin C supplementation in nondeficient patients would improve bone healing.
Other research has demonstrated a link between vitamin C and mesenchymal cell differentiation. Mohan and colleagues32 used an sfx mouse model to show that vitamin C deficiency results in decreased bone formation secondary to impaired osteoblast differentiation, diminished bone density, and development of spontaneous fractures. The authors indicated that not only is vitamin C essential for maintenance of differentiated functions of osteoblasts, but deficiency during early active growth may affect peak BMD levels in humans. Additional studies have demonstrated the role of vitamin C in endochondral bone formation through both induction of osteoblast differentiation and modulation of gene expression in hypertrophic chondrocytes.33-36 Chronic vitamin C deficiency has been found to depress osteoblast function and differentiation of chondrocytes.37 More recently, Kim and colleagues38 examined the effect of vitamin C insufficiency in Gulo-deficient mice, which are unable to synthesize ascorbic acid. Ascorbic acid insufficiency over 4 weeks led to decreased plasma levels of osteocalcin and bone formation in vivo as well as significantly diminished metaphyseal trabecular bone. Despite all the evidence demonstrating the importance of vitamin C in bone formation and maintenance, many of the underlying processes in this relationship have yet to be determined.
Bone Mineral Density
Several observational studies have found a positive association between vitamin C intake and BMD in postmenopausal women. In a retrospective, cross-sectional study by Hall and Greendale,39 a positive association was found between vitamin C intake and BMD of the femoral neck in 775 participants in the Postmenopausal Estrogen/Progestin Interventions trial. After calcium intake, physical activity level, smoking, estrogen use, age, and body mass index were adjusted for, each 100-mg increase in dietary vitamin C was associated with a 0.017 g/cm2 increase in BMD. Wang and colleagues40 found a positive association between dietary vitamin C intake and femoral neck BMD in a retrospective analysis of 125 postmenopausal Mexican American women. Other observational studies have reported that decreased intake of vitamin C is associated with osteoporosis41 and increased rates of BMD loss42 and that supplementation with vitamin C may suppress bone resorption in postmenopausal women.43
The results of these studies contrast with the findings of Leveille and colleagues,44 who examined the relationship between dietary vitamin C and hip BMD in 1892 postmenopausal women. Although the authors found that women (age, 55-64 years) using vitamin C supplements for more than 10 years had an average BMD 6.7% higher than that of nonusers, they did not find any association between dietary vitamin C intake and BMD. Moreover, NHANES III also found inconsistent associations between vitamin C and BMD among 13,080 adults surveyed in the United States.45 Although for premenopausal women dietary ascorbic acid was associated with increased BMD, for postmenopausal women with a history of smoking and estrogen replacement, it was actually associated with lower BMD values. For other subgroups in the study, the relationship was also inconsistent or nonlinear.
The exact mechanism by which ascorbic acid contributes to BMD is not fully delineated. However, it likely is related to the known role of vitamin C in collagen formation, bone matrix development, osteoblast differentiation, and its antioxidant effects limiting bone resorption.44,46
Hip Fractures
Besides demonstrating positive effects of vitamin C on bone healing and BMD, epidemiologic studies have found evidence of a protective effect of vitamin C on hip fracture risk. In a study of the Swedish Mammography cohort, 66,651 women (age, 40-76 years) were prospectively followed.47 The authors found that the odds ratio (OR) for hip fractures among smokers with a low intake of vitamin E (median intake, ≤6.2 mg/d) was 3.0 (95% CI, 1.6-5.4) and for vitamin C (median intake, ≤67 mg/d) was 3.0 (95% CI, 1.6-5.6). Moreover, in smokers with a low intake of both vitamins E and C, OR increased to 4.9 (95% CI, 2.2-11.0). In addition, the Utah Study of Nutrition and Bone Health matched 1215 cases of hip fractures in patients who had ever smoked (age, >50 years) with 1349 controls and found that vitamin C intake above 159 mg/d had a significant protective effect on the incidence of hip fracture; however, a graded relationship was not observed.48 Despite the inconsistencies in the NHANES III study regarding the relationship between vitamin C and BMD, Simon and Hudes45 found that serum vitamin C was associated with lower risk for self-reported fracture in postmenopausal women who had ever smoked and had a history of estrogen therapy (OR, 0.51; 95% CI, 0.36-0.70). Finally, Sahni and colleagues49 followed 958 Framingham cohort men and women (mean age, 75 years) over 17 years and found that those in the highest tertile of total vitamin C intake (median, 313 mg/d) had significantly fewer hip fractures and nonvertebral fractures compared with those in the lowest tertile of intake (median, 94 mg/d). Dietary vitamin C intake was not associated with fracture risk in this study.
Complex Regional Pain Syndrome
Type 1 CRPS is a debilitating condition characterized by severe pain, swelling, and vasomotor instability. It is commonly precipitated by an injury or surgery to an extremity and is a dreaded sequelae in orthopedics,50 with incidence rates of 10% to 22% in wrist fractures51-53 and 10% after foot and ankle surgery.54 Although the pathophysiology of CRPS remains unknown, dysregulation and increased permeability of the vasculature caused by free radicals are thought to play an important role.55 In dermal burns, high doses of vitamin C therapy slowed progression of vascular permeability and therefore reduced extravascular leakage of fluids and protein.56,57 The ability of vitamin C to prevent CRPS has been studied in only a handful of trials.
In a double-blind trial, Zollinger and colleagues51 randomized 127 conservatively treated distal radius fractures to receive either vitamin C 500 mg or placebo daily for 50 days starting on day of injury. Incidence of CRPS (using the diagnostic criteria proposed by Veldman and colleagues58) at 1-year follow-up was 22% in the placebo group and 7% in the vitamin C group (95% CI for difference, 2%-26%). Complaints while wearing the cast and fracture type increased the risk for developing CRPS. This initial study was followed up by a prospective, randomized, double-blind multicenter trial by the same authors,52 who had 416 patients with 427 wrist fractures receive either placebo or vitamin C 200 mg/d, 500 mg/d, or 1500 mg/d for 50 days. This follow-up study included both operative (11%) and nonoperative (89%) distal radius fractures. Incidence of CRPS was 10.1% in the placebo group and 2.4% in the vitamin C group (P < .002). Although there was an appreciable drop in the relative risk (RR) of developing CRPS between the vitamin C 200-mg/d and 500-mg/d groups (0.41-0.17), there was no additional benefit in the 1500-mg/d group. Pooling the data for these 2 randomized trials showed that the overall RR for developing CRPS was lower with vitamin C supplementation (RR, 0.28; 95% CI, 0.14-0.56; P = .0003).59
Results of the 2 trials by Zollinger and colleagues51,52 have been met with several concerns.60-62 As a corollary to the unclear etiology of CRPS, several different sets of diagnostic criteria exist, and the criteria are somewhat subjective and imprecise. Although both trials used the Veldman criteria,58 the incidence of CRPS in the placebo group dropped unexpectedly between trials, from 22% to 10.1%, and the results may have been different had other criteria been used. Moreover, the idea that toxic oxygen radicals have a role in CRPS and that vitamin C can scavenge these radicals is based on limited data.61 In the absence of a clear pathophysiologic explanation, some surgeons have been reluctant to treat patients with vitamin C supplementation.
Cazeneuve and colleagues53 also studied the effect of vitamin C supplementation on CRPS in patients with distal radius fractures treated with reduction and intrafocal pinning. Group 1 consisted of 100 patients (treated from 1995 to 1998) who did not receive vitamin C supplementation, and group 2 consisted of 95 patients (treated from 1998 to 2002) who received vitamin C 1000 mg/d for 45 days starting on day of fracture. Patients were followed for up to 90 days after surgery. Incidence of CRPS type 1 was 10% in the untreated group and 2.1% in the group that received vitamin C supplementation.
Vitamin C prophylaxis for CRPS has also been studied in foot and ankle surgery. Besse and colleagues54 prospectively compared 2 chronologically successive groups that received (235 feet) or did not receive (185 feet) vitamin C 1000-mg/d supplementation for 45 days. Incidence of CRPS type 1 as diagnosed with International Association for the Study of Pain (IASP) criteria dropped from 9.6% to 1.7% with vitamin C supplementation. In a case series, Zollinger and colleagues63 examined CRPS type 1 rates after performing cementless total trapeziometacarpal semiconstrained joint prosthesis implantations for trapeziometacarpal arthritis. Forty implantations were performed in 34 patients. All patients received vitamin C 500 mg/d for CRPS prevention starting 2 days before surgery for 50 days. There were no cases of CRPS in the postoperative period, according to Veldman or IASP criteria. Although the results of the studies by Cazeneuve and colleagues53 and Besse and colleagues54 agree with those of the distal radius fracture trials by Zollinger and colleagues,51,52 the quasi-experimental design and the lack of blinding and randomization temper the conclusions that can be drawn because of the risk for significant bias.
In a recent systematic review examining the effectiveness of vitamin C supplementation in preventing CRPS in trauma and surgery in the extremities, Shibuya and colleagues64 concluded that taking at least 500 mg of vitamin C daily for 45 to 50 days after injury or surgery may help decrease the incidence of CRPS after a traumatic event.
Osteoarthritis
Damage caused by free radicals has long been thought to play an important role in osteoarthritis (OA).65-67 A cross-sectional study in knee OA found that amounts of joint fluid antioxidants were lower in patients with severe arthritis than in those with intact cartilage, further implicating free radicals in the pathophysiology of OA.68 Use of vitamin C for prophylaxis against development or progression of OA is therefore a hot research topic. Thus far, animal studies have had mixed results—several showing a chondroprotective effect of vitamin C69,70 and others finding either no effect or even a positive association with the development of arthritis.71
The literature on human subjects, chiefly observational studies, is just as controversial. Wang and colleagues40 found vitamin C intake associated with both a 50% risk reduction of bone marrow lesions on magnetic resonance imaging over a 10-year interval (OR, 0.5; 95% CI, 0.29-0.87) and inversely associated with the tibial plateau bone area. Similarly, the Clearwater Osteoarthritis Study, which followed 1023 patients (age, >40 years), showed that participants who took vitamin C supplements were 11% less likely to develop radiographic evidence of OA (RR, 0.89; 95% CI, 0.85-0.93).72 Nonetheless, other studies have failed to show such associations73 or have demonstrated the opposite effect. Chaganti and colleagues74 analyzed levels of vitamins C and E in the Multicenter Osteoarthritis Study (MOST) cohort of 3026 men and women (age, 50-79 years) and found higher vitamin levels were not protective against incidence of radiographic whole-knee OA and may even have been associated with increased risk.
Conclusion
Vitamin C is an essential micronutrient and a powerful water-soluble antioxidant in numerous biochemical pathways that influence bone health. It has been implicated in the biology of fracture healing, and vitamin C supplementation has been proposed as prophylaxis against hip fractures based on observational data. Results of 2 high-quality double-blind randomized trials support use of vitamin C as prophylaxis against CRPS in wrist fractures treated conservatively and operatively; the evidence for foot and ankle surgery is weaker. Use of vitamin C in OA prevention has tremendous potential, though animal and human study results are controversial. Heterogeneous results and lack of prospective trials preclude any recommendation at this time.
L-ascorbic acid, more commonly know as vitamin C, is an essential micronutrient used in numerous metabolic pathways. It functions physiologically as a water-soluble antioxidant by virtue of its high reducing power, playing a key role in the function of leukocytes, protein metabolism, and production of neurotransmitters.1-3 Vitamin C also contributes to musculoskeletal health through biosynthesis of carnitine and collagen4 and enhancement of intestinal absorption of dietary iron5 from plants and vegetables. Unlike most animals, humans are unable to synthesize this essential vitamin and therefore require intake from natural dietary sources or supplements.6 The ability of vitamin C to prevent or treat disease has been an area of research interest since the vitamin was identified and isolated by Szent-Györgyi in the 1930s.7-16 Research in orthopedic surgery has focused on the effects of vitamin C on fracture healing, its potential use in preventing complex regional pain syndrome (CRPS), and its role in the pathophysiology of osteoarthritis. In this article, we review the basics of vitamin C metabolism and summarize the evidence surrounding the role of vitamin C supplementation in orthopedics.
Sources and Metabolism
Vitamin C is found naturally in many fruits and vegetables (Table 1) and is a common fortification in cereals, juices, and multivitamins. Daily recommended intake (Table 2) depends on age and smoking status. Absorption occurs in the distal small intestine, with blood plasma vitamin C concentrations reflecting dietary intake. Pharmacokinetic studies have shown that vitamin C concentrations are tightly regulated through absorption, tissue accumulation, and renal resorption, with plasma concentrations rarely exceeding 100 μmol/L without additional supplementation.17 Although the usual dietary doses of 100 mg/d (adult) are almost completely absorbed, producing a plasma concentration of 60 μmol/L, higher intake results in an increasingly smaller fraction absorbed.1,18 Intake of more than 1000 mg/d results in less than 50% absorption19 (unmetabolized vitamin C is excreted in stool and urine1). Even at higher doses, vitamin C has low toxicity3; the most common complaints are diarrhea, nausea, and abdominal cramps caused by the osmotic effect of unabsorbed vitamin C in the gastrointestinal tract.1
Vitamin C Deficiency
The relationship between vitamin C deficiency and the development of scurvy has been documented for centuries. Symptoms are described in the ancient Egyptian, Greek, and Roman literature.20 Ascorbic acid is essential for normal collagen function, as it is a required cofactor for enzymatic transfer of hydroxyl groups to select proline and lysine residues during procollagen formation. Hydroxylysine contributes to the intermolecular cross-links in collagen, and hydroxyproline stabilizes the triple-helix structure of collagen.21 Insufficient vitamin C during this process results in collagen that is non-cross-linked, nonhelical, structurally unstable, and weak.21 Clinical manifestations of scurvy stem from an underlying impairment of collagen production causing a systemic decrease in connective tissue integrity, capillary fragility, poor wound healing, fatigue, myalgias, arthritis, and even death.22 Vitamin C deficiency has also been implicated as a cause of diffuse bleeding in surgical patients with normal coagulation parameters secondary to capillary fragility.23 In the United States, the 2003–2004 National Health and Nutrition Examination Survey (NHANES) measured serum vitamin C concentrations in 7277 noninstitutionalized patients 6 years old or older.24 Age-adjusted incidence of subnormal serum vitamin C levels (<28 μmol/L) was 19.6%, and incidence of frank vitamin C deficiency (<11.4 μmol/L) was 7.1%. Reported rates of vitamin C deficiency in hospitalized patients are much higher, with 47% to 60% having subnormal values (<28 μmol/L) and 17% to 19% being vitamin C–deficient (<11.4 μmol/L).22,25 Identified risk factors for hypovitaminosis C include advanced age, obesity, low socioeconomic status, unemployment, male sex, and concomitant alcohol and tobacco consumption.22,24,25
Fracture Healing and Prevention
The effects of vitamin C deficiency on bone healing have been studied with animal models as early as the 1940s.26,27 Early experiments using guinea pigs demonstrated failure of bone graft incorporation, delayed collagen maturation, and decreased collagen and callus formation in scorbutic animals compared with controls that received vitamin C supplementation.26,27 Based on his work with guinea pigs, Bourne26 reported in 1942 that vitamin C deficiency significantly inhibited the reparative process in damaged bone and that patients with fractures should receive vitamin C supplementation. Building on this early research, Yilmaz and colleagues28 found faster histologic healing for tibia fractures in a rat model for animals that received a single injection of vitamin C 0.5 mg/kg compared with a nonscorbutic control group, and Sarisözen and colleagues29 showed significantly accelerated histologic bone formation and mineralization at the fracture site for rats that received vitamin C supplementation. Moreover, Kipp and colleagues30 found that scorbutic guinea pigs had lower bone mineral density (BMD), decreased bone mineral content, and impaired collagen synthesis of articular cartilage and tendons compared with nondeficient controls.
Besides promoting bone formation, vitamin C improves the mechanical strength of callus formation. Alcantara-Martos and colleagues31 used an osteogenic disorder Shionogi (ODS) rat model to examine the effects of vitamin C intake on femoral fracture healing. This particular animal model is unable to produce its own vitamin C. The groups with lower serum vitamin C levels demonstrated lower mechanical resistance of the fracture callus to torsional loads 5 weeks after fracture. Moreover, the group that received vitamin C supplementation showed higher histologic grade of callus formation and demonstrated faster healing rates. The authors suggested that subclinical vitamin C deficiency can delay fracture healing and that vitamin C supplementation in nondeficient patients would improve bone healing.
Other research has demonstrated a link between vitamin C and mesenchymal cell differentiation. Mohan and colleagues32 used an sfx mouse model to show that vitamin C deficiency results in decreased bone formation secondary to impaired osteoblast differentiation, diminished bone density, and development of spontaneous fractures. The authors indicated that not only is vitamin C essential for maintenance of differentiated functions of osteoblasts, but deficiency during early active growth may affect peak BMD levels in humans. Additional studies have demonstrated the role of vitamin C in endochondral bone formation through both induction of osteoblast differentiation and modulation of gene expression in hypertrophic chondrocytes.33-36 Chronic vitamin C deficiency has been found to depress osteoblast function and differentiation of chondrocytes.37 More recently, Kim and colleagues38 examined the effect of vitamin C insufficiency in Gulo-deficient mice, which are unable to synthesize ascorbic acid. Ascorbic acid insufficiency over 4 weeks led to decreased plasma levels of osteocalcin and bone formation in vivo as well as significantly diminished metaphyseal trabecular bone. Despite all the evidence demonstrating the importance of vitamin C in bone formation and maintenance, many of the underlying processes in this relationship have yet to be determined.
Bone Mineral Density
Several observational studies have found a positive association between vitamin C intake and BMD in postmenopausal women. In a retrospective, cross-sectional study by Hall and Greendale,39 a positive association was found between vitamin C intake and BMD of the femoral neck in 775 participants in the Postmenopausal Estrogen/Progestin Interventions trial. After calcium intake, physical activity level, smoking, estrogen use, age, and body mass index were adjusted for, each 100-mg increase in dietary vitamin C was associated with a 0.017 g/cm2 increase in BMD. Wang and colleagues40 found a positive association between dietary vitamin C intake and femoral neck BMD in a retrospective analysis of 125 postmenopausal Mexican American women. Other observational studies have reported that decreased intake of vitamin C is associated with osteoporosis41 and increased rates of BMD loss42 and that supplementation with vitamin C may suppress bone resorption in postmenopausal women.43
The results of these studies contrast with the findings of Leveille and colleagues,44 who examined the relationship between dietary vitamin C and hip BMD in 1892 postmenopausal women. Although the authors found that women (age, 55-64 years) using vitamin C supplements for more than 10 years had an average BMD 6.7% higher than that of nonusers, they did not find any association between dietary vitamin C intake and BMD. Moreover, NHANES III also found inconsistent associations between vitamin C and BMD among 13,080 adults surveyed in the United States.45 Although for premenopausal women dietary ascorbic acid was associated with increased BMD, for postmenopausal women with a history of smoking and estrogen replacement, it was actually associated with lower BMD values. For other subgroups in the study, the relationship was also inconsistent or nonlinear.
The exact mechanism by which ascorbic acid contributes to BMD is not fully delineated. However, it likely is related to the known role of vitamin C in collagen formation, bone matrix development, osteoblast differentiation, and its antioxidant effects limiting bone resorption.44,46
Hip Fractures
Besides demonstrating positive effects of vitamin C on bone healing and BMD, epidemiologic studies have found evidence of a protective effect of vitamin C on hip fracture risk. In a study of the Swedish Mammography cohort, 66,651 women (age, 40-76 years) were prospectively followed.47 The authors found that the odds ratio (OR) for hip fractures among smokers with a low intake of vitamin E (median intake, ≤6.2 mg/d) was 3.0 (95% CI, 1.6-5.4) and for vitamin C (median intake, ≤67 mg/d) was 3.0 (95% CI, 1.6-5.6). Moreover, in smokers with a low intake of both vitamins E and C, OR increased to 4.9 (95% CI, 2.2-11.0). In addition, the Utah Study of Nutrition and Bone Health matched 1215 cases of hip fractures in patients who had ever smoked (age, >50 years) with 1349 controls and found that vitamin C intake above 159 mg/d had a significant protective effect on the incidence of hip fracture; however, a graded relationship was not observed.48 Despite the inconsistencies in the NHANES III study regarding the relationship between vitamin C and BMD, Simon and Hudes45 found that serum vitamin C was associated with lower risk for self-reported fracture in postmenopausal women who had ever smoked and had a history of estrogen therapy (OR, 0.51; 95% CI, 0.36-0.70). Finally, Sahni and colleagues49 followed 958 Framingham cohort men and women (mean age, 75 years) over 17 years and found that those in the highest tertile of total vitamin C intake (median, 313 mg/d) had significantly fewer hip fractures and nonvertebral fractures compared with those in the lowest tertile of intake (median, 94 mg/d). Dietary vitamin C intake was not associated with fracture risk in this study.
Complex Regional Pain Syndrome
Type 1 CRPS is a debilitating condition characterized by severe pain, swelling, and vasomotor instability. It is commonly precipitated by an injury or surgery to an extremity and is a dreaded sequelae in orthopedics,50 with incidence rates of 10% to 22% in wrist fractures51-53 and 10% after foot and ankle surgery.54 Although the pathophysiology of CRPS remains unknown, dysregulation and increased permeability of the vasculature caused by free radicals are thought to play an important role.55 In dermal burns, high doses of vitamin C therapy slowed progression of vascular permeability and therefore reduced extravascular leakage of fluids and protein.56,57 The ability of vitamin C to prevent CRPS has been studied in only a handful of trials.
In a double-blind trial, Zollinger and colleagues51 randomized 127 conservatively treated distal radius fractures to receive either vitamin C 500 mg or placebo daily for 50 days starting on day of injury. Incidence of CRPS (using the diagnostic criteria proposed by Veldman and colleagues58) at 1-year follow-up was 22% in the placebo group and 7% in the vitamin C group (95% CI for difference, 2%-26%). Complaints while wearing the cast and fracture type increased the risk for developing CRPS. This initial study was followed up by a prospective, randomized, double-blind multicenter trial by the same authors,52 who had 416 patients with 427 wrist fractures receive either placebo or vitamin C 200 mg/d, 500 mg/d, or 1500 mg/d for 50 days. This follow-up study included both operative (11%) and nonoperative (89%) distal radius fractures. Incidence of CRPS was 10.1% in the placebo group and 2.4% in the vitamin C group (P < .002). Although there was an appreciable drop in the relative risk (RR) of developing CRPS between the vitamin C 200-mg/d and 500-mg/d groups (0.41-0.17), there was no additional benefit in the 1500-mg/d group. Pooling the data for these 2 randomized trials showed that the overall RR for developing CRPS was lower with vitamin C supplementation (RR, 0.28; 95% CI, 0.14-0.56; P = .0003).59
Results of the 2 trials by Zollinger and colleagues51,52 have been met with several concerns.60-62 As a corollary to the unclear etiology of CRPS, several different sets of diagnostic criteria exist, and the criteria are somewhat subjective and imprecise. Although both trials used the Veldman criteria,58 the incidence of CRPS in the placebo group dropped unexpectedly between trials, from 22% to 10.1%, and the results may have been different had other criteria been used. Moreover, the idea that toxic oxygen radicals have a role in CRPS and that vitamin C can scavenge these radicals is based on limited data.61 In the absence of a clear pathophysiologic explanation, some surgeons have been reluctant to treat patients with vitamin C supplementation.
Cazeneuve and colleagues53 also studied the effect of vitamin C supplementation on CRPS in patients with distal radius fractures treated with reduction and intrafocal pinning. Group 1 consisted of 100 patients (treated from 1995 to 1998) who did not receive vitamin C supplementation, and group 2 consisted of 95 patients (treated from 1998 to 2002) who received vitamin C 1000 mg/d for 45 days starting on day of fracture. Patients were followed for up to 90 days after surgery. Incidence of CRPS type 1 was 10% in the untreated group and 2.1% in the group that received vitamin C supplementation.
Vitamin C prophylaxis for CRPS has also been studied in foot and ankle surgery. Besse and colleagues54 prospectively compared 2 chronologically successive groups that received (235 feet) or did not receive (185 feet) vitamin C 1000-mg/d supplementation for 45 days. Incidence of CRPS type 1 as diagnosed with International Association for the Study of Pain (IASP) criteria dropped from 9.6% to 1.7% with vitamin C supplementation. In a case series, Zollinger and colleagues63 examined CRPS type 1 rates after performing cementless total trapeziometacarpal semiconstrained joint prosthesis implantations for trapeziometacarpal arthritis. Forty implantations were performed in 34 patients. All patients received vitamin C 500 mg/d for CRPS prevention starting 2 days before surgery for 50 days. There were no cases of CRPS in the postoperative period, according to Veldman or IASP criteria. Although the results of the studies by Cazeneuve and colleagues53 and Besse and colleagues54 agree with those of the distal radius fracture trials by Zollinger and colleagues,51,52 the quasi-experimental design and the lack of blinding and randomization temper the conclusions that can be drawn because of the risk for significant bias.
In a recent systematic review examining the effectiveness of vitamin C supplementation in preventing CRPS in trauma and surgery in the extremities, Shibuya and colleagues64 concluded that taking at least 500 mg of vitamin C daily for 45 to 50 days after injury or surgery may help decrease the incidence of CRPS after a traumatic event.
Osteoarthritis
Damage caused by free radicals has long been thought to play an important role in osteoarthritis (OA).65-67 A cross-sectional study in knee OA found that amounts of joint fluid antioxidants were lower in patients with severe arthritis than in those with intact cartilage, further implicating free radicals in the pathophysiology of OA.68 Use of vitamin C for prophylaxis against development or progression of OA is therefore a hot research topic. Thus far, animal studies have had mixed results—several showing a chondroprotective effect of vitamin C69,70 and others finding either no effect or even a positive association with the development of arthritis.71
The literature on human subjects, chiefly observational studies, is just as controversial. Wang and colleagues40 found vitamin C intake associated with both a 50% risk reduction of bone marrow lesions on magnetic resonance imaging over a 10-year interval (OR, 0.5; 95% CI, 0.29-0.87) and inversely associated with the tibial plateau bone area. Similarly, the Clearwater Osteoarthritis Study, which followed 1023 patients (age, >40 years), showed that participants who took vitamin C supplements were 11% less likely to develop radiographic evidence of OA (RR, 0.89; 95% CI, 0.85-0.93).72 Nonetheless, other studies have failed to show such associations73 or have demonstrated the opposite effect. Chaganti and colleagues74 analyzed levels of vitamins C and E in the Multicenter Osteoarthritis Study (MOST) cohort of 3026 men and women (age, 50-79 years) and found higher vitamin levels were not protective against incidence of radiographic whole-knee OA and may even have been associated with increased risk.
Conclusion
Vitamin C is an essential micronutrient and a powerful water-soluble antioxidant in numerous biochemical pathways that influence bone health. It has been implicated in the biology of fracture healing, and vitamin C supplementation has been proposed as prophylaxis against hip fractures based on observational data. Results of 2 high-quality double-blind randomized trials support use of vitamin C as prophylaxis against CRPS in wrist fractures treated conservatively and operatively; the evidence for foot and ankle surgery is weaker. Use of vitamin C in OA prevention has tremendous potential, though animal and human study results are controversial. Heterogeneous results and lack of prospective trials preclude any recommendation at this time.
1. Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002;5(2):66-74.
2. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86(16):6377-6381.
3. Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100(6):637-640.
4. Padh H. Vitamin C: newer insights into its biochemical functions. Nutr Rev. 1991;49(3):65-70.
5. Gershoff SN. Vitamin C (ascorbic acid): new roles, new requirements? Nutr Rev. 1993;51(11):313-326.
6. Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137(10):2171-2184.
7. Szent-Györgyi A. On the function of hexuronic acid in the respiration of the cabbage leaf. J Biol Chem. 1931;90(1):385-393.
8. Svirbely JL, Szent-Györgyi A. The chemical nature of vitamin C. Biochem J. 1933;27(1):279-285.
9. Pauling L. Vitamin C and the Common Cold. San Francisco, CA: Freeman; 1970.
10. Spittle CR. Atherosclerosis and vitamin C. Lancet. 1971;2(7737):1280-1281.
11. Chappell LC, Seed PT, Briley AL, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354(9181):810-816.
12. Block G. Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr. 1991;53(1 suppl):270S-282S.
13. Creagan ET, Moertel CG, O’Fallon JR, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979;301(13):687-690.
14. Hemila H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;1:CD000980.
15. Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH; Vitamins in Pre-eclampsia (VIP) Trial Consortium. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367(9517):1145-1154.
16. Roberts JM, Myatt L, Spong CY, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362(14):1282-1291.
17. Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78-88.
18. Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281(15):1415-1423.
19. Glatthaar BE, Hornig DH, Moser U. The role of ascorbic acid in carcinogenesis. Adv Exp Med Biol. 1986;206:357-377.
20. Carpenter KJ. The History of Scurvy and Vitamin C. New York, NY: Cambridge University Press; 1986.
21. Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78(5):2879-2882.
22. Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14(7):419-425.
23. Blee TH, Cogbill TH, Lambert PJ. Hemorrhage associated with vitamin C deficiency in surgical patients. Surgery. 2002;131(4):408-412.
24. Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90(5):1252-1263.
25. Gan R, Eintracht S, Hoffer LJ. Vitamin C deficiency in a university teaching hospital. J Am Coll Nutr. 2008;27(3):428-433.
26. Bourne G. The effect of graded doses of vitamin C upon the regeneration of bone in guinea-pigs on a scorbutic diet. J Physiol. 1942;101(3):327-336.
27. Bourne GH. The relative importance of periosteum and endosteum in bone healing and the relationship of vitamin C to their activities. Proc R Soc Med. 1944;37(6):275-279.
28. Yilmaz C, Erdemli E, Selek H, Kinik H, Arikan M, Erdemli B. The contribution of vitamin C to healing of experimental fractures. Arch Orthop Trauma Surg. 2001;121(7):426-428.
29. Sarisözen B, Durak K, Dinçer G, Bilgen OF. The effects of vitamins E and C on fracture healing in rats. J Int Med Res. 2002;30(3):309-313.
30. Kipp DE, McElvain M, Kimmel DB, Akhter MP, Robinson RG, Lukert BP. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone. 1996;18(3):281-288.
31. Alcantara-Martos T, Delgado-Martinez AD, Vega MV, Carrascal MT, Munuera-Martinez L. Effect of vitamin C on fracture healing in elderly osteogenic disorder Shionogi rats. J Bone Joint Surg Br. 2007;89(3):402-407.
32. Mohan S, Kapoor A, Singgih A, et al. Spontaneous fractures in the mouse mutant sfx are caused by deletion of the gulonolactone oxidase gene, causing vitamin C deficiency. J Bone Miner Res. 2005;20(9):1597-1610.
33. Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol. 1990;143(2):213-221.
34. Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7(2):235-246.
35. Leboy PS, Vaias L, Uschmann B, Golub E, Adams SL, Pacifici M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J Biol Chem. 1989;264(29):17281-17286.
36. Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid–dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol Endocrinol. 1997;11(8):1103-1113.
37. Sakamoto Y, Takano Y. Morphological influence of ascorbic acid deficiency on endochondral ossification in osteogenic disorder Shionogi rat. Anat Rec. 2002;268(2):93-104.
38. Kim W, Bae S, Kim H, et al. Ascorbic acid insufficiency induces the severe defect on bone formation via the down-regulation of osteocalcin production. Anat Cell Biol. 2013;46(4):254-261.
39. Hall SL, Greendale GA. The relation of dietary vitamin C intake to bone mineral density: results from the PEPI study. Calcif Tissue Int. 1998;63(3):183-189.
40. Wang Y, Hodge AM, Wluka AE, et al. Effect of antioxidants on knee cartilage and bone in healthy, middle-aged subjects: a cross-sectional study. Arthritis Res Ther. 2007;9(4):R66.
41. Maggio D, Barabani M, Pierandrei M, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88(4):1523-1527.
42. Kaptoge S, Welch A, McTaggart A, et al. Effects of dietary nutrients and food groups on bone loss from the proximal femur in men and women in the 7th and 8th decades of age. Osteoporosis Int. 2003;14(5):418-428.
43. Pasco JA, Henry MJ, Wilkinson LK, Nicholson GC, Schneider HG, Kotowicz MA. Antioxidant vitamin supplements and markers of bone turnover in a community sample of nonsmoking women. J Womens Health. 2006;15(3):295-300.
44. Leveille SG, LaCroix AZ, Koepsell TD, Beresford SA, Van Belle G, Buchner DM. Dietary vitamin C and bone mineral density in postmenopausal women in Washington state, USA. J Epidemiol Community Health. 1997;51(5):479-485.
45. Simon JA, Hudes ES. Relation of ascorbic acid to bone mineral density and self-reported fractures among US adults. Am J Epidemiol. 2001;154(5):427-433.
46. Wolf RL, Cauley JA, Pettinger M, et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: results from the Women’s Health Initiative. Am J Clin Nutr. 2005;82(3):581-588.
47. Melhus H, Michaelsson K, Holmberg L, Wolk A, Ljunghall S. Smoking, antioxidant vitamins, and the risk of hip fracture. J Bone Miner Res. 1999;14(1):129-135.
48. Zhang J, Munger RG, West NA, Cutler DR, Wengreen HJ, Corcoran CD. Antioxidant intake and risk of osteoporotic hip fracture in Utah: an effect modified by smoking status. Am J Epidemiol. 2006;163(1):9-17.
49. Sahni S, Hannan MT, Blumberg J, Cupples LA, Kiel DP, Tucker KL. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: a 17-year follow-up from the Framingham Osteoporosis Study. J Bone Miner Res. 2009;24(6):1086-1094.
50. Rho RH, Brewer RP, Lamer TJ, Wilson PR. Complex regional pain syndrome. Mayo Clin Proc. 2002;77(2):174-180.
51. Zollinger PE, Tuinebreijer WE, Kreis RW, Breederveld RS. Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: a randomised trial. Lancet. 1999;354(9195):2025-2028.
52. Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose–response study. J Bone Joint Surg Am. 2007;89(7):1424-1431.
53. Cazeneuve JF, Leborgne JM, Kermad K, Hassan Y. Vitamin C and prevention of reflex sympathetic dystrophy following surgical management of distal radius fractures [in French]. Acta Orthop Belg. 2002;68(5):481-484.
54. Besse JL, Gadeyne S, Galand-Desme S, Lerat JL, Moyen B. Effect of vitamin C on prevention of complex regional pain syndrome type I in foot and ankle surgery. Foot Ankle Surg. 2009;15(4):179-182.
55. Goris RJ, Dongen LM, Winters HA. Are toxic oxygen radicals involved in the pathogenesis of reflex sympathetic dystrophy? Free Radic Res Commun. 1987;3(1-5):13-18.
56. Matsuda T, Tanaka H, Shimazaki S, et al. High-dose vitamin C therapy for extensive deep dermal burns. Burns. 1992;18(2):127-131.
57. Matsuda T, Tanaka H, Hanumadass M, et al. Effects of high-dose vitamin C administration on postburn microvascular fluid and protein flux. J Burn Care Rehabil. 1992;13(5):560-566.
58. Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342(8878):1012-1016.
59. Zollinger PE. The administration of vitamin C in prevention of CRPS-I after distal radial fractures and hand surgery—a review of two RCTs and one observational prospective study. Open Conference Proc J. 2011;2:1-4.
60. Rogers BA, Ricketts DM. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2008;90(2):447-448.
61. Amadio PC. Vitamin C reduced the incidence of reflex sympathetic dystrophy after wrist fracture. J Bone Joint Surg Am. 2000;82(6):873.
62. Frolke JP. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2007;89(11):2550-2551.
63. Zollinger PE, Unal H, Ellis ML, Tuinebreijer WE. Clinical results of 40 consecutive basal thumb prostheses and no CRPS type I after vitamin C prophylaxis. Open Orthop J. 2010;4:62-66.
64. Shibuya N, Humphers JM, Agarwal MR, Jupiter DC. Efficacy and safety of high-dose vitamin C on complex regional pain syndrome in extremity trauma and surgery—systematic review and meta-analysis. J Foot Ankle Surg. 2013;52(1):62-66.
65. Henrotin Y, Deby-Dupont G, Deby C, De Bruyn M, Lamy M, Franchimont P. Production of active oxygen species by isolated human chondrocytes. Br J Rheumatol. 1993;32(7):562-567.
66. McAlindon TE, Jacques P, Zhang Y, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39(4):648-656.
67. Kaiki G, Tsuji H, Yonezawa T, et al. Osteoarthrosis induced by intra-articular hydrogen peroxide injection and running load. J Orthop Res. 1990;8(5):731-740.
68. Regan EA, Bowler RP, Crapo JD. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthritis Cartilage. 2008;16(4):515-521.
69. Meacock SC, Bodmer JL, Billingham ME. Experimental osteoarthritis in guinea-pigs. J Exp Pathol. 1990;71(2):279-293.
70. Kurz B, Jost B, Schunke M. Dietary vitamins and selenium diminish the development of mechanically induced osteoarthritis and increase the expression of antioxidative enzymes in the knee joint of STR/1N mice. Osteoarthritis Cartilage. 2002;10(2):119-126.
71. Kraus VB, Huebner JL, Stabler T, et al. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum. 2004;50(6):1822-1831.
72. Peregoy J, Wilder FV. The effects of vitamin C supplementation on incident and progressive knee osteoarthritis: a longitudinal study. Public Health Nutr. 2011;14(4):709-715.
73. Hill J, Bird HA. Failure of selenium-ace to improve osteoarthritis. Br J Rheumatol. 1990;29(3):211-213.
74. Chaganti RK, Tolstykh I, Javaid MK, et al; Multicenter Osteoarthritis Study Group (MOST). High plasma levels of vitamin C and E are associated with incident radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):190-196.
75. US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference. Release 26. http://www.ars.usda.gov/Services/docs.htm?docid=24936. Published August 2013. Revised November 2013. Accessed May 14, 2015.
76. National Institutes of Health, Office of Dietary Supplements. Vitamin C: fact sheet for health professionals. National Institutes of Health website. http://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/. Reviewed June 5, 2013. Accessed May 14, 2015.
77. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000.
1. Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002;5(2):66-74.
2. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86(16):6377-6381.
3. Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100(6):637-640.
4. Padh H. Vitamin C: newer insights into its biochemical functions. Nutr Rev. 1991;49(3):65-70.
5. Gershoff SN. Vitamin C (ascorbic acid): new roles, new requirements? Nutr Rev. 1993;51(11):313-326.
6. Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137(10):2171-2184.
7. Szent-Györgyi A. On the function of hexuronic acid in the respiration of the cabbage leaf. J Biol Chem. 1931;90(1):385-393.
8. Svirbely JL, Szent-Györgyi A. The chemical nature of vitamin C. Biochem J. 1933;27(1):279-285.
9. Pauling L. Vitamin C and the Common Cold. San Francisco, CA: Freeman; 1970.
10. Spittle CR. Atherosclerosis and vitamin C. Lancet. 1971;2(7737):1280-1281.
11. Chappell LC, Seed PT, Briley AL, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354(9181):810-816.
12. Block G. Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr. 1991;53(1 suppl):270S-282S.
13. Creagan ET, Moertel CG, O’Fallon JR, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979;301(13):687-690.
14. Hemila H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;1:CD000980.
15. Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH; Vitamins in Pre-eclampsia (VIP) Trial Consortium. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367(9517):1145-1154.
16. Roberts JM, Myatt L, Spong CY, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362(14):1282-1291.
17. Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78-88.
18. Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281(15):1415-1423.
19. Glatthaar BE, Hornig DH, Moser U. The role of ascorbic acid in carcinogenesis. Adv Exp Med Biol. 1986;206:357-377.
20. Carpenter KJ. The History of Scurvy and Vitamin C. New York, NY: Cambridge University Press; 1986.
21. Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78(5):2879-2882.
22. Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14(7):419-425.
23. Blee TH, Cogbill TH, Lambert PJ. Hemorrhage associated with vitamin C deficiency in surgical patients. Surgery. 2002;131(4):408-412.
24. Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90(5):1252-1263.
25. Gan R, Eintracht S, Hoffer LJ. Vitamin C deficiency in a university teaching hospital. J Am Coll Nutr. 2008;27(3):428-433.
26. Bourne G. The effect of graded doses of vitamin C upon the regeneration of bone in guinea-pigs on a scorbutic diet. J Physiol. 1942;101(3):327-336.
27. Bourne GH. The relative importance of periosteum and endosteum in bone healing and the relationship of vitamin C to their activities. Proc R Soc Med. 1944;37(6):275-279.
28. Yilmaz C, Erdemli E, Selek H, Kinik H, Arikan M, Erdemli B. The contribution of vitamin C to healing of experimental fractures. Arch Orthop Trauma Surg. 2001;121(7):426-428.
29. Sarisözen B, Durak K, Dinçer G, Bilgen OF. The effects of vitamins E and C on fracture healing in rats. J Int Med Res. 2002;30(3):309-313.
30. Kipp DE, McElvain M, Kimmel DB, Akhter MP, Robinson RG, Lukert BP. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone. 1996;18(3):281-288.
31. Alcantara-Martos T, Delgado-Martinez AD, Vega MV, Carrascal MT, Munuera-Martinez L. Effect of vitamin C on fracture healing in elderly osteogenic disorder Shionogi rats. J Bone Joint Surg Br. 2007;89(3):402-407.
32. Mohan S, Kapoor A, Singgih A, et al. Spontaneous fractures in the mouse mutant sfx are caused by deletion of the gulonolactone oxidase gene, causing vitamin C deficiency. J Bone Miner Res. 2005;20(9):1597-1610.
33. Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol. 1990;143(2):213-221.
34. Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7(2):235-246.
35. Leboy PS, Vaias L, Uschmann B, Golub E, Adams SL, Pacifici M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J Biol Chem. 1989;264(29):17281-17286.
36. Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid–dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol Endocrinol. 1997;11(8):1103-1113.
37. Sakamoto Y, Takano Y. Morphological influence of ascorbic acid deficiency on endochondral ossification in osteogenic disorder Shionogi rat. Anat Rec. 2002;268(2):93-104.
38. Kim W, Bae S, Kim H, et al. Ascorbic acid insufficiency induces the severe defect on bone formation via the down-regulation of osteocalcin production. Anat Cell Biol. 2013;46(4):254-261.
39. Hall SL, Greendale GA. The relation of dietary vitamin C intake to bone mineral density: results from the PEPI study. Calcif Tissue Int. 1998;63(3):183-189.
40. Wang Y, Hodge AM, Wluka AE, et al. Effect of antioxidants on knee cartilage and bone in healthy, middle-aged subjects: a cross-sectional study. Arthritis Res Ther. 2007;9(4):R66.
41. Maggio D, Barabani M, Pierandrei M, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88(4):1523-1527.
42. Kaptoge S, Welch A, McTaggart A, et al. Effects of dietary nutrients and food groups on bone loss from the proximal femur in men and women in the 7th and 8th decades of age. Osteoporosis Int. 2003;14(5):418-428.
43. Pasco JA, Henry MJ, Wilkinson LK, Nicholson GC, Schneider HG, Kotowicz MA. Antioxidant vitamin supplements and markers of bone turnover in a community sample of nonsmoking women. J Womens Health. 2006;15(3):295-300.
44. Leveille SG, LaCroix AZ, Koepsell TD, Beresford SA, Van Belle G, Buchner DM. Dietary vitamin C and bone mineral density in postmenopausal women in Washington state, USA. J Epidemiol Community Health. 1997;51(5):479-485.
45. Simon JA, Hudes ES. Relation of ascorbic acid to bone mineral density and self-reported fractures among US adults. Am J Epidemiol. 2001;154(5):427-433.
46. Wolf RL, Cauley JA, Pettinger M, et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: results from the Women’s Health Initiative. Am J Clin Nutr. 2005;82(3):581-588.
47. Melhus H, Michaelsson K, Holmberg L, Wolk A, Ljunghall S. Smoking, antioxidant vitamins, and the risk of hip fracture. J Bone Miner Res. 1999;14(1):129-135.
48. Zhang J, Munger RG, West NA, Cutler DR, Wengreen HJ, Corcoran CD. Antioxidant intake and risk of osteoporotic hip fracture in Utah: an effect modified by smoking status. Am J Epidemiol. 2006;163(1):9-17.
49. Sahni S, Hannan MT, Blumberg J, Cupples LA, Kiel DP, Tucker KL. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: a 17-year follow-up from the Framingham Osteoporosis Study. J Bone Miner Res. 2009;24(6):1086-1094.
50. Rho RH, Brewer RP, Lamer TJ, Wilson PR. Complex regional pain syndrome. Mayo Clin Proc. 2002;77(2):174-180.
51. Zollinger PE, Tuinebreijer WE, Kreis RW, Breederveld RS. Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: a randomised trial. Lancet. 1999;354(9195):2025-2028.
52. Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose–response study. J Bone Joint Surg Am. 2007;89(7):1424-1431.
53. Cazeneuve JF, Leborgne JM, Kermad K, Hassan Y. Vitamin C and prevention of reflex sympathetic dystrophy following surgical management of distal radius fractures [in French]. Acta Orthop Belg. 2002;68(5):481-484.
54. Besse JL, Gadeyne S, Galand-Desme S, Lerat JL, Moyen B. Effect of vitamin C on prevention of complex regional pain syndrome type I in foot and ankle surgery. Foot Ankle Surg. 2009;15(4):179-182.
55. Goris RJ, Dongen LM, Winters HA. Are toxic oxygen radicals involved in the pathogenesis of reflex sympathetic dystrophy? Free Radic Res Commun. 1987;3(1-5):13-18.
56. Matsuda T, Tanaka H, Shimazaki S, et al. High-dose vitamin C therapy for extensive deep dermal burns. Burns. 1992;18(2):127-131.
57. Matsuda T, Tanaka H, Hanumadass M, et al. Effects of high-dose vitamin C administration on postburn microvascular fluid and protein flux. J Burn Care Rehabil. 1992;13(5):560-566.
58. Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342(8878):1012-1016.
59. Zollinger PE. The administration of vitamin C in prevention of CRPS-I after distal radial fractures and hand surgery—a review of two RCTs and one observational prospective study. Open Conference Proc J. 2011;2:1-4.
60. Rogers BA, Ricketts DM. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2008;90(2):447-448.
61. Amadio PC. Vitamin C reduced the incidence of reflex sympathetic dystrophy after wrist fracture. J Bone Joint Surg Am. 2000;82(6):873.
62. Frolke JP. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2007;89(11):2550-2551.
63. Zollinger PE, Unal H, Ellis ML, Tuinebreijer WE. Clinical results of 40 consecutive basal thumb prostheses and no CRPS type I after vitamin C prophylaxis. Open Orthop J. 2010;4:62-66.
64. Shibuya N, Humphers JM, Agarwal MR, Jupiter DC. Efficacy and safety of high-dose vitamin C on complex regional pain syndrome in extremity trauma and surgery—systematic review and meta-analysis. J Foot Ankle Surg. 2013;52(1):62-66.
65. Henrotin Y, Deby-Dupont G, Deby C, De Bruyn M, Lamy M, Franchimont P. Production of active oxygen species by isolated human chondrocytes. Br J Rheumatol. 1993;32(7):562-567.
66. McAlindon TE, Jacques P, Zhang Y, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39(4):648-656.
67. Kaiki G, Tsuji H, Yonezawa T, et al. Osteoarthrosis induced by intra-articular hydrogen peroxide injection and running load. J Orthop Res. 1990;8(5):731-740.
68. Regan EA, Bowler RP, Crapo JD. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthritis Cartilage. 2008;16(4):515-521.
69. Meacock SC, Bodmer JL, Billingham ME. Experimental osteoarthritis in guinea-pigs. J Exp Pathol. 1990;71(2):279-293.
70. Kurz B, Jost B, Schunke M. Dietary vitamins and selenium diminish the development of mechanically induced osteoarthritis and increase the expression of antioxidative enzymes in the knee joint of STR/1N mice. Osteoarthritis Cartilage. 2002;10(2):119-126.
71. Kraus VB, Huebner JL, Stabler T, et al. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum. 2004;50(6):1822-1831.
72. Peregoy J, Wilder FV. The effects of vitamin C supplementation on incident and progressive knee osteoarthritis: a longitudinal study. Public Health Nutr. 2011;14(4):709-715.
73. Hill J, Bird HA. Failure of selenium-ace to improve osteoarthritis. Br J Rheumatol. 1990;29(3):211-213.
74. Chaganti RK, Tolstykh I, Javaid MK, et al; Multicenter Osteoarthritis Study Group (MOST). High plasma levels of vitamin C and E are associated with incident radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):190-196.
75. US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference. Release 26. http://www.ars.usda.gov/Services/docs.htm?docid=24936. Published August 2013. Revised November 2013. Accessed May 14, 2015.
76. National Institutes of Health, Office of Dietary Supplements. Vitamin C: fact sheet for health professionals. National Institutes of Health website. http://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/. Reviewed June 5, 2013. Accessed May 14, 2015.
77. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000.
Fish Oil and Osteoarthritis: Current Evidence
First-line treatments for osteoarthritis (OA) are targeted at the inflammatory reaction that occurs after breakdown of articular cartilage through regular use of nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections, or surgical intervention. Associated activity restrictions and chronic pain have spurred a search for alternative treatments, commonly daily supplements such as glucosamine, chondroitin, and fish oil, to name a select few of the innumerable products reported to benefit patients with OA.
Background
Fish oil is 1 of the 2 most popular supplements among patients with OA. However, its effectiveness and precise benefit are still debated,1,2 and there is confusion about the definition of the product, the nature of investigations into its effectiveness, and the standardization of research unique to OA. Most fish oil research relates to patients with rheumatoid arthritis (RA). The anti-inflammatory benefits seen in patients with RA are generally applied to characterize fish oils as anti-inflammatory agents with a logical benefit in reducing OA symptoms. However, there is a dearth of independent and focused clinical results justifying that assumption. Further, lack of federal regulation of the supplement industry hinders conducting generalizable studies regarding medical benefit in a regulated and verified dose and form.3
The benefits of fish oil in RA treatment are well supported and accepted. In patients with RA, daily fish oil supplementation has been shown to reduce use of other medications and improve pain scores reported by both physicians and patients.4-10 The clinical efficacy of fish oil use in RA has been determined to be “reasonably strong,” with multiple studies confirming suppression of inflammatory cytokines in vitro and in vivo.11,12 The mechanism by which the inflammatory processes are augmented by fish oil supplementation suggests potential benefit to patients with OA, though review articles as recent as 2011 have concluded that research in that capacity is not sufficient to warrant recommendation.13,14
Most studies of OA-specific use of fish oils have been conducted in in vitro models. Treatment of bovine chondrocytes with omega-3 fatty acids causes reductions in inflammatory markers induced by interleukin 1, one of several proinflammatory cytokines that induce inflammation in OA at the gene and plasma levels, and these reductions have been reproduced.15-17 Although a preventive benefit was found in a study of pig medial collateral ligament fibroblasts, findings of later studies have been inconsistent.18 It also appears that fish oils may alter lipid composition in membranes, favoring incorporation of anti-inflammatory precursor n-3 fatty acids over proinflammatory precursor n-6 fatty acids in these model systems.19,20
Animal in vivo models have also been used to describe the effects of fish oil supplementation on OA. Assessment of dogs with OA before and after supplementation with the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) revealed improvement in clinical signs observed by owners, improvement in weight-bearing measured by veterinary clinicians, and decreased use of NSAIDs.21-24
Fish oil studies using osteoarthritic cartilage samples harvested during surgical procedures have demonstrated results consistent with other model systems described thus far. They have demonstrated a dose-dependent decrease in induced inflammatory destruction of tissue associated with fish oil supplementation. In addition, finding a lack of cellular toxicity, they have validated the safety of supplements.25,26 Proposed but unproven mechanisms for the anti-inflammatory actions of EPA and DHA include competition with n-6 fatty acids; presence of resolvins (anti-inflammatory molecules derived from EPA and DHA); presence of n-3 products that compete with proinflammatory molecules for receptors; reduction in gene expression of cytokines, cyclo-oxygenase 2, and degrading proteinases; interference in the signaling pathways of inflammation; and reduction in lymphocyte proliferation.26,27
Reduction in the n-6/n-3 ratio has been correlated with reduced inflammatory conditions such as OA, stemming from the epidemiologic evidence that higher n-3 intake in Eastern diets and lower intake of n-6 result in a lower incidence of these diseases.18,28,29 Studies have found sufficient evidence to suggest that this ratio has a role in OA, though not sufficient to recommend supplement use over diet modification.19 One study demonstrated an ability to favorably alter bone marrow lipid composition with n-3 fatty acid supplementation.10
The evidence leads to a conclusion of anti-inflammatory benefits from fish oils in these abstracted models. The multitude of basic science studies conducted on the anti-inflammatory properties of omega-3 fatty acids, only briefly reviewed here, supports the potential benefits colloquially ascribed to fish oil in the treatment of OA yet also implies the need for human clinical trials to address these properties clinically.
We reviewed the literature to address claims that fish oil supplementation can prevent or decrease severity of OA. We hypothesized there would be insufficient clinical studies to justify recommending supplementation to patients. Of note, the degree of heterogeneity in the evidence precluded performing a meta-analysis with any statistical validity.
Literature Review
In the PubMed database, we targeted the subject of fish oils and OA by using search terms that included omega-3, DHA, EPA, and alpha-linolenic acid. The MedLine and Google Scholar databases were searched as well. Results were limited to those reported in English and involving human subjects and clinical trials; results were excluded if they primarily involved patients with RA. Studies cited or mentioned in articles found through the PubMed search were evaluated according to the criteria mentioned, such that all relevant articles available at time of search are thought to be included, and these articles represent a reasonable presentation of the available evidence.
Findings
Our search revealed 6 clinical trials in which omega-3–containing supplements were used in the treatment of human OA with differing endpoints. We reviewed these trials in detail. One study, which used alteration of bone marrow lipids as an endpoint, was included for completeness of the evaluation of the relevant evidence.20 In addition, the study by Wang and colleagues,30 who assessed patients without clinical evidence of OA for development of bone marrow lesions, was reviewed. This study was deemed relevant to examine the process by which n-3 fatty acids alter knee structure, as subsequent risk of OA has not been elucidated, and effects on bone marrow lesions may indeed have a direct impact on the OA process. Results of the trials that were identified were varied between no significant difference in OA symptoms between treatment and control groups, implied benefits, and substantial benefits.
The first clinical study of omega-3 supplementation in OA treatment was conducted in 1992.31 The study compared 10 g of cod liver oil (containing 786 mg of EPA) with 10 g of olive oil, both taken daily over 24 weeks by 86 patients with OA. Effects were assessed by NSAID use (recorded in patient diary) and pain score (evaluated by clinician) every 4 weeks. The trial found no significant difference in effects between the oils.
Wang and colleagues30 used a food questionnaire to measure the n-3 intake of 293 healthy adults and quantified their bone marrow lesions after 10 years in an effort to describe how n-3 intake correlates with development of OA or pre-OA lesions. Higher intake of n-6 fatty acids was positively associated with presence of bone marrow lesions; n-3 intake had no association.
In a study of 84 patients who had joint replacement, Pritchett20 evaluated lipid alterations resulting from a regimen of 3 g of fish oil containing 11% DHA daily for a 6-month trial period, measuring lipids before and after the trial period. Pritchett20 found a 20% increase in long-chain fatty acids and a corresponding decrease in saturated fatty acids, as measured in bone marrow.
The supplement Phytalgic (Phythea Laboratories), which is advertised for OA, includes n-3 fatty acids, n-6 fatty acids, extract from Urtica dioica (the common nettle), zinc, and vitamin E. In a study by Jacquet and colleagues,32 this supplement was given 3 times daily over 3 separate 4-week periods to 81 patients with knee or hip OA. Measuring NSAID use with patient diaries and assessing pain with the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index every 4 weeks for 12 weeks, the authors found a significant decrease in NSAID use and, according to WOMAC results, a more than 50% reduction in pain and stiffness, and improved function.
One study compared the effects of glucosamine with and without omega-3 fatty acids in 182 patients with knee or hip OA.33 Each day, patients took 500 mg of glucosamine plus 3 capsules each containing either 444 mg of omega-3 fatty acids or 444 mg of an oil mixture. Pain was assessed with visual analog scale and the WOMAC scale 3 times over the 26-week study. More than 90% reductions in morning stiffness and pain were found for the combination of fish oil and glucosamine.
The Multicenter Osteoarthritis Study (MOST), published in February 2012, demonstrated that plasma levels of n-3 and n-6 polyunsaturated fatty acids (PUFAs) may be related to knee structural findings.34 This study confirmed that dietary modification of n-3 and n-6 PUFAs altered plasma concentration predictably. Higher DHA intake was associated with less evidence of OA on patellofemoral cartilage, though no association was found on tibiofemoral cartilage.34
Discussion
The lack of human clinical trials detailing the effects of fish oil supplementation in patients with OA is arguably the most significant hindrance to fish oil being routinely recommended. Since 1992, only 6 studies have addressed this topic, and their endpoints and results were inconsistent. These interventional trials had their limitations, including short duration, insufficient dosage, inappropriate n-3 choice, dietary interactions, genotype, and medication interactions.18 The present review is limited as well, by the quantity of evidence on the topic and by the focus (of the majority of the studies) on short-term alterations in pain and mobility instead of on disease-modifying potential. Short-term evaluation is unlikely to capture such an effect, which may require long-term supplementation to become evident.
The results of the study by Stammers and colleagues31 must be examined critically, as the likelihood of detection bias is high. Highly subjective assessments of effect, lack of standardized NSAID treatments, and limitations in patient numbers and disease severity raise concerns about validity. In addition, confounding variables (eg, medication interactions, alternative treatments, olive oil use) undermine the design. It is therefore difficult to interpret the results of this trial.
The study by Wang and colleagues30 did not involve supplementation, and intake was assessed only with food frequency questionnaires. It is therefore difficult to apply their results or findings to this review. In addition, the authors did not obtain baseline magnetic resonance imaging for comparison with that obtained at study completion—that is, they did not address any subclinical disease before dietary recording.
Pritchett20 acknowledged study limitations of small sample size and use of 1 subject as both patient and control. Although the study seemed to demonstrate that omega-3 supplementation augmented the lipid profile of joints, it did not directly demonstrate improvement in or prevention of OA. Identification of bone marrow lesions is not definitive proof of OA but an alteration that may correlate with development. The logical supposition is that altering the local environment may alter development of disease within that environment, though this is not proven.
An article reviewing the Phytalgic study highlighted the suspect nature of its results—claims that the supplement is 76% more effective than gold-standard corticosteroid injection.35 Also highlighted were lack of confirmed mechanism, questionable control, detection bias caused by aftertaste, and the high attrition rate in the placebo group. It is difficult to apply these results to fish oil supplementation, as Phytalgic contains other potentially confounding substances.
Of note, the findings of MOST were observational; n-3 and n-6 levels were not altered or supplemented. Altered disease process was demonstrated in patellofemoral cartilage but not in tibiofemoral cartilage in the same patient. The inconsistencies may be explained by the observational nature of the study and the lack of supplementation that would have produced a more significant increase in n-3 PUFA levels and thus more uniform conclusions, if in fact n-3 PUFAs were the significant factor in the altered cartilage structure. Although supportive of a preventive or disease-altering benefit, the results do not speak to supplementation.
Perhaps the most convincing evidence supporting fish oil for OA comes from a 2009 study by Gruenwald and colleagues.33 However, this 2-supplement study addressing synergy was financed by Seven Seas, a company with industry ties. The study was not placebo-controlled and was registered only after completion. The authors omitted baseline values, apparently did not correct for baseline in the statistical analysis, and did not report the distribution of results. The implication is that the results were overstated, or that, at minimum, the supporting data were not reported. Nevertheless, this study demonstrated benefits consistent with the animal and human laboratory studies. However, research is needed to repeat and validate these results, elucidate the mechanism of action, and quantify the benefit unique to fish oil.
Conclusion
Despite the overwhelming popularity of fish oil supplements and the assumption of benefit for patients with arthritis, there appears to be insufficient clinical evidence to justify use of fish oils in the treatment or prevention of OA. Possible efficacy in laboratory and animal studies has yet to be sufficiently observed and verified in clinical trials. Although it is impossible to refute the promise of these agents as beneficial adjuncts to anti-inflammatory regimens, there remains a need for significant, well-designed clinical trials to evaluate the efficacy, safety, and clinical parameters of omega-3 fatty acids in a standardized form before they can in good faith be recommended to patients with OA.
1. Jordan KM, Sawyer S, Coakley HE, Smith HE, Cooper C, Arden NK. The use of conventional and complementary treatments for knee osteoarthritis in the community. Rheumatology. 2003;43(3):381-384.
2. Vista ES, Lau CS. What about supplements for osteoarthritis? A critical and evidenced-based review. Int J Rheum Dis. 2011;14(2):152-158.
3. European Food Safety Authority Panel on Biological Hazards (BIOHAZ). Scientific opinion on fish oil for human consumption. Food hygiene, including rancidity. EFSA J. 2010;8(10):1874.
4. Berbert AA, Kondo CR, Almendra CL, Matsuo T, Dichi I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition. 2005;21(2):131-136.
5. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 suppl):1505S-1519S.
6. Calder PC, Zurier RB. Polyunsaturated fatty acids and rheumatoid arthritis. Curr Opin Clin Nutr Metab Care. 2001;4(2):115-121.
7. Kremer JM. Effects of modulation of inflammatory and immune parameters in patients with rheumatic and inflammatory disease receiving dietary supplementation of n-3 and n-6 fatty acids. Lipids. 1996;31(suppl):S243-S247.
8. Kremer JM, Jubiz W, Michalek A, et al. Fish oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987:106(4):497-503.
9. Kremer JM, Lawrence DA, Jubiz W, et al. Dietary fish oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. 1990;33(6):810-820.
10. Nielsen GL, Faarvang KL, Thomsen BS, et al. The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a randomized, double blind trial. Eur J Clin Invest. 1992;22(10):687-691.
11. Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129(1-2):210-223.
12. van der Tempel H, Tulleken JE, Limburg PC, Muskiet FA, van Rijswijk MH. Effects of fish oil supplementation in rheumatoid arthritis. Ann Rheum Dis. 1990;49(2):76-80.
13. Rosenbaum CC, O’Mathúna DP, Chavez M, Shields K. Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern Ther Health Med. 2010;16(2):32-40.
14. Sanghi D, Avasthi S, Srivastava RN, Singh A. Nutritional factors and osteoarthritis: a review article. Internet J Med Update. 2009;4(1).
15. Curtis CL, Hughes CE, Flannery CR, Little CB, Harwood JL, Caterson B. n-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J Biol Chem. 2000;275(2):721-724.
16. Curtis CL, Rees SG, Cramp J, et al. Effects of fatty acids on cartilage metabolism. Proc Nutr Soc. 2002;61(3):381-389.
17. Zainal Z, Longman AJ, Hurst S, et al. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthritis Cartilage. 2009;17(7):896-905.
18. Hankenson KD, Watkins BA, Schoenlein IA, Allen KG, Turek JJ. Omega-3 fatty acids enhance ligament fibroblast collagen formation in association with changes in interleukin-6 production. Proc Soc Exp Biol Med. 2000;223(1):88-95.
19. Melanson KJ. Diet, nutrition and osteoarthritis. Am J Lifestyle Med. 2007;1(4):260-263.
20. Pritchett JW. Statins and dietary fish oils improve lipid composition in bone marrow and joints. Clin Orthop Relat Res. 2007;(456):233-237.
21. Roush JK, Cross AR, Renberg WC, et al. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(1):67-73.
22. Roush JK, Dodd CE, Fritsch DA, et al. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J Am Vet Med Assoc. 2010;236(1):59-66.
23. Fritsch DA, Allen TA, Dodd CE, et al. A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(5):535-539.
24. Fritsch DA, Allen TA, Dodd CE, et al. Dose-titration effects of fish oil in osteoarthritic dogs. J Vet Intern Med. 2010;24(5):1020-1026.
25. Curtis CL, Rees SG, Little CB, et al. Pathologic indicators of degradation and inflammation in human osteoarthritic cartilage are abrogated by exposure to n-3 fatty acids. Arthritis Rheum. 2002;46(6):1544-1553.
26. Shen CL, Dunn DM, Henry JH, Li Y, Watkins BA. Decreased production of inflammatory mediators in human osteoarthritic chondrocytes by conjugated linoleic acids. Lipids. 2004;39(2):161-166.
27. Hurst S, Zainal Z, Caterson B, Hughes CE, Harwood JL. Dietary fatty acids and arthritis. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4-6):315-318.
28. Cleland LG, Hill CL, James MJ. Diet and arthritis. Baillieres Clin Rheumatol. 1995;9(4):771-785.
29. Maresz K, Meus K, Porwolik B. Krill oil: background and benefits. Int Sci Health Found. 2010;1-11.
30. Wang Y, Wluka AE, Hodge AM, et al. Effect of fatty acids on bone marrow lesions and knee cartilage in healthy, middle-aged subjects without clinical knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(5):579-583.
31. Stammers T, Sibbald B, Freeling P. Efficacy of cod liver oil as an adjunct to non-steroidal anti-inflammatory drug treatment in the management of osteoarthritis in general practice. Ann Rheum Dis. 1992;51(1):128-129.
32. Jacquet A, Girodet PO, Pariente A, Forest K, Mallet L, Moore N. Phytalgic, a food supplement, vs placebo in patients with osteoarthritis of the knee or hip: a randomised double-blind placebo-controlled clinical trial. Arthritis Res Ther. 2009;11(6):R192.
33. Gruenwald J, Petzold E, Busch R, Petzold HP, Graubaum HJ. Effect of glucosamine sulfate with or without omega-3 fatty acids in patients with osteoarthritis. Adv Ther. 2009;26(9):858-871.
34. Baker KR, Matthan NR, Lichtenstein AH, et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis Cartilage. 2012;20(5):382-387.
35. Christensen R, Bliddal H. Is Phytalgic® a goldmine for osteoarthritis patients or is there something fishy about this neutraceutical? A summary of findings and risk-of-bias assessment. Arthritis Res Ther. 2010;12(1):105.
First-line treatments for osteoarthritis (OA) are targeted at the inflammatory reaction that occurs after breakdown of articular cartilage through regular use of nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections, or surgical intervention. Associated activity restrictions and chronic pain have spurred a search for alternative treatments, commonly daily supplements such as glucosamine, chondroitin, and fish oil, to name a select few of the innumerable products reported to benefit patients with OA.
Background
Fish oil is 1 of the 2 most popular supplements among patients with OA. However, its effectiveness and precise benefit are still debated,1,2 and there is confusion about the definition of the product, the nature of investigations into its effectiveness, and the standardization of research unique to OA. Most fish oil research relates to patients with rheumatoid arthritis (RA). The anti-inflammatory benefits seen in patients with RA are generally applied to characterize fish oils as anti-inflammatory agents with a logical benefit in reducing OA symptoms. However, there is a dearth of independent and focused clinical results justifying that assumption. Further, lack of federal regulation of the supplement industry hinders conducting generalizable studies regarding medical benefit in a regulated and verified dose and form.3
The benefits of fish oil in RA treatment are well supported and accepted. In patients with RA, daily fish oil supplementation has been shown to reduce use of other medications and improve pain scores reported by both physicians and patients.4-10 The clinical efficacy of fish oil use in RA has been determined to be “reasonably strong,” with multiple studies confirming suppression of inflammatory cytokines in vitro and in vivo.11,12 The mechanism by which the inflammatory processes are augmented by fish oil supplementation suggests potential benefit to patients with OA, though review articles as recent as 2011 have concluded that research in that capacity is not sufficient to warrant recommendation.13,14
Most studies of OA-specific use of fish oils have been conducted in in vitro models. Treatment of bovine chondrocytes with omega-3 fatty acids causes reductions in inflammatory markers induced by interleukin 1, one of several proinflammatory cytokines that induce inflammation in OA at the gene and plasma levels, and these reductions have been reproduced.15-17 Although a preventive benefit was found in a study of pig medial collateral ligament fibroblasts, findings of later studies have been inconsistent.18 It also appears that fish oils may alter lipid composition in membranes, favoring incorporation of anti-inflammatory precursor n-3 fatty acids over proinflammatory precursor n-6 fatty acids in these model systems.19,20
Animal in vivo models have also been used to describe the effects of fish oil supplementation on OA. Assessment of dogs with OA before and after supplementation with the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) revealed improvement in clinical signs observed by owners, improvement in weight-bearing measured by veterinary clinicians, and decreased use of NSAIDs.21-24
Fish oil studies using osteoarthritic cartilage samples harvested during surgical procedures have demonstrated results consistent with other model systems described thus far. They have demonstrated a dose-dependent decrease in induced inflammatory destruction of tissue associated with fish oil supplementation. In addition, finding a lack of cellular toxicity, they have validated the safety of supplements.25,26 Proposed but unproven mechanisms for the anti-inflammatory actions of EPA and DHA include competition with n-6 fatty acids; presence of resolvins (anti-inflammatory molecules derived from EPA and DHA); presence of n-3 products that compete with proinflammatory molecules for receptors; reduction in gene expression of cytokines, cyclo-oxygenase 2, and degrading proteinases; interference in the signaling pathways of inflammation; and reduction in lymphocyte proliferation.26,27
Reduction in the n-6/n-3 ratio has been correlated with reduced inflammatory conditions such as OA, stemming from the epidemiologic evidence that higher n-3 intake in Eastern diets and lower intake of n-6 result in a lower incidence of these diseases.18,28,29 Studies have found sufficient evidence to suggest that this ratio has a role in OA, though not sufficient to recommend supplement use over diet modification.19 One study demonstrated an ability to favorably alter bone marrow lipid composition with n-3 fatty acid supplementation.10
The evidence leads to a conclusion of anti-inflammatory benefits from fish oils in these abstracted models. The multitude of basic science studies conducted on the anti-inflammatory properties of omega-3 fatty acids, only briefly reviewed here, supports the potential benefits colloquially ascribed to fish oil in the treatment of OA yet also implies the need for human clinical trials to address these properties clinically.
We reviewed the literature to address claims that fish oil supplementation can prevent or decrease severity of OA. We hypothesized there would be insufficient clinical studies to justify recommending supplementation to patients. Of note, the degree of heterogeneity in the evidence precluded performing a meta-analysis with any statistical validity.
Literature Review
In the PubMed database, we targeted the subject of fish oils and OA by using search terms that included omega-3, DHA, EPA, and alpha-linolenic acid. The MedLine and Google Scholar databases were searched as well. Results were limited to those reported in English and involving human subjects and clinical trials; results were excluded if they primarily involved patients with RA. Studies cited or mentioned in articles found through the PubMed search were evaluated according to the criteria mentioned, such that all relevant articles available at time of search are thought to be included, and these articles represent a reasonable presentation of the available evidence.
Findings
Our search revealed 6 clinical trials in which omega-3–containing supplements were used in the treatment of human OA with differing endpoints. We reviewed these trials in detail. One study, which used alteration of bone marrow lipids as an endpoint, was included for completeness of the evaluation of the relevant evidence.20 In addition, the study by Wang and colleagues,30 who assessed patients without clinical evidence of OA for development of bone marrow lesions, was reviewed. This study was deemed relevant to examine the process by which n-3 fatty acids alter knee structure, as subsequent risk of OA has not been elucidated, and effects on bone marrow lesions may indeed have a direct impact on the OA process. Results of the trials that were identified were varied between no significant difference in OA symptoms between treatment and control groups, implied benefits, and substantial benefits.
The first clinical study of omega-3 supplementation in OA treatment was conducted in 1992.31 The study compared 10 g of cod liver oil (containing 786 mg of EPA) with 10 g of olive oil, both taken daily over 24 weeks by 86 patients with OA. Effects were assessed by NSAID use (recorded in patient diary) and pain score (evaluated by clinician) every 4 weeks. The trial found no significant difference in effects between the oils.
Wang and colleagues30 used a food questionnaire to measure the n-3 intake of 293 healthy adults and quantified their bone marrow lesions after 10 years in an effort to describe how n-3 intake correlates with development of OA or pre-OA lesions. Higher intake of n-6 fatty acids was positively associated with presence of bone marrow lesions; n-3 intake had no association.
In a study of 84 patients who had joint replacement, Pritchett20 evaluated lipid alterations resulting from a regimen of 3 g of fish oil containing 11% DHA daily for a 6-month trial period, measuring lipids before and after the trial period. Pritchett20 found a 20% increase in long-chain fatty acids and a corresponding decrease in saturated fatty acids, as measured in bone marrow.
The supplement Phytalgic (Phythea Laboratories), which is advertised for OA, includes n-3 fatty acids, n-6 fatty acids, extract from Urtica dioica (the common nettle), zinc, and vitamin E. In a study by Jacquet and colleagues,32 this supplement was given 3 times daily over 3 separate 4-week periods to 81 patients with knee or hip OA. Measuring NSAID use with patient diaries and assessing pain with the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index every 4 weeks for 12 weeks, the authors found a significant decrease in NSAID use and, according to WOMAC results, a more than 50% reduction in pain and stiffness, and improved function.
One study compared the effects of glucosamine with and without omega-3 fatty acids in 182 patients with knee or hip OA.33 Each day, patients took 500 mg of glucosamine plus 3 capsules each containing either 444 mg of omega-3 fatty acids or 444 mg of an oil mixture. Pain was assessed with visual analog scale and the WOMAC scale 3 times over the 26-week study. More than 90% reductions in morning stiffness and pain were found for the combination of fish oil and glucosamine.
The Multicenter Osteoarthritis Study (MOST), published in February 2012, demonstrated that plasma levels of n-3 and n-6 polyunsaturated fatty acids (PUFAs) may be related to knee structural findings.34 This study confirmed that dietary modification of n-3 and n-6 PUFAs altered plasma concentration predictably. Higher DHA intake was associated with less evidence of OA on patellofemoral cartilage, though no association was found on tibiofemoral cartilage.34
Discussion
The lack of human clinical trials detailing the effects of fish oil supplementation in patients with OA is arguably the most significant hindrance to fish oil being routinely recommended. Since 1992, only 6 studies have addressed this topic, and their endpoints and results were inconsistent. These interventional trials had their limitations, including short duration, insufficient dosage, inappropriate n-3 choice, dietary interactions, genotype, and medication interactions.18 The present review is limited as well, by the quantity of evidence on the topic and by the focus (of the majority of the studies) on short-term alterations in pain and mobility instead of on disease-modifying potential. Short-term evaluation is unlikely to capture such an effect, which may require long-term supplementation to become evident.
The results of the study by Stammers and colleagues31 must be examined critically, as the likelihood of detection bias is high. Highly subjective assessments of effect, lack of standardized NSAID treatments, and limitations in patient numbers and disease severity raise concerns about validity. In addition, confounding variables (eg, medication interactions, alternative treatments, olive oil use) undermine the design. It is therefore difficult to interpret the results of this trial.
The study by Wang and colleagues30 did not involve supplementation, and intake was assessed only with food frequency questionnaires. It is therefore difficult to apply their results or findings to this review. In addition, the authors did not obtain baseline magnetic resonance imaging for comparison with that obtained at study completion—that is, they did not address any subclinical disease before dietary recording.
Pritchett20 acknowledged study limitations of small sample size and use of 1 subject as both patient and control. Although the study seemed to demonstrate that omega-3 supplementation augmented the lipid profile of joints, it did not directly demonstrate improvement in or prevention of OA. Identification of bone marrow lesions is not definitive proof of OA but an alteration that may correlate with development. The logical supposition is that altering the local environment may alter development of disease within that environment, though this is not proven.
An article reviewing the Phytalgic study highlighted the suspect nature of its results—claims that the supplement is 76% more effective than gold-standard corticosteroid injection.35 Also highlighted were lack of confirmed mechanism, questionable control, detection bias caused by aftertaste, and the high attrition rate in the placebo group. It is difficult to apply these results to fish oil supplementation, as Phytalgic contains other potentially confounding substances.
Of note, the findings of MOST were observational; n-3 and n-6 levels were not altered or supplemented. Altered disease process was demonstrated in patellofemoral cartilage but not in tibiofemoral cartilage in the same patient. The inconsistencies may be explained by the observational nature of the study and the lack of supplementation that would have produced a more significant increase in n-3 PUFA levels and thus more uniform conclusions, if in fact n-3 PUFAs were the significant factor in the altered cartilage structure. Although supportive of a preventive or disease-altering benefit, the results do not speak to supplementation.
Perhaps the most convincing evidence supporting fish oil for OA comes from a 2009 study by Gruenwald and colleagues.33 However, this 2-supplement study addressing synergy was financed by Seven Seas, a company with industry ties. The study was not placebo-controlled and was registered only after completion. The authors omitted baseline values, apparently did not correct for baseline in the statistical analysis, and did not report the distribution of results. The implication is that the results were overstated, or that, at minimum, the supporting data were not reported. Nevertheless, this study demonstrated benefits consistent with the animal and human laboratory studies. However, research is needed to repeat and validate these results, elucidate the mechanism of action, and quantify the benefit unique to fish oil.
Conclusion
Despite the overwhelming popularity of fish oil supplements and the assumption of benefit for patients with arthritis, there appears to be insufficient clinical evidence to justify use of fish oils in the treatment or prevention of OA. Possible efficacy in laboratory and animal studies has yet to be sufficiently observed and verified in clinical trials. Although it is impossible to refute the promise of these agents as beneficial adjuncts to anti-inflammatory regimens, there remains a need for significant, well-designed clinical trials to evaluate the efficacy, safety, and clinical parameters of omega-3 fatty acids in a standardized form before they can in good faith be recommended to patients with OA.
First-line treatments for osteoarthritis (OA) are targeted at the inflammatory reaction that occurs after breakdown of articular cartilage through regular use of nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections, or surgical intervention. Associated activity restrictions and chronic pain have spurred a search for alternative treatments, commonly daily supplements such as glucosamine, chondroitin, and fish oil, to name a select few of the innumerable products reported to benefit patients with OA.
Background
Fish oil is 1 of the 2 most popular supplements among patients with OA. However, its effectiveness and precise benefit are still debated,1,2 and there is confusion about the definition of the product, the nature of investigations into its effectiveness, and the standardization of research unique to OA. Most fish oil research relates to patients with rheumatoid arthritis (RA). The anti-inflammatory benefits seen in patients with RA are generally applied to characterize fish oils as anti-inflammatory agents with a logical benefit in reducing OA symptoms. However, there is a dearth of independent and focused clinical results justifying that assumption. Further, lack of federal regulation of the supplement industry hinders conducting generalizable studies regarding medical benefit in a regulated and verified dose and form.3
The benefits of fish oil in RA treatment are well supported and accepted. In patients with RA, daily fish oil supplementation has been shown to reduce use of other medications and improve pain scores reported by both physicians and patients.4-10 The clinical efficacy of fish oil use in RA has been determined to be “reasonably strong,” with multiple studies confirming suppression of inflammatory cytokines in vitro and in vivo.11,12 The mechanism by which the inflammatory processes are augmented by fish oil supplementation suggests potential benefit to patients with OA, though review articles as recent as 2011 have concluded that research in that capacity is not sufficient to warrant recommendation.13,14
Most studies of OA-specific use of fish oils have been conducted in in vitro models. Treatment of bovine chondrocytes with omega-3 fatty acids causes reductions in inflammatory markers induced by interleukin 1, one of several proinflammatory cytokines that induce inflammation in OA at the gene and plasma levels, and these reductions have been reproduced.15-17 Although a preventive benefit was found in a study of pig medial collateral ligament fibroblasts, findings of later studies have been inconsistent.18 It also appears that fish oils may alter lipid composition in membranes, favoring incorporation of anti-inflammatory precursor n-3 fatty acids over proinflammatory precursor n-6 fatty acids in these model systems.19,20
Animal in vivo models have also been used to describe the effects of fish oil supplementation on OA. Assessment of dogs with OA before and after supplementation with the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) revealed improvement in clinical signs observed by owners, improvement in weight-bearing measured by veterinary clinicians, and decreased use of NSAIDs.21-24
Fish oil studies using osteoarthritic cartilage samples harvested during surgical procedures have demonstrated results consistent with other model systems described thus far. They have demonstrated a dose-dependent decrease in induced inflammatory destruction of tissue associated with fish oil supplementation. In addition, finding a lack of cellular toxicity, they have validated the safety of supplements.25,26 Proposed but unproven mechanisms for the anti-inflammatory actions of EPA and DHA include competition with n-6 fatty acids; presence of resolvins (anti-inflammatory molecules derived from EPA and DHA); presence of n-3 products that compete with proinflammatory molecules for receptors; reduction in gene expression of cytokines, cyclo-oxygenase 2, and degrading proteinases; interference in the signaling pathways of inflammation; and reduction in lymphocyte proliferation.26,27
Reduction in the n-6/n-3 ratio has been correlated with reduced inflammatory conditions such as OA, stemming from the epidemiologic evidence that higher n-3 intake in Eastern diets and lower intake of n-6 result in a lower incidence of these diseases.18,28,29 Studies have found sufficient evidence to suggest that this ratio has a role in OA, though not sufficient to recommend supplement use over diet modification.19 One study demonstrated an ability to favorably alter bone marrow lipid composition with n-3 fatty acid supplementation.10
The evidence leads to a conclusion of anti-inflammatory benefits from fish oils in these abstracted models. The multitude of basic science studies conducted on the anti-inflammatory properties of omega-3 fatty acids, only briefly reviewed here, supports the potential benefits colloquially ascribed to fish oil in the treatment of OA yet also implies the need for human clinical trials to address these properties clinically.
We reviewed the literature to address claims that fish oil supplementation can prevent or decrease severity of OA. We hypothesized there would be insufficient clinical studies to justify recommending supplementation to patients. Of note, the degree of heterogeneity in the evidence precluded performing a meta-analysis with any statistical validity.
Literature Review
In the PubMed database, we targeted the subject of fish oils and OA by using search terms that included omega-3, DHA, EPA, and alpha-linolenic acid. The MedLine and Google Scholar databases were searched as well. Results were limited to those reported in English and involving human subjects and clinical trials; results were excluded if they primarily involved patients with RA. Studies cited or mentioned in articles found through the PubMed search were evaluated according to the criteria mentioned, such that all relevant articles available at time of search are thought to be included, and these articles represent a reasonable presentation of the available evidence.
Findings
Our search revealed 6 clinical trials in which omega-3–containing supplements were used in the treatment of human OA with differing endpoints. We reviewed these trials in detail. One study, which used alteration of bone marrow lipids as an endpoint, was included for completeness of the evaluation of the relevant evidence.20 In addition, the study by Wang and colleagues,30 who assessed patients without clinical evidence of OA for development of bone marrow lesions, was reviewed. This study was deemed relevant to examine the process by which n-3 fatty acids alter knee structure, as subsequent risk of OA has not been elucidated, and effects on bone marrow lesions may indeed have a direct impact on the OA process. Results of the trials that were identified were varied between no significant difference in OA symptoms between treatment and control groups, implied benefits, and substantial benefits.
The first clinical study of omega-3 supplementation in OA treatment was conducted in 1992.31 The study compared 10 g of cod liver oil (containing 786 mg of EPA) with 10 g of olive oil, both taken daily over 24 weeks by 86 patients with OA. Effects were assessed by NSAID use (recorded in patient diary) and pain score (evaluated by clinician) every 4 weeks. The trial found no significant difference in effects between the oils.
Wang and colleagues30 used a food questionnaire to measure the n-3 intake of 293 healthy adults and quantified their bone marrow lesions after 10 years in an effort to describe how n-3 intake correlates with development of OA or pre-OA lesions. Higher intake of n-6 fatty acids was positively associated with presence of bone marrow lesions; n-3 intake had no association.
In a study of 84 patients who had joint replacement, Pritchett20 evaluated lipid alterations resulting from a regimen of 3 g of fish oil containing 11% DHA daily for a 6-month trial period, measuring lipids before and after the trial period. Pritchett20 found a 20% increase in long-chain fatty acids and a corresponding decrease in saturated fatty acids, as measured in bone marrow.
The supplement Phytalgic (Phythea Laboratories), which is advertised for OA, includes n-3 fatty acids, n-6 fatty acids, extract from Urtica dioica (the common nettle), zinc, and vitamin E. In a study by Jacquet and colleagues,32 this supplement was given 3 times daily over 3 separate 4-week periods to 81 patients with knee or hip OA. Measuring NSAID use with patient diaries and assessing pain with the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index every 4 weeks for 12 weeks, the authors found a significant decrease in NSAID use and, according to WOMAC results, a more than 50% reduction in pain and stiffness, and improved function.
One study compared the effects of glucosamine with and without omega-3 fatty acids in 182 patients with knee or hip OA.33 Each day, patients took 500 mg of glucosamine plus 3 capsules each containing either 444 mg of omega-3 fatty acids or 444 mg of an oil mixture. Pain was assessed with visual analog scale and the WOMAC scale 3 times over the 26-week study. More than 90% reductions in morning stiffness and pain were found for the combination of fish oil and glucosamine.
The Multicenter Osteoarthritis Study (MOST), published in February 2012, demonstrated that plasma levels of n-3 and n-6 polyunsaturated fatty acids (PUFAs) may be related to knee structural findings.34 This study confirmed that dietary modification of n-3 and n-6 PUFAs altered plasma concentration predictably. Higher DHA intake was associated with less evidence of OA on patellofemoral cartilage, though no association was found on tibiofemoral cartilage.34
Discussion
The lack of human clinical trials detailing the effects of fish oil supplementation in patients with OA is arguably the most significant hindrance to fish oil being routinely recommended. Since 1992, only 6 studies have addressed this topic, and their endpoints and results were inconsistent. These interventional trials had their limitations, including short duration, insufficient dosage, inappropriate n-3 choice, dietary interactions, genotype, and medication interactions.18 The present review is limited as well, by the quantity of evidence on the topic and by the focus (of the majority of the studies) on short-term alterations in pain and mobility instead of on disease-modifying potential. Short-term evaluation is unlikely to capture such an effect, which may require long-term supplementation to become evident.
The results of the study by Stammers and colleagues31 must be examined critically, as the likelihood of detection bias is high. Highly subjective assessments of effect, lack of standardized NSAID treatments, and limitations in patient numbers and disease severity raise concerns about validity. In addition, confounding variables (eg, medication interactions, alternative treatments, olive oil use) undermine the design. It is therefore difficult to interpret the results of this trial.
The study by Wang and colleagues30 did not involve supplementation, and intake was assessed only with food frequency questionnaires. It is therefore difficult to apply their results or findings to this review. In addition, the authors did not obtain baseline magnetic resonance imaging for comparison with that obtained at study completion—that is, they did not address any subclinical disease before dietary recording.
Pritchett20 acknowledged study limitations of small sample size and use of 1 subject as both patient and control. Although the study seemed to demonstrate that omega-3 supplementation augmented the lipid profile of joints, it did not directly demonstrate improvement in or prevention of OA. Identification of bone marrow lesions is not definitive proof of OA but an alteration that may correlate with development. The logical supposition is that altering the local environment may alter development of disease within that environment, though this is not proven.
An article reviewing the Phytalgic study highlighted the suspect nature of its results—claims that the supplement is 76% more effective than gold-standard corticosteroid injection.35 Also highlighted were lack of confirmed mechanism, questionable control, detection bias caused by aftertaste, and the high attrition rate in the placebo group. It is difficult to apply these results to fish oil supplementation, as Phytalgic contains other potentially confounding substances.
Of note, the findings of MOST were observational; n-3 and n-6 levels were not altered or supplemented. Altered disease process was demonstrated in patellofemoral cartilage but not in tibiofemoral cartilage in the same patient. The inconsistencies may be explained by the observational nature of the study and the lack of supplementation that would have produced a more significant increase in n-3 PUFA levels and thus more uniform conclusions, if in fact n-3 PUFAs were the significant factor in the altered cartilage structure. Although supportive of a preventive or disease-altering benefit, the results do not speak to supplementation.
Perhaps the most convincing evidence supporting fish oil for OA comes from a 2009 study by Gruenwald and colleagues.33 However, this 2-supplement study addressing synergy was financed by Seven Seas, a company with industry ties. The study was not placebo-controlled and was registered only after completion. The authors omitted baseline values, apparently did not correct for baseline in the statistical analysis, and did not report the distribution of results. The implication is that the results were overstated, or that, at minimum, the supporting data were not reported. Nevertheless, this study demonstrated benefits consistent with the animal and human laboratory studies. However, research is needed to repeat and validate these results, elucidate the mechanism of action, and quantify the benefit unique to fish oil.
Conclusion
Despite the overwhelming popularity of fish oil supplements and the assumption of benefit for patients with arthritis, there appears to be insufficient clinical evidence to justify use of fish oils in the treatment or prevention of OA. Possible efficacy in laboratory and animal studies has yet to be sufficiently observed and verified in clinical trials. Although it is impossible to refute the promise of these agents as beneficial adjuncts to anti-inflammatory regimens, there remains a need for significant, well-designed clinical trials to evaluate the efficacy, safety, and clinical parameters of omega-3 fatty acids in a standardized form before they can in good faith be recommended to patients with OA.
1. Jordan KM, Sawyer S, Coakley HE, Smith HE, Cooper C, Arden NK. The use of conventional and complementary treatments for knee osteoarthritis in the community. Rheumatology. 2003;43(3):381-384.
2. Vista ES, Lau CS. What about supplements for osteoarthritis? A critical and evidenced-based review. Int J Rheum Dis. 2011;14(2):152-158.
3. European Food Safety Authority Panel on Biological Hazards (BIOHAZ). Scientific opinion on fish oil for human consumption. Food hygiene, including rancidity. EFSA J. 2010;8(10):1874.
4. Berbert AA, Kondo CR, Almendra CL, Matsuo T, Dichi I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition. 2005;21(2):131-136.
5. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 suppl):1505S-1519S.
6. Calder PC, Zurier RB. Polyunsaturated fatty acids and rheumatoid arthritis. Curr Opin Clin Nutr Metab Care. 2001;4(2):115-121.
7. Kremer JM. Effects of modulation of inflammatory and immune parameters in patients with rheumatic and inflammatory disease receiving dietary supplementation of n-3 and n-6 fatty acids. Lipids. 1996;31(suppl):S243-S247.
8. Kremer JM, Jubiz W, Michalek A, et al. Fish oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987:106(4):497-503.
9. Kremer JM, Lawrence DA, Jubiz W, et al. Dietary fish oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. 1990;33(6):810-820.
10. Nielsen GL, Faarvang KL, Thomsen BS, et al. The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a randomized, double blind trial. Eur J Clin Invest. 1992;22(10):687-691.
11. Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129(1-2):210-223.
12. van der Tempel H, Tulleken JE, Limburg PC, Muskiet FA, van Rijswijk MH. Effects of fish oil supplementation in rheumatoid arthritis. Ann Rheum Dis. 1990;49(2):76-80.
13. Rosenbaum CC, O’Mathúna DP, Chavez M, Shields K. Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern Ther Health Med. 2010;16(2):32-40.
14. Sanghi D, Avasthi S, Srivastava RN, Singh A. Nutritional factors and osteoarthritis: a review article. Internet J Med Update. 2009;4(1).
15. Curtis CL, Hughes CE, Flannery CR, Little CB, Harwood JL, Caterson B. n-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J Biol Chem. 2000;275(2):721-724.
16. Curtis CL, Rees SG, Cramp J, et al. Effects of fatty acids on cartilage metabolism. Proc Nutr Soc. 2002;61(3):381-389.
17. Zainal Z, Longman AJ, Hurst S, et al. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthritis Cartilage. 2009;17(7):896-905.
18. Hankenson KD, Watkins BA, Schoenlein IA, Allen KG, Turek JJ. Omega-3 fatty acids enhance ligament fibroblast collagen formation in association with changes in interleukin-6 production. Proc Soc Exp Biol Med. 2000;223(1):88-95.
19. Melanson KJ. Diet, nutrition and osteoarthritis. Am J Lifestyle Med. 2007;1(4):260-263.
20. Pritchett JW. Statins and dietary fish oils improve lipid composition in bone marrow and joints. Clin Orthop Relat Res. 2007;(456):233-237.
21. Roush JK, Cross AR, Renberg WC, et al. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(1):67-73.
22. Roush JK, Dodd CE, Fritsch DA, et al. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J Am Vet Med Assoc. 2010;236(1):59-66.
23. Fritsch DA, Allen TA, Dodd CE, et al. A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(5):535-539.
24. Fritsch DA, Allen TA, Dodd CE, et al. Dose-titration effects of fish oil in osteoarthritic dogs. J Vet Intern Med. 2010;24(5):1020-1026.
25. Curtis CL, Rees SG, Little CB, et al. Pathologic indicators of degradation and inflammation in human osteoarthritic cartilage are abrogated by exposure to n-3 fatty acids. Arthritis Rheum. 2002;46(6):1544-1553.
26. Shen CL, Dunn DM, Henry JH, Li Y, Watkins BA. Decreased production of inflammatory mediators in human osteoarthritic chondrocytes by conjugated linoleic acids. Lipids. 2004;39(2):161-166.
27. Hurst S, Zainal Z, Caterson B, Hughes CE, Harwood JL. Dietary fatty acids and arthritis. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4-6):315-318.
28. Cleland LG, Hill CL, James MJ. Diet and arthritis. Baillieres Clin Rheumatol. 1995;9(4):771-785.
29. Maresz K, Meus K, Porwolik B. Krill oil: background and benefits. Int Sci Health Found. 2010;1-11.
30. Wang Y, Wluka AE, Hodge AM, et al. Effect of fatty acids on bone marrow lesions and knee cartilage in healthy, middle-aged subjects without clinical knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(5):579-583.
31. Stammers T, Sibbald B, Freeling P. Efficacy of cod liver oil as an adjunct to non-steroidal anti-inflammatory drug treatment in the management of osteoarthritis in general practice. Ann Rheum Dis. 1992;51(1):128-129.
32. Jacquet A, Girodet PO, Pariente A, Forest K, Mallet L, Moore N. Phytalgic, a food supplement, vs placebo in patients with osteoarthritis of the knee or hip: a randomised double-blind placebo-controlled clinical trial. Arthritis Res Ther. 2009;11(6):R192.
33. Gruenwald J, Petzold E, Busch R, Petzold HP, Graubaum HJ. Effect of glucosamine sulfate with or without omega-3 fatty acids in patients with osteoarthritis. Adv Ther. 2009;26(9):858-871.
34. Baker KR, Matthan NR, Lichtenstein AH, et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis Cartilage. 2012;20(5):382-387.
35. Christensen R, Bliddal H. Is Phytalgic® a goldmine for osteoarthritis patients or is there something fishy about this neutraceutical? A summary of findings and risk-of-bias assessment. Arthritis Res Ther. 2010;12(1):105.
1. Jordan KM, Sawyer S, Coakley HE, Smith HE, Cooper C, Arden NK. The use of conventional and complementary treatments for knee osteoarthritis in the community. Rheumatology. 2003;43(3):381-384.
2. Vista ES, Lau CS. What about supplements for osteoarthritis? A critical and evidenced-based review. Int J Rheum Dis. 2011;14(2):152-158.
3. European Food Safety Authority Panel on Biological Hazards (BIOHAZ). Scientific opinion on fish oil for human consumption. Food hygiene, including rancidity. EFSA J. 2010;8(10):1874.
4. Berbert AA, Kondo CR, Almendra CL, Matsuo T, Dichi I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition. 2005;21(2):131-136.
5. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 suppl):1505S-1519S.
6. Calder PC, Zurier RB. Polyunsaturated fatty acids and rheumatoid arthritis. Curr Opin Clin Nutr Metab Care. 2001;4(2):115-121.
7. Kremer JM. Effects of modulation of inflammatory and immune parameters in patients with rheumatic and inflammatory disease receiving dietary supplementation of n-3 and n-6 fatty acids. Lipids. 1996;31(suppl):S243-S247.
8. Kremer JM, Jubiz W, Michalek A, et al. Fish oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987:106(4):497-503.
9. Kremer JM, Lawrence DA, Jubiz W, et al. Dietary fish oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. 1990;33(6):810-820.
10. Nielsen GL, Faarvang KL, Thomsen BS, et al. The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a randomized, double blind trial. Eur J Clin Invest. 1992;22(10):687-691.
11. Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129(1-2):210-223.
12. van der Tempel H, Tulleken JE, Limburg PC, Muskiet FA, van Rijswijk MH. Effects of fish oil supplementation in rheumatoid arthritis. Ann Rheum Dis. 1990;49(2):76-80.
13. Rosenbaum CC, O’Mathúna DP, Chavez M, Shields K. Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern Ther Health Med. 2010;16(2):32-40.
14. Sanghi D, Avasthi S, Srivastava RN, Singh A. Nutritional factors and osteoarthritis: a review article. Internet J Med Update. 2009;4(1).
15. Curtis CL, Hughes CE, Flannery CR, Little CB, Harwood JL, Caterson B. n-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J Biol Chem. 2000;275(2):721-724.
16. Curtis CL, Rees SG, Cramp J, et al. Effects of fatty acids on cartilage metabolism. Proc Nutr Soc. 2002;61(3):381-389.
17. Zainal Z, Longman AJ, Hurst S, et al. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthritis Cartilage. 2009;17(7):896-905.
18. Hankenson KD, Watkins BA, Schoenlein IA, Allen KG, Turek JJ. Omega-3 fatty acids enhance ligament fibroblast collagen formation in association with changes in interleukin-6 production. Proc Soc Exp Biol Med. 2000;223(1):88-95.
19. Melanson KJ. Diet, nutrition and osteoarthritis. Am J Lifestyle Med. 2007;1(4):260-263.
20. Pritchett JW. Statins and dietary fish oils improve lipid composition in bone marrow and joints. Clin Orthop Relat Res. 2007;(456):233-237.
21. Roush JK, Cross AR, Renberg WC, et al. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(1):67-73.
22. Roush JK, Dodd CE, Fritsch DA, et al. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J Am Vet Med Assoc. 2010;236(1):59-66.
23. Fritsch DA, Allen TA, Dodd CE, et al. A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(5):535-539.
24. Fritsch DA, Allen TA, Dodd CE, et al. Dose-titration effects of fish oil in osteoarthritic dogs. J Vet Intern Med. 2010;24(5):1020-1026.
25. Curtis CL, Rees SG, Little CB, et al. Pathologic indicators of degradation and inflammation in human osteoarthritic cartilage are abrogated by exposure to n-3 fatty acids. Arthritis Rheum. 2002;46(6):1544-1553.
26. Shen CL, Dunn DM, Henry JH, Li Y, Watkins BA. Decreased production of inflammatory mediators in human osteoarthritic chondrocytes by conjugated linoleic acids. Lipids. 2004;39(2):161-166.
27. Hurst S, Zainal Z, Caterson B, Hughes CE, Harwood JL. Dietary fatty acids and arthritis. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4-6):315-318.
28. Cleland LG, Hill CL, James MJ. Diet and arthritis. Baillieres Clin Rheumatol. 1995;9(4):771-785.
29. Maresz K, Meus K, Porwolik B. Krill oil: background and benefits. Int Sci Health Found. 2010;1-11.
30. Wang Y, Wluka AE, Hodge AM, et al. Effect of fatty acids on bone marrow lesions and knee cartilage in healthy, middle-aged subjects without clinical knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(5):579-583.
31. Stammers T, Sibbald B, Freeling P. Efficacy of cod liver oil as an adjunct to non-steroidal anti-inflammatory drug treatment in the management of osteoarthritis in general practice. Ann Rheum Dis. 1992;51(1):128-129.
32. Jacquet A, Girodet PO, Pariente A, Forest K, Mallet L, Moore N. Phytalgic, a food supplement, vs placebo in patients with osteoarthritis of the knee or hip: a randomised double-blind placebo-controlled clinical trial. Arthritis Res Ther. 2009;11(6):R192.
33. Gruenwald J, Petzold E, Busch R, Petzold HP, Graubaum HJ. Effect of glucosamine sulfate with or without omega-3 fatty acids in patients with osteoarthritis. Adv Ther. 2009;26(9):858-871.
34. Baker KR, Matthan NR, Lichtenstein AH, et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis Cartilage. 2012;20(5):382-387.
35. Christensen R, Bliddal H. Is Phytalgic® a goldmine for osteoarthritis patients or is there something fishy about this neutraceutical? A summary of findings and risk-of-bias assessment. Arthritis Res Ther. 2010;12(1):105.