User login
Arthroscopic knee surgery offers no lasting pain benefit
Arthroscopic knee surgery in middle-aged or older patients with knee pain is associated with greater harm than good, according to a meta-analysis.
The analysis of nine randomized, controlled trials in 1,270 patients of arthroscopic surgery involving partial meniscectomy, debridement, or both showed a small but statistically significant benefit from the procedure – comparable to the pain-relieving effects of paracetamol – but which falls below significance after 12 months.
However, there were no significant improvements in knee function, and there were significant increases in the risk of deep vein thrombosis (4.13 events per 1,000 procedures) as well as infection, pulmonary embolism, and death, according to the paper published online June 16 in BMJ.
“Thus, middle-aged patients with knee pain and meniscal tears should be considered as having early-stage osteoarthritis and be treated according to clinical guidelines for knee osteoarthritis, starting with information, exercise, and often weight loss,” wrote Dr. Jonas B. Thorlund of the University of Southern Denmark, Odense, and coauthors (BMJ 2015, June 16 [doi:10.1136/bmj.h2747]).
The study was supported by the Swedish Research Council. One author declared personal fees from several pharmaceutical and medical companies and relevant journal editorship, while another declared fees from lectureships and books.
While randomized trials, uncontrolled observational studies, and surgeons’ own observations suggest that patients improve after arthroscopy, robust and bias-free trials that use placebo controls show that active treatment works no better than control treatment.
We may be close to a tipping point where the weight of evidence against arthroscopic knee surgery for pain is enough to overcome concerns about the quality of the studies, confirmation bias, and vested interests. When that point is reached, we should anticipate a swift reversal of established practice.
Dr. Andy Carr is from the Oxford University Institute of Musculoskeletal Sciences at the NIHR Oxford Musculoskeletal Biomedical Research Unit, Oxford, England. These comments are taken from an accompanying editorial (BMJ 2015, June 16 [doi:10.1136/bmj.h2983]). He declared research grants from NIHR and Arthritis Research UK.
While randomized trials, uncontrolled observational studies, and surgeons’ own observations suggest that patients improve after arthroscopy, robust and bias-free trials that use placebo controls show that active treatment works no better than control treatment.
We may be close to a tipping point where the weight of evidence against arthroscopic knee surgery for pain is enough to overcome concerns about the quality of the studies, confirmation bias, and vested interests. When that point is reached, we should anticipate a swift reversal of established practice.
Dr. Andy Carr is from the Oxford University Institute of Musculoskeletal Sciences at the NIHR Oxford Musculoskeletal Biomedical Research Unit, Oxford, England. These comments are taken from an accompanying editorial (BMJ 2015, June 16 [doi:10.1136/bmj.h2983]). He declared research grants from NIHR and Arthritis Research UK.
While randomized trials, uncontrolled observational studies, and surgeons’ own observations suggest that patients improve after arthroscopy, robust and bias-free trials that use placebo controls show that active treatment works no better than control treatment.
We may be close to a tipping point where the weight of evidence against arthroscopic knee surgery for pain is enough to overcome concerns about the quality of the studies, confirmation bias, and vested interests. When that point is reached, we should anticipate a swift reversal of established practice.
Dr. Andy Carr is from the Oxford University Institute of Musculoskeletal Sciences at the NIHR Oxford Musculoskeletal Biomedical Research Unit, Oxford, England. These comments are taken from an accompanying editorial (BMJ 2015, June 16 [doi:10.1136/bmj.h2983]). He declared research grants from NIHR and Arthritis Research UK.
Arthroscopic knee surgery in middle-aged or older patients with knee pain is associated with greater harm than good, according to a meta-analysis.
The analysis of nine randomized, controlled trials in 1,270 patients of arthroscopic surgery involving partial meniscectomy, debridement, or both showed a small but statistically significant benefit from the procedure – comparable to the pain-relieving effects of paracetamol – but which falls below significance after 12 months.
However, there were no significant improvements in knee function, and there were significant increases in the risk of deep vein thrombosis (4.13 events per 1,000 procedures) as well as infection, pulmonary embolism, and death, according to the paper published online June 16 in BMJ.
“Thus, middle-aged patients with knee pain and meniscal tears should be considered as having early-stage osteoarthritis and be treated according to clinical guidelines for knee osteoarthritis, starting with information, exercise, and often weight loss,” wrote Dr. Jonas B. Thorlund of the University of Southern Denmark, Odense, and coauthors (BMJ 2015, June 16 [doi:10.1136/bmj.h2747]).
The study was supported by the Swedish Research Council. One author declared personal fees from several pharmaceutical and medical companies and relevant journal editorship, while another declared fees from lectureships and books.
Arthroscopic knee surgery in middle-aged or older patients with knee pain is associated with greater harm than good, according to a meta-analysis.
The analysis of nine randomized, controlled trials in 1,270 patients of arthroscopic surgery involving partial meniscectomy, debridement, or both showed a small but statistically significant benefit from the procedure – comparable to the pain-relieving effects of paracetamol – but which falls below significance after 12 months.
However, there were no significant improvements in knee function, and there were significant increases in the risk of deep vein thrombosis (4.13 events per 1,000 procedures) as well as infection, pulmonary embolism, and death, according to the paper published online June 16 in BMJ.
“Thus, middle-aged patients with knee pain and meniscal tears should be considered as having early-stage osteoarthritis and be treated according to clinical guidelines for knee osteoarthritis, starting with information, exercise, and often weight loss,” wrote Dr. Jonas B. Thorlund of the University of Southern Denmark, Odense, and coauthors (BMJ 2015, June 16 [doi:10.1136/bmj.h2747]).
The study was supported by the Swedish Research Council. One author declared personal fees from several pharmaceutical and medical companies and relevant journal editorship, while another declared fees from lectureships and books.
FROM BMJ
Key clinical point: The potential harms of arthroscopic knee surgery in middle-aged or older patients with knee pain outweigh the benefits.
Major finding: Arthroscopic knee surgery is associated with a small but significant improvement in symptoms that disappears after 12 months.
Data source: Meta-analysis of nine randomized, controlled trials in 1,270 patients.
Disclosures: The study was supported by the Swedish Research Council. One author declared personal fees from several pharmaceutical and medical companies and relevant journal editorship, while another declared fees from lectureships and books.
Comparison of Carpal Tunnel Release Methods and Complications
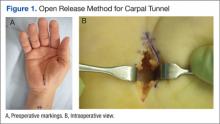
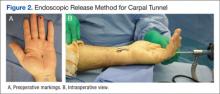
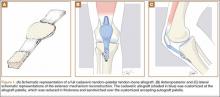
Carpal tunnel release is one of the most common hand surgeries performed at the North Florida/South Georgia Veterans Health System (NFSGVHS). Depending on surgeon experience and comfort level, surgeries are performed through either the traditional open method or the endoscopic method, single or double port (Figures 1 and 2). The advantage of the endoscopic method is faster recovery and return to work; however, the endoscopic method requires more expensive equipment and a steeper learning curve for surgeons. Complications are uncommon but can create unsatisfactory patient experiences because of costly lost workdays and long travel distances to the medical facility.
The purpose of this study was to compare the endoscopic method with the open carpal tunnel release method to determine whether there was an increased complication risk. Researchers anticipated that this information would help surgeons better inform patients of operative risks and prompt changes in NFSGVHS treatment plans to improve the quality of veteran care.
Methods
An Institutional Review Board- approved (#647-2011) retrospective review was done of patients who had carpal tunnel surgery performed by the NFSGVHS plastic surgery service from January 1, 2005, to December 31, 2010. Surgeries included in the review took place at the Malcom Randall VAMC in Gainesville and at the Lake City VAMC, both in Florida. Most of the surgeries included in the study were performed by a resident or fellow under the supervision of an attending physician. Eight different attending surgeons staffed the operations. Seven were board-certified or board-eligible plastic surgeons, 2 had advanced hand fellowship training, and 1 was a general surgeon with hand fellowship training. All hand fellowship-trained surgeons were in their first year of practice at the time of the study.
Only primary carpal tunnel releases were included in the study. Exclusion criteria included patients who were operated on by a service section other than the plastic surgery service (orthopedics or neurosurgery) and hands on which other procedures were performed during the same operation. Charts were reviewed for up to 1 year post surgery. Complications that required intervention were recorded. Researchers did not include pillar tenderness or an increase in occupational therapy visits as complications, due to the wide variety of patient tolerance to postoperative pain and varying motivation to return to work and daily routine.
Methods of release were endoscopic, open, or endoscopic converted to open. All but 6 of the completed endoscopic surgeries were performed using the double port Chow technique. The other 6 endoscopic surgeries were performed using the single port Agee technique at the distal wrist crease. There were 3 endoscopic converted to open cases that were performed using a single port, proximally-based technique in the midpalm. This method was abandoned after 3 unsuccessful endoscopic attempts, 1 resulting in digital nerve injury despite interactive cadaver labs prior to operative experience.
Endoscopic surgeries converted to open were recorded as open surgeries, because the patients had the full invasive experience. Researchers used the chi-square test and P value < .05 to compare the different methods of carpal tunnel release with identified complications.
Results and Complications
A total of 584 hands belonging to 452 patients were included in the study. Patients included 395 men and 57 women aged from 33 to 91 years. There were 271 endoscopic releases, 228 open releases, and 85 endoscopic converted to open releases. The NFSGVHS conversion rate was 23.7%. Complications in the converted cases (n = 4) were included in the open release results.
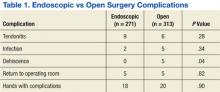
There were 40 complications in 38 hands. The overall complication rate was 6.5%. Complications noted were tendonitis presenting as De Quervain disease or trigger finger (9 endoscopic surgeries; 6 open surgeries), infection (2 endoscopic surgeries; 6 open surgeries), wound dehiscence (5 open surgeries), nerve injury (1 open surgery), respiratory distress (1 endoscopic), complex regional pain syndrome (1 open surgery), and scheduled returns to the operating room (OR) for recurrent, ongoing, or worsening symptoms (5 endoscopic surgeries; 5 open surgeries). Complications with an n > 1 were evaluated for statistical significance with P value < .05 (Table 1).
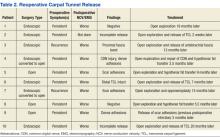
The NFSGVHS study had 10 patients return to the OR for open exploration (Table 2). Nine of these patients went back to the OR based on symptoms consistent with nerve conduction studies that had deteriorated compared with their preoperative studies. One endoscopic case was brought back to the OR for a suspected nerve injury without nerve conduction studies. Findings during reoperation included scar adhesions, incomplete release of ligaments, digital nerve injury, and negative explorations.
Two hypothenar fat transfers were performed to prevent scar adhesions in cases that had originally been open releases.1 Two of the open cases were endoscopic converted to open cases. One went back to the OR with a suspected nerve injury. Dense adhesions and an injured common digital nerve were identified and repaired. The second converted case that went back to the OR had a suspected, but unconfirmed, nerve injury to the motor branch. The diagnosis and treatment were delayed for more than a year due to the patient having other pressing medical and family concerns. An exploration found significant scar adhesions, and an opponensplasty was performed.
One patient had respiratory insufficiency secondary to chemical pneumonitis. The patient was sedated during an endoscopic carpal tunnel release, aspirated, and kept intubated in the intensive care unit until the morning after surgery.
An early complex regional pain syndrome diagnosis was made in a patient with underlying neuropathy and a preoperative “profound” median neuropathies diagnosis at the wrist with underlying peripheral neuropathy found on nerve conduction studies. The patient experienced an unusual amount of postoperative pain and edema after an uncomplicated open carpal tunnel release. This was treated with rapid intervention using anti- inflammatories and hand therapy. The patient also started a regimen of skin care, edema management, neuroreeducation, and contrast baths. Symptoms responded within a week.
There were 12 wound complications: 10 in open and 2 in endoscopic surgeries. Total wound complications were equally split between patients with and without diabetes. Infection and dehiscence were noted. Sutures were removed an average of 9.6 days after surgery in the patients whose wounds broke down. A statistically significant relationship was found only between the open method of release and wound dehiscence (P < .05).
There was no statistically significant difference in the overall complication rate in the NFSGVHS population when comparing endoscopic with open carpal tunnel release or when comparing the risk of postoperative tendonitis, wound infection, or return to the OR.
Discussion
Carpal tunnel syndrome was documented by James Paget in mid-19th century in reference to a distal radius fracture.2 It is the most common peripheral nerve compression, with an incidence ranging from 1 to 3 cases per 1,000 subjects per year and a prevalence of 50 cases per 1,000 subjects per year.3 In an active-duty U.S. military population, the incidence of carpal tunnel syndrome is 3.98 per 1,000 person years.4
Related: Risk Factors for Postoperative Complications in Trigger Finger Release
The endoscopic method of release was first introduced in 1989 by Okutsu and colleagues.5 About 500,000 carpal tunnel releases are now performed in the U.S. every year, with 50,000 performed endoscopically.3 There were 185 carpal tunnel releases (56 endoscopic and 129 open) performed at the NFSGVHS in 2012.6 The minimally invasive procedure was designed to preserve the overlying skin and fascia, promoting an earlier return to work and daily activities. This is particularly relevant for manual workers who desire rapid return of grip strength. Multiple published reports have found more rapid recovery based on a reduction in scar tenderness, increase in grip strength, or return to work.7-13 Patients seem to have equivalent results over the long term, ranging from 3 months to 1 year.7,8,13-15 Return to work was not evaluated in this study, because many patients were either retired or not working steadily.
The endoscopic method was criticized after its introduction due to its potential increase in major structural injury to the median nerve, ulnar nerve, palmar arch, ulnar artery, or flexor tendons.16 A meta-analysis found improved outcomes but a statistically significant higher complication rate in endoscopic, compared with open release (2.2% in endoscopic vs 1.2% in open).16 Referral patterns have found iatrogenic nerve injury in patients referred by surgeons without formal hand fellowship training.17 There is a wide variety of background training for surgeons who may offer carpal tunnel release, including plastic surgery, orthopedics, general surgery, and neurosurgery.
Major structural injuries were reported by hand surgeons using both open and endoscopic methods in a questionnaire sent to members of the American Society for Surgery of the Hand, indicating that either approach demands respect.18 A large review of the literature from 1966 to 2001 by Benson and colleagues found that the endoscopic approach was not more likely to produce injury to tendons, arteries, or nerves compared with the open approach and actually had a lower rate of structural damage (0.49% vs 0.19%).19 Researchers who conducted this study confirmed one common digital nerve injury in an endoscopic converted to open technique, using a distally-based port with the blade not being deployed via the endoscopic method. The endoscopic method has been found to have a higher rate of reversible nerve injury (neuropraxia) compared with the open technique.7,10,19
The NFSGVHS results found a higher rate of wound dehiscence. More frequent wound site complications, particularly infection, hypertrophic scar, and scar tenderness have been noted using the open method.3,8,20 This is probably due to the deeper and slightly larger incision used for the open method compared with the smaller and shallower incisions used for the endoscopic release.
There is the inevitable learning curve for the endoscopic release due to the more complicated nature of the procedure. The NFSGVHS conversion rate was 23.7% over the 5-year period from 2005 to 2010. All 3 fellowship- trained hand surgeons were in their first year of practice at the time of the study, so the authors anticipate a lower conversion rate in forthcoming studies. The NFSGVHS researchers did not consider converting to an open technique to be a complication and believe it is appropriate to teach plastic surgery residents and fellows to have a low threshold to convert when visualization is not optimal and the potential for significant injury exists. The learning curve and a higher conversion rate have been acknowledged by Beck and colleagues with no increase in morbidity.21
The authors anticipated finding an increased rate of tendonitis in the endoscopic method, as found by Goshtasby and colleagues, where trigger finger was found more frequently in the endoscopic patients.22 The NFSGVHS study found that the number of patients presenting for steroid injections to treat postoperative tendonitis in the hand and wrist was not statistically significant when comparing the 2 surgical methods of release (3.3% in endoscopic vs 1.9% in open; P = .28).
The NFSGVHS rate of return to the OR within a year of surgery was 1.7%. The researchers from NFSGHVS anticipated a higher rate of return to the OR for ongoing symptoms secondary to incomplete release of the transverse carpal ligament. Published studies have found an intact retinaculum to be a cause of persistent symptoms when smaller incisions are used.23,24 Five endoscopic cases and 5 open cases eventually returned to the OR for carpal tunnel exploration. Two of the patients were classified as recurrent, because they had improvement of symptoms initially but presented > 6 months later with new symptoms. Eight of the patients were classified as persistent, because they did not have an extended period of relief of preoperative symptoms (Table 2).25 There was no statistically significant difference in return to the OR in the 2 study groups. The NFSGVHS researchers did note a trend in more incomplete nerve releases in the endoscopic group and more scar adhesions as the etiology of symptoms in the open group who went back to surgery.
Published studies have found no difference in overall complication rates when comparing the open with the endoscopic method of release, which is consistent with NFSGVHS data.8,11,12,26
A limitation of the current retrospective study is the large number of providers who both operated on the patients and documented their postoperative findings. The strength of the study is that VA patients tend to stay within the VISN for their health care so postoperative problems will be identified and routed to the plastic surgery service for evaluation and treatment.
Clinical implications for the NFSGVHS practice are that surgeons can confidently offer both the open and endoscopic surgeries without an overall risk of increased complications to patients. Patients who are identified as higher risk for wound dehiscence, such as those who place an unusual amount of pressure on their palms due to assisted walking devices or are at a higher risk of falling onto the surgical site, will be steered toward an endoscopic surgery. The NFSGVHS began a splinting protocol in the early postoperative period that was not previously used on those select patients who have open carpal tunnel releases.
Conclusion
Wound dehiscence was the only statistically significant complication found in the NFSGVHS veteran population when comparing open with endoscopic carpal tunnel release. This can potentially be prevented in future patients by delaying the removal of sutures and prolonging the use of a protective dressing in patients who undergo open release. There was not a statistically significant increase in overall complications when using the minimally invasive method of release, which is consistent with existing literature.
Acknowledgement
This material is the result of work supported with resources and the use of facilities at the Malcom Randall VAMC.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Chrysopoulo MT, Greenberg JA, Kleinman WB. The hypothenar fat pad transposition flap: a modified surgical technique. Tech Hand Up Extrem Surg. 2006;10(3):150-156.
2. Paget J. Lectures on Surgical Pathology Delivered at the Royal College of Surgeons of England. London, England: Longman, Green, Brown, and Longmans; 1853.
3. Mintalucci DJ, Leinberry CF Jr. Open versus endoscopic carpal tunnel release. Orthop Clin North Am. 2012;43(4):431-437.
4. Wolf JM, Mountcastle S, Owens BD. Incidence of carpal tunnel syndrome in the US military population. Hand (NY). 2009;4(3):289-293.
5. Okutsu I, Ninomiya S, Takatori Y, Ugawa Y. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
6. U.S. Department of Veterans Affairs. Health Information Systems and Technology Architecture Database, Ambulatory Surgical Case Load Report, 2012. Accessed March 14, 2013.
7. Larsen MB, Sørensen AI, Crone KL, Weis T, Boeckstyns ME. Carpal tunnel release: a randomized comparison of three surgical methods. J Hand Surg Eur Vol. 2013;38(6):646-650.
8. Malhotra R, Kiran EK, Dua A, Mallinath SG, Bhan S. Endoscopic versus open carpal tunnel release: a short-term comparative study. Indian J Orthop. 2007;41(1):57-61.
9. Sabesan VJ, Pedrotty D, Urbaniak JR, Aldridge JM 3rd. An evidence-based review of a single surgeon’s experience with endoscopic carpal tunnel release. J Surg Orthop Adv. 2012;21(3):117-121.
10. Thoma A, Veltri K, Haines T, Duku E. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
11. Tian Y, Zhao H, Wang T. Prospective comparison of endoscopic and open surgical methods for carpal tunnel syndrome. Chin Med Sci J. 2007;22(2):104-107.
12. Trumble TE, Diao E, Abrams RA, Gilbert-Anderson MM. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84-A(7):1107-1115.
13. Vasiliadis HS, Xenakis TA, Mitsionis G, Paschos N, Georgoulis A. Endoscopic versus open carpal tunnel release. Arthroscopy. 2010:26(1):26-33.
14. Macdermid JC, Richards RS, Roth JH, Ross DC, King GJ. Endoscopic versus open carpal tunnel release: a randomized trial. J Hand Surg Am. 2003;28(3):475-480.
15. Aslani HR, Alizadeh K, Eajazi A, et al. Comparison of carpal tunnel release with three different techniques. Clin Neurol Neurosurg. 2012;114(7):965-968.
16. Kohanzadeh S, Herrera FA, Dobke M. Outcomes of open and endoscopic carpal tunnel release: a meta-analysis. Hand (NY). 2012;7(3):247-251.
17. Azari KK, Spiess AM, Buterbaugh GA, Imbriglia JE. Major nerve injuries associated with carpal tunnel release. Plast Reconstr Surg. 2007;119(6):1977-1978.
18. Palmer AK, Toivonen DA. Complications of endoscopic and open carpal tunnel release. J Hand Surg Am. 1999;24(3):561-565.
19. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
20. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
21. Beck JD, Deegan JH, Rhoades D, Klena JC. Results of endoscopic carpal tunnel release relative to surgeon experience with the Agee technique. J Hand Surg Am. 2011;36(1):61-64.
22. Goshtasby PH, Wheeler DR, Moy OJ. Risk factors for trigger finger occurrence after carpal tunnel release. Hand Surg. 2010;15(2):81-87.
23. Assmus H, Dombert T, Staub F. Reoperations for CTS because of recurrence or for correction [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):306-311.
24. Frik A, Baumeister RG. Re-intervention after carpal tunnel release [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):312-316.
25. Jones NF, Ahn HC, Eo S. Revision surgery for persistent and recurrent carpal tunnel syndrome and for failed carpal tunnel release. Plast Reconstr Surg. 2012;129(3):683-692.
26. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002:84(3):375-379.
Carpal tunnel release is one of the most common hand surgeries performed at the North Florida/South Georgia Veterans Health System (NFSGVHS). Depending on surgeon experience and comfort level, surgeries are performed through either the traditional open method or the endoscopic method, single or double port (Figures 1 and 2). The advantage of the endoscopic method is faster recovery and return to work; however, the endoscopic method requires more expensive equipment and a steeper learning curve for surgeons. Complications are uncommon but can create unsatisfactory patient experiences because of costly lost workdays and long travel distances to the medical facility.
The purpose of this study was to compare the endoscopic method with the open carpal tunnel release method to determine whether there was an increased complication risk. Researchers anticipated that this information would help surgeons better inform patients of operative risks and prompt changes in NFSGVHS treatment plans to improve the quality of veteran care.
Methods
An Institutional Review Board- approved (#647-2011) retrospective review was done of patients who had carpal tunnel surgery performed by the NFSGVHS plastic surgery service from January 1, 2005, to December 31, 2010. Surgeries included in the review took place at the Malcom Randall VAMC in Gainesville and at the Lake City VAMC, both in Florida. Most of the surgeries included in the study were performed by a resident or fellow under the supervision of an attending physician. Eight different attending surgeons staffed the operations. Seven were board-certified or board-eligible plastic surgeons, 2 had advanced hand fellowship training, and 1 was a general surgeon with hand fellowship training. All hand fellowship-trained surgeons were in their first year of practice at the time of the study.
Only primary carpal tunnel releases were included in the study. Exclusion criteria included patients who were operated on by a service section other than the plastic surgery service (orthopedics or neurosurgery) and hands on which other procedures were performed during the same operation. Charts were reviewed for up to 1 year post surgery. Complications that required intervention were recorded. Researchers did not include pillar tenderness or an increase in occupational therapy visits as complications, due to the wide variety of patient tolerance to postoperative pain and varying motivation to return to work and daily routine.
Methods of release were endoscopic, open, or endoscopic converted to open. All but 6 of the completed endoscopic surgeries were performed using the double port Chow technique. The other 6 endoscopic surgeries were performed using the single port Agee technique at the distal wrist crease. There were 3 endoscopic converted to open cases that were performed using a single port, proximally-based technique in the midpalm. This method was abandoned after 3 unsuccessful endoscopic attempts, 1 resulting in digital nerve injury despite interactive cadaver labs prior to operative experience.
Endoscopic surgeries converted to open were recorded as open surgeries, because the patients had the full invasive experience. Researchers used the chi-square test and P value < .05 to compare the different methods of carpal tunnel release with identified complications.
Results and Complications
A total of 584 hands belonging to 452 patients were included in the study. Patients included 395 men and 57 women aged from 33 to 91 years. There were 271 endoscopic releases, 228 open releases, and 85 endoscopic converted to open releases. The NFSGVHS conversion rate was 23.7%. Complications in the converted cases (n = 4) were included in the open release results.
There were 40 complications in 38 hands. The overall complication rate was 6.5%. Complications noted were tendonitis presenting as De Quervain disease or trigger finger (9 endoscopic surgeries; 6 open surgeries), infection (2 endoscopic surgeries; 6 open surgeries), wound dehiscence (5 open surgeries), nerve injury (1 open surgery), respiratory distress (1 endoscopic), complex regional pain syndrome (1 open surgery), and scheduled returns to the operating room (OR) for recurrent, ongoing, or worsening symptoms (5 endoscopic surgeries; 5 open surgeries). Complications with an n > 1 were evaluated for statistical significance with P value < .05 (Table 1).
The NFSGVHS study had 10 patients return to the OR for open exploration (Table 2). Nine of these patients went back to the OR based on symptoms consistent with nerve conduction studies that had deteriorated compared with their preoperative studies. One endoscopic case was brought back to the OR for a suspected nerve injury without nerve conduction studies. Findings during reoperation included scar adhesions, incomplete release of ligaments, digital nerve injury, and negative explorations.
Two hypothenar fat transfers were performed to prevent scar adhesions in cases that had originally been open releases.1 Two of the open cases were endoscopic converted to open cases. One went back to the OR with a suspected nerve injury. Dense adhesions and an injured common digital nerve were identified and repaired. The second converted case that went back to the OR had a suspected, but unconfirmed, nerve injury to the motor branch. The diagnosis and treatment were delayed for more than a year due to the patient having other pressing medical and family concerns. An exploration found significant scar adhesions, and an opponensplasty was performed.
One patient had respiratory insufficiency secondary to chemical pneumonitis. The patient was sedated during an endoscopic carpal tunnel release, aspirated, and kept intubated in the intensive care unit until the morning after surgery.
An early complex regional pain syndrome diagnosis was made in a patient with underlying neuropathy and a preoperative “profound” median neuropathies diagnosis at the wrist with underlying peripheral neuropathy found on nerve conduction studies. The patient experienced an unusual amount of postoperative pain and edema after an uncomplicated open carpal tunnel release. This was treated with rapid intervention using anti- inflammatories and hand therapy. The patient also started a regimen of skin care, edema management, neuroreeducation, and contrast baths. Symptoms responded within a week.
There were 12 wound complications: 10 in open and 2 in endoscopic surgeries. Total wound complications were equally split between patients with and without diabetes. Infection and dehiscence were noted. Sutures were removed an average of 9.6 days after surgery in the patients whose wounds broke down. A statistically significant relationship was found only between the open method of release and wound dehiscence (P < .05).
There was no statistically significant difference in the overall complication rate in the NFSGVHS population when comparing endoscopic with open carpal tunnel release or when comparing the risk of postoperative tendonitis, wound infection, or return to the OR.
Discussion
Carpal tunnel syndrome was documented by James Paget in mid-19th century in reference to a distal radius fracture.2 It is the most common peripheral nerve compression, with an incidence ranging from 1 to 3 cases per 1,000 subjects per year and a prevalence of 50 cases per 1,000 subjects per year.3 In an active-duty U.S. military population, the incidence of carpal tunnel syndrome is 3.98 per 1,000 person years.4
Related: Risk Factors for Postoperative Complications in Trigger Finger Release
The endoscopic method of release was first introduced in 1989 by Okutsu and colleagues.5 About 500,000 carpal tunnel releases are now performed in the U.S. every year, with 50,000 performed endoscopically.3 There were 185 carpal tunnel releases (56 endoscopic and 129 open) performed at the NFSGVHS in 2012.6 The minimally invasive procedure was designed to preserve the overlying skin and fascia, promoting an earlier return to work and daily activities. This is particularly relevant for manual workers who desire rapid return of grip strength. Multiple published reports have found more rapid recovery based on a reduction in scar tenderness, increase in grip strength, or return to work.7-13 Patients seem to have equivalent results over the long term, ranging from 3 months to 1 year.7,8,13-15 Return to work was not evaluated in this study, because many patients were either retired or not working steadily.
The endoscopic method was criticized after its introduction due to its potential increase in major structural injury to the median nerve, ulnar nerve, palmar arch, ulnar artery, or flexor tendons.16 A meta-analysis found improved outcomes but a statistically significant higher complication rate in endoscopic, compared with open release (2.2% in endoscopic vs 1.2% in open).16 Referral patterns have found iatrogenic nerve injury in patients referred by surgeons without formal hand fellowship training.17 There is a wide variety of background training for surgeons who may offer carpal tunnel release, including plastic surgery, orthopedics, general surgery, and neurosurgery.
Major structural injuries were reported by hand surgeons using both open and endoscopic methods in a questionnaire sent to members of the American Society for Surgery of the Hand, indicating that either approach demands respect.18 A large review of the literature from 1966 to 2001 by Benson and colleagues found that the endoscopic approach was not more likely to produce injury to tendons, arteries, or nerves compared with the open approach and actually had a lower rate of structural damage (0.49% vs 0.19%).19 Researchers who conducted this study confirmed one common digital nerve injury in an endoscopic converted to open technique, using a distally-based port with the blade not being deployed via the endoscopic method. The endoscopic method has been found to have a higher rate of reversible nerve injury (neuropraxia) compared with the open technique.7,10,19
The NFSGVHS results found a higher rate of wound dehiscence. More frequent wound site complications, particularly infection, hypertrophic scar, and scar tenderness have been noted using the open method.3,8,20 This is probably due to the deeper and slightly larger incision used for the open method compared with the smaller and shallower incisions used for the endoscopic release.
There is the inevitable learning curve for the endoscopic release due to the more complicated nature of the procedure. The NFSGVHS conversion rate was 23.7% over the 5-year period from 2005 to 2010. All 3 fellowship- trained hand surgeons were in their first year of practice at the time of the study, so the authors anticipate a lower conversion rate in forthcoming studies. The NFSGVHS researchers did not consider converting to an open technique to be a complication and believe it is appropriate to teach plastic surgery residents and fellows to have a low threshold to convert when visualization is not optimal and the potential for significant injury exists. The learning curve and a higher conversion rate have been acknowledged by Beck and colleagues with no increase in morbidity.21
The authors anticipated finding an increased rate of tendonitis in the endoscopic method, as found by Goshtasby and colleagues, where trigger finger was found more frequently in the endoscopic patients.22 The NFSGVHS study found that the number of patients presenting for steroid injections to treat postoperative tendonitis in the hand and wrist was not statistically significant when comparing the 2 surgical methods of release (3.3% in endoscopic vs 1.9% in open; P = .28).
The NFSGVHS rate of return to the OR within a year of surgery was 1.7%. The researchers from NFSGHVS anticipated a higher rate of return to the OR for ongoing symptoms secondary to incomplete release of the transverse carpal ligament. Published studies have found an intact retinaculum to be a cause of persistent symptoms when smaller incisions are used.23,24 Five endoscopic cases and 5 open cases eventually returned to the OR for carpal tunnel exploration. Two of the patients were classified as recurrent, because they had improvement of symptoms initially but presented > 6 months later with new symptoms. Eight of the patients were classified as persistent, because they did not have an extended period of relief of preoperative symptoms (Table 2).25 There was no statistically significant difference in return to the OR in the 2 study groups. The NFSGVHS researchers did note a trend in more incomplete nerve releases in the endoscopic group and more scar adhesions as the etiology of symptoms in the open group who went back to surgery.
Published studies have found no difference in overall complication rates when comparing the open with the endoscopic method of release, which is consistent with NFSGVHS data.8,11,12,26
A limitation of the current retrospective study is the large number of providers who both operated on the patients and documented their postoperative findings. The strength of the study is that VA patients tend to stay within the VISN for their health care so postoperative problems will be identified and routed to the plastic surgery service for evaluation and treatment.
Clinical implications for the NFSGVHS practice are that surgeons can confidently offer both the open and endoscopic surgeries without an overall risk of increased complications to patients. Patients who are identified as higher risk for wound dehiscence, such as those who place an unusual amount of pressure on their palms due to assisted walking devices or are at a higher risk of falling onto the surgical site, will be steered toward an endoscopic surgery. The NFSGVHS began a splinting protocol in the early postoperative period that was not previously used on those select patients who have open carpal tunnel releases.
Conclusion
Wound dehiscence was the only statistically significant complication found in the NFSGVHS veteran population when comparing open with endoscopic carpal tunnel release. This can potentially be prevented in future patients by delaying the removal of sutures and prolonging the use of a protective dressing in patients who undergo open release. There was not a statistically significant increase in overall complications when using the minimally invasive method of release, which is consistent with existing literature.
Acknowledgement
This material is the result of work supported with resources and the use of facilities at the Malcom Randall VAMC.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Carpal tunnel release is one of the most common hand surgeries performed at the North Florida/South Georgia Veterans Health System (NFSGVHS). Depending on surgeon experience and comfort level, surgeries are performed through either the traditional open method or the endoscopic method, single or double port (Figures 1 and 2). The advantage of the endoscopic method is faster recovery and return to work; however, the endoscopic method requires more expensive equipment and a steeper learning curve for surgeons. Complications are uncommon but can create unsatisfactory patient experiences because of costly lost workdays and long travel distances to the medical facility.
The purpose of this study was to compare the endoscopic method with the open carpal tunnel release method to determine whether there was an increased complication risk. Researchers anticipated that this information would help surgeons better inform patients of operative risks and prompt changes in NFSGVHS treatment plans to improve the quality of veteran care.
Methods
An Institutional Review Board- approved (#647-2011) retrospective review was done of patients who had carpal tunnel surgery performed by the NFSGVHS plastic surgery service from January 1, 2005, to December 31, 2010. Surgeries included in the review took place at the Malcom Randall VAMC in Gainesville and at the Lake City VAMC, both in Florida. Most of the surgeries included in the study were performed by a resident or fellow under the supervision of an attending physician. Eight different attending surgeons staffed the operations. Seven were board-certified or board-eligible plastic surgeons, 2 had advanced hand fellowship training, and 1 was a general surgeon with hand fellowship training. All hand fellowship-trained surgeons were in their first year of practice at the time of the study.
Only primary carpal tunnel releases were included in the study. Exclusion criteria included patients who were operated on by a service section other than the plastic surgery service (orthopedics or neurosurgery) and hands on which other procedures were performed during the same operation. Charts were reviewed for up to 1 year post surgery. Complications that required intervention were recorded. Researchers did not include pillar tenderness or an increase in occupational therapy visits as complications, due to the wide variety of patient tolerance to postoperative pain and varying motivation to return to work and daily routine.
Methods of release were endoscopic, open, or endoscopic converted to open. All but 6 of the completed endoscopic surgeries were performed using the double port Chow technique. The other 6 endoscopic surgeries were performed using the single port Agee technique at the distal wrist crease. There were 3 endoscopic converted to open cases that were performed using a single port, proximally-based technique in the midpalm. This method was abandoned after 3 unsuccessful endoscopic attempts, 1 resulting in digital nerve injury despite interactive cadaver labs prior to operative experience.
Endoscopic surgeries converted to open were recorded as open surgeries, because the patients had the full invasive experience. Researchers used the chi-square test and P value < .05 to compare the different methods of carpal tunnel release with identified complications.
Results and Complications
A total of 584 hands belonging to 452 patients were included in the study. Patients included 395 men and 57 women aged from 33 to 91 years. There were 271 endoscopic releases, 228 open releases, and 85 endoscopic converted to open releases. The NFSGVHS conversion rate was 23.7%. Complications in the converted cases (n = 4) were included in the open release results.
There were 40 complications in 38 hands. The overall complication rate was 6.5%. Complications noted were tendonitis presenting as De Quervain disease or trigger finger (9 endoscopic surgeries; 6 open surgeries), infection (2 endoscopic surgeries; 6 open surgeries), wound dehiscence (5 open surgeries), nerve injury (1 open surgery), respiratory distress (1 endoscopic), complex regional pain syndrome (1 open surgery), and scheduled returns to the operating room (OR) for recurrent, ongoing, or worsening symptoms (5 endoscopic surgeries; 5 open surgeries). Complications with an n > 1 were evaluated for statistical significance with P value < .05 (Table 1).
The NFSGVHS study had 10 patients return to the OR for open exploration (Table 2). Nine of these patients went back to the OR based on symptoms consistent with nerve conduction studies that had deteriorated compared with their preoperative studies. One endoscopic case was brought back to the OR for a suspected nerve injury without nerve conduction studies. Findings during reoperation included scar adhesions, incomplete release of ligaments, digital nerve injury, and negative explorations.
Two hypothenar fat transfers were performed to prevent scar adhesions in cases that had originally been open releases.1 Two of the open cases were endoscopic converted to open cases. One went back to the OR with a suspected nerve injury. Dense adhesions and an injured common digital nerve were identified and repaired. The second converted case that went back to the OR had a suspected, but unconfirmed, nerve injury to the motor branch. The diagnosis and treatment were delayed for more than a year due to the patient having other pressing medical and family concerns. An exploration found significant scar adhesions, and an opponensplasty was performed.
One patient had respiratory insufficiency secondary to chemical pneumonitis. The patient was sedated during an endoscopic carpal tunnel release, aspirated, and kept intubated in the intensive care unit until the morning after surgery.
An early complex regional pain syndrome diagnosis was made in a patient with underlying neuropathy and a preoperative “profound” median neuropathies diagnosis at the wrist with underlying peripheral neuropathy found on nerve conduction studies. The patient experienced an unusual amount of postoperative pain and edema after an uncomplicated open carpal tunnel release. This was treated with rapid intervention using anti- inflammatories and hand therapy. The patient also started a regimen of skin care, edema management, neuroreeducation, and contrast baths. Symptoms responded within a week.
There were 12 wound complications: 10 in open and 2 in endoscopic surgeries. Total wound complications were equally split between patients with and without diabetes. Infection and dehiscence were noted. Sutures were removed an average of 9.6 days after surgery in the patients whose wounds broke down. A statistically significant relationship was found only between the open method of release and wound dehiscence (P < .05).
There was no statistically significant difference in the overall complication rate in the NFSGVHS population when comparing endoscopic with open carpal tunnel release or when comparing the risk of postoperative tendonitis, wound infection, or return to the OR.
Discussion
Carpal tunnel syndrome was documented by James Paget in mid-19th century in reference to a distal radius fracture.2 It is the most common peripheral nerve compression, with an incidence ranging from 1 to 3 cases per 1,000 subjects per year and a prevalence of 50 cases per 1,000 subjects per year.3 In an active-duty U.S. military population, the incidence of carpal tunnel syndrome is 3.98 per 1,000 person years.4
Related: Risk Factors for Postoperative Complications in Trigger Finger Release
The endoscopic method of release was first introduced in 1989 by Okutsu and colleagues.5 About 500,000 carpal tunnel releases are now performed in the U.S. every year, with 50,000 performed endoscopically.3 There were 185 carpal tunnel releases (56 endoscopic and 129 open) performed at the NFSGVHS in 2012.6 The minimally invasive procedure was designed to preserve the overlying skin and fascia, promoting an earlier return to work and daily activities. This is particularly relevant for manual workers who desire rapid return of grip strength. Multiple published reports have found more rapid recovery based on a reduction in scar tenderness, increase in grip strength, or return to work.7-13 Patients seem to have equivalent results over the long term, ranging from 3 months to 1 year.7,8,13-15 Return to work was not evaluated in this study, because many patients were either retired or not working steadily.
The endoscopic method was criticized after its introduction due to its potential increase in major structural injury to the median nerve, ulnar nerve, palmar arch, ulnar artery, or flexor tendons.16 A meta-analysis found improved outcomes but a statistically significant higher complication rate in endoscopic, compared with open release (2.2% in endoscopic vs 1.2% in open).16 Referral patterns have found iatrogenic nerve injury in patients referred by surgeons without formal hand fellowship training.17 There is a wide variety of background training for surgeons who may offer carpal tunnel release, including plastic surgery, orthopedics, general surgery, and neurosurgery.
Major structural injuries were reported by hand surgeons using both open and endoscopic methods in a questionnaire sent to members of the American Society for Surgery of the Hand, indicating that either approach demands respect.18 A large review of the literature from 1966 to 2001 by Benson and colleagues found that the endoscopic approach was not more likely to produce injury to tendons, arteries, or nerves compared with the open approach and actually had a lower rate of structural damage (0.49% vs 0.19%).19 Researchers who conducted this study confirmed one common digital nerve injury in an endoscopic converted to open technique, using a distally-based port with the blade not being deployed via the endoscopic method. The endoscopic method has been found to have a higher rate of reversible nerve injury (neuropraxia) compared with the open technique.7,10,19
The NFSGVHS results found a higher rate of wound dehiscence. More frequent wound site complications, particularly infection, hypertrophic scar, and scar tenderness have been noted using the open method.3,8,20 This is probably due to the deeper and slightly larger incision used for the open method compared with the smaller and shallower incisions used for the endoscopic release.
There is the inevitable learning curve for the endoscopic release due to the more complicated nature of the procedure. The NFSGVHS conversion rate was 23.7% over the 5-year period from 2005 to 2010. All 3 fellowship- trained hand surgeons were in their first year of practice at the time of the study, so the authors anticipate a lower conversion rate in forthcoming studies. The NFSGVHS researchers did not consider converting to an open technique to be a complication and believe it is appropriate to teach plastic surgery residents and fellows to have a low threshold to convert when visualization is not optimal and the potential for significant injury exists. The learning curve and a higher conversion rate have been acknowledged by Beck and colleagues with no increase in morbidity.21
The authors anticipated finding an increased rate of tendonitis in the endoscopic method, as found by Goshtasby and colleagues, where trigger finger was found more frequently in the endoscopic patients.22 The NFSGVHS study found that the number of patients presenting for steroid injections to treat postoperative tendonitis in the hand and wrist was not statistically significant when comparing the 2 surgical methods of release (3.3% in endoscopic vs 1.9% in open; P = .28).
The NFSGVHS rate of return to the OR within a year of surgery was 1.7%. The researchers from NFSGHVS anticipated a higher rate of return to the OR for ongoing symptoms secondary to incomplete release of the transverse carpal ligament. Published studies have found an intact retinaculum to be a cause of persistent symptoms when smaller incisions are used.23,24 Five endoscopic cases and 5 open cases eventually returned to the OR for carpal tunnel exploration. Two of the patients were classified as recurrent, because they had improvement of symptoms initially but presented > 6 months later with new symptoms. Eight of the patients were classified as persistent, because they did not have an extended period of relief of preoperative symptoms (Table 2).25 There was no statistically significant difference in return to the OR in the 2 study groups. The NFSGVHS researchers did note a trend in more incomplete nerve releases in the endoscopic group and more scar adhesions as the etiology of symptoms in the open group who went back to surgery.
Published studies have found no difference in overall complication rates when comparing the open with the endoscopic method of release, which is consistent with NFSGVHS data.8,11,12,26
A limitation of the current retrospective study is the large number of providers who both operated on the patients and documented their postoperative findings. The strength of the study is that VA patients tend to stay within the VISN for their health care so postoperative problems will be identified and routed to the plastic surgery service for evaluation and treatment.
Clinical implications for the NFSGVHS practice are that surgeons can confidently offer both the open and endoscopic surgeries without an overall risk of increased complications to patients. Patients who are identified as higher risk for wound dehiscence, such as those who place an unusual amount of pressure on their palms due to assisted walking devices or are at a higher risk of falling onto the surgical site, will be steered toward an endoscopic surgery. The NFSGVHS began a splinting protocol in the early postoperative period that was not previously used on those select patients who have open carpal tunnel releases.
Conclusion
Wound dehiscence was the only statistically significant complication found in the NFSGVHS veteran population when comparing open with endoscopic carpal tunnel release. This can potentially be prevented in future patients by delaying the removal of sutures and prolonging the use of a protective dressing in patients who undergo open release. There was not a statistically significant increase in overall complications when using the minimally invasive method of release, which is consistent with existing literature.
Acknowledgement
This material is the result of work supported with resources and the use of facilities at the Malcom Randall VAMC.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Chrysopoulo MT, Greenberg JA, Kleinman WB. The hypothenar fat pad transposition flap: a modified surgical technique. Tech Hand Up Extrem Surg. 2006;10(3):150-156.
2. Paget J. Lectures on Surgical Pathology Delivered at the Royal College of Surgeons of England. London, England: Longman, Green, Brown, and Longmans; 1853.
3. Mintalucci DJ, Leinberry CF Jr. Open versus endoscopic carpal tunnel release. Orthop Clin North Am. 2012;43(4):431-437.
4. Wolf JM, Mountcastle S, Owens BD. Incidence of carpal tunnel syndrome in the US military population. Hand (NY). 2009;4(3):289-293.
5. Okutsu I, Ninomiya S, Takatori Y, Ugawa Y. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
6. U.S. Department of Veterans Affairs. Health Information Systems and Technology Architecture Database, Ambulatory Surgical Case Load Report, 2012. Accessed March 14, 2013.
7. Larsen MB, Sørensen AI, Crone KL, Weis T, Boeckstyns ME. Carpal tunnel release: a randomized comparison of three surgical methods. J Hand Surg Eur Vol. 2013;38(6):646-650.
8. Malhotra R, Kiran EK, Dua A, Mallinath SG, Bhan S. Endoscopic versus open carpal tunnel release: a short-term comparative study. Indian J Orthop. 2007;41(1):57-61.
9. Sabesan VJ, Pedrotty D, Urbaniak JR, Aldridge JM 3rd. An evidence-based review of a single surgeon’s experience with endoscopic carpal tunnel release. J Surg Orthop Adv. 2012;21(3):117-121.
10. Thoma A, Veltri K, Haines T, Duku E. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
11. Tian Y, Zhao H, Wang T. Prospective comparison of endoscopic and open surgical methods for carpal tunnel syndrome. Chin Med Sci J. 2007;22(2):104-107.
12. Trumble TE, Diao E, Abrams RA, Gilbert-Anderson MM. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84-A(7):1107-1115.
13. Vasiliadis HS, Xenakis TA, Mitsionis G, Paschos N, Georgoulis A. Endoscopic versus open carpal tunnel release. Arthroscopy. 2010:26(1):26-33.
14. Macdermid JC, Richards RS, Roth JH, Ross DC, King GJ. Endoscopic versus open carpal tunnel release: a randomized trial. J Hand Surg Am. 2003;28(3):475-480.
15. Aslani HR, Alizadeh K, Eajazi A, et al. Comparison of carpal tunnel release with three different techniques. Clin Neurol Neurosurg. 2012;114(7):965-968.
16. Kohanzadeh S, Herrera FA, Dobke M. Outcomes of open and endoscopic carpal tunnel release: a meta-analysis. Hand (NY). 2012;7(3):247-251.
17. Azari KK, Spiess AM, Buterbaugh GA, Imbriglia JE. Major nerve injuries associated with carpal tunnel release. Plast Reconstr Surg. 2007;119(6):1977-1978.
18. Palmer AK, Toivonen DA. Complications of endoscopic and open carpal tunnel release. J Hand Surg Am. 1999;24(3):561-565.
19. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
20. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
21. Beck JD, Deegan JH, Rhoades D, Klena JC. Results of endoscopic carpal tunnel release relative to surgeon experience with the Agee technique. J Hand Surg Am. 2011;36(1):61-64.
22. Goshtasby PH, Wheeler DR, Moy OJ. Risk factors for trigger finger occurrence after carpal tunnel release. Hand Surg. 2010;15(2):81-87.
23. Assmus H, Dombert T, Staub F. Reoperations for CTS because of recurrence or for correction [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):306-311.
24. Frik A, Baumeister RG. Re-intervention after carpal tunnel release [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):312-316.
25. Jones NF, Ahn HC, Eo S. Revision surgery for persistent and recurrent carpal tunnel syndrome and for failed carpal tunnel release. Plast Reconstr Surg. 2012;129(3):683-692.
26. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002:84(3):375-379.
1. Chrysopoulo MT, Greenberg JA, Kleinman WB. The hypothenar fat pad transposition flap: a modified surgical technique. Tech Hand Up Extrem Surg. 2006;10(3):150-156.
2. Paget J. Lectures on Surgical Pathology Delivered at the Royal College of Surgeons of England. London, England: Longman, Green, Brown, and Longmans; 1853.
3. Mintalucci DJ, Leinberry CF Jr. Open versus endoscopic carpal tunnel release. Orthop Clin North Am. 2012;43(4):431-437.
4. Wolf JM, Mountcastle S, Owens BD. Incidence of carpal tunnel syndrome in the US military population. Hand (NY). 2009;4(3):289-293.
5. Okutsu I, Ninomiya S, Takatori Y, Ugawa Y. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
6. U.S. Department of Veterans Affairs. Health Information Systems and Technology Architecture Database, Ambulatory Surgical Case Load Report, 2012. Accessed March 14, 2013.
7. Larsen MB, Sørensen AI, Crone KL, Weis T, Boeckstyns ME. Carpal tunnel release: a randomized comparison of three surgical methods. J Hand Surg Eur Vol. 2013;38(6):646-650.
8. Malhotra R, Kiran EK, Dua A, Mallinath SG, Bhan S. Endoscopic versus open carpal tunnel release: a short-term comparative study. Indian J Orthop. 2007;41(1):57-61.
9. Sabesan VJ, Pedrotty D, Urbaniak JR, Aldridge JM 3rd. An evidence-based review of a single surgeon’s experience with endoscopic carpal tunnel release. J Surg Orthop Adv. 2012;21(3):117-121.
10. Thoma A, Veltri K, Haines T, Duku E. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
11. Tian Y, Zhao H, Wang T. Prospective comparison of endoscopic and open surgical methods for carpal tunnel syndrome. Chin Med Sci J. 2007;22(2):104-107.
12. Trumble TE, Diao E, Abrams RA, Gilbert-Anderson MM. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84-A(7):1107-1115.
13. Vasiliadis HS, Xenakis TA, Mitsionis G, Paschos N, Georgoulis A. Endoscopic versus open carpal tunnel release. Arthroscopy. 2010:26(1):26-33.
14. Macdermid JC, Richards RS, Roth JH, Ross DC, King GJ. Endoscopic versus open carpal tunnel release: a randomized trial. J Hand Surg Am. 2003;28(3):475-480.
15. Aslani HR, Alizadeh K, Eajazi A, et al. Comparison of carpal tunnel release with three different techniques. Clin Neurol Neurosurg. 2012;114(7):965-968.
16. Kohanzadeh S, Herrera FA, Dobke M. Outcomes of open and endoscopic carpal tunnel release: a meta-analysis. Hand (NY). 2012;7(3):247-251.
17. Azari KK, Spiess AM, Buterbaugh GA, Imbriglia JE. Major nerve injuries associated with carpal tunnel release. Plast Reconstr Surg. 2007;119(6):1977-1978.
18. Palmer AK, Toivonen DA. Complications of endoscopic and open carpal tunnel release. J Hand Surg Am. 1999;24(3):561-565.
19. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
20. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
21. Beck JD, Deegan JH, Rhoades D, Klena JC. Results of endoscopic carpal tunnel release relative to surgeon experience with the Agee technique. J Hand Surg Am. 2011;36(1):61-64.
22. Goshtasby PH, Wheeler DR, Moy OJ. Risk factors for trigger finger occurrence after carpal tunnel release. Hand Surg. 2010;15(2):81-87.
23. Assmus H, Dombert T, Staub F. Reoperations for CTS because of recurrence or for correction [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):306-311.
24. Frik A, Baumeister RG. Re-intervention after carpal tunnel release [article in German]. Handchir Mikrochir Plast Chir. 2006;38(5):312-316.
25. Jones NF, Ahn HC, Eo S. Revision surgery for persistent and recurrent carpal tunnel syndrome and for failed carpal tunnel release. Plast Reconstr Surg. 2012;129(3):683-692.
26. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002:84(3):375-379.
An Important Use of a National Joint Registry
An Important Use of a National Joint Registry
I enjoyed the 2 articles on the issue of “Orthopedic Registries” by Dr. Sarmiento and Dr. Mont and colleagues in the April 2015 issue of The American Journal of Orthopedics (pages 159-162). Both authors have valid points, but I think they both miss what is to me the most important use of a national registry. It is for identifying an old prosthesis.
Many times in my 35-plus years of practice, I have seen patients that need revision hips or knees that were initially done 15 or 20 years ago. It would be extremely helpful if the physician could call the registry with the patient’s name, Social Security number, birth date, and approximate date of surgery to find out what prosthesis was used—specifically, the size and manufacturer. So often the implanting surgeon has retired and the hospital where the patient thinks he or she had the surgery is closed or cannot find old records.
James C. Cobey, MD, MPH, FACS
Washington, DC
Authors’ Responses
Dr. Cobey should be congratulated for expressing his sincere concern and suggestion regarding the national registry dealing with long-term follow-up of total joint implants.
However, I think that the registry must maintain a consistent evaluation criterion throughout. Needless to say, adherence to it is essential when addressing revision surgery. Dr. Cobey’s proposal would allow a possibly large number of patients to enter the registry without meeting the established criterion. They would enter without having provided truly relevant information, such as history of infection, trauma, fracture, recurrent dislocations, wear, lysis, etc, which are the most common conditions leading to revision surgery. The data from patients entering with only the minimal information proposed by Dr. Cobey—date of birth, size of the prosthesis, and name of the manufacturer—is meaningless. It could even be harmful by trivializing and weakening whatever sound goals the national registry hopes to reach.
On the other hand, if Dr. Cobey’s suggestion is favorably considered by the registry’s leaders and its value is felt to be potentially significant, the issue should be seriously studied and debated prior to its implementation.
Augusto Sarmiento, MD
Coral Gables, FL
We would like to thank Dr. Cobey for his comments and thoughts regarding the American Joint Replacement Registry (AJRR). We wholeheartedly agree that an important purpose of this effort is to provide hospital staff and surgeons with as much information as possible regarding our patients. Incorporating information on previous surgeries, and specifically, previous prostheses that have been implanted, is no exception.
The registry is a process that requires the gradual accumulation of data. The AJRR has collected level I data, which, from a 2011 article in AAOS Now, “is an institutional responsibility and includes several core data elements, such as patient data (name, sex, date of birth, social security number, ICD-9 code for diagnosis), surgeon data (name, number of surgeries performed), procedure data (ICD-I code for type of surgery, date of surgery, patient age at surgery, laterality, implant), and hospital data (name, address, number of surgeries performed there). Each patient, surgeon, and hospital has a unique identifier, which enables index procedures to be linked to subsequent events, permits patients to access their own information, allows data to be linked to other databases, and helps maintain confidentiality.”1 Therefore, it would certainly be possible for a surgeon to collect the data that Dr. Cobey has mentioned, which would be “extremely helpful.”
In addition, as the AJRR continues to evolve its component element database, identification of implants will become easier. Also, collaborative efforts are underway with the International Society of Arthroplasty Registries (ISAR) to expand and harmonize data collection, including the recognition of implants.2 The US Food and Drug Administration has also proposed the incorporation of unique device identifiers into patient medical records, although this is a concept that remains in debate with the Centers for Medicare & Medicaid Services (CMS).3
We would like to thank Dr. Sarmiento and Dr. Cobey for their contributions to this discussion, and we welcome any ongoing suggestions and queries to improve the development of the AJRR.
Randa K. Elmallah, MD
Baltimore, MD
Bryan D. Springer, MD
Charlotte, NC
Michael A. Mont, MD
Baltimore, MD
1. Porucznik MA. AJRR completes data collection pilot project. AAOS Now. 2011;5(8). http://www.aaos.org/news/aaosnow/aug11/advocacy1.asp. Accessed May 5, 2015.
2. McKee J. Arthroplasty registries expand around the world. AAOS Now. 2014;8(4). http://www.aaos.org/news/aaosnow/apr14/research6.asp. Accessed May 5, 2015.
3. Enriquez J. FDA, CMS at odds over unique device identification (UDI) implementation. Med Device Online. http://www.meddeviceonline.com/doc/fda-cms-at-odds-over-unique-device-identification-udi-implementation-0001. Published March 12, 2015. Accessed May 5, 2015.
An Important Use of a National Joint Registry
I enjoyed the 2 articles on the issue of “Orthopedic Registries” by Dr. Sarmiento and Dr. Mont and colleagues in the April 2015 issue of The American Journal of Orthopedics (pages 159-162). Both authors have valid points, but I think they both miss what is to me the most important use of a national registry. It is for identifying an old prosthesis.
Many times in my 35-plus years of practice, I have seen patients that need revision hips or knees that were initially done 15 or 20 years ago. It would be extremely helpful if the physician could call the registry with the patient’s name, Social Security number, birth date, and approximate date of surgery to find out what prosthesis was used—specifically, the size and manufacturer. So often the implanting surgeon has retired and the hospital where the patient thinks he or she had the surgery is closed or cannot find old records.
James C. Cobey, MD, MPH, FACS
Washington, DC
Authors’ Responses
Dr. Cobey should be congratulated for expressing his sincere concern and suggestion regarding the national registry dealing with long-term follow-up of total joint implants.
However, I think that the registry must maintain a consistent evaluation criterion throughout. Needless to say, adherence to it is essential when addressing revision surgery. Dr. Cobey’s proposal would allow a possibly large number of patients to enter the registry without meeting the established criterion. They would enter without having provided truly relevant information, such as history of infection, trauma, fracture, recurrent dislocations, wear, lysis, etc, which are the most common conditions leading to revision surgery. The data from patients entering with only the minimal information proposed by Dr. Cobey—date of birth, size of the prosthesis, and name of the manufacturer—is meaningless. It could even be harmful by trivializing and weakening whatever sound goals the national registry hopes to reach.
On the other hand, if Dr. Cobey’s suggestion is favorably considered by the registry’s leaders and its value is felt to be potentially significant, the issue should be seriously studied and debated prior to its implementation.
Augusto Sarmiento, MD
Coral Gables, FL
We would like to thank Dr. Cobey for his comments and thoughts regarding the American Joint Replacement Registry (AJRR). We wholeheartedly agree that an important purpose of this effort is to provide hospital staff and surgeons with as much information as possible regarding our patients. Incorporating information on previous surgeries, and specifically, previous prostheses that have been implanted, is no exception.
The registry is a process that requires the gradual accumulation of data. The AJRR has collected level I data, which, from a 2011 article in AAOS Now, “is an institutional responsibility and includes several core data elements, such as patient data (name, sex, date of birth, social security number, ICD-9 code for diagnosis), surgeon data (name, number of surgeries performed), procedure data (ICD-I code for type of surgery, date of surgery, patient age at surgery, laterality, implant), and hospital data (name, address, number of surgeries performed there). Each patient, surgeon, and hospital has a unique identifier, which enables index procedures to be linked to subsequent events, permits patients to access their own information, allows data to be linked to other databases, and helps maintain confidentiality.”1 Therefore, it would certainly be possible for a surgeon to collect the data that Dr. Cobey has mentioned, which would be “extremely helpful.”
In addition, as the AJRR continues to evolve its component element database, identification of implants will become easier. Also, collaborative efforts are underway with the International Society of Arthroplasty Registries (ISAR) to expand and harmonize data collection, including the recognition of implants.2 The US Food and Drug Administration has also proposed the incorporation of unique device identifiers into patient medical records, although this is a concept that remains in debate with the Centers for Medicare & Medicaid Services (CMS).3
We would like to thank Dr. Sarmiento and Dr. Cobey for their contributions to this discussion, and we welcome any ongoing suggestions and queries to improve the development of the AJRR.
Randa K. Elmallah, MD
Baltimore, MD
Bryan D. Springer, MD
Charlotte, NC
Michael A. Mont, MD
Baltimore, MD
An Important Use of a National Joint Registry
I enjoyed the 2 articles on the issue of “Orthopedic Registries” by Dr. Sarmiento and Dr. Mont and colleagues in the April 2015 issue of The American Journal of Orthopedics (pages 159-162). Both authors have valid points, but I think they both miss what is to me the most important use of a national registry. It is for identifying an old prosthesis.
Many times in my 35-plus years of practice, I have seen patients that need revision hips or knees that were initially done 15 or 20 years ago. It would be extremely helpful if the physician could call the registry with the patient’s name, Social Security number, birth date, and approximate date of surgery to find out what prosthesis was used—specifically, the size and manufacturer. So often the implanting surgeon has retired and the hospital where the patient thinks he or she had the surgery is closed or cannot find old records.
James C. Cobey, MD, MPH, FACS
Washington, DC
Authors’ Responses
Dr. Cobey should be congratulated for expressing his sincere concern and suggestion regarding the national registry dealing with long-term follow-up of total joint implants.
However, I think that the registry must maintain a consistent evaluation criterion throughout. Needless to say, adherence to it is essential when addressing revision surgery. Dr. Cobey’s proposal would allow a possibly large number of patients to enter the registry without meeting the established criterion. They would enter without having provided truly relevant information, such as history of infection, trauma, fracture, recurrent dislocations, wear, lysis, etc, which are the most common conditions leading to revision surgery. The data from patients entering with only the minimal information proposed by Dr. Cobey—date of birth, size of the prosthesis, and name of the manufacturer—is meaningless. It could even be harmful by trivializing and weakening whatever sound goals the national registry hopes to reach.
On the other hand, if Dr. Cobey’s suggestion is favorably considered by the registry’s leaders and its value is felt to be potentially significant, the issue should be seriously studied and debated prior to its implementation.
Augusto Sarmiento, MD
Coral Gables, FL
We would like to thank Dr. Cobey for his comments and thoughts regarding the American Joint Replacement Registry (AJRR). We wholeheartedly agree that an important purpose of this effort is to provide hospital staff and surgeons with as much information as possible regarding our patients. Incorporating information on previous surgeries, and specifically, previous prostheses that have been implanted, is no exception.
The registry is a process that requires the gradual accumulation of data. The AJRR has collected level I data, which, from a 2011 article in AAOS Now, “is an institutional responsibility and includes several core data elements, such as patient data (name, sex, date of birth, social security number, ICD-9 code for diagnosis), surgeon data (name, number of surgeries performed), procedure data (ICD-I code for type of surgery, date of surgery, patient age at surgery, laterality, implant), and hospital data (name, address, number of surgeries performed there). Each patient, surgeon, and hospital has a unique identifier, which enables index procedures to be linked to subsequent events, permits patients to access their own information, allows data to be linked to other databases, and helps maintain confidentiality.”1 Therefore, it would certainly be possible for a surgeon to collect the data that Dr. Cobey has mentioned, which would be “extremely helpful.”
In addition, as the AJRR continues to evolve its component element database, identification of implants will become easier. Also, collaborative efforts are underway with the International Society of Arthroplasty Registries (ISAR) to expand and harmonize data collection, including the recognition of implants.2 The US Food and Drug Administration has also proposed the incorporation of unique device identifiers into patient medical records, although this is a concept that remains in debate with the Centers for Medicare & Medicaid Services (CMS).3
We would like to thank Dr. Sarmiento and Dr. Cobey for their contributions to this discussion, and we welcome any ongoing suggestions and queries to improve the development of the AJRR.
Randa K. Elmallah, MD
Baltimore, MD
Bryan D. Springer, MD
Charlotte, NC
Michael A. Mont, MD
Baltimore, MD
1. Porucznik MA. AJRR completes data collection pilot project. AAOS Now. 2011;5(8). http://www.aaos.org/news/aaosnow/aug11/advocacy1.asp. Accessed May 5, 2015.
2. McKee J. Arthroplasty registries expand around the world. AAOS Now. 2014;8(4). http://www.aaos.org/news/aaosnow/apr14/research6.asp. Accessed May 5, 2015.
3. Enriquez J. FDA, CMS at odds over unique device identification (UDI) implementation. Med Device Online. http://www.meddeviceonline.com/doc/fda-cms-at-odds-over-unique-device-identification-udi-implementation-0001. Published March 12, 2015. Accessed May 5, 2015.
1. Porucznik MA. AJRR completes data collection pilot project. AAOS Now. 2011;5(8). http://www.aaos.org/news/aaosnow/aug11/advocacy1.asp. Accessed May 5, 2015.
2. McKee J. Arthroplasty registries expand around the world. AAOS Now. 2014;8(4). http://www.aaos.org/news/aaosnow/apr14/research6.asp. Accessed May 5, 2015.
3. Enriquez J. FDA, CMS at odds over unique device identification (UDI) implementation. Med Device Online. http://www.meddeviceonline.com/doc/fda-cms-at-odds-over-unique-device-identification-udi-implementation-0001. Published March 12, 2015. Accessed May 5, 2015.
Knee Extensor Mechanism Reconstruction With Complete Extensor Allograft After Failure of Patellar Tendon Repair
The extensor mechanism of the knee comprises the quadriceps tendon, the patella, and the patellar tendon. The extensor mechanism may be damaged by injury to these structures, with consequences such as the inability to actively extend the knee and hemarthrosis.1,2 Disruption of this mechanism is rare, and the most common injury pattern is an eccentric contraction of the quadriceps tendon on a flexed knee causing a tendon (quadriceps or patellar) rupture or a patella fracture.1,2
Patellar tendon ruptures are more common in persons younger than 40 years.1 Treatment is surgical, regardless of age and physical activity. In the acute setting, repair can be end-to-end suture or transosseous tunnel insertion. End-to-end suturing is difficult in chronic patellar tendon ruptures because of patella alta secondary to quadriceps contraction.3 Treatment options for chronic ruptures may involve transpatellar traction4 or tendon reinforcement with fascia lata, a semitendinosus band, or synthetic materials.3-5 Alternatively, tendon autograft and allografts have also been recommended, especially in extreme situations.1,6 Furthermore, animal experiments have shown that a compact platelet-rich fibrin scaffold (CPFS) has the potential to accelerate healing of patellar tendon defects and to act as a bioscaffold for graft augmentation.7
We describe the case of a 30-year-old man who underwent extensor mechanism reconstruction with cadaveric tendon–patellar tendon–bone allograft for failure of an infected primary end-to-end repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old healthy man landed on an empty glass fish tank, resulting in a traumatic right-knee arthrotomy. On initial evaluation, the patient had a negative straight-leg-raise test and impaired knee extension. The patient was taken urgently to the operating room for irrigation and débridement and concurrent repair of the patellar tendon laceration. Antibiotic prophylaxis with 2 g of intravenous (IV) cefazolin was given in the emergency room.
Intraoperatively, after visualizing the patellar tendon laceration and excluding any associated chondral lesions, we proceeded with extensive débridement and irrigation using 9 L of normal saline pulse lavage. After we achieved a clean site, we proceeded to repair the patellar tendon using No. 2 FiberWire sutures (Arthrex, Naples, Florida) with a classic Krackow repair8 consisting of 2 sutures run in a 4-row fashion through the patella and the patellar tendon. The suture was securely tightened and then tested for stability to at least 90° of knee flexion. The retinaculum was repaired using No. 0 Vicryl sutures (Ethicon, Somerville, New Jersey). After wound closure and dressing, the patient was placed in a hinged knee brace locked in extension at all times after surgery. Antibiotic treatment with IV cefazolin was administered for 48 hours.
Postoperative management consisted of weight-bearing as tolerated on the operative limb and appropriate deep venous thrombosis prophylaxis. The patient followed up in clinic 2 weeks and 4 weeks after surgery. At 4 weeks, the patient was noted to have a secondary wound infection with superficial dehiscence and serosanguineous drainage. No wound opening was noticed, and local wound care was performed with a 1-week course of oral cephalexin. The patient was scheduled to follow up a few weeks later but did not follow up for a year.
At 1-year follow-up, the patient reported that he had had a steady progression of his knee range of motion (ROM) with decreased pain. However, over time, the patient noted subjective instability of the knee, with frequent falls occurring close to his 1-year follow-up. Examination of his knee showed that his active ROM ranged from 20° in extension to 120° in flexion, with a weak extensor mechanism. Passively, his knee could be brought to full extension. His incision was well healed, but it had an area of bogginess in the middle. Radiographs showed patella alta on the affected knee, with a lengthening of the patellar tendon of 7.70 cm on the right compared with 5.18 cm on the left. Magnetic resonance imaging (MRI) showed moderate-to-severe patellar tendinosis with small fluid pockets around the surgical material and evidence of acute patellar enthesopathy. The laboratory values showed a white blood cell count of 7580/μL (normal, 4500-11,000/μL), an erythrocyte sedimentation rate of 2 mm/h (normal, 1-15 mm/h), and a C-reactive protein level of 1.93 mg/dL (normal, 0.00-0.29 mg/dL). Based on the clinical examination and imaging findings, there was a concern for a possible chronic deep-tissue infection, in addition to failure of the primary patellar tendon repair. Operative versus nonoperative management options were discussed with the patient, and he elected to undergo surgery.
During surgery, the patellar laxity was confirmed, and the patellar tendon was noticed to be chronically thickened and surrounded by unhealthy tissue. Initially, an extensive soft-tissue débridement was performed, and all patellar tendon loculations visualized on the preoperative MRI were drained; a solid purulent-like fluid was expressed. Unfortunately, the extensive and required débridement did not allow the preservation of the patellar tendon. Appropriate cultures were taken and sent for immediate Gram-stain analysis, which returned negative. Tissue samples from the patellar tendon were also sent to the pathology department for analysis. Intraoperatively, the infrapatellar defect was filled temporarily with a tobramycin cement spacer mixed with 2 g of vancomycin in a manner similar to that of the Masquelet technique used for infected long-bone nonunions with bone loss.9,10 This technique is a 2-stage procedure that promotes the formation of a biologic membrane that allows bone healing in the reconstruction of long-bone defects. The first stage consists of a radical débridement with soft-tissue repair by flaps when needed, with the insertion of a polymethylmethacrylate cement spacer into the bone defect. The second stage is usually performed 6 to 8 weeks later, with removal of the spacer and preservation of the induced membrane, which is filled with iliac crest bone autograft augmented (if necessary) with demineralized allograft.
The incision was closed primarily, and after surgery, the patient was allowed to bear weight as tolerated in a hinged knee brace locked in extension. Final laboratory analysis from cultures and tissue samples revealed acute and chronic inflammation with more than 20 neutrophils per high-powered field. No organisms grew from aerobic, anaerobic, fungal, or mycobacterial cultures. The infectious disease service was consulted and recommended oral cephalexin.
Because all cultures were negative, all laboratory examinations did not indicate any residual infections, and no bony involvement was noticed intraoperatively or in the preoperative knee MRI, we decided to proceed with the second stage of the Masquelet technique after 2 weeks. The patient returned to the operating room for final reconstruction of his patellar tendon using a custom-ordered cadaveric tendon–patellar tendon–bone allograft, the length of which was determined by measuring the contralateral patellar tendon, ie, 5.18 cm (Figure 1A). The previous anterior knee incision was reopened and extended distally past the tibial tuberosity and proximally toward the quadriceps tendon. The antibiotic spacer was removed. We proceeded with a repeat irrigation and débridement and the allograft transfer. The selected allograft was customized by reducing the tibial bone component to an approximately 1×2-cm bone block and by reducing the allograft patellar thickness with an oscillating saw, leaving an approximately 2-mm thick patellar bone graft attached to the patellar tendon. In a similar technique using an oscillating saw, we shaved off the anterior cortex of the patient’s patella to accommodate, in a sandwich fashion, the patellar allograft. Proximally, the quadriceps tendon insertion was split longitudinally and partially separated from the superior pole of the patellar tendon to allow seating and fixation of the modified quadriceps allograft tendon component.
We proceeded with the fixation of the allograft first distally on the patella. The anterior cortex of the tibial tuberosity was resected to allow the perfect seating of the bone block allograft. The graft was secured with a 4.0-mm fully threaded cancellous lag screw and reinforced with a 2.4-mm, 3-hole T-volar buttress plate (Synthes, Paoli, Pennsylvania). The plate was contoured to better fit the patient’s tibia. We sutured the patellar allograft tendon to the patella using two No. 2-0 FiberWire sutures in Krackow suture technique8 (Figures 1B, 1C). We obtained good fixation of the patellar tendon, and the distance between the patellar insertion and the inferior patellar pole was the same as before surgery: 5.57 cm and comparable to the contralateral side (Figures 2A-2C). The patellar allograft and autograft sandwich were secured with additional No. 2-0 FiberWire sutures, and the quadriceps allograft and autograft were secured with the cross-stitch technique with the same material. Fine suturing of the quadriceps tendon was done with No. 0 Vicryl sutures. After the fixation was completed, we tested the stability of the reconstruction and found good flexion up to 120°.
The postoperative protocol consisted of weight-bearing as tolerated in full extension and passive knee ROM, using a continuous passive ROM machine from 0° to 45° for the first 4 weeks, followed by active ROM, increased as tolerated, during the next 8 weeks.
The patient was seen in clinic 3 and 9 months after surgery. At the 3-month follow-up appointment, the patient’s examination showed knee ROM from 0° extension to 130° of flexion, no secondary infection signs, and radiographic evidence of a well-healing patellar allograft with symmetric patellar tendon length to the contralateral side. At 9-month follow-up, the patient’s active ROM was from 0° extension to 140° flexion (Figures 3A, 3B), and he had returned to his preinjury level of functioning.
Discussion
This case report describes the successful reconstruction of a patellar tendon defect with cadaveric tendon–patellar tendon–bone allograft. Extensor mechanism injuries are uncommon in general, and the incidence of patellar tendon injury is higher in men than in women.2 Patellar tendon tears occur frequently in active patients younger than 40 years, usually as a result of sudden quadriceps contraction with the knee slightly flexed.1 Treatment of patellar tendon injury is surgical, and functional outcomes for patients with this injury are equivalent to those of patients with quadriceps tendon injuries or patellar fractures.2 Acute patellar tendon tears can be repaired by end-to-end suturing or transosseous tunnel insertion in the tibia or patella.1 Reinforcement is often added between the patella and tibial tuberosity, using a semitendinosus band or wire.1 End-to-end suture is performed using a thick resorbable suture. It is important to avoid patella alta during suturing, comparing the position of the patella with the contralateral patella with the knee in 45° of flexion. In proximal avulsion, the tendon is anchored to the bone by 2 thick nonresorbable sutures through 2 parallel bone tunnels to the proximal pole of the patella. Distal avulsion is rare in adults, but it can be managed by using staples or suture anchors.1
End-to-end suturing of chronic patellar tendon defects is difficult more than 45 days after injury primarily because of difficulties in correcting patella alta secondary to the upward force exerted by the quadriceps tendon.1,3 Extreme situations similar to the case we present warrant Achilles or patellar tendon allograft for reconstruction of the extensor mechanism.1,3,6,9
Extensor mechanism allograft also provides an effective remedy for severe quadriceps deficiency caused by loss of the patella, patellar tendon, and quadriceps tendon in total knee arthroplasty.10 However, in such cases, late failure is common, and major quadriceps deficiency occurs after removal of the allograft material.10 To improve outcome, a novel technique using the medial gastrocnemius muscle transferred to the muscular portion of the vastus medialis and lateralis flaps provides a secure and strong closure of the anterior knee, thereby restoring the extensor mechanism of the knee.10
Patellar tendon reconstruction with allograft tissue has been successfully used, especially in cases related to chronic patellar tendon ruptures11 and total knee arthroplasty.6,12-14 Crossett and colleagues12 showed that, at 2-year follow-up, the average knee score for pain, ROM, and stability had improved from 26 points (range, 6-39 points) before surgery to 81 points (range, 40-92 points). The average knee score for function had also improved: 14 points (range, 0-35 points) before surgery to 53 points (range, 30-90 points).12 Primary repair may succeed in early intervention, but in an established rupture, allograft reconstruction is often necessary. Achilles tendon is the preferred allograft, with the calcaneus fragment embedded into the proximal tibia as a new tubercle and the tendon sutured into the remaining extensor mechanism.1,11 The repair is further protected using a cable loop from the superior pole of the patella to a drill hole in the upper tibia.9 Techniques have also been described involving passage of the proximal aspect of the allograft tendon through patellar bone tunnels and suture fixation to the native quadriceps tendon.11,15 However, in our technique, we shaved off the anterior cortex of the patient’s patella to allow a sandwich-type over-position of the allograft to secure fixation to the patella.
Another alternative to allograft reconstruction involves biocompatible scaffolds. Such scaffolds incorporate the use of platelets in a fibrin framework. A CPFS, produced from blood and calcium gluconate to improve healing of patellar tendon defects, has been described in animal studies.7 In the rabbit model, CPFS acts as a provisional bioscaffold that can accelerate healing of an injured patellar tendon repair, potentially secondary to several growth factors derived from platelets.7 Platelets are biocompatible sources of growth factors, and CPFS can act as a scaffold to restore the mechanical integrity of injured soft tissue.7,16 In addition, CPFS can act to lower donor-site morbidity associated with harvesting tissue autograft.7 However, to our knowledge, such scaffolds have not been used in human trials. The LARS biocompatible ligament (Corin Group PLC, Cirencester, United Kingdom), currently not approved by the US Food and Drug Administration, is used for reconstructions of isolated or multiple knee ligament injuries.17 This graft requires the presence of healthy tissue with good blood supply from which new tendon or ligament can grow in. Sometimes it is also used for extensor mechanism reconstruction after radical tumor resection around the knee; however, good results are achieved in only 59% of cases,18 and to our knowledge, only 1 case of primary repair of a patellar tendon rupture has been published.19
Techniques involving the use of tendon–patellar tendon–bone graft with fixation via the sandwich-type over-position of the allograft for chronic patellar tendon rupture have not been described in the literature. In our patient, given the extensive patellar tendon lesion and inflammation with chronic tissue degeneration, there was no option but to use allograft. To improve the patient’s outcome, we chose the strongest possible allograft, tendon–patellar tendon–bone graft.
Conclusion
Revision patellar tendon reconstruction is a challenging, but necessary, procedure to restore the extensor mechanism of the knee, especially in young, active individuals. Various options to reconstruct the tissue defects are available. Our patient was successfully treated with a tendon–patellar tendon–bone allograft reconstruction.
1. Saragaglia D, Pison A, Rubens-Duval B. Acute and old ruptures of the extensor apparatus of the knee in adults (excluding knee replacement). Orthop Traumatol Surg Res. 2013;99(1 suppl):S67-S76.
2. Tejwani NC, Lekic N, Bechtel C, Montero N, Egol KA. Outcomes after knee joint extensor mechanism disruptions: is it better to fracture the patella or rupture the tendon? J Orthop Trauma. 2012;26(11):648-651.
3. Ecker ML, Lotke PA, Glazer RM. Late reconstruction of the patellar tendon. J Bone Joint Surg Am. 1979;61(6):884-886.
4. Siwek CW, Rao JP. Ruptures of the extensor mechanism of the knee joint. J Bone Joint Surg Am. 1981;63(6):932-937.
5. Levy M, Goldstein J, Rosner M. A method of repair for quadriceps tendon or patellar ligament (tendon) ruptures without cast immobilization. Preliminary report. Clin Orthop Relat Res. 1987;218:297-301.
6. Burks RT, Edelson RH. Allograft reconstruction of the patellar ligament. A case report. J Bone Joint Surg Am. 1994;76(7):1077-1079.
7. Matsunaga D, Akizuki S, Takizawa T, Omae S, Kato H. Compact platelet-rich fibrin scaffold to improve healing of patellar tendon defects and for medial collateral ligament reconstruction. Knee. 2013;20(6):545-550.
8. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
9. Brooks P. Extensor mechanism ruptures. Orthopedics. 2009;32(9):683-684.
10. Whiteside LA. Surgical technique: muscle transfer restores extensor function after failed patella-patellar tendon allograft. Clin Orthop Relat Res. 2014;472(1):218-226.
11. Farmer K, Cosgarea AJ. Procedure 25. Acute and chronic patellar tendon ruptures. In: Miller MD, Cole BJ, Cosgarea AJ, Sekiya JK, eds. Operative Techniques: Sports Knee Surgery. Philadelphia, PA: Saunders (Elsevier); 2008:397-417.
12. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
13. Lahav A, Burks RT, Scholl MD. Allograft reconstruction of the patellar tendon: 12-year follow-up. Am J Orthop. 2004;33(12):623-624.
14. Yoo JH, Chang JD, Seo YJ, Baek SW. Reconstruction of a patellar tendon with Achilles tendon allograft for severe patellar infera--a case report. Knee. 2011;18(5):350-353.
15. Saldua NS, Mazurek MT. Procedure 37. Quadriceps and patellar tendon repair. In: Reider B, Terry MA, Provencher MT, eds. Operative Techniques: Sports Medicine Surgery. Philadelphia, PA: Saunders (Elsevier); 2010:623-640.
16. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4-15.
17. Ibrahim SAR, Ahmad FHF, Salah M, Al Misfer ARK, Ghaffer SA, Khirat S. Surgical management of traumatic knee dislocation. Arthroscopy. 2008;24(2):178-187.
18. Dominkus M, Sabeti M, Toma C, Abdolvahab F, Trieb K, Kotz RI. Reconstructing the extensor apparatus with a new polyester ligament. Clin Orthop Relat Res. 2006;453:328-334.
19. Naim S, Gougoulias N, Griffiths D. Patellar tendon reconstruction using LARS ligament: surgical technique and case report. Strategies Trauma Limb Reconstr. 2011;6(1):39-41.
The extensor mechanism of the knee comprises the quadriceps tendon, the patella, and the patellar tendon. The extensor mechanism may be damaged by injury to these structures, with consequences such as the inability to actively extend the knee and hemarthrosis.1,2 Disruption of this mechanism is rare, and the most common injury pattern is an eccentric contraction of the quadriceps tendon on a flexed knee causing a tendon (quadriceps or patellar) rupture or a patella fracture.1,2
Patellar tendon ruptures are more common in persons younger than 40 years.1 Treatment is surgical, regardless of age and physical activity. In the acute setting, repair can be end-to-end suture or transosseous tunnel insertion. End-to-end suturing is difficult in chronic patellar tendon ruptures because of patella alta secondary to quadriceps contraction.3 Treatment options for chronic ruptures may involve transpatellar traction4 or tendon reinforcement with fascia lata, a semitendinosus band, or synthetic materials.3-5 Alternatively, tendon autograft and allografts have also been recommended, especially in extreme situations.1,6 Furthermore, animal experiments have shown that a compact platelet-rich fibrin scaffold (CPFS) has the potential to accelerate healing of patellar tendon defects and to act as a bioscaffold for graft augmentation.7
We describe the case of a 30-year-old man who underwent extensor mechanism reconstruction with cadaveric tendon–patellar tendon–bone allograft for failure of an infected primary end-to-end repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old healthy man landed on an empty glass fish tank, resulting in a traumatic right-knee arthrotomy. On initial evaluation, the patient had a negative straight-leg-raise test and impaired knee extension. The patient was taken urgently to the operating room for irrigation and débridement and concurrent repair of the patellar tendon laceration. Antibiotic prophylaxis with 2 g of intravenous (IV) cefazolin was given in the emergency room.
Intraoperatively, after visualizing the patellar tendon laceration and excluding any associated chondral lesions, we proceeded with extensive débridement and irrigation using 9 L of normal saline pulse lavage. After we achieved a clean site, we proceeded to repair the patellar tendon using No. 2 FiberWire sutures (Arthrex, Naples, Florida) with a classic Krackow repair8 consisting of 2 sutures run in a 4-row fashion through the patella and the patellar tendon. The suture was securely tightened and then tested for stability to at least 90° of knee flexion. The retinaculum was repaired using No. 0 Vicryl sutures (Ethicon, Somerville, New Jersey). After wound closure and dressing, the patient was placed in a hinged knee brace locked in extension at all times after surgery. Antibiotic treatment with IV cefazolin was administered for 48 hours.
Postoperative management consisted of weight-bearing as tolerated on the operative limb and appropriate deep venous thrombosis prophylaxis. The patient followed up in clinic 2 weeks and 4 weeks after surgery. At 4 weeks, the patient was noted to have a secondary wound infection with superficial dehiscence and serosanguineous drainage. No wound opening was noticed, and local wound care was performed with a 1-week course of oral cephalexin. The patient was scheduled to follow up a few weeks later but did not follow up for a year.
At 1-year follow-up, the patient reported that he had had a steady progression of his knee range of motion (ROM) with decreased pain. However, over time, the patient noted subjective instability of the knee, with frequent falls occurring close to his 1-year follow-up. Examination of his knee showed that his active ROM ranged from 20° in extension to 120° in flexion, with a weak extensor mechanism. Passively, his knee could be brought to full extension. His incision was well healed, but it had an area of bogginess in the middle. Radiographs showed patella alta on the affected knee, with a lengthening of the patellar tendon of 7.70 cm on the right compared with 5.18 cm on the left. Magnetic resonance imaging (MRI) showed moderate-to-severe patellar tendinosis with small fluid pockets around the surgical material and evidence of acute patellar enthesopathy. The laboratory values showed a white blood cell count of 7580/μL (normal, 4500-11,000/μL), an erythrocyte sedimentation rate of 2 mm/h (normal, 1-15 mm/h), and a C-reactive protein level of 1.93 mg/dL (normal, 0.00-0.29 mg/dL). Based on the clinical examination and imaging findings, there was a concern for a possible chronic deep-tissue infection, in addition to failure of the primary patellar tendon repair. Operative versus nonoperative management options were discussed with the patient, and he elected to undergo surgery.
During surgery, the patellar laxity was confirmed, and the patellar tendon was noticed to be chronically thickened and surrounded by unhealthy tissue. Initially, an extensive soft-tissue débridement was performed, and all patellar tendon loculations visualized on the preoperative MRI were drained; a solid purulent-like fluid was expressed. Unfortunately, the extensive and required débridement did not allow the preservation of the patellar tendon. Appropriate cultures were taken and sent for immediate Gram-stain analysis, which returned negative. Tissue samples from the patellar tendon were also sent to the pathology department for analysis. Intraoperatively, the infrapatellar defect was filled temporarily with a tobramycin cement spacer mixed with 2 g of vancomycin in a manner similar to that of the Masquelet technique used for infected long-bone nonunions with bone loss.9,10 This technique is a 2-stage procedure that promotes the formation of a biologic membrane that allows bone healing in the reconstruction of long-bone defects. The first stage consists of a radical débridement with soft-tissue repair by flaps when needed, with the insertion of a polymethylmethacrylate cement spacer into the bone defect. The second stage is usually performed 6 to 8 weeks later, with removal of the spacer and preservation of the induced membrane, which is filled with iliac crest bone autograft augmented (if necessary) with demineralized allograft.
The incision was closed primarily, and after surgery, the patient was allowed to bear weight as tolerated in a hinged knee brace locked in extension. Final laboratory analysis from cultures and tissue samples revealed acute and chronic inflammation with more than 20 neutrophils per high-powered field. No organisms grew from aerobic, anaerobic, fungal, or mycobacterial cultures. The infectious disease service was consulted and recommended oral cephalexin.
Because all cultures were negative, all laboratory examinations did not indicate any residual infections, and no bony involvement was noticed intraoperatively or in the preoperative knee MRI, we decided to proceed with the second stage of the Masquelet technique after 2 weeks. The patient returned to the operating room for final reconstruction of his patellar tendon using a custom-ordered cadaveric tendon–patellar tendon–bone allograft, the length of which was determined by measuring the contralateral patellar tendon, ie, 5.18 cm (Figure 1A). The previous anterior knee incision was reopened and extended distally past the tibial tuberosity and proximally toward the quadriceps tendon. The antibiotic spacer was removed. We proceeded with a repeat irrigation and débridement and the allograft transfer. The selected allograft was customized by reducing the tibial bone component to an approximately 1×2-cm bone block and by reducing the allograft patellar thickness with an oscillating saw, leaving an approximately 2-mm thick patellar bone graft attached to the patellar tendon. In a similar technique using an oscillating saw, we shaved off the anterior cortex of the patient’s patella to accommodate, in a sandwich fashion, the patellar allograft. Proximally, the quadriceps tendon insertion was split longitudinally and partially separated from the superior pole of the patellar tendon to allow seating and fixation of the modified quadriceps allograft tendon component.
We proceeded with the fixation of the allograft first distally on the patella. The anterior cortex of the tibial tuberosity was resected to allow the perfect seating of the bone block allograft. The graft was secured with a 4.0-mm fully threaded cancellous lag screw and reinforced with a 2.4-mm, 3-hole T-volar buttress plate (Synthes, Paoli, Pennsylvania). The plate was contoured to better fit the patient’s tibia. We sutured the patellar allograft tendon to the patella using two No. 2-0 FiberWire sutures in Krackow suture technique8 (Figures 1B, 1C). We obtained good fixation of the patellar tendon, and the distance between the patellar insertion and the inferior patellar pole was the same as before surgery: 5.57 cm and comparable to the contralateral side (Figures 2A-2C). The patellar allograft and autograft sandwich were secured with additional No. 2-0 FiberWire sutures, and the quadriceps allograft and autograft were secured with the cross-stitch technique with the same material. Fine suturing of the quadriceps tendon was done with No. 0 Vicryl sutures. After the fixation was completed, we tested the stability of the reconstruction and found good flexion up to 120°.
The postoperative protocol consisted of weight-bearing as tolerated in full extension and passive knee ROM, using a continuous passive ROM machine from 0° to 45° for the first 4 weeks, followed by active ROM, increased as tolerated, during the next 8 weeks.
The patient was seen in clinic 3 and 9 months after surgery. At the 3-month follow-up appointment, the patient’s examination showed knee ROM from 0° extension to 130° of flexion, no secondary infection signs, and radiographic evidence of a well-healing patellar allograft with symmetric patellar tendon length to the contralateral side. At 9-month follow-up, the patient’s active ROM was from 0° extension to 140° flexion (Figures 3A, 3B), and he had returned to his preinjury level of functioning.
Discussion
This case report describes the successful reconstruction of a patellar tendon defect with cadaveric tendon–patellar tendon–bone allograft. Extensor mechanism injuries are uncommon in general, and the incidence of patellar tendon injury is higher in men than in women.2 Patellar tendon tears occur frequently in active patients younger than 40 years, usually as a result of sudden quadriceps contraction with the knee slightly flexed.1 Treatment of patellar tendon injury is surgical, and functional outcomes for patients with this injury are equivalent to those of patients with quadriceps tendon injuries or patellar fractures.2 Acute patellar tendon tears can be repaired by end-to-end suturing or transosseous tunnel insertion in the tibia or patella.1 Reinforcement is often added between the patella and tibial tuberosity, using a semitendinosus band or wire.1 End-to-end suture is performed using a thick resorbable suture. It is important to avoid patella alta during suturing, comparing the position of the patella with the contralateral patella with the knee in 45° of flexion. In proximal avulsion, the tendon is anchored to the bone by 2 thick nonresorbable sutures through 2 parallel bone tunnels to the proximal pole of the patella. Distal avulsion is rare in adults, but it can be managed by using staples or suture anchors.1
End-to-end suturing of chronic patellar tendon defects is difficult more than 45 days after injury primarily because of difficulties in correcting patella alta secondary to the upward force exerted by the quadriceps tendon.1,3 Extreme situations similar to the case we present warrant Achilles or patellar tendon allograft for reconstruction of the extensor mechanism.1,3,6,9
Extensor mechanism allograft also provides an effective remedy for severe quadriceps deficiency caused by loss of the patella, patellar tendon, and quadriceps tendon in total knee arthroplasty.10 However, in such cases, late failure is common, and major quadriceps deficiency occurs after removal of the allograft material.10 To improve outcome, a novel technique using the medial gastrocnemius muscle transferred to the muscular portion of the vastus medialis and lateralis flaps provides a secure and strong closure of the anterior knee, thereby restoring the extensor mechanism of the knee.10
Patellar tendon reconstruction with allograft tissue has been successfully used, especially in cases related to chronic patellar tendon ruptures11 and total knee arthroplasty.6,12-14 Crossett and colleagues12 showed that, at 2-year follow-up, the average knee score for pain, ROM, and stability had improved from 26 points (range, 6-39 points) before surgery to 81 points (range, 40-92 points). The average knee score for function had also improved: 14 points (range, 0-35 points) before surgery to 53 points (range, 30-90 points).12 Primary repair may succeed in early intervention, but in an established rupture, allograft reconstruction is often necessary. Achilles tendon is the preferred allograft, with the calcaneus fragment embedded into the proximal tibia as a new tubercle and the tendon sutured into the remaining extensor mechanism.1,11 The repair is further protected using a cable loop from the superior pole of the patella to a drill hole in the upper tibia.9 Techniques have also been described involving passage of the proximal aspect of the allograft tendon through patellar bone tunnels and suture fixation to the native quadriceps tendon.11,15 However, in our technique, we shaved off the anterior cortex of the patient’s patella to allow a sandwich-type over-position of the allograft to secure fixation to the patella.
Another alternative to allograft reconstruction involves biocompatible scaffolds. Such scaffolds incorporate the use of platelets in a fibrin framework. A CPFS, produced from blood and calcium gluconate to improve healing of patellar tendon defects, has been described in animal studies.7 In the rabbit model, CPFS acts as a provisional bioscaffold that can accelerate healing of an injured patellar tendon repair, potentially secondary to several growth factors derived from platelets.7 Platelets are biocompatible sources of growth factors, and CPFS can act as a scaffold to restore the mechanical integrity of injured soft tissue.7,16 In addition, CPFS can act to lower donor-site morbidity associated with harvesting tissue autograft.7 However, to our knowledge, such scaffolds have not been used in human trials. The LARS biocompatible ligament (Corin Group PLC, Cirencester, United Kingdom), currently not approved by the US Food and Drug Administration, is used for reconstructions of isolated or multiple knee ligament injuries.17 This graft requires the presence of healthy tissue with good blood supply from which new tendon or ligament can grow in. Sometimes it is also used for extensor mechanism reconstruction after radical tumor resection around the knee; however, good results are achieved in only 59% of cases,18 and to our knowledge, only 1 case of primary repair of a patellar tendon rupture has been published.19
Techniques involving the use of tendon–patellar tendon–bone graft with fixation via the sandwich-type over-position of the allograft for chronic patellar tendon rupture have not been described in the literature. In our patient, given the extensive patellar tendon lesion and inflammation with chronic tissue degeneration, there was no option but to use allograft. To improve the patient’s outcome, we chose the strongest possible allograft, tendon–patellar tendon–bone graft.
Conclusion
Revision patellar tendon reconstruction is a challenging, but necessary, procedure to restore the extensor mechanism of the knee, especially in young, active individuals. Various options to reconstruct the tissue defects are available. Our patient was successfully treated with a tendon–patellar tendon–bone allograft reconstruction.
The extensor mechanism of the knee comprises the quadriceps tendon, the patella, and the patellar tendon. The extensor mechanism may be damaged by injury to these structures, with consequences such as the inability to actively extend the knee and hemarthrosis.1,2 Disruption of this mechanism is rare, and the most common injury pattern is an eccentric contraction of the quadriceps tendon on a flexed knee causing a tendon (quadriceps or patellar) rupture or a patella fracture.1,2
Patellar tendon ruptures are more common in persons younger than 40 years.1 Treatment is surgical, regardless of age and physical activity. In the acute setting, repair can be end-to-end suture or transosseous tunnel insertion. End-to-end suturing is difficult in chronic patellar tendon ruptures because of patella alta secondary to quadriceps contraction.3 Treatment options for chronic ruptures may involve transpatellar traction4 or tendon reinforcement with fascia lata, a semitendinosus band, or synthetic materials.3-5 Alternatively, tendon autograft and allografts have also been recommended, especially in extreme situations.1,6 Furthermore, animal experiments have shown that a compact platelet-rich fibrin scaffold (CPFS) has the potential to accelerate healing of patellar tendon defects and to act as a bioscaffold for graft augmentation.7
We describe the case of a 30-year-old man who underwent extensor mechanism reconstruction with cadaveric tendon–patellar tendon–bone allograft for failure of an infected primary end-to-end repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old healthy man landed on an empty glass fish tank, resulting in a traumatic right-knee arthrotomy. On initial evaluation, the patient had a negative straight-leg-raise test and impaired knee extension. The patient was taken urgently to the operating room for irrigation and débridement and concurrent repair of the patellar tendon laceration. Antibiotic prophylaxis with 2 g of intravenous (IV) cefazolin was given in the emergency room.
Intraoperatively, after visualizing the patellar tendon laceration and excluding any associated chondral lesions, we proceeded with extensive débridement and irrigation using 9 L of normal saline pulse lavage. After we achieved a clean site, we proceeded to repair the patellar tendon using No. 2 FiberWire sutures (Arthrex, Naples, Florida) with a classic Krackow repair8 consisting of 2 sutures run in a 4-row fashion through the patella and the patellar tendon. The suture was securely tightened and then tested for stability to at least 90° of knee flexion. The retinaculum was repaired using No. 0 Vicryl sutures (Ethicon, Somerville, New Jersey). After wound closure and dressing, the patient was placed in a hinged knee brace locked in extension at all times after surgery. Antibiotic treatment with IV cefazolin was administered for 48 hours.
Postoperative management consisted of weight-bearing as tolerated on the operative limb and appropriate deep venous thrombosis prophylaxis. The patient followed up in clinic 2 weeks and 4 weeks after surgery. At 4 weeks, the patient was noted to have a secondary wound infection with superficial dehiscence and serosanguineous drainage. No wound opening was noticed, and local wound care was performed with a 1-week course of oral cephalexin. The patient was scheduled to follow up a few weeks later but did not follow up for a year.
At 1-year follow-up, the patient reported that he had had a steady progression of his knee range of motion (ROM) with decreased pain. However, over time, the patient noted subjective instability of the knee, with frequent falls occurring close to his 1-year follow-up. Examination of his knee showed that his active ROM ranged from 20° in extension to 120° in flexion, with a weak extensor mechanism. Passively, his knee could be brought to full extension. His incision was well healed, but it had an area of bogginess in the middle. Radiographs showed patella alta on the affected knee, with a lengthening of the patellar tendon of 7.70 cm on the right compared with 5.18 cm on the left. Magnetic resonance imaging (MRI) showed moderate-to-severe patellar tendinosis with small fluid pockets around the surgical material and evidence of acute patellar enthesopathy. The laboratory values showed a white blood cell count of 7580/μL (normal, 4500-11,000/μL), an erythrocyte sedimentation rate of 2 mm/h (normal, 1-15 mm/h), and a C-reactive protein level of 1.93 mg/dL (normal, 0.00-0.29 mg/dL). Based on the clinical examination and imaging findings, there was a concern for a possible chronic deep-tissue infection, in addition to failure of the primary patellar tendon repair. Operative versus nonoperative management options were discussed with the patient, and he elected to undergo surgery.
During surgery, the patellar laxity was confirmed, and the patellar tendon was noticed to be chronically thickened and surrounded by unhealthy tissue. Initially, an extensive soft-tissue débridement was performed, and all patellar tendon loculations visualized on the preoperative MRI were drained; a solid purulent-like fluid was expressed. Unfortunately, the extensive and required débridement did not allow the preservation of the patellar tendon. Appropriate cultures were taken and sent for immediate Gram-stain analysis, which returned negative. Tissue samples from the patellar tendon were also sent to the pathology department for analysis. Intraoperatively, the infrapatellar defect was filled temporarily with a tobramycin cement spacer mixed with 2 g of vancomycin in a manner similar to that of the Masquelet technique used for infected long-bone nonunions with bone loss.9,10 This technique is a 2-stage procedure that promotes the formation of a biologic membrane that allows bone healing in the reconstruction of long-bone defects. The first stage consists of a radical débridement with soft-tissue repair by flaps when needed, with the insertion of a polymethylmethacrylate cement spacer into the bone defect. The second stage is usually performed 6 to 8 weeks later, with removal of the spacer and preservation of the induced membrane, which is filled with iliac crest bone autograft augmented (if necessary) with demineralized allograft.
The incision was closed primarily, and after surgery, the patient was allowed to bear weight as tolerated in a hinged knee brace locked in extension. Final laboratory analysis from cultures and tissue samples revealed acute and chronic inflammation with more than 20 neutrophils per high-powered field. No organisms grew from aerobic, anaerobic, fungal, or mycobacterial cultures. The infectious disease service was consulted and recommended oral cephalexin.
Because all cultures were negative, all laboratory examinations did not indicate any residual infections, and no bony involvement was noticed intraoperatively or in the preoperative knee MRI, we decided to proceed with the second stage of the Masquelet technique after 2 weeks. The patient returned to the operating room for final reconstruction of his patellar tendon using a custom-ordered cadaveric tendon–patellar tendon–bone allograft, the length of which was determined by measuring the contralateral patellar tendon, ie, 5.18 cm (Figure 1A). The previous anterior knee incision was reopened and extended distally past the tibial tuberosity and proximally toward the quadriceps tendon. The antibiotic spacer was removed. We proceeded with a repeat irrigation and débridement and the allograft transfer. The selected allograft was customized by reducing the tibial bone component to an approximately 1×2-cm bone block and by reducing the allograft patellar thickness with an oscillating saw, leaving an approximately 2-mm thick patellar bone graft attached to the patellar tendon. In a similar technique using an oscillating saw, we shaved off the anterior cortex of the patient’s patella to accommodate, in a sandwich fashion, the patellar allograft. Proximally, the quadriceps tendon insertion was split longitudinally and partially separated from the superior pole of the patellar tendon to allow seating and fixation of the modified quadriceps allograft tendon component.
We proceeded with the fixation of the allograft first distally on the patella. The anterior cortex of the tibial tuberosity was resected to allow the perfect seating of the bone block allograft. The graft was secured with a 4.0-mm fully threaded cancellous lag screw and reinforced with a 2.4-mm, 3-hole T-volar buttress plate (Synthes, Paoli, Pennsylvania). The plate was contoured to better fit the patient’s tibia. We sutured the patellar allograft tendon to the patella using two No. 2-0 FiberWire sutures in Krackow suture technique8 (Figures 1B, 1C). We obtained good fixation of the patellar tendon, and the distance between the patellar insertion and the inferior patellar pole was the same as before surgery: 5.57 cm and comparable to the contralateral side (Figures 2A-2C). The patellar allograft and autograft sandwich were secured with additional No. 2-0 FiberWire sutures, and the quadriceps allograft and autograft were secured with the cross-stitch technique with the same material. Fine suturing of the quadriceps tendon was done with No. 0 Vicryl sutures. After the fixation was completed, we tested the stability of the reconstruction and found good flexion up to 120°.
The postoperative protocol consisted of weight-bearing as tolerated in full extension and passive knee ROM, using a continuous passive ROM machine from 0° to 45° for the first 4 weeks, followed by active ROM, increased as tolerated, during the next 8 weeks.
The patient was seen in clinic 3 and 9 months after surgery. At the 3-month follow-up appointment, the patient’s examination showed knee ROM from 0° extension to 130° of flexion, no secondary infection signs, and radiographic evidence of a well-healing patellar allograft with symmetric patellar tendon length to the contralateral side. At 9-month follow-up, the patient’s active ROM was from 0° extension to 140° flexion (Figures 3A, 3B), and he had returned to his preinjury level of functioning.
Discussion
This case report describes the successful reconstruction of a patellar tendon defect with cadaveric tendon–patellar tendon–bone allograft. Extensor mechanism injuries are uncommon in general, and the incidence of patellar tendon injury is higher in men than in women.2 Patellar tendon tears occur frequently in active patients younger than 40 years, usually as a result of sudden quadriceps contraction with the knee slightly flexed.1 Treatment of patellar tendon injury is surgical, and functional outcomes for patients with this injury are equivalent to those of patients with quadriceps tendon injuries or patellar fractures.2 Acute patellar tendon tears can be repaired by end-to-end suturing or transosseous tunnel insertion in the tibia or patella.1 Reinforcement is often added between the patella and tibial tuberosity, using a semitendinosus band or wire.1 End-to-end suture is performed using a thick resorbable suture. It is important to avoid patella alta during suturing, comparing the position of the patella with the contralateral patella with the knee in 45° of flexion. In proximal avulsion, the tendon is anchored to the bone by 2 thick nonresorbable sutures through 2 parallel bone tunnels to the proximal pole of the patella. Distal avulsion is rare in adults, but it can be managed by using staples or suture anchors.1
End-to-end suturing of chronic patellar tendon defects is difficult more than 45 days after injury primarily because of difficulties in correcting patella alta secondary to the upward force exerted by the quadriceps tendon.1,3 Extreme situations similar to the case we present warrant Achilles or patellar tendon allograft for reconstruction of the extensor mechanism.1,3,6,9
Extensor mechanism allograft also provides an effective remedy for severe quadriceps deficiency caused by loss of the patella, patellar tendon, and quadriceps tendon in total knee arthroplasty.10 However, in such cases, late failure is common, and major quadriceps deficiency occurs after removal of the allograft material.10 To improve outcome, a novel technique using the medial gastrocnemius muscle transferred to the muscular portion of the vastus medialis and lateralis flaps provides a secure and strong closure of the anterior knee, thereby restoring the extensor mechanism of the knee.10
Patellar tendon reconstruction with allograft tissue has been successfully used, especially in cases related to chronic patellar tendon ruptures11 and total knee arthroplasty.6,12-14 Crossett and colleagues12 showed that, at 2-year follow-up, the average knee score for pain, ROM, and stability had improved from 26 points (range, 6-39 points) before surgery to 81 points (range, 40-92 points). The average knee score for function had also improved: 14 points (range, 0-35 points) before surgery to 53 points (range, 30-90 points).12 Primary repair may succeed in early intervention, but in an established rupture, allograft reconstruction is often necessary. Achilles tendon is the preferred allograft, with the calcaneus fragment embedded into the proximal tibia as a new tubercle and the tendon sutured into the remaining extensor mechanism.1,11 The repair is further protected using a cable loop from the superior pole of the patella to a drill hole in the upper tibia.9 Techniques have also been described involving passage of the proximal aspect of the allograft tendon through patellar bone tunnels and suture fixation to the native quadriceps tendon.11,15 However, in our technique, we shaved off the anterior cortex of the patient’s patella to allow a sandwich-type over-position of the allograft to secure fixation to the patella.
Another alternative to allograft reconstruction involves biocompatible scaffolds. Such scaffolds incorporate the use of platelets in a fibrin framework. A CPFS, produced from blood and calcium gluconate to improve healing of patellar tendon defects, has been described in animal studies.7 In the rabbit model, CPFS acts as a provisional bioscaffold that can accelerate healing of an injured patellar tendon repair, potentially secondary to several growth factors derived from platelets.7 Platelets are biocompatible sources of growth factors, and CPFS can act as a scaffold to restore the mechanical integrity of injured soft tissue.7,16 In addition, CPFS can act to lower donor-site morbidity associated with harvesting tissue autograft.7 However, to our knowledge, such scaffolds have not been used in human trials. The LARS biocompatible ligament (Corin Group PLC, Cirencester, United Kingdom), currently not approved by the US Food and Drug Administration, is used for reconstructions of isolated or multiple knee ligament injuries.17 This graft requires the presence of healthy tissue with good blood supply from which new tendon or ligament can grow in. Sometimes it is also used for extensor mechanism reconstruction after radical tumor resection around the knee; however, good results are achieved in only 59% of cases,18 and to our knowledge, only 1 case of primary repair of a patellar tendon rupture has been published.19
Techniques involving the use of tendon–patellar tendon–bone graft with fixation via the sandwich-type over-position of the allograft for chronic patellar tendon rupture have not been described in the literature. In our patient, given the extensive patellar tendon lesion and inflammation with chronic tissue degeneration, there was no option but to use allograft. To improve the patient’s outcome, we chose the strongest possible allograft, tendon–patellar tendon–bone graft.
Conclusion
Revision patellar tendon reconstruction is a challenging, but necessary, procedure to restore the extensor mechanism of the knee, especially in young, active individuals. Various options to reconstruct the tissue defects are available. Our patient was successfully treated with a tendon–patellar tendon–bone allograft reconstruction.
1. Saragaglia D, Pison A, Rubens-Duval B. Acute and old ruptures of the extensor apparatus of the knee in adults (excluding knee replacement). Orthop Traumatol Surg Res. 2013;99(1 suppl):S67-S76.
2. Tejwani NC, Lekic N, Bechtel C, Montero N, Egol KA. Outcomes after knee joint extensor mechanism disruptions: is it better to fracture the patella or rupture the tendon? J Orthop Trauma. 2012;26(11):648-651.
3. Ecker ML, Lotke PA, Glazer RM. Late reconstruction of the patellar tendon. J Bone Joint Surg Am. 1979;61(6):884-886.
4. Siwek CW, Rao JP. Ruptures of the extensor mechanism of the knee joint. J Bone Joint Surg Am. 1981;63(6):932-937.
5. Levy M, Goldstein J, Rosner M. A method of repair for quadriceps tendon or patellar ligament (tendon) ruptures without cast immobilization. Preliminary report. Clin Orthop Relat Res. 1987;218:297-301.
6. Burks RT, Edelson RH. Allograft reconstruction of the patellar ligament. A case report. J Bone Joint Surg Am. 1994;76(7):1077-1079.
7. Matsunaga D, Akizuki S, Takizawa T, Omae S, Kato H. Compact platelet-rich fibrin scaffold to improve healing of patellar tendon defects and for medial collateral ligament reconstruction. Knee. 2013;20(6):545-550.
8. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
9. Brooks P. Extensor mechanism ruptures. Orthopedics. 2009;32(9):683-684.
10. Whiteside LA. Surgical technique: muscle transfer restores extensor function after failed patella-patellar tendon allograft. Clin Orthop Relat Res. 2014;472(1):218-226.
11. Farmer K, Cosgarea AJ. Procedure 25. Acute and chronic patellar tendon ruptures. In: Miller MD, Cole BJ, Cosgarea AJ, Sekiya JK, eds. Operative Techniques: Sports Knee Surgery. Philadelphia, PA: Saunders (Elsevier); 2008:397-417.
12. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
13. Lahav A, Burks RT, Scholl MD. Allograft reconstruction of the patellar tendon: 12-year follow-up. Am J Orthop. 2004;33(12):623-624.
14. Yoo JH, Chang JD, Seo YJ, Baek SW. Reconstruction of a patellar tendon with Achilles tendon allograft for severe patellar infera--a case report. Knee. 2011;18(5):350-353.
15. Saldua NS, Mazurek MT. Procedure 37. Quadriceps and patellar tendon repair. In: Reider B, Terry MA, Provencher MT, eds. Operative Techniques: Sports Medicine Surgery. Philadelphia, PA: Saunders (Elsevier); 2010:623-640.
16. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4-15.
17. Ibrahim SAR, Ahmad FHF, Salah M, Al Misfer ARK, Ghaffer SA, Khirat S. Surgical management of traumatic knee dislocation. Arthroscopy. 2008;24(2):178-187.
18. Dominkus M, Sabeti M, Toma C, Abdolvahab F, Trieb K, Kotz RI. Reconstructing the extensor apparatus with a new polyester ligament. Clin Orthop Relat Res. 2006;453:328-334.
19. Naim S, Gougoulias N, Griffiths D. Patellar tendon reconstruction using LARS ligament: surgical technique and case report. Strategies Trauma Limb Reconstr. 2011;6(1):39-41.
1. Saragaglia D, Pison A, Rubens-Duval B. Acute and old ruptures of the extensor apparatus of the knee in adults (excluding knee replacement). Orthop Traumatol Surg Res. 2013;99(1 suppl):S67-S76.
2. Tejwani NC, Lekic N, Bechtel C, Montero N, Egol KA. Outcomes after knee joint extensor mechanism disruptions: is it better to fracture the patella or rupture the tendon? J Orthop Trauma. 2012;26(11):648-651.
3. Ecker ML, Lotke PA, Glazer RM. Late reconstruction of the patellar tendon. J Bone Joint Surg Am. 1979;61(6):884-886.
4. Siwek CW, Rao JP. Ruptures of the extensor mechanism of the knee joint. J Bone Joint Surg Am. 1981;63(6):932-937.
5. Levy M, Goldstein J, Rosner M. A method of repair for quadriceps tendon or patellar ligament (tendon) ruptures without cast immobilization. Preliminary report. Clin Orthop Relat Res. 1987;218:297-301.
6. Burks RT, Edelson RH. Allograft reconstruction of the patellar ligament. A case report. J Bone Joint Surg Am. 1994;76(7):1077-1079.
7. Matsunaga D, Akizuki S, Takizawa T, Omae S, Kato H. Compact platelet-rich fibrin scaffold to improve healing of patellar tendon defects and for medial collateral ligament reconstruction. Knee. 2013;20(6):545-550.
8. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
9. Brooks P. Extensor mechanism ruptures. Orthopedics. 2009;32(9):683-684.
10. Whiteside LA. Surgical technique: muscle transfer restores extensor function after failed patella-patellar tendon allograft. Clin Orthop Relat Res. 2014;472(1):218-226.
11. Farmer K, Cosgarea AJ. Procedure 25. Acute and chronic patellar tendon ruptures. In: Miller MD, Cole BJ, Cosgarea AJ, Sekiya JK, eds. Operative Techniques: Sports Knee Surgery. Philadelphia, PA: Saunders (Elsevier); 2008:397-417.
12. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
13. Lahav A, Burks RT, Scholl MD. Allograft reconstruction of the patellar tendon: 12-year follow-up. Am J Orthop. 2004;33(12):623-624.
14. Yoo JH, Chang JD, Seo YJ, Baek SW. Reconstruction of a patellar tendon with Achilles tendon allograft for severe patellar infera--a case report. Knee. 2011;18(5):350-353.
15. Saldua NS, Mazurek MT. Procedure 37. Quadriceps and patellar tendon repair. In: Reider B, Terry MA, Provencher MT, eds. Operative Techniques: Sports Medicine Surgery. Philadelphia, PA: Saunders (Elsevier); 2010:623-640.
16. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4-15.
17. Ibrahim SAR, Ahmad FHF, Salah M, Al Misfer ARK, Ghaffer SA, Khirat S. Surgical management of traumatic knee dislocation. Arthroscopy. 2008;24(2):178-187.
18. Dominkus M, Sabeti M, Toma C, Abdolvahab F, Trieb K, Kotz RI. Reconstructing the extensor apparatus with a new polyester ligament. Clin Orthop Relat Res. 2006;453:328-334.
19. Naim S, Gougoulias N, Griffiths D. Patellar tendon reconstruction using LARS ligament: surgical technique and case report. Strategies Trauma Limb Reconstr. 2011;6(1):39-41.
Intra-Articular Dislocation of the Patella With Associated Hoffa Fracture in a Skeletally Immature Patient
In 1887, Midelfart1 first reported on an intra-articular dislocation of the patella, and since then approximately 50 cases have been reported in the worldwide literature.2 Also known as an inferior patellar dislocation, these rare traumatic events occur when the patella dislocates intra-articularly. Because the patella commonly rotates about its horizontal axis, the articular surface is facing proximally or distally. The patella becomes lodged within the trochlea and locks the knee joint. Most cases described in the literature involved adolescent boys, with the patella difficult to reduce. Most patients required open reduction, while those who underwent successful closed reduction often needed general anesthesia.3
Similarly, coronal shear fractures of the femoral condyle (ie, Hoffa fractures) are an uncommon fracture pattern typically seen in adults. These fractures are even more infrequent in skeletally immature patients, with fewer than 5 cases documented in the literature.4-7 In our case report, we present a 14-year-old boy with a coronal shear fracture of the femoral condyle associated with an intra-articular patellar dislocation. To our knowledge, this constellation of injuries has not been reported. Additionally, closed reduction of the patella was successful after intra-articular lidocaine injection, without the need for sedation or general anesthesia. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy presented to our institution after sustaining a direct blow to his left knee. The injury occurred as he jumped and landed on a flexed knee while playing with friends. The patient was unable to ambulate after the injury, and his left knee was locked in a slightly flexed position. Examination in the emergency department showed the knee to be held in approximately 60º of flexion, with an obvious bony prominence noted anteriorly over the femoral condyles. The patient was unable to perform a straight leg raise or any active range of motion (ROM) at the knee. Radiographs performed with the knee maintained in flexion confirmed that the patella was displaced into the knee joint and was rotated with the articular surface facing distally. Also noted was a coronal shear fracture of the lateral femoral condyle (Figures 1A, 1B).
The patient received pain medication and an intra-articular lidocaine injection prior to a reduction attempt by the orthopedic resident. With the patient supine, the hip was gently flexed to relax the quadriceps muscle. As the knee was flexed up to 110º, the prominent patella was gripped between the thumb and fingers to gently free and elevate the patella out of the intercondylar notch.
After reduction, an immediate return of normal patellar contour and patellofemoral tracking was observed as the knee was gently extended. There was no obvious defect to the patellar or quadriceps tendons, and the patient was able to perform a straight-leg raise, confirming the integrity of the extensor mechanism. Radiographs performed after the reduction confirmed relocation of the patella in correct anatomic position, as well as a lateral femoral condyle fracture (Figures 2A, 2B). Magnetic resonance imaging (MRI) of the knee confirmed no full-thickness quadriceps or patellar tendon tear. A computed tomography (CT) scan of the knee showed a comminuted fracture of the lateral femoral condyle in the coronal plane, as well as multiple bone fragments within the joint (Figures 3A, 3B). The patient was placed in a bulky soft dressing and underwent open reduction and internal fixation of the fracture.
A 10-cm incision was made over the anterior aspect of the knee, and after dissection to the level of the retinaculum, a lateral parapatellar arthrotomy was performed. The patella was retracted medially to identify and free the fracture fragments. The fracture fragments were provisionally reduced and stabilized with three 0.065-in Kirschner wires. An area of osteochondral impaction proximal to the fracture was elevated and allograft bone was incorporated below the articular surface (Figures 4A, 4B). Rigid fixation of the fracture was achieved using 3 screws (2 Bio-Compression Screws [Arthrex Inc., Naples, Florida] and 1 Synthes cannulated screw [Synthes, West Chester, Pennsylvania]). The screws were placed in posteroanterior (PA) direction and inserted into the weight-bearing articular surface of the femoral condyle (Figures 4C, 4D). The screws were countersunk, and stable fixation with compression of the fracture was achieved. Reduction and screw position were verified with fluoroscopic views. The wound was closed in layers, and the patient was discharged home the next day.
Postoperatively, the patient was non-weight-bearing on the affected limb with a hinged-knee brace to allow for knee ROM exercises immediately. He was also given a continuous passive motion device to maintain knee motion. At the 6-week mark, the patient’s fracture alignment appeared to be well-maintained and showed interval healing. Clinically, the patient was noted to have limited knee ROM. The decision was made to take the patient to the operating room primarily for a manipulation under anesthesia and resection of scar tissue from postoperative arthrofibrosis. Arthroscopic screw removal was also planned as a secondary procedure at the same time in order to prevent the possibility of chondral injury from screw migration. During the procedure, the patient was noted to have improved ROM from 5º to 85º premanipulation to 5º to 110º postoperatively. At 3 months after the initial injury, the patient was allowed to begin progressive weight-bearing on the left knee. At most recent follow-up, after 12 months, the patient was able to ambulate and bear weight on the left leg without pain. Plain radiographs show a well-healed fracture with no evidence of collapse of the femoral condyle (Figures 5A, 5B). His active ROM of the left knee was 5º to 110º without pain (Figures 5C, 5D).
Discussion
In the vast majority of patellar dislocations, the patella dislocates laterally over the trochlear groove. Inferior, or intra-articular, dislocations of the patella are rare. The mechanism of injury is usually a blow onto the patella with a flexed knee. The 2 groups commonly involved are adolescent boys and the elderly.8,9 In young men, it is thought that lax patellar attachments place adolescents at higher risk for this type of injury.10-12 While patella fractures and frank extensor mechanism ruptures are uncommon in this age group, the same mechanism of injury can lead to stripping of the deep fibers of the patellar tendon from the superior pole of the patella.3,13 The intact superficial fibers of the tendon allow the patella to hinge and displace into the joint.14
Inferior dislocations of the patella are classified into 2 types based on the orientation of the articular surface and the presence of osteophytes.15 Type I inferior dislocations occur after a direct blow to a flexed knee forces the superior pole of the patella into the intercondylar notch. Type II dislocations are caused by osteophytes on the superior pole of the patella that become wedged in the intercondylar notch and dislocate the patella inferiorly. In type I dislocations, the patella is rotated in the horizontal plane and the articular surface often faces inferiorly, but type II dislocations do not involve rotation of the articular surface. Type II injuries are seen more commonly in the elderly.
Our patient was able to tolerate a closed reduction of the patella after an intra-articular lidocaine injection, and a successful reduction was achieved without great difficulty. However, the majority of reports describe the need for an open reduction of inferior patellar dislocations.3,8 When closed reductions were a success, they were performed under general anesthesia or conscious sedation.3 It is thought that the difficulty of reduction results from the tension of the quadriceps muscle pulling the patella superiorly into intercondylar notch.11,16 However, successful closed reduction may be more likely in patients with less patellar rotation and entrapment within the intercondylar notch, as well as in patients whose knee is near full extension at presentation.17-19 Successful closed reduction is also seen in elderly patients, where dislocation is generally caused by less forceful impact and held by osteophytes. In these patients, the knee is commonly held in extension.12,15,20-22
The fracture pattern seen in this case also shows a rare fracture in skeletally immature patients, with only a few case reports in the literature. Isolated coronal plane femur fractures account for 0.65% of all femur fractures and are usually seen in adults after high-energy trauma.23 In the skeletally immature, the fracture can occur with lower-energy mechanisms. The typical mechanism is thought to be a shearing force to the femur caused by an axial load to the knee in 90° or more of flexion.4,24 A CT scan is recommended for better identification of the fracture and to plan treatment.25,26 Because of their intra-articular nature and tenuous blood supply, Hoffa fractures tend to do poorly with nonoperative treatment and are prone to displacement and nonunion.27,28 The goal of operative treatment is to obtain anatomic reduction and rigid fixation. While operative fixation techniques are varied, screw fixation with multiple smaller diameter screws has equal pullout strength compared to larger screws and may minimize damage to the articular cartilage.29-31 By preserving blood supply to the fracture, and allowing for early active mobilization, operative treatment generally provides good long-term functional outcomes in these fracture patterns.24
Conclusion
We describe a case in which the patella of an adolescent boy dislocated inferiorly into the knee joint, with an associated coronal shear fracture of the lateral femoral condyle. To our knowledge, this constellation of injuries has not been reported. For this uncommon injury pattern, we recommend a sequential treatment algorithm to minimize morbidity. We recommend first attempting a closed reduction of the patella with adequate pain control to avoid the morbidity associated with general anesthesia. After a successful reduction, an advanced imaging study (eg, MRI) is advisable to assess for concomitant soft-tissue injuries and preoperative planning, if necessary. The mechanism of injury and force required to cause a patellar dislocation of this nature leaves a high likelihood of other injuries. When a fracture is noted on plain radiographs after reduction, a CT scan can provide important information for planning surgical fixation of the fracture. Even in a skeletally immature patient, the principle of direct reduction and stable interfragmentary fixation of an articular fracture is critical for long-term function, even after a significant trauma to the knee.
1. Midelfart V. En sjelden luxation of patella. Norsk Magazin for Laegevidenskaben. 1887;4:588.
2. Kramer DE, Simoni MK. Horizontal intra-articular patellar dislocation resulting in quadriceps avulsion and medial patellofemoral ligament tear: a case report. J Pediatr Orthop B. 2013;22(4):329-332.
3. van den Broek TA, Moll PJ. Horizontal rotation of the patella. A case report with review of the literature. Acta Orthop Scand. 1985;56(5):436-438.
4. Flanagin BA, Cruz AI, Medvecky MJ. Hoffa fracture in a 14-year-old. Orthopedics. 2011;34(2):138.
5. Strauss E, Nelson JM, Abdelwahab IF. Fracture of the lateral femoral condyle. A case report. Bull Hosp Jt Dis Orthop Inst. 1984;44(1):86-90.
6. Biau DJ, Schranz PJ. Transverse Hoffa’s or deep osteochondral fracture? An unusual fracture of the lateral femoral condyle in a child. Injury. 2005;36(7):862-865.
7. McDonough PW, Bernstein RM. Nonunion of a Hoffa fracture in a child. J Orthop Trauma. 2000;14(7):519-521.
8. Brady TA, Russell D. Interarticular horizontal dislocation of the patella. A case report. J Bone Joint Surg Am. 1965;47(7):1393-1396.
9. Yuguero M, Gonzalez JA, Carma A, Huguet J. Intra-articular patellar dislocation. Orthopedics. 2003;26(5):517-518.
10. Frangakis EK. Intra-articular dislocation of the patella. A case report. J Bone Joint Surg Am. 1974;56(2):423-424.
11. Nanda R, Yadav RS, Thakur M. Intra-articular dislocation of the patella. J Trauma. 2000;48(1):159-160.
12. Choudhary RK, Tice JW. Intra-articular dislocation of the patella with incomplete rotation--two case reports and a review of the literature. Knee. 2004;11(2):125-127.
13. Chatziantoniou I, Diakos G, Pantelelli M. Horizontal dislocation of the patella. Case report. EEXOT. 2008;59(2):112-114.
14. McHugh G, Ryan E, Cleary M, Kenny P, O’Flanagan S, Keogh P. Intra-articular dislocation of the patella. Case Rep Orthop. 2013;2013:535803.
15. Bankes MJ, Eastwood DM. Inferior dislocation of the patella in the degenerate knee. Injury. 2002;33(6):528-529.
16. Theodorides A, Guo S, Case R. Intra-articular dislocation of the patella: A case report and review of the literature. Injury Extra. 2010;41(10):103-105.
17. Dimentberg RA. Intra-articular dislocation of the patella: case report and literature review. Clin J Sport Med. 1997;7(2):126-128.
18. Morin WD, Steadman JR. Case report of a successful closed reduction without anesthesia. Clin Orthop. 1993(297):179-181.
19. Murakami Y. Intra-articular dislocation of the patella. A case report. Clin Orthop. 1982;171:137-139.
20. Joshi RP. Inferior dislocation of the patella. Injury. 1997;28(5-6):389-390.
21. Garner JP, Pike JM, George CD. Intra-articular dislocation of the patella: two cases and literature review. J Trauma. 1999;47(4):780-783.
22. McCarthy TA, Quinn B, Pegum JM. Inferior dislocation of the patella: an unusual cause of a locked knee. Ir J Med Sci. 2001;170(3):209-210.
23. Manfredini M, Gildone A, Ferrante R, Bernasconi S, Massari L. Unicondylar femoral fractures: therapeutic strategy and long-term results. A review of 23 patients. Acta Orthop Belg. 2001;67(2):132-138.
24. Holmes SM, Bomback D, Baumgaertner MR. Coronal fractures of the femoral condyle: a brief report of five cases. J Orthop Trauma. 2004;18(5):316-319.
25. Nork SE, Segina DN, Aflatoon K, et al. The association between supracondylar-intercondylar distal femoral fractures and coronal plane fractures. J Bone Joint Surg Am. 2005;87(3):564-569.
26. Allmann KH, Altehoefer C, Wildanger G, et al. Hoffa fracture--a radiologic diagnostic approach. J Belge Radiol. 1996;79(5):201-202.
27. Oztürk A, Ozkan Y, Ozdemir RM. Nonunion of a Hoffa fracture in an adult. Chir Organi Mov. 2009;93(3):183-185.
28. Lewis SL, Pozo JL, Muirhead-Allwood WF. Coronal fractures of the lateral femoral condyle. J Bone Joint Surg Br. 1989;71(1):118-120.
29. Arastu MH, Kokke MC, Duffy PJ, Korley RE, Buckley RE. Coronal plane partial articular fractures of the distal femoral condyle: current concepts in management. Bone Joint J. 2013;95-B(9):1165-1171.
30. Westmoreland GL, McLaurin TM, Hutton WC. Screw pullout strength: a biomechanical comparison of large-fragment and small-fragment fixation in the tibial plateau. J Orthop Trauma. 2002;16(3):178-181.
31. Jarit GJ, Kummer FJ, Gibber MJ, Egol KA. A mechanical evaluation of two fixation methods using cancellous screws for coronal fractures of the lateral condyle of the distal femur (OTA type 33B). J Orthop Trauma. 2006;20(4):273-276.
In 1887, Midelfart1 first reported on an intra-articular dislocation of the patella, and since then approximately 50 cases have been reported in the worldwide literature.2 Also known as an inferior patellar dislocation, these rare traumatic events occur when the patella dislocates intra-articularly. Because the patella commonly rotates about its horizontal axis, the articular surface is facing proximally or distally. The patella becomes lodged within the trochlea and locks the knee joint. Most cases described in the literature involved adolescent boys, with the patella difficult to reduce. Most patients required open reduction, while those who underwent successful closed reduction often needed general anesthesia.3
Similarly, coronal shear fractures of the femoral condyle (ie, Hoffa fractures) are an uncommon fracture pattern typically seen in adults. These fractures are even more infrequent in skeletally immature patients, with fewer than 5 cases documented in the literature.4-7 In our case report, we present a 14-year-old boy with a coronal shear fracture of the femoral condyle associated with an intra-articular patellar dislocation. To our knowledge, this constellation of injuries has not been reported. Additionally, closed reduction of the patella was successful after intra-articular lidocaine injection, without the need for sedation or general anesthesia. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy presented to our institution after sustaining a direct blow to his left knee. The injury occurred as he jumped and landed on a flexed knee while playing with friends. The patient was unable to ambulate after the injury, and his left knee was locked in a slightly flexed position. Examination in the emergency department showed the knee to be held in approximately 60º of flexion, with an obvious bony prominence noted anteriorly over the femoral condyles. The patient was unable to perform a straight leg raise or any active range of motion (ROM) at the knee. Radiographs performed with the knee maintained in flexion confirmed that the patella was displaced into the knee joint and was rotated with the articular surface facing distally. Also noted was a coronal shear fracture of the lateral femoral condyle (Figures 1A, 1B).
The patient received pain medication and an intra-articular lidocaine injection prior to a reduction attempt by the orthopedic resident. With the patient supine, the hip was gently flexed to relax the quadriceps muscle. As the knee was flexed up to 110º, the prominent patella was gripped between the thumb and fingers to gently free and elevate the patella out of the intercondylar notch.
After reduction, an immediate return of normal patellar contour and patellofemoral tracking was observed as the knee was gently extended. There was no obvious defect to the patellar or quadriceps tendons, and the patient was able to perform a straight-leg raise, confirming the integrity of the extensor mechanism. Radiographs performed after the reduction confirmed relocation of the patella in correct anatomic position, as well as a lateral femoral condyle fracture (Figures 2A, 2B). Magnetic resonance imaging (MRI) of the knee confirmed no full-thickness quadriceps or patellar tendon tear. A computed tomography (CT) scan of the knee showed a comminuted fracture of the lateral femoral condyle in the coronal plane, as well as multiple bone fragments within the joint (Figures 3A, 3B). The patient was placed in a bulky soft dressing and underwent open reduction and internal fixation of the fracture.
A 10-cm incision was made over the anterior aspect of the knee, and after dissection to the level of the retinaculum, a lateral parapatellar arthrotomy was performed. The patella was retracted medially to identify and free the fracture fragments. The fracture fragments were provisionally reduced and stabilized with three 0.065-in Kirschner wires. An area of osteochondral impaction proximal to the fracture was elevated and allograft bone was incorporated below the articular surface (Figures 4A, 4B). Rigid fixation of the fracture was achieved using 3 screws (2 Bio-Compression Screws [Arthrex Inc., Naples, Florida] and 1 Synthes cannulated screw [Synthes, West Chester, Pennsylvania]). The screws were placed in posteroanterior (PA) direction and inserted into the weight-bearing articular surface of the femoral condyle (Figures 4C, 4D). The screws were countersunk, and stable fixation with compression of the fracture was achieved. Reduction and screw position were verified with fluoroscopic views. The wound was closed in layers, and the patient was discharged home the next day.
Postoperatively, the patient was non-weight-bearing on the affected limb with a hinged-knee brace to allow for knee ROM exercises immediately. He was also given a continuous passive motion device to maintain knee motion. At the 6-week mark, the patient’s fracture alignment appeared to be well-maintained and showed interval healing. Clinically, the patient was noted to have limited knee ROM. The decision was made to take the patient to the operating room primarily for a manipulation under anesthesia and resection of scar tissue from postoperative arthrofibrosis. Arthroscopic screw removal was also planned as a secondary procedure at the same time in order to prevent the possibility of chondral injury from screw migration. During the procedure, the patient was noted to have improved ROM from 5º to 85º premanipulation to 5º to 110º postoperatively. At 3 months after the initial injury, the patient was allowed to begin progressive weight-bearing on the left knee. At most recent follow-up, after 12 months, the patient was able to ambulate and bear weight on the left leg without pain. Plain radiographs show a well-healed fracture with no evidence of collapse of the femoral condyle (Figures 5A, 5B). His active ROM of the left knee was 5º to 110º without pain (Figures 5C, 5D).
Discussion
In the vast majority of patellar dislocations, the patella dislocates laterally over the trochlear groove. Inferior, or intra-articular, dislocations of the patella are rare. The mechanism of injury is usually a blow onto the patella with a flexed knee. The 2 groups commonly involved are adolescent boys and the elderly.8,9 In young men, it is thought that lax patellar attachments place adolescents at higher risk for this type of injury.10-12 While patella fractures and frank extensor mechanism ruptures are uncommon in this age group, the same mechanism of injury can lead to stripping of the deep fibers of the patellar tendon from the superior pole of the patella.3,13 The intact superficial fibers of the tendon allow the patella to hinge and displace into the joint.14
Inferior dislocations of the patella are classified into 2 types based on the orientation of the articular surface and the presence of osteophytes.15 Type I inferior dislocations occur after a direct blow to a flexed knee forces the superior pole of the patella into the intercondylar notch. Type II dislocations are caused by osteophytes on the superior pole of the patella that become wedged in the intercondylar notch and dislocate the patella inferiorly. In type I dislocations, the patella is rotated in the horizontal plane and the articular surface often faces inferiorly, but type II dislocations do not involve rotation of the articular surface. Type II injuries are seen more commonly in the elderly.
Our patient was able to tolerate a closed reduction of the patella after an intra-articular lidocaine injection, and a successful reduction was achieved without great difficulty. However, the majority of reports describe the need for an open reduction of inferior patellar dislocations.3,8 When closed reductions were a success, they were performed under general anesthesia or conscious sedation.3 It is thought that the difficulty of reduction results from the tension of the quadriceps muscle pulling the patella superiorly into intercondylar notch.11,16 However, successful closed reduction may be more likely in patients with less patellar rotation and entrapment within the intercondylar notch, as well as in patients whose knee is near full extension at presentation.17-19 Successful closed reduction is also seen in elderly patients, where dislocation is generally caused by less forceful impact and held by osteophytes. In these patients, the knee is commonly held in extension.12,15,20-22
The fracture pattern seen in this case also shows a rare fracture in skeletally immature patients, with only a few case reports in the literature. Isolated coronal plane femur fractures account for 0.65% of all femur fractures and are usually seen in adults after high-energy trauma.23 In the skeletally immature, the fracture can occur with lower-energy mechanisms. The typical mechanism is thought to be a shearing force to the femur caused by an axial load to the knee in 90° or more of flexion.4,24 A CT scan is recommended for better identification of the fracture and to plan treatment.25,26 Because of their intra-articular nature and tenuous blood supply, Hoffa fractures tend to do poorly with nonoperative treatment and are prone to displacement and nonunion.27,28 The goal of operative treatment is to obtain anatomic reduction and rigid fixation. While operative fixation techniques are varied, screw fixation with multiple smaller diameter screws has equal pullout strength compared to larger screws and may minimize damage to the articular cartilage.29-31 By preserving blood supply to the fracture, and allowing for early active mobilization, operative treatment generally provides good long-term functional outcomes in these fracture patterns.24
Conclusion
We describe a case in which the patella of an adolescent boy dislocated inferiorly into the knee joint, with an associated coronal shear fracture of the lateral femoral condyle. To our knowledge, this constellation of injuries has not been reported. For this uncommon injury pattern, we recommend a sequential treatment algorithm to minimize morbidity. We recommend first attempting a closed reduction of the patella with adequate pain control to avoid the morbidity associated with general anesthesia. After a successful reduction, an advanced imaging study (eg, MRI) is advisable to assess for concomitant soft-tissue injuries and preoperative planning, if necessary. The mechanism of injury and force required to cause a patellar dislocation of this nature leaves a high likelihood of other injuries. When a fracture is noted on plain radiographs after reduction, a CT scan can provide important information for planning surgical fixation of the fracture. Even in a skeletally immature patient, the principle of direct reduction and stable interfragmentary fixation of an articular fracture is critical for long-term function, even after a significant trauma to the knee.
In 1887, Midelfart1 first reported on an intra-articular dislocation of the patella, and since then approximately 50 cases have been reported in the worldwide literature.2 Also known as an inferior patellar dislocation, these rare traumatic events occur when the patella dislocates intra-articularly. Because the patella commonly rotates about its horizontal axis, the articular surface is facing proximally or distally. The patella becomes lodged within the trochlea and locks the knee joint. Most cases described in the literature involved adolescent boys, with the patella difficult to reduce. Most patients required open reduction, while those who underwent successful closed reduction often needed general anesthesia.3
Similarly, coronal shear fractures of the femoral condyle (ie, Hoffa fractures) are an uncommon fracture pattern typically seen in adults. These fractures are even more infrequent in skeletally immature patients, with fewer than 5 cases documented in the literature.4-7 In our case report, we present a 14-year-old boy with a coronal shear fracture of the femoral condyle associated with an intra-articular patellar dislocation. To our knowledge, this constellation of injuries has not been reported. Additionally, closed reduction of the patella was successful after intra-articular lidocaine injection, without the need for sedation or general anesthesia. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy presented to our institution after sustaining a direct blow to his left knee. The injury occurred as he jumped and landed on a flexed knee while playing with friends. The patient was unable to ambulate after the injury, and his left knee was locked in a slightly flexed position. Examination in the emergency department showed the knee to be held in approximately 60º of flexion, with an obvious bony prominence noted anteriorly over the femoral condyles. The patient was unable to perform a straight leg raise or any active range of motion (ROM) at the knee. Radiographs performed with the knee maintained in flexion confirmed that the patella was displaced into the knee joint and was rotated with the articular surface facing distally. Also noted was a coronal shear fracture of the lateral femoral condyle (Figures 1A, 1B).
The patient received pain medication and an intra-articular lidocaine injection prior to a reduction attempt by the orthopedic resident. With the patient supine, the hip was gently flexed to relax the quadriceps muscle. As the knee was flexed up to 110º, the prominent patella was gripped between the thumb and fingers to gently free and elevate the patella out of the intercondylar notch.
After reduction, an immediate return of normal patellar contour and patellofemoral tracking was observed as the knee was gently extended. There was no obvious defect to the patellar or quadriceps tendons, and the patient was able to perform a straight-leg raise, confirming the integrity of the extensor mechanism. Radiographs performed after the reduction confirmed relocation of the patella in correct anatomic position, as well as a lateral femoral condyle fracture (Figures 2A, 2B). Magnetic resonance imaging (MRI) of the knee confirmed no full-thickness quadriceps or patellar tendon tear. A computed tomography (CT) scan of the knee showed a comminuted fracture of the lateral femoral condyle in the coronal plane, as well as multiple bone fragments within the joint (Figures 3A, 3B). The patient was placed in a bulky soft dressing and underwent open reduction and internal fixation of the fracture.
A 10-cm incision was made over the anterior aspect of the knee, and after dissection to the level of the retinaculum, a lateral parapatellar arthrotomy was performed. The patella was retracted medially to identify and free the fracture fragments. The fracture fragments were provisionally reduced and stabilized with three 0.065-in Kirschner wires. An area of osteochondral impaction proximal to the fracture was elevated and allograft bone was incorporated below the articular surface (Figures 4A, 4B). Rigid fixation of the fracture was achieved using 3 screws (2 Bio-Compression Screws [Arthrex Inc., Naples, Florida] and 1 Synthes cannulated screw [Synthes, West Chester, Pennsylvania]). The screws were placed in posteroanterior (PA) direction and inserted into the weight-bearing articular surface of the femoral condyle (Figures 4C, 4D). The screws were countersunk, and stable fixation with compression of the fracture was achieved. Reduction and screw position were verified with fluoroscopic views. The wound was closed in layers, and the patient was discharged home the next day.
Postoperatively, the patient was non-weight-bearing on the affected limb with a hinged-knee brace to allow for knee ROM exercises immediately. He was also given a continuous passive motion device to maintain knee motion. At the 6-week mark, the patient’s fracture alignment appeared to be well-maintained and showed interval healing. Clinically, the patient was noted to have limited knee ROM. The decision was made to take the patient to the operating room primarily for a manipulation under anesthesia and resection of scar tissue from postoperative arthrofibrosis. Arthroscopic screw removal was also planned as a secondary procedure at the same time in order to prevent the possibility of chondral injury from screw migration. During the procedure, the patient was noted to have improved ROM from 5º to 85º premanipulation to 5º to 110º postoperatively. At 3 months after the initial injury, the patient was allowed to begin progressive weight-bearing on the left knee. At most recent follow-up, after 12 months, the patient was able to ambulate and bear weight on the left leg without pain. Plain radiographs show a well-healed fracture with no evidence of collapse of the femoral condyle (Figures 5A, 5B). His active ROM of the left knee was 5º to 110º without pain (Figures 5C, 5D).
Discussion
In the vast majority of patellar dislocations, the patella dislocates laterally over the trochlear groove. Inferior, or intra-articular, dislocations of the patella are rare. The mechanism of injury is usually a blow onto the patella with a flexed knee. The 2 groups commonly involved are adolescent boys and the elderly.8,9 In young men, it is thought that lax patellar attachments place adolescents at higher risk for this type of injury.10-12 While patella fractures and frank extensor mechanism ruptures are uncommon in this age group, the same mechanism of injury can lead to stripping of the deep fibers of the patellar tendon from the superior pole of the patella.3,13 The intact superficial fibers of the tendon allow the patella to hinge and displace into the joint.14
Inferior dislocations of the patella are classified into 2 types based on the orientation of the articular surface and the presence of osteophytes.15 Type I inferior dislocations occur after a direct blow to a flexed knee forces the superior pole of the patella into the intercondylar notch. Type II dislocations are caused by osteophytes on the superior pole of the patella that become wedged in the intercondylar notch and dislocate the patella inferiorly. In type I dislocations, the patella is rotated in the horizontal plane and the articular surface often faces inferiorly, but type II dislocations do not involve rotation of the articular surface. Type II injuries are seen more commonly in the elderly.
Our patient was able to tolerate a closed reduction of the patella after an intra-articular lidocaine injection, and a successful reduction was achieved without great difficulty. However, the majority of reports describe the need for an open reduction of inferior patellar dislocations.3,8 When closed reductions were a success, they were performed under general anesthesia or conscious sedation.3 It is thought that the difficulty of reduction results from the tension of the quadriceps muscle pulling the patella superiorly into intercondylar notch.11,16 However, successful closed reduction may be more likely in patients with less patellar rotation and entrapment within the intercondylar notch, as well as in patients whose knee is near full extension at presentation.17-19 Successful closed reduction is also seen in elderly patients, where dislocation is generally caused by less forceful impact and held by osteophytes. In these patients, the knee is commonly held in extension.12,15,20-22
The fracture pattern seen in this case also shows a rare fracture in skeletally immature patients, with only a few case reports in the literature. Isolated coronal plane femur fractures account for 0.65% of all femur fractures and are usually seen in adults after high-energy trauma.23 In the skeletally immature, the fracture can occur with lower-energy mechanisms. The typical mechanism is thought to be a shearing force to the femur caused by an axial load to the knee in 90° or more of flexion.4,24 A CT scan is recommended for better identification of the fracture and to plan treatment.25,26 Because of their intra-articular nature and tenuous blood supply, Hoffa fractures tend to do poorly with nonoperative treatment and are prone to displacement and nonunion.27,28 The goal of operative treatment is to obtain anatomic reduction and rigid fixation. While operative fixation techniques are varied, screw fixation with multiple smaller diameter screws has equal pullout strength compared to larger screws and may minimize damage to the articular cartilage.29-31 By preserving blood supply to the fracture, and allowing for early active mobilization, operative treatment generally provides good long-term functional outcomes in these fracture patterns.24
Conclusion
We describe a case in which the patella of an adolescent boy dislocated inferiorly into the knee joint, with an associated coronal shear fracture of the lateral femoral condyle. To our knowledge, this constellation of injuries has not been reported. For this uncommon injury pattern, we recommend a sequential treatment algorithm to minimize morbidity. We recommend first attempting a closed reduction of the patella with adequate pain control to avoid the morbidity associated with general anesthesia. After a successful reduction, an advanced imaging study (eg, MRI) is advisable to assess for concomitant soft-tissue injuries and preoperative planning, if necessary. The mechanism of injury and force required to cause a patellar dislocation of this nature leaves a high likelihood of other injuries. When a fracture is noted on plain radiographs after reduction, a CT scan can provide important information for planning surgical fixation of the fracture. Even in a skeletally immature patient, the principle of direct reduction and stable interfragmentary fixation of an articular fracture is critical for long-term function, even after a significant trauma to the knee.
1. Midelfart V. En sjelden luxation of patella. Norsk Magazin for Laegevidenskaben. 1887;4:588.
2. Kramer DE, Simoni MK. Horizontal intra-articular patellar dislocation resulting in quadriceps avulsion and medial patellofemoral ligament tear: a case report. J Pediatr Orthop B. 2013;22(4):329-332.
3. van den Broek TA, Moll PJ. Horizontal rotation of the patella. A case report with review of the literature. Acta Orthop Scand. 1985;56(5):436-438.
4. Flanagin BA, Cruz AI, Medvecky MJ. Hoffa fracture in a 14-year-old. Orthopedics. 2011;34(2):138.
5. Strauss E, Nelson JM, Abdelwahab IF. Fracture of the lateral femoral condyle. A case report. Bull Hosp Jt Dis Orthop Inst. 1984;44(1):86-90.
6. Biau DJ, Schranz PJ. Transverse Hoffa’s or deep osteochondral fracture? An unusual fracture of the lateral femoral condyle in a child. Injury. 2005;36(7):862-865.
7. McDonough PW, Bernstein RM. Nonunion of a Hoffa fracture in a child. J Orthop Trauma. 2000;14(7):519-521.
8. Brady TA, Russell D. Interarticular horizontal dislocation of the patella. A case report. J Bone Joint Surg Am. 1965;47(7):1393-1396.
9. Yuguero M, Gonzalez JA, Carma A, Huguet J. Intra-articular patellar dislocation. Orthopedics. 2003;26(5):517-518.
10. Frangakis EK. Intra-articular dislocation of the patella. A case report. J Bone Joint Surg Am. 1974;56(2):423-424.
11. Nanda R, Yadav RS, Thakur M. Intra-articular dislocation of the patella. J Trauma. 2000;48(1):159-160.
12. Choudhary RK, Tice JW. Intra-articular dislocation of the patella with incomplete rotation--two case reports and a review of the literature. Knee. 2004;11(2):125-127.
13. Chatziantoniou I, Diakos G, Pantelelli M. Horizontal dislocation of the patella. Case report. EEXOT. 2008;59(2):112-114.
14. McHugh G, Ryan E, Cleary M, Kenny P, O’Flanagan S, Keogh P. Intra-articular dislocation of the patella. Case Rep Orthop. 2013;2013:535803.
15. Bankes MJ, Eastwood DM. Inferior dislocation of the patella in the degenerate knee. Injury. 2002;33(6):528-529.
16. Theodorides A, Guo S, Case R. Intra-articular dislocation of the patella: A case report and review of the literature. Injury Extra. 2010;41(10):103-105.
17. Dimentberg RA. Intra-articular dislocation of the patella: case report and literature review. Clin J Sport Med. 1997;7(2):126-128.
18. Morin WD, Steadman JR. Case report of a successful closed reduction without anesthesia. Clin Orthop. 1993(297):179-181.
19. Murakami Y. Intra-articular dislocation of the patella. A case report. Clin Orthop. 1982;171:137-139.
20. Joshi RP. Inferior dislocation of the patella. Injury. 1997;28(5-6):389-390.
21. Garner JP, Pike JM, George CD. Intra-articular dislocation of the patella: two cases and literature review. J Trauma. 1999;47(4):780-783.
22. McCarthy TA, Quinn B, Pegum JM. Inferior dislocation of the patella: an unusual cause of a locked knee. Ir J Med Sci. 2001;170(3):209-210.
23. Manfredini M, Gildone A, Ferrante R, Bernasconi S, Massari L. Unicondylar femoral fractures: therapeutic strategy and long-term results. A review of 23 patients. Acta Orthop Belg. 2001;67(2):132-138.
24. Holmes SM, Bomback D, Baumgaertner MR. Coronal fractures of the femoral condyle: a brief report of five cases. J Orthop Trauma. 2004;18(5):316-319.
25. Nork SE, Segina DN, Aflatoon K, et al. The association between supracondylar-intercondylar distal femoral fractures and coronal plane fractures. J Bone Joint Surg Am. 2005;87(3):564-569.
26. Allmann KH, Altehoefer C, Wildanger G, et al. Hoffa fracture--a radiologic diagnostic approach. J Belge Radiol. 1996;79(5):201-202.
27. Oztürk A, Ozkan Y, Ozdemir RM. Nonunion of a Hoffa fracture in an adult. Chir Organi Mov. 2009;93(3):183-185.
28. Lewis SL, Pozo JL, Muirhead-Allwood WF. Coronal fractures of the lateral femoral condyle. J Bone Joint Surg Br. 1989;71(1):118-120.
29. Arastu MH, Kokke MC, Duffy PJ, Korley RE, Buckley RE. Coronal plane partial articular fractures of the distal femoral condyle: current concepts in management. Bone Joint J. 2013;95-B(9):1165-1171.
30. Westmoreland GL, McLaurin TM, Hutton WC. Screw pullout strength: a biomechanical comparison of large-fragment and small-fragment fixation in the tibial plateau. J Orthop Trauma. 2002;16(3):178-181.
31. Jarit GJ, Kummer FJ, Gibber MJ, Egol KA. A mechanical evaluation of two fixation methods using cancellous screws for coronal fractures of the lateral condyle of the distal femur (OTA type 33B). J Orthop Trauma. 2006;20(4):273-276.
1. Midelfart V. En sjelden luxation of patella. Norsk Magazin for Laegevidenskaben. 1887;4:588.
2. Kramer DE, Simoni MK. Horizontal intra-articular patellar dislocation resulting in quadriceps avulsion and medial patellofemoral ligament tear: a case report. J Pediatr Orthop B. 2013;22(4):329-332.
3. van den Broek TA, Moll PJ. Horizontal rotation of the patella. A case report with review of the literature. Acta Orthop Scand. 1985;56(5):436-438.
4. Flanagin BA, Cruz AI, Medvecky MJ. Hoffa fracture in a 14-year-old. Orthopedics. 2011;34(2):138.
5. Strauss E, Nelson JM, Abdelwahab IF. Fracture of the lateral femoral condyle. A case report. Bull Hosp Jt Dis Orthop Inst. 1984;44(1):86-90.
6. Biau DJ, Schranz PJ. Transverse Hoffa’s or deep osteochondral fracture? An unusual fracture of the lateral femoral condyle in a child. Injury. 2005;36(7):862-865.
7. McDonough PW, Bernstein RM. Nonunion of a Hoffa fracture in a child. J Orthop Trauma. 2000;14(7):519-521.
8. Brady TA, Russell D. Interarticular horizontal dislocation of the patella. A case report. J Bone Joint Surg Am. 1965;47(7):1393-1396.
9. Yuguero M, Gonzalez JA, Carma A, Huguet J. Intra-articular patellar dislocation. Orthopedics. 2003;26(5):517-518.
10. Frangakis EK. Intra-articular dislocation of the patella. A case report. J Bone Joint Surg Am. 1974;56(2):423-424.
11. Nanda R, Yadav RS, Thakur M. Intra-articular dislocation of the patella. J Trauma. 2000;48(1):159-160.
12. Choudhary RK, Tice JW. Intra-articular dislocation of the patella with incomplete rotation--two case reports and a review of the literature. Knee. 2004;11(2):125-127.
13. Chatziantoniou I, Diakos G, Pantelelli M. Horizontal dislocation of the patella. Case report. EEXOT. 2008;59(2):112-114.
14. McHugh G, Ryan E, Cleary M, Kenny P, O’Flanagan S, Keogh P. Intra-articular dislocation of the patella. Case Rep Orthop. 2013;2013:535803.
15. Bankes MJ, Eastwood DM. Inferior dislocation of the patella in the degenerate knee. Injury. 2002;33(6):528-529.
16. Theodorides A, Guo S, Case R. Intra-articular dislocation of the patella: A case report and review of the literature. Injury Extra. 2010;41(10):103-105.
17. Dimentberg RA. Intra-articular dislocation of the patella: case report and literature review. Clin J Sport Med. 1997;7(2):126-128.
18. Morin WD, Steadman JR. Case report of a successful closed reduction without anesthesia. Clin Orthop. 1993(297):179-181.
19. Murakami Y. Intra-articular dislocation of the patella. A case report. Clin Orthop. 1982;171:137-139.
20. Joshi RP. Inferior dislocation of the patella. Injury. 1997;28(5-6):389-390.
21. Garner JP, Pike JM, George CD. Intra-articular dislocation of the patella: two cases and literature review. J Trauma. 1999;47(4):780-783.
22. McCarthy TA, Quinn B, Pegum JM. Inferior dislocation of the patella: an unusual cause of a locked knee. Ir J Med Sci. 2001;170(3):209-210.
23. Manfredini M, Gildone A, Ferrante R, Bernasconi S, Massari L. Unicondylar femoral fractures: therapeutic strategy and long-term results. A review of 23 patients. Acta Orthop Belg. 2001;67(2):132-138.
24. Holmes SM, Bomback D, Baumgaertner MR. Coronal fractures of the femoral condyle: a brief report of five cases. J Orthop Trauma. 2004;18(5):316-319.
25. Nork SE, Segina DN, Aflatoon K, et al. The association between supracondylar-intercondylar distal femoral fractures and coronal plane fractures. J Bone Joint Surg Am. 2005;87(3):564-569.
26. Allmann KH, Altehoefer C, Wildanger G, et al. Hoffa fracture--a radiologic diagnostic approach. J Belge Radiol. 1996;79(5):201-202.
27. Oztürk A, Ozkan Y, Ozdemir RM. Nonunion of a Hoffa fracture in an adult. Chir Organi Mov. 2009;93(3):183-185.
28. Lewis SL, Pozo JL, Muirhead-Allwood WF. Coronal fractures of the lateral femoral condyle. J Bone Joint Surg Br. 1989;71(1):118-120.
29. Arastu MH, Kokke MC, Duffy PJ, Korley RE, Buckley RE. Coronal plane partial articular fractures of the distal femoral condyle: current concepts in management. Bone Joint J. 2013;95-B(9):1165-1171.
30. Westmoreland GL, McLaurin TM, Hutton WC. Screw pullout strength: a biomechanical comparison of large-fragment and small-fragment fixation in the tibial plateau. J Orthop Trauma. 2002;16(3):178-181.
31. Jarit GJ, Kummer FJ, Gibber MJ, Egol KA. A mechanical evaluation of two fixation methods using cancellous screws for coronal fractures of the lateral condyle of the distal femur (OTA type 33B). J Orthop Trauma. 2006;20(4):273-276.
The Effect of Arthroscopic Rotator Interval Closure on Glenohumeral Volume
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
Spontaneous Osteonecrosis of Knee After Arthroscopy Is Not Necessarily Related to the Procedure
The term spontaneous osteonecrosis of the knee was first used by Ahlbäck1 in 1968. This term, and the acronym SONK (sometimes SPONK2), has subsequently been used by other authors to refer to an apparent osteonecrosis of the knee, most commonly occurring within the medial femoral condyle. SONK typically occurs in older women who usually do not have the typical osteonecrosis risk factors, such as steroid use, sickle-cell anemia, and excessive alcohol intake. Furthermore, the radiologic appearance of SONK differs from the typical avascular necrosis findings seen with radiography and magnetic resonance imaging (MRI). In particular, on MRI, the abnormality of SONK does not have the typical serpiginous margin of bone infarction, or the double-line sign indicating both sclerosis and granulation tissue.3 SONK is normally seen as a line of signal intensity on T1- and T2-weighted sequences; this line is adjacent to or parallels the subchondral bone with an adjacent area of extensive edema.
There is dispute over the cause of SONK. Yamamoto and Bullough4 proposed the lesion is in part a subchondral insufficiency fracture and staged it into 4 parts. Histologic findings suggest at least some SONK lesions are subchondral insufficiency fractures.5 Brahme and colleagues6 were the first to describe SONK occurring after arthroscopy, and others have documented this finding. The condition has also been referred to as osteonecrosis in the postoperative knee.7-13 An association of postoperative SONK with cartilage loss and meniscal tear has been proposed.7-13
We reviewed the clinical, radiologic, and MRI findings in 11 patients with evidence of postarthroscopy SONK to try to identify any risk factors that might predispose them to poor outcomes. Our study population consisted of 11 patients (12 knees) with SONK; 6 of the knees had the lesion before knee arthroscopy, and the other 6 developed the lesion after arthroscopy. We also considered MRI findings in a group of 11 age- and sex-matched patients who underwent knee arthroscopy and did not have or develop SONK. We reviewed the preoperative MRI findings of both groups for meniscal tear, meniscal extrusion, and cartilage loss. We had 2 hypotheses. First, patients with preoperative MRI findings of SONK would have articular cartilage changes, posterior root degeneration, and meniscal extrusion similar to those of patients who developed SONK after arthroscopy. Second, an age- and sex-matched group of patients who underwent arthroscopy and did not develop SONK would be similar in articular cartilage changes, posterior root degeneration or tear, and meniscal extrusion.
Materials and Methods
With institutional review board approval and waived informed consent, we reviewed all imaging studies, particularly the radiographs and MRI studies, of 11 patients (12 knees) who either had SONK before arthroscopy or developed it after arthroscopy. In all these cases, arthroscopy was performed to alleviate mechanical symptoms associated with meniscal tear.
On subsequent review by a musculoskeletal radiologist, 6 patients with SONK had an identifiable lesion before surgery. All patients’ symptoms had not improved with an earlier trial of conservative management. All preoperative and postoperative radiologic and MRI findings were reviewed. The patient group was assembled by writing to all the orthopedic surgeons who performed arthroscopy at our institution and asking for SONK cases seen in their practices. All but 2 cases were performed by a surgeon who treated a predominantly older, less active population. Clinical notes were reviewed for outcomes, and the musculoskeletal radiologist reviewed all radiologic studies. The 4 men and 7 women in the SONK group (1 woman had bilateral knee lesions) ranged in age from 43 to 74 years (mean, 63.8 years), and the 4 men and 7 women in the control group were age-matched to 43 to 75 years (mean, 63.6 years). The controls were chosen from a pool of patients who underwent knee arthroscopy at our institution.
MRI was performed using General Electric 1-T, 1.5-T, or 3-T magnets (GE Healthcare, Milwaukee, Wisconsin) or using Philips 1.5-T or open 0.7-T magnets (Philips Healthcare, Andover, Massachusetts). Imaging included sagittal and coronal proton density–weighted sequences and coronal and axial fat-suppressed T2-weighted sequences. SONK was diagnosed when a low signal line adjacent to the subchondral bone plate on the femoral or tibial condyles was present with an adjacent area of bone marrow edema in the respective condyle or when there was depression of the subchondral bone plate with adjacent edema. The MRI studies were reviewed for lesion location, and medial meniscus and lateral meniscus were reviewed for tear. Type of meniscal tear (horizontal cleavage, radial, complex degenerative) was documented, as was meniscal extrusion. The meniscus was regarded as extruded if the body extended more than 3 mm from the joint margin. Cartilage in the medial and lateral compartment was reviewed according to a modified Noyes scale listing 0 as normal, 1 as internal changes only, 2A as 1% to 49% cartilage loss, 2B as 50% to 90% loss of articular cartilage, 3A as 100% articular cartilage loss with subchondral bone plate intact, and 3B as 100% articular cartilage loss with ulcerated subchondral bone plate.14 Osteoarthritic severity was similarly classified using the Kellgren-Lawrence scale,15 where grade 0 is normal; grade 1 is unlikely to have narrowing of the joint space but potentially has osteophytic lipping; grade 2 has both definite narrowing of the joint space and osteophytes; grade 3 has narrowing of the joint space and multiple osteophytes, some sclerosis, and possible deformity of bone contour; and grade 4 has marked narrowing of the joint space, large osteophytes, severe sclerosis, and definite deformity of bone contour. Follow-up clinical notes and radiologic studies were reviewed in the assessment of patient outcomes.
All statistical analyses were performed with SAS 9.2 software (SAS Institute, Cary, North Carolina). Age data were evaluated with the Shapiro-Wilk test and graphical displays and were found to violate normality assumptions, so they are presented as medians and ranges; other variables are presented as count and column percentages. The Wilcoxon rank sum test was used to compare the 2 groups’ age distributions. Fisher exact tests were used to compare proportions between the 2 groups for the other variables. Statistical significance was set at P < .05.
Results
Table 1 lists the demographics and imaging characteristics of the 11 patients—6 had SONK before arthroscopy and 6 developed it after arthroscopy. Comparison of the 11 patients with SONK and the 11 controls is summarized with P values in Table 2. Representative cases that either presented before surgery or developed after surgery are shown in Figures 1 to 4. There were 6 prearthroscopy lesions and 6 postarthroscopy lesions—all 12 in the medial femoral condyle. Eleven of the 12 knees had a medial meniscal tear, and 1 knee had both medial and lateral meniscal tears. In 8 of the 12 knees, the lateral meniscus was normal; in 2 knees, it had mild degeneration; and, in 1 knee, it had a complex tear. Assessment of hyaline cartilage revealed medial cartilage loss ranging from 2A to 3B (median, 2B) in the patients with SONK, and lateral cartilage loss ranging from 0 to 2A (median, 0). At surgery, all knees had a partial medial meniscectomy, and 6 had a partial lateral meniscectomy. Ten of the 12 knees had chondroplasty, 9 patellar and 5 of the medial femoral condyle. Only 4 of the 11 patients with follow-up of more than 1 year went on to joint replacement. Six of the 12 had follow-up of more than 2 years. Of the 6 patients without an identifiable SONK lesion on MRI before arthroscopy, 4 had mild to moderate knee pain 0.5, 2.4, 3.5, and 4 years after surgery. For the other 2 patients, knee replacement was performed 1.5 and 1.8 years after surgery. Of the 6 patients with prearthroscopy SONK, 4 had mild to moderate knee pain 1.5, 3.7, 6.5, and 6.8 years after surgery; the other 2 had knee replacement 0.5 and 1.8 years after surgery. Articular cartilage degeneration and meniscal extrusion were similar (Table 1). In the control group, there was only 1 knee replacement, at 3 years, and the other 11 were functioning 2.6 to 5 years later. The longer follow-up resulted from selection of appropriate controls from the same year. Of the 6 SONK lesions found on preoperative MRI, 3 were read by the interpreting radiologist before surgery as possible SONK lesions, 2 were read as insufficiency fractures, and 1 was read as a possible insufficiency fracture.
Discussion
SONK is well described as a complication of arthroscopic knee surgery. However, this condition more commonly appears spontaneously in a population that has not had surgery. It has become clear that the term SONK may be misleading.16 In a recent series of postoperative subchondral fractures reported by MacDessi and colleagues,5 the average age of patients included in their study was 64 years. Pathologic analysis revealed subchondral fracture with callus formation in all cases. Only 2 knees had evidence of osteonecrosis, which appeared to be secondary to the fracture. Based on these findings, the authors concluded that “further investigation into the etiology of this condition is warranted.” A prominent association with medial meniscal tear has been noted, with the medial femoral condyle predominantly affected. As already mentioned, SONK differs from classical avascular necrosis on several points, including lack of the typical avascular osteonecrosis risk factors and absence of the serpiginous margin and double-line sign seen with typical bone infarction. In addition, the SONK lesions seen on radiographs and MRIs of the knee typically are in the medial femoral condyle and are very different from the typical area of infarction seen in patients with known risk factors for secondary osteonecrosis.
The cause of SONK is not known. Of more importance from a medicolegal standpoint is that these lesions are not necessarily related to arthroscopy.17 Interestingly, Pape and colleagues17 noted that some of the lesions they studied may have been present before surgery, which is what we found in 6 (50%) of the SONK knees in our study. Our data thus support the proposition that some SONK lesions are present before arthroscopy, and some cases of so-called postarthroscopy SONK may in fact have been progressing before surgery.
Our data also reinforce the importance of radiologist–orthopedic surgeon communication regarding the presence of SONK. We emphasize the importance of communicating the MRI findings clearly, whether the lesion is called SONK, SPONK, or insufficiency fracture. The orthopedic surgeons in our series may have been unaware of the presence of these lesions before arthroscopic meniscectomy, given the wide variety of terms being used in radiologic reports.
The natural history of spontaneous osteonecrosis of the medial tibial plateau has also been studied.18 There were 3 outcome patterns—acute extensive collapse of the medial tibial plateau, rapid progression to varying degrees of osteoarthritis, and complete resolution. It has been shown that resolution of SONK can occur in the early stages of the disease, within several months, but often the changes progress to bone destruction and articular cartilage collapse.19
In our series of patients, there was a female predominance, and mean age was 64 years. We investigated cartilage loss, meniscal tear, and meniscal extrusion to see if we could predict outcomes in patients who had the lesion before arthroscopy and if we could predict who might be at risk for developing the lesion after arthroscopy. Type of surgical procedure was also reviewed. For the sake of simplicity, we divided the follow-up patients into 2 groups: those managed with conservative treatment, which we deemed a reasonable outcome, and those who subsequently required knee joint replacement, which we deemed a poor outcome. As seen from our representative cases, both groups had patients with cartilage loss, meniscal tear, and meniscal extrusion to varying degrees. There were no risk factors pointing to a reasonable or poor outcome. In the group of patients with prearthroscopy lesions, we found the same problem. We were unable to identify a risk factor that might suggest a poor rather than a reasonable outcome. We must also emphasize that, in our review of patient charts, we could find no other causes for osteonecrosis. In particular, arthroscopic causes of acute chondral loss (eg, thermal wash, laser, bupivacaine pain pumps, epinephrine in irrigant) were not identified.
This study consisted of a series of cases managed at our institution over the past 8 years. Our data and this study had several limitations:
We may have been unable to identify other SONK cases that belonged in the group from our institution. In addition, we had only 11 patients for comparison with patients without SONK. Likewise, there were only 6 knees each in the prearthroscopy and postarthroscopy SONK groups. We also used images obtained from 1-T, 1.5-T, and 3-T closed MRI devices and one 0.7-T open device. These were, however, at the same institution.
Timing of our imaging was not uniform. In particular, in 3 of the patients who developed SONK after arthroscopy, preoperative MRI studies were performed quite some time before surgery. However, in these patients, more recent preoperative radiographs did not show any evidence of lesions. It can also be seen that postarthroscopy follow-up of patients varied. It is possible that, on longer follow-up, some of the cases we classified as having a reasonable outcome may have gone on to require total knee arthroplasty. One could argue that, in the patient who developed SONK within 1 year after surgery (Figure 4), the lesion was not related to the surgery. However, this patient’s radiographs 3 months after surgery did not show the SONK lesion but clearly showed prominent medial joint space narrowing—a new finding.
Only 1 musculoskeletal radiologist evaluated the radiographs, MRIs, and tomosynthesis (similar to computed tomography) studies for this investigation.
This lesion is not common, thus giving us a small group to analyze.
Despite our data limitations and the retrospective nature of this study, we compiled a reasonably representative sample of surgical SONK patients that matches other samples reported in the literature. Unfortunately, we could not identify any risk factors pointing to the likelihood of developing SONK or any risk factors pointing to either a reasonable or a poor prognosis in these patients. The etiology of the lesion remains an enigma. Our finding 6 cases of prearthroscopy lesions that did not necessarily result in a poor outcome, combined with our inability to identify any risk factors for SONK, points to the lack of a causal relationship with arthroscopy.
1. Ahlbäck S. Osteoarthritis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
2. Juréus J, Lindstrand A, Geijer M, Robertsson O, Tägil M. The natural course of spontaneous osteonecrosis of the knee (SPONK): a 1- to 27-year follow-up of 40 patients. Acta Orthop. 2013;84(4):410-414.
3. Zurlo JV. The double-line sign. Radiology. 1999;212(2):541-542.
4. Yamamoto T, Bullough PG. Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Joint Surg Am. 2000;82(6):858-866.
5. MacDessi SJ, Brophy RH, Bullough PG, Windsor RE, Sculco TP. Subchondral fracture following arthroscopic knee surgery. A series of eight cases. J Bone Joint Surg Am. 2008;90(5):1007-1012.
6. Brahme SK, Fox JM, Ferkel RD, Friedman MJ, Flannigan BD, Resnick DL. Osteonecrosis of the knee after arthroscopic surgery: diagnosis with MR imaging. Radiology. 1991;178(3):851-853.
7. Faletti C, Robba T, de Petro P. Postmeniscectomy osteonecrosis. Arthroscopy. 2002;18(1):91-94.
8. Johnson TC, Evans JA, Gilley JA, DeLee JC. Osteonecrosis of the knee after arthroscopic surgery for meniscal tears and chondral lesions. Arthroscopy. 2000;16(3):254-261.
9. al-Kaar M, Garcia J, Fritschy D, Bonvin JC. Aseptic osteonecrosis of the femoral condyle after meniscectomy by the arthroscopic approach. J Radiol. 1997;78(4):283-288.
10. DeFalco RA, Ricci AR, Balduini FC. Osteonecrosis of the knee after arthroscopic meniscectomy and chondroplasty: a case report and literature review. Am J Sports Med. 2003;31(6):1013-1016.
11. Kusayama T. Idiopathic osteonecrosis of the femoral condyle after meniscectomy. Tokai J Exp Clin Med. 2003;28(4):145-150.
12. Prues-Latour V, Bonvin JC, Fritschy D. Nine cases of osteonecrosis in elderly patients following arthroscopic meniscectomy. Knee Surg Sports Traumatol Arthrosc. 1998;6(3):142-147.
13. Santori N, Condello V, Adriani E, Mariani PP. Osteonecrosis after arthroscopic medial meniscectomy. Arthroscopy. 1995;11(2):220-224.
14. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
15. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
16. Kidwai AS, Hemphill SD, Griffiths HJ. Radiologic case study. Spontaneous osteonecrosis of the knee reclassified as insufficiency fracture. Orthopedics. 2005;28(3):236, 333-236.
17. Pape D, Lorbach O, Anagnostakos K, Kohn D. Osteonecrosis in the postarthroscopic knee. Orthopade. 2008;37(11):1099-1107.
18. Satku K, Kumar VP, Chacha PB. Stress fractures around the knee in elderly patients. A cause of acute pain in the knee. J Bone Joint Surg Am. 1990;72(6):918-922.
19. Soucacos PN, Xenakis TH, Beris AE, Soucacos PK, Georgoulis A. Idiopathic osteonecrosis of the medial femoral condyle. Classification and treatment. Clin Orthop. 1997;(341):82-89.
The term spontaneous osteonecrosis of the knee was first used by Ahlbäck1 in 1968. This term, and the acronym SONK (sometimes SPONK2), has subsequently been used by other authors to refer to an apparent osteonecrosis of the knee, most commonly occurring within the medial femoral condyle. SONK typically occurs in older women who usually do not have the typical osteonecrosis risk factors, such as steroid use, sickle-cell anemia, and excessive alcohol intake. Furthermore, the radiologic appearance of SONK differs from the typical avascular necrosis findings seen with radiography and magnetic resonance imaging (MRI). In particular, on MRI, the abnormality of SONK does not have the typical serpiginous margin of bone infarction, or the double-line sign indicating both sclerosis and granulation tissue.3 SONK is normally seen as a line of signal intensity on T1- and T2-weighted sequences; this line is adjacent to or parallels the subchondral bone with an adjacent area of extensive edema.
There is dispute over the cause of SONK. Yamamoto and Bullough4 proposed the lesion is in part a subchondral insufficiency fracture and staged it into 4 parts. Histologic findings suggest at least some SONK lesions are subchondral insufficiency fractures.5 Brahme and colleagues6 were the first to describe SONK occurring after arthroscopy, and others have documented this finding. The condition has also been referred to as osteonecrosis in the postoperative knee.7-13 An association of postoperative SONK with cartilage loss and meniscal tear has been proposed.7-13
We reviewed the clinical, radiologic, and MRI findings in 11 patients with evidence of postarthroscopy SONK to try to identify any risk factors that might predispose them to poor outcomes. Our study population consisted of 11 patients (12 knees) with SONK; 6 of the knees had the lesion before knee arthroscopy, and the other 6 developed the lesion after arthroscopy. We also considered MRI findings in a group of 11 age- and sex-matched patients who underwent knee arthroscopy and did not have or develop SONK. We reviewed the preoperative MRI findings of both groups for meniscal tear, meniscal extrusion, and cartilage loss. We had 2 hypotheses. First, patients with preoperative MRI findings of SONK would have articular cartilage changes, posterior root degeneration, and meniscal extrusion similar to those of patients who developed SONK after arthroscopy. Second, an age- and sex-matched group of patients who underwent arthroscopy and did not develop SONK would be similar in articular cartilage changes, posterior root degeneration or tear, and meniscal extrusion.
Materials and Methods
With institutional review board approval and waived informed consent, we reviewed all imaging studies, particularly the radiographs and MRI studies, of 11 patients (12 knees) who either had SONK before arthroscopy or developed it after arthroscopy. In all these cases, arthroscopy was performed to alleviate mechanical symptoms associated with meniscal tear.
On subsequent review by a musculoskeletal radiologist, 6 patients with SONK had an identifiable lesion before surgery. All patients’ symptoms had not improved with an earlier trial of conservative management. All preoperative and postoperative radiologic and MRI findings were reviewed. The patient group was assembled by writing to all the orthopedic surgeons who performed arthroscopy at our institution and asking for SONK cases seen in their practices. All but 2 cases were performed by a surgeon who treated a predominantly older, less active population. Clinical notes were reviewed for outcomes, and the musculoskeletal radiologist reviewed all radiologic studies. The 4 men and 7 women in the SONK group (1 woman had bilateral knee lesions) ranged in age from 43 to 74 years (mean, 63.8 years), and the 4 men and 7 women in the control group were age-matched to 43 to 75 years (mean, 63.6 years). The controls were chosen from a pool of patients who underwent knee arthroscopy at our institution.
MRI was performed using General Electric 1-T, 1.5-T, or 3-T magnets (GE Healthcare, Milwaukee, Wisconsin) or using Philips 1.5-T or open 0.7-T magnets (Philips Healthcare, Andover, Massachusetts). Imaging included sagittal and coronal proton density–weighted sequences and coronal and axial fat-suppressed T2-weighted sequences. SONK was diagnosed when a low signal line adjacent to the subchondral bone plate on the femoral or tibial condyles was present with an adjacent area of bone marrow edema in the respective condyle or when there was depression of the subchondral bone plate with adjacent edema. The MRI studies were reviewed for lesion location, and medial meniscus and lateral meniscus were reviewed for tear. Type of meniscal tear (horizontal cleavage, radial, complex degenerative) was documented, as was meniscal extrusion. The meniscus was regarded as extruded if the body extended more than 3 mm from the joint margin. Cartilage in the medial and lateral compartment was reviewed according to a modified Noyes scale listing 0 as normal, 1 as internal changes only, 2A as 1% to 49% cartilage loss, 2B as 50% to 90% loss of articular cartilage, 3A as 100% articular cartilage loss with subchondral bone plate intact, and 3B as 100% articular cartilage loss with ulcerated subchondral bone plate.14 Osteoarthritic severity was similarly classified using the Kellgren-Lawrence scale,15 where grade 0 is normal; grade 1 is unlikely to have narrowing of the joint space but potentially has osteophytic lipping; grade 2 has both definite narrowing of the joint space and osteophytes; grade 3 has narrowing of the joint space and multiple osteophytes, some sclerosis, and possible deformity of bone contour; and grade 4 has marked narrowing of the joint space, large osteophytes, severe sclerosis, and definite deformity of bone contour. Follow-up clinical notes and radiologic studies were reviewed in the assessment of patient outcomes.
All statistical analyses were performed with SAS 9.2 software (SAS Institute, Cary, North Carolina). Age data were evaluated with the Shapiro-Wilk test and graphical displays and were found to violate normality assumptions, so they are presented as medians and ranges; other variables are presented as count and column percentages. The Wilcoxon rank sum test was used to compare the 2 groups’ age distributions. Fisher exact tests were used to compare proportions between the 2 groups for the other variables. Statistical significance was set at P < .05.
Results
Table 1 lists the demographics and imaging characteristics of the 11 patients—6 had SONK before arthroscopy and 6 developed it after arthroscopy. Comparison of the 11 patients with SONK and the 11 controls is summarized with P values in Table 2. Representative cases that either presented before surgery or developed after surgery are shown in Figures 1 to 4. There were 6 prearthroscopy lesions and 6 postarthroscopy lesions—all 12 in the medial femoral condyle. Eleven of the 12 knees had a medial meniscal tear, and 1 knee had both medial and lateral meniscal tears. In 8 of the 12 knees, the lateral meniscus was normal; in 2 knees, it had mild degeneration; and, in 1 knee, it had a complex tear. Assessment of hyaline cartilage revealed medial cartilage loss ranging from 2A to 3B (median, 2B) in the patients with SONK, and lateral cartilage loss ranging from 0 to 2A (median, 0). At surgery, all knees had a partial medial meniscectomy, and 6 had a partial lateral meniscectomy. Ten of the 12 knees had chondroplasty, 9 patellar and 5 of the medial femoral condyle. Only 4 of the 11 patients with follow-up of more than 1 year went on to joint replacement. Six of the 12 had follow-up of more than 2 years. Of the 6 patients without an identifiable SONK lesion on MRI before arthroscopy, 4 had mild to moderate knee pain 0.5, 2.4, 3.5, and 4 years after surgery. For the other 2 patients, knee replacement was performed 1.5 and 1.8 years after surgery. Of the 6 patients with prearthroscopy SONK, 4 had mild to moderate knee pain 1.5, 3.7, 6.5, and 6.8 years after surgery; the other 2 had knee replacement 0.5 and 1.8 years after surgery. Articular cartilage degeneration and meniscal extrusion were similar (Table 1). In the control group, there was only 1 knee replacement, at 3 years, and the other 11 were functioning 2.6 to 5 years later. The longer follow-up resulted from selection of appropriate controls from the same year. Of the 6 SONK lesions found on preoperative MRI, 3 were read by the interpreting radiologist before surgery as possible SONK lesions, 2 were read as insufficiency fractures, and 1 was read as a possible insufficiency fracture.
Discussion
SONK is well described as a complication of arthroscopic knee surgery. However, this condition more commonly appears spontaneously in a population that has not had surgery. It has become clear that the term SONK may be misleading.16 In a recent series of postoperative subchondral fractures reported by MacDessi and colleagues,5 the average age of patients included in their study was 64 years. Pathologic analysis revealed subchondral fracture with callus formation in all cases. Only 2 knees had evidence of osteonecrosis, which appeared to be secondary to the fracture. Based on these findings, the authors concluded that “further investigation into the etiology of this condition is warranted.” A prominent association with medial meniscal tear has been noted, with the medial femoral condyle predominantly affected. As already mentioned, SONK differs from classical avascular necrosis on several points, including lack of the typical avascular osteonecrosis risk factors and absence of the serpiginous margin and double-line sign seen with typical bone infarction. In addition, the SONK lesions seen on radiographs and MRIs of the knee typically are in the medial femoral condyle and are very different from the typical area of infarction seen in patients with known risk factors for secondary osteonecrosis.
The cause of SONK is not known. Of more importance from a medicolegal standpoint is that these lesions are not necessarily related to arthroscopy.17 Interestingly, Pape and colleagues17 noted that some of the lesions they studied may have been present before surgery, which is what we found in 6 (50%) of the SONK knees in our study. Our data thus support the proposition that some SONK lesions are present before arthroscopy, and some cases of so-called postarthroscopy SONK may in fact have been progressing before surgery.
Our data also reinforce the importance of radiologist–orthopedic surgeon communication regarding the presence of SONK. We emphasize the importance of communicating the MRI findings clearly, whether the lesion is called SONK, SPONK, or insufficiency fracture. The orthopedic surgeons in our series may have been unaware of the presence of these lesions before arthroscopic meniscectomy, given the wide variety of terms being used in radiologic reports.
The natural history of spontaneous osteonecrosis of the medial tibial plateau has also been studied.18 There were 3 outcome patterns—acute extensive collapse of the medial tibial plateau, rapid progression to varying degrees of osteoarthritis, and complete resolution. It has been shown that resolution of SONK can occur in the early stages of the disease, within several months, but often the changes progress to bone destruction and articular cartilage collapse.19
In our series of patients, there was a female predominance, and mean age was 64 years. We investigated cartilage loss, meniscal tear, and meniscal extrusion to see if we could predict outcomes in patients who had the lesion before arthroscopy and if we could predict who might be at risk for developing the lesion after arthroscopy. Type of surgical procedure was also reviewed. For the sake of simplicity, we divided the follow-up patients into 2 groups: those managed with conservative treatment, which we deemed a reasonable outcome, and those who subsequently required knee joint replacement, which we deemed a poor outcome. As seen from our representative cases, both groups had patients with cartilage loss, meniscal tear, and meniscal extrusion to varying degrees. There were no risk factors pointing to a reasonable or poor outcome. In the group of patients with prearthroscopy lesions, we found the same problem. We were unable to identify a risk factor that might suggest a poor rather than a reasonable outcome. We must also emphasize that, in our review of patient charts, we could find no other causes for osteonecrosis. In particular, arthroscopic causes of acute chondral loss (eg, thermal wash, laser, bupivacaine pain pumps, epinephrine in irrigant) were not identified.
This study consisted of a series of cases managed at our institution over the past 8 years. Our data and this study had several limitations:
We may have been unable to identify other SONK cases that belonged in the group from our institution. In addition, we had only 11 patients for comparison with patients without SONK. Likewise, there were only 6 knees each in the prearthroscopy and postarthroscopy SONK groups. We also used images obtained from 1-T, 1.5-T, and 3-T closed MRI devices and one 0.7-T open device. These were, however, at the same institution.
Timing of our imaging was not uniform. In particular, in 3 of the patients who developed SONK after arthroscopy, preoperative MRI studies were performed quite some time before surgery. However, in these patients, more recent preoperative radiographs did not show any evidence of lesions. It can also be seen that postarthroscopy follow-up of patients varied. It is possible that, on longer follow-up, some of the cases we classified as having a reasonable outcome may have gone on to require total knee arthroplasty. One could argue that, in the patient who developed SONK within 1 year after surgery (Figure 4), the lesion was not related to the surgery. However, this patient’s radiographs 3 months after surgery did not show the SONK lesion but clearly showed prominent medial joint space narrowing—a new finding.
Only 1 musculoskeletal radiologist evaluated the radiographs, MRIs, and tomosynthesis (similar to computed tomography) studies for this investigation.
This lesion is not common, thus giving us a small group to analyze.
Despite our data limitations and the retrospective nature of this study, we compiled a reasonably representative sample of surgical SONK patients that matches other samples reported in the literature. Unfortunately, we could not identify any risk factors pointing to the likelihood of developing SONK or any risk factors pointing to either a reasonable or a poor prognosis in these patients. The etiology of the lesion remains an enigma. Our finding 6 cases of prearthroscopy lesions that did not necessarily result in a poor outcome, combined with our inability to identify any risk factors for SONK, points to the lack of a causal relationship with arthroscopy.
The term spontaneous osteonecrosis of the knee was first used by Ahlbäck1 in 1968. This term, and the acronym SONK (sometimes SPONK2), has subsequently been used by other authors to refer to an apparent osteonecrosis of the knee, most commonly occurring within the medial femoral condyle. SONK typically occurs in older women who usually do not have the typical osteonecrosis risk factors, such as steroid use, sickle-cell anemia, and excessive alcohol intake. Furthermore, the radiologic appearance of SONK differs from the typical avascular necrosis findings seen with radiography and magnetic resonance imaging (MRI). In particular, on MRI, the abnormality of SONK does not have the typical serpiginous margin of bone infarction, or the double-line sign indicating both sclerosis and granulation tissue.3 SONK is normally seen as a line of signal intensity on T1- and T2-weighted sequences; this line is adjacent to or parallels the subchondral bone with an adjacent area of extensive edema.
There is dispute over the cause of SONK. Yamamoto and Bullough4 proposed the lesion is in part a subchondral insufficiency fracture and staged it into 4 parts. Histologic findings suggest at least some SONK lesions are subchondral insufficiency fractures.5 Brahme and colleagues6 were the first to describe SONK occurring after arthroscopy, and others have documented this finding. The condition has also been referred to as osteonecrosis in the postoperative knee.7-13 An association of postoperative SONK with cartilage loss and meniscal tear has been proposed.7-13
We reviewed the clinical, radiologic, and MRI findings in 11 patients with evidence of postarthroscopy SONK to try to identify any risk factors that might predispose them to poor outcomes. Our study population consisted of 11 patients (12 knees) with SONK; 6 of the knees had the lesion before knee arthroscopy, and the other 6 developed the lesion after arthroscopy. We also considered MRI findings in a group of 11 age- and sex-matched patients who underwent knee arthroscopy and did not have or develop SONK. We reviewed the preoperative MRI findings of both groups for meniscal tear, meniscal extrusion, and cartilage loss. We had 2 hypotheses. First, patients with preoperative MRI findings of SONK would have articular cartilage changes, posterior root degeneration, and meniscal extrusion similar to those of patients who developed SONK after arthroscopy. Second, an age- and sex-matched group of patients who underwent arthroscopy and did not develop SONK would be similar in articular cartilage changes, posterior root degeneration or tear, and meniscal extrusion.
Materials and Methods
With institutional review board approval and waived informed consent, we reviewed all imaging studies, particularly the radiographs and MRI studies, of 11 patients (12 knees) who either had SONK before arthroscopy or developed it after arthroscopy. In all these cases, arthroscopy was performed to alleviate mechanical symptoms associated with meniscal tear.
On subsequent review by a musculoskeletal radiologist, 6 patients with SONK had an identifiable lesion before surgery. All patients’ symptoms had not improved with an earlier trial of conservative management. All preoperative and postoperative radiologic and MRI findings were reviewed. The patient group was assembled by writing to all the orthopedic surgeons who performed arthroscopy at our institution and asking for SONK cases seen in their practices. All but 2 cases were performed by a surgeon who treated a predominantly older, less active population. Clinical notes were reviewed for outcomes, and the musculoskeletal radiologist reviewed all radiologic studies. The 4 men and 7 women in the SONK group (1 woman had bilateral knee lesions) ranged in age from 43 to 74 years (mean, 63.8 years), and the 4 men and 7 women in the control group were age-matched to 43 to 75 years (mean, 63.6 years). The controls were chosen from a pool of patients who underwent knee arthroscopy at our institution.
MRI was performed using General Electric 1-T, 1.5-T, or 3-T magnets (GE Healthcare, Milwaukee, Wisconsin) or using Philips 1.5-T or open 0.7-T magnets (Philips Healthcare, Andover, Massachusetts). Imaging included sagittal and coronal proton density–weighted sequences and coronal and axial fat-suppressed T2-weighted sequences. SONK was diagnosed when a low signal line adjacent to the subchondral bone plate on the femoral or tibial condyles was present with an adjacent area of bone marrow edema in the respective condyle or when there was depression of the subchondral bone plate with adjacent edema. The MRI studies were reviewed for lesion location, and medial meniscus and lateral meniscus were reviewed for tear. Type of meniscal tear (horizontal cleavage, radial, complex degenerative) was documented, as was meniscal extrusion. The meniscus was regarded as extruded if the body extended more than 3 mm from the joint margin. Cartilage in the medial and lateral compartment was reviewed according to a modified Noyes scale listing 0 as normal, 1 as internal changes only, 2A as 1% to 49% cartilage loss, 2B as 50% to 90% loss of articular cartilage, 3A as 100% articular cartilage loss with subchondral bone plate intact, and 3B as 100% articular cartilage loss with ulcerated subchondral bone plate.14 Osteoarthritic severity was similarly classified using the Kellgren-Lawrence scale,15 where grade 0 is normal; grade 1 is unlikely to have narrowing of the joint space but potentially has osteophytic lipping; grade 2 has both definite narrowing of the joint space and osteophytes; grade 3 has narrowing of the joint space and multiple osteophytes, some sclerosis, and possible deformity of bone contour; and grade 4 has marked narrowing of the joint space, large osteophytes, severe sclerosis, and definite deformity of bone contour. Follow-up clinical notes and radiologic studies were reviewed in the assessment of patient outcomes.
All statistical analyses were performed with SAS 9.2 software (SAS Institute, Cary, North Carolina). Age data were evaluated with the Shapiro-Wilk test and graphical displays and were found to violate normality assumptions, so they are presented as medians and ranges; other variables are presented as count and column percentages. The Wilcoxon rank sum test was used to compare the 2 groups’ age distributions. Fisher exact tests were used to compare proportions between the 2 groups for the other variables. Statistical significance was set at P < .05.
Results
Table 1 lists the demographics and imaging characteristics of the 11 patients—6 had SONK before arthroscopy and 6 developed it after arthroscopy. Comparison of the 11 patients with SONK and the 11 controls is summarized with P values in Table 2. Representative cases that either presented before surgery or developed after surgery are shown in Figures 1 to 4. There were 6 prearthroscopy lesions and 6 postarthroscopy lesions—all 12 in the medial femoral condyle. Eleven of the 12 knees had a medial meniscal tear, and 1 knee had both medial and lateral meniscal tears. In 8 of the 12 knees, the lateral meniscus was normal; in 2 knees, it had mild degeneration; and, in 1 knee, it had a complex tear. Assessment of hyaline cartilage revealed medial cartilage loss ranging from 2A to 3B (median, 2B) in the patients with SONK, and lateral cartilage loss ranging from 0 to 2A (median, 0). At surgery, all knees had a partial medial meniscectomy, and 6 had a partial lateral meniscectomy. Ten of the 12 knees had chondroplasty, 9 patellar and 5 of the medial femoral condyle. Only 4 of the 11 patients with follow-up of more than 1 year went on to joint replacement. Six of the 12 had follow-up of more than 2 years. Of the 6 patients without an identifiable SONK lesion on MRI before arthroscopy, 4 had mild to moderate knee pain 0.5, 2.4, 3.5, and 4 years after surgery. For the other 2 patients, knee replacement was performed 1.5 and 1.8 years after surgery. Of the 6 patients with prearthroscopy SONK, 4 had mild to moderate knee pain 1.5, 3.7, 6.5, and 6.8 years after surgery; the other 2 had knee replacement 0.5 and 1.8 years after surgery. Articular cartilage degeneration and meniscal extrusion were similar (Table 1). In the control group, there was only 1 knee replacement, at 3 years, and the other 11 were functioning 2.6 to 5 years later. The longer follow-up resulted from selection of appropriate controls from the same year. Of the 6 SONK lesions found on preoperative MRI, 3 were read by the interpreting radiologist before surgery as possible SONK lesions, 2 were read as insufficiency fractures, and 1 was read as a possible insufficiency fracture.
Discussion
SONK is well described as a complication of arthroscopic knee surgery. However, this condition more commonly appears spontaneously in a population that has not had surgery. It has become clear that the term SONK may be misleading.16 In a recent series of postoperative subchondral fractures reported by MacDessi and colleagues,5 the average age of patients included in their study was 64 years. Pathologic analysis revealed subchondral fracture with callus formation in all cases. Only 2 knees had evidence of osteonecrosis, which appeared to be secondary to the fracture. Based on these findings, the authors concluded that “further investigation into the etiology of this condition is warranted.” A prominent association with medial meniscal tear has been noted, with the medial femoral condyle predominantly affected. As already mentioned, SONK differs from classical avascular necrosis on several points, including lack of the typical avascular osteonecrosis risk factors and absence of the serpiginous margin and double-line sign seen with typical bone infarction. In addition, the SONK lesions seen on radiographs and MRIs of the knee typically are in the medial femoral condyle and are very different from the typical area of infarction seen in patients with known risk factors for secondary osteonecrosis.
The cause of SONK is not known. Of more importance from a medicolegal standpoint is that these lesions are not necessarily related to arthroscopy.17 Interestingly, Pape and colleagues17 noted that some of the lesions they studied may have been present before surgery, which is what we found in 6 (50%) of the SONK knees in our study. Our data thus support the proposition that some SONK lesions are present before arthroscopy, and some cases of so-called postarthroscopy SONK may in fact have been progressing before surgery.
Our data also reinforce the importance of radiologist–orthopedic surgeon communication regarding the presence of SONK. We emphasize the importance of communicating the MRI findings clearly, whether the lesion is called SONK, SPONK, or insufficiency fracture. The orthopedic surgeons in our series may have been unaware of the presence of these lesions before arthroscopic meniscectomy, given the wide variety of terms being used in radiologic reports.
The natural history of spontaneous osteonecrosis of the medial tibial plateau has also been studied.18 There were 3 outcome patterns—acute extensive collapse of the medial tibial plateau, rapid progression to varying degrees of osteoarthritis, and complete resolution. It has been shown that resolution of SONK can occur in the early stages of the disease, within several months, but often the changes progress to bone destruction and articular cartilage collapse.19
In our series of patients, there was a female predominance, and mean age was 64 years. We investigated cartilage loss, meniscal tear, and meniscal extrusion to see if we could predict outcomes in patients who had the lesion before arthroscopy and if we could predict who might be at risk for developing the lesion after arthroscopy. Type of surgical procedure was also reviewed. For the sake of simplicity, we divided the follow-up patients into 2 groups: those managed with conservative treatment, which we deemed a reasonable outcome, and those who subsequently required knee joint replacement, which we deemed a poor outcome. As seen from our representative cases, both groups had patients with cartilage loss, meniscal tear, and meniscal extrusion to varying degrees. There were no risk factors pointing to a reasonable or poor outcome. In the group of patients with prearthroscopy lesions, we found the same problem. We were unable to identify a risk factor that might suggest a poor rather than a reasonable outcome. We must also emphasize that, in our review of patient charts, we could find no other causes for osteonecrosis. In particular, arthroscopic causes of acute chondral loss (eg, thermal wash, laser, bupivacaine pain pumps, epinephrine in irrigant) were not identified.
This study consisted of a series of cases managed at our institution over the past 8 years. Our data and this study had several limitations:
We may have been unable to identify other SONK cases that belonged in the group from our institution. In addition, we had only 11 patients for comparison with patients without SONK. Likewise, there were only 6 knees each in the prearthroscopy and postarthroscopy SONK groups. We also used images obtained from 1-T, 1.5-T, and 3-T closed MRI devices and one 0.7-T open device. These were, however, at the same institution.
Timing of our imaging was not uniform. In particular, in 3 of the patients who developed SONK after arthroscopy, preoperative MRI studies were performed quite some time before surgery. However, in these patients, more recent preoperative radiographs did not show any evidence of lesions. It can also be seen that postarthroscopy follow-up of patients varied. It is possible that, on longer follow-up, some of the cases we classified as having a reasonable outcome may have gone on to require total knee arthroplasty. One could argue that, in the patient who developed SONK within 1 year after surgery (Figure 4), the lesion was not related to the surgery. However, this patient’s radiographs 3 months after surgery did not show the SONK lesion but clearly showed prominent medial joint space narrowing—a new finding.
Only 1 musculoskeletal radiologist evaluated the radiographs, MRIs, and tomosynthesis (similar to computed tomography) studies for this investigation.
This lesion is not common, thus giving us a small group to analyze.
Despite our data limitations and the retrospective nature of this study, we compiled a reasonably representative sample of surgical SONK patients that matches other samples reported in the literature. Unfortunately, we could not identify any risk factors pointing to the likelihood of developing SONK or any risk factors pointing to either a reasonable or a poor prognosis in these patients. The etiology of the lesion remains an enigma. Our finding 6 cases of prearthroscopy lesions that did not necessarily result in a poor outcome, combined with our inability to identify any risk factors for SONK, points to the lack of a causal relationship with arthroscopy.
1. Ahlbäck S. Osteoarthritis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
2. Juréus J, Lindstrand A, Geijer M, Robertsson O, Tägil M. The natural course of spontaneous osteonecrosis of the knee (SPONK): a 1- to 27-year follow-up of 40 patients. Acta Orthop. 2013;84(4):410-414.
3. Zurlo JV. The double-line sign. Radiology. 1999;212(2):541-542.
4. Yamamoto T, Bullough PG. Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Joint Surg Am. 2000;82(6):858-866.
5. MacDessi SJ, Brophy RH, Bullough PG, Windsor RE, Sculco TP. Subchondral fracture following arthroscopic knee surgery. A series of eight cases. J Bone Joint Surg Am. 2008;90(5):1007-1012.
6. Brahme SK, Fox JM, Ferkel RD, Friedman MJ, Flannigan BD, Resnick DL. Osteonecrosis of the knee after arthroscopic surgery: diagnosis with MR imaging. Radiology. 1991;178(3):851-853.
7. Faletti C, Robba T, de Petro P. Postmeniscectomy osteonecrosis. Arthroscopy. 2002;18(1):91-94.
8. Johnson TC, Evans JA, Gilley JA, DeLee JC. Osteonecrosis of the knee after arthroscopic surgery for meniscal tears and chondral lesions. Arthroscopy. 2000;16(3):254-261.
9. al-Kaar M, Garcia J, Fritschy D, Bonvin JC. Aseptic osteonecrosis of the femoral condyle after meniscectomy by the arthroscopic approach. J Radiol. 1997;78(4):283-288.
10. DeFalco RA, Ricci AR, Balduini FC. Osteonecrosis of the knee after arthroscopic meniscectomy and chondroplasty: a case report and literature review. Am J Sports Med. 2003;31(6):1013-1016.
11. Kusayama T. Idiopathic osteonecrosis of the femoral condyle after meniscectomy. Tokai J Exp Clin Med. 2003;28(4):145-150.
12. Prues-Latour V, Bonvin JC, Fritschy D. Nine cases of osteonecrosis in elderly patients following arthroscopic meniscectomy. Knee Surg Sports Traumatol Arthrosc. 1998;6(3):142-147.
13. Santori N, Condello V, Adriani E, Mariani PP. Osteonecrosis after arthroscopic medial meniscectomy. Arthroscopy. 1995;11(2):220-224.
14. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
15. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
16. Kidwai AS, Hemphill SD, Griffiths HJ. Radiologic case study. Spontaneous osteonecrosis of the knee reclassified as insufficiency fracture. Orthopedics. 2005;28(3):236, 333-236.
17. Pape D, Lorbach O, Anagnostakos K, Kohn D. Osteonecrosis in the postarthroscopic knee. Orthopade. 2008;37(11):1099-1107.
18. Satku K, Kumar VP, Chacha PB. Stress fractures around the knee in elderly patients. A cause of acute pain in the knee. J Bone Joint Surg Am. 1990;72(6):918-922.
19. Soucacos PN, Xenakis TH, Beris AE, Soucacos PK, Georgoulis A. Idiopathic osteonecrosis of the medial femoral condyle. Classification and treatment. Clin Orthop. 1997;(341):82-89.
1. Ahlbäck S. Osteoarthritis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
2. Juréus J, Lindstrand A, Geijer M, Robertsson O, Tägil M. The natural course of spontaneous osteonecrosis of the knee (SPONK): a 1- to 27-year follow-up of 40 patients. Acta Orthop. 2013;84(4):410-414.
3. Zurlo JV. The double-line sign. Radiology. 1999;212(2):541-542.
4. Yamamoto T, Bullough PG. Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Joint Surg Am. 2000;82(6):858-866.
5. MacDessi SJ, Brophy RH, Bullough PG, Windsor RE, Sculco TP. Subchondral fracture following arthroscopic knee surgery. A series of eight cases. J Bone Joint Surg Am. 2008;90(5):1007-1012.
6. Brahme SK, Fox JM, Ferkel RD, Friedman MJ, Flannigan BD, Resnick DL. Osteonecrosis of the knee after arthroscopic surgery: diagnosis with MR imaging. Radiology. 1991;178(3):851-853.
7. Faletti C, Robba T, de Petro P. Postmeniscectomy osteonecrosis. Arthroscopy. 2002;18(1):91-94.
8. Johnson TC, Evans JA, Gilley JA, DeLee JC. Osteonecrosis of the knee after arthroscopic surgery for meniscal tears and chondral lesions. Arthroscopy. 2000;16(3):254-261.
9. al-Kaar M, Garcia J, Fritschy D, Bonvin JC. Aseptic osteonecrosis of the femoral condyle after meniscectomy by the arthroscopic approach. J Radiol. 1997;78(4):283-288.
10. DeFalco RA, Ricci AR, Balduini FC. Osteonecrosis of the knee after arthroscopic meniscectomy and chondroplasty: a case report and literature review. Am J Sports Med. 2003;31(6):1013-1016.
11. Kusayama T. Idiopathic osteonecrosis of the femoral condyle after meniscectomy. Tokai J Exp Clin Med. 2003;28(4):145-150.
12. Prues-Latour V, Bonvin JC, Fritschy D. Nine cases of osteonecrosis in elderly patients following arthroscopic meniscectomy. Knee Surg Sports Traumatol Arthrosc. 1998;6(3):142-147.
13. Santori N, Condello V, Adriani E, Mariani PP. Osteonecrosis after arthroscopic medial meniscectomy. Arthroscopy. 1995;11(2):220-224.
14. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
15. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
16. Kidwai AS, Hemphill SD, Griffiths HJ. Radiologic case study. Spontaneous osteonecrosis of the knee reclassified as insufficiency fracture. Orthopedics. 2005;28(3):236, 333-236.
17. Pape D, Lorbach O, Anagnostakos K, Kohn D. Osteonecrosis in the postarthroscopic knee. Orthopade. 2008;37(11):1099-1107.
18. Satku K, Kumar VP, Chacha PB. Stress fractures around the knee in elderly patients. A cause of acute pain in the knee. J Bone Joint Surg Am. 1990;72(6):918-922.
19. Soucacos PN, Xenakis TH, Beris AE, Soucacos PK, Georgoulis A. Idiopathic osteonecrosis of the medial femoral condyle. Classification and treatment. Clin Orthop. 1997;(341):82-89.
Incidence and Injury Types in Motorcycle Collisions Involving Deer in Western New York
The combination of urban sprawl and a large deer population has caused deer–motor vehicle collisions to become a major concern over the past few decades. According to State Farm Insurance industry data, New York State drivers in 2010-2011 had a 1 in 149.5 likelihood of colliding with a deer over the year, compared with a national average of 1 in 183.4.1 Reports from the Midwest have highlighted the frequency and severity of this type of accident.2-4 Frequent performance of orthopedic procedures in this subset of trauma patients prompted a local review to determine the frequency and severity of injuries. This series differs from the Midwest studies in the existence of a universal helmet law for all motorcyclists and passengers in New York State. Other studies looking at this type of accident were performed in states, including Minnesota and Wisconsin, that require helmets only for riders younger than 18 years or persons with an instructional permit.5
The Erie County Medical Center (ECMC) is a level I trauma center located in Buffalo, New York, and serves much of western New York, as well as part of northwestern Pennsylvania and, occasionally, southern Ontario, Canada. Because the ECMC receives almost all major trauma cases in the region, we had sufficient records to explore the incidence and the severity of deer–motorcycle accidents in these regions. In addition to adding to the limited data analyzing crash outcomes, we also looked at the numbers and proportions of motorcycle accidents attributable to deer and compared these with results from studies from different geographical regions. Because the number of registered motorcycles in Erie Country is among the highest in New York State, and because of the increased severity of motorcycle–deer collisions relative to other motor vehicle–deer collisions, this issue has both safety and financial considerations.
Materials and Methods
A retrospective review of records from ECMC was performed to capture all records from motorcycle accidents from May 2007 through June 2011. The population was identified to include only motorcycle accidents that were caused by collision with deer.
Injury severity was standardized using the Injury Severity Score (ISS), and the level of consciousness on arrival was standardized using the Glasgow Coma Scale (GCS). Chart abstraction included patient age, identification of the patient as driver or passenger of the motorcycle, use of helmet, time of year, types of injuries, length of hospital stay, and whether the patient lost consciousness. Patient age was also abstracted for the entire initial screen of all motorcycle accidents regardless of mechanism.
Statistical analysis was done using SPSS (IBM SPSS Statistics for Windows, Version 19.0; IBM Corp., Armonk, New York). Continuous data were analyzed using the appropriate descriptive statistics. Comparisons were made using Student t test, and a 0.05 level of significance was accepted.
Results
The initial screening of the trauma database returned 487 patients who had been involved in a motorcycle accident; of these, 39 patients were in an accident that involved a deer. According to one medical record, the spouse of a patient was a passenger who was dead at the scene, although there was no separate medical record for this person; this person was included in our data. Therefore, our total study population numbered 40 patients involved in 36 accidents, with 36 drivers and 4 passengers; 35 were men and 5 were women, with the women accounting for all 4 passengers and 1 driver. The mean (SD) patient age for deer–motorcycle collisions was 48.9 (8.9) years (range, 21-64 years). This was significantly higher than the mean (SD) age for all motorcycle accidents from the ECMC trauma database, which was 41.9 (13.9) years (range, 17-79 years) (P < .002).
The majority of accidents (31; 86%) with deer occurred during the months of May through September, with the most occurring in June (11; 31%). There was only 1 (3%) in October, 3 (8%) in November, and 1 (3%) in January. The number of collisions per year averaged 9.75, with a range of 8 to 12 from 2007-2010. (The year 2011 was omitted because data were collected before the year was complete). The presence or absence of helmet use was recorded in 22 cases. Of these, 21 patients had been wearing a helmet (95%), and only 1 patient was unhelmeted. Among all riders involved in motorcycle accidents from the trauma database, the presence or absence of a helmet was recorded in 271 cases. Of these, 262 (97%) were wearing a helmet. The average length of hospital stay was 6 days, with 6 patients having stays that were 10 days or longer, and the longest stay was 31 days. Thirty-three medical records noted whether the patient described loss of consciousness after the accident; of these, 14 (42%) claimed loss of consciousness and the remaining 19 (58%) denied any loss of consciousness after the accident. The mean (SD) ISS for deer–motorcycle collisions was 17.1 (9.8), and the mean (SD) GCS was 14.3 (2.5).
Chest, orthopedic, and head injuries were the most common injuries seen in deer–motorcycle collisions (Table). Head injuries, including the 1 patient who was confirmed to not have been wearing a helmet, accounted for 15.0% of the total injuries. This patient also had a longer length of stay at 19 days than the average of 6 days. Rib fractures were the most common injury, occurring in 20 (50%) patients. The 1 recorded fatality was the passenger of a patient who was dead at the scene.
Twenty-five (62.5%) patients in this series had injuries that are traditionally treated by orthopedic trauma surgeons, including scapular, clavicle, pelvic, and extremity fractures. Upper and lower extremity injuries occurred 10 (8.3%) and 15 (12.5%) times respectively, with the lower extremity injuries including long bone fractures, foot and ankle fractures, and 1 lower extremity traumatic amputation. Fourteen (35%) patients underwent one or more orthopedic surgical procedures.
Discussion
Although animal–vehicle collisions have been described in the literature, comparatively little data are available for the subset of animal–motorcycle accidents. This is an important gap considering that fatalities in collisions with animals were 6 times more likely to be persons riding motorcycles, although animal collisions are more common with other vehicles.6
Smoot and colleagues2 also reported that motorcycle collisions with deer tend to result in a higher injury severity than collisions of other vehicles with deer. According to reports for Midwestern regions, motorcycle-versus-deer accidents are a significant problem, causing a large number of serious injuries as well as creating the financial burden of vehicle damage and medical costs.2,3 However, the overall data are limited, and there is not much detailed information available for western New York.
Because of the large number of motorcyclists in New York State, it is important to consider accident data in this subset of the population. In 2010, 340,260 motorcycles were registered in New York State, with Erie County having the second highest number (21,745) of motorcycles registered.7 These numbers increased to 345,820 and 22,183 motorcycles, respectively, in 2011.8 In that year, the number of police-reported motorcycle accidents in New York decreased to 4855 from 5047 accidents in 2010, although both numbers are increased from 4647 accidents in 2009.9-11 Despite the decrease in total police-reported motorcycle accidents from 2010 to 2011, the trend in motorcycle accidents involving an animal’s action has steadily increased from 313 (6.7%) in 2009 to 335 (6.6%) in 2010 to 401 (8.3%) in 2011.9-11 Although these data from the New York State Department of Motor Vehicles are not further broken down by animal species, it can be reasonably surmised that most of these are caused by deer. This inference is supported by data from Bramati and colleagues4 showing that 81% of animal–vehicle collisions involved deer, as well as by the Wildlife-Vehicle Collision Reduction Study that showed deer were involved in 54.4% of animal–vehicle collisions in California and more than 90% of animal–vehicle collisions in Illinois and Minnesota.4,12 These studies predominantly comprised collisions involving animals capable of causing substantial property damage on impact, such as deer or larger animals. This, along with the evidence of higher ISS seen in motorcyclists in deer-related traffic injuries,2 supports the intuitive thought that motorcyclists are at increased risk for injury and fatality relative to other motor vehicles involved in accidents.
Williams and Wells13 reviewed 147 fatal wildlife–vehicle fatalities from 9 regions and found that the 2 most common fatalities were the motorcycle driver or passenger after striking an animal or an object. Jones14 also reported that the most common fatal wildlife–vehicle crashes involved motorcycles, as did fatal-accident reporting system data in the Wildlife-Vehicle Collision Reduction Study, which confirmed that approximately 30% of fatal crashes with animals involved motorcycles.12
Interestingly, the age of patients involved in motorcycle–deer collisions tends to be higher than that of patients involved in other motorcycle accidents. The numbers in our study reflect results in other study populations that suggest motorcycle riders who collide with deer are generally older than riders in other accidents who are more likely to be younger.4 One explanation is that younger riders may drive faster and more recklessly than older and experienced riders, resulting in an increased number of accidents unrelated to deer. Another consideration places younger drivers less commonly on roads where wildlife crashes more often occur (ie, roads that are rural, 2-lane).
Helmet use, when reported, was very high in our study population, most likely as a result of New York State’s mandatory helmet law for motorcyclists. Our data showed that more than 95% of patients whose charts documented helmet usage were wearing helmets at the time of the collision, compared with a Wisconsin study showing that only 29% of patients were wearing helmets.3 This may explain the proportion of head injuries in our study being 15.0% compared with the 29.5% in the Wisconsin study.3 Although both datasets involved a limited number of patients, the results suggest that mandatory helmet laws are effective in preventing head injuries. Also, the only patient in our study who was confirmed to have not been wearing a helmet had a much longer length of hospital stay than the average patient (19 vs 6 days). William and Wells13 found that 65% of motorcyclists killed in collisions with animals were not wearing helmets, and they believed that many of these fatalities could have been prevented with helmet use. Again, these limited data suggest the effectiveness of mandatory helmet use.
Two other factors, season and time of day, are important to consider in motorcycle collisions with deer. According to our data, 86% of these collisions occur in the warmer months, May through September, peaking in June. This is similar to findings from the Wisconsin study showing June and July as the peak months for deer–motorcycle collisions and a study in Minnesota where 61% of these crashes occurred in the summer months.2,3 These data most likely indicate increased motorcycle traffic in favorable weather conditions. Although time of accident could not be determined through our retrospective review, multiple studies have shown that the majority of collisions with deer tend to be between dusk and dawn. Smoot and colleagues2 found that 56% of vehicle collisions with deer occurred between 5 pm and midnight, with 80% between 5 pm and 6 am.2 Similarly, Nelson and colleagues3 found that 54.5% of collisions happened in a 4-hour period, from 6 pm to 10 pm. These data indicate that motorcycle operators should be especially vigilant in the morning and evening hours when deer may be more active.
Other than driver awareness and vigilance, prevention efforts can involve wildlife fencing, alert systems, and deer-culling programs. Fences are used extensively, most commonly on larger thoroughfares, and have been shown effective in reducing wildlife vehicle crashes by 80% to 90%.12 Animal detection systems using sensors to detect large wildlife approaching the roadway can activate warning signs to alert approaching drivers. Such systems have been installed in more than 30 locations in North America and Europe with variable effectiveness.12 However, there are typically no standards or guidelines for the collection of data about wildlife–vehicle crashes. Data are collected inconsistently and often haphazardly, and methods vary between states and agencies. Some transportation agencies do not collect this type of data at all. Without reliable, consistent data, it is difficult to identify road sections where mitigation methods may be required, to select appropriate mitigation measures, or to evaluate whether that effort is making a difference.
Culling systems for deer populations are frequently discussed, often in suburban as well as rural settings. Recreational hunting ordinances, higher limits on the number of females a hunter can bag, and occasional use of professional shooters can be applicable in less rural areas. Their effectiveness is debatable and tends to be time-limited.
Conclusion
This study highlights the fairly common occurrence and relative severity of deer–motorcycle crashes in an upstate New York setting, approximating published series from the Midwest. Helmet laws may lower rates of head injury in motorcycle–wildlife crashes. Finally, there are no fender benders when the chosen vehicle sports no fenders, so motorcyclists need to be especially vigilant in order to avoid collisions with deer and other wildlife.
1. Likelihood of collision with deer (amended 2010-2011). State Farm website. https://static1.st8fm.com/en_US/content_pages/1/pdf/us/likelihood-of-collision-2011.pdf. Accessed April 29, 2015.
2. Smoot DL, Zielinski MD, Cullinane DC, Jenkins DH, Schiller HJ, Sawyer MD. Patterns in deer-related traffic injuries over a decade: the Mayo Clinic experience. Scand J Trauma Resusc Emerg Med. 2010;18:46.
3. Nelson RS, Gustafson PT, Szlabick RE. Motorcycle collisions involving white-tailed deer in central and northern Wisconsin: a rural trauma center experience. J Trauma. 2006;60(6):1297-1300.
4. Bramati PS, Heinert LF, Narloch LB, Hostetter J, Finkielman JD. Animal-related motorcycle collisions in North Dakota. Wilderness Environ Med. 2012;23(1):65-69.
5. Save lives, save money – how does your state measure up. Injury Prevention & Control: Motor Vehicle Safety. Centers for Disease Control and Prevention website. http://www.cdc.gov/motorvehiclesafety/mc/states/index.html. Updated June 13, 2012. Accessed April 23, 2015.
6. Langley RL, Higgins SA, Herrin KB. Risk factors associated with fatal animal-vehicle collisions in the United States, 1995-2004. Wilderness Environ Med. 2006;17(4):229-239.
7. Vehicle registrations in force – 2010. Archives of Statistical Summaries. New York State Department of Motor Vehicles website. http://dmv.ny.gov/statistic/regin10.pdf. Accessed May 11, 2015.
8. Vehicle registrations in force – 2011. Archives of Statistical Summaries. New York State Department of Motor Vehicles website. http://dmv.ny.gov/statistic/regin11.pdf. Accessed May 11, 2015.
9. Summary of motorcycle crashes – 2011. Archives of Statistical Summaries. New York State Department of Motor Vehicles website. http://dmv.ny.gov/statistic/2011MotorcycleCrashSummary.pdf. Accessed April 23, 2015.
10. Summary of motorcycle accidents – 2010. Archives of Statistical Summaries. New York State Department of Motor Vehicles website. dmv.ny.gov/statistic/2010MotorcycleAccSummary.pdf. Accessed April 23, 2015.
11. Summary of motorcycle accidents – 2009. Archives of Statistical Summaries. New York State Department of Motor Vehicles website. dmv.ny.gov/statistic/2009MotorcycleSummary.pdf. Accessed April 23, 2015.
12. Huijser MP, McGowen P, Fuller J, et al; Federal Highway Administration. Wildlife-Vehicle Collision Reduction Study: Report to Congress. Report no. FHWA-HRT-08-034. Washington, DC: US Department of Transportation, Federal Highway Administration; 2008. http://www.fhwa.dot.gov/publications/research/safety/08034/08034.pdf. Accessed April 23, 2015.
13. Williams AF, Wells JK. Characteristics of vehicle-animal crashes in which vehicle occupants are killed. Traffic Inj Prev. 2005;6(1):56-59.
14. Jones M. Deer-vehicle crash injuries, fatalities reach all-time high in Wisconsin. Milwaukee Journal Sentinel. April 14, 2000:1B-2B.
The combination of urban sprawl and a large deer population has caused deer–motor vehicle collisions to become a major concern over the past few decades. According to State Farm Insurance industry data, New York State drivers in 2010-2011 had a 1 in 149.5 likelihood of colliding with a deer over the year, compared with a national average of 1 in 183.4.1 Reports from the Midwest have highlighted the frequency and severity of this type of accident.2-4 Frequent performance of orthopedic procedures in this subset of trauma patients prompted a local review to determine the frequency and severity of injuries. This series differs from the Midwest studies in the existence of a universal helmet law for all motorcyclists and passengers in New York State. Other studies looking at this type of accident were performed in states, including Minnesota and Wisconsin, that require helmets only for riders younger than 18 years or persons with an instructional permit.5
The Erie County Medical Center (ECMC) is a level I trauma center located in Buffalo, New York, and serves much of western New York, as well as part of northwestern Pennsylvania and, occasionally, southern Ontario, Canada. Because the ECMC receives almost all major trauma cases in the region, we had sufficient records to explore the incidence and the severity of deer–motorcycle accidents in these regions. In addition to adding to the limited data analyzing crash outcomes, we also looked at the numbers and proportions of motorcycle accidents attributable to deer and compared these with results from studies from different geographical regions. Because the number of registered motorcycles in Erie Country is among the highest in New York State, and because of the increased severity of motorcycle–deer collisions relative to other motor vehicle–deer collisions, this issue has both safety and financial considerations.
Materials and Methods
A retrospective review of records from ECMC was performed to capture all records from motorcycle accidents from May 2007 through June 2011. The population was identified to include only motorcycle accidents that were caused by collision with deer.
Injury severity was standardized using the Injury Severity Score (ISS), and the level of consciousness on arrival was standardized using the Glasgow Coma Scale (GCS). Chart abstraction included patient age, identification of the patient as driver or passenger of the motorcycle, use of helmet, time of year, types of injuries, length of hospital stay, and whether the patient lost consciousness. Patient age was also abstracted for the entire initial screen of all motorcycle accidents regardless of mechanism.
Statistical analysis was done using SPSS (IBM SPSS Statistics for Windows, Version 19.0; IBM Corp., Armonk, New York). Continuous data were analyzed using the appropriate descriptive statistics. Comparisons were made using Student t test, and a 0.05 level of significance was accepted.
Results
The initial screening of the trauma database returned 487 patients who had been involved in a motorcycle accident; of these, 39 patients were in an accident that involved a deer. According to one medical record, the spouse of a patient was a passenger who was dead at the scene, although there was no separate medical record for this person; this person was included in our data. Therefore, our total study population numbered 40 patients involved in 36 accidents, with 36 drivers and 4 passengers; 35 were men and 5 were women, with the women accounting for all 4 passengers and 1 driver. The mean (SD) patient age for deer–motorcycle collisions was 48.9 (8.9) years (range, 21-64 years). This was significantly higher than the mean (SD) age for all motorcycle accidents from the ECMC trauma database, which was 41.9 (13.9) years (range, 17-79 years) (P < .002).
The majority of accidents (31; 86%) with deer occurred during the months of May through September, with the most occurring in June (11; 31%). There was only 1 (3%) in October, 3 (8%) in November, and 1 (3%) in January. The number of collisions per year averaged 9.75, with a range of 8 to 12 from 2007-2010. (The year 2011 was omitted because data were collected before the year was complete). The presence or absence of helmet use was recorded in 22 cases. Of these, 21 patients had been wearing a helmet (95%), and only 1 patient was unhelmeted. Among all riders involved in motorcycle accidents from the trauma database, the presence or absence of a helmet was recorded in 271 cases. Of these, 262 (97%) were wearing a helmet. The average length of hospital stay was 6 days, with 6 patients having stays that were 10 days or longer, and the longest stay was 31 days. Thirty-three medical records noted whether the patient described loss of consciousness after the accident; of these, 14 (42%) claimed loss of consciousness and the remaining 19 (58%) denied any loss of consciousness after the accident. The mean (SD) ISS for deer–motorcycle collisions was 17.1 (9.8), and the mean (SD) GCS was 14.3 (2.5).
Chest, orthopedic, and head injuries were the most common injuries seen in deer–motorcycle collisions (Table). Head injuries, including the 1 patient who was confirmed to not have been wearing a helmet, accounted for 15.0% of the total injuries. This patient also had a longer length of stay at 19 days than the average of 6 days. Rib fractures were the most common injury, occurring in 20 (50%) patients. The 1 recorded fatality was the passenger of a patient who was dead at the scene.
Twenty-five (62.5%) patients in this series had injuries that are traditionally treated by orthopedic trauma surgeons, including scapular, clavicle, pelvic, and extremity fractures. Upper and lower extremity injuries occurred 10 (8.3%) and 15 (12.5%) times respectively, with the lower extremity injuries including long bone fractures, foot and ankle fractures, and 1 lower extremity traumatic amputation. Fourteen (35%) patients underwent one or more orthopedic surgical procedures.
Discussion
Although animal–vehicle collisions have been described in the literature, comparatively little data are available for the subset of animal–motorcycle accidents. This is an important gap considering that fatalities in collisions with animals were 6 times more likely to be persons riding motorcycles, although animal collisions are more common with other vehicles.6
Smoot and colleagues2 also reported that motorcycle collisions with deer tend to result in a higher injury severity than collisions of other vehicles with deer. According to reports for Midwestern regions, motorcycle-versus-deer accidents are a significant problem, causing a large number of serious injuries as well as creating the financial burden of vehicle damage and medical costs.2,3 However, the overall data are limited, and there is not much detailed information available for western New York.
Because of the large number of motorcyclists in New York State, it is important to consider accident data in this subset of the population. In 2010, 340,260 motorcycles were registered in New York State, with Erie County having the second highest number (21,745) of motorcycles registered.7 These numbers increased to 345,820 and 22,183 motorcycles, respectively, in 2011.8 In that year, the number of police-reported motorcycle accidents in New York decreased to 4855 from 5047 accidents in 2010, although both numbers are increased from 4647 accidents in 2009.9-11 Despite the decrease in total police-reported motorcycle accidents from 2010 to 2011, the trend in motorcycle accidents involving an animal’s action has steadily increased from 313 (6.7%) in 2009 to 335 (6.6%) in 2010 to 401 (8.3%) in 2011.9-11 Although these data from the New York State Department of Motor Vehicles are not further broken down by animal species, it can be reasonably surmised that most of these are caused by deer. This inference is supported by data from Bramati and colleagues4 showing that 81% of animal–vehicle collisions involved deer, as well as by the Wildlife-Vehicle Collision Reduction Study that showed deer were involved in 54.4% of animal–vehicle collisions in California and more than 90% of animal–vehicle collisions in Illinois and Minnesota.4,12 These studies predominantly comprised collisions involving animals capable of causing substantial property damage on impact, such as deer or larger animals. This, along with the evidence of higher ISS seen in motorcyclists in deer-related traffic injuries,2 supports the intuitive thought that motorcyclists are at increased risk for injury and fatality relative to other motor vehicles involved in accidents.
Williams and Wells13 reviewed 147 fatal wildlife–vehicle fatalities from 9 regions and found that the 2 most common fatalities were the motorcycle driver or passenger after striking an animal or an object. Jones14 also reported that the most common fatal wildlife–vehicle crashes involved motorcycles, as did fatal-accident reporting system data in the Wildlife-Vehicle Collision Reduction Study, which confirmed that approximately 30% of fatal crashes with animals involved motorcycles.12
Interestingly, the age of patients involved in motorcycle–deer collisions tends to be higher than that of patients involved in other motorcycle accidents. The numbers in our study reflect results in other study populations that suggest motorcycle riders who collide with deer are generally older than riders in other accidents who are more likely to be younger.4 One explanation is that younger riders may drive faster and more recklessly than older and experienced riders, resulting in an increased number of accidents unrelated to deer. Another consideration places younger drivers less commonly on roads where wildlife crashes more often occur (ie, roads that are rural, 2-lane).
Helmet use, when reported, was very high in our study population, most likely as a result of New York State’s mandatory helmet law for motorcyclists. Our data showed that more than 95% of patients whose charts documented helmet usage were wearing helmets at the time of the collision, compared with a Wisconsin study showing that only 29% of patients were wearing helmets.3 This may explain the proportion of head injuries in our study being 15.0% compared with the 29.5% in the Wisconsin study.3 Although both datasets involved a limited number of patients, the results suggest that mandatory helmet laws are effective in preventing head injuries. Also, the only patient in our study who was confirmed to have not been wearing a helmet had a much longer length of hospital stay than the average patient (19 vs 6 days). William and Wells13 found that 65% of motorcyclists killed in collisions with animals were not wearing helmets, and they believed that many of these fatalities could have been prevented with helmet use. Again, these limited data suggest the effectiveness of mandatory helmet use.
Two other factors, season and time of day, are important to consider in motorcycle collisions with deer. According to our data, 86% of these collisions occur in the warmer months, May through September, peaking in June. This is similar to findings from the Wisconsin study showing June and July as the peak months for deer–motorcycle collisions and a study in Minnesota where 61% of these crashes occurred in the summer months.2,3 These data most likely indicate increased motorcycle traffic in favorable weather conditions. Although time of accident could not be determined through our retrospective review, multiple studies have shown that the majority of collisions with deer tend to be between dusk and dawn. Smoot and colleagues2 found that 56% of vehicle collisions with deer occurred between 5 pm and midnight, with 80% between 5 pm and 6 am.2 Similarly, Nelson and colleagues3 found that 54.5% of collisions happened in a 4-hour period, from 6 pm to 10 pm. These data indicate that motorcycle operators should be especially vigilant in the morning and evening hours when deer may be more active.
Other than driver awareness and vigilance, prevention efforts can involve wildlife fencing, alert systems, and deer-culling programs. Fences are used extensively, most commonly on larger thoroughfares, and have been shown effective in reducing wildlife vehicle crashes by 80% to 90%.12 Animal detection systems using sensors to detect large wildlife approaching the roadway can activate warning signs to alert approaching drivers. Such systems have been installed in more than 30 locations in North America and Europe with variable effectiveness.12 However, there are typically no standards or guidelines for the collection of data about wildlife–vehicle crashes. Data are collected inconsistently and often haphazardly, and methods vary between states and agencies. Some transportation agencies do not collect this type of data at all. Without reliable, consistent data, it is difficult to identify road sections where mitigation methods may be required, to select appropriate mitigation measures, or to evaluate whether that effort is making a difference.
Culling systems for deer populations are frequently discussed, often in suburban as well as rural settings. Recreational hunting ordinances, higher limits on the number of females a hunter can bag, and occasional use of professional shooters can be applicable in less rural areas. Their effectiveness is debatable and tends to be time-limited.
Conclusion
This study highlights the fairly common occurrence and relative severity of deer–motorcycle crashes in an upstate New York setting, approximating published series from the Midwest. Helmet laws may lower rates of head injury in motorcycle–wildlife crashes. Finally, there are no fender benders when the chosen vehicle sports no fenders, so motorcyclists need to be especially vigilant in order to avoid collisions with deer and other wildlife.
The combination of urban sprawl and a large deer population has caused deer–motor vehicle collisions to become a major concern over the past few decades. According to State Farm Insurance industry data, New York State drivers in 2010-2011 had a 1 in 149.5 likelihood of colliding with a deer over the year, compared with a national average of 1 in 183.4.1 Reports from the Midwest have highlighted the frequency and severity of this type of accident.2-4 Frequent performance of orthopedic procedures in this subset of trauma patients prompted a local review to determine the frequency and severity of injuries. This series differs from the Midwest studies in the existence of a universal helmet law for all motorcyclists and passengers in New York State. Other studies looking at this type of accident were performed in states, including Minnesota and Wisconsin, that require helmets only for riders younger than 18 years or persons with an instructional permit.5
The Erie County Medical Center (ECMC) is a level I trauma center located in Buffalo, New York, and serves much of western New York, as well as part of northwestern Pennsylvania and, occasionally, southern Ontario, Canada. Because the ECMC receives almost all major trauma cases in the region, we had sufficient records to explore the incidence and the severity of deer–motorcycle accidents in these regions. In addition to adding to the limited data analyzing crash outcomes, we also looked at the numbers and proportions of motorcycle accidents attributable to deer and compared these with results from studies from different geographical regions. Because the number of registered motorcycles in Erie Country is among the highest in New York State, and because of the increased severity of motorcycle–deer collisions relative to other motor vehicle–deer collisions, this issue has both safety and financial considerations.
Materials and Methods
A retrospective review of records from ECMC was performed to capture all records from motorcycle accidents from May 2007 through June 2011. The population was identified to include only motorcycle accidents that were caused by collision with deer.
Injury severity was standardized using the Injury Severity Score (ISS), and the level of consciousness on arrival was standardized using the Glasgow Coma Scale (GCS). Chart abstraction included patient age, identification of the patient as driver or passenger of the motorcycle, use of helmet, time of year, types of injuries, length of hospital stay, and whether the patient lost consciousness. Patient age was also abstracted for the entire initial screen of all motorcycle accidents regardless of mechanism.
Statistical analysis was done using SPSS (IBM SPSS Statistics for Windows, Version 19.0; IBM Corp., Armonk, New York). Continuous data were analyzed using the appropriate descriptive statistics. Comparisons were made using Student t test, and a 0.05 level of significance was accepted.
Results
The initial screening of the trauma database returned 487 patients who had been involved in a motorcycle accident; of these, 39 patients were in an accident that involved a deer. According to one medical record, the spouse of a patient was a passenger who was dead at the scene, although there was no separate medical record for this person; this person was included in our data. Therefore, our total study population numbered 40 patients involved in 36 accidents, with 36 drivers and 4 passengers; 35 were men and 5 were women, with the women accounting for all 4 passengers and 1 driver. The mean (SD) patient age for deer–motorcycle collisions was 48.9 (8.9) years (range, 21-64 years). This was significantly higher than the mean (SD) age for all motorcycle accidents from the ECMC trauma database, which was 41.9 (13.9) years (range, 17-79 years) (P < .002).
The majority of accidents (31; 86%) with deer occurred during the months of May through September, with the most occurring in June (11; 31%). There was only 1 (3%) in October, 3 (8%) in November, and 1 (3%) in January. The number of collisions per year averaged 9.75, with a range of 8 to 12 from 2007-2010. (The year 2011 was omitted because data were collected before the year was complete). The presence or absence of helmet use was recorded in 22 cases. Of these, 21 patients had been wearing a helmet (95%), and only 1 patient was unhelmeted. Among all riders involved in motorcycle accidents from the trauma database, the presence or absence of a helmet was recorded in 271 cases. Of these, 262 (97%) were wearing a helmet. The average length of hospital stay was 6 days, with 6 patients having stays that were 10 days or longer, and the longest stay was 31 days. Thirty-three medical records noted whether the patient described loss of consciousness after the accident; of these, 14 (42%) claimed loss of consciousness and the remaining 19 (58%) denied any loss of consciousness after the accident. The mean (SD) ISS for deer–motorcycle collisions was 17.1 (9.8), and the mean (SD) GCS was 14.3 (2.5).
Chest, orthopedic, and head injuries were the most common injuries seen in deer–motorcycle collisions (Table). Head injuries, including the 1 patient who was confirmed to not have been wearing a helmet, accounted for 15.0% of the total injuries. This patient also had a longer length of stay at 19 days than the average of 6 days. Rib fractures were the most common injury, occurring in 20 (50%) patients. The 1 recorded fatality was the passenger of a patient who was dead at the scene.
Twenty-five (62.5%) patients in this series had injuries that are traditionally treated by orthopedic trauma surgeons, including scapular, clavicle, pelvic, and extremity fractures. Upper and lower extremity injuries occurred 10 (8.3%) and 15 (12.5%) times respectively, with the lower extremity injuries including long bone fractures, foot and ankle fractures, and 1 lower extremity traumatic amputation. Fourteen (35%) patients underwent one or more orthopedic surgical procedures.
Discussion
Although animal–vehicle collisions have been described in the literature, comparatively little data are available for the subset of animal–motorcycle accidents. This is an important gap considering that fatalities in collisions with animals were 6 times more likely to be persons riding motorcycles, although animal collisions are more common with other vehicles.6
Smoot and colleagues2 also reported that motorcycle collisions with deer tend to result in a higher injury severity than collisions of other vehicles with deer. According to reports for Midwestern regions, motorcycle-versus-deer accidents are a significant problem, causing a large number of serious injuries as well as creating the financial burden of vehicle damage and medical costs.2,3 However, the overall data are limited, and there is not much detailed information available for western New York.
Because of the large number of motorcyclists in New York State, it is important to consider accident data in this subset of the population. In 2010, 340,260 motorcycles were registered in New York State, with Erie County having the second highest number (21,745) of motorcycles registered.7 These numbers increased to 345,820 and 22,183 motorcycles, respectively, in 2011.8 In that year, the number of police-reported motorcycle accidents in New York decreased to 4855 from 5047 accidents in 2010, although both numbers are increased from 4647 accidents in 2009.9-11 Despite the decrease in total police-reported motorcycle accidents from 2010 to 2011, the trend in motorcycle accidents involving an animal’s action has steadily increased from 313 (6.7%) in 2009 to 335 (6.6%) in 2010 to 401 (8.3%) in 2011.9-11 Although these data from the New York State Department of Motor Vehicles are not further broken down by animal species, it can be reasonably surmised that most of these are caused by deer. This inference is supported by data from Bramati and colleagues4 showing that 81% of animal–vehicle collisions involved deer, as well as by the Wildlife-Vehicle Collision Reduction Study that showed deer were involved in 54.4% of animal–vehicle collisions in California and more than 90% of animal–vehicle collisions in Illinois and Minnesota.4,12 These studies predominantly comprised collisions involving animals capable of causing substantial property damage on impact, such as deer or larger animals. This, along with the evidence of higher ISS seen in motorcyclists in deer-related traffic injuries,2 supports the intuitive thought that motorcyclists are at increased risk for injury and fatality relative to other motor vehicles involved in accidents.
Williams and Wells13 reviewed 147 fatal wildlife–vehicle fatalities from 9 regions and found that the 2 most common fatalities were the motorcycle driver or passenger after striking an animal or an object. Jones14 also reported that the most common fatal wildlife–vehicle crashes involved motorcycles, as did fatal-accident reporting system data in the Wildlife-Vehicle Collision Reduction Study, which confirmed that approximately 30% of fatal crashes with animals involved motorcycles.12
Interestingly, the age of patients involved in motorcycle–deer collisions tends to be higher than that of patients involved in other motorcycle accidents. The numbers in our study reflect results in other study populations that suggest motorcycle riders who collide with deer are generally older than riders in other accidents who are more likely to be younger.4 One explanation is that younger riders may drive faster and more recklessly than older and experienced riders, resulting in an increased number of accidents unrelated to deer. Another consideration places younger drivers less commonly on roads where wildlife crashes more often occur (ie, roads that are rural, 2-lane).
Helmet use, when reported, was very high in our study population, most likely as a result of New York State’s mandatory helmet law for motorcyclists. Our data showed that more than 95% of patients whose charts documented helmet usage were wearing helmets at the time of the collision, compared with a Wisconsin study showing that only 29% of patients were wearing helmets.3 This may explain the proportion of head injuries in our study being 15.0% compared with the 29.5% in the Wisconsin study.3 Although both datasets involved a limited number of patients, the results suggest that mandatory helmet laws are effective in preventing head injuries. Also, the only patient in our study who was confirmed to have not been wearing a helmet had a much longer length of hospital stay than the average patient (19 vs 6 days). William and Wells13 found that 65% of motorcyclists killed in collisions with animals were not wearing helmets, and they believed that many of these fatalities could have been prevented with helmet use. Again, these limited data suggest the effectiveness of mandatory helmet use.
Two other factors, season and time of day, are important to consider in motorcycle collisions with deer. According to our data, 86% of these collisions occur in the warmer months, May through September, peaking in June. This is similar to findings from the Wisconsin study showing June and July as the peak months for deer–motorcycle collisions and a study in Minnesota where 61% of these crashes occurred in the summer months.2,3 These data most likely indicate increased motorcycle traffic in favorable weather conditions. Although time of accident could not be determined through our retrospective review, multiple studies have shown that the majority of collisions with deer tend to be between dusk and dawn. Smoot and colleagues2 found that 56% of vehicle collisions with deer occurred between 5 pm and midnight, with 80% between 5 pm and 6 am.2 Similarly, Nelson and colleagues3 found that 54.5% of collisions happened in a 4-hour period, from 6 pm to 10 pm. These data indicate that motorcycle operators should be especially vigilant in the morning and evening hours when deer may be more active.
Other than driver awareness and vigilance, prevention efforts can involve wildlife fencing, alert systems, and deer-culling programs. Fences are used extensively, most commonly on larger thoroughfares, and have been shown effective in reducing wildlife vehicle crashes by 80% to 90%.12 Animal detection systems using sensors to detect large wildlife approaching the roadway can activate warning signs to alert approaching drivers. Such systems have been installed in more than 30 locations in North America and Europe with variable effectiveness.12 However, there are typically no standards or guidelines for the collection of data about wildlife–vehicle crashes. Data are collected inconsistently and often haphazardly, and methods vary between states and agencies. Some transportation agencies do not collect this type of data at all. Without reliable, consistent data, it is difficult to identify road sections where mitigation methods may be required, to select appropriate mitigation measures, or to evaluate whether that effort is making a difference.
Culling systems for deer populations are frequently discussed, often in suburban as well as rural settings. Recreational hunting ordinances, higher limits on the number of females a hunter can bag, and occasional use of professional shooters can be applicable in less rural areas. Their effectiveness is debatable and tends to be time-limited.
Conclusion
This study highlights the fairly common occurrence and relative severity of deer–motorcycle crashes in an upstate New York setting, approximating published series from the Midwest. Helmet laws may lower rates of head injury in motorcycle–wildlife crashes. Finally, there are no fender benders when the chosen vehicle sports no fenders, so motorcyclists need to be especially vigilant in order to avoid collisions with deer and other wildlife.
1. Likelihood of collision with deer (amended 2010-2011). State Farm website. https://static1.st8fm.com/en_US/content_pages/1/pdf/us/likelihood-of-collision-2011.pdf. Accessed April 29, 2015.
2. Smoot DL, Zielinski MD, Cullinane DC, Jenkins DH, Schiller HJ, Sawyer MD. Patterns in deer-related traffic injuries over a decade: the Mayo Clinic experience. Scand J Trauma Resusc Emerg Med. 2010;18:46.
3. Nelson RS, Gustafson PT, Szlabick RE. Motorcycle collisions involving white-tailed deer in central and northern Wisconsin: a rural trauma center experience. J Trauma. 2006;60(6):1297-1300.
4. Bramati PS, Heinert LF, Narloch LB, Hostetter J, Finkielman JD. Animal-related motorcycle collisions in North Dakota. Wilderness Environ Med. 2012;23(1):65-69.
5. Save lives, save money – how does your state measure up. Injury Prevention & Control: Motor Vehicle Safety. Centers for Disease Control and Prevention website. http://www.cdc.gov/motorvehiclesafety/mc/states/index.html. Updated June 13, 2012. Accessed April 23, 2015.
6. Langley RL, Higgins SA, Herrin KB. Risk factors associated with fatal animal-vehicle collisions in the United States, 1995-2004. Wilderness Environ Med. 2006;17(4):229-239.
7. Vehicle registrations in force – 2010. Archives of Statistical Summaries. New York State Department of Motor Vehicles website. http://dmv.ny.gov/statistic/regin10.pdf. Accessed May 11, 2015.
8. Vehicle registrations in force – 2011. Archives of Statistical Summaries. New York State Department of Motor Vehicles website. http://dmv.ny.gov/statistic/regin11.pdf. Accessed May 11, 2015.
9. Summary of motorcycle crashes – 2011. Archives of Statistical Summaries. New York State Department of Motor Vehicles website. http://dmv.ny.gov/statistic/2011MotorcycleCrashSummary.pdf. Accessed April 23, 2015.
10. Summary of motorcycle accidents – 2010. Archives of Statistical Summaries. New York State Department of Motor Vehicles website. dmv.ny.gov/statistic/2010MotorcycleAccSummary.pdf. Accessed April 23, 2015.
11. Summary of motorcycle accidents – 2009. Archives of Statistical Summaries. New York State Department of Motor Vehicles website. dmv.ny.gov/statistic/2009MotorcycleSummary.pdf. Accessed April 23, 2015.
12. Huijser MP, McGowen P, Fuller J, et al; Federal Highway Administration. Wildlife-Vehicle Collision Reduction Study: Report to Congress. Report no. FHWA-HRT-08-034. Washington, DC: US Department of Transportation, Federal Highway Administration; 2008. http://www.fhwa.dot.gov/publications/research/safety/08034/08034.pdf. Accessed April 23, 2015.
13. Williams AF, Wells JK. Characteristics of vehicle-animal crashes in which vehicle occupants are killed. Traffic Inj Prev. 2005;6(1):56-59.
14. Jones M. Deer-vehicle crash injuries, fatalities reach all-time high in Wisconsin. Milwaukee Journal Sentinel. April 14, 2000:1B-2B.
1. Likelihood of collision with deer (amended 2010-2011). State Farm website. https://static1.st8fm.com/en_US/content_pages/1/pdf/us/likelihood-of-collision-2011.pdf. Accessed April 29, 2015.
2. Smoot DL, Zielinski MD, Cullinane DC, Jenkins DH, Schiller HJ, Sawyer MD. Patterns in deer-related traffic injuries over a decade: the Mayo Clinic experience. Scand J Trauma Resusc Emerg Med. 2010;18:46.
3. Nelson RS, Gustafson PT, Szlabick RE. Motorcycle collisions involving white-tailed deer in central and northern Wisconsin: a rural trauma center experience. J Trauma. 2006;60(6):1297-1300.
4. Bramati PS, Heinert LF, Narloch LB, Hostetter J, Finkielman JD. Animal-related motorcycle collisions in North Dakota. Wilderness Environ Med. 2012;23(1):65-69.
5. Save lives, save money – how does your state measure up. Injury Prevention & Control: Motor Vehicle Safety. Centers for Disease Control and Prevention website. http://www.cdc.gov/motorvehiclesafety/mc/states/index.html. Updated June 13, 2012. Accessed April 23, 2015.
6. Langley RL, Higgins SA, Herrin KB. Risk factors associated with fatal animal-vehicle collisions in the United States, 1995-2004. Wilderness Environ Med. 2006;17(4):229-239.
7. Vehicle registrations in force – 2010. Archives of Statistical Summaries. New York State Department of Motor Vehicles website. http://dmv.ny.gov/statistic/regin10.pdf. Accessed May 11, 2015.
8. Vehicle registrations in force – 2011. Archives of Statistical Summaries. New York State Department of Motor Vehicles website. http://dmv.ny.gov/statistic/regin11.pdf. Accessed May 11, 2015.
9. Summary of motorcycle crashes – 2011. Archives of Statistical Summaries. New York State Department of Motor Vehicles website. http://dmv.ny.gov/statistic/2011MotorcycleCrashSummary.pdf. Accessed April 23, 2015.
10. Summary of motorcycle accidents – 2010. Archives of Statistical Summaries. New York State Department of Motor Vehicles website. dmv.ny.gov/statistic/2010MotorcycleAccSummary.pdf. Accessed April 23, 2015.
11. Summary of motorcycle accidents – 2009. Archives of Statistical Summaries. New York State Department of Motor Vehicles website. dmv.ny.gov/statistic/2009MotorcycleSummary.pdf. Accessed April 23, 2015.
12. Huijser MP, McGowen P, Fuller J, et al; Federal Highway Administration. Wildlife-Vehicle Collision Reduction Study: Report to Congress. Report no. FHWA-HRT-08-034. Washington, DC: US Department of Transportation, Federal Highway Administration; 2008. http://www.fhwa.dot.gov/publications/research/safety/08034/08034.pdf. Accessed April 23, 2015.
13. Williams AF, Wells JK. Characteristics of vehicle-animal crashes in which vehicle occupants are killed. Traffic Inj Prev. 2005;6(1):56-59.
14. Jones M. Deer-vehicle crash injuries, fatalities reach all-time high in Wisconsin. Milwaukee Journal Sentinel. April 14, 2000:1B-2B.
Mortality Rates Associated With Odontoid and Subaxial Cervical Spine Fractures
Mortality rate is an important indicator of the severity of traumatic injuries, and these values have been described for different orthopedic injuries and fractures. Studies have identified 3 distinct trends in patient survival when compared with the age- and sex-matched uninjured population:
1. Hip fractures bring about a transient increase in mortality relative to age-matched controls that normalizes after a few months to 1 year.1-10
2. Thoracic and lumbar compression fractures are associated with an ongoing, lifelong increase in mortality rate relative to age-matched controls without an initial marked upswing.11-15
3. Certain injuries such as isolated rib or wrist fractures do not adversely affect survival relative to age-matched controls.12,16-18
Understanding the mortality patterns after these injuries can help guide management and even facilitate the development of appropriate treatment algorithms.19-21 While studies have examined mortality in specific odontoid fracture types,22 such mortality trends have not been broadly established in persons with cervical spine fractures.
Cervical spine fractures are common: 60% of spine fractures localize to this region,23-26 and this equates to 2% to 3% of all blunt-trauma patients.27,28 These injuries can lead to devastating consequences, including neurologic compromise, permanent disability, and death.29-31
Studies have estimated that up to 20% of cervical fractures involve the odontoid process.23-26 These injuries are more common among the elderly population because of their greater prevalence of osteoporosis and likelihood of falling.32 Because of demographic similarities to those of the hip fracture population, a survival analysis of all odontoid fractures is particularly interesting. Published odontoid mortality rates vary significantly, with reports ranging from 13% to 44%.22,33-35 Unfortunately, these studies largely evaluated survival rates specific to an individual treatment modality, such as nonoperative compared with operative, or specific to certain odontoid fracture types (eg, type II). Additionally, studies have generally only considered survivorship during initial hospitalization, have been specific to a constrained age group, or have been based solely on inpatient records that do not permit the longer-term follow-up critical to determining the effect of odontoid fractures on overall mortality.36-39
Likewise, mortality rates after fractures of the subaxial spine (ie, the motion segments between C3 and C7) have yet to be established. In 1 study, the mortality risk of a cohort of elderly patients with cervical fractures appeared to be elevated for the first 6 to 12 months after the traumatic event.40 However, the sample size was too small to examine mortality beyond 1 year.
In this context, the purpose of the current study was to determine the mortality rates at several time points (3 months, 1 year, and 2 years) of patients 50 years or older (start of the second mode of the bimodal age distribution of odontoid fractures41-44) with fractures of the odontoid and subaxial cervical spine. A secondary purpose of this study was to compare survival rates of these 2 cohorts relative to each other and to the general population.
Materials and Methods
Identification of Cervical Fractures and Collection of Demographic Information
This protocol was approved by the human investigation committee of our institution. Every computed tomography (CT) scan of the cervical spine performed in the emergency department (ED) of an academic hospital between November 27, 1997, and December 31, 2006, was identified. Since the threshold for obtaining a CT scan of a patient with suspected cervical spine trauma is relatively low, it was assumed that virtually all acute cervical spine fractures during this time period would be successfully identified through this approach.
Radiology reports for all identified CT scans were reviewed for any findings consistent with acute fractures and/or dislocations of the cervical spine (Figure 1). Every study noted to be positive or equivocal for cervical trauma was directly visualized, including those that did not specifically mention the presence or absence of an injury. Scans with no signs of acute trauma or that showed fractures caused by a pathologic process or penetrating mechanism (eg, metastatic lesions or gunshot wounds) were omitted from this series. Finally, relevant demographic information, such as the medical record number, age, gender, and date of study, was recorded for every subject in this group.
Fracture Classification
Next, the level and the type of cervical injury were documented for each patient. Fractures were segregated according to their involvement with the odontoid or the subaxial vertebrae.
Odontoid fractures were categorized into type I (limited to the tip), type II (across the base of the process) and type III (through the base with extension into the C2 vertebral body).45,46 Since many systems for classifying subaxial cervical spine trauma require a subjective inference of the injury mechanism, which is difficult to ascertain from imaging studies alone, all of these fractures were pooled together.
A preliminary survey of the data indicated that the odontoid fractures appeared to exhibit a bimodal age distribution, with the beginning of the second cluster occurring around age 50 years (Figure 2). As noted above, this has been shown in previous studies.41-44 As a consequence, the mortalities of those older than 50 years became the focus of this study. To control for comorbid conditions, mechanism of injury, and to allow for more direct comparison with the odontoid fractures in this study, the same age demarcation was used for subaxial cervical fractures.
Mortality Data
The mortality status of every patient diagnosed with an acute cervical injury at our institution between November 27, 1997, and December 31, 2006, was determined by referencing the National Death Index (NDI). The NDI is a computerized database of death records maintained by the National Center for Health Statistics (NCHS). The time window for the current study was selected because we had access to NDI information only through 2007 at the time of this study. Social Security numbers (SSNs), which were available for approximately half of the subjects, were used to search the NDI catalog. For individuals whose SSNs were unavailable, patient names and birthdates were considered to be sufficient to confirm a true match. Our center’s medical records of this cohort were also examined to verify whether any had died during their initial hospitalizations and to substantiate the NDI data. Finally, patient deaths were categorized as trauma (eg, motor vehicle accident, fall from a height) or medical comorbidity (eg, diabetes mellitus, cancer, congestive heart failure), based on information in the NDI listing.
Age- and Sex-Matched Controls
Age- and sex-matched controls were determined from the Wide-ranging Online Data for Epidemiologic Research (WONDER) application distributed by the Centers for Disease Control and Prevention (http://wonder.cdc.gov). Composite mortality data from the state in which the study was performed was obtained for the years between 1999 and 2007, and this information was further stratified according to gender and age to estimate the mortality rates and construct survival curves for each group. Controls were used to establish a standardized mortality ratio (SMR) for subjects 50 years and older, a value that compares the number of observed deaths with the figure expected for matched populations from the general population.
Statistical Methods
Statistical analyses were performed by using both SAS 9.2 (SAS Institute Inc., Cary, North Carolina) and R (version 2.9; www.r-project.org, Auckland, New Zealand). Relevant comparisons were planned, and all tests were 2-sided. The Wilcoxon rank sum test was applied to compare the survival times of patients with odontoid fractures with different documented causes of death, and Pearson χ2 test was used to compare the age distributions of odontoid and subaxial fractures. Survival rates at 3 months, 1 year, and 2 years were estimated from Kaplan-Meier curves. The relative survival of these cohorts was compared by completing a 2-sample log-rank test. In addition, a 1-sample log-rank test was implemented to compare the mortality from either odontoid or subaxial cervical spine fractures with that of the age- and gender-matched general population. Statistical significance was defined as a 2-sided α error of less than 0.05 (P < .05).
Results
Fifty-nine patients were diagnosed with odontoid fractures (28 men, 31 women), and 233 patients were diagnosed with subaxial cervical spine fractures (168 men, 65 women).
Odontoid Fracture Patients
Odontoid fracture patients exhibited a distinct bimodal age distribution (Figure 2). In the younger population, there were 14 subjects, 3 of whom died within days of the injury (mean, 12 days; 78.6% survival). At 2-year follow-up, there were no further deaths. The fractures that caused death were high-energy injuries, and only early deaths occurred in these cases.
Because of the significant bimodal age distribution, it was believed these cohorts could not be directly compared. As a result, the remaining analysis focused on the older age group. In the older population mode (50 years and older) were 45 patients with odontoid fractures. Of the 12 subjects who died after odontoid fracture, 5 were assigned a trauma code as the cause of death, while a medical comorbidity code was assigned for the remaining 7. Mean survival time of those who died secondary to trauma was significantly shorter than the medical comorbidity group (P = .025).
In the cohort of subjects older than 50 years, 3-month, 1-year, and 2-year survival rates were 84.4%, 82.2%, and 72.9%, respectively. Figure 2 shows the 1- and 2-year follow-up data by age group.
Analysis was performed relative to gender. Of male patients (n = 22), the 3-month, 1-year, and 2-year survival rates were 72.7%, 72.7%, and 62.7%, respectively. Among women (n = 23), the 3-month, 1-year, and 2-year survival rates were 95.7%, 91.3%, and 82.6%, respectively.
Figure 3 shows the Kaplan-Meier survival curves of the older patients with odontoid fractures. A comparison of the curves for each gender showed no significant disparities between the male and female survival (Figure 3A, P = .124). Compared with age-matched male counterparts, the survival of male subjects with odontoid fractures was significantly worse (Figure 3B, P < .001). Men experienced an initial acute decline in survival, with the remainder of the survival curve matching that of the general male population. In contrast, odontoid fractures did not adversely affect female survival compared with the matched population (Figure 3C, P = .568).
The 2-year SMR of 2.98 for men showed that odontoid fractures led to greater mortality compared with a sex- and age-matched population. This means that men older than 50 years who sustained an odontoid fracture had nearly 3 times the mortality rate after 2 years compared with a normal, matched population; this increase is attributed to the 3-month time point that subsequently normalized. The female rate was 1.33 times that of a matched population, a difference that is not statistically significant.
Subaxial Fracture Patients
Of the 91 patients older than 50 years with subaxial fractures, 3-month, 1-year, and 2-year survival rates were 87.9%, 85.7%, and 85.7%, respectively. Figure 4 shows the 1- and 2-year follow-up data by age group.
Gender-specific analysis was performed. For men (n = 58), the 3-month, 1-year, and 2-year survival rates were 87.9%, 84.5%, and 84.5%, respectively. Among women (n = 33), 4 deaths were recorded at all time points (87.9% survival).
Figure 5 shows Kaplan-Meier survival curves for the older population with subaxial fractures. A comparison of the curves between genders again showed no significant differences between male and female survival (P = .683, Figure 5A). Compared with age- and gender-matched counterparts, men showed decreased relative survival (P < .0001, Figure 5B), whereas subaxial fractures did not decrease female survival (P = .554, Figure 5C).
The 2-year SMR of 2.90 for men showed higher mortality rates relative to sex- and age-matched controls. Men who were both 50 years old and sustained a subaxial fracture were 2.9 times as likely to die within 2 years of follow-up compared with their counterparts. Similar to odontoid fractures, this increase occurred by the 3-month time point and subsequently normalized. The female rate, which was 1.34 times that of the uninjured population, was not statistically significant.
Comparison of Odontoid and Subaxial Fracture Patients
The survival of subaxial injuries was not significantly different from that of odontoid fractures (P = .113, Figure 6A). When analyzed by gender and controlled for age, the rates in both male (P = .347, Figure 6B) and female (P = .643, Figure 6C) patients did not differ between fracture types.
Discussion
The US population is aging rapidly, with the demographic older than 65 years predicted to more than double in size between 2010 and 2050.47 As our elderly population grows, the incidence of age-related injuries will rise accordingly. An understanding of mortality risks associated with different fractures will not only assist practitioners in advising patients regarding prognosis but may also lead to improvements in clinical care.19,48-50 While we know cervical spine trauma is associated with significant morbidity,29-31 little is known about associated moderate-term mortality rates that can be compared with other known injury patterns, such as hip fractures or osteoporotic compression fractures.
An interesting finding of the present study is the bimodal age distribution of the 59 odontoid fractures (Figure 2). The 14 patients younger than 50 years included 3 individuals who died, all within days of their presentation from severe multisystem trauma. This is consistent with the determination that high-energy forces are required to fracture the odontoid process in younger individuals.38,45,46,51,52 Given the severity of their nonspinal injuries, the cause of death was likely not primarily related to their odontoid fractures. Also in line with previous studies, the majority (76%) of odontoid fractures were documented in subjects older than 50 years.32,53,54 Within our cohort older than 50 years, the deaths appear to be spread evenly across age groups and do not seem to be skewed by the oldest portion of the population (Figure 2).
Our gender-specific analyses revealed that older men with odontoid injuries exhibited higher mortality compared with an age-matched male cohort, with 6 of the 8 deaths occurring within 3 months. However, after this exaggerated decline in survival, the rate normalized towards general population mortality rates (Figure 3B). As in the younger cohort, these earlier deaths were largely attributable to multisystem trauma, whereas medical comorbidities were implicated in those who died later. In contrast, the Kaplan-Meier curve of older women with odontoid fractures closely approximates that of age-matched women at every time point (Figure 3C), indicating that these injuries do not decrease survival as they do in their male counterparts.
When comparing the survival of older patients with subaxial cervical spine fractures with that of gender- and age-matched controls, the mortality rates of women were, once again, essentially equivalent. However, the survival of older men was significantly compromised by these injuries. In men, 7 of the 9 deaths were within 3 months, with the remaining 2 deaths occurring within 7 months. Nevertheless, beyond this initial period of elevated mortality, the survival curve again stabilized and paralleled that of the general population. As with odontoid fractures, there was no sustained increase in the mortality of male patients who lived at least 3 months after injury.
The mortality rates of odontoid and subaxial fractures were also compared in the older population. When controlled for age, there was no difference in mortality rates between these 2 groups. When individually analyzed in both men and women, the mortality rates of both fracture types matched those of the general population at all time points.
It is useful to contextualize our findings alongside the mortality of older individuals with other fracture types. Based on our results, we believe that the survival curves of geriatric men with odontoid or subaxial cervical spine fractures most closely resemble the characteristic pattern seen in hip fractures. Hip fractures have shown an early spike in mortality by as much as 8% to 49% in the first 6 to 12 months that returns to baseline after 1 year.1-10 This presumably reflects the natural history of these injuries in response to appropriate therapeutic interventions. Interestingly, the male mortality rates for both odontoid and subaxial cervical spine fractures in this study are largely analogous to those reported by various hip fracture surveys.1,5,55-58 In contrast, similar to prior studies of rib or wrist fractures, older women with these cervical spine fractures did not show a survival decrease after their injuries.12,16-18
While the reasons underlying the differential effects of cervical fractures on the mortality of men and women have not been established, one explanation is that the female geriatric population is relatively more osteoporotic; thus, cervical injuries may occur after lower-energy forces, leading to less severe associated trauma that could otherwise decrease survival. Another explanation is that men are more likely to be involved in high-energy accidents,59,60 thus decreasing their overall survival after injury.
This investigation is not without limitations. Our primary concern is the determination of survival. The NDI maintained by the NCHS is an extremely reliable tool regularly employed by epidemiologists to collect mortality data. However, it is possible that deaths may have been missed. We believe this number would be small, because the NDI database provided multiple probable matches that were carefully compared with supplemental personal information. It is also possible that deaths that were not appropriately registered with the NDI are not represented in this series. Another limitation lies in the determination of controls. As with any case–control study, the patients sustaining these odontoid fractures may differ in some significant way from the average population. A final limitation is that a small portion of patients in the study have only 1-year follow-up, because patient data was collected through 2006, although access to NDI data ended in 2007.
Conclusion
Our results indicate that the survival of older men with either odontoid or subaxial cervical spine fractures shares many of the same mortality characteristics as hip fractures, with diminished survival in the first 3 months that normalizes to the survival rate of the age-matched population. Interestingly, and perhaps because of disparate rates of osteoporosis and traumatic forces, the mortality rates in the female cohort were similar to that of the age-matched general population at all time points. These trends were nearly identical for both odontoid and subaxial cervical fractures.
1. Gennarelli TA, Champion HR, Sacco WJ, Copes WS, Alves WM. Mortality of patients with head injury and extracranial injury treated in trauma centers. J Trauma. 1989;29(9):1193-1201; discussion 1201-1202.
2. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology (Oxford). 2000;39(4):346-349.
3. Gerrelts BD, Petersen EU, Mabry J, Petersen SR. Delayed diagnosis of cervical spine injuries. J Trauma. 1991;31(12):1622-1626.
4. Giannoudis PV, Mehta SS, Tsiridis E. Incidence and outcome of whiplash injury after multiple trauma. Spine. 2007;32(7):776-781.
5. Goldberg W, Mueller C, Panacek E, et al. Distribution and patterns of blunt traumatic cervical spine injury. Ann Emerg Med. 2001;38(1):17-21.
6. Grauer JN, Shafi B, Hilibrand AS, et al. Proposal of a modified, treatment-oriented classification of odontoid fractures. Spine J. 2005;5(2):123-129.
7. Greene KA, Dickman CA, Marciano FF, Drabier JB, Hadley MN, Sonntag VK. Acute axis fractures. Analysis of management and outcome in 340 consecutive cases. Spine. 1997;22(16):1843-1852.
8. Gulli B, Templeman D. Compartment syndrome of the lower extremity. Orthop Clin North Am. 1994;25(4):677-684.
9. Guthkelch AN, Fleischer AS. Patterns of cervical spine injury and their associated lesions. West J Med. 1987;147(4):428-431.
10. Hackl W, Hausberger K, Sailer R, Ulmer H, Gassner R. Prevalence of cervical spine injuries in patients with facial trauma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(4):370-376.
11. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
12. Garabige V, Giraud P, De Rycke Y, et al. [Impact of nutrition management in patients with head and neck cancers treated with irradiation: is the nutritional intervention useful?]. Cancer Radiother. 2007;11(3):111-116.
13. Garbuz DS, Leitch K, Wright JG. The treatment of supracondylar fractures in children with an absent radial pulse. J Pediatr Orthop. 1996;16(5):594-596.
14. Henderson RL, Reid DC, Saboe LA. Multiple noncontiguous spine fractures. Spine. 1991;16(2):128-131.
15. Henrikson B. Supracondylar fracture of the humerus in children. A late review of end-results with special reference to the cause of deformity, disability and complications. Acta Chir Scand Suppl. 1966;369:1-72.
16. De Boeck H, De Smet P, Penders W, De Rydt D. Supracondylar elbow fractures with impaction of the medial condyle in children. J Pediatr Orthop. 1995;15(4):444-448.
17. Gelberman RH, Panagis JS, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part I: The extraosseous vascularity. J Hand Surg Am. 1983;8(4):367-375.
18. Hu J, Liao Q, Long W. Diagnosis and treatment of multiple-level noncontiguous spinal fractures. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2005;19(6):424-426.
19. Eleraky MA, Theodore N, Adams M, Rekate HL, Sonntag VK. Pediatric cervical spine injuries: report of 102 cases and review of the literature. J Neurosurg. 2000;92(1 suppl):12-17.
20. Ioannidis G, Papaioannou A, Hopman WM, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ. 2009;181(5):265-271.
21. Husby J, Sorensen KH. Fracture of the odontoid process of the axis. Acta Orthop Scand. 1974;45(2):182-192.
22. Schoenfeld AJ, Bono CM, Reichmann WM, et al. Type II odontoid fractures of the cervical spine: do treatment type and medical comorbidities affect mortality in elderly patients? Spine. 2011;36(11):879-885.
23. Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc. 2000;48(3):241-249.
24. Fassett DR, Dailey AT, Vaccaro AR. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21(4):252-258.
25. Ippolito E, Caterini R, Scola E. Supracondylar fractures of the humerus in children. Analysis at maturity of fifty-three patients treated conservatively. J Bone Joint Surg Am. 1986;68(3):333-344.
26. Spence KF Jr, Decker S, Sell KW. Bursting atlantal fracture associated with rupture of the transverse ligament. J Bone Joint Surg Am. 1970;52(3):543-549.
27. Irwin ZN, Arthur M, Mullins RJ, Hart RA. Variations in injury patterns, treatment, and outcome for spinal fracture and paralysis in adult versus geriatric patients. Spine. 2004;29(7):796-802.
28. Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int. 1998;8(3):291-297.
29. Iyengar SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and pinning of type III displaced supracondylar fractures of the humerus in children: a comparative study. J Orthop Trauma. 1999;13(1):51-55.
30. Jackson AP, Haak MH, Khan N, Meyer PR. Cervical spine injuries in the elderly: acute postoperative mortality. Spine. 2005;30(13):1524-1527.
31. Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Race and sex differences in mortality following fracture of the hip. Am J Public Health. 1992;82(8):1147-1150.
32. Fisher ES, Baron JA, Malenka DJ, et al. Hip fracture incidence and mortality in New England. Epidemiology. 1991;2(2):116-122.
33. Chapman J, Smith JS, Kopjar B, et al. The AOSpine North America Geriatric Odontoid Fracture Mortality Study: a retrospective review of mortality outcomes for operative versus nonoperative treatment of 322 patients with long-term follow-up. Spine. 2013;38:1098-1104.
34. Denault A, Bains I, Moghadam K, Hu RW, Swamy G. Evaluation of mortality following an odontoid fracture in the octogenarian population. J Bone Joint Surg Br. 2011;93(Supp IV):585.
35. Molinari WJ III, Molinari RW, Khera OA, Gruhn WL. Functional outcomes, morbidity, mortality, and fracture healing in 58 consecutive patients with geriatric odontoid fracture treated with cervical collar or posterior fusion. Global Spine J. 2013;3(1):21-32.
36. Hanigan WC, Powell FC, Elwood PW, Henderson JP. Odontoid fractures in elderly patients. J Neurosurg. 1993;78(1):32-35.
37. Korres DS, Boscainos PJ, Papagelopoulos PJ, Psycharis I, Goudelis G, Nikolopoulos K. Multiple level noncontiguous fractures of the spine. Clin Orthop. 2003;411:95-102.
38. Leet AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3 supracondylar humerus fractures in children. J Pediatr Orthop. 2002;22(2):203-207.
39. Leone A, Cerase A, Colosimo C, Lauro L, Puca A, Marano P. Occipital condylar fractures: a review. Radiology. 2000;216(3):635-644.
40. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799-1809.
41. Müller EJ, Wick M, Russe O, Muhr G. Management of odontoid fractures in the elderly. Eur Spine J. 1999;8(5):360-365.
42. Pepin JW, Bourne RB, Hawkins RJ. Odontoid fractures, with special reference to the elderly patient. Clin Orthop. 1985;193:178-183.
43. Ryan MD, Henderson JJ. The epidemiology of fractures and fracture-dislocations of the cervical spine. Injury. 1992;23(1):38-40.
44. Butler JS, Dolan RT, Burbridge M, et al. The long-term functional outcome of type II odontoid fractures managed non-operatively. Eur Spine J. 2010;19(10):1635-1642.
45. Levine AM, Edwards CC. The management of traumatic spondylolisthesis of the axis. J Bone Joint Surg Am. 1985;67(2):217-226.
46. Lowery DW, Wald MM, Browne BJ, Tigges S, Hoffman JR, Mover WR; NEXUS Group. Epidemiology of cervical spine injury victims. Ann Emerg Med. 2001;38(1):12-16.
47. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-16. http://www.prb.org/pdf11/aging-in-america.pdf. Published February 2011. Accessed April 22, 2015.
48. Holly LT, Kelly DF, Counelis GJ, Blinman T, McArthur DL, Cryer HG. Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors, and injury characteristics. J Neurosurg. 2002;96(3 suppl):285-291.
49. Holmes JF, Akkinepalli R. Computed tomography versus plain radiography to screen for cervical spine injury: a meta-analysis. J Trauma. 2005;58(5):902-905.
50. Hove LM. Epidemiology of scaphoid fractures in Bergen, Norway. Scand J Plast Reconstr Surg Hand Surg. 1999;33(4):423-426.
51. Lu-Yao G, Baron Ja, Barrett Ja, Fisher Es. Treatment and survival among elderly Americans with hip fractures: a population-based study. Am J Public Health. 1994;84(8):1287-1291.
52. Lu-Yao GL, Keller RB, Littenberg B, Wennberg JE. Outcomes after displaced fractures of the femoral neck. A meta-analysis of one hundred and six published reports. J Bone Joint Surg Am. 1994;76(1):15-25.
53. Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159(11):1215-1220.
54. Levine AM, Edwards CC. Fractures of the atlas. J Bone Joint Surg Am. 1991;73(5):680-691.
55. Maak TG, Grauer JN. The contemporary treatment of odontoid injuries. Spine. 2006;31(11 Suppl):S53-S60; discussion S61.
56. Magaziner J, Fredman L, Hawkes W, et al. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol. 2003;157(11):1023-1031.
57. Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55(9):M498-M507.
58. Malham GM, Ackland HM, Jones R, Williamson OD, Varma DK. Occipital condyle fractures: incidence and clinical follow-up at a level 1 trauma centre. Emerg Radiol. 2009;16(4):291-297.
59. Probst C, Zelle B, Panzica M, et al. Clinical re-examination 10 or more years after polytrauma: is there a gender related difference? J Trauma. 2010;68(3):706-711.
60. Holbrook TL, Hoyt DB, Anderson JP. The importance of gender on outcome after major trauma: functional and psychologic outcomes in women versus men. J Trauma. 2001;50(2):270-273.
Mortality rate is an important indicator of the severity of traumatic injuries, and these values have been described for different orthopedic injuries and fractures. Studies have identified 3 distinct trends in patient survival when compared with the age- and sex-matched uninjured population:
1. Hip fractures bring about a transient increase in mortality relative to age-matched controls that normalizes after a few months to 1 year.1-10
2. Thoracic and lumbar compression fractures are associated with an ongoing, lifelong increase in mortality rate relative to age-matched controls without an initial marked upswing.11-15
3. Certain injuries such as isolated rib or wrist fractures do not adversely affect survival relative to age-matched controls.12,16-18
Understanding the mortality patterns after these injuries can help guide management and even facilitate the development of appropriate treatment algorithms.19-21 While studies have examined mortality in specific odontoid fracture types,22 such mortality trends have not been broadly established in persons with cervical spine fractures.
Cervical spine fractures are common: 60% of spine fractures localize to this region,23-26 and this equates to 2% to 3% of all blunt-trauma patients.27,28 These injuries can lead to devastating consequences, including neurologic compromise, permanent disability, and death.29-31
Studies have estimated that up to 20% of cervical fractures involve the odontoid process.23-26 These injuries are more common among the elderly population because of their greater prevalence of osteoporosis and likelihood of falling.32 Because of demographic similarities to those of the hip fracture population, a survival analysis of all odontoid fractures is particularly interesting. Published odontoid mortality rates vary significantly, with reports ranging from 13% to 44%.22,33-35 Unfortunately, these studies largely evaluated survival rates specific to an individual treatment modality, such as nonoperative compared with operative, or specific to certain odontoid fracture types (eg, type II). Additionally, studies have generally only considered survivorship during initial hospitalization, have been specific to a constrained age group, or have been based solely on inpatient records that do not permit the longer-term follow-up critical to determining the effect of odontoid fractures on overall mortality.36-39
Likewise, mortality rates after fractures of the subaxial spine (ie, the motion segments between C3 and C7) have yet to be established. In 1 study, the mortality risk of a cohort of elderly patients with cervical fractures appeared to be elevated for the first 6 to 12 months after the traumatic event.40 However, the sample size was too small to examine mortality beyond 1 year.
In this context, the purpose of the current study was to determine the mortality rates at several time points (3 months, 1 year, and 2 years) of patients 50 years or older (start of the second mode of the bimodal age distribution of odontoid fractures41-44) with fractures of the odontoid and subaxial cervical spine. A secondary purpose of this study was to compare survival rates of these 2 cohorts relative to each other and to the general population.
Materials and Methods
Identification of Cervical Fractures and Collection of Demographic Information
This protocol was approved by the human investigation committee of our institution. Every computed tomography (CT) scan of the cervical spine performed in the emergency department (ED) of an academic hospital between November 27, 1997, and December 31, 2006, was identified. Since the threshold for obtaining a CT scan of a patient with suspected cervical spine trauma is relatively low, it was assumed that virtually all acute cervical spine fractures during this time period would be successfully identified through this approach.
Radiology reports for all identified CT scans were reviewed for any findings consistent with acute fractures and/or dislocations of the cervical spine (Figure 1). Every study noted to be positive or equivocal for cervical trauma was directly visualized, including those that did not specifically mention the presence or absence of an injury. Scans with no signs of acute trauma or that showed fractures caused by a pathologic process or penetrating mechanism (eg, metastatic lesions or gunshot wounds) were omitted from this series. Finally, relevant demographic information, such as the medical record number, age, gender, and date of study, was recorded for every subject in this group.
Fracture Classification
Next, the level and the type of cervical injury were documented for each patient. Fractures were segregated according to their involvement with the odontoid or the subaxial vertebrae.
Odontoid fractures were categorized into type I (limited to the tip), type II (across the base of the process) and type III (through the base with extension into the C2 vertebral body).45,46 Since many systems for classifying subaxial cervical spine trauma require a subjective inference of the injury mechanism, which is difficult to ascertain from imaging studies alone, all of these fractures were pooled together.
A preliminary survey of the data indicated that the odontoid fractures appeared to exhibit a bimodal age distribution, with the beginning of the second cluster occurring around age 50 years (Figure 2). As noted above, this has been shown in previous studies.41-44 As a consequence, the mortalities of those older than 50 years became the focus of this study. To control for comorbid conditions, mechanism of injury, and to allow for more direct comparison with the odontoid fractures in this study, the same age demarcation was used for subaxial cervical fractures.
Mortality Data
The mortality status of every patient diagnosed with an acute cervical injury at our institution between November 27, 1997, and December 31, 2006, was determined by referencing the National Death Index (NDI). The NDI is a computerized database of death records maintained by the National Center for Health Statistics (NCHS). The time window for the current study was selected because we had access to NDI information only through 2007 at the time of this study. Social Security numbers (SSNs), which were available for approximately half of the subjects, were used to search the NDI catalog. For individuals whose SSNs were unavailable, patient names and birthdates were considered to be sufficient to confirm a true match. Our center’s medical records of this cohort were also examined to verify whether any had died during their initial hospitalizations and to substantiate the NDI data. Finally, patient deaths were categorized as trauma (eg, motor vehicle accident, fall from a height) or medical comorbidity (eg, diabetes mellitus, cancer, congestive heart failure), based on information in the NDI listing.
Age- and Sex-Matched Controls
Age- and sex-matched controls were determined from the Wide-ranging Online Data for Epidemiologic Research (WONDER) application distributed by the Centers for Disease Control and Prevention (http://wonder.cdc.gov). Composite mortality data from the state in which the study was performed was obtained for the years between 1999 and 2007, and this information was further stratified according to gender and age to estimate the mortality rates and construct survival curves for each group. Controls were used to establish a standardized mortality ratio (SMR) for subjects 50 years and older, a value that compares the number of observed deaths with the figure expected for matched populations from the general population.
Statistical Methods
Statistical analyses were performed by using both SAS 9.2 (SAS Institute Inc., Cary, North Carolina) and R (version 2.9; www.r-project.org, Auckland, New Zealand). Relevant comparisons were planned, and all tests were 2-sided. The Wilcoxon rank sum test was applied to compare the survival times of patients with odontoid fractures with different documented causes of death, and Pearson χ2 test was used to compare the age distributions of odontoid and subaxial fractures. Survival rates at 3 months, 1 year, and 2 years were estimated from Kaplan-Meier curves. The relative survival of these cohorts was compared by completing a 2-sample log-rank test. In addition, a 1-sample log-rank test was implemented to compare the mortality from either odontoid or subaxial cervical spine fractures with that of the age- and gender-matched general population. Statistical significance was defined as a 2-sided α error of less than 0.05 (P < .05).
Results
Fifty-nine patients were diagnosed with odontoid fractures (28 men, 31 women), and 233 patients were diagnosed with subaxial cervical spine fractures (168 men, 65 women).
Odontoid Fracture Patients
Odontoid fracture patients exhibited a distinct bimodal age distribution (Figure 2). In the younger population, there were 14 subjects, 3 of whom died within days of the injury (mean, 12 days; 78.6% survival). At 2-year follow-up, there were no further deaths. The fractures that caused death were high-energy injuries, and only early deaths occurred in these cases.
Because of the significant bimodal age distribution, it was believed these cohorts could not be directly compared. As a result, the remaining analysis focused on the older age group. In the older population mode (50 years and older) were 45 patients with odontoid fractures. Of the 12 subjects who died after odontoid fracture, 5 were assigned a trauma code as the cause of death, while a medical comorbidity code was assigned for the remaining 7. Mean survival time of those who died secondary to trauma was significantly shorter than the medical comorbidity group (P = .025).
In the cohort of subjects older than 50 years, 3-month, 1-year, and 2-year survival rates were 84.4%, 82.2%, and 72.9%, respectively. Figure 2 shows the 1- and 2-year follow-up data by age group.
Analysis was performed relative to gender. Of male patients (n = 22), the 3-month, 1-year, and 2-year survival rates were 72.7%, 72.7%, and 62.7%, respectively. Among women (n = 23), the 3-month, 1-year, and 2-year survival rates were 95.7%, 91.3%, and 82.6%, respectively.
Figure 3 shows the Kaplan-Meier survival curves of the older patients with odontoid fractures. A comparison of the curves for each gender showed no significant disparities between the male and female survival (Figure 3A, P = .124). Compared with age-matched male counterparts, the survival of male subjects with odontoid fractures was significantly worse (Figure 3B, P < .001). Men experienced an initial acute decline in survival, with the remainder of the survival curve matching that of the general male population. In contrast, odontoid fractures did not adversely affect female survival compared with the matched population (Figure 3C, P = .568).
The 2-year SMR of 2.98 for men showed that odontoid fractures led to greater mortality compared with a sex- and age-matched population. This means that men older than 50 years who sustained an odontoid fracture had nearly 3 times the mortality rate after 2 years compared with a normal, matched population; this increase is attributed to the 3-month time point that subsequently normalized. The female rate was 1.33 times that of a matched population, a difference that is not statistically significant.
Subaxial Fracture Patients
Of the 91 patients older than 50 years with subaxial fractures, 3-month, 1-year, and 2-year survival rates were 87.9%, 85.7%, and 85.7%, respectively. Figure 4 shows the 1- and 2-year follow-up data by age group.
Gender-specific analysis was performed. For men (n = 58), the 3-month, 1-year, and 2-year survival rates were 87.9%, 84.5%, and 84.5%, respectively. Among women (n = 33), 4 deaths were recorded at all time points (87.9% survival).
Figure 5 shows Kaplan-Meier survival curves for the older population with subaxial fractures. A comparison of the curves between genders again showed no significant differences between male and female survival (P = .683, Figure 5A). Compared with age- and gender-matched counterparts, men showed decreased relative survival (P < .0001, Figure 5B), whereas subaxial fractures did not decrease female survival (P = .554, Figure 5C).
The 2-year SMR of 2.90 for men showed higher mortality rates relative to sex- and age-matched controls. Men who were both 50 years old and sustained a subaxial fracture were 2.9 times as likely to die within 2 years of follow-up compared with their counterparts. Similar to odontoid fractures, this increase occurred by the 3-month time point and subsequently normalized. The female rate, which was 1.34 times that of the uninjured population, was not statistically significant.
Comparison of Odontoid and Subaxial Fracture Patients
The survival of subaxial injuries was not significantly different from that of odontoid fractures (P = .113, Figure 6A). When analyzed by gender and controlled for age, the rates in both male (P = .347, Figure 6B) and female (P = .643, Figure 6C) patients did not differ between fracture types.
Discussion
The US population is aging rapidly, with the demographic older than 65 years predicted to more than double in size between 2010 and 2050.47 As our elderly population grows, the incidence of age-related injuries will rise accordingly. An understanding of mortality risks associated with different fractures will not only assist practitioners in advising patients regarding prognosis but may also lead to improvements in clinical care.19,48-50 While we know cervical spine trauma is associated with significant morbidity,29-31 little is known about associated moderate-term mortality rates that can be compared with other known injury patterns, such as hip fractures or osteoporotic compression fractures.
An interesting finding of the present study is the bimodal age distribution of the 59 odontoid fractures (Figure 2). The 14 patients younger than 50 years included 3 individuals who died, all within days of their presentation from severe multisystem trauma. This is consistent with the determination that high-energy forces are required to fracture the odontoid process in younger individuals.38,45,46,51,52 Given the severity of their nonspinal injuries, the cause of death was likely not primarily related to their odontoid fractures. Also in line with previous studies, the majority (76%) of odontoid fractures were documented in subjects older than 50 years.32,53,54 Within our cohort older than 50 years, the deaths appear to be spread evenly across age groups and do not seem to be skewed by the oldest portion of the population (Figure 2).
Our gender-specific analyses revealed that older men with odontoid injuries exhibited higher mortality compared with an age-matched male cohort, with 6 of the 8 deaths occurring within 3 months. However, after this exaggerated decline in survival, the rate normalized towards general population mortality rates (Figure 3B). As in the younger cohort, these earlier deaths were largely attributable to multisystem trauma, whereas medical comorbidities were implicated in those who died later. In contrast, the Kaplan-Meier curve of older women with odontoid fractures closely approximates that of age-matched women at every time point (Figure 3C), indicating that these injuries do not decrease survival as they do in their male counterparts.
When comparing the survival of older patients with subaxial cervical spine fractures with that of gender- and age-matched controls, the mortality rates of women were, once again, essentially equivalent. However, the survival of older men was significantly compromised by these injuries. In men, 7 of the 9 deaths were within 3 months, with the remaining 2 deaths occurring within 7 months. Nevertheless, beyond this initial period of elevated mortality, the survival curve again stabilized and paralleled that of the general population. As with odontoid fractures, there was no sustained increase in the mortality of male patients who lived at least 3 months after injury.
The mortality rates of odontoid and subaxial fractures were also compared in the older population. When controlled for age, there was no difference in mortality rates between these 2 groups. When individually analyzed in both men and women, the mortality rates of both fracture types matched those of the general population at all time points.
It is useful to contextualize our findings alongside the mortality of older individuals with other fracture types. Based on our results, we believe that the survival curves of geriatric men with odontoid or subaxial cervical spine fractures most closely resemble the characteristic pattern seen in hip fractures. Hip fractures have shown an early spike in mortality by as much as 8% to 49% in the first 6 to 12 months that returns to baseline after 1 year.1-10 This presumably reflects the natural history of these injuries in response to appropriate therapeutic interventions. Interestingly, the male mortality rates for both odontoid and subaxial cervical spine fractures in this study are largely analogous to those reported by various hip fracture surveys.1,5,55-58 In contrast, similar to prior studies of rib or wrist fractures, older women with these cervical spine fractures did not show a survival decrease after their injuries.12,16-18
While the reasons underlying the differential effects of cervical fractures on the mortality of men and women have not been established, one explanation is that the female geriatric population is relatively more osteoporotic; thus, cervical injuries may occur after lower-energy forces, leading to less severe associated trauma that could otherwise decrease survival. Another explanation is that men are more likely to be involved in high-energy accidents,59,60 thus decreasing their overall survival after injury.
This investigation is not without limitations. Our primary concern is the determination of survival. The NDI maintained by the NCHS is an extremely reliable tool regularly employed by epidemiologists to collect mortality data. However, it is possible that deaths may have been missed. We believe this number would be small, because the NDI database provided multiple probable matches that were carefully compared with supplemental personal information. It is also possible that deaths that were not appropriately registered with the NDI are not represented in this series. Another limitation lies in the determination of controls. As with any case–control study, the patients sustaining these odontoid fractures may differ in some significant way from the average population. A final limitation is that a small portion of patients in the study have only 1-year follow-up, because patient data was collected through 2006, although access to NDI data ended in 2007.
Conclusion
Our results indicate that the survival of older men with either odontoid or subaxial cervical spine fractures shares many of the same mortality characteristics as hip fractures, with diminished survival in the first 3 months that normalizes to the survival rate of the age-matched population. Interestingly, and perhaps because of disparate rates of osteoporosis and traumatic forces, the mortality rates in the female cohort were similar to that of the age-matched general population at all time points. These trends were nearly identical for both odontoid and subaxial cervical fractures.
Mortality rate is an important indicator of the severity of traumatic injuries, and these values have been described for different orthopedic injuries and fractures. Studies have identified 3 distinct trends in patient survival when compared with the age- and sex-matched uninjured population:
1. Hip fractures bring about a transient increase in mortality relative to age-matched controls that normalizes after a few months to 1 year.1-10
2. Thoracic and lumbar compression fractures are associated with an ongoing, lifelong increase in mortality rate relative to age-matched controls without an initial marked upswing.11-15
3. Certain injuries such as isolated rib or wrist fractures do not adversely affect survival relative to age-matched controls.12,16-18
Understanding the mortality patterns after these injuries can help guide management and even facilitate the development of appropriate treatment algorithms.19-21 While studies have examined mortality in specific odontoid fracture types,22 such mortality trends have not been broadly established in persons with cervical spine fractures.
Cervical spine fractures are common: 60% of spine fractures localize to this region,23-26 and this equates to 2% to 3% of all blunt-trauma patients.27,28 These injuries can lead to devastating consequences, including neurologic compromise, permanent disability, and death.29-31
Studies have estimated that up to 20% of cervical fractures involve the odontoid process.23-26 These injuries are more common among the elderly population because of their greater prevalence of osteoporosis and likelihood of falling.32 Because of demographic similarities to those of the hip fracture population, a survival analysis of all odontoid fractures is particularly interesting. Published odontoid mortality rates vary significantly, with reports ranging from 13% to 44%.22,33-35 Unfortunately, these studies largely evaluated survival rates specific to an individual treatment modality, such as nonoperative compared with operative, or specific to certain odontoid fracture types (eg, type II). Additionally, studies have generally only considered survivorship during initial hospitalization, have been specific to a constrained age group, or have been based solely on inpatient records that do not permit the longer-term follow-up critical to determining the effect of odontoid fractures on overall mortality.36-39
Likewise, mortality rates after fractures of the subaxial spine (ie, the motion segments between C3 and C7) have yet to be established. In 1 study, the mortality risk of a cohort of elderly patients with cervical fractures appeared to be elevated for the first 6 to 12 months after the traumatic event.40 However, the sample size was too small to examine mortality beyond 1 year.
In this context, the purpose of the current study was to determine the mortality rates at several time points (3 months, 1 year, and 2 years) of patients 50 years or older (start of the second mode of the bimodal age distribution of odontoid fractures41-44) with fractures of the odontoid and subaxial cervical spine. A secondary purpose of this study was to compare survival rates of these 2 cohorts relative to each other and to the general population.
Materials and Methods
Identification of Cervical Fractures and Collection of Demographic Information
This protocol was approved by the human investigation committee of our institution. Every computed tomography (CT) scan of the cervical spine performed in the emergency department (ED) of an academic hospital between November 27, 1997, and December 31, 2006, was identified. Since the threshold for obtaining a CT scan of a patient with suspected cervical spine trauma is relatively low, it was assumed that virtually all acute cervical spine fractures during this time period would be successfully identified through this approach.
Radiology reports for all identified CT scans were reviewed for any findings consistent with acute fractures and/or dislocations of the cervical spine (Figure 1). Every study noted to be positive or equivocal for cervical trauma was directly visualized, including those that did not specifically mention the presence or absence of an injury. Scans with no signs of acute trauma or that showed fractures caused by a pathologic process or penetrating mechanism (eg, metastatic lesions or gunshot wounds) were omitted from this series. Finally, relevant demographic information, such as the medical record number, age, gender, and date of study, was recorded for every subject in this group.
Fracture Classification
Next, the level and the type of cervical injury were documented for each patient. Fractures were segregated according to their involvement with the odontoid or the subaxial vertebrae.
Odontoid fractures were categorized into type I (limited to the tip), type II (across the base of the process) and type III (through the base with extension into the C2 vertebral body).45,46 Since many systems for classifying subaxial cervical spine trauma require a subjective inference of the injury mechanism, which is difficult to ascertain from imaging studies alone, all of these fractures were pooled together.
A preliminary survey of the data indicated that the odontoid fractures appeared to exhibit a bimodal age distribution, with the beginning of the second cluster occurring around age 50 years (Figure 2). As noted above, this has been shown in previous studies.41-44 As a consequence, the mortalities of those older than 50 years became the focus of this study. To control for comorbid conditions, mechanism of injury, and to allow for more direct comparison with the odontoid fractures in this study, the same age demarcation was used for subaxial cervical fractures.
Mortality Data
The mortality status of every patient diagnosed with an acute cervical injury at our institution between November 27, 1997, and December 31, 2006, was determined by referencing the National Death Index (NDI). The NDI is a computerized database of death records maintained by the National Center for Health Statistics (NCHS). The time window for the current study was selected because we had access to NDI information only through 2007 at the time of this study. Social Security numbers (SSNs), which were available for approximately half of the subjects, were used to search the NDI catalog. For individuals whose SSNs were unavailable, patient names and birthdates were considered to be sufficient to confirm a true match. Our center’s medical records of this cohort were also examined to verify whether any had died during their initial hospitalizations and to substantiate the NDI data. Finally, patient deaths were categorized as trauma (eg, motor vehicle accident, fall from a height) or medical comorbidity (eg, diabetes mellitus, cancer, congestive heart failure), based on information in the NDI listing.
Age- and Sex-Matched Controls
Age- and sex-matched controls were determined from the Wide-ranging Online Data for Epidemiologic Research (WONDER) application distributed by the Centers for Disease Control and Prevention (http://wonder.cdc.gov). Composite mortality data from the state in which the study was performed was obtained for the years between 1999 and 2007, and this information was further stratified according to gender and age to estimate the mortality rates and construct survival curves for each group. Controls were used to establish a standardized mortality ratio (SMR) for subjects 50 years and older, a value that compares the number of observed deaths with the figure expected for matched populations from the general population.
Statistical Methods
Statistical analyses were performed by using both SAS 9.2 (SAS Institute Inc., Cary, North Carolina) and R (version 2.9; www.r-project.org, Auckland, New Zealand). Relevant comparisons were planned, and all tests were 2-sided. The Wilcoxon rank sum test was applied to compare the survival times of patients with odontoid fractures with different documented causes of death, and Pearson χ2 test was used to compare the age distributions of odontoid and subaxial fractures. Survival rates at 3 months, 1 year, and 2 years were estimated from Kaplan-Meier curves. The relative survival of these cohorts was compared by completing a 2-sample log-rank test. In addition, a 1-sample log-rank test was implemented to compare the mortality from either odontoid or subaxial cervical spine fractures with that of the age- and gender-matched general population. Statistical significance was defined as a 2-sided α error of less than 0.05 (P < .05).
Results
Fifty-nine patients were diagnosed with odontoid fractures (28 men, 31 women), and 233 patients were diagnosed with subaxial cervical spine fractures (168 men, 65 women).
Odontoid Fracture Patients
Odontoid fracture patients exhibited a distinct bimodal age distribution (Figure 2). In the younger population, there were 14 subjects, 3 of whom died within days of the injury (mean, 12 days; 78.6% survival). At 2-year follow-up, there were no further deaths. The fractures that caused death were high-energy injuries, and only early deaths occurred in these cases.
Because of the significant bimodal age distribution, it was believed these cohorts could not be directly compared. As a result, the remaining analysis focused on the older age group. In the older population mode (50 years and older) were 45 patients with odontoid fractures. Of the 12 subjects who died after odontoid fracture, 5 were assigned a trauma code as the cause of death, while a medical comorbidity code was assigned for the remaining 7. Mean survival time of those who died secondary to trauma was significantly shorter than the medical comorbidity group (P = .025).
In the cohort of subjects older than 50 years, 3-month, 1-year, and 2-year survival rates were 84.4%, 82.2%, and 72.9%, respectively. Figure 2 shows the 1- and 2-year follow-up data by age group.
Analysis was performed relative to gender. Of male patients (n = 22), the 3-month, 1-year, and 2-year survival rates were 72.7%, 72.7%, and 62.7%, respectively. Among women (n = 23), the 3-month, 1-year, and 2-year survival rates were 95.7%, 91.3%, and 82.6%, respectively.
Figure 3 shows the Kaplan-Meier survival curves of the older patients with odontoid fractures. A comparison of the curves for each gender showed no significant disparities between the male and female survival (Figure 3A, P = .124). Compared with age-matched male counterparts, the survival of male subjects with odontoid fractures was significantly worse (Figure 3B, P < .001). Men experienced an initial acute decline in survival, with the remainder of the survival curve matching that of the general male population. In contrast, odontoid fractures did not adversely affect female survival compared with the matched population (Figure 3C, P = .568).
The 2-year SMR of 2.98 for men showed that odontoid fractures led to greater mortality compared with a sex- and age-matched population. This means that men older than 50 years who sustained an odontoid fracture had nearly 3 times the mortality rate after 2 years compared with a normal, matched population; this increase is attributed to the 3-month time point that subsequently normalized. The female rate was 1.33 times that of a matched population, a difference that is not statistically significant.
Subaxial Fracture Patients
Of the 91 patients older than 50 years with subaxial fractures, 3-month, 1-year, and 2-year survival rates were 87.9%, 85.7%, and 85.7%, respectively. Figure 4 shows the 1- and 2-year follow-up data by age group.
Gender-specific analysis was performed. For men (n = 58), the 3-month, 1-year, and 2-year survival rates were 87.9%, 84.5%, and 84.5%, respectively. Among women (n = 33), 4 deaths were recorded at all time points (87.9% survival).
Figure 5 shows Kaplan-Meier survival curves for the older population with subaxial fractures. A comparison of the curves between genders again showed no significant differences between male and female survival (P = .683, Figure 5A). Compared with age- and gender-matched counterparts, men showed decreased relative survival (P < .0001, Figure 5B), whereas subaxial fractures did not decrease female survival (P = .554, Figure 5C).
The 2-year SMR of 2.90 for men showed higher mortality rates relative to sex- and age-matched controls. Men who were both 50 years old and sustained a subaxial fracture were 2.9 times as likely to die within 2 years of follow-up compared with their counterparts. Similar to odontoid fractures, this increase occurred by the 3-month time point and subsequently normalized. The female rate, which was 1.34 times that of the uninjured population, was not statistically significant.
Comparison of Odontoid and Subaxial Fracture Patients
The survival of subaxial injuries was not significantly different from that of odontoid fractures (P = .113, Figure 6A). When analyzed by gender and controlled for age, the rates in both male (P = .347, Figure 6B) and female (P = .643, Figure 6C) patients did not differ between fracture types.
Discussion
The US population is aging rapidly, with the demographic older than 65 years predicted to more than double in size between 2010 and 2050.47 As our elderly population grows, the incidence of age-related injuries will rise accordingly. An understanding of mortality risks associated with different fractures will not only assist practitioners in advising patients regarding prognosis but may also lead to improvements in clinical care.19,48-50 While we know cervical spine trauma is associated with significant morbidity,29-31 little is known about associated moderate-term mortality rates that can be compared with other known injury patterns, such as hip fractures or osteoporotic compression fractures.
An interesting finding of the present study is the bimodal age distribution of the 59 odontoid fractures (Figure 2). The 14 patients younger than 50 years included 3 individuals who died, all within days of their presentation from severe multisystem trauma. This is consistent with the determination that high-energy forces are required to fracture the odontoid process in younger individuals.38,45,46,51,52 Given the severity of their nonspinal injuries, the cause of death was likely not primarily related to their odontoid fractures. Also in line with previous studies, the majority (76%) of odontoid fractures were documented in subjects older than 50 years.32,53,54 Within our cohort older than 50 years, the deaths appear to be spread evenly across age groups and do not seem to be skewed by the oldest portion of the population (Figure 2).
Our gender-specific analyses revealed that older men with odontoid injuries exhibited higher mortality compared with an age-matched male cohort, with 6 of the 8 deaths occurring within 3 months. However, after this exaggerated decline in survival, the rate normalized towards general population mortality rates (Figure 3B). As in the younger cohort, these earlier deaths were largely attributable to multisystem trauma, whereas medical comorbidities were implicated in those who died later. In contrast, the Kaplan-Meier curve of older women with odontoid fractures closely approximates that of age-matched women at every time point (Figure 3C), indicating that these injuries do not decrease survival as they do in their male counterparts.
When comparing the survival of older patients with subaxial cervical spine fractures with that of gender- and age-matched controls, the mortality rates of women were, once again, essentially equivalent. However, the survival of older men was significantly compromised by these injuries. In men, 7 of the 9 deaths were within 3 months, with the remaining 2 deaths occurring within 7 months. Nevertheless, beyond this initial period of elevated mortality, the survival curve again stabilized and paralleled that of the general population. As with odontoid fractures, there was no sustained increase in the mortality of male patients who lived at least 3 months after injury.
The mortality rates of odontoid and subaxial fractures were also compared in the older population. When controlled for age, there was no difference in mortality rates between these 2 groups. When individually analyzed in both men and women, the mortality rates of both fracture types matched those of the general population at all time points.
It is useful to contextualize our findings alongside the mortality of older individuals with other fracture types. Based on our results, we believe that the survival curves of geriatric men with odontoid or subaxial cervical spine fractures most closely resemble the characteristic pattern seen in hip fractures. Hip fractures have shown an early spike in mortality by as much as 8% to 49% in the first 6 to 12 months that returns to baseline after 1 year.1-10 This presumably reflects the natural history of these injuries in response to appropriate therapeutic interventions. Interestingly, the male mortality rates for both odontoid and subaxial cervical spine fractures in this study are largely analogous to those reported by various hip fracture surveys.1,5,55-58 In contrast, similar to prior studies of rib or wrist fractures, older women with these cervical spine fractures did not show a survival decrease after their injuries.12,16-18
While the reasons underlying the differential effects of cervical fractures on the mortality of men and women have not been established, one explanation is that the female geriatric population is relatively more osteoporotic; thus, cervical injuries may occur after lower-energy forces, leading to less severe associated trauma that could otherwise decrease survival. Another explanation is that men are more likely to be involved in high-energy accidents,59,60 thus decreasing their overall survival after injury.
This investigation is not without limitations. Our primary concern is the determination of survival. The NDI maintained by the NCHS is an extremely reliable tool regularly employed by epidemiologists to collect mortality data. However, it is possible that deaths may have been missed. We believe this number would be small, because the NDI database provided multiple probable matches that were carefully compared with supplemental personal information. It is also possible that deaths that were not appropriately registered with the NDI are not represented in this series. Another limitation lies in the determination of controls. As with any case–control study, the patients sustaining these odontoid fractures may differ in some significant way from the average population. A final limitation is that a small portion of patients in the study have only 1-year follow-up, because patient data was collected through 2006, although access to NDI data ended in 2007.
Conclusion
Our results indicate that the survival of older men with either odontoid or subaxial cervical spine fractures shares many of the same mortality characteristics as hip fractures, with diminished survival in the first 3 months that normalizes to the survival rate of the age-matched population. Interestingly, and perhaps because of disparate rates of osteoporosis and traumatic forces, the mortality rates in the female cohort were similar to that of the age-matched general population at all time points. These trends were nearly identical for both odontoid and subaxial cervical fractures.
1. Gennarelli TA, Champion HR, Sacco WJ, Copes WS, Alves WM. Mortality of patients with head injury and extracranial injury treated in trauma centers. J Trauma. 1989;29(9):1193-1201; discussion 1201-1202.
2. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology (Oxford). 2000;39(4):346-349.
3. Gerrelts BD, Petersen EU, Mabry J, Petersen SR. Delayed diagnosis of cervical spine injuries. J Trauma. 1991;31(12):1622-1626.
4. Giannoudis PV, Mehta SS, Tsiridis E. Incidence and outcome of whiplash injury after multiple trauma. Spine. 2007;32(7):776-781.
5. Goldberg W, Mueller C, Panacek E, et al. Distribution and patterns of blunt traumatic cervical spine injury. Ann Emerg Med. 2001;38(1):17-21.
6. Grauer JN, Shafi B, Hilibrand AS, et al. Proposal of a modified, treatment-oriented classification of odontoid fractures. Spine J. 2005;5(2):123-129.
7. Greene KA, Dickman CA, Marciano FF, Drabier JB, Hadley MN, Sonntag VK. Acute axis fractures. Analysis of management and outcome in 340 consecutive cases. Spine. 1997;22(16):1843-1852.
8. Gulli B, Templeman D. Compartment syndrome of the lower extremity. Orthop Clin North Am. 1994;25(4):677-684.
9. Guthkelch AN, Fleischer AS. Patterns of cervical spine injury and their associated lesions. West J Med. 1987;147(4):428-431.
10. Hackl W, Hausberger K, Sailer R, Ulmer H, Gassner R. Prevalence of cervical spine injuries in patients with facial trauma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(4):370-376.
11. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
12. Garabige V, Giraud P, De Rycke Y, et al. [Impact of nutrition management in patients with head and neck cancers treated with irradiation: is the nutritional intervention useful?]. Cancer Radiother. 2007;11(3):111-116.
13. Garbuz DS, Leitch K, Wright JG. The treatment of supracondylar fractures in children with an absent radial pulse. J Pediatr Orthop. 1996;16(5):594-596.
14. Henderson RL, Reid DC, Saboe LA. Multiple noncontiguous spine fractures. Spine. 1991;16(2):128-131.
15. Henrikson B. Supracondylar fracture of the humerus in children. A late review of end-results with special reference to the cause of deformity, disability and complications. Acta Chir Scand Suppl. 1966;369:1-72.
16. De Boeck H, De Smet P, Penders W, De Rydt D. Supracondylar elbow fractures with impaction of the medial condyle in children. J Pediatr Orthop. 1995;15(4):444-448.
17. Gelberman RH, Panagis JS, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part I: The extraosseous vascularity. J Hand Surg Am. 1983;8(4):367-375.
18. Hu J, Liao Q, Long W. Diagnosis and treatment of multiple-level noncontiguous spinal fractures. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2005;19(6):424-426.
19. Eleraky MA, Theodore N, Adams M, Rekate HL, Sonntag VK. Pediatric cervical spine injuries: report of 102 cases and review of the literature. J Neurosurg. 2000;92(1 suppl):12-17.
20. Ioannidis G, Papaioannou A, Hopman WM, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ. 2009;181(5):265-271.
21. Husby J, Sorensen KH. Fracture of the odontoid process of the axis. Acta Orthop Scand. 1974;45(2):182-192.
22. Schoenfeld AJ, Bono CM, Reichmann WM, et al. Type II odontoid fractures of the cervical spine: do treatment type and medical comorbidities affect mortality in elderly patients? Spine. 2011;36(11):879-885.
23. Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc. 2000;48(3):241-249.
24. Fassett DR, Dailey AT, Vaccaro AR. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21(4):252-258.
25. Ippolito E, Caterini R, Scola E. Supracondylar fractures of the humerus in children. Analysis at maturity of fifty-three patients treated conservatively. J Bone Joint Surg Am. 1986;68(3):333-344.
26. Spence KF Jr, Decker S, Sell KW. Bursting atlantal fracture associated with rupture of the transverse ligament. J Bone Joint Surg Am. 1970;52(3):543-549.
27. Irwin ZN, Arthur M, Mullins RJ, Hart RA. Variations in injury patterns, treatment, and outcome for spinal fracture and paralysis in adult versus geriatric patients. Spine. 2004;29(7):796-802.
28. Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int. 1998;8(3):291-297.
29. Iyengar SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and pinning of type III displaced supracondylar fractures of the humerus in children: a comparative study. J Orthop Trauma. 1999;13(1):51-55.
30. Jackson AP, Haak MH, Khan N, Meyer PR. Cervical spine injuries in the elderly: acute postoperative mortality. Spine. 2005;30(13):1524-1527.
31. Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Race and sex differences in mortality following fracture of the hip. Am J Public Health. 1992;82(8):1147-1150.
32. Fisher ES, Baron JA, Malenka DJ, et al. Hip fracture incidence and mortality in New England. Epidemiology. 1991;2(2):116-122.
33. Chapman J, Smith JS, Kopjar B, et al. The AOSpine North America Geriatric Odontoid Fracture Mortality Study: a retrospective review of mortality outcomes for operative versus nonoperative treatment of 322 patients with long-term follow-up. Spine. 2013;38:1098-1104.
34. Denault A, Bains I, Moghadam K, Hu RW, Swamy G. Evaluation of mortality following an odontoid fracture in the octogenarian population. J Bone Joint Surg Br. 2011;93(Supp IV):585.
35. Molinari WJ III, Molinari RW, Khera OA, Gruhn WL. Functional outcomes, morbidity, mortality, and fracture healing in 58 consecutive patients with geriatric odontoid fracture treated with cervical collar or posterior fusion. Global Spine J. 2013;3(1):21-32.
36. Hanigan WC, Powell FC, Elwood PW, Henderson JP. Odontoid fractures in elderly patients. J Neurosurg. 1993;78(1):32-35.
37. Korres DS, Boscainos PJ, Papagelopoulos PJ, Psycharis I, Goudelis G, Nikolopoulos K. Multiple level noncontiguous fractures of the spine. Clin Orthop. 2003;411:95-102.
38. Leet AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3 supracondylar humerus fractures in children. J Pediatr Orthop. 2002;22(2):203-207.
39. Leone A, Cerase A, Colosimo C, Lauro L, Puca A, Marano P. Occipital condylar fractures: a review. Radiology. 2000;216(3):635-644.
40. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799-1809.
41. Müller EJ, Wick M, Russe O, Muhr G. Management of odontoid fractures in the elderly. Eur Spine J. 1999;8(5):360-365.
42. Pepin JW, Bourne RB, Hawkins RJ. Odontoid fractures, with special reference to the elderly patient. Clin Orthop. 1985;193:178-183.
43. Ryan MD, Henderson JJ. The epidemiology of fractures and fracture-dislocations of the cervical spine. Injury. 1992;23(1):38-40.
44. Butler JS, Dolan RT, Burbridge M, et al. The long-term functional outcome of type II odontoid fractures managed non-operatively. Eur Spine J. 2010;19(10):1635-1642.
45. Levine AM, Edwards CC. The management of traumatic spondylolisthesis of the axis. J Bone Joint Surg Am. 1985;67(2):217-226.
46. Lowery DW, Wald MM, Browne BJ, Tigges S, Hoffman JR, Mover WR; NEXUS Group. Epidemiology of cervical spine injury victims. Ann Emerg Med. 2001;38(1):12-16.
47. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-16. http://www.prb.org/pdf11/aging-in-america.pdf. Published February 2011. Accessed April 22, 2015.
48. Holly LT, Kelly DF, Counelis GJ, Blinman T, McArthur DL, Cryer HG. Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors, and injury characteristics. J Neurosurg. 2002;96(3 suppl):285-291.
49. Holmes JF, Akkinepalli R. Computed tomography versus plain radiography to screen for cervical spine injury: a meta-analysis. J Trauma. 2005;58(5):902-905.
50. Hove LM. Epidemiology of scaphoid fractures in Bergen, Norway. Scand J Plast Reconstr Surg Hand Surg. 1999;33(4):423-426.
51. Lu-Yao G, Baron Ja, Barrett Ja, Fisher Es. Treatment and survival among elderly Americans with hip fractures: a population-based study. Am J Public Health. 1994;84(8):1287-1291.
52. Lu-Yao GL, Keller RB, Littenberg B, Wennberg JE. Outcomes after displaced fractures of the femoral neck. A meta-analysis of one hundred and six published reports. J Bone Joint Surg Am. 1994;76(1):15-25.
53. Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159(11):1215-1220.
54. Levine AM, Edwards CC. Fractures of the atlas. J Bone Joint Surg Am. 1991;73(5):680-691.
55. Maak TG, Grauer JN. The contemporary treatment of odontoid injuries. Spine. 2006;31(11 Suppl):S53-S60; discussion S61.
56. Magaziner J, Fredman L, Hawkes W, et al. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol. 2003;157(11):1023-1031.
57. Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55(9):M498-M507.
58. Malham GM, Ackland HM, Jones R, Williamson OD, Varma DK. Occipital condyle fractures: incidence and clinical follow-up at a level 1 trauma centre. Emerg Radiol. 2009;16(4):291-297.
59. Probst C, Zelle B, Panzica M, et al. Clinical re-examination 10 or more years after polytrauma: is there a gender related difference? J Trauma. 2010;68(3):706-711.
60. Holbrook TL, Hoyt DB, Anderson JP. The importance of gender on outcome after major trauma: functional and psychologic outcomes in women versus men. J Trauma. 2001;50(2):270-273.
1. Gennarelli TA, Champion HR, Sacco WJ, Copes WS, Alves WM. Mortality of patients with head injury and extracranial injury treated in trauma centers. J Trauma. 1989;29(9):1193-1201; discussion 1201-1202.
2. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology (Oxford). 2000;39(4):346-349.
3. Gerrelts BD, Petersen EU, Mabry J, Petersen SR. Delayed diagnosis of cervical spine injuries. J Trauma. 1991;31(12):1622-1626.
4. Giannoudis PV, Mehta SS, Tsiridis E. Incidence and outcome of whiplash injury after multiple trauma. Spine. 2007;32(7):776-781.
5. Goldberg W, Mueller C, Panacek E, et al. Distribution and patterns of blunt traumatic cervical spine injury. Ann Emerg Med. 2001;38(1):17-21.
6. Grauer JN, Shafi B, Hilibrand AS, et al. Proposal of a modified, treatment-oriented classification of odontoid fractures. Spine J. 2005;5(2):123-129.
7. Greene KA, Dickman CA, Marciano FF, Drabier JB, Hadley MN, Sonntag VK. Acute axis fractures. Analysis of management and outcome in 340 consecutive cases. Spine. 1997;22(16):1843-1852.
8. Gulli B, Templeman D. Compartment syndrome of the lower extremity. Orthop Clin North Am. 1994;25(4):677-684.
9. Guthkelch AN, Fleischer AS. Patterns of cervical spine injury and their associated lesions. West J Med. 1987;147(4):428-431.
10. Hackl W, Hausberger K, Sailer R, Ulmer H, Gassner R. Prevalence of cervical spine injuries in patients with facial trauma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(4):370-376.
11. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
12. Garabige V, Giraud P, De Rycke Y, et al. [Impact of nutrition management in patients with head and neck cancers treated with irradiation: is the nutritional intervention useful?]. Cancer Radiother. 2007;11(3):111-116.
13. Garbuz DS, Leitch K, Wright JG. The treatment of supracondylar fractures in children with an absent radial pulse. J Pediatr Orthop. 1996;16(5):594-596.
14. Henderson RL, Reid DC, Saboe LA. Multiple noncontiguous spine fractures. Spine. 1991;16(2):128-131.
15. Henrikson B. Supracondylar fracture of the humerus in children. A late review of end-results with special reference to the cause of deformity, disability and complications. Acta Chir Scand Suppl. 1966;369:1-72.
16. De Boeck H, De Smet P, Penders W, De Rydt D. Supracondylar elbow fractures with impaction of the medial condyle in children. J Pediatr Orthop. 1995;15(4):444-448.
17. Gelberman RH, Panagis JS, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part I: The extraosseous vascularity. J Hand Surg Am. 1983;8(4):367-375.
18. Hu J, Liao Q, Long W. Diagnosis and treatment of multiple-level noncontiguous spinal fractures. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2005;19(6):424-426.
19. Eleraky MA, Theodore N, Adams M, Rekate HL, Sonntag VK. Pediatric cervical spine injuries: report of 102 cases and review of the literature. J Neurosurg. 2000;92(1 suppl):12-17.
20. Ioannidis G, Papaioannou A, Hopman WM, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ. 2009;181(5):265-271.
21. Husby J, Sorensen KH. Fracture of the odontoid process of the axis. Acta Orthop Scand. 1974;45(2):182-192.
22. Schoenfeld AJ, Bono CM, Reichmann WM, et al. Type II odontoid fractures of the cervical spine: do treatment type and medical comorbidities affect mortality in elderly patients? Spine. 2011;36(11):879-885.
23. Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc. 2000;48(3):241-249.
24. Fassett DR, Dailey AT, Vaccaro AR. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21(4):252-258.
25. Ippolito E, Caterini R, Scola E. Supracondylar fractures of the humerus in children. Analysis at maturity of fifty-three patients treated conservatively. J Bone Joint Surg Am. 1986;68(3):333-344.
26. Spence KF Jr, Decker S, Sell KW. Bursting atlantal fracture associated with rupture of the transverse ligament. J Bone Joint Surg Am. 1970;52(3):543-549.
27. Irwin ZN, Arthur M, Mullins RJ, Hart RA. Variations in injury patterns, treatment, and outcome for spinal fracture and paralysis in adult versus geriatric patients. Spine. 2004;29(7):796-802.
28. Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int. 1998;8(3):291-297.
29. Iyengar SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and pinning of type III displaced supracondylar fractures of the humerus in children: a comparative study. J Orthop Trauma. 1999;13(1):51-55.
30. Jackson AP, Haak MH, Khan N, Meyer PR. Cervical spine injuries in the elderly: acute postoperative mortality. Spine. 2005;30(13):1524-1527.
31. Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Race and sex differences in mortality following fracture of the hip. Am J Public Health. 1992;82(8):1147-1150.
32. Fisher ES, Baron JA, Malenka DJ, et al. Hip fracture incidence and mortality in New England. Epidemiology. 1991;2(2):116-122.
33. Chapman J, Smith JS, Kopjar B, et al. The AOSpine North America Geriatric Odontoid Fracture Mortality Study: a retrospective review of mortality outcomes for operative versus nonoperative treatment of 322 patients with long-term follow-up. Spine. 2013;38:1098-1104.
34. Denault A, Bains I, Moghadam K, Hu RW, Swamy G. Evaluation of mortality following an odontoid fracture in the octogenarian population. J Bone Joint Surg Br. 2011;93(Supp IV):585.
35. Molinari WJ III, Molinari RW, Khera OA, Gruhn WL. Functional outcomes, morbidity, mortality, and fracture healing in 58 consecutive patients with geriatric odontoid fracture treated with cervical collar or posterior fusion. Global Spine J. 2013;3(1):21-32.
36. Hanigan WC, Powell FC, Elwood PW, Henderson JP. Odontoid fractures in elderly patients. J Neurosurg. 1993;78(1):32-35.
37. Korres DS, Boscainos PJ, Papagelopoulos PJ, Psycharis I, Goudelis G, Nikolopoulos K. Multiple level noncontiguous fractures of the spine. Clin Orthop. 2003;411:95-102.
38. Leet AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3 supracondylar humerus fractures in children. J Pediatr Orthop. 2002;22(2):203-207.
39. Leone A, Cerase A, Colosimo C, Lauro L, Puca A, Marano P. Occipital condylar fractures: a review. Radiology. 2000;216(3):635-644.
40. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799-1809.
41. Müller EJ, Wick M, Russe O, Muhr G. Management of odontoid fractures in the elderly. Eur Spine J. 1999;8(5):360-365.
42. Pepin JW, Bourne RB, Hawkins RJ. Odontoid fractures, with special reference to the elderly patient. Clin Orthop. 1985;193:178-183.
43. Ryan MD, Henderson JJ. The epidemiology of fractures and fracture-dislocations of the cervical spine. Injury. 1992;23(1):38-40.
44. Butler JS, Dolan RT, Burbridge M, et al. The long-term functional outcome of type II odontoid fractures managed non-operatively. Eur Spine J. 2010;19(10):1635-1642.
45. Levine AM, Edwards CC. The management of traumatic spondylolisthesis of the axis. J Bone Joint Surg Am. 1985;67(2):217-226.
46. Lowery DW, Wald MM, Browne BJ, Tigges S, Hoffman JR, Mover WR; NEXUS Group. Epidemiology of cervical spine injury victims. Ann Emerg Med. 2001;38(1):12-16.
47. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-16. http://www.prb.org/pdf11/aging-in-america.pdf. Published February 2011. Accessed April 22, 2015.
48. Holly LT, Kelly DF, Counelis GJ, Blinman T, McArthur DL, Cryer HG. Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors, and injury characteristics. J Neurosurg. 2002;96(3 suppl):285-291.
49. Holmes JF, Akkinepalli R. Computed tomography versus plain radiography to screen for cervical spine injury: a meta-analysis. J Trauma. 2005;58(5):902-905.
50. Hove LM. Epidemiology of scaphoid fractures in Bergen, Norway. Scand J Plast Reconstr Surg Hand Surg. 1999;33(4):423-426.
51. Lu-Yao G, Baron Ja, Barrett Ja, Fisher Es. Treatment and survival among elderly Americans with hip fractures: a population-based study. Am J Public Health. 1994;84(8):1287-1291.
52. Lu-Yao GL, Keller RB, Littenberg B, Wennberg JE. Outcomes after displaced fractures of the femoral neck. A meta-analysis of one hundred and six published reports. J Bone Joint Surg Am. 1994;76(1):15-25.
53. Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159(11):1215-1220.
54. Levine AM, Edwards CC. Fractures of the atlas. J Bone Joint Surg Am. 1991;73(5):680-691.
55. Maak TG, Grauer JN. The contemporary treatment of odontoid injuries. Spine. 2006;31(11 Suppl):S53-S60; discussion S61.
56. Magaziner J, Fredman L, Hawkes W, et al. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol. 2003;157(11):1023-1031.
57. Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55(9):M498-M507.
58. Malham GM, Ackland HM, Jones R, Williamson OD, Varma DK. Occipital condyle fractures: incidence and clinical follow-up at a level 1 trauma centre. Emerg Radiol. 2009;16(4):291-297.
59. Probst C, Zelle B, Panzica M, et al. Clinical re-examination 10 or more years after polytrauma: is there a gender related difference? J Trauma. 2010;68(3):706-711.
60. Holbrook TL, Hoyt DB, Anderson JP. The importance of gender on outcome after major trauma: functional and psychologic outcomes in women versus men. J Trauma. 2001;50(2):270-273.
Is Life Expectancy Different for Patients Beginning Osteoporosis Treatment?
A new trial has found that life expectancy of newly diagnosed and treated osteoporosis patients is in excess of 15 years in women younger than 75, and in men younger than 60, according to a study published online ahead of print May 21 in Journal of Bone and Mineral Research.
“How best to treat patients with osteoporosis is a really simple issue when it comes to beginning treatment, but deciding how long to treat for is really very challenging,” said lead author Bo Abrahamsen, MD, PhD, Professor and Consultant Endocrinologist at Glostrup Hospital in Copenhagen.
Researchers conducted an observational study in Danish national registries tracking prescriptions for osteoporosis drugs, comorbid conditions, and deaths. Investigators included 58,637 patients and 225,084 age- and gender-matched control subjects. Information on deaths until the end of 2013 was retrieved, providing a follow-up period of 10 to 17 years.
In men younger than 80 and women younger than 60, the relative risk of dying declined from being strongly increased in the first year to a stable but elevated level in subsequent years. In women older than 65 through 70, there was only a small elevation in risk in the first year of treatment followed by lower than background mortality.
The residual life expectancy of a 50-year-old man beginning osteoporosis treatment was estimated to be 18.2 years; for a 75-year-old man it was 7.5 years. Estimates in women were 26.4 years and 13.5 years for the same age groups, respectively.
According to the researchers, their findings show an excess mortality in men and in women below age 70 who are treated for osteoporosis, compared with the background population. This excess risk, they said, is more pronounced in the first few years on treatment. The average life expectancy of osteoporosis patients is in excess of 15 years in women below the age of 75 and in men below the age of 60, highlighting the importance of developing tools for long-term management.
“The present study shows that most of the patients we treat have a long life expectancy. Therefore it is absolutely vital that we are not complacent but develop evidence-based strategies for the long-term management of osteoporosis,” stated Dr. Abrahamsen.
Suggested Reading
Abrahamsen B, Osmond C, Cooper C. Life expectancy in patients treated for osteoporosis: observational cohort study using national Danish prescription data. J Bone Miner Res. 2015 May 21. [Epub ahead of print]
A new trial has found that life expectancy of newly diagnosed and treated osteoporosis patients is in excess of 15 years in women younger than 75, and in men younger than 60, according to a study published online ahead of print May 21 in Journal of Bone and Mineral Research.
“How best to treat patients with osteoporosis is a really simple issue when it comes to beginning treatment, but deciding how long to treat for is really very challenging,” said lead author Bo Abrahamsen, MD, PhD, Professor and Consultant Endocrinologist at Glostrup Hospital in Copenhagen.
Researchers conducted an observational study in Danish national registries tracking prescriptions for osteoporosis drugs, comorbid conditions, and deaths. Investigators included 58,637 patients and 225,084 age- and gender-matched control subjects. Information on deaths until the end of 2013 was retrieved, providing a follow-up period of 10 to 17 years.
In men younger than 80 and women younger than 60, the relative risk of dying declined from being strongly increased in the first year to a stable but elevated level in subsequent years. In women older than 65 through 70, there was only a small elevation in risk in the first year of treatment followed by lower than background mortality.
The residual life expectancy of a 50-year-old man beginning osteoporosis treatment was estimated to be 18.2 years; for a 75-year-old man it was 7.5 years. Estimates in women were 26.4 years and 13.5 years for the same age groups, respectively.
According to the researchers, their findings show an excess mortality in men and in women below age 70 who are treated for osteoporosis, compared with the background population. This excess risk, they said, is more pronounced in the first few years on treatment. The average life expectancy of osteoporosis patients is in excess of 15 years in women below the age of 75 and in men below the age of 60, highlighting the importance of developing tools for long-term management.
“The present study shows that most of the patients we treat have a long life expectancy. Therefore it is absolutely vital that we are not complacent but develop evidence-based strategies for the long-term management of osteoporosis,” stated Dr. Abrahamsen.
A new trial has found that life expectancy of newly diagnosed and treated osteoporosis patients is in excess of 15 years in women younger than 75, and in men younger than 60, according to a study published online ahead of print May 21 in Journal of Bone and Mineral Research.
“How best to treat patients with osteoporosis is a really simple issue when it comes to beginning treatment, but deciding how long to treat for is really very challenging,” said lead author Bo Abrahamsen, MD, PhD, Professor and Consultant Endocrinologist at Glostrup Hospital in Copenhagen.
Researchers conducted an observational study in Danish national registries tracking prescriptions for osteoporosis drugs, comorbid conditions, and deaths. Investigators included 58,637 patients and 225,084 age- and gender-matched control subjects. Information on deaths until the end of 2013 was retrieved, providing a follow-up period of 10 to 17 years.
In men younger than 80 and women younger than 60, the relative risk of dying declined from being strongly increased in the first year to a stable but elevated level in subsequent years. In women older than 65 through 70, there was only a small elevation in risk in the first year of treatment followed by lower than background mortality.
The residual life expectancy of a 50-year-old man beginning osteoporosis treatment was estimated to be 18.2 years; for a 75-year-old man it was 7.5 years. Estimates in women were 26.4 years and 13.5 years for the same age groups, respectively.
According to the researchers, their findings show an excess mortality in men and in women below age 70 who are treated for osteoporosis, compared with the background population. This excess risk, they said, is more pronounced in the first few years on treatment. The average life expectancy of osteoporosis patients is in excess of 15 years in women below the age of 75 and in men below the age of 60, highlighting the importance of developing tools for long-term management.
“The present study shows that most of the patients we treat have a long life expectancy. Therefore it is absolutely vital that we are not complacent but develop evidence-based strategies for the long-term management of osteoporosis,” stated Dr. Abrahamsen.
Suggested Reading
Abrahamsen B, Osmond C, Cooper C. Life expectancy in patients treated for osteoporosis: observational cohort study using national Danish prescription data. J Bone Miner Res. 2015 May 21. [Epub ahead of print]
Suggested Reading
Abrahamsen B, Osmond C, Cooper C. Life expectancy in patients treated for osteoporosis: observational cohort study using national Danish prescription data. J Bone Miner Res. 2015 May 21. [Epub ahead of print]