User login
Cognitive Decline Minimal After Endocrine + CDK4/6 Inhibition in BC
“Patients who are diagnosed with advanced breast cancer and start their first-line treatment already show cognitive impairments due to their previous treatments. And luckily, our results show that during first-line treatment for advanced breast cancer with endocrine therapy, with or without a CDK4/6 inhibitor, further cognitive decline is minimal,” lead investigator Maryse Luijendijk, said during her presentation at the annual meeting of the American Society of Clinical Oncology (ASCO).
“It is well known that cancer patients can experience cognitive problems, such as memory loss, problems with concentration or with planning, during or following their treatment,” explained Ms. Luijendijk, a PhD candidate in the department of Psychosocial Research and Epidemiology at the Netherlands Cancer Institute, in Amsterdam. “Much is known about the effects of chemotherapy or irradiation to the brain, but evidence into endocrine therapy is scarce, which is surprising because cognitive effects are biologically plausible.
“We know that estrogen plays an important role in neuronal functioning and that certain types of endocrine therapies are able to cross the blood-brain barrier, where they may interact with estrogen receptors distributed widely throughout the brain … We know that CDK4/6 inhibitors may either negatively affect cognitive function by increased fatigue due to cytokine release or by interrupting the cell cycle of healthy cells, or positively, as they have been associated with reduced inflammation and remyelination.”
Initial results of the SONIA trial, reported at ASCO last year, examined overall and progression-free survival in patients with HR-positive, HER2-negative metastatic breast cancer and no prior treatment for advanced disease. Findings for those who were randomized to treatment with nonsteroidal aromatase inhibition either with or without the addition of CDK4/6 inhibitors showed no between-group differences, explained Ms. Luijendijk.
The new results, described as being from the SONIA-EfFECT (Evaluation of cognitive functioning in patients with metastatic breast cancer treated with endocrine or combined therapy) trial, were based on the authors investigating cognitive functioning in the same cohort used in the SONIA trial plus a control group.
In SONIA-EfFECT, patients who participated in SONIA were asked to identify a female relative or friend without cancer to serve as a cancer-free control. Members of the 130-patient control group were matched for age, education, and computer use.
Participants in the SONIA trial and control group were asked to complete the Amsterdam Cognition Scan, an online neuropsychological test battery at baseline and again after 9 months of treatment. Of those patients from SONIA, 130 had received first-line treatment with aromatase inhibitors with CDK4/6 inhibition (Arm A) and 130 had received aromatase inhibitors without CDK4/6 inhibition (Arm B).
Baseline assessments for SONIA-EfFECT were completed for 260 patients from SONIA and the full 130-person control group. Follow-up assessments were completed for 119 members of the control group and 199 patients from the original SONIA trial (108 from Arm A, and 91 from Arm B). Patients from SONIA who switched to second-line treatment within 9 months were not retested.
Patients in both SONIA arms performed significantly worse than the controls on the domains of verbal memory, working memory, processing speed, executive function, and motor function. In both patient arms and the controls, standardized regression-based change scores showed limited decline in cognitive function over the 9-month interval. Minimal differences in cognitive change were observed between the patients treated with and without CDK4/6 inhibitors, and between patients and the controls, according to the abstract for SONIA-EfFECT, published in the program for the annual meeting of ASCO.
“At baseline, patients show worse cognitive function across all domains compared to the controls. And as expected, there were no differences between the two treatment arms,” Ms. Luijendijk explained. After 9 months of treatment, the testing showed limited further decline among patients, “and even some improvement on some tests,” with minimal differences between treatment arms “implying that cognitive function does not need to be an aspect when deciding on treatment.”
Ms. Luijendijk reported no relevant disclosures.
“Patients who are diagnosed with advanced breast cancer and start their first-line treatment already show cognitive impairments due to their previous treatments. And luckily, our results show that during first-line treatment for advanced breast cancer with endocrine therapy, with or without a CDK4/6 inhibitor, further cognitive decline is minimal,” lead investigator Maryse Luijendijk, said during her presentation at the annual meeting of the American Society of Clinical Oncology (ASCO).
“It is well known that cancer patients can experience cognitive problems, such as memory loss, problems with concentration or with planning, during or following their treatment,” explained Ms. Luijendijk, a PhD candidate in the department of Psychosocial Research and Epidemiology at the Netherlands Cancer Institute, in Amsterdam. “Much is known about the effects of chemotherapy or irradiation to the brain, but evidence into endocrine therapy is scarce, which is surprising because cognitive effects are biologically plausible.
“We know that estrogen plays an important role in neuronal functioning and that certain types of endocrine therapies are able to cross the blood-brain barrier, where they may interact with estrogen receptors distributed widely throughout the brain … We know that CDK4/6 inhibitors may either negatively affect cognitive function by increased fatigue due to cytokine release or by interrupting the cell cycle of healthy cells, or positively, as they have been associated with reduced inflammation and remyelination.”
Initial results of the SONIA trial, reported at ASCO last year, examined overall and progression-free survival in patients with HR-positive, HER2-negative metastatic breast cancer and no prior treatment for advanced disease. Findings for those who were randomized to treatment with nonsteroidal aromatase inhibition either with or without the addition of CDK4/6 inhibitors showed no between-group differences, explained Ms. Luijendijk.
The new results, described as being from the SONIA-EfFECT (Evaluation of cognitive functioning in patients with metastatic breast cancer treated with endocrine or combined therapy) trial, were based on the authors investigating cognitive functioning in the same cohort used in the SONIA trial plus a control group.
In SONIA-EfFECT, patients who participated in SONIA were asked to identify a female relative or friend without cancer to serve as a cancer-free control. Members of the 130-patient control group were matched for age, education, and computer use.
Participants in the SONIA trial and control group were asked to complete the Amsterdam Cognition Scan, an online neuropsychological test battery at baseline and again after 9 months of treatment. Of those patients from SONIA, 130 had received first-line treatment with aromatase inhibitors with CDK4/6 inhibition (Arm A) and 130 had received aromatase inhibitors without CDK4/6 inhibition (Arm B).
Baseline assessments for SONIA-EfFECT were completed for 260 patients from SONIA and the full 130-person control group. Follow-up assessments were completed for 119 members of the control group and 199 patients from the original SONIA trial (108 from Arm A, and 91 from Arm B). Patients from SONIA who switched to second-line treatment within 9 months were not retested.
Patients in both SONIA arms performed significantly worse than the controls on the domains of verbal memory, working memory, processing speed, executive function, and motor function. In both patient arms and the controls, standardized regression-based change scores showed limited decline in cognitive function over the 9-month interval. Minimal differences in cognitive change were observed between the patients treated with and without CDK4/6 inhibitors, and between patients and the controls, according to the abstract for SONIA-EfFECT, published in the program for the annual meeting of ASCO.
“At baseline, patients show worse cognitive function across all domains compared to the controls. And as expected, there were no differences between the two treatment arms,” Ms. Luijendijk explained. After 9 months of treatment, the testing showed limited further decline among patients, “and even some improvement on some tests,” with minimal differences between treatment arms “implying that cognitive function does not need to be an aspect when deciding on treatment.”
Ms. Luijendijk reported no relevant disclosures.
“Patients who are diagnosed with advanced breast cancer and start their first-line treatment already show cognitive impairments due to their previous treatments. And luckily, our results show that during first-line treatment for advanced breast cancer with endocrine therapy, with or without a CDK4/6 inhibitor, further cognitive decline is minimal,” lead investigator Maryse Luijendijk, said during her presentation at the annual meeting of the American Society of Clinical Oncology (ASCO).
“It is well known that cancer patients can experience cognitive problems, such as memory loss, problems with concentration or with planning, during or following their treatment,” explained Ms. Luijendijk, a PhD candidate in the department of Psychosocial Research and Epidemiology at the Netherlands Cancer Institute, in Amsterdam. “Much is known about the effects of chemotherapy or irradiation to the brain, but evidence into endocrine therapy is scarce, which is surprising because cognitive effects are biologically plausible.
“We know that estrogen plays an important role in neuronal functioning and that certain types of endocrine therapies are able to cross the blood-brain barrier, where they may interact with estrogen receptors distributed widely throughout the brain … We know that CDK4/6 inhibitors may either negatively affect cognitive function by increased fatigue due to cytokine release or by interrupting the cell cycle of healthy cells, or positively, as they have been associated with reduced inflammation and remyelination.”
Initial results of the SONIA trial, reported at ASCO last year, examined overall and progression-free survival in patients with HR-positive, HER2-negative metastatic breast cancer and no prior treatment for advanced disease. Findings for those who were randomized to treatment with nonsteroidal aromatase inhibition either with or without the addition of CDK4/6 inhibitors showed no between-group differences, explained Ms. Luijendijk.
The new results, described as being from the SONIA-EfFECT (Evaluation of cognitive functioning in patients with metastatic breast cancer treated with endocrine or combined therapy) trial, were based on the authors investigating cognitive functioning in the same cohort used in the SONIA trial plus a control group.
In SONIA-EfFECT, patients who participated in SONIA were asked to identify a female relative or friend without cancer to serve as a cancer-free control. Members of the 130-patient control group were matched for age, education, and computer use.
Participants in the SONIA trial and control group were asked to complete the Amsterdam Cognition Scan, an online neuropsychological test battery at baseline and again after 9 months of treatment. Of those patients from SONIA, 130 had received first-line treatment with aromatase inhibitors with CDK4/6 inhibition (Arm A) and 130 had received aromatase inhibitors without CDK4/6 inhibition (Arm B).
Baseline assessments for SONIA-EfFECT were completed for 260 patients from SONIA and the full 130-person control group. Follow-up assessments were completed for 119 members of the control group and 199 patients from the original SONIA trial (108 from Arm A, and 91 from Arm B). Patients from SONIA who switched to second-line treatment within 9 months were not retested.
Patients in both SONIA arms performed significantly worse than the controls on the domains of verbal memory, working memory, processing speed, executive function, and motor function. In both patient arms and the controls, standardized regression-based change scores showed limited decline in cognitive function over the 9-month interval. Minimal differences in cognitive change were observed between the patients treated with and without CDK4/6 inhibitors, and between patients and the controls, according to the abstract for SONIA-EfFECT, published in the program for the annual meeting of ASCO.
“At baseline, patients show worse cognitive function across all domains compared to the controls. And as expected, there were no differences between the two treatment arms,” Ms. Luijendijk explained. After 9 months of treatment, the testing showed limited further decline among patients, “and even some improvement on some tests,” with minimal differences between treatment arms “implying that cognitive function does not need to be an aspect when deciding on treatment.”
Ms. Luijendijk reported no relevant disclosures.
FROM ASCO 2024
Does Extended Postop Follow-Up Improve Survival in Gastric Cancer?
TOPLINE:
METHODOLOGY:
- Currently, postgastrectomy cancer surveillance typically lasts 5 years, although some centers now monitor patients beyond this point.
- To investigate the potential benefit of extended surveillance, researchers used Korean National Health Insurance claims data to identify 40,468 patients with gastric cancer who were disease free 5 years after gastrectomy — 14,294 received extended regular follow-up visits and 26,174 did not.
- The extended regular follow-up group was defined as having endoscopy or abdominopelvic CT between 2 months and 2 years before diagnosis of late recurrence or gastric remnant cancer and having two or more examinations between 5.5 and 8.5 years after gastrectomy. Late recurrence was a recurrence diagnosed 5 years after gastrectomy.
- Researchers used Cox proportional hazards regression to evaluate the independent association between follow-up and overall and postrecurrence survival rates.
TAKEAWAY:
- Overall, 5 years postgastrectomy, the incidence of late recurrence or gastric remnant cancer was 7.8% — 4.0% between 5 and 10 years (1610 of 40,468 patients) and 9.4% after 10 years (1528 of 16,287 patients).
- Regular follow-up beyond 5 years was associated with a significant reduction in overall mortality — from 49.4% to 36.9% at 15 years (P < .001). Overall survival after late recurrence or gastric remnant cancer also improved significantly with extended regular follow-up, with the 5-year postrecurrence survival rate increasing from 32.7% to 71.1% (P < .001).
- The combination of endoscopy and abdominopelvic CT provided the highest 5-year postrecurrence survival rate (74.5%), compared with endoscopy alone (54.5%) or CT alone (47.1%).
- A time interval of more than 2 years between a previous endoscopy or abdominopelvic CT and diagnosis of late recurrence or gastric remnant cancer significantly decreased postrecurrence survival (hazard ratio [HR], 1.72 for endoscopy and HR, 1.48 for abdominopelvic CT).
IN PRACTICE:
“These findings suggest that extended regular follow-up after 5 years post gastrectomy should be implemented clinically and that current practice and value of follow-up protocols in postoperative care of patients with gastric cancer be reconsidered,” the authors concluded.
The authors of an accompanying commentary cautioned that, while the study “successfully establishes groundwork for extending surveillance of gastric cancer in high-risk populations, more work is needed to strategically identify those who would benefit most from extended surveillance.”
SOURCE:
The study, with first author Ju-Hee Lee, MD, PhD, Department of Surgery, Hanyang University College of Medicine, Seoul, South Korea, and accompanying commentary were published online on June 18 in JAMA Surgery.
LIMITATIONS:
Recurrent cancer and gastric remnant cancer could not be distinguished from each other because clinical records were not analyzed. The claims database lacked detailed clinical information on individual patients, including cancer stages, and a separate analysis of tumor markers could not be performed.
DISCLOSURES:
The study was funded by a grant from the Korean Gastric Cancer Association. The study authors and commentary authors reported no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Currently, postgastrectomy cancer surveillance typically lasts 5 years, although some centers now monitor patients beyond this point.
- To investigate the potential benefit of extended surveillance, researchers used Korean National Health Insurance claims data to identify 40,468 patients with gastric cancer who were disease free 5 years after gastrectomy — 14,294 received extended regular follow-up visits and 26,174 did not.
- The extended regular follow-up group was defined as having endoscopy or abdominopelvic CT between 2 months and 2 years before diagnosis of late recurrence or gastric remnant cancer and having two or more examinations between 5.5 and 8.5 years after gastrectomy. Late recurrence was a recurrence diagnosed 5 years after gastrectomy.
- Researchers used Cox proportional hazards regression to evaluate the independent association between follow-up and overall and postrecurrence survival rates.
TAKEAWAY:
- Overall, 5 years postgastrectomy, the incidence of late recurrence or gastric remnant cancer was 7.8% — 4.0% between 5 and 10 years (1610 of 40,468 patients) and 9.4% after 10 years (1528 of 16,287 patients).
- Regular follow-up beyond 5 years was associated with a significant reduction in overall mortality — from 49.4% to 36.9% at 15 years (P < .001). Overall survival after late recurrence or gastric remnant cancer also improved significantly with extended regular follow-up, with the 5-year postrecurrence survival rate increasing from 32.7% to 71.1% (P < .001).
- The combination of endoscopy and abdominopelvic CT provided the highest 5-year postrecurrence survival rate (74.5%), compared with endoscopy alone (54.5%) or CT alone (47.1%).
- A time interval of more than 2 years between a previous endoscopy or abdominopelvic CT and diagnosis of late recurrence or gastric remnant cancer significantly decreased postrecurrence survival (hazard ratio [HR], 1.72 for endoscopy and HR, 1.48 for abdominopelvic CT).
IN PRACTICE:
“These findings suggest that extended regular follow-up after 5 years post gastrectomy should be implemented clinically and that current practice and value of follow-up protocols in postoperative care of patients with gastric cancer be reconsidered,” the authors concluded.
The authors of an accompanying commentary cautioned that, while the study “successfully establishes groundwork for extending surveillance of gastric cancer in high-risk populations, more work is needed to strategically identify those who would benefit most from extended surveillance.”
SOURCE:
The study, with first author Ju-Hee Lee, MD, PhD, Department of Surgery, Hanyang University College of Medicine, Seoul, South Korea, and accompanying commentary were published online on June 18 in JAMA Surgery.
LIMITATIONS:
Recurrent cancer and gastric remnant cancer could not be distinguished from each other because clinical records were not analyzed. The claims database lacked detailed clinical information on individual patients, including cancer stages, and a separate analysis of tumor markers could not be performed.
DISCLOSURES:
The study was funded by a grant from the Korean Gastric Cancer Association. The study authors and commentary authors reported no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Currently, postgastrectomy cancer surveillance typically lasts 5 years, although some centers now monitor patients beyond this point.
- To investigate the potential benefit of extended surveillance, researchers used Korean National Health Insurance claims data to identify 40,468 patients with gastric cancer who were disease free 5 years after gastrectomy — 14,294 received extended regular follow-up visits and 26,174 did not.
- The extended regular follow-up group was defined as having endoscopy or abdominopelvic CT between 2 months and 2 years before diagnosis of late recurrence or gastric remnant cancer and having two or more examinations between 5.5 and 8.5 years after gastrectomy. Late recurrence was a recurrence diagnosed 5 years after gastrectomy.
- Researchers used Cox proportional hazards regression to evaluate the independent association between follow-up and overall and postrecurrence survival rates.
TAKEAWAY:
- Overall, 5 years postgastrectomy, the incidence of late recurrence or gastric remnant cancer was 7.8% — 4.0% between 5 and 10 years (1610 of 40,468 patients) and 9.4% after 10 years (1528 of 16,287 patients).
- Regular follow-up beyond 5 years was associated with a significant reduction in overall mortality — from 49.4% to 36.9% at 15 years (P < .001). Overall survival after late recurrence or gastric remnant cancer also improved significantly with extended regular follow-up, with the 5-year postrecurrence survival rate increasing from 32.7% to 71.1% (P < .001).
- The combination of endoscopy and abdominopelvic CT provided the highest 5-year postrecurrence survival rate (74.5%), compared with endoscopy alone (54.5%) or CT alone (47.1%).
- A time interval of more than 2 years between a previous endoscopy or abdominopelvic CT and diagnosis of late recurrence or gastric remnant cancer significantly decreased postrecurrence survival (hazard ratio [HR], 1.72 for endoscopy and HR, 1.48 for abdominopelvic CT).
IN PRACTICE:
“These findings suggest that extended regular follow-up after 5 years post gastrectomy should be implemented clinically and that current practice and value of follow-up protocols in postoperative care of patients with gastric cancer be reconsidered,” the authors concluded.
The authors of an accompanying commentary cautioned that, while the study “successfully establishes groundwork for extending surveillance of gastric cancer in high-risk populations, more work is needed to strategically identify those who would benefit most from extended surveillance.”
SOURCE:
The study, with first author Ju-Hee Lee, MD, PhD, Department of Surgery, Hanyang University College of Medicine, Seoul, South Korea, and accompanying commentary were published online on June 18 in JAMA Surgery.
LIMITATIONS:
Recurrent cancer and gastric remnant cancer could not be distinguished from each other because clinical records were not analyzed. The claims database lacked detailed clinical information on individual patients, including cancer stages, and a separate analysis of tumor markers could not be performed.
DISCLOSURES:
The study was funded by a grant from the Korean Gastric Cancer Association. The study authors and commentary authors reported no conflicts of interest.
A version of this article appeared on Medscape.com.
Factors Linked to Complete Response, Survival in Pancreatic Cancer
TOPLINE:
a multicenter cohort study found. Several factors, including treatment type and tumor features, influenced the outcomes.
METHODOLOGY:
- Preoperative chemo(radio)therapy is increasingly used in patients with localized pancreatic adenocarcinoma and may improve the chance of a pathologic complete response. Achieving a pathologic complete response is associated with improved overall survival.
- However, the evidence on pathologic complete response is based on large national databases or small single-center series. Multicenter studies with in-depth data about complete response are lacking.
- In the current analysis, researchers investigated the incidence and factors associated with pathologic complete response after preoperative chemo(radio)therapy among 1758 patients (mean age, 64 years; 50% men) with localized pancreatic adenocarcinoma who underwent resection after two or more cycles of chemotherapy (with or without radiotherapy).
- Patients were treated at 19 centers in eight countries. The median follow-up was 19 months. Pathologic complete response was defined as the absence of vital tumor cells in the patient’s sampled pancreas specimen after resection.
- Factors associated with overall survival and pathologic complete response were investigated with Cox proportional hazards and logistic regression models, respectively.
TAKEAWAY:
- Researchers found that the rate of pathologic complete response was 4.8% in patients who received chemo(radio)therapy before pancreatic cancer resection.
- Having a pathologic complete response was associated with a 54% lower risk for death (hazard ratio, 0.46). At 5 years, the overall survival rate was 63% in patients with a pathologic complete response vs 30% in patients without one.
- More patients who received preoperative modified FOLFIRINOX achieved a pathologic complete response (58.8% vs 44.7%). Other factors associated with pathologic complete response included tumors located in the pancreatic head (odds ratio [OR], 2.51), tumors > 40 mm at diagnosis (OR, 2.58), partial or complete radiologic response (OR, 13.0), and normal(ized) serum carbohydrate antigen 19-9 after preoperative therapy (OR, 3.76).
- Preoperative radiotherapy (OR, 2.03) and preoperative stereotactic body radiotherapy (OR, 8.91) were also associated with a pathologic complete response; however, preoperative radiotherapy did not improve overall survival, and preoperative stereotactic body radiotherapy was independently associated with worse overall survival. These findings suggest that a pathologic complete response might not always reflect an optimal disease response.
IN PRACTICE:
Although pathologic complete response does not reflect cure, it is associated with better overall survival, the authors wrote. Factors associated with a pathologic complete response may inform treatment decisions.
SOURCE:
The study, with first author Thomas F. Stoop, MD, University of Amsterdam, the Netherlands, was published online on June 18 in JAMA Network Open.
LIMITATIONS:
The study had several limitations. The sample size and the limited number of events precluded comparative subanalyses, as well as a more detailed stratification for preoperative chemotherapy regimens. Information about patients’ race and the presence of BRCA germline mutations, both of which seem to be relevant to the chance of achieving a major pathologic response, was not collected or available.
DISCLOSURES:
No specific funding was noted. Several coauthors have industry relationships outside of the submitted work.
A version of this article first appeared on Medscape.com.
TOPLINE:
a multicenter cohort study found. Several factors, including treatment type and tumor features, influenced the outcomes.
METHODOLOGY:
- Preoperative chemo(radio)therapy is increasingly used in patients with localized pancreatic adenocarcinoma and may improve the chance of a pathologic complete response. Achieving a pathologic complete response is associated with improved overall survival.
- However, the evidence on pathologic complete response is based on large national databases or small single-center series. Multicenter studies with in-depth data about complete response are lacking.
- In the current analysis, researchers investigated the incidence and factors associated with pathologic complete response after preoperative chemo(radio)therapy among 1758 patients (mean age, 64 years; 50% men) with localized pancreatic adenocarcinoma who underwent resection after two or more cycles of chemotherapy (with or without radiotherapy).
- Patients were treated at 19 centers in eight countries. The median follow-up was 19 months. Pathologic complete response was defined as the absence of vital tumor cells in the patient’s sampled pancreas specimen after resection.
- Factors associated with overall survival and pathologic complete response were investigated with Cox proportional hazards and logistic regression models, respectively.
TAKEAWAY:
- Researchers found that the rate of pathologic complete response was 4.8% in patients who received chemo(radio)therapy before pancreatic cancer resection.
- Having a pathologic complete response was associated with a 54% lower risk for death (hazard ratio, 0.46). At 5 years, the overall survival rate was 63% in patients with a pathologic complete response vs 30% in patients without one.
- More patients who received preoperative modified FOLFIRINOX achieved a pathologic complete response (58.8% vs 44.7%). Other factors associated with pathologic complete response included tumors located in the pancreatic head (odds ratio [OR], 2.51), tumors > 40 mm at diagnosis (OR, 2.58), partial or complete radiologic response (OR, 13.0), and normal(ized) serum carbohydrate antigen 19-9 after preoperative therapy (OR, 3.76).
- Preoperative radiotherapy (OR, 2.03) and preoperative stereotactic body radiotherapy (OR, 8.91) were also associated with a pathologic complete response; however, preoperative radiotherapy did not improve overall survival, and preoperative stereotactic body radiotherapy was independently associated with worse overall survival. These findings suggest that a pathologic complete response might not always reflect an optimal disease response.
IN PRACTICE:
Although pathologic complete response does not reflect cure, it is associated with better overall survival, the authors wrote. Factors associated with a pathologic complete response may inform treatment decisions.
SOURCE:
The study, with first author Thomas F. Stoop, MD, University of Amsterdam, the Netherlands, was published online on June 18 in JAMA Network Open.
LIMITATIONS:
The study had several limitations. The sample size and the limited number of events precluded comparative subanalyses, as well as a more detailed stratification for preoperative chemotherapy regimens. Information about patients’ race and the presence of BRCA germline mutations, both of which seem to be relevant to the chance of achieving a major pathologic response, was not collected or available.
DISCLOSURES:
No specific funding was noted. Several coauthors have industry relationships outside of the submitted work.
A version of this article first appeared on Medscape.com.
TOPLINE:
a multicenter cohort study found. Several factors, including treatment type and tumor features, influenced the outcomes.
METHODOLOGY:
- Preoperative chemo(radio)therapy is increasingly used in patients with localized pancreatic adenocarcinoma and may improve the chance of a pathologic complete response. Achieving a pathologic complete response is associated with improved overall survival.
- However, the evidence on pathologic complete response is based on large national databases or small single-center series. Multicenter studies with in-depth data about complete response are lacking.
- In the current analysis, researchers investigated the incidence and factors associated with pathologic complete response after preoperative chemo(radio)therapy among 1758 patients (mean age, 64 years; 50% men) with localized pancreatic adenocarcinoma who underwent resection after two or more cycles of chemotherapy (with or without radiotherapy).
- Patients were treated at 19 centers in eight countries. The median follow-up was 19 months. Pathologic complete response was defined as the absence of vital tumor cells in the patient’s sampled pancreas specimen after resection.
- Factors associated with overall survival and pathologic complete response were investigated with Cox proportional hazards and logistic regression models, respectively.
TAKEAWAY:
- Researchers found that the rate of pathologic complete response was 4.8% in patients who received chemo(radio)therapy before pancreatic cancer resection.
- Having a pathologic complete response was associated with a 54% lower risk for death (hazard ratio, 0.46). At 5 years, the overall survival rate was 63% in patients with a pathologic complete response vs 30% in patients without one.
- More patients who received preoperative modified FOLFIRINOX achieved a pathologic complete response (58.8% vs 44.7%). Other factors associated with pathologic complete response included tumors located in the pancreatic head (odds ratio [OR], 2.51), tumors > 40 mm at diagnosis (OR, 2.58), partial or complete radiologic response (OR, 13.0), and normal(ized) serum carbohydrate antigen 19-9 after preoperative therapy (OR, 3.76).
- Preoperative radiotherapy (OR, 2.03) and preoperative stereotactic body radiotherapy (OR, 8.91) were also associated with a pathologic complete response; however, preoperative radiotherapy did not improve overall survival, and preoperative stereotactic body radiotherapy was independently associated with worse overall survival. These findings suggest that a pathologic complete response might not always reflect an optimal disease response.
IN PRACTICE:
Although pathologic complete response does not reflect cure, it is associated with better overall survival, the authors wrote. Factors associated with a pathologic complete response may inform treatment decisions.
SOURCE:
The study, with first author Thomas F. Stoop, MD, University of Amsterdam, the Netherlands, was published online on June 18 in JAMA Network Open.
LIMITATIONS:
The study had several limitations. The sample size and the limited number of events precluded comparative subanalyses, as well as a more detailed stratification for preoperative chemotherapy regimens. Information about patients’ race and the presence of BRCA germline mutations, both of which seem to be relevant to the chance of achieving a major pathologic response, was not collected or available.
DISCLOSURES:
No specific funding was noted. Several coauthors have industry relationships outside of the submitted work.
A version of this article first appeared on Medscape.com.
Managing Cancer in Pregnancy: Improvements and Considerations
Introduction: Tremendous Progress on Cancer Extends to Cancer in Pregnancy
The biomedical research enterprise that took shape in the United States after World War II has had numerous positive effects, including significant progress made during the past 75-plus years in the diagnosis, prevention, and treatment of cancer.
President Franklin D. Roosevelt’s 1944 request of Dr. Vannevar Bush, director of the then Office of Scientific Research and Development, to organize a program that would advance and apply scientific knowledge for times of peace — just as it been advanced and applied in times of war — culminated in a historic report, Science – The Endless Frontier. Presented in 1945 to President Harry S. Truman, this report helped fuel decades of broad, bold, and coordinated government-sponsored biomedical research aimed at addressing disease and improving the health of the American people (National Science Foundation, 1945).
Discoveries made from research in basic and translational sciences deepened our knowledge of the cellular and molecular underpinnings of cancer, leading to advances in chemotherapy, radiotherapy, and other treatment approaches as well as continual refinements in their application. Similarly, our diagnostic armamentarium has significantly improved.
As a result, we have reduced both the incidence and mortality of cancer. Today, some cancers can be prevented. Others can be reversed or put in remission. Granted, progress has been variable, with some cancers such as ovarian cancer still having relatively low survival rates. Much more needs to be done. Overall, however, the positive effects of the U.S. biomedical research enterprise on cancer are evident. According to the National Cancer Institute’s most recent report on the status of cancer, death rates from cancer fell 1.9% per year on average in females from 2015 to 2019 (Cancer. 2022 Oct 22. doi: 10.1002/cncr.34479).
It is not only patients whose cancer occurs outside of pregnancy who have benefited. When treatment is appropriately selected and timing considerations are made, patients whose cancer is diagnosed during pregnancy — and their children — can have good outcomes.
To explain how the management of cancer in pregnancy has improved, we have invited Gautam G. Rao, MD, gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to write this installment of the Master Class in Obstetrics. As Dr. Rao explains, radiation is not as dangerous to the fetus as once thought, and the safety of many chemotherapeutic regimens in pregnancy has been documented. Obstetricians can and should counsel patients, he explains, about the likelihood of good maternal and fetal outcomes.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Managing Cancer in Pregnancy
Cancer can cause fear and distress for any patient, but when cancer is diagnosed during pregnancy, an expectant mother fears not only for her own health but for the health of her unborn child. Fortunately, ob.gyn.s and multidisciplinary teams have good reason to reassure patients about the likelihood of good outcomes.
Cancer treatment in pregnancy has improved with advancements in imaging and chemotherapy, and while maternal and fetal outcomes of prenatal cancer treatment are not well reported, evidence acquired in recent years from case series and retrospective studies shows that most imaging studies and procedural diagnostic tests – and many treatments – can be performed safely in pregnancy.
Decades ago, we avoided CT scans during pregnancy because of concerns about radiation exposure to the fetus, leaving some patients without an accurate staging of their cancer. Today, we have evidence that a CT scan is generally safe in pregnancy. Similarly, the safety of many chemotherapeutic regimens in pregnancy has been documented in recent decades,and the use of chemotherapy during pregnancy has increased progressively. Radiation is also commonly utilized in the management of cancers that may occur during pregnancy, such as breast cancer.1
Considerations of timing are often central to decision-making; chemotherapy and radiotherapy are generally avoided in the first trimester to prevent structural fetal anomalies, for instance, and delaying cancer treatment is often warranted when the patient is a few weeks away from delivery. On occasion, iatrogenic preterm birth is considered when the risks to the mother of delaying a necessary cancer treatment outweigh the risks to the fetus of prematurity.1
Pregnancy termination is rarely indicated, however, and information gathered over the past 2 decades suggests that fetal and placental metastases are rare.1 There is broad agreement that prenatal treatment of cancer in pregnancy should adhere as much as possible to protocols and guidelines for nonpregnant patients and that treatment delays driven by fear of fetal anomalies and miscarriage are unnecessary.
Cancer Incidence, Use of Diagnostic Imaging
Data on the incidence of cancer in pregnancy comes from population-based cancer registries, and unfortunately, these data are not standardized and are often incomplete. Many studies include cancer diagnosed up to 1 year after pregnancy, and some include preinvasive disease. Estimates therefore vary considerably (see Table 1 for a sampling of estimates incidences.)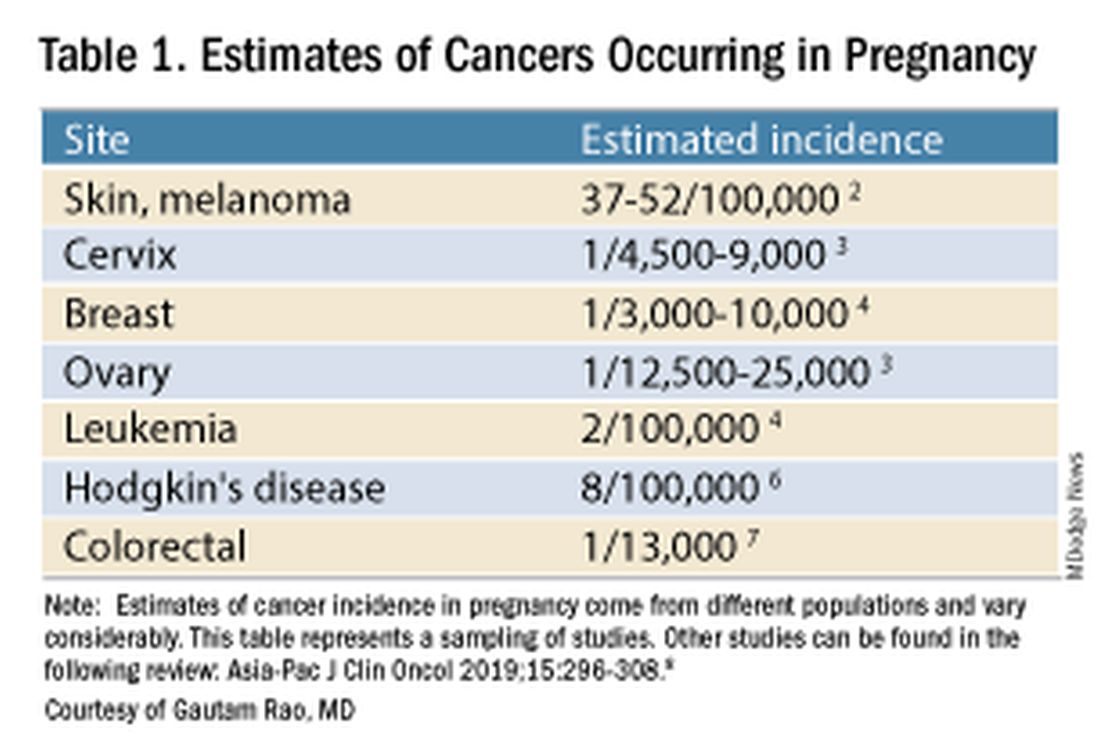
It has been reported, and often cited in the literature, that invasive malignancy complicates one in 1,000 pregnancies and that the incidence of cancer in pregnancy (invasive and noninvasive malignancies) has been rising over time.8 Increasing maternal age is believed to be playing a role in this rise; as women delay childbearing, they enter the age range in which some cancers become more common. Additionally, improvements in screening and diagnostics have led to earlier cancer detection. The incidence of ovarian neoplasms found during pregnancy has increased, for instance, with the routine use of diagnostic ultrasound in pregnancy.1
Among the studies showing an increased incidence of pregnancy-associated cancer is a population-based study in Australia, which found that from 1994 to 2007 the crude incidence of pregnancy-associated cancer increased from 112.3 to 191.5 per 100,000 pregnancies (P < .001).9 A cohort study in the United States documented an increase in incidence from 75.0 per 100,000 pregnancies in 2002 to 138.5 per 100,000 pregnancies in 2012.10
Overall, the literature shows us that the skin, cervix, and breast are also common sites for malignancy during pregnancy.1 According to a 2022 review, breast cancer during pregnancy is less often hormone receptor–positive and more frequently triple negative compared with age-matched controls.11 The frequencies of other pregnancy-associated cancers appear overall to be similar to that of cancer occurring in all women across their reproductive years.1
Too often, diagnosis is delayed because cancer symptoms can be masked by or can mimic normal physiological changes in pregnancy. For instance, breast cancer can be difficult to diagnose during pregnancy and lactation due to anatomic changes in the breast parenchyma. Several studies published in the 1990s showed that breast cancer presents at a more advanced stage in pregnant patients than in nonpregnant patients because of this delay.1 Skin changes suggestive of melanoma can be attributed to hyperpigmentation of pregnancy, for instance. Several observational studies have suggested that thicker melanomas found in pregnancy may be because of delayed diagnosis.8
It is important that we thoroughly investigate signs and symptoms suggestive of a malignancy and not automatically attribute these symptoms to the pregnancy itself. Cervical biopsy of a mass or lesion suspicious for cervical cancer can be done safely during pregnancy and should not be delayed or deferred.
Fetal radiation exposure from radiologic examinations has long been a concern, but we know today that while the imaging modality should be chosen to minimize fetal radiation exposure, CT scans and even PET scans should be performed if these exams are deemed best for evaluation. Embryonic exposure to a dose of less than 50 mGy is rarely if at all associated with fetal malformations or miscarriage and a radiation dose of 100 mGy may be considered a floor for consideration of therapeutic termination of pregnancy.1,8
CT exams are associated with a fetal dose far less than 50 mGy (see Table 2 for radiation doses).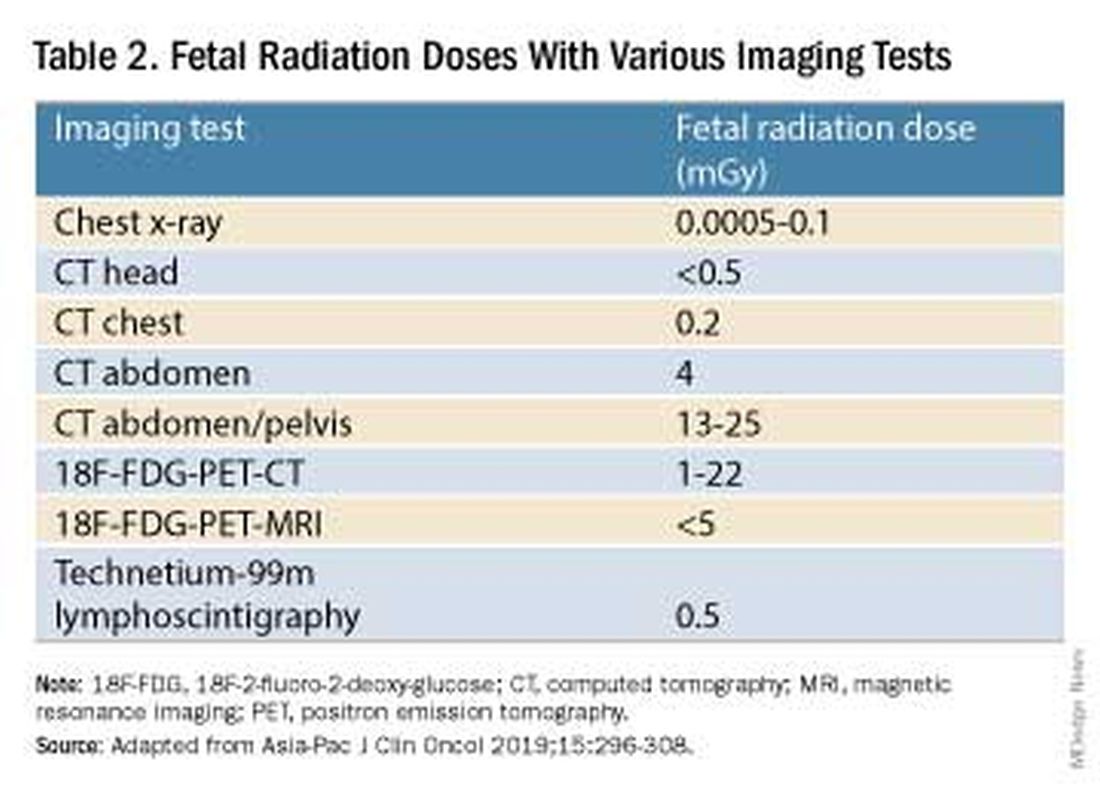
Magnetic resonance imaging with a magnet strength of 3 Tesla or less in any trimester is not associated with an increased risk of harm to the fetus or in early childhood, but the contrast agent gadolinium should be avoided in pregnancy as it has been associated with an increased risk of stillbirth, neonatal death, and childhood inflammatory, rheumatologic, and infiltrative skin lesions.1,8,12
Chemotherapy, Surgery, and Radiation in Pregnancy
The management of cancer during pregnancy requires a multidisciplinary team including medical, gynecologic, or radiation oncologists, and maternal-fetal medicine specialists (Figure 1). Prematurity and low birth weight are frequent complications for fetuses exposed to chemotherapy, although there is some uncertainty as to whether the treatment is causative. However, congenital anomalies no longer are a major concern, provided that drugs are appropriately selected and that fetal exposure occurs during the second or third trimester.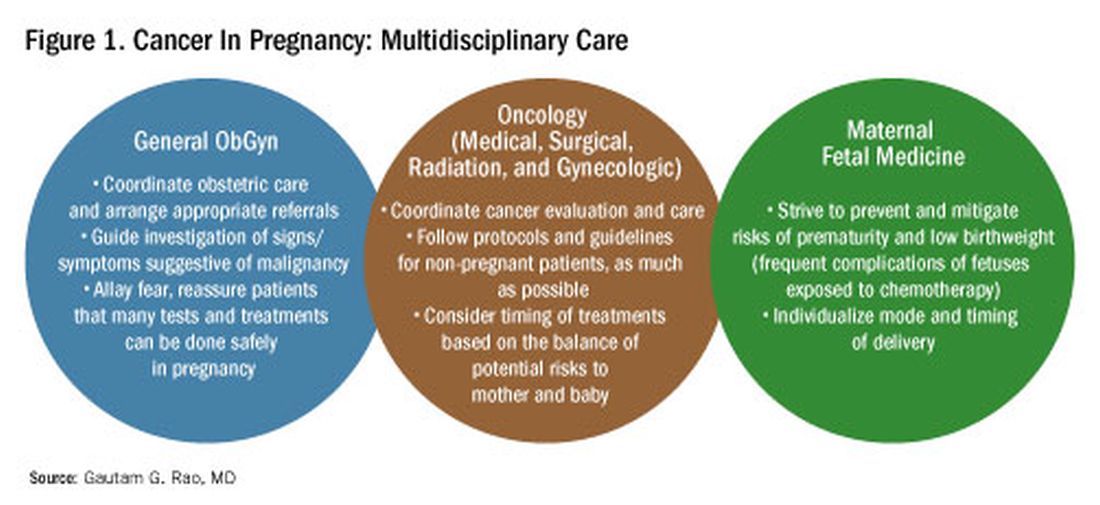
For instance, alkylating agents including cisplatin (an important drug in the management of gynecologic malignancies) have been associated with congenital anomalies in the first trimester but not in the second and third trimesters, and a variety of antimetabolites — excluding methotrexate and aminopterin — similarly have been shown to be relatively safe when used after the first trimester.1
Small studies have shown no long-term effects of chemotherapy exposure on postnatal growth and long-term neurologic/neurocognitive function,1 but this is an area that needs more research.
Also in need of investigation is the safety of newer agents in pregnancy. Data are limited on the use of new targeted treatments, monoclonal antibodies, and immunotherapies in pregnancy and their effects on the fetus, with current knowledge coming mainly from single case reports.13
Until more is learned — a challenge given that pregnant women are generally excluded from clinical trials — management teams are generally postponing use of these therapies until after delivery. Considering the pace of new developments revolutionizing cancer treatment, this topic will likely get more complex and confusing before we begin acquiring sufficient knowledge.
The timing of surgery for malignancy in pregnancy is similarly based on the balance of maternal and fetal risks, including the risk of maternal disease progression, the risk of preterm delivery, and the prevention of fetal metastases. In general, the safest time is the second trimester.
Maternal surgery in the third trimester may be associated with a risk of premature labor and altered uteroplacental perfusion. A 2005 systematic review of 12,452 women who underwent nonobstetric surgery during pregnancy provides some reassurance, however; compared with the general obstetric population, there was no increase in the rate of miscarriage or major birth defects.14
Radiotherapy used to be contraindicated in pregnancy but many experts today believe it can be safely utilized provided the uterus is out of field and is protected from scattered radiation. The head, neck, and breast, for instance, can be treated with newer radiotherapies, including stereotactic ablative radiation therapy.8 Patients with advanced cervical cancer often receive chemotherapy during pregnancy to slow metastatic growth followed by definitive treatment with postpartum radiation or surgery.
More research is needed, but available data on maternal outcomes are encouraging. For instance, there appear to be no significant differences in short- and long-term complications or survival between women who are pregnant and nonpregnant when treated for invasive cervical cancer.8 Similarly, while earlier studies of breast cancer diagnosed during pregnancy suggested a poor prognosis, data now show similar prognoses for pregnant and nonpregnant patients when controlled for stage.1
Dr. Rao is a gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported no relevant disclosures.
References
1. Rao GG. Chapter 42. Clinical Obstetrics: The Fetus & Mother, 4th ed. Reece EA et al. (eds): 2021.
2. Bannister-Tyrrell M et al. Aust N Z J Obstet Gynaecol. 2014;55:116-122.
3. Oehler MK et al. Aust N Z J Obstet Gynaecol. 2003;43(6):414-420.
4. Ruiz R et al. Breast. 2017;35:136-141. doi: 10.1016/j.breast.2017.07.008.
5. Nolan S et al. Am J Obstet Gynecol. 2019;220(1):S480. doi: 10.1016/j.ajog.2018.11.752.
6. El-Messidi A et al. J Perinat Med. 2015;43(6):683-688. doi: 10.1515/jpm-2014-0133.
7. Pellino G et al. Eur J Gastroenterol Hepatol. 2017;29(7):743-753. doi: 10.1097/MEG.0000000000000863.
8. Eastwood-Wilshere N et al. Asia-Pac J Clin Oncol. 2019;15:296-308.
9. Lee YY et al. BJOG. 2012;119(13):1572-1582.
10. Cottreau CM et al. J Womens Health (Larchmt). 2019 Feb;28(2):250-257.
11. Boere I et al. Best Pract Res Clin Obstet Gynaecol. 2022;82:46-59.
12. Ray JG et al. JAMA 2016;316(9):952-961.
13. Schwab R et al. Cancers. (Basel) 2021;13(12):3048.
14. Cohen-Kerem et al. Am J Surg. 2005;190(3):467-473.
Introduction: Tremendous Progress on Cancer Extends to Cancer in Pregnancy
The biomedical research enterprise that took shape in the United States after World War II has had numerous positive effects, including significant progress made during the past 75-plus years in the diagnosis, prevention, and treatment of cancer.
President Franklin D. Roosevelt’s 1944 request of Dr. Vannevar Bush, director of the then Office of Scientific Research and Development, to organize a program that would advance and apply scientific knowledge for times of peace — just as it been advanced and applied in times of war — culminated in a historic report, Science – The Endless Frontier. Presented in 1945 to President Harry S. Truman, this report helped fuel decades of broad, bold, and coordinated government-sponsored biomedical research aimed at addressing disease and improving the health of the American people (National Science Foundation, 1945).
Discoveries made from research in basic and translational sciences deepened our knowledge of the cellular and molecular underpinnings of cancer, leading to advances in chemotherapy, radiotherapy, and other treatment approaches as well as continual refinements in their application. Similarly, our diagnostic armamentarium has significantly improved.
As a result, we have reduced both the incidence and mortality of cancer. Today, some cancers can be prevented. Others can be reversed or put in remission. Granted, progress has been variable, with some cancers such as ovarian cancer still having relatively low survival rates. Much more needs to be done. Overall, however, the positive effects of the U.S. biomedical research enterprise on cancer are evident. According to the National Cancer Institute’s most recent report on the status of cancer, death rates from cancer fell 1.9% per year on average in females from 2015 to 2019 (Cancer. 2022 Oct 22. doi: 10.1002/cncr.34479).
It is not only patients whose cancer occurs outside of pregnancy who have benefited. When treatment is appropriately selected and timing considerations are made, patients whose cancer is diagnosed during pregnancy — and their children — can have good outcomes.
To explain how the management of cancer in pregnancy has improved, we have invited Gautam G. Rao, MD, gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to write this installment of the Master Class in Obstetrics. As Dr. Rao explains, radiation is not as dangerous to the fetus as once thought, and the safety of many chemotherapeutic regimens in pregnancy has been documented. Obstetricians can and should counsel patients, he explains, about the likelihood of good maternal and fetal outcomes.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Managing Cancer in Pregnancy
Cancer can cause fear and distress for any patient, but when cancer is diagnosed during pregnancy, an expectant mother fears not only for her own health but for the health of her unborn child. Fortunately, ob.gyn.s and multidisciplinary teams have good reason to reassure patients about the likelihood of good outcomes.
Cancer treatment in pregnancy has improved with advancements in imaging and chemotherapy, and while maternal and fetal outcomes of prenatal cancer treatment are not well reported, evidence acquired in recent years from case series and retrospective studies shows that most imaging studies and procedural diagnostic tests – and many treatments – can be performed safely in pregnancy.
Decades ago, we avoided CT scans during pregnancy because of concerns about radiation exposure to the fetus, leaving some patients without an accurate staging of their cancer. Today, we have evidence that a CT scan is generally safe in pregnancy. Similarly, the safety of many chemotherapeutic regimens in pregnancy has been documented in recent decades,and the use of chemotherapy during pregnancy has increased progressively. Radiation is also commonly utilized in the management of cancers that may occur during pregnancy, such as breast cancer.1
Considerations of timing are often central to decision-making; chemotherapy and radiotherapy are generally avoided in the first trimester to prevent structural fetal anomalies, for instance, and delaying cancer treatment is often warranted when the patient is a few weeks away from delivery. On occasion, iatrogenic preterm birth is considered when the risks to the mother of delaying a necessary cancer treatment outweigh the risks to the fetus of prematurity.1
Pregnancy termination is rarely indicated, however, and information gathered over the past 2 decades suggests that fetal and placental metastases are rare.1 There is broad agreement that prenatal treatment of cancer in pregnancy should adhere as much as possible to protocols and guidelines for nonpregnant patients and that treatment delays driven by fear of fetal anomalies and miscarriage are unnecessary.
Cancer Incidence, Use of Diagnostic Imaging
Data on the incidence of cancer in pregnancy comes from population-based cancer registries, and unfortunately, these data are not standardized and are often incomplete. Many studies include cancer diagnosed up to 1 year after pregnancy, and some include preinvasive disease. Estimates therefore vary considerably (see Table 1 for a sampling of estimates incidences.)
It has been reported, and often cited in the literature, that invasive malignancy complicates one in 1,000 pregnancies and that the incidence of cancer in pregnancy (invasive and noninvasive malignancies) has been rising over time.8 Increasing maternal age is believed to be playing a role in this rise; as women delay childbearing, they enter the age range in which some cancers become more common. Additionally, improvements in screening and diagnostics have led to earlier cancer detection. The incidence of ovarian neoplasms found during pregnancy has increased, for instance, with the routine use of diagnostic ultrasound in pregnancy.1
Among the studies showing an increased incidence of pregnancy-associated cancer is a population-based study in Australia, which found that from 1994 to 2007 the crude incidence of pregnancy-associated cancer increased from 112.3 to 191.5 per 100,000 pregnancies (P < .001).9 A cohort study in the United States documented an increase in incidence from 75.0 per 100,000 pregnancies in 2002 to 138.5 per 100,000 pregnancies in 2012.10
Overall, the literature shows us that the skin, cervix, and breast are also common sites for malignancy during pregnancy.1 According to a 2022 review, breast cancer during pregnancy is less often hormone receptor–positive and more frequently triple negative compared with age-matched controls.11 The frequencies of other pregnancy-associated cancers appear overall to be similar to that of cancer occurring in all women across their reproductive years.1
Too often, diagnosis is delayed because cancer symptoms can be masked by or can mimic normal physiological changes in pregnancy. For instance, breast cancer can be difficult to diagnose during pregnancy and lactation due to anatomic changes in the breast parenchyma. Several studies published in the 1990s showed that breast cancer presents at a more advanced stage in pregnant patients than in nonpregnant patients because of this delay.1 Skin changes suggestive of melanoma can be attributed to hyperpigmentation of pregnancy, for instance. Several observational studies have suggested that thicker melanomas found in pregnancy may be because of delayed diagnosis.8
It is important that we thoroughly investigate signs and symptoms suggestive of a malignancy and not automatically attribute these symptoms to the pregnancy itself. Cervical biopsy of a mass or lesion suspicious for cervical cancer can be done safely during pregnancy and should not be delayed or deferred.
Fetal radiation exposure from radiologic examinations has long been a concern, but we know today that while the imaging modality should be chosen to minimize fetal radiation exposure, CT scans and even PET scans should be performed if these exams are deemed best for evaluation. Embryonic exposure to a dose of less than 50 mGy is rarely if at all associated with fetal malformations or miscarriage and a radiation dose of 100 mGy may be considered a floor for consideration of therapeutic termination of pregnancy.1,8
CT exams are associated with a fetal dose far less than 50 mGy (see Table 2 for radiation doses).
Magnetic resonance imaging with a magnet strength of 3 Tesla or less in any trimester is not associated with an increased risk of harm to the fetus or in early childhood, but the contrast agent gadolinium should be avoided in pregnancy as it has been associated with an increased risk of stillbirth, neonatal death, and childhood inflammatory, rheumatologic, and infiltrative skin lesions.1,8,12
Chemotherapy, Surgery, and Radiation in Pregnancy
The management of cancer during pregnancy requires a multidisciplinary team including medical, gynecologic, or radiation oncologists, and maternal-fetal medicine specialists (Figure 1). Prematurity and low birth weight are frequent complications for fetuses exposed to chemotherapy, although there is some uncertainty as to whether the treatment is causative. However, congenital anomalies no longer are a major concern, provided that drugs are appropriately selected and that fetal exposure occurs during the second or third trimester.
For instance, alkylating agents including cisplatin (an important drug in the management of gynecologic malignancies) have been associated with congenital anomalies in the first trimester but not in the second and third trimesters, and a variety of antimetabolites — excluding methotrexate and aminopterin — similarly have been shown to be relatively safe when used after the first trimester.1
Small studies have shown no long-term effects of chemotherapy exposure on postnatal growth and long-term neurologic/neurocognitive function,1 but this is an area that needs more research.
Also in need of investigation is the safety of newer agents in pregnancy. Data are limited on the use of new targeted treatments, monoclonal antibodies, and immunotherapies in pregnancy and their effects on the fetus, with current knowledge coming mainly from single case reports.13
Until more is learned — a challenge given that pregnant women are generally excluded from clinical trials — management teams are generally postponing use of these therapies until after delivery. Considering the pace of new developments revolutionizing cancer treatment, this topic will likely get more complex and confusing before we begin acquiring sufficient knowledge.
The timing of surgery for malignancy in pregnancy is similarly based on the balance of maternal and fetal risks, including the risk of maternal disease progression, the risk of preterm delivery, and the prevention of fetal metastases. In general, the safest time is the second trimester.
Maternal surgery in the third trimester may be associated with a risk of premature labor and altered uteroplacental perfusion. A 2005 systematic review of 12,452 women who underwent nonobstetric surgery during pregnancy provides some reassurance, however; compared with the general obstetric population, there was no increase in the rate of miscarriage or major birth defects.14
Radiotherapy used to be contraindicated in pregnancy but many experts today believe it can be safely utilized provided the uterus is out of field and is protected from scattered radiation. The head, neck, and breast, for instance, can be treated with newer radiotherapies, including stereotactic ablative radiation therapy.8 Patients with advanced cervical cancer often receive chemotherapy during pregnancy to slow metastatic growth followed by definitive treatment with postpartum radiation or surgery.
More research is needed, but available data on maternal outcomes are encouraging. For instance, there appear to be no significant differences in short- and long-term complications or survival between women who are pregnant and nonpregnant when treated for invasive cervical cancer.8 Similarly, while earlier studies of breast cancer diagnosed during pregnancy suggested a poor prognosis, data now show similar prognoses for pregnant and nonpregnant patients when controlled for stage.1
Dr. Rao is a gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported no relevant disclosures.
References
1. Rao GG. Chapter 42. Clinical Obstetrics: The Fetus & Mother, 4th ed. Reece EA et al. (eds): 2021.
2. Bannister-Tyrrell M et al. Aust N Z J Obstet Gynaecol. 2014;55:116-122.
3. Oehler MK et al. Aust N Z J Obstet Gynaecol. 2003;43(6):414-420.
4. Ruiz R et al. Breast. 2017;35:136-141. doi: 10.1016/j.breast.2017.07.008.
5. Nolan S et al. Am J Obstet Gynecol. 2019;220(1):S480. doi: 10.1016/j.ajog.2018.11.752.
6. El-Messidi A et al. J Perinat Med. 2015;43(6):683-688. doi: 10.1515/jpm-2014-0133.
7. Pellino G et al. Eur J Gastroenterol Hepatol. 2017;29(7):743-753. doi: 10.1097/MEG.0000000000000863.
8. Eastwood-Wilshere N et al. Asia-Pac J Clin Oncol. 2019;15:296-308.
9. Lee YY et al. BJOG. 2012;119(13):1572-1582.
10. Cottreau CM et al. J Womens Health (Larchmt). 2019 Feb;28(2):250-257.
11. Boere I et al. Best Pract Res Clin Obstet Gynaecol. 2022;82:46-59.
12. Ray JG et al. JAMA 2016;316(9):952-961.
13. Schwab R et al. Cancers. (Basel) 2021;13(12):3048.
14. Cohen-Kerem et al. Am J Surg. 2005;190(3):467-473.
Introduction: Tremendous Progress on Cancer Extends to Cancer in Pregnancy
The biomedical research enterprise that took shape in the United States after World War II has had numerous positive effects, including significant progress made during the past 75-plus years in the diagnosis, prevention, and treatment of cancer.
President Franklin D. Roosevelt’s 1944 request of Dr. Vannevar Bush, director of the then Office of Scientific Research and Development, to organize a program that would advance and apply scientific knowledge for times of peace — just as it been advanced and applied in times of war — culminated in a historic report, Science – The Endless Frontier. Presented in 1945 to President Harry S. Truman, this report helped fuel decades of broad, bold, and coordinated government-sponsored biomedical research aimed at addressing disease and improving the health of the American people (National Science Foundation, 1945).
Discoveries made from research in basic and translational sciences deepened our knowledge of the cellular and molecular underpinnings of cancer, leading to advances in chemotherapy, radiotherapy, and other treatment approaches as well as continual refinements in their application. Similarly, our diagnostic armamentarium has significantly improved.
As a result, we have reduced both the incidence and mortality of cancer. Today, some cancers can be prevented. Others can be reversed or put in remission. Granted, progress has been variable, with some cancers such as ovarian cancer still having relatively low survival rates. Much more needs to be done. Overall, however, the positive effects of the U.S. biomedical research enterprise on cancer are evident. According to the National Cancer Institute’s most recent report on the status of cancer, death rates from cancer fell 1.9% per year on average in females from 2015 to 2019 (Cancer. 2022 Oct 22. doi: 10.1002/cncr.34479).
It is not only patients whose cancer occurs outside of pregnancy who have benefited. When treatment is appropriately selected and timing considerations are made, patients whose cancer is diagnosed during pregnancy — and their children — can have good outcomes.
To explain how the management of cancer in pregnancy has improved, we have invited Gautam G. Rao, MD, gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to write this installment of the Master Class in Obstetrics. As Dr. Rao explains, radiation is not as dangerous to the fetus as once thought, and the safety of many chemotherapeutic regimens in pregnancy has been documented. Obstetricians can and should counsel patients, he explains, about the likelihood of good maternal and fetal outcomes.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Managing Cancer in Pregnancy
Cancer can cause fear and distress for any patient, but when cancer is diagnosed during pregnancy, an expectant mother fears not only for her own health but for the health of her unborn child. Fortunately, ob.gyn.s and multidisciplinary teams have good reason to reassure patients about the likelihood of good outcomes.
Cancer treatment in pregnancy has improved with advancements in imaging and chemotherapy, and while maternal and fetal outcomes of prenatal cancer treatment are not well reported, evidence acquired in recent years from case series and retrospective studies shows that most imaging studies and procedural diagnostic tests – and many treatments – can be performed safely in pregnancy.
Decades ago, we avoided CT scans during pregnancy because of concerns about radiation exposure to the fetus, leaving some patients without an accurate staging of their cancer. Today, we have evidence that a CT scan is generally safe in pregnancy. Similarly, the safety of many chemotherapeutic regimens in pregnancy has been documented in recent decades,and the use of chemotherapy during pregnancy has increased progressively. Radiation is also commonly utilized in the management of cancers that may occur during pregnancy, such as breast cancer.1
Considerations of timing are often central to decision-making; chemotherapy and radiotherapy are generally avoided in the first trimester to prevent structural fetal anomalies, for instance, and delaying cancer treatment is often warranted when the patient is a few weeks away from delivery. On occasion, iatrogenic preterm birth is considered when the risks to the mother of delaying a necessary cancer treatment outweigh the risks to the fetus of prematurity.1
Pregnancy termination is rarely indicated, however, and information gathered over the past 2 decades suggests that fetal and placental metastases are rare.1 There is broad agreement that prenatal treatment of cancer in pregnancy should adhere as much as possible to protocols and guidelines for nonpregnant patients and that treatment delays driven by fear of fetal anomalies and miscarriage are unnecessary.
Cancer Incidence, Use of Diagnostic Imaging
Data on the incidence of cancer in pregnancy comes from population-based cancer registries, and unfortunately, these data are not standardized and are often incomplete. Many studies include cancer diagnosed up to 1 year after pregnancy, and some include preinvasive disease. Estimates therefore vary considerably (see Table 1 for a sampling of estimates incidences.)
It has been reported, and often cited in the literature, that invasive malignancy complicates one in 1,000 pregnancies and that the incidence of cancer in pregnancy (invasive and noninvasive malignancies) has been rising over time.8 Increasing maternal age is believed to be playing a role in this rise; as women delay childbearing, they enter the age range in which some cancers become more common. Additionally, improvements in screening and diagnostics have led to earlier cancer detection. The incidence of ovarian neoplasms found during pregnancy has increased, for instance, with the routine use of diagnostic ultrasound in pregnancy.1
Among the studies showing an increased incidence of pregnancy-associated cancer is a population-based study in Australia, which found that from 1994 to 2007 the crude incidence of pregnancy-associated cancer increased from 112.3 to 191.5 per 100,000 pregnancies (P < .001).9 A cohort study in the United States documented an increase in incidence from 75.0 per 100,000 pregnancies in 2002 to 138.5 per 100,000 pregnancies in 2012.10
Overall, the literature shows us that the skin, cervix, and breast are also common sites for malignancy during pregnancy.1 According to a 2022 review, breast cancer during pregnancy is less often hormone receptor–positive and more frequently triple negative compared with age-matched controls.11 The frequencies of other pregnancy-associated cancers appear overall to be similar to that of cancer occurring in all women across their reproductive years.1
Too often, diagnosis is delayed because cancer symptoms can be masked by or can mimic normal physiological changes in pregnancy. For instance, breast cancer can be difficult to diagnose during pregnancy and lactation due to anatomic changes in the breast parenchyma. Several studies published in the 1990s showed that breast cancer presents at a more advanced stage in pregnant patients than in nonpregnant patients because of this delay.1 Skin changes suggestive of melanoma can be attributed to hyperpigmentation of pregnancy, for instance. Several observational studies have suggested that thicker melanomas found in pregnancy may be because of delayed diagnosis.8
It is important that we thoroughly investigate signs and symptoms suggestive of a malignancy and not automatically attribute these symptoms to the pregnancy itself. Cervical biopsy of a mass or lesion suspicious for cervical cancer can be done safely during pregnancy and should not be delayed or deferred.
Fetal radiation exposure from radiologic examinations has long been a concern, but we know today that while the imaging modality should be chosen to minimize fetal radiation exposure, CT scans and even PET scans should be performed if these exams are deemed best for evaluation. Embryonic exposure to a dose of less than 50 mGy is rarely if at all associated with fetal malformations or miscarriage and a radiation dose of 100 mGy may be considered a floor for consideration of therapeutic termination of pregnancy.1,8
CT exams are associated with a fetal dose far less than 50 mGy (see Table 2 for radiation doses).
Magnetic resonance imaging with a magnet strength of 3 Tesla or less in any trimester is not associated with an increased risk of harm to the fetus or in early childhood, but the contrast agent gadolinium should be avoided in pregnancy as it has been associated with an increased risk of stillbirth, neonatal death, and childhood inflammatory, rheumatologic, and infiltrative skin lesions.1,8,12
Chemotherapy, Surgery, and Radiation in Pregnancy
The management of cancer during pregnancy requires a multidisciplinary team including medical, gynecologic, or radiation oncologists, and maternal-fetal medicine specialists (Figure 1). Prematurity and low birth weight are frequent complications for fetuses exposed to chemotherapy, although there is some uncertainty as to whether the treatment is causative. However, congenital anomalies no longer are a major concern, provided that drugs are appropriately selected and that fetal exposure occurs during the second or third trimester.
For instance, alkylating agents including cisplatin (an important drug in the management of gynecologic malignancies) have been associated with congenital anomalies in the first trimester but not in the second and third trimesters, and a variety of antimetabolites — excluding methotrexate and aminopterin — similarly have been shown to be relatively safe when used after the first trimester.1
Small studies have shown no long-term effects of chemotherapy exposure on postnatal growth and long-term neurologic/neurocognitive function,1 but this is an area that needs more research.
Also in need of investigation is the safety of newer agents in pregnancy. Data are limited on the use of new targeted treatments, monoclonal antibodies, and immunotherapies in pregnancy and their effects on the fetus, with current knowledge coming mainly from single case reports.13
Until more is learned — a challenge given that pregnant women are generally excluded from clinical trials — management teams are generally postponing use of these therapies until after delivery. Considering the pace of new developments revolutionizing cancer treatment, this topic will likely get more complex and confusing before we begin acquiring sufficient knowledge.
The timing of surgery for malignancy in pregnancy is similarly based on the balance of maternal and fetal risks, including the risk of maternal disease progression, the risk of preterm delivery, and the prevention of fetal metastases. In general, the safest time is the second trimester.
Maternal surgery in the third trimester may be associated with a risk of premature labor and altered uteroplacental perfusion. A 2005 systematic review of 12,452 women who underwent nonobstetric surgery during pregnancy provides some reassurance, however; compared with the general obstetric population, there was no increase in the rate of miscarriage or major birth defects.14
Radiotherapy used to be contraindicated in pregnancy but many experts today believe it can be safely utilized provided the uterus is out of field and is protected from scattered radiation. The head, neck, and breast, for instance, can be treated with newer radiotherapies, including stereotactic ablative radiation therapy.8 Patients with advanced cervical cancer often receive chemotherapy during pregnancy to slow metastatic growth followed by definitive treatment with postpartum radiation or surgery.
More research is needed, but available data on maternal outcomes are encouraging. For instance, there appear to be no significant differences in short- and long-term complications or survival between women who are pregnant and nonpregnant when treated for invasive cervical cancer.8 Similarly, while earlier studies of breast cancer diagnosed during pregnancy suggested a poor prognosis, data now show similar prognoses for pregnant and nonpregnant patients when controlled for stage.1
Dr. Rao is a gynecologic oncologist and associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported no relevant disclosures.
References
1. Rao GG. Chapter 42. Clinical Obstetrics: The Fetus & Mother, 4th ed. Reece EA et al. (eds): 2021.
2. Bannister-Tyrrell M et al. Aust N Z J Obstet Gynaecol. 2014;55:116-122.
3. Oehler MK et al. Aust N Z J Obstet Gynaecol. 2003;43(6):414-420.
4. Ruiz R et al. Breast. 2017;35:136-141. doi: 10.1016/j.breast.2017.07.008.
5. Nolan S et al. Am J Obstet Gynecol. 2019;220(1):S480. doi: 10.1016/j.ajog.2018.11.752.
6. El-Messidi A et al. J Perinat Med. 2015;43(6):683-688. doi: 10.1515/jpm-2014-0133.
7. Pellino G et al. Eur J Gastroenterol Hepatol. 2017;29(7):743-753. doi: 10.1097/MEG.0000000000000863.
8. Eastwood-Wilshere N et al. Asia-Pac J Clin Oncol. 2019;15:296-308.
9. Lee YY et al. BJOG. 2012;119(13):1572-1582.
10. Cottreau CM et al. J Womens Health (Larchmt). 2019 Feb;28(2):250-257.
11. Boere I et al. Best Pract Res Clin Obstet Gynaecol. 2022;82:46-59.
12. Ray JG et al. JAMA 2016;316(9):952-961.
13. Schwab R et al. Cancers. (Basel) 2021;13(12):3048.
14. Cohen-Kerem et al. Am J Surg. 2005;190(3):467-473.
Genetics and Lifestyle Choices Can Affect Early Prostate Cancer Deaths
TOPLINE:
METHODOLOGY:
- About one third of men die from prostate cancer before age 75, highlighting the need for prevention strategies that target high-risk populations.
- In the current study, researchers analyzed data from two prospective cohort studies — the Malmö Diet and Cancer Study (MDCS) and the Health Professionals Follow-Up Study (HPFS) — which included 19,607 men with a median age at inclusion of 59 years (MDCS) and 65.1 years (HPFS) followed from 1991 to 2019.
- Participants were categorized by genetic risk and lifestyle score. Genetic risk was defined using a multiancestry polygenic risk score (PRS) for overall prostate cancer that included 400 genetic risk variants.
- A healthy lifestyle score was defined as 3-6, while an unhealthy lifestyle score was 0-2. Lifestyle factors included smoking, weight, physical activity, and diet.
- The researchers calculated hazard ratios (HRs) for the association between genetic and lifestyle factors and prostate cancer death.
TAKEAWAY:
- Combining the PRS and family history of cancer, 67% of men overall (13,186 of 19,607) were considered to have higher genetic risk, and about 30% overall had an unhealthy lifestyle score of 0-2.
- Men at higher genetic risk accounted for 88% (94 of 107) of early prostate cancer deaths.
- Compared with men at lower genetic risk, those at higher genetic risk had more than a threefold higher rate of early prostate cancer death (HR, 3.26) and more than a twofold increased rate of late prostate cancer death (HR, 2.26) as well as a higher lifetime risk for prostate cancer death.
- Among men at higher genetic risk, an unhealthy lifestyle was associated with a higher risk of early prostate cancer death, with smoking and a BMI of ≥ 30 being significant factors. Depending on the definition of a healthy lifestyle, the researchers estimated that 22%-36% of early prostate cancer deaths among men at higher genetic risk might be preventable.
IN PRACTICE:
“Based on data from two prospective cohort studies, this analysis provides evidence for targeting men at increased genetic risk with prevention strategies aimed at reducing premature deaths from prostate cancer,” the researchers concluded.
SOURCE:
The study, with first author Anna Plym, PhD, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet in Stockholm, Sweden, was published online on July 3 in JAMA Network Open.
LIMITATIONS:
Differences in prostate cancer testing and treatment may account for some of the observed association between a healthy lifestyle and prostate cancer death. This analysis provides an estimate of what is achievable in terms of prevention had everyone adopted a healthy lifestyle. The authors only considered factors at study entry, which would not include changes that happen later.
DISCLOSURES:
The study authors reported several disclosures. Fredrik Wiklund, PhD, received grants from GE Healthcare, personal fees from Janssen, Varian Medical Systems, and WebMD, and stock options and personal fees from Cortechs Labs outside the submitted work. Adam S. Kibel, MD, received personal fees from Janssen, Pfizer, Bristol Myers Squibb, Cellvax, Merck, and Roche and served as a consultant for Bristol Myers Squibb and Candel outside the submitted work. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- About one third of men die from prostate cancer before age 75, highlighting the need for prevention strategies that target high-risk populations.
- In the current study, researchers analyzed data from two prospective cohort studies — the Malmö Diet and Cancer Study (MDCS) and the Health Professionals Follow-Up Study (HPFS) — which included 19,607 men with a median age at inclusion of 59 years (MDCS) and 65.1 years (HPFS) followed from 1991 to 2019.
- Participants were categorized by genetic risk and lifestyle score. Genetic risk was defined using a multiancestry polygenic risk score (PRS) for overall prostate cancer that included 400 genetic risk variants.
- A healthy lifestyle score was defined as 3-6, while an unhealthy lifestyle score was 0-2. Lifestyle factors included smoking, weight, physical activity, and diet.
- The researchers calculated hazard ratios (HRs) for the association between genetic and lifestyle factors and prostate cancer death.
TAKEAWAY:
- Combining the PRS and family history of cancer, 67% of men overall (13,186 of 19,607) were considered to have higher genetic risk, and about 30% overall had an unhealthy lifestyle score of 0-2.
- Men at higher genetic risk accounted for 88% (94 of 107) of early prostate cancer deaths.
- Compared with men at lower genetic risk, those at higher genetic risk had more than a threefold higher rate of early prostate cancer death (HR, 3.26) and more than a twofold increased rate of late prostate cancer death (HR, 2.26) as well as a higher lifetime risk for prostate cancer death.
- Among men at higher genetic risk, an unhealthy lifestyle was associated with a higher risk of early prostate cancer death, with smoking and a BMI of ≥ 30 being significant factors. Depending on the definition of a healthy lifestyle, the researchers estimated that 22%-36% of early prostate cancer deaths among men at higher genetic risk might be preventable.
IN PRACTICE:
“Based on data from two prospective cohort studies, this analysis provides evidence for targeting men at increased genetic risk with prevention strategies aimed at reducing premature deaths from prostate cancer,” the researchers concluded.
SOURCE:
The study, with first author Anna Plym, PhD, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet in Stockholm, Sweden, was published online on July 3 in JAMA Network Open.
LIMITATIONS:
Differences in prostate cancer testing and treatment may account for some of the observed association between a healthy lifestyle and prostate cancer death. This analysis provides an estimate of what is achievable in terms of prevention had everyone adopted a healthy lifestyle. The authors only considered factors at study entry, which would not include changes that happen later.
DISCLOSURES:
The study authors reported several disclosures. Fredrik Wiklund, PhD, received grants from GE Healthcare, personal fees from Janssen, Varian Medical Systems, and WebMD, and stock options and personal fees from Cortechs Labs outside the submitted work. Adam S. Kibel, MD, received personal fees from Janssen, Pfizer, Bristol Myers Squibb, Cellvax, Merck, and Roche and served as a consultant for Bristol Myers Squibb and Candel outside the submitted work. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- About one third of men die from prostate cancer before age 75, highlighting the need for prevention strategies that target high-risk populations.
- In the current study, researchers analyzed data from two prospective cohort studies — the Malmö Diet and Cancer Study (MDCS) and the Health Professionals Follow-Up Study (HPFS) — which included 19,607 men with a median age at inclusion of 59 years (MDCS) and 65.1 years (HPFS) followed from 1991 to 2019.
- Participants were categorized by genetic risk and lifestyle score. Genetic risk was defined using a multiancestry polygenic risk score (PRS) for overall prostate cancer that included 400 genetic risk variants.
- A healthy lifestyle score was defined as 3-6, while an unhealthy lifestyle score was 0-2. Lifestyle factors included smoking, weight, physical activity, and diet.
- The researchers calculated hazard ratios (HRs) for the association between genetic and lifestyle factors and prostate cancer death.
TAKEAWAY:
- Combining the PRS and family history of cancer, 67% of men overall (13,186 of 19,607) were considered to have higher genetic risk, and about 30% overall had an unhealthy lifestyle score of 0-2.
- Men at higher genetic risk accounted for 88% (94 of 107) of early prostate cancer deaths.
- Compared with men at lower genetic risk, those at higher genetic risk had more than a threefold higher rate of early prostate cancer death (HR, 3.26) and more than a twofold increased rate of late prostate cancer death (HR, 2.26) as well as a higher lifetime risk for prostate cancer death.
- Among men at higher genetic risk, an unhealthy lifestyle was associated with a higher risk of early prostate cancer death, with smoking and a BMI of ≥ 30 being significant factors. Depending on the definition of a healthy lifestyle, the researchers estimated that 22%-36% of early prostate cancer deaths among men at higher genetic risk might be preventable.
IN PRACTICE:
“Based on data from two prospective cohort studies, this analysis provides evidence for targeting men at increased genetic risk with prevention strategies aimed at reducing premature deaths from prostate cancer,” the researchers concluded.
SOURCE:
The study, with first author Anna Plym, PhD, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet in Stockholm, Sweden, was published online on July 3 in JAMA Network Open.
LIMITATIONS:
Differences in prostate cancer testing and treatment may account for some of the observed association between a healthy lifestyle and prostate cancer death. This analysis provides an estimate of what is achievable in terms of prevention had everyone adopted a healthy lifestyle. The authors only considered factors at study entry, which would not include changes that happen later.
DISCLOSURES:
The study authors reported several disclosures. Fredrik Wiklund, PhD, received grants from GE Healthcare, personal fees from Janssen, Varian Medical Systems, and WebMD, and stock options and personal fees from Cortechs Labs outside the submitted work. Adam S. Kibel, MD, received personal fees from Janssen, Pfizer, Bristol Myers Squibb, Cellvax, Merck, and Roche and served as a consultant for Bristol Myers Squibb and Candel outside the submitted work. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
ASCO 2024: An Expert’s Top Hematology Highlights
Research presented at the annual meeting of the American Society of Clinical Oncology (ASCO) has the potential to change practice — and assumptions — about acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and blood cancer as a whole, according to the chief science officer of the American Cancer Society.
In an interview following the conference, Arif H. Kamal, MD, MBA, MHS, who practices hematology-oncology at Duke University, Durham, North Carolina, recapped several landmark studies and discussed their lessons for clinicians.
Question: You’ve highlighted a randomized, multisite clinical trialled by a researcher from Massachusetts General Hospital in Boston. The researchers enrolled 115 adult patients with AML or high-risk myelodysplastic syndrome (MDS) who were receiving non–intensive care to usual care or regular meetings with palliative care clinicians (monthly as outpatients and at least twice weekly as inpatients). Among those who died (61.7%), those in the intervention group had their end-of-life preferences documented much earlier (41 days before death vs. 1.5 days, P < .001). They were also more likely to have documented end-of-life care preferences (96.5% vs. 68.4%, P < .001) and less likely to have been hospitalized within the last month of life (70.6% vs. 91.9%, P = .031). Why did this study strike you as especially important?
Dr. Kamal: A few studies have now shown better outcomes in hematology after the use of early palliative care. This has been shown not only in transplant patients but also in non-transplant patients with hematologic malignancies. As a result, you’re seeing a shift toward regular integration of palliative care.
The historical concern has been that palliative care takes the foot off the gas pedal. Another way to look at it is that palliative care helps keep the foot on the gas pedal.
Q: Should the focus be on all hematologic cancer patients or just on those who are more severe cases or whose illness is terminal?
Dr. Kamal: The focus is on patients with acute progressive leukemias rather than those with indolent, long-standing lymphomas. This a reflection of severity and complexity: In leukemia, you can be someone really sick all of a sudden and require intensive treatment.
Q: What’s new about this kind of research?
Dr. Kamal: We’re learning how palliative care is valuable in all cancers, but particularly in blood cancers, where it has historically not been studied. The groundbreaking studies in palliative care over the last 20 years have largely been in solid tumors such as lung cancers and colorectal cancers.
Q: What is unique about the patient experience in hematologic cancers compared to solid tumor cancers?
Dr. Kamal: Blood cancers are a relatively new place to integrate palliative care, but what we’re finding is that it may be even more needed than in solid tumors in terms of improving outcomes.
In pancreatic cancer, you may not know if something is going to work, but it is going to take you months to figure it out. In leukemia, there can be a lot of dynamism: You’re going to find out in a matter of days. You have to be able to pivot really quickly to the next thing, go to transplant very quickly and urgently, or make a decision to pursue supportive care.
This really compresses the normal issues like uncertainty and emotional anxiety that a pancreatic cancer patient may process over a year. Leukemic patients may need to process that over 2, 3, or 4 weeks. Palliative care can be there to help the patient to process options.
Q: You also highlighted the industry-funded phase 3 ASC4FIRST study into asciminib (Scemblix) in newly diagnosed patients with CML. The trial was led by a researcher from the South Australian Health and Medical Research Institute and the University of Adelaide, Australia. Asciminib, a STAMP inhibitor, is FDA-approved for certain CML indications. In an intention-to-treat analysis, the new study finds better major molecular response at 48 weeks for the drug vs. investigator-selected tyrosine kinase inhibitors (67.7% vs. 49.0%, P < .001). What do these findings tell you?
Dr. Kamal: CML has been a disease where you had Gleevec — imatinib — and additional options that were all in the second-line or third-line setting after failure. Now, you’re seeing durable responses across the board: an expansion of options and potentially new options in the first-line setting.
[Editor’s note: For more about asciminib, check commentaries from physicians who spoke to Medscape and ASCO Daily News.]
Q: What makes this drug unique?
Dr. Kamal: CML was the leader in helping us to understand that if you identify a mutation, you can create a medication against it. Now, what we’re finding out is that there are other ways to work around mutations. Asciminib is not affected by the most common mutations that lend to drug resistance in the classic drugs that target BCR-ABL cells like imatinib.
Q: Finally, you spotlighted a retrospective study led by researchers at Case Western Reserve University that explored rates of obesity-related cancers — including multiple myeloma — in patients with BMI ≥ 35 who took glucagon-like protein-1 receptor agonists (GLP-1 RAs) or underwent bariatric surgery. Both strategies were linked to lower risk of the cancers vs. no intervention (GLP-1 RAs, hazard ratio [HR] = 0.61; 95% CI 0.46-0.81, and bariatric surgery, HR = 0.78; 95% CI 0.67-0.91). What did you learn from this research?
Dr. Kamal: When we think about risk reduction for cancer, we generally think about hormone-driven cancers. Blood cancers are not typically hormone-driven.
This study is hinting at that idea that healthy weight across the board will reduce your cancer risk even in blood cancers, and pharmacologic interventions to reduce your weight may also reduce that cancer risk.
Q: So weight-loss drugs such as Ozempic could potentially lower the risk of hematologic cancer?
Dr. Kamal: We’re going to need more data on this, and you wouldn’t take it for that reason. But there may be a story here that says get to a healthy weight — it doesn’t matter how you do it — and your risk of all cancers goes down.
Dr. Kamal has no disclosures to report.
Research presented at the annual meeting of the American Society of Clinical Oncology (ASCO) has the potential to change practice — and assumptions — about acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and blood cancer as a whole, according to the chief science officer of the American Cancer Society.
In an interview following the conference, Arif H. Kamal, MD, MBA, MHS, who practices hematology-oncology at Duke University, Durham, North Carolina, recapped several landmark studies and discussed their lessons for clinicians.
Question: You’ve highlighted a randomized, multisite clinical trialled by a researcher from Massachusetts General Hospital in Boston. The researchers enrolled 115 adult patients with AML or high-risk myelodysplastic syndrome (MDS) who were receiving non–intensive care to usual care or regular meetings with palliative care clinicians (monthly as outpatients and at least twice weekly as inpatients). Among those who died (61.7%), those in the intervention group had their end-of-life preferences documented much earlier (41 days before death vs. 1.5 days, P < .001). They were also more likely to have documented end-of-life care preferences (96.5% vs. 68.4%, P < .001) and less likely to have been hospitalized within the last month of life (70.6% vs. 91.9%, P = .031). Why did this study strike you as especially important?
Dr. Kamal: A few studies have now shown better outcomes in hematology after the use of early palliative care. This has been shown not only in transplant patients but also in non-transplant patients with hematologic malignancies. As a result, you’re seeing a shift toward regular integration of palliative care.
The historical concern has been that palliative care takes the foot off the gas pedal. Another way to look at it is that palliative care helps keep the foot on the gas pedal.
Q: Should the focus be on all hematologic cancer patients or just on those who are more severe cases or whose illness is terminal?
Dr. Kamal: The focus is on patients with acute progressive leukemias rather than those with indolent, long-standing lymphomas. This a reflection of severity and complexity: In leukemia, you can be someone really sick all of a sudden and require intensive treatment.
Q: What’s new about this kind of research?
Dr. Kamal: We’re learning how palliative care is valuable in all cancers, but particularly in blood cancers, where it has historically not been studied. The groundbreaking studies in palliative care over the last 20 years have largely been in solid tumors such as lung cancers and colorectal cancers.
Q: What is unique about the patient experience in hematologic cancers compared to solid tumor cancers?
Dr. Kamal: Blood cancers are a relatively new place to integrate palliative care, but what we’re finding is that it may be even more needed than in solid tumors in terms of improving outcomes.
In pancreatic cancer, you may not know if something is going to work, but it is going to take you months to figure it out. In leukemia, there can be a lot of dynamism: You’re going to find out in a matter of days. You have to be able to pivot really quickly to the next thing, go to transplant very quickly and urgently, or make a decision to pursue supportive care.
This really compresses the normal issues like uncertainty and emotional anxiety that a pancreatic cancer patient may process over a year. Leukemic patients may need to process that over 2, 3, or 4 weeks. Palliative care can be there to help the patient to process options.
Q: You also highlighted the industry-funded phase 3 ASC4FIRST study into asciminib (Scemblix) in newly diagnosed patients with CML. The trial was led by a researcher from the South Australian Health and Medical Research Institute and the University of Adelaide, Australia. Asciminib, a STAMP inhibitor, is FDA-approved for certain CML indications. In an intention-to-treat analysis, the new study finds better major molecular response at 48 weeks for the drug vs. investigator-selected tyrosine kinase inhibitors (67.7% vs. 49.0%, P < .001). What do these findings tell you?
Dr. Kamal: CML has been a disease where you had Gleevec — imatinib — and additional options that were all in the second-line or third-line setting after failure. Now, you’re seeing durable responses across the board: an expansion of options and potentially new options in the first-line setting.
[Editor’s note: For more about asciminib, check commentaries from physicians who spoke to Medscape and ASCO Daily News.]
Q: What makes this drug unique?
Dr. Kamal: CML was the leader in helping us to understand that if you identify a mutation, you can create a medication against it. Now, what we’re finding out is that there are other ways to work around mutations. Asciminib is not affected by the most common mutations that lend to drug resistance in the classic drugs that target BCR-ABL cells like imatinib.
Q: Finally, you spotlighted a retrospective study led by researchers at Case Western Reserve University that explored rates of obesity-related cancers — including multiple myeloma — in patients with BMI ≥ 35 who took glucagon-like protein-1 receptor agonists (GLP-1 RAs) or underwent bariatric surgery. Both strategies were linked to lower risk of the cancers vs. no intervention (GLP-1 RAs, hazard ratio [HR] = 0.61; 95% CI 0.46-0.81, and bariatric surgery, HR = 0.78; 95% CI 0.67-0.91). What did you learn from this research?
Dr. Kamal: When we think about risk reduction for cancer, we generally think about hormone-driven cancers. Blood cancers are not typically hormone-driven.
This study is hinting at that idea that healthy weight across the board will reduce your cancer risk even in blood cancers, and pharmacologic interventions to reduce your weight may also reduce that cancer risk.
Q: So weight-loss drugs such as Ozempic could potentially lower the risk of hematologic cancer?
Dr. Kamal: We’re going to need more data on this, and you wouldn’t take it for that reason. But there may be a story here that says get to a healthy weight — it doesn’t matter how you do it — and your risk of all cancers goes down.
Dr. Kamal has no disclosures to report.
Research presented at the annual meeting of the American Society of Clinical Oncology (ASCO) has the potential to change practice — and assumptions — about acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and blood cancer as a whole, according to the chief science officer of the American Cancer Society.
In an interview following the conference, Arif H. Kamal, MD, MBA, MHS, who practices hematology-oncology at Duke University, Durham, North Carolina, recapped several landmark studies and discussed their lessons for clinicians.
Question: You’ve highlighted a randomized, multisite clinical trialled by a researcher from Massachusetts General Hospital in Boston. The researchers enrolled 115 adult patients with AML or high-risk myelodysplastic syndrome (MDS) who were receiving non–intensive care to usual care or regular meetings with palliative care clinicians (monthly as outpatients and at least twice weekly as inpatients). Among those who died (61.7%), those in the intervention group had their end-of-life preferences documented much earlier (41 days before death vs. 1.5 days, P < .001). They were also more likely to have documented end-of-life care preferences (96.5% vs. 68.4%, P < .001) and less likely to have been hospitalized within the last month of life (70.6% vs. 91.9%, P = .031). Why did this study strike you as especially important?
Dr. Kamal: A few studies have now shown better outcomes in hematology after the use of early palliative care. This has been shown not only in transplant patients but also in non-transplant patients with hematologic malignancies. As a result, you’re seeing a shift toward regular integration of palliative care.
The historical concern has been that palliative care takes the foot off the gas pedal. Another way to look at it is that palliative care helps keep the foot on the gas pedal.
Q: Should the focus be on all hematologic cancer patients or just on those who are more severe cases or whose illness is terminal?
Dr. Kamal: The focus is on patients with acute progressive leukemias rather than those with indolent, long-standing lymphomas. This a reflection of severity and complexity: In leukemia, you can be someone really sick all of a sudden and require intensive treatment.
Q: What’s new about this kind of research?
Dr. Kamal: We’re learning how palliative care is valuable in all cancers, but particularly in blood cancers, where it has historically not been studied. The groundbreaking studies in palliative care over the last 20 years have largely been in solid tumors such as lung cancers and colorectal cancers.
Q: What is unique about the patient experience in hematologic cancers compared to solid tumor cancers?
Dr. Kamal: Blood cancers are a relatively new place to integrate palliative care, but what we’re finding is that it may be even more needed than in solid tumors in terms of improving outcomes.
In pancreatic cancer, you may not know if something is going to work, but it is going to take you months to figure it out. In leukemia, there can be a lot of dynamism: You’re going to find out in a matter of days. You have to be able to pivot really quickly to the next thing, go to transplant very quickly and urgently, or make a decision to pursue supportive care.
This really compresses the normal issues like uncertainty and emotional anxiety that a pancreatic cancer patient may process over a year. Leukemic patients may need to process that over 2, 3, or 4 weeks. Palliative care can be there to help the patient to process options.
Q: You also highlighted the industry-funded phase 3 ASC4FIRST study into asciminib (Scemblix) in newly diagnosed patients with CML. The trial was led by a researcher from the South Australian Health and Medical Research Institute and the University of Adelaide, Australia. Asciminib, a STAMP inhibitor, is FDA-approved for certain CML indications. In an intention-to-treat analysis, the new study finds better major molecular response at 48 weeks for the drug vs. investigator-selected tyrosine kinase inhibitors (67.7% vs. 49.0%, P < .001). What do these findings tell you?
Dr. Kamal: CML has been a disease where you had Gleevec — imatinib — and additional options that were all in the second-line or third-line setting after failure. Now, you’re seeing durable responses across the board: an expansion of options and potentially new options in the first-line setting.
[Editor’s note: For more about asciminib, check commentaries from physicians who spoke to Medscape and ASCO Daily News.]
Q: What makes this drug unique?
Dr. Kamal: CML was the leader in helping us to understand that if you identify a mutation, you can create a medication against it. Now, what we’re finding out is that there are other ways to work around mutations. Asciminib is not affected by the most common mutations that lend to drug resistance in the classic drugs that target BCR-ABL cells like imatinib.
Q: Finally, you spotlighted a retrospective study led by researchers at Case Western Reserve University that explored rates of obesity-related cancers — including multiple myeloma — in patients with BMI ≥ 35 who took glucagon-like protein-1 receptor agonists (GLP-1 RAs) or underwent bariatric surgery. Both strategies were linked to lower risk of the cancers vs. no intervention (GLP-1 RAs, hazard ratio [HR] = 0.61; 95% CI 0.46-0.81, and bariatric surgery, HR = 0.78; 95% CI 0.67-0.91). What did you learn from this research?
Dr. Kamal: When we think about risk reduction for cancer, we generally think about hormone-driven cancers. Blood cancers are not typically hormone-driven.
This study is hinting at that idea that healthy weight across the board will reduce your cancer risk even in blood cancers, and pharmacologic interventions to reduce your weight may also reduce that cancer risk.
Q: So weight-loss drugs such as Ozempic could potentially lower the risk of hematologic cancer?
Dr. Kamal: We’re going to need more data on this, and you wouldn’t take it for that reason. But there may be a story here that says get to a healthy weight — it doesn’t matter how you do it — and your risk of all cancers goes down.
Dr. Kamal has no disclosures to report.
Eribulin Similar to Taxane When Paired With Dual HER2 Blockade in BC
The results of this multicenter, randomized, open-label, parallel-group, phase 3 Japanese trial suggest that patients who cannot tolerate the standard taxane-based regimen have a new option for treatment.
“Our study is the first to show the non-inferiority of eribulin to a taxane, when used in combination with dual HER2 blockade as first-line treatment for this population,” lead author Toshinari Yamashita, MD, PhD, from the Kanagawa Cancer Center, in Kanagawa, Japan, said at the annual meeting of the American Society of Clinical Oncology.
“To our knowledge, noninferiority of eribulin to a taxane when used in combination with dual HER2 blockade has not been investigated,” Dr. Yamashita said.
“The combination of trastuzumab, pertuzumab, and taxane is a current standard first-line therapy for recurrent or metastatic HER2-positive breast cancer,” explained Dr. Yamashita. “However, because of taxane-induced toxicity, the development of less toxic but equally effective alternatives are needed.
“Because its efficacy is comparable to that of the current standard regimen, the combination of eribulin, trastuzumab, and pertuzumab is one of the options for first-line treatment of how to fight locally advanced or metastatic breast cancer,” he continued.
Study Results and Methods
The trial enrolled 446 patients, mean age 56 years, all of whom had locally advanced or metastatic breast cancer and no prior use of chemotherapy, excluding T-DM1. Patients who had received hormonal or HER2 therapy alone or the combination, as treatment for recurrence, were also eligible.
They were randomized 1:1 to receive a 21-day chemotherapy cycle of either (i) eribulin (1.4 mg/m2 on days 1 and 8), or (ii) a taxane (docetaxel 75 mg/m2 on day 1 or paclitaxel 80 mg/m2 on days 1, 8 and 15), each being administered in combination with a dual HER2 blockade of trastuzumab plus pertuzumab.
Baseline characteristics of both groups were well balanced, with 257 (57.6%) having ER-positive disease, 292 (65.5%) visceral metastasis, and 263 (59%) with de novo stage 4 disease, explained Dr. Yamashita.
For the primary endpoint, the median progression-free survival (PFS) was 14 versus 12.9 months in the eribulin and taxane group, respectively (hazard ratio [HR] 0.95, P = .6817), confirming non-inferiority of the study regimen, he reported.
The clinical benefit rate was similar between the two groups, with an objective response rate of 76.8% in the eribulin group and 75.2% in the taxane group.
Median OS was 65.3 months in the taxane group, but has not been reached in the study group (HR 1.09).
In terms of side-effects, the incidence of treatment-emergent adverse events was similar between the eribulin and taxane groups (58.9% vs 59.2%, respectively, for grade 3 or higher).
“Skin-related adverse events (62.4% vs 40.6%), diarrhea (54.1% vs 36.6%), and edema (42.2% vs 8.5%) tend to be more common with taxane, whereas neutropenia (61.6% vs 30.7%) and peripheral neuropathy (61.2% vs 52.8%) tend to be more common with eribulin use,” he said.
Overall, “these results suggest that eribulin is less toxic chemotherapeutic partner for dual HER2 blockade and can be used for a longer,” he said.
Findings Are a ‘Clinical Pearl’
Harold Burstein, MD, PhD, a breast cancer expert at Dana-Farber Cancer Institute and professor at Harvard Medical School in Boston, described the findings as “a nice clinical pearl,” because some patients do not tolerate taxane therapy. “In such cases, you can substitute eribulin, which is usually tolerated without allergic hypersensitivity issues,” he said in an interview.
Eribulin has specific properties that “could make it a perfect candidate” as an adjunct to standard treatment regimens across different breast cancer subtypes, observed Wynne Wijaya, MD an oncology researcher at the University of Oxford, England, and Universitas Gadjah Mada, in Yogyakarta, Indonesia, in a recent review (World J Exp Med. 2024;14[2]:92558).
Dr. Wijaya, who was not involved in this study, said in an interview that the findings have important implications.
“This encouraging result adds eribulin as another option in the first line treatment regimen for patients with HER2-positive, locally advanced or metastatic breast cancer, especially in terms of side effects/toxicities,” she said. “As clinicians, we can offer to tailor the choice of therapy between eribulin versus taxane in the regimen based on [which side effects patients are better able to tolerate]. It would also be interesting and worthwhile to conduct similar trials in different types of populations to provide more robust evidence.”
Eisai Co. funded the research. Dr. Yamashita disclosed ties with AstraZeneca, Chugai Pharma, Daiichi Sankyo, Eisai, Kyowa Hakko Kiri, Lilly, MSD, Pfizer, Taiho, Gilead Sciences, Nihonkayaku, Ono Yakuhin, and Seagen. Dr. Burstein disclosed a research grant from National Cancer Institute. Dr. Wijaya had no relevant disclosures.
The results of this multicenter, randomized, open-label, parallel-group, phase 3 Japanese trial suggest that patients who cannot tolerate the standard taxane-based regimen have a new option for treatment.
“Our study is the first to show the non-inferiority of eribulin to a taxane, when used in combination with dual HER2 blockade as first-line treatment for this population,” lead author Toshinari Yamashita, MD, PhD, from the Kanagawa Cancer Center, in Kanagawa, Japan, said at the annual meeting of the American Society of Clinical Oncology.
“To our knowledge, noninferiority of eribulin to a taxane when used in combination with dual HER2 blockade has not been investigated,” Dr. Yamashita said.
“The combination of trastuzumab, pertuzumab, and taxane is a current standard first-line therapy for recurrent or metastatic HER2-positive breast cancer,” explained Dr. Yamashita. “However, because of taxane-induced toxicity, the development of less toxic but equally effective alternatives are needed.
“Because its efficacy is comparable to that of the current standard regimen, the combination of eribulin, trastuzumab, and pertuzumab is one of the options for first-line treatment of how to fight locally advanced or metastatic breast cancer,” he continued.
Study Results and Methods
The trial enrolled 446 patients, mean age 56 years, all of whom had locally advanced or metastatic breast cancer and no prior use of chemotherapy, excluding T-DM1. Patients who had received hormonal or HER2 therapy alone or the combination, as treatment for recurrence, were also eligible.
They were randomized 1:1 to receive a 21-day chemotherapy cycle of either (i) eribulin (1.4 mg/m2 on days 1 and 8), or (ii) a taxane (docetaxel 75 mg/m2 on day 1 or paclitaxel 80 mg/m2 on days 1, 8 and 15), each being administered in combination with a dual HER2 blockade of trastuzumab plus pertuzumab.
Baseline characteristics of both groups were well balanced, with 257 (57.6%) having ER-positive disease, 292 (65.5%) visceral metastasis, and 263 (59%) with de novo stage 4 disease, explained Dr. Yamashita.
For the primary endpoint, the median progression-free survival (PFS) was 14 versus 12.9 months in the eribulin and taxane group, respectively (hazard ratio [HR] 0.95, P = .6817), confirming non-inferiority of the study regimen, he reported.
The clinical benefit rate was similar between the two groups, with an objective response rate of 76.8% in the eribulin group and 75.2% in the taxane group.
Median OS was 65.3 months in the taxane group, but has not been reached in the study group (HR 1.09).
In terms of side-effects, the incidence of treatment-emergent adverse events was similar between the eribulin and taxane groups (58.9% vs 59.2%, respectively, for grade 3 or higher).
“Skin-related adverse events (62.4% vs 40.6%), diarrhea (54.1% vs 36.6%), and edema (42.2% vs 8.5%) tend to be more common with taxane, whereas neutropenia (61.6% vs 30.7%) and peripheral neuropathy (61.2% vs 52.8%) tend to be more common with eribulin use,” he said.
Overall, “these results suggest that eribulin is less toxic chemotherapeutic partner for dual HER2 blockade and can be used for a longer,” he said.
Findings Are a ‘Clinical Pearl’
Harold Burstein, MD, PhD, a breast cancer expert at Dana-Farber Cancer Institute and professor at Harvard Medical School in Boston, described the findings as “a nice clinical pearl,” because some patients do not tolerate taxane therapy. “In such cases, you can substitute eribulin, which is usually tolerated without allergic hypersensitivity issues,” he said in an interview.
Eribulin has specific properties that “could make it a perfect candidate” as an adjunct to standard treatment regimens across different breast cancer subtypes, observed Wynne Wijaya, MD an oncology researcher at the University of Oxford, England, and Universitas Gadjah Mada, in Yogyakarta, Indonesia, in a recent review (World J Exp Med. 2024;14[2]:92558).
Dr. Wijaya, who was not involved in this study, said in an interview that the findings have important implications.
“This encouraging result adds eribulin as another option in the first line treatment regimen for patients with HER2-positive, locally advanced or metastatic breast cancer, especially in terms of side effects/toxicities,” she said. “As clinicians, we can offer to tailor the choice of therapy between eribulin versus taxane in the regimen based on [which side effects patients are better able to tolerate]. It would also be interesting and worthwhile to conduct similar trials in different types of populations to provide more robust evidence.”
Eisai Co. funded the research. Dr. Yamashita disclosed ties with AstraZeneca, Chugai Pharma, Daiichi Sankyo, Eisai, Kyowa Hakko Kiri, Lilly, MSD, Pfizer, Taiho, Gilead Sciences, Nihonkayaku, Ono Yakuhin, and Seagen. Dr. Burstein disclosed a research grant from National Cancer Institute. Dr. Wijaya had no relevant disclosures.
The results of this multicenter, randomized, open-label, parallel-group, phase 3 Japanese trial suggest that patients who cannot tolerate the standard taxane-based regimen have a new option for treatment.
“Our study is the first to show the non-inferiority of eribulin to a taxane, when used in combination with dual HER2 blockade as first-line treatment for this population,” lead author Toshinari Yamashita, MD, PhD, from the Kanagawa Cancer Center, in Kanagawa, Japan, said at the annual meeting of the American Society of Clinical Oncology.
“To our knowledge, noninferiority of eribulin to a taxane when used in combination with dual HER2 blockade has not been investigated,” Dr. Yamashita said.
“The combination of trastuzumab, pertuzumab, and taxane is a current standard first-line therapy for recurrent or metastatic HER2-positive breast cancer,” explained Dr. Yamashita. “However, because of taxane-induced toxicity, the development of less toxic but equally effective alternatives are needed.
“Because its efficacy is comparable to that of the current standard regimen, the combination of eribulin, trastuzumab, and pertuzumab is one of the options for first-line treatment of how to fight locally advanced or metastatic breast cancer,” he continued.
Study Results and Methods
The trial enrolled 446 patients, mean age 56 years, all of whom had locally advanced or metastatic breast cancer and no prior use of chemotherapy, excluding T-DM1. Patients who had received hormonal or HER2 therapy alone or the combination, as treatment for recurrence, were also eligible.
They were randomized 1:1 to receive a 21-day chemotherapy cycle of either (i) eribulin (1.4 mg/m2 on days 1 and 8), or (ii) a taxane (docetaxel 75 mg/m2 on day 1 or paclitaxel 80 mg/m2 on days 1, 8 and 15), each being administered in combination with a dual HER2 blockade of trastuzumab plus pertuzumab.
Baseline characteristics of both groups were well balanced, with 257 (57.6%) having ER-positive disease, 292 (65.5%) visceral metastasis, and 263 (59%) with de novo stage 4 disease, explained Dr. Yamashita.
For the primary endpoint, the median progression-free survival (PFS) was 14 versus 12.9 months in the eribulin and taxane group, respectively (hazard ratio [HR] 0.95, P = .6817), confirming non-inferiority of the study regimen, he reported.
The clinical benefit rate was similar between the two groups, with an objective response rate of 76.8% in the eribulin group and 75.2% in the taxane group.
Median OS was 65.3 months in the taxane group, but has not been reached in the study group (HR 1.09).
In terms of side-effects, the incidence of treatment-emergent adverse events was similar between the eribulin and taxane groups (58.9% vs 59.2%, respectively, for grade 3 or higher).
“Skin-related adverse events (62.4% vs 40.6%), diarrhea (54.1% vs 36.6%), and edema (42.2% vs 8.5%) tend to be more common with taxane, whereas neutropenia (61.6% vs 30.7%) and peripheral neuropathy (61.2% vs 52.8%) tend to be more common with eribulin use,” he said.
Overall, “these results suggest that eribulin is less toxic chemotherapeutic partner for dual HER2 blockade and can be used for a longer,” he said.
Findings Are a ‘Clinical Pearl’
Harold Burstein, MD, PhD, a breast cancer expert at Dana-Farber Cancer Institute and professor at Harvard Medical School in Boston, described the findings as “a nice clinical pearl,” because some patients do not tolerate taxane therapy. “In such cases, you can substitute eribulin, which is usually tolerated without allergic hypersensitivity issues,” he said in an interview.
Eribulin has specific properties that “could make it a perfect candidate” as an adjunct to standard treatment regimens across different breast cancer subtypes, observed Wynne Wijaya, MD an oncology researcher at the University of Oxford, England, and Universitas Gadjah Mada, in Yogyakarta, Indonesia, in a recent review (World J Exp Med. 2024;14[2]:92558).
Dr. Wijaya, who was not involved in this study, said in an interview that the findings have important implications.
“This encouraging result adds eribulin as another option in the first line treatment regimen for patients with HER2-positive, locally advanced or metastatic breast cancer, especially in terms of side effects/toxicities,” she said. “As clinicians, we can offer to tailor the choice of therapy between eribulin versus taxane in the regimen based on [which side effects patients are better able to tolerate]. It would also be interesting and worthwhile to conduct similar trials in different types of populations to provide more robust evidence.”
Eisai Co. funded the research. Dr. Yamashita disclosed ties with AstraZeneca, Chugai Pharma, Daiichi Sankyo, Eisai, Kyowa Hakko Kiri, Lilly, MSD, Pfizer, Taiho, Gilead Sciences, Nihonkayaku, Ono Yakuhin, and Seagen. Dr. Burstein disclosed a research grant from National Cancer Institute. Dr. Wijaya had no relevant disclosures.
FROM ASCO 2024
PFS Benefits Seen With Palbociclib + Endocrine Therapy in Breast Cancer
“The combination of palbociclib plus exemestane plus leuprolide showed a consistent significant improvement in PFS [progression-free survival] compared to the capecitabine arm,” Yeon Hee Park, MD, PhD, from Samsung Medical Center, Sungkyunkwan University, Seoul, South Korea, reported at the annual meeting of the American Society of Clinical Oncology.
Study Methods and Results
Young-PEARL, a prospective, multicenter, open-label, randomized phase 2 study, included 184 patients, median age 44 years, who had relapsed or progressed during previous tamoxifen therapy, with one line of previous chemotherapy for mBC allowed. Patients were randomized to palbociclib plus endocrine therapy (oral palbociclib 125 mg per day for 21 days every 4 weeks, oral exemestane 25 mg per day for 28 days, plus leuprolide 3.75 mg subcutaneously every 4 weeks) or chemotherapy (oral capecitabine 1250 mg/m2, twice daily for 2 weeks every 3 weeks).
Previously published initial results (Lancet Oncol. 2019 Dec;20[12]:1750-1759) for the primary endpoint showed a median PFS of 20.1 months in the palbociclib group versus 14.4 months in the capecitabine group, (hazard ratio [HR] 0.659, P = .0235) after median follow-up of 17 months.
Updated results showed this benefit was maintained after a median of 54 months, with a PFS of 19.5 months in the palbociclib arm, versus 14 months in capecitabine arm (HR 0.744, P = .0357), Dr. Park reported. However, this PFS benefit did not lead to an overall survival (OS) benefit, with median OS being similar: 54.8 versus 57.8 months in the palbociclib and capecitabine groups, respectively (HR = 1.02, P = .92).
To explore why PFS — but not OS — was better in the palbociclib arm, the researchers conducted a multivariate analysis which showed that going on to an additional CDK4/6 inhibitor treatment after the end of the study was as an independent variable favoring OS. Because more patients in the capecitabine arm received a post-study CDK4/6 inhibitor (49.3%) compared with in the palbociclib group (15%), this weighted the OS to the capecitabine arm, Dr. Park explained in an interview.
“In the capecitabine arm, excluding post-study CDK4/6 inhibitor use, the median OS was 38.8 months.” This was inferior to the 49 months OS seen in the palbociclib arm (P = .065), she said.
“As expected, hematologic toxicity was more common in the palbociclib arm compared with in the capecitabine arm,” Dr. Park said (92% vs 86%), with neutropenia topping the list [of all adverse events] (65.2% vs 27.9%, all grades). However, “most [adverse events] were not that serious,” Dr. Park said. Arthralgia was more common in the palbociclib arm (25% vs 7%), and diarrhea and hand-foot syndrome were more common in the capecitabine arm (15.2% vs 39.5% and 79.1% vs 2.2%).
Study Validates Endocrine Therapy + CDK4/6 Inhibitor as First Line
Commenting on Young-PEARL in an interview, Harold Burstein, MD, PhD, said, “The point of this study was to compare whether upfront chemotherapy would be better than upfront hormonal therapy for patients who had metastatic ER positive breast cancer.”
“This is the first study in probably 20 years that has compared these two approaches, and it validated that for the vast majority of patients with ER positive metastatic breast cancer, the appropriate first treatment is endocrine therapy with a CDK4/6 inhibitor,” continued Dr. Burstein, a breast cancer expert at Dana-Farber Cancer Institute, and professor at Harvard Medical School in Boston.
Dr. Park disclosed honoraria from AstraZeneca, Daiichi Sankyo, Eisai, Lilly, MSD, Novartis, Pfizer, and Roche; consulting or advisory roles for AstraZeneca, Boryung, Daiichi Sankyo, Eisai, Gilead Sciences, Lilly, Menarini, MSD, Novartis, Pfizer, and Roche; research funding from AstraZeneca, Gencurix, Genome Insight, NGeneBio, Pfizer; and Roche; and travel/accommodations/expenses from Gilead. Dr. Burstein disclosed a research grant from the National Cancer Institute.
“The combination of palbociclib plus exemestane plus leuprolide showed a consistent significant improvement in PFS [progression-free survival] compared to the capecitabine arm,” Yeon Hee Park, MD, PhD, from Samsung Medical Center, Sungkyunkwan University, Seoul, South Korea, reported at the annual meeting of the American Society of Clinical Oncology.
Study Methods and Results
Young-PEARL, a prospective, multicenter, open-label, randomized phase 2 study, included 184 patients, median age 44 years, who had relapsed or progressed during previous tamoxifen therapy, with one line of previous chemotherapy for mBC allowed. Patients were randomized to palbociclib plus endocrine therapy (oral palbociclib 125 mg per day for 21 days every 4 weeks, oral exemestane 25 mg per day for 28 days, plus leuprolide 3.75 mg subcutaneously every 4 weeks) or chemotherapy (oral capecitabine 1250 mg/m2, twice daily for 2 weeks every 3 weeks).
Previously published initial results (Lancet Oncol. 2019 Dec;20[12]:1750-1759) for the primary endpoint showed a median PFS of 20.1 months in the palbociclib group versus 14.4 months in the capecitabine group, (hazard ratio [HR] 0.659, P = .0235) after median follow-up of 17 months.
Updated results showed this benefit was maintained after a median of 54 months, with a PFS of 19.5 months in the palbociclib arm, versus 14 months in capecitabine arm (HR 0.744, P = .0357), Dr. Park reported. However, this PFS benefit did not lead to an overall survival (OS) benefit, with median OS being similar: 54.8 versus 57.8 months in the palbociclib and capecitabine groups, respectively (HR = 1.02, P = .92).
To explore why PFS — but not OS — was better in the palbociclib arm, the researchers conducted a multivariate analysis which showed that going on to an additional CDK4/6 inhibitor treatment after the end of the study was as an independent variable favoring OS. Because more patients in the capecitabine arm received a post-study CDK4/6 inhibitor (49.3%) compared with in the palbociclib group (15%), this weighted the OS to the capecitabine arm, Dr. Park explained in an interview.
“In the capecitabine arm, excluding post-study CDK4/6 inhibitor use, the median OS was 38.8 months.” This was inferior to the 49 months OS seen in the palbociclib arm (P = .065), she said.
“As expected, hematologic toxicity was more common in the palbociclib arm compared with in the capecitabine arm,” Dr. Park said (92% vs 86%), with neutropenia topping the list [of all adverse events] (65.2% vs 27.9%, all grades). However, “most [adverse events] were not that serious,” Dr. Park said. Arthralgia was more common in the palbociclib arm (25% vs 7%), and diarrhea and hand-foot syndrome were more common in the capecitabine arm (15.2% vs 39.5% and 79.1% vs 2.2%).
Study Validates Endocrine Therapy + CDK4/6 Inhibitor as First Line
Commenting on Young-PEARL in an interview, Harold Burstein, MD, PhD, said, “The point of this study was to compare whether upfront chemotherapy would be better than upfront hormonal therapy for patients who had metastatic ER positive breast cancer.”
“This is the first study in probably 20 years that has compared these two approaches, and it validated that for the vast majority of patients with ER positive metastatic breast cancer, the appropriate first treatment is endocrine therapy with a CDK4/6 inhibitor,” continued Dr. Burstein, a breast cancer expert at Dana-Farber Cancer Institute, and professor at Harvard Medical School in Boston.
Dr. Park disclosed honoraria from AstraZeneca, Daiichi Sankyo, Eisai, Lilly, MSD, Novartis, Pfizer, and Roche; consulting or advisory roles for AstraZeneca, Boryung, Daiichi Sankyo, Eisai, Gilead Sciences, Lilly, Menarini, MSD, Novartis, Pfizer, and Roche; research funding from AstraZeneca, Gencurix, Genome Insight, NGeneBio, Pfizer; and Roche; and travel/accommodations/expenses from Gilead. Dr. Burstein disclosed a research grant from the National Cancer Institute.
“The combination of palbociclib plus exemestane plus leuprolide showed a consistent significant improvement in PFS [progression-free survival] compared to the capecitabine arm,” Yeon Hee Park, MD, PhD, from Samsung Medical Center, Sungkyunkwan University, Seoul, South Korea, reported at the annual meeting of the American Society of Clinical Oncology.
Study Methods and Results
Young-PEARL, a prospective, multicenter, open-label, randomized phase 2 study, included 184 patients, median age 44 years, who had relapsed or progressed during previous tamoxifen therapy, with one line of previous chemotherapy for mBC allowed. Patients were randomized to palbociclib plus endocrine therapy (oral palbociclib 125 mg per day for 21 days every 4 weeks, oral exemestane 25 mg per day for 28 days, plus leuprolide 3.75 mg subcutaneously every 4 weeks) or chemotherapy (oral capecitabine 1250 mg/m2, twice daily for 2 weeks every 3 weeks).
Previously published initial results (Lancet Oncol. 2019 Dec;20[12]:1750-1759) for the primary endpoint showed a median PFS of 20.1 months in the palbociclib group versus 14.4 months in the capecitabine group, (hazard ratio [HR] 0.659, P = .0235) after median follow-up of 17 months.
Updated results showed this benefit was maintained after a median of 54 months, with a PFS of 19.5 months in the palbociclib arm, versus 14 months in capecitabine arm (HR 0.744, P = .0357), Dr. Park reported. However, this PFS benefit did not lead to an overall survival (OS) benefit, with median OS being similar: 54.8 versus 57.8 months in the palbociclib and capecitabine groups, respectively (HR = 1.02, P = .92).
To explore why PFS — but not OS — was better in the palbociclib arm, the researchers conducted a multivariate analysis which showed that going on to an additional CDK4/6 inhibitor treatment after the end of the study was as an independent variable favoring OS. Because more patients in the capecitabine arm received a post-study CDK4/6 inhibitor (49.3%) compared with in the palbociclib group (15%), this weighted the OS to the capecitabine arm, Dr. Park explained in an interview.
“In the capecitabine arm, excluding post-study CDK4/6 inhibitor use, the median OS was 38.8 months.” This was inferior to the 49 months OS seen in the palbociclib arm (P = .065), she said.
“As expected, hematologic toxicity was more common in the palbociclib arm compared with in the capecitabine arm,” Dr. Park said (92% vs 86%), with neutropenia topping the list [of all adverse events] (65.2% vs 27.9%, all grades). However, “most [adverse events] were not that serious,” Dr. Park said. Arthralgia was more common in the palbociclib arm (25% vs 7%), and diarrhea and hand-foot syndrome were more common in the capecitabine arm (15.2% vs 39.5% and 79.1% vs 2.2%).
Study Validates Endocrine Therapy + CDK4/6 Inhibitor as First Line
Commenting on Young-PEARL in an interview, Harold Burstein, MD, PhD, said, “The point of this study was to compare whether upfront chemotherapy would be better than upfront hormonal therapy for patients who had metastatic ER positive breast cancer.”
“This is the first study in probably 20 years that has compared these two approaches, and it validated that for the vast majority of patients with ER positive metastatic breast cancer, the appropriate first treatment is endocrine therapy with a CDK4/6 inhibitor,” continued Dr. Burstein, a breast cancer expert at Dana-Farber Cancer Institute, and professor at Harvard Medical School in Boston.
Dr. Park disclosed honoraria from AstraZeneca, Daiichi Sankyo, Eisai, Lilly, MSD, Novartis, Pfizer, and Roche; consulting or advisory roles for AstraZeneca, Boryung, Daiichi Sankyo, Eisai, Gilead Sciences, Lilly, Menarini, MSD, Novartis, Pfizer, and Roche; research funding from AstraZeneca, Gencurix, Genome Insight, NGeneBio, Pfizer; and Roche; and travel/accommodations/expenses from Gilead. Dr. Burstein disclosed a research grant from the National Cancer Institute.
FROM ASCO 2024
New Canadian BC Guidelines Emphasize Personal Choice
The draft guidelines stem from a review of more than 165 recent randomized controlled trials, observational studies, mathematical models, and other data.
The guideline working group included four breast cancer experts (a medical oncologist, a radiation oncologist, a surgical oncologist, and a radiologist), three patient partners, six family physicians, a nurse practitioner, evidence review teams, and other experts.
To avoid potential conflicts of interest, the oncologists provided input but did not vote on the final recommendations, Guylène Thériault, MD, a family physician and chair of the task force and Breast Cancer Working Group, said in an interview.
The guideline recommends that, after the potential benefits and harms of screening have been considered, mammography should be accessible every 2-3 years to women (ie, people assigned female at birth) between ages 40 and 74 years who are at average or moderately increased risk.
Women with a personal or extensive family history of breast cancer or genetic mutations that would increase breast cancer risk; those who have symptoms, such as a lump; those who feel they may be at high risk; and those who are transgender women should consult a healthcare provider about appropriate options, according to the updated guidelines, which do not apply to these patients.
The draft guidelines were published online on May 30 and are open for public comment until August 30.
‘Three Big Questions’
To develop the guidelines, the work group asked “three big questions,” said Dr. Thériault. The first was the effectiveness of breast cancer screening for women aged 40 years and over. For this question, this systematic review, unlike the 2018 guideline update, included not only randomized trials but also observational data to ensure that the work group considered all available data.
“The second question was about comparative effectiveness,” which is something the United States considered for the latest US Preventive Services Task Force (USPSTF) update, said Dr. Thériault. The USPSTF asked questions such as “What happens if we start screening patients at age 40 years? Or at age 50 years? What happens if we stop at age 74 years? Or if we use different tests such as 3D versus digital mammography?”
The Canadian Task Force relied on the evidence that the USPSTF found after grading it with its own criteria, she said. The results were similar, and so are the recommendations in this area. “For example, we don’t recommend supplementary screening for women with dense breasts because there are no studies to inform patient-oriented benefits.”
The third question was about the values and preferences of women regarding breast cancer screening, which is something the United States didn’t examine. “We had looked at that issue in 2018, and this time around, even though we expanded the type of studies, we got the same message: That there are differences between women in their 40s and those who are age 50 years and over.”
“The majority of women in their 40s think that the harms outweigh the benefits and are not interested in screening,” said Dr. Thériault. “But when I say the majority, that’s not every woman. So, we had to recognize that there is variability. And the majority, but not all, of women ages 50-74 years thinks the benefits are higher than the harms. That’s why we say in our recommendation that from ages 40 to 74, it’s a personal choice.”
Responding to Objections
Not surprisingly, the task force has heard objections to its draft guidelines. The first is that women aged 40-49 years are being denied mammograms, said Michelle Nadler, MD, a medical oncologist at Princess Margaret Cancer Centre in Toronto, Canada. “This [objection] has attained a lot of media coverage, which is unfortunate, because people who have not read the guidelines may believe this is true. The guidelines clearly state that an eligible, informed woman of this age group who wants a screening mammogram should receive one.”
The second commonly heard objection is that the task force is overestimating the harms of screening, such as anxiety and overdiagnosis, she said. But an outcome of “anxiety” was not factored into the guideline. Overdiagnosis was calculated on the basis of the literature, and estimates were converted to a common denominator so that they could be compared, said Dr. Nadler. The same was true of benefits.
Another objection was that screening could mean less need for chemotherapy or full axillary dissection, Dr. Nadler said. However, the task force did not find any primary studies that evaluated these outcomes.
Critics also said that the recommendations do not account for racial or ethnic variations. Although more research is likely needed in this area, “the task force states that individuals should be informed of all of their breast cancer risk factors, including race/ethnicity, and that this should be factored into decisions about screening,” said Dr. Nadler.
“I was very surprised that the task force was accused by some parties of paternalism,” added René Wittmer, MD, adjunct clinical professor of family medicine at the University of Montreal and chair of Choosing Wisely Quebec, Montreal, Canada. “In my opinion, the importance they place on shared decision-making is contrary to medical paternalism and aims to empower women to make a decision that fits with their values and preferences.”
Nevertheless, the inclusion of modeling studies and observational trials “may cause the potential benefits to be amplified, compared with what is seen in randomized controlled trials,” he said in an interview.
Decision Aids Help
Once the guidelines are finalized, decision aids will be available to patients and providers to help guide screening discussions, said Dr. Nadler. “Primary care providers need to be aware of an individual’s personal risk factors for breast cancer to know if they are at average, above average, or high lifetime risk of breast cancer. These guidelines do not apply to those with > 20% lifetime risk of breast cancer.”
“The standards for risk communication are in absolute numbers over a common denominator,” she noted. “This is how primary care providers discuss other important primary care topics like smoking cessation, cardiovascular disease (and decisions about statin medications), and osteoporosis risk. These same standards should apply for breast cancer screening.”
Furthermore, she said, providers “should be aware that individuals from marginalized communities may benefit from more than one conversation until they are able to make a decision about screening that is right for them.”
“There is good evidence showing that most advances we’ve seen in breast cancer outcomes (ie, reduction in breast cancer mortality) are likely due to improvements in treatment, not screening,” said Dr. Wittmer. “In fact, mortality reductions are seen even in age groups or countries where there is no routine screening. This means that women benefit from advances in treatments, whether they choose to get screened or not.”
‘Mammography Saves Lives’
Commenting on the updated guidelines, Janie Lee, MD, professor of radiology at the University of Washington School of Medicine and director of breast imaging at the Fred Hutchinson Cancer Center, both in Seattle, said: “For the USPSTF, benefits of life years gained were also considered, in addition to breast cancer deaths averted. To save more lives from breast cancer, guidelines may focus on screening women at older ages, when annual rates of breast cancer are higher.” By contrast, when thinking in terms of years of life saved, focusing on screening younger women, who have more years of life left, increases benefits. “Both are important outcomes that we want to improve with effective screening.”
That said, “we should follow the guidelines of our specific national organizations,” she continued. “Overall populations and healthcare systems are different between the US and Canada.”
For example, “the USPSTF specifically highlighted the potential for reducing breast cancer mortality in Black women, who are more likely to develop biologically aggressive tumors that are diagnosed at more advanced stages, when making updated recommendations earlier this year,” she said. “The Canadian guidelines did not make specific recommendations by race or ethnicity group, instead highlighting the need for more research on the impact of screening in these groups.”
In addition, “screening every year versus every other year is more routine in the US compared with Canada,” she noted. And nonmedical factors that influence health and that may influence access to medical care and timely diagnosis of breast cancer “may be different between our two countries.”
“The most important take-home message is that the scientific evidence is strong that screening mammography saves lives,” said Dr. Lee. “These new recommendations will hopefully result in more early diagnoses of breast cancer and save more lives. Screening works best when it’s used regularly, regardless of how frequently you return. Once you start screening, please urge your patients to plan to return.”
Dr. Nadler disclosed speaker honoraria and consulting fees from Novartis and Exact Sciences outside the scope of this interview and innovation funding from the NSH/UHN AMO Innovation Fund Competition for Developing and Implementing a Consensus Recommendation for Breast Cancer Screening Best Practices. Dr. Thériault is chair of the task force and chair of the working group for the draft guidelines. Dr. Wittmer is chair of Choosing Wisely Quebec. Dr. Lee reported no relevant financial relationships related to her interview.
A version of this article appeared on Medscape.com.
The draft guidelines stem from a review of more than 165 recent randomized controlled trials, observational studies, mathematical models, and other data.
The guideline working group included four breast cancer experts (a medical oncologist, a radiation oncologist, a surgical oncologist, and a radiologist), three patient partners, six family physicians, a nurse practitioner, evidence review teams, and other experts.
To avoid potential conflicts of interest, the oncologists provided input but did not vote on the final recommendations, Guylène Thériault, MD, a family physician and chair of the task force and Breast Cancer Working Group, said in an interview.
The guideline recommends that, after the potential benefits and harms of screening have been considered, mammography should be accessible every 2-3 years to women (ie, people assigned female at birth) between ages 40 and 74 years who are at average or moderately increased risk.
Women with a personal or extensive family history of breast cancer or genetic mutations that would increase breast cancer risk; those who have symptoms, such as a lump; those who feel they may be at high risk; and those who are transgender women should consult a healthcare provider about appropriate options, according to the updated guidelines, which do not apply to these patients.
The draft guidelines were published online on May 30 and are open for public comment until August 30.
‘Three Big Questions’
To develop the guidelines, the work group asked “three big questions,” said Dr. Thériault. The first was the effectiveness of breast cancer screening for women aged 40 years and over. For this question, this systematic review, unlike the 2018 guideline update, included not only randomized trials but also observational data to ensure that the work group considered all available data.
“The second question was about comparative effectiveness,” which is something the United States considered for the latest US Preventive Services Task Force (USPSTF) update, said Dr. Thériault. The USPSTF asked questions such as “What happens if we start screening patients at age 40 years? Or at age 50 years? What happens if we stop at age 74 years? Or if we use different tests such as 3D versus digital mammography?”
The Canadian Task Force relied on the evidence that the USPSTF found after grading it with its own criteria, she said. The results were similar, and so are the recommendations in this area. “For example, we don’t recommend supplementary screening for women with dense breasts because there are no studies to inform patient-oriented benefits.”
The third question was about the values and preferences of women regarding breast cancer screening, which is something the United States didn’t examine. “We had looked at that issue in 2018, and this time around, even though we expanded the type of studies, we got the same message: That there are differences between women in their 40s and those who are age 50 years and over.”
“The majority of women in their 40s think that the harms outweigh the benefits and are not interested in screening,” said Dr. Thériault. “But when I say the majority, that’s not every woman. So, we had to recognize that there is variability. And the majority, but not all, of women ages 50-74 years thinks the benefits are higher than the harms. That’s why we say in our recommendation that from ages 40 to 74, it’s a personal choice.”
Responding to Objections
Not surprisingly, the task force has heard objections to its draft guidelines. The first is that women aged 40-49 years are being denied mammograms, said Michelle Nadler, MD, a medical oncologist at Princess Margaret Cancer Centre in Toronto, Canada. “This [objection] has attained a lot of media coverage, which is unfortunate, because people who have not read the guidelines may believe this is true. The guidelines clearly state that an eligible, informed woman of this age group who wants a screening mammogram should receive one.”
The second commonly heard objection is that the task force is overestimating the harms of screening, such as anxiety and overdiagnosis, she said. But an outcome of “anxiety” was not factored into the guideline. Overdiagnosis was calculated on the basis of the literature, and estimates were converted to a common denominator so that they could be compared, said Dr. Nadler. The same was true of benefits.
Another objection was that screening could mean less need for chemotherapy or full axillary dissection, Dr. Nadler said. However, the task force did not find any primary studies that evaluated these outcomes.
Critics also said that the recommendations do not account for racial or ethnic variations. Although more research is likely needed in this area, “the task force states that individuals should be informed of all of their breast cancer risk factors, including race/ethnicity, and that this should be factored into decisions about screening,” said Dr. Nadler.
“I was very surprised that the task force was accused by some parties of paternalism,” added René Wittmer, MD, adjunct clinical professor of family medicine at the University of Montreal and chair of Choosing Wisely Quebec, Montreal, Canada. “In my opinion, the importance they place on shared decision-making is contrary to medical paternalism and aims to empower women to make a decision that fits with their values and preferences.”
Nevertheless, the inclusion of modeling studies and observational trials “may cause the potential benefits to be amplified, compared with what is seen in randomized controlled trials,” he said in an interview.
Decision Aids Help
Once the guidelines are finalized, decision aids will be available to patients and providers to help guide screening discussions, said Dr. Nadler. “Primary care providers need to be aware of an individual’s personal risk factors for breast cancer to know if they are at average, above average, or high lifetime risk of breast cancer. These guidelines do not apply to those with > 20% lifetime risk of breast cancer.”
“The standards for risk communication are in absolute numbers over a common denominator,” she noted. “This is how primary care providers discuss other important primary care topics like smoking cessation, cardiovascular disease (and decisions about statin medications), and osteoporosis risk. These same standards should apply for breast cancer screening.”
Furthermore, she said, providers “should be aware that individuals from marginalized communities may benefit from more than one conversation until they are able to make a decision about screening that is right for them.”
“There is good evidence showing that most advances we’ve seen in breast cancer outcomes (ie, reduction in breast cancer mortality) are likely due to improvements in treatment, not screening,” said Dr. Wittmer. “In fact, mortality reductions are seen even in age groups or countries where there is no routine screening. This means that women benefit from advances in treatments, whether they choose to get screened or not.”
‘Mammography Saves Lives’
Commenting on the updated guidelines, Janie Lee, MD, professor of radiology at the University of Washington School of Medicine and director of breast imaging at the Fred Hutchinson Cancer Center, both in Seattle, said: “For the USPSTF, benefits of life years gained were also considered, in addition to breast cancer deaths averted. To save more lives from breast cancer, guidelines may focus on screening women at older ages, when annual rates of breast cancer are higher.” By contrast, when thinking in terms of years of life saved, focusing on screening younger women, who have more years of life left, increases benefits. “Both are important outcomes that we want to improve with effective screening.”
That said, “we should follow the guidelines of our specific national organizations,” she continued. “Overall populations and healthcare systems are different between the US and Canada.”
For example, “the USPSTF specifically highlighted the potential for reducing breast cancer mortality in Black women, who are more likely to develop biologically aggressive tumors that are diagnosed at more advanced stages, when making updated recommendations earlier this year,” she said. “The Canadian guidelines did not make specific recommendations by race or ethnicity group, instead highlighting the need for more research on the impact of screening in these groups.”
In addition, “screening every year versus every other year is more routine in the US compared with Canada,” she noted. And nonmedical factors that influence health and that may influence access to medical care and timely diagnosis of breast cancer “may be different between our two countries.”
“The most important take-home message is that the scientific evidence is strong that screening mammography saves lives,” said Dr. Lee. “These new recommendations will hopefully result in more early diagnoses of breast cancer and save more lives. Screening works best when it’s used regularly, regardless of how frequently you return. Once you start screening, please urge your patients to plan to return.”
Dr. Nadler disclosed speaker honoraria and consulting fees from Novartis and Exact Sciences outside the scope of this interview and innovation funding from the NSH/UHN AMO Innovation Fund Competition for Developing and Implementing a Consensus Recommendation for Breast Cancer Screening Best Practices. Dr. Thériault is chair of the task force and chair of the working group for the draft guidelines. Dr. Wittmer is chair of Choosing Wisely Quebec. Dr. Lee reported no relevant financial relationships related to her interview.
A version of this article appeared on Medscape.com.
The draft guidelines stem from a review of more than 165 recent randomized controlled trials, observational studies, mathematical models, and other data.
The guideline working group included four breast cancer experts (a medical oncologist, a radiation oncologist, a surgical oncologist, and a radiologist), three patient partners, six family physicians, a nurse practitioner, evidence review teams, and other experts.
To avoid potential conflicts of interest, the oncologists provided input but did not vote on the final recommendations, Guylène Thériault, MD, a family physician and chair of the task force and Breast Cancer Working Group, said in an interview.
The guideline recommends that, after the potential benefits and harms of screening have been considered, mammography should be accessible every 2-3 years to women (ie, people assigned female at birth) between ages 40 and 74 years who are at average or moderately increased risk.
Women with a personal or extensive family history of breast cancer or genetic mutations that would increase breast cancer risk; those who have symptoms, such as a lump; those who feel they may be at high risk; and those who are transgender women should consult a healthcare provider about appropriate options, according to the updated guidelines, which do not apply to these patients.
The draft guidelines were published online on May 30 and are open for public comment until August 30.
‘Three Big Questions’
To develop the guidelines, the work group asked “three big questions,” said Dr. Thériault. The first was the effectiveness of breast cancer screening for women aged 40 years and over. For this question, this systematic review, unlike the 2018 guideline update, included not only randomized trials but also observational data to ensure that the work group considered all available data.
“The second question was about comparative effectiveness,” which is something the United States considered for the latest US Preventive Services Task Force (USPSTF) update, said Dr. Thériault. The USPSTF asked questions such as “What happens if we start screening patients at age 40 years? Or at age 50 years? What happens if we stop at age 74 years? Or if we use different tests such as 3D versus digital mammography?”
The Canadian Task Force relied on the evidence that the USPSTF found after grading it with its own criteria, she said. The results were similar, and so are the recommendations in this area. “For example, we don’t recommend supplementary screening for women with dense breasts because there are no studies to inform patient-oriented benefits.”
The third question was about the values and preferences of women regarding breast cancer screening, which is something the United States didn’t examine. “We had looked at that issue in 2018, and this time around, even though we expanded the type of studies, we got the same message: That there are differences between women in their 40s and those who are age 50 years and over.”
“The majority of women in their 40s think that the harms outweigh the benefits and are not interested in screening,” said Dr. Thériault. “But when I say the majority, that’s not every woman. So, we had to recognize that there is variability. And the majority, but not all, of women ages 50-74 years thinks the benefits are higher than the harms. That’s why we say in our recommendation that from ages 40 to 74, it’s a personal choice.”
Responding to Objections
Not surprisingly, the task force has heard objections to its draft guidelines. The first is that women aged 40-49 years are being denied mammograms, said Michelle Nadler, MD, a medical oncologist at Princess Margaret Cancer Centre in Toronto, Canada. “This [objection] has attained a lot of media coverage, which is unfortunate, because people who have not read the guidelines may believe this is true. The guidelines clearly state that an eligible, informed woman of this age group who wants a screening mammogram should receive one.”
The second commonly heard objection is that the task force is overestimating the harms of screening, such as anxiety and overdiagnosis, she said. But an outcome of “anxiety” was not factored into the guideline. Overdiagnosis was calculated on the basis of the literature, and estimates were converted to a common denominator so that they could be compared, said Dr. Nadler. The same was true of benefits.
Another objection was that screening could mean less need for chemotherapy or full axillary dissection, Dr. Nadler said. However, the task force did not find any primary studies that evaluated these outcomes.
Critics also said that the recommendations do not account for racial or ethnic variations. Although more research is likely needed in this area, “the task force states that individuals should be informed of all of their breast cancer risk factors, including race/ethnicity, and that this should be factored into decisions about screening,” said Dr. Nadler.
“I was very surprised that the task force was accused by some parties of paternalism,” added René Wittmer, MD, adjunct clinical professor of family medicine at the University of Montreal and chair of Choosing Wisely Quebec, Montreal, Canada. “In my opinion, the importance they place on shared decision-making is contrary to medical paternalism and aims to empower women to make a decision that fits with their values and preferences.”
Nevertheless, the inclusion of modeling studies and observational trials “may cause the potential benefits to be amplified, compared with what is seen in randomized controlled trials,” he said in an interview.
Decision Aids Help
Once the guidelines are finalized, decision aids will be available to patients and providers to help guide screening discussions, said Dr. Nadler. “Primary care providers need to be aware of an individual’s personal risk factors for breast cancer to know if they are at average, above average, or high lifetime risk of breast cancer. These guidelines do not apply to those with > 20% lifetime risk of breast cancer.”
“The standards for risk communication are in absolute numbers over a common denominator,” she noted. “This is how primary care providers discuss other important primary care topics like smoking cessation, cardiovascular disease (and decisions about statin medications), and osteoporosis risk. These same standards should apply for breast cancer screening.”
Furthermore, she said, providers “should be aware that individuals from marginalized communities may benefit from more than one conversation until they are able to make a decision about screening that is right for them.”
“There is good evidence showing that most advances we’ve seen in breast cancer outcomes (ie, reduction in breast cancer mortality) are likely due to improvements in treatment, not screening,” said Dr. Wittmer. “In fact, mortality reductions are seen even in age groups or countries where there is no routine screening. This means that women benefit from advances in treatments, whether they choose to get screened or not.”
‘Mammography Saves Lives’
Commenting on the updated guidelines, Janie Lee, MD, professor of radiology at the University of Washington School of Medicine and director of breast imaging at the Fred Hutchinson Cancer Center, both in Seattle, said: “For the USPSTF, benefits of life years gained were also considered, in addition to breast cancer deaths averted. To save more lives from breast cancer, guidelines may focus on screening women at older ages, when annual rates of breast cancer are higher.” By contrast, when thinking in terms of years of life saved, focusing on screening younger women, who have more years of life left, increases benefits. “Both are important outcomes that we want to improve with effective screening.”
That said, “we should follow the guidelines of our specific national organizations,” she continued. “Overall populations and healthcare systems are different between the US and Canada.”
For example, “the USPSTF specifically highlighted the potential for reducing breast cancer mortality in Black women, who are more likely to develop biologically aggressive tumors that are diagnosed at more advanced stages, when making updated recommendations earlier this year,” she said. “The Canadian guidelines did not make specific recommendations by race or ethnicity group, instead highlighting the need for more research on the impact of screening in these groups.”
In addition, “screening every year versus every other year is more routine in the US compared with Canada,” she noted. And nonmedical factors that influence health and that may influence access to medical care and timely diagnosis of breast cancer “may be different between our two countries.”
“The most important take-home message is that the scientific evidence is strong that screening mammography saves lives,” said Dr. Lee. “These new recommendations will hopefully result in more early diagnoses of breast cancer and save more lives. Screening works best when it’s used regularly, regardless of how frequently you return. Once you start screening, please urge your patients to plan to return.”
Dr. Nadler disclosed speaker honoraria and consulting fees from Novartis and Exact Sciences outside the scope of this interview and innovation funding from the NSH/UHN AMO Innovation Fund Competition for Developing and Implementing a Consensus Recommendation for Breast Cancer Screening Best Practices. Dr. Thériault is chair of the task force and chair of the working group for the draft guidelines. Dr. Wittmer is chair of Choosing Wisely Quebec. Dr. Lee reported no relevant financial relationships related to her interview.
A version of this article appeared on Medscape.com.
Should Cancer Trial Eligibility Become More Inclusive?
The study, published online in Clinical Cancer Research, highlighted the potential benefits of broadening eligibility criteria for clinical trials.
“It is well known that results in an ‘ideal’ population do not always translate to the real-world population,” senior author Hans Gelderblom, MD, chair of the Department of Medical Oncology at the Leiden University Medical Center, Leiden, the Netherlands, said in a press release. “Eligibility criteria are often too strict, and educated exemptions by experienced investigators can help individual patients, especially in a last-resort trial.”
Although experts have expressed interest in improving trial inclusivity, it’s unclear how doing so might impact treatment safety and efficacy.
In the Drug Rediscovery Protocol (DRUP), Dr. Gelderblom and colleagues examined the impact of broadening trial eligibility on patient outcomes. DRUP is an ongoing Dutch national, multicenter, pan-cancer, nonrandomized clinical trial in which patients are treated off-label with approved molecularly targeted or immunotherapies.
In the trial, 1019 patients with treatment-refractory disease were matched to one of the available study drugs based on their tumor molecular profile and enrolled in parallel cohorts. Cohorts were defined by tumor type, molecular profile, and study drug.
Among these patients, 82 patients — 8% of the cohort — were granted waivers to participate. Most waivers (45%) were granted as exceptions to general- or drug-related eligibility criteria, often because of out-of-range lab results. Other categories included treatment and testing exceptions, as well as out-of-window testing.
The researchers then compared safety and efficacy outcomes between the 82 participants granted waivers and the 937 who did not receive waivers.
Overall, Dr. Gelderblom’s team found that the rate of serious adverse events was similar between patients who received a waiver and those who did not: 39% vs 41%, respectively.
A relationship between waivers and serious adverse events was deemed “unlikely” for 86% of patients and “possible” for 14%. In two cases concerning a direct relationship, for instance, patients who received waivers for decreased hemoglobin levels developed anemia.
The rate of clinical benefit — defined as an objective response or stable disease for at least 16 weeks — was similar between the groups. Overall, 40% of patients who received a waiver (33 of 82) had a clinical benefit vs 33% of patients without a waiver (P = .43). Median overall survival for patients that received a waiver was also similar — 11 months in the waiver group and 8 months in the nonwaiver group (hazard ratio, 0.87; P = .33).
“Safety and clinical benefit were preserved in patients for whom a waiver was granted,” the authors concluded.
The study had several limitations. The diversity of cancer types, treatments, and reasons for protocol exemptions precluded subgroup analyses. In addition, because the decision to grant waivers depended in large part on the likelihood of clinical benefit, “it is possible that patients who received waivers were positively selected for clinical benefit compared with the general study population,” the authors wrote.
So, “although the clinical benefit rate of the patient group for whom a waiver was granted appears to be slightly higher, this difference might be explained by the selection process of the central study team, in which each waiver request was carefully considered, weighing the risks and potential benefits for the patient in question,” the authors explained.
Overall, “these findings advocate for a broader and more inclusive design when establishing novel trials, paving the way for a more effective and tailored application of cancer therapies in patients with advanced or refractory disease,” Dr. Gelderblom said.
Commenting on the study, Bishal Gyawali, MD, PhD, said that “relaxing eligibility criteria is important, and I support this. Trials should include patients that are more representative of the real-world, so that results are generalizable.”
However, “the paper overemphasized efficacy,” said Dr. Gyawali, from Queen’s University, Kingston, Ontario, Canada. The sample size of waiver-granted patients was small, plus “the clinical benefit rate is not a marker of efficacy.
“The response rate is somewhat better, but for a heterogeneous study with multiple targets and drugs, it is difficult to say much about treatment effects here,” Dr. Gyawali added. Overall, “we shouldn’t read too much into treatment benefits based on these numbers.”
Funding for the study was provided by the Stelvio for Life Foundation, the Dutch Cancer Society, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, pharma&, Eisai Co., Ipsen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. Dr. Gelderblom declared no conflicts of interest, and Dr. Gyawali declared no conflicts of interest related to his comment.
A version of this article appeared on Medscape.com.
The study, published online in Clinical Cancer Research, highlighted the potential benefits of broadening eligibility criteria for clinical trials.
“It is well known that results in an ‘ideal’ population do not always translate to the real-world population,” senior author Hans Gelderblom, MD, chair of the Department of Medical Oncology at the Leiden University Medical Center, Leiden, the Netherlands, said in a press release. “Eligibility criteria are often too strict, and educated exemptions by experienced investigators can help individual patients, especially in a last-resort trial.”
Although experts have expressed interest in improving trial inclusivity, it’s unclear how doing so might impact treatment safety and efficacy.
In the Drug Rediscovery Protocol (DRUP), Dr. Gelderblom and colleagues examined the impact of broadening trial eligibility on patient outcomes. DRUP is an ongoing Dutch national, multicenter, pan-cancer, nonrandomized clinical trial in which patients are treated off-label with approved molecularly targeted or immunotherapies.
In the trial, 1019 patients with treatment-refractory disease were matched to one of the available study drugs based on their tumor molecular profile and enrolled in parallel cohorts. Cohorts were defined by tumor type, molecular profile, and study drug.
Among these patients, 82 patients — 8% of the cohort — were granted waivers to participate. Most waivers (45%) were granted as exceptions to general- or drug-related eligibility criteria, often because of out-of-range lab results. Other categories included treatment and testing exceptions, as well as out-of-window testing.
The researchers then compared safety and efficacy outcomes between the 82 participants granted waivers and the 937 who did not receive waivers.
Overall, Dr. Gelderblom’s team found that the rate of serious adverse events was similar between patients who received a waiver and those who did not: 39% vs 41%, respectively.
A relationship between waivers and serious adverse events was deemed “unlikely” for 86% of patients and “possible” for 14%. In two cases concerning a direct relationship, for instance, patients who received waivers for decreased hemoglobin levels developed anemia.
The rate of clinical benefit — defined as an objective response or stable disease for at least 16 weeks — was similar between the groups. Overall, 40% of patients who received a waiver (33 of 82) had a clinical benefit vs 33% of patients without a waiver (P = .43). Median overall survival for patients that received a waiver was also similar — 11 months in the waiver group and 8 months in the nonwaiver group (hazard ratio, 0.87; P = .33).
“Safety and clinical benefit were preserved in patients for whom a waiver was granted,” the authors concluded.
The study had several limitations. The diversity of cancer types, treatments, and reasons for protocol exemptions precluded subgroup analyses. In addition, because the decision to grant waivers depended in large part on the likelihood of clinical benefit, “it is possible that patients who received waivers were positively selected for clinical benefit compared with the general study population,” the authors wrote.
So, “although the clinical benefit rate of the patient group for whom a waiver was granted appears to be slightly higher, this difference might be explained by the selection process of the central study team, in which each waiver request was carefully considered, weighing the risks and potential benefits for the patient in question,” the authors explained.
Overall, “these findings advocate for a broader and more inclusive design when establishing novel trials, paving the way for a more effective and tailored application of cancer therapies in patients with advanced or refractory disease,” Dr. Gelderblom said.
Commenting on the study, Bishal Gyawali, MD, PhD, said that “relaxing eligibility criteria is important, and I support this. Trials should include patients that are more representative of the real-world, so that results are generalizable.”
However, “the paper overemphasized efficacy,” said Dr. Gyawali, from Queen’s University, Kingston, Ontario, Canada. The sample size of waiver-granted patients was small, plus “the clinical benefit rate is not a marker of efficacy.
“The response rate is somewhat better, but for a heterogeneous study with multiple targets and drugs, it is difficult to say much about treatment effects here,” Dr. Gyawali added. Overall, “we shouldn’t read too much into treatment benefits based on these numbers.”
Funding for the study was provided by the Stelvio for Life Foundation, the Dutch Cancer Society, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, pharma&, Eisai Co., Ipsen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. Dr. Gelderblom declared no conflicts of interest, and Dr. Gyawali declared no conflicts of interest related to his comment.
A version of this article appeared on Medscape.com.
The study, published online in Clinical Cancer Research, highlighted the potential benefits of broadening eligibility criteria for clinical trials.
“It is well known that results in an ‘ideal’ population do not always translate to the real-world population,” senior author Hans Gelderblom, MD, chair of the Department of Medical Oncology at the Leiden University Medical Center, Leiden, the Netherlands, said in a press release. “Eligibility criteria are often too strict, and educated exemptions by experienced investigators can help individual patients, especially in a last-resort trial.”
Although experts have expressed interest in improving trial inclusivity, it’s unclear how doing so might impact treatment safety and efficacy.
In the Drug Rediscovery Protocol (DRUP), Dr. Gelderblom and colleagues examined the impact of broadening trial eligibility on patient outcomes. DRUP is an ongoing Dutch national, multicenter, pan-cancer, nonrandomized clinical trial in which patients are treated off-label with approved molecularly targeted or immunotherapies.
In the trial, 1019 patients with treatment-refractory disease were matched to one of the available study drugs based on their tumor molecular profile and enrolled in parallel cohorts. Cohorts were defined by tumor type, molecular profile, and study drug.
Among these patients, 82 patients — 8% of the cohort — were granted waivers to participate. Most waivers (45%) were granted as exceptions to general- or drug-related eligibility criteria, often because of out-of-range lab results. Other categories included treatment and testing exceptions, as well as out-of-window testing.
The researchers then compared safety and efficacy outcomes between the 82 participants granted waivers and the 937 who did not receive waivers.
Overall, Dr. Gelderblom’s team found that the rate of serious adverse events was similar between patients who received a waiver and those who did not: 39% vs 41%, respectively.
A relationship between waivers and serious adverse events was deemed “unlikely” for 86% of patients and “possible” for 14%. In two cases concerning a direct relationship, for instance, patients who received waivers for decreased hemoglobin levels developed anemia.
The rate of clinical benefit — defined as an objective response or stable disease for at least 16 weeks — was similar between the groups. Overall, 40% of patients who received a waiver (33 of 82) had a clinical benefit vs 33% of patients without a waiver (P = .43). Median overall survival for patients that received a waiver was also similar — 11 months in the waiver group and 8 months in the nonwaiver group (hazard ratio, 0.87; P = .33).
“Safety and clinical benefit were preserved in patients for whom a waiver was granted,” the authors concluded.
The study had several limitations. The diversity of cancer types, treatments, and reasons for protocol exemptions precluded subgroup analyses. In addition, because the decision to grant waivers depended in large part on the likelihood of clinical benefit, “it is possible that patients who received waivers were positively selected for clinical benefit compared with the general study population,” the authors wrote.
So, “although the clinical benefit rate of the patient group for whom a waiver was granted appears to be slightly higher, this difference might be explained by the selection process of the central study team, in which each waiver request was carefully considered, weighing the risks and potential benefits for the patient in question,” the authors explained.
Overall, “these findings advocate for a broader and more inclusive design when establishing novel trials, paving the way for a more effective and tailored application of cancer therapies in patients with advanced or refractory disease,” Dr. Gelderblom said.
Commenting on the study, Bishal Gyawali, MD, PhD, said that “relaxing eligibility criteria is important, and I support this. Trials should include patients that are more representative of the real-world, so that results are generalizable.”
However, “the paper overemphasized efficacy,” said Dr. Gyawali, from Queen’s University, Kingston, Ontario, Canada. The sample size of waiver-granted patients was small, plus “the clinical benefit rate is not a marker of efficacy.
“The response rate is somewhat better, but for a heterogeneous study with multiple targets and drugs, it is difficult to say much about treatment effects here,” Dr. Gyawali added. Overall, “we shouldn’t read too much into treatment benefits based on these numbers.”
Funding for the study was provided by the Stelvio for Life Foundation, the Dutch Cancer Society, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, pharma&, Eisai Co., Ipsen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. Dr. Gelderblom declared no conflicts of interest, and Dr. Gyawali declared no conflicts of interest related to his comment.
A version of this article appeared on Medscape.com.


