User login
Study Reports Safety Data in Children on JAK Inhibitors
TOPLINE:
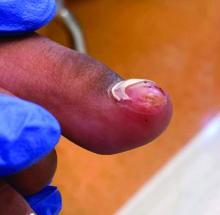
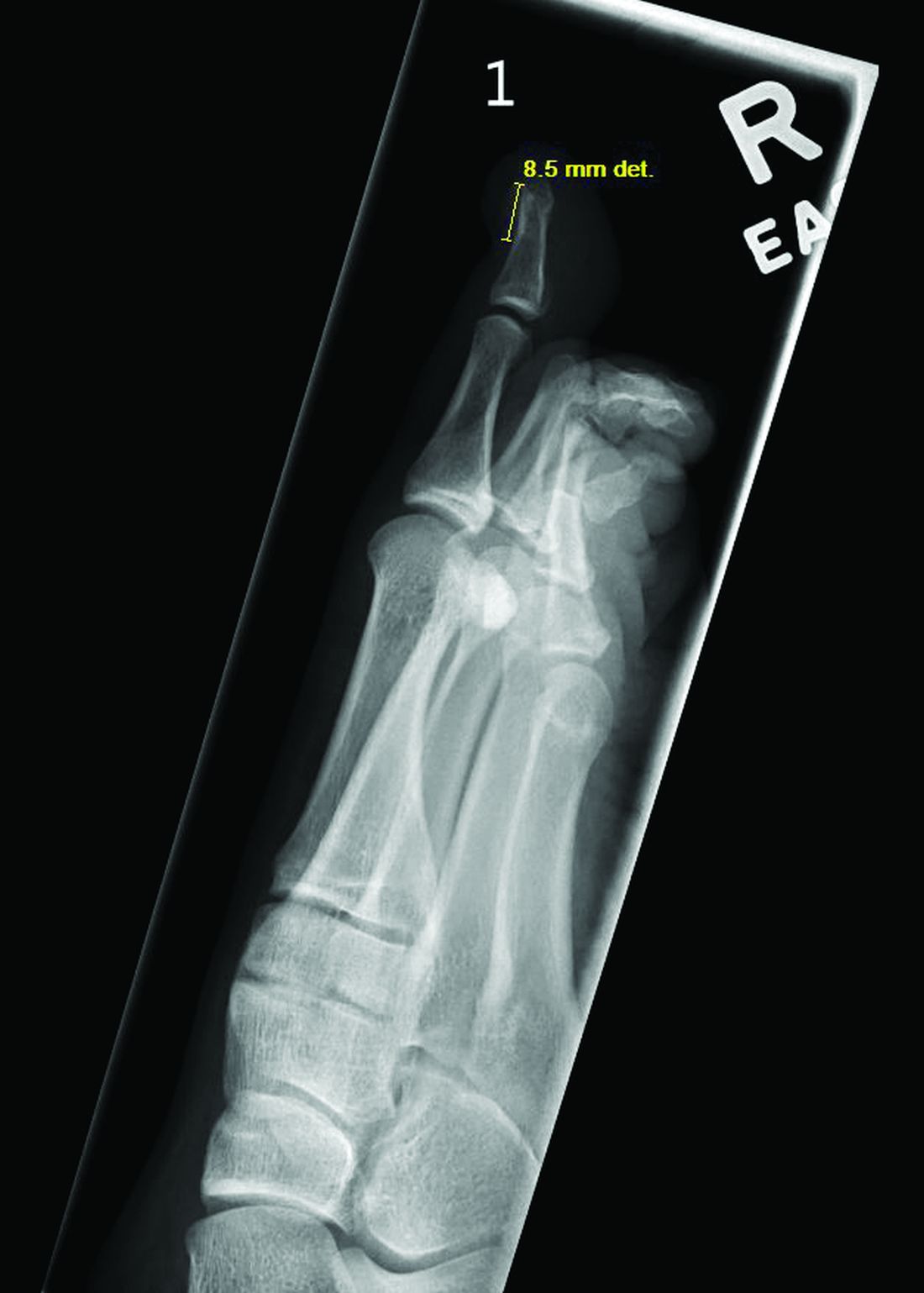
which also found that acne was the most common skin-related AE in children, and serious AEs were less common.
METHODOLOGY:
- Researchers analyzed 399,649 AEs in 133,216 adult patients and 2883 AEs in 955 pediatric patients (age, < 18 years) from November 2011 to February 2023 using the US Food and Drug Administration Adverse Event Reporting System and the Canada Vigilance Adverse Reaction Online Database.
- AEs were categorized on the basis of the Medical Dictionary for Regulatory Activities system organ class.
- Five JAK inhibitors approved for use in children were included in the study: Baricitinib, upadacitinib, abrocitinib, ruxolitinib, and tofacitinib.
TAKEAWAY:
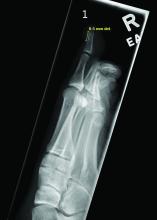
- The most frequently reported AEs in children were blood and lymphatic system disorders, including neutropenia, thrombocytopenia, and anemia (24%); viral, fungal, and bacterial infections, such as pneumonia and sepsis (17.2%); constitutional symptoms and administrative concerns, including pyrexia and fatigue (15.7%); gastrointestinal disorders, such as vomiting and abdominal pain (13.6%); and respiratory disorders, such as cough and respiratory distress (5.3%).
- In adults, the most common AEs were viral, fungal, and bacterial infections (16.8%); constitutional symptoms and administrative concerns (13.5%); musculoskeletal and connective tissue disorders (7.04%); and gastrointestinal (5.8%) and nervous system (5%) disorders.
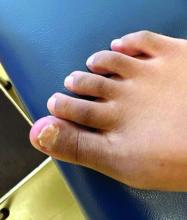
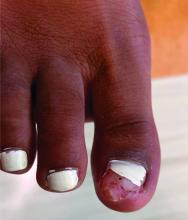
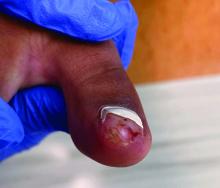
- Acne (30.6%), atopic dermatitis (22.2%), and psoriasis (16.7%) were the most common skin and subcutaneous tissue AEs reported in children. Skin and subcutaneous AEs were more common with upadacitinib (21.1%), abrocitinib (9.1%), and tofacitinib (6.3%) in children.
- Serious AEs included in the boxed warning for JAK inhibitors — serious infection, mortality, malignancy, cardiovascular events, and thrombosis — were similar for baricitinib in children (4 of 49 patients, 8.2%) and adults (325 of 3707, 8.8%). For other JAK inhibitors, absolute numbers of these AEs in children were small and rates were lower in children than in adults.
IN PRACTICE:
“This information can support customized treatment and minimize the potential for undesired or intolerable AEs,” the authors wrote.
SOURCE:
This study was led by Sahithi Talasila, BS, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and was published online in Pediatric Dermatology.
LIMITATIONS:
Pharmacovigilance registries did not fully capture the complete range of AEs because of potential reporting bias or recall bias. Additionally, events lacking sufficient objective evidence were underreported, while common AEs associated with JAK inhibitor therapy were overreported.
DISCLOSURES:
No specific funding sources for the study were reported. One author reported being a consultant, one reported serving as a principal investigator in clinical trials, and another reported serving on data and safety monitoring boards of various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which also found that acne was the most common skin-related AE in children, and serious AEs were less common.
METHODOLOGY:
- Researchers analyzed 399,649 AEs in 133,216 adult patients and 2883 AEs in 955 pediatric patients (age, < 18 years) from November 2011 to February 2023 using the US Food and Drug Administration Adverse Event Reporting System and the Canada Vigilance Adverse Reaction Online Database.
- AEs were categorized on the basis of the Medical Dictionary for Regulatory Activities system organ class.
- Five JAK inhibitors approved for use in children were included in the study: Baricitinib, upadacitinib, abrocitinib, ruxolitinib, and tofacitinib.
TAKEAWAY:
- The most frequently reported AEs in children were blood and lymphatic system disorders, including neutropenia, thrombocytopenia, and anemia (24%); viral, fungal, and bacterial infections, such as pneumonia and sepsis (17.2%); constitutional symptoms and administrative concerns, including pyrexia and fatigue (15.7%); gastrointestinal disorders, such as vomiting and abdominal pain (13.6%); and respiratory disorders, such as cough and respiratory distress (5.3%).
- In adults, the most common AEs were viral, fungal, and bacterial infections (16.8%); constitutional symptoms and administrative concerns (13.5%); musculoskeletal and connective tissue disorders (7.04%); and gastrointestinal (5.8%) and nervous system (5%) disorders.
- Acne (30.6%), atopic dermatitis (22.2%), and psoriasis (16.7%) were the most common skin and subcutaneous tissue AEs reported in children. Skin and subcutaneous AEs were more common with upadacitinib (21.1%), abrocitinib (9.1%), and tofacitinib (6.3%) in children.
- Serious AEs included in the boxed warning for JAK inhibitors — serious infection, mortality, malignancy, cardiovascular events, and thrombosis — were similar for baricitinib in children (4 of 49 patients, 8.2%) and adults (325 of 3707, 8.8%). For other JAK inhibitors, absolute numbers of these AEs in children were small and rates were lower in children than in adults.
IN PRACTICE:
“This information can support customized treatment and minimize the potential for undesired or intolerable AEs,” the authors wrote.
SOURCE:
This study was led by Sahithi Talasila, BS, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and was published online in Pediatric Dermatology.
LIMITATIONS:
Pharmacovigilance registries did not fully capture the complete range of AEs because of potential reporting bias or recall bias. Additionally, events lacking sufficient objective evidence were underreported, while common AEs associated with JAK inhibitor therapy were overreported.
DISCLOSURES:
No specific funding sources for the study were reported. One author reported being a consultant, one reported serving as a principal investigator in clinical trials, and another reported serving on data and safety monitoring boards of various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which also found that acne was the most common skin-related AE in children, and serious AEs were less common.
METHODOLOGY:
- Researchers analyzed 399,649 AEs in 133,216 adult patients and 2883 AEs in 955 pediatric patients (age, < 18 years) from November 2011 to February 2023 using the US Food and Drug Administration Adverse Event Reporting System and the Canada Vigilance Adverse Reaction Online Database.
- AEs were categorized on the basis of the Medical Dictionary for Regulatory Activities system organ class.
- Five JAK inhibitors approved for use in children were included in the study: Baricitinib, upadacitinib, abrocitinib, ruxolitinib, and tofacitinib.
TAKEAWAY:
- The most frequently reported AEs in children were blood and lymphatic system disorders, including neutropenia, thrombocytopenia, and anemia (24%); viral, fungal, and bacterial infections, such as pneumonia and sepsis (17.2%); constitutional symptoms and administrative concerns, including pyrexia and fatigue (15.7%); gastrointestinal disorders, such as vomiting and abdominal pain (13.6%); and respiratory disorders, such as cough and respiratory distress (5.3%).
- In adults, the most common AEs were viral, fungal, and bacterial infections (16.8%); constitutional symptoms and administrative concerns (13.5%); musculoskeletal and connective tissue disorders (7.04%); and gastrointestinal (5.8%) and nervous system (5%) disorders.
- Acne (30.6%), atopic dermatitis (22.2%), and psoriasis (16.7%) were the most common skin and subcutaneous tissue AEs reported in children. Skin and subcutaneous AEs were more common with upadacitinib (21.1%), abrocitinib (9.1%), and tofacitinib (6.3%) in children.
- Serious AEs included in the boxed warning for JAK inhibitors — serious infection, mortality, malignancy, cardiovascular events, and thrombosis — were similar for baricitinib in children (4 of 49 patients, 8.2%) and adults (325 of 3707, 8.8%). For other JAK inhibitors, absolute numbers of these AEs in children were small and rates were lower in children than in adults.
IN PRACTICE:
“This information can support customized treatment and minimize the potential for undesired or intolerable AEs,” the authors wrote.
SOURCE:
This study was led by Sahithi Talasila, BS, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and was published online in Pediatric Dermatology.
LIMITATIONS:
Pharmacovigilance registries did not fully capture the complete range of AEs because of potential reporting bias or recall bias. Additionally, events lacking sufficient objective evidence were underreported, while common AEs associated with JAK inhibitor therapy were overreported.
DISCLOSURES:
No specific funding sources for the study were reported. One author reported being a consultant, one reported serving as a principal investigator in clinical trials, and another reported serving on data and safety monitoring boards of various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Oropouche Virus
The pediatrician’s first patient of the day was a 15-year-old boy complaining of fever, chills, and profound arthralgias. His exam, including a careful assessment of his joints, yielded no clues, and the pediatrician was ready to diagnose this as a routine viral illness. An additional bit of history provided by the patient’s mother prompted the pediatrician to pause and reconsider.
“A week ago, we returned from a visit to Cuba,” the mother reported. “Could this be Oropouche virus infection?”
Oropouche virus disease is an arboviral disease caused by the Oropouche virus (OROV). It is transmitted to humans through midge or mosquito bites. Although largely unknown to most United States clinicians until recently, this vector-borne virus is not new. The first human Oropouche virus infection was identified in Trinidad and Tobago in 1955 and since then, there have been intermittent outbreaks in the Amazon region. In recent months, though, the epidemiology of Oropouche virus infections has changed. Infections are being identified in new geographic areas, including Cuba. According to the Pan American Health Organization, 506 cases of Oropouche virus infection have been identified in Cuba since May 27, 2024.
Two deaths from Oropouche virus infection have been reported in previously healthy people. Evolving data suggests adverse outcomes associated with vertical transmission during pregnancy. One fetal death and child with congenital anomalies have been reported in Brazil. Additional fetal deaths, miscarriages, and congenital anomalies are under investigation.
Travel-associated cases have been reported in the United States. As of September 10, 2024, 52 Oropouche virus disease cases had been reported from five states in the United States. The Centers for Disease Control and Prevention confirmed that the first 31 of these cases were travelers returning from Cuba. The CDC issued a health advisory on August 16, 2024: Increased Oropouche Virus Activity and Associated Risk to Travelers.
The pediatrician quickly reviewed the signs and symptoms of Oropouche virus infection. Disease typically presents as an abrupt onset of fever, severe headache, chills, myalgia, and arthralgia 3 to 10 days after the bite of infected mosquito. Some patients develop a maculopapular rash that starts on the trunk and spreads to the extremities. Meningitis and encephalitis develop in less than 1 in 20 people. The symptoms of Oropouche virus infection overlap with those of other arboviruses such as dengue, chikungunya, and Zika viruses. The disease can also mimic malaria or rickettsial infection. Approximately 60% of people with Oropouche virus infection experience a recurrence of symptoms within days to weeks of the initial resolution of symptoms.
Testing for Oropouche virus infection is available through the CDC’s Arbovirus Diagnostic Laboratory. In people who are acutely ill, reverse transcription-polymerase chain reaction testing can be used to identify the virus in serum and cerebrospinal fluid. Serologic testing is also available for people who have been symptomatic for at least 6 days.
The pediatrician contacted his local health department to discuss the possibility of Oropouche virus infection. After reviewing the case definition, public health authorities recommended laboratory testing for Oropouche virus, dengue, and Zika virus.
Back in the exam room, the pediatrician provided anticipatory guidance to the patient and his mother. There are no antiviral medications to treat Oropouche virus infection, so the pediatrician recommended supportive care, including acetaminophen for fever and pain. He also advised avoiding aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) until dengue could be ruled out to reduce the risk of bleeding. After confirming that no one else in the home was sick with similar symptoms, he counseled about prevention strategies.
To date, transmission of Oropouche virus in the United States has not been documented, but vectors potentially capable of transmitting the virus are present in some areas of the United States. When people who are infected with Oropouche are bitten, they can spread the virus through their blood to biting midges or mosquitoes. The insects can then spread the virus to other people. To reduce to potential for local transmission, people who are sick with suspected Oropouche virus infection are advised to avoid biting-midge and mosquito bites for the first week of their illness. Any person who has recently traveled to an area where Oropouche virus transmission is occurring should also avoid insect bites for 3 weeks after returning home to account for the potential incubation period of the virus. This includes wearing an EPA-registered insect repellent.
A suspect case is a patient who has been in an area with documented or suspected OROV circulation* within 2 weeks of initial symptom onset (as patients may experience recurrent symptoms) and the following:
- Abrupt onset of reported fever, headache, and one or more of the following: myalgia, arthralgia, photophobia, retro-orbital/eye pain, or signs and symptoms of neuroinvasive disease (eg, stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis).
- Tested negative for other possible diseases, in particular dengue.†
- Absence of a more likely clinical explanation.
*If concern exists for local transmission in a nonendemic area, consider if the patient shared an exposure location with a person with confirmed OROV infection, lives in an area where travel-related cases have been identified, or has known vector exposure (eg, mosquitoes or biting midges).
†If strong suspicion of OROV disease exists based on the patient’s clinical features and history of travel to an area with virus circulation, do not wait on negative testing before sending specimens to CDC.
Adapted from: Centers for Disease Control and Prevention. Response to Oropouche Virus Disease Cases in U.S. States and Territories in the Americas. Available at: https.//www.cdc.gov/oropouche/media/pdfs/2024/09/response-to-oropouche-virus-disease.pdf
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at [email protected]. (Also [email protected])
The pediatrician’s first patient of the day was a 15-year-old boy complaining of fever, chills, and profound arthralgias. His exam, including a careful assessment of his joints, yielded no clues, and the pediatrician was ready to diagnose this as a routine viral illness. An additional bit of history provided by the patient’s mother prompted the pediatrician to pause and reconsider.
“A week ago, we returned from a visit to Cuba,” the mother reported. “Could this be Oropouche virus infection?”
Oropouche virus disease is an arboviral disease caused by the Oropouche virus (OROV). It is transmitted to humans through midge or mosquito bites. Although largely unknown to most United States clinicians until recently, this vector-borne virus is not new. The first human Oropouche virus infection was identified in Trinidad and Tobago in 1955 and since then, there have been intermittent outbreaks in the Amazon region. In recent months, though, the epidemiology of Oropouche virus infections has changed. Infections are being identified in new geographic areas, including Cuba. According to the Pan American Health Organization, 506 cases of Oropouche virus infection have been identified in Cuba since May 27, 2024.
Two deaths from Oropouche virus infection have been reported in previously healthy people. Evolving data suggests adverse outcomes associated with vertical transmission during pregnancy. One fetal death and child with congenital anomalies have been reported in Brazil. Additional fetal deaths, miscarriages, and congenital anomalies are under investigation.
Travel-associated cases have been reported in the United States. As of September 10, 2024, 52 Oropouche virus disease cases had been reported from five states in the United States. The Centers for Disease Control and Prevention confirmed that the first 31 of these cases were travelers returning from Cuba. The CDC issued a health advisory on August 16, 2024: Increased Oropouche Virus Activity and Associated Risk to Travelers.
The pediatrician quickly reviewed the signs and symptoms of Oropouche virus infection. Disease typically presents as an abrupt onset of fever, severe headache, chills, myalgia, and arthralgia 3 to 10 days after the bite of infected mosquito. Some patients develop a maculopapular rash that starts on the trunk and spreads to the extremities. Meningitis and encephalitis develop in less than 1 in 20 people. The symptoms of Oropouche virus infection overlap with those of other arboviruses such as dengue, chikungunya, and Zika viruses. The disease can also mimic malaria or rickettsial infection. Approximately 60% of people with Oropouche virus infection experience a recurrence of symptoms within days to weeks of the initial resolution of symptoms.
Testing for Oropouche virus infection is available through the CDC’s Arbovirus Diagnostic Laboratory. In people who are acutely ill, reverse transcription-polymerase chain reaction testing can be used to identify the virus in serum and cerebrospinal fluid. Serologic testing is also available for people who have been symptomatic for at least 6 days.
The pediatrician contacted his local health department to discuss the possibility of Oropouche virus infection. After reviewing the case definition, public health authorities recommended laboratory testing for Oropouche virus, dengue, and Zika virus.
Back in the exam room, the pediatrician provided anticipatory guidance to the patient and his mother. There are no antiviral medications to treat Oropouche virus infection, so the pediatrician recommended supportive care, including acetaminophen for fever and pain. He also advised avoiding aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) until dengue could be ruled out to reduce the risk of bleeding. After confirming that no one else in the home was sick with similar symptoms, he counseled about prevention strategies.
To date, transmission of Oropouche virus in the United States has not been documented, but vectors potentially capable of transmitting the virus are present in some areas of the United States. When people who are infected with Oropouche are bitten, they can spread the virus through their blood to biting midges or mosquitoes. The insects can then spread the virus to other people. To reduce to potential for local transmission, people who are sick with suspected Oropouche virus infection are advised to avoid biting-midge and mosquito bites for the first week of their illness. Any person who has recently traveled to an area where Oropouche virus transmission is occurring should also avoid insect bites for 3 weeks after returning home to account for the potential incubation period of the virus. This includes wearing an EPA-registered insect repellent.
A suspect case is a patient who has been in an area with documented or suspected OROV circulation* within 2 weeks of initial symptom onset (as patients may experience recurrent symptoms) and the following:
- Abrupt onset of reported fever, headache, and one or more of the following: myalgia, arthralgia, photophobia, retro-orbital/eye pain, or signs and symptoms of neuroinvasive disease (eg, stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis).
- Tested negative for other possible diseases, in particular dengue.†
- Absence of a more likely clinical explanation.
*If concern exists for local transmission in a nonendemic area, consider if the patient shared an exposure location with a person with confirmed OROV infection, lives in an area where travel-related cases have been identified, or has known vector exposure (eg, mosquitoes or biting midges).
†If strong suspicion of OROV disease exists based on the patient’s clinical features and history of travel to an area with virus circulation, do not wait on negative testing before sending specimens to CDC.
Adapted from: Centers for Disease Control and Prevention. Response to Oropouche Virus Disease Cases in U.S. States and Territories in the Americas. Available at: https.//www.cdc.gov/oropouche/media/pdfs/2024/09/response-to-oropouche-virus-disease.pdf
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at [email protected]. (Also [email protected])
The pediatrician’s first patient of the day was a 15-year-old boy complaining of fever, chills, and profound arthralgias. His exam, including a careful assessment of his joints, yielded no clues, and the pediatrician was ready to diagnose this as a routine viral illness. An additional bit of history provided by the patient’s mother prompted the pediatrician to pause and reconsider.
“A week ago, we returned from a visit to Cuba,” the mother reported. “Could this be Oropouche virus infection?”
Oropouche virus disease is an arboviral disease caused by the Oropouche virus (OROV). It is transmitted to humans through midge or mosquito bites. Although largely unknown to most United States clinicians until recently, this vector-borne virus is not new. The first human Oropouche virus infection was identified in Trinidad and Tobago in 1955 and since then, there have been intermittent outbreaks in the Amazon region. In recent months, though, the epidemiology of Oropouche virus infections has changed. Infections are being identified in new geographic areas, including Cuba. According to the Pan American Health Organization, 506 cases of Oropouche virus infection have been identified in Cuba since May 27, 2024.
Two deaths from Oropouche virus infection have been reported in previously healthy people. Evolving data suggests adverse outcomes associated with vertical transmission during pregnancy. One fetal death and child with congenital anomalies have been reported in Brazil. Additional fetal deaths, miscarriages, and congenital anomalies are under investigation.
Travel-associated cases have been reported in the United States. As of September 10, 2024, 52 Oropouche virus disease cases had been reported from five states in the United States. The Centers for Disease Control and Prevention confirmed that the first 31 of these cases were travelers returning from Cuba. The CDC issued a health advisory on August 16, 2024: Increased Oropouche Virus Activity and Associated Risk to Travelers.
The pediatrician quickly reviewed the signs and symptoms of Oropouche virus infection. Disease typically presents as an abrupt onset of fever, severe headache, chills, myalgia, and arthralgia 3 to 10 days after the bite of infected mosquito. Some patients develop a maculopapular rash that starts on the trunk and spreads to the extremities. Meningitis and encephalitis develop in less than 1 in 20 people. The symptoms of Oropouche virus infection overlap with those of other arboviruses such as dengue, chikungunya, and Zika viruses. The disease can also mimic malaria or rickettsial infection. Approximately 60% of people with Oropouche virus infection experience a recurrence of symptoms within days to weeks of the initial resolution of symptoms.
Testing for Oropouche virus infection is available through the CDC’s Arbovirus Diagnostic Laboratory. In people who are acutely ill, reverse transcription-polymerase chain reaction testing can be used to identify the virus in serum and cerebrospinal fluid. Serologic testing is also available for people who have been symptomatic for at least 6 days.
The pediatrician contacted his local health department to discuss the possibility of Oropouche virus infection. After reviewing the case definition, public health authorities recommended laboratory testing for Oropouche virus, dengue, and Zika virus.
Back in the exam room, the pediatrician provided anticipatory guidance to the patient and his mother. There are no antiviral medications to treat Oropouche virus infection, so the pediatrician recommended supportive care, including acetaminophen for fever and pain. He also advised avoiding aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) until dengue could be ruled out to reduce the risk of bleeding. After confirming that no one else in the home was sick with similar symptoms, he counseled about prevention strategies.
To date, transmission of Oropouche virus in the United States has not been documented, but vectors potentially capable of transmitting the virus are present in some areas of the United States. When people who are infected with Oropouche are bitten, they can spread the virus through their blood to biting midges or mosquitoes. The insects can then spread the virus to other people. To reduce to potential for local transmission, people who are sick with suspected Oropouche virus infection are advised to avoid biting-midge and mosquito bites for the first week of their illness. Any person who has recently traveled to an area where Oropouche virus transmission is occurring should also avoid insect bites for 3 weeks after returning home to account for the potential incubation period of the virus. This includes wearing an EPA-registered insect repellent.
A suspect case is a patient who has been in an area with documented or suspected OROV circulation* within 2 weeks of initial symptom onset (as patients may experience recurrent symptoms) and the following:
- Abrupt onset of reported fever, headache, and one or more of the following: myalgia, arthralgia, photophobia, retro-orbital/eye pain, or signs and symptoms of neuroinvasive disease (eg, stiff neck, altered mental status, seizures, limb weakness, or cerebrospinal fluid pleocytosis).
- Tested negative for other possible diseases, in particular dengue.†
- Absence of a more likely clinical explanation.
*If concern exists for local transmission in a nonendemic area, consider if the patient shared an exposure location with a person with confirmed OROV infection, lives in an area where travel-related cases have been identified, or has known vector exposure (eg, mosquitoes or biting midges).
†If strong suspicion of OROV disease exists based on the patient’s clinical features and history of travel to an area with virus circulation, do not wait on negative testing before sending specimens to CDC.
Adapted from: Centers for Disease Control and Prevention. Response to Oropouche Virus Disease Cases in U.S. States and Territories in the Americas. Available at: https.//www.cdc.gov/oropouche/media/pdfs/2024/09/response-to-oropouche-virus-disease.pdf
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at [email protected]. (Also [email protected])
Stress Management
With the changing leaves and cooling temperatures, early autumn also brings the excitement of the new school year. While returning to sports, mastering new subjects, and spending time with old and new friends is exhilarating, this season can also be a time of intense stress.
For those high school students who are especially ambitious, the school year presents the challenge of a very high stakes performance, one whose success will be measured by admission to a prized college. Not only are there classes to study for, but schedules are packed with a maximum number of subjects, a maximum number of Advanced Placement courses and a maximum number of impressive extra-curricular activities. Varsity sports practice, SAT prep, Debate Club, volunteer hours, and on and on.
What is often missing is enough time for sleep, socializing, exploring new interests, and unwinding. When you hear your patients (or parents) describing the intense stress of their overloaded schedules compounded by a sense that “I have no choice,” you have an opportunity to complicate their thinking. Introduce the idea that there are smart approaches to performing your best under stress. Pushing themselves relentlessly will inevitably lead to burnout and exhaustion. This approach will help them learn to make wise choices and will better serve their healthy development.
Start by acknowledging the stress of high-stakes performance. Telling your patients that they need to lower the temperature by not putting so much pressure on themselves is likely to be experienced as a lack of confidence in them and is unlikely to get any traction. Instead, ask your patients what matters to them the most: Is it admission to the college of their choice? Achieving a certain score or GPA? Is it their competitiveness and drive to win? There is no wrong answer, but it is helpful for them to be able to reflect on what matters to them. Are they hoping to impress someone else? Are they worried about their future financial health and convinced that getting into a certain college will secure their financial success? Do they think this matters more to their parents than to themselves? Or have they discovered an intense interest in theoretical physics and want to be able to study at Caltech? If their ambition is meaningfully connected to an authentic interest or to their emerging identity, their sense of purpose will be much deeper and able to sustain them.
Even with talent and a strong sense of purpose, performing well is very difficult and demanding. It is important to consider the cycle of performance as including preparation, performance itself, and effective rest and recovery, just as with athletic performance. Whether the performance is the SATs, an AP test, a debate or big game, there were probably hours of preparation for every hour of performance. Help them to consider the importance of this practice or preparation time, and how to use that time effectively. Are they able to work in environments where there are few distractions? Do they have the support or useful feedback they need? How are they able to know when it is time for a break or when they are ready? It can be helpful for them to appreciate whether preparation or performance is more challenging for them, as the former requires focus and patience, while the latter requires courage and tenacity. If they are aware of which is harder for them, they can be thoughtful about how to effectively handle those challenges.
What can be most valuable for your patients is hearing from their pediatricians that they need to have time protected for rest and recharging, and not only for preparation and performance. Any athlete knows that failing to do so will lead to exhaustion and injury, and performance inevitably suffers. Rest is unwinding and slowing down, and a restful activity will leave them feeling calm, relaxed, and ready for sleep. A recharging activity is one that leaves them feeling refreshed and energized. Some common restful activities are a hot bath or shower, a distracting activity such as watching a show or surfing the web, playing a simple video game or puzzle or listening to music. Some recharging activities are creative ones (making art or music), engaging in hobbies, reading, or talking with a good friend. A few activities — sleep, exercise, and mindfulness meditation, are powerful in that they pack both rest and recharge into the same activity. Your patients should be discovering and learning which activities they find restful or recharging. The college application process or preparing for a varsity tryout will both add stress and give them an opportunity to learn what rests and recharges them. They should aim to have a list of at least five effective strategies that they can turn to when it’s time to rest or to recharge. Help them turn their work ethic to building a deeper well of self-knowledge that will serve them when they face challenges in high school or when they are on their own in college. This time of stress can be a time of growth, too.
Of course, remind your patients that this is a critical time to focus on basic self-care: They need consistently adequate, restful sleep, good nutrition, and physical activity. They will benefit from regular time in nature and time spent with friends that nourish them. They can find ways to compound these activities: Go for a walk with a friend, eat dinner with family, play a relaxing game while enjoying music. Lastly, ask your patients what is the last new thing they tried. It is easy to become so focused on an ambitious project that there is no time for exploration and play. Play is important throughout life, but adolescents are actively discovering their interests, talents, tastes, and values. To do this they need to be trying things that are new and maybe less purpose-driven. I call this type of activity “senseless fun.” Splashing in the pool is senseless fun, swimming laps is purposeful exercise that my contribute to recharging, and competing in a swim meet is often more on the stressful side. As they discover new talents, deeply engaging interests, what relaxes and recharges them, they will be learning who they are. Regardless of the outcome of a test, a big game, or where they go to college, it is this emerging knowledge about themselves that will carry them into adulthood. The pediatrician’s goal: Encouraging aspiration, exploration, and self-awareness in the context of giving permission for rest, recharging, and senseless fun.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
With the changing leaves and cooling temperatures, early autumn also brings the excitement of the new school year. While returning to sports, mastering new subjects, and spending time with old and new friends is exhilarating, this season can also be a time of intense stress.
For those high school students who are especially ambitious, the school year presents the challenge of a very high stakes performance, one whose success will be measured by admission to a prized college. Not only are there classes to study for, but schedules are packed with a maximum number of subjects, a maximum number of Advanced Placement courses and a maximum number of impressive extra-curricular activities. Varsity sports practice, SAT prep, Debate Club, volunteer hours, and on and on.
What is often missing is enough time for sleep, socializing, exploring new interests, and unwinding. When you hear your patients (or parents) describing the intense stress of their overloaded schedules compounded by a sense that “I have no choice,” you have an opportunity to complicate their thinking. Introduce the idea that there are smart approaches to performing your best under stress. Pushing themselves relentlessly will inevitably lead to burnout and exhaustion. This approach will help them learn to make wise choices and will better serve their healthy development.
Start by acknowledging the stress of high-stakes performance. Telling your patients that they need to lower the temperature by not putting so much pressure on themselves is likely to be experienced as a lack of confidence in them and is unlikely to get any traction. Instead, ask your patients what matters to them the most: Is it admission to the college of their choice? Achieving a certain score or GPA? Is it their competitiveness and drive to win? There is no wrong answer, but it is helpful for them to be able to reflect on what matters to them. Are they hoping to impress someone else? Are they worried about their future financial health and convinced that getting into a certain college will secure their financial success? Do they think this matters more to their parents than to themselves? Or have they discovered an intense interest in theoretical physics and want to be able to study at Caltech? If their ambition is meaningfully connected to an authentic interest or to their emerging identity, their sense of purpose will be much deeper and able to sustain them.
Even with talent and a strong sense of purpose, performing well is very difficult and demanding. It is important to consider the cycle of performance as including preparation, performance itself, and effective rest and recovery, just as with athletic performance. Whether the performance is the SATs, an AP test, a debate or big game, there were probably hours of preparation for every hour of performance. Help them to consider the importance of this practice or preparation time, and how to use that time effectively. Are they able to work in environments where there are few distractions? Do they have the support or useful feedback they need? How are they able to know when it is time for a break or when they are ready? It can be helpful for them to appreciate whether preparation or performance is more challenging for them, as the former requires focus and patience, while the latter requires courage and tenacity. If they are aware of which is harder for them, they can be thoughtful about how to effectively handle those challenges.
What can be most valuable for your patients is hearing from their pediatricians that they need to have time protected for rest and recharging, and not only for preparation and performance. Any athlete knows that failing to do so will lead to exhaustion and injury, and performance inevitably suffers. Rest is unwinding and slowing down, and a restful activity will leave them feeling calm, relaxed, and ready for sleep. A recharging activity is one that leaves them feeling refreshed and energized. Some common restful activities are a hot bath or shower, a distracting activity such as watching a show or surfing the web, playing a simple video game or puzzle or listening to music. Some recharging activities are creative ones (making art or music), engaging in hobbies, reading, or talking with a good friend. A few activities — sleep, exercise, and mindfulness meditation, are powerful in that they pack both rest and recharge into the same activity. Your patients should be discovering and learning which activities they find restful or recharging. The college application process or preparing for a varsity tryout will both add stress and give them an opportunity to learn what rests and recharges them. They should aim to have a list of at least five effective strategies that they can turn to when it’s time to rest or to recharge. Help them turn their work ethic to building a deeper well of self-knowledge that will serve them when they face challenges in high school or when they are on their own in college. This time of stress can be a time of growth, too.
Of course, remind your patients that this is a critical time to focus on basic self-care: They need consistently adequate, restful sleep, good nutrition, and physical activity. They will benefit from regular time in nature and time spent with friends that nourish them. They can find ways to compound these activities: Go for a walk with a friend, eat dinner with family, play a relaxing game while enjoying music. Lastly, ask your patients what is the last new thing they tried. It is easy to become so focused on an ambitious project that there is no time for exploration and play. Play is important throughout life, but adolescents are actively discovering their interests, talents, tastes, and values. To do this they need to be trying things that are new and maybe less purpose-driven. I call this type of activity “senseless fun.” Splashing in the pool is senseless fun, swimming laps is purposeful exercise that my contribute to recharging, and competing in a swim meet is often more on the stressful side. As they discover new talents, deeply engaging interests, what relaxes and recharges them, they will be learning who they are. Regardless of the outcome of a test, a big game, or where they go to college, it is this emerging knowledge about themselves that will carry them into adulthood. The pediatrician’s goal: Encouraging aspiration, exploration, and self-awareness in the context of giving permission for rest, recharging, and senseless fun.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
With the changing leaves and cooling temperatures, early autumn also brings the excitement of the new school year. While returning to sports, mastering new subjects, and spending time with old and new friends is exhilarating, this season can also be a time of intense stress.
For those high school students who are especially ambitious, the school year presents the challenge of a very high stakes performance, one whose success will be measured by admission to a prized college. Not only are there classes to study for, but schedules are packed with a maximum number of subjects, a maximum number of Advanced Placement courses and a maximum number of impressive extra-curricular activities. Varsity sports practice, SAT prep, Debate Club, volunteer hours, and on and on.
What is often missing is enough time for sleep, socializing, exploring new interests, and unwinding. When you hear your patients (or parents) describing the intense stress of their overloaded schedules compounded by a sense that “I have no choice,” you have an opportunity to complicate their thinking. Introduce the idea that there are smart approaches to performing your best under stress. Pushing themselves relentlessly will inevitably lead to burnout and exhaustion. This approach will help them learn to make wise choices and will better serve their healthy development.
Start by acknowledging the stress of high-stakes performance. Telling your patients that they need to lower the temperature by not putting so much pressure on themselves is likely to be experienced as a lack of confidence in them and is unlikely to get any traction. Instead, ask your patients what matters to them the most: Is it admission to the college of their choice? Achieving a certain score or GPA? Is it their competitiveness and drive to win? There is no wrong answer, but it is helpful for them to be able to reflect on what matters to them. Are they hoping to impress someone else? Are they worried about their future financial health and convinced that getting into a certain college will secure their financial success? Do they think this matters more to their parents than to themselves? Or have they discovered an intense interest in theoretical physics and want to be able to study at Caltech? If their ambition is meaningfully connected to an authentic interest or to their emerging identity, their sense of purpose will be much deeper and able to sustain them.
Even with talent and a strong sense of purpose, performing well is very difficult and demanding. It is important to consider the cycle of performance as including preparation, performance itself, and effective rest and recovery, just as with athletic performance. Whether the performance is the SATs, an AP test, a debate or big game, there were probably hours of preparation for every hour of performance. Help them to consider the importance of this practice or preparation time, and how to use that time effectively. Are they able to work in environments where there are few distractions? Do they have the support or useful feedback they need? How are they able to know when it is time for a break or when they are ready? It can be helpful for them to appreciate whether preparation or performance is more challenging for them, as the former requires focus and patience, while the latter requires courage and tenacity. If they are aware of which is harder for them, they can be thoughtful about how to effectively handle those challenges.
What can be most valuable for your patients is hearing from their pediatricians that they need to have time protected for rest and recharging, and not only for preparation and performance. Any athlete knows that failing to do so will lead to exhaustion and injury, and performance inevitably suffers. Rest is unwinding and slowing down, and a restful activity will leave them feeling calm, relaxed, and ready for sleep. A recharging activity is one that leaves them feeling refreshed and energized. Some common restful activities are a hot bath or shower, a distracting activity such as watching a show or surfing the web, playing a simple video game or puzzle or listening to music. Some recharging activities are creative ones (making art or music), engaging in hobbies, reading, or talking with a good friend. A few activities — sleep, exercise, and mindfulness meditation, are powerful in that they pack both rest and recharge into the same activity. Your patients should be discovering and learning which activities they find restful or recharging. The college application process or preparing for a varsity tryout will both add stress and give them an opportunity to learn what rests and recharges them. They should aim to have a list of at least five effective strategies that they can turn to when it’s time to rest or to recharge. Help them turn their work ethic to building a deeper well of self-knowledge that will serve them when they face challenges in high school or when they are on their own in college. This time of stress can be a time of growth, too.
Of course, remind your patients that this is a critical time to focus on basic self-care: They need consistently adequate, restful sleep, good nutrition, and physical activity. They will benefit from regular time in nature and time spent with friends that nourish them. They can find ways to compound these activities: Go for a walk with a friend, eat dinner with family, play a relaxing game while enjoying music. Lastly, ask your patients what is the last new thing they tried. It is easy to become so focused on an ambitious project that there is no time for exploration and play. Play is important throughout life, but adolescents are actively discovering their interests, talents, tastes, and values. To do this they need to be trying things that are new and maybe less purpose-driven. I call this type of activity “senseless fun.” Splashing in the pool is senseless fun, swimming laps is purposeful exercise that my contribute to recharging, and competing in a swim meet is often more on the stressful side. As they discover new talents, deeply engaging interests, what relaxes and recharges them, they will be learning who they are. Regardless of the outcome of a test, a big game, or where they go to college, it is this emerging knowledge about themselves that will carry them into adulthood. The pediatrician’s goal: Encouraging aspiration, exploration, and self-awareness in the context of giving permission for rest, recharging, and senseless fun.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Is It Time for Universal Suicide Screening?
US suicide rates have reached alarming levels, with data from Centers for Disease Control and Prevention (CDC) showing a 37% increase from 2000 to 2022. Nearly 49,000 people died by suicide in 2022 alone, translating to one death every 11 minutes.
The increase in suicide rates has prompted calls for expansion of universal suicide screening, in which all individuals in medical or mental health care settings are screened for suicide risk, regardless of the purpose for their visit. But the psychiatric field is split on the issue, with some experts citing false positives and a lack of mental health care resources for those deemed at risk.
In 2022, when the US Preventative Services Task Force released its recommendations on suicide prevention, first in children and adolescents, and then in adults, the authors said there was insufficient evidence to support universal suicide screening.
Proponents of the practice pushed back on that finding, arguing that universal suicide screening could help identify those at high risk who might otherwise go undiagnosed, leading to earlier, potentially lifesaving, intervention.
So, what is the case for — and against — universal screening?
Sounding an Alert
The introduction of universal screening was driven by a confluence of factors that began with a 1999 report by then-US Surgeon General David Satcher, MD. This was followed by a report in 2016 from the Joint Commission on Detecting and Treating Suicidal Ideation that called for healthcare organizations to improve detection and treatment of suicidal ideation in all healthcare care settings.
Data from the alert showed that a significant number of people who died by suicide had a healthcare visit before their death. Half had seen a clinician a month before their death; nearly 30% had a medical visit just the week before — all with no detection of increased suicide risk.
It was that sort of finding that led Parkland Health and Hospital System in Dallas to become the first US hospital to implement universal suicide screening. Since the program launched in 2015, the system has screened more than 4.3 million patients in its emergency department, inpatient units, and 20 primary care clinics.
“Since the program began, we’ve completed between 40,000 to 50,000 screenings per month,” said Kimberly Roaten, PhD, associate chief quality and safety officer for behavioral health at Parkland Health.
Clinicians at Parkland use the five-item Ask Suicide-Screening Questions to assess suicidal intent, a commonly used tool that was originally developed for use in pediatric emergency rooms (ERs). The tool, which takes about 20 seconds to administer, has since been validated in both children and adults.
Based on a patient’s response, a clinical decision support system integrated into the electronic health record classifies suicide risk as none, moderate, or high.
Patients identified as moderate risk are offered a more in-depth assessment with a mental health clinician, though participation is not mandatory, said Dr. Roaten. Those at high risk receive a more thorough evaluation.
The proportion of ER patients at Parkland who screen positive for any suicidal intent has consistently remained at about 7%, and at 2% in the primary care clinics, she said.
To better understand what the program may have had on suicide prevention, Dr. Roaten is leading a National Institute of Mental Health–funded study to link a decade of mortality data from the state of Texas to patient data from Parkland Health. Investigators will analyze information about patients identified at risk for suicide, those patients’ characteristics, and who dies by suicide.
Universal Screening Expands
Other health systems have adopted universal suicide screening including the Indian Health Service and the US Veterans Health Administration. Universal suicide screening is also in place in a growing number of primary care practices and hospitals throughout the United States and will be mandatory for patients aged 12 years and older in all acute care hospitals in California beginning in 2025.
There is also a push for universal screening to be coordinated through local, state, and federal government, nonprofit, and private sectors. The National Action Alliance for Suicide Prevention is charged with advancing the White House’s 2024 National Strategy for Suicide Prevention, a 10-year plan to address gaps in suicide prevention in the United States.
Sarah Brummett, JD, director of the National Action Alliance for Suicide Prevention’s executive committee, said that universal suicide screening is part of the 2024 strategy. “We know there are barriers to universal screening, and so it’s important to recognize what they are so we can address them,” said Ms. Brummett.
Barriers may include adequate staffing, or a system in place to triage patients who screen positive.
At Parkland, cost and workload have been minimal, Dr. Roaten said. “We built a model that only dedicates our highest-value resources to the most at-risk patients.”
She also noted that relief may be on the horizon for health systems where cost is an obstacle to universal screening and subsequent intervention. “There are efforts at the federal level to increase funding for suicide assessment and crisis response,” she said.
Pushback on Universal Screening
Universal suicide screening has its detractors, including critics who say expansion is unlikely to reduce suicide rates.
“The issue with suicidal ideation is that it is very dynamic. Suicidal ideation changes very quickly — sometimes within hours,” said Craig Bryan, PsyD, professor of psychiatry and behavioral health at Ohio State University in Columbus, Ohio.
Universal screening can also lead to false positives, where a patient who screens positive for suicidal ideation has no actual intention of attempting suicide, potentially creating unnecessary concern and burden on health care resources, Dr. Bryan noted.
“What do you do with everyone who screens positive?” Dr. Bryan said. “I’ve spoken with leaders of many health systems in the United States, and there is pushback against universal screening because they don’t have enough mental health resources to handle all of the referrals.”
Suicide screening also doesn’t predict who will die by suicide, Dr. Bryan added. It only identifies those willing to disclose suicidal thoughts. There is a significant number of people without mental illness who may never seek medical care, so “the warning signs we’re teaching people to recognize — depression, anxiety, and substance abuse — might not be evident in these individuals,” he said.
“Life sideswipes them suddenly, and they go from 0 to 60 ... and they may have access to a highly lethal method [of suicide] which weaponizes that moment of despair,” said Dr. Bryan. No amount of screening could possibly predict those types of suicides, he added.
Paul Nestadt, MD, associate professor of psychiatry and behavioral sciences at Johns Hopkins School of Medicine, agrees with Dr. Bryan and noted there isn’t a strong correlation between suicidal ideation and death by suicide.
“Suicidal thoughts are very common, but suicide is a rare event,” he said.
He cited a study that showed that two thirds of individuals who died by suicide had denied experiencing suicidal thoughts when asked, and half of them died within 2 days of this denial. Other research suggests that as many as 98% of people who express suicidal ideation do not die by suicide, Dr. Nestadt said.
A Public Health Issue
If universal screening is not the answer to predicting and preventing suicide, what is? One way would be to approach suicide as a public health issue, Dr. Nestadt said.
“How did we reduce the rate of motor vehicle deaths? We didn’t test each driver’s reaction time behind the wheel,” he said. “Instead, we passed seatbelt and airbag legislation, implemented federal speed limits, and as a result, the number of motor vehicle fatalities decreased.”
Dr. Nestadt is an advocate for stronger gun safety legislation, which has proven effective in reducing suicide rates. A study published this year showed that states with child access prevention laws, negligent storage laws, and mandatory waiting periods for gun purchases reported fewer suicide deaths than those without that legislation.
Other measures might be applied in cases of extreme individual suicide risk, including extreme risk protection orders, also known as “red flag” laws, he added. This type of legislation provides a pathway for law enforcement to temporarily remove firearms from individuals who pose a risk to themselves or others.
“These have been shown to be very effective in saving lives,” Dr. Nestadt said.
Dr. Nestadt and others are also using machine learning models to predict suicide risk. Those identified as high-risk may be flagged on their electronic medical record. Ideally, when the algorithm becomes more accurate at predicting suicide, anyone treating this patient can then decide if action is needed, said Dr. Nestadt.
In his work with suicidal military personnel, Dr. Bryan and his colleagues established a brief form of cognitive behavioral therapy (BCBT) to help participants challenge cognitive distortions and build coping strategies to deal with feel with intense feelings of distress. Data show that BCBT reduced suicide attempts among active-duty soldiers by 60% compared with standard mental health treatment. It has since been shown to work in civilians as well.
Dr. Bryan is also researching fluctuations in the wish to live versus the wish to die relative to one another and mapping the trajectory of risk states along the way.
The goal is that these and other suicide prevention strategies currently under study by his team and others will help stem the rise in suicide deaths.
“Overall, we need to train mental health providers to implement suicide prevention therapies and establish suicide risk programs,” Dr. Bryan said. “But until we build one of these suicide prevention interventions to scale, we’re putting the cart before the horse.”
Dr. Roaten, Ms. Brummett, Dr. Bryan, and Dr. Nestadt reported no relevant disclosures.
A version of this article appeared on Medscape.com.
US suicide rates have reached alarming levels, with data from Centers for Disease Control and Prevention (CDC) showing a 37% increase from 2000 to 2022. Nearly 49,000 people died by suicide in 2022 alone, translating to one death every 11 minutes.
The increase in suicide rates has prompted calls for expansion of universal suicide screening, in which all individuals in medical or mental health care settings are screened for suicide risk, regardless of the purpose for their visit. But the psychiatric field is split on the issue, with some experts citing false positives and a lack of mental health care resources for those deemed at risk.
In 2022, when the US Preventative Services Task Force released its recommendations on suicide prevention, first in children and adolescents, and then in adults, the authors said there was insufficient evidence to support universal suicide screening.
Proponents of the practice pushed back on that finding, arguing that universal suicide screening could help identify those at high risk who might otherwise go undiagnosed, leading to earlier, potentially lifesaving, intervention.
So, what is the case for — and against — universal screening?
Sounding an Alert
The introduction of universal screening was driven by a confluence of factors that began with a 1999 report by then-US Surgeon General David Satcher, MD. This was followed by a report in 2016 from the Joint Commission on Detecting and Treating Suicidal Ideation that called for healthcare organizations to improve detection and treatment of suicidal ideation in all healthcare care settings.
Data from the alert showed that a significant number of people who died by suicide had a healthcare visit before their death. Half had seen a clinician a month before their death; nearly 30% had a medical visit just the week before — all with no detection of increased suicide risk.
It was that sort of finding that led Parkland Health and Hospital System in Dallas to become the first US hospital to implement universal suicide screening. Since the program launched in 2015, the system has screened more than 4.3 million patients in its emergency department, inpatient units, and 20 primary care clinics.
“Since the program began, we’ve completed between 40,000 to 50,000 screenings per month,” said Kimberly Roaten, PhD, associate chief quality and safety officer for behavioral health at Parkland Health.
Clinicians at Parkland use the five-item Ask Suicide-Screening Questions to assess suicidal intent, a commonly used tool that was originally developed for use in pediatric emergency rooms (ERs). The tool, which takes about 20 seconds to administer, has since been validated in both children and adults.
Based on a patient’s response, a clinical decision support system integrated into the electronic health record classifies suicide risk as none, moderate, or high.
Patients identified as moderate risk are offered a more in-depth assessment with a mental health clinician, though participation is not mandatory, said Dr. Roaten. Those at high risk receive a more thorough evaluation.
The proportion of ER patients at Parkland who screen positive for any suicidal intent has consistently remained at about 7%, and at 2% in the primary care clinics, she said.
To better understand what the program may have had on suicide prevention, Dr. Roaten is leading a National Institute of Mental Health–funded study to link a decade of mortality data from the state of Texas to patient data from Parkland Health. Investigators will analyze information about patients identified at risk for suicide, those patients’ characteristics, and who dies by suicide.
Universal Screening Expands
Other health systems have adopted universal suicide screening including the Indian Health Service and the US Veterans Health Administration. Universal suicide screening is also in place in a growing number of primary care practices and hospitals throughout the United States and will be mandatory for patients aged 12 years and older in all acute care hospitals in California beginning in 2025.
There is also a push for universal screening to be coordinated through local, state, and federal government, nonprofit, and private sectors. The National Action Alliance for Suicide Prevention is charged with advancing the White House’s 2024 National Strategy for Suicide Prevention, a 10-year plan to address gaps in suicide prevention in the United States.
Sarah Brummett, JD, director of the National Action Alliance for Suicide Prevention’s executive committee, said that universal suicide screening is part of the 2024 strategy. “We know there are barriers to universal screening, and so it’s important to recognize what they are so we can address them,” said Ms. Brummett.
Barriers may include adequate staffing, or a system in place to triage patients who screen positive.
At Parkland, cost and workload have been minimal, Dr. Roaten said. “We built a model that only dedicates our highest-value resources to the most at-risk patients.”
She also noted that relief may be on the horizon for health systems where cost is an obstacle to universal screening and subsequent intervention. “There are efforts at the federal level to increase funding for suicide assessment and crisis response,” she said.
Pushback on Universal Screening
Universal suicide screening has its detractors, including critics who say expansion is unlikely to reduce suicide rates.
“The issue with suicidal ideation is that it is very dynamic. Suicidal ideation changes very quickly — sometimes within hours,” said Craig Bryan, PsyD, professor of psychiatry and behavioral health at Ohio State University in Columbus, Ohio.
Universal screening can also lead to false positives, where a patient who screens positive for suicidal ideation has no actual intention of attempting suicide, potentially creating unnecessary concern and burden on health care resources, Dr. Bryan noted.
“What do you do with everyone who screens positive?” Dr. Bryan said. “I’ve spoken with leaders of many health systems in the United States, and there is pushback against universal screening because they don’t have enough mental health resources to handle all of the referrals.”
Suicide screening also doesn’t predict who will die by suicide, Dr. Bryan added. It only identifies those willing to disclose suicidal thoughts. There is a significant number of people without mental illness who may never seek medical care, so “the warning signs we’re teaching people to recognize — depression, anxiety, and substance abuse — might not be evident in these individuals,” he said.
“Life sideswipes them suddenly, and they go from 0 to 60 ... and they may have access to a highly lethal method [of suicide] which weaponizes that moment of despair,” said Dr. Bryan. No amount of screening could possibly predict those types of suicides, he added.
Paul Nestadt, MD, associate professor of psychiatry and behavioral sciences at Johns Hopkins School of Medicine, agrees with Dr. Bryan and noted there isn’t a strong correlation between suicidal ideation and death by suicide.
“Suicidal thoughts are very common, but suicide is a rare event,” he said.
He cited a study that showed that two thirds of individuals who died by suicide had denied experiencing suicidal thoughts when asked, and half of them died within 2 days of this denial. Other research suggests that as many as 98% of people who express suicidal ideation do not die by suicide, Dr. Nestadt said.
A Public Health Issue
If universal screening is not the answer to predicting and preventing suicide, what is? One way would be to approach suicide as a public health issue, Dr. Nestadt said.
“How did we reduce the rate of motor vehicle deaths? We didn’t test each driver’s reaction time behind the wheel,” he said. “Instead, we passed seatbelt and airbag legislation, implemented federal speed limits, and as a result, the number of motor vehicle fatalities decreased.”
Dr. Nestadt is an advocate for stronger gun safety legislation, which has proven effective in reducing suicide rates. A study published this year showed that states with child access prevention laws, negligent storage laws, and mandatory waiting periods for gun purchases reported fewer suicide deaths than those without that legislation.
Other measures might be applied in cases of extreme individual suicide risk, including extreme risk protection orders, also known as “red flag” laws, he added. This type of legislation provides a pathway for law enforcement to temporarily remove firearms from individuals who pose a risk to themselves or others.
“These have been shown to be very effective in saving lives,” Dr. Nestadt said.
Dr. Nestadt and others are also using machine learning models to predict suicide risk. Those identified as high-risk may be flagged on their electronic medical record. Ideally, when the algorithm becomes more accurate at predicting suicide, anyone treating this patient can then decide if action is needed, said Dr. Nestadt.
In his work with suicidal military personnel, Dr. Bryan and his colleagues established a brief form of cognitive behavioral therapy (BCBT) to help participants challenge cognitive distortions and build coping strategies to deal with feel with intense feelings of distress. Data show that BCBT reduced suicide attempts among active-duty soldiers by 60% compared with standard mental health treatment. It has since been shown to work in civilians as well.
Dr. Bryan is also researching fluctuations in the wish to live versus the wish to die relative to one another and mapping the trajectory of risk states along the way.
The goal is that these and other suicide prevention strategies currently under study by his team and others will help stem the rise in suicide deaths.
“Overall, we need to train mental health providers to implement suicide prevention therapies and establish suicide risk programs,” Dr. Bryan said. “But until we build one of these suicide prevention interventions to scale, we’re putting the cart before the horse.”
Dr. Roaten, Ms. Brummett, Dr. Bryan, and Dr. Nestadt reported no relevant disclosures.
A version of this article appeared on Medscape.com.
US suicide rates have reached alarming levels, with data from Centers for Disease Control and Prevention (CDC) showing a 37% increase from 2000 to 2022. Nearly 49,000 people died by suicide in 2022 alone, translating to one death every 11 minutes.
The increase in suicide rates has prompted calls for expansion of universal suicide screening, in which all individuals in medical or mental health care settings are screened for suicide risk, regardless of the purpose for their visit. But the psychiatric field is split on the issue, with some experts citing false positives and a lack of mental health care resources for those deemed at risk.
In 2022, when the US Preventative Services Task Force released its recommendations on suicide prevention, first in children and adolescents, and then in adults, the authors said there was insufficient evidence to support universal suicide screening.
Proponents of the practice pushed back on that finding, arguing that universal suicide screening could help identify those at high risk who might otherwise go undiagnosed, leading to earlier, potentially lifesaving, intervention.
So, what is the case for — and against — universal screening?
Sounding an Alert
The introduction of universal screening was driven by a confluence of factors that began with a 1999 report by then-US Surgeon General David Satcher, MD. This was followed by a report in 2016 from the Joint Commission on Detecting and Treating Suicidal Ideation that called for healthcare organizations to improve detection and treatment of suicidal ideation in all healthcare care settings.
Data from the alert showed that a significant number of people who died by suicide had a healthcare visit before their death. Half had seen a clinician a month before their death; nearly 30% had a medical visit just the week before — all with no detection of increased suicide risk.
It was that sort of finding that led Parkland Health and Hospital System in Dallas to become the first US hospital to implement universal suicide screening. Since the program launched in 2015, the system has screened more than 4.3 million patients in its emergency department, inpatient units, and 20 primary care clinics.
“Since the program began, we’ve completed between 40,000 to 50,000 screenings per month,” said Kimberly Roaten, PhD, associate chief quality and safety officer for behavioral health at Parkland Health.
Clinicians at Parkland use the five-item Ask Suicide-Screening Questions to assess suicidal intent, a commonly used tool that was originally developed for use in pediatric emergency rooms (ERs). The tool, which takes about 20 seconds to administer, has since been validated in both children and adults.
Based on a patient’s response, a clinical decision support system integrated into the electronic health record classifies suicide risk as none, moderate, or high.
Patients identified as moderate risk are offered a more in-depth assessment with a mental health clinician, though participation is not mandatory, said Dr. Roaten. Those at high risk receive a more thorough evaluation.
The proportion of ER patients at Parkland who screen positive for any suicidal intent has consistently remained at about 7%, and at 2% in the primary care clinics, she said.
To better understand what the program may have had on suicide prevention, Dr. Roaten is leading a National Institute of Mental Health–funded study to link a decade of mortality data from the state of Texas to patient data from Parkland Health. Investigators will analyze information about patients identified at risk for suicide, those patients’ characteristics, and who dies by suicide.
Universal Screening Expands
Other health systems have adopted universal suicide screening including the Indian Health Service and the US Veterans Health Administration. Universal suicide screening is also in place in a growing number of primary care practices and hospitals throughout the United States and will be mandatory for patients aged 12 years and older in all acute care hospitals in California beginning in 2025.
There is also a push for universal screening to be coordinated through local, state, and federal government, nonprofit, and private sectors. The National Action Alliance for Suicide Prevention is charged with advancing the White House’s 2024 National Strategy for Suicide Prevention, a 10-year plan to address gaps in suicide prevention in the United States.
Sarah Brummett, JD, director of the National Action Alliance for Suicide Prevention’s executive committee, said that universal suicide screening is part of the 2024 strategy. “We know there are barriers to universal screening, and so it’s important to recognize what they are so we can address them,” said Ms. Brummett.
Barriers may include adequate staffing, or a system in place to triage patients who screen positive.
At Parkland, cost and workload have been minimal, Dr. Roaten said. “We built a model that only dedicates our highest-value resources to the most at-risk patients.”
She also noted that relief may be on the horizon for health systems where cost is an obstacle to universal screening and subsequent intervention. “There are efforts at the federal level to increase funding for suicide assessment and crisis response,” she said.
Pushback on Universal Screening
Universal suicide screening has its detractors, including critics who say expansion is unlikely to reduce suicide rates.
“The issue with suicidal ideation is that it is very dynamic. Suicidal ideation changes very quickly — sometimes within hours,” said Craig Bryan, PsyD, professor of psychiatry and behavioral health at Ohio State University in Columbus, Ohio.
Universal screening can also lead to false positives, where a patient who screens positive for suicidal ideation has no actual intention of attempting suicide, potentially creating unnecessary concern and burden on health care resources, Dr. Bryan noted.
“What do you do with everyone who screens positive?” Dr. Bryan said. “I’ve spoken with leaders of many health systems in the United States, and there is pushback against universal screening because they don’t have enough mental health resources to handle all of the referrals.”
Suicide screening also doesn’t predict who will die by suicide, Dr. Bryan added. It only identifies those willing to disclose suicidal thoughts. There is a significant number of people without mental illness who may never seek medical care, so “the warning signs we’re teaching people to recognize — depression, anxiety, and substance abuse — might not be evident in these individuals,” he said.
“Life sideswipes them suddenly, and they go from 0 to 60 ... and they may have access to a highly lethal method [of suicide] which weaponizes that moment of despair,” said Dr. Bryan. No amount of screening could possibly predict those types of suicides, he added.
Paul Nestadt, MD, associate professor of psychiatry and behavioral sciences at Johns Hopkins School of Medicine, agrees with Dr. Bryan and noted there isn’t a strong correlation between suicidal ideation and death by suicide.
“Suicidal thoughts are very common, but suicide is a rare event,” he said.
He cited a study that showed that two thirds of individuals who died by suicide had denied experiencing suicidal thoughts when asked, and half of them died within 2 days of this denial. Other research suggests that as many as 98% of people who express suicidal ideation do not die by suicide, Dr. Nestadt said.
A Public Health Issue
If universal screening is not the answer to predicting and preventing suicide, what is? One way would be to approach suicide as a public health issue, Dr. Nestadt said.
“How did we reduce the rate of motor vehicle deaths? We didn’t test each driver’s reaction time behind the wheel,” he said. “Instead, we passed seatbelt and airbag legislation, implemented federal speed limits, and as a result, the number of motor vehicle fatalities decreased.”
Dr. Nestadt is an advocate for stronger gun safety legislation, which has proven effective in reducing suicide rates. A study published this year showed that states with child access prevention laws, negligent storage laws, and mandatory waiting periods for gun purchases reported fewer suicide deaths than those without that legislation.
Other measures might be applied in cases of extreme individual suicide risk, including extreme risk protection orders, also known as “red flag” laws, he added. This type of legislation provides a pathway for law enforcement to temporarily remove firearms from individuals who pose a risk to themselves or others.
“These have been shown to be very effective in saving lives,” Dr. Nestadt said.
Dr. Nestadt and others are also using machine learning models to predict suicide risk. Those identified as high-risk may be flagged on their electronic medical record. Ideally, when the algorithm becomes more accurate at predicting suicide, anyone treating this patient can then decide if action is needed, said Dr. Nestadt.
In his work with suicidal military personnel, Dr. Bryan and his colleagues established a brief form of cognitive behavioral therapy (BCBT) to help participants challenge cognitive distortions and build coping strategies to deal with feel with intense feelings of distress. Data show that BCBT reduced suicide attempts among active-duty soldiers by 60% compared with standard mental health treatment. It has since been shown to work in civilians as well.
Dr. Bryan is also researching fluctuations in the wish to live versus the wish to die relative to one another and mapping the trajectory of risk states along the way.
The goal is that these and other suicide prevention strategies currently under study by his team and others will help stem the rise in suicide deaths.
“Overall, we need to train mental health providers to implement suicide prevention therapies and establish suicide risk programs,” Dr. Bryan said. “But until we build one of these suicide prevention interventions to scale, we’re putting the cart before the horse.”
Dr. Roaten, Ms. Brummett, Dr. Bryan, and Dr. Nestadt reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Playing the ‘Doctor’ Card: A Lesson in Three Hypotheticals
Scenario I. Let’s say you wake with a collection of symptoms. None of them is concerning, but the combination seems a bit unusual, or at least confusing. You would like to speak to your PCP, whom you have known for a long time, and ask for either reassurance or advice on whether you should make an appointment. However, your experience with the front office’s organization tells you that the quick 4-minute conversation you’re looking for is not going to happen easily.
You have that robotic phone message memorized. It begins suggesting that you think you have an emergency to call 911. Then it reminds you that if have a question about COVID to press “2,” which will take you to a recorded message and eventually link you to a triage nurse if the recording doesn’t answer your questions. If you need a prescription refill you should press “3.” If you are a doctor’s office and wish speak to the doctor press “4.” If you know you need an appointment press “5.” And finally if you have a question press “6” and leave a message and a nurse will get back to you before the end of the day.
The good news is that your PCP’s office is good to its word and will return your call the same day, but the bad news is that it is likely to be well into the afternoon. And, while you don’t consider your symptoms life-threatening, you don’t want getting an answer to be an exercise in schedule disruption.
You were a doctor before you retired and you still have an “office.” It’s really more of a combination den and studio. So, technically you are a doctor’s office wanting to speak to the doctor. And, you know that pressing “4” will get you the answer you are looking for in a matter of minutes.
Scenario II. Your spouse, or your aunt, or the elderly widow next door asks you to accompany her at an upcoming doctor’s visit because she had been having trouble understanding the physician’s plan regarding further diagnosis and possible treatment. She believes having you along as kind of an interpreter/advocate would be a big help. Do you agree and do you make any stipulations?
Scenario III. Your PCP has referred you to a specialist. You are filling out the previsit form(s). Do you list your occupation as “retired physician” or just “retired”? Or just leave it blank?
Whether you deserve it or not, graduating from medical school has conferred on you a specialness in the eyes of many people. It is assumed you are smarter than the average bear and in taking the Hippocratic oath you have joined an elite club. And, with that membership comes some special undefined privileges.
But with that specialness there are are some downsides. For example, in some states being a physician once allowed you to have a license plate with “MD” in the number sequence. Sometimes that helped you avoid the occasional parking ticket. That is until folks realized the “MD” made you a target for car thieves and drug seekers who mistakenly believe we all carry drugs in our glove compartments.
So what about that first scenario? Do you press “4” to jump yourself to the head of the queue and avoid the inconvenience of having to wait for a reasonably timely response from your PCP? After all, you are fellow physicians and you’ve known her for a decade or two. If you are retired is your time any more valuable than that of her other patients? If you are still in active practice you can argue that getting special attention will benefit your patients. But, if it’s a weekend and you are off it’s a bit harder to rationalize special treatment. Playing the doctor card in this situation is your own decision but you must be prepared to shoulder the perceptions by your PCP and her staff as well as your own sense of fairness.
The other two scenarios are much different. In neither are you risking the impression that you are asking for a favor. But, they each have their downsides. In the second scenario you are doing someone a favor to act as an interpreter. How could this have downside? Unfortunately, what happens too often in situations like this is that when the patient’s physician learns that you are a fellow physician, the rest of the visit becomes a dialogue in doctor-speak between the two physicians with the patient sitting by as an observer. In the end this discussion may benefit the patient by creating a treatment plan that the patient can understand either because they overheard it or more likely because you eventually explained it to them.
On the other the hand, this doctor-to-doctor chat has done nothing to build a doctor-patient relationship that had obviously been lacking something. In situations like this it is probably better to keep the doctor card up your sleeve to be played at the end of the visit or maybe not at all. Before agreeing to be an interpreter/advocate, ask the patient to avoid mentioning that you are a physician. Instead, ask that she introduce you as a friend or relative that she has asked to come along to serve as a memory bank. During the visit it may be helpful to occasionally interject and suggest that the patient ask a question that hasn’t been adequately addressed. While some physicians may be upset when they belatedly find you have not revealed up front that you are a physician, I find this a harmless omission that has the benefit of improving patient care.
The final scenario — in which you are the patient — is likely to occur more often as you get older. When filling out a previsit form, I often simply put retired or leave it blank. But, how I answer the question often seems to be irrelevant because I have learned that physicians and their staff read those boilerplate forms so cursorily that even when I report my status as “retired physician” everyone seems surprised if and when it later comes to light.
My rationale in keeping the doctor card close to my vest in these situations is that I want to be addressed without any assumptions regarding my medical knowledge, which in my situation is well over half a century old and spotty at best. I don’t want my physicians to say “I’m sure you understand.” Because I often don’t. I would like them to learn about who I am just as I hope they would other patients. I won’t be offended if they “talk down” to me. If this specialist is as good as I’ve heard she is, I want to hear her full performance, not one edited for fellow and former physicians.
It doesn’t arrive gold edged with a list of special privileges. If it comes with any extras, they are risks that must be avoided.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Scenario I. Let’s say you wake with a collection of symptoms. None of them is concerning, but the combination seems a bit unusual, or at least confusing. You would like to speak to your PCP, whom you have known for a long time, and ask for either reassurance or advice on whether you should make an appointment. However, your experience with the front office’s organization tells you that the quick 4-minute conversation you’re looking for is not going to happen easily.
You have that robotic phone message memorized. It begins suggesting that you think you have an emergency to call 911. Then it reminds you that if have a question about COVID to press “2,” which will take you to a recorded message and eventually link you to a triage nurse if the recording doesn’t answer your questions. If you need a prescription refill you should press “3.” If you are a doctor’s office and wish speak to the doctor press “4.” If you know you need an appointment press “5.” And finally if you have a question press “6” and leave a message and a nurse will get back to you before the end of the day.
The good news is that your PCP’s office is good to its word and will return your call the same day, but the bad news is that it is likely to be well into the afternoon. And, while you don’t consider your symptoms life-threatening, you don’t want getting an answer to be an exercise in schedule disruption.
You were a doctor before you retired and you still have an “office.” It’s really more of a combination den and studio. So, technically you are a doctor’s office wanting to speak to the doctor. And, you know that pressing “4” will get you the answer you are looking for in a matter of minutes.
Scenario II. Your spouse, or your aunt, or the elderly widow next door asks you to accompany her at an upcoming doctor’s visit because she had been having trouble understanding the physician’s plan regarding further diagnosis and possible treatment. She believes having you along as kind of an interpreter/advocate would be a big help. Do you agree and do you make any stipulations?
Scenario III. Your PCP has referred you to a specialist. You are filling out the previsit form(s). Do you list your occupation as “retired physician” or just “retired”? Or just leave it blank?
Whether you deserve it or not, graduating from medical school has conferred on you a specialness in the eyes of many people. It is assumed you are smarter than the average bear and in taking the Hippocratic oath you have joined an elite club. And, with that membership comes some special undefined privileges.
But with that specialness there are are some downsides. For example, in some states being a physician once allowed you to have a license plate with “MD” in the number sequence. Sometimes that helped you avoid the occasional parking ticket. That is until folks realized the “MD” made you a target for car thieves and drug seekers who mistakenly believe we all carry drugs in our glove compartments.
So what about that first scenario? Do you press “4” to jump yourself to the head of the queue and avoid the inconvenience of having to wait for a reasonably timely response from your PCP? After all, you are fellow physicians and you’ve known her for a decade or two. If you are retired is your time any more valuable than that of her other patients? If you are still in active practice you can argue that getting special attention will benefit your patients. But, if it’s a weekend and you are off it’s a bit harder to rationalize special treatment. Playing the doctor card in this situation is your own decision but you must be prepared to shoulder the perceptions by your PCP and her staff as well as your own sense of fairness.
The other two scenarios are much different. In neither are you risking the impression that you are asking for a favor. But, they each have their downsides. In the second scenario you are doing someone a favor to act as an interpreter. How could this have downside? Unfortunately, what happens too often in situations like this is that when the patient’s physician learns that you are a fellow physician, the rest of the visit becomes a dialogue in doctor-speak between the two physicians with the patient sitting by as an observer. In the end this discussion may benefit the patient by creating a treatment plan that the patient can understand either because they overheard it or more likely because you eventually explained it to them.
On the other the hand, this doctor-to-doctor chat has done nothing to build a doctor-patient relationship that had obviously been lacking something. In situations like this it is probably better to keep the doctor card up your sleeve to be played at the end of the visit or maybe not at all. Before agreeing to be an interpreter/advocate, ask the patient to avoid mentioning that you are a physician. Instead, ask that she introduce you as a friend or relative that she has asked to come along to serve as a memory bank. During the visit it may be helpful to occasionally interject and suggest that the patient ask a question that hasn’t been adequately addressed. While some physicians may be upset when they belatedly find you have not revealed up front that you are a physician, I find this a harmless omission that has the benefit of improving patient care.
The final scenario — in which you are the patient — is likely to occur more often as you get older. When filling out a previsit form, I often simply put retired or leave it blank. But, how I answer the question often seems to be irrelevant because I have learned that physicians and their staff read those boilerplate forms so cursorily that even when I report my status as “retired physician” everyone seems surprised if and when it later comes to light.
My rationale in keeping the doctor card close to my vest in these situations is that I want to be addressed without any assumptions regarding my medical knowledge, which in my situation is well over half a century old and spotty at best. I don’t want my physicians to say “I’m sure you understand.” Because I often don’t. I would like them to learn about who I am just as I hope they would other patients. I won’t be offended if they “talk down” to me. If this specialist is as good as I’ve heard she is, I want to hear her full performance, not one edited for fellow and former physicians.
It doesn’t arrive gold edged with a list of special privileges. If it comes with any extras, they are risks that must be avoided.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Scenario I. Let’s say you wake with a collection of symptoms. None of them is concerning, but the combination seems a bit unusual, or at least confusing. You would like to speak to your PCP, whom you have known for a long time, and ask for either reassurance or advice on whether you should make an appointment. However, your experience with the front office’s organization tells you that the quick 4-minute conversation you’re looking for is not going to happen easily.
You have that robotic phone message memorized. It begins suggesting that you think you have an emergency to call 911. Then it reminds you that if have a question about COVID to press “2,” which will take you to a recorded message and eventually link you to a triage nurse if the recording doesn’t answer your questions. If you need a prescription refill you should press “3.” If you are a doctor’s office and wish speak to the doctor press “4.” If you know you need an appointment press “5.” And finally if you have a question press “6” and leave a message and a nurse will get back to you before the end of the day.
The good news is that your PCP’s office is good to its word and will return your call the same day, but the bad news is that it is likely to be well into the afternoon. And, while you don’t consider your symptoms life-threatening, you don’t want getting an answer to be an exercise in schedule disruption.
You were a doctor before you retired and you still have an “office.” It’s really more of a combination den and studio. So, technically you are a doctor’s office wanting to speak to the doctor. And, you know that pressing “4” will get you the answer you are looking for in a matter of minutes.
Scenario II. Your spouse, or your aunt, or the elderly widow next door asks you to accompany her at an upcoming doctor’s visit because she had been having trouble understanding the physician’s plan regarding further diagnosis and possible treatment. She believes having you along as kind of an interpreter/advocate would be a big help. Do you agree and do you make any stipulations?
Scenario III. Your PCP has referred you to a specialist. You are filling out the previsit form(s). Do you list your occupation as “retired physician” or just “retired”? Or just leave it blank?
Whether you deserve it or not, graduating from medical school has conferred on you a specialness in the eyes of many people. It is assumed you are smarter than the average bear and in taking the Hippocratic oath you have joined an elite club. And, with that membership comes some special undefined privileges.
But with that specialness there are are some downsides. For example, in some states being a physician once allowed you to have a license plate with “MD” in the number sequence. Sometimes that helped you avoid the occasional parking ticket. That is until folks realized the “MD” made you a target for car thieves and drug seekers who mistakenly believe we all carry drugs in our glove compartments.
So what about that first scenario? Do you press “4” to jump yourself to the head of the queue and avoid the inconvenience of having to wait for a reasonably timely response from your PCP? After all, you are fellow physicians and you’ve known her for a decade or two. If you are retired is your time any more valuable than that of her other patients? If you are still in active practice you can argue that getting special attention will benefit your patients. But, if it’s a weekend and you are off it’s a bit harder to rationalize special treatment. Playing the doctor card in this situation is your own decision but you must be prepared to shoulder the perceptions by your PCP and her staff as well as your own sense of fairness.
The other two scenarios are much different. In neither are you risking the impression that you are asking for a favor. But, they each have their downsides. In the second scenario you are doing someone a favor to act as an interpreter. How could this have downside? Unfortunately, what happens too often in situations like this is that when the patient’s physician learns that you are a fellow physician, the rest of the visit becomes a dialogue in doctor-speak between the two physicians with the patient sitting by as an observer. In the end this discussion may benefit the patient by creating a treatment plan that the patient can understand either because they overheard it or more likely because you eventually explained it to them.
On the other the hand, this doctor-to-doctor chat has done nothing to build a doctor-patient relationship that had obviously been lacking something. In situations like this it is probably better to keep the doctor card up your sleeve to be played at the end of the visit or maybe not at all. Before agreeing to be an interpreter/advocate, ask the patient to avoid mentioning that you are a physician. Instead, ask that she introduce you as a friend or relative that she has asked to come along to serve as a memory bank. During the visit it may be helpful to occasionally interject and suggest that the patient ask a question that hasn’t been adequately addressed. While some physicians may be upset when they belatedly find you have not revealed up front that you are a physician, I find this a harmless omission that has the benefit of improving patient care.
The final scenario — in which you are the patient — is likely to occur more often as you get older. When filling out a previsit form, I often simply put retired or leave it blank. But, how I answer the question often seems to be irrelevant because I have learned that physicians and their staff read those boilerplate forms so cursorily that even when I report my status as “retired physician” everyone seems surprised if and when it later comes to light.
My rationale in keeping the doctor card close to my vest in these situations is that I want to be addressed without any assumptions regarding my medical knowledge, which in my situation is well over half a century old and spotty at best. I don’t want my physicians to say “I’m sure you understand.” Because I often don’t. I would like them to learn about who I am just as I hope they would other patients. I won’t be offended if they “talk down” to me. If this specialist is as good as I’ve heard she is, I want to hear her full performance, not one edited for fellow and former physicians.
It doesn’t arrive gold edged with a list of special privileges. If it comes with any extras, they are risks that must be avoided.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
FDA Approves IL-13 inhibitor for Atopic Dermatitis
The that is not well controlled, despite treatment with topical prescription therapies.
The recommended initial starting dose of lebrikizumab consists of 500 mg (two 250 mg injections) at baseline and week 2, followed by 250 mg every 2 weeks until week 16 or later when adequate clinical response is achieved. Then, maintenance dosing is recommended with one monthly injection (250 mg every 4 weeks). Children aged 12-17 years must weigh at least 88 pounds (40 kg) to be eligible for lebrikizumab treatment.
According to a press release from Lilly, which has been developing lebrikizumab, approval was based on results from the ADvocate 1, ADvocate 2, and ADhere studies, which included over 1000 adults and children aged 12 and older with moderate to severe AD. The primary endpoint for these studies was evaluated at 16 weeks and measured clear or almost clear skin (IGA score of 0 or 1).
According to Lilly, 38% of people in ADvocate 1 and 2 who took lebrikizumab achieved clear or almost-clear skin at 16 weeks, compared with 12% of those in the placebo arm, and 10% experienced these results as early as 4 weeks. Of those treated with lebrikizumab who experienced clear or almost-clear skin at week 16, 77% maintained those results at 1 year on the once-monthly dose. In addition, on average, 43% of those on lebrikizumab experienced relief of itch at 16 weeks, compared with 12% of those on placebo, according to the press release.
The most common side effects of lebrikizumab observed in the clinical trials include eye and eyelid inflammation, such as redness, swelling, and itching; injection-site reactions; and herpes zoster (shingles).
Lebrikizumab was approved in Japan in January 2024, and by the European Commission in 2023.
A version of this article first appeared on Medscape.com.
The that is not well controlled, despite treatment with topical prescription therapies.
The recommended initial starting dose of lebrikizumab consists of 500 mg (two 250 mg injections) at baseline and week 2, followed by 250 mg every 2 weeks until week 16 or later when adequate clinical response is achieved. Then, maintenance dosing is recommended with one monthly injection (250 mg every 4 weeks). Children aged 12-17 years must weigh at least 88 pounds (40 kg) to be eligible for lebrikizumab treatment.
According to a press release from Lilly, which has been developing lebrikizumab, approval was based on results from the ADvocate 1, ADvocate 2, and ADhere studies, which included over 1000 adults and children aged 12 and older with moderate to severe AD. The primary endpoint for these studies was evaluated at 16 weeks and measured clear or almost clear skin (IGA score of 0 or 1).
According to Lilly, 38% of people in ADvocate 1 and 2 who took lebrikizumab achieved clear or almost-clear skin at 16 weeks, compared with 12% of those in the placebo arm, and 10% experienced these results as early as 4 weeks. Of those treated with lebrikizumab who experienced clear or almost-clear skin at week 16, 77% maintained those results at 1 year on the once-monthly dose. In addition, on average, 43% of those on lebrikizumab experienced relief of itch at 16 weeks, compared with 12% of those on placebo, according to the press release.
The most common side effects of lebrikizumab observed in the clinical trials include eye and eyelid inflammation, such as redness, swelling, and itching; injection-site reactions; and herpes zoster (shingles).
Lebrikizumab was approved in Japan in January 2024, and by the European Commission in 2023.
A version of this article first appeared on Medscape.com.
The that is not well controlled, despite treatment with topical prescription therapies.
The recommended initial starting dose of lebrikizumab consists of 500 mg (two 250 mg injections) at baseline and week 2, followed by 250 mg every 2 weeks until week 16 or later when adequate clinical response is achieved. Then, maintenance dosing is recommended with one monthly injection (250 mg every 4 weeks). Children aged 12-17 years must weigh at least 88 pounds (40 kg) to be eligible for lebrikizumab treatment.
According to a press release from Lilly, which has been developing lebrikizumab, approval was based on results from the ADvocate 1, ADvocate 2, and ADhere studies, which included over 1000 adults and children aged 12 and older with moderate to severe AD. The primary endpoint for these studies was evaluated at 16 weeks and measured clear or almost clear skin (IGA score of 0 or 1).
According to Lilly, 38% of people in ADvocate 1 and 2 who took lebrikizumab achieved clear or almost-clear skin at 16 weeks, compared with 12% of those in the placebo arm, and 10% experienced these results as early as 4 weeks. Of those treated with lebrikizumab who experienced clear or almost-clear skin at week 16, 77% maintained those results at 1 year on the once-monthly dose. In addition, on average, 43% of those on lebrikizumab experienced relief of itch at 16 weeks, compared with 12% of those on placebo, according to the press release.
The most common side effects of lebrikizumab observed in the clinical trials include eye and eyelid inflammation, such as redness, swelling, and itching; injection-site reactions; and herpes zoster (shingles).
Lebrikizumab was approved in Japan in January 2024, and by the European Commission in 2023.
A version of this article first appeared on Medscape.com.
A 14-Year-Old Female Presents With a Growth Under Her Toenail
BY XOCHITL LONGSTAFF, BS; ANGELINA LABIB, MD; AND DAWN EICHENFIELD, MD, PHD
Diagnosis: Subungual bony exostosis
The patient was referred to orthopedics for further evaluation and ultimately underwent excisional surgery. At her most recent follow-up visit with orthopedic surgery, her new nail was observed to be growing well.
Subungual exostosis, also known as Dupuytren’s exostosis, is a benign osteocartilaginous tumor that classically presents as a bony growth at the dorsal aspect of the distal phalanx of the great toe, near the nail bed. The pathogenesis remains unclear, but suggested etiologies include prior trauma, infection, and hereditary abnormalities.1
Clinically, lesions can be painful and may be associated with skin ulceration. The location at the dorsal distal great toe is a key distinguishing feature. Physical exam reveals a firm, fixed nodule with a hyperkeratotic smooth surface.2
Radiographic evaluation, particularly with a lateral view, is often diagnostic. The classic radiographic finding in subungual exostosis is an osseous structure connected to the distal phalanx, with a hazy periphery representing a fibrocartilage cap.
Treatment involves complete marginal excision. The complications from surgical excision are minimal, with the most common being recurrence.3 However, the recurrence rate is also generally low, around 4%.1
Ms. Longstaff is currently completing a research year as a Pediatric Clinical Research Fellow at University of California San Diego (UCSD) Rady Children’s Hospital prior to finishing her final year at the David Geffen School of Medicine at the University of California, Los Angeles. Dr. Labib is the Post-Doctoral Pediatric Clinical Research Fellow at UCSD Rady Children’s Hospital. Dr. Eichenfield is a dermatologist at Rady Children’s Hospital–San Diego and assistant clinical professor at UCSD.
References
1. Alabdullrahman LW et al. Osteochondroma. In: StatPearls [Internet]. 2024 Feb 26. https://www.ncbi.nlm.nih.gov/books/NBK544296/#.
2. DaCambra MP et al. Clin Orthop Relat Res. 2014 Apr;472(4):1251-9. doi: 10.1007/s11999-013-3345-4.
3. Womack ME et al. J Am Acad Orthop Surg Glob Res Rev. 2022 Mar 22;6(3):e21.00239. doi: 10.5435/JAAOSGlobal-D-21-00239.
BY XOCHITL LONGSTAFF, BS; ANGELINA LABIB, MD; AND DAWN EICHENFIELD, MD, PHD
Diagnosis: Subungual bony exostosis
The patient was referred to orthopedics for further evaluation and ultimately underwent excisional surgery. At her most recent follow-up visit with orthopedic surgery, her new nail was observed to be growing well.
Subungual exostosis, also known as Dupuytren’s exostosis, is a benign osteocartilaginous tumor that classically presents as a bony growth at the dorsal aspect of the distal phalanx of the great toe, near the nail bed. The pathogenesis remains unclear, but suggested etiologies include prior trauma, infection, and hereditary abnormalities.1
Clinically, lesions can be painful and may be associated with skin ulceration. The location at the dorsal distal great toe is a key distinguishing feature. Physical exam reveals a firm, fixed nodule with a hyperkeratotic smooth surface.2
Radiographic evaluation, particularly with a lateral view, is often diagnostic. The classic radiographic finding in subungual exostosis is an osseous structure connected to the distal phalanx, with a hazy periphery representing a fibrocartilage cap.
Treatment involves complete marginal excision. The complications from surgical excision are minimal, with the most common being recurrence.3 However, the recurrence rate is also generally low, around 4%.1
Ms. Longstaff is currently completing a research year as a Pediatric Clinical Research Fellow at University of California San Diego (UCSD) Rady Children’s Hospital prior to finishing her final year at the David Geffen School of Medicine at the University of California, Los Angeles. Dr. Labib is the Post-Doctoral Pediatric Clinical Research Fellow at UCSD Rady Children’s Hospital. Dr. Eichenfield is a dermatologist at Rady Children’s Hospital–San Diego and assistant clinical professor at UCSD.
References
1. Alabdullrahman LW et al. Osteochondroma. In: StatPearls [Internet]. 2024 Feb 26. https://www.ncbi.nlm.nih.gov/books/NBK544296/#.
2. DaCambra MP et al. Clin Orthop Relat Res. 2014 Apr;472(4):1251-9. doi: 10.1007/s11999-013-3345-4.
3. Womack ME et al. J Am Acad Orthop Surg Glob Res Rev. 2022 Mar 22;6(3):e21.00239. doi: 10.5435/JAAOSGlobal-D-21-00239.
BY XOCHITL LONGSTAFF, BS; ANGELINA LABIB, MD; AND DAWN EICHENFIELD, MD, PHD
Diagnosis: Subungual bony exostosis
The patient was referred to orthopedics for further evaluation and ultimately underwent excisional surgery. At her most recent follow-up visit with orthopedic surgery, her new nail was observed to be growing well.
Subungual exostosis, also known as Dupuytren’s exostosis, is a benign osteocartilaginous tumor that classically presents as a bony growth at the dorsal aspect of the distal phalanx of the great toe, near the nail bed. The pathogenesis remains unclear, but suggested etiologies include prior trauma, infection, and hereditary abnormalities.1
Clinically, lesions can be painful and may be associated with skin ulceration. The location at the dorsal distal great toe is a key distinguishing feature. Physical exam reveals a firm, fixed nodule with a hyperkeratotic smooth surface.2
Radiographic evaluation, particularly with a lateral view, is often diagnostic. The classic radiographic finding in subungual exostosis is an osseous structure connected to the distal phalanx, with a hazy periphery representing a fibrocartilage cap.
Treatment involves complete marginal excision. The complications from surgical excision are minimal, with the most common being recurrence.3 However, the recurrence rate is also generally low, around 4%.1
Ms. Longstaff is currently completing a research year as a Pediatric Clinical Research Fellow at University of California San Diego (UCSD) Rady Children’s Hospital prior to finishing her final year at the David Geffen School of Medicine at the University of California, Los Angeles. Dr. Labib is the Post-Doctoral Pediatric Clinical Research Fellow at UCSD Rady Children’s Hospital. Dr. Eichenfield is a dermatologist at Rady Children’s Hospital–San Diego and assistant clinical professor at UCSD.
References
1. Alabdullrahman LW et al. Osteochondroma. In: StatPearls [Internet]. 2024 Feb 26. https://www.ncbi.nlm.nih.gov/books/NBK544296/#.
2. DaCambra MP et al. Clin Orthop Relat Res. 2014 Apr;472(4):1251-9. doi: 10.1007/s11999-013-3345-4.
3. Womack ME et al. J Am Acad Orthop Surg Glob Res Rev. 2022 Mar 22;6(3):e21.00239. doi: 10.5435/JAAOSGlobal-D-21-00239.
A 14-year-old healthy female presents with a painful nodule under her great toenail. The nodule had been present for 2 months and there was no preceding trauma. Three days prior to presentation, her nail cracked and bled after bumping her toe. The toe is painful to palpation. Given the associated pain, the patient visited urgent care and was prescribed cephalexin and acetaminophen.
Physical examination reveals a skin-colored subungual nodule with hypertrophic tissue originating from the nail bed of the right great toe, but no thickening of the nail plate (Figures 1-3).
The Surgeon General’s Advisory on Parental Mental Health: Implications for Pediatric Practice
As child psychiatrists and pediatricians, our mission extends beyond treating the physical health of children; it encompasses understanding the intricate web of factors that influence a child’s overall well-being. A recent advisory from U.S. Surgeon General Dr. Vivek Murthy has brought to light a critical issue that demands our attention: the declining mental health of parents and its profound impact on children. As providers who depend heavily on parental involvement to manage the needs of our pediatric patients, addressing parental mental health can be a crucial step in safeguarding the mental health of children.
The Surgeon General’s Advisory: A Call to Action
On August 28, 2024, the U.S. Surgeon General issued an advisory highlighting the significant stressors impacting parents and caregivers, and the broader implications for children’s mental health. The advisory emphasizes the bidirectional relationship between parental and child mental health, urging healthcare providers, policymakers, and communities to prioritize support for parents. It stresses that the mental health of parents is not only vital for their well-being but also plays a critical role in shaping the emotional and psychological development of their children.1
The Link Between Parental and Child Mental Health
Research shows that a parent’s mental health directly influences the child’s emotional and behavioral outcomes. Children of parents with untreated mental health conditions, such as depression, anxiety, trauma, or chronic stress, are at a significantly higher risk of developing similar conditions. This risk is mediated through various mechanisms, including genetic predisposition, compromised parent-child interactions, and exposure to adverse environments.
1. Parental Depression and Child Outcomes: Parental depression, particularly maternal depression, has been extensively studied and is strongly associated with emotional and behavioral problems in children. Children of depressed parents are more likely to experience anxiety, depression, and resulting academic difficulties. Depressed parents may struggle with consistent and positive parenting, which can disrupt the development of secure attachments and emotional regulation in children.2-4
2. Anxiety and Parenting Styles: Parental anxiety can influence parenting styles, often leading to overprotectiveness, inconsistency, or heightened criticism. These behaviors, in turn, can cultivate anxiety in children, creating a cycle that perpetuates mental health challenges across generations. Children raised in environments where anxiety is pervasive may learn to view the world as threatening, contributing to hypervigilance and stress.5
3. Impact of Chronic Stress on Parenting: Chronic stress experienced by parents, often due to financial hardship, lack of social support, or work-life imbalance, can impair their ability to engage in responsive and nurturing parenting. This, in turn, can affect children’s ability to meaningfully engage with parents to form secure attachments. Further, chronic stress can negatively impact the quality of parent-child interactions and fuel the cycle of rupture with limited opportunity for repair. The advisory stresses the need to address these systemic stressors as part of a broader public health strategy to support families.1
Implications for Pediatric Practice
Pediatricians are often the first point of contact for families navigating mental health challenges. The Surgeon General’s advisory highlights the need for pediatricians to adopt a holistic approach that considers the mental health of the entire family, not just the child. This can be challenging with the average follow-up appointment time of 16 minutes, though many of the recommendations take this logistical hurdle into consideration:
1. Screening for Parental Mental Health: Incorporating routine screening for parental mental health into pediatric visits can be a powerful tool. Questions about parental stress, depression (especially postpartum depression), and anxiety should be integrated into well-child visits, especially in families where children present with emotional or behavioral difficulties. By identifying at-risk parents early, timely referrals to mental health services can be secured.
2. Providing Resources and Referrals: Offering resources and referrals to parents who may be struggling can positively impact the entire family. This includes connecting families with mental health professionals, parenting support groups, or community resources that can alleviate stressors such as food insecurity or lack of childcare. Having a list of local mental health resources available in your practice can empower parents to seek the help they need.
3. Promoting Positive Parenting Practices: Guidance on positive parenting practices, stress management, and self-care can make a significant difference in the mental health of parents and their children. Workshops or educational materials on topics like mindfulness, managing work-life balance, and fostering healthy communication within the family can be valuable and high-yield additions to pediatric care.
4. Collaborative Care Models: Collaborative care models, where pediatricians work closely with child psychiatrists, psychologists, and social workers, can provide comprehensive support to families. This integrated approach ensures that both children and their parents receive the care they need, promoting better outcomes for the entire family unit.
Addressing Broader Systemic Issues
The advisory also calls for systemic changes that extend beyond the clinic. Policy changes such as expanding access to paid family leave, affordable childcare, and mental health services are essential to creating an environment where parents can thrive. As pediatricians, advocating for these changes at the local and national level can amplify the overall impact on families.
1. Advocating for Paid Family Leave: Paid family leave allows parents to bond with their children and attend to their own mental health needs without the added pressure of financial instability. Supporting policies that provide adequate paid leave can pave the way for a successful and healthy return to work and have long-term benefits for family health.
2. Expanding Mental Health Services: Increasing access to mental health services, especially in underserved communities, is crucial. Pediatricians can play a role by partnering with local mental health providers to offer integrated care within their practices or community settings.
3. Community Support Programs: The creation of community support programs that offer parenting classes, stress management workshops, and peer support groups can help reduce the isolation and stress that many parents feel. Pediatricians can collaborate with community organizations to promote these resources to families.
Conclusion
The Surgeon General’s advisory serves as a timely reminder of the interconnectedness of parental and child mental health. Pediatricians have a unique opportunity to influence not only the health of their pediatric patients, but also the well-being of their families. By recognizing and addressing the mental health needs of parents, we can break the cycle of stress and mental illness that affects so many families, ensuring a healthier future for the next generation.
Let us embrace this call to action and work together to create a supportive environment where all parents and children can thrive.
Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. American Hospital Association. Surgeon General Issues Advisory on Mental Health and Well-Being of Parents. American Hospital Association. 2024 Sep 4.
2. Goodman SH, Gotlib IH. Risk for Psychopathology in the Children of Depressed Mothers: A Developmental Model for Understanding Mechanisms of Transmission. Psychol Rev. 1999;106(3):458-490. doi: 10.1037/0033-295X.106.3.458.
3. Lovejoy MC et al. Maternal Depression and Parenting Behavior: A Meta-Analytic Review. Clin Psychol Rev. 2000;20(5):561-592. doi: 10.1016/s0272-7358(98)00100-7.
4. Weissman MM et al. Offspring of Depressed Parents: 20 Years Later. Am J Psychiatry. 2006;163(6):1001-1008. doi: 10.1176/ajp.2006.163.6.1001.
5. Smith KE, Pollak SD. Early Life Stress and Development: Potential Mechanisms for Adverse Outcomes. J Neurodev Disord. 2020;12(1):3-14. doi: 10.1186/s11689-020-09337-y.
As child psychiatrists and pediatricians, our mission extends beyond treating the physical health of children; it encompasses understanding the intricate web of factors that influence a child’s overall well-being. A recent advisory from U.S. Surgeon General Dr. Vivek Murthy has brought to light a critical issue that demands our attention: the declining mental health of parents and its profound impact on children. As providers who depend heavily on parental involvement to manage the needs of our pediatric patients, addressing parental mental health can be a crucial step in safeguarding the mental health of children.
The Surgeon General’s Advisory: A Call to Action
On August 28, 2024, the U.S. Surgeon General issued an advisory highlighting the significant stressors impacting parents and caregivers, and the broader implications for children’s mental health. The advisory emphasizes the bidirectional relationship between parental and child mental health, urging healthcare providers, policymakers, and communities to prioritize support for parents. It stresses that the mental health of parents is not only vital for their well-being but also plays a critical role in shaping the emotional and psychological development of their children.1
The Link Between Parental and Child Mental Health
Research shows that a parent’s mental health directly influences the child’s emotional and behavioral outcomes. Children of parents with untreated mental health conditions, such as depression, anxiety, trauma, or chronic stress, are at a significantly higher risk of developing similar conditions. This risk is mediated through various mechanisms, including genetic predisposition, compromised parent-child interactions, and exposure to adverse environments.
1. Parental Depression and Child Outcomes: Parental depression, particularly maternal depression, has been extensively studied and is strongly associated with emotional and behavioral problems in children. Children of depressed parents are more likely to experience anxiety, depression, and resulting academic difficulties. Depressed parents may struggle with consistent and positive parenting, which can disrupt the development of secure attachments and emotional regulation in children.2-4
2. Anxiety and Parenting Styles: Parental anxiety can influence parenting styles, often leading to overprotectiveness, inconsistency, or heightened criticism. These behaviors, in turn, can cultivate anxiety in children, creating a cycle that perpetuates mental health challenges across generations. Children raised in environments where anxiety is pervasive may learn to view the world as threatening, contributing to hypervigilance and stress.5
3. Impact of Chronic Stress on Parenting: Chronic stress experienced by parents, often due to financial hardship, lack of social support, or work-life imbalance, can impair their ability to engage in responsive and nurturing parenting. This, in turn, can affect children’s ability to meaningfully engage with parents to form secure attachments. Further, chronic stress can negatively impact the quality of parent-child interactions and fuel the cycle of rupture with limited opportunity for repair. The advisory stresses the need to address these systemic stressors as part of a broader public health strategy to support families.1
Implications for Pediatric Practice
Pediatricians are often the first point of contact for families navigating mental health challenges. The Surgeon General’s advisory highlights the need for pediatricians to adopt a holistic approach that considers the mental health of the entire family, not just the child. This can be challenging with the average follow-up appointment time of 16 minutes, though many of the recommendations take this logistical hurdle into consideration:
1. Screening for Parental Mental Health: Incorporating routine screening for parental mental health into pediatric visits can be a powerful tool. Questions about parental stress, depression (especially postpartum depression), and anxiety should be integrated into well-child visits, especially in families where children present with emotional or behavioral difficulties. By identifying at-risk parents early, timely referrals to mental health services can be secured.
2. Providing Resources and Referrals: Offering resources and referrals to parents who may be struggling can positively impact the entire family. This includes connecting families with mental health professionals, parenting support groups, or community resources that can alleviate stressors such as food insecurity or lack of childcare. Having a list of local mental health resources available in your practice can empower parents to seek the help they need.
3. Promoting Positive Parenting Practices: Guidance on positive parenting practices, stress management, and self-care can make a significant difference in the mental health of parents and their children. Workshops or educational materials on topics like mindfulness, managing work-life balance, and fostering healthy communication within the family can be valuable and high-yield additions to pediatric care.
4. Collaborative Care Models: Collaborative care models, where pediatricians work closely with child psychiatrists, psychologists, and social workers, can provide comprehensive support to families. This integrated approach ensures that both children and their parents receive the care they need, promoting better outcomes for the entire family unit.
Addressing Broader Systemic Issues
The advisory also calls for systemic changes that extend beyond the clinic. Policy changes such as expanding access to paid family leave, affordable childcare, and mental health services are essential to creating an environment where parents can thrive. As pediatricians, advocating for these changes at the local and national level can amplify the overall impact on families.
1. Advocating for Paid Family Leave: Paid family leave allows parents to bond with their children and attend to their own mental health needs without the added pressure of financial instability. Supporting policies that provide adequate paid leave can pave the way for a successful and healthy return to work and have long-term benefits for family health.
2. Expanding Mental Health Services: Increasing access to mental health services, especially in underserved communities, is crucial. Pediatricians can play a role by partnering with local mental health providers to offer integrated care within their practices or community settings.
3. Community Support Programs: The creation of community support programs that offer parenting classes, stress management workshops, and peer support groups can help reduce the isolation and stress that many parents feel. Pediatricians can collaborate with community organizations to promote these resources to families.
Conclusion
The Surgeon General’s advisory serves as a timely reminder of the interconnectedness of parental and child mental health. Pediatricians have a unique opportunity to influence not only the health of their pediatric patients, but also the well-being of their families. By recognizing and addressing the mental health needs of parents, we can break the cycle of stress and mental illness that affects so many families, ensuring a healthier future for the next generation.
Let us embrace this call to action and work together to create a supportive environment where all parents and children can thrive.
Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. American Hospital Association. Surgeon General Issues Advisory on Mental Health and Well-Being of Parents. American Hospital Association. 2024 Sep 4.
2. Goodman SH, Gotlib IH. Risk for Psychopathology in the Children of Depressed Mothers: A Developmental Model for Understanding Mechanisms of Transmission. Psychol Rev. 1999;106(3):458-490. doi: 10.1037/0033-295X.106.3.458.
3. Lovejoy MC et al. Maternal Depression and Parenting Behavior: A Meta-Analytic Review. Clin Psychol Rev. 2000;20(5):561-592. doi: 10.1016/s0272-7358(98)00100-7.
4. Weissman MM et al. Offspring of Depressed Parents: 20 Years Later. Am J Psychiatry. 2006;163(6):1001-1008. doi: 10.1176/ajp.2006.163.6.1001.
5. Smith KE, Pollak SD. Early Life Stress and Development: Potential Mechanisms for Adverse Outcomes. J Neurodev Disord. 2020;12(1):3-14. doi: 10.1186/s11689-020-09337-y.
As child psychiatrists and pediatricians, our mission extends beyond treating the physical health of children; it encompasses understanding the intricate web of factors that influence a child’s overall well-being. A recent advisory from U.S. Surgeon General Dr. Vivek Murthy has brought to light a critical issue that demands our attention: the declining mental health of parents and its profound impact on children. As providers who depend heavily on parental involvement to manage the needs of our pediatric patients, addressing parental mental health can be a crucial step in safeguarding the mental health of children.
The Surgeon General’s Advisory: A Call to Action
On August 28, 2024, the U.S. Surgeon General issued an advisory highlighting the significant stressors impacting parents and caregivers, and the broader implications for children’s mental health. The advisory emphasizes the bidirectional relationship between parental and child mental health, urging healthcare providers, policymakers, and communities to prioritize support for parents. It stresses that the mental health of parents is not only vital for their well-being but also plays a critical role in shaping the emotional and psychological development of their children.1
The Link Between Parental and Child Mental Health
Research shows that a parent’s mental health directly influences the child’s emotional and behavioral outcomes. Children of parents with untreated mental health conditions, such as depression, anxiety, trauma, or chronic stress, are at a significantly higher risk of developing similar conditions. This risk is mediated through various mechanisms, including genetic predisposition, compromised parent-child interactions, and exposure to adverse environments.
1. Parental Depression and Child Outcomes: Parental depression, particularly maternal depression, has been extensively studied and is strongly associated with emotional and behavioral problems in children. Children of depressed parents are more likely to experience anxiety, depression, and resulting academic difficulties. Depressed parents may struggle with consistent and positive parenting, which can disrupt the development of secure attachments and emotional regulation in children.2-4
2. Anxiety and Parenting Styles: Parental anxiety can influence parenting styles, often leading to overprotectiveness, inconsistency, or heightened criticism. These behaviors, in turn, can cultivate anxiety in children, creating a cycle that perpetuates mental health challenges across generations. Children raised in environments where anxiety is pervasive may learn to view the world as threatening, contributing to hypervigilance and stress.5
3. Impact of Chronic Stress on Parenting: Chronic stress experienced by parents, often due to financial hardship, lack of social support, or work-life imbalance, can impair their ability to engage in responsive and nurturing parenting. This, in turn, can affect children’s ability to meaningfully engage with parents to form secure attachments. Further, chronic stress can negatively impact the quality of parent-child interactions and fuel the cycle of rupture with limited opportunity for repair. The advisory stresses the need to address these systemic stressors as part of a broader public health strategy to support families.1
Implications for Pediatric Practice
Pediatricians are often the first point of contact for families navigating mental health challenges. The Surgeon General’s advisory highlights the need for pediatricians to adopt a holistic approach that considers the mental health of the entire family, not just the child. This can be challenging with the average follow-up appointment time of 16 minutes, though many of the recommendations take this logistical hurdle into consideration:
1. Screening for Parental Mental Health: Incorporating routine screening for parental mental health into pediatric visits can be a powerful tool. Questions about parental stress, depression (especially postpartum depression), and anxiety should be integrated into well-child visits, especially in families where children present with emotional or behavioral difficulties. By identifying at-risk parents early, timely referrals to mental health services can be secured.
2. Providing Resources and Referrals: Offering resources and referrals to parents who may be struggling can positively impact the entire family. This includes connecting families with mental health professionals, parenting support groups, or community resources that can alleviate stressors such as food insecurity or lack of childcare. Having a list of local mental health resources available in your practice can empower parents to seek the help they need.
3. Promoting Positive Parenting Practices: Guidance on positive parenting practices, stress management, and self-care can make a significant difference in the mental health of parents and their children. Workshops or educational materials on topics like mindfulness, managing work-life balance, and fostering healthy communication within the family can be valuable and high-yield additions to pediatric care.
4. Collaborative Care Models: Collaborative care models, where pediatricians work closely with child psychiatrists, psychologists, and social workers, can provide comprehensive support to families. This integrated approach ensures that both children and their parents receive the care they need, promoting better outcomes for the entire family unit.
Addressing Broader Systemic Issues
The advisory also calls for systemic changes that extend beyond the clinic. Policy changes such as expanding access to paid family leave, affordable childcare, and mental health services are essential to creating an environment where parents can thrive. As pediatricians, advocating for these changes at the local and national level can amplify the overall impact on families.
1. Advocating for Paid Family Leave: Paid family leave allows parents to bond with their children and attend to their own mental health needs without the added pressure of financial instability. Supporting policies that provide adequate paid leave can pave the way for a successful and healthy return to work and have long-term benefits for family health.
2. Expanding Mental Health Services: Increasing access to mental health services, especially in underserved communities, is crucial. Pediatricians can play a role by partnering with local mental health providers to offer integrated care within their practices or community settings.
3. Community Support Programs: The creation of community support programs that offer parenting classes, stress management workshops, and peer support groups can help reduce the isolation and stress that many parents feel. Pediatricians can collaborate with community organizations to promote these resources to families.
Conclusion
The Surgeon General’s advisory serves as a timely reminder of the interconnectedness of parental and child mental health. Pediatricians have a unique opportunity to influence not only the health of their pediatric patients, but also the well-being of their families. By recognizing and addressing the mental health needs of parents, we can break the cycle of stress and mental illness that affects so many families, ensuring a healthier future for the next generation.
Let us embrace this call to action and work together to create a supportive environment where all parents and children can thrive.
Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. American Hospital Association. Surgeon General Issues Advisory on Mental Health and Well-Being of Parents. American Hospital Association. 2024 Sep 4.
2. Goodman SH, Gotlib IH. Risk for Psychopathology in the Children of Depressed Mothers: A Developmental Model for Understanding Mechanisms of Transmission. Psychol Rev. 1999;106(3):458-490. doi: 10.1037/0033-295X.106.3.458.
3. Lovejoy MC et al. Maternal Depression and Parenting Behavior: A Meta-Analytic Review. Clin Psychol Rev. 2000;20(5):561-592. doi: 10.1016/s0272-7358(98)00100-7.
4. Weissman MM et al. Offspring of Depressed Parents: 20 Years Later. Am J Psychiatry. 2006;163(6):1001-1008. doi: 10.1176/ajp.2006.163.6.1001.
5. Smith KE, Pollak SD. Early Life Stress and Development: Potential Mechanisms for Adverse Outcomes. J Neurodev Disord. 2020;12(1):3-14. doi: 10.1186/s11689-020-09337-y.
Enhanced Care for Pediatric Patients With Generalized Lichen Planus: Diagnosis and Treatment Tips
Practice Gap
Lichen planus (LP) is an inflammatory cutaneous disorder. Although it often is characterized by the 6 Ps—pruritic, polygonal, planar, purple, papules, and plaques with a predilection for the wrists and ankles—the presentation can vary in morphology and distribution.1-5 With an incidence of approximately 1% in the general population, LP is undoubtedly uncommon.1 Its prevalence in the pediatric population is especially low, with only 2% to 3% of cases manifesting in individuals younger than 20 years.2
Generalized LP (also referred to as eruptive or exanthematous LP) is a rarely reported clinical subtype in which lesions are disseminated or spread rapidly.5 The rarity of generalized LP in children often leads to misdiagnosis or delayed treatment, impacting the patient’s quality of life. Thus, there is a need for heightened awareness among clinicians on the variable presentation of LP in the pediatric population. Incorporating a punch biopsy for the diagnosis of LP when lesions manifest as widespread, erythematous to violaceous, flat-topped papules or plaques, along with the addition of an intramuscular (IM) injection in the treatment plan, improves overall patient outcomes.
Tools and Techniques
A detailed physical examination followed by a punch biopsy was critical for the diagnosis of generalized LP in a 7-year-old Black girl. The examination revealed a widespread distribution of dark, violaceous, polygonal, shiny, flat-topped, firm papules coalescing into plaques across the entire body, with a greater predilection for the legs and overlying joints (Figure, A). Some lesions exhibited fine, silver-white, reticular patterns consistent with Wickham striae. Notably, there was no involvement of the scalp, nails, or mucosal surfaces.
The patient had no relevant medical or family history of skin disease and no recent history of illness. She previously was treated by a pediatrician with triamcinolone cream 0.1%, a course of oral cephalexin, and oral cetirizine 10 mg once daily without relief of symptoms.
Although the clinical presentation was consistent with LP, the differential diagnosis included lichen simplex chronicus, atopic dermatitis, psoriasis, and generalized granuloma annulare. To address the need for early recognition of LP in pediatric patients, a punch biopsy of a lesion on the left anterior thigh was performed and showed lichenoid interface dermatitis—a pivotal finding in distinguishing LP from other conditions in the differential.
Given the patient’s age and severity of the LP, a combination of topical and systemic therapies was prescribed—clobetasol cream 0.025% twice daily and 1 injection of 0.5 cc of IM triamcinolone acetonide 40 mg/mL. This regimen was guided by the efficacy of IM injections in providing prompt symptomatic relief, particularly for patients with extensive disease or for those whose condition is refractory to topical treatments.6 Our patient achieved remarkable improvement at 2-week follow-up (Figure, B), without any observed adverse effects. At that time, the patient’s mother refused further systemic treatment and opted for only the topical therapy as well as natural light therapy.
Practice Implications
Timely and accurate diagnosis of LP in pediatric patients, especially those with skin of color, is crucial. Early intervention is especially important in mitigating the risk for chronic symptoms and preventing potential scarring, which tends to be more pronounced and challenging to treat in individuals with darker skin tones.7 Although not present in our patient, it is important to note that LP can affect the face (including the eyelids) as well as the palms and soles in pediatric patients with skin of color.
The most common approach to management of pediatric LP involves the use of a topical corticosteroid and an oral antihistamine, but the recalcitrant and generalized distribution of lesions warrants the administration of a systemic corticosteroid regardless of the patient’s age.6 In our patient, prompt administration of low-dose IM triamcinolone was both crucial and beneficial. Although an underutilized approach, IM triamcinolone helps to prevent the progression of lesions to the scalp, nails, and mucosa while also reducing inflammation and pruritus in glabrous skin.8
Triamcinolone acetonide injections—administered at concentrations of 5 to 40 mg/mL—directly into the lesion (0.5–1 cc per 2 cm2) are highly effective in managing recalcitrant thickened lesions such as those seen in hypertrophic LP and palmoplantar LP.6 This treatment is particularly beneficial when lesions are unresponsive to topical therapies. Administered every 3 to 6 weeks, these injections provide rapid symptom relief, typically within 72 hours,6 while also contributing to the reduction of lesion size and thickness over time. The concentration of triamcinolone acetonide should be selected based on the lesion’s severity, with higher concentrations reserved for thicker, more resistant lesions. More frequent injections may be warranted in cases in which rapid lesion reduction is necessary, while less frequent sessions may suffice for maintenance therapy. It is important to follow patients closely for adverse effects, such as signs of local skin atrophy or hypopigmentation, and to adjust the dose or frequency accordingly. To mitigate these risks, consider using the lowest effective concentration and rotating injection sites if treating multiple lesions. Additionally, combining intralesional corticosteroids with topical therapies can enhance outcomes, particularly in cases in which monotherapy is insufficient.
Patients should be monitored vigilantly for complications of LP. The risk for postinflammatory hyperpigmentation is a particular concern for patients with skin of color. Other complications of untreated LP include nail deformities and scarring alopecia.9 Regular and thorough follow-ups every few months to monitor scalp, mucosal, and genital involvement are essential to manage this risk effectively.
Furthermore, patient education is key. Informing patients and their caregivers about the nature of LP, the available treatment options, and the importance of ongoing follow-up can help to enhance treatment adherence and improve overall outcomes.
- Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/NEJMcp1103641
- Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423-427. doi:10.1046/j.1365-4362.2002.01522.x
- George J, Murray T, Bain M. Generalized, eruptive lichen planus in a pediatric patient. Contemp Pediatr. 2022;39:32-34.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. Updated June 1, 2023. Accessed August 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK526126/
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Mutalik SD, Belgaumkar VA, Rasal YD. Current perspectives in the treatment of childhood lichen planus. Indian J Paediatr Dermatol. 2021;22:316-325. doi:10.4103/ijpd.ijpd_165_20
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi:10.1155/2014/742826
Practice Gap
Lichen planus (LP) is an inflammatory cutaneous disorder. Although it often is characterized by the 6 Ps—pruritic, polygonal, planar, purple, papules, and plaques with a predilection for the wrists and ankles—the presentation can vary in morphology and distribution.1-5 With an incidence of approximately 1% in the general population, LP is undoubtedly uncommon.1 Its prevalence in the pediatric population is especially low, with only 2% to 3% of cases manifesting in individuals younger than 20 years.2
Generalized LP (also referred to as eruptive or exanthematous LP) is a rarely reported clinical subtype in which lesions are disseminated or spread rapidly.5 The rarity of generalized LP in children often leads to misdiagnosis or delayed treatment, impacting the patient’s quality of life. Thus, there is a need for heightened awareness among clinicians on the variable presentation of LP in the pediatric population. Incorporating a punch biopsy for the diagnosis of LP when lesions manifest as widespread, erythematous to violaceous, flat-topped papules or plaques, along with the addition of an intramuscular (IM) injection in the treatment plan, improves overall patient outcomes.
Tools and Techniques
A detailed physical examination followed by a punch biopsy was critical for the diagnosis of generalized LP in a 7-year-old Black girl. The examination revealed a widespread distribution of dark, violaceous, polygonal, shiny, flat-topped, firm papules coalescing into plaques across the entire body, with a greater predilection for the legs and overlying joints (Figure, A). Some lesions exhibited fine, silver-white, reticular patterns consistent with Wickham striae. Notably, there was no involvement of the scalp, nails, or mucosal surfaces.

The patient had no relevant medical or family history of skin disease and no recent history of illness. She previously was treated by a pediatrician with triamcinolone cream 0.1%, a course of oral cephalexin, and oral cetirizine 10 mg once daily without relief of symptoms.
Although the clinical presentation was consistent with LP, the differential diagnosis included lichen simplex chronicus, atopic dermatitis, psoriasis, and generalized granuloma annulare. To address the need for early recognition of LP in pediatric patients, a punch biopsy of a lesion on the left anterior thigh was performed and showed lichenoid interface dermatitis—a pivotal finding in distinguishing LP from other conditions in the differential.
Given the patient’s age and severity of the LP, a combination of topical and systemic therapies was prescribed—clobetasol cream 0.025% twice daily and 1 injection of 0.5 cc of IM triamcinolone acetonide 40 mg/mL. This regimen was guided by the efficacy of IM injections in providing prompt symptomatic relief, particularly for patients with extensive disease or for those whose condition is refractory to topical treatments.6 Our patient achieved remarkable improvement at 2-week follow-up (Figure, B), without any observed adverse effects. At that time, the patient’s mother refused further systemic treatment and opted for only the topical therapy as well as natural light therapy.
Practice Implications
Timely and accurate diagnosis of LP in pediatric patients, especially those with skin of color, is crucial. Early intervention is especially important in mitigating the risk for chronic symptoms and preventing potential scarring, which tends to be more pronounced and challenging to treat in individuals with darker skin tones.7 Although not present in our patient, it is important to note that LP can affect the face (including the eyelids) as well as the palms and soles in pediatric patients with skin of color.
The most common approach to management of pediatric LP involves the use of a topical corticosteroid and an oral antihistamine, but the recalcitrant and generalized distribution of lesions warrants the administration of a systemic corticosteroid regardless of the patient’s age.6 In our patient, prompt administration of low-dose IM triamcinolone was both crucial and beneficial. Although an underutilized approach, IM triamcinolone helps to prevent the progression of lesions to the scalp, nails, and mucosa while also reducing inflammation and pruritus in glabrous skin.8
Triamcinolone acetonide injections—administered at concentrations of 5 to 40 mg/mL—directly into the lesion (0.5–1 cc per 2 cm2) are highly effective in managing recalcitrant thickened lesions such as those seen in hypertrophic LP and palmoplantar LP.6 This treatment is particularly beneficial when lesions are unresponsive to topical therapies. Administered every 3 to 6 weeks, these injections provide rapid symptom relief, typically within 72 hours,6 while also contributing to the reduction of lesion size and thickness over time. The concentration of triamcinolone acetonide should be selected based on the lesion’s severity, with higher concentrations reserved for thicker, more resistant lesions. More frequent injections may be warranted in cases in which rapid lesion reduction is necessary, while less frequent sessions may suffice for maintenance therapy. It is important to follow patients closely for adverse effects, such as signs of local skin atrophy or hypopigmentation, and to adjust the dose or frequency accordingly. To mitigate these risks, consider using the lowest effective concentration and rotating injection sites if treating multiple lesions. Additionally, combining intralesional corticosteroids with topical therapies can enhance outcomes, particularly in cases in which monotherapy is insufficient.
Patients should be monitored vigilantly for complications of LP. The risk for postinflammatory hyperpigmentation is a particular concern for patients with skin of color. Other complications of untreated LP include nail deformities and scarring alopecia.9 Regular and thorough follow-ups every few months to monitor scalp, mucosal, and genital involvement are essential to manage this risk effectively.
Furthermore, patient education is key. Informing patients and their caregivers about the nature of LP, the available treatment options, and the importance of ongoing follow-up can help to enhance treatment adherence and improve overall outcomes.
Practice Gap
Lichen planus (LP) is an inflammatory cutaneous disorder. Although it often is characterized by the 6 Ps—pruritic, polygonal, planar, purple, papules, and plaques with a predilection for the wrists and ankles—the presentation can vary in morphology and distribution.1-5 With an incidence of approximately 1% in the general population, LP is undoubtedly uncommon.1 Its prevalence in the pediatric population is especially low, with only 2% to 3% of cases manifesting in individuals younger than 20 years.2
Generalized LP (also referred to as eruptive or exanthematous LP) is a rarely reported clinical subtype in which lesions are disseminated or spread rapidly.5 The rarity of generalized LP in children often leads to misdiagnosis or delayed treatment, impacting the patient’s quality of life. Thus, there is a need for heightened awareness among clinicians on the variable presentation of LP in the pediatric population. Incorporating a punch biopsy for the diagnosis of LP when lesions manifest as widespread, erythematous to violaceous, flat-topped papules or plaques, along with the addition of an intramuscular (IM) injection in the treatment plan, improves overall patient outcomes.
Tools and Techniques
A detailed physical examination followed by a punch biopsy was critical for the diagnosis of generalized LP in a 7-year-old Black girl. The examination revealed a widespread distribution of dark, violaceous, polygonal, shiny, flat-topped, firm papules coalescing into plaques across the entire body, with a greater predilection for the legs and overlying joints (Figure, A). Some lesions exhibited fine, silver-white, reticular patterns consistent with Wickham striae. Notably, there was no involvement of the scalp, nails, or mucosal surfaces.

The patient had no relevant medical or family history of skin disease and no recent history of illness. She previously was treated by a pediatrician with triamcinolone cream 0.1%, a course of oral cephalexin, and oral cetirizine 10 mg once daily without relief of symptoms.
Although the clinical presentation was consistent with LP, the differential diagnosis included lichen simplex chronicus, atopic dermatitis, psoriasis, and generalized granuloma annulare. To address the need for early recognition of LP in pediatric patients, a punch biopsy of a lesion on the left anterior thigh was performed and showed lichenoid interface dermatitis—a pivotal finding in distinguishing LP from other conditions in the differential.
Given the patient’s age and severity of the LP, a combination of topical and systemic therapies was prescribed—clobetasol cream 0.025% twice daily and 1 injection of 0.5 cc of IM triamcinolone acetonide 40 mg/mL. This regimen was guided by the efficacy of IM injections in providing prompt symptomatic relief, particularly for patients with extensive disease or for those whose condition is refractory to topical treatments.6 Our patient achieved remarkable improvement at 2-week follow-up (Figure, B), without any observed adverse effects. At that time, the patient’s mother refused further systemic treatment and opted for only the topical therapy as well as natural light therapy.
Practice Implications
Timely and accurate diagnosis of LP in pediatric patients, especially those with skin of color, is crucial. Early intervention is especially important in mitigating the risk for chronic symptoms and preventing potential scarring, which tends to be more pronounced and challenging to treat in individuals with darker skin tones.7 Although not present in our patient, it is important to note that LP can affect the face (including the eyelids) as well as the palms and soles in pediatric patients with skin of color.
The most common approach to management of pediatric LP involves the use of a topical corticosteroid and an oral antihistamine, but the recalcitrant and generalized distribution of lesions warrants the administration of a systemic corticosteroid regardless of the patient’s age.6 In our patient, prompt administration of low-dose IM triamcinolone was both crucial and beneficial. Although an underutilized approach, IM triamcinolone helps to prevent the progression of lesions to the scalp, nails, and mucosa while also reducing inflammation and pruritus in glabrous skin.8
Triamcinolone acetonide injections—administered at concentrations of 5 to 40 mg/mL—directly into the lesion (0.5–1 cc per 2 cm2) are highly effective in managing recalcitrant thickened lesions such as those seen in hypertrophic LP and palmoplantar LP.6 This treatment is particularly beneficial when lesions are unresponsive to topical therapies. Administered every 3 to 6 weeks, these injections provide rapid symptom relief, typically within 72 hours,6 while also contributing to the reduction of lesion size and thickness over time. The concentration of triamcinolone acetonide should be selected based on the lesion’s severity, with higher concentrations reserved for thicker, more resistant lesions. More frequent injections may be warranted in cases in which rapid lesion reduction is necessary, while less frequent sessions may suffice for maintenance therapy. It is important to follow patients closely for adverse effects, such as signs of local skin atrophy or hypopigmentation, and to adjust the dose or frequency accordingly. To mitigate these risks, consider using the lowest effective concentration and rotating injection sites if treating multiple lesions. Additionally, combining intralesional corticosteroids with topical therapies can enhance outcomes, particularly in cases in which monotherapy is insufficient.
Patients should be monitored vigilantly for complications of LP. The risk for postinflammatory hyperpigmentation is a particular concern for patients with skin of color. Other complications of untreated LP include nail deformities and scarring alopecia.9 Regular and thorough follow-ups every few months to monitor scalp, mucosal, and genital involvement are essential to manage this risk effectively.
Furthermore, patient education is key. Informing patients and their caregivers about the nature of LP, the available treatment options, and the importance of ongoing follow-up can help to enhance treatment adherence and improve overall outcomes.
- Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/NEJMcp1103641
- Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423-427. doi:10.1046/j.1365-4362.2002.01522.x
- George J, Murray T, Bain M. Generalized, eruptive lichen planus in a pediatric patient. Contemp Pediatr. 2022;39:32-34.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. Updated June 1, 2023. Accessed August 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK526126/
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Mutalik SD, Belgaumkar VA, Rasal YD. Current perspectives in the treatment of childhood lichen planus. Indian J Paediatr Dermatol. 2021;22:316-325. doi:10.4103/ijpd.ijpd_165_20
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi:10.1155/2014/742826
- Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/NEJMcp1103641
- Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423-427. doi:10.1046/j.1365-4362.2002.01522.x
- George J, Murray T, Bain M. Generalized, eruptive lichen planus in a pediatric patient. Contemp Pediatr. 2022;39:32-34.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. Updated June 1, 2023. Accessed August 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK526126/
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Mutalik SD, Belgaumkar VA, Rasal YD. Current perspectives in the treatment of childhood lichen planus. Indian J Paediatr Dermatol. 2021;22:316-325. doi:10.4103/ijpd.ijpd_165_20
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi:10.1155/2014/742826
Can Antihistamines Trigger Seizures in Young Kids?
TOPLINE:
new research shows. The risk appears to be most pronounced in children aged 6-24 months.
METHODOLOGY:
- Researchers in Korea used a self-controlled case-crossover design to assess the risk for seizures associated with prescriptions of first-generation antihistamines.
- They analyzed data from 11,729 children who had a seizure event (an emergency department visit with a diagnosis of epilepsy, status epilepticus, or convulsion) and had previously received a prescription for a first-generation antihistamine, including chlorpheniramine maleate, mequitazine, oxatomide, piprinhydrinate, or hydroxyzine hydrochloride.
- Prescriptions during the 15 days before a seizure were considered to have been received during a hazard period, whereas earlier prescriptions were considered to have been received during a control period.
- The researchers excluded patients with febrile seizures.
TAKEAWAY:
- In an adjusted analysis, a prescription for an antihistamine during the hazard period was associated with a 22% higher risk for seizures in children (adjusted odds ratio, 1.22; 95% CI, 1.13-1.31).
- The seizure risk was significant in children aged 6-24 months, with an adjusted odds ratio of 1.49 (95% CI, 1.31-1.70).
- For older children, the risk was not statistically significant.
IN PRACTICE:
“The study underscores a substantial increase in seizure risk associated with antihistamine prescription among children aged 6-24 months,” the authors of the study wrote. “We are not aware of any other studies that have pointed out the increased risk of seizures with first-generation antihistamines in this particular age group. ... The benefits and risks of antihistamine use should always be carefully considered, especially when prescribing H1 antihistamines to vulnerable infants.”
The findings raise a host of questions for clinicians, including how a “relatively small risk” should translate into practice, and whether the risk may be attenuated with newer antihistamines, wrote Frank Max Charles Besag, MB, ChB, with East London NHS Foundation Trust in England, in an editorial accompanying the study. “It would be reasonable to inform families that at least one study has suggested a relatively small increase in the risk of seizures with first-generation antihistamines, adding that there are still too few data to draw any firm conclusions and also providing families with the information on what to do if the child were to have a seizure.”
SOURCE:
Seonkyeong Rhie, MD, and Man Yong Han, MD, both with the Department of Pediatrics at CHA University School of Medicine, in Seongnam, South Korea, were the corresponding authors on the study. The research was published online in JAMA Network Open.
LIMITATIONS:
The researchers did not have details about seizure symptoms, did not include children seen in outpatient clinics, and were unable to verify the actual intake of the prescribed antihistamines. Although second-generation antihistamines may be less likely to cross the blood-brain barrier, one newer medication, desloratadine, has been associated with seizures.
DISCLOSURES:
The study was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, the Ministry of Health and Welfare, Republic of Korea.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
new research shows. The risk appears to be most pronounced in children aged 6-24 months.
METHODOLOGY:
- Researchers in Korea used a self-controlled case-crossover design to assess the risk for seizures associated with prescriptions of first-generation antihistamines.
- They analyzed data from 11,729 children who had a seizure event (an emergency department visit with a diagnosis of epilepsy, status epilepticus, or convulsion) and had previously received a prescription for a first-generation antihistamine, including chlorpheniramine maleate, mequitazine, oxatomide, piprinhydrinate, or hydroxyzine hydrochloride.
- Prescriptions during the 15 days before a seizure were considered to have been received during a hazard period, whereas earlier prescriptions were considered to have been received during a control period.
- The researchers excluded patients with febrile seizures.
TAKEAWAY:
- In an adjusted analysis, a prescription for an antihistamine during the hazard period was associated with a 22% higher risk for seizures in children (adjusted odds ratio, 1.22; 95% CI, 1.13-1.31).
- The seizure risk was significant in children aged 6-24 months, with an adjusted odds ratio of 1.49 (95% CI, 1.31-1.70).
- For older children, the risk was not statistically significant.
IN PRACTICE:
“The study underscores a substantial increase in seizure risk associated with antihistamine prescription among children aged 6-24 months,” the authors of the study wrote. “We are not aware of any other studies that have pointed out the increased risk of seizures with first-generation antihistamines in this particular age group. ... The benefits and risks of antihistamine use should always be carefully considered, especially when prescribing H1 antihistamines to vulnerable infants.”
The findings raise a host of questions for clinicians, including how a “relatively small risk” should translate into practice, and whether the risk may be attenuated with newer antihistamines, wrote Frank Max Charles Besag, MB, ChB, with East London NHS Foundation Trust in England, in an editorial accompanying the study. “It would be reasonable to inform families that at least one study has suggested a relatively small increase in the risk of seizures with first-generation antihistamines, adding that there are still too few data to draw any firm conclusions and also providing families with the information on what to do if the child were to have a seizure.”
SOURCE:
Seonkyeong Rhie, MD, and Man Yong Han, MD, both with the Department of Pediatrics at CHA University School of Medicine, in Seongnam, South Korea, were the corresponding authors on the study. The research was published online in JAMA Network Open.
LIMITATIONS:
The researchers did not have details about seizure symptoms, did not include children seen in outpatient clinics, and were unable to verify the actual intake of the prescribed antihistamines. Although second-generation antihistamines may be less likely to cross the blood-brain barrier, one newer medication, desloratadine, has been associated with seizures.
DISCLOSURES:
The study was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, the Ministry of Health and Welfare, Republic of Korea.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
new research shows. The risk appears to be most pronounced in children aged 6-24 months.
METHODOLOGY:
- Researchers in Korea used a self-controlled case-crossover design to assess the risk for seizures associated with prescriptions of first-generation antihistamines.
- They analyzed data from 11,729 children who had a seizure event (an emergency department visit with a diagnosis of epilepsy, status epilepticus, or convulsion) and had previously received a prescription for a first-generation antihistamine, including chlorpheniramine maleate, mequitazine, oxatomide, piprinhydrinate, or hydroxyzine hydrochloride.
- Prescriptions during the 15 days before a seizure were considered to have been received during a hazard period, whereas earlier prescriptions were considered to have been received during a control period.
- The researchers excluded patients with febrile seizures.
TAKEAWAY:
- In an adjusted analysis, a prescription for an antihistamine during the hazard period was associated with a 22% higher risk for seizures in children (adjusted odds ratio, 1.22; 95% CI, 1.13-1.31).
- The seizure risk was significant in children aged 6-24 months, with an adjusted odds ratio of 1.49 (95% CI, 1.31-1.70).
- For older children, the risk was not statistically significant.
IN PRACTICE:
“The study underscores a substantial increase in seizure risk associated with antihistamine prescription among children aged 6-24 months,” the authors of the study wrote. “We are not aware of any other studies that have pointed out the increased risk of seizures with first-generation antihistamines in this particular age group. ... The benefits and risks of antihistamine use should always be carefully considered, especially when prescribing H1 antihistamines to vulnerable infants.”
The findings raise a host of questions for clinicians, including how a “relatively small risk” should translate into practice, and whether the risk may be attenuated with newer antihistamines, wrote Frank Max Charles Besag, MB, ChB, with East London NHS Foundation Trust in England, in an editorial accompanying the study. “It would be reasonable to inform families that at least one study has suggested a relatively small increase in the risk of seizures with first-generation antihistamines, adding that there are still too few data to draw any firm conclusions and also providing families with the information on what to do if the child were to have a seizure.”
SOURCE:
Seonkyeong Rhie, MD, and Man Yong Han, MD, both with the Department of Pediatrics at CHA University School of Medicine, in Seongnam, South Korea, were the corresponding authors on the study. The research was published online in JAMA Network Open.
LIMITATIONS:
The researchers did not have details about seizure symptoms, did not include children seen in outpatient clinics, and were unable to verify the actual intake of the prescribed antihistamines. Although second-generation antihistamines may be less likely to cross the blood-brain barrier, one newer medication, desloratadine, has been associated with seizures.
DISCLOSURES:
The study was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, the Ministry of Health and Welfare, Republic of Korea.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.