User login
Long COVID symptoms reported by 6% of pediatric patients
The prevalence of long COVID in children has been unclear, and is complicated by the lack of a consistent definition, said Anna Funk, PhD, an epidemiologist at the University of Calgary (Alba.), during her online presentation of the findings at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
In the several small studies conducted to date, rates range from 0% to 67% 2-4 months after infection, Dr. Funk reported.
To examine prevalence, she and her colleagues, as part of the Pediatric Emergency Research Network (PERN) global research consortium, assessed more than 10,500 children who were screened for SARS-CoV-2 when they presented to the ED at 1 of 41 study sites in 10 countries – Australia, Canada, Indonesia, the United States, plus three countries in Latin America and three in Western Europe – from March 2020 to June 15, 2021.
PERN researchers are following up with the more than 3,100 children who tested positive 14, 30, and 90 days after testing, tracking respiratory, neurologic, and psychobehavioral sequelae.
Dr. Funk presented data on the 1,884 children who tested positive for SARS-CoV-2 before Jan. 20, 2021, and who had completed 90-day follow-up; 447 of those children were hospitalized and 1,437 were not.
Symptoms were reported more often by children admitted to the hospital than not admitted (9.8% vs. 4.6%). Common persistent symptoms were respiratory in 2% of cases, systemic (such as fatigue and fever) in 2%, neurologic (such as headache, seizures, and continued loss of taste or smell) in 1%, and psychological (such as new-onset depression and anxiety) in 1%.
“This study provides the first good epidemiological data on persistent symptoms among SARS-CoV-2–infected children, regardless of severity,” said Kevin Messacar, MD, a pediatric infectious disease clinician and researcher at Children’s Hospital Colorado in Aurora, who was not involved in the study.
And the findings show that, although severe COVID and chronic symptoms are less common in children than in adults, they are “not nonexistent and need to be taken seriously,” he said in an interview.
After adjustment for country of enrollment, children aged 10-17 years were more likely to experience persistent symptoms than children younger than 1 year (odds ratio, 2.4; P = .002).
Hospitalized children were more than twice as likely to experience persistent symptoms as nonhospitalized children (OR, 2.5; P < .001). And children who presented to the ED with at least seven symptoms were four times more likely to have long-term symptoms than those who presented with fewer symptoms (OR, 4.02; P = .01).
‘Some reassurance’
“Given that COVID is new and is known to have acute cardiac and neurologic effects, particularly in children with [multisystem inflammatory syndrome], there were initially concerns about persistent cardiovascular and neurologic effects in any infected child,” Dr. Messacar explained. “These data provide some reassurance that this is uncommon among children with mild or moderate infections who are not hospitalized.”
But “the risk is not zero,” he added. “Getting children vaccinated when it is available to them and taking precautions to prevent unvaccinated children getting COVID is the best way to reduce the risk of severe disease or persistent symptoms.”
The study was limited by its lack of data on variants, reliance on self-reported symptoms, and a population drawn solely from EDs, Dr. Funk acknowledged.
No external funding source was noted. Dr. Messacar and Dr. Funk disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The prevalence of long COVID in children has been unclear, and is complicated by the lack of a consistent definition, said Anna Funk, PhD, an epidemiologist at the University of Calgary (Alba.), during her online presentation of the findings at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
In the several small studies conducted to date, rates range from 0% to 67% 2-4 months after infection, Dr. Funk reported.
To examine prevalence, she and her colleagues, as part of the Pediatric Emergency Research Network (PERN) global research consortium, assessed more than 10,500 children who were screened for SARS-CoV-2 when they presented to the ED at 1 of 41 study sites in 10 countries – Australia, Canada, Indonesia, the United States, plus three countries in Latin America and three in Western Europe – from March 2020 to June 15, 2021.
PERN researchers are following up with the more than 3,100 children who tested positive 14, 30, and 90 days after testing, tracking respiratory, neurologic, and psychobehavioral sequelae.
Dr. Funk presented data on the 1,884 children who tested positive for SARS-CoV-2 before Jan. 20, 2021, and who had completed 90-day follow-up; 447 of those children were hospitalized and 1,437 were not.
Symptoms were reported more often by children admitted to the hospital than not admitted (9.8% vs. 4.6%). Common persistent symptoms were respiratory in 2% of cases, systemic (such as fatigue and fever) in 2%, neurologic (such as headache, seizures, and continued loss of taste or smell) in 1%, and psychological (such as new-onset depression and anxiety) in 1%.
“This study provides the first good epidemiological data on persistent symptoms among SARS-CoV-2–infected children, regardless of severity,” said Kevin Messacar, MD, a pediatric infectious disease clinician and researcher at Children’s Hospital Colorado in Aurora, who was not involved in the study.
And the findings show that, although severe COVID and chronic symptoms are less common in children than in adults, they are “not nonexistent and need to be taken seriously,” he said in an interview.
After adjustment for country of enrollment, children aged 10-17 years were more likely to experience persistent symptoms than children younger than 1 year (odds ratio, 2.4; P = .002).
Hospitalized children were more than twice as likely to experience persistent symptoms as nonhospitalized children (OR, 2.5; P < .001). And children who presented to the ED with at least seven symptoms were four times more likely to have long-term symptoms than those who presented with fewer symptoms (OR, 4.02; P = .01).
‘Some reassurance’
“Given that COVID is new and is known to have acute cardiac and neurologic effects, particularly in children with [multisystem inflammatory syndrome], there were initially concerns about persistent cardiovascular and neurologic effects in any infected child,” Dr. Messacar explained. “These data provide some reassurance that this is uncommon among children with mild or moderate infections who are not hospitalized.”
But “the risk is not zero,” he added. “Getting children vaccinated when it is available to them and taking precautions to prevent unvaccinated children getting COVID is the best way to reduce the risk of severe disease or persistent symptoms.”
The study was limited by its lack of data on variants, reliance on self-reported symptoms, and a population drawn solely from EDs, Dr. Funk acknowledged.
No external funding source was noted. Dr. Messacar and Dr. Funk disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The prevalence of long COVID in children has been unclear, and is complicated by the lack of a consistent definition, said Anna Funk, PhD, an epidemiologist at the University of Calgary (Alba.), during her online presentation of the findings at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
In the several small studies conducted to date, rates range from 0% to 67% 2-4 months after infection, Dr. Funk reported.
To examine prevalence, she and her colleagues, as part of the Pediatric Emergency Research Network (PERN) global research consortium, assessed more than 10,500 children who were screened for SARS-CoV-2 when they presented to the ED at 1 of 41 study sites in 10 countries – Australia, Canada, Indonesia, the United States, plus three countries in Latin America and three in Western Europe – from March 2020 to June 15, 2021.
PERN researchers are following up with the more than 3,100 children who tested positive 14, 30, and 90 days after testing, tracking respiratory, neurologic, and psychobehavioral sequelae.
Dr. Funk presented data on the 1,884 children who tested positive for SARS-CoV-2 before Jan. 20, 2021, and who had completed 90-day follow-up; 447 of those children were hospitalized and 1,437 were not.
Symptoms were reported more often by children admitted to the hospital than not admitted (9.8% vs. 4.6%). Common persistent symptoms were respiratory in 2% of cases, systemic (such as fatigue and fever) in 2%, neurologic (such as headache, seizures, and continued loss of taste or smell) in 1%, and psychological (such as new-onset depression and anxiety) in 1%.
“This study provides the first good epidemiological data on persistent symptoms among SARS-CoV-2–infected children, regardless of severity,” said Kevin Messacar, MD, a pediatric infectious disease clinician and researcher at Children’s Hospital Colorado in Aurora, who was not involved in the study.
And the findings show that, although severe COVID and chronic symptoms are less common in children than in adults, they are “not nonexistent and need to be taken seriously,” he said in an interview.
After adjustment for country of enrollment, children aged 10-17 years were more likely to experience persistent symptoms than children younger than 1 year (odds ratio, 2.4; P = .002).
Hospitalized children were more than twice as likely to experience persistent symptoms as nonhospitalized children (OR, 2.5; P < .001). And children who presented to the ED with at least seven symptoms were four times more likely to have long-term symptoms than those who presented with fewer symptoms (OR, 4.02; P = .01).
‘Some reassurance’
“Given that COVID is new and is known to have acute cardiac and neurologic effects, particularly in children with [multisystem inflammatory syndrome], there were initially concerns about persistent cardiovascular and neurologic effects in any infected child,” Dr. Messacar explained. “These data provide some reassurance that this is uncommon among children with mild or moderate infections who are not hospitalized.”
But “the risk is not zero,” he added. “Getting children vaccinated when it is available to them and taking precautions to prevent unvaccinated children getting COVID is the best way to reduce the risk of severe disease or persistent symptoms.”
The study was limited by its lack of data on variants, reliance on self-reported symptoms, and a population drawn solely from EDs, Dr. Funk acknowledged.
No external funding source was noted. Dr. Messacar and Dr. Funk disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New agents for youth-onset type 2 diabetes ‘finally in sight’
There are limited treatment options for children and youth with type 2 diabetes, but a few novel therapies beyond metformin are on the horizon, experts said at the annual scientific sessions of the American Diabetes Association.
“Type 2 diabetes in youth only emerged as a well-recognized pediatric medical problem in the 1990s and the first decade of the 21st century,” session chair Kenneth C. Copeland, MD, said in an interview.
“Fortunately, a number of clinical trials of antidiabetic pharmacologic agents in diabetic youth have now been completed, demonstrating both safety and efficacy, and at long last, a ... variety of agents are finally in sight,” he noted.
Type 2 diabetes in youth is profoundly different from type 2 diabetes in adults, added Dr. Copeland, pediatrics professor emeritus, University of Oklahoma, Oklahoma City. In youth, its course is typically aggressive and refractive to treatment.
Concerted efforts at lifestyle intervention are important but insufficient, and a response to metformin, even when initiated at diagnosis, is often short lived, he added.
Because of the rapid glycemic deterioration that is typical of type 2 diabetes in youth and leads to the full array of diabetic complications, early aggressive pharmacologic treatment is indicated.
“We all look forward to this next decade ushering in new treatment options, spanning the spectrum from obesity prevention to complex pharmacologic intervention,” Dr. Copeland summarized.
Increasing prevalence of T2D in youth, limited therapies
Rates of type 2 diabetes in youth continue to increase, especially among non-White groups, and most of these individuals have less than optimal diabetes control, Elvira Isganaitis, MD, MPH, a pediatric endocrinologist at the Joslin Diabetes Center and assistant professor of pediatrics at Harvard Medical School, both in Boston, told the meeting.
Although the Food and Drug Administration has approved more than 25 drugs to treat type 2 diabetes in adults, “unfortunately,” metformin is the only oral medication approved to treat the disease in a pediatric population, “and a majority of youth either do not respond to it or do not tolerate it,” she said in an interview.
Dr. Copeland observed that “the TODAY study demonstrated conclusively that, despite an often dramatic initial improvement in glycemic control upon initiation of pharmacologic and lifestyle intervention, this initial response was followed by a rapid deterioration of beta-cell function and glycemic failure, indicating that additional pharmacologic agents were sorely needed for this population.”
The RISE study also showed that, compared with adults, youth had more rapid beta-cell deterioration despite treatment.
Until the June 2019 FDA approval of the injectable glucagonlike peptide–1 receptor agonist liraglutide (Victoza, Novo Nordisk) for children 10 years or older, “except for insulin, metformin was the only antidiabetic medication available for use in youth, severely limiting treatment options,” he added.
Liraglutide ‘a huge breakthrough,’ other options on the horizon
The FDA approval of liraglutide was “a huge breakthrough” as the first noninsulin drug for pediatric type 2 diabetes since metformin was approved for pediatric use in 2000, Dr. Isganaitis said.
The ELLIPSE study, on which the approval was based, showed liraglutide was effective at lowering hemoglobin A1c and was generally well tolerated, although it was associated with a higher incidence of gastrointestinal symptoms.
In December 2020, the FDA also approved liraglutide (Saxenda) for the treatment of obesity in youth age 12 and older (at a dose of 3 mg as opposed to the 1.8-mg dose of liraglutide [Victoza]), “which is wonderful news considering that the majority of pediatric patients with type 2 diabetes also have obesity,” Dr. Isganaitis added.
“The results of studies of liraglutide on glycemia in diabetic youth are impressive, with both an additional benefit of weight loss and without unacceptable identified risks or side effects,” Dr. Copeland concurred.
Waiting in the wings
Dr. Isganaitis reported that a few phase 3 clinical trials of other therapies for pediatric patients with type 2 diabetes are in the wings.
The 24-week phase 3 T2GO clinical trial of the sodium-glucose cotransporter 2 inhibitor dapagliflozin (AstraZeneca) versus placebo in 72 patients with type 2 diabetes aged 10-24 years was completed in April 2020, and the data are being analyzed.
An AstraZeneca-sponsored phase 3 trial of the safety and efficacy of a weekly injection of the GLP-1 receptor agonist exenatide in 10- to 17-year-olds with type 2 diabetes (n = 82) has also been completed and data are being analyzed.
A Takeda-sponsored phase 3 pediatric study of the dipeptidyl peptidase–4 inhibitor alogliptin in 10- to 17-year-olds with type 2 diabetes (n = 150) is estimated to be completed by February 2022.
And the phase 3 DINAMO trial, sponsored by Boehringer Ingelheim, which is evaluating the efficacy and safety of the SGLT2 inhibitor empagliflozin (10 mg/25 mg) versus the DPP-4 inhibitor linagliptin (5 mg) versus placebo over 26 weeks in 10- to 17-year-olds with type 2 diabetes (estimated 186 participants), is expected to be completed in May 2023.
“I hope that these medications will demonstrate efficacy and allow pediatric patients with type 2 diabetes to have more treatment options,” Dr. Isganaitis concluded.
Type 2 diabetes more aggressive than type 1 diabetes in kids
According to Dr. Isganaitis, “there is a widely held misconception among the general public and even among some physicians that type 2 diabetes is somehow less worrisome or ‘milder’ than a diagnosis of type 1 diabetes.”
However, the risk of complications and severe morbidity is higher with a diagnosis of type 2 diabetes versus type 1 diabetes in a child, so “this condition needs to be managed intensively with a multidisciplinary team including pediatric endocrinology, nutrition [support], diabetes educators, and mental health support,” she emphasized.
Many people also believe that “type 2 diabetes in kids is a ‘lifestyle disease,’ ” she continued, “but in fact, there is a strong role for genetics.”
The ADA Presidents’ Select Abstract “paints a picture of youth-onset type 2 diabetes as a disease intermediate in extremity between monogenic diabetes [caused by mutations in a single gene] and type 2 diabetes [caused by multiple genes and lifestyle factors such as obesity], in which genetic variants in both insulin secretion and insulin response pathways are implicated.”
Along the same lines, Dr. Isganaitis presented an oral abstract at the meeting that showed that, among youth with newly diagnosed type 2 diabetes, those whose mothers had diabetes had faster disease progression and earlier onset of diabetes complications.
Dr. Isganaitis has reported no relevant financial relationships. Dr. Copeland has reported serving on data monitoring committees for Boehringer Ingelheim and Novo Nordisk, and on an advisory committee for a research study for Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
There are limited treatment options for children and youth with type 2 diabetes, but a few novel therapies beyond metformin are on the horizon, experts said at the annual scientific sessions of the American Diabetes Association.
“Type 2 diabetes in youth only emerged as a well-recognized pediatric medical problem in the 1990s and the first decade of the 21st century,” session chair Kenneth C. Copeland, MD, said in an interview.
“Fortunately, a number of clinical trials of antidiabetic pharmacologic agents in diabetic youth have now been completed, demonstrating both safety and efficacy, and at long last, a ... variety of agents are finally in sight,” he noted.
Type 2 diabetes in youth is profoundly different from type 2 diabetes in adults, added Dr. Copeland, pediatrics professor emeritus, University of Oklahoma, Oklahoma City. In youth, its course is typically aggressive and refractive to treatment.
Concerted efforts at lifestyle intervention are important but insufficient, and a response to metformin, even when initiated at diagnosis, is often short lived, he added.
Because of the rapid glycemic deterioration that is typical of type 2 diabetes in youth and leads to the full array of diabetic complications, early aggressive pharmacologic treatment is indicated.
“We all look forward to this next decade ushering in new treatment options, spanning the spectrum from obesity prevention to complex pharmacologic intervention,” Dr. Copeland summarized.
Increasing prevalence of T2D in youth, limited therapies
Rates of type 2 diabetes in youth continue to increase, especially among non-White groups, and most of these individuals have less than optimal diabetes control, Elvira Isganaitis, MD, MPH, a pediatric endocrinologist at the Joslin Diabetes Center and assistant professor of pediatrics at Harvard Medical School, both in Boston, told the meeting.
Although the Food and Drug Administration has approved more than 25 drugs to treat type 2 diabetes in adults, “unfortunately,” metformin is the only oral medication approved to treat the disease in a pediatric population, “and a majority of youth either do not respond to it or do not tolerate it,” she said in an interview.
Dr. Copeland observed that “the TODAY study demonstrated conclusively that, despite an often dramatic initial improvement in glycemic control upon initiation of pharmacologic and lifestyle intervention, this initial response was followed by a rapid deterioration of beta-cell function and glycemic failure, indicating that additional pharmacologic agents were sorely needed for this population.”
The RISE study also showed that, compared with adults, youth had more rapid beta-cell deterioration despite treatment.
Until the June 2019 FDA approval of the injectable glucagonlike peptide–1 receptor agonist liraglutide (Victoza, Novo Nordisk) for children 10 years or older, “except for insulin, metformin was the only antidiabetic medication available for use in youth, severely limiting treatment options,” he added.
Liraglutide ‘a huge breakthrough,’ other options on the horizon
The FDA approval of liraglutide was “a huge breakthrough” as the first noninsulin drug for pediatric type 2 diabetes since metformin was approved for pediatric use in 2000, Dr. Isganaitis said.
The ELLIPSE study, on which the approval was based, showed liraglutide was effective at lowering hemoglobin A1c and was generally well tolerated, although it was associated with a higher incidence of gastrointestinal symptoms.
In December 2020, the FDA also approved liraglutide (Saxenda) for the treatment of obesity in youth age 12 and older (at a dose of 3 mg as opposed to the 1.8-mg dose of liraglutide [Victoza]), “which is wonderful news considering that the majority of pediatric patients with type 2 diabetes also have obesity,” Dr. Isganaitis added.
“The results of studies of liraglutide on glycemia in diabetic youth are impressive, with both an additional benefit of weight loss and without unacceptable identified risks or side effects,” Dr. Copeland concurred.
Waiting in the wings
Dr. Isganaitis reported that a few phase 3 clinical trials of other therapies for pediatric patients with type 2 diabetes are in the wings.
The 24-week phase 3 T2GO clinical trial of the sodium-glucose cotransporter 2 inhibitor dapagliflozin (AstraZeneca) versus placebo in 72 patients with type 2 diabetes aged 10-24 years was completed in April 2020, and the data are being analyzed.
An AstraZeneca-sponsored phase 3 trial of the safety and efficacy of a weekly injection of the GLP-1 receptor agonist exenatide in 10- to 17-year-olds with type 2 diabetes (n = 82) has also been completed and data are being analyzed.
A Takeda-sponsored phase 3 pediatric study of the dipeptidyl peptidase–4 inhibitor alogliptin in 10- to 17-year-olds with type 2 diabetes (n = 150) is estimated to be completed by February 2022.
And the phase 3 DINAMO trial, sponsored by Boehringer Ingelheim, which is evaluating the efficacy and safety of the SGLT2 inhibitor empagliflozin (10 mg/25 mg) versus the DPP-4 inhibitor linagliptin (5 mg) versus placebo over 26 weeks in 10- to 17-year-olds with type 2 diabetes (estimated 186 participants), is expected to be completed in May 2023.
“I hope that these medications will demonstrate efficacy and allow pediatric patients with type 2 diabetes to have more treatment options,” Dr. Isganaitis concluded.
Type 2 diabetes more aggressive than type 1 diabetes in kids
According to Dr. Isganaitis, “there is a widely held misconception among the general public and even among some physicians that type 2 diabetes is somehow less worrisome or ‘milder’ than a diagnosis of type 1 diabetes.”
However, the risk of complications and severe morbidity is higher with a diagnosis of type 2 diabetes versus type 1 diabetes in a child, so “this condition needs to be managed intensively with a multidisciplinary team including pediatric endocrinology, nutrition [support], diabetes educators, and mental health support,” she emphasized.
Many people also believe that “type 2 diabetes in kids is a ‘lifestyle disease,’ ” she continued, “but in fact, there is a strong role for genetics.”
The ADA Presidents’ Select Abstract “paints a picture of youth-onset type 2 diabetes as a disease intermediate in extremity between monogenic diabetes [caused by mutations in a single gene] and type 2 diabetes [caused by multiple genes and lifestyle factors such as obesity], in which genetic variants in both insulin secretion and insulin response pathways are implicated.”
Along the same lines, Dr. Isganaitis presented an oral abstract at the meeting that showed that, among youth with newly diagnosed type 2 diabetes, those whose mothers had diabetes had faster disease progression and earlier onset of diabetes complications.
Dr. Isganaitis has reported no relevant financial relationships. Dr. Copeland has reported serving on data monitoring committees for Boehringer Ingelheim and Novo Nordisk, and on an advisory committee for a research study for Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
There are limited treatment options for children and youth with type 2 diabetes, but a few novel therapies beyond metformin are on the horizon, experts said at the annual scientific sessions of the American Diabetes Association.
“Type 2 diabetes in youth only emerged as a well-recognized pediatric medical problem in the 1990s and the first decade of the 21st century,” session chair Kenneth C. Copeland, MD, said in an interview.
“Fortunately, a number of clinical trials of antidiabetic pharmacologic agents in diabetic youth have now been completed, demonstrating both safety and efficacy, and at long last, a ... variety of agents are finally in sight,” he noted.
Type 2 diabetes in youth is profoundly different from type 2 diabetes in adults, added Dr. Copeland, pediatrics professor emeritus, University of Oklahoma, Oklahoma City. In youth, its course is typically aggressive and refractive to treatment.
Concerted efforts at lifestyle intervention are important but insufficient, and a response to metformin, even when initiated at diagnosis, is often short lived, he added.
Because of the rapid glycemic deterioration that is typical of type 2 diabetes in youth and leads to the full array of diabetic complications, early aggressive pharmacologic treatment is indicated.
“We all look forward to this next decade ushering in new treatment options, spanning the spectrum from obesity prevention to complex pharmacologic intervention,” Dr. Copeland summarized.
Increasing prevalence of T2D in youth, limited therapies
Rates of type 2 diabetes in youth continue to increase, especially among non-White groups, and most of these individuals have less than optimal diabetes control, Elvira Isganaitis, MD, MPH, a pediatric endocrinologist at the Joslin Diabetes Center and assistant professor of pediatrics at Harvard Medical School, both in Boston, told the meeting.
Although the Food and Drug Administration has approved more than 25 drugs to treat type 2 diabetes in adults, “unfortunately,” metformin is the only oral medication approved to treat the disease in a pediatric population, “and a majority of youth either do not respond to it or do not tolerate it,” she said in an interview.
Dr. Copeland observed that “the TODAY study demonstrated conclusively that, despite an often dramatic initial improvement in glycemic control upon initiation of pharmacologic and lifestyle intervention, this initial response was followed by a rapid deterioration of beta-cell function and glycemic failure, indicating that additional pharmacologic agents were sorely needed for this population.”
The RISE study also showed that, compared with adults, youth had more rapid beta-cell deterioration despite treatment.
Until the June 2019 FDA approval of the injectable glucagonlike peptide–1 receptor agonist liraglutide (Victoza, Novo Nordisk) for children 10 years or older, “except for insulin, metformin was the only antidiabetic medication available for use in youth, severely limiting treatment options,” he added.
Liraglutide ‘a huge breakthrough,’ other options on the horizon
The FDA approval of liraglutide was “a huge breakthrough” as the first noninsulin drug for pediatric type 2 diabetes since metformin was approved for pediatric use in 2000, Dr. Isganaitis said.
The ELLIPSE study, on which the approval was based, showed liraglutide was effective at lowering hemoglobin A1c and was generally well tolerated, although it was associated with a higher incidence of gastrointestinal symptoms.
In December 2020, the FDA also approved liraglutide (Saxenda) for the treatment of obesity in youth age 12 and older (at a dose of 3 mg as opposed to the 1.8-mg dose of liraglutide [Victoza]), “which is wonderful news considering that the majority of pediatric patients with type 2 diabetes also have obesity,” Dr. Isganaitis added.
“The results of studies of liraglutide on glycemia in diabetic youth are impressive, with both an additional benefit of weight loss and without unacceptable identified risks or side effects,” Dr. Copeland concurred.
Waiting in the wings
Dr. Isganaitis reported that a few phase 3 clinical trials of other therapies for pediatric patients with type 2 diabetes are in the wings.
The 24-week phase 3 T2GO clinical trial of the sodium-glucose cotransporter 2 inhibitor dapagliflozin (AstraZeneca) versus placebo in 72 patients with type 2 diabetes aged 10-24 years was completed in April 2020, and the data are being analyzed.
An AstraZeneca-sponsored phase 3 trial of the safety and efficacy of a weekly injection of the GLP-1 receptor agonist exenatide in 10- to 17-year-olds with type 2 diabetes (n = 82) has also been completed and data are being analyzed.
A Takeda-sponsored phase 3 pediatric study of the dipeptidyl peptidase–4 inhibitor alogliptin in 10- to 17-year-olds with type 2 diabetes (n = 150) is estimated to be completed by February 2022.
And the phase 3 DINAMO trial, sponsored by Boehringer Ingelheim, which is evaluating the efficacy and safety of the SGLT2 inhibitor empagliflozin (10 mg/25 mg) versus the DPP-4 inhibitor linagliptin (5 mg) versus placebo over 26 weeks in 10- to 17-year-olds with type 2 diabetes (estimated 186 participants), is expected to be completed in May 2023.
“I hope that these medications will demonstrate efficacy and allow pediatric patients with type 2 diabetes to have more treatment options,” Dr. Isganaitis concluded.
Type 2 diabetes more aggressive than type 1 diabetes in kids
According to Dr. Isganaitis, “there is a widely held misconception among the general public and even among some physicians that type 2 diabetes is somehow less worrisome or ‘milder’ than a diagnosis of type 1 diabetes.”
However, the risk of complications and severe morbidity is higher with a diagnosis of type 2 diabetes versus type 1 diabetes in a child, so “this condition needs to be managed intensively with a multidisciplinary team including pediatric endocrinology, nutrition [support], diabetes educators, and mental health support,” she emphasized.
Many people also believe that “type 2 diabetes in kids is a ‘lifestyle disease,’ ” she continued, “but in fact, there is a strong role for genetics.”
The ADA Presidents’ Select Abstract “paints a picture of youth-onset type 2 diabetes as a disease intermediate in extremity between monogenic diabetes [caused by mutations in a single gene] and type 2 diabetes [caused by multiple genes and lifestyle factors such as obesity], in which genetic variants in both insulin secretion and insulin response pathways are implicated.”
Along the same lines, Dr. Isganaitis presented an oral abstract at the meeting that showed that, among youth with newly diagnosed type 2 diabetes, those whose mothers had diabetes had faster disease progression and earlier onset of diabetes complications.
Dr. Isganaitis has reported no relevant financial relationships. Dr. Copeland has reported serving on data monitoring committees for Boehringer Ingelheim and Novo Nordisk, and on an advisory committee for a research study for Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
Rising rates of T1D in children: Is COVID to blame?
In early 2020, the COVID-19 pandemic changed everything about life as we know it, with widespread shutdowns across the globe. The U.S. health care system quickly adapted, pivoting to telehealth visits when able and proactively managing outpatient conditions to prevent overwhelming hospital resources and utilization. Meanwhile, at my practice, the typical rate of about one new-onset pediatric type 1 diabetes (T1D) case per week increased to about two per week.
However, the new diabetes cases continued to accumulate, and I saw more patients being diagnosed who did not have a known family history of autoimmunity. I began to ask friends at other centers whether they were noticing the same trend.
One colleague documented a 36% increase in her large center compared with the previous year. Another noted a 40% rise at his children’s hospital. We observed that there was often a respiratory illness reported several weeks before presenting with T1D. Sometimes the child was known to be COVID-positive. Sometimes the child had not been tested. Sometimes we suspected that COVID had been a preceding illness and then found negative SARS-CoV-2 antibodies – but we were not certain whether the result was meaningful given the time lapsed since infection.
Soon, reports emerged of large increases in severe diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state at initial presentation, a trend reported in other countries.
Is COVID-19 a trigger for T1D?
There is known precedent for increased risk for T1D after viral infections in patients who are already genetically susceptible. Mechanisms of immune-mediated islet cell failure would make sense following SARS-CoV-2 infection; direct islet toxicity was noted with SARS-CoV-1 and has been suspected with SARS-CoV-2 but not proven. Some have suggested that hypercoagulability with COVID-19 may lead to ischemic damage to the pancreas.
With multiple potential pathways for islet damage, increases in insulin-dependent diabetes would logically follow. Still, whether this is the case remains unclear. There is not yet definitive evidence that there is uptake of SARS-CoV-2 via receptors in the pancreatic beta cells.
Our current understanding of T1D pathogenesis is that susceptible individuals develop autoimmunity in response to an environmental trigger, with beta-cell failure developing over months to years. Perhaps vulnerable patients with genetic risk for pancreatic autoimmunity were stressed by SARS-CoV-2 infection and were diagnosed earlier than they might have been, showing some lead-time bias. Adult patients with COVID-19 demonstrated hyperglycemia that has been reversible in some cases, like the stress hyperglycemia seen with other infections and surgery in response to proinflammatory states.
The true question seems to be whether there is a unique type of diabetes related to direct viral toxicity. Do newly diagnosed patients have measurable traditional antibodies, like anti-glutamic acid decarboxylase or anti-islet cell antibodies? Is there proof of preceding SARS-CoV-2 infection? In the new cases that I thought were unusual at first glance, I found typical pancreatic autoimmunity and negative SARS-CoV-2 antibodies. The small cohorts reported thus far have had similar findings.
A stronger case can be made for the risk of developing diabetes (types 1 and 2) with rapid weight gain. Another marked pattern that pediatric endocrinologists have observed has been increased weight gain in children with closed schools, decreased activity, and more social isolation. I have seen weight change as great as 100 lb in a teen over the past year; 30- to 50-lb weight increases over the course of the pandemic have been common. Considering the “accelerator hypothesis” of faster onset of type 2 diabetes with rapid weight gain, implications for hastening of T1D with weight gain have also been considered. The full impact of these dramatic weight changes will take time to understand.
The true story may not emerge for years
Anecdotes and theoretical concerns may give us pause, but they are far from scientific truth. Efforts are underway to explore this perceived trend with international registries, including the CoviDIAB Registry as well as T1D Exchange. The true story may not emerge until years have passed to see the cumulative fallout of COVID-19. Regardless, these troubling observations should be considered as pandemic safeguards continue to loosen.
While pediatric mortality from COVID-19 has been relatively low (though sadly not zero), some have placed too little focus on possible morbidity. Long-term effects like long COVID and neuropsychiatric sequelae are becoming evident in all populations, including children. If a lifelong illness like diabetes can be directly linked to COVID-19, protecting children from infection with measures like masks becomes all the more crucial until vaccines are more readily available. Despite our rapid progress with understanding COVID-19 disease, there is still much left to learn.
A version of this article first appeared on Medscape.com.
In early 2020, the COVID-19 pandemic changed everything about life as we know it, with widespread shutdowns across the globe. The U.S. health care system quickly adapted, pivoting to telehealth visits when able and proactively managing outpatient conditions to prevent overwhelming hospital resources and utilization. Meanwhile, at my practice, the typical rate of about one new-onset pediatric type 1 diabetes (T1D) case per week increased to about two per week.
However, the new diabetes cases continued to accumulate, and I saw more patients being diagnosed who did not have a known family history of autoimmunity. I began to ask friends at other centers whether they were noticing the same trend.
One colleague documented a 36% increase in her large center compared with the previous year. Another noted a 40% rise at his children’s hospital. We observed that there was often a respiratory illness reported several weeks before presenting with T1D. Sometimes the child was known to be COVID-positive. Sometimes the child had not been tested. Sometimes we suspected that COVID had been a preceding illness and then found negative SARS-CoV-2 antibodies – but we were not certain whether the result was meaningful given the time lapsed since infection.
Soon, reports emerged of large increases in severe diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state at initial presentation, a trend reported in other countries.
Is COVID-19 a trigger for T1D?
There is known precedent for increased risk for T1D after viral infections in patients who are already genetically susceptible. Mechanisms of immune-mediated islet cell failure would make sense following SARS-CoV-2 infection; direct islet toxicity was noted with SARS-CoV-1 and has been suspected with SARS-CoV-2 but not proven. Some have suggested that hypercoagulability with COVID-19 may lead to ischemic damage to the pancreas.
With multiple potential pathways for islet damage, increases in insulin-dependent diabetes would logically follow. Still, whether this is the case remains unclear. There is not yet definitive evidence that there is uptake of SARS-CoV-2 via receptors in the pancreatic beta cells.
Our current understanding of T1D pathogenesis is that susceptible individuals develop autoimmunity in response to an environmental trigger, with beta-cell failure developing over months to years. Perhaps vulnerable patients with genetic risk for pancreatic autoimmunity were stressed by SARS-CoV-2 infection and were diagnosed earlier than they might have been, showing some lead-time bias. Adult patients with COVID-19 demonstrated hyperglycemia that has been reversible in some cases, like the stress hyperglycemia seen with other infections and surgery in response to proinflammatory states.
The true question seems to be whether there is a unique type of diabetes related to direct viral toxicity. Do newly diagnosed patients have measurable traditional antibodies, like anti-glutamic acid decarboxylase or anti-islet cell antibodies? Is there proof of preceding SARS-CoV-2 infection? In the new cases that I thought were unusual at first glance, I found typical pancreatic autoimmunity and negative SARS-CoV-2 antibodies. The small cohorts reported thus far have had similar findings.
A stronger case can be made for the risk of developing diabetes (types 1 and 2) with rapid weight gain. Another marked pattern that pediatric endocrinologists have observed has been increased weight gain in children with closed schools, decreased activity, and more social isolation. I have seen weight change as great as 100 lb in a teen over the past year; 30- to 50-lb weight increases over the course of the pandemic have been common. Considering the “accelerator hypothesis” of faster onset of type 2 diabetes with rapid weight gain, implications for hastening of T1D with weight gain have also been considered. The full impact of these dramatic weight changes will take time to understand.
The true story may not emerge for years
Anecdotes and theoretical concerns may give us pause, but they are far from scientific truth. Efforts are underway to explore this perceived trend with international registries, including the CoviDIAB Registry as well as T1D Exchange. The true story may not emerge until years have passed to see the cumulative fallout of COVID-19. Regardless, these troubling observations should be considered as pandemic safeguards continue to loosen.
While pediatric mortality from COVID-19 has been relatively low (though sadly not zero), some have placed too little focus on possible morbidity. Long-term effects like long COVID and neuropsychiatric sequelae are becoming evident in all populations, including children. If a lifelong illness like diabetes can be directly linked to COVID-19, protecting children from infection with measures like masks becomes all the more crucial until vaccines are more readily available. Despite our rapid progress with understanding COVID-19 disease, there is still much left to learn.
A version of this article first appeared on Medscape.com.
In early 2020, the COVID-19 pandemic changed everything about life as we know it, with widespread shutdowns across the globe. The U.S. health care system quickly adapted, pivoting to telehealth visits when able and proactively managing outpatient conditions to prevent overwhelming hospital resources and utilization. Meanwhile, at my practice, the typical rate of about one new-onset pediatric type 1 diabetes (T1D) case per week increased to about two per week.
However, the new diabetes cases continued to accumulate, and I saw more patients being diagnosed who did not have a known family history of autoimmunity. I began to ask friends at other centers whether they were noticing the same trend.
One colleague documented a 36% increase in her large center compared with the previous year. Another noted a 40% rise at his children’s hospital. We observed that there was often a respiratory illness reported several weeks before presenting with T1D. Sometimes the child was known to be COVID-positive. Sometimes the child had not been tested. Sometimes we suspected that COVID had been a preceding illness and then found negative SARS-CoV-2 antibodies – but we were not certain whether the result was meaningful given the time lapsed since infection.
Soon, reports emerged of large increases in severe diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state at initial presentation, a trend reported in other countries.
Is COVID-19 a trigger for T1D?
There is known precedent for increased risk for T1D after viral infections in patients who are already genetically susceptible. Mechanisms of immune-mediated islet cell failure would make sense following SARS-CoV-2 infection; direct islet toxicity was noted with SARS-CoV-1 and has been suspected with SARS-CoV-2 but not proven. Some have suggested that hypercoagulability with COVID-19 may lead to ischemic damage to the pancreas.
With multiple potential pathways for islet damage, increases in insulin-dependent diabetes would logically follow. Still, whether this is the case remains unclear. There is not yet definitive evidence that there is uptake of SARS-CoV-2 via receptors in the pancreatic beta cells.
Our current understanding of T1D pathogenesis is that susceptible individuals develop autoimmunity in response to an environmental trigger, with beta-cell failure developing over months to years. Perhaps vulnerable patients with genetic risk for pancreatic autoimmunity were stressed by SARS-CoV-2 infection and were diagnosed earlier than they might have been, showing some lead-time bias. Adult patients with COVID-19 demonstrated hyperglycemia that has been reversible in some cases, like the stress hyperglycemia seen with other infections and surgery in response to proinflammatory states.
The true question seems to be whether there is a unique type of diabetes related to direct viral toxicity. Do newly diagnosed patients have measurable traditional antibodies, like anti-glutamic acid decarboxylase or anti-islet cell antibodies? Is there proof of preceding SARS-CoV-2 infection? In the new cases that I thought were unusual at first glance, I found typical pancreatic autoimmunity and negative SARS-CoV-2 antibodies. The small cohorts reported thus far have had similar findings.
A stronger case can be made for the risk of developing diabetes (types 1 and 2) with rapid weight gain. Another marked pattern that pediatric endocrinologists have observed has been increased weight gain in children with closed schools, decreased activity, and more social isolation. I have seen weight change as great as 100 lb in a teen over the past year; 30- to 50-lb weight increases over the course of the pandemic have been common. Considering the “accelerator hypothesis” of faster onset of type 2 diabetes with rapid weight gain, implications for hastening of T1D with weight gain have also been considered. The full impact of these dramatic weight changes will take time to understand.
The true story may not emerge for years
Anecdotes and theoretical concerns may give us pause, but they are far from scientific truth. Efforts are underway to explore this perceived trend with international registries, including the CoviDIAB Registry as well as T1D Exchange. The true story may not emerge until years have passed to see the cumulative fallout of COVID-19. Regardless, these troubling observations should be considered as pandemic safeguards continue to loosen.
While pediatric mortality from COVID-19 has been relatively low (though sadly not zero), some have placed too little focus on possible morbidity. Long-term effects like long COVID and neuropsychiatric sequelae are becoming evident in all populations, including children. If a lifelong illness like diabetes can be directly linked to COVID-19, protecting children from infection with measures like masks becomes all the more crucial until vaccines are more readily available. Despite our rapid progress with understanding COVID-19 disease, there is still much left to learn.
A version of this article first appeared on Medscape.com.
Children and COVID: New vaccinations drop as the case count rises
With only a quarter of all children aged 12-15 years fully vaccinated against COVID-19, first vaccinations continued to drop and new cases for all children rose for the second consecutive week.

Just under 25% of children aged 12-15 had completed the vaccine regimen as of July 12, and just over one-third (33.5%) had received at least one dose. Meanwhile, that age group represented 11.5% of people who initiated vaccination during the 2 weeks ending July 12, down from 12.1% a week earlier, the Centers for Disease Control and Prevention said. The total number of new vaccinations for the week ending July 12 was just over 201,000, compared with 307,000 for the previous week.
New cases of COVID-19, however, were on the rise in children. The 19,000 new cases reported for the week ending July 8 were up from 12,000 a week earlier and 8,000 the week before that, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That report also shows that children made up 22.3% of all new cases during the week of July 2-8, compared with 16.8% the previous week, and that there were nine deaths in children that same week, the most since March. COVID-related deaths among children total 344 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting such data by age. “It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states,” the two groups noted.
Such data are available from the CDC’s COVID Data Tracker, however, and they show that children aged 16-17 years, who became eligible for COVID vaccination before the younger age group, are further ahead in the process. Among the older children, almost 46% had gotten at least one dose and 37% were fully vaccinated by July 12.
With only a quarter of all children aged 12-15 years fully vaccinated against COVID-19, first vaccinations continued to drop and new cases for all children rose for the second consecutive week.

Just under 25% of children aged 12-15 had completed the vaccine regimen as of July 12, and just over one-third (33.5%) had received at least one dose. Meanwhile, that age group represented 11.5% of people who initiated vaccination during the 2 weeks ending July 12, down from 12.1% a week earlier, the Centers for Disease Control and Prevention said. The total number of new vaccinations for the week ending July 12 was just over 201,000, compared with 307,000 for the previous week.
New cases of COVID-19, however, were on the rise in children. The 19,000 new cases reported for the week ending July 8 were up from 12,000 a week earlier and 8,000 the week before that, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That report also shows that children made up 22.3% of all new cases during the week of July 2-8, compared with 16.8% the previous week, and that there were nine deaths in children that same week, the most since March. COVID-related deaths among children total 344 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting such data by age. “It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states,” the two groups noted.
Such data are available from the CDC’s COVID Data Tracker, however, and they show that children aged 16-17 years, who became eligible for COVID vaccination before the younger age group, are further ahead in the process. Among the older children, almost 46% had gotten at least one dose and 37% were fully vaccinated by July 12.
With only a quarter of all children aged 12-15 years fully vaccinated against COVID-19, first vaccinations continued to drop and new cases for all children rose for the second consecutive week.

Just under 25% of children aged 12-15 had completed the vaccine regimen as of July 12, and just over one-third (33.5%) had received at least one dose. Meanwhile, that age group represented 11.5% of people who initiated vaccination during the 2 weeks ending July 12, down from 12.1% a week earlier, the Centers for Disease Control and Prevention said. The total number of new vaccinations for the week ending July 12 was just over 201,000, compared with 307,000 for the previous week.
New cases of COVID-19, however, were on the rise in children. The 19,000 new cases reported for the week ending July 8 were up from 12,000 a week earlier and 8,000 the week before that, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That report also shows that children made up 22.3% of all new cases during the week of July 2-8, compared with 16.8% the previous week, and that there were nine deaths in children that same week, the most since March. COVID-related deaths among children total 344 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting such data by age. “It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states,” the two groups noted.
Such data are available from the CDC’s COVID Data Tracker, however, and they show that children aged 16-17 years, who became eligible for COVID vaccination before the younger age group, are further ahead in the process. Among the older children, almost 46% had gotten at least one dose and 37% were fully vaccinated by July 12.
Respiratory infection– and asthma-prone children
Some children are more susceptible to viral and bacterial respiratory infections in the first few years of life than others. However, the factors contributing to this susceptibility are incompletely understood. The pathogenesis, development, severity, and clinical outcomes of respiratory infections are largely dependent on the resident composition of the nasopharyngeal microbiome and immune defense.1
Respiratory infections caused by bacteria and/or viruses are a leading cause of death in children in the United States and worldwide. The well-recognized, predominant causative bacteria are Streptococcus pneumoniae (pneumococcus), nontypeable Haemophilus influenzae (Hflu), and Moraxella catarrhalis (Mcat). Respiratory infections caused by these pathogens result in considerable morbidity, mortality, and account for high health care costs. The clinical and laboratory group that I lead in Rochester, N.Y., has been studying acute otitis media (AOM) etiology, epidemiology, pathogenesis, prevention, and treatment for over 3 decades. Our research findings are likely applicable and generalizable to understanding the pathogenesis and immune response to other infectious diseases induced by pneumococcus, Hflu, and Mcat since they are also key pathogens causing sinusitis and lung infections.
Previous immunologic analysis of children with AOM by our group provided clarity in differences between infection-prone children manifest as otitis prone (OP; often referred to in our publications as stringently defined OP because of the stringent diagnostic requirement of tympanocentesis-proven etiology of infection) and non-OP children. We showed that about 90% of OP children have deficient immune responses following nasopharyngeal colonization and AOM, demonstrated by inadequate innate responses and adaptive immune responses.2 Many of these children also showed an increased propensity to viral upper respiratory infection and 30% fail to produce protective antibody responses after injection of routine pediatric vaccines.3,4
In this column, I want to share new information regarding differences in the nasopharyngeal microbiome of children who are respiratory infection prone versus those who are non–respiratory infection prone and children with asthma versus those who do not exhibit that clinical phenotype. We performed a retrospective analysis of clinical samples collected from 358 children, aged 6 months to 5 years, from our prospectively enrolled cohort in Rochester, N.Y., to determine associations between AOM and other childhood respiratory illnesses and nasopharyngeal microbiota. In order to define subgroups of children within the cohort, we used a statistical method called unsupervised clustering analysis to see if relatively unique groups of children could be discerned. The overall cohort successfully clustered into two groups, showing marked differences in the prevalence of respiratory infections and asthma.5 We termed the two clinical phenotypes infection and asthma prone (n = 99, 28% of the children) and non–infection and asthma prone (n = 259, 72% of the children). Infection- and asthma-prone children were significantly more likely to experience recurrent AOM, influenza, sinusitis, pneumonia, asthma, and allergic rhinitis, compared with non–infection- and asthma-prone children (Figure).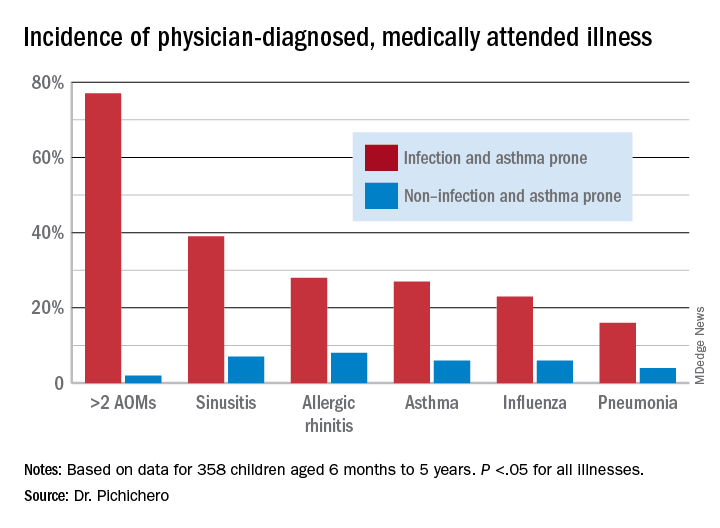
The two groups did not experience significantly different rates of eczema, food allergy, skin infections, urinary tract infections, or acute gastroenteritis, suggesting a common thread involving the respiratory tract that did not cross over to the gastrointestinal, skin, or urinary tract. We found that age at first nasopharyngeal colonization with any of the three bacterial respiratory pathogens (pneumococcus, Hflu, or Mcat) was significantly associated with the respiratory infection– and asthma-prone clinical phenotype. Specifically, respiratory infection– and asthma-prone children experienced colonization at a significantly earlier age than nonprone children did for all three bacteria. In an analysis of individual conditions, early Mcat colonization significantly associated with pneumonia, sinusitis, and asthma susceptibility; Hflu with pneumonia, sinusitis, influenza, and allergic rhinitis; and pneumococcus with sinusitis.
Since early colonization with the three bacterial respiratory pathogens was strongly associated with respiratory illnesses and asthma, nasopharyngeal microbiome analysis was performed on an available subset of samples. Bacterial diversity trended lower in infection- and asthma-prone children, consistent with dysbiosis in the respiratory infection– and asthma-prone clinical phenotype. Nine different bacteria genera were found to be differentially abundant when comparing respiratory infection– and asthma-prone and nonprone children, pointing the way to possible interventions to make the respiratory infection– and asthma-prone child nasopharyngeal microbiome more like the nonprone child.
As I have written previously in this column, recent accumulating data have shed light on the importance of the human microbiome in modulating immune homeostasis and disease susceptibility.6 My group is working toward generating new knowledge for the long-term goal of identifying new therapeutic strategies to facilitate a protective, diverse nasopharyngeal microbiome (with appropriately tuned intranasal probiotics) to prevent respiratory pathogen colonization and/or subsequent progression to respiratory infection and asthma. Also, vaccines directed against colonization-enhancing members of the microbiome may provide a means to indirectly control respiratory pathogen nasopharyngeal colonization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Contact him at [email protected]
References
1. Man WH et al. Nat Rev Microbiol. 2017;15(5):259-70.
2. Pichichero ME. J Infect. 2020;80(6):614-22.
3. Ren D et al. Clin Infect Dis. 2019;68(9):1566-74.
4. Pichichero ME et al. Pediatr Infect Dis J. 2013;32(11):1163-8.
5. Chapman T et al. PLoS One. 2020 Dec 11;15(12).
6. Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124:4162-5.
Some children are more susceptible to viral and bacterial respiratory infections in the first few years of life than others. However, the factors contributing to this susceptibility are incompletely understood. The pathogenesis, development, severity, and clinical outcomes of respiratory infections are largely dependent on the resident composition of the nasopharyngeal microbiome and immune defense.1
Respiratory infections caused by bacteria and/or viruses are a leading cause of death in children in the United States and worldwide. The well-recognized, predominant causative bacteria are Streptococcus pneumoniae (pneumococcus), nontypeable Haemophilus influenzae (Hflu), and Moraxella catarrhalis (Mcat). Respiratory infections caused by these pathogens result in considerable morbidity, mortality, and account for high health care costs. The clinical and laboratory group that I lead in Rochester, N.Y., has been studying acute otitis media (AOM) etiology, epidemiology, pathogenesis, prevention, and treatment for over 3 decades. Our research findings are likely applicable and generalizable to understanding the pathogenesis and immune response to other infectious diseases induced by pneumococcus, Hflu, and Mcat since they are also key pathogens causing sinusitis and lung infections.
Previous immunologic analysis of children with AOM by our group provided clarity in differences between infection-prone children manifest as otitis prone (OP; often referred to in our publications as stringently defined OP because of the stringent diagnostic requirement of tympanocentesis-proven etiology of infection) and non-OP children. We showed that about 90% of OP children have deficient immune responses following nasopharyngeal colonization and AOM, demonstrated by inadequate innate responses and adaptive immune responses.2 Many of these children also showed an increased propensity to viral upper respiratory infection and 30% fail to produce protective antibody responses after injection of routine pediatric vaccines.3,4
In this column, I want to share new information regarding differences in the nasopharyngeal microbiome of children who are respiratory infection prone versus those who are non–respiratory infection prone and children with asthma versus those who do not exhibit that clinical phenotype. We performed a retrospective analysis of clinical samples collected from 358 children, aged 6 months to 5 years, from our prospectively enrolled cohort in Rochester, N.Y., to determine associations between AOM and other childhood respiratory illnesses and nasopharyngeal microbiota. In order to define subgroups of children within the cohort, we used a statistical method called unsupervised clustering analysis to see if relatively unique groups of children could be discerned. The overall cohort successfully clustered into two groups, showing marked differences in the prevalence of respiratory infections and asthma.5 We termed the two clinical phenotypes infection and asthma prone (n = 99, 28% of the children) and non–infection and asthma prone (n = 259, 72% of the children). Infection- and asthma-prone children were significantly more likely to experience recurrent AOM, influenza, sinusitis, pneumonia, asthma, and allergic rhinitis, compared with non–infection- and asthma-prone children (Figure).
The two groups did not experience significantly different rates of eczema, food allergy, skin infections, urinary tract infections, or acute gastroenteritis, suggesting a common thread involving the respiratory tract that did not cross over to the gastrointestinal, skin, or urinary tract. We found that age at first nasopharyngeal colonization with any of the three bacterial respiratory pathogens (pneumococcus, Hflu, or Mcat) was significantly associated with the respiratory infection– and asthma-prone clinical phenotype. Specifically, respiratory infection– and asthma-prone children experienced colonization at a significantly earlier age than nonprone children did for all three bacteria. In an analysis of individual conditions, early Mcat colonization significantly associated with pneumonia, sinusitis, and asthma susceptibility; Hflu with pneumonia, sinusitis, influenza, and allergic rhinitis; and pneumococcus with sinusitis.
Since early colonization with the three bacterial respiratory pathogens was strongly associated with respiratory illnesses and asthma, nasopharyngeal microbiome analysis was performed on an available subset of samples. Bacterial diversity trended lower in infection- and asthma-prone children, consistent with dysbiosis in the respiratory infection– and asthma-prone clinical phenotype. Nine different bacteria genera were found to be differentially abundant when comparing respiratory infection– and asthma-prone and nonprone children, pointing the way to possible interventions to make the respiratory infection– and asthma-prone child nasopharyngeal microbiome more like the nonprone child.
As I have written previously in this column, recent accumulating data have shed light on the importance of the human microbiome in modulating immune homeostasis and disease susceptibility.6 My group is working toward generating new knowledge for the long-term goal of identifying new therapeutic strategies to facilitate a protective, diverse nasopharyngeal microbiome (with appropriately tuned intranasal probiotics) to prevent respiratory pathogen colonization and/or subsequent progression to respiratory infection and asthma. Also, vaccines directed against colonization-enhancing members of the microbiome may provide a means to indirectly control respiratory pathogen nasopharyngeal colonization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Contact him at [email protected]
References
1. Man WH et al. Nat Rev Microbiol. 2017;15(5):259-70.
2. Pichichero ME. J Infect. 2020;80(6):614-22.
3. Ren D et al. Clin Infect Dis. 2019;68(9):1566-74.
4. Pichichero ME et al. Pediatr Infect Dis J. 2013;32(11):1163-8.
5. Chapman T et al. PLoS One. 2020 Dec 11;15(12).
6. Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124:4162-5.
Some children are more susceptible to viral and bacterial respiratory infections in the first few years of life than others. However, the factors contributing to this susceptibility are incompletely understood. The pathogenesis, development, severity, and clinical outcomes of respiratory infections are largely dependent on the resident composition of the nasopharyngeal microbiome and immune defense.1
Respiratory infections caused by bacteria and/or viruses are a leading cause of death in children in the United States and worldwide. The well-recognized, predominant causative bacteria are Streptococcus pneumoniae (pneumococcus), nontypeable Haemophilus influenzae (Hflu), and Moraxella catarrhalis (Mcat). Respiratory infections caused by these pathogens result in considerable morbidity, mortality, and account for high health care costs. The clinical and laboratory group that I lead in Rochester, N.Y., has been studying acute otitis media (AOM) etiology, epidemiology, pathogenesis, prevention, and treatment for over 3 decades. Our research findings are likely applicable and generalizable to understanding the pathogenesis and immune response to other infectious diseases induced by pneumococcus, Hflu, and Mcat since they are also key pathogens causing sinusitis and lung infections.
Previous immunologic analysis of children with AOM by our group provided clarity in differences between infection-prone children manifest as otitis prone (OP; often referred to in our publications as stringently defined OP because of the stringent diagnostic requirement of tympanocentesis-proven etiology of infection) and non-OP children. We showed that about 90% of OP children have deficient immune responses following nasopharyngeal colonization and AOM, demonstrated by inadequate innate responses and adaptive immune responses.2 Many of these children also showed an increased propensity to viral upper respiratory infection and 30% fail to produce protective antibody responses after injection of routine pediatric vaccines.3,4
In this column, I want to share new information regarding differences in the nasopharyngeal microbiome of children who are respiratory infection prone versus those who are non–respiratory infection prone and children with asthma versus those who do not exhibit that clinical phenotype. We performed a retrospective analysis of clinical samples collected from 358 children, aged 6 months to 5 years, from our prospectively enrolled cohort in Rochester, N.Y., to determine associations between AOM and other childhood respiratory illnesses and nasopharyngeal microbiota. In order to define subgroups of children within the cohort, we used a statistical method called unsupervised clustering analysis to see if relatively unique groups of children could be discerned. The overall cohort successfully clustered into two groups, showing marked differences in the prevalence of respiratory infections and asthma.5 We termed the two clinical phenotypes infection and asthma prone (n = 99, 28% of the children) and non–infection and asthma prone (n = 259, 72% of the children). Infection- and asthma-prone children were significantly more likely to experience recurrent AOM, influenza, sinusitis, pneumonia, asthma, and allergic rhinitis, compared with non–infection- and asthma-prone children (Figure).
The two groups did not experience significantly different rates of eczema, food allergy, skin infections, urinary tract infections, or acute gastroenteritis, suggesting a common thread involving the respiratory tract that did not cross over to the gastrointestinal, skin, or urinary tract. We found that age at first nasopharyngeal colonization with any of the three bacterial respiratory pathogens (pneumococcus, Hflu, or Mcat) was significantly associated with the respiratory infection– and asthma-prone clinical phenotype. Specifically, respiratory infection– and asthma-prone children experienced colonization at a significantly earlier age than nonprone children did for all three bacteria. In an analysis of individual conditions, early Mcat colonization significantly associated with pneumonia, sinusitis, and asthma susceptibility; Hflu with pneumonia, sinusitis, influenza, and allergic rhinitis; and pneumococcus with sinusitis.
Since early colonization with the three bacterial respiratory pathogens was strongly associated with respiratory illnesses and asthma, nasopharyngeal microbiome analysis was performed on an available subset of samples. Bacterial diversity trended lower in infection- and asthma-prone children, consistent with dysbiosis in the respiratory infection– and asthma-prone clinical phenotype. Nine different bacteria genera were found to be differentially abundant when comparing respiratory infection– and asthma-prone and nonprone children, pointing the way to possible interventions to make the respiratory infection– and asthma-prone child nasopharyngeal microbiome more like the nonprone child.
As I have written previously in this column, recent accumulating data have shed light on the importance of the human microbiome in modulating immune homeostasis and disease susceptibility.6 My group is working toward generating new knowledge for the long-term goal of identifying new therapeutic strategies to facilitate a protective, diverse nasopharyngeal microbiome (with appropriately tuned intranasal probiotics) to prevent respiratory pathogen colonization and/or subsequent progression to respiratory infection and asthma. Also, vaccines directed against colonization-enhancing members of the microbiome may provide a means to indirectly control respiratory pathogen nasopharyngeal colonization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Contact him at [email protected]
References
1. Man WH et al. Nat Rev Microbiol. 2017;15(5):259-70.
2. Pichichero ME. J Infect. 2020;80(6):614-22.
3. Ren D et al. Clin Infect Dis. 2019;68(9):1566-74.
4. Pichichero ME et al. Pediatr Infect Dis J. 2013;32(11):1163-8.
5. Chapman T et al. PLoS One. 2020 Dec 11;15(12).
6. Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124:4162-5.
Nadolol bests propranolol for infantile hemangioma treatment out to 52 weeks
of 71 patients showed.
“In clinical practice, we notice that nadolol works very well in terms of controlling the size and the appearance of the hemangioma,” lead study author Elena Pope, MD, MSc, said during the annual meeting of the Society for Pediatric Dermatology. Hence, she and her colleagues were interested in comparing their clinical experience with the standard treatment with propranolol, and designed a prospective, randomized, controlled, double-blinded study, with the aim of proving that “nadolol is noninferior to propranolol, with a margin of noninferiority of 10%.”
Between 2016 and 2020, Dr. Pope and colleagues at two academic Canadian pediatric dermatology centers enrolled 71 infants aged 1-6 months with significant hemangioma that had either the potential for functional impairment or cosmetic deformity, defined as a lesion greater than 1.5 cm on the face or greater than 3 cm on another body part. Treatment consisted of oral propranolol or nadolol in escalating doses up to 2 mg/kg per day. “The blinding portion of the study was for 24 weeks with a follow-up up to 52 weeks,” said Dr. Pope, professor of pediatrics at the University of Toronto and section head of pediatric dermatology at The Hospital for Sick Children, also in Toronto. “After the unblinding at 24 weeks, patients were allowed to switch their intervention if they were not happy with the results.”
Of the 71 patients, 35 received nadolol and 36 received propranolol. The two groups were similar in terms of clinical and demographic characteristics. Their mean age at enrollment was 3.15 months, 80% were female, 61% were White, 20% were Asian, and the rest were from other ethnic backgrounds.
At 24 weeks, the researchers found that the mean size involution was 97.94% in the nadolol group and 89.14% in the propranolol group (P = .005), while the mean color fading on the visual analogue scale (VAS) was 94.47% in the nadolol group and 80.54% in the propranolol group (P < .001). At 52 weeks, the mean size involution was 99.63% in the nadolol group and 93.63% in the propranolol group (P = .001), while the mean VAS color fading was 97.34% in the nadolol group and 87.23% in the propranolol group (P = .001).
According to Dr. Pope, Kaplan-Meir analysis showed that patients in the propranolol group responded slower to treatment (P = .019), while safety data was similar between the two groups. For example, between weeks 25 and 52, 84.2% of patients in the nadolol group experienced an adverse event, compared with 74.2% of patients in the propranolol group (P = .466). The most common respiratory adverse event was upper respiratory tract infection, which affected 87.5% of patients in the nadolol group, compared with 100% of patients in the propranolol group (P = 0.341).
The most common gastrointestinal adverse event was diarrhea, which affected 66.7% of patients in both groups. One patient in the propranolol group was admitted to the hospital with pneumonia and fully recovered. The incident was not suspected to be related to the medication.
“We believe that this data backs up our clinical experience and it may offer an alternative treatment in other centers where patients experience propranolol unresponsiveness, side effects, or intolerance, or where a fast response is needed,” Dr. Pope said. As for the potential cost implications, “nadolol is cheaper than the Hemangiol but comparable with the compounded formulation of propranolol.”
Concern over the safety of nadolol was raised in a case report published in Pediatrics in 2020. Authors from Alberta reported the case of a 10-week-old girl who was started on nadolol for infantile hemangioma, died 7 weeks later, and was found to have an elevated postmortem cardiac blood nadolol level of 0.94 mg/L. “The infant had no bowel movements for 10 days before her death, which we hypothesize contributed to nadolol toxicity,” the authors wrote.
In a reply to the authors in the same issue of Pediatrics, Dr. Pope, Cathryn Sibbald, MD, and Erin Chung, PhD, pointed out that postmortem redistribution of medications “is complex and measured postmortem cardiac blood concentrations may be significantly higher than the true blood nadolol concentration at the time of death due to significant diffusion from the peripheral tissues.”
They added that the report did not address “other potential errors such as in compounding, dispensing, and administration of the solution,” they wrote, adding: “Finally, we are aware of a Canadian case of death in an infant receiving propranolol, although the cause of death in that case was unable to be determined (ISMP Canada 2016 Safety Bulletin).We agree with the authors that careful consideration of the risks and benefits of beta-blocker therapy should be employed, parents need to be informed when to discontinue therapy and that further research into the pharmacokinetics and pharmacogenetics of beta-blockers are warranted.”
Following publication of the case report in Pediatrics, Dr. Pope said that the only change she made in her practice was to ask families to temporarily discontinue nadolol if their child had constipation for more than 5 days.
The study was supported by a grant from Physician Services, Inc. Dr. Pope reported having no financial disclosures.
of 71 patients showed.
“In clinical practice, we notice that nadolol works very well in terms of controlling the size and the appearance of the hemangioma,” lead study author Elena Pope, MD, MSc, said during the annual meeting of the Society for Pediatric Dermatology. Hence, she and her colleagues were interested in comparing their clinical experience with the standard treatment with propranolol, and designed a prospective, randomized, controlled, double-blinded study, with the aim of proving that “nadolol is noninferior to propranolol, with a margin of noninferiority of 10%.”
Between 2016 and 2020, Dr. Pope and colleagues at two academic Canadian pediatric dermatology centers enrolled 71 infants aged 1-6 months with significant hemangioma that had either the potential for functional impairment or cosmetic deformity, defined as a lesion greater than 1.5 cm on the face or greater than 3 cm on another body part. Treatment consisted of oral propranolol or nadolol in escalating doses up to 2 mg/kg per day. “The blinding portion of the study was for 24 weeks with a follow-up up to 52 weeks,” said Dr. Pope, professor of pediatrics at the University of Toronto and section head of pediatric dermatology at The Hospital for Sick Children, also in Toronto. “After the unblinding at 24 weeks, patients were allowed to switch their intervention if they were not happy with the results.”
Of the 71 patients, 35 received nadolol and 36 received propranolol. The two groups were similar in terms of clinical and demographic characteristics. Their mean age at enrollment was 3.15 months, 80% were female, 61% were White, 20% were Asian, and the rest were from other ethnic backgrounds.
At 24 weeks, the researchers found that the mean size involution was 97.94% in the nadolol group and 89.14% in the propranolol group (P = .005), while the mean color fading on the visual analogue scale (VAS) was 94.47% in the nadolol group and 80.54% in the propranolol group (P < .001). At 52 weeks, the mean size involution was 99.63% in the nadolol group and 93.63% in the propranolol group (P = .001), while the mean VAS color fading was 97.34% in the nadolol group and 87.23% in the propranolol group (P = .001).
According to Dr. Pope, Kaplan-Meir analysis showed that patients in the propranolol group responded slower to treatment (P = .019), while safety data was similar between the two groups. For example, between weeks 25 and 52, 84.2% of patients in the nadolol group experienced an adverse event, compared with 74.2% of patients in the propranolol group (P = .466). The most common respiratory adverse event was upper respiratory tract infection, which affected 87.5% of patients in the nadolol group, compared with 100% of patients in the propranolol group (P = 0.341).
The most common gastrointestinal adverse event was diarrhea, which affected 66.7% of patients in both groups. One patient in the propranolol group was admitted to the hospital with pneumonia and fully recovered. The incident was not suspected to be related to the medication.
“We believe that this data backs up our clinical experience and it may offer an alternative treatment in other centers where patients experience propranolol unresponsiveness, side effects, or intolerance, or where a fast response is needed,” Dr. Pope said. As for the potential cost implications, “nadolol is cheaper than the Hemangiol but comparable with the compounded formulation of propranolol.”
Concern over the safety of nadolol was raised in a case report published in Pediatrics in 2020. Authors from Alberta reported the case of a 10-week-old girl who was started on nadolol for infantile hemangioma, died 7 weeks later, and was found to have an elevated postmortem cardiac blood nadolol level of 0.94 mg/L. “The infant had no bowel movements for 10 days before her death, which we hypothesize contributed to nadolol toxicity,” the authors wrote.
In a reply to the authors in the same issue of Pediatrics, Dr. Pope, Cathryn Sibbald, MD, and Erin Chung, PhD, pointed out that postmortem redistribution of medications “is complex and measured postmortem cardiac blood concentrations may be significantly higher than the true blood nadolol concentration at the time of death due to significant diffusion from the peripheral tissues.”
They added that the report did not address “other potential errors such as in compounding, dispensing, and administration of the solution,” they wrote, adding: “Finally, we are aware of a Canadian case of death in an infant receiving propranolol, although the cause of death in that case was unable to be determined (ISMP Canada 2016 Safety Bulletin).We agree with the authors that careful consideration of the risks and benefits of beta-blocker therapy should be employed, parents need to be informed when to discontinue therapy and that further research into the pharmacokinetics and pharmacogenetics of beta-blockers are warranted.”
Following publication of the case report in Pediatrics, Dr. Pope said that the only change she made in her practice was to ask families to temporarily discontinue nadolol if their child had constipation for more than 5 days.
The study was supported by a grant from Physician Services, Inc. Dr. Pope reported having no financial disclosures.
of 71 patients showed.
“In clinical practice, we notice that nadolol works very well in terms of controlling the size and the appearance of the hemangioma,” lead study author Elena Pope, MD, MSc, said during the annual meeting of the Society for Pediatric Dermatology. Hence, she and her colleagues were interested in comparing their clinical experience with the standard treatment with propranolol, and designed a prospective, randomized, controlled, double-blinded study, with the aim of proving that “nadolol is noninferior to propranolol, with a margin of noninferiority of 10%.”
Between 2016 and 2020, Dr. Pope and colleagues at two academic Canadian pediatric dermatology centers enrolled 71 infants aged 1-6 months with significant hemangioma that had either the potential for functional impairment or cosmetic deformity, defined as a lesion greater than 1.5 cm on the face or greater than 3 cm on another body part. Treatment consisted of oral propranolol or nadolol in escalating doses up to 2 mg/kg per day. “The blinding portion of the study was for 24 weeks with a follow-up up to 52 weeks,” said Dr. Pope, professor of pediatrics at the University of Toronto and section head of pediatric dermatology at The Hospital for Sick Children, also in Toronto. “After the unblinding at 24 weeks, patients were allowed to switch their intervention if they were not happy with the results.”
Of the 71 patients, 35 received nadolol and 36 received propranolol. The two groups were similar in terms of clinical and demographic characteristics. Their mean age at enrollment was 3.15 months, 80% were female, 61% were White, 20% were Asian, and the rest were from other ethnic backgrounds.
At 24 weeks, the researchers found that the mean size involution was 97.94% in the nadolol group and 89.14% in the propranolol group (P = .005), while the mean color fading on the visual analogue scale (VAS) was 94.47% in the nadolol group and 80.54% in the propranolol group (P < .001). At 52 weeks, the mean size involution was 99.63% in the nadolol group and 93.63% in the propranolol group (P = .001), while the mean VAS color fading was 97.34% in the nadolol group and 87.23% in the propranolol group (P = .001).
According to Dr. Pope, Kaplan-Meir analysis showed that patients in the propranolol group responded slower to treatment (P = .019), while safety data was similar between the two groups. For example, between weeks 25 and 52, 84.2% of patients in the nadolol group experienced an adverse event, compared with 74.2% of patients in the propranolol group (P = .466). The most common respiratory adverse event was upper respiratory tract infection, which affected 87.5% of patients in the nadolol group, compared with 100% of patients in the propranolol group (P = 0.341).
The most common gastrointestinal adverse event was diarrhea, which affected 66.7% of patients in both groups. One patient in the propranolol group was admitted to the hospital with pneumonia and fully recovered. The incident was not suspected to be related to the medication.
“We believe that this data backs up our clinical experience and it may offer an alternative treatment in other centers where patients experience propranolol unresponsiveness, side effects, or intolerance, or where a fast response is needed,” Dr. Pope said. As for the potential cost implications, “nadolol is cheaper than the Hemangiol but comparable with the compounded formulation of propranolol.”
Concern over the safety of nadolol was raised in a case report published in Pediatrics in 2020. Authors from Alberta reported the case of a 10-week-old girl who was started on nadolol for infantile hemangioma, died 7 weeks later, and was found to have an elevated postmortem cardiac blood nadolol level of 0.94 mg/L. “The infant had no bowel movements for 10 days before her death, which we hypothesize contributed to nadolol toxicity,” the authors wrote.
In a reply to the authors in the same issue of Pediatrics, Dr. Pope, Cathryn Sibbald, MD, and Erin Chung, PhD, pointed out that postmortem redistribution of medications “is complex and measured postmortem cardiac blood concentrations may be significantly higher than the true blood nadolol concentration at the time of death due to significant diffusion from the peripheral tissues.”
They added that the report did not address “other potential errors such as in compounding, dispensing, and administration of the solution,” they wrote, adding: “Finally, we are aware of a Canadian case of death in an infant receiving propranolol, although the cause of death in that case was unable to be determined (ISMP Canada 2016 Safety Bulletin).We agree with the authors that careful consideration of the risks and benefits of beta-blocker therapy should be employed, parents need to be informed when to discontinue therapy and that further research into the pharmacokinetics and pharmacogenetics of beta-blockers are warranted.”
Following publication of the case report in Pediatrics, Dr. Pope said that the only change she made in her practice was to ask families to temporarily discontinue nadolol if their child had constipation for more than 5 days.
The study was supported by a grant from Physician Services, Inc. Dr. Pope reported having no financial disclosures.
FROM SPD 2021
Married docs remove girl’s lethal facial tumor in ‘excruciatingly difficult’ procedure
In 2019, doctors in London saw a 5-year old girl from rural Ethiopia with an enormous tumor extending from her cheek to her lower jaw. Her name was Negalem and the tumor was a vascular malformation, a life-threatening web of tangled blood vessels.
Surgery to remove it was impossible, the doctors told the foundation advocating for the girl. The child would never make it off the operating table. After a closer examination, the London group still declined to do the procedure, but told the child’s parents and advocates that if anyone was going to attempt this, they’d need to get the little girl to New York.
In New York City, on 64th St. in Manhattan, is the Vascular Birthmark Institute, founded by Milton Waner, MD, who has exclusively treated hemangiomas and vascular malformations for the last 30 years. “I’m the only person in the [United] States whose practice is exclusively [treating] vascular anomalies,” Dr. Waner said in an interview.
Dr. Waner has assembled a multidisciplinary team of experts at the institute’s offices in Lenox Hill – including his wife Teresa O, MD, a facial plastic and reconstructive surgeon and neurospecialist. “People often ask how the hell do you spend so much time with your spouse?” Dr. Waner says. “We work extremely well together. We complement each other.”
Dr. O and Dr. Waner each manage half of the cases at VBI. And in January they received an email about Negalem. After corresponding with the child’s advocate and reviewing images,
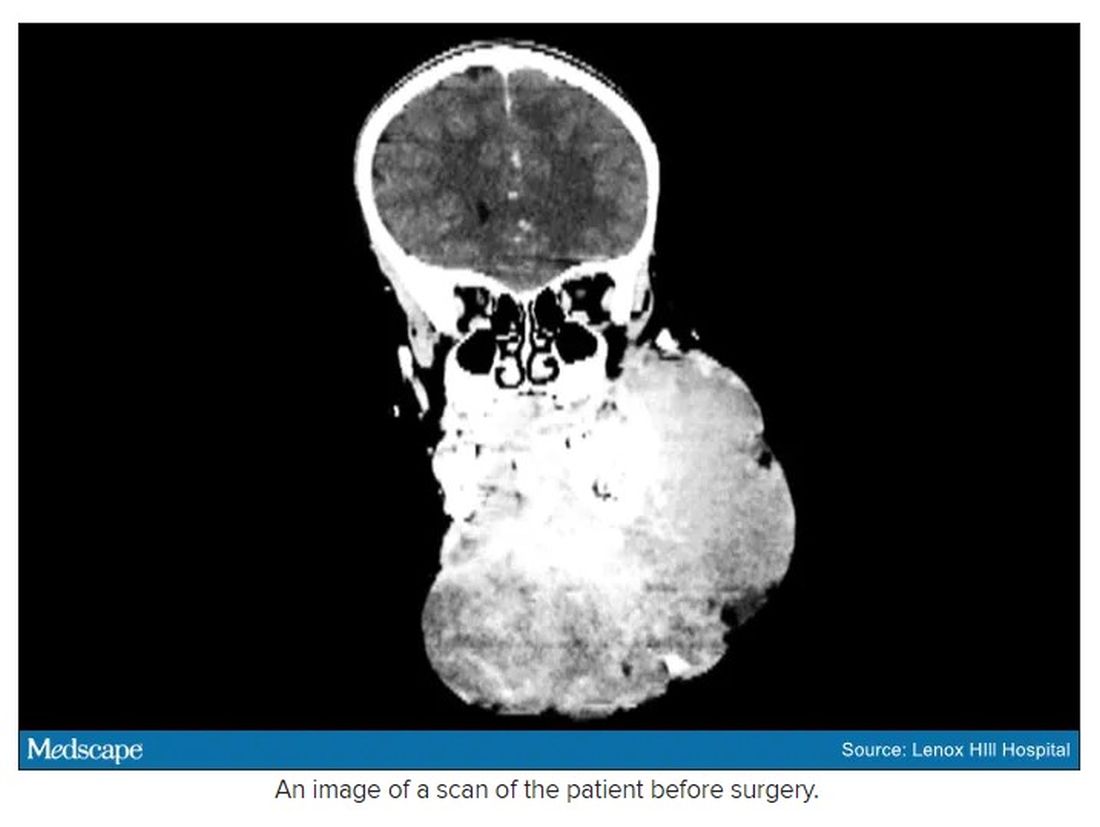
The challenge with vascular malformations in children, Dr. Waner said, is that they have a fraction of the blood an adult has. Where adults have an average of 5 L of blood, a child this age has only 1 L. To lose 200 or 300 mL of blood, “that’s 20% or 30% of their blood volume,” Dr. Waner said. So the removal of such a mass, which requires a meticulous dissection around many blood vessels, carries a high risk of the child bleeding out.
There were some logistical hurdles, but the patient arrived in Manhattan in mid-June, at no cost to her family. The medical visa was organized by a volunteer who also work for USAID. Healing the Children Northeast paid for her travel and the Waner Kids Foundation paid for her hotel stay. Lenox Hill Hospital and Northwell Health covered all hospital costs and postsurgery care. And Dr. O and Dr. Waner did the planning, consult visits, and procedure pro bono.
The surgery was possible because of the generosity of several organizations, but the two surgeons still had a limited time to remove the mass. Under different circumstances, and with the luxury of more time, the patient would have undergone several rounds of sclerotherapy. This procedure, done by interventional radiologists, involves injecting a toxin into the blood vessels, which causes them to clot. Done prior to surgery it can help limit bleeding risk.
On June 23, the morning of the surgery, the patient underwent one round of sclerotherapy. However, it didn’t have the intended effect, Dr. Waner said, “because the lesion was just so massive.”
The team had planned several of their moves ahead of time. But this isn’t the sort of surgery you’d find in a textbook. Because it’s such a unique field, Dr. Waner and Dr. O have developed many of their own techniques along the way. This patient was much like the cases they treat every day, only “several orders of magnitudes greater,” Dr. Waner said. “On a scale of 1 to 10 she was a 12.”
The morning of the surgery, “I was very apprehensive,” Dr. Waner recalled. He vividly remembers the girl’s father repeatedly kissing her to say goodbye as she lay on the operating table, fully aware that this procedure was a life-threatening one. And from the beginning there were challenges, like getting her under anesthesia when the anatomy of her mouth, deformed by the tumor, didn’t allow the anesthesiologists to use their typical tubing. Then, once the skin was removed, it became clear how dilated and tangled the involved blood vessels were. There were many vital structures tangled in the anomaly. “The jugular vein was right there. The carotid artery was right there,” Dr. Waner said. It was extremely difficult to delineate and preserve them, he said.
“That’s why we really took our time. We just went very slowly and deliberately,” Dr. O said. The blood vessels were so dilated that their only option was to move painstakingly slow – otherwise a small nick could be devastating.
But even with the slow pace the surgery was “excruciatingly difficult,” Dr. Waner said. And early on in the dissection he wasn’t quite sure they’d make it out. The sclerotherapy hadn’t done much to prevent bleeding. “At one point every millimeter or 2 that we advanced we got into some bleeding,” Dr. Waner said. “Brisk bleeding.”
Once they got into the surgery they also realized that the growth had adhered to the jaw bone. “There were vessels traversing into the bone, which were hard to control,” Dr. O said.
But finally, both doctors realized they’d be able to remove it. With the lesion removed they began the work of reconstruction and reanimation.
The child’s jaw and cheek bone had grown beyond their normal size to support the growth. They had to shave them down to achieve facial symmetry. The tumor had also inhibited much of the child’s facial nerve control. With it gone, Dr. O began the work of finding all the facial nerve branches and assembling them to reanimate the child’s face.
Before medicine, Dr. O trained as an architect, which, according to Dr. Waner, has equipped her with very good spatial awareness – a valuable skill in the surgical reconstruction phase. After seeing a lecture by Dr. Waner, she immediately saw a fit for her unique interest and skill set. She did fellowship training with Dr. Waner in vascular anomalies, and then went on to specialize in facial nerve reanimation. The proof of Dr. O’s expertise is Negalem’s new, beautiful smile, Dr. Waner said.
The surgery drew out over 8 hours, as long as a day of surgeries for the two doctors. When Dr. O finally walked into the waiting room to inform the family of the success, the first words out of the father’s mouth were: “Is my daughter alive?”
A growth like Negalem had is not compatible with a normal life. Dr. Waner’s mantra is that every child has the right to look normal. But this case went beyond aesthetics. If the growth hadn’t been removed, the child was expected to live only 4-6 more years, Dr. Waner said. Without the surgery, she could have suffocated, starved without the ability to swallow, or suffered a fatal bleed.
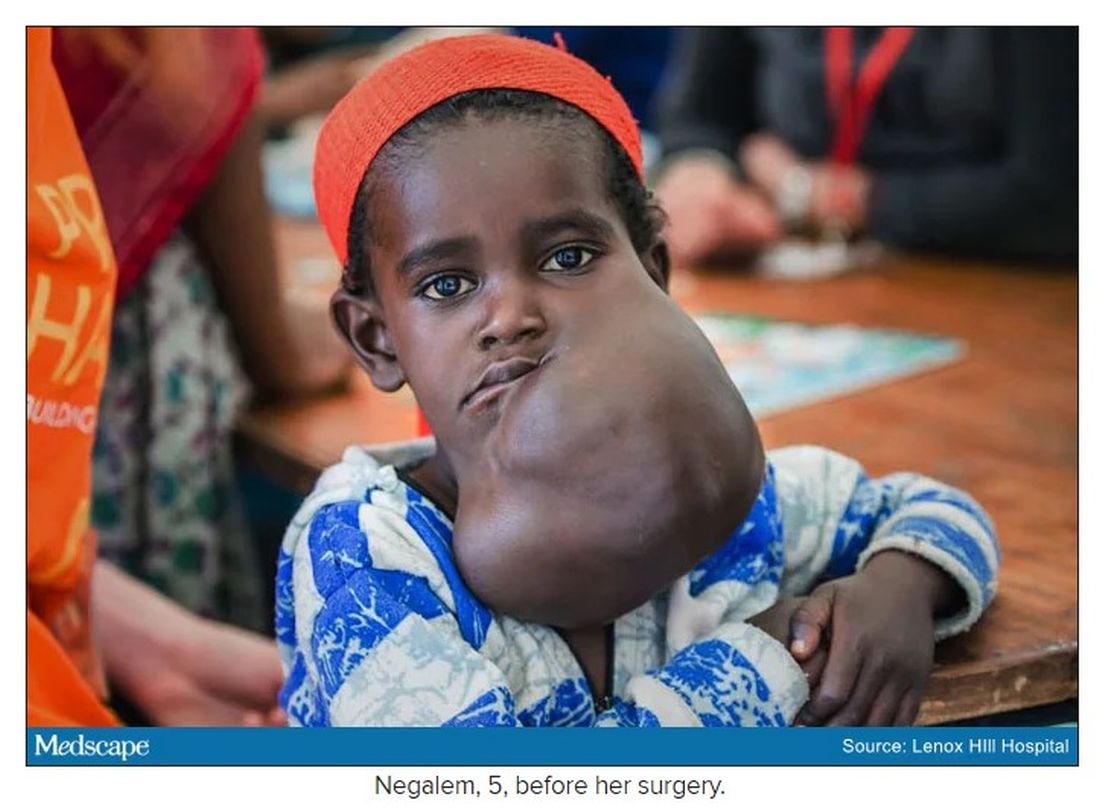
Dr. O and Dr. Waner are uniquely equipped to do this kind of work, but both are adamant that treating vascular anomalies is a multidisciplinary, multimodal approach. Specialties in anesthesiology, radiology, lasers, facial nerves – they are all critical to these procedures. And often patients with these kinds of lesions require medical and radiologic interventions in addition to surgery. In this particular case, from logistics to post op, “it was a lot of teamwork,” Dr. O said, “a lot of international teams coming together.”
Though extremely difficult, “in the end the result was exactly what we wanted,” Dr. Waner said. Negalem can live a normal life. And as for the surgical duo, both feel very fortunate to do this work. Dr. O said, “I’m honored to have found this specialty and to be able to train with and work with Milton. I’m so happy to do what I do every day.”
A version of this article first appeared on Medscape.com.
In 2019, doctors in London saw a 5-year old girl from rural Ethiopia with an enormous tumor extending from her cheek to her lower jaw. Her name was Negalem and the tumor was a vascular malformation, a life-threatening web of tangled blood vessels.
Surgery to remove it was impossible, the doctors told the foundation advocating for the girl. The child would never make it off the operating table. After a closer examination, the London group still declined to do the procedure, but told the child’s parents and advocates that if anyone was going to attempt this, they’d need to get the little girl to New York.
In New York City, on 64th St. in Manhattan, is the Vascular Birthmark Institute, founded by Milton Waner, MD, who has exclusively treated hemangiomas and vascular malformations for the last 30 years. “I’m the only person in the [United] States whose practice is exclusively [treating] vascular anomalies,” Dr. Waner said in an interview.
Dr. Waner has assembled a multidisciplinary team of experts at the institute’s offices in Lenox Hill – including his wife Teresa O, MD, a facial plastic and reconstructive surgeon and neurospecialist. “People often ask how the hell do you spend so much time with your spouse?” Dr. Waner says. “We work extremely well together. We complement each other.”
Dr. O and Dr. Waner each manage half of the cases at VBI. And in January they received an email about Negalem. After corresponding with the child’s advocate and reviewing images,

The challenge with vascular malformations in children, Dr. Waner said, is that they have a fraction of the blood an adult has. Where adults have an average of 5 L of blood, a child this age has only 1 L. To lose 200 or 300 mL of blood, “that’s 20% or 30% of their blood volume,” Dr. Waner said. So the removal of such a mass, which requires a meticulous dissection around many blood vessels, carries a high risk of the child bleeding out.
There were some logistical hurdles, but the patient arrived in Manhattan in mid-June, at no cost to her family. The medical visa was organized by a volunteer who also work for USAID. Healing the Children Northeast paid for her travel and the Waner Kids Foundation paid for her hotel stay. Lenox Hill Hospital and Northwell Health covered all hospital costs and postsurgery care. And Dr. O and Dr. Waner did the planning, consult visits, and procedure pro bono.
The surgery was possible because of the generosity of several organizations, but the two surgeons still had a limited time to remove the mass. Under different circumstances, and with the luxury of more time, the patient would have undergone several rounds of sclerotherapy. This procedure, done by interventional radiologists, involves injecting a toxin into the blood vessels, which causes them to clot. Done prior to surgery it can help limit bleeding risk.
On June 23, the morning of the surgery, the patient underwent one round of sclerotherapy. However, it didn’t have the intended effect, Dr. Waner said, “because the lesion was just so massive.”
The team had planned several of their moves ahead of time. But this isn’t the sort of surgery you’d find in a textbook. Because it’s such a unique field, Dr. Waner and Dr. O have developed many of their own techniques along the way. This patient was much like the cases they treat every day, only “several orders of magnitudes greater,” Dr. Waner said. “On a scale of 1 to 10 she was a 12.”
The morning of the surgery, “I was very apprehensive,” Dr. Waner recalled. He vividly remembers the girl’s father repeatedly kissing her to say goodbye as she lay on the operating table, fully aware that this procedure was a life-threatening one. And from the beginning there were challenges, like getting her under anesthesia when the anatomy of her mouth, deformed by the tumor, didn’t allow the anesthesiologists to use their typical tubing. Then, once the skin was removed, it became clear how dilated and tangled the involved blood vessels were. There were many vital structures tangled in the anomaly. “The jugular vein was right there. The carotid artery was right there,” Dr. Waner said. It was extremely difficult to delineate and preserve them, he said.

“That’s why we really took our time. We just went very slowly and deliberately,” Dr. O said. The blood vessels were so dilated that their only option was to move painstakingly slow – otherwise a small nick could be devastating.
But even with the slow pace the surgery was “excruciatingly difficult,” Dr. Waner said. And early on in the dissection he wasn’t quite sure they’d make it out. The sclerotherapy hadn’t done much to prevent bleeding. “At one point every millimeter or 2 that we advanced we got into some bleeding,” Dr. Waner said. “Brisk bleeding.”
Once they got into the surgery they also realized that the growth had adhered to the jaw bone. “There were vessels traversing into the bone, which were hard to control,” Dr. O said.
But finally, both doctors realized they’d be able to remove it. With the lesion removed they began the work of reconstruction and reanimation.
The child’s jaw and cheek bone had grown beyond their normal size to support the growth. They had to shave them down to achieve facial symmetry. The tumor had also inhibited much of the child’s facial nerve control. With it gone, Dr. O began the work of finding all the facial nerve branches and assembling them to reanimate the child’s face.
Before medicine, Dr. O trained as an architect, which, according to Dr. Waner, has equipped her with very good spatial awareness – a valuable skill in the surgical reconstruction phase. After seeing a lecture by Dr. Waner, she immediately saw a fit for her unique interest and skill set. She did fellowship training with Dr. Waner in vascular anomalies, and then went on to specialize in facial nerve reanimation. The proof of Dr. O’s expertise is Negalem’s new, beautiful smile, Dr. Waner said.
The surgery drew out over 8 hours, as long as a day of surgeries for the two doctors. When Dr. O finally walked into the waiting room to inform the family of the success, the first words out of the father’s mouth were: “Is my daughter alive?”
A growth like Negalem had is not compatible with a normal life. Dr. Waner’s mantra is that every child has the right to look normal. But this case went beyond aesthetics. If the growth hadn’t been removed, the child was expected to live only 4-6 more years, Dr. Waner said. Without the surgery, she could have suffocated, starved without the ability to swallow, or suffered a fatal bleed.

Dr. O and Dr. Waner are uniquely equipped to do this kind of work, but both are adamant that treating vascular anomalies is a multidisciplinary, multimodal approach. Specialties in anesthesiology, radiology, lasers, facial nerves – they are all critical to these procedures. And often patients with these kinds of lesions require medical and radiologic interventions in addition to surgery. In this particular case, from logistics to post op, “it was a lot of teamwork,” Dr. O said, “a lot of international teams coming together.”
Though extremely difficult, “in the end the result was exactly what we wanted,” Dr. Waner said. Negalem can live a normal life. And as for the surgical duo, both feel very fortunate to do this work. Dr. O said, “I’m honored to have found this specialty and to be able to train with and work with Milton. I’m so happy to do what I do every day.”
A version of this article first appeared on Medscape.com.
In 2019, doctors in London saw a 5-year old girl from rural Ethiopia with an enormous tumor extending from her cheek to her lower jaw. Her name was Negalem and the tumor was a vascular malformation, a life-threatening web of tangled blood vessels.
Surgery to remove it was impossible, the doctors told the foundation advocating for the girl. The child would never make it off the operating table. After a closer examination, the London group still declined to do the procedure, but told the child’s parents and advocates that if anyone was going to attempt this, they’d need to get the little girl to New York.
In New York City, on 64th St. in Manhattan, is the Vascular Birthmark Institute, founded by Milton Waner, MD, who has exclusively treated hemangiomas and vascular malformations for the last 30 years. “I’m the only person in the [United] States whose practice is exclusively [treating] vascular anomalies,” Dr. Waner said in an interview.
Dr. Waner has assembled a multidisciplinary team of experts at the institute’s offices in Lenox Hill – including his wife Teresa O, MD, a facial plastic and reconstructive surgeon and neurospecialist. “People often ask how the hell do you spend so much time with your spouse?” Dr. Waner says. “We work extremely well together. We complement each other.”
Dr. O and Dr. Waner each manage half of the cases at VBI. And in January they received an email about Negalem. After corresponding with the child’s advocate and reviewing images,

The challenge with vascular malformations in children, Dr. Waner said, is that they have a fraction of the blood an adult has. Where adults have an average of 5 L of blood, a child this age has only 1 L. To lose 200 or 300 mL of blood, “that’s 20% or 30% of their blood volume,” Dr. Waner said. So the removal of such a mass, which requires a meticulous dissection around many blood vessels, carries a high risk of the child bleeding out.
There were some logistical hurdles, but the patient arrived in Manhattan in mid-June, at no cost to her family. The medical visa was organized by a volunteer who also work for USAID. Healing the Children Northeast paid for her travel and the Waner Kids Foundation paid for her hotel stay. Lenox Hill Hospital and Northwell Health covered all hospital costs and postsurgery care. And Dr. O and Dr. Waner did the planning, consult visits, and procedure pro bono.
The surgery was possible because of the generosity of several organizations, but the two surgeons still had a limited time to remove the mass. Under different circumstances, and with the luxury of more time, the patient would have undergone several rounds of sclerotherapy. This procedure, done by interventional radiologists, involves injecting a toxin into the blood vessels, which causes them to clot. Done prior to surgery it can help limit bleeding risk.
On June 23, the morning of the surgery, the patient underwent one round of sclerotherapy. However, it didn’t have the intended effect, Dr. Waner said, “because the lesion was just so massive.”
The team had planned several of their moves ahead of time. But this isn’t the sort of surgery you’d find in a textbook. Because it’s such a unique field, Dr. Waner and Dr. O have developed many of their own techniques along the way. This patient was much like the cases they treat every day, only “several orders of magnitudes greater,” Dr. Waner said. “On a scale of 1 to 10 she was a 12.”
The morning of the surgery, “I was very apprehensive,” Dr. Waner recalled. He vividly remembers the girl’s father repeatedly kissing her to say goodbye as she lay on the operating table, fully aware that this procedure was a life-threatening one. And from the beginning there were challenges, like getting her under anesthesia when the anatomy of her mouth, deformed by the tumor, didn’t allow the anesthesiologists to use their typical tubing. Then, once the skin was removed, it became clear how dilated and tangled the involved blood vessels were. There were many vital structures tangled in the anomaly. “The jugular vein was right there. The carotid artery was right there,” Dr. Waner said. It was extremely difficult to delineate and preserve them, he said.

“That’s why we really took our time. We just went very slowly and deliberately,” Dr. O said. The blood vessels were so dilated that their only option was to move painstakingly slow – otherwise a small nick could be devastating.
But even with the slow pace the surgery was “excruciatingly difficult,” Dr. Waner said. And early on in the dissection he wasn’t quite sure they’d make it out. The sclerotherapy hadn’t done much to prevent bleeding. “At one point every millimeter or 2 that we advanced we got into some bleeding,” Dr. Waner said. “Brisk bleeding.”
Once they got into the surgery they also realized that the growth had adhered to the jaw bone. “There were vessels traversing into the bone, which were hard to control,” Dr. O said.
But finally, both doctors realized they’d be able to remove it. With the lesion removed they began the work of reconstruction and reanimation.
The child’s jaw and cheek bone had grown beyond their normal size to support the growth. They had to shave them down to achieve facial symmetry. The tumor had also inhibited much of the child’s facial nerve control. With it gone, Dr. O began the work of finding all the facial nerve branches and assembling them to reanimate the child’s face.
Before medicine, Dr. O trained as an architect, which, according to Dr. Waner, has equipped her with very good spatial awareness – a valuable skill in the surgical reconstruction phase. After seeing a lecture by Dr. Waner, she immediately saw a fit for her unique interest and skill set. She did fellowship training with Dr. Waner in vascular anomalies, and then went on to specialize in facial nerve reanimation. The proof of Dr. O’s expertise is Negalem’s new, beautiful smile, Dr. Waner said.
The surgery drew out over 8 hours, as long as a day of surgeries for the two doctors. When Dr. O finally walked into the waiting room to inform the family of the success, the first words out of the father’s mouth were: “Is my daughter alive?”
A growth like Negalem had is not compatible with a normal life. Dr. Waner’s mantra is that every child has the right to look normal. But this case went beyond aesthetics. If the growth hadn’t been removed, the child was expected to live only 4-6 more years, Dr. Waner said. Without the surgery, she could have suffocated, starved without the ability to swallow, or suffered a fatal bleed.

Dr. O and Dr. Waner are uniquely equipped to do this kind of work, but both are adamant that treating vascular anomalies is a multidisciplinary, multimodal approach. Specialties in anesthesiology, radiology, lasers, facial nerves – they are all critical to these procedures. And often patients with these kinds of lesions require medical and radiologic interventions in addition to surgery. In this particular case, from logistics to post op, “it was a lot of teamwork,” Dr. O said, “a lot of international teams coming together.”
Though extremely difficult, “in the end the result was exactly what we wanted,” Dr. Waner said. Negalem can live a normal life. And as for the surgical duo, both feel very fortunate to do this work. Dr. O said, “I’m honored to have found this specialty and to be able to train with and work with Milton. I’m so happy to do what I do every day.”
A version of this article first appeared on Medscape.com.
Study eyes impact of isotretinoin on triglycerides, other lab measures
.
“Isotretinoin is a very effective treatment for severe acne,” Varsha Parthasarathy said at the annual meeting of the Society for Pediatric Dermatology. “However, initiating this medication requires a complex process of laboratory testing,” which includes human chorionic gonadotropin pregnancy testing, because isotretinoin is a teratogen, as well as lipid labs and liver function tests, she noted. “Importantly, triglycerides are measured due to an association in adults between isotretinoin and hypertriglyceridemia-associated pancreatitis. However, these findings in children are limited to case reports, as are findings of retinoid-induced hepatotoxicity.”
To identify the role of isotretinoin on changes in lipids, aspartate aminotransferase (AST), and alanine aminotransferase (ALT), and to determine the impact on treatment course, Ms. Parthasarathy, a 4-year medical student at George Washington University, Washington, and colleagues retrospectively reviewed the charts of 130 patients aged 12-21 years who were cared for at Children’s National Hospital between January 2012 and October 2020. Nearly two-thirds (65%) were male, their average age was 16 years, and the mean time to obtain follow-up labs after starting isotretinoin was 3.25 months.
Between baseline and follow-up, the researchers observed increases in total cholesterol, triglycerides, and LDL (P less than .001 for all associations) and a decrease in HDL (P = .001), but there were no significant changes in AST or ALT levels. These findings were consistent with prior studies in adults examining the utility of these laboratory tests, most notably a 2016 study by Timothy J. Hansen, MD, and colleagues.
Among the 13 patients with elevated triglycerides at baseline, 9 (69%) were overweight or obese. Of the 20 patients with elevated triglycerides at follow-up, 11 patients (55%) were obese. At follow-up, 11 patients had levels of 200-500 mg/dL (grade I elevation), and 2 patients had levels of 501-1,000 mg/dL (grade II elevation). Isotretinoin was stopped in the latter two patients, who also had obesity as a risk factor for their hypertriglyceridemia.
“None of these patients had clinical sequelae from their hypertriglyceridemia, such as pancreatitis at baseline or follow-up,” Ms. Parthasarathy said. “However, since pancreatitis would be expected to be exceedingly rare, the sample size may be limited in identifying this adverse effect.”
She noted that while isotretinoin might cause a significant increase in lipid levels, the mean levels remained within normal limits at both baseline and follow-up. “Of the patients with elevated triglycerides at baseline and follow-up, obesity may have been a potential risk factor,” she said. “This could suggest a possible strategy for reduced testing in nonobese isotretinoin patients, which can be further explored in larger study populations.”
In addition, “there was a lack of significant change in AST and ALT in this study and adult studies, as well as minimal evidence for pediatric retinoid-induced hepatotoxicity, which raises the question of the necessity of baseline and follow-up comprehensive metabolic panel testing,” Ms. Parthasarathy added. “Clinicians must weigh the laboratory values with the costs of laboratory testing, including opportunity costs such as time, monetary costs, and the discomfort of testing for pediatric patients.”
The study’s senior author was A. Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital, Washington. The researchers reported having no relevant financial disclosures.
.
“Isotretinoin is a very effective treatment for severe acne,” Varsha Parthasarathy said at the annual meeting of the Society for Pediatric Dermatology. “However, initiating this medication requires a complex process of laboratory testing,” which includes human chorionic gonadotropin pregnancy testing, because isotretinoin is a teratogen, as well as lipid labs and liver function tests, she noted. “Importantly, triglycerides are measured due to an association in adults between isotretinoin and hypertriglyceridemia-associated pancreatitis. However, these findings in children are limited to case reports, as are findings of retinoid-induced hepatotoxicity.”
To identify the role of isotretinoin on changes in lipids, aspartate aminotransferase (AST), and alanine aminotransferase (ALT), and to determine the impact on treatment course, Ms. Parthasarathy, a 4-year medical student at George Washington University, Washington, and colleagues retrospectively reviewed the charts of 130 patients aged 12-21 years who were cared for at Children’s National Hospital between January 2012 and October 2020. Nearly two-thirds (65%) were male, their average age was 16 years, and the mean time to obtain follow-up labs after starting isotretinoin was 3.25 months.
Between baseline and follow-up, the researchers observed increases in total cholesterol, triglycerides, and LDL (P less than .001 for all associations) and a decrease in HDL (P = .001), but there were no significant changes in AST or ALT levels. These findings were consistent with prior studies in adults examining the utility of these laboratory tests, most notably a 2016 study by Timothy J. Hansen, MD, and colleagues.
Among the 13 patients with elevated triglycerides at baseline, 9 (69%) were overweight or obese. Of the 20 patients with elevated triglycerides at follow-up, 11 patients (55%) were obese. At follow-up, 11 patients had levels of 200-500 mg/dL (grade I elevation), and 2 patients had levels of 501-1,000 mg/dL (grade II elevation). Isotretinoin was stopped in the latter two patients, who also had obesity as a risk factor for their hypertriglyceridemia.
“None of these patients had clinical sequelae from their hypertriglyceridemia, such as pancreatitis at baseline or follow-up,” Ms. Parthasarathy said. “However, since pancreatitis would be expected to be exceedingly rare, the sample size may be limited in identifying this adverse effect.”
She noted that while isotretinoin might cause a significant increase in lipid levels, the mean levels remained within normal limits at both baseline and follow-up. “Of the patients with elevated triglycerides at baseline and follow-up, obesity may have been a potential risk factor,” she said. “This could suggest a possible strategy for reduced testing in nonobese isotretinoin patients, which can be further explored in larger study populations.”
In addition, “there was a lack of significant change in AST and ALT in this study and adult studies, as well as minimal evidence for pediatric retinoid-induced hepatotoxicity, which raises the question of the necessity of baseline and follow-up comprehensive metabolic panel testing,” Ms. Parthasarathy added. “Clinicians must weigh the laboratory values with the costs of laboratory testing, including opportunity costs such as time, monetary costs, and the discomfort of testing for pediatric patients.”
The study’s senior author was A. Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital, Washington. The researchers reported having no relevant financial disclosures.
.
“Isotretinoin is a very effective treatment for severe acne,” Varsha Parthasarathy said at the annual meeting of the Society for Pediatric Dermatology. “However, initiating this medication requires a complex process of laboratory testing,” which includes human chorionic gonadotropin pregnancy testing, because isotretinoin is a teratogen, as well as lipid labs and liver function tests, she noted. “Importantly, triglycerides are measured due to an association in adults between isotretinoin and hypertriglyceridemia-associated pancreatitis. However, these findings in children are limited to case reports, as are findings of retinoid-induced hepatotoxicity.”
To identify the role of isotretinoin on changes in lipids, aspartate aminotransferase (AST), and alanine aminotransferase (ALT), and to determine the impact on treatment course, Ms. Parthasarathy, a 4-year medical student at George Washington University, Washington, and colleagues retrospectively reviewed the charts of 130 patients aged 12-21 years who were cared for at Children’s National Hospital between January 2012 and October 2020. Nearly two-thirds (65%) were male, their average age was 16 years, and the mean time to obtain follow-up labs after starting isotretinoin was 3.25 months.
Between baseline and follow-up, the researchers observed increases in total cholesterol, triglycerides, and LDL (P less than .001 for all associations) and a decrease in HDL (P = .001), but there were no significant changes in AST or ALT levels. These findings were consistent with prior studies in adults examining the utility of these laboratory tests, most notably a 2016 study by Timothy J. Hansen, MD, and colleagues.
Among the 13 patients with elevated triglycerides at baseline, 9 (69%) were overweight or obese. Of the 20 patients with elevated triglycerides at follow-up, 11 patients (55%) were obese. At follow-up, 11 patients had levels of 200-500 mg/dL (grade I elevation), and 2 patients had levels of 501-1,000 mg/dL (grade II elevation). Isotretinoin was stopped in the latter two patients, who also had obesity as a risk factor for their hypertriglyceridemia.
“None of these patients had clinical sequelae from their hypertriglyceridemia, such as pancreatitis at baseline or follow-up,” Ms. Parthasarathy said. “However, since pancreatitis would be expected to be exceedingly rare, the sample size may be limited in identifying this adverse effect.”
She noted that while isotretinoin might cause a significant increase in lipid levels, the mean levels remained within normal limits at both baseline and follow-up. “Of the patients with elevated triglycerides at baseline and follow-up, obesity may have been a potential risk factor,” she said. “This could suggest a possible strategy for reduced testing in nonobese isotretinoin patients, which can be further explored in larger study populations.”
In addition, “there was a lack of significant change in AST and ALT in this study and adult studies, as well as minimal evidence for pediatric retinoid-induced hepatotoxicity, which raises the question of the necessity of baseline and follow-up comprehensive metabolic panel testing,” Ms. Parthasarathy added. “Clinicians must weigh the laboratory values with the costs of laboratory testing, including opportunity costs such as time, monetary costs, and the discomfort of testing for pediatric patients.”
The study’s senior author was A. Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital, Washington. The researchers reported having no relevant financial disclosures.
FROM SPD 2021
Isotretinoin benefits similar in overweight, obese adolescents, and those in normal weight range
a retrospective cohort study found.
“Oral isotretinoin is among the most effective treatments for acne and is indicated for the treatment of severe acne or when first-line regimens have failed,” Maggie Tallmadge said at the annual meeting of the Society for Pediatric Dermatology. In adolescents with acne, isotretinoin is prescribed at a dose of 0.5-1 mg/kg per day “with the goal of reaching a cumulative dose of 120-150 mg/kg and clinical clearance with durable remission,” she said. “Most providers do not prescribe a daily dose over 80 mg due to perceived increased risk of side effects, including xerosis, cheilitis, liver dysfunction, and acne flare. However, many adolescents weigh over 80 kg and are therefore effectively underdosed, prolonging treatment time and possibly increasing the risk of side effects due to prolonged therapy.”
To evaluate differences in treatment courses among normal-weight, overweight, and obese adolescents, and the efficacy and safety of treatment, Ms. Tallmadge, a third-year medical student at the Medical College of Wisconsin, Milwaukee, and colleagues completed a retrospective chart review of 550 dermatology patients at Children’s Wisconsin, also in Milwaukee, who completed at least 2 months of isotretinoin treatment for acne when they were between the ages of 10 and 24, from November 2012 to January 2020. They collected data on age, weight, height, daily dose, cumulative dose, time to acne clearance, side effects, and acne recurrence after treatment, and classified patients as normal weight, overweight, or obese based on their body mass index for age percentile.
Of the 550 patients, 367 (67%) were normal weight, 101 (18%) were overweight, and 82 (15%) were obese. The median age of those in the normal-weight and overweight groups was 16, and was 15 in the obese group.
There was were significant differences in the median cumulative dose in each weight group: 143.7 mg/kg for normal-weight patients, 138.2 mg/kg for overweight patients, and 140.6 mg/kg for obese patients (P < .001).
“Despite achieving different cumulative doses, there was no difference in acne clearance, relapse, and most side effects among the three [body mass index] cohorts,” Ms. Tallmadge said. “Thus, it appears that current treatment strategies may be appropriate for overweight and obese adolescents.”
The proportion of patients with acne clearance did not differ significantly among the three groups of patients: 62% who were in the normal weight range, 60% who were overweight, and 59% who were obese had clearance of facial acne with treatment (P = .84).
Of patients whose treatment course was completed by the time of data collection, the proportion with acne recurrences was similar between the three groups: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients (P > .05). Of patients whose treatment course was completed by the time of data collection, there was no significant differences in acne recurrence: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients.
However, the proportion of patients reporting headaches differed significantly between the groups: 29% of normal-weight patients, compared with 40% of both overweight and obese patients (P = .035). The researchers also observed a significant positive correlation between increased BMI and increased triglyceride and ALT levels during treatment (P < .001 for both associations), yet no elevations required clinical action.
Funding for the study was provided by the MCW Medical Student Summer Research Program and the American Acne & Rosacea Society.
a retrospective cohort study found.
“Oral isotretinoin is among the most effective treatments for acne and is indicated for the treatment of severe acne or when first-line regimens have failed,” Maggie Tallmadge said at the annual meeting of the Society for Pediatric Dermatology. In adolescents with acne, isotretinoin is prescribed at a dose of 0.5-1 mg/kg per day “with the goal of reaching a cumulative dose of 120-150 mg/kg and clinical clearance with durable remission,” she said. “Most providers do not prescribe a daily dose over 80 mg due to perceived increased risk of side effects, including xerosis, cheilitis, liver dysfunction, and acne flare. However, many adolescents weigh over 80 kg and are therefore effectively underdosed, prolonging treatment time and possibly increasing the risk of side effects due to prolonged therapy.”
To evaluate differences in treatment courses among normal-weight, overweight, and obese adolescents, and the efficacy and safety of treatment, Ms. Tallmadge, a third-year medical student at the Medical College of Wisconsin, Milwaukee, and colleagues completed a retrospective chart review of 550 dermatology patients at Children’s Wisconsin, also in Milwaukee, who completed at least 2 months of isotretinoin treatment for acne when they were between the ages of 10 and 24, from November 2012 to January 2020. They collected data on age, weight, height, daily dose, cumulative dose, time to acne clearance, side effects, and acne recurrence after treatment, and classified patients as normal weight, overweight, or obese based on their body mass index for age percentile.
Of the 550 patients, 367 (67%) were normal weight, 101 (18%) were overweight, and 82 (15%) were obese. The median age of those in the normal-weight and overweight groups was 16, and was 15 in the obese group.
There was were significant differences in the median cumulative dose in each weight group: 143.7 mg/kg for normal-weight patients, 138.2 mg/kg for overweight patients, and 140.6 mg/kg for obese patients (P < .001).
“Despite achieving different cumulative doses, there was no difference in acne clearance, relapse, and most side effects among the three [body mass index] cohorts,” Ms. Tallmadge said. “Thus, it appears that current treatment strategies may be appropriate for overweight and obese adolescents.”
The proportion of patients with acne clearance did not differ significantly among the three groups of patients: 62% who were in the normal weight range, 60% who were overweight, and 59% who were obese had clearance of facial acne with treatment (P = .84).
Of patients whose treatment course was completed by the time of data collection, the proportion with acne recurrences was similar between the three groups: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients (P > .05). Of patients whose treatment course was completed by the time of data collection, there was no significant differences in acne recurrence: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients.
However, the proportion of patients reporting headaches differed significantly between the groups: 29% of normal-weight patients, compared with 40% of both overweight and obese patients (P = .035). The researchers also observed a significant positive correlation between increased BMI and increased triglyceride and ALT levels during treatment (P < .001 for both associations), yet no elevations required clinical action.
Funding for the study was provided by the MCW Medical Student Summer Research Program and the American Acne & Rosacea Society.
a retrospective cohort study found.
“Oral isotretinoin is among the most effective treatments for acne and is indicated for the treatment of severe acne or when first-line regimens have failed,” Maggie Tallmadge said at the annual meeting of the Society for Pediatric Dermatology. In adolescents with acne, isotretinoin is prescribed at a dose of 0.5-1 mg/kg per day “with the goal of reaching a cumulative dose of 120-150 mg/kg and clinical clearance with durable remission,” she said. “Most providers do not prescribe a daily dose over 80 mg due to perceived increased risk of side effects, including xerosis, cheilitis, liver dysfunction, and acne flare. However, many adolescents weigh over 80 kg and are therefore effectively underdosed, prolonging treatment time and possibly increasing the risk of side effects due to prolonged therapy.”
To evaluate differences in treatment courses among normal-weight, overweight, and obese adolescents, and the efficacy and safety of treatment, Ms. Tallmadge, a third-year medical student at the Medical College of Wisconsin, Milwaukee, and colleagues completed a retrospective chart review of 550 dermatology patients at Children’s Wisconsin, also in Milwaukee, who completed at least 2 months of isotretinoin treatment for acne when they were between the ages of 10 and 24, from November 2012 to January 2020. They collected data on age, weight, height, daily dose, cumulative dose, time to acne clearance, side effects, and acne recurrence after treatment, and classified patients as normal weight, overweight, or obese based on their body mass index for age percentile.
Of the 550 patients, 367 (67%) were normal weight, 101 (18%) were overweight, and 82 (15%) were obese. The median age of those in the normal-weight and overweight groups was 16, and was 15 in the obese group.
There was were significant differences in the median cumulative dose in each weight group: 143.7 mg/kg for normal-weight patients, 138.2 mg/kg for overweight patients, and 140.6 mg/kg for obese patients (P < .001).
“Despite achieving different cumulative doses, there was no difference in acne clearance, relapse, and most side effects among the three [body mass index] cohorts,” Ms. Tallmadge said. “Thus, it appears that current treatment strategies may be appropriate for overweight and obese adolescents.”
The proportion of patients with acne clearance did not differ significantly among the three groups of patients: 62% who were in the normal weight range, 60% who were overweight, and 59% who were obese had clearance of facial acne with treatment (P = .84).
Of patients whose treatment course was completed by the time of data collection, the proportion with acne recurrences was similar between the three groups: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients (P > .05). Of patients whose treatment course was completed by the time of data collection, there was no significant differences in acne recurrence: 25% of normal-weight patients, 27% of overweight patients, and 35% of obese patients.
However, the proportion of patients reporting headaches differed significantly between the groups: 29% of normal-weight patients, compared with 40% of both overweight and obese patients (P = .035). The researchers also observed a significant positive correlation between increased BMI and increased triglyceride and ALT levels during treatment (P < .001 for both associations), yet no elevations required clinical action.
Funding for the study was provided by the MCW Medical Student Summer Research Program and the American Acne & Rosacea Society.
FROM SPD 2021
Sublingual immunotherapy: Where does it stand?
Sublingual immunotherapy (SLIT) emerged over a century ago as a gentler alternative to allergy shots. It uses the same antigens found in allergy shots, delivering them through tablets or drops under the tongue rather than by injecting them into the skin.
Yet injection immunotherapy has been the mainstay of allergy treatment in the United States. Allergy shots are “the bread and butter, keeping the lights on at allergy practices,” said allergist Sakina Bajowala, MD, of Kaneland Allergy and Asthma Center, in the Chicago area. So even “when environmental SLIT showed quite clearly that it had efficacy, people were so slow to adapt.”
SLIT – a daily treatment that builds protection from allergens gradually over years with few side effects – is popular around the globe, particularly for environmental allergies. But only a handful of clinics offer food SLIT. Even though recent trials in peanut-allergic children show that SLIT is far safer than oral immunotherapy and about as effective as the Food and Drug Administration–approved peanut-allergy product and has lasting benefits for toddlers, many allergists lack experience with customized immunotherapies and hesitate to offer an unregulated treatment for which the evidence base is still emerging.
Why hasn’t food allergy SLIT caught on?
One issue is that there is scant evidence from randomized, controlled trials. The treatments that clinics offer often hinge on insurance coverage, and increasingly, insurers only cover FDA-approved products. FDA approval requires thousands of patients being enrolled in long, expensive studies to prove the treatment’s merit. In a similar vein, doctors are trained to question methods that lack a strong publication base, for good reason.
Yet SLIT caught the attention of pioneering physicians who were intrigued by this “low-and-slow” immune-modifying approach, despite limited published evidence, and they sought real-world experience.
The late physician David Morris, MD, came across SLIT in the 1960s while searching for alternative ways to help mold-allergic farmers who were suffering terrible side effects from allergy shots. Dr. Morris attended conferences, learned more about sublingual techniques, got board certified in allergy, and opened Allergy Associates of La Crosse (Wis.), in 1970 to offer SLIT as a treatment for food and environmental allergies.
Dr. Morris and colleagues developed a protocol to create custom SLIT drops tailored to individual patients’ clinical histories and allergy test results. The method has been used to treat more than 200,000 patients. It has been used by allergist Nikhila Schroeder, MD, MEng, who learned SLIT methods while treating nearly 1,000 patients at Allergy Associates. In 2018, she opened her own direct-care SLIT practice, Allergenuity Health, in the Charlotte metropolitan area of North Carolina (see part 2 of this series).
Dr. Bajowala’s clinic offers SLIT in addition to oral immunotherapy (OIT). She was encouraged by the recent toddler SLIT data but wondered whether it would translate to a real-world setting. According to her calculations, the published protocol – according to which participants receive up to 4 mg/d over 6 months and continue receiving a daily maintenance dose of 4 mg for 3 years – would cost $10,000 per patient.
With this dosing regimen, the intervention is unaffordable, Dr. Bajowala said. And “there’s no way to make it cheaper because that’s the raw materials cost. It does not include labor or bottles or profit at all. That’s just $10,000 in peanut extract.”
Owing to cost, Dr. Bajowala’s clinic generally uses SLIT as a bridge to OIT. Her food allergy patients receive up to 1 mg/d and remain at that dose for a month or so before transitioning to OIT, “for which the supplies are orders of magnitude cheaper,” she said.
Dr. Schroeder said there is evidence for efficacy at microgram and even nanogram dosing – much lower than used in the recent food SLIT trials. Maintenance doses range from 50 ng/d to 25 mcg/d for environmental SLIT and 4-37 mcg/d for food SLIT, she said. The La Crosse method uses even lower dose ranges.
However, dosing information is not readily available, Dr. Schroeder noted. She has spent years scrutinizing articles and compiling information from allergen extract suppliers – all the while treating hundreds of SLIT patients. “I have had to expend a lot of time and effort,” said Dr. Schroeder. “It’s really hard to explain quickly.”
In the published literature, SLIT dosing recommendations vary widely. According to a 2007 analysis, environmental allergy symptoms improved with doses over a 1,000-fold range. What’s more, success did not scale with increased dosing and seemed to depend more on frequency and duration of treatment.
There are fewer studies regarding food SLIT. The most promising data come from recent trials of peanut-allergic children led by Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill. Still, “I am nervous to tell people to go do this based on 150 kids at one site,” Dr. Kim said. “We need to have a gigantic study across multiple sites that actually confirms what we have found in our single center.”
Because there are few published trials of food SLIT, confusion about which doses are optimal, how early to start, and how long the benefits last will be a barrier for many clinicians, said Douglas Mack, MD, FRCPC, assistant clinical professor in pediatrics at McMaster University, Hamilton, Ont.
Much could be learned from Allergy Associates of La Crosse, Allergenuity Health, and other clinics with SLIT experience involving thousands of patients. But that real-world data are messy and difficult to publish. Plus, it is hard for private allergists to find time to review charts, analyze data, and draft papers alongside seeing patients and running a clinic – especially without students and interns, who typically assist with academic research, Dr. Schroeder said.
Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern University, Chicago, and colleagues worked with a La Crosse team 6 or 7 years ago to try to analyze and publish SLIT outcomes for 121 peanut-allergic children who were treated for food and environmental allergies at the Wisconsin clinic. The researchers had hoped to publish an article describing caregiver-reported and clinical outcomes.
Among 73 caregivers who responded to a survey, more than half reported improved eczema, asthma, and environmental allergy symptoms, and virtually all families said SLIT calmed anxieties and minimized fear of allergic reactions. However, the clinical outcomes – skinprick test results, immune changes, and oral food challenges – were not as robust. And the data were incomplete. Some patients had traveled to La Crosse for SLIT drops but underwent skin and blood testing with their local allergist. Compiling records is “so much harder when you’re not doing a prospective clinical trial,” Dr. Gupta said.
The caregiver-reported outcomes were presented as a poster at the 2015 annual meeting of the American College of Allergy, Asthma, and Immunology and the 2016 annual meeting of the Pediatric Academic Society, said Jeff Kessler, MBA, FACHE, who is practice executive at La Crosse. However, with only self-reported data and no convincing lab metrics, the findings were never submitted for publication.
Others are eager to see clearer proof that SLIT works at doses lower than those published in the most recent trials. “If we can get efficacy with lower doses, that means we can increase accessibility, because we can lower the cost,” Dr. Bajowala said.
Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins University, Baltimore, has a pending grant proposal for a multifood trial of SLIT. “It’s a big missing piece,” he said.
Dr. Mack said that in Canada there was “almost an instant change in group think” when the Canadian Society of Allergy and Clinical Immunology published guidelines in support of OIT. With the new guidelines, “people are less concerned about liability. Once they start getting into OIT, I think you’re going to see SLIT coming right along for the ride.”
The shift will be slower in the United States, which has 20 times as many practicing allergists as Canada. Nevertheless, “I totally think SLIT has a place at the table,” Dr. Mack said. “I hope we start to see more high-quality data and people start to use it and experiment with it a bit and see how it works.”
A version of this article first appeared on Medscape.com. This is part three of a three-part series. Part one is here. Part two is here.
Sublingual immunotherapy (SLIT) emerged over a century ago as a gentler alternative to allergy shots. It uses the same antigens found in allergy shots, delivering them through tablets or drops under the tongue rather than by injecting them into the skin.
Yet injection immunotherapy has been the mainstay of allergy treatment in the United States. Allergy shots are “the bread and butter, keeping the lights on at allergy practices,” said allergist Sakina Bajowala, MD, of Kaneland Allergy and Asthma Center, in the Chicago area. So even “when environmental SLIT showed quite clearly that it had efficacy, people were so slow to adapt.”
SLIT – a daily treatment that builds protection from allergens gradually over years with few side effects – is popular around the globe, particularly for environmental allergies. But only a handful of clinics offer food SLIT. Even though recent trials in peanut-allergic children show that SLIT is far safer than oral immunotherapy and about as effective as the Food and Drug Administration–approved peanut-allergy product and has lasting benefits for toddlers, many allergists lack experience with customized immunotherapies and hesitate to offer an unregulated treatment for which the evidence base is still emerging.
Why hasn’t food allergy SLIT caught on?
One issue is that there is scant evidence from randomized, controlled trials. The treatments that clinics offer often hinge on insurance coverage, and increasingly, insurers only cover FDA-approved products. FDA approval requires thousands of patients being enrolled in long, expensive studies to prove the treatment’s merit. In a similar vein, doctors are trained to question methods that lack a strong publication base, for good reason.
Yet SLIT caught the attention of pioneering physicians who were intrigued by this “low-and-slow” immune-modifying approach, despite limited published evidence, and they sought real-world experience.
The late physician David Morris, MD, came across SLIT in the 1960s while searching for alternative ways to help mold-allergic farmers who were suffering terrible side effects from allergy shots. Dr. Morris attended conferences, learned more about sublingual techniques, got board certified in allergy, and opened Allergy Associates of La Crosse (Wis.), in 1970 to offer SLIT as a treatment for food and environmental allergies.
Dr. Morris and colleagues developed a protocol to create custom SLIT drops tailored to individual patients’ clinical histories and allergy test results. The method has been used to treat more than 200,000 patients. It has been used by allergist Nikhila Schroeder, MD, MEng, who learned SLIT methods while treating nearly 1,000 patients at Allergy Associates. In 2018, she opened her own direct-care SLIT practice, Allergenuity Health, in the Charlotte metropolitan area of North Carolina (see part 2 of this series).
Dr. Bajowala’s clinic offers SLIT in addition to oral immunotherapy (OIT). She was encouraged by the recent toddler SLIT data but wondered whether it would translate to a real-world setting. According to her calculations, the published protocol – according to which participants receive up to 4 mg/d over 6 months and continue receiving a daily maintenance dose of 4 mg for 3 years – would cost $10,000 per patient.
With this dosing regimen, the intervention is unaffordable, Dr. Bajowala said. And “there’s no way to make it cheaper because that’s the raw materials cost. It does not include labor or bottles or profit at all. That’s just $10,000 in peanut extract.”
Owing to cost, Dr. Bajowala’s clinic generally uses SLIT as a bridge to OIT. Her food allergy patients receive up to 1 mg/d and remain at that dose for a month or so before transitioning to OIT, “for which the supplies are orders of magnitude cheaper,” she said.
Dr. Schroeder said there is evidence for efficacy at microgram and even nanogram dosing – much lower than used in the recent food SLIT trials. Maintenance doses range from 50 ng/d to 25 mcg/d for environmental SLIT and 4-37 mcg/d for food SLIT, she said. The La Crosse method uses even lower dose ranges.
However, dosing information is not readily available, Dr. Schroeder noted. She has spent years scrutinizing articles and compiling information from allergen extract suppliers – all the while treating hundreds of SLIT patients. “I have had to expend a lot of time and effort,” said Dr. Schroeder. “It’s really hard to explain quickly.”
In the published literature, SLIT dosing recommendations vary widely. According to a 2007 analysis, environmental allergy symptoms improved with doses over a 1,000-fold range. What’s more, success did not scale with increased dosing and seemed to depend more on frequency and duration of treatment.
There are fewer studies regarding food SLIT. The most promising data come from recent trials of peanut-allergic children led by Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill. Still, “I am nervous to tell people to go do this based on 150 kids at one site,” Dr. Kim said. “We need to have a gigantic study across multiple sites that actually confirms what we have found in our single center.”
Because there are few published trials of food SLIT, confusion about which doses are optimal, how early to start, and how long the benefits last will be a barrier for many clinicians, said Douglas Mack, MD, FRCPC, assistant clinical professor in pediatrics at McMaster University, Hamilton, Ont.
Much could be learned from Allergy Associates of La Crosse, Allergenuity Health, and other clinics with SLIT experience involving thousands of patients. But that real-world data are messy and difficult to publish. Plus, it is hard for private allergists to find time to review charts, analyze data, and draft papers alongside seeing patients and running a clinic – especially without students and interns, who typically assist with academic research, Dr. Schroeder said.
Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern University, Chicago, and colleagues worked with a La Crosse team 6 or 7 years ago to try to analyze and publish SLIT outcomes for 121 peanut-allergic children who were treated for food and environmental allergies at the Wisconsin clinic. The researchers had hoped to publish an article describing caregiver-reported and clinical outcomes.
Among 73 caregivers who responded to a survey, more than half reported improved eczema, asthma, and environmental allergy symptoms, and virtually all families said SLIT calmed anxieties and minimized fear of allergic reactions. However, the clinical outcomes – skinprick test results, immune changes, and oral food challenges – were not as robust. And the data were incomplete. Some patients had traveled to La Crosse for SLIT drops but underwent skin and blood testing with their local allergist. Compiling records is “so much harder when you’re not doing a prospective clinical trial,” Dr. Gupta said.
The caregiver-reported outcomes were presented as a poster at the 2015 annual meeting of the American College of Allergy, Asthma, and Immunology and the 2016 annual meeting of the Pediatric Academic Society, said Jeff Kessler, MBA, FACHE, who is practice executive at La Crosse. However, with only self-reported data and no convincing lab metrics, the findings were never submitted for publication.
Others are eager to see clearer proof that SLIT works at doses lower than those published in the most recent trials. “If we can get efficacy with lower doses, that means we can increase accessibility, because we can lower the cost,” Dr. Bajowala said.
Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins University, Baltimore, has a pending grant proposal for a multifood trial of SLIT. “It’s a big missing piece,” he said.
Dr. Mack said that in Canada there was “almost an instant change in group think” when the Canadian Society of Allergy and Clinical Immunology published guidelines in support of OIT. With the new guidelines, “people are less concerned about liability. Once they start getting into OIT, I think you’re going to see SLIT coming right along for the ride.”
The shift will be slower in the United States, which has 20 times as many practicing allergists as Canada. Nevertheless, “I totally think SLIT has a place at the table,” Dr. Mack said. “I hope we start to see more high-quality data and people start to use it and experiment with it a bit and see how it works.”
A version of this article first appeared on Medscape.com. This is part three of a three-part series. Part one is here. Part two is here.
Sublingual immunotherapy (SLIT) emerged over a century ago as a gentler alternative to allergy shots. It uses the same antigens found in allergy shots, delivering them through tablets or drops under the tongue rather than by injecting them into the skin.
Yet injection immunotherapy has been the mainstay of allergy treatment in the United States. Allergy shots are “the bread and butter, keeping the lights on at allergy practices,” said allergist Sakina Bajowala, MD, of Kaneland Allergy and Asthma Center, in the Chicago area. So even “when environmental SLIT showed quite clearly that it had efficacy, people were so slow to adapt.”
SLIT – a daily treatment that builds protection from allergens gradually over years with few side effects – is popular around the globe, particularly for environmental allergies. But only a handful of clinics offer food SLIT. Even though recent trials in peanut-allergic children show that SLIT is far safer than oral immunotherapy and about as effective as the Food and Drug Administration–approved peanut-allergy product and has lasting benefits for toddlers, many allergists lack experience with customized immunotherapies and hesitate to offer an unregulated treatment for which the evidence base is still emerging.
Why hasn’t food allergy SLIT caught on?
One issue is that there is scant evidence from randomized, controlled trials. The treatments that clinics offer often hinge on insurance coverage, and increasingly, insurers only cover FDA-approved products. FDA approval requires thousands of patients being enrolled in long, expensive studies to prove the treatment’s merit. In a similar vein, doctors are trained to question methods that lack a strong publication base, for good reason.
Yet SLIT caught the attention of pioneering physicians who were intrigued by this “low-and-slow” immune-modifying approach, despite limited published evidence, and they sought real-world experience.
The late physician David Morris, MD, came across SLIT in the 1960s while searching for alternative ways to help mold-allergic farmers who were suffering terrible side effects from allergy shots. Dr. Morris attended conferences, learned more about sublingual techniques, got board certified in allergy, and opened Allergy Associates of La Crosse (Wis.), in 1970 to offer SLIT as a treatment for food and environmental allergies.
Dr. Morris and colleagues developed a protocol to create custom SLIT drops tailored to individual patients’ clinical histories and allergy test results. The method has been used to treat more than 200,000 patients. It has been used by allergist Nikhila Schroeder, MD, MEng, who learned SLIT methods while treating nearly 1,000 patients at Allergy Associates. In 2018, she opened her own direct-care SLIT practice, Allergenuity Health, in the Charlotte metropolitan area of North Carolina (see part 2 of this series).
Dr. Bajowala’s clinic offers SLIT in addition to oral immunotherapy (OIT). She was encouraged by the recent toddler SLIT data but wondered whether it would translate to a real-world setting. According to her calculations, the published protocol – according to which participants receive up to 4 mg/d over 6 months and continue receiving a daily maintenance dose of 4 mg for 3 years – would cost $10,000 per patient.
With this dosing regimen, the intervention is unaffordable, Dr. Bajowala said. And “there’s no way to make it cheaper because that’s the raw materials cost. It does not include labor or bottles or profit at all. That’s just $10,000 in peanut extract.”
Owing to cost, Dr. Bajowala’s clinic generally uses SLIT as a bridge to OIT. Her food allergy patients receive up to 1 mg/d and remain at that dose for a month or so before transitioning to OIT, “for which the supplies are orders of magnitude cheaper,” she said.
Dr. Schroeder said there is evidence for efficacy at microgram and even nanogram dosing – much lower than used in the recent food SLIT trials. Maintenance doses range from 50 ng/d to 25 mcg/d for environmental SLIT and 4-37 mcg/d for food SLIT, she said. The La Crosse method uses even lower dose ranges.
However, dosing information is not readily available, Dr. Schroeder noted. She has spent years scrutinizing articles and compiling information from allergen extract suppliers – all the while treating hundreds of SLIT patients. “I have had to expend a lot of time and effort,” said Dr. Schroeder. “It’s really hard to explain quickly.”
In the published literature, SLIT dosing recommendations vary widely. According to a 2007 analysis, environmental allergy symptoms improved with doses over a 1,000-fold range. What’s more, success did not scale with increased dosing and seemed to depend more on frequency and duration of treatment.
There are fewer studies regarding food SLIT. The most promising data come from recent trials of peanut-allergic children led by Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill. Still, “I am nervous to tell people to go do this based on 150 kids at one site,” Dr. Kim said. “We need to have a gigantic study across multiple sites that actually confirms what we have found in our single center.”
Because there are few published trials of food SLIT, confusion about which doses are optimal, how early to start, and how long the benefits last will be a barrier for many clinicians, said Douglas Mack, MD, FRCPC, assistant clinical professor in pediatrics at McMaster University, Hamilton, Ont.
Much could be learned from Allergy Associates of La Crosse, Allergenuity Health, and other clinics with SLIT experience involving thousands of patients. But that real-world data are messy and difficult to publish. Plus, it is hard for private allergists to find time to review charts, analyze data, and draft papers alongside seeing patients and running a clinic – especially without students and interns, who typically assist with academic research, Dr. Schroeder said.
Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern University, Chicago, and colleagues worked with a La Crosse team 6 or 7 years ago to try to analyze and publish SLIT outcomes for 121 peanut-allergic children who were treated for food and environmental allergies at the Wisconsin clinic. The researchers had hoped to publish an article describing caregiver-reported and clinical outcomes.
Among 73 caregivers who responded to a survey, more than half reported improved eczema, asthma, and environmental allergy symptoms, and virtually all families said SLIT calmed anxieties and minimized fear of allergic reactions. However, the clinical outcomes – skinprick test results, immune changes, and oral food challenges – were not as robust. And the data were incomplete. Some patients had traveled to La Crosse for SLIT drops but underwent skin and blood testing with their local allergist. Compiling records is “so much harder when you’re not doing a prospective clinical trial,” Dr. Gupta said.
The caregiver-reported outcomes were presented as a poster at the 2015 annual meeting of the American College of Allergy, Asthma, and Immunology and the 2016 annual meeting of the Pediatric Academic Society, said Jeff Kessler, MBA, FACHE, who is practice executive at La Crosse. However, with only self-reported data and no convincing lab metrics, the findings were never submitted for publication.
Others are eager to see clearer proof that SLIT works at doses lower than those published in the most recent trials. “If we can get efficacy with lower doses, that means we can increase accessibility, because we can lower the cost,” Dr. Bajowala said.
Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins University, Baltimore, has a pending grant proposal for a multifood trial of SLIT. “It’s a big missing piece,” he said.
Dr. Mack said that in Canada there was “almost an instant change in group think” when the Canadian Society of Allergy and Clinical Immunology published guidelines in support of OIT. With the new guidelines, “people are less concerned about liability. Once they start getting into OIT, I think you’re going to see SLIT coming right along for the ride.”
The shift will be slower in the United States, which has 20 times as many practicing allergists as Canada. Nevertheless, “I totally think SLIT has a place at the table,” Dr. Mack said. “I hope we start to see more high-quality data and people start to use it and experiment with it a bit and see how it works.”
A version of this article first appeared on Medscape.com. This is part three of a three-part series. Part one is here. Part two is here.












