User login
Vaping and pregnancy: Inhaled toxins among reasons for pause
Researchers are trying to understand how e-cigarette use affects pregnancy and birth outcomes. This question may become more relevant as younger vapers, among whom the devices gained considerable popularity, start having children.
Limited emerging data from animal experiments and human epidemiologic studies suggest that vaping may have negative effects on fertility and pregnancy. “Even if these impacts are less severe than conventional smoking, we really should be thinking about alternate options that may be safer for our patients than inhalation of this aerosol,” said Blair J. Wylie, MD, MPH, a maternal-fetal medicine physician at Beth Israel Deaconess Medical Center in Boston.
Dr. Wylie reviewed what is known about vaping, including chemicals other than nicotine that have been detected in vape aerosols, and pregnancy at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“There’s a lot we don’t know,” she said. “These products were only introduced recently, in 2003. They are marketed aggressively to our youth and have gained tremendous popularity among that population. And it’s only a matter of time, I think, before we see a lot of use in our own patient population.”
In a separate study presented at the ACOG meeting, Nicole Izhakoff, a researcher at Florida International University, Miami, and colleagues evaluated the association between e-cigarette use during pregnancy and unfavorable birth outcomes, such as preterm birth, low birth weight, or extended hospital stay for the newborn.
The investigators used 2016-2017 survey data from the Pregnancy Risk Assessment Monitoring System. In all, 71,940 women completed the survey, including 859 who reported e-cigarette use during pregnancy.
After adjusting for age, race, ethnicity, insurance, maternal education, prenatal care, abuse during pregnancy, and complications during pregnancy, the researchers estimated that the odds of an unfavorable birth outcome were 62% greater among women who used e-cigarettes during pregnancy, compared with those who did not.
The researchers lacked information about simultaneous use of alcohol, traditional tobacco, or other drugs, however.
“Physicians of all subspecialties, especially those of obstetrics-gynecology and pediatrics, need to increase the implementation of screening for past or current e-cigarette use in at-risk patients,” Ms. Izhakoff and coauthors concluded. “Further research regarding the long-term health effects of e-cigarettes is warranted.”
Dr. Wylie coauthored another study related to this topic that was published online May 24, 2021, in the Journal of Maternal-Fetal & Neonatal Medicine.
The researchers examined birth weights of children whose mothers use e-cigarettes alone, those whose mothers used both e-cigarettes and conventional cigarettes, and those whose mothers smoked conventional cigarettes only. Their estimates were imprecise, but signaled that e-cigarette use may reduce birth weight. The use of e-cigarettes alone appeared to have less of an impact on birth weight than the dual use of conventional cigarettes and e-cigarettes did.
Dr. Wylie cautioned that outcomes like birth weight are “pretty crude measures of whether an exposure is okay or not in pregnancy. Many of these toxins that we know that are in the aerosols can cause harm, but they may not be reflected in the absolute value of the birth weight.”
In addition, clinicians should avoid focusing on the wrong question when caring for patients.
“I think the wrong question is: Is vaping safer than smoking?” Dr. Wylie said in an interview. “Metals are going into your lungs. Plastics are going into your lungs. It is hard for me to think that we are going to identify that as our champion smoking cessation strategy in pregnancy.”
Rapidly changing landscape
Answering the question of which is safer is a challenge anyway because researchers likely have incomplete information about who vapes, who smokes, and who does both.
Still, the new research illustrates that “people are starting to think about this and beginning to do some analysis that is really hypothesis generating at this point,” Dr. Wylie said. Such studies may prompt clinicians to ask their patients about e-cigarette use. “Marijuana is sort of a similar thing where patients’ perception of safety, because things are legal, can lead to use during pregnancy without ... letting their care teams know,” she said. “Things are changing so rapidly in terms of what’s available to people to use that we need to stay on top of that as obstetricians and ask the right questions and try to understand what the risks are and potential benefits.”
Dr. Wylie is an obstetric consultant to the New England Pediatric Environmental Health Specialty Unit, which is where she heard pediatricians discussing widespread e-cigarette use among youth. It occurred to her that some of these teens eventually would be seeing obstetricians. She also saw parallels to prior research she conducted that focused on household air pollution or cooking from wood-burning fires in Africa.
“What is frightening, I think, about these electronic cigarettes is that you’re heating this liquid to extraordinarily high temperatures to create the vapor,” and the extreme heat vaporizes plastics and metals as well as nicotine, Dr. Wylie said.
An ACOG committee opinion discusses approaches to smoking and vaping cessation such as counseling, behavioral therapy, and medication.
The publication also lists a host of elements have been isolated from vape aerosol, including “carbonyl compounds (formaldehyde, acetaldehyde, acetone, and acrolein); volatile organic compounds (benzene and toluene); nitrosamines; particulate matter; and heavy metals such as copper, lead, zinc, and tin.”
In addition to the nicotine in e-cigarette liquids, which is harmful in itself, there is “all of this other company that it keeps,” including solvent byproducts, known carcinogens, and lung irritants, Dr. Wylie said. Fine particulate matter “can land in the small airways and cause inflammation, even translocate into the systemic circulation and cause systemic inflammation.”
The use of flavoring “likely alters perceptions of harm” and contributes to the popularity of vaping, Dr. Wylie noted. At the same time, the use of flavoring also has little regulatory oversight. Flavors usually are approved for marketing based on safety for ingestion, but that may not translate into safety for inhalation.
Parsing the health effects
People who vape have increased cough, wheezing, and phlegm production, compared with people who do not vape. Vaping also may worsen underlying lung disease like asthma. Lung function on spirometry decreases after e-cigarette use, studies have shown.
In 2019, researchers described e-cigarette or vaping product use–related acute lung injury (EVALI), which has caused more than 60 deaths in the United States. The condition may be related to vitamin E acetate, a component that had been used in some liquids used by patients with EVALI.
And the nicotine in e-cigarettes can accelerate atherogenesis and affect blood pressure, heart rate, and arterial stiffness.
Initially introduced as a smoking cessation tool, e-cigarettes now often are used on their own or in addition to cigarettes, rather than strictly for smoking cessation.
A Cochrane review suggests that e-cigarettes may be more effective than other approaches to smoking cessation. But “the effect is modest at best,” Dr. Wylie said. Among 100 people attempting to quit cigarette smoking, there might four to six more quitters with the use of e-cigarettes as a smoking cessation intervention, compared with other approaches.
Animal models provide other reasons for caution. One experiment in mice showed that exposure to e-cigarette aerosol impaired implantation and fetal health. The results suggest “that there might be some negative impacts across generations,” Dr. Wylie said.
Another study has suggested the possibility that women who currently use e-cigarettes may have slightly diminished fecundability. The results were not statistically significant, but the study “gives us pause about whether there could be some impact on early pregnancy and fertility,” Dr. Wylie said.
In mouse models, prenatal exposure to e-cigarette aerosol has decreased fetal weight and length, altered neurodevelopment and neuroregulatory gene expression, and increased proinflammatory cytokines. E-cigarette aerosol also has caused birth defects in zebrafish and facial clefting in frogs. Whether and how these data relate to human pregnancy is unclear.
While e-cigarette ads may convey a sense of style and harmlessness, clinicians have reasons to worry about the effects. “We have to be a little bit more cautious when we are talking about this with our patients,” Dr. Wylie said.
Dr. Wylie had no relevant financial disclosures. She is a Society for Maternal-Fetal Medicine board member and receives grant support related to research of household air pollution and pregnancy, prenatal pesticide exposure, preeclampsia in low income settings, and malaria during pregnancy. Ms. Izhakoff and coauthors had no disclosures.
Researchers are trying to understand how e-cigarette use affects pregnancy and birth outcomes. This question may become more relevant as younger vapers, among whom the devices gained considerable popularity, start having children.
Limited emerging data from animal experiments and human epidemiologic studies suggest that vaping may have negative effects on fertility and pregnancy. “Even if these impacts are less severe than conventional smoking, we really should be thinking about alternate options that may be safer for our patients than inhalation of this aerosol,” said Blair J. Wylie, MD, MPH, a maternal-fetal medicine physician at Beth Israel Deaconess Medical Center in Boston.
Dr. Wylie reviewed what is known about vaping, including chemicals other than nicotine that have been detected in vape aerosols, and pregnancy at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“There’s a lot we don’t know,” she said. “These products were only introduced recently, in 2003. They are marketed aggressively to our youth and have gained tremendous popularity among that population. And it’s only a matter of time, I think, before we see a lot of use in our own patient population.”
In a separate study presented at the ACOG meeting, Nicole Izhakoff, a researcher at Florida International University, Miami, and colleagues evaluated the association between e-cigarette use during pregnancy and unfavorable birth outcomes, such as preterm birth, low birth weight, or extended hospital stay for the newborn.
The investigators used 2016-2017 survey data from the Pregnancy Risk Assessment Monitoring System. In all, 71,940 women completed the survey, including 859 who reported e-cigarette use during pregnancy.
After adjusting for age, race, ethnicity, insurance, maternal education, prenatal care, abuse during pregnancy, and complications during pregnancy, the researchers estimated that the odds of an unfavorable birth outcome were 62% greater among women who used e-cigarettes during pregnancy, compared with those who did not.
The researchers lacked information about simultaneous use of alcohol, traditional tobacco, or other drugs, however.
“Physicians of all subspecialties, especially those of obstetrics-gynecology and pediatrics, need to increase the implementation of screening for past or current e-cigarette use in at-risk patients,” Ms. Izhakoff and coauthors concluded. “Further research regarding the long-term health effects of e-cigarettes is warranted.”
Dr. Wylie coauthored another study related to this topic that was published online May 24, 2021, in the Journal of Maternal-Fetal & Neonatal Medicine.
The researchers examined birth weights of children whose mothers use e-cigarettes alone, those whose mothers used both e-cigarettes and conventional cigarettes, and those whose mothers smoked conventional cigarettes only. Their estimates were imprecise, but signaled that e-cigarette use may reduce birth weight. The use of e-cigarettes alone appeared to have less of an impact on birth weight than the dual use of conventional cigarettes and e-cigarettes did.
Dr. Wylie cautioned that outcomes like birth weight are “pretty crude measures of whether an exposure is okay or not in pregnancy. Many of these toxins that we know that are in the aerosols can cause harm, but they may not be reflected in the absolute value of the birth weight.”
In addition, clinicians should avoid focusing on the wrong question when caring for patients.
“I think the wrong question is: Is vaping safer than smoking?” Dr. Wylie said in an interview. “Metals are going into your lungs. Plastics are going into your lungs. It is hard for me to think that we are going to identify that as our champion smoking cessation strategy in pregnancy.”
Rapidly changing landscape
Answering the question of which is safer is a challenge anyway because researchers likely have incomplete information about who vapes, who smokes, and who does both.
Still, the new research illustrates that “people are starting to think about this and beginning to do some analysis that is really hypothesis generating at this point,” Dr. Wylie said. Such studies may prompt clinicians to ask their patients about e-cigarette use. “Marijuana is sort of a similar thing where patients’ perception of safety, because things are legal, can lead to use during pregnancy without ... letting their care teams know,” she said. “Things are changing so rapidly in terms of what’s available to people to use that we need to stay on top of that as obstetricians and ask the right questions and try to understand what the risks are and potential benefits.”
Dr. Wylie is an obstetric consultant to the New England Pediatric Environmental Health Specialty Unit, which is where she heard pediatricians discussing widespread e-cigarette use among youth. It occurred to her that some of these teens eventually would be seeing obstetricians. She also saw parallels to prior research she conducted that focused on household air pollution or cooking from wood-burning fires in Africa.
“What is frightening, I think, about these electronic cigarettes is that you’re heating this liquid to extraordinarily high temperatures to create the vapor,” and the extreme heat vaporizes plastics and metals as well as nicotine, Dr. Wylie said.
An ACOG committee opinion discusses approaches to smoking and vaping cessation such as counseling, behavioral therapy, and medication.
The publication also lists a host of elements have been isolated from vape aerosol, including “carbonyl compounds (formaldehyde, acetaldehyde, acetone, and acrolein); volatile organic compounds (benzene and toluene); nitrosamines; particulate matter; and heavy metals such as copper, lead, zinc, and tin.”
In addition to the nicotine in e-cigarette liquids, which is harmful in itself, there is “all of this other company that it keeps,” including solvent byproducts, known carcinogens, and lung irritants, Dr. Wylie said. Fine particulate matter “can land in the small airways and cause inflammation, even translocate into the systemic circulation and cause systemic inflammation.”
The use of flavoring “likely alters perceptions of harm” and contributes to the popularity of vaping, Dr. Wylie noted. At the same time, the use of flavoring also has little regulatory oversight. Flavors usually are approved for marketing based on safety for ingestion, but that may not translate into safety for inhalation.
Parsing the health effects
People who vape have increased cough, wheezing, and phlegm production, compared with people who do not vape. Vaping also may worsen underlying lung disease like asthma. Lung function on spirometry decreases after e-cigarette use, studies have shown.
In 2019, researchers described e-cigarette or vaping product use–related acute lung injury (EVALI), which has caused more than 60 deaths in the United States. The condition may be related to vitamin E acetate, a component that had been used in some liquids used by patients with EVALI.
And the nicotine in e-cigarettes can accelerate atherogenesis and affect blood pressure, heart rate, and arterial stiffness.
Initially introduced as a smoking cessation tool, e-cigarettes now often are used on their own or in addition to cigarettes, rather than strictly for smoking cessation.
A Cochrane review suggests that e-cigarettes may be more effective than other approaches to smoking cessation. But “the effect is modest at best,” Dr. Wylie said. Among 100 people attempting to quit cigarette smoking, there might four to six more quitters with the use of e-cigarettes as a smoking cessation intervention, compared with other approaches.
Animal models provide other reasons for caution. One experiment in mice showed that exposure to e-cigarette aerosol impaired implantation and fetal health. The results suggest “that there might be some negative impacts across generations,” Dr. Wylie said.
Another study has suggested the possibility that women who currently use e-cigarettes may have slightly diminished fecundability. The results were not statistically significant, but the study “gives us pause about whether there could be some impact on early pregnancy and fertility,” Dr. Wylie said.
In mouse models, prenatal exposure to e-cigarette aerosol has decreased fetal weight and length, altered neurodevelopment and neuroregulatory gene expression, and increased proinflammatory cytokines. E-cigarette aerosol also has caused birth defects in zebrafish and facial clefting in frogs. Whether and how these data relate to human pregnancy is unclear.
While e-cigarette ads may convey a sense of style and harmlessness, clinicians have reasons to worry about the effects. “We have to be a little bit more cautious when we are talking about this with our patients,” Dr. Wylie said.
Dr. Wylie had no relevant financial disclosures. She is a Society for Maternal-Fetal Medicine board member and receives grant support related to research of household air pollution and pregnancy, prenatal pesticide exposure, preeclampsia in low income settings, and malaria during pregnancy. Ms. Izhakoff and coauthors had no disclosures.
Researchers are trying to understand how e-cigarette use affects pregnancy and birth outcomes. This question may become more relevant as younger vapers, among whom the devices gained considerable popularity, start having children.
Limited emerging data from animal experiments and human epidemiologic studies suggest that vaping may have negative effects on fertility and pregnancy. “Even if these impacts are less severe than conventional smoking, we really should be thinking about alternate options that may be safer for our patients than inhalation of this aerosol,” said Blair J. Wylie, MD, MPH, a maternal-fetal medicine physician at Beth Israel Deaconess Medical Center in Boston.
Dr. Wylie reviewed what is known about vaping, including chemicals other than nicotine that have been detected in vape aerosols, and pregnancy at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“There’s a lot we don’t know,” she said. “These products were only introduced recently, in 2003. They are marketed aggressively to our youth and have gained tremendous popularity among that population. And it’s only a matter of time, I think, before we see a lot of use in our own patient population.”
In a separate study presented at the ACOG meeting, Nicole Izhakoff, a researcher at Florida International University, Miami, and colleagues evaluated the association between e-cigarette use during pregnancy and unfavorable birth outcomes, such as preterm birth, low birth weight, or extended hospital stay for the newborn.
The investigators used 2016-2017 survey data from the Pregnancy Risk Assessment Monitoring System. In all, 71,940 women completed the survey, including 859 who reported e-cigarette use during pregnancy.
After adjusting for age, race, ethnicity, insurance, maternal education, prenatal care, abuse during pregnancy, and complications during pregnancy, the researchers estimated that the odds of an unfavorable birth outcome were 62% greater among women who used e-cigarettes during pregnancy, compared with those who did not.
The researchers lacked information about simultaneous use of alcohol, traditional tobacco, or other drugs, however.
“Physicians of all subspecialties, especially those of obstetrics-gynecology and pediatrics, need to increase the implementation of screening for past or current e-cigarette use in at-risk patients,” Ms. Izhakoff and coauthors concluded. “Further research regarding the long-term health effects of e-cigarettes is warranted.”
Dr. Wylie coauthored another study related to this topic that was published online May 24, 2021, in the Journal of Maternal-Fetal & Neonatal Medicine.
The researchers examined birth weights of children whose mothers use e-cigarettes alone, those whose mothers used both e-cigarettes and conventional cigarettes, and those whose mothers smoked conventional cigarettes only. Their estimates were imprecise, but signaled that e-cigarette use may reduce birth weight. The use of e-cigarettes alone appeared to have less of an impact on birth weight than the dual use of conventional cigarettes and e-cigarettes did.
Dr. Wylie cautioned that outcomes like birth weight are “pretty crude measures of whether an exposure is okay or not in pregnancy. Many of these toxins that we know that are in the aerosols can cause harm, but they may not be reflected in the absolute value of the birth weight.”
In addition, clinicians should avoid focusing on the wrong question when caring for patients.
“I think the wrong question is: Is vaping safer than smoking?” Dr. Wylie said in an interview. “Metals are going into your lungs. Plastics are going into your lungs. It is hard for me to think that we are going to identify that as our champion smoking cessation strategy in pregnancy.”
Rapidly changing landscape
Answering the question of which is safer is a challenge anyway because researchers likely have incomplete information about who vapes, who smokes, and who does both.
Still, the new research illustrates that “people are starting to think about this and beginning to do some analysis that is really hypothesis generating at this point,” Dr. Wylie said. Such studies may prompt clinicians to ask their patients about e-cigarette use. “Marijuana is sort of a similar thing where patients’ perception of safety, because things are legal, can lead to use during pregnancy without ... letting their care teams know,” she said. “Things are changing so rapidly in terms of what’s available to people to use that we need to stay on top of that as obstetricians and ask the right questions and try to understand what the risks are and potential benefits.”
Dr. Wylie is an obstetric consultant to the New England Pediatric Environmental Health Specialty Unit, which is where she heard pediatricians discussing widespread e-cigarette use among youth. It occurred to her that some of these teens eventually would be seeing obstetricians. She also saw parallels to prior research she conducted that focused on household air pollution or cooking from wood-burning fires in Africa.
“What is frightening, I think, about these electronic cigarettes is that you’re heating this liquid to extraordinarily high temperatures to create the vapor,” and the extreme heat vaporizes plastics and metals as well as nicotine, Dr. Wylie said.
An ACOG committee opinion discusses approaches to smoking and vaping cessation such as counseling, behavioral therapy, and medication.
The publication also lists a host of elements have been isolated from vape aerosol, including “carbonyl compounds (formaldehyde, acetaldehyde, acetone, and acrolein); volatile organic compounds (benzene and toluene); nitrosamines; particulate matter; and heavy metals such as copper, lead, zinc, and tin.”
In addition to the nicotine in e-cigarette liquids, which is harmful in itself, there is “all of this other company that it keeps,” including solvent byproducts, known carcinogens, and lung irritants, Dr. Wylie said. Fine particulate matter “can land in the small airways and cause inflammation, even translocate into the systemic circulation and cause systemic inflammation.”
The use of flavoring “likely alters perceptions of harm” and contributes to the popularity of vaping, Dr. Wylie noted. At the same time, the use of flavoring also has little regulatory oversight. Flavors usually are approved for marketing based on safety for ingestion, but that may not translate into safety for inhalation.
Parsing the health effects
People who vape have increased cough, wheezing, and phlegm production, compared with people who do not vape. Vaping also may worsen underlying lung disease like asthma. Lung function on spirometry decreases after e-cigarette use, studies have shown.
In 2019, researchers described e-cigarette or vaping product use–related acute lung injury (EVALI), which has caused more than 60 deaths in the United States. The condition may be related to vitamin E acetate, a component that had been used in some liquids used by patients with EVALI.
And the nicotine in e-cigarettes can accelerate atherogenesis and affect blood pressure, heart rate, and arterial stiffness.
Initially introduced as a smoking cessation tool, e-cigarettes now often are used on their own or in addition to cigarettes, rather than strictly for smoking cessation.
A Cochrane review suggests that e-cigarettes may be more effective than other approaches to smoking cessation. But “the effect is modest at best,” Dr. Wylie said. Among 100 people attempting to quit cigarette smoking, there might four to six more quitters with the use of e-cigarettes as a smoking cessation intervention, compared with other approaches.
Animal models provide other reasons for caution. One experiment in mice showed that exposure to e-cigarette aerosol impaired implantation and fetal health. The results suggest “that there might be some negative impacts across generations,” Dr. Wylie said.
Another study has suggested the possibility that women who currently use e-cigarettes may have slightly diminished fecundability. The results were not statistically significant, but the study “gives us pause about whether there could be some impact on early pregnancy and fertility,” Dr. Wylie said.
In mouse models, prenatal exposure to e-cigarette aerosol has decreased fetal weight and length, altered neurodevelopment and neuroregulatory gene expression, and increased proinflammatory cytokines. E-cigarette aerosol also has caused birth defects in zebrafish and facial clefting in frogs. Whether and how these data relate to human pregnancy is unclear.
While e-cigarette ads may convey a sense of style and harmlessness, clinicians have reasons to worry about the effects. “We have to be a little bit more cautious when we are talking about this with our patients,” Dr. Wylie said.
Dr. Wylie had no relevant financial disclosures. She is a Society for Maternal-Fetal Medicine board member and receives grant support related to research of household air pollution and pregnancy, prenatal pesticide exposure, preeclampsia in low income settings, and malaria during pregnancy. Ms. Izhakoff and coauthors had no disclosures.
FROM ACOG 2021
FDA panel endorses teplizumab for delaying type 1 diabetes
in at-risk individuals.
The 10-7 vote of the FDA’s endocrinologic and metabolic drugs advisory committee on May 27 reflected a difficult decision-making process on the part of many members to weigh the benefits of a potential 2-year delay in the onset of type 1 diabetes against both observed and theoretical risks, as well as what most considered to be insufficient data.
Regardless of their vote, nearly all panel members advised the FDA that the company should be required to conduct at least one additional larger long-term efficacy and safety trial to satisfy what they felt were major gaps in the data. Some advised that use of the drug be restricted to a very narrow group of recipients until efficacy and safety can be better established.
If approved, teplizumab, which interferes with T cell–mediated autoimmune destruction of pancreatic beta cells, would be the first disease-modifying therapy for impeding progression of type 1 diabetes. The proposed indication is for individuals who have two or more type 1 diabetes-associated autoantibodies and subclinical dysglycemia.
That “stage 2” or “at-risk” condition is associated with a nearly 100% lifetime risk of progression to clinical (“stage 3”) type 1 diabetes and a 75% risk of developing the disease within 5 years. As of now, most such individuals are first-degree relatives of people with type 1 diabetes identified through TrialNet.
What’s the evidence to support approval so far?
In 2019, a pivotal phase 2 randomized, placebo-controlled TN-10 trial involving 76 at-risk children and adults ages 8 years and older showed that a single 14-day treatment of daily intravenous infusions of teplizumab in 44 patients resulted in a significant median 2-year delay to onset of clinical type 1 diabetes, compared with 32 who received placebo. Further follow-up data continue to show that fewer patients who received teplizumab have progressed to clinical type 1 diabetes.
While most advisory panelists agreed that the TN-10 study demonstrated efficacy, several also said that the sample size was insufficient and at least one additional randomized trial should be conducted to replicate the findings.
Although the FDA typically requires companies to demonstrate a drug’s effectiveness with at least two separate clinical trials, the agency allows companies to substitute other forms of data for a second randomized clinical trial, such as study results for the drug in a closely related condition, mechanistic data, or knowledge of other drugs from the same class.
In this case, Provention’s submission included as “confirmatory” evidence a meta-analysis of data from five earlier randomized trials (three placebo controlled, two open label) of a total 942 individuals with newly diagnosed type 1 diabetes (“stage 3”) who received either one or two 14-day teplizumab courses (n = 729) or placebo. These showed consistent preservation of C-peptide, a surrogate marker of beta-cell function, along with lower mean insulin use.
Several panel members expressed dissatisfaction with those confirmatory data, noting the patient population was different from those for which the company is currently seeking the indication, and that C-peptide is an inadequate endpoint for demonstrating efficacy.
Safety: Adverse events mostly transient, but unanswered questions
Adverse events reported in at least 10% of teplizumab recipients included lymphopenia (76.8% vs. 9.4% placebo; relative risk, 8.2), leukopenia (82.1% vs. 24.1%; RR, 3.4), and rash (44.5% vs. 9.0%; RR, 4.9).
“Most adverse events related to teplizumab were mechanism-based, predictable, transient, and manageable,” Chief Medical Officer of Provention Bio, Eleanor Ramos, MD, said.
Among other safety issues that concerned the panel, diabetic ketoacidosis (DKA) was seen in 2.3% of 773 teplizumab recipients with new-onset type 1 diabetes versus just 1% among the 245 controls, a significant, nearly sixfold increase. No DKA occurred in the TN-10 trial. No clear explanation was offered for the imbalance in the meta-analysis.
Cytokine release syndrome occurred in 0.6% of patients who received teplizumab versus no controls, and infections in 3.4% versus 2.0%, respectively.
Approximately 10% of patients were not able to complete the treatment course because of protocol-directed withdrawal criteria, which included elevations in bilirubin or liver enzymes, or drops in platelet count, neutrophils, or hemoglobin, FDA reviewer Lauren Wood Heickman, MD, noted.
There was only one malignancy, a melanoma in a patient with a preexisting lesion, but malignancy is a theoretical concern with long-term immunosuppression, Dr. Heickman said.
Despite the concerns about the data, panel members expressed unanimous appreciation for the 18 people who spoke during public comments attesting to the lifelong burdens involved in living with type 1 diabetes who urged the FDA to approve teplizumab.
Many of them noted that even a 2-year reprieve from the burden of constant attention to managing blood glucose can make a major difference in the life of a young person. The speakers included physicians, parents of children with type 1 diabetes, adults who have the condition themselves and who worry about their children getting it, and researchers in the field.
Panel members describe ‘struggle’ with vote decision
Panel member Michael Blaha, MD, of Johns Hopkins University, Baltimore, voted in favor of teplizumab approval. However, he said, “I was very conflicted on this one and my ‘yes’ is very qualified. In my opinion the risk-benefit is very narrow, and I would only approve this drug for the exact indication of the trial. ... Patients who don’t fit the criteria could hopefully be enrolled in a second confirmatory trial.”
He also advised an extensive Risk Evaluation and Mitigation Strategies program to look for both short- and long-term adverse effects.
“My overall take on this is that I do think it’s a promising paradigm-shifting therapy that really needs to move forward, at least scientifically. I’m excited about it, but I have a lot of skepticism about the entire body of data to make any more than the most narrow of approval,” Dr. Blaha said.
Susan S. Ellenberg, PhD, professor of biostatistics, medical ethics, and health policy at the University of Pennsylvania, Philadelphia, voted yes but also with difficulty.
“I really struggled with it. ... I was pushed by the very encouraging results of what is admittedly a very small study and something I can’t feel is completely definitive. But I would not like to deny the kind of people that we heard from today the opportunity to weigh their own risks and benefits to try this. And I would certainly agree that a very, very rigorous postmarketing program, preferably including another controlled trial, should be carried out.”
But David M. Nathan, MD, director of the Diabetes Center and Clinical Research Center, Massachusetts General Hospital, Boston, voted no.
“I struggled with this vote, tremendously, having listened carefully to the patients with type 1 diabetes ... but that said, having done clinical research for 40 years in type 1 diabetes, I think we need more data, both in terms of efficacy and of safety. I would hate a number of years down the road to figure out that we actually caused more harm than good, especially keeping in mind that the treatment of type 1 diabetes is evolving rapidly.”
A different perspective came from Mara L. Becker, MD, vice chair of the department of pediatric rheumatology at Duke University, Durham, N.C. She voted yes, pointing out that she’s accustomed to prescribing biologics for chronic conditions in children.
“I was unconflicted in my vote, which was yes. I thought the data ... were convincing and the need is great. I would support a label for children [aged 8 years] and older with at least stage 2 disease ... and I would require postmarketing safety surveillance to understand what the long-term side effects could be, but I would still be in favor of it.”
FDA advisory panel committee members are vetted for conflicts of interest and waivers granted for participation if necessary; none were granted for this meeting.
A version of this article first appeared on Medscape.com.
in at-risk individuals.
The 10-7 vote of the FDA’s endocrinologic and metabolic drugs advisory committee on May 27 reflected a difficult decision-making process on the part of many members to weigh the benefits of a potential 2-year delay in the onset of type 1 diabetes against both observed and theoretical risks, as well as what most considered to be insufficient data.
Regardless of their vote, nearly all panel members advised the FDA that the company should be required to conduct at least one additional larger long-term efficacy and safety trial to satisfy what they felt were major gaps in the data. Some advised that use of the drug be restricted to a very narrow group of recipients until efficacy and safety can be better established.
If approved, teplizumab, which interferes with T cell–mediated autoimmune destruction of pancreatic beta cells, would be the first disease-modifying therapy for impeding progression of type 1 diabetes. The proposed indication is for individuals who have two or more type 1 diabetes-associated autoantibodies and subclinical dysglycemia.
That “stage 2” or “at-risk” condition is associated with a nearly 100% lifetime risk of progression to clinical (“stage 3”) type 1 diabetes and a 75% risk of developing the disease within 5 years. As of now, most such individuals are first-degree relatives of people with type 1 diabetes identified through TrialNet.
What’s the evidence to support approval so far?
In 2019, a pivotal phase 2 randomized, placebo-controlled TN-10 trial involving 76 at-risk children and adults ages 8 years and older showed that a single 14-day treatment of daily intravenous infusions of teplizumab in 44 patients resulted in a significant median 2-year delay to onset of clinical type 1 diabetes, compared with 32 who received placebo. Further follow-up data continue to show that fewer patients who received teplizumab have progressed to clinical type 1 diabetes.
While most advisory panelists agreed that the TN-10 study demonstrated efficacy, several also said that the sample size was insufficient and at least one additional randomized trial should be conducted to replicate the findings.
Although the FDA typically requires companies to demonstrate a drug’s effectiveness with at least two separate clinical trials, the agency allows companies to substitute other forms of data for a second randomized clinical trial, such as study results for the drug in a closely related condition, mechanistic data, or knowledge of other drugs from the same class.
In this case, Provention’s submission included as “confirmatory” evidence a meta-analysis of data from five earlier randomized trials (three placebo controlled, two open label) of a total 942 individuals with newly diagnosed type 1 diabetes (“stage 3”) who received either one or two 14-day teplizumab courses (n = 729) or placebo. These showed consistent preservation of C-peptide, a surrogate marker of beta-cell function, along with lower mean insulin use.
Several panel members expressed dissatisfaction with those confirmatory data, noting the patient population was different from those for which the company is currently seeking the indication, and that C-peptide is an inadequate endpoint for demonstrating efficacy.
Safety: Adverse events mostly transient, but unanswered questions
Adverse events reported in at least 10% of teplizumab recipients included lymphopenia (76.8% vs. 9.4% placebo; relative risk, 8.2), leukopenia (82.1% vs. 24.1%; RR, 3.4), and rash (44.5% vs. 9.0%; RR, 4.9).
“Most adverse events related to teplizumab were mechanism-based, predictable, transient, and manageable,” Chief Medical Officer of Provention Bio, Eleanor Ramos, MD, said.
Among other safety issues that concerned the panel, diabetic ketoacidosis (DKA) was seen in 2.3% of 773 teplizumab recipients with new-onset type 1 diabetes versus just 1% among the 245 controls, a significant, nearly sixfold increase. No DKA occurred in the TN-10 trial. No clear explanation was offered for the imbalance in the meta-analysis.
Cytokine release syndrome occurred in 0.6% of patients who received teplizumab versus no controls, and infections in 3.4% versus 2.0%, respectively.
Approximately 10% of patients were not able to complete the treatment course because of protocol-directed withdrawal criteria, which included elevations in bilirubin or liver enzymes, or drops in platelet count, neutrophils, or hemoglobin, FDA reviewer Lauren Wood Heickman, MD, noted.
There was only one malignancy, a melanoma in a patient with a preexisting lesion, but malignancy is a theoretical concern with long-term immunosuppression, Dr. Heickman said.
Despite the concerns about the data, panel members expressed unanimous appreciation for the 18 people who spoke during public comments attesting to the lifelong burdens involved in living with type 1 diabetes who urged the FDA to approve teplizumab.
Many of them noted that even a 2-year reprieve from the burden of constant attention to managing blood glucose can make a major difference in the life of a young person. The speakers included physicians, parents of children with type 1 diabetes, adults who have the condition themselves and who worry about their children getting it, and researchers in the field.
Panel members describe ‘struggle’ with vote decision
Panel member Michael Blaha, MD, of Johns Hopkins University, Baltimore, voted in favor of teplizumab approval. However, he said, “I was very conflicted on this one and my ‘yes’ is very qualified. In my opinion the risk-benefit is very narrow, and I would only approve this drug for the exact indication of the trial. ... Patients who don’t fit the criteria could hopefully be enrolled in a second confirmatory trial.”
He also advised an extensive Risk Evaluation and Mitigation Strategies program to look for both short- and long-term adverse effects.
“My overall take on this is that I do think it’s a promising paradigm-shifting therapy that really needs to move forward, at least scientifically. I’m excited about it, but I have a lot of skepticism about the entire body of data to make any more than the most narrow of approval,” Dr. Blaha said.
Susan S. Ellenberg, PhD, professor of biostatistics, medical ethics, and health policy at the University of Pennsylvania, Philadelphia, voted yes but also with difficulty.
“I really struggled with it. ... I was pushed by the very encouraging results of what is admittedly a very small study and something I can’t feel is completely definitive. But I would not like to deny the kind of people that we heard from today the opportunity to weigh their own risks and benefits to try this. And I would certainly agree that a very, very rigorous postmarketing program, preferably including another controlled trial, should be carried out.”
But David M. Nathan, MD, director of the Diabetes Center and Clinical Research Center, Massachusetts General Hospital, Boston, voted no.
“I struggled with this vote, tremendously, having listened carefully to the patients with type 1 diabetes ... but that said, having done clinical research for 40 years in type 1 diabetes, I think we need more data, both in terms of efficacy and of safety. I would hate a number of years down the road to figure out that we actually caused more harm than good, especially keeping in mind that the treatment of type 1 diabetes is evolving rapidly.”
A different perspective came from Mara L. Becker, MD, vice chair of the department of pediatric rheumatology at Duke University, Durham, N.C. She voted yes, pointing out that she’s accustomed to prescribing biologics for chronic conditions in children.
“I was unconflicted in my vote, which was yes. I thought the data ... were convincing and the need is great. I would support a label for children [aged 8 years] and older with at least stage 2 disease ... and I would require postmarketing safety surveillance to understand what the long-term side effects could be, but I would still be in favor of it.”
FDA advisory panel committee members are vetted for conflicts of interest and waivers granted for participation if necessary; none were granted for this meeting.
A version of this article first appeared on Medscape.com.
in at-risk individuals.
The 10-7 vote of the FDA’s endocrinologic and metabolic drugs advisory committee on May 27 reflected a difficult decision-making process on the part of many members to weigh the benefits of a potential 2-year delay in the onset of type 1 diabetes against both observed and theoretical risks, as well as what most considered to be insufficient data.
Regardless of their vote, nearly all panel members advised the FDA that the company should be required to conduct at least one additional larger long-term efficacy and safety trial to satisfy what they felt were major gaps in the data. Some advised that use of the drug be restricted to a very narrow group of recipients until efficacy and safety can be better established.
If approved, teplizumab, which interferes with T cell–mediated autoimmune destruction of pancreatic beta cells, would be the first disease-modifying therapy for impeding progression of type 1 diabetes. The proposed indication is for individuals who have two or more type 1 diabetes-associated autoantibodies and subclinical dysglycemia.
That “stage 2” or “at-risk” condition is associated with a nearly 100% lifetime risk of progression to clinical (“stage 3”) type 1 diabetes and a 75% risk of developing the disease within 5 years. As of now, most such individuals are first-degree relatives of people with type 1 diabetes identified through TrialNet.
What’s the evidence to support approval so far?
In 2019, a pivotal phase 2 randomized, placebo-controlled TN-10 trial involving 76 at-risk children and adults ages 8 years and older showed that a single 14-day treatment of daily intravenous infusions of teplizumab in 44 patients resulted in a significant median 2-year delay to onset of clinical type 1 diabetes, compared with 32 who received placebo. Further follow-up data continue to show that fewer patients who received teplizumab have progressed to clinical type 1 diabetes.
While most advisory panelists agreed that the TN-10 study demonstrated efficacy, several also said that the sample size was insufficient and at least one additional randomized trial should be conducted to replicate the findings.
Although the FDA typically requires companies to demonstrate a drug’s effectiveness with at least two separate clinical trials, the agency allows companies to substitute other forms of data for a second randomized clinical trial, such as study results for the drug in a closely related condition, mechanistic data, or knowledge of other drugs from the same class.
In this case, Provention’s submission included as “confirmatory” evidence a meta-analysis of data from five earlier randomized trials (three placebo controlled, two open label) of a total 942 individuals with newly diagnosed type 1 diabetes (“stage 3”) who received either one or two 14-day teplizumab courses (n = 729) or placebo. These showed consistent preservation of C-peptide, a surrogate marker of beta-cell function, along with lower mean insulin use.
Several panel members expressed dissatisfaction with those confirmatory data, noting the patient population was different from those for which the company is currently seeking the indication, and that C-peptide is an inadequate endpoint for demonstrating efficacy.
Safety: Adverse events mostly transient, but unanswered questions
Adverse events reported in at least 10% of teplizumab recipients included lymphopenia (76.8% vs. 9.4% placebo; relative risk, 8.2), leukopenia (82.1% vs. 24.1%; RR, 3.4), and rash (44.5% vs. 9.0%; RR, 4.9).
“Most adverse events related to teplizumab were mechanism-based, predictable, transient, and manageable,” Chief Medical Officer of Provention Bio, Eleanor Ramos, MD, said.
Among other safety issues that concerned the panel, diabetic ketoacidosis (DKA) was seen in 2.3% of 773 teplizumab recipients with new-onset type 1 diabetes versus just 1% among the 245 controls, a significant, nearly sixfold increase. No DKA occurred in the TN-10 trial. No clear explanation was offered for the imbalance in the meta-analysis.
Cytokine release syndrome occurred in 0.6% of patients who received teplizumab versus no controls, and infections in 3.4% versus 2.0%, respectively.
Approximately 10% of patients were not able to complete the treatment course because of protocol-directed withdrawal criteria, which included elevations in bilirubin or liver enzymes, or drops in platelet count, neutrophils, or hemoglobin, FDA reviewer Lauren Wood Heickman, MD, noted.
There was only one malignancy, a melanoma in a patient with a preexisting lesion, but malignancy is a theoretical concern with long-term immunosuppression, Dr. Heickman said.
Despite the concerns about the data, panel members expressed unanimous appreciation for the 18 people who spoke during public comments attesting to the lifelong burdens involved in living with type 1 diabetes who urged the FDA to approve teplizumab.
Many of them noted that even a 2-year reprieve from the burden of constant attention to managing blood glucose can make a major difference in the life of a young person. The speakers included physicians, parents of children with type 1 diabetes, adults who have the condition themselves and who worry about their children getting it, and researchers in the field.
Panel members describe ‘struggle’ with vote decision
Panel member Michael Blaha, MD, of Johns Hopkins University, Baltimore, voted in favor of teplizumab approval. However, he said, “I was very conflicted on this one and my ‘yes’ is very qualified. In my opinion the risk-benefit is very narrow, and I would only approve this drug for the exact indication of the trial. ... Patients who don’t fit the criteria could hopefully be enrolled in a second confirmatory trial.”
He also advised an extensive Risk Evaluation and Mitigation Strategies program to look for both short- and long-term adverse effects.
“My overall take on this is that I do think it’s a promising paradigm-shifting therapy that really needs to move forward, at least scientifically. I’m excited about it, but I have a lot of skepticism about the entire body of data to make any more than the most narrow of approval,” Dr. Blaha said.
Susan S. Ellenberg, PhD, professor of biostatistics, medical ethics, and health policy at the University of Pennsylvania, Philadelphia, voted yes but also with difficulty.
“I really struggled with it. ... I was pushed by the very encouraging results of what is admittedly a very small study and something I can’t feel is completely definitive. But I would not like to deny the kind of people that we heard from today the opportunity to weigh their own risks and benefits to try this. And I would certainly agree that a very, very rigorous postmarketing program, preferably including another controlled trial, should be carried out.”
But David M. Nathan, MD, director of the Diabetes Center and Clinical Research Center, Massachusetts General Hospital, Boston, voted no.
“I struggled with this vote, tremendously, having listened carefully to the patients with type 1 diabetes ... but that said, having done clinical research for 40 years in type 1 diabetes, I think we need more data, both in terms of efficacy and of safety. I would hate a number of years down the road to figure out that we actually caused more harm than good, especially keeping in mind that the treatment of type 1 diabetes is evolving rapidly.”
A different perspective came from Mara L. Becker, MD, vice chair of the department of pediatric rheumatology at Duke University, Durham, N.C. She voted yes, pointing out that she’s accustomed to prescribing biologics for chronic conditions in children.
“I was unconflicted in my vote, which was yes. I thought the data ... were convincing and the need is great. I would support a label for children [aged 8 years] and older with at least stage 2 disease ... and I would require postmarketing safety surveillance to understand what the long-term side effects could be, but I would still be in favor of it.”
FDA advisory panel committee members are vetted for conflicts of interest and waivers granted for participation if necessary; none were granted for this meeting.
A version of this article first appeared on Medscape.com.
Sustained long-term benefit of gene therapy for SMA
, new long-term follow-up data show. At a median of 5.2 years since receiving the approved therapeutic dose, onasemnogene abeparvovec provided “sustained, durable efficacy, with all patients alive and without the need for permanent ventilation,” reported Jerry Mendell, MD, with the Center for Gene Therapy, Nationwide Children’s Hospital, Columbus, Ohio, and colleagues.
The study was published online May 17 in JAMA Neurology.
Single infusion
SMA is a rare genetic disease that can lead to paralysis, breathing difficulty, and death. The disorder is caused by a mutation in the survival motor neuron 1 (SMN1) gene, which encodes the SMN protein critical for maintenance and function of motor neurons.
In 2019, Zolgensma was approved in the United States for children with SMA and younger than 2 years of age.
Zolgensma is an adeno-associated virus vector-based gene therapy that addresses the genetic root cause of SMA by replacing the defective or missing SMN1 gene to halt disease progression. A single, one-time intravenous infusion results in expression of the SMN protein motor neurons, which improves chances of survival, as well as muscle movement and function.
In the phase 1 START study, 15 infants with SMA type 1 were treated with either a low or therapeutic dose of Zolgensma at Nationwide Children’s Hospital between 2014 and 2017.
The START long-term follow-up study (START LTFU) is an ongoing, observational study assessing safety and durability of response over 15 years in 13 of the infants; three infants received the low dose and 10 received the approved high dose.
Prior to baseline, four patients (40%) in the therapeutic dose cohort required noninvasive ventilatory support, and six (60%) did not require regular ventilatory support, which did not change in long-term follow-up.
All 10 patients who received the therapeutic dose remained alive and without the need for permanent ventilation up to 6.2 years after dosing, Dr. Mendell and colleagues report.
These patients also maintained previously acquired motor milestones. Two patients attained the new milestone of “standing with assistance” without the use of nusinersen (Spinraza, Biogen).
Serious adverse events occurred in eight patients (62%), none of which resulted in study discontinuation or death. The most common serious adverse events were related to the underlying SMA disease process and included acute respiratory failure (31%), pneumonia (31%), dehydration (23%), respiratory distress (15%), and bronchiolitis (15%).
Importantly, the investigators noted, no new safety signals or “adverse events of special interest” emerged during follow-up, including liver function enzyme elevations, new incidences of malignancy or hematologic disorders, and new incidences or exacerbations of existing neurologic or autoimmune disorders.
The investigators acknowledged that this follow-up study is limited by the small sample size of the patient population and confounded by treatment with nusinersen in several patients. “However, given that the two patients who acquired the new motor milestone of standing with assistance did not receive nusinersen at any time, this benefit can be attributed solely to onasemnogene abeparvovec,” Dr. Mendell and colleagues said.
The study was supported by Novartis Gene Therapies. Dr. Mendell and several co-investigators have disclosed financial relationships with the company.
A version of this article first appeared on Medscape.com.
, new long-term follow-up data show. At a median of 5.2 years since receiving the approved therapeutic dose, onasemnogene abeparvovec provided “sustained, durable efficacy, with all patients alive and without the need for permanent ventilation,” reported Jerry Mendell, MD, with the Center for Gene Therapy, Nationwide Children’s Hospital, Columbus, Ohio, and colleagues.
The study was published online May 17 in JAMA Neurology.
Single infusion
SMA is a rare genetic disease that can lead to paralysis, breathing difficulty, and death. The disorder is caused by a mutation in the survival motor neuron 1 (SMN1) gene, which encodes the SMN protein critical for maintenance and function of motor neurons.
In 2019, Zolgensma was approved in the United States for children with SMA and younger than 2 years of age.
Zolgensma is an adeno-associated virus vector-based gene therapy that addresses the genetic root cause of SMA by replacing the defective or missing SMN1 gene to halt disease progression. A single, one-time intravenous infusion results in expression of the SMN protein motor neurons, which improves chances of survival, as well as muscle movement and function.
In the phase 1 START study, 15 infants with SMA type 1 were treated with either a low or therapeutic dose of Zolgensma at Nationwide Children’s Hospital between 2014 and 2017.
The START long-term follow-up study (START LTFU) is an ongoing, observational study assessing safety and durability of response over 15 years in 13 of the infants; three infants received the low dose and 10 received the approved high dose.
Prior to baseline, four patients (40%) in the therapeutic dose cohort required noninvasive ventilatory support, and six (60%) did not require regular ventilatory support, which did not change in long-term follow-up.
All 10 patients who received the therapeutic dose remained alive and without the need for permanent ventilation up to 6.2 years after dosing, Dr. Mendell and colleagues report.
These patients also maintained previously acquired motor milestones. Two patients attained the new milestone of “standing with assistance” without the use of nusinersen (Spinraza, Biogen).
Serious adverse events occurred in eight patients (62%), none of which resulted in study discontinuation or death. The most common serious adverse events were related to the underlying SMA disease process and included acute respiratory failure (31%), pneumonia (31%), dehydration (23%), respiratory distress (15%), and bronchiolitis (15%).
Importantly, the investigators noted, no new safety signals or “adverse events of special interest” emerged during follow-up, including liver function enzyme elevations, new incidences of malignancy or hematologic disorders, and new incidences or exacerbations of existing neurologic or autoimmune disorders.
The investigators acknowledged that this follow-up study is limited by the small sample size of the patient population and confounded by treatment with nusinersen in several patients. “However, given that the two patients who acquired the new motor milestone of standing with assistance did not receive nusinersen at any time, this benefit can be attributed solely to onasemnogene abeparvovec,” Dr. Mendell and colleagues said.
The study was supported by Novartis Gene Therapies. Dr. Mendell and several co-investigators have disclosed financial relationships with the company.
A version of this article first appeared on Medscape.com.
, new long-term follow-up data show. At a median of 5.2 years since receiving the approved therapeutic dose, onasemnogene abeparvovec provided “sustained, durable efficacy, with all patients alive and without the need for permanent ventilation,” reported Jerry Mendell, MD, with the Center for Gene Therapy, Nationwide Children’s Hospital, Columbus, Ohio, and colleagues.
The study was published online May 17 in JAMA Neurology.
Single infusion
SMA is a rare genetic disease that can lead to paralysis, breathing difficulty, and death. The disorder is caused by a mutation in the survival motor neuron 1 (SMN1) gene, which encodes the SMN protein critical for maintenance and function of motor neurons.
In 2019, Zolgensma was approved in the United States for children with SMA and younger than 2 years of age.
Zolgensma is an adeno-associated virus vector-based gene therapy that addresses the genetic root cause of SMA by replacing the defective or missing SMN1 gene to halt disease progression. A single, one-time intravenous infusion results in expression of the SMN protein motor neurons, which improves chances of survival, as well as muscle movement and function.
In the phase 1 START study, 15 infants with SMA type 1 were treated with either a low or therapeutic dose of Zolgensma at Nationwide Children’s Hospital between 2014 and 2017.
The START long-term follow-up study (START LTFU) is an ongoing, observational study assessing safety and durability of response over 15 years in 13 of the infants; three infants received the low dose and 10 received the approved high dose.
Prior to baseline, four patients (40%) in the therapeutic dose cohort required noninvasive ventilatory support, and six (60%) did not require regular ventilatory support, which did not change in long-term follow-up.
All 10 patients who received the therapeutic dose remained alive and without the need for permanent ventilation up to 6.2 years after dosing, Dr. Mendell and colleagues report.
These patients also maintained previously acquired motor milestones. Two patients attained the new milestone of “standing with assistance” without the use of nusinersen (Spinraza, Biogen).
Serious adverse events occurred in eight patients (62%), none of which resulted in study discontinuation or death. The most common serious adverse events were related to the underlying SMA disease process and included acute respiratory failure (31%), pneumonia (31%), dehydration (23%), respiratory distress (15%), and bronchiolitis (15%).
Importantly, the investigators noted, no new safety signals or “adverse events of special interest” emerged during follow-up, including liver function enzyme elevations, new incidences of malignancy or hematologic disorders, and new incidences or exacerbations of existing neurologic or autoimmune disorders.
The investigators acknowledged that this follow-up study is limited by the small sample size of the patient population and confounded by treatment with nusinersen in several patients. “However, given that the two patients who acquired the new motor milestone of standing with assistance did not receive nusinersen at any time, this benefit can be attributed solely to onasemnogene abeparvovec,” Dr. Mendell and colleagues said.
The study was supported by Novartis Gene Therapies. Dr. Mendell and several co-investigators have disclosed financial relationships with the company.
A version of this article first appeared on Medscape.com.
The COVID-19 pandemic and changes in pediatric respiratory and nonrespiratory illnesses
The COVID-19 pandemic upended the U.S. health care market and disrupted much of what was thought to be consistent and necessary hospital-based care for children. Early in the pandemic, clinics closed, elective surgeries were delayed, and well visits were postponed. Mitigation strategies were launched nationwide to limit the spread of SARS-CoV-2 including mask mandates, social distancing, shelter-in-place orders, and school closures. While these measures were enacted to target COVID-19, a potential off-target effect was reductions in transmission of other respiratory illness, and potentially nonrespiratory infectious illnesses and conditions exacerbated by acute infections.1 These measures have heavily impacted the pediatric population, wherein respiratory infections are common, and also because daycares and school can be hubs for disease transmission.2
To evaluate the effect of the COVID-19 pandemic on pediatric health care utilization, we performed a multicenter, cross-sectional study of 44 children’s hospitals using the Pediatric Health Information System (PHIS) database.3 Children aged 2 months to 18 years discharged from a PHIS hospital with nonsurgical diagnoses from Jan. 1 to Sept. 30 over a 4-year period (2017-2020) were included in the study. The primary exposure was the 2020 COVID-19 pandemic, which was divided into three study periods: pre–COVID-19 (January–February 2020), early COVID-19 (March-April 2020), and COVID-19 (May-September 2020). The primary outcomes were the observed-to-expected ratio of respiratory and nonrespiratory illness encounters of the study period, compared with the 3 years prior to the pandemic. For these calculations, the expected encounters for each period was derived from the same calendar periods from prepandemic years (2017-2019).
A total of 9,051,980 pediatric encounters were included in the analyses: 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. We found a 42% reduction in overall encounters during the COVID-19 period, compared with the 3 years prior to the pandemic, with a greater reduction in respiratory, compared with nonrespiratory illnesses, which decreased 62% and 38%, respectively. These reductions were consistent across geographic and encounter type (ED vs. hospitalization). The frequency of hospital-based encounters for common pediatric respiratory illnesses was substantially reduced, with reductions in asthma exacerbations (down 76%), pneumonia (down 81%), croup (down 84%), influenza (down 87%) and bronchiolitis (down 91%). Differences in both respiratory and nonrespiratory illnesses varied by age, with larger reductions found in children aged less than 12 years. While adolescent (children aged over 12 years) encounters diminished during the early COVID period for both respiratory and nonrespiratory illnesses, their encounters returned to previous levels faster than those from younger children. For respiratory illnesses, hospital-based adolescents encounters had returned to prepandemic levels by the end of the study period (September 2020).
These findings warrant consideration as relaxation of SARS-CoV-2 mitigation are contemplated. Encounters for respiratory and nonrespiratory illnesses declined less and recovered faster in adolescents, compared with younger children. The underlying contributors to this trend are likely multifactorial. For example, respiratory illnesses such as croup and bronchiolitis are more common in younger children and adolescents may be more likely to transmit SARS-CoV-2, compared with younger age groups.4,5 However, adolescents may have had less strict adherence to social distancing measures.6 Future efforts to halt transmission of SARS-CoV-2, as well as other respiratory pathogens, should inform mitigation efforts in the adolescent population with considerations of the intensity of social mixing in different pediatric age groups.
While reductions in encounters caused by respiratory illnesses were substantial, more modest but similar age-based trends were seen in nonrespiratory illnesses. Yet, reduced transmission of infectious agents may not fully explain these findings. For example, it is possible that families sought care for mild to moderate nonrespiratory illness in clinics or via telehealth rather than the EDs.7 Provided there were no unintended negative consequences, such transition of care to non-ED settings would suggest there was overutilization of hospital resources prior to the pandemic. Additional assessments would be helpful to examine this more closely and to clarify the long-term impact of those transitions.
It is also possible that the pandemic effects on financial, social, and family stress may have led to increases in some pediatric health care encounters, such as those for mental health conditions,8 nonaccidental trauma or inability to adhere to treatment because of lack of resources.9,10 Additional study on the evolution and distribution of social and stress-related illnesses is critical to maintain and improve the health of children and adolescents.
The COVID-19 pandemic resulted in rapid and marked changes to both communicable and noncommunicable illnesses and care-seeking behaviors. Some of these findings are encouraging, such as large reductions in respiratory and nonrespiratory illnesses. However, other trends may be harbingers of negative health consequences of the pandemic, such as increases in health care utilization later in the pandemic. Further study of the evolving pandemic’s effects on disease and health care utilization is needed to benefit our children now and during the next pandemic.
Dr. Antoon is an assistant professor of pediatrics at Vanderbilt University and a pediatric hospitalist at the Monroe Carroll Jr. Children’s Hospital at Vanderbilt, both in Nashville, Tenn.
References
1. Kenyon CC et al. Initial effects of the COVID-19 pandemic on pediatric asthma emergency department utilization. J Allergy Clin Immunol Pract. 2020 Sep;8(8):2774-6.e1. doi: 10.1016/j.jaip.2020.05.045.
2. Luca G et al. The impact of regular school closure on seasonal influenza epidemics: A data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. doi: 10.1186/s12879-017-2934-3.
3. Antoon JW et al. The COVID-19 Pandemic and changes in healthcare utilization for pediatric respiratory and nonrespiratory illnesses in the United States. J Hosp Med. 2021 Mar 8. doi: 10.12788/jhm.3608.
4. Park YJ et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020 Oct;26(10):2465-8. doi: 10.3201/eid2610.201315.
5. Davies NG et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020 Aug;26(8):1205-11. doi: 10.1038/s41591-020-0962-9.
6. Andrews JL et al. Peer influence in adolescence: Public health implications for COVID-19. Trends Cogn Sci. 2020;24(8):585-7. doi: 10.1016/j.tics.2020.05.001.
7. Taquechel K et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020 Nov-Dec;8(10):3378-87.e11. doi: 10.1016/j.jaip.2020.07.057.
8. Hill RM et al. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. 2021;147(3):e2020029280. doi: 10.1542/peds.2020-029280.
9. Sharma S et al. COVID-19: Differences in sentinel injury and child abuse reporting during a pandemic. Child Abuse Negl. 2020 Dec;110:104709. doi: 10.1016/j.chiabu.2020.104709.
10. Lauren BN et al. Predictors of households at risk for food insecurity in the United States during the COVID-19 pandemic. Public Health Nutr. 2021 Jan 27. doi: 10.1017/S1368980021000355.
The COVID-19 pandemic upended the U.S. health care market and disrupted much of what was thought to be consistent and necessary hospital-based care for children. Early in the pandemic, clinics closed, elective surgeries were delayed, and well visits were postponed. Mitigation strategies were launched nationwide to limit the spread of SARS-CoV-2 including mask mandates, social distancing, shelter-in-place orders, and school closures. While these measures were enacted to target COVID-19, a potential off-target effect was reductions in transmission of other respiratory illness, and potentially nonrespiratory infectious illnesses and conditions exacerbated by acute infections.1 These measures have heavily impacted the pediatric population, wherein respiratory infections are common, and also because daycares and school can be hubs for disease transmission.2
To evaluate the effect of the COVID-19 pandemic on pediatric health care utilization, we performed a multicenter, cross-sectional study of 44 children’s hospitals using the Pediatric Health Information System (PHIS) database.3 Children aged 2 months to 18 years discharged from a PHIS hospital with nonsurgical diagnoses from Jan. 1 to Sept. 30 over a 4-year period (2017-2020) were included in the study. The primary exposure was the 2020 COVID-19 pandemic, which was divided into three study periods: pre–COVID-19 (January–February 2020), early COVID-19 (March-April 2020), and COVID-19 (May-September 2020). The primary outcomes were the observed-to-expected ratio of respiratory and nonrespiratory illness encounters of the study period, compared with the 3 years prior to the pandemic. For these calculations, the expected encounters for each period was derived from the same calendar periods from prepandemic years (2017-2019).
A total of 9,051,980 pediatric encounters were included in the analyses: 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. We found a 42% reduction in overall encounters during the COVID-19 period, compared with the 3 years prior to the pandemic, with a greater reduction in respiratory, compared with nonrespiratory illnesses, which decreased 62% and 38%, respectively. These reductions were consistent across geographic and encounter type (ED vs. hospitalization). The frequency of hospital-based encounters for common pediatric respiratory illnesses was substantially reduced, with reductions in asthma exacerbations (down 76%), pneumonia (down 81%), croup (down 84%), influenza (down 87%) and bronchiolitis (down 91%). Differences in both respiratory and nonrespiratory illnesses varied by age, with larger reductions found in children aged less than 12 years. While adolescent (children aged over 12 years) encounters diminished during the early COVID period for both respiratory and nonrespiratory illnesses, their encounters returned to previous levels faster than those from younger children. For respiratory illnesses, hospital-based adolescents encounters had returned to prepandemic levels by the end of the study period (September 2020).
These findings warrant consideration as relaxation of SARS-CoV-2 mitigation are contemplated. Encounters for respiratory and nonrespiratory illnesses declined less and recovered faster in adolescents, compared with younger children. The underlying contributors to this trend are likely multifactorial. For example, respiratory illnesses such as croup and bronchiolitis are more common in younger children and adolescents may be more likely to transmit SARS-CoV-2, compared with younger age groups.4,5 However, adolescents may have had less strict adherence to social distancing measures.6 Future efforts to halt transmission of SARS-CoV-2, as well as other respiratory pathogens, should inform mitigation efforts in the adolescent population with considerations of the intensity of social mixing in different pediatric age groups.
While reductions in encounters caused by respiratory illnesses were substantial, more modest but similar age-based trends were seen in nonrespiratory illnesses. Yet, reduced transmission of infectious agents may not fully explain these findings. For example, it is possible that families sought care for mild to moderate nonrespiratory illness in clinics or via telehealth rather than the EDs.7 Provided there were no unintended negative consequences, such transition of care to non-ED settings would suggest there was overutilization of hospital resources prior to the pandemic. Additional assessments would be helpful to examine this more closely and to clarify the long-term impact of those transitions.
It is also possible that the pandemic effects on financial, social, and family stress may have led to increases in some pediatric health care encounters, such as those for mental health conditions,8 nonaccidental trauma or inability to adhere to treatment because of lack of resources.9,10 Additional study on the evolution and distribution of social and stress-related illnesses is critical to maintain and improve the health of children and adolescents.
The COVID-19 pandemic resulted in rapid and marked changes to both communicable and noncommunicable illnesses and care-seeking behaviors. Some of these findings are encouraging, such as large reductions in respiratory and nonrespiratory illnesses. However, other trends may be harbingers of negative health consequences of the pandemic, such as increases in health care utilization later in the pandemic. Further study of the evolving pandemic’s effects on disease and health care utilization is needed to benefit our children now and during the next pandemic.
Dr. Antoon is an assistant professor of pediatrics at Vanderbilt University and a pediatric hospitalist at the Monroe Carroll Jr. Children’s Hospital at Vanderbilt, both in Nashville, Tenn.
References
1. Kenyon CC et al. Initial effects of the COVID-19 pandemic on pediatric asthma emergency department utilization. J Allergy Clin Immunol Pract. 2020 Sep;8(8):2774-6.e1. doi: 10.1016/j.jaip.2020.05.045.
2. Luca G et al. The impact of regular school closure on seasonal influenza epidemics: A data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. doi: 10.1186/s12879-017-2934-3.
3. Antoon JW et al. The COVID-19 Pandemic and changes in healthcare utilization for pediatric respiratory and nonrespiratory illnesses in the United States. J Hosp Med. 2021 Mar 8. doi: 10.12788/jhm.3608.
4. Park YJ et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020 Oct;26(10):2465-8. doi: 10.3201/eid2610.201315.
5. Davies NG et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020 Aug;26(8):1205-11. doi: 10.1038/s41591-020-0962-9.
6. Andrews JL et al. Peer influence in adolescence: Public health implications for COVID-19. Trends Cogn Sci. 2020;24(8):585-7. doi: 10.1016/j.tics.2020.05.001.
7. Taquechel K et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020 Nov-Dec;8(10):3378-87.e11. doi: 10.1016/j.jaip.2020.07.057.
8. Hill RM et al. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. 2021;147(3):e2020029280. doi: 10.1542/peds.2020-029280.
9. Sharma S et al. COVID-19: Differences in sentinel injury and child abuse reporting during a pandemic. Child Abuse Negl. 2020 Dec;110:104709. doi: 10.1016/j.chiabu.2020.104709.
10. Lauren BN et al. Predictors of households at risk for food insecurity in the United States during the COVID-19 pandemic. Public Health Nutr. 2021 Jan 27. doi: 10.1017/S1368980021000355.
The COVID-19 pandemic upended the U.S. health care market and disrupted much of what was thought to be consistent and necessary hospital-based care for children. Early in the pandemic, clinics closed, elective surgeries were delayed, and well visits were postponed. Mitigation strategies were launched nationwide to limit the spread of SARS-CoV-2 including mask mandates, social distancing, shelter-in-place orders, and school closures. While these measures were enacted to target COVID-19, a potential off-target effect was reductions in transmission of other respiratory illness, and potentially nonrespiratory infectious illnesses and conditions exacerbated by acute infections.1 These measures have heavily impacted the pediatric population, wherein respiratory infections are common, and also because daycares and school can be hubs for disease transmission.2
To evaluate the effect of the COVID-19 pandemic on pediatric health care utilization, we performed a multicenter, cross-sectional study of 44 children’s hospitals using the Pediatric Health Information System (PHIS) database.3 Children aged 2 months to 18 years discharged from a PHIS hospital with nonsurgical diagnoses from Jan. 1 to Sept. 30 over a 4-year period (2017-2020) were included in the study. The primary exposure was the 2020 COVID-19 pandemic, which was divided into three study periods: pre–COVID-19 (January–February 2020), early COVID-19 (March-April 2020), and COVID-19 (May-September 2020). The primary outcomes were the observed-to-expected ratio of respiratory and nonrespiratory illness encounters of the study period, compared with the 3 years prior to the pandemic. For these calculations, the expected encounters for each period was derived from the same calendar periods from prepandemic years (2017-2019).
A total of 9,051,980 pediatric encounters were included in the analyses: 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. We found a 42% reduction in overall encounters during the COVID-19 period, compared with the 3 years prior to the pandemic, with a greater reduction in respiratory, compared with nonrespiratory illnesses, which decreased 62% and 38%, respectively. These reductions were consistent across geographic and encounter type (ED vs. hospitalization). The frequency of hospital-based encounters for common pediatric respiratory illnesses was substantially reduced, with reductions in asthma exacerbations (down 76%), pneumonia (down 81%), croup (down 84%), influenza (down 87%) and bronchiolitis (down 91%). Differences in both respiratory and nonrespiratory illnesses varied by age, with larger reductions found in children aged less than 12 years. While adolescent (children aged over 12 years) encounters diminished during the early COVID period for both respiratory and nonrespiratory illnesses, their encounters returned to previous levels faster than those from younger children. For respiratory illnesses, hospital-based adolescents encounters had returned to prepandemic levels by the end of the study period (September 2020).
These findings warrant consideration as relaxation of SARS-CoV-2 mitigation are contemplated. Encounters for respiratory and nonrespiratory illnesses declined less and recovered faster in adolescents, compared with younger children. The underlying contributors to this trend are likely multifactorial. For example, respiratory illnesses such as croup and bronchiolitis are more common in younger children and adolescents may be more likely to transmit SARS-CoV-2, compared with younger age groups.4,5 However, adolescents may have had less strict adherence to social distancing measures.6 Future efforts to halt transmission of SARS-CoV-2, as well as other respiratory pathogens, should inform mitigation efforts in the adolescent population with considerations of the intensity of social mixing in different pediatric age groups.
While reductions in encounters caused by respiratory illnesses were substantial, more modest but similar age-based trends were seen in nonrespiratory illnesses. Yet, reduced transmission of infectious agents may not fully explain these findings. For example, it is possible that families sought care for mild to moderate nonrespiratory illness in clinics or via telehealth rather than the EDs.7 Provided there were no unintended negative consequences, such transition of care to non-ED settings would suggest there was overutilization of hospital resources prior to the pandemic. Additional assessments would be helpful to examine this more closely and to clarify the long-term impact of those transitions.
It is also possible that the pandemic effects on financial, social, and family stress may have led to increases in some pediatric health care encounters, such as those for mental health conditions,8 nonaccidental trauma or inability to adhere to treatment because of lack of resources.9,10 Additional study on the evolution and distribution of social and stress-related illnesses is critical to maintain and improve the health of children and adolescents.
The COVID-19 pandemic resulted in rapid and marked changes to both communicable and noncommunicable illnesses and care-seeking behaviors. Some of these findings are encouraging, such as large reductions in respiratory and nonrespiratory illnesses. However, other trends may be harbingers of negative health consequences of the pandemic, such as increases in health care utilization later in the pandemic. Further study of the evolving pandemic’s effects on disease and health care utilization is needed to benefit our children now and during the next pandemic.
Dr. Antoon is an assistant professor of pediatrics at Vanderbilt University and a pediatric hospitalist at the Monroe Carroll Jr. Children’s Hospital at Vanderbilt, both in Nashville, Tenn.
References
1. Kenyon CC et al. Initial effects of the COVID-19 pandemic on pediatric asthma emergency department utilization. J Allergy Clin Immunol Pract. 2020 Sep;8(8):2774-6.e1. doi: 10.1016/j.jaip.2020.05.045.
2. Luca G et al. The impact of regular school closure on seasonal influenza epidemics: A data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. doi: 10.1186/s12879-017-2934-3.
3. Antoon JW et al. The COVID-19 Pandemic and changes in healthcare utilization for pediatric respiratory and nonrespiratory illnesses in the United States. J Hosp Med. 2021 Mar 8. doi: 10.12788/jhm.3608.
4. Park YJ et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020 Oct;26(10):2465-8. doi: 10.3201/eid2610.201315.
5. Davies NG et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020 Aug;26(8):1205-11. doi: 10.1038/s41591-020-0962-9.
6. Andrews JL et al. Peer influence in adolescence: Public health implications for COVID-19. Trends Cogn Sci. 2020;24(8):585-7. doi: 10.1016/j.tics.2020.05.001.
7. Taquechel K et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020 Nov-Dec;8(10):3378-87.e11. doi: 10.1016/j.jaip.2020.07.057.
8. Hill RM et al. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. 2021;147(3):e2020029280. doi: 10.1542/peds.2020-029280.
9. Sharma S et al. COVID-19: Differences in sentinel injury and child abuse reporting during a pandemic. Child Abuse Negl. 2020 Dec;110:104709. doi: 10.1016/j.chiabu.2020.104709.
10. Lauren BN et al. Predictors of households at risk for food insecurity in the United States during the COVID-19 pandemic. Public Health Nutr. 2021 Jan 27. doi: 10.1017/S1368980021000355.
New allergy guidelines call for end to food bans in schools
Children with food allergies often require diligent monitoring and a restricted diet to reduce allergic attacks, but there is little evidence available to support so-called “food bans” at schools and childcare centers.
Instead, a new practice guideline published earlier this month in the Journal of Allergy and Clinical Immunology calls for better allergy management training for staff, as well as increased epinephrine availability in educational environments. The guidelines were developed by an international panel of clinicians, school personnel, and parents.
The guidance at a glance
Rather than creating site-wide food prohibitions on nuts, dairy, and other allergenic foods, the practice guidance recommends centers and schools use “common-sense approaches” to reduce allergic reaction risk among school-aged children. According to the guideline authors, these strategies could include promoting handwashing, providing adult supervision during snacks and meals, and cleaning surfaces where food is either eaten or prepared.
Additionally, the new evidence-based guidance calls for schools and childcare centers to teach school personnel to recognize, prevent, and respond appropriately to food-related allergic reactions when they do occur.
The guidance also recommends that educational institutions require an up-to-date allergy ‘action plan’ from parents, designed for their children with allergies. These action plans can be integrated into the training of teachers and nurses to help manage potential allergic reactions.
Moreover, the guidance suggests schools should keep unassigned epinephrine autoinjectors in stock, both on site and even when traveling, where laws permit, rather than requiring students with allergies to bring in their own autoinjectors. Ultimately, this represents a more proactive approach to treating anaphylaxis, particularly in settings where treatment is urgently needed, such as when students are away from campus and participating in a school-designated trip or event.
Expert perspectives
Jennifer A. Dantzer, MD, MHS, allergist-immunologist and assistant professor of pediatrics at Johns Hopkins University, Baltimore, told this news organization via email that the practice guidelines offer an important starting point for ensuring quality of life of students, parents, and other school personnel.
While the Centers for Disease Control and Prevention published voluntary guidance for managing food allergies in schools back in 2013, there has since “been a lack of universal policies and procedures to manage the risk of allergic reactions in schools,” explained Dr. Dantzer. “The new guidelines are a good first step of using available evidence and all the key stakeholders, clinicians, school personnel, and families to figure out the best way to keep children with food allergies safe at school.”
Dr. Dantzer wasn’t involved in the creation of the new practice guidelines, but she shared how her clinical experience reinforces the need for the evidence-based recommendations. “Every single week we talk with families, both in clinic and in our research studies, about living with food allergies, and we recognize that every child is different,” she said. “We constantly work to advocate for each individual child with food allergies.”
Pediatric allergist Malika Gupta, MBBS, MD, said in an interview via email that the guidelines could assist in the creation of new nationwide policies for food allergy management at schools. “Also, the guidelines are labeled ‘conditional,’ which gives policymakers the ability to adapt to their specific circumstances and individuals, as well as make modifications according to regional trends,” she added.
Dr. Gupta, a clinical assistant professor in the Division of Allergy and Clinical Immunology at the University of Michigan, Ann Arbor, echoed the guideline panel’s sentiments regarding food bans, explaining that prohibiting certain foods could lend a “false sense of security” and could also “promote bullying and a sense of isolation for the food-allergic child.” In spite of the lack of evidence supporting food bans, Dr. Gupta noted that these bans can give families a sense of control and security. Ideally, more research should be performed to determine whether food bans actually work, she added.
In addition to promoting the new guidelines, allergists and pediatricians can also implement proactive allergy reaction mitigation strategies that work with school systems, according to Dr. Gupta. “In-clinic, we ensure all families have food allergy action plans for school and current epinephrine auto-injectors,” she said. “We also often have our food allergy nurses educate schools when food allergy awareness is a concern.”
Many of the 25 authors of the food allergy guidelines disclosed relevant financial relationships. The full list is with the original article. According to a footnote within the guidelines, “Panel members who were deemed to have a real, perceived, or potential conflict of interest were asked to abstain from voting on recommendations related to that interest.”
A version of this article first appeared on Medscape.com.
Children with food allergies often require diligent monitoring and a restricted diet to reduce allergic attacks, but there is little evidence available to support so-called “food bans” at schools and childcare centers.
Instead, a new practice guideline published earlier this month in the Journal of Allergy and Clinical Immunology calls for better allergy management training for staff, as well as increased epinephrine availability in educational environments. The guidelines were developed by an international panel of clinicians, school personnel, and parents.
The guidance at a glance
Rather than creating site-wide food prohibitions on nuts, dairy, and other allergenic foods, the practice guidance recommends centers and schools use “common-sense approaches” to reduce allergic reaction risk among school-aged children. According to the guideline authors, these strategies could include promoting handwashing, providing adult supervision during snacks and meals, and cleaning surfaces where food is either eaten or prepared.
Additionally, the new evidence-based guidance calls for schools and childcare centers to teach school personnel to recognize, prevent, and respond appropriately to food-related allergic reactions when they do occur.
The guidance also recommends that educational institutions require an up-to-date allergy ‘action plan’ from parents, designed for their children with allergies. These action plans can be integrated into the training of teachers and nurses to help manage potential allergic reactions.
Moreover, the guidance suggests schools should keep unassigned epinephrine autoinjectors in stock, both on site and even when traveling, where laws permit, rather than requiring students with allergies to bring in their own autoinjectors. Ultimately, this represents a more proactive approach to treating anaphylaxis, particularly in settings where treatment is urgently needed, such as when students are away from campus and participating in a school-designated trip or event.
Expert perspectives
Jennifer A. Dantzer, MD, MHS, allergist-immunologist and assistant professor of pediatrics at Johns Hopkins University, Baltimore, told this news organization via email that the practice guidelines offer an important starting point for ensuring quality of life of students, parents, and other school personnel.
While the Centers for Disease Control and Prevention published voluntary guidance for managing food allergies in schools back in 2013, there has since “been a lack of universal policies and procedures to manage the risk of allergic reactions in schools,” explained Dr. Dantzer. “The new guidelines are a good first step of using available evidence and all the key stakeholders, clinicians, school personnel, and families to figure out the best way to keep children with food allergies safe at school.”
Dr. Dantzer wasn’t involved in the creation of the new practice guidelines, but she shared how her clinical experience reinforces the need for the evidence-based recommendations. “Every single week we talk with families, both in clinic and in our research studies, about living with food allergies, and we recognize that every child is different,” she said. “We constantly work to advocate for each individual child with food allergies.”
Pediatric allergist Malika Gupta, MBBS, MD, said in an interview via email that the guidelines could assist in the creation of new nationwide policies for food allergy management at schools. “Also, the guidelines are labeled ‘conditional,’ which gives policymakers the ability to adapt to their specific circumstances and individuals, as well as make modifications according to regional trends,” she added.
Dr. Gupta, a clinical assistant professor in the Division of Allergy and Clinical Immunology at the University of Michigan, Ann Arbor, echoed the guideline panel’s sentiments regarding food bans, explaining that prohibiting certain foods could lend a “false sense of security” and could also “promote bullying and a sense of isolation for the food-allergic child.” In spite of the lack of evidence supporting food bans, Dr. Gupta noted that these bans can give families a sense of control and security. Ideally, more research should be performed to determine whether food bans actually work, she added.
In addition to promoting the new guidelines, allergists and pediatricians can also implement proactive allergy reaction mitigation strategies that work with school systems, according to Dr. Gupta. “In-clinic, we ensure all families have food allergy action plans for school and current epinephrine auto-injectors,” she said. “We also often have our food allergy nurses educate schools when food allergy awareness is a concern.”
Many of the 25 authors of the food allergy guidelines disclosed relevant financial relationships. The full list is with the original article. According to a footnote within the guidelines, “Panel members who were deemed to have a real, perceived, or potential conflict of interest were asked to abstain from voting on recommendations related to that interest.”
A version of this article first appeared on Medscape.com.
Children with food allergies often require diligent monitoring and a restricted diet to reduce allergic attacks, but there is little evidence available to support so-called “food bans” at schools and childcare centers.
Instead, a new practice guideline published earlier this month in the Journal of Allergy and Clinical Immunology calls for better allergy management training for staff, as well as increased epinephrine availability in educational environments. The guidelines were developed by an international panel of clinicians, school personnel, and parents.
The guidance at a glance
Rather than creating site-wide food prohibitions on nuts, dairy, and other allergenic foods, the practice guidance recommends centers and schools use “common-sense approaches” to reduce allergic reaction risk among school-aged children. According to the guideline authors, these strategies could include promoting handwashing, providing adult supervision during snacks and meals, and cleaning surfaces where food is either eaten or prepared.
Additionally, the new evidence-based guidance calls for schools and childcare centers to teach school personnel to recognize, prevent, and respond appropriately to food-related allergic reactions when they do occur.
The guidance also recommends that educational institutions require an up-to-date allergy ‘action plan’ from parents, designed for their children with allergies. These action plans can be integrated into the training of teachers and nurses to help manage potential allergic reactions.
Moreover, the guidance suggests schools should keep unassigned epinephrine autoinjectors in stock, both on site and even when traveling, where laws permit, rather than requiring students with allergies to bring in their own autoinjectors. Ultimately, this represents a more proactive approach to treating anaphylaxis, particularly in settings where treatment is urgently needed, such as when students are away from campus and participating in a school-designated trip or event.
Expert perspectives
Jennifer A. Dantzer, MD, MHS, allergist-immunologist and assistant professor of pediatrics at Johns Hopkins University, Baltimore, told this news organization via email that the practice guidelines offer an important starting point for ensuring quality of life of students, parents, and other school personnel.
While the Centers for Disease Control and Prevention published voluntary guidance for managing food allergies in schools back in 2013, there has since “been a lack of universal policies and procedures to manage the risk of allergic reactions in schools,” explained Dr. Dantzer. “The new guidelines are a good first step of using available evidence and all the key stakeholders, clinicians, school personnel, and families to figure out the best way to keep children with food allergies safe at school.”
Dr. Dantzer wasn’t involved in the creation of the new practice guidelines, but she shared how her clinical experience reinforces the need for the evidence-based recommendations. “Every single week we talk with families, both in clinic and in our research studies, about living with food allergies, and we recognize that every child is different,” she said. “We constantly work to advocate for each individual child with food allergies.”
Pediatric allergist Malika Gupta, MBBS, MD, said in an interview via email that the guidelines could assist in the creation of new nationwide policies for food allergy management at schools. “Also, the guidelines are labeled ‘conditional,’ which gives policymakers the ability to adapt to their specific circumstances and individuals, as well as make modifications according to regional trends,” she added.
Dr. Gupta, a clinical assistant professor in the Division of Allergy and Clinical Immunology at the University of Michigan, Ann Arbor, echoed the guideline panel’s sentiments regarding food bans, explaining that prohibiting certain foods could lend a “false sense of security” and could also “promote bullying and a sense of isolation for the food-allergic child.” In spite of the lack of evidence supporting food bans, Dr. Gupta noted that these bans can give families a sense of control and security. Ideally, more research should be performed to determine whether food bans actually work, she added.
In addition to promoting the new guidelines, allergists and pediatricians can also implement proactive allergy reaction mitigation strategies that work with school systems, according to Dr. Gupta. “In-clinic, we ensure all families have food allergy action plans for school and current epinephrine auto-injectors,” she said. “We also often have our food allergy nurses educate schools when food allergy awareness is a concern.”
Many of the 25 authors of the food allergy guidelines disclosed relevant financial relationships. The full list is with the original article. According to a footnote within the guidelines, “Panel members who were deemed to have a real, perceived, or potential conflict of interest were asked to abstain from voting on recommendations related to that interest.”
A version of this article first appeared on Medscape.com.
COVID-19 vaccination rate rising quickly among adolescents
With nearly half of all Americans having received at least one dose of a COVID-19 vaccine, the youngest eligible group is beginning to overcome its late start, according to data from the Centers for Disease Control and Prevention.
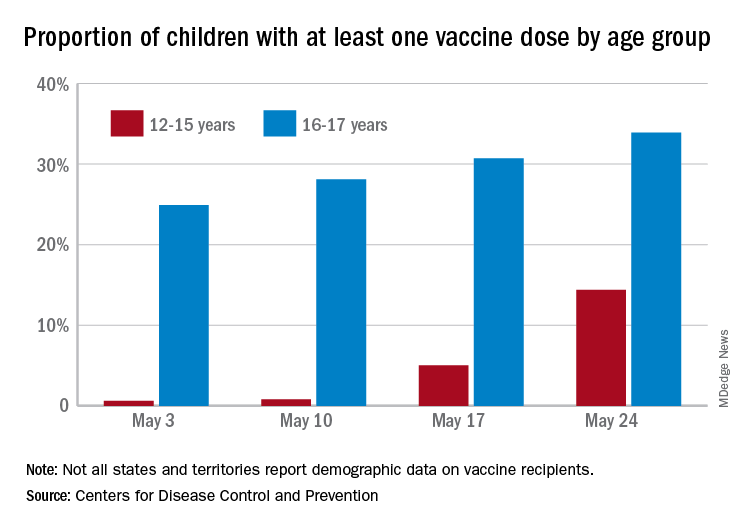
As of May 24, 49.4% of the U.S. population – that’s almost 164 million people – has received at least one dose of vaccine. The corresponding figure for children aged 12-15 years is 14.4%, but that’s up from only 0.6% just 3 weeks before. Among children aged 16-17, who’ve been getting vaccinated since early April in some states, the proportion receiving at least one dose went from 24.9% to 33.9% over those same 3 weeks, the CDC said on its COVID Data Tracker site.
The comparatively rapid increase among the younger group of eligible children can be seen over the last 14 days. To put that into perspective, only those aged 25-39 years were higher at 21.9%, while 18-24 (12.1%), 40-49 (13.4%), 50-64 (18.2%), 65-74 (5.3%), and ≥75 (2.9%) were all lower.
The 12- to 15-year-olds are further behind when it comes to full vaccination status, however, with just 0.6% having received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 21.6% for those aged 16-17 years. Children aged 12-15 make up 5% of the total U.S. population but just 0.1% of all those who have been fully vaccinated versus 2.5% and 1.4%, respectively, for those aged 16-17, the CDC reported.
With nearly half of all Americans having received at least one dose of a COVID-19 vaccine, the youngest eligible group is beginning to overcome its late start, according to data from the Centers for Disease Control and Prevention.

As of May 24, 49.4% of the U.S. population – that’s almost 164 million people – has received at least one dose of vaccine. The corresponding figure for children aged 12-15 years is 14.4%, but that’s up from only 0.6% just 3 weeks before. Among children aged 16-17, who’ve been getting vaccinated since early April in some states, the proportion receiving at least one dose went from 24.9% to 33.9% over those same 3 weeks, the CDC said on its COVID Data Tracker site.
The comparatively rapid increase among the younger group of eligible children can be seen over the last 14 days. To put that into perspective, only those aged 25-39 years were higher at 21.9%, while 18-24 (12.1%), 40-49 (13.4%), 50-64 (18.2%), 65-74 (5.3%), and ≥75 (2.9%) were all lower.
The 12- to 15-year-olds are further behind when it comes to full vaccination status, however, with just 0.6% having received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 21.6% for those aged 16-17 years. Children aged 12-15 make up 5% of the total U.S. population but just 0.1% of all those who have been fully vaccinated versus 2.5% and 1.4%, respectively, for those aged 16-17, the CDC reported.
With nearly half of all Americans having received at least one dose of a COVID-19 vaccine, the youngest eligible group is beginning to overcome its late start, according to data from the Centers for Disease Control and Prevention.

As of May 24, 49.4% of the U.S. population – that’s almost 164 million people – has received at least one dose of vaccine. The corresponding figure for children aged 12-15 years is 14.4%, but that’s up from only 0.6% just 3 weeks before. Among children aged 16-17, who’ve been getting vaccinated since early April in some states, the proportion receiving at least one dose went from 24.9% to 33.9% over those same 3 weeks, the CDC said on its COVID Data Tracker site.
The comparatively rapid increase among the younger group of eligible children can be seen over the last 14 days. To put that into perspective, only those aged 25-39 years were higher at 21.9%, while 18-24 (12.1%), 40-49 (13.4%), 50-64 (18.2%), 65-74 (5.3%), and ≥75 (2.9%) were all lower.
The 12- to 15-year-olds are further behind when it comes to full vaccination status, however, with just 0.6% having received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 21.6% for those aged 16-17 years. Children aged 12-15 make up 5% of the total U.S. population but just 0.1% of all those who have been fully vaccinated versus 2.5% and 1.4%, respectively, for those aged 16-17, the CDC reported.
Skip routine probiotics for preemies, AAP says
The American Academy of Pediatrics now recommends against the routine administration of probiotics to preterm infants, particularly the most vulnerable (those whose birth weight is <1,000 g), for the treatment or prevention of necrotizing enterocolitis (NEC) and late-onset sepsis.
Although probiotics are increasingly given to preterm infants, the AAP notes that the data on their safety and efficacy are inconsistent. In addition, the supplements are not subject to approval by the Food and Drug Administration.
Therefore, the academy advises clinicians to use extreme caution in selecting preterm neonates to receive these microorganisms and recommends obtaining informed consent from parents after carefully discussing the risks. It also recommends that centers using probiotics conduct surveillance, inasmuch as probiotics can alter a center’s flora, potentially affecting all patients. Such centers should also carefully document outcomes, adverse events, and safety.
The AAP’s clinical report, published online May 24 in Pediatrics, highlights wide differences between commercially available formulations and a lack of regulatory standards in this country.
Absent FDA-approved drug labeling, these nutritional supplements cannot be marketed as treatment or prophylaxis, but that has scarcely stopped their use. “Despite lack of availability of a pharmaceutical-grade product, the number of preterm infants receiving probiotics in the United States and Canada is steadily increasing,” wrote Brenda Poindexter, MD, FAAP, chief of neonatology at Children’s Healthcare of Atlanta, and members of the AAP’s Committee on Fetus and Newborn.
Analyses of U.S. collaborative databases indicate that approximately 10% of neonates of extremely low gestational age receive a probiotic preparation in the neonatal intensive care unit (NICU). The use of these preparations varies widely across institutions.
“NEC is a devastating morbidity of prematurity, and it’s multifactorial. Some babies only given mother’s milk still get NEC, and the decision to use these products is a very nuanced one,” Dr. Poindexter said in an interview. “I suspect some people will disagree with the report, and we tried to give folks some wiggle room.”
Evidence from other countries suggests that probiotics can be protective against NEC, she added, “so not to have a reliable product in this country is very frustrating.”
Dr. Poindexter and colleagues pointed to a 2015 study that found that only 1 of 16 commercial products tested contained the exact organisms listed on their labels. One product contained none of the species listed on the label.
In light of increasing use, the AAP emphasizes the need for development of pharmaceutical-grade probiotics that would be rigorously evaluated for safety and efficacy.
The infant microbiome
Over the past decade, the gut microbiome has been increasingly recognized as a factor relevant to health and disease in preterm infants, the authors noted. Differences in the intestinal microbiota between full-term and preterm infants are substantial. The microbiome of preterm infants tends to include fewer bacterial species and greater proportions of potentially pathogenic strains.
Evidence of benefit from probiotics has been mixed. Some studies and pooled systematic analyses suggest a significant benefit. However, Dr. Poindexter and colleagues noted that some researchers express concerns about the study methods used, such as pooling results from trials that tested different probiotic strains or that had few infants in the highest risk category.
Whereas the potential for probiotic-related infection appears low, there does seem to be some risk for sepsis associated with colonization by a strain in a given product or from contamination with a pathogen during manufacturing, the report explains.
At least one trial found that a third of infants randomly assigned to receive placebo showed evidence of the probiotic strain.
“However, it may be difficult to distinguish the change in the infant from the change in the resident flora of the NICU,” the AAP panel wrote.
In addition, there have recently been several recalls of dietary supplement–grade probiotics for contamination, which have raised concerns. Pathogens include Salmonella, Rhizopus, and Penicillium species. Fatal gastrointestinal mucormycosis has also been reported in a preterm infant who received ABC Dophilus powder that was contaminated with the microfungus Rhizopus oryzae.
Other safety considerations, according to the authors, are the unknown longer-term effect of probiotics on preterm infants and the unknown impact of microorganisms on the microbiome over time.
Last year, the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition published a position paper with a conditional recommendation for selected probiotics to reduce NEC rates. “These guidelines would be applicable in the U.S. if we had products manufactured in a way that could guarantee that what’s on the label is in the bottle,” Dr. Poindexter said.
Asked for her perspective on the AAP clinical report, Erica Wymore, MD, assistant professor of neonatal and perinatal medicine at the University of Colorado at Denver, Aurora, called it “an excellent review of the current literature” that shows the inadequacy of data on the composition, dosage, timing, duration, and use of single-strain vs. multiple-strain probiotics to reduce NEC. Her clinical center, Children’s Hospital Colorado, does not administer probiotics to preterm babies.
Although guidelines can improve outcomes, said Dr. Wymore, who was not involved in the AAP report, improvement observed with probiotics results from more stringent care in centers that experience high NEC rates. “It’s hard to know if it’s due to the probiotics if they already have a high rate of NEC,” Dr. Wymore said.
She echoed the AAP’s position and stressed the need for extreme caution in giving these products to vulnerable infants with immature immune systems. Before that can be safely done, she said, “we need more FDA oversight of product composition [and] pharmaceutical-grade products, and more studies to determine efficacy.”
Added Dr. Poindexter: “Hopefully, this report will inform clinicians of the risks of using non–pharmaceutical-grade products and encourage industry to actually develop probiotics for neonates that we can feel comfortable using.”
The report received no external funding. Dr. Poindexter and Dr. Wymore have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The American Academy of Pediatrics now recommends against the routine administration of probiotics to preterm infants, particularly the most vulnerable (those whose birth weight is <1,000 g), for the treatment or prevention of necrotizing enterocolitis (NEC) and late-onset sepsis.
Although probiotics are increasingly given to preterm infants, the AAP notes that the data on their safety and efficacy are inconsistent. In addition, the supplements are not subject to approval by the Food and Drug Administration.
Therefore, the academy advises clinicians to use extreme caution in selecting preterm neonates to receive these microorganisms and recommends obtaining informed consent from parents after carefully discussing the risks. It also recommends that centers using probiotics conduct surveillance, inasmuch as probiotics can alter a center’s flora, potentially affecting all patients. Such centers should also carefully document outcomes, adverse events, and safety.
The AAP’s clinical report, published online May 24 in Pediatrics, highlights wide differences between commercially available formulations and a lack of regulatory standards in this country.
Absent FDA-approved drug labeling, these nutritional supplements cannot be marketed as treatment or prophylaxis, but that has scarcely stopped their use. “Despite lack of availability of a pharmaceutical-grade product, the number of preterm infants receiving probiotics in the United States and Canada is steadily increasing,” wrote Brenda Poindexter, MD, FAAP, chief of neonatology at Children’s Healthcare of Atlanta, and members of the AAP’s Committee on Fetus and Newborn.
Analyses of U.S. collaborative databases indicate that approximately 10% of neonates of extremely low gestational age receive a probiotic preparation in the neonatal intensive care unit (NICU). The use of these preparations varies widely across institutions.
“NEC is a devastating morbidity of prematurity, and it’s multifactorial. Some babies only given mother’s milk still get NEC, and the decision to use these products is a very nuanced one,” Dr. Poindexter said in an interview. “I suspect some people will disagree with the report, and we tried to give folks some wiggle room.”
Evidence from other countries suggests that probiotics can be protective against NEC, she added, “so not to have a reliable product in this country is very frustrating.”
Dr. Poindexter and colleagues pointed to a 2015 study that found that only 1 of 16 commercial products tested contained the exact organisms listed on their labels. One product contained none of the species listed on the label.
In light of increasing use, the AAP emphasizes the need for development of pharmaceutical-grade probiotics that would be rigorously evaluated for safety and efficacy.
The infant microbiome
Over the past decade, the gut microbiome has been increasingly recognized as a factor relevant to health and disease in preterm infants, the authors noted. Differences in the intestinal microbiota between full-term and preterm infants are substantial. The microbiome of preterm infants tends to include fewer bacterial species and greater proportions of potentially pathogenic strains.
Evidence of benefit from probiotics has been mixed. Some studies and pooled systematic analyses suggest a significant benefit. However, Dr. Poindexter and colleagues noted that some researchers express concerns about the study methods used, such as pooling results from trials that tested different probiotic strains or that had few infants in the highest risk category.
Whereas the potential for probiotic-related infection appears low, there does seem to be some risk for sepsis associated with colonization by a strain in a given product or from contamination with a pathogen during manufacturing, the report explains.
At least one trial found that a third of infants randomly assigned to receive placebo showed evidence of the probiotic strain.
“However, it may be difficult to distinguish the change in the infant from the change in the resident flora of the NICU,” the AAP panel wrote.
In addition, there have recently been several recalls of dietary supplement–grade probiotics for contamination, which have raised concerns. Pathogens include Salmonella, Rhizopus, and Penicillium species. Fatal gastrointestinal mucormycosis has also been reported in a preterm infant who received ABC Dophilus powder that was contaminated with the microfungus Rhizopus oryzae.
Other safety considerations, according to the authors, are the unknown longer-term effect of probiotics on preterm infants and the unknown impact of microorganisms on the microbiome over time.
Last year, the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition published a position paper with a conditional recommendation for selected probiotics to reduce NEC rates. “These guidelines would be applicable in the U.S. if we had products manufactured in a way that could guarantee that what’s on the label is in the bottle,” Dr. Poindexter said.
Asked for her perspective on the AAP clinical report, Erica Wymore, MD, assistant professor of neonatal and perinatal medicine at the University of Colorado at Denver, Aurora, called it “an excellent review of the current literature” that shows the inadequacy of data on the composition, dosage, timing, duration, and use of single-strain vs. multiple-strain probiotics to reduce NEC. Her clinical center, Children’s Hospital Colorado, does not administer probiotics to preterm babies.
Although guidelines can improve outcomes, said Dr. Wymore, who was not involved in the AAP report, improvement observed with probiotics results from more stringent care in centers that experience high NEC rates. “It’s hard to know if it’s due to the probiotics if they already have a high rate of NEC,” Dr. Wymore said.
She echoed the AAP’s position and stressed the need for extreme caution in giving these products to vulnerable infants with immature immune systems. Before that can be safely done, she said, “we need more FDA oversight of product composition [and] pharmaceutical-grade products, and more studies to determine efficacy.”
Added Dr. Poindexter: “Hopefully, this report will inform clinicians of the risks of using non–pharmaceutical-grade products and encourage industry to actually develop probiotics for neonates that we can feel comfortable using.”
The report received no external funding. Dr. Poindexter and Dr. Wymore have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The American Academy of Pediatrics now recommends against the routine administration of probiotics to preterm infants, particularly the most vulnerable (those whose birth weight is <1,000 g), for the treatment or prevention of necrotizing enterocolitis (NEC) and late-onset sepsis.
Although probiotics are increasingly given to preterm infants, the AAP notes that the data on their safety and efficacy are inconsistent. In addition, the supplements are not subject to approval by the Food and Drug Administration.
Therefore, the academy advises clinicians to use extreme caution in selecting preterm neonates to receive these microorganisms and recommends obtaining informed consent from parents after carefully discussing the risks. It also recommends that centers using probiotics conduct surveillance, inasmuch as probiotics can alter a center’s flora, potentially affecting all patients. Such centers should also carefully document outcomes, adverse events, and safety.
The AAP’s clinical report, published online May 24 in Pediatrics, highlights wide differences between commercially available formulations and a lack of regulatory standards in this country.
Absent FDA-approved drug labeling, these nutritional supplements cannot be marketed as treatment or prophylaxis, but that has scarcely stopped their use. “Despite lack of availability of a pharmaceutical-grade product, the number of preterm infants receiving probiotics in the United States and Canada is steadily increasing,” wrote Brenda Poindexter, MD, FAAP, chief of neonatology at Children’s Healthcare of Atlanta, and members of the AAP’s Committee on Fetus and Newborn.
Analyses of U.S. collaborative databases indicate that approximately 10% of neonates of extremely low gestational age receive a probiotic preparation in the neonatal intensive care unit (NICU). The use of these preparations varies widely across institutions.
“NEC is a devastating morbidity of prematurity, and it’s multifactorial. Some babies only given mother’s milk still get NEC, and the decision to use these products is a very nuanced one,” Dr. Poindexter said in an interview. “I suspect some people will disagree with the report, and we tried to give folks some wiggle room.”
Evidence from other countries suggests that probiotics can be protective against NEC, she added, “so not to have a reliable product in this country is very frustrating.”
Dr. Poindexter and colleagues pointed to a 2015 study that found that only 1 of 16 commercial products tested contained the exact organisms listed on their labels. One product contained none of the species listed on the label.
In light of increasing use, the AAP emphasizes the need for development of pharmaceutical-grade probiotics that would be rigorously evaluated for safety and efficacy.
The infant microbiome
Over the past decade, the gut microbiome has been increasingly recognized as a factor relevant to health and disease in preterm infants, the authors noted. Differences in the intestinal microbiota between full-term and preterm infants are substantial. The microbiome of preterm infants tends to include fewer bacterial species and greater proportions of potentially pathogenic strains.
Evidence of benefit from probiotics has been mixed. Some studies and pooled systematic analyses suggest a significant benefit. However, Dr. Poindexter and colleagues noted that some researchers express concerns about the study methods used, such as pooling results from trials that tested different probiotic strains or that had few infants in the highest risk category.
Whereas the potential for probiotic-related infection appears low, there does seem to be some risk for sepsis associated with colonization by a strain in a given product or from contamination with a pathogen during manufacturing, the report explains.
At least one trial found that a third of infants randomly assigned to receive placebo showed evidence of the probiotic strain.
“However, it may be difficult to distinguish the change in the infant from the change in the resident flora of the NICU,” the AAP panel wrote.
In addition, there have recently been several recalls of dietary supplement–grade probiotics for contamination, which have raised concerns. Pathogens include Salmonella, Rhizopus, and Penicillium species. Fatal gastrointestinal mucormycosis has also been reported in a preterm infant who received ABC Dophilus powder that was contaminated with the microfungus Rhizopus oryzae.
Other safety considerations, according to the authors, are the unknown longer-term effect of probiotics on preterm infants and the unknown impact of microorganisms on the microbiome over time.
Last year, the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition published a position paper with a conditional recommendation for selected probiotics to reduce NEC rates. “These guidelines would be applicable in the U.S. if we had products manufactured in a way that could guarantee that what’s on the label is in the bottle,” Dr. Poindexter said.
Asked for her perspective on the AAP clinical report, Erica Wymore, MD, assistant professor of neonatal and perinatal medicine at the University of Colorado at Denver, Aurora, called it “an excellent review of the current literature” that shows the inadequacy of data on the composition, dosage, timing, duration, and use of single-strain vs. multiple-strain probiotics to reduce NEC. Her clinical center, Children’s Hospital Colorado, does not administer probiotics to preterm babies.
Although guidelines can improve outcomes, said Dr. Wymore, who was not involved in the AAP report, improvement observed with probiotics results from more stringent care in centers that experience high NEC rates. “It’s hard to know if it’s due to the probiotics if they already have a high rate of NEC,” Dr. Wymore said.
She echoed the AAP’s position and stressed the need for extreme caution in giving these products to vulnerable infants with immature immune systems. Before that can be safely done, she said, “we need more FDA oversight of product composition [and] pharmaceutical-grade products, and more studies to determine efficacy.”
Added Dr. Poindexter: “Hopefully, this report will inform clinicians of the risks of using non–pharmaceutical-grade products and encourage industry to actually develop probiotics for neonates that we can feel comfortable using.”
The report received no external funding. Dr. Poindexter and Dr. Wymore have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adversity accelerates aging at early ages, now measurable in real-time
Adversity in early life – whether preterm birth or socioeconomic disadvantage in childhood – accelerates aging, according to two recent studies, but underlying mechanisms remain unclear, and methods of investigation continue to evolve.
While one study used an established epigenetic clock to measure biological age among adults with extremely low birth weight, the other showcased a relatively new tool to measure pace of biological aging in disadvantaged children, suggesting that the metric may one day serve as a real-time measure of interventional efficacy.
These findings build upon previous studies that have demonstrated a correlation between biological age, also known as methylation age, and an increased risk of health problems later in life, according to Daniel A. Notterman, MD, professor of molecular biology at Princeton (N.J.) University.
“Finding that a person’s methylation age is greater than their chronological age has been taken as evidence of increased ‘biological age’ and perhaps a tendency to greater future morbidity,” Dr. Notterman wrote in a Pediatrics editorial. “Indeed, methylation age is advanced in association with a number of childhood and midlife adversities as well as morbidities such as atherosclerosis, cancer, and obesity.”
Extremely low birth weight associated with faster aging in men
For some individuals, accelerated biological aging begins at birth, or even in utero, according to Ryan J. Van Lieshout, MD, PhD, Canada Research Chair in the Perinatal Programming of Mental Disorders and the Albert Einstein/Irving Zucker Chair in Neuroscience at McMaster University, Hamilton, Ont., and colleagues.
The investigators conducted a study involving 45 extremely low birth weight (ELBW) survivors and 49 individuals born at normal birth weight. All participants were drawn from a longitudinal study conducted between 1977 and 1982 that assessed advances in neonatal intensive care. Controls were recruited at 8 years of age and matched with ELBW survivors based on family socioeconomic status, sex, and age. Follow-up continued through adulthood, allowing for the present trial to compare data from ages 8, 30, and 35.
Using samples of buccal epithelial cells, the investigators measured biological age with the Horvath epigenetic clock, the most commonly used tool of its kind, which measures cytosine-5 methylation at 353 cytosine-phosphate-guanine sites. Results were adjusted for a variety of covariates, such as smoking status, body mass index, number of chronic health conditions, and others.
Between groups, ELBW survivors trended toward older biological age, compared with adults born at normal birth weight (29.0 vs. 27.9 years), a difference that was not statistically significant. Further analysis, however, showed a significant sex-based difference between groups: Male survivors of ELBW, in adulthood, were almost 5 years biologically older than men born at normal birth weight (31.4 vs. 26.9 years; P = .01).
“[W]e provide preliminary evidence of a new link between ELBW and accelerated biological aging among men,” the investigators concluded.
In an accompanying editorial, Pam Factor-Litvak, PhD, vice chair of epidemiology at Columbia University, New York, wrote, “The findings are intriguing and open many questions for further study.”
Dr. Factor-Litvak noted that it remains unclear whether differences in biological aging were present at birth.
“[D]ifferences would provide evidence that accelerated aging begins during the in utero period, perhaps because of maternal undernutrition, stress, or another exposure,” Dr. Factor-Litvak wrote. “[R]eductions in chronic stress levels, which may begin for neonates with ELBW in utero and in the first hours of life, may provide an opportunity for interventions,” she added.
According to Calvin J. Hobel, MD, professor of pediatrics at Cedars-Sinai and professor of obstetrics and gynecology at University of California, Los Angeles, who has been studying preterm birth for more than 40 years, interventions may need to begin even earlier.
“The only way to prevent preterm birth is to do it before women get pregnant,” Dr. Hobel said in an interview. “The reason for preterm birth and poor fetal growth is the fact that the mother has early cardiovascular disease – unrecognized.”
Compared with women who give birth to full-term infants, women who give birth to preterm infants typically have increased blood pressure, Dr. Hobel said. Although these elevations in blood pressure are generally asymptomatic and not high enough to be classified as hypertensive, they impact umbilical artery vascular resistance starting at 28 weeks of gestation.
“In utero, [preterm infants] are programmed for increased vascular resistance and increased risk of cardiovascular disease,” Dr. Hobel said.
Regarding the effects of ELBW in men versus women, Dr. Hobel suggested that dissimilar neuroendocrine systems between sexes may protect females from adverse outcomes, although exact mechanisms remain elusive.
Measuring the impact of socioeconomic status on biological aging, now in real-time
A second study, by Laurel Raffington, PhD, of the University of Texas at Austin, and colleagues, evaluated the relationship between socioeconomic disadvantage in childhood and pace of biological aging.
To do so, they used the DunedinPoAm DNA methylation algorithm, a relatively new tool that was developed by analyzing changes in organ system integrity over time among adults with the same chronological age.
“Whereas epigenetic clocks quantify the amount of aging that has already occurred up to the time of measurement, DunedinPoAm quantifies how fast an individual is aging,” Dr. Raffington and colleagues wrote. “In other words, whereas epigenetic clocks tell you what time it is, pace-of-aging measures tell you how fast the clock is ticking.”
The investigators measured pace of aging in 600 children and adolescents (8-18 years of age) from the Texas Twin Project, “an ongoing longitudinal study that includes the collection of salivary samples.” The final dataset included 457 participants who identified as White, 77 who identified as Latinx, and 61 who identified as both White and Latinx.
The investigators evaluated pace of aging compared with family-level and neighborhood-level socioeconomic status, and tested for confounding by tobacco exposure, BMI, and pubertal development.
This analysis revealed that children experiencing socioeconomic disadvantage were aging more quickly than their peers, in terms of both family-level and neighborhood-level inequity (both levels, r = 0.18; P = .001).
Children who identified as Latinx aged faster than did those who identified as White only or White and Latinx, “consistent with higher levels of disadvantage in this group,” the investigators wrote. “Thus, our findings are consistent with observations that racial and/or ethnic socioeconomic disparities are an important contributor to racial and/or ethnic disparities in health.”
Higher BMI, greater tobacco exposure, and more advanced pubertal development were also associated with more rapid aging. After adjustment for these covariates, however, the significant correlation between socioeconomic disadvantage and rapid aging remained, the investigators noted.
“Our results suggest that salivary DNA methylation measures of pace of aging may provide a surrogate or intermediate endpoint for understanding the health impacts of [childhood] interventions,” the investigators concluded. “Such applications may prove particularly useful for evaluating the effectiveness of health-promoting interventions in at-risk groups.”
Still, more work is needed to understand exactly how socioeconomic disadvantage is associated with accelerated aging.
“Ultimately, not only longitudinal repeated-measures studies but also natural experiment studies and randomized controlled trials of social programs are needed to establish causal effects of social disadvantage on DunedinPoAm-measured pace of aging and to establish DunedinPoAm as a mediator of the process through which childhood disadvantage leads to aging-related health conditions,” the investigators wrote.
In his editorial, Dr. Notterman emphasized this point.
“[I]t is worth remembering that associations with either methylation age or pace of aging and health or longevity may represent the effect of an exposure on both the measure and the outcome of interest rather than a causal pathway that runs from the exposure (low socioeconomic status, adversity) to health outcome (i.e., cancer, vascular disease),” he wrote.
Paul Chung, MD, professor and chair of health systems science at Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, Calif., and adjunct professor at the University of California, Los Angeles, called the findings “preliminary,” but noted that confirmation through further research could “fill in some really important gaps.
“Right now, to some degree, we’re at a little bit of an impasse,” Dr. Chung said.
Adverse childhood experiences are “associated very strongly” with mental and physical health issues, Dr. Chung said, “but we don’t know exactly why, and because of that, it’s really hard to come up with social policy solutions that aren’t anything but extremely sort of blunt-ended. We just say, ‘Well, I guess you gotta fix everything.’ And it’s a hard place to be, I think, in the field.”
Although the present study doesn’t resolve this issue, Dr. Chung suggested that the findings “really open the door to a lot of really exciting research that could have a lot of impacts on practice and policy.”
“Sometimes the only way to get people to pay attention enough to generate the level of excitement that would allow you to even do these sorts of studies ... is to generate some initial exploratory data that makes people perk up their ears, and makes people go, ‘Hey, wow, maybe we should be looking into this.’ ”
The study by Dr. Raffington and colleagues was funded by the National Institutes of Health and the Jacobs Foundation, with additional support from the German Research Foundation, Russell Sage Foundation Biology and Social Science Grant, the Canadian Institute for Advanced Research Child and Brain Development Network, and others. The study by Dr. Lieshout and colleagues was supported by Canadian Institutes of Health Research. Dr. Factor-Litvak and Dr. Notterman reported funding from the National Institutes of Health. All of the investigators and interviewees reported no conflicts of interest.
Adversity in early life – whether preterm birth or socioeconomic disadvantage in childhood – accelerates aging, according to two recent studies, but underlying mechanisms remain unclear, and methods of investigation continue to evolve.
While one study used an established epigenetic clock to measure biological age among adults with extremely low birth weight, the other showcased a relatively new tool to measure pace of biological aging in disadvantaged children, suggesting that the metric may one day serve as a real-time measure of interventional efficacy.
These findings build upon previous studies that have demonstrated a correlation between biological age, also known as methylation age, and an increased risk of health problems later in life, according to Daniel A. Notterman, MD, professor of molecular biology at Princeton (N.J.) University.
“Finding that a person’s methylation age is greater than their chronological age has been taken as evidence of increased ‘biological age’ and perhaps a tendency to greater future morbidity,” Dr. Notterman wrote in a Pediatrics editorial. “Indeed, methylation age is advanced in association with a number of childhood and midlife adversities as well as morbidities such as atherosclerosis, cancer, and obesity.”
Extremely low birth weight associated with faster aging in men
For some individuals, accelerated biological aging begins at birth, or even in utero, according to Ryan J. Van Lieshout, MD, PhD, Canada Research Chair in the Perinatal Programming of Mental Disorders and the Albert Einstein/Irving Zucker Chair in Neuroscience at McMaster University, Hamilton, Ont., and colleagues.
The investigators conducted a study involving 45 extremely low birth weight (ELBW) survivors and 49 individuals born at normal birth weight. All participants were drawn from a longitudinal study conducted between 1977 and 1982 that assessed advances in neonatal intensive care. Controls were recruited at 8 years of age and matched with ELBW survivors based on family socioeconomic status, sex, and age. Follow-up continued through adulthood, allowing for the present trial to compare data from ages 8, 30, and 35.
Using samples of buccal epithelial cells, the investigators measured biological age with the Horvath epigenetic clock, the most commonly used tool of its kind, which measures cytosine-5 methylation at 353 cytosine-phosphate-guanine sites. Results were adjusted for a variety of covariates, such as smoking status, body mass index, number of chronic health conditions, and others.
Between groups, ELBW survivors trended toward older biological age, compared with adults born at normal birth weight (29.0 vs. 27.9 years), a difference that was not statistically significant. Further analysis, however, showed a significant sex-based difference between groups: Male survivors of ELBW, in adulthood, were almost 5 years biologically older than men born at normal birth weight (31.4 vs. 26.9 years; P = .01).
“[W]e provide preliminary evidence of a new link between ELBW and accelerated biological aging among men,” the investigators concluded.
In an accompanying editorial, Pam Factor-Litvak, PhD, vice chair of epidemiology at Columbia University, New York, wrote, “The findings are intriguing and open many questions for further study.”
Dr. Factor-Litvak noted that it remains unclear whether differences in biological aging were present at birth.
“[D]ifferences would provide evidence that accelerated aging begins during the in utero period, perhaps because of maternal undernutrition, stress, or another exposure,” Dr. Factor-Litvak wrote. “[R]eductions in chronic stress levels, which may begin for neonates with ELBW in utero and in the first hours of life, may provide an opportunity for interventions,” she added.
According to Calvin J. Hobel, MD, professor of pediatrics at Cedars-Sinai and professor of obstetrics and gynecology at University of California, Los Angeles, who has been studying preterm birth for more than 40 years, interventions may need to begin even earlier.
“The only way to prevent preterm birth is to do it before women get pregnant,” Dr. Hobel said in an interview. “The reason for preterm birth and poor fetal growth is the fact that the mother has early cardiovascular disease – unrecognized.”
Compared with women who give birth to full-term infants, women who give birth to preterm infants typically have increased blood pressure, Dr. Hobel said. Although these elevations in blood pressure are generally asymptomatic and not high enough to be classified as hypertensive, they impact umbilical artery vascular resistance starting at 28 weeks of gestation.
“In utero, [preterm infants] are programmed for increased vascular resistance and increased risk of cardiovascular disease,” Dr. Hobel said.
Regarding the effects of ELBW in men versus women, Dr. Hobel suggested that dissimilar neuroendocrine systems between sexes may protect females from adverse outcomes, although exact mechanisms remain elusive.
Measuring the impact of socioeconomic status on biological aging, now in real-time
A second study, by Laurel Raffington, PhD, of the University of Texas at Austin, and colleagues, evaluated the relationship between socioeconomic disadvantage in childhood and pace of biological aging.
To do so, they used the DunedinPoAm DNA methylation algorithm, a relatively new tool that was developed by analyzing changes in organ system integrity over time among adults with the same chronological age.
“Whereas epigenetic clocks quantify the amount of aging that has already occurred up to the time of measurement, DunedinPoAm quantifies how fast an individual is aging,” Dr. Raffington and colleagues wrote. “In other words, whereas epigenetic clocks tell you what time it is, pace-of-aging measures tell you how fast the clock is ticking.”
The investigators measured pace of aging in 600 children and adolescents (8-18 years of age) from the Texas Twin Project, “an ongoing longitudinal study that includes the collection of salivary samples.” The final dataset included 457 participants who identified as White, 77 who identified as Latinx, and 61 who identified as both White and Latinx.
The investigators evaluated pace of aging compared with family-level and neighborhood-level socioeconomic status, and tested for confounding by tobacco exposure, BMI, and pubertal development.
This analysis revealed that children experiencing socioeconomic disadvantage were aging more quickly than their peers, in terms of both family-level and neighborhood-level inequity (both levels, r = 0.18; P = .001).
Children who identified as Latinx aged faster than did those who identified as White only or White and Latinx, “consistent with higher levels of disadvantage in this group,” the investigators wrote. “Thus, our findings are consistent with observations that racial and/or ethnic socioeconomic disparities are an important contributor to racial and/or ethnic disparities in health.”
Higher BMI, greater tobacco exposure, and more advanced pubertal development were also associated with more rapid aging. After adjustment for these covariates, however, the significant correlation between socioeconomic disadvantage and rapid aging remained, the investigators noted.
“Our results suggest that salivary DNA methylation measures of pace of aging may provide a surrogate or intermediate endpoint for understanding the health impacts of [childhood] interventions,” the investigators concluded. “Such applications may prove particularly useful for evaluating the effectiveness of health-promoting interventions in at-risk groups.”
Still, more work is needed to understand exactly how socioeconomic disadvantage is associated with accelerated aging.
“Ultimately, not only longitudinal repeated-measures studies but also natural experiment studies and randomized controlled trials of social programs are needed to establish causal effects of social disadvantage on DunedinPoAm-measured pace of aging and to establish DunedinPoAm as a mediator of the process through which childhood disadvantage leads to aging-related health conditions,” the investigators wrote.
In his editorial, Dr. Notterman emphasized this point.
“[I]t is worth remembering that associations with either methylation age or pace of aging and health or longevity may represent the effect of an exposure on both the measure and the outcome of interest rather than a causal pathway that runs from the exposure (low socioeconomic status, adversity) to health outcome (i.e., cancer, vascular disease),” he wrote.
Paul Chung, MD, professor and chair of health systems science at Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, Calif., and adjunct professor at the University of California, Los Angeles, called the findings “preliminary,” but noted that confirmation through further research could “fill in some really important gaps.
“Right now, to some degree, we’re at a little bit of an impasse,” Dr. Chung said.
Adverse childhood experiences are “associated very strongly” with mental and physical health issues, Dr. Chung said, “but we don’t know exactly why, and because of that, it’s really hard to come up with social policy solutions that aren’t anything but extremely sort of blunt-ended. We just say, ‘Well, I guess you gotta fix everything.’ And it’s a hard place to be, I think, in the field.”
Although the present study doesn’t resolve this issue, Dr. Chung suggested that the findings “really open the door to a lot of really exciting research that could have a lot of impacts on practice and policy.”
“Sometimes the only way to get people to pay attention enough to generate the level of excitement that would allow you to even do these sorts of studies ... is to generate some initial exploratory data that makes people perk up their ears, and makes people go, ‘Hey, wow, maybe we should be looking into this.’ ”
The study by Dr. Raffington and colleagues was funded by the National Institutes of Health and the Jacobs Foundation, with additional support from the German Research Foundation, Russell Sage Foundation Biology and Social Science Grant, the Canadian Institute for Advanced Research Child and Brain Development Network, and others. The study by Dr. Lieshout and colleagues was supported by Canadian Institutes of Health Research. Dr. Factor-Litvak and Dr. Notterman reported funding from the National Institutes of Health. All of the investigators and interviewees reported no conflicts of interest.
Adversity in early life – whether preterm birth or socioeconomic disadvantage in childhood – accelerates aging, according to two recent studies, but underlying mechanisms remain unclear, and methods of investigation continue to evolve.
While one study used an established epigenetic clock to measure biological age among adults with extremely low birth weight, the other showcased a relatively new tool to measure pace of biological aging in disadvantaged children, suggesting that the metric may one day serve as a real-time measure of interventional efficacy.
These findings build upon previous studies that have demonstrated a correlation between biological age, also known as methylation age, and an increased risk of health problems later in life, according to Daniel A. Notterman, MD, professor of molecular biology at Princeton (N.J.) University.
“Finding that a person’s methylation age is greater than their chronological age has been taken as evidence of increased ‘biological age’ and perhaps a tendency to greater future morbidity,” Dr. Notterman wrote in a Pediatrics editorial. “Indeed, methylation age is advanced in association with a number of childhood and midlife adversities as well as morbidities such as atherosclerosis, cancer, and obesity.”
Extremely low birth weight associated with faster aging in men
For some individuals, accelerated biological aging begins at birth, or even in utero, according to Ryan J. Van Lieshout, MD, PhD, Canada Research Chair in the Perinatal Programming of Mental Disorders and the Albert Einstein/Irving Zucker Chair in Neuroscience at McMaster University, Hamilton, Ont., and colleagues.
The investigators conducted a study involving 45 extremely low birth weight (ELBW) survivors and 49 individuals born at normal birth weight. All participants were drawn from a longitudinal study conducted between 1977 and 1982 that assessed advances in neonatal intensive care. Controls were recruited at 8 years of age and matched with ELBW survivors based on family socioeconomic status, sex, and age. Follow-up continued through adulthood, allowing for the present trial to compare data from ages 8, 30, and 35.
Using samples of buccal epithelial cells, the investigators measured biological age with the Horvath epigenetic clock, the most commonly used tool of its kind, which measures cytosine-5 methylation at 353 cytosine-phosphate-guanine sites. Results were adjusted for a variety of covariates, such as smoking status, body mass index, number of chronic health conditions, and others.
Between groups, ELBW survivors trended toward older biological age, compared with adults born at normal birth weight (29.0 vs. 27.9 years), a difference that was not statistically significant. Further analysis, however, showed a significant sex-based difference between groups: Male survivors of ELBW, in adulthood, were almost 5 years biologically older than men born at normal birth weight (31.4 vs. 26.9 years; P = .01).
“[W]e provide preliminary evidence of a new link between ELBW and accelerated biological aging among men,” the investigators concluded.
In an accompanying editorial, Pam Factor-Litvak, PhD, vice chair of epidemiology at Columbia University, New York, wrote, “The findings are intriguing and open many questions for further study.”
Dr. Factor-Litvak noted that it remains unclear whether differences in biological aging were present at birth.
“[D]ifferences would provide evidence that accelerated aging begins during the in utero period, perhaps because of maternal undernutrition, stress, or another exposure,” Dr. Factor-Litvak wrote. “[R]eductions in chronic stress levels, which may begin for neonates with ELBW in utero and in the first hours of life, may provide an opportunity for interventions,” she added.
According to Calvin J. Hobel, MD, professor of pediatrics at Cedars-Sinai and professor of obstetrics and gynecology at University of California, Los Angeles, who has been studying preterm birth for more than 40 years, interventions may need to begin even earlier.
“The only way to prevent preterm birth is to do it before women get pregnant,” Dr. Hobel said in an interview. “The reason for preterm birth and poor fetal growth is the fact that the mother has early cardiovascular disease – unrecognized.”
Compared with women who give birth to full-term infants, women who give birth to preterm infants typically have increased blood pressure, Dr. Hobel said. Although these elevations in blood pressure are generally asymptomatic and not high enough to be classified as hypertensive, they impact umbilical artery vascular resistance starting at 28 weeks of gestation.
“In utero, [preterm infants] are programmed for increased vascular resistance and increased risk of cardiovascular disease,” Dr. Hobel said.
Regarding the effects of ELBW in men versus women, Dr. Hobel suggested that dissimilar neuroendocrine systems between sexes may protect females from adverse outcomes, although exact mechanisms remain elusive.
Measuring the impact of socioeconomic status on biological aging, now in real-time
A second study, by Laurel Raffington, PhD, of the University of Texas at Austin, and colleagues, evaluated the relationship between socioeconomic disadvantage in childhood and pace of biological aging.
To do so, they used the DunedinPoAm DNA methylation algorithm, a relatively new tool that was developed by analyzing changes in organ system integrity over time among adults with the same chronological age.
“Whereas epigenetic clocks quantify the amount of aging that has already occurred up to the time of measurement, DunedinPoAm quantifies how fast an individual is aging,” Dr. Raffington and colleagues wrote. “In other words, whereas epigenetic clocks tell you what time it is, pace-of-aging measures tell you how fast the clock is ticking.”
The investigators measured pace of aging in 600 children and adolescents (8-18 years of age) from the Texas Twin Project, “an ongoing longitudinal study that includes the collection of salivary samples.” The final dataset included 457 participants who identified as White, 77 who identified as Latinx, and 61 who identified as both White and Latinx.
The investigators evaluated pace of aging compared with family-level and neighborhood-level socioeconomic status, and tested for confounding by tobacco exposure, BMI, and pubertal development.
This analysis revealed that children experiencing socioeconomic disadvantage were aging more quickly than their peers, in terms of both family-level and neighborhood-level inequity (both levels, r = 0.18; P = .001).
Children who identified as Latinx aged faster than did those who identified as White only or White and Latinx, “consistent with higher levels of disadvantage in this group,” the investigators wrote. “Thus, our findings are consistent with observations that racial and/or ethnic socioeconomic disparities are an important contributor to racial and/or ethnic disparities in health.”
Higher BMI, greater tobacco exposure, and more advanced pubertal development were also associated with more rapid aging. After adjustment for these covariates, however, the significant correlation between socioeconomic disadvantage and rapid aging remained, the investigators noted.
“Our results suggest that salivary DNA methylation measures of pace of aging may provide a surrogate or intermediate endpoint for understanding the health impacts of [childhood] interventions,” the investigators concluded. “Such applications may prove particularly useful for evaluating the effectiveness of health-promoting interventions in at-risk groups.”
Still, more work is needed to understand exactly how socioeconomic disadvantage is associated with accelerated aging.
“Ultimately, not only longitudinal repeated-measures studies but also natural experiment studies and randomized controlled trials of social programs are needed to establish causal effects of social disadvantage on DunedinPoAm-measured pace of aging and to establish DunedinPoAm as a mediator of the process through which childhood disadvantage leads to aging-related health conditions,” the investigators wrote.
In his editorial, Dr. Notterman emphasized this point.
“[I]t is worth remembering that associations with either methylation age or pace of aging and health or longevity may represent the effect of an exposure on both the measure and the outcome of interest rather than a causal pathway that runs from the exposure (low socioeconomic status, adversity) to health outcome (i.e., cancer, vascular disease),” he wrote.
Paul Chung, MD, professor and chair of health systems science at Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, Calif., and adjunct professor at the University of California, Los Angeles, called the findings “preliminary,” but noted that confirmation through further research could “fill in some really important gaps.
“Right now, to some degree, we’re at a little bit of an impasse,” Dr. Chung said.
Adverse childhood experiences are “associated very strongly” with mental and physical health issues, Dr. Chung said, “but we don’t know exactly why, and because of that, it’s really hard to come up with social policy solutions that aren’t anything but extremely sort of blunt-ended. We just say, ‘Well, I guess you gotta fix everything.’ And it’s a hard place to be, I think, in the field.”
Although the present study doesn’t resolve this issue, Dr. Chung suggested that the findings “really open the door to a lot of really exciting research that could have a lot of impacts on practice and policy.”
“Sometimes the only way to get people to pay attention enough to generate the level of excitement that would allow you to even do these sorts of studies ... is to generate some initial exploratory data that makes people perk up their ears, and makes people go, ‘Hey, wow, maybe we should be looking into this.’ ”
The study by Dr. Raffington and colleagues was funded by the National Institutes of Health and the Jacobs Foundation, with additional support from the German Research Foundation, Russell Sage Foundation Biology and Social Science Grant, the Canadian Institute for Advanced Research Child and Brain Development Network, and others. The study by Dr. Lieshout and colleagues was supported by Canadian Institutes of Health Research. Dr. Factor-Litvak and Dr. Notterman reported funding from the National Institutes of Health. All of the investigators and interviewees reported no conflicts of interest.
FROM PEDIATRICS
Some things pediatric hospitalists do for no reason
Converge 2021 session
High Value Care in Pediatrics – Things We Do for No Reason
Presenter
Ricardo Quinonez, MD, FAAP, FHM
Session summary
Dr. Ricardo Quinonez, associate professor of pediatrics at Baylor College of Medicine and chief of pediatric hospital medicine at Texas Children’s Hospital, both in Houston, presented key topics in pediatric hospital medicine with low-value care management practices which are not supported by recent literature. This session was a continuation of the popular lecture series first presented at the Society of Hospital Medicine annual conference and the “Choosing Wisely: Things We Do for No Reason” article series in the Journal of Hospital Medicine.
Dr. Quinonez began by discussing high flow nasal cannula (HFNC) in bronchiolitis. At first, early observational studies showed a decrease in intubation rate for children placed on HFNC, which resulted in its high utilization. Randomized, controlled trials (RCTs) later showed that early initiation of HFNC did not affect rates of transfer to the ICU, duration of oxygen need, or length of stay.
He then discussed the treatment of symptomatic spontaneous pneumothorax in children, which is often managed by hospital admission, needle aspiration and chest tube placement, and serial chest x-rays. Instead, recent literature supports an ambulatory approach by placing a device with an 8 French catheter with one way Heimlich valve. After placement, a chest x-ray is performed and if the pneumothorax is stable, the patient is discharged with plans for serial chest x-rays as an outpatient. The device is removed after re-expansion of the lung.
Dr. Quinonez then discussed the frequent pediatric complaint of constipation. He stated that abdominal x-rays for evaluation of “stool burden” are not reliable, and x-rays are recommended against in both U.S. and British guidelines. Furthermore, a high-fiber diet is often recommended as a treatment for constipation. However, after review of recent RCTs and cohort studies, no relationship between a low-fiber diet and constipation was seen. Instead, genetics likely plays a large part in causing constipation.
Lastly, Dr. Quinonez discussed electrolyte testing in children with acute gastroenteritis. Electrolyte testing is commonly performed, yet testing patterns vary greatly across children’s hospitals. One quality improvement project found that after decreasing electrolyte testing by more than a third during hospitalizations, no change in readmission rate or renal replacement therapy was reported.
Key takeaways
- Early use of high flow nasal cannula in bronchiolitis does not affect rates of transfer to the ICU or length of stay.
- Abdominal x-rays to assess for constipation are not recommended and are not reliable in measuring stool burden.
- A low-fiber diet does not cause constipation.
- Quality improvement projects can help physicians “choose wisely” and decrease things we do for no reason.
Dr. Tantoco is an academic med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, Chicago.
Converge 2021 session
High Value Care in Pediatrics – Things We Do for No Reason
Presenter
Ricardo Quinonez, MD, FAAP, FHM
Session summary
Dr. Ricardo Quinonez, associate professor of pediatrics at Baylor College of Medicine and chief of pediatric hospital medicine at Texas Children’s Hospital, both in Houston, presented key topics in pediatric hospital medicine with low-value care management practices which are not supported by recent literature. This session was a continuation of the popular lecture series first presented at the Society of Hospital Medicine annual conference and the “Choosing Wisely: Things We Do for No Reason” article series in the Journal of Hospital Medicine.
Dr. Quinonez began by discussing high flow nasal cannula (HFNC) in bronchiolitis. At first, early observational studies showed a decrease in intubation rate for children placed on HFNC, which resulted in its high utilization. Randomized, controlled trials (RCTs) later showed that early initiation of HFNC did not affect rates of transfer to the ICU, duration of oxygen need, or length of stay.
He then discussed the treatment of symptomatic spontaneous pneumothorax in children, which is often managed by hospital admission, needle aspiration and chest tube placement, and serial chest x-rays. Instead, recent literature supports an ambulatory approach by placing a device with an 8 French catheter with one way Heimlich valve. After placement, a chest x-ray is performed and if the pneumothorax is stable, the patient is discharged with plans for serial chest x-rays as an outpatient. The device is removed after re-expansion of the lung.
Dr. Quinonez then discussed the frequent pediatric complaint of constipation. He stated that abdominal x-rays for evaluation of “stool burden” are not reliable, and x-rays are recommended against in both U.S. and British guidelines. Furthermore, a high-fiber diet is often recommended as a treatment for constipation. However, after review of recent RCTs and cohort studies, no relationship between a low-fiber diet and constipation was seen. Instead, genetics likely plays a large part in causing constipation.
Lastly, Dr. Quinonez discussed electrolyte testing in children with acute gastroenteritis. Electrolyte testing is commonly performed, yet testing patterns vary greatly across children’s hospitals. One quality improvement project found that after decreasing electrolyte testing by more than a third during hospitalizations, no change in readmission rate or renal replacement therapy was reported.
Key takeaways
- Early use of high flow nasal cannula in bronchiolitis does not affect rates of transfer to the ICU or length of stay.
- Abdominal x-rays to assess for constipation are not recommended and are not reliable in measuring stool burden.
- A low-fiber diet does not cause constipation.
- Quality improvement projects can help physicians “choose wisely” and decrease things we do for no reason.
Dr. Tantoco is an academic med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, Chicago.
Converge 2021 session
High Value Care in Pediatrics – Things We Do for No Reason
Presenter
Ricardo Quinonez, MD, FAAP, FHM
Session summary
Dr. Ricardo Quinonez, associate professor of pediatrics at Baylor College of Medicine and chief of pediatric hospital medicine at Texas Children’s Hospital, both in Houston, presented key topics in pediatric hospital medicine with low-value care management practices which are not supported by recent literature. This session was a continuation of the popular lecture series first presented at the Society of Hospital Medicine annual conference and the “Choosing Wisely: Things We Do for No Reason” article series in the Journal of Hospital Medicine.
Dr. Quinonez began by discussing high flow nasal cannula (HFNC) in bronchiolitis. At first, early observational studies showed a decrease in intubation rate for children placed on HFNC, which resulted in its high utilization. Randomized, controlled trials (RCTs) later showed that early initiation of HFNC did not affect rates of transfer to the ICU, duration of oxygen need, or length of stay.
He then discussed the treatment of symptomatic spontaneous pneumothorax in children, which is often managed by hospital admission, needle aspiration and chest tube placement, and serial chest x-rays. Instead, recent literature supports an ambulatory approach by placing a device with an 8 French catheter with one way Heimlich valve. After placement, a chest x-ray is performed and if the pneumothorax is stable, the patient is discharged with plans for serial chest x-rays as an outpatient. The device is removed after re-expansion of the lung.
Dr. Quinonez then discussed the frequent pediatric complaint of constipation. He stated that abdominal x-rays for evaluation of “stool burden” are not reliable, and x-rays are recommended against in both U.S. and British guidelines. Furthermore, a high-fiber diet is often recommended as a treatment for constipation. However, after review of recent RCTs and cohort studies, no relationship between a low-fiber diet and constipation was seen. Instead, genetics likely plays a large part in causing constipation.
Lastly, Dr. Quinonez discussed electrolyte testing in children with acute gastroenteritis. Electrolyte testing is commonly performed, yet testing patterns vary greatly across children’s hospitals. One quality improvement project found that after decreasing electrolyte testing by more than a third during hospitalizations, no change in readmission rate or renal replacement therapy was reported.
Key takeaways
- Early use of high flow nasal cannula in bronchiolitis does not affect rates of transfer to the ICU or length of stay.
- Abdominal x-rays to assess for constipation are not recommended and are not reliable in measuring stool burden.
- A low-fiber diet does not cause constipation.
- Quality improvement projects can help physicians “choose wisely” and decrease things we do for no reason.
Dr. Tantoco is an academic med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, Chicago.
FROM SHM CONVERGE 2021
Hospital outcomes for children with MIS-C unaffected by initial presentation site
Length of hospital stay and the need for intensive care for pediatric COVID-19 patients with multisystem inflammatory syndrome in children was not significantly different for those who presented first as outpatients or emergency patients, based on data from 34 children.
Multisystem inflammatory syndrome in children (MIS-C) can be challenging to diagnose, as the key characteristics of fever, elevated inflammatory markers, and involvement of at least two organ systems often overlap with other illnesses, said Erin B. Treemarcki, DO, of the University of Utah, Salt Lake City, and colleagues.
“Primary care and urgent care providers are often the first point of health care for children with symptoms of MIS-C,” the researchers wrote. In a study (Poster 142) presented at the annual meeting of the Pediatric Academic Societies, held virtually, the researchers conducted a retrospective review of 34 patients younger than 21 years who were hospitalized with MIS-C at a single center between April 2020 and December 2020. The average age of the patients was 7.9 years, 68% were male, 82% were White, and 53% first presented to an outpatient clinic.
Sixteen patients presented to an emergency department and 18 presented to an ambulatory setting. The length of hospitalization ranged from 3 to 16 days with a median of 6 days, and the PICU stay ranged from 1 to 10 days with a median of 2 days.
Overall, the length of hospital stay and rate of PICU admission were not significantly different between the emergency presentation and outpatient presentation groups. Twenty-four patients entered the PICU, 13 at admission and 11 as transfers. However, the median number of days of symptoms prior to admission was significantly higher for outpatient cases (6 days vs. 4 days, P = .03).
One patient was readmitted to the hospital within 30 days for aseptic meningitis, and none of the patients died.
Initial symptoms were not significantly different for outpatient vs. emergency department patients. The most common initial manifestations of MIS-C included fever (100%), gastrointestinal symptoms (85%), and mucocutaneous symptoms (88%). Mucocutaneous symptoms included rash, oral mucosal changes, conjunctivitis, and hand/foot edema. In addition, 65% of the patients met at least 3 criteria for Kawasaki disease, the researchers noted.
The most common elevated labs at presentation regardless of setting were D-dimer (100%), C-reactive protein (97%), ferritin (97%), procalcitonin (97%), and serum IL-6 (94%).
The study findings were limited by the small sample size and focus on data from a single center. However, the results emphasize the varied presentations of MIS-C and the importance that both primary care and urgent care providers know the signs, as they are often the first point of health care for children with MIS-C, the researchers noted.
Keep looking for factors that put children at risk
“MIS-C is probably the most serious complication of COVID in children, so we as pediatricians on the front line need to know what it looks like,” Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was surprised by the study finding that children’s length of hospital stay was not affected by presentation setting.
“I would have thought the kids presenting in an outpatient setting would take longer to diagnose, and therefore have a longer hospital stay,” she noted. Instead, the take-home message is that whether the MIS-C diagnosis occurs in the outpatient or emergency setting, the length of stay is the same, and that the most common symptoms are fever, gastrointestinal, mucocutaneous, and cardiac symptoms regardless of initial presentation setting, she said.
More research is needed, and future studies should examine “any potential underlying factors making these particular kids susceptible to MIS-C,” Dr. Kinsella added.
The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts, but serves on the Pediatric News Editorial Advisory Board.
Length of hospital stay and the need for intensive care for pediatric COVID-19 patients with multisystem inflammatory syndrome in children was not significantly different for those who presented first as outpatients or emergency patients, based on data from 34 children.
Multisystem inflammatory syndrome in children (MIS-C) can be challenging to diagnose, as the key characteristics of fever, elevated inflammatory markers, and involvement of at least two organ systems often overlap with other illnesses, said Erin B. Treemarcki, DO, of the University of Utah, Salt Lake City, and colleagues.
“Primary care and urgent care providers are often the first point of health care for children with symptoms of MIS-C,” the researchers wrote. In a study (Poster 142) presented at the annual meeting of the Pediatric Academic Societies, held virtually, the researchers conducted a retrospective review of 34 patients younger than 21 years who were hospitalized with MIS-C at a single center between April 2020 and December 2020. The average age of the patients was 7.9 years, 68% were male, 82% were White, and 53% first presented to an outpatient clinic.
Sixteen patients presented to an emergency department and 18 presented to an ambulatory setting. The length of hospitalization ranged from 3 to 16 days with a median of 6 days, and the PICU stay ranged from 1 to 10 days with a median of 2 days.
Overall, the length of hospital stay and rate of PICU admission were not significantly different between the emergency presentation and outpatient presentation groups. Twenty-four patients entered the PICU, 13 at admission and 11 as transfers. However, the median number of days of symptoms prior to admission was significantly higher for outpatient cases (6 days vs. 4 days, P = .03).
One patient was readmitted to the hospital within 30 days for aseptic meningitis, and none of the patients died.
Initial symptoms were not significantly different for outpatient vs. emergency department patients. The most common initial manifestations of MIS-C included fever (100%), gastrointestinal symptoms (85%), and mucocutaneous symptoms (88%). Mucocutaneous symptoms included rash, oral mucosal changes, conjunctivitis, and hand/foot edema. In addition, 65% of the patients met at least 3 criteria for Kawasaki disease, the researchers noted.
The most common elevated labs at presentation regardless of setting were D-dimer (100%), C-reactive protein (97%), ferritin (97%), procalcitonin (97%), and serum IL-6 (94%).
The study findings were limited by the small sample size and focus on data from a single center. However, the results emphasize the varied presentations of MIS-C and the importance that both primary care and urgent care providers know the signs, as they are often the first point of health care for children with MIS-C, the researchers noted.
Keep looking for factors that put children at risk
“MIS-C is probably the most serious complication of COVID in children, so we as pediatricians on the front line need to know what it looks like,” Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was surprised by the study finding that children’s length of hospital stay was not affected by presentation setting.
“I would have thought the kids presenting in an outpatient setting would take longer to diagnose, and therefore have a longer hospital stay,” she noted. Instead, the take-home message is that whether the MIS-C diagnosis occurs in the outpatient or emergency setting, the length of stay is the same, and that the most common symptoms are fever, gastrointestinal, mucocutaneous, and cardiac symptoms regardless of initial presentation setting, she said.
More research is needed, and future studies should examine “any potential underlying factors making these particular kids susceptible to MIS-C,” Dr. Kinsella added.
The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts, but serves on the Pediatric News Editorial Advisory Board.
Length of hospital stay and the need for intensive care for pediatric COVID-19 patients with multisystem inflammatory syndrome in children was not significantly different for those who presented first as outpatients or emergency patients, based on data from 34 children.
Multisystem inflammatory syndrome in children (MIS-C) can be challenging to diagnose, as the key characteristics of fever, elevated inflammatory markers, and involvement of at least two organ systems often overlap with other illnesses, said Erin B. Treemarcki, DO, of the University of Utah, Salt Lake City, and colleagues.
“Primary care and urgent care providers are often the first point of health care for children with symptoms of MIS-C,” the researchers wrote. In a study (Poster 142) presented at the annual meeting of the Pediatric Academic Societies, held virtually, the researchers conducted a retrospective review of 34 patients younger than 21 years who were hospitalized with MIS-C at a single center between April 2020 and December 2020. The average age of the patients was 7.9 years, 68% were male, 82% were White, and 53% first presented to an outpatient clinic.
Sixteen patients presented to an emergency department and 18 presented to an ambulatory setting. The length of hospitalization ranged from 3 to 16 days with a median of 6 days, and the PICU stay ranged from 1 to 10 days with a median of 2 days.
Overall, the length of hospital stay and rate of PICU admission were not significantly different between the emergency presentation and outpatient presentation groups. Twenty-four patients entered the PICU, 13 at admission and 11 as transfers. However, the median number of days of symptoms prior to admission was significantly higher for outpatient cases (6 days vs. 4 days, P = .03).
One patient was readmitted to the hospital within 30 days for aseptic meningitis, and none of the patients died.
Initial symptoms were not significantly different for outpatient vs. emergency department patients. The most common initial manifestations of MIS-C included fever (100%), gastrointestinal symptoms (85%), and mucocutaneous symptoms (88%). Mucocutaneous symptoms included rash, oral mucosal changes, conjunctivitis, and hand/foot edema. In addition, 65% of the patients met at least 3 criteria for Kawasaki disease, the researchers noted.
The most common elevated labs at presentation regardless of setting were D-dimer (100%), C-reactive protein (97%), ferritin (97%), procalcitonin (97%), and serum IL-6 (94%).
The study findings were limited by the small sample size and focus on data from a single center. However, the results emphasize the varied presentations of MIS-C and the importance that both primary care and urgent care providers know the signs, as they are often the first point of health care for children with MIS-C, the researchers noted.
Keep looking for factors that put children at risk
“MIS-C is probably the most serious complication of COVID in children, so we as pediatricians on the front line need to know what it looks like,” Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was surprised by the study finding that children’s length of hospital stay was not affected by presentation setting.
“I would have thought the kids presenting in an outpatient setting would take longer to diagnose, and therefore have a longer hospital stay,” she noted. Instead, the take-home message is that whether the MIS-C diagnosis occurs in the outpatient or emergency setting, the length of stay is the same, and that the most common symptoms are fever, gastrointestinal, mucocutaneous, and cardiac symptoms regardless of initial presentation setting, she said.
More research is needed, and future studies should examine “any potential underlying factors making these particular kids susceptible to MIS-C,” Dr. Kinsella added.
The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts, but serves on the Pediatric News Editorial Advisory Board.
FROM PAS 2021









