User login
Coping with postpandemic school hesitancy
As the protective effect of the vaccines becomes increasingly apparent, a large number of school systems are beginning to return to prepandemic in-school learning. But anecdotal reports from around the country are making it clear that some children or their families are hesitant to return to the old norm of face to face learning (Goldstein D. “Schools Are Open, but Many Families Remain Hesitant to Return.” New York Times. 2021 May 9). The possible explanations for this hesitancy include a broad list that goes well beyond the obvious concern about the child contracting COVID-19.
I hear from my grandchildren that remote learning has for the most part been unpleasant and lacked the rigor of their in-class experiences. But, they admit that they have found that, in some situations, they prefer the environment at home because it is less distracting. They also acknowledge that, while they miss seeing their friends, at times the isolation has allowed them to be more efficient. Of course, their observations must be viewed in light of their personalities and the support provided by their parents. For these motivated teenagers, the bottom line is that they would prefer to be in school.
However, for the children who have always been a bit ambivalent about school either because they were anxious in social situations or because they found the academics too challenging, one can easily understand why they might prefer to remain in a less-intimidating home environment. For them, missing their friends may have little draw because they may not have had any friends. And, the negative feedback and bullying they have received at school is too overwhelming. A teenager for whom the pandemic has offered the out-of-school free time to explore her independence, feel more like an adult, and enjoy the benefits of having a job may be hesitant to return to the restrictions imposed by what she sees as the childishness of in-school learning.
Compounding the problem is the risk avoidance posture of some school systems and the hesitancy of some teachers to return to an environment that they continue to view as unsafe despite the evidence of the effectiveness of the vaccines and the minimal threat of in-school spread. It is going to be interesting to see how school administrators and politicians deal with this level of institutional hesitancy. Some schools may take what might be considered a hard-line approach and eliminate remote learning completely.
Regardless of how swiftly and thoughtfully schools return to in-class learning, a large number of children will eventually be faced with the stark reality of returning to a place in which they had felt painfully uncomfortable in the past. Pediatricians must be prepared to see this current wave of school hesitancy morph into a full-fledged tsunami of school refusals.
Successful management of a family whose child finds school too challenging emotionally has always required a combination of careful attention to the possible medical causes of the child’s complaints, consultation with a mental health practitioner, and thoughtful coordination with educators sensitive to the child’s school-generated distress.
It has never been easy to reassure the family of a child with frequent headaches or belly pain that his symptoms have no physical basis and then gently point out that the stress of school attendance may be a contributing factor. Some families who buy into the association may be fortunate enough to be able to offer their child home schooling as a solution to school refusal. But this strategy often requires that one parent remain home and has the temperament and the skills to teach.
Now that we have all seen that remote learning has the potential to work in a crisis, will some parents begin to demand it for their children with school refusal? Who will pay for it? I think you and I would prefer to see a solution that targeted therapeutic interventions aimed at getting the child back in school. But you and I also know those strategies don’t always work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As the protective effect of the vaccines becomes increasingly apparent, a large number of school systems are beginning to return to prepandemic in-school learning. But anecdotal reports from around the country are making it clear that some children or their families are hesitant to return to the old norm of face to face learning (Goldstein D. “Schools Are Open, but Many Families Remain Hesitant to Return.” New York Times. 2021 May 9). The possible explanations for this hesitancy include a broad list that goes well beyond the obvious concern about the child contracting COVID-19.
I hear from my grandchildren that remote learning has for the most part been unpleasant and lacked the rigor of their in-class experiences. But, they admit that they have found that, in some situations, they prefer the environment at home because it is less distracting. They also acknowledge that, while they miss seeing their friends, at times the isolation has allowed them to be more efficient. Of course, their observations must be viewed in light of their personalities and the support provided by their parents. For these motivated teenagers, the bottom line is that they would prefer to be in school.
However, for the children who have always been a bit ambivalent about school either because they were anxious in social situations or because they found the academics too challenging, one can easily understand why they might prefer to remain in a less-intimidating home environment. For them, missing their friends may have little draw because they may not have had any friends. And, the negative feedback and bullying they have received at school is too overwhelming. A teenager for whom the pandemic has offered the out-of-school free time to explore her independence, feel more like an adult, and enjoy the benefits of having a job may be hesitant to return to the restrictions imposed by what she sees as the childishness of in-school learning.
Compounding the problem is the risk avoidance posture of some school systems and the hesitancy of some teachers to return to an environment that they continue to view as unsafe despite the evidence of the effectiveness of the vaccines and the minimal threat of in-school spread. It is going to be interesting to see how school administrators and politicians deal with this level of institutional hesitancy. Some schools may take what might be considered a hard-line approach and eliminate remote learning completely.
Regardless of how swiftly and thoughtfully schools return to in-class learning, a large number of children will eventually be faced with the stark reality of returning to a place in which they had felt painfully uncomfortable in the past. Pediatricians must be prepared to see this current wave of school hesitancy morph into a full-fledged tsunami of school refusals.
Successful management of a family whose child finds school too challenging emotionally has always required a combination of careful attention to the possible medical causes of the child’s complaints, consultation with a mental health practitioner, and thoughtful coordination with educators sensitive to the child’s school-generated distress.
It has never been easy to reassure the family of a child with frequent headaches or belly pain that his symptoms have no physical basis and then gently point out that the stress of school attendance may be a contributing factor. Some families who buy into the association may be fortunate enough to be able to offer their child home schooling as a solution to school refusal. But this strategy often requires that one parent remain home and has the temperament and the skills to teach.
Now that we have all seen that remote learning has the potential to work in a crisis, will some parents begin to demand it for their children with school refusal? Who will pay for it? I think you and I would prefer to see a solution that targeted therapeutic interventions aimed at getting the child back in school. But you and I also know those strategies don’t always work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As the protective effect of the vaccines becomes increasingly apparent, a large number of school systems are beginning to return to prepandemic in-school learning. But anecdotal reports from around the country are making it clear that some children or their families are hesitant to return to the old norm of face to face learning (Goldstein D. “Schools Are Open, but Many Families Remain Hesitant to Return.” New York Times. 2021 May 9). The possible explanations for this hesitancy include a broad list that goes well beyond the obvious concern about the child contracting COVID-19.
I hear from my grandchildren that remote learning has for the most part been unpleasant and lacked the rigor of their in-class experiences. But, they admit that they have found that, in some situations, they prefer the environment at home because it is less distracting. They also acknowledge that, while they miss seeing their friends, at times the isolation has allowed them to be more efficient. Of course, their observations must be viewed in light of their personalities and the support provided by their parents. For these motivated teenagers, the bottom line is that they would prefer to be in school.
However, for the children who have always been a bit ambivalent about school either because they were anxious in social situations or because they found the academics too challenging, one can easily understand why they might prefer to remain in a less-intimidating home environment. For them, missing their friends may have little draw because they may not have had any friends. And, the negative feedback and bullying they have received at school is too overwhelming. A teenager for whom the pandemic has offered the out-of-school free time to explore her independence, feel more like an adult, and enjoy the benefits of having a job may be hesitant to return to the restrictions imposed by what she sees as the childishness of in-school learning.
Compounding the problem is the risk avoidance posture of some school systems and the hesitancy of some teachers to return to an environment that they continue to view as unsafe despite the evidence of the effectiveness of the vaccines and the minimal threat of in-school spread. It is going to be interesting to see how school administrators and politicians deal with this level of institutional hesitancy. Some schools may take what might be considered a hard-line approach and eliminate remote learning completely.
Regardless of how swiftly and thoughtfully schools return to in-class learning, a large number of children will eventually be faced with the stark reality of returning to a place in which they had felt painfully uncomfortable in the past. Pediatricians must be prepared to see this current wave of school hesitancy morph into a full-fledged tsunami of school refusals.
Successful management of a family whose child finds school too challenging emotionally has always required a combination of careful attention to the possible medical causes of the child’s complaints, consultation with a mental health practitioner, and thoughtful coordination with educators sensitive to the child’s school-generated distress.
It has never been easy to reassure the family of a child with frequent headaches or belly pain that his symptoms have no physical basis and then gently point out that the stress of school attendance may be a contributing factor. Some families who buy into the association may be fortunate enough to be able to offer their child home schooling as a solution to school refusal. But this strategy often requires that one parent remain home and has the temperament and the skills to teach.
Now that we have all seen that remote learning has the potential to work in a crisis, will some parents begin to demand it for their children with school refusal? Who will pay for it? I think you and I would prefer to see a solution that targeted therapeutic interventions aimed at getting the child back in school. But you and I also know those strategies don’t always work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Mother-to-infant COVID-19 transmission is unlikely
Mothers with a history of COVID-19 exposure during pregnancy are not likely to transmit the infection to their newborns, based on data from more than 2,000 women.
“Uncertainty at the onset of the COVID-19 pandemic led to varying postnatal care recommendations for newborns exposed to SARS-CoV-2 in utero,” said Margaret H. Kyle, of Columbia University, New York, and colleagues.
The Columbia University Irving Medical Center, an early epicenter of the pandemic, allowed rooming-in and encouraged direct breastfeeding between infected mothers and their newborns while adopting extensive safety measures, the researchers said.
In a study presented at the virtual meeting of the Pediatric Academic Societies (Poster 141), the researchers conducted a retrospective chart review of all newborns born at the medical center from March 22, 2020, through August 7, 2020. The study was part of Columbia University’s ongoing COVID-19 Mother Baby Outcomes (COMBO) initiative to “describe the health and well-being of mother-infant dyads with and without prenatal SARS-CoV-2 infections,” according to the researchers.
During the study period, the researchers identified newborns of 327 women who tested positive for COVID-19 at any point during pregnancy and compared them to newborns of 2,125 unexposed women. Demographics were similar between the groups.
Overall, the total test positivity was 0.7% for exposed newborns; 1.0% tested positive on an initial test, and 0% were positive on retest. During the newborn hospital stay and a 2-week follow-up, 0% of all newborns showed clinical evidence of infection.
No significant differences were noted between exposed and unexposed newborns in clinical outcomes including gestational age, mode of delivery, 5-minute Apgar score, heart rate, respiratory rate, or temperature. Although more infants of COVID-19–exposed mothers compared with unexposed mothers had an emergency department visit within the first 14 days of life (6% vs. 3%, P = .002), none of the infants was diagnosed with COVID-19 during these visits. Cough, fever, congestion, or bilirubin were more frequent reasons for emergency department visits in the exposed infants compared with unexposed infants, but these differences were not significant.
The study findings were limited by several factors, including the retrospective design and the limited follow-up period to only the first 2 weeks of life, the researchers noted. In addition, perinatal transmission rates were available only for the 202 newborns who were followed up in the hospital system, they said. However, the results suggest that the risk of mother-to-newborn vertical transmission of COVID-19 remains low, even when mothers are breastfeeding and infants are rooming in, they concluded.
Study supports safety of rooming in
The study is important because of the value of mother and infant bonding, Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview. “We know maternal and infant bonding and breastfeeding are extremely important in the first few days of life,” she said. “Initially, COVID-positive moms were separated from their babies during this important time.” Dr. Kinsella said she was not surprised by the study findings, as they reflect other research that newborns have not been getting infected with COVID-19 from their mothers.
Consequently, the take-home message is that newborns can room in with their mothers in the hospital setting, and they are at low risk for COVID-19 regardless of the mother’s exposure history, said Dr. Kinsella. Looking ahead, future areas of research could include examining SARS-CoV-2 antibodies in newborns, she noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
Mothers with a history of COVID-19 exposure during pregnancy are not likely to transmit the infection to their newborns, based on data from more than 2,000 women.
“Uncertainty at the onset of the COVID-19 pandemic led to varying postnatal care recommendations for newborns exposed to SARS-CoV-2 in utero,” said Margaret H. Kyle, of Columbia University, New York, and colleagues.
The Columbia University Irving Medical Center, an early epicenter of the pandemic, allowed rooming-in and encouraged direct breastfeeding between infected mothers and their newborns while adopting extensive safety measures, the researchers said.
In a study presented at the virtual meeting of the Pediatric Academic Societies (Poster 141), the researchers conducted a retrospective chart review of all newborns born at the medical center from March 22, 2020, through August 7, 2020. The study was part of Columbia University’s ongoing COVID-19 Mother Baby Outcomes (COMBO) initiative to “describe the health and well-being of mother-infant dyads with and without prenatal SARS-CoV-2 infections,” according to the researchers.
During the study period, the researchers identified newborns of 327 women who tested positive for COVID-19 at any point during pregnancy and compared them to newborns of 2,125 unexposed women. Demographics were similar between the groups.
Overall, the total test positivity was 0.7% for exposed newborns; 1.0% tested positive on an initial test, and 0% were positive on retest. During the newborn hospital stay and a 2-week follow-up, 0% of all newborns showed clinical evidence of infection.
No significant differences were noted between exposed and unexposed newborns in clinical outcomes including gestational age, mode of delivery, 5-minute Apgar score, heart rate, respiratory rate, or temperature. Although more infants of COVID-19–exposed mothers compared with unexposed mothers had an emergency department visit within the first 14 days of life (6% vs. 3%, P = .002), none of the infants was diagnosed with COVID-19 during these visits. Cough, fever, congestion, or bilirubin were more frequent reasons for emergency department visits in the exposed infants compared with unexposed infants, but these differences were not significant.
The study findings were limited by several factors, including the retrospective design and the limited follow-up period to only the first 2 weeks of life, the researchers noted. In addition, perinatal transmission rates were available only for the 202 newborns who were followed up in the hospital system, they said. However, the results suggest that the risk of mother-to-newborn vertical transmission of COVID-19 remains low, even when mothers are breastfeeding and infants are rooming in, they concluded.
Study supports safety of rooming in
The study is important because of the value of mother and infant bonding, Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview. “We know maternal and infant bonding and breastfeeding are extremely important in the first few days of life,” she said. “Initially, COVID-positive moms were separated from their babies during this important time.” Dr. Kinsella said she was not surprised by the study findings, as they reflect other research that newborns have not been getting infected with COVID-19 from their mothers.
Consequently, the take-home message is that newborns can room in with their mothers in the hospital setting, and they are at low risk for COVID-19 regardless of the mother’s exposure history, said Dr. Kinsella. Looking ahead, future areas of research could include examining SARS-CoV-2 antibodies in newborns, she noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
Mothers with a history of COVID-19 exposure during pregnancy are not likely to transmit the infection to their newborns, based on data from more than 2,000 women.
“Uncertainty at the onset of the COVID-19 pandemic led to varying postnatal care recommendations for newborns exposed to SARS-CoV-2 in utero,” said Margaret H. Kyle, of Columbia University, New York, and colleagues.
The Columbia University Irving Medical Center, an early epicenter of the pandemic, allowed rooming-in and encouraged direct breastfeeding between infected mothers and their newborns while adopting extensive safety measures, the researchers said.
In a study presented at the virtual meeting of the Pediatric Academic Societies (Poster 141), the researchers conducted a retrospective chart review of all newborns born at the medical center from March 22, 2020, through August 7, 2020. The study was part of Columbia University’s ongoing COVID-19 Mother Baby Outcomes (COMBO) initiative to “describe the health and well-being of mother-infant dyads with and without prenatal SARS-CoV-2 infections,” according to the researchers.
During the study period, the researchers identified newborns of 327 women who tested positive for COVID-19 at any point during pregnancy and compared them to newborns of 2,125 unexposed women. Demographics were similar between the groups.
Overall, the total test positivity was 0.7% for exposed newborns; 1.0% tested positive on an initial test, and 0% were positive on retest. During the newborn hospital stay and a 2-week follow-up, 0% of all newborns showed clinical evidence of infection.
No significant differences were noted between exposed and unexposed newborns in clinical outcomes including gestational age, mode of delivery, 5-minute Apgar score, heart rate, respiratory rate, or temperature. Although more infants of COVID-19–exposed mothers compared with unexposed mothers had an emergency department visit within the first 14 days of life (6% vs. 3%, P = .002), none of the infants was diagnosed with COVID-19 during these visits. Cough, fever, congestion, or bilirubin were more frequent reasons for emergency department visits in the exposed infants compared with unexposed infants, but these differences were not significant.
The study findings were limited by several factors, including the retrospective design and the limited follow-up period to only the first 2 weeks of life, the researchers noted. In addition, perinatal transmission rates were available only for the 202 newborns who were followed up in the hospital system, they said. However, the results suggest that the risk of mother-to-newborn vertical transmission of COVID-19 remains low, even when mothers are breastfeeding and infants are rooming in, they concluded.
Study supports safety of rooming in
The study is important because of the value of mother and infant bonding, Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview. “We know maternal and infant bonding and breastfeeding are extremely important in the first few days of life,” she said. “Initially, COVID-positive moms were separated from their babies during this important time.” Dr. Kinsella said she was not surprised by the study findings, as they reflect other research that newborns have not been getting infected with COVID-19 from their mothers.
Consequently, the take-home message is that newborns can room in with their mothers in the hospital setting, and they are at low risk for COVID-19 regardless of the mother’s exposure history, said Dr. Kinsella. Looking ahead, future areas of research could include examining SARS-CoV-2 antibodies in newborns, she noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
FROM PAS 2021
COVID-19 in children: Weekly cases drop to 6-month low
Just 1 week after it looked like the COVID-19 situation in children might be taking another turn for the worse, the number of new pediatric cases dropped to its lowest level since October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVID-19 report. During the week of April 30 to May 6 – the same week Rhode Island reported a large backlog of cases and increased its total by 30% – the number of new cases went up slightly after 2 weeks of declines.
Other positive indicators come in the form of the proportion of cases occurring in children. The cumulative percentage of cases in children since the start of the pandemic remained at 14.0% for a second consecutive week, and the proportion of new cases in children held at 24.0% and did not increase for the first time in 6 weeks, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of child COVID-19 cases reported in these jurisdictions is now up to 3.9 million, for a cumulative rate of 5,187 cases per 100,000 children in the United States. Among the states, total counts range from a low of 4,070 in Hawaii to 475,619 in California. Hawaii also has the lowest rate at 1,357 per 100,000 children, while the highest, 9,778 per 100,000, can be found in Rhode Island, the AAP and CHA said.
Deaths in children continue to accumulate at a relatively slow pace, with two more added during the week of May 7-13, bringing the total to 308 for the entire pandemic in 43 states, New York City, Puerto Rico, and Guam. Children’s share of the mortality burden is currently 0.06%, a figure that has not changed since mid-December, and the death rate for children with COVID-19 is 0.01%, according to the report.
Almost two-thirds (65%) of all deaths have occurred in just nine states – Arizona (31), California (21), Colorado (13), Georgia (10), Illinois (18), Maryland (10), Pennsylvania (10), Tennessee (10), and Texas (52) – and New York City (24), while eight states have not reported any deaths yet, the two groups said.
Just 1 week after it looked like the COVID-19 situation in children might be taking another turn for the worse, the number of new pediatric cases dropped to its lowest level since October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVID-19 report. During the week of April 30 to May 6 – the same week Rhode Island reported a large backlog of cases and increased its total by 30% – the number of new cases went up slightly after 2 weeks of declines.
Other positive indicators come in the form of the proportion of cases occurring in children. The cumulative percentage of cases in children since the start of the pandemic remained at 14.0% for a second consecutive week, and the proportion of new cases in children held at 24.0% and did not increase for the first time in 6 weeks, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of child COVID-19 cases reported in these jurisdictions is now up to 3.9 million, for a cumulative rate of 5,187 cases per 100,000 children in the United States. Among the states, total counts range from a low of 4,070 in Hawaii to 475,619 in California. Hawaii also has the lowest rate at 1,357 per 100,000 children, while the highest, 9,778 per 100,000, can be found in Rhode Island, the AAP and CHA said.
Deaths in children continue to accumulate at a relatively slow pace, with two more added during the week of May 7-13, bringing the total to 308 for the entire pandemic in 43 states, New York City, Puerto Rico, and Guam. Children’s share of the mortality burden is currently 0.06%, a figure that has not changed since mid-December, and the death rate for children with COVID-19 is 0.01%, according to the report.
Almost two-thirds (65%) of all deaths have occurred in just nine states – Arizona (31), California (21), Colorado (13), Georgia (10), Illinois (18), Maryland (10), Pennsylvania (10), Tennessee (10), and Texas (52) – and New York City (24), while eight states have not reported any deaths yet, the two groups said.
Just 1 week after it looked like the COVID-19 situation in children might be taking another turn for the worse, the number of new pediatric cases dropped to its lowest level since October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVID-19 report. During the week of April 30 to May 6 – the same week Rhode Island reported a large backlog of cases and increased its total by 30% – the number of new cases went up slightly after 2 weeks of declines.
Other positive indicators come in the form of the proportion of cases occurring in children. The cumulative percentage of cases in children since the start of the pandemic remained at 14.0% for a second consecutive week, and the proportion of new cases in children held at 24.0% and did not increase for the first time in 6 weeks, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of child COVID-19 cases reported in these jurisdictions is now up to 3.9 million, for a cumulative rate of 5,187 cases per 100,000 children in the United States. Among the states, total counts range from a low of 4,070 in Hawaii to 475,619 in California. Hawaii also has the lowest rate at 1,357 per 100,000 children, while the highest, 9,778 per 100,000, can be found in Rhode Island, the AAP and CHA said.
Deaths in children continue to accumulate at a relatively slow pace, with two more added during the week of May 7-13, bringing the total to 308 for the entire pandemic in 43 states, New York City, Puerto Rico, and Guam. Children’s share of the mortality burden is currently 0.06%, a figure that has not changed since mid-December, and the death rate for children with COVID-19 is 0.01%, according to the report.
Almost two-thirds (65%) of all deaths have occurred in just nine states – Arizona (31), California (21), Colorado (13), Georgia (10), Illinois (18), Maryland (10), Pennsylvania (10), Tennessee (10), and Texas (52) – and New York City (24), while eight states have not reported any deaths yet, the two groups said.
Heavy cannabis use in pregnancy correlates with risks to infant
Cannabis use that interferes with a woman’s ability to function during pregnancy is a risk factor for severe health problems in the child, new research indicates.
Pregnant women with cannabis use disorder are more likely to have children with low birth weights and children who die within 1 year of birth, compared with matched controls, according to a study published online in Addiction.
The death rate among infants exposed to prenatal cannabis use disorder was 0.98%, compared with 0.75% among infants whose mothers did not have this diagnosis.
Cannabis use disorder during pregnancy “has increased dramatically in the past two decades,” but few studies have examined the health impacts on offspring, study author Yuyan Shi, PhD, said in an interview. “It is particularly concerning in states with cannabis legalization where cannabis is increasingly available.”
Dr. Shi, a researcher at the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, and colleagues analyzed data from more than 4.8 million mothers who delivered a live singleton birth in California between 2001 and 2012 and their infants. They focused on 20,237 mothers who had a diagnosis of cannabis use disorder at delivery. The disorder is defined by continued use of the drug despite impairments in physical, psychological, and social functioning.
The researchers matched mothers with cannabis use disorder 1:2 to mothers who did not have this diagnosis. They aimed to balance factors such as maternal age, educational attainment, health insurance, physical and mental health conditions, prenatal care, and alcohol and opioid use disorder.
An increasingly common diagnosis
Over the study period, the rate of cannabis use disorder increased from 2.8 cases per 1,000 deliveries in 2001 to 6.9 cases per 1,000 deliveries in 2012.
Cannabis use disorder was associated with increased odds of preterm birth (odds ratio, 1.06), small for gestational age (OR, 1.13), low birth weight (OR, 1.13), and death within 1 year of birth (OR, 1.35), according to the researchers’ estimates. Cannabis use disorder was associated with lower odds of hospitalization within 1 year of birth, however (OR, 0.91).
“The most notable observation is that exposed infants were 35% more likely to die within 1 year of birth than unexposed infants,” Dr. Shi and colleagues wrote. More research is needed to understand the causes of death at different stages of infancy, they said.
The results “imply that cannabis use disorder screening as well as appropriate education, counseling, or referral to substance abuse treatment services should be encouraged among pregnant women,” Dr. Shi said.
The study does not establish that cannabis use disorder causes adverse effects, and it is not clear how the results might apply to mothers who use cannabis but do not meet diagnostic criteria for the disorder, the authors noted.
“Presumably the health consequences of mothers who use cannabis but do not meet the criteria ... are less severe than mothers with cannabis use disorder,” Dr. Shi said. “Unfortunately, no research has been conducted to test this hypothesis.”
Enough data to recommend abstaining
Many clinicians may not feel equipped to make a diagnosis of cannabis use disorder, said Jamie Lo, MD, assistant professor of obstetrics and gynecology at Oregon Health and Science University in Portland.
Although many clinicians ask patients about substance use in general, specifically screening for cannabis use is not necessarily routine practice. “I think people are starting to adopt that, but it probably will take a little bit of time,” Dr. Lo said.
Dr. Lo, who was not involved in the study, researches the effects of marijuana during pregnancy.
Confounding factors such as frequent co-use of tobacco have so far made it “difficult to suss out” whether observed effects are directly from cannabis use, other substances or exposures, or a combination, said Dr. Lo. The possibility that stigma may lead to inaccurate self-reporting poses another challenge. And the range of cannabis delivery devices further complicates matters.
“It is hard to compare smoking a bowl versus a joint versus using the oils or CBD or edibles,” Dr. Lo said. The data regarding cigarettes and alcohol are cleaner and more precise, in comparison.
Still, federal agencies and professional societies agree that “what we do know is enough to recommend that pregnant women abstain from using cannabis during pregnancy,” Dr. Lo said.
The National Institute on Drug Abuse, which funded the study, said the results add to the evidence that prenatal exposure to cannabis may be associated with poor birth outcomes and infant health.
“While we cannot establish that cannabis use caused negative outcomes in this study, these data reinforce the case for caution around using cannabis during pregnancy,” Nora D. Volkow, MD, the director of the agency, said in a news release.
“Careful analysis of data like these is one way we can responsibly study how cannabis use affects the developing child, all while a natural experiment is playing out across our country in places where cannabis is becoming widely available to pregnant consumers.”
The study authors and Dr. Lo had no disclosures.
Cannabis use that interferes with a woman’s ability to function during pregnancy is a risk factor for severe health problems in the child, new research indicates.
Pregnant women with cannabis use disorder are more likely to have children with low birth weights and children who die within 1 year of birth, compared with matched controls, according to a study published online in Addiction.
The death rate among infants exposed to prenatal cannabis use disorder was 0.98%, compared with 0.75% among infants whose mothers did not have this diagnosis.
Cannabis use disorder during pregnancy “has increased dramatically in the past two decades,” but few studies have examined the health impacts on offspring, study author Yuyan Shi, PhD, said in an interview. “It is particularly concerning in states with cannabis legalization where cannabis is increasingly available.”
Dr. Shi, a researcher at the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, and colleagues analyzed data from more than 4.8 million mothers who delivered a live singleton birth in California between 2001 and 2012 and their infants. They focused on 20,237 mothers who had a diagnosis of cannabis use disorder at delivery. The disorder is defined by continued use of the drug despite impairments in physical, psychological, and social functioning.
The researchers matched mothers with cannabis use disorder 1:2 to mothers who did not have this diagnosis. They aimed to balance factors such as maternal age, educational attainment, health insurance, physical and mental health conditions, prenatal care, and alcohol and opioid use disorder.
An increasingly common diagnosis
Over the study period, the rate of cannabis use disorder increased from 2.8 cases per 1,000 deliveries in 2001 to 6.9 cases per 1,000 deliveries in 2012.
Cannabis use disorder was associated with increased odds of preterm birth (odds ratio, 1.06), small for gestational age (OR, 1.13), low birth weight (OR, 1.13), and death within 1 year of birth (OR, 1.35), according to the researchers’ estimates. Cannabis use disorder was associated with lower odds of hospitalization within 1 year of birth, however (OR, 0.91).
“The most notable observation is that exposed infants were 35% more likely to die within 1 year of birth than unexposed infants,” Dr. Shi and colleagues wrote. More research is needed to understand the causes of death at different stages of infancy, they said.
The results “imply that cannabis use disorder screening as well as appropriate education, counseling, or referral to substance abuse treatment services should be encouraged among pregnant women,” Dr. Shi said.
The study does not establish that cannabis use disorder causes adverse effects, and it is not clear how the results might apply to mothers who use cannabis but do not meet diagnostic criteria for the disorder, the authors noted.
“Presumably the health consequences of mothers who use cannabis but do not meet the criteria ... are less severe than mothers with cannabis use disorder,” Dr. Shi said. “Unfortunately, no research has been conducted to test this hypothesis.”
Enough data to recommend abstaining
Many clinicians may not feel equipped to make a diagnosis of cannabis use disorder, said Jamie Lo, MD, assistant professor of obstetrics and gynecology at Oregon Health and Science University in Portland.
Although many clinicians ask patients about substance use in general, specifically screening for cannabis use is not necessarily routine practice. “I think people are starting to adopt that, but it probably will take a little bit of time,” Dr. Lo said.
Dr. Lo, who was not involved in the study, researches the effects of marijuana during pregnancy.
Confounding factors such as frequent co-use of tobacco have so far made it “difficult to suss out” whether observed effects are directly from cannabis use, other substances or exposures, or a combination, said Dr. Lo. The possibility that stigma may lead to inaccurate self-reporting poses another challenge. And the range of cannabis delivery devices further complicates matters.
“It is hard to compare smoking a bowl versus a joint versus using the oils or CBD or edibles,” Dr. Lo said. The data regarding cigarettes and alcohol are cleaner and more precise, in comparison.
Still, federal agencies and professional societies agree that “what we do know is enough to recommend that pregnant women abstain from using cannabis during pregnancy,” Dr. Lo said.
The National Institute on Drug Abuse, which funded the study, said the results add to the evidence that prenatal exposure to cannabis may be associated with poor birth outcomes and infant health.
“While we cannot establish that cannabis use caused negative outcomes in this study, these data reinforce the case for caution around using cannabis during pregnancy,” Nora D. Volkow, MD, the director of the agency, said in a news release.
“Careful analysis of data like these is one way we can responsibly study how cannabis use affects the developing child, all while a natural experiment is playing out across our country in places where cannabis is becoming widely available to pregnant consumers.”
The study authors and Dr. Lo had no disclosures.
Cannabis use that interferes with a woman’s ability to function during pregnancy is a risk factor for severe health problems in the child, new research indicates.
Pregnant women with cannabis use disorder are more likely to have children with low birth weights and children who die within 1 year of birth, compared with matched controls, according to a study published online in Addiction.
The death rate among infants exposed to prenatal cannabis use disorder was 0.98%, compared with 0.75% among infants whose mothers did not have this diagnosis.
Cannabis use disorder during pregnancy “has increased dramatically in the past two decades,” but few studies have examined the health impacts on offspring, study author Yuyan Shi, PhD, said in an interview. “It is particularly concerning in states with cannabis legalization where cannabis is increasingly available.”
Dr. Shi, a researcher at the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, and colleagues analyzed data from more than 4.8 million mothers who delivered a live singleton birth in California between 2001 and 2012 and their infants. They focused on 20,237 mothers who had a diagnosis of cannabis use disorder at delivery. The disorder is defined by continued use of the drug despite impairments in physical, psychological, and social functioning.
The researchers matched mothers with cannabis use disorder 1:2 to mothers who did not have this diagnosis. They aimed to balance factors such as maternal age, educational attainment, health insurance, physical and mental health conditions, prenatal care, and alcohol and opioid use disorder.
An increasingly common diagnosis
Over the study period, the rate of cannabis use disorder increased from 2.8 cases per 1,000 deliveries in 2001 to 6.9 cases per 1,000 deliveries in 2012.
Cannabis use disorder was associated with increased odds of preterm birth (odds ratio, 1.06), small for gestational age (OR, 1.13), low birth weight (OR, 1.13), and death within 1 year of birth (OR, 1.35), according to the researchers’ estimates. Cannabis use disorder was associated with lower odds of hospitalization within 1 year of birth, however (OR, 0.91).
“The most notable observation is that exposed infants were 35% more likely to die within 1 year of birth than unexposed infants,” Dr. Shi and colleagues wrote. More research is needed to understand the causes of death at different stages of infancy, they said.
The results “imply that cannabis use disorder screening as well as appropriate education, counseling, or referral to substance abuse treatment services should be encouraged among pregnant women,” Dr. Shi said.
The study does not establish that cannabis use disorder causes adverse effects, and it is not clear how the results might apply to mothers who use cannabis but do not meet diagnostic criteria for the disorder, the authors noted.
“Presumably the health consequences of mothers who use cannabis but do not meet the criteria ... are less severe than mothers with cannabis use disorder,” Dr. Shi said. “Unfortunately, no research has been conducted to test this hypothesis.”
Enough data to recommend abstaining
Many clinicians may not feel equipped to make a diagnosis of cannabis use disorder, said Jamie Lo, MD, assistant professor of obstetrics and gynecology at Oregon Health and Science University in Portland.
Although many clinicians ask patients about substance use in general, specifically screening for cannabis use is not necessarily routine practice. “I think people are starting to adopt that, but it probably will take a little bit of time,” Dr. Lo said.
Dr. Lo, who was not involved in the study, researches the effects of marijuana during pregnancy.
Confounding factors such as frequent co-use of tobacco have so far made it “difficult to suss out” whether observed effects are directly from cannabis use, other substances or exposures, or a combination, said Dr. Lo. The possibility that stigma may lead to inaccurate self-reporting poses another challenge. And the range of cannabis delivery devices further complicates matters.
“It is hard to compare smoking a bowl versus a joint versus using the oils or CBD or edibles,” Dr. Lo said. The data regarding cigarettes and alcohol are cleaner and more precise, in comparison.
Still, federal agencies and professional societies agree that “what we do know is enough to recommend that pregnant women abstain from using cannabis during pregnancy,” Dr. Lo said.
The National Institute on Drug Abuse, which funded the study, said the results add to the evidence that prenatal exposure to cannabis may be associated with poor birth outcomes and infant health.
“While we cannot establish that cannabis use caused negative outcomes in this study, these data reinforce the case for caution around using cannabis during pregnancy,” Nora D. Volkow, MD, the director of the agency, said in a news release.
“Careful analysis of data like these is one way we can responsibly study how cannabis use affects the developing child, all while a natural experiment is playing out across our country in places where cannabis is becoming widely available to pregnant consumers.”
The study authors and Dr. Lo had no disclosures.
Infants with UTI do not have an increased risk of bacterial meningitis
The decision to perform a spinal tap procedure in infants to determine whether they have bacterial meningitis should not be guided by abnormal urinalysis results alone, according to new research published in JAMA Network Open.
The findings suggest febrile infants with positive urinalysis results do not have a higher risk of bacterial meningitis than those with negative urinalysis results.
Nearly 1 in 100,000 people are diagnosed with bacterial meningitis in the United States each year, according to Boston Children’s Hospital. Infants have an increased risk for bacterial meningitis, compared with those in other age groups, according to the Centers for Disease Control and Prevention. However, rates of the infectious disease have been declining in the United States since the late 1990s.
Researchers of the current study said published guidelines and quality initiatives recommend performing a lumbar puncture on febrile infants with positive urinalysis results to exclude bacterial meningitis as a cause.
“It really raises the question of should we be doing everything we’re doing?” study author Brett Burstein, MD, PhD, MPH, said in an interview. “What we conclude here is that, contrary to all the published guidelines, this invasive strategy for testing in well-appearing infants should not be guided by the urinalysis results. That’s a major departure.”
The study adds to growing research that questions whether a lumbar puncture in infants with fever and a positive urinalysis results should be routinely required.
“[Our findings] certainly goes against 30 years of clinical decisions, rules, and guidelines,” Dr. Burstein said. “We think they’re very important and they stand to change practice because approximately 500 infants will undergo these invasive procedures to not miss that needle in the haystack.”
Dr. Burstein, a clinician-scientist in pediatric emergency medicine at Montreal Children’s Hospital, led a team of researchers to perform a meta-analysis of 48 studies, including data from more than 25,000 infants.
Researchers found that the prevalence of bacterial meningitis in well-appearing febrile infants aged 29-60 days with a positive urinalysis results was 0.44%, compared with 0.50% of infants with negative urinalysis results.
Instead of relying on urinalysis results alone, Dr. Burstein suggests doctors use other stratifying biomarkers to decide whether they should perform a lumbar puncture.
“If you’ve done blood testing, for example, and your blood test results suggest serious infection, that should guide the decision to go on to invasive testing,” Dr. Burstein said. “You can use your urinary tract infection information in combination now with blood results.”
This means that, if infants have reassuring blood results, despite having a urinary tract infection, they do not need invasive testing, according to Dr. Burstein.
Some of the risks involved with invasive lumbar puncture testing include infection, bleeding, respiratory problems, as well as pain for the infant and parental anxiety.
Paul Aronson, MD, MHS, of Yale University, New Haven, Conn., who was not involved in the study, said in an interview that he has personally moved away from routine lumbar puncture in infants with a positive urinalysis, but added that many doctors have not.
Dr. Aronson said that, although there have been previous studies on this topic, what sets Dr. Burstein’s study apart is the fact that it has a “tightly defined” group of patients, which are infants aged between 29 and 60 days. He also said it is helpful that the study compared the prevalence of meningitis between infants who had positive urinalysis results with those who had negative results.
“The study compared positive urinalyses to negative analyses, which in the meta-analysis form had not been done previously,” Dr. Aronson said. “And so I think this [current study] probably provides some of the strongest evidence.”
No relevant financial relationships were reported.
The decision to perform a spinal tap procedure in infants to determine whether they have bacterial meningitis should not be guided by abnormal urinalysis results alone, according to new research published in JAMA Network Open.
The findings suggest febrile infants with positive urinalysis results do not have a higher risk of bacterial meningitis than those with negative urinalysis results.
Nearly 1 in 100,000 people are diagnosed with bacterial meningitis in the United States each year, according to Boston Children’s Hospital. Infants have an increased risk for bacterial meningitis, compared with those in other age groups, according to the Centers for Disease Control and Prevention. However, rates of the infectious disease have been declining in the United States since the late 1990s.
Researchers of the current study said published guidelines and quality initiatives recommend performing a lumbar puncture on febrile infants with positive urinalysis results to exclude bacterial meningitis as a cause.
“It really raises the question of should we be doing everything we’re doing?” study author Brett Burstein, MD, PhD, MPH, said in an interview. “What we conclude here is that, contrary to all the published guidelines, this invasive strategy for testing in well-appearing infants should not be guided by the urinalysis results. That’s a major departure.”
The study adds to growing research that questions whether a lumbar puncture in infants with fever and a positive urinalysis results should be routinely required.
“[Our findings] certainly goes against 30 years of clinical decisions, rules, and guidelines,” Dr. Burstein said. “We think they’re very important and they stand to change practice because approximately 500 infants will undergo these invasive procedures to not miss that needle in the haystack.”
Dr. Burstein, a clinician-scientist in pediatric emergency medicine at Montreal Children’s Hospital, led a team of researchers to perform a meta-analysis of 48 studies, including data from more than 25,000 infants.
Researchers found that the prevalence of bacterial meningitis in well-appearing febrile infants aged 29-60 days with a positive urinalysis results was 0.44%, compared with 0.50% of infants with negative urinalysis results.
Instead of relying on urinalysis results alone, Dr. Burstein suggests doctors use other stratifying biomarkers to decide whether they should perform a lumbar puncture.
“If you’ve done blood testing, for example, and your blood test results suggest serious infection, that should guide the decision to go on to invasive testing,” Dr. Burstein said. “You can use your urinary tract infection information in combination now with blood results.”
This means that, if infants have reassuring blood results, despite having a urinary tract infection, they do not need invasive testing, according to Dr. Burstein.
Some of the risks involved with invasive lumbar puncture testing include infection, bleeding, respiratory problems, as well as pain for the infant and parental anxiety.
Paul Aronson, MD, MHS, of Yale University, New Haven, Conn., who was not involved in the study, said in an interview that he has personally moved away from routine lumbar puncture in infants with a positive urinalysis, but added that many doctors have not.
Dr. Aronson said that, although there have been previous studies on this topic, what sets Dr. Burstein’s study apart is the fact that it has a “tightly defined” group of patients, which are infants aged between 29 and 60 days. He also said it is helpful that the study compared the prevalence of meningitis between infants who had positive urinalysis results with those who had negative results.
“The study compared positive urinalyses to negative analyses, which in the meta-analysis form had not been done previously,” Dr. Aronson said. “And so I think this [current study] probably provides some of the strongest evidence.”
No relevant financial relationships were reported.
The decision to perform a spinal tap procedure in infants to determine whether they have bacterial meningitis should not be guided by abnormal urinalysis results alone, according to new research published in JAMA Network Open.
The findings suggest febrile infants with positive urinalysis results do not have a higher risk of bacterial meningitis than those with negative urinalysis results.
Nearly 1 in 100,000 people are diagnosed with bacterial meningitis in the United States each year, according to Boston Children’s Hospital. Infants have an increased risk for bacterial meningitis, compared with those in other age groups, according to the Centers for Disease Control and Prevention. However, rates of the infectious disease have been declining in the United States since the late 1990s.
Researchers of the current study said published guidelines and quality initiatives recommend performing a lumbar puncture on febrile infants with positive urinalysis results to exclude bacterial meningitis as a cause.
“It really raises the question of should we be doing everything we’re doing?” study author Brett Burstein, MD, PhD, MPH, said in an interview. “What we conclude here is that, contrary to all the published guidelines, this invasive strategy for testing in well-appearing infants should not be guided by the urinalysis results. That’s a major departure.”
The study adds to growing research that questions whether a lumbar puncture in infants with fever and a positive urinalysis results should be routinely required.
“[Our findings] certainly goes against 30 years of clinical decisions, rules, and guidelines,” Dr. Burstein said. “We think they’re very important and they stand to change practice because approximately 500 infants will undergo these invasive procedures to not miss that needle in the haystack.”
Dr. Burstein, a clinician-scientist in pediatric emergency medicine at Montreal Children’s Hospital, led a team of researchers to perform a meta-analysis of 48 studies, including data from more than 25,000 infants.
Researchers found that the prevalence of bacterial meningitis in well-appearing febrile infants aged 29-60 days with a positive urinalysis results was 0.44%, compared with 0.50% of infants with negative urinalysis results.
Instead of relying on urinalysis results alone, Dr. Burstein suggests doctors use other stratifying biomarkers to decide whether they should perform a lumbar puncture.
“If you’ve done blood testing, for example, and your blood test results suggest serious infection, that should guide the decision to go on to invasive testing,” Dr. Burstein said. “You can use your urinary tract infection information in combination now with blood results.”
This means that, if infants have reassuring blood results, despite having a urinary tract infection, they do not need invasive testing, according to Dr. Burstein.
Some of the risks involved with invasive lumbar puncture testing include infection, bleeding, respiratory problems, as well as pain for the infant and parental anxiety.
Paul Aronson, MD, MHS, of Yale University, New Haven, Conn., who was not involved in the study, said in an interview that he has personally moved away from routine lumbar puncture in infants with a positive urinalysis, but added that many doctors have not.
Dr. Aronson said that, although there have been previous studies on this topic, what sets Dr. Burstein’s study apart is the fact that it has a “tightly defined” group of patients, which are infants aged between 29 and 60 days. He also said it is helpful that the study compared the prevalence of meningitis between infants who had positive urinalysis results with those who had negative results.
“The study compared positive urinalyses to negative analyses, which in the meta-analysis form had not been done previously,” Dr. Aronson said. “And so I think this [current study] probably provides some of the strongest evidence.”
No relevant financial relationships were reported.
FROM JAMA NETWORK OPEN
55 new chemicals found in pregnant women, their newborns
Fifty-five chemicals never before reported in humans were found in pregnant women, according to a study from the University of California, San Francisco. The chemicals likely come from consumer products or industrial sources, researchers say.
Findings were published online in Environmental Science and Technology.
Co-first authors Aolin Wang, PhD, and Dimitri Panagopoulos Abrahamsson, PhD, postdoctoral fellows in UCSF’s obstetrics and gynecology department, and colleagues found 109 chemicals in the blood of pregnant women, including 42 “mystery chemicals” whose sources and uses are not known.
The chemicals were also found in their newborns, tests from umbilical cord blood show, suggesting the chemicals cross through the placenta.
Among the chemicals, 40 are used as plasticizers, 28 are used in cosmetics, another 25 are used in consumer products, 29 as pharmaceuticals, 23 as pesticides, three as flame retardants, and seven are PFAS [per- and polyfluoroalkyl substances] compounds used in multiple applications including carpeting and upholstery, the authors report.
Senior author Tracey Woodruff, PhD, MPH, characterized their discoveries as “disturbing.”
She told this news organization that it’s not only frustrating to know the chemicals are present but to know so little about them.
“We know it’s a chemical registered to be manufactured, and it’s used in commerce, but we don’t know where,” she explained. “That’s very disturbing, that we can’t trace them, and that shows a failure in public policy and government.”
“Exposures are occurring without our consent,” said Ms. Woodruff, a former U.S. Environmental Protection Agency scientist, who directs the Program on Reproductive Health and the Environment (PRHE) and the Environmental Research and Translation for Health (EaRTH) Center, both at UCSF.
She said researchers know from previous studies that when the U.S. government acts to remove harmful chemicals from the marketplace, the levels of those chemicals measured in people drop.
“Examples include lead, certain PFAS, flame retardant chemicals, and certain phthalates,” she said. “So public policies can be effective in preventing exposures that can be harmful.”
Technological advances led to the discoveries
The team used high-resolution mass spectrometry (HRMS) to identify human-made chemicals in people.
Dr. Abrahamsson said in an interview that the technology is relatively new in research and had not previously been used to scan for chemicals in pregnant women and their infants.
Because scientists often study what other scientists have studied, he said, the same chemicals tend to get attention. The wider scope made possible by the new technology helps illumine where to focus future research, he said.
A benefit of the technology is that now researchers don’t have to know which chemicals they are looking for when they scan blood samples, but they can observe whatever appears, he said.
Ms. Woodruff said, “We hope this is further data and evidence that support government policies that require industries to tell us where they are using their chemicals and how we might be exposed to them.”
She said this research will also help identify which chemicals to prioritize for monitoring in the environment.
Average age of the women in the study was 32 years. Nearly half were Hispanic; 37% were non-Hispanic Whites; and 17% were non-Hispanic Asians, Pacific Islanders, and African Americans. Half of the participants were born outside the United States and had lived in the U.S. for an average 22 years.
Sean Palfrey, MD, a professor of clinical pediatrics and public health at Boston University, said more chemical discoveries like these will come as technology continues to evolve.
Dr. Palfrey, who was not involved in the study, agrees with the authors that there is a lack of oversight as to what substances are used in products.
“Our industrial regulations are very poor and therefore our industries get away with using new and untested substances in their products,” he told this news organization.
“This lack of regulation is really important when it results in us not recognizing that known and serious toxins are being put into foods or other products, or when a new class of toxin has been invented which is a serious poison. Most of the toxins, though, are discovered in products in very low levels,” he said.
Dr. Palfrey said, however, that focus should stay on the known and serious toxins that seep into the environment from common products.
“It has taken us decades to ban certain flame retardants from home products,” he said. “TOSCA [the Toxic Substances Control Act passed by Congress in 1976] was too limited when it was passed decades ago and is now fearfully out of date. Unless we discover a COVID among the toxins discovered in studies like this, we should focus on the big stuff.”
The authors and Dr. Palfrey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fifty-five chemicals never before reported in humans were found in pregnant women, according to a study from the University of California, San Francisco. The chemicals likely come from consumer products or industrial sources, researchers say.
Findings were published online in Environmental Science and Technology.
Co-first authors Aolin Wang, PhD, and Dimitri Panagopoulos Abrahamsson, PhD, postdoctoral fellows in UCSF’s obstetrics and gynecology department, and colleagues found 109 chemicals in the blood of pregnant women, including 42 “mystery chemicals” whose sources and uses are not known.
The chemicals were also found in their newborns, tests from umbilical cord blood show, suggesting the chemicals cross through the placenta.
Among the chemicals, 40 are used as plasticizers, 28 are used in cosmetics, another 25 are used in consumer products, 29 as pharmaceuticals, 23 as pesticides, three as flame retardants, and seven are PFAS [per- and polyfluoroalkyl substances] compounds used in multiple applications including carpeting and upholstery, the authors report.
Senior author Tracey Woodruff, PhD, MPH, characterized their discoveries as “disturbing.”
She told this news organization that it’s not only frustrating to know the chemicals are present but to know so little about them.
“We know it’s a chemical registered to be manufactured, and it’s used in commerce, but we don’t know where,” she explained. “That’s very disturbing, that we can’t trace them, and that shows a failure in public policy and government.”
“Exposures are occurring without our consent,” said Ms. Woodruff, a former U.S. Environmental Protection Agency scientist, who directs the Program on Reproductive Health and the Environment (PRHE) and the Environmental Research and Translation for Health (EaRTH) Center, both at UCSF.
She said researchers know from previous studies that when the U.S. government acts to remove harmful chemicals from the marketplace, the levels of those chemicals measured in people drop.
“Examples include lead, certain PFAS, flame retardant chemicals, and certain phthalates,” she said. “So public policies can be effective in preventing exposures that can be harmful.”
Technological advances led to the discoveries
The team used high-resolution mass spectrometry (HRMS) to identify human-made chemicals in people.
Dr. Abrahamsson said in an interview that the technology is relatively new in research and had not previously been used to scan for chemicals in pregnant women and their infants.
Because scientists often study what other scientists have studied, he said, the same chemicals tend to get attention. The wider scope made possible by the new technology helps illumine where to focus future research, he said.
A benefit of the technology is that now researchers don’t have to know which chemicals they are looking for when they scan blood samples, but they can observe whatever appears, he said.
Ms. Woodruff said, “We hope this is further data and evidence that support government policies that require industries to tell us where they are using their chemicals and how we might be exposed to them.”
She said this research will also help identify which chemicals to prioritize for monitoring in the environment.
Average age of the women in the study was 32 years. Nearly half were Hispanic; 37% were non-Hispanic Whites; and 17% were non-Hispanic Asians, Pacific Islanders, and African Americans. Half of the participants were born outside the United States and had lived in the U.S. for an average 22 years.
Sean Palfrey, MD, a professor of clinical pediatrics and public health at Boston University, said more chemical discoveries like these will come as technology continues to evolve.
Dr. Palfrey, who was not involved in the study, agrees with the authors that there is a lack of oversight as to what substances are used in products.
“Our industrial regulations are very poor and therefore our industries get away with using new and untested substances in their products,” he told this news organization.
“This lack of regulation is really important when it results in us not recognizing that known and serious toxins are being put into foods or other products, or when a new class of toxin has been invented which is a serious poison. Most of the toxins, though, are discovered in products in very low levels,” he said.
Dr. Palfrey said, however, that focus should stay on the known and serious toxins that seep into the environment from common products.
“It has taken us decades to ban certain flame retardants from home products,” he said. “TOSCA [the Toxic Substances Control Act passed by Congress in 1976] was too limited when it was passed decades ago and is now fearfully out of date. Unless we discover a COVID among the toxins discovered in studies like this, we should focus on the big stuff.”
The authors and Dr. Palfrey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fifty-five chemicals never before reported in humans were found in pregnant women, according to a study from the University of California, San Francisco. The chemicals likely come from consumer products or industrial sources, researchers say.
Findings were published online in Environmental Science and Technology.
Co-first authors Aolin Wang, PhD, and Dimitri Panagopoulos Abrahamsson, PhD, postdoctoral fellows in UCSF’s obstetrics and gynecology department, and colleagues found 109 chemicals in the blood of pregnant women, including 42 “mystery chemicals” whose sources and uses are not known.
The chemicals were also found in their newborns, tests from umbilical cord blood show, suggesting the chemicals cross through the placenta.
Among the chemicals, 40 are used as plasticizers, 28 are used in cosmetics, another 25 are used in consumer products, 29 as pharmaceuticals, 23 as pesticides, three as flame retardants, and seven are PFAS [per- and polyfluoroalkyl substances] compounds used in multiple applications including carpeting and upholstery, the authors report.
Senior author Tracey Woodruff, PhD, MPH, characterized their discoveries as “disturbing.”
She told this news organization that it’s not only frustrating to know the chemicals are present but to know so little about them.
“We know it’s a chemical registered to be manufactured, and it’s used in commerce, but we don’t know where,” she explained. “That’s very disturbing, that we can’t trace them, and that shows a failure in public policy and government.”
“Exposures are occurring without our consent,” said Ms. Woodruff, a former U.S. Environmental Protection Agency scientist, who directs the Program on Reproductive Health and the Environment (PRHE) and the Environmental Research and Translation for Health (EaRTH) Center, both at UCSF.
She said researchers know from previous studies that when the U.S. government acts to remove harmful chemicals from the marketplace, the levels of those chemicals measured in people drop.
“Examples include lead, certain PFAS, flame retardant chemicals, and certain phthalates,” she said. “So public policies can be effective in preventing exposures that can be harmful.”
Technological advances led to the discoveries
The team used high-resolution mass spectrometry (HRMS) to identify human-made chemicals in people.
Dr. Abrahamsson said in an interview that the technology is relatively new in research and had not previously been used to scan for chemicals in pregnant women and their infants.
Because scientists often study what other scientists have studied, he said, the same chemicals tend to get attention. The wider scope made possible by the new technology helps illumine where to focus future research, he said.
A benefit of the technology is that now researchers don’t have to know which chemicals they are looking for when they scan blood samples, but they can observe whatever appears, he said.
Ms. Woodruff said, “We hope this is further data and evidence that support government policies that require industries to tell us where they are using their chemicals and how we might be exposed to them.”
She said this research will also help identify which chemicals to prioritize for monitoring in the environment.
Average age of the women in the study was 32 years. Nearly half were Hispanic; 37% were non-Hispanic Whites; and 17% were non-Hispanic Asians, Pacific Islanders, and African Americans. Half of the participants were born outside the United States and had lived in the U.S. for an average 22 years.
Sean Palfrey, MD, a professor of clinical pediatrics and public health at Boston University, said more chemical discoveries like these will come as technology continues to evolve.
Dr. Palfrey, who was not involved in the study, agrees with the authors that there is a lack of oversight as to what substances are used in products.
“Our industrial regulations are very poor and therefore our industries get away with using new and untested substances in their products,” he told this news organization.
“This lack of regulation is really important when it results in us not recognizing that known and serious toxins are being put into foods or other products, or when a new class of toxin has been invented which is a serious poison. Most of the toxins, though, are discovered in products in very low levels,” he said.
Dr. Palfrey said, however, that focus should stay on the known and serious toxins that seep into the environment from common products.
“It has taken us decades to ban certain flame retardants from home products,” he said. “TOSCA [the Toxic Substances Control Act passed by Congress in 1976] was too limited when it was passed decades ago and is now fearfully out of date. Unless we discover a COVID among the toxins discovered in studies like this, we should focus on the big stuff.”
The authors and Dr. Palfrey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
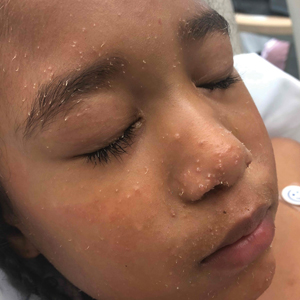
Widespread Hyperkeratotic Papules in a Transplant Recipient
The Diagnosis: Trichodysplasia Spinulosa
Trichodysplasia spinulosa has been described in case reports over the last several decades, with its causative virus trichodysplasia spinulosa-associated polyomavirus (TSPyV) identified in 2010 by van der Meijden et al.1 Trichodysplasia spinulosa-associated polyomavirus is a small, nonenveloped, double-stranded DNA virus in the Polyomaviridae family, among several other known cutaneous polyomaviruses including Merkel cell polyomavirus, human polyomavirus (HPyV) 6, HPyV7, HPyV10, and possibly HPyV13.2 The primary target of TSPyV is follicular keratinocytes, and it is believed to cause trichodysplasia spinulosa by primary infection rather than by reactivation. Trichodysplasia spinulosa presents in immunosuppressed patients as a folliculocentric eruption of papules with keratinous spines on the face, often with concurrent alopecia, eventually spreading to the trunk and extremities.3 The diagnosis often is clinical, but a biopsy may be performed for histopathologic confirmation. Alternatively, lesional spicules can be painlessly collected manually and submitted for viral polymerase chain reaction (PCR).4 The diagnosis of trichodysplasia spinulosa can be difficult due to similarities with other more common conditions such as keratosis pilaris, milia, filiform warts, or lichen spinulosus.
Similar to trichodysplasia spinulosa, keratosis pilaris also presents with folliculocentric and often erythematous papules.5 Keratosis pilaris most frequently affects the posterior upper arms and thighs but also may affect the cheeks, as seen in trichodysplasia spinulosa. Differentiation between the 2 diagnoses can be made on a clinical basis, as keratosis pilaris lacks the characteristic keratinous spines and often spares the central face and nose, locations that commonly are affected in trichodysplasia spinulosa.3
Milia typically appear as white to yellow papules, often on the cheeks, eyelids, nose, and chin.6 Given their predilection for the face, milia can appear similarly to trichodysplasia spinulosa. Differentiation can be made clinically, as milia typically are not as numerous as the spiculed papules seen in trichodysplasia spinulosa. Morphologically, milia will present as smooth, dome-shaped papules as opposed to the keratinous spicules seen in trichodysplasia spinulosa. The diagnosis of milia can be confirmed by incision and removal of the white chalky keratin core, a feature absent in trichodysplasia spinulosa.
Filiform warts are benign epidermal proliferations caused by human papillomavirus infection that manifest as flesh-colored, verrucous, hyperkeratotic papules.7 They can appear on virtually any skin surface, including the face, and thus may be mistaken for trichodysplasia spinulosa. Close inspection usually will reveal tiny black dots that represent thrombosed capillaries, a feature lacking in trichodysplasia spinulosa. In long-standing lesions or immunocompromised patients, confluent verrucous plaques may develop.8 Diagnosis of filiform warts can be confirmed with biopsy, which will demonstrate a compact stratum corneum, coarse hypergranulosis, and papillomatosis curving inward, while biopsy of a trichodysplasia spinulosa lesion would show polyomavirus infection of the hair follicle and characteristic eosinophilic inclusion bodies.9
Lichen spinulosus may appear as multiple folliculocentric scaly papules with hairlike horny spines.10 Lichen spinulosus differs from trichodysplasia spinulosa in that it commonly appears on the neck, abdomen, trochanteric region, arms, elbows, or knees. Lichen spinulosus also classically appears as a concrete cluster of papules, often localized to a certain region, in contrast to trichodysplasia spinulosa, which will be widespread, often spreading over time. Finally, clinical history may help differentiate the 2 entities. Lichen spinulosus most often appears in children and adolescents and often has an indolent course, typically resolving during puberty, while trichodysplasia spinulosa is seen in immunocompromised patients.
In our patient, the dermatology team made a diagnosis of trichodysplasia spinulosa based on the characteristic clinical presentation, which was confirmed after approximately 10 lesional spicules were removed by tissue forceps and submitted for PCR analysis showing TSPyV (Figure). Two other cases utilized spicule PCR analysis for confirmation of TSPyV.11,12 This technique may represent a viable option for diagnostic confirmation in pediatric cases.
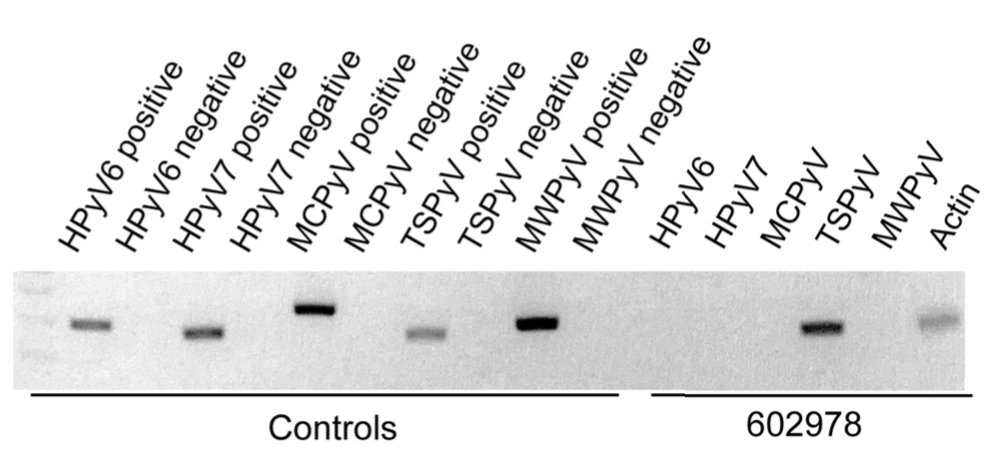
Although some articles have examined the molecular and biologic features of trichodysplasia spinulosa, literature on clinical presentation and management is limited to isolated case reports with no comprehensive studies to establish a standardized treatment. Of these reports, oral valganciclovir 900 mg daily, topical retinoids, cidofovir cream 1% to 3%, and decreasing or altering the immunosuppressive regimen all have been noted to provide clinical improvement.13,14 Other therapies including leflunomide and routine manual extraction of spicules also have shown effectiveness in the treatment of trichodysplasia spinulosa.15
In our patient, treatment included decreasing immunosuppression, as she was getting recurrent sinus and upper respiratory infections. Mycophenolate mofetil was discontinued, and the patient was continued solely on tacrolimus therapy. She demonstrated notable improvement after 3 months, with approximately 50% clearance of the eruption. A mutual decision was made at that visit to initiate therapy with compounded cidofovir cream 1% daily to the lesions until the next follow-up visit. Unfortunately, the patient did not return for her scheduled dermatology visits and was lost to long-term follow-up.
Acknowledgment
We thank Richard C. Wang, MD, PhD (Dallas, Texas), for his dermatologic expertise and assistance in analysis of lesional samples for TSPyV.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromised patient. PLoS Pathog. 2010;6:E1001024.
- Sheu JC, Tran J, Rady PL, et al. Polyomaviruses of the skin: integrating molecular and clinical advances in an emerging class of viruses. Br J Dermatol. 2019;180:1302-1311.
- Sperling LC, Tomaszewski MM, Thomas DA. Viral-associated trichodysplasia in patients who are immunocompromised. J Am Acad Dermatol. 2004;50:318-322.
- Wu JH, Nguyen HP, Rady PL, et al. Molecular insight into the viral biology and clinical features of trichodysplasia spinulosa. Br J Dermatol. 2016;174:490-498.
- Hwang S, Schwartz RA. Keratosis pilaris: a common follicular hyperkeratosis. Cutis. 2008;82:177-180.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- Micali G, Dall'Oglio F, Nasca MR, et al. Management of cutaneous warts: an evidence-based approach. Am J Clin Dermatol. 2004;5:311-317.
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018.
- Elston DM, Ferringer T, Ko CJ. Dermatopathology. 3rd ed. Elsevier; 2018.
- Tilly JJ, Drolet BA, Esterly NB. Lichenoid eruptions in children. J Am Acad Dermatol. 2004;51:606-624.
- Chamseddin BH, Tran BAPD, Lee EE, et al. Trichodysplasia spinulosa in a child: identification of trichodysplasia spinulosa-associated polyomavirus in skin, serum, and urine. Pediatr Dermatol. 2019;36:723-724.
- Sonstegard A, Grossman M, Garg A. Trichodysplasia spinulosa in a kidney transplant recipient. JAMA Dermatol. 2021;157:105.
- Leitenberger JJ, Abdelmalek M, Wang RC, et al. Two cases of trichodysplasia spinulosa responsive to compounded topical cidofovir 3% cream. JAAD Case Rep. 2015;1:S33-S35.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Nguyen KD, Chamseddin BH, Cockerell CJ, et al. The biology and clinical features of cutaneous polyomaviruses. J Invest Dermatol. 2019;139:285-292.
The Diagnosis: Trichodysplasia Spinulosa
Trichodysplasia spinulosa has been described in case reports over the last several decades, with its causative virus trichodysplasia spinulosa-associated polyomavirus (TSPyV) identified in 2010 by van der Meijden et al.1 Trichodysplasia spinulosa-associated polyomavirus is a small, nonenveloped, double-stranded DNA virus in the Polyomaviridae family, among several other known cutaneous polyomaviruses including Merkel cell polyomavirus, human polyomavirus (HPyV) 6, HPyV7, HPyV10, and possibly HPyV13.2 The primary target of TSPyV is follicular keratinocytes, and it is believed to cause trichodysplasia spinulosa by primary infection rather than by reactivation. Trichodysplasia spinulosa presents in immunosuppressed patients as a folliculocentric eruption of papules with keratinous spines on the face, often with concurrent alopecia, eventually spreading to the trunk and extremities.3 The diagnosis often is clinical, but a biopsy may be performed for histopathologic confirmation. Alternatively, lesional spicules can be painlessly collected manually and submitted for viral polymerase chain reaction (PCR).4 The diagnosis of trichodysplasia spinulosa can be difficult due to similarities with other more common conditions such as keratosis pilaris, milia, filiform warts, or lichen spinulosus.
Similar to trichodysplasia spinulosa, keratosis pilaris also presents with folliculocentric and often erythematous papules.5 Keratosis pilaris most frequently affects the posterior upper arms and thighs but also may affect the cheeks, as seen in trichodysplasia spinulosa. Differentiation between the 2 diagnoses can be made on a clinical basis, as keratosis pilaris lacks the characteristic keratinous spines and often spares the central face and nose, locations that commonly are affected in trichodysplasia spinulosa.3
Milia typically appear as white to yellow papules, often on the cheeks, eyelids, nose, and chin.6 Given their predilection for the face, milia can appear similarly to trichodysplasia spinulosa. Differentiation can be made clinically, as milia typically are not as numerous as the spiculed papules seen in trichodysplasia spinulosa. Morphologically, milia will present as smooth, dome-shaped papules as opposed to the keratinous spicules seen in trichodysplasia spinulosa. The diagnosis of milia can be confirmed by incision and removal of the white chalky keratin core, a feature absent in trichodysplasia spinulosa.
Filiform warts are benign epidermal proliferations caused by human papillomavirus infection that manifest as flesh-colored, verrucous, hyperkeratotic papules.7 They can appear on virtually any skin surface, including the face, and thus may be mistaken for trichodysplasia spinulosa. Close inspection usually will reveal tiny black dots that represent thrombosed capillaries, a feature lacking in trichodysplasia spinulosa. In long-standing lesions or immunocompromised patients, confluent verrucous plaques may develop.8 Diagnosis of filiform warts can be confirmed with biopsy, which will demonstrate a compact stratum corneum, coarse hypergranulosis, and papillomatosis curving inward, while biopsy of a trichodysplasia spinulosa lesion would show polyomavirus infection of the hair follicle and characteristic eosinophilic inclusion bodies.9
Lichen spinulosus may appear as multiple folliculocentric scaly papules with hairlike horny spines.10 Lichen spinulosus differs from trichodysplasia spinulosa in that it commonly appears on the neck, abdomen, trochanteric region, arms, elbows, or knees. Lichen spinulosus also classically appears as a concrete cluster of papules, often localized to a certain region, in contrast to trichodysplasia spinulosa, which will be widespread, often spreading over time. Finally, clinical history may help differentiate the 2 entities. Lichen spinulosus most often appears in children and adolescents and often has an indolent course, typically resolving during puberty, while trichodysplasia spinulosa is seen in immunocompromised patients.
In our patient, the dermatology team made a diagnosis of trichodysplasia spinulosa based on the characteristic clinical presentation, which was confirmed after approximately 10 lesional spicules were removed by tissue forceps and submitted for PCR analysis showing TSPyV (Figure). Two other cases utilized spicule PCR analysis for confirmation of TSPyV.11,12 This technique may represent a viable option for diagnostic confirmation in pediatric cases.

Although some articles have examined the molecular and biologic features of trichodysplasia spinulosa, literature on clinical presentation and management is limited to isolated case reports with no comprehensive studies to establish a standardized treatment. Of these reports, oral valganciclovir 900 mg daily, topical retinoids, cidofovir cream 1% to 3%, and decreasing or altering the immunosuppressive regimen all have been noted to provide clinical improvement.13,14 Other therapies including leflunomide and routine manual extraction of spicules also have shown effectiveness in the treatment of trichodysplasia spinulosa.15
In our patient, treatment included decreasing immunosuppression, as she was getting recurrent sinus and upper respiratory infections. Mycophenolate mofetil was discontinued, and the patient was continued solely on tacrolimus therapy. She demonstrated notable improvement after 3 months, with approximately 50% clearance of the eruption. A mutual decision was made at that visit to initiate therapy with compounded cidofovir cream 1% daily to the lesions until the next follow-up visit. Unfortunately, the patient did not return for her scheduled dermatology visits and was lost to long-term follow-up.
Acknowledgment
We thank Richard C. Wang, MD, PhD (Dallas, Texas), for his dermatologic expertise and assistance in analysis of lesional samples for TSPyV.
The Diagnosis: Trichodysplasia Spinulosa
Trichodysplasia spinulosa has been described in case reports over the last several decades, with its causative virus trichodysplasia spinulosa-associated polyomavirus (TSPyV) identified in 2010 by van der Meijden et al.1 Trichodysplasia spinulosa-associated polyomavirus is a small, nonenveloped, double-stranded DNA virus in the Polyomaviridae family, among several other known cutaneous polyomaviruses including Merkel cell polyomavirus, human polyomavirus (HPyV) 6, HPyV7, HPyV10, and possibly HPyV13.2 The primary target of TSPyV is follicular keratinocytes, and it is believed to cause trichodysplasia spinulosa by primary infection rather than by reactivation. Trichodysplasia spinulosa presents in immunosuppressed patients as a folliculocentric eruption of papules with keratinous spines on the face, often with concurrent alopecia, eventually spreading to the trunk and extremities.3 The diagnosis often is clinical, but a biopsy may be performed for histopathologic confirmation. Alternatively, lesional spicules can be painlessly collected manually and submitted for viral polymerase chain reaction (PCR).4 The diagnosis of trichodysplasia spinulosa can be difficult due to similarities with other more common conditions such as keratosis pilaris, milia, filiform warts, or lichen spinulosus.
Similar to trichodysplasia spinulosa, keratosis pilaris also presents with folliculocentric and often erythematous papules.5 Keratosis pilaris most frequently affects the posterior upper arms and thighs but also may affect the cheeks, as seen in trichodysplasia spinulosa. Differentiation between the 2 diagnoses can be made on a clinical basis, as keratosis pilaris lacks the characteristic keratinous spines and often spares the central face and nose, locations that commonly are affected in trichodysplasia spinulosa.3
Milia typically appear as white to yellow papules, often on the cheeks, eyelids, nose, and chin.6 Given their predilection for the face, milia can appear similarly to trichodysplasia spinulosa. Differentiation can be made clinically, as milia typically are not as numerous as the spiculed papules seen in trichodysplasia spinulosa. Morphologically, milia will present as smooth, dome-shaped papules as opposed to the keratinous spicules seen in trichodysplasia spinulosa. The diagnosis of milia can be confirmed by incision and removal of the white chalky keratin core, a feature absent in trichodysplasia spinulosa.
Filiform warts are benign epidermal proliferations caused by human papillomavirus infection that manifest as flesh-colored, verrucous, hyperkeratotic papules.7 They can appear on virtually any skin surface, including the face, and thus may be mistaken for trichodysplasia spinulosa. Close inspection usually will reveal tiny black dots that represent thrombosed capillaries, a feature lacking in trichodysplasia spinulosa. In long-standing lesions or immunocompromised patients, confluent verrucous plaques may develop.8 Diagnosis of filiform warts can be confirmed with biopsy, which will demonstrate a compact stratum corneum, coarse hypergranulosis, and papillomatosis curving inward, while biopsy of a trichodysplasia spinulosa lesion would show polyomavirus infection of the hair follicle and characteristic eosinophilic inclusion bodies.9
Lichen spinulosus may appear as multiple folliculocentric scaly papules with hairlike horny spines.10 Lichen spinulosus differs from trichodysplasia spinulosa in that it commonly appears on the neck, abdomen, trochanteric region, arms, elbows, or knees. Lichen spinulosus also classically appears as a concrete cluster of papules, often localized to a certain region, in contrast to trichodysplasia spinulosa, which will be widespread, often spreading over time. Finally, clinical history may help differentiate the 2 entities. Lichen spinulosus most often appears in children and adolescents and often has an indolent course, typically resolving during puberty, while trichodysplasia spinulosa is seen in immunocompromised patients.
In our patient, the dermatology team made a diagnosis of trichodysplasia spinulosa based on the characteristic clinical presentation, which was confirmed after approximately 10 lesional spicules were removed by tissue forceps and submitted for PCR analysis showing TSPyV (Figure). Two other cases utilized spicule PCR analysis for confirmation of TSPyV.11,12 This technique may represent a viable option for diagnostic confirmation in pediatric cases.

Although some articles have examined the molecular and biologic features of trichodysplasia spinulosa, literature on clinical presentation and management is limited to isolated case reports with no comprehensive studies to establish a standardized treatment. Of these reports, oral valganciclovir 900 mg daily, topical retinoids, cidofovir cream 1% to 3%, and decreasing or altering the immunosuppressive regimen all have been noted to provide clinical improvement.13,14 Other therapies including leflunomide and routine manual extraction of spicules also have shown effectiveness in the treatment of trichodysplasia spinulosa.15
In our patient, treatment included decreasing immunosuppression, as she was getting recurrent sinus and upper respiratory infections. Mycophenolate mofetil was discontinued, and the patient was continued solely on tacrolimus therapy. She demonstrated notable improvement after 3 months, with approximately 50% clearance of the eruption. A mutual decision was made at that visit to initiate therapy with compounded cidofovir cream 1% daily to the lesions until the next follow-up visit. Unfortunately, the patient did not return for her scheduled dermatology visits and was lost to long-term follow-up.
Acknowledgment
We thank Richard C. Wang, MD, PhD (Dallas, Texas), for his dermatologic expertise and assistance in analysis of lesional samples for TSPyV.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromised patient. PLoS Pathog. 2010;6:E1001024.
- Sheu JC, Tran J, Rady PL, et al. Polyomaviruses of the skin: integrating molecular and clinical advances in an emerging class of viruses. Br J Dermatol. 2019;180:1302-1311.
- Sperling LC, Tomaszewski MM, Thomas DA. Viral-associated trichodysplasia in patients who are immunocompromised. J Am Acad Dermatol. 2004;50:318-322.
- Wu JH, Nguyen HP, Rady PL, et al. Molecular insight into the viral biology and clinical features of trichodysplasia spinulosa. Br J Dermatol. 2016;174:490-498.
- Hwang S, Schwartz RA. Keratosis pilaris: a common follicular hyperkeratosis. Cutis. 2008;82:177-180.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- Micali G, Dall'Oglio F, Nasca MR, et al. Management of cutaneous warts: an evidence-based approach. Am J Clin Dermatol. 2004;5:311-317.
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018.
- Elston DM, Ferringer T, Ko CJ. Dermatopathology. 3rd ed. Elsevier; 2018.
- Tilly JJ, Drolet BA, Esterly NB. Lichenoid eruptions in children. J Am Acad Dermatol. 2004;51:606-624.
- Chamseddin BH, Tran BAPD, Lee EE, et al. Trichodysplasia spinulosa in a child: identification of trichodysplasia spinulosa-associated polyomavirus in skin, serum, and urine. Pediatr Dermatol. 2019;36:723-724.
- Sonstegard A, Grossman M, Garg A. Trichodysplasia spinulosa in a kidney transplant recipient. JAMA Dermatol. 2021;157:105.
- Leitenberger JJ, Abdelmalek M, Wang RC, et al. Two cases of trichodysplasia spinulosa responsive to compounded topical cidofovir 3% cream. JAAD Case Rep. 2015;1:S33-S35.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Nguyen KD, Chamseddin BH, Cockerell CJ, et al. The biology and clinical features of cutaneous polyomaviruses. J Invest Dermatol. 2019;139:285-292.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromised patient. PLoS Pathog. 2010;6:E1001024.
- Sheu JC, Tran J, Rady PL, et al. Polyomaviruses of the skin: integrating molecular and clinical advances in an emerging class of viruses. Br J Dermatol. 2019;180:1302-1311.
- Sperling LC, Tomaszewski MM, Thomas DA. Viral-associated trichodysplasia in patients who are immunocompromised. J Am Acad Dermatol. 2004;50:318-322.
- Wu JH, Nguyen HP, Rady PL, et al. Molecular insight into the viral biology and clinical features of trichodysplasia spinulosa. Br J Dermatol. 2016;174:490-498.
- Hwang S, Schwartz RA. Keratosis pilaris: a common follicular hyperkeratosis. Cutis. 2008;82:177-180.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- Micali G, Dall'Oglio F, Nasca MR, et al. Management of cutaneous warts: an evidence-based approach. Am J Clin Dermatol. 2004;5:311-317.
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018.
- Elston DM, Ferringer T, Ko CJ. Dermatopathology. 3rd ed. Elsevier; 2018.
- Tilly JJ, Drolet BA, Esterly NB. Lichenoid eruptions in children. J Am Acad Dermatol. 2004;51:606-624.
- Chamseddin BH, Tran BAPD, Lee EE, et al. Trichodysplasia spinulosa in a child: identification of trichodysplasia spinulosa-associated polyomavirus in skin, serum, and urine. Pediatr Dermatol. 2019;36:723-724.
- Sonstegard A, Grossman M, Garg A. Trichodysplasia spinulosa in a kidney transplant recipient. JAMA Dermatol. 2021;157:105.
- Leitenberger JJ, Abdelmalek M, Wang RC, et al. Two cases of trichodysplasia spinulosa responsive to compounded topical cidofovir 3% cream. JAAD Case Rep. 2015;1:S33-S35.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Nguyen KD, Chamseddin BH, Cockerell CJ, et al. The biology and clinical features of cutaneous polyomaviruses. J Invest Dermatol. 2019;139:285-292.
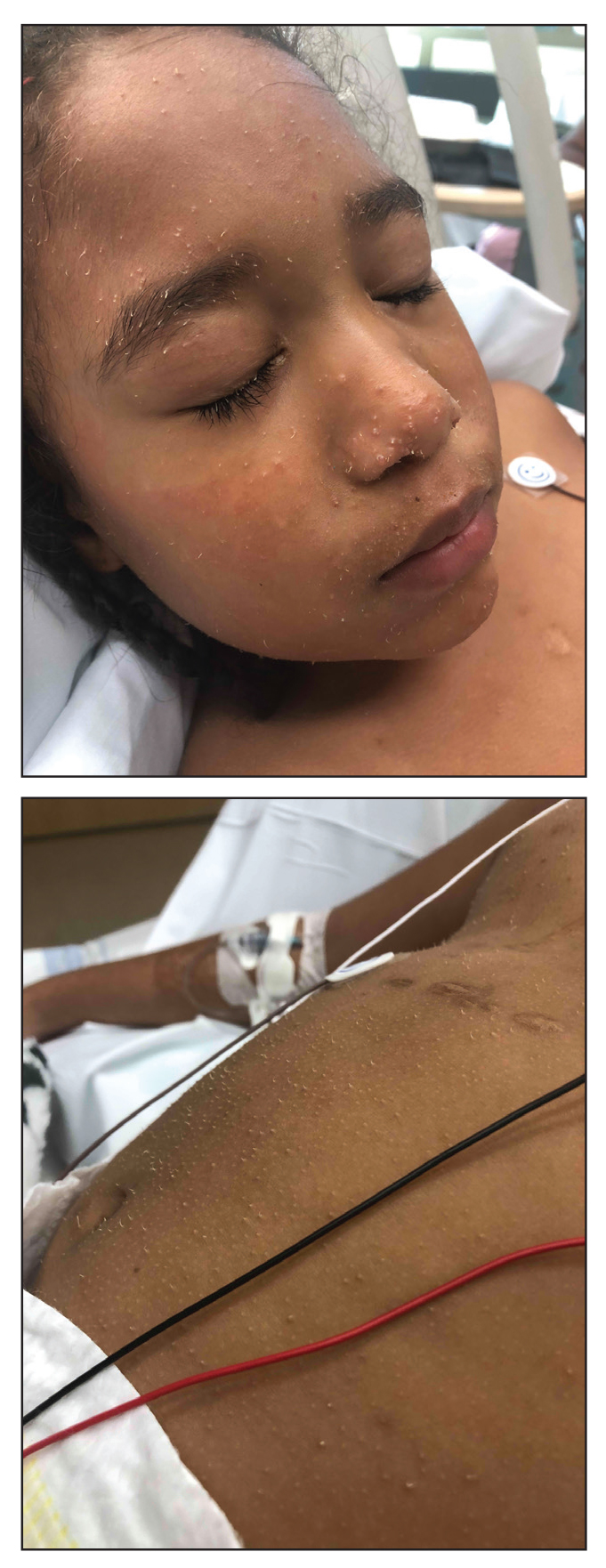
A 4-year-old girl with a history of cardiac transplantation 1 year prior for dilated cardiomyopathy presented to the dermatology consultation service with widespread hyperkeratotic papules of 2 months’ duration. The eruption initially had appeared on the face with subsequent involvement of the trunk and extremities. Her immunosuppressive medications included oral tacrolimus and mycophenolate mofetil. No over-the-counter or prescription treatments had been used for the eruption; the patient’s mother had been manually extracting the spicules from the nose, cheeks, and forehead with tweezers. The lesions were asymptomatic with only mild follicular erythema. Physical examination revealed multiple folliculocentric keratinous spicules on the nose, cheeks, forehead (top), trunk (bottom), arms, and legs.
PHM groups issue Choosing Wisely® recommendations
SHM members involved from the start
The Choosing Wisely® Pediatric Hospital Medicine (PHM) recommendations were published in January 2021. The initial Choosing Wisely® PHM recommendations were released in 2012 and the 2021 recommendations were the result of an extensive and years-long process. The Choosing Wisely® campaign, an initiative led by the American Board of Internal Medicine, was developed to enhance clinician-patient conversations, promoting care that is evidenced based, free from harm, and truly necessary.
The campaign has been embraced by the entire medical community, with more than 70 professional medical societies releasing recommendations. With its emphasis on high value care and eliminating medical waste, it is no surprise that the Choosing Wisely® campaign has found a home in a pediatric hospital medicine community that prides itself on those very traits. This article sheds light on the recommendation development process and identifies challenges and opportunities for implementation across the country.
The Choosing Wisely® process started with the selection of a committee. This group comprised nine members, with equal representation from all three societies affiliated with PHM: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics’ Section on Hospital Medicine (AAP SOHM), and the Academic Pediatric Association (APA). Members of the committee intentionally represented a wide spectrum of practice variability, geography, and clinical experience.
The SHM members of the group were: James O’Callaghan, MD, FAAP, SFHM, pediatric hospitalist at Seattle Children’s Hospital and clinical professor of pediatrics at the University of Washington School of Medicine; Vivian Lee, MD, clinical pediatric hospitalist at Children’s Hospital of Los Angeles and associate professor of pediatrics at USC Keck School of Medicine; and Francisco Alvarez, MD, pediatric hospitalist at Lucile Packard Children’s Hospital, Palo Alto, Calif., and clinical associate professor of pediatrics at Stanford (Calif.) University.
According to Dr. O’Callaghan, it was important that the Choosing Wisely® recommendations come from the broader PHM community, reflecting the community’s priorities.
The committee started the process by asking the broader PHM community to submit ideas for consideration, via SHM’s HMX and the AAP SOHM listserv. The PHM community responded with more than 400 submissions.
Dr. Alvarez said the committee organized and trimmed the initial submissions, removing redundancy, into approximately 200 distinct recommendations. After initial literature review, the committee focused on approximately 70 recommendations. At that point, each member undertook an extensive literature review of the topics.
Once every potential recommendation had received a thorough review, Dr. Lee said, the committee underwent a modified Delphi process to evaluate the list. In this process, each member ranked the recommendations on validity – a measure of the quality of evidence supporting a topic – and feasibility – a measure of the PHM community’s ability to influence compliance.
At the end of this objective process, Dr. O’Callaghan said, the committee chose the five recommendations that received the highest total scores. While there were spirited discussions regarding the data available for each recommendation, all three SHM members of the committee agreed that the objective process played itself out.
Now that the Choosing Wisely® recommendations have been published, the PHM community is challenged to implement these recommendations to spur change for the care of hospitalized children throughout the country. Given the variety that exists in PHM, specifically in practice settings, it may be a daunting task. Dr. O’Callaghan said that differing opinions among physicians in a group may be a challenge to implementing change. “These recommendations allow for those conversations” to take place, he said. Dr. Lee said she hopes these recommendations provide a national panel opinion of the evidence to help support hospitalists in management discussions with others in a hospital – such as subspecialists or emergency department physicians – to increase high value care.
Since the nature of hospital medicine is one of collaboration, these recommendations will allow pediatric hospitalists to lead change throughout their hospitals and health care systems. However, it may not be a quick task. Dr. Alvarez estimates it may take 10-15 years until these recommendations are fully implemented throughout the country. However, there is reason to be optimistic, as the initial PHM Choosing Wisely® recommendations from 2012 have been broadly accepted and now represent national standards of care.
While the road ahead may be long and filled with challenges, the path forward has been clearly delineated, and the PHM community is grateful for the work done by members of the Choosing Wisely® Pediatric Hospital Medicine committee.
Dr. Casey is a pediatric hospitalist at Joe DiMaggio Children’s Hospital in Hollywood, Fla., and a member of the Society of Hospital Medicine’s Pediatric Special Interest Group’s Executive Council.
SHM members involved from the start
SHM members involved from the start
The Choosing Wisely® Pediatric Hospital Medicine (PHM) recommendations were published in January 2021. The initial Choosing Wisely® PHM recommendations were released in 2012 and the 2021 recommendations were the result of an extensive and years-long process. The Choosing Wisely® campaign, an initiative led by the American Board of Internal Medicine, was developed to enhance clinician-patient conversations, promoting care that is evidenced based, free from harm, and truly necessary.
The campaign has been embraced by the entire medical community, with more than 70 professional medical societies releasing recommendations. With its emphasis on high value care and eliminating medical waste, it is no surprise that the Choosing Wisely® campaign has found a home in a pediatric hospital medicine community that prides itself on those very traits. This article sheds light on the recommendation development process and identifies challenges and opportunities for implementation across the country.
The Choosing Wisely® process started with the selection of a committee. This group comprised nine members, with equal representation from all three societies affiliated with PHM: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics’ Section on Hospital Medicine (AAP SOHM), and the Academic Pediatric Association (APA). Members of the committee intentionally represented a wide spectrum of practice variability, geography, and clinical experience.
The SHM members of the group were: James O’Callaghan, MD, FAAP, SFHM, pediatric hospitalist at Seattle Children’s Hospital and clinical professor of pediatrics at the University of Washington School of Medicine; Vivian Lee, MD, clinical pediatric hospitalist at Children’s Hospital of Los Angeles and associate professor of pediatrics at USC Keck School of Medicine; and Francisco Alvarez, MD, pediatric hospitalist at Lucile Packard Children’s Hospital, Palo Alto, Calif., and clinical associate professor of pediatrics at Stanford (Calif.) University.
According to Dr. O’Callaghan, it was important that the Choosing Wisely® recommendations come from the broader PHM community, reflecting the community’s priorities.
The committee started the process by asking the broader PHM community to submit ideas for consideration, via SHM’s HMX and the AAP SOHM listserv. The PHM community responded with more than 400 submissions.
Dr. Alvarez said the committee organized and trimmed the initial submissions, removing redundancy, into approximately 200 distinct recommendations. After initial literature review, the committee focused on approximately 70 recommendations. At that point, each member undertook an extensive literature review of the topics.
Once every potential recommendation had received a thorough review, Dr. Lee said, the committee underwent a modified Delphi process to evaluate the list. In this process, each member ranked the recommendations on validity – a measure of the quality of evidence supporting a topic – and feasibility – a measure of the PHM community’s ability to influence compliance.
At the end of this objective process, Dr. O’Callaghan said, the committee chose the five recommendations that received the highest total scores. While there were spirited discussions regarding the data available for each recommendation, all three SHM members of the committee agreed that the objective process played itself out.
Now that the Choosing Wisely® recommendations have been published, the PHM community is challenged to implement these recommendations to spur change for the care of hospitalized children throughout the country. Given the variety that exists in PHM, specifically in practice settings, it may be a daunting task. Dr. O’Callaghan said that differing opinions among physicians in a group may be a challenge to implementing change. “These recommendations allow for those conversations” to take place, he said. Dr. Lee said she hopes these recommendations provide a national panel opinion of the evidence to help support hospitalists in management discussions with others in a hospital – such as subspecialists or emergency department physicians – to increase high value care.
Since the nature of hospital medicine is one of collaboration, these recommendations will allow pediatric hospitalists to lead change throughout their hospitals and health care systems. However, it may not be a quick task. Dr. Alvarez estimates it may take 10-15 years until these recommendations are fully implemented throughout the country. However, there is reason to be optimistic, as the initial PHM Choosing Wisely® recommendations from 2012 have been broadly accepted and now represent national standards of care.
While the road ahead may be long and filled with challenges, the path forward has been clearly delineated, and the PHM community is grateful for the work done by members of the Choosing Wisely® Pediatric Hospital Medicine committee.
Dr. Casey is a pediatric hospitalist at Joe DiMaggio Children’s Hospital in Hollywood, Fla., and a member of the Society of Hospital Medicine’s Pediatric Special Interest Group’s Executive Council.
The Choosing Wisely® Pediatric Hospital Medicine (PHM) recommendations were published in January 2021. The initial Choosing Wisely® PHM recommendations were released in 2012 and the 2021 recommendations were the result of an extensive and years-long process. The Choosing Wisely® campaign, an initiative led by the American Board of Internal Medicine, was developed to enhance clinician-patient conversations, promoting care that is evidenced based, free from harm, and truly necessary.
The campaign has been embraced by the entire medical community, with more than 70 professional medical societies releasing recommendations. With its emphasis on high value care and eliminating medical waste, it is no surprise that the Choosing Wisely® campaign has found a home in a pediatric hospital medicine community that prides itself on those very traits. This article sheds light on the recommendation development process and identifies challenges and opportunities for implementation across the country.
The Choosing Wisely® process started with the selection of a committee. This group comprised nine members, with equal representation from all three societies affiliated with PHM: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics’ Section on Hospital Medicine (AAP SOHM), and the Academic Pediatric Association (APA). Members of the committee intentionally represented a wide spectrum of practice variability, geography, and clinical experience.
The SHM members of the group were: James O’Callaghan, MD, FAAP, SFHM, pediatric hospitalist at Seattle Children’s Hospital and clinical professor of pediatrics at the University of Washington School of Medicine; Vivian Lee, MD, clinical pediatric hospitalist at Children’s Hospital of Los Angeles and associate professor of pediatrics at USC Keck School of Medicine; and Francisco Alvarez, MD, pediatric hospitalist at Lucile Packard Children’s Hospital, Palo Alto, Calif., and clinical associate professor of pediatrics at Stanford (Calif.) University.
According to Dr. O’Callaghan, it was important that the Choosing Wisely® recommendations come from the broader PHM community, reflecting the community’s priorities.
The committee started the process by asking the broader PHM community to submit ideas for consideration, via SHM’s HMX and the AAP SOHM listserv. The PHM community responded with more than 400 submissions.
Dr. Alvarez said the committee organized and trimmed the initial submissions, removing redundancy, into approximately 200 distinct recommendations. After initial literature review, the committee focused on approximately 70 recommendations. At that point, each member undertook an extensive literature review of the topics.
Once every potential recommendation had received a thorough review, Dr. Lee said, the committee underwent a modified Delphi process to evaluate the list. In this process, each member ranked the recommendations on validity – a measure of the quality of evidence supporting a topic – and feasibility – a measure of the PHM community’s ability to influence compliance.
At the end of this objective process, Dr. O’Callaghan said, the committee chose the five recommendations that received the highest total scores. While there were spirited discussions regarding the data available for each recommendation, all three SHM members of the committee agreed that the objective process played itself out.
Now that the Choosing Wisely® recommendations have been published, the PHM community is challenged to implement these recommendations to spur change for the care of hospitalized children throughout the country. Given the variety that exists in PHM, specifically in practice settings, it may be a daunting task. Dr. O’Callaghan said that differing opinions among physicians in a group may be a challenge to implementing change. “These recommendations allow for those conversations” to take place, he said. Dr. Lee said she hopes these recommendations provide a national panel opinion of the evidence to help support hospitalists in management discussions with others in a hospital – such as subspecialists or emergency department physicians – to increase high value care.
Since the nature of hospital medicine is one of collaboration, these recommendations will allow pediatric hospitalists to lead change throughout their hospitals and health care systems. However, it may not be a quick task. Dr. Alvarez estimates it may take 10-15 years until these recommendations are fully implemented throughout the country. However, there is reason to be optimistic, as the initial PHM Choosing Wisely® recommendations from 2012 have been broadly accepted and now represent national standards of care.
While the road ahead may be long and filled with challenges, the path forward has been clearly delineated, and the PHM community is grateful for the work done by members of the Choosing Wisely® Pediatric Hospital Medicine committee.
Dr. Casey is a pediatric hospitalist at Joe DiMaggio Children’s Hospital in Hollywood, Fla., and a member of the Society of Hospital Medicine’s Pediatric Special Interest Group’s Executive Council.
Ear tubes no better than antibiotics for otitis media in young kids
The debate over tympanostomy tubes versus antibiotics for recurrent acute otitis media (AOM) in young children is long-standing. Now, results of a randomized controlled trial show that tubes do not significantly lower the rate of episodes, compared with antibiotics, and medical management doesn’t increase antibiotic resistance.
“We found no evidence of microbial resistance from treating with antibiotics. If there’s not an impact on resistance, why take unnecessary chances on complications of surgery?” lead author Alejandro Hoberman, MD, from Children’s Hospital of Pittsburgh, said in an interview.
The study by Dr. Hoberman and colleagues was published May 13 in the New England Journal of Medicine.
AOM is the most frequent condition diagnosed in children in the United States after the common cold, affecting five of six children younger than 3 years. It is the leading indication for antimicrobial treatment, and tympanostomy tube insertion is the most frequently performed pediatric operation after the newborn period.
Randomized controlled clinical trials were conducted in the 1980s, but by the 1990s, questions of overuse arose. The American Academy of Otolaryngology–Head and Neck Surgery Foundation published the first clinical practice guidelines in 2013.
Parents must weigh the pros and cons. The use of tubes may avoid or delay the next round of drugs, but tubes cost more and introduce small risks (anesthesia, refractory otorrhea, tube blockage, premature dislocation or extrusion, and mild conductive hearing loss).
“We addressed issues that plagued older studies – a longer-term follow-up of 2 years, validated diagnoses of infection to determine eligibility – and used rating scales to measure quality of life,” Dr. Hoberman said.
The researchers randomly assigned children to receive antibiotics or tubes. To be eligible, children had to be 6-35 months of age and have had at least three episodes of AOM within 6 months or at least four episodes within 12 months, including at least one within the preceding 6 months.
The primary outcome was the mean number of episodes of AOM per child-year. Children were assessed at 8-week intervals and within 48 hours of developing symptoms of ear infection. The medically treated children received oral amoxicillin or, if that was ineffective, intramuscular ceftriaxone.
Criteria for determining treatment failure included persistent otorrhea, tympanic membrane perforation, antibiotic-associated diarrhea, reaction to anesthesia, and recurrence of AOM at a frequency equal to the frequency before antibiotic treatment.
In comparing tympanostomy tubes with antibiotics, Dr. Hoberman said, “We were unable to show benefit in the rate of ear infections per child per year over a 2-year period.” As expected, the infection rate fell by about half from the first year to the second in all children.
Overall, the investigators found “no substantial differences between treatment groups” with regard to AOM frequency, percentage of severe episodes, extent of antimicrobial resistance, quality of life for the children, and parental stress.
In an intention-to-treat analysis, the rate of AOM episodes per child-year during the study was 1.48 ± 0.08 for tubes and 1.56 ± 0.08 for antibiotics (P = .66).
However, randomization was not maintained in the intention-to-treat arm. Ten percent (13 of 129) of the children slated to receive tubes didn’t get them because of parental request. Conversely, 16% (54 of 121) of children in the antibiotic group received tubes, 35 (29%) of them in accordance with the trial protocol because of frequent recurrences, and 19 (16%) at parental request.
In a per-protocol analysis, rates of AOM episodes per child-year were 1.47 ± 0.08 for tubes and 1.72 ± 0.11 for antibiotics.
Tubes were associated with longer time until the first ear infection post placement, at a median of 4.34 months, compared with 2.33 months for children who received antibiotics. A smaller percentage of children in the tube group had treatment failure than in the antibiotic group (45% vs. 62%). Children who received tubes also had fewer days per year with symptoms in comparison with the children in the antibiotic group (mean, 2.00 ± 0.29 days vs. 8.33 ± 0.59 days).
The frequency distribution of AOM episodes, the percentage of severe episodes, and antimicrobial resistance detected in respiratory specimens were the same for both groups.
“Hoberman and colleagues add to our knowledge of managing children with recurrent ear infections with a large and rigorous clinical trial showing comparable efficacy of tympanostomy tube insertion, with antibiotic eardrops for new infections versus watchful waiting, with intermittent oral antibiotics, if further ear infections occur,” said Richard M. Rosenfeld, MD, MPH, MBA, distinguished professor and chairman, department of otolaryngology, SUNY Downstate Medical Center, New York.
However, in an accompanying editorial, Ellen R. Wald, MD, from the University of Wisconsin, Madison, pointed out that the sample size was smaller than desired, owing to participants switching groups.
In addition, Dr. Rosenfeld, who was the lead author of the 2013 guidelines, said the study likely underestimates the impact of tubes “because about two-thirds of the children who received them did not have persistent middle-ear fluid at baseline and would not have been candidates for tubes based on the current national guideline on tube indications.”
“Both tubes and intermittent antibiotic therapy are effective for managing recurrent AOM, and parents of children with persistent middle-ear effusion should engage in shared decision-making with their physician to decide on the best management option,” said Dr. Rosenfeld. “When in doubt, watchful waiting is appropriate because many children with recurrent AOM do better over time.”
Dr. Hoberman owns stock in Kaizen Bioscience and holds patents on devices to diagnose and treat AOM. One coauthor consults for Merck. Dr. Wald and Dr. Rosenfeld report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The debate over tympanostomy tubes versus antibiotics for recurrent acute otitis media (AOM) in young children is long-standing. Now, results of a randomized controlled trial show that tubes do not significantly lower the rate of episodes, compared with antibiotics, and medical management doesn’t increase antibiotic resistance.
“We found no evidence of microbial resistance from treating with antibiotics. If there’s not an impact on resistance, why take unnecessary chances on complications of surgery?” lead author Alejandro Hoberman, MD, from Children’s Hospital of Pittsburgh, said in an interview.
The study by Dr. Hoberman and colleagues was published May 13 in the New England Journal of Medicine.
AOM is the most frequent condition diagnosed in children in the United States after the common cold, affecting five of six children younger than 3 years. It is the leading indication for antimicrobial treatment, and tympanostomy tube insertion is the most frequently performed pediatric operation after the newborn period.
Randomized controlled clinical trials were conducted in the 1980s, but by the 1990s, questions of overuse arose. The American Academy of Otolaryngology–Head and Neck Surgery Foundation published the first clinical practice guidelines in 2013.
Parents must weigh the pros and cons. The use of tubes may avoid or delay the next round of drugs, but tubes cost more and introduce small risks (anesthesia, refractory otorrhea, tube blockage, premature dislocation or extrusion, and mild conductive hearing loss).
“We addressed issues that plagued older studies – a longer-term follow-up of 2 years, validated diagnoses of infection to determine eligibility – and used rating scales to measure quality of life,” Dr. Hoberman said.
The researchers randomly assigned children to receive antibiotics or tubes. To be eligible, children had to be 6-35 months of age and have had at least three episodes of AOM within 6 months or at least four episodes within 12 months, including at least one within the preceding 6 months.
The primary outcome was the mean number of episodes of AOM per child-year. Children were assessed at 8-week intervals and within 48 hours of developing symptoms of ear infection. The medically treated children received oral amoxicillin or, if that was ineffective, intramuscular ceftriaxone.
Criteria for determining treatment failure included persistent otorrhea, tympanic membrane perforation, antibiotic-associated diarrhea, reaction to anesthesia, and recurrence of AOM at a frequency equal to the frequency before antibiotic treatment.
In comparing tympanostomy tubes with antibiotics, Dr. Hoberman said, “We were unable to show benefit in the rate of ear infections per child per year over a 2-year period.” As expected, the infection rate fell by about half from the first year to the second in all children.
Overall, the investigators found “no substantial differences between treatment groups” with regard to AOM frequency, percentage of severe episodes, extent of antimicrobial resistance, quality of life for the children, and parental stress.
In an intention-to-treat analysis, the rate of AOM episodes per child-year during the study was 1.48 ± 0.08 for tubes and 1.56 ± 0.08 for antibiotics (P = .66).
However, randomization was not maintained in the intention-to-treat arm. Ten percent (13 of 129) of the children slated to receive tubes didn’t get them because of parental request. Conversely, 16% (54 of 121) of children in the antibiotic group received tubes, 35 (29%) of them in accordance with the trial protocol because of frequent recurrences, and 19 (16%) at parental request.
In a per-protocol analysis, rates of AOM episodes per child-year were 1.47 ± 0.08 for tubes and 1.72 ± 0.11 for antibiotics.
Tubes were associated with longer time until the first ear infection post placement, at a median of 4.34 months, compared with 2.33 months for children who received antibiotics. A smaller percentage of children in the tube group had treatment failure than in the antibiotic group (45% vs. 62%). Children who received tubes also had fewer days per year with symptoms in comparison with the children in the antibiotic group (mean, 2.00 ± 0.29 days vs. 8.33 ± 0.59 days).
The frequency distribution of AOM episodes, the percentage of severe episodes, and antimicrobial resistance detected in respiratory specimens were the same for both groups.
“Hoberman and colleagues add to our knowledge of managing children with recurrent ear infections with a large and rigorous clinical trial showing comparable efficacy of tympanostomy tube insertion, with antibiotic eardrops for new infections versus watchful waiting, with intermittent oral antibiotics, if further ear infections occur,” said Richard M. Rosenfeld, MD, MPH, MBA, distinguished professor and chairman, department of otolaryngology, SUNY Downstate Medical Center, New York.
However, in an accompanying editorial, Ellen R. Wald, MD, from the University of Wisconsin, Madison, pointed out that the sample size was smaller than desired, owing to participants switching groups.
In addition, Dr. Rosenfeld, who was the lead author of the 2013 guidelines, said the study likely underestimates the impact of tubes “because about two-thirds of the children who received them did not have persistent middle-ear fluid at baseline and would not have been candidates for tubes based on the current national guideline on tube indications.”
“Both tubes and intermittent antibiotic therapy are effective for managing recurrent AOM, and parents of children with persistent middle-ear effusion should engage in shared decision-making with their physician to decide on the best management option,” said Dr. Rosenfeld. “When in doubt, watchful waiting is appropriate because many children with recurrent AOM do better over time.”
Dr. Hoberman owns stock in Kaizen Bioscience and holds patents on devices to diagnose and treat AOM. One coauthor consults for Merck. Dr. Wald and Dr. Rosenfeld report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The debate over tympanostomy tubes versus antibiotics for recurrent acute otitis media (AOM) in young children is long-standing. Now, results of a randomized controlled trial show that tubes do not significantly lower the rate of episodes, compared with antibiotics, and medical management doesn’t increase antibiotic resistance.
“We found no evidence of microbial resistance from treating with antibiotics. If there’s not an impact on resistance, why take unnecessary chances on complications of surgery?” lead author Alejandro Hoberman, MD, from Children’s Hospital of Pittsburgh, said in an interview.
The study by Dr. Hoberman and colleagues was published May 13 in the New England Journal of Medicine.
AOM is the most frequent condition diagnosed in children in the United States after the common cold, affecting five of six children younger than 3 years. It is the leading indication for antimicrobial treatment, and tympanostomy tube insertion is the most frequently performed pediatric operation after the newborn period.
Randomized controlled clinical trials were conducted in the 1980s, but by the 1990s, questions of overuse arose. The American Academy of Otolaryngology–Head and Neck Surgery Foundation published the first clinical practice guidelines in 2013.
Parents must weigh the pros and cons. The use of tubes may avoid or delay the next round of drugs, but tubes cost more and introduce small risks (anesthesia, refractory otorrhea, tube blockage, premature dislocation or extrusion, and mild conductive hearing loss).
“We addressed issues that plagued older studies – a longer-term follow-up of 2 years, validated diagnoses of infection to determine eligibility – and used rating scales to measure quality of life,” Dr. Hoberman said.
The researchers randomly assigned children to receive antibiotics or tubes. To be eligible, children had to be 6-35 months of age and have had at least three episodes of AOM within 6 months or at least four episodes within 12 months, including at least one within the preceding 6 months.
The primary outcome was the mean number of episodes of AOM per child-year. Children were assessed at 8-week intervals and within 48 hours of developing symptoms of ear infection. The medically treated children received oral amoxicillin or, if that was ineffective, intramuscular ceftriaxone.
Criteria for determining treatment failure included persistent otorrhea, tympanic membrane perforation, antibiotic-associated diarrhea, reaction to anesthesia, and recurrence of AOM at a frequency equal to the frequency before antibiotic treatment.
In comparing tympanostomy tubes with antibiotics, Dr. Hoberman said, “We were unable to show benefit in the rate of ear infections per child per year over a 2-year period.” As expected, the infection rate fell by about half from the first year to the second in all children.
Overall, the investigators found “no substantial differences between treatment groups” with regard to AOM frequency, percentage of severe episodes, extent of antimicrobial resistance, quality of life for the children, and parental stress.
In an intention-to-treat analysis, the rate of AOM episodes per child-year during the study was 1.48 ± 0.08 for tubes and 1.56 ± 0.08 for antibiotics (P = .66).
However, randomization was not maintained in the intention-to-treat arm. Ten percent (13 of 129) of the children slated to receive tubes didn’t get them because of parental request. Conversely, 16% (54 of 121) of children in the antibiotic group received tubes, 35 (29%) of them in accordance with the trial protocol because of frequent recurrences, and 19 (16%) at parental request.
In a per-protocol analysis, rates of AOM episodes per child-year were 1.47 ± 0.08 for tubes and 1.72 ± 0.11 for antibiotics.
Tubes were associated with longer time until the first ear infection post placement, at a median of 4.34 months, compared with 2.33 months for children who received antibiotics. A smaller percentage of children in the tube group had treatment failure than in the antibiotic group (45% vs. 62%). Children who received tubes also had fewer days per year with symptoms in comparison with the children in the antibiotic group (mean, 2.00 ± 0.29 days vs. 8.33 ± 0.59 days).
The frequency distribution of AOM episodes, the percentage of severe episodes, and antimicrobial resistance detected in respiratory specimens were the same for both groups.
“Hoberman and colleagues add to our knowledge of managing children with recurrent ear infections with a large and rigorous clinical trial showing comparable efficacy of tympanostomy tube insertion, with antibiotic eardrops for new infections versus watchful waiting, with intermittent oral antibiotics, if further ear infections occur,” said Richard M. Rosenfeld, MD, MPH, MBA, distinguished professor and chairman, department of otolaryngology, SUNY Downstate Medical Center, New York.
However, in an accompanying editorial, Ellen R. Wald, MD, from the University of Wisconsin, Madison, pointed out that the sample size was smaller than desired, owing to participants switching groups.
In addition, Dr. Rosenfeld, who was the lead author of the 2013 guidelines, said the study likely underestimates the impact of tubes “because about two-thirds of the children who received them did not have persistent middle-ear fluid at baseline and would not have been candidates for tubes based on the current national guideline on tube indications.”
“Both tubes and intermittent antibiotic therapy are effective for managing recurrent AOM, and parents of children with persistent middle-ear effusion should engage in shared decision-making with their physician to decide on the best management option,” said Dr. Rosenfeld. “When in doubt, watchful waiting is appropriate because many children with recurrent AOM do better over time.”
Dr. Hoberman owns stock in Kaizen Bioscience and holds patents on devices to diagnose and treat AOM. One coauthor consults for Merck. Dr. Wald and Dr. Rosenfeld report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low-risk preterm infants may not need antibiotics
Selective use of antibiotics based on birth circumstances may reduce unnecessary antibiotic exposure for preterm infants at risk of early-onset sepsis, based on data from 340 preterm infants at a single center.
Preterm infants born because of preterm labor, premature rupture of membranes, and/or intraamniotic infection (IAI) are considered at increased risk for early-onset sepsis, and current management strategies include a blood culture and initiation of empirical antibiotics, said Kirtan Patel, MD, of Texas A&M University, Dallas, and colleagues in a poster (# 1720) presented at the Pediatric Academic Societies annual meeting.
However, this blanket approach “may increase the unnecessary early antibiotic exposure in preterm infants possibly leading to future adverse health outcomes,” and physicians are advised to review the risks and benefits, Dr. Patel said.
Data from previous studies suggest that preterm infants born as a result of preterm labor and/or premature rupture of membranes with adequate Group B Streptococcus (GBS) intrapartum antibiotic prophylaxis and no indication of IAI may be managed without empiric antibiotics because the early-onset sepsis risk in these infants is much lower than the ones born through IAI and inadequate GBS intrapartum antibiotic prophylaxis.
To better identify preterm birth circumstances in which antibiotics might be avoided, the researchers conducted a retrospective cohort study of preterm infants born at 28-34 weeks’ gestation during the period from Jan. 1, 2015, to Dec. 31, 2018. These infants were in the low-risk category of preterm birth because of preterm labor or premature rupture of membranes, with no IAI and adequate GBS intrapartum antibiotic prophylaxis, and no signs of cardiovascular or respiratory instability after birth. Of these, 157 (46.2%) received empiric antibiotics soon after birth and 183 infants (53.8%) did not receive empiric antibiotics.
The mean gestational age and birth weight were significantly lower in the empiric antibiotic group, but after correcting for these variables, the factors with the greatest influence on the initiation of antibiotics were maternal intrapartum antibiotic prophylaxis (odds ratio, 3.13); premature rupture of membranes (OR, 3.75); use of continuous positive airway pressure (CPAP) in the delivery room (OR, 1.84); CPAP on admission to the neonatal intensive care unit (OR, 1.94); drawing a blood culture (OR, 13.72); and a complete blood count with immature to total neutrophil ratio greater than 0.2 (OR, 3.84).
Three infants (2%) in the antibiotics group had culture-positive early-onset sepsis with Escherichia coli, compared with no infants in the no-antibiotics group. No differences in short-term hospital outcomes appeared between the two groups. The study was limited in part by the retrospective design and sample size, the researchers noted.
However, the results support a selective approach to antibiotics for preterm infants, taking various birth circumstances into account, they said.
Further risk factor identification could curb antibiotic use
In this study, empiric antibiotics were cast as a wide net to avoid missing serious infections in a few patients, said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“It is interesting in this retrospective review of 340 preterm infants that the three newborns that did have serious bacterial infection were correctly given empiric antibiotics from the start,” Dr. Joos noted. “The authors were very effective at elucidating the possible factors that go into starting or not starting empiric antibiotics, although there may be other factors in the clinician’s judgment that are being missed. … More studies are needed on this topic,” Dr. Joos said. “Further research examining how the septic newborns differ from the nonseptic ones could help to even further narrow the use of empiric antibiotics,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
Selective use of antibiotics based on birth circumstances may reduce unnecessary antibiotic exposure for preterm infants at risk of early-onset sepsis, based on data from 340 preterm infants at a single center.
Preterm infants born because of preterm labor, premature rupture of membranes, and/or intraamniotic infection (IAI) are considered at increased risk for early-onset sepsis, and current management strategies include a blood culture and initiation of empirical antibiotics, said Kirtan Patel, MD, of Texas A&M University, Dallas, and colleagues in a poster (# 1720) presented at the Pediatric Academic Societies annual meeting.
However, this blanket approach “may increase the unnecessary early antibiotic exposure in preterm infants possibly leading to future adverse health outcomes,” and physicians are advised to review the risks and benefits, Dr. Patel said.
Data from previous studies suggest that preterm infants born as a result of preterm labor and/or premature rupture of membranes with adequate Group B Streptococcus (GBS) intrapartum antibiotic prophylaxis and no indication of IAI may be managed without empiric antibiotics because the early-onset sepsis risk in these infants is much lower than the ones born through IAI and inadequate GBS intrapartum antibiotic prophylaxis.
To better identify preterm birth circumstances in which antibiotics might be avoided, the researchers conducted a retrospective cohort study of preterm infants born at 28-34 weeks’ gestation during the period from Jan. 1, 2015, to Dec. 31, 2018. These infants were in the low-risk category of preterm birth because of preterm labor or premature rupture of membranes, with no IAI and adequate GBS intrapartum antibiotic prophylaxis, and no signs of cardiovascular or respiratory instability after birth. Of these, 157 (46.2%) received empiric antibiotics soon after birth and 183 infants (53.8%) did not receive empiric antibiotics.
The mean gestational age and birth weight were significantly lower in the empiric antibiotic group, but after correcting for these variables, the factors with the greatest influence on the initiation of antibiotics were maternal intrapartum antibiotic prophylaxis (odds ratio, 3.13); premature rupture of membranes (OR, 3.75); use of continuous positive airway pressure (CPAP) in the delivery room (OR, 1.84); CPAP on admission to the neonatal intensive care unit (OR, 1.94); drawing a blood culture (OR, 13.72); and a complete blood count with immature to total neutrophil ratio greater than 0.2 (OR, 3.84).
Three infants (2%) in the antibiotics group had culture-positive early-onset sepsis with Escherichia coli, compared with no infants in the no-antibiotics group. No differences in short-term hospital outcomes appeared between the two groups. The study was limited in part by the retrospective design and sample size, the researchers noted.
However, the results support a selective approach to antibiotics for preterm infants, taking various birth circumstances into account, they said.
Further risk factor identification could curb antibiotic use
In this study, empiric antibiotics were cast as a wide net to avoid missing serious infections in a few patients, said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“It is interesting in this retrospective review of 340 preterm infants that the three newborns that did have serious bacterial infection were correctly given empiric antibiotics from the start,” Dr. Joos noted. “The authors were very effective at elucidating the possible factors that go into starting or not starting empiric antibiotics, although there may be other factors in the clinician’s judgment that are being missed. … More studies are needed on this topic,” Dr. Joos said. “Further research examining how the septic newborns differ from the nonseptic ones could help to even further narrow the use of empiric antibiotics,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
Selective use of antibiotics based on birth circumstances may reduce unnecessary antibiotic exposure for preterm infants at risk of early-onset sepsis, based on data from 340 preterm infants at a single center.
Preterm infants born because of preterm labor, premature rupture of membranes, and/or intraamniotic infection (IAI) are considered at increased risk for early-onset sepsis, and current management strategies include a blood culture and initiation of empirical antibiotics, said Kirtan Patel, MD, of Texas A&M University, Dallas, and colleagues in a poster (# 1720) presented at the Pediatric Academic Societies annual meeting.
However, this blanket approach “may increase the unnecessary early antibiotic exposure in preterm infants possibly leading to future adverse health outcomes,” and physicians are advised to review the risks and benefits, Dr. Patel said.
Data from previous studies suggest that preterm infants born as a result of preterm labor and/or premature rupture of membranes with adequate Group B Streptococcus (GBS) intrapartum antibiotic prophylaxis and no indication of IAI may be managed without empiric antibiotics because the early-onset sepsis risk in these infants is much lower than the ones born through IAI and inadequate GBS intrapartum antibiotic prophylaxis.
To better identify preterm birth circumstances in which antibiotics might be avoided, the researchers conducted a retrospective cohort study of preterm infants born at 28-34 weeks’ gestation during the period from Jan. 1, 2015, to Dec. 31, 2018. These infants were in the low-risk category of preterm birth because of preterm labor or premature rupture of membranes, with no IAI and adequate GBS intrapartum antibiotic prophylaxis, and no signs of cardiovascular or respiratory instability after birth. Of these, 157 (46.2%) received empiric antibiotics soon after birth and 183 infants (53.8%) did not receive empiric antibiotics.
The mean gestational age and birth weight were significantly lower in the empiric antibiotic group, but after correcting for these variables, the factors with the greatest influence on the initiation of antibiotics were maternal intrapartum antibiotic prophylaxis (odds ratio, 3.13); premature rupture of membranes (OR, 3.75); use of continuous positive airway pressure (CPAP) in the delivery room (OR, 1.84); CPAP on admission to the neonatal intensive care unit (OR, 1.94); drawing a blood culture (OR, 13.72); and a complete blood count with immature to total neutrophil ratio greater than 0.2 (OR, 3.84).
Three infants (2%) in the antibiotics group had culture-positive early-onset sepsis with Escherichia coli, compared with no infants in the no-antibiotics group. No differences in short-term hospital outcomes appeared between the two groups. The study was limited in part by the retrospective design and sample size, the researchers noted.
However, the results support a selective approach to antibiotics for preterm infants, taking various birth circumstances into account, they said.
Further risk factor identification could curb antibiotic use
In this study, empiric antibiotics were cast as a wide net to avoid missing serious infections in a few patients, said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“It is interesting in this retrospective review of 340 preterm infants that the three newborns that did have serious bacterial infection were correctly given empiric antibiotics from the start,” Dr. Joos noted. “The authors were very effective at elucidating the possible factors that go into starting or not starting empiric antibiotics, although there may be other factors in the clinician’s judgment that are being missed. … More studies are needed on this topic,” Dr. Joos said. “Further research examining how the septic newborns differ from the nonseptic ones could help to even further narrow the use of empiric antibiotics,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
FROM PAS 2021