User login
U.S. and African programs aim to improve understanding, treatment of sickle cell disease
Researchers are leading several programs designed to serve the sickle cell community in the United States and sub-Saharan Africa, officials at the National Heart, Lung, and Blood Institute (NHLBI) said during a recent webinar.
One program based in the United States is focused on building a registry for patients with sickle cell disease (SCD) and conducting studies designed to improve SCD care. Another program involves building “an information-sharing network and patient-powered registry” in the United States.
The programs in sub-Saharan Africa were designed to establish a database of SCD patients, optimize the use of hydroxyurea in children with SCD, and aid genomic studies of SCD.
W. Keith Hoots, MD, director of the Division of Blood Diseases and Resources at NHLBI, began the webinar with an overview of the programs in sub-Saharan Africa. He described four programs with sites in nine countries (Angola, Cameroon, Democratic Republic of Congo, Ghana, Kenya, Nigeria, South Africa, Tanzania, and Uganda).
SPARCO and SADaCC
Dr. Hoots outlined the scope the Sickle Pan-African Research Consortium (SPARCO) and the Sickle Africa Data Coordinating Center (SADaCC), both part of the Sickle In Africa consortium.
A major goal of SPARCO and SADaCC is to create a Research Electronic Data Capture database that encompasses SCD patients in sub-Saharan Africa. As of April 2019, the database included 6,578 patients. The target is 13,000 patients.
Other goals of SPARCO and SADaCC are to “harmonize” SCD phenotype definitions and ontologies, create clinical guidelines for SCD management in sub-Saharan Africa, plan future cohort studies, and develop programs for newborn screening, infection prevention, and increased use of hydroxyurea.
“So far, they’re well along in establishing a registry and a database system,” Dr. Hoots said. “They’ve agreed on the database elements, phenotype definitions, and ontologies, they’ve developed some regionally appropriate clinical management guidelines, and they’ve begun skills development on the ground at all respective sites.”
REACH
Another program Dr. Hoots discussed is Realizing Effectiveness Across Continents With Hydroxyurea (REACH), a phase 1/2 pilot study of hydroxyurea in children (aged 1-10 years) with SCD in sub-Saharan Africa.
The goals of REACH are to determine the optimal dose of hydroxyurea in this population; teach African physicians how to administer hydroxyurea; assess the safety, feasibility, and benefits of hydroxyurea; study variability in response to hydroxyurea; gather data for the Research Electronic Data Capture database; and establish a research infrastructure for future collaborations.
Results from more than 600 children enrolled in REACH were presented at the 2018 annual meeting of the American Society of Hematology and simultaneously published in the New England Journal of Medicine (N Engl J Med. 2019 Jan 10; 380[2]:121-31).
SickleGenAfrica
SickleGenAfrica is part of the H3Africa consortium and aims to “build capacity for genomic research in Africa,” Dr. Hoots said.
Under this program, researchers will conduct three studies to test the hypothesis that genetic variation affects the defense against hemolysis and organ damage in patients with SCD. The researchers will study existing cohorts of SCD patients including children and adults.
Other goals of SickleGenAfrica are to establish a molecular hematology and sickle cell mouse core, an SCD biorepository core, a bioinformatics core, and an administrative core for the coordination of activities. The program will also be used to train “future science leaders” in SCD research, Dr. Hoots said.
SCDIC
Cheryl Anne Boyce, PhD, chief of the Implementation Science Branch at the Center for Translation Research and Implementation Science at NHLBI, discussed the United States–based Sickle Cell Disease Implementation Consortium (SCDIC).
“The goals of the consortium are to develop a registry in collaboration with other centers and the NHLBI, as well as a needs-based community assessment of the barriers to care for subjects with sickle cell disease,” Dr. Boyce said. “We also wanted to design implementation research studies that address the identified barriers to care.”
Dr. Boyce said the SCDIC’s registry is open to patients aged 15-45 years who have a confirmed SCD diagnosis, speak English, and are able to consent to and complete a survey. The registry has enrolled almost 2,400 patients from eight centers over 18 months.
The SCDIC has also performed a needs assessment that prompted the development of three implementation research studies. The first study involves using mobile health interventions to, ideally, increase patient adherence to hydroxyurea and improve provider knowledge of hydroxyurea.
With the second study, researchers aim to improve the care of SCD patients in the emergency department by using an inpatient portal. The goals of the third study are to establish a standard definition for unaffiliated patients, conduct a needs assessment for this group, and develop an intervention that can provide these patients with guideline-based SCD care.
Get Connected
Kim Smith-Whitley, MD, director of the Comprehensive Sickle Cell Center at the Children’s Hospital of Philadelphia and a board member of the Sickle Cell Disease Association of America (SCDAA), described Get Connected, “an information-sharing network and patient-powered registry” created by SCDAA.
Dr. Smith-Whitley said one purpose of Get Connected is to provide a network that facilitates “the distribution of information related to clinical care, research, health services, health policy, and advocacy.”
The network is open to families living with SCD and sickle cell trait, SCDAA member organizations, health care providers, clinical researchers, and community-based organizations.
Get Connected also includes a registry for SCD patients that stores information on their diagnosis and treatment, as well as online communities that can be used to share information and provide psychosocial support.
Thus far, Get Connected has enrolled 6,329 individuals. This includes 5,100 children and adults with SCD, 652 children and adults with sickle cell trait, and 577 nonpatients.
The webinar presenters did not disclose any conflicts of interest.
Researchers are leading several programs designed to serve the sickle cell community in the United States and sub-Saharan Africa, officials at the National Heart, Lung, and Blood Institute (NHLBI) said during a recent webinar.
One program based in the United States is focused on building a registry for patients with sickle cell disease (SCD) and conducting studies designed to improve SCD care. Another program involves building “an information-sharing network and patient-powered registry” in the United States.
The programs in sub-Saharan Africa were designed to establish a database of SCD patients, optimize the use of hydroxyurea in children with SCD, and aid genomic studies of SCD.
W. Keith Hoots, MD, director of the Division of Blood Diseases and Resources at NHLBI, began the webinar with an overview of the programs in sub-Saharan Africa. He described four programs with sites in nine countries (Angola, Cameroon, Democratic Republic of Congo, Ghana, Kenya, Nigeria, South Africa, Tanzania, and Uganda).
SPARCO and SADaCC
Dr. Hoots outlined the scope the Sickle Pan-African Research Consortium (SPARCO) and the Sickle Africa Data Coordinating Center (SADaCC), both part of the Sickle In Africa consortium.
A major goal of SPARCO and SADaCC is to create a Research Electronic Data Capture database that encompasses SCD patients in sub-Saharan Africa. As of April 2019, the database included 6,578 patients. The target is 13,000 patients.
Other goals of SPARCO and SADaCC are to “harmonize” SCD phenotype definitions and ontologies, create clinical guidelines for SCD management in sub-Saharan Africa, plan future cohort studies, and develop programs for newborn screening, infection prevention, and increased use of hydroxyurea.
“So far, they’re well along in establishing a registry and a database system,” Dr. Hoots said. “They’ve agreed on the database elements, phenotype definitions, and ontologies, they’ve developed some regionally appropriate clinical management guidelines, and they’ve begun skills development on the ground at all respective sites.”
REACH
Another program Dr. Hoots discussed is Realizing Effectiveness Across Continents With Hydroxyurea (REACH), a phase 1/2 pilot study of hydroxyurea in children (aged 1-10 years) with SCD in sub-Saharan Africa.
The goals of REACH are to determine the optimal dose of hydroxyurea in this population; teach African physicians how to administer hydroxyurea; assess the safety, feasibility, and benefits of hydroxyurea; study variability in response to hydroxyurea; gather data for the Research Electronic Data Capture database; and establish a research infrastructure for future collaborations.
Results from more than 600 children enrolled in REACH were presented at the 2018 annual meeting of the American Society of Hematology and simultaneously published in the New England Journal of Medicine (N Engl J Med. 2019 Jan 10; 380[2]:121-31).
SickleGenAfrica
SickleGenAfrica is part of the H3Africa consortium and aims to “build capacity for genomic research in Africa,” Dr. Hoots said.
Under this program, researchers will conduct three studies to test the hypothesis that genetic variation affects the defense against hemolysis and organ damage in patients with SCD. The researchers will study existing cohorts of SCD patients including children and adults.
Other goals of SickleGenAfrica are to establish a molecular hematology and sickle cell mouse core, an SCD biorepository core, a bioinformatics core, and an administrative core for the coordination of activities. The program will also be used to train “future science leaders” in SCD research, Dr. Hoots said.
SCDIC
Cheryl Anne Boyce, PhD, chief of the Implementation Science Branch at the Center for Translation Research and Implementation Science at NHLBI, discussed the United States–based Sickle Cell Disease Implementation Consortium (SCDIC).
“The goals of the consortium are to develop a registry in collaboration with other centers and the NHLBI, as well as a needs-based community assessment of the barriers to care for subjects with sickle cell disease,” Dr. Boyce said. “We also wanted to design implementation research studies that address the identified barriers to care.”
Dr. Boyce said the SCDIC’s registry is open to patients aged 15-45 years who have a confirmed SCD diagnosis, speak English, and are able to consent to and complete a survey. The registry has enrolled almost 2,400 patients from eight centers over 18 months.
The SCDIC has also performed a needs assessment that prompted the development of three implementation research studies. The first study involves using mobile health interventions to, ideally, increase patient adherence to hydroxyurea and improve provider knowledge of hydroxyurea.
With the second study, researchers aim to improve the care of SCD patients in the emergency department by using an inpatient portal. The goals of the third study are to establish a standard definition for unaffiliated patients, conduct a needs assessment for this group, and develop an intervention that can provide these patients with guideline-based SCD care.
Get Connected
Kim Smith-Whitley, MD, director of the Comprehensive Sickle Cell Center at the Children’s Hospital of Philadelphia and a board member of the Sickle Cell Disease Association of America (SCDAA), described Get Connected, “an information-sharing network and patient-powered registry” created by SCDAA.
Dr. Smith-Whitley said one purpose of Get Connected is to provide a network that facilitates “the distribution of information related to clinical care, research, health services, health policy, and advocacy.”
The network is open to families living with SCD and sickle cell trait, SCDAA member organizations, health care providers, clinical researchers, and community-based organizations.
Get Connected also includes a registry for SCD patients that stores information on their diagnosis and treatment, as well as online communities that can be used to share information and provide psychosocial support.
Thus far, Get Connected has enrolled 6,329 individuals. This includes 5,100 children and adults with SCD, 652 children and adults with sickle cell trait, and 577 nonpatients.
The webinar presenters did not disclose any conflicts of interest.
Researchers are leading several programs designed to serve the sickle cell community in the United States and sub-Saharan Africa, officials at the National Heart, Lung, and Blood Institute (NHLBI) said during a recent webinar.
One program based in the United States is focused on building a registry for patients with sickle cell disease (SCD) and conducting studies designed to improve SCD care. Another program involves building “an information-sharing network and patient-powered registry” in the United States.
The programs in sub-Saharan Africa were designed to establish a database of SCD patients, optimize the use of hydroxyurea in children with SCD, and aid genomic studies of SCD.
W. Keith Hoots, MD, director of the Division of Blood Diseases and Resources at NHLBI, began the webinar with an overview of the programs in sub-Saharan Africa. He described four programs with sites in nine countries (Angola, Cameroon, Democratic Republic of Congo, Ghana, Kenya, Nigeria, South Africa, Tanzania, and Uganda).
SPARCO and SADaCC
Dr. Hoots outlined the scope the Sickle Pan-African Research Consortium (SPARCO) and the Sickle Africa Data Coordinating Center (SADaCC), both part of the Sickle In Africa consortium.
A major goal of SPARCO and SADaCC is to create a Research Electronic Data Capture database that encompasses SCD patients in sub-Saharan Africa. As of April 2019, the database included 6,578 patients. The target is 13,000 patients.
Other goals of SPARCO and SADaCC are to “harmonize” SCD phenotype definitions and ontologies, create clinical guidelines for SCD management in sub-Saharan Africa, plan future cohort studies, and develop programs for newborn screening, infection prevention, and increased use of hydroxyurea.
“So far, they’re well along in establishing a registry and a database system,” Dr. Hoots said. “They’ve agreed on the database elements, phenotype definitions, and ontologies, they’ve developed some regionally appropriate clinical management guidelines, and they’ve begun skills development on the ground at all respective sites.”
REACH
Another program Dr. Hoots discussed is Realizing Effectiveness Across Continents With Hydroxyurea (REACH), a phase 1/2 pilot study of hydroxyurea in children (aged 1-10 years) with SCD in sub-Saharan Africa.
The goals of REACH are to determine the optimal dose of hydroxyurea in this population; teach African physicians how to administer hydroxyurea; assess the safety, feasibility, and benefits of hydroxyurea; study variability in response to hydroxyurea; gather data for the Research Electronic Data Capture database; and establish a research infrastructure for future collaborations.
Results from more than 600 children enrolled in REACH were presented at the 2018 annual meeting of the American Society of Hematology and simultaneously published in the New England Journal of Medicine (N Engl J Med. 2019 Jan 10; 380[2]:121-31).
SickleGenAfrica
SickleGenAfrica is part of the H3Africa consortium and aims to “build capacity for genomic research in Africa,” Dr. Hoots said.
Under this program, researchers will conduct three studies to test the hypothesis that genetic variation affects the defense against hemolysis and organ damage in patients with SCD. The researchers will study existing cohorts of SCD patients including children and adults.
Other goals of SickleGenAfrica are to establish a molecular hematology and sickle cell mouse core, an SCD biorepository core, a bioinformatics core, and an administrative core for the coordination of activities. The program will also be used to train “future science leaders” in SCD research, Dr. Hoots said.
SCDIC
Cheryl Anne Boyce, PhD, chief of the Implementation Science Branch at the Center for Translation Research and Implementation Science at NHLBI, discussed the United States–based Sickle Cell Disease Implementation Consortium (SCDIC).
“The goals of the consortium are to develop a registry in collaboration with other centers and the NHLBI, as well as a needs-based community assessment of the barriers to care for subjects with sickle cell disease,” Dr. Boyce said. “We also wanted to design implementation research studies that address the identified barriers to care.”
Dr. Boyce said the SCDIC’s registry is open to patients aged 15-45 years who have a confirmed SCD diagnosis, speak English, and are able to consent to and complete a survey. The registry has enrolled almost 2,400 patients from eight centers over 18 months.
The SCDIC has also performed a needs assessment that prompted the development of three implementation research studies. The first study involves using mobile health interventions to, ideally, increase patient adherence to hydroxyurea and improve provider knowledge of hydroxyurea.
With the second study, researchers aim to improve the care of SCD patients in the emergency department by using an inpatient portal. The goals of the third study are to establish a standard definition for unaffiliated patients, conduct a needs assessment for this group, and develop an intervention that can provide these patients with guideline-based SCD care.
Get Connected
Kim Smith-Whitley, MD, director of the Comprehensive Sickle Cell Center at the Children’s Hospital of Philadelphia and a board member of the Sickle Cell Disease Association of America (SCDAA), described Get Connected, “an information-sharing network and patient-powered registry” created by SCDAA.
Dr. Smith-Whitley said one purpose of Get Connected is to provide a network that facilitates “the distribution of information related to clinical care, research, health services, health policy, and advocacy.”
The network is open to families living with SCD and sickle cell trait, SCDAA member organizations, health care providers, clinical researchers, and community-based organizations.
Get Connected also includes a registry for SCD patients that stores information on their diagnosis and treatment, as well as online communities that can be used to share information and provide psychosocial support.
Thus far, Get Connected has enrolled 6,329 individuals. This includes 5,100 children and adults with SCD, 652 children and adults with sickle cell trait, and 577 nonpatients.
The webinar presenters did not disclose any conflicts of interest.
Pediatric HSCT recipients still risking sunburn
Young people who have received allogeneic hematopoietic stem cell transplants (HSCTs) are more likely to wear hats, sunscreen and other sun protection, but still intentionally tan and experience sunburn at the same rate as their peers, new research suggests.
In a survey‐based, cross‐sectional cohort study, researchers compared sun-protection behaviors and sun exposure in 85 children aged 21 years and younger who had undergone HSCT and 85 age-, sex-, and skin type–matched controls. The findings were published in Pediatric Dermatology.
HSCT recipients have a higher risk of long-term complications such as skin cancer, for which sun exposure is a major modifiable environmental risk factor.
“Therefore, consistent sun avoidance and protection as well as regular dermatologic evaluations are important for HSCT recipients,” wrote Edward B. Li, PhD, from Harvard Medical School, Boston, and coauthors.
The survey found no significant difference between the transplant and control group in the amount of intentional sun exposure, such as the amount of time spent outside on weekdays and weekends during the peak sun intensity hours of 10 a.m. and 4 p.m. More than one in five transplant recipients (21.2%) reported spending at least 3 hours a day outside between 10 a.m. and 4 p.m. on weekdays, as did 36.5% of transplant recipients on weekends.
There were also no significant differences between the two groups in terms of time spent tanning, either in the sun or in a tanning bed. Additionally, a similar number of transplant recipients and controls experienced one or more red or painful sunburns in the past year (25.9% vs. 27.1%).
However, transplant patients did practice better sun protection behaviors than did the control group, with 60% reporting that they always wore sunscreen, compared with 29.4% of controls. The transplant recipients were also significantly more likely to wear sunglasses and a hat and to stay in the shade or use an umbrella.
“While these data may reflect that HSCT patients are not practicing adequate sun avoidance, it may also suggest that these long‐term survivors are able to enjoy being outdoors as much as their peers and have a similar desire to have a tanned appearance,” the researchers wrote. “While a healthy and active lifestyle should be encouraged for all children, our results emphasize the need for pediatric HSCT survivors to be educated on their increased risk for UV‐related skin cancers, counseled on avoidance of intentional tanning, and advised on the importance of sun protection behaviors in an effort to improve long-term outcomes.”
The researchers noted that transplant recipients were significantly more likely to have had a full body skin exam from a health care professional than were individuals in the control group (61.2% vs. 4.7%) and were more likely to have done a self-check or been checked by a partner in the previous year.
The study was supported by the Society for Pediatric Dermatology, the Dermatology Foundation, the National Institutes of Health, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. One author declared a financial interest in a company developing a dermatological product. No other conflicts of interest were declared.
SOURCE: Li EB et al. Pediatr Dermatol. 2019 Aug 13. doi: 10.1111/pde.13984.
Young people who have received allogeneic hematopoietic stem cell transplants (HSCTs) are more likely to wear hats, sunscreen and other sun protection, but still intentionally tan and experience sunburn at the same rate as their peers, new research suggests.
In a survey‐based, cross‐sectional cohort study, researchers compared sun-protection behaviors and sun exposure in 85 children aged 21 years and younger who had undergone HSCT and 85 age-, sex-, and skin type–matched controls. The findings were published in Pediatric Dermatology.
HSCT recipients have a higher risk of long-term complications such as skin cancer, for which sun exposure is a major modifiable environmental risk factor.
“Therefore, consistent sun avoidance and protection as well as regular dermatologic evaluations are important for HSCT recipients,” wrote Edward B. Li, PhD, from Harvard Medical School, Boston, and coauthors.
The survey found no significant difference between the transplant and control group in the amount of intentional sun exposure, such as the amount of time spent outside on weekdays and weekends during the peak sun intensity hours of 10 a.m. and 4 p.m. More than one in five transplant recipients (21.2%) reported spending at least 3 hours a day outside between 10 a.m. and 4 p.m. on weekdays, as did 36.5% of transplant recipients on weekends.
There were also no significant differences between the two groups in terms of time spent tanning, either in the sun or in a tanning bed. Additionally, a similar number of transplant recipients and controls experienced one or more red or painful sunburns in the past year (25.9% vs. 27.1%).
However, transplant patients did practice better sun protection behaviors than did the control group, with 60% reporting that they always wore sunscreen, compared with 29.4% of controls. The transplant recipients were also significantly more likely to wear sunglasses and a hat and to stay in the shade or use an umbrella.
“While these data may reflect that HSCT patients are not practicing adequate sun avoidance, it may also suggest that these long‐term survivors are able to enjoy being outdoors as much as their peers and have a similar desire to have a tanned appearance,” the researchers wrote. “While a healthy and active lifestyle should be encouraged for all children, our results emphasize the need for pediatric HSCT survivors to be educated on their increased risk for UV‐related skin cancers, counseled on avoidance of intentional tanning, and advised on the importance of sun protection behaviors in an effort to improve long-term outcomes.”
The researchers noted that transplant recipients were significantly more likely to have had a full body skin exam from a health care professional than were individuals in the control group (61.2% vs. 4.7%) and were more likely to have done a self-check or been checked by a partner in the previous year.
The study was supported by the Society for Pediatric Dermatology, the Dermatology Foundation, the National Institutes of Health, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. One author declared a financial interest in a company developing a dermatological product. No other conflicts of interest were declared.
SOURCE: Li EB et al. Pediatr Dermatol. 2019 Aug 13. doi: 10.1111/pde.13984.
Young people who have received allogeneic hematopoietic stem cell transplants (HSCTs) are more likely to wear hats, sunscreen and other sun protection, but still intentionally tan and experience sunburn at the same rate as their peers, new research suggests.
In a survey‐based, cross‐sectional cohort study, researchers compared sun-protection behaviors and sun exposure in 85 children aged 21 years and younger who had undergone HSCT and 85 age-, sex-, and skin type–matched controls. The findings were published in Pediatric Dermatology.
HSCT recipients have a higher risk of long-term complications such as skin cancer, for which sun exposure is a major modifiable environmental risk factor.
“Therefore, consistent sun avoidance and protection as well as regular dermatologic evaluations are important for HSCT recipients,” wrote Edward B. Li, PhD, from Harvard Medical School, Boston, and coauthors.
The survey found no significant difference between the transplant and control group in the amount of intentional sun exposure, such as the amount of time spent outside on weekdays and weekends during the peak sun intensity hours of 10 a.m. and 4 p.m. More than one in five transplant recipients (21.2%) reported spending at least 3 hours a day outside between 10 a.m. and 4 p.m. on weekdays, as did 36.5% of transplant recipients on weekends.
There were also no significant differences between the two groups in terms of time spent tanning, either in the sun or in a tanning bed. Additionally, a similar number of transplant recipients and controls experienced one or more red or painful sunburns in the past year (25.9% vs. 27.1%).
However, transplant patients did practice better sun protection behaviors than did the control group, with 60% reporting that they always wore sunscreen, compared with 29.4% of controls. The transplant recipients were also significantly more likely to wear sunglasses and a hat and to stay in the shade or use an umbrella.
“While these data may reflect that HSCT patients are not practicing adequate sun avoidance, it may also suggest that these long‐term survivors are able to enjoy being outdoors as much as their peers and have a similar desire to have a tanned appearance,” the researchers wrote. “While a healthy and active lifestyle should be encouraged for all children, our results emphasize the need for pediatric HSCT survivors to be educated on their increased risk for UV‐related skin cancers, counseled on avoidance of intentional tanning, and advised on the importance of sun protection behaviors in an effort to improve long-term outcomes.”
The researchers noted that transplant recipients were significantly more likely to have had a full body skin exam from a health care professional than were individuals in the control group (61.2% vs. 4.7%) and were more likely to have done a self-check or been checked by a partner in the previous year.
The study was supported by the Society for Pediatric Dermatology, the Dermatology Foundation, the National Institutes of Health, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. One author declared a financial interest in a company developing a dermatological product. No other conflicts of interest were declared.
SOURCE: Li EB et al. Pediatr Dermatol. 2019 Aug 13. doi: 10.1111/pde.13984.
FROM PEDIATRIC DERMATOLOGY
Wrong cuff size throws off pediatric BP by 5 mm Hg
NEW ORLEANS – according to investigators from Columbia University in New York.
There are five cuff sizes in pediatrics, depending on a child’s arm circumference. Ideally, it’s measured beforehand so the right cuff size is used, but that doesn’t always happen in everyday practice.
Sometimes, clinicians just estimate arm size before choosing a cuff or opt for the medium-sized cuff in most kids; other times, the correct size has gone missing, said lead investigator Ruchi Gupta Mahajan, MD, a pediatric nephrology fellow at Columbia.
For those situations, she and her colleagues wanted to quantify how much the wrong cuff size throws off blood pressure readings in children, something that’s been done before in adult medicine, but not in pediatrics.
The idea was to give clinicians a decent estimate of true blood pressure even when the cuff isn’t quite right, something that’s particularly important with an increasing emphasis on catching hypertension as early as possible in children, she said.
After her subjects sat quietly for 10 minutes, Dr. Mahajan took automated blood pressure readings on 137 children; once with the right cuff size, once with a cuff one size too small, and once with a cuff one size too big, with a minute apart between readings.
The children were aged 4-12 years old and were in the office for wellness visits. None of them had heart or kidney disease, and none were on steroids or any other medications that affect blood pressure. There were a few more boys than girls, and almost all the children were Hispanic.
Overall, systolic blood pressure was an average of 5 mm Hg less with the larger cuff and 5 mm Hg more with the smaller cuff. The finding was the same in both girls and boys, and it held across age groups and in under, over, and normal weight children.
“I was really surprised there was no difference between ages, 4-12 years of age, its just a single number: 5. [Even] if [you] don’t have the appropriate cuff size,” the finding means that it’s still possible to have a good estimate of blood pressure, Dr. Mahajan said at the joint scientific sessions of the American Heart Association (AHA) Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Meanwhile, cuff size didn’t have any statistically significant effect on diastolic pressure.
There was no outside funding for the study and Dr. Mahajan reported having no disclosures.
SOURCE: Mahajan RG et al. Joint Hypertension 2019, Abstract P182.
NEW ORLEANS – according to investigators from Columbia University in New York.
There are five cuff sizes in pediatrics, depending on a child’s arm circumference. Ideally, it’s measured beforehand so the right cuff size is used, but that doesn’t always happen in everyday practice.
Sometimes, clinicians just estimate arm size before choosing a cuff or opt for the medium-sized cuff in most kids; other times, the correct size has gone missing, said lead investigator Ruchi Gupta Mahajan, MD, a pediatric nephrology fellow at Columbia.
For those situations, she and her colleagues wanted to quantify how much the wrong cuff size throws off blood pressure readings in children, something that’s been done before in adult medicine, but not in pediatrics.
The idea was to give clinicians a decent estimate of true blood pressure even when the cuff isn’t quite right, something that’s particularly important with an increasing emphasis on catching hypertension as early as possible in children, she said.
After her subjects sat quietly for 10 minutes, Dr. Mahajan took automated blood pressure readings on 137 children; once with the right cuff size, once with a cuff one size too small, and once with a cuff one size too big, with a minute apart between readings.
The children were aged 4-12 years old and were in the office for wellness visits. None of them had heart or kidney disease, and none were on steroids or any other medications that affect blood pressure. There were a few more boys than girls, and almost all the children were Hispanic.
Overall, systolic blood pressure was an average of 5 mm Hg less with the larger cuff and 5 mm Hg more with the smaller cuff. The finding was the same in both girls and boys, and it held across age groups and in under, over, and normal weight children.
“I was really surprised there was no difference between ages, 4-12 years of age, its just a single number: 5. [Even] if [you] don’t have the appropriate cuff size,” the finding means that it’s still possible to have a good estimate of blood pressure, Dr. Mahajan said at the joint scientific sessions of the American Heart Association (AHA) Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Meanwhile, cuff size didn’t have any statistically significant effect on diastolic pressure.
There was no outside funding for the study and Dr. Mahajan reported having no disclosures.
SOURCE: Mahajan RG et al. Joint Hypertension 2019, Abstract P182.
NEW ORLEANS – according to investigators from Columbia University in New York.
There are five cuff sizes in pediatrics, depending on a child’s arm circumference. Ideally, it’s measured beforehand so the right cuff size is used, but that doesn’t always happen in everyday practice.
Sometimes, clinicians just estimate arm size before choosing a cuff or opt for the medium-sized cuff in most kids; other times, the correct size has gone missing, said lead investigator Ruchi Gupta Mahajan, MD, a pediatric nephrology fellow at Columbia.
For those situations, she and her colleagues wanted to quantify how much the wrong cuff size throws off blood pressure readings in children, something that’s been done before in adult medicine, but not in pediatrics.
The idea was to give clinicians a decent estimate of true blood pressure even when the cuff isn’t quite right, something that’s particularly important with an increasing emphasis on catching hypertension as early as possible in children, she said.
After her subjects sat quietly for 10 minutes, Dr. Mahajan took automated blood pressure readings on 137 children; once with the right cuff size, once with a cuff one size too small, and once with a cuff one size too big, with a minute apart between readings.
The children were aged 4-12 years old and were in the office for wellness visits. None of them had heart or kidney disease, and none were on steroids or any other medications that affect blood pressure. There were a few more boys than girls, and almost all the children were Hispanic.
Overall, systolic blood pressure was an average of 5 mm Hg less with the larger cuff and 5 mm Hg more with the smaller cuff. The finding was the same in both girls and boys, and it held across age groups and in under, over, and normal weight children.
“I was really surprised there was no difference between ages, 4-12 years of age, its just a single number: 5. [Even] if [you] don’t have the appropriate cuff size,” the finding means that it’s still possible to have a good estimate of blood pressure, Dr. Mahajan said at the joint scientific sessions of the American Heart Association (AHA) Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Meanwhile, cuff size didn’t have any statistically significant effect on diastolic pressure.
There was no outside funding for the study and Dr. Mahajan reported having no disclosures.
SOURCE: Mahajan RG et al. Joint Hypertension 2019, Abstract P182.
REPORTING FROM JOINT HYPERTENSION 2019
No ‘one size fits all’ approach to managing severe pediatric psoriasis
AUSTIN, TEX. – The way Kelly M. Cordoro sees it, the most for which patient.
“You can look up the dosing and frequency of these drugs; all of that’s available,” she said at the annual meeting of the Society for Pediatric Dermatology. “But how do we think about which drug for which patient? What are the considerations?”
Dr. Cordoro, professor of dermatology and pediatrics at the University of California, San Francisco, described psoriasis as an autoamplifying inflammatory cascade involving innate and adaptive immunity and noted that various components of that cascade represent treatment targets. “We don’t have a true comprehension of the pathophysiology of psoriasis, but as we learn the pathways, we’re targeting them,” she said. “You can target keratinocyte proliferation with drugs like retinoids and phototherapy. You can broadly target T cells, neutrophils, and dendritic cells with methotrexate, cyclosporine, and phototherapy. The newer drugs target the cytokine milieu, including TNF [tumor necrosis factor]–alpha, IL [interleukin]–17A, IL-12, and IL-23.”
There is no one right answer for which drug to prescribe, she continued, except in the cases of certain comorbidities, contraindications, and genetic variants. “For example, if a patient has psoriatic arthritis, then you have methotrexate and all of the biologics that might be disease modifying,” she said. “If a patient has inflammatory bowel disease, it’s critical to know that IL-17 inhibitors will flare that disease, but anti-TNF and IL-12 and IL-23 inhibitors are okay. If a patient has liver and kidney disease, you want to avoid methotrexate and cyclosporine. If there’s a female of childbearing potential you want to be very cautious with using retinoids. I think the harder question for us is, How about the rest of the patients?”
In addition to a drug’s mechanism of action, patient- and family-related factors play a role in deciding which agent to use. For example, does the patient prefer an oral or an injectable agent? Is the patient able to travel to a phototherapy center? Is it feasible for the family to manage visits for lab work and direct clinical monitoring? Does the family have a high level of health literacy and are you communicating with them in ways that facilitate shared decision making?
“The best way to choose a systemic therapy is to develop an individualized assessment of overall disease burden,” said Dr. Cordoro, who is also division chief of pediatric dermatology at UCSF. “Include psychological burden and subjective data in addition to objective measures like body surface area. Look for triggers. Infants are more commonly affected by viral infections and, in a subset, monogenic forms of psoriasis such as deficiency of interleukin 1 receptor antagonist [DIRA]. In general, we try to take a conservative approach in the developing child. As children hit early adolescence and become post pubertal, you have to start thinking about the psychosocial impact [of psoriasis], and we have to start treating patients with the consideration that chronic uncontrolled inflammation can potentially lead to comorbidities down the road. We see this in adults with severe psoriasis and early onset cardiovascular disease, the so-called psoriatic march from chronic inflammation to cardiovascular disease.”
Dr. Cordoro advises clinicians to rethink the conventional “therapeutic ladder” concept and embrace the idea of “finding the right tool for the job right now.” If a patient presents with a flare from a known trigger such as a strep infection, “maybe you want to treat with something more conservative,” she said. “Once you treat, and if the trigger has been managed, they might be better. But some patients will need the most aggressive treatment right out of the gate.”
Tried and true systemic therapies for psoriasis include methotrexate, cyclosporine, acitretin, and phototherapy, but none is approved by the Food and Drug Administration for use in children. “These drugs have decades of experience behind them,” Dr. Cordoro said. “Methotrexate is slow to start but has a sustained profile, so if you can get the patient to respond, that response tends to persist. Methotrexate also prevents the formation of antidrug antibodies, which is important if you are considering use of a biologic agent later on.”
Cyclosporine is best if you need a rapid rescue drug to get the disease under control before moving on to other options. “One in four patients relapse once cyclosporine is discontinued, so the benefit may not be as sustained as with methotrexate,” she said. “Acitretin is a really nice choice when you can’t or don’t want to immunosuppress the patient, and phototherapy is good if you can get it. The advantages of systemic therapies are that they’re easy on, easy off, and you can combine medications in severe situations. Almost all of these drugs can be combined with another, with few exceptions. I would caution that over immunosuppression is the biggest risk ... so this must be done carefully and only when necessary.”
Biologic agents such as TNF inhibitors and IL-12/23 inhibitors are playing an increasing role in pediatric psoriasis. They can be expensive and difficult for some insurance plans to cover, but offer the convenience of better efficacy and less frequent lab monitoring than conventional systemics. In the United States, etanercept and ustekinumab are approved for moderate to severe pediatric plaque psoriasis in patients as young as age 4 and 12 years, respectively. TNF inhibitors have accumulated the most data in children, while data are accumulating in trials of IL-17 inhibitors, IL-23 inhibitors, and PDE4 inhibitors.
“These drugs have reassuring safety profiles; low rates of infection and adverse reactions,” Dr. Cordoro said of biologic agents. “They’ve changed the landscape completely because now the expectation is complete or near-complete clearance. In contrast to the systemic agents, which may be started and stopped repeatedly, you need to think about continuous therapy, because these drugs are immunogenic,” she noted. “Whether antibodies against them become neutralizing or not is a different case. If a patient does have antibodies, it does not mean you have to stop the drug. Dose escalation can help. Increasing frequency of use of the drug can help, but patients will develop antibodies and it may result in loss of efficacy or reactions to the drug,” she added.
“When you’re thinking about using a biologic agent, think about patients who are chronic, moderate to severe, and who will need more long-term therapy. Most importantly, treatment should be individualized, as there is no ‘one size fits all’ approach.”
Dr. Cordoro disclosed that she is a member of the Celgene Corporation Scientific Steering Committee.
AUSTIN, TEX. – The way Kelly M. Cordoro sees it, the most for which patient.
“You can look up the dosing and frequency of these drugs; all of that’s available,” she said at the annual meeting of the Society for Pediatric Dermatology. “But how do we think about which drug for which patient? What are the considerations?”
Dr. Cordoro, professor of dermatology and pediatrics at the University of California, San Francisco, described psoriasis as an autoamplifying inflammatory cascade involving innate and adaptive immunity and noted that various components of that cascade represent treatment targets. “We don’t have a true comprehension of the pathophysiology of psoriasis, but as we learn the pathways, we’re targeting them,” she said. “You can target keratinocyte proliferation with drugs like retinoids and phototherapy. You can broadly target T cells, neutrophils, and dendritic cells with methotrexate, cyclosporine, and phototherapy. The newer drugs target the cytokine milieu, including TNF [tumor necrosis factor]–alpha, IL [interleukin]–17A, IL-12, and IL-23.”
There is no one right answer for which drug to prescribe, she continued, except in the cases of certain comorbidities, contraindications, and genetic variants. “For example, if a patient has psoriatic arthritis, then you have methotrexate and all of the biologics that might be disease modifying,” she said. “If a patient has inflammatory bowel disease, it’s critical to know that IL-17 inhibitors will flare that disease, but anti-TNF and IL-12 and IL-23 inhibitors are okay. If a patient has liver and kidney disease, you want to avoid methotrexate and cyclosporine. If there’s a female of childbearing potential you want to be very cautious with using retinoids. I think the harder question for us is, How about the rest of the patients?”
In addition to a drug’s mechanism of action, patient- and family-related factors play a role in deciding which agent to use. For example, does the patient prefer an oral or an injectable agent? Is the patient able to travel to a phototherapy center? Is it feasible for the family to manage visits for lab work and direct clinical monitoring? Does the family have a high level of health literacy and are you communicating with them in ways that facilitate shared decision making?
“The best way to choose a systemic therapy is to develop an individualized assessment of overall disease burden,” said Dr. Cordoro, who is also division chief of pediatric dermatology at UCSF. “Include psychological burden and subjective data in addition to objective measures like body surface area. Look for triggers. Infants are more commonly affected by viral infections and, in a subset, monogenic forms of psoriasis such as deficiency of interleukin 1 receptor antagonist [DIRA]. In general, we try to take a conservative approach in the developing child. As children hit early adolescence and become post pubertal, you have to start thinking about the psychosocial impact [of psoriasis], and we have to start treating patients with the consideration that chronic uncontrolled inflammation can potentially lead to comorbidities down the road. We see this in adults with severe psoriasis and early onset cardiovascular disease, the so-called psoriatic march from chronic inflammation to cardiovascular disease.”
Dr. Cordoro advises clinicians to rethink the conventional “therapeutic ladder” concept and embrace the idea of “finding the right tool for the job right now.” If a patient presents with a flare from a known trigger such as a strep infection, “maybe you want to treat with something more conservative,” she said. “Once you treat, and if the trigger has been managed, they might be better. But some patients will need the most aggressive treatment right out of the gate.”
Tried and true systemic therapies for psoriasis include methotrexate, cyclosporine, acitretin, and phototherapy, but none is approved by the Food and Drug Administration for use in children. “These drugs have decades of experience behind them,” Dr. Cordoro said. “Methotrexate is slow to start but has a sustained profile, so if you can get the patient to respond, that response tends to persist. Methotrexate also prevents the formation of antidrug antibodies, which is important if you are considering use of a biologic agent later on.”
Cyclosporine is best if you need a rapid rescue drug to get the disease under control before moving on to other options. “One in four patients relapse once cyclosporine is discontinued, so the benefit may not be as sustained as with methotrexate,” she said. “Acitretin is a really nice choice when you can’t or don’t want to immunosuppress the patient, and phototherapy is good if you can get it. The advantages of systemic therapies are that they’re easy on, easy off, and you can combine medications in severe situations. Almost all of these drugs can be combined with another, with few exceptions. I would caution that over immunosuppression is the biggest risk ... so this must be done carefully and only when necessary.”
Biologic agents such as TNF inhibitors and IL-12/23 inhibitors are playing an increasing role in pediatric psoriasis. They can be expensive and difficult for some insurance plans to cover, but offer the convenience of better efficacy and less frequent lab monitoring than conventional systemics. In the United States, etanercept and ustekinumab are approved for moderate to severe pediatric plaque psoriasis in patients as young as age 4 and 12 years, respectively. TNF inhibitors have accumulated the most data in children, while data are accumulating in trials of IL-17 inhibitors, IL-23 inhibitors, and PDE4 inhibitors.
“These drugs have reassuring safety profiles; low rates of infection and adverse reactions,” Dr. Cordoro said of biologic agents. “They’ve changed the landscape completely because now the expectation is complete or near-complete clearance. In contrast to the systemic agents, which may be started and stopped repeatedly, you need to think about continuous therapy, because these drugs are immunogenic,” she noted. “Whether antibodies against them become neutralizing or not is a different case. If a patient does have antibodies, it does not mean you have to stop the drug. Dose escalation can help. Increasing frequency of use of the drug can help, but patients will develop antibodies and it may result in loss of efficacy or reactions to the drug,” she added.
“When you’re thinking about using a biologic agent, think about patients who are chronic, moderate to severe, and who will need more long-term therapy. Most importantly, treatment should be individualized, as there is no ‘one size fits all’ approach.”
Dr. Cordoro disclosed that she is a member of the Celgene Corporation Scientific Steering Committee.
AUSTIN, TEX. – The way Kelly M. Cordoro sees it, the most for which patient.
“You can look up the dosing and frequency of these drugs; all of that’s available,” she said at the annual meeting of the Society for Pediatric Dermatology. “But how do we think about which drug for which patient? What are the considerations?”
Dr. Cordoro, professor of dermatology and pediatrics at the University of California, San Francisco, described psoriasis as an autoamplifying inflammatory cascade involving innate and adaptive immunity and noted that various components of that cascade represent treatment targets. “We don’t have a true comprehension of the pathophysiology of psoriasis, but as we learn the pathways, we’re targeting them,” she said. “You can target keratinocyte proliferation with drugs like retinoids and phototherapy. You can broadly target T cells, neutrophils, and dendritic cells with methotrexate, cyclosporine, and phototherapy. The newer drugs target the cytokine milieu, including TNF [tumor necrosis factor]–alpha, IL [interleukin]–17A, IL-12, and IL-23.”
There is no one right answer for which drug to prescribe, she continued, except in the cases of certain comorbidities, contraindications, and genetic variants. “For example, if a patient has psoriatic arthritis, then you have methotrexate and all of the biologics that might be disease modifying,” she said. “If a patient has inflammatory bowel disease, it’s critical to know that IL-17 inhibitors will flare that disease, but anti-TNF and IL-12 and IL-23 inhibitors are okay. If a patient has liver and kidney disease, you want to avoid methotrexate and cyclosporine. If there’s a female of childbearing potential you want to be very cautious with using retinoids. I think the harder question for us is, How about the rest of the patients?”
In addition to a drug’s mechanism of action, patient- and family-related factors play a role in deciding which agent to use. For example, does the patient prefer an oral or an injectable agent? Is the patient able to travel to a phototherapy center? Is it feasible for the family to manage visits for lab work and direct clinical monitoring? Does the family have a high level of health literacy and are you communicating with them in ways that facilitate shared decision making?
“The best way to choose a systemic therapy is to develop an individualized assessment of overall disease burden,” said Dr. Cordoro, who is also division chief of pediatric dermatology at UCSF. “Include psychological burden and subjective data in addition to objective measures like body surface area. Look for triggers. Infants are more commonly affected by viral infections and, in a subset, monogenic forms of psoriasis such as deficiency of interleukin 1 receptor antagonist [DIRA]. In general, we try to take a conservative approach in the developing child. As children hit early adolescence and become post pubertal, you have to start thinking about the psychosocial impact [of psoriasis], and we have to start treating patients with the consideration that chronic uncontrolled inflammation can potentially lead to comorbidities down the road. We see this in adults with severe psoriasis and early onset cardiovascular disease, the so-called psoriatic march from chronic inflammation to cardiovascular disease.”
Dr. Cordoro advises clinicians to rethink the conventional “therapeutic ladder” concept and embrace the idea of “finding the right tool for the job right now.” If a patient presents with a flare from a known trigger such as a strep infection, “maybe you want to treat with something more conservative,” she said. “Once you treat, and if the trigger has been managed, they might be better. But some patients will need the most aggressive treatment right out of the gate.”
Tried and true systemic therapies for psoriasis include methotrexate, cyclosporine, acitretin, and phototherapy, but none is approved by the Food and Drug Administration for use in children. “These drugs have decades of experience behind them,” Dr. Cordoro said. “Methotrexate is slow to start but has a sustained profile, so if you can get the patient to respond, that response tends to persist. Methotrexate also prevents the formation of antidrug antibodies, which is important if you are considering use of a biologic agent later on.”
Cyclosporine is best if you need a rapid rescue drug to get the disease under control before moving on to other options. “One in four patients relapse once cyclosporine is discontinued, so the benefit may not be as sustained as with methotrexate,” she said. “Acitretin is a really nice choice when you can’t or don’t want to immunosuppress the patient, and phototherapy is good if you can get it. The advantages of systemic therapies are that they’re easy on, easy off, and you can combine medications in severe situations. Almost all of these drugs can be combined with another, with few exceptions. I would caution that over immunosuppression is the biggest risk ... so this must be done carefully and only when necessary.”
Biologic agents such as TNF inhibitors and IL-12/23 inhibitors are playing an increasing role in pediatric psoriasis. They can be expensive and difficult for some insurance plans to cover, but offer the convenience of better efficacy and less frequent lab monitoring than conventional systemics. In the United States, etanercept and ustekinumab are approved for moderate to severe pediatric plaque psoriasis in patients as young as age 4 and 12 years, respectively. TNF inhibitors have accumulated the most data in children, while data are accumulating in trials of IL-17 inhibitors, IL-23 inhibitors, and PDE4 inhibitors.
“These drugs have reassuring safety profiles; low rates of infection and adverse reactions,” Dr. Cordoro said of biologic agents. “They’ve changed the landscape completely because now the expectation is complete or near-complete clearance. In contrast to the systemic agents, which may be started and stopped repeatedly, you need to think about continuous therapy, because these drugs are immunogenic,” she noted. “Whether antibodies against them become neutralizing or not is a different case. If a patient does have antibodies, it does not mean you have to stop the drug. Dose escalation can help. Increasing frequency of use of the drug can help, but patients will develop antibodies and it may result in loss of efficacy or reactions to the drug,” she added.
“When you’re thinking about using a biologic agent, think about patients who are chronic, moderate to severe, and who will need more long-term therapy. Most importantly, treatment should be individualized, as there is no ‘one size fits all’ approach.”
Dr. Cordoro disclosed that she is a member of the Celgene Corporation Scientific Steering Committee.
EXPERT ANALYSIS FROM SPD 2019
Vape lung disease cases exceed 400, 3 dead
Vitamin E acetate is one possible culprit in the mysterious vaping-associated lung disease that has killed three patients, sickened 450, and baffled clinicians and investigators all summer.
Another death may be linked to the disorder, officials said during a joint press briefing held by the Centers for Disease Control and Prevention and the Food and Drug Administration. In all, 450 potential cases have been reported and e-cigarette use confirmed in 215. Cases have occurred in 33 states and one territory. A total of 84% of the patients reported having used tetrahydrocannabinol (THC) products in e-cigarette devices.
A preliminary report on the situation by Jennifer Layden, MD, of the department of public health in Illinois and colleagues – including a preliminary case definition – was simultaneously released in the New England Journal of Medicine (2019 Sep 6. doi: 10.1056/NEJMoa1911614).
No single device or substance was common to all the cases, leading officials to issue a blanket warning against e-cigarettes, especially those containing THC.
“We believe a chemical exposure is likely related, but more information is needed to determine what substances. Some labs have identified vitamin E acetate in some samples,” said Dana Meaney-Delman, MD, MPH, incident manager, CDC 2019 Lung Injury Response. “Continued investigation is needed to identify the risk associated with a specific product or substance.”
Besides vitamin E acetate, federal labs are looking at other cannabinoids, cutting agents, diluting agents, pesticides, opioids, and toxins.
Officials also issued a general warning about the products. Youths, young people, and pregnant women should never use e-cigarettes, they cautioned, and no one should buy them from a noncertified source, a street vendor, or a social contact. Even cartridges originally obtained from a certified source should never have been altered in any way.
Dr. Layden and colleagues reported that bilateral lung infiltrates was characterized in 98% of the 53 patients hospitalized with the recently reported e-cigarette–induced lung injury. Nonspecific constitutional symptoms, including fever, chills, weight loss, and fatigue, were present in all of the patients.
Patients may show some symptoms days or even weeks before acute respiratory failure develops, and many had sought medical help before that. All presented with bilateral lung infiltrates, part of an evolving case definition. Many complained of nonspecific constitutional symptoms, including fever, chills, gastrointestinal symptoms, and weight loss. Of the patients who underwent bronchoscopy, many were diagnosed as having lipoid pneumonia, a rare condition characterized by lipid-laden macrophages.
“We don’t know the significance of the lipid-containing macrophages, and we don’t know if the lipids are endogenous or exogenous,” Dr. Meaney-Delman said.
The incidence of such cases appears to be rising rapidly, Dr. Layden noted. An epidemiologic review of cases in Illinois found that the mean monthly rate of visits related to severe respiratory illness in June-August was twice that observed during the same months last year.
SOURCE: Layden JE et al. N Engl J Med. 2019 Sep 6. doi: 1 0.1056/NEJMoa1911614.
Vitamin E acetate is one possible culprit in the mysterious vaping-associated lung disease that has killed three patients, sickened 450, and baffled clinicians and investigators all summer.
Another death may be linked to the disorder, officials said during a joint press briefing held by the Centers for Disease Control and Prevention and the Food and Drug Administration. In all, 450 potential cases have been reported and e-cigarette use confirmed in 215. Cases have occurred in 33 states and one territory. A total of 84% of the patients reported having used tetrahydrocannabinol (THC) products in e-cigarette devices.
A preliminary report on the situation by Jennifer Layden, MD, of the department of public health in Illinois and colleagues – including a preliminary case definition – was simultaneously released in the New England Journal of Medicine (2019 Sep 6. doi: 10.1056/NEJMoa1911614).
No single device or substance was common to all the cases, leading officials to issue a blanket warning against e-cigarettes, especially those containing THC.
“We believe a chemical exposure is likely related, but more information is needed to determine what substances. Some labs have identified vitamin E acetate in some samples,” said Dana Meaney-Delman, MD, MPH, incident manager, CDC 2019 Lung Injury Response. “Continued investigation is needed to identify the risk associated with a specific product or substance.”
Besides vitamin E acetate, federal labs are looking at other cannabinoids, cutting agents, diluting agents, pesticides, opioids, and toxins.
Officials also issued a general warning about the products. Youths, young people, and pregnant women should never use e-cigarettes, they cautioned, and no one should buy them from a noncertified source, a street vendor, or a social contact. Even cartridges originally obtained from a certified source should never have been altered in any way.
Dr. Layden and colleagues reported that bilateral lung infiltrates was characterized in 98% of the 53 patients hospitalized with the recently reported e-cigarette–induced lung injury. Nonspecific constitutional symptoms, including fever, chills, weight loss, and fatigue, were present in all of the patients.
Patients may show some symptoms days or even weeks before acute respiratory failure develops, and many had sought medical help before that. All presented with bilateral lung infiltrates, part of an evolving case definition. Many complained of nonspecific constitutional symptoms, including fever, chills, gastrointestinal symptoms, and weight loss. Of the patients who underwent bronchoscopy, many were diagnosed as having lipoid pneumonia, a rare condition characterized by lipid-laden macrophages.
“We don’t know the significance of the lipid-containing macrophages, and we don’t know if the lipids are endogenous or exogenous,” Dr. Meaney-Delman said.
The incidence of such cases appears to be rising rapidly, Dr. Layden noted. An epidemiologic review of cases in Illinois found that the mean monthly rate of visits related to severe respiratory illness in June-August was twice that observed during the same months last year.
SOURCE: Layden JE et al. N Engl J Med. 2019 Sep 6. doi: 1 0.1056/NEJMoa1911614.
Vitamin E acetate is one possible culprit in the mysterious vaping-associated lung disease that has killed three patients, sickened 450, and baffled clinicians and investigators all summer.
Another death may be linked to the disorder, officials said during a joint press briefing held by the Centers for Disease Control and Prevention and the Food and Drug Administration. In all, 450 potential cases have been reported and e-cigarette use confirmed in 215. Cases have occurred in 33 states and one territory. A total of 84% of the patients reported having used tetrahydrocannabinol (THC) products in e-cigarette devices.
A preliminary report on the situation by Jennifer Layden, MD, of the department of public health in Illinois and colleagues – including a preliminary case definition – was simultaneously released in the New England Journal of Medicine (2019 Sep 6. doi: 10.1056/NEJMoa1911614).
No single device or substance was common to all the cases, leading officials to issue a blanket warning against e-cigarettes, especially those containing THC.
“We believe a chemical exposure is likely related, but more information is needed to determine what substances. Some labs have identified vitamin E acetate in some samples,” said Dana Meaney-Delman, MD, MPH, incident manager, CDC 2019 Lung Injury Response. “Continued investigation is needed to identify the risk associated with a specific product or substance.”
Besides vitamin E acetate, federal labs are looking at other cannabinoids, cutting agents, diluting agents, pesticides, opioids, and toxins.
Officials also issued a general warning about the products. Youths, young people, and pregnant women should never use e-cigarettes, they cautioned, and no one should buy them from a noncertified source, a street vendor, or a social contact. Even cartridges originally obtained from a certified source should never have been altered in any way.
Dr. Layden and colleagues reported that bilateral lung infiltrates was characterized in 98% of the 53 patients hospitalized with the recently reported e-cigarette–induced lung injury. Nonspecific constitutional symptoms, including fever, chills, weight loss, and fatigue, were present in all of the patients.
Patients may show some symptoms days or even weeks before acute respiratory failure develops, and many had sought medical help before that. All presented with bilateral lung infiltrates, part of an evolving case definition. Many complained of nonspecific constitutional symptoms, including fever, chills, gastrointestinal symptoms, and weight loss. Of the patients who underwent bronchoscopy, many were diagnosed as having lipoid pneumonia, a rare condition characterized by lipid-laden macrophages.
“We don’t know the significance of the lipid-containing macrophages, and we don’t know if the lipids are endogenous or exogenous,” Dr. Meaney-Delman said.
The incidence of such cases appears to be rising rapidly, Dr. Layden noted. An epidemiologic review of cases in Illinois found that the mean monthly rate of visits related to severe respiratory illness in June-August was twice that observed during the same months last year.
SOURCE: Layden JE et al. N Engl J Med. 2019 Sep 6. doi: 1 0.1056/NEJMoa1911614.
FROM A CDC TELECONFERENCE AND NEJM
8-year-old boy • palpable purpura on the legs with arthralgia • absence of coagulopathy • upper respiratory infection • Dx?
THE CASE
An 8-year-old boy presented to his family physician (FP) with pharyngitis, nasal drainage, and a dry cough of 3 days’ duration. He denied any fever, chills, vomiting, or diarrhea. He had no sick contacts or prior history of streptococcal pharyngitis, but a rapid strep test was positive. No throat culture was performed at this time. The patient was started on amoxicillin 250 mg 3 times daily for 10 days.
On Day 7 of symptoms, the patient presented to the emergency department with elbow and knee pain, as well as mild swelling and purpura of his legs of 3 days’ duration. He was normotensive and reported no abdominal pain. A laboratory workup, including a complete blood cell count and differential, prothrombin time, partial thromboplastin time, comprehensive metabolic panel, creatinine kinase test, urinalysis, and chest radiograph, was normal, but his erythrocyte sedimentation rate (ESR) was mildly elevated at 22 mm/h (reference range, 0–20 mm/h). The patient was discharged on acetaminophen 15 mg/kg every 4 hours as needed for pain.
THE DIAGNOSIS
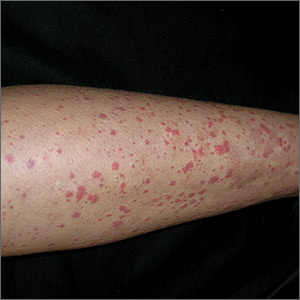
Based on the distinctive palpable purpura on the legs, arthralgia, upper respiratory infection, and lack of thrombocytopenia and coagulopathy, a presumptive diagnosis of Henoch-Schönlein purpura (HSP) was made.
On Day 9 of symptoms, the patient returned to his FP’s office because the arthralgia persisted in his ankles, knees, and hips. He had developed lower back pain, but the pharyngitis and upper respiratory symptoms had resolved. On physical examination, he was normotensive with a normal abdominal exam. The patient reported that it hurt to move his wrists, hands, elbows, shoulders, knees, and ankles. He also had mild swelling in his left wrist, hand, and ankle. The paraspinal muscles in the lower thoracic and lumbar back were mildly tender to palpation. A complete metabolic panel and urinalysis were normal. Dermatologic examination revealed discrete purpuric lesions ranging from 1 to 8 mm in diameter on the child’s shins, thighs, and buttocks. Urinalysis, blood urea nitrogen, and creatinine kinase were normal. His ESR remained mildly elevated at 24 mm/h. Since there was no evidence of glomerulonephritis, ibuprofen 10 mg/kg every 8 hours as needed was added for pain management.
The child was brought back to his FP on Day 18 for a scheduled follow-up visit. The parents reported that his arthralgia was improved during the day, but by the evening, his knees and ankles hurt so much that they had to carry him to the bathroom. On physical examination, he still had palpable purpura of the legs. There was no swelling, but his joints were still tender to palpation. His parents were reminded to give him ibuprofen after school to control evening pain. Over the next 2 weeks, the patient showed gradual improvement, and by Day 33 the rash and all of the associated symptoms had resolved.
DISCUSSION
Clinical presentation. HSP is an IgA immune complex vasculitis in which abnormal glycosylation of IgA creates large immune complexes that are deposited in the walls of the skin capillaries and arterioles. The primary clinical finding in HSP is a distinctive nonthrombocytopenic purpuric rash that is not associated with coagulopathy and is characterized by reddish purple macules that progress to palpable purpura with petechiae (
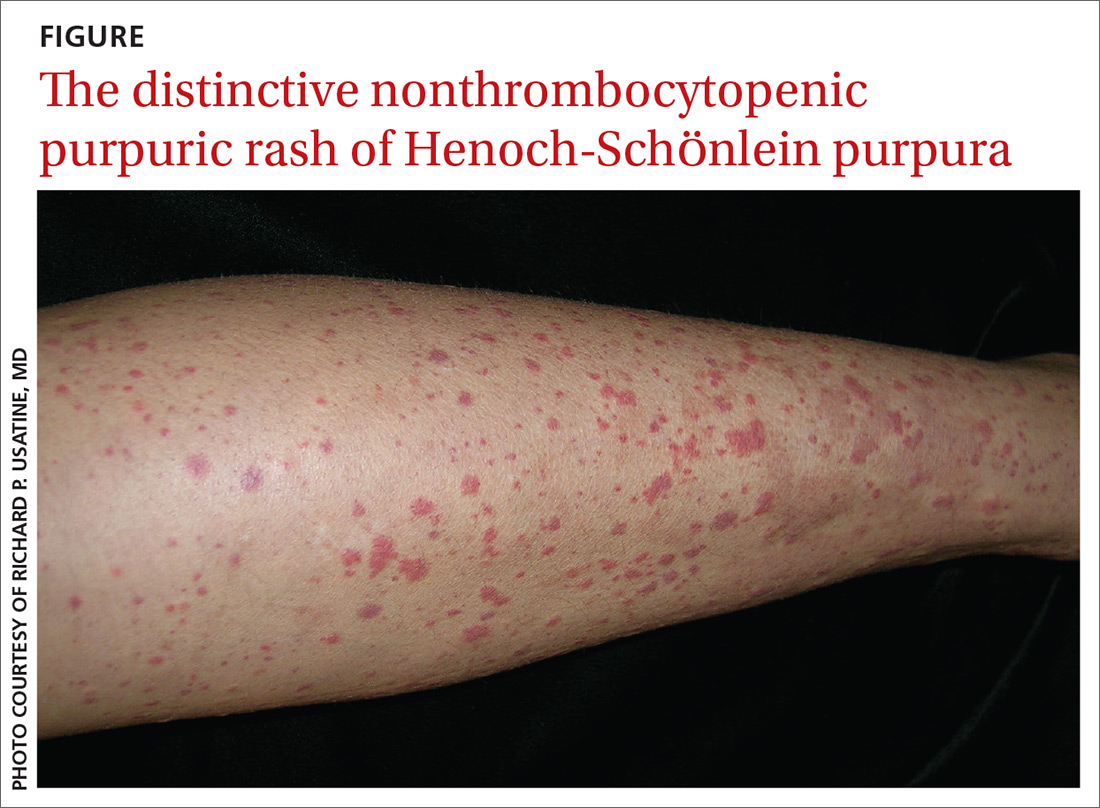
A preceding upper respiratory infection has been found in 37% of patients,1 and in patients with renal complications, 20% to 50% have been found to have a group A Streptococcus infection.2 Other associations include food allergies, cold exposure, insect bites, and drug allergies.
Continue to: HSP vasculitis causes...
HSP vasculitis causes abdominal pain in 50% to 75% of patients due to proximal small-bowel submucosal hemorrhage and bowel wall edema.3 In children with HSP, 20% to 55% have been shown to develop renal disease,4 which can range in severity from microscopic hematuria to nephrotic syndrome.3 To ensure prompt treatment of renal manifestations, renal function should be monitored regularly via blood pressure and urinalysis during the course of HSP and after resolution. Renal disease associated with HSP can be acute or chronic.
This case was different because our patient did not exhibit all elements of the classic tetrad of HSP, which includes the characteristic rash, abdominal pain, renal involvement, and arthralgia.
Incidence. HSP is more common in children than adults, with average annual incidence rates of 20/100,000 and 70/100,000 in children in the United States and Asia, respectively.5 While 90% of HSP cases occur in children < 10 years, the peak incidence is at 6 years of age.6 Complications from HSP are more common in adults than in children.7 Caucasian and Asian populations have a 3- to 4-times higher prevalence of HSP than black populations. The male-to-female ratio is 2 to 1.6
The diagnosis of HSP is usually made clinically, based on the distinctive rash, which typically is symmetrical, involving the buttocks, lower legs, elbows, and/or knees. HSP also can be confirmed via skin biopsy and/or direct immunofluorescence, which can identify the presence of IgA in the vessel walls.
The presence of 3 or more of the following criteria also suggests HSP: palpable purpura, bowel angina, gastrointestinal (GI) bleeding, hematuria, ≤ 20 years of age at onset, and no medications prior to presentation of symptoms (87% of cases correctly classified). Fewer than 3 of these factors favor hypersensitivity vasculitis (74% of cases correctly classified).8
Continue to: The differential diagnosis
The differential diagnosis for HSP includes polyarteritis nodosa, a vasculitis with a different characteristic rash; acute abdomen, distinguished by the absence of purpura or arthralgia; meningococcemia, in which fever and meningeal signs may occur; hypersensitivity vasculitis, which arises due to prior exposure to medications or food allergens; and thrombocytopenic purpura, which is characterized by low platelet count.9
Treatment focuses on pain management
In the absence of renal disease, HSP commonly is treated with naproxen for pain management (dosage for children < 2 years of age: 5-7 mg/kg orally every 8-12 hours; dosage for children ≥ 2 years of age, adolescents, and adults: 10-20 mg/kg/d divided into 2 doses; maximum adolescent and adult dose is 1500 mg/d for 3 days followed by a maximum of 1000 mg/d thereafter).
For patients of all ages with severe pain and those with GI effects limiting oral intake of medication, use oral prednisone (1-2 mg/kg/d [maximum dose, 60-80 mg/d]) or intravenous methylprednisolone (0.8-1.6 mg/kg/d [maximum dose, 64 mg/d). Glucocorticoids may then be tapered slowly over 4 to 8 weeks to avoid rebound since they help with inflammation but do not shorten the course of disease. Steroids can ease GI and joint symptoms in HSP but will not improve the rash.
THE TAKEAWAY
The classic tetrad of HSP includes the characteristic rash, abdominal pain, renal involvement, and arthralgia. Diagnosis usually is made clinically, but skin biopsy and direct immunofluorescence can confirm small vessel vasculitis with IgA deposits. More severe manifestations of HSP such as renal disease, hemorrhage, severe anemia, signs of intestinal obstruction, or peritonitis require rapid subspecialty referral.
CORRESPONDENCE
Rachel Bramson, MD, Department of Primary Care, Baylor Scott and White Health, University Clinic, 1700 University Drive, College Station, TX 77840; [email protected]
1. Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
2. LaConti JJ, Donet JA, Cho-Vega JH, et al. Henoch-Schönlein Purpura with adalimumab therapy for ulcerative colitis: a case report and review of the literature [published online July 27, 2016]. Case Rep Rheumatol. 2016;2016:2812980.
3. Trnka P. Henoch-Schönlein purpura in children. J Paediatr Child Health. 2013;49:995-1003.
4. Audemard-Verger A, Pillebout E, Guillevin L, et al. IgA vasculitis (Henoch-Shönlein purpura) in adults: diagnostic and therapeutic aspects. Autoimmun Rev. 2015;14:579-585.
5. Chen J, Mao J. Henoch-Schönlein purpura nephritis in children: incidence, pathogenesis and management. World J Pediatr. 2015;11:29-34.
6. Michel B, Hunder G, Bloch D, et al. Hypersensitivity vasculitis and Henoch-Schönlein purpura: a comparison between the 2 disorders. J Rheumatol. 1992;19:721-728.
7. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
8. Yang YH, Yu HH, Chiang BL. The diagnosis and classification of Henoch-Schönlein purpura: an updated review. Autoimmun Rev. 2014;13:355-358.
9. Floege J, Feehally J. Treatment of IgA nephropathy and Henoch-Schönlein nephritis. Nat Rev Nephrol. 2013;9:320-327.
THE CASE
An 8-year-old boy presented to his family physician (FP) with pharyngitis, nasal drainage, and a dry cough of 3 days’ duration. He denied any fever, chills, vomiting, or diarrhea. He had no sick contacts or prior history of streptococcal pharyngitis, but a rapid strep test was positive. No throat culture was performed at this time. The patient was started on amoxicillin 250 mg 3 times daily for 10 days.
On Day 7 of symptoms, the patient presented to the emergency department with elbow and knee pain, as well as mild swelling and purpura of his legs of 3 days’ duration. He was normotensive and reported no abdominal pain. A laboratory workup, including a complete blood cell count and differential, prothrombin time, partial thromboplastin time, comprehensive metabolic panel, creatinine kinase test, urinalysis, and chest radiograph, was normal, but his erythrocyte sedimentation rate (ESR) was mildly elevated at 22 mm/h (reference range, 0–20 mm/h). The patient was discharged on acetaminophen 15 mg/kg every 4 hours as needed for pain.
THE DIAGNOSIS
Based on the distinctive palpable purpura on the legs, arthralgia, upper respiratory infection, and lack of thrombocytopenia and coagulopathy, a presumptive diagnosis of Henoch-Schönlein purpura (HSP) was made.
On Day 9 of symptoms, the patient returned to his FP’s office because the arthralgia persisted in his ankles, knees, and hips. He had developed lower back pain, but the pharyngitis and upper respiratory symptoms had resolved. On physical examination, he was normotensive with a normal abdominal exam. The patient reported that it hurt to move his wrists, hands, elbows, shoulders, knees, and ankles. He also had mild swelling in his left wrist, hand, and ankle. The paraspinal muscles in the lower thoracic and lumbar back were mildly tender to palpation. A complete metabolic panel and urinalysis were normal. Dermatologic examination revealed discrete purpuric lesions ranging from 1 to 8 mm in diameter on the child’s shins, thighs, and buttocks. Urinalysis, blood urea nitrogen, and creatinine kinase were normal. His ESR remained mildly elevated at 24 mm/h. Since there was no evidence of glomerulonephritis, ibuprofen 10 mg/kg every 8 hours as needed was added for pain management.
The child was brought back to his FP on Day 18 for a scheduled follow-up visit. The parents reported that his arthralgia was improved during the day, but by the evening, his knees and ankles hurt so much that they had to carry him to the bathroom. On physical examination, he still had palpable purpura of the legs. There was no swelling, but his joints were still tender to palpation. His parents were reminded to give him ibuprofen after school to control evening pain. Over the next 2 weeks, the patient showed gradual improvement, and by Day 33 the rash and all of the associated symptoms had resolved.
DISCUSSION
Clinical presentation. HSP is an IgA immune complex vasculitis in which abnormal glycosylation of IgA creates large immune complexes that are deposited in the walls of the skin capillaries and arterioles. The primary clinical finding in HSP is a distinctive nonthrombocytopenic purpuric rash that is not associated with coagulopathy and is characterized by reddish purple macules that progress to palpable purpura with petechiae (

A preceding upper respiratory infection has been found in 37% of patients,1 and in patients with renal complications, 20% to 50% have been found to have a group A Streptococcus infection.2 Other associations include food allergies, cold exposure, insect bites, and drug allergies.
Continue to: HSP vasculitis causes...
HSP vasculitis causes abdominal pain in 50% to 75% of patients due to proximal small-bowel submucosal hemorrhage and bowel wall edema.3 In children with HSP, 20% to 55% have been shown to develop renal disease,4 which can range in severity from microscopic hematuria to nephrotic syndrome.3 To ensure prompt treatment of renal manifestations, renal function should be monitored regularly via blood pressure and urinalysis during the course of HSP and after resolution. Renal disease associated with HSP can be acute or chronic.
This case was different because our patient did not exhibit all elements of the classic tetrad of HSP, which includes the characteristic rash, abdominal pain, renal involvement, and arthralgia.
Incidence. HSP is more common in children than adults, with average annual incidence rates of 20/100,000 and 70/100,000 in children in the United States and Asia, respectively.5 While 90% of HSP cases occur in children < 10 years, the peak incidence is at 6 years of age.6 Complications from HSP are more common in adults than in children.7 Caucasian and Asian populations have a 3- to 4-times higher prevalence of HSP than black populations. The male-to-female ratio is 2 to 1.6
The diagnosis of HSP is usually made clinically, based on the distinctive rash, which typically is symmetrical, involving the buttocks, lower legs, elbows, and/or knees. HSP also can be confirmed via skin biopsy and/or direct immunofluorescence, which can identify the presence of IgA in the vessel walls.
The presence of 3 or more of the following criteria also suggests HSP: palpable purpura, bowel angina, gastrointestinal (GI) bleeding, hematuria, ≤ 20 years of age at onset, and no medications prior to presentation of symptoms (87% of cases correctly classified). Fewer than 3 of these factors favor hypersensitivity vasculitis (74% of cases correctly classified).8
Continue to: The differential diagnosis
The differential diagnosis for HSP includes polyarteritis nodosa, a vasculitis with a different characteristic rash; acute abdomen, distinguished by the absence of purpura or arthralgia; meningococcemia, in which fever and meningeal signs may occur; hypersensitivity vasculitis, which arises due to prior exposure to medications or food allergens; and thrombocytopenic purpura, which is characterized by low platelet count.9
Treatment focuses on pain management
In the absence of renal disease, HSP commonly is treated with naproxen for pain management (dosage for children < 2 years of age: 5-7 mg/kg orally every 8-12 hours; dosage for children ≥ 2 years of age, adolescents, and adults: 10-20 mg/kg/d divided into 2 doses; maximum adolescent and adult dose is 1500 mg/d for 3 days followed by a maximum of 1000 mg/d thereafter).
For patients of all ages with severe pain and those with GI effects limiting oral intake of medication, use oral prednisone (1-2 mg/kg/d [maximum dose, 60-80 mg/d]) or intravenous methylprednisolone (0.8-1.6 mg/kg/d [maximum dose, 64 mg/d). Glucocorticoids may then be tapered slowly over 4 to 8 weeks to avoid rebound since they help with inflammation but do not shorten the course of disease. Steroids can ease GI and joint symptoms in HSP but will not improve the rash.
THE TAKEAWAY
The classic tetrad of HSP includes the characteristic rash, abdominal pain, renal involvement, and arthralgia. Diagnosis usually is made clinically, but skin biopsy and direct immunofluorescence can confirm small vessel vasculitis with IgA deposits. More severe manifestations of HSP such as renal disease, hemorrhage, severe anemia, signs of intestinal obstruction, or peritonitis require rapid subspecialty referral.
CORRESPONDENCE
Rachel Bramson, MD, Department of Primary Care, Baylor Scott and White Health, University Clinic, 1700 University Drive, College Station, TX 77840; [email protected]
THE CASE
An 8-year-old boy presented to his family physician (FP) with pharyngitis, nasal drainage, and a dry cough of 3 days’ duration. He denied any fever, chills, vomiting, or diarrhea. He had no sick contacts or prior history of streptococcal pharyngitis, but a rapid strep test was positive. No throat culture was performed at this time. The patient was started on amoxicillin 250 mg 3 times daily for 10 days.
On Day 7 of symptoms, the patient presented to the emergency department with elbow and knee pain, as well as mild swelling and purpura of his legs of 3 days’ duration. He was normotensive and reported no abdominal pain. A laboratory workup, including a complete blood cell count and differential, prothrombin time, partial thromboplastin time, comprehensive metabolic panel, creatinine kinase test, urinalysis, and chest radiograph, was normal, but his erythrocyte sedimentation rate (ESR) was mildly elevated at 22 mm/h (reference range, 0–20 mm/h). The patient was discharged on acetaminophen 15 mg/kg every 4 hours as needed for pain.
THE DIAGNOSIS
Based on the distinctive palpable purpura on the legs, arthralgia, upper respiratory infection, and lack of thrombocytopenia and coagulopathy, a presumptive diagnosis of Henoch-Schönlein purpura (HSP) was made.
On Day 9 of symptoms, the patient returned to his FP’s office because the arthralgia persisted in his ankles, knees, and hips. He had developed lower back pain, but the pharyngitis and upper respiratory symptoms had resolved. On physical examination, he was normotensive with a normal abdominal exam. The patient reported that it hurt to move his wrists, hands, elbows, shoulders, knees, and ankles. He also had mild swelling in his left wrist, hand, and ankle. The paraspinal muscles in the lower thoracic and lumbar back were mildly tender to palpation. A complete metabolic panel and urinalysis were normal. Dermatologic examination revealed discrete purpuric lesions ranging from 1 to 8 mm in diameter on the child’s shins, thighs, and buttocks. Urinalysis, blood urea nitrogen, and creatinine kinase were normal. His ESR remained mildly elevated at 24 mm/h. Since there was no evidence of glomerulonephritis, ibuprofen 10 mg/kg every 8 hours as needed was added for pain management.
The child was brought back to his FP on Day 18 for a scheduled follow-up visit. The parents reported that his arthralgia was improved during the day, but by the evening, his knees and ankles hurt so much that they had to carry him to the bathroom. On physical examination, he still had palpable purpura of the legs. There was no swelling, but his joints were still tender to palpation. His parents were reminded to give him ibuprofen after school to control evening pain. Over the next 2 weeks, the patient showed gradual improvement, and by Day 33 the rash and all of the associated symptoms had resolved.
DISCUSSION
Clinical presentation. HSP is an IgA immune complex vasculitis in which abnormal glycosylation of IgA creates large immune complexes that are deposited in the walls of the skin capillaries and arterioles. The primary clinical finding in HSP is a distinctive nonthrombocytopenic purpuric rash that is not associated with coagulopathy and is characterized by reddish purple macules that progress to palpable purpura with petechiae (

A preceding upper respiratory infection has been found in 37% of patients,1 and in patients with renal complications, 20% to 50% have been found to have a group A Streptococcus infection.2 Other associations include food allergies, cold exposure, insect bites, and drug allergies.
Continue to: HSP vasculitis causes...
HSP vasculitis causes abdominal pain in 50% to 75% of patients due to proximal small-bowel submucosal hemorrhage and bowel wall edema.3 In children with HSP, 20% to 55% have been shown to develop renal disease,4 which can range in severity from microscopic hematuria to nephrotic syndrome.3 To ensure prompt treatment of renal manifestations, renal function should be monitored regularly via blood pressure and urinalysis during the course of HSP and after resolution. Renal disease associated with HSP can be acute or chronic.
This case was different because our patient did not exhibit all elements of the classic tetrad of HSP, which includes the characteristic rash, abdominal pain, renal involvement, and arthralgia.
Incidence. HSP is more common in children than adults, with average annual incidence rates of 20/100,000 and 70/100,000 in children in the United States and Asia, respectively.5 While 90% of HSP cases occur in children < 10 years, the peak incidence is at 6 years of age.6 Complications from HSP are more common in adults than in children.7 Caucasian and Asian populations have a 3- to 4-times higher prevalence of HSP than black populations. The male-to-female ratio is 2 to 1.6
The diagnosis of HSP is usually made clinically, based on the distinctive rash, which typically is symmetrical, involving the buttocks, lower legs, elbows, and/or knees. HSP also can be confirmed via skin biopsy and/or direct immunofluorescence, which can identify the presence of IgA in the vessel walls.
The presence of 3 or more of the following criteria also suggests HSP: palpable purpura, bowel angina, gastrointestinal (GI) bleeding, hematuria, ≤ 20 years of age at onset, and no medications prior to presentation of symptoms (87% of cases correctly classified). Fewer than 3 of these factors favor hypersensitivity vasculitis (74% of cases correctly classified).8
Continue to: The differential diagnosis
The differential diagnosis for HSP includes polyarteritis nodosa, a vasculitis with a different characteristic rash; acute abdomen, distinguished by the absence of purpura or arthralgia; meningococcemia, in which fever and meningeal signs may occur; hypersensitivity vasculitis, which arises due to prior exposure to medications or food allergens; and thrombocytopenic purpura, which is characterized by low platelet count.9
Treatment focuses on pain management
In the absence of renal disease, HSP commonly is treated with naproxen for pain management (dosage for children < 2 years of age: 5-7 mg/kg orally every 8-12 hours; dosage for children ≥ 2 years of age, adolescents, and adults: 10-20 mg/kg/d divided into 2 doses; maximum adolescent and adult dose is 1500 mg/d for 3 days followed by a maximum of 1000 mg/d thereafter).
For patients of all ages with severe pain and those with GI effects limiting oral intake of medication, use oral prednisone (1-2 mg/kg/d [maximum dose, 60-80 mg/d]) or intravenous methylprednisolone (0.8-1.6 mg/kg/d [maximum dose, 64 mg/d). Glucocorticoids may then be tapered slowly over 4 to 8 weeks to avoid rebound since they help with inflammation but do not shorten the course of disease. Steroids can ease GI and joint symptoms in HSP but will not improve the rash.
THE TAKEAWAY
The classic tetrad of HSP includes the characteristic rash, abdominal pain, renal involvement, and arthralgia. Diagnosis usually is made clinically, but skin biopsy and direct immunofluorescence can confirm small vessel vasculitis with IgA deposits. More severe manifestations of HSP such as renal disease, hemorrhage, severe anemia, signs of intestinal obstruction, or peritonitis require rapid subspecialty referral.
CORRESPONDENCE
Rachel Bramson, MD, Department of Primary Care, Baylor Scott and White Health, University Clinic, 1700 University Drive, College Station, TX 77840; [email protected]
1. Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
2. LaConti JJ, Donet JA, Cho-Vega JH, et al. Henoch-Schönlein Purpura with adalimumab therapy for ulcerative colitis: a case report and review of the literature [published online July 27, 2016]. Case Rep Rheumatol. 2016;2016:2812980.
3. Trnka P. Henoch-Schönlein purpura in children. J Paediatr Child Health. 2013;49:995-1003.
4. Audemard-Verger A, Pillebout E, Guillevin L, et al. IgA vasculitis (Henoch-Shönlein purpura) in adults: diagnostic and therapeutic aspects. Autoimmun Rev. 2015;14:579-585.
5. Chen J, Mao J. Henoch-Schönlein purpura nephritis in children: incidence, pathogenesis and management. World J Pediatr. 2015;11:29-34.
6. Michel B, Hunder G, Bloch D, et al. Hypersensitivity vasculitis and Henoch-Schönlein purpura: a comparison between the 2 disorders. J Rheumatol. 1992;19:721-728.
7. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
8. Yang YH, Yu HH, Chiang BL. The diagnosis and classification of Henoch-Schönlein purpura: an updated review. Autoimmun Rev. 2014;13:355-358.
9. Floege J, Feehally J. Treatment of IgA nephropathy and Henoch-Schönlein nephritis. Nat Rev Nephrol. 2013;9:320-327.
1. Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
2. LaConti JJ, Donet JA, Cho-Vega JH, et al. Henoch-Schönlein Purpura with adalimumab therapy for ulcerative colitis: a case report and review of the literature [published online July 27, 2016]. Case Rep Rheumatol. 2016;2016:2812980.
3. Trnka P. Henoch-Schönlein purpura in children. J Paediatr Child Health. 2013;49:995-1003.
4. Audemard-Verger A, Pillebout E, Guillevin L, et al. IgA vasculitis (Henoch-Shönlein purpura) in adults: diagnostic and therapeutic aspects. Autoimmun Rev. 2015;14:579-585.
5. Chen J, Mao J. Henoch-Schönlein purpura nephritis in children: incidence, pathogenesis and management. World J Pediatr. 2015;11:29-34.
6. Michel B, Hunder G, Bloch D, et al. Hypersensitivity vasculitis and Henoch-Schönlein purpura: a comparison between the 2 disorders. J Rheumatol. 1992;19:721-728.
7. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
8. Yang YH, Yu HH, Chiang BL. The diagnosis and classification of Henoch-Schönlein purpura: an updated review. Autoimmun Rev. 2014;13:355-358.
9. Floege J, Feehally J. Treatment of IgA nephropathy and Henoch-Schönlein nephritis. Nat Rev Nephrol. 2013;9:320-327.
New report cites mental health challenges faced by separated immigrant children
Care providers encountered significant challenges when addressing the mental health needs of unaccompanied immigrant children in federal custody, including overwhelming caseloads and the deteriorating mental health of some patients, according to a new report by the Office of Inspector General (OIG).
In the report, released Sept. 3, the OIG outlined findings from its analysis of 45 Office of Refugee Resettlement (ORR) facilities between August and September 2018. The U.S. Department of Health & Human Services ORR is the legal custodian of unaccompanied immigrant children in its care who have no parent or legal guardian available. This includes children who arrive in the United States unaccompanied and children who are separated from their parents or guardians by immigration authorities after arriving in the country.
For the analysis, OIG investigators collected data from interviews with mental health clinicians, medical coordinators, facility leadership, and ORR federal field specialists at the 45 selected facilities.
Investigators recorded numerous serious challenges experienced by providers when attempting to provide mental health care to the children. Namely, they cited overwhelming patient caseloads, and difficulty accessing external mental health clinicians and referring children to providers within ORR’s network, according to the OIG’s report.
Mental health clinicians reported that the high caseloads hurt their ability to build rapport with young patients – and allowed less time for counseling and less frequent sessions for children with greater needs. The heavy caseloads were generated by heightened immigration enforcement beginning in 2017, and the separation of many more families at the border and more children being placed in federal custody, according to the report.
In addition, providers reported challenges when addressing the mental health needs of children who had experienced significant trauma before coming into federal custody. This included trauma that occurred while the children lived in the countries of origin, trauma during their journey to the United States, and trauma upon their arrival in the United States.
Separation from parents and a chaotic reunification process added to the trauma that children had already experienced, providers reported, and put extreme pressure on facility staff. Separated children exhibited “more fear, feelings of abandonment, and posttraumatic stress than did children who were not separated,” according to the findings. Separated children also experienced elevated feelings of anxiety and loss as a consequence of unexpected separation from loved ones.
Also, facilities reported that longer lengths of stay resulted in deteriorating mental health for some children and increased demands on staff. Facilities reported that children who stayed in federal custody for longer periods experienced more stress, anxiety, and behavioral issues. According to the facilities, the longer stays resulted in higher levels of defiance, hopelessness, and frustration among children – in addition to more instances of self-harm and suicidal ideation.
It is not surprising that the OIG study reflects that mental health services at facilities for unaccompanied minors are understaffed, undertrained, and overwhelmed, said Craig L. Katz, MD, a clinical professor of psychiatry at Mount Sinai in New York.
“In some sense, this can probably be said for most of the U.S. and definitely the world when it comes to child mental health services,” Dr. Katz said in an interview. “But, what’s especially tough to stomach about this shortfall at these facilities is that they encompass an immensely high-risk population – an inevitably highly, if not multiply traumatized population of children who lack primary caregivers.”
Dr. Katz was coinvestigator of a recent study that assessed the mental health of children held at a U.S. immigration detention center through the Parent-Report Strengths and Difficulties Questionnaire. Among the 425 children evaluated, many demonstrated elevated scores for emotional problems, peer problems, and total difficulties, according to the June 2018 study, published in Social Science & Medicine (2019 Jun; 230:303-8). Younger children (aged 4-8 years) demonstrated more difficulties associated with conduct, hyperactivity, and total difficulties, compared with older children, the study found.
Children who had been forcibly separated from their mothers demonstrated significantly more emotional problems and total difficulties, compared with those who had never been separated. Of 150 children who completed the Posttraumatic Stress Disorder Reaction Index, 17% had a probable diagnosis of PTSD, results found.
Dr. Katz said the OIG reached the same basic conclusion as his quantitative study – that separated minors appear to have even greater mental health problems than do fellow unaccompanied minors.
“In our study, we found that children in family detention had greater mental health problems than [did] American community samples but that formerly separated children who had been reunited with their mothers had even more health problems than their fellow detainees,” Dr. Katz said. “Something about being separated per U.S. policy was especially pernicious, which we knew in our hearts; but now in this study and ours, we know empirically.”
As long as the United States continues to detain children, the psychological harm created by such detainments is likely to continue, said Kim A. Baranowski, PhD, a psychologist and lecturer at Columbia University in New York. At a minimum, unaccompanied minors should have access to highly trained licensed clinicians who can respond to their immediate mental health needs within the initial hours and days following their arrival in the United States, and such children should be released rapidly from government custody and reunited with their families, said Dr. Baranowski, a coauthor of the Social Science & Medicine study.
“We need to effectively support their integration into the community, and connect children and their families with linguistically, culturally, and developmentally appropriate trauma-informed pro bono treatment services that respond to their experiences” of premigration, migration, and postmigration stressors, “as well as potential exposure to trauma,” she said in an interview.
The OIG issued several recommendations for practical steps that ORR can take to assist facilities and better provide mental health care to immigrant children in federal custody. The agency advised that the ORR should provide facilities with evidence-based guidance on addressing trauma in short-term therapy and that the ORR also should develop strategies for overcoming challenges to hiring and retaining qualified mental health clinicians.
The Office of Inspector General also suggested that facilities consider maximum caseloads for individual clinicians. Finally, the OIG recommends that ORR address gaps in options for children who require more specialized treatment and that the office take reasonable steps to minimize the length of time that children remain in custody.
[email protected]
*This article was updated 9/5/2019.
Care providers encountered significant challenges when addressing the mental health needs of unaccompanied immigrant children in federal custody, including overwhelming caseloads and the deteriorating mental health of some patients, according to a new report by the Office of Inspector General (OIG).
In the report, released Sept. 3, the OIG outlined findings from its analysis of 45 Office of Refugee Resettlement (ORR) facilities between August and September 2018. The U.S. Department of Health & Human Services ORR is the legal custodian of unaccompanied immigrant children in its care who have no parent or legal guardian available. This includes children who arrive in the United States unaccompanied and children who are separated from their parents or guardians by immigration authorities after arriving in the country.
For the analysis, OIG investigators collected data from interviews with mental health clinicians, medical coordinators, facility leadership, and ORR federal field specialists at the 45 selected facilities.
Investigators recorded numerous serious challenges experienced by providers when attempting to provide mental health care to the children. Namely, they cited overwhelming patient caseloads, and difficulty accessing external mental health clinicians and referring children to providers within ORR’s network, according to the OIG’s report.
Mental health clinicians reported that the high caseloads hurt their ability to build rapport with young patients – and allowed less time for counseling and less frequent sessions for children with greater needs. The heavy caseloads were generated by heightened immigration enforcement beginning in 2017, and the separation of many more families at the border and more children being placed in federal custody, according to the report.
In addition, providers reported challenges when addressing the mental health needs of children who had experienced significant trauma before coming into federal custody. This included trauma that occurred while the children lived in the countries of origin, trauma during their journey to the United States, and trauma upon their arrival in the United States.
Separation from parents and a chaotic reunification process added to the trauma that children had already experienced, providers reported, and put extreme pressure on facility staff. Separated children exhibited “more fear, feelings of abandonment, and posttraumatic stress than did children who were not separated,” according to the findings. Separated children also experienced elevated feelings of anxiety and loss as a consequence of unexpected separation from loved ones.
Also, facilities reported that longer lengths of stay resulted in deteriorating mental health for some children and increased demands on staff. Facilities reported that children who stayed in federal custody for longer periods experienced more stress, anxiety, and behavioral issues. According to the facilities, the longer stays resulted in higher levels of defiance, hopelessness, and frustration among children – in addition to more instances of self-harm and suicidal ideation.
It is not surprising that the OIG study reflects that mental health services at facilities for unaccompanied minors are understaffed, undertrained, and overwhelmed, said Craig L. Katz, MD, a clinical professor of psychiatry at Mount Sinai in New York.
“In some sense, this can probably be said for most of the U.S. and definitely the world when it comes to child mental health services,” Dr. Katz said in an interview. “But, what’s especially tough to stomach about this shortfall at these facilities is that they encompass an immensely high-risk population – an inevitably highly, if not multiply traumatized population of children who lack primary caregivers.”
Dr. Katz was coinvestigator of a recent study that assessed the mental health of children held at a U.S. immigration detention center through the Parent-Report Strengths and Difficulties Questionnaire. Among the 425 children evaluated, many demonstrated elevated scores for emotional problems, peer problems, and total difficulties, according to the June 2018 study, published in Social Science & Medicine (2019 Jun; 230:303-8). Younger children (aged 4-8 years) demonstrated more difficulties associated with conduct, hyperactivity, and total difficulties, compared with older children, the study found.
Children who had been forcibly separated from their mothers demonstrated significantly more emotional problems and total difficulties, compared with those who had never been separated. Of 150 children who completed the Posttraumatic Stress Disorder Reaction Index, 17% had a probable diagnosis of PTSD, results found.
Dr. Katz said the OIG reached the same basic conclusion as his quantitative study – that separated minors appear to have even greater mental health problems than do fellow unaccompanied minors.
“In our study, we found that children in family detention had greater mental health problems than [did] American community samples but that formerly separated children who had been reunited with their mothers had even more health problems than their fellow detainees,” Dr. Katz said. “Something about being separated per U.S. policy was especially pernicious, which we knew in our hearts; but now in this study and ours, we know empirically.”
As long as the United States continues to detain children, the psychological harm created by such detainments is likely to continue, said Kim A. Baranowski, PhD, a psychologist and lecturer at Columbia University in New York. At a minimum, unaccompanied minors should have access to highly trained licensed clinicians who can respond to their immediate mental health needs within the initial hours and days following their arrival in the United States, and such children should be released rapidly from government custody and reunited with their families, said Dr. Baranowski, a coauthor of the Social Science & Medicine study.
“We need to effectively support their integration into the community, and connect children and their families with linguistically, culturally, and developmentally appropriate trauma-informed pro bono treatment services that respond to their experiences” of premigration, migration, and postmigration stressors, “as well as potential exposure to trauma,” she said in an interview.
The OIG issued several recommendations for practical steps that ORR can take to assist facilities and better provide mental health care to immigrant children in federal custody. The agency advised that the ORR should provide facilities with evidence-based guidance on addressing trauma in short-term therapy and that the ORR also should develop strategies for overcoming challenges to hiring and retaining qualified mental health clinicians.
The Office of Inspector General also suggested that facilities consider maximum caseloads for individual clinicians. Finally, the OIG recommends that ORR address gaps in options for children who require more specialized treatment and that the office take reasonable steps to minimize the length of time that children remain in custody.
[email protected]
*This article was updated 9/5/2019.
Care providers encountered significant challenges when addressing the mental health needs of unaccompanied immigrant children in federal custody, including overwhelming caseloads and the deteriorating mental health of some patients, according to a new report by the Office of Inspector General (OIG).
In the report, released Sept. 3, the OIG outlined findings from its analysis of 45 Office of Refugee Resettlement (ORR) facilities between August and September 2018. The U.S. Department of Health & Human Services ORR is the legal custodian of unaccompanied immigrant children in its care who have no parent or legal guardian available. This includes children who arrive in the United States unaccompanied and children who are separated from their parents or guardians by immigration authorities after arriving in the country.
For the analysis, OIG investigators collected data from interviews with mental health clinicians, medical coordinators, facility leadership, and ORR federal field specialists at the 45 selected facilities.
Investigators recorded numerous serious challenges experienced by providers when attempting to provide mental health care to the children. Namely, they cited overwhelming patient caseloads, and difficulty accessing external mental health clinicians and referring children to providers within ORR’s network, according to the OIG’s report.
Mental health clinicians reported that the high caseloads hurt their ability to build rapport with young patients – and allowed less time for counseling and less frequent sessions for children with greater needs. The heavy caseloads were generated by heightened immigration enforcement beginning in 2017, and the separation of many more families at the border and more children being placed in federal custody, according to the report.
In addition, providers reported challenges when addressing the mental health needs of children who had experienced significant trauma before coming into federal custody. This included trauma that occurred while the children lived in the countries of origin, trauma during their journey to the United States, and trauma upon their arrival in the United States.
Separation from parents and a chaotic reunification process added to the trauma that children had already experienced, providers reported, and put extreme pressure on facility staff. Separated children exhibited “more fear, feelings of abandonment, and posttraumatic stress than did children who were not separated,” according to the findings. Separated children also experienced elevated feelings of anxiety and loss as a consequence of unexpected separation from loved ones.
Also, facilities reported that longer lengths of stay resulted in deteriorating mental health for some children and increased demands on staff. Facilities reported that children who stayed in federal custody for longer periods experienced more stress, anxiety, and behavioral issues. According to the facilities, the longer stays resulted in higher levels of defiance, hopelessness, and frustration among children – in addition to more instances of self-harm and suicidal ideation.
It is not surprising that the OIG study reflects that mental health services at facilities for unaccompanied minors are understaffed, undertrained, and overwhelmed, said Craig L. Katz, MD, a clinical professor of psychiatry at Mount Sinai in New York.
“In some sense, this can probably be said for most of the U.S. and definitely the world when it comes to child mental health services,” Dr. Katz said in an interview. “But, what’s especially tough to stomach about this shortfall at these facilities is that they encompass an immensely high-risk population – an inevitably highly, if not multiply traumatized population of children who lack primary caregivers.”
Dr. Katz was coinvestigator of a recent study that assessed the mental health of children held at a U.S. immigration detention center through the Parent-Report Strengths and Difficulties Questionnaire. Among the 425 children evaluated, many demonstrated elevated scores for emotional problems, peer problems, and total difficulties, according to the June 2018 study, published in Social Science & Medicine (2019 Jun; 230:303-8). Younger children (aged 4-8 years) demonstrated more difficulties associated with conduct, hyperactivity, and total difficulties, compared with older children, the study found.
Children who had been forcibly separated from their mothers demonstrated significantly more emotional problems and total difficulties, compared with those who had never been separated. Of 150 children who completed the Posttraumatic Stress Disorder Reaction Index, 17% had a probable diagnosis of PTSD, results found.
Dr. Katz said the OIG reached the same basic conclusion as his quantitative study – that separated minors appear to have even greater mental health problems than do fellow unaccompanied minors.
“In our study, we found that children in family detention had greater mental health problems than [did] American community samples but that formerly separated children who had been reunited with their mothers had even more health problems than their fellow detainees,” Dr. Katz said. “Something about being separated per U.S. policy was especially pernicious, which we knew in our hearts; but now in this study and ours, we know empirically.”
As long as the United States continues to detain children, the psychological harm created by such detainments is likely to continue, said Kim A. Baranowski, PhD, a psychologist and lecturer at Columbia University in New York. At a minimum, unaccompanied minors should have access to highly trained licensed clinicians who can respond to their immediate mental health needs within the initial hours and days following their arrival in the United States, and such children should be released rapidly from government custody and reunited with their families, said Dr. Baranowski, a coauthor of the Social Science & Medicine study.
“We need to effectively support their integration into the community, and connect children and their families with linguistically, culturally, and developmentally appropriate trauma-informed pro bono treatment services that respond to their experiences” of premigration, migration, and postmigration stressors, “as well as potential exposure to trauma,” she said in an interview.
The OIG issued several recommendations for practical steps that ORR can take to assist facilities and better provide mental health care to immigrant children in federal custody. The agency advised that the ORR should provide facilities with evidence-based guidance on addressing trauma in short-term therapy and that the ORR also should develop strategies for overcoming challenges to hiring and retaining qualified mental health clinicians.
The Office of Inspector General also suggested that facilities consider maximum caseloads for individual clinicians. Finally, the OIG recommends that ORR address gaps in options for children who require more specialized treatment and that the office take reasonable steps to minimize the length of time that children remain in custody.
[email protected]
*This article was updated 9/5/2019.
Michigan becomes first state to ban flavored e-cigarettes
The state health agency is expected to issue rules outlining the ban within the next 30 days. The emergency ban will be in effect for 6 months, with the possibility of a 6-month extension while state health regulators craft rules to set in place a permanent ban.
The ban will also prohibit “misleading marketing of vaping products, including the use of terms like ‘clean,’ ‘safe,’ and ‘healthy,’ that perpetuate beliefs that these products are harmless,” according to a statement issued by Gov. Whitmer.
Companies selling vaping products “are using candy flavors to hook children on nicotine and misleading claims to promote the belief that these products are safe,” she said in a statement. “That ends today. Our kids deserve leaders who are going to fight to protect them. These bold steps will finally put an end to these irresponsible and deceptive practices and protect Michiganders’ public health.”
The ban also will cover mint- and menthol-flavors in addition to sweet flavors but will not ban tobacco-flavored e-cigarette products.
The American Academy of Pediatrics, American Heart Association, American Lung Association, American Cancer Society Cancer Action Network and other organizations praised the action taken by the state, calling the steps “necessary and appropriate.”
“The need for action is even more urgent in light of the recent outbreak of severe lung illness associated with e-cigarette use and the failure of the U.S. Food and Drug Administration to take strong regulatory action such as prohibiting the sale of the flavored products nationwide that have attracted shocking numbers of our nation’s youth,” the organizations said in a statement.
The groups noted that “health authorities are investigating reports of severe respiratory illness associated with e-cigarette use in at least 215 people ... in 25 states,” adding that many are youth and young adults.
The U.S. Department of Health & Human Services Secretary Alex Azar said in an Aug. 30 statement that the federal government is “using every tool we have to get to the bottom of this deeply concerning outbreak of illness in Americans who use e-cigarettes. More broadly, we will continue using every regulatory and enforcement power we have to stop the epidemic of youth e-cigarette use.”
HHS noted that no single substance or e-cigarette product has been consistently associated with the reports of illness. The agency called upon clinicians to report any new cases as appropriate to their state and local health departments.
Gov. Whitmer earlier this year signed bills that clarify that it is illegal to sell nontraditional nicotine products to minors, but the governor’s statement notes her criticism that the bills did not go far enough to protect the state’s youth, necessitating this further action.
The state health agency is expected to issue rules outlining the ban within the next 30 days. The emergency ban will be in effect for 6 months, with the possibility of a 6-month extension while state health regulators craft rules to set in place a permanent ban.
The ban will also prohibit “misleading marketing of vaping products, including the use of terms like ‘clean,’ ‘safe,’ and ‘healthy,’ that perpetuate beliefs that these products are harmless,” according to a statement issued by Gov. Whitmer.
Companies selling vaping products “are using candy flavors to hook children on nicotine and misleading claims to promote the belief that these products are safe,” she said in a statement. “That ends today. Our kids deserve leaders who are going to fight to protect them. These bold steps will finally put an end to these irresponsible and deceptive practices and protect Michiganders’ public health.”
The ban also will cover mint- and menthol-flavors in addition to sweet flavors but will not ban tobacco-flavored e-cigarette products.
The American Academy of Pediatrics, American Heart Association, American Lung Association, American Cancer Society Cancer Action Network and other organizations praised the action taken by the state, calling the steps “necessary and appropriate.”
“The need for action is even more urgent in light of the recent outbreak of severe lung illness associated with e-cigarette use and the failure of the U.S. Food and Drug Administration to take strong regulatory action such as prohibiting the sale of the flavored products nationwide that have attracted shocking numbers of our nation’s youth,” the organizations said in a statement.
The groups noted that “health authorities are investigating reports of severe respiratory illness associated with e-cigarette use in at least 215 people ... in 25 states,” adding that many are youth and young adults.
The U.S. Department of Health & Human Services Secretary Alex Azar said in an Aug. 30 statement that the federal government is “using every tool we have to get to the bottom of this deeply concerning outbreak of illness in Americans who use e-cigarettes. More broadly, we will continue using every regulatory and enforcement power we have to stop the epidemic of youth e-cigarette use.”
HHS noted that no single substance or e-cigarette product has been consistently associated with the reports of illness. The agency called upon clinicians to report any new cases as appropriate to their state and local health departments.
Gov. Whitmer earlier this year signed bills that clarify that it is illegal to sell nontraditional nicotine products to minors, but the governor’s statement notes her criticism that the bills did not go far enough to protect the state’s youth, necessitating this further action.
The state health agency is expected to issue rules outlining the ban within the next 30 days. The emergency ban will be in effect for 6 months, with the possibility of a 6-month extension while state health regulators craft rules to set in place a permanent ban.
The ban will also prohibit “misleading marketing of vaping products, including the use of terms like ‘clean,’ ‘safe,’ and ‘healthy,’ that perpetuate beliefs that these products are harmless,” according to a statement issued by Gov. Whitmer.
Companies selling vaping products “are using candy flavors to hook children on nicotine and misleading claims to promote the belief that these products are safe,” she said in a statement. “That ends today. Our kids deserve leaders who are going to fight to protect them. These bold steps will finally put an end to these irresponsible and deceptive practices and protect Michiganders’ public health.”
The ban also will cover mint- and menthol-flavors in addition to sweet flavors but will not ban tobacco-flavored e-cigarette products.
The American Academy of Pediatrics, American Heart Association, American Lung Association, American Cancer Society Cancer Action Network and other organizations praised the action taken by the state, calling the steps “necessary and appropriate.”
“The need for action is even more urgent in light of the recent outbreak of severe lung illness associated with e-cigarette use and the failure of the U.S. Food and Drug Administration to take strong regulatory action such as prohibiting the sale of the flavored products nationwide that have attracted shocking numbers of our nation’s youth,” the organizations said in a statement.
The groups noted that “health authorities are investigating reports of severe respiratory illness associated with e-cigarette use in at least 215 people ... in 25 states,” adding that many are youth and young adults.
The U.S. Department of Health & Human Services Secretary Alex Azar said in an Aug. 30 statement that the federal government is “using every tool we have to get to the bottom of this deeply concerning outbreak of illness in Americans who use e-cigarettes. More broadly, we will continue using every regulatory and enforcement power we have to stop the epidemic of youth e-cigarette use.”
HHS noted that no single substance or e-cigarette product has been consistently associated with the reports of illness. The agency called upon clinicians to report any new cases as appropriate to their state and local health departments.
Gov. Whitmer earlier this year signed bills that clarify that it is illegal to sell nontraditional nicotine products to minors, but the governor’s statement notes her criticism that the bills did not go far enough to protect the state’s youth, necessitating this further action.
Quality of Life in Patients With Atopic Dermatitis
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease typically with childhood onset. In some cases, the condition persists, but AD usually resolves by the time a child reaches adulthood. Prevalence is difficult to estimate but, in developed countries, is approximately 15% to 30% among children and 2% to 10% among adults.1
Atopic dermatitis is characterized by chronically itchy dry skin, weeping erythematous papules and plaques, and lichenification. Furthermore, AD often is associated with other atopic diseases, such as food allergy, allergic rhinitis, and bronchial asthma.
In this article, we review the literature on the quality of life (QOL) of patients with AD. Our goals are to discuss the most common methods for measuring QOL in AD and how to use them; highlight specific alterations of QOL in AD; and review data about QOL of children with AD, which is underrepresented in the medical literature, as studies tend to focus on adults. In addition, we address the importance of assessing QOL in patients with AD due to the psychological burden of the disease.
Quality of Life
The harmful effects of AD can include a range of areas, including
Because QOL is an important instrument used in many AD studies, we call attention to the work of the Harmonising Outcome Measures for Eczema (HOME) initiative, which established a core outcome set for all AD clinical trials to enable comparison of results of individual studies.2 Quality of life was identified in HOME as one of 4 basic outcome measures that should be included in every AD trial (the others are clinician-reported signs, patient-reported symptoms, and long-term control).3 According to the recent agreement, the following QOL instruments should be used: Dermatology Life Quality Index (DLQI) for adults, Children’s Dermatology Life Quality Index (CDLQI) for children, and Infants’ Dermatitis Quality of Life Index (IDQOL) for infants.4
In dermatology, these instruments can be divided into 3 basic categories: generic, dermatology specific, and disease specific.5 Generic QOL questionnaires are beneficial when comparing the QOL of an AD patient to patients with other conditions or to healthy individuals. On the other hand, dermatology-specific and AD-specific methods are more effective instruments for detecting impairments linked directly to the disease and, therefore, are more sensitive to changes in QOL.5 Some of the most frequently used QOL measures5,6 for AD along with their key attributes are
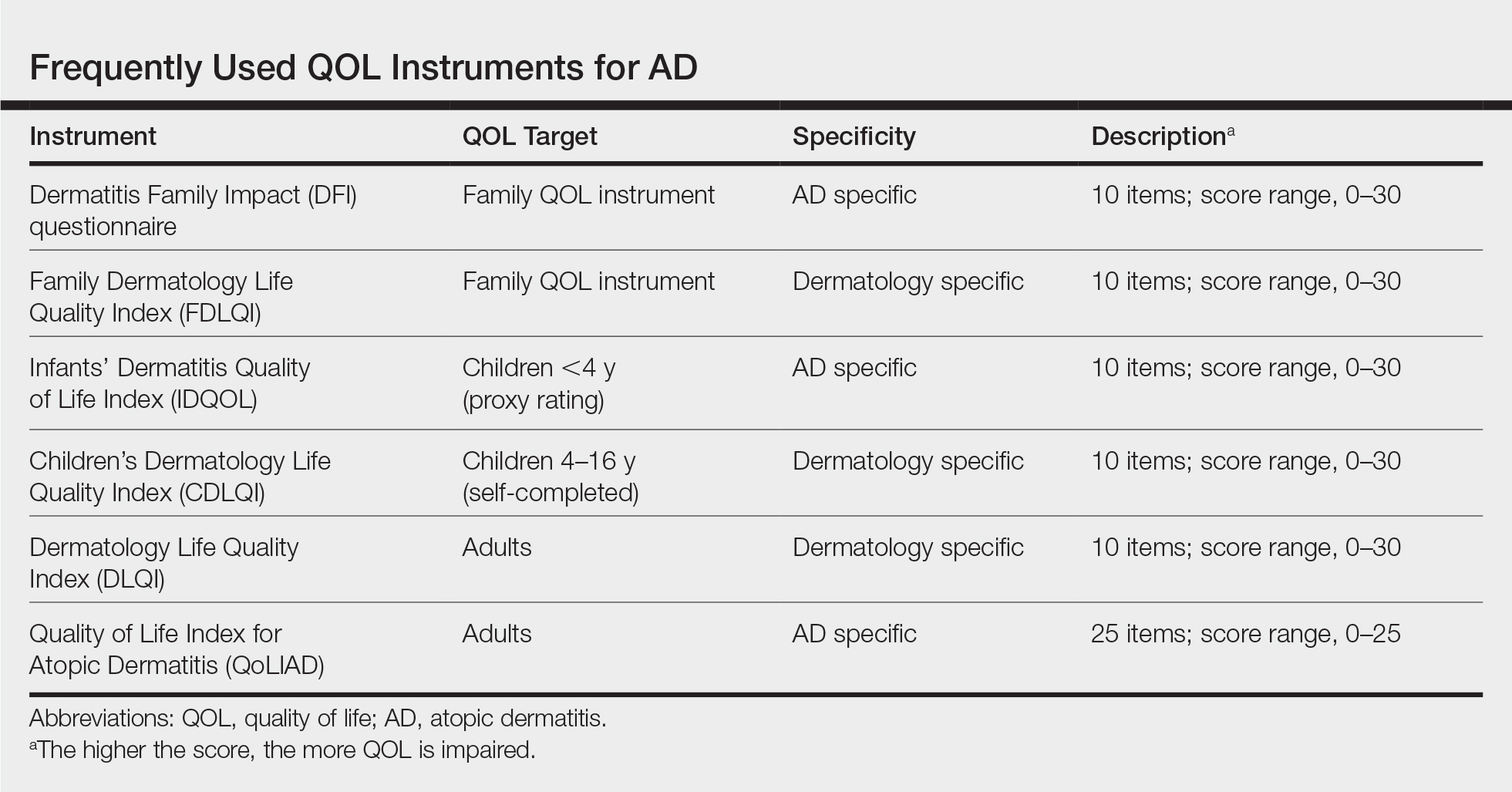
Given that AD is a chronic disease that requires constant care, parents/guardians or the partner of the patient usually are affected as well. To detect this effect, the Family Dermatology Life Quality Index (FDLQI), a dermatology-specific instrument, measures the QOL in family members of dermatology patients.7 The Dermatitis Family Impact (DFI)8 is a disease-specific method for assessing how having a child with AD can impact the QOL of family members; it is a 10-item questionnaire completed by an adult family member. The FDLQI7 and DFI8 both help to understand the secondary impact of the disease.
In contrast, several other methods that also are administered by a parent/guardian assess how the parent perceives the QOL of their child with AD; these methods are essential for small children and infants who cannot answer questions themselves. The IDQOL9 was designed to assess the QOL of patients younger than 4 years using a parent-completed questionnaire. For older children and adolescents aged 4 to 16 years, the CDLQI10 is a widely used instrument; the questionnaire is completed by the child and is available in a cartoon format.10
For patients older than 16 years, 2 important instruments are the DLQI, a generic dermatology instrument, and the Quality of Life Index for Atopic Dermatitis (QoLIAD).11
Clearly it can be troublesome for researchers and clinicians to find the most suitable instrument to evaluate QOL in AD patients. To make this task easier, the European Academy of Dermatology and Venereology Task Force released a position paper with the following recommendations: (1) only validated instruments should be used, and (2) their use should be based on the age of the patients for which the instruments were designed. It is reommended that researchers use a combination of a generic and a dermatology-specific or AD-specific instrument, whereas clinicians should apply a dermatology-specific or AD-specific method, or both.5
Alterations of QOL in AD
Sleep Disturbance in AD
Sleep disorders observed in AD include difficulty falling asleep, frequent waking episodes, shorter sleep duration, and feelings of inadequate sleep, which often result in impairment of daily activity.12,13 Correlation has been found between sleep quality and QOL in both children and adults.14 Approximately 60% of children affected by AD experience a sleep disturbance,15 which seems to correlate well with disease severity.16 A US study found that adults with AD are more likely to experience a sleep disturbance, which often affects daytime functioning and work productivity.13
Financial Aspects and Impact on Work
The financial burden of AD is extensive.17 There are direct medical costs, including medication, visits to the physician, alternative therapies, and nonprescription products. Patients tend to spend relevant money on such items as moisturizers, bath products, antihistamines, topical steroids, and topical antibiotics.18,19 However, it seems that most of the cost of AD is due to indirect and nonmedical costs, including transportation to medical visits; loss of work days; extra childcare; and expenditures associated with lifestyle changes,19,20 such as modifying diet, wearing special clothes, using special bed linens, and purchasing special household items (eg, anti–dust mite vacuum cleaner, humidifier, new carpeting).17,19
Absenteeism from work often is a consequence of physician appointments; in addition, parents/guardians of a child with AD often miss work due to medical care. Even at work, patients (or parents/guardians) often experience decreased work productivity (so-called presenteeism) due to loss of sleep and anxiety.21 In addressing the effects of AD on work life, a systematic literature review found that AD strongly affects sick leave and might have an impact on job choice and change or loss of job.22
Furthermore, according to Su et al,23 the costs of AD are related to disease severity. Moreover, their data suggest that among chronic childhood diseases, the financial burden of AD is greater than the cost of asthma and similar to the cost of diabetes mellitus.23
Association Between QOL and Disease Severity
A large observational study found that improvement in AD severity was followed by an increase in QOL.24 A positive correlation between disease severity and QOL has been found in other studies,25,26 though no correlation or only moderate correlation also has been reported.27 Apparently, in addition to QOL, disease severity scores are substantial parameters in the evaluation of distress caused by AD; the HOME initiative has identified clinician-reported signs and patient-reported symptoms as 2 of 4 core outcomes domains to include in all future AD clinical trials.3 For measuring symptoms, the Patient-Oriented Eczema Measure (POEM) is the recommended instrument.28 Regarding clinical signs, the HOME group named the Eczema Area and Severity Index (EASI) as the preferred instrument.29
Psychological Burden
Stress is a triggering factor for AD, but the connection between skin and mind appears bidirectional. The biological reaction to stress probably lowers the itch threshold and disrupts the skin barrier.30 The Global Burden of Disease Study showed that skin diseases are the fourth leading cause of nonfatal disease burden.31 There are several factors—pruritus, scratch, and pain—that can all lead to sleep deprivation and daytime fatigue. Based on our experience, if lesions develop on visible areas, patients can feel stigmatized, which restricts their social life.
The most common psychological comorbidities of AD are anxiety and depression. In a cross-sectional, multicenter study, there was a significantly higher prevalence of depression (P<.001) and anxiety disorder (P=.02) among patients with common skin diseases compared to a control group.32 In a study that assessed AD patients, researchers found a higher risk of depression and anxiety.33 Suicidal ideation also is more common in the population with AD32,34; a study showed that the risk of suicidal ideation in adolescents was nearly 4-fold in patients with itching skin lesions compared to those without itch.34
According to Linnet and Jemec,35 mental and psychological comorbidities of AD are associated with lower QOL, not with clinical severity. As a result, to improve QOL in AD, one should take care of both dermatological and psychological problems. It has been demonstrated that psychological interventions, such as autogenic training, cognitive-behavioral therapy, relaxation techniques, habit reversal training,36 and hypnotherapy37 might be helpful in individual cases; educational interventions also are recommended.36 With these adjuvant therapies, psychological status, unpleasant clinical symptoms, and QOL could be improved, though further studies are needed to confirm these benefits.
Conclusion
Atopic dermatitis places a notable burden on patients and their families. The degree of burden is probably related to disease severity. For measuring QOL, researchers and clinicians should use validated methods suited to the age of the patients for which they were designed. More studies are needed to assess the effects of different treatments on QOL. Besides pharmacotherapy, psychotherapy and educational programs might be beneficial for improving QOL, another important area to be studied.
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483-1494.
- Schmitt J, Williams H; HOME Development Group. Harmonising Outcome Measures for Eczema (HOME). report from the First International Consensus Meeting (HOME 1), 24 July 2010, Munich, Germany. Br J Dermatol. 2010;163:1166-1168.
- Schmitt J, Spuls P, Boers M, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy. 2012;67:1111-1117.
- Quality of Life (QoL). Harmonising Outcome Measures for Eczema (HOME) website. http://www.homeforeczema.org/research/quality-of-life.aspx. Accessed August 18, 2019.
Chernyshov PV, Tomas-Aragones L, Manolache L, et al; EADV Quality of Life Task Force. Quality of life measurement in atopic dermatitis. Position paper of the European Academy of Dermatology and Venereology (EADV) Task Force on quality of life. J Eur Acad Dermatol Venereol. 2017;31:576-593. - Hill MK, Kheirandish Pishkenari A, Braunberger TL, et al. Recent trends in disease severity and quality of life instruments for patients with atopic dermatitis: a systematic review. J Am Acad Dermatol. 2016;75:906-917.
- Basra MK, Sue-Ho R, Finlay AY. The Family Dermatology Life Quality Index: measuring the secondary impact of skin disease. Br J Dermatol. 2007;156:528-538.
- Dodington SR, Basra MK, Finlay AY, et al. The Dermatitis Family Impact questionnaire: a review of its measurement properties and clinical application. Br J Dermatol. 2013;169:31-46.
- Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol. 2001;144:104-110.
- Holme SA, Man I, Sharpe JL, et al. The Children’s Dermatology Life Quality Index: validation of the cartoon version. Br J Dermatol. 2003;148:285-290.
- Whalley D, McKenna SP, Dewar AL, et al. A new instrument for assessing quality of life in atopic dermatitis: international development of the Quality of Life Index for Atopic Dermatitis (QoLIAD). Br J Dermatol. 2004;150:274-283.
- Jeon C, Yan D, Nakamura M, et al. Frequency and management of sleep disturbance in adults with atopic dermatitis: a systematic review. Dermatol Ther (Heidelb). 2017;7:349-364.
- Yu SH, Attarian H, Zee P, et al. Burden of sleep and fatigue in US adults with atopic dermatitis. Dermatitis. 2016;27:50-58.
- Kong TS, Han TY, Lee JH, et al. Correlation between severity of atopic dermatitis and sleep quality in children and adults. Ann Dermatol. 2016;28:321-326.
- Fishbein AB, Mueller K, Kruse L, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case-control study. J Am Acad Dermatol. 2018;78:336-341.
- Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159:745-750.
- Emerson RM, Williams HC, Allen BR. What is the cost of atopic dermatitis in preschool children? Br J Dermatol. 2001;144:514-522.
- Filanovsky MG, Pootongkam S, Tamburro JE, et al. The financial and emotional impact of atopic dermatitis on children and their families. J Pediatr. 2016;169:284-290.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Drucker AM, Wang AR, Qureshi AA. Research gaps in quality of life and economic burden of atopic dermatitis: the National Eczema Association Burden of Disease Audit. JAMA Dermatol. 2016;152:873-874.
- Nørreslet LB, Ebbehøj NE, Ellekilde Bonde JP, et al. The impact of atopic dermatitis on work life—a systematic review. J Eur Acad Dermatol Venereol. 2018;32:23-38.
- Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
- Coutanceau C, Stalder JF. Analysis of correlations between patient-oriented SCORAD (PO-SCORAD) and other assessment scores of atopic dermatitis severity and quality of life. Dermatology. 2014;229:248-255.
- Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol. 2004;150:284-290.
- van Valburg RW, Willemsen MG, Dirven-Meijer PC, et al. Quality of life measurement and its relationship to disease severity in children with atopic dermatitis in general practice. Acta Derm Venereol. 2011;91:147-151.
- Haeck IM, ten Berge O, van Velsen SG, et al. Moderate correlation between quality of life and disease activity in adult patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2012;26:236-241.
- Spuls PI, Gerbens LAA, Simpson E, et al; HOME initiative collaborators. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. Br J Dermatol. 2017;176:979-984.
- Schmitt J, Spuls PI, Thomas KS, et al; HOME initiative collaborators. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol. 2014;134:800-807.
- Oh SH, Bae BG, Park CO, et al. Association of stress with symptoms of atopic dermatitis. Acta Derm Venereol. 2010;90:582-588.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Cheng CM, Hsu JW, Huang KL, et al. Risk of developing major depressive disorder and anxiety disorders among adolescents and adults with atopic dermatitis: a nationwide longitudinal study. J Affect Disord. 2015;178:60-65.
Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854. - Linnet J, Jemec GB. An assessment of anxiety and dermatology life quality in patients with atopic dermatitis. Br J Dermatol. 1999;140:268-272.
- Ring J, Alomar A, Bieber T, et al; European Dermatology Forum; European Academy of Dermatology and Venereology; European Task Force on Atopic Dermatitis; European Federation of Allergy; European Society of Pediatric Dermatology; Global Allergy and Asthma European Network. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol. 2012;26:1176-1193.
- Perczel K, Gál J. Hypnotherapy of atopic dermatitis in an adult. case report. Orv Hetil. 2016;157:111-115.
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease typically with childhood onset. In some cases, the condition persists, but AD usually resolves by the time a child reaches adulthood. Prevalence is difficult to estimate but, in developed countries, is approximately 15% to 30% among children and 2% to 10% among adults.1
Atopic dermatitis is characterized by chronically itchy dry skin, weeping erythematous papules and plaques, and lichenification. Furthermore, AD often is associated with other atopic diseases, such as food allergy, allergic rhinitis, and bronchial asthma.
In this article, we review the literature on the quality of life (QOL) of patients with AD. Our goals are to discuss the most common methods for measuring QOL in AD and how to use them; highlight specific alterations of QOL in AD; and review data about QOL of children with AD, which is underrepresented in the medical literature, as studies tend to focus on adults. In addition, we address the importance of assessing QOL in patients with AD due to the psychological burden of the disease.
Quality of Life
The harmful effects of AD can include a range of areas, including
Because QOL is an important instrument used in many AD studies, we call attention to the work of the Harmonising Outcome Measures for Eczema (HOME) initiative, which established a core outcome set for all AD clinical trials to enable comparison of results of individual studies.2 Quality of life was identified in HOME as one of 4 basic outcome measures that should be included in every AD trial (the others are clinician-reported signs, patient-reported symptoms, and long-term control).3 According to the recent agreement, the following QOL instruments should be used: Dermatology Life Quality Index (DLQI) for adults, Children’s Dermatology Life Quality Index (CDLQI) for children, and Infants’ Dermatitis Quality of Life Index (IDQOL) for infants.4
In dermatology, these instruments can be divided into 3 basic categories: generic, dermatology specific, and disease specific.5 Generic QOL questionnaires are beneficial when comparing the QOL of an AD patient to patients with other conditions or to healthy individuals. On the other hand, dermatology-specific and AD-specific methods are more effective instruments for detecting impairments linked directly to the disease and, therefore, are more sensitive to changes in QOL.5 Some of the most frequently used QOL measures5,6 for AD along with their key attributes are

Given that AD is a chronic disease that requires constant care, parents/guardians or the partner of the patient usually are affected as well. To detect this effect, the Family Dermatology Life Quality Index (FDLQI), a dermatology-specific instrument, measures the QOL in family members of dermatology patients.7 The Dermatitis Family Impact (DFI)8 is a disease-specific method for assessing how having a child with AD can impact the QOL of family members; it is a 10-item questionnaire completed by an adult family member. The FDLQI7 and DFI8 both help to understand the secondary impact of the disease.
In contrast, several other methods that also are administered by a parent/guardian assess how the parent perceives the QOL of their child with AD; these methods are essential for small children and infants who cannot answer questions themselves. The IDQOL9 was designed to assess the QOL of patients younger than 4 years using a parent-completed questionnaire. For older children and adolescents aged 4 to 16 years, the CDLQI10 is a widely used instrument; the questionnaire is completed by the child and is available in a cartoon format.10
For patients older than 16 years, 2 important instruments are the DLQI, a generic dermatology instrument, and the Quality of Life Index for Atopic Dermatitis (QoLIAD).11
Clearly it can be troublesome for researchers and clinicians to find the most suitable instrument to evaluate QOL in AD patients. To make this task easier, the European Academy of Dermatology and Venereology Task Force released a position paper with the following recommendations: (1) only validated instruments should be used, and (2) their use should be based on the age of the patients for which the instruments were designed. It is reommended that researchers use a combination of a generic and a dermatology-specific or AD-specific instrument, whereas clinicians should apply a dermatology-specific or AD-specific method, or both.5
Alterations of QOL in AD
Sleep Disturbance in AD
Sleep disorders observed in AD include difficulty falling asleep, frequent waking episodes, shorter sleep duration, and feelings of inadequate sleep, which often result in impairment of daily activity.12,13 Correlation has been found between sleep quality and QOL in both children and adults.14 Approximately 60% of children affected by AD experience a sleep disturbance,15 which seems to correlate well with disease severity.16 A US study found that adults with AD are more likely to experience a sleep disturbance, which often affects daytime functioning and work productivity.13
Financial Aspects and Impact on Work
The financial burden of AD is extensive.17 There are direct medical costs, including medication, visits to the physician, alternative therapies, and nonprescription products. Patients tend to spend relevant money on such items as moisturizers, bath products, antihistamines, topical steroids, and topical antibiotics.18,19 However, it seems that most of the cost of AD is due to indirect and nonmedical costs, including transportation to medical visits; loss of work days; extra childcare; and expenditures associated with lifestyle changes,19,20 such as modifying diet, wearing special clothes, using special bed linens, and purchasing special household items (eg, anti–dust mite vacuum cleaner, humidifier, new carpeting).17,19
Absenteeism from work often is a consequence of physician appointments; in addition, parents/guardians of a child with AD often miss work due to medical care. Even at work, patients (or parents/guardians) often experience decreased work productivity (so-called presenteeism) due to loss of sleep and anxiety.21 In addressing the effects of AD on work life, a systematic literature review found that AD strongly affects sick leave and might have an impact on job choice and change or loss of job.22
Furthermore, according to Su et al,23 the costs of AD are related to disease severity. Moreover, their data suggest that among chronic childhood diseases, the financial burden of AD is greater than the cost of asthma and similar to the cost of diabetes mellitus.23
Association Between QOL and Disease Severity
A large observational study found that improvement in AD severity was followed by an increase in QOL.24 A positive correlation between disease severity and QOL has been found in other studies,25,26 though no correlation or only moderate correlation also has been reported.27 Apparently, in addition to QOL, disease severity scores are substantial parameters in the evaluation of distress caused by AD; the HOME initiative has identified clinician-reported signs and patient-reported symptoms as 2 of 4 core outcomes domains to include in all future AD clinical trials.3 For measuring symptoms, the Patient-Oriented Eczema Measure (POEM) is the recommended instrument.28 Regarding clinical signs, the HOME group named the Eczema Area and Severity Index (EASI) as the preferred instrument.29
Psychological Burden
Stress is a triggering factor for AD, but the connection between skin and mind appears bidirectional. The biological reaction to stress probably lowers the itch threshold and disrupts the skin barrier.30 The Global Burden of Disease Study showed that skin diseases are the fourth leading cause of nonfatal disease burden.31 There are several factors—pruritus, scratch, and pain—that can all lead to sleep deprivation and daytime fatigue. Based on our experience, if lesions develop on visible areas, patients can feel stigmatized, which restricts their social life.
The most common psychological comorbidities of AD are anxiety and depression. In a cross-sectional, multicenter study, there was a significantly higher prevalence of depression (P<.001) and anxiety disorder (P=.02) among patients with common skin diseases compared to a control group.32 In a study that assessed AD patients, researchers found a higher risk of depression and anxiety.33 Suicidal ideation also is more common in the population with AD32,34; a study showed that the risk of suicidal ideation in adolescents was nearly 4-fold in patients with itching skin lesions compared to those without itch.34
According to Linnet and Jemec,35 mental and psychological comorbidities of AD are associated with lower QOL, not with clinical severity. As a result, to improve QOL in AD, one should take care of both dermatological and psychological problems. It has been demonstrated that psychological interventions, such as autogenic training, cognitive-behavioral therapy, relaxation techniques, habit reversal training,36 and hypnotherapy37 might be helpful in individual cases; educational interventions also are recommended.36 With these adjuvant therapies, psychological status, unpleasant clinical symptoms, and QOL could be improved, though further studies are needed to confirm these benefits.
Conclusion
Atopic dermatitis places a notable burden on patients and their families. The degree of burden is probably related to disease severity. For measuring QOL, researchers and clinicians should use validated methods suited to the age of the patients for which they were designed. More studies are needed to assess the effects of different treatments on QOL. Besides pharmacotherapy, psychotherapy and educational programs might be beneficial for improving QOL, another important area to be studied.
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease typically with childhood onset. In some cases, the condition persists, but AD usually resolves by the time a child reaches adulthood. Prevalence is difficult to estimate but, in developed countries, is approximately 15% to 30% among children and 2% to 10% among adults.1
Atopic dermatitis is characterized by chronically itchy dry skin, weeping erythematous papules and plaques, and lichenification. Furthermore, AD often is associated with other atopic diseases, such as food allergy, allergic rhinitis, and bronchial asthma.
In this article, we review the literature on the quality of life (QOL) of patients with AD. Our goals are to discuss the most common methods for measuring QOL in AD and how to use them; highlight specific alterations of QOL in AD; and review data about QOL of children with AD, which is underrepresented in the medical literature, as studies tend to focus on adults. In addition, we address the importance of assessing QOL in patients with AD due to the psychological burden of the disease.
Quality of Life
The harmful effects of AD can include a range of areas, including
Because QOL is an important instrument used in many AD studies, we call attention to the work of the Harmonising Outcome Measures for Eczema (HOME) initiative, which established a core outcome set for all AD clinical trials to enable comparison of results of individual studies.2 Quality of life was identified in HOME as one of 4 basic outcome measures that should be included in every AD trial (the others are clinician-reported signs, patient-reported symptoms, and long-term control).3 According to the recent agreement, the following QOL instruments should be used: Dermatology Life Quality Index (DLQI) for adults, Children’s Dermatology Life Quality Index (CDLQI) for children, and Infants’ Dermatitis Quality of Life Index (IDQOL) for infants.4
In dermatology, these instruments can be divided into 3 basic categories: generic, dermatology specific, and disease specific.5 Generic QOL questionnaires are beneficial when comparing the QOL of an AD patient to patients with other conditions or to healthy individuals. On the other hand, dermatology-specific and AD-specific methods are more effective instruments for detecting impairments linked directly to the disease and, therefore, are more sensitive to changes in QOL.5 Some of the most frequently used QOL measures5,6 for AD along with their key attributes are

Given that AD is a chronic disease that requires constant care, parents/guardians or the partner of the patient usually are affected as well. To detect this effect, the Family Dermatology Life Quality Index (FDLQI), a dermatology-specific instrument, measures the QOL in family members of dermatology patients.7 The Dermatitis Family Impact (DFI)8 is a disease-specific method for assessing how having a child with AD can impact the QOL of family members; it is a 10-item questionnaire completed by an adult family member. The FDLQI7 and DFI8 both help to understand the secondary impact of the disease.
In contrast, several other methods that also are administered by a parent/guardian assess how the parent perceives the QOL of their child with AD; these methods are essential for small children and infants who cannot answer questions themselves. The IDQOL9 was designed to assess the QOL of patients younger than 4 years using a parent-completed questionnaire. For older children and adolescents aged 4 to 16 years, the CDLQI10 is a widely used instrument; the questionnaire is completed by the child and is available in a cartoon format.10
For patients older than 16 years, 2 important instruments are the DLQI, a generic dermatology instrument, and the Quality of Life Index for Atopic Dermatitis (QoLIAD).11
Clearly it can be troublesome for researchers and clinicians to find the most suitable instrument to evaluate QOL in AD patients. To make this task easier, the European Academy of Dermatology and Venereology Task Force released a position paper with the following recommendations: (1) only validated instruments should be used, and (2) their use should be based on the age of the patients for which the instruments were designed. It is reommended that researchers use a combination of a generic and a dermatology-specific or AD-specific instrument, whereas clinicians should apply a dermatology-specific or AD-specific method, or both.5
Alterations of QOL in AD
Sleep Disturbance in AD
Sleep disorders observed in AD include difficulty falling asleep, frequent waking episodes, shorter sleep duration, and feelings of inadequate sleep, which often result in impairment of daily activity.12,13 Correlation has been found between sleep quality and QOL in both children and adults.14 Approximately 60% of children affected by AD experience a sleep disturbance,15 which seems to correlate well with disease severity.16 A US study found that adults with AD are more likely to experience a sleep disturbance, which often affects daytime functioning and work productivity.13
Financial Aspects and Impact on Work
The financial burden of AD is extensive.17 There are direct medical costs, including medication, visits to the physician, alternative therapies, and nonprescription products. Patients tend to spend relevant money on such items as moisturizers, bath products, antihistamines, topical steroids, and topical antibiotics.18,19 However, it seems that most of the cost of AD is due to indirect and nonmedical costs, including transportation to medical visits; loss of work days; extra childcare; and expenditures associated with lifestyle changes,19,20 such as modifying diet, wearing special clothes, using special bed linens, and purchasing special household items (eg, anti–dust mite vacuum cleaner, humidifier, new carpeting).17,19
Absenteeism from work often is a consequence of physician appointments; in addition, parents/guardians of a child with AD often miss work due to medical care. Even at work, patients (or parents/guardians) often experience decreased work productivity (so-called presenteeism) due to loss of sleep and anxiety.21 In addressing the effects of AD on work life, a systematic literature review found that AD strongly affects sick leave and might have an impact on job choice and change or loss of job.22
Furthermore, according to Su et al,23 the costs of AD are related to disease severity. Moreover, their data suggest that among chronic childhood diseases, the financial burden of AD is greater than the cost of asthma and similar to the cost of diabetes mellitus.23
Association Between QOL and Disease Severity
A large observational study found that improvement in AD severity was followed by an increase in QOL.24 A positive correlation between disease severity and QOL has been found in other studies,25,26 though no correlation or only moderate correlation also has been reported.27 Apparently, in addition to QOL, disease severity scores are substantial parameters in the evaluation of distress caused by AD; the HOME initiative has identified clinician-reported signs and patient-reported symptoms as 2 of 4 core outcomes domains to include in all future AD clinical trials.3 For measuring symptoms, the Patient-Oriented Eczema Measure (POEM) is the recommended instrument.28 Regarding clinical signs, the HOME group named the Eczema Area and Severity Index (EASI) as the preferred instrument.29
Psychological Burden
Stress is a triggering factor for AD, but the connection between skin and mind appears bidirectional. The biological reaction to stress probably lowers the itch threshold and disrupts the skin barrier.30 The Global Burden of Disease Study showed that skin diseases are the fourth leading cause of nonfatal disease burden.31 There are several factors—pruritus, scratch, and pain—that can all lead to sleep deprivation and daytime fatigue. Based on our experience, if lesions develop on visible areas, patients can feel stigmatized, which restricts their social life.
The most common psychological comorbidities of AD are anxiety and depression. In a cross-sectional, multicenter study, there was a significantly higher prevalence of depression (P<.001) and anxiety disorder (P=.02) among patients with common skin diseases compared to a control group.32 In a study that assessed AD patients, researchers found a higher risk of depression and anxiety.33 Suicidal ideation also is more common in the population with AD32,34; a study showed that the risk of suicidal ideation in adolescents was nearly 4-fold in patients with itching skin lesions compared to those without itch.34
According to Linnet and Jemec,35 mental and psychological comorbidities of AD are associated with lower QOL, not with clinical severity. As a result, to improve QOL in AD, one should take care of both dermatological and psychological problems. It has been demonstrated that psychological interventions, such as autogenic training, cognitive-behavioral therapy, relaxation techniques, habit reversal training,36 and hypnotherapy37 might be helpful in individual cases; educational interventions also are recommended.36 With these adjuvant therapies, psychological status, unpleasant clinical symptoms, and QOL could be improved, though further studies are needed to confirm these benefits.
Conclusion
Atopic dermatitis places a notable burden on patients and their families. The degree of burden is probably related to disease severity. For measuring QOL, researchers and clinicians should use validated methods suited to the age of the patients for which they were designed. More studies are needed to assess the effects of different treatments on QOL. Besides pharmacotherapy, psychotherapy and educational programs might be beneficial for improving QOL, another important area to be studied.
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483-1494.
- Schmitt J, Williams H; HOME Development Group. Harmonising Outcome Measures for Eczema (HOME). report from the First International Consensus Meeting (HOME 1), 24 July 2010, Munich, Germany. Br J Dermatol. 2010;163:1166-1168.
- Schmitt J, Spuls P, Boers M, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy. 2012;67:1111-1117.
- Quality of Life (QoL). Harmonising Outcome Measures for Eczema (HOME) website. http://www.homeforeczema.org/research/quality-of-life.aspx. Accessed August 18, 2019.
Chernyshov PV, Tomas-Aragones L, Manolache L, et al; EADV Quality of Life Task Force. Quality of life measurement in atopic dermatitis. Position paper of the European Academy of Dermatology and Venereology (EADV) Task Force on quality of life. J Eur Acad Dermatol Venereol. 2017;31:576-593. - Hill MK, Kheirandish Pishkenari A, Braunberger TL, et al. Recent trends in disease severity and quality of life instruments for patients with atopic dermatitis: a systematic review. J Am Acad Dermatol. 2016;75:906-917.
- Basra MK, Sue-Ho R, Finlay AY. The Family Dermatology Life Quality Index: measuring the secondary impact of skin disease. Br J Dermatol. 2007;156:528-538.
- Dodington SR, Basra MK, Finlay AY, et al. The Dermatitis Family Impact questionnaire: a review of its measurement properties and clinical application. Br J Dermatol. 2013;169:31-46.
- Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol. 2001;144:104-110.
- Holme SA, Man I, Sharpe JL, et al. The Children’s Dermatology Life Quality Index: validation of the cartoon version. Br J Dermatol. 2003;148:285-290.
- Whalley D, McKenna SP, Dewar AL, et al. A new instrument for assessing quality of life in atopic dermatitis: international development of the Quality of Life Index for Atopic Dermatitis (QoLIAD). Br J Dermatol. 2004;150:274-283.
- Jeon C, Yan D, Nakamura M, et al. Frequency and management of sleep disturbance in adults with atopic dermatitis: a systematic review. Dermatol Ther (Heidelb). 2017;7:349-364.
- Yu SH, Attarian H, Zee P, et al. Burden of sleep and fatigue in US adults with atopic dermatitis. Dermatitis. 2016;27:50-58.
- Kong TS, Han TY, Lee JH, et al. Correlation between severity of atopic dermatitis and sleep quality in children and adults. Ann Dermatol. 2016;28:321-326.
- Fishbein AB, Mueller K, Kruse L, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case-control study. J Am Acad Dermatol. 2018;78:336-341.
- Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159:745-750.
- Emerson RM, Williams HC, Allen BR. What is the cost of atopic dermatitis in preschool children? Br J Dermatol. 2001;144:514-522.
- Filanovsky MG, Pootongkam S, Tamburro JE, et al. The financial and emotional impact of atopic dermatitis on children and their families. J Pediatr. 2016;169:284-290.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Drucker AM, Wang AR, Qureshi AA. Research gaps in quality of life and economic burden of atopic dermatitis: the National Eczema Association Burden of Disease Audit. JAMA Dermatol. 2016;152:873-874.
- Nørreslet LB, Ebbehøj NE, Ellekilde Bonde JP, et al. The impact of atopic dermatitis on work life—a systematic review. J Eur Acad Dermatol Venereol. 2018;32:23-38.
- Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
- Coutanceau C, Stalder JF. Analysis of correlations between patient-oriented SCORAD (PO-SCORAD) and other assessment scores of atopic dermatitis severity and quality of life. Dermatology. 2014;229:248-255.
- Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol. 2004;150:284-290.
- van Valburg RW, Willemsen MG, Dirven-Meijer PC, et al. Quality of life measurement and its relationship to disease severity in children with atopic dermatitis in general practice. Acta Derm Venereol. 2011;91:147-151.
- Haeck IM, ten Berge O, van Velsen SG, et al. Moderate correlation between quality of life and disease activity in adult patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2012;26:236-241.
- Spuls PI, Gerbens LAA, Simpson E, et al; HOME initiative collaborators. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. Br J Dermatol. 2017;176:979-984.
- Schmitt J, Spuls PI, Thomas KS, et al; HOME initiative collaborators. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol. 2014;134:800-807.
- Oh SH, Bae BG, Park CO, et al. Association of stress with symptoms of atopic dermatitis. Acta Derm Venereol. 2010;90:582-588.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Cheng CM, Hsu JW, Huang KL, et al. Risk of developing major depressive disorder and anxiety disorders among adolescents and adults with atopic dermatitis: a nationwide longitudinal study. J Affect Disord. 2015;178:60-65.
Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854. - Linnet J, Jemec GB. An assessment of anxiety and dermatology life quality in patients with atopic dermatitis. Br J Dermatol. 1999;140:268-272.
- Ring J, Alomar A, Bieber T, et al; European Dermatology Forum; European Academy of Dermatology and Venereology; European Task Force on Atopic Dermatitis; European Federation of Allergy; European Society of Pediatric Dermatology; Global Allergy and Asthma European Network. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol. 2012;26:1176-1193.
- Perczel K, Gál J. Hypnotherapy of atopic dermatitis in an adult. case report. Orv Hetil. 2016;157:111-115.
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483-1494.
- Schmitt J, Williams H; HOME Development Group. Harmonising Outcome Measures for Eczema (HOME). report from the First International Consensus Meeting (HOME 1), 24 July 2010, Munich, Germany. Br J Dermatol. 2010;163:1166-1168.
- Schmitt J, Spuls P, Boers M, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy. 2012;67:1111-1117.
- Quality of Life (QoL). Harmonising Outcome Measures for Eczema (HOME) website. http://www.homeforeczema.org/research/quality-of-life.aspx. Accessed August 18, 2019.
Chernyshov PV, Tomas-Aragones L, Manolache L, et al; EADV Quality of Life Task Force. Quality of life measurement in atopic dermatitis. Position paper of the European Academy of Dermatology and Venereology (EADV) Task Force on quality of life. J Eur Acad Dermatol Venereol. 2017;31:576-593. - Hill MK, Kheirandish Pishkenari A, Braunberger TL, et al. Recent trends in disease severity and quality of life instruments for patients with atopic dermatitis: a systematic review. J Am Acad Dermatol. 2016;75:906-917.
- Basra MK, Sue-Ho R, Finlay AY. The Family Dermatology Life Quality Index: measuring the secondary impact of skin disease. Br J Dermatol. 2007;156:528-538.
- Dodington SR, Basra MK, Finlay AY, et al. The Dermatitis Family Impact questionnaire: a review of its measurement properties and clinical application. Br J Dermatol. 2013;169:31-46.
- Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol. 2001;144:104-110.
- Holme SA, Man I, Sharpe JL, et al. The Children’s Dermatology Life Quality Index: validation of the cartoon version. Br J Dermatol. 2003;148:285-290.
- Whalley D, McKenna SP, Dewar AL, et al. A new instrument for assessing quality of life in atopic dermatitis: international development of the Quality of Life Index for Atopic Dermatitis (QoLIAD). Br J Dermatol. 2004;150:274-283.
- Jeon C, Yan D, Nakamura M, et al. Frequency and management of sleep disturbance in adults with atopic dermatitis: a systematic review. Dermatol Ther (Heidelb). 2017;7:349-364.
- Yu SH, Attarian H, Zee P, et al. Burden of sleep and fatigue in US adults with atopic dermatitis. Dermatitis. 2016;27:50-58.
- Kong TS, Han TY, Lee JH, et al. Correlation between severity of atopic dermatitis and sleep quality in children and adults. Ann Dermatol. 2016;28:321-326.
- Fishbein AB, Mueller K, Kruse L, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case-control study. J Am Acad Dermatol. 2018;78:336-341.
- Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159:745-750.
- Emerson RM, Williams HC, Allen BR. What is the cost of atopic dermatitis in preschool children? Br J Dermatol. 2001;144:514-522.
- Filanovsky MG, Pootongkam S, Tamburro JE, et al. The financial and emotional impact of atopic dermatitis on children and their families. J Pediatr. 2016;169:284-290.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Drucker AM, Wang AR, Qureshi AA. Research gaps in quality of life and economic burden of atopic dermatitis: the National Eczema Association Burden of Disease Audit. JAMA Dermatol. 2016;152:873-874.
- Nørreslet LB, Ebbehøj NE, Ellekilde Bonde JP, et al. The impact of atopic dermatitis on work life—a systematic review. J Eur Acad Dermatol Venereol. 2018;32:23-38.
- Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
- Coutanceau C, Stalder JF. Analysis of correlations between patient-oriented SCORAD (PO-SCORAD) and other assessment scores of atopic dermatitis severity and quality of life. Dermatology. 2014;229:248-255.
- Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol. 2004;150:284-290.
- van Valburg RW, Willemsen MG, Dirven-Meijer PC, et al. Quality of life measurement and its relationship to disease severity in children with atopic dermatitis in general practice. Acta Derm Venereol. 2011;91:147-151.
- Haeck IM, ten Berge O, van Velsen SG, et al. Moderate correlation between quality of life and disease activity in adult patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2012;26:236-241.
- Spuls PI, Gerbens LAA, Simpson E, et al; HOME initiative collaborators. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. Br J Dermatol. 2017;176:979-984.
- Schmitt J, Spuls PI, Thomas KS, et al; HOME initiative collaborators. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol. 2014;134:800-807.
- Oh SH, Bae BG, Park CO, et al. Association of stress with symptoms of atopic dermatitis. Acta Derm Venereol. 2010;90:582-588.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Cheng CM, Hsu JW, Huang KL, et al. Risk of developing major depressive disorder and anxiety disorders among adolescents and adults with atopic dermatitis: a nationwide longitudinal study. J Affect Disord. 2015;178:60-65.
Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854. - Linnet J, Jemec GB. An assessment of anxiety and dermatology life quality in patients with atopic dermatitis. Br J Dermatol. 1999;140:268-272.
- Ring J, Alomar A, Bieber T, et al; European Dermatology Forum; European Academy of Dermatology and Venereology; European Task Force on Atopic Dermatitis; European Federation of Allergy; European Society of Pediatric Dermatology; Global Allergy and Asthma European Network. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol. 2012;26:1176-1193.
- Perczel K, Gál J. Hypnotherapy of atopic dermatitis in an adult. case report. Orv Hetil. 2016;157:111-115.
Practice Points
- For assessing quality of life (QOL) in atopic dermatitis (AD), it is recommended that researchers use a combination of a generic and a dermatology-specific or AD-specific instrument, whereas clinicians should apply a dermatology-specific or an AD-specific method or both.
- Anxiety and depression are common comorbidities in AD; patients also may need psychological support.
- Patient education is key for improving QOL in AD.
- Financial aspects of the treatment of AD should be taken into consideration because AD requires constant care, which puts a financial burden on patients.
Measles outbreak in New York City has ended
The measles outbreak in New York City, the largest in the nation this year, has officially ended, Mayor Bill de Blasio and city health officials announced Sept. 3.
“Ending the measles outbreak required extensive collaboration with community organizations and Jewish leaders. They helped encourage vaccinations and achieve record immunization levels in parts of Brooklyn,” Mayor de Blasio said in a written statement. “As we head back to school this week, we just remain vigilant. To keep our children and communities safe, I urge all New Yorkers to get vaccinated. It’s the best defense we have.”
A measles outbreak is considered to be over when 42 days, or two incubation periods, have elapsed since the last affected persons in the area were no longer infectious. “That time period has now passed for the people most recently infected with measles and reported,” the city health department said in the statement.
Since the outbreak began in October of last year, 654 individuals were diagnosed with measles in the five boroughs of New York, although 72% occurred in the Williamsburg neighborhood of Brooklyn. according to the health department. The majority of affected people were under 18 years of age (80%), and most were either unvaccinated (73%) or incompletely vaccinated (7%).
The end of the measles outbreak also brings an end to the public health emergency that was declared on April 9 for parts of Brooklyn, the statement noted.
“Vaccination coverage has increased significantly since the emergency order, which has been supported by community-led efforts. We are grateful to the New Yorkers who shared the truth about vaccines and protected the health of their friends and neighbors through this outbreak,” city health commissioner Dr. Oxiris Barbot said in the statement.
The measles outbreak in New York City, the largest in the nation this year, has officially ended, Mayor Bill de Blasio and city health officials announced Sept. 3.
“Ending the measles outbreak required extensive collaboration with community organizations and Jewish leaders. They helped encourage vaccinations and achieve record immunization levels in parts of Brooklyn,” Mayor de Blasio said in a written statement. “As we head back to school this week, we just remain vigilant. To keep our children and communities safe, I urge all New Yorkers to get vaccinated. It’s the best defense we have.”
A measles outbreak is considered to be over when 42 days, or two incubation periods, have elapsed since the last affected persons in the area were no longer infectious. “That time period has now passed for the people most recently infected with measles and reported,” the city health department said in the statement.
Since the outbreak began in October of last year, 654 individuals were diagnosed with measles in the five boroughs of New York, although 72% occurred in the Williamsburg neighborhood of Brooklyn. according to the health department. The majority of affected people were under 18 years of age (80%), and most were either unvaccinated (73%) or incompletely vaccinated (7%).
The end of the measles outbreak also brings an end to the public health emergency that was declared on April 9 for parts of Brooklyn, the statement noted.
“Vaccination coverage has increased significantly since the emergency order, which has been supported by community-led efforts. We are grateful to the New Yorkers who shared the truth about vaccines and protected the health of their friends and neighbors through this outbreak,” city health commissioner Dr. Oxiris Barbot said in the statement.
The measles outbreak in New York City, the largest in the nation this year, has officially ended, Mayor Bill de Blasio and city health officials announced Sept. 3.
“Ending the measles outbreak required extensive collaboration with community organizations and Jewish leaders. They helped encourage vaccinations and achieve record immunization levels in parts of Brooklyn,” Mayor de Blasio said in a written statement. “As we head back to school this week, we just remain vigilant. To keep our children and communities safe, I urge all New Yorkers to get vaccinated. It’s the best defense we have.”
A measles outbreak is considered to be over when 42 days, or two incubation periods, have elapsed since the last affected persons in the area were no longer infectious. “That time period has now passed for the people most recently infected with measles and reported,” the city health department said in the statement.
Since the outbreak began in October of last year, 654 individuals were diagnosed with measles in the five boroughs of New York, although 72% occurred in the Williamsburg neighborhood of Brooklyn. according to the health department. The majority of affected people were under 18 years of age (80%), and most were either unvaccinated (73%) or incompletely vaccinated (7%).
The end of the measles outbreak also brings an end to the public health emergency that was declared on April 9 for parts of Brooklyn, the statement noted.
“Vaccination coverage has increased significantly since the emergency order, which has been supported by community-led efforts. We are grateful to the New Yorkers who shared the truth about vaccines and protected the health of their friends and neighbors through this outbreak,” city health commissioner Dr. Oxiris Barbot said in the statement.