User login
HFNC 12 L/min on floor cuts down on bronchiolitis ICU transfers
SEATTLE – ICU transfers for acute bronchiolitis dropped 63% at Johns Hopkins All Children’s Hospital in St. Petersburg, Fla., after the high-flow nasal cannula limit on the floor was raised from 6 L/min to 12 L/min, and treatment was started in the emergency department, according to a presentation at Pediatric Hospital Medicine.
A year before the change was made in April 2018, there were 17 transfers among 249 bronchiolitis patients treated on the floor, a transfer rate of 6.8%. In the year after the change, there were eight among 319 patients, a transfer rate of 2.5%. Raising the limit to 12 L/min prevented an estimated 14 transfers, for a total savings of almost $250,000, said pediatric hospitalist and assistant professor Shaila Siraj, MD.
The change was made after Dr. Siraj and her colleagues noticed that when children topped out at 6 L, they sometimes only needed a slightly higher flow rate in the ICU, maybe 8 L or 10 L, for a short while before they came back to the floor. Given the safety of high-flow nasal cannula (HFNC), the ICU transfer often seemed like a waste of time and resources.
“As hospitalists, we felt we could safely take care of these patients,” Dr. Siraj said.
So she and her colleague pediatric critical care specialist Anthony Sochet, MD, also an assistant professor of pediatrics, reviewed over a year’s worth of data at All Children’s. They found that 12 L/min – roughly 1.5 L/kg/min – was the cutoff that best discriminated between patients who needed intubation and those who did not, “so that’s what we chose,” Dr. Sochet said.
For simplicity, they broke limits down by age: A maximum flow rate of 8 L/min for children up to 6 months old; 10 L for children aged 6-12 months; and up to 12 L/min for children age 12-24 months. The fraction of inspired oxygen remained the same at 50%. Children were started at maximum flows, then weaned down as they improved. Respiratory assessments were made at least every 4 hours.
The changes were part of a larger revision of the hospital’s pathway for uncomplicated bronchiolitis in children up to 2 years old; it was a joint effort involving nurses, respiratory therapists, and pediatric hospitalists, and ED and ICU teams.
Early initiation in the ED was “probably one of the most important” changes; it kept children from wearing out as they struggled to breath. Kids often start to improve right away, but when then don’t after 30-60 minutes, it’s an indication that they should probably be triaged to the ICU for possible intubation, Dr. Siraj said.
Dr. Sochet was careful to note that institutions have to assess their own situations before taking similar steps. “Not everyone has a tertiary care ICU staffed 24 and 7,” he said.
“You have to ask what floor resources you have, what’s your ability to escalate when you need to. Use data from your own institution to guide where you pick your cutoffs. Adequate staffing is really about respiratory [therapist]/nursing ratios, not the physicians,” he said.
In addition, “in an otherwise healthy child that just has [HFNC] for bronchiolitis, there is absolutely no reason why you should be withholding feeds.” Fed children will feel better and do better, he said.
The presenters had no disclosures.
SEATTLE – ICU transfers for acute bronchiolitis dropped 63% at Johns Hopkins All Children’s Hospital in St. Petersburg, Fla., after the high-flow nasal cannula limit on the floor was raised from 6 L/min to 12 L/min, and treatment was started in the emergency department, according to a presentation at Pediatric Hospital Medicine.
A year before the change was made in April 2018, there were 17 transfers among 249 bronchiolitis patients treated on the floor, a transfer rate of 6.8%. In the year after the change, there were eight among 319 patients, a transfer rate of 2.5%. Raising the limit to 12 L/min prevented an estimated 14 transfers, for a total savings of almost $250,000, said pediatric hospitalist and assistant professor Shaila Siraj, MD.
The change was made after Dr. Siraj and her colleagues noticed that when children topped out at 6 L, they sometimes only needed a slightly higher flow rate in the ICU, maybe 8 L or 10 L, for a short while before they came back to the floor. Given the safety of high-flow nasal cannula (HFNC), the ICU transfer often seemed like a waste of time and resources.
“As hospitalists, we felt we could safely take care of these patients,” Dr. Siraj said.
So she and her colleague pediatric critical care specialist Anthony Sochet, MD, also an assistant professor of pediatrics, reviewed over a year’s worth of data at All Children’s. They found that 12 L/min – roughly 1.5 L/kg/min – was the cutoff that best discriminated between patients who needed intubation and those who did not, “so that’s what we chose,” Dr. Sochet said.
For simplicity, they broke limits down by age: A maximum flow rate of 8 L/min for children up to 6 months old; 10 L for children aged 6-12 months; and up to 12 L/min for children age 12-24 months. The fraction of inspired oxygen remained the same at 50%. Children were started at maximum flows, then weaned down as they improved. Respiratory assessments were made at least every 4 hours.
The changes were part of a larger revision of the hospital’s pathway for uncomplicated bronchiolitis in children up to 2 years old; it was a joint effort involving nurses, respiratory therapists, and pediatric hospitalists, and ED and ICU teams.
Early initiation in the ED was “probably one of the most important” changes; it kept children from wearing out as they struggled to breath. Kids often start to improve right away, but when then don’t after 30-60 minutes, it’s an indication that they should probably be triaged to the ICU for possible intubation, Dr. Siraj said.
Dr. Sochet was careful to note that institutions have to assess their own situations before taking similar steps. “Not everyone has a tertiary care ICU staffed 24 and 7,” he said.
“You have to ask what floor resources you have, what’s your ability to escalate when you need to. Use data from your own institution to guide where you pick your cutoffs. Adequate staffing is really about respiratory [therapist]/nursing ratios, not the physicians,” he said.
In addition, “in an otherwise healthy child that just has [HFNC] for bronchiolitis, there is absolutely no reason why you should be withholding feeds.” Fed children will feel better and do better, he said.
The presenters had no disclosures.
SEATTLE – ICU transfers for acute bronchiolitis dropped 63% at Johns Hopkins All Children’s Hospital in St. Petersburg, Fla., after the high-flow nasal cannula limit on the floor was raised from 6 L/min to 12 L/min, and treatment was started in the emergency department, according to a presentation at Pediatric Hospital Medicine.
A year before the change was made in April 2018, there were 17 transfers among 249 bronchiolitis patients treated on the floor, a transfer rate of 6.8%. In the year after the change, there were eight among 319 patients, a transfer rate of 2.5%. Raising the limit to 12 L/min prevented an estimated 14 transfers, for a total savings of almost $250,000, said pediatric hospitalist and assistant professor Shaila Siraj, MD.
The change was made after Dr. Siraj and her colleagues noticed that when children topped out at 6 L, they sometimes only needed a slightly higher flow rate in the ICU, maybe 8 L or 10 L, for a short while before they came back to the floor. Given the safety of high-flow nasal cannula (HFNC), the ICU transfer often seemed like a waste of time and resources.
“As hospitalists, we felt we could safely take care of these patients,” Dr. Siraj said.
So she and her colleague pediatric critical care specialist Anthony Sochet, MD, also an assistant professor of pediatrics, reviewed over a year’s worth of data at All Children’s. They found that 12 L/min – roughly 1.5 L/kg/min – was the cutoff that best discriminated between patients who needed intubation and those who did not, “so that’s what we chose,” Dr. Sochet said.
For simplicity, they broke limits down by age: A maximum flow rate of 8 L/min for children up to 6 months old; 10 L for children aged 6-12 months; and up to 12 L/min for children age 12-24 months. The fraction of inspired oxygen remained the same at 50%. Children were started at maximum flows, then weaned down as they improved. Respiratory assessments were made at least every 4 hours.
The changes were part of a larger revision of the hospital’s pathway for uncomplicated bronchiolitis in children up to 2 years old; it was a joint effort involving nurses, respiratory therapists, and pediatric hospitalists, and ED and ICU teams.
Early initiation in the ED was “probably one of the most important” changes; it kept children from wearing out as they struggled to breath. Kids often start to improve right away, but when then don’t after 30-60 minutes, it’s an indication that they should probably be triaged to the ICU for possible intubation, Dr. Siraj said.
Dr. Sochet was careful to note that institutions have to assess their own situations before taking similar steps. “Not everyone has a tertiary care ICU staffed 24 and 7,” he said.
“You have to ask what floor resources you have, what’s your ability to escalate when you need to. Use data from your own institution to guide where you pick your cutoffs. Adequate staffing is really about respiratory [therapist]/nursing ratios, not the physicians,” he said.
In addition, “in an otherwise healthy child that just has [HFNC] for bronchiolitis, there is absolutely no reason why you should be withholding feeds.” Fed children will feel better and do better, he said.
The presenters had no disclosures.
REPORTING FROM PHM 2019
Key clinical point:
Major finding: ICU transfers dropped 63% after the floor limit was raised from 6 L/min to 12 L/min.
Study details: Before/after quality improvement project
Disclosures: There was no external funding, and the presenters had no disclosures.
Cephalosporins remain empiric therapy for skin infections in pediatric AD
“Clindamycin, tetracyclines, or TMP‐SMX can be considered in patients suspected to have, or with a history of, MRSA [methicillin‐resistant S. aureus] infection,” wrote Cristopher C. Briscoe, MD, of the Washington University School of Medicine in St. Louis, Missouri, and his coauthors. The study was published in Pediatric Dermatology.
To determine the optimal empiric antibiotic for pediatric AD patients with skin infections, the researchers analyzed skin cultures from 106 patients seen at Saint Louis Children’s Hospital (SLCH). The results were also compared to cultures from pediatric patients who presented at the SLCH emergency department (ED) with S. aureus skin abscesses.
Of the 170 cultures that grew S. aureus, 130 (77.8%) grew MSSA, and 37 (22.2%) grew MRSA. Three of the cultures grew both. The prevalence of MRSA in the cohort differed from the prevalence in the ED patients (44%). The prevalence of either infection did not differ significantly by age, sex or race, though the average number of cultures in African American patients topped the average for Caucasian patients (1.8 vs. 1.2, P less than .003).
All patients with MSSA – in both the cohort and the ED – proved 100% susceptible to cefazolin. Cohort patients with MSSA saw lower susceptibility to doxycycline compared to the ED patients (89.4% vs. 97%), as did MRSA cohort patients to trimethoprim‐sulfamethoxazole (92% vs. 98%).
“When a patient with AD walks into your office and looks like they have an infection of their eczema, your go-to antibiotic is going to be one that targets MSSA,” said coauthor Carrie Coughlin, MD, of the Washington University School of Medicine in an interview. “You’ll still do a culture to prove or disprove that assumption, but it gives you a guide to help make that patient better in the short term while you work things up.”
“Also, remember that MSSA is not ‘better’ to have than MRSA,” she added. “You can now see some of the virulence factors from MRSA strains in MSSA strains, so treating both of them is important.”
The authors acknowledged their study’s limitations, including the limited generalizability of a single-center design and a lack of information as to the body sites from which the cultures were obtained. They were also unable to reliably determine prior antibiotic exposure, noting that “future work could examine whether prior exposure differed significantly in the MRSA and MSSA groups.”
The study was funded by grants from the Agency for Healthcare Research and Quality. The authors reported no conflicts of interest.
SOURCE: Briscoe CC et al. Pediatr Dermatol. 2019 May 24. doi: 10.1111/pde.13867.
“Clindamycin, tetracyclines, or TMP‐SMX can be considered in patients suspected to have, or with a history of, MRSA [methicillin‐resistant S. aureus] infection,” wrote Cristopher C. Briscoe, MD, of the Washington University School of Medicine in St. Louis, Missouri, and his coauthors. The study was published in Pediatric Dermatology.
To determine the optimal empiric antibiotic for pediatric AD patients with skin infections, the researchers analyzed skin cultures from 106 patients seen at Saint Louis Children’s Hospital (SLCH). The results were also compared to cultures from pediatric patients who presented at the SLCH emergency department (ED) with S. aureus skin abscesses.
Of the 170 cultures that grew S. aureus, 130 (77.8%) grew MSSA, and 37 (22.2%) grew MRSA. Three of the cultures grew both. The prevalence of MRSA in the cohort differed from the prevalence in the ED patients (44%). The prevalence of either infection did not differ significantly by age, sex or race, though the average number of cultures in African American patients topped the average for Caucasian patients (1.8 vs. 1.2, P less than .003).
All patients with MSSA – in both the cohort and the ED – proved 100% susceptible to cefazolin. Cohort patients with MSSA saw lower susceptibility to doxycycline compared to the ED patients (89.4% vs. 97%), as did MRSA cohort patients to trimethoprim‐sulfamethoxazole (92% vs. 98%).
“When a patient with AD walks into your office and looks like they have an infection of their eczema, your go-to antibiotic is going to be one that targets MSSA,” said coauthor Carrie Coughlin, MD, of the Washington University School of Medicine in an interview. “You’ll still do a culture to prove or disprove that assumption, but it gives you a guide to help make that patient better in the short term while you work things up.”
“Also, remember that MSSA is not ‘better’ to have than MRSA,” she added. “You can now see some of the virulence factors from MRSA strains in MSSA strains, so treating both of them is important.”
The authors acknowledged their study’s limitations, including the limited generalizability of a single-center design and a lack of information as to the body sites from which the cultures were obtained. They were also unable to reliably determine prior antibiotic exposure, noting that “future work could examine whether prior exposure differed significantly in the MRSA and MSSA groups.”
The study was funded by grants from the Agency for Healthcare Research and Quality. The authors reported no conflicts of interest.
SOURCE: Briscoe CC et al. Pediatr Dermatol. 2019 May 24. doi: 10.1111/pde.13867.
“Clindamycin, tetracyclines, or TMP‐SMX can be considered in patients suspected to have, or with a history of, MRSA [methicillin‐resistant S. aureus] infection,” wrote Cristopher C. Briscoe, MD, of the Washington University School of Medicine in St. Louis, Missouri, and his coauthors. The study was published in Pediatric Dermatology.
To determine the optimal empiric antibiotic for pediatric AD patients with skin infections, the researchers analyzed skin cultures from 106 patients seen at Saint Louis Children’s Hospital (SLCH). The results were also compared to cultures from pediatric patients who presented at the SLCH emergency department (ED) with S. aureus skin abscesses.
Of the 170 cultures that grew S. aureus, 130 (77.8%) grew MSSA, and 37 (22.2%) grew MRSA. Three of the cultures grew both. The prevalence of MRSA in the cohort differed from the prevalence in the ED patients (44%). The prevalence of either infection did not differ significantly by age, sex or race, though the average number of cultures in African American patients topped the average for Caucasian patients (1.8 vs. 1.2, P less than .003).
All patients with MSSA – in both the cohort and the ED – proved 100% susceptible to cefazolin. Cohort patients with MSSA saw lower susceptibility to doxycycline compared to the ED patients (89.4% vs. 97%), as did MRSA cohort patients to trimethoprim‐sulfamethoxazole (92% vs. 98%).
“When a patient with AD walks into your office and looks like they have an infection of their eczema, your go-to antibiotic is going to be one that targets MSSA,” said coauthor Carrie Coughlin, MD, of the Washington University School of Medicine in an interview. “You’ll still do a culture to prove or disprove that assumption, but it gives you a guide to help make that patient better in the short term while you work things up.”
“Also, remember that MSSA is not ‘better’ to have than MRSA,” she added. “You can now see some of the virulence factors from MRSA strains in MSSA strains, so treating both of them is important.”
The authors acknowledged their study’s limitations, including the limited generalizability of a single-center design and a lack of information as to the body sites from which the cultures were obtained. They were also unable to reliably determine prior antibiotic exposure, noting that “future work could examine whether prior exposure differed significantly in the MRSA and MSSA groups.”
The study was funded by grants from the Agency for Healthcare Research and Quality. The authors reported no conflicts of interest.
SOURCE: Briscoe CC et al. Pediatr Dermatol. 2019 May 24. doi: 10.1111/pde.13867.
FROM PEDIATRIC DERMATOLOGY
Antidepressants for pediatric patients
Major depressive disorder (MDD) is a significant pediatric health problem, with a lifetime prevalence as high as 20% by the end of adolescence.1-3 Major depressive disorder in adolescence is associated with significant morbidity, including poor social functioning, school difficulties, early pregnancy, and increased risk of physical illness and substance abuse.4-6 It is also linked with significant mortality, with increased risk for suicide, which is now the second leading cause of death in individuals age 10 to 24 years.1,7,8
As their name suggests, antidepressants comprise a group of medications that are used to treat MDD; they are also, however, first-line agents for generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD) in adults. Anxiety disorders (including GAD and other anxiety diagnoses) and PTSD are also common in childhood and adolescence with a combined lifetime prevalence ranging from 15% to 30%.9,10 These disorders are also associated with increased risk of suicide.11 For all of these disorders, depending on the severity of presentation and the preference of the patient, treatments are often a combination of psychotherapy and psychopharmacology.
Clinicians face several challenges when considering antidepressants for pediatric patients. Pediatricians and psychiatrists need to understand whether these medications work in children and adolescents, and whether there are unique developmental safety and tolerability issues. The evidence base in child psychiatry is considerably smaller compared with that of adult psychiatry. From this more limited evidence base also came the controversial “black-box” warning regarding a risk of emergent suicidality when starting antidepressants that accompanies all antidepressants for pediatric, but not adult, patients. This warning has had major effects on clinical encounters with children experiencing depression, including altering clinician prescribing behavior.12
Do antidepressants work in children?
Selective serotonin reuptake inhibitors. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly used class of antidepressants in both children and adults.13 While only a few SSRIs are FDA-approved for pediatric indications, the lack of FDA approval is typically related to a lack of sufficient testing in randomized controlled trials (RCTs) for specific pediatric indications, rather than to demonstrable differences in efficacy between antidepressant agents. Since there is currently no data to suggest inferiority of one agent compared to another in children or adults,14,15 efficacy data will be discussed here as applied to the class of SSRIs, generalizing from RCTs conducted on individual drugs. Table 1 lists FDA indications and dosing information for individual antidepressants.
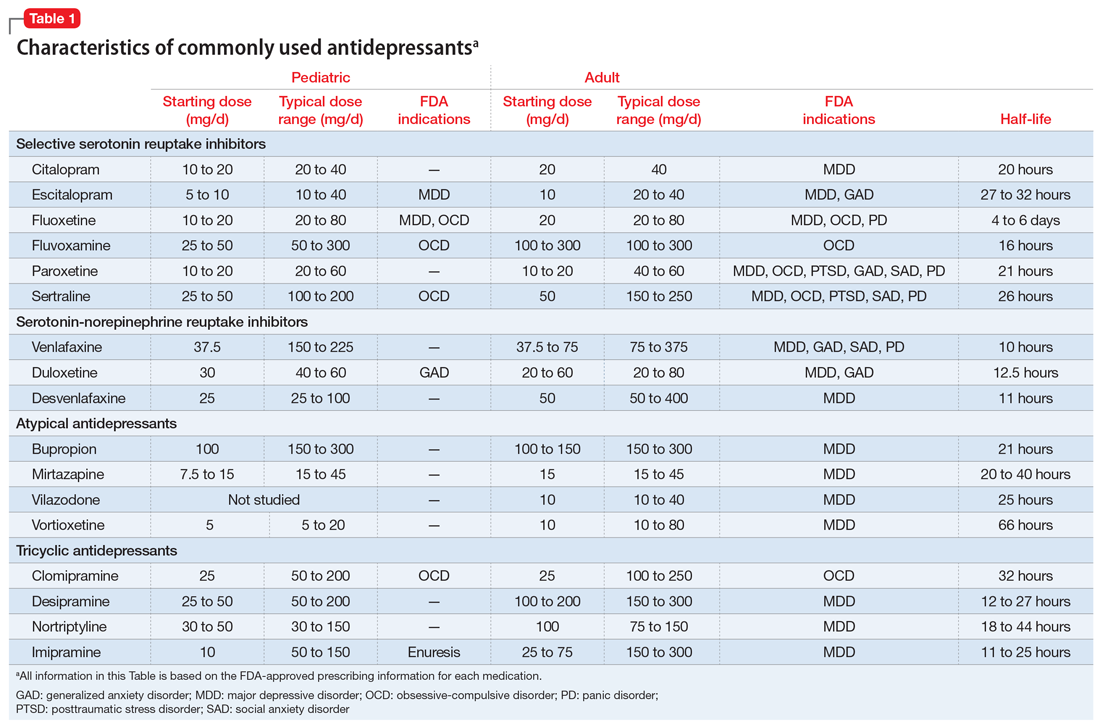
There is strong evidence that SSRIs are effective for treating pediatric anxiety disorders (eg, social anxiety disorder and GAD)16 and OCD,17 with numbers needed to treat (NNT) between 3 and 5. For both of these disorders, SSRIs combined with cognitive-behavioral therapy (CBT) have the highest likelihood of improving symptoms or achieving remission.17,18
Selective serotonin reuptake inhibitors are also effective for treating pediatric MDD; however, the literature is more complex for this disorder compared to GAD and OCD as there are considerable differences in effect sizes between National Institute of Mental Health (NIMH)–funded studies and industry-sponsored trials.13 The major NIMH-sponsored adolescent depression trial, TADS (Treatment for Adolescents and Depression Study), showed that SSRIs (fluoxetine in this case) were quite effective, with an NNT of 4 over the acute phase (12 weeks).19 Ultimately, approximately 80% of adolescents improved over 9 months. Many industry-sponsored trials for MDD in pediatric patients had large placebo response rates (approximately 60%), which resulted in smaller between-group differences, and estimates of an NNT closer to 12,13 which has muddied the waters in meta-analyses that include all trials.20 Improvement in depressive symptoms also appears to be bolstered by concomitant CBT in MDD,19 but not as robustly as in GAD and OCD. While the full benefit of SSRIs for depression may take as long as 8 weeks, a meta-analysis of depression studies of pediatric patients suggests that significant benefits from placebo are observed as early as 2 weeks, and that further treatment gains are minimal after 4 weeks.15 Thus, we recommend at least a 4- to 6-week trial at therapeutic dosing before deeming a medication a treatment failure.
Continue to: Posttraumatic stress disorder...
Posttraumatic stress disorder is a fourth disorder in which SSRIs are a first-line treatment in adults. The data for using SSRIs to treat pediatric patients with PTSD is scant, with only a few RCTs, and no large NIMH-funded trials. Randomized controlled trials have not demonstrated significant differences between SSRIs and placebo21,22 and thus the current first-line recommendation in pediatric PTSD remains trauma-focused therapy, with good evidence for trauma-focused CBT.23 Practically speaking, there can be considerable overlap of PTSD, depression, and anxiety symptoms in children,23 and children with a history of trauma who also have comorbid MDD may benefit from medication if their symptoms persist despite an adequate trial of psychotherapy.
Taken together, the current evidence suggests that SSRIs are often effective in pediatric GAD, OCD, and MDD, with low NNTs (ranging from 3 to 5 based on NIMH-funded trials) for all of these disorders; there is not yet sufficient evidence of efficacy in pediatric patients with PTSD.
Fluoxetine has been studied more intensively than other SSRIs (for example, it was the antidepressant used in the TADS trial), and thus has the largest evidence base. For this reason, fluoxetine is often considered the first of the first-line options. Additionally, fluoxetine has a longer half-life than other antidepressants, which may make it more effective in situations where patients are likely to miss doses, and results in a lower risk of withdrawal symptoms when stopped due to “self-tapering.”
SNRIs and atypical antidepressants. Other antidepressants commonly used in pediatric patients but with far less evidence of efficacy include serotonin-norepinephrine reuptake inhibitors (SNRIs) and the atypical antidepressants bupropion and mirtazapine. The SNRI duloxetine is FDA-approved for treating GAD in children age 7 to 17, but there are no other pediatric indications for duloxetine, or for the other SNRIs.
In general, adverse effect profiles are worse for SNRIs compared to SSRIs, further limiting their utility. While there are no pediatric studies demonstrating SNRI efficacy for neuropathic pain, good data exists in adults.24 Thus, an SNRI could be a reasonable option if a pediatric patient has failed prior adequate SSRI trials and also has comorbid neuropathic pain.
Continue to: Neither bupropion nor mirtazapine...
Neither bupropion nor mirtazapine have undergone rigorous testing in pediatric patients, and therefore these agents should generally be considered only once other first-line treatments have failed. Bupropion has been evaluated for attention-deficit/hyperactivity disorder (ADHD)25 and for adolescent smoking cessation.26 However, the evidence is weak, and bupropion is not considered a first-line option for children and adolescents.
Tricyclic antidepressants. Randomized controlled trials have demonstrated that tricyclic antidepressants (TCAs) are efficacious for treating several pediatric conditions; however, their significant side effect profile, their monitoring requirements, as well as their lethality in overdose has left them replaced by SSRIs in most cases. That said, they can be appropriate in refractory ADHD (desipramine27,28) and refractory OCD (clomipramine is FDA-approved for this indication29); they are considered a third-line treatment for enuresis.30
Why did my patient stop the medication?
Common adverse effects. Although the greatest benefit of antidepressant medications compared with placebo is achieved relatively early on in treatment, it generally takes time for these benefits to accrue and become clinically apparent.15,31 By contrast, most adverse effects of antidepressants present and are at their most severe early in treatment. The combination of early adverse effects and delayed efficacy leads many patients, families, and clinicians to discontinue medications before they have an adequate chance to work. Thus, it is imperative to provide psychoeducation before starting a medication about the typical time-course of improvement and adverse effects (Table 2).
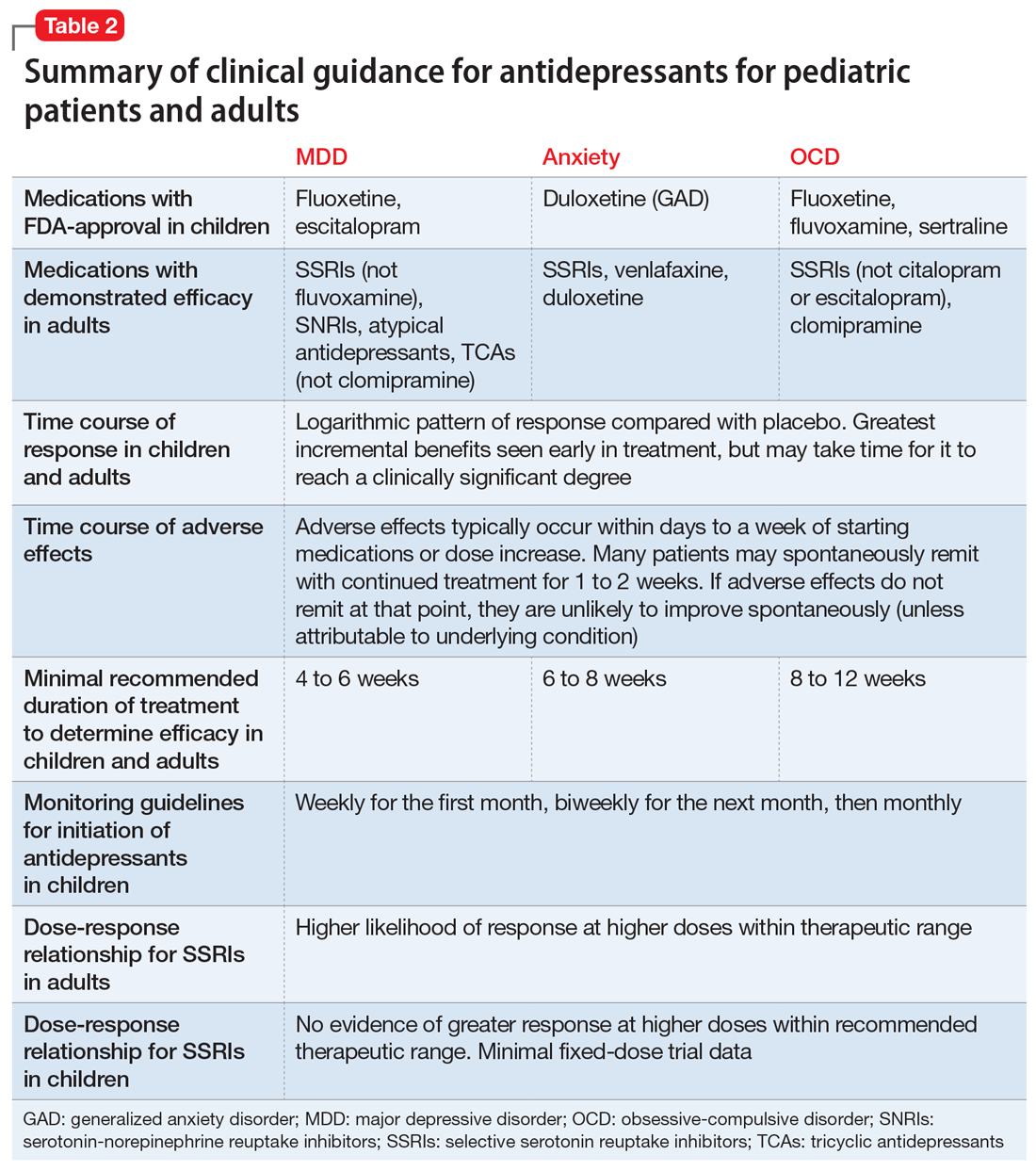
Adverse effects of SSRIs often appear or worsen transiently during initiation of a medication, during a dose increase,32 or, theoretically, with the addition of a medication that interferes with SSRI metabolism (eg, cimetidine inhibition of cytochrome P450 2D6).33 If families are prepared for this phenomenon and the therapeutic alliance is adequate, adverse effects can be tolerated to allow for a full medication trial. Common adverse effects of SSRIs include sleep problems (insomnia/sedation), gastrointestinal upset, sexual dysfunction, dry mouth, and hyperhidrosis. Although SSRIs differ somewhat in the frequency of these effects, as a class, they are more similar than different. Adequate psychoeducation is especially imperative in the treatment of OCD and anxiety disorders, where there is limited evidence of efficacy for any non-serotonergic antidepressants.
Serotonin-norepinephrine reuptake inhibitors are not considered first-line medications because of the reduced evidence base compared to SSRIs and their enhanced adverse effect profiles. Because SNRIs partially share a mechanism of action with SSRIs, they also share portions of the adverse effects profile. However, SNRIs have the additional adverse effect of hypertension, which is related to their noradrenergic activity. Thus, it is reasonable to obtain a baseline blood pressure before initiating an SNRI, as well as periodically after initiation and during dose increases, particularly if the patient has other risk factors for hypertension.34
Continue to: Although TCAs have efficacy...
Although TCAs have efficacy in some pediatric disorders,27-29,35 their adverse effect profile limits their use. Tricyclic antidepressants are highly anticholinergic (causing dizziness secondary to orthostatic hypotension, dry mouth, and urinary retention) and antihistaminergic (causing sedation and weight gain). Additionally, TCAs lower the seizure threshold and have adverse cardiac effects relating to their anti-alpha-1 adrenergic activity, resulting in dose-dependent increases in the QTc and cardiac toxicity in overdose that could lead to arrhythmia and death. These medications have their place, but their use requires careful informed consent, clear treatment goals, and baseline and periodic cardiac monitoring (via electrocardiogram).
Serious adverse effects. Clinicians may be hesitant to prescribe antidepressants for pediatric patients because of the potential for more serious adverse effects, including severe behavioral activation syndromes, serotonin syndrome, and emergent suicidality. However, current FDA-approved antidepressants arguably have one of the most positive risk/benefit profiles of any orally-administered medication approved for pediatric patients. Having a strong understanding of the evidence is critical to evaluating when it is appropriate to prescribe an antidepressant, how to properly monitor the patient, and how to obtain accurate informed consent.
Pediatric behavioral activation syndrome. Many clinicians report that children receiving antidepressants experience a pediatric behavioral activation syndrome, which exists along a spectrum from mild activation, increased energy, insomnia, or irritability up through more severe presentations of agitation, hyperactivity, or possibly mania. A recent meta-analysis suggested a positive association between antidepressant use and activation events on the milder end of this spectrum in pediatric patients with non-OCD anxiety disorders,16 and it is thought that compared with adolescents, younger children are more susceptible to activation adverse effects.36 The likelihood of activation events has been associated with higher antidepressant plasma levels,37 suggesting that dose or individual differences in metabolism may play a role. At the severe end of the spectrum, the risk of induction of mania in pediatric patients with depression or anxiety is relatively rare (<2%) and not statistically different from placebo in RCTs of pediatric participants.38 Meta-analyses of larger randomized, placebo-controlled trials of adults do not support the idea that SSRIs and other second-generation antidepressants carry an increased risk of mania compared with placebo.39,40 Children or adolescents with bona fide bipolar disorder (ie, patients who have had observed mania that meets all DSM-5 criteria) should be treated with a mood-stabilizing agent or antipsychotic if prescribed an antidepressant.41 These clear-cut cases are, however, relatively rare, and more often clinicians are confronted with ambiguous cases that include a family history of bipolar disorder along with “softer” symptoms of irritability, intrusiveness, or aggression. In these children, SSRIs may be appropriate for depressive, OCD, or anxiety symptoms, and should be strongly considered before prescribing antipsychotics or mood stabilizers, as long as initiated with proper monitoring.
Serotonin syndrome is a life-threatening condition caused by excess synaptic serotonin. It is characterized by confusion, sweating, diarrhea, hypertension, hyperthermia, and tachycardia. At its most severe, serotonin syndrome can result in seizures, arrhythmias, and death. The risk of serotonin syndrome is very low when using an SSRI as monotherapy. Risk increases with polypharmacy, particularly unexamined polypharmacy when multiple serotonergic agents are inadvertently on board. Commonly used serotonergic agents include other antidepressants, migraine medications (eg, triptans), some pain medications, and the cough suppressant dextromethorphan.
The easiest way to mitigate the risk of serotonin syndrome is to use an interaction index computer program, which can help ensure that the interacting agents are not prescribed without first discussing the risks and benefits. It is important to teach adolescents that certain recreational drugs are highly serotonergic and can cause serious interactions with antidepressants. For example, recreational use of dextromethorphan or 3,4-methylenedioxymethamphetamine (MDMA; commonly known as “ecstasy”) has been associated with serotonin syndrome in adolescents taking antidepressant medications.42,43
Continue to: Suicidality
Suicidality. The black-box warning regarding a risk of emergent suicidality when starting antidepressant treatment in children is controversial.44 The prospect that a medication intended to ameliorate depression might instead risk increasing suicidal thinking is alarming to parents and clinicians alike. To appropriately weigh and discuss the risks and benefits with families, it is important to understand the data upon which the warning is based.
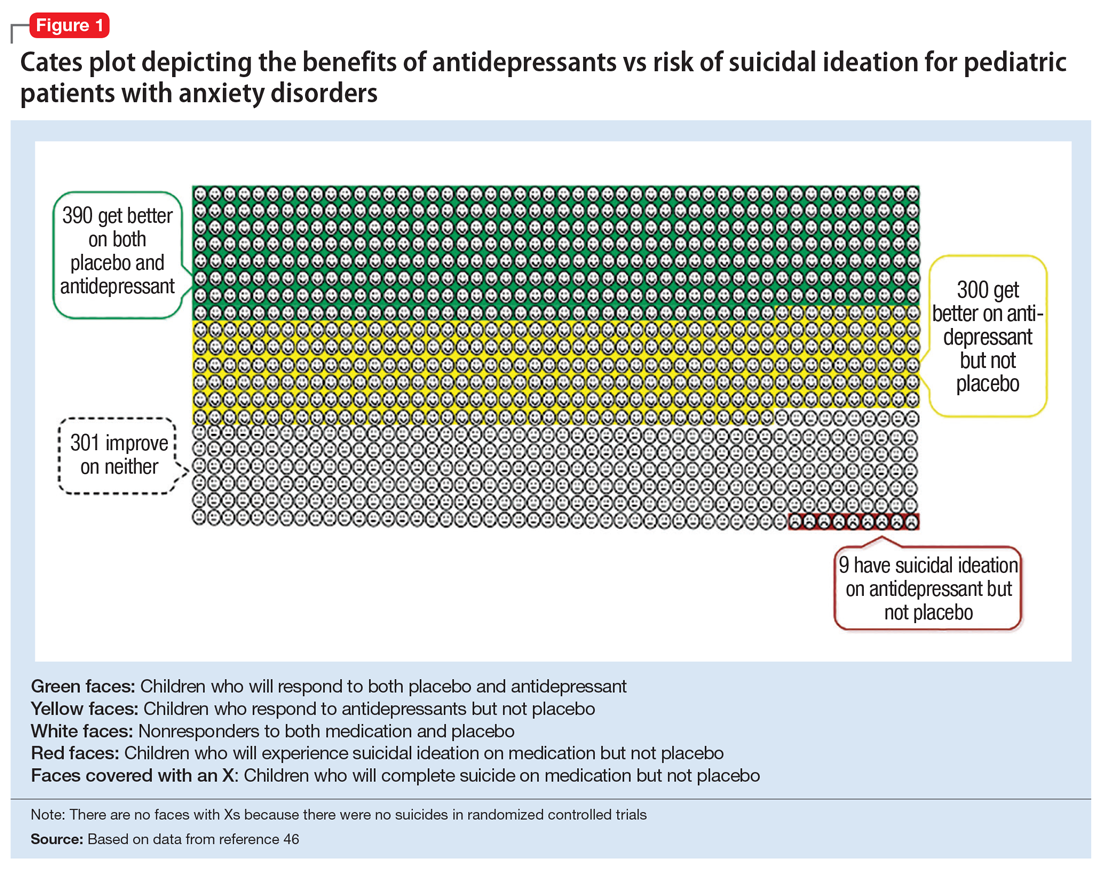
In 2004, the FDA commissioned a review of 23 antidepressant trials, both published and unpublished, pooling studies across multiple indications (MDD, OCD, anxiety, and ADHD) and multiple antidepressant classes. This meta-analysis, which included nearly 4,400 pediatric patients, found a small but statistically significant increase in spontaneously-reported suicidal thoughts or actions, with a risk difference of 1% (95% confidence interval [CI], 1% to 2%).45 These data suggest that if one treats 100 pediatric patients, 1 to 2 of them may experience short-term increases in suicidal thinking or behavior.45 There were no differences in suicidal thinking when assessed systematically (ie, when all subjects reported symptoms of suicidal ideation on structured rating scales), and there were no completed suicides.45 A subsequent analysis that included 27 pediatric trials suggested an even lower, although still significant, risk difference (<1%), yielding a number needed to harm (NNH) of 143.46 Thus, with low NNT for efficacy (3 to 6) and relatively high NNH for emergent suicidal thoughts or behaviors (100 to 143), for many patients the benefits will outweigh the risks.
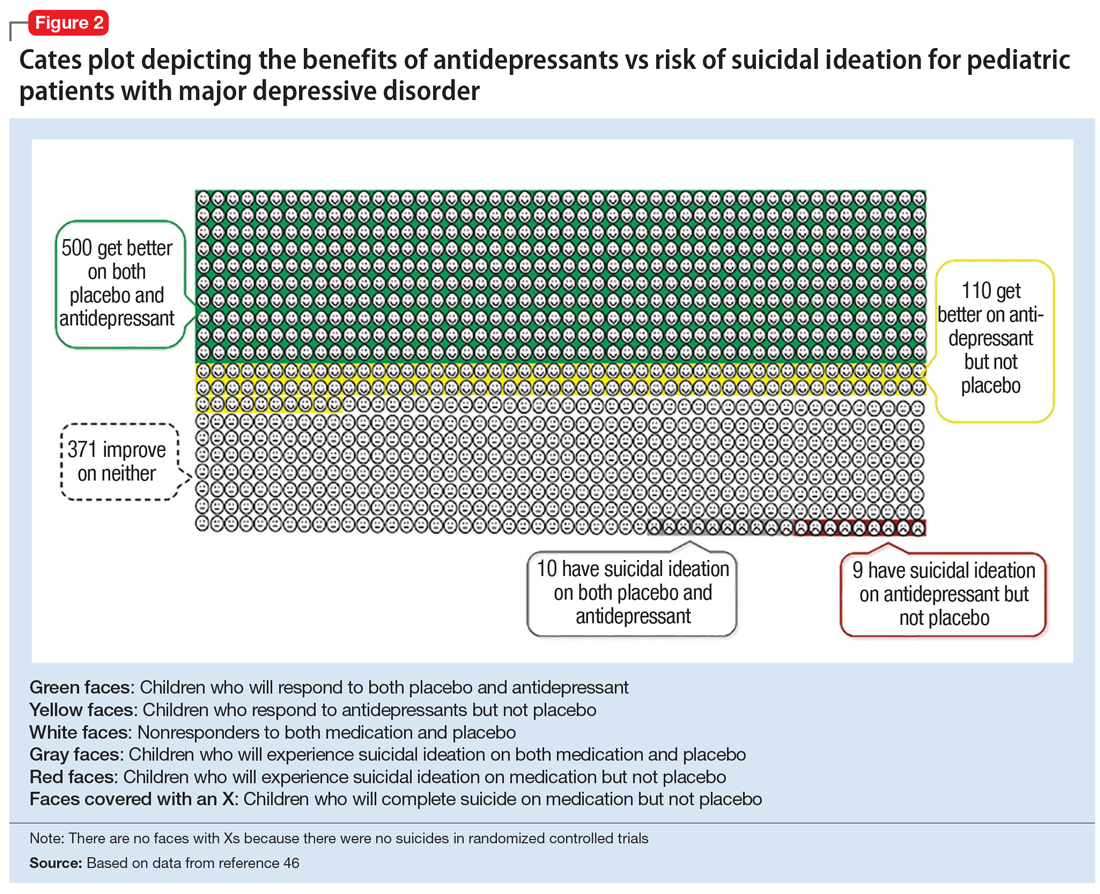
Figure 1, Figure 2, and Figure 3 are Cates plots that depict the absolute benefits of antidepressants compared with the risk of suicidality for pediatric patients with MDD, OCD, and anxiety disorders. Recent meta-analyses have suggested that the increased risk of suicidality in antidepressant trials is specific to studies that included children and adolescents, and is not observed in adult studies. A meta-analysis of 70 trials involving 18,526 participants suggested that the odds ratio of suicidality in trials of children and adolescents was 2.39 (95% CI, 1.31 to 4.33) compared with 0.81 (95% CI, 0.51 to 1.28) in adults.47 Additionally, a network meta-analysis exclusively focusing on pediatric antidepressant trials in MDD reported significantly higher suicidality-related adverse events in venlafaxine trials compared with placebo, duloxetine, and several SSRIs (fluoxetine, paroxetine, and escitalopram).20 These data should be interpreted with caution as differences in suicidality detected between agents is quite possibly related to differences in the method of assessment between trials, as opposed to actual differences in risk between agents.
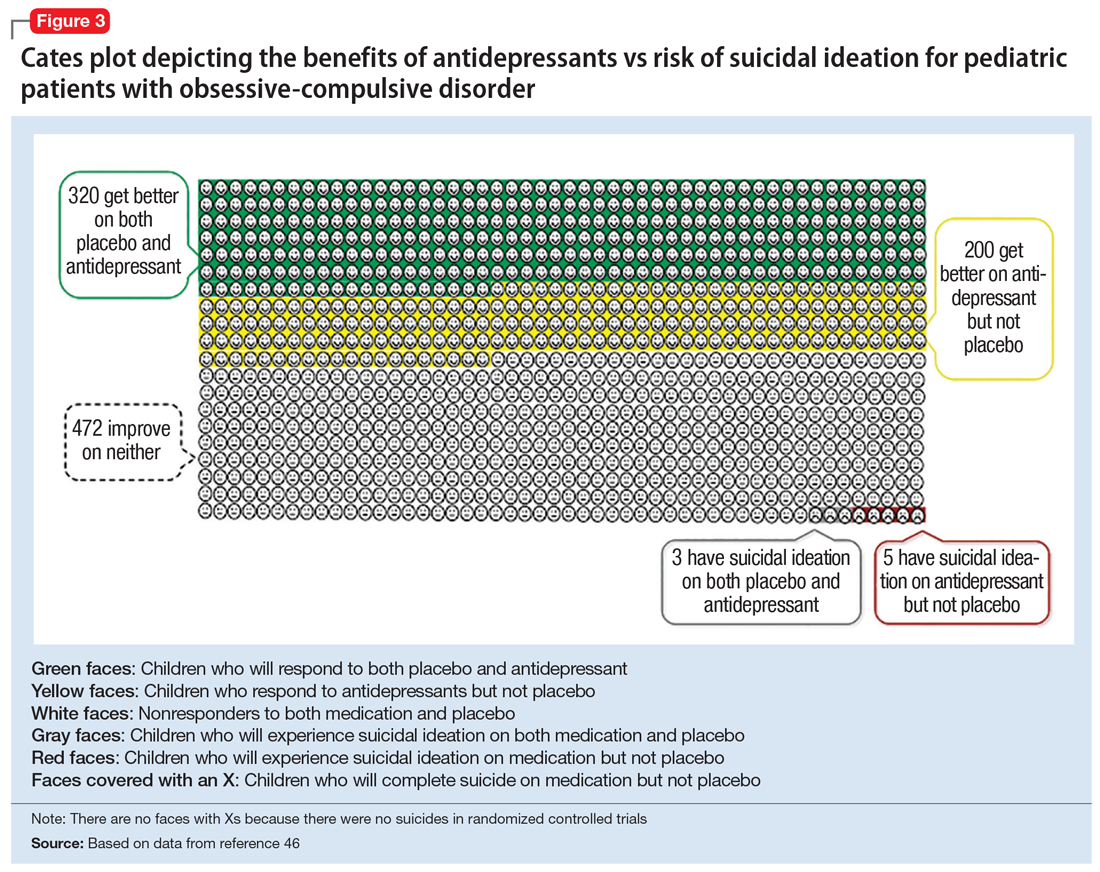
Epidemiologic data further support the use of antidepressants in pediatric patients, showing that antidepressant use is associated with decreased teen suicide attempts and completions,48 and the decline in prescriptions that occurred following the black-box warning was accompanied by a 14% increase in teen suicides.49 Multiple hypotheses have been proposed to explain the pediatric clinical trial findings. One idea is that potential adverse effects of activation, or the intended effects of restoring the motivation, energy, and social engagement that is often impaired in depression, increases the likelihood of thinking about suicide or acting on thoughts. Another theory is that reporting of suicidality may be increased, rather than increased de novo suicidality itself. Antidepressants are effective for treating pediatric anxiety disorders, including social anxiety disorder,16 which could result in more willingness to report. Also, the manner in which adverse effects are generally ascertained in trials might have led to increased spontaneous reporting. In many trials, investigators ask whether participants have any adverse effects in general, and inquire about specific adverse effects only if the family answers affirmatively. Thus, the increased rate of other adverse effects associated with antidepressants (sleep problems, gastrointestinal upset, dry mouth, etc.) might trigger a specific question regarding suicidal ideation, which the child or family then may be more likely to report. Alternatively, any type of psychiatric treatment could increase an individual’s propensity to report; in adolescent psychotherapy trials, non-medic
Continue to: How long should the antidepressant be continued?
How long should the antidepressant be continued?
Many patients are concerned about how long they may be taking medication, and whether they will be taking an antidepressant “forever.” A treatment course can be broken into an acute phase, wherein remission is achieved and maintained for 6 to 8 weeks. This is followed by a continuation phase, with the goal of relapse prevention, lasting 16 to 20 weeks. The length of the last phase—the maintenance phase—depends both on the child’s history, the underlying therapeutic indication, the adverse effect burden experienced, and the family’s preferences/values. In general, for a first depressive episode, after treating for 1 year, a trial of discontinuation can be attempted with close monitoring. For a second depressive episode, we recommend 2 years of remission on antidepressant therapy before attempting discontinuation. In patients who have had 3 depressive episodes, or have had episodes of high severity, we recommend continuing antidepressant treatment indefinitely. Although much less well studied, the risk of relapse following SSRI discontinuation appears much more significant in OCD, whereas anxiety disorders and MDD have a relatively comparable risk.52
In general, stopping an antidepressant should be done carefully and slowly.
What to do when first-line treatments fail
Adequate psychotherapy? To determine whether children are receiving adequate CBT, ask:
- if the child receives homework from psychotherapy
- if the parents are included in treatment
- if therapy has involved identifying thought patterns that may be contributing to the child’s illness, and
- if the therapist has ever exposed the child to a challenge likely to produce anxiety or distress in a supervised environment and has developed an exposure hierarchy (for conditions with primarily exposure-based therapies, such as OCD or anxiety disorders).
If a family is not receiving most of these elements in psychotherapy, this is a good indicator that they may not be receiving evidence-based CBT.
Continue to: Adequate pharmacotherapy?
Adequate pharmacotherapy? Similarly, when determining the adequacy of previous pharmacotherapy, it is critical to determine whether the child received an adequate dose of medications (at least the FDA-recommended minimum dose) for an adequate duration of time at therapeutic dosing (at least 6 weeks for MDD, 8 weeks for anxiety disorders, and 8 to 12 weeks for pediatric patients with OCD), and that the child actually took the medication regularly during that period. Patient compliance can typically be tracked through checking refill requests or intervals through the patient’s pharmacy. Ensuring proper delivery of first-line treatments is imperative because (1) the adverse effects associated with second-line treatments are often more substantial; (2) the cost in terms of time and money is considerably higher with second-line treatments, and; (3) the evidence regarding the benefits of these treatments is much less certain.
Inadequate dosing is a common reason for non-response in pediatric patients. Therapeutic dose ranges for common antidepressants are displayed in Table 1. Many clinicians underdose antidepressants for pediatric patients initially (and often throughout treatment) because they fear that the typical dose titration used in clinical trials will increase the risk of adverse effects compared with more conservative dosing. There is limited evidence to suggest that this underdosing strategy is likely to be successful; adverse effects attributable to these medications are modest, and most that are experienced early in treatment (eg, headache, increased anxiety or irritability, sleep problems, gastrointestinal upset) are self-limiting and may be coincidental rather than medication-induced. Furthermore, there is no evidence for efficacy of subtherapeutic dosing in children in the acute phase of treatment or for preventing relapse.14 Thus, from an efficacy standpoint, a medication trial has not officially begun until the therapeutic dose range is reached.
Once dosing is within the therapeutic range, however, pediatric data differs from the adult literature. In most adult psychiatric conditions, higher doses of SSRIs within the therapeutic range are associated with an increased response rate.14,54 In pediatrics, there are few fixed dose trials, and once within the recommended therapeutic range, minimal data supports an association between higher dosing and higher efficacy.14 In general, pediatric guidelines are silent regarding optimal dosing of SSRIs within the recommended dose range, and higher antidepressant doses often result in a more significant adverse effect burden for children. One exception is pediatric OCD, where, similar to adults, the guidelines suggest that higher dosing of SSRIs often is required to induce a therapeutic response as compared to MDD and GAD.31,55
If a child does not respond to adequate first-line treatment (or has a treatment history that cannot be fully verified), repeating these first-line interventions carries little risk and can be quite effective. For example, 60% of adolescents with MDD who did not initially respond to an SSRI demonstrated a significant response when prescribed a second SSRI or venlafaxine (with or without CBT).56
When pediatric patients continue to experience significantly distressing and/or debilitating symptoms (particularly in MDD) despite multiple trials of antidepressants and psychotherapy, practitioners should consider a careful referral to interventional psychiatry services, which can include the more intensive treatments of electroconvulsive therapy, repetitive transcranial magnetic stimulation, or ketamine (see Box 1). Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and under-utilized clinically in pediatric populations.
Continue to: Antidepressants in general...
Antidepressants in general, and SSRIs in particular, are the first-line pharmacotherapy for pediatric anxiety, OCD, and MDD. For PTSD, although they are a first-line treatment in adults, their efficacy has not been demonstrated in children and adolescents. Antidepressants are generally safe, well-tolerated, and effective, with low NNTs (3 to 5 for anxiety and OCD; 4 to 12 in MDD, depending on whether industry trials are included). It is important that clinicians and families be educated about possible adverse effects and their time course in order to anticipate difficulties, ensure adequate informed consent, and monitor appropriately. The black-box warning regarding treatment-emergent suicidal thoughts or behaviors must be discussed (for suggested talking points, see Box 2). The NNH is large (100 to 143), and for many patients, the benefits will outweigh the risks. For pediatric patients who fail to respond to multiple adequate trials of antidepressants and psychotherapy, referrals for interventional psychiatry consultation should be considered.
Bottom Line
Although the evidence base for prescribing antidepressants for children and adolescents is smaller compared to the adult literature, properly understanding and prescribing these agents, and explaining their risks and benefits to families, can make a major difference in patient compliance, satisfaction, and outcomes. Antidepressants (particularly selective serotonin reuptake inhibitors) are the firstline pharmacologic intervention for pediatric patients with anxiety disorders, obsessive-compulsive disorder, or major depressive disorder.
Related Resource
- Bonin L, Moreland CS. Overview of prevention and treatment for pediatric depression. https://www.uptodate.com/contents/overview-of-prevention-and-treatment-for-pediatric-depression. Last reviewed July 2019.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cimetidine • Tagamet
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Box 1
Continuing severe depression is associated with reduced educational attainment and quality of life, as well as increased risk of substance abuse and suicide,1,2 which is the second leading cause of death in individuals age 10 to 24 years.3 Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and underutilized in pediatric patients. Interventional antidepressants in adults include electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), and, most recently, ketamine.
Electroconvulsive therapy is the most effective therapy available for depression in adults, alleviating depressive symptoms in treatment-refractory patients and outperforming both pharmacotherapy4 and rTMS.5 Despite its track record of effectiveness and safety in adults, ECT continues to suffer considerable stigma.4 Cognitive adverse effects and memory problems in adults are generally self-limited, and some aspects of cognition actually improve after ECT as depression lifts.6 The combination of stigma and the concern about possible cognitive adverse effects during periods of brain development have likely impeded the rigorous testing of ECT in treatment-refractory pediatric patients. Several case series and other retrospective analyses suggest, however, that ECT has strong efficacy and limited adverse effects in adolescents who have severe depression or psychotic symptoms.7-9 Despite these positive preliminary data in pediatric patients, and a large body of literature in adults, no controlled trials of ECT have been conducted in the pediatric population, and it remains a rarely used treatment in severe pediatric mental illness.
Repetitive transcranial magnetic stimulation is a technique in which magnetic stimulation is used to activate the left dorsolateral prefrontal cortex (DLPFC), a target thought to be important in the pathophysiology of MDD. Repetitive transcranial magnetic stimulation is FDAapproved to treat medication-refractory major depressive disorder (MDD) in adults, and has been shown to be effective as both a monotherapy10 and an adjunctive treatment.11 The estimated number needed to treat (NNT) for rTMS ranges from 6 to 8, which is quite effective, although less so than ECT (and probably initial pharmacotherapy).5 Similar to ECT, however, there are no large randomized controlled trials (RCTs) in children or adolescents. Pilot RCTs12 and open trials13 suggest that DLPFC rTMS may be effective as an adjunctive treatment, speeding or augmenting response to a selective serotonin reuptake inhibitor in adolescents with MDD. Larger trials studying rTMS in pediatric patients are needed. While rTMS is generally well tolerated, disadvantages include the time-consuming schedule (the initial treatment is typically 5 days/week for several weeks) and local adverse effects of headache and scalp pain.
Ketamine, which traditionally is used as a dissociative anesthetic, is a rapidly emerging novel treatment in adult treatment-refractory MDD. It acts quickly (within hours to days) and cause significant improvement in difficult symptoms such as anhedonia14 and suicidal ideation.15 In adult studies, ketamine has a robust average effect size of >1.2, and an NNT ranging from 3 to 5 in medication-refractory patients.16,17 Ketamine is a glutamatergic modulator, acting outside of the monoamine neurochemical systems traditionally targeted by standard antidepressants.16 The efficacy of ketamine in treatment-refractory adults is impressive, but the effects of a single treatment are ephemeral, dissipating within 1 to 2 weeks, which has led to significant discussion surrounding optimal dosing strategies.16 Although small RCTs in pediatric patients are currently underway, at this time, the only evidence for ketamine for pediatric MDD is based on case series/report data18,19 which was positive.
For all of these interventional modalities, it is critical to refer children with treatmentrefractory disorders to interventionists who have appropriate experience and monitoring capabilities.
References
1. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
2. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
3. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
4. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and metaanalysis. Lancet. 2003;361(9360):799-808.
5. Berlim MT, Van den Eynde F, Daskalakis ZJ. Efficacy and acceptability of high frequency repetitive transcranial magnetic stimulation (rTMS) versus electroconvulsive therapy (ECT) for major depression: a systematic review and meta-analysis of randomized trials. Depress Anxiety. 2013;30(7):614-623.
6. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
7. Jacob P, Gogi PK, Srinath S, et al. Review of electroconvulsive therapy practice from a tertiary child and adolescent psychiatry centre. Asian J Psychiatr. 2014;12(1):95-99.
8. Zhand N, Courtney DB, Flament MF. Use of electroconvulsive therapy in adolescents with treatment-resistant depressive disorders: a case series. J ECT. 2015;31(4):238-245.
9. Puffer CC, Wall CA, Huxsahl JE, et al. A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol. 2016;26(7):632-636.
10. Berlim MT, van den Eynde F, Tovar-Perdomo S, et al. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225-239.
11. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
12. Huang ML, Luo BY, Hu JB, et al. Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: a double-blind, randomized, sham-controlled trial. Aust N Z J Psychiatry. 2012;46(3):257-264.
13. Wall CA, Croarkin PE, Sim LA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry. 2011;72(9):1263-1269.
14. Lally N, Nugent AC, Luckenbaugh DA, et al. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105.
15. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161-166.
16. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
17. McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693-704.
18. Dwyer JB, Beyer C, Wilkinson ST, et al. Ketamine as a treatment for adolescent depression: a case report. J Am Acad Child Adolesc Psychiatry. 2017;56(4):352-354.
19. Cullen KR, Amatya P, Roback MG, et al. Intravenous ketamine for adolescents with treatment-resistant depression: an open-label study. J Child Adolesc Psychopharmacol. 2018;28(7):437-444.
Box 2
Efficacy
- Selective serotonin reuptake inhibitors are the most effective pharmacologic treatment we have for pediatric depression, OCD, and anxiety
- More than one-half of children who are prescribed SSRIs have a significant improvement, regardless of condition
- Based on current estimates, we need to treat 4 to 6 children with an SSRI to find one that will improve who would not improve with placebo
- The clinical benefits of SSRIs generally take a while to accrue; therefore, it is advisable to take the medication for at least 2 to 3 months before concluding that it is ineffective
- In addition to medication, evidence-based psychotherapies provide significant benefit for pediatric depression, OCD, and anxiety
Tolerability
- Most commonly prescribed pediatric antidepressants have been used safely in children for 2 to 3 decades. The safety profiles of SSRIs are among the best of any medications used for children and adolescents
- While many children get better when taking these medications, it’s important that we also talk about potential adverse effects. Some children will experience sleep problems (either sleepier than usual or difficulty sleeping), changes in energy levels, headache, gastrointestinal upset, and dry mouth. These are most likely at the beginning of treatment, or when we increase the dose; they usually are time-limited and go away on their own
- Often adverse effects occur first and the benefits come later. Because it may take at least a few weeks to start to see the mood/anxiety benefits, it’s important for us to talk about any adverse effects your child experiences and remember that they usually are short-lived
Suicidality
- The FDA placed a “black-box” warning on antidepressants after pediatric studies found a small but statistically significant increased risk of reporting suicidal thoughts or behaviors over the short-term compared with placebo
- The increased risk of spontaneously reporting suicidal ideation was quite small. Studies suggested that one would need to treat 100 to 140 children to see 1 child report suicidal ideation compared to placebo. Suicidal ideation is a common symptom in children with depression and anxiety
- Studies found no increased risk when suicidal ideation was systematically assessed using structured rating scales
- In the studies evaluated, there were no completed suicides by patients taking medication or placebo
- Population studies show that higher rates of antidepressant prescriptions are associated with lower rates of attempted and completed teen suicide, which underscores that in general, these medicines treat the underlying causes of suicidality
- No scientific consensus exists on whether these medications are truly associated with an increased risk of new-onset suicidal ideation, or if this association is due to other factors (eg, improvement in anxiety and depressive symptoms that make patients more comfortable to report suicidal ideation spontaneously)
- Regardless, the FDA recommends frequent monitoring of children for suicidal thoughts when these medications are started. This should be done anyway in children experiencing depression and anxiety, and it’s why we will plan to have more frequent appointments as the medication is initiated
OCD: obsessive-compulsive disorder; SSRIs: selective serotonin reuptake inhibitors
1. Williams SB, O’Connor EA, Eder M, et al. Screening for child and adolescent depression in primary care settings: a systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2009;123(4):e716-e735. doi: 10.1542/peds.2008-2415.
2. Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002-1014.
3. Lewinsohn PM, Clarke GN, Seeley JR, et al. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. J Am Acad Child Adolesc Psychiatry. 1994;33(6):809-818.
4. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
5. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
6. Keenan-Miller D, Hammen CL, Brennan PA. Health outcomes related to early adolescent depression. J Adolesc Health. 2007; 41(3): 256-62.
7. Shaffer D, Gould MS, Fisher P, et al. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53(4):339-348.
8. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
9. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
10. Wittchen HU, Nelson CB, Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol Med. 1998;28(1):109-126.
11. Foley DL, Goldston DB, Costello EJ, et al. Proximal psychiatric risk factors for suicidality in youth: the Great Smoky Mountains Study. Arch Gen Psychiatry. 2006;63(9):1017-1024.
12. Cheung A, Sacks D, Dewa CS, et al. Pediatric prescribing practices and the FDA black-box warning on antidepressants. J Dev Behav Pediatr. 2008 29(3):213-215.
13. Walkup JT. Antidepressant efficacy for depression in children and adolescents: industry- and NIMH-funded studies. Am J Psychiatry. 2017;174(5):430-437.
14. Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173(2):174-183.
15. Varigonda AL, Jakubovski E, Taylor MJ, et al. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(7):557-564.
16. Strawn JR, Welge JA, Wehry AM, et al. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149-157.
17. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;280(20):1752-1756.
18. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
19. Kennard BD, Silva SG, Tonev S, et al. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48(2):186-195.
20. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388(10047):881-890.
21. Cohen JA, Mannarino AP, Perel JM, et al. A pilot randomized controlled trial of combined trauma-focused CBT and sertraline for childhood PTSD symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46(7):811-819.
22. Robb AS, Cueva JE, Sporn J, et al. Sertraline treatment of children and adolescents with posttraumatic stress disorder: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2010;20(6):463-471.
23. Diehle J, Opmeer BC, Boer F, et al. Trauma-focused cognitive behavioral therapy or eye movement desensitization and reprocessing: what works in children with posttraumatic stress symptoms? A randomized controlled trial. Eur Child Adolesc Psychiatry. 2015;24(2):227-236.
24. Aiyer R, Barkin RL, Bhatia A. Treatment of neuropathic pain with venlafaxine: a systematic review. Pain Med. 2017;18(10):1999-2012.
25. Barrickman LL, Perry PJ, Allen AJ, et al. Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34(5):649-657.
26. Monuteaux MC, Spencer TJ, Faraone SV, et al. A randomized, placebo-controlled clinical trial of bupropion for the prevention of smoking in children and adolescents with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2007;68(7):1094-1101.
27. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
28. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656.
29. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder--a multicenter trial. J Am Acad Child Adolesc Psychiatry. 1992;31(1):45-49.
30. Caldwell PH, Sureshkumar P, Wong WC. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2016;(1):CD002117.
31. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(10):851-859.e2. doi: 10.1016/j.jaac.2016.07.768.
32. Walkup J, Labellarte M. Complications of SSRI treatment. J Child Adolesc Psychopharmacol. 2001;11(1):1-4.
33. Leo RJ, Lichter DG, Hershey LA. Parkinsonism associated with fluoxetine and cimetidine: a case report. J Geriatr Psychiatry Neurol. 1995;8(4):231-233.
34. Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283-293.
35. Bernstein GA, Borchardt CM, Perwien AR, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry. 2000;39(3): 276-283.
36. Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1-2):159-169.
37. Reinblatt SP, DosReis S, Walkup JT, et al. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119-126.
38. Goldsmith M, Singh M, Chang K. Antidepressants and psychostimulants in pediatric populations: is there an association with mania? Paediatr Drugs. 2011;13(4): 225-243.
39. Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
40. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
41. McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):107-125.
42. Dobry Y, Rice T, Sher L. Ecstasy use and serotonin syndrome: a neglected danger to adolescents and young adults prescribed selective serotonin reuptake inhibitors. Int J Adolesc Med Health. 2013; 25(3):193-199.
43. Schwartz AR, Pizon AF, Brooks DE. Dextromethorphan-induced serotonin syndrome. Clin Toxicol (Phila). 2008;46(8):771-773.
44. Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164(9):1356-1363.
45. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332-339.
46. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696.
47. Sharma T, Guski LS, Freund N, et al. Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ. 2016;352: i65. doi: https://doi.org/10.1136/bmj.i65.
48. Olfson M, Shaffer D, Marcus SC, et al. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry. 2003;60(10):978-982.
49. Garland JE, Kutcher S, Virani A, et al. Update on the Use of SSRIs and SNRIs with children and adolescents in clinical practice. J Can Acad Child Adolesc Psychiatry. 2016;25(1):4-10.
50. Bridge JA, Barbe RP, Birmaher B, et al. Emergent suicidality in a clinical psychotherapy trial for adolescent depression. Am J Psychiatry. 2005;162(11):2173-2175.
51. Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503-1526.
52. Ravizza L, Maina G, Bogetto F, et al. Long term treatment of obsessive-compulsive disorder. CNS Drugs. 1998;10(4):247-255.
53. Hosenbocus S, Chahal R. SSRIs and SNRIs: a review of the discontinuation syndrome in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2011;20(1):60-67.
54. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855.
55. Issari Y, Jakubovski E, Bartley CA, et al. Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis. J Clin Psychiatry. 2016; 77(5):e605-e611. doi: 10.4088/JCP.14r09758.
56. Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901-913.
Major depressive disorder (MDD) is a significant pediatric health problem, with a lifetime prevalence as high as 20% by the end of adolescence.1-3 Major depressive disorder in adolescence is associated with significant morbidity, including poor social functioning, school difficulties, early pregnancy, and increased risk of physical illness and substance abuse.4-6 It is also linked with significant mortality, with increased risk for suicide, which is now the second leading cause of death in individuals age 10 to 24 years.1,7,8
As their name suggests, antidepressants comprise a group of medications that are used to treat MDD; they are also, however, first-line agents for generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD) in adults. Anxiety disorders (including GAD and other anxiety diagnoses) and PTSD are also common in childhood and adolescence with a combined lifetime prevalence ranging from 15% to 30%.9,10 These disorders are also associated with increased risk of suicide.11 For all of these disorders, depending on the severity of presentation and the preference of the patient, treatments are often a combination of psychotherapy and psychopharmacology.
Clinicians face several challenges when considering antidepressants for pediatric patients. Pediatricians and psychiatrists need to understand whether these medications work in children and adolescents, and whether there are unique developmental safety and tolerability issues. The evidence base in child psychiatry is considerably smaller compared with that of adult psychiatry. From this more limited evidence base also came the controversial “black-box” warning regarding a risk of emergent suicidality when starting antidepressants that accompanies all antidepressants for pediatric, but not adult, patients. This warning has had major effects on clinical encounters with children experiencing depression, including altering clinician prescribing behavior.12
Do antidepressants work in children?
Selective serotonin reuptake inhibitors. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly used class of antidepressants in both children and adults.13 While only a few SSRIs are FDA-approved for pediatric indications, the lack of FDA approval is typically related to a lack of sufficient testing in randomized controlled trials (RCTs) for specific pediatric indications, rather than to demonstrable differences in efficacy between antidepressant agents. Since there is currently no data to suggest inferiority of one agent compared to another in children or adults,14,15 efficacy data will be discussed here as applied to the class of SSRIs, generalizing from RCTs conducted on individual drugs. Table 1 lists FDA indications and dosing information for individual antidepressants.

There is strong evidence that SSRIs are effective for treating pediatric anxiety disorders (eg, social anxiety disorder and GAD)16 and OCD,17 with numbers needed to treat (NNT) between 3 and 5. For both of these disorders, SSRIs combined with cognitive-behavioral therapy (CBT) have the highest likelihood of improving symptoms or achieving remission.17,18
Selective serotonin reuptake inhibitors are also effective for treating pediatric MDD; however, the literature is more complex for this disorder compared to GAD and OCD as there are considerable differences in effect sizes between National Institute of Mental Health (NIMH)–funded studies and industry-sponsored trials.13 The major NIMH-sponsored adolescent depression trial, TADS (Treatment for Adolescents and Depression Study), showed that SSRIs (fluoxetine in this case) were quite effective, with an NNT of 4 over the acute phase (12 weeks).19 Ultimately, approximately 80% of adolescents improved over 9 months. Many industry-sponsored trials for MDD in pediatric patients had large placebo response rates (approximately 60%), which resulted in smaller between-group differences, and estimates of an NNT closer to 12,13 which has muddied the waters in meta-analyses that include all trials.20 Improvement in depressive symptoms also appears to be bolstered by concomitant CBT in MDD,19 but not as robustly as in GAD and OCD. While the full benefit of SSRIs for depression may take as long as 8 weeks, a meta-analysis of depression studies of pediatric patients suggests that significant benefits from placebo are observed as early as 2 weeks, and that further treatment gains are minimal after 4 weeks.15 Thus, we recommend at least a 4- to 6-week trial at therapeutic dosing before deeming a medication a treatment failure.
Continue to: Posttraumatic stress disorder...
Posttraumatic stress disorder is a fourth disorder in which SSRIs are a first-line treatment in adults. The data for using SSRIs to treat pediatric patients with PTSD is scant, with only a few RCTs, and no large NIMH-funded trials. Randomized controlled trials have not demonstrated significant differences between SSRIs and placebo21,22 and thus the current first-line recommendation in pediatric PTSD remains trauma-focused therapy, with good evidence for trauma-focused CBT.23 Practically speaking, there can be considerable overlap of PTSD, depression, and anxiety symptoms in children,23 and children with a history of trauma who also have comorbid MDD may benefit from medication if their symptoms persist despite an adequate trial of psychotherapy.
Taken together, the current evidence suggests that SSRIs are often effective in pediatric GAD, OCD, and MDD, with low NNTs (ranging from 3 to 5 based on NIMH-funded trials) for all of these disorders; there is not yet sufficient evidence of efficacy in pediatric patients with PTSD.
Fluoxetine has been studied more intensively than other SSRIs (for example, it was the antidepressant used in the TADS trial), and thus has the largest evidence base. For this reason, fluoxetine is often considered the first of the first-line options. Additionally, fluoxetine has a longer half-life than other antidepressants, which may make it more effective in situations where patients are likely to miss doses, and results in a lower risk of withdrawal symptoms when stopped due to “self-tapering.”
SNRIs and atypical antidepressants. Other antidepressants commonly used in pediatric patients but with far less evidence of efficacy include serotonin-norepinephrine reuptake inhibitors (SNRIs) and the atypical antidepressants bupropion and mirtazapine. The SNRI duloxetine is FDA-approved for treating GAD in children age 7 to 17, but there are no other pediatric indications for duloxetine, or for the other SNRIs.
In general, adverse effect profiles are worse for SNRIs compared to SSRIs, further limiting their utility. While there are no pediatric studies demonstrating SNRI efficacy for neuropathic pain, good data exists in adults.24 Thus, an SNRI could be a reasonable option if a pediatric patient has failed prior adequate SSRI trials and also has comorbid neuropathic pain.
Continue to: Neither bupropion nor mirtazapine...
Neither bupropion nor mirtazapine have undergone rigorous testing in pediatric patients, and therefore these agents should generally be considered only once other first-line treatments have failed. Bupropion has been evaluated for attention-deficit/hyperactivity disorder (ADHD)25 and for adolescent smoking cessation.26 However, the evidence is weak, and bupropion is not considered a first-line option for children and adolescents.
Tricyclic antidepressants. Randomized controlled trials have demonstrated that tricyclic antidepressants (TCAs) are efficacious for treating several pediatric conditions; however, their significant side effect profile, their monitoring requirements, as well as their lethality in overdose has left them replaced by SSRIs in most cases. That said, they can be appropriate in refractory ADHD (desipramine27,28) and refractory OCD (clomipramine is FDA-approved for this indication29); they are considered a third-line treatment for enuresis.30
Why did my patient stop the medication?
Common adverse effects. Although the greatest benefit of antidepressant medications compared with placebo is achieved relatively early on in treatment, it generally takes time for these benefits to accrue and become clinically apparent.15,31 By contrast, most adverse effects of antidepressants present and are at their most severe early in treatment. The combination of early adverse effects and delayed efficacy leads many patients, families, and clinicians to discontinue medications before they have an adequate chance to work. Thus, it is imperative to provide psychoeducation before starting a medication about the typical time-course of improvement and adverse effects (Table 2).

Adverse effects of SSRIs often appear or worsen transiently during initiation of a medication, during a dose increase,32 or, theoretically, with the addition of a medication that interferes with SSRI metabolism (eg, cimetidine inhibition of cytochrome P450 2D6).33 If families are prepared for this phenomenon and the therapeutic alliance is adequate, adverse effects can be tolerated to allow for a full medication trial. Common adverse effects of SSRIs include sleep problems (insomnia/sedation), gastrointestinal upset, sexual dysfunction, dry mouth, and hyperhidrosis. Although SSRIs differ somewhat in the frequency of these effects, as a class, they are more similar than different. Adequate psychoeducation is especially imperative in the treatment of OCD and anxiety disorders, where there is limited evidence of efficacy for any non-serotonergic antidepressants.
Serotonin-norepinephrine reuptake inhibitors are not considered first-line medications because of the reduced evidence base compared to SSRIs and their enhanced adverse effect profiles. Because SNRIs partially share a mechanism of action with SSRIs, they also share portions of the adverse effects profile. However, SNRIs have the additional adverse effect of hypertension, which is related to their noradrenergic activity. Thus, it is reasonable to obtain a baseline blood pressure before initiating an SNRI, as well as periodically after initiation and during dose increases, particularly if the patient has other risk factors for hypertension.34
Continue to: Although TCAs have efficacy...
Although TCAs have efficacy in some pediatric disorders,27-29,35 their adverse effect profile limits their use. Tricyclic antidepressants are highly anticholinergic (causing dizziness secondary to orthostatic hypotension, dry mouth, and urinary retention) and antihistaminergic (causing sedation and weight gain). Additionally, TCAs lower the seizure threshold and have adverse cardiac effects relating to their anti-alpha-1 adrenergic activity, resulting in dose-dependent increases in the QTc and cardiac toxicity in overdose that could lead to arrhythmia and death. These medications have their place, but their use requires careful informed consent, clear treatment goals, and baseline and periodic cardiac monitoring (via electrocardiogram).
Serious adverse effects. Clinicians may be hesitant to prescribe antidepressants for pediatric patients because of the potential for more serious adverse effects, including severe behavioral activation syndromes, serotonin syndrome, and emergent suicidality. However, current FDA-approved antidepressants arguably have one of the most positive risk/benefit profiles of any orally-administered medication approved for pediatric patients. Having a strong understanding of the evidence is critical to evaluating when it is appropriate to prescribe an antidepressant, how to properly monitor the patient, and how to obtain accurate informed consent.
Pediatric behavioral activation syndrome. Many clinicians report that children receiving antidepressants experience a pediatric behavioral activation syndrome, which exists along a spectrum from mild activation, increased energy, insomnia, or irritability up through more severe presentations of agitation, hyperactivity, or possibly mania. A recent meta-analysis suggested a positive association between antidepressant use and activation events on the milder end of this spectrum in pediatric patients with non-OCD anxiety disorders,16 and it is thought that compared with adolescents, younger children are more susceptible to activation adverse effects.36 The likelihood of activation events has been associated with higher antidepressant plasma levels,37 suggesting that dose or individual differences in metabolism may play a role. At the severe end of the spectrum, the risk of induction of mania in pediatric patients with depression or anxiety is relatively rare (<2%) and not statistically different from placebo in RCTs of pediatric participants.38 Meta-analyses of larger randomized, placebo-controlled trials of adults do not support the idea that SSRIs and other second-generation antidepressants carry an increased risk of mania compared with placebo.39,40 Children or adolescents with bona fide bipolar disorder (ie, patients who have had observed mania that meets all DSM-5 criteria) should be treated with a mood-stabilizing agent or antipsychotic if prescribed an antidepressant.41 These clear-cut cases are, however, relatively rare, and more often clinicians are confronted with ambiguous cases that include a family history of bipolar disorder along with “softer” symptoms of irritability, intrusiveness, or aggression. In these children, SSRIs may be appropriate for depressive, OCD, or anxiety symptoms, and should be strongly considered before prescribing antipsychotics or mood stabilizers, as long as initiated with proper monitoring.
Serotonin syndrome is a life-threatening condition caused by excess synaptic serotonin. It is characterized by confusion, sweating, diarrhea, hypertension, hyperthermia, and tachycardia. At its most severe, serotonin syndrome can result in seizures, arrhythmias, and death. The risk of serotonin syndrome is very low when using an SSRI as monotherapy. Risk increases with polypharmacy, particularly unexamined polypharmacy when multiple serotonergic agents are inadvertently on board. Commonly used serotonergic agents include other antidepressants, migraine medications (eg, triptans), some pain medications, and the cough suppressant dextromethorphan.
The easiest way to mitigate the risk of serotonin syndrome is to use an interaction index computer program, which can help ensure that the interacting agents are not prescribed without first discussing the risks and benefits. It is important to teach adolescents that certain recreational drugs are highly serotonergic and can cause serious interactions with antidepressants. For example, recreational use of dextromethorphan or 3,4-methylenedioxymethamphetamine (MDMA; commonly known as “ecstasy”) has been associated with serotonin syndrome in adolescents taking antidepressant medications.42,43
Continue to: Suicidality
Suicidality. The black-box warning regarding a risk of emergent suicidality when starting antidepressant treatment in children is controversial.44 The prospect that a medication intended to ameliorate depression might instead risk increasing suicidal thinking is alarming to parents and clinicians alike. To appropriately weigh and discuss the risks and benefits with families, it is important to understand the data upon which the warning is based.

In 2004, the FDA commissioned a review of 23 antidepressant trials, both published and unpublished, pooling studies across multiple indications (MDD, OCD, anxiety, and ADHD) and multiple antidepressant classes. This meta-analysis, which included nearly 4,400 pediatric patients, found a small but statistically significant increase in spontaneously-reported suicidal thoughts or actions, with a risk difference of 1% (95% confidence interval [CI], 1% to 2%).45 These data suggest that if one treats 100 pediatric patients, 1 to 2 of them may experience short-term increases in suicidal thinking or behavior.45 There were no differences in suicidal thinking when assessed systematically (ie, when all subjects reported symptoms of suicidal ideation on structured rating scales), and there were no completed suicides.45 A subsequent analysis that included 27 pediatric trials suggested an even lower, although still significant, risk difference (<1%), yielding a number needed to harm (NNH) of 143.46 Thus, with low NNT for efficacy (3 to 6) and relatively high NNH for emergent suicidal thoughts or behaviors (100 to 143), for many patients the benefits will outweigh the risks.

Figure 1, Figure 2, and Figure 3 are Cates plots that depict the absolute benefits of antidepressants compared with the risk of suicidality for pediatric patients with MDD, OCD, and anxiety disorders. Recent meta-analyses have suggested that the increased risk of suicidality in antidepressant trials is specific to studies that included children and adolescents, and is not observed in adult studies. A meta-analysis of 70 trials involving 18,526 participants suggested that the odds ratio of suicidality in trials of children and adolescents was 2.39 (95% CI, 1.31 to 4.33) compared with 0.81 (95% CI, 0.51 to 1.28) in adults.47 Additionally, a network meta-analysis exclusively focusing on pediatric antidepressant trials in MDD reported significantly higher suicidality-related adverse events in venlafaxine trials compared with placebo, duloxetine, and several SSRIs (fluoxetine, paroxetine, and escitalopram).20 These data should be interpreted with caution as differences in suicidality detected between agents is quite possibly related to differences in the method of assessment between trials, as opposed to actual differences in risk between agents.

Epidemiologic data further support the use of antidepressants in pediatric patients, showing that antidepressant use is associated with decreased teen suicide attempts and completions,48 and the decline in prescriptions that occurred following the black-box warning was accompanied by a 14% increase in teen suicides.49 Multiple hypotheses have been proposed to explain the pediatric clinical trial findings. One idea is that potential adverse effects of activation, or the intended effects of restoring the motivation, energy, and social engagement that is often impaired in depression, increases the likelihood of thinking about suicide or acting on thoughts. Another theory is that reporting of suicidality may be increased, rather than increased de novo suicidality itself. Antidepressants are effective for treating pediatric anxiety disorders, including social anxiety disorder,16 which could result in more willingness to report. Also, the manner in which adverse effects are generally ascertained in trials might have led to increased spontaneous reporting. In many trials, investigators ask whether participants have any adverse effects in general, and inquire about specific adverse effects only if the family answers affirmatively. Thus, the increased rate of other adverse effects associated with antidepressants (sleep problems, gastrointestinal upset, dry mouth, etc.) might trigger a specific question regarding suicidal ideation, which the child or family then may be more likely to report. Alternatively, any type of psychiatric treatment could increase an individual’s propensity to report; in adolescent psychotherapy trials, non-medic
Continue to: How long should the antidepressant be continued?
How long should the antidepressant be continued?
Many patients are concerned about how long they may be taking medication, and whether they will be taking an antidepressant “forever.” A treatment course can be broken into an acute phase, wherein remission is achieved and maintained for 6 to 8 weeks. This is followed by a continuation phase, with the goal of relapse prevention, lasting 16 to 20 weeks. The length of the last phase—the maintenance phase—depends both on the child’s history, the underlying therapeutic indication, the adverse effect burden experienced, and the family’s preferences/values. In general, for a first depressive episode, after treating for 1 year, a trial of discontinuation can be attempted with close monitoring. For a second depressive episode, we recommend 2 years of remission on antidepressant therapy before attempting discontinuation. In patients who have had 3 depressive episodes, or have had episodes of high severity, we recommend continuing antidepressant treatment indefinitely. Although much less well studied, the risk of relapse following SSRI discontinuation appears much more significant in OCD, whereas anxiety disorders and MDD have a relatively comparable risk.52
In general, stopping an antidepressant should be done carefully and slowly.
What to do when first-line treatments fail
Adequate psychotherapy? To determine whether children are receiving adequate CBT, ask:
- if the child receives homework from psychotherapy
- if the parents are included in treatment
- if therapy has involved identifying thought patterns that may be contributing to the child’s illness, and
- if the therapist has ever exposed the child to a challenge likely to produce anxiety or distress in a supervised environment and has developed an exposure hierarchy (for conditions with primarily exposure-based therapies, such as OCD or anxiety disorders).
If a family is not receiving most of these elements in psychotherapy, this is a good indicator that they may not be receiving evidence-based CBT.
Continue to: Adequate pharmacotherapy?
Adequate pharmacotherapy? Similarly, when determining the adequacy of previous pharmacotherapy, it is critical to determine whether the child received an adequate dose of medications (at least the FDA-recommended minimum dose) for an adequate duration of time at therapeutic dosing (at least 6 weeks for MDD, 8 weeks for anxiety disorders, and 8 to 12 weeks for pediatric patients with OCD), and that the child actually took the medication regularly during that period. Patient compliance can typically be tracked through checking refill requests or intervals through the patient’s pharmacy. Ensuring proper delivery of first-line treatments is imperative because (1) the adverse effects associated with second-line treatments are often more substantial; (2) the cost in terms of time and money is considerably higher with second-line treatments, and; (3) the evidence regarding the benefits of these treatments is much less certain.
Inadequate dosing is a common reason for non-response in pediatric patients. Therapeutic dose ranges for common antidepressants are displayed in Table 1. Many clinicians underdose antidepressants for pediatric patients initially (and often throughout treatment) because they fear that the typical dose titration used in clinical trials will increase the risk of adverse effects compared with more conservative dosing. There is limited evidence to suggest that this underdosing strategy is likely to be successful; adverse effects attributable to these medications are modest, and most that are experienced early in treatment (eg, headache, increased anxiety or irritability, sleep problems, gastrointestinal upset) are self-limiting and may be coincidental rather than medication-induced. Furthermore, there is no evidence for efficacy of subtherapeutic dosing in children in the acute phase of treatment or for preventing relapse.14 Thus, from an efficacy standpoint, a medication trial has not officially begun until the therapeutic dose range is reached.
Once dosing is within the therapeutic range, however, pediatric data differs from the adult literature. In most adult psychiatric conditions, higher doses of SSRIs within the therapeutic range are associated with an increased response rate.14,54 In pediatrics, there are few fixed dose trials, and once within the recommended therapeutic range, minimal data supports an association between higher dosing and higher efficacy.14 In general, pediatric guidelines are silent regarding optimal dosing of SSRIs within the recommended dose range, and higher antidepressant doses often result in a more significant adverse effect burden for children. One exception is pediatric OCD, where, similar to adults, the guidelines suggest that higher dosing of SSRIs often is required to induce a therapeutic response as compared to MDD and GAD.31,55
If a child does not respond to adequate first-line treatment (or has a treatment history that cannot be fully verified), repeating these first-line interventions carries little risk and can be quite effective. For example, 60% of adolescents with MDD who did not initially respond to an SSRI demonstrated a significant response when prescribed a second SSRI or venlafaxine (with or without CBT).56
When pediatric patients continue to experience significantly distressing and/or debilitating symptoms (particularly in MDD) despite multiple trials of antidepressants and psychotherapy, practitioners should consider a careful referral to interventional psychiatry services, which can include the more intensive treatments of electroconvulsive therapy, repetitive transcranial magnetic stimulation, or ketamine (see Box 1). Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and under-utilized clinically in pediatric populations.
Continue to: Antidepressants in general...
Antidepressants in general, and SSRIs in particular, are the first-line pharmacotherapy for pediatric anxiety, OCD, and MDD. For PTSD, although they are a first-line treatment in adults, their efficacy has not been demonstrated in children and adolescents. Antidepressants are generally safe, well-tolerated, and effective, with low NNTs (3 to 5 for anxiety and OCD; 4 to 12 in MDD, depending on whether industry trials are included). It is important that clinicians and families be educated about possible adverse effects and their time course in order to anticipate difficulties, ensure adequate informed consent, and monitor appropriately. The black-box warning regarding treatment-emergent suicidal thoughts or behaviors must be discussed (for suggested talking points, see Box 2). The NNH is large (100 to 143), and for many patients, the benefits will outweigh the risks. For pediatric patients who fail to respond to multiple adequate trials of antidepressants and psychotherapy, referrals for interventional psychiatry consultation should be considered.
Bottom Line
Although the evidence base for prescribing antidepressants for children and adolescents is smaller compared to the adult literature, properly understanding and prescribing these agents, and explaining their risks and benefits to families, can make a major difference in patient compliance, satisfaction, and outcomes. Antidepressants (particularly selective serotonin reuptake inhibitors) are the firstline pharmacologic intervention for pediatric patients with anxiety disorders, obsessive-compulsive disorder, or major depressive disorder.
Related Resource
- Bonin L, Moreland CS. Overview of prevention and treatment for pediatric depression. https://www.uptodate.com/contents/overview-of-prevention-and-treatment-for-pediatric-depression. Last reviewed July 2019.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cimetidine • Tagamet
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Box 1
Continuing severe depression is associated with reduced educational attainment and quality of life, as well as increased risk of substance abuse and suicide,1,2 which is the second leading cause of death in individuals age 10 to 24 years.3 Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and underutilized in pediatric patients. Interventional antidepressants in adults include electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), and, most recently, ketamine.
Electroconvulsive therapy is the most effective therapy available for depression in adults, alleviating depressive symptoms in treatment-refractory patients and outperforming both pharmacotherapy4 and rTMS.5 Despite its track record of effectiveness and safety in adults, ECT continues to suffer considerable stigma.4 Cognitive adverse effects and memory problems in adults are generally self-limited, and some aspects of cognition actually improve after ECT as depression lifts.6 The combination of stigma and the concern about possible cognitive adverse effects during periods of brain development have likely impeded the rigorous testing of ECT in treatment-refractory pediatric patients. Several case series and other retrospective analyses suggest, however, that ECT has strong efficacy and limited adverse effects in adolescents who have severe depression or psychotic symptoms.7-9 Despite these positive preliminary data in pediatric patients, and a large body of literature in adults, no controlled trials of ECT have been conducted in the pediatric population, and it remains a rarely used treatment in severe pediatric mental illness.
Repetitive transcranial magnetic stimulation is a technique in which magnetic stimulation is used to activate the left dorsolateral prefrontal cortex (DLPFC), a target thought to be important in the pathophysiology of MDD. Repetitive transcranial magnetic stimulation is FDAapproved to treat medication-refractory major depressive disorder (MDD) in adults, and has been shown to be effective as both a monotherapy10 and an adjunctive treatment.11 The estimated number needed to treat (NNT) for rTMS ranges from 6 to 8, which is quite effective, although less so than ECT (and probably initial pharmacotherapy).5 Similar to ECT, however, there are no large randomized controlled trials (RCTs) in children or adolescents. Pilot RCTs12 and open trials13 suggest that DLPFC rTMS may be effective as an adjunctive treatment, speeding or augmenting response to a selective serotonin reuptake inhibitor in adolescents with MDD. Larger trials studying rTMS in pediatric patients are needed. While rTMS is generally well tolerated, disadvantages include the time-consuming schedule (the initial treatment is typically 5 days/week for several weeks) and local adverse effects of headache and scalp pain.
Ketamine, which traditionally is used as a dissociative anesthetic, is a rapidly emerging novel treatment in adult treatment-refractory MDD. It acts quickly (within hours to days) and cause significant improvement in difficult symptoms such as anhedonia14 and suicidal ideation.15 In adult studies, ketamine has a robust average effect size of >1.2, and an NNT ranging from 3 to 5 in medication-refractory patients.16,17 Ketamine is a glutamatergic modulator, acting outside of the monoamine neurochemical systems traditionally targeted by standard antidepressants.16 The efficacy of ketamine in treatment-refractory adults is impressive, but the effects of a single treatment are ephemeral, dissipating within 1 to 2 weeks, which has led to significant discussion surrounding optimal dosing strategies.16 Although small RCTs in pediatric patients are currently underway, at this time, the only evidence for ketamine for pediatric MDD is based on case series/report data18,19 which was positive.
For all of these interventional modalities, it is critical to refer children with treatmentrefractory disorders to interventionists who have appropriate experience and monitoring capabilities.
References
1. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
2. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
3. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
4. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and metaanalysis. Lancet. 2003;361(9360):799-808.
5. Berlim MT, Van den Eynde F, Daskalakis ZJ. Efficacy and acceptability of high frequency repetitive transcranial magnetic stimulation (rTMS) versus electroconvulsive therapy (ECT) for major depression: a systematic review and meta-analysis of randomized trials. Depress Anxiety. 2013;30(7):614-623.
6. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
7. Jacob P, Gogi PK, Srinath S, et al. Review of electroconvulsive therapy practice from a tertiary child and adolescent psychiatry centre. Asian J Psychiatr. 2014;12(1):95-99.
8. Zhand N, Courtney DB, Flament MF. Use of electroconvulsive therapy in adolescents with treatment-resistant depressive disorders: a case series. J ECT. 2015;31(4):238-245.
9. Puffer CC, Wall CA, Huxsahl JE, et al. A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol. 2016;26(7):632-636.
10. Berlim MT, van den Eynde F, Tovar-Perdomo S, et al. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225-239.
11. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
12. Huang ML, Luo BY, Hu JB, et al. Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: a double-blind, randomized, sham-controlled trial. Aust N Z J Psychiatry. 2012;46(3):257-264.
13. Wall CA, Croarkin PE, Sim LA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry. 2011;72(9):1263-1269.
14. Lally N, Nugent AC, Luckenbaugh DA, et al. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105.
15. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161-166.
16. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
17. McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693-704.
18. Dwyer JB, Beyer C, Wilkinson ST, et al. Ketamine as a treatment for adolescent depression: a case report. J Am Acad Child Adolesc Psychiatry. 2017;56(4):352-354.
19. Cullen KR, Amatya P, Roback MG, et al. Intravenous ketamine for adolescents with treatment-resistant depression: an open-label study. J Child Adolesc Psychopharmacol. 2018;28(7):437-444.
Box 2
Efficacy
- Selective serotonin reuptake inhibitors are the most effective pharmacologic treatment we have for pediatric depression, OCD, and anxiety
- More than one-half of children who are prescribed SSRIs have a significant improvement, regardless of condition
- Based on current estimates, we need to treat 4 to 6 children with an SSRI to find one that will improve who would not improve with placebo
- The clinical benefits of SSRIs generally take a while to accrue; therefore, it is advisable to take the medication for at least 2 to 3 months before concluding that it is ineffective
- In addition to medication, evidence-based psychotherapies provide significant benefit for pediatric depression, OCD, and anxiety
Tolerability
- Most commonly prescribed pediatric antidepressants have been used safely in children for 2 to 3 decades. The safety profiles of SSRIs are among the best of any medications used for children and adolescents
- While many children get better when taking these medications, it’s important that we also talk about potential adverse effects. Some children will experience sleep problems (either sleepier than usual or difficulty sleeping), changes in energy levels, headache, gastrointestinal upset, and dry mouth. These are most likely at the beginning of treatment, or when we increase the dose; they usually are time-limited and go away on their own
- Often adverse effects occur first and the benefits come later. Because it may take at least a few weeks to start to see the mood/anxiety benefits, it’s important for us to talk about any adverse effects your child experiences and remember that they usually are short-lived
Suicidality
- The FDA placed a “black-box” warning on antidepressants after pediatric studies found a small but statistically significant increased risk of reporting suicidal thoughts or behaviors over the short-term compared with placebo
- The increased risk of spontaneously reporting suicidal ideation was quite small. Studies suggested that one would need to treat 100 to 140 children to see 1 child report suicidal ideation compared to placebo. Suicidal ideation is a common symptom in children with depression and anxiety
- Studies found no increased risk when suicidal ideation was systematically assessed using structured rating scales
- In the studies evaluated, there were no completed suicides by patients taking medication or placebo
- Population studies show that higher rates of antidepressant prescriptions are associated with lower rates of attempted and completed teen suicide, which underscores that in general, these medicines treat the underlying causes of suicidality
- No scientific consensus exists on whether these medications are truly associated with an increased risk of new-onset suicidal ideation, or if this association is due to other factors (eg, improvement in anxiety and depressive symptoms that make patients more comfortable to report suicidal ideation spontaneously)
- Regardless, the FDA recommends frequent monitoring of children for suicidal thoughts when these medications are started. This should be done anyway in children experiencing depression and anxiety, and it’s why we will plan to have more frequent appointments as the medication is initiated
OCD: obsessive-compulsive disorder; SSRIs: selective serotonin reuptake inhibitors
Major depressive disorder (MDD) is a significant pediatric health problem, with a lifetime prevalence as high as 20% by the end of adolescence.1-3 Major depressive disorder in adolescence is associated with significant morbidity, including poor social functioning, school difficulties, early pregnancy, and increased risk of physical illness and substance abuse.4-6 It is also linked with significant mortality, with increased risk for suicide, which is now the second leading cause of death in individuals age 10 to 24 years.1,7,8
As their name suggests, antidepressants comprise a group of medications that are used to treat MDD; they are also, however, first-line agents for generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD) in adults. Anxiety disorders (including GAD and other anxiety diagnoses) and PTSD are also common in childhood and adolescence with a combined lifetime prevalence ranging from 15% to 30%.9,10 These disorders are also associated with increased risk of suicide.11 For all of these disorders, depending on the severity of presentation and the preference of the patient, treatments are often a combination of psychotherapy and psychopharmacology.
Clinicians face several challenges when considering antidepressants for pediatric patients. Pediatricians and psychiatrists need to understand whether these medications work in children and adolescents, and whether there are unique developmental safety and tolerability issues. The evidence base in child psychiatry is considerably smaller compared with that of adult psychiatry. From this more limited evidence base also came the controversial “black-box” warning regarding a risk of emergent suicidality when starting antidepressants that accompanies all antidepressants for pediatric, but not adult, patients. This warning has had major effects on clinical encounters with children experiencing depression, including altering clinician prescribing behavior.12
Do antidepressants work in children?
Selective serotonin reuptake inhibitors. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly used class of antidepressants in both children and adults.13 While only a few SSRIs are FDA-approved for pediatric indications, the lack of FDA approval is typically related to a lack of sufficient testing in randomized controlled trials (RCTs) for specific pediatric indications, rather than to demonstrable differences in efficacy between antidepressant agents. Since there is currently no data to suggest inferiority of one agent compared to another in children or adults,14,15 efficacy data will be discussed here as applied to the class of SSRIs, generalizing from RCTs conducted on individual drugs. Table 1 lists FDA indications and dosing information for individual antidepressants.

There is strong evidence that SSRIs are effective for treating pediatric anxiety disorders (eg, social anxiety disorder and GAD)16 and OCD,17 with numbers needed to treat (NNT) between 3 and 5. For both of these disorders, SSRIs combined with cognitive-behavioral therapy (CBT) have the highest likelihood of improving symptoms or achieving remission.17,18
Selective serotonin reuptake inhibitors are also effective for treating pediatric MDD; however, the literature is more complex for this disorder compared to GAD and OCD as there are considerable differences in effect sizes between National Institute of Mental Health (NIMH)–funded studies and industry-sponsored trials.13 The major NIMH-sponsored adolescent depression trial, TADS (Treatment for Adolescents and Depression Study), showed that SSRIs (fluoxetine in this case) were quite effective, with an NNT of 4 over the acute phase (12 weeks).19 Ultimately, approximately 80% of adolescents improved over 9 months. Many industry-sponsored trials for MDD in pediatric patients had large placebo response rates (approximately 60%), which resulted in smaller between-group differences, and estimates of an NNT closer to 12,13 which has muddied the waters in meta-analyses that include all trials.20 Improvement in depressive symptoms also appears to be bolstered by concomitant CBT in MDD,19 but not as robustly as in GAD and OCD. While the full benefit of SSRIs for depression may take as long as 8 weeks, a meta-analysis of depression studies of pediatric patients suggests that significant benefits from placebo are observed as early as 2 weeks, and that further treatment gains are minimal after 4 weeks.15 Thus, we recommend at least a 4- to 6-week trial at therapeutic dosing before deeming a medication a treatment failure.
Continue to: Posttraumatic stress disorder...
Posttraumatic stress disorder is a fourth disorder in which SSRIs are a first-line treatment in adults. The data for using SSRIs to treat pediatric patients with PTSD is scant, with only a few RCTs, and no large NIMH-funded trials. Randomized controlled trials have not demonstrated significant differences between SSRIs and placebo21,22 and thus the current first-line recommendation in pediatric PTSD remains trauma-focused therapy, with good evidence for trauma-focused CBT.23 Practically speaking, there can be considerable overlap of PTSD, depression, and anxiety symptoms in children,23 and children with a history of trauma who also have comorbid MDD may benefit from medication if their symptoms persist despite an adequate trial of psychotherapy.
Taken together, the current evidence suggests that SSRIs are often effective in pediatric GAD, OCD, and MDD, with low NNTs (ranging from 3 to 5 based on NIMH-funded trials) for all of these disorders; there is not yet sufficient evidence of efficacy in pediatric patients with PTSD.
Fluoxetine has been studied more intensively than other SSRIs (for example, it was the antidepressant used in the TADS trial), and thus has the largest evidence base. For this reason, fluoxetine is often considered the first of the first-line options. Additionally, fluoxetine has a longer half-life than other antidepressants, which may make it more effective in situations where patients are likely to miss doses, and results in a lower risk of withdrawal symptoms when stopped due to “self-tapering.”
SNRIs and atypical antidepressants. Other antidepressants commonly used in pediatric patients but with far less evidence of efficacy include serotonin-norepinephrine reuptake inhibitors (SNRIs) and the atypical antidepressants bupropion and mirtazapine. The SNRI duloxetine is FDA-approved for treating GAD in children age 7 to 17, but there are no other pediatric indications for duloxetine, or for the other SNRIs.
In general, adverse effect profiles are worse for SNRIs compared to SSRIs, further limiting their utility. While there are no pediatric studies demonstrating SNRI efficacy for neuropathic pain, good data exists in adults.24 Thus, an SNRI could be a reasonable option if a pediatric patient has failed prior adequate SSRI trials and also has comorbid neuropathic pain.
Continue to: Neither bupropion nor mirtazapine...
Neither bupropion nor mirtazapine have undergone rigorous testing in pediatric patients, and therefore these agents should generally be considered only once other first-line treatments have failed. Bupropion has been evaluated for attention-deficit/hyperactivity disorder (ADHD)25 and for adolescent smoking cessation.26 However, the evidence is weak, and bupropion is not considered a first-line option for children and adolescents.
Tricyclic antidepressants. Randomized controlled trials have demonstrated that tricyclic antidepressants (TCAs) are efficacious for treating several pediatric conditions; however, their significant side effect profile, their monitoring requirements, as well as their lethality in overdose has left them replaced by SSRIs in most cases. That said, they can be appropriate in refractory ADHD (desipramine27,28) and refractory OCD (clomipramine is FDA-approved for this indication29); they are considered a third-line treatment for enuresis.30
Why did my patient stop the medication?
Common adverse effects. Although the greatest benefit of antidepressant medications compared with placebo is achieved relatively early on in treatment, it generally takes time for these benefits to accrue and become clinically apparent.15,31 By contrast, most adverse effects of antidepressants present and are at their most severe early in treatment. The combination of early adverse effects and delayed efficacy leads many patients, families, and clinicians to discontinue medications before they have an adequate chance to work. Thus, it is imperative to provide psychoeducation before starting a medication about the typical time-course of improvement and adverse effects (Table 2).

Adverse effects of SSRIs often appear or worsen transiently during initiation of a medication, during a dose increase,32 or, theoretically, with the addition of a medication that interferes with SSRI metabolism (eg, cimetidine inhibition of cytochrome P450 2D6).33 If families are prepared for this phenomenon and the therapeutic alliance is adequate, adverse effects can be tolerated to allow for a full medication trial. Common adverse effects of SSRIs include sleep problems (insomnia/sedation), gastrointestinal upset, sexual dysfunction, dry mouth, and hyperhidrosis. Although SSRIs differ somewhat in the frequency of these effects, as a class, they are more similar than different. Adequate psychoeducation is especially imperative in the treatment of OCD and anxiety disorders, where there is limited evidence of efficacy for any non-serotonergic antidepressants.
Serotonin-norepinephrine reuptake inhibitors are not considered first-line medications because of the reduced evidence base compared to SSRIs and their enhanced adverse effect profiles. Because SNRIs partially share a mechanism of action with SSRIs, they also share portions of the adverse effects profile. However, SNRIs have the additional adverse effect of hypertension, which is related to their noradrenergic activity. Thus, it is reasonable to obtain a baseline blood pressure before initiating an SNRI, as well as periodically after initiation and during dose increases, particularly if the patient has other risk factors for hypertension.34
Continue to: Although TCAs have efficacy...
Although TCAs have efficacy in some pediatric disorders,27-29,35 their adverse effect profile limits their use. Tricyclic antidepressants are highly anticholinergic (causing dizziness secondary to orthostatic hypotension, dry mouth, and urinary retention) and antihistaminergic (causing sedation and weight gain). Additionally, TCAs lower the seizure threshold and have adverse cardiac effects relating to their anti-alpha-1 adrenergic activity, resulting in dose-dependent increases in the QTc and cardiac toxicity in overdose that could lead to arrhythmia and death. These medications have their place, but their use requires careful informed consent, clear treatment goals, and baseline and periodic cardiac monitoring (via electrocardiogram).
Serious adverse effects. Clinicians may be hesitant to prescribe antidepressants for pediatric patients because of the potential for more serious adverse effects, including severe behavioral activation syndromes, serotonin syndrome, and emergent suicidality. However, current FDA-approved antidepressants arguably have one of the most positive risk/benefit profiles of any orally-administered medication approved for pediatric patients. Having a strong understanding of the evidence is critical to evaluating when it is appropriate to prescribe an antidepressant, how to properly monitor the patient, and how to obtain accurate informed consent.
Pediatric behavioral activation syndrome. Many clinicians report that children receiving antidepressants experience a pediatric behavioral activation syndrome, which exists along a spectrum from mild activation, increased energy, insomnia, or irritability up through more severe presentations of agitation, hyperactivity, or possibly mania. A recent meta-analysis suggested a positive association between antidepressant use and activation events on the milder end of this spectrum in pediatric patients with non-OCD anxiety disorders,16 and it is thought that compared with adolescents, younger children are more susceptible to activation adverse effects.36 The likelihood of activation events has been associated with higher antidepressant plasma levels,37 suggesting that dose or individual differences in metabolism may play a role. At the severe end of the spectrum, the risk of induction of mania in pediatric patients with depression or anxiety is relatively rare (<2%) and not statistically different from placebo in RCTs of pediatric participants.38 Meta-analyses of larger randomized, placebo-controlled trials of adults do not support the idea that SSRIs and other second-generation antidepressants carry an increased risk of mania compared with placebo.39,40 Children or adolescents with bona fide bipolar disorder (ie, patients who have had observed mania that meets all DSM-5 criteria) should be treated with a mood-stabilizing agent or antipsychotic if prescribed an antidepressant.41 These clear-cut cases are, however, relatively rare, and more often clinicians are confronted with ambiguous cases that include a family history of bipolar disorder along with “softer” symptoms of irritability, intrusiveness, or aggression. In these children, SSRIs may be appropriate for depressive, OCD, or anxiety symptoms, and should be strongly considered before prescribing antipsychotics or mood stabilizers, as long as initiated with proper monitoring.
Serotonin syndrome is a life-threatening condition caused by excess synaptic serotonin. It is characterized by confusion, sweating, diarrhea, hypertension, hyperthermia, and tachycardia. At its most severe, serotonin syndrome can result in seizures, arrhythmias, and death. The risk of serotonin syndrome is very low when using an SSRI as monotherapy. Risk increases with polypharmacy, particularly unexamined polypharmacy when multiple serotonergic agents are inadvertently on board. Commonly used serotonergic agents include other antidepressants, migraine medications (eg, triptans), some pain medications, and the cough suppressant dextromethorphan.
The easiest way to mitigate the risk of serotonin syndrome is to use an interaction index computer program, which can help ensure that the interacting agents are not prescribed without first discussing the risks and benefits. It is important to teach adolescents that certain recreational drugs are highly serotonergic and can cause serious interactions with antidepressants. For example, recreational use of dextromethorphan or 3,4-methylenedioxymethamphetamine (MDMA; commonly known as “ecstasy”) has been associated with serotonin syndrome in adolescents taking antidepressant medications.42,43
Continue to: Suicidality
Suicidality. The black-box warning regarding a risk of emergent suicidality when starting antidepressant treatment in children is controversial.44 The prospect that a medication intended to ameliorate depression might instead risk increasing suicidal thinking is alarming to parents and clinicians alike. To appropriately weigh and discuss the risks and benefits with families, it is important to understand the data upon which the warning is based.

In 2004, the FDA commissioned a review of 23 antidepressant trials, both published and unpublished, pooling studies across multiple indications (MDD, OCD, anxiety, and ADHD) and multiple antidepressant classes. This meta-analysis, which included nearly 4,400 pediatric patients, found a small but statistically significant increase in spontaneously-reported suicidal thoughts or actions, with a risk difference of 1% (95% confidence interval [CI], 1% to 2%).45 These data suggest that if one treats 100 pediatric patients, 1 to 2 of them may experience short-term increases in suicidal thinking or behavior.45 There were no differences in suicidal thinking when assessed systematically (ie, when all subjects reported symptoms of suicidal ideation on structured rating scales), and there were no completed suicides.45 A subsequent analysis that included 27 pediatric trials suggested an even lower, although still significant, risk difference (<1%), yielding a number needed to harm (NNH) of 143.46 Thus, with low NNT for efficacy (3 to 6) and relatively high NNH for emergent suicidal thoughts or behaviors (100 to 143), for many patients the benefits will outweigh the risks.

Figure 1, Figure 2, and Figure 3 are Cates plots that depict the absolute benefits of antidepressants compared with the risk of suicidality for pediatric patients with MDD, OCD, and anxiety disorders. Recent meta-analyses have suggested that the increased risk of suicidality in antidepressant trials is specific to studies that included children and adolescents, and is not observed in adult studies. A meta-analysis of 70 trials involving 18,526 participants suggested that the odds ratio of suicidality in trials of children and adolescents was 2.39 (95% CI, 1.31 to 4.33) compared with 0.81 (95% CI, 0.51 to 1.28) in adults.47 Additionally, a network meta-analysis exclusively focusing on pediatric antidepressant trials in MDD reported significantly higher suicidality-related adverse events in venlafaxine trials compared with placebo, duloxetine, and several SSRIs (fluoxetine, paroxetine, and escitalopram).20 These data should be interpreted with caution as differences in suicidality detected between agents is quite possibly related to differences in the method of assessment between trials, as opposed to actual differences in risk between agents.

Epidemiologic data further support the use of antidepressants in pediatric patients, showing that antidepressant use is associated with decreased teen suicide attempts and completions,48 and the decline in prescriptions that occurred following the black-box warning was accompanied by a 14% increase in teen suicides.49 Multiple hypotheses have been proposed to explain the pediatric clinical trial findings. One idea is that potential adverse effects of activation, or the intended effects of restoring the motivation, energy, and social engagement that is often impaired in depression, increases the likelihood of thinking about suicide or acting on thoughts. Another theory is that reporting of suicidality may be increased, rather than increased de novo suicidality itself. Antidepressants are effective for treating pediatric anxiety disorders, including social anxiety disorder,16 which could result in more willingness to report. Also, the manner in which adverse effects are generally ascertained in trials might have led to increased spontaneous reporting. In many trials, investigators ask whether participants have any adverse effects in general, and inquire about specific adverse effects only if the family answers affirmatively. Thus, the increased rate of other adverse effects associated with antidepressants (sleep problems, gastrointestinal upset, dry mouth, etc.) might trigger a specific question regarding suicidal ideation, which the child or family then may be more likely to report. Alternatively, any type of psychiatric treatment could increase an individual’s propensity to report; in adolescent psychotherapy trials, non-medic
Continue to: How long should the antidepressant be continued?
How long should the antidepressant be continued?
Many patients are concerned about how long they may be taking medication, and whether they will be taking an antidepressant “forever.” A treatment course can be broken into an acute phase, wherein remission is achieved and maintained for 6 to 8 weeks. This is followed by a continuation phase, with the goal of relapse prevention, lasting 16 to 20 weeks. The length of the last phase—the maintenance phase—depends both on the child’s history, the underlying therapeutic indication, the adverse effect burden experienced, and the family’s preferences/values. In general, for a first depressive episode, after treating for 1 year, a trial of discontinuation can be attempted with close monitoring. For a second depressive episode, we recommend 2 years of remission on antidepressant therapy before attempting discontinuation. In patients who have had 3 depressive episodes, or have had episodes of high severity, we recommend continuing antidepressant treatment indefinitely. Although much less well studied, the risk of relapse following SSRI discontinuation appears much more significant in OCD, whereas anxiety disorders and MDD have a relatively comparable risk.52
In general, stopping an antidepressant should be done carefully and slowly.
What to do when first-line treatments fail
Adequate psychotherapy? To determine whether children are receiving adequate CBT, ask:
- if the child receives homework from psychotherapy
- if the parents are included in treatment
- if therapy has involved identifying thought patterns that may be contributing to the child’s illness, and
- if the therapist has ever exposed the child to a challenge likely to produce anxiety or distress in a supervised environment and has developed an exposure hierarchy (for conditions with primarily exposure-based therapies, such as OCD or anxiety disorders).
If a family is not receiving most of these elements in psychotherapy, this is a good indicator that they may not be receiving evidence-based CBT.
Continue to: Adequate pharmacotherapy?
Adequate pharmacotherapy? Similarly, when determining the adequacy of previous pharmacotherapy, it is critical to determine whether the child received an adequate dose of medications (at least the FDA-recommended minimum dose) for an adequate duration of time at therapeutic dosing (at least 6 weeks for MDD, 8 weeks for anxiety disorders, and 8 to 12 weeks for pediatric patients with OCD), and that the child actually took the medication regularly during that period. Patient compliance can typically be tracked through checking refill requests or intervals through the patient’s pharmacy. Ensuring proper delivery of first-line treatments is imperative because (1) the adverse effects associated with second-line treatments are often more substantial; (2) the cost in terms of time and money is considerably higher with second-line treatments, and; (3) the evidence regarding the benefits of these treatments is much less certain.
Inadequate dosing is a common reason for non-response in pediatric patients. Therapeutic dose ranges for common antidepressants are displayed in Table 1. Many clinicians underdose antidepressants for pediatric patients initially (and often throughout treatment) because they fear that the typical dose titration used in clinical trials will increase the risk of adverse effects compared with more conservative dosing. There is limited evidence to suggest that this underdosing strategy is likely to be successful; adverse effects attributable to these medications are modest, and most that are experienced early in treatment (eg, headache, increased anxiety or irritability, sleep problems, gastrointestinal upset) are self-limiting and may be coincidental rather than medication-induced. Furthermore, there is no evidence for efficacy of subtherapeutic dosing in children in the acute phase of treatment or for preventing relapse.14 Thus, from an efficacy standpoint, a medication trial has not officially begun until the therapeutic dose range is reached.
Once dosing is within the therapeutic range, however, pediatric data differs from the adult literature. In most adult psychiatric conditions, higher doses of SSRIs within the therapeutic range are associated with an increased response rate.14,54 In pediatrics, there are few fixed dose trials, and once within the recommended therapeutic range, minimal data supports an association between higher dosing and higher efficacy.14 In general, pediatric guidelines are silent regarding optimal dosing of SSRIs within the recommended dose range, and higher antidepressant doses often result in a more significant adverse effect burden for children. One exception is pediatric OCD, where, similar to adults, the guidelines suggest that higher dosing of SSRIs often is required to induce a therapeutic response as compared to MDD and GAD.31,55
If a child does not respond to adequate first-line treatment (or has a treatment history that cannot be fully verified), repeating these first-line interventions carries little risk and can be quite effective. For example, 60% of adolescents with MDD who did not initially respond to an SSRI demonstrated a significant response when prescribed a second SSRI or venlafaxine (with or without CBT).56
When pediatric patients continue to experience significantly distressing and/or debilitating symptoms (particularly in MDD) despite multiple trials of antidepressants and psychotherapy, practitioners should consider a careful referral to interventional psychiatry services, which can include the more intensive treatments of electroconvulsive therapy, repetitive transcranial magnetic stimulation, or ketamine (see Box 1). Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and under-utilized clinically in pediatric populations.
Continue to: Antidepressants in general...
Antidepressants in general, and SSRIs in particular, are the first-line pharmacotherapy for pediatric anxiety, OCD, and MDD. For PTSD, although they are a first-line treatment in adults, their efficacy has not been demonstrated in children and adolescents. Antidepressants are generally safe, well-tolerated, and effective, with low NNTs (3 to 5 for anxiety and OCD; 4 to 12 in MDD, depending on whether industry trials are included). It is important that clinicians and families be educated about possible adverse effects and their time course in order to anticipate difficulties, ensure adequate informed consent, and monitor appropriately. The black-box warning regarding treatment-emergent suicidal thoughts or behaviors must be discussed (for suggested talking points, see Box 2). The NNH is large (100 to 143), and for many patients, the benefits will outweigh the risks. For pediatric patients who fail to respond to multiple adequate trials of antidepressants and psychotherapy, referrals for interventional psychiatry consultation should be considered.
Bottom Line
Although the evidence base for prescribing antidepressants for children and adolescents is smaller compared to the adult literature, properly understanding and prescribing these agents, and explaining their risks and benefits to families, can make a major difference in patient compliance, satisfaction, and outcomes. Antidepressants (particularly selective serotonin reuptake inhibitors) are the firstline pharmacologic intervention for pediatric patients with anxiety disorders, obsessive-compulsive disorder, or major depressive disorder.
Related Resource
- Bonin L, Moreland CS. Overview of prevention and treatment for pediatric depression. https://www.uptodate.com/contents/overview-of-prevention-and-treatment-for-pediatric-depression. Last reviewed July 2019.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Cimetidine • Tagamet
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Box 1
Continuing severe depression is associated with reduced educational attainment and quality of life, as well as increased risk of substance abuse and suicide,1,2 which is the second leading cause of death in individuals age 10 to 24 years.3 Given the substantial morbidity and mortality associated with adolescent depression, interventional psychiatry treatments are under-researched and underutilized in pediatric patients. Interventional antidepressants in adults include electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), and, most recently, ketamine.
Electroconvulsive therapy is the most effective therapy available for depression in adults, alleviating depressive symptoms in treatment-refractory patients and outperforming both pharmacotherapy4 and rTMS.5 Despite its track record of effectiveness and safety in adults, ECT continues to suffer considerable stigma.4 Cognitive adverse effects and memory problems in adults are generally self-limited, and some aspects of cognition actually improve after ECT as depression lifts.6 The combination of stigma and the concern about possible cognitive adverse effects during periods of brain development have likely impeded the rigorous testing of ECT in treatment-refractory pediatric patients. Several case series and other retrospective analyses suggest, however, that ECT has strong efficacy and limited adverse effects in adolescents who have severe depression or psychotic symptoms.7-9 Despite these positive preliminary data in pediatric patients, and a large body of literature in adults, no controlled trials of ECT have been conducted in the pediatric population, and it remains a rarely used treatment in severe pediatric mental illness.
Repetitive transcranial magnetic stimulation is a technique in which magnetic stimulation is used to activate the left dorsolateral prefrontal cortex (DLPFC), a target thought to be important in the pathophysiology of MDD. Repetitive transcranial magnetic stimulation is FDAapproved to treat medication-refractory major depressive disorder (MDD) in adults, and has been shown to be effective as both a monotherapy10 and an adjunctive treatment.11 The estimated number needed to treat (NNT) for rTMS ranges from 6 to 8, which is quite effective, although less so than ECT (and probably initial pharmacotherapy).5 Similar to ECT, however, there are no large randomized controlled trials (RCTs) in children or adolescents. Pilot RCTs12 and open trials13 suggest that DLPFC rTMS may be effective as an adjunctive treatment, speeding or augmenting response to a selective serotonin reuptake inhibitor in adolescents with MDD. Larger trials studying rTMS in pediatric patients are needed. While rTMS is generally well tolerated, disadvantages include the time-consuming schedule (the initial treatment is typically 5 days/week for several weeks) and local adverse effects of headache and scalp pain.
Ketamine, which traditionally is used as a dissociative anesthetic, is a rapidly emerging novel treatment in adult treatment-refractory MDD. It acts quickly (within hours to days) and cause significant improvement in difficult symptoms such as anhedonia14 and suicidal ideation.15 In adult studies, ketamine has a robust average effect size of >1.2, and an NNT ranging from 3 to 5 in medication-refractory patients.16,17 Ketamine is a glutamatergic modulator, acting outside of the monoamine neurochemical systems traditionally targeted by standard antidepressants.16 The efficacy of ketamine in treatment-refractory adults is impressive, but the effects of a single treatment are ephemeral, dissipating within 1 to 2 weeks, which has led to significant discussion surrounding optimal dosing strategies.16 Although small RCTs in pediatric patients are currently underway, at this time, the only evidence for ketamine for pediatric MDD is based on case series/report data18,19 which was positive.
For all of these interventional modalities, it is critical to refer children with treatmentrefractory disorders to interventionists who have appropriate experience and monitoring capabilities.
References
1. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
2. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
3. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
4. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and metaanalysis. Lancet. 2003;361(9360):799-808.
5. Berlim MT, Van den Eynde F, Daskalakis ZJ. Efficacy and acceptability of high frequency repetitive transcranial magnetic stimulation (rTMS) versus electroconvulsive therapy (ECT) for major depression: a systematic review and meta-analysis of randomized trials. Depress Anxiety. 2013;30(7):614-623.
6. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
7. Jacob P, Gogi PK, Srinath S, et al. Review of electroconvulsive therapy practice from a tertiary child and adolescent psychiatry centre. Asian J Psychiatr. 2014;12(1):95-99.
8. Zhand N, Courtney DB, Flament MF. Use of electroconvulsive therapy in adolescents with treatment-resistant depressive disorders: a case series. J ECT. 2015;31(4):238-245.
9. Puffer CC, Wall CA, Huxsahl JE, et al. A 20 year practice review of electroconvulsive therapy for adolescents. J Child Adolesc Psychopharmacol. 2016;26(7):632-636.
10. Berlim MT, van den Eynde F, Tovar-Perdomo S, et al. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225-239.
11. Liu B, Zhang Y, Zhang L, et al. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
12. Huang ML, Luo BY, Hu JB, et al. Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: a double-blind, randomized, sham-controlled trial. Aust N Z J Psychiatry. 2012;46(3):257-264.
13. Wall CA, Croarkin PE, Sim LA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry. 2011;72(9):1263-1269.
14. Lally N, Nugent AC, Luckenbaugh DA, et al. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105.
15. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161-166.
16. Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
17. McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693-704.
18. Dwyer JB, Beyer C, Wilkinson ST, et al. Ketamine as a treatment for adolescent depression: a case report. J Am Acad Child Adolesc Psychiatry. 2017;56(4):352-354.
19. Cullen KR, Amatya P, Roback MG, et al. Intravenous ketamine for adolescents with treatment-resistant depression: an open-label study. J Child Adolesc Psychopharmacol. 2018;28(7):437-444.
Box 2
Efficacy
- Selective serotonin reuptake inhibitors are the most effective pharmacologic treatment we have for pediatric depression, OCD, and anxiety
- More than one-half of children who are prescribed SSRIs have a significant improvement, regardless of condition
- Based on current estimates, we need to treat 4 to 6 children with an SSRI to find one that will improve who would not improve with placebo
- The clinical benefits of SSRIs generally take a while to accrue; therefore, it is advisable to take the medication for at least 2 to 3 months before concluding that it is ineffective
- In addition to medication, evidence-based psychotherapies provide significant benefit for pediatric depression, OCD, and anxiety
Tolerability
- Most commonly prescribed pediatric antidepressants have been used safely in children for 2 to 3 decades. The safety profiles of SSRIs are among the best of any medications used for children and adolescents
- While many children get better when taking these medications, it’s important that we also talk about potential adverse effects. Some children will experience sleep problems (either sleepier than usual or difficulty sleeping), changes in energy levels, headache, gastrointestinal upset, and dry mouth. These are most likely at the beginning of treatment, or when we increase the dose; they usually are time-limited and go away on their own
- Often adverse effects occur first and the benefits come later. Because it may take at least a few weeks to start to see the mood/anxiety benefits, it’s important for us to talk about any adverse effects your child experiences and remember that they usually are short-lived
Suicidality
- The FDA placed a “black-box” warning on antidepressants after pediatric studies found a small but statistically significant increased risk of reporting suicidal thoughts or behaviors over the short-term compared with placebo
- The increased risk of spontaneously reporting suicidal ideation was quite small. Studies suggested that one would need to treat 100 to 140 children to see 1 child report suicidal ideation compared to placebo. Suicidal ideation is a common symptom in children with depression and anxiety
- Studies found no increased risk when suicidal ideation was systematically assessed using structured rating scales
- In the studies evaluated, there were no completed suicides by patients taking medication or placebo
- Population studies show that higher rates of antidepressant prescriptions are associated with lower rates of attempted and completed teen suicide, which underscores that in general, these medicines treat the underlying causes of suicidality
- No scientific consensus exists on whether these medications are truly associated with an increased risk of new-onset suicidal ideation, or if this association is due to other factors (eg, improvement in anxiety and depressive symptoms that make patients more comfortable to report suicidal ideation spontaneously)
- Regardless, the FDA recommends frequent monitoring of children for suicidal thoughts when these medications are started. This should be done anyway in children experiencing depression and anxiety, and it’s why we will plan to have more frequent appointments as the medication is initiated
OCD: obsessive-compulsive disorder; SSRIs: selective serotonin reuptake inhibitors
1. Williams SB, O’Connor EA, Eder M, et al. Screening for child and adolescent depression in primary care settings: a systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2009;123(4):e716-e735. doi: 10.1542/peds.2008-2415.
2. Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002-1014.
3. Lewinsohn PM, Clarke GN, Seeley JR, et al. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. J Am Acad Child Adolesc Psychiatry. 1994;33(6):809-818.
4. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
5. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
6. Keenan-Miller D, Hammen CL, Brennan PA. Health outcomes related to early adolescent depression. J Adolesc Health. 2007; 41(3): 256-62.
7. Shaffer D, Gould MS, Fisher P, et al. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53(4):339-348.
8. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
9. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
10. Wittchen HU, Nelson CB, Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol Med. 1998;28(1):109-126.
11. Foley DL, Goldston DB, Costello EJ, et al. Proximal psychiatric risk factors for suicidality in youth: the Great Smoky Mountains Study. Arch Gen Psychiatry. 2006;63(9):1017-1024.
12. Cheung A, Sacks D, Dewa CS, et al. Pediatric prescribing practices and the FDA black-box warning on antidepressants. J Dev Behav Pediatr. 2008 29(3):213-215.
13. Walkup JT. Antidepressant efficacy for depression in children and adolescents: industry- and NIMH-funded studies. Am J Psychiatry. 2017;174(5):430-437.
14. Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173(2):174-183.
15. Varigonda AL, Jakubovski E, Taylor MJ, et al. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(7):557-564.
16. Strawn JR, Welge JA, Wehry AM, et al. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149-157.
17. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;280(20):1752-1756.
18. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
19. Kennard BD, Silva SG, Tonev S, et al. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48(2):186-195.
20. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388(10047):881-890.
21. Cohen JA, Mannarino AP, Perel JM, et al. A pilot randomized controlled trial of combined trauma-focused CBT and sertraline for childhood PTSD symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46(7):811-819.
22. Robb AS, Cueva JE, Sporn J, et al. Sertraline treatment of children and adolescents with posttraumatic stress disorder: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2010;20(6):463-471.
23. Diehle J, Opmeer BC, Boer F, et al. Trauma-focused cognitive behavioral therapy or eye movement desensitization and reprocessing: what works in children with posttraumatic stress symptoms? A randomized controlled trial. Eur Child Adolesc Psychiatry. 2015;24(2):227-236.
24. Aiyer R, Barkin RL, Bhatia A. Treatment of neuropathic pain with venlafaxine: a systematic review. Pain Med. 2017;18(10):1999-2012.
25. Barrickman LL, Perry PJ, Allen AJ, et al. Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34(5):649-657.
26. Monuteaux MC, Spencer TJ, Faraone SV, et al. A randomized, placebo-controlled clinical trial of bupropion for the prevention of smoking in children and adolescents with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2007;68(7):1094-1101.
27. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
28. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656.
29. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder--a multicenter trial. J Am Acad Child Adolesc Psychiatry. 1992;31(1):45-49.
30. Caldwell PH, Sureshkumar P, Wong WC. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2016;(1):CD002117.
31. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(10):851-859.e2. doi: 10.1016/j.jaac.2016.07.768.
32. Walkup J, Labellarte M. Complications of SSRI treatment. J Child Adolesc Psychopharmacol. 2001;11(1):1-4.
33. Leo RJ, Lichter DG, Hershey LA. Parkinsonism associated with fluoxetine and cimetidine: a case report. J Geriatr Psychiatry Neurol. 1995;8(4):231-233.
34. Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283-293.
35. Bernstein GA, Borchardt CM, Perwien AR, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry. 2000;39(3): 276-283.
36. Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1-2):159-169.
37. Reinblatt SP, DosReis S, Walkup JT, et al. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119-126.
38. Goldsmith M, Singh M, Chang K. Antidepressants and psychostimulants in pediatric populations: is there an association with mania? Paediatr Drugs. 2011;13(4): 225-243.
39. Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
40. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
41. McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):107-125.
42. Dobry Y, Rice T, Sher L. Ecstasy use and serotonin syndrome: a neglected danger to adolescents and young adults prescribed selective serotonin reuptake inhibitors. Int J Adolesc Med Health. 2013; 25(3):193-199.
43. Schwartz AR, Pizon AF, Brooks DE. Dextromethorphan-induced serotonin syndrome. Clin Toxicol (Phila). 2008;46(8):771-773.
44. Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164(9):1356-1363.
45. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332-339.
46. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696.
47. Sharma T, Guski LS, Freund N, et al. Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ. 2016;352: i65. doi: https://doi.org/10.1136/bmj.i65.
48. Olfson M, Shaffer D, Marcus SC, et al. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry. 2003;60(10):978-982.
49. Garland JE, Kutcher S, Virani A, et al. Update on the Use of SSRIs and SNRIs with children and adolescents in clinical practice. J Can Acad Child Adolesc Psychiatry. 2016;25(1):4-10.
50. Bridge JA, Barbe RP, Birmaher B, et al. Emergent suicidality in a clinical psychotherapy trial for adolescent depression. Am J Psychiatry. 2005;162(11):2173-2175.
51. Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503-1526.
52. Ravizza L, Maina G, Bogetto F, et al. Long term treatment of obsessive-compulsive disorder. CNS Drugs. 1998;10(4):247-255.
53. Hosenbocus S, Chahal R. SSRIs and SNRIs: a review of the discontinuation syndrome in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2011;20(1):60-67.
54. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855.
55. Issari Y, Jakubovski E, Bartley CA, et al. Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis. J Clin Psychiatry. 2016; 77(5):e605-e611. doi: 10.4088/JCP.14r09758.
56. Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901-913.
1. Williams SB, O’Connor EA, Eder M, et al. Screening for child and adolescent depression in primary care settings: a systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2009;123(4):e716-e735. doi: 10.1542/peds.2008-2415.
2. Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002-1014.
3. Lewinsohn PM, Clarke GN, Seeley JR, et al. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. J Am Acad Child Adolesc Psychiatry. 1994;33(6):809-818.
4. Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. JAMA.1999;281(18):1707-1713.
5. Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225-231.
6. Keenan-Miller D, Hammen CL, Brennan PA. Health outcomes related to early adolescent depression. J Adolesc Health. 2007; 41(3): 256-62.
7. Shaffer D, Gould MS, Fisher P, et al. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53(4):339-348.
8. Centers for Disease Control and Prevention. National Vital Statistics System. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race and sex: United States, 1999-2015. https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm. Published October 23, 2017. Accessed May 2, 2019.
9. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
10. Wittchen HU, Nelson CB, Lachner G. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol Med. 1998;28(1):109-126.
11. Foley DL, Goldston DB, Costello EJ, et al. Proximal psychiatric risk factors for suicidality in youth: the Great Smoky Mountains Study. Arch Gen Psychiatry. 2006;63(9):1017-1024.
12. Cheung A, Sacks D, Dewa CS, et al. Pediatric prescribing practices and the FDA black-box warning on antidepressants. J Dev Behav Pediatr. 2008 29(3):213-215.
13. Walkup JT. Antidepressant efficacy for depression in children and adolescents: industry- and NIMH-funded studies. Am J Psychiatry. 2017;174(5):430-437.
14. Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173(2):174-183.
15. Varigonda AL, Jakubovski E, Taylor MJ, et al. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(7):557-564.
16. Strawn JR, Welge JA, Wehry AM, et al. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149-157.
17. March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;280(20):1752-1756.
18. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
19. Kennard BD, Silva SG, Tonev S, et al. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48(2):186-195.
20. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388(10047):881-890.
21. Cohen JA, Mannarino AP, Perel JM, et al. A pilot randomized controlled trial of combined trauma-focused CBT and sertraline for childhood PTSD symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46(7):811-819.
22. Robb AS, Cueva JE, Sporn J, et al. Sertraline treatment of children and adolescents with posttraumatic stress disorder: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2010;20(6):463-471.
23. Diehle J, Opmeer BC, Boer F, et al. Trauma-focused cognitive behavioral therapy or eye movement desensitization and reprocessing: what works in children with posttraumatic stress symptoms? A randomized controlled trial. Eur Child Adolesc Psychiatry. 2015;24(2):227-236.
24. Aiyer R, Barkin RL, Bhatia A. Treatment of neuropathic pain with venlafaxine: a systematic review. Pain Med. 2017;18(10):1999-2012.
25. Barrickman LL, Perry PJ, Allen AJ, et al. Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34(5):649-657.
26. Monuteaux MC, Spencer TJ, Faraone SV, et al. A randomized, placebo-controlled clinical trial of bupropion for the prevention of smoking in children and adolescents with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2007;68(7):1094-1101.
27. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
28. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59(7):649-656.
29. DeVeaugh-Geiss J, Moroz G, Biederman J, et al. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder--a multicenter trial. J Am Acad Child Adolesc Psychiatry. 1992;31(1):45-49.
30. Caldwell PH, Sureshkumar P, Wong WC. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2016;(1):CD002117.
31. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(10):851-859.e2. doi: 10.1016/j.jaac.2016.07.768.
32. Walkup J, Labellarte M. Complications of SSRI treatment. J Child Adolesc Psychopharmacol. 2001;11(1):1-4.
33. Leo RJ, Lichter DG, Hershey LA. Parkinsonism associated with fluoxetine and cimetidine: a case report. J Geriatr Psychiatry Neurol. 1995;8(4):231-233.
34. Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283-293.
35. Bernstein GA, Borchardt CM, Perwien AR, et al. Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry. 2000;39(3): 276-283.
36. Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1-2):159-169.
37. Reinblatt SP, DosReis S, Walkup JT, et al. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119-126.
38. Goldsmith M, Singh M, Chang K. Antidepressants and psychostimulants in pediatric populations: is there an association with mania? Paediatr Drugs. 2011;13(4): 225-243.
39. Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
40. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
41. McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):107-125.
42. Dobry Y, Rice T, Sher L. Ecstasy use and serotonin syndrome: a neglected danger to adolescents and young adults prescribed selective serotonin reuptake inhibitors. Int J Adolesc Med Health. 2013; 25(3):193-199.
43. Schwartz AR, Pizon AF, Brooks DE. Dextromethorphan-induced serotonin syndrome. Clin Toxicol (Phila). 2008;46(8):771-773.
44. Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164(9):1356-1363.
45. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332-339.
46. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696.
47. Sharma T, Guski LS, Freund N, et al. Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ. 2016;352: i65. doi: https://doi.org/10.1136/bmj.i65.
48. Olfson M, Shaffer D, Marcus SC, et al. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry. 2003;60(10):978-982.
49. Garland JE, Kutcher S, Virani A, et al. Update on the Use of SSRIs and SNRIs with children and adolescents in clinical practice. J Can Acad Child Adolesc Psychiatry. 2016;25(1):4-10.
50. Bridge JA, Barbe RP, Birmaher B, et al. Emergent suicidality in a clinical psychotherapy trial for adolescent depression. Am J Psychiatry. 2005;162(11):2173-2175.
51. Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503-1526.
52. Ravizza L, Maina G, Bogetto F, et al. Long term treatment of obsessive-compulsive disorder. CNS Drugs. 1998;10(4):247-255.
53. Hosenbocus S, Chahal R. SSRIs and SNRIs: a review of the discontinuation syndrome in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2011;20(1):60-67.
54. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855.
55. Issari Y, Jakubovski E, Bartley CA, et al. Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis. J Clin Psychiatry. 2016; 77(5):e605-e611. doi: 10.4088/JCP.14r09758.
56. Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901-913.
Consider cyclosporine a go-to for refractory atopic dermatitis in kids
AUSTIN, TEX. – For
Cyclosporine “works quickly, and it’s very reliable,” Dr. Lio said at the annual meeting of the Society for Pediatric Dermatology. “In my experience, more than 90% of patients will see significant improvement, but there are real risks, including hypertension, kidney damage, monthly blood work, tremor, hypertrichosis, gum hypertrophy, and cancer/infection risk.”
To mitigate those risks, Dr. Lio, of the departments of dermatology and pediatrics at Northwestern University, Chicago, prescribes cyclosporine for 3-6 months at a dose of 5 mg/kg per day with a cap of 300 mg per day to “to cool things down.” He then transitions patients to phototherapy or mycophenolate right away. “Those are my two favorites,” he said. “Methotrexate can also be used, but I rarely use azathioprine.
“If you do this, you avoid most of the major risks and you can put people in a remission. More than half of the time, maybe two-thirds of the time, I get them into at least a relative remission,” Dr. Lio said.
While patients are on cyclosporine, blood pressure should be monitored each week for 4 weeks, and then monthly, he said. Draws for complete blood count, liver function tests, comprehensive metabolic panel, uric acid, and lipids should be performed monthly for 3 months, then every 8 weeks, he advised. Dr. Lio typically maintains the cyclosporine for 3 months, then tapers patients off the drug.
Patients who continue to struggle for relief might try taking cyclosporine on weekends only, a concept reported by Spanish investigators in 2015 (Pediatr Dermatol 2015 32[4]:551-2). “This involves twice-daily full dosing just on Saturdays and Sundays,” Dr. Lio said. “I now have quite a few patients doing well with this approach.”
Dr. Lio disclosed having financial ties to numerous pharmaceutical companies but none related to cyclosporine.
AUSTIN, TEX. – For
Cyclosporine “works quickly, and it’s very reliable,” Dr. Lio said at the annual meeting of the Society for Pediatric Dermatology. “In my experience, more than 90% of patients will see significant improvement, but there are real risks, including hypertension, kidney damage, monthly blood work, tremor, hypertrichosis, gum hypertrophy, and cancer/infection risk.”
To mitigate those risks, Dr. Lio, of the departments of dermatology and pediatrics at Northwestern University, Chicago, prescribes cyclosporine for 3-6 months at a dose of 5 mg/kg per day with a cap of 300 mg per day to “to cool things down.” He then transitions patients to phototherapy or mycophenolate right away. “Those are my two favorites,” he said. “Methotrexate can also be used, but I rarely use azathioprine.
“If you do this, you avoid most of the major risks and you can put people in a remission. More than half of the time, maybe two-thirds of the time, I get them into at least a relative remission,” Dr. Lio said.
While patients are on cyclosporine, blood pressure should be monitored each week for 4 weeks, and then monthly, he said. Draws for complete blood count, liver function tests, comprehensive metabolic panel, uric acid, and lipids should be performed monthly for 3 months, then every 8 weeks, he advised. Dr. Lio typically maintains the cyclosporine for 3 months, then tapers patients off the drug.
Patients who continue to struggle for relief might try taking cyclosporine on weekends only, a concept reported by Spanish investigators in 2015 (Pediatr Dermatol 2015 32[4]:551-2). “This involves twice-daily full dosing just on Saturdays and Sundays,” Dr. Lio said. “I now have quite a few patients doing well with this approach.”
Dr. Lio disclosed having financial ties to numerous pharmaceutical companies but none related to cyclosporine.
AUSTIN, TEX. – For
Cyclosporine “works quickly, and it’s very reliable,” Dr. Lio said at the annual meeting of the Society for Pediatric Dermatology. “In my experience, more than 90% of patients will see significant improvement, but there are real risks, including hypertension, kidney damage, monthly blood work, tremor, hypertrichosis, gum hypertrophy, and cancer/infection risk.”
To mitigate those risks, Dr. Lio, of the departments of dermatology and pediatrics at Northwestern University, Chicago, prescribes cyclosporine for 3-6 months at a dose of 5 mg/kg per day with a cap of 300 mg per day to “to cool things down.” He then transitions patients to phototherapy or mycophenolate right away. “Those are my two favorites,” he said. “Methotrexate can also be used, but I rarely use azathioprine.
“If you do this, you avoid most of the major risks and you can put people in a remission. More than half of the time, maybe two-thirds of the time, I get them into at least a relative remission,” Dr. Lio said.
While patients are on cyclosporine, blood pressure should be monitored each week for 4 weeks, and then monthly, he said. Draws for complete blood count, liver function tests, comprehensive metabolic panel, uric acid, and lipids should be performed monthly for 3 months, then every 8 weeks, he advised. Dr. Lio typically maintains the cyclosporine for 3 months, then tapers patients off the drug.
Patients who continue to struggle for relief might try taking cyclosporine on weekends only, a concept reported by Spanish investigators in 2015 (Pediatr Dermatol 2015 32[4]:551-2). “This involves twice-daily full dosing just on Saturdays and Sundays,” Dr. Lio said. “I now have quite a few patients doing well with this approach.”
Dr. Lio disclosed having financial ties to numerous pharmaceutical companies but none related to cyclosporine.
REPORTING FROM SPD 2019
Cerliponase alfa continues to impress for CLN2 disease
BANGKOK – Biweekly cerliponase alfa continued to show durable and clinically important therapeutic benefit in children with neuronal ceroid lipofuscinosis type 2 (CLN2) disease at the 3-year mark in an ongoing international study, Marina Trivisano, MD, reported at the International Epilepsy Congress.
Cerliponase alfa, approved under the trade name Brineura by the Food and Drug Administration and European Commission, is a recombinant human tripeptidyl peptidase 1 designed as enzyme replacement therapy delivered by a surgically implanted intraventricular infusion device in children with this rare lysosomal storage disease, a form of Batten disease, she explained at the congress sponsored by the International League Against Epilepsy.
When both healthy parents carry one defective gene, each of their children has a one in four chance of inheriting this devastating disease that causes rapidly progressive dementia. CLN2 disease typically reveals itself when a child reaches about 3 years of age, with seizures, language delay, or loss of acquired language being the most common first indications.
Of 23 patients enrolled in the open-label study, 21 remained participants at 3 years of follow-up. The two dropouts weren’t caused by treatment-related adverse events, but rather by the formidable logistic challenges posed because the treatment – 300 mg of cerliponase alfa delivered by intraventricular infusion over a 4-hour period every 2 weeks – was available only at five medical centers located in Rome; London; New York; Hamburg, Germany; and Columbus, Ohio.
At 3 years of follow-up, 83% of patients met the primary study endpoint, defined as the absence of a 2-point or greater decline in the motor-language score on the 0-6 CLN2 Clinical Rating Scale. This was a success rate 12 times greater than in 42 historical controls. Indeed, at 3 years the cerliponase alfa–treated patients had an average CLN2 Clinical Rating Scale motor-language score 3.8 points better than the historical controls, reported Dr. Trivisano, a pediatric neurologist at Bambino Gesu Children’s Hospital in Rome.
Side effects included several cases of device failure, infection, and hypersensitivity reactions.
In an earlier report based upon 96 weeks of follow-up, the mean rate of decline in the motor-language score was 0.27 points per 48 weeks in treated patients, compared with 2.12 points in the historical controls (N Engl J Med. 2018 May 17;378[20]:1898-1907).
The study was funded by BioMarin Pharmaceutical, which markets Brineura. Dr. Trivisano was a subinvestigator in the trial.
SOURCE: Trivisano M et al. IEC 2019, Abstract P333.
BANGKOK – Biweekly cerliponase alfa continued to show durable and clinically important therapeutic benefit in children with neuronal ceroid lipofuscinosis type 2 (CLN2) disease at the 3-year mark in an ongoing international study, Marina Trivisano, MD, reported at the International Epilepsy Congress.
Cerliponase alfa, approved under the trade name Brineura by the Food and Drug Administration and European Commission, is a recombinant human tripeptidyl peptidase 1 designed as enzyme replacement therapy delivered by a surgically implanted intraventricular infusion device in children with this rare lysosomal storage disease, a form of Batten disease, she explained at the congress sponsored by the International League Against Epilepsy.
When both healthy parents carry one defective gene, each of their children has a one in four chance of inheriting this devastating disease that causes rapidly progressive dementia. CLN2 disease typically reveals itself when a child reaches about 3 years of age, with seizures, language delay, or loss of acquired language being the most common first indications.
Of 23 patients enrolled in the open-label study, 21 remained participants at 3 years of follow-up. The two dropouts weren’t caused by treatment-related adverse events, but rather by the formidable logistic challenges posed because the treatment – 300 mg of cerliponase alfa delivered by intraventricular infusion over a 4-hour period every 2 weeks – was available only at five medical centers located in Rome; London; New York; Hamburg, Germany; and Columbus, Ohio.
At 3 years of follow-up, 83% of patients met the primary study endpoint, defined as the absence of a 2-point or greater decline in the motor-language score on the 0-6 CLN2 Clinical Rating Scale. This was a success rate 12 times greater than in 42 historical controls. Indeed, at 3 years the cerliponase alfa–treated patients had an average CLN2 Clinical Rating Scale motor-language score 3.8 points better than the historical controls, reported Dr. Trivisano, a pediatric neurologist at Bambino Gesu Children’s Hospital in Rome.
Side effects included several cases of device failure, infection, and hypersensitivity reactions.
In an earlier report based upon 96 weeks of follow-up, the mean rate of decline in the motor-language score was 0.27 points per 48 weeks in treated patients, compared with 2.12 points in the historical controls (N Engl J Med. 2018 May 17;378[20]:1898-1907).
The study was funded by BioMarin Pharmaceutical, which markets Brineura. Dr. Trivisano was a subinvestigator in the trial.
SOURCE: Trivisano M et al. IEC 2019, Abstract P333.
BANGKOK – Biweekly cerliponase alfa continued to show durable and clinically important therapeutic benefit in children with neuronal ceroid lipofuscinosis type 2 (CLN2) disease at the 3-year mark in an ongoing international study, Marina Trivisano, MD, reported at the International Epilepsy Congress.
Cerliponase alfa, approved under the trade name Brineura by the Food and Drug Administration and European Commission, is a recombinant human tripeptidyl peptidase 1 designed as enzyme replacement therapy delivered by a surgically implanted intraventricular infusion device in children with this rare lysosomal storage disease, a form of Batten disease, she explained at the congress sponsored by the International League Against Epilepsy.
When both healthy parents carry one defective gene, each of their children has a one in four chance of inheriting this devastating disease that causes rapidly progressive dementia. CLN2 disease typically reveals itself when a child reaches about 3 years of age, with seizures, language delay, or loss of acquired language being the most common first indications.
Of 23 patients enrolled in the open-label study, 21 remained participants at 3 years of follow-up. The two dropouts weren’t caused by treatment-related adverse events, but rather by the formidable logistic challenges posed because the treatment – 300 mg of cerliponase alfa delivered by intraventricular infusion over a 4-hour period every 2 weeks – was available only at five medical centers located in Rome; London; New York; Hamburg, Germany; and Columbus, Ohio.
At 3 years of follow-up, 83% of patients met the primary study endpoint, defined as the absence of a 2-point or greater decline in the motor-language score on the 0-6 CLN2 Clinical Rating Scale. This was a success rate 12 times greater than in 42 historical controls. Indeed, at 3 years the cerliponase alfa–treated patients had an average CLN2 Clinical Rating Scale motor-language score 3.8 points better than the historical controls, reported Dr. Trivisano, a pediatric neurologist at Bambino Gesu Children’s Hospital in Rome.
Side effects included several cases of device failure, infection, and hypersensitivity reactions.
In an earlier report based upon 96 weeks of follow-up, the mean rate of decline in the motor-language score was 0.27 points per 48 weeks in treated patients, compared with 2.12 points in the historical controls (N Engl J Med. 2018 May 17;378[20]:1898-1907).
The study was funded by BioMarin Pharmaceutical, which markets Brineura. Dr. Trivisano was a subinvestigator in the trial.
SOURCE: Trivisano M et al. IEC 2019, Abstract P333.
REPORTING FROM IEC 2019
Combo therapy outcomes for West syndrome prove no better than monotherapy
BANGKOK – Hiroki Nariai, MD, declared at the International Epilepsy Congress.
West syndrome, or infantile spasms with a hypsarrhythmic EEG, is a severe infantile epileptic encephalopathy. It has high morbidity and mortality, and it’s challenging to treat. So neurologists and pediatricians were thrilled by an earlier preliminary report from an open-label, randomized, controlled trial conducted by the International Collaborative Infantile Spasms Study (ICISS) investigators. They reported that a hormonal therapy and vigabatrin (Sabril) combination provided significantly better seizure control between days 14 and 42 of treatment than hormonal therapy alone, albeit at the cost of more side effects (Lancet Neurol. 2017 Jan;16[1]:33-42).
However, a sobering update from the 377-infant study conducted in Australia, Switzerland, Germany, New Zealand, and the United Kingdom concluded that combination therapy didn’t result in improved developmental or epilepsy outcomes at 18 months, Dr. Nariai said at the congress sponsored by the International League Against Epilepsy.
“We still have inconclusive evidence to support the routine use of combination therapy. Clearly we need a better disease-modifying therapy because our best results with hormonal therapy or vigabatrin are only a 50%-70% response rate. And having a biomarker to guide early therapy and follow treatment response would help in establishing a better therapy,” commented Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
He wasn’t involved in the international trial. He is, however, active in the search for a biomarker that would aid in speedier diagnosis of West syndrome, which in turn would allow for earlier treatment and, potentially, better outcomes. Indeed, Dr. Nariai has done pioneering work in identifying several EEG abnormalities readily measurable noninvasively using scalp electrodes that show considerable promise in this regard. These candidate biomarkers include ictal or interictal high-frequency oscillations at 80 Hz or more, along with cross-frequency coupling of high-frequency oscillations and delta-wave activity.
The primary endpoint in the ICISS study was developmental outcome at 18 months as evaluated using the Vineland Adaptive Behavior Scales composite score. The mean score was 73.9 in the combination therapy group and closely similar at 72.7 in the children on hormonal therapy alone. At 18 months, 30% of children in the combination therapy group carried a diagnosis of epilepsy, as did 29.2% of controls randomized to either high-dose oral steroids or intramuscular depot tetracosactide. About 15% of children randomized to combination therapy still had spasms at 18 months, as did 15.7% on hormonal therapy alone (Lancet Child Adolesc Health. 2018 Oct;2[10]:715-25).
The chief side effects of hormonal therapy included hypertension, hypoglycemia, and immunosuppression. Vigabatrin’s side effects included dose- and duration-dependent peripheral vision loss, movement disorders, and undesirable MRI signal changes.
Dr. Nariai observed that, even though hormonal therapy is widely used as first-line therapy in West syndrome, it remains surrounded by important unanswered questions.
“We don’t have head-to-head comparative studies of ACTH versus high-dose steroids, the optimal dosing protocol is not established, and we really don’t even know the mechanism of action for hormonal therapy and vigabatrin,” he said.
The study was sponsored by the U.K. National Institute of Health Research and other noncommercial entities. Dr. Nariai reported having no financial conflicts regarding his presentation.
BANGKOK – Hiroki Nariai, MD, declared at the International Epilepsy Congress.
West syndrome, or infantile spasms with a hypsarrhythmic EEG, is a severe infantile epileptic encephalopathy. It has high morbidity and mortality, and it’s challenging to treat. So neurologists and pediatricians were thrilled by an earlier preliminary report from an open-label, randomized, controlled trial conducted by the International Collaborative Infantile Spasms Study (ICISS) investigators. They reported that a hormonal therapy and vigabatrin (Sabril) combination provided significantly better seizure control between days 14 and 42 of treatment than hormonal therapy alone, albeit at the cost of more side effects (Lancet Neurol. 2017 Jan;16[1]:33-42).
However, a sobering update from the 377-infant study conducted in Australia, Switzerland, Germany, New Zealand, and the United Kingdom concluded that combination therapy didn’t result in improved developmental or epilepsy outcomes at 18 months, Dr. Nariai said at the congress sponsored by the International League Against Epilepsy.
“We still have inconclusive evidence to support the routine use of combination therapy. Clearly we need a better disease-modifying therapy because our best results with hormonal therapy or vigabatrin are only a 50%-70% response rate. And having a biomarker to guide early therapy and follow treatment response would help in establishing a better therapy,” commented Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
He wasn’t involved in the international trial. He is, however, active in the search for a biomarker that would aid in speedier diagnosis of West syndrome, which in turn would allow for earlier treatment and, potentially, better outcomes. Indeed, Dr. Nariai has done pioneering work in identifying several EEG abnormalities readily measurable noninvasively using scalp electrodes that show considerable promise in this regard. These candidate biomarkers include ictal or interictal high-frequency oscillations at 80 Hz or more, along with cross-frequency coupling of high-frequency oscillations and delta-wave activity.
The primary endpoint in the ICISS study was developmental outcome at 18 months as evaluated using the Vineland Adaptive Behavior Scales composite score. The mean score was 73.9 in the combination therapy group and closely similar at 72.7 in the children on hormonal therapy alone. At 18 months, 30% of children in the combination therapy group carried a diagnosis of epilepsy, as did 29.2% of controls randomized to either high-dose oral steroids or intramuscular depot tetracosactide. About 15% of children randomized to combination therapy still had spasms at 18 months, as did 15.7% on hormonal therapy alone (Lancet Child Adolesc Health. 2018 Oct;2[10]:715-25).
The chief side effects of hormonal therapy included hypertension, hypoglycemia, and immunosuppression. Vigabatrin’s side effects included dose- and duration-dependent peripheral vision loss, movement disorders, and undesirable MRI signal changes.
Dr. Nariai observed that, even though hormonal therapy is widely used as first-line therapy in West syndrome, it remains surrounded by important unanswered questions.
“We don’t have head-to-head comparative studies of ACTH versus high-dose steroids, the optimal dosing protocol is not established, and we really don’t even know the mechanism of action for hormonal therapy and vigabatrin,” he said.
The study was sponsored by the U.K. National Institute of Health Research and other noncommercial entities. Dr. Nariai reported having no financial conflicts regarding his presentation.
BANGKOK – Hiroki Nariai, MD, declared at the International Epilepsy Congress.
West syndrome, or infantile spasms with a hypsarrhythmic EEG, is a severe infantile epileptic encephalopathy. It has high morbidity and mortality, and it’s challenging to treat. So neurologists and pediatricians were thrilled by an earlier preliminary report from an open-label, randomized, controlled trial conducted by the International Collaborative Infantile Spasms Study (ICISS) investigators. They reported that a hormonal therapy and vigabatrin (Sabril) combination provided significantly better seizure control between days 14 and 42 of treatment than hormonal therapy alone, albeit at the cost of more side effects (Lancet Neurol. 2017 Jan;16[1]:33-42).
However, a sobering update from the 377-infant study conducted in Australia, Switzerland, Germany, New Zealand, and the United Kingdom concluded that combination therapy didn’t result in improved developmental or epilepsy outcomes at 18 months, Dr. Nariai said at the congress sponsored by the International League Against Epilepsy.
“We still have inconclusive evidence to support the routine use of combination therapy. Clearly we need a better disease-modifying therapy because our best results with hormonal therapy or vigabatrin are only a 50%-70% response rate. And having a biomarker to guide early therapy and follow treatment response would help in establishing a better therapy,” commented Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
He wasn’t involved in the international trial. He is, however, active in the search for a biomarker that would aid in speedier diagnosis of West syndrome, which in turn would allow for earlier treatment and, potentially, better outcomes. Indeed, Dr. Nariai has done pioneering work in identifying several EEG abnormalities readily measurable noninvasively using scalp electrodes that show considerable promise in this regard. These candidate biomarkers include ictal or interictal high-frequency oscillations at 80 Hz or more, along with cross-frequency coupling of high-frequency oscillations and delta-wave activity.
The primary endpoint in the ICISS study was developmental outcome at 18 months as evaluated using the Vineland Adaptive Behavior Scales composite score. The mean score was 73.9 in the combination therapy group and closely similar at 72.7 in the children on hormonal therapy alone. At 18 months, 30% of children in the combination therapy group carried a diagnosis of epilepsy, as did 29.2% of controls randomized to either high-dose oral steroids or intramuscular depot tetracosactide. About 15% of children randomized to combination therapy still had spasms at 18 months, as did 15.7% on hormonal therapy alone (Lancet Child Adolesc Health. 2018 Oct;2[10]:715-25).
The chief side effects of hormonal therapy included hypertension, hypoglycemia, and immunosuppression. Vigabatrin’s side effects included dose- and duration-dependent peripheral vision loss, movement disorders, and undesirable MRI signal changes.
Dr. Nariai observed that, even though hormonal therapy is widely used as first-line therapy in West syndrome, it remains surrounded by important unanswered questions.
“We don’t have head-to-head comparative studies of ACTH versus high-dose steroids, the optimal dosing protocol is not established, and we really don’t even know the mechanism of action for hormonal therapy and vigabatrin,” he said.
The study was sponsored by the U.K. National Institute of Health Research and other noncommercial entities. Dr. Nariai reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM IEC 2019
Decision making regarding vaccines varies among accepters, deniers, partial accepters
Parents who accepted, denied, or partially accepted participation in the Dutch National Immunization Program reached their decisions through different methods, according to Kim A.G.J. Romijnders of the National Institute for Public Health and the Environment in Bilthoven, the Netherlands, and associates.
For the study published in Vaccine, the investigators conducted a series of 12 focus groups: 3 with accepters (n = 19), 3 with deniers (n =12), and 6 with partial accepters (n =24); in the partial accepters groups, there were three groups with parents delaying vaccination and three with parents refusing some vaccinations. Three-quarters of participants were women, the average age was 39 years, and 96% had at least university education. Parents were asked about their knowledge, attitudes, deliberation, and information needs regarding childhood vaccination.
Vaccine accepters regarded the decision to vaccinate their children as self-evident, but deniers and partial accepters reported conducting extensive deliberation on the pros and cons of vaccination. Deniers and partial accepters, in general, perceived fewer risks of vaccine-preventable diseases, more risks of vaccine side effects, less social support from their environment, less trust in child welfare centers, and provided less information than accepters.
The investigators noted that This process alienated them from their child vaccine provider, with trust being lost when the provider either refused or was unable to answer questions. Partial accepters also reported a lack of social support from friends, family, and providers regarding partial vaccine acceptance.
“The findings can facilitate informed decision making among parents by promoting an open dialogue at the [child welfare center], and improving the type and form of information presented. An open dialogue between parents and [child vaccine providers] may increase deliberation among parents, strengthen positive attitudes, prevent misperceptions, and resolve decisional conflict,” the investigators concluded.
The study was supported by the Dutch National Institute for Public Health and the Environment; the authors reported no conflicts of interest.
SOURCE: Romijnders KAGJ et al. Vaccine. 2019 Aug 2. doi: 10.1016/j.vaccine.2019.07.060.
Parents who accepted, denied, or partially accepted participation in the Dutch National Immunization Program reached their decisions through different methods, according to Kim A.G.J. Romijnders of the National Institute for Public Health and the Environment in Bilthoven, the Netherlands, and associates.
For the study published in Vaccine, the investigators conducted a series of 12 focus groups: 3 with accepters (n = 19), 3 with deniers (n =12), and 6 with partial accepters (n =24); in the partial accepters groups, there were three groups with parents delaying vaccination and three with parents refusing some vaccinations. Three-quarters of participants were women, the average age was 39 years, and 96% had at least university education. Parents were asked about their knowledge, attitudes, deliberation, and information needs regarding childhood vaccination.
Vaccine accepters regarded the decision to vaccinate their children as self-evident, but deniers and partial accepters reported conducting extensive deliberation on the pros and cons of vaccination. Deniers and partial accepters, in general, perceived fewer risks of vaccine-preventable diseases, more risks of vaccine side effects, less social support from their environment, less trust in child welfare centers, and provided less information than accepters.
The investigators noted that This process alienated them from their child vaccine provider, with trust being lost when the provider either refused or was unable to answer questions. Partial accepters also reported a lack of social support from friends, family, and providers regarding partial vaccine acceptance.
“The findings can facilitate informed decision making among parents by promoting an open dialogue at the [child welfare center], and improving the type and form of information presented. An open dialogue between parents and [child vaccine providers] may increase deliberation among parents, strengthen positive attitudes, prevent misperceptions, and resolve decisional conflict,” the investigators concluded.
The study was supported by the Dutch National Institute for Public Health and the Environment; the authors reported no conflicts of interest.
SOURCE: Romijnders KAGJ et al. Vaccine. 2019 Aug 2. doi: 10.1016/j.vaccine.2019.07.060.
Parents who accepted, denied, or partially accepted participation in the Dutch National Immunization Program reached their decisions through different methods, according to Kim A.G.J. Romijnders of the National Institute for Public Health and the Environment in Bilthoven, the Netherlands, and associates.
For the study published in Vaccine, the investigators conducted a series of 12 focus groups: 3 with accepters (n = 19), 3 with deniers (n =12), and 6 with partial accepters (n =24); in the partial accepters groups, there were three groups with parents delaying vaccination and three with parents refusing some vaccinations. Three-quarters of participants were women, the average age was 39 years, and 96% had at least university education. Parents were asked about their knowledge, attitudes, deliberation, and information needs regarding childhood vaccination.
Vaccine accepters regarded the decision to vaccinate their children as self-evident, but deniers and partial accepters reported conducting extensive deliberation on the pros and cons of vaccination. Deniers and partial accepters, in general, perceived fewer risks of vaccine-preventable diseases, more risks of vaccine side effects, less social support from their environment, less trust in child welfare centers, and provided less information than accepters.
The investigators noted that This process alienated them from their child vaccine provider, with trust being lost when the provider either refused or was unable to answer questions. Partial accepters also reported a lack of social support from friends, family, and providers regarding partial vaccine acceptance.
“The findings can facilitate informed decision making among parents by promoting an open dialogue at the [child welfare center], and improving the type and form of information presented. An open dialogue between parents and [child vaccine providers] may increase deliberation among parents, strengthen positive attitudes, prevent misperceptions, and resolve decisional conflict,” the investigators concluded.
The study was supported by the Dutch National Institute for Public Health and the Environment; the authors reported no conflicts of interest.
SOURCE: Romijnders KAGJ et al. Vaccine. 2019 Aug 2. doi: 10.1016/j.vaccine.2019.07.060.
FROM VACCINE
Tandem transplants provide EFS edge in pediatric neuroblastoma
For young patients with high-risk neuroblastoma, an intensive consolidation regimen with tandem autologous stem cell transplants was associated with significantly better event-free survival, compared with single-transplant consolidation, results of a randomized trial show.
Among 355 patients with high-risk neuroblastoma, the 3-year event-free survival (EFS) rate was 61.6% for patients randomized to tandem (sequential) autologous stem cell transplants, compared with 48.4% for patients randomized to a single transplant (P = .006), reported Julie R. Park, MD from Seattle Children’s Hospital in Washington, and coinvestigators in the Children’s Oncology Group’s ANBL0532 trial.
“Results of the current study are consistent with earlier trials demonstrating that induction chemotherapy followed by consolidation with autologous transplant improved EFS, compared with less intensive consolidation, and that further intensification of consolidation benefits some patients,” they wrote in JAMA.
But of the 652 patients enrolled in the study, only 355 were actually randomized. Although the randomization rate was slightly higher than anticipated, the authors acknowledged that the results may not apply to all patients with high-risk neuroblastoma.
Patients eligible for the trial included those with International Neuroblastoma Staging System (INSS) stage 4 neuroblastoma aged older than 18 months; INSS stage 3 neuroblastoma aged older than 18 months with International Neuroblastoma Pathology Classification of unfavorable histology; INSS stage 2, 3, 4, or 4S neuroblastoma with MYCN amplification; and INSS stage 4 neuroblastoma diagnosed from age 12-18 months whose tumors showed any unfavorable features. Patients initially diagnosed with non–high-risk neuroblastoma (including stage 1) who had not received chemotherapy and whose disease had progressed to high-risk neuroblastoma were also eligible.
Following induction with two cycles of topotecan and cyclophosphamide, patients underwent peripheral blood stem cell collection, followed by four alternating cycles of cisplatin and etoposide and doxorubicin and cyclophosphamide, and vincristine.
For those patients who did not have primary tumors resected at diagnosis, resection was performed after the fourth or fifth cycle.
Those patients who after induction had no disease progression, no uncontrolled infection, sufficient stem cell levels, and adequate organ function were then eligible for randomization. One patient did not receive any therapy, 27 were nonrandomly assigned to single transplant, 62 were not eligible for randomization, and 207 were not randomized because of physician or family preference.
Of the remaining patients (median age at diagnosis, 36.1 months) 176 were randomized to receive tandem transplant with thiotepa and cyclophosphamide followed by dose-reduced carboplatin, etoposide, and melphalan conditioning, and 179 were randomized to single transplant with standard-dose carboplatin, etoposide, and melphalan.
A total of 17 patients died on study from toxicity; 7 during induction and 10 during consolidation. Significant transplant-related toxicities included mucositis in 11.7% of tandem-transplant patients and 15.4% of single-transplant patients, and infections in 17.8% versus 18.3%, respectively.
As noted before, 3-year EFS from the time of randomization, the primary endpoint, was higher for patients in the tandem-transplant arm (61.6% vs. 48.4%, P = .006).
The median duration of follow-up after randomization for patients without relapse, disease progression, second malignancy, or death was 5.6 years.
A post hoc analysis of the randomized patients showed a 3-year overall survival rate of 71.6%, which did not differ significantly between the study arms (74.1% for the tandem-transplant group vs. 68.1% for the single-transplant group). The analysis also showed that 3-year EFS and overall survival was higher in the tandem- versus single-transplant groups among 250 patients who also received immunotherapy with isotretinoin plus an anti-GD2 chimeric antibody and cytokines.
The trial was supported by grants from the National Institutes of Health, National Cancer Institute, National Clinical Trials Network Operations Center, and St. Baldrick’s Foundation. Dr. Park reported no relevant disclosures. Multiple coauthors disclosed grants or personal feeds outside the submitted work.
SOURCE: Park JR et al. JAMA. 2019;322(8):746-55.
The ANBL0532 trial also does not address the important question as to whether tandem high-dose chemotherapy with autologous stem cell transplant results in benefit for all-comers with high-risk neuroblastoma, because just over half of the eligible patients underwent randomization. Although characteristics of the entire cohort and the randomized cohort were similar with respect to age, stage, tumor histology, and MYCN status, there may be differences unrelated to widely accepted neuroblastoma risk variables. A separate but important challenge in interpretation of these results, as with any clinical trial results, is to understand the generalizability of findings to patient populations who may not be enrolling in trials. Previous work in pediatric oncology showed that trial enrollment correlated with race, age, and zip code, and it is difficult to know whether the results of the ANBL0532 trial are applicable to patient groups who may not be well represented.
An additional challenge is that even though a difference in event-free survival was detected between groups assigned to receive single versus tandem transplant, and a difference in overall survival was detected in a post hoc analysis of patients who received immunotherapy, no difference in overall survival was detected in the overall randomized cohort. Overall survival was evaluated as a secondary outcome but the trial was not powered to detect a difference in overall survival. Moreover, as noted by the authors, overall survival can be influenced by therapies delivered after relapse. This is particularly relevant in an era in which relapse therapies have been shown to induce responses, including periods of remission.
Remarks from Rochelle Bagatell, MD, from the University of Pennsylvania, Philadelphia, and Meredith S. Irwin, MD, from the Hospital for Sick Children in Toronto, are adapted and condensed from an editorial accompanying the study by Park et al. Dr. Bagatell is the vice chair of the Children’s Oncology Group Neuroblastoma Disease Committee. Dr. Irwin reported receiving personal fees from Bayer Canada outside the submitted work and is the vice chair of the Children’s Oncology Group Neuroblastoma Biology Committee. Neither Dr. Bagatell nor Dr. Irwin was involved in the design of the ANBL0532 trial or in the analysis of the results.
The ANBL0532 trial also does not address the important question as to whether tandem high-dose chemotherapy with autologous stem cell transplant results in benefit for all-comers with high-risk neuroblastoma, because just over half of the eligible patients underwent randomization. Although characteristics of the entire cohort and the randomized cohort were similar with respect to age, stage, tumor histology, and MYCN status, there may be differences unrelated to widely accepted neuroblastoma risk variables. A separate but important challenge in interpretation of these results, as with any clinical trial results, is to understand the generalizability of findings to patient populations who may not be enrolling in trials. Previous work in pediatric oncology showed that trial enrollment correlated with race, age, and zip code, and it is difficult to know whether the results of the ANBL0532 trial are applicable to patient groups who may not be well represented.
An additional challenge is that even though a difference in event-free survival was detected between groups assigned to receive single versus tandem transplant, and a difference in overall survival was detected in a post hoc analysis of patients who received immunotherapy, no difference in overall survival was detected in the overall randomized cohort. Overall survival was evaluated as a secondary outcome but the trial was not powered to detect a difference in overall survival. Moreover, as noted by the authors, overall survival can be influenced by therapies delivered after relapse. This is particularly relevant in an era in which relapse therapies have been shown to induce responses, including periods of remission.
Remarks from Rochelle Bagatell, MD, from the University of Pennsylvania, Philadelphia, and Meredith S. Irwin, MD, from the Hospital for Sick Children in Toronto, are adapted and condensed from an editorial accompanying the study by Park et al. Dr. Bagatell is the vice chair of the Children’s Oncology Group Neuroblastoma Disease Committee. Dr. Irwin reported receiving personal fees from Bayer Canada outside the submitted work and is the vice chair of the Children’s Oncology Group Neuroblastoma Biology Committee. Neither Dr. Bagatell nor Dr. Irwin was involved in the design of the ANBL0532 trial or in the analysis of the results.
The ANBL0532 trial also does not address the important question as to whether tandem high-dose chemotherapy with autologous stem cell transplant results in benefit for all-comers with high-risk neuroblastoma, because just over half of the eligible patients underwent randomization. Although characteristics of the entire cohort and the randomized cohort were similar with respect to age, stage, tumor histology, and MYCN status, there may be differences unrelated to widely accepted neuroblastoma risk variables. A separate but important challenge in interpretation of these results, as with any clinical trial results, is to understand the generalizability of findings to patient populations who may not be enrolling in trials. Previous work in pediatric oncology showed that trial enrollment correlated with race, age, and zip code, and it is difficult to know whether the results of the ANBL0532 trial are applicable to patient groups who may not be well represented.
An additional challenge is that even though a difference in event-free survival was detected between groups assigned to receive single versus tandem transplant, and a difference in overall survival was detected in a post hoc analysis of patients who received immunotherapy, no difference in overall survival was detected in the overall randomized cohort. Overall survival was evaluated as a secondary outcome but the trial was not powered to detect a difference in overall survival. Moreover, as noted by the authors, overall survival can be influenced by therapies delivered after relapse. This is particularly relevant in an era in which relapse therapies have been shown to induce responses, including periods of remission.
Remarks from Rochelle Bagatell, MD, from the University of Pennsylvania, Philadelphia, and Meredith S. Irwin, MD, from the Hospital for Sick Children in Toronto, are adapted and condensed from an editorial accompanying the study by Park et al. Dr. Bagatell is the vice chair of the Children’s Oncology Group Neuroblastoma Disease Committee. Dr. Irwin reported receiving personal fees from Bayer Canada outside the submitted work and is the vice chair of the Children’s Oncology Group Neuroblastoma Biology Committee. Neither Dr. Bagatell nor Dr. Irwin was involved in the design of the ANBL0532 trial or in the analysis of the results.
For young patients with high-risk neuroblastoma, an intensive consolidation regimen with tandem autologous stem cell transplants was associated with significantly better event-free survival, compared with single-transplant consolidation, results of a randomized trial show.
Among 355 patients with high-risk neuroblastoma, the 3-year event-free survival (EFS) rate was 61.6% for patients randomized to tandem (sequential) autologous stem cell transplants, compared with 48.4% for patients randomized to a single transplant (P = .006), reported Julie R. Park, MD from Seattle Children’s Hospital in Washington, and coinvestigators in the Children’s Oncology Group’s ANBL0532 trial.
“Results of the current study are consistent with earlier trials demonstrating that induction chemotherapy followed by consolidation with autologous transplant improved EFS, compared with less intensive consolidation, and that further intensification of consolidation benefits some patients,” they wrote in JAMA.
But of the 652 patients enrolled in the study, only 355 were actually randomized. Although the randomization rate was slightly higher than anticipated, the authors acknowledged that the results may not apply to all patients with high-risk neuroblastoma.
Patients eligible for the trial included those with International Neuroblastoma Staging System (INSS) stage 4 neuroblastoma aged older than 18 months; INSS stage 3 neuroblastoma aged older than 18 months with International Neuroblastoma Pathology Classification of unfavorable histology; INSS stage 2, 3, 4, or 4S neuroblastoma with MYCN amplification; and INSS stage 4 neuroblastoma diagnosed from age 12-18 months whose tumors showed any unfavorable features. Patients initially diagnosed with non–high-risk neuroblastoma (including stage 1) who had not received chemotherapy and whose disease had progressed to high-risk neuroblastoma were also eligible.
Following induction with two cycles of topotecan and cyclophosphamide, patients underwent peripheral blood stem cell collection, followed by four alternating cycles of cisplatin and etoposide and doxorubicin and cyclophosphamide, and vincristine.
For those patients who did not have primary tumors resected at diagnosis, resection was performed after the fourth or fifth cycle.
Those patients who after induction had no disease progression, no uncontrolled infection, sufficient stem cell levels, and adequate organ function were then eligible for randomization. One patient did not receive any therapy, 27 were nonrandomly assigned to single transplant, 62 were not eligible for randomization, and 207 were not randomized because of physician or family preference.
Of the remaining patients (median age at diagnosis, 36.1 months) 176 were randomized to receive tandem transplant with thiotepa and cyclophosphamide followed by dose-reduced carboplatin, etoposide, and melphalan conditioning, and 179 were randomized to single transplant with standard-dose carboplatin, etoposide, and melphalan.
A total of 17 patients died on study from toxicity; 7 during induction and 10 during consolidation. Significant transplant-related toxicities included mucositis in 11.7% of tandem-transplant patients and 15.4% of single-transplant patients, and infections in 17.8% versus 18.3%, respectively.
As noted before, 3-year EFS from the time of randomization, the primary endpoint, was higher for patients in the tandem-transplant arm (61.6% vs. 48.4%, P = .006).
The median duration of follow-up after randomization for patients without relapse, disease progression, second malignancy, or death was 5.6 years.
A post hoc analysis of the randomized patients showed a 3-year overall survival rate of 71.6%, which did not differ significantly between the study arms (74.1% for the tandem-transplant group vs. 68.1% for the single-transplant group). The analysis also showed that 3-year EFS and overall survival was higher in the tandem- versus single-transplant groups among 250 patients who also received immunotherapy with isotretinoin plus an anti-GD2 chimeric antibody and cytokines.
The trial was supported by grants from the National Institutes of Health, National Cancer Institute, National Clinical Trials Network Operations Center, and St. Baldrick’s Foundation. Dr. Park reported no relevant disclosures. Multiple coauthors disclosed grants or personal feeds outside the submitted work.
SOURCE: Park JR et al. JAMA. 2019;322(8):746-55.
For young patients with high-risk neuroblastoma, an intensive consolidation regimen with tandem autologous stem cell transplants was associated with significantly better event-free survival, compared with single-transplant consolidation, results of a randomized trial show.
Among 355 patients with high-risk neuroblastoma, the 3-year event-free survival (EFS) rate was 61.6% for patients randomized to tandem (sequential) autologous stem cell transplants, compared with 48.4% for patients randomized to a single transplant (P = .006), reported Julie R. Park, MD from Seattle Children’s Hospital in Washington, and coinvestigators in the Children’s Oncology Group’s ANBL0532 trial.
“Results of the current study are consistent with earlier trials demonstrating that induction chemotherapy followed by consolidation with autologous transplant improved EFS, compared with less intensive consolidation, and that further intensification of consolidation benefits some patients,” they wrote in JAMA.
But of the 652 patients enrolled in the study, only 355 were actually randomized. Although the randomization rate was slightly higher than anticipated, the authors acknowledged that the results may not apply to all patients with high-risk neuroblastoma.
Patients eligible for the trial included those with International Neuroblastoma Staging System (INSS) stage 4 neuroblastoma aged older than 18 months; INSS stage 3 neuroblastoma aged older than 18 months with International Neuroblastoma Pathology Classification of unfavorable histology; INSS stage 2, 3, 4, or 4S neuroblastoma with MYCN amplification; and INSS stage 4 neuroblastoma diagnosed from age 12-18 months whose tumors showed any unfavorable features. Patients initially diagnosed with non–high-risk neuroblastoma (including stage 1) who had not received chemotherapy and whose disease had progressed to high-risk neuroblastoma were also eligible.
Following induction with two cycles of topotecan and cyclophosphamide, patients underwent peripheral blood stem cell collection, followed by four alternating cycles of cisplatin and etoposide and doxorubicin and cyclophosphamide, and vincristine.
For those patients who did not have primary tumors resected at diagnosis, resection was performed after the fourth or fifth cycle.
Those patients who after induction had no disease progression, no uncontrolled infection, sufficient stem cell levels, and adequate organ function were then eligible for randomization. One patient did not receive any therapy, 27 were nonrandomly assigned to single transplant, 62 were not eligible for randomization, and 207 were not randomized because of physician or family preference.
Of the remaining patients (median age at diagnosis, 36.1 months) 176 were randomized to receive tandem transplant with thiotepa and cyclophosphamide followed by dose-reduced carboplatin, etoposide, and melphalan conditioning, and 179 were randomized to single transplant with standard-dose carboplatin, etoposide, and melphalan.
A total of 17 patients died on study from toxicity; 7 during induction and 10 during consolidation. Significant transplant-related toxicities included mucositis in 11.7% of tandem-transplant patients and 15.4% of single-transplant patients, and infections in 17.8% versus 18.3%, respectively.
As noted before, 3-year EFS from the time of randomization, the primary endpoint, was higher for patients in the tandem-transplant arm (61.6% vs. 48.4%, P = .006).
The median duration of follow-up after randomization for patients without relapse, disease progression, second malignancy, or death was 5.6 years.
A post hoc analysis of the randomized patients showed a 3-year overall survival rate of 71.6%, which did not differ significantly between the study arms (74.1% for the tandem-transplant group vs. 68.1% for the single-transplant group). The analysis also showed that 3-year EFS and overall survival was higher in the tandem- versus single-transplant groups among 250 patients who also received immunotherapy with isotretinoin plus an anti-GD2 chimeric antibody and cytokines.
The trial was supported by grants from the National Institutes of Health, National Cancer Institute, National Clinical Trials Network Operations Center, and St. Baldrick’s Foundation. Dr. Park reported no relevant disclosures. Multiple coauthors disclosed grants or personal feeds outside the submitted work.
SOURCE: Park JR et al. JAMA. 2019;322(8):746-55.
FROM JAMA
Before the die is cast
When asked about my decision to choose pediatrics over the other specialty opportunities I was being offered, I have always answered that my choice was primarily based on my desire to work with children. That affinity certainly didn’t stem from my experience with my sister who is 7 years my junior. By her own admission, she was a bratty little thing and a major annoyance during my journey through adolescence. However, during the summers of high school and college I worked as a lifeguard, and one of my duties was to teach swimming classes. The joy and reward of watching children overcome their fear of the water and become competent swimmers left a positive impression, which was in stark contrast to the few classes of adult nonswimmers my coworkers and I taught. Our success rate with adults was pretty close to zero.
If I was going to spend my time and effort becoming a physician, I decided I wanted to be working with patients with the high potential for positive change and ones who had yet to accumulate a several decades long list of bad health habits. I wanted to be practicing in situations well before the die had been cast.
With this background in mind, you can understand why I was drawn to a recent article in the Harvard Gazette titled “Social spending on kids yields the biggest bang for the buck,” by Clea Simon. The article describes a recent study by Opportunity Insights, a Harvard-based institute of policy analysts and social scientists (“A Unified Welfare Analysis of Government Policies” by Nathaniel Hendren, PhD, and Ben Sprung-Keyser). Using computer algorithms capable of mining large pools of data, the researchers looked at 133 government policy changes over the last 50 years and compared the long-term results of those changes by assessing dollars spent against those returned in the form of tax revenue.
The Harvard article quotes Dr. Hendren as saying, This association was most impressive for children who came from lower-income families. This was especially true for programs that aimed at improving child health and increasing educational attainment.
Of course, these observations don’t come as a surprise to those of us who have accepted the challenge of improving the health of children. But it’s always nice to hear some new data that warms our hearts and reinforces our commitment to building healthy communities by focusing our efforts on its youngest members.
However, the paper did provide a finding that disappointed me. This big data analysis revealed that programs aimed at encouraging young people to attend college produced higher future earnings than did those focused on job training. I guess this shouldn’t be much of a surprise, but I believe we have been overemphasizing college track programs when we should be destigmatizing a career path in one of the trades. It may be that job training has been poorly done or at least not flexible enough to meet the changing demands of industry.
The investigators were surprised that their analysis demonstrated that policy changes targeted at children through their middle and high school years and even into college yielded return on investment at least as great if not greater than some successful preschool programs. Dr. Hendren responded to this finding by observing that “it’s never too late.” However, I think his comment deserves the loud and clear caveat, “as long as we are still talking about children.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
When asked about my decision to choose pediatrics over the other specialty opportunities I was being offered, I have always answered that my choice was primarily based on my desire to work with children. That affinity certainly didn’t stem from my experience with my sister who is 7 years my junior. By her own admission, she was a bratty little thing and a major annoyance during my journey through adolescence. However, during the summers of high school and college I worked as a lifeguard, and one of my duties was to teach swimming classes. The joy and reward of watching children overcome their fear of the water and become competent swimmers left a positive impression, which was in stark contrast to the few classes of adult nonswimmers my coworkers and I taught. Our success rate with adults was pretty close to zero.
If I was going to spend my time and effort becoming a physician, I decided I wanted to be working with patients with the high potential for positive change and ones who had yet to accumulate a several decades long list of bad health habits. I wanted to be practicing in situations well before the die had been cast.
With this background in mind, you can understand why I was drawn to a recent article in the Harvard Gazette titled “Social spending on kids yields the biggest bang for the buck,” by Clea Simon. The article describes a recent study by Opportunity Insights, a Harvard-based institute of policy analysts and social scientists (“A Unified Welfare Analysis of Government Policies” by Nathaniel Hendren, PhD, and Ben Sprung-Keyser). Using computer algorithms capable of mining large pools of data, the researchers looked at 133 government policy changes over the last 50 years and compared the long-term results of those changes by assessing dollars spent against those returned in the form of tax revenue.
The Harvard article quotes Dr. Hendren as saying, This association was most impressive for children who came from lower-income families. This was especially true for programs that aimed at improving child health and increasing educational attainment.
Of course, these observations don’t come as a surprise to those of us who have accepted the challenge of improving the health of children. But it’s always nice to hear some new data that warms our hearts and reinforces our commitment to building healthy communities by focusing our efforts on its youngest members.
However, the paper did provide a finding that disappointed me. This big data analysis revealed that programs aimed at encouraging young people to attend college produced higher future earnings than did those focused on job training. I guess this shouldn’t be much of a surprise, but I believe we have been overemphasizing college track programs when we should be destigmatizing a career path in one of the trades. It may be that job training has been poorly done or at least not flexible enough to meet the changing demands of industry.
The investigators were surprised that their analysis demonstrated that policy changes targeted at children through their middle and high school years and even into college yielded return on investment at least as great if not greater than some successful preschool programs. Dr. Hendren responded to this finding by observing that “it’s never too late.” However, I think his comment deserves the loud and clear caveat, “as long as we are still talking about children.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
When asked about my decision to choose pediatrics over the other specialty opportunities I was being offered, I have always answered that my choice was primarily based on my desire to work with children. That affinity certainly didn’t stem from my experience with my sister who is 7 years my junior. By her own admission, she was a bratty little thing and a major annoyance during my journey through adolescence. However, during the summers of high school and college I worked as a lifeguard, and one of my duties was to teach swimming classes. The joy and reward of watching children overcome their fear of the water and become competent swimmers left a positive impression, which was in stark contrast to the few classes of adult nonswimmers my coworkers and I taught. Our success rate with adults was pretty close to zero.
If I was going to spend my time and effort becoming a physician, I decided I wanted to be working with patients with the high potential for positive change and ones who had yet to accumulate a several decades long list of bad health habits. I wanted to be practicing in situations well before the die had been cast.
With this background in mind, you can understand why I was drawn to a recent article in the Harvard Gazette titled “Social spending on kids yields the biggest bang for the buck,” by Clea Simon. The article describes a recent study by Opportunity Insights, a Harvard-based institute of policy analysts and social scientists (“A Unified Welfare Analysis of Government Policies” by Nathaniel Hendren, PhD, and Ben Sprung-Keyser). Using computer algorithms capable of mining large pools of data, the researchers looked at 133 government policy changes over the last 50 years and compared the long-term results of those changes by assessing dollars spent against those returned in the form of tax revenue.
The Harvard article quotes Dr. Hendren as saying, This association was most impressive for children who came from lower-income families. This was especially true for programs that aimed at improving child health and increasing educational attainment.
Of course, these observations don’t come as a surprise to those of us who have accepted the challenge of improving the health of children. But it’s always nice to hear some new data that warms our hearts and reinforces our commitment to building healthy communities by focusing our efforts on its youngest members.
However, the paper did provide a finding that disappointed me. This big data analysis revealed that programs aimed at encouraging young people to attend college produced higher future earnings than did those focused on job training. I guess this shouldn’t be much of a surprise, but I believe we have been overemphasizing college track programs when we should be destigmatizing a career path in one of the trades. It may be that job training has been poorly done or at least not flexible enough to meet the changing demands of industry.
The investigators were surprised that their analysis demonstrated that policy changes targeted at children through their middle and high school years and even into college yielded return on investment at least as great if not greater than some successful preschool programs. Dr. Hendren responded to this finding by observing that “it’s never too late.” However, I think his comment deserves the loud and clear caveat, “as long as we are still talking about children.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
MenB vaccination coverage higher in those receiving MenB-4C
While meningococcal group B (MenB) vaccination remains suboptimal in the United States, completion was significantly higher for the MenB-4C (Bexsero) vaccine, compared with the other vaccine option, MenB-FHbp (Trumenba), according to Elizabeth Packnett of IBM Watson Health in Bethesda, Md., and associates.
In a study published in Vaccine, the investigators retrospectively analyzed 65,205 (36,118 received MenB-4C; 29,087 received MenB-FHbp) commercially insured individuals from the MarketScan Commercial Claims and Encounters Database during Jan. 1, 2015–Feb. 28, 2018, as well as 13,535 (10,153 received MenB-4C; 3,382 received MenB-FHbp) Medicaid-covered individuals from the Medicaid Multi-State Database during Jan. 1, 2015–Dec. 31, 2017.
The rate of vaccine completion in the MarketScan database within 15 months of initiation was 63% for MenB-4C and 52% for MenB-FHbp, and dosing schedule adherence was 62% for MenB-4C and 18% for MenB-FHbp. The median time to completion among those who finished vaccination was 68 days for MenB-4C versus 258 days for MenB-FHbp.
In the Medicaid database, the rate of vaccine completion within 15 months of initiation was 49% for MenB-4C and 31% for MenB-FHbp; dosing schedule adherence was 48% and 8%, respectively. Median time to vaccine completion was 88 days for MenB-4C versus 309 days for MenB-FHbp.
“The observations of improved completion and schedule adherence rates for MenB-4C, compared with MenB-FHbp, were consistent across both the commercial and Medicaid populations, and persisted after adjusting for individual factors in multivariable analyses, suggesting that the results were not skewed by population differences in demographic or other characteristics,” the investigators noted, adding that the significant difference in completion and schedule adherence between vaccines likely reflects the MenB-4C flexible dosing schedule.
The study was funded by GlaxoSmithKline, the maker of MenB-4C, and four coauthors reported being employed by the company. Five coauthors were employed by IBM Watson Health, which conducted the study.
SOURCE: Packnett E et al. Vaccine. 2019 Aug 20. doi: 10.1016/j.vaccine.2019.06.065.
While meningococcal group B (MenB) vaccination remains suboptimal in the United States, completion was significantly higher for the MenB-4C (Bexsero) vaccine, compared with the other vaccine option, MenB-FHbp (Trumenba), according to Elizabeth Packnett of IBM Watson Health in Bethesda, Md., and associates.
In a study published in Vaccine, the investigators retrospectively analyzed 65,205 (36,118 received MenB-4C; 29,087 received MenB-FHbp) commercially insured individuals from the MarketScan Commercial Claims and Encounters Database during Jan. 1, 2015–Feb. 28, 2018, as well as 13,535 (10,153 received MenB-4C; 3,382 received MenB-FHbp) Medicaid-covered individuals from the Medicaid Multi-State Database during Jan. 1, 2015–Dec. 31, 2017.
The rate of vaccine completion in the MarketScan database within 15 months of initiation was 63% for MenB-4C and 52% for MenB-FHbp, and dosing schedule adherence was 62% for MenB-4C and 18% for MenB-FHbp. The median time to completion among those who finished vaccination was 68 days for MenB-4C versus 258 days for MenB-FHbp.
In the Medicaid database, the rate of vaccine completion within 15 months of initiation was 49% for MenB-4C and 31% for MenB-FHbp; dosing schedule adherence was 48% and 8%, respectively. Median time to vaccine completion was 88 days for MenB-4C versus 309 days for MenB-FHbp.
“The observations of improved completion and schedule adherence rates for MenB-4C, compared with MenB-FHbp, were consistent across both the commercial and Medicaid populations, and persisted after adjusting for individual factors in multivariable analyses, suggesting that the results were not skewed by population differences in demographic or other characteristics,” the investigators noted, adding that the significant difference in completion and schedule adherence between vaccines likely reflects the MenB-4C flexible dosing schedule.
The study was funded by GlaxoSmithKline, the maker of MenB-4C, and four coauthors reported being employed by the company. Five coauthors were employed by IBM Watson Health, which conducted the study.
SOURCE: Packnett E et al. Vaccine. 2019 Aug 20. doi: 10.1016/j.vaccine.2019.06.065.
While meningococcal group B (MenB) vaccination remains suboptimal in the United States, completion was significantly higher for the MenB-4C (Bexsero) vaccine, compared with the other vaccine option, MenB-FHbp (Trumenba), according to Elizabeth Packnett of IBM Watson Health in Bethesda, Md., and associates.
In a study published in Vaccine, the investigators retrospectively analyzed 65,205 (36,118 received MenB-4C; 29,087 received MenB-FHbp) commercially insured individuals from the MarketScan Commercial Claims and Encounters Database during Jan. 1, 2015–Feb. 28, 2018, as well as 13,535 (10,153 received MenB-4C; 3,382 received MenB-FHbp) Medicaid-covered individuals from the Medicaid Multi-State Database during Jan. 1, 2015–Dec. 31, 2017.
The rate of vaccine completion in the MarketScan database within 15 months of initiation was 63% for MenB-4C and 52% for MenB-FHbp, and dosing schedule adherence was 62% for MenB-4C and 18% for MenB-FHbp. The median time to completion among those who finished vaccination was 68 days for MenB-4C versus 258 days for MenB-FHbp.
In the Medicaid database, the rate of vaccine completion within 15 months of initiation was 49% for MenB-4C and 31% for MenB-FHbp; dosing schedule adherence was 48% and 8%, respectively. Median time to vaccine completion was 88 days for MenB-4C versus 309 days for MenB-FHbp.
“The observations of improved completion and schedule adherence rates for MenB-4C, compared with MenB-FHbp, were consistent across both the commercial and Medicaid populations, and persisted after adjusting for individual factors in multivariable analyses, suggesting that the results were not skewed by population differences in demographic or other characteristics,” the investigators noted, adding that the significant difference in completion and schedule adherence between vaccines likely reflects the MenB-4C flexible dosing schedule.
The study was funded by GlaxoSmithKline, the maker of MenB-4C, and four coauthors reported being employed by the company. Five coauthors were employed by IBM Watson Health, which conducted the study.
SOURCE: Packnett E et al. Vaccine. 2019 Aug 20. doi: 10.1016/j.vaccine.2019.06.065.
FROM VACCINE