User login
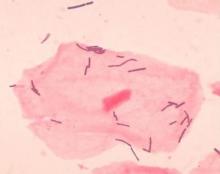
Diverse vaginal microbiome may signal risk for preterm birth
in an analysis of approximately 12,000 samples, according to a study published in Nature Medicine.
Preterm births, defined as less than 37 weeks’ gestation, remain the second most common cause of neonatal death worldwide, but few strategies exist to prevent and predict preterm birth (PTB) wrote Jennifer M. Fettweis, MD, of Virginia Commonwealth University, Richmond, and her colleagues. In the United States, women of African ancestry are at significantly greater risk for PTB.
A highly diverse vaginal microbiome is thought to be associated with an increased risk of inflammation, infection, and PTB, “however, many asymptomatic healthy women have diverse vaginal microbiota,” the researchers said.
To identify vaginal microbiota distinct to women who experienced PTB, the researchers analyzed data from the Multi-Omic Microbiome Study: Pregnancy Initiative (MOMS-PI), part of the National Institutes of Health–sponsored Integrative Human Microbiome Project. The MOMS-PI study included 12,039 samples of vaginal flora from 597 pregnancies; the analysis included 45 singleton pregnancies that met the criteria for spontaneous PTB (23-36 weeks, 6 days of gestation) and 90 case-matched full-term singleton pregnancies (greater than or equal to 39 weeks). Approximately 78% of the women were of African descent in both groups, and their average age was 26 years in both groups.
Overall, the diversity of the vaginal microbiome was greater among women who experienced PTB, compared with term birth (TB). Women who experienced PTB had less Lactobacillus crispatus, but more bacterial vaginosis–associated bacterium-1 (BVAB1), Prevotella cluster 2, and Sneathia amnii, compared with TB women.
Of note, vaginal cytokine data showed that proinflammatory cytokines, which may be associated with the induction of labor, may be prompted by inflammation in the vaginal microbiome, Dr. Fettweis and her associates said. “We observed that vaginal IP-10/CXCL10 levels were inversely correlated with BVAB1 in PTB, inversely correlated with L. crispatus in TB, and positively correlated with L. iners in TB, suggesting complex host-microbiome interactions in pregnancy,” they said.
“Further studies are needed to determine whether the signatures of PTB reported in the present study replicate in other cohorts of women of African ancestry, to examine whether the observed differences in vaginal microbiome composition between women of different ancestries has a direct causal link to the ethnic and racial disparities in PTB rates, and to establish whether population-specific microbial markers can be ultimately integrated into a generalizable spectrum of vaginal microbiome states linked to the risk for PTB,” Dr. Fettweis and her associates said.
In a companion study also published in Nature Medicine, Myrna G. Serrano, MD, also of Virginia Commonwealth University, and her colleagues as part of the MOMS-PI initially determined that vaginal microbiome profiles varied between 613 pregnant and 1,969 nonpregnant women in that “pregnant women had significantly higher prevalence of the four most common Lactobacillus vagitypes (L. crispatus, L. iners, L. gasseri, and L. jensenii) and a commensurately lower prevalence of vagitypes dominated by other taxa.” The primary driver of the differences was L. iners.
They then compared vaginal microbiome data from 300 pregnant and 300 nonpregnant case-matched women of African, Hispanic, or European ancestry, as well as 90 pregnant women (49 of African ancestry and 41 of European) ancestry.
In the subset of 300 pregnant and 300 nonpregnant women, the vaginal microbiome of the pregnant women overall became more dominated by Lactobacillus early in pregnancy. Further stratification by race showed that pregnant women of African and Hispanic ancestry had significantly higher levels of four types of Lactobacillus than their nonpregnant counterparts, but no significant difference was seen between pregnant and nonpregnant women of European ancestry.
“It appears that changes occurring during pregnancy may render the reproductive tracts of women of all racial backgrounds more hospitable to taxa of Lactobacillus and less favorable for Gardnerella vaginalis and other taxa associated with BV [bacterial vaginosis] and dysbiosis,” the researchers said.
“Interestingly, BVAB1, which has been associated with dysbiotic vaginal conditions and risk of PTB, and which is present as a major vagitype largely in women of African ancestry, is not noticeably decreased in prevalence in pregnancy,” Dr. Serrano and her associates said. “Thus, BVAB1, for reasons yet to be determined, is apparently resistant to factors sculpting the microbiome in pregnant women, possibly explaining in part the enhanced risk for PTB experienced by women of African ancestry.”
In a look at the 49 pregnant women of African ancestry and 41 of European ancestry, those of African ancestry had “significantly lower representation of the L. crispatus, L. gasseri and L. jensenii vagitypes, and higher representation of L. iners and BVAB1 vagitypes. Variability in women of African ancestry was driven by BVAB1 and L. iners, whereas variability in women of non-African ancestry was driven by L. crispatus and L. iners. Again, pregnancy had no significant effect on prevalence of the BVAB1 vagitype. Prevalence of Lactobacillus-dominated profiles in women of African ancestry was lower in the first than in later trimesters, whereas women of European ancestry had a higher prevalence of Lactobacillus vagitypes throughout pregnancy.”
The presence of vaginal microbiome profiles associated with adverse pregnancy outcomes highlights the need for further studies that take advantage of this information, Dr. Serrano and her associates said. “That the vaginal microbiomes known to confer higher risk of poor health and adverse outcomes of pregnancy are more highly associated with women of African and Hispanic ancestry, but that pregnancy tends to drive these microbiomes toward more favorable microbiota, suggests that an external intervention that favors this trend might be beneficial for these populations,” they concluded. “What remains is to verify the most favorable microbiome and the most effective strategy for intervention.”
Dr. Fettweis had no financial conflicts to disclose; two coauthors are full-time employees at Pacific Biosciences. Dr. Serrano and her coauthors had no relevant financial disclosures. Dr. Serrano’s study received grants from the National Institutes of Health and other sources, as well as support from the Common Fund, the National Center for Complementary and Integrative Health, the Office of Research on Women’s Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases.
SOURCES: Fettweis J et al. Nature Medicine 2019 May 29. doi: 10.1038/s41591-019-0450-2; Serrano M et al. Nature Medicine. 2019 May 29. doi: 10.1038/s41591-019-0465-8.
in an analysis of approximately 12,000 samples, according to a study published in Nature Medicine.
Preterm births, defined as less than 37 weeks’ gestation, remain the second most common cause of neonatal death worldwide, but few strategies exist to prevent and predict preterm birth (PTB) wrote Jennifer M. Fettweis, MD, of Virginia Commonwealth University, Richmond, and her colleagues. In the United States, women of African ancestry are at significantly greater risk for PTB.
A highly diverse vaginal microbiome is thought to be associated with an increased risk of inflammation, infection, and PTB, “however, many asymptomatic healthy women have diverse vaginal microbiota,” the researchers said.
To identify vaginal microbiota distinct to women who experienced PTB, the researchers analyzed data from the Multi-Omic Microbiome Study: Pregnancy Initiative (MOMS-PI), part of the National Institutes of Health–sponsored Integrative Human Microbiome Project. The MOMS-PI study included 12,039 samples of vaginal flora from 597 pregnancies; the analysis included 45 singleton pregnancies that met the criteria for spontaneous PTB (23-36 weeks, 6 days of gestation) and 90 case-matched full-term singleton pregnancies (greater than or equal to 39 weeks). Approximately 78% of the women were of African descent in both groups, and their average age was 26 years in both groups.
Overall, the diversity of the vaginal microbiome was greater among women who experienced PTB, compared with term birth (TB). Women who experienced PTB had less Lactobacillus crispatus, but more bacterial vaginosis–associated bacterium-1 (BVAB1), Prevotella cluster 2, and Sneathia amnii, compared with TB women.
Of note, vaginal cytokine data showed that proinflammatory cytokines, which may be associated with the induction of labor, may be prompted by inflammation in the vaginal microbiome, Dr. Fettweis and her associates said. “We observed that vaginal IP-10/CXCL10 levels were inversely correlated with BVAB1 in PTB, inversely correlated with L. crispatus in TB, and positively correlated with L. iners in TB, suggesting complex host-microbiome interactions in pregnancy,” they said.
“Further studies are needed to determine whether the signatures of PTB reported in the present study replicate in other cohorts of women of African ancestry, to examine whether the observed differences in vaginal microbiome composition between women of different ancestries has a direct causal link to the ethnic and racial disparities in PTB rates, and to establish whether population-specific microbial markers can be ultimately integrated into a generalizable spectrum of vaginal microbiome states linked to the risk for PTB,” Dr. Fettweis and her associates said.
In a companion study also published in Nature Medicine, Myrna G. Serrano, MD, also of Virginia Commonwealth University, and her colleagues as part of the MOMS-PI initially determined that vaginal microbiome profiles varied between 613 pregnant and 1,969 nonpregnant women in that “pregnant women had significantly higher prevalence of the four most common Lactobacillus vagitypes (L. crispatus, L. iners, L. gasseri, and L. jensenii) and a commensurately lower prevalence of vagitypes dominated by other taxa.” The primary driver of the differences was L. iners.
They then compared vaginal microbiome data from 300 pregnant and 300 nonpregnant case-matched women of African, Hispanic, or European ancestry, as well as 90 pregnant women (49 of African ancestry and 41 of European) ancestry.
In the subset of 300 pregnant and 300 nonpregnant women, the vaginal microbiome of the pregnant women overall became more dominated by Lactobacillus early in pregnancy. Further stratification by race showed that pregnant women of African and Hispanic ancestry had significantly higher levels of four types of Lactobacillus than their nonpregnant counterparts, but no significant difference was seen between pregnant and nonpregnant women of European ancestry.
“It appears that changes occurring during pregnancy may render the reproductive tracts of women of all racial backgrounds more hospitable to taxa of Lactobacillus and less favorable for Gardnerella vaginalis and other taxa associated with BV [bacterial vaginosis] and dysbiosis,” the researchers said.
“Interestingly, BVAB1, which has been associated with dysbiotic vaginal conditions and risk of PTB, and which is present as a major vagitype largely in women of African ancestry, is not noticeably decreased in prevalence in pregnancy,” Dr. Serrano and her associates said. “Thus, BVAB1, for reasons yet to be determined, is apparently resistant to factors sculpting the microbiome in pregnant women, possibly explaining in part the enhanced risk for PTB experienced by women of African ancestry.”
In a look at the 49 pregnant women of African ancestry and 41 of European ancestry, those of African ancestry had “significantly lower representation of the L. crispatus, L. gasseri and L. jensenii vagitypes, and higher representation of L. iners and BVAB1 vagitypes. Variability in women of African ancestry was driven by BVAB1 and L. iners, whereas variability in women of non-African ancestry was driven by L. crispatus and L. iners. Again, pregnancy had no significant effect on prevalence of the BVAB1 vagitype. Prevalence of Lactobacillus-dominated profiles in women of African ancestry was lower in the first than in later trimesters, whereas women of European ancestry had a higher prevalence of Lactobacillus vagitypes throughout pregnancy.”
The presence of vaginal microbiome profiles associated with adverse pregnancy outcomes highlights the need for further studies that take advantage of this information, Dr. Serrano and her associates said. “That the vaginal microbiomes known to confer higher risk of poor health and adverse outcomes of pregnancy are more highly associated with women of African and Hispanic ancestry, but that pregnancy tends to drive these microbiomes toward more favorable microbiota, suggests that an external intervention that favors this trend might be beneficial for these populations,” they concluded. “What remains is to verify the most favorable microbiome and the most effective strategy for intervention.”
Dr. Fettweis had no financial conflicts to disclose; two coauthors are full-time employees at Pacific Biosciences. Dr. Serrano and her coauthors had no relevant financial disclosures. Dr. Serrano’s study received grants from the National Institutes of Health and other sources, as well as support from the Common Fund, the National Center for Complementary and Integrative Health, the Office of Research on Women’s Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases.
SOURCES: Fettweis J et al. Nature Medicine 2019 May 29. doi: 10.1038/s41591-019-0450-2; Serrano M et al. Nature Medicine. 2019 May 29. doi: 10.1038/s41591-019-0465-8.
in an analysis of approximately 12,000 samples, according to a study published in Nature Medicine.
Preterm births, defined as less than 37 weeks’ gestation, remain the second most common cause of neonatal death worldwide, but few strategies exist to prevent and predict preterm birth (PTB) wrote Jennifer M. Fettweis, MD, of Virginia Commonwealth University, Richmond, and her colleagues. In the United States, women of African ancestry are at significantly greater risk for PTB.
A highly diverse vaginal microbiome is thought to be associated with an increased risk of inflammation, infection, and PTB, “however, many asymptomatic healthy women have diverse vaginal microbiota,” the researchers said.
To identify vaginal microbiota distinct to women who experienced PTB, the researchers analyzed data from the Multi-Omic Microbiome Study: Pregnancy Initiative (MOMS-PI), part of the National Institutes of Health–sponsored Integrative Human Microbiome Project. The MOMS-PI study included 12,039 samples of vaginal flora from 597 pregnancies; the analysis included 45 singleton pregnancies that met the criteria for spontaneous PTB (23-36 weeks, 6 days of gestation) and 90 case-matched full-term singleton pregnancies (greater than or equal to 39 weeks). Approximately 78% of the women were of African descent in both groups, and their average age was 26 years in both groups.
Overall, the diversity of the vaginal microbiome was greater among women who experienced PTB, compared with term birth (TB). Women who experienced PTB had less Lactobacillus crispatus, but more bacterial vaginosis–associated bacterium-1 (BVAB1), Prevotella cluster 2, and Sneathia amnii, compared with TB women.
Of note, vaginal cytokine data showed that proinflammatory cytokines, which may be associated with the induction of labor, may be prompted by inflammation in the vaginal microbiome, Dr. Fettweis and her associates said. “We observed that vaginal IP-10/CXCL10 levels were inversely correlated with BVAB1 in PTB, inversely correlated with L. crispatus in TB, and positively correlated with L. iners in TB, suggesting complex host-microbiome interactions in pregnancy,” they said.
“Further studies are needed to determine whether the signatures of PTB reported in the present study replicate in other cohorts of women of African ancestry, to examine whether the observed differences in vaginal microbiome composition between women of different ancestries has a direct causal link to the ethnic and racial disparities in PTB rates, and to establish whether population-specific microbial markers can be ultimately integrated into a generalizable spectrum of vaginal microbiome states linked to the risk for PTB,” Dr. Fettweis and her associates said.
In a companion study also published in Nature Medicine, Myrna G. Serrano, MD, also of Virginia Commonwealth University, and her colleagues as part of the MOMS-PI initially determined that vaginal microbiome profiles varied between 613 pregnant and 1,969 nonpregnant women in that “pregnant women had significantly higher prevalence of the four most common Lactobacillus vagitypes (L. crispatus, L. iners, L. gasseri, and L. jensenii) and a commensurately lower prevalence of vagitypes dominated by other taxa.” The primary driver of the differences was L. iners.
They then compared vaginal microbiome data from 300 pregnant and 300 nonpregnant case-matched women of African, Hispanic, or European ancestry, as well as 90 pregnant women (49 of African ancestry and 41 of European) ancestry.
In the subset of 300 pregnant and 300 nonpregnant women, the vaginal microbiome of the pregnant women overall became more dominated by Lactobacillus early in pregnancy. Further stratification by race showed that pregnant women of African and Hispanic ancestry had significantly higher levels of four types of Lactobacillus than their nonpregnant counterparts, but no significant difference was seen between pregnant and nonpregnant women of European ancestry.
“It appears that changes occurring during pregnancy may render the reproductive tracts of women of all racial backgrounds more hospitable to taxa of Lactobacillus and less favorable for Gardnerella vaginalis and other taxa associated with BV [bacterial vaginosis] and dysbiosis,” the researchers said.
“Interestingly, BVAB1, which has been associated with dysbiotic vaginal conditions and risk of PTB, and which is present as a major vagitype largely in women of African ancestry, is not noticeably decreased in prevalence in pregnancy,” Dr. Serrano and her associates said. “Thus, BVAB1, for reasons yet to be determined, is apparently resistant to factors sculpting the microbiome in pregnant women, possibly explaining in part the enhanced risk for PTB experienced by women of African ancestry.”
In a look at the 49 pregnant women of African ancestry and 41 of European ancestry, those of African ancestry had “significantly lower representation of the L. crispatus, L. gasseri and L. jensenii vagitypes, and higher representation of L. iners and BVAB1 vagitypes. Variability in women of African ancestry was driven by BVAB1 and L. iners, whereas variability in women of non-African ancestry was driven by L. crispatus and L. iners. Again, pregnancy had no significant effect on prevalence of the BVAB1 vagitype. Prevalence of Lactobacillus-dominated profiles in women of African ancestry was lower in the first than in later trimesters, whereas women of European ancestry had a higher prevalence of Lactobacillus vagitypes throughout pregnancy.”
The presence of vaginal microbiome profiles associated with adverse pregnancy outcomes highlights the need for further studies that take advantage of this information, Dr. Serrano and her associates said. “That the vaginal microbiomes known to confer higher risk of poor health and adverse outcomes of pregnancy are more highly associated with women of African and Hispanic ancestry, but that pregnancy tends to drive these microbiomes toward more favorable microbiota, suggests that an external intervention that favors this trend might be beneficial for these populations,” they concluded. “What remains is to verify the most favorable microbiome and the most effective strategy for intervention.”
Dr. Fettweis had no financial conflicts to disclose; two coauthors are full-time employees at Pacific Biosciences. Dr. Serrano and her coauthors had no relevant financial disclosures. Dr. Serrano’s study received grants from the National Institutes of Health and other sources, as well as support from the Common Fund, the National Center for Complementary and Integrative Health, the Office of Research on Women’s Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases.
SOURCES: Fettweis J et al. Nature Medicine 2019 May 29. doi: 10.1038/s41591-019-0450-2; Serrano M et al. Nature Medicine. 2019 May 29. doi: 10.1038/s41591-019-0465-8.
FROM NATURE MEDICINE
Pediatric MS often goes untreated in the year after diagnosis
SEATTLE – Females may be more likely than males to receive DMT during this time, said Chinmay Deshpande, PhD, at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Pediatric onset of MS occurs in 3-5% of patients with the disease, and the median age of pediatric onset is 15 years. This population tends to have a high relapse rate and may develop disability at a younger age, said Dr. Deshpande, associate director of health economics and outcomes research at Novartis. “There have been very few studies done on this population, especially in the clinical trial setting. ... Physicians face considerable uncertainty in how to treat these patients.”
Observational data
To assess the proportion of patients with pediatric MS who receive DMT in the year after diagnosis, Dr. Deshpande and colleagues analyzed retrospective observational data from the Truven Health Marketscan Commercial and Encounters administrative claims databases. They studied patients who received an MS diagnosis between Jan. 1, 2010, and Dec. 31, 2016. In addition, they examined which DMTs were used as first-line therapies, whether prescribing patterns changed between 2010 and 2017, and time to treatment discontinuation or switch.
The databases included data from more than 182,000 patients with two or more claims of MS diagnosis. After including only patients age 17 years or younger at the index diagnosis date who had insurance during the 6 months prior to the index date and 12 months after the index date and who did not use DMT during the 6 months prior to the index date, 288 patients remained in the analysis. Patients had an average age of about 14 years.
The primary outcome was the proportion of patients who started DMT in the year after MS diagnosis. “The proportion of untreated patients within their first year of diagnosis was around 65%,” said Dr. Deshpande. On average, treated patients were slightly older than untreated patients (15.0 years vs. 13.3 years). Among treated patients, 75% were female, and 25% were male. Overall, however, 61% were female and 39% were male. The difference in treatment by gender was surprising and the reason for it is not understood, Dr. Deshpande said. One possibility is that the difference relates to earlier maturation in females, but that is only a hypothesis, he said.
Glatiramer acetate and interferon beta-1a were first-line DMTs for 48% and 30.6% of the treated patients, respectively. Dimethyl fumarate (7.1%), natalizumab (5.1%), fingolimod (4.1%), interferon beta-1b (4.1%), and peginterferon beta-1a (1%) also were used as first-line therapy.
Twenty percent of patients who received DMT switched to another medication during the follow-up period. The median time of switching was within 6 months of starting first-line therapy. Most patients who discontinued DMT – that is, they did not have any DMT for 60 days after stopping their first DMT – did so within 10 months of diagnosis.
Use of newer medications
Overall, the use of glatiramer acetate and interferons has decreased over time, and while the use of newer DMTs has increased, the trend is not consistent. “With the growing uptake of newer oral and infusible DMTs over the recent years, there is a need to increase treatment awareness in the pediatric MS population and to inform currently approved treatment options to the prescribers,” Dr. Deshpande and colleagues said.
The claims database is generalizable and nationally representative, but it does not include clinical or MRI data. “It’s hard to understand the reasoning why they discontinued or why they are switching,” Dr. Deshpande said. In addition, the sample size was relatively small, and the results should be interpreted accordingly, he said.
Novartis funded the study, and Dr. Deshpande and a coauthor are employees of Novartis. Other coauthors reported consulting fees from Novartis, as well as consulting fees and grant funding from other pharmaceutical companies.
SOURCE: Greenberg B et al. CMSC 2019, Abstract DXM02.
SEATTLE – Females may be more likely than males to receive DMT during this time, said Chinmay Deshpande, PhD, at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Pediatric onset of MS occurs in 3-5% of patients with the disease, and the median age of pediatric onset is 15 years. This population tends to have a high relapse rate and may develop disability at a younger age, said Dr. Deshpande, associate director of health economics and outcomes research at Novartis. “There have been very few studies done on this population, especially in the clinical trial setting. ... Physicians face considerable uncertainty in how to treat these patients.”
Observational data
To assess the proportion of patients with pediatric MS who receive DMT in the year after diagnosis, Dr. Deshpande and colleagues analyzed retrospective observational data from the Truven Health Marketscan Commercial and Encounters administrative claims databases. They studied patients who received an MS diagnosis between Jan. 1, 2010, and Dec. 31, 2016. In addition, they examined which DMTs were used as first-line therapies, whether prescribing patterns changed between 2010 and 2017, and time to treatment discontinuation or switch.
The databases included data from more than 182,000 patients with two or more claims of MS diagnosis. After including only patients age 17 years or younger at the index diagnosis date who had insurance during the 6 months prior to the index date and 12 months after the index date and who did not use DMT during the 6 months prior to the index date, 288 patients remained in the analysis. Patients had an average age of about 14 years.
The primary outcome was the proportion of patients who started DMT in the year after MS diagnosis. “The proportion of untreated patients within their first year of diagnosis was around 65%,” said Dr. Deshpande. On average, treated patients were slightly older than untreated patients (15.0 years vs. 13.3 years). Among treated patients, 75% were female, and 25% were male. Overall, however, 61% were female and 39% were male. The difference in treatment by gender was surprising and the reason for it is not understood, Dr. Deshpande said. One possibility is that the difference relates to earlier maturation in females, but that is only a hypothesis, he said.
Glatiramer acetate and interferon beta-1a were first-line DMTs for 48% and 30.6% of the treated patients, respectively. Dimethyl fumarate (7.1%), natalizumab (5.1%), fingolimod (4.1%), interferon beta-1b (4.1%), and peginterferon beta-1a (1%) also were used as first-line therapy.
Twenty percent of patients who received DMT switched to another medication during the follow-up period. The median time of switching was within 6 months of starting first-line therapy. Most patients who discontinued DMT – that is, they did not have any DMT for 60 days after stopping their first DMT – did so within 10 months of diagnosis.
Use of newer medications
Overall, the use of glatiramer acetate and interferons has decreased over time, and while the use of newer DMTs has increased, the trend is not consistent. “With the growing uptake of newer oral and infusible DMTs over the recent years, there is a need to increase treatment awareness in the pediatric MS population and to inform currently approved treatment options to the prescribers,” Dr. Deshpande and colleagues said.
The claims database is generalizable and nationally representative, but it does not include clinical or MRI data. “It’s hard to understand the reasoning why they discontinued or why they are switching,” Dr. Deshpande said. In addition, the sample size was relatively small, and the results should be interpreted accordingly, he said.
Novartis funded the study, and Dr. Deshpande and a coauthor are employees of Novartis. Other coauthors reported consulting fees from Novartis, as well as consulting fees and grant funding from other pharmaceutical companies.
SOURCE: Greenberg B et al. CMSC 2019, Abstract DXM02.
SEATTLE – Females may be more likely than males to receive DMT during this time, said Chinmay Deshpande, PhD, at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Pediatric onset of MS occurs in 3-5% of patients with the disease, and the median age of pediatric onset is 15 years. This population tends to have a high relapse rate and may develop disability at a younger age, said Dr. Deshpande, associate director of health economics and outcomes research at Novartis. “There have been very few studies done on this population, especially in the clinical trial setting. ... Physicians face considerable uncertainty in how to treat these patients.”
Observational data
To assess the proportion of patients with pediatric MS who receive DMT in the year after diagnosis, Dr. Deshpande and colleagues analyzed retrospective observational data from the Truven Health Marketscan Commercial and Encounters administrative claims databases. They studied patients who received an MS diagnosis between Jan. 1, 2010, and Dec. 31, 2016. In addition, they examined which DMTs were used as first-line therapies, whether prescribing patterns changed between 2010 and 2017, and time to treatment discontinuation or switch.
The databases included data from more than 182,000 patients with two or more claims of MS diagnosis. After including only patients age 17 years or younger at the index diagnosis date who had insurance during the 6 months prior to the index date and 12 months after the index date and who did not use DMT during the 6 months prior to the index date, 288 patients remained in the analysis. Patients had an average age of about 14 years.
The primary outcome was the proportion of patients who started DMT in the year after MS diagnosis. “The proportion of untreated patients within their first year of diagnosis was around 65%,” said Dr. Deshpande. On average, treated patients were slightly older than untreated patients (15.0 years vs. 13.3 years). Among treated patients, 75% were female, and 25% were male. Overall, however, 61% were female and 39% were male. The difference in treatment by gender was surprising and the reason for it is not understood, Dr. Deshpande said. One possibility is that the difference relates to earlier maturation in females, but that is only a hypothesis, he said.
Glatiramer acetate and interferon beta-1a were first-line DMTs for 48% and 30.6% of the treated patients, respectively. Dimethyl fumarate (7.1%), natalizumab (5.1%), fingolimod (4.1%), interferon beta-1b (4.1%), and peginterferon beta-1a (1%) also were used as first-line therapy.
Twenty percent of patients who received DMT switched to another medication during the follow-up period. The median time of switching was within 6 months of starting first-line therapy. Most patients who discontinued DMT – that is, they did not have any DMT for 60 days after stopping their first DMT – did so within 10 months of diagnosis.
Use of newer medications
Overall, the use of glatiramer acetate and interferons has decreased over time, and while the use of newer DMTs has increased, the trend is not consistent. “With the growing uptake of newer oral and infusible DMTs over the recent years, there is a need to increase treatment awareness in the pediatric MS population and to inform currently approved treatment options to the prescribers,” Dr. Deshpande and colleagues said.
The claims database is generalizable and nationally representative, but it does not include clinical or MRI data. “It’s hard to understand the reasoning why they discontinued or why they are switching,” Dr. Deshpande said. In addition, the sample size was relatively small, and the results should be interpreted accordingly, he said.
Novartis funded the study, and Dr. Deshpande and a coauthor are employees of Novartis. Other coauthors reported consulting fees from Novartis, as well as consulting fees and grant funding from other pharmaceutical companies.
SOURCE: Greenberg B et al. CMSC 2019, Abstract DXM02.
REPORTING FROM CMSC 2019
A warning song to keep our children safe
Pay heed to “The House of the Rising Sun”
“There is a house in New Orleans. They call the Rising Sun. And it’s been the ruin of many a poor boy. And, God, I know I’m one.”
The 1960s rock band the Animals will tell you a tale to convince you to get vaccinated. Don’t believe me? Follow along.
The first hints of the song “House of the Rising Sun” rolled out of the hills of Appalachia.
Somewhere in the Golden Triangle, far away from New Orleans, where Virginia, Kentucky, and Tennessee rise in quiet desolation, a warning song about a tailor and a drunk emerged. Sometime around the Civil War, a hint of a tune began. Over the next century, it evolved, until it became cemented in rock culture 50 years ago by The Animals, existing as the version played most commonly today.
In the mid-19th century, medicine shows rambled through the South, stopping in places like Noetown or Daisy. The small towns would empty out for the day to see the entertainers, singers, and jugglers perform. Hundreds gathered in the hot summer day, the entertainment solely a pretext for the traveling doctors to sell their wares, the snake oil, and cure-alls, as well as various patent medicines.
These were isolated towns, with no deliveries, few visitors, and the railroad yet to arrive. Frequently, the only news from outside came from these caravans of entertainers and con men who swept into town. They were like Professor Marvel from The Wizard of Oz, or a current-day Dr. Oz, luring the crowd with false advertising, selling colored water, and then disappearing before you realized you were duped. Today, traveling doctors of the same ilk convince parents to not vaccinate their children, tell them to visit stem cell centers that claim false cures, and offer them a shiny object with one hand while taking their cash with the other.
Yet, there was a positive development in the wake of these patent medicine shows: the entertainment lingered. New songs traveled the same journeys as these medicine shows – new earworms that would then be warbled in the local bars, while doing chores around the barn, or simply during walks on the Appalachian trails.
In 1937, Alan Lomax arrived in Noetown, Ky., with a microphone and an acetate record and recorded the voice of 16-year-old Georgia Turner singing “House of the Rising Sun.” She didn’t know where she heard that song, but most likely picked it up at the medicine show.
One of those singers was Clarence Ashley, who would croon about the Rising Sun Blues. He sang with Doc Cloud and Doc Hauer, who offered tonics for whatever ailed you. Perhaps Georgia Turner heard the song in the early 1900s as well. Her 1937 version contains the lyrics most closely related to the Animals’ tune.
Lomax spent the 1940s gathering songs around the Appalachian South. He put these songs into a songbook and spread them throughout the country. He would also return to New York City and gather in a room with legendary folk singers. They would hear these new lyrics, new sounds, and make them their own.
In that room would be Lead Belly, Pete Seeger, Woody Guthrie, and Josh White, the fathers of folk music. The music Lomax pulled out of the mountains in small towns would become new again in the guitars and harmonicas of the Greenwich Village singers and musicians. Pete Seeger performed with the Weavers, named because they would weave songs from the past into new versions.
“House of the Rising Sun” was woven into the folk music landscape, evolving and growing. Josh White is credited with changing the song from a major key into the minor key we know today. Bob Dylan sang a version. And then in 1964, Eric Burdon and The Animals released their version, which became the standard. An arpeggio guitar opening, the rhythm sped up, a louder sound, and that minor key provides an emotional wallop for this warning song.
Numerous covers followed, including a beautiful version of “Amazing Grace”, sung to the tune of “House of the Rising Sun” by the Blind Boys of Alabama.
The song endures for its melody as well as for its lyrics. This was a warning song, a universal song, “not to do what I have done.” The small towns in Kentucky may have heard of the sinful ways of New Orleans and would spread the message with these songs to avoid the brothels, the drink, and the broken marriages that would reverberate with visits to the Crescent City.
“House of the Rising Sun” is one of the most covered songs, traveling wide and far, no longer with the need for a medicine show. It was a pivotal moment in rock ‘n roll, turning folk music into rock music. The Animals became huge because of this song, and their version became the standard on which all subsequent covers based their version. It made Bob Dylan’s older version seem quaint.
The song has been in my head for a while now. My wife is hoping writing about it will keep it from being played in our household any more. There are various reasons it has been resonating with me, including the following:
- It traces the origins of folk music and the importance of people like Lomax and Guthrie to collect and save Americana.
- The magic of musical evolution – a reminder of how art is built on the work of those who came before, each version with its unique personality.
- The release of “House of the Rising Sun” was a seminal, transformative moment when folk became rock music.
- The lasting power of warning songs.
- The hucksters that enabled this song to be kept alive.
That last one has really stuck with me. The medicine shows are an important part of American history. For instance, Coca-Cola started as one of those patent medicines; it was one of the many concoctions of the Atlanta pharmacist John Stith Pemberton, sold to treat all that ails us. Dr. Pepper, too, was a medicine in a sugary bottle – another that often contained alcohol or cocaine. Society wants a cure-all, and the marketing and selling done during these medicine shows offered placebos.
The hucksters exist in various forms today, selling detoxifications, magic diet cures, psychic powers of healing, or convincing parents that their kids don’t need vaccines. We need a warning song that goes viral to keep our children safe. We are blessed to be in a world without smallpox, almost rid of polio, and we have the knowledge and opportunity to rid the world of other preventable illnesses. Measles was declared eliminated in the United States in 2000; now, outbreaks emerge in every news cycle.
The CDC admits they have not been targeting misinformation well. How can we spread the science, the truth, the message faster than the lies? Better marketing? The answer may be through stories and narratives and song, with the backing of good science. “House of the Rising Sun” is a warning song. Maybe we need more. We need that deep history, that long trail to remind us of the world before vaccines, when everyone knew someone, either in their own household or next door, who succumbed to one of the childhood illnesses.
Let the “House of the Rising Sun” play on. Create a new version, and let that message reverberate, too.
Tell your children; they need to be vaccinated.
Dr. Messler is a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. He previously chaired SHM’s Quality and Patient Safety Committee and has been active in several SHM mentoring programs, most recently with Project BOOST and Glycemic Control. This article appeared originally in SHM's official blog The Hospital Leader. Read more recent posts here.
Pay heed to “The House of the Rising Sun”
Pay heed to “The House of the Rising Sun”
“There is a house in New Orleans. They call the Rising Sun. And it’s been the ruin of many a poor boy. And, God, I know I’m one.”
The 1960s rock band the Animals will tell you a tale to convince you to get vaccinated. Don’t believe me? Follow along.
The first hints of the song “House of the Rising Sun” rolled out of the hills of Appalachia.
Somewhere in the Golden Triangle, far away from New Orleans, where Virginia, Kentucky, and Tennessee rise in quiet desolation, a warning song about a tailor and a drunk emerged. Sometime around the Civil War, a hint of a tune began. Over the next century, it evolved, until it became cemented in rock culture 50 years ago by The Animals, existing as the version played most commonly today.
In the mid-19th century, medicine shows rambled through the South, stopping in places like Noetown or Daisy. The small towns would empty out for the day to see the entertainers, singers, and jugglers perform. Hundreds gathered in the hot summer day, the entertainment solely a pretext for the traveling doctors to sell their wares, the snake oil, and cure-alls, as well as various patent medicines.
These were isolated towns, with no deliveries, few visitors, and the railroad yet to arrive. Frequently, the only news from outside came from these caravans of entertainers and con men who swept into town. They were like Professor Marvel from The Wizard of Oz, or a current-day Dr. Oz, luring the crowd with false advertising, selling colored water, and then disappearing before you realized you were duped. Today, traveling doctors of the same ilk convince parents to not vaccinate their children, tell them to visit stem cell centers that claim false cures, and offer them a shiny object with one hand while taking their cash with the other.
Yet, there was a positive development in the wake of these patent medicine shows: the entertainment lingered. New songs traveled the same journeys as these medicine shows – new earworms that would then be warbled in the local bars, while doing chores around the barn, or simply during walks on the Appalachian trails.
In 1937, Alan Lomax arrived in Noetown, Ky., with a microphone and an acetate record and recorded the voice of 16-year-old Georgia Turner singing “House of the Rising Sun.” She didn’t know where she heard that song, but most likely picked it up at the medicine show.
One of those singers was Clarence Ashley, who would croon about the Rising Sun Blues. He sang with Doc Cloud and Doc Hauer, who offered tonics for whatever ailed you. Perhaps Georgia Turner heard the song in the early 1900s as well. Her 1937 version contains the lyrics most closely related to the Animals’ tune.
Lomax spent the 1940s gathering songs around the Appalachian South. He put these songs into a songbook and spread them throughout the country. He would also return to New York City and gather in a room with legendary folk singers. They would hear these new lyrics, new sounds, and make them their own.
In that room would be Lead Belly, Pete Seeger, Woody Guthrie, and Josh White, the fathers of folk music. The music Lomax pulled out of the mountains in small towns would become new again in the guitars and harmonicas of the Greenwich Village singers and musicians. Pete Seeger performed with the Weavers, named because they would weave songs from the past into new versions.
“House of the Rising Sun” was woven into the folk music landscape, evolving and growing. Josh White is credited with changing the song from a major key into the minor key we know today. Bob Dylan sang a version. And then in 1964, Eric Burdon and The Animals released their version, which became the standard. An arpeggio guitar opening, the rhythm sped up, a louder sound, and that minor key provides an emotional wallop for this warning song.
Numerous covers followed, including a beautiful version of “Amazing Grace”, sung to the tune of “House of the Rising Sun” by the Blind Boys of Alabama.
The song endures for its melody as well as for its lyrics. This was a warning song, a universal song, “not to do what I have done.” The small towns in Kentucky may have heard of the sinful ways of New Orleans and would spread the message with these songs to avoid the brothels, the drink, and the broken marriages that would reverberate with visits to the Crescent City.
“House of the Rising Sun” is one of the most covered songs, traveling wide and far, no longer with the need for a medicine show. It was a pivotal moment in rock ‘n roll, turning folk music into rock music. The Animals became huge because of this song, and their version became the standard on which all subsequent covers based their version. It made Bob Dylan’s older version seem quaint.
The song has been in my head for a while now. My wife is hoping writing about it will keep it from being played in our household any more. There are various reasons it has been resonating with me, including the following:
- It traces the origins of folk music and the importance of people like Lomax and Guthrie to collect and save Americana.
- The magic of musical evolution – a reminder of how art is built on the work of those who came before, each version with its unique personality.
- The release of “House of the Rising Sun” was a seminal, transformative moment when folk became rock music.
- The lasting power of warning songs.
- The hucksters that enabled this song to be kept alive.
That last one has really stuck with me. The medicine shows are an important part of American history. For instance, Coca-Cola started as one of those patent medicines; it was one of the many concoctions of the Atlanta pharmacist John Stith Pemberton, sold to treat all that ails us. Dr. Pepper, too, was a medicine in a sugary bottle – another that often contained alcohol or cocaine. Society wants a cure-all, and the marketing and selling done during these medicine shows offered placebos.
The hucksters exist in various forms today, selling detoxifications, magic diet cures, psychic powers of healing, or convincing parents that their kids don’t need vaccines. We need a warning song that goes viral to keep our children safe. We are blessed to be in a world without smallpox, almost rid of polio, and we have the knowledge and opportunity to rid the world of other preventable illnesses. Measles was declared eliminated in the United States in 2000; now, outbreaks emerge in every news cycle.
The CDC admits they have not been targeting misinformation well. How can we spread the science, the truth, the message faster than the lies? Better marketing? The answer may be through stories and narratives and song, with the backing of good science. “House of the Rising Sun” is a warning song. Maybe we need more. We need that deep history, that long trail to remind us of the world before vaccines, when everyone knew someone, either in their own household or next door, who succumbed to one of the childhood illnesses.
Let the “House of the Rising Sun” play on. Create a new version, and let that message reverberate, too.
Tell your children; they need to be vaccinated.
Dr. Messler is a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. He previously chaired SHM’s Quality and Patient Safety Committee and has been active in several SHM mentoring programs, most recently with Project BOOST and Glycemic Control. This article appeared originally in SHM's official blog The Hospital Leader. Read more recent posts here.
“There is a house in New Orleans. They call the Rising Sun. And it’s been the ruin of many a poor boy. And, God, I know I’m one.”
The 1960s rock band the Animals will tell you a tale to convince you to get vaccinated. Don’t believe me? Follow along.
The first hints of the song “House of the Rising Sun” rolled out of the hills of Appalachia.
Somewhere in the Golden Triangle, far away from New Orleans, where Virginia, Kentucky, and Tennessee rise in quiet desolation, a warning song about a tailor and a drunk emerged. Sometime around the Civil War, a hint of a tune began. Over the next century, it evolved, until it became cemented in rock culture 50 years ago by The Animals, existing as the version played most commonly today.
In the mid-19th century, medicine shows rambled through the South, stopping in places like Noetown or Daisy. The small towns would empty out for the day to see the entertainers, singers, and jugglers perform. Hundreds gathered in the hot summer day, the entertainment solely a pretext for the traveling doctors to sell their wares, the snake oil, and cure-alls, as well as various patent medicines.
These were isolated towns, with no deliveries, few visitors, and the railroad yet to arrive. Frequently, the only news from outside came from these caravans of entertainers and con men who swept into town. They were like Professor Marvel from The Wizard of Oz, or a current-day Dr. Oz, luring the crowd with false advertising, selling colored water, and then disappearing before you realized you were duped. Today, traveling doctors of the same ilk convince parents to not vaccinate their children, tell them to visit stem cell centers that claim false cures, and offer them a shiny object with one hand while taking their cash with the other.
Yet, there was a positive development in the wake of these patent medicine shows: the entertainment lingered. New songs traveled the same journeys as these medicine shows – new earworms that would then be warbled in the local bars, while doing chores around the barn, or simply during walks on the Appalachian trails.
In 1937, Alan Lomax arrived in Noetown, Ky., with a microphone and an acetate record and recorded the voice of 16-year-old Georgia Turner singing “House of the Rising Sun.” She didn’t know where she heard that song, but most likely picked it up at the medicine show.
One of those singers was Clarence Ashley, who would croon about the Rising Sun Blues. He sang with Doc Cloud and Doc Hauer, who offered tonics for whatever ailed you. Perhaps Georgia Turner heard the song in the early 1900s as well. Her 1937 version contains the lyrics most closely related to the Animals’ tune.
Lomax spent the 1940s gathering songs around the Appalachian South. He put these songs into a songbook and spread them throughout the country. He would also return to New York City and gather in a room with legendary folk singers. They would hear these new lyrics, new sounds, and make them their own.
In that room would be Lead Belly, Pete Seeger, Woody Guthrie, and Josh White, the fathers of folk music. The music Lomax pulled out of the mountains in small towns would become new again in the guitars and harmonicas of the Greenwich Village singers and musicians. Pete Seeger performed with the Weavers, named because they would weave songs from the past into new versions.
“House of the Rising Sun” was woven into the folk music landscape, evolving and growing. Josh White is credited with changing the song from a major key into the minor key we know today. Bob Dylan sang a version. And then in 1964, Eric Burdon and The Animals released their version, which became the standard. An arpeggio guitar opening, the rhythm sped up, a louder sound, and that minor key provides an emotional wallop for this warning song.
Numerous covers followed, including a beautiful version of “Amazing Grace”, sung to the tune of “House of the Rising Sun” by the Blind Boys of Alabama.
The song endures for its melody as well as for its lyrics. This was a warning song, a universal song, “not to do what I have done.” The small towns in Kentucky may have heard of the sinful ways of New Orleans and would spread the message with these songs to avoid the brothels, the drink, and the broken marriages that would reverberate with visits to the Crescent City.
“House of the Rising Sun” is one of the most covered songs, traveling wide and far, no longer with the need for a medicine show. It was a pivotal moment in rock ‘n roll, turning folk music into rock music. The Animals became huge because of this song, and their version became the standard on which all subsequent covers based their version. It made Bob Dylan’s older version seem quaint.
The song has been in my head for a while now. My wife is hoping writing about it will keep it from being played in our household any more. There are various reasons it has been resonating with me, including the following:
- It traces the origins of folk music and the importance of people like Lomax and Guthrie to collect and save Americana.
- The magic of musical evolution – a reminder of how art is built on the work of those who came before, each version with its unique personality.
- The release of “House of the Rising Sun” was a seminal, transformative moment when folk became rock music.
- The lasting power of warning songs.
- The hucksters that enabled this song to be kept alive.
That last one has really stuck with me. The medicine shows are an important part of American history. For instance, Coca-Cola started as one of those patent medicines; it was one of the many concoctions of the Atlanta pharmacist John Stith Pemberton, sold to treat all that ails us. Dr. Pepper, too, was a medicine in a sugary bottle – another that often contained alcohol or cocaine. Society wants a cure-all, and the marketing and selling done during these medicine shows offered placebos.
The hucksters exist in various forms today, selling detoxifications, magic diet cures, psychic powers of healing, or convincing parents that their kids don’t need vaccines. We need a warning song that goes viral to keep our children safe. We are blessed to be in a world without smallpox, almost rid of polio, and we have the knowledge and opportunity to rid the world of other preventable illnesses. Measles was declared eliminated in the United States in 2000; now, outbreaks emerge in every news cycle.
The CDC admits they have not been targeting misinformation well. How can we spread the science, the truth, the message faster than the lies? Better marketing? The answer may be through stories and narratives and song, with the backing of good science. “House of the Rising Sun” is a warning song. Maybe we need more. We need that deep history, that long trail to remind us of the world before vaccines, when everyone knew someone, either in their own household or next door, who succumbed to one of the childhood illnesses.
Let the “House of the Rising Sun” play on. Create a new version, and let that message reverberate, too.
Tell your children; they need to be vaccinated.
Dr. Messler is a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. He previously chaired SHM’s Quality and Patient Safety Committee and has been active in several SHM mentoring programs, most recently with Project BOOST and Glycemic Control. This article appeared originally in SHM's official blog The Hospital Leader. Read more recent posts here.
The urge to move
When you have a few spare minutes on your lunch break, walk by the grade school playground in your neighborhood. Even at a quick glance you will notice that almost all the children are in motion – running, chasing, or being chased. Don’t linger too long or make repeat visits because unfortunately your presence may raise suspicions about your motives. But, even on your brief visit, you will also notice that there are a few children who are sitting down either chatting with a classmate or playing by themselves. If despite my caution you returned several days in a row, you would have noticed that the sedentary outliers tend to be the same children.
Some of the children playing alone simply may be shy loners or socially inept. But I’ve always suspected that there are some people who come in the world genetically predisposed to being sedentary. You can try to make the environment more enticing and stimulating, but the children predestined to be inactive will choose to sit and watch. Not surprisingly, most of those less active children are predestined to be overweight and obese.
At least as young children we seem to be driven to be active, and it is the few outliers who are sedentary. A recent investigation from the department of health and kinesiology at Texas A&M University at College Station is beginning to shed some light on when in our evolutionary history the urge to be active was incorporated into our genome (PLOS ONE. 2019 Apr 29. doi: 10.1371/journal.pone.0216155). The researchers found that snippets of DNA already known to be associated with levels of activity emerged in our ancestors before we were Homo sapiens about 500,000 years ago. This finding surprised the investigators who had suspected that this incorporation of a gene sequence driving activity was more likely to have occurred ten thousand years ago when subsistence farming and its physical demands first appeared.
The authors now postulate that the drive to be active coincided as pre–Homo sapiens grew larger and moved from a treed landscape into the open savanna (“To Move Is to Thrive. It’s in Our Genes” by Gretchen Reynolds. The New York Times, May 15, 2019). As J. Timothy Lightfoot, the senior investigator, observed, “If you were lazy then, you did not survive.”
Our observation of a playground in contact motion is probably evidence that those snippets of DNA still are buried in our genome. However, it is abundantly clear that in North America one doesn’t need to be active to survive, at least in the sense of being reproductively fit. It only takes a few us who must be physically active to grow and build things that we in the sedentary majority can buy or trade for.
There are some of us who have inherited some DNA snippets that drive us to be active post early childhood. My father walked two or three times a day until a few months before his death at 92, and not because someone told him it do it for his health. Like him, I just feel better if I have spent a couple of hours being active every day.
The challenge for us as pediatricians is to help families create environments that foster continued activity by discouraging sedentary entertainments and modeling active lifestyles. For example, simple things like choosing a spot at the periphery of the parking lot instead of close to the store. Choosing stairs instead of the elevator. Of course, anything you will be doing is artificial because the truth is we don’t need to be active to survive even though the urge to move is deeply rooted in our genes.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
When you have a few spare minutes on your lunch break, walk by the grade school playground in your neighborhood. Even at a quick glance you will notice that almost all the children are in motion – running, chasing, or being chased. Don’t linger too long or make repeat visits because unfortunately your presence may raise suspicions about your motives. But, even on your brief visit, you will also notice that there are a few children who are sitting down either chatting with a classmate or playing by themselves. If despite my caution you returned several days in a row, you would have noticed that the sedentary outliers tend to be the same children.
Some of the children playing alone simply may be shy loners or socially inept. But I’ve always suspected that there are some people who come in the world genetically predisposed to being sedentary. You can try to make the environment more enticing and stimulating, but the children predestined to be inactive will choose to sit and watch. Not surprisingly, most of those less active children are predestined to be overweight and obese.
At least as young children we seem to be driven to be active, and it is the few outliers who are sedentary. A recent investigation from the department of health and kinesiology at Texas A&M University at College Station is beginning to shed some light on when in our evolutionary history the urge to be active was incorporated into our genome (PLOS ONE. 2019 Apr 29. doi: 10.1371/journal.pone.0216155). The researchers found that snippets of DNA already known to be associated with levels of activity emerged in our ancestors before we were Homo sapiens about 500,000 years ago. This finding surprised the investigators who had suspected that this incorporation of a gene sequence driving activity was more likely to have occurred ten thousand years ago when subsistence farming and its physical demands first appeared.
The authors now postulate that the drive to be active coincided as pre–Homo sapiens grew larger and moved from a treed landscape into the open savanna (“To Move Is to Thrive. It’s in Our Genes” by Gretchen Reynolds. The New York Times, May 15, 2019). As J. Timothy Lightfoot, the senior investigator, observed, “If you were lazy then, you did not survive.”
Our observation of a playground in contact motion is probably evidence that those snippets of DNA still are buried in our genome. However, it is abundantly clear that in North America one doesn’t need to be active to survive, at least in the sense of being reproductively fit. It only takes a few us who must be physically active to grow and build things that we in the sedentary majority can buy or trade for.
There are some of us who have inherited some DNA snippets that drive us to be active post early childhood. My father walked two or three times a day until a few months before his death at 92, and not because someone told him it do it for his health. Like him, I just feel better if I have spent a couple of hours being active every day.
The challenge for us as pediatricians is to help families create environments that foster continued activity by discouraging sedentary entertainments and modeling active lifestyles. For example, simple things like choosing a spot at the periphery of the parking lot instead of close to the store. Choosing stairs instead of the elevator. Of course, anything you will be doing is artificial because the truth is we don’t need to be active to survive even though the urge to move is deeply rooted in our genes.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
When you have a few spare minutes on your lunch break, walk by the grade school playground in your neighborhood. Even at a quick glance you will notice that almost all the children are in motion – running, chasing, or being chased. Don’t linger too long or make repeat visits because unfortunately your presence may raise suspicions about your motives. But, even on your brief visit, you will also notice that there are a few children who are sitting down either chatting with a classmate or playing by themselves. If despite my caution you returned several days in a row, you would have noticed that the sedentary outliers tend to be the same children.
Some of the children playing alone simply may be shy loners or socially inept. But I’ve always suspected that there are some people who come in the world genetically predisposed to being sedentary. You can try to make the environment more enticing and stimulating, but the children predestined to be inactive will choose to sit and watch. Not surprisingly, most of those less active children are predestined to be overweight and obese.
At least as young children we seem to be driven to be active, and it is the few outliers who are sedentary. A recent investigation from the department of health and kinesiology at Texas A&M University at College Station is beginning to shed some light on when in our evolutionary history the urge to be active was incorporated into our genome (PLOS ONE. 2019 Apr 29. doi: 10.1371/journal.pone.0216155). The researchers found that snippets of DNA already known to be associated with levels of activity emerged in our ancestors before we were Homo sapiens about 500,000 years ago. This finding surprised the investigators who had suspected that this incorporation of a gene sequence driving activity was more likely to have occurred ten thousand years ago when subsistence farming and its physical demands first appeared.
The authors now postulate that the drive to be active coincided as pre–Homo sapiens grew larger and moved from a treed landscape into the open savanna (“To Move Is to Thrive. It’s in Our Genes” by Gretchen Reynolds. The New York Times, May 15, 2019). As J. Timothy Lightfoot, the senior investigator, observed, “If you were lazy then, you did not survive.”
Our observation of a playground in contact motion is probably evidence that those snippets of DNA still are buried in our genome. However, it is abundantly clear that in North America one doesn’t need to be active to survive, at least in the sense of being reproductively fit. It only takes a few us who must be physically active to grow and build things that we in the sedentary majority can buy or trade for.
There are some of us who have inherited some DNA snippets that drive us to be active post early childhood. My father walked two or three times a day until a few months before his death at 92, and not because someone told him it do it for his health. Like him, I just feel better if I have spent a couple of hours being active every day.
The challenge for us as pediatricians is to help families create environments that foster continued activity by discouraging sedentary entertainments and modeling active lifestyles. For example, simple things like choosing a spot at the periphery of the parking lot instead of close to the store. Choosing stairs instead of the elevator. Of course, anything you will be doing is artificial because the truth is we don’t need to be active to survive even though the urge to move is deeply rooted in our genes.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Pediatricians report low knowledge, comfort discussing e-cigarettes
BALTIMORE – according to a recent study.
“Providers are aware of the increased prevalence, harms [of e-cigs] and [the] positive impact of counseling teens about e-cigs,” said Allison Heinly, MD, of Hasbro Children’s Hospital in Providence, R.I., and her colleagues. But, “providers are less likely to ask, advise, or assist parents [and teens] regarding e-cig use, compared to tobacco, and are less comfortable doing so.” The researchers presented their findings at the Pediatric Academic Societies annual meeting.
A variety of concerns exist regarding ingredients in e-cigarettes, Dr. Heinly noted, including nicotine, volatile organic compounds, carcinogenic chemicals, flavorings, and ultra-fine particles.
Dr. Heinly and her associates aimed to assess pediatricians’ knowledge, attitudes, and behaviors toward both teens’ and parents’ use of e-cigarettes, as well as the barrier pediatricians perceived when it came to screening and counseling those who use e-cigarettes.
Among 69 providers at a large Northeastern urban academic primary care clinic who received surveys, 62 responded, primarily residents (84%). The respondents included 44 pediatric residents, eight triple-board residents, and 10 attending physicians.
The researchers collapsed “most of the time”/“always” and “some of the time”/“never” responses into two categories.
Most of the respondents (82%) knew e-cigarettes are the most common tobacco product that youth use, and nearly all (97%) believed e-cigarettes were addictive and harmful to users’ health. In addition, most (79%) believed using e-cigarettes could be a pathway toward students beginning to use other drugs.
Even though respondents believed counseling teens about use of tobacco or e-cigarettes can reduce the likelihood that they will start using them, providers were much less likely to discuss e-cigarettes than tobacco with teens.
Nearly all the doctors (97%) reported asking teens about their use of tobacco, but only about half (52%) asked about e-cigarette use (P less than .001). And only about one in five doctors (21%) reported counseling teens about using e-cigarettes, compared with 47% of those who advised teens regarding tobacco use (P = .002).
Over a third of responding physicians (37%) reported helping adolescent patients quit using tobacco, but just 7% reported doing so with e-cigarettes (P less than .001).
Doctors overwhelmingly reported feeling comfortable talking about tobacco with teens (98%), but fewer felt comfortable discussing e-cigarettes (77%; P less than .001). Respondents similarly were less comfortable discussing e-cigarettes (55%) than tobacco (87%) with parents (P less than .001).
Very few pediatricians asked parents about their use of e-cigarettes (5%) or advised them about e-cigarettes’ harms (7%), and even fewer reported helping parents quit using them (2%). By contrast, more than half of pediatricians (60%) asked parents about smoking or advised them about tobacco use harms (52%), and nearly one-third (31%) reported helping parents quit smoking (P less than .001 for all comparisons).
The biggest barrier to discussing e-cigarettes with families was, as with discussing tobacco, not having enough time. But about twice as many respondents cited insufficient knowledge as a barrier for e-cigarettes as for tobacco (P = .003). A small percentage of respondents (less than 20%) also reported feeling unsure about the harm of e-cigarettes (P = .001).
Lack of training was a significant barrier to physicians’ discussion of e-cigarettes as well. Many more physicians reported receiving training in medical school on tobacco and traditional cigarettes (78%) than on e-cigarettes (13%), possibly because of how recently e-cigarettes have become widely available (P less than .001).
More physicians reported receiving training related to e-cigarettes during residency (36%), but it still fell well short of how many reported other tobacco and smoking training during residency (61%; P = .001).
The findings “emphasize the importance of increasing training about e-cig counseling,” Dr. Heinly and her associates concluded.
The researchers noted no external funding or disclosures.
BALTIMORE – according to a recent study.
“Providers are aware of the increased prevalence, harms [of e-cigs] and [the] positive impact of counseling teens about e-cigs,” said Allison Heinly, MD, of Hasbro Children’s Hospital in Providence, R.I., and her colleagues. But, “providers are less likely to ask, advise, or assist parents [and teens] regarding e-cig use, compared to tobacco, and are less comfortable doing so.” The researchers presented their findings at the Pediatric Academic Societies annual meeting.
A variety of concerns exist regarding ingredients in e-cigarettes, Dr. Heinly noted, including nicotine, volatile organic compounds, carcinogenic chemicals, flavorings, and ultra-fine particles.
Dr. Heinly and her associates aimed to assess pediatricians’ knowledge, attitudes, and behaviors toward both teens’ and parents’ use of e-cigarettes, as well as the barrier pediatricians perceived when it came to screening and counseling those who use e-cigarettes.
Among 69 providers at a large Northeastern urban academic primary care clinic who received surveys, 62 responded, primarily residents (84%). The respondents included 44 pediatric residents, eight triple-board residents, and 10 attending physicians.
The researchers collapsed “most of the time”/“always” and “some of the time”/“never” responses into two categories.
Most of the respondents (82%) knew e-cigarettes are the most common tobacco product that youth use, and nearly all (97%) believed e-cigarettes were addictive and harmful to users’ health. In addition, most (79%) believed using e-cigarettes could be a pathway toward students beginning to use other drugs.
Even though respondents believed counseling teens about use of tobacco or e-cigarettes can reduce the likelihood that they will start using them, providers were much less likely to discuss e-cigarettes than tobacco with teens.
Nearly all the doctors (97%) reported asking teens about their use of tobacco, but only about half (52%) asked about e-cigarette use (P less than .001). And only about one in five doctors (21%) reported counseling teens about using e-cigarettes, compared with 47% of those who advised teens regarding tobacco use (P = .002).
Over a third of responding physicians (37%) reported helping adolescent patients quit using tobacco, but just 7% reported doing so with e-cigarettes (P less than .001).
Doctors overwhelmingly reported feeling comfortable talking about tobacco with teens (98%), but fewer felt comfortable discussing e-cigarettes (77%; P less than .001). Respondents similarly were less comfortable discussing e-cigarettes (55%) than tobacco (87%) with parents (P less than .001).
Very few pediatricians asked parents about their use of e-cigarettes (5%) or advised them about e-cigarettes’ harms (7%), and even fewer reported helping parents quit using them (2%). By contrast, more than half of pediatricians (60%) asked parents about smoking or advised them about tobacco use harms (52%), and nearly one-third (31%) reported helping parents quit smoking (P less than .001 for all comparisons).
The biggest barrier to discussing e-cigarettes with families was, as with discussing tobacco, not having enough time. But about twice as many respondents cited insufficient knowledge as a barrier for e-cigarettes as for tobacco (P = .003). A small percentage of respondents (less than 20%) also reported feeling unsure about the harm of e-cigarettes (P = .001).
Lack of training was a significant barrier to physicians’ discussion of e-cigarettes as well. Many more physicians reported receiving training in medical school on tobacco and traditional cigarettes (78%) than on e-cigarettes (13%), possibly because of how recently e-cigarettes have become widely available (P less than .001).
More physicians reported receiving training related to e-cigarettes during residency (36%), but it still fell well short of how many reported other tobacco and smoking training during residency (61%; P = .001).
The findings “emphasize the importance of increasing training about e-cig counseling,” Dr. Heinly and her associates concluded.
The researchers noted no external funding or disclosures.
BALTIMORE – according to a recent study.
“Providers are aware of the increased prevalence, harms [of e-cigs] and [the] positive impact of counseling teens about e-cigs,” said Allison Heinly, MD, of Hasbro Children’s Hospital in Providence, R.I., and her colleagues. But, “providers are less likely to ask, advise, or assist parents [and teens] regarding e-cig use, compared to tobacco, and are less comfortable doing so.” The researchers presented their findings at the Pediatric Academic Societies annual meeting.
A variety of concerns exist regarding ingredients in e-cigarettes, Dr. Heinly noted, including nicotine, volatile organic compounds, carcinogenic chemicals, flavorings, and ultra-fine particles.
Dr. Heinly and her associates aimed to assess pediatricians’ knowledge, attitudes, and behaviors toward both teens’ and parents’ use of e-cigarettes, as well as the barrier pediatricians perceived when it came to screening and counseling those who use e-cigarettes.
Among 69 providers at a large Northeastern urban academic primary care clinic who received surveys, 62 responded, primarily residents (84%). The respondents included 44 pediatric residents, eight triple-board residents, and 10 attending physicians.
The researchers collapsed “most of the time”/“always” and “some of the time”/“never” responses into two categories.
Most of the respondents (82%) knew e-cigarettes are the most common tobacco product that youth use, and nearly all (97%) believed e-cigarettes were addictive and harmful to users’ health. In addition, most (79%) believed using e-cigarettes could be a pathway toward students beginning to use other drugs.
Even though respondents believed counseling teens about use of tobacco or e-cigarettes can reduce the likelihood that they will start using them, providers were much less likely to discuss e-cigarettes than tobacco with teens.
Nearly all the doctors (97%) reported asking teens about their use of tobacco, but only about half (52%) asked about e-cigarette use (P less than .001). And only about one in five doctors (21%) reported counseling teens about using e-cigarettes, compared with 47% of those who advised teens regarding tobacco use (P = .002).
Over a third of responding physicians (37%) reported helping adolescent patients quit using tobacco, but just 7% reported doing so with e-cigarettes (P less than .001).
Doctors overwhelmingly reported feeling comfortable talking about tobacco with teens (98%), but fewer felt comfortable discussing e-cigarettes (77%; P less than .001). Respondents similarly were less comfortable discussing e-cigarettes (55%) than tobacco (87%) with parents (P less than .001).
Very few pediatricians asked parents about their use of e-cigarettes (5%) or advised them about e-cigarettes’ harms (7%), and even fewer reported helping parents quit using them (2%). By contrast, more than half of pediatricians (60%) asked parents about smoking or advised them about tobacco use harms (52%), and nearly one-third (31%) reported helping parents quit smoking (P less than .001 for all comparisons).
The biggest barrier to discussing e-cigarettes with families was, as with discussing tobacco, not having enough time. But about twice as many respondents cited insufficient knowledge as a barrier for e-cigarettes as for tobacco (P = .003). A small percentage of respondents (less than 20%) also reported feeling unsure about the harm of e-cigarettes (P = .001).
Lack of training was a significant barrier to physicians’ discussion of e-cigarettes as well. Many more physicians reported receiving training in medical school on tobacco and traditional cigarettes (78%) than on e-cigarettes (13%), possibly because of how recently e-cigarettes have become widely available (P less than .001).
More physicians reported receiving training related to e-cigarettes during residency (36%), but it still fell well short of how many reported other tobacco and smoking training during residency (61%; P = .001).
The findings “emphasize the importance of increasing training about e-cig counseling,” Dr. Heinly and her associates concluded.
The researchers noted no external funding or disclosures.
REPORTING FROM PAS 2019
Key clinical point: Physicians report less training and less comfort when discussing e-cigarettes with teens and parents than when discussing tobacco products.
Major finding: 7% of physicians reported helping adolescent patients quit using e-cigarettes, compared with 37% helping with quitting tobacco use (P less than .001).
Study details: The findings are based on a cross-sectional survey of 62 pediatric residents and attendings at a large urban academic primary care clinic in the Northeast.
Disclosures: The researchers noted no external funding or disclosures.
Timolol shortens propranolol use in infantile hemangioma
according to a study published in Pediatric Dermatology.
Diana B. Mannschreck, BSN, of Johns Hopkins University, Baltimore, and colleagues performed a retrospective chart review of 559 patients with infantile hemangioma seen in the dermatology clinic at Johns Hopkins between December 2008 and January 2018. Patients received any of five courses of treatment, including oral propranolol followed by topical timolol, propranolol only, and timolol only. Of the courses evaluated, propranolol followed by timolol had the shortest duration of propranolol therapy – a median of 2.2 months shorter than propranolol-only therapy (P = .0006). This sequential regimen also was associated with no reinitiations of propranolol therapy following tapering, whereas 13% of those receiving propranolol alone had to reinitiate it after tapering.
This is of interest because oral beta-blockers, including propranolol, have been associated with rare but serious adverse events, such as bronchospasm, hypotension, and hypoglycemia.
Limitations of the study include its retrospective and single-center nature. There was no funding or disclosure information given.
SOURCE: Mannschreck DB et al. Pediatr Dermatol. 2019 Apr 9. doi: 10.1111/pde.13816.
according to a study published in Pediatric Dermatology.
Diana B. Mannschreck, BSN, of Johns Hopkins University, Baltimore, and colleagues performed a retrospective chart review of 559 patients with infantile hemangioma seen in the dermatology clinic at Johns Hopkins between December 2008 and January 2018. Patients received any of five courses of treatment, including oral propranolol followed by topical timolol, propranolol only, and timolol only. Of the courses evaluated, propranolol followed by timolol had the shortest duration of propranolol therapy – a median of 2.2 months shorter than propranolol-only therapy (P = .0006). This sequential regimen also was associated with no reinitiations of propranolol therapy following tapering, whereas 13% of those receiving propranolol alone had to reinitiate it after tapering.
This is of interest because oral beta-blockers, including propranolol, have been associated with rare but serious adverse events, such as bronchospasm, hypotension, and hypoglycemia.
Limitations of the study include its retrospective and single-center nature. There was no funding or disclosure information given.
SOURCE: Mannschreck DB et al. Pediatr Dermatol. 2019 Apr 9. doi: 10.1111/pde.13816.
according to a study published in Pediatric Dermatology.
Diana B. Mannschreck, BSN, of Johns Hopkins University, Baltimore, and colleagues performed a retrospective chart review of 559 patients with infantile hemangioma seen in the dermatology clinic at Johns Hopkins between December 2008 and January 2018. Patients received any of five courses of treatment, including oral propranolol followed by topical timolol, propranolol only, and timolol only. Of the courses evaluated, propranolol followed by timolol had the shortest duration of propranolol therapy – a median of 2.2 months shorter than propranolol-only therapy (P = .0006). This sequential regimen also was associated with no reinitiations of propranolol therapy following tapering, whereas 13% of those receiving propranolol alone had to reinitiate it after tapering.
This is of interest because oral beta-blockers, including propranolol, have been associated with rare but serious adverse events, such as bronchospasm, hypotension, and hypoglycemia.
Limitations of the study include its retrospective and single-center nature. There was no funding or disclosure information given.
SOURCE: Mannschreck DB et al. Pediatr Dermatol. 2019 Apr 9. doi: 10.1111/pde.13816.
FROM PEDIATRIC DERMATOLOGY
Lack of inhaler at school a major barrier to asthma care
BALTIMORE – frequently because the parent did not provide an inhaler or did not provide a written order for one, according to new research. Only seven U.S. states have laws allowing schools to stock albuterol for students.
“Most students only have access to this lifesaving medication when they bring a personal inhaler,” Alexandra M. Sims, MD, of Children’s National Hospital in Washington and colleagues wrote in their abstract at the annual meeting of Pediatric Academic Societies. “Interventions that address medication availability may be an important step in removing obstacles to asthma care in school.”
One such option is a stock inhaler available for any students to use. National guidelines from the Centers for Disease Control and Prevention recommend that students with asthma have access to inhaled albuterol at school, yet most states do not have legislation related to albuterol stocking in schools, according to the Asthma and Allergy Foundation of America.
Not having access to rescue inhaler medication at school contributes to lost class time and referrals to the emergency department, the authors note in their background information. Yet, “in most U.S. jurisdictions, including the school district we examined, students need both a personal albuterol inhaler and a physician order to receive medication at school.”
To determine what barriers exist regarding students’ asthma care in schools, the authors sent 166 school nurses in an urban school district an anonymous survey during the 2015-2016 school year. The survey asked about 21 factors that could delay or prevent students from returning to class and asked nurses’ agreement or disagreement with 25 additional statements.
The 130 respondents made up a 78% response rate. The institutions represented by the nurses included 44% elementary schools, 9% middle schools, 16% high schools, and 32% other (such as those who may serve multiple schools).
The majority of respondents (72%) agreed that asthma is one of the biggest health problems students face, particularly among middle and high school students (P less than .05). Most (74%) also said an albuterol inhaler at school could reduce the likelihood of students with asthma needing to leave school early.
The largest barrier to students returning to class was parents not providing an albuterol inhaler and/or a written order for an inhaler despite a request from the nurse, according to 69% of the respondents (P less than .05). In high schools in particular, another barrier was students simply not bringing their inhaler to school even though they usually carry one (P less than .01).
Only 15% of nurses saw disease severity as a significant barrier, and 17% cited the staff not adequately recognizing a student’s symptoms.
The researchers did not note use of external funding or author disclosures.
BALTIMORE – frequently because the parent did not provide an inhaler or did not provide a written order for one, according to new research. Only seven U.S. states have laws allowing schools to stock albuterol for students.
“Most students only have access to this lifesaving medication when they bring a personal inhaler,” Alexandra M. Sims, MD, of Children’s National Hospital in Washington and colleagues wrote in their abstract at the annual meeting of Pediatric Academic Societies. “Interventions that address medication availability may be an important step in removing obstacles to asthma care in school.”
One such option is a stock inhaler available for any students to use. National guidelines from the Centers for Disease Control and Prevention recommend that students with asthma have access to inhaled albuterol at school, yet most states do not have legislation related to albuterol stocking in schools, according to the Asthma and Allergy Foundation of America.
Not having access to rescue inhaler medication at school contributes to lost class time and referrals to the emergency department, the authors note in their background information. Yet, “in most U.S. jurisdictions, including the school district we examined, students need both a personal albuterol inhaler and a physician order to receive medication at school.”
To determine what barriers exist regarding students’ asthma care in schools, the authors sent 166 school nurses in an urban school district an anonymous survey during the 2015-2016 school year. The survey asked about 21 factors that could delay or prevent students from returning to class and asked nurses’ agreement or disagreement with 25 additional statements.
The 130 respondents made up a 78% response rate. The institutions represented by the nurses included 44% elementary schools, 9% middle schools, 16% high schools, and 32% other (such as those who may serve multiple schools).
The majority of respondents (72%) agreed that asthma is one of the biggest health problems students face, particularly among middle and high school students (P less than .05). Most (74%) also said an albuterol inhaler at school could reduce the likelihood of students with asthma needing to leave school early.
The largest barrier to students returning to class was parents not providing an albuterol inhaler and/or a written order for an inhaler despite a request from the nurse, according to 69% of the respondents (P less than .05). In high schools in particular, another barrier was students simply not bringing their inhaler to school even though they usually carry one (P less than .01).
Only 15% of nurses saw disease severity as a significant barrier, and 17% cited the staff not adequately recognizing a student’s symptoms.
The researchers did not note use of external funding or author disclosures.
BALTIMORE – frequently because the parent did not provide an inhaler or did not provide a written order for one, according to new research. Only seven U.S. states have laws allowing schools to stock albuterol for students.
“Most students only have access to this lifesaving medication when they bring a personal inhaler,” Alexandra M. Sims, MD, of Children’s National Hospital in Washington and colleagues wrote in their abstract at the annual meeting of Pediatric Academic Societies. “Interventions that address medication availability may be an important step in removing obstacles to asthma care in school.”
One such option is a stock inhaler available for any students to use. National guidelines from the Centers for Disease Control and Prevention recommend that students with asthma have access to inhaled albuterol at school, yet most states do not have legislation related to albuterol stocking in schools, according to the Asthma and Allergy Foundation of America.
Not having access to rescue inhaler medication at school contributes to lost class time and referrals to the emergency department, the authors note in their background information. Yet, “in most U.S. jurisdictions, including the school district we examined, students need both a personal albuterol inhaler and a physician order to receive medication at school.”
To determine what barriers exist regarding students’ asthma care in schools, the authors sent 166 school nurses in an urban school district an anonymous survey during the 2015-2016 school year. The survey asked about 21 factors that could delay or prevent students from returning to class and asked nurses’ agreement or disagreement with 25 additional statements.
The 130 respondents made up a 78% response rate. The institutions represented by the nurses included 44% elementary schools, 9% middle schools, 16% high schools, and 32% other (such as those who may serve multiple schools).
The majority of respondents (72%) agreed that asthma is one of the biggest health problems students face, particularly among middle and high school students (P less than .05). Most (74%) also said an albuterol inhaler at school could reduce the likelihood of students with asthma needing to leave school early.
The largest barrier to students returning to class was parents not providing an albuterol inhaler and/or a written order for an inhaler despite a request from the nurse, according to 69% of the respondents (P less than .05). In high schools in particular, another barrier was students simply not bringing their inhaler to school even though they usually carry one (P less than .01).
Only 15% of nurses saw disease severity as a significant barrier, and 17% cited the staff not adequately recognizing a student’s symptoms.
The researchers did not note use of external funding or author disclosures.
REPORTING FROM PAS 2019
Some “slime”-related contact dermatitis is allergic
The viscous homemade children’s plaything known as “slime” has been associated with allergic, as well as irritant, contact dermatitis of the hands thanks to an array of possible compounds with which it can be made, according to a case report in Pediatric Dermatology. The report details many possible compounds causing the dermatitis reactions seen by health care professionals.
In the case, which was reported by L. Elizabeth Anderson, MD, of the Children’s Hospital of Philadelphia and colleagues, an 11-year-old girl with a history of atopic dermatitis presented with hand dermatitis that was suspected to be related to playing with slime. After her dermatitis failed to respond to strong topical steroids, she was referred for patch testing, with positivity for methylchloroisothiazolinone/methylisothiazolinone (MCI/MI). After all contact with any products containing MCI/MI was eliminated, her hand dermatitis cleared, and bodywide atopic dermatitis improved some as well.
MCI/MI and MI are among the most commonly suspected culprits in cases of slime-related contact dermatitis. Although most cases are irritant contact dermatitis, some are allergic and can be detected using patch tests. MCI/MI is included in the T.R.U.E. Test, but according to the case report, 37% of patients with allergy to MI alone will not have positive response with the T.R.U.E. Test because of the low concentrations of MI in that test. The authors of this case report also listed many other the potential allergens in popular slime recipes; however, many are not included in the T.R.U.E. Test.
“While the T.R.U.E. Test does not capture most of the potential allergens in popular slime recipes, the recently published Pediatric Baseline Patch Test Series by Yu et al. [Dermatitis. 2018;29:206-12] does and is recommended for use in patients suspected of having dermatitis secondary to slime,” Dr. Anderson and associates wrote.
SOURCE: Anderson LE et al. Pediatr Dermatol. 2019 Mar 13. doi: 10.1111/pde.13792.
The viscous homemade children’s plaything known as “slime” has been associated with allergic, as well as irritant, contact dermatitis of the hands thanks to an array of possible compounds with which it can be made, according to a case report in Pediatric Dermatology. The report details many possible compounds causing the dermatitis reactions seen by health care professionals.
In the case, which was reported by L. Elizabeth Anderson, MD, of the Children’s Hospital of Philadelphia and colleagues, an 11-year-old girl with a history of atopic dermatitis presented with hand dermatitis that was suspected to be related to playing with slime. After her dermatitis failed to respond to strong topical steroids, she was referred for patch testing, with positivity for methylchloroisothiazolinone/methylisothiazolinone (MCI/MI). After all contact with any products containing MCI/MI was eliminated, her hand dermatitis cleared, and bodywide atopic dermatitis improved some as well.
MCI/MI and MI are among the most commonly suspected culprits in cases of slime-related contact dermatitis. Although most cases are irritant contact dermatitis, some are allergic and can be detected using patch tests. MCI/MI is included in the T.R.U.E. Test, but according to the case report, 37% of patients with allergy to MI alone will not have positive response with the T.R.U.E. Test because of the low concentrations of MI in that test. The authors of this case report also listed many other the potential allergens in popular slime recipes; however, many are not included in the T.R.U.E. Test.
“While the T.R.U.E. Test does not capture most of the potential allergens in popular slime recipes, the recently published Pediatric Baseline Patch Test Series by Yu et al. [Dermatitis. 2018;29:206-12] does and is recommended for use in patients suspected of having dermatitis secondary to slime,” Dr. Anderson and associates wrote.
SOURCE: Anderson LE et al. Pediatr Dermatol. 2019 Mar 13. doi: 10.1111/pde.13792.
The viscous homemade children’s plaything known as “slime” has been associated with allergic, as well as irritant, contact dermatitis of the hands thanks to an array of possible compounds with which it can be made, according to a case report in Pediatric Dermatology. The report details many possible compounds causing the dermatitis reactions seen by health care professionals.
In the case, which was reported by L. Elizabeth Anderson, MD, of the Children’s Hospital of Philadelphia and colleagues, an 11-year-old girl with a history of atopic dermatitis presented with hand dermatitis that was suspected to be related to playing with slime. After her dermatitis failed to respond to strong topical steroids, she was referred for patch testing, with positivity for methylchloroisothiazolinone/methylisothiazolinone (MCI/MI). After all contact with any products containing MCI/MI was eliminated, her hand dermatitis cleared, and bodywide atopic dermatitis improved some as well.
MCI/MI and MI are among the most commonly suspected culprits in cases of slime-related contact dermatitis. Although most cases are irritant contact dermatitis, some are allergic and can be detected using patch tests. MCI/MI is included in the T.R.U.E. Test, but according to the case report, 37% of patients with allergy to MI alone will not have positive response with the T.R.U.E. Test because of the low concentrations of MI in that test. The authors of this case report also listed many other the potential allergens in popular slime recipes; however, many are not included in the T.R.U.E. Test.
“While the T.R.U.E. Test does not capture most of the potential allergens in popular slime recipes, the recently published Pediatric Baseline Patch Test Series by Yu et al. [Dermatitis. 2018;29:206-12] does and is recommended for use in patients suspected of having dermatitis secondary to slime,” Dr. Anderson and associates wrote.
SOURCE: Anderson LE et al. Pediatr Dermatol. 2019 Mar 13. doi: 10.1111/pde.13792.
FROM PEDIATRIC DERMATOLOGY
Consider measles vaccine booster in HIV-positive patients
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
REPORTING FROM ESPID 2019
Breastfeeding protects against intussusception
LJUBLJANA, SLOVENIA – in a German case-control study.
Two other potent risk factors for intussusception in children less than 1 year old were identified: a family history of intussusception, and an episode of gastroenteritis, Doris F. Oberle, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Oberle, of the Paul Ehrlich Institute in Langen, Germany, presented a retrospective study of 116 meticulously validated cases of intussusception in infancy treated at 19 German pediatric centers during 2010-2014 and 272 controls matched by birth month, sex, and location. A standardized interview was conducted with the parents of all study participants.
Rotavirus vaccine was added to the German national vaccination schedule in 2013. In a multivariate logistic regression analysis, the risk of intussusception was increased by 5.4-fold following the first dose of the vaccine, compared with nonrecipients. However, subsequent doses of rotavirus vaccine were not associated with any excess risk.
In addition, a family history of intussusception was linked to a 4.2-fold increased risk, while an episode of gastroenteritis during the first year of life was associated with a 4.7-fold elevated risk.
In a novel finding, breastfeeding was independently associated with a 44% reduction in the risk of intussusception, compared with that of bottle-fed babies.
The most common presenting signs and symptoms of intussusception were vomiting, abdominal pain, hematochezia, pallor, and reduced appetite, each present in at least half of affected infants.
Dr. Oberle reported having no financial conflicts regarding her study, supported by the Paul Ehrlich Institute.
LJUBLJANA, SLOVENIA – in a German case-control study.
Two other potent risk factors for intussusception in children less than 1 year old were identified: a family history of intussusception, and an episode of gastroenteritis, Doris F. Oberle, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Oberle, of the Paul Ehrlich Institute in Langen, Germany, presented a retrospective study of 116 meticulously validated cases of intussusception in infancy treated at 19 German pediatric centers during 2010-2014 and 272 controls matched by birth month, sex, and location. A standardized interview was conducted with the parents of all study participants.
Rotavirus vaccine was added to the German national vaccination schedule in 2013. In a multivariate logistic regression analysis, the risk of intussusception was increased by 5.4-fold following the first dose of the vaccine, compared with nonrecipients. However, subsequent doses of rotavirus vaccine were not associated with any excess risk.
In addition, a family history of intussusception was linked to a 4.2-fold increased risk, while an episode of gastroenteritis during the first year of life was associated with a 4.7-fold elevated risk.
In a novel finding, breastfeeding was independently associated with a 44% reduction in the risk of intussusception, compared with that of bottle-fed babies.
The most common presenting signs and symptoms of intussusception were vomiting, abdominal pain, hematochezia, pallor, and reduced appetite, each present in at least half of affected infants.
Dr. Oberle reported having no financial conflicts regarding her study, supported by the Paul Ehrlich Institute.
LJUBLJANA, SLOVENIA – in a German case-control study.
Two other potent risk factors for intussusception in children less than 1 year old were identified: a family history of intussusception, and an episode of gastroenteritis, Doris F. Oberle, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Oberle, of the Paul Ehrlich Institute in Langen, Germany, presented a retrospective study of 116 meticulously validated cases of intussusception in infancy treated at 19 German pediatric centers during 2010-2014 and 272 controls matched by birth month, sex, and location. A standardized interview was conducted with the parents of all study participants.
Rotavirus vaccine was added to the German national vaccination schedule in 2013. In a multivariate logistic regression analysis, the risk of intussusception was increased by 5.4-fold following the first dose of the vaccine, compared with nonrecipients. However, subsequent doses of rotavirus vaccine were not associated with any excess risk.
In addition, a family history of intussusception was linked to a 4.2-fold increased risk, while an episode of gastroenteritis during the first year of life was associated with a 4.7-fold elevated risk.
In a novel finding, breastfeeding was independently associated with a 44% reduction in the risk of intussusception, compared with that of bottle-fed babies.
The most common presenting signs and symptoms of intussusception were vomiting, abdominal pain, hematochezia, pallor, and reduced appetite, each present in at least half of affected infants.
Dr. Oberle reported having no financial conflicts regarding her study, supported by the Paul Ehrlich Institute.
REPORTING FROM ESPID 2019