User login
How to have ‘the talk’ with vaccine skeptics
LJUBLJANA, SLOVENIA – An effective strategy in helping vaccine skeptics to come around to accepting immunizations for their children is to pivot the conversation away from vaccine safety and focus instead on the disease itself and its potential consequences, Saad B. Omer, MBBS, PhD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Why do we cede ground by focusing too much on the vaccine itself? I call it the disease salience approach,” said Dr. Omer, professor of global health, epidemiology, and pediatrics at Emory University in Atlanta.
It’s a strategy guided by developments in social psychology, persuasion theory, and communication theory. But if applied incorrectly, the disease salience approach can backfire, causing behavioral paralysis and an inability to act, he cautioned.
Dr. Omer explained that it’s a matter of framing.
“Always include a solution to promote self-efficacy and response-efficacy. After you inform parents of disease risks, provide them with actions they can take. Now readdress the vaccine, pointing out that this is the single best way to protect yourself and your baby,” he said. “The lesson is that since vaccines are a social norm, reframe nonvaccination as an active act, rather than vaccination as an active act.”
Don’t attempt to wow parents with statistics on how vaccine complication rates are dwarfed by the disease risk if left unvaccinated, he advised. Studies have shown that‘s generally not effective. What actually works is to provide narratives of disease severity.
“We are excellent linguists, but really, really poor statisticians,” Dr. Omer observed.
Is it ethical to talk to parents about disease risks to influence their behavior? Absolutely, in his view.
“We’re not selling toothpaste. We are in the business of life-saving vaccines. And I would submit that if it’s done correctly it’s entirely ethical to talk about the disease, and sometimes even the severe risks of the disease, instead of the vaccine,” said Dr. Omer.
If parents cite a myth about vaccines, it’s necessary to address it head on without lingering on it. But debunking a myth is tricky because people tend to remember negative information they received earlier.
“If you’re going to debunk a myth, clearly label it as a myth in the headline as you introduce it. State why it’s not true. Replace the myth with the best alternative explanation. Think of it like a blank space where the myth used to reside. That space needs to be filled with an alternative explanation or the myth will come back,” Dr. Omer said.
He is a coauthor of a book titled, ‘The Clinician’s Vaccine Safety Resource Guide: Optimizing Prevention of Vaccine-Preventable Diseases Across the Lifespan.’
LJUBLJANA, SLOVENIA – An effective strategy in helping vaccine skeptics to come around to accepting immunizations for their children is to pivot the conversation away from vaccine safety and focus instead on the disease itself and its potential consequences, Saad B. Omer, MBBS, PhD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Why do we cede ground by focusing too much on the vaccine itself? I call it the disease salience approach,” said Dr. Omer, professor of global health, epidemiology, and pediatrics at Emory University in Atlanta.
It’s a strategy guided by developments in social psychology, persuasion theory, and communication theory. But if applied incorrectly, the disease salience approach can backfire, causing behavioral paralysis and an inability to act, he cautioned.
Dr. Omer explained that it’s a matter of framing.
“Always include a solution to promote self-efficacy and response-efficacy. After you inform parents of disease risks, provide them with actions they can take. Now readdress the vaccine, pointing out that this is the single best way to protect yourself and your baby,” he said. “The lesson is that since vaccines are a social norm, reframe nonvaccination as an active act, rather than vaccination as an active act.”
Don’t attempt to wow parents with statistics on how vaccine complication rates are dwarfed by the disease risk if left unvaccinated, he advised. Studies have shown that‘s generally not effective. What actually works is to provide narratives of disease severity.
“We are excellent linguists, but really, really poor statisticians,” Dr. Omer observed.
Is it ethical to talk to parents about disease risks to influence their behavior? Absolutely, in his view.
“We’re not selling toothpaste. We are in the business of life-saving vaccines. And I would submit that if it’s done correctly it’s entirely ethical to talk about the disease, and sometimes even the severe risks of the disease, instead of the vaccine,” said Dr. Omer.
If parents cite a myth about vaccines, it’s necessary to address it head on without lingering on it. But debunking a myth is tricky because people tend to remember negative information they received earlier.
“If you’re going to debunk a myth, clearly label it as a myth in the headline as you introduce it. State why it’s not true. Replace the myth with the best alternative explanation. Think of it like a blank space where the myth used to reside. That space needs to be filled with an alternative explanation or the myth will come back,” Dr. Omer said.
He is a coauthor of a book titled, ‘The Clinician’s Vaccine Safety Resource Guide: Optimizing Prevention of Vaccine-Preventable Diseases Across the Lifespan.’
LJUBLJANA, SLOVENIA – An effective strategy in helping vaccine skeptics to come around to accepting immunizations for their children is to pivot the conversation away from vaccine safety and focus instead on the disease itself and its potential consequences, Saad B. Omer, MBBS, PhD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Why do we cede ground by focusing too much on the vaccine itself? I call it the disease salience approach,” said Dr. Omer, professor of global health, epidemiology, and pediatrics at Emory University in Atlanta.
It’s a strategy guided by developments in social psychology, persuasion theory, and communication theory. But if applied incorrectly, the disease salience approach can backfire, causing behavioral paralysis and an inability to act, he cautioned.
Dr. Omer explained that it’s a matter of framing.
“Always include a solution to promote self-efficacy and response-efficacy. After you inform parents of disease risks, provide them with actions they can take. Now readdress the vaccine, pointing out that this is the single best way to protect yourself and your baby,” he said. “The lesson is that since vaccines are a social norm, reframe nonvaccination as an active act, rather than vaccination as an active act.”
Don’t attempt to wow parents with statistics on how vaccine complication rates are dwarfed by the disease risk if left unvaccinated, he advised. Studies have shown that‘s generally not effective. What actually works is to provide narratives of disease severity.
“We are excellent linguists, but really, really poor statisticians,” Dr. Omer observed.
Is it ethical to talk to parents about disease risks to influence their behavior? Absolutely, in his view.
“We’re not selling toothpaste. We are in the business of life-saving vaccines. And I would submit that if it’s done correctly it’s entirely ethical to talk about the disease, and sometimes even the severe risks of the disease, instead of the vaccine,” said Dr. Omer.
If parents cite a myth about vaccines, it’s necessary to address it head on without lingering on it. But debunking a myth is tricky because people tend to remember negative information they received earlier.
“If you’re going to debunk a myth, clearly label it as a myth in the headline as you introduce it. State why it’s not true. Replace the myth with the best alternative explanation. Think of it like a blank space where the myth used to reside. That space needs to be filled with an alternative explanation or the myth will come back,” Dr. Omer said.
He is a coauthor of a book titled, ‘The Clinician’s Vaccine Safety Resource Guide: Optimizing Prevention of Vaccine-Preventable Diseases Across the Lifespan.’
EXPERT ANALYSIS FROM ESPID 2019
Scabies rates plummeted with community mass drug administration
MILAN – In a region where scabies is endemic, a , findings that may have implications for future treatment of scabies or other infestations in other regions, dermatologist Margot Whitfield, MD, said at the World Congress of Dermatology.
“Mass drug administration is highly effective and safe in the treatment of endemic scabies,” she said.
Using a strategy of directly observed treatment (DOT) with oral ivermectin or topical permethrin for all residents of two separate island groups in Fiji, Dr. Whitfield, together with epidemiologist Lucia Romani, PhD, both of the University of New South Wales, Sydney, and coinvestigators, demonstrated large and sustained decreases in the rates of scabies and impetigo (N Engl J Med. 2015 Dec 10;373[24]:2305-13).
Across study arms, which included a usual care arm, the baseline rate for scabies ranged from 30% to 40%. With usual care, the rate dropped from 36.6% to 18.8% at the end of 12 months, a relative reduction of 49%. However, the 15.8% prevalence rate 12 months after permethrin DOT (from 41.7%), and the 1.9% rate 12 months after ivermectin DOT (from 32.1%) – reductions of 62% and 94%, respectively – represented much larger decreases, “especially since these reductions were seen without any further interventions,” Dr. Whitfield said. “This was extremely exciting, and a game-changer as far as the management of endemic scabies is concerned.”
At baseline, impetigo rates hovered around 20%-25%, and usual care resulted in a 32% reduction at 12 months. With permethrin DOT, the impetigo rate dropped by 54%; with ivermectin DOT, the impetigo rate dropped by 67%. “The community level of impetigo went down, purely as a result of treating the scabies,” Dr. Whitfield said.
The outcomes of this study, she noted, “have contributed to the global discussion of the treatment of scabies.”
Two years after the mass drug administration (MDA) campaign, scabies prevalence remained much lower than at baseline, with clinical scabies diagnosed in 15.2% of the usual care group, 13.5% of the permethrin group, and just 3.6% of the ivermectin group. “The exciting thing for us was that these levels ... were able to be sustained at 2 years,” Dr. Whitfield noted.
The islands that had received ivermectin saw a continued decline in impetigo prevalence as well: By 24 months, impetigo was seen in 2.6% of participants in that arm.
Scabies is a neglected – but highly treatable – tropical disease, she noted. It is associated with intense pruritus, which results in reduced quality of life, and excoriations predispose those affected to bacterial superinfections, commonly impetigo in the young.
In Fiji, the scabies mite infests nearly 40% of those aged 5-9 years, and over one-third of those younger than 5 years. Rates drop steeply with increasing age and then climb again for the elderly; still, prevalence tops 10% for all Fijian age groups, Dr. Whitfield pointed out. Overall, scabies prevalence is 23% in Fiji, with resultant impetigo affecting 19% of the population.
Providing more details about the study, she said that she and her collaborators – working in conjunction with the Fijian Ministry of Health – took advantage of the geography of the island country, whose 850,000 residents live on 300 islands, to compare mass drug treatment with either ivermectin or permethrin with usual care. “We actually didn’t look for ‘infected scabies,’ ” she explained. “We looked for scabies as one outcome, and infection as another.”
The study was designed to take advantage of lessons from previous public health work addressing filariasis and soil-transmitted helminths, and addressed the following question: In Fiji, could a single round of MDA for scabies control lead to sustained reductions in scabies and impetigo prevalence 12 months later, compared with standard care?
The study applied standard-of-care scabies treatment to residents of one island; here, all residents of the island were assessed for scabies, and those who received a clinical diagnosis of scabies, along with family members and close contacts, were treated. Another group of three small islands received permethrin MDA. A third pair of neighboring islands received ivermectin MDA.
For one MDA arm, island residents received oral ivermectin via DOT. A second DOT dose was administered for those with clinically diagnosed scabies. For pregnant and breastfeeding women, children weighing less than 15 kg, and those with ivermectin hypersensitivity, permethrin was used, Dr. Whitfield said.
The individuals in the permethrin MDA arm received one topical dose via DOT, with a second round of topical permethrin for those with topical scabies.
In all, 803 Fijians were assigned to receive standard of care, 532 permethrin MDA, and 716 ivermectin MDA. Of these, 623 received ivermectin DOT, and 93 received permethrin. In all, DOT was achieved for 96% of those receiving the first dose. At baseline, 230 patients had scabies, with 200 receiving ivermectin and 30 permethrin; the DOT rate was 100% for the second dose.
For the permethrin arm, just 307 of 532 participants (58%) had DOT, though all were given permethrin. Scabies was present at baseline for 222 participants, and of these, 181 had DOT. “It’s much easier to do the direct observed therapy with an oral medication than with a cream,” Dr. Whitfield said. Data were not collected for the Fijians who received usual care at community health centers.
Outcomes were clinically determined via the child skin assessment algorithm of the World Health Organization’s International Management of Childhood Illness (IMCI) guidelines.
Dr. Whitfield acknowledged that the study was not a true cluster-randomized trial, and differences existed between the communities studies. Also, “dermatoscopy was not a practical option” for this real-world trial in a resource-limited setting, but validated clinical criteria were used, she said.
Going forward, she and her colleagues are continuing to track durability of reduced scabies rates, as well as downstream sequelae such as impetigo and septicemia. Also, “we need to see whether this community- and island-based project could be scaled up to a national or regional level,” she said.
The burden of disease from scabies globally is probably underestimated, and changing migration patterns may bring endemic scabies to the doorsteps of more developed nations, prompting consideration of MDA as a strategy in expanded circumstances.
Dr. Whitfield reported that she had no relevant conflicts of interest.
MILAN – In a region where scabies is endemic, a , findings that may have implications for future treatment of scabies or other infestations in other regions, dermatologist Margot Whitfield, MD, said at the World Congress of Dermatology.
“Mass drug administration is highly effective and safe in the treatment of endemic scabies,” she said.
Using a strategy of directly observed treatment (DOT) with oral ivermectin or topical permethrin for all residents of two separate island groups in Fiji, Dr. Whitfield, together with epidemiologist Lucia Romani, PhD, both of the University of New South Wales, Sydney, and coinvestigators, demonstrated large and sustained decreases in the rates of scabies and impetigo (N Engl J Med. 2015 Dec 10;373[24]:2305-13).
Across study arms, which included a usual care arm, the baseline rate for scabies ranged from 30% to 40%. With usual care, the rate dropped from 36.6% to 18.8% at the end of 12 months, a relative reduction of 49%. However, the 15.8% prevalence rate 12 months after permethrin DOT (from 41.7%), and the 1.9% rate 12 months after ivermectin DOT (from 32.1%) – reductions of 62% and 94%, respectively – represented much larger decreases, “especially since these reductions were seen without any further interventions,” Dr. Whitfield said. “This was extremely exciting, and a game-changer as far as the management of endemic scabies is concerned.”
At baseline, impetigo rates hovered around 20%-25%, and usual care resulted in a 32% reduction at 12 months. With permethrin DOT, the impetigo rate dropped by 54%; with ivermectin DOT, the impetigo rate dropped by 67%. “The community level of impetigo went down, purely as a result of treating the scabies,” Dr. Whitfield said.
The outcomes of this study, she noted, “have contributed to the global discussion of the treatment of scabies.”
Two years after the mass drug administration (MDA) campaign, scabies prevalence remained much lower than at baseline, with clinical scabies diagnosed in 15.2% of the usual care group, 13.5% of the permethrin group, and just 3.6% of the ivermectin group. “The exciting thing for us was that these levels ... were able to be sustained at 2 years,” Dr. Whitfield noted.
The islands that had received ivermectin saw a continued decline in impetigo prevalence as well: By 24 months, impetigo was seen in 2.6% of participants in that arm.
Scabies is a neglected – but highly treatable – tropical disease, she noted. It is associated with intense pruritus, which results in reduced quality of life, and excoriations predispose those affected to bacterial superinfections, commonly impetigo in the young.
In Fiji, the scabies mite infests nearly 40% of those aged 5-9 years, and over one-third of those younger than 5 years. Rates drop steeply with increasing age and then climb again for the elderly; still, prevalence tops 10% for all Fijian age groups, Dr. Whitfield pointed out. Overall, scabies prevalence is 23% in Fiji, with resultant impetigo affecting 19% of the population.
Providing more details about the study, she said that she and her collaborators – working in conjunction with the Fijian Ministry of Health – took advantage of the geography of the island country, whose 850,000 residents live on 300 islands, to compare mass drug treatment with either ivermectin or permethrin with usual care. “We actually didn’t look for ‘infected scabies,’ ” she explained. “We looked for scabies as one outcome, and infection as another.”
The study was designed to take advantage of lessons from previous public health work addressing filariasis and soil-transmitted helminths, and addressed the following question: In Fiji, could a single round of MDA for scabies control lead to sustained reductions in scabies and impetigo prevalence 12 months later, compared with standard care?
The study applied standard-of-care scabies treatment to residents of one island; here, all residents of the island were assessed for scabies, and those who received a clinical diagnosis of scabies, along with family members and close contacts, were treated. Another group of three small islands received permethrin MDA. A third pair of neighboring islands received ivermectin MDA.
For one MDA arm, island residents received oral ivermectin via DOT. A second DOT dose was administered for those with clinically diagnosed scabies. For pregnant and breastfeeding women, children weighing less than 15 kg, and those with ivermectin hypersensitivity, permethrin was used, Dr. Whitfield said.
The individuals in the permethrin MDA arm received one topical dose via DOT, with a second round of topical permethrin for those with topical scabies.
In all, 803 Fijians were assigned to receive standard of care, 532 permethrin MDA, and 716 ivermectin MDA. Of these, 623 received ivermectin DOT, and 93 received permethrin. In all, DOT was achieved for 96% of those receiving the first dose. At baseline, 230 patients had scabies, with 200 receiving ivermectin and 30 permethrin; the DOT rate was 100% for the second dose.
For the permethrin arm, just 307 of 532 participants (58%) had DOT, though all were given permethrin. Scabies was present at baseline for 222 participants, and of these, 181 had DOT. “It’s much easier to do the direct observed therapy with an oral medication than with a cream,” Dr. Whitfield said. Data were not collected for the Fijians who received usual care at community health centers.
Outcomes were clinically determined via the child skin assessment algorithm of the World Health Organization’s International Management of Childhood Illness (IMCI) guidelines.
Dr. Whitfield acknowledged that the study was not a true cluster-randomized trial, and differences existed between the communities studies. Also, “dermatoscopy was not a practical option” for this real-world trial in a resource-limited setting, but validated clinical criteria were used, she said.
Going forward, she and her colleagues are continuing to track durability of reduced scabies rates, as well as downstream sequelae such as impetigo and septicemia. Also, “we need to see whether this community- and island-based project could be scaled up to a national or regional level,” she said.
The burden of disease from scabies globally is probably underestimated, and changing migration patterns may bring endemic scabies to the doorsteps of more developed nations, prompting consideration of MDA as a strategy in expanded circumstances.
Dr. Whitfield reported that she had no relevant conflicts of interest.
MILAN – In a region where scabies is endemic, a , findings that may have implications for future treatment of scabies or other infestations in other regions, dermatologist Margot Whitfield, MD, said at the World Congress of Dermatology.
“Mass drug administration is highly effective and safe in the treatment of endemic scabies,” she said.
Using a strategy of directly observed treatment (DOT) with oral ivermectin or topical permethrin for all residents of two separate island groups in Fiji, Dr. Whitfield, together with epidemiologist Lucia Romani, PhD, both of the University of New South Wales, Sydney, and coinvestigators, demonstrated large and sustained decreases in the rates of scabies and impetigo (N Engl J Med. 2015 Dec 10;373[24]:2305-13).
Across study arms, which included a usual care arm, the baseline rate for scabies ranged from 30% to 40%. With usual care, the rate dropped from 36.6% to 18.8% at the end of 12 months, a relative reduction of 49%. However, the 15.8% prevalence rate 12 months after permethrin DOT (from 41.7%), and the 1.9% rate 12 months after ivermectin DOT (from 32.1%) – reductions of 62% and 94%, respectively – represented much larger decreases, “especially since these reductions were seen without any further interventions,” Dr. Whitfield said. “This was extremely exciting, and a game-changer as far as the management of endemic scabies is concerned.”
At baseline, impetigo rates hovered around 20%-25%, and usual care resulted in a 32% reduction at 12 months. With permethrin DOT, the impetigo rate dropped by 54%; with ivermectin DOT, the impetigo rate dropped by 67%. “The community level of impetigo went down, purely as a result of treating the scabies,” Dr. Whitfield said.
The outcomes of this study, she noted, “have contributed to the global discussion of the treatment of scabies.”
Two years after the mass drug administration (MDA) campaign, scabies prevalence remained much lower than at baseline, with clinical scabies diagnosed in 15.2% of the usual care group, 13.5% of the permethrin group, and just 3.6% of the ivermectin group. “The exciting thing for us was that these levels ... were able to be sustained at 2 years,” Dr. Whitfield noted.
The islands that had received ivermectin saw a continued decline in impetigo prevalence as well: By 24 months, impetigo was seen in 2.6% of participants in that arm.
Scabies is a neglected – but highly treatable – tropical disease, she noted. It is associated with intense pruritus, which results in reduced quality of life, and excoriations predispose those affected to bacterial superinfections, commonly impetigo in the young.
In Fiji, the scabies mite infests nearly 40% of those aged 5-9 years, and over one-third of those younger than 5 years. Rates drop steeply with increasing age and then climb again for the elderly; still, prevalence tops 10% for all Fijian age groups, Dr. Whitfield pointed out. Overall, scabies prevalence is 23% in Fiji, with resultant impetigo affecting 19% of the population.
Providing more details about the study, she said that she and her collaborators – working in conjunction with the Fijian Ministry of Health – took advantage of the geography of the island country, whose 850,000 residents live on 300 islands, to compare mass drug treatment with either ivermectin or permethrin with usual care. “We actually didn’t look for ‘infected scabies,’ ” she explained. “We looked for scabies as one outcome, and infection as another.”
The study was designed to take advantage of lessons from previous public health work addressing filariasis and soil-transmitted helminths, and addressed the following question: In Fiji, could a single round of MDA for scabies control lead to sustained reductions in scabies and impetigo prevalence 12 months later, compared with standard care?
The study applied standard-of-care scabies treatment to residents of one island; here, all residents of the island were assessed for scabies, and those who received a clinical diagnosis of scabies, along with family members and close contacts, were treated. Another group of three small islands received permethrin MDA. A third pair of neighboring islands received ivermectin MDA.
For one MDA arm, island residents received oral ivermectin via DOT. A second DOT dose was administered for those with clinically diagnosed scabies. For pregnant and breastfeeding women, children weighing less than 15 kg, and those with ivermectin hypersensitivity, permethrin was used, Dr. Whitfield said.
The individuals in the permethrin MDA arm received one topical dose via DOT, with a second round of topical permethrin for those with topical scabies.
In all, 803 Fijians were assigned to receive standard of care, 532 permethrin MDA, and 716 ivermectin MDA. Of these, 623 received ivermectin DOT, and 93 received permethrin. In all, DOT was achieved for 96% of those receiving the first dose. At baseline, 230 patients had scabies, with 200 receiving ivermectin and 30 permethrin; the DOT rate was 100% for the second dose.
For the permethrin arm, just 307 of 532 participants (58%) had DOT, though all were given permethrin. Scabies was present at baseline for 222 participants, and of these, 181 had DOT. “It’s much easier to do the direct observed therapy with an oral medication than with a cream,” Dr. Whitfield said. Data were not collected for the Fijians who received usual care at community health centers.
Outcomes were clinically determined via the child skin assessment algorithm of the World Health Organization’s International Management of Childhood Illness (IMCI) guidelines.
Dr. Whitfield acknowledged that the study was not a true cluster-randomized trial, and differences existed between the communities studies. Also, “dermatoscopy was not a practical option” for this real-world trial in a resource-limited setting, but validated clinical criteria were used, she said.
Going forward, she and her colleagues are continuing to track durability of reduced scabies rates, as well as downstream sequelae such as impetigo and septicemia. Also, “we need to see whether this community- and island-based project could be scaled up to a national or regional level,” she said.
The burden of disease from scabies globally is probably underestimated, and changing migration patterns may bring endemic scabies to the doorsteps of more developed nations, prompting consideration of MDA as a strategy in expanded circumstances.
Dr. Whitfield reported that she had no relevant conflicts of interest.
EXPERT ANALYSIS FROM WCD2019
In MS, children aren’t just little adults
SEATTLE – Multiple sclerosis (MS) presents quite differently in children than adults, a neurologist told colleagues, but many treatments are the same.
“We need to treat them when they’re young, perhaps, to prevent disability later on,” said Jennifer Graves, MD, PhD, director of neuroimmunology research at the University of California, San Diego, and director of the Rady Children’s Pediatric MS Clinic.
Fortunately, “they tend to respond to any DMT [disease-modifying therapy] you give them,” said Dr. Graves, who spoke at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Unlike the adult version, pediatric MS is almost never progressive, she said, and the relapsing form affects 85%-99% of those with the condition.
And children with MS suffer from high relapse rates. “In some of the more recent studies, the annual relapse rates have been two to even five to six times higher than in adults,” she related.
For acute relapses, Dr. Graves recommends IV methylprednisolone as a first-line treatment. High-dose oral steroids can be appropriate in teenagers, and plasma exchange is a second-line option.
DMT does work in children. “They’re just so inflammatory, having so much activity, that they tend to respond to anything,” she noted.
With few exceptions, DMTs have been tested in children, she said. But the trials in children have tended to be small, and only fingolimod (Gilenya) is Food and Drug Administration–approved for pediatric patients (aged 10-18 years).
What’s the best DMT for kids? “There is evidence that the higher-potency drugs may work better in some patients,” Dr. Graves said. “I diagnosed a 4 year old with MS. With an annual relapse rate of 6, I had no hesitancy about jumping to the highest-potency agent I had available.”
She cautioned, however, that there may never be deep insight into the best choices because of it is not feasible to study all the available DMTs in children.
Dr. Graves offered these tips about treating children with MS:
- Be prepared to spend long periods educating children and their parents. “You think you spend a long time with your adult patients? Double it,” she said. “Most new patient appointments will be 90 minutes at least. Negotiate that time for the patients in your clinic who are very young.”
- Don’t separate kids from parents. “I discourage parents from asking their children to leave the room. Everyone should know what the choices are as much as possible and having the children on the same page can help with compliance,” Dr. Graves said.
- Consider unique safety considerations. “Children are less likely to be JC [John Cunningham] virus antibody-positive than adults, and we can feel more confident with agents that cause PML [progressive multifocal leukoencephalopathy],” she said. “But they’re more likely to convert on our watch.”
- Consider mentioning sexual function to teens. As adolescents, “they’re unsure of sexual function to begin with, and they often don’t know if they’re normal in terms of sexual function.” Don’t forget that contraceptives may be appropriate because some MS drugs are teratogenic. “Have a lengthy and realistic conversation about birth control,” Dr. Graves said, “and maybe ask the parents to leave the room.”
Dr. Graves disclosed receiving speaking honoraria from Novartis and Sanofi Genzyme, and grants from Biogen and Genentech.
SEATTLE – Multiple sclerosis (MS) presents quite differently in children than adults, a neurologist told colleagues, but many treatments are the same.
“We need to treat them when they’re young, perhaps, to prevent disability later on,” said Jennifer Graves, MD, PhD, director of neuroimmunology research at the University of California, San Diego, and director of the Rady Children’s Pediatric MS Clinic.
Fortunately, “they tend to respond to any DMT [disease-modifying therapy] you give them,” said Dr. Graves, who spoke at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Unlike the adult version, pediatric MS is almost never progressive, she said, and the relapsing form affects 85%-99% of those with the condition.
And children with MS suffer from high relapse rates. “In some of the more recent studies, the annual relapse rates have been two to even five to six times higher than in adults,” she related.
For acute relapses, Dr. Graves recommends IV methylprednisolone as a first-line treatment. High-dose oral steroids can be appropriate in teenagers, and plasma exchange is a second-line option.
DMT does work in children. “They’re just so inflammatory, having so much activity, that they tend to respond to anything,” she noted.
With few exceptions, DMTs have been tested in children, she said. But the trials in children have tended to be small, and only fingolimod (Gilenya) is Food and Drug Administration–approved for pediatric patients (aged 10-18 years).
What’s the best DMT for kids? “There is evidence that the higher-potency drugs may work better in some patients,” Dr. Graves said. “I diagnosed a 4 year old with MS. With an annual relapse rate of 6, I had no hesitancy about jumping to the highest-potency agent I had available.”
She cautioned, however, that there may never be deep insight into the best choices because of it is not feasible to study all the available DMTs in children.
Dr. Graves offered these tips about treating children with MS:
- Be prepared to spend long periods educating children and their parents. “You think you spend a long time with your adult patients? Double it,” she said. “Most new patient appointments will be 90 minutes at least. Negotiate that time for the patients in your clinic who are very young.”
- Don’t separate kids from parents. “I discourage parents from asking their children to leave the room. Everyone should know what the choices are as much as possible and having the children on the same page can help with compliance,” Dr. Graves said.
- Consider unique safety considerations. “Children are less likely to be JC [John Cunningham] virus antibody-positive than adults, and we can feel more confident with agents that cause PML [progressive multifocal leukoencephalopathy],” she said. “But they’re more likely to convert on our watch.”
- Consider mentioning sexual function to teens. As adolescents, “they’re unsure of sexual function to begin with, and they often don’t know if they’re normal in terms of sexual function.” Don’t forget that contraceptives may be appropriate because some MS drugs are teratogenic. “Have a lengthy and realistic conversation about birth control,” Dr. Graves said, “and maybe ask the parents to leave the room.”
Dr. Graves disclosed receiving speaking honoraria from Novartis and Sanofi Genzyme, and grants from Biogen and Genentech.
SEATTLE – Multiple sclerosis (MS) presents quite differently in children than adults, a neurologist told colleagues, but many treatments are the same.
“We need to treat them when they’re young, perhaps, to prevent disability later on,” said Jennifer Graves, MD, PhD, director of neuroimmunology research at the University of California, San Diego, and director of the Rady Children’s Pediatric MS Clinic.
Fortunately, “they tend to respond to any DMT [disease-modifying therapy] you give them,” said Dr. Graves, who spoke at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Unlike the adult version, pediatric MS is almost never progressive, she said, and the relapsing form affects 85%-99% of those with the condition.
And children with MS suffer from high relapse rates. “In some of the more recent studies, the annual relapse rates have been two to even five to six times higher than in adults,” she related.
For acute relapses, Dr. Graves recommends IV methylprednisolone as a first-line treatment. High-dose oral steroids can be appropriate in teenagers, and plasma exchange is a second-line option.
DMT does work in children. “They’re just so inflammatory, having so much activity, that they tend to respond to anything,” she noted.
With few exceptions, DMTs have been tested in children, she said. But the trials in children have tended to be small, and only fingolimod (Gilenya) is Food and Drug Administration–approved for pediatric patients (aged 10-18 years).
What’s the best DMT for kids? “There is evidence that the higher-potency drugs may work better in some patients,” Dr. Graves said. “I diagnosed a 4 year old with MS. With an annual relapse rate of 6, I had no hesitancy about jumping to the highest-potency agent I had available.”
She cautioned, however, that there may never be deep insight into the best choices because of it is not feasible to study all the available DMTs in children.
Dr. Graves offered these tips about treating children with MS:
- Be prepared to spend long periods educating children and their parents. “You think you spend a long time with your adult patients? Double it,” she said. “Most new patient appointments will be 90 minutes at least. Negotiate that time for the patients in your clinic who are very young.”
- Don’t separate kids from parents. “I discourage parents from asking their children to leave the room. Everyone should know what the choices are as much as possible and having the children on the same page can help with compliance,” Dr. Graves said.
- Consider unique safety considerations. “Children are less likely to be JC [John Cunningham] virus antibody-positive than adults, and we can feel more confident with agents that cause PML [progressive multifocal leukoencephalopathy],” she said. “But they’re more likely to convert on our watch.”
- Consider mentioning sexual function to teens. As adolescents, “they’re unsure of sexual function to begin with, and they often don’t know if they’re normal in terms of sexual function.” Don’t forget that contraceptives may be appropriate because some MS drugs are teratogenic. “Have a lengthy and realistic conversation about birth control,” Dr. Graves said, “and maybe ask the parents to leave the room.”
Dr. Graves disclosed receiving speaking honoraria from Novartis and Sanofi Genzyme, and grants from Biogen and Genentech.
EXPERT ANALYSIS FROM CMSC 2019
Reducing pediatric RSV burden is top priority
LJUBLJANA, SLOVENIA – Prevention or early effective treatment of respiratory syncytial virus (RSV) infection in infants and small children holds the promise of sharply reduced burdens of both acute otitis media (AOM) and pneumonia, Terho Heikkinen, MD, PhD, predicted in the Bill Marshall Award Lecture presented at the annual meeting of the European Society for Paediatric Infectious Diseases (ESPID).
RSV is by far the hottest virus in the world,” declared Dr. Heikkinen, professor of pediatrics at the University of Turku (Finland).
“A lot of progress is being made with respect to RSV. This increased understanding holds great promise for new interventions,” he explained. “Lots of different types of vaccines are being developed, monoclonal antibodies, antivirals. So
Today influenza is the only respiratory viral infection that’s preventable via vaccine or effectively treatable using antiviral drugs. That situation has to change, as Dr. Heikkinen demonstrated early in his career; RSV is the respiratory virus that’s most likely to invade the middle ear during AOM. It’s much more ototropic than influenza, parainfluenza, enteroviruses, or adenoviruses (N Engl J Med. 1999 Jan 28;340[4]:260-4), he noted.
The Bill Marshall Award and Lecture, ESPID’s most prestigious award, is given annually to an individual recognized as having significantly advanced the field of pediatric infectious diseases. Dr. Heikkinen was singled out for his decades of work establishing that viruses, including RSV, play a key role in AOM, which had traditionally been regarded as a bacterial infection. He and his coinvestigators demonstrated that in about two-thirds of cases, AOM is actually caused by a combination of bacteria and viruses, which explains why patients’ clinical response to antibiotic therapy for AOM often is poor. They also described the chain of events whereby viral infection of the upper airway epithelium triggers an inflammatory response in the nasopharynx, with resultant Eustachian tube dysfunction and negative middle ear pressure, which in turn encourages microbial invasion of the middle ear. Moreover, they showed that the peak incidence of AOM isn’t on day 1 after onset of upper respiratory infection symptoms, but on day 3 or 4.
“What this tells us is that, once a child has a viral respiratory infection, there is a certain window of opportunity to try to prevent the development of the complication if we have the right tools in place,” Dr. Heikkinen said.
He and his colleagues put this lesson to good use nearly a decade ago in a randomized, double-blind trial in which they showed that giving oseltamivir (Tamiflu) within 12 hours after onset of influenza symptoms in children aged 1-3 years reduced the subsequent incidence of AOM by 85%, compared with placebo (Clin Infect Dis. 2010 Oct 15;51[8]:887-94).
These observations paved the way for the ongoing intensive research effort exploring ways of preventing AOM through interventions at two different levels: by developing viral vaccines to prevent a healthy child from contracting the viral upper respiratory infection that precedes AOM and by coming up with antiviral drugs or bacterial vaccines to prevent a upper respiratory infection from evolving into AOM.
The same applies to pneumonia. Other investigators showed years ago that both respiratory viruses and bacteria were present in two-thirds of sputum samples obtained from children with community-acquired pneumonia (Clin Microbiol Infect. 2012 Mar;18[3]:300-7).
RSV is the top cause of hospitalization for acute respiratory infection – pneumonia and bronchiolitis – in infants. Worldwide, it’s estimated that RSV accounts for more than 33 million episodes of pneumonia annually, with 3.2 million hospitalizations and 118,200 deaths.
Beyond the hospital, however, Dr. Heikkinen and colleagues conducted a prospective cohort study in Turku over the course of two consecutive respiratory infection seasons in which they captured the huge burden of RSV as an outpatient illness. It hit hardest in children younger than 3 years, in whom the average annual incidence of RSV infection was 275 cases per 1,000 children. In that youngest age population, RSV upper respiratory infection was followed by AOM 58% of the time, with antibiotics prescribed in 66% of the cases of this complication of RSV illness. The mean duration of RSV illness was greatest in this young age group, at 13 days, and it was associated with parental absenteeism from work at a rate of 136 days per 100 children with RSV illness.
Moreover, while AOM occurred less frequently in children aged 3-6 years, 46% of the cases were attributed to a preceding RSV infection, which led to antibiotic treatment nearly half of the time (J Infect Dis. 2017 Jan 1;215[1]:17-23). This documentation has spurred further efforts to develop RSV vaccines and antivirals.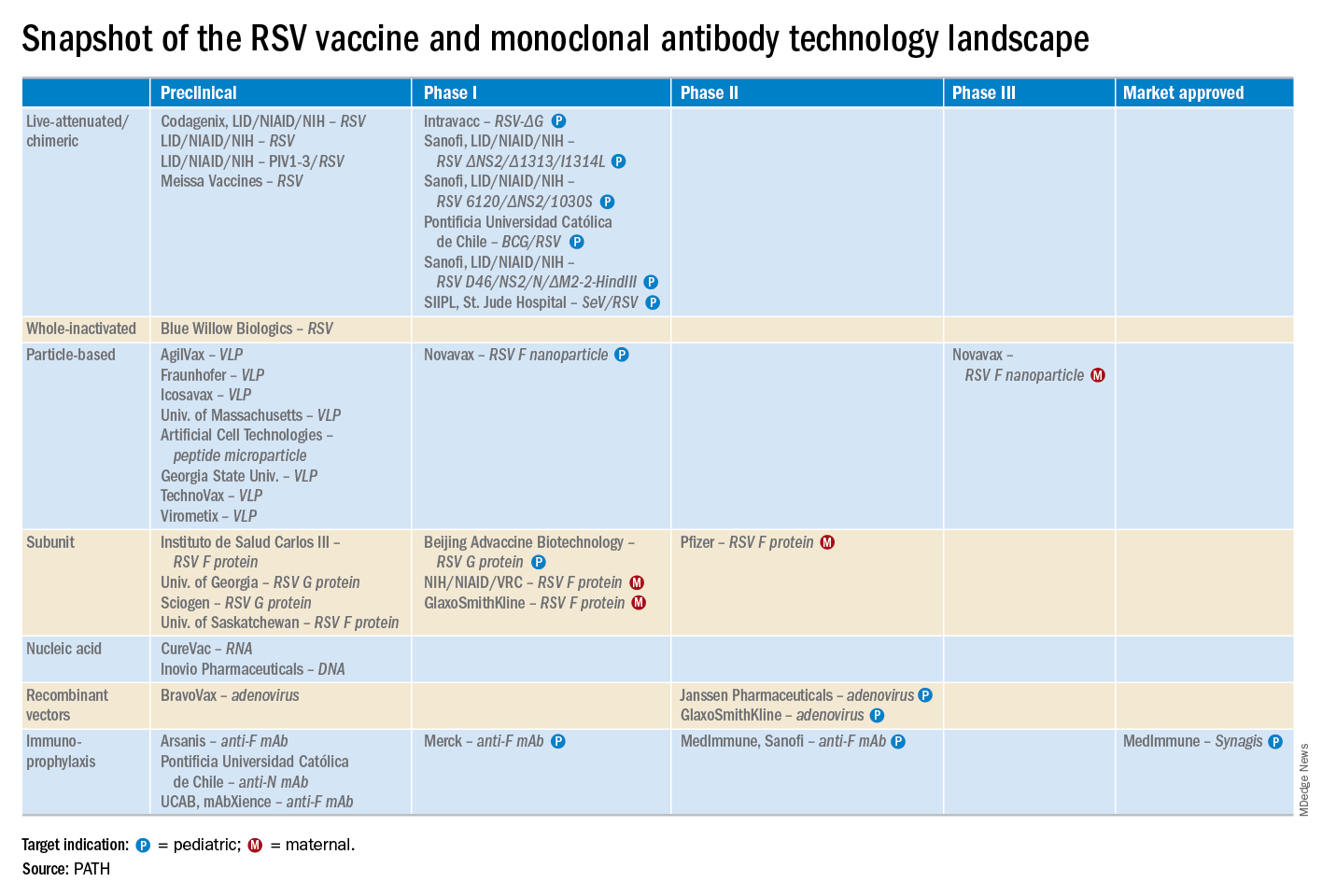
He reported serving as a consultant to a half-dozen pharmaceutical companies, as well as having received research funding from Janssen, GlaxoSmithKline, and Novavax.
LJUBLJANA, SLOVENIA – Prevention or early effective treatment of respiratory syncytial virus (RSV) infection in infants and small children holds the promise of sharply reduced burdens of both acute otitis media (AOM) and pneumonia, Terho Heikkinen, MD, PhD, predicted in the Bill Marshall Award Lecture presented at the annual meeting of the European Society for Paediatric Infectious Diseases (ESPID).
RSV is by far the hottest virus in the world,” declared Dr. Heikkinen, professor of pediatrics at the University of Turku (Finland).
“A lot of progress is being made with respect to RSV. This increased understanding holds great promise for new interventions,” he explained. “Lots of different types of vaccines are being developed, monoclonal antibodies, antivirals. So
Today influenza is the only respiratory viral infection that’s preventable via vaccine or effectively treatable using antiviral drugs. That situation has to change, as Dr. Heikkinen demonstrated early in his career; RSV is the respiratory virus that’s most likely to invade the middle ear during AOM. It’s much more ototropic than influenza, parainfluenza, enteroviruses, or adenoviruses (N Engl J Med. 1999 Jan 28;340[4]:260-4), he noted.
The Bill Marshall Award and Lecture, ESPID’s most prestigious award, is given annually to an individual recognized as having significantly advanced the field of pediatric infectious diseases. Dr. Heikkinen was singled out for his decades of work establishing that viruses, including RSV, play a key role in AOM, which had traditionally been regarded as a bacterial infection. He and his coinvestigators demonstrated that in about two-thirds of cases, AOM is actually caused by a combination of bacteria and viruses, which explains why patients’ clinical response to antibiotic therapy for AOM often is poor. They also described the chain of events whereby viral infection of the upper airway epithelium triggers an inflammatory response in the nasopharynx, with resultant Eustachian tube dysfunction and negative middle ear pressure, which in turn encourages microbial invasion of the middle ear. Moreover, they showed that the peak incidence of AOM isn’t on day 1 after onset of upper respiratory infection symptoms, but on day 3 or 4.
“What this tells us is that, once a child has a viral respiratory infection, there is a certain window of opportunity to try to prevent the development of the complication if we have the right tools in place,” Dr. Heikkinen said.
He and his colleagues put this lesson to good use nearly a decade ago in a randomized, double-blind trial in which they showed that giving oseltamivir (Tamiflu) within 12 hours after onset of influenza symptoms in children aged 1-3 years reduced the subsequent incidence of AOM by 85%, compared with placebo (Clin Infect Dis. 2010 Oct 15;51[8]:887-94).
These observations paved the way for the ongoing intensive research effort exploring ways of preventing AOM through interventions at two different levels: by developing viral vaccines to prevent a healthy child from contracting the viral upper respiratory infection that precedes AOM and by coming up with antiviral drugs or bacterial vaccines to prevent a upper respiratory infection from evolving into AOM.
The same applies to pneumonia. Other investigators showed years ago that both respiratory viruses and bacteria were present in two-thirds of sputum samples obtained from children with community-acquired pneumonia (Clin Microbiol Infect. 2012 Mar;18[3]:300-7).
RSV is the top cause of hospitalization for acute respiratory infection – pneumonia and bronchiolitis – in infants. Worldwide, it’s estimated that RSV accounts for more than 33 million episodes of pneumonia annually, with 3.2 million hospitalizations and 118,200 deaths.
Beyond the hospital, however, Dr. Heikkinen and colleagues conducted a prospective cohort study in Turku over the course of two consecutive respiratory infection seasons in which they captured the huge burden of RSV as an outpatient illness. It hit hardest in children younger than 3 years, in whom the average annual incidence of RSV infection was 275 cases per 1,000 children. In that youngest age population, RSV upper respiratory infection was followed by AOM 58% of the time, with antibiotics prescribed in 66% of the cases of this complication of RSV illness. The mean duration of RSV illness was greatest in this young age group, at 13 days, and it was associated with parental absenteeism from work at a rate of 136 days per 100 children with RSV illness.
Moreover, while AOM occurred less frequently in children aged 3-6 years, 46% of the cases were attributed to a preceding RSV infection, which led to antibiotic treatment nearly half of the time (J Infect Dis. 2017 Jan 1;215[1]:17-23). This documentation has spurred further efforts to develop RSV vaccines and antivirals.
He reported serving as a consultant to a half-dozen pharmaceutical companies, as well as having received research funding from Janssen, GlaxoSmithKline, and Novavax.
LJUBLJANA, SLOVENIA – Prevention or early effective treatment of respiratory syncytial virus (RSV) infection in infants and small children holds the promise of sharply reduced burdens of both acute otitis media (AOM) and pneumonia, Terho Heikkinen, MD, PhD, predicted in the Bill Marshall Award Lecture presented at the annual meeting of the European Society for Paediatric Infectious Diseases (ESPID).
RSV is by far the hottest virus in the world,” declared Dr. Heikkinen, professor of pediatrics at the University of Turku (Finland).
“A lot of progress is being made with respect to RSV. This increased understanding holds great promise for new interventions,” he explained. “Lots of different types of vaccines are being developed, monoclonal antibodies, antivirals. So
Today influenza is the only respiratory viral infection that’s preventable via vaccine or effectively treatable using antiviral drugs. That situation has to change, as Dr. Heikkinen demonstrated early in his career; RSV is the respiratory virus that’s most likely to invade the middle ear during AOM. It’s much more ototropic than influenza, parainfluenza, enteroviruses, or adenoviruses (N Engl J Med. 1999 Jan 28;340[4]:260-4), he noted.
The Bill Marshall Award and Lecture, ESPID’s most prestigious award, is given annually to an individual recognized as having significantly advanced the field of pediatric infectious diseases. Dr. Heikkinen was singled out for his decades of work establishing that viruses, including RSV, play a key role in AOM, which had traditionally been regarded as a bacterial infection. He and his coinvestigators demonstrated that in about two-thirds of cases, AOM is actually caused by a combination of bacteria and viruses, which explains why patients’ clinical response to antibiotic therapy for AOM often is poor. They also described the chain of events whereby viral infection of the upper airway epithelium triggers an inflammatory response in the nasopharynx, with resultant Eustachian tube dysfunction and negative middle ear pressure, which in turn encourages microbial invasion of the middle ear. Moreover, they showed that the peak incidence of AOM isn’t on day 1 after onset of upper respiratory infection symptoms, but on day 3 or 4.
“What this tells us is that, once a child has a viral respiratory infection, there is a certain window of opportunity to try to prevent the development of the complication if we have the right tools in place,” Dr. Heikkinen said.
He and his colleagues put this lesson to good use nearly a decade ago in a randomized, double-blind trial in which they showed that giving oseltamivir (Tamiflu) within 12 hours after onset of influenza symptoms in children aged 1-3 years reduced the subsequent incidence of AOM by 85%, compared with placebo (Clin Infect Dis. 2010 Oct 15;51[8]:887-94).
These observations paved the way for the ongoing intensive research effort exploring ways of preventing AOM through interventions at two different levels: by developing viral vaccines to prevent a healthy child from contracting the viral upper respiratory infection that precedes AOM and by coming up with antiviral drugs or bacterial vaccines to prevent a upper respiratory infection from evolving into AOM.
The same applies to pneumonia. Other investigators showed years ago that both respiratory viruses and bacteria were present in two-thirds of sputum samples obtained from children with community-acquired pneumonia (Clin Microbiol Infect. 2012 Mar;18[3]:300-7).
RSV is the top cause of hospitalization for acute respiratory infection – pneumonia and bronchiolitis – in infants. Worldwide, it’s estimated that RSV accounts for more than 33 million episodes of pneumonia annually, with 3.2 million hospitalizations and 118,200 deaths.
Beyond the hospital, however, Dr. Heikkinen and colleagues conducted a prospective cohort study in Turku over the course of two consecutive respiratory infection seasons in which they captured the huge burden of RSV as an outpatient illness. It hit hardest in children younger than 3 years, in whom the average annual incidence of RSV infection was 275 cases per 1,000 children. In that youngest age population, RSV upper respiratory infection was followed by AOM 58% of the time, with antibiotics prescribed in 66% of the cases of this complication of RSV illness. The mean duration of RSV illness was greatest in this young age group, at 13 days, and it was associated with parental absenteeism from work at a rate of 136 days per 100 children with RSV illness.
Moreover, while AOM occurred less frequently in children aged 3-6 years, 46% of the cases were attributed to a preceding RSV infection, which led to antibiotic treatment nearly half of the time (J Infect Dis. 2017 Jan 1;215[1]:17-23). This documentation has spurred further efforts to develop RSV vaccines and antivirals.
He reported serving as a consultant to a half-dozen pharmaceutical companies, as well as having received research funding from Janssen, GlaxoSmithKline, and Novavax.
EXPERT ANALYSIS FROM ESPID 2019
Waning pertussis immunity may be linked to acellular vaccine
A large Kaiser Permanente study paints a nuanced picture of the acellular pertussis vaccine, with more cases occurring in fully vaccinated children, but the highest risk of disease occurring among the under- and unvaccinated.
Among nearly half a million children, the unvaccinated were 13 times more likely to develop pertussis than fully vaccinated children, Ousseny Zerbo, PhD, of Kaiser Permanente Northern California in Oakland and colleagues wrote in Pediatrics. But 82% of cases occurred in fully vaccinated children and just 5% in undervaccinated children – and rates increased in both groups the farther they were in time from the last vaccination.
“Within our study population, greater than 80% of pertussis cases occurred among age-appropriately vaccinated children,” the team wrote. “Children who were further away from their last DTaP dose were at increased risk of pertussis, even after controlling for undervaccination. Our results suggest that, in this population, possibly in conjunction with other factors not addressed in this study, suboptimal vaccine efficacy and waning [immunity] played a major role in recent pertussis epidemics.”
The results are consistent with several prior studies, including one finding that the odds of the disease increased by 33% for every additional year after the third or fifth DTaP dose (Pediatrics. 2015;135[2]:331-43).
The current study comprised 469,982 children aged between 3 months and 11 years, who were followed for a mean of 4.6 years. Over the entire study period, there were 738 lab-confirmed pertussis cases. Most of these (515; 70%) occurred in fully vaccinated children. Another 99 (13%) occurred in unvaccinated children, 36 (5%) in undervaccinated children, and 88 (12%) in fully vaccinated plus one dose.
In a multivariate analysis, the risk of pertussis was 13 times higher among the unvaccinated (adjusted hazard ratio, 13) and almost 2 times higher among the undervaccinated (aHR, 1.9), compared with fully vaccinated children. Those who had been fully vaccinated and received a booster had the lowest risk, about half that of fully vaccinated children (aHR, 0.48).
Risk varied according to age, but also was significantly higher among unvaccinated children at each time point. Risk ranged from 4 times higher among those aged 3-5 months to 23 times higher among those aged 19-84 months. Undervaccinated children aged 5-7 months and 19-84 months also were at significantly increased risk for pertussis, compared with fully vaccinated children. Children who were fully vaccinated plus one dose had a significantly reduced risk at 7-19 months and at 19-84 months, compared with the fully vaccinated reference group.
“Across all follow-up and all age groups, VE [vaccine effectiveness] was 86% ... for undervaccinated children, compared with unvaccinated children,” Dr. Zerbo and associates wrote. “VE was even higher for fully vaccinated children [93%] and for those who were fully vaccinated plus one dose [96%].”
But VE waned as time progressed farther from the last DTaP dose. The multivariate model found more than a 100% increased risk for those whose last DTaP was at least 3 years past, compared with less than 1 year past (aHR, 2.58).
The model also found time-bound risk increases among fully vaccinated children, with a more than 300% increased risk among those at least 6 years out from the last DTaP dose, compared with 3 years out (aHR, 4.66).
The results indicate that other factors besides adherence to the recommended vaccine schedule may be at work in recent pertussis outbreaks.
“Although waning immunity is clearly an important factor driving pertussis epidemics in recent years, other factors that we did not evaluate in this study might also contribute to pertussis epidemics individually or in synergy,” Dr. Zerbo and associates wrote. “Results from studies in baboons suggest that the acellular pertussis vaccines are unable to prevent colonization, carriage, and transmission. If this is also true for humans, this could contribute to pertussis epidemics. The causes of recent pertussis epidemics are complex, and we were only able to address some aspects in our study.”
The study was funded by Kaiser Permanente Northern California, the National Institutes of Health, and in part by a National Institute of Allergy and Infectious Diseases grant. One coauthor reported receiving research grant support from Sanofi Pasteur, Novartis, GlaxoSmithKline, Merck, MedImmune, Pfizer, and Dynavax for unrelated studies; the other authors reported no relevant financial disclosures.
SOURCE: Zerbo O et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3466.
Fixing one problem with the pertussis vaccine seemed to have created another, Kathryn M. Edwards, MD, wrote in an accompanying editorial.
The current acellular vaccine was approved in 1997. It was considered a less reactive substitute for the previous whole-cell vaccine, which was associated with injection site pain, swelling, fever, and febrile seizures, Dr. Edwards wrote. “For about a decade, all seemed to be going well with pertussis control. Serological methods were employed to diagnose pertussis infections in adolescents and adults, and polymerase chain reaction methods were devised to more accurately detect pertussis organisms. Thus, the burden of pertussis disease was increasingly appreciated as the diagnostic methods improved.”
But things soon changed. There were pertussis outbreaks, some of them quite large. The increasing disease rates showed that protection conferred by the acellular vaccine waned much more quickly than that conferred by the whole-cell vaccine. “In the current study, Zerbo et al. add to the body of evidence documenting the increase in pertussis risk with time after DTaP vaccination,” she noted.
This has several practical implications, Dr. Edwards wrote.
“First, given the markedly increased risk of pertussis in unvaccinated and undervaccinated children, universal DTaP vaccination should be strongly recommended. Second, the addition of maternal Tdap vaccination administered during pregnancy has been shown to significantly reduce infant disease before primary immunization and should remain the standard,” Dr. Edwards wrote.
More problematic is how to address the waning DTaP immunity now seen. “One option presented [at an international meeting] was a live-attenuated pertussis vaccine administered intranasally that would stimulate local immune responses and prevent colonization with pertussis organisms. This vaccine is currently being studied in adults and might provide a solution for waning immunity seen with DTaP vaccine,” she noted.
Another possibility is adding the live vaccine to the current DTaP, which should, in theory, stimulate more long-lasting immunity. But numerous safety studies in young children would be necessary before adopting such an approach, Dr. Edwards wrote.
Adding more antigens to the acellular vaccine also might work, and investigational vaccines like this are in development.
Studies in animals and humans show that acellular vaccines “generate functionally different T-cell responses than those seen after whole-cell vaccines, with the whole cell vaccines generating more protective T-cell responses. Studies are ongoing to determine if adjuvants can be added to acellular vaccines to modify their T-cell responses to a more protective immune response or whether the T-cell response remains fixed once primed with DTaP vaccine,” she wrote.
Dr. Edwards is a pediatric infectious disease specialist at Vanderbilt University, Nashville, Tenn. She wrote an editorial to accompany Zerbo et al (Pediatrics. 2019. doi: 10.1542/peds.2019-1276). She reported no financial disclosures, and received no funding to write the editorial.
Fixing one problem with the pertussis vaccine seemed to have created another, Kathryn M. Edwards, MD, wrote in an accompanying editorial.
The current acellular vaccine was approved in 1997. It was considered a less reactive substitute for the previous whole-cell vaccine, which was associated with injection site pain, swelling, fever, and febrile seizures, Dr. Edwards wrote. “For about a decade, all seemed to be going well with pertussis control. Serological methods were employed to diagnose pertussis infections in adolescents and adults, and polymerase chain reaction methods were devised to more accurately detect pertussis organisms. Thus, the burden of pertussis disease was increasingly appreciated as the diagnostic methods improved.”
But things soon changed. There were pertussis outbreaks, some of them quite large. The increasing disease rates showed that protection conferred by the acellular vaccine waned much more quickly than that conferred by the whole-cell vaccine. “In the current study, Zerbo et al. add to the body of evidence documenting the increase in pertussis risk with time after DTaP vaccination,” she noted.
This has several practical implications, Dr. Edwards wrote.
“First, given the markedly increased risk of pertussis in unvaccinated and undervaccinated children, universal DTaP vaccination should be strongly recommended. Second, the addition of maternal Tdap vaccination administered during pregnancy has been shown to significantly reduce infant disease before primary immunization and should remain the standard,” Dr. Edwards wrote.
More problematic is how to address the waning DTaP immunity now seen. “One option presented [at an international meeting] was a live-attenuated pertussis vaccine administered intranasally that would stimulate local immune responses and prevent colonization with pertussis organisms. This vaccine is currently being studied in adults and might provide a solution for waning immunity seen with DTaP vaccine,” she noted.
Another possibility is adding the live vaccine to the current DTaP, which should, in theory, stimulate more long-lasting immunity. But numerous safety studies in young children would be necessary before adopting such an approach, Dr. Edwards wrote.
Adding more antigens to the acellular vaccine also might work, and investigational vaccines like this are in development.
Studies in animals and humans show that acellular vaccines “generate functionally different T-cell responses than those seen after whole-cell vaccines, with the whole cell vaccines generating more protective T-cell responses. Studies are ongoing to determine if adjuvants can be added to acellular vaccines to modify their T-cell responses to a more protective immune response or whether the T-cell response remains fixed once primed with DTaP vaccine,” she wrote.
Dr. Edwards is a pediatric infectious disease specialist at Vanderbilt University, Nashville, Tenn. She wrote an editorial to accompany Zerbo et al (Pediatrics. 2019. doi: 10.1542/peds.2019-1276). She reported no financial disclosures, and received no funding to write the editorial.
Fixing one problem with the pertussis vaccine seemed to have created another, Kathryn M. Edwards, MD, wrote in an accompanying editorial.
The current acellular vaccine was approved in 1997. It was considered a less reactive substitute for the previous whole-cell vaccine, which was associated with injection site pain, swelling, fever, and febrile seizures, Dr. Edwards wrote. “For about a decade, all seemed to be going well with pertussis control. Serological methods were employed to diagnose pertussis infections in adolescents and adults, and polymerase chain reaction methods were devised to more accurately detect pertussis organisms. Thus, the burden of pertussis disease was increasingly appreciated as the diagnostic methods improved.”
But things soon changed. There were pertussis outbreaks, some of them quite large. The increasing disease rates showed that protection conferred by the acellular vaccine waned much more quickly than that conferred by the whole-cell vaccine. “In the current study, Zerbo et al. add to the body of evidence documenting the increase in pertussis risk with time after DTaP vaccination,” she noted.
This has several practical implications, Dr. Edwards wrote.
“First, given the markedly increased risk of pertussis in unvaccinated and undervaccinated children, universal DTaP vaccination should be strongly recommended. Second, the addition of maternal Tdap vaccination administered during pregnancy has been shown to significantly reduce infant disease before primary immunization and should remain the standard,” Dr. Edwards wrote.
More problematic is how to address the waning DTaP immunity now seen. “One option presented [at an international meeting] was a live-attenuated pertussis vaccine administered intranasally that would stimulate local immune responses and prevent colonization with pertussis organisms. This vaccine is currently being studied in adults and might provide a solution for waning immunity seen with DTaP vaccine,” she noted.
Another possibility is adding the live vaccine to the current DTaP, which should, in theory, stimulate more long-lasting immunity. But numerous safety studies in young children would be necessary before adopting such an approach, Dr. Edwards wrote.
Adding more antigens to the acellular vaccine also might work, and investigational vaccines like this are in development.
Studies in animals and humans show that acellular vaccines “generate functionally different T-cell responses than those seen after whole-cell vaccines, with the whole cell vaccines generating more protective T-cell responses. Studies are ongoing to determine if adjuvants can be added to acellular vaccines to modify their T-cell responses to a more protective immune response or whether the T-cell response remains fixed once primed with DTaP vaccine,” she wrote.
Dr. Edwards is a pediatric infectious disease specialist at Vanderbilt University, Nashville, Tenn. She wrote an editorial to accompany Zerbo et al (Pediatrics. 2019. doi: 10.1542/peds.2019-1276). She reported no financial disclosures, and received no funding to write the editorial.
A large Kaiser Permanente study paints a nuanced picture of the acellular pertussis vaccine, with more cases occurring in fully vaccinated children, but the highest risk of disease occurring among the under- and unvaccinated.
Among nearly half a million children, the unvaccinated were 13 times more likely to develop pertussis than fully vaccinated children, Ousseny Zerbo, PhD, of Kaiser Permanente Northern California in Oakland and colleagues wrote in Pediatrics. But 82% of cases occurred in fully vaccinated children and just 5% in undervaccinated children – and rates increased in both groups the farther they were in time from the last vaccination.
“Within our study population, greater than 80% of pertussis cases occurred among age-appropriately vaccinated children,” the team wrote. “Children who were further away from their last DTaP dose were at increased risk of pertussis, even after controlling for undervaccination. Our results suggest that, in this population, possibly in conjunction with other factors not addressed in this study, suboptimal vaccine efficacy and waning [immunity] played a major role in recent pertussis epidemics.”
The results are consistent with several prior studies, including one finding that the odds of the disease increased by 33% for every additional year after the third or fifth DTaP dose (Pediatrics. 2015;135[2]:331-43).
The current study comprised 469,982 children aged between 3 months and 11 years, who were followed for a mean of 4.6 years. Over the entire study period, there were 738 lab-confirmed pertussis cases. Most of these (515; 70%) occurred in fully vaccinated children. Another 99 (13%) occurred in unvaccinated children, 36 (5%) in undervaccinated children, and 88 (12%) in fully vaccinated plus one dose.
In a multivariate analysis, the risk of pertussis was 13 times higher among the unvaccinated (adjusted hazard ratio, 13) and almost 2 times higher among the undervaccinated (aHR, 1.9), compared with fully vaccinated children. Those who had been fully vaccinated and received a booster had the lowest risk, about half that of fully vaccinated children (aHR, 0.48).
Risk varied according to age, but also was significantly higher among unvaccinated children at each time point. Risk ranged from 4 times higher among those aged 3-5 months to 23 times higher among those aged 19-84 months. Undervaccinated children aged 5-7 months and 19-84 months also were at significantly increased risk for pertussis, compared with fully vaccinated children. Children who were fully vaccinated plus one dose had a significantly reduced risk at 7-19 months and at 19-84 months, compared with the fully vaccinated reference group.
“Across all follow-up and all age groups, VE [vaccine effectiveness] was 86% ... for undervaccinated children, compared with unvaccinated children,” Dr. Zerbo and associates wrote. “VE was even higher for fully vaccinated children [93%] and for those who were fully vaccinated plus one dose [96%].”
But VE waned as time progressed farther from the last DTaP dose. The multivariate model found more than a 100% increased risk for those whose last DTaP was at least 3 years past, compared with less than 1 year past (aHR, 2.58).
The model also found time-bound risk increases among fully vaccinated children, with a more than 300% increased risk among those at least 6 years out from the last DTaP dose, compared with 3 years out (aHR, 4.66).
The results indicate that other factors besides adherence to the recommended vaccine schedule may be at work in recent pertussis outbreaks.
“Although waning immunity is clearly an important factor driving pertussis epidemics in recent years, other factors that we did not evaluate in this study might also contribute to pertussis epidemics individually or in synergy,” Dr. Zerbo and associates wrote. “Results from studies in baboons suggest that the acellular pertussis vaccines are unable to prevent colonization, carriage, and transmission. If this is also true for humans, this could contribute to pertussis epidemics. The causes of recent pertussis epidemics are complex, and we were only able to address some aspects in our study.”
The study was funded by Kaiser Permanente Northern California, the National Institutes of Health, and in part by a National Institute of Allergy and Infectious Diseases grant. One coauthor reported receiving research grant support from Sanofi Pasteur, Novartis, GlaxoSmithKline, Merck, MedImmune, Pfizer, and Dynavax for unrelated studies; the other authors reported no relevant financial disclosures.
SOURCE: Zerbo O et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3466.
A large Kaiser Permanente study paints a nuanced picture of the acellular pertussis vaccine, with more cases occurring in fully vaccinated children, but the highest risk of disease occurring among the under- and unvaccinated.
Among nearly half a million children, the unvaccinated were 13 times more likely to develop pertussis than fully vaccinated children, Ousseny Zerbo, PhD, of Kaiser Permanente Northern California in Oakland and colleagues wrote in Pediatrics. But 82% of cases occurred in fully vaccinated children and just 5% in undervaccinated children – and rates increased in both groups the farther they were in time from the last vaccination.
“Within our study population, greater than 80% of pertussis cases occurred among age-appropriately vaccinated children,” the team wrote. “Children who were further away from their last DTaP dose were at increased risk of pertussis, even after controlling for undervaccination. Our results suggest that, in this population, possibly in conjunction with other factors not addressed in this study, suboptimal vaccine efficacy and waning [immunity] played a major role in recent pertussis epidemics.”
The results are consistent with several prior studies, including one finding that the odds of the disease increased by 33% for every additional year after the third or fifth DTaP dose (Pediatrics. 2015;135[2]:331-43).
The current study comprised 469,982 children aged between 3 months and 11 years, who were followed for a mean of 4.6 years. Over the entire study period, there were 738 lab-confirmed pertussis cases. Most of these (515; 70%) occurred in fully vaccinated children. Another 99 (13%) occurred in unvaccinated children, 36 (5%) in undervaccinated children, and 88 (12%) in fully vaccinated plus one dose.
In a multivariate analysis, the risk of pertussis was 13 times higher among the unvaccinated (adjusted hazard ratio, 13) and almost 2 times higher among the undervaccinated (aHR, 1.9), compared with fully vaccinated children. Those who had been fully vaccinated and received a booster had the lowest risk, about half that of fully vaccinated children (aHR, 0.48).
Risk varied according to age, but also was significantly higher among unvaccinated children at each time point. Risk ranged from 4 times higher among those aged 3-5 months to 23 times higher among those aged 19-84 months. Undervaccinated children aged 5-7 months and 19-84 months also were at significantly increased risk for pertussis, compared with fully vaccinated children. Children who were fully vaccinated plus one dose had a significantly reduced risk at 7-19 months and at 19-84 months, compared with the fully vaccinated reference group.
“Across all follow-up and all age groups, VE [vaccine effectiveness] was 86% ... for undervaccinated children, compared with unvaccinated children,” Dr. Zerbo and associates wrote. “VE was even higher for fully vaccinated children [93%] and for those who were fully vaccinated plus one dose [96%].”
But VE waned as time progressed farther from the last DTaP dose. The multivariate model found more than a 100% increased risk for those whose last DTaP was at least 3 years past, compared with less than 1 year past (aHR, 2.58).
The model also found time-bound risk increases among fully vaccinated children, with a more than 300% increased risk among those at least 6 years out from the last DTaP dose, compared with 3 years out (aHR, 4.66).
The results indicate that other factors besides adherence to the recommended vaccine schedule may be at work in recent pertussis outbreaks.
“Although waning immunity is clearly an important factor driving pertussis epidemics in recent years, other factors that we did not evaluate in this study might also contribute to pertussis epidemics individually or in synergy,” Dr. Zerbo and associates wrote. “Results from studies in baboons suggest that the acellular pertussis vaccines are unable to prevent colonization, carriage, and transmission. If this is also true for humans, this could contribute to pertussis epidemics. The causes of recent pertussis epidemics are complex, and we were only able to address some aspects in our study.”
The study was funded by Kaiser Permanente Northern California, the National Institutes of Health, and in part by a National Institute of Allergy and Infectious Diseases grant. One coauthor reported receiving research grant support from Sanofi Pasteur, Novartis, GlaxoSmithKline, Merck, MedImmune, Pfizer, and Dynavax for unrelated studies; the other authors reported no relevant financial disclosures.
SOURCE: Zerbo O et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3466.
FROM PEDIATRICS
United States now over 1,000 measles cases this year
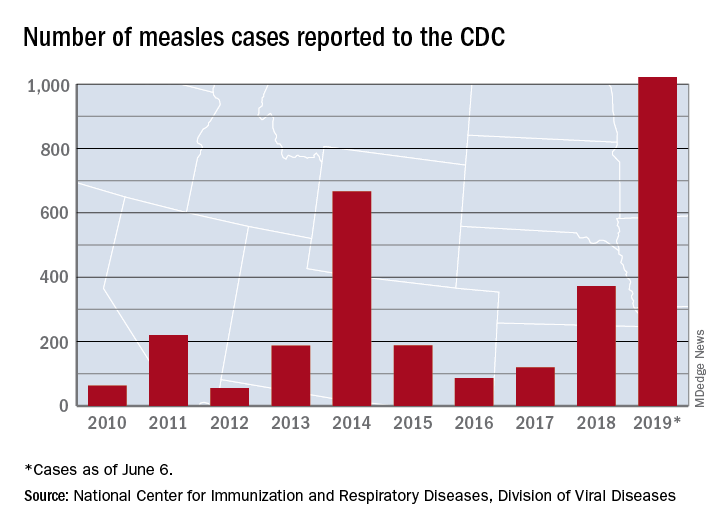
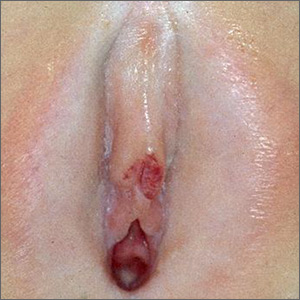
The 41 new cases reported for the week ending June 6 bring the total for the year to 1,022, the CDC reported June 10, and that is more than any year since 1992, when there were 2,237 cases.
Idaho and Virginia reported their first cases of 2019, which makes a total of 28 states with measles cases this year. The Idaho case was reported in Latah County and is the state’s first since 2001. In Virginia, health officials are investigating possible contacts with an infected individual at Dulles International Airport and two other locations on June 2 and 4.
Outbreaks in Georgia, Maryland, and Michigan have ended, while seven others continue in California (Butte, Los Angeles, and Sacramento Counties), New York (Rockland County and New York City), Pennsylvania, and Washington, the CDC said. New York City has the largest outbreak this year with 509 cases through June 3, most of them occurring in Brooklyn.

The 41 new cases reported for the week ending June 6 bring the total for the year to 1,022, the CDC reported June 10, and that is more than any year since 1992, when there were 2,237 cases.
Idaho and Virginia reported their first cases of 2019, which makes a total of 28 states with measles cases this year. The Idaho case was reported in Latah County and is the state’s first since 2001. In Virginia, health officials are investigating possible contacts with an infected individual at Dulles International Airport and two other locations on June 2 and 4.
Outbreaks in Georgia, Maryland, and Michigan have ended, while seven others continue in California (Butte, Los Angeles, and Sacramento Counties), New York (Rockland County and New York City), Pennsylvania, and Washington, the CDC said. New York City has the largest outbreak this year with 509 cases through June 3, most of them occurring in Brooklyn.

The 41 new cases reported for the week ending June 6 bring the total for the year to 1,022, the CDC reported June 10, and that is more than any year since 1992, when there were 2,237 cases.
Idaho and Virginia reported their first cases of 2019, which makes a total of 28 states with measles cases this year. The Idaho case was reported in Latah County and is the state’s first since 2001. In Virginia, health officials are investigating possible contacts with an infected individual at Dulles International Airport and two other locations on June 2 and 4.
Outbreaks in Georgia, Maryland, and Michigan have ended, while seven others continue in California (Butte, Los Angeles, and Sacramento Counties), New York (Rockland County and New York City), Pennsylvania, and Washington, the CDC said. New York City has the largest outbreak this year with 509 cases through June 3, most of them occurring in Brooklyn.
Discharge before noon: An appropriate metric for efficiency?
I first heard the term “discharge before noon” (DCBN) as a third-year medical student starting my internal medicine rotation. The basic idea made sense: Get patients out of the hospital early so rooms can be cleaned more quickly and new patients wouldn’t have to wait so long in the ED.
It quickly became apparent, however, that a lot of moving parts had to align perfectly for DCBN. Even if we prioritized rounding on dischargeable patients (starting 8-9 a.m. depending on the service/day), they still needed prescriptions filled, normal clothes to wear, and a way to get home, which wasn’t easy to coordinate while we were still trying to see all the other patients.
Fast forward through 5 years of residency/fellowship experience and DCBN seems even more unrealistic in hospitalized pediatric patients. As a simple example, discharge criteria for dehydration (one of the most common reasons for pediatric hospitalization) include demonstrating the ability to drink enough liquids to stay hydrated. Who’s going to force children to stay up all night sipping fluids (plus changing all those diapers or taking them to the bathroom)? If the child stays on intravenous fluids overnight, we have to monitor at least through breakfast, likely lunch, thus making DCBN nearly impossible.
In a January 2019 article in the Journal of Hospital Medicine, Hailey James, MHA, (@Haileyjms on Twitter) and her colleagues demonstrated an association between DCBN and decreased length of stay (LOS) for medical but not surgical pediatric discharges.1 This made them question if DCBN is an appropriate metric for discharge efficiency, as well as workflow differences between services. Many hospitals, however, still try to push DCBN as a goal (see Destino et al in the same January 2019 issue of JHM2), which could potentially lead to people trying to game the system.
How does your institution try to make discharge processes more efficient? Is it actually possible to do everything more quickly without sacrificing quality or trainee education? Whether your patients are kids, adults, or both, there are likely many issues in common where we could all learn from each other.
We discussed this topic in #JHMChat on April 15 on Twitter. New to Twitter or not familiar with #JHMChat? Since October 2015, JHM has reviewed and discussed dozens of articles spanning a wide variety of topics related to caring for hospitalized patients. All are welcome to join, including medical students, residents, nurses, practicing hospitalists, and more. It’s a great opportunity to virtually meet and learn from others while earning free CME.
To participate in future chats, type #JHMChat in the search box on the top right corner of your Twitter homepage, click on the “Latest” tab at the top left to see the most recent tweets, and join the conversation (don’t forget the hashtag)!
Dr. Chen is a pediatric hospital medicine fellow at Rady Children’s Hospital, University of California, San Diego. She is one of the cofounders/moderators of #PHMFellowJC, serves as a fellow district representative for the American Academy of Pediatrics, and is an active #tweetiatrician at @DrJenChen4kids. This article appeared originally in SHM's official blog The Hospital Leader. Read more recent posts here.
References
1. James HJ et al. The Association of Discharge Before Noon and Length of Stay in Hospitalized Pediatric Patients. J Hosp Med. 2019;14(1):28-32. doi: 10.12788/jhm.3111.
2. Destino L et al. Improving Patient Flow: Analysis of an Initiative to Improve Early Discharge. J Hosp Med. 2019;14(1):22-7. doi: 10.12788/jhm.3133.
I first heard the term “discharge before noon” (DCBN) as a third-year medical student starting my internal medicine rotation. The basic idea made sense: Get patients out of the hospital early so rooms can be cleaned more quickly and new patients wouldn’t have to wait so long in the ED.
It quickly became apparent, however, that a lot of moving parts had to align perfectly for DCBN. Even if we prioritized rounding on dischargeable patients (starting 8-9 a.m. depending on the service/day), they still needed prescriptions filled, normal clothes to wear, and a way to get home, which wasn’t easy to coordinate while we were still trying to see all the other patients.
Fast forward through 5 years of residency/fellowship experience and DCBN seems even more unrealistic in hospitalized pediatric patients. As a simple example, discharge criteria for dehydration (one of the most common reasons for pediatric hospitalization) include demonstrating the ability to drink enough liquids to stay hydrated. Who’s going to force children to stay up all night sipping fluids (plus changing all those diapers or taking them to the bathroom)? If the child stays on intravenous fluids overnight, we have to monitor at least through breakfast, likely lunch, thus making DCBN nearly impossible.
In a January 2019 article in the Journal of Hospital Medicine, Hailey James, MHA, (@Haileyjms on Twitter) and her colleagues demonstrated an association between DCBN and decreased length of stay (LOS) for medical but not surgical pediatric discharges.1 This made them question if DCBN is an appropriate metric for discharge efficiency, as well as workflow differences between services. Many hospitals, however, still try to push DCBN as a goal (see Destino et al in the same January 2019 issue of JHM2), which could potentially lead to people trying to game the system.
How does your institution try to make discharge processes more efficient? Is it actually possible to do everything more quickly without sacrificing quality or trainee education? Whether your patients are kids, adults, or both, there are likely many issues in common where we could all learn from each other.
We discussed this topic in #JHMChat on April 15 on Twitter. New to Twitter or not familiar with #JHMChat? Since October 2015, JHM has reviewed and discussed dozens of articles spanning a wide variety of topics related to caring for hospitalized patients. All are welcome to join, including medical students, residents, nurses, practicing hospitalists, and more. It’s a great opportunity to virtually meet and learn from others while earning free CME.
To participate in future chats, type #JHMChat in the search box on the top right corner of your Twitter homepage, click on the “Latest” tab at the top left to see the most recent tweets, and join the conversation (don’t forget the hashtag)!
Dr. Chen is a pediatric hospital medicine fellow at Rady Children’s Hospital, University of California, San Diego. She is one of the cofounders/moderators of #PHMFellowJC, serves as a fellow district representative for the American Academy of Pediatrics, and is an active #tweetiatrician at @DrJenChen4kids. This article appeared originally in SHM's official blog The Hospital Leader. Read more recent posts here.
References
1. James HJ et al. The Association of Discharge Before Noon and Length of Stay in Hospitalized Pediatric Patients. J Hosp Med. 2019;14(1):28-32. doi: 10.12788/jhm.3111.
2. Destino L et al. Improving Patient Flow: Analysis of an Initiative to Improve Early Discharge. J Hosp Med. 2019;14(1):22-7. doi: 10.12788/jhm.3133.
I first heard the term “discharge before noon” (DCBN) as a third-year medical student starting my internal medicine rotation. The basic idea made sense: Get patients out of the hospital early so rooms can be cleaned more quickly and new patients wouldn’t have to wait so long in the ED.
It quickly became apparent, however, that a lot of moving parts had to align perfectly for DCBN. Even if we prioritized rounding on dischargeable patients (starting 8-9 a.m. depending on the service/day), they still needed prescriptions filled, normal clothes to wear, and a way to get home, which wasn’t easy to coordinate while we were still trying to see all the other patients.
Fast forward through 5 years of residency/fellowship experience and DCBN seems even more unrealistic in hospitalized pediatric patients. As a simple example, discharge criteria for dehydration (one of the most common reasons for pediatric hospitalization) include demonstrating the ability to drink enough liquids to stay hydrated. Who’s going to force children to stay up all night sipping fluids (plus changing all those diapers or taking them to the bathroom)? If the child stays on intravenous fluids overnight, we have to monitor at least through breakfast, likely lunch, thus making DCBN nearly impossible.
In a January 2019 article in the Journal of Hospital Medicine, Hailey James, MHA, (@Haileyjms on Twitter) and her colleagues demonstrated an association between DCBN and decreased length of stay (LOS) for medical but not surgical pediatric discharges.1 This made them question if DCBN is an appropriate metric for discharge efficiency, as well as workflow differences between services. Many hospitals, however, still try to push DCBN as a goal (see Destino et al in the same January 2019 issue of JHM2), which could potentially lead to people trying to game the system.
How does your institution try to make discharge processes more efficient? Is it actually possible to do everything more quickly without sacrificing quality or trainee education? Whether your patients are kids, adults, or both, there are likely many issues in common where we could all learn from each other.
We discussed this topic in #JHMChat on April 15 on Twitter. New to Twitter or not familiar with #JHMChat? Since October 2015, JHM has reviewed and discussed dozens of articles spanning a wide variety of topics related to caring for hospitalized patients. All are welcome to join, including medical students, residents, nurses, practicing hospitalists, and more. It’s a great opportunity to virtually meet and learn from others while earning free CME.
To participate in future chats, type #JHMChat in the search box on the top right corner of your Twitter homepage, click on the “Latest” tab at the top left to see the most recent tweets, and join the conversation (don’t forget the hashtag)!
Dr. Chen is a pediatric hospital medicine fellow at Rady Children’s Hospital, University of California, San Diego. She is one of the cofounders/moderators of #PHMFellowJC, serves as a fellow district representative for the American Academy of Pediatrics, and is an active #tweetiatrician at @DrJenChen4kids. This article appeared originally in SHM's official blog The Hospital Leader. Read more recent posts here.
References
1. James HJ et al. The Association of Discharge Before Noon and Length of Stay in Hospitalized Pediatric Patients. J Hosp Med. 2019;14(1):28-32. doi: 10.12788/jhm.3111.
2. Destino L et al. Improving Patient Flow: Analysis of an Initiative to Improve Early Discharge. J Hosp Med. 2019;14(1):22-7. doi: 10.12788/jhm.3133.
4-year-old girl • genital discomfort and dysuria • clitoral hood swelling • Blood blister on the labia minora • Dx?
THE CASE
A 4-year-old girl presented to her pediatrician with genital discomfort and dysuria of 6 months’ duration. The patient’s mother said that 3 days earlier, she noticed a tear near the child’s clitoris and a scab on the labia minora that the mother attributed to minor trauma from scratching. The pediatrician was concerned about genital trauma from sexual abuse and referred the patient to the emergency department, where a report with child protective services (CPS) was filed. The mother reported that the patient and her 8-year-old sibling spent 3 to 4 hours a day with a babysitter, who was always supervised, and the parents had no concerns about possible sexual abuse.
Physical examination by our institution’s Child Protection Team revealed clitoral hood swelling with subepithelial hemorrhages, a blood blister on the right labia minora, a fissure and subepithelial hemorrhages on the posterior fourchette, and a thin depigmented figure-of-eight lesion around the vulva and anus.
THE DIAGNOSIS
Since the clinical findings were consistent with prepubertal lichen sclerosus (LS), the CPS case was closed and the patient was referred to Pediatric Gynecology. Treatment with high-potency topical steroids was initiated with clobetasol ointment 0.05% twice daily for 2 weeks, then once daily for 2 weeks. She was then switched to triamcinolone ointment 0.01% twice daily for 2 weeks, then once daily for 2 weeks. These treatments were enough to stop the LS flare and decrease the anogenital itching.
DISCUSSION
Lichen sclerosus is a chronic inflammatory skin disease that primarily presents in the anogenital region; however, extragenital lesions on the upper extremities, thighs, and breasts have been reported in 15% to 20% of patients.1 Lichen sclerosus most commonly affects females as a result of low estrogen and may occur during puberty or following menopause, but it also is seen in males.1,2 The estimated prevalence of LS in prepubertal girls is 1 in 900.3 The effects of increased estrogen exposure on LS during puberty are not entirely clear. Lichen sclerosus previously was thought to improve with puberty, since it is not as common in women of reproductive age; however, studies have shown persistent symptoms after menarche in some patients.4-6
The pathogenesis of LS is multifactorial, likely with an autoimmune component, as it often is associated with other autoimmune findings such as thyroiditis, alopecia, pernicious anemia, and vitiligo.2 Diagnosis of prepubertal LS usually is made based on a review of the patient’s history and clinical examination. Presenting symptoms may include pruritus, skin irritation, vulvar pain, dysuria, bleeding from excoriations, fissures, and constipation.1,3,7
On physical examination, LS can present on the anogenital skin as smooth white spots or wrinkled, blotchy, atrophic patches. The skin around the vaginal opening and anus is thin and often is described as resembling parchment or cigarette paper in a figure-of-eight pattern (FIGURE 1A). Vulvar and anal fissures and subepithelial hemorrhages with the appearance of blood blisters also can be found (FIGURE 1B).8 Affected areas are fragile and susceptible to minor trauma, which may result in bruising or bleeding (FIGURE 1C).
Over time, scarring can occur and may result in disruption of the anogenital architecture—specifically loss of the labia minora, narrowing of the introitus, and burying of the clitoris.1,2 These changes can be similar to the scarring seen in postmenopausal women with LS.
Continue to: The differential diagnosis...
The differential diagnosis for prepubertal LS includes vitiligo, lichen planus, lichen simplex chronicus, psoriasis, eczema, vulvovaginitis, contact dermatitis, and trauma.2,7 On average, it takes 1 to 2 years after onset of symptoms before a correct diagnosis of prepubertal LS is made, and trauma and/or sexual abuse often are first suspected.7,9 For clinicians who are unfamiliar with prepubertal LS, the clinical findings of anogenital bruising and bleeding understandably may be suggestive of abuse. It is important to note that diagnosis of LS does not preclude the possibility of sexual abuse; in some cases, LS can be triggered or exacerbated by anogenital trauma, known as the Koebner phenomenon.2
Treatment. After the diagnosis of prepubertal LS is established, the goals of treatment are to provide symptom relief and prevent scarring of the external genitalia. To our knowledge, there have been no randomized controlled trials for treatment of LS in prepubertal girls. In general, acute symptoms are treated with high-potency topical steroids, such as clobetasol propionate or betamethasone valerate, and treatment regimens are variable.7
LS has an unpredictable clinical course and there often are recurrences that require repeat courses of topical steroids.9 Since concurrent bacterial infection is common,10 genital cultures should be obtained prior to initiation of topical steroids if an infection is suspected.
Topical calcineurin inhibitors have been used successfully, but proof of their effectiveness is limited to case reports in the literature.7 Surgical treatment of LS typically is reserved for complications associated with symptomatic adhesions that are refractory to medical management.7,11 Vulvar hygiene is paramount to symptom control, and topical emollients can be used to manage minor irritation.7,8 In our patient, clobetasol and triamcinolone ointments were enough to stop the LS flare and decrease the anogenital itching.
THE TAKEAWAY
Although LS has very characteristic skin findings, the diagnosis continues to be challenging for physicians who are unfamiliar with this condition. Failure to recognize prepubertal LS not only delays diagnosis and treatment but also may lead to repeated genital examinations and investigation by CPS for suspected sexual abuse. As with any genital complaint in a prepubertal girl, diagnosis of LS should not preclude appropriate screening for sexual abuse. Although providers should be vigilant about potential sexual abuse, familiarity with skin conditions that mimic genital trauma is essential.
CORRESPONDENCE
Monica Rosen, MD, L4000 Women’s Hospital, 1500 E Medical Center Drive, SPC 5276 Ann Arbor, MI 48109; [email protected]
1. Powell JJ, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777-1783.
2. Murphy R. Lichen sclerosus. Dermatol Clin. 2010;28:707-715.
3. Powell J, Wojnarowska F. Childhood vulvar lichen sclerosus: an increasingly common problem. J Am Acad Dermatol. 2001;44:803-806.
4. Powell J, Wojnarowska F. Childhood vulvar lichen sclerosus. The course after puberty. J Reprod Med. 2002;47:706-709.
5. Smith SD, Fischer G. Childhood onset vulvar lichen sclerosus does not resolve at puberty: a prospective case series. Pediatr Dermatol. 2009;26:725-729.
6. Focseneanu MA, Gupta M, Squires KC, et al. The course of lichen sclerosus diagnosed prior to puberty. J Pediatr Adolesc Gynecol. 2013;26:153-155.
7. Bercaw-Pratt JL, Boardman LA, Simms-Cendan JS. Clinical recommendation: pediatric lichen sclerosus. J Pediatr Adolesc Gynecol. 2014;27:111-116.
8. Jenny C, Kirby P, Fuquay D. Genital lichen sclerosus mistaken for child sexual abuse. Pediatrics. 1989;83:597-599.
9. Dendrinos ML, Quint EH. Lichen sclerosus in children and adolescents. Curr Opin Obstet Gynecol. 2013;25:370-374.
10. Lagerstedt M, Karvinen K, Joki-Erkkila M, et al. Childhood lichen sclerosus—a challenge for clinicians. Pediatr Dermatol. 2013;30:444-450.
11. Gurumurthy M, Morah N, Gioffre G, et al. The surgical management of complications of vulval lichen sclerosus. Eur J Obstet Gynecol Reprod Biol. 2012;162:79-82.
THE CASE
A 4-year-old girl presented to her pediatrician with genital discomfort and dysuria of 6 months’ duration. The patient’s mother said that 3 days earlier, she noticed a tear near the child’s clitoris and a scab on the labia minora that the mother attributed to minor trauma from scratching. The pediatrician was concerned about genital trauma from sexual abuse and referred the patient to the emergency department, where a report with child protective services (CPS) was filed. The mother reported that the patient and her 8-year-old sibling spent 3 to 4 hours a day with a babysitter, who was always supervised, and the parents had no concerns about possible sexual abuse.
Physical examination by our institution’s Child Protection Team revealed clitoral hood swelling with subepithelial hemorrhages, a blood blister on the right labia minora, a fissure and subepithelial hemorrhages on the posterior fourchette, and a thin depigmented figure-of-eight lesion around the vulva and anus.
THE DIAGNOSIS
Since the clinical findings were consistent with prepubertal lichen sclerosus (LS), the CPS case was closed and the patient was referred to Pediatric Gynecology. Treatment with high-potency topical steroids was initiated with clobetasol ointment 0.05% twice daily for 2 weeks, then once daily for 2 weeks. She was then switched to triamcinolone ointment 0.01% twice daily for 2 weeks, then once daily for 2 weeks. These treatments were enough to stop the LS flare and decrease the anogenital itching.
DISCUSSION
Lichen sclerosus is a chronic inflammatory skin disease that primarily presents in the anogenital region; however, extragenital lesions on the upper extremities, thighs, and breasts have been reported in 15% to 20% of patients.1 Lichen sclerosus most commonly affects females as a result of low estrogen and may occur during puberty or following menopause, but it also is seen in males.1,2 The estimated prevalence of LS in prepubertal girls is 1 in 900.3 The effects of increased estrogen exposure on LS during puberty are not entirely clear. Lichen sclerosus previously was thought to improve with puberty, since it is not as common in women of reproductive age; however, studies have shown persistent symptoms after menarche in some patients.4-6
The pathogenesis of LS is multifactorial, likely with an autoimmune component, as it often is associated with other autoimmune findings such as thyroiditis, alopecia, pernicious anemia, and vitiligo.2 Diagnosis of prepubertal LS usually is made based on a review of the patient’s history and clinical examination. Presenting symptoms may include pruritus, skin irritation, vulvar pain, dysuria, bleeding from excoriations, fissures, and constipation.1,3,7
On physical examination, LS can present on the anogenital skin as smooth white spots or wrinkled, blotchy, atrophic patches. The skin around the vaginal opening and anus is thin and often is described as resembling parchment or cigarette paper in a figure-of-eight pattern (FIGURE 1A). Vulvar and anal fissures and subepithelial hemorrhages with the appearance of blood blisters also can be found (FIGURE 1B).8 Affected areas are fragile and susceptible to minor trauma, which may result in bruising or bleeding (FIGURE 1C).

Over time, scarring can occur and may result in disruption of the anogenital architecture—specifically loss of the labia minora, narrowing of the introitus, and burying of the clitoris.1,2 These changes can be similar to the scarring seen in postmenopausal women with LS.
Continue to: The differential diagnosis...
The differential diagnosis for prepubertal LS includes vitiligo, lichen planus, lichen simplex chronicus, psoriasis, eczema, vulvovaginitis, contact dermatitis, and trauma.2,7 On average, it takes 1 to 2 years after onset of symptoms before a correct diagnosis of prepubertal LS is made, and trauma and/or sexual abuse often are first suspected.7,9 For clinicians who are unfamiliar with prepubertal LS, the clinical findings of anogenital bruising and bleeding understandably may be suggestive of abuse. It is important to note that diagnosis of LS does not preclude the possibility of sexual abuse; in some cases, LS can be triggered or exacerbated by anogenital trauma, known as the Koebner phenomenon.2
Treatment. After the diagnosis of prepubertal LS is established, the goals of treatment are to provide symptom relief and prevent scarring of the external genitalia. To our knowledge, there have been no randomized controlled trials for treatment of LS in prepubertal girls. In general, acute symptoms are treated with high-potency topical steroids, such as clobetasol propionate or betamethasone valerate, and treatment regimens are variable.7
LS has an unpredictable clinical course and there often are recurrences that require repeat courses of topical steroids.9 Since concurrent bacterial infection is common,10 genital cultures should be obtained prior to initiation of topical steroids if an infection is suspected.
Topical calcineurin inhibitors have been used successfully, but proof of their effectiveness is limited to case reports in the literature.7 Surgical treatment of LS typically is reserved for complications associated with symptomatic adhesions that are refractory to medical management.7,11 Vulvar hygiene is paramount to symptom control, and topical emollients can be used to manage minor irritation.7,8 In our patient, clobetasol and triamcinolone ointments were enough to stop the LS flare and decrease the anogenital itching.
THE TAKEAWAY
Although LS has very characteristic skin findings, the diagnosis continues to be challenging for physicians who are unfamiliar with this condition. Failure to recognize prepubertal LS not only delays diagnosis and treatment but also may lead to repeated genital examinations and investigation by CPS for suspected sexual abuse. As with any genital complaint in a prepubertal girl, diagnosis of LS should not preclude appropriate screening for sexual abuse. Although providers should be vigilant about potential sexual abuse, familiarity with skin conditions that mimic genital trauma is essential.
CORRESPONDENCE
Monica Rosen, MD, L4000 Women’s Hospital, 1500 E Medical Center Drive, SPC 5276 Ann Arbor, MI 48109; [email protected]
THE CASE
A 4-year-old girl presented to her pediatrician with genital discomfort and dysuria of 6 months’ duration. The patient’s mother said that 3 days earlier, she noticed a tear near the child’s clitoris and a scab on the labia minora that the mother attributed to minor trauma from scratching. The pediatrician was concerned about genital trauma from sexual abuse and referred the patient to the emergency department, where a report with child protective services (CPS) was filed. The mother reported that the patient and her 8-year-old sibling spent 3 to 4 hours a day with a babysitter, who was always supervised, and the parents had no concerns about possible sexual abuse.
Physical examination by our institution’s Child Protection Team revealed clitoral hood swelling with subepithelial hemorrhages, a blood blister on the right labia minora, a fissure and subepithelial hemorrhages on the posterior fourchette, and a thin depigmented figure-of-eight lesion around the vulva and anus.
THE DIAGNOSIS
Since the clinical findings were consistent with prepubertal lichen sclerosus (LS), the CPS case was closed and the patient was referred to Pediatric Gynecology. Treatment with high-potency topical steroids was initiated with clobetasol ointment 0.05% twice daily for 2 weeks, then once daily for 2 weeks. She was then switched to triamcinolone ointment 0.01% twice daily for 2 weeks, then once daily for 2 weeks. These treatments were enough to stop the LS flare and decrease the anogenital itching.
DISCUSSION
Lichen sclerosus is a chronic inflammatory skin disease that primarily presents in the anogenital region; however, extragenital lesions on the upper extremities, thighs, and breasts have been reported in 15% to 20% of patients.1 Lichen sclerosus most commonly affects females as a result of low estrogen and may occur during puberty or following menopause, but it also is seen in males.1,2 The estimated prevalence of LS in prepubertal girls is 1 in 900.3 The effects of increased estrogen exposure on LS during puberty are not entirely clear. Lichen sclerosus previously was thought to improve with puberty, since it is not as common in women of reproductive age; however, studies have shown persistent symptoms after menarche in some patients.4-6
The pathogenesis of LS is multifactorial, likely with an autoimmune component, as it often is associated with other autoimmune findings such as thyroiditis, alopecia, pernicious anemia, and vitiligo.2 Diagnosis of prepubertal LS usually is made based on a review of the patient’s history and clinical examination. Presenting symptoms may include pruritus, skin irritation, vulvar pain, dysuria, bleeding from excoriations, fissures, and constipation.1,3,7
On physical examination, LS can present on the anogenital skin as smooth white spots or wrinkled, blotchy, atrophic patches. The skin around the vaginal opening and anus is thin and often is described as resembling parchment or cigarette paper in a figure-of-eight pattern (FIGURE 1A). Vulvar and anal fissures and subepithelial hemorrhages with the appearance of blood blisters also can be found (FIGURE 1B).8 Affected areas are fragile and susceptible to minor trauma, which may result in bruising or bleeding (FIGURE 1C).

Over time, scarring can occur and may result in disruption of the anogenital architecture—specifically loss of the labia minora, narrowing of the introitus, and burying of the clitoris.1,2 These changes can be similar to the scarring seen in postmenopausal women with LS.
Continue to: The differential diagnosis...
The differential diagnosis for prepubertal LS includes vitiligo, lichen planus, lichen simplex chronicus, psoriasis, eczema, vulvovaginitis, contact dermatitis, and trauma.2,7 On average, it takes 1 to 2 years after onset of symptoms before a correct diagnosis of prepubertal LS is made, and trauma and/or sexual abuse often are first suspected.7,9 For clinicians who are unfamiliar with prepubertal LS, the clinical findings of anogenital bruising and bleeding understandably may be suggestive of abuse. It is important to note that diagnosis of LS does not preclude the possibility of sexual abuse; in some cases, LS can be triggered or exacerbated by anogenital trauma, known as the Koebner phenomenon.2
Treatment. After the diagnosis of prepubertal LS is established, the goals of treatment are to provide symptom relief and prevent scarring of the external genitalia. To our knowledge, there have been no randomized controlled trials for treatment of LS in prepubertal girls. In general, acute symptoms are treated with high-potency topical steroids, such as clobetasol propionate or betamethasone valerate, and treatment regimens are variable.7
LS has an unpredictable clinical course and there often are recurrences that require repeat courses of topical steroids.9 Since concurrent bacterial infection is common,10 genital cultures should be obtained prior to initiation of topical steroids if an infection is suspected.
Topical calcineurin inhibitors have been used successfully, but proof of their effectiveness is limited to case reports in the literature.7 Surgical treatment of LS typically is reserved for complications associated with symptomatic adhesions that are refractory to medical management.7,11 Vulvar hygiene is paramount to symptom control, and topical emollients can be used to manage minor irritation.7,8 In our patient, clobetasol and triamcinolone ointments were enough to stop the LS flare and decrease the anogenital itching.
THE TAKEAWAY
Although LS has very characteristic skin findings, the diagnosis continues to be challenging for physicians who are unfamiliar with this condition. Failure to recognize prepubertal LS not only delays diagnosis and treatment but also may lead to repeated genital examinations and investigation by CPS for suspected sexual abuse. As with any genital complaint in a prepubertal girl, diagnosis of LS should not preclude appropriate screening for sexual abuse. Although providers should be vigilant about potential sexual abuse, familiarity with skin conditions that mimic genital trauma is essential.
CORRESPONDENCE
Monica Rosen, MD, L4000 Women’s Hospital, 1500 E Medical Center Drive, SPC 5276 Ann Arbor, MI 48109; [email protected]
1. Powell JJ, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777-1783.
2. Murphy R. Lichen sclerosus. Dermatol Clin. 2010;28:707-715.
3. Powell J, Wojnarowska F. Childhood vulvar lichen sclerosus: an increasingly common problem. J Am Acad Dermatol. 2001;44:803-806.
4. Powell J, Wojnarowska F. Childhood vulvar lichen sclerosus. The course after puberty. J Reprod Med. 2002;47:706-709.
5. Smith SD, Fischer G. Childhood onset vulvar lichen sclerosus does not resolve at puberty: a prospective case series. Pediatr Dermatol. 2009;26:725-729.
6. Focseneanu MA, Gupta M, Squires KC, et al. The course of lichen sclerosus diagnosed prior to puberty. J Pediatr Adolesc Gynecol. 2013;26:153-155.
7. Bercaw-Pratt JL, Boardman LA, Simms-Cendan JS. Clinical recommendation: pediatric lichen sclerosus. J Pediatr Adolesc Gynecol. 2014;27:111-116.
8. Jenny C, Kirby P, Fuquay D. Genital lichen sclerosus mistaken for child sexual abuse. Pediatrics. 1989;83:597-599.
9. Dendrinos ML, Quint EH. Lichen sclerosus in children and adolescents. Curr Opin Obstet Gynecol. 2013;25:370-374.
10. Lagerstedt M, Karvinen K, Joki-Erkkila M, et al. Childhood lichen sclerosus—a challenge for clinicians. Pediatr Dermatol. 2013;30:444-450.
11. Gurumurthy M, Morah N, Gioffre G, et al. The surgical management of complications of vulval lichen sclerosus. Eur J Obstet Gynecol Reprod Biol. 2012;162:79-82.
1. Powell JJ, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777-1783.
2. Murphy R. Lichen sclerosus. Dermatol Clin. 2010;28:707-715.
3. Powell J, Wojnarowska F. Childhood vulvar lichen sclerosus: an increasingly common problem. J Am Acad Dermatol. 2001;44:803-806.
4. Powell J, Wojnarowska F. Childhood vulvar lichen sclerosus. The course after puberty. J Reprod Med. 2002;47:706-709.
5. Smith SD, Fischer G. Childhood onset vulvar lichen sclerosus does not resolve at puberty: a prospective case series. Pediatr Dermatol. 2009;26:725-729.
6. Focseneanu MA, Gupta M, Squires KC, et al. The course of lichen sclerosus diagnosed prior to puberty. J Pediatr Adolesc Gynecol. 2013;26:153-155.
7. Bercaw-Pratt JL, Boardman LA, Simms-Cendan JS. Clinical recommendation: pediatric lichen sclerosus. J Pediatr Adolesc Gynecol. 2014;27:111-116.
8. Jenny C, Kirby P, Fuquay D. Genital lichen sclerosus mistaken for child sexual abuse. Pediatrics. 1989;83:597-599.
9. Dendrinos ML, Quint EH. Lichen sclerosus in children and adolescents. Curr Opin Obstet Gynecol. 2013;25:370-374.
10. Lagerstedt M, Karvinen K, Joki-Erkkila M, et al. Childhood lichen sclerosus—a challenge for clinicians. Pediatr Dermatol. 2013;30:444-450.
11. Gurumurthy M, Morah N, Gioffre G, et al. The surgical management of complications of vulval lichen sclerosus. Eur J Obstet Gynecol Reprod Biol. 2012;162:79-82.
Varicella vaccine delivers doubled benefit to children
than those in unvaccinated children.
The benefit became largely apparent after children received the second vaccination in the recommended series, and persisted throughout childhood, Sheila Weinmann, PhD, of Kaiser Permanente Northern California, Oakland, and colleagues said.*
The analysis included 6.37 million children in the Kaiser Permanente database, 50% of whom were vaccinated for all or some of the study period stretching from 2003 to 2014. Overall, the crude lab-confirmed herpes zoster (HZ) incidence rate was 74/100,000 person-years. When stratified by vaccine status, the crude rate of HZ among vaccinated children was 78% lower than among unvaccinated children (38 vs. 170 cases per 100,000 person years).
Herpes zoster was more common among girls than boys and up to six times more common in immunosuppressed children than in nonimmunosuppressed children.
The authors also found that unvaccinated children benefited from the high rate of vaccination around them. Although the HZ rate was always lower among vaccinated children, the rate among unvaccinated children fell sharply after 2007.
“The trend of decreasing HZ incidence among children who were unvaccinated is likely due to a lack of primary VZV [varicella-zoster virus] infection resulting from herd immunity in a highly vaccinated population,” Dr. Weinmann and her associates said.
There was some variability among age groups, especially among the youngest who were not fully vaccinated.
“In the group aged 1-2 years, the confirmation-adjusted HZ rate among children who were vaccinated was 70% higher than among those who were unvaccinated,” the authors said. In the “older groups, HZ rates were significantly higher in children who were unvaccinated than in those who were vaccinated,” the researchers noted.
The highest incidence was among vaccinated 1-year-olds, who had a 140% higher risk of HZ than did unvaccinated 1-year-olds. But this risk elevation disappeared by age 2 years. For everyone else, aged 2-17 years, the rate of HZ remained significantly lower in vaccinated children.
“Among the small number of children vaccinated at 11 months of age (for whom the vaccine is not recommended), the HZ incidence rate was significantly higher than in children vaccinated at 1 year of age and older. Similarly, children who contract wild-type varicella infection at younger than 1 year of age also have a higher risk of HZ (relative risk, 13.5). The immature adaptive T-cell response in children less than 1 year of age appears less able to contain VZV as a latent infection, compared with older children.
“Our findings for 11-month-olds who were vaccinated should be interpreted with caution because this population included only three cases of HZ and could have included children participating in a prelicensure study with a vaccine formulation different from Varivax,” Dr. Weinmann and her associates said.
Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
SOURCE: Weinmann S et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-2917.
* This article was updated 6/14/2019
The finding of a 78% lower incidence of zoster in varicella-vaccinated children is nothing short of “remarkable,” Anne A Gershon, MD, wrote in an accompanying editorial.
But the benefit could be in jeopardy, as parents question the safety and effectiveness of all vaccines, she wrote.
“That the varicella vaccine prevents not only varicella but zoster as well is an exciting dual benefit from the varicella vaccine, further improving the health of children by immunization,” Dr. Gershon said. “Additional studies will be necessary to show the mechanism for the protection against zoster (viral, immunologic, or both), how long this benefit lasts, and whether additional doses of some form of VZV [varicella-zoster virus] vaccine will be more useful.”
But, she suggested, in a time when cases of clinical varicella are dwindling, so is public awareness of the vaccine’s benefit. Clinical varicella is worse for adults than it is for children.
“Efforts to immunize all children against chickenpox must continue to be made to protect our population from wild-type VZV. Fortunately, antiviral therapy is also available for individuals who are unvaccinated and develop varicella or zoster, but immunization is, as usual, preferable,” Dr. Gershon concluded.
Dr. Gershon, a pediatric infectious disease specialist, is a professor of pediatrics at Columbia University, New York. She wrote a commentary to accompany the article by Weinmann et al. (Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3561). Dr. Gershon had no relevant financial disclosures. The commentary was funded by the National Institutes of Health.
The finding of a 78% lower incidence of zoster in varicella-vaccinated children is nothing short of “remarkable,” Anne A Gershon, MD, wrote in an accompanying editorial.
But the benefit could be in jeopardy, as parents question the safety and effectiveness of all vaccines, she wrote.
“That the varicella vaccine prevents not only varicella but zoster as well is an exciting dual benefit from the varicella vaccine, further improving the health of children by immunization,” Dr. Gershon said. “Additional studies will be necessary to show the mechanism for the protection against zoster (viral, immunologic, or both), how long this benefit lasts, and whether additional doses of some form of VZV [varicella-zoster virus] vaccine will be more useful.”
But, she suggested, in a time when cases of clinical varicella are dwindling, so is public awareness of the vaccine’s benefit. Clinical varicella is worse for adults than it is for children.
“Efforts to immunize all children against chickenpox must continue to be made to protect our population from wild-type VZV. Fortunately, antiviral therapy is also available for individuals who are unvaccinated and develop varicella or zoster, but immunization is, as usual, preferable,” Dr. Gershon concluded.
Dr. Gershon, a pediatric infectious disease specialist, is a professor of pediatrics at Columbia University, New York. She wrote a commentary to accompany the article by Weinmann et al. (Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3561). Dr. Gershon had no relevant financial disclosures. The commentary was funded by the National Institutes of Health.
The finding of a 78% lower incidence of zoster in varicella-vaccinated children is nothing short of “remarkable,” Anne A Gershon, MD, wrote in an accompanying editorial.
But the benefit could be in jeopardy, as parents question the safety and effectiveness of all vaccines, she wrote.
“That the varicella vaccine prevents not only varicella but zoster as well is an exciting dual benefit from the varicella vaccine, further improving the health of children by immunization,” Dr. Gershon said. “Additional studies will be necessary to show the mechanism for the protection against zoster (viral, immunologic, or both), how long this benefit lasts, and whether additional doses of some form of VZV [varicella-zoster virus] vaccine will be more useful.”
But, she suggested, in a time when cases of clinical varicella are dwindling, so is public awareness of the vaccine’s benefit. Clinical varicella is worse for adults than it is for children.
“Efforts to immunize all children against chickenpox must continue to be made to protect our population from wild-type VZV. Fortunately, antiviral therapy is also available for individuals who are unvaccinated and develop varicella or zoster, but immunization is, as usual, preferable,” Dr. Gershon concluded.
Dr. Gershon, a pediatric infectious disease specialist, is a professor of pediatrics at Columbia University, New York. She wrote a commentary to accompany the article by Weinmann et al. (Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3561). Dr. Gershon had no relevant financial disclosures. The commentary was funded by the National Institutes of Health.
than those in unvaccinated children.
The benefit became largely apparent after children received the second vaccination in the recommended series, and persisted throughout childhood, Sheila Weinmann, PhD, of Kaiser Permanente Northern California, Oakland, and colleagues said.*
The analysis included 6.37 million children in the Kaiser Permanente database, 50% of whom were vaccinated for all or some of the study period stretching from 2003 to 2014. Overall, the crude lab-confirmed herpes zoster (HZ) incidence rate was 74/100,000 person-years. When stratified by vaccine status, the crude rate of HZ among vaccinated children was 78% lower than among unvaccinated children (38 vs. 170 cases per 100,000 person years).
Herpes zoster was more common among girls than boys and up to six times more common in immunosuppressed children than in nonimmunosuppressed children.
The authors also found that unvaccinated children benefited from the high rate of vaccination around them. Although the HZ rate was always lower among vaccinated children, the rate among unvaccinated children fell sharply after 2007.
“The trend of decreasing HZ incidence among children who were unvaccinated is likely due to a lack of primary VZV [varicella-zoster virus] infection resulting from herd immunity in a highly vaccinated population,” Dr. Weinmann and her associates said.
There was some variability among age groups, especially among the youngest who were not fully vaccinated.
“In the group aged 1-2 years, the confirmation-adjusted HZ rate among children who were vaccinated was 70% higher than among those who were unvaccinated,” the authors said. In the “older groups, HZ rates were significantly higher in children who were unvaccinated than in those who were vaccinated,” the researchers noted.
The highest incidence was among vaccinated 1-year-olds, who had a 140% higher risk of HZ than did unvaccinated 1-year-olds. But this risk elevation disappeared by age 2 years. For everyone else, aged 2-17 years, the rate of HZ remained significantly lower in vaccinated children.
“Among the small number of children vaccinated at 11 months of age (for whom the vaccine is not recommended), the HZ incidence rate was significantly higher than in children vaccinated at 1 year of age and older. Similarly, children who contract wild-type varicella infection at younger than 1 year of age also have a higher risk of HZ (relative risk, 13.5). The immature adaptive T-cell response in children less than 1 year of age appears less able to contain VZV as a latent infection, compared with older children.
“Our findings for 11-month-olds who were vaccinated should be interpreted with caution because this population included only three cases of HZ and could have included children participating in a prelicensure study with a vaccine formulation different from Varivax,” Dr. Weinmann and her associates said.
Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
SOURCE: Weinmann S et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-2917.
* This article was updated 6/14/2019
than those in unvaccinated children.
The benefit became largely apparent after children received the second vaccination in the recommended series, and persisted throughout childhood, Sheila Weinmann, PhD, of Kaiser Permanente Northern California, Oakland, and colleagues said.*
The analysis included 6.37 million children in the Kaiser Permanente database, 50% of whom were vaccinated for all or some of the study period stretching from 2003 to 2014. Overall, the crude lab-confirmed herpes zoster (HZ) incidence rate was 74/100,000 person-years. When stratified by vaccine status, the crude rate of HZ among vaccinated children was 78% lower than among unvaccinated children (38 vs. 170 cases per 100,000 person years).
Herpes zoster was more common among girls than boys and up to six times more common in immunosuppressed children than in nonimmunosuppressed children.
The authors also found that unvaccinated children benefited from the high rate of vaccination around them. Although the HZ rate was always lower among vaccinated children, the rate among unvaccinated children fell sharply after 2007.
“The trend of decreasing HZ incidence among children who were unvaccinated is likely due to a lack of primary VZV [varicella-zoster virus] infection resulting from herd immunity in a highly vaccinated population,” Dr. Weinmann and her associates said.
There was some variability among age groups, especially among the youngest who were not fully vaccinated.
“In the group aged 1-2 years, the confirmation-adjusted HZ rate among children who were vaccinated was 70% higher than among those who were unvaccinated,” the authors said. In the “older groups, HZ rates were significantly higher in children who were unvaccinated than in those who were vaccinated,” the researchers noted.
The highest incidence was among vaccinated 1-year-olds, who had a 140% higher risk of HZ than did unvaccinated 1-year-olds. But this risk elevation disappeared by age 2 years. For everyone else, aged 2-17 years, the rate of HZ remained significantly lower in vaccinated children.
“Among the small number of children vaccinated at 11 months of age (for whom the vaccine is not recommended), the HZ incidence rate was significantly higher than in children vaccinated at 1 year of age and older. Similarly, children who contract wild-type varicella infection at younger than 1 year of age also have a higher risk of HZ (relative risk, 13.5). The immature adaptive T-cell response in children less than 1 year of age appears less able to contain VZV as a latent infection, compared with older children.
“Our findings for 11-month-olds who were vaccinated should be interpreted with caution because this population included only three cases of HZ and could have included children participating in a prelicensure study with a vaccine formulation different from Varivax,” Dr. Weinmann and her associates said.
Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
SOURCE: Weinmann S et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-2917.
* This article was updated 6/14/2019
FROM PEDIATRICS
Key clinical point: Varicella vaccine is preventing pediatric zoster among children aged 2-17 years.
Major finding: Varicella-vaccinated children have a 78% lower incidence of pediatric zoster than do unvaccinated children.
Study details: The population-based cohort study included more than 6.3 million children.
Disclosures: Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
Source: Weinmann S et al. Pediatrics. 2019. doi: 10.1542/peds.2018-2917.
New help for peanut allergies
Breakthrough therapy holds potential
When it comes to anaphylaxis episodes leading to pediatric intensive care–unit stays, peanuts are the most common culprit. Now the results of a recent clinical trial may lead to approval of the first oral medication to ameliorate reactions in children with severe peanut allergies.
After 6 months of treatment and 6 months of maintenance therapy, two-thirds of the 372 children who received this treatment could ingest the equivalent of two peanuts without allergic symptoms. Just 4% of the 124 children given a placebo powder were able to consume that amount of peanut without reacting. The treatment was not effective for the small number of adults in the study.
This trial of the drug, called AR101 and developed by Aimmune Therapeutics, was published in Nov. 2018 in the New England Journal of Medicine. The company has submitted a biologics license application to the U.S. Food and Drug Administration, and because the drug has been designated a breakthrough therapy, it will go through an accelerated approval process. It could be on the market by the end of 2019.
Reference
1. Rabin RC. New Peanut Allergy Drug Shows ‘Lifesaving’ Potential. New York Times. Nov. 18, 2018. https://www.nytimes.com/2018/11/18/well/live/new-peanut-allergy-drug-shows-lifesaving-potential.html. Accessed Nov. 26, 2018.
Breakthrough therapy holds potential
Breakthrough therapy holds potential
When it comes to anaphylaxis episodes leading to pediatric intensive care–unit stays, peanuts are the most common culprit. Now the results of a recent clinical trial may lead to approval of the first oral medication to ameliorate reactions in children with severe peanut allergies.
After 6 months of treatment and 6 months of maintenance therapy, two-thirds of the 372 children who received this treatment could ingest the equivalent of two peanuts without allergic symptoms. Just 4% of the 124 children given a placebo powder were able to consume that amount of peanut without reacting. The treatment was not effective for the small number of adults in the study.
This trial of the drug, called AR101 and developed by Aimmune Therapeutics, was published in Nov. 2018 in the New England Journal of Medicine. The company has submitted a biologics license application to the U.S. Food and Drug Administration, and because the drug has been designated a breakthrough therapy, it will go through an accelerated approval process. It could be on the market by the end of 2019.
Reference
1. Rabin RC. New Peanut Allergy Drug Shows ‘Lifesaving’ Potential. New York Times. Nov. 18, 2018. https://www.nytimes.com/2018/11/18/well/live/new-peanut-allergy-drug-shows-lifesaving-potential.html. Accessed Nov. 26, 2018.
When it comes to anaphylaxis episodes leading to pediatric intensive care–unit stays, peanuts are the most common culprit. Now the results of a recent clinical trial may lead to approval of the first oral medication to ameliorate reactions in children with severe peanut allergies.
After 6 months of treatment and 6 months of maintenance therapy, two-thirds of the 372 children who received this treatment could ingest the equivalent of two peanuts without allergic symptoms. Just 4% of the 124 children given a placebo powder were able to consume that amount of peanut without reacting. The treatment was not effective for the small number of adults in the study.
This trial of the drug, called AR101 and developed by Aimmune Therapeutics, was published in Nov. 2018 in the New England Journal of Medicine. The company has submitted a biologics license application to the U.S. Food and Drug Administration, and because the drug has been designated a breakthrough therapy, it will go through an accelerated approval process. It could be on the market by the end of 2019.
Reference
1. Rabin RC. New Peanut Allergy Drug Shows ‘Lifesaving’ Potential. New York Times. Nov. 18, 2018. https://www.nytimes.com/2018/11/18/well/live/new-peanut-allergy-drug-shows-lifesaving-potential.html. Accessed Nov. 26, 2018.