User login
Study evaluating in utero treatment for hypohidrotic ectodermal dysplasia seeks enrollees
A multicenter, international phase 2 trial known as EDELIFE is underway to investigate the safety and efficacy of an in utero treatment for developing males with X-linked hypohidrotic ectodermal dysplasia (XLHED).
This condition is caused by mutations in the gene coding for ectodysplasin A (EDA), a protein that signals the epithelial-mesenchymal transition during embryogenesis. EDA loss or dysfunction precludes binding to its endogenous EDA1 receptor (EDAR), and downstream development of teeth, hair, nails, and skin adnexae, most notably eccrine glands.
The treatment, ER004, is a first-in-class signaling protein EDA replacement molecule now under investigation by the EspeRare Foundation, with support from the Pierre Fabre Foundation. The pioneering clinical trial is evaluating the delivery of ER004 protein replacement in utero to affected fetuses, allowing antenatal binding to the EDAR. According to the EDELIFE web site, when ER004 is administered to XLHED-affected males in utero, it “should act as a replacement for the missing EDA and trigger the process that leads to the normal development of a baby’s skin, teeth, hair, and sweat glands, leading to better formation of these structures.”
The protein is delivered into the amniotic fluid via a needle and syringe under ultrasound guidance. In a report on this treatment used in a pair of affected twins and a third XLHED-affected male published in 2018, the authors reported that the three babies were able to sweat normally after birth, “and XLHED-related illness had not developed by 14-22 months of age.”
The goal of the prospective, open-label, genotype match–controlled EDELIFE trial is to confirm the efficacy and safety results for ER004 in a larger group of boys, and to determine if it can lead to robust, and long-lasting improvement in XLHED-associated defects.
In the United States, the first pregnant woman to join the study received the treatment in February 2023 at Washington University in St. Louis. Other clinical sites are located in France, Germany, Italy, Spain, and the United Kingdom. Led by principal investigator Holm Schneider, MD, of the University Erlanger-Nurnberg (Germany), researchers are seeking to enroll mothers aged 18 years and older who are genetically confirmed carriers of the XLHED mutation and pregnant with a boy or considering pregnancy. The control group will include XLHED-affected males, 6 months to 60 years old, who are blood relatives of the pregnant woman participating in the study.
“This is an unprecedented approach to preventing a significant morbidity affecting boys with XLHED, and a potential model for in utero correction of genetic defects involving embryogenesis,” Elaine Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, said in an interview. Dr. Siegfried, who has served on the scientific advisory board of the National Foundation for Ectodermal Dysplasias since 1997, added that many years of effort “has finally yielded sufficient funding and identified an international network of experts to support this ambitious trial. We are now seeking participation of the most important collaborators: mothers willing to help establish safety and efficacy of this approach.”
Mary Fete, MSN, RN, executive director of the NFED, said that the EDELIFE clinical trial “provides enormous hope for our families affected by XLHED. It’s extraordinary to think that the baby boys affected by XLHED who have received ER004 are sweating normally and have other improved symptoms. The NFED is proud to have begun and fostered the research for 30-plus years that developed ER004.”
Dr. Siegfried is a member of the independent data monitoring committee for the EDELIFE trial.
Clinicians treating affected families or potentially eligible subjects are encouraged to contact the trial investigators at this link.
A multicenter, international phase 2 trial known as EDELIFE is underway to investigate the safety and efficacy of an in utero treatment for developing males with X-linked hypohidrotic ectodermal dysplasia (XLHED).
This condition is caused by mutations in the gene coding for ectodysplasin A (EDA), a protein that signals the epithelial-mesenchymal transition during embryogenesis. EDA loss or dysfunction precludes binding to its endogenous EDA1 receptor (EDAR), and downstream development of teeth, hair, nails, and skin adnexae, most notably eccrine glands.
The treatment, ER004, is a first-in-class signaling protein EDA replacement molecule now under investigation by the EspeRare Foundation, with support from the Pierre Fabre Foundation. The pioneering clinical trial is evaluating the delivery of ER004 protein replacement in utero to affected fetuses, allowing antenatal binding to the EDAR. According to the EDELIFE web site, when ER004 is administered to XLHED-affected males in utero, it “should act as a replacement for the missing EDA and trigger the process that leads to the normal development of a baby’s skin, teeth, hair, and sweat glands, leading to better formation of these structures.”
The protein is delivered into the amniotic fluid via a needle and syringe under ultrasound guidance. In a report on this treatment used in a pair of affected twins and a third XLHED-affected male published in 2018, the authors reported that the three babies were able to sweat normally after birth, “and XLHED-related illness had not developed by 14-22 months of age.”
The goal of the prospective, open-label, genotype match–controlled EDELIFE trial is to confirm the efficacy and safety results for ER004 in a larger group of boys, and to determine if it can lead to robust, and long-lasting improvement in XLHED-associated defects.
In the United States, the first pregnant woman to join the study received the treatment in February 2023 at Washington University in St. Louis. Other clinical sites are located in France, Germany, Italy, Spain, and the United Kingdom. Led by principal investigator Holm Schneider, MD, of the University Erlanger-Nurnberg (Germany), researchers are seeking to enroll mothers aged 18 years and older who are genetically confirmed carriers of the XLHED mutation and pregnant with a boy or considering pregnancy. The control group will include XLHED-affected males, 6 months to 60 years old, who are blood relatives of the pregnant woman participating in the study.
“This is an unprecedented approach to preventing a significant morbidity affecting boys with XLHED, and a potential model for in utero correction of genetic defects involving embryogenesis,” Elaine Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, said in an interview. Dr. Siegfried, who has served on the scientific advisory board of the National Foundation for Ectodermal Dysplasias since 1997, added that many years of effort “has finally yielded sufficient funding and identified an international network of experts to support this ambitious trial. We are now seeking participation of the most important collaborators: mothers willing to help establish safety and efficacy of this approach.”
Mary Fete, MSN, RN, executive director of the NFED, said that the EDELIFE clinical trial “provides enormous hope for our families affected by XLHED. It’s extraordinary to think that the baby boys affected by XLHED who have received ER004 are sweating normally and have other improved symptoms. The NFED is proud to have begun and fostered the research for 30-plus years that developed ER004.”
Dr. Siegfried is a member of the independent data monitoring committee for the EDELIFE trial.
Clinicians treating affected families or potentially eligible subjects are encouraged to contact the trial investigators at this link.
A multicenter, international phase 2 trial known as EDELIFE is underway to investigate the safety and efficacy of an in utero treatment for developing males with X-linked hypohidrotic ectodermal dysplasia (XLHED).
This condition is caused by mutations in the gene coding for ectodysplasin A (EDA), a protein that signals the epithelial-mesenchymal transition during embryogenesis. EDA loss or dysfunction precludes binding to its endogenous EDA1 receptor (EDAR), and downstream development of teeth, hair, nails, and skin adnexae, most notably eccrine glands.
The treatment, ER004, is a first-in-class signaling protein EDA replacement molecule now under investigation by the EspeRare Foundation, with support from the Pierre Fabre Foundation. The pioneering clinical trial is evaluating the delivery of ER004 protein replacement in utero to affected fetuses, allowing antenatal binding to the EDAR. According to the EDELIFE web site, when ER004 is administered to XLHED-affected males in utero, it “should act as a replacement for the missing EDA and trigger the process that leads to the normal development of a baby’s skin, teeth, hair, and sweat glands, leading to better formation of these structures.”
The protein is delivered into the amniotic fluid via a needle and syringe under ultrasound guidance. In a report on this treatment used in a pair of affected twins and a third XLHED-affected male published in 2018, the authors reported that the three babies were able to sweat normally after birth, “and XLHED-related illness had not developed by 14-22 months of age.”
The goal of the prospective, open-label, genotype match–controlled EDELIFE trial is to confirm the efficacy and safety results for ER004 in a larger group of boys, and to determine if it can lead to robust, and long-lasting improvement in XLHED-associated defects.
In the United States, the first pregnant woman to join the study received the treatment in February 2023 at Washington University in St. Louis. Other clinical sites are located in France, Germany, Italy, Spain, and the United Kingdom. Led by principal investigator Holm Schneider, MD, of the University Erlanger-Nurnberg (Germany), researchers are seeking to enroll mothers aged 18 years and older who are genetically confirmed carriers of the XLHED mutation and pregnant with a boy or considering pregnancy. The control group will include XLHED-affected males, 6 months to 60 years old, who are blood relatives of the pregnant woman participating in the study.
“This is an unprecedented approach to preventing a significant morbidity affecting boys with XLHED, and a potential model for in utero correction of genetic defects involving embryogenesis,” Elaine Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, said in an interview. Dr. Siegfried, who has served on the scientific advisory board of the National Foundation for Ectodermal Dysplasias since 1997, added that many years of effort “has finally yielded sufficient funding and identified an international network of experts to support this ambitious trial. We are now seeking participation of the most important collaborators: mothers willing to help establish safety and efficacy of this approach.”
Mary Fete, MSN, RN, executive director of the NFED, said that the EDELIFE clinical trial “provides enormous hope for our families affected by XLHED. It’s extraordinary to think that the baby boys affected by XLHED who have received ER004 are sweating normally and have other improved symptoms. The NFED is proud to have begun and fostered the research for 30-plus years that developed ER004.”
Dr. Siegfried is a member of the independent data monitoring committee for the EDELIFE trial.
Clinicians treating affected families or potentially eligible subjects are encouraged to contact the trial investigators at this link.
Innovations in pediatric chronic pain management
At the new Walnut Creek Clinic in the East Bay of the San Francisco Bay area, kids get a “Comfort Promise.”
The clinic extends the work of the Stad Center for Pediatric Pain, Palliative & Integrative Medicine beyond the locations in University of California San Francisco Benioff Children’s Hospitals in San Francisco and Oakland.
At Walnut Creek, clinical acupuncturists, massage therapists, and specialists in hypnosis complement advanced medical care with integrative techniques.
The “Comfort Promise” program, which is being rolled out at that clinic and other UCSF pediatric clinics through the end of 2024, is the clinicians’ pledge to do everything in their power to make tests, infusions, and vaccinations “practically pain free.”
Needle sticks, for example, can be a common source of pain and anxiety for kids. Techniques to minimize pain vary by age. Among the ways the clinicians minimize needle pain for a child 6- to 12-years-old are:
- Giving the child control options to pick which arm; and watch the injection, pause it, or stop it with a communication sign.
- Introducing memory shaping by asking the child about the experience afterward and presenting it in a positive way by praising the acts of sitting still, breathing deeply, or being brave.
- Using distractors such as asking the child to hold a favorite item from home, storytelling, coloring, singing, or using breathing exercises.
Stefan Friedrichsdorf, MD, chief of the UCSF division of pediatric pain, palliative & integrative medicine, said in a statement: “For kids with chronic pain, complex pain medications can cause more harm than benefit. Our goal is to combine exercise and physical therapy with integrative medicine and skills-based psychotherapy to help them become pain free in their everyday life.”
Bundling appointments for early impact
At Lurie Children’s Hospital of Chicago, the chronic pain treatment program bundles visits with experts in several disciplines, include social workers, psychologists, and physical therapists, in addition to the medical team, so that patients can complete a first round of visits with multiple specialists in a short period, as opposed to several months.
Natalie Weatherred, APRN-NP, CPNP-PC, a pediatric nurse practitioner in anesthesiology and the pain clinic coordinator, said in an interview that the up-front visits involve between four and eight follow-up sessions in a short period with everybody in the multidisciplinary team “to really help jump-start their pain treatment.”
She pointed out that many families come from distant parts of the state or beyond so the bundled appointments are also important for easing burden on families.
Sarah Duggan, APRN-NP, CPNP-PC, also a pediatric nurse practitioner in anesthesiology at Lurie’s, pointed out that patients at their clinic often have other chronic conditions as well, such as such as postural orthostatic tachycardia syndrome so the care integration is particularly important.
“We can get them the appropriate care that they need and the resources they need, much sooner than we would have been able to do 5 or 10 years ago,” Ms. Duggan said.
Virtual reality distraction instead of sedation
Henry Huang, MD, anesthesiologist and pain physician at Texas Children’s Hospital, Houston, said a special team there collaborates with the Chariot Program at Stanford (Calif.) University and incorporates virtual reality to distract children from pain and anxiety and harness their imaginations during induction for anesthesia, intravenous placement, and vaccinations.
“At our institution we’ve been recruiting patients to do a proof of concept to do virtual reality distraction for pain procedures, such as nerve blocks or steroid injections,” Dr. Huang said.
Traditionally, kids would have received oral or intravenous sedation to help them cope with the fear and pain.
“We’ve been successful in several cases without relying on any sedation,” he said. “The next target is to expand that to the chronic pain population.”
The distraction techniques are promising for a wide range of ages, he said, and the programming is tailored to the child’s ability to interact with the technology.
He said he is also part of a group promoting use of ultrasound instead of x-rays to guide injections to the spine and chest to reduce children’s exposure to radiation. His group is helping teach these methods to other clinicians nationally.
Dr. Huang said the most important development in chronic pediatric pain has been the growth of rehab centers that include the medical team, and practitioners from psychology as well as occupational and physical therapy.
“More and more hospitals are recognizing the importance of these pain rehab centers,” he said.
The problem, Dr. Huang said, is that these programs have always been resource intensive and involve highly specialized clinicians. The cost and the limited number of specialists make it difficult for widespread rollout.
“That’s always been the challenge from the pediatric pain world,” he said.
Recognizing the complexity of kids’ chronic pain
Angela Garcia, MD, a consulting physician for pediatric rehabilitation medicine at UPMC Children’s Hospital of Pittsburgh said
Techniques such as biofeedback and acupuncture are becoming more mainstream in pediatric chronic care, she said.
At the UPMC clinic, children and their families talk with a care team about their values and what they want to accomplish in managing the child’s pain. They ask what the pain is preventing the child from doing.
“Their goals really are our goals,” she said.
She said she also refers almost all patients to one of the center’s pain psychologists.
“Pain is biopsychosocial,” she said. “We want to make sure we’re addressing how to cope with pain.”
Dr. Garcia said she hopes nutritional therapy is one of the next approaches the clinic will incorporate, particularly surrounding how dietary changes can reduce inflammation “and heal the body from the inside out.”
She said the hospital is also looking at developing an inpatient pain program for kids whose functioning has changed so drastically that they need more intensive therapies.
Whatever the treatment approach, she said, addressing the pain early is critical.
“There is an increased risk of a child with chronic pain becoming an adult with chronic pain,” Dr. Garcia pointed out, “and that can lead to a decrease in the ability to participate in society.”
Ms. Weatherred, Ms. Duggan, Dr. Huang, and Dr. Garcia reported no relevant financial relationships.
At the new Walnut Creek Clinic in the East Bay of the San Francisco Bay area, kids get a “Comfort Promise.”
The clinic extends the work of the Stad Center for Pediatric Pain, Palliative & Integrative Medicine beyond the locations in University of California San Francisco Benioff Children’s Hospitals in San Francisco and Oakland.
At Walnut Creek, clinical acupuncturists, massage therapists, and specialists in hypnosis complement advanced medical care with integrative techniques.
The “Comfort Promise” program, which is being rolled out at that clinic and other UCSF pediatric clinics through the end of 2024, is the clinicians’ pledge to do everything in their power to make tests, infusions, and vaccinations “practically pain free.”
Needle sticks, for example, can be a common source of pain and anxiety for kids. Techniques to minimize pain vary by age. Among the ways the clinicians minimize needle pain for a child 6- to 12-years-old are:
- Giving the child control options to pick which arm; and watch the injection, pause it, or stop it with a communication sign.
- Introducing memory shaping by asking the child about the experience afterward and presenting it in a positive way by praising the acts of sitting still, breathing deeply, or being brave.
- Using distractors such as asking the child to hold a favorite item from home, storytelling, coloring, singing, or using breathing exercises.
Stefan Friedrichsdorf, MD, chief of the UCSF division of pediatric pain, palliative & integrative medicine, said in a statement: “For kids with chronic pain, complex pain medications can cause more harm than benefit. Our goal is to combine exercise and physical therapy with integrative medicine and skills-based psychotherapy to help them become pain free in their everyday life.”
Bundling appointments for early impact
At Lurie Children’s Hospital of Chicago, the chronic pain treatment program bundles visits with experts in several disciplines, include social workers, psychologists, and physical therapists, in addition to the medical team, so that patients can complete a first round of visits with multiple specialists in a short period, as opposed to several months.
Natalie Weatherred, APRN-NP, CPNP-PC, a pediatric nurse practitioner in anesthesiology and the pain clinic coordinator, said in an interview that the up-front visits involve between four and eight follow-up sessions in a short period with everybody in the multidisciplinary team “to really help jump-start their pain treatment.”
She pointed out that many families come from distant parts of the state or beyond so the bundled appointments are also important for easing burden on families.
Sarah Duggan, APRN-NP, CPNP-PC, also a pediatric nurse practitioner in anesthesiology at Lurie’s, pointed out that patients at their clinic often have other chronic conditions as well, such as such as postural orthostatic tachycardia syndrome so the care integration is particularly important.
“We can get them the appropriate care that they need and the resources they need, much sooner than we would have been able to do 5 or 10 years ago,” Ms. Duggan said.
Virtual reality distraction instead of sedation
Henry Huang, MD, anesthesiologist and pain physician at Texas Children’s Hospital, Houston, said a special team there collaborates with the Chariot Program at Stanford (Calif.) University and incorporates virtual reality to distract children from pain and anxiety and harness their imaginations during induction for anesthesia, intravenous placement, and vaccinations.
“At our institution we’ve been recruiting patients to do a proof of concept to do virtual reality distraction for pain procedures, such as nerve blocks or steroid injections,” Dr. Huang said.
Traditionally, kids would have received oral or intravenous sedation to help them cope with the fear and pain.
“We’ve been successful in several cases without relying on any sedation,” he said. “The next target is to expand that to the chronic pain population.”
The distraction techniques are promising for a wide range of ages, he said, and the programming is tailored to the child’s ability to interact with the technology.
He said he is also part of a group promoting use of ultrasound instead of x-rays to guide injections to the spine and chest to reduce children’s exposure to radiation. His group is helping teach these methods to other clinicians nationally.
Dr. Huang said the most important development in chronic pediatric pain has been the growth of rehab centers that include the medical team, and practitioners from psychology as well as occupational and physical therapy.
“More and more hospitals are recognizing the importance of these pain rehab centers,” he said.
The problem, Dr. Huang said, is that these programs have always been resource intensive and involve highly specialized clinicians. The cost and the limited number of specialists make it difficult for widespread rollout.
“That’s always been the challenge from the pediatric pain world,” he said.
Recognizing the complexity of kids’ chronic pain
Angela Garcia, MD, a consulting physician for pediatric rehabilitation medicine at UPMC Children’s Hospital of Pittsburgh said
Techniques such as biofeedback and acupuncture are becoming more mainstream in pediatric chronic care, she said.
At the UPMC clinic, children and their families talk with a care team about their values and what they want to accomplish in managing the child’s pain. They ask what the pain is preventing the child from doing.
“Their goals really are our goals,” she said.
She said she also refers almost all patients to one of the center’s pain psychologists.
“Pain is biopsychosocial,” she said. “We want to make sure we’re addressing how to cope with pain.”
Dr. Garcia said she hopes nutritional therapy is one of the next approaches the clinic will incorporate, particularly surrounding how dietary changes can reduce inflammation “and heal the body from the inside out.”
She said the hospital is also looking at developing an inpatient pain program for kids whose functioning has changed so drastically that they need more intensive therapies.
Whatever the treatment approach, she said, addressing the pain early is critical.
“There is an increased risk of a child with chronic pain becoming an adult with chronic pain,” Dr. Garcia pointed out, “and that can lead to a decrease in the ability to participate in society.”
Ms. Weatherred, Ms. Duggan, Dr. Huang, and Dr. Garcia reported no relevant financial relationships.
At the new Walnut Creek Clinic in the East Bay of the San Francisco Bay area, kids get a “Comfort Promise.”
The clinic extends the work of the Stad Center for Pediatric Pain, Palliative & Integrative Medicine beyond the locations in University of California San Francisco Benioff Children’s Hospitals in San Francisco and Oakland.
At Walnut Creek, clinical acupuncturists, massage therapists, and specialists in hypnosis complement advanced medical care with integrative techniques.
The “Comfort Promise” program, which is being rolled out at that clinic and other UCSF pediatric clinics through the end of 2024, is the clinicians’ pledge to do everything in their power to make tests, infusions, and vaccinations “practically pain free.”
Needle sticks, for example, can be a common source of pain and anxiety for kids. Techniques to minimize pain vary by age. Among the ways the clinicians minimize needle pain for a child 6- to 12-years-old are:
- Giving the child control options to pick which arm; and watch the injection, pause it, or stop it with a communication sign.
- Introducing memory shaping by asking the child about the experience afterward and presenting it in a positive way by praising the acts of sitting still, breathing deeply, or being brave.
- Using distractors such as asking the child to hold a favorite item from home, storytelling, coloring, singing, or using breathing exercises.
Stefan Friedrichsdorf, MD, chief of the UCSF division of pediatric pain, palliative & integrative medicine, said in a statement: “For kids with chronic pain, complex pain medications can cause more harm than benefit. Our goal is to combine exercise and physical therapy with integrative medicine and skills-based psychotherapy to help them become pain free in their everyday life.”
Bundling appointments for early impact
At Lurie Children’s Hospital of Chicago, the chronic pain treatment program bundles visits with experts in several disciplines, include social workers, psychologists, and physical therapists, in addition to the medical team, so that patients can complete a first round of visits with multiple specialists in a short period, as opposed to several months.
Natalie Weatherred, APRN-NP, CPNP-PC, a pediatric nurse practitioner in anesthesiology and the pain clinic coordinator, said in an interview that the up-front visits involve between four and eight follow-up sessions in a short period with everybody in the multidisciplinary team “to really help jump-start their pain treatment.”
She pointed out that many families come from distant parts of the state or beyond so the bundled appointments are also important for easing burden on families.
Sarah Duggan, APRN-NP, CPNP-PC, also a pediatric nurse practitioner in anesthesiology at Lurie’s, pointed out that patients at their clinic often have other chronic conditions as well, such as such as postural orthostatic tachycardia syndrome so the care integration is particularly important.
“We can get them the appropriate care that they need and the resources they need, much sooner than we would have been able to do 5 or 10 years ago,” Ms. Duggan said.
Virtual reality distraction instead of sedation
Henry Huang, MD, anesthesiologist and pain physician at Texas Children’s Hospital, Houston, said a special team there collaborates with the Chariot Program at Stanford (Calif.) University and incorporates virtual reality to distract children from pain and anxiety and harness their imaginations during induction for anesthesia, intravenous placement, and vaccinations.
“At our institution we’ve been recruiting patients to do a proof of concept to do virtual reality distraction for pain procedures, such as nerve blocks or steroid injections,” Dr. Huang said.
Traditionally, kids would have received oral or intravenous sedation to help them cope with the fear and pain.
“We’ve been successful in several cases without relying on any sedation,” he said. “The next target is to expand that to the chronic pain population.”
The distraction techniques are promising for a wide range of ages, he said, and the programming is tailored to the child’s ability to interact with the technology.
He said he is also part of a group promoting use of ultrasound instead of x-rays to guide injections to the spine and chest to reduce children’s exposure to radiation. His group is helping teach these methods to other clinicians nationally.
Dr. Huang said the most important development in chronic pediatric pain has been the growth of rehab centers that include the medical team, and practitioners from psychology as well as occupational and physical therapy.
“More and more hospitals are recognizing the importance of these pain rehab centers,” he said.
The problem, Dr. Huang said, is that these programs have always been resource intensive and involve highly specialized clinicians. The cost and the limited number of specialists make it difficult for widespread rollout.
“That’s always been the challenge from the pediatric pain world,” he said.
Recognizing the complexity of kids’ chronic pain
Angela Garcia, MD, a consulting physician for pediatric rehabilitation medicine at UPMC Children’s Hospital of Pittsburgh said
Techniques such as biofeedback and acupuncture are becoming more mainstream in pediatric chronic care, she said.
At the UPMC clinic, children and their families talk with a care team about their values and what they want to accomplish in managing the child’s pain. They ask what the pain is preventing the child from doing.
“Their goals really are our goals,” she said.
She said she also refers almost all patients to one of the center’s pain psychologists.
“Pain is biopsychosocial,” she said. “We want to make sure we’re addressing how to cope with pain.”
Dr. Garcia said she hopes nutritional therapy is one of the next approaches the clinic will incorporate, particularly surrounding how dietary changes can reduce inflammation “and heal the body from the inside out.”
She said the hospital is also looking at developing an inpatient pain program for kids whose functioning has changed so drastically that they need more intensive therapies.
Whatever the treatment approach, she said, addressing the pain early is critical.
“There is an increased risk of a child with chronic pain becoming an adult with chronic pain,” Dr. Garcia pointed out, “and that can lead to a decrease in the ability to participate in society.”
Ms. Weatherred, Ms. Duggan, Dr. Huang, and Dr. Garcia reported no relevant financial relationships.
Perinatal psychiatry: 5 key principles
Perinatal mood and anxiety disorders are the most common complication of pregnancy and childbirth.1 Mental health concerns are a leading cause of maternal mortality in the United States, which has rising maternal mortality rates and glaring racial and socioeconomic disparities.2 Inconsistent perinatal psychiatry training likely contributes to perceived discomfort of patients who are pregnant.3 This is why it is critical for all psychiatrists to understand the principles of perinatal psychiatry. Here is a brief description of 5 key principles.
1. Discuss preconception planning
Reproductive life planning should occur with all patients who are capable of becoming pregnant. This planning should include not just a risks/benefits analysis and anticipatory planning regarding medications but also a discussion of prior perinatal symptoms, pregnancy intentions and contraception (especially in light of increasingly limited access to abortion), and the bidirectional nature of pregnancy and mental health conditions.
The acronym PATH provides a framework for these conversations:
- Pregnancy Attitudes: “Do you think you might like to have (more) children at some point?”
- Timing: “If considering future parenthood, when do you think that might be?”
- How important is prevention: “How important is it to you to prevent pregnancy (until then)?”4
2. Focus on perinatal mental health
Discussion often centers on medication risks to the fetus at the expense of considering risks of under- or nontreatment for both members of the dyad. Undertreating perinatal mental health conditions results in dual exposures (medication and illness), and untreated illness is associated with negative effects on obstetric and neonatal outcomes and the well-being of the parent and offspring.1
3. Resist experimentation
It is common for clinicians to reflexively switch patients who are pregnant from an effective medication to one viewed as the “safest” or “best” because it has more data. This exposes the fetus to 2 medications and the dyad to potential symptoms of the illness. Decisions about medication changes should instead be made on an individual basis considering the risks and benefits of all exposures as well as the patient’s current symptoms, previous treatment, and family history.
4. Collaborate and communicate
Despite effective interventions, many perinatal mental health conditions go untreated.1 Normalize perinatal mental health symptoms with patients to reduce stigma and barriers to disclosure, and respect their decisions regarding perinatal medication use. Proper communication with the obstetric team ensures appropriate perinatal mental health screening and fetal monitoring (eg, possible fetal growth ultrasounds for a patient taking prazosin, or assessing for neonatal adaptation syndrome if there is selective serotonin reuptake inhibitor exposure in utero).
5. Recognize your limitations
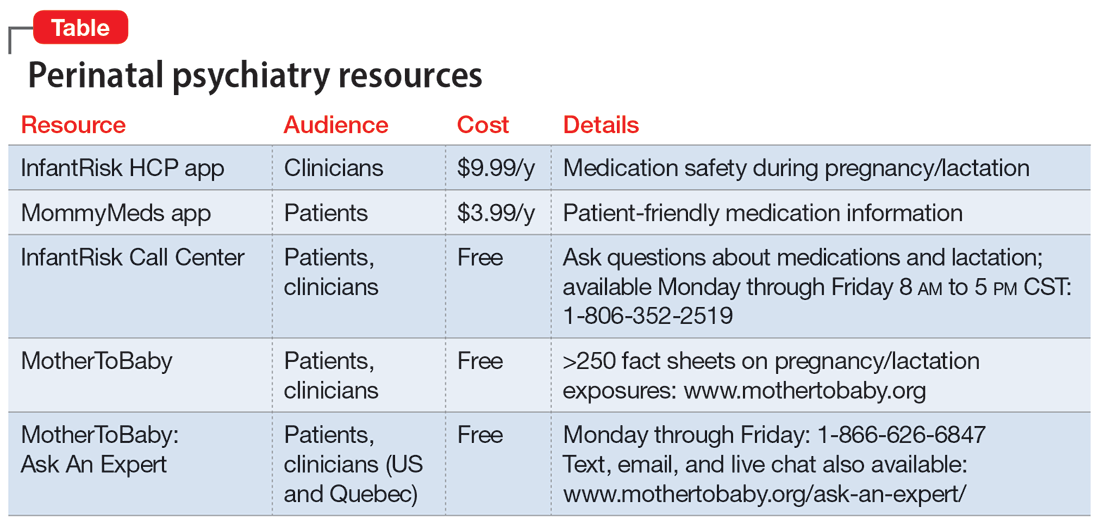
Our understanding of psychotropics’ teratogenicity is constantly evolving, and we must recognize when we don’t know something. In addition to medication databases such as Reprotox (https://reprotox.org/) and LactMed (https://www.ncbi.nlm.nih.gov/books/NBK501922/), several perinatal psychiatry resources are available for both patients and clinicians (Table). Additionally, Postpartum Support International maintains a National Perinatal Consult Line (1-877-499-4773) as well as a list of state perinatal psychiatry access lines (https://www.postpartum.net/professionals/state-perinatal-psychiatry-access-lines/) for clinicians. The Massachusetts General Hospital Center for Women’s Mental Health (https://womensmentalhealth.org) is also a helpful resource for clinicians.
1. Luca DL, Garlow N, Staatz C, et al. Societal costs of untreated perinatal mood and anxiety disorders in the United States. Mathematica Policy Research. April 29, 2019. Accessed July 13, 2023. https://www.mathematica.org/publications/societal-costs-of-untreated-perinatal-mood-and-anxiety-disorders-in-the-united-states
2. Singh GK. Trends and social inequalities in maternal mortality in the United States, 1969-2018. Int J MCH AIDS. 2021;10(1):29-42. doi:10.21106/ijma.444
3. Weinreb L, Byatt N, Moore Simas TA, et al. What happens to mental health treatment during pregnancy? Women’s experience with prescribing providers. Psychiatr Q. 2014;85(3):349-355. doi:10.1007/s11126-014-9293-7
4. Callegari LS, Aiken AR, Dehlendorf C, et al. Addressing potential pitfalls of reproductive life planning with patient-centered counseling. Am J Obstet Gynecol. 2017;216(2):129-134. doi:10.1016/j.ajog.2016.10.004
Perinatal mood and anxiety disorders are the most common complication of pregnancy and childbirth.1 Mental health concerns are a leading cause of maternal mortality in the United States, which has rising maternal mortality rates and glaring racial and socioeconomic disparities.2 Inconsistent perinatal psychiatry training likely contributes to perceived discomfort of patients who are pregnant.3 This is why it is critical for all psychiatrists to understand the principles of perinatal psychiatry. Here is a brief description of 5 key principles.
1. Discuss preconception planning
Reproductive life planning should occur with all patients who are capable of becoming pregnant. This planning should include not just a risks/benefits analysis and anticipatory planning regarding medications but also a discussion of prior perinatal symptoms, pregnancy intentions and contraception (especially in light of increasingly limited access to abortion), and the bidirectional nature of pregnancy and mental health conditions.
The acronym PATH provides a framework for these conversations:
- Pregnancy Attitudes: “Do you think you might like to have (more) children at some point?”
- Timing: “If considering future parenthood, when do you think that might be?”
- How important is prevention: “How important is it to you to prevent pregnancy (until then)?”4
2. Focus on perinatal mental health
Discussion often centers on medication risks to the fetus at the expense of considering risks of under- or nontreatment for both members of the dyad. Undertreating perinatal mental health conditions results in dual exposures (medication and illness), and untreated illness is associated with negative effects on obstetric and neonatal outcomes and the well-being of the parent and offspring.1
3. Resist experimentation
It is common for clinicians to reflexively switch patients who are pregnant from an effective medication to one viewed as the “safest” or “best” because it has more data. This exposes the fetus to 2 medications and the dyad to potential symptoms of the illness. Decisions about medication changes should instead be made on an individual basis considering the risks and benefits of all exposures as well as the patient’s current symptoms, previous treatment, and family history.
4. Collaborate and communicate
Despite effective interventions, many perinatal mental health conditions go untreated.1 Normalize perinatal mental health symptoms with patients to reduce stigma and barriers to disclosure, and respect their decisions regarding perinatal medication use. Proper communication with the obstetric team ensures appropriate perinatal mental health screening and fetal monitoring (eg, possible fetal growth ultrasounds for a patient taking prazosin, or assessing for neonatal adaptation syndrome if there is selective serotonin reuptake inhibitor exposure in utero).
5. Recognize your limitations
Our understanding of psychotropics’ teratogenicity is constantly evolving, and we must recognize when we don’t know something. In addition to medication databases such as Reprotox (https://reprotox.org/) and LactMed (https://www.ncbi.nlm.nih.gov/books/NBK501922/), several perinatal psychiatry resources are available for both patients and clinicians (Table). Additionally, Postpartum Support International maintains a National Perinatal Consult Line (1-877-499-4773) as well as a list of state perinatal psychiatry access lines (https://www.postpartum.net/professionals/state-perinatal-psychiatry-access-lines/) for clinicians. The Massachusetts General Hospital Center for Women’s Mental Health (https://womensmentalhealth.org) is also a helpful resource for clinicians.

Perinatal mood and anxiety disorders are the most common complication of pregnancy and childbirth.1 Mental health concerns are a leading cause of maternal mortality in the United States, which has rising maternal mortality rates and glaring racial and socioeconomic disparities.2 Inconsistent perinatal psychiatry training likely contributes to perceived discomfort of patients who are pregnant.3 This is why it is critical for all psychiatrists to understand the principles of perinatal psychiatry. Here is a brief description of 5 key principles.
1. Discuss preconception planning
Reproductive life planning should occur with all patients who are capable of becoming pregnant. This planning should include not just a risks/benefits analysis and anticipatory planning regarding medications but also a discussion of prior perinatal symptoms, pregnancy intentions and contraception (especially in light of increasingly limited access to abortion), and the bidirectional nature of pregnancy and mental health conditions.
The acronym PATH provides a framework for these conversations:
- Pregnancy Attitudes: “Do you think you might like to have (more) children at some point?”
- Timing: “If considering future parenthood, when do you think that might be?”
- How important is prevention: “How important is it to you to prevent pregnancy (until then)?”4
2. Focus on perinatal mental health
Discussion often centers on medication risks to the fetus at the expense of considering risks of under- or nontreatment for both members of the dyad. Undertreating perinatal mental health conditions results in dual exposures (medication and illness), and untreated illness is associated with negative effects on obstetric and neonatal outcomes and the well-being of the parent and offspring.1
3. Resist experimentation
It is common for clinicians to reflexively switch patients who are pregnant from an effective medication to one viewed as the “safest” or “best” because it has more data. This exposes the fetus to 2 medications and the dyad to potential symptoms of the illness. Decisions about medication changes should instead be made on an individual basis considering the risks and benefits of all exposures as well as the patient’s current symptoms, previous treatment, and family history.
4. Collaborate and communicate
Despite effective interventions, many perinatal mental health conditions go untreated.1 Normalize perinatal mental health symptoms with patients to reduce stigma and barriers to disclosure, and respect their decisions regarding perinatal medication use. Proper communication with the obstetric team ensures appropriate perinatal mental health screening and fetal monitoring (eg, possible fetal growth ultrasounds for a patient taking prazosin, or assessing for neonatal adaptation syndrome if there is selective serotonin reuptake inhibitor exposure in utero).
5. Recognize your limitations
Our understanding of psychotropics’ teratogenicity is constantly evolving, and we must recognize when we don’t know something. In addition to medication databases such as Reprotox (https://reprotox.org/) and LactMed (https://www.ncbi.nlm.nih.gov/books/NBK501922/), several perinatal psychiatry resources are available for both patients and clinicians (Table). Additionally, Postpartum Support International maintains a National Perinatal Consult Line (1-877-499-4773) as well as a list of state perinatal psychiatry access lines (https://www.postpartum.net/professionals/state-perinatal-psychiatry-access-lines/) for clinicians. The Massachusetts General Hospital Center for Women’s Mental Health (https://womensmentalhealth.org) is also a helpful resource for clinicians.

1. Luca DL, Garlow N, Staatz C, et al. Societal costs of untreated perinatal mood and anxiety disorders in the United States. Mathematica Policy Research. April 29, 2019. Accessed July 13, 2023. https://www.mathematica.org/publications/societal-costs-of-untreated-perinatal-mood-and-anxiety-disorders-in-the-united-states
2. Singh GK. Trends and social inequalities in maternal mortality in the United States, 1969-2018. Int J MCH AIDS. 2021;10(1):29-42. doi:10.21106/ijma.444
3. Weinreb L, Byatt N, Moore Simas TA, et al. What happens to mental health treatment during pregnancy? Women’s experience with prescribing providers. Psychiatr Q. 2014;85(3):349-355. doi:10.1007/s11126-014-9293-7
4. Callegari LS, Aiken AR, Dehlendorf C, et al. Addressing potential pitfalls of reproductive life planning with patient-centered counseling. Am J Obstet Gynecol. 2017;216(2):129-134. doi:10.1016/j.ajog.2016.10.004
1. Luca DL, Garlow N, Staatz C, et al. Societal costs of untreated perinatal mood and anxiety disorders in the United States. Mathematica Policy Research. April 29, 2019. Accessed July 13, 2023. https://www.mathematica.org/publications/societal-costs-of-untreated-perinatal-mood-and-anxiety-disorders-in-the-united-states
2. Singh GK. Trends and social inequalities in maternal mortality in the United States, 1969-2018. Int J MCH AIDS. 2021;10(1):29-42. doi:10.21106/ijma.444
3. Weinreb L, Byatt N, Moore Simas TA, et al. What happens to mental health treatment during pregnancy? Women’s experience with prescribing providers. Psychiatr Q. 2014;85(3):349-355. doi:10.1007/s11126-014-9293-7
4. Callegari LS, Aiken AR, Dehlendorf C, et al. Addressing potential pitfalls of reproductive life planning with patient-centered counseling. Am J Obstet Gynecol. 2017;216(2):129-134. doi:10.1016/j.ajog.2016.10.004
Reassuring data on stimulants for ADHD in kids and later substance abuse
“Throughout rigorous analyses, and after accounting for more than 70 variables in this longitudinal sample of children with ADHD taking stimulants, we did not find an association with later substance use,” lead investigator Brooke Molina, PhD, director of the youth and family research program at the University of Pittsburgh, said in an interview.
The findings were published online in JAMA Psychiatry.
Protective effect?
Owing to symptoms of impulsivity inherent to ADHD, the disorder itself carries a risk for elevated substance use, the investigators note.
They speculate that this may be why some previous research suggests prescription stimulants reduce the risk of subsequent substance use disorder. However, other studies have found no such protective link.
To shed more light on the issue, the investigators used data from the Multimodal Treatment Study of ADHD, a multicenter, 14-month randomized clinical trial of medication and behavioral therapy for children with ADHD. However, for the purposes of the present study, investigators focused only on stimulant use in children.
At the time of recruitment, the children were aged 7-9 and had been diagnosed with ADHD between 1994 and 1996.
Investigators assessed the participants prior to randomization, at months 3 and 9, and at the end of treatment. They were then followed for 16 years and were assessed at years 2, 3, 6, 8, 10, 12, 14, and 16 until a mean age of 25.
During 12-, 14-, and 16-year follow-up, participants completed a questionnaire on their use of alcohol, marijuana, cigarettes, and several illicit and prescription drugs.
Investigators collected information on participants’ stimulant treatment via the Services for Children and Adolescents Parent Interview until they reached age 18. After that, participants reported their own stimulant treatment.
A total of 579 participants were included in the analysis. Of these, 61% were White, 20% were Black, and 8% were Hispanic.
Decline in stimulant use over time
The analysis showed that stimulant use declined “precipitously” over time – from 60% at the 2- and 3-year assessments to an average of 7% during early adulthood.
The investigators also found that for some participants, substance use increased steadily through adolescence and remained stable through early adulthood. For instance, 36.5% of the adolescents in the total cohort reported smoking tobacco daily, and 29.6% reported using marijuana every week.
In addition, approximately 21% of the participants indulged in heavy drinking at least once a week, and 6% reported “other” substance use, which included sedative misuse, heroin, inhalants, hallucinogens, or other substances taken to “get high.”
After accounting for developmental trends in substance use in the sample through adolescence into early adulthood with several rigorous statistical models, the researchers found no association between current or prior stimulant treatment and cigarette, marijuana, alcohol, or other substance use, with one exception.
While cumulative stimulant treatment was associated with increased heavy drinking, the effect size of this association was small. Each additional year of cumulative stimulant use was estimated to increase participants’ likelihood of any binge drinking/drunkenness vs. none in the past year by 4% (95% confidence interval, 0.01-0.08; P =.03).
When the investigators used a causal analytic method to account for age and other time-varying characteristics, including household income, behavior problems, and parental support, there was no evidence that current (B range, –0.62-0.34) or prior stimulant treatment (B range, –0.06-0.70) or their interaction (B range, –0.49-0.86) was associated with substance use in adulthood.
Dr. Molina noted that although participants were recruited from multiple sites, the sample may not be generalizable because children and parents who present for an intensive treatment study such as this are not necessarily representative of the general ADHD population.
Reassuring findings
In a comment, Julie Schweitzer, PhD, professor of psychiatry and behavioral sciences at the University of California, Davis, said she hopes the study findings will quell the stigma surrounding stimulant use by children with ADHD.
“Parents’ fears that stimulant use will lead to a substance use disorder inhibits them from bringing their children for an ADHD evaluation, thus reducing the likelihood that they will receive timely treatment,” Dr. Schweitzer said.
“While stimulant medication is the first-line treatment most often recommended for most persons with ADHD, by not following through on evaluations, parents also miss the opportunity to learn about nonpharmacological strategies that might also be helpful to help cope with ADHD symptoms and its potential co-occurring challenges,” she added.
Dr. Schweitzer also noted that many parents hope their children will outgrow the symptoms without realizing that by not obtaining an evaluation and treatment for their child, there is an associated cost, including less than optimal academic performance, social relationships, and emotional health.
The Multimodal Treatment Study of Children with ADHD was a National Institute of Mental Health cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and under a National Institute on Drug Abuse contract followed by a data analysis grant. Dr. Molina reported grants from the NIMH and the National Institute on Drug Abuse during the conduct of the study.
A version of this article first appeared on Medscape.com.
“Throughout rigorous analyses, and after accounting for more than 70 variables in this longitudinal sample of children with ADHD taking stimulants, we did not find an association with later substance use,” lead investigator Brooke Molina, PhD, director of the youth and family research program at the University of Pittsburgh, said in an interview.
The findings were published online in JAMA Psychiatry.
Protective effect?
Owing to symptoms of impulsivity inherent to ADHD, the disorder itself carries a risk for elevated substance use, the investigators note.
They speculate that this may be why some previous research suggests prescription stimulants reduce the risk of subsequent substance use disorder. However, other studies have found no such protective link.
To shed more light on the issue, the investigators used data from the Multimodal Treatment Study of ADHD, a multicenter, 14-month randomized clinical trial of medication and behavioral therapy for children with ADHD. However, for the purposes of the present study, investigators focused only on stimulant use in children.
At the time of recruitment, the children were aged 7-9 and had been diagnosed with ADHD between 1994 and 1996.
Investigators assessed the participants prior to randomization, at months 3 and 9, and at the end of treatment. They were then followed for 16 years and were assessed at years 2, 3, 6, 8, 10, 12, 14, and 16 until a mean age of 25.
During 12-, 14-, and 16-year follow-up, participants completed a questionnaire on their use of alcohol, marijuana, cigarettes, and several illicit and prescription drugs.
Investigators collected information on participants’ stimulant treatment via the Services for Children and Adolescents Parent Interview until they reached age 18. After that, participants reported their own stimulant treatment.
A total of 579 participants were included in the analysis. Of these, 61% were White, 20% were Black, and 8% were Hispanic.
Decline in stimulant use over time
The analysis showed that stimulant use declined “precipitously” over time – from 60% at the 2- and 3-year assessments to an average of 7% during early adulthood.
The investigators also found that for some participants, substance use increased steadily through adolescence and remained stable through early adulthood. For instance, 36.5% of the adolescents in the total cohort reported smoking tobacco daily, and 29.6% reported using marijuana every week.
In addition, approximately 21% of the participants indulged in heavy drinking at least once a week, and 6% reported “other” substance use, which included sedative misuse, heroin, inhalants, hallucinogens, or other substances taken to “get high.”
After accounting for developmental trends in substance use in the sample through adolescence into early adulthood with several rigorous statistical models, the researchers found no association between current or prior stimulant treatment and cigarette, marijuana, alcohol, or other substance use, with one exception.
While cumulative stimulant treatment was associated with increased heavy drinking, the effect size of this association was small. Each additional year of cumulative stimulant use was estimated to increase participants’ likelihood of any binge drinking/drunkenness vs. none in the past year by 4% (95% confidence interval, 0.01-0.08; P =.03).
When the investigators used a causal analytic method to account for age and other time-varying characteristics, including household income, behavior problems, and parental support, there was no evidence that current (B range, –0.62-0.34) or prior stimulant treatment (B range, –0.06-0.70) or their interaction (B range, –0.49-0.86) was associated with substance use in adulthood.
Dr. Molina noted that although participants were recruited from multiple sites, the sample may not be generalizable because children and parents who present for an intensive treatment study such as this are not necessarily representative of the general ADHD population.
Reassuring findings
In a comment, Julie Schweitzer, PhD, professor of psychiatry and behavioral sciences at the University of California, Davis, said she hopes the study findings will quell the stigma surrounding stimulant use by children with ADHD.
“Parents’ fears that stimulant use will lead to a substance use disorder inhibits them from bringing their children for an ADHD evaluation, thus reducing the likelihood that they will receive timely treatment,” Dr. Schweitzer said.
“While stimulant medication is the first-line treatment most often recommended for most persons with ADHD, by not following through on evaluations, parents also miss the opportunity to learn about nonpharmacological strategies that might also be helpful to help cope with ADHD symptoms and its potential co-occurring challenges,” she added.
Dr. Schweitzer also noted that many parents hope their children will outgrow the symptoms without realizing that by not obtaining an evaluation and treatment for their child, there is an associated cost, including less than optimal academic performance, social relationships, and emotional health.
The Multimodal Treatment Study of Children with ADHD was a National Institute of Mental Health cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and under a National Institute on Drug Abuse contract followed by a data analysis grant. Dr. Molina reported grants from the NIMH and the National Institute on Drug Abuse during the conduct of the study.
A version of this article first appeared on Medscape.com.
“Throughout rigorous analyses, and after accounting for more than 70 variables in this longitudinal sample of children with ADHD taking stimulants, we did not find an association with later substance use,” lead investigator Brooke Molina, PhD, director of the youth and family research program at the University of Pittsburgh, said in an interview.
The findings were published online in JAMA Psychiatry.
Protective effect?
Owing to symptoms of impulsivity inherent to ADHD, the disorder itself carries a risk for elevated substance use, the investigators note.
They speculate that this may be why some previous research suggests prescription stimulants reduce the risk of subsequent substance use disorder. However, other studies have found no such protective link.
To shed more light on the issue, the investigators used data from the Multimodal Treatment Study of ADHD, a multicenter, 14-month randomized clinical trial of medication and behavioral therapy for children with ADHD. However, for the purposes of the present study, investigators focused only on stimulant use in children.
At the time of recruitment, the children were aged 7-9 and had been diagnosed with ADHD between 1994 and 1996.
Investigators assessed the participants prior to randomization, at months 3 and 9, and at the end of treatment. They were then followed for 16 years and were assessed at years 2, 3, 6, 8, 10, 12, 14, and 16 until a mean age of 25.
During 12-, 14-, and 16-year follow-up, participants completed a questionnaire on their use of alcohol, marijuana, cigarettes, and several illicit and prescription drugs.
Investigators collected information on participants’ stimulant treatment via the Services for Children and Adolescents Parent Interview until they reached age 18. After that, participants reported their own stimulant treatment.
A total of 579 participants were included in the analysis. Of these, 61% were White, 20% were Black, and 8% were Hispanic.
Decline in stimulant use over time
The analysis showed that stimulant use declined “precipitously” over time – from 60% at the 2- and 3-year assessments to an average of 7% during early adulthood.
The investigators also found that for some participants, substance use increased steadily through adolescence and remained stable through early adulthood. For instance, 36.5% of the adolescents in the total cohort reported smoking tobacco daily, and 29.6% reported using marijuana every week.
In addition, approximately 21% of the participants indulged in heavy drinking at least once a week, and 6% reported “other” substance use, which included sedative misuse, heroin, inhalants, hallucinogens, or other substances taken to “get high.”
After accounting for developmental trends in substance use in the sample through adolescence into early adulthood with several rigorous statistical models, the researchers found no association between current or prior stimulant treatment and cigarette, marijuana, alcohol, or other substance use, with one exception.
While cumulative stimulant treatment was associated with increased heavy drinking, the effect size of this association was small. Each additional year of cumulative stimulant use was estimated to increase participants’ likelihood of any binge drinking/drunkenness vs. none in the past year by 4% (95% confidence interval, 0.01-0.08; P =.03).
When the investigators used a causal analytic method to account for age and other time-varying characteristics, including household income, behavior problems, and parental support, there was no evidence that current (B range, –0.62-0.34) or prior stimulant treatment (B range, –0.06-0.70) or their interaction (B range, –0.49-0.86) was associated with substance use in adulthood.
Dr. Molina noted that although participants were recruited from multiple sites, the sample may not be generalizable because children and parents who present for an intensive treatment study such as this are not necessarily representative of the general ADHD population.
Reassuring findings
In a comment, Julie Schweitzer, PhD, professor of psychiatry and behavioral sciences at the University of California, Davis, said she hopes the study findings will quell the stigma surrounding stimulant use by children with ADHD.
“Parents’ fears that stimulant use will lead to a substance use disorder inhibits them from bringing their children for an ADHD evaluation, thus reducing the likelihood that they will receive timely treatment,” Dr. Schweitzer said.
“While stimulant medication is the first-line treatment most often recommended for most persons with ADHD, by not following through on evaluations, parents also miss the opportunity to learn about nonpharmacological strategies that might also be helpful to help cope with ADHD symptoms and its potential co-occurring challenges,” she added.
Dr. Schweitzer also noted that many parents hope their children will outgrow the symptoms without realizing that by not obtaining an evaluation and treatment for their child, there is an associated cost, including less than optimal academic performance, social relationships, and emotional health.
The Multimodal Treatment Study of Children with ADHD was a National Institute of Mental Health cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and under a National Institute on Drug Abuse contract followed by a data analysis grant. Dr. Molina reported grants from the NIMH and the National Institute on Drug Abuse during the conduct of the study.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Concussion may not affect IQ in children
data suggest.
In a multicenter study of almost 900 children with concussion or orthopedic injury, differences between groups in full-scale IQ (Cohen’s d = 0.13) and matrix reasoning scores (d = 0.16) were small.
“We draw the inference that IQ scores are unchanged, in the sense that they’re not different from [those of] kids with other types of injuries that don’t involve the brain,” said study author Keith Owen Yeates, PhD, Ronald and Irene Ward Chair in Pediatric Brain Injury and a professor of psychology at the University of Calgary (Alta.).
The study was published in Pediatrics.
A representative sample
The investigators analyzed data from two prospective cohort studies of children who were treated for concussion or mild orthopedic injury at two hospitals in the United States and five in Canada. Participants were aged 8-17 years and were recruited within 24 hours of the index event. Patients in the United States completed IQ and performance validity testing at 3-18 days after injury. Patients in Canada did so at 3 months after injury. The study used the short-form IQ test. The investigators included 866 children in their analysis.
Using linear modeling, Bayesian analysis, and multigroup factor analysis, the researchers found “very small group differences” in full-scale IQ scores between the two groups. Mean IQ was 104.95 for the concussion group and 106.08 for the orthopedic-injury group. Matrix reasoning scores were 52.28 and 53.81 for the concussion and orthopedic-injury groups, respectively.
Vocabulary scores did not differ between the two groups (53.25 for the concussion group and 53.27 for the orthopedic-injury group).
The study population is “pretty representative” from a demographic perspective, although it was predominantly White, said Dr. Yeates. “On the other hand, we did look at socioeconomic status, and that didn’t seem to alter the findings at all.”
The sample size is one of the study’s strengths, said Dr. Yeates. “Having 866 kids is far larger, I think, than just about any other study out there.” Drawing from seven children’s hospitals in North America is another strength. “Previous studies, in addition to having smaller samples, were from a single site and often recruited from a clinic population, not a representative group for a general population of kids with concussion.”
The findings must be interpreted precisely, however. “We don’t have actual preinjury data, so the more precise way of describing the findings is to say they’re not different from kids who are very similar to them demographically, have the same risk factors for injuries, and had a similar experience of a traumatic injury,” said Dr. Yeates. “The IQ scores for both groups are smack dab in the average range.”
Overall, the results are encouraging. “There’s been a lot of bad news in the media and in the science about concussion that worries patients, so it’s nice to be able to provide a little bit of balance,” said Dr. Yeates. “The message I give parents is that most kids recover within 2-4 weeks, and we’re much better now at predicting who’s going to [recover] and who isn’t, and that helps, too, so that we can focus our intervention on kids who are most at risk.”
Some children will have persisting symptoms, but evidence-based treatments are lacking. “I think that’ll be a really important direction for the future,” said Dr. Yeates.
Graduated return
Commenting on the findings, Michael Esser, MD, a pediatric neurologist at Alberta Children’s Hospital, Calgary, and an associate professor in pediatrics at the University of Calgary, said that they can help allay parents’ concerns about concussions. “It can also be of help for clinicians who want to have evidence to reassure families and promote a graduated return to activities. In particular, the study would support the philosophy of a graduated return to school or work, after a brief period of rest, following concussion.” Dr. Esser did not participate in the study.
The research is also noteworthy because it acknowledges that the differences in the design and methodology used in prior studies may explain the apparent disagreement over how concussion may influence cognitive function.
“This is an important message,” said Dr. Esser. “Families struggle with determining the merit of a lot of information due to the myriad of social media comments about concussion and the risk for cognitive impairment. Therefore, it is important that conclusions with a significant implication are evaluated with a variety of approaches.”
The study received funding from the National Institutes of Health and the Canadian Institutes for Health Research. Dr. Yeates disclosed relationships with the American Psychological Association, Guilford Press, and Cambridge University Press. He has received grant funding from the Canadian Institutes of Health Research, the National Institutes of Health, Brain Canada Foundation, and the National Football League Scientific Advisory Board. He also has relationships with the National Institute for Child Health and Human Development, National Institute of Neurologic Disorders and Stroke, National Pediatric Rehabilitation Resource Center, Center for Pediatric Rehabilitation, and Virginia Tech University. Dr. Esser had no relevant relationships to disclose.
A version of this article appeared on Medscape.com.
data suggest.
In a multicenter study of almost 900 children with concussion or orthopedic injury, differences between groups in full-scale IQ (Cohen’s d = 0.13) and matrix reasoning scores (d = 0.16) were small.
“We draw the inference that IQ scores are unchanged, in the sense that they’re not different from [those of] kids with other types of injuries that don’t involve the brain,” said study author Keith Owen Yeates, PhD, Ronald and Irene Ward Chair in Pediatric Brain Injury and a professor of psychology at the University of Calgary (Alta.).
The study was published in Pediatrics.
A representative sample
The investigators analyzed data from two prospective cohort studies of children who were treated for concussion or mild orthopedic injury at two hospitals in the United States and five in Canada. Participants were aged 8-17 years and were recruited within 24 hours of the index event. Patients in the United States completed IQ and performance validity testing at 3-18 days after injury. Patients in Canada did so at 3 months after injury. The study used the short-form IQ test. The investigators included 866 children in their analysis.
Using linear modeling, Bayesian analysis, and multigroup factor analysis, the researchers found “very small group differences” in full-scale IQ scores between the two groups. Mean IQ was 104.95 for the concussion group and 106.08 for the orthopedic-injury group. Matrix reasoning scores were 52.28 and 53.81 for the concussion and orthopedic-injury groups, respectively.
Vocabulary scores did not differ between the two groups (53.25 for the concussion group and 53.27 for the orthopedic-injury group).
The study population is “pretty representative” from a demographic perspective, although it was predominantly White, said Dr. Yeates. “On the other hand, we did look at socioeconomic status, and that didn’t seem to alter the findings at all.”
The sample size is one of the study’s strengths, said Dr. Yeates. “Having 866 kids is far larger, I think, than just about any other study out there.” Drawing from seven children’s hospitals in North America is another strength. “Previous studies, in addition to having smaller samples, were from a single site and often recruited from a clinic population, not a representative group for a general population of kids with concussion.”
The findings must be interpreted precisely, however. “We don’t have actual preinjury data, so the more precise way of describing the findings is to say they’re not different from kids who are very similar to them demographically, have the same risk factors for injuries, and had a similar experience of a traumatic injury,” said Dr. Yeates. “The IQ scores for both groups are smack dab in the average range.”
Overall, the results are encouraging. “There’s been a lot of bad news in the media and in the science about concussion that worries patients, so it’s nice to be able to provide a little bit of balance,” said Dr. Yeates. “The message I give parents is that most kids recover within 2-4 weeks, and we’re much better now at predicting who’s going to [recover] and who isn’t, and that helps, too, so that we can focus our intervention on kids who are most at risk.”
Some children will have persisting symptoms, but evidence-based treatments are lacking. “I think that’ll be a really important direction for the future,” said Dr. Yeates.
Graduated return
Commenting on the findings, Michael Esser, MD, a pediatric neurologist at Alberta Children’s Hospital, Calgary, and an associate professor in pediatrics at the University of Calgary, said that they can help allay parents’ concerns about concussions. “It can also be of help for clinicians who want to have evidence to reassure families and promote a graduated return to activities. In particular, the study would support the philosophy of a graduated return to school or work, after a brief period of rest, following concussion.” Dr. Esser did not participate in the study.
The research is also noteworthy because it acknowledges that the differences in the design and methodology used in prior studies may explain the apparent disagreement over how concussion may influence cognitive function.
“This is an important message,” said Dr. Esser. “Families struggle with determining the merit of a lot of information due to the myriad of social media comments about concussion and the risk for cognitive impairment. Therefore, it is important that conclusions with a significant implication are evaluated with a variety of approaches.”
The study received funding from the National Institutes of Health and the Canadian Institutes for Health Research. Dr. Yeates disclosed relationships with the American Psychological Association, Guilford Press, and Cambridge University Press. He has received grant funding from the Canadian Institutes of Health Research, the National Institutes of Health, Brain Canada Foundation, and the National Football League Scientific Advisory Board. He also has relationships with the National Institute for Child Health and Human Development, National Institute of Neurologic Disorders and Stroke, National Pediatric Rehabilitation Resource Center, Center for Pediatric Rehabilitation, and Virginia Tech University. Dr. Esser had no relevant relationships to disclose.
A version of this article appeared on Medscape.com.
data suggest.
In a multicenter study of almost 900 children with concussion or orthopedic injury, differences between groups in full-scale IQ (Cohen’s d = 0.13) and matrix reasoning scores (d = 0.16) were small.
“We draw the inference that IQ scores are unchanged, in the sense that they’re not different from [those of] kids with other types of injuries that don’t involve the brain,” said study author Keith Owen Yeates, PhD, Ronald and Irene Ward Chair in Pediatric Brain Injury and a professor of psychology at the University of Calgary (Alta.).
The study was published in Pediatrics.
A representative sample
The investigators analyzed data from two prospective cohort studies of children who were treated for concussion or mild orthopedic injury at two hospitals in the United States and five in Canada. Participants were aged 8-17 years and were recruited within 24 hours of the index event. Patients in the United States completed IQ and performance validity testing at 3-18 days after injury. Patients in Canada did so at 3 months after injury. The study used the short-form IQ test. The investigators included 866 children in their analysis.
Using linear modeling, Bayesian analysis, and multigroup factor analysis, the researchers found “very small group differences” in full-scale IQ scores between the two groups. Mean IQ was 104.95 for the concussion group and 106.08 for the orthopedic-injury group. Matrix reasoning scores were 52.28 and 53.81 for the concussion and orthopedic-injury groups, respectively.
Vocabulary scores did not differ between the two groups (53.25 for the concussion group and 53.27 for the orthopedic-injury group).
The study population is “pretty representative” from a demographic perspective, although it was predominantly White, said Dr. Yeates. “On the other hand, we did look at socioeconomic status, and that didn’t seem to alter the findings at all.”
The sample size is one of the study’s strengths, said Dr. Yeates. “Having 866 kids is far larger, I think, than just about any other study out there.” Drawing from seven children’s hospitals in North America is another strength. “Previous studies, in addition to having smaller samples, were from a single site and often recruited from a clinic population, not a representative group for a general population of kids with concussion.”
The findings must be interpreted precisely, however. “We don’t have actual preinjury data, so the more precise way of describing the findings is to say they’re not different from kids who are very similar to them demographically, have the same risk factors for injuries, and had a similar experience of a traumatic injury,” said Dr. Yeates. “The IQ scores for both groups are smack dab in the average range.”
Overall, the results are encouraging. “There’s been a lot of bad news in the media and in the science about concussion that worries patients, so it’s nice to be able to provide a little bit of balance,” said Dr. Yeates. “The message I give parents is that most kids recover within 2-4 weeks, and we’re much better now at predicting who’s going to [recover] and who isn’t, and that helps, too, so that we can focus our intervention on kids who are most at risk.”
Some children will have persisting symptoms, but evidence-based treatments are lacking. “I think that’ll be a really important direction for the future,” said Dr. Yeates.
Graduated return
Commenting on the findings, Michael Esser, MD, a pediatric neurologist at Alberta Children’s Hospital, Calgary, and an associate professor in pediatrics at the University of Calgary, said that they can help allay parents’ concerns about concussions. “It can also be of help for clinicians who want to have evidence to reassure families and promote a graduated return to activities. In particular, the study would support the philosophy of a graduated return to school or work, after a brief period of rest, following concussion.” Dr. Esser did not participate in the study.
The research is also noteworthy because it acknowledges that the differences in the design and methodology used in prior studies may explain the apparent disagreement over how concussion may influence cognitive function.
“This is an important message,” said Dr. Esser. “Families struggle with determining the merit of a lot of information due to the myriad of social media comments about concussion and the risk for cognitive impairment. Therefore, it is important that conclusions with a significant implication are evaluated with a variety of approaches.”
The study received funding from the National Institutes of Health and the Canadian Institutes for Health Research. Dr. Yeates disclosed relationships with the American Psychological Association, Guilford Press, and Cambridge University Press. He has received grant funding from the Canadian Institutes of Health Research, the National Institutes of Health, Brain Canada Foundation, and the National Football League Scientific Advisory Board. He also has relationships with the National Institute for Child Health and Human Development, National Institute of Neurologic Disorders and Stroke, National Pediatric Rehabilitation Resource Center, Center for Pediatric Rehabilitation, and Virginia Tech University. Dr. Esser had no relevant relationships to disclose.
A version of this article appeared on Medscape.com.
FROM PEDIATRICS
Exercise program boosted physical, but not mental, health in young children with overweight
A defined exercise program significantly improved cardiometabolic health and body composition in children with overweight and obesity, but no effect was seen on mental health, based on data from 92 children.
Childhood obesity is associated with negative health outcomes including type 2 diabetes, cardiovascular disease, and mental health disorders, and exercise is considered essential to treatment, wrote Jairo H. Migueles, PhD, of the University of Granada, Spain, and colleagues. However, the effect on children with obesity and overweight of an exercise program on physical and mental health, including within-individual changes, has not been well studied, they said.
In a study published in JAMA Network Open, the researchers reviewed data from 36 girls and 56 boys with overweight or obesity who were randomized to a 20-week exercise program with aerobic and resistance elements, or waitlisted to serve as controls. The participants ranged in age from 8 to 11 years with a mean age of 10 years. The data were collected between Nov. 1, 2014, and June 30, 2016, as part of a parallel-group randomized clinical trial. The exercise program consisted of three to five 90-minute exercise sessions per week for 20 weeks, and the control children continued their usual routines.
The main cardiometabolic outcomes measured in the study were divided into three categories: body composition, physical fitness, and traditional risk factors (waist circumference, blood lipid levels, glucose levels, insulin levels, and blood pressure).
A cardiometabolic risk score was defined by z score. The researchers also added cardiorespiratory fitness (CRF) to the cardiometabolic risk score. Mental health was assessed using composite standardized scores for psychological well-being and poor mental health.
After 20 weeks, cardiometabolic risk scores decreased by approximately 0.38 standard deviations in the exercise group compared with the control group. In addition, specific measures of cardiometabolic health improved significantly from baseline in the exercise group compared with control children for low-density lipoprotein (change of –7.00 mg/dL), body mass index (–5.9 kg/m2), fat mass index (−0.67), and visceral adipose tissue (31.44 g).
Cardiorespiratory fitness improved by 2.75 laps in the exercise group compared with control children. In addition, significantly more children in the exercise group showed meaningful changes (defined as individual changes of at least 0.2 SDs) compared with control children in measures of fat mass index (37 vs. 17, P < .001) and CRF performance (30 vs. 17, P = .03).
However, no significant effects appeared on mental health outcomes in exercisers, the researchers noted.
The reduction in cardiometabolic score was attributable mainly to improvements in cardiovascular fitness, blood lipid levels, and total and visceral adiposity, the researchers wrote in their discussion. The lack of changes in mental health measures may be a result of the healthy mental state of the children at the study outset, they said. “The null effect on mental health outcomes needs to be further investigated, including, among other things, whether the instruments are sensitive enough to detect changes and whether there is a ceiling effect in young children who might be mentally healthy overall,” they wrote.
The findings were limited by several factors, including the relatively small sample size and lack of blinding for some evaluators. However, the results show the potential of exercise programs to affect meaningful change and improve cardiometabolic health in overweight and obese children, although more research is needed to explore the effects of larger-scale and longer-lasting public health interventions combining exercise and other health behaviors such as diet, the researchers concluded.
Bottom line: Exercise works
The increasing rates of overweight and obesity in children in the United States have “significant downstream consequences that include increased risk of metabolic disease, including diabetes and hypertension, as well as increased rates of anxiety and depression,” Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, said in an interview.
Therefore, the effect of interventions such as exercise training on outcomes is important, he said.
The current study findings are “what you would hope for and expect – improvement in cardiometabolic parameters and fitness,” said Dr. Skolnik. “It was encouraging to see the effect of this relatively short duration of intervention has a clear positive effect on weight, BMI, and cardiometabolic parameters,” he said. “The real benefit, of course, comes from sustaining these habits over a long period of time.”
The lack of improvement in mental health is not surprising given the small study population “who did not have a high rate of mental health problems to begin with,” Dr. Skolnik added.
Barriers to promoting exercise programs for obese and overweight children in primary care are many, Dr. Skolnik said, including “having the motivation and funding to create programs like this so they are readily available to youth.”
However, the key message from the current study is simple and straightforward, according to Dr. Skolnik. “Exercise works! It works to improve fitness, cardiometabolic parameters, and weight control,” he said.
“There is always room for more research,” Dr. Skolnik added. The questions now are not about whether exercise benefits health; they are about figuring out how to implement the known benefits of exercise into daily living for all children, athletes and nonathletes alike, he said. “We need to find nonjudgmental ways to encourage exercise as a part of routine daily healthy living, up there with brushing teeth every day,” he emphasized.
The study was supported by grants from the Spanish Ministry of Economy and Competitiveness and El Fondo Europeo de Desarrollo Regional (FEDER) and by the MCIN (Ministerio de Ciencia e Innovación) / AEI (Agencia Estatal de Investigación. The researchers and Dr. Skolnik had no financial conflicts to disclose. Dr. Skolnik serves on the editorial advisory board of Family Practice News.
A defined exercise program significantly improved cardiometabolic health and body composition in children with overweight and obesity, but no effect was seen on mental health, based on data from 92 children.
Childhood obesity is associated with negative health outcomes including type 2 diabetes, cardiovascular disease, and mental health disorders, and exercise is considered essential to treatment, wrote Jairo H. Migueles, PhD, of the University of Granada, Spain, and colleagues. However, the effect on children with obesity and overweight of an exercise program on physical and mental health, including within-individual changes, has not been well studied, they said.
In a study published in JAMA Network Open, the researchers reviewed data from 36 girls and 56 boys with overweight or obesity who were randomized to a 20-week exercise program with aerobic and resistance elements, or waitlisted to serve as controls. The participants ranged in age from 8 to 11 years with a mean age of 10 years. The data were collected between Nov. 1, 2014, and June 30, 2016, as part of a parallel-group randomized clinical trial. The exercise program consisted of three to five 90-minute exercise sessions per week for 20 weeks, and the control children continued their usual routines.
The main cardiometabolic outcomes measured in the study were divided into three categories: body composition, physical fitness, and traditional risk factors (waist circumference, blood lipid levels, glucose levels, insulin levels, and blood pressure).
A cardiometabolic risk score was defined by z score. The researchers also added cardiorespiratory fitness (CRF) to the cardiometabolic risk score. Mental health was assessed using composite standardized scores for psychological well-being and poor mental health.
After 20 weeks, cardiometabolic risk scores decreased by approximately 0.38 standard deviations in the exercise group compared with the control group. In addition, specific measures of cardiometabolic health improved significantly from baseline in the exercise group compared with control children for low-density lipoprotein (change of –7.00 mg/dL), body mass index (–5.9 kg/m2), fat mass index (−0.67), and visceral adipose tissue (31.44 g).
Cardiorespiratory fitness improved by 2.75 laps in the exercise group compared with control children. In addition, significantly more children in the exercise group showed meaningful changes (defined as individual changes of at least 0.2 SDs) compared with control children in measures of fat mass index (37 vs. 17, P < .001) and CRF performance (30 vs. 17, P = .03).
However, no significant effects appeared on mental health outcomes in exercisers, the researchers noted.
The reduction in cardiometabolic score was attributable mainly to improvements in cardiovascular fitness, blood lipid levels, and total and visceral adiposity, the researchers wrote in their discussion. The lack of changes in mental health measures may be a result of the healthy mental state of the children at the study outset, they said. “The null effect on mental health outcomes needs to be further investigated, including, among other things, whether the instruments are sensitive enough to detect changes and whether there is a ceiling effect in young children who might be mentally healthy overall,” they wrote.
The findings were limited by several factors, including the relatively small sample size and lack of blinding for some evaluators. However, the results show the potential of exercise programs to affect meaningful change and improve cardiometabolic health in overweight and obese children, although more research is needed to explore the effects of larger-scale and longer-lasting public health interventions combining exercise and other health behaviors such as diet, the researchers concluded.
Bottom line: Exercise works
The increasing rates of overweight and obesity in children in the United States have “significant downstream consequences that include increased risk of metabolic disease, including diabetes and hypertension, as well as increased rates of anxiety and depression,” Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, said in an interview.
Therefore, the effect of interventions such as exercise training on outcomes is important, he said.
The current study findings are “what you would hope for and expect – improvement in cardiometabolic parameters and fitness,” said Dr. Skolnik. “It was encouraging to see the effect of this relatively short duration of intervention has a clear positive effect on weight, BMI, and cardiometabolic parameters,” he said. “The real benefit, of course, comes from sustaining these habits over a long period of time.”
The lack of improvement in mental health is not surprising given the small study population “who did not have a high rate of mental health problems to begin with,” Dr. Skolnik added.
Barriers to promoting exercise programs for obese and overweight children in primary care are many, Dr. Skolnik said, including “having the motivation and funding to create programs like this so they are readily available to youth.”
However, the key message from the current study is simple and straightforward, according to Dr. Skolnik. “Exercise works! It works to improve fitness, cardiometabolic parameters, and weight control,” he said.
“There is always room for more research,” Dr. Skolnik added. The questions now are not about whether exercise benefits health; they are about figuring out how to implement the known benefits of exercise into daily living for all children, athletes and nonathletes alike, he said. “We need to find nonjudgmental ways to encourage exercise as a part of routine daily healthy living, up there with brushing teeth every day,” he emphasized.
The study was supported by grants from the Spanish Ministry of Economy and Competitiveness and El Fondo Europeo de Desarrollo Regional (FEDER) and by the MCIN (Ministerio de Ciencia e Innovación) / AEI (Agencia Estatal de Investigación. The researchers and Dr. Skolnik had no financial conflicts to disclose. Dr. Skolnik serves on the editorial advisory board of Family Practice News.
A defined exercise program significantly improved cardiometabolic health and body composition in children with overweight and obesity, but no effect was seen on mental health, based on data from 92 children.
Childhood obesity is associated with negative health outcomes including type 2 diabetes, cardiovascular disease, and mental health disorders, and exercise is considered essential to treatment, wrote Jairo H. Migueles, PhD, of the University of Granada, Spain, and colleagues. However, the effect on children with obesity and overweight of an exercise program on physical and mental health, including within-individual changes, has not been well studied, they said.
In a study published in JAMA Network Open, the researchers reviewed data from 36 girls and 56 boys with overweight or obesity who were randomized to a 20-week exercise program with aerobic and resistance elements, or waitlisted to serve as controls. The participants ranged in age from 8 to 11 years with a mean age of 10 years. The data were collected between Nov. 1, 2014, and June 30, 2016, as part of a parallel-group randomized clinical trial. The exercise program consisted of three to five 90-minute exercise sessions per week for 20 weeks, and the control children continued their usual routines.
The main cardiometabolic outcomes measured in the study were divided into three categories: body composition, physical fitness, and traditional risk factors (waist circumference, blood lipid levels, glucose levels, insulin levels, and blood pressure).
A cardiometabolic risk score was defined by z score. The researchers also added cardiorespiratory fitness (CRF) to the cardiometabolic risk score. Mental health was assessed using composite standardized scores for psychological well-being and poor mental health.
After 20 weeks, cardiometabolic risk scores decreased by approximately 0.38 standard deviations in the exercise group compared with the control group. In addition, specific measures of cardiometabolic health improved significantly from baseline in the exercise group compared with control children for low-density lipoprotein (change of –7.00 mg/dL), body mass index (–5.9 kg/m2), fat mass index (−0.67), and visceral adipose tissue (31.44 g).
Cardiorespiratory fitness improved by 2.75 laps in the exercise group compared with control children. In addition, significantly more children in the exercise group showed meaningful changes (defined as individual changes of at least 0.2 SDs) compared with control children in measures of fat mass index (37 vs. 17, P < .001) and CRF performance (30 vs. 17, P = .03).
However, no significant effects appeared on mental health outcomes in exercisers, the researchers noted.
The reduction in cardiometabolic score was attributable mainly to improvements in cardiovascular fitness, blood lipid levels, and total and visceral adiposity, the researchers wrote in their discussion. The lack of changes in mental health measures may be a result of the healthy mental state of the children at the study outset, they said. “The null effect on mental health outcomes needs to be further investigated, including, among other things, whether the instruments are sensitive enough to detect changes and whether there is a ceiling effect in young children who might be mentally healthy overall,” they wrote.
The findings were limited by several factors, including the relatively small sample size and lack of blinding for some evaluators. However, the results show the potential of exercise programs to affect meaningful change and improve cardiometabolic health in overweight and obese children, although more research is needed to explore the effects of larger-scale and longer-lasting public health interventions combining exercise and other health behaviors such as diet, the researchers concluded.
Bottom line: Exercise works
The increasing rates of overweight and obesity in children in the United States have “significant downstream consequences that include increased risk of metabolic disease, including diabetes and hypertension, as well as increased rates of anxiety and depression,” Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, said in an interview.
Therefore, the effect of interventions such as exercise training on outcomes is important, he said.
The current study findings are “what you would hope for and expect – improvement in cardiometabolic parameters and fitness,” said Dr. Skolnik. “It was encouraging to see the effect of this relatively short duration of intervention has a clear positive effect on weight, BMI, and cardiometabolic parameters,” he said. “The real benefit, of course, comes from sustaining these habits over a long period of time.”
The lack of improvement in mental health is not surprising given the small study population “who did not have a high rate of mental health problems to begin with,” Dr. Skolnik added.
Barriers to promoting exercise programs for obese and overweight children in primary care are many, Dr. Skolnik said, including “having the motivation and funding to create programs like this so they are readily available to youth.”
However, the key message from the current study is simple and straightforward, according to Dr. Skolnik. “Exercise works! It works to improve fitness, cardiometabolic parameters, and weight control,” he said.
“There is always room for more research,” Dr. Skolnik added. The questions now are not about whether exercise benefits health; they are about figuring out how to implement the known benefits of exercise into daily living for all children, athletes and nonathletes alike, he said. “We need to find nonjudgmental ways to encourage exercise as a part of routine daily healthy living, up there with brushing teeth every day,” he emphasized.
The study was supported by grants from the Spanish Ministry of Economy and Competitiveness and El Fondo Europeo de Desarrollo Regional (FEDER) and by the MCIN (Ministerio de Ciencia e Innovación) / AEI (Agencia Estatal de Investigación. The researchers and Dr. Skolnik had no financial conflicts to disclose. Dr. Skolnik serves on the editorial advisory board of Family Practice News.
FROM JAMA NETWORK OPEN
Case series supports targeted drugs in treatment of alopecia in children with AD
in children with AA and concomitant atopy.
It was only a little over a year ago that the JAK inhibitor baricitinib became the first systemic therapy approved by the Food and Drug Administration for AA in adults. In June 2023, the JAK inhibitor ritlecitinib was approved for severe AA in patients as young as 12 years of age, but there is accumulating evidence that dupilumab, which binds to the interleukin-4 receptor, might be an option for even younger children with AA.
Of those who have worked with dupilumab for controlling AA in children, Brittany Craiglow, MD, an adjunct associate professor of dermatology at Yale University, New Haven, Conn., updated a case series at the recent MedscapeLive! Annual Women’s and Pediatric Dermatology Seminar in Baltimore. A series of six children with AA treated with dupilumab was published 2 years ago in JAAD Case Reports.
Even in 2021, her case series was not the first report of benefit from dupilumab in children with AA, but instead contributed to a “growing body of literature” supporting the potential benefit in the setting of concomitant atopy, Dr. Craiglow, one of the authors of the series, said in an interview.
Of the six patients in that series, five had improvement and four had complete regrowth with dupilumab, whether as a monotherapy or in combination with other agents. The children ranged in age from 7 to 12 years. The age range at the time of AA onset was 3-11 years. All had atopic dermatitis (AD) and most had additional atopic conditions, such as food allergies or asthma.
Since publication, Dr. Craiglow has successfully treated many more patients with dupilumab, either as monotherapy or in combination with oral minoxidil, corticosteroids, and/or a topical JAK inhibitor. Dupilumab, which is approved for the treatment of AD in children as young as 6 months of age, has been well tolerated.
“Oral minoxidil is often a great adjuvant treatment in patients with AA and should be used unless there are contraindications,” based on the initial and subsequent experience treating AA with dupilumab, said Dr. Craiglow.
“Topical steroids can be used in combination with dupilumab and minoxidil, but in general dupilumab should not be combined with an oral JAK inhibitor,” she added.
Now, with the approval of ritlecitinib, Dr. Craiglow said this JAK inhibitor will become a first-line therapy in children 12 years or older with severe, persistent AA, but she considers a trial of dupilumab reasonable in younger children, given the controlled studies of safety for atopic diseases.
“I would say that dupilumab could be considered in the following clinical scenarios: children under 12 with AA and concomitant atopy, such as atopic dermatitis, asthma, allergies, and/or elevated IgE; and children over the age of 12 with concomitant atopy who either have a contraindication to a JAK inhibitor or whose families have reservations about or are unwilling to take one,” Dr. Craiglow said.
In older children, she believes that dupilumab has “a much lower chance of being effective” than an oral JAK inhibitor like ritlecitinib, but it circumvents the potential safety issues of JAK inhibitors that have been observed in adults.
With ritlecitinib providing an on-label option for AA in older children, Dr. Craiglow suggested it might be easier to obtain third-party coverage for dupilumab as an alternative to a JAK inhibitor for AA in patients younger than 12, particularly when there is an indication for a concomitant atopic condition and a rationale, such as a concern about relative safety.
Two years ago, when Dr. Craiglow and her coinvestigator published their six-patient case series, a second case series was published about the same time by investigators at the University of Pennsylvania, Philadelphia, in the Journal of the American Academy of Dermatology. This series of 16 pediatric patients with AA on dupilumab was more heterogeneous, but four of six patients with active disease and more than 4 months of follow-up had improvement in AA, including total regrowth. The improvement was concentrated in patients with moderate to severe AD at the time of treatment.
Based on this series, the authors, led by Leslie Castelo-Soccio, MD, PhD, who is now an attending physician in the Dermatology Branch of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, Md., concluded that dupilumab “may be a therapeutic option for AA” when traditional therapies have failed, “especially in patients with concurrent AD or asthma, for which the benefits of dupilumab are clear.”
When contacted about where this therapy might fit on the basis of her case series and the update on Dr. Craiglow’s experience, Dr. Castelo-Soccio, like Dr. Craiglow, stressed the importance of employing this therapy selectively.
“I do think that dupilumab is a reasonable option for AA in children with atopy and IgE levels greater than 200 IU/mL, especially if treatment is for atopic dermatitis or asthma as well,” she said.
Many clinicians, including Dr. Craiglow, have experience with oral JAK inhibitors in children younger than 12. Indeed, a recently published case study associated oral abrocitinib, a JAK inhibitor approved for moderate to severe AD in patients ages 12 and older, with hair regrowth in an 11-year-old child who had persistent AA for more than 6 years despite numerous conventional therapies.
However, the advantage of dupilumab in younger children is the greater evidence of safety, providing a level of reassurance for a treatment that is commonly used for severe atopic diseases but does not have a specific indication for AA, according to Dr. Craiglow.
Dr. Craiglow disclosed being a speaker for AbbVie and a speaker and consultant for Eli Lilly, Incyte, Pfizer, Regeneron, and Sanofi Genzyme. Dr. Castelo-Soccio had no disclosures.
in children with AA and concomitant atopy.
It was only a little over a year ago that the JAK inhibitor baricitinib became the first systemic therapy approved by the Food and Drug Administration for AA in adults. In June 2023, the JAK inhibitor ritlecitinib was approved for severe AA in patients as young as 12 years of age, but there is accumulating evidence that dupilumab, which binds to the interleukin-4 receptor, might be an option for even younger children with AA.
Of those who have worked with dupilumab for controlling AA in children, Brittany Craiglow, MD, an adjunct associate professor of dermatology at Yale University, New Haven, Conn., updated a case series at the recent MedscapeLive! Annual Women’s and Pediatric Dermatology Seminar in Baltimore. A series of six children with AA treated with dupilumab was published 2 years ago in JAAD Case Reports.
Even in 2021, her case series was not the first report of benefit from dupilumab in children with AA, but instead contributed to a “growing body of literature” supporting the potential benefit in the setting of concomitant atopy, Dr. Craiglow, one of the authors of the series, said in an interview.
Of the six patients in that series, five had improvement and four had complete regrowth with dupilumab, whether as a monotherapy or in combination with other agents. The children ranged in age from 7 to 12 years. The age range at the time of AA onset was 3-11 years. All had atopic dermatitis (AD) and most had additional atopic conditions, such as food allergies or asthma.
Since publication, Dr. Craiglow has successfully treated many more patients with dupilumab, either as monotherapy or in combination with oral minoxidil, corticosteroids, and/or a topical JAK inhibitor. Dupilumab, which is approved for the treatment of AD in children as young as 6 months of age, has been well tolerated.
“Oral minoxidil is often a great adjuvant treatment in patients with AA and should be used unless there are contraindications,” based on the initial and subsequent experience treating AA with dupilumab, said Dr. Craiglow.
“Topical steroids can be used in combination with dupilumab and minoxidil, but in general dupilumab should not be combined with an oral JAK inhibitor,” she added.
Now, with the approval of ritlecitinib, Dr. Craiglow said this JAK inhibitor will become a first-line therapy in children 12 years or older with severe, persistent AA, but she considers a trial of dupilumab reasonable in younger children, given the controlled studies of safety for atopic diseases.
“I would say that dupilumab could be considered in the following clinical scenarios: children under 12 with AA and concomitant atopy, such as atopic dermatitis, asthma, allergies, and/or elevated IgE; and children over the age of 12 with concomitant atopy who either have a contraindication to a JAK inhibitor or whose families have reservations about or are unwilling to take one,” Dr. Craiglow said.
In older children, she believes that dupilumab has “a much lower chance of being effective” than an oral JAK inhibitor like ritlecitinib, but it circumvents the potential safety issues of JAK inhibitors that have been observed in adults.
With ritlecitinib providing an on-label option for AA in older children, Dr. Craiglow suggested it might be easier to obtain third-party coverage for dupilumab as an alternative to a JAK inhibitor for AA in patients younger than 12, particularly when there is an indication for a concomitant atopic condition and a rationale, such as a concern about relative safety.
Two years ago, when Dr. Craiglow and her coinvestigator published their six-patient case series, a second case series was published about the same time by investigators at the University of Pennsylvania, Philadelphia, in the Journal of the American Academy of Dermatology. This series of 16 pediatric patients with AA on dupilumab was more heterogeneous, but four of six patients with active disease and more than 4 months of follow-up had improvement in AA, including total regrowth. The improvement was concentrated in patients with moderate to severe AD at the time of treatment.
Based on this series, the authors, led by Leslie Castelo-Soccio, MD, PhD, who is now an attending physician in the Dermatology Branch of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, Md., concluded that dupilumab “may be a therapeutic option for AA” when traditional therapies have failed, “especially in patients with concurrent AD or asthma, for which the benefits of dupilumab are clear.”
When contacted about where this therapy might fit on the basis of her case series and the update on Dr. Craiglow’s experience, Dr. Castelo-Soccio, like Dr. Craiglow, stressed the importance of employing this therapy selectively.
“I do think that dupilumab is a reasonable option for AA in children with atopy and IgE levels greater than 200 IU/mL, especially if treatment is for atopic dermatitis or asthma as well,” she said.
Many clinicians, including Dr. Craiglow, have experience with oral JAK inhibitors in children younger than 12. Indeed, a recently published case study associated oral abrocitinib, a JAK inhibitor approved for moderate to severe AD in patients ages 12 and older, with hair regrowth in an 11-year-old child who had persistent AA for more than 6 years despite numerous conventional therapies.
However, the advantage of dupilumab in younger children is the greater evidence of safety, providing a level of reassurance for a treatment that is commonly used for severe atopic diseases but does not have a specific indication for AA, according to Dr. Craiglow.
Dr. Craiglow disclosed being a speaker for AbbVie and a speaker and consultant for Eli Lilly, Incyte, Pfizer, Regeneron, and Sanofi Genzyme. Dr. Castelo-Soccio had no disclosures.
in children with AA and concomitant atopy.
It was only a little over a year ago that the JAK inhibitor baricitinib became the first systemic therapy approved by the Food and Drug Administration for AA in adults. In June 2023, the JAK inhibitor ritlecitinib was approved for severe AA in patients as young as 12 years of age, but there is accumulating evidence that dupilumab, which binds to the interleukin-4 receptor, might be an option for even younger children with AA.
Of those who have worked with dupilumab for controlling AA in children, Brittany Craiglow, MD, an adjunct associate professor of dermatology at Yale University, New Haven, Conn., updated a case series at the recent MedscapeLive! Annual Women’s and Pediatric Dermatology Seminar in Baltimore. A series of six children with AA treated with dupilumab was published 2 years ago in JAAD Case Reports.
Even in 2021, her case series was not the first report of benefit from dupilumab in children with AA, but instead contributed to a “growing body of literature” supporting the potential benefit in the setting of concomitant atopy, Dr. Craiglow, one of the authors of the series, said in an interview.
Of the six patients in that series, five had improvement and four had complete regrowth with dupilumab, whether as a monotherapy or in combination with other agents. The children ranged in age from 7 to 12 years. The age range at the time of AA onset was 3-11 years. All had atopic dermatitis (AD) and most had additional atopic conditions, such as food allergies or asthma.
Since publication, Dr. Craiglow has successfully treated many more patients with dupilumab, either as monotherapy or in combination with oral minoxidil, corticosteroids, and/or a topical JAK inhibitor. Dupilumab, which is approved for the treatment of AD in children as young as 6 months of age, has been well tolerated.
“Oral minoxidil is often a great adjuvant treatment in patients with AA and should be used unless there are contraindications,” based on the initial and subsequent experience treating AA with dupilumab, said Dr. Craiglow.
“Topical steroids can be used in combination with dupilumab and minoxidil, but in general dupilumab should not be combined with an oral JAK inhibitor,” she added.
Now, with the approval of ritlecitinib, Dr. Craiglow said this JAK inhibitor will become a first-line therapy in children 12 years or older with severe, persistent AA, but she considers a trial of dupilumab reasonable in younger children, given the controlled studies of safety for atopic diseases.
“I would say that dupilumab could be considered in the following clinical scenarios: children under 12 with AA and concomitant atopy, such as atopic dermatitis, asthma, allergies, and/or elevated IgE; and children over the age of 12 with concomitant atopy who either have a contraindication to a JAK inhibitor or whose families have reservations about or are unwilling to take one,” Dr. Craiglow said.
In older children, she believes that dupilumab has “a much lower chance of being effective” than an oral JAK inhibitor like ritlecitinib, but it circumvents the potential safety issues of JAK inhibitors that have been observed in adults.
With ritlecitinib providing an on-label option for AA in older children, Dr. Craiglow suggested it might be easier to obtain third-party coverage for dupilumab as an alternative to a JAK inhibitor for AA in patients younger than 12, particularly when there is an indication for a concomitant atopic condition and a rationale, such as a concern about relative safety.
Two years ago, when Dr. Craiglow and her coinvestigator published their six-patient case series, a second case series was published about the same time by investigators at the University of Pennsylvania, Philadelphia, in the Journal of the American Academy of Dermatology. This series of 16 pediatric patients with AA on dupilumab was more heterogeneous, but four of six patients with active disease and more than 4 months of follow-up had improvement in AA, including total regrowth. The improvement was concentrated in patients with moderate to severe AD at the time of treatment.
Based on this series, the authors, led by Leslie Castelo-Soccio, MD, PhD, who is now an attending physician in the Dermatology Branch of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, Md., concluded that dupilumab “may be a therapeutic option for AA” when traditional therapies have failed, “especially in patients with concurrent AD or asthma, for which the benefits of dupilumab are clear.”
When contacted about where this therapy might fit on the basis of her case series and the update on Dr. Craiglow’s experience, Dr. Castelo-Soccio, like Dr. Craiglow, stressed the importance of employing this therapy selectively.
“I do think that dupilumab is a reasonable option for AA in children with atopy and IgE levels greater than 200 IU/mL, especially if treatment is for atopic dermatitis or asthma as well,” she said.
Many clinicians, including Dr. Craiglow, have experience with oral JAK inhibitors in children younger than 12. Indeed, a recently published case study associated oral abrocitinib, a JAK inhibitor approved for moderate to severe AD in patients ages 12 and older, with hair regrowth in an 11-year-old child who had persistent AA for more than 6 years despite numerous conventional therapies.
However, the advantage of dupilumab in younger children is the greater evidence of safety, providing a level of reassurance for a treatment that is commonly used for severe atopic diseases but does not have a specific indication for AA, according to Dr. Craiglow.
Dr. Craiglow disclosed being a speaker for AbbVie and a speaker and consultant for Eli Lilly, Incyte, Pfizer, Regeneron, and Sanofi Genzyme. Dr. Castelo-Soccio had no disclosures.
New guidelines for laser treatment of cutaneous vascular anomalies
A new practice guideline is setting a standard for doctors who use lasers to treat cutaneous vascular anomalies.
Poor treatment has been an issue in this field because no uniform guidelines existed to inform practice, according to a press release from the American Society for Laser Medicine and Surgery.
The laser treatment settings can vary based on the type and location of the birthmark and also the patient’s skin type, which has resulted in an inconsistent approach from clinicians, according to the release.
“For decades, I have observed adverse outcomes from the improper laser treatment of vascular birthmarks,” Linda Rozell-Shannon, PhD, president and founder of the Vascular Birthmarks Foundation said in a statement from ASLMS. “As a result of these guidelines, patient outcomes will be improved.”
The guideline, published on the ASLMS website along with supporting videos, was jointly developed by ASLMS, VBF, and an international group of clinicians, marking the first consensus guideline on laser treatments for cutaneous vascular anomalies. It details 32 best practice directives for various scenarios, including advice on safety considerations, additional testing, and when to refer.
“It is important to realize that just because someone is board certified does not mean they are skilled in treating all conditions or using all lasers,” Paul Friedman, MD, a dermatologist in Houston, and former president of ASLMS, said in the ASLMS statement.
Vascular birthmarks are a common condition affecting up to 14% of children, according to VBF. Most are hemangiomas, a buildup of blood vessels that usually appears at birth or within a month after birth. Laser therapy reduces the size and color of the anomalies.
Support for this initiative was provided by Candela Medical.
A version of this article first appeared on Medscape.com.
A new practice guideline is setting a standard for doctors who use lasers to treat cutaneous vascular anomalies.
Poor treatment has been an issue in this field because no uniform guidelines existed to inform practice, according to a press release from the American Society for Laser Medicine and Surgery.
The laser treatment settings can vary based on the type and location of the birthmark and also the patient’s skin type, which has resulted in an inconsistent approach from clinicians, according to the release.
“For decades, I have observed adverse outcomes from the improper laser treatment of vascular birthmarks,” Linda Rozell-Shannon, PhD, president and founder of the Vascular Birthmarks Foundation said in a statement from ASLMS. “As a result of these guidelines, patient outcomes will be improved.”
The guideline, published on the ASLMS website along with supporting videos, was jointly developed by ASLMS, VBF, and an international group of clinicians, marking the first consensus guideline on laser treatments for cutaneous vascular anomalies. It details 32 best practice directives for various scenarios, including advice on safety considerations, additional testing, and when to refer.
“It is important to realize that just because someone is board certified does not mean they are skilled in treating all conditions or using all lasers,” Paul Friedman, MD, a dermatologist in Houston, and former president of ASLMS, said in the ASLMS statement.
Vascular birthmarks are a common condition affecting up to 14% of children, according to VBF. Most are hemangiomas, a buildup of blood vessels that usually appears at birth or within a month after birth. Laser therapy reduces the size and color of the anomalies.
Support for this initiative was provided by Candela Medical.
A version of this article first appeared on Medscape.com.
A new practice guideline is setting a standard for doctors who use lasers to treat cutaneous vascular anomalies.
Poor treatment has been an issue in this field because no uniform guidelines existed to inform practice, according to a press release from the American Society for Laser Medicine and Surgery.
The laser treatment settings can vary based on the type and location of the birthmark and also the patient’s skin type, which has resulted in an inconsistent approach from clinicians, according to the release.
“For decades, I have observed adverse outcomes from the improper laser treatment of vascular birthmarks,” Linda Rozell-Shannon, PhD, president and founder of the Vascular Birthmarks Foundation said in a statement from ASLMS. “As a result of these guidelines, patient outcomes will be improved.”
The guideline, published on the ASLMS website along with supporting videos, was jointly developed by ASLMS, VBF, and an international group of clinicians, marking the first consensus guideline on laser treatments for cutaneous vascular anomalies. It details 32 best practice directives for various scenarios, including advice on safety considerations, additional testing, and when to refer.
“It is important to realize that just because someone is board certified does not mean they are skilled in treating all conditions or using all lasers,” Paul Friedman, MD, a dermatologist in Houston, and former president of ASLMS, said in the ASLMS statement.
Vascular birthmarks are a common condition affecting up to 14% of children, according to VBF. Most are hemangiomas, a buildup of blood vessels that usually appears at birth or within a month after birth. Laser therapy reduces the size and color of the anomalies.
Support for this initiative was provided by Candela Medical.
A version of this article first appeared on Medscape.com.
Progress seen on five fronts for substantially improving treatment of epidermolysis bullosa
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
AT SPD 2023
Does screening kids with acute sinusitis symptoms for bacterial infection cut unnecessary antibiotic use?
Testing children with acute sinusitis symptoms for specific bacteria may dramatically decrease unnecessary antibiotic use, new research suggests.
The study, published in JAMA, found that children with positive nasopharyngeal tests for one or more of Haemophilus influenzae, Streptococcus pneumoniae, or Moraxella catarrhalis had better resolution of symptoms with antibiotics than those without these bacteria.
If antibiotic use was limited to children with H. influenzae or S. pneumoniae in their nasopharynx at the time of diagnosis, antibiotic use would decrease by 53%, according to the study authors.
Sinusitis is common in children, and symptoms are similar with uncomplicated viral upper respiratory infections.
“We have not had a good way to predict which children will benefit from antibiotics,” said Nader Shaikh, MD, MPH, professor of pediatrics and clinical and translational science at the University of Pittsburgh, and the lead study author. “When a child comes in with a sore throat, we test for strep. If the test is positive, we prescribe antibiotics.”
Dr. Shaikh and his colleagues found that the same approach – swabbing the nose and testing for various bacteria – worked for children with sinusitis.
“Children who tested negative for bacteria did not benefit from antibiotics,” Dr. Shaikh said.
In the double-blind clinical trial, Dr. Shaikh and his colleagues randomized 510 children between ages 2 and 11 with acute sinusitis at six academic primary care offices over a 6-year period. Almost two-thirds of participants were between ages 2 and 5, around half were male, and around half were White. All participants had an initial score of nine or higher on the validated Pediatric Rhinosinusitis Symptom Scale (PRSS).
For 10 days, 254 children received oral amoxicillin (90 mg/kg/day) and clavulanate (6.4mg/kg/day) and 256 received placebo.
In children receiving antibiotics, symptoms resolved over a median of 7 days, compared with 9 days for those given placebo (P = .003).
Children without detected nasopharyngeal pathogens did not benefit from antibiotics as much as those with the pathogens, the researchers found. Among those with pathogens, the mean symptom burden score was 1.95 points lower in the group that received antibiotics, compared with the group that received placebo. For those without pathogens, there was a 0.88-point difference between the antibiotic and placebo groups (P = .02).
The researchers also took nasal swabs at the first and final study visits and tested for S. pneumoniae, H. influenzae, and M. catarrhalis. During that time, parents or caregivers used the PRSS to assess their child’s symptoms, and they recorded the nasal discharge color. Nasal discharge color, Dr. Shaikh and colleagues found, was not linked with antibiotic effect.
Welcome findings
Pediatricians and primary care providers face a significant clinical dilemma when they consider using antibiotics with upper respiratory tract infections (URTIs), according to John H. Greinwald Jr., MD, professor in the department of pediatrics at Cincinnati Children’s Hospital Medical Center.
“These findings certainly make sense because most respiratory infections in children are viral,” Dr. Greinwald said. “The investigators follow the appropriate clinical guidelines for considering antibiotic use in patients with URTIs, which include URTI symptoms lasting longer than 10 days or symptoms initially getting better, then worsening again day 6 through 10.”
Not only is antibiotic resistance a major public health concern, but the drugs can have side effects such as diarrhea, and their long-term effects on the microbiome are unknown.
“Differentiating who has acute sinusitis from who has a viral infection is difficult for primary care providers,” said Eelam A. Adil, MD, MBA, assistant professor of otolaryngology at Harvard Medical School in Boston.
The findings may help clinicians be more selective with antibiotic prescriptions, according to Jacob G. Eide, MD, a head and neck surgeon at Henry Ford Health in Detroit.
“However, we do not want to deny antibiotics when they are beneficial,” Dr. Eide said. “And the difficulty and costs involved in developing the tests need to be considered.”
Dr. Shaikh and his team are studying ways to bring nasal testing into clinical practice, potentially utilizing commercially available molecular testing and rapid antigen tests that work like COVID-19 at-home tests. They are also exploring if other biomarkers in nasal discharge may indicate the presence of bacteria.
All study authors as well as outside experts reported no relevant financial relationships. The study was supported by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
Testing children with acute sinusitis symptoms for specific bacteria may dramatically decrease unnecessary antibiotic use, new research suggests.
The study, published in JAMA, found that children with positive nasopharyngeal tests for one or more of Haemophilus influenzae, Streptococcus pneumoniae, or Moraxella catarrhalis had better resolution of symptoms with antibiotics than those without these bacteria.
If antibiotic use was limited to children with H. influenzae or S. pneumoniae in their nasopharynx at the time of diagnosis, antibiotic use would decrease by 53%, according to the study authors.
Sinusitis is common in children, and symptoms are similar with uncomplicated viral upper respiratory infections.
“We have not had a good way to predict which children will benefit from antibiotics,” said Nader Shaikh, MD, MPH, professor of pediatrics and clinical and translational science at the University of Pittsburgh, and the lead study author. “When a child comes in with a sore throat, we test for strep. If the test is positive, we prescribe antibiotics.”
Dr. Shaikh and his colleagues found that the same approach – swabbing the nose and testing for various bacteria – worked for children with sinusitis.
“Children who tested negative for bacteria did not benefit from antibiotics,” Dr. Shaikh said.
In the double-blind clinical trial, Dr. Shaikh and his colleagues randomized 510 children between ages 2 and 11 with acute sinusitis at six academic primary care offices over a 6-year period. Almost two-thirds of participants were between ages 2 and 5, around half were male, and around half were White. All participants had an initial score of nine or higher on the validated Pediatric Rhinosinusitis Symptom Scale (PRSS).
For 10 days, 254 children received oral amoxicillin (90 mg/kg/day) and clavulanate (6.4mg/kg/day) and 256 received placebo.
In children receiving antibiotics, symptoms resolved over a median of 7 days, compared with 9 days for those given placebo (P = .003).
Children without detected nasopharyngeal pathogens did not benefit from antibiotics as much as those with the pathogens, the researchers found. Among those with pathogens, the mean symptom burden score was 1.95 points lower in the group that received antibiotics, compared with the group that received placebo. For those without pathogens, there was a 0.88-point difference between the antibiotic and placebo groups (P = .02).
The researchers also took nasal swabs at the first and final study visits and tested for S. pneumoniae, H. influenzae, and M. catarrhalis. During that time, parents or caregivers used the PRSS to assess their child’s symptoms, and they recorded the nasal discharge color. Nasal discharge color, Dr. Shaikh and colleagues found, was not linked with antibiotic effect.
Welcome findings
Pediatricians and primary care providers face a significant clinical dilemma when they consider using antibiotics with upper respiratory tract infections (URTIs), according to John H. Greinwald Jr., MD, professor in the department of pediatrics at Cincinnati Children’s Hospital Medical Center.
“These findings certainly make sense because most respiratory infections in children are viral,” Dr. Greinwald said. “The investigators follow the appropriate clinical guidelines for considering antibiotic use in patients with URTIs, which include URTI symptoms lasting longer than 10 days or symptoms initially getting better, then worsening again day 6 through 10.”
Not only is antibiotic resistance a major public health concern, but the drugs can have side effects such as diarrhea, and their long-term effects on the microbiome are unknown.
“Differentiating who has acute sinusitis from who has a viral infection is difficult for primary care providers,” said Eelam A. Adil, MD, MBA, assistant professor of otolaryngology at Harvard Medical School in Boston.
The findings may help clinicians be more selective with antibiotic prescriptions, according to Jacob G. Eide, MD, a head and neck surgeon at Henry Ford Health in Detroit.
“However, we do not want to deny antibiotics when they are beneficial,” Dr. Eide said. “And the difficulty and costs involved in developing the tests need to be considered.”
Dr. Shaikh and his team are studying ways to bring nasal testing into clinical practice, potentially utilizing commercially available molecular testing and rapid antigen tests that work like COVID-19 at-home tests. They are also exploring if other biomarkers in nasal discharge may indicate the presence of bacteria.
All study authors as well as outside experts reported no relevant financial relationships. The study was supported by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
Testing children with acute sinusitis symptoms for specific bacteria may dramatically decrease unnecessary antibiotic use, new research suggests.
The study, published in JAMA, found that children with positive nasopharyngeal tests for one or more of Haemophilus influenzae, Streptococcus pneumoniae, or Moraxella catarrhalis had better resolution of symptoms with antibiotics than those without these bacteria.
If antibiotic use was limited to children with H. influenzae or S. pneumoniae in their nasopharynx at the time of diagnosis, antibiotic use would decrease by 53%, according to the study authors.
Sinusitis is common in children, and symptoms are similar with uncomplicated viral upper respiratory infections.
“We have not had a good way to predict which children will benefit from antibiotics,” said Nader Shaikh, MD, MPH, professor of pediatrics and clinical and translational science at the University of Pittsburgh, and the lead study author. “When a child comes in with a sore throat, we test for strep. If the test is positive, we prescribe antibiotics.”
Dr. Shaikh and his colleagues found that the same approach – swabbing the nose and testing for various bacteria – worked for children with sinusitis.
“Children who tested negative for bacteria did not benefit from antibiotics,” Dr. Shaikh said.
In the double-blind clinical trial, Dr. Shaikh and his colleagues randomized 510 children between ages 2 and 11 with acute sinusitis at six academic primary care offices over a 6-year period. Almost two-thirds of participants were between ages 2 and 5, around half were male, and around half were White. All participants had an initial score of nine or higher on the validated Pediatric Rhinosinusitis Symptom Scale (PRSS).
For 10 days, 254 children received oral amoxicillin (90 mg/kg/day) and clavulanate (6.4mg/kg/day) and 256 received placebo.
In children receiving antibiotics, symptoms resolved over a median of 7 days, compared with 9 days for those given placebo (P = .003).
Children without detected nasopharyngeal pathogens did not benefit from antibiotics as much as those with the pathogens, the researchers found. Among those with pathogens, the mean symptom burden score was 1.95 points lower in the group that received antibiotics, compared with the group that received placebo. For those without pathogens, there was a 0.88-point difference between the antibiotic and placebo groups (P = .02).
The researchers also took nasal swabs at the first and final study visits and tested for S. pneumoniae, H. influenzae, and M. catarrhalis. During that time, parents or caregivers used the PRSS to assess their child’s symptoms, and they recorded the nasal discharge color. Nasal discharge color, Dr. Shaikh and colleagues found, was not linked with antibiotic effect.
Welcome findings
Pediatricians and primary care providers face a significant clinical dilemma when they consider using antibiotics with upper respiratory tract infections (URTIs), according to John H. Greinwald Jr., MD, professor in the department of pediatrics at Cincinnati Children’s Hospital Medical Center.
“These findings certainly make sense because most respiratory infections in children are viral,” Dr. Greinwald said. “The investigators follow the appropriate clinical guidelines for considering antibiotic use in patients with URTIs, which include URTI symptoms lasting longer than 10 days or symptoms initially getting better, then worsening again day 6 through 10.”
Not only is antibiotic resistance a major public health concern, but the drugs can have side effects such as diarrhea, and their long-term effects on the microbiome are unknown.
“Differentiating who has acute sinusitis from who has a viral infection is difficult for primary care providers,” said Eelam A. Adil, MD, MBA, assistant professor of otolaryngology at Harvard Medical School in Boston.
The findings may help clinicians be more selective with antibiotic prescriptions, according to Jacob G. Eide, MD, a head and neck surgeon at Henry Ford Health in Detroit.
“However, we do not want to deny antibiotics when they are beneficial,” Dr. Eide said. “And the difficulty and costs involved in developing the tests need to be considered.”
Dr. Shaikh and his team are studying ways to bring nasal testing into clinical practice, potentially utilizing commercially available molecular testing and rapid antigen tests that work like COVID-19 at-home tests. They are also exploring if other biomarkers in nasal discharge may indicate the presence of bacteria.
All study authors as well as outside experts reported no relevant financial relationships. The study was supported by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
FROM JAMA





