User login
Scarred med student inspired by dermatologist who treated her
It’s not uncommon for a medical student to change specialty plans. For Jamie Harris, a second-year student at the University of Florida School of Medicine, Gainesville, that decision came as the result of a vicious dog and an empathetic doctor.
, a New York dermatologist whose approach involves early and aggressive treatment. After treating her, Dr. Bhanusali offered to have Ms. Harris shadow him.
She returned to school to shadow other dermatologists and to research the specialty before taking Dr. Bhanusali up on his offer. Ms. Harris sat in on procedures and meetings with patients and studied Dr. Bhanusali’s approach to the specialty. “I just fell in love with dermatology,” Ms. Harris told this news organization. “I knew that what I wanted for my own career was exactly how he runs his practice and how he treats patients.”
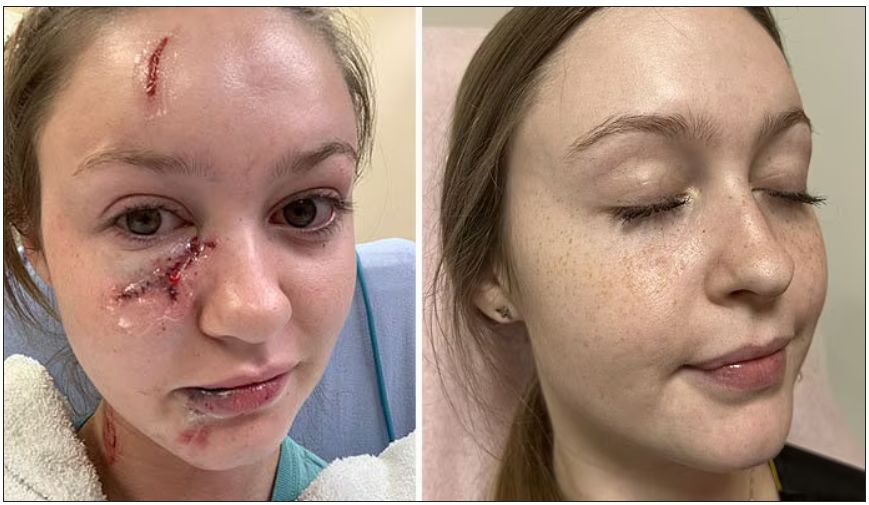
Life-changing injury
In 2020, Ms. Harris was a sophomore in the University of Florida’s medical honors program, an accelerated track that allows students to earn both a bachelor of science degree and a doctor of medicine degree in 7 years. She had finished studying at a friend’s apartment and was watching television when the rescue dog the friend adopted lunged at Ms. Harris, biting her on the face. “I was just cowering in the corner of the couch,” she recalls. “I didn’t go into fight-or-flight mode; I just went into hide mode.”
After receiving stitches in the emergency department, she visited several dermatologists and plastic surgeons for further treatment. There was scarring from her forehead to her chin, which was particularly severe on her upper cheek just under her eye. But because there was no infection or medical problems, the doctors turned her away. “They said, ‘OK, you look great.’ I did not look great,” she said.
Ms. Harris’ doctors advised her to wait a year before starting treatment for the scarring, a traditional approach. She was frustrated. “At the time, I was interested in becoming a pediatrician and thought, ‘No kid is going to want me as their doctor.’ ” But she accepted the medical advice – until her mother remembered a news story she’d seen.
Bridger Walker, a 6-year-old Wyoming boy, made headlines when he saved his younger sister from a dog that was attacking, but he was bitten multiple times as a result. Dr. Bhanusali treated the boy’s scarring.
Ms. Harris and her mother contacted the doctor, and after meeting via Zoom, Dr. Bhanusali agreed to treat her right away. He used lasers to resurface the skin, which created a suitable foundation for the scar cream, and he administered steroid injections to soften the scar tissue.
‘I see you’
Dr. Bhansali said he was impressed with the young student he treated. “There’s curiosity, and then there’s genuine passion. She has the latter,” he said in an interview. “Having gone through this, she will understand the value of research and keeping up with the literature and that just because something is being done a certain way today doesn’t mean it has to be that way tomorrow.”
Ms. Harris agrees that the experience will make her a better dermatologist. “One of the best parts about dermatology is that you can see your results in real time and really see what’s working and what’s not working. The potential for innovation is just amazing.”
But Ms. Harris believes she also gained empathy with dermatology patients. “I know exactly what it’s like to look in the mirror and not even recognize yourself, just have your eyes go straight to one thing and feel like the whole world is staring at you,” she said. “I’ll be able to reassure people that no matter what their concern is, whether it’s eczema or acne, whether it’s one pimple, I see you, and I know exactly how that feels.”
A version of this article first appeared on Medscape.com.
It’s not uncommon for a medical student to change specialty plans. For Jamie Harris, a second-year student at the University of Florida School of Medicine, Gainesville, that decision came as the result of a vicious dog and an empathetic doctor.
, a New York dermatologist whose approach involves early and aggressive treatment. After treating her, Dr. Bhanusali offered to have Ms. Harris shadow him.
She returned to school to shadow other dermatologists and to research the specialty before taking Dr. Bhanusali up on his offer. Ms. Harris sat in on procedures and meetings with patients and studied Dr. Bhanusali’s approach to the specialty. “I just fell in love with dermatology,” Ms. Harris told this news organization. “I knew that what I wanted for my own career was exactly how he runs his practice and how he treats patients.”

Life-changing injury
In 2020, Ms. Harris was a sophomore in the University of Florida’s medical honors program, an accelerated track that allows students to earn both a bachelor of science degree and a doctor of medicine degree in 7 years. She had finished studying at a friend’s apartment and was watching television when the rescue dog the friend adopted lunged at Ms. Harris, biting her on the face. “I was just cowering in the corner of the couch,” she recalls. “I didn’t go into fight-or-flight mode; I just went into hide mode.”
After receiving stitches in the emergency department, she visited several dermatologists and plastic surgeons for further treatment. There was scarring from her forehead to her chin, which was particularly severe on her upper cheek just under her eye. But because there was no infection or medical problems, the doctors turned her away. “They said, ‘OK, you look great.’ I did not look great,” she said.
Ms. Harris’ doctors advised her to wait a year before starting treatment for the scarring, a traditional approach. She was frustrated. “At the time, I was interested in becoming a pediatrician and thought, ‘No kid is going to want me as their doctor.’ ” But she accepted the medical advice – until her mother remembered a news story she’d seen.
Bridger Walker, a 6-year-old Wyoming boy, made headlines when he saved his younger sister from a dog that was attacking, but he was bitten multiple times as a result. Dr. Bhanusali treated the boy’s scarring.
Ms. Harris and her mother contacted the doctor, and after meeting via Zoom, Dr. Bhanusali agreed to treat her right away. He used lasers to resurface the skin, which created a suitable foundation for the scar cream, and he administered steroid injections to soften the scar tissue.
‘I see you’
Dr. Bhansali said he was impressed with the young student he treated. “There’s curiosity, and then there’s genuine passion. She has the latter,” he said in an interview. “Having gone through this, she will understand the value of research and keeping up with the literature and that just because something is being done a certain way today doesn’t mean it has to be that way tomorrow.”
Ms. Harris agrees that the experience will make her a better dermatologist. “One of the best parts about dermatology is that you can see your results in real time and really see what’s working and what’s not working. The potential for innovation is just amazing.”
But Ms. Harris believes she also gained empathy with dermatology patients. “I know exactly what it’s like to look in the mirror and not even recognize yourself, just have your eyes go straight to one thing and feel like the whole world is staring at you,” she said. “I’ll be able to reassure people that no matter what their concern is, whether it’s eczema or acne, whether it’s one pimple, I see you, and I know exactly how that feels.”
A version of this article first appeared on Medscape.com.
It’s not uncommon for a medical student to change specialty plans. For Jamie Harris, a second-year student at the University of Florida School of Medicine, Gainesville, that decision came as the result of a vicious dog and an empathetic doctor.
, a New York dermatologist whose approach involves early and aggressive treatment. After treating her, Dr. Bhanusali offered to have Ms. Harris shadow him.
She returned to school to shadow other dermatologists and to research the specialty before taking Dr. Bhanusali up on his offer. Ms. Harris sat in on procedures and meetings with patients and studied Dr. Bhanusali’s approach to the specialty. “I just fell in love with dermatology,” Ms. Harris told this news organization. “I knew that what I wanted for my own career was exactly how he runs his practice and how he treats patients.”

Life-changing injury
In 2020, Ms. Harris was a sophomore in the University of Florida’s medical honors program, an accelerated track that allows students to earn both a bachelor of science degree and a doctor of medicine degree in 7 years. She had finished studying at a friend’s apartment and was watching television when the rescue dog the friend adopted lunged at Ms. Harris, biting her on the face. “I was just cowering in the corner of the couch,” she recalls. “I didn’t go into fight-or-flight mode; I just went into hide mode.”
After receiving stitches in the emergency department, she visited several dermatologists and plastic surgeons for further treatment. There was scarring from her forehead to her chin, which was particularly severe on her upper cheek just under her eye. But because there was no infection or medical problems, the doctors turned her away. “They said, ‘OK, you look great.’ I did not look great,” she said.
Ms. Harris’ doctors advised her to wait a year before starting treatment for the scarring, a traditional approach. She was frustrated. “At the time, I was interested in becoming a pediatrician and thought, ‘No kid is going to want me as their doctor.’ ” But she accepted the medical advice – until her mother remembered a news story she’d seen.
Bridger Walker, a 6-year-old Wyoming boy, made headlines when he saved his younger sister from a dog that was attacking, but he was bitten multiple times as a result. Dr. Bhanusali treated the boy’s scarring.
Ms. Harris and her mother contacted the doctor, and after meeting via Zoom, Dr. Bhanusali agreed to treat her right away. He used lasers to resurface the skin, which created a suitable foundation for the scar cream, and he administered steroid injections to soften the scar tissue.
‘I see you’
Dr. Bhansali said he was impressed with the young student he treated. “There’s curiosity, and then there’s genuine passion. She has the latter,” he said in an interview. “Having gone through this, she will understand the value of research and keeping up with the literature and that just because something is being done a certain way today doesn’t mean it has to be that way tomorrow.”
Ms. Harris agrees that the experience will make her a better dermatologist. “One of the best parts about dermatology is that you can see your results in real time and really see what’s working and what’s not working. The potential for innovation is just amazing.”
But Ms. Harris believes she also gained empathy with dermatology patients. “I know exactly what it’s like to look in the mirror and not even recognize yourself, just have your eyes go straight to one thing and feel like the whole world is staring at you,” she said. “I’ll be able to reassure people that no matter what their concern is, whether it’s eczema or acne, whether it’s one pimple, I see you, and I know exactly how that feels.”
A version of this article first appeared on Medscape.com.
Most children with ADHD are not receiving treatment
Just more than one-third (34.8%) had received either treatment.
Researchers, led by Mark Olfson, MD, MPH, Elizabeth K. Dollard Professor of Psychiatry, Medicine and Law and professor of epidemiology at New York State Psychiatric Institute and Columbia University Department of Psychiatry, New York, also found that girls were much less likely to get medications.
In this cross-sectional sample taken from 11, 723 children in the Adolescent Brain and Cognitive Development Study, 1,206 children aged 9 and 10 years had parent-reported ADHD, and of those children, 15.7% of boys and 7% of girls were currently receiving ADHD medications. The parents reported the children met ADHD criteria according to the Diagnostic and Statistical Manual of Mental Disorders.
Findings were published online in JAMA Network Open.
Diagnoses have doubled but treatment numbers lag
Report authors noted that the percentage of U.S. children whose parents report their child has been diagnosed with ADHD has nearly doubled over 2 decades from 5.5% in 1999 to 9.8% in 2018. That has led to misperceptions among professionals and the public that the disorder is overdiagnosed and overtreated, the authors wrote.
However, they wrote, “a focus on the increasing numbers of children treated for ADHD does not give a sense of what fraction of children in the population with ADHD receive treatment.”
Higher uptake at lower income and education levels
Researchers also found that, contrary to popular belief, children with ADHD from families with lower educational levels and lower income were more likely than those with higher educational levels and higher incomes to have received outpatient mental health care.
Among children with ADHD whose parents did not have a high school education, 32.2% of children were receiving medications while among children of parents with a bachelor’s degree 11.5% received medications.
Among children from families with incomes of less than $25 000, 36.5% were receiving outpatient mental health care, compared with 20.1% of those from families with incomes of $75,000 or more.
“These patterns suggest that attitudinal rather than socioeconomic factors often impede the flow of children with ADHD into treatment,” they wrote.
Black children less likely to receive medications
The researchers found that substantially more White children (14.8% [104 of 759]) than Black children (9.4% [22 of 206]), received medication, a finding consistent with previous research.
“Population-based racial and ethnic gradients exist in prescriptions for stimulants and other controlled substances, with the highest rates in majority-White areas,” the authors wrote. “As a result of structural racism, Black parents’ perspectives might further influence ADHD management decisions through mistrust in clinicians and concerns over safety and efficacy of stimulants.”
“Physician efforts to recognize and manage their own implicit biases, together with patient-centered clinical approaches that promote shared decision-making,” might help narrow the treatment gap, the authors wrote. That includes talking with Black parents about their knowledge and beliefs concerning managing ADHD, they added.
Confirming diagnosis critical
The authors noted that not all children with parent-reported ADHD need treatment or would benefit from it.
Lenard Adler, MD, director of the adult ADHD program and professor of Psychiatry and Child and Adolescent Psychiatry at New York University Langone Health, who was not part of the current study, said this research emphasizes the urgency of clinical diagnosis.
Dr. Adler was part of a team of researchers that found similar low numbers for treatment among adults with ADHD.
The current results highlight that “we want to get the diagnosis correct so that people who receive a diagnosis actually have it and, if they do, that they have access to care. Because the consequences for not having treatment for ADHD are significant,” Dr. Adler said.
He urged physicians who diagnose ADHD to make follow-up part of the care plan or these treatment gaps will persist.
The authors wrote that the results suggest a need to increase availability for mental health services and better communicate symptoms among parents, teachers, and primary care providers.
The authors declare no relevant financial relationships. Dr. Adler has consulted with Supernus Pharmaceuticals and Otsuka Pharmaceuticals, has done research with Takeda, and has received royalty payments from NYU for licensing of ADHD training materials.
Just more than one-third (34.8%) had received either treatment.
Researchers, led by Mark Olfson, MD, MPH, Elizabeth K. Dollard Professor of Psychiatry, Medicine and Law and professor of epidemiology at New York State Psychiatric Institute and Columbia University Department of Psychiatry, New York, also found that girls were much less likely to get medications.
In this cross-sectional sample taken from 11, 723 children in the Adolescent Brain and Cognitive Development Study, 1,206 children aged 9 and 10 years had parent-reported ADHD, and of those children, 15.7% of boys and 7% of girls were currently receiving ADHD medications. The parents reported the children met ADHD criteria according to the Diagnostic and Statistical Manual of Mental Disorders.
Findings were published online in JAMA Network Open.
Diagnoses have doubled but treatment numbers lag
Report authors noted that the percentage of U.S. children whose parents report their child has been diagnosed with ADHD has nearly doubled over 2 decades from 5.5% in 1999 to 9.8% in 2018. That has led to misperceptions among professionals and the public that the disorder is overdiagnosed and overtreated, the authors wrote.
However, they wrote, “a focus on the increasing numbers of children treated for ADHD does not give a sense of what fraction of children in the population with ADHD receive treatment.”
Higher uptake at lower income and education levels
Researchers also found that, contrary to popular belief, children with ADHD from families with lower educational levels and lower income were more likely than those with higher educational levels and higher incomes to have received outpatient mental health care.
Among children with ADHD whose parents did not have a high school education, 32.2% of children were receiving medications while among children of parents with a bachelor’s degree 11.5% received medications.
Among children from families with incomes of less than $25 000, 36.5% were receiving outpatient mental health care, compared with 20.1% of those from families with incomes of $75,000 or more.
“These patterns suggest that attitudinal rather than socioeconomic factors often impede the flow of children with ADHD into treatment,” they wrote.
Black children less likely to receive medications
The researchers found that substantially more White children (14.8% [104 of 759]) than Black children (9.4% [22 of 206]), received medication, a finding consistent with previous research.
“Population-based racial and ethnic gradients exist in prescriptions for stimulants and other controlled substances, with the highest rates in majority-White areas,” the authors wrote. “As a result of structural racism, Black parents’ perspectives might further influence ADHD management decisions through mistrust in clinicians and concerns over safety and efficacy of stimulants.”
“Physician efforts to recognize and manage their own implicit biases, together with patient-centered clinical approaches that promote shared decision-making,” might help narrow the treatment gap, the authors wrote. That includes talking with Black parents about their knowledge and beliefs concerning managing ADHD, they added.
Confirming diagnosis critical
The authors noted that not all children with parent-reported ADHD need treatment or would benefit from it.
Lenard Adler, MD, director of the adult ADHD program and professor of Psychiatry and Child and Adolescent Psychiatry at New York University Langone Health, who was not part of the current study, said this research emphasizes the urgency of clinical diagnosis.
Dr. Adler was part of a team of researchers that found similar low numbers for treatment among adults with ADHD.
The current results highlight that “we want to get the diagnosis correct so that people who receive a diagnosis actually have it and, if they do, that they have access to care. Because the consequences for not having treatment for ADHD are significant,” Dr. Adler said.
He urged physicians who diagnose ADHD to make follow-up part of the care plan or these treatment gaps will persist.
The authors wrote that the results suggest a need to increase availability for mental health services and better communicate symptoms among parents, teachers, and primary care providers.
The authors declare no relevant financial relationships. Dr. Adler has consulted with Supernus Pharmaceuticals and Otsuka Pharmaceuticals, has done research with Takeda, and has received royalty payments from NYU for licensing of ADHD training materials.
Just more than one-third (34.8%) had received either treatment.
Researchers, led by Mark Olfson, MD, MPH, Elizabeth K. Dollard Professor of Psychiatry, Medicine and Law and professor of epidemiology at New York State Psychiatric Institute and Columbia University Department of Psychiatry, New York, also found that girls were much less likely to get medications.
In this cross-sectional sample taken from 11, 723 children in the Adolescent Brain and Cognitive Development Study, 1,206 children aged 9 and 10 years had parent-reported ADHD, and of those children, 15.7% of boys and 7% of girls were currently receiving ADHD medications. The parents reported the children met ADHD criteria according to the Diagnostic and Statistical Manual of Mental Disorders.
Findings were published online in JAMA Network Open.
Diagnoses have doubled but treatment numbers lag
Report authors noted that the percentage of U.S. children whose parents report their child has been diagnosed with ADHD has nearly doubled over 2 decades from 5.5% in 1999 to 9.8% in 2018. That has led to misperceptions among professionals and the public that the disorder is overdiagnosed and overtreated, the authors wrote.
However, they wrote, “a focus on the increasing numbers of children treated for ADHD does not give a sense of what fraction of children in the population with ADHD receive treatment.”
Higher uptake at lower income and education levels
Researchers also found that, contrary to popular belief, children with ADHD from families with lower educational levels and lower income were more likely than those with higher educational levels and higher incomes to have received outpatient mental health care.
Among children with ADHD whose parents did not have a high school education, 32.2% of children were receiving medications while among children of parents with a bachelor’s degree 11.5% received medications.
Among children from families with incomes of less than $25 000, 36.5% were receiving outpatient mental health care, compared with 20.1% of those from families with incomes of $75,000 or more.
“These patterns suggest that attitudinal rather than socioeconomic factors often impede the flow of children with ADHD into treatment,” they wrote.
Black children less likely to receive medications
The researchers found that substantially more White children (14.8% [104 of 759]) than Black children (9.4% [22 of 206]), received medication, a finding consistent with previous research.
“Population-based racial and ethnic gradients exist in prescriptions for stimulants and other controlled substances, with the highest rates in majority-White areas,” the authors wrote. “As a result of structural racism, Black parents’ perspectives might further influence ADHD management decisions through mistrust in clinicians and concerns over safety and efficacy of stimulants.”
“Physician efforts to recognize and manage their own implicit biases, together with patient-centered clinical approaches that promote shared decision-making,” might help narrow the treatment gap, the authors wrote. That includes talking with Black parents about their knowledge and beliefs concerning managing ADHD, they added.
Confirming diagnosis critical
The authors noted that not all children with parent-reported ADHD need treatment or would benefit from it.
Lenard Adler, MD, director of the adult ADHD program and professor of Psychiatry and Child and Adolescent Psychiatry at New York University Langone Health, who was not part of the current study, said this research emphasizes the urgency of clinical diagnosis.
Dr. Adler was part of a team of researchers that found similar low numbers for treatment among adults with ADHD.
The current results highlight that “we want to get the diagnosis correct so that people who receive a diagnosis actually have it and, if they do, that they have access to care. Because the consequences for not having treatment for ADHD are significant,” Dr. Adler said.
He urged physicians who diagnose ADHD to make follow-up part of the care plan or these treatment gaps will persist.
The authors wrote that the results suggest a need to increase availability for mental health services and better communicate symptoms among parents, teachers, and primary care providers.
The authors declare no relevant financial relationships. Dr. Adler has consulted with Supernus Pharmaceuticals and Otsuka Pharmaceuticals, has done research with Takeda, and has received royalty payments from NYU for licensing of ADHD training materials.
FROM JAMA NETWORK OPEN
Contact allergens lurk in diabetes devices
in a presentation at the annual meeting of the American Contact Dermatitis Society.
Advanced technologies used for the management of diabetes fall into three main categories, said Dr. Chen, of the department of dermatology, Stanford University, Redwood City, Calif. Continuous glucose monitoring (CGM) devices, which are worn on the body, collect glucose measurements. Continuous subcutaneous insulin infusion (CSII) devices are attached to the body via an infusion set and are now available as tubing-free patch pumps that are attached directly to the skin via a catheter. Glucose-responsive insulin delivery systems combine the sensing and delivery features of the other two types of devices.
Once thought to be rare, reports of skin complications related to diabetes devices have been increasing in recent years, she said. Some reports suggest that at any given time, skin complications may affect as many as one quarter to one half of patients who use these devices, “so this is an important issue,” she emphasized. “Skin reactions are a major factor in device discontinuation, so we as clinicians need to be really proactive about treating these reactions.”
Risk factors for skin complications related to diabetes devices include sensitization to the adhesive used with the devices, as well as prolonged exposure to the device, Dr. Chen said. Younger age also appears to be a risk factor, as is a compromised skin barrier in the area where the device is used.
Unfortunately, obtaining details on the specific adhesives and the raw materials used in these devices, so as to customize patch testing, remains a challenge, she said. “Patch testing initially was often negative to commercially available allergens, even while patients were testing positive to pieces of device adhesive,” she noted.
Consider isobornyl acrylate
An article published in 2017 in Contact Dermatitis was “a major breakthrough” in that it identified isobornyl acrylate (IBOA) as an allergen in connection with the Freestyle Libre, a CGM device that was relatively new at the time. The finding was serendipitous, Dr. Chen said. A patient being treated for suspected allergic contact dermatitis in connection with use of a Freestyle Libre device was tested for IBOA accidentally, after the nurse administering the patch test thought that this was part of the standard acrylate series, she explained.
Subsequently, researchers identified 15 patients who had experienced reactions to the Freestyle Libre; 12 of 13 patients who were patch tested for IBOA tested positive. IBOA was found throughout the device, particularly where the top and bottom plastic components were connected, Dr. Chen said. This suggested that the IBOA was in the device housing and had diffused into the adhesive that attached the device to the skin.
An article published in 2018 in the Journal of Diabetes Science described three patients who developed severe allergic contact dermatitis from IBOA while using a CGM device, Dr. Chen said. The investigators confirmed that there were no reactions to the adhesive itself, again suggesting that IBOA had diffused into the adhesive from other parts of the device.
Although the authors were bound by a confidentiality agreement regarding the individual adhesive components, “the authors noted most of the acrylates in the adhesive were not present in commercially available acrylate series for patch testing,” she said.
IBOA, the ACDS’ Allergen of the Year in 2020, is common in sealants, glues, and adhesives, Dr. Chen said. Although IBOA had been reported infrequently as an allergen, it has now been identified as a “potential culprit” behind skin reactions in many diabetes devices, including CSII and CGM devices, she added.
In addition, N,N-dimethylacrylamide (DMAA) is an allergen that has been identified in several diabetes devices and often occurs with IBOA in medical-grade UV-cured adhesives, Dr. Chen noted. Other allergens identified in diabetes devices include colophony, which is present in many adhesives, as well as other acrylates and epoxy resin.
Diabetes devices are constantly evolving. IBOA is no longer found in Freestyle Libre devices. It is important that clinicians stay up to date with the medical literature and advocate for partnership with device manufacturers, she emphasized.
Patch testing
When diabetes devices are suspected as the source of allergic contact dermatitis, a minimum of a baseline series that contains colophony at a concentration of 20% in petrolatum should be carried out, Dr. Chen said. Commercialized patch test trays, which include plastics, glues, acrylates, epoxy resins/isocyanates, and colophony derivatives, should be ideal. “Personal-care products should be included if they are potentially relevant,” she added.
Dr. Chen shared tables published in Contact Dermatitis in 2021 with examples of screening test series. She said to consider including screening for other allergens more recently discovered in diabetes devices, including 2,2’-methylenebis(6-tert-butyl-4-methylphenol) monoacrylate (MBPA) 1.5% pet; dipropylene glycol diacrylate (DPGDA) 0.1% pet; and butylated hydroxytoluene (BHT) 2% pet.
Testing for monomethyl ether of hydroquinone should also be considered; this may be included in the test preparations for IBOA and DMAA.
Management strategies
For patients who experience skin reactions to their diabetes devices, consideration may given to relocating the device to another area of skin or changing sensors more frequently, according to Dr. Chen.
For some patients, the reaction can be managed with corticosteroid cream, ointment, solution, or nasal spray. Topical antibiotics or topical antihistamines can be helpful, as can barrier dressings, solutions, or sprays, she said. The best solution is to change to a device that does not have the culprit allergen, “but that is difficult, since we don’t know what is in these devices,” she added. Good alternatives include the Eversense CGM device or devices that have been demonstrated not to contain IBOA, such as the Freestyle Libre 2 or the newer version of the Omnipod, an insulin delivery system
Looking ahead, Dr. Chen said that “mandatory labeling is needed, as devices with the same name may have different compositions, depending on the date of manufacture.” Allergens relevant to people with diabetes are constantly evolving, and many are still unidentified, so clinicians and manufacturers need to work together to identify the culprit allergens and their sources, she said.
Dr. Chen has served as principal investigator or subinvestigator for Amgen, AbbVie, and Sanofi Regeneron and as a consultant for Purity Brands.
A version of this article first appeared on Medscape.com.
in a presentation at the annual meeting of the American Contact Dermatitis Society.
Advanced technologies used for the management of diabetes fall into three main categories, said Dr. Chen, of the department of dermatology, Stanford University, Redwood City, Calif. Continuous glucose monitoring (CGM) devices, which are worn on the body, collect glucose measurements. Continuous subcutaneous insulin infusion (CSII) devices are attached to the body via an infusion set and are now available as tubing-free patch pumps that are attached directly to the skin via a catheter. Glucose-responsive insulin delivery systems combine the sensing and delivery features of the other two types of devices.
Once thought to be rare, reports of skin complications related to diabetes devices have been increasing in recent years, she said. Some reports suggest that at any given time, skin complications may affect as many as one quarter to one half of patients who use these devices, “so this is an important issue,” she emphasized. “Skin reactions are a major factor in device discontinuation, so we as clinicians need to be really proactive about treating these reactions.”
Risk factors for skin complications related to diabetes devices include sensitization to the adhesive used with the devices, as well as prolonged exposure to the device, Dr. Chen said. Younger age also appears to be a risk factor, as is a compromised skin barrier in the area where the device is used.
Unfortunately, obtaining details on the specific adhesives and the raw materials used in these devices, so as to customize patch testing, remains a challenge, she said. “Patch testing initially was often negative to commercially available allergens, even while patients were testing positive to pieces of device adhesive,” she noted.
Consider isobornyl acrylate
An article published in 2017 in Contact Dermatitis was “a major breakthrough” in that it identified isobornyl acrylate (IBOA) as an allergen in connection with the Freestyle Libre, a CGM device that was relatively new at the time. The finding was serendipitous, Dr. Chen said. A patient being treated for suspected allergic contact dermatitis in connection with use of a Freestyle Libre device was tested for IBOA accidentally, after the nurse administering the patch test thought that this was part of the standard acrylate series, she explained.
Subsequently, researchers identified 15 patients who had experienced reactions to the Freestyle Libre; 12 of 13 patients who were patch tested for IBOA tested positive. IBOA was found throughout the device, particularly where the top and bottom plastic components were connected, Dr. Chen said. This suggested that the IBOA was in the device housing and had diffused into the adhesive that attached the device to the skin.
An article published in 2018 in the Journal of Diabetes Science described three patients who developed severe allergic contact dermatitis from IBOA while using a CGM device, Dr. Chen said. The investigators confirmed that there were no reactions to the adhesive itself, again suggesting that IBOA had diffused into the adhesive from other parts of the device.
Although the authors were bound by a confidentiality agreement regarding the individual adhesive components, “the authors noted most of the acrylates in the adhesive were not present in commercially available acrylate series for patch testing,” she said.
IBOA, the ACDS’ Allergen of the Year in 2020, is common in sealants, glues, and adhesives, Dr. Chen said. Although IBOA had been reported infrequently as an allergen, it has now been identified as a “potential culprit” behind skin reactions in many diabetes devices, including CSII and CGM devices, she added.
In addition, N,N-dimethylacrylamide (DMAA) is an allergen that has been identified in several diabetes devices and often occurs with IBOA in medical-grade UV-cured adhesives, Dr. Chen noted. Other allergens identified in diabetes devices include colophony, which is present in many adhesives, as well as other acrylates and epoxy resin.
Diabetes devices are constantly evolving. IBOA is no longer found in Freestyle Libre devices. It is important that clinicians stay up to date with the medical literature and advocate for partnership with device manufacturers, she emphasized.
Patch testing
When diabetes devices are suspected as the source of allergic contact dermatitis, a minimum of a baseline series that contains colophony at a concentration of 20% in petrolatum should be carried out, Dr. Chen said. Commercialized patch test trays, which include plastics, glues, acrylates, epoxy resins/isocyanates, and colophony derivatives, should be ideal. “Personal-care products should be included if they are potentially relevant,” she added.
Dr. Chen shared tables published in Contact Dermatitis in 2021 with examples of screening test series. She said to consider including screening for other allergens more recently discovered in diabetes devices, including 2,2’-methylenebis(6-tert-butyl-4-methylphenol) monoacrylate (MBPA) 1.5% pet; dipropylene glycol diacrylate (DPGDA) 0.1% pet; and butylated hydroxytoluene (BHT) 2% pet.
Testing for monomethyl ether of hydroquinone should also be considered; this may be included in the test preparations for IBOA and DMAA.
Management strategies
For patients who experience skin reactions to their diabetes devices, consideration may given to relocating the device to another area of skin or changing sensors more frequently, according to Dr. Chen.
For some patients, the reaction can be managed with corticosteroid cream, ointment, solution, or nasal spray. Topical antibiotics or topical antihistamines can be helpful, as can barrier dressings, solutions, or sprays, she said. The best solution is to change to a device that does not have the culprit allergen, “but that is difficult, since we don’t know what is in these devices,” she added. Good alternatives include the Eversense CGM device or devices that have been demonstrated not to contain IBOA, such as the Freestyle Libre 2 or the newer version of the Omnipod, an insulin delivery system
Looking ahead, Dr. Chen said that “mandatory labeling is needed, as devices with the same name may have different compositions, depending on the date of manufacture.” Allergens relevant to people with diabetes are constantly evolving, and many are still unidentified, so clinicians and manufacturers need to work together to identify the culprit allergens and their sources, she said.
Dr. Chen has served as principal investigator or subinvestigator for Amgen, AbbVie, and Sanofi Regeneron and as a consultant for Purity Brands.
A version of this article first appeared on Medscape.com.
in a presentation at the annual meeting of the American Contact Dermatitis Society.
Advanced technologies used for the management of diabetes fall into three main categories, said Dr. Chen, of the department of dermatology, Stanford University, Redwood City, Calif. Continuous glucose monitoring (CGM) devices, which are worn on the body, collect glucose measurements. Continuous subcutaneous insulin infusion (CSII) devices are attached to the body via an infusion set and are now available as tubing-free patch pumps that are attached directly to the skin via a catheter. Glucose-responsive insulin delivery systems combine the sensing and delivery features of the other two types of devices.
Once thought to be rare, reports of skin complications related to diabetes devices have been increasing in recent years, she said. Some reports suggest that at any given time, skin complications may affect as many as one quarter to one half of patients who use these devices, “so this is an important issue,” she emphasized. “Skin reactions are a major factor in device discontinuation, so we as clinicians need to be really proactive about treating these reactions.”
Risk factors for skin complications related to diabetes devices include sensitization to the adhesive used with the devices, as well as prolonged exposure to the device, Dr. Chen said. Younger age also appears to be a risk factor, as is a compromised skin barrier in the area where the device is used.
Unfortunately, obtaining details on the specific adhesives and the raw materials used in these devices, so as to customize patch testing, remains a challenge, she said. “Patch testing initially was often negative to commercially available allergens, even while patients were testing positive to pieces of device adhesive,” she noted.
Consider isobornyl acrylate
An article published in 2017 in Contact Dermatitis was “a major breakthrough” in that it identified isobornyl acrylate (IBOA) as an allergen in connection with the Freestyle Libre, a CGM device that was relatively new at the time. The finding was serendipitous, Dr. Chen said. A patient being treated for suspected allergic contact dermatitis in connection with use of a Freestyle Libre device was tested for IBOA accidentally, after the nurse administering the patch test thought that this was part of the standard acrylate series, she explained.
Subsequently, researchers identified 15 patients who had experienced reactions to the Freestyle Libre; 12 of 13 patients who were patch tested for IBOA tested positive. IBOA was found throughout the device, particularly where the top and bottom plastic components were connected, Dr. Chen said. This suggested that the IBOA was in the device housing and had diffused into the adhesive that attached the device to the skin.
An article published in 2018 in the Journal of Diabetes Science described three patients who developed severe allergic contact dermatitis from IBOA while using a CGM device, Dr. Chen said. The investigators confirmed that there were no reactions to the adhesive itself, again suggesting that IBOA had diffused into the adhesive from other parts of the device.
Although the authors were bound by a confidentiality agreement regarding the individual adhesive components, “the authors noted most of the acrylates in the adhesive were not present in commercially available acrylate series for patch testing,” she said.
IBOA, the ACDS’ Allergen of the Year in 2020, is common in sealants, glues, and adhesives, Dr. Chen said. Although IBOA had been reported infrequently as an allergen, it has now been identified as a “potential culprit” behind skin reactions in many diabetes devices, including CSII and CGM devices, she added.
In addition, N,N-dimethylacrylamide (DMAA) is an allergen that has been identified in several diabetes devices and often occurs with IBOA in medical-grade UV-cured adhesives, Dr. Chen noted. Other allergens identified in diabetes devices include colophony, which is present in many adhesives, as well as other acrylates and epoxy resin.
Diabetes devices are constantly evolving. IBOA is no longer found in Freestyle Libre devices. It is important that clinicians stay up to date with the medical literature and advocate for partnership with device manufacturers, she emphasized.
Patch testing
When diabetes devices are suspected as the source of allergic contact dermatitis, a minimum of a baseline series that contains colophony at a concentration of 20% in petrolatum should be carried out, Dr. Chen said. Commercialized patch test trays, which include plastics, glues, acrylates, epoxy resins/isocyanates, and colophony derivatives, should be ideal. “Personal-care products should be included if they are potentially relevant,” she added.
Dr. Chen shared tables published in Contact Dermatitis in 2021 with examples of screening test series. She said to consider including screening for other allergens more recently discovered in diabetes devices, including 2,2’-methylenebis(6-tert-butyl-4-methylphenol) monoacrylate (MBPA) 1.5% pet; dipropylene glycol diacrylate (DPGDA) 0.1% pet; and butylated hydroxytoluene (BHT) 2% pet.
Testing for monomethyl ether of hydroquinone should also be considered; this may be included in the test preparations for IBOA and DMAA.
Management strategies
For patients who experience skin reactions to their diabetes devices, consideration may given to relocating the device to another area of skin or changing sensors more frequently, according to Dr. Chen.
For some patients, the reaction can be managed with corticosteroid cream, ointment, solution, or nasal spray. Topical antibiotics or topical antihistamines can be helpful, as can barrier dressings, solutions, or sprays, she said. The best solution is to change to a device that does not have the culprit allergen, “but that is difficult, since we don’t know what is in these devices,” she added. Good alternatives include the Eversense CGM device or devices that have been demonstrated not to contain IBOA, such as the Freestyle Libre 2 or the newer version of the Omnipod, an insulin delivery system
Looking ahead, Dr. Chen said that “mandatory labeling is needed, as devices with the same name may have different compositions, depending on the date of manufacture.” Allergens relevant to people with diabetes are constantly evolving, and many are still unidentified, so clinicians and manufacturers need to work together to identify the culprit allergens and their sources, she said.
Dr. Chen has served as principal investigator or subinvestigator for Amgen, AbbVie, and Sanofi Regeneron and as a consultant for Purity Brands.
A version of this article first appeared on Medscape.com.
FROM ACDS 2023
Beware the hidden allergens in nutritional supplements
, Alison Ehrlich, MD, said at the annual meeting of the American Contact Dermatitis Society.
Allergens may be hidden in a range of supplement products, from colorings in vitamin C powders to some vitamins used in hair products and other products.
“In general, our patients do not tell us what supplements they are taking,” said Dr. Ehrlich, a dermatologist who practices in Washington, D.C. Antiaging, sleep, and weight loss/weight control supplements are among the most popular, she said.
Surveys have shown that many patients do not discuss supplement use with their health care providers, in part because they believe their providers would disapprove of supplement use, and patients are not educated about supplements, she said. “This is definitely an area that we should try to learn more about,” she added.
Current regulations regarding dietary supplements stem from the Dietary Supplement Health and Education Act of 1994, which defined dietary supplements as distinct from meals but regulated them as a category of food, not as medications. Dietary supplements can be vitamins, minerals, herbs, and extracts, Dr. Ehrlich said.
“There is not a lot of safety wrapped around how supplements come onto the market,” she explained. “It is not the manufacturer’s responsibility to test these products and make sure they are safe. When they get pulled off the market, it is because safety reports are getting back to the FDA.”
Consequently, a detailed history of supplement use is important, as it may reveal possible allergens as the cause of previously unidentified reactions, she said.
Dr. Ehrlich shared a case involving a patient who claimed to have had a reaction to a “Prevage-like” product that was labeled as a crepe repair cream. Listed among the product’s ingredients was idebenone, a synthetic version of the popular antioxidant known as Coenzyme Q.
Be wary of vitamins
Another potential source of allergy is vitamin C supplements, which became especially popular during the pandemic as people sought additional immune system support, Dr. Ehrlich noted. “What kind of vitamin C product our patients are taking is important,” she said. For example, some vitamin C powders contain coloring agents, such as carmine. Some also contain gelatin, which may cause an allergic reaction in individuals with alpha-gal syndrome, she added.
In general, water-soluble vitamins such as vitamins B1 to B9, B12, and C are more likely to cause an immediate reaction, Dr. Ehrlich said. Fat-soluble vitamins, such as vitamins A, D, E, and K, are more likely to cause a delayed reaction of allergic contact dermatitis.
Dr. Ehrlich described some unusual reactions to vitamins that have been reported, including a systemic allergy associated with vitamin B1 (thiamine), burning mouth syndrome associated with vitamin B3 (nicotinate), contact urticaria associated with vitamin B5 (panthenol), systemic allergy and generalized ACD associated with vitamin E (tocopherol), and erythema multiforme–like ACD associated with vitamin K1.
Notably, vitamin B5 has been associated with ACD as an ingredient in hair products, moisturizers, and wound care products, as well as B-complex vitamins and fortified foods, Dr. Ehrlich said.
Herbs and spices can act as allergens as well. Turmeric is a spice that has become a popular supplement ingredient, she said. Turmeric and curcumin (found in turmeric) can be used as a dye for its yellow color as well as a flavoring but has been associated with allergic reactions. Another popular herbal supplement, ginkgo biloba, has been marketed as a product that improves memory and cognition. It is available in pill form and in herbal teas.
“It’s really important to think about what herbal products our patients are taking, and not just in pill form,” Dr. Ehrlich said. “We need to expand our thoughts on what the herbs are in.”
Consider food additives as allergens
Food additives, in the form of colorants, preservatives, or flavoring agents, can cause allergic reactions, Dr. Ehrlich noted.
The question of whether food-additive contact sensitivity has a role in the occurrence of atopic dermatitis (AD) in children remains unclear, she said. However, a study published in 2020 found that 62% of children with AD had positive patch test reactions to at least one food-additive allergen, compared with 20% of children without AD. The additives responsible for the most reactions were azorubine (24.4%); formic acid (15.6%); and carmine, cochineal red, and amaranth (13.3% for each).
Common colorant culprits in allergic reactions include carmine, annatto, tartrazine, and spices (such as paprika and saffron), Dr. Ehrlich said. Carmine is used in meat to prevent photo-oxidation and to preserve a red color, and it has other uses as well, she said. Carmine has been associated with ACD, AD flares, and immediate hypersensitivity. Annatto is used in foods, including processed foods, butter, and cheese, to provide a yellow color. It is also found in some lipsticks and has been associated with urticaria and angioedema, she noted.
Food preservatives that have been associated with allergic reactions include butylated hydroxyanisole and sulfites, Dr. Ehrlich said. Sulfites are used to prevent food from turning brown, and it may be present in dried fruit, fruit juice, molasses, pickled foods, vinegar, and wine.
Reports of ACD in response to sodium metabisulfite have been increasing, she noted. Other sulfite reactions may occur with exposure to other products, such as cosmetics, body washes, and swimming pool water, she said.
Awareness of allergens in supplements is important “because the number of our patients taking supplements for different reasons is increasing” and allergens in supplements could account for flares, Dr. Ehrlich said. Clinicians should encourage patients to tell them what supplements they use. Clinicians should review the ingredients in these supplements with their patients to identify potential allergens that may be causing reactions, she advised.
Dr. Ehrlich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, Alison Ehrlich, MD, said at the annual meeting of the American Contact Dermatitis Society.
Allergens may be hidden in a range of supplement products, from colorings in vitamin C powders to some vitamins used in hair products and other products.
“In general, our patients do not tell us what supplements they are taking,” said Dr. Ehrlich, a dermatologist who practices in Washington, D.C. Antiaging, sleep, and weight loss/weight control supplements are among the most popular, she said.
Surveys have shown that many patients do not discuss supplement use with their health care providers, in part because they believe their providers would disapprove of supplement use, and patients are not educated about supplements, she said. “This is definitely an area that we should try to learn more about,” she added.
Current regulations regarding dietary supplements stem from the Dietary Supplement Health and Education Act of 1994, which defined dietary supplements as distinct from meals but regulated them as a category of food, not as medications. Dietary supplements can be vitamins, minerals, herbs, and extracts, Dr. Ehrlich said.
“There is not a lot of safety wrapped around how supplements come onto the market,” she explained. “It is not the manufacturer’s responsibility to test these products and make sure they are safe. When they get pulled off the market, it is because safety reports are getting back to the FDA.”
Consequently, a detailed history of supplement use is important, as it may reveal possible allergens as the cause of previously unidentified reactions, she said.
Dr. Ehrlich shared a case involving a patient who claimed to have had a reaction to a “Prevage-like” product that was labeled as a crepe repair cream. Listed among the product’s ingredients was idebenone, a synthetic version of the popular antioxidant known as Coenzyme Q.
Be wary of vitamins
Another potential source of allergy is vitamin C supplements, which became especially popular during the pandemic as people sought additional immune system support, Dr. Ehrlich noted. “What kind of vitamin C product our patients are taking is important,” she said. For example, some vitamin C powders contain coloring agents, such as carmine. Some also contain gelatin, which may cause an allergic reaction in individuals with alpha-gal syndrome, she added.
In general, water-soluble vitamins such as vitamins B1 to B9, B12, and C are more likely to cause an immediate reaction, Dr. Ehrlich said. Fat-soluble vitamins, such as vitamins A, D, E, and K, are more likely to cause a delayed reaction of allergic contact dermatitis.
Dr. Ehrlich described some unusual reactions to vitamins that have been reported, including a systemic allergy associated with vitamin B1 (thiamine), burning mouth syndrome associated with vitamin B3 (nicotinate), contact urticaria associated with vitamin B5 (panthenol), systemic allergy and generalized ACD associated with vitamin E (tocopherol), and erythema multiforme–like ACD associated with vitamin K1.
Notably, vitamin B5 has been associated with ACD as an ingredient in hair products, moisturizers, and wound care products, as well as B-complex vitamins and fortified foods, Dr. Ehrlich said.
Herbs and spices can act as allergens as well. Turmeric is a spice that has become a popular supplement ingredient, she said. Turmeric and curcumin (found in turmeric) can be used as a dye for its yellow color as well as a flavoring but has been associated with allergic reactions. Another popular herbal supplement, ginkgo biloba, has been marketed as a product that improves memory and cognition. It is available in pill form and in herbal teas.
“It’s really important to think about what herbal products our patients are taking, and not just in pill form,” Dr. Ehrlich said. “We need to expand our thoughts on what the herbs are in.”
Consider food additives as allergens
Food additives, in the form of colorants, preservatives, or flavoring agents, can cause allergic reactions, Dr. Ehrlich noted.
The question of whether food-additive contact sensitivity has a role in the occurrence of atopic dermatitis (AD) in children remains unclear, she said. However, a study published in 2020 found that 62% of children with AD had positive patch test reactions to at least one food-additive allergen, compared with 20% of children without AD. The additives responsible for the most reactions were azorubine (24.4%); formic acid (15.6%); and carmine, cochineal red, and amaranth (13.3% for each).
Common colorant culprits in allergic reactions include carmine, annatto, tartrazine, and spices (such as paprika and saffron), Dr. Ehrlich said. Carmine is used in meat to prevent photo-oxidation and to preserve a red color, and it has other uses as well, she said. Carmine has been associated with ACD, AD flares, and immediate hypersensitivity. Annatto is used in foods, including processed foods, butter, and cheese, to provide a yellow color. It is also found in some lipsticks and has been associated with urticaria and angioedema, she noted.
Food preservatives that have been associated with allergic reactions include butylated hydroxyanisole and sulfites, Dr. Ehrlich said. Sulfites are used to prevent food from turning brown, and it may be present in dried fruit, fruit juice, molasses, pickled foods, vinegar, and wine.
Reports of ACD in response to sodium metabisulfite have been increasing, she noted. Other sulfite reactions may occur with exposure to other products, such as cosmetics, body washes, and swimming pool water, she said.
Awareness of allergens in supplements is important “because the number of our patients taking supplements for different reasons is increasing” and allergens in supplements could account for flares, Dr. Ehrlich said. Clinicians should encourage patients to tell them what supplements they use. Clinicians should review the ingredients in these supplements with their patients to identify potential allergens that may be causing reactions, she advised.
Dr. Ehrlich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, Alison Ehrlich, MD, said at the annual meeting of the American Contact Dermatitis Society.
Allergens may be hidden in a range of supplement products, from colorings in vitamin C powders to some vitamins used in hair products and other products.
“In general, our patients do not tell us what supplements they are taking,” said Dr. Ehrlich, a dermatologist who practices in Washington, D.C. Antiaging, sleep, and weight loss/weight control supplements are among the most popular, she said.
Surveys have shown that many patients do not discuss supplement use with their health care providers, in part because they believe their providers would disapprove of supplement use, and patients are not educated about supplements, she said. “This is definitely an area that we should try to learn more about,” she added.
Current regulations regarding dietary supplements stem from the Dietary Supplement Health and Education Act of 1994, which defined dietary supplements as distinct from meals but regulated them as a category of food, not as medications. Dietary supplements can be vitamins, minerals, herbs, and extracts, Dr. Ehrlich said.
“There is not a lot of safety wrapped around how supplements come onto the market,” she explained. “It is not the manufacturer’s responsibility to test these products and make sure they are safe. When they get pulled off the market, it is because safety reports are getting back to the FDA.”
Consequently, a detailed history of supplement use is important, as it may reveal possible allergens as the cause of previously unidentified reactions, she said.
Dr. Ehrlich shared a case involving a patient who claimed to have had a reaction to a “Prevage-like” product that was labeled as a crepe repair cream. Listed among the product’s ingredients was idebenone, a synthetic version of the popular antioxidant known as Coenzyme Q.
Be wary of vitamins
Another potential source of allergy is vitamin C supplements, which became especially popular during the pandemic as people sought additional immune system support, Dr. Ehrlich noted. “What kind of vitamin C product our patients are taking is important,” she said. For example, some vitamin C powders contain coloring agents, such as carmine. Some also contain gelatin, which may cause an allergic reaction in individuals with alpha-gal syndrome, she added.
In general, water-soluble vitamins such as vitamins B1 to B9, B12, and C are more likely to cause an immediate reaction, Dr. Ehrlich said. Fat-soluble vitamins, such as vitamins A, D, E, and K, are more likely to cause a delayed reaction of allergic contact dermatitis.
Dr. Ehrlich described some unusual reactions to vitamins that have been reported, including a systemic allergy associated with vitamin B1 (thiamine), burning mouth syndrome associated with vitamin B3 (nicotinate), contact urticaria associated with vitamin B5 (panthenol), systemic allergy and generalized ACD associated with vitamin E (tocopherol), and erythema multiforme–like ACD associated with vitamin K1.
Notably, vitamin B5 has been associated with ACD as an ingredient in hair products, moisturizers, and wound care products, as well as B-complex vitamins and fortified foods, Dr. Ehrlich said.
Herbs and spices can act as allergens as well. Turmeric is a spice that has become a popular supplement ingredient, she said. Turmeric and curcumin (found in turmeric) can be used as a dye for its yellow color as well as a flavoring but has been associated with allergic reactions. Another popular herbal supplement, ginkgo biloba, has been marketed as a product that improves memory and cognition. It is available in pill form and in herbal teas.
“It’s really important to think about what herbal products our patients are taking, and not just in pill form,” Dr. Ehrlich said. “We need to expand our thoughts on what the herbs are in.”
Consider food additives as allergens
Food additives, in the form of colorants, preservatives, or flavoring agents, can cause allergic reactions, Dr. Ehrlich noted.
The question of whether food-additive contact sensitivity has a role in the occurrence of atopic dermatitis (AD) in children remains unclear, she said. However, a study published in 2020 found that 62% of children with AD had positive patch test reactions to at least one food-additive allergen, compared with 20% of children without AD. The additives responsible for the most reactions were azorubine (24.4%); formic acid (15.6%); and carmine, cochineal red, and amaranth (13.3% for each).
Common colorant culprits in allergic reactions include carmine, annatto, tartrazine, and spices (such as paprika and saffron), Dr. Ehrlich said. Carmine is used in meat to prevent photo-oxidation and to preserve a red color, and it has other uses as well, she said. Carmine has been associated with ACD, AD flares, and immediate hypersensitivity. Annatto is used in foods, including processed foods, butter, and cheese, to provide a yellow color. It is also found in some lipsticks and has been associated with urticaria and angioedema, she noted.
Food preservatives that have been associated with allergic reactions include butylated hydroxyanisole and sulfites, Dr. Ehrlich said. Sulfites are used to prevent food from turning brown, and it may be present in dried fruit, fruit juice, molasses, pickled foods, vinegar, and wine.
Reports of ACD in response to sodium metabisulfite have been increasing, she noted. Other sulfite reactions may occur with exposure to other products, such as cosmetics, body washes, and swimming pool water, she said.
Awareness of allergens in supplements is important “because the number of our patients taking supplements for different reasons is increasing” and allergens in supplements could account for flares, Dr. Ehrlich said. Clinicians should encourage patients to tell them what supplements they use. Clinicians should review the ingredients in these supplements with their patients to identify potential allergens that may be causing reactions, she advised.
Dr. Ehrlich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACDS 2023
Autism and bone health: What you need to know
Many years ago, at the conclusion of a talk I gave on bone health in teens with anorexia nervosa, I was approached by a colleague, Ann Neumeyer, MD, medical director of the Lurie Center for Autism at Massachusetts General Hospital, Boston, who asked about bone health in children with autism spectrum disorder (ASD).
When I explained that there was little information about bone health in this patient population, she suggested that we learn and investigate together. Ann explained that she had observed that some of her patients with ASD had suffered fractures with minimal trauma, raising her concern about their bone health.
This was the beginning of a partnership that led us down the path of many grant submissions, some of which were funded and others that were not, to explore and investigate bone outcomes in children with ASD.
This applies to prepubertal children as well as older children and adolescents. One study showed that 28% and 33% of children with ASD 8-14 years old had very low bone density (z scores of ≤ –2) at the spine and hip, respectively, compared with 0% of typically developing controls.
Studies that have used sophisticated imaging techniques to determine bone strength have shown that it is lower at the forearm and lower leg in children with ASD versus neurotypical children.
These findings are of particular concern during the childhood and teenage years when bone is typically accrued at a rapid rate. A normal rate of bone accrual at this time of life is essential for optimal bone health in later life. While children with ASD gain bone mass at a similar rate as neurotypical controls, they start at a deficit and seem unable to “catch up.”
Further, people with ASD are more prone to certain kinds of fracture than those without the condition. For example, both children and adults with ASD have a high risk for hip fracture, while adult women with ASD have a higher risk for forearm and spine fractures. There is some protection against forearm fractures in children and adult men, probably because of markedly lower levels of physical activity, which would reduce fall risk.
Many of Ann’s patients with ASD had unusual or restricted diets, low levels of physical activity, and were on multiple medications. We have since learned that some factors that contribute to low bone density in ASD include lower levels of weight-bearing physical activity; lower muscle mass; low muscle tone; suboptimal dietary calcium and vitamin D intake; lower vitamin D levels; higher levels of the hormone cortisol, which has deleterious effects on bone; and use of medications that can lower bone density.
In order to mitigate the risk for low bone density and fractures, it is important to optimize physical activity while considering the child’s ability to safely engage in weight-bearing sports.
High-impact sports like gymnastics and jumping, or cross-impact sports like soccer, basketball, field hockey, and lacrosse, are particularly useful in this context, but many patients with ASD are not able to easily engage in typical team sports.
For such children, a prescribed amount of time spent walking, as well as weight and resistance training, could be helpful. The latter would also help increase muscle mass, a key modulator of bone health.
Other strategies include ensuring sufficient intake of calcium and vitamin D through diet and supplements. This can be a particular challenge for children with ASD on specialized diets, such as a gluten-free or dairy-free diet, which are deficient in calcium and vitamin D. Health care providers should check for intake of dairy and dairy products, as well as serum vitamin D levels, and prescribe supplements as needed.
All children should get at least 600 IUs of vitamin D and 1,000-1,300 mg of elemental calcium daily. That said, many with ASD need much higher quantities of vitamin D (1,000-4,000 IUs or more) to maintain levels in the normal range. This is particularly true for dark-skinned children and children with obesity, as well as those who have medical disorders that cause malabsorption.
Higher cortisol levels in the ASD patient population are harder to manage. Efforts to ease anxiety and depression may help reduce cortisol levels. Medications such as protein pump inhibitors and glucocorticosteroids can compromise bone health.
In addition, certain antipsychotics can cause marked elevations in prolactin which, in turn, can lower levels of estrogen and testosterone, which are very important for bone health. In such cases, the clinician should consider switching patients to a different, less detrimental medication or adjust the current medication so that patients receive the lowest possible effective dose.
Obesity is associated with increased fracture risk and with suboptimal bone accrual during childhood, so ensuring a healthy diet is important. This includes avoiding sugary beverages and reducing intake of processed food and juice.
Sometimes, particularly when a child has low bone density and a history of several low-trauma fractures, medications such as bisphosphonates should be considered to increase bone density.
Above all, as physicians who manage ASD, it is essential that we raise awareness about bone health among our colleagues, patients, and their families to help mitigate fracture risk.
Madhusmita Misra, MD, MPH, is chief of the Division of Pediatric Endocrinology at Mass General for Children, Boston.
A version of this article first appeared on Medscape.com.
Many years ago, at the conclusion of a talk I gave on bone health in teens with anorexia nervosa, I was approached by a colleague, Ann Neumeyer, MD, medical director of the Lurie Center for Autism at Massachusetts General Hospital, Boston, who asked about bone health in children with autism spectrum disorder (ASD).
When I explained that there was little information about bone health in this patient population, she suggested that we learn and investigate together. Ann explained that she had observed that some of her patients with ASD had suffered fractures with minimal trauma, raising her concern about their bone health.
This was the beginning of a partnership that led us down the path of many grant submissions, some of which were funded and others that were not, to explore and investigate bone outcomes in children with ASD.
This applies to prepubertal children as well as older children and adolescents. One study showed that 28% and 33% of children with ASD 8-14 years old had very low bone density (z scores of ≤ –2) at the spine and hip, respectively, compared with 0% of typically developing controls.
Studies that have used sophisticated imaging techniques to determine bone strength have shown that it is lower at the forearm and lower leg in children with ASD versus neurotypical children.
These findings are of particular concern during the childhood and teenage years when bone is typically accrued at a rapid rate. A normal rate of bone accrual at this time of life is essential for optimal bone health in later life. While children with ASD gain bone mass at a similar rate as neurotypical controls, they start at a deficit and seem unable to “catch up.”
Further, people with ASD are more prone to certain kinds of fracture than those without the condition. For example, both children and adults with ASD have a high risk for hip fracture, while adult women with ASD have a higher risk for forearm and spine fractures. There is some protection against forearm fractures in children and adult men, probably because of markedly lower levels of physical activity, which would reduce fall risk.
Many of Ann’s patients with ASD had unusual or restricted diets, low levels of physical activity, and were on multiple medications. We have since learned that some factors that contribute to low bone density in ASD include lower levels of weight-bearing physical activity; lower muscle mass; low muscle tone; suboptimal dietary calcium and vitamin D intake; lower vitamin D levels; higher levels of the hormone cortisol, which has deleterious effects on bone; and use of medications that can lower bone density.
In order to mitigate the risk for low bone density and fractures, it is important to optimize physical activity while considering the child’s ability to safely engage in weight-bearing sports.
High-impact sports like gymnastics and jumping, or cross-impact sports like soccer, basketball, field hockey, and lacrosse, are particularly useful in this context, but many patients with ASD are not able to easily engage in typical team sports.
For such children, a prescribed amount of time spent walking, as well as weight and resistance training, could be helpful. The latter would also help increase muscle mass, a key modulator of bone health.
Other strategies include ensuring sufficient intake of calcium and vitamin D through diet and supplements. This can be a particular challenge for children with ASD on specialized diets, such as a gluten-free or dairy-free diet, which are deficient in calcium and vitamin D. Health care providers should check for intake of dairy and dairy products, as well as serum vitamin D levels, and prescribe supplements as needed.
All children should get at least 600 IUs of vitamin D and 1,000-1,300 mg of elemental calcium daily. That said, many with ASD need much higher quantities of vitamin D (1,000-4,000 IUs or more) to maintain levels in the normal range. This is particularly true for dark-skinned children and children with obesity, as well as those who have medical disorders that cause malabsorption.
Higher cortisol levels in the ASD patient population are harder to manage. Efforts to ease anxiety and depression may help reduce cortisol levels. Medications such as protein pump inhibitors and glucocorticosteroids can compromise bone health.
In addition, certain antipsychotics can cause marked elevations in prolactin which, in turn, can lower levels of estrogen and testosterone, which are very important for bone health. In such cases, the clinician should consider switching patients to a different, less detrimental medication or adjust the current medication so that patients receive the lowest possible effective dose.
Obesity is associated with increased fracture risk and with suboptimal bone accrual during childhood, so ensuring a healthy diet is important. This includes avoiding sugary beverages and reducing intake of processed food and juice.
Sometimes, particularly when a child has low bone density and a history of several low-trauma fractures, medications such as bisphosphonates should be considered to increase bone density.
Above all, as physicians who manage ASD, it is essential that we raise awareness about bone health among our colleagues, patients, and their families to help mitigate fracture risk.
Madhusmita Misra, MD, MPH, is chief of the Division of Pediatric Endocrinology at Mass General for Children, Boston.
A version of this article first appeared on Medscape.com.
Many years ago, at the conclusion of a talk I gave on bone health in teens with anorexia nervosa, I was approached by a colleague, Ann Neumeyer, MD, medical director of the Lurie Center for Autism at Massachusetts General Hospital, Boston, who asked about bone health in children with autism spectrum disorder (ASD).
When I explained that there was little information about bone health in this patient population, she suggested that we learn and investigate together. Ann explained that she had observed that some of her patients with ASD had suffered fractures with minimal trauma, raising her concern about their bone health.
This was the beginning of a partnership that led us down the path of many grant submissions, some of which were funded and others that were not, to explore and investigate bone outcomes in children with ASD.
This applies to prepubertal children as well as older children and adolescents. One study showed that 28% and 33% of children with ASD 8-14 years old had very low bone density (z scores of ≤ –2) at the spine and hip, respectively, compared with 0% of typically developing controls.
Studies that have used sophisticated imaging techniques to determine bone strength have shown that it is lower at the forearm and lower leg in children with ASD versus neurotypical children.
These findings are of particular concern during the childhood and teenage years when bone is typically accrued at a rapid rate. A normal rate of bone accrual at this time of life is essential for optimal bone health in later life. While children with ASD gain bone mass at a similar rate as neurotypical controls, they start at a deficit and seem unable to “catch up.”
Further, people with ASD are more prone to certain kinds of fracture than those without the condition. For example, both children and adults with ASD have a high risk for hip fracture, while adult women with ASD have a higher risk for forearm and spine fractures. There is some protection against forearm fractures in children and adult men, probably because of markedly lower levels of physical activity, which would reduce fall risk.
Many of Ann’s patients with ASD had unusual or restricted diets, low levels of physical activity, and were on multiple medications. We have since learned that some factors that contribute to low bone density in ASD include lower levels of weight-bearing physical activity; lower muscle mass; low muscle tone; suboptimal dietary calcium and vitamin D intake; lower vitamin D levels; higher levels of the hormone cortisol, which has deleterious effects on bone; and use of medications that can lower bone density.
In order to mitigate the risk for low bone density and fractures, it is important to optimize physical activity while considering the child’s ability to safely engage in weight-bearing sports.
High-impact sports like gymnastics and jumping, or cross-impact sports like soccer, basketball, field hockey, and lacrosse, are particularly useful in this context, but many patients with ASD are not able to easily engage in typical team sports.
For such children, a prescribed amount of time spent walking, as well as weight and resistance training, could be helpful. The latter would also help increase muscle mass, a key modulator of bone health.
Other strategies include ensuring sufficient intake of calcium and vitamin D through diet and supplements. This can be a particular challenge for children with ASD on specialized diets, such as a gluten-free or dairy-free diet, which are deficient in calcium and vitamin D. Health care providers should check for intake of dairy and dairy products, as well as serum vitamin D levels, and prescribe supplements as needed.
All children should get at least 600 IUs of vitamin D and 1,000-1,300 mg of elemental calcium daily. That said, many with ASD need much higher quantities of vitamin D (1,000-4,000 IUs or more) to maintain levels in the normal range. This is particularly true for dark-skinned children and children with obesity, as well as those who have medical disorders that cause malabsorption.
Higher cortisol levels in the ASD patient population are harder to manage. Efforts to ease anxiety and depression may help reduce cortisol levels. Medications such as protein pump inhibitors and glucocorticosteroids can compromise bone health.
In addition, certain antipsychotics can cause marked elevations in prolactin which, in turn, can lower levels of estrogen and testosterone, which are very important for bone health. In such cases, the clinician should consider switching patients to a different, less detrimental medication or adjust the current medication so that patients receive the lowest possible effective dose.
Obesity is associated with increased fracture risk and with suboptimal bone accrual during childhood, so ensuring a healthy diet is important. This includes avoiding sugary beverages and reducing intake of processed food and juice.
Sometimes, particularly when a child has low bone density and a history of several low-trauma fractures, medications such as bisphosphonates should be considered to increase bone density.
Above all, as physicians who manage ASD, it is essential that we raise awareness about bone health among our colleagues, patients, and their families to help mitigate fracture risk.
Madhusmita Misra, MD, MPH, is chief of the Division of Pediatric Endocrinology at Mass General for Children, Boston.
A version of this article first appeared on Medscape.com.
Long-term impact of childhood trauma explained
WASHINGTON –
“We already knew childhood trauma is associated with the later development of depressive and anxiety disorders, but it’s been unclear what makes sufferers of early trauma more likely to develop these psychiatric conditions,” study investigator Erika Kuzminskaite, PhD candidate, department of psychiatry, Amsterdam University Medical Center (UMC), the Netherlands, told this news organization.
“The evidence now points to unbalanced stress systems as a possible cause of this vulnerability, and now the most important question is, how we can develop preventive interventions,” she added.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Elevated cortisol, inflammation
The study included 2,779 adults from the Netherlands Study of Depression and Anxiety (NESDA). Two thirds of participants were female.
Participants retrospectively reported childhood trauma, defined as emotional, physical, or sexual abuse or emotional or physical neglect, before the age of 18 years. Severe trauma was defined as multiple types or increased frequency of abuse.
Of the total cohort, 48% reported experiencing some childhood trauma – 21% reported severe trauma, 27% reported mild trauma, and 42% reported no childhood trauma.
Among those with trauma, 89% had a current or remitted anxiety or depressive disorder, and 11% had no psychiatric sequelae. Among participants who reported no trauma, 68% had a current or remitted disorder, and 32% had no psychiatric disorders.
At baseline, researchers assessed markers of major bodily stress systems, including the hypothalamic-pituitary-adrenal (HPA) axis, the immune-inflammatory system, and the autonomic nervous system (ANS). They examined these markers separately and cumulatively.
In one model, investigators found that levels of cortisol and inflammation were significantly elevated in those with severe childhood trauma compared to those with no childhood trauma. The effects were largest for the cumulative markers for HPA-axis, inflammation, and all stress system markers (Cohen’s d = 0.23, 0.12, and 0.25, respectively). There was no association with ANS markers.
The results were partially explained by lifestyle, said Ms. Kuzminskaite, who noted that people with severe childhood trauma tend to have a higher body mass index, smoke more, and have other unhealthy habits that may represent a “coping” mechanism for trauma.
Those who experienced childhood trauma also have higher rates of other disorders, including asthma, diabetes, and cardiovascular disease. Ms. Kuzminskaite noted that people with childhood trauma have at least double the risk of cancer in later life.
When researchers adjusted for lifestyle factors and chronic conditions, the association for cortisol was reduced and that for inflammation disappeared. However, the cumulative inflammatory markers remained significant.
Another model examined lipopolysaccharide-stimulated (LPS) immune-inflammatory markers by childhood trauma severity. This provides a more “dynamic” measure of stress systems than looking only at static circulating levels in the blood, as was done in the first model, said Ms. Kuzminskaite.
“These levels should theoretically be more affected by experiences such as childhood trauma and they are also less sensitive to lifestyle.”
Here, researchers found significant positive associations with childhood trauma, especially severe trauma, after adjusting for lifestyle and health-related covariates (cumulative index d = 0.19).
“Almost all people with childhood trauma, especially severe trauma, had LPS-stimulated cytokines upregulated,” said Ms. Kuzminskaite. “So again, there is this dysregulation of immune system functioning in these subjects.”
And again, the strongest effect was for the cumulative index of all cytokines, she said.
Personalized interventions
Ms. Kuzminskaite noted the importance of learning the impact of early trauma on stress responses. “The goal is to eventually have personalized interventions for people with depression or anxiety related to childhood trauma, or even preventative interventions. If we know, for example, something is going wrong with a patient’s stress systems, we can suggest some therapeutic targets.”
Investigators in Amsterdam are examining the efficacy of mifepristone, which blocks progesterone and is used along with misoprostol for medication abortions and to treat high blood sugar. “The drug is supposed to reset the stress system functioning,” said Ms. Kuzminskaite.
It’s still important to target unhealthy lifestyle habits “that are really impacting the functioning of the stress systems,” she said. Lifestyle interventions could improve the efficacy of treatments for depression, for example, she added.
Luana Marques, PhD, associate professor, department of psychiatry, Harvard Medical School, Boston, said such research is important.
“It reveals the potentially extensive and long-lasting impact of childhood trauma on functioning. The findings underscore the importance of equipping at-risk and trauma-exposed youth with evidence-based skills for managing stress,” she said.
No conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
WASHINGTON –
“We already knew childhood trauma is associated with the later development of depressive and anxiety disorders, but it’s been unclear what makes sufferers of early trauma more likely to develop these psychiatric conditions,” study investigator Erika Kuzminskaite, PhD candidate, department of psychiatry, Amsterdam University Medical Center (UMC), the Netherlands, told this news organization.
“The evidence now points to unbalanced stress systems as a possible cause of this vulnerability, and now the most important question is, how we can develop preventive interventions,” she added.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Elevated cortisol, inflammation
The study included 2,779 adults from the Netherlands Study of Depression and Anxiety (NESDA). Two thirds of participants were female.
Participants retrospectively reported childhood trauma, defined as emotional, physical, or sexual abuse or emotional or physical neglect, before the age of 18 years. Severe trauma was defined as multiple types or increased frequency of abuse.
Of the total cohort, 48% reported experiencing some childhood trauma – 21% reported severe trauma, 27% reported mild trauma, and 42% reported no childhood trauma.
Among those with trauma, 89% had a current or remitted anxiety or depressive disorder, and 11% had no psychiatric sequelae. Among participants who reported no trauma, 68% had a current or remitted disorder, and 32% had no psychiatric disorders.
At baseline, researchers assessed markers of major bodily stress systems, including the hypothalamic-pituitary-adrenal (HPA) axis, the immune-inflammatory system, and the autonomic nervous system (ANS). They examined these markers separately and cumulatively.
In one model, investigators found that levels of cortisol and inflammation were significantly elevated in those with severe childhood trauma compared to those with no childhood trauma. The effects were largest for the cumulative markers for HPA-axis, inflammation, and all stress system markers (Cohen’s d = 0.23, 0.12, and 0.25, respectively). There was no association with ANS markers.
The results were partially explained by lifestyle, said Ms. Kuzminskaite, who noted that people with severe childhood trauma tend to have a higher body mass index, smoke more, and have other unhealthy habits that may represent a “coping” mechanism for trauma.
Those who experienced childhood trauma also have higher rates of other disorders, including asthma, diabetes, and cardiovascular disease. Ms. Kuzminskaite noted that people with childhood trauma have at least double the risk of cancer in later life.
When researchers adjusted for lifestyle factors and chronic conditions, the association for cortisol was reduced and that for inflammation disappeared. However, the cumulative inflammatory markers remained significant.
Another model examined lipopolysaccharide-stimulated (LPS) immune-inflammatory markers by childhood trauma severity. This provides a more “dynamic” measure of stress systems than looking only at static circulating levels in the blood, as was done in the first model, said Ms. Kuzminskaite.
“These levels should theoretically be more affected by experiences such as childhood trauma and they are also less sensitive to lifestyle.”
Here, researchers found significant positive associations with childhood trauma, especially severe trauma, after adjusting for lifestyle and health-related covariates (cumulative index d = 0.19).
“Almost all people with childhood trauma, especially severe trauma, had LPS-stimulated cytokines upregulated,” said Ms. Kuzminskaite. “So again, there is this dysregulation of immune system functioning in these subjects.”
And again, the strongest effect was for the cumulative index of all cytokines, she said.
Personalized interventions
Ms. Kuzminskaite noted the importance of learning the impact of early trauma on stress responses. “The goal is to eventually have personalized interventions for people with depression or anxiety related to childhood trauma, or even preventative interventions. If we know, for example, something is going wrong with a patient’s stress systems, we can suggest some therapeutic targets.”
Investigators in Amsterdam are examining the efficacy of mifepristone, which blocks progesterone and is used along with misoprostol for medication abortions and to treat high blood sugar. “The drug is supposed to reset the stress system functioning,” said Ms. Kuzminskaite.
It’s still important to target unhealthy lifestyle habits “that are really impacting the functioning of the stress systems,” she said. Lifestyle interventions could improve the efficacy of treatments for depression, for example, she added.
Luana Marques, PhD, associate professor, department of psychiatry, Harvard Medical School, Boston, said such research is important.
“It reveals the potentially extensive and long-lasting impact of childhood trauma on functioning. The findings underscore the importance of equipping at-risk and trauma-exposed youth with evidence-based skills for managing stress,” she said.
No conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
WASHINGTON –
“We already knew childhood trauma is associated with the later development of depressive and anxiety disorders, but it’s been unclear what makes sufferers of early trauma more likely to develop these psychiatric conditions,” study investigator Erika Kuzminskaite, PhD candidate, department of psychiatry, Amsterdam University Medical Center (UMC), the Netherlands, told this news organization.
“The evidence now points to unbalanced stress systems as a possible cause of this vulnerability, and now the most important question is, how we can develop preventive interventions,” she added.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Elevated cortisol, inflammation
The study included 2,779 adults from the Netherlands Study of Depression and Anxiety (NESDA). Two thirds of participants were female.
Participants retrospectively reported childhood trauma, defined as emotional, physical, or sexual abuse or emotional or physical neglect, before the age of 18 years. Severe trauma was defined as multiple types or increased frequency of abuse.
Of the total cohort, 48% reported experiencing some childhood trauma – 21% reported severe trauma, 27% reported mild trauma, and 42% reported no childhood trauma.
Among those with trauma, 89% had a current or remitted anxiety or depressive disorder, and 11% had no psychiatric sequelae. Among participants who reported no trauma, 68% had a current or remitted disorder, and 32% had no psychiatric disorders.
At baseline, researchers assessed markers of major bodily stress systems, including the hypothalamic-pituitary-adrenal (HPA) axis, the immune-inflammatory system, and the autonomic nervous system (ANS). They examined these markers separately and cumulatively.
In one model, investigators found that levels of cortisol and inflammation were significantly elevated in those with severe childhood trauma compared to those with no childhood trauma. The effects were largest for the cumulative markers for HPA-axis, inflammation, and all stress system markers (Cohen’s d = 0.23, 0.12, and 0.25, respectively). There was no association with ANS markers.
The results were partially explained by lifestyle, said Ms. Kuzminskaite, who noted that people with severe childhood trauma tend to have a higher body mass index, smoke more, and have other unhealthy habits that may represent a “coping” mechanism for trauma.
Those who experienced childhood trauma also have higher rates of other disorders, including asthma, diabetes, and cardiovascular disease. Ms. Kuzminskaite noted that people with childhood trauma have at least double the risk of cancer in later life.
When researchers adjusted for lifestyle factors and chronic conditions, the association for cortisol was reduced and that for inflammation disappeared. However, the cumulative inflammatory markers remained significant.
Another model examined lipopolysaccharide-stimulated (LPS) immune-inflammatory markers by childhood trauma severity. This provides a more “dynamic” measure of stress systems than looking only at static circulating levels in the blood, as was done in the first model, said Ms. Kuzminskaite.
“These levels should theoretically be more affected by experiences such as childhood trauma and they are also less sensitive to lifestyle.”
Here, researchers found significant positive associations with childhood trauma, especially severe trauma, after adjusting for lifestyle and health-related covariates (cumulative index d = 0.19).
“Almost all people with childhood trauma, especially severe trauma, had LPS-stimulated cytokines upregulated,” said Ms. Kuzminskaite. “So again, there is this dysregulation of immune system functioning in these subjects.”
And again, the strongest effect was for the cumulative index of all cytokines, she said.
Personalized interventions
Ms. Kuzminskaite noted the importance of learning the impact of early trauma on stress responses. “The goal is to eventually have personalized interventions for people with depression or anxiety related to childhood trauma, or even preventative interventions. If we know, for example, something is going wrong with a patient’s stress systems, we can suggest some therapeutic targets.”
Investigators in Amsterdam are examining the efficacy of mifepristone, which blocks progesterone and is used along with misoprostol for medication abortions and to treat high blood sugar. “The drug is supposed to reset the stress system functioning,” said Ms. Kuzminskaite.
It’s still important to target unhealthy lifestyle habits “that are really impacting the functioning of the stress systems,” she said. Lifestyle interventions could improve the efficacy of treatments for depression, for example, she added.
Luana Marques, PhD, associate professor, department of psychiatry, Harvard Medical School, Boston, said such research is important.
“It reveals the potentially extensive and long-lasting impact of childhood trauma on functioning. The findings underscore the importance of equipping at-risk and trauma-exposed youth with evidence-based skills for managing stress,” she said.
No conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
AT ADAA 2023
“Terrific progress”: Adding blinatumomab for infant leukemia
.
Two-year disease-free and overall survival measures, as well as the percentage of children who had complete minimal residual disease (MRD) responses, were substantially higher among the 30 infants in the study than in historical controls treated with the same chemotherapy backbone in an earlier trial, Interfant-06.
“These outcome data are very promising, given the poor survival and lack of improvements in outcomes among infants with KMT2A-rearranged ALL in recent decades,” said the investigators, led by Inge M. van der Sluis, MD, PhD, a hematologist-oncologist at Princess Maxima Center for Pediatric Oncology in Utrecht, the Netherlands.
“The low incidence of relapse after treatment with blinatumomab is remarkable, given that in historical controls relapses occur frequently and early during therapy,” the investigators stated. Although the “follow-up time was relatively short” in the study, “it included the period historically defined” as being at high risk of relapse, they said.
The team suggested that future research should assess whether infants benefit from multiple courses of blinatumomab, rather than the one course used in the study, and whether blinatumomab plus chemotherapy can replace stem cell transplants for high-risk infants.
Pediatric community responds
There was excitement on Twitter about the results; a number of pediatric blood cancer specialists were impressed and posted the study on that platform. Comments included, “Wow! After years of stagnation, a huge step forward for infant leukemia” and “great news for infant lymphoblastic leukemia.”
Akshay Sharma, MBBS, a pediatric bone marrow transplant and cellular therapy specialist at St. Jude Children’s Research Hospital, Memphis, also posted. He said in an interview that the findings are “very exciting.”
The “outcomes of children diagnosed with leukemia in their infancy, particularly if they have a KMT2A rearrangement, have been dismal. This is terrific progress and a testament to the role that immunotherapy and novel agents will be playing in treatment of several malignant diseases in the decade to come,” he said.
Another poster, Pratik “Tik” Patel, MD, a pediatric hematology/oncology fellow at Emory University in Atlanta, told this news organization that the study “is welcome news to pediatric oncologists” and highlights “the success in incorporating newer immune-based therapeutics upfront in treatment rather than in relapsed/refractory settings.”
The National Cancer Institute–funded Children’s Oncology Group is thinking the same way. The group is launching a large, randomized trial to test if adding blinatumomab to chemotherapy upfront for B-cell acute lymphoblastic leukemia and lymphoblastic lymphoma improves outcomes in children and young adults aged 1-31 years. Results are due after 2029.
Study details
Blinatumomab is an expensive “T-cell engager” that helps cytotoxic CD3+T cells link to and destroy leukemic CD19+ B cells. Past studies have shown that it’s safe and works in older children and adults with B-lineage ALL after intensive chemotherapy, but until now the approach hadn’t been tested in infants, the investigators said.
The 30 subjects in the study were under a year old and newly diagnosed with KMT2A-rearranged ALL. They were treated with the Interfant-06 chemotherapy regimen – cytosine arabinoside and other agents – plus one postinduction course of blinatumomab at 15 micrograms/m2 per day as a 4-week continuous infusion. Eight of nine high-risk patients had allogeneic hematopoietic stem cell transplants.
Overall survival was 93.3% over a median follow up of 26.3 months, substantially higher than the 65.8% in the Interfant-06 trial. Two-year disease-free survival was 81.6% versus 49.4% in Interfant-06.
Sixteen patients (53%) were MRD negative after blinatumomab infusion and 12 (40%) had low levels of MRD. All of the children who continued chemotherapy went on to become MRD negative.
There were no permanent blinatumomab discontinuations and no treatment related deaths. Serious toxic effects were consistent with those in older patients and included four fevers, four infections, and one case each of hypertension and vomiting.
There were no cases of severe cytokine release syndrome (CRS) because of the low tumor burden of the subjects. Likewise, there were no obvious neurologic adverse events – like CRS, a particular concern with blinatumomab – but “we cannot rule out underreporting of mild neurologic symptoms that may have been unrecognized in infants,” the investigators said.
Patients who relapsed in the study had CNS involvement at relapse. “This underscores the need for adequate intrathecal chemotherapy during the blinatumomab infusion, because the efficacy of blinatumomab for the treatment of CNS leukemia may be limited,” they said.
The work was supported by Amgen, the maker of blinatumomab, as well as the Princess Maxima Center Foundation, the Danish Childhood Cancer Foundation, and others. Dr. Sluis is a consultant and researcher for Amgen. Five other authors were also consultants/advisers/researchers for the company. Dr. Sharma and Dr. Patel didn’t have any relevant disclosures.
.
Two-year disease-free and overall survival measures, as well as the percentage of children who had complete minimal residual disease (MRD) responses, were substantially higher among the 30 infants in the study than in historical controls treated with the same chemotherapy backbone in an earlier trial, Interfant-06.
“These outcome data are very promising, given the poor survival and lack of improvements in outcomes among infants with KMT2A-rearranged ALL in recent decades,” said the investigators, led by Inge M. van der Sluis, MD, PhD, a hematologist-oncologist at Princess Maxima Center for Pediatric Oncology in Utrecht, the Netherlands.
“The low incidence of relapse after treatment with blinatumomab is remarkable, given that in historical controls relapses occur frequently and early during therapy,” the investigators stated. Although the “follow-up time was relatively short” in the study, “it included the period historically defined” as being at high risk of relapse, they said.
The team suggested that future research should assess whether infants benefit from multiple courses of blinatumomab, rather than the one course used in the study, and whether blinatumomab plus chemotherapy can replace stem cell transplants for high-risk infants.
Pediatric community responds
There was excitement on Twitter about the results; a number of pediatric blood cancer specialists were impressed and posted the study on that platform. Comments included, “Wow! After years of stagnation, a huge step forward for infant leukemia” and “great news for infant lymphoblastic leukemia.”
Akshay Sharma, MBBS, a pediatric bone marrow transplant and cellular therapy specialist at St. Jude Children’s Research Hospital, Memphis, also posted. He said in an interview that the findings are “very exciting.”
The “outcomes of children diagnosed with leukemia in their infancy, particularly if they have a KMT2A rearrangement, have been dismal. This is terrific progress and a testament to the role that immunotherapy and novel agents will be playing in treatment of several malignant diseases in the decade to come,” he said.
Another poster, Pratik “Tik” Patel, MD, a pediatric hematology/oncology fellow at Emory University in Atlanta, told this news organization that the study “is welcome news to pediatric oncologists” and highlights “the success in incorporating newer immune-based therapeutics upfront in treatment rather than in relapsed/refractory settings.”
The National Cancer Institute–funded Children’s Oncology Group is thinking the same way. The group is launching a large, randomized trial to test if adding blinatumomab to chemotherapy upfront for B-cell acute lymphoblastic leukemia and lymphoblastic lymphoma improves outcomes in children and young adults aged 1-31 years. Results are due after 2029.
Study details
Blinatumomab is an expensive “T-cell engager” that helps cytotoxic CD3+T cells link to and destroy leukemic CD19+ B cells. Past studies have shown that it’s safe and works in older children and adults with B-lineage ALL after intensive chemotherapy, but until now the approach hadn’t been tested in infants, the investigators said.
The 30 subjects in the study were under a year old and newly diagnosed with KMT2A-rearranged ALL. They were treated with the Interfant-06 chemotherapy regimen – cytosine arabinoside and other agents – plus one postinduction course of blinatumomab at 15 micrograms/m2 per day as a 4-week continuous infusion. Eight of nine high-risk patients had allogeneic hematopoietic stem cell transplants.
Overall survival was 93.3% over a median follow up of 26.3 months, substantially higher than the 65.8% in the Interfant-06 trial. Two-year disease-free survival was 81.6% versus 49.4% in Interfant-06.
Sixteen patients (53%) were MRD negative after blinatumomab infusion and 12 (40%) had low levels of MRD. All of the children who continued chemotherapy went on to become MRD negative.
There were no permanent blinatumomab discontinuations and no treatment related deaths. Serious toxic effects were consistent with those in older patients and included four fevers, four infections, and one case each of hypertension and vomiting.
There were no cases of severe cytokine release syndrome (CRS) because of the low tumor burden of the subjects. Likewise, there were no obvious neurologic adverse events – like CRS, a particular concern with blinatumomab – but “we cannot rule out underreporting of mild neurologic symptoms that may have been unrecognized in infants,” the investigators said.
Patients who relapsed in the study had CNS involvement at relapse. “This underscores the need for adequate intrathecal chemotherapy during the blinatumomab infusion, because the efficacy of blinatumomab for the treatment of CNS leukemia may be limited,” they said.
The work was supported by Amgen, the maker of blinatumomab, as well as the Princess Maxima Center Foundation, the Danish Childhood Cancer Foundation, and others. Dr. Sluis is a consultant and researcher for Amgen. Five other authors were also consultants/advisers/researchers for the company. Dr. Sharma and Dr. Patel didn’t have any relevant disclosures.
.
Two-year disease-free and overall survival measures, as well as the percentage of children who had complete minimal residual disease (MRD) responses, were substantially higher among the 30 infants in the study than in historical controls treated with the same chemotherapy backbone in an earlier trial, Interfant-06.
“These outcome data are very promising, given the poor survival and lack of improvements in outcomes among infants with KMT2A-rearranged ALL in recent decades,” said the investigators, led by Inge M. van der Sluis, MD, PhD, a hematologist-oncologist at Princess Maxima Center for Pediatric Oncology in Utrecht, the Netherlands.
“The low incidence of relapse after treatment with blinatumomab is remarkable, given that in historical controls relapses occur frequently and early during therapy,” the investigators stated. Although the “follow-up time was relatively short” in the study, “it included the period historically defined” as being at high risk of relapse, they said.
The team suggested that future research should assess whether infants benefit from multiple courses of blinatumomab, rather than the one course used in the study, and whether blinatumomab plus chemotherapy can replace stem cell transplants for high-risk infants.
Pediatric community responds
There was excitement on Twitter about the results; a number of pediatric blood cancer specialists were impressed and posted the study on that platform. Comments included, “Wow! After years of stagnation, a huge step forward for infant leukemia” and “great news for infant lymphoblastic leukemia.”
Akshay Sharma, MBBS, a pediatric bone marrow transplant and cellular therapy specialist at St. Jude Children’s Research Hospital, Memphis, also posted. He said in an interview that the findings are “very exciting.”
The “outcomes of children diagnosed with leukemia in their infancy, particularly if they have a KMT2A rearrangement, have been dismal. This is terrific progress and a testament to the role that immunotherapy and novel agents will be playing in treatment of several malignant diseases in the decade to come,” he said.
Another poster, Pratik “Tik” Patel, MD, a pediatric hematology/oncology fellow at Emory University in Atlanta, told this news organization that the study “is welcome news to pediatric oncologists” and highlights “the success in incorporating newer immune-based therapeutics upfront in treatment rather than in relapsed/refractory settings.”
The National Cancer Institute–funded Children’s Oncology Group is thinking the same way. The group is launching a large, randomized trial to test if adding blinatumomab to chemotherapy upfront for B-cell acute lymphoblastic leukemia and lymphoblastic lymphoma improves outcomes in children and young adults aged 1-31 years. Results are due after 2029.
Study details
Blinatumomab is an expensive “T-cell engager” that helps cytotoxic CD3+T cells link to and destroy leukemic CD19+ B cells. Past studies have shown that it’s safe and works in older children and adults with B-lineage ALL after intensive chemotherapy, but until now the approach hadn’t been tested in infants, the investigators said.
The 30 subjects in the study were under a year old and newly diagnosed with KMT2A-rearranged ALL. They were treated with the Interfant-06 chemotherapy regimen – cytosine arabinoside and other agents – plus one postinduction course of blinatumomab at 15 micrograms/m2 per day as a 4-week continuous infusion. Eight of nine high-risk patients had allogeneic hematopoietic stem cell transplants.
Overall survival was 93.3% over a median follow up of 26.3 months, substantially higher than the 65.8% in the Interfant-06 trial. Two-year disease-free survival was 81.6% versus 49.4% in Interfant-06.
Sixteen patients (53%) were MRD negative after blinatumomab infusion and 12 (40%) had low levels of MRD. All of the children who continued chemotherapy went on to become MRD negative.
There were no permanent blinatumomab discontinuations and no treatment related deaths. Serious toxic effects were consistent with those in older patients and included four fevers, four infections, and one case each of hypertension and vomiting.
There were no cases of severe cytokine release syndrome (CRS) because of the low tumor burden of the subjects. Likewise, there were no obvious neurologic adverse events – like CRS, a particular concern with blinatumomab – but “we cannot rule out underreporting of mild neurologic symptoms that may have been unrecognized in infants,” the investigators said.
Patients who relapsed in the study had CNS involvement at relapse. “This underscores the need for adequate intrathecal chemotherapy during the blinatumomab infusion, because the efficacy of blinatumomab for the treatment of CNS leukemia may be limited,” they said.
The work was supported by Amgen, the maker of blinatumomab, as well as the Princess Maxima Center Foundation, the Danish Childhood Cancer Foundation, and others. Dr. Sluis is a consultant and researcher for Amgen. Five other authors were also consultants/advisers/researchers for the company. Dr. Sharma and Dr. Patel didn’t have any relevant disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Survey reveals room for improvement in teen substance use screening
WASHINGTON – , Deepa Camenga, MD, said in a presentation at the 2023 Pediatric Academic Societies annual meeting.
The American Academy of Pediatrics recommends universal screening for substance use in adolescents during annual health visits, but current screening rates and practices among primary care pediatricians in the United States are unknown, said Dr. Camenga, an associate professor at Yale University, New Haven, Conn.
Uniformity in screening is lacking
Dr. Camenga presented data from the 2021 AAP Periodic Survey, which included 1,683 nonretired AAP members in the United States. Residents were excluded. The current analysis included 471 pediatricians who reported providing health supervision to adolescents. Overall, 284 of the 471 included respondents (60%) reported always screening adolescent patients for substance use during a health supervision visit. Of these, 42% reported using a standardized screening instrument, Dr. Camenga said.
The majority (70%) of pediatricians who used a standardized screening tool opted for the CRAFFT tool (Car, Relax, Alone, Forget, Friends, Trouble) designed for ages 12-21 years. Another 21% reported using an unspecified screening tool, 4% used RAAPS (Rapid Assessment for Adolescent Preventive Services), 3% used S2BI (Screening to Brief Intervention), and 1% used BSTAD (Brief Screener for Tobacco, Alcohol, and other Drugs).
A total of 77% of respondents reported screening their adolescent patients for substance use without a parent or guardian present. Approximately half (52%) used paper-based screening, 22% used electronic screening, 21% used verbal screening, and 6% reported other methods.
A total of 68% and 70% of respondents, respectively, agreed or strongly agreed that top barriers to screening were the lack of an onsite provider for counseling and the lack of readily available treatment options. Other reported barriers included lack of knowledge or information, patient reluctance to discuss substance use, too many other priorities during the visit, and inadequate payment. Only 6% of respondents strongly agreed that lack of time was a barrier, said Dr. Camenga.
Screening frequency and screening practices varied by geographic region, Dr. Camenga said. Pediatricians in the South and Midwest were only half as likely as those in the Northeast to report always screening adolescents for substance use (adjusted odds ratio, 0.43 and 0.53, respectively; P < .05). Similarly, compared with pediatricians in the Northeast, those in the South, Midwest, and West were significantly less likely to report using a standardized instrument for substance use screening (aOR, 0.53, 0.24, and 0.52, respectively; P < 0.001 for all).
The disparities in screening by geographic region show that there is room for improvement in this area, said Dr. Camenga. Systems-level interventions such as treatment financing and access to telehealth services could improve primary care access to substance use treatment professionals, she said.
At the practice level, embedding screening and referral tools into electronic health records could potentially improve screening rates. Many primary care pediatricians do not receive training in identifying and assessing substance use in their patients, or in first-line treatment, Dr. Camenga said.
“We have to invest in a ‘train the trainer’ type of model,” she emphasized.
Data highlight regional resource gaps
The current study is important because it highlights potential missed opportunities to screen adolescents for substance use, said Sarah Yale, MD, assistant professor of pediatrics at the Medical College of Wisconsin, Milwaukee, in an interview. Dr. Yale said that the disparities in screening by region are interesting and should serve as a focus for resource investment because the lack of specialists for referral and treatment options in these areas is likely a contributing factor.
However, lack of training also plays a role, said Dr. Yale, who was not involved in the study but served as a moderator of the presentation session at the meeting. Many pediatricians in practice have not been trained in substance use screening, and the fact that many of those who did try to screen were not using a standardized screening tool indicates a need for provider education, she said. The take-home message for clinicians is to find ways to include substance use screening in the care of their adolescent patients. Additionally, more research is needed to assess how best to integrate screening tools into visits, whether on paper, electronically, or verbally, and to include training on substance use screening during pediatric medical training.
The survey was conducted by the American Academy of Pediatrics Research Division. This year’s survey was supported by the Conrad N. Hilton Foundation. Dr. Camenga had no financial conflicts to disclose. Dr. Yale had no financial conflicts to disclose.
WASHINGTON – , Deepa Camenga, MD, said in a presentation at the 2023 Pediatric Academic Societies annual meeting.
The American Academy of Pediatrics recommends universal screening for substance use in adolescents during annual health visits, but current screening rates and practices among primary care pediatricians in the United States are unknown, said Dr. Camenga, an associate professor at Yale University, New Haven, Conn.
Uniformity in screening is lacking
Dr. Camenga presented data from the 2021 AAP Periodic Survey, which included 1,683 nonretired AAP members in the United States. Residents were excluded. The current analysis included 471 pediatricians who reported providing health supervision to adolescents. Overall, 284 of the 471 included respondents (60%) reported always screening adolescent patients for substance use during a health supervision visit. Of these, 42% reported using a standardized screening instrument, Dr. Camenga said.
The majority (70%) of pediatricians who used a standardized screening tool opted for the CRAFFT tool (Car, Relax, Alone, Forget, Friends, Trouble) designed for ages 12-21 years. Another 21% reported using an unspecified screening tool, 4% used RAAPS (Rapid Assessment for Adolescent Preventive Services), 3% used S2BI (Screening to Brief Intervention), and 1% used BSTAD (Brief Screener for Tobacco, Alcohol, and other Drugs).
A total of 77% of respondents reported screening their adolescent patients for substance use without a parent or guardian present. Approximately half (52%) used paper-based screening, 22% used electronic screening, 21% used verbal screening, and 6% reported other methods.
A total of 68% and 70% of respondents, respectively, agreed or strongly agreed that top barriers to screening were the lack of an onsite provider for counseling and the lack of readily available treatment options. Other reported barriers included lack of knowledge or information, patient reluctance to discuss substance use, too many other priorities during the visit, and inadequate payment. Only 6% of respondents strongly agreed that lack of time was a barrier, said Dr. Camenga.
Screening frequency and screening practices varied by geographic region, Dr. Camenga said. Pediatricians in the South and Midwest were only half as likely as those in the Northeast to report always screening adolescents for substance use (adjusted odds ratio, 0.43 and 0.53, respectively; P < .05). Similarly, compared with pediatricians in the Northeast, those in the South, Midwest, and West were significantly less likely to report using a standardized instrument for substance use screening (aOR, 0.53, 0.24, and 0.52, respectively; P < 0.001 for all).
The disparities in screening by geographic region show that there is room for improvement in this area, said Dr. Camenga. Systems-level interventions such as treatment financing and access to telehealth services could improve primary care access to substance use treatment professionals, she said.
At the practice level, embedding screening and referral tools into electronic health records could potentially improve screening rates. Many primary care pediatricians do not receive training in identifying and assessing substance use in their patients, or in first-line treatment, Dr. Camenga said.
“We have to invest in a ‘train the trainer’ type of model,” she emphasized.
Data highlight regional resource gaps
The current study is important because it highlights potential missed opportunities to screen adolescents for substance use, said Sarah Yale, MD, assistant professor of pediatrics at the Medical College of Wisconsin, Milwaukee, in an interview. Dr. Yale said that the disparities in screening by region are interesting and should serve as a focus for resource investment because the lack of specialists for referral and treatment options in these areas is likely a contributing factor.
However, lack of training also plays a role, said Dr. Yale, who was not involved in the study but served as a moderator of the presentation session at the meeting. Many pediatricians in practice have not been trained in substance use screening, and the fact that many of those who did try to screen were not using a standardized screening tool indicates a need for provider education, she said. The take-home message for clinicians is to find ways to include substance use screening in the care of their adolescent patients. Additionally, more research is needed to assess how best to integrate screening tools into visits, whether on paper, electronically, or verbally, and to include training on substance use screening during pediatric medical training.
The survey was conducted by the American Academy of Pediatrics Research Division. This year’s survey was supported by the Conrad N. Hilton Foundation. Dr. Camenga had no financial conflicts to disclose. Dr. Yale had no financial conflicts to disclose.
WASHINGTON – , Deepa Camenga, MD, said in a presentation at the 2023 Pediatric Academic Societies annual meeting.
The American Academy of Pediatrics recommends universal screening for substance use in adolescents during annual health visits, but current screening rates and practices among primary care pediatricians in the United States are unknown, said Dr. Camenga, an associate professor at Yale University, New Haven, Conn.
Uniformity in screening is lacking
Dr. Camenga presented data from the 2021 AAP Periodic Survey, which included 1,683 nonretired AAP members in the United States. Residents were excluded. The current analysis included 471 pediatricians who reported providing health supervision to adolescents. Overall, 284 of the 471 included respondents (60%) reported always screening adolescent patients for substance use during a health supervision visit. Of these, 42% reported using a standardized screening instrument, Dr. Camenga said.
The majority (70%) of pediatricians who used a standardized screening tool opted for the CRAFFT tool (Car, Relax, Alone, Forget, Friends, Trouble) designed for ages 12-21 years. Another 21% reported using an unspecified screening tool, 4% used RAAPS (Rapid Assessment for Adolescent Preventive Services), 3% used S2BI (Screening to Brief Intervention), and 1% used BSTAD (Brief Screener for Tobacco, Alcohol, and other Drugs).
A total of 77% of respondents reported screening their adolescent patients for substance use without a parent or guardian present. Approximately half (52%) used paper-based screening, 22% used electronic screening, 21% used verbal screening, and 6% reported other methods.
A total of 68% and 70% of respondents, respectively, agreed or strongly agreed that top barriers to screening were the lack of an onsite provider for counseling and the lack of readily available treatment options. Other reported barriers included lack of knowledge or information, patient reluctance to discuss substance use, too many other priorities during the visit, and inadequate payment. Only 6% of respondents strongly agreed that lack of time was a barrier, said Dr. Camenga.
Screening frequency and screening practices varied by geographic region, Dr. Camenga said. Pediatricians in the South and Midwest were only half as likely as those in the Northeast to report always screening adolescents for substance use (adjusted odds ratio, 0.43 and 0.53, respectively; P < .05). Similarly, compared with pediatricians in the Northeast, those in the South, Midwest, and West were significantly less likely to report using a standardized instrument for substance use screening (aOR, 0.53, 0.24, and 0.52, respectively; P < 0.001 for all).
The disparities in screening by geographic region show that there is room for improvement in this area, said Dr. Camenga. Systems-level interventions such as treatment financing and access to telehealth services could improve primary care access to substance use treatment professionals, she said.
At the practice level, embedding screening and referral tools into electronic health records could potentially improve screening rates. Many primary care pediatricians do not receive training in identifying and assessing substance use in their patients, or in first-line treatment, Dr. Camenga said.
“We have to invest in a ‘train the trainer’ type of model,” she emphasized.
Data highlight regional resource gaps
The current study is important because it highlights potential missed opportunities to screen adolescents for substance use, said Sarah Yale, MD, assistant professor of pediatrics at the Medical College of Wisconsin, Milwaukee, in an interview. Dr. Yale said that the disparities in screening by region are interesting and should serve as a focus for resource investment because the lack of specialists for referral and treatment options in these areas is likely a contributing factor.
However, lack of training also plays a role, said Dr. Yale, who was not involved in the study but served as a moderator of the presentation session at the meeting. Many pediatricians in practice have not been trained in substance use screening, and the fact that many of those who did try to screen were not using a standardized screening tool indicates a need for provider education, she said. The take-home message for clinicians is to find ways to include substance use screening in the care of their adolescent patients. Additionally, more research is needed to assess how best to integrate screening tools into visits, whether on paper, electronically, or verbally, and to include training on substance use screening during pediatric medical training.
The survey was conducted by the American Academy of Pediatrics Research Division. This year’s survey was supported by the Conrad N. Hilton Foundation. Dr. Camenga had no financial conflicts to disclose. Dr. Yale had no financial conflicts to disclose.
AT PAS 2023
Step count–heart rate link confirmed in children
, according to a study presented at the Pediatric Academic Societies annual meeting.
The new findings provide a new means for pediatricians to measure physical fitness, the researchers said.
“It really changes the way we evaluate kids’ fitness and gives us a new method of judging physical fitness other than body mass index,” said Susan Gasparino, MD, an instructor in pediatrics at the University of Rochester (N.Y.) Medical Center, who led the study.
Using data from the 2005 to 2006 National Health and Nutrition Examination Survey, Dr. Gasparino and her colleagues examined the association between resting heart rate (RHR) and step count among 899 children and 1,640 adolescents aged 6-19 years.
In the adolescent group, the mean RHR was 74.9 among those who walked more than 10,000 steps per day (n = 414) and 79.3 for those whose step counts fell below that cutoff (n = 1,226) (P < .001). For each additional 1,000 steps per day, RHR decreased by an average of 0.7 beats per minute in this group (P < .001).
In the younger age group, mean RHR was 85.3 among children who took more than 10,000 steps per day (n = 447) and 86.3 among those who did not reach that threshold (n = 452) (P = .29). For each additional 1,000 steps per day, RHR decreased by an average of 0.3 bpm in this group (P = .02)
Dr. Gasparino said next steps in research could include controlling for confounders, such as baseline anxiety and medications that could blunt the heart rate.
Broader implications
If similar results bear out in future studies, monitoring RHR could be incorporated into fitness programs for children and adolescents. Doing so could obviate “the need for intensive treadmill assessments using VO2max, time-consuming and emotionally fraught school-based physical fitness tests, and the fear and potential shame of the scale,” the researchers said.
Dr. Gasparino said measuring RHR during a 3-minute step test could help organizations and governments determine whether fitness programs are improving cardiovascular and overall health and could help them direct “funding and resources to the programs that are effective.” Such a test could also be incorporated into pediatrician wellness checks, she noted.
“It’s an exciting development, and [RHR measurement] holds a lot of promise as a clinical tool that can be applicable in a lot of settings,” said Nicholas M. Edwards, MD, MPH, a sports medicine pediatrician and an associate professor of orthopedics at the University of Minnesota in Minneapolis.
Dr. Edwards said that, because measurement of fitness in clinical settings is difficult, finding ways to “assess fitness in the office with the equipment already at hand would be a superb development.”
If use of RHR to measure fitness “is validated in a clinical setting,” Dr. Edwards said, “I think adoption would be a natural next step.”
Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, according to a study presented at the Pediatric Academic Societies annual meeting.
The new findings provide a new means for pediatricians to measure physical fitness, the researchers said.
“It really changes the way we evaluate kids’ fitness and gives us a new method of judging physical fitness other than body mass index,” said Susan Gasparino, MD, an instructor in pediatrics at the University of Rochester (N.Y.) Medical Center, who led the study.
Using data from the 2005 to 2006 National Health and Nutrition Examination Survey, Dr. Gasparino and her colleagues examined the association between resting heart rate (RHR) and step count among 899 children and 1,640 adolescents aged 6-19 years.
In the adolescent group, the mean RHR was 74.9 among those who walked more than 10,000 steps per day (n = 414) and 79.3 for those whose step counts fell below that cutoff (n = 1,226) (P < .001). For each additional 1,000 steps per day, RHR decreased by an average of 0.7 beats per minute in this group (P < .001).
In the younger age group, mean RHR was 85.3 among children who took more than 10,000 steps per day (n = 447) and 86.3 among those who did not reach that threshold (n = 452) (P = .29). For each additional 1,000 steps per day, RHR decreased by an average of 0.3 bpm in this group (P = .02)
Dr. Gasparino said next steps in research could include controlling for confounders, such as baseline anxiety and medications that could blunt the heart rate.
Broader implications
If similar results bear out in future studies, monitoring RHR could be incorporated into fitness programs for children and adolescents. Doing so could obviate “the need for intensive treadmill assessments using VO2max, time-consuming and emotionally fraught school-based physical fitness tests, and the fear and potential shame of the scale,” the researchers said.
Dr. Gasparino said measuring RHR during a 3-minute step test could help organizations and governments determine whether fitness programs are improving cardiovascular and overall health and could help them direct “funding and resources to the programs that are effective.” Such a test could also be incorporated into pediatrician wellness checks, she noted.
“It’s an exciting development, and [RHR measurement] holds a lot of promise as a clinical tool that can be applicable in a lot of settings,” said Nicholas M. Edwards, MD, MPH, a sports medicine pediatrician and an associate professor of orthopedics at the University of Minnesota in Minneapolis.
Dr. Edwards said that, because measurement of fitness in clinical settings is difficult, finding ways to “assess fitness in the office with the equipment already at hand would be a superb development.”
If use of RHR to measure fitness “is validated in a clinical setting,” Dr. Edwards said, “I think adoption would be a natural next step.”
Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, according to a study presented at the Pediatric Academic Societies annual meeting.
The new findings provide a new means for pediatricians to measure physical fitness, the researchers said.
“It really changes the way we evaluate kids’ fitness and gives us a new method of judging physical fitness other than body mass index,” said Susan Gasparino, MD, an instructor in pediatrics at the University of Rochester (N.Y.) Medical Center, who led the study.
Using data from the 2005 to 2006 National Health and Nutrition Examination Survey, Dr. Gasparino and her colleagues examined the association between resting heart rate (RHR) and step count among 899 children and 1,640 adolescents aged 6-19 years.
In the adolescent group, the mean RHR was 74.9 among those who walked more than 10,000 steps per day (n = 414) and 79.3 for those whose step counts fell below that cutoff (n = 1,226) (P < .001). For each additional 1,000 steps per day, RHR decreased by an average of 0.7 beats per minute in this group (P < .001).
In the younger age group, mean RHR was 85.3 among children who took more than 10,000 steps per day (n = 447) and 86.3 among those who did not reach that threshold (n = 452) (P = .29). For each additional 1,000 steps per day, RHR decreased by an average of 0.3 bpm in this group (P = .02)
Dr. Gasparino said next steps in research could include controlling for confounders, such as baseline anxiety and medications that could blunt the heart rate.
Broader implications
If similar results bear out in future studies, monitoring RHR could be incorporated into fitness programs for children and adolescents. Doing so could obviate “the need for intensive treadmill assessments using VO2max, time-consuming and emotionally fraught school-based physical fitness tests, and the fear and potential shame of the scale,” the researchers said.
Dr. Gasparino said measuring RHR during a 3-minute step test could help organizations and governments determine whether fitness programs are improving cardiovascular and overall health and could help them direct “funding and resources to the programs that are effective.” Such a test could also be incorporated into pediatrician wellness checks, she noted.
“It’s an exciting development, and [RHR measurement] holds a lot of promise as a clinical tool that can be applicable in a lot of settings,” said Nicholas M. Edwards, MD, MPH, a sports medicine pediatrician and an associate professor of orthopedics at the University of Minnesota in Minneapolis.
Dr. Edwards said that, because measurement of fitness in clinical settings is difficult, finding ways to “assess fitness in the office with the equipment already at hand would be a superb development.”
If use of RHR to measure fitness “is validated in a clinical setting,” Dr. Edwards said, “I think adoption would be a natural next step.”
Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM PAS 2023
Gender-diverse teens face barriers to physical activity
WASHINGTON – in a poster presented at the Pediatric Academic Societies annual meeting. Other barriers included body dissatisfaction and discomfort or pain from binding or tucking, based on data from 160 individuals.
Previous studies suggest that gender-diverse teens have lower levels of physical activity than cisgender teens, but data on the specific barriers to physical activity reported by gender-diverse adolescents are lacking, according to Karishma Desai, BA, a medical student at Northwestern University, Chicago, and colleagues.
The researchers reviewed data from adolescents aged 13-18 years who identified as transgender or nonbinary and lived in the United States. Participants were recruited through flyers, wallet cards, email, and social media. They completed an online survey that included questions on preferred types of physical activity and potential barriers to physical activity. Major barriers were defined as items that “almost always” or “always” got in the way of physical activity.
Overall, 51% of the participants identified as female/transfeminine, 31% as male/transmasculine, 9% as genderqueer or agender, 8% as nonbinary, and 1% as unsure. A total of 86 participants were assigned male at birth, 73 were assigned female, and 1 was assigned intersex or other. Nearly all of the participants (96%) had begun social transition; approximately half (48%) reported using a chest binder, and 75% had been or were currently taking gender-affirming hormones.
Potential negative judgment from others was the top barrier to physical activity (cited by 39% of participants), followed by body dissatisfaction from gender dysphoria (38%) and discomfort with the available options for locker rooms or changing rooms (38%). Approximately one-third (36%) of respondents reported physical discomfort or pain from binding or tucking as a barrier to physical activity, and 34% cited discomfort with requirements for a physical activity uniform or athletic clothing at school. Other gender-diverse specific barriers to physical activity included bullying related to being transgender (31%) and the inability to participate in a group of choice because of gender identity (24%).
In addition, participants cited general barriers to physical activity including bullying related to weight (33%), dissatisfaction with weight or size (31%), and bullying in general or for reasons other than gender status (29%).
However, more than 50% of respondents said they were comfortable or very comfortable (4 or 5 on a 5-point Likert Scale) with physical activity in the settings of coed or all-gender teams (61%) or engaging in individual activities (71%). By contrast, 36% were comfortable or very comfortable with a team, group, or class that aligned with sex assignment at birth.
The majority of participants (81%) were comfortable or very comfortable with their homes or a private location as a setting for physical activity, 54% with a public space such as a park, and 43% with a school setting.
Increasing gender congruence was the biggest facilitator of physical activity, reported by 53% of participants, the researchers noted. Other facilitators of physical activity included increasing body satisfaction (43%), staying healthy to avoid long-term health problems in the future (43%), and staying healthy to prepare for gender-related surgery in the future (18%).
The study findings were limited by the use of self-reports and the use of a convenience sample, as well as the lack of data on race, the researchers noted. However, the results suggest that access to all-gender teams, standardizing physical activity clothing, and increasing inclusive facilities may promote greater physical activity participation by gender-diverse adolescents, and offering private or individual options may increase comfort with physical activity, they concluded.
Study provides teens’ perspectives
The current study is especially timely given the recent passage by the U.S. House of Representatives of the anti-trans sports bill preventing transgender women and girls from playing on sports teams “consistent with their gender identity,” said Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin in Milwaukee, in an interview. Ms. Thew was not involved in the current study.
“The House bill seeks to amend federal law to require that sex shall be recognized based solely on a person’s reproductive biology and genetics at birth, for the purpose of determining compliance with Title IX in athletics,” Ms. Thew said.
“Despite political responses to sports participation for transgender adolescents, we have not heard the perspective of the teens themselves,” she emphasized. “It is imperative for parents, coaches, and clinicians to hear the adolescents’ concerns so they can advocate for the students and provide the needed support.” In addition, Ms. Thew noted, “these concerns may also provide overdue changes to the required uniforms described for specific sports.”
Ms. Thew said she was surprised by the finding of transgender teens’ comfort with coed teams and individual activities, both of which may be opportunities to promote physical activity for transgender adolescents.
However, she added that she was not surprised by some of the results. “Many transgender adolescents experience the discomfort and further body dysmorphia of being put into gender-conforming attire such as swimwear, spandex shorts for female volleyball players, or field hockey skirts, for example.”
Although many schools are establishing safe, comfortable places for all adolescents to change clothing prior to physical education and sports participation, “resources are limited, and students and parents need to advocate within the school system,” Ms. Thew noted.
“We as a society, including athletic clothing makers, need to hear the testimony of transgender adolescents on the discomfort from body modifications to better support and innovate attire to meet their needs,” she added.
“The take-home message for clinicians is twofold,” said Ms. Thew. “Clinicians need to advocate for transgender patients to have the same opportunities as all teens when it comes to sports participation and physical activity. Also, clinicians need to ask all adolescents about their comfort in participating in physical activity both on club/school teams and independently,” she said. “If barriers are identified, clinicians need to work to support the adolescent with alternative activities/attire that will promote healthy physical activities for overall health.”
The current study also suggests that transgender adolescents who may have interest in, but discomfort with, physical activity should be redirected to coed or individual sports available in their communities, Ms. Thew added.
More research is needed on innovative sports attire that would improve comfort for transgender adolescents and thereby encourage physical activity, Ms. Thew told this news organization. More data also are needed on which sports transgender adolescents participate in and why, and how these activities might be promoted, she said.
Finally, more research will be needed to examine the impact of the recent House bills on physical activity for transgender youth, Ms. Thew said.
The study was supported by the Potocsnak Family Division of Adolescent and Young Adult Medicine at Ann and Robert H. Lurie’s Children’s Hospital of Chicago. The researchers had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose, but she serves on the Editorial Advisory Board of Pediatric News.
WASHINGTON – in a poster presented at the Pediatric Academic Societies annual meeting. Other barriers included body dissatisfaction and discomfort or pain from binding or tucking, based on data from 160 individuals.
Previous studies suggest that gender-diverse teens have lower levels of physical activity than cisgender teens, but data on the specific barriers to physical activity reported by gender-diverse adolescents are lacking, according to Karishma Desai, BA, a medical student at Northwestern University, Chicago, and colleagues.
The researchers reviewed data from adolescents aged 13-18 years who identified as transgender or nonbinary and lived in the United States. Participants were recruited through flyers, wallet cards, email, and social media. They completed an online survey that included questions on preferred types of physical activity and potential barriers to physical activity. Major barriers were defined as items that “almost always” or “always” got in the way of physical activity.
Overall, 51% of the participants identified as female/transfeminine, 31% as male/transmasculine, 9% as genderqueer or agender, 8% as nonbinary, and 1% as unsure. A total of 86 participants were assigned male at birth, 73 were assigned female, and 1 was assigned intersex or other. Nearly all of the participants (96%) had begun social transition; approximately half (48%) reported using a chest binder, and 75% had been or were currently taking gender-affirming hormones.
Potential negative judgment from others was the top barrier to physical activity (cited by 39% of participants), followed by body dissatisfaction from gender dysphoria (38%) and discomfort with the available options for locker rooms or changing rooms (38%). Approximately one-third (36%) of respondents reported physical discomfort or pain from binding or tucking as a barrier to physical activity, and 34% cited discomfort with requirements for a physical activity uniform or athletic clothing at school. Other gender-diverse specific barriers to physical activity included bullying related to being transgender (31%) and the inability to participate in a group of choice because of gender identity (24%).
In addition, participants cited general barriers to physical activity including bullying related to weight (33%), dissatisfaction with weight or size (31%), and bullying in general or for reasons other than gender status (29%).
However, more than 50% of respondents said they were comfortable or very comfortable (4 or 5 on a 5-point Likert Scale) with physical activity in the settings of coed or all-gender teams (61%) or engaging in individual activities (71%). By contrast, 36% were comfortable or very comfortable with a team, group, or class that aligned with sex assignment at birth.
The majority of participants (81%) were comfortable or very comfortable with their homes or a private location as a setting for physical activity, 54% with a public space such as a park, and 43% with a school setting.
Increasing gender congruence was the biggest facilitator of physical activity, reported by 53% of participants, the researchers noted. Other facilitators of physical activity included increasing body satisfaction (43%), staying healthy to avoid long-term health problems in the future (43%), and staying healthy to prepare for gender-related surgery in the future (18%).
The study findings were limited by the use of self-reports and the use of a convenience sample, as well as the lack of data on race, the researchers noted. However, the results suggest that access to all-gender teams, standardizing physical activity clothing, and increasing inclusive facilities may promote greater physical activity participation by gender-diverse adolescents, and offering private or individual options may increase comfort with physical activity, they concluded.
Study provides teens’ perspectives
The current study is especially timely given the recent passage by the U.S. House of Representatives of the anti-trans sports bill preventing transgender women and girls from playing on sports teams “consistent with their gender identity,” said Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin in Milwaukee, in an interview. Ms. Thew was not involved in the current study.
“The House bill seeks to amend federal law to require that sex shall be recognized based solely on a person’s reproductive biology and genetics at birth, for the purpose of determining compliance with Title IX in athletics,” Ms. Thew said.
“Despite political responses to sports participation for transgender adolescents, we have not heard the perspective of the teens themselves,” she emphasized. “It is imperative for parents, coaches, and clinicians to hear the adolescents’ concerns so they can advocate for the students and provide the needed support.” In addition, Ms. Thew noted, “these concerns may also provide overdue changes to the required uniforms described for specific sports.”
Ms. Thew said she was surprised by the finding of transgender teens’ comfort with coed teams and individual activities, both of which may be opportunities to promote physical activity for transgender adolescents.
However, she added that she was not surprised by some of the results. “Many transgender adolescents experience the discomfort and further body dysmorphia of being put into gender-conforming attire such as swimwear, spandex shorts for female volleyball players, or field hockey skirts, for example.”
Although many schools are establishing safe, comfortable places for all adolescents to change clothing prior to physical education and sports participation, “resources are limited, and students and parents need to advocate within the school system,” Ms. Thew noted.
“We as a society, including athletic clothing makers, need to hear the testimony of transgender adolescents on the discomfort from body modifications to better support and innovate attire to meet their needs,” she added.
“The take-home message for clinicians is twofold,” said Ms. Thew. “Clinicians need to advocate for transgender patients to have the same opportunities as all teens when it comes to sports participation and physical activity. Also, clinicians need to ask all adolescents about their comfort in participating in physical activity both on club/school teams and independently,” she said. “If barriers are identified, clinicians need to work to support the adolescent with alternative activities/attire that will promote healthy physical activities for overall health.”
The current study also suggests that transgender adolescents who may have interest in, but discomfort with, physical activity should be redirected to coed or individual sports available in their communities, Ms. Thew added.
More research is needed on innovative sports attire that would improve comfort for transgender adolescents and thereby encourage physical activity, Ms. Thew told this news organization. More data also are needed on which sports transgender adolescents participate in and why, and how these activities might be promoted, she said.
Finally, more research will be needed to examine the impact of the recent House bills on physical activity for transgender youth, Ms. Thew said.
The study was supported by the Potocsnak Family Division of Adolescent and Young Adult Medicine at Ann and Robert H. Lurie’s Children’s Hospital of Chicago. The researchers had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose, but she serves on the Editorial Advisory Board of Pediatric News.
WASHINGTON – in a poster presented at the Pediatric Academic Societies annual meeting. Other barriers included body dissatisfaction and discomfort or pain from binding or tucking, based on data from 160 individuals.
Previous studies suggest that gender-diverse teens have lower levels of physical activity than cisgender teens, but data on the specific barriers to physical activity reported by gender-diverse adolescents are lacking, according to Karishma Desai, BA, a medical student at Northwestern University, Chicago, and colleagues.
The researchers reviewed data from adolescents aged 13-18 years who identified as transgender or nonbinary and lived in the United States. Participants were recruited through flyers, wallet cards, email, and social media. They completed an online survey that included questions on preferred types of physical activity and potential barriers to physical activity. Major barriers were defined as items that “almost always” or “always” got in the way of physical activity.
Overall, 51% of the participants identified as female/transfeminine, 31% as male/transmasculine, 9% as genderqueer or agender, 8% as nonbinary, and 1% as unsure. A total of 86 participants were assigned male at birth, 73 were assigned female, and 1 was assigned intersex or other. Nearly all of the participants (96%) had begun social transition; approximately half (48%) reported using a chest binder, and 75% had been or were currently taking gender-affirming hormones.
Potential negative judgment from others was the top barrier to physical activity (cited by 39% of participants), followed by body dissatisfaction from gender dysphoria (38%) and discomfort with the available options for locker rooms or changing rooms (38%). Approximately one-third (36%) of respondents reported physical discomfort or pain from binding or tucking as a barrier to physical activity, and 34% cited discomfort with requirements for a physical activity uniform or athletic clothing at school. Other gender-diverse specific barriers to physical activity included bullying related to being transgender (31%) and the inability to participate in a group of choice because of gender identity (24%).
In addition, participants cited general barriers to physical activity including bullying related to weight (33%), dissatisfaction with weight or size (31%), and bullying in general or for reasons other than gender status (29%).
However, more than 50% of respondents said they were comfortable or very comfortable (4 or 5 on a 5-point Likert Scale) with physical activity in the settings of coed or all-gender teams (61%) or engaging in individual activities (71%). By contrast, 36% were comfortable or very comfortable with a team, group, or class that aligned with sex assignment at birth.
The majority of participants (81%) were comfortable or very comfortable with their homes or a private location as a setting for physical activity, 54% with a public space such as a park, and 43% with a school setting.
Increasing gender congruence was the biggest facilitator of physical activity, reported by 53% of participants, the researchers noted. Other facilitators of physical activity included increasing body satisfaction (43%), staying healthy to avoid long-term health problems in the future (43%), and staying healthy to prepare for gender-related surgery in the future (18%).
The study findings were limited by the use of self-reports and the use of a convenience sample, as well as the lack of data on race, the researchers noted. However, the results suggest that access to all-gender teams, standardizing physical activity clothing, and increasing inclusive facilities may promote greater physical activity participation by gender-diverse adolescents, and offering private or individual options may increase comfort with physical activity, they concluded.
Study provides teens’ perspectives
The current study is especially timely given the recent passage by the U.S. House of Representatives of the anti-trans sports bill preventing transgender women and girls from playing on sports teams “consistent with their gender identity,” said Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin in Milwaukee, in an interview. Ms. Thew was not involved in the current study.
“The House bill seeks to amend federal law to require that sex shall be recognized based solely on a person’s reproductive biology and genetics at birth, for the purpose of determining compliance with Title IX in athletics,” Ms. Thew said.
“Despite political responses to sports participation for transgender adolescents, we have not heard the perspective of the teens themselves,” she emphasized. “It is imperative for parents, coaches, and clinicians to hear the adolescents’ concerns so they can advocate for the students and provide the needed support.” In addition, Ms. Thew noted, “these concerns may also provide overdue changes to the required uniforms described for specific sports.”
Ms. Thew said she was surprised by the finding of transgender teens’ comfort with coed teams and individual activities, both of which may be opportunities to promote physical activity for transgender adolescents.
However, she added that she was not surprised by some of the results. “Many transgender adolescents experience the discomfort and further body dysmorphia of being put into gender-conforming attire such as swimwear, spandex shorts for female volleyball players, or field hockey skirts, for example.”
Although many schools are establishing safe, comfortable places for all adolescents to change clothing prior to physical education and sports participation, “resources are limited, and students and parents need to advocate within the school system,” Ms. Thew noted.
“We as a society, including athletic clothing makers, need to hear the testimony of transgender adolescents on the discomfort from body modifications to better support and innovate attire to meet their needs,” she added.
“The take-home message for clinicians is twofold,” said Ms. Thew. “Clinicians need to advocate for transgender patients to have the same opportunities as all teens when it comes to sports participation and physical activity. Also, clinicians need to ask all adolescents about their comfort in participating in physical activity both on club/school teams and independently,” she said. “If barriers are identified, clinicians need to work to support the adolescent with alternative activities/attire that will promote healthy physical activities for overall health.”
The current study also suggests that transgender adolescents who may have interest in, but discomfort with, physical activity should be redirected to coed or individual sports available in their communities, Ms. Thew added.
More research is needed on innovative sports attire that would improve comfort for transgender adolescents and thereby encourage physical activity, Ms. Thew told this news organization. More data also are needed on which sports transgender adolescents participate in and why, and how these activities might be promoted, she said.
Finally, more research will be needed to examine the impact of the recent House bills on physical activity for transgender youth, Ms. Thew said.
The study was supported by the Potocsnak Family Division of Adolescent and Young Adult Medicine at Ann and Robert H. Lurie’s Children’s Hospital of Chicago. The researchers had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose, but she serves on the Editorial Advisory Board of Pediatric News.
AT PAS 2023








