User login
Scalp Nodule Associated With Hair Loss
The Diagnosis: Alopecic and Aseptic Nodule of the Scalp
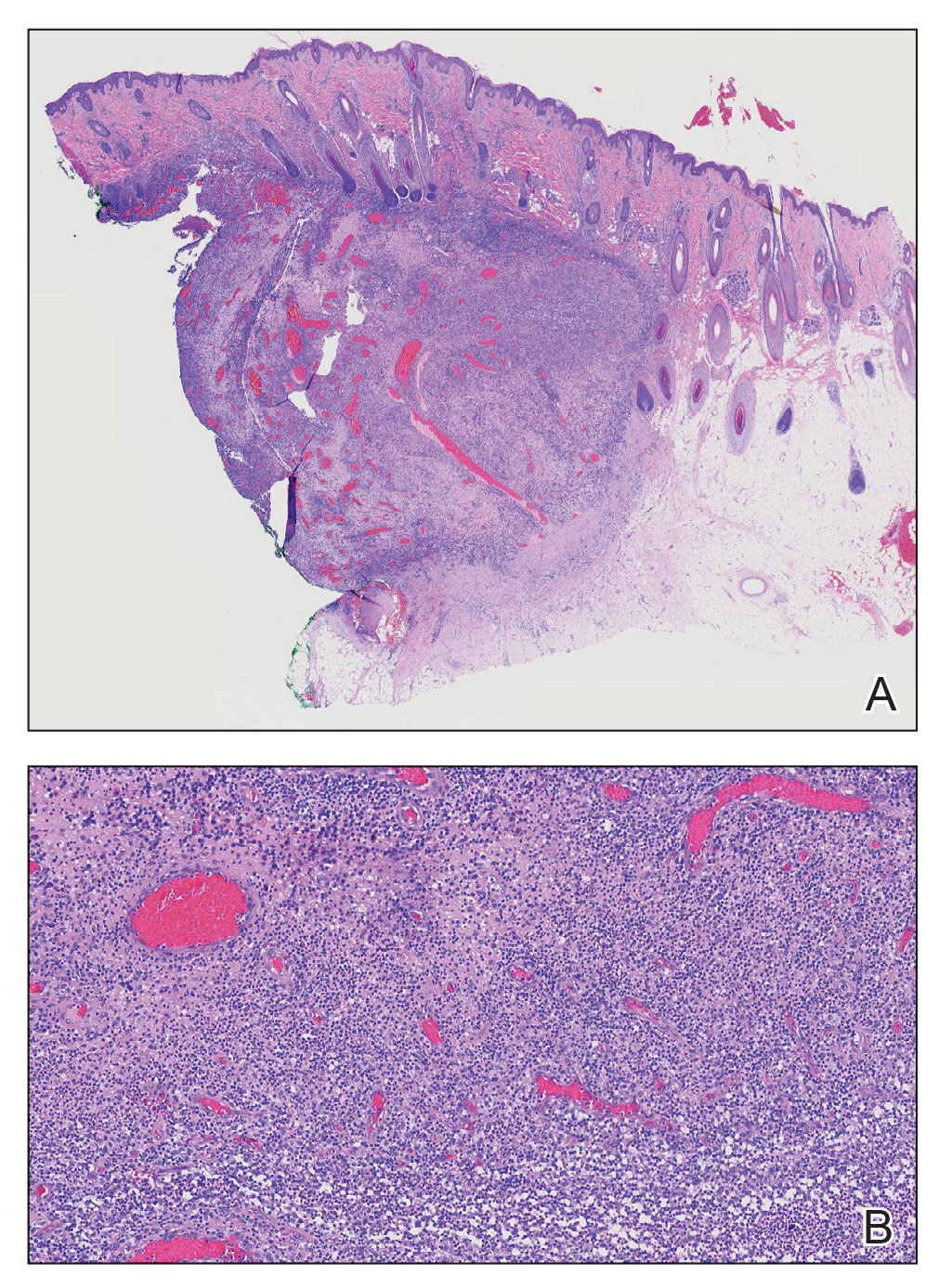
Alopecic and aseptic nodule of the scalp (AANS) is an underdiagnosed condition presenting with one or few inflammatory nodules on the scalp with overlying nonscarring alopecia. The nodules can be soft, fluctuant, or firm and are characterized by negative fungal and bacterial stains as well as cultures.1 Trichoscopic features such as black or yellow dots, fine vellus hairs, and broken hairs have been reported.1-3 Dilated follicular openings may be seen and are termed the Eastern pancake sign, as they resemble the bubble cavities formed during the cooking of atayef.2 The histologic features of AANS often are nonspecific but show a nodular or pseudocystic, lymphohistiocytic to acute inflammatory component centered in the dermis.1 Granulomatous inflammation or isolated giant cells have been reported within the deep dermis.1,4 In our patient, histopathology revealed admixed acute and granulomatous inflammation within the deep dermis (Figure). Treatment of AANS includes oral antibiotics such as doxycycline, intralesional corticosteroids, or excision.1
Although the etiology of AANS currently is unclear, a process of follicular plugging or a deep folliculitis sparing the bulge stem cells has been theorized. Young males are disproportionately affected.1 It is uncertain how much overlap there is, if any, between AANS and pseudocyst of the scalp, the latter of which primarily is reported in the Japanese literature and demonstrates alopecic nodules between the forehead and vertex of the scalp with pseudocystic architecture and granulomatous infiltration on histopathology.4-7
There are several clinical and histologic differences between AANS and other diagnoses in the differential. Dermoid cysts tend to present at birth, with 70% of cases presenting before the age of 6 years, and without overlying skin changes.8 They represent a benign entrapment of ectoderm along embryonic closure lines during development.9 Histologic examination typically will show a squamous-lined cyst within the dermis with associated adnexal structures.10 Cylindromas are benign neoplasms of eccrine sweat glands named after the histologic presentation of cylinder-shaped basaloid cell populations when cross-sectioned.11,12 When cylindromas coalesce on the scalp, they form a distinctive morphology sometimes loosely resembling a turban, giving them the previously more common name turban tumors.11,13 Cylindromas appear as slow-growing protuberant tumors that are erythematous or flesh colored. Cylindromas are 9 times more common in females.13 Pilar cysts have a stratified squamous epithelium lining with a palisaded outer layer and are derived from the outer root sheath of hair follicles.14 Clinically, pilar cysts are smooth mobile cysts that favor skin with a dense concentration of hair follicles.14,15 On palpation, pilar cysts are firm due to their keratinous contents and typically are nontender unless inflamed.15 Lipomas are benign mesenchymal tumors with mature adipocytes that often appear as subcutaneous nodules without overlying skin changes, though they can involve deep fascia. On palpation, lipomas generally are soft, mobile, and nontender.16
- Bellinato F, Maurelli M, Colato C, et al. Alopecic and aseptic nodules of the scalp: a new case with a systematic review of the literature [published online May 1, 2021]. Clin Case Rep. 2021;9:E04153. doi:10.1002/ccr3.4153
- Lázaro-Simó AI, Sancho MI, Quintana-Codina M, et al. Alopecic and aseptic nodules of the scalp with trichoscopic and ultrasonographic findings. Indian J Dermatol. 2017;62:515-518.
- Garrido-Colmenero C, Arias-Santiago S, Aneiros Fernández J, et al. Trichoscopy and ultrasonography features of aseptic and alopecic nodules of the scalp. J Eur Acad Dermatol Venereol. 2016;30:507-509. doi:10.1111/jdv.12903
- Seol JE, Park IH, Kim DH, et al. Alopecic and aseptic nodules of the scalp/pseudocyst of the scalp: clinicopathological and therapeutic analyses in 11 Korean patients. Dermatology. 2016;232:165-170.
- Lee SS, Kim SY, Im M, et al. Pseudocyst of the scalp. Ann Dermatol. 2011;23(suppl 2):S267-S269.
- Eisenberg EL. Alopecia-associated pseudocyst of the scalp. J Am Acad Dermatol. 2012;67:E114-E116.
- Tsuruta D, Hayashi A, Kobayashi H, et al. Pseudocyst of the scalp. Dermatology. 2005;210:333-335.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Julapalli MR, Cohen BA, Hollier LH, et al. Congenital, ill-defined, yellowish plaque: the nasal dermoid. Pediatr Dermatol. 2006;23:556-559.
- Reissis D, Pfaff MJ, Patel A, et al. Craniofacial dermoid cysts: histological analysis and inter-site comparison. Yale J Biol Med. 2014;87:349-357.
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61.
- Albores-Saavedra J, Heard SC, McLaren B, et al. Cylindroma (dermal analog tumor) of the breast: a comparison with cylindroma of the skin and adenoid cystic carcinoma of the breast. Am J Clin Pathol. 2005;123:866-873.
- Myers DJ, Fillman EP. Cylindroma. StatPearls. StatPearls Publishing; 2022.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. doi:10.4103/1947-2714.107532
- Al Aboud DM, Yarrarapu SNS, Patel BC. Pilar cyst. StatPearls. StatPearls Publishing; 2022. 16. Kolb L, Yarrarapu SNS, Ameer MA, et al. Lipoma. StatPearls. StatPearls Publishing; 2022.
The Diagnosis: Alopecic and Aseptic Nodule of the Scalp
Alopecic and aseptic nodule of the scalp (AANS) is an underdiagnosed condition presenting with one or few inflammatory nodules on the scalp with overlying nonscarring alopecia. The nodules can be soft, fluctuant, or firm and are characterized by negative fungal and bacterial stains as well as cultures.1 Trichoscopic features such as black or yellow dots, fine vellus hairs, and broken hairs have been reported.1-3 Dilated follicular openings may be seen and are termed the Eastern pancake sign, as they resemble the bubble cavities formed during the cooking of atayef.2 The histologic features of AANS often are nonspecific but show a nodular or pseudocystic, lymphohistiocytic to acute inflammatory component centered in the dermis.1 Granulomatous inflammation or isolated giant cells have been reported within the deep dermis.1,4 In our patient, histopathology revealed admixed acute and granulomatous inflammation within the deep dermis (Figure). Treatment of AANS includes oral antibiotics such as doxycycline, intralesional corticosteroids, or excision.1

Although the etiology of AANS currently is unclear, a process of follicular plugging or a deep folliculitis sparing the bulge stem cells has been theorized. Young males are disproportionately affected.1 It is uncertain how much overlap there is, if any, between AANS and pseudocyst of the scalp, the latter of which primarily is reported in the Japanese literature and demonstrates alopecic nodules between the forehead and vertex of the scalp with pseudocystic architecture and granulomatous infiltration on histopathology.4-7
There are several clinical and histologic differences between AANS and other diagnoses in the differential. Dermoid cysts tend to present at birth, with 70% of cases presenting before the age of 6 years, and without overlying skin changes.8 They represent a benign entrapment of ectoderm along embryonic closure lines during development.9 Histologic examination typically will show a squamous-lined cyst within the dermis with associated adnexal structures.10 Cylindromas are benign neoplasms of eccrine sweat glands named after the histologic presentation of cylinder-shaped basaloid cell populations when cross-sectioned.11,12 When cylindromas coalesce on the scalp, they form a distinctive morphology sometimes loosely resembling a turban, giving them the previously more common name turban tumors.11,13 Cylindromas appear as slow-growing protuberant tumors that are erythematous or flesh colored. Cylindromas are 9 times more common in females.13 Pilar cysts have a stratified squamous epithelium lining with a palisaded outer layer and are derived from the outer root sheath of hair follicles.14 Clinically, pilar cysts are smooth mobile cysts that favor skin with a dense concentration of hair follicles.14,15 On palpation, pilar cysts are firm due to their keratinous contents and typically are nontender unless inflamed.15 Lipomas are benign mesenchymal tumors with mature adipocytes that often appear as subcutaneous nodules without overlying skin changes, though they can involve deep fascia. On palpation, lipomas generally are soft, mobile, and nontender.16
The Diagnosis: Alopecic and Aseptic Nodule of the Scalp
Alopecic and aseptic nodule of the scalp (AANS) is an underdiagnosed condition presenting with one or few inflammatory nodules on the scalp with overlying nonscarring alopecia. The nodules can be soft, fluctuant, or firm and are characterized by negative fungal and bacterial stains as well as cultures.1 Trichoscopic features such as black or yellow dots, fine vellus hairs, and broken hairs have been reported.1-3 Dilated follicular openings may be seen and are termed the Eastern pancake sign, as they resemble the bubble cavities formed during the cooking of atayef.2 The histologic features of AANS often are nonspecific but show a nodular or pseudocystic, lymphohistiocytic to acute inflammatory component centered in the dermis.1 Granulomatous inflammation or isolated giant cells have been reported within the deep dermis.1,4 In our patient, histopathology revealed admixed acute and granulomatous inflammation within the deep dermis (Figure). Treatment of AANS includes oral antibiotics such as doxycycline, intralesional corticosteroids, or excision.1

Although the etiology of AANS currently is unclear, a process of follicular plugging or a deep folliculitis sparing the bulge stem cells has been theorized. Young males are disproportionately affected.1 It is uncertain how much overlap there is, if any, between AANS and pseudocyst of the scalp, the latter of which primarily is reported in the Japanese literature and demonstrates alopecic nodules between the forehead and vertex of the scalp with pseudocystic architecture and granulomatous infiltration on histopathology.4-7
There are several clinical and histologic differences between AANS and other diagnoses in the differential. Dermoid cysts tend to present at birth, with 70% of cases presenting before the age of 6 years, and without overlying skin changes.8 They represent a benign entrapment of ectoderm along embryonic closure lines during development.9 Histologic examination typically will show a squamous-lined cyst within the dermis with associated adnexal structures.10 Cylindromas are benign neoplasms of eccrine sweat glands named after the histologic presentation of cylinder-shaped basaloid cell populations when cross-sectioned.11,12 When cylindromas coalesce on the scalp, they form a distinctive morphology sometimes loosely resembling a turban, giving them the previously more common name turban tumors.11,13 Cylindromas appear as slow-growing protuberant tumors that are erythematous or flesh colored. Cylindromas are 9 times more common in females.13 Pilar cysts have a stratified squamous epithelium lining with a palisaded outer layer and are derived from the outer root sheath of hair follicles.14 Clinically, pilar cysts are smooth mobile cysts that favor skin with a dense concentration of hair follicles.14,15 On palpation, pilar cysts are firm due to their keratinous contents and typically are nontender unless inflamed.15 Lipomas are benign mesenchymal tumors with mature adipocytes that often appear as subcutaneous nodules without overlying skin changes, though they can involve deep fascia. On palpation, lipomas generally are soft, mobile, and nontender.16
- Bellinato F, Maurelli M, Colato C, et al. Alopecic and aseptic nodules of the scalp: a new case with a systematic review of the literature [published online May 1, 2021]. Clin Case Rep. 2021;9:E04153. doi:10.1002/ccr3.4153
- Lázaro-Simó AI, Sancho MI, Quintana-Codina M, et al. Alopecic and aseptic nodules of the scalp with trichoscopic and ultrasonographic findings. Indian J Dermatol. 2017;62:515-518.
- Garrido-Colmenero C, Arias-Santiago S, Aneiros Fernández J, et al. Trichoscopy and ultrasonography features of aseptic and alopecic nodules of the scalp. J Eur Acad Dermatol Venereol. 2016;30:507-509. doi:10.1111/jdv.12903
- Seol JE, Park IH, Kim DH, et al. Alopecic and aseptic nodules of the scalp/pseudocyst of the scalp: clinicopathological and therapeutic analyses in 11 Korean patients. Dermatology. 2016;232:165-170.
- Lee SS, Kim SY, Im M, et al. Pseudocyst of the scalp. Ann Dermatol. 2011;23(suppl 2):S267-S269.
- Eisenberg EL. Alopecia-associated pseudocyst of the scalp. J Am Acad Dermatol. 2012;67:E114-E116.
- Tsuruta D, Hayashi A, Kobayashi H, et al. Pseudocyst of the scalp. Dermatology. 2005;210:333-335.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Julapalli MR, Cohen BA, Hollier LH, et al. Congenital, ill-defined, yellowish plaque: the nasal dermoid. Pediatr Dermatol. 2006;23:556-559.
- Reissis D, Pfaff MJ, Patel A, et al. Craniofacial dermoid cysts: histological analysis and inter-site comparison. Yale J Biol Med. 2014;87:349-357.
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61.
- Albores-Saavedra J, Heard SC, McLaren B, et al. Cylindroma (dermal analog tumor) of the breast: a comparison with cylindroma of the skin and adenoid cystic carcinoma of the breast. Am J Clin Pathol. 2005;123:866-873.
- Myers DJ, Fillman EP. Cylindroma. StatPearls. StatPearls Publishing; 2022.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. doi:10.4103/1947-2714.107532
- Al Aboud DM, Yarrarapu SNS, Patel BC. Pilar cyst. StatPearls. StatPearls Publishing; 2022. 16. Kolb L, Yarrarapu SNS, Ameer MA, et al. Lipoma. StatPearls. StatPearls Publishing; 2022.
- Bellinato F, Maurelli M, Colato C, et al. Alopecic and aseptic nodules of the scalp: a new case with a systematic review of the literature [published online May 1, 2021]. Clin Case Rep. 2021;9:E04153. doi:10.1002/ccr3.4153
- Lázaro-Simó AI, Sancho MI, Quintana-Codina M, et al. Alopecic and aseptic nodules of the scalp with trichoscopic and ultrasonographic findings. Indian J Dermatol. 2017;62:515-518.
- Garrido-Colmenero C, Arias-Santiago S, Aneiros Fernández J, et al. Trichoscopy and ultrasonography features of aseptic and alopecic nodules of the scalp. J Eur Acad Dermatol Venereol. 2016;30:507-509. doi:10.1111/jdv.12903
- Seol JE, Park IH, Kim DH, et al. Alopecic and aseptic nodules of the scalp/pseudocyst of the scalp: clinicopathological and therapeutic analyses in 11 Korean patients. Dermatology. 2016;232:165-170.
- Lee SS, Kim SY, Im M, et al. Pseudocyst of the scalp. Ann Dermatol. 2011;23(suppl 2):S267-S269.
- Eisenberg EL. Alopecia-associated pseudocyst of the scalp. J Am Acad Dermatol. 2012;67:E114-E116.
- Tsuruta D, Hayashi A, Kobayashi H, et al. Pseudocyst of the scalp. Dermatology. 2005;210:333-335.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Julapalli MR, Cohen BA, Hollier LH, et al. Congenital, ill-defined, yellowish plaque: the nasal dermoid. Pediatr Dermatol. 2006;23:556-559.
- Reissis D, Pfaff MJ, Patel A, et al. Craniofacial dermoid cysts: histological analysis and inter-site comparison. Yale J Biol Med. 2014;87:349-357.
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61.
- Albores-Saavedra J, Heard SC, McLaren B, et al. Cylindroma (dermal analog tumor) of the breast: a comparison with cylindroma of the skin and adenoid cystic carcinoma of the breast. Am J Clin Pathol. 2005;123:866-873.
- Myers DJ, Fillman EP. Cylindroma. StatPearls. StatPearls Publishing; 2022.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. doi:10.4103/1947-2714.107532
- Al Aboud DM, Yarrarapu SNS, Patel BC. Pilar cyst. StatPearls. StatPearls Publishing; 2022. 16. Kolb L, Yarrarapu SNS, Ameer MA, et al. Lipoma. StatPearls. StatPearls Publishing; 2022.
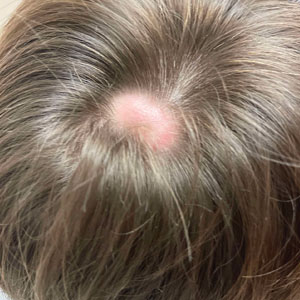
A 9-year-old boy presented with a soft subcutaneous nodule with overlying alopecia on the right parietal scalp of 5 months’ duration that had grown in size, became increasingly alopecic, and was complicated by intermittent pain. An excisional biopsy of the nodule revealed deep dermal mixed inflammation with scattered granulomas. No foreign material, definitive cystic spaces, or cyst wall lining was identified. Special stains including periodic acid– Schiff, Fite acid-fast, and Twort Gram were negative for infectious organisms. His postoperative course was uneventful, and no recurrence of the nodule was reported.

UTI imaging falls short in some primary care settings
WASHINGTON –
“Timely imaging is recommended after febrile UTI (fUTI) in young children to identify treatable urologic conditions,” wrote Jonathan Hatoun, MD, of Boston Children’s Hospital, and colleagues in a poster presented at the Pediatric Academic Societies annual meeting.
The American Academy of Pediatrics (AAP) currently recommends renal-bladder ultrasound (RBUS) after fUTI with voiding cystourethrogram (VCUG) after abnormal RBUS or second fUTI, but data on clinician adherence to these recommendations are limited, the researchers said.
To characterize practice patterns regarding fUTI, the researchers reviewed data from children younger than 24 months of age with fUTI who were treated at a primary care network in Massachusetts in 2019. The definition of fUTI was temperature of 38° C or higher, positive urinalysis, and more than 50,000 CFU on urine culture. The median age of the patients was 9 months; 84% were female.
In a multivariate analysis, post-UTI imaging followed the AAP guidelines in 82 cases (69.5%). The main reasons for nonadherence were lack of RBUS in 21 patients, VCUG despite normal RBUS in 9 patients, no VCUG after abnormal RBUS in 4 patients, and no VCUG after a second fUTI in 2 patients.
Overall, nonadherence was a result of not ordering a recommended study in 23% of cases (errors of omission) and ordering an unnecessary study in 8% of cases (errors of commission).
Commercial insurance, larger number of providers in practice, and younger provider age were significant independent predictors of adherence (odds ratios 2.82, 1.38, and 0.96, respectively).
The findings were limited by the use of data from a single center; however, the results suggest that targeted training may improve guideline adherence, the researchers wrote. Additional research and quality improvement studies are needed to understand and address the impact of insurance on guideline adherence for imaging after febrile UTIs, they noted.
Provider education is essential to continued quality of care
When it comes to febrile UTIs, “it is important to stay focused on the quality of care being provided, as opposed to the usual benchmark of quantity of care,” Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, said in an interview.
“This is a very simple but interesting study on provider compliance with practice guidelines,” said Dr. Joos, who was not involved in the study. “I was surprised that the providers did so well in ordering the correct imaging in 70% of the cases,” he said.
Of particular interest, Dr. Joos noted, was that “the authors also showed that older providers and those working in smaller practices are less likely to comply with this particular imaging guideline. This can be summed up as the ‘I didn’t know the guideline’ effect.”
To improve quality of care, “more research and effort should be directed at updating providers when strong new evidence changes previous practices and guidelines,” Dr. Joos told this news organization.
The study received no outside funding. The researchers and Dr. Joos had no financial conflicts to disclose.
WASHINGTON –
“Timely imaging is recommended after febrile UTI (fUTI) in young children to identify treatable urologic conditions,” wrote Jonathan Hatoun, MD, of Boston Children’s Hospital, and colleagues in a poster presented at the Pediatric Academic Societies annual meeting.
The American Academy of Pediatrics (AAP) currently recommends renal-bladder ultrasound (RBUS) after fUTI with voiding cystourethrogram (VCUG) after abnormal RBUS or second fUTI, but data on clinician adherence to these recommendations are limited, the researchers said.
To characterize practice patterns regarding fUTI, the researchers reviewed data from children younger than 24 months of age with fUTI who were treated at a primary care network in Massachusetts in 2019. The definition of fUTI was temperature of 38° C or higher, positive urinalysis, and more than 50,000 CFU on urine culture. The median age of the patients was 9 months; 84% were female.
In a multivariate analysis, post-UTI imaging followed the AAP guidelines in 82 cases (69.5%). The main reasons for nonadherence were lack of RBUS in 21 patients, VCUG despite normal RBUS in 9 patients, no VCUG after abnormal RBUS in 4 patients, and no VCUG after a second fUTI in 2 patients.
Overall, nonadherence was a result of not ordering a recommended study in 23% of cases (errors of omission) and ordering an unnecessary study in 8% of cases (errors of commission).
Commercial insurance, larger number of providers in practice, and younger provider age were significant independent predictors of adherence (odds ratios 2.82, 1.38, and 0.96, respectively).
The findings were limited by the use of data from a single center; however, the results suggest that targeted training may improve guideline adherence, the researchers wrote. Additional research and quality improvement studies are needed to understand and address the impact of insurance on guideline adherence for imaging after febrile UTIs, they noted.
Provider education is essential to continued quality of care
When it comes to febrile UTIs, “it is important to stay focused on the quality of care being provided, as opposed to the usual benchmark of quantity of care,” Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, said in an interview.
“This is a very simple but interesting study on provider compliance with practice guidelines,” said Dr. Joos, who was not involved in the study. “I was surprised that the providers did so well in ordering the correct imaging in 70% of the cases,” he said.
Of particular interest, Dr. Joos noted, was that “the authors also showed that older providers and those working in smaller practices are less likely to comply with this particular imaging guideline. This can be summed up as the ‘I didn’t know the guideline’ effect.”
To improve quality of care, “more research and effort should be directed at updating providers when strong new evidence changes previous practices and guidelines,” Dr. Joos told this news organization.
The study received no outside funding. The researchers and Dr. Joos had no financial conflicts to disclose.
WASHINGTON –
“Timely imaging is recommended after febrile UTI (fUTI) in young children to identify treatable urologic conditions,” wrote Jonathan Hatoun, MD, of Boston Children’s Hospital, and colleagues in a poster presented at the Pediatric Academic Societies annual meeting.
The American Academy of Pediatrics (AAP) currently recommends renal-bladder ultrasound (RBUS) after fUTI with voiding cystourethrogram (VCUG) after abnormal RBUS or second fUTI, but data on clinician adherence to these recommendations are limited, the researchers said.
To characterize practice patterns regarding fUTI, the researchers reviewed data from children younger than 24 months of age with fUTI who were treated at a primary care network in Massachusetts in 2019. The definition of fUTI was temperature of 38° C or higher, positive urinalysis, and more than 50,000 CFU on urine culture. The median age of the patients was 9 months; 84% were female.
In a multivariate analysis, post-UTI imaging followed the AAP guidelines in 82 cases (69.5%). The main reasons for nonadherence were lack of RBUS in 21 patients, VCUG despite normal RBUS in 9 patients, no VCUG after abnormal RBUS in 4 patients, and no VCUG after a second fUTI in 2 patients.
Overall, nonadherence was a result of not ordering a recommended study in 23% of cases (errors of omission) and ordering an unnecessary study in 8% of cases (errors of commission).
Commercial insurance, larger number of providers in practice, and younger provider age were significant independent predictors of adherence (odds ratios 2.82, 1.38, and 0.96, respectively).
The findings were limited by the use of data from a single center; however, the results suggest that targeted training may improve guideline adherence, the researchers wrote. Additional research and quality improvement studies are needed to understand and address the impact of insurance on guideline adherence for imaging after febrile UTIs, they noted.
Provider education is essential to continued quality of care
When it comes to febrile UTIs, “it is important to stay focused on the quality of care being provided, as opposed to the usual benchmark of quantity of care,” Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, said in an interview.
“This is a very simple but interesting study on provider compliance with practice guidelines,” said Dr. Joos, who was not involved in the study. “I was surprised that the providers did so well in ordering the correct imaging in 70% of the cases,” he said.
Of particular interest, Dr. Joos noted, was that “the authors also showed that older providers and those working in smaller practices are less likely to comply with this particular imaging guideline. This can be summed up as the ‘I didn’t know the guideline’ effect.”
To improve quality of care, “more research and effort should be directed at updating providers when strong new evidence changes previous practices and guidelines,” Dr. Joos told this news organization.
The study received no outside funding. The researchers and Dr. Joos had no financial conflicts to disclose.
AT PAS 2023
Four profiles help identify kids at risk for suicide
The profiles were developed from their study of children and adolescents aged 5-18 years who had been admitted with a neuropsychiatric event to two children’s hospitals.
The researchers used Bayesian regression to identify the profiles developed from 32 covariates: age, sex, and 30 mental health diagnostic groups from April 2016 to March 2020. The profiles include low-, moderate-, high- and very-high-risk categories.
The study, led by Mert Sekmen with the division of hospital medicine at Monroe Carell Jr. Children’s Hospital, and a student at Vanderbilt University Medical Center in Nashville, Tenn., included 1,098 children, average age 14. Of those, 406 (37%) were diagnosed with a self-harm event.
Traditionally, single diagnoses have been linked with risk of self-harm, independent of other comorbidities, but this study gauges risk for a set of diagnoses.
Findings were published online in Pediatrics.
The risk groups were described as follows:
- Low risk. (45% of the study population; median risk of 0.04 (interquartile range, 0.03-0.04; odds ratio, 0.08). The group included children aged 5-9 years with a non–mental health diagnosis, and without mood, behavioral, psychotic, developmental, trauma, or substance-related disorders.
- Moderate risk. (8% of the study group). This group had the same risk as the baseline risk for the entire cohort (37%) and served as the reference group, with a median risk of 0.30 (IQR, 0.27-0.33). This profile was characterized by several mood disorders and behavioral disorders but without depressive disorders.
- High risk. (36%) This group had an average risk of 0.69 (IQR, 0.67-0.71; OR, 5.09). This profile included female adolescents ages 14-17 with depression and anxiety in conjunction with substance- and trauma-related disorders. Personality and eating disorders were significant in this group. Importantly, the authors wrote, the high-risk group did not include behavioral and developmental disorders.
- Very high risk. (11%) The very-high-risk profile had the highest average risk of 0.79 (IQR, 0.73-0.79; OR, 7.21) and included male children aged 10-13. This profile, like the high-risk profile, included anxiety and depressive disorders. The very-high-risk profile differed from the high-risk with its inclusion of bipolar disorder; attention-deficit/hyperactivity disorder; and trauma-related and developmental disorders such as autism spectrum disorder or intellectual disability, along with conduct disorders. Neither the high- nor the very-high-risk profiles included a concurrent non–mental health diagnosis.
Differences by sex
The authors explained some of the differences by sex. They noted that in a study of children aged 5-11, deaths by suicide were more prevalent among boys. A mental health diagnosis was identified in 31%, the most common being ADHD, depression, and other unspecified co-occurring disorders.
“The very-high-risk group also reflects a concerning rise in death by suicide among (males) aged 10-13, who have seen rates nearly triple from 2007 to 2017,” the authors wrote.
The authors pointed out that, although incidence of anxiety and depressive disorders between male and female children is much the same before adolescence, “female adolescents are twice as likely to be diagnosed with either disorder during adolescence. Girls also have higher rates of suicidal ideation and attempts after puberty.”
Eating disorders were also included in the high-risk profile. A study showed that emergency department visits for adolescent girls attempting suicide were 51% higher from February to March 2021, compared with the same period in the pre-COVID-19 year 2019.
Jason Lewis, PhD, psychologist and section director of mood, anxiety and trauma disorders in the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia, who was not part of the research team, said the “constellations of risk factors put into acuity levels” helps to better project risk than knowing the risk associated with a particular diagnosis.
Gap closing between young children, adolescents
Dr. Lewis said he was surprised by the young age of 10-13 among the boys in the highest-risk category. That speaks to the differences from standard thinking this paper points out, he said. “Generally, we think about adolescents as being at the highest risk of suicide death and suicidal behavior,” he said.
Dr. Lewis said it’s important to note that the authors acknowledge these profiles are not static. He gave an example that the rate of suicide deaths among females is rising.
“As things like that change, some of these risk profiles will change as well.”
Dr. Lewis said the profiles may be especially helpful to medical providers in emergency departments or those making discharge decisions who don’t have an ongoing relationship with a patient.
The information could also help educators and lay people, “think about suicide in the youth population in ways we don’t normally think about it,” Dr. Lewis said.
Covariates considered for profiles were determined through expert consensus between pediatric psychiatrists, general pediatricians, pediatric hospitalists, pediatric complex care physicians, and pediatric pharmacoepidemiologists.
Age was broken into three groups: 5-9 years, 10-13 years, and 14-17 years based on Centers for Disease Control and Prevention reporting and previous studies that showed significant increases in suicide rates in these age-based subgroups.
Results are preliminary
The authors note that the profiles were developed using data from 1,000 children with neuropsychiatric complaints at two academic children’s hospitals and are thus preliminary.
“Future studies should focus on validating these risk profiles in a larger, more heterogeneous population of children and adolescents,” the authors write.
They also acknowledge that they were not able to include factors such as medication use, previous suicidal behavior, and family and social support, which also factor into risk.
The study authors and Dr. Lewis report no relevant financial relationships.
The profiles were developed from their study of children and adolescents aged 5-18 years who had been admitted with a neuropsychiatric event to two children’s hospitals.
The researchers used Bayesian regression to identify the profiles developed from 32 covariates: age, sex, and 30 mental health diagnostic groups from April 2016 to March 2020. The profiles include low-, moderate-, high- and very-high-risk categories.
The study, led by Mert Sekmen with the division of hospital medicine at Monroe Carell Jr. Children’s Hospital, and a student at Vanderbilt University Medical Center in Nashville, Tenn., included 1,098 children, average age 14. Of those, 406 (37%) were diagnosed with a self-harm event.
Traditionally, single diagnoses have been linked with risk of self-harm, independent of other comorbidities, but this study gauges risk for a set of diagnoses.
Findings were published online in Pediatrics.
The risk groups were described as follows:
- Low risk. (45% of the study population; median risk of 0.04 (interquartile range, 0.03-0.04; odds ratio, 0.08). The group included children aged 5-9 years with a non–mental health diagnosis, and without mood, behavioral, psychotic, developmental, trauma, or substance-related disorders.
- Moderate risk. (8% of the study group). This group had the same risk as the baseline risk for the entire cohort (37%) and served as the reference group, with a median risk of 0.30 (IQR, 0.27-0.33). This profile was characterized by several mood disorders and behavioral disorders but without depressive disorders.
- High risk. (36%) This group had an average risk of 0.69 (IQR, 0.67-0.71; OR, 5.09). This profile included female adolescents ages 14-17 with depression and anxiety in conjunction with substance- and trauma-related disorders. Personality and eating disorders were significant in this group. Importantly, the authors wrote, the high-risk group did not include behavioral and developmental disorders.
- Very high risk. (11%) The very-high-risk profile had the highest average risk of 0.79 (IQR, 0.73-0.79; OR, 7.21) and included male children aged 10-13. This profile, like the high-risk profile, included anxiety and depressive disorders. The very-high-risk profile differed from the high-risk with its inclusion of bipolar disorder; attention-deficit/hyperactivity disorder; and trauma-related and developmental disorders such as autism spectrum disorder or intellectual disability, along with conduct disorders. Neither the high- nor the very-high-risk profiles included a concurrent non–mental health diagnosis.
Differences by sex
The authors explained some of the differences by sex. They noted that in a study of children aged 5-11, deaths by suicide were more prevalent among boys. A mental health diagnosis was identified in 31%, the most common being ADHD, depression, and other unspecified co-occurring disorders.
“The very-high-risk group also reflects a concerning rise in death by suicide among (males) aged 10-13, who have seen rates nearly triple from 2007 to 2017,” the authors wrote.
The authors pointed out that, although incidence of anxiety and depressive disorders between male and female children is much the same before adolescence, “female adolescents are twice as likely to be diagnosed with either disorder during adolescence. Girls also have higher rates of suicidal ideation and attempts after puberty.”
Eating disorders were also included in the high-risk profile. A study showed that emergency department visits for adolescent girls attempting suicide were 51% higher from February to March 2021, compared with the same period in the pre-COVID-19 year 2019.
Jason Lewis, PhD, psychologist and section director of mood, anxiety and trauma disorders in the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia, who was not part of the research team, said the “constellations of risk factors put into acuity levels” helps to better project risk than knowing the risk associated with a particular diagnosis.
Gap closing between young children, adolescents
Dr. Lewis said he was surprised by the young age of 10-13 among the boys in the highest-risk category. That speaks to the differences from standard thinking this paper points out, he said. “Generally, we think about adolescents as being at the highest risk of suicide death and suicidal behavior,” he said.
Dr. Lewis said it’s important to note that the authors acknowledge these profiles are not static. He gave an example that the rate of suicide deaths among females is rising.
“As things like that change, some of these risk profiles will change as well.”
Dr. Lewis said the profiles may be especially helpful to medical providers in emergency departments or those making discharge decisions who don’t have an ongoing relationship with a patient.
The information could also help educators and lay people, “think about suicide in the youth population in ways we don’t normally think about it,” Dr. Lewis said.
Covariates considered for profiles were determined through expert consensus between pediatric psychiatrists, general pediatricians, pediatric hospitalists, pediatric complex care physicians, and pediatric pharmacoepidemiologists.
Age was broken into three groups: 5-9 years, 10-13 years, and 14-17 years based on Centers for Disease Control and Prevention reporting and previous studies that showed significant increases in suicide rates in these age-based subgroups.
Results are preliminary
The authors note that the profiles were developed using data from 1,000 children with neuropsychiatric complaints at two academic children’s hospitals and are thus preliminary.
“Future studies should focus on validating these risk profiles in a larger, more heterogeneous population of children and adolescents,” the authors write.
They also acknowledge that they were not able to include factors such as medication use, previous suicidal behavior, and family and social support, which also factor into risk.
The study authors and Dr. Lewis report no relevant financial relationships.
The profiles were developed from their study of children and adolescents aged 5-18 years who had been admitted with a neuropsychiatric event to two children’s hospitals.
The researchers used Bayesian regression to identify the profiles developed from 32 covariates: age, sex, and 30 mental health diagnostic groups from April 2016 to March 2020. The profiles include low-, moderate-, high- and very-high-risk categories.
The study, led by Mert Sekmen with the division of hospital medicine at Monroe Carell Jr. Children’s Hospital, and a student at Vanderbilt University Medical Center in Nashville, Tenn., included 1,098 children, average age 14. Of those, 406 (37%) were diagnosed with a self-harm event.
Traditionally, single diagnoses have been linked with risk of self-harm, independent of other comorbidities, but this study gauges risk for a set of diagnoses.
Findings were published online in Pediatrics.
The risk groups were described as follows:
- Low risk. (45% of the study population; median risk of 0.04 (interquartile range, 0.03-0.04; odds ratio, 0.08). The group included children aged 5-9 years with a non–mental health diagnosis, and without mood, behavioral, psychotic, developmental, trauma, or substance-related disorders.
- Moderate risk. (8% of the study group). This group had the same risk as the baseline risk for the entire cohort (37%) and served as the reference group, with a median risk of 0.30 (IQR, 0.27-0.33). This profile was characterized by several mood disorders and behavioral disorders but without depressive disorders.
- High risk. (36%) This group had an average risk of 0.69 (IQR, 0.67-0.71; OR, 5.09). This profile included female adolescents ages 14-17 with depression and anxiety in conjunction with substance- and trauma-related disorders. Personality and eating disorders were significant in this group. Importantly, the authors wrote, the high-risk group did not include behavioral and developmental disorders.
- Very high risk. (11%) The very-high-risk profile had the highest average risk of 0.79 (IQR, 0.73-0.79; OR, 7.21) and included male children aged 10-13. This profile, like the high-risk profile, included anxiety and depressive disorders. The very-high-risk profile differed from the high-risk with its inclusion of bipolar disorder; attention-deficit/hyperactivity disorder; and trauma-related and developmental disorders such as autism spectrum disorder or intellectual disability, along with conduct disorders. Neither the high- nor the very-high-risk profiles included a concurrent non–mental health diagnosis.
Differences by sex
The authors explained some of the differences by sex. They noted that in a study of children aged 5-11, deaths by suicide were more prevalent among boys. A mental health diagnosis was identified in 31%, the most common being ADHD, depression, and other unspecified co-occurring disorders.
“The very-high-risk group also reflects a concerning rise in death by suicide among (males) aged 10-13, who have seen rates nearly triple from 2007 to 2017,” the authors wrote.
The authors pointed out that, although incidence of anxiety and depressive disorders between male and female children is much the same before adolescence, “female adolescents are twice as likely to be diagnosed with either disorder during adolescence. Girls also have higher rates of suicidal ideation and attempts after puberty.”
Eating disorders were also included in the high-risk profile. A study showed that emergency department visits for adolescent girls attempting suicide were 51% higher from February to March 2021, compared with the same period in the pre-COVID-19 year 2019.
Jason Lewis, PhD, psychologist and section director of mood, anxiety and trauma disorders in the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia, who was not part of the research team, said the “constellations of risk factors put into acuity levels” helps to better project risk than knowing the risk associated with a particular diagnosis.
Gap closing between young children, adolescents
Dr. Lewis said he was surprised by the young age of 10-13 among the boys in the highest-risk category. That speaks to the differences from standard thinking this paper points out, he said. “Generally, we think about adolescents as being at the highest risk of suicide death and suicidal behavior,” he said.
Dr. Lewis said it’s important to note that the authors acknowledge these profiles are not static. He gave an example that the rate of suicide deaths among females is rising.
“As things like that change, some of these risk profiles will change as well.”
Dr. Lewis said the profiles may be especially helpful to medical providers in emergency departments or those making discharge decisions who don’t have an ongoing relationship with a patient.
The information could also help educators and lay people, “think about suicide in the youth population in ways we don’t normally think about it,” Dr. Lewis said.
Covariates considered for profiles were determined through expert consensus between pediatric psychiatrists, general pediatricians, pediatric hospitalists, pediatric complex care physicians, and pediatric pharmacoepidemiologists.
Age was broken into three groups: 5-9 years, 10-13 years, and 14-17 years based on Centers for Disease Control and Prevention reporting and previous studies that showed significant increases in suicide rates in these age-based subgroups.
Results are preliminary
The authors note that the profiles were developed using data from 1,000 children with neuropsychiatric complaints at two academic children’s hospitals and are thus preliminary.
“Future studies should focus on validating these risk profiles in a larger, more heterogeneous population of children and adolescents,” the authors write.
They also acknowledge that they were not able to include factors such as medication use, previous suicidal behavior, and family and social support, which also factor into risk.
The study authors and Dr. Lewis report no relevant financial relationships.
FROM PEDIATRICS
New hope for adult children with ‘failure to launch’ syndrome
WASHINGTON – , a new pilot study shows.
Known as failure to launch (FTL) syndrome, the criteria for this condition include the absence of a neurodevelopmental, mental, or intellectual condition, difficulty adapting to the challenges of adulthood, and living with or at the expense of parents.
Results suggest that the program benefits families dealing with FTL, said study investigator Uri Berger, PhD, postdoctoral associate, Yale Child Study Center Anxiety and Mood Disorders Program, New Haven, Conn.
“If you encounter parents who are say 50-60 years old who have a child with FTL, you can tell them there’s something they can do; there’s work they can do even if their child is refusing to go to therapy,” he said.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Anxious, isolated
Estimates suggest that there are 3.3 million physically able adults with FTL and that the disorder may be on the rise. These individuals often present with mental health symptoms including anxiety, depression, and suicidality, and tend to be socially isolated.
The investigators noted that intervening is often challenging because individuals with the syndrome are frequently noncompliant with therapy, and currently there is no standard of care.
“The longer you’re isolated, the harder it is getting out of your cocoon, and when these adult children get to the point where they seek help, they’re less likely to comply,” he said. However, he noted, this is not because they are lazy; it’s that they’re “very, very anxious.”
Parents and other family members are also negatively affected. Dr. Berger noted that 15% of parents of a child with FTL equate their caregiver burden with having a family member with a chronic physical illness. “It’s huge; parents go through hell and it’s very hard on them. Many believe it is their fault and they feel a lot of shame.”
Supportive Parenting for Anxious Childhood Emotions (SPACE) is a manualized, parent-based program for childhood anxiety and obsessive-compulsive disorder. It has been tested in clinical trials and found to be noninferior to cognitive behavioral therapy for childhood anxiety.
The research adapted it to treat FTL. SPACE-FTL focuses on reducing parents’ family accommodation (FA), a descriptor for a child’s excessive dependence on their parents to help them avoid anxiety-provoking situations.
The study examined the feasibility, acceptability, and treatment satisfaction and its effect on adult child psychopathology symptoms, parents’ FA, and the paternal burden of caring for adult children.
The study included parents (mean age, 59.46 years; 85% female) of 40 adult children with FTL (mean age, 23.51 years; 20% female) from across the United States.
Parents were randomized to a 13-week wait-list or the SPACE-FTL program, which involves 13-20 therapy sessions, depending on the need. The average number of sessions in the study was 15. The program has five key components:
- Providing information emphasizing FTL as not a character flaw but a problem with anxiety.
- Helping parents identify how they accommodate their child’s behavior, and facilitating an environment that encourages independence.
- Getting parents to show acceptance and confidence in their child who’s trying to overcome anxiety when, for example, they seek employment, instead of being overprotective and demanding.
- Focusing on change nonconfrontationally.
- Involving other family, community members, and professionals who can support the parent, child, or both.
The recruitment, treatment sessions, and assessments were all done online. Most participants rated the intervention as highly satisfactory on the Client Satisfaction Questionnaire (CSQ-8; mean score, 27.7 out of a maximum of 32). About 60% of the offspring no longer met full criteria for FTL (P < .001; Cohen’s D = 1.76).
All children of the wait-listed parents still met criteria for FTL.
FTL symptoms decreased significantly in the offspring of the intervention group, as seen in both in the Adult Entitled Dependence Scale (AED; P < .05; Cohen’s D = 0.84); and the Adaptive Behaviors Scale (ABS; P < .05; Cohen’s D = 0.70).
There was no change in anxiety as assessed by the Adult Behavior Checklist (ABCL). But Dr. Berger noted that child anxiety is difficult to assess through parental report.
“This population is self-isolating and parents sometimes don’t know what’s going on,” and ABCL measures may not be “as sensitive as we would have liked them to be,” Dr. Berger said.
Parental burden was significantly decreased as measured by the Zarit Burden Interview (ZBI; P < .05; Cohen’s D = 0.70). In addition, family accommodation decreased significantly as determined by the Family Accommodation Scale–Anxiety (FASA; P < .05; Cohen’s D = 0.70).
Innovative work
In a comment, Jonathan E. Alpert, MD, PhD, chair, department of psychiatry and behavioral sciences, and professor of psychiatry, neuroscience, and pediatrics, Albert Einstein College of Medicine, New York, described the program as “innovative.”
He noted that the SPACE-FTL approach provides parents with education and skills to reduce behaviors that reinforce their child’s avoidance of independent activities. Such behaviors “may inadvertently contribute to the adult child remaining stuck,” he said.
“Through its involvement of parents and use of a structured approach, SPACE-FTL is a very interesting step toward more evidence-based therapies.”
However, he noted that the number of study participants is still “very low” and further work is needed to better characterize this condition and develop effective therapies.
He noted that parents of adult children with FTL should not be judged or blamed. “They have been living with a worrisome problem for years and are simply doing their best to cope as any of us would do.”
In addition, he noted that some adult children aren’t capable of launching because of a serious mental illness or substance use disorder that needs treatment.
It’s unclear just how many adult children have FTL, as the condition lacks formal, agreed-upon clinical and research criteria and a reliable evidence base for treatment, Dr. Alpert said.
“Whatever the actual numbers of FTL, my anecdotal clinical experience suggests that it is a very common problem which is understudied.”
He added that the definitions of FTL should include cultural context. In some groups, it’s quite normal for adults in their 20s, 30s, or even older to live with their parents, Dr. Alpert said.
Dr. Berger and Dr. Albert report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON – , a new pilot study shows.
Known as failure to launch (FTL) syndrome, the criteria for this condition include the absence of a neurodevelopmental, mental, or intellectual condition, difficulty adapting to the challenges of adulthood, and living with or at the expense of parents.
Results suggest that the program benefits families dealing with FTL, said study investigator Uri Berger, PhD, postdoctoral associate, Yale Child Study Center Anxiety and Mood Disorders Program, New Haven, Conn.
“If you encounter parents who are say 50-60 years old who have a child with FTL, you can tell them there’s something they can do; there’s work they can do even if their child is refusing to go to therapy,” he said.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Anxious, isolated
Estimates suggest that there are 3.3 million physically able adults with FTL and that the disorder may be on the rise. These individuals often present with mental health symptoms including anxiety, depression, and suicidality, and tend to be socially isolated.
The investigators noted that intervening is often challenging because individuals with the syndrome are frequently noncompliant with therapy, and currently there is no standard of care.
“The longer you’re isolated, the harder it is getting out of your cocoon, and when these adult children get to the point where they seek help, they’re less likely to comply,” he said. However, he noted, this is not because they are lazy; it’s that they’re “very, very anxious.”
Parents and other family members are also negatively affected. Dr. Berger noted that 15% of parents of a child with FTL equate their caregiver burden with having a family member with a chronic physical illness. “It’s huge; parents go through hell and it’s very hard on them. Many believe it is their fault and they feel a lot of shame.”
Supportive Parenting for Anxious Childhood Emotions (SPACE) is a manualized, parent-based program for childhood anxiety and obsessive-compulsive disorder. It has been tested in clinical trials and found to be noninferior to cognitive behavioral therapy for childhood anxiety.
The research adapted it to treat FTL. SPACE-FTL focuses on reducing parents’ family accommodation (FA), a descriptor for a child’s excessive dependence on their parents to help them avoid anxiety-provoking situations.
The study examined the feasibility, acceptability, and treatment satisfaction and its effect on adult child psychopathology symptoms, parents’ FA, and the paternal burden of caring for adult children.
The study included parents (mean age, 59.46 years; 85% female) of 40 adult children with FTL (mean age, 23.51 years; 20% female) from across the United States.
Parents were randomized to a 13-week wait-list or the SPACE-FTL program, which involves 13-20 therapy sessions, depending on the need. The average number of sessions in the study was 15. The program has five key components:
- Providing information emphasizing FTL as not a character flaw but a problem with anxiety.
- Helping parents identify how they accommodate their child’s behavior, and facilitating an environment that encourages independence.
- Getting parents to show acceptance and confidence in their child who’s trying to overcome anxiety when, for example, they seek employment, instead of being overprotective and demanding.
- Focusing on change nonconfrontationally.
- Involving other family, community members, and professionals who can support the parent, child, or both.
The recruitment, treatment sessions, and assessments were all done online. Most participants rated the intervention as highly satisfactory on the Client Satisfaction Questionnaire (CSQ-8; mean score, 27.7 out of a maximum of 32). About 60% of the offspring no longer met full criteria for FTL (P < .001; Cohen’s D = 1.76).
All children of the wait-listed parents still met criteria for FTL.
FTL symptoms decreased significantly in the offspring of the intervention group, as seen in both in the Adult Entitled Dependence Scale (AED; P < .05; Cohen’s D = 0.84); and the Adaptive Behaviors Scale (ABS; P < .05; Cohen’s D = 0.70).
There was no change in anxiety as assessed by the Adult Behavior Checklist (ABCL). But Dr. Berger noted that child anxiety is difficult to assess through parental report.
“This population is self-isolating and parents sometimes don’t know what’s going on,” and ABCL measures may not be “as sensitive as we would have liked them to be,” Dr. Berger said.
Parental burden was significantly decreased as measured by the Zarit Burden Interview (ZBI; P < .05; Cohen’s D = 0.70). In addition, family accommodation decreased significantly as determined by the Family Accommodation Scale–Anxiety (FASA; P < .05; Cohen’s D = 0.70).
Innovative work
In a comment, Jonathan E. Alpert, MD, PhD, chair, department of psychiatry and behavioral sciences, and professor of psychiatry, neuroscience, and pediatrics, Albert Einstein College of Medicine, New York, described the program as “innovative.”
He noted that the SPACE-FTL approach provides parents with education and skills to reduce behaviors that reinforce their child’s avoidance of independent activities. Such behaviors “may inadvertently contribute to the adult child remaining stuck,” he said.
“Through its involvement of parents and use of a structured approach, SPACE-FTL is a very interesting step toward more evidence-based therapies.”
However, he noted that the number of study participants is still “very low” and further work is needed to better characterize this condition and develop effective therapies.
He noted that parents of adult children with FTL should not be judged or blamed. “They have been living with a worrisome problem for years and are simply doing their best to cope as any of us would do.”
In addition, he noted that some adult children aren’t capable of launching because of a serious mental illness or substance use disorder that needs treatment.
It’s unclear just how many adult children have FTL, as the condition lacks formal, agreed-upon clinical and research criteria and a reliable evidence base for treatment, Dr. Alpert said.
“Whatever the actual numbers of FTL, my anecdotal clinical experience suggests that it is a very common problem which is understudied.”
He added that the definitions of FTL should include cultural context. In some groups, it’s quite normal for adults in their 20s, 30s, or even older to live with their parents, Dr. Alpert said.
Dr. Berger and Dr. Albert report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON – , a new pilot study shows.
Known as failure to launch (FTL) syndrome, the criteria for this condition include the absence of a neurodevelopmental, mental, or intellectual condition, difficulty adapting to the challenges of adulthood, and living with or at the expense of parents.
Results suggest that the program benefits families dealing with FTL, said study investigator Uri Berger, PhD, postdoctoral associate, Yale Child Study Center Anxiety and Mood Disorders Program, New Haven, Conn.
“If you encounter parents who are say 50-60 years old who have a child with FTL, you can tell them there’s something they can do; there’s work they can do even if their child is refusing to go to therapy,” he said.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Anxious, isolated
Estimates suggest that there are 3.3 million physically able adults with FTL and that the disorder may be on the rise. These individuals often present with mental health symptoms including anxiety, depression, and suicidality, and tend to be socially isolated.
The investigators noted that intervening is often challenging because individuals with the syndrome are frequently noncompliant with therapy, and currently there is no standard of care.
“The longer you’re isolated, the harder it is getting out of your cocoon, and when these adult children get to the point where they seek help, they’re less likely to comply,” he said. However, he noted, this is not because they are lazy; it’s that they’re “very, very anxious.”
Parents and other family members are also negatively affected. Dr. Berger noted that 15% of parents of a child with FTL equate their caregiver burden with having a family member with a chronic physical illness. “It’s huge; parents go through hell and it’s very hard on them. Many believe it is their fault and they feel a lot of shame.”
Supportive Parenting for Anxious Childhood Emotions (SPACE) is a manualized, parent-based program for childhood anxiety and obsessive-compulsive disorder. It has been tested in clinical trials and found to be noninferior to cognitive behavioral therapy for childhood anxiety.
The research adapted it to treat FTL. SPACE-FTL focuses on reducing parents’ family accommodation (FA), a descriptor for a child’s excessive dependence on their parents to help them avoid anxiety-provoking situations.
The study examined the feasibility, acceptability, and treatment satisfaction and its effect on adult child psychopathology symptoms, parents’ FA, and the paternal burden of caring for adult children.
The study included parents (mean age, 59.46 years; 85% female) of 40 adult children with FTL (mean age, 23.51 years; 20% female) from across the United States.
Parents were randomized to a 13-week wait-list or the SPACE-FTL program, which involves 13-20 therapy sessions, depending on the need. The average number of sessions in the study was 15. The program has five key components:
- Providing information emphasizing FTL as not a character flaw but a problem with anxiety.
- Helping parents identify how they accommodate their child’s behavior, and facilitating an environment that encourages independence.
- Getting parents to show acceptance and confidence in their child who’s trying to overcome anxiety when, for example, they seek employment, instead of being overprotective and demanding.
- Focusing on change nonconfrontationally.
- Involving other family, community members, and professionals who can support the parent, child, or both.
The recruitment, treatment sessions, and assessments were all done online. Most participants rated the intervention as highly satisfactory on the Client Satisfaction Questionnaire (CSQ-8; mean score, 27.7 out of a maximum of 32). About 60% of the offspring no longer met full criteria for FTL (P < .001; Cohen’s D = 1.76).
All children of the wait-listed parents still met criteria for FTL.
FTL symptoms decreased significantly in the offspring of the intervention group, as seen in both in the Adult Entitled Dependence Scale (AED; P < .05; Cohen’s D = 0.84); and the Adaptive Behaviors Scale (ABS; P < .05; Cohen’s D = 0.70).
There was no change in anxiety as assessed by the Adult Behavior Checklist (ABCL). But Dr. Berger noted that child anxiety is difficult to assess through parental report.
“This population is self-isolating and parents sometimes don’t know what’s going on,” and ABCL measures may not be “as sensitive as we would have liked them to be,” Dr. Berger said.
Parental burden was significantly decreased as measured by the Zarit Burden Interview (ZBI; P < .05; Cohen’s D = 0.70). In addition, family accommodation decreased significantly as determined by the Family Accommodation Scale–Anxiety (FASA; P < .05; Cohen’s D = 0.70).
Innovative work
In a comment, Jonathan E. Alpert, MD, PhD, chair, department of psychiatry and behavioral sciences, and professor of psychiatry, neuroscience, and pediatrics, Albert Einstein College of Medicine, New York, described the program as “innovative.”
He noted that the SPACE-FTL approach provides parents with education and skills to reduce behaviors that reinforce their child’s avoidance of independent activities. Such behaviors “may inadvertently contribute to the adult child remaining stuck,” he said.
“Through its involvement of parents and use of a structured approach, SPACE-FTL is a very interesting step toward more evidence-based therapies.”
However, he noted that the number of study participants is still “very low” and further work is needed to better characterize this condition and develop effective therapies.
He noted that parents of adult children with FTL should not be judged or blamed. “They have been living with a worrisome problem for years and are simply doing their best to cope as any of us would do.”
In addition, he noted that some adult children aren’t capable of launching because of a serious mental illness or substance use disorder that needs treatment.
It’s unclear just how many adult children have FTL, as the condition lacks formal, agreed-upon clinical and research criteria and a reliable evidence base for treatment, Dr. Alpert said.
“Whatever the actual numbers of FTL, my anecdotal clinical experience suggests that it is a very common problem which is understudied.”
He added that the definitions of FTL should include cultural context. In some groups, it’s quite normal for adults in their 20s, 30s, or even older to live with their parents, Dr. Alpert said.
Dr. Berger and Dr. Albert report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ADAA 2023
Remote weight monitoring minimizes office visits for newborns
WASHINGTON, D.C. – according to a new study presented at the Pediatric Academic Societies annual meeting.
The pilot trial compared the frequency of office visits for healthy babies born at 37 weeks’ gestation or later. One group of 20 infants had their weight monitored at home by parents, and another group of 20 infants received usual care, which included two in-person office visits over the first 6 weeks of life.
Researchers found that visits for infants in the intervention group decreased by 25% after the first week of life and by 23% after the second week.
The remote method can help alert physicians earlier to insufficient weight because parents report gains or losses three times a week over the 6 weeks, resulting in more data for providers.
“You’re going to see fewer visits with people who have scales because the docs are getting the information they need, which is: ‘Is this baby doing okay or not?’ ” said Diane DiTomasso, PhD, RN, a professor at the University of Rhode Island, South Kingstown, who was not involved with the study. “I think it’s a very necessary study because, to my knowledge, nobody has done a randomized controlled trial on this topic.”
Keeping infants at home can also protect babies from infections they might catch in the clinic.
“There are a lot of other kids in an office setting, and kids like touching things,” said Anirudha Das, MD, MPH, a neonatologist at Cleveland Clinic Children’s and the lead author of the study. “When there are a lot of other kids, there are a lot of viruses. It’s a very dangerous environment.”
Parents in the intervention group were given scales and asked to enter their infant’s weight into a patient portal app three times per week for 6 weeks. Physicians then determined if in-office visits were necessary.
The benefits of home weight checks can include helping to allow for breastfeeding for a longer duration.
Weight is more closely monitored for breastfed infants. Waiting weeks for office checks can heighten parental anxiety and lead to prematurely stopping breastfeeding. With regular at-home checks, parents receive up-to-date information from physicians that can alleviate concerns and empower them with more control over the process, according to Dr. DiTomasso.
Breastfeeding is associated with a lower risk for cardiovascular disease, diabetes, obesity, cancer in later life, and a lower risk of breast cancer for breastfeeding parents.
Office weight checks can also alleviate a significant and unnecessary burden for parents, Dr. Das said.
“You shouldn’t have to put your baby in a car, possibly in freezing temperatures, hire someone to take care of your other kids, drive to the hospital, pay for parking, and walk to the office for a weight check,” Dr. Das said.
Dr. Das noted that, because of technical errors, parents weren’t able to use remote monitoring and had in-person visits during the first 5 days of life. The intervention group had more visits during that period than the usual-care group.
The study was funded by the American Academy of Pediatrics. The authors and Dr. Das reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
WASHINGTON, D.C. – according to a new study presented at the Pediatric Academic Societies annual meeting.
The pilot trial compared the frequency of office visits for healthy babies born at 37 weeks’ gestation or later. One group of 20 infants had their weight monitored at home by parents, and another group of 20 infants received usual care, which included two in-person office visits over the first 6 weeks of life.
Researchers found that visits for infants in the intervention group decreased by 25% after the first week of life and by 23% after the second week.
The remote method can help alert physicians earlier to insufficient weight because parents report gains or losses three times a week over the 6 weeks, resulting in more data for providers.
“You’re going to see fewer visits with people who have scales because the docs are getting the information they need, which is: ‘Is this baby doing okay or not?’ ” said Diane DiTomasso, PhD, RN, a professor at the University of Rhode Island, South Kingstown, who was not involved with the study. “I think it’s a very necessary study because, to my knowledge, nobody has done a randomized controlled trial on this topic.”
Keeping infants at home can also protect babies from infections they might catch in the clinic.
“There are a lot of other kids in an office setting, and kids like touching things,” said Anirudha Das, MD, MPH, a neonatologist at Cleveland Clinic Children’s and the lead author of the study. “When there are a lot of other kids, there are a lot of viruses. It’s a very dangerous environment.”
Parents in the intervention group were given scales and asked to enter their infant’s weight into a patient portal app three times per week for 6 weeks. Physicians then determined if in-office visits were necessary.
The benefits of home weight checks can include helping to allow for breastfeeding for a longer duration.
Weight is more closely monitored for breastfed infants. Waiting weeks for office checks can heighten parental anxiety and lead to prematurely stopping breastfeeding. With regular at-home checks, parents receive up-to-date information from physicians that can alleviate concerns and empower them with more control over the process, according to Dr. DiTomasso.
Breastfeeding is associated with a lower risk for cardiovascular disease, diabetes, obesity, cancer in later life, and a lower risk of breast cancer for breastfeeding parents.
Office weight checks can also alleviate a significant and unnecessary burden for parents, Dr. Das said.
“You shouldn’t have to put your baby in a car, possibly in freezing temperatures, hire someone to take care of your other kids, drive to the hospital, pay for parking, and walk to the office for a weight check,” Dr. Das said.
Dr. Das noted that, because of technical errors, parents weren’t able to use remote monitoring and had in-person visits during the first 5 days of life. The intervention group had more visits during that period than the usual-care group.
The study was funded by the American Academy of Pediatrics. The authors and Dr. Das reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
WASHINGTON, D.C. – according to a new study presented at the Pediatric Academic Societies annual meeting.
The pilot trial compared the frequency of office visits for healthy babies born at 37 weeks’ gestation or later. One group of 20 infants had their weight monitored at home by parents, and another group of 20 infants received usual care, which included two in-person office visits over the first 6 weeks of life.
Researchers found that visits for infants in the intervention group decreased by 25% after the first week of life and by 23% after the second week.
The remote method can help alert physicians earlier to insufficient weight because parents report gains or losses three times a week over the 6 weeks, resulting in more data for providers.
“You’re going to see fewer visits with people who have scales because the docs are getting the information they need, which is: ‘Is this baby doing okay or not?’ ” said Diane DiTomasso, PhD, RN, a professor at the University of Rhode Island, South Kingstown, who was not involved with the study. “I think it’s a very necessary study because, to my knowledge, nobody has done a randomized controlled trial on this topic.”
Keeping infants at home can also protect babies from infections they might catch in the clinic.
“There are a lot of other kids in an office setting, and kids like touching things,” said Anirudha Das, MD, MPH, a neonatologist at Cleveland Clinic Children’s and the lead author of the study. “When there are a lot of other kids, there are a lot of viruses. It’s a very dangerous environment.”
Parents in the intervention group were given scales and asked to enter their infant’s weight into a patient portal app three times per week for 6 weeks. Physicians then determined if in-office visits were necessary.
The benefits of home weight checks can include helping to allow for breastfeeding for a longer duration.
Weight is more closely monitored for breastfed infants. Waiting weeks for office checks can heighten parental anxiety and lead to prematurely stopping breastfeeding. With regular at-home checks, parents receive up-to-date information from physicians that can alleviate concerns and empower them with more control over the process, according to Dr. DiTomasso.
Breastfeeding is associated with a lower risk for cardiovascular disease, diabetes, obesity, cancer in later life, and a lower risk of breast cancer for breastfeeding parents.
Office weight checks can also alleviate a significant and unnecessary burden for parents, Dr. Das said.
“You shouldn’t have to put your baby in a car, possibly in freezing temperatures, hire someone to take care of your other kids, drive to the hospital, pay for parking, and walk to the office for a weight check,” Dr. Das said.
Dr. Das noted that, because of technical errors, parents weren’t able to use remote monitoring and had in-person visits during the first 5 days of life. The intervention group had more visits during that period than the usual-care group.
The study was funded by the American Academy of Pediatrics. The authors and Dr. Das reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AT PAS 2023
Should youth with type 1 diabetes use closed-loop systems?
Would closed-loop systems be a good option for young patients with type 1 diabetes?
International and French recommendations on closed-loop systems state that the use of an “artificial pancreas” should be reserved for adults who are fully engaged with their treatment. This means that young patients, especially adolescents, who are less likely to comply with treatment and are more likely to experience suboptimal blood glucose control, are often excluded from the use of such systems for managing their diabetes.
Several recent studies seem to call this approach into question.
One such study, which was presented at a Francophone Diabetes Society conference and was published in Nature Communications, showed that adolescents with poorly controlled diabetes who were equipped with closed-loop systems gained IQ points and reasoning capacity and experienced a reduction in edematous tissue in the brain cortex. Furthermore, with the closed-loop system, patients spent 13% more time in a target range, and there was a significant reduction in time spent in hyperglycemia.
In the same vein, a small prospective study published in Diabetes Care showed that the closed-loop system with the Minimed 780G pump improved glycemic control for 20 young patients with type 1 diabetes aged 13-25 years whose diabetes was poorly controlled (hemoglobin A1c ≥ 8.5%). At the end of the 3-month study period, the average A1c had decreased from 10.5% (±2.1%) to 7.6% (±1.1%), an average decrease of 2.9%. The time spent in target A1c, which was set from 0.70 g/L to 1.80 g/L, was increased by almost 40%.
With respect to very young children, a study published in The New England Journal of Medicine also showed a favorable risk-benefit ratio for closed-loop systems. The trial, which enrolled 102 children aged 2 years to less than 6 years who had type 1 diabetes, showed that the amount of time that the glucose level was within the target range during the 13-week study period was higher (+3 hours) for those who had been randomly assigned to receive the hybrid closed-loop system (n = 68) than for those who had received the standard treatment (n = 34), either with an insulin pump or multiple daily injections or a Dexcom G6 continuous glucose monitoring device.
A previous study carried out by the Paris Public Hospital System had already shown that the French Diabeloop system could reduce episodes of hypoglycemia and achieve good glycemic control for prepubescent children (n = 21; aged 6-12 years) with type 1 diabetes in real-life conditions.
Eric Renard, MD, PhD, head of the department of endocrinology and diabetes at Lapeyronie Hospital in Montpellier, France, was not surprised at the findings from the study, especially in adolescents with poorly controlled diabetes.
“We have already seen studies in which those patients who had the most poorly controlled diabetes at the start were the ones who improved the most with the closed-loop system, by at least 20% in terms of time in target. These findings resonate with what I see in my clinic,” said Dr. Renard in an interview.
“In my experience, these young adolescents, who neglected their diabetes when they had no devices to help control it, when they had to inject themselves, et cetera ... well, they’re just not the same people when they’re put on a closed-loop system,” he added. “They rise to the challenge, and for the first time, they succeed without making a huge effort, since the algorithm does what they weren’t doing. It’s astonishing to see near-total engagement in these young people when explaining the technology to them and saying, ‘Let’s give it a go.’ These are the very same youngsters who didn’t want to hear about their diabetes in the past. They are delighted and once again involved in managing their condition.”
That’s why Dr. Renard recommends keeping an open mind when considering treatment options for young patients with poorly controlled type 1 diabetes.
“When young people have very poorly controlled diabetes, they risk having cardiovascular complications and damaging their retinas and kidneys,” he said. “If we can get them from 25% to 45% time in target, even if that hasn’t been easy to achieve, this will help save their blood vessels! The only thing we have to be careful of is that we don’t set up a closed-loop system in someone who doesn’t want one. But, if it can manage to spark the interest of a young patient, in most cases, it’s beneficial.”
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
Would closed-loop systems be a good option for young patients with type 1 diabetes?
International and French recommendations on closed-loop systems state that the use of an “artificial pancreas” should be reserved for adults who are fully engaged with their treatment. This means that young patients, especially adolescents, who are less likely to comply with treatment and are more likely to experience suboptimal blood glucose control, are often excluded from the use of such systems for managing their diabetes.
Several recent studies seem to call this approach into question.
One such study, which was presented at a Francophone Diabetes Society conference and was published in Nature Communications, showed that adolescents with poorly controlled diabetes who were equipped with closed-loop systems gained IQ points and reasoning capacity and experienced a reduction in edematous tissue in the brain cortex. Furthermore, with the closed-loop system, patients spent 13% more time in a target range, and there was a significant reduction in time spent in hyperglycemia.
In the same vein, a small prospective study published in Diabetes Care showed that the closed-loop system with the Minimed 780G pump improved glycemic control for 20 young patients with type 1 diabetes aged 13-25 years whose diabetes was poorly controlled (hemoglobin A1c ≥ 8.5%). At the end of the 3-month study period, the average A1c had decreased from 10.5% (±2.1%) to 7.6% (±1.1%), an average decrease of 2.9%. The time spent in target A1c, which was set from 0.70 g/L to 1.80 g/L, was increased by almost 40%.
With respect to very young children, a study published in The New England Journal of Medicine also showed a favorable risk-benefit ratio for closed-loop systems. The trial, which enrolled 102 children aged 2 years to less than 6 years who had type 1 diabetes, showed that the amount of time that the glucose level was within the target range during the 13-week study period was higher (+3 hours) for those who had been randomly assigned to receive the hybrid closed-loop system (n = 68) than for those who had received the standard treatment (n = 34), either with an insulin pump or multiple daily injections or a Dexcom G6 continuous glucose monitoring device.
A previous study carried out by the Paris Public Hospital System had already shown that the French Diabeloop system could reduce episodes of hypoglycemia and achieve good glycemic control for prepubescent children (n = 21; aged 6-12 years) with type 1 diabetes in real-life conditions.
Eric Renard, MD, PhD, head of the department of endocrinology and diabetes at Lapeyronie Hospital in Montpellier, France, was not surprised at the findings from the study, especially in adolescents with poorly controlled diabetes.
“We have already seen studies in which those patients who had the most poorly controlled diabetes at the start were the ones who improved the most with the closed-loop system, by at least 20% in terms of time in target. These findings resonate with what I see in my clinic,” said Dr. Renard in an interview.
“In my experience, these young adolescents, who neglected their diabetes when they had no devices to help control it, when they had to inject themselves, et cetera ... well, they’re just not the same people when they’re put on a closed-loop system,” he added. “They rise to the challenge, and for the first time, they succeed without making a huge effort, since the algorithm does what they weren’t doing. It’s astonishing to see near-total engagement in these young people when explaining the technology to them and saying, ‘Let’s give it a go.’ These are the very same youngsters who didn’t want to hear about their diabetes in the past. They are delighted and once again involved in managing their condition.”
That’s why Dr. Renard recommends keeping an open mind when considering treatment options for young patients with poorly controlled type 1 diabetes.
“When young people have very poorly controlled diabetes, they risk having cardiovascular complications and damaging their retinas and kidneys,” he said. “If we can get them from 25% to 45% time in target, even if that hasn’t been easy to achieve, this will help save their blood vessels! The only thing we have to be careful of is that we don’t set up a closed-loop system in someone who doesn’t want one. But, if it can manage to spark the interest of a young patient, in most cases, it’s beneficial.”
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
Would closed-loop systems be a good option for young patients with type 1 diabetes?
International and French recommendations on closed-loop systems state that the use of an “artificial pancreas” should be reserved for adults who are fully engaged with their treatment. This means that young patients, especially adolescents, who are less likely to comply with treatment and are more likely to experience suboptimal blood glucose control, are often excluded from the use of such systems for managing their diabetes.
Several recent studies seem to call this approach into question.
One such study, which was presented at a Francophone Diabetes Society conference and was published in Nature Communications, showed that adolescents with poorly controlled diabetes who were equipped with closed-loop systems gained IQ points and reasoning capacity and experienced a reduction in edematous tissue in the brain cortex. Furthermore, with the closed-loop system, patients spent 13% more time in a target range, and there was a significant reduction in time spent in hyperglycemia.
In the same vein, a small prospective study published in Diabetes Care showed that the closed-loop system with the Minimed 780G pump improved glycemic control for 20 young patients with type 1 diabetes aged 13-25 years whose diabetes was poorly controlled (hemoglobin A1c ≥ 8.5%). At the end of the 3-month study period, the average A1c had decreased from 10.5% (±2.1%) to 7.6% (±1.1%), an average decrease of 2.9%. The time spent in target A1c, which was set from 0.70 g/L to 1.80 g/L, was increased by almost 40%.
With respect to very young children, a study published in The New England Journal of Medicine also showed a favorable risk-benefit ratio for closed-loop systems. The trial, which enrolled 102 children aged 2 years to less than 6 years who had type 1 diabetes, showed that the amount of time that the glucose level was within the target range during the 13-week study period was higher (+3 hours) for those who had been randomly assigned to receive the hybrid closed-loop system (n = 68) than for those who had received the standard treatment (n = 34), either with an insulin pump or multiple daily injections or a Dexcom G6 continuous glucose monitoring device.
A previous study carried out by the Paris Public Hospital System had already shown that the French Diabeloop system could reduce episodes of hypoglycemia and achieve good glycemic control for prepubescent children (n = 21; aged 6-12 years) with type 1 diabetes in real-life conditions.
Eric Renard, MD, PhD, head of the department of endocrinology and diabetes at Lapeyronie Hospital in Montpellier, France, was not surprised at the findings from the study, especially in adolescents with poorly controlled diabetes.
“We have already seen studies in which those patients who had the most poorly controlled diabetes at the start were the ones who improved the most with the closed-loop system, by at least 20% in terms of time in target. These findings resonate with what I see in my clinic,” said Dr. Renard in an interview.
“In my experience, these young adolescents, who neglected their diabetes when they had no devices to help control it, when they had to inject themselves, et cetera ... well, they’re just not the same people when they’re put on a closed-loop system,” he added. “They rise to the challenge, and for the first time, they succeed without making a huge effort, since the algorithm does what they weren’t doing. It’s astonishing to see near-total engagement in these young people when explaining the technology to them and saying, ‘Let’s give it a go.’ These are the very same youngsters who didn’t want to hear about their diabetes in the past. They are delighted and once again involved in managing their condition.”
That’s why Dr. Renard recommends keeping an open mind when considering treatment options for young patients with poorly controlled type 1 diabetes.
“When young people have very poorly controlled diabetes, they risk having cardiovascular complications and damaging their retinas and kidneys,” he said. “If we can get them from 25% to 45% time in target, even if that hasn’t been easy to achieve, this will help save their blood vessels! The only thing we have to be careful of is that we don’t set up a closed-loop system in someone who doesn’t want one. But, if it can manage to spark the interest of a young patient, in most cases, it’s beneficial.”
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
Repeated CTs in childhood linked with increased cancer risk
In a population-based case-control study that included more than 85,000 participants, researchers found a ninefold increased risk of intracranial tumors among children who received four or more CT scans.
The results “indicate that judicious CT usage and radiation-reducing techniques should be advocated,” Yu-Hsuan Joni Shao, PhD, professor of biomedical informatics at Taipei (Taiwan) Medical University, and colleagues wrote.
The study was published in the Canadian Medical Association Journal.
Dose-response relationship
The investigators used the National Health Insurance Research Database in Taiwan to identify 7,807 patients under age 25 years with intracranial tumors (grades I-IV), leukemia, non-Hodgkin lymphomas, or Hodgkin lymphomas that had been diagnosed in a 14-year span between the years 2000 and 2013. They matched each case with 10 control participants without cancer by sex, date of birth, and date of entry into the cohort.
Radiation exposure was calculated for each patient according to number and type of CT scans received and an estimated organ-specific cumulative dose based on previously published models. The investigators excluded patients from the analysis if they had a diagnosis of any malignant disease before the study period or if they had any cancer-predisposing conditions, such as Down syndrome (which entails an increased risk of leukemia) or immunodeficiency (which may require multiple CT scans).
Compared with no exposure, exposure to a single pediatric CT scan was not associated with increased cancer risk. Exposure to two to three CT scans, however, was associated with an increased risk for intracranial tumour (adjusted odds ratio, 2.36), but not for leukemia, non-Hodgkin lymphoma, or Hodgkin lymphoma. Exposure to four or more CT scans was associated with increased risk for intracranial tumor (aOR, 9.01), leukemia (aOR, 4.80), and non-Hodgkin lymphoma (aOR, 6.76), but not for Hodgkin lymphoma.
The researchers also found a dose-response relationship. Participants in the top quintile of cumulative brain radiation dose had a significantly higher risk for intracranial tumor, compared with nonexposed participants (aOR, 3.61), although this relationship was not seen with the other cancers.
Age at exposure was also a significant factor. Children exposed to four or more CT scans at or before age 6 years had the highest risk for cancer (aOR, 22.95), followed by the same number of scans in those aged 7-12 years (aOR, 5.69) and those aged 13-18 years (aOR, 3.20).
The authors noted that, although these cancers are uncommon in children, “our work reinforces the importance of radiation protection strategies, addressed by the International Atomic Energy Agency. Unnecessary CT scans should be avoided, and special attention should be paid to patients who require repeated CT scans. Parents and pediatric patients should be well informed on risks and benefits before radiological procedures and encouraged to participate in decision-making around imaging.”
True risks underestimated?
Commenting on the findings, Rebecca Smith-Bindman, MD, a radiologist at the University of California, San Francisco, and an expert on the impact of CT scans on patient outcomes, said that she trusts the authors’ overall findings. But “because of the direction of their biases,” the study design “doesn’t let me accept their conclusion that one CT does not elevate the risk.
“It’s an interesting study that found the risk of brain cancer is more than doubled in children who undergo two or more CT scans, but in many ways, their assumptions will underestimate the true risk,” said Dr. Smith-Bindman, who is a professor of epidemiology and biostatistics at UCSF. She said reasons for this include the fact that the investigators used estimated, rather than actual radiation doses; that their estimates “reflect doses far lower than we have found actually occur in clinical practice”; that they do not differentiate between a low-dose or a high-dose CT; and that that they include a long, 3-year lag during which leukemia can develop after a CT scan.
“They did a lot of really well-done adjustments to ensure that they were not overestimating risk,” said Dr. Smith-Bindman. “They made sure to delete children who had cancer susceptibility syndrome, they included a lag of 3 years, assuming that there could be hidden cancers for up to 3 years after the first imaging study when they might have had a preexisting cancer. These are decisions that ensure that any cancer risk they find is real, but it also means that the risks that are estimated are almost certainly an underestimate of the true risks.”
The study was conducted without external funding. The authors declared no relevant financial relationships. Dr. Smith-Bindman is a cofounder of Alara Imaging, a company focused on collecting and reporting radiation dose information associated with CT.
A version of this article first appeared on Medscape.com.
In a population-based case-control study that included more than 85,000 participants, researchers found a ninefold increased risk of intracranial tumors among children who received four or more CT scans.
The results “indicate that judicious CT usage and radiation-reducing techniques should be advocated,” Yu-Hsuan Joni Shao, PhD, professor of biomedical informatics at Taipei (Taiwan) Medical University, and colleagues wrote.
The study was published in the Canadian Medical Association Journal.
Dose-response relationship
The investigators used the National Health Insurance Research Database in Taiwan to identify 7,807 patients under age 25 years with intracranial tumors (grades I-IV), leukemia, non-Hodgkin lymphomas, or Hodgkin lymphomas that had been diagnosed in a 14-year span between the years 2000 and 2013. They matched each case with 10 control participants without cancer by sex, date of birth, and date of entry into the cohort.
Radiation exposure was calculated for each patient according to number and type of CT scans received and an estimated organ-specific cumulative dose based on previously published models. The investigators excluded patients from the analysis if they had a diagnosis of any malignant disease before the study period or if they had any cancer-predisposing conditions, such as Down syndrome (which entails an increased risk of leukemia) or immunodeficiency (which may require multiple CT scans).
Compared with no exposure, exposure to a single pediatric CT scan was not associated with increased cancer risk. Exposure to two to three CT scans, however, was associated with an increased risk for intracranial tumour (adjusted odds ratio, 2.36), but not for leukemia, non-Hodgkin lymphoma, or Hodgkin lymphoma. Exposure to four or more CT scans was associated with increased risk for intracranial tumor (aOR, 9.01), leukemia (aOR, 4.80), and non-Hodgkin lymphoma (aOR, 6.76), but not for Hodgkin lymphoma.
The researchers also found a dose-response relationship. Participants in the top quintile of cumulative brain radiation dose had a significantly higher risk for intracranial tumor, compared with nonexposed participants (aOR, 3.61), although this relationship was not seen with the other cancers.
Age at exposure was also a significant factor. Children exposed to four or more CT scans at or before age 6 years had the highest risk for cancer (aOR, 22.95), followed by the same number of scans in those aged 7-12 years (aOR, 5.69) and those aged 13-18 years (aOR, 3.20).
The authors noted that, although these cancers are uncommon in children, “our work reinforces the importance of radiation protection strategies, addressed by the International Atomic Energy Agency. Unnecessary CT scans should be avoided, and special attention should be paid to patients who require repeated CT scans. Parents and pediatric patients should be well informed on risks and benefits before radiological procedures and encouraged to participate in decision-making around imaging.”
True risks underestimated?
Commenting on the findings, Rebecca Smith-Bindman, MD, a radiologist at the University of California, San Francisco, and an expert on the impact of CT scans on patient outcomes, said that she trusts the authors’ overall findings. But “because of the direction of their biases,” the study design “doesn’t let me accept their conclusion that one CT does not elevate the risk.
“It’s an interesting study that found the risk of brain cancer is more than doubled in children who undergo two or more CT scans, but in many ways, their assumptions will underestimate the true risk,” said Dr. Smith-Bindman, who is a professor of epidemiology and biostatistics at UCSF. She said reasons for this include the fact that the investigators used estimated, rather than actual radiation doses; that their estimates “reflect doses far lower than we have found actually occur in clinical practice”; that they do not differentiate between a low-dose or a high-dose CT; and that that they include a long, 3-year lag during which leukemia can develop after a CT scan.
“They did a lot of really well-done adjustments to ensure that they were not overestimating risk,” said Dr. Smith-Bindman. “They made sure to delete children who had cancer susceptibility syndrome, they included a lag of 3 years, assuming that there could be hidden cancers for up to 3 years after the first imaging study when they might have had a preexisting cancer. These are decisions that ensure that any cancer risk they find is real, but it also means that the risks that are estimated are almost certainly an underestimate of the true risks.”
The study was conducted without external funding. The authors declared no relevant financial relationships. Dr. Smith-Bindman is a cofounder of Alara Imaging, a company focused on collecting and reporting radiation dose information associated with CT.
A version of this article first appeared on Medscape.com.
In a population-based case-control study that included more than 85,000 participants, researchers found a ninefold increased risk of intracranial tumors among children who received four or more CT scans.
The results “indicate that judicious CT usage and radiation-reducing techniques should be advocated,” Yu-Hsuan Joni Shao, PhD, professor of biomedical informatics at Taipei (Taiwan) Medical University, and colleagues wrote.
The study was published in the Canadian Medical Association Journal.
Dose-response relationship
The investigators used the National Health Insurance Research Database in Taiwan to identify 7,807 patients under age 25 years with intracranial tumors (grades I-IV), leukemia, non-Hodgkin lymphomas, or Hodgkin lymphomas that had been diagnosed in a 14-year span between the years 2000 and 2013. They matched each case with 10 control participants without cancer by sex, date of birth, and date of entry into the cohort.
Radiation exposure was calculated for each patient according to number and type of CT scans received and an estimated organ-specific cumulative dose based on previously published models. The investigators excluded patients from the analysis if they had a diagnosis of any malignant disease before the study period or if they had any cancer-predisposing conditions, such as Down syndrome (which entails an increased risk of leukemia) or immunodeficiency (which may require multiple CT scans).
Compared with no exposure, exposure to a single pediatric CT scan was not associated with increased cancer risk. Exposure to two to three CT scans, however, was associated with an increased risk for intracranial tumour (adjusted odds ratio, 2.36), but not for leukemia, non-Hodgkin lymphoma, or Hodgkin lymphoma. Exposure to four or more CT scans was associated with increased risk for intracranial tumor (aOR, 9.01), leukemia (aOR, 4.80), and non-Hodgkin lymphoma (aOR, 6.76), but not for Hodgkin lymphoma.
The researchers also found a dose-response relationship. Participants in the top quintile of cumulative brain radiation dose had a significantly higher risk for intracranial tumor, compared with nonexposed participants (aOR, 3.61), although this relationship was not seen with the other cancers.
Age at exposure was also a significant factor. Children exposed to four or more CT scans at or before age 6 years had the highest risk for cancer (aOR, 22.95), followed by the same number of scans in those aged 7-12 years (aOR, 5.69) and those aged 13-18 years (aOR, 3.20).
The authors noted that, although these cancers are uncommon in children, “our work reinforces the importance of radiation protection strategies, addressed by the International Atomic Energy Agency. Unnecessary CT scans should be avoided, and special attention should be paid to patients who require repeated CT scans. Parents and pediatric patients should be well informed on risks and benefits before radiological procedures and encouraged to participate in decision-making around imaging.”
True risks underestimated?
Commenting on the findings, Rebecca Smith-Bindman, MD, a radiologist at the University of California, San Francisco, and an expert on the impact of CT scans on patient outcomes, said that she trusts the authors’ overall findings. But “because of the direction of their biases,” the study design “doesn’t let me accept their conclusion that one CT does not elevate the risk.
“It’s an interesting study that found the risk of brain cancer is more than doubled in children who undergo two or more CT scans, but in many ways, their assumptions will underestimate the true risk,” said Dr. Smith-Bindman, who is a professor of epidemiology and biostatistics at UCSF. She said reasons for this include the fact that the investigators used estimated, rather than actual radiation doses; that their estimates “reflect doses far lower than we have found actually occur in clinical practice”; that they do not differentiate between a low-dose or a high-dose CT; and that that they include a long, 3-year lag during which leukemia can develop after a CT scan.
“They did a lot of really well-done adjustments to ensure that they were not overestimating risk,” said Dr. Smith-Bindman. “They made sure to delete children who had cancer susceptibility syndrome, they included a lag of 3 years, assuming that there could be hidden cancers for up to 3 years after the first imaging study when they might have had a preexisting cancer. These are decisions that ensure that any cancer risk they find is real, but it also means that the risks that are estimated are almost certainly an underestimate of the true risks.”
The study was conducted without external funding. The authors declared no relevant financial relationships. Dr. Smith-Bindman is a cofounder of Alara Imaging, a company focused on collecting and reporting radiation dose information associated with CT.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
‘Shocking’ data on what’s really in melatonin gummies
New data may explain the recent massive jump in pediatric hospitalizations.
Thenvestigators found that consuming some products as directed could expose consumers, including children, to doses that are 40-130 times greater than what’s recommended.
“The results were quite shocking,” lead researcher Pieter Cohen, MD, with Harvard Medical School, Boston, and Cambridge Health Alliance, Somerville, Mass., said in an interview.
“Melatonin gummies contained up to 347% more melatonin than what was listed on the label, and some products also contained cannabidiol; in one brand of melatonin gummies, there was zero melatonin, just CBD,” Dr. Cohen said.
The study was published online in JAMA.
530% jump in pediatric hospitalizations
Melatonin products are not approved by the Food and Drug Administration but are sold over the counter or online.
Previous research from JAMA has shown the use of melatonin has increased over the past 2 decades among people of all ages.
With increased use has come a spike in reports of melatonin overdose, calls to poison control centers, and related ED visits for children.
Federal data show the number of U.S. children who unintentionally ingested melatonin supplements jumped 530% from 2012 to 2021. More than 4,000 of the reported ingestions led to a hospital stay; 287 children required intensive care, and two children died.
It was unclear why melatonin supplements were causing these harms, which led Dr. Cohen’s team to analyze 25 unique brands of “melatonin” gummies purchased online.
One product didn’t contain any melatonin but did contain 31.3 mg of CBD.
In the remaining products, the quantity of melatonin ranged from 1.3 mg to 13.1 mg per serving. The actual quantity of melatonin ranged from 74% to 347% of the labeled quantity, the researchers found.
They note that for a young adult who takes as little as 0.1-0.3 mg of melatonin, plasma concentrations can increase into the normal night-time range.
Of the 25 products (88%) analyzed, 22 were inaccurately labeled, and only 3 (12%) contained a quantity of melatonin that was within 10% (plus or minus) of the declared quantity.
Five products listed CBD as an ingredient. The listed quantity ranged from 10.6 mg to 31.3 mg per serving, although the actual quantity of CBD ranged from 104% to 118% of the labeled quantity.
Inquire about use in kids
A limitation of the study is that only one sample of each brand was analyzed, and only gummies were analyzed. It is not known whether the results are generalizable to melatonin products sold as tablets and capsules in the United States or whether the quantity of melatonin within an individual brand may vary from batch to batch.
A recent study from Canada showed similar results. In an analysis of 16 Canadian melatonin brands, the actual dose of melatonin ranged from 17% to 478% of the declared quantity.
It’s estimated that more than 1% of all U.S. children use melatonin supplements, most commonly for sleep, stress, and relaxation.
“Given new research as to the excessive quantities of melatonin in gummies, caution should be used if considering their use,” said Dr. Cohen.
“It’s important to inquire about melatonin use when caring for children, particularly when parents express concerns about their child’s sleep,” he added.
The American Academy of Sleep Medicine recently issued a health advisory encouraging parents to talk to a health care professional before giving melatonin or any supplement to children.
Children don’t need melatonin
Commenting on the study, Michael Breus, PhD, clinical psychologist and founder of TheSleepDoctor.com, agreed that analyzing only one sample of each brand is a key limitation “because supplements are made in batches, and gummies in particular are difficult to distribute the active ingredient evenly.
“But even with that being said, 88% of them were labeled incorrectly, so even if there were a few single-sample issues, I kind of doubt its all of them,” Dr. Breus said.
“Kids as a general rule do not need melatonin. Their brains make almost four times the necessary amount already. If you start giving kids pills to help them sleep, then they start to have a pill problem, causing another issue,” Dr. Breus added.
“Most children’s falling asleep and staying sleep issues can be treated with behavioral measures like cognitive-behavioral therapy for insomnia,” he said.
The study had no specific funding. Dr. Cohen has received research support from Consumers Union and PEW Charitable Trusts and royalties from UptoDate. Dr. Breus disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New data may explain the recent massive jump in pediatric hospitalizations.
Thenvestigators found that consuming some products as directed could expose consumers, including children, to doses that are 40-130 times greater than what’s recommended.
“The results were quite shocking,” lead researcher Pieter Cohen, MD, with Harvard Medical School, Boston, and Cambridge Health Alliance, Somerville, Mass., said in an interview.
“Melatonin gummies contained up to 347% more melatonin than what was listed on the label, and some products also contained cannabidiol; in one brand of melatonin gummies, there was zero melatonin, just CBD,” Dr. Cohen said.
The study was published online in JAMA.
530% jump in pediatric hospitalizations
Melatonin products are not approved by the Food and Drug Administration but are sold over the counter or online.
Previous research from JAMA has shown the use of melatonin has increased over the past 2 decades among people of all ages.
With increased use has come a spike in reports of melatonin overdose, calls to poison control centers, and related ED visits for children.
Federal data show the number of U.S. children who unintentionally ingested melatonin supplements jumped 530% from 2012 to 2021. More than 4,000 of the reported ingestions led to a hospital stay; 287 children required intensive care, and two children died.
It was unclear why melatonin supplements were causing these harms, which led Dr. Cohen’s team to analyze 25 unique brands of “melatonin” gummies purchased online.
One product didn’t contain any melatonin but did contain 31.3 mg of CBD.
In the remaining products, the quantity of melatonin ranged from 1.3 mg to 13.1 mg per serving. The actual quantity of melatonin ranged from 74% to 347% of the labeled quantity, the researchers found.
They note that for a young adult who takes as little as 0.1-0.3 mg of melatonin, plasma concentrations can increase into the normal night-time range.
Of the 25 products (88%) analyzed, 22 were inaccurately labeled, and only 3 (12%) contained a quantity of melatonin that was within 10% (plus or minus) of the declared quantity.
Five products listed CBD as an ingredient. The listed quantity ranged from 10.6 mg to 31.3 mg per serving, although the actual quantity of CBD ranged from 104% to 118% of the labeled quantity.
Inquire about use in kids
A limitation of the study is that only one sample of each brand was analyzed, and only gummies were analyzed. It is not known whether the results are generalizable to melatonin products sold as tablets and capsules in the United States or whether the quantity of melatonin within an individual brand may vary from batch to batch.
A recent study from Canada showed similar results. In an analysis of 16 Canadian melatonin brands, the actual dose of melatonin ranged from 17% to 478% of the declared quantity.
It’s estimated that more than 1% of all U.S. children use melatonin supplements, most commonly for sleep, stress, and relaxation.
“Given new research as to the excessive quantities of melatonin in gummies, caution should be used if considering their use,” said Dr. Cohen.
“It’s important to inquire about melatonin use when caring for children, particularly when parents express concerns about their child’s sleep,” he added.
The American Academy of Sleep Medicine recently issued a health advisory encouraging parents to talk to a health care professional before giving melatonin or any supplement to children.
Children don’t need melatonin
Commenting on the study, Michael Breus, PhD, clinical psychologist and founder of TheSleepDoctor.com, agreed that analyzing only one sample of each brand is a key limitation “because supplements are made in batches, and gummies in particular are difficult to distribute the active ingredient evenly.
“But even with that being said, 88% of them were labeled incorrectly, so even if there were a few single-sample issues, I kind of doubt its all of them,” Dr. Breus said.
“Kids as a general rule do not need melatonin. Their brains make almost four times the necessary amount already. If you start giving kids pills to help them sleep, then they start to have a pill problem, causing another issue,” Dr. Breus added.
“Most children’s falling asleep and staying sleep issues can be treated with behavioral measures like cognitive-behavioral therapy for insomnia,” he said.
The study had no specific funding. Dr. Cohen has received research support from Consumers Union and PEW Charitable Trusts and royalties from UptoDate. Dr. Breus disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New data may explain the recent massive jump in pediatric hospitalizations.
Thenvestigators found that consuming some products as directed could expose consumers, including children, to doses that are 40-130 times greater than what’s recommended.
“The results were quite shocking,” lead researcher Pieter Cohen, MD, with Harvard Medical School, Boston, and Cambridge Health Alliance, Somerville, Mass., said in an interview.
“Melatonin gummies contained up to 347% more melatonin than what was listed on the label, and some products also contained cannabidiol; in one brand of melatonin gummies, there was zero melatonin, just CBD,” Dr. Cohen said.
The study was published online in JAMA.
530% jump in pediatric hospitalizations
Melatonin products are not approved by the Food and Drug Administration but are sold over the counter or online.
Previous research from JAMA has shown the use of melatonin has increased over the past 2 decades among people of all ages.
With increased use has come a spike in reports of melatonin overdose, calls to poison control centers, and related ED visits for children.
Federal data show the number of U.S. children who unintentionally ingested melatonin supplements jumped 530% from 2012 to 2021. More than 4,000 of the reported ingestions led to a hospital stay; 287 children required intensive care, and two children died.
It was unclear why melatonin supplements were causing these harms, which led Dr. Cohen’s team to analyze 25 unique brands of “melatonin” gummies purchased online.
One product didn’t contain any melatonin but did contain 31.3 mg of CBD.
In the remaining products, the quantity of melatonin ranged from 1.3 mg to 13.1 mg per serving. The actual quantity of melatonin ranged from 74% to 347% of the labeled quantity, the researchers found.
They note that for a young adult who takes as little as 0.1-0.3 mg of melatonin, plasma concentrations can increase into the normal night-time range.
Of the 25 products (88%) analyzed, 22 were inaccurately labeled, and only 3 (12%) contained a quantity of melatonin that was within 10% (plus or minus) of the declared quantity.
Five products listed CBD as an ingredient. The listed quantity ranged from 10.6 mg to 31.3 mg per serving, although the actual quantity of CBD ranged from 104% to 118% of the labeled quantity.
Inquire about use in kids
A limitation of the study is that only one sample of each brand was analyzed, and only gummies were analyzed. It is not known whether the results are generalizable to melatonin products sold as tablets and capsules in the United States or whether the quantity of melatonin within an individual brand may vary from batch to batch.
A recent study from Canada showed similar results. In an analysis of 16 Canadian melatonin brands, the actual dose of melatonin ranged from 17% to 478% of the declared quantity.
It’s estimated that more than 1% of all U.S. children use melatonin supplements, most commonly for sleep, stress, and relaxation.
“Given new research as to the excessive quantities of melatonin in gummies, caution should be used if considering their use,” said Dr. Cohen.
“It’s important to inquire about melatonin use when caring for children, particularly when parents express concerns about their child’s sleep,” he added.
The American Academy of Sleep Medicine recently issued a health advisory encouraging parents to talk to a health care professional before giving melatonin or any supplement to children.
Children don’t need melatonin
Commenting on the study, Michael Breus, PhD, clinical psychologist and founder of TheSleepDoctor.com, agreed that analyzing only one sample of each brand is a key limitation “because supplements are made in batches, and gummies in particular are difficult to distribute the active ingredient evenly.
“But even with that being said, 88% of them were labeled incorrectly, so even if there were a few single-sample issues, I kind of doubt its all of them,” Dr. Breus said.
“Kids as a general rule do not need melatonin. Their brains make almost four times the necessary amount already. If you start giving kids pills to help them sleep, then they start to have a pill problem, causing another issue,” Dr. Breus added.
“Most children’s falling asleep and staying sleep issues can be treated with behavioral measures like cognitive-behavioral therapy for insomnia,” he said.
The study had no specific funding. Dr. Cohen has received research support from Consumers Union and PEW Charitable Trusts and royalties from UptoDate. Dr. Breus disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Walnuts linked to improved attention, psychological maturity in teens
, new research shows. Adolescents who consumed walnuts for at least 100 days showed improved sustained attention and fluid intelligence as well as a reduction in symptoms of attension deficit hyperactivity disorder, compared with matched controls who did not consume the nuts. However, there were no statistically significant changes between the groups in other parameters, such as working memory and executive function.
Clinicians should advise adolescents “to eat a handful of walnuts three times a week for the rest of their lives. They may have a healthier brain with better cognitive function,” said senior investigator Jordi Julvez, PhD, group leader at the Institute of Health Research Pere Virgili, Barcelona, and associated researcher at the Barcelona Institute for Global Health.
The study was published online in eClinicalMedicine.
Rich source of omega-3s
Adolescence is “a period of refinement of brain connectivity and complex behaviors,” the investigators noted.
Previous research suggests polyunsaturated fatty acids are key in central nervous system architecture and function during times of neural development, with three specific PUFAs playing an “essential developmental role.”
Two omega-3 fatty acids – docosahexaenoic acid and eicosapentaenoic acid – are PUFAs that must be obtained through diet, mainly from seafood. Walnuts are “among the richest sources” of plant-derived omega-3 fatty acids, particularly alpha-linolenic acid (ALA), a precursor for longer-chain EPA and DHA.
ALA independently “has positive effects on brain function and plasticity,” the authors wrote. In addition, walnut constituents – particularly polyphenols and other bioactive compounds – “may act synergistically with ALA to foster brain health.”
Earlier small studies have found positive associations between walnut consumption and cognitive function in children, adolescents, and young adults, but to date, no randomized controlled trial has focused on the effect of walnut consumption on adolescent neuropsychological function.
The researchers studied 771 healthy adolescents (aged 11-16 years, mean age 14) drawn from 12 Spanish high schools. Participants were instructed to follow healthy eating recommendations and were randomly assigned 1:1 to the intervention (n = 386) or the control group (n = 385).
At baseline and after 6 months, they completed neuropsychological tests and behavioral rating scales. The Attention Network Test assessed attention, and the N-back test was used to assess working memory. The Tests of Primary Mental Abilities assessed fluid intelligence. Risky decision-making was tested using the Roulettes Task.
Fruit and nuts
Participants also completed the Strengths and Difficulties Questionnaire, which provided a total score of problem behavior. Teachers filled out the ADHD DSM-IV form list to provide additional information about ADHD behaviors.
The intervention group received 30 grams/day of raw California walnut kernels to incorporate into their daily diet. It is estimated that this walnut contains about 9 g of ALA per 100 g.
All participants received a seasonal fruit calendar and were asked to eat at least one piece of seasonal fruit daily.
Parents reported their child’s daily walnut consumption, with adherence defined as 100 or more days of eating walnuts during the 6-month period.
All main analyses were based on an intention-to-treat method (participants were analyzed according to their original group assignment, regardless of their adherence to the intervention).
The researchers also conducted a secondary per-protocol analysis, comparing the intervention and control groups to estimate the effect if all participants had adhered to their assigned intervention. They censored data for participants who reported eating walnuts for less than 100 days during the 6-month trial period.
Secondary outcomes included changes in height, weight, waist circumference, and BMI, as well as red blood cell proportions of omega-3 fatty acids (DHA, EPA, and ALA) at baseline and after 6 months.
Adherence counts
Most participants had “medium” or “high” levels of adherence to the Mediterranean diet, with “no meaningful differences” at baseline between the intervention and control groups in lifestyle characteristics or mean scores in all primary endpoints.
In the ITT analysis, there were no statistically significant differences in primary outcomes between the groups following the intervention. As for secondary outcomes, the RBC ALA significantly increased in the walnuts group but not the control group (coefficient, 0.04%; 95% confidence interval, 0.03%-0.06%; P < .0001).
However, there were differences in primary outcomes between the groups in the per-protocol analysis: The adherence-adjusted effect on improvement in attention score was −11.26 ms; 95% CI, −19.92 to −2.60; P = .011) for the intervention versus the control group.
The per-protocol analysis showed other differences: an improvement in fluid intelligence score (1.78; 95% CI, 0.90 - 2.67; P < .0001) and a reduction in ADHD symptom score (−2.18; 95% CI, −3.70 to −0.67; P = .0050).
“Overall, no significant differences were found in the intervention group in relation to the control group,” Dr. Julvez said in a news release. “But if the adherence factor is considered, then positive results are observed, since participants who most closely followed the guidelines – in terms of the recommended dose of walnuts and the number of days of consumption – did show improvements in the neuropsychological functions evaluated.”
Adolescence “is a time of great biological changes. Hormonal transformation occurs, which in turn is responsible for stimulating the synaptic growth of the frontal lobe,” he continued, adding that this brain region “enables neuropsychological maturation of more complex emotional and cognitive functions.”
“Neurons that are well nourished with these types of fatty acids will be able to grow and form new, stronger synapses,” he said.
Food as medicine
Uma Naidoo, MD, director of nutritional and lifestyle psychiatry at Massachusetts General Hospital, Boston, “commends” the researchers for conducting an RCT with a “robust” sample size and said she is “excited to see research like this furthering functional nutrition for mental health,” as she believes that “food is medicine.”
Dr. Naidoo, a professional chef, nutritional biologist, and author of the book “This Is Your Brain on Food,” said the findings “align” with her own approach to nutritional psychiatry and are also “in line” with her clinical practice.
However, although these results are “promising,” more research is needed across more diverse populations to “make sure these results are truly generalizable,” said Dr. Naidoo, a faculty member at Harvard Medical School, Boston, who was not involved with the study.
She “envisions a future where the research is so advanced that we can ‘dose’ these healthy whole foods for specific psychiatric symptoms and conditions.”
This study was supported by Instituto de Salud Carlos III (co-funded by European Union Regional Development Fund “A way to make Europe”). The California Walnut Commission has given support by supplying the walnuts for free for the Walnuts Smart Snack Dietary Intervention Trial. Dr. Julvez holds a Miguel Servet-II contract awarded by the Instituto de Salud Carlos III (co-funded by European Union Social Fund). The other authors’ disclosures are listed in the original article. Dr. Naidoo reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Adolescents who consumed walnuts for at least 100 days showed improved sustained attention and fluid intelligence as well as a reduction in symptoms of attension deficit hyperactivity disorder, compared with matched controls who did not consume the nuts. However, there were no statistically significant changes between the groups in other parameters, such as working memory and executive function.
Clinicians should advise adolescents “to eat a handful of walnuts three times a week for the rest of their lives. They may have a healthier brain with better cognitive function,” said senior investigator Jordi Julvez, PhD, group leader at the Institute of Health Research Pere Virgili, Barcelona, and associated researcher at the Barcelona Institute for Global Health.
The study was published online in eClinicalMedicine.
Rich source of omega-3s
Adolescence is “a period of refinement of brain connectivity and complex behaviors,” the investigators noted.
Previous research suggests polyunsaturated fatty acids are key in central nervous system architecture and function during times of neural development, with three specific PUFAs playing an “essential developmental role.”
Two omega-3 fatty acids – docosahexaenoic acid and eicosapentaenoic acid – are PUFAs that must be obtained through diet, mainly from seafood. Walnuts are “among the richest sources” of plant-derived omega-3 fatty acids, particularly alpha-linolenic acid (ALA), a precursor for longer-chain EPA and DHA.
ALA independently “has positive effects on brain function and plasticity,” the authors wrote. In addition, walnut constituents – particularly polyphenols and other bioactive compounds – “may act synergistically with ALA to foster brain health.”
Earlier small studies have found positive associations between walnut consumption and cognitive function in children, adolescents, and young adults, but to date, no randomized controlled trial has focused on the effect of walnut consumption on adolescent neuropsychological function.
The researchers studied 771 healthy adolescents (aged 11-16 years, mean age 14) drawn from 12 Spanish high schools. Participants were instructed to follow healthy eating recommendations and were randomly assigned 1:1 to the intervention (n = 386) or the control group (n = 385).
At baseline and after 6 months, they completed neuropsychological tests and behavioral rating scales. The Attention Network Test assessed attention, and the N-back test was used to assess working memory. The Tests of Primary Mental Abilities assessed fluid intelligence. Risky decision-making was tested using the Roulettes Task.
Fruit and nuts
Participants also completed the Strengths and Difficulties Questionnaire, which provided a total score of problem behavior. Teachers filled out the ADHD DSM-IV form list to provide additional information about ADHD behaviors.
The intervention group received 30 grams/day of raw California walnut kernels to incorporate into their daily diet. It is estimated that this walnut contains about 9 g of ALA per 100 g.
All participants received a seasonal fruit calendar and were asked to eat at least one piece of seasonal fruit daily.
Parents reported their child’s daily walnut consumption, with adherence defined as 100 or more days of eating walnuts during the 6-month period.
All main analyses were based on an intention-to-treat method (participants were analyzed according to their original group assignment, regardless of their adherence to the intervention).
The researchers also conducted a secondary per-protocol analysis, comparing the intervention and control groups to estimate the effect if all participants had adhered to their assigned intervention. They censored data for participants who reported eating walnuts for less than 100 days during the 6-month trial period.
Secondary outcomes included changes in height, weight, waist circumference, and BMI, as well as red blood cell proportions of omega-3 fatty acids (DHA, EPA, and ALA) at baseline and after 6 months.
Adherence counts
Most participants had “medium” or “high” levels of adherence to the Mediterranean diet, with “no meaningful differences” at baseline between the intervention and control groups in lifestyle characteristics or mean scores in all primary endpoints.
In the ITT analysis, there were no statistically significant differences in primary outcomes between the groups following the intervention. As for secondary outcomes, the RBC ALA significantly increased in the walnuts group but not the control group (coefficient, 0.04%; 95% confidence interval, 0.03%-0.06%; P < .0001).
However, there were differences in primary outcomes between the groups in the per-protocol analysis: The adherence-adjusted effect on improvement in attention score was −11.26 ms; 95% CI, −19.92 to −2.60; P = .011) for the intervention versus the control group.
The per-protocol analysis showed other differences: an improvement in fluid intelligence score (1.78; 95% CI, 0.90 - 2.67; P < .0001) and a reduction in ADHD symptom score (−2.18; 95% CI, −3.70 to −0.67; P = .0050).
“Overall, no significant differences were found in the intervention group in relation to the control group,” Dr. Julvez said in a news release. “But if the adherence factor is considered, then positive results are observed, since participants who most closely followed the guidelines – in terms of the recommended dose of walnuts and the number of days of consumption – did show improvements in the neuropsychological functions evaluated.”
Adolescence “is a time of great biological changes. Hormonal transformation occurs, which in turn is responsible for stimulating the synaptic growth of the frontal lobe,” he continued, adding that this brain region “enables neuropsychological maturation of more complex emotional and cognitive functions.”
“Neurons that are well nourished with these types of fatty acids will be able to grow and form new, stronger synapses,” he said.
Food as medicine
Uma Naidoo, MD, director of nutritional and lifestyle psychiatry at Massachusetts General Hospital, Boston, “commends” the researchers for conducting an RCT with a “robust” sample size and said she is “excited to see research like this furthering functional nutrition for mental health,” as she believes that “food is medicine.”
Dr. Naidoo, a professional chef, nutritional biologist, and author of the book “This Is Your Brain on Food,” said the findings “align” with her own approach to nutritional psychiatry and are also “in line” with her clinical practice.
However, although these results are “promising,” more research is needed across more diverse populations to “make sure these results are truly generalizable,” said Dr. Naidoo, a faculty member at Harvard Medical School, Boston, who was not involved with the study.
She “envisions a future where the research is so advanced that we can ‘dose’ these healthy whole foods for specific psychiatric symptoms and conditions.”
This study was supported by Instituto de Salud Carlos III (co-funded by European Union Regional Development Fund “A way to make Europe”). The California Walnut Commission has given support by supplying the walnuts for free for the Walnuts Smart Snack Dietary Intervention Trial. Dr. Julvez holds a Miguel Servet-II contract awarded by the Instituto de Salud Carlos III (co-funded by European Union Social Fund). The other authors’ disclosures are listed in the original article. Dr. Naidoo reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Adolescents who consumed walnuts for at least 100 days showed improved sustained attention and fluid intelligence as well as a reduction in symptoms of attension deficit hyperactivity disorder, compared with matched controls who did not consume the nuts. However, there were no statistically significant changes between the groups in other parameters, such as working memory and executive function.
Clinicians should advise adolescents “to eat a handful of walnuts three times a week for the rest of their lives. They may have a healthier brain with better cognitive function,” said senior investigator Jordi Julvez, PhD, group leader at the Institute of Health Research Pere Virgili, Barcelona, and associated researcher at the Barcelona Institute for Global Health.
The study was published online in eClinicalMedicine.
Rich source of omega-3s
Adolescence is “a period of refinement of brain connectivity and complex behaviors,” the investigators noted.
Previous research suggests polyunsaturated fatty acids are key in central nervous system architecture and function during times of neural development, with three specific PUFAs playing an “essential developmental role.”
Two omega-3 fatty acids – docosahexaenoic acid and eicosapentaenoic acid – are PUFAs that must be obtained through diet, mainly from seafood. Walnuts are “among the richest sources” of plant-derived omega-3 fatty acids, particularly alpha-linolenic acid (ALA), a precursor for longer-chain EPA and DHA.
ALA independently “has positive effects on brain function and plasticity,” the authors wrote. In addition, walnut constituents – particularly polyphenols and other bioactive compounds – “may act synergistically with ALA to foster brain health.”
Earlier small studies have found positive associations between walnut consumption and cognitive function in children, adolescents, and young adults, but to date, no randomized controlled trial has focused on the effect of walnut consumption on adolescent neuropsychological function.
The researchers studied 771 healthy adolescents (aged 11-16 years, mean age 14) drawn from 12 Spanish high schools. Participants were instructed to follow healthy eating recommendations and were randomly assigned 1:1 to the intervention (n = 386) or the control group (n = 385).
At baseline and after 6 months, they completed neuropsychological tests and behavioral rating scales. The Attention Network Test assessed attention, and the N-back test was used to assess working memory. The Tests of Primary Mental Abilities assessed fluid intelligence. Risky decision-making was tested using the Roulettes Task.
Fruit and nuts
Participants also completed the Strengths and Difficulties Questionnaire, which provided a total score of problem behavior. Teachers filled out the ADHD DSM-IV form list to provide additional information about ADHD behaviors.
The intervention group received 30 grams/day of raw California walnut kernels to incorporate into their daily diet. It is estimated that this walnut contains about 9 g of ALA per 100 g.
All participants received a seasonal fruit calendar and were asked to eat at least one piece of seasonal fruit daily.
Parents reported their child’s daily walnut consumption, with adherence defined as 100 or more days of eating walnuts during the 6-month period.
All main analyses were based on an intention-to-treat method (participants were analyzed according to their original group assignment, regardless of their adherence to the intervention).
The researchers also conducted a secondary per-protocol analysis, comparing the intervention and control groups to estimate the effect if all participants had adhered to their assigned intervention. They censored data for participants who reported eating walnuts for less than 100 days during the 6-month trial period.
Secondary outcomes included changes in height, weight, waist circumference, and BMI, as well as red blood cell proportions of omega-3 fatty acids (DHA, EPA, and ALA) at baseline and after 6 months.
Adherence counts
Most participants had “medium” or “high” levels of adherence to the Mediterranean diet, with “no meaningful differences” at baseline between the intervention and control groups in lifestyle characteristics or mean scores in all primary endpoints.
In the ITT analysis, there were no statistically significant differences in primary outcomes between the groups following the intervention. As for secondary outcomes, the RBC ALA significantly increased in the walnuts group but not the control group (coefficient, 0.04%; 95% confidence interval, 0.03%-0.06%; P < .0001).
However, there were differences in primary outcomes between the groups in the per-protocol analysis: The adherence-adjusted effect on improvement in attention score was −11.26 ms; 95% CI, −19.92 to −2.60; P = .011) for the intervention versus the control group.
The per-protocol analysis showed other differences: an improvement in fluid intelligence score (1.78; 95% CI, 0.90 - 2.67; P < .0001) and a reduction in ADHD symptom score (−2.18; 95% CI, −3.70 to −0.67; P = .0050).
“Overall, no significant differences were found in the intervention group in relation to the control group,” Dr. Julvez said in a news release. “But if the adherence factor is considered, then positive results are observed, since participants who most closely followed the guidelines – in terms of the recommended dose of walnuts and the number of days of consumption – did show improvements in the neuropsychological functions evaluated.”
Adolescence “is a time of great biological changes. Hormonal transformation occurs, which in turn is responsible for stimulating the synaptic growth of the frontal lobe,” he continued, adding that this brain region “enables neuropsychological maturation of more complex emotional and cognitive functions.”
“Neurons that are well nourished with these types of fatty acids will be able to grow and form new, stronger synapses,” he said.
Food as medicine
Uma Naidoo, MD, director of nutritional and lifestyle psychiatry at Massachusetts General Hospital, Boston, “commends” the researchers for conducting an RCT with a “robust” sample size and said she is “excited to see research like this furthering functional nutrition for mental health,” as she believes that “food is medicine.”
Dr. Naidoo, a professional chef, nutritional biologist, and author of the book “This Is Your Brain on Food,” said the findings “align” with her own approach to nutritional psychiatry and are also “in line” with her clinical practice.
However, although these results are “promising,” more research is needed across more diverse populations to “make sure these results are truly generalizable,” said Dr. Naidoo, a faculty member at Harvard Medical School, Boston, who was not involved with the study.
She “envisions a future where the research is so advanced that we can ‘dose’ these healthy whole foods for specific psychiatric symptoms and conditions.”
This study was supported by Instituto de Salud Carlos III (co-funded by European Union Regional Development Fund “A way to make Europe”). The California Walnut Commission has given support by supplying the walnuts for free for the Walnuts Smart Snack Dietary Intervention Trial. Dr. Julvez holds a Miguel Servet-II contract awarded by the Instituto de Salud Carlos III (co-funded by European Union Social Fund). The other authors’ disclosures are listed in the original article. Dr. Naidoo reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECLINICALMEDICINE
Meta-analysis examines cancer risk concern for JAK inhibitors
MANCHESTER, ENGLAND – Janus kinase (JAK) inhibitors may be associated with a higher risk for cancer relative to tumor necrosis factor (TNF) inhibitors, according to a meta-analysis reported at the annual meeting of the British Society for Rheumatology.
Looking at all phase 2, 3, and 4 trials and long-term extension studies across the indications of rheumatoid arthritis, psoriatic arthritis, psoriasis, axial spondyloarthritis, inflammatory bowel disease, and atopic dermatitis, the risk ratio for any cancer developing was 1.63 when compared with anti-TNF therapy (95% confidence interval, 1.27-2.09).
By comparison, JAK inhibitor use was not significantly associated with any greater risk for cancer than methotrexate (RR, 1.06; 95% confidence interval, 0.58-1.94) or placebo (RR, 1.16; 95% CI, 0.75-1.80).
“Our data suggests that rather than JAK inhibitors necessarily being harmful, it could be more a case of TNF inhibitors being protective,” said Christopher Stovin, MBChB, a specialist registrar in rheumatology at the Princess Royal University Hospital, King’s College Hospital NHS Trust, London.
“We should stress that these are rare events in our study, roughly around 1 in every 100 patient-years of exposure,” Dr. Stovin said.
“Despite having over 80,000 years of patient exposure, the median follow-up duration for JAK inhibitors was still only 118 weeks, which for cancers [that] obviously have long latency periods is still a relatively small duration of time,” the researcher added.
“People worry about the drugs. But there is a possibility that [a] disturbed immune system plays a role per se in development of cancers,” consultant rheumatologist Anurag Bharadwaj, MD, DM, said in an interview.
“Although there are studies which attribute increased risk of cancer to different DMARDs [disease-modifying antirheumatic drugs] and biologics like TNF, but on other hand, it’s maybe that we are giving these drugs to patients who have got more serious immunological disease,” suggested Bharadwaj, who serves as the clinical lead for rheumatology at Basildon (England) Hospital, Mid & South Essex Foundation Trust.
“So, a possibility may be that the more severe or the more active the immunological inflammatory disease, the higher the chance of cancer, and these are the patients who go for the stronger medications,” Dr. Bharadwaj said.
There is an “immunological window of opportunity” when treating these inflammatory diseases, said Dr. Bharadwaj, noting that the first few months of treatment are vital. “For all immunological diseases, the more quickly you bring the immunological abnormality down, the chances of long-term complications go down, including [possibly that the] chances of cancer go down, chances of cardiovascular disease go down, and chances of lung disease go down. Hit it early, hit it hard.”
Concern over a possible higher risk for cancer with JAK inhibitors than with TNF inhibitors was raised following the release of data from the ORAL Surveillance trial, a postmarketing trial of tofacitinib (Xeljanz) that had been mandated by the Food and Drug Administration.
“This was a study looking at the coprimary endpoints of malignancy and major adverse cardiovascular events, and it was enriched with patients over the age of 50, with one additional cardiac risk factor, designed to amplify the detection of these rare events,” Dr. Stovin said.
“There was a signal of an increased risk of malignancy in the tofacitinib group, and this led to the FDA issuing a [boxed warning for all licensed JAK inhibitors] at that time,” he added.
Dr. Stovin and colleagues aimed to determine what, if any, cancer risk was associated with all available JAK inhibitors relative to placebo, TNF inhibitors, and methotrexate.
In all, data from 62 randomized controlled trials and 14 long-term extension studies were included in the meta-analysis, accounting for 82,366 patient years of follow-up. The JAK inhibitors analyzed included tofacitinib, baricitinib (Olumiant), upadacitinib (Rinvoq), filgotinib (Jyseleca), and peficitinib (Smyraf). (Filgotinib and peficitinib have not been approved by the FDA.)
The researchers performed sensitivity analyses that excluded cancers detected within the first 6 months of treatment, the use of higher than licensed JAK inhibitor doses, and patients with non-rheumatoid arthritis diagnoses, but the results remained largely unchanged, Dr. Stovin reported.
“Perhaps not surprisingly, when we removed ORAL Surveillance” from the analysis comparing JAK inhibitors and TNF inhibitors, “we lost statistical significance,” he said.
“Longitudinal observational data is needed but currently remains limited,” Dr. Stovin concluded.
Dr. Stovin and Dr. Bharadwaj reported no relevant financial relationships. The meta-analysis was independently supported. Dr. Bharadwaj was not involved in the study and provided comment ahead of the presentation.
A version of this article first appeared on Medscape.com.
MANCHESTER, ENGLAND – Janus kinase (JAK) inhibitors may be associated with a higher risk for cancer relative to tumor necrosis factor (TNF) inhibitors, according to a meta-analysis reported at the annual meeting of the British Society for Rheumatology.
Looking at all phase 2, 3, and 4 trials and long-term extension studies across the indications of rheumatoid arthritis, psoriatic arthritis, psoriasis, axial spondyloarthritis, inflammatory bowel disease, and atopic dermatitis, the risk ratio for any cancer developing was 1.63 when compared with anti-TNF therapy (95% confidence interval, 1.27-2.09).
By comparison, JAK inhibitor use was not significantly associated with any greater risk for cancer than methotrexate (RR, 1.06; 95% confidence interval, 0.58-1.94) or placebo (RR, 1.16; 95% CI, 0.75-1.80).
“Our data suggests that rather than JAK inhibitors necessarily being harmful, it could be more a case of TNF inhibitors being protective,” said Christopher Stovin, MBChB, a specialist registrar in rheumatology at the Princess Royal University Hospital, King’s College Hospital NHS Trust, London.
“We should stress that these are rare events in our study, roughly around 1 in every 100 patient-years of exposure,” Dr. Stovin said.
“Despite having over 80,000 years of patient exposure, the median follow-up duration for JAK inhibitors was still only 118 weeks, which for cancers [that] obviously have long latency periods is still a relatively small duration of time,” the researcher added.
“People worry about the drugs. But there is a possibility that [a] disturbed immune system plays a role per se in development of cancers,” consultant rheumatologist Anurag Bharadwaj, MD, DM, said in an interview.
“Although there are studies which attribute increased risk of cancer to different DMARDs [disease-modifying antirheumatic drugs] and biologics like TNF, but on other hand, it’s maybe that we are giving these drugs to patients who have got more serious immunological disease,” suggested Bharadwaj, who serves as the clinical lead for rheumatology at Basildon (England) Hospital, Mid & South Essex Foundation Trust.
“So, a possibility may be that the more severe or the more active the immunological inflammatory disease, the higher the chance of cancer, and these are the patients who go for the stronger medications,” Dr. Bharadwaj said.
There is an “immunological window of opportunity” when treating these inflammatory diseases, said Dr. Bharadwaj, noting that the first few months of treatment are vital. “For all immunological diseases, the more quickly you bring the immunological abnormality down, the chances of long-term complications go down, including [possibly that the] chances of cancer go down, chances of cardiovascular disease go down, and chances of lung disease go down. Hit it early, hit it hard.”
Concern over a possible higher risk for cancer with JAK inhibitors than with TNF inhibitors was raised following the release of data from the ORAL Surveillance trial, a postmarketing trial of tofacitinib (Xeljanz) that had been mandated by the Food and Drug Administration.
“This was a study looking at the coprimary endpoints of malignancy and major adverse cardiovascular events, and it was enriched with patients over the age of 50, with one additional cardiac risk factor, designed to amplify the detection of these rare events,” Dr. Stovin said.
“There was a signal of an increased risk of malignancy in the tofacitinib group, and this led to the FDA issuing a [boxed warning for all licensed JAK inhibitors] at that time,” he added.
Dr. Stovin and colleagues aimed to determine what, if any, cancer risk was associated with all available JAK inhibitors relative to placebo, TNF inhibitors, and methotrexate.
In all, data from 62 randomized controlled trials and 14 long-term extension studies were included in the meta-analysis, accounting for 82,366 patient years of follow-up. The JAK inhibitors analyzed included tofacitinib, baricitinib (Olumiant), upadacitinib (Rinvoq), filgotinib (Jyseleca), and peficitinib (Smyraf). (Filgotinib and peficitinib have not been approved by the FDA.)
The researchers performed sensitivity analyses that excluded cancers detected within the first 6 months of treatment, the use of higher than licensed JAK inhibitor doses, and patients with non-rheumatoid arthritis diagnoses, but the results remained largely unchanged, Dr. Stovin reported.
“Perhaps not surprisingly, when we removed ORAL Surveillance” from the analysis comparing JAK inhibitors and TNF inhibitors, “we lost statistical significance,” he said.
“Longitudinal observational data is needed but currently remains limited,” Dr. Stovin concluded.
Dr. Stovin and Dr. Bharadwaj reported no relevant financial relationships. The meta-analysis was independently supported. Dr. Bharadwaj was not involved in the study and provided comment ahead of the presentation.
A version of this article first appeared on Medscape.com.
MANCHESTER, ENGLAND – Janus kinase (JAK) inhibitors may be associated with a higher risk for cancer relative to tumor necrosis factor (TNF) inhibitors, according to a meta-analysis reported at the annual meeting of the British Society for Rheumatology.
Looking at all phase 2, 3, and 4 trials and long-term extension studies across the indications of rheumatoid arthritis, psoriatic arthritis, psoriasis, axial spondyloarthritis, inflammatory bowel disease, and atopic dermatitis, the risk ratio for any cancer developing was 1.63 when compared with anti-TNF therapy (95% confidence interval, 1.27-2.09).
By comparison, JAK inhibitor use was not significantly associated with any greater risk for cancer than methotrexate (RR, 1.06; 95% confidence interval, 0.58-1.94) or placebo (RR, 1.16; 95% CI, 0.75-1.80).
“Our data suggests that rather than JAK inhibitors necessarily being harmful, it could be more a case of TNF inhibitors being protective,” said Christopher Stovin, MBChB, a specialist registrar in rheumatology at the Princess Royal University Hospital, King’s College Hospital NHS Trust, London.
“We should stress that these are rare events in our study, roughly around 1 in every 100 patient-years of exposure,” Dr. Stovin said.
“Despite having over 80,000 years of patient exposure, the median follow-up duration for JAK inhibitors was still only 118 weeks, which for cancers [that] obviously have long latency periods is still a relatively small duration of time,” the researcher added.
“People worry about the drugs. But there is a possibility that [a] disturbed immune system plays a role per se in development of cancers,” consultant rheumatologist Anurag Bharadwaj, MD, DM, said in an interview.
“Although there are studies which attribute increased risk of cancer to different DMARDs [disease-modifying antirheumatic drugs] and biologics like TNF, but on other hand, it’s maybe that we are giving these drugs to patients who have got more serious immunological disease,” suggested Bharadwaj, who serves as the clinical lead for rheumatology at Basildon (England) Hospital, Mid & South Essex Foundation Trust.
“So, a possibility may be that the more severe or the more active the immunological inflammatory disease, the higher the chance of cancer, and these are the patients who go for the stronger medications,” Dr. Bharadwaj said.
There is an “immunological window of opportunity” when treating these inflammatory diseases, said Dr. Bharadwaj, noting that the first few months of treatment are vital. “For all immunological diseases, the more quickly you bring the immunological abnormality down, the chances of long-term complications go down, including [possibly that the] chances of cancer go down, chances of cardiovascular disease go down, and chances of lung disease go down. Hit it early, hit it hard.”
Concern over a possible higher risk for cancer with JAK inhibitors than with TNF inhibitors was raised following the release of data from the ORAL Surveillance trial, a postmarketing trial of tofacitinib (Xeljanz) that had been mandated by the Food and Drug Administration.
“This was a study looking at the coprimary endpoints of malignancy and major adverse cardiovascular events, and it was enriched with patients over the age of 50, with one additional cardiac risk factor, designed to amplify the detection of these rare events,” Dr. Stovin said.
“There was a signal of an increased risk of malignancy in the tofacitinib group, and this led to the FDA issuing a [boxed warning for all licensed JAK inhibitors] at that time,” he added.
Dr. Stovin and colleagues aimed to determine what, if any, cancer risk was associated with all available JAK inhibitors relative to placebo, TNF inhibitors, and methotrexate.
In all, data from 62 randomized controlled trials and 14 long-term extension studies were included in the meta-analysis, accounting for 82,366 patient years of follow-up. The JAK inhibitors analyzed included tofacitinib, baricitinib (Olumiant), upadacitinib (Rinvoq), filgotinib (Jyseleca), and peficitinib (Smyraf). (Filgotinib and peficitinib have not been approved by the FDA.)
The researchers performed sensitivity analyses that excluded cancers detected within the first 6 months of treatment, the use of higher than licensed JAK inhibitor doses, and patients with non-rheumatoid arthritis diagnoses, but the results remained largely unchanged, Dr. Stovin reported.
“Perhaps not surprisingly, when we removed ORAL Surveillance” from the analysis comparing JAK inhibitors and TNF inhibitors, “we lost statistical significance,” he said.
“Longitudinal observational data is needed but currently remains limited,” Dr. Stovin concluded.
Dr. Stovin and Dr. Bharadwaj reported no relevant financial relationships. The meta-analysis was independently supported. Dr. Bharadwaj was not involved in the study and provided comment ahead of the presentation.
A version of this article first appeared on Medscape.com.
AT BSR 2023