User login
14-year-old boy • aching midsternal pain following a basketball injury • worsening pain with direct pressure and when the patient sneezed • Dx?
THE CASE
A 14-year-old boy sought care at our clinic for persistent chest pain after being hit in the chest with a teammate’s shoulder during a basketball game 3 weeks earlier. He had aching midsternal chest pain that worsened with direct pressure and when he sneezed, twisted, or bent forward. There was no bruising or swelling.
On examination, the patient demonstrated normal perfusion and normal work of breathing. He had focal tenderness with palpation at the manubrium with no noticeable step-off, and mild tenderness at the adjacent costochondral junctions and over his pectoral muscles. His sternal pain along the proximal sternum was reproducible with a weighted wall push-up. Although the patient maintained full range of motion in his upper extremities, he did have sternal pain with flexion, abduction, and external rotation of the bilateral upper extremities against resistance. Anteroposterior (AP) and lateral chest radiographs were unremarkable.
THE DIAGNOSIS
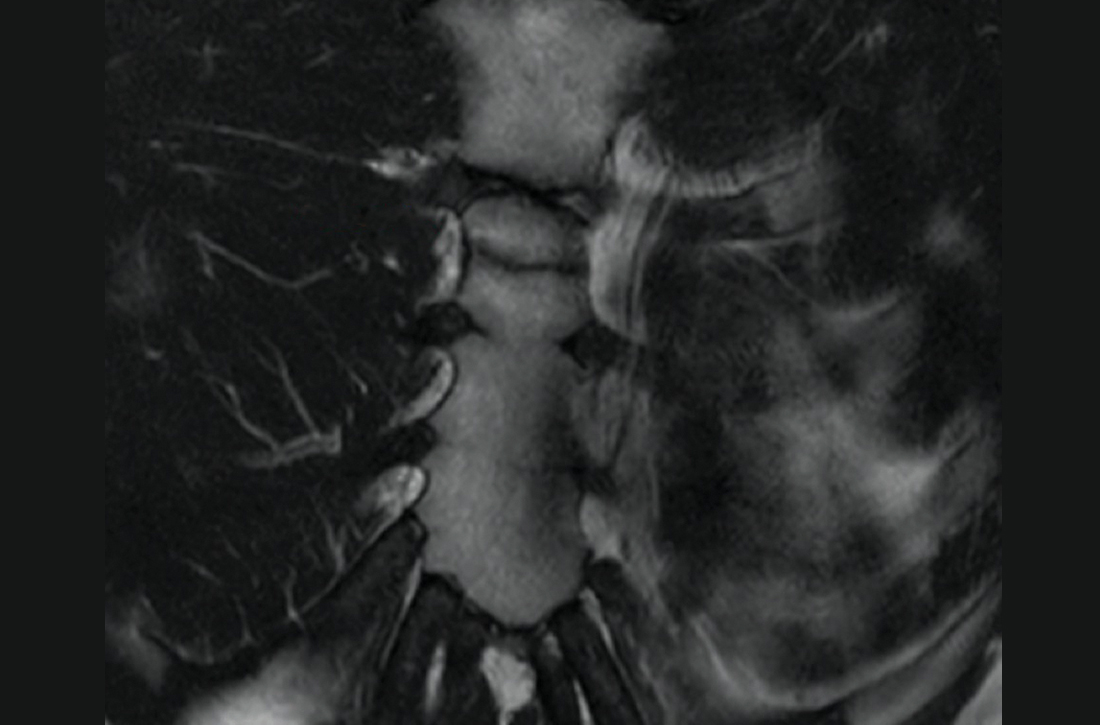
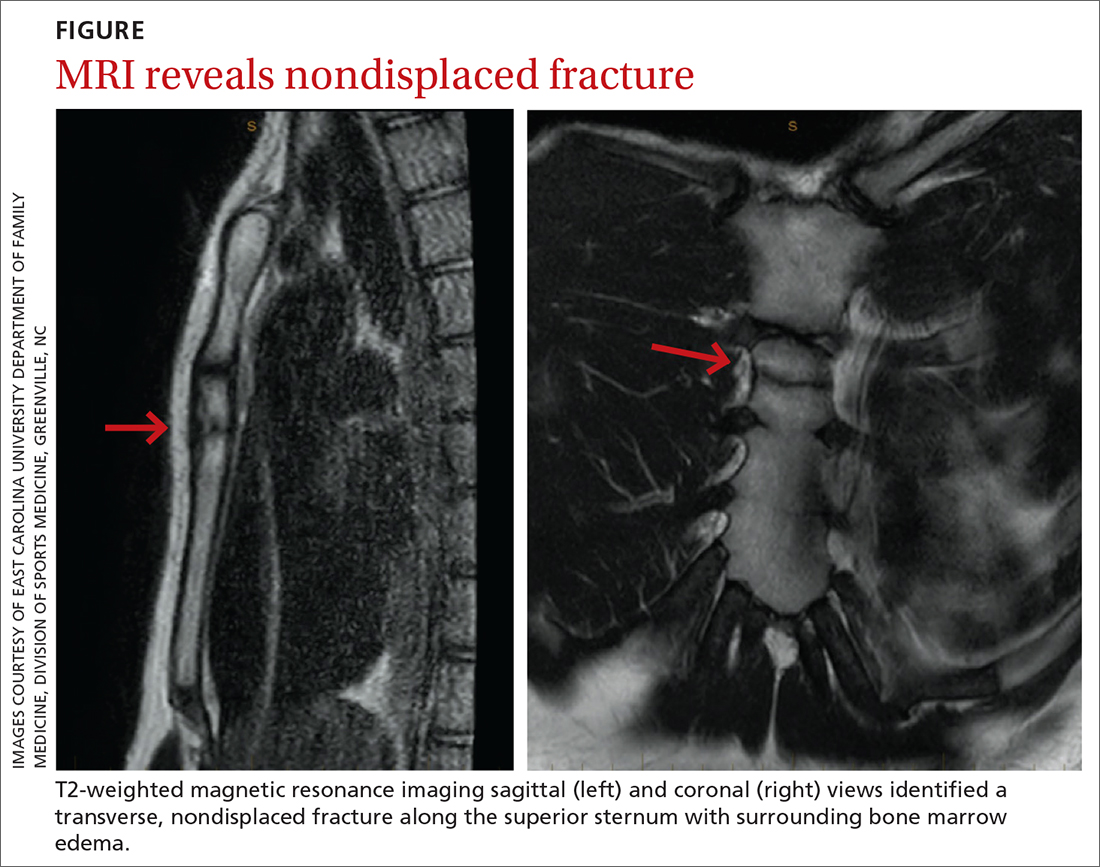
The unremarkable chest radiographs prompted further investigation with a diagnostic ultrasound, which revealed a small cortical defect with overlying anechoic fluid collection in the area of focal tenderness. T2-weighted magnetic resonance imaging (MRI) of the chest was performed; it revealed a transverse, nondisplaced fracture of the superior body of the sternum with surrounding bone marrow edema (FIGURE).
DISCUSSION
Fractures of the sternum comprise < 1% of traumatic fractures and have a low mortality rate (0.7%).1,2 The rarity of these fractures is attributed to the ribs’ elastic recoil, which protects the chest wall from anterior forces.1,3 These fractures are even more unusual in children due to the increased elasticity of their chest walls.4-6 Thus, it takes a significant amount of force for a child’s sternum to fracture.
While isolated sternum fractures can occur, two-thirds of sternum fractures are nonisolated and are associated with injuries to surrounding structures (including the heart, lungs, and vasculature) or fractures of the ribs and spine.2,3 Most often, these injuries are caused by significant blunt trauma to the anterior chest, rapid deceleration, or flexion-compression injury.2,3 They are typically transverse and localized, with 70% of fractures occurring in the mid-body and 17.6% at the manubriosternal joint.1,3,6
Athletes with a sternal fracture typically present as our patient did, with a history of blunt force trauma to the chest and with pain and tenderness over the anterior midline of the chest that increases with respiration or movement.1 A physical examination that includes chest palpation and auscultation of the heart and lungs must be performed to rule out damage to intrathoracic structures and assess the patient’s cardiac and pulmonary stability. An electrocardiogram should be performed to confirm that there are no cardiovascular complications.3,4
Initial imaging should include AP and lateral chest radiographs because any displacement will occur in the sagittal plane.1,2,4-6 If the radiograph shows no clear pathology, follow up with computed tomography, ultrasound, MRI, or technetium bone scans to gain additional information.1 Diagnosis of sternal fractures is especially difficult in children due to the presence of ossification centers for bone growth, which may be misinterpreted as a sternal fracture in the absence of a proper understanding of sternal development.5,6 On ultrasound, sternal fractures appear as a sharp step-off in the cortex, whereas in the absence of fracture, there is no cortical step-off and the cartilaginous plate between ossification centers appears in line with the cortex.7
Continue to: A self-limiting injury that requires proper pain control
A self-limiting injury that requires proper pain control
Isolated sternal fractures are typically self-limiting with a good prognosis.2 These injuries are managed supportively with rest, ice, and analgesics1; proper pain control is crucial to prevent respiratory compromise.8
Complete recovery for most patients occurs in 10 to 12 weeks.9 Recovery periods longer than 12 weeks are associated with nonisolated sternal fractures that are complicated by soft-tissue injury, injuries to the chest wall (such as sternoclavicular joint dislocation, usually from a fall on the shoulder), or fracture nonunion.1,2,5
Anterior sternoclavicular joint dislocations and stable posterior dislocations are managed with closed reduction and immobilization in a figure-of-eight brace.1 Operative management is reserved for patients with displaced fractures, sternal deformity, chest wall instability, respiratory insufficiency, uncontrolled pain, or fracture nonunion.1,3,8
A return-to-play protocol can begin once the patient is asymptomatic.1 The timeframe for a full return to play can vary from 6 weeks to 6 months, depending on the severity of the fracture.1 This process is guided by how quickly the symptoms resolve and by radiographic stability.9
Our patient was followed every 3 to 4 weeks and started physical therapy 6 weeks after his injury occurred. He was held from play for 10 weeks and gradually returned to play; he returned to full-contact activity after tolerating a practice without pain.
THE TAKEAWAY
Children typically have greater chest wall elasticity, and thus, it is unusual for them to sustain a sternal fracture. Diagnosis in children is complicated by the presence of ossification centers for bone growth on imaging. In this case, the fracture was first noticed on ultrasound and confirmed with MRI. Since these fractures can be associated with damage to surrounding structures, additional injuries should be considered when evaluating a patient with a sternum fracture.
CORRESPONDENCE
Catherine Romaine, East Carolina University, Brody School of Medicine, 600 Moye Boulevard, Greenville, NC 27834; [email protected]
1. Alent J, Narducci DM, Moran B, et al. Sternal injuries in sport: a review of the literature. Sports Med. 2018;48:2715-2724. doi: 10.1007/s40279-018-0990-5
2. Khoriati A-A, Rajakulasingam R, Shah R. Sternal fractures and their management. J Emerg Trauma Shock. 2013;6:113-116. doi: 10.4103/0974-2700.110763
3. Athanassiadi K, Gerazounis M, Moustardas M, et al. Sternal fractures: retrospective analysis of 100 cases. World J Surg. 2002;26:1243-1246. doi: 10.1007/s00268-002-6511-5
4. Ferguson LP, Wilkinson AG, Beattie TF. Fracture of the sternum in children. Emerg Med J. 2003;20:518-520. doi: 10.1136/emj.20.6.518
5. Ramgopal S, Shaffiey SA, Conti KA. Pediatric sternal fractures from a Level 1 trauma center. J Pediatr Surg. 2019;54:1628-1631. doi: 10.1016/j.jpedsurg.2018.08.040
6. Sesia SB, Prüfer F, Mayr J. Sternal fracture in children: diagnosis by ultrasonography. European J Pediatr Surg Rep. 2017;5:e39-e42. doi: 10.1055/s-0037-1606197
7. Nickson C, Rippey J. Ultrasonography of sternal fractures. Australas J Ultrasound Med. 2011;14:6-11. doi: 10.1002/j.2205-0140.2011.tb00131.x
8. Bauman ZM, Yanala U, Waibel BH, et al. Sternal fixation for isolated traumatic sternal fractures improves pain and upper extremity range of motion. Eur J Trauma Emerg Surg. 2022;48:225-230. doi: 10.1007/s00068-020-01568-x
9. Culp B, Hurbanek JG, Novak J, et al. Acute traumatic sternum fracture in a female college hockey player. Orthopedics. 2010;33:683. doi: 10.3928/01477447-20100722-17
THE CASE
A 14-year-old boy sought care at our clinic for persistent chest pain after being hit in the chest with a teammate’s shoulder during a basketball game 3 weeks earlier. He had aching midsternal chest pain that worsened with direct pressure and when he sneezed, twisted, or bent forward. There was no bruising or swelling.
On examination, the patient demonstrated normal perfusion and normal work of breathing. He had focal tenderness with palpation at the manubrium with no noticeable step-off, and mild tenderness at the adjacent costochondral junctions and over his pectoral muscles. His sternal pain along the proximal sternum was reproducible with a weighted wall push-up. Although the patient maintained full range of motion in his upper extremities, he did have sternal pain with flexion, abduction, and external rotation of the bilateral upper extremities against resistance. Anteroposterior (AP) and lateral chest radiographs were unremarkable.
THE DIAGNOSIS
The unremarkable chest radiographs prompted further investigation with a diagnostic ultrasound, which revealed a small cortical defect with overlying anechoic fluid collection in the area of focal tenderness. T2-weighted magnetic resonance imaging (MRI) of the chest was performed; it revealed a transverse, nondisplaced fracture of the superior body of the sternum with surrounding bone marrow edema (FIGURE).

DISCUSSION
Fractures of the sternum comprise < 1% of traumatic fractures and have a low mortality rate (0.7%).1,2 The rarity of these fractures is attributed to the ribs’ elastic recoil, which protects the chest wall from anterior forces.1,3 These fractures are even more unusual in children due to the increased elasticity of their chest walls.4-6 Thus, it takes a significant amount of force for a child’s sternum to fracture.
While isolated sternum fractures can occur, two-thirds of sternum fractures are nonisolated and are associated with injuries to surrounding structures (including the heart, lungs, and vasculature) or fractures of the ribs and spine.2,3 Most often, these injuries are caused by significant blunt trauma to the anterior chest, rapid deceleration, or flexion-compression injury.2,3 They are typically transverse and localized, with 70% of fractures occurring in the mid-body and 17.6% at the manubriosternal joint.1,3,6
Athletes with a sternal fracture typically present as our patient did, with a history of blunt force trauma to the chest and with pain and tenderness over the anterior midline of the chest that increases with respiration or movement.1 A physical examination that includes chest palpation and auscultation of the heart and lungs must be performed to rule out damage to intrathoracic structures and assess the patient’s cardiac and pulmonary stability. An electrocardiogram should be performed to confirm that there are no cardiovascular complications.3,4
Initial imaging should include AP and lateral chest radiographs because any displacement will occur in the sagittal plane.1,2,4-6 If the radiograph shows no clear pathology, follow up with computed tomography, ultrasound, MRI, or technetium bone scans to gain additional information.1 Diagnosis of sternal fractures is especially difficult in children due to the presence of ossification centers for bone growth, which may be misinterpreted as a sternal fracture in the absence of a proper understanding of sternal development.5,6 On ultrasound, sternal fractures appear as a sharp step-off in the cortex, whereas in the absence of fracture, there is no cortical step-off and the cartilaginous plate between ossification centers appears in line with the cortex.7
Continue to: A self-limiting injury that requires proper pain control
A self-limiting injury that requires proper pain control
Isolated sternal fractures are typically self-limiting with a good prognosis.2 These injuries are managed supportively with rest, ice, and analgesics1; proper pain control is crucial to prevent respiratory compromise.8
Complete recovery for most patients occurs in 10 to 12 weeks.9 Recovery periods longer than 12 weeks are associated with nonisolated sternal fractures that are complicated by soft-tissue injury, injuries to the chest wall (such as sternoclavicular joint dislocation, usually from a fall on the shoulder), or fracture nonunion.1,2,5
Anterior sternoclavicular joint dislocations and stable posterior dislocations are managed with closed reduction and immobilization in a figure-of-eight brace.1 Operative management is reserved for patients with displaced fractures, sternal deformity, chest wall instability, respiratory insufficiency, uncontrolled pain, or fracture nonunion.1,3,8
A return-to-play protocol can begin once the patient is asymptomatic.1 The timeframe for a full return to play can vary from 6 weeks to 6 months, depending on the severity of the fracture.1 This process is guided by how quickly the symptoms resolve and by radiographic stability.9
Our patient was followed every 3 to 4 weeks and started physical therapy 6 weeks after his injury occurred. He was held from play for 10 weeks and gradually returned to play; he returned to full-contact activity after tolerating a practice without pain.
THE TAKEAWAY
Children typically have greater chest wall elasticity, and thus, it is unusual for them to sustain a sternal fracture. Diagnosis in children is complicated by the presence of ossification centers for bone growth on imaging. In this case, the fracture was first noticed on ultrasound and confirmed with MRI. Since these fractures can be associated with damage to surrounding structures, additional injuries should be considered when evaluating a patient with a sternum fracture.
CORRESPONDENCE
Catherine Romaine, East Carolina University, Brody School of Medicine, 600 Moye Boulevard, Greenville, NC 27834; [email protected]
THE CASE
A 14-year-old boy sought care at our clinic for persistent chest pain after being hit in the chest with a teammate’s shoulder during a basketball game 3 weeks earlier. He had aching midsternal chest pain that worsened with direct pressure and when he sneezed, twisted, or bent forward. There was no bruising or swelling.
On examination, the patient demonstrated normal perfusion and normal work of breathing. He had focal tenderness with palpation at the manubrium with no noticeable step-off, and mild tenderness at the adjacent costochondral junctions and over his pectoral muscles. His sternal pain along the proximal sternum was reproducible with a weighted wall push-up. Although the patient maintained full range of motion in his upper extremities, he did have sternal pain with flexion, abduction, and external rotation of the bilateral upper extremities against resistance. Anteroposterior (AP) and lateral chest radiographs were unremarkable.
THE DIAGNOSIS
The unremarkable chest radiographs prompted further investigation with a diagnostic ultrasound, which revealed a small cortical defect with overlying anechoic fluid collection in the area of focal tenderness. T2-weighted magnetic resonance imaging (MRI) of the chest was performed; it revealed a transverse, nondisplaced fracture of the superior body of the sternum with surrounding bone marrow edema (FIGURE).

DISCUSSION
Fractures of the sternum comprise < 1% of traumatic fractures and have a low mortality rate (0.7%).1,2 The rarity of these fractures is attributed to the ribs’ elastic recoil, which protects the chest wall from anterior forces.1,3 These fractures are even more unusual in children due to the increased elasticity of their chest walls.4-6 Thus, it takes a significant amount of force for a child’s sternum to fracture.
While isolated sternum fractures can occur, two-thirds of sternum fractures are nonisolated and are associated with injuries to surrounding structures (including the heart, lungs, and vasculature) or fractures of the ribs and spine.2,3 Most often, these injuries are caused by significant blunt trauma to the anterior chest, rapid deceleration, or flexion-compression injury.2,3 They are typically transverse and localized, with 70% of fractures occurring in the mid-body and 17.6% at the manubriosternal joint.1,3,6
Athletes with a sternal fracture typically present as our patient did, with a history of blunt force trauma to the chest and with pain and tenderness over the anterior midline of the chest that increases with respiration or movement.1 A physical examination that includes chest palpation and auscultation of the heart and lungs must be performed to rule out damage to intrathoracic structures and assess the patient’s cardiac and pulmonary stability. An electrocardiogram should be performed to confirm that there are no cardiovascular complications.3,4
Initial imaging should include AP and lateral chest radiographs because any displacement will occur in the sagittal plane.1,2,4-6 If the radiograph shows no clear pathology, follow up with computed tomography, ultrasound, MRI, or technetium bone scans to gain additional information.1 Diagnosis of sternal fractures is especially difficult in children due to the presence of ossification centers for bone growth, which may be misinterpreted as a sternal fracture in the absence of a proper understanding of sternal development.5,6 On ultrasound, sternal fractures appear as a sharp step-off in the cortex, whereas in the absence of fracture, there is no cortical step-off and the cartilaginous plate between ossification centers appears in line with the cortex.7
Continue to: A self-limiting injury that requires proper pain control
A self-limiting injury that requires proper pain control
Isolated sternal fractures are typically self-limiting with a good prognosis.2 These injuries are managed supportively with rest, ice, and analgesics1; proper pain control is crucial to prevent respiratory compromise.8
Complete recovery for most patients occurs in 10 to 12 weeks.9 Recovery periods longer than 12 weeks are associated with nonisolated sternal fractures that are complicated by soft-tissue injury, injuries to the chest wall (such as sternoclavicular joint dislocation, usually from a fall on the shoulder), or fracture nonunion.1,2,5
Anterior sternoclavicular joint dislocations and stable posterior dislocations are managed with closed reduction and immobilization in a figure-of-eight brace.1 Operative management is reserved for patients with displaced fractures, sternal deformity, chest wall instability, respiratory insufficiency, uncontrolled pain, or fracture nonunion.1,3,8
A return-to-play protocol can begin once the patient is asymptomatic.1 The timeframe for a full return to play can vary from 6 weeks to 6 months, depending on the severity of the fracture.1 This process is guided by how quickly the symptoms resolve and by radiographic stability.9
Our patient was followed every 3 to 4 weeks and started physical therapy 6 weeks after his injury occurred. He was held from play for 10 weeks and gradually returned to play; he returned to full-contact activity after tolerating a practice without pain.
THE TAKEAWAY
Children typically have greater chest wall elasticity, and thus, it is unusual for them to sustain a sternal fracture. Diagnosis in children is complicated by the presence of ossification centers for bone growth on imaging. In this case, the fracture was first noticed on ultrasound and confirmed with MRI. Since these fractures can be associated with damage to surrounding structures, additional injuries should be considered when evaluating a patient with a sternum fracture.
CORRESPONDENCE
Catherine Romaine, East Carolina University, Brody School of Medicine, 600 Moye Boulevard, Greenville, NC 27834; [email protected]
1. Alent J, Narducci DM, Moran B, et al. Sternal injuries in sport: a review of the literature. Sports Med. 2018;48:2715-2724. doi: 10.1007/s40279-018-0990-5
2. Khoriati A-A, Rajakulasingam R, Shah R. Sternal fractures and their management. J Emerg Trauma Shock. 2013;6:113-116. doi: 10.4103/0974-2700.110763
3. Athanassiadi K, Gerazounis M, Moustardas M, et al. Sternal fractures: retrospective analysis of 100 cases. World J Surg. 2002;26:1243-1246. doi: 10.1007/s00268-002-6511-5
4. Ferguson LP, Wilkinson AG, Beattie TF. Fracture of the sternum in children. Emerg Med J. 2003;20:518-520. doi: 10.1136/emj.20.6.518
5. Ramgopal S, Shaffiey SA, Conti KA. Pediatric sternal fractures from a Level 1 trauma center. J Pediatr Surg. 2019;54:1628-1631. doi: 10.1016/j.jpedsurg.2018.08.040
6. Sesia SB, Prüfer F, Mayr J. Sternal fracture in children: diagnosis by ultrasonography. European J Pediatr Surg Rep. 2017;5:e39-e42. doi: 10.1055/s-0037-1606197
7. Nickson C, Rippey J. Ultrasonography of sternal fractures. Australas J Ultrasound Med. 2011;14:6-11. doi: 10.1002/j.2205-0140.2011.tb00131.x
8. Bauman ZM, Yanala U, Waibel BH, et al. Sternal fixation for isolated traumatic sternal fractures improves pain and upper extremity range of motion. Eur J Trauma Emerg Surg. 2022;48:225-230. doi: 10.1007/s00068-020-01568-x
9. Culp B, Hurbanek JG, Novak J, et al. Acute traumatic sternum fracture in a female college hockey player. Orthopedics. 2010;33:683. doi: 10.3928/01477447-20100722-17
1. Alent J, Narducci DM, Moran B, et al. Sternal injuries in sport: a review of the literature. Sports Med. 2018;48:2715-2724. doi: 10.1007/s40279-018-0990-5
2. Khoriati A-A, Rajakulasingam R, Shah R. Sternal fractures and their management. J Emerg Trauma Shock. 2013;6:113-116. doi: 10.4103/0974-2700.110763
3. Athanassiadi K, Gerazounis M, Moustardas M, et al. Sternal fractures: retrospective analysis of 100 cases. World J Surg. 2002;26:1243-1246. doi: 10.1007/s00268-002-6511-5
4. Ferguson LP, Wilkinson AG, Beattie TF. Fracture of the sternum in children. Emerg Med J. 2003;20:518-520. doi: 10.1136/emj.20.6.518
5. Ramgopal S, Shaffiey SA, Conti KA. Pediatric sternal fractures from a Level 1 trauma center. J Pediatr Surg. 2019;54:1628-1631. doi: 10.1016/j.jpedsurg.2018.08.040
6. Sesia SB, Prüfer F, Mayr J. Sternal fracture in children: diagnosis by ultrasonography. European J Pediatr Surg Rep. 2017;5:e39-e42. doi: 10.1055/s-0037-1606197
7. Nickson C, Rippey J. Ultrasonography of sternal fractures. Australas J Ultrasound Med. 2011;14:6-11. doi: 10.1002/j.2205-0140.2011.tb00131.x
8. Bauman ZM, Yanala U, Waibel BH, et al. Sternal fixation for isolated traumatic sternal fractures improves pain and upper extremity range of motion. Eur J Trauma Emerg Surg. 2022;48:225-230. doi: 10.1007/s00068-020-01568-x
9. Culp B, Hurbanek JG, Novak J, et al. Acute traumatic sternum fracture in a female college hockey player. Orthopedics. 2010;33:683. doi: 10.3928/01477447-20100722-17
‘Sugar tax’ prevented thousands of girls becoming obese
The introduction of the soft drinks industry levy (SDIL) – dubbed the ‘sugar tax’ – in England was followed by a drop in the number of older primary school girls succumbing to obesity, according to researchers from the Universities of Cambridge, Oxford, and Bath, with colleagues at the London School of Hygiene and Tropical Medicine.
The study, published in PLOS Medicine, has led to calls to extend the levy to other unhealthy foods and drinks
Obesity has become a global public health problem, the researchers said. In England, around 10% of 4- to 5-year-old children and 20% of 10- to 11-year-olds were recorded as obese in 2020. Childhood obesity is associated with depression in children and the adults into which they maturate, as well as with serious health problems in later life including high blood pressure and type 2 diabetes.
In the United Kingdom, young people consume significantly more added sugars than are recommended – by late adolescence, typically 70 g of added sugar per day, more than double the recommended 30g. The team said that sugar-sweetened beverages (SSB) are the primary sources of dietary added sugars in children, with high consumption commonly observed in more deprived areas where obesity prevalence is also highest.
Protecting children from excessive sugar
The two-tier SDIL on drinks manufacturers was implemented in April 2018 and aimed to protect children from excessive sugar consumption and tackle childhood obesity by incentivizing reformulation of SSBs in the U.K. with reduced sugar content.
To assess the effects of SDIL, the researchers used data from the National Child Measurement Programme on over 1 million children at ages 4 to 5 years (reception class) and 10 to 11 years (school year 6) in state-maintained English primary schools. The surveillance program includes annual repeat cross-sectional measurements, enabling the researchers to examine trajectories in monthly prevalence of obesity from September 2013 to November 2019, 19 months after the implementation of the SDIL.
Taking account of previous trends in obesity levels, they estimated both absolute and relative changes in obesity prevalence, both overall and by sex and deprivation, and compared obesity levels after the SDIL with predicted levels had the tax not been introduced, controlling for children’s sex and the level of deprivation of their school area.
Although they found no significant association with obesity levels in reception-age children or year-6 boys, they noted an overall absolute reduction in obesity prevalence of 1.6 percentage points (PPs) (95% confidence interval, 1.1-2.1) in 10- to 11-year-old (year 6) girls. This equated to an 8% relative reduction in obesity rates compared with a counterfactual estimated from the trend prior to the SDIL announcement in March 2016, adjusted for temporal variations in obesity prevalence.
The researchers estimated that this was equivalent to preventing 5,234 cases of obesity per year in this group of year-6 girls alone.
Obesity reductions greatest in most deprived areas
Reductions were greatest in girls whose schools were in the most deprived areas, where children are known to consume the largest amount of sugary drinks. The greatest reductions in obesity were observed in the two most deprived quintiles – such that in the lowest quintile the absolute obesity prevalence reduction was 2.4 PP (95% CI, 1.6-3.2), equivalent to a 9% reduction in those living in the most deprived areas.
There are several reasons why the sugar tax did not lead to changes in levels of obesity among the younger children, the researchers said. Very young children consume fewer sugar-sweetened drinks than older children, so the soft drinks levy would have had a smaller effect. Also, fruit juices are not included in the levy, but contribute similar amounts of sugar in young children’s diets as do sugar-sweetened beverages.
Advertising may impact consumption in boys
It’s also unclear why the sugar tax might affect obesity prevalence in girls and boys differently, they said, especially since boys are higher consumers of sugar-sweetened beverages. One explanation is the possible impact of advertising – numerous studies have found that boys are often exposed to more food advertising than girls, both through higher levels of TV viewing and in how adverts are framed. Physical activity is often used to promote junk food and boys, compared with girls, have been shown to be more likely to believe that energy-dense junk foods depicted in adverts will boost physical performance, and so are more likely to choose energy-dense, nutrient-poor products following celebrity endorsements.
Tax ‘led to positive health impacts’
“Our findings suggest that the U.K. SDIL led to positive health impacts in the form of reduced obesity levels in girls aged 10-11 years,” the authors said. However: “Additional strategies beyond SSB taxation will be needed to reduce obesity prevalence overall, and particularly in older boys and younger children.”
Dr. Nina Rogers from the MRC Epidemiology Unit at Cambridge (England), who led the study, said: “We urgently need to find ways to tackle the increasing numbers of children living with obesity, otherwise we risk our children growing up to face significant health problems. That was one reason why the U.K.’s SDIL was introduced, and the evidence so far is promising. We’ve shown for the first time that it is likely to have helped prevent thousands of children each year becoming obese.
“It isn’t a straightforward picture, though, as it was mainly older girls who benefited. But the fact that we saw the biggest difference among girls from areas of high deprivation is important and is a step towards reducing the health inequalities they face.”
Although the researchers found an association rather than a causal link, this study adds to previous findings that the levy was associated with a substantial reduction in the amount of sugar in soft drinks.
Senior author Professor Jean Adams from the MRC Epidemiology Unit said: “We know that consuming too many sugary drinks contributes to obesity and that the U.K. soft drinks levy led to a drop in the amount of sugar in soft drinks available in the U.K., so it makes sense that we also see a drop in cases of obesity, although we only found this in girls. Children from more deprived backgrounds tend to consume the largest amount of sugary drinks, and it was among girls in this group that we saw the biggest change.”
Tom Sanders, professor emeritus of nutrition and dietetics at King’s College London, said: “The claim that the soft drink levy might have prevented 5,000 children from becoming obese is speculative because it is based on an association not actual measurements of consumption.”
He added that: “As well as continuing to discourage the consumption of sugar sweetened beverages and sweets, wider recognition should be given to foods such as biscuits [and] deep-fried foods (crisps, corn snacks, chips) that make [a] bigger contribution to excess calorie intake in children. Tackling poverty, however, is probably [the] best way to improve the diets of socially deprived children.”
Government ‘should learn from this success’
Asked to comment by this news organization, Katharine Jenner, director of the Obesity Health Alliance, said: “Government should be heartened that their soft drinks policy is already improving the health of young girls, regardless of where they live. The government should learn from this success, especially when compared with the many unsuccessful attempts to persuade industry to change their products voluntarily. They must now press ahead with policies that make it easier for everyone to eat a healthier diet, including extending the soft drinks industry levy to include other less healthy foods and drinks and measures to take junk food out of the spotlight.
“The research notes that numerous studies have found that boys are often exposed to more food advertising content than girls, negating the impact of the soft drinks levy [so] we need restriction on junk food marketing now, to put healthy food back in the spotlight.”
The research was supported by the National Institute of Health and Care Research and the Medical Research Council.
A version of this article originally appeared on MedscapeUK.
The introduction of the soft drinks industry levy (SDIL) – dubbed the ‘sugar tax’ – in England was followed by a drop in the number of older primary school girls succumbing to obesity, according to researchers from the Universities of Cambridge, Oxford, and Bath, with colleagues at the London School of Hygiene and Tropical Medicine.
The study, published in PLOS Medicine, has led to calls to extend the levy to other unhealthy foods and drinks
Obesity has become a global public health problem, the researchers said. In England, around 10% of 4- to 5-year-old children and 20% of 10- to 11-year-olds were recorded as obese in 2020. Childhood obesity is associated with depression in children and the adults into which they maturate, as well as with serious health problems in later life including high blood pressure and type 2 diabetes.
In the United Kingdom, young people consume significantly more added sugars than are recommended – by late adolescence, typically 70 g of added sugar per day, more than double the recommended 30g. The team said that sugar-sweetened beverages (SSB) are the primary sources of dietary added sugars in children, with high consumption commonly observed in more deprived areas where obesity prevalence is also highest.
Protecting children from excessive sugar
The two-tier SDIL on drinks manufacturers was implemented in April 2018 and aimed to protect children from excessive sugar consumption and tackle childhood obesity by incentivizing reformulation of SSBs in the U.K. with reduced sugar content.
To assess the effects of SDIL, the researchers used data from the National Child Measurement Programme on over 1 million children at ages 4 to 5 years (reception class) and 10 to 11 years (school year 6) in state-maintained English primary schools. The surveillance program includes annual repeat cross-sectional measurements, enabling the researchers to examine trajectories in monthly prevalence of obesity from September 2013 to November 2019, 19 months after the implementation of the SDIL.
Taking account of previous trends in obesity levels, they estimated both absolute and relative changes in obesity prevalence, both overall and by sex and deprivation, and compared obesity levels after the SDIL with predicted levels had the tax not been introduced, controlling for children’s sex and the level of deprivation of their school area.
Although they found no significant association with obesity levels in reception-age children or year-6 boys, they noted an overall absolute reduction in obesity prevalence of 1.6 percentage points (PPs) (95% confidence interval, 1.1-2.1) in 10- to 11-year-old (year 6) girls. This equated to an 8% relative reduction in obesity rates compared with a counterfactual estimated from the trend prior to the SDIL announcement in March 2016, adjusted for temporal variations in obesity prevalence.
The researchers estimated that this was equivalent to preventing 5,234 cases of obesity per year in this group of year-6 girls alone.
Obesity reductions greatest in most deprived areas
Reductions were greatest in girls whose schools were in the most deprived areas, where children are known to consume the largest amount of sugary drinks. The greatest reductions in obesity were observed in the two most deprived quintiles – such that in the lowest quintile the absolute obesity prevalence reduction was 2.4 PP (95% CI, 1.6-3.2), equivalent to a 9% reduction in those living in the most deprived areas.
There are several reasons why the sugar tax did not lead to changes in levels of obesity among the younger children, the researchers said. Very young children consume fewer sugar-sweetened drinks than older children, so the soft drinks levy would have had a smaller effect. Also, fruit juices are not included in the levy, but contribute similar amounts of sugar in young children’s diets as do sugar-sweetened beverages.
Advertising may impact consumption in boys
It’s also unclear why the sugar tax might affect obesity prevalence in girls and boys differently, they said, especially since boys are higher consumers of sugar-sweetened beverages. One explanation is the possible impact of advertising – numerous studies have found that boys are often exposed to more food advertising than girls, both through higher levels of TV viewing and in how adverts are framed. Physical activity is often used to promote junk food and boys, compared with girls, have been shown to be more likely to believe that energy-dense junk foods depicted in adverts will boost physical performance, and so are more likely to choose energy-dense, nutrient-poor products following celebrity endorsements.
Tax ‘led to positive health impacts’
“Our findings suggest that the U.K. SDIL led to positive health impacts in the form of reduced obesity levels in girls aged 10-11 years,” the authors said. However: “Additional strategies beyond SSB taxation will be needed to reduce obesity prevalence overall, and particularly in older boys and younger children.”
Dr. Nina Rogers from the MRC Epidemiology Unit at Cambridge (England), who led the study, said: “We urgently need to find ways to tackle the increasing numbers of children living with obesity, otherwise we risk our children growing up to face significant health problems. That was one reason why the U.K.’s SDIL was introduced, and the evidence so far is promising. We’ve shown for the first time that it is likely to have helped prevent thousands of children each year becoming obese.
“It isn’t a straightforward picture, though, as it was mainly older girls who benefited. But the fact that we saw the biggest difference among girls from areas of high deprivation is important and is a step towards reducing the health inequalities they face.”
Although the researchers found an association rather than a causal link, this study adds to previous findings that the levy was associated with a substantial reduction in the amount of sugar in soft drinks.
Senior author Professor Jean Adams from the MRC Epidemiology Unit said: “We know that consuming too many sugary drinks contributes to obesity and that the U.K. soft drinks levy led to a drop in the amount of sugar in soft drinks available in the U.K., so it makes sense that we also see a drop in cases of obesity, although we only found this in girls. Children from more deprived backgrounds tend to consume the largest amount of sugary drinks, and it was among girls in this group that we saw the biggest change.”
Tom Sanders, professor emeritus of nutrition and dietetics at King’s College London, said: “The claim that the soft drink levy might have prevented 5,000 children from becoming obese is speculative because it is based on an association not actual measurements of consumption.”
He added that: “As well as continuing to discourage the consumption of sugar sweetened beverages and sweets, wider recognition should be given to foods such as biscuits [and] deep-fried foods (crisps, corn snacks, chips) that make [a] bigger contribution to excess calorie intake in children. Tackling poverty, however, is probably [the] best way to improve the diets of socially deprived children.”
Government ‘should learn from this success’
Asked to comment by this news organization, Katharine Jenner, director of the Obesity Health Alliance, said: “Government should be heartened that their soft drinks policy is already improving the health of young girls, regardless of where they live. The government should learn from this success, especially when compared with the many unsuccessful attempts to persuade industry to change their products voluntarily. They must now press ahead with policies that make it easier for everyone to eat a healthier diet, including extending the soft drinks industry levy to include other less healthy foods and drinks and measures to take junk food out of the spotlight.
“The research notes that numerous studies have found that boys are often exposed to more food advertising content than girls, negating the impact of the soft drinks levy [so] we need restriction on junk food marketing now, to put healthy food back in the spotlight.”
The research was supported by the National Institute of Health and Care Research and the Medical Research Council.
A version of this article originally appeared on MedscapeUK.
The introduction of the soft drinks industry levy (SDIL) – dubbed the ‘sugar tax’ – in England was followed by a drop in the number of older primary school girls succumbing to obesity, according to researchers from the Universities of Cambridge, Oxford, and Bath, with colleagues at the London School of Hygiene and Tropical Medicine.
The study, published in PLOS Medicine, has led to calls to extend the levy to other unhealthy foods and drinks
Obesity has become a global public health problem, the researchers said. In England, around 10% of 4- to 5-year-old children and 20% of 10- to 11-year-olds were recorded as obese in 2020. Childhood obesity is associated with depression in children and the adults into which they maturate, as well as with serious health problems in later life including high blood pressure and type 2 diabetes.
In the United Kingdom, young people consume significantly more added sugars than are recommended – by late adolescence, typically 70 g of added sugar per day, more than double the recommended 30g. The team said that sugar-sweetened beverages (SSB) are the primary sources of dietary added sugars in children, with high consumption commonly observed in more deprived areas where obesity prevalence is also highest.
Protecting children from excessive sugar
The two-tier SDIL on drinks manufacturers was implemented in April 2018 and aimed to protect children from excessive sugar consumption and tackle childhood obesity by incentivizing reformulation of SSBs in the U.K. with reduced sugar content.
To assess the effects of SDIL, the researchers used data from the National Child Measurement Programme on over 1 million children at ages 4 to 5 years (reception class) and 10 to 11 years (school year 6) in state-maintained English primary schools. The surveillance program includes annual repeat cross-sectional measurements, enabling the researchers to examine trajectories in monthly prevalence of obesity from September 2013 to November 2019, 19 months after the implementation of the SDIL.
Taking account of previous trends in obesity levels, they estimated both absolute and relative changes in obesity prevalence, both overall and by sex and deprivation, and compared obesity levels after the SDIL with predicted levels had the tax not been introduced, controlling for children’s sex and the level of deprivation of their school area.
Although they found no significant association with obesity levels in reception-age children or year-6 boys, they noted an overall absolute reduction in obesity prevalence of 1.6 percentage points (PPs) (95% confidence interval, 1.1-2.1) in 10- to 11-year-old (year 6) girls. This equated to an 8% relative reduction in obesity rates compared with a counterfactual estimated from the trend prior to the SDIL announcement in March 2016, adjusted for temporal variations in obesity prevalence.
The researchers estimated that this was equivalent to preventing 5,234 cases of obesity per year in this group of year-6 girls alone.
Obesity reductions greatest in most deprived areas
Reductions were greatest in girls whose schools were in the most deprived areas, where children are known to consume the largest amount of sugary drinks. The greatest reductions in obesity were observed in the two most deprived quintiles – such that in the lowest quintile the absolute obesity prevalence reduction was 2.4 PP (95% CI, 1.6-3.2), equivalent to a 9% reduction in those living in the most deprived areas.
There are several reasons why the sugar tax did not lead to changes in levels of obesity among the younger children, the researchers said. Very young children consume fewer sugar-sweetened drinks than older children, so the soft drinks levy would have had a smaller effect. Also, fruit juices are not included in the levy, but contribute similar amounts of sugar in young children’s diets as do sugar-sweetened beverages.
Advertising may impact consumption in boys
It’s also unclear why the sugar tax might affect obesity prevalence in girls and boys differently, they said, especially since boys are higher consumers of sugar-sweetened beverages. One explanation is the possible impact of advertising – numerous studies have found that boys are often exposed to more food advertising than girls, both through higher levels of TV viewing and in how adverts are framed. Physical activity is often used to promote junk food and boys, compared with girls, have been shown to be more likely to believe that energy-dense junk foods depicted in adverts will boost physical performance, and so are more likely to choose energy-dense, nutrient-poor products following celebrity endorsements.
Tax ‘led to positive health impacts’
“Our findings suggest that the U.K. SDIL led to positive health impacts in the form of reduced obesity levels in girls aged 10-11 years,” the authors said. However: “Additional strategies beyond SSB taxation will be needed to reduce obesity prevalence overall, and particularly in older boys and younger children.”
Dr. Nina Rogers from the MRC Epidemiology Unit at Cambridge (England), who led the study, said: “We urgently need to find ways to tackle the increasing numbers of children living with obesity, otherwise we risk our children growing up to face significant health problems. That was one reason why the U.K.’s SDIL was introduced, and the evidence so far is promising. We’ve shown for the first time that it is likely to have helped prevent thousands of children each year becoming obese.
“It isn’t a straightforward picture, though, as it was mainly older girls who benefited. But the fact that we saw the biggest difference among girls from areas of high deprivation is important and is a step towards reducing the health inequalities they face.”
Although the researchers found an association rather than a causal link, this study adds to previous findings that the levy was associated with a substantial reduction in the amount of sugar in soft drinks.
Senior author Professor Jean Adams from the MRC Epidemiology Unit said: “We know that consuming too many sugary drinks contributes to obesity and that the U.K. soft drinks levy led to a drop in the amount of sugar in soft drinks available in the U.K., so it makes sense that we also see a drop in cases of obesity, although we only found this in girls. Children from more deprived backgrounds tend to consume the largest amount of sugary drinks, and it was among girls in this group that we saw the biggest change.”
Tom Sanders, professor emeritus of nutrition and dietetics at King’s College London, said: “The claim that the soft drink levy might have prevented 5,000 children from becoming obese is speculative because it is based on an association not actual measurements of consumption.”
He added that: “As well as continuing to discourage the consumption of sugar sweetened beverages and sweets, wider recognition should be given to foods such as biscuits [and] deep-fried foods (crisps, corn snacks, chips) that make [a] bigger contribution to excess calorie intake in children. Tackling poverty, however, is probably [the] best way to improve the diets of socially deprived children.”
Government ‘should learn from this success’
Asked to comment by this news organization, Katharine Jenner, director of the Obesity Health Alliance, said: “Government should be heartened that their soft drinks policy is already improving the health of young girls, regardless of where they live. The government should learn from this success, especially when compared with the many unsuccessful attempts to persuade industry to change their products voluntarily. They must now press ahead with policies that make it easier for everyone to eat a healthier diet, including extending the soft drinks industry levy to include other less healthy foods and drinks and measures to take junk food out of the spotlight.
“The research notes that numerous studies have found that boys are often exposed to more food advertising content than girls, negating the impact of the soft drinks levy [so] we need restriction on junk food marketing now, to put healthy food back in the spotlight.”
The research was supported by the National Institute of Health and Care Research and the Medical Research Council.
A version of this article originally appeared on MedscapeUK.
Infant with red eyelid lesion
A 4-MONTH-OLD HISPANIC INFANT was brought to her pediatrician by her parents for evaluation of a dark red lesion over her right eyelid. The mother said that the lesion appeared when the child was 4 weeks old and started as a small red dot. As the baby grew, so did the red dot. The mother said the lesion appeared redder and darker when the baby got fussy and cried. The mother noted that some of the child’s eyelashes on the affected eyelid had fallen out. The infant was still able to use her eyes to follow the movements of her parents and siblings.
The mother denied any complications during pregnancy and delivered the child vaginally. No one else in the family had a similar lesion. When asked, the mother said that when her daughter was born, she was missing hair on her scalp and had dark spots on her lower backside. The mother had taken the baby to all wellness checks. The child was up to date on her vaccines, had no known drug allergies, and was otherwise healthy.
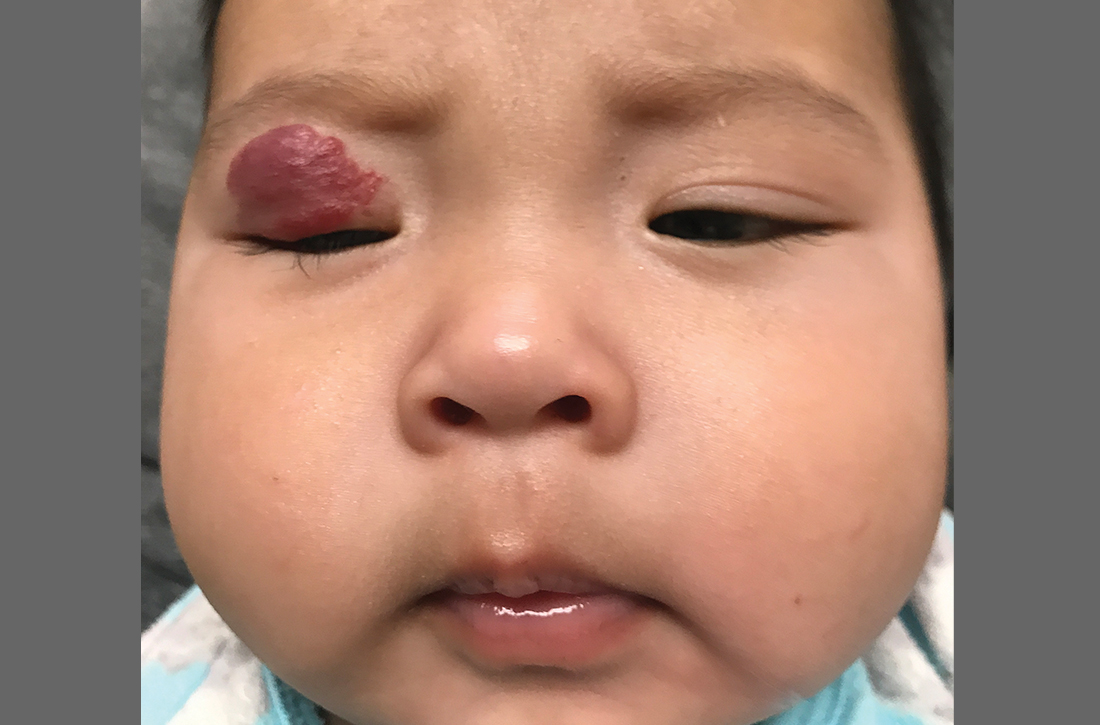
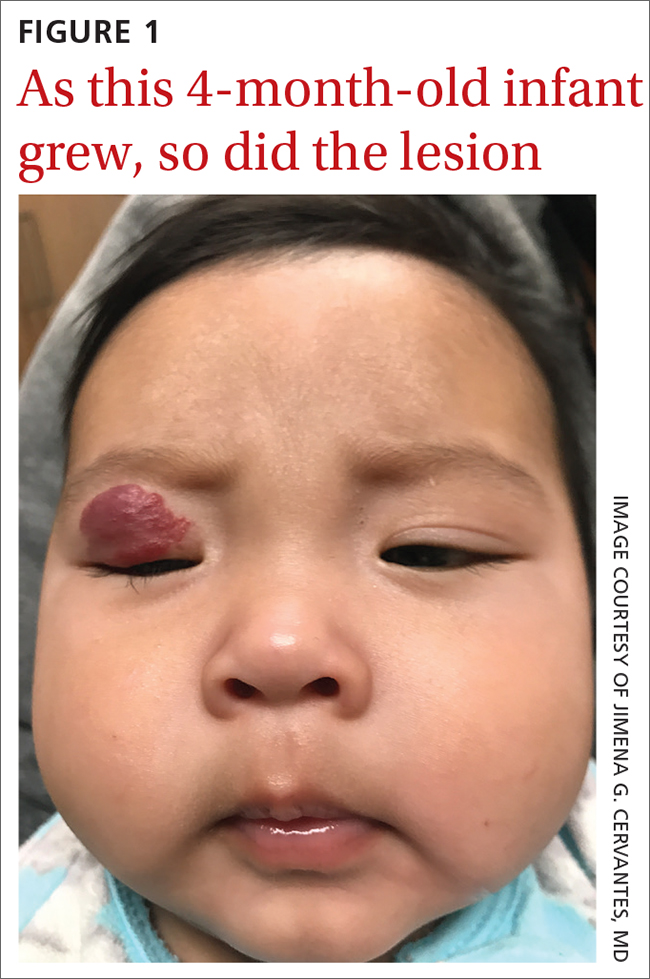
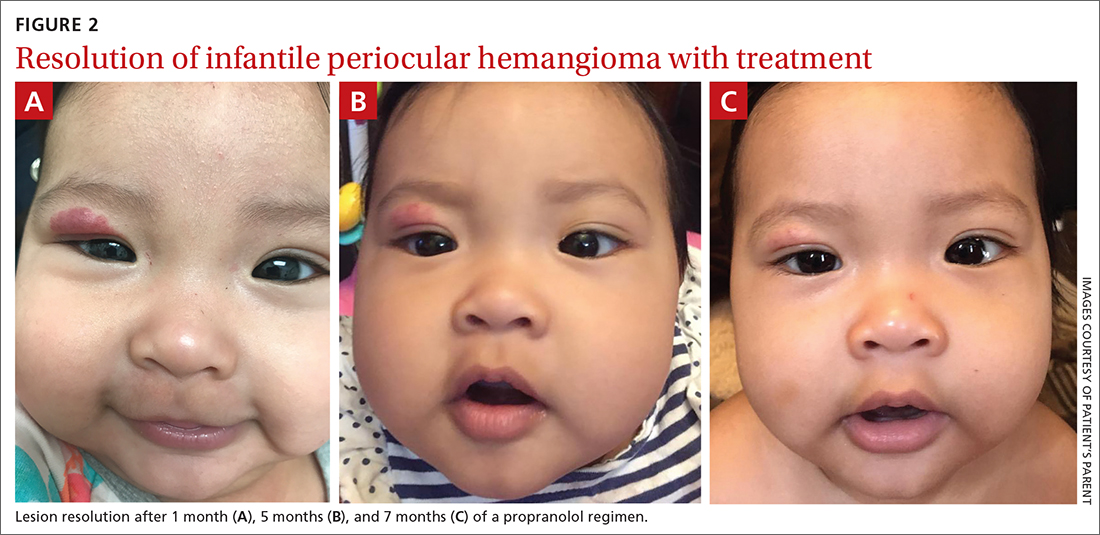
The pediatrician referred the baby to our skin clinic for further evaluation and treatment of the right eyelid lesion. Skin examination showed a 2.1-cm focal/localized, vascular, violaceous/dark red plaque over the right upper eyelid with an irregular border causing mild drooping of the right eyelid and some missing eyelashes (FIGURE 1). Multiple hyperpigmented patches on the upper and lower back were clinically consistent with Mongolian spots. Hair thinning was observed on the posterior and left posterior scalp.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Infantile hemangioma
The diagnosis of an infantile hemangioma was made clinically, based on the lesion’s appearance and when it became noticeable (during the child’s first few weeks of life).
Infantile hemangiomas are the most common benign tumors of infancy, and the majority are not present at birth.1,2 Infantile periocular hemangioma, which our patient had, is typically unilateral and involves the upper eyelid.1 Infantile hemangiomas appear in the first few weeks of life with an area of pallor and later a faint red patch, which the mother first noted in our patient. Lesions grow rapidly in the first 3 to 6 months.2 Superficial lesions appear as bright red papules or patches that may have a flat or rough surface and are sharply demarcated, while deep lesions tend to be bluish and dome shaped.1,2
Infantile hemangiomas continue to grow until 9 to 12 months of age, at which time the growth rate slows to parallel the growth of the child. Involution typically begins by the time the child is 1 year old. Most infantile hemangiomas do not improve significantly after 3.5 years of age.3
Differential includes congenital hemangiomas, pyogenic granulomas
Clinical presentation, histology, and lesion evolution distinguish infantile hemangioma from other diagnoses, notably the following:
Congenital hemangiomas (CH) are fully formed vascular tumors present at birth; they occur less frequently than infantile hemangiomas. CHs are divided into 2 categories: rapidly involuting CHs and noninvoluting CHs.4
Continue to: Pyogenic granulomas
Pyogenic granulomas are usually small (< 1 cm), sessile or pedunculated red papules or nodules. They are friable, bleed easily, and grow rapidly.
Capillary malformations can manifest at birth as flat, red/purple, cutaneous patches with irregular borders that are painless and can spontaneously bleed; they can be found in any part of the body but mainly occur in the cervicofacial area.5 Capillary malformations are commonly known as stork bites on the nape of the neck or angel kisses if found on the forehead. Lateral lesions, known as port wine stains, persist and do not resolve without treatment.5
Tufted angioma and kaposiform hemangioendothelioma manifest as expanding ecchymotic firm masses with purpura and accompanying lymphedema.4 Magnetic resonance imaging, including magnetic resonance angiography, is recommended for management and treatment.4
Venous malformations can be noted at birth as a dark blue or purple discoloration and manifest as a deep mass.5 Venous malformations grow with the patient and have a rapid growth phase during puberty, pregnancy, or traumatic injury.5
Arteriovenous malformations (AVMs) may be present at birth as a slight blush hypervascular lesion. AVMs can be quiescent for many years and grow with the patient. AVMs have a palpable warmth, pulse, or thrill due to high vascular flow.5
Continue to: Individualize treatment when it's needed
Individualize treatment when it’s needed
The majority of infantile hemangiomas do not require treatment because they can resolve spontaneously over time.2 That said, children with periocular infantile hemangiomas may require treatment because the lesions may result in amblyopia and visual impairment if not properly treated.6 Treatment should be individualized, depending on the size, rate of growth, morphology, number, and location of the lesions; existing or potential complications; benefits and adverse events associated with the treatment; age of the patient; level of parental concern; and the physician’s comfort level with the various treatment options.
Predictive factors for ocular complications in patients with periocular infantile hemangiomas are diameter > 1 cm, a deep component, and upper eyelid involvement. Patients at risk for ocular complications should be promptly referred to an ophthalmologist, and treatment should be strongly considered.6 Currently, oral propranolol is the treatment of choice for high-risk and complicated infantile hemangiomas.2 This is a very safe treatment. Only rarely do the following adverse effects occur: bronchospasm, bradycardia, hypotension, nightmares, cold hands, and hypoglycemia. If these adverse effects do occur, they are reversible with discontinuation of propranolol. Hypoglycemia can be prevented by giving propranolol during or right after feeding.
Our patient was started on propranolol 1 mg/kg/d for 1 month. The medication was administered by syringe for precise measurement. After the initial dose was tolerated, this was increased to 2 mg/kg/d for 1 month, then continued sequentially another month on 2.5 mg/kg/d, 2 months on 3 mg/kg/d, and finally 2 months on 3.4 mg/kg/d. All doses were divided twice per day between feedings.
After 7 months of total treatment time (FIGURE 2), we began titrating down the patient’s dose over the next several months. After 3 months, treatment was stopped altogether. At the time treatment was completed, only a faint pink blush remained.
1. Tavakoli M, Yadegari S, Mosallaei M, et al. Infantile periocular hemangioma. J Ophthalmic Vis Res. 2017;12:205-211. doi: 10.4103/jovr.jovr_66_17
2. Leung AKC, Lam JM, Leong KF, et al. Infantile hemangioma: an updated review. Curr Pediatr Rev. 2021;17:55-69. doi: 10.2174/1573396316666200508100038
3. Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624. doi: 10.1097/PRS.0b013e31825dc129
4. Wildgruber M, Sadick M, Müller-Wille R, et al. Vascular tumors in infants and adolescents. Insights Imaging. 2019;10:30. doi: 10.1186/s13244-019-0718-6
5. Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr. 2012;2012:645678. doi: 10.1155/2012/645678
6. Samuelov L, Kinori M, Rychlik K, et al. Risk factors for ocular complications in periocular infantile hemangiomas. Pediatr Dermatol. 2018;35:458-462. doi: 10.1111/pde.13525
A 4-MONTH-OLD HISPANIC INFANT was brought to her pediatrician by her parents for evaluation of a dark red lesion over her right eyelid. The mother said that the lesion appeared when the child was 4 weeks old and started as a small red dot. As the baby grew, so did the red dot. The mother said the lesion appeared redder and darker when the baby got fussy and cried. The mother noted that some of the child’s eyelashes on the affected eyelid had fallen out. The infant was still able to use her eyes to follow the movements of her parents and siblings.
The mother denied any complications during pregnancy and delivered the child vaginally. No one else in the family had a similar lesion. When asked, the mother said that when her daughter was born, she was missing hair on her scalp and had dark spots on her lower backside. The mother had taken the baby to all wellness checks. The child was up to date on her vaccines, had no known drug allergies, and was otherwise healthy.
The pediatrician referred the baby to our skin clinic for further evaluation and treatment of the right eyelid lesion. Skin examination showed a 2.1-cm focal/localized, vascular, violaceous/dark red plaque over the right upper eyelid with an irregular border causing mild drooping of the right eyelid and some missing eyelashes (FIGURE 1). Multiple hyperpigmented patches on the upper and lower back were clinically consistent with Mongolian spots. Hair thinning was observed on the posterior and left posterior scalp.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Infantile hemangioma
The diagnosis of an infantile hemangioma was made clinically, based on the lesion’s appearance and when it became noticeable (during the child’s first few weeks of life).
Infantile hemangiomas are the most common benign tumors of infancy, and the majority are not present at birth.1,2 Infantile periocular hemangioma, which our patient had, is typically unilateral and involves the upper eyelid.1 Infantile hemangiomas appear in the first few weeks of life with an area of pallor and later a faint red patch, which the mother first noted in our patient. Lesions grow rapidly in the first 3 to 6 months.2 Superficial lesions appear as bright red papules or patches that may have a flat or rough surface and are sharply demarcated, while deep lesions tend to be bluish and dome shaped.1,2
Infantile hemangiomas continue to grow until 9 to 12 months of age, at which time the growth rate slows to parallel the growth of the child. Involution typically begins by the time the child is 1 year old. Most infantile hemangiomas do not improve significantly after 3.5 years of age.3
Differential includes congenital hemangiomas, pyogenic granulomas
Clinical presentation, histology, and lesion evolution distinguish infantile hemangioma from other diagnoses, notably the following:
Congenital hemangiomas (CH) are fully formed vascular tumors present at birth; they occur less frequently than infantile hemangiomas. CHs are divided into 2 categories: rapidly involuting CHs and noninvoluting CHs.4
Continue to: Pyogenic granulomas
Pyogenic granulomas are usually small (< 1 cm), sessile or pedunculated red papules or nodules. They are friable, bleed easily, and grow rapidly.
Capillary malformations can manifest at birth as flat, red/purple, cutaneous patches with irregular borders that are painless and can spontaneously bleed; they can be found in any part of the body but mainly occur in the cervicofacial area.5 Capillary malformations are commonly known as stork bites on the nape of the neck or angel kisses if found on the forehead. Lateral lesions, known as port wine stains, persist and do not resolve without treatment.5
Tufted angioma and kaposiform hemangioendothelioma manifest as expanding ecchymotic firm masses with purpura and accompanying lymphedema.4 Magnetic resonance imaging, including magnetic resonance angiography, is recommended for management and treatment.4
Venous malformations can be noted at birth as a dark blue or purple discoloration and manifest as a deep mass.5 Venous malformations grow with the patient and have a rapid growth phase during puberty, pregnancy, or traumatic injury.5
Arteriovenous malformations (AVMs) may be present at birth as a slight blush hypervascular lesion. AVMs can be quiescent for many years and grow with the patient. AVMs have a palpable warmth, pulse, or thrill due to high vascular flow.5
Continue to: Individualize treatment when it's needed
Individualize treatment when it’s needed
The majority of infantile hemangiomas do not require treatment because they can resolve spontaneously over time.2 That said, children with periocular infantile hemangiomas may require treatment because the lesions may result in amblyopia and visual impairment if not properly treated.6 Treatment should be individualized, depending on the size, rate of growth, morphology, number, and location of the lesions; existing or potential complications; benefits and adverse events associated with the treatment; age of the patient; level of parental concern; and the physician’s comfort level with the various treatment options.
Predictive factors for ocular complications in patients with periocular infantile hemangiomas are diameter > 1 cm, a deep component, and upper eyelid involvement. Patients at risk for ocular complications should be promptly referred to an ophthalmologist, and treatment should be strongly considered.6 Currently, oral propranolol is the treatment of choice for high-risk and complicated infantile hemangiomas.2 This is a very safe treatment. Only rarely do the following adverse effects occur: bronchospasm, bradycardia, hypotension, nightmares, cold hands, and hypoglycemia. If these adverse effects do occur, they are reversible with discontinuation of propranolol. Hypoglycemia can be prevented by giving propranolol during or right after feeding.
Our patient was started on propranolol 1 mg/kg/d for 1 month. The medication was administered by syringe for precise measurement. After the initial dose was tolerated, this was increased to 2 mg/kg/d for 1 month, then continued sequentially another month on 2.5 mg/kg/d, 2 months on 3 mg/kg/d, and finally 2 months on 3.4 mg/kg/d. All doses were divided twice per day between feedings.
After 7 months of total treatment time (FIGURE 2), we began titrating down the patient’s dose over the next several months. After 3 months, treatment was stopped altogether. At the time treatment was completed, only a faint pink blush remained.

A 4-MONTH-OLD HISPANIC INFANT was brought to her pediatrician by her parents for evaluation of a dark red lesion over her right eyelid. The mother said that the lesion appeared when the child was 4 weeks old and started as a small red dot. As the baby grew, so did the red dot. The mother said the lesion appeared redder and darker when the baby got fussy and cried. The mother noted that some of the child’s eyelashes on the affected eyelid had fallen out. The infant was still able to use her eyes to follow the movements of her parents and siblings.
The mother denied any complications during pregnancy and delivered the child vaginally. No one else in the family had a similar lesion. When asked, the mother said that when her daughter was born, she was missing hair on her scalp and had dark spots on her lower backside. The mother had taken the baby to all wellness checks. The child was up to date on her vaccines, had no known drug allergies, and was otherwise healthy.
The pediatrician referred the baby to our skin clinic for further evaluation and treatment of the right eyelid lesion. Skin examination showed a 2.1-cm focal/localized, vascular, violaceous/dark red plaque over the right upper eyelid with an irregular border causing mild drooping of the right eyelid and some missing eyelashes (FIGURE 1). Multiple hyperpigmented patches on the upper and lower back were clinically consistent with Mongolian spots. Hair thinning was observed on the posterior and left posterior scalp.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Infantile hemangioma
The diagnosis of an infantile hemangioma was made clinically, based on the lesion’s appearance and when it became noticeable (during the child’s first few weeks of life).
Infantile hemangiomas are the most common benign tumors of infancy, and the majority are not present at birth.1,2 Infantile periocular hemangioma, which our patient had, is typically unilateral and involves the upper eyelid.1 Infantile hemangiomas appear in the first few weeks of life with an area of pallor and later a faint red patch, which the mother first noted in our patient. Lesions grow rapidly in the first 3 to 6 months.2 Superficial lesions appear as bright red papules or patches that may have a flat or rough surface and are sharply demarcated, while deep lesions tend to be bluish and dome shaped.1,2
Infantile hemangiomas continue to grow until 9 to 12 months of age, at which time the growth rate slows to parallel the growth of the child. Involution typically begins by the time the child is 1 year old. Most infantile hemangiomas do not improve significantly after 3.5 years of age.3
Differential includes congenital hemangiomas, pyogenic granulomas
Clinical presentation, histology, and lesion evolution distinguish infantile hemangioma from other diagnoses, notably the following:
Congenital hemangiomas (CH) are fully formed vascular tumors present at birth; they occur less frequently than infantile hemangiomas. CHs are divided into 2 categories: rapidly involuting CHs and noninvoluting CHs.4
Continue to: Pyogenic granulomas
Pyogenic granulomas are usually small (< 1 cm), sessile or pedunculated red papules or nodules. They are friable, bleed easily, and grow rapidly.
Capillary malformations can manifest at birth as flat, red/purple, cutaneous patches with irregular borders that are painless and can spontaneously bleed; they can be found in any part of the body but mainly occur in the cervicofacial area.5 Capillary malformations are commonly known as stork bites on the nape of the neck or angel kisses if found on the forehead. Lateral lesions, known as port wine stains, persist and do not resolve without treatment.5
Tufted angioma and kaposiform hemangioendothelioma manifest as expanding ecchymotic firm masses with purpura and accompanying lymphedema.4 Magnetic resonance imaging, including magnetic resonance angiography, is recommended for management and treatment.4
Venous malformations can be noted at birth as a dark blue or purple discoloration and manifest as a deep mass.5 Venous malformations grow with the patient and have a rapid growth phase during puberty, pregnancy, or traumatic injury.5
Arteriovenous malformations (AVMs) may be present at birth as a slight blush hypervascular lesion. AVMs can be quiescent for many years and grow with the patient. AVMs have a palpable warmth, pulse, or thrill due to high vascular flow.5
Continue to: Individualize treatment when it's needed
Individualize treatment when it’s needed
The majority of infantile hemangiomas do not require treatment because they can resolve spontaneously over time.2 That said, children with periocular infantile hemangiomas may require treatment because the lesions may result in amblyopia and visual impairment if not properly treated.6 Treatment should be individualized, depending on the size, rate of growth, morphology, number, and location of the lesions; existing or potential complications; benefits and adverse events associated with the treatment; age of the patient; level of parental concern; and the physician’s comfort level with the various treatment options.
Predictive factors for ocular complications in patients with periocular infantile hemangiomas are diameter > 1 cm, a deep component, and upper eyelid involvement. Patients at risk for ocular complications should be promptly referred to an ophthalmologist, and treatment should be strongly considered.6 Currently, oral propranolol is the treatment of choice for high-risk and complicated infantile hemangiomas.2 This is a very safe treatment. Only rarely do the following adverse effects occur: bronchospasm, bradycardia, hypotension, nightmares, cold hands, and hypoglycemia. If these adverse effects do occur, they are reversible with discontinuation of propranolol. Hypoglycemia can be prevented by giving propranolol during or right after feeding.
Our patient was started on propranolol 1 mg/kg/d for 1 month. The medication was administered by syringe for precise measurement. After the initial dose was tolerated, this was increased to 2 mg/kg/d for 1 month, then continued sequentially another month on 2.5 mg/kg/d, 2 months on 3 mg/kg/d, and finally 2 months on 3.4 mg/kg/d. All doses were divided twice per day between feedings.
After 7 months of total treatment time (FIGURE 2), we began titrating down the patient’s dose over the next several months. After 3 months, treatment was stopped altogether. At the time treatment was completed, only a faint pink blush remained.

1. Tavakoli M, Yadegari S, Mosallaei M, et al. Infantile periocular hemangioma. J Ophthalmic Vis Res. 2017;12:205-211. doi: 10.4103/jovr.jovr_66_17
2. Leung AKC, Lam JM, Leong KF, et al. Infantile hemangioma: an updated review. Curr Pediatr Rev. 2021;17:55-69. doi: 10.2174/1573396316666200508100038
3. Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624. doi: 10.1097/PRS.0b013e31825dc129
4. Wildgruber M, Sadick M, Müller-Wille R, et al. Vascular tumors in infants and adolescents. Insights Imaging. 2019;10:30. doi: 10.1186/s13244-019-0718-6
5. Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr. 2012;2012:645678. doi: 10.1155/2012/645678
6. Samuelov L, Kinori M, Rychlik K, et al. Risk factors for ocular complications in periocular infantile hemangiomas. Pediatr Dermatol. 2018;35:458-462. doi: 10.1111/pde.13525
1. Tavakoli M, Yadegari S, Mosallaei M, et al. Infantile periocular hemangioma. J Ophthalmic Vis Res. 2017;12:205-211. doi: 10.4103/jovr.jovr_66_17
2. Leung AKC, Lam JM, Leong KF, et al. Infantile hemangioma: an updated review. Curr Pediatr Rev. 2021;17:55-69. doi: 10.2174/1573396316666200508100038
3. Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624. doi: 10.1097/PRS.0b013e31825dc129
4. Wildgruber M, Sadick M, Müller-Wille R, et al. Vascular tumors in infants and adolescents. Insights Imaging. 2019;10:30. doi: 10.1186/s13244-019-0718-6
5. Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr. 2012;2012:645678. doi: 10.1155/2012/645678
6. Samuelov L, Kinori M, Rychlik K, et al. Risk factors for ocular complications in periocular infantile hemangiomas. Pediatr Dermatol. 2018;35:458-462. doi: 10.1111/pde.13525
Weight bias affects views of kids’ obesity recommendations
Apparently, offering children effective treatments for a chronic disease that markedly increases their risk for other chronic diseases, regularly erodes their quality of life, and is the No. 1 target of school-based bullying is wrong.
At least that’s my take watching the coverage of the recent American Academy of Pediatrics new pediatric obesity treatment guidelines that, gasp, suggest that children whose severity of obesity warrants medication or surgeries be offered medication or surgery. Because it’s wiser to not try to treat the obesity that›s contributing to a child’s type 2 diabetes, hypertension, fatty liver disease, or reduced quality of life?
The reaction isn’t surprising. Some of those who are up in arms about it have clinical or research careers dependent on championing their own favorite dietary strategies as if they are more effective and reproducible than decades of uniformly disappointing studies proving that they’re not. Others are upset because, for reasons that at times may be personal and at times may be conflicted, they believe that obesity should not be treated and/or that sustained weight loss is impossible. But overarchingly, probably the bulk of the hoopla stems from obesity being seen as a moral failing. Because the notion that those who suffer with obesity are themselves to blame has been the prevailing societal view for decades, if not centuries.
Working with families of children with obesity severe enough for them to seek help, it’s clear that if desire were sufficient to will it away, we wouldn’t need treatment guidelines let alone medications or surgery. Near uniformly, parents describe their children being bullied consequent to and being deeply self-conscious of their weight.
And what would those who think children shouldn’t be offered reproducibly effective treatment for obesity have them do about it? Many seem to think it would be preferable for kids to be placed on formal diets and, of course, that they should go out and play more. And though I’m all for encouraging the improvement of a child’s dietary quality and activity level, anyone suggesting those as panaceas for childhood obesity haven’t a clue. Not to mention the fact that, in most cases, improving overall dietary quality, something worthwhile at any weight, isn’t the dietary goal being recommended. Instead, the prescription seems to be restrictive dieting coupled with overexercising, which, unlike appropriately and thoughtfully informed and utilized medication, may increase a child’s risk of maladaptive thinking around food and fitness as well as disordered eating, not to mention challenge their self-esteem if their lifestyle results are underwhelming.
This brings us to one of the most bizarre takes on this whole business – that medications will be pushed and used when not necessary. No doubt that at times, that may occur, but the issue is that of a clinician’s overzealous prescribing and not of the treatment options or indications. Consider childhood asthma. There is no worry or uproar that children with mild asthma that isn’t having an impact on their quality of life or markedly risking their health will be placed on multiple inhaled steroids and treatments. Why? Because clinicians have been taught how to dispassionately evaluate treatment needs for asthma, monitor disease course, and not simply prescribe everything in our armamentarium.
Shocking, I know, but as is the case with every other medical condition, I think doctors are capable of learning and following an algorithm covering the indications and options for the treatment of childhood obesity.
How that looks also mirrors what’s seen with any other chronic noncommunicable disease with varied severity and impact. Doctors will evaluate each child with obesity to see whether it’s having a detrimental effect on their health or quality of life. They will monitor their patients’ obesity to see if it’s worsening and will, when necessary, undertake investigations to rule out its potential contribution to common comorbidities like type 2 diabetes, hypertension, and fatty liver disease. And, when appropriate, they will provide information on available treatment options – from lifestyle to medication to surgery and the risks, benefits, and realistic expectations associated with each – and then, without judgment, support their patients’ treatment choices because blame-free informed discussion and supportive prescription of care is, in fact, the distillation of our jobs.
If people are looking to be outraged rather than focusing their outrage on what we now need to do about childhood obesity, they should instead look to what got us here: our obesogenic environment. We and our children are swimming against a torrential current of cheap ultraprocessed calories being pushed upon us by a broken societal food culture that values convenience and simultaneously embraces the notion that knowledge is a match versus the thousands of genes and dozens of hormones that increasingly sophisticated food industry marketers and scientists prey upon. When dealing with torrential currents, we need to do more than just recommend swimming lessons.
Like asthma, which may be exacerbated by pollution in our environment both outdoors and indoors, childhood obesity is a modern-day environmentally influenced disease with varied penetrance that does not always require active treatment. Like asthma, childhood obesity is not a disease that children choose to have; it’s not a disease that can be willed away; and it’s not a disease that responds uniformly, dramatically, or enduringly to diet and exercise. Finally, literally and figuratively, like asthma, for childhood obesity, we thankfully now have a number of effective treatment options that we can offer, and it’s only our societal weight bias that leads to thinking that’s anything but great.
A version of this article first appeared on Medscape.com.
Apparently, offering children effective treatments for a chronic disease that markedly increases their risk for other chronic diseases, regularly erodes their quality of life, and is the No. 1 target of school-based bullying is wrong.
At least that’s my take watching the coverage of the recent American Academy of Pediatrics new pediatric obesity treatment guidelines that, gasp, suggest that children whose severity of obesity warrants medication or surgeries be offered medication or surgery. Because it’s wiser to not try to treat the obesity that›s contributing to a child’s type 2 diabetes, hypertension, fatty liver disease, or reduced quality of life?
The reaction isn’t surprising. Some of those who are up in arms about it have clinical or research careers dependent on championing their own favorite dietary strategies as if they are more effective and reproducible than decades of uniformly disappointing studies proving that they’re not. Others are upset because, for reasons that at times may be personal and at times may be conflicted, they believe that obesity should not be treated and/or that sustained weight loss is impossible. But overarchingly, probably the bulk of the hoopla stems from obesity being seen as a moral failing. Because the notion that those who suffer with obesity are themselves to blame has been the prevailing societal view for decades, if not centuries.
Working with families of children with obesity severe enough for them to seek help, it’s clear that if desire were sufficient to will it away, we wouldn’t need treatment guidelines let alone medications or surgery. Near uniformly, parents describe their children being bullied consequent to and being deeply self-conscious of their weight.
And what would those who think children shouldn’t be offered reproducibly effective treatment for obesity have them do about it? Many seem to think it would be preferable for kids to be placed on formal diets and, of course, that they should go out and play more. And though I’m all for encouraging the improvement of a child’s dietary quality and activity level, anyone suggesting those as panaceas for childhood obesity haven’t a clue. Not to mention the fact that, in most cases, improving overall dietary quality, something worthwhile at any weight, isn’t the dietary goal being recommended. Instead, the prescription seems to be restrictive dieting coupled with overexercising, which, unlike appropriately and thoughtfully informed and utilized medication, may increase a child’s risk of maladaptive thinking around food and fitness as well as disordered eating, not to mention challenge their self-esteem if their lifestyle results are underwhelming.
This brings us to one of the most bizarre takes on this whole business – that medications will be pushed and used when not necessary. No doubt that at times, that may occur, but the issue is that of a clinician’s overzealous prescribing and not of the treatment options or indications. Consider childhood asthma. There is no worry or uproar that children with mild asthma that isn’t having an impact on their quality of life or markedly risking their health will be placed on multiple inhaled steroids and treatments. Why? Because clinicians have been taught how to dispassionately evaluate treatment needs for asthma, monitor disease course, and not simply prescribe everything in our armamentarium.
Shocking, I know, but as is the case with every other medical condition, I think doctors are capable of learning and following an algorithm covering the indications and options for the treatment of childhood obesity.
How that looks also mirrors what’s seen with any other chronic noncommunicable disease with varied severity and impact. Doctors will evaluate each child with obesity to see whether it’s having a detrimental effect on their health or quality of life. They will monitor their patients’ obesity to see if it’s worsening and will, when necessary, undertake investigations to rule out its potential contribution to common comorbidities like type 2 diabetes, hypertension, and fatty liver disease. And, when appropriate, they will provide information on available treatment options – from lifestyle to medication to surgery and the risks, benefits, and realistic expectations associated with each – and then, without judgment, support their patients’ treatment choices because blame-free informed discussion and supportive prescription of care is, in fact, the distillation of our jobs.
If people are looking to be outraged rather than focusing their outrage on what we now need to do about childhood obesity, they should instead look to what got us here: our obesogenic environment. We and our children are swimming against a torrential current of cheap ultraprocessed calories being pushed upon us by a broken societal food culture that values convenience and simultaneously embraces the notion that knowledge is a match versus the thousands of genes and dozens of hormones that increasingly sophisticated food industry marketers and scientists prey upon. When dealing with torrential currents, we need to do more than just recommend swimming lessons.
Like asthma, which may be exacerbated by pollution in our environment both outdoors and indoors, childhood obesity is a modern-day environmentally influenced disease with varied penetrance that does not always require active treatment. Like asthma, childhood obesity is not a disease that children choose to have; it’s not a disease that can be willed away; and it’s not a disease that responds uniformly, dramatically, or enduringly to diet and exercise. Finally, literally and figuratively, like asthma, for childhood obesity, we thankfully now have a number of effective treatment options that we can offer, and it’s only our societal weight bias that leads to thinking that’s anything but great.
A version of this article first appeared on Medscape.com.
Apparently, offering children effective treatments for a chronic disease that markedly increases their risk for other chronic diseases, regularly erodes their quality of life, and is the No. 1 target of school-based bullying is wrong.
At least that’s my take watching the coverage of the recent American Academy of Pediatrics new pediatric obesity treatment guidelines that, gasp, suggest that children whose severity of obesity warrants medication or surgeries be offered medication or surgery. Because it’s wiser to not try to treat the obesity that›s contributing to a child’s type 2 diabetes, hypertension, fatty liver disease, or reduced quality of life?
The reaction isn’t surprising. Some of those who are up in arms about it have clinical or research careers dependent on championing their own favorite dietary strategies as if they are more effective and reproducible than decades of uniformly disappointing studies proving that they’re not. Others are upset because, for reasons that at times may be personal and at times may be conflicted, they believe that obesity should not be treated and/or that sustained weight loss is impossible. But overarchingly, probably the bulk of the hoopla stems from obesity being seen as a moral failing. Because the notion that those who suffer with obesity are themselves to blame has been the prevailing societal view for decades, if not centuries.
Working with families of children with obesity severe enough for them to seek help, it’s clear that if desire were sufficient to will it away, we wouldn’t need treatment guidelines let alone medications or surgery. Near uniformly, parents describe their children being bullied consequent to and being deeply self-conscious of their weight.
And what would those who think children shouldn’t be offered reproducibly effective treatment for obesity have them do about it? Many seem to think it would be preferable for kids to be placed on formal diets and, of course, that they should go out and play more. And though I’m all for encouraging the improvement of a child’s dietary quality and activity level, anyone suggesting those as panaceas for childhood obesity haven’t a clue. Not to mention the fact that, in most cases, improving overall dietary quality, something worthwhile at any weight, isn’t the dietary goal being recommended. Instead, the prescription seems to be restrictive dieting coupled with overexercising, which, unlike appropriately and thoughtfully informed and utilized medication, may increase a child’s risk of maladaptive thinking around food and fitness as well as disordered eating, not to mention challenge their self-esteem if their lifestyle results are underwhelming.
This brings us to one of the most bizarre takes on this whole business – that medications will be pushed and used when not necessary. No doubt that at times, that may occur, but the issue is that of a clinician’s overzealous prescribing and not of the treatment options or indications. Consider childhood asthma. There is no worry or uproar that children with mild asthma that isn’t having an impact on their quality of life or markedly risking their health will be placed on multiple inhaled steroids and treatments. Why? Because clinicians have been taught how to dispassionately evaluate treatment needs for asthma, monitor disease course, and not simply prescribe everything in our armamentarium.
Shocking, I know, but as is the case with every other medical condition, I think doctors are capable of learning and following an algorithm covering the indications and options for the treatment of childhood obesity.
How that looks also mirrors what’s seen with any other chronic noncommunicable disease with varied severity and impact. Doctors will evaluate each child with obesity to see whether it’s having a detrimental effect on their health or quality of life. They will monitor their patients’ obesity to see if it’s worsening and will, when necessary, undertake investigations to rule out its potential contribution to common comorbidities like type 2 diabetes, hypertension, and fatty liver disease. And, when appropriate, they will provide information on available treatment options – from lifestyle to medication to surgery and the risks, benefits, and realistic expectations associated with each – and then, without judgment, support their patients’ treatment choices because blame-free informed discussion and supportive prescription of care is, in fact, the distillation of our jobs.
If people are looking to be outraged rather than focusing their outrage on what we now need to do about childhood obesity, they should instead look to what got us here: our obesogenic environment. We and our children are swimming against a torrential current of cheap ultraprocessed calories being pushed upon us by a broken societal food culture that values convenience and simultaneously embraces the notion that knowledge is a match versus the thousands of genes and dozens of hormones that increasingly sophisticated food industry marketers and scientists prey upon. When dealing with torrential currents, we need to do more than just recommend swimming lessons.
Like asthma, which may be exacerbated by pollution in our environment both outdoors and indoors, childhood obesity is a modern-day environmentally influenced disease with varied penetrance that does not always require active treatment. Like asthma, childhood obesity is not a disease that children choose to have; it’s not a disease that can be willed away; and it’s not a disease that responds uniformly, dramatically, or enduringly to diet and exercise. Finally, literally and figuratively, like asthma, for childhood obesity, we thankfully now have a number of effective treatment options that we can offer, and it’s only our societal weight bias that leads to thinking that’s anything but great.
A version of this article first appeared on Medscape.com.
Tips and tools to help you manage ADHD in children, adolescents
THE CASE
James B* is a 7-year-old Black child who presented to his primary care physician (PCP) for a well-child visit. During preventive health screening, James’ mother expressed concerns about his behavior, characterizing him as immature, aggressive, destructive, and occasionally self-loathing. She described him as physically uncoordinated, struggling to keep up with his peers in sports, and tiring after 20 minutes of activity. James slept 10 hours nightly but was often restless and snored intermittently. As a second grader, his academic achievement was not progressing, and he had become increasingly inattentive at home and at school. James’ mother offered several examples of his fighting with his siblings, noncompliance with morning routines, and avoidance of learning activities. Additionally, his mother expressed concern that James, as a Black child, might eventually be unfairly labeled as a problem child by his teachers or held back a grade level in school.
Although James did not have a family history of developmental delays or learning disorders, he had not met any milestones on time for gross or fine motor, language, cognitive, and social-emotional skills. James had a history of chronic otitis media, for which pressure equalizer tubes were inserted at age 2 years. He had not had any major physical injuries, psychological trauma, recent life transitions, or adverse childhood events. When asked, James’ mother acknowledged symptoms of maternal depression but alluded to faith-based reasons for not seeking treatment for herself.
James’ physical examination was unremarkable. His height, weight, and vitals were all within normal limits. However, he had some difficulty with verbal articulation and expression and showed signs of a possible vocal tic. Based on James’ presentation, his PCP suspected attention-deficit/hyperactivity disorder (ADHD), as well as neurodevelopmental delays.
The PCP gave James’ mother the Strengths and Difficulties Questionnaire to complete and the Vanderbilt Assessment Scales for her and James’ teacher to fill out independently and return to the clinic. The PCP also instructed James’ mother on how to use a sleep diary to maintain a 1-month log of his sleep patterns and habits. The PCP consulted the integrated behavioral health clinician (IBHC; a clinical social worker embedded in the primary care clinic) and made a warm handoff for the IBHC to further assess James’ maladaptive behaviors and interactions.
●
* The patient’s name has been changed to protect his identity.
James is one of more than 6 million children, ages 3 to 17 years, in the United States who live with ADHD.1,2 ADHD is the most common neurodevelopmental disorder among children, and it affects multiple cognitive and behavioral domains throughout the lifespan.3 Children with ADHD often initially present in primary care settings; thus, PCPs are well positioned to diagnose the disorder and provide longitudinal treatment. This Behavioral Health Consult reviews clinical assessment and practice guidelines, as well as treatment recommendations applicable across different areas of influence—individual, family, community, and systems—for PCPs and IBHCs to use in managing ADHD in children.
ADHD features can vary by age and sex
ADHD is a persistent pattern of inattention or hyperactivity and impulsivity interfering with functioning or development in childhood and functioning later in adulthood. ADHD symptoms manifest prior to age 12 years and must occur in 2 or more settings.4 Symptoms should not be better explained by another psychiatric disorder or occur exclusively during the course of another disorder (TABLE 1).4
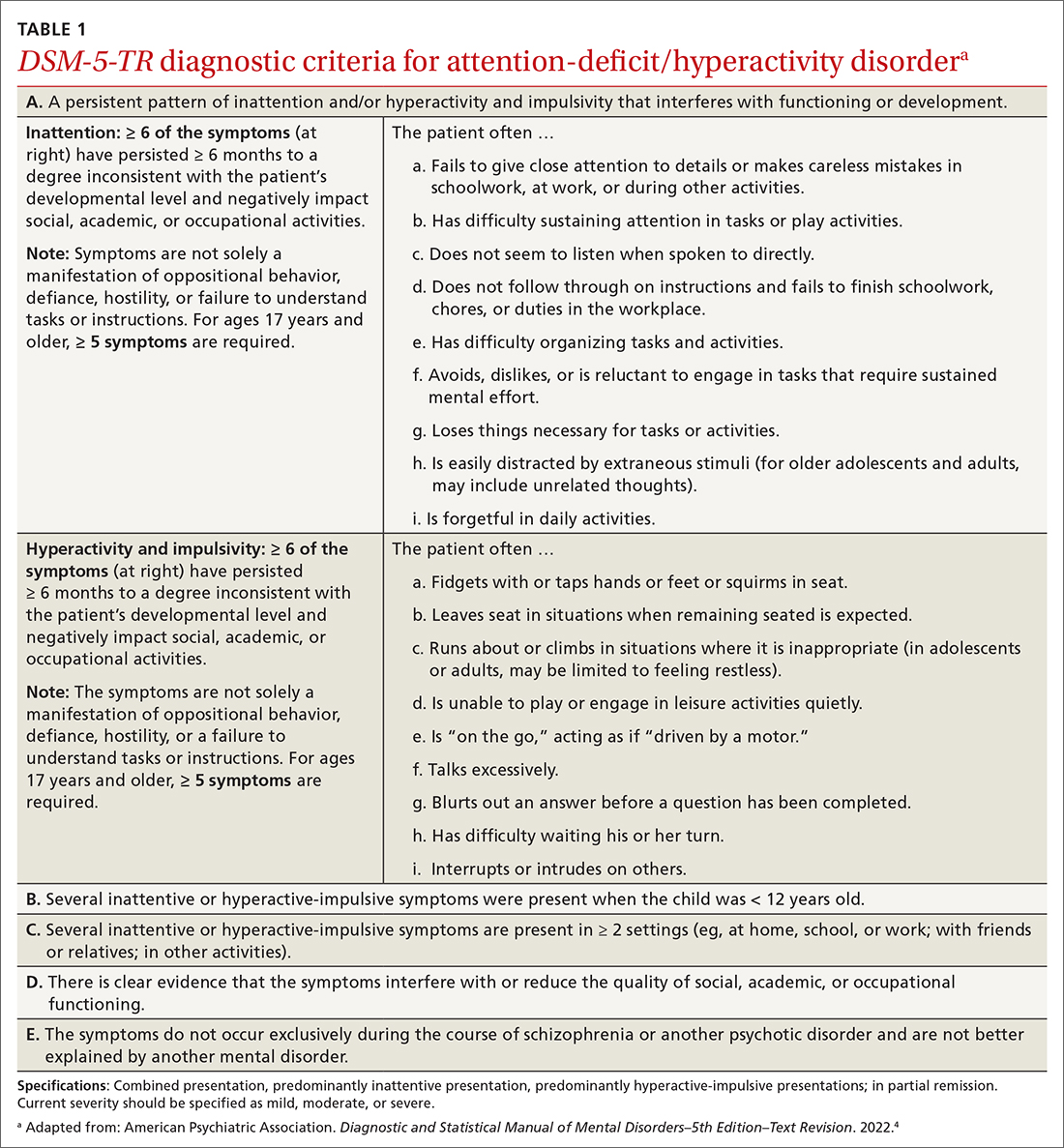
The rate of heritability is high, with significant incidence among first-degree relatives.4 Children with ADHD show executive functioning deficits in 1 or more cognitive domains (eg, visuospatial, memory, inhibitions, decision making, and reward regulation).4,5 The prevalence of ADHD nationally is approximately 9.8% (2.2%, ages 3-5 years; 10%, ages 6-11 years; 13.2%, ages 12-17 years) in children and adolescents; worldwide prevalence is 7.2%.1,6 It persists among 2.6% to 6.8% of adults worldwide.7
Research has shown that boys ages 6 to 11 years are significantly more likely than girls to exhibit attention-getting, externalizing behaviors or conduct problems (eg, hyperactivity, impulsivity, disruption, aggression).1,6 On the other hand, girls ages 12 to 17 years tend to display internalized (eg, depressed mood, anxiety, low self-esteem) or inattentive behaviors, which clinicians and educators may assess as less severe and warranting fewer supportive measures.1
The prevalence of ADHD and its associated factors, which evolve through maturation, underscore the importance of persistent, patient-centered, and collaborative PCP and IBHC clinical management.
Continue to: Begin with a screening tool, move to a clinical interview
Begin with a screening tool, move to a clinical interview
When caregivers express concerns about their child’s behavior, focus, mood, learning, and socialization, consider initiating a multimodal evaluation for ADHD.5,8 Embarking on an ADHD assessment can require extended or multiple visits to arrive at the diagnosis, followed by still more visits to confirm a course of care and adjust medications. The integrative care approach described in the patient case and elaborated on later in this article can help facilitate assessment and treatment of ADHD.9
Signs of ADHD may be observed at initial screening using a tool such as the Ages & Stages Questionnaire (https://agesandstages.com/products-pricing/asq3/) to reveal indications of norm deviations or delays commensurate with ADHD.10 However, to substantiate the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision criteria for an accurate diagnosis,4 the American Academy of Pediatrics (AAP) clinical practice guidelines require a thorough clinical interview, administration of a standardized assessment tool, and review of objective reports in conjunction with a physical examination and psychosocial evaluation.6 Standardized measures of psychological, neurocognitive, and academic achievement reported by caregivers and collateral contacts (eg, teachers, counselors, coaches, care providers) are needed to maximize data objectivity and symptom accuracy across settings (TABLE 210-17). Additionally, periodic reassessment is recommended to validate changes in diagnostic subtype and treatment plans due to the chronic and dynamic nature of ADHD.
Consider comorbidities and alternate diagnoses
The diagnostic possibility of ADHD should also prompt consideration of other childhood disorders due to the high potential for comorbidities.4,6 In a 2016 study, approximately 64% of
Various medical disorders may manifest with similar signs or symptoms to ADHD, such as thyroid disorders, seizure disorders, adverse drug effects, anemia, genetic anomalies, and others.6,19
If there are behavioral concerns or developmental delays associated with tall stature for age or pubertal or testicular development anomalies, consult a geneticist and a developmental pediatrician for targeted testing and neurodevelopmental assessment, respectively. For example, ADHD is a common comorbidity among boys who also have XYY syndrome (Jacobs syndrome). However, due to the variability of symptoms and severity, XYY syndrome often goes undiagnosed, leaving a host of compounding pervasive and developmental problems untreated. Overall, more than two-thirds of patients with ADHD and a co-occurring condition are either inaccurately diagnosed or not referred for additional assessment and adjunct treatment.21
Continue to: Risks that arise over time
Risks that arise over time. As ADHD persists, adolescents are at greater risk for psychiatric comorbidities, suicidality, and functional impairments (eg, risky behaviors, occupational problems, truancy, delinquency, and poor self-esteem).4,8 Adolescents with internalized behaviors are more likely to experience comorbid depressive disorders with increased risk for self-harm.4,5,8 As adolescents age and their sense of autonomy increases, there is a tendency among those who have received a diagnosis of ADHD to minimize symptoms and decrease the frequency of routine clinic visits along with medication use and treatment compliance.3 Additionally, abuse, misuse, and misappropriation of stimulants among teens and young adults are commonplace.
Wide-scope, multidisciplinary evaluation and close clinical management reduce the potential for imprecise diagnoses, particularly at critical developmental junctures. AAP suggests that PCPs can treat mild and moderate cases of ADHD, but if the treating clinician does not have adequate training, experience, time, or clinical support to manage this condition, early referral is warranted.6
A guide to pharmacotherapy
Approximately 77% of children ages 2 to 17 years with a diagnosis of ADHD receive any form of treatment.2 Treatment for ADHD can include behavioral therapy and medication.2 AAP clinical practice guidelines caution against prescribing medications for children younger than 6 years, relying instead on caregiver-, teacher-, or clinician-administered behavioral strategies and parental training in behavioral modification. For children and adolescents between ages 6 and 18 years, first-line treatment includes pharmacotherapy balanced with behavioral therapy, academic modifications, and educational supports (eg, 504 Plan, individualized education plan [IEP]).6
Psychostimulants are preferred. These agents (eg, methylphenidate, amphetamine) remain the most efficacious class of medications to reduce hyperactivity and inattentiveness and to improve function. While long-acting psychostimulants are associated with better medication adherence and adverse-effect tolerance than are short-acting forms, the latter offer more flexibility in dosing. Start by titrating any stimulant to the lowest effective dose; reassess monthly until potential rebound effects stabilize.
Due to potential adverse effects of this class of medication, screen for any family history or personal risk for structural or electrical cardiac anomalies before starting pharmacotherapy. If any such risks exist, arrange for further cardiac evaluation before initiating medication.6 Adverse effects of stimulants include reduced appetite, gastrointestinal symptoms, headaches, anxiousness, parasomnia, tachycardia, and hypertension.
Continue to: Once medication is stabilized...
Once medication is stabilized, monitor treatment 2 to 3 times per year thereafter; watch for longer-term adverse effects such as weight loss, decreased growth rate, and psychiatric comorbidities including the Food and Drug Administration (FDA)’s black box warning of increased risk for suicidality.5,6,22
Other options. The optimal duration of psychostimulant use remains debatable, as existing evidence does not support its long-term use (10 years) over other interventions, such as nonstimulants and nonmedicinal therapies.22 Although backed by less evidence, additional medications indicated for the treatment of ADHD include: (1) atomoxetine, a selective norepinephrine reuptake inhibitor, and (2) the selective alpha-2 adrenergic agonists, extended-release guanfacine and extended-release clonidine (third-line agent).22
Adverse effects of these FDA-approved medications are similar to those observed in stimulant medications. Evaluation of cardiac risks is recommended before starting nonstimulant medications. The alpha-2 adrenergic agonists may also be used as adjunct therapies to stimulants. Before stopping an alpha-2 adrenergic agonist, taper the dosage slowly to avoid the risk for rebound hypertension.6,23 Given the wide variety of medication options and variability of effects, it may be necessary to try different medications as children grow and their symptoms and capacity to manage them change. Additional guidance on FDA-approved medications is available at www.ADHDMedicationGuide.com.
How multilevel care coordination can work
As with other chronic or developmental conditions, the treatment of ADHD requires an interdisciplinary perspective. Continuous, comprehensive case management can help patients overcome obstacles to wellness by balancing the resolution of problems with the development of resilience. Well-documented collaboration of subspecialists, educators, and other stakeholders engaged in ADHD care at multiple levels (individual, family, community, and health care system) increases the likelihood of meaningful, sustainable gains. Using a patient-centered medical home framework, IBHCs or other allied health professionals embedded in, or co-located with, primary care settings can be key to accessing evidence-based treatments that include: psycho-education and mindfulness-based stress reduction training for caregivers24,25; occupational,26 cognitive behavioral,27 or family therapies28,29; neuro-feedback; computer-based attention training; group- or community-based interventions; and academic and social supports.5,8
Treatment approaches that capitalize on children’s neurologic and psychological plasticity and fortify self-efficacy with developmentally appropriate tools empower them to surmount ADHD symptoms over time.23 Facilitating children’s resilience within a developmental framework and health system’s capacities with socio-culturally relevant approaches, consultation, and research can optimize outcomes and mitigate pervasiveness into adulthood. While the patient is at the center of treatment, it is important to consider the family, school, and communities in which the child lives, learns, and plays. PCPs and IBHCs together can consider a “try and track” method to follow progress, changes, and outcomes over time. With this method, the physician can employ approaches that focus on the patient, caregiver, or the caregiver–child interaction (TABLE 3).
Continue to: Assess patients' needs and the resources available
Assess patients’ needs and the resources available throughout the system of care beyond the primary care setting. Stay abreast of hospital policies, health care insurance coverage, and community- and school-based health programs, and any gaps in adequate and equitable assessment and treatment. For example, while clinical recommendations include psychiatric care, health insurance availability or limits in coverage may dissuade caregivers from seeking help or limit initial or long-term access to resources for help.30 Integrating or advocating for clinic support resources or staffing to assist patients in navigating and mitigating challenges may lessen the management burden and increase the likelihood and longevity of favorable health outcomes.
Steps to ensuring health care equity
Among children of historically marginalized and racial and ethnic minority groups or those of populations affected by health disparities, ADHD symptoms and needs are often masked by structural biases that lead to inequitable care and outcomes, as well as treatment misprioritization or delays.31 In particular, evidence has shown that recognition and diagnostic specificity of ADHD and comorbidities, not prevalence, vary more widely among minority than among nonminority populations,32 contributing to the 23% of children with ADHD who receive no treatment at all.2
Understand caregiver concerns. This diagnosis discrepancy is correlated with symptom rating sensitivities (eg, reliability, perception, accuracy) among informants and how caregivers observe, perceive, appreciate, understand, and report behaviors. This discrepancy is also related to cultural belief differences, physician–patient communication variants, and a litany of other socioeconomic determinants.2,4,31 Caregivers from some cultural, ethnic, or socioeconomic backgrounds may be doubtful of psychiatric assessment, diagnoses, treatment, or medication, and that can impact how children are engaged in clinical and educational settings from the outset.31 In the case we described, James’ mother was initially hesitant to explore psychotropic medications and was concerned about stigmatization within the school system. She also seemed to avoid psychiatric treatment for her own depressive symptoms due to cultural and religious beliefs.
Health care provider concerns. Some PCPs may hesitate to explore medications due to limited knowledge and skill in dosing and titrating based on a child’s age, stage, and symptoms, and a perceived lack of competence in managing ADHD. This, too, can indirectly perpetuate existing health disparities. Furthermore, ADHD symptoms may be deemed a secondary or tertiary concern if other complex or urgent medical or undifferentiated developmental problems manifest.
Compounding matters is the limited dissemination of empiric research articles (including randomized controlled trials with representative samples) and limited education on the effectiveness and safety of psychopharmacologic interventions across the lifespan and different cultural and ethnic groups.4 Consequently, patients who struggle with unmanaged ADHD symptoms are more likely to have chronic mental health disorders, maladaptive behaviors, and other co-occurring conditions contributing to the complexity of individual needs, health care burdens, or justice system involvement; this is particularly true for those of racial and ethnic minorities.33
Continue to: Impact of the COVID-19 pandemic
Impact of the COVID-19 pandemic. Patients—particularly those in minority or health disparity populations—who under normal circumstances might have been hesitant to seek help may have felt even more reluctant to do so during the COVID-19 pandemic. We have not yet learned the degree to which limited availability of preventive health care services, decreased routine visits, and fluctuating insurance coverage has impacted the diagnosis, management, or severity of childhood disorders during the past 2 years. Reports of national findings indicate that prolonged periods out of school and reduced daily structure were associated with increased disruptions in mood, sleep, and appetite, particularly among children with pre-existing pathologies. Evidence suggests that school-aged children experienced more anxiety, regressive behaviors, and parasomnias than they did before the pandemic, while adolescents experienced more isolation and depressive symptoms.34,35
However, there remains a paucity of large-scale or representative studies that use an intersectional lens to examine the influence of COVID-19 on children with ADHD. Therefore, PCPs and IBHCs should refocus attention on possibly undiagnosed, stagnated, or regressed ADHD cases, as well as the adults who care for them. (See “5 ways to overcome Tx barriers and promote health equity.”)
SIDEBAR
5 ways to overcome Tx barriers and promote health equitya
1. Inquire about cultural or ethnic beliefs and behaviors and socioeconomic barriers.
2. Establish trust or assuage mistrust by exploring and dispelling misinformation.
3. Offer accessible, feasible, and sustainable evidence-based interventions.
4. Encourage autonomy and selfdetermination throughout the health care process.
5. Connect caregivers and children with clinical, community, and school-based resources and coordinators.
a These recommendations are based on the authors’ combined clinical experience.
THE CASE
During a follow-up visit 1 month later, the PCP confirmed the clinical impression of ADHD combined presentation with a clinical interview and review of the Strengths and Difficulties Questionnaire completed by James’ mother and the Vanderbilt Assessment Scales completed by James’ mother and teacher. The sleep diary indicated potential problems and apneas worthy of consults for pulmonary function testing, a sleep study, and otolaryngology examination. The PCP informed James’ mother on sleep hygiene strategies and ADHD medication options. She indicated that she wanted to pursue the referrals and behavioral modifications before starting any medication trial.
The PCP referred James to a developmental pediatrician for in-depth assessment of his overall development, learning, and functioning. The developmental pediatrician ultimately confirmed the diagnosis of ADHD, as well as motor and speech delays warranting physical, occupational, and speech therapies. The developmental pediatrician also referred James for targeted genetic testing because she suspected a genetic disorder (eg, XYY syndrome).
The PCP reconnected James and his mother to the IBHC to facilitate subspecialty and school-based care coordination and to provide in-office and home-based interventions. The IBHC assessed James’ emotional dysregulation and impulsivity as adversely impacting his interpersonal relationships and planned to address these issues with behavioral and parent–child interaction therapies and skills training during the course of 6 to 12 visits. James’ mother was encouraged to engage his teacher on his academic performance and to initiate a 504 Plan or IEP for in-school accommodations and support. The IBHC aided in tracking his assessments, referrals, follow-ups, access barriers, and treatment goals.
After 6 months, James had made only modest progress, and his mother requested that he begin a trial of medication. Based on his weight, symptoms, behavior patterns, and sleep habits, the PCP prescribed extended-release dexmethylphenidate 10 mg each morning, then extended-release clonidine 0.1 mg nightly. With team-based clinical management of pharmacologic, behavioral, physical, speech, and occupational therapies, James’ behavior and sleep improved, and the signs of a vocal tic diminished.
By the next school year, James demonstrated a marked improvement in impulse control, attention, and academic functioning. He followed up with the PCP at least quarterly for reassessment of his symptoms, growth, and experience of adverse effects, and to titrate medications accordingly. James and his mother continued to work closely with the IBHC monthly to engage interventions and to monitor his progress at home and school.
CORRESPONDENCE
Sundania J. W. Wonnum, PhD, LCSW, National Institute on Minority Health and Health Disparities, 6707 Democracy Boulevard, Suite 800, Bethesda, MD 20892; [email protected]
1. Bitsko RH, Claussen AH, Lichstein J, et al. Mental health surveillance among children—United States, 2013-2019. MMWR Suppl. 2022;71:1-42. doi: 10.15585/mmwr.su7102a1
2. Danielson ML, Holbrook JR, Blumberg SJ, et al. State-level estimates of the prevalence of parent-reported ADHD diagnosis and treatment among U.S. children and adolescents, 2016 to 2019. J Atten Disord. 2022;26:1685-1697. doi: 10.1177/10870547221099961
3. Faraone SV, Banaschewski T, Coghill D, et al. The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. 2021;128:789-818. doi: 10.1016/j.neubiorev.2021.01.022
4. American Psychiatric Association
5. Brahmbhatt K, Hilty DM, Mina H, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: a concise review. J Adolesc Health. 2016;59:135-143. doi: 10.1016/j.jadohealth.2016.03.025
6. Wolraich ML, Hagan JF, Allan C, et al. AAP Subcommittee on Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics. 2019;144:e20192528. doi: 10.1542/peds.2019-2528
7. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi: 10.7189/jogh.11.04009
8. Chang JG, Cimino FM, Gossa W. ADHD in children: common questions and answers. Am Fam Physician. 2020;102:592-602.
9. Asarnow JR, Rozenman M, Wiblin J, et al. Integrated medical-behavioral care compared with usual primary care for child and adolescent behavioral health: a meta-analysis. JAMA Pediatr. 2015;169:929-937. doi: 10.1001/jamapediatrics.2015.1141
10. Squires J, Bricker D. Ages & Stages Questionnaires®. 3rd ed (ASQ®-3). Paul H. Brookes Publishing Co., Inc; 2009.
11. DuPaul GJ, Barkley RA. Situational variability of attention problems: psychometric properties of the Revised Home and School Situations Questionnaires. J Clin Child Psychol. 1992;21:178-188. doi.org/10.1207/s15374424jccp2102_10
12. Merenda PF. BASC: behavior assessment system for children. Meas Eval Counsel Develop. 1996;28:229-232.
13. Conners CK. Conners, 3rd ed manual. Multi-Health Systems. 2008.
14. Achenbach TM. The Child Behavior Checklist and related instruments. In: Maruish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Lawrence Erlbaum Associates Publishers; 1999:429-466.
15. Goodman R. The extended version of the Strengths and Difficulties Questionnaire as a guide to child psychiatric caseness and consequent burden. J Child Psychol Psychiatry. 1999;40:791-799.
16. Wolraich ML, Lambert W, Doffing MA, et al. Psychometric properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a referred population. J Pediatr Psychol. 2003;28:559-567. doi: 10.1093/jpepsy/jsg046
17. Sparrow SS, Cicchetti DV. The Vineland Adaptive Behavior Scales. In: Newmark CS, ed. Major Psychological Assessment Instruments. Vol 2. Allyn & Bacon; 2003:199-231.
18. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47:199-212. doi: 10.1080/15374416.2017.1417860
19. Ghriwati NA, Langberg JM, Gardner W, et al. Impact of mental health comorbidities on the community-based pediatric treatment and outcomes of children with attention deficit hyperactivity disorder. J Dev Behav Ped. 2017;38:20-28. doi: 10.1097/DBP.0000000000000359
20. Niclasen J, Obel C, Homøe P, et al. Associations between otitis media and child behavioural and learning difficulties: results from a Danish Cohort. Int J Ped Otorhinolaryngol. 2016;84:12-20. doi: 10.1016/j.ijporl.2016.02.017
21. Ross JL Roeltgen DP Kushner H, et al. Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. doi: 10.1542/peds.2011-0719
22. Mechler K, Banaschewski T, Hohmann S, et al. Evidence-based pharmacological treatment options for ADHD in children and adolescents. Pharmacol Ther. 2022;230:107940. doi: 10.1016/j.pharmthera.2021.107940
23. Mishra J, Merzenich MM, Sagar R. Accessible online neuroplasticity-targeted training for children with ADHD. Child Adolesc Psychiatry Ment Health. 2013;7:38. doi: 10.1186/1753-2000-7-38
24. Neece CL. Mindfulness-based stress reduction for parents of young children with developmental delays: implications for parental mental health and child behavior problems. J Applied Res Intellect Disabil. 2014;27:174-186. doi: 10.1111/jar.12064
25. Petcharat M, Liehr P. Mindfulness training for parents of children with special needs: guidance for nurses in mental health practice. J Child Adolesc Psychiatr Nursing. 2017;30:35-46. doi: 10.1111/jcap.12169
26. Hahn-Markowitz J, Burger I, Manor I, et al. Efficacy of cognitive-functional (Cog-Fun) occupational therapy intervention among children with ADHD: an RCT. J Atten Disord. 2020;24:655-666. doi: 10.1177/1087054716666955
27. Young Z, Moghaddam N, Tickle A. The efficacy of cognitive behavioral therapy for adults with ADHD: a systematic review and meta-analysis of randomized controlled trials. J Atten Disord. 2020;24:875-888.
28. Carr AW, Bean RA, Nelson KF. Childhood attention-deficit hyperactivity disorder: family therapy from an attachment based perspective. Child Youth Serv Rev. 2020;119:105666.
29. Robin AL. Family therapy for adolescents with ADHD. Child Adolesc Psychiatr Clin N Am. 2014;23:747-756. doi: 10.1016/j.chc.2014.06.001
30. Cattoi B, Alpern I, Katz JS, et al. The adverse health outcomes, economic burden, and public health implications of unmanaged attention deficit hyperactivity disorder (ADHD): a call to action resulting from CHADD summit, Washington, DC, October 17, 2019. J Atten Disord. 2022;26:807-808. doi: 10.1177/10870547211036754
31. Hinojosa MS, Hinojosa R, Nguyen J. Shared decision making and treatment for minority children with ADHD. J Transcult Nurs. 2020;31:135-143. doi: 10.1177/1043659619853021
32. Slobodin O, Masalha R. Challenges in ADHD care for ethnic minority children: a review of the current literature. Transcult Psychiatry. 2020;57:468-483. doi: 10.1177/1363461520902885
33. Retz W, Ginsberg Y, Turner D, et al. Attention-deficit/hyperactivity disorder (ADHD), antisociality and delinquent behavior over the lifespan. Neurosci Biobehav Rev. 2021;120:236-248. doi: 10.1016/j.neubiorev.2020.11.025
34. Del Sol Calderon P, Izquierdo A, Garcia Moreno M. Effects of the pandemic on the mental health of children and adolescents. Review and current scientific evidence of the SARS-COV2 pandemic. Eur Psychiatry. 2021;64:S223-S224. doi: 10.1192/j.eurpsy.2021.597
35. Insa I, Alda JA. Attention deficit hyperactivity disorder (ADHD) & COVID-19: attention deficit hyperactivity disorder: consequences of the 1st wave. Eur Psychiatry. 2021;64:S660. doi: 10.1192/j.eurpsy.2021.1752
THE CASE
James B* is a 7-year-old Black child who presented to his primary care physician (PCP) for a well-child visit. During preventive health screening, James’ mother expressed concerns about his behavior, characterizing him as immature, aggressive, destructive, and occasionally self-loathing. She described him as physically uncoordinated, struggling to keep up with his peers in sports, and tiring after 20 minutes of activity. James slept 10 hours nightly but was often restless and snored intermittently. As a second grader, his academic achievement was not progressing, and he had become increasingly inattentive at home and at school. James’ mother offered several examples of his fighting with his siblings, noncompliance with morning routines, and avoidance of learning activities. Additionally, his mother expressed concern that James, as a Black child, might eventually be unfairly labeled as a problem child by his teachers or held back a grade level in school.
Although James did not have a family history of developmental delays or learning disorders, he had not met any milestones on time for gross or fine motor, language, cognitive, and social-emotional skills. James had a history of chronic otitis media, for which pressure equalizer tubes were inserted at age 2 years. He had not had any major physical injuries, psychological trauma, recent life transitions, or adverse childhood events. When asked, James’ mother acknowledged symptoms of maternal depression but alluded to faith-based reasons for not seeking treatment for herself.
James’ physical examination was unremarkable. His height, weight, and vitals were all within normal limits. However, he had some difficulty with verbal articulation and expression and showed signs of a possible vocal tic. Based on James’ presentation, his PCP suspected attention-deficit/hyperactivity disorder (ADHD), as well as neurodevelopmental delays.
The PCP gave James’ mother the Strengths and Difficulties Questionnaire to complete and the Vanderbilt Assessment Scales for her and James’ teacher to fill out independently and return to the clinic. The PCP also instructed James’ mother on how to use a sleep diary to maintain a 1-month log of his sleep patterns and habits. The PCP consulted the integrated behavioral health clinician (IBHC; a clinical social worker embedded in the primary care clinic) and made a warm handoff for the IBHC to further assess James’ maladaptive behaviors and interactions.
●
* The patient’s name has been changed to protect his identity.
James is one of more than 6 million children, ages 3 to 17 years, in the United States who live with ADHD.1,2 ADHD is the most common neurodevelopmental disorder among children, and it affects multiple cognitive and behavioral domains throughout the lifespan.3 Children with ADHD often initially present in primary care settings; thus, PCPs are well positioned to diagnose the disorder and provide longitudinal treatment. This Behavioral Health Consult reviews clinical assessment and practice guidelines, as well as treatment recommendations applicable across different areas of influence—individual, family, community, and systems—for PCPs and IBHCs to use in managing ADHD in children.
ADHD features can vary by age and sex
ADHD is a persistent pattern of inattention or hyperactivity and impulsivity interfering with functioning or development in childhood and functioning later in adulthood. ADHD symptoms manifest prior to age 12 years and must occur in 2 or more settings.4 Symptoms should not be better explained by another psychiatric disorder or occur exclusively during the course of another disorder (TABLE 1).4

The rate of heritability is high, with significant incidence among first-degree relatives.4 Children with ADHD show executive functioning deficits in 1 or more cognitive domains (eg, visuospatial, memory, inhibitions, decision making, and reward regulation).4,5 The prevalence of ADHD nationally is approximately 9.8% (2.2%, ages 3-5 years; 10%, ages 6-11 years; 13.2%, ages 12-17 years) in children and adolescents; worldwide prevalence is 7.2%.1,6 It persists among 2.6% to 6.8% of adults worldwide.7
Research has shown that boys ages 6 to 11 years are significantly more likely than girls to exhibit attention-getting, externalizing behaviors or conduct problems (eg, hyperactivity, impulsivity, disruption, aggression).1,6 On the other hand, girls ages 12 to 17 years tend to display internalized (eg, depressed mood, anxiety, low self-esteem) or inattentive behaviors, which clinicians and educators may assess as less severe and warranting fewer supportive measures.1
The prevalence of ADHD and its associated factors, which evolve through maturation, underscore the importance of persistent, patient-centered, and collaborative PCP and IBHC clinical management.
Continue to: Begin with a screening tool, move to a clinical interview
Begin with a screening tool, move to a clinical interview
When caregivers express concerns about their child’s behavior, focus, mood, learning, and socialization, consider initiating a multimodal evaluation for ADHD.5,8 Embarking on an ADHD assessment can require extended or multiple visits to arrive at the diagnosis, followed by still more visits to confirm a course of care and adjust medications. The integrative care approach described in the patient case and elaborated on later in this article can help facilitate assessment and treatment of ADHD.9
Signs of ADHD may be observed at initial screening using a tool such as the Ages & Stages Questionnaire (https://agesandstages.com/products-pricing/asq3/) to reveal indications of norm deviations or delays commensurate with ADHD.10 However, to substantiate the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision criteria for an accurate diagnosis,4 the American Academy of Pediatrics (AAP) clinical practice guidelines require a thorough clinical interview, administration of a standardized assessment tool, and review of objective reports in conjunction with a physical examination and psychosocial evaluation.6 Standardized measures of psychological, neurocognitive, and academic achievement reported by caregivers and collateral contacts (eg, teachers, counselors, coaches, care providers) are needed to maximize data objectivity and symptom accuracy across settings (TABLE 210-17). Additionally, periodic reassessment is recommended to validate changes in diagnostic subtype and treatment plans due to the chronic and dynamic nature of ADHD.

Consider comorbidities and alternate diagnoses
The diagnostic possibility of ADHD should also prompt consideration of other childhood disorders due to the high potential for comorbidities.4,6 In a 2016 study, approximately 64% of
Various medical disorders may manifest with similar signs or symptoms to ADHD, such as thyroid disorders, seizure disorders, adverse drug effects, anemia, genetic anomalies, and others.6,19
If there are behavioral concerns or developmental delays associated with tall stature for age or pubertal or testicular development anomalies, consult a geneticist and a developmental pediatrician for targeted testing and neurodevelopmental assessment, respectively. For example, ADHD is a common comorbidity among boys who also have XYY syndrome (Jacobs syndrome). However, due to the variability of symptoms and severity, XYY syndrome often goes undiagnosed, leaving a host of compounding pervasive and developmental problems untreated. Overall, more than two-thirds of patients with ADHD and a co-occurring condition are either inaccurately diagnosed or not referred for additional assessment and adjunct treatment.21
Continue to: Risks that arise over time
Risks that arise over time. As ADHD persists, adolescents are at greater risk for psychiatric comorbidities, suicidality, and functional impairments (eg, risky behaviors, occupational problems, truancy, delinquency, and poor self-esteem).4,8 Adolescents with internalized behaviors are more likely to experience comorbid depressive disorders with increased risk for self-harm.4,5,8 As adolescents age and their sense of autonomy increases, there is a tendency among those who have received a diagnosis of ADHD to minimize symptoms and decrease the frequency of routine clinic visits along with medication use and treatment compliance.3 Additionally, abuse, misuse, and misappropriation of stimulants among teens and young adults are commonplace.
Wide-scope, multidisciplinary evaluation and close clinical management reduce the potential for imprecise diagnoses, particularly at critical developmental junctures. AAP suggests that PCPs can treat mild and moderate cases of ADHD, but if the treating clinician does not have adequate training, experience, time, or clinical support to manage this condition, early referral is warranted.6
A guide to pharmacotherapy
Approximately 77% of children ages 2 to 17 years with a diagnosis of ADHD receive any form of treatment.2 Treatment for ADHD can include behavioral therapy and medication.2 AAP clinical practice guidelines caution against prescribing medications for children younger than 6 years, relying instead on caregiver-, teacher-, or clinician-administered behavioral strategies and parental training in behavioral modification. For children and adolescents between ages 6 and 18 years, first-line treatment includes pharmacotherapy balanced with behavioral therapy, academic modifications, and educational supports (eg, 504 Plan, individualized education plan [IEP]).6
Psychostimulants are preferred. These agents (eg, methylphenidate, amphetamine) remain the most efficacious class of medications to reduce hyperactivity and inattentiveness and to improve function. While long-acting psychostimulants are associated with better medication adherence and adverse-effect tolerance than are short-acting forms, the latter offer more flexibility in dosing. Start by titrating any stimulant to the lowest effective dose; reassess monthly until potential rebound effects stabilize.
Due to potential adverse effects of this class of medication, screen for any family history or personal risk for structural or electrical cardiac anomalies before starting pharmacotherapy. If any such risks exist, arrange for further cardiac evaluation before initiating medication.6 Adverse effects of stimulants include reduced appetite, gastrointestinal symptoms, headaches, anxiousness, parasomnia, tachycardia, and hypertension.
Continue to: Once medication is stabilized...
Once medication is stabilized, monitor treatment 2 to 3 times per year thereafter; watch for longer-term adverse effects such as weight loss, decreased growth rate, and psychiatric comorbidities including the Food and Drug Administration (FDA)’s black box warning of increased risk for suicidality.5,6,22
Other options. The optimal duration of psychostimulant use remains debatable, as existing evidence does not support its long-term use (10 years) over other interventions, such as nonstimulants and nonmedicinal therapies.22 Although backed by less evidence, additional medications indicated for the treatment of ADHD include: (1) atomoxetine, a selective norepinephrine reuptake inhibitor, and (2) the selective alpha-2 adrenergic agonists, extended-release guanfacine and extended-release clonidine (third-line agent).22
Adverse effects of these FDA-approved medications are similar to those observed in stimulant medications. Evaluation of cardiac risks is recommended before starting nonstimulant medications. The alpha-2 adrenergic agonists may also be used as adjunct therapies to stimulants. Before stopping an alpha-2 adrenergic agonist, taper the dosage slowly to avoid the risk for rebound hypertension.6,23 Given the wide variety of medication options and variability of effects, it may be necessary to try different medications as children grow and their symptoms and capacity to manage them change. Additional guidance on FDA-approved medications is available at www.ADHDMedicationGuide.com.
How multilevel care coordination can work
As with other chronic or developmental conditions, the treatment of ADHD requires an interdisciplinary perspective. Continuous, comprehensive case management can help patients overcome obstacles to wellness by balancing the resolution of problems with the development of resilience. Well-documented collaboration of subspecialists, educators, and other stakeholders engaged in ADHD care at multiple levels (individual, family, community, and health care system) increases the likelihood of meaningful, sustainable gains. Using a patient-centered medical home framework, IBHCs or other allied health professionals embedded in, or co-located with, primary care settings can be key to accessing evidence-based treatments that include: psycho-education and mindfulness-based stress reduction training for caregivers24,25; occupational,26 cognitive behavioral,27 or family therapies28,29; neuro-feedback; computer-based attention training; group- or community-based interventions; and academic and social supports.5,8
Treatment approaches that capitalize on children’s neurologic and psychological plasticity and fortify self-efficacy with developmentally appropriate tools empower them to surmount ADHD symptoms over time.23 Facilitating children’s resilience within a developmental framework and health system’s capacities with socio-culturally relevant approaches, consultation, and research can optimize outcomes and mitigate pervasiveness into adulthood. While the patient is at the center of treatment, it is important to consider the family, school, and communities in which the child lives, learns, and plays. PCPs and IBHCs together can consider a “try and track” method to follow progress, changes, and outcomes over time. With this method, the physician can employ approaches that focus on the patient, caregiver, or the caregiver–child interaction (TABLE 3).

Continue to: Assess patients' needs and the resources available
Assess patients’ needs and the resources available throughout the system of care beyond the primary care setting. Stay abreast of hospital policies, health care insurance coverage, and community- and school-based health programs, and any gaps in adequate and equitable assessment and treatment. For example, while clinical recommendations include psychiatric care, health insurance availability or limits in coverage may dissuade caregivers from seeking help or limit initial or long-term access to resources for help.30 Integrating or advocating for clinic support resources or staffing to assist patients in navigating and mitigating challenges may lessen the management burden and increase the likelihood and longevity of favorable health outcomes.
Steps to ensuring health care equity
Among children of historically marginalized and racial and ethnic minority groups or those of populations affected by health disparities, ADHD symptoms and needs are often masked by structural biases that lead to inequitable care and outcomes, as well as treatment misprioritization or delays.31 In particular, evidence has shown that recognition and diagnostic specificity of ADHD and comorbidities, not prevalence, vary more widely among minority than among nonminority populations,32 contributing to the 23% of children with ADHD who receive no treatment at all.2
Understand caregiver concerns. This diagnosis discrepancy is correlated with symptom rating sensitivities (eg, reliability, perception, accuracy) among informants and how caregivers observe, perceive, appreciate, understand, and report behaviors. This discrepancy is also related to cultural belief differences, physician–patient communication variants, and a litany of other socioeconomic determinants.2,4,31 Caregivers from some cultural, ethnic, or socioeconomic backgrounds may be doubtful of psychiatric assessment, diagnoses, treatment, or medication, and that can impact how children are engaged in clinical and educational settings from the outset.31 In the case we described, James’ mother was initially hesitant to explore psychotropic medications and was concerned about stigmatization within the school system. She also seemed to avoid psychiatric treatment for her own depressive symptoms due to cultural and religious beliefs.
Health care provider concerns. Some PCPs may hesitate to explore medications due to limited knowledge and skill in dosing and titrating based on a child’s age, stage, and symptoms, and a perceived lack of competence in managing ADHD. This, too, can indirectly perpetuate existing health disparities. Furthermore, ADHD symptoms may be deemed a secondary or tertiary concern if other complex or urgent medical or undifferentiated developmental problems manifest.
Compounding matters is the limited dissemination of empiric research articles (including randomized controlled trials with representative samples) and limited education on the effectiveness and safety of psychopharmacologic interventions across the lifespan and different cultural and ethnic groups.4 Consequently, patients who struggle with unmanaged ADHD symptoms are more likely to have chronic mental health disorders, maladaptive behaviors, and other co-occurring conditions contributing to the complexity of individual needs, health care burdens, or justice system involvement; this is particularly true for those of racial and ethnic minorities.33
Continue to: Impact of the COVID-19 pandemic
Impact of the COVID-19 pandemic. Patients—particularly those in minority or health disparity populations—who under normal circumstances might have been hesitant to seek help may have felt even more reluctant to do so during the COVID-19 pandemic. We have not yet learned the degree to which limited availability of preventive health care services, decreased routine visits, and fluctuating insurance coverage has impacted the diagnosis, management, or severity of childhood disorders during the past 2 years. Reports of national findings indicate that prolonged periods out of school and reduced daily structure were associated with increased disruptions in mood, sleep, and appetite, particularly among children with pre-existing pathologies. Evidence suggests that school-aged children experienced more anxiety, regressive behaviors, and parasomnias than they did before the pandemic, while adolescents experienced more isolation and depressive symptoms.34,35
However, there remains a paucity of large-scale or representative studies that use an intersectional lens to examine the influence of COVID-19 on children with ADHD. Therefore, PCPs and IBHCs should refocus attention on possibly undiagnosed, stagnated, or regressed ADHD cases, as well as the adults who care for them. (See “5 ways to overcome Tx barriers and promote health equity.”)
SIDEBAR
5 ways to overcome Tx barriers and promote health equitya
1. Inquire about cultural or ethnic beliefs and behaviors and socioeconomic barriers.
2. Establish trust or assuage mistrust by exploring and dispelling misinformation.
3. Offer accessible, feasible, and sustainable evidence-based interventions.
4. Encourage autonomy and selfdetermination throughout the health care process.
5. Connect caregivers and children with clinical, community, and school-based resources and coordinators.
a These recommendations are based on the authors’ combined clinical experience.
THE CASE
During a follow-up visit 1 month later, the PCP confirmed the clinical impression of ADHD combined presentation with a clinical interview and review of the Strengths and Difficulties Questionnaire completed by James’ mother and the Vanderbilt Assessment Scales completed by James’ mother and teacher. The sleep diary indicated potential problems and apneas worthy of consults for pulmonary function testing, a sleep study, and otolaryngology examination. The PCP informed James’ mother on sleep hygiene strategies and ADHD medication options. She indicated that she wanted to pursue the referrals and behavioral modifications before starting any medication trial.
The PCP referred James to a developmental pediatrician for in-depth assessment of his overall development, learning, and functioning. The developmental pediatrician ultimately confirmed the diagnosis of ADHD, as well as motor and speech delays warranting physical, occupational, and speech therapies. The developmental pediatrician also referred James for targeted genetic testing because she suspected a genetic disorder (eg, XYY syndrome).
The PCP reconnected James and his mother to the IBHC to facilitate subspecialty and school-based care coordination and to provide in-office and home-based interventions. The IBHC assessed James’ emotional dysregulation and impulsivity as adversely impacting his interpersonal relationships and planned to address these issues with behavioral and parent–child interaction therapies and skills training during the course of 6 to 12 visits. James’ mother was encouraged to engage his teacher on his academic performance and to initiate a 504 Plan or IEP for in-school accommodations and support. The IBHC aided in tracking his assessments, referrals, follow-ups, access barriers, and treatment goals.
After 6 months, James had made only modest progress, and his mother requested that he begin a trial of medication. Based on his weight, symptoms, behavior patterns, and sleep habits, the PCP prescribed extended-release dexmethylphenidate 10 mg each morning, then extended-release clonidine 0.1 mg nightly. With team-based clinical management of pharmacologic, behavioral, physical, speech, and occupational therapies, James’ behavior and sleep improved, and the signs of a vocal tic diminished.
By the next school year, James demonstrated a marked improvement in impulse control, attention, and academic functioning. He followed up with the PCP at least quarterly for reassessment of his symptoms, growth, and experience of adverse effects, and to titrate medications accordingly. James and his mother continued to work closely with the IBHC monthly to engage interventions and to monitor his progress at home and school.
CORRESPONDENCE
Sundania J. W. Wonnum, PhD, LCSW, National Institute on Minority Health and Health Disparities, 6707 Democracy Boulevard, Suite 800, Bethesda, MD 20892; [email protected]
THE CASE
James B* is a 7-year-old Black child who presented to his primary care physician (PCP) for a well-child visit. During preventive health screening, James’ mother expressed concerns about his behavior, characterizing him as immature, aggressive, destructive, and occasionally self-loathing. She described him as physically uncoordinated, struggling to keep up with his peers in sports, and tiring after 20 minutes of activity. James slept 10 hours nightly but was often restless and snored intermittently. As a second grader, his academic achievement was not progressing, and he had become increasingly inattentive at home and at school. James’ mother offered several examples of his fighting with his siblings, noncompliance with morning routines, and avoidance of learning activities. Additionally, his mother expressed concern that James, as a Black child, might eventually be unfairly labeled as a problem child by his teachers or held back a grade level in school.
Although James did not have a family history of developmental delays or learning disorders, he had not met any milestones on time for gross or fine motor, language, cognitive, and social-emotional skills. James had a history of chronic otitis media, for which pressure equalizer tubes were inserted at age 2 years. He had not had any major physical injuries, psychological trauma, recent life transitions, or adverse childhood events. When asked, James’ mother acknowledged symptoms of maternal depression but alluded to faith-based reasons for not seeking treatment for herself.
James’ physical examination was unremarkable. His height, weight, and vitals were all within normal limits. However, he had some difficulty with verbal articulation and expression and showed signs of a possible vocal tic. Based on James’ presentation, his PCP suspected attention-deficit/hyperactivity disorder (ADHD), as well as neurodevelopmental delays.
The PCP gave James’ mother the Strengths and Difficulties Questionnaire to complete and the Vanderbilt Assessment Scales for her and James’ teacher to fill out independently and return to the clinic. The PCP also instructed James’ mother on how to use a sleep diary to maintain a 1-month log of his sleep patterns and habits. The PCP consulted the integrated behavioral health clinician (IBHC; a clinical social worker embedded in the primary care clinic) and made a warm handoff for the IBHC to further assess James’ maladaptive behaviors and interactions.
●
* The patient’s name has been changed to protect his identity.
James is one of more than 6 million children, ages 3 to 17 years, in the United States who live with ADHD.1,2 ADHD is the most common neurodevelopmental disorder among children, and it affects multiple cognitive and behavioral domains throughout the lifespan.3 Children with ADHD often initially present in primary care settings; thus, PCPs are well positioned to diagnose the disorder and provide longitudinal treatment. This Behavioral Health Consult reviews clinical assessment and practice guidelines, as well as treatment recommendations applicable across different areas of influence—individual, family, community, and systems—for PCPs and IBHCs to use in managing ADHD in children.
ADHD features can vary by age and sex
ADHD is a persistent pattern of inattention or hyperactivity and impulsivity interfering with functioning or development in childhood and functioning later in adulthood. ADHD symptoms manifest prior to age 12 years and must occur in 2 or more settings.4 Symptoms should not be better explained by another psychiatric disorder or occur exclusively during the course of another disorder (TABLE 1).4

The rate of heritability is high, with significant incidence among first-degree relatives.4 Children with ADHD show executive functioning deficits in 1 or more cognitive domains (eg, visuospatial, memory, inhibitions, decision making, and reward regulation).4,5 The prevalence of ADHD nationally is approximately 9.8% (2.2%, ages 3-5 years; 10%, ages 6-11 years; 13.2%, ages 12-17 years) in children and adolescents; worldwide prevalence is 7.2%.1,6 It persists among 2.6% to 6.8% of adults worldwide.7
Research has shown that boys ages 6 to 11 years are significantly more likely than girls to exhibit attention-getting, externalizing behaviors or conduct problems (eg, hyperactivity, impulsivity, disruption, aggression).1,6 On the other hand, girls ages 12 to 17 years tend to display internalized (eg, depressed mood, anxiety, low self-esteem) or inattentive behaviors, which clinicians and educators may assess as less severe and warranting fewer supportive measures.1
The prevalence of ADHD and its associated factors, which evolve through maturation, underscore the importance of persistent, patient-centered, and collaborative PCP and IBHC clinical management.
Continue to: Begin with a screening tool, move to a clinical interview
Begin with a screening tool, move to a clinical interview
When caregivers express concerns about their child’s behavior, focus, mood, learning, and socialization, consider initiating a multimodal evaluation for ADHD.5,8 Embarking on an ADHD assessment can require extended or multiple visits to arrive at the diagnosis, followed by still more visits to confirm a course of care and adjust medications. The integrative care approach described in the patient case and elaborated on later in this article can help facilitate assessment and treatment of ADHD.9
Signs of ADHD may be observed at initial screening using a tool such as the Ages & Stages Questionnaire (https://agesandstages.com/products-pricing/asq3/) to reveal indications of norm deviations or delays commensurate with ADHD.10 However, to substantiate the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision criteria for an accurate diagnosis,4 the American Academy of Pediatrics (AAP) clinical practice guidelines require a thorough clinical interview, administration of a standardized assessment tool, and review of objective reports in conjunction with a physical examination and psychosocial evaluation.6 Standardized measures of psychological, neurocognitive, and academic achievement reported by caregivers and collateral contacts (eg, teachers, counselors, coaches, care providers) are needed to maximize data objectivity and symptom accuracy across settings (TABLE 210-17). Additionally, periodic reassessment is recommended to validate changes in diagnostic subtype and treatment plans due to the chronic and dynamic nature of ADHD.

Consider comorbidities and alternate diagnoses
The diagnostic possibility of ADHD should also prompt consideration of other childhood disorders due to the high potential for comorbidities.4,6 In a 2016 study, approximately 64% of
Various medical disorders may manifest with similar signs or symptoms to ADHD, such as thyroid disorders, seizure disorders, adverse drug effects, anemia, genetic anomalies, and others.6,19
If there are behavioral concerns or developmental delays associated with tall stature for age or pubertal or testicular development anomalies, consult a geneticist and a developmental pediatrician for targeted testing and neurodevelopmental assessment, respectively. For example, ADHD is a common comorbidity among boys who also have XYY syndrome (Jacobs syndrome). However, due to the variability of symptoms and severity, XYY syndrome often goes undiagnosed, leaving a host of compounding pervasive and developmental problems untreated. Overall, more than two-thirds of patients with ADHD and a co-occurring condition are either inaccurately diagnosed or not referred for additional assessment and adjunct treatment.21
Continue to: Risks that arise over time
Risks that arise over time. As ADHD persists, adolescents are at greater risk for psychiatric comorbidities, suicidality, and functional impairments (eg, risky behaviors, occupational problems, truancy, delinquency, and poor self-esteem).4,8 Adolescents with internalized behaviors are more likely to experience comorbid depressive disorders with increased risk for self-harm.4,5,8 As adolescents age and their sense of autonomy increases, there is a tendency among those who have received a diagnosis of ADHD to minimize symptoms and decrease the frequency of routine clinic visits along with medication use and treatment compliance.3 Additionally, abuse, misuse, and misappropriation of stimulants among teens and young adults are commonplace.
Wide-scope, multidisciplinary evaluation and close clinical management reduce the potential for imprecise diagnoses, particularly at critical developmental junctures. AAP suggests that PCPs can treat mild and moderate cases of ADHD, but if the treating clinician does not have adequate training, experience, time, or clinical support to manage this condition, early referral is warranted.6
A guide to pharmacotherapy
Approximately 77% of children ages 2 to 17 years with a diagnosis of ADHD receive any form of treatment.2 Treatment for ADHD can include behavioral therapy and medication.2 AAP clinical practice guidelines caution against prescribing medications for children younger than 6 years, relying instead on caregiver-, teacher-, or clinician-administered behavioral strategies and parental training in behavioral modification. For children and adolescents between ages 6 and 18 years, first-line treatment includes pharmacotherapy balanced with behavioral therapy, academic modifications, and educational supports (eg, 504 Plan, individualized education plan [IEP]).6
Psychostimulants are preferred. These agents (eg, methylphenidate, amphetamine) remain the most efficacious class of medications to reduce hyperactivity and inattentiveness and to improve function. While long-acting psychostimulants are associated with better medication adherence and adverse-effect tolerance than are short-acting forms, the latter offer more flexibility in dosing. Start by titrating any stimulant to the lowest effective dose; reassess monthly until potential rebound effects stabilize.
Due to potential adverse effects of this class of medication, screen for any family history or personal risk for structural or electrical cardiac anomalies before starting pharmacotherapy. If any such risks exist, arrange for further cardiac evaluation before initiating medication.6 Adverse effects of stimulants include reduced appetite, gastrointestinal symptoms, headaches, anxiousness, parasomnia, tachycardia, and hypertension.
Continue to: Once medication is stabilized...
Once medication is stabilized, monitor treatment 2 to 3 times per year thereafter; watch for longer-term adverse effects such as weight loss, decreased growth rate, and psychiatric comorbidities including the Food and Drug Administration (FDA)’s black box warning of increased risk for suicidality.5,6,22
Other options. The optimal duration of psychostimulant use remains debatable, as existing evidence does not support its long-term use (10 years) over other interventions, such as nonstimulants and nonmedicinal therapies.22 Although backed by less evidence, additional medications indicated for the treatment of ADHD include: (1) atomoxetine, a selective norepinephrine reuptake inhibitor, and (2) the selective alpha-2 adrenergic agonists, extended-release guanfacine and extended-release clonidine (third-line agent).22
Adverse effects of these FDA-approved medications are similar to those observed in stimulant medications. Evaluation of cardiac risks is recommended before starting nonstimulant medications. The alpha-2 adrenergic agonists may also be used as adjunct therapies to stimulants. Before stopping an alpha-2 adrenergic agonist, taper the dosage slowly to avoid the risk for rebound hypertension.6,23 Given the wide variety of medication options and variability of effects, it may be necessary to try different medications as children grow and their symptoms and capacity to manage them change. Additional guidance on FDA-approved medications is available at www.ADHDMedicationGuide.com.
How multilevel care coordination can work
As with other chronic or developmental conditions, the treatment of ADHD requires an interdisciplinary perspective. Continuous, comprehensive case management can help patients overcome obstacles to wellness by balancing the resolution of problems with the development of resilience. Well-documented collaboration of subspecialists, educators, and other stakeholders engaged in ADHD care at multiple levels (individual, family, community, and health care system) increases the likelihood of meaningful, sustainable gains. Using a patient-centered medical home framework, IBHCs or other allied health professionals embedded in, or co-located with, primary care settings can be key to accessing evidence-based treatments that include: psycho-education and mindfulness-based stress reduction training for caregivers24,25; occupational,26 cognitive behavioral,27 or family therapies28,29; neuro-feedback; computer-based attention training; group- or community-based interventions; and academic and social supports.5,8
Treatment approaches that capitalize on children’s neurologic and psychological plasticity and fortify self-efficacy with developmentally appropriate tools empower them to surmount ADHD symptoms over time.23 Facilitating children’s resilience within a developmental framework and health system’s capacities with socio-culturally relevant approaches, consultation, and research can optimize outcomes and mitigate pervasiveness into adulthood. While the patient is at the center of treatment, it is important to consider the family, school, and communities in which the child lives, learns, and plays. PCPs and IBHCs together can consider a “try and track” method to follow progress, changes, and outcomes over time. With this method, the physician can employ approaches that focus on the patient, caregiver, or the caregiver–child interaction (TABLE 3).

Continue to: Assess patients' needs and the resources available
Assess patients’ needs and the resources available throughout the system of care beyond the primary care setting. Stay abreast of hospital policies, health care insurance coverage, and community- and school-based health programs, and any gaps in adequate and equitable assessment and treatment. For example, while clinical recommendations include psychiatric care, health insurance availability or limits in coverage may dissuade caregivers from seeking help or limit initial or long-term access to resources for help.30 Integrating or advocating for clinic support resources or staffing to assist patients in navigating and mitigating challenges may lessen the management burden and increase the likelihood and longevity of favorable health outcomes.
Steps to ensuring health care equity
Among children of historically marginalized and racial and ethnic minority groups or those of populations affected by health disparities, ADHD symptoms and needs are often masked by structural biases that lead to inequitable care and outcomes, as well as treatment misprioritization or delays.31 In particular, evidence has shown that recognition and diagnostic specificity of ADHD and comorbidities, not prevalence, vary more widely among minority than among nonminority populations,32 contributing to the 23% of children with ADHD who receive no treatment at all.2
Understand caregiver concerns. This diagnosis discrepancy is correlated with symptom rating sensitivities (eg, reliability, perception, accuracy) among informants and how caregivers observe, perceive, appreciate, understand, and report behaviors. This discrepancy is also related to cultural belief differences, physician–patient communication variants, and a litany of other socioeconomic determinants.2,4,31 Caregivers from some cultural, ethnic, or socioeconomic backgrounds may be doubtful of psychiatric assessment, diagnoses, treatment, or medication, and that can impact how children are engaged in clinical and educational settings from the outset.31 In the case we described, James’ mother was initially hesitant to explore psychotropic medications and was concerned about stigmatization within the school system. She also seemed to avoid psychiatric treatment for her own depressive symptoms due to cultural and religious beliefs.
Health care provider concerns. Some PCPs may hesitate to explore medications due to limited knowledge and skill in dosing and titrating based on a child’s age, stage, and symptoms, and a perceived lack of competence in managing ADHD. This, too, can indirectly perpetuate existing health disparities. Furthermore, ADHD symptoms may be deemed a secondary or tertiary concern if other complex or urgent medical or undifferentiated developmental problems manifest.
Compounding matters is the limited dissemination of empiric research articles (including randomized controlled trials with representative samples) and limited education on the effectiveness and safety of psychopharmacologic interventions across the lifespan and different cultural and ethnic groups.4 Consequently, patients who struggle with unmanaged ADHD symptoms are more likely to have chronic mental health disorders, maladaptive behaviors, and other co-occurring conditions contributing to the complexity of individual needs, health care burdens, or justice system involvement; this is particularly true for those of racial and ethnic minorities.33
Continue to: Impact of the COVID-19 pandemic
Impact of the COVID-19 pandemic. Patients—particularly those in minority or health disparity populations—who under normal circumstances might have been hesitant to seek help may have felt even more reluctant to do so during the COVID-19 pandemic. We have not yet learned the degree to which limited availability of preventive health care services, decreased routine visits, and fluctuating insurance coverage has impacted the diagnosis, management, or severity of childhood disorders during the past 2 years. Reports of national findings indicate that prolonged periods out of school and reduced daily structure were associated with increased disruptions in mood, sleep, and appetite, particularly among children with pre-existing pathologies. Evidence suggests that school-aged children experienced more anxiety, regressive behaviors, and parasomnias than they did before the pandemic, while adolescents experienced more isolation and depressive symptoms.34,35
However, there remains a paucity of large-scale or representative studies that use an intersectional lens to examine the influence of COVID-19 on children with ADHD. Therefore, PCPs and IBHCs should refocus attention on possibly undiagnosed, stagnated, or regressed ADHD cases, as well as the adults who care for them. (See “5 ways to overcome Tx barriers and promote health equity.”)
SIDEBAR
5 ways to overcome Tx barriers and promote health equitya
1. Inquire about cultural or ethnic beliefs and behaviors and socioeconomic barriers.
2. Establish trust or assuage mistrust by exploring and dispelling misinformation.
3. Offer accessible, feasible, and sustainable evidence-based interventions.
4. Encourage autonomy and selfdetermination throughout the health care process.
5. Connect caregivers and children with clinical, community, and school-based resources and coordinators.
a These recommendations are based on the authors’ combined clinical experience.
THE CASE
During a follow-up visit 1 month later, the PCP confirmed the clinical impression of ADHD combined presentation with a clinical interview and review of the Strengths and Difficulties Questionnaire completed by James’ mother and the Vanderbilt Assessment Scales completed by James’ mother and teacher. The sleep diary indicated potential problems and apneas worthy of consults for pulmonary function testing, a sleep study, and otolaryngology examination. The PCP informed James’ mother on sleep hygiene strategies and ADHD medication options. She indicated that she wanted to pursue the referrals and behavioral modifications before starting any medication trial.
The PCP referred James to a developmental pediatrician for in-depth assessment of his overall development, learning, and functioning. The developmental pediatrician ultimately confirmed the diagnosis of ADHD, as well as motor and speech delays warranting physical, occupational, and speech therapies. The developmental pediatrician also referred James for targeted genetic testing because she suspected a genetic disorder (eg, XYY syndrome).
The PCP reconnected James and his mother to the IBHC to facilitate subspecialty and school-based care coordination and to provide in-office and home-based interventions. The IBHC assessed James’ emotional dysregulation and impulsivity as adversely impacting his interpersonal relationships and planned to address these issues with behavioral and parent–child interaction therapies and skills training during the course of 6 to 12 visits. James’ mother was encouraged to engage his teacher on his academic performance and to initiate a 504 Plan or IEP for in-school accommodations and support. The IBHC aided in tracking his assessments, referrals, follow-ups, access barriers, and treatment goals.
After 6 months, James had made only modest progress, and his mother requested that he begin a trial of medication. Based on his weight, symptoms, behavior patterns, and sleep habits, the PCP prescribed extended-release dexmethylphenidate 10 mg each morning, then extended-release clonidine 0.1 mg nightly. With team-based clinical management of pharmacologic, behavioral, physical, speech, and occupational therapies, James’ behavior and sleep improved, and the signs of a vocal tic diminished.
By the next school year, James demonstrated a marked improvement in impulse control, attention, and academic functioning. He followed up with the PCP at least quarterly for reassessment of his symptoms, growth, and experience of adverse effects, and to titrate medications accordingly. James and his mother continued to work closely with the IBHC monthly to engage interventions and to monitor his progress at home and school.
CORRESPONDENCE
Sundania J. W. Wonnum, PhD, LCSW, National Institute on Minority Health and Health Disparities, 6707 Democracy Boulevard, Suite 800, Bethesda, MD 20892; [email protected]
1. Bitsko RH, Claussen AH, Lichstein J, et al. Mental health surveillance among children—United States, 2013-2019. MMWR Suppl. 2022;71:1-42. doi: 10.15585/mmwr.su7102a1
2. Danielson ML, Holbrook JR, Blumberg SJ, et al. State-level estimates of the prevalence of parent-reported ADHD diagnosis and treatment among U.S. children and adolescents, 2016 to 2019. J Atten Disord. 2022;26:1685-1697. doi: 10.1177/10870547221099961
3. Faraone SV, Banaschewski T, Coghill D, et al. The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. 2021;128:789-818. doi: 10.1016/j.neubiorev.2021.01.022
4. American Psychiatric Association
5. Brahmbhatt K, Hilty DM, Mina H, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: a concise review. J Adolesc Health. 2016;59:135-143. doi: 10.1016/j.jadohealth.2016.03.025
6. Wolraich ML, Hagan JF, Allan C, et al. AAP Subcommittee on Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics. 2019;144:e20192528. doi: 10.1542/peds.2019-2528
7. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi: 10.7189/jogh.11.04009
8. Chang JG, Cimino FM, Gossa W. ADHD in children: common questions and answers. Am Fam Physician. 2020;102:592-602.
9. Asarnow JR, Rozenman M, Wiblin J, et al. Integrated medical-behavioral care compared with usual primary care for child and adolescent behavioral health: a meta-analysis. JAMA Pediatr. 2015;169:929-937. doi: 10.1001/jamapediatrics.2015.1141
10. Squires J, Bricker D. Ages & Stages Questionnaires®. 3rd ed (ASQ®-3). Paul H. Brookes Publishing Co., Inc; 2009.
11. DuPaul GJ, Barkley RA. Situational variability of attention problems: psychometric properties of the Revised Home and School Situations Questionnaires. J Clin Child Psychol. 1992;21:178-188. doi.org/10.1207/s15374424jccp2102_10
12. Merenda PF. BASC: behavior assessment system for children. Meas Eval Counsel Develop. 1996;28:229-232.
13. Conners CK. Conners, 3rd ed manual. Multi-Health Systems. 2008.
14. Achenbach TM. The Child Behavior Checklist and related instruments. In: Maruish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Lawrence Erlbaum Associates Publishers; 1999:429-466.
15. Goodman R. The extended version of the Strengths and Difficulties Questionnaire as a guide to child psychiatric caseness and consequent burden. J Child Psychol Psychiatry. 1999;40:791-799.
16. Wolraich ML, Lambert W, Doffing MA, et al. Psychometric properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a referred population. J Pediatr Psychol. 2003;28:559-567. doi: 10.1093/jpepsy/jsg046
17. Sparrow SS, Cicchetti DV. The Vineland Adaptive Behavior Scales. In: Newmark CS, ed. Major Psychological Assessment Instruments. Vol 2. Allyn & Bacon; 2003:199-231.
18. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47:199-212. doi: 10.1080/15374416.2017.1417860
19. Ghriwati NA, Langberg JM, Gardner W, et al. Impact of mental health comorbidities on the community-based pediatric treatment and outcomes of children with attention deficit hyperactivity disorder. J Dev Behav Ped. 2017;38:20-28. doi: 10.1097/DBP.0000000000000359
20. Niclasen J, Obel C, Homøe P, et al. Associations between otitis media and child behavioural and learning difficulties: results from a Danish Cohort. Int J Ped Otorhinolaryngol. 2016;84:12-20. doi: 10.1016/j.ijporl.2016.02.017
21. Ross JL Roeltgen DP Kushner H, et al. Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. doi: 10.1542/peds.2011-0719
22. Mechler K, Banaschewski T, Hohmann S, et al. Evidence-based pharmacological treatment options for ADHD in children and adolescents. Pharmacol Ther. 2022;230:107940. doi: 10.1016/j.pharmthera.2021.107940
23. Mishra J, Merzenich MM, Sagar R. Accessible online neuroplasticity-targeted training for children with ADHD. Child Adolesc Psychiatry Ment Health. 2013;7:38. doi: 10.1186/1753-2000-7-38
24. Neece CL. Mindfulness-based stress reduction for parents of young children with developmental delays: implications for parental mental health and child behavior problems. J Applied Res Intellect Disabil. 2014;27:174-186. doi: 10.1111/jar.12064
25. Petcharat M, Liehr P. Mindfulness training for parents of children with special needs: guidance for nurses in mental health practice. J Child Adolesc Psychiatr Nursing. 2017;30:35-46. doi: 10.1111/jcap.12169
26. Hahn-Markowitz J, Burger I, Manor I, et al. Efficacy of cognitive-functional (Cog-Fun) occupational therapy intervention among children with ADHD: an RCT. J Atten Disord. 2020;24:655-666. doi: 10.1177/1087054716666955
27. Young Z, Moghaddam N, Tickle A. The efficacy of cognitive behavioral therapy for adults with ADHD: a systematic review and meta-analysis of randomized controlled trials. J Atten Disord. 2020;24:875-888.
28. Carr AW, Bean RA, Nelson KF. Childhood attention-deficit hyperactivity disorder: family therapy from an attachment based perspective. Child Youth Serv Rev. 2020;119:105666.
29. Robin AL. Family therapy for adolescents with ADHD. Child Adolesc Psychiatr Clin N Am. 2014;23:747-756. doi: 10.1016/j.chc.2014.06.001
30. Cattoi B, Alpern I, Katz JS, et al. The adverse health outcomes, economic burden, and public health implications of unmanaged attention deficit hyperactivity disorder (ADHD): a call to action resulting from CHADD summit, Washington, DC, October 17, 2019. J Atten Disord. 2022;26:807-808. doi: 10.1177/10870547211036754
31. Hinojosa MS, Hinojosa R, Nguyen J. Shared decision making and treatment for minority children with ADHD. J Transcult Nurs. 2020;31:135-143. doi: 10.1177/1043659619853021
32. Slobodin O, Masalha R. Challenges in ADHD care for ethnic minority children: a review of the current literature. Transcult Psychiatry. 2020;57:468-483. doi: 10.1177/1363461520902885
33. Retz W, Ginsberg Y, Turner D, et al. Attention-deficit/hyperactivity disorder (ADHD), antisociality and delinquent behavior over the lifespan. Neurosci Biobehav Rev. 2021;120:236-248. doi: 10.1016/j.neubiorev.2020.11.025
34. Del Sol Calderon P, Izquierdo A, Garcia Moreno M. Effects of the pandemic on the mental health of children and adolescents. Review and current scientific evidence of the SARS-COV2 pandemic. Eur Psychiatry. 2021;64:S223-S224. doi: 10.1192/j.eurpsy.2021.597
35. Insa I, Alda JA. Attention deficit hyperactivity disorder (ADHD) & COVID-19: attention deficit hyperactivity disorder: consequences of the 1st wave. Eur Psychiatry. 2021;64:S660. doi: 10.1192/j.eurpsy.2021.1752
1. Bitsko RH, Claussen AH, Lichstein J, et al. Mental health surveillance among children—United States, 2013-2019. MMWR Suppl. 2022;71:1-42. doi: 10.15585/mmwr.su7102a1
2. Danielson ML, Holbrook JR, Blumberg SJ, et al. State-level estimates of the prevalence of parent-reported ADHD diagnosis and treatment among U.S. children and adolescents, 2016 to 2019. J Atten Disord. 2022;26:1685-1697. doi: 10.1177/10870547221099961
3. Faraone SV, Banaschewski T, Coghill D, et al. The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. 2021;128:789-818. doi: 10.1016/j.neubiorev.2021.01.022
4. American Psychiatric Association
5. Brahmbhatt K, Hilty DM, Mina H, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: a concise review. J Adolesc Health. 2016;59:135-143. doi: 10.1016/j.jadohealth.2016.03.025
6. Wolraich ML, Hagan JF, Allan C, et al. AAP Subcommittee on Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics. 2019;144:e20192528. doi: 10.1542/peds.2019-2528
7. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi: 10.7189/jogh.11.04009
8. Chang JG, Cimino FM, Gossa W. ADHD in children: common questions and answers. Am Fam Physician. 2020;102:592-602.
9. Asarnow JR, Rozenman M, Wiblin J, et al. Integrated medical-behavioral care compared with usual primary care for child and adolescent behavioral health: a meta-analysis. JAMA Pediatr. 2015;169:929-937. doi: 10.1001/jamapediatrics.2015.1141
10. Squires J, Bricker D. Ages & Stages Questionnaires®. 3rd ed (ASQ®-3). Paul H. Brookes Publishing Co., Inc; 2009.
11. DuPaul GJ, Barkley RA. Situational variability of attention problems: psychometric properties of the Revised Home and School Situations Questionnaires. J Clin Child Psychol. 1992;21:178-188. doi.org/10.1207/s15374424jccp2102_10
12. Merenda PF. BASC: behavior assessment system for children. Meas Eval Counsel Develop. 1996;28:229-232.
13. Conners CK. Conners, 3rd ed manual. Multi-Health Systems. 2008.
14. Achenbach TM. The Child Behavior Checklist and related instruments. In: Maruish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Lawrence Erlbaum Associates Publishers; 1999:429-466.
15. Goodman R. The extended version of the Strengths and Difficulties Questionnaire as a guide to child psychiatric caseness and consequent burden. J Child Psychol Psychiatry. 1999;40:791-799.
16. Wolraich ML, Lambert W, Doffing MA, et al. Psychometric properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a referred population. J Pediatr Psychol. 2003;28:559-567. doi: 10.1093/jpepsy/jsg046
17. Sparrow SS, Cicchetti DV. The Vineland Adaptive Behavior Scales. In: Newmark CS, ed. Major Psychological Assessment Instruments. Vol 2. Allyn & Bacon; 2003:199-231.
18. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47:199-212. doi: 10.1080/15374416.2017.1417860
19. Ghriwati NA, Langberg JM, Gardner W, et al. Impact of mental health comorbidities on the community-based pediatric treatment and outcomes of children with attention deficit hyperactivity disorder. J Dev Behav Ped. 2017;38:20-28. doi: 10.1097/DBP.0000000000000359
20. Niclasen J, Obel C, Homøe P, et al. Associations between otitis media and child behavioural and learning difficulties: results from a Danish Cohort. Int J Ped Otorhinolaryngol. 2016;84:12-20. doi: 10.1016/j.ijporl.2016.02.017
21. Ross JL Roeltgen DP Kushner H, et al. Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. doi: 10.1542/peds.2011-0719
22. Mechler K, Banaschewski T, Hohmann S, et al. Evidence-based pharmacological treatment options for ADHD in children and adolescents. Pharmacol Ther. 2022;230:107940. doi: 10.1016/j.pharmthera.2021.107940
23. Mishra J, Merzenich MM, Sagar R. Accessible online neuroplasticity-targeted training for children with ADHD. Child Adolesc Psychiatry Ment Health. 2013;7:38. doi: 10.1186/1753-2000-7-38
24. Neece CL. Mindfulness-based stress reduction for parents of young children with developmental delays: implications for parental mental health and child behavior problems. J Applied Res Intellect Disabil. 2014;27:174-186. doi: 10.1111/jar.12064
25. Petcharat M, Liehr P. Mindfulness training for parents of children with special needs: guidance for nurses in mental health practice. J Child Adolesc Psychiatr Nursing. 2017;30:35-46. doi: 10.1111/jcap.12169
26. Hahn-Markowitz J, Burger I, Manor I, et al. Efficacy of cognitive-functional (Cog-Fun) occupational therapy intervention among children with ADHD: an RCT. J Atten Disord. 2020;24:655-666. doi: 10.1177/1087054716666955
27. Young Z, Moghaddam N, Tickle A. The efficacy of cognitive behavioral therapy for adults with ADHD: a systematic review and meta-analysis of randomized controlled trials. J Atten Disord. 2020;24:875-888.
28. Carr AW, Bean RA, Nelson KF. Childhood attention-deficit hyperactivity disorder: family therapy from an attachment based perspective. Child Youth Serv Rev. 2020;119:105666.
29. Robin AL. Family therapy for adolescents with ADHD. Child Adolesc Psychiatr Clin N Am. 2014;23:747-756. doi: 10.1016/j.chc.2014.06.001
30. Cattoi B, Alpern I, Katz JS, et al. The adverse health outcomes, economic burden, and public health implications of unmanaged attention deficit hyperactivity disorder (ADHD): a call to action resulting from CHADD summit, Washington, DC, October 17, 2019. J Atten Disord. 2022;26:807-808. doi: 10.1177/10870547211036754
31. Hinojosa MS, Hinojosa R, Nguyen J. Shared decision making and treatment for minority children with ADHD. J Transcult Nurs. 2020;31:135-143. doi: 10.1177/1043659619853021
32. Slobodin O, Masalha R. Challenges in ADHD care for ethnic minority children: a review of the current literature. Transcult Psychiatry. 2020;57:468-483. doi: 10.1177/1363461520902885
33. Retz W, Ginsberg Y, Turner D, et al. Attention-deficit/hyperactivity disorder (ADHD), antisociality and delinquent behavior over the lifespan. Neurosci Biobehav Rev. 2021;120:236-248. doi: 10.1016/j.neubiorev.2020.11.025
34. Del Sol Calderon P, Izquierdo A, Garcia Moreno M. Effects of the pandemic on the mental health of children and adolescents. Review and current scientific evidence of the SARS-COV2 pandemic. Eur Psychiatry. 2021;64:S223-S224. doi: 10.1192/j.eurpsy.2021.597
35. Insa I, Alda JA. Attention deficit hyperactivity disorder (ADHD) & COVID-19: attention deficit hyperactivity disorder: consequences of the 1st wave. Eur Psychiatry. 2021;64:S660. doi: 10.1192/j.eurpsy.2021.1752
75 years: A look back on the fascinating history of methotrexate and folate antagonists
If you could go back in time 75 years and tell Dr. Sidney Farber, the developer of methotrexate for cancer therapy, that 21st-century medicine would utilize his specially designed drug more in rheumatology than oncology, he might be surprised. He might scratch his head even more, hearing of his drug sparking interest in still other medical fields, like cardiology.
But drug repurposing is not so uncommon. One classic example is aspirin. Once the most common pain medication and used also in rheumatology, aspirin now finds a range of applications, from colorectal cancer to the prevention of cardiovascular and cerebrovascular thrombosis. Minoxidil is another example, developed for hypertension but used today mostly to stop hair loss. Perhaps most ironic is thalidomide, utilized today for leprosy and multiple myeloma, yet actually contraindicated for its original application, nausea of pregnancy.
Methotrexate, thus, has much in common with other medical treatments, and yet its origin story is as unique and as fascinating as the story of Dr. Farber himself. While this is a rheumatology article, it’s also a story about the origin of a particular rheumatologic treatment, and so the story of that origin will take us mostly through a discussion of hematologic malignancy and of the clinical researcher who dared search for a cure.
Born in 1903, in Buffalo, New York, third of fourteen children of Jewish immigrants from Poland, Dr. Farber grew up in a household that was crowded but academically rigorous. His father, Simon, routinely brought home textbooks, assigning each child a book to read and on which to write a report. His mother, Matilda, was as devoted as her husband to raising the children to succeed in their adopted new country. Upstairs, the children were permitted to speak Yiddish, but downstairs they were required to use only English and German.
As a teen, Dr. Farber lived through the 1918 influenza pandemic that killed at least 50 million people worldwide, including more than 2,000 Buffalonians. This probably helped motivate him to study medicine, but with antisemitism overt in the America of the early 1920s, securing admission to a U.S. medical school was close to impossible. So, in what now seems like the greatest of ironies, Dr. Farber began medical studies in Germany, then transferred for the second year to a U.S. program that seemed adequate – Harvard Medical School, from which he graduated in 1927. From there, he trained as a pathologist, focusing ultimately on pediatric pathology. But, frustrated by case after case of malignancy, whose young victims he’d often have to autopsy, Dr. Farber decided that he wanted to advance the pitiful state of cancer therapeutics, especially for hematologic malignancy.
This was a tall order in the 1930s and early 1940s, when cancer therapeutics consisted only of surgical resection and very primitive forms of radiation therapy. Applicable only to neoplasia that was localized, these options were useless against malignancies in the blood, like acute lymphoblastic leukemia (ALL), but by January 1948 there was at least one glimmer of hope. At that time, one patient with ALL, 2-year-old Robert Sandler, was too ill to join his twin brother Elliott for snow play outside their home in the Dorchester section of Boston. Diagnosed back in August, Robert had suffered multiple episodes of fever, anemia, and thrombocytopenia. His illness had enlarged his spleen dramatically and caused pathologic bone fractures with excruciating bone pain, and for a while he couldn’t walk because of pressure on his lower spinal cord. All of this was the result of uncontrolled mitosis and cell division of lymphoblasts, immature lymphocytes. By December, these out-of-control cells had elevated the boy’s white blood cell count to a peak of 70,000/mcL, more than six times the high end of the normal range (4,500-11,000/mcL). This had happened despite treatment with an experimental drug, developed at Boston Children’s Hospital by Dr. Farber and his team, working on the assumption that inhibition of folate metabolism should slow the growth of tumor cells. On Dec. 28, however, Dr. Farber had switched the child to a new drug with a chemical structure just slightly different from the other agent’s.
Merely another chemical modification in a series of attempts by the research team, the new drug, aminopterin, was not expected to do anything dramatic, but Dr. Farber and the team had come such a long way since the middle of 1947, when he’d actually done the opposite of what he was doing now. On the basis of British research from India showing folic acid deficiency as the basis of a common type of anemia in malnourished people, Dr. Farber had reasoned that children with leukemia, who also suffered from anemia, might also benefit from folic acid supplementation. Even without prior rodent testing, Dr. Farber had tried giving the nutrient to patients with ALL, a strategy made possible by the presence of a spectacular chemist working on folic acid synthesis at Farber’s own hospital to help combat folate deficiency. Born into a poor Brahmin family in India, the chemist, Dr. Yellapragada SubbaRow, had begun life with so much stacked against him as to appear even less likely during childhood than the young Dr. Farber to grow up to make major contributions to medicine. Going through childhood with death all around him, Dr. SubbaRow was motivated to study medicine, but getting into medical school had been an uphill fight, given his family’s economic difficulty. Knowing that he’d also face discrimination on account of his low status after receiving admission to a medical program, SubbaRow could have made things a bit easier for himself by living within the norms of the British Imperial system, but as a supporter of Mohandas Gandhi’s nationalist movement, he boycotted British goods. As a medical student, this meant doing things like wearing Indian-made surgical gloves, instead of the English products that were expected of the students. Such actions led Dr. SubbaRow to receive a kind of second-rate medical degree, rather than the prestigious MBBS.
The political situation also led Dr. SubbaRow to emigrate to the United States, where, ironically, his medical degree initially was taken less seriously than it had been taken in his British-occupied homeland. He thus worked in the capacity of a hospital night porter at Peter Bent Brigham Hospital (the future Brigham and Women’s Hospital), doing menial tasks like changing sheets to make ends meet. He studied, however, and made enough of an impression to gain admission to the same institution that also admitted Farber through the backdoor, Harvard Medical School. This launched him into a research career in which he not only would be instrumental in developing folate antagonists and other classes of drugs, but also would make him the codiscoverer of the role of creatine phosphate and ATP in cellular energy metabolism. Sadly, even after obtaining his top-notch American credentials and contributing through his research to what you might say is a good chunk of the biochemistry pathways that first year medical students memorize without ever learning who discovered them, Dr. SubbaRow still faced prejudice for the rest of his life, which turned out to last only until the age of 53. To add insult to injury, he is rarely remembered for his role.
Dr. Farber proceeded with the folic acid supplementation idea in patients with ALL, even though ALL caused a hypoproliferative anemia, whereas anemia from folate deficiency was megaloblastic, meaning that erythrocytes were produced but they were oversized and dysfunctional. Tragically, folic acid had accelerated the disease process in children with ALL, but the process of chemical experimentation aimed at synthesizing folate also produced some compounds that mimicked chemical precursors of folate in a way that made them antifolates, inhibitors of folate metabolism. If folic acid made lymphoblasts grow faster, Dr. Farber had reasoned that antifolates should inhibit their growth. He thus asked the chemistry lab to focus on folate inhibitors. Testing aminopterin, beginning with young Robert Sandler at the end of December, is what proved his hypothesis correct. By late January, aminopterin had brought the child’s WBC count down to the realm of 12,000, just slightly above normal, with symptoms and signs abating as well, and by February, the child could play with his twin brother. It was not a cure; malignant lymphoblasts still showed on microscopy of Robert’s blood. While he and some 15 other children whom Dr. Farber treated in this early trial would all succumb to ALL, they experienced remission lasting several months.
This was a big deal because the concept of chemotherapy was based only on serendipitous observations of WBC counts dropping in soldiers exposed to nitrogen mustard gas during World War I and during an incident in World War II, yet aminopterin had been designed from the ground up. Though difficult to synthesize in quantities, there was no reason for Dr. Farber’s team not to keep tweaking the drug, and so they did. Replacing one hydrogen atom with a methyl group, they turned it into methotrexate.
Proving easier to synthesize and less toxic, methotrexate would become a workhorse for chemotherapy over the next couple of decades, but the capability of both methotrexate and aminopterin to blunt the growth of white blood cells and other cells did not go unnoticed outside the realm of oncology. As early as the 1950s, dermatologists were using aminopterin to treat psoriasis. This led to the approval of methotrexate for psoriasis in 1972.
Meanwhile, like oncology, infectious diseases, aviation medicine, and so many other areas of practice, rheumatology had gotten a major boost from research stemming from World War II. During the war, Dr. Philip Hench of the Mayo Clinic developed cortisone, which pilots used to stay alert and energetic during trans-Atlantic flights. But it turned out that cortisone had a powerful immunosuppressive effect that dramatically improved rheumatoid arthritis, leading Dr. Hench to receive the Nobel Prize in Physiology or Medicine in 1950. By the end of the 1950s, however, the significant side effects of long-term corticosteroid therapy were very clear, so over the next few decades there was a major effort to develop different treatments for RA and other rheumatologic diseases.
Top on the list of such agents was methotrexate, developed for RA in part by Dr. Michael Weinblatt of Brigham and Women’s Hospital in Boston. In the 1980s, Dr. Weinblatt published the first clinical trial showing the benefits of methotrexate for RA patients. This has since developed into a standard treatment, noticeably different from the original malignancy application in that it is a low-dose regimen. Patients taking methotrexate for RA typically receive no more than 25 mg per week orally, and often much less. Rheumatology today includes expertise in keeping long-term methotrexate therapy safe by monitoring liver function and through other routine tests. The routine nature of the therapy has brought methotrexate to the point of beckoning in a realm that Dr. Farber might not have predicted in his wildest imagination: cardiology. This is on account of the growing appreciation of the inflammatory process in the pathophysiology of atherosclerotic heart disease.
Meanwhile, being an antimetabolite, harmful to rapidly dividing cells, the danger of methotrexate to the embryo and fetus was recognized early. This made methotrexate off-limits to pregnant women, yet it also has made the drug useful as an abortifacient. Though not as good for medication abortion in unwanted but thriving pregnancies, where mifepristone/misoprostol has become the regimen of choice, methotrexate has become a workhorse in other obstetrical settings, such as for ending ectopic pregnancy.
Looking at the present and into the future, the potential for this very old medication looks wide open, as if it could go in any direction, so let’s wind up the discussion with the thought that we may be in for some surprises. Rather than jumping deeply into any rheumatologic issue, we spent most of this article weaving through other medical issues, but does this not make today’s story fairly analogous to rheumatology itself?
Dr. Warmflash is a physician from Portland, Ore. He reported no conflicts of interest.
This story was updated 2/10/2023.
A version of this article first appeared on Medscape.com.
If you could go back in time 75 years and tell Dr. Sidney Farber, the developer of methotrexate for cancer therapy, that 21st-century medicine would utilize his specially designed drug more in rheumatology than oncology, he might be surprised. He might scratch his head even more, hearing of his drug sparking interest in still other medical fields, like cardiology.
But drug repurposing is not so uncommon. One classic example is aspirin. Once the most common pain medication and used also in rheumatology, aspirin now finds a range of applications, from colorectal cancer to the prevention of cardiovascular and cerebrovascular thrombosis. Minoxidil is another example, developed for hypertension but used today mostly to stop hair loss. Perhaps most ironic is thalidomide, utilized today for leprosy and multiple myeloma, yet actually contraindicated for its original application, nausea of pregnancy.
Methotrexate, thus, has much in common with other medical treatments, and yet its origin story is as unique and as fascinating as the story of Dr. Farber himself. While this is a rheumatology article, it’s also a story about the origin of a particular rheumatologic treatment, and so the story of that origin will take us mostly through a discussion of hematologic malignancy and of the clinical researcher who dared search for a cure.
Born in 1903, in Buffalo, New York, third of fourteen children of Jewish immigrants from Poland, Dr. Farber grew up in a household that was crowded but academically rigorous. His father, Simon, routinely brought home textbooks, assigning each child a book to read and on which to write a report. His mother, Matilda, was as devoted as her husband to raising the children to succeed in their adopted new country. Upstairs, the children were permitted to speak Yiddish, but downstairs they were required to use only English and German.
As a teen, Dr. Farber lived through the 1918 influenza pandemic that killed at least 50 million people worldwide, including more than 2,000 Buffalonians. This probably helped motivate him to study medicine, but with antisemitism overt in the America of the early 1920s, securing admission to a U.S. medical school was close to impossible. So, in what now seems like the greatest of ironies, Dr. Farber began medical studies in Germany, then transferred for the second year to a U.S. program that seemed adequate – Harvard Medical School, from which he graduated in 1927. From there, he trained as a pathologist, focusing ultimately on pediatric pathology. But, frustrated by case after case of malignancy, whose young victims he’d often have to autopsy, Dr. Farber decided that he wanted to advance the pitiful state of cancer therapeutics, especially for hematologic malignancy.
This was a tall order in the 1930s and early 1940s, when cancer therapeutics consisted only of surgical resection and very primitive forms of radiation therapy. Applicable only to neoplasia that was localized, these options were useless against malignancies in the blood, like acute lymphoblastic leukemia (ALL), but by January 1948 there was at least one glimmer of hope. At that time, one patient with ALL, 2-year-old Robert Sandler, was too ill to join his twin brother Elliott for snow play outside their home in the Dorchester section of Boston. Diagnosed back in August, Robert had suffered multiple episodes of fever, anemia, and thrombocytopenia. His illness had enlarged his spleen dramatically and caused pathologic bone fractures with excruciating bone pain, and for a while he couldn’t walk because of pressure on his lower spinal cord. All of this was the result of uncontrolled mitosis and cell division of lymphoblasts, immature lymphocytes. By December, these out-of-control cells had elevated the boy’s white blood cell count to a peak of 70,000/mcL, more than six times the high end of the normal range (4,500-11,000/mcL). This had happened despite treatment with an experimental drug, developed at Boston Children’s Hospital by Dr. Farber and his team, working on the assumption that inhibition of folate metabolism should slow the growth of tumor cells. On Dec. 28, however, Dr. Farber had switched the child to a new drug with a chemical structure just slightly different from the other agent’s.
Merely another chemical modification in a series of attempts by the research team, the new drug, aminopterin, was not expected to do anything dramatic, but Dr. Farber and the team had come such a long way since the middle of 1947, when he’d actually done the opposite of what he was doing now. On the basis of British research from India showing folic acid deficiency as the basis of a common type of anemia in malnourished people, Dr. Farber had reasoned that children with leukemia, who also suffered from anemia, might also benefit from folic acid supplementation. Even without prior rodent testing, Dr. Farber had tried giving the nutrient to patients with ALL, a strategy made possible by the presence of a spectacular chemist working on folic acid synthesis at Farber’s own hospital to help combat folate deficiency. Born into a poor Brahmin family in India, the chemist, Dr. Yellapragada SubbaRow, had begun life with so much stacked against him as to appear even less likely during childhood than the young Dr. Farber to grow up to make major contributions to medicine. Going through childhood with death all around him, Dr. SubbaRow was motivated to study medicine, but getting into medical school had been an uphill fight, given his family’s economic difficulty. Knowing that he’d also face discrimination on account of his low status after receiving admission to a medical program, SubbaRow could have made things a bit easier for himself by living within the norms of the British Imperial system, but as a supporter of Mohandas Gandhi’s nationalist movement, he boycotted British goods. As a medical student, this meant doing things like wearing Indian-made surgical gloves, instead of the English products that were expected of the students. Such actions led Dr. SubbaRow to receive a kind of second-rate medical degree, rather than the prestigious MBBS.
The political situation also led Dr. SubbaRow to emigrate to the United States, where, ironically, his medical degree initially was taken less seriously than it had been taken in his British-occupied homeland. He thus worked in the capacity of a hospital night porter at Peter Bent Brigham Hospital (the future Brigham and Women’s Hospital), doing menial tasks like changing sheets to make ends meet. He studied, however, and made enough of an impression to gain admission to the same institution that also admitted Farber through the backdoor, Harvard Medical School. This launched him into a research career in which he not only would be instrumental in developing folate antagonists and other classes of drugs, but also would make him the codiscoverer of the role of creatine phosphate and ATP in cellular energy metabolism. Sadly, even after obtaining his top-notch American credentials and contributing through his research to what you might say is a good chunk of the biochemistry pathways that first year medical students memorize without ever learning who discovered them, Dr. SubbaRow still faced prejudice for the rest of his life, which turned out to last only until the age of 53. To add insult to injury, he is rarely remembered for his role.
Dr. Farber proceeded with the folic acid supplementation idea in patients with ALL, even though ALL caused a hypoproliferative anemia, whereas anemia from folate deficiency was megaloblastic, meaning that erythrocytes were produced but they were oversized and dysfunctional. Tragically, folic acid had accelerated the disease process in children with ALL, but the process of chemical experimentation aimed at synthesizing folate also produced some compounds that mimicked chemical precursors of folate in a way that made them antifolates, inhibitors of folate metabolism. If folic acid made lymphoblasts grow faster, Dr. Farber had reasoned that antifolates should inhibit their growth. He thus asked the chemistry lab to focus on folate inhibitors. Testing aminopterin, beginning with young Robert Sandler at the end of December, is what proved his hypothesis correct. By late January, aminopterin had brought the child’s WBC count down to the realm of 12,000, just slightly above normal, with symptoms and signs abating as well, and by February, the child could play with his twin brother. It was not a cure; malignant lymphoblasts still showed on microscopy of Robert’s blood. While he and some 15 other children whom Dr. Farber treated in this early trial would all succumb to ALL, they experienced remission lasting several months.
This was a big deal because the concept of chemotherapy was based only on serendipitous observations of WBC counts dropping in soldiers exposed to nitrogen mustard gas during World War I and during an incident in World War II, yet aminopterin had been designed from the ground up. Though difficult to synthesize in quantities, there was no reason for Dr. Farber’s team not to keep tweaking the drug, and so they did. Replacing one hydrogen atom with a methyl group, they turned it into methotrexate.
Proving easier to synthesize and less toxic, methotrexate would become a workhorse for chemotherapy over the next couple of decades, but the capability of both methotrexate and aminopterin to blunt the growth of white blood cells and other cells did not go unnoticed outside the realm of oncology. As early as the 1950s, dermatologists were using aminopterin to treat psoriasis. This led to the approval of methotrexate for psoriasis in 1972.
Meanwhile, like oncology, infectious diseases, aviation medicine, and so many other areas of practice, rheumatology had gotten a major boost from research stemming from World War II. During the war, Dr. Philip Hench of the Mayo Clinic developed cortisone, which pilots used to stay alert and energetic during trans-Atlantic flights. But it turned out that cortisone had a powerful immunosuppressive effect that dramatically improved rheumatoid arthritis, leading Dr. Hench to receive the Nobel Prize in Physiology or Medicine in 1950. By the end of the 1950s, however, the significant side effects of long-term corticosteroid therapy were very clear, so over the next few decades there was a major effort to develop different treatments for RA and other rheumatologic diseases.
Top on the list of such agents was methotrexate, developed for RA in part by Dr. Michael Weinblatt of Brigham and Women’s Hospital in Boston. In the 1980s, Dr. Weinblatt published the first clinical trial showing the benefits of methotrexate for RA patients. This has since developed into a standard treatment, noticeably different from the original malignancy application in that it is a low-dose regimen. Patients taking methotrexate for RA typically receive no more than 25 mg per week orally, and often much less. Rheumatology today includes expertise in keeping long-term methotrexate therapy safe by monitoring liver function and through other routine tests. The routine nature of the therapy has brought methotrexate to the point of beckoning in a realm that Dr. Farber might not have predicted in his wildest imagination: cardiology. This is on account of the growing appreciation of the inflammatory process in the pathophysiology of atherosclerotic heart disease.
Meanwhile, being an antimetabolite, harmful to rapidly dividing cells, the danger of methotrexate to the embryo and fetus was recognized early. This made methotrexate off-limits to pregnant women, yet it also has made the drug useful as an abortifacient. Though not as good for medication abortion in unwanted but thriving pregnancies, where mifepristone/misoprostol has become the regimen of choice, methotrexate has become a workhorse in other obstetrical settings, such as for ending ectopic pregnancy.
Looking at the present and into the future, the potential for this very old medication looks wide open, as if it could go in any direction, so let’s wind up the discussion with the thought that we may be in for some surprises. Rather than jumping deeply into any rheumatologic issue, we spent most of this article weaving through other medical issues, but does this not make today’s story fairly analogous to rheumatology itself?
Dr. Warmflash is a physician from Portland, Ore. He reported no conflicts of interest.
This story was updated 2/10/2023.
A version of this article first appeared on Medscape.com.
If you could go back in time 75 years and tell Dr. Sidney Farber, the developer of methotrexate for cancer therapy, that 21st-century medicine would utilize his specially designed drug more in rheumatology than oncology, he might be surprised. He might scratch his head even more, hearing of his drug sparking interest in still other medical fields, like cardiology.
But drug repurposing is not so uncommon. One classic example is aspirin. Once the most common pain medication and used also in rheumatology, aspirin now finds a range of applications, from colorectal cancer to the prevention of cardiovascular and cerebrovascular thrombosis. Minoxidil is another example, developed for hypertension but used today mostly to stop hair loss. Perhaps most ironic is thalidomide, utilized today for leprosy and multiple myeloma, yet actually contraindicated for its original application, nausea of pregnancy.
Methotrexate, thus, has much in common with other medical treatments, and yet its origin story is as unique and as fascinating as the story of Dr. Farber himself. While this is a rheumatology article, it’s also a story about the origin of a particular rheumatologic treatment, and so the story of that origin will take us mostly through a discussion of hematologic malignancy and of the clinical researcher who dared search for a cure.
Born in 1903, in Buffalo, New York, third of fourteen children of Jewish immigrants from Poland, Dr. Farber grew up in a household that was crowded but academically rigorous. His father, Simon, routinely brought home textbooks, assigning each child a book to read and on which to write a report. His mother, Matilda, was as devoted as her husband to raising the children to succeed in their adopted new country. Upstairs, the children were permitted to speak Yiddish, but downstairs they were required to use only English and German.
As a teen, Dr. Farber lived through the 1918 influenza pandemic that killed at least 50 million people worldwide, including more than 2,000 Buffalonians. This probably helped motivate him to study medicine, but with antisemitism overt in the America of the early 1920s, securing admission to a U.S. medical school was close to impossible. So, in what now seems like the greatest of ironies, Dr. Farber began medical studies in Germany, then transferred for the second year to a U.S. program that seemed adequate – Harvard Medical School, from which he graduated in 1927. From there, he trained as a pathologist, focusing ultimately on pediatric pathology. But, frustrated by case after case of malignancy, whose young victims he’d often have to autopsy, Dr. Farber decided that he wanted to advance the pitiful state of cancer therapeutics, especially for hematologic malignancy.
This was a tall order in the 1930s and early 1940s, when cancer therapeutics consisted only of surgical resection and very primitive forms of radiation therapy. Applicable only to neoplasia that was localized, these options were useless against malignancies in the blood, like acute lymphoblastic leukemia (ALL), but by January 1948 there was at least one glimmer of hope. At that time, one patient with ALL, 2-year-old Robert Sandler, was too ill to join his twin brother Elliott for snow play outside their home in the Dorchester section of Boston. Diagnosed back in August, Robert had suffered multiple episodes of fever, anemia, and thrombocytopenia. His illness had enlarged his spleen dramatically and caused pathologic bone fractures with excruciating bone pain, and for a while he couldn’t walk because of pressure on his lower spinal cord. All of this was the result of uncontrolled mitosis and cell division of lymphoblasts, immature lymphocytes. By December, these out-of-control cells had elevated the boy’s white blood cell count to a peak of 70,000/mcL, more than six times the high end of the normal range (4,500-11,000/mcL). This had happened despite treatment with an experimental drug, developed at Boston Children’s Hospital by Dr. Farber and his team, working on the assumption that inhibition of folate metabolism should slow the growth of tumor cells. On Dec. 28, however, Dr. Farber had switched the child to a new drug with a chemical structure just slightly different from the other agent’s.
Merely another chemical modification in a series of attempts by the research team, the new drug, aminopterin, was not expected to do anything dramatic, but Dr. Farber and the team had come such a long way since the middle of 1947, when he’d actually done the opposite of what he was doing now. On the basis of British research from India showing folic acid deficiency as the basis of a common type of anemia in malnourished people, Dr. Farber had reasoned that children with leukemia, who also suffered from anemia, might also benefit from folic acid supplementation. Even without prior rodent testing, Dr. Farber had tried giving the nutrient to patients with ALL, a strategy made possible by the presence of a spectacular chemist working on folic acid synthesis at Farber’s own hospital to help combat folate deficiency. Born into a poor Brahmin family in India, the chemist, Dr. Yellapragada SubbaRow, had begun life with so much stacked against him as to appear even less likely during childhood than the young Dr. Farber to grow up to make major contributions to medicine. Going through childhood with death all around him, Dr. SubbaRow was motivated to study medicine, but getting into medical school had been an uphill fight, given his family’s economic difficulty. Knowing that he’d also face discrimination on account of his low status after receiving admission to a medical program, SubbaRow could have made things a bit easier for himself by living within the norms of the British Imperial system, but as a supporter of Mohandas Gandhi’s nationalist movement, he boycotted British goods. As a medical student, this meant doing things like wearing Indian-made surgical gloves, instead of the English products that were expected of the students. Such actions led Dr. SubbaRow to receive a kind of second-rate medical degree, rather than the prestigious MBBS.
The political situation also led Dr. SubbaRow to emigrate to the United States, where, ironically, his medical degree initially was taken less seriously than it had been taken in his British-occupied homeland. He thus worked in the capacity of a hospital night porter at Peter Bent Brigham Hospital (the future Brigham and Women’s Hospital), doing menial tasks like changing sheets to make ends meet. He studied, however, and made enough of an impression to gain admission to the same institution that also admitted Farber through the backdoor, Harvard Medical School. This launched him into a research career in which he not only would be instrumental in developing folate antagonists and other classes of drugs, but also would make him the codiscoverer of the role of creatine phosphate and ATP in cellular energy metabolism. Sadly, even after obtaining his top-notch American credentials and contributing through his research to what you might say is a good chunk of the biochemistry pathways that first year medical students memorize without ever learning who discovered them, Dr. SubbaRow still faced prejudice for the rest of his life, which turned out to last only until the age of 53. To add insult to injury, he is rarely remembered for his role.
Dr. Farber proceeded with the folic acid supplementation idea in patients with ALL, even though ALL caused a hypoproliferative anemia, whereas anemia from folate deficiency was megaloblastic, meaning that erythrocytes were produced but they were oversized and dysfunctional. Tragically, folic acid had accelerated the disease process in children with ALL, but the process of chemical experimentation aimed at synthesizing folate also produced some compounds that mimicked chemical precursors of folate in a way that made them antifolates, inhibitors of folate metabolism. If folic acid made lymphoblasts grow faster, Dr. Farber had reasoned that antifolates should inhibit their growth. He thus asked the chemistry lab to focus on folate inhibitors. Testing aminopterin, beginning with young Robert Sandler at the end of December, is what proved his hypothesis correct. By late January, aminopterin had brought the child’s WBC count down to the realm of 12,000, just slightly above normal, with symptoms and signs abating as well, and by February, the child could play with his twin brother. It was not a cure; malignant lymphoblasts still showed on microscopy of Robert’s blood. While he and some 15 other children whom Dr. Farber treated in this early trial would all succumb to ALL, they experienced remission lasting several months.
This was a big deal because the concept of chemotherapy was based only on serendipitous observations of WBC counts dropping in soldiers exposed to nitrogen mustard gas during World War I and during an incident in World War II, yet aminopterin had been designed from the ground up. Though difficult to synthesize in quantities, there was no reason for Dr. Farber’s team not to keep tweaking the drug, and so they did. Replacing one hydrogen atom with a methyl group, they turned it into methotrexate.
Proving easier to synthesize and less toxic, methotrexate would become a workhorse for chemotherapy over the next couple of decades, but the capability of both methotrexate and aminopterin to blunt the growth of white blood cells and other cells did not go unnoticed outside the realm of oncology. As early as the 1950s, dermatologists were using aminopterin to treat psoriasis. This led to the approval of methotrexate for psoriasis in 1972.
Meanwhile, like oncology, infectious diseases, aviation medicine, and so many other areas of practice, rheumatology had gotten a major boost from research stemming from World War II. During the war, Dr. Philip Hench of the Mayo Clinic developed cortisone, which pilots used to stay alert and energetic during trans-Atlantic flights. But it turned out that cortisone had a powerful immunosuppressive effect that dramatically improved rheumatoid arthritis, leading Dr. Hench to receive the Nobel Prize in Physiology or Medicine in 1950. By the end of the 1950s, however, the significant side effects of long-term corticosteroid therapy were very clear, so over the next few decades there was a major effort to develop different treatments for RA and other rheumatologic diseases.
Top on the list of such agents was methotrexate, developed for RA in part by Dr. Michael Weinblatt of Brigham and Women’s Hospital in Boston. In the 1980s, Dr. Weinblatt published the first clinical trial showing the benefits of methotrexate for RA patients. This has since developed into a standard treatment, noticeably different from the original malignancy application in that it is a low-dose regimen. Patients taking methotrexate for RA typically receive no more than 25 mg per week orally, and often much less. Rheumatology today includes expertise in keeping long-term methotrexate therapy safe by monitoring liver function and through other routine tests. The routine nature of the therapy has brought methotrexate to the point of beckoning in a realm that Dr. Farber might not have predicted in his wildest imagination: cardiology. This is on account of the growing appreciation of the inflammatory process in the pathophysiology of atherosclerotic heart disease.
Meanwhile, being an antimetabolite, harmful to rapidly dividing cells, the danger of methotrexate to the embryo and fetus was recognized early. This made methotrexate off-limits to pregnant women, yet it also has made the drug useful as an abortifacient. Though not as good for medication abortion in unwanted but thriving pregnancies, where mifepristone/misoprostol has become the regimen of choice, methotrexate has become a workhorse in other obstetrical settings, such as for ending ectopic pregnancy.
Looking at the present and into the future, the potential for this very old medication looks wide open, as if it could go in any direction, so let’s wind up the discussion with the thought that we may be in for some surprises. Rather than jumping deeply into any rheumatologic issue, we spent most of this article weaving through other medical issues, but does this not make today’s story fairly analogous to rheumatology itself?
Dr. Warmflash is a physician from Portland, Ore. He reported no conflicts of interest.
This story was updated 2/10/2023.
A version of this article first appeared on Medscape.com.
Pediatricians, specialists largely agree on ASD diagnoses
General pediatricians and a multidisciplinary team of specialists agreed most of the time on which children should be diagnosed with autism spectrum disorder (ASD), data from a new study suggest.
But when it came to ruling out ASD, the agreement rate was much lower.
The study by Melanie Penner, MSc, MD, with the Autism Research Centre at Bloorview Research Institute, Toronto, and colleagues found that 89% of the time when a physician determined a child had ASD, the multidisciplinary team agreed. But when a pediatrician thought a child did not have ASD, the multidisciplinary team agreed only 60% of the time. The study was published in JAMA Network Open.
Multidisciplinary team model can’t keep up with demand
The findings are important as many guidelines recommend multidisciplinary teams (MDTs) for all ASD diagnostic assessment. However, the resources for this model can’t meet the demand of children needing a diagnosis and can lead to long waits for ASD therapies.
In Canada, the researchers note, the average wait time from referral to receipt of ASD diagnosis has been reported as 7 months and “has likely lengthened since the COVID-19 pandemic.”
Jennifer Gerdts, PhD, an attending psychologist at the Seattle Children’s Autism Center, said in an interview that the wait there for diagnosis in children older than 4 is “multiple years,” a length of time that’s common across the United States. Meanwhile, in many states families can’t access services without a diagnosis.
Expanding capacity with diagnoses by general pediatricians may improve access, but the diagnostic accuracy is critical.
Dr. Gerdts, who was not part of the study, said this research is “hugely important in the work that is under way to build community capacity for diagnostic evaluation.”
She said this study shows that not all diagnoses need the resources of a multiple-disciplinary team and that “pediatricians can do it, too, and they can do it pretty accurately.” Dr. Gerdts evaluates children for autism and helps train pediatricians to make diagnoses.
Pediatricians, specialist team completed blinded assessments
The 17 pediatricians in the study and the specialist team independently completed blinded assessment and each recorded a decision on whether the child had ASD. The prospective diagnostic study was conducted in a specialist assessment center in Toronto and in general pediatrician practices in Ontario from June 2016 to March 2020.
Children were younger than 5.5 years, did not have an ASD diagnosis and were referred because there was a development concern. The pediatricians referred 106 children (75% boys; average age, 3.5 years). More than half (57%) of the participating children were from minority racial and ethnic groups.
The children were randomly assigned to two groups: One included children who had their MDT visits before their pediatrician assessment and the other group included those who had their MDT visits after their pediatrician assessment.
The MDT diagnosed more than two-thirds of the children (68%) with ASD.
Sensitivity and specificity of the pediatrician assessments, compared with that of the specialist team, were 0.75 (95% confidence interval, 0.67-0.83) and 0.79 (95% CI, 0.62-0.91), respectively.
A look at pediatricians’ accuracy
Pediatricians reported the decisions they would have made had the child not been in the study.
- In 69% of the true-positive cases, pediatricians would have given an ASD diagnosis.
- In 44% of true-negative cases, they would have told the family the child did not have autism; in 30% of those case, they would give alternative diagnoses (most commonly ADHD and language delay).
- The pediatrician would have diagnosed ASD in only one of the seven false-positive cases and would refer those patients to a subspecialist 71% of the time.
- In false-negative cases, the pediatrician would incorrectly tell the family the child does not have autism 44% of the time.
Regarding the false-negative cases, the authors wrote, “more caution is needed for pediatricians when definitively ruling out ASD, which might result in diagnostic delays.”
Confidence is key
Physician confidence was also correlated with accuracy.
The authors wrote: “Among true-positive cases (MDT and pediatrician agree the child has ASD), the pediatrician was certain or very certain 80% of the time (43 cases) and the MDT was certain or very certain 96% of the time (52 cases). As such, if pediatricians conferred ASD diagnoses when feeling certain or very certain, they would make 46 correct diagnoses and 2 incorrect diagnoses.”
The high accuracy of diagnosis when physicians are confident suggests “listening to that sense of certainty is important,” Dr. Gerdts said. Conversely, these numbers show when a physician is uncertain about diagnosing ASD, they should listen to that instinct, too, and refer.
The results of the study support having general pediatricians diagnose and move forward with their patients when the signs of ASD are more definitive, saving the less-certain cases for the more resource-intensive teams to diagnose. Many states are moving toward that “tiered” system, Dr. Gerdts said.
“For many, and in fact most children, general pediatricians are pretty accurate when making an autism diagnosis,” she said.
“Let’s get [general pediatricians] confident in recognizing when this is outside their skill and ability level,” she said. “If you’re not sure, it is better to refer them on than to misdiagnose them.”
The important missing piece she said is how to support them “so they don’t feel pressure to make that call,” Dr. Gerdts said.
This project was funded by a grant from the Bloorview Research Institute, a grant from the Canadian Institutes of Health Research and a grant from the Canadian Institutes of Health. Three coauthors consult for and receive grants from several pharmaceutical companies and other organizations. Dr. Gerdts declared no relevant financial relationships.
General pediatricians and a multidisciplinary team of specialists agreed most of the time on which children should be diagnosed with autism spectrum disorder (ASD), data from a new study suggest.
But when it came to ruling out ASD, the agreement rate was much lower.
The study by Melanie Penner, MSc, MD, with the Autism Research Centre at Bloorview Research Institute, Toronto, and colleagues found that 89% of the time when a physician determined a child had ASD, the multidisciplinary team agreed. But when a pediatrician thought a child did not have ASD, the multidisciplinary team agreed only 60% of the time. The study was published in JAMA Network Open.
Multidisciplinary team model can’t keep up with demand
The findings are important as many guidelines recommend multidisciplinary teams (MDTs) for all ASD diagnostic assessment. However, the resources for this model can’t meet the demand of children needing a diagnosis and can lead to long waits for ASD therapies.
In Canada, the researchers note, the average wait time from referral to receipt of ASD diagnosis has been reported as 7 months and “has likely lengthened since the COVID-19 pandemic.”
Jennifer Gerdts, PhD, an attending psychologist at the Seattle Children’s Autism Center, said in an interview that the wait there for diagnosis in children older than 4 is “multiple years,” a length of time that’s common across the United States. Meanwhile, in many states families can’t access services without a diagnosis.
Expanding capacity with diagnoses by general pediatricians may improve access, but the diagnostic accuracy is critical.
Dr. Gerdts, who was not part of the study, said this research is “hugely important in the work that is under way to build community capacity for diagnostic evaluation.”
She said this study shows that not all diagnoses need the resources of a multiple-disciplinary team and that “pediatricians can do it, too, and they can do it pretty accurately.” Dr. Gerdts evaluates children for autism and helps train pediatricians to make diagnoses.
Pediatricians, specialist team completed blinded assessments
The 17 pediatricians in the study and the specialist team independently completed blinded assessment and each recorded a decision on whether the child had ASD. The prospective diagnostic study was conducted in a specialist assessment center in Toronto and in general pediatrician practices in Ontario from June 2016 to March 2020.
Children were younger than 5.5 years, did not have an ASD diagnosis and were referred because there was a development concern. The pediatricians referred 106 children (75% boys; average age, 3.5 years). More than half (57%) of the participating children were from minority racial and ethnic groups.
The children were randomly assigned to two groups: One included children who had their MDT visits before their pediatrician assessment and the other group included those who had their MDT visits after their pediatrician assessment.
The MDT diagnosed more than two-thirds of the children (68%) with ASD.
Sensitivity and specificity of the pediatrician assessments, compared with that of the specialist team, were 0.75 (95% confidence interval, 0.67-0.83) and 0.79 (95% CI, 0.62-0.91), respectively.
A look at pediatricians’ accuracy
Pediatricians reported the decisions they would have made had the child not been in the study.
- In 69% of the true-positive cases, pediatricians would have given an ASD diagnosis.
- In 44% of true-negative cases, they would have told the family the child did not have autism; in 30% of those case, they would give alternative diagnoses (most commonly ADHD and language delay).
- The pediatrician would have diagnosed ASD in only one of the seven false-positive cases and would refer those patients to a subspecialist 71% of the time.
- In false-negative cases, the pediatrician would incorrectly tell the family the child does not have autism 44% of the time.
Regarding the false-negative cases, the authors wrote, “more caution is needed for pediatricians when definitively ruling out ASD, which might result in diagnostic delays.”
Confidence is key
Physician confidence was also correlated with accuracy.
The authors wrote: “Among true-positive cases (MDT and pediatrician agree the child has ASD), the pediatrician was certain or very certain 80% of the time (43 cases) and the MDT was certain or very certain 96% of the time (52 cases). As such, if pediatricians conferred ASD diagnoses when feeling certain or very certain, they would make 46 correct diagnoses and 2 incorrect diagnoses.”
The high accuracy of diagnosis when physicians are confident suggests “listening to that sense of certainty is important,” Dr. Gerdts said. Conversely, these numbers show when a physician is uncertain about diagnosing ASD, they should listen to that instinct, too, and refer.
The results of the study support having general pediatricians diagnose and move forward with their patients when the signs of ASD are more definitive, saving the less-certain cases for the more resource-intensive teams to diagnose. Many states are moving toward that “tiered” system, Dr. Gerdts said.
“For many, and in fact most children, general pediatricians are pretty accurate when making an autism diagnosis,” she said.
“Let’s get [general pediatricians] confident in recognizing when this is outside their skill and ability level,” she said. “If you’re not sure, it is better to refer them on than to misdiagnose them.”
The important missing piece she said is how to support them “so they don’t feel pressure to make that call,” Dr. Gerdts said.
This project was funded by a grant from the Bloorview Research Institute, a grant from the Canadian Institutes of Health Research and a grant from the Canadian Institutes of Health. Three coauthors consult for and receive grants from several pharmaceutical companies and other organizations. Dr. Gerdts declared no relevant financial relationships.
General pediatricians and a multidisciplinary team of specialists agreed most of the time on which children should be diagnosed with autism spectrum disorder (ASD), data from a new study suggest.
But when it came to ruling out ASD, the agreement rate was much lower.
The study by Melanie Penner, MSc, MD, with the Autism Research Centre at Bloorview Research Institute, Toronto, and colleagues found that 89% of the time when a physician determined a child had ASD, the multidisciplinary team agreed. But when a pediatrician thought a child did not have ASD, the multidisciplinary team agreed only 60% of the time. The study was published in JAMA Network Open.
Multidisciplinary team model can’t keep up with demand
The findings are important as many guidelines recommend multidisciplinary teams (MDTs) for all ASD diagnostic assessment. However, the resources for this model can’t meet the demand of children needing a diagnosis and can lead to long waits for ASD therapies.
In Canada, the researchers note, the average wait time from referral to receipt of ASD diagnosis has been reported as 7 months and “has likely lengthened since the COVID-19 pandemic.”
Jennifer Gerdts, PhD, an attending psychologist at the Seattle Children’s Autism Center, said in an interview that the wait there for diagnosis in children older than 4 is “multiple years,” a length of time that’s common across the United States. Meanwhile, in many states families can’t access services without a diagnosis.
Expanding capacity with diagnoses by general pediatricians may improve access, but the diagnostic accuracy is critical.
Dr. Gerdts, who was not part of the study, said this research is “hugely important in the work that is under way to build community capacity for diagnostic evaluation.”
She said this study shows that not all diagnoses need the resources of a multiple-disciplinary team and that “pediatricians can do it, too, and they can do it pretty accurately.” Dr. Gerdts evaluates children for autism and helps train pediatricians to make diagnoses.
Pediatricians, specialist team completed blinded assessments
The 17 pediatricians in the study and the specialist team independently completed blinded assessment and each recorded a decision on whether the child had ASD. The prospective diagnostic study was conducted in a specialist assessment center in Toronto and in general pediatrician practices in Ontario from June 2016 to March 2020.
Children were younger than 5.5 years, did not have an ASD diagnosis and were referred because there was a development concern. The pediatricians referred 106 children (75% boys; average age, 3.5 years). More than half (57%) of the participating children were from minority racial and ethnic groups.
The children were randomly assigned to two groups: One included children who had their MDT visits before their pediatrician assessment and the other group included those who had their MDT visits after their pediatrician assessment.
The MDT diagnosed more than two-thirds of the children (68%) with ASD.
Sensitivity and specificity of the pediatrician assessments, compared with that of the specialist team, were 0.75 (95% confidence interval, 0.67-0.83) and 0.79 (95% CI, 0.62-0.91), respectively.
A look at pediatricians’ accuracy
Pediatricians reported the decisions they would have made had the child not been in the study.
- In 69% of the true-positive cases, pediatricians would have given an ASD diagnosis.
- In 44% of true-negative cases, they would have told the family the child did not have autism; in 30% of those case, they would give alternative diagnoses (most commonly ADHD and language delay).
- The pediatrician would have diagnosed ASD in only one of the seven false-positive cases and would refer those patients to a subspecialist 71% of the time.
- In false-negative cases, the pediatrician would incorrectly tell the family the child does not have autism 44% of the time.
Regarding the false-negative cases, the authors wrote, “more caution is needed for pediatricians when definitively ruling out ASD, which might result in diagnostic delays.”
Confidence is key
Physician confidence was also correlated with accuracy.
The authors wrote: “Among true-positive cases (MDT and pediatrician agree the child has ASD), the pediatrician was certain or very certain 80% of the time (43 cases) and the MDT was certain or very certain 96% of the time (52 cases). As such, if pediatricians conferred ASD diagnoses when feeling certain or very certain, they would make 46 correct diagnoses and 2 incorrect diagnoses.”
The high accuracy of diagnosis when physicians are confident suggests “listening to that sense of certainty is important,” Dr. Gerdts said. Conversely, these numbers show when a physician is uncertain about diagnosing ASD, they should listen to that instinct, too, and refer.
The results of the study support having general pediatricians diagnose and move forward with their patients when the signs of ASD are more definitive, saving the less-certain cases for the more resource-intensive teams to diagnose. Many states are moving toward that “tiered” system, Dr. Gerdts said.
“For many, and in fact most children, general pediatricians are pretty accurate when making an autism diagnosis,” she said.
“Let’s get [general pediatricians] confident in recognizing when this is outside their skill and ability level,” she said. “If you’re not sure, it is better to refer them on than to misdiagnose them.”
The important missing piece she said is how to support them “so they don’t feel pressure to make that call,” Dr. Gerdts said.
This project was funded by a grant from the Bloorview Research Institute, a grant from the Canadian Institutes of Health Research and a grant from the Canadian Institutes of Health. Three coauthors consult for and receive grants from several pharmaceutical companies and other organizations. Dr. Gerdts declared no relevant financial relationships.
FROM JAMA NETWORK OPEN
FDA okays Tidepool Loop app to help guide insulin delivery
The Food and Drug Administration has cleared the Tidepool Loop, a mobile application for use with compatible continuous glucose monitors (CGMs) and insulin pumps to enable automated insulin delivery.
Indicated for people with type 1 diabetes ages 6 years and up, the app algorithm was developed by the diabetes startup Tidepool, which already hosts a cloud-based platform for users to download and review data from different glucose meters, insulin pumps, and CGM systems. The Tidepool Loop project arose from patient-led, open-source initiatives to enable interoperability between the devices.
“The [FDA] authorization of the Tidepool Loop is a huge win for the type 1 diabetes (T1D) community and is a vital step towards a world where people with T1D can choose the pump, CGM, and algorithm that are best for them – and have all three work together seamlessly,” Aaron Kowalski, PhD, CEO of the advocacy organization JDRF, said in a statement.
JDRF helped support preclinical and clinical research in the development of the Loop algorithm, along with The Leona M. and Harry B. Helmsley Charitable Trust, the Tullman Foundation, and partnerships with device makers and donations from the T1D community.
Available by prescription only, the Tidepool app is for single patient use. It works with designated “integrated CGMs” and “alternate controller enabled pumps” to automatically increase, decrease, or suspend insulin delivery, based on the glucose readings and predicted values. The app can also recommend correction doses, which the user can confirm.
According to an FDA statement:“Tidepool Loop’s algorithm technology is designed to be compatible with other individual interoperable devices that meet prespecified acceptance criteria set forth in a validation and integration plan provided by the sponsor and cleared by the FDA as part of the premarket submission.”
Tidepool is finalizing agreements with the various device manufacturers “to create a seamless experience for both physicians prescribing Tidepool Loop and the patients using it,” according to a company statement.
Tidepool’s initial launch device partners have not yet been announced, but the company “has a development partnership with Dexcom and other yet-to-be-named medical device companies for future inclusion of their components with the Tidepool Loop platform,” the statement says.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared the Tidepool Loop, a mobile application for use with compatible continuous glucose monitors (CGMs) and insulin pumps to enable automated insulin delivery.
Indicated for people with type 1 diabetes ages 6 years and up, the app algorithm was developed by the diabetes startup Tidepool, which already hosts a cloud-based platform for users to download and review data from different glucose meters, insulin pumps, and CGM systems. The Tidepool Loop project arose from patient-led, open-source initiatives to enable interoperability between the devices.
“The [FDA] authorization of the Tidepool Loop is a huge win for the type 1 diabetes (T1D) community and is a vital step towards a world where people with T1D can choose the pump, CGM, and algorithm that are best for them – and have all three work together seamlessly,” Aaron Kowalski, PhD, CEO of the advocacy organization JDRF, said in a statement.
JDRF helped support preclinical and clinical research in the development of the Loop algorithm, along with The Leona M. and Harry B. Helmsley Charitable Trust, the Tullman Foundation, and partnerships with device makers and donations from the T1D community.
Available by prescription only, the Tidepool app is for single patient use. It works with designated “integrated CGMs” and “alternate controller enabled pumps” to automatically increase, decrease, or suspend insulin delivery, based on the glucose readings and predicted values. The app can also recommend correction doses, which the user can confirm.
According to an FDA statement:“Tidepool Loop’s algorithm technology is designed to be compatible with other individual interoperable devices that meet prespecified acceptance criteria set forth in a validation and integration plan provided by the sponsor and cleared by the FDA as part of the premarket submission.”
Tidepool is finalizing agreements with the various device manufacturers “to create a seamless experience for both physicians prescribing Tidepool Loop and the patients using it,” according to a company statement.
Tidepool’s initial launch device partners have not yet been announced, but the company “has a development partnership with Dexcom and other yet-to-be-named medical device companies for future inclusion of their components with the Tidepool Loop platform,” the statement says.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared the Tidepool Loop, a mobile application for use with compatible continuous glucose monitors (CGMs) and insulin pumps to enable automated insulin delivery.
Indicated for people with type 1 diabetes ages 6 years and up, the app algorithm was developed by the diabetes startup Tidepool, which already hosts a cloud-based platform for users to download and review data from different glucose meters, insulin pumps, and CGM systems. The Tidepool Loop project arose from patient-led, open-source initiatives to enable interoperability between the devices.
“The [FDA] authorization of the Tidepool Loop is a huge win for the type 1 diabetes (T1D) community and is a vital step towards a world where people with T1D can choose the pump, CGM, and algorithm that are best for them – and have all three work together seamlessly,” Aaron Kowalski, PhD, CEO of the advocacy organization JDRF, said in a statement.
JDRF helped support preclinical and clinical research in the development of the Loop algorithm, along with The Leona M. and Harry B. Helmsley Charitable Trust, the Tullman Foundation, and partnerships with device makers and donations from the T1D community.
Available by prescription only, the Tidepool app is for single patient use. It works with designated “integrated CGMs” and “alternate controller enabled pumps” to automatically increase, decrease, or suspend insulin delivery, based on the glucose readings and predicted values. The app can also recommend correction doses, which the user can confirm.
According to an FDA statement:“Tidepool Loop’s algorithm technology is designed to be compatible with other individual interoperable devices that meet prespecified acceptance criteria set forth in a validation and integration plan provided by the sponsor and cleared by the FDA as part of the premarket submission.”
Tidepool is finalizing agreements with the various device manufacturers “to create a seamless experience for both physicians prescribing Tidepool Loop and the patients using it,” according to a company statement.
Tidepool’s initial launch device partners have not yet been announced, but the company “has a development partnership with Dexcom and other yet-to-be-named medical device companies for future inclusion of their components with the Tidepool Loop platform,” the statement says.
A version of this article first appeared on Medscape.com.
Children with autism but no intellectual disability may be falling through the cracks
Approximately two out of three children with autism spectrum disorder (ASD) do not have concurrent intellectual disability, according to a population study of ASD trends.
Intellectual functioning remains the best predictor of functional outcomes in kids with ASD, and missing those with no cognitive impairment (ASD-N) can prevent intervention and affect future achievement.
Furthermore, while the study found that ASD-N increased among all demographic subgroups from 2000 to 2016, it also observed widespread health disparities in identifying ASD-N, especially in Black, Hispanic, and underprivileged children.
“ASD is a major public health concern and prevalence estimates are likely to continue to rise as disparities are reduced and ASD identification is improved,” wrote researchers led by Josephine Shenouda, DrPH, MS, of Rutgers School of Public Health in Piscataway, N.J., in Pediatrics .
The study period saw a surprising 500% increase in the prevalence of ASD-N and a 200% increase in the prevalence of cognitive impairment–associated ASD-I , with higher rates across all sex, race, ethnicity, and socioeconomic subgroups. The five- and twofold respective increases are consistent with previous research.
“To a large degree, the rise in autism estimates has been driven by individuals without intellectual disability,” Dr. Shenouda said in an interview. “The best way to address increasing autism and to affect disparities in autism identification is through universal autism screening during the toddler period. And different metrics of functional outcomes need to be developed to understand the expression of autism better.”
Her group had previously seen autism estimates of approximately 1% in 2000 rise to 3% by 2016 but had noted variations, with some communities exceeding 5% for autism estimates. “That led to the question of why, and we saw that in areas with high estimates, we are identifying more children with autism without intellectual disability,” she said. “We wanted to know if the increase over time was equally distributed among children with autism with and without intellectual disability.”
A study in disparities
The cross-sectional study examined data from active ASD surveillance by the CDC’s Autism and Developmental Disabilities Monitoring Network in 8-year-olds residing in the New York/New Jersey Metropolitan Area. Overall, 4,661 children were identified with ASD, with ASD-I affecting 1,505 (32.3%), and ASD-N affecting 2,764 (59.3%). Non-Hispanic Black children who were affected numbered 946 (20.3%), while 1,230 (26.4%) were Hispanic, and 2,114 (45.4%) were non-Hispanic White.
Notably, Black children were 30% less likely to be identified with ASD-N compared with White children, and children residing in affluent areas were 80% more likely to be identified with ASD-N versus those in underserved areas. Furthermore, a greater proportion of children with ASD-I resided in vulnerable areas compared with their counterparts with ASD-N.
While males had a higher prevalence compared with females regardless of intellectual disability status, male-to-female ratios were slightly lower among ASD-I compared with ASD-N cases.
Commenting on the study but not involved in it, Barbara J. Howard, MD, an assistant professor of medicine at Johns Hopkins University, Baltimore, said the increasing gap in identifying ASD-N according to race, ethnicity, and socioeconomic status measures probably reflects greater parental awareness of ASD and access to diagnostic services in White families and those of higher socioeconomic status. “There were no racial, ethnicity, or socioeconomic status differences in the prevalence of the more obvious and impairing ASD-I in the sample, but its prevalence was also increasing over this period,” she said.
Although the greater recognition of the less impairing ASD-N is important for optimal outcomes through intervention, the increasing discrepancies mean that more children generally and more marginalized children specifically are not being diagnosed or served. “There should be no differences in prevalence by these characteristics,” Dr. Howard said. “The striking inequity for non-White children and those of lower socioeconomic status in being diagnosed with ASD-N and thus qualifying for intervention that could improve their long-term functioning is likely also compounded by service, educational, and social disadvantages they may experience.”
In light of these disparities, an accompanying editorial by Emily Hotez, PhD, of the University of California, Los Angeles, and Lindsay Shea, DrPH, of the A.J. Drexel Autism Institute at Drexel University, Philadelphia, argues that social determinants of health (SDOH) should be prioritized in the public health surveillance of autism since these factors potentially contribute to the general underdiagnosis of autism in minority groups and merit more attention from pediatricians. While SDOH affects many nonautistic conditions, it may be even more important for families dealing with the stressors and isolation associated with autism, the commentators said. “Our commentary speaks to the utility of increasing SDOH surveillance in improving our understanding of autistic individuals’ needs, experiences, and priorities on a population level,” Dr. Hotez said in an interview. She added that integrating SDOH surveillance into pediatricians’ workflows will lead to improvements in clinical practice and patient care in the long term.
“Specifically, increased uptake of universal SDOH screening and referral practices will allow pediatricians to more proactively link autistic children and families, particularly those from marginalized groups, with much-needed health-promoting services and supports.” She cautioned, however, that while most providers believe universal SDOH screening is important, fewer report that screening is feasible or feel prepared to address families’ social needs when they are identified.
This study was supported by the Centers for Disease Control and Prevention and the National Institutes of Health/National Institute of Environmental Health Sciences. The authors had no conflicts of interest to disclose. The commentators had no potential conflicts of interest to disclose. Dr. Howard disclosed no competing interests relevant to her comments.
Approximately two out of three children with autism spectrum disorder (ASD) do not have concurrent intellectual disability, according to a population study of ASD trends.
Intellectual functioning remains the best predictor of functional outcomes in kids with ASD, and missing those with no cognitive impairment (ASD-N) can prevent intervention and affect future achievement.
Furthermore, while the study found that ASD-N increased among all demographic subgroups from 2000 to 2016, it also observed widespread health disparities in identifying ASD-N, especially in Black, Hispanic, and underprivileged children.
“ASD is a major public health concern and prevalence estimates are likely to continue to rise as disparities are reduced and ASD identification is improved,” wrote researchers led by Josephine Shenouda, DrPH, MS, of Rutgers School of Public Health in Piscataway, N.J., in Pediatrics .
The study period saw a surprising 500% increase in the prevalence of ASD-N and a 200% increase in the prevalence of cognitive impairment–associated ASD-I , with higher rates across all sex, race, ethnicity, and socioeconomic subgroups. The five- and twofold respective increases are consistent with previous research.
“To a large degree, the rise in autism estimates has been driven by individuals without intellectual disability,” Dr. Shenouda said in an interview. “The best way to address increasing autism and to affect disparities in autism identification is through universal autism screening during the toddler period. And different metrics of functional outcomes need to be developed to understand the expression of autism better.”
Her group had previously seen autism estimates of approximately 1% in 2000 rise to 3% by 2016 but had noted variations, with some communities exceeding 5% for autism estimates. “That led to the question of why, and we saw that in areas with high estimates, we are identifying more children with autism without intellectual disability,” she said. “We wanted to know if the increase over time was equally distributed among children with autism with and without intellectual disability.”
A study in disparities
The cross-sectional study examined data from active ASD surveillance by the CDC’s Autism and Developmental Disabilities Monitoring Network in 8-year-olds residing in the New York/New Jersey Metropolitan Area. Overall, 4,661 children were identified with ASD, with ASD-I affecting 1,505 (32.3%), and ASD-N affecting 2,764 (59.3%). Non-Hispanic Black children who were affected numbered 946 (20.3%), while 1,230 (26.4%) were Hispanic, and 2,114 (45.4%) were non-Hispanic White.
Notably, Black children were 30% less likely to be identified with ASD-N compared with White children, and children residing in affluent areas were 80% more likely to be identified with ASD-N versus those in underserved areas. Furthermore, a greater proportion of children with ASD-I resided in vulnerable areas compared with their counterparts with ASD-N.
While males had a higher prevalence compared with females regardless of intellectual disability status, male-to-female ratios were slightly lower among ASD-I compared with ASD-N cases.
Commenting on the study but not involved in it, Barbara J. Howard, MD, an assistant professor of medicine at Johns Hopkins University, Baltimore, said the increasing gap in identifying ASD-N according to race, ethnicity, and socioeconomic status measures probably reflects greater parental awareness of ASD and access to diagnostic services in White families and those of higher socioeconomic status. “There were no racial, ethnicity, or socioeconomic status differences in the prevalence of the more obvious and impairing ASD-I in the sample, but its prevalence was also increasing over this period,” she said.
Although the greater recognition of the less impairing ASD-N is important for optimal outcomes through intervention, the increasing discrepancies mean that more children generally and more marginalized children specifically are not being diagnosed or served. “There should be no differences in prevalence by these characteristics,” Dr. Howard said. “The striking inequity for non-White children and those of lower socioeconomic status in being diagnosed with ASD-N and thus qualifying for intervention that could improve their long-term functioning is likely also compounded by service, educational, and social disadvantages they may experience.”
In light of these disparities, an accompanying editorial by Emily Hotez, PhD, of the University of California, Los Angeles, and Lindsay Shea, DrPH, of the A.J. Drexel Autism Institute at Drexel University, Philadelphia, argues that social determinants of health (SDOH) should be prioritized in the public health surveillance of autism since these factors potentially contribute to the general underdiagnosis of autism in minority groups and merit more attention from pediatricians. While SDOH affects many nonautistic conditions, it may be even more important for families dealing with the stressors and isolation associated with autism, the commentators said. “Our commentary speaks to the utility of increasing SDOH surveillance in improving our understanding of autistic individuals’ needs, experiences, and priorities on a population level,” Dr. Hotez said in an interview. She added that integrating SDOH surveillance into pediatricians’ workflows will lead to improvements in clinical practice and patient care in the long term.
“Specifically, increased uptake of universal SDOH screening and referral practices will allow pediatricians to more proactively link autistic children and families, particularly those from marginalized groups, with much-needed health-promoting services and supports.” She cautioned, however, that while most providers believe universal SDOH screening is important, fewer report that screening is feasible or feel prepared to address families’ social needs when they are identified.
This study was supported by the Centers for Disease Control and Prevention and the National Institutes of Health/National Institute of Environmental Health Sciences. The authors had no conflicts of interest to disclose. The commentators had no potential conflicts of interest to disclose. Dr. Howard disclosed no competing interests relevant to her comments.
Approximately two out of three children with autism spectrum disorder (ASD) do not have concurrent intellectual disability, according to a population study of ASD trends.
Intellectual functioning remains the best predictor of functional outcomes in kids with ASD, and missing those with no cognitive impairment (ASD-N) can prevent intervention and affect future achievement.
Furthermore, while the study found that ASD-N increased among all demographic subgroups from 2000 to 2016, it also observed widespread health disparities in identifying ASD-N, especially in Black, Hispanic, and underprivileged children.
“ASD is a major public health concern and prevalence estimates are likely to continue to rise as disparities are reduced and ASD identification is improved,” wrote researchers led by Josephine Shenouda, DrPH, MS, of Rutgers School of Public Health in Piscataway, N.J., in Pediatrics .
The study period saw a surprising 500% increase in the prevalence of ASD-N and a 200% increase in the prevalence of cognitive impairment–associated ASD-I , with higher rates across all sex, race, ethnicity, and socioeconomic subgroups. The five- and twofold respective increases are consistent with previous research.
“To a large degree, the rise in autism estimates has been driven by individuals without intellectual disability,” Dr. Shenouda said in an interview. “The best way to address increasing autism and to affect disparities in autism identification is through universal autism screening during the toddler period. And different metrics of functional outcomes need to be developed to understand the expression of autism better.”
Her group had previously seen autism estimates of approximately 1% in 2000 rise to 3% by 2016 but had noted variations, with some communities exceeding 5% for autism estimates. “That led to the question of why, and we saw that in areas with high estimates, we are identifying more children with autism without intellectual disability,” she said. “We wanted to know if the increase over time was equally distributed among children with autism with and without intellectual disability.”
A study in disparities
The cross-sectional study examined data from active ASD surveillance by the CDC’s Autism and Developmental Disabilities Monitoring Network in 8-year-olds residing in the New York/New Jersey Metropolitan Area. Overall, 4,661 children were identified with ASD, with ASD-I affecting 1,505 (32.3%), and ASD-N affecting 2,764 (59.3%). Non-Hispanic Black children who were affected numbered 946 (20.3%), while 1,230 (26.4%) were Hispanic, and 2,114 (45.4%) were non-Hispanic White.
Notably, Black children were 30% less likely to be identified with ASD-N compared with White children, and children residing in affluent areas were 80% more likely to be identified with ASD-N versus those in underserved areas. Furthermore, a greater proportion of children with ASD-I resided in vulnerable areas compared with their counterparts with ASD-N.
While males had a higher prevalence compared with females regardless of intellectual disability status, male-to-female ratios were slightly lower among ASD-I compared with ASD-N cases.
Commenting on the study but not involved in it, Barbara J. Howard, MD, an assistant professor of medicine at Johns Hopkins University, Baltimore, said the increasing gap in identifying ASD-N according to race, ethnicity, and socioeconomic status measures probably reflects greater parental awareness of ASD and access to diagnostic services in White families and those of higher socioeconomic status. “There were no racial, ethnicity, or socioeconomic status differences in the prevalence of the more obvious and impairing ASD-I in the sample, but its prevalence was also increasing over this period,” she said.
Although the greater recognition of the less impairing ASD-N is important for optimal outcomes through intervention, the increasing discrepancies mean that more children generally and more marginalized children specifically are not being diagnosed or served. “There should be no differences in prevalence by these characteristics,” Dr. Howard said. “The striking inequity for non-White children and those of lower socioeconomic status in being diagnosed with ASD-N and thus qualifying for intervention that could improve their long-term functioning is likely also compounded by service, educational, and social disadvantages they may experience.”
In light of these disparities, an accompanying editorial by Emily Hotez, PhD, of the University of California, Los Angeles, and Lindsay Shea, DrPH, of the A.J. Drexel Autism Institute at Drexel University, Philadelphia, argues that social determinants of health (SDOH) should be prioritized in the public health surveillance of autism since these factors potentially contribute to the general underdiagnosis of autism in minority groups and merit more attention from pediatricians. While SDOH affects many nonautistic conditions, it may be even more important for families dealing with the stressors and isolation associated with autism, the commentators said. “Our commentary speaks to the utility of increasing SDOH surveillance in improving our understanding of autistic individuals’ needs, experiences, and priorities on a population level,” Dr. Hotez said in an interview. She added that integrating SDOH surveillance into pediatricians’ workflows will lead to improvements in clinical practice and patient care in the long term.
“Specifically, increased uptake of universal SDOH screening and referral practices will allow pediatricians to more proactively link autistic children and families, particularly those from marginalized groups, with much-needed health-promoting services and supports.” She cautioned, however, that while most providers believe universal SDOH screening is important, fewer report that screening is feasible or feel prepared to address families’ social needs when they are identified.
This study was supported by the Centers for Disease Control and Prevention and the National Institutes of Health/National Institute of Environmental Health Sciences. The authors had no conflicts of interest to disclose. The commentators had no potential conflicts of interest to disclose. Dr. Howard disclosed no competing interests relevant to her comments.
FROM PEDIATRICS
Outdoor play may mitigate screen time’s risk to brain development
Watching a screen more than an hour a day as a toddler is directly linked with poorer communication and daily living skills at age 4, but outdoor play may lessen some of the effects, new research suggests.
The results point to outdoor play as a potential targeted intervention to counter suboptimal brain development in young children who are watching screens at increasingly younger ages.
The findings were published online in JAMA Pediatrics.
The researchers first investigated whether higher screen time (more than 1 hour a day on a device or watching television) at age 2 years is associated with neurodevelopmental outcomes at age 4.
They found the 885 children in the sample from the Japanese Hamamatsu Birth Cohort Study for Mothers and Children who had more screen time had lower scores on communication and daily living skills than children who watched less than an hour a day.
Scores were based on the Vineland Adaptive Behavior Scale according to parent responses to questions. The children included were born between December 2007 and March 2012 and were followed from 18 months to 4 years.
After finding the connection between screen time and lower scores, the researchers investigated whether outdoor play (at least 30 minutes a day) introduced at a 2 years and 8 months made a difference. They considered 6 or 7 days per week frequent outdoor play.
Outdoor play mitigated poorer daily living scores
The researchers found that the outdoor play intervention mitigated 18% of the association between high screen time and lower daily living scores but did not mitigate the lower communication scores.
They also found that more screen time at age 2 was significantly linked with infrequent outdoor play at age 32 months (odds ratio, 2.03; 95% confidence interval, 1.48-2.76).
The associations were consistent after taking into account factors including a child’s sex, parental education, and any autism spectrum disorder symptoms at age 18 months.
The authors noted that neurodevelopment concerns with screen use are particularly troubling as the age for use is getting younger.
A recent meta-analysis found that 75% of children younger than 2 use or watch screens, even though guidelines recommend against any screen time before 2.
In addition, the “COVID-19 pandemic led to children having more screen time, less outdoor play, and less physical activity, putting them at potentially greater risk for neurodevelopmental problems,” the authors noted.
“What is concerning is that data show screen time has not decreased after seclusion measures were lifted,” they added.
Proven benefits for outdoor play
Jennifer Cross, MD,* assistant professor and section chief for developmental pediatrics at Weill Cornell Medicine, New York, who was not part of the study, said the mitigation properties of outdoor play were something she hadn’t seen before but the concept makes sense.
“The overwhelming evidence is that screen time is not helpful for young children under the age of 2,” she said.
Outdoor play, on the other hand, has proven benefits.
“Physical activity has been shown to be good for physical and mental health so there’s no reason to believe it wouldn’t be good for 2-and-a-half-year olds,” Dr. Cross said. “It’s also good for developmental health. You want them to be engaged in imaginative play and be interactive.”
“[Outdoor play] gets them away from screens and gives them an opportunity to experience another environment and work on their motor skills and motor planning,” she added. “Exercise will change, briefly, the way our brains process information.”
Dr. Cross added that a lot of motor skills are involved in daily living skills, such as feeding, dressing, and toileting.
Screen time is increasing
The authors acknowledged that screen time may be underestimated by parents. They also noted that they did not have access to what children were watching on the screens.
“This should have been collected because the effect of high screen time differs depending on the type of program,” the authors wrote.
They added that children born in the 2020s may have been exposed to more screen time than the children reared in the early 2010s in this study.
Dr. Cross said screen use in the 2020s may be higher than estimated here and higher in certain populations globally, so it’s not easy to tell if the intervention in this study would have the same mitigating effect on a real-world population.
However, she said, the effect of outdoor play is always going to be helpful for brain development and there’s no downside.
“Exercise is just as important for little kids as it is for grown-ups,” she said.
The authors reported no relevant financial disclosures. Dr. Cross reported no relevant financial disclosures.
*Dr. Jennifer Cross is the correct name, not Dr. Jennifer Frost. The correction was made on Jan. 27, 2023.
Watching a screen more than an hour a day as a toddler is directly linked with poorer communication and daily living skills at age 4, but outdoor play may lessen some of the effects, new research suggests.
The results point to outdoor play as a potential targeted intervention to counter suboptimal brain development in young children who are watching screens at increasingly younger ages.
The findings were published online in JAMA Pediatrics.
The researchers first investigated whether higher screen time (more than 1 hour a day on a device or watching television) at age 2 years is associated with neurodevelopmental outcomes at age 4.
They found the 885 children in the sample from the Japanese Hamamatsu Birth Cohort Study for Mothers and Children who had more screen time had lower scores on communication and daily living skills than children who watched less than an hour a day.
Scores were based on the Vineland Adaptive Behavior Scale according to parent responses to questions. The children included were born between December 2007 and March 2012 and were followed from 18 months to 4 years.
After finding the connection between screen time and lower scores, the researchers investigated whether outdoor play (at least 30 minutes a day) introduced at a 2 years and 8 months made a difference. They considered 6 or 7 days per week frequent outdoor play.
Outdoor play mitigated poorer daily living scores
The researchers found that the outdoor play intervention mitigated 18% of the association between high screen time and lower daily living scores but did not mitigate the lower communication scores.
They also found that more screen time at age 2 was significantly linked with infrequent outdoor play at age 32 months (odds ratio, 2.03; 95% confidence interval, 1.48-2.76).
The associations were consistent after taking into account factors including a child’s sex, parental education, and any autism spectrum disorder symptoms at age 18 months.
The authors noted that neurodevelopment concerns with screen use are particularly troubling as the age for use is getting younger.
A recent meta-analysis found that 75% of children younger than 2 use or watch screens, even though guidelines recommend against any screen time before 2.
In addition, the “COVID-19 pandemic led to children having more screen time, less outdoor play, and less physical activity, putting them at potentially greater risk for neurodevelopmental problems,” the authors noted.
“What is concerning is that data show screen time has not decreased after seclusion measures were lifted,” they added.
Proven benefits for outdoor play
Jennifer Cross, MD,* assistant professor and section chief for developmental pediatrics at Weill Cornell Medicine, New York, who was not part of the study, said the mitigation properties of outdoor play were something she hadn’t seen before but the concept makes sense.
“The overwhelming evidence is that screen time is not helpful for young children under the age of 2,” she said.
Outdoor play, on the other hand, has proven benefits.
“Physical activity has been shown to be good for physical and mental health so there’s no reason to believe it wouldn’t be good for 2-and-a-half-year olds,” Dr. Cross said. “It’s also good for developmental health. You want them to be engaged in imaginative play and be interactive.”
“[Outdoor play] gets them away from screens and gives them an opportunity to experience another environment and work on their motor skills and motor planning,” she added. “Exercise will change, briefly, the way our brains process information.”
Dr. Cross added that a lot of motor skills are involved in daily living skills, such as feeding, dressing, and toileting.
Screen time is increasing
The authors acknowledged that screen time may be underestimated by parents. They also noted that they did not have access to what children were watching on the screens.
“This should have been collected because the effect of high screen time differs depending on the type of program,” the authors wrote.
They added that children born in the 2020s may have been exposed to more screen time than the children reared in the early 2010s in this study.
Dr. Cross said screen use in the 2020s may be higher than estimated here and higher in certain populations globally, so it’s not easy to tell if the intervention in this study would have the same mitigating effect on a real-world population.
However, she said, the effect of outdoor play is always going to be helpful for brain development and there’s no downside.
“Exercise is just as important for little kids as it is for grown-ups,” she said.
The authors reported no relevant financial disclosures. Dr. Cross reported no relevant financial disclosures.
*Dr. Jennifer Cross is the correct name, not Dr. Jennifer Frost. The correction was made on Jan. 27, 2023.
Watching a screen more than an hour a day as a toddler is directly linked with poorer communication and daily living skills at age 4, but outdoor play may lessen some of the effects, new research suggests.
The results point to outdoor play as a potential targeted intervention to counter suboptimal brain development in young children who are watching screens at increasingly younger ages.
The findings were published online in JAMA Pediatrics.
The researchers first investigated whether higher screen time (more than 1 hour a day on a device or watching television) at age 2 years is associated with neurodevelopmental outcomes at age 4.
They found the 885 children in the sample from the Japanese Hamamatsu Birth Cohort Study for Mothers and Children who had more screen time had lower scores on communication and daily living skills than children who watched less than an hour a day.
Scores were based on the Vineland Adaptive Behavior Scale according to parent responses to questions. The children included were born between December 2007 and March 2012 and were followed from 18 months to 4 years.
After finding the connection between screen time and lower scores, the researchers investigated whether outdoor play (at least 30 minutes a day) introduced at a 2 years and 8 months made a difference. They considered 6 or 7 days per week frequent outdoor play.
Outdoor play mitigated poorer daily living scores
The researchers found that the outdoor play intervention mitigated 18% of the association between high screen time and lower daily living scores but did not mitigate the lower communication scores.
They also found that more screen time at age 2 was significantly linked with infrequent outdoor play at age 32 months (odds ratio, 2.03; 95% confidence interval, 1.48-2.76).
The associations were consistent after taking into account factors including a child’s sex, parental education, and any autism spectrum disorder symptoms at age 18 months.
The authors noted that neurodevelopment concerns with screen use are particularly troubling as the age for use is getting younger.
A recent meta-analysis found that 75% of children younger than 2 use or watch screens, even though guidelines recommend against any screen time before 2.
In addition, the “COVID-19 pandemic led to children having more screen time, less outdoor play, and less physical activity, putting them at potentially greater risk for neurodevelopmental problems,” the authors noted.
“What is concerning is that data show screen time has not decreased after seclusion measures were lifted,” they added.
Proven benefits for outdoor play
Jennifer Cross, MD,* assistant professor and section chief for developmental pediatrics at Weill Cornell Medicine, New York, who was not part of the study, said the mitigation properties of outdoor play were something she hadn’t seen before but the concept makes sense.
“The overwhelming evidence is that screen time is not helpful for young children under the age of 2,” she said.
Outdoor play, on the other hand, has proven benefits.
“Physical activity has been shown to be good for physical and mental health so there’s no reason to believe it wouldn’t be good for 2-and-a-half-year olds,” Dr. Cross said. “It’s also good for developmental health. You want them to be engaged in imaginative play and be interactive.”
“[Outdoor play] gets them away from screens and gives them an opportunity to experience another environment and work on their motor skills and motor planning,” she added. “Exercise will change, briefly, the way our brains process information.”
Dr. Cross added that a lot of motor skills are involved in daily living skills, such as feeding, dressing, and toileting.
Screen time is increasing
The authors acknowledged that screen time may be underestimated by parents. They also noted that they did not have access to what children were watching on the screens.
“This should have been collected because the effect of high screen time differs depending on the type of program,” the authors wrote.
They added that children born in the 2020s may have been exposed to more screen time than the children reared in the early 2010s in this study.
Dr. Cross said screen use in the 2020s may be higher than estimated here and higher in certain populations globally, so it’s not easy to tell if the intervention in this study would have the same mitigating effect on a real-world population.
However, she said, the effect of outdoor play is always going to be helpful for brain development and there’s no downside.
“Exercise is just as important for little kids as it is for grown-ups,” she said.
The authors reported no relevant financial disclosures. Dr. Cross reported no relevant financial disclosures.
*Dr. Jennifer Cross is the correct name, not Dr. Jennifer Frost. The correction was made on Jan. 27, 2023.
FROM JAMA PEDIATRICS