User login
Childhood behavioral, emotional problems linked to poor economic and social outcomes in adulthood
Children with chronically elevated externalizing symptoms, such as behavioral problems, or internalizing symptoms, such as mental health concerns, have an increased risk for poor economic and social outcomes in adulthood, data from a new study suggest.
Children with comorbid externalizing and internalizing symptoms were especially vulnerable to long-term economic and social exclusion.
“Research has mostly studied the outcomes of children with either behavioral problems or depression-anxiety problems. However, comorbidity is the rule rather than the exception in clinical practice,” senior author Massimilliano Orri, PhD, an assistant professor of psychiatry at McGill University and clinical psychologist with the Douglas Mental Health University Institute, both in Montreal, said in an interview.
“Our findings are important, as they show that comorbidity between externalizing and internalizing problems is associated with real-life outcomes that profoundly influence a youth’s chances to participate in society later in life,” he said.
The study was published in JAMA Network Open.
Analyzing associations
Dr. Orri and colleagues analyzed data for 3,017 children in the Quebec Longitudinal Study of Kindergarten Children, a population-based birth cohort that enrolled participants in 1986-1987 and 1987-1988 while they were attending kindergarten. The sample included 2,000 children selected at random and 1,017 children who scored at or above the 80th percentile for disruptive behavior problems.
The research team looked at the association between childhood behavioral profiles and economic and social outcomes for ages 19-37 years, including employment earnings, receipt of welfare, intimate partnerships, and having children living in the household. They obtained the outcome data from participants’ tax returns for 1998-2017.
During enrollment in the study, the children’s teachers assessed behavioral symptoms annually for ages 6-12 years using the Social Behavior Questionnaire. Based on the assessments, the research team categorized the students as having no or low symptoms, high externalizing symptoms only (such as hyperactivity, impulsivity, aggression, and rule violation), high internalizing symptoms only (such as anxiety, depression, worry, and social withdrawal), or comorbid symptoms. They looked at other variables as well, including the child’s sex, the parents’ age at the birth of their first child, the parents’ years of education, family structure, and the parents’ household income.
Among the 3,017 participants, 45.4% of children had no or low symptoms, 29.2% had high externalizing symptoms, 11.7% had high internalizing symptoms, and 13.7% had comorbid symptoms. About 53% were boys, and 47% were girls.
In general, boys were more likely to exhibit high externalizing symptoms, and girls were more likely to exhibit high internalizing symptoms. In the comorbid group, about 82% were boys, and they were more likely to have younger mothers, come from households with lower earnings when they were ages 3-5 years, and have a nonintact family at age 6 years.
The average age at follow-up was 37 years. Participants earned an average of $32,800 per year at ages 33-37 years (between 2013 and 2017). During the 20 years of follow-up, participants received welfare support for about 1.5 years, had an intimate partner for 7.4 years, and had children living in the household for 11 years.
Overall, participants in the high externalizing and high internalizing symptom profiles – and especially those in the comorbid profile – had lower earnings and a higher incidence of annual welfare receipt across early adulthood, compared with participants with low or no symptoms. They were also less likely to have an intimate partner or have children living in the household. Participants with a comorbid symptom profile earned $15,031 less per year and had a 3.79-times higher incidence of annual welfare receipt.
Lower earnings
Across the sample, men were more likely to have higher earnings and less likely to receive welfare each year, but they also were less likely to have an intimate partner or have children in the household. Among those with the high externalizing profile, men were significantly less likely to receive welfare. Among the comorbid profile, men were less likely to have children in the household.
Compared with the no-symptom or low-symptom profile, those in the high externalizing profile earned $5,904 less per year and had a two-times–higher incidence of welfare receipt. Those in the high internalizing profile earned $8,473 less per year, had a 2.07-times higher incidence of welfare receipt, and had a lower incidence of intimate partnership.
Compared with the high externalizing profile, those in the comorbid profile earned $9,126 less per year, had a higher incidence of annual welfare receipt, had a lower incidence of intimate partnership, and were less likely to have children in the household. Similarly, compared with the high internalizing profile, those in the comorbid profile earned $6,558 less per year and were more likely to exhibit the other poor long-term outcomes. Participants in the high internalizing profile earned $2,568 less per year than those in the high externalizing profile.
During a 40-year working career, the estimated lost personal employment earnings were $140,515 for the high externalizing profile, $201,657 for the high internalizing profile, and $357,737 for the comorbid profile, compared with those in the no-symptom or low-symptom profile.
“We know that children with externalizing and internalizing symptoms can have many problems in the short term – like social difficulties and lower education attainment – but it’s important to also understand the potential long-term outcomes,” study author Francis Vergunst, DPhil/PhD, an associate professor of child psychosocial difficulties at the University of Oslo, told this news organization.
“For example, when people have insufficient income, are forced to seek welfare support, or lack the social support structure that comes from an intimate partnership, it can have profound consequences for their mental health and well-being – and for society as a whole,” he said. “Understanding this helps to build the case for early prevention programs that can reduce childhood externalizing and internalizing problems and improve long-term outcomes.”
Several mechanisms could explain the associations found across the childhood symptom profiles, the study authors wrote. For instance, children with early behavior problems may be more likely to engage in risky adolescent activities, such as substance use, delinquent peer affiliations, and academic underachievement, which affects their transition to adulthood and accumulation of social and economic capital throughout life. Those with comorbid symptoms likely experience a compounded effect.
Future studies should investigate how to intervene effectively to support children, particularly those with comorbid externalizing and internalizing symptoms, the study authors write.
“Currently, most published studies focus on children with either externalizing or internalizing problems (and these programs can be effective, especially for externalizing problems), but we know very little about how to improve long-term outcomes for children with comorbid symptoms,” Dr. Vergunst said. “Given the large costs of these problems for individuals and society, this is a critical area for further research.”
‘Solid evidence’
Commenting on the findings, Ian Colman, PhD, a professor of epidemiology and public health and director of the Applied Psychiatric Epidemiology Across the Life course (APEAL) lab at the University of Ottawa, said, “Research like this provides solid evidence that if we do not provide appropriate supports for children who are struggling with their mental health or related behaviors, then these children are more likely to face a life of social and economic exclusion.”
Dr. Colman, who wasn’t involved with this study, has researched long-term psychosocial outcomes among adolescents with depression, as well as those with externalizing behaviors. He and colleagues have found poorer outcomes among those who exhibit mild or severe difficulties during childhood.
“Studying the long-term outcomes associated with child and adolescent mental and behavioral disorders gives us an idea of how concerned we should be about their future,” he said.
Dr. Vergunst was funded by the Canadian Institute of Health Research and Fonds de Recherche du Quebec Santé postdoctoral fellowships. Dr. Orri and Dr. Colman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children with chronically elevated externalizing symptoms, such as behavioral problems, or internalizing symptoms, such as mental health concerns, have an increased risk for poor economic and social outcomes in adulthood, data from a new study suggest.
Children with comorbid externalizing and internalizing symptoms were especially vulnerable to long-term economic and social exclusion.
“Research has mostly studied the outcomes of children with either behavioral problems or depression-anxiety problems. However, comorbidity is the rule rather than the exception in clinical practice,” senior author Massimilliano Orri, PhD, an assistant professor of psychiatry at McGill University and clinical psychologist with the Douglas Mental Health University Institute, both in Montreal, said in an interview.
“Our findings are important, as they show that comorbidity between externalizing and internalizing problems is associated with real-life outcomes that profoundly influence a youth’s chances to participate in society later in life,” he said.
The study was published in JAMA Network Open.
Analyzing associations
Dr. Orri and colleagues analyzed data for 3,017 children in the Quebec Longitudinal Study of Kindergarten Children, a population-based birth cohort that enrolled participants in 1986-1987 and 1987-1988 while they were attending kindergarten. The sample included 2,000 children selected at random and 1,017 children who scored at or above the 80th percentile for disruptive behavior problems.
The research team looked at the association between childhood behavioral profiles and economic and social outcomes for ages 19-37 years, including employment earnings, receipt of welfare, intimate partnerships, and having children living in the household. They obtained the outcome data from participants’ tax returns for 1998-2017.
During enrollment in the study, the children’s teachers assessed behavioral symptoms annually for ages 6-12 years using the Social Behavior Questionnaire. Based on the assessments, the research team categorized the students as having no or low symptoms, high externalizing symptoms only (such as hyperactivity, impulsivity, aggression, and rule violation), high internalizing symptoms only (such as anxiety, depression, worry, and social withdrawal), or comorbid symptoms. They looked at other variables as well, including the child’s sex, the parents’ age at the birth of their first child, the parents’ years of education, family structure, and the parents’ household income.
Among the 3,017 participants, 45.4% of children had no or low symptoms, 29.2% had high externalizing symptoms, 11.7% had high internalizing symptoms, and 13.7% had comorbid symptoms. About 53% were boys, and 47% were girls.
In general, boys were more likely to exhibit high externalizing symptoms, and girls were more likely to exhibit high internalizing symptoms. In the comorbid group, about 82% were boys, and they were more likely to have younger mothers, come from households with lower earnings when they were ages 3-5 years, and have a nonintact family at age 6 years.
The average age at follow-up was 37 years. Participants earned an average of $32,800 per year at ages 33-37 years (between 2013 and 2017). During the 20 years of follow-up, participants received welfare support for about 1.5 years, had an intimate partner for 7.4 years, and had children living in the household for 11 years.
Overall, participants in the high externalizing and high internalizing symptom profiles – and especially those in the comorbid profile – had lower earnings and a higher incidence of annual welfare receipt across early adulthood, compared with participants with low or no symptoms. They were also less likely to have an intimate partner or have children living in the household. Participants with a comorbid symptom profile earned $15,031 less per year and had a 3.79-times higher incidence of annual welfare receipt.
Lower earnings
Across the sample, men were more likely to have higher earnings and less likely to receive welfare each year, but they also were less likely to have an intimate partner or have children in the household. Among those with the high externalizing profile, men were significantly less likely to receive welfare. Among the comorbid profile, men were less likely to have children in the household.
Compared with the no-symptom or low-symptom profile, those in the high externalizing profile earned $5,904 less per year and had a two-times–higher incidence of welfare receipt. Those in the high internalizing profile earned $8,473 less per year, had a 2.07-times higher incidence of welfare receipt, and had a lower incidence of intimate partnership.
Compared with the high externalizing profile, those in the comorbid profile earned $9,126 less per year, had a higher incidence of annual welfare receipt, had a lower incidence of intimate partnership, and were less likely to have children in the household. Similarly, compared with the high internalizing profile, those in the comorbid profile earned $6,558 less per year and were more likely to exhibit the other poor long-term outcomes. Participants in the high internalizing profile earned $2,568 less per year than those in the high externalizing profile.
During a 40-year working career, the estimated lost personal employment earnings were $140,515 for the high externalizing profile, $201,657 for the high internalizing profile, and $357,737 for the comorbid profile, compared with those in the no-symptom or low-symptom profile.
“We know that children with externalizing and internalizing symptoms can have many problems in the short term – like social difficulties and lower education attainment – but it’s important to also understand the potential long-term outcomes,” study author Francis Vergunst, DPhil/PhD, an associate professor of child psychosocial difficulties at the University of Oslo, told this news organization.
“For example, when people have insufficient income, are forced to seek welfare support, or lack the social support structure that comes from an intimate partnership, it can have profound consequences for their mental health and well-being – and for society as a whole,” he said. “Understanding this helps to build the case for early prevention programs that can reduce childhood externalizing and internalizing problems and improve long-term outcomes.”
Several mechanisms could explain the associations found across the childhood symptom profiles, the study authors wrote. For instance, children with early behavior problems may be more likely to engage in risky adolescent activities, such as substance use, delinquent peer affiliations, and academic underachievement, which affects their transition to adulthood and accumulation of social and economic capital throughout life. Those with comorbid symptoms likely experience a compounded effect.
Future studies should investigate how to intervene effectively to support children, particularly those with comorbid externalizing and internalizing symptoms, the study authors write.
“Currently, most published studies focus on children with either externalizing or internalizing problems (and these programs can be effective, especially for externalizing problems), but we know very little about how to improve long-term outcomes for children with comorbid symptoms,” Dr. Vergunst said. “Given the large costs of these problems for individuals and society, this is a critical area for further research.”
‘Solid evidence’
Commenting on the findings, Ian Colman, PhD, a professor of epidemiology and public health and director of the Applied Psychiatric Epidemiology Across the Life course (APEAL) lab at the University of Ottawa, said, “Research like this provides solid evidence that if we do not provide appropriate supports for children who are struggling with their mental health or related behaviors, then these children are more likely to face a life of social and economic exclusion.”
Dr. Colman, who wasn’t involved with this study, has researched long-term psychosocial outcomes among adolescents with depression, as well as those with externalizing behaviors. He and colleagues have found poorer outcomes among those who exhibit mild or severe difficulties during childhood.
“Studying the long-term outcomes associated with child and adolescent mental and behavioral disorders gives us an idea of how concerned we should be about their future,” he said.
Dr. Vergunst was funded by the Canadian Institute of Health Research and Fonds de Recherche du Quebec Santé postdoctoral fellowships. Dr. Orri and Dr. Colman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children with chronically elevated externalizing symptoms, such as behavioral problems, or internalizing symptoms, such as mental health concerns, have an increased risk for poor economic and social outcomes in adulthood, data from a new study suggest.
Children with comorbid externalizing and internalizing symptoms were especially vulnerable to long-term economic and social exclusion.
“Research has mostly studied the outcomes of children with either behavioral problems or depression-anxiety problems. However, comorbidity is the rule rather than the exception in clinical practice,” senior author Massimilliano Orri, PhD, an assistant professor of psychiatry at McGill University and clinical psychologist with the Douglas Mental Health University Institute, both in Montreal, said in an interview.
“Our findings are important, as they show that comorbidity between externalizing and internalizing problems is associated with real-life outcomes that profoundly influence a youth’s chances to participate in society later in life,” he said.
The study was published in JAMA Network Open.
Analyzing associations
Dr. Orri and colleagues analyzed data for 3,017 children in the Quebec Longitudinal Study of Kindergarten Children, a population-based birth cohort that enrolled participants in 1986-1987 and 1987-1988 while they were attending kindergarten. The sample included 2,000 children selected at random and 1,017 children who scored at or above the 80th percentile for disruptive behavior problems.
The research team looked at the association between childhood behavioral profiles and economic and social outcomes for ages 19-37 years, including employment earnings, receipt of welfare, intimate partnerships, and having children living in the household. They obtained the outcome data from participants’ tax returns for 1998-2017.
During enrollment in the study, the children’s teachers assessed behavioral symptoms annually for ages 6-12 years using the Social Behavior Questionnaire. Based on the assessments, the research team categorized the students as having no or low symptoms, high externalizing symptoms only (such as hyperactivity, impulsivity, aggression, and rule violation), high internalizing symptoms only (such as anxiety, depression, worry, and social withdrawal), or comorbid symptoms. They looked at other variables as well, including the child’s sex, the parents’ age at the birth of their first child, the parents’ years of education, family structure, and the parents’ household income.
Among the 3,017 participants, 45.4% of children had no or low symptoms, 29.2% had high externalizing symptoms, 11.7% had high internalizing symptoms, and 13.7% had comorbid symptoms. About 53% were boys, and 47% were girls.
In general, boys were more likely to exhibit high externalizing symptoms, and girls were more likely to exhibit high internalizing symptoms. In the comorbid group, about 82% were boys, and they were more likely to have younger mothers, come from households with lower earnings when they were ages 3-5 years, and have a nonintact family at age 6 years.
The average age at follow-up was 37 years. Participants earned an average of $32,800 per year at ages 33-37 years (between 2013 and 2017). During the 20 years of follow-up, participants received welfare support for about 1.5 years, had an intimate partner for 7.4 years, and had children living in the household for 11 years.
Overall, participants in the high externalizing and high internalizing symptom profiles – and especially those in the comorbid profile – had lower earnings and a higher incidence of annual welfare receipt across early adulthood, compared with participants with low or no symptoms. They were also less likely to have an intimate partner or have children living in the household. Participants with a comorbid symptom profile earned $15,031 less per year and had a 3.79-times higher incidence of annual welfare receipt.
Lower earnings
Across the sample, men were more likely to have higher earnings and less likely to receive welfare each year, but they also were less likely to have an intimate partner or have children in the household. Among those with the high externalizing profile, men were significantly less likely to receive welfare. Among the comorbid profile, men were less likely to have children in the household.
Compared with the no-symptom or low-symptom profile, those in the high externalizing profile earned $5,904 less per year and had a two-times–higher incidence of welfare receipt. Those in the high internalizing profile earned $8,473 less per year, had a 2.07-times higher incidence of welfare receipt, and had a lower incidence of intimate partnership.
Compared with the high externalizing profile, those in the comorbid profile earned $9,126 less per year, had a higher incidence of annual welfare receipt, had a lower incidence of intimate partnership, and were less likely to have children in the household. Similarly, compared with the high internalizing profile, those in the comorbid profile earned $6,558 less per year and were more likely to exhibit the other poor long-term outcomes. Participants in the high internalizing profile earned $2,568 less per year than those in the high externalizing profile.
During a 40-year working career, the estimated lost personal employment earnings were $140,515 for the high externalizing profile, $201,657 for the high internalizing profile, and $357,737 for the comorbid profile, compared with those in the no-symptom or low-symptom profile.
“We know that children with externalizing and internalizing symptoms can have many problems in the short term – like social difficulties and lower education attainment – but it’s important to also understand the potential long-term outcomes,” study author Francis Vergunst, DPhil/PhD, an associate professor of child psychosocial difficulties at the University of Oslo, told this news organization.
“For example, when people have insufficient income, are forced to seek welfare support, or lack the social support structure that comes from an intimate partnership, it can have profound consequences for their mental health and well-being – and for society as a whole,” he said. “Understanding this helps to build the case for early prevention programs that can reduce childhood externalizing and internalizing problems and improve long-term outcomes.”
Several mechanisms could explain the associations found across the childhood symptom profiles, the study authors wrote. For instance, children with early behavior problems may be more likely to engage in risky adolescent activities, such as substance use, delinquent peer affiliations, and academic underachievement, which affects their transition to adulthood and accumulation of social and economic capital throughout life. Those with comorbid symptoms likely experience a compounded effect.
Future studies should investigate how to intervene effectively to support children, particularly those with comorbid externalizing and internalizing symptoms, the study authors write.
“Currently, most published studies focus on children with either externalizing or internalizing problems (and these programs can be effective, especially for externalizing problems), but we know very little about how to improve long-term outcomes for children with comorbid symptoms,” Dr. Vergunst said. “Given the large costs of these problems for individuals and society, this is a critical area for further research.”
‘Solid evidence’
Commenting on the findings, Ian Colman, PhD, a professor of epidemiology and public health and director of the Applied Psychiatric Epidemiology Across the Life course (APEAL) lab at the University of Ottawa, said, “Research like this provides solid evidence that if we do not provide appropriate supports for children who are struggling with their mental health or related behaviors, then these children are more likely to face a life of social and economic exclusion.”
Dr. Colman, who wasn’t involved with this study, has researched long-term psychosocial outcomes among adolescents with depression, as well as those with externalizing behaviors. He and colleagues have found poorer outcomes among those who exhibit mild or severe difficulties during childhood.
“Studying the long-term outcomes associated with child and adolescent mental and behavioral disorders gives us an idea of how concerned we should be about their future,” he said.
Dr. Vergunst was funded by the Canadian Institute of Health Research and Fonds de Recherche du Quebec Santé postdoctoral fellowships. Dr. Orri and Dr. Colman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Why do GI symptoms persist in some children with celiac disease?
FROM WORLD JOURNAL OF GASTROENTEROLOGY
Developing FGIDs may be linked to caloric intake and percentage of food fat, but it does not change between a GFD with processed foods or a GFD with natural products.
These are the main findings of a study run jointly by the “Federico II” University of Naples and the “Luigi Vanvitelli” University of Campania, the results of which were published in the World Journal of Gastroenterology.
Unlike in previous studies, the criteria used in this study (the Rome IV criteria) allowed investigators to diagnose FGIDs even when other organic diseases, such as celiac disease or chronic inflammatory bowel disease, were present. The evidence obtained shows that adult individuals with celiac disease are at an increased risk for functional abdominal pain, even if they adhere well to a GFD. The researchers at the University of Campania wanted to determine the prevalence of FGIDs in the pediatric age group, which has been a poorly explored area.
The study authors enrolled 104 pediatric patients (aged 1-18 years) who had been diagnosed with celiac disease. The patients were randomly divided into two groups. Group A (n = 55) received a controlled GFD with processed foods (diet 1), and group B (n = 49) received a controlled GFD with > 60% natural products (diet 2). The presence of FGIDs was assessed at diagnosis (T0) and after 12 months (T1), and any potential link to the type of diet was analyzed.
The number of symptomatic children at enrollment was 30 of 55 (54.5%) in group A and 25 of 49 (51%) in group B. After 12 months, despite negative serology for celiac disease, the prevalence of FGIDs was 10/55 (18%) in group A and 8/49 (16.3%) in group B. There was no statistically significant difference between the two groups at T1. The most common disorder was functional constipation, followed by postprandial distress syndrome. At T1, the macro- and micronutrient intake was similar between the two groups, with no significant differences in nutrient analysis. However, in both groups, the prevalence of FGIDs was lower in patients who were consuming fewer calories (odds ratio [OR], 0.99; 95% confidence interval [CI], 0.99-1.00) and fat (OR, 0.33; 95% CI, 0.65-0.95). The figure was very close to being statistically significant (P = .055).
“This is the first study to show that the presence of functional GI symptoms in children with celiac disease on a GFD are possibly related to higher caloric and fat intake,” wrote the study authors. “It remains to be determined whether the risk is due to the persistence of a chronic inflammatory process or to nutritional factors. Long-term monitoring studies will assist in determining the natural history of these functional symptoms.”
The study authors reported having no relevant financial conflicts.
This article was translated from Univadis Italy and a version appeared on Medscape.com.
FROM WORLD JOURNAL OF GASTROENTEROLOGY
Developing FGIDs may be linked to caloric intake and percentage of food fat, but it does not change between a GFD with processed foods or a GFD with natural products.
These are the main findings of a study run jointly by the “Federico II” University of Naples and the “Luigi Vanvitelli” University of Campania, the results of which were published in the World Journal of Gastroenterology.
Unlike in previous studies, the criteria used in this study (the Rome IV criteria) allowed investigators to diagnose FGIDs even when other organic diseases, such as celiac disease or chronic inflammatory bowel disease, were present. The evidence obtained shows that adult individuals with celiac disease are at an increased risk for functional abdominal pain, even if they adhere well to a GFD. The researchers at the University of Campania wanted to determine the prevalence of FGIDs in the pediatric age group, which has been a poorly explored area.
The study authors enrolled 104 pediatric patients (aged 1-18 years) who had been diagnosed with celiac disease. The patients were randomly divided into two groups. Group A (n = 55) received a controlled GFD with processed foods (diet 1), and group B (n = 49) received a controlled GFD with > 60% natural products (diet 2). The presence of FGIDs was assessed at diagnosis (T0) and after 12 months (T1), and any potential link to the type of diet was analyzed.
The number of symptomatic children at enrollment was 30 of 55 (54.5%) in group A and 25 of 49 (51%) in group B. After 12 months, despite negative serology for celiac disease, the prevalence of FGIDs was 10/55 (18%) in group A and 8/49 (16.3%) in group B. There was no statistically significant difference between the two groups at T1. The most common disorder was functional constipation, followed by postprandial distress syndrome. At T1, the macro- and micronutrient intake was similar between the two groups, with no significant differences in nutrient analysis. However, in both groups, the prevalence of FGIDs was lower in patients who were consuming fewer calories (odds ratio [OR], 0.99; 95% confidence interval [CI], 0.99-1.00) and fat (OR, 0.33; 95% CI, 0.65-0.95). The figure was very close to being statistically significant (P = .055).
“This is the first study to show that the presence of functional GI symptoms in children with celiac disease on a GFD are possibly related to higher caloric and fat intake,” wrote the study authors. “It remains to be determined whether the risk is due to the persistence of a chronic inflammatory process or to nutritional factors. Long-term monitoring studies will assist in determining the natural history of these functional symptoms.”
The study authors reported having no relevant financial conflicts.
This article was translated from Univadis Italy and a version appeared on Medscape.com.
FROM WORLD JOURNAL OF GASTROENTEROLOGY
Developing FGIDs may be linked to caloric intake and percentage of food fat, but it does not change between a GFD with processed foods or a GFD with natural products.
These are the main findings of a study run jointly by the “Federico II” University of Naples and the “Luigi Vanvitelli” University of Campania, the results of which were published in the World Journal of Gastroenterology.
Unlike in previous studies, the criteria used in this study (the Rome IV criteria) allowed investigators to diagnose FGIDs even when other organic diseases, such as celiac disease or chronic inflammatory bowel disease, were present. The evidence obtained shows that adult individuals with celiac disease are at an increased risk for functional abdominal pain, even if they adhere well to a GFD. The researchers at the University of Campania wanted to determine the prevalence of FGIDs in the pediatric age group, which has been a poorly explored area.
The study authors enrolled 104 pediatric patients (aged 1-18 years) who had been diagnosed with celiac disease. The patients were randomly divided into two groups. Group A (n = 55) received a controlled GFD with processed foods (diet 1), and group B (n = 49) received a controlled GFD with > 60% natural products (diet 2). The presence of FGIDs was assessed at diagnosis (T0) and after 12 months (T1), and any potential link to the type of diet was analyzed.
The number of symptomatic children at enrollment was 30 of 55 (54.5%) in group A and 25 of 49 (51%) in group B. After 12 months, despite negative serology for celiac disease, the prevalence of FGIDs was 10/55 (18%) in group A and 8/49 (16.3%) in group B. There was no statistically significant difference between the two groups at T1. The most common disorder was functional constipation, followed by postprandial distress syndrome. At T1, the macro- and micronutrient intake was similar between the two groups, with no significant differences in nutrient analysis. However, in both groups, the prevalence of FGIDs was lower in patients who were consuming fewer calories (odds ratio [OR], 0.99; 95% confidence interval [CI], 0.99-1.00) and fat (OR, 0.33; 95% CI, 0.65-0.95). The figure was very close to being statistically significant (P = .055).
“This is the first study to show that the presence of functional GI symptoms in children with celiac disease on a GFD are possibly related to higher caloric and fat intake,” wrote the study authors. “It remains to be determined whether the risk is due to the persistence of a chronic inflammatory process or to nutritional factors. Long-term monitoring studies will assist in determining the natural history of these functional symptoms.”
The study authors reported having no relevant financial conflicts.
This article was translated from Univadis Italy and a version appeared on Medscape.com.
Hope for catching infants with CP early
A new prognostic tool may help identify infants with cerebral palsy (CP) earlier, allowing them to receive therapies to improve later outcomes.
Researchers from Canada used 12 clinical variables to predict the condition. The tool accurately predicted 75% of CP cases. The study was published in JAMA Pediatrics.
The prevalence of CP in the United States is 2-3 children per 1,000, a rate that has been relatively unchanged for decades. Although recent innovations in diagnosis using motor scores and MRI scans have aided in diagnosis, these techniques have historically been reserved only for infants who were cared for in neonatal intensive care units, were born prematurely, or who had other neurologic risk factors, such as birth defects.
The tool identified 2.4 times more children with CP than would have been detected using current diagnostic methods, according to the researchers.
“We developed the prediction tool to try to make these findings accessible to any health care provider, which will hopefully help break down the long-held perception that CP is usually related to prematurity or a difficult delivery,” said Mary Dunbar, MD, an author of the study. “We know that about half of children with CP aren’t premature and didn’t have a particularly difficult birth.”
The bedside tool weighs factors such as the use by mothers of illicit drugs and tobacco; the presence of diabetes and preeclampsia during pregnancy; whether the infant is male; birth weight; and the number of miscarriages the mother had prior to the birth. The tool also factors in results from a test that measures how well the infant is adjusting to life outside the womb.
Dr. Dunbar and colleagues compared 1,265 infants with CP from the Canadian Cerebral Palsy Registry from 2003 to 2019 with a control group of 1,985 children without CP from the Alberta Pregnancy Outcomes and Nutrition longitudinal study.
The study authors hope that the prognostic tool can be integrated into existing newborn screenings and completed by nurses or physicians as part of routine care.
“Its cost is low especially in comparison to MRI and specialized neurological assessments,” said Sarah Taylor, MD, section chief of neonatal-perinatal medicine at Yale New Haven Children’s Hospital in New Haven, Conn. Health systems and doctors may be more apt to adopt the tool, since it does not require specialized equipment or training.
Surprising findings
Several clinical variables independently increased the risk of CP, including independent 5-minute Apgar test scores of <6, chorioamnionitis, and illicit drug use during the pregnancy. Dr. Dunbar and colleagues recommend that primary care clinicians provide enhanced surveillance for these infants.
“I think there are also really important public health implications to address maternal and reproductive health to support pregnant people, since this study shows that common pregnancy conditions that are potentially treatable may additively contribute to cerebral palsy risk,” said Dr. Dunbar, a pediatric neurologist and assistant professor at the University of Calgary (Alta.)
For infants identified as being at risk, the study authors also suggest that doctors conduct focused examinations for CP at 3-, 6- and 12-month well-baby visits. If results of an examination are abnormal, doctors can advise the caregiver to conduct an early expert evaluation for a general movements assessment. Interventions for children with CP usually start in the first few years of life and can include occupational therapy, use of orthotic devices, and medication.
Dr. Dunbar and colleagues acknowledge that the test is not perfect and that additional work is needed.
“As helpful as the prediction tool may be to identify cases of CP early, we know there are still a minority of CP cases that it won’t catch because they don’t have any of the known risk factors,” Dr. Dunbar said. “We’re currently working on further research about this unique group.”
The researchers cited several limitations to the dataset used in the study, including a control group that was skewed toward older patients and persons of higher socioeconomic status. In addition, the data included a greater proportion of White women than the average Canadian population.
The Canadian Cerebral Palsy Registry was supported by the NeuroDevNet, KidsBrainHealth, the Harvey Guyda Chair of McGill University, Montreal Children’s Hospital, and the Public Health Agency of Canada. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new prognostic tool may help identify infants with cerebral palsy (CP) earlier, allowing them to receive therapies to improve later outcomes.
Researchers from Canada used 12 clinical variables to predict the condition. The tool accurately predicted 75% of CP cases. The study was published in JAMA Pediatrics.
The prevalence of CP in the United States is 2-3 children per 1,000, a rate that has been relatively unchanged for decades. Although recent innovations in diagnosis using motor scores and MRI scans have aided in diagnosis, these techniques have historically been reserved only for infants who were cared for in neonatal intensive care units, were born prematurely, or who had other neurologic risk factors, such as birth defects.
The tool identified 2.4 times more children with CP than would have been detected using current diagnostic methods, according to the researchers.
“We developed the prediction tool to try to make these findings accessible to any health care provider, which will hopefully help break down the long-held perception that CP is usually related to prematurity or a difficult delivery,” said Mary Dunbar, MD, an author of the study. “We know that about half of children with CP aren’t premature and didn’t have a particularly difficult birth.”
The bedside tool weighs factors such as the use by mothers of illicit drugs and tobacco; the presence of diabetes and preeclampsia during pregnancy; whether the infant is male; birth weight; and the number of miscarriages the mother had prior to the birth. The tool also factors in results from a test that measures how well the infant is adjusting to life outside the womb.
Dr. Dunbar and colleagues compared 1,265 infants with CP from the Canadian Cerebral Palsy Registry from 2003 to 2019 with a control group of 1,985 children without CP from the Alberta Pregnancy Outcomes and Nutrition longitudinal study.
The study authors hope that the prognostic tool can be integrated into existing newborn screenings and completed by nurses or physicians as part of routine care.
“Its cost is low especially in comparison to MRI and specialized neurological assessments,” said Sarah Taylor, MD, section chief of neonatal-perinatal medicine at Yale New Haven Children’s Hospital in New Haven, Conn. Health systems and doctors may be more apt to adopt the tool, since it does not require specialized equipment or training.
Surprising findings
Several clinical variables independently increased the risk of CP, including independent 5-minute Apgar test scores of <6, chorioamnionitis, and illicit drug use during the pregnancy. Dr. Dunbar and colleagues recommend that primary care clinicians provide enhanced surveillance for these infants.
“I think there are also really important public health implications to address maternal and reproductive health to support pregnant people, since this study shows that common pregnancy conditions that are potentially treatable may additively contribute to cerebral palsy risk,” said Dr. Dunbar, a pediatric neurologist and assistant professor at the University of Calgary (Alta.)
For infants identified as being at risk, the study authors also suggest that doctors conduct focused examinations for CP at 3-, 6- and 12-month well-baby visits. If results of an examination are abnormal, doctors can advise the caregiver to conduct an early expert evaluation for a general movements assessment. Interventions for children with CP usually start in the first few years of life and can include occupational therapy, use of orthotic devices, and medication.
Dr. Dunbar and colleagues acknowledge that the test is not perfect and that additional work is needed.
“As helpful as the prediction tool may be to identify cases of CP early, we know there are still a minority of CP cases that it won’t catch because they don’t have any of the known risk factors,” Dr. Dunbar said. “We’re currently working on further research about this unique group.”
The researchers cited several limitations to the dataset used in the study, including a control group that was skewed toward older patients and persons of higher socioeconomic status. In addition, the data included a greater proportion of White women than the average Canadian population.
The Canadian Cerebral Palsy Registry was supported by the NeuroDevNet, KidsBrainHealth, the Harvey Guyda Chair of McGill University, Montreal Children’s Hospital, and the Public Health Agency of Canada. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new prognostic tool may help identify infants with cerebral palsy (CP) earlier, allowing them to receive therapies to improve later outcomes.
Researchers from Canada used 12 clinical variables to predict the condition. The tool accurately predicted 75% of CP cases. The study was published in JAMA Pediatrics.
The prevalence of CP in the United States is 2-3 children per 1,000, a rate that has been relatively unchanged for decades. Although recent innovations in diagnosis using motor scores and MRI scans have aided in diagnosis, these techniques have historically been reserved only for infants who were cared for in neonatal intensive care units, were born prematurely, or who had other neurologic risk factors, such as birth defects.
The tool identified 2.4 times more children with CP than would have been detected using current diagnostic methods, according to the researchers.
“We developed the prediction tool to try to make these findings accessible to any health care provider, which will hopefully help break down the long-held perception that CP is usually related to prematurity or a difficult delivery,” said Mary Dunbar, MD, an author of the study. “We know that about half of children with CP aren’t premature and didn’t have a particularly difficult birth.”
The bedside tool weighs factors such as the use by mothers of illicit drugs and tobacco; the presence of diabetes and preeclampsia during pregnancy; whether the infant is male; birth weight; and the number of miscarriages the mother had prior to the birth. The tool also factors in results from a test that measures how well the infant is adjusting to life outside the womb.
Dr. Dunbar and colleagues compared 1,265 infants with CP from the Canadian Cerebral Palsy Registry from 2003 to 2019 with a control group of 1,985 children without CP from the Alberta Pregnancy Outcomes and Nutrition longitudinal study.
The study authors hope that the prognostic tool can be integrated into existing newborn screenings and completed by nurses or physicians as part of routine care.
“Its cost is low especially in comparison to MRI and specialized neurological assessments,” said Sarah Taylor, MD, section chief of neonatal-perinatal medicine at Yale New Haven Children’s Hospital in New Haven, Conn. Health systems and doctors may be more apt to adopt the tool, since it does not require specialized equipment or training.
Surprising findings
Several clinical variables independently increased the risk of CP, including independent 5-minute Apgar test scores of <6, chorioamnionitis, and illicit drug use during the pregnancy. Dr. Dunbar and colleagues recommend that primary care clinicians provide enhanced surveillance for these infants.
“I think there are also really important public health implications to address maternal and reproductive health to support pregnant people, since this study shows that common pregnancy conditions that are potentially treatable may additively contribute to cerebral palsy risk,” said Dr. Dunbar, a pediatric neurologist and assistant professor at the University of Calgary (Alta.)
For infants identified as being at risk, the study authors also suggest that doctors conduct focused examinations for CP at 3-, 6- and 12-month well-baby visits. If results of an examination are abnormal, doctors can advise the caregiver to conduct an early expert evaluation for a general movements assessment. Interventions for children with CP usually start in the first few years of life and can include occupational therapy, use of orthotic devices, and medication.
Dr. Dunbar and colleagues acknowledge that the test is not perfect and that additional work is needed.
“As helpful as the prediction tool may be to identify cases of CP early, we know there are still a minority of CP cases that it won’t catch because they don’t have any of the known risk factors,” Dr. Dunbar said. “We’re currently working on further research about this unique group.”
The researchers cited several limitations to the dataset used in the study, including a control group that was skewed toward older patients and persons of higher socioeconomic status. In addition, the data included a greater proportion of White women than the average Canadian population.
The Canadian Cerebral Palsy Registry was supported by the NeuroDevNet, KidsBrainHealth, the Harvey Guyda Chair of McGill University, Montreal Children’s Hospital, and the Public Health Agency of Canada. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
‘Concerning’ uptick in pediatric antipsychotic prescribing
“This study demonstrates a concerning trend in antipsychotic prescribing in children and adolescents,” study investigator Matthias Pierce, PhD, senior research fellow at the University of Manchester (England) Center for Women’s Mental Health, who jointly led the study, said in a news release.
“We do not think the changes in prescribing necessarily relate to changes in clinical need; rather, it may be more likely to reflect changes in prescribing practice by clinicians,” Dr. Pierce said.
The study was published online in The Lancet Psychiatry.
Increase in long-term use
Between 2000 and 2019, prescriptions for antipsychotics nearly doubled from 0.06% to 0.11%.
The investigators note that the U.K.’s National Institute for Health and Care Excellence has approved the use of some antipsychotics in patients younger than age 18 with schizophrenia, bipolar disorder, and severely aggressive behavior attributable to conduct disorder.
However, these data suggest antipsychotics are being prescribed for an increasingly broad range of conditions, most commonly autism, but also for attention-deficit/ hyperactivity disorder, tic disorders like Tourrette syndrome, and learning difficulties.
“Broadening use of antipsychotics in developing young people begs questions about their safety over time and demands more research on this topic,” senior author Kathryn Abel, MBBS, PhD, from the University of Manchester said in the news release.
During the study period, antipsychotic prescribing in primary care increased by an average of 3.3% per year and the rate of first prescriptions increased by 2.2% per year.
The data also suggest that more children and adolescents are taking these powerful drugs for longer periods of time. The proportion receiving antipsychotics for at least 6 months after an initial prescription rose from 41.9% in 2000 to 62.8% in 2018.
Prescribing inequities
From 2009 onwards, more than 90% of prescriptions were for atypical antipsychotics.
Over time, risperidone dominated, with more than 60% of all prescriptions, followed by aripiprazole, quetiapine, olanzapine, and haloperidol as the most prescribed antipsychotics.
Boys and older children aged 15-18 years were most likely to receive an antipsychotic. However, the increasing trends were evident in all groups.
The data also point to inequities in prescribing as a result of deprivation levels, with typical antipsychotics prescribed more frequently in more deprived areas over time.
Dr. Pierce said he hopes this study will “help clinicians to evaluate the prescribing of antipsychotics to children more fully and will encourage them to consider better access to alternatives.”
Dr. Abel noted that antipsychotic medications “continue to have a valuable role in the treatment of serious mental illness. These findings represent a descriptive account of antipsychotic prescribing to children and adolescents in the U.K. today and provide a window onto current practice.”
Findings are no surprise
Emily Simonoff, MD, professor of child and adolescent psychiatry, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, offered perspective on the study in a statement from the U.K. nonprofit Science Media Centre.
“To clinicians, it will not be surprising that the authors demonstrate an increase in rates of prescriptions over that time period, as there has been a steadily emerging evidence base for the benefits of this group of medication for a range of different indications, which has been further supported by new licensing indications and recommendations from NICE,” Dr. Simonoff said.
For example, “there is good evidence for their benefits for other conditions such as irritability in autism spectrum disorder.
“However, it should also be noted that NICE recommendations for their use in many conditions is as part of a multimodal treatment plan, for example including psychological or behavioral interventions. It’s unclear from the study whether such recommendations were being followed or medication was being used on its own,” she added.
Dr. Simonoff also said it’s “reassuring” that prescribing rates remain very low in the youngest children and notes that the authors “rightly highlight the need for high-quality, longer-term studies on efficacy and, most importantly, adverse effects. This should be a research priority.”
The study had no funding. The authors report no relevant financial relationships. Dr. Simonoff is a member of the NICE guideline development group for the management of autism and has published on the efficacy of antipsychotic medication for irritability in autism.
A version of this article first appeared on Medscape.com.
“This study demonstrates a concerning trend in antipsychotic prescribing in children and adolescents,” study investigator Matthias Pierce, PhD, senior research fellow at the University of Manchester (England) Center for Women’s Mental Health, who jointly led the study, said in a news release.
“We do not think the changes in prescribing necessarily relate to changes in clinical need; rather, it may be more likely to reflect changes in prescribing practice by clinicians,” Dr. Pierce said.
The study was published online in The Lancet Psychiatry.
Increase in long-term use
Between 2000 and 2019, prescriptions for antipsychotics nearly doubled from 0.06% to 0.11%.
The investigators note that the U.K.’s National Institute for Health and Care Excellence has approved the use of some antipsychotics in patients younger than age 18 with schizophrenia, bipolar disorder, and severely aggressive behavior attributable to conduct disorder.
However, these data suggest antipsychotics are being prescribed for an increasingly broad range of conditions, most commonly autism, but also for attention-deficit/ hyperactivity disorder, tic disorders like Tourrette syndrome, and learning difficulties.
“Broadening use of antipsychotics in developing young people begs questions about their safety over time and demands more research on this topic,” senior author Kathryn Abel, MBBS, PhD, from the University of Manchester said in the news release.
During the study period, antipsychotic prescribing in primary care increased by an average of 3.3% per year and the rate of first prescriptions increased by 2.2% per year.
The data also suggest that more children and adolescents are taking these powerful drugs for longer periods of time. The proportion receiving antipsychotics for at least 6 months after an initial prescription rose from 41.9% in 2000 to 62.8% in 2018.
Prescribing inequities
From 2009 onwards, more than 90% of prescriptions were for atypical antipsychotics.
Over time, risperidone dominated, with more than 60% of all prescriptions, followed by aripiprazole, quetiapine, olanzapine, and haloperidol as the most prescribed antipsychotics.
Boys and older children aged 15-18 years were most likely to receive an antipsychotic. However, the increasing trends were evident in all groups.
The data also point to inequities in prescribing as a result of deprivation levels, with typical antipsychotics prescribed more frequently in more deprived areas over time.
Dr. Pierce said he hopes this study will “help clinicians to evaluate the prescribing of antipsychotics to children more fully and will encourage them to consider better access to alternatives.”
Dr. Abel noted that antipsychotic medications “continue to have a valuable role in the treatment of serious mental illness. These findings represent a descriptive account of antipsychotic prescribing to children and adolescents in the U.K. today and provide a window onto current practice.”
Findings are no surprise
Emily Simonoff, MD, professor of child and adolescent psychiatry, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, offered perspective on the study in a statement from the U.K. nonprofit Science Media Centre.
“To clinicians, it will not be surprising that the authors demonstrate an increase in rates of prescriptions over that time period, as there has been a steadily emerging evidence base for the benefits of this group of medication for a range of different indications, which has been further supported by new licensing indications and recommendations from NICE,” Dr. Simonoff said.
For example, “there is good evidence for their benefits for other conditions such as irritability in autism spectrum disorder.
“However, it should also be noted that NICE recommendations for their use in many conditions is as part of a multimodal treatment plan, for example including psychological or behavioral interventions. It’s unclear from the study whether such recommendations were being followed or medication was being used on its own,” she added.
Dr. Simonoff also said it’s “reassuring” that prescribing rates remain very low in the youngest children and notes that the authors “rightly highlight the need for high-quality, longer-term studies on efficacy and, most importantly, adverse effects. This should be a research priority.”
The study had no funding. The authors report no relevant financial relationships. Dr. Simonoff is a member of the NICE guideline development group for the management of autism and has published on the efficacy of antipsychotic medication for irritability in autism.
A version of this article first appeared on Medscape.com.
“This study demonstrates a concerning trend in antipsychotic prescribing in children and adolescents,” study investigator Matthias Pierce, PhD, senior research fellow at the University of Manchester (England) Center for Women’s Mental Health, who jointly led the study, said in a news release.
“We do not think the changes in prescribing necessarily relate to changes in clinical need; rather, it may be more likely to reflect changes in prescribing practice by clinicians,” Dr. Pierce said.
The study was published online in The Lancet Psychiatry.
Increase in long-term use
Between 2000 and 2019, prescriptions for antipsychotics nearly doubled from 0.06% to 0.11%.
The investigators note that the U.K.’s National Institute for Health and Care Excellence has approved the use of some antipsychotics in patients younger than age 18 with schizophrenia, bipolar disorder, and severely aggressive behavior attributable to conduct disorder.
However, these data suggest antipsychotics are being prescribed for an increasingly broad range of conditions, most commonly autism, but also for attention-deficit/ hyperactivity disorder, tic disorders like Tourrette syndrome, and learning difficulties.
“Broadening use of antipsychotics in developing young people begs questions about their safety over time and demands more research on this topic,” senior author Kathryn Abel, MBBS, PhD, from the University of Manchester said in the news release.
During the study period, antipsychotic prescribing in primary care increased by an average of 3.3% per year and the rate of first prescriptions increased by 2.2% per year.
The data also suggest that more children and adolescents are taking these powerful drugs for longer periods of time. The proportion receiving antipsychotics for at least 6 months after an initial prescription rose from 41.9% in 2000 to 62.8% in 2018.
Prescribing inequities
From 2009 onwards, more than 90% of prescriptions were for atypical antipsychotics.
Over time, risperidone dominated, with more than 60% of all prescriptions, followed by aripiprazole, quetiapine, olanzapine, and haloperidol as the most prescribed antipsychotics.
Boys and older children aged 15-18 years were most likely to receive an antipsychotic. However, the increasing trends were evident in all groups.
The data also point to inequities in prescribing as a result of deprivation levels, with typical antipsychotics prescribed more frequently in more deprived areas over time.
Dr. Pierce said he hopes this study will “help clinicians to evaluate the prescribing of antipsychotics to children more fully and will encourage them to consider better access to alternatives.”
Dr. Abel noted that antipsychotic medications “continue to have a valuable role in the treatment of serious mental illness. These findings represent a descriptive account of antipsychotic prescribing to children and adolescents in the U.K. today and provide a window onto current practice.”
Findings are no surprise
Emily Simonoff, MD, professor of child and adolescent psychiatry, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, offered perspective on the study in a statement from the U.K. nonprofit Science Media Centre.
“To clinicians, it will not be surprising that the authors demonstrate an increase in rates of prescriptions over that time period, as there has been a steadily emerging evidence base for the benefits of this group of medication for a range of different indications, which has been further supported by new licensing indications and recommendations from NICE,” Dr. Simonoff said.
For example, “there is good evidence for their benefits for other conditions such as irritability in autism spectrum disorder.
“However, it should also be noted that NICE recommendations for their use in many conditions is as part of a multimodal treatment plan, for example including psychological or behavioral interventions. It’s unclear from the study whether such recommendations were being followed or medication was being used on its own,” she added.
Dr. Simonoff also said it’s “reassuring” that prescribing rates remain very low in the youngest children and notes that the authors “rightly highlight the need for high-quality, longer-term studies on efficacy and, most importantly, adverse effects. This should be a research priority.”
The study had no funding. The authors report no relevant financial relationships. Dr. Simonoff is a member of the NICE guideline development group for the management of autism and has published on the efficacy of antipsychotic medication for irritability in autism.
A version of this article first appeared on Medscape.com.
FROM THE LANCET PSYCHIATRY
Kids with concussions may benefit from early return to school
The timing for return to school after a concussion has been the subject of guidelines, but data on how the timing of school returns affects later symptom burdens are limited, Christopher G. Vaughan, PhD, of Children’s National Hospital, Rockville, Md., and colleagues wrote.
Examining how the timing of return to school (RTS) affects later symptoms is needed to inform early postinjury management, they said.
In the new study published in JAMA Network Open, the researchers identified 1,630 children and teens aged 5-18 years who were treated for concussions at nine Canadian pediatric EDs. The primary outcome was symptom burden at 14 days post concussion, based on the Post-Concussion Symptom Inventory (PCSI). Early RTS was defined as missing fewer than 3 days of school post concussion.
Overall, the mean number of missed school days was 3.74 (excluding weekends). When divided by age, the mean number of missed days was 2.61 for children aged 5-7 years, 3.26 for those aged 8-12 years, and 4.71 for those aged 13-18 years.
Slightly more than half (53.7%) of the participants had an early RTS of 2 missed days or fewer. Later RTS was most common in the oldest age group, followed by the middle and younger age groups.
The researchers used a propensity score–matched analysis to determine associations. At 14 days, an early RTS was associated with reduced symptoms among 8- to 12-year-olds and 13- to 18-year-olds, though not in the youngest patients aged 5-7 years. In addition, the researchers created quantiles based on initial symptom ratings.
For the youngest age group, the association between early RTS and reduced symptoms at day 14 was higher among those with lower initial symptoms.
For the two older groups, the association was higher for those with higher initial symptoms (based on the PCSI).
The findings that earlier RTS was associated with a lower symptom burden at day 14 for those with higher levels of symptoms at baseline was surprising, but the mechanisms of the timing and effect of RTS requires more study, the researchers wrote in their discussion.
The effect of early RTS on symptoms may be in part related to factors such as “the benefits of socialization, reduced stress from not missing too much school, maintaining or returning to a normal sleep-wake schedule, and returning to light to moderate physical activity (gym class and recreational activities),” the researchers noted.
Another study related to recovery and concussion recently appeared in Neurology. In that study, the authors found that those athletes who took a longer time to recover from a sports-related concussion could still return to play with additional time off, but the methods and populations differed from the current study, which focused on RTS rather than returning to play.
The current study findings were limited by several factors including the lack of randomization for RTS timing and a lack of data on the variety of potential supports and accommodations students received, the researchers noted.
However, the results were strengthened by the large size and diverse nature of the concussions, and the roughly equal representation of boys and girls, they said.
Although randomized trials are needed to determine the best timing for RTS, the current study suggests that RTS within 2 days of a concussion is associated with improved symptoms, “and may directly or indirectly promote faster recovery,” they concluded.
Early return remains feasible for most children and teens
“Return to school can be a complicated issue for children and teens with concussions,” said Caitlyn Mooney, MD, a pediatrician and specialist in sports medicine at the University of Texas Health Science Center, San Antonio, said in an interview. Although much research has focused on diagnosis and return to sport after a concussion, there has been less focus on returning to school and learning. Various issues post concussion can make schooling difficult, and students may experience trouble with vision, concentration, sleep, headaches, and more.
Despite this knowledge, studies that specifically address recommended school protocols are limited, Dr. Mooney said. “Additionally, all concussions are different; while some students will need minimal help to return and succeed in school, others may need individualized learning plans and accommodations for school.” A return to school ideally would be a team-based approach with input from the parent, patient, physician, and educators.
“The theory of cognitive rest stems from the idea that a concussion causes metabolic dysfunction in the brain, and that increasing the metabolic demands of the brain can result in symptoms and a delayed return to school,” said Dr. Mooney.
Evidence suggests that those who start resting early after a concussion improve more quickly, “but there has been ongoing discussion over the years of what is the correct balance of cognitive rest to returning to modified activity,” she said. “This has led to the current general recommendation of rest for 24-48 hours followed by a gradual return to school as tolerated.”
Although the current study is large, it is limited by the lack of randomization, Dr. Mooney noted, therefore conclusions cannot be made that the cause of the improved symptoms is a quicker return to school.
However, the results support data from previous studies, in that both of the older age groups showed less disease burden at 14 days after an earlier return to school, she said.
“With prolonged absences, adolescents get isolated at home away from friends, and they may have increased mood symptoms. Additionally, I have found a high number of my patients who do not go to school as quickly have more sleep disturbance, which seems to increase symptoms such as difficulty concentrating or headaches,” she said. “It seems like the students do benefit from a routine schedule even if they have to have some accommodations at school, especially older students who may have more stress about missing school and falling behind on schoolwork.”
The message for pediatricians is that return to school should be individualized, Dr. Mooney said.
Although the current study does not dictate the optimal return to school, the results support those of previous studies in showing that, after 1-2 days of rest, an early return does not harm children and teens and may improve symptoms in many cases, she said. “In my experience, sometimes schools find it easier to keep the student at home rather than manage rest or special accommodations,” but the current study suggests that delaying return to school may not be the right choice for many patients.
“I hope this study empowers clinicians to advocate for these students, that the right place for them is in the classroom even with rest, extra time, or other accommodations,” said Dr. Mooney.
“Each concussion should be evaluated and treated individually; there will likely be a few who may need to stay home for a longer period of time, but this study suggests that the majority of students will suffer no ill effects from returning to the normal routine after a 2-day rest,” she noted.
The study was supported by the Canadian Institutes for Health Research. Dr. Vaughan and several coauthors disclosed being authors of the Postconcussion Symptom Inventory outside of the current study. Dr. Mooney had no financial conflicts to disclose.
The timing for return to school after a concussion has been the subject of guidelines, but data on how the timing of school returns affects later symptom burdens are limited, Christopher G. Vaughan, PhD, of Children’s National Hospital, Rockville, Md., and colleagues wrote.
Examining how the timing of return to school (RTS) affects later symptoms is needed to inform early postinjury management, they said.
In the new study published in JAMA Network Open, the researchers identified 1,630 children and teens aged 5-18 years who were treated for concussions at nine Canadian pediatric EDs. The primary outcome was symptom burden at 14 days post concussion, based on the Post-Concussion Symptom Inventory (PCSI). Early RTS was defined as missing fewer than 3 days of school post concussion.
Overall, the mean number of missed school days was 3.74 (excluding weekends). When divided by age, the mean number of missed days was 2.61 for children aged 5-7 years, 3.26 for those aged 8-12 years, and 4.71 for those aged 13-18 years.
Slightly more than half (53.7%) of the participants had an early RTS of 2 missed days or fewer. Later RTS was most common in the oldest age group, followed by the middle and younger age groups.
The researchers used a propensity score–matched analysis to determine associations. At 14 days, an early RTS was associated with reduced symptoms among 8- to 12-year-olds and 13- to 18-year-olds, though not in the youngest patients aged 5-7 years. In addition, the researchers created quantiles based on initial symptom ratings.
For the youngest age group, the association between early RTS and reduced symptoms at day 14 was higher among those with lower initial symptoms.
For the two older groups, the association was higher for those with higher initial symptoms (based on the PCSI).
The findings that earlier RTS was associated with a lower symptom burden at day 14 for those with higher levels of symptoms at baseline was surprising, but the mechanisms of the timing and effect of RTS requires more study, the researchers wrote in their discussion.
The effect of early RTS on symptoms may be in part related to factors such as “the benefits of socialization, reduced stress from not missing too much school, maintaining or returning to a normal sleep-wake schedule, and returning to light to moderate physical activity (gym class and recreational activities),” the researchers noted.
Another study related to recovery and concussion recently appeared in Neurology. In that study, the authors found that those athletes who took a longer time to recover from a sports-related concussion could still return to play with additional time off, but the methods and populations differed from the current study, which focused on RTS rather than returning to play.
The current study findings were limited by several factors including the lack of randomization for RTS timing and a lack of data on the variety of potential supports and accommodations students received, the researchers noted.
However, the results were strengthened by the large size and diverse nature of the concussions, and the roughly equal representation of boys and girls, they said.
Although randomized trials are needed to determine the best timing for RTS, the current study suggests that RTS within 2 days of a concussion is associated with improved symptoms, “and may directly or indirectly promote faster recovery,” they concluded.
Early return remains feasible for most children and teens
“Return to school can be a complicated issue for children and teens with concussions,” said Caitlyn Mooney, MD, a pediatrician and specialist in sports medicine at the University of Texas Health Science Center, San Antonio, said in an interview. Although much research has focused on diagnosis and return to sport after a concussion, there has been less focus on returning to school and learning. Various issues post concussion can make schooling difficult, and students may experience trouble with vision, concentration, sleep, headaches, and more.
Despite this knowledge, studies that specifically address recommended school protocols are limited, Dr. Mooney said. “Additionally, all concussions are different; while some students will need minimal help to return and succeed in school, others may need individualized learning plans and accommodations for school.” A return to school ideally would be a team-based approach with input from the parent, patient, physician, and educators.
“The theory of cognitive rest stems from the idea that a concussion causes metabolic dysfunction in the brain, and that increasing the metabolic demands of the brain can result in symptoms and a delayed return to school,” said Dr. Mooney.
Evidence suggests that those who start resting early after a concussion improve more quickly, “but there has been ongoing discussion over the years of what is the correct balance of cognitive rest to returning to modified activity,” she said. “This has led to the current general recommendation of rest for 24-48 hours followed by a gradual return to school as tolerated.”
Although the current study is large, it is limited by the lack of randomization, Dr. Mooney noted, therefore conclusions cannot be made that the cause of the improved symptoms is a quicker return to school.
However, the results support data from previous studies, in that both of the older age groups showed less disease burden at 14 days after an earlier return to school, she said.
“With prolonged absences, adolescents get isolated at home away from friends, and they may have increased mood symptoms. Additionally, I have found a high number of my patients who do not go to school as quickly have more sleep disturbance, which seems to increase symptoms such as difficulty concentrating or headaches,” she said. “It seems like the students do benefit from a routine schedule even if they have to have some accommodations at school, especially older students who may have more stress about missing school and falling behind on schoolwork.”
The message for pediatricians is that return to school should be individualized, Dr. Mooney said.
Although the current study does not dictate the optimal return to school, the results support those of previous studies in showing that, after 1-2 days of rest, an early return does not harm children and teens and may improve symptoms in many cases, she said. “In my experience, sometimes schools find it easier to keep the student at home rather than manage rest or special accommodations,” but the current study suggests that delaying return to school may not be the right choice for many patients.
“I hope this study empowers clinicians to advocate for these students, that the right place for them is in the classroom even with rest, extra time, or other accommodations,” said Dr. Mooney.
“Each concussion should be evaluated and treated individually; there will likely be a few who may need to stay home for a longer period of time, but this study suggests that the majority of students will suffer no ill effects from returning to the normal routine after a 2-day rest,” she noted.
The study was supported by the Canadian Institutes for Health Research. Dr. Vaughan and several coauthors disclosed being authors of the Postconcussion Symptom Inventory outside of the current study. Dr. Mooney had no financial conflicts to disclose.
The timing for return to school after a concussion has been the subject of guidelines, but data on how the timing of school returns affects later symptom burdens are limited, Christopher G. Vaughan, PhD, of Children’s National Hospital, Rockville, Md., and colleagues wrote.
Examining how the timing of return to school (RTS) affects later symptoms is needed to inform early postinjury management, they said.
In the new study published in JAMA Network Open, the researchers identified 1,630 children and teens aged 5-18 years who were treated for concussions at nine Canadian pediatric EDs. The primary outcome was symptom burden at 14 days post concussion, based on the Post-Concussion Symptom Inventory (PCSI). Early RTS was defined as missing fewer than 3 days of school post concussion.
Overall, the mean number of missed school days was 3.74 (excluding weekends). When divided by age, the mean number of missed days was 2.61 for children aged 5-7 years, 3.26 for those aged 8-12 years, and 4.71 for those aged 13-18 years.
Slightly more than half (53.7%) of the participants had an early RTS of 2 missed days or fewer. Later RTS was most common in the oldest age group, followed by the middle and younger age groups.
The researchers used a propensity score–matched analysis to determine associations. At 14 days, an early RTS was associated with reduced symptoms among 8- to 12-year-olds and 13- to 18-year-olds, though not in the youngest patients aged 5-7 years. In addition, the researchers created quantiles based on initial symptom ratings.
For the youngest age group, the association between early RTS and reduced symptoms at day 14 was higher among those with lower initial symptoms.
For the two older groups, the association was higher for those with higher initial symptoms (based on the PCSI).
The findings that earlier RTS was associated with a lower symptom burden at day 14 for those with higher levels of symptoms at baseline was surprising, but the mechanisms of the timing and effect of RTS requires more study, the researchers wrote in their discussion.
The effect of early RTS on symptoms may be in part related to factors such as “the benefits of socialization, reduced stress from not missing too much school, maintaining or returning to a normal sleep-wake schedule, and returning to light to moderate physical activity (gym class and recreational activities),” the researchers noted.
Another study related to recovery and concussion recently appeared in Neurology. In that study, the authors found that those athletes who took a longer time to recover from a sports-related concussion could still return to play with additional time off, but the methods and populations differed from the current study, which focused on RTS rather than returning to play.
The current study findings were limited by several factors including the lack of randomization for RTS timing and a lack of data on the variety of potential supports and accommodations students received, the researchers noted.
However, the results were strengthened by the large size and diverse nature of the concussions, and the roughly equal representation of boys and girls, they said.
Although randomized trials are needed to determine the best timing for RTS, the current study suggests that RTS within 2 days of a concussion is associated with improved symptoms, “and may directly or indirectly promote faster recovery,” they concluded.
Early return remains feasible for most children and teens
“Return to school can be a complicated issue for children and teens with concussions,” said Caitlyn Mooney, MD, a pediatrician and specialist in sports medicine at the University of Texas Health Science Center, San Antonio, said in an interview. Although much research has focused on diagnosis and return to sport after a concussion, there has been less focus on returning to school and learning. Various issues post concussion can make schooling difficult, and students may experience trouble with vision, concentration, sleep, headaches, and more.
Despite this knowledge, studies that specifically address recommended school protocols are limited, Dr. Mooney said. “Additionally, all concussions are different; while some students will need minimal help to return and succeed in school, others may need individualized learning plans and accommodations for school.” A return to school ideally would be a team-based approach with input from the parent, patient, physician, and educators.
“The theory of cognitive rest stems from the idea that a concussion causes metabolic dysfunction in the brain, and that increasing the metabolic demands of the brain can result in symptoms and a delayed return to school,” said Dr. Mooney.
Evidence suggests that those who start resting early after a concussion improve more quickly, “but there has been ongoing discussion over the years of what is the correct balance of cognitive rest to returning to modified activity,” she said. “This has led to the current general recommendation of rest for 24-48 hours followed by a gradual return to school as tolerated.”
Although the current study is large, it is limited by the lack of randomization, Dr. Mooney noted, therefore conclusions cannot be made that the cause of the improved symptoms is a quicker return to school.
However, the results support data from previous studies, in that both of the older age groups showed less disease burden at 14 days after an earlier return to school, she said.
“With prolonged absences, adolescents get isolated at home away from friends, and they may have increased mood symptoms. Additionally, I have found a high number of my patients who do not go to school as quickly have more sleep disturbance, which seems to increase symptoms such as difficulty concentrating or headaches,” she said. “It seems like the students do benefit from a routine schedule even if they have to have some accommodations at school, especially older students who may have more stress about missing school and falling behind on schoolwork.”
The message for pediatricians is that return to school should be individualized, Dr. Mooney said.
Although the current study does not dictate the optimal return to school, the results support those of previous studies in showing that, after 1-2 days of rest, an early return does not harm children and teens and may improve symptoms in many cases, she said. “In my experience, sometimes schools find it easier to keep the student at home rather than manage rest or special accommodations,” but the current study suggests that delaying return to school may not be the right choice for many patients.
“I hope this study empowers clinicians to advocate for these students, that the right place for them is in the classroom even with rest, extra time, or other accommodations,” said Dr. Mooney.
“Each concussion should be evaluated and treated individually; there will likely be a few who may need to stay home for a longer period of time, but this study suggests that the majority of students will suffer no ill effects from returning to the normal routine after a 2-day rest,” she noted.
The study was supported by the Canadian Institutes for Health Research. Dr. Vaughan and several coauthors disclosed being authors of the Postconcussion Symptom Inventory outside of the current study. Dr. Mooney had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Congenital CMV linked to pediatric hyperdiploid ALL
Children with hyperdiploid acute lymphoblastic leukemia (ALL) are much more likely to also have congenital cytomegalovirus (CMV) infection, according to an analysis published in JAMA Network Open.
Although researchers found no association between ALL and congenital CMV infection overall, pediatric patients diagnosed with hyperdiploid ALL had sixfold greater odds of being positive for congenital CMV than cancer-free controls.
“These findings suggest mixed evidence for an association between congenital CMV infection and ALL” and that “a CMV-ALL association may be specific to hyperdiploid ALL,” said investigators, led by Jennifer Geris, PhD, a postdoctoral associate at Baylor College of Medicine, Houston.
A growing body of evidence suggests that CMV, a member of the herpesvirus family, may be a risk factor for ALL. Although the mechanism remains unclear, congenital CMV may encourage proliferation of CD34+ hematopoietic progenitor cells in bone marrow that are vulnerable to oncogenic transformation.
Two prior independent studies have suggested that prenatal CMV infection is associated with an increased risk of childhood ALL. However, given how common CMV infection is (more than 80% seropositivity worldwide) and the relatively rarity of pediatric ALL, Joseph Wiemels, PhD, argued in an accompanying editorial that CMV can’t be a direct cause of leukemia.
“Instead, CMV may play a supportive role” with infection in some infants altering immune function in a way that increases vulnerability to more direct causes of ALL, explained Dr. Wiemels, professor of population and public health sciences at the University of Southern California, Los Angeles. In other words, “exposure to CMV early rather than fulminant infection” at birth “may be the key epidemiologic feature.”
In the current study, Dr. Geris and colleagues tested dried newborn blood spots from 1189 children with ALL and 4,756 controls matched on age, sex, and mother’s race and ethnicity for the presence of cytomegalovirus at birth. Children were born in Michigan on or after Oct. 1, 1987.
Across the entire study population, congenital CMV was detected in 6 ALL cases (0.5%) and 21 controls (0.4%), with no difference in the odds of congenital CMV infection between the two groups. Among subjects positive for congenital CMV, it was not clear who had fulminant, clinically recognized disease and who did not.
Overall, 2 of 74 cases (2.7%) of hyperdiploid ALL were positive for congenital CMV. Compared with all controls in an unmatched analysis, those with hyperdiploid ALL were 6.26 times more likely to be CMV positive.
Overall, the investigators concluded that the current findings, in combination with previous evidence showing a similar connection, “strongly suggest CMV is associated specifically to hyperdiploid ALL.”
Although “the evidence supporting an association between CMV and ALL is tantalizing and mounting rapidly,” Dr. Wiemels noted that “much additional research attention is required to mechanistically describe pathways by which CMV may influence leukemia before the virus could be considered a potential target for prevention or clinical management of ALL.”
“We are still in the early chapters of the book describing the role of CMV and ALL,” but the virus might emerge as a clinical target “with much future promise for the health and well-being of our children,” he said.
The work was funded by the National Institutes of Health, the University of Minnesota, and the Department of Defense. The investigators and editorialist have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children with hyperdiploid acute lymphoblastic leukemia (ALL) are much more likely to also have congenital cytomegalovirus (CMV) infection, according to an analysis published in JAMA Network Open.
Although researchers found no association between ALL and congenital CMV infection overall, pediatric patients diagnosed with hyperdiploid ALL had sixfold greater odds of being positive for congenital CMV than cancer-free controls.
“These findings suggest mixed evidence for an association between congenital CMV infection and ALL” and that “a CMV-ALL association may be specific to hyperdiploid ALL,” said investigators, led by Jennifer Geris, PhD, a postdoctoral associate at Baylor College of Medicine, Houston.
A growing body of evidence suggests that CMV, a member of the herpesvirus family, may be a risk factor for ALL. Although the mechanism remains unclear, congenital CMV may encourage proliferation of CD34+ hematopoietic progenitor cells in bone marrow that are vulnerable to oncogenic transformation.
Two prior independent studies have suggested that prenatal CMV infection is associated with an increased risk of childhood ALL. However, given how common CMV infection is (more than 80% seropositivity worldwide) and the relatively rarity of pediatric ALL, Joseph Wiemels, PhD, argued in an accompanying editorial that CMV can’t be a direct cause of leukemia.
“Instead, CMV may play a supportive role” with infection in some infants altering immune function in a way that increases vulnerability to more direct causes of ALL, explained Dr. Wiemels, professor of population and public health sciences at the University of Southern California, Los Angeles. In other words, “exposure to CMV early rather than fulminant infection” at birth “may be the key epidemiologic feature.”
In the current study, Dr. Geris and colleagues tested dried newborn blood spots from 1189 children with ALL and 4,756 controls matched on age, sex, and mother’s race and ethnicity for the presence of cytomegalovirus at birth. Children were born in Michigan on or after Oct. 1, 1987.
Across the entire study population, congenital CMV was detected in 6 ALL cases (0.5%) and 21 controls (0.4%), with no difference in the odds of congenital CMV infection between the two groups. Among subjects positive for congenital CMV, it was not clear who had fulminant, clinically recognized disease and who did not.
Overall, 2 of 74 cases (2.7%) of hyperdiploid ALL were positive for congenital CMV. Compared with all controls in an unmatched analysis, those with hyperdiploid ALL were 6.26 times more likely to be CMV positive.
Overall, the investigators concluded that the current findings, in combination with previous evidence showing a similar connection, “strongly suggest CMV is associated specifically to hyperdiploid ALL.”
Although “the evidence supporting an association between CMV and ALL is tantalizing and mounting rapidly,” Dr. Wiemels noted that “much additional research attention is required to mechanistically describe pathways by which CMV may influence leukemia before the virus could be considered a potential target for prevention or clinical management of ALL.”
“We are still in the early chapters of the book describing the role of CMV and ALL,” but the virus might emerge as a clinical target “with much future promise for the health and well-being of our children,” he said.
The work was funded by the National Institutes of Health, the University of Minnesota, and the Department of Defense. The investigators and editorialist have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children with hyperdiploid acute lymphoblastic leukemia (ALL) are much more likely to also have congenital cytomegalovirus (CMV) infection, according to an analysis published in JAMA Network Open.
Although researchers found no association between ALL and congenital CMV infection overall, pediatric patients diagnosed with hyperdiploid ALL had sixfold greater odds of being positive for congenital CMV than cancer-free controls.
“These findings suggest mixed evidence for an association between congenital CMV infection and ALL” and that “a CMV-ALL association may be specific to hyperdiploid ALL,” said investigators, led by Jennifer Geris, PhD, a postdoctoral associate at Baylor College of Medicine, Houston.
A growing body of evidence suggests that CMV, a member of the herpesvirus family, may be a risk factor for ALL. Although the mechanism remains unclear, congenital CMV may encourage proliferation of CD34+ hematopoietic progenitor cells in bone marrow that are vulnerable to oncogenic transformation.
Two prior independent studies have suggested that prenatal CMV infection is associated with an increased risk of childhood ALL. However, given how common CMV infection is (more than 80% seropositivity worldwide) and the relatively rarity of pediatric ALL, Joseph Wiemels, PhD, argued in an accompanying editorial that CMV can’t be a direct cause of leukemia.
“Instead, CMV may play a supportive role” with infection in some infants altering immune function in a way that increases vulnerability to more direct causes of ALL, explained Dr. Wiemels, professor of population and public health sciences at the University of Southern California, Los Angeles. In other words, “exposure to CMV early rather than fulminant infection” at birth “may be the key epidemiologic feature.”
In the current study, Dr. Geris and colleagues tested dried newborn blood spots from 1189 children with ALL and 4,756 controls matched on age, sex, and mother’s race and ethnicity for the presence of cytomegalovirus at birth. Children were born in Michigan on or after Oct. 1, 1987.
Across the entire study population, congenital CMV was detected in 6 ALL cases (0.5%) and 21 controls (0.4%), with no difference in the odds of congenital CMV infection between the two groups. Among subjects positive for congenital CMV, it was not clear who had fulminant, clinically recognized disease and who did not.
Overall, 2 of 74 cases (2.7%) of hyperdiploid ALL were positive for congenital CMV. Compared with all controls in an unmatched analysis, those with hyperdiploid ALL were 6.26 times more likely to be CMV positive.
Overall, the investigators concluded that the current findings, in combination with previous evidence showing a similar connection, “strongly suggest CMV is associated specifically to hyperdiploid ALL.”
Although “the evidence supporting an association between CMV and ALL is tantalizing and mounting rapidly,” Dr. Wiemels noted that “much additional research attention is required to mechanistically describe pathways by which CMV may influence leukemia before the virus could be considered a potential target for prevention or clinical management of ALL.”
“We are still in the early chapters of the book describing the role of CMV and ALL,” but the virus might emerge as a clinical target “with much future promise for the health and well-being of our children,” he said.
The work was funded by the National Institutes of Health, the University of Minnesota, and the Department of Defense. The investigators and editorialist have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
A toddler presents with a dark line on a fingernail
Given the over 1-year history of an unchanging longitudinal band of pigment without extension to the proximal or lateral nailfolds or any other nail findings, the most likely diagnosis is benign longitudinal melanonychia.
Longitudinal melanonychia, also known as melanonychia striata, describes a brown to black streak of pigment extending from the nail matrix to the free edge of the nail.1,2
This disorder can occur secondary to a wide variety of benign and pathologic causes including lentigines, nevi, melanoma, chronic trauma, inflammatory skin diseases, systemic diseases, iatrogenic causes, and genetic syndromes.3 In melanocytic causes of longitudinal melanonychia, either melanocytic activation or hyperplasia drive pigmentary development leading to the brown to black band seen in the nail.4 Benign causes of longitudinal melanonychia include benign melanocyte activation, lentigo, and benign nevus.1
What’s the differential diagnosis?
The differential diagnosis for longitudinal melanonychia can include a wide variety of local and systemic causes. For our discussion, we will limit our differential to other locally involved disorders of the nail including subungual melanoma, subungual hematoma, onychomycosis, and glomus tumor.
Subungual melanoma is a rare subtype of acral lentiginous melanoma that most often presents as longitudinal melanonychia. Subungual melanoma is more common in those aged 50-70 years, individuals with personal or family history of melanoma or dysplastic nevus syndrome, and persons with African American, Native American, and Asian descent. Longitudinal melanonychia features that can be concerning for subungual melanoma include the presence of multiple colors, width greater than or equal to 3 mm, blurry borders, rapid increase in size, and extension to the proximal or lateral nailfolds (Hutchinson’s sign). Biopsy is required to make the diagnosis of subungual melanoma but is not necessary for melanonychia without atypical features.
Treatment of subungual melanoma depends on disease stage and can range from wide local excision of the nail apparatus to amputation of the affected digit and management with a medical oncologist. Given the absence of concerning neoplastic findings or personal or family history of melanoma, subungual melanoma is unlikely in this patient.
Subungual hematoma is an accumulation of blood underneath the nail plate that is typically the result of acute or chronic trauma to the distal phalanx. It can present as purple, red, pink, brown, or black discoloration under the nail plate and is most commonly found on the first toe. With acute trauma, pain is usually present upon initial injury. Subungual hematomas typically resolve on their own with normal nail growth. The absence of a history of trauma or pain, and the linear appearance of the lesion in our patient are inconsistent with a subungual hematoma.
Onychomycosis is a fungal infection of the nail caused by dermatophytes, nondermatophytes, or yeasts. It may present with longitudinal melanonychia; however, it more often presents with other nail abnormalities such as nail thickening, yellow discoloration, onycholysis, splitting, subungual hyperkeratosis, and nail plate destruction, which are not present in this patient. Furthermore, onychomycosis is more common in adults than children. Diagnosis is usually made with potassium hydroxide (KOH) preparations, histopathologic examination of nail clippings with a periodic acid-Schiff stain, fungal culture, or PCR.
Glomus tumor is a rare, benign neoplasm originating from cells of the glomus body. It is often found in the subungual region, in addition to other areas rich in glomus bodies such as the fingertips, palms, wrists, and forearms. Subungual glomus tumors present as a red, purple, or blueish lesions under the nail plate. Distal notching or an overlying longitudinal fissure may be present. Subungual glomus tumors are typically associated with pinpoint tenderness, paroxysmal pain, and cold sensitivity, features that are not present in our patient. The history and examination of our patient are much more consistent with benign longitudinal melanonychia.
It appears that melanoma associated with longitudinal melanonychia is very rare in children. According to one review published in 2020, only 12 cases of pediatric subungual melanoma have been reported.5 Recent series have observed longitudinal melanonychia in large sets of children, with findings that demonstrate that the vast majority of longitudinal melanonychia either stops progressing or regresses. These investigations therefore recommend serial observation of longitudinal melanonychia except in rare circumstances.6,7
Given the lack of troubling findings or concerning history, our patient was managed with observation. On follow-up 6 months later, he was found to have no change in his nail pigmentation.
Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology; Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Mannava KA et al. Hand Surg. 2013;18(1):133-9.
2. Leung AKC et al. Int J Dermatol. 2019;58(11):1239-45.
3. Andre J and Lateur N. Dermatol Clin. 2006;24(3):329-39.
4. Lee DK and Lipner SR. Ann Med. 2022;54(1):694-712.
5. Smith RJ and Rubin AI. Curr Opin Pediatr. 2020;32(4):506-15. .
6. Matsui Y et al. J Am Acad Dermatol. 2022;86(4):946-8.
7. Lee JS et al. J Am Acad Dermatol. 2022;87(2):366-72.
Given the over 1-year history of an unchanging longitudinal band of pigment without extension to the proximal or lateral nailfolds or any other nail findings, the most likely diagnosis is benign longitudinal melanonychia.
Longitudinal melanonychia, also known as melanonychia striata, describes a brown to black streak of pigment extending from the nail matrix to the free edge of the nail.1,2
This disorder can occur secondary to a wide variety of benign and pathologic causes including lentigines, nevi, melanoma, chronic trauma, inflammatory skin diseases, systemic diseases, iatrogenic causes, and genetic syndromes.3 In melanocytic causes of longitudinal melanonychia, either melanocytic activation or hyperplasia drive pigmentary development leading to the brown to black band seen in the nail.4 Benign causes of longitudinal melanonychia include benign melanocyte activation, lentigo, and benign nevus.1
What’s the differential diagnosis?
The differential diagnosis for longitudinal melanonychia can include a wide variety of local and systemic causes. For our discussion, we will limit our differential to other locally involved disorders of the nail including subungual melanoma, subungual hematoma, onychomycosis, and glomus tumor.
Subungual melanoma is a rare subtype of acral lentiginous melanoma that most often presents as longitudinal melanonychia. Subungual melanoma is more common in those aged 50-70 years, individuals with personal or family history of melanoma or dysplastic nevus syndrome, and persons with African American, Native American, and Asian descent. Longitudinal melanonychia features that can be concerning for subungual melanoma include the presence of multiple colors, width greater than or equal to 3 mm, blurry borders, rapid increase in size, and extension to the proximal or lateral nailfolds (Hutchinson’s sign). Biopsy is required to make the diagnosis of subungual melanoma but is not necessary for melanonychia without atypical features.
Treatment of subungual melanoma depends on disease stage and can range from wide local excision of the nail apparatus to amputation of the affected digit and management with a medical oncologist. Given the absence of concerning neoplastic findings or personal or family history of melanoma, subungual melanoma is unlikely in this patient.
Subungual hematoma is an accumulation of blood underneath the nail plate that is typically the result of acute or chronic trauma to the distal phalanx. It can present as purple, red, pink, brown, or black discoloration under the nail plate and is most commonly found on the first toe. With acute trauma, pain is usually present upon initial injury. Subungual hematomas typically resolve on their own with normal nail growth. The absence of a history of trauma or pain, and the linear appearance of the lesion in our patient are inconsistent with a subungual hematoma.
Onychomycosis is a fungal infection of the nail caused by dermatophytes, nondermatophytes, or yeasts. It may present with longitudinal melanonychia; however, it more often presents with other nail abnormalities such as nail thickening, yellow discoloration, onycholysis, splitting, subungual hyperkeratosis, and nail plate destruction, which are not present in this patient. Furthermore, onychomycosis is more common in adults than children. Diagnosis is usually made with potassium hydroxide (KOH) preparations, histopathologic examination of nail clippings with a periodic acid-Schiff stain, fungal culture, or PCR.
Glomus tumor is a rare, benign neoplasm originating from cells of the glomus body. It is often found in the subungual region, in addition to other areas rich in glomus bodies such as the fingertips, palms, wrists, and forearms. Subungual glomus tumors present as a red, purple, or blueish lesions under the nail plate. Distal notching or an overlying longitudinal fissure may be present. Subungual glomus tumors are typically associated with pinpoint tenderness, paroxysmal pain, and cold sensitivity, features that are not present in our patient. The history and examination of our patient are much more consistent with benign longitudinal melanonychia.
It appears that melanoma associated with longitudinal melanonychia is very rare in children. According to one review published in 2020, only 12 cases of pediatric subungual melanoma have been reported.5 Recent series have observed longitudinal melanonychia in large sets of children, with findings that demonstrate that the vast majority of longitudinal melanonychia either stops progressing or regresses. These investigations therefore recommend serial observation of longitudinal melanonychia except in rare circumstances.6,7
Given the lack of troubling findings or concerning history, our patient was managed with observation. On follow-up 6 months later, he was found to have no change in his nail pigmentation.
Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology; Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Mannava KA et al. Hand Surg. 2013;18(1):133-9.
2. Leung AKC et al. Int J Dermatol. 2019;58(11):1239-45.
3. Andre J and Lateur N. Dermatol Clin. 2006;24(3):329-39.
4. Lee DK and Lipner SR. Ann Med. 2022;54(1):694-712.
5. Smith RJ and Rubin AI. Curr Opin Pediatr. 2020;32(4):506-15. .
6. Matsui Y et al. J Am Acad Dermatol. 2022;86(4):946-8.
7. Lee JS et al. J Am Acad Dermatol. 2022;87(2):366-72.
Given the over 1-year history of an unchanging longitudinal band of pigment without extension to the proximal or lateral nailfolds or any other nail findings, the most likely diagnosis is benign longitudinal melanonychia.
Longitudinal melanonychia, also known as melanonychia striata, describes a brown to black streak of pigment extending from the nail matrix to the free edge of the nail.1,2
This disorder can occur secondary to a wide variety of benign and pathologic causes including lentigines, nevi, melanoma, chronic trauma, inflammatory skin diseases, systemic diseases, iatrogenic causes, and genetic syndromes.3 In melanocytic causes of longitudinal melanonychia, either melanocytic activation or hyperplasia drive pigmentary development leading to the brown to black band seen in the nail.4 Benign causes of longitudinal melanonychia include benign melanocyte activation, lentigo, and benign nevus.1
What’s the differential diagnosis?
The differential diagnosis for longitudinal melanonychia can include a wide variety of local and systemic causes. For our discussion, we will limit our differential to other locally involved disorders of the nail including subungual melanoma, subungual hematoma, onychomycosis, and glomus tumor.
Subungual melanoma is a rare subtype of acral lentiginous melanoma that most often presents as longitudinal melanonychia. Subungual melanoma is more common in those aged 50-70 years, individuals with personal or family history of melanoma or dysplastic nevus syndrome, and persons with African American, Native American, and Asian descent. Longitudinal melanonychia features that can be concerning for subungual melanoma include the presence of multiple colors, width greater than or equal to 3 mm, blurry borders, rapid increase in size, and extension to the proximal or lateral nailfolds (Hutchinson’s sign). Biopsy is required to make the diagnosis of subungual melanoma but is not necessary for melanonychia without atypical features.
Treatment of subungual melanoma depends on disease stage and can range from wide local excision of the nail apparatus to amputation of the affected digit and management with a medical oncologist. Given the absence of concerning neoplastic findings or personal or family history of melanoma, subungual melanoma is unlikely in this patient.
Subungual hematoma is an accumulation of blood underneath the nail plate that is typically the result of acute or chronic trauma to the distal phalanx. It can present as purple, red, pink, brown, or black discoloration under the nail plate and is most commonly found on the first toe. With acute trauma, pain is usually present upon initial injury. Subungual hematomas typically resolve on their own with normal nail growth. The absence of a history of trauma or pain, and the linear appearance of the lesion in our patient are inconsistent with a subungual hematoma.
Onychomycosis is a fungal infection of the nail caused by dermatophytes, nondermatophytes, or yeasts. It may present with longitudinal melanonychia; however, it more often presents with other nail abnormalities such as nail thickening, yellow discoloration, onycholysis, splitting, subungual hyperkeratosis, and nail plate destruction, which are not present in this patient. Furthermore, onychomycosis is more common in adults than children. Diagnosis is usually made with potassium hydroxide (KOH) preparations, histopathologic examination of nail clippings with a periodic acid-Schiff stain, fungal culture, or PCR.
Glomus tumor is a rare, benign neoplasm originating from cells of the glomus body. It is often found in the subungual region, in addition to other areas rich in glomus bodies such as the fingertips, palms, wrists, and forearms. Subungual glomus tumors present as a red, purple, or blueish lesions under the nail plate. Distal notching or an overlying longitudinal fissure may be present. Subungual glomus tumors are typically associated with pinpoint tenderness, paroxysmal pain, and cold sensitivity, features that are not present in our patient. The history and examination of our patient are much more consistent with benign longitudinal melanonychia.
It appears that melanoma associated with longitudinal melanonychia is very rare in children. According to one review published in 2020, only 12 cases of pediatric subungual melanoma have been reported.5 Recent series have observed longitudinal melanonychia in large sets of children, with findings that demonstrate that the vast majority of longitudinal melanonychia either stops progressing or regresses. These investigations therefore recommend serial observation of longitudinal melanonychia except in rare circumstances.6,7
Given the lack of troubling findings or concerning history, our patient was managed with observation. On follow-up 6 months later, he was found to have no change in his nail pigmentation.
Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology; Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Mannava KA et al. Hand Surg. 2013;18(1):133-9.
2. Leung AKC et al. Int J Dermatol. 2019;58(11):1239-45.
3. Andre J and Lateur N. Dermatol Clin. 2006;24(3):329-39.
4. Lee DK and Lipner SR. Ann Med. 2022;54(1):694-712.
5. Smith RJ and Rubin AI. Curr Opin Pediatr. 2020;32(4):506-15. .
6. Matsui Y et al. J Am Acad Dermatol. 2022;86(4):946-8.
7. Lee JS et al. J Am Acad Dermatol. 2022;87(2):366-72.
Examination findings reveal a 2-mm brown longitudinal band on the radial aspect of the right thumbnail that does not extend into the proximal or lateral nailfolds. The rest of the skin and nail exam is unremarkable.
CDC frets over further dip in kindergarten vaccination rates
The percentage of kindergarteners in the United States who have received routine vaccines to protect against illnesses such as measles, whooping cough, and polio has declined for 2 straight years, a new study has found.
Drops in vaccine coverage leave communities more susceptible to outbreaks of vaccine-preventable diseases, such as those that occurred in 2022, public health officials said.
Coverage for four vaccines – against measles, mumps, and rubella (MMR); diphtheria, tetanus, and acellular pertussis (DTaP); poliovirus; and varicella – among kindergarten students was about 95% in 2019-2020.
The rate fell to 94% the following year.
For the 2021-2022 school year, coverage dropped another point, to 93%, according to the report, published online in Morbidity and Mortality Weekly Report.
The rate of vaccination overall remains high, but about 250,000 kindergarten students may not be protected against measles, the researchers estimate. Measles, which is highly infectious, can lead to serious illness and even death in children who have not been vaccinated against the virus.
“In 2022, two communities in the United States responded to outbreaks of measles where children have been hospitalized,” Georgina Peacock, MD, MPH, director of the immunization services division of the Centers for Disease Control and Prevention, said in a media briefing about the report. “One community reported a case of paralytic polio in an unvaccinated person. These outbreaks were preventable. The best way to prevent these diseases and their devastating impact on children is through vaccination.”
Exemptions steady
For the new study, Ranee Seither, MPH, with the CDC’s National Center for Immunization and Respiratory Diseases and her colleagues analyzed data reported by states to estimate nationwide coverage for the four routine vaccines.
The number of students with exemptions remained low, at 2.6%, but another 3.9% who were without exemptions were not up to date with the MMR vaccine, the investigators report.
In a separate study, researchers found that vaccination coverage for 2-year-olds has increased. Approximately 70% of children were up to date with a seven-vaccine series by age 24 months. The coverage rate was higher for children born during 2018-2019 than for those born during 2016-2017.
Although the COVID-19 pandemic was not associated with decreased vaccination rates in this younger age group overall, coverage fell by 4-5 percentage points for children living below the poverty level or in rural areas, according to the study.
In addition, uninsured children were eight times more likely than those with private insurance to not be vaccinated by their second birthday, the researchers found.
Strategies to increase vaccination coverage include enforcing school vaccination requirements and holding vaccination clinics at schools, the CDC said.
“Providers should review children’s histories and recommend needed vaccinations during every clinical encounter and address parental hesitancy to help reduce disparities and ensure that all children are protected from vaccine-preventable diseases,” the agency said.
To that end, the agency launched an initiative this week called Let’s RISE (Routine Immunizations on Schedule for Everyone) to provide clinicians with resources to help patients get on track with their immunizations.
Hundreds of thousands unprotected
MMR vaccination coverage for kindergartners is the lowest it has been in over a decade, Dr. Peacock noted. Decreased coverage for kindergarten students might be tied to pandemic-related disruptions in health care systems and schools, she said. School administrators and parents may have been less focused on routine vaccination paperwork amid the return to in-person learning, for instance.
Hesitancy about COVID vaccines could be affecting routine vaccinations. “That’s something that we are watching very closely,” Dr. Peacock said.
The 2-point decrease in vaccination coverage “translates to hundreds of thousands of children starting school without being fully protected” against preventable diseases that can spread easily in classrooms, Sean O’Leary, MD, chair of the American Academy of Pediatrics’ Committee on Infectious Diseases, said.
Despite the drop in coverage, Dr. O’Leary said he saw some encouraging signs in the data: Nonmedical exemptions for kindergarten students have not increased. And the vast majority of parents are still having their children vaccinated. At the same time, the reports highlight a need to address child poverty and improve vaccine access in rural areas, he said.
A version of this article first appeared on Medscape.com.
The percentage of kindergarteners in the United States who have received routine vaccines to protect against illnesses such as measles, whooping cough, and polio has declined for 2 straight years, a new study has found.
Drops in vaccine coverage leave communities more susceptible to outbreaks of vaccine-preventable diseases, such as those that occurred in 2022, public health officials said.
Coverage for four vaccines – against measles, mumps, and rubella (MMR); diphtheria, tetanus, and acellular pertussis (DTaP); poliovirus; and varicella – among kindergarten students was about 95% in 2019-2020.
The rate fell to 94% the following year.
For the 2021-2022 school year, coverage dropped another point, to 93%, according to the report, published online in Morbidity and Mortality Weekly Report.
The rate of vaccination overall remains high, but about 250,000 kindergarten students may not be protected against measles, the researchers estimate. Measles, which is highly infectious, can lead to serious illness and even death in children who have not been vaccinated against the virus.
“In 2022, two communities in the United States responded to outbreaks of measles where children have been hospitalized,” Georgina Peacock, MD, MPH, director of the immunization services division of the Centers for Disease Control and Prevention, said in a media briefing about the report. “One community reported a case of paralytic polio in an unvaccinated person. These outbreaks were preventable. The best way to prevent these diseases and their devastating impact on children is through vaccination.”
Exemptions steady
For the new study, Ranee Seither, MPH, with the CDC’s National Center for Immunization and Respiratory Diseases and her colleagues analyzed data reported by states to estimate nationwide coverage for the four routine vaccines.
The number of students with exemptions remained low, at 2.6%, but another 3.9% who were without exemptions were not up to date with the MMR vaccine, the investigators report.
In a separate study, researchers found that vaccination coverage for 2-year-olds has increased. Approximately 70% of children were up to date with a seven-vaccine series by age 24 months. The coverage rate was higher for children born during 2018-2019 than for those born during 2016-2017.
Although the COVID-19 pandemic was not associated with decreased vaccination rates in this younger age group overall, coverage fell by 4-5 percentage points for children living below the poverty level or in rural areas, according to the study.
In addition, uninsured children were eight times more likely than those with private insurance to not be vaccinated by their second birthday, the researchers found.
Strategies to increase vaccination coverage include enforcing school vaccination requirements and holding vaccination clinics at schools, the CDC said.
“Providers should review children’s histories and recommend needed vaccinations during every clinical encounter and address parental hesitancy to help reduce disparities and ensure that all children are protected from vaccine-preventable diseases,” the agency said.
To that end, the agency launched an initiative this week called Let’s RISE (Routine Immunizations on Schedule for Everyone) to provide clinicians with resources to help patients get on track with their immunizations.
Hundreds of thousands unprotected
MMR vaccination coverage for kindergartners is the lowest it has been in over a decade, Dr. Peacock noted. Decreased coverage for kindergarten students might be tied to pandemic-related disruptions in health care systems and schools, she said. School administrators and parents may have been less focused on routine vaccination paperwork amid the return to in-person learning, for instance.
Hesitancy about COVID vaccines could be affecting routine vaccinations. “That’s something that we are watching very closely,” Dr. Peacock said.
The 2-point decrease in vaccination coverage “translates to hundreds of thousands of children starting school without being fully protected” against preventable diseases that can spread easily in classrooms, Sean O’Leary, MD, chair of the American Academy of Pediatrics’ Committee on Infectious Diseases, said.
Despite the drop in coverage, Dr. O’Leary said he saw some encouraging signs in the data: Nonmedical exemptions for kindergarten students have not increased. And the vast majority of parents are still having their children vaccinated. At the same time, the reports highlight a need to address child poverty and improve vaccine access in rural areas, he said.
A version of this article first appeared on Medscape.com.
The percentage of kindergarteners in the United States who have received routine vaccines to protect against illnesses such as measles, whooping cough, and polio has declined for 2 straight years, a new study has found.
Drops in vaccine coverage leave communities more susceptible to outbreaks of vaccine-preventable diseases, such as those that occurred in 2022, public health officials said.
Coverage for four vaccines – against measles, mumps, and rubella (MMR); diphtheria, tetanus, and acellular pertussis (DTaP); poliovirus; and varicella – among kindergarten students was about 95% in 2019-2020.
The rate fell to 94% the following year.
For the 2021-2022 school year, coverage dropped another point, to 93%, according to the report, published online in Morbidity and Mortality Weekly Report.
The rate of vaccination overall remains high, but about 250,000 kindergarten students may not be protected against measles, the researchers estimate. Measles, which is highly infectious, can lead to serious illness and even death in children who have not been vaccinated against the virus.
“In 2022, two communities in the United States responded to outbreaks of measles where children have been hospitalized,” Georgina Peacock, MD, MPH, director of the immunization services division of the Centers for Disease Control and Prevention, said in a media briefing about the report. “One community reported a case of paralytic polio in an unvaccinated person. These outbreaks were preventable. The best way to prevent these diseases and their devastating impact on children is through vaccination.”
Exemptions steady
For the new study, Ranee Seither, MPH, with the CDC’s National Center for Immunization and Respiratory Diseases and her colleagues analyzed data reported by states to estimate nationwide coverage for the four routine vaccines.
The number of students with exemptions remained low, at 2.6%, but another 3.9% who were without exemptions were not up to date with the MMR vaccine, the investigators report.
In a separate study, researchers found that vaccination coverage for 2-year-olds has increased. Approximately 70% of children were up to date with a seven-vaccine series by age 24 months. The coverage rate was higher for children born during 2018-2019 than for those born during 2016-2017.
Although the COVID-19 pandemic was not associated with decreased vaccination rates in this younger age group overall, coverage fell by 4-5 percentage points for children living below the poverty level or in rural areas, according to the study.
In addition, uninsured children were eight times more likely than those with private insurance to not be vaccinated by their second birthday, the researchers found.
Strategies to increase vaccination coverage include enforcing school vaccination requirements and holding vaccination clinics at schools, the CDC said.
“Providers should review children’s histories and recommend needed vaccinations during every clinical encounter and address parental hesitancy to help reduce disparities and ensure that all children are protected from vaccine-preventable diseases,” the agency said.
To that end, the agency launched an initiative this week called Let’s RISE (Routine Immunizations on Schedule for Everyone) to provide clinicians with resources to help patients get on track with their immunizations.
Hundreds of thousands unprotected
MMR vaccination coverage for kindergartners is the lowest it has been in over a decade, Dr. Peacock noted. Decreased coverage for kindergarten students might be tied to pandemic-related disruptions in health care systems and schools, she said. School administrators and parents may have been less focused on routine vaccination paperwork amid the return to in-person learning, for instance.
Hesitancy about COVID vaccines could be affecting routine vaccinations. “That’s something that we are watching very closely,” Dr. Peacock said.
The 2-point decrease in vaccination coverage “translates to hundreds of thousands of children starting school without being fully protected” against preventable diseases that can spread easily in classrooms, Sean O’Leary, MD, chair of the American Academy of Pediatrics’ Committee on Infectious Diseases, said.
Despite the drop in coverage, Dr. O’Leary said he saw some encouraging signs in the data: Nonmedical exemptions for kindergarten students have not increased. And the vast majority of parents are still having their children vaccinated. At the same time, the reports highlight a need to address child poverty and improve vaccine access in rural areas, he said.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
AD outcomes improved with lebrikizumab and topical steroids
, according to results of the 16-week phase 3 ADhere trial.
“Lebrikizumab, a monoclonal antibody inhibiting interleukin-13, combined with TCS was associated with reduced overall disease severity of moderate to severe AD in adolescents and adults, and had a safety profile consistent with previous lebrikizumab AD studies,” noted lead author Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, and coauthors in their article on the study, which was published in JAMA Dermatology.
The double-blind trial, conducted at 54 sites across Germany, Poland, Canada, and the United States, included 211 patients, mean age 37.2 years, of whom 48.8% were female and roughly 22% were adolescents. Almost 15% were Asian, and about 13% were Black.
At baseline, participants had a score of 16 or higher on the Eczema Area and Severity Index (EASI), a score of 3 or higher on the Investigator’s Global Assessment (IGA) scale, AD covering a body surface area of 10% or greater, and a history of inadequate response to treatment with topical medications.
After a minimum 1-week washout period from topical and systemic therapy, participants were randomized in a 2:1 ratio to receive lebrikizumab plus TCS (n = 145) or placebo plus TCS (n = 66) for 16 weeks.
Lebrikizumab or placebo was administered by subcutaneous injection every 2 weeks; the loading and week-2 doses of lebrikizumab were 500 mg, followed by 250 mg thereafter. All patients were instructed to use low- to mid-potency TCS at their own discretion. Study sites provided a mid-potency TCS (triamcinolone acetonide 0.1% cream) and a low-potency TCS (hydrocortisone 1% cream), with topical calcineurin inhibitors permitted for sensitive skin areas.
Primary outcomes at 16 weeks included a 2-point or more reduction in IGA score from baseline and EASI-75 response. Patients in the lebrikizumab arm had superior responses on both of these outcomes, with statistical significance achieved as early as week 8 and week 4, respectively, and maintained through week 16. Specifically, 41.2% of those treated with lebrikizumab had an IGA reduction of 2 points or more, compared with 22.1% of those receiving placebo plus TCS (P = .01), and the proportion of patients achieving EASI-75 responses was 69.5% vs. 42.2%, respectively (P < .001).
Patients treated with lebrikizumab also showed statistically significant improvements, compared with TCS alone in all key secondary endpoints, “including skin clearance, improvement in itch, itch interference on sleep, and enhanced QoL [quality of life],” noted the authors. “This study captured the clinical benefit of lebrikizumab through the combined end point of physician-assessed clinical sign of skin clearance (EASI-75) and patient-reported outcome of improvement in itch (Pruritus NRS).”
The percentage of patients who achieved the combined endpoint was more than double for the lebrikizumab plus TCS group vs. the group on TCS alone, indicating that patients treated with lebrikizumab plus TCS “were more likely to experience improvement in skin symptoms and itch,” the investigators added.
The authors noted that most treatment-emergent adverse events “were nonserious, mild, or moderate in severity, and did not lead to study discontinuation.” These included conjunctivitis (4.8%), headache (4.8%), hypertension (2.8%), injection-site reactions (2.8%), and herpes infection (3.4%) – all of which occurred in 1.5% or less of patients in the placebo group.
“The higher incidence of conjunctivitis has also been reported in other biologics inhibiting IL [interleukin]–13 and/or IL-4 signaling, as well as lebrikizumab monotherapy studies,” they noted. The 4.8% rate of conjunctivitis reported in the combination study, they added, is “compared with 7.5% frequency in 16-week data from the lebrikizumab monotherapy studies. Although the mechanism remains unclear, it has been reported that conjunctival goblet cell scarcity due to IL-13 and IL-4 inhibition, and subsequent effects on the homeostasis of the conjunctival mucosal surface, results in ocular AEs [adverse events].”
“This truly is a time of great hope and promise for our patients with AD,” commented Zelma Chiesa Fuxench, MD, who was not involved in the study. “The advent of newer, targeted therapeutic agents for AD continues to revolutionize the treatment experience for our patients, offering the possibility of greater AD disease control with a favorable risk profile and less need for blood work monitoring compared to traditional systemic agents.”
On the basis of the study results, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview that “lebrikizumab represents an additional option in the treatment armamentarium for providers who care for patients with AD.” She added that, “while head-to-head trials comparing lebrikizumab to dupilumab, the first FDA-approved biologic for AD, would be beneficial, to the best of my knowledge this data is currently lacking. However, based on the results of this study, we would expect lebrikizumab to work at least similarly to dupilumab, based on the reported improvements in IGA and EASI score.”
Additionally, lebrikizumab showed a favorable safety profile, “with most treatment-emergent adverse effects reported as nonserious and not leading to drug discontinuation,” she said. “Of interest to clinicians may be the reported rates of conjunctivitis in this study. Rates of conjunctivitis for lebrikizumab appear to be lower than those reported in the LIBERTY AD CHRONOS study for dupilumab – a finding that merits further scrutiny in my opinion, as this one of the most frequent treatment-emergent adverse events that I encounter in my clinical practice.”
The study was funded by Dermira, a subsidiary of Eli Lilly. Dr. Simpson reported personal fees and grants from multiple sources, including Dermira and Eli Lilly, the companies developing lebrikizumab. Several authors were employees of Eli Lilly. Dr. Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
A version of this article first appeared on Medscape.com.
, according to results of the 16-week phase 3 ADhere trial.
“Lebrikizumab, a monoclonal antibody inhibiting interleukin-13, combined with TCS was associated with reduced overall disease severity of moderate to severe AD in adolescents and adults, and had a safety profile consistent with previous lebrikizumab AD studies,” noted lead author Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, and coauthors in their article on the study, which was published in JAMA Dermatology.
The double-blind trial, conducted at 54 sites across Germany, Poland, Canada, and the United States, included 211 patients, mean age 37.2 years, of whom 48.8% were female and roughly 22% were adolescents. Almost 15% were Asian, and about 13% were Black.
At baseline, participants had a score of 16 or higher on the Eczema Area and Severity Index (EASI), a score of 3 or higher on the Investigator’s Global Assessment (IGA) scale, AD covering a body surface area of 10% or greater, and a history of inadequate response to treatment with topical medications.
After a minimum 1-week washout period from topical and systemic therapy, participants were randomized in a 2:1 ratio to receive lebrikizumab plus TCS (n = 145) or placebo plus TCS (n = 66) for 16 weeks.
Lebrikizumab or placebo was administered by subcutaneous injection every 2 weeks; the loading and week-2 doses of lebrikizumab were 500 mg, followed by 250 mg thereafter. All patients were instructed to use low- to mid-potency TCS at their own discretion. Study sites provided a mid-potency TCS (triamcinolone acetonide 0.1% cream) and a low-potency TCS (hydrocortisone 1% cream), with topical calcineurin inhibitors permitted for sensitive skin areas.
Primary outcomes at 16 weeks included a 2-point or more reduction in IGA score from baseline and EASI-75 response. Patients in the lebrikizumab arm had superior responses on both of these outcomes, with statistical significance achieved as early as week 8 and week 4, respectively, and maintained through week 16. Specifically, 41.2% of those treated with lebrikizumab had an IGA reduction of 2 points or more, compared with 22.1% of those receiving placebo plus TCS (P = .01), and the proportion of patients achieving EASI-75 responses was 69.5% vs. 42.2%, respectively (P < .001).
Patients treated with lebrikizumab also showed statistically significant improvements, compared with TCS alone in all key secondary endpoints, “including skin clearance, improvement in itch, itch interference on sleep, and enhanced QoL [quality of life],” noted the authors. “This study captured the clinical benefit of lebrikizumab through the combined end point of physician-assessed clinical sign of skin clearance (EASI-75) and patient-reported outcome of improvement in itch (Pruritus NRS).”
The percentage of patients who achieved the combined endpoint was more than double for the lebrikizumab plus TCS group vs. the group on TCS alone, indicating that patients treated with lebrikizumab plus TCS “were more likely to experience improvement in skin symptoms and itch,” the investigators added.
The authors noted that most treatment-emergent adverse events “were nonserious, mild, or moderate in severity, and did not lead to study discontinuation.” These included conjunctivitis (4.8%), headache (4.8%), hypertension (2.8%), injection-site reactions (2.8%), and herpes infection (3.4%) – all of which occurred in 1.5% or less of patients in the placebo group.
“The higher incidence of conjunctivitis has also been reported in other biologics inhibiting IL [interleukin]–13 and/or IL-4 signaling, as well as lebrikizumab monotherapy studies,” they noted. The 4.8% rate of conjunctivitis reported in the combination study, they added, is “compared with 7.5% frequency in 16-week data from the lebrikizumab monotherapy studies. Although the mechanism remains unclear, it has been reported that conjunctival goblet cell scarcity due to IL-13 and IL-4 inhibition, and subsequent effects on the homeostasis of the conjunctival mucosal surface, results in ocular AEs [adverse events].”
“This truly is a time of great hope and promise for our patients with AD,” commented Zelma Chiesa Fuxench, MD, who was not involved in the study. “The advent of newer, targeted therapeutic agents for AD continues to revolutionize the treatment experience for our patients, offering the possibility of greater AD disease control with a favorable risk profile and less need for blood work monitoring compared to traditional systemic agents.”
On the basis of the study results, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview that “lebrikizumab represents an additional option in the treatment armamentarium for providers who care for patients with AD.” She added that, “while head-to-head trials comparing lebrikizumab to dupilumab, the first FDA-approved biologic for AD, would be beneficial, to the best of my knowledge this data is currently lacking. However, based on the results of this study, we would expect lebrikizumab to work at least similarly to dupilumab, based on the reported improvements in IGA and EASI score.”
Additionally, lebrikizumab showed a favorable safety profile, “with most treatment-emergent adverse effects reported as nonserious and not leading to drug discontinuation,” she said. “Of interest to clinicians may be the reported rates of conjunctivitis in this study. Rates of conjunctivitis for lebrikizumab appear to be lower than those reported in the LIBERTY AD CHRONOS study for dupilumab – a finding that merits further scrutiny in my opinion, as this one of the most frequent treatment-emergent adverse events that I encounter in my clinical practice.”
The study was funded by Dermira, a subsidiary of Eli Lilly. Dr. Simpson reported personal fees and grants from multiple sources, including Dermira and Eli Lilly, the companies developing lebrikizumab. Several authors were employees of Eli Lilly. Dr. Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
A version of this article first appeared on Medscape.com.
, according to results of the 16-week phase 3 ADhere trial.
“Lebrikizumab, a monoclonal antibody inhibiting interleukin-13, combined with TCS was associated with reduced overall disease severity of moderate to severe AD in adolescents and adults, and had a safety profile consistent with previous lebrikizumab AD studies,” noted lead author Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, and coauthors in their article on the study, which was published in JAMA Dermatology.
The double-blind trial, conducted at 54 sites across Germany, Poland, Canada, and the United States, included 211 patients, mean age 37.2 years, of whom 48.8% were female and roughly 22% were adolescents. Almost 15% were Asian, and about 13% were Black.
At baseline, participants had a score of 16 or higher on the Eczema Area and Severity Index (EASI), a score of 3 or higher on the Investigator’s Global Assessment (IGA) scale, AD covering a body surface area of 10% or greater, and a history of inadequate response to treatment with topical medications.
After a minimum 1-week washout period from topical and systemic therapy, participants were randomized in a 2:1 ratio to receive lebrikizumab plus TCS (n = 145) or placebo plus TCS (n = 66) for 16 weeks.
Lebrikizumab or placebo was administered by subcutaneous injection every 2 weeks; the loading and week-2 doses of lebrikizumab were 500 mg, followed by 250 mg thereafter. All patients were instructed to use low- to mid-potency TCS at their own discretion. Study sites provided a mid-potency TCS (triamcinolone acetonide 0.1% cream) and a low-potency TCS (hydrocortisone 1% cream), with topical calcineurin inhibitors permitted for sensitive skin areas.
Primary outcomes at 16 weeks included a 2-point or more reduction in IGA score from baseline and EASI-75 response. Patients in the lebrikizumab arm had superior responses on both of these outcomes, with statistical significance achieved as early as week 8 and week 4, respectively, and maintained through week 16. Specifically, 41.2% of those treated with lebrikizumab had an IGA reduction of 2 points or more, compared with 22.1% of those receiving placebo plus TCS (P = .01), and the proportion of patients achieving EASI-75 responses was 69.5% vs. 42.2%, respectively (P < .001).
Patients treated with lebrikizumab also showed statistically significant improvements, compared with TCS alone in all key secondary endpoints, “including skin clearance, improvement in itch, itch interference on sleep, and enhanced QoL [quality of life],” noted the authors. “This study captured the clinical benefit of lebrikizumab through the combined end point of physician-assessed clinical sign of skin clearance (EASI-75) and patient-reported outcome of improvement in itch (Pruritus NRS).”
The percentage of patients who achieved the combined endpoint was more than double for the lebrikizumab plus TCS group vs. the group on TCS alone, indicating that patients treated with lebrikizumab plus TCS “were more likely to experience improvement in skin symptoms and itch,” the investigators added.
The authors noted that most treatment-emergent adverse events “were nonserious, mild, or moderate in severity, and did not lead to study discontinuation.” These included conjunctivitis (4.8%), headache (4.8%), hypertension (2.8%), injection-site reactions (2.8%), and herpes infection (3.4%) – all of which occurred in 1.5% or less of patients in the placebo group.
“The higher incidence of conjunctivitis has also been reported in other biologics inhibiting IL [interleukin]–13 and/or IL-4 signaling, as well as lebrikizumab monotherapy studies,” they noted. The 4.8% rate of conjunctivitis reported in the combination study, they added, is “compared with 7.5% frequency in 16-week data from the lebrikizumab monotherapy studies. Although the mechanism remains unclear, it has been reported that conjunctival goblet cell scarcity due to IL-13 and IL-4 inhibition, and subsequent effects on the homeostasis of the conjunctival mucosal surface, results in ocular AEs [adverse events].”
“This truly is a time of great hope and promise for our patients with AD,” commented Zelma Chiesa Fuxench, MD, who was not involved in the study. “The advent of newer, targeted therapeutic agents for AD continues to revolutionize the treatment experience for our patients, offering the possibility of greater AD disease control with a favorable risk profile and less need for blood work monitoring compared to traditional systemic agents.”
On the basis of the study results, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview that “lebrikizumab represents an additional option in the treatment armamentarium for providers who care for patients with AD.” She added that, “while head-to-head trials comparing lebrikizumab to dupilumab, the first FDA-approved biologic for AD, would be beneficial, to the best of my knowledge this data is currently lacking. However, based on the results of this study, we would expect lebrikizumab to work at least similarly to dupilumab, based on the reported improvements in IGA and EASI score.”
Additionally, lebrikizumab showed a favorable safety profile, “with most treatment-emergent adverse effects reported as nonserious and not leading to drug discontinuation,” she said. “Of interest to clinicians may be the reported rates of conjunctivitis in this study. Rates of conjunctivitis for lebrikizumab appear to be lower than those reported in the LIBERTY AD CHRONOS study for dupilumab – a finding that merits further scrutiny in my opinion, as this one of the most frequent treatment-emergent adverse events that I encounter in my clinical practice.”
The study was funded by Dermira, a subsidiary of Eli Lilly. Dr. Simpson reported personal fees and grants from multiple sources, including Dermira and Eli Lilly, the companies developing lebrikizumab. Several authors were employees of Eli Lilly. Dr. Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Children and COVID: ED visits and hospitalizations start to fall again
Emergency department visits and hospitalizations for COVID-19 in children appear to be following the declining trend set by weekly cases since early December, based on data from the Centers for Disease Control and Prevention.
. New cases took a different path that had the weekly total falling through November before taking a big jump during the week of Nov. 27 to Dec. 3 – the count doubled from 30,000 the previous week to 63,000 – and then decreased again, the CDC reported.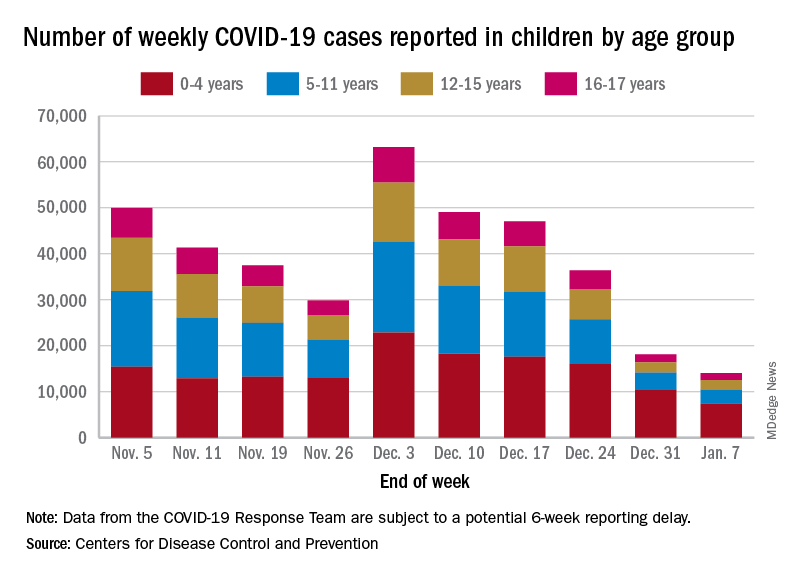
The proportion of ED visits with COVID, which was down to 1.0% of all ED visits (7-day average) for children aged 0-4 years on Nov. 4, was up to 3.2% on Jan. 3 but slipped to 2.5% as of Jan. 10. The patterns for older children are similar, with some differences in timing and lower peaks (1.7% for 12- to 15-year-olds and 1.9% for those aged 16-17), according to the CDC’s COVID Data Tracker.
The trend for new hospital admissions of children with confirmed COVID showed a similar rise through December, and the latest data for the very beginning of January suggest an even faster drop, although there is more of a reporting lag with hospitalization data, compared with ED visits, the CDC noted.
The most current data (Dec. 30 to Jan. 5) available from the American Academy of Pediatrics and the Children’s Hospital Association show less volatility in the number of weekly cases through November and December, with the peak being about 48,000 in mid-December. The AAP/CHA totals for the last 2 weeks, however, were both higher than the CDC’s corresponding counts, which are more preliminary and subject to revision.
The CDC puts the total number of COVID cases in children at 16.7 million – about 17.2% of all cases – as of Jan. 11, with 1,981 deaths reported so far. The AAP and CHA are not tracking deaths, but their case total as of Jan. 5 was 15.2 million, which represents 18.1% of cases in all ages. The AAP/CHA report is based on data reported publicly by an ever-decreasing number of states and territories.
Emergency department visits and hospitalizations for COVID-19 in children appear to be following the declining trend set by weekly cases since early December, based on data from the Centers for Disease Control and Prevention.
. New cases took a different path that had the weekly total falling through November before taking a big jump during the week of Nov. 27 to Dec. 3 – the count doubled from 30,000 the previous week to 63,000 – and then decreased again, the CDC reported.
The proportion of ED visits with COVID, which was down to 1.0% of all ED visits (7-day average) for children aged 0-4 years on Nov. 4, was up to 3.2% on Jan. 3 but slipped to 2.5% as of Jan. 10. The patterns for older children are similar, with some differences in timing and lower peaks (1.7% for 12- to 15-year-olds and 1.9% for those aged 16-17), according to the CDC’s COVID Data Tracker.
The trend for new hospital admissions of children with confirmed COVID showed a similar rise through December, and the latest data for the very beginning of January suggest an even faster drop, although there is more of a reporting lag with hospitalization data, compared with ED visits, the CDC noted.
The most current data (Dec. 30 to Jan. 5) available from the American Academy of Pediatrics and the Children’s Hospital Association show less volatility in the number of weekly cases through November and December, with the peak being about 48,000 in mid-December. The AAP/CHA totals for the last 2 weeks, however, were both higher than the CDC’s corresponding counts, which are more preliminary and subject to revision.
The CDC puts the total number of COVID cases in children at 16.7 million – about 17.2% of all cases – as of Jan. 11, with 1,981 deaths reported so far. The AAP and CHA are not tracking deaths, but their case total as of Jan. 5 was 15.2 million, which represents 18.1% of cases in all ages. The AAP/CHA report is based on data reported publicly by an ever-decreasing number of states and territories.
Emergency department visits and hospitalizations for COVID-19 in children appear to be following the declining trend set by weekly cases since early December, based on data from the Centers for Disease Control and Prevention.
. New cases took a different path that had the weekly total falling through November before taking a big jump during the week of Nov. 27 to Dec. 3 – the count doubled from 30,000 the previous week to 63,000 – and then decreased again, the CDC reported.
The proportion of ED visits with COVID, which was down to 1.0% of all ED visits (7-day average) for children aged 0-4 years on Nov. 4, was up to 3.2% on Jan. 3 but slipped to 2.5% as of Jan. 10. The patterns for older children are similar, with some differences in timing and lower peaks (1.7% for 12- to 15-year-olds and 1.9% for those aged 16-17), according to the CDC’s COVID Data Tracker.
The trend for new hospital admissions of children with confirmed COVID showed a similar rise through December, and the latest data for the very beginning of January suggest an even faster drop, although there is more of a reporting lag with hospitalization data, compared with ED visits, the CDC noted.
The most current data (Dec. 30 to Jan. 5) available from the American Academy of Pediatrics and the Children’s Hospital Association show less volatility in the number of weekly cases through November and December, with the peak being about 48,000 in mid-December. The AAP/CHA totals for the last 2 weeks, however, were both higher than the CDC’s corresponding counts, which are more preliminary and subject to revision.
The CDC puts the total number of COVID cases in children at 16.7 million – about 17.2% of all cases – as of Jan. 11, with 1,981 deaths reported so far. The AAP and CHA are not tracking deaths, but their case total as of Jan. 5 was 15.2 million, which represents 18.1% of cases in all ages. The AAP/CHA report is based on data reported publicly by an ever-decreasing number of states and territories.