User login
Microneedling plus 10% TCA peels bests CO2 laser alone for infraorbital dark circles
In a study of patients with mild to moderate infraorbital dark circles, treatment with carbon dioxide laser resurfacing did not produce a significant improvement in infraorbital hyperpigmentation. However, the combination of microneedling and 10% trichloroacetic acid peels did.
The finding comes from what is believed to be the first head-to-head comparison of the two procedures for infraorbital dark circles, which are a common cosmetic concern with increased age.
During a late-breaking abstract session at the virtual annual meeting of the American Academy of Dermatology, lead study author Banu Farabi, MD, said that dark circles seen in the periorbital area are defined as bilateral, round homogeneous pigmented macules whose etiology is thought to be multifactorial. Available treatments include bleaching creams, topical retinoids, chemical peels, lasers, autologous fat transplantation, injectable fillers, and blepharoplasty.
“Microneedling has been recently suggested as an effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi said. “This technique is based on creating microchannels that can stimulate the production of subcutaneous collagen and elastin. It also enhances the revascularization and fibroblast activity, which increases the skin thickness and gives a shiny appearance to the skin. The fractional CO2 has also been introduced as an effective procedure to remove infraorbital dark circles. However, there are some potential complications with that therapy.”
For the current study, Dr. Farabi, of the department of dermatology at Ankara (Turkey) University, and Mohamad Goldust, MD, of University Hospital Basel (Switzerland), They used the handheld Automatic Microneedle Therapy System-Handhold from MCure. After creating microchannels, the investigators topically applied 10% trichloroacetic acid peels to each infraorbital area and waited for 5 minutes.
In the carbon dioxide laser group, a Lutronic CO2 laser was used with a pulse energy of 10 J/cm2, a 100-microsecond pulse rate, 30 W of power, and a pulse width of 4 mm. The treatment outcome was assessed with the patient’s satisfaction and the physician’s judgment, which were no response, partial response, and complete response. Patients in both study groups were followed up for blinded-investigator assessment of infraorbital hyperpigmentation, adverse events, and improvement, compared with baseline.
The mean age of patients was 40 years, with a range between 27 and 58 years. About one-third of patients in each group had Fitzpatrick skin types II, III, and IV, respectively. In the blinded investigator assessment, the laser-resurfacing procedure did not demonstrate a significant improvement in infraorbital hyperpigmentation at day 90 (P = .24). However, the combination of microneedling and 10% trichloroacetic acid peels significantly improved infraorbital hyperpigmentation by day 90, with improvement maintained through day 180 (P = .012 and .002, respectively).
Adverse events were mild and temporary in both groups. In the laser-resurfacing group, 7 of the patients (22.5%) developed transient infraorbital hyperpigmentation postoperatively that lasted 4 weeks. In the combination treatment group, 18 patients (58%) developed transient erythema that lasted for up to 1 week.
“We suggest using microneedling plus 10% [trichloroacetic acid] as a cost-effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi concluded.
The researchers reported having no financial disclosures.
In a study of patients with mild to moderate infraorbital dark circles, treatment with carbon dioxide laser resurfacing did not produce a significant improvement in infraorbital hyperpigmentation. However, the combination of microneedling and 10% trichloroacetic acid peels did.
The finding comes from what is believed to be the first head-to-head comparison of the two procedures for infraorbital dark circles, which are a common cosmetic concern with increased age.
During a late-breaking abstract session at the virtual annual meeting of the American Academy of Dermatology, lead study author Banu Farabi, MD, said that dark circles seen in the periorbital area are defined as bilateral, round homogeneous pigmented macules whose etiology is thought to be multifactorial. Available treatments include bleaching creams, topical retinoids, chemical peels, lasers, autologous fat transplantation, injectable fillers, and blepharoplasty.
“Microneedling has been recently suggested as an effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi said. “This technique is based on creating microchannels that can stimulate the production of subcutaneous collagen and elastin. It also enhances the revascularization and fibroblast activity, which increases the skin thickness and gives a shiny appearance to the skin. The fractional CO2 has also been introduced as an effective procedure to remove infraorbital dark circles. However, there are some potential complications with that therapy.”
For the current study, Dr. Farabi, of the department of dermatology at Ankara (Turkey) University, and Mohamad Goldust, MD, of University Hospital Basel (Switzerland), They used the handheld Automatic Microneedle Therapy System-Handhold from MCure. After creating microchannels, the investigators topically applied 10% trichloroacetic acid peels to each infraorbital area and waited for 5 minutes.
In the carbon dioxide laser group, a Lutronic CO2 laser was used with a pulse energy of 10 J/cm2, a 100-microsecond pulse rate, 30 W of power, and a pulse width of 4 mm. The treatment outcome was assessed with the patient’s satisfaction and the physician’s judgment, which were no response, partial response, and complete response. Patients in both study groups were followed up for blinded-investigator assessment of infraorbital hyperpigmentation, adverse events, and improvement, compared with baseline.
The mean age of patients was 40 years, with a range between 27 and 58 years. About one-third of patients in each group had Fitzpatrick skin types II, III, and IV, respectively. In the blinded investigator assessment, the laser-resurfacing procedure did not demonstrate a significant improvement in infraorbital hyperpigmentation at day 90 (P = .24). However, the combination of microneedling and 10% trichloroacetic acid peels significantly improved infraorbital hyperpigmentation by day 90, with improvement maintained through day 180 (P = .012 and .002, respectively).
Adverse events were mild and temporary in both groups. In the laser-resurfacing group, 7 of the patients (22.5%) developed transient infraorbital hyperpigmentation postoperatively that lasted 4 weeks. In the combination treatment group, 18 patients (58%) developed transient erythema that lasted for up to 1 week.
“We suggest using microneedling plus 10% [trichloroacetic acid] as a cost-effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi concluded.
The researchers reported having no financial disclosures.
In a study of patients with mild to moderate infraorbital dark circles, treatment with carbon dioxide laser resurfacing did not produce a significant improvement in infraorbital hyperpigmentation. However, the combination of microneedling and 10% trichloroacetic acid peels did.
The finding comes from what is believed to be the first head-to-head comparison of the two procedures for infraorbital dark circles, which are a common cosmetic concern with increased age.
During a late-breaking abstract session at the virtual annual meeting of the American Academy of Dermatology, lead study author Banu Farabi, MD, said that dark circles seen in the periorbital area are defined as bilateral, round homogeneous pigmented macules whose etiology is thought to be multifactorial. Available treatments include bleaching creams, topical retinoids, chemical peels, lasers, autologous fat transplantation, injectable fillers, and blepharoplasty.
“Microneedling has been recently suggested as an effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi said. “This technique is based on creating microchannels that can stimulate the production of subcutaneous collagen and elastin. It also enhances the revascularization and fibroblast activity, which increases the skin thickness and gives a shiny appearance to the skin. The fractional CO2 has also been introduced as an effective procedure to remove infraorbital dark circles. However, there are some potential complications with that therapy.”
For the current study, Dr. Farabi, of the department of dermatology at Ankara (Turkey) University, and Mohamad Goldust, MD, of University Hospital Basel (Switzerland), They used the handheld Automatic Microneedle Therapy System-Handhold from MCure. After creating microchannels, the investigators topically applied 10% trichloroacetic acid peels to each infraorbital area and waited for 5 minutes.
In the carbon dioxide laser group, a Lutronic CO2 laser was used with a pulse energy of 10 J/cm2, a 100-microsecond pulse rate, 30 W of power, and a pulse width of 4 mm. The treatment outcome was assessed with the patient’s satisfaction and the physician’s judgment, which were no response, partial response, and complete response. Patients in both study groups were followed up for blinded-investigator assessment of infraorbital hyperpigmentation, adverse events, and improvement, compared with baseline.
The mean age of patients was 40 years, with a range between 27 and 58 years. About one-third of patients in each group had Fitzpatrick skin types II, III, and IV, respectively. In the blinded investigator assessment, the laser-resurfacing procedure did not demonstrate a significant improvement in infraorbital hyperpigmentation at day 90 (P = .24). However, the combination of microneedling and 10% trichloroacetic acid peels significantly improved infraorbital hyperpigmentation by day 90, with improvement maintained through day 180 (P = .012 and .002, respectively).
Adverse events were mild and temporary in both groups. In the laser-resurfacing group, 7 of the patients (22.5%) developed transient infraorbital hyperpigmentation postoperatively that lasted 4 weeks. In the combination treatment group, 18 patients (58%) developed transient erythema that lasted for up to 1 week.
“We suggest using microneedling plus 10% [trichloroacetic acid] as a cost-effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi concluded.
The researchers reported having no financial disclosures.
FROM AAD 20
Multiethnic Training in Residency: A Survey of Dermatology Residents
Dermatologic treatment of patients with skin of color offers specific challenges. Studies have reported structural, morphologic, and physiologic distinctions among different ethnic groups,1 which may account for distinct clinical presentations of skin disease seen in patients with skin of color. Patients with skin of color are at increased risk for specific dermatologic conditions, such as postinflammatory hyperpigmentation, keloid development, and central centrifugal cicatricial alopecia.2,3 Furthermore, although skin cancer is less prevalent in patients with skin of color, it often presents at a more advanced stage and with a worse prognosis compared to white patients.4
Prior studies have demonstrated the need for increased exposure, education, and training in diseases pertaining to skin of color in US dermatology residency programs.6-8 The aim of this study was to assess if dermatologists in-training feel that their residency curriculum sufficiently educates them on the needs of patients with skin of color.
Methods
A 10-question anonymous survey was emailed to 109 dermatology residency programs to evaluate the attitudes of dermatology residents about their exposure to patients with skin of color and their skin-of-color curriculum. The study included individuals 18 years or older who were current residents in a dermatology program accredited by the Accreditation Council for Graduate Medical Education.
Results
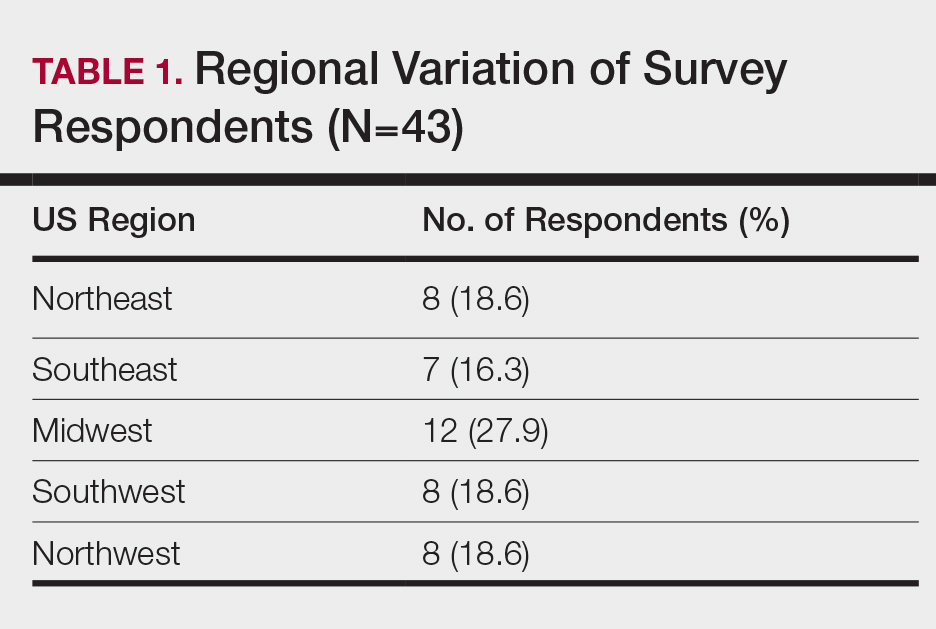
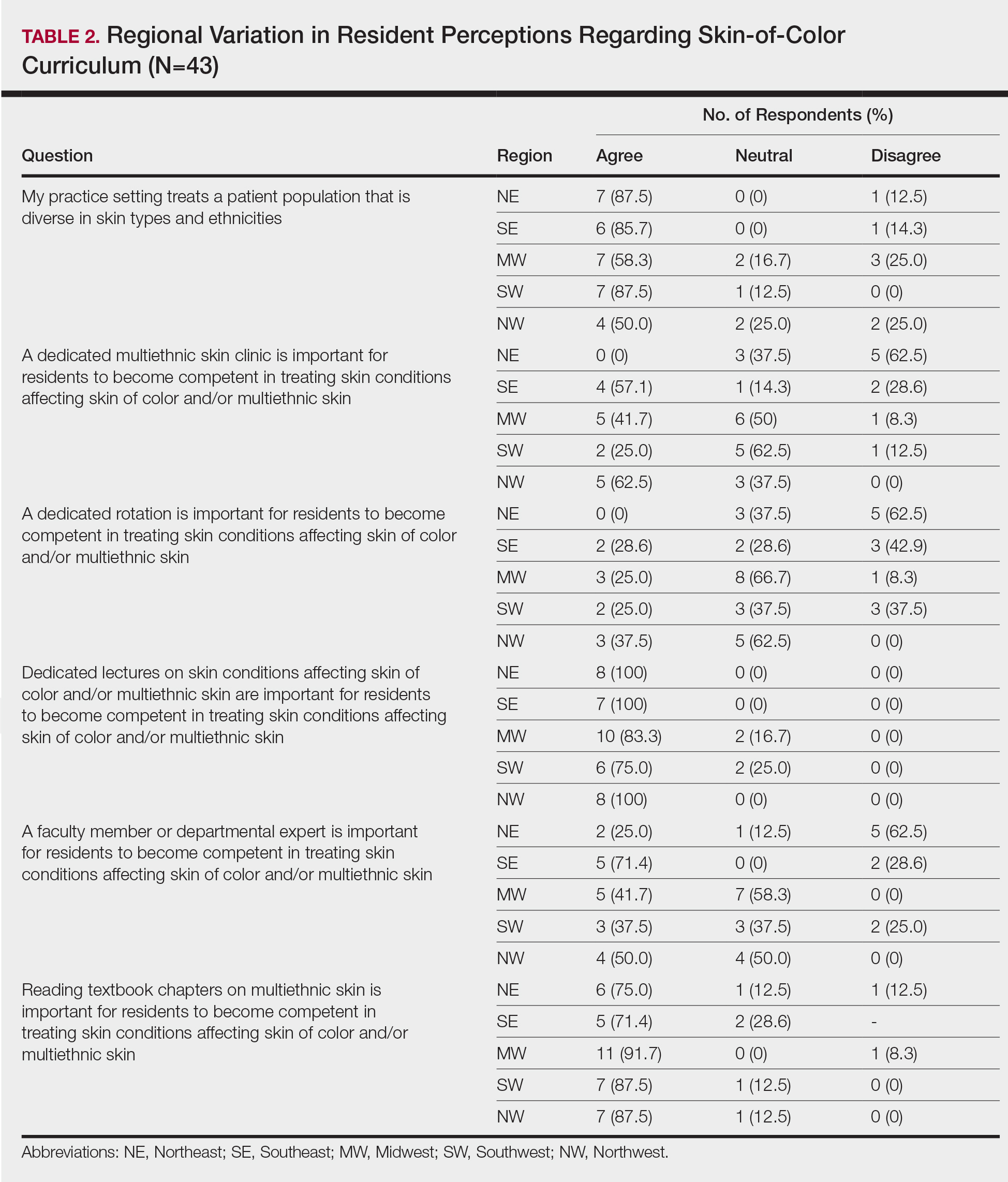
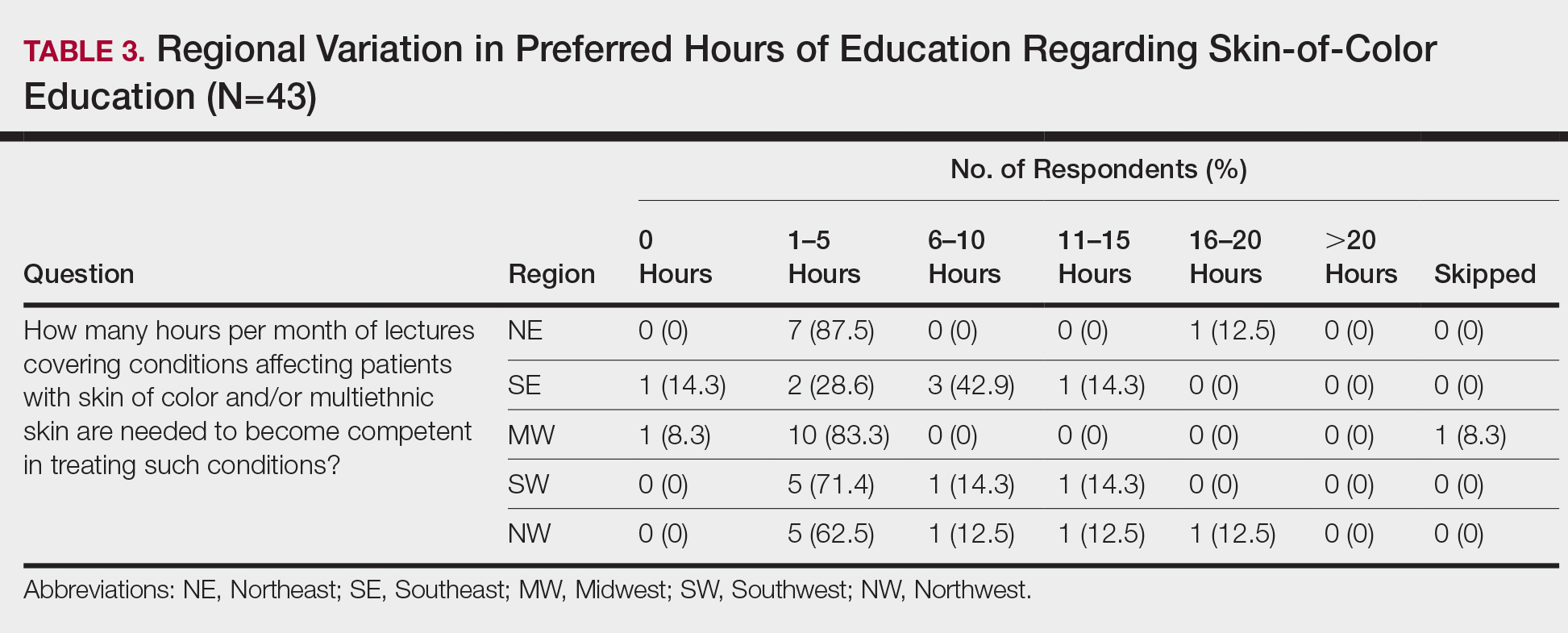
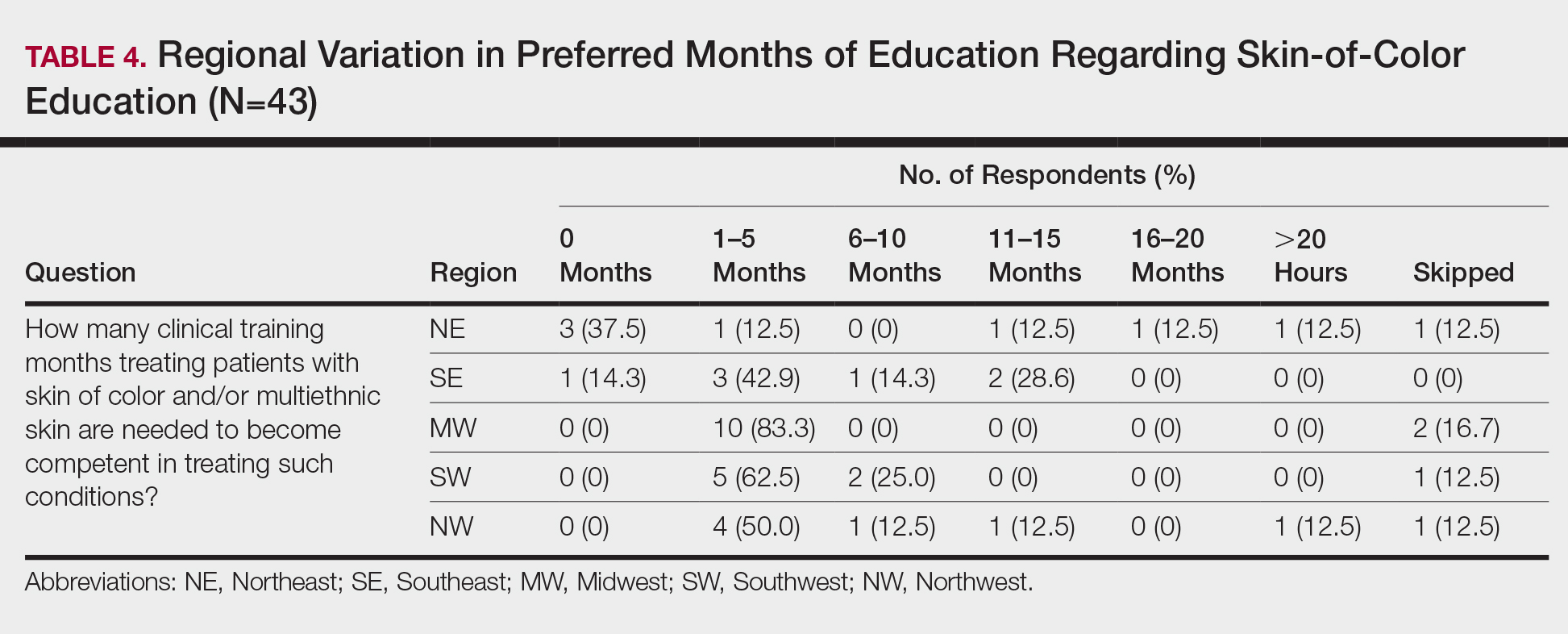
When asked the number of hours of lecture per month necessary to gain competence in conditions affecting patients with skin of color, 67% agreed that 1 to 5 hours was sufficient (Table 3). There were significant differences in the responses between the NE and SE (P=.024) and the SE and MW (P=.007). Of all respondents, 53% reported 1 to 5 months of clinical training are needed to gain competence in treating conditions affecting patients with skin of color, with significant differences in responses between the NE and MW (P<.001), the NE and SW (P=.019), and the SE and MW (P=.015)(Table 4).
Comment
Responses varied by practicing region
Although interactive lectures and textbook readings are important for obtaining a foundational understanding of dermatologic disease, they cannot substitute for clinical interactions and hands-on experience treating patients with skin of color.9 Not only do clinical interactions encourage independent reading and the study of encountered diagnoses, but intercommunication with patients may have a more profound and lasting impact on residents’ education.
Different regions of the United States have varying distributions of patients with skin of color, and dermatology residency program training reflects these disparities.6 In areas of less diversity, dermatology residents examine, diagnose, and treat substantially fewer patients with skin of color. The desire for more diverse training supports the prior findings of Nijhawan et al6 and is reflected in the responses we received in our study, whereby residents from the less ethnically diversified regions of the MW and NW were more likely to agree that clinics and rotations were necessary for training in preparation to sufficiently address the needs of patients with skin of color.
One way to compensate for the lack of ethnic diversity encountered in areas such as the MW and NW would be to develop educational programs featuring experts on skin of color.6 These specialists would not only train dermatology residents in areas of the country currently lacking ethnic diversity but also expand the expertise for treating patients with skin of color. Additionally, dedicated multiethnic skin clinics and externships devoted solely to treating patients with skin of color could be encouraged for residency training.6 Finally, community outreach through volunteer clinics may provide residents exposure to patients with skin of color seeking dermatologic care.10
This study was limited by the small number of respondents, but we were able to extract important trends and data from the collected responses. It is possible that respondents felt strongly about topics involving patients with skin of color, and the results were skewed to reflect individual bias. Additional limitations included not asking respondents for program names and population density (eg, urban, suburban, rural). Future studies should be directed toward analyzing how the diversity of the local population influences training in patients with skin of color, comparing program directors’ perceptions with residents’ perceptions on training in skin of color, and assessing patient perception of residents’ training in skin of color.
Conclusion
In the last decade it has become increasingly apparent that the US population is diversifying and that patients with skin of color will comprise a substantial proportion of the future population,8,11 which emphasizes the need for dermatology residency programs to ensure that residents receive adequate training and exposure to patients with skin of color as well as the distinct skin diseases seen more commonly in these populations.12
- Luther N, Darvin ME, Sterry W, et al. Ethnic differences in skin physiology, hair follicle morphology and follicular penetration. Skin Pharmacol Physiol. 2012;25:182-191.
- Shokeen D. Postinflammatory hyperpigmentation in patients with skin of color. Cutis. 2016;97:E9-E11.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Women’s Dermatol. 2017;3:S21-S37.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Colby SL, Ortman JM; US Census Bureau. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. https://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 13, 2020.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Ernst H, Colthorpe K. The efficacy of interactive lecturing for students with diverse science backgrounds. Adv Physiol Educ. 2007;31:41-44.
- Allday E. UCSF opens ‘skin of color’ dermatology clinic to address disparity in care. San Francisco Chronicle. March 20, 2019. https://www.sfchronicle.com/health/article/UCSF-opens-skin-of-color-dermatology-clinic-13704387.php. Accessed May 13, 2020.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Enos CW, Harvey VM. From bench to bedside: the Hampton University Skin of Color Research Institute 2015 Skin of Color Symposium. J Investig Dermatol Symp Proc. 2017;18:S29-S30.
Dermatologic treatment of patients with skin of color offers specific challenges. Studies have reported structural, morphologic, and physiologic distinctions among different ethnic groups,1 which may account for distinct clinical presentations of skin disease seen in patients with skin of color. Patients with skin of color are at increased risk for specific dermatologic conditions, such as postinflammatory hyperpigmentation, keloid development, and central centrifugal cicatricial alopecia.2,3 Furthermore, although skin cancer is less prevalent in patients with skin of color, it often presents at a more advanced stage and with a worse prognosis compared to white patients.4
Prior studies have demonstrated the need for increased exposure, education, and training in diseases pertaining to skin of color in US dermatology residency programs.6-8 The aim of this study was to assess if dermatologists in-training feel that their residency curriculum sufficiently educates them on the needs of patients with skin of color.
Methods
A 10-question anonymous survey was emailed to 109 dermatology residency programs to evaluate the attitudes of dermatology residents about their exposure to patients with skin of color and their skin-of-color curriculum. The study included individuals 18 years or older who were current residents in a dermatology program accredited by the Accreditation Council for Graduate Medical Education.
Results


When asked the number of hours of lecture per month necessary to gain competence in conditions affecting patients with skin of color, 67% agreed that 1 to 5 hours was sufficient (Table 3). There were significant differences in the responses between the NE and SE (P=.024) and the SE and MW (P=.007). Of all respondents, 53% reported 1 to 5 months of clinical training are needed to gain competence in treating conditions affecting patients with skin of color, with significant differences in responses between the NE and MW (P<.001), the NE and SW (P=.019), and the SE and MW (P=.015)(Table 4).


Comment
Responses varied by practicing region
Although interactive lectures and textbook readings are important for obtaining a foundational understanding of dermatologic disease, they cannot substitute for clinical interactions and hands-on experience treating patients with skin of color.9 Not only do clinical interactions encourage independent reading and the study of encountered diagnoses, but intercommunication with patients may have a more profound and lasting impact on residents’ education.
Different regions of the United States have varying distributions of patients with skin of color, and dermatology residency program training reflects these disparities.6 In areas of less diversity, dermatology residents examine, diagnose, and treat substantially fewer patients with skin of color. The desire for more diverse training supports the prior findings of Nijhawan et al6 and is reflected in the responses we received in our study, whereby residents from the less ethnically diversified regions of the MW and NW were more likely to agree that clinics and rotations were necessary for training in preparation to sufficiently address the needs of patients with skin of color.
One way to compensate for the lack of ethnic diversity encountered in areas such as the MW and NW would be to develop educational programs featuring experts on skin of color.6 These specialists would not only train dermatology residents in areas of the country currently lacking ethnic diversity but also expand the expertise for treating patients with skin of color. Additionally, dedicated multiethnic skin clinics and externships devoted solely to treating patients with skin of color could be encouraged for residency training.6 Finally, community outreach through volunteer clinics may provide residents exposure to patients with skin of color seeking dermatologic care.10
This study was limited by the small number of respondents, but we were able to extract important trends and data from the collected responses. It is possible that respondents felt strongly about topics involving patients with skin of color, and the results were skewed to reflect individual bias. Additional limitations included not asking respondents for program names and population density (eg, urban, suburban, rural). Future studies should be directed toward analyzing how the diversity of the local population influences training in patients with skin of color, comparing program directors’ perceptions with residents’ perceptions on training in skin of color, and assessing patient perception of residents’ training in skin of color.
Conclusion
In the last decade it has become increasingly apparent that the US population is diversifying and that patients with skin of color will comprise a substantial proportion of the future population,8,11 which emphasizes the need for dermatology residency programs to ensure that residents receive adequate training and exposure to patients with skin of color as well as the distinct skin diseases seen more commonly in these populations.12
Dermatologic treatment of patients with skin of color offers specific challenges. Studies have reported structural, morphologic, and physiologic distinctions among different ethnic groups,1 which may account for distinct clinical presentations of skin disease seen in patients with skin of color. Patients with skin of color are at increased risk for specific dermatologic conditions, such as postinflammatory hyperpigmentation, keloid development, and central centrifugal cicatricial alopecia.2,3 Furthermore, although skin cancer is less prevalent in patients with skin of color, it often presents at a more advanced stage and with a worse prognosis compared to white patients.4
Prior studies have demonstrated the need for increased exposure, education, and training in diseases pertaining to skin of color in US dermatology residency programs.6-8 The aim of this study was to assess if dermatologists in-training feel that their residency curriculum sufficiently educates them on the needs of patients with skin of color.
Methods
A 10-question anonymous survey was emailed to 109 dermatology residency programs to evaluate the attitudes of dermatology residents about their exposure to patients with skin of color and their skin-of-color curriculum. The study included individuals 18 years or older who were current residents in a dermatology program accredited by the Accreditation Council for Graduate Medical Education.
Results


When asked the number of hours of lecture per month necessary to gain competence in conditions affecting patients with skin of color, 67% agreed that 1 to 5 hours was sufficient (Table 3). There were significant differences in the responses between the NE and SE (P=.024) and the SE and MW (P=.007). Of all respondents, 53% reported 1 to 5 months of clinical training are needed to gain competence in treating conditions affecting patients with skin of color, with significant differences in responses between the NE and MW (P<.001), the NE and SW (P=.019), and the SE and MW (P=.015)(Table 4).


Comment
Responses varied by practicing region
Although interactive lectures and textbook readings are important for obtaining a foundational understanding of dermatologic disease, they cannot substitute for clinical interactions and hands-on experience treating patients with skin of color.9 Not only do clinical interactions encourage independent reading and the study of encountered diagnoses, but intercommunication with patients may have a more profound and lasting impact on residents’ education.
Different regions of the United States have varying distributions of patients with skin of color, and dermatology residency program training reflects these disparities.6 In areas of less diversity, dermatology residents examine, diagnose, and treat substantially fewer patients with skin of color. The desire for more diverse training supports the prior findings of Nijhawan et al6 and is reflected in the responses we received in our study, whereby residents from the less ethnically diversified regions of the MW and NW were more likely to agree that clinics and rotations were necessary for training in preparation to sufficiently address the needs of patients with skin of color.
One way to compensate for the lack of ethnic diversity encountered in areas such as the MW and NW would be to develop educational programs featuring experts on skin of color.6 These specialists would not only train dermatology residents in areas of the country currently lacking ethnic diversity but also expand the expertise for treating patients with skin of color. Additionally, dedicated multiethnic skin clinics and externships devoted solely to treating patients with skin of color could be encouraged for residency training.6 Finally, community outreach through volunteer clinics may provide residents exposure to patients with skin of color seeking dermatologic care.10
This study was limited by the small number of respondents, but we were able to extract important trends and data from the collected responses. It is possible that respondents felt strongly about topics involving patients with skin of color, and the results were skewed to reflect individual bias. Additional limitations included not asking respondents for program names and population density (eg, urban, suburban, rural). Future studies should be directed toward analyzing how the diversity of the local population influences training in patients with skin of color, comparing program directors’ perceptions with residents’ perceptions on training in skin of color, and assessing patient perception of residents’ training in skin of color.
Conclusion
In the last decade it has become increasingly apparent that the US population is diversifying and that patients with skin of color will comprise a substantial proportion of the future population,8,11 which emphasizes the need for dermatology residency programs to ensure that residents receive adequate training and exposure to patients with skin of color as well as the distinct skin diseases seen more commonly in these populations.12
- Luther N, Darvin ME, Sterry W, et al. Ethnic differences in skin physiology, hair follicle morphology and follicular penetration. Skin Pharmacol Physiol. 2012;25:182-191.
- Shokeen D. Postinflammatory hyperpigmentation in patients with skin of color. Cutis. 2016;97:E9-E11.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Women’s Dermatol. 2017;3:S21-S37.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Colby SL, Ortman JM; US Census Bureau. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. https://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 13, 2020.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Ernst H, Colthorpe K. The efficacy of interactive lecturing for students with diverse science backgrounds. Adv Physiol Educ. 2007;31:41-44.
- Allday E. UCSF opens ‘skin of color’ dermatology clinic to address disparity in care. San Francisco Chronicle. March 20, 2019. https://www.sfchronicle.com/health/article/UCSF-opens-skin-of-color-dermatology-clinic-13704387.php. Accessed May 13, 2020.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Enos CW, Harvey VM. From bench to bedside: the Hampton University Skin of Color Research Institute 2015 Skin of Color Symposium. J Investig Dermatol Symp Proc. 2017;18:S29-S30.
- Luther N, Darvin ME, Sterry W, et al. Ethnic differences in skin physiology, hair follicle morphology and follicular penetration. Skin Pharmacol Physiol. 2012;25:182-191.
- Shokeen D. Postinflammatory hyperpigmentation in patients with skin of color. Cutis. 2016;97:E9-E11.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Women’s Dermatol. 2017;3:S21-S37.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Colby SL, Ortman JM; US Census Bureau. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. https://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 13, 2020.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Ernst H, Colthorpe K. The efficacy of interactive lecturing for students with diverse science backgrounds. Adv Physiol Educ. 2007;31:41-44.
- Allday E. UCSF opens ‘skin of color’ dermatology clinic to address disparity in care. San Francisco Chronicle. March 20, 2019. https://www.sfchronicle.com/health/article/UCSF-opens-skin-of-color-dermatology-clinic-13704387.php. Accessed May 13, 2020.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Enos CW, Harvey VM. From bench to bedside: the Hampton University Skin of Color Research Institute 2015 Skin of Color Symposium. J Investig Dermatol Symp Proc. 2017;18:S29-S30.
Practice Points
- To treat the ever-changing demographics of patients in the United States, dermatologists must receive adequate exposure and education regarding dermatologic conditions in patients from various ethnic backgrounds.
- Dermatology residents from less diverse regions are more likely to agree that dedicated clinics and rotations are important to gain competence compared to those from more diverse regions.
- In areas with less diversity, dedicated multiethnic skin clinics and faculty may be more important for assuring an adequate residency experience.
Comment on “Racial Limitations of Fitzpatrick Skin Type”
To the Editor:
It is with great interest that I read the article by Ware et al,1 “Racial Limitations of Fitzpatrick Skin Type.” Within my own department, the issue of the appropriateness of using Fitzpatrick skin type (FST) as a surrogate to describe skin color has been raised with mixed responses.
As in many dermatology residency programs across the country, first-year dermatology residents are asked to describe the morphology of a lesion/eruption seen on a patient during Grand Rounds. Preceding the morphologic description, many providers describe the appearance of the patient including their skin color, as constitutive skin color can impact understanding of the morphologic descriptions, favor different diagnoses based on disease epidemiology, and guide subsequent treatment recommendations.2,3 During one of my first Grand Rounds as an early dermatology resident, a patient was described as a “well-appearing brown boy,” which led to a lively discussion regarding the terms that should be used to describe skin color, with some in the audience preferring FST, others including myself preferring degree of pigmentation (eg, light, moderate, dark), and lastly others preferring an inferred ethnicity based on the patient’s appearance. One audience member commented, “I am brown, therefore I think it is fine to say ‘brown boy,’” which adds to findings from Ware et al1 that there may be differences in what providers prefer to utilize to describe a patient’s skin color based on their own constitutive skin color.
I inquired with 2 other first-year dermatology residents with skin of color at other programs. When asked what terminology they use to describe a patient for Grand Rounds or in clinic, one resident replied, “It’s stylistic but if it’s your one liner [for assessment and plan] use their ethnicity [whereas] if it’s [for] a physical exam use their Fitzpatrick skin type.” The other resident replied, “I use Fitzpatrick skin type even though it’s technically subjective and therefore not appropriate for use within objective data, such as the physical exam, however it’s a language that most colleagues understand as a substitute for skin color.” I also raised the same question to an attending dermatologist at a primarily skin-of-color community hospital. She replied, “I think when unsure about ethnicity, Fitzpatrick type is an appropriate way to describe someone. It’s not really correct to say [a patient’s ethnicity] when you don’t know for sure.”
Unfortunately, as Ware and colleagues1 indicated, there is no consensus by which to objectively classify nonwhite skin color. Within the dermatology literature, it has been proposed that race should not be used to express skin color, and this article proposes that FST is an inappropriate surrogate for race/ethnicity.4 Although I agree that appropriate use of FST should be emphasized in training, is there a vocabulary that Ware et al1 recommend we use instead? Does the Skin of Color Society have suggestions on preferred language among its members? Finally, what efforts are being made to develop “culturally appropriate and clinically relevant methods for describing skin of color,” as the authors stated, within our own Skin of Color Society, or to whom does this responsibility ultimately fall?
References
1. Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
2. Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
3. Kelly AP, Taylor SC, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill Education; 2016.
4. Bigby M, Thaler D. Describing patients’ “race” in clinical presentations should be abandoned. J Am Acad Dermatol. 2006;54:1074-1076.
Author’s Response
My colleagues and I thank Dr. Pimentel for his insights regarding the article, “Racial Limitations of Fitzpatrick Skin Type.”1 The conundrum on how to appropriately categorize skin color for descriptive and epidemiologic purposes continues to remain unsolved today. However, attempts have been made in the past. For example, in September 2006, Dr. Susan C. Taylor (Philadelphia, Pennsylvania), formed and chaired a workshop session titled “A New Classification System for All Skin Types.” Dermatology leaders with skin of color expertise were invited from around the world for a weekend in New York, New York, to brainstorm a new skin color classification system. This endeavor did not produce any successful alternatives, but it has remained a pertinent topic of discussion in academic dermatology, including the Skin of Color Society, since then.
When unsure about ethnicity, my colleagues and I continue to advocate that the Fitzpatrick scale is not an appropriate substitute to describe skin color. This usage of Fitzpatrick skin type (FST) perpetuates the idea that the Fitzpatrick scale is a suitable proxy to describe ethnicity or race, which it is not. It is important to remember that race is a social classification construct, not a biological one.2 The topic of race in contemporary culture undoubtedly invokes strong emotional connotations. The language around race is constantly evolving. I would argue that fear and discomfort of using incorrect racial language promotes the inappropriate use of FST, as the FST may be perceived as a more scientific and pseudoapplicable form of classification. To gain knowledge about a patient’s ethnicity/race to assess epidemiologic ethnic trends, we recommend asking the patient in an intake form or during consultation to self-identify his/her ethnicity or race,3 which takes the guesswork out for providers. However, caution must be exercised to avoid using race and ethnicity to later describe skin color.
Until a more culturally and medically relevant method of skin color classification is created, my colleagues and I recommend using basic color adjectives such as brown, black, pink, tan, or white supplemented with light, medium, or dark predescriptors. For example, “A 35-year-old self-identified African American woman with a dark brown skin hue presents with a 2-week flare of itchy, dark purple plaques with white scale on the scalp and extensor surfaces of the knees and elbows.” These basic descriptions for constitutive skin color conjure ample visual information for the listener/reader to understand morphologic descriptions, presentation of erythema, changes in pigmentation, and more. For a more specific skin color classification, we recommend developing a user-friendly Pantone-like color system to classify constitutive skin color.4
Jessica E. Dawson, MD
From the University of Washington School of Medicine, Seattle.
The author reports no conflict of interest.
Correspondence: Jessica E. Dawson, MD, University of Washington School of Medicine, 1959 NE Pacific St, Seattle, WA 98195 ([email protected]).
References
1. Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
2. Ifekwunigwe JO, Wagner JK, Yu JH, et al. A qualitative analysis of how anthropologists interpret the race construct. Am Anthropol. 2017;119:422-434.
3. Hasnain-Wynia R, Baker DW. Obtaining data on patient race, ethnicity, and primary language in health care organizations: current challenges and proposed solutions. Health Serv Res. 2006;41:1501-1518.
4. What is the Pantone color system? Pantone website. https://www.pantone.com/color-systems/pantone-color-systems-explained. Accesed May 13, 2020.
To the Editor:
It is with great interest that I read the article by Ware et al,1 “Racial Limitations of Fitzpatrick Skin Type.” Within my own department, the issue of the appropriateness of using Fitzpatrick skin type (FST) as a surrogate to describe skin color has been raised with mixed responses.
As in many dermatology residency programs across the country, first-year dermatology residents are asked to describe the morphology of a lesion/eruption seen on a patient during Grand Rounds. Preceding the morphologic description, many providers describe the appearance of the patient including their skin color, as constitutive skin color can impact understanding of the morphologic descriptions, favor different diagnoses based on disease epidemiology, and guide subsequent treatment recommendations.2,3 During one of my first Grand Rounds as an early dermatology resident, a patient was described as a “well-appearing brown boy,” which led to a lively discussion regarding the terms that should be used to describe skin color, with some in the audience preferring FST, others including myself preferring degree of pigmentation (eg, light, moderate, dark), and lastly others preferring an inferred ethnicity based on the patient’s appearance. One audience member commented, “I am brown, therefore I think it is fine to say ‘brown boy,’” which adds to findings from Ware et al1 that there may be differences in what providers prefer to utilize to describe a patient’s skin color based on their own constitutive skin color.
I inquired with 2 other first-year dermatology residents with skin of color at other programs. When asked what terminology they use to describe a patient for Grand Rounds or in clinic, one resident replied, “It’s stylistic but if it’s your one liner [for assessment and plan] use their ethnicity [whereas] if it’s [for] a physical exam use their Fitzpatrick skin type.” The other resident replied, “I use Fitzpatrick skin type even though it’s technically subjective and therefore not appropriate for use within objective data, such as the physical exam, however it’s a language that most colleagues understand as a substitute for skin color.” I also raised the same question to an attending dermatologist at a primarily skin-of-color community hospital. She replied, “I think when unsure about ethnicity, Fitzpatrick type is an appropriate way to describe someone. It’s not really correct to say [a patient’s ethnicity] when you don’t know for sure.”
Unfortunately, as Ware and colleagues1 indicated, there is no consensus by which to objectively classify nonwhite skin color. Within the dermatology literature, it has been proposed that race should not be used to express skin color, and this article proposes that FST is an inappropriate surrogate for race/ethnicity.4 Although I agree that appropriate use of FST should be emphasized in training, is there a vocabulary that Ware et al1 recommend we use instead? Does the Skin of Color Society have suggestions on preferred language among its members? Finally, what efforts are being made to develop “culturally appropriate and clinically relevant methods for describing skin of color,” as the authors stated, within our own Skin of Color Society, or to whom does this responsibility ultimately fall?
References
1. Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
2. Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
3. Kelly AP, Taylor SC, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill Education; 2016.
4. Bigby M, Thaler D. Describing patients’ “race” in clinical presentations should be abandoned. J Am Acad Dermatol. 2006;54:1074-1076.
Author’s Response
My colleagues and I thank Dr. Pimentel for his insights regarding the article, “Racial Limitations of Fitzpatrick Skin Type.”1 The conundrum on how to appropriately categorize skin color for descriptive and epidemiologic purposes continues to remain unsolved today. However, attempts have been made in the past. For example, in September 2006, Dr. Susan C. Taylor (Philadelphia, Pennsylvania), formed and chaired a workshop session titled “A New Classification System for All Skin Types.” Dermatology leaders with skin of color expertise were invited from around the world for a weekend in New York, New York, to brainstorm a new skin color classification system. This endeavor did not produce any successful alternatives, but it has remained a pertinent topic of discussion in academic dermatology, including the Skin of Color Society, since then.
When unsure about ethnicity, my colleagues and I continue to advocate that the Fitzpatrick scale is not an appropriate substitute to describe skin color. This usage of Fitzpatrick skin type (FST) perpetuates the idea that the Fitzpatrick scale is a suitable proxy to describe ethnicity or race, which it is not. It is important to remember that race is a social classification construct, not a biological one.2 The topic of race in contemporary culture undoubtedly invokes strong emotional connotations. The language around race is constantly evolving. I would argue that fear and discomfort of using incorrect racial language promotes the inappropriate use of FST, as the FST may be perceived as a more scientific and pseudoapplicable form of classification. To gain knowledge about a patient’s ethnicity/race to assess epidemiologic ethnic trends, we recommend asking the patient in an intake form or during consultation to self-identify his/her ethnicity or race,3 which takes the guesswork out for providers. However, caution must be exercised to avoid using race and ethnicity to later describe skin color.
Until a more culturally and medically relevant method of skin color classification is created, my colleagues and I recommend using basic color adjectives such as brown, black, pink, tan, or white supplemented with light, medium, or dark predescriptors. For example, “A 35-year-old self-identified African American woman with a dark brown skin hue presents with a 2-week flare of itchy, dark purple plaques with white scale on the scalp and extensor surfaces of the knees and elbows.” These basic descriptions for constitutive skin color conjure ample visual information for the listener/reader to understand morphologic descriptions, presentation of erythema, changes in pigmentation, and more. For a more specific skin color classification, we recommend developing a user-friendly Pantone-like color system to classify constitutive skin color.4
Jessica E. Dawson, MD
From the University of Washington School of Medicine, Seattle.
The author reports no conflict of interest.
Correspondence: Jessica E. Dawson, MD, University of Washington School of Medicine, 1959 NE Pacific St, Seattle, WA 98195 ([email protected]).
References
1. Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
2. Ifekwunigwe JO, Wagner JK, Yu JH, et al. A qualitative analysis of how anthropologists interpret the race construct. Am Anthropol. 2017;119:422-434.
3. Hasnain-Wynia R, Baker DW. Obtaining data on patient race, ethnicity, and primary language in health care organizations: current challenges and proposed solutions. Health Serv Res. 2006;41:1501-1518.
4. What is the Pantone color system? Pantone website. https://www.pantone.com/color-systems/pantone-color-systems-explained. Accesed May 13, 2020.
To the Editor:
It is with great interest that I read the article by Ware et al,1 “Racial Limitations of Fitzpatrick Skin Type.” Within my own department, the issue of the appropriateness of using Fitzpatrick skin type (FST) as a surrogate to describe skin color has been raised with mixed responses.
As in many dermatology residency programs across the country, first-year dermatology residents are asked to describe the morphology of a lesion/eruption seen on a patient during Grand Rounds. Preceding the morphologic description, many providers describe the appearance of the patient including their skin color, as constitutive skin color can impact understanding of the morphologic descriptions, favor different diagnoses based on disease epidemiology, and guide subsequent treatment recommendations.2,3 During one of my first Grand Rounds as an early dermatology resident, a patient was described as a “well-appearing brown boy,” which led to a lively discussion regarding the terms that should be used to describe skin color, with some in the audience preferring FST, others including myself preferring degree of pigmentation (eg, light, moderate, dark), and lastly others preferring an inferred ethnicity based on the patient’s appearance. One audience member commented, “I am brown, therefore I think it is fine to say ‘brown boy,’” which adds to findings from Ware et al1 that there may be differences in what providers prefer to utilize to describe a patient’s skin color based on their own constitutive skin color.
I inquired with 2 other first-year dermatology residents with skin of color at other programs. When asked what terminology they use to describe a patient for Grand Rounds or in clinic, one resident replied, “It’s stylistic but if it’s your one liner [for assessment and plan] use their ethnicity [whereas] if it’s [for] a physical exam use their Fitzpatrick skin type.” The other resident replied, “I use Fitzpatrick skin type even though it’s technically subjective and therefore not appropriate for use within objective data, such as the physical exam, however it’s a language that most colleagues understand as a substitute for skin color.” I also raised the same question to an attending dermatologist at a primarily skin-of-color community hospital. She replied, “I think when unsure about ethnicity, Fitzpatrick type is an appropriate way to describe someone. It’s not really correct to say [a patient’s ethnicity] when you don’t know for sure.”
Unfortunately, as Ware and colleagues1 indicated, there is no consensus by which to objectively classify nonwhite skin color. Within the dermatology literature, it has been proposed that race should not be used to express skin color, and this article proposes that FST is an inappropriate surrogate for race/ethnicity.4 Although I agree that appropriate use of FST should be emphasized in training, is there a vocabulary that Ware et al1 recommend we use instead? Does the Skin of Color Society have suggestions on preferred language among its members? Finally, what efforts are being made to develop “culturally appropriate and clinically relevant methods for describing skin of color,” as the authors stated, within our own Skin of Color Society, or to whom does this responsibility ultimately fall?
References
1. Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
2. Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
3. Kelly AP, Taylor SC, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill Education; 2016.
4. Bigby M, Thaler D. Describing patients’ “race” in clinical presentations should be abandoned. J Am Acad Dermatol. 2006;54:1074-1076.
Author’s Response
My colleagues and I thank Dr. Pimentel for his insights regarding the article, “Racial Limitations of Fitzpatrick Skin Type.”1 The conundrum on how to appropriately categorize skin color for descriptive and epidemiologic purposes continues to remain unsolved today. However, attempts have been made in the past. For example, in September 2006, Dr. Susan C. Taylor (Philadelphia, Pennsylvania), formed and chaired a workshop session titled “A New Classification System for All Skin Types.” Dermatology leaders with skin of color expertise were invited from around the world for a weekend in New York, New York, to brainstorm a new skin color classification system. This endeavor did not produce any successful alternatives, but it has remained a pertinent topic of discussion in academic dermatology, including the Skin of Color Society, since then.
When unsure about ethnicity, my colleagues and I continue to advocate that the Fitzpatrick scale is not an appropriate substitute to describe skin color. This usage of Fitzpatrick skin type (FST) perpetuates the idea that the Fitzpatrick scale is a suitable proxy to describe ethnicity or race, which it is not. It is important to remember that race is a social classification construct, not a biological one.2 The topic of race in contemporary culture undoubtedly invokes strong emotional connotations. The language around race is constantly evolving. I would argue that fear and discomfort of using incorrect racial language promotes the inappropriate use of FST, as the FST may be perceived as a more scientific and pseudoapplicable form of classification. To gain knowledge about a patient’s ethnicity/race to assess epidemiologic ethnic trends, we recommend asking the patient in an intake form or during consultation to self-identify his/her ethnicity or race,3 which takes the guesswork out for providers. However, caution must be exercised to avoid using race and ethnicity to later describe skin color.
Until a more culturally and medically relevant method of skin color classification is created, my colleagues and I recommend using basic color adjectives such as brown, black, pink, tan, or white supplemented with light, medium, or dark predescriptors. For example, “A 35-year-old self-identified African American woman with a dark brown skin hue presents with a 2-week flare of itchy, dark purple plaques with white scale on the scalp and extensor surfaces of the knees and elbows.” These basic descriptions for constitutive skin color conjure ample visual information for the listener/reader to understand morphologic descriptions, presentation of erythema, changes in pigmentation, and more. For a more specific skin color classification, we recommend developing a user-friendly Pantone-like color system to classify constitutive skin color.4
Jessica E. Dawson, MD
From the University of Washington School of Medicine, Seattle.
The author reports no conflict of interest.
Correspondence: Jessica E. Dawson, MD, University of Washington School of Medicine, 1959 NE Pacific St, Seattle, WA 98195 ([email protected]).
References
1. Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
2. Ifekwunigwe JO, Wagner JK, Yu JH, et al. A qualitative analysis of how anthropologists interpret the race construct. Am Anthropol. 2017;119:422-434.
3. Hasnain-Wynia R, Baker DW. Obtaining data on patient race, ethnicity, and primary language in health care organizations: current challenges and proposed solutions. Health Serv Res. 2006;41:1501-1518.
4. What is the Pantone color system? Pantone website. https://www.pantone.com/color-systems/pantone-color-systems-explained. Accesed May 13, 2020.
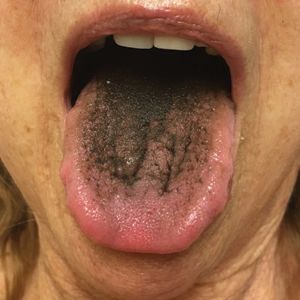
Asymptomatic Transient Lingual Hyperpigmentation
The Diagnosis: Pseudo-Black Hairy Tongue
Pseudo-black hairy tongue is a benign and painless disorder characterized by transient hyperpigmentation of the tongue with a substance that can be easily scraped off. In this case, the patient's lingual discoloration was secondary to the ingestion of bismuth salicylate. The phenomenon is thought to occur due to a reaction between bismuth and sulfur-containing compounds in the saliva, resulting in the characteristic black substance on the surface of the tongue that nestles between the lingual papillae.1 An associated feature may include black stools. Other etiologic factors involved in pseudo-black hairy tongue include food coloring, tobacco, and other drugs such antibiotics and antidepressants.2
The differential diagnosis of lingual hyperpigmentation includes lingua villosa nigra (also known as black hairy tongue), pigmented fungiform papillae of the tongue, acanthosis nigricans, and oral hairy leukoplakia. Lingua villosa nigra is a similar condition in which individuals present with a black tongue; however, the tongue also appears hairy. The tongue may appear as other colors such as brown, yellow, or green. Patients additionally may have symptoms of burning, dysgeusia, halitosis, or gagging. Poor oral hygiene, xerostomia, use of tobacco or alcohol, and different medications including antibiotics and antipsychotic medications increase the risk for developing lingua villosa nigra.2,3 This condition is distinguished from pseudo-black hairy tongue by proliferation and elongation of the filiform papillae.3 Pigmented fungiform papillae of the tongue is a normal variant of tongue morphology, is more common in individuals with darker skin types, and primarily affects the lateral aspect and apex of the tongue.4 Acanthosis nigricans can appear in the oral cavity as multiple pigmented papillary lesions on the dorsal and lateral regions of the tongue and frequently involves the lips; this condition may be associated with metabolic disorders or underlying malignancy.2,3 Oral hairy leukoplakia is caused by Epstein-Barr virus infection and typically presents as white plaques on the dorsal and ventral surfaces of the tongue; this condition largely is found in immunocompromised patients.5
In our patient there was an acute onset of tongue discoloration associated with ingestion of bismuth salicylate, no hypertrophy or lengthening of the lingual papillae, and no involvement of the patient's lips, which was consistent with the diagnosis of pseudo-black hairy tongue. Pseudo-black hairy tongue is transient and treated by discontinuation of offending agents and proper hygiene practices.
- Bradley B, Singleton M, Lin Wan Po A. Bismuth toxicity--a reassessment. J Clin Pharm Ther. 1989;14:423-441.
- Gurvits GE, Tan A. Black hairy tongue syndrome. World J Gastroenterol. 2014;20:10845-10850.
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569.
- Mangold AR, Torgerson RR, Rogers RS. Diseases of the tongue. Clin Dermatol. 2016;34:458-469.
- Husak R, Garbe C, Orfanos CE. Oral hairy leukoplakia in 71 HIV-seropositive patients: clinical symptoms, relation to immunologic status, and prognostic significance. J Am Acad Dermatol. 1996;35:928-934.
The Diagnosis: Pseudo-Black Hairy Tongue
Pseudo-black hairy tongue is a benign and painless disorder characterized by transient hyperpigmentation of the tongue with a substance that can be easily scraped off. In this case, the patient's lingual discoloration was secondary to the ingestion of bismuth salicylate. The phenomenon is thought to occur due to a reaction between bismuth and sulfur-containing compounds in the saliva, resulting in the characteristic black substance on the surface of the tongue that nestles between the lingual papillae.1 An associated feature may include black stools. Other etiologic factors involved in pseudo-black hairy tongue include food coloring, tobacco, and other drugs such antibiotics and antidepressants.2
The differential diagnosis of lingual hyperpigmentation includes lingua villosa nigra (also known as black hairy tongue), pigmented fungiform papillae of the tongue, acanthosis nigricans, and oral hairy leukoplakia. Lingua villosa nigra is a similar condition in which individuals present with a black tongue; however, the tongue also appears hairy. The tongue may appear as other colors such as brown, yellow, or green. Patients additionally may have symptoms of burning, dysgeusia, halitosis, or gagging. Poor oral hygiene, xerostomia, use of tobacco or alcohol, and different medications including antibiotics and antipsychotic medications increase the risk for developing lingua villosa nigra.2,3 This condition is distinguished from pseudo-black hairy tongue by proliferation and elongation of the filiform papillae.3 Pigmented fungiform papillae of the tongue is a normal variant of tongue morphology, is more common in individuals with darker skin types, and primarily affects the lateral aspect and apex of the tongue.4 Acanthosis nigricans can appear in the oral cavity as multiple pigmented papillary lesions on the dorsal and lateral regions of the tongue and frequently involves the lips; this condition may be associated with metabolic disorders or underlying malignancy.2,3 Oral hairy leukoplakia is caused by Epstein-Barr virus infection and typically presents as white plaques on the dorsal and ventral surfaces of the tongue; this condition largely is found in immunocompromised patients.5
In our patient there was an acute onset of tongue discoloration associated with ingestion of bismuth salicylate, no hypertrophy or lengthening of the lingual papillae, and no involvement of the patient's lips, which was consistent with the diagnosis of pseudo-black hairy tongue. Pseudo-black hairy tongue is transient and treated by discontinuation of offending agents and proper hygiene practices.
The Diagnosis: Pseudo-Black Hairy Tongue
Pseudo-black hairy tongue is a benign and painless disorder characterized by transient hyperpigmentation of the tongue with a substance that can be easily scraped off. In this case, the patient's lingual discoloration was secondary to the ingestion of bismuth salicylate. The phenomenon is thought to occur due to a reaction between bismuth and sulfur-containing compounds in the saliva, resulting in the characteristic black substance on the surface of the tongue that nestles between the lingual papillae.1 An associated feature may include black stools. Other etiologic factors involved in pseudo-black hairy tongue include food coloring, tobacco, and other drugs such antibiotics and antidepressants.2
The differential diagnosis of lingual hyperpigmentation includes lingua villosa nigra (also known as black hairy tongue), pigmented fungiform papillae of the tongue, acanthosis nigricans, and oral hairy leukoplakia. Lingua villosa nigra is a similar condition in which individuals present with a black tongue; however, the tongue also appears hairy. The tongue may appear as other colors such as brown, yellow, or green. Patients additionally may have symptoms of burning, dysgeusia, halitosis, or gagging. Poor oral hygiene, xerostomia, use of tobacco or alcohol, and different medications including antibiotics and antipsychotic medications increase the risk for developing lingua villosa nigra.2,3 This condition is distinguished from pseudo-black hairy tongue by proliferation and elongation of the filiform papillae.3 Pigmented fungiform papillae of the tongue is a normal variant of tongue morphology, is more common in individuals with darker skin types, and primarily affects the lateral aspect and apex of the tongue.4 Acanthosis nigricans can appear in the oral cavity as multiple pigmented papillary lesions on the dorsal and lateral regions of the tongue and frequently involves the lips; this condition may be associated with metabolic disorders or underlying malignancy.2,3 Oral hairy leukoplakia is caused by Epstein-Barr virus infection and typically presents as white plaques on the dorsal and ventral surfaces of the tongue; this condition largely is found in immunocompromised patients.5
In our patient there was an acute onset of tongue discoloration associated with ingestion of bismuth salicylate, no hypertrophy or lengthening of the lingual papillae, and no involvement of the patient's lips, which was consistent with the diagnosis of pseudo-black hairy tongue. Pseudo-black hairy tongue is transient and treated by discontinuation of offending agents and proper hygiene practices.
- Bradley B, Singleton M, Lin Wan Po A. Bismuth toxicity--a reassessment. J Clin Pharm Ther. 1989;14:423-441.
- Gurvits GE, Tan A. Black hairy tongue syndrome. World J Gastroenterol. 2014;20:10845-10850.
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569.
- Mangold AR, Torgerson RR, Rogers RS. Diseases of the tongue. Clin Dermatol. 2016;34:458-469.
- Husak R, Garbe C, Orfanos CE. Oral hairy leukoplakia in 71 HIV-seropositive patients: clinical symptoms, relation to immunologic status, and prognostic significance. J Am Acad Dermatol. 1996;35:928-934.
- Bradley B, Singleton M, Lin Wan Po A. Bismuth toxicity--a reassessment. J Clin Pharm Ther. 1989;14:423-441.
- Gurvits GE, Tan A. Black hairy tongue syndrome. World J Gastroenterol. 2014;20:10845-10850.
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569.
- Mangold AR, Torgerson RR, Rogers RS. Diseases of the tongue. Clin Dermatol. 2016;34:458-469.
- Husak R, Garbe C, Orfanos CE. Oral hairy leukoplakia in 71 HIV-seropositive patients: clinical symptoms, relation to immunologic status, and prognostic significance. J Am Acad Dermatol. 1996;35:928-934.
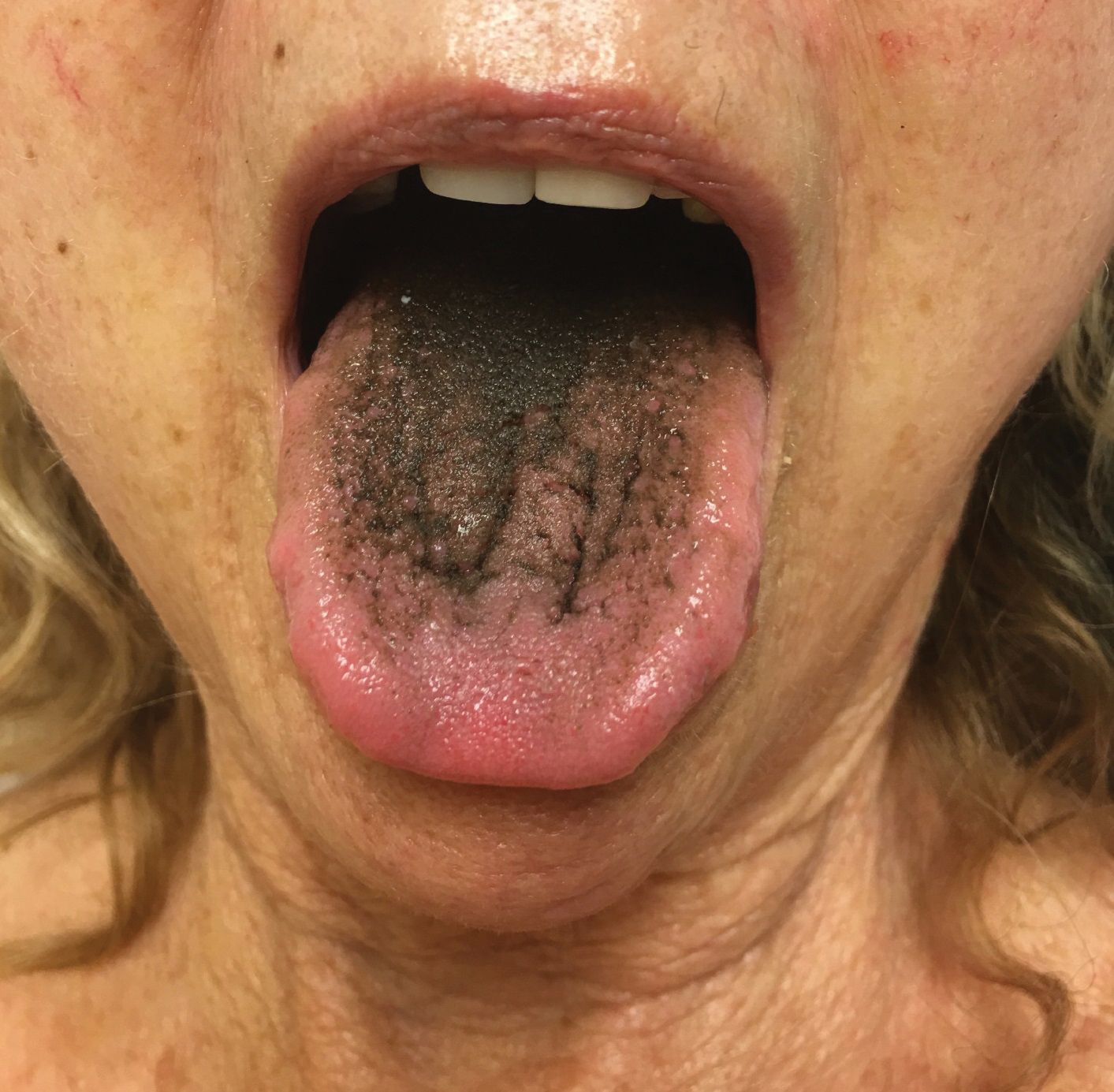
A 77-year-old woman incidentally was noted to have black discoloration of the tongue during a routine dermatologic examination. The patient was unaware of the tongue discoloration and reported that her tongue appeared normal the day prior. The tongue was asymptomatic. Clinical examination revealed black hyperpigmentation on the dorsal aspect of the tongue without appreciable hypertrophy or hyperkeratosis of the filiform papillae. The patient had a half-pack daily smoking habit for many years but had abstained from any smoking or tobacco use for the last 15 years. The patient endorsed good oral hygiene. Upon further questioning, the patient revealed that she had ingested 1 tablet of bismuth salicylate the prior night to relieve postprandial dyspepsia. A cotton-tipped applicator was rubbed gently against the affected area and removed some of the black pigment.
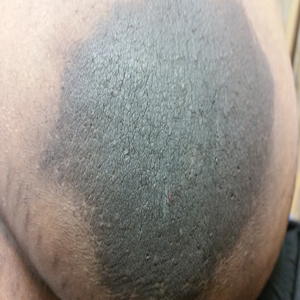
Sunless Tanner Caused Persistent Hyperpigmented Patches on the Hands
To the Editor:
The use of sunless tanners has become an alternative for individuals who wish to have tan skin without exposure to UV radiation.1 We present a case of a patient who experienced persistent hyperpigmented patches on the hands months after the use of a sunless tanner containing dihydroxyacetone (DHA), a carbohydrate that reacts with amino acids in the stratum corneum to produce pigments called melanoidins. The hyperpigmentation caused by DHA is due to the Maillard reaction, which is the nonenzymatic glycation of amino groups of proteins by the carbonyl groups of sugar.2 Many sunless tanners contain DHA at varying concentrations. Dermatologists should be aware of the benefits and potential side effects of these alternative products so that they can appropriately counsel patients.
A 20-year-old woman with no history of skin disease presented for evaluation of hyperpigmented patches on the dorsal hands of several months’ duration. Physical examination revealed ill-defined hyperpigmented patches on the dorsal fingers without associated scale or erythema (Figure 1). She had a remote history of Hodgkin lymphoma treated with chemotherapy and was in remission for 5 years prior to the current presentation. Her hematologists referred her to dermatology for evaluation, as they did not believe the patches could be related to her chemotherapy given that she had completed the treatment years before.

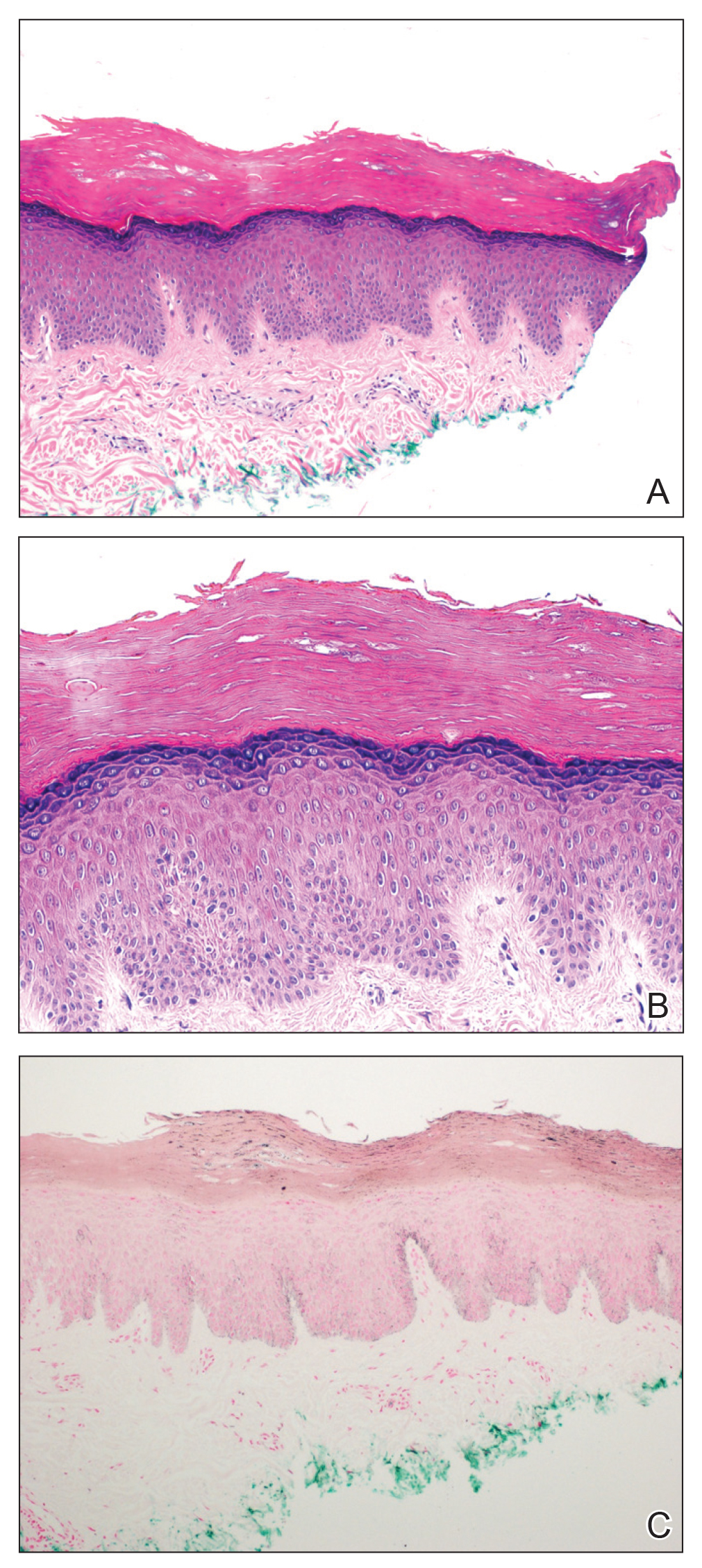
A punch biopsy of one of the patches was obtained to elucidate the origin of the hyperpigmentation, which had no obvious triggers according to the patient. Histopathologic examination revealed hyperpigmented parakeratosis and lentiginous hyperplasia along with pigmentation of the stratum corneum (Figures 2A and 2B) with black pigment, which stained positive with Fontana-Masson (Figure 2C).
Upon further questioning, it was revealed that our patient had used a sunless tanner 3 months prior to the development of the pigmented patches. She also used urea cream to hasten exfoliation, which resulted in lighter but still apparent hyperpigmentation at follow-up 6 months after the initial presentation.
There has been a rapid growth of the sunless tanning industry in the last several years due to effective public education against UV tanning. Generally, patients apply the sunless tanner and notice an increase in tan within the following 48 hours. Typically, the tan progressively fades with the normal skin exfoliation over the span of weeks. Although most of the DHA binds proteins in the stratum corneum, the US Food and Drug Administration released a report speculating that approximately 11% of the compound reaches the epidermis and dermis.3 There are limited data regarding the effects of the compound should it pass the stratum corneum into the living skin cells.
Products with DHA only confer a sun protection factor of approximately 34; although patients may appear tan, they have no actual decreased risk for sunburn after use. Reports have shown that the use of sunless tanners containing DHA can alter the appearance of melanocytic lesions clinically and has caused pseudochromhidrosis on the palms.3,5,6 A study performed on a human keratinocyte cell line, HaCaT, showed that DHA can induce DNA damage, cell-cycle block, and apoptosis.7 In addition, as described in our case, patients may experience prolonged hyperpigmentation after use.
This case demonstrates the potential for persistent hyperpigmentation months after the use of sunless tanners containing DHA. Asking patients specific questions regarding their history of tanning product use is essential in identifying the pathology. Although a skin biopsy may not be strictly indicated, it may aid diagnosis, especially when the history is unclear. As more dermatologists support the use of sunless tanner, we must be aware of this possible outcome, especially on more cosmetically sensitive areas such as the fingers in this patient. Clinicians should be aware that the US Food and Drug Administration recommends avoiding contact with mucous membranes when applying products containing DHA and also recommends use of a test spot prior to treating the entire body with the product.8 Patients must not only be educated on the benefits of using sunless tanners but on the potential side effects with use of these products as well.
- Garone M, Howard J, Fabrikant J. A review of common tanning methods. J Clin Aesthet Dermatol. 2015;8:43-47.
- Finot PA. Nonenzymatic browning products: physiologic effects and metabolic transit in relation to chemical structure. a review. Diabetes. 1982;31:22-28.
- Yourick JJ, Koenig ML, Yourick DL, et al. Fate of chemicals in skin after dermal application: does the in vitro skin reservoir affect the estimate of systemic absorption? Toxicol Appl Pharmacol. 2004;195:309-320.
- Nguyen B, Kochevar I. Influence of hydration on dihydroxyacetone-induced pigmentation of stratum corneum. J Invest Dermatol. 2003;120:655-661.
- Takita Y, Ichimiya M, Yamaguchi M, et al. A case of pseudochromhidrosis due to dihydroxyacetone. J Dermatol. 2006;33:230-231.
- Yoshida R, Kobayashi S, Amagai M, et al. Brown palm pseudochromhidrosis. Contact Dermatitis. 2002;46:237-238.
- Petersen AB, Wulf HC, Gniadecki R, et al. Dihydroxyacetone, the active browning ingredient in sunless tanning lotions, induces DNA damage, cell-cycle block and apoptosis in cultured HaCaT keratinocytes. Mutat Res. 2004;560:173-186.
- US Food and Drug Administration. Sunless tanners & bronzers. FDA website. http://www.fda.gov/Cosmetics/ProductsIngredients
/Products/ucm134064.htm. Updated March 6, 2018. Accessed April 23, 2020
To the Editor:
The use of sunless tanners has become an alternative for individuals who wish to have tan skin without exposure to UV radiation.1 We present a case of a patient who experienced persistent hyperpigmented patches on the hands months after the use of a sunless tanner containing dihydroxyacetone (DHA), a carbohydrate that reacts with amino acids in the stratum corneum to produce pigments called melanoidins. The hyperpigmentation caused by DHA is due to the Maillard reaction, which is the nonenzymatic glycation of amino groups of proteins by the carbonyl groups of sugar.2 Many sunless tanners contain DHA at varying concentrations. Dermatologists should be aware of the benefits and potential side effects of these alternative products so that they can appropriately counsel patients.
A 20-year-old woman with no history of skin disease presented for evaluation of hyperpigmented patches on the dorsal hands of several months’ duration. Physical examination revealed ill-defined hyperpigmented patches on the dorsal fingers without associated scale or erythema (Figure 1). She had a remote history of Hodgkin lymphoma treated with chemotherapy and was in remission for 5 years prior to the current presentation. Her hematologists referred her to dermatology for evaluation, as they did not believe the patches could be related to her chemotherapy given that she had completed the treatment years before.

A punch biopsy of one of the patches was obtained to elucidate the origin of the hyperpigmentation, which had no obvious triggers according to the patient. Histopathologic examination revealed hyperpigmented parakeratosis and lentiginous hyperplasia along with pigmentation of the stratum corneum (Figures 2A and 2B) with black pigment, which stained positive with Fontana-Masson (Figure 2C).

Upon further questioning, it was revealed that our patient had used a sunless tanner 3 months prior to the development of the pigmented patches. She also used urea cream to hasten exfoliation, which resulted in lighter but still apparent hyperpigmentation at follow-up 6 months after the initial presentation.
There has been a rapid growth of the sunless tanning industry in the last several years due to effective public education against UV tanning. Generally, patients apply the sunless tanner and notice an increase in tan within the following 48 hours. Typically, the tan progressively fades with the normal skin exfoliation over the span of weeks. Although most of the DHA binds proteins in the stratum corneum, the US Food and Drug Administration released a report speculating that approximately 11% of the compound reaches the epidermis and dermis.3 There are limited data regarding the effects of the compound should it pass the stratum corneum into the living skin cells.
Products with DHA only confer a sun protection factor of approximately 34; although patients may appear tan, they have no actual decreased risk for sunburn after use. Reports have shown that the use of sunless tanners containing DHA can alter the appearance of melanocytic lesions clinically and has caused pseudochromhidrosis on the palms.3,5,6 A study performed on a human keratinocyte cell line, HaCaT, showed that DHA can induce DNA damage, cell-cycle block, and apoptosis.7 In addition, as described in our case, patients may experience prolonged hyperpigmentation after use.
This case demonstrates the potential for persistent hyperpigmentation months after the use of sunless tanners containing DHA. Asking patients specific questions regarding their history of tanning product use is essential in identifying the pathology. Although a skin biopsy may not be strictly indicated, it may aid diagnosis, especially when the history is unclear. As more dermatologists support the use of sunless tanner, we must be aware of this possible outcome, especially on more cosmetically sensitive areas such as the fingers in this patient. Clinicians should be aware that the US Food and Drug Administration recommends avoiding contact with mucous membranes when applying products containing DHA and also recommends use of a test spot prior to treating the entire body with the product.8 Patients must not only be educated on the benefits of using sunless tanners but on the potential side effects with use of these products as well.
To the Editor:
The use of sunless tanners has become an alternative for individuals who wish to have tan skin without exposure to UV radiation.1 We present a case of a patient who experienced persistent hyperpigmented patches on the hands months after the use of a sunless tanner containing dihydroxyacetone (DHA), a carbohydrate that reacts with amino acids in the stratum corneum to produce pigments called melanoidins. The hyperpigmentation caused by DHA is due to the Maillard reaction, which is the nonenzymatic glycation of amino groups of proteins by the carbonyl groups of sugar.2 Many sunless tanners contain DHA at varying concentrations. Dermatologists should be aware of the benefits and potential side effects of these alternative products so that they can appropriately counsel patients.
A 20-year-old woman with no history of skin disease presented for evaluation of hyperpigmented patches on the dorsal hands of several months’ duration. Physical examination revealed ill-defined hyperpigmented patches on the dorsal fingers without associated scale or erythema (Figure 1). She had a remote history of Hodgkin lymphoma treated with chemotherapy and was in remission for 5 years prior to the current presentation. Her hematologists referred her to dermatology for evaluation, as they did not believe the patches could be related to her chemotherapy given that she had completed the treatment years before.

A punch biopsy of one of the patches was obtained to elucidate the origin of the hyperpigmentation, which had no obvious triggers according to the patient. Histopathologic examination revealed hyperpigmented parakeratosis and lentiginous hyperplasia along with pigmentation of the stratum corneum (Figures 2A and 2B) with black pigment, which stained positive with Fontana-Masson (Figure 2C).

Upon further questioning, it was revealed that our patient had used a sunless tanner 3 months prior to the development of the pigmented patches. She also used urea cream to hasten exfoliation, which resulted in lighter but still apparent hyperpigmentation at follow-up 6 months after the initial presentation.
There has been a rapid growth of the sunless tanning industry in the last several years due to effective public education against UV tanning. Generally, patients apply the sunless tanner and notice an increase in tan within the following 48 hours. Typically, the tan progressively fades with the normal skin exfoliation over the span of weeks. Although most of the DHA binds proteins in the stratum corneum, the US Food and Drug Administration released a report speculating that approximately 11% of the compound reaches the epidermis and dermis.3 There are limited data regarding the effects of the compound should it pass the stratum corneum into the living skin cells.
Products with DHA only confer a sun protection factor of approximately 34; although patients may appear tan, they have no actual decreased risk for sunburn after use. Reports have shown that the use of sunless tanners containing DHA can alter the appearance of melanocytic lesions clinically and has caused pseudochromhidrosis on the palms.3,5,6 A study performed on a human keratinocyte cell line, HaCaT, showed that DHA can induce DNA damage, cell-cycle block, and apoptosis.7 In addition, as described in our case, patients may experience prolonged hyperpigmentation after use.
This case demonstrates the potential for persistent hyperpigmentation months after the use of sunless tanners containing DHA. Asking patients specific questions regarding their history of tanning product use is essential in identifying the pathology. Although a skin biopsy may not be strictly indicated, it may aid diagnosis, especially when the history is unclear. As more dermatologists support the use of sunless tanner, we must be aware of this possible outcome, especially on more cosmetically sensitive areas such as the fingers in this patient. Clinicians should be aware that the US Food and Drug Administration recommends avoiding contact with mucous membranes when applying products containing DHA and also recommends use of a test spot prior to treating the entire body with the product.8 Patients must not only be educated on the benefits of using sunless tanners but on the potential side effects with use of these products as well.
- Garone M, Howard J, Fabrikant J. A review of common tanning methods. J Clin Aesthet Dermatol. 2015;8:43-47.
- Finot PA. Nonenzymatic browning products: physiologic effects and metabolic transit in relation to chemical structure. a review. Diabetes. 1982;31:22-28.
- Yourick JJ, Koenig ML, Yourick DL, et al. Fate of chemicals in skin after dermal application: does the in vitro skin reservoir affect the estimate of systemic absorption? Toxicol Appl Pharmacol. 2004;195:309-320.
- Nguyen B, Kochevar I. Influence of hydration on dihydroxyacetone-induced pigmentation of stratum corneum. J Invest Dermatol. 2003;120:655-661.
- Takita Y, Ichimiya M, Yamaguchi M, et al. A case of pseudochromhidrosis due to dihydroxyacetone. J Dermatol. 2006;33:230-231.
- Yoshida R, Kobayashi S, Amagai M, et al. Brown palm pseudochromhidrosis. Contact Dermatitis. 2002;46:237-238.
- Petersen AB, Wulf HC, Gniadecki R, et al. Dihydroxyacetone, the active browning ingredient in sunless tanning lotions, induces DNA damage, cell-cycle block and apoptosis in cultured HaCaT keratinocytes. Mutat Res. 2004;560:173-186.
- US Food and Drug Administration. Sunless tanners & bronzers. FDA website. http://www.fda.gov/Cosmetics/ProductsIngredients
/Products/ucm134064.htm. Updated March 6, 2018. Accessed April 23, 2020
- Garone M, Howard J, Fabrikant J. A review of common tanning methods. J Clin Aesthet Dermatol. 2015;8:43-47.
- Finot PA. Nonenzymatic browning products: physiologic effects and metabolic transit in relation to chemical structure. a review. Diabetes. 1982;31:22-28.
- Yourick JJ, Koenig ML, Yourick DL, et al. Fate of chemicals in skin after dermal application: does the in vitro skin reservoir affect the estimate of systemic absorption? Toxicol Appl Pharmacol. 2004;195:309-320.
- Nguyen B, Kochevar I. Influence of hydration on dihydroxyacetone-induced pigmentation of stratum corneum. J Invest Dermatol. 2003;120:655-661.
- Takita Y, Ichimiya M, Yamaguchi M, et al. A case of pseudochromhidrosis due to dihydroxyacetone. J Dermatol. 2006;33:230-231.
- Yoshida R, Kobayashi S, Amagai M, et al. Brown palm pseudochromhidrosis. Contact Dermatitis. 2002;46:237-238.
- Petersen AB, Wulf HC, Gniadecki R, et al. Dihydroxyacetone, the active browning ingredient in sunless tanning lotions, induces DNA damage, cell-cycle block and apoptosis in cultured HaCaT keratinocytes. Mutat Res. 2004;560:173-186.
- US Food and Drug Administration. Sunless tanners & bronzers. FDA website. http://www.fda.gov/Cosmetics/ProductsIngredients
/Products/ucm134064.htm. Updated March 6, 2018. Accessed April 23, 2020
Practice Points
- Patient education on the benefits and risks associated with sunless tanners is critical when using these products.
- Sunless tanners containing dihydroxyacetone potentially can lead to persistent hyperpigmented patches on areas of contact.
- Skin biopsy showing hyperpigmented parakeratosis along with pigmentation of the stratum corneum can aid in diagnosis.
Vitiligo: To Biopsy or Not To Biopsy?
The histopathologic diagnosis of vitiligo is classically understood as the absence of melanocytes and melanin in the skin biopsy.1 It is difficult for a pathologist to establish the absolute absence of melanocytes and melanin in a skin biopsy. Therefore, we need to take into consideration many variables when we face the possibility to biopsy a vitiligo lesion.
The basis of the clinical diagnosis of vitiligo is the appearance of achromic lesions in periorificial and acral areas; however, sometimes it is difficult to differentiate between an achromic or hypochromic lesion. Although Wood light is of great help in these circumstances, it still can be difficult to make the diagnosis with certainty.
In other cases, the lesions do not present a classic distribution of vitiligo, and other differential diagnoses are considered. For example, if we see a single hypochromic or achromic lesion in a young child, then the main differential diagnosis would be achromic nevus. If there are multiple lesions, then we may consider progressive macular hypomelanosis, postinflammatory hypopigmentation, and hypopigmented mycosis fungoides. In genital lesions, the differential diagnosis between initial lichen sclerosus and vitiligo also can be considered. Finally, we must always bear in mind that both sarcoidosis and Hansen disease can appear as achromic or hypochromic lesions.
The histologic diagnosis of vitiligo in a completely constituted lesion implies the total loss of melanocytes and melanin in the epidermis. Additional histologic findings are described at the edge of the advanced border, such as the presence of melanocytes that have increased in size with large dendrites and lymphoid infiltrate. In perilesional skin, vacuolated keratinocytes and Langerhans cells have increased in number and repositioned in the basal layer, with visible degeneration of nerves and sweat glands. Lymphocytes also can be found in contact with the melanocytes.2 It is important to note that in addition to these histologic findings, it is common to find spongiosis, mononuclear superficial perivascular inflammatory infiltrate, and melanophages in biopsies of vitiligo.3
Given that ensuring the absence of melanocytes is central to diagnosis and melanocytes can be difficult to identify or differentiate from repositioned Langerhans cells in the basal layer with hematoxylin and eosin stain, immunohistochemical techniques must be performed every time we are dealing with vitiligo biopsies. Although there are no studies comparing the diagnostic value of the different immunohistochemical techniques in vitiligo, dihydroxyphenylalanine (DOPA) seems to be a good option, as it will only mark active melanocytes. Human melanoma black 45 (HMB-45), anti-TYRP1 (Mel-5), and antimelanoma gp 100 antibody (NKI/beteb) also have been used. Some authors recommend the use of pan melanoma because it includes 3 markers—HMB-45, tyrosinase, and Mart-1. Currently, SRY-related HMG-box10 (SOX10) seems to be a good option, as it is a nuclear marker that makes it easier to differentiate melanocytes from pigmented keratinocytes.4
Establishing a complete absence of melanocytes in the lesions or finding there are melanocytes but they are inactivated is key to evaluating the pathogenesis of vitiligo and directly affects the histologic diagnosis and eventually even the treatment. Le Poole et al5 used a panel of 17 monoclonal antibodies and a polyclonal antibody in lesions of 12 patients with vitiligo without identifying the presence of melanocytes. They concluded that there are no melanocytes in lesions of vitiligo.5
In a subsequent study with a larger number of patients, Kim et al2 found melanocytes that marked with NKI/beteb and Mart-1 in 12 of 100 patients with vitiligo. They also showed melanocytes by electron microscopy in lesional skin of 1 of 3 patients with vitiligo.2 Tobin et al6 managed to grow melanocytes from skin with vitiligo and confirmed the presence of melanin in basal keratinocytes of lesions of stable vitiligo. From this evidence we can conclude that the absence of melanocytes and melanin in the epidermis confirms the diagnosis of vitiligo; however, the opposite is not true—that is, the presence of melanocytes or melanin in a skin biopsy does not rule out the diagnosis of vitiligo.
Taking this information into consideration, we can understand that if our differential diagnosis is a dermatosis that requires the evaluation of the number of melanocytes as a fundamental diagnostic clue (eg, postinflammatory hypopigmentation), the biopsy will probably not be useful. On the other hand, when our differential diagnosis has characteristic diagnostic findings independent of the number of melanocytes or the presence of melanin, the biopsy will be useful (eg, hypopigmented mycosis fungoides).
Thus, we can understand why the histologic differentiation between vitiligo,
In all the differentials named, the solution to the diagnostic doubt is not based on the histologic findings but on the clinical evolution of the patients. In cases of vitiligo, the lesions will become more evident in the evolution. They will eventually disappear in pityriasis alba, postinflammatory hypopigmentation, and progressive macular hypopigmentation and will remain unchanged in nevus depigmentosus. It is important, especially when we are dealing with concerned parents/guardians, to convey the importance of assessing the evolution of the disease as the main diagnostic procedure. Even though a biopsy is minimally invasive, it is usually stressful on children, it may leave sequelae, and above all it will not contribute to the diagnosis in this clinical context.
There are other clinical circumstances in the scenario of hypochromic or achromic lesions in which the biopsy will be useful: If we consider an initial genital lichen sclerosus vs vitiligo. In lichen sclerosus the biopsy will show dermal hyalinosis and interphase changes; absence of both will support vitiligo. If we need to differentiate hypopigmented mycosis fungoides from vitiligo, we will find an infiltrate of pleomorphic lymphocytes in the epidermis and dermis in the former and an absence of these findings in vitiligo. Finally, if we find granulomas in a biopsy of an achromic or hypopigmented lesion, we may be dealing with hypopigmented sarcoidosis or Hansen disease.
It also is important to choose the best site to perform the biopsy to have the best chance at diagnosing vitiligo histologically. As already described, in the edges and in the perilesional skin we can find remnant melanocytes, Langerhans cells, and interphase changes that do not allow us to clearly evaluate the main change that is the loss of melanocytes and melanin. In fact, a biopsy of the edge of a vitiligo macula can lead to confusion. For example, if the differential diagnosis is lichen sclerosus and the image we see in the biopsy of the edge of a vitiligo lesion is an interface reaction, we can interpret it as a finding that favors lichen sclerosus. In this way, it is better to biopsy the center of a well-constituted vitiligo lesion where we have the best chance to assess the absence of melanin and melanocytes.
The vitiligo differential diagnosis can be divided into 2 groups: entities that are difficult to differentiate from vitiligo histologically (ie, pityriasis alba, postinflammatory hypopigmentation, progressive macular hypopigmentation, nevus depigmentosus) and entities that are easily distinguishable from vitiligo histologically (ie, lichen sclerosus, mycosis fungoides, sarcoidosis, leprosy). If our differential diagnosis was found in the first group, the final diagnosis should be based on the evolution of the patient. If it was in the second group, a biopsy of the center of the lesion will be useful and may allow us to reach a definitive diagnosis.
- Weedon D. Weedon´s Skin Pathology. 3rd edition. Churchill Livingston. 2009.
- Kim YC, Kim YJ, Kang HY, et al. Histopathologic features in vitiligo. Am J Dermatopathol. 2008;30:112-116.
- Yadav AK, Singh P, Khunger N. Clinicopathologic analysis of stable and unstable vitiligo: a study of 66 cases. Am J Dermatopathol. 2016;38:608-613.
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part i. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 201165:473-491.
- Le Poole IC, van der Wijngaard RM, Westerhof W, et al. Presence or absence of melanocytes in vitiligo lesions: an immunohistochemical investigation. J Invest Dermatol. 1993;100:816-822.
- Tobin DJ, Swanson NN, Pittelkow MR, et al. Melanocytes are not absent in lesional skin of long duration vitiligo. J Pathol. 2000;191:407-416.
- Vargas-Ocampo F. Pityriasis alba: a histologic study. Int J Dermatol. 1993:32:870-873.
- Xu AE, Huang B, Li YW, et al. Clinical, histopathological and ultrastructural characteristics of naevus depigmentosus. Clin Exp Dermatol. 2008;33:400-405.
The histopathologic diagnosis of vitiligo is classically understood as the absence of melanocytes and melanin in the skin biopsy.1 It is difficult for a pathologist to establish the absolute absence of melanocytes and melanin in a skin biopsy. Therefore, we need to take into consideration many variables when we face the possibility to biopsy a vitiligo lesion.
The basis of the clinical diagnosis of vitiligo is the appearance of achromic lesions in periorificial and acral areas; however, sometimes it is difficult to differentiate between an achromic or hypochromic lesion. Although Wood light is of great help in these circumstances, it still can be difficult to make the diagnosis with certainty.
In other cases, the lesions do not present a classic distribution of vitiligo, and other differential diagnoses are considered. For example, if we see a single hypochromic or achromic lesion in a young child, then the main differential diagnosis would be achromic nevus. If there are multiple lesions, then we may consider progressive macular hypomelanosis, postinflammatory hypopigmentation, and hypopigmented mycosis fungoides. In genital lesions, the differential diagnosis between initial lichen sclerosus and vitiligo also can be considered. Finally, we must always bear in mind that both sarcoidosis and Hansen disease can appear as achromic or hypochromic lesions.
The histologic diagnosis of vitiligo in a completely constituted lesion implies the total loss of melanocytes and melanin in the epidermis. Additional histologic findings are described at the edge of the advanced border, such as the presence of melanocytes that have increased in size with large dendrites and lymphoid infiltrate. In perilesional skin, vacuolated keratinocytes and Langerhans cells have increased in number and repositioned in the basal layer, with visible degeneration of nerves and sweat glands. Lymphocytes also can be found in contact with the melanocytes.2 It is important to note that in addition to these histologic findings, it is common to find spongiosis, mononuclear superficial perivascular inflammatory infiltrate, and melanophages in biopsies of vitiligo.3
Given that ensuring the absence of melanocytes is central to diagnosis and melanocytes can be difficult to identify or differentiate from repositioned Langerhans cells in the basal layer with hematoxylin and eosin stain, immunohistochemical techniques must be performed every time we are dealing with vitiligo biopsies. Although there are no studies comparing the diagnostic value of the different immunohistochemical techniques in vitiligo, dihydroxyphenylalanine (DOPA) seems to be a good option, as it will only mark active melanocytes. Human melanoma black 45 (HMB-45), anti-TYRP1 (Mel-5), and antimelanoma gp 100 antibody (NKI/beteb) also have been used. Some authors recommend the use of pan melanoma because it includes 3 markers—HMB-45, tyrosinase, and Mart-1. Currently, SRY-related HMG-box10 (SOX10) seems to be a good option, as it is a nuclear marker that makes it easier to differentiate melanocytes from pigmented keratinocytes.4
Establishing a complete absence of melanocytes in the lesions or finding there are melanocytes but they are inactivated is key to evaluating the pathogenesis of vitiligo and directly affects the histologic diagnosis and eventually even the treatment. Le Poole et al5 used a panel of 17 monoclonal antibodies and a polyclonal antibody in lesions of 12 patients with vitiligo without identifying the presence of melanocytes. They concluded that there are no melanocytes in lesions of vitiligo.5
In a subsequent study with a larger number of patients, Kim et al2 found melanocytes that marked with NKI/beteb and Mart-1 in 12 of 100 patients with vitiligo. They also showed melanocytes by electron microscopy in lesional skin of 1 of 3 patients with vitiligo.2 Tobin et al6 managed to grow melanocytes from skin with vitiligo and confirmed the presence of melanin in basal keratinocytes of lesions of stable vitiligo. From this evidence we can conclude that the absence of melanocytes and melanin in the epidermis confirms the diagnosis of vitiligo; however, the opposite is not true—that is, the presence of melanocytes or melanin in a skin biopsy does not rule out the diagnosis of vitiligo.
Taking this information into consideration, we can understand that if our differential diagnosis is a dermatosis that requires the evaluation of the number of melanocytes as a fundamental diagnostic clue (eg, postinflammatory hypopigmentation), the biopsy will probably not be useful. On the other hand, when our differential diagnosis has characteristic diagnostic findings independent of the number of melanocytes or the presence of melanin, the biopsy will be useful (eg, hypopigmented mycosis fungoides).
Thus, we can understand why the histologic differentiation between vitiligo,
In all the differentials named, the solution to the diagnostic doubt is not based on the histologic findings but on the clinical evolution of the patients. In cases of vitiligo, the lesions will become more evident in the evolution. They will eventually disappear in pityriasis alba, postinflammatory hypopigmentation, and progressive macular hypopigmentation and will remain unchanged in nevus depigmentosus. It is important, especially when we are dealing with concerned parents/guardians, to convey the importance of assessing the evolution of the disease as the main diagnostic procedure. Even though a biopsy is minimally invasive, it is usually stressful on children, it may leave sequelae, and above all it will not contribute to the diagnosis in this clinical context.
There are other clinical circumstances in the scenario of hypochromic or achromic lesions in which the biopsy will be useful: If we consider an initial genital lichen sclerosus vs vitiligo. In lichen sclerosus the biopsy will show dermal hyalinosis and interphase changes; absence of both will support vitiligo. If we need to differentiate hypopigmented mycosis fungoides from vitiligo, we will find an infiltrate of pleomorphic lymphocytes in the epidermis and dermis in the former and an absence of these findings in vitiligo. Finally, if we find granulomas in a biopsy of an achromic or hypopigmented lesion, we may be dealing with hypopigmented sarcoidosis or Hansen disease.
It also is important to choose the best site to perform the biopsy to have the best chance at diagnosing vitiligo histologically. As already described, in the edges and in the perilesional skin we can find remnant melanocytes, Langerhans cells, and interphase changes that do not allow us to clearly evaluate the main change that is the loss of melanocytes and melanin. In fact, a biopsy of the edge of a vitiligo macula can lead to confusion. For example, if the differential diagnosis is lichen sclerosus and the image we see in the biopsy of the edge of a vitiligo lesion is an interface reaction, we can interpret it as a finding that favors lichen sclerosus. In this way, it is better to biopsy the center of a well-constituted vitiligo lesion where we have the best chance to assess the absence of melanin and melanocytes.
The vitiligo differential diagnosis can be divided into 2 groups: entities that are difficult to differentiate from vitiligo histologically (ie, pityriasis alba, postinflammatory hypopigmentation, progressive macular hypopigmentation, nevus depigmentosus) and entities that are easily distinguishable from vitiligo histologically (ie, lichen sclerosus, mycosis fungoides, sarcoidosis, leprosy). If our differential diagnosis was found in the first group, the final diagnosis should be based on the evolution of the patient. If it was in the second group, a biopsy of the center of the lesion will be useful and may allow us to reach a definitive diagnosis.
The histopathologic diagnosis of vitiligo is classically understood as the absence of melanocytes and melanin in the skin biopsy.1 It is difficult for a pathologist to establish the absolute absence of melanocytes and melanin in a skin biopsy. Therefore, we need to take into consideration many variables when we face the possibility to biopsy a vitiligo lesion.
The basis of the clinical diagnosis of vitiligo is the appearance of achromic lesions in periorificial and acral areas; however, sometimes it is difficult to differentiate between an achromic or hypochromic lesion. Although Wood light is of great help in these circumstances, it still can be difficult to make the diagnosis with certainty.
In other cases, the lesions do not present a classic distribution of vitiligo, and other differential diagnoses are considered. For example, if we see a single hypochromic or achromic lesion in a young child, then the main differential diagnosis would be achromic nevus. If there are multiple lesions, then we may consider progressive macular hypomelanosis, postinflammatory hypopigmentation, and hypopigmented mycosis fungoides. In genital lesions, the differential diagnosis between initial lichen sclerosus and vitiligo also can be considered. Finally, we must always bear in mind that both sarcoidosis and Hansen disease can appear as achromic or hypochromic lesions.
The histologic diagnosis of vitiligo in a completely constituted lesion implies the total loss of melanocytes and melanin in the epidermis. Additional histologic findings are described at the edge of the advanced border, such as the presence of melanocytes that have increased in size with large dendrites and lymphoid infiltrate. In perilesional skin, vacuolated keratinocytes and Langerhans cells have increased in number and repositioned in the basal layer, with visible degeneration of nerves and sweat glands. Lymphocytes also can be found in contact with the melanocytes.2 It is important to note that in addition to these histologic findings, it is common to find spongiosis, mononuclear superficial perivascular inflammatory infiltrate, and melanophages in biopsies of vitiligo.3
Given that ensuring the absence of melanocytes is central to diagnosis and melanocytes can be difficult to identify or differentiate from repositioned Langerhans cells in the basal layer with hematoxylin and eosin stain, immunohistochemical techniques must be performed every time we are dealing with vitiligo biopsies. Although there are no studies comparing the diagnostic value of the different immunohistochemical techniques in vitiligo, dihydroxyphenylalanine (DOPA) seems to be a good option, as it will only mark active melanocytes. Human melanoma black 45 (HMB-45), anti-TYRP1 (Mel-5), and antimelanoma gp 100 antibody (NKI/beteb) also have been used. Some authors recommend the use of pan melanoma because it includes 3 markers—HMB-45, tyrosinase, and Mart-1. Currently, SRY-related HMG-box10 (SOX10) seems to be a good option, as it is a nuclear marker that makes it easier to differentiate melanocytes from pigmented keratinocytes.4
Establishing a complete absence of melanocytes in the lesions or finding there are melanocytes but they are inactivated is key to evaluating the pathogenesis of vitiligo and directly affects the histologic diagnosis and eventually even the treatment. Le Poole et al5 used a panel of 17 monoclonal antibodies and a polyclonal antibody in lesions of 12 patients with vitiligo without identifying the presence of melanocytes. They concluded that there are no melanocytes in lesions of vitiligo.5
In a subsequent study with a larger number of patients, Kim et al2 found melanocytes that marked with NKI/beteb and Mart-1 in 12 of 100 patients with vitiligo. They also showed melanocytes by electron microscopy in lesional skin of 1 of 3 patients with vitiligo.2 Tobin et al6 managed to grow melanocytes from skin with vitiligo and confirmed the presence of melanin in basal keratinocytes of lesions of stable vitiligo. From this evidence we can conclude that the absence of melanocytes and melanin in the epidermis confirms the diagnosis of vitiligo; however, the opposite is not true—that is, the presence of melanocytes or melanin in a skin biopsy does not rule out the diagnosis of vitiligo.
Taking this information into consideration, we can understand that if our differential diagnosis is a dermatosis that requires the evaluation of the number of melanocytes as a fundamental diagnostic clue (eg, postinflammatory hypopigmentation), the biopsy will probably not be useful. On the other hand, when our differential diagnosis has characteristic diagnostic findings independent of the number of melanocytes or the presence of melanin, the biopsy will be useful (eg, hypopigmented mycosis fungoides).
Thus, we can understand why the histologic differentiation between vitiligo,
In all the differentials named, the solution to the diagnostic doubt is not based on the histologic findings but on the clinical evolution of the patients. In cases of vitiligo, the lesions will become more evident in the evolution. They will eventually disappear in pityriasis alba, postinflammatory hypopigmentation, and progressive macular hypopigmentation and will remain unchanged in nevus depigmentosus. It is important, especially when we are dealing with concerned parents/guardians, to convey the importance of assessing the evolution of the disease as the main diagnostic procedure. Even though a biopsy is minimally invasive, it is usually stressful on children, it may leave sequelae, and above all it will not contribute to the diagnosis in this clinical context.
There are other clinical circumstances in the scenario of hypochromic or achromic lesions in which the biopsy will be useful: If we consider an initial genital lichen sclerosus vs vitiligo. In lichen sclerosus the biopsy will show dermal hyalinosis and interphase changes; absence of both will support vitiligo. If we need to differentiate hypopigmented mycosis fungoides from vitiligo, we will find an infiltrate of pleomorphic lymphocytes in the epidermis and dermis in the former and an absence of these findings in vitiligo. Finally, if we find granulomas in a biopsy of an achromic or hypopigmented lesion, we may be dealing with hypopigmented sarcoidosis or Hansen disease.
It also is important to choose the best site to perform the biopsy to have the best chance at diagnosing vitiligo histologically. As already described, in the edges and in the perilesional skin we can find remnant melanocytes, Langerhans cells, and interphase changes that do not allow us to clearly evaluate the main change that is the loss of melanocytes and melanin. In fact, a biopsy of the edge of a vitiligo macula can lead to confusion. For example, if the differential diagnosis is lichen sclerosus and the image we see in the biopsy of the edge of a vitiligo lesion is an interface reaction, we can interpret it as a finding that favors lichen sclerosus. In this way, it is better to biopsy the center of a well-constituted vitiligo lesion where we have the best chance to assess the absence of melanin and melanocytes.
The vitiligo differential diagnosis can be divided into 2 groups: entities that are difficult to differentiate from vitiligo histologically (ie, pityriasis alba, postinflammatory hypopigmentation, progressive macular hypopigmentation, nevus depigmentosus) and entities that are easily distinguishable from vitiligo histologically (ie, lichen sclerosus, mycosis fungoides, sarcoidosis, leprosy). If our differential diagnosis was found in the first group, the final diagnosis should be based on the evolution of the patient. If it was in the second group, a biopsy of the center of the lesion will be useful and may allow us to reach a definitive diagnosis.
- Weedon D. Weedon´s Skin Pathology. 3rd edition. Churchill Livingston. 2009.
- Kim YC, Kim YJ, Kang HY, et al. Histopathologic features in vitiligo. Am J Dermatopathol. 2008;30:112-116.
- Yadav AK, Singh P, Khunger N. Clinicopathologic analysis of stable and unstable vitiligo: a study of 66 cases. Am J Dermatopathol. 2016;38:608-613.
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part i. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 201165:473-491.
- Le Poole IC, van der Wijngaard RM, Westerhof W, et al. Presence or absence of melanocytes in vitiligo lesions: an immunohistochemical investigation. J Invest Dermatol. 1993;100:816-822.
- Tobin DJ, Swanson NN, Pittelkow MR, et al. Melanocytes are not absent in lesional skin of long duration vitiligo. J Pathol. 2000;191:407-416.
- Vargas-Ocampo F. Pityriasis alba: a histologic study. Int J Dermatol. 1993:32:870-873.
- Xu AE, Huang B, Li YW, et al. Clinical, histopathological and ultrastructural characteristics of naevus depigmentosus. Clin Exp Dermatol. 2008;33:400-405.
- Weedon D. Weedon´s Skin Pathology. 3rd edition. Churchill Livingston. 2009.
- Kim YC, Kim YJ, Kang HY, et al. Histopathologic features in vitiligo. Am J Dermatopathol. 2008;30:112-116.
- Yadav AK, Singh P, Khunger N. Clinicopathologic analysis of stable and unstable vitiligo: a study of 66 cases. Am J Dermatopathol. 2016;38:608-613.
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part i. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 201165:473-491.
- Le Poole IC, van der Wijngaard RM, Westerhof W, et al. Presence or absence of melanocytes in vitiligo lesions: an immunohistochemical investigation. J Invest Dermatol. 1993;100:816-822.
- Tobin DJ, Swanson NN, Pittelkow MR, et al. Melanocytes are not absent in lesional skin of long duration vitiligo. J Pathol. 2000;191:407-416.
- Vargas-Ocampo F. Pityriasis alba: a histologic study. Int J Dermatol. 1993:32:870-873.
- Xu AE, Huang B, Li YW, et al. Clinical, histopathological and ultrastructural characteristics of naevus depigmentosus. Clin Exp Dermatol. 2008;33:400-405.
Oral propranolol shown safe in PHACE
LAHAINA, HAWAII – Reassuring evidence of the safety of oral propranolol for treatment of complicated infantile hemangiomas in patients with PHACE syndrome comes from a recent multicenter study.
Oral propranolol is now well-ensconced as first-line therapy for complicated infantile hemangiomas in otherwise healthy children. However, the beta-blocker’s use in PHACE (Posterior fossa malformations, Hemangiomas, Arterial anomalies, Cardiac defects, and Eye abnormalities) syndrome has been controversial, with concerns raised by some that it might raise the risk for arterial ischemic stroke. Not so, Moise L. Levy, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“I’m not suggesting you use propranolol with reckless abandon in this population, but this stroke concern is something that should be put to bed based on this study,” advised Dr. Levy, professor of dermatology and pediatrics at Dell Medical School in Austin, Tex., and physician-in-chief at Dell Children’s Medical Center.
PHACE syndrome is characterized by large, thick, plaque-like hemangiomas greater than 5 cm in size, most commonly on the face, although they can be located elsewhere.
“There was concern that if you found severely altered cerebrovascular arterial flow and you put a kid on a beta-blocker you might be causing some harm. But what I will tell you is that in this recently published paper this was not in fact an issue,” he said.
Dr. Levy was not an investigator in the multicenter retrospective study, which included 76 patients with PHACE syndrome treated for infantile hemangioma with oral propranolol at 0.3 mg/kg per dose or more at 11 academic tertiary care pediatric dermatology clinics. Treatment started at a median age of 56 days.
There were no strokes, TIAs, cardiovascular events, or other significant problems associated with treatment. Twenty-nine children experienced mild adverse events: minor gastrointestinal or respiratory symptoms, and sleep disturbances were threefold more frequent than reported with placebo in another study. The investigators noted that the safety experience in their PHACE syndrome population compared favorably with that in 726 infants without PHACE syndrome who received oral propranolol for hemangiomas, where the incidence of serious adverse events on treatment was 0.4% (JAMA Dermatol. 2019 Dec 11. doi: 10.1001/jamadermatol.2019.3839).
‘Hemangiomas – but we were taught that they go away’
Dr. Levy gave a shout-out to the American Academy of Pediatrics for publishing interdisciplinary expert consensus-based practice guidelines for the management of infantile hemangiomas, which he praised as “quite well done” (Pediatrics. 2019 Jan;143[1]. pii: e20183475. doi: 10.1542/peds.2018-3475).
Following release of the guidelines last year, he and other pediatric vascular anomalies experts saw an uptick in referrals from general pediatricians, which has since tapered off.
“It’s probably like for all of us: We read an article, it’s fresh on the mind, then you forget about the article and what you’ve read. So we need a little reinforcement from a learning perspective. This is a great article,” he said.
The guidelines debunk as myth the classic teaching that infantile hemangiomas go away. Explicit information is provided about the high-risk anatomic sites warranting consideration for early referral, including the periocular, lumbosacral, and perineal areas, the lip, and lower face.
“The major point is early identification of those lesions requiring evaluation and intervention. Hemangiomas generally speaking are at their ultimate size by 3-5 months of age. The bottom line is if you think something needs to be done, please send that patient, or act upon that patient, sooner rather than later. I can’t tell you how many cases of hemangiomas I’ve seen when the kid is 18 months of age, 3 years of age, 5 years, with a large area of redundant skin, scarring, or something of that sort, and it would have been really nice to have seen them earlier and acted upon them then,” the pediatric dermatologist said.
The guidelines recommend intervention or referral by 1 month of age, ideally. Guidance is provided about the use of oral propranolol as first-line therapy.
“Propranolol is something that has been a real game changer for us,” he noted. “Many people continue to be worried about side effects in using this, particularly in the young childhood population, but this paper shows pretty clearly that hypotension or bradycardia is not a real concern. I never hospitalize these patients for propranolol therapy except in high-risk populations: very preemie, any history of breathing problems. We check the blood pressure and heart rate at baseline, again at 7-10 days, and at every visit. We’ve never found any significant drop in blood pressure.”
Dr. Levy reported financial relationships with half a dozen pharmaceutical companies, none relevant to his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Reassuring evidence of the safety of oral propranolol for treatment of complicated infantile hemangiomas in patients with PHACE syndrome comes from a recent multicenter study.
Oral propranolol is now well-ensconced as first-line therapy for complicated infantile hemangiomas in otherwise healthy children. However, the beta-blocker’s use in PHACE (Posterior fossa malformations, Hemangiomas, Arterial anomalies, Cardiac defects, and Eye abnormalities) syndrome has been controversial, with concerns raised by some that it might raise the risk for arterial ischemic stroke. Not so, Moise L. Levy, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“I’m not suggesting you use propranolol with reckless abandon in this population, but this stroke concern is something that should be put to bed based on this study,” advised Dr. Levy, professor of dermatology and pediatrics at Dell Medical School in Austin, Tex., and physician-in-chief at Dell Children’s Medical Center.
PHACE syndrome is characterized by large, thick, plaque-like hemangiomas greater than 5 cm in size, most commonly on the face, although they can be located elsewhere.
“There was concern that if you found severely altered cerebrovascular arterial flow and you put a kid on a beta-blocker you might be causing some harm. But what I will tell you is that in this recently published paper this was not in fact an issue,” he said.
Dr. Levy was not an investigator in the multicenter retrospective study, which included 76 patients with PHACE syndrome treated for infantile hemangioma with oral propranolol at 0.3 mg/kg per dose or more at 11 academic tertiary care pediatric dermatology clinics. Treatment started at a median age of 56 days.
There were no strokes, TIAs, cardiovascular events, or other significant problems associated with treatment. Twenty-nine children experienced mild adverse events: minor gastrointestinal or respiratory symptoms, and sleep disturbances were threefold more frequent than reported with placebo in another study. The investigators noted that the safety experience in their PHACE syndrome population compared favorably with that in 726 infants without PHACE syndrome who received oral propranolol for hemangiomas, where the incidence of serious adverse events on treatment was 0.4% (JAMA Dermatol. 2019 Dec 11. doi: 10.1001/jamadermatol.2019.3839).
‘Hemangiomas – but we were taught that they go away’
Dr. Levy gave a shout-out to the American Academy of Pediatrics for publishing interdisciplinary expert consensus-based practice guidelines for the management of infantile hemangiomas, which he praised as “quite well done” (Pediatrics. 2019 Jan;143[1]. pii: e20183475. doi: 10.1542/peds.2018-3475).
Following release of the guidelines last year, he and other pediatric vascular anomalies experts saw an uptick in referrals from general pediatricians, which has since tapered off.
“It’s probably like for all of us: We read an article, it’s fresh on the mind, then you forget about the article and what you’ve read. So we need a little reinforcement from a learning perspective. This is a great article,” he said.
The guidelines debunk as myth the classic teaching that infantile hemangiomas go away. Explicit information is provided about the high-risk anatomic sites warranting consideration for early referral, including the periocular, lumbosacral, and perineal areas, the lip, and lower face.
“The major point is early identification of those lesions requiring evaluation and intervention. Hemangiomas generally speaking are at their ultimate size by 3-5 months of age. The bottom line is if you think something needs to be done, please send that patient, or act upon that patient, sooner rather than later. I can’t tell you how many cases of hemangiomas I’ve seen when the kid is 18 months of age, 3 years of age, 5 years, with a large area of redundant skin, scarring, or something of that sort, and it would have been really nice to have seen them earlier and acted upon them then,” the pediatric dermatologist said.
The guidelines recommend intervention or referral by 1 month of age, ideally. Guidance is provided about the use of oral propranolol as first-line therapy.
“Propranolol is something that has been a real game changer for us,” he noted. “Many people continue to be worried about side effects in using this, particularly in the young childhood population, but this paper shows pretty clearly that hypotension or bradycardia is not a real concern. I never hospitalize these patients for propranolol therapy except in high-risk populations: very preemie, any history of breathing problems. We check the blood pressure and heart rate at baseline, again at 7-10 days, and at every visit. We’ve never found any significant drop in blood pressure.”
Dr. Levy reported financial relationships with half a dozen pharmaceutical companies, none relevant to his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Reassuring evidence of the safety of oral propranolol for treatment of complicated infantile hemangiomas in patients with PHACE syndrome comes from a recent multicenter study.
Oral propranolol is now well-ensconced as first-line therapy for complicated infantile hemangiomas in otherwise healthy children. However, the beta-blocker’s use in PHACE (Posterior fossa malformations, Hemangiomas, Arterial anomalies, Cardiac defects, and Eye abnormalities) syndrome has been controversial, with concerns raised by some that it might raise the risk for arterial ischemic stroke. Not so, Moise L. Levy, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“I’m not suggesting you use propranolol with reckless abandon in this population, but this stroke concern is something that should be put to bed based on this study,” advised Dr. Levy, professor of dermatology and pediatrics at Dell Medical School in Austin, Tex., and physician-in-chief at Dell Children’s Medical Center.
PHACE syndrome is characterized by large, thick, plaque-like hemangiomas greater than 5 cm in size, most commonly on the face, although they can be located elsewhere.
“There was concern that if you found severely altered cerebrovascular arterial flow and you put a kid on a beta-blocker you might be causing some harm. But what I will tell you is that in this recently published paper this was not in fact an issue,” he said.
Dr. Levy was not an investigator in the multicenter retrospective study, which included 76 patients with PHACE syndrome treated for infantile hemangioma with oral propranolol at 0.3 mg/kg per dose or more at 11 academic tertiary care pediatric dermatology clinics. Treatment started at a median age of 56 days.
There were no strokes, TIAs, cardiovascular events, or other significant problems associated with treatment. Twenty-nine children experienced mild adverse events: minor gastrointestinal or respiratory symptoms, and sleep disturbances were threefold more frequent than reported with placebo in another study. The investigators noted that the safety experience in their PHACE syndrome population compared favorably with that in 726 infants without PHACE syndrome who received oral propranolol for hemangiomas, where the incidence of serious adverse events on treatment was 0.4% (JAMA Dermatol. 2019 Dec 11. doi: 10.1001/jamadermatol.2019.3839).
‘Hemangiomas – but we were taught that they go away’
Dr. Levy gave a shout-out to the American Academy of Pediatrics for publishing interdisciplinary expert consensus-based practice guidelines for the management of infantile hemangiomas, which he praised as “quite well done” (Pediatrics. 2019 Jan;143[1]. pii: e20183475. doi: 10.1542/peds.2018-3475).
Following release of the guidelines last year, he and other pediatric vascular anomalies experts saw an uptick in referrals from general pediatricians, which has since tapered off.
“It’s probably like for all of us: We read an article, it’s fresh on the mind, then you forget about the article and what you’ve read. So we need a little reinforcement from a learning perspective. This is a great article,” he said.
The guidelines debunk as myth the classic teaching that infantile hemangiomas go away. Explicit information is provided about the high-risk anatomic sites warranting consideration for early referral, including the periocular, lumbosacral, and perineal areas, the lip, and lower face.
“The major point is early identification of those lesions requiring evaluation and intervention. Hemangiomas generally speaking are at their ultimate size by 3-5 months of age. The bottom line is if you think something needs to be done, please send that patient, or act upon that patient, sooner rather than later. I can’t tell you how many cases of hemangiomas I’ve seen when the kid is 18 months of age, 3 years of age, 5 years, with a large area of redundant skin, scarring, or something of that sort, and it would have been really nice to have seen them earlier and acted upon them then,” the pediatric dermatologist said.
The guidelines recommend intervention or referral by 1 month of age, ideally. Guidance is provided about the use of oral propranolol as first-line therapy.
“Propranolol is something that has been a real game changer for us,” he noted. “Many people continue to be worried about side effects in using this, particularly in the young childhood population, but this paper shows pretty clearly that hypotension or bradycardia is not a real concern. I never hospitalize these patients for propranolol therapy except in high-risk populations: very preemie, any history of breathing problems. We check the blood pressure and heart rate at baseline, again at 7-10 days, and at every visit. We’ve never found any significant drop in blood pressure.”
Dr. Levy reported financial relationships with half a dozen pharmaceutical companies, none relevant to his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Localized Acanthosis Nigricans at the Site of Repetitive Insulin Injections
To the Editor:
Acanthosis nigricans (AN) is characterized by asymptomatic, hyperpigmented, velvety plaques that can occur anywhere on the body but most often present on the skin of the neck, axillae, groin, and other body folds.1-12 Although there are 5 subtypes, benign AN is the most common and is related to insulin resistance.1-4 Insulin can bind to insulinlike growth factor 1 (IGF-1) on keratinocytes, stimulating their proliferation. In type 2 diabetes mellitus, endogenous insulin levels are high enough to bind IGF-1 and activate keratinocytes, leading to the development of AN. Insulin injections have been associated with cutaneous side effects including lipoatrophy, lipohypertrophy, and postinflammatory hyperpigmentation.3 Acanthosis nigricans at insulin injection sites is a rare clinical condition.
A 64-year-old man presented for evaluation of a growing asymptomatic lesion on the abdomen of 6 years’ duration. He had a 17-year history of type 2 diabetes mellitus treated with insulin injections for 14 years after failing oral hypoglycemic agents. The patient reported injecting at the same site on the abdomen for the last 14 years. Physical examination revealed a lichenified, hyperpigmented, verrucous plaque on the lower abdomen under the umbilicus (Figure 1). No similar skin lesions were observed elsewhere on the body. A punch biopsy of the plaque showed hyperkeratosis, papillomatosis, acanthosis, and hyperpigmentation, which was consistent with AN (Figure 2). The patient was instructed to rotate injection sites and avoid the affected area. Over-the-counter ammonium lactate cream applied twice daily to the affected site also was recommended. After 2 months of treatment with this regimen, the patient reported softening and lightening of the lesion on the abdomen.
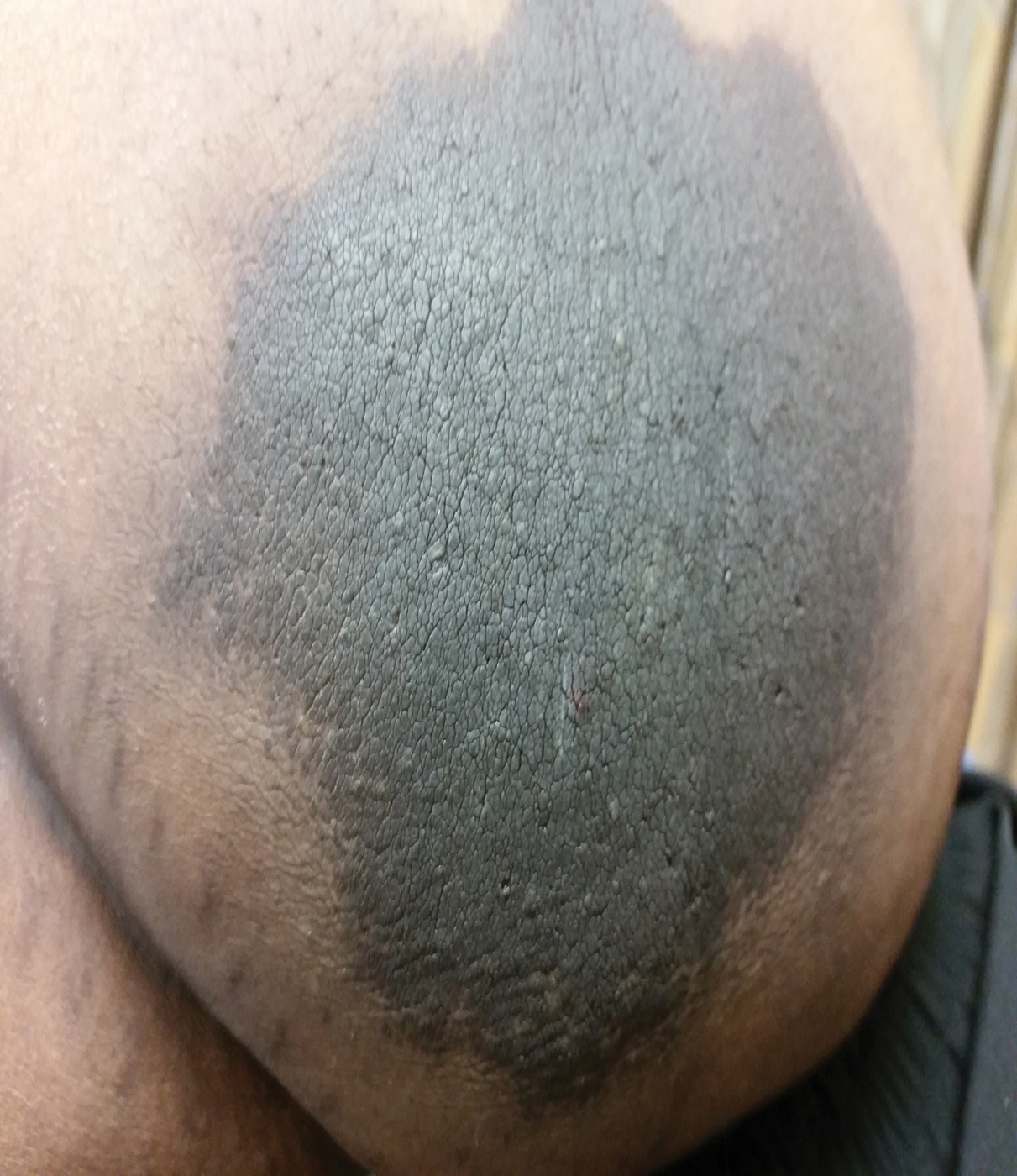

A PubMed search of articles indexed for MEDLINE for all English-language studies with human participants using the terms acanthosis nigricans and insulin injections yielded 20 results. Of them, 13 discussed localized AN at insulin injection sites: 12 case reports (Table)1-12 and 1 observational study in a group of diabetic patients.13
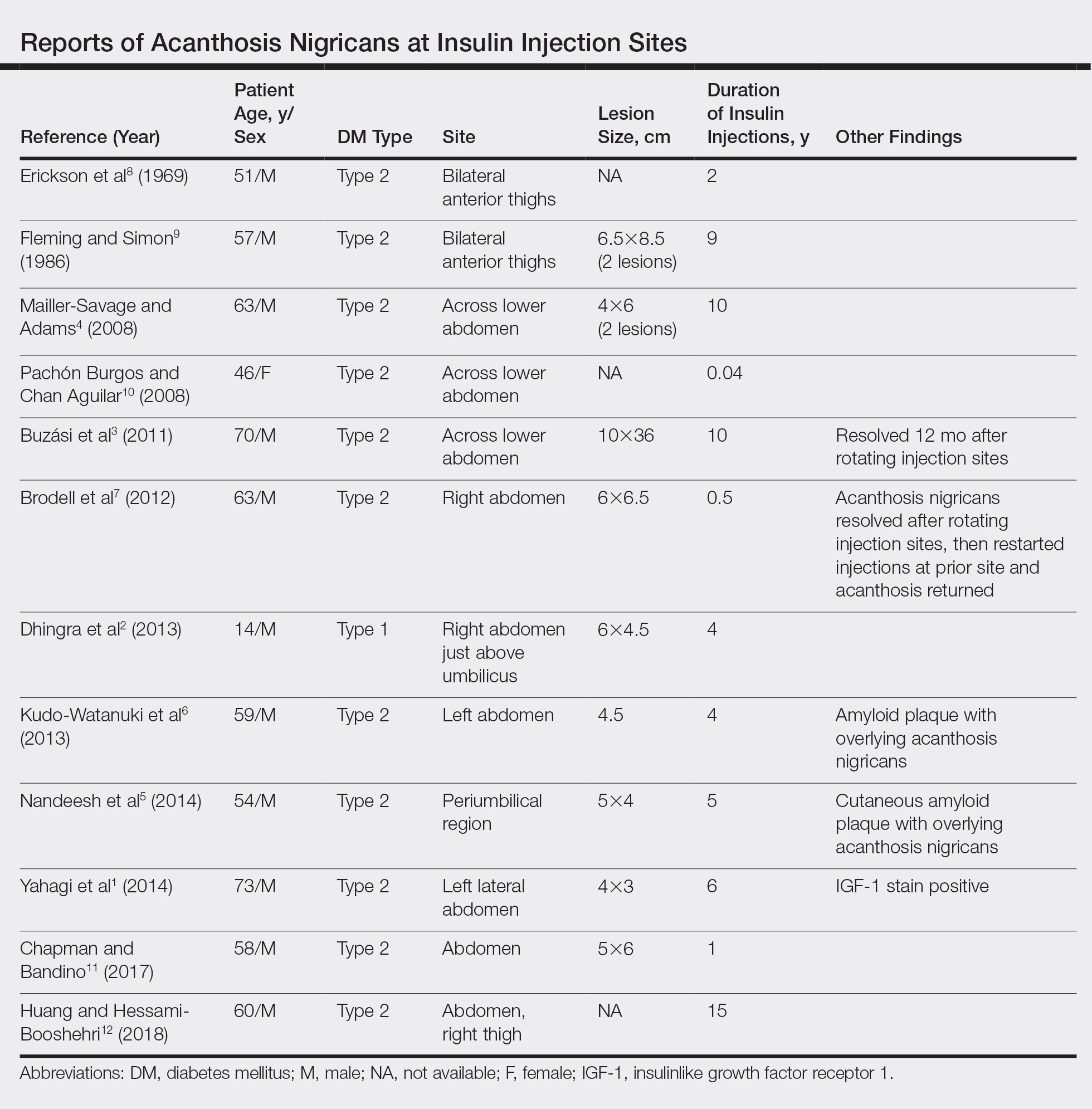
In the observational study, 500 diabetic patients were examined for insulin injection-site dermatoses and only 2 had localized injection-site AN. No other information was provided for these 2 patients.13 In the 12 published case reports,1-12 all patients did not rotate sites for their insulin injections and repeatedly injected into the affected area. The abdomen was the most commonly affected site, seen in 83% (10/12) of cases, while 25% (3/12) involved the thighs. All but 1 patient had type 2 diabetes mellitus. In 2 patients, “amyloid” was noted on the biopsy report in addition to changes consistent with AN. At least 2 patients had clearance after rotating injection sites.3,12
It has been suggested that localized AN at insulin injection sites develops through similar mechanisms as benign AN. Contributing factors to the development of benign AN may be IGF-1, fibroblast growth factor, and epidermal growth factor.1-3 Insulin is similar in structure to IGF-1 and can bind to IGF-1 receptors at high enough concentrations. Insulinlike growth factor 1 receptors are present on keratinocytes and fibroblasts, which proliferate upon activation, leading to the development of AN.1-3 Localized AN is thought to occur when repetitive insulin at the injection site saturates IGF-1 receptors on local keratinocytes.
Based on our patient and prior reports in the literature, localized AN is an uncommon cutaneous complication of insulin injections. Physicians should ask about repetitive insulin injections in the same site when localized AN occurs in patients with diabetes mellitus on insulin therapy. They also should discuss the importance of rotating sites for insulin adminstration to prevent the development of cutaneous complications including AN.
- Yahagi E, Mabuchi T, Nuruki H, et al. Case of exogenous insulin-derived acanthosis nigricans caused by insulin injections. Tokai J Exp Clin Med. 2014;39:5-9.
- Dhingra M, Garg G, Gupta M, et al. Exogenous insulin-derived acanthosis nigricans: could it be a cause of increased insulin requirement? Dermatol Online J. 2013;19:9.
- Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94:E34-E36.
- Mailler-Savage EA, Adams BB. Exogenous insulin-derived acanthosis nigricans. Arch Dermatol. 2008;144:126-127.
- Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57:127-129.
- Kudo-Watanuki S, Kurihara E, Yamamoto K, et al. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25-29.
- Brodell JD Jr, Cannella JD, Helms SE. Case report: acanthosis nigricans resulting from repetitive same-site insulin injections. J Drugs Dermatol. 2012;11:E85-E87.
- Erickson L, Lipschutz DE, Wrigley W, et al. A peculiar cutaneous reaction to repeated injections of insulin. JAMA. 1969;209:934-935.
- Fleming MG, Simon SI. Cutaneous insulin reaction resembling acanthosis nigricans. Arch Dermatol. 1986;122:1054-1056.
- Pachon Burgos A, Chan Aguilar MP. Visual vignette. hyperpigmented hyperkeratotic cutaneous insulin reaction that resembles acanthosis nigricans with lipohypertrophy. Endocr Pract. 2008;14:514.
- Chapman SE, Bandino JP. A verrucous plaque on the abdomen: challenge. Am J Dermatopathol. 2017;39:E163.
- Huang Y, Hessami-Booshehri M. Acanthosis nigricans at sites of insulin injection in a man with diabetes. CMAJ. 2018;190:E1390.
- Sawatkar GU, Kanwar AJ, Dogra S, et al. Spectrum of cutaneous manifestations of type 1 diabetes mellitus in 500 South Asian patients. Br J Dermatol. 2014;171:1402-1406.
To the Editor:
Acanthosis nigricans (AN) is characterized by asymptomatic, hyperpigmented, velvety plaques that can occur anywhere on the body but most often present on the skin of the neck, axillae, groin, and other body folds.1-12 Although there are 5 subtypes, benign AN is the most common and is related to insulin resistance.1-4 Insulin can bind to insulinlike growth factor 1 (IGF-1) on keratinocytes, stimulating their proliferation. In type 2 diabetes mellitus, endogenous insulin levels are high enough to bind IGF-1 and activate keratinocytes, leading to the development of AN. Insulin injections have been associated with cutaneous side effects including lipoatrophy, lipohypertrophy, and postinflammatory hyperpigmentation.3 Acanthosis nigricans at insulin injection sites is a rare clinical condition.
A 64-year-old man presented for evaluation of a growing asymptomatic lesion on the abdomen of 6 years’ duration. He had a 17-year history of type 2 diabetes mellitus treated with insulin injections for 14 years after failing oral hypoglycemic agents. The patient reported injecting at the same site on the abdomen for the last 14 years. Physical examination revealed a lichenified, hyperpigmented, verrucous plaque on the lower abdomen under the umbilicus (Figure 1). No similar skin lesions were observed elsewhere on the body. A punch biopsy of the plaque showed hyperkeratosis, papillomatosis, acanthosis, and hyperpigmentation, which was consistent with AN (Figure 2). The patient was instructed to rotate injection sites and avoid the affected area. Over-the-counter ammonium lactate cream applied twice daily to the affected site also was recommended. After 2 months of treatment with this regimen, the patient reported softening and lightening of the lesion on the abdomen.


A PubMed search of articles indexed for MEDLINE for all English-language studies with human participants using the terms acanthosis nigricans and insulin injections yielded 20 results. Of them, 13 discussed localized AN at insulin injection sites: 12 case reports (Table)1-12 and 1 observational study in a group of diabetic patients.13

In the observational study, 500 diabetic patients were examined for insulin injection-site dermatoses and only 2 had localized injection-site AN. No other information was provided for these 2 patients.13 In the 12 published case reports,1-12 all patients did not rotate sites for their insulin injections and repeatedly injected into the affected area. The abdomen was the most commonly affected site, seen in 83% (10/12) of cases, while 25% (3/12) involved the thighs. All but 1 patient had type 2 diabetes mellitus. In 2 patients, “amyloid” was noted on the biopsy report in addition to changes consistent with AN. At least 2 patients had clearance after rotating injection sites.3,12
It has been suggested that localized AN at insulin injection sites develops through similar mechanisms as benign AN. Contributing factors to the development of benign AN may be IGF-1, fibroblast growth factor, and epidermal growth factor.1-3 Insulin is similar in structure to IGF-1 and can bind to IGF-1 receptors at high enough concentrations. Insulinlike growth factor 1 receptors are present on keratinocytes and fibroblasts, which proliferate upon activation, leading to the development of AN.1-3 Localized AN is thought to occur when repetitive insulin at the injection site saturates IGF-1 receptors on local keratinocytes.
Based on our patient and prior reports in the literature, localized AN is an uncommon cutaneous complication of insulin injections. Physicians should ask about repetitive insulin injections in the same site when localized AN occurs in patients with diabetes mellitus on insulin therapy. They also should discuss the importance of rotating sites for insulin adminstration to prevent the development of cutaneous complications including AN.
To the Editor:
Acanthosis nigricans (AN) is characterized by asymptomatic, hyperpigmented, velvety plaques that can occur anywhere on the body but most often present on the skin of the neck, axillae, groin, and other body folds.1-12 Although there are 5 subtypes, benign AN is the most common and is related to insulin resistance.1-4 Insulin can bind to insulinlike growth factor 1 (IGF-1) on keratinocytes, stimulating their proliferation. In type 2 diabetes mellitus, endogenous insulin levels are high enough to bind IGF-1 and activate keratinocytes, leading to the development of AN. Insulin injections have been associated with cutaneous side effects including lipoatrophy, lipohypertrophy, and postinflammatory hyperpigmentation.3 Acanthosis nigricans at insulin injection sites is a rare clinical condition.
A 64-year-old man presented for evaluation of a growing asymptomatic lesion on the abdomen of 6 years’ duration. He had a 17-year history of type 2 diabetes mellitus treated with insulin injections for 14 years after failing oral hypoglycemic agents. The patient reported injecting at the same site on the abdomen for the last 14 years. Physical examination revealed a lichenified, hyperpigmented, verrucous plaque on the lower abdomen under the umbilicus (Figure 1). No similar skin lesions were observed elsewhere on the body. A punch biopsy of the plaque showed hyperkeratosis, papillomatosis, acanthosis, and hyperpigmentation, which was consistent with AN (Figure 2). The patient was instructed to rotate injection sites and avoid the affected area. Over-the-counter ammonium lactate cream applied twice daily to the affected site also was recommended. After 2 months of treatment with this regimen, the patient reported softening and lightening of the lesion on the abdomen.


A PubMed search of articles indexed for MEDLINE for all English-language studies with human participants using the terms acanthosis nigricans and insulin injections yielded 20 results. Of them, 13 discussed localized AN at insulin injection sites: 12 case reports (Table)1-12 and 1 observational study in a group of diabetic patients.13

In the observational study, 500 diabetic patients were examined for insulin injection-site dermatoses and only 2 had localized injection-site AN. No other information was provided for these 2 patients.13 In the 12 published case reports,1-12 all patients did not rotate sites for their insulin injections and repeatedly injected into the affected area. The abdomen was the most commonly affected site, seen in 83% (10/12) of cases, while 25% (3/12) involved the thighs. All but 1 patient had type 2 diabetes mellitus. In 2 patients, “amyloid” was noted on the biopsy report in addition to changes consistent with AN. At least 2 patients had clearance after rotating injection sites.3,12
It has been suggested that localized AN at insulin injection sites develops through similar mechanisms as benign AN. Contributing factors to the development of benign AN may be IGF-1, fibroblast growth factor, and epidermal growth factor.1-3 Insulin is similar in structure to IGF-1 and can bind to IGF-1 receptors at high enough concentrations. Insulinlike growth factor 1 receptors are present on keratinocytes and fibroblasts, which proliferate upon activation, leading to the development of AN.1-3 Localized AN is thought to occur when repetitive insulin at the injection site saturates IGF-1 receptors on local keratinocytes.
Based on our patient and prior reports in the literature, localized AN is an uncommon cutaneous complication of insulin injections. Physicians should ask about repetitive insulin injections in the same site when localized AN occurs in patients with diabetes mellitus on insulin therapy. They also should discuss the importance of rotating sites for insulin adminstration to prevent the development of cutaneous complications including AN.
- Yahagi E, Mabuchi T, Nuruki H, et al. Case of exogenous insulin-derived acanthosis nigricans caused by insulin injections. Tokai J Exp Clin Med. 2014;39:5-9.
- Dhingra M, Garg G, Gupta M, et al. Exogenous insulin-derived acanthosis nigricans: could it be a cause of increased insulin requirement? Dermatol Online J. 2013;19:9.
- Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94:E34-E36.
- Mailler-Savage EA, Adams BB. Exogenous insulin-derived acanthosis nigricans. Arch Dermatol. 2008;144:126-127.
- Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57:127-129.
- Kudo-Watanuki S, Kurihara E, Yamamoto K, et al. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25-29.
- Brodell JD Jr, Cannella JD, Helms SE. Case report: acanthosis nigricans resulting from repetitive same-site insulin injections. J Drugs Dermatol. 2012;11:E85-E87.
- Erickson L, Lipschutz DE, Wrigley W, et al. A peculiar cutaneous reaction to repeated injections of insulin. JAMA. 1969;209:934-935.
- Fleming MG, Simon SI. Cutaneous insulin reaction resembling acanthosis nigricans. Arch Dermatol. 1986;122:1054-1056.
- Pachon Burgos A, Chan Aguilar MP. Visual vignette. hyperpigmented hyperkeratotic cutaneous insulin reaction that resembles acanthosis nigricans with lipohypertrophy. Endocr Pract. 2008;14:514.
- Chapman SE, Bandino JP. A verrucous plaque on the abdomen: challenge. Am J Dermatopathol. 2017;39:E163.
- Huang Y, Hessami-Booshehri M. Acanthosis nigricans at sites of insulin injection in a man with diabetes. CMAJ. 2018;190:E1390.
- Sawatkar GU, Kanwar AJ, Dogra S, et al. Spectrum of cutaneous manifestations of type 1 diabetes mellitus in 500 South Asian patients. Br J Dermatol. 2014;171:1402-1406.
- Yahagi E, Mabuchi T, Nuruki H, et al. Case of exogenous insulin-derived acanthosis nigricans caused by insulin injections. Tokai J Exp Clin Med. 2014;39:5-9.
- Dhingra M, Garg G, Gupta M, et al. Exogenous insulin-derived acanthosis nigricans: could it be a cause of increased insulin requirement? Dermatol Online J. 2013;19:9.
- Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94:E34-E36.
- Mailler-Savage EA, Adams BB. Exogenous insulin-derived acanthosis nigricans. Arch Dermatol. 2008;144:126-127.
- Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57:127-129.
- Kudo-Watanuki S, Kurihara E, Yamamoto K, et al. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25-29.
- Brodell JD Jr, Cannella JD, Helms SE. Case report: acanthosis nigricans resulting from repetitive same-site insulin injections. J Drugs Dermatol. 2012;11:E85-E87.
- Erickson L, Lipschutz DE, Wrigley W, et al. A peculiar cutaneous reaction to repeated injections of insulin. JAMA. 1969;209:934-935.
- Fleming MG, Simon SI. Cutaneous insulin reaction resembling acanthosis nigricans. Arch Dermatol. 1986;122:1054-1056.
- Pachon Burgos A, Chan Aguilar MP. Visual vignette. hyperpigmented hyperkeratotic cutaneous insulin reaction that resembles acanthosis nigricans with lipohypertrophy. Endocr Pract. 2008;14:514.
- Chapman SE, Bandino JP. A verrucous plaque on the abdomen: challenge. Am J Dermatopathol. 2017;39:E163.
- Huang Y, Hessami-Booshehri M. Acanthosis nigricans at sites of insulin injection in a man with diabetes. CMAJ. 2018;190:E1390.
- Sawatkar GU, Kanwar AJ, Dogra S, et al. Spectrum of cutaneous manifestations of type 1 diabetes mellitus in 500 South Asian patients. Br J Dermatol. 2014;171:1402-1406.
Practice Points
- Benign acanthosis nigricans (AN) is most often related to insulin resistance and presents as asymptomatic, hyperpigmented, velvety plaques on the neck, axillae, groin, and other body folds.
- Benign AN related to insulin resistance occurs when insulin binds to insulinlike growth factor 1 on keratinocytes and stimulates proliferations.
- Although insulin injections have been associated with several cutaneous side effects, including lipoatrophy, lipohypertrophy, and postinflammatory hyperpigmentation, localized AN is an uncommonly reported cutaneous adverse effect.
Unilateral Vesicular Eruption in a Neonate
The Diagnosis: Incontinentia Pigmenti
The patient was diagnosed clinically with the vesicular stage of incontinentia pigmenti (IP), a rare, X-linked dominant neuroectodermal dysplasia that usually is lethal in males. The genetic mutation has been identified in the IKBKG gene (inhibitor of nuclear factor κB; formally NEMO), which leads to a truncated and defective nuclear factor κB. Female infants survive and display characteristic findings on examination due to X-inactivation leading to mosaicism.1 Worldwide, there are approximately 27.6 new cases of IP per year. Although it is heritable, the majority (65%-75%) of cases are due to sporadic mutations, with the remaining minority (25%-35%) representing familial disease.1
Cutaneous findings of IP classically progress through 4 stages, though individual patients often do not develop the characteristic lesions of each of the 4 stages. The vesicular stage (stage 1) presented in our patient (quiz image). This stage presents within 2 weeks of birth in 90% of patients and typically disappears when the patient is approximately 4 months of age.1-3 Although the clinical presentation is striking, it is essential to rule out herpes simplex virus infection, which can mimic vesicular IP. Localized herpes simplex virus is most commonly seen in clusters on the scalp and often is not present at birth. Alternatively, IP is most often seen on the extremities in bands or whorls of distribution along Blaschko lines,4 as in this patient.
Stage 2 (the verrucous stage) presents with verrucous papules or pustules in a similar blaschkoid distribution. Areas previously involved in stage 1 are not always the same areas affected in stage 2. Approximately 70% of patients develop stage 2 lesions, usually at 2 to 6 weeks of age.1-3 Erythema toxicum neonatorum presents in the first week of life with pustules often on the trunk or extremities, but these lesions are not confined to Blaschko lines, differentiating it from IP.4
The third stage (hyperpigmented stage) lends the disease its name and occurs in 90% to 95% of patients with IP. Linear and whorled hyperpigmentation develops in early infancy and can either persist or fade by adolescence.1 Pustules and hyperpigmentation in transient neonatal pustular melanosis may be similar to this stage of IP, but the distribution is more variable and progression to other lesions is not seen.5
The fourth and final stage is the hypopigmented stage, whereby blaschkoid linear and whorled lines of hypopigmentation with or without both atrophy and alopecia develop in 75% of patients. This is the last finding, beginning in adolescence and often persisting into adulthood.1 Goltz syndrome is another X-linked dominant disorder with features similar to IP. Verrucous and atrophic lesions along Blaschko lines are reminiscent of the second and fourth stages of IP but are differentiated in Goltz syndrome because they present concurrently rather than in sequential stages such as IP. Similar extracutaneous organs are affected such as the eyes, teeth, and nails; however, Goltz syndrome may be associated with more distinguishing systemic signs such as sweating and skeletal abnormalities.6
Given its unique appearance, physicians usually diagnose IP clinically after identification of characteristic linear lesions along the lines of Blaschko in an infant or neonate. Skin biopsy is confirmatory, which would differ depending on the stage of disease biopsied. The vesicular stage is characterized by eosinophilic spongiosis and is differentiated from other items on the histologic differential diagnosis by the presence of dyskeratosis.7 Genetic testing is available and should be performed along with a physical examination of the mother for counseling purposes.1
Proper diagnosis is critical because of the potential multisystem nature of the disease with implications for longitudinal care and prognosis in patients. As in other neurocutaneous disease, IP can affect the hair, nails, teeth, central nervous system, and eyes. All IP patients receive a referral to ophthalmology at the time of diagnosis for a dilated fundus examination, with repeat examinations every several months initially--every 3 months for a year, every 6 months from 1 to 3 years of age--and annually thereafter. Dental evaluation should occur at 6 months of age or whenever the first tooth erupts.1 Mental retardation, seizures, and developmental delay can occur and usually are evident in the first year of life. Patients should have developmental milestones closely monitored and be referred to appropriate specialists if signs or symptoms develop consistent with neurologic involvement.1
- Greene-Roethke C. Incontinentia pigmenti: a summary review of this rare ectodermal dysplasia with neurologic manifestations, including treatment protocols. J Pediatr Health Care. 2017;31:e45-e52.
- Shah KN. Incontinentia pigmenti clinical presentation. Medscape. https://emedicine.medscape.com/article/1114205-clinical. Updated March 5, 2019. Accessed August 2, 2019.
- Poziomczyk CS, Recuero JK, Bringhenti L, et al. Incontinentia pigmenti. An Bras Dermatol. 2014;89:23-36.
- Mathes E, Howard RM. Vesicular, pustular, and bullous lesions in the newborn and infant. UpToDate. https://www.uptodate.com/contents/vesicular-pustular-and-bullous-lesions-in-the-newborn-and-infant. Updated December 3, 2018. Accessed February 20, 2020.
- Ghosh S. Neonatal pustular dermatosis: an overview. Indian J Dermatol. 2015;60:211.
- Temple IK, MacDowall P, Baraitser M, et al. Focal dermal hypoplasia (Goltz syndrome). J Med Genet. 1990;27:180-187.
- Ferringer T. Genodermatoses. In: Elston D, Ferringer T, Ko CJ, et al, eds. Dermatology. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2014:208-213.
The Diagnosis: Incontinentia Pigmenti
The patient was diagnosed clinically with the vesicular stage of incontinentia pigmenti (IP), a rare, X-linked dominant neuroectodermal dysplasia that usually is lethal in males. The genetic mutation has been identified in the IKBKG gene (inhibitor of nuclear factor κB; formally NEMO), which leads to a truncated and defective nuclear factor κB. Female infants survive and display characteristic findings on examination due to X-inactivation leading to mosaicism.1 Worldwide, there are approximately 27.6 new cases of IP per year. Although it is heritable, the majority (65%-75%) of cases are due to sporadic mutations, with the remaining minority (25%-35%) representing familial disease.1
Cutaneous findings of IP classically progress through 4 stages, though individual patients often do not develop the characteristic lesions of each of the 4 stages. The vesicular stage (stage 1) presented in our patient (quiz image). This stage presents within 2 weeks of birth in 90% of patients and typically disappears when the patient is approximately 4 months of age.1-3 Although the clinical presentation is striking, it is essential to rule out herpes simplex virus infection, which can mimic vesicular IP. Localized herpes simplex virus is most commonly seen in clusters on the scalp and often is not present at birth. Alternatively, IP is most often seen on the extremities in bands or whorls of distribution along Blaschko lines,4 as in this patient.
Stage 2 (the verrucous stage) presents with verrucous papules or pustules in a similar blaschkoid distribution. Areas previously involved in stage 1 are not always the same areas affected in stage 2. Approximately 70% of patients develop stage 2 lesions, usually at 2 to 6 weeks of age.1-3 Erythema toxicum neonatorum presents in the first week of life with pustules often on the trunk or extremities, but these lesions are not confined to Blaschko lines, differentiating it from IP.4
The third stage (hyperpigmented stage) lends the disease its name and occurs in 90% to 95% of patients with IP. Linear and whorled hyperpigmentation develops in early infancy and can either persist or fade by adolescence.1 Pustules and hyperpigmentation in transient neonatal pustular melanosis may be similar to this stage of IP, but the distribution is more variable and progression to other lesions is not seen.5
The fourth and final stage is the hypopigmented stage, whereby blaschkoid linear and whorled lines of hypopigmentation with or without both atrophy and alopecia develop in 75% of patients. This is the last finding, beginning in adolescence and often persisting into adulthood.1 Goltz syndrome is another X-linked dominant disorder with features similar to IP. Verrucous and atrophic lesions along Blaschko lines are reminiscent of the second and fourth stages of IP but are differentiated in Goltz syndrome because they present concurrently rather than in sequential stages such as IP. Similar extracutaneous organs are affected such as the eyes, teeth, and nails; however, Goltz syndrome may be associated with more distinguishing systemic signs such as sweating and skeletal abnormalities.6
Given its unique appearance, physicians usually diagnose IP clinically after identification of characteristic linear lesions along the lines of Blaschko in an infant or neonate. Skin biopsy is confirmatory, which would differ depending on the stage of disease biopsied. The vesicular stage is characterized by eosinophilic spongiosis and is differentiated from other items on the histologic differential diagnosis by the presence of dyskeratosis.7 Genetic testing is available and should be performed along with a physical examination of the mother for counseling purposes.1
Proper diagnosis is critical because of the potential multisystem nature of the disease with implications for longitudinal care and prognosis in patients. As in other neurocutaneous disease, IP can affect the hair, nails, teeth, central nervous system, and eyes. All IP patients receive a referral to ophthalmology at the time of diagnosis for a dilated fundus examination, with repeat examinations every several months initially--every 3 months for a year, every 6 months from 1 to 3 years of age--and annually thereafter. Dental evaluation should occur at 6 months of age or whenever the first tooth erupts.1 Mental retardation, seizures, and developmental delay can occur and usually are evident in the first year of life. Patients should have developmental milestones closely monitored and be referred to appropriate specialists if signs or symptoms develop consistent with neurologic involvement.1
The Diagnosis: Incontinentia Pigmenti
The patient was diagnosed clinically with the vesicular stage of incontinentia pigmenti (IP), a rare, X-linked dominant neuroectodermal dysplasia that usually is lethal in males. The genetic mutation has been identified in the IKBKG gene (inhibitor of nuclear factor κB; formally NEMO), which leads to a truncated and defective nuclear factor κB. Female infants survive and display characteristic findings on examination due to X-inactivation leading to mosaicism.1 Worldwide, there are approximately 27.6 new cases of IP per year. Although it is heritable, the majority (65%-75%) of cases are due to sporadic mutations, with the remaining minority (25%-35%) representing familial disease.1
Cutaneous findings of IP classically progress through 4 stages, though individual patients often do not develop the characteristic lesions of each of the 4 stages. The vesicular stage (stage 1) presented in our patient (quiz image). This stage presents within 2 weeks of birth in 90% of patients and typically disappears when the patient is approximately 4 months of age.1-3 Although the clinical presentation is striking, it is essential to rule out herpes simplex virus infection, which can mimic vesicular IP. Localized herpes simplex virus is most commonly seen in clusters on the scalp and often is not present at birth. Alternatively, IP is most often seen on the extremities in bands or whorls of distribution along Blaschko lines,4 as in this patient.
Stage 2 (the verrucous stage) presents with verrucous papules or pustules in a similar blaschkoid distribution. Areas previously involved in stage 1 are not always the same areas affected in stage 2. Approximately 70% of patients develop stage 2 lesions, usually at 2 to 6 weeks of age.1-3 Erythema toxicum neonatorum presents in the first week of life with pustules often on the trunk or extremities, but these lesions are not confined to Blaschko lines, differentiating it from IP.4
The third stage (hyperpigmented stage) lends the disease its name and occurs in 90% to 95% of patients with IP. Linear and whorled hyperpigmentation develops in early infancy and can either persist or fade by adolescence.1 Pustules and hyperpigmentation in transient neonatal pustular melanosis may be similar to this stage of IP, but the distribution is more variable and progression to other lesions is not seen.5
The fourth and final stage is the hypopigmented stage, whereby blaschkoid linear and whorled lines of hypopigmentation with or without both atrophy and alopecia develop in 75% of patients. This is the last finding, beginning in adolescence and often persisting into adulthood.1 Goltz syndrome is another X-linked dominant disorder with features similar to IP. Verrucous and atrophic lesions along Blaschko lines are reminiscent of the second and fourth stages of IP but are differentiated in Goltz syndrome because they present concurrently rather than in sequential stages such as IP. Similar extracutaneous organs are affected such as the eyes, teeth, and nails; however, Goltz syndrome may be associated with more distinguishing systemic signs such as sweating and skeletal abnormalities.6
Given its unique appearance, physicians usually diagnose IP clinically after identification of characteristic linear lesions along the lines of Blaschko in an infant or neonate. Skin biopsy is confirmatory, which would differ depending on the stage of disease biopsied. The vesicular stage is characterized by eosinophilic spongiosis and is differentiated from other items on the histologic differential diagnosis by the presence of dyskeratosis.7 Genetic testing is available and should be performed along with a physical examination of the mother for counseling purposes.1
Proper diagnosis is critical because of the potential multisystem nature of the disease with implications for longitudinal care and prognosis in patients. As in other neurocutaneous disease, IP can affect the hair, nails, teeth, central nervous system, and eyes. All IP patients receive a referral to ophthalmology at the time of diagnosis for a dilated fundus examination, with repeat examinations every several months initially--every 3 months for a year, every 6 months from 1 to 3 years of age--and annually thereafter. Dental evaluation should occur at 6 months of age or whenever the first tooth erupts.1 Mental retardation, seizures, and developmental delay can occur and usually are evident in the first year of life. Patients should have developmental milestones closely monitored and be referred to appropriate specialists if signs or symptoms develop consistent with neurologic involvement.1
- Greene-Roethke C. Incontinentia pigmenti: a summary review of this rare ectodermal dysplasia with neurologic manifestations, including treatment protocols. J Pediatr Health Care. 2017;31:e45-e52.
- Shah KN. Incontinentia pigmenti clinical presentation. Medscape. https://emedicine.medscape.com/article/1114205-clinical. Updated March 5, 2019. Accessed August 2, 2019.
- Poziomczyk CS, Recuero JK, Bringhenti L, et al. Incontinentia pigmenti. An Bras Dermatol. 2014;89:23-36.
- Mathes E, Howard RM. Vesicular, pustular, and bullous lesions in the newborn and infant. UpToDate. https://www.uptodate.com/contents/vesicular-pustular-and-bullous-lesions-in-the-newborn-and-infant. Updated December 3, 2018. Accessed February 20, 2020.
- Ghosh S. Neonatal pustular dermatosis: an overview. Indian J Dermatol. 2015;60:211.
- Temple IK, MacDowall P, Baraitser M, et al. Focal dermal hypoplasia (Goltz syndrome). J Med Genet. 1990;27:180-187.
- Ferringer T. Genodermatoses. In: Elston D, Ferringer T, Ko CJ, et al, eds. Dermatology. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2014:208-213.
- Greene-Roethke C. Incontinentia pigmenti: a summary review of this rare ectodermal dysplasia with neurologic manifestations, including treatment protocols. J Pediatr Health Care. 2017;31:e45-e52.
- Shah KN. Incontinentia pigmenti clinical presentation. Medscape. https://emedicine.medscape.com/article/1114205-clinical. Updated March 5, 2019. Accessed August 2, 2019.
- Poziomczyk CS, Recuero JK, Bringhenti L, et al. Incontinentia pigmenti. An Bras Dermatol. 2014;89:23-36.
- Mathes E, Howard RM. Vesicular, pustular, and bullous lesions in the newborn and infant. UpToDate. https://www.uptodate.com/contents/vesicular-pustular-and-bullous-lesions-in-the-newborn-and-infant. Updated December 3, 2018. Accessed February 20, 2020.
- Ghosh S. Neonatal pustular dermatosis: an overview. Indian J Dermatol. 2015;60:211.
- Temple IK, MacDowall P, Baraitser M, et al. Focal dermal hypoplasia (Goltz syndrome). J Med Genet. 1990;27:180-187.
- Ferringer T. Genodermatoses. In: Elston D, Ferringer T, Ko CJ, et al, eds. Dermatology. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2014:208-213.

A 4-day-old female neonate presented to the dermatology clinic with a vesicular eruption on the left leg of 1 day's duration. The eruption was asymptomatic without any extracutaneous findings. This term infant was born without complication, and the mother denied any symptoms consistent with herpes simplex virus infection. Physical examination revealed yellow-red vesicles on an erythematous base in a blaschkoid distribution on the left leg. The rest of the examination was unremarkable. Herpes simplex virus polymerase chain reaction testing was negative.
Vitiligo tied to lower risk of internal malignancies
LAHAINA, HAWAII – from South Korea, Iltefat Hamzavi, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Previous studies by Dr. Hamzavi and others have established that vitiligo patients have a reduced risk of melanoma and perhaps nonmelanoma skin cancers as well. But the South Korean national study of 101,078 vitiligo patients matched by age and sex to twice as many vitiligo-free controls was the first large examination of the association between vitiligo and internal malignancies. The findings suggest that immunosurveillance in patients with the disease is not merely a skin-deep phenomenon, noted Dr. Hamzavi, of the MultiCultural Dermatology Center at Henry Ford Hospital in Detroit.
“Vitiligo is probably a systemic disease in which there may be increased immunosurveillance. The point here is that as we suppress the disease, we have to be careful that we’re not going to increase cancer rates,” the dermatologist explained in an interview. “This is big data, and something to be aware of, but don’t act on it yet in clinical practice. I just want people to be aware that all of these autoimmune diseases are there for a reason. There are lower rates of melanoma and internal cancers in patients who have vitiligo, but what that means for our new therapies that are coming up we don’t know yet.”
He predicted that the study will open up an active new research domain, but it will take time to find definitive answers as to whether emerging immunomodulatory therapies for patients with vitiligo might, in some instances, increase their current favorably lower risk of internal malignancies. In the meantime, physicians interested in treating vitiligo off label with, for example, Janus kinase (JAK) inhibitors will want to be particularly cautious in patients with a strong history of skin cancer or internal malignancies.
The retrospective, population-based study utilized data from the Korean National Health Insurance claims database. The investigators found that the incidence rate of internal malignancies was 612.9 per 100,000 person-years in the vitiligo group and 708.9 per 100,000 person-years in controls, for a statistically significant and clinically meaningful 14% relative risk reduction after adjustment for age, sex, and comorbid conditions.
Among the most striking organ-specific findings: the vitiligo group had a 38% relative risk reduction in colorectal cancer, a 25% reduction in the risk of lung cancer, and a 38% decrease in ovarian cancer. In contrast, they had a 20% increase in the risk of thyroid cancer (J Clin Oncol. 2019 Apr 10;37[11]:903-11).
Despite the fact that vitiligo is a common disease that affects 0.5%-1% of the population worldwide, for decades it has been something of a pharmacotherapeutic backwater. That’s changed recently and in dramatic fashion as a result of new understanding of the disease pathogenesis. The JAK inhibitors are now under active investigation for the treatment of vitiligo. Indeed, ruxolitinib cream, a potent JAK-1 and -2 inhibitor, is now in phase 3 investigation following a highly successful phase 2 trial. Interleukin-15 blockade is another promising avenue.
Dr. Hamzavi reported serving as a consultant to AbbVie, Aclaris, Novartis, and Pfizer, and receiving research funding from Estee Lauder, Clinuvel Pharmaceuticals, Incyte, and Pfizer. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – from South Korea, Iltefat Hamzavi, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Previous studies by Dr. Hamzavi and others have established that vitiligo patients have a reduced risk of melanoma and perhaps nonmelanoma skin cancers as well. But the South Korean national study of 101,078 vitiligo patients matched by age and sex to twice as many vitiligo-free controls was the first large examination of the association between vitiligo and internal malignancies. The findings suggest that immunosurveillance in patients with the disease is not merely a skin-deep phenomenon, noted Dr. Hamzavi, of the MultiCultural Dermatology Center at Henry Ford Hospital in Detroit.
“Vitiligo is probably a systemic disease in which there may be increased immunosurveillance. The point here is that as we suppress the disease, we have to be careful that we’re not going to increase cancer rates,” the dermatologist explained in an interview. “This is big data, and something to be aware of, but don’t act on it yet in clinical practice. I just want people to be aware that all of these autoimmune diseases are there for a reason. There are lower rates of melanoma and internal cancers in patients who have vitiligo, but what that means for our new therapies that are coming up we don’t know yet.”
He predicted that the study will open up an active new research domain, but it will take time to find definitive answers as to whether emerging immunomodulatory therapies for patients with vitiligo might, in some instances, increase their current favorably lower risk of internal malignancies. In the meantime, physicians interested in treating vitiligo off label with, for example, Janus kinase (JAK) inhibitors will want to be particularly cautious in patients with a strong history of skin cancer or internal malignancies.
The retrospective, population-based study utilized data from the Korean National Health Insurance claims database. The investigators found that the incidence rate of internal malignancies was 612.9 per 100,000 person-years in the vitiligo group and 708.9 per 100,000 person-years in controls, for a statistically significant and clinically meaningful 14% relative risk reduction after adjustment for age, sex, and comorbid conditions.
Among the most striking organ-specific findings: the vitiligo group had a 38% relative risk reduction in colorectal cancer, a 25% reduction in the risk of lung cancer, and a 38% decrease in ovarian cancer. In contrast, they had a 20% increase in the risk of thyroid cancer (J Clin Oncol. 2019 Apr 10;37[11]:903-11).
Despite the fact that vitiligo is a common disease that affects 0.5%-1% of the population worldwide, for decades it has been something of a pharmacotherapeutic backwater. That’s changed recently and in dramatic fashion as a result of new understanding of the disease pathogenesis. The JAK inhibitors are now under active investigation for the treatment of vitiligo. Indeed, ruxolitinib cream, a potent JAK-1 and -2 inhibitor, is now in phase 3 investigation following a highly successful phase 2 trial. Interleukin-15 blockade is another promising avenue.
Dr. Hamzavi reported serving as a consultant to AbbVie, Aclaris, Novartis, and Pfizer, and receiving research funding from Estee Lauder, Clinuvel Pharmaceuticals, Incyte, and Pfizer. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – from South Korea, Iltefat Hamzavi, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Previous studies by Dr. Hamzavi and others have established that vitiligo patients have a reduced risk of melanoma and perhaps nonmelanoma skin cancers as well. But the South Korean national study of 101,078 vitiligo patients matched by age and sex to twice as many vitiligo-free controls was the first large examination of the association between vitiligo and internal malignancies. The findings suggest that immunosurveillance in patients with the disease is not merely a skin-deep phenomenon, noted Dr. Hamzavi, of the MultiCultural Dermatology Center at Henry Ford Hospital in Detroit.
“Vitiligo is probably a systemic disease in which there may be increased immunosurveillance. The point here is that as we suppress the disease, we have to be careful that we’re not going to increase cancer rates,” the dermatologist explained in an interview. “This is big data, and something to be aware of, but don’t act on it yet in clinical practice. I just want people to be aware that all of these autoimmune diseases are there for a reason. There are lower rates of melanoma and internal cancers in patients who have vitiligo, but what that means for our new therapies that are coming up we don’t know yet.”
He predicted that the study will open up an active new research domain, but it will take time to find definitive answers as to whether emerging immunomodulatory therapies for patients with vitiligo might, in some instances, increase their current favorably lower risk of internal malignancies. In the meantime, physicians interested in treating vitiligo off label with, for example, Janus kinase (JAK) inhibitors will want to be particularly cautious in patients with a strong history of skin cancer or internal malignancies.
The retrospective, population-based study utilized data from the Korean National Health Insurance claims database. The investigators found that the incidence rate of internal malignancies was 612.9 per 100,000 person-years in the vitiligo group and 708.9 per 100,000 person-years in controls, for a statistically significant and clinically meaningful 14% relative risk reduction after adjustment for age, sex, and comorbid conditions.
Among the most striking organ-specific findings: the vitiligo group had a 38% relative risk reduction in colorectal cancer, a 25% reduction in the risk of lung cancer, and a 38% decrease in ovarian cancer. In contrast, they had a 20% increase in the risk of thyroid cancer (J Clin Oncol. 2019 Apr 10;37[11]:903-11).
Despite the fact that vitiligo is a common disease that affects 0.5%-1% of the population worldwide, for decades it has been something of a pharmacotherapeutic backwater. That’s changed recently and in dramatic fashion as a result of new understanding of the disease pathogenesis. The JAK inhibitors are now under active investigation for the treatment of vitiligo. Indeed, ruxolitinib cream, a potent JAK-1 and -2 inhibitor, is now in phase 3 investigation following a highly successful phase 2 trial. Interleukin-15 blockade is another promising avenue.
Dr. Hamzavi reported serving as a consultant to AbbVie, Aclaris, Novartis, and Pfizer, and receiving research funding from Estee Lauder, Clinuvel Pharmaceuticals, Incyte, and Pfizer. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR