User login
Racial Limitations of Fitzpatrick Skin Type
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
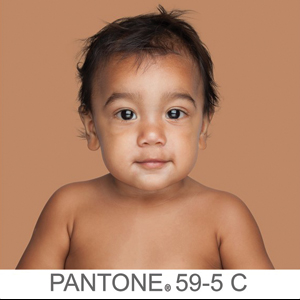
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.

© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20
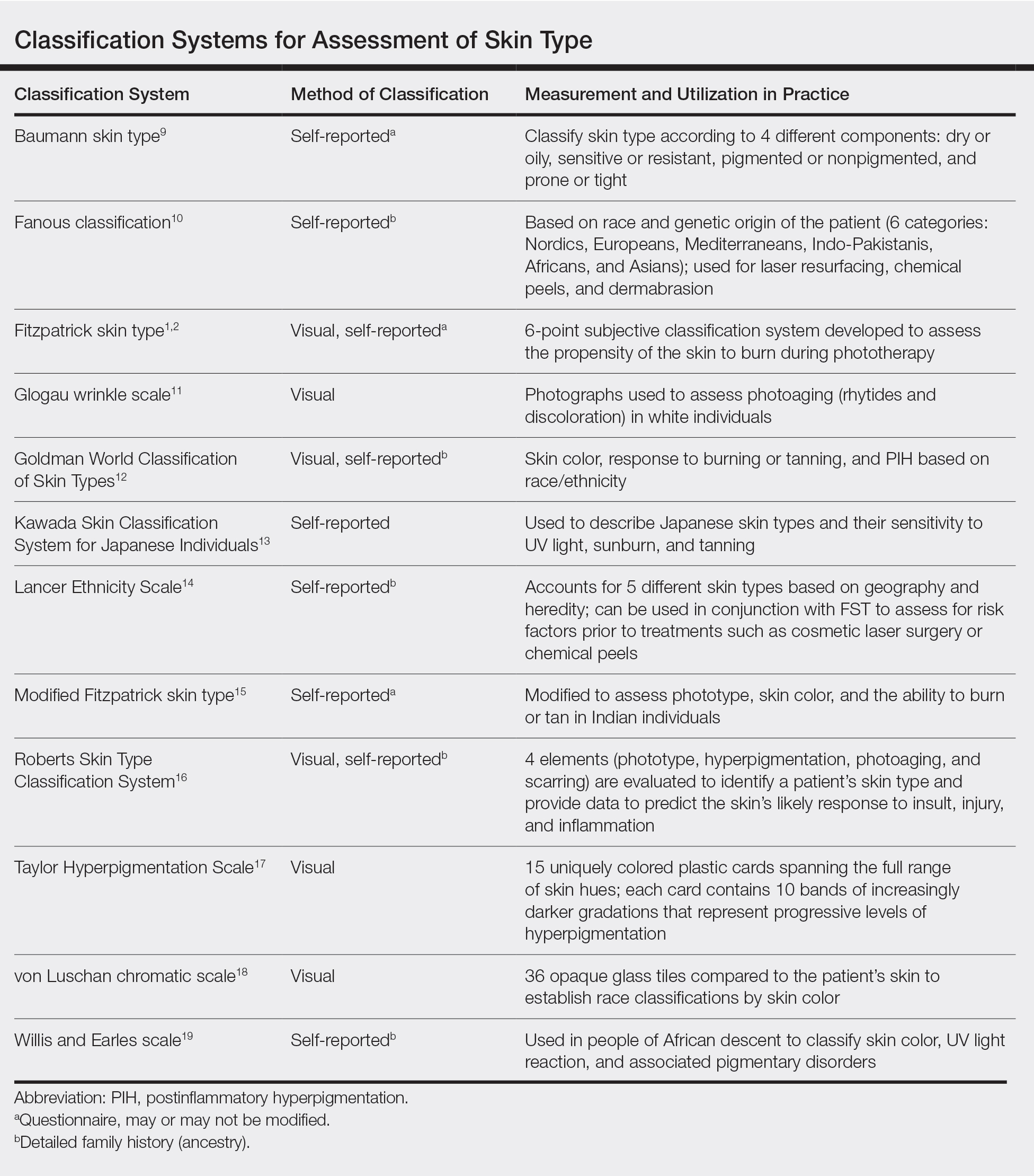
Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.

© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20

Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.

© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20

Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
Practice Points
- Medical providers should be cognizant of conflating race and ethnicity with Fitzpatrick skin type (FST).
- Misuse of FST may occur more frequently among physicians who do not identify as having skin of color.
- Although alternative skin type classification systems have been proposed, more clinically relevant methods for describing skin of color need to be developed.
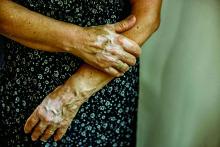
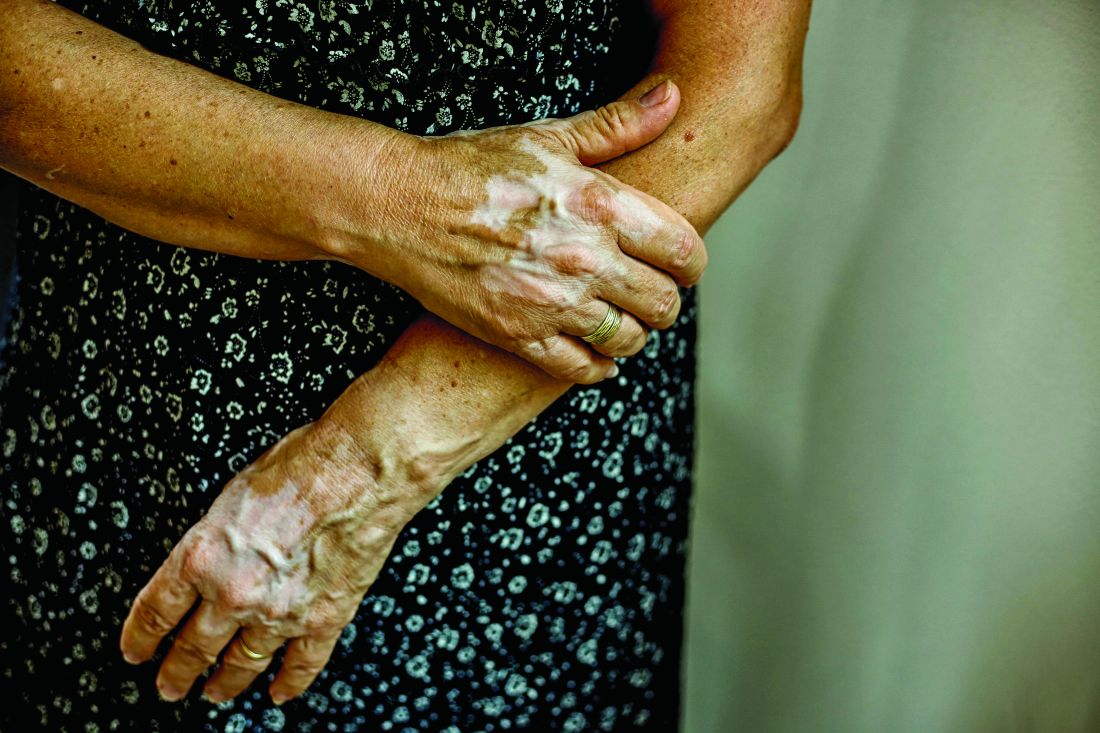
New Barbie lineup includes a doll with vitiligo
A new line of Barbie dolls unveiled by Mattel earlier this month includes one with vitiligo, much to the delight of clinicians who treat children and adolescents with the condition.
“When I see young children and adolescents with vitiligo, it is very common for me to feel their emotional suffering from their skin condition,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center in Dallas said in an interview. “Kids can be cruel. Name calling, social ostracizing, [and] effects on self-esteem are all things I have seen amongst my patients and their families in their own struggles with vitiligo.”
According to a brand communications representative from toymaker Mattel, which began manufacturing Barbie dolls in 1959, the company worked with a board-certified dermatologist to include a doll with vitiligo in its 2020 “Fashionistas” line. “As we continue to redefine what it means to be a ‘Barbie’ or look like Barbie, offering a doll with vitiligo in our main doll line allows kids to play out even more stories they see in the world around them,” the representative wrote in an email message. Other dolls debuting as part of the lineup include one with no hair, one with a darker skin tone that uses a gold prosthetic limb, and a Ken doll with long rooted hair (think Jeff Spicoli in “Fast Times at Ridgemont High,” but about six inches longer).
Such efforts to celebrate diversity and inclusiveness go far in helping children and young adults to embrace their skin and their own identities, said Dr. Desai, the immediate past president of the Skin of Color Society and a member of the American Academy of Dermatology board of directors. “One nuance, perhaps even more important, is that the Barbie can help to break down barriers, create awareness, and potentially even reduce bullying, stigma, and lack of knowledge about vitiligo amongst the general public who don’t understand vitiligo,” he said. “I hope the public and social media will embrace this new Barbie. Who knows? Pretty soon, vitiligo may no longer be a ‘thing’ that causes ‘stares’ and ‘glares.’ ”
Referring to the Barbie with no hair in the new line of dolls, the Mattel statement said, “ if a girl is experiencing hair loss for any reason, she can see herself reflected in the line.”
In 2019, Mattel introduced a lineup of Barbie dolls reflecting permanent disabilities, including one with a prosthetic limb. For that effort, the company collaborated with then-12-year-old Jordan Reeves, the “Born Just Right” coauthor “who is on a mission to build creative solutions that help kids with disabilities, to create a play experience that is as representative as possible,” the Mattel representative wrote.
A new line of Barbie dolls unveiled by Mattel earlier this month includes one with vitiligo, much to the delight of clinicians who treat children and adolescents with the condition.
“When I see young children and adolescents with vitiligo, it is very common for me to feel their emotional suffering from their skin condition,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center in Dallas said in an interview. “Kids can be cruel. Name calling, social ostracizing, [and] effects on self-esteem are all things I have seen amongst my patients and their families in their own struggles with vitiligo.”
According to a brand communications representative from toymaker Mattel, which began manufacturing Barbie dolls in 1959, the company worked with a board-certified dermatologist to include a doll with vitiligo in its 2020 “Fashionistas” line. “As we continue to redefine what it means to be a ‘Barbie’ or look like Barbie, offering a doll with vitiligo in our main doll line allows kids to play out even more stories they see in the world around them,” the representative wrote in an email message. Other dolls debuting as part of the lineup include one with no hair, one with a darker skin tone that uses a gold prosthetic limb, and a Ken doll with long rooted hair (think Jeff Spicoli in “Fast Times at Ridgemont High,” but about six inches longer).
Such efforts to celebrate diversity and inclusiveness go far in helping children and young adults to embrace their skin and their own identities, said Dr. Desai, the immediate past president of the Skin of Color Society and a member of the American Academy of Dermatology board of directors. “One nuance, perhaps even more important, is that the Barbie can help to break down barriers, create awareness, and potentially even reduce bullying, stigma, and lack of knowledge about vitiligo amongst the general public who don’t understand vitiligo,” he said. “I hope the public and social media will embrace this new Barbie. Who knows? Pretty soon, vitiligo may no longer be a ‘thing’ that causes ‘stares’ and ‘glares.’ ”
Referring to the Barbie with no hair in the new line of dolls, the Mattel statement said, “ if a girl is experiencing hair loss for any reason, she can see herself reflected in the line.”
In 2019, Mattel introduced a lineup of Barbie dolls reflecting permanent disabilities, including one with a prosthetic limb. For that effort, the company collaborated with then-12-year-old Jordan Reeves, the “Born Just Right” coauthor “who is on a mission to build creative solutions that help kids with disabilities, to create a play experience that is as representative as possible,” the Mattel representative wrote.
A new line of Barbie dolls unveiled by Mattel earlier this month includes one with vitiligo, much to the delight of clinicians who treat children and adolescents with the condition.
“When I see young children and adolescents with vitiligo, it is very common for me to feel their emotional suffering from their skin condition,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center in Dallas said in an interview. “Kids can be cruel. Name calling, social ostracizing, [and] effects on self-esteem are all things I have seen amongst my patients and their families in their own struggles with vitiligo.”
According to a brand communications representative from toymaker Mattel, which began manufacturing Barbie dolls in 1959, the company worked with a board-certified dermatologist to include a doll with vitiligo in its 2020 “Fashionistas” line. “As we continue to redefine what it means to be a ‘Barbie’ or look like Barbie, offering a doll with vitiligo in our main doll line allows kids to play out even more stories they see in the world around them,” the representative wrote in an email message. Other dolls debuting as part of the lineup include one with no hair, one with a darker skin tone that uses a gold prosthetic limb, and a Ken doll with long rooted hair (think Jeff Spicoli in “Fast Times at Ridgemont High,” but about six inches longer).
Such efforts to celebrate diversity and inclusiveness go far in helping children and young adults to embrace their skin and their own identities, said Dr. Desai, the immediate past president of the Skin of Color Society and a member of the American Academy of Dermatology board of directors. “One nuance, perhaps even more important, is that the Barbie can help to break down barriers, create awareness, and potentially even reduce bullying, stigma, and lack of knowledge about vitiligo amongst the general public who don’t understand vitiligo,” he said. “I hope the public and social media will embrace this new Barbie. Who knows? Pretty soon, vitiligo may no longer be a ‘thing’ that causes ‘stares’ and ‘glares.’ ”
Referring to the Barbie with no hair in the new line of dolls, the Mattel statement said, “ if a girl is experiencing hair loss for any reason, she can see herself reflected in the line.”
In 2019, Mattel introduced a lineup of Barbie dolls reflecting permanent disabilities, including one with a prosthetic limb. For that effort, the company collaborated with then-12-year-old Jordan Reeves, the “Born Just Right” coauthor “who is on a mission to build creative solutions that help kids with disabilities, to create a play experience that is as representative as possible,” the Mattel representative wrote.
Albinism awareness goes global in dermatologists’ nonprofit work
A dermatologist-led nonprofit organization has entered into a
Representatives from the New York–based NYDG Foundation, including dermatologist David Colbert, MD, recently signed the agreement with the United Nations High Commissioner for Human Rights. At the center of the inclusivity efforts is the foundation’s ColorFull campaign, which aims to shape a collective response to the discrimination and violence that individuals with albinism face around the world.
“We really need to build more inclusive and communal health care systems for all. Partnering with the United Nations will help us to reach our goals and build stronger bonds with those health care providers working with one of the most marginalized and vulnerable groups in Africa,” Dr. Colbert said in an interview.
Stylish images of individuals with albinism, including prominent model Diandra Forrest, anchor the ColorFull campaign’s messaging; Ms. Forrest is featured in a video posted by the United Nations in November announcing the joint human rights campaign. Because the consequences of albinism can be deadly serious for affected individuals in many parts of the world, awareness is desperately needed, participants in NYDG’s work and in Standing Voice, another nonprofit that provides resources for people with albinism in East Africa, emphasized in interviews.
Striving to do good work
Stephan Bognar, a seasoned leader of international nonprofits, has teamed up with Dr. Colbert, NYDG Foundation’s founding physician, to craft the international campaign to raise awareness of albinism and increase acceptance of those with the condition. “You don’t always have to stand alone to break down the walls of exclusion. The fight for social justice and human rights for persons with albinism requires a collective responsibility,” Mr. Bognar said in an interview.
Dr. Colbert, senior partner of the New York Dermatology Group, a large Manhattan-based practice, founded the nonprofit when he became involved in wound-care efforts in Haiti following the 2010 earthquake. The foundation has since supported such philanthropic efforts as helping people with albinism, offering scholarships, and raising awareness of the importance of sun protection among youth athletes.
“One day, 3 years ago or so, I was reading the New York Times and I came across this article – it was called ‘The Hunted,’ ” Dr. Colbert recalled. “It was something I knew nothing about. In Eastern Africa, people with albinism are often hunted down for body parts and their lives are at risk” from being hunted and murdered – but also because their body parts are used for witchcraft and magic, he noted.
“I was captivated by that, and I remember I called Stephan, and I said, ‘I have a project for you.’ ” Because of extensive previous work with international nongovernmental organizations and the United Nations, Mr. Bognar, who is now the executive director of the NYDG Foundation, “had the pedigree to make things happen instead of spinning our wheels,” Dr. Colbert said.
Albinism is more common by a factor of about 10 in certain sub-Saharan African populations in Tanzania and Malawi, compared with worldwide prevalence. The condition is stigmatized, but people with albinism are also believed to possess some magical powers. People with albinism are attacked, maimed, and even killed for their body parts, which are used by traditional “witch doctors” in ceremonies designed to generate wealth and good fortune. Raping a woman with albinism is thought by some to cure HIV/AIDS and infertility.
If African individuals with albinism escapes these horrors, they are still at high risk of developing a disfiguring, or even fatal, skin cancer. Even in higher-resource countries and in places farther from the equator, though, people with albinism still need stringent sun-exposure precautions and frequent dermatologic surveillance.
Philanthropic work in dermatology
Despite his busy practice, Dr. Colbert said he has found great satisfaction in pursuing philanthropic work. For physicians considering similar efforts, he said that genuine engagement with the issue is critical and global travel isn’t necessary to make a real difference.
“I think that, first, this should be something that you’re interested in and that you have the means to make some impact,” Dr. Colbert said. “Doing something doesn’t need to be a global campaign. You don’t need to have a home run – every little thing counts. Catching one squamous cell cancer on one patient with albinism makes a difference. But if you want to go bigger, you have to look at your community and see who has the resources and who might also be interested” in a cause you’re passionate about.
He added that a busy physician shouldn’t expect to do it all. “You have to find the right partner because we as physicians are taking care of our patients and paying the rent, so taking on a partner who is trained to do that can ... help you achieve what you envision.”
Though the NYDG Foundation has funded trips to Africa and participates in teledermatology there, Dr. Colbert said that the awareness campaign the NYDG is cosponsoring with the United Nations is of fundamental importance as well. “This is a really great example of the positive impact that social media can have on our society – in a good way, instead of a negative or self-serving way,” he said.
“I think that the ColorFull campaign will normalize the idea of people who are living without melanin in their skin. It keeps it out of the realm of ‘Don’t say anything.’ People don’t know what it means, so if we bring out the science, and show successful people who have normal lives, who have children, and we explain what it is, it demystifies it – and everybody wins. ... We’re all just people, no matter how many melanin granules we have.”
Dr. Colbert reported that he has no relevant conflicts of interest.
Standing Voice also provides resources in East Africa
The work of other nongovernmental organizations is also making a difference for people in East Africa with albinism.
Standing Voice is a United Kingdom–based nonprofit that provides education and resources that include sunscreen, as well as assessment and treatment of skin conditions for people with albinism in Tanzania and Malawi.
This and other work by Standing Voice were on display in an exhibit at the World Congress of Dermatology meeting in Milan in June 2019. In an interview at the meeting, Dr. Sharp, who spent his childhood in East Africa, contrasted access to dermatology care in the United States and United Kingdom with that in Africa, where an entire country may have hardly more than a few dermatologists.
“I go about three times a year, for about a week,” explained Dr. Sharp. “I’ll do a workshop to teach basic skin surgery techniques – excisions and biopsies. Very simple stuff. I’ll teach skin grafting as well because some of these patients have large lesions that won’t close directly,” he said. “On the whole, we like to use good grafts, rather than flaps, because often a local flap is just moving sun-damaged skin.”

Many patients have to travel great distances to reach a facility where general anesthesia and a full operating room suite are available, resources that are in high demand in resource-restricted African nations, according to Dr. Sharp. Teaching African practitioners regional anesthesia techniques that can be used for skin cancer surgery also helps ensure that more patients with albinism and squamous cell carcinoma can be treated – and treated closer to home.

Dr. Sharp reported that he has no relevant conflicts of interest.
A dermatologist-led nonprofit organization has entered into a
Representatives from the New York–based NYDG Foundation, including dermatologist David Colbert, MD, recently signed the agreement with the United Nations High Commissioner for Human Rights. At the center of the inclusivity efforts is the foundation’s ColorFull campaign, which aims to shape a collective response to the discrimination and violence that individuals with albinism face around the world.
“We really need to build more inclusive and communal health care systems for all. Partnering with the United Nations will help us to reach our goals and build stronger bonds with those health care providers working with one of the most marginalized and vulnerable groups in Africa,” Dr. Colbert said in an interview.
Stylish images of individuals with albinism, including prominent model Diandra Forrest, anchor the ColorFull campaign’s messaging; Ms. Forrest is featured in a video posted by the United Nations in November announcing the joint human rights campaign. Because the consequences of albinism can be deadly serious for affected individuals in many parts of the world, awareness is desperately needed, participants in NYDG’s work and in Standing Voice, another nonprofit that provides resources for people with albinism in East Africa, emphasized in interviews.
Striving to do good work
Stephan Bognar, a seasoned leader of international nonprofits, has teamed up with Dr. Colbert, NYDG Foundation’s founding physician, to craft the international campaign to raise awareness of albinism and increase acceptance of those with the condition. “You don’t always have to stand alone to break down the walls of exclusion. The fight for social justice and human rights for persons with albinism requires a collective responsibility,” Mr. Bognar said in an interview.
Dr. Colbert, senior partner of the New York Dermatology Group, a large Manhattan-based practice, founded the nonprofit when he became involved in wound-care efforts in Haiti following the 2010 earthquake. The foundation has since supported such philanthropic efforts as helping people with albinism, offering scholarships, and raising awareness of the importance of sun protection among youth athletes.
“One day, 3 years ago or so, I was reading the New York Times and I came across this article – it was called ‘The Hunted,’ ” Dr. Colbert recalled. “It was something I knew nothing about. In Eastern Africa, people with albinism are often hunted down for body parts and their lives are at risk” from being hunted and murdered – but also because their body parts are used for witchcraft and magic, he noted.
“I was captivated by that, and I remember I called Stephan, and I said, ‘I have a project for you.’ ” Because of extensive previous work with international nongovernmental organizations and the United Nations, Mr. Bognar, who is now the executive director of the NYDG Foundation, “had the pedigree to make things happen instead of spinning our wheels,” Dr. Colbert said.
Albinism is more common by a factor of about 10 in certain sub-Saharan African populations in Tanzania and Malawi, compared with worldwide prevalence. The condition is stigmatized, but people with albinism are also believed to possess some magical powers. People with albinism are attacked, maimed, and even killed for their body parts, which are used by traditional “witch doctors” in ceremonies designed to generate wealth and good fortune. Raping a woman with albinism is thought by some to cure HIV/AIDS and infertility.
If African individuals with albinism escapes these horrors, they are still at high risk of developing a disfiguring, or even fatal, skin cancer. Even in higher-resource countries and in places farther from the equator, though, people with albinism still need stringent sun-exposure precautions and frequent dermatologic surveillance.
Philanthropic work in dermatology
Despite his busy practice, Dr. Colbert said he has found great satisfaction in pursuing philanthropic work. For physicians considering similar efforts, he said that genuine engagement with the issue is critical and global travel isn’t necessary to make a real difference.
“I think that, first, this should be something that you’re interested in and that you have the means to make some impact,” Dr. Colbert said. “Doing something doesn’t need to be a global campaign. You don’t need to have a home run – every little thing counts. Catching one squamous cell cancer on one patient with albinism makes a difference. But if you want to go bigger, you have to look at your community and see who has the resources and who might also be interested” in a cause you’re passionate about.
He added that a busy physician shouldn’t expect to do it all. “You have to find the right partner because we as physicians are taking care of our patients and paying the rent, so taking on a partner who is trained to do that can ... help you achieve what you envision.”
Though the NYDG Foundation has funded trips to Africa and participates in teledermatology there, Dr. Colbert said that the awareness campaign the NYDG is cosponsoring with the United Nations is of fundamental importance as well. “This is a really great example of the positive impact that social media can have on our society – in a good way, instead of a negative or self-serving way,” he said.
“I think that the ColorFull campaign will normalize the idea of people who are living without melanin in their skin. It keeps it out of the realm of ‘Don’t say anything.’ People don’t know what it means, so if we bring out the science, and show successful people who have normal lives, who have children, and we explain what it is, it demystifies it – and everybody wins. ... We’re all just people, no matter how many melanin granules we have.”
Dr. Colbert reported that he has no relevant conflicts of interest.
Standing Voice also provides resources in East Africa
The work of other nongovernmental organizations is also making a difference for people in East Africa with albinism.
Standing Voice is a United Kingdom–based nonprofit that provides education and resources that include sunscreen, as well as assessment and treatment of skin conditions for people with albinism in Tanzania and Malawi.

This and other work by Standing Voice were on display in an exhibit at the World Congress of Dermatology meeting in Milan in June 2019. In an interview at the meeting, Dr. Sharp, who spent his childhood in East Africa, contrasted access to dermatology care in the United States and United Kingdom with that in Africa, where an entire country may have hardly more than a few dermatologists.

“I go about three times a year, for about a week,” explained Dr. Sharp. “I’ll do a workshop to teach basic skin surgery techniques – excisions and biopsies. Very simple stuff. I’ll teach skin grafting as well because some of these patients have large lesions that won’t close directly,” he said. “On the whole, we like to use good grafts, rather than flaps, because often a local flap is just moving sun-damaged skin.”

Many patients have to travel great distances to reach a facility where general anesthesia and a full operating room suite are available, resources that are in high demand in resource-restricted African nations, according to Dr. Sharp. Teaching African practitioners regional anesthesia techniques that can be used for skin cancer surgery also helps ensure that more patients with albinism and squamous cell carcinoma can be treated – and treated closer to home.

Dr. Sharp reported that he has no relevant conflicts of interest.
A dermatologist-led nonprofit organization has entered into a
Representatives from the New York–based NYDG Foundation, including dermatologist David Colbert, MD, recently signed the agreement with the United Nations High Commissioner for Human Rights. At the center of the inclusivity efforts is the foundation’s ColorFull campaign, which aims to shape a collective response to the discrimination and violence that individuals with albinism face around the world.
“We really need to build more inclusive and communal health care systems for all. Partnering with the United Nations will help us to reach our goals and build stronger bonds with those health care providers working with one of the most marginalized and vulnerable groups in Africa,” Dr. Colbert said in an interview.
Stylish images of individuals with albinism, including prominent model Diandra Forrest, anchor the ColorFull campaign’s messaging; Ms. Forrest is featured in a video posted by the United Nations in November announcing the joint human rights campaign. Because the consequences of albinism can be deadly serious for affected individuals in many parts of the world, awareness is desperately needed, participants in NYDG’s work and in Standing Voice, another nonprofit that provides resources for people with albinism in East Africa, emphasized in interviews.
Striving to do good work
Stephan Bognar, a seasoned leader of international nonprofits, has teamed up with Dr. Colbert, NYDG Foundation’s founding physician, to craft the international campaign to raise awareness of albinism and increase acceptance of those with the condition. “You don’t always have to stand alone to break down the walls of exclusion. The fight for social justice and human rights for persons with albinism requires a collective responsibility,” Mr. Bognar said in an interview.
Dr. Colbert, senior partner of the New York Dermatology Group, a large Manhattan-based practice, founded the nonprofit when he became involved in wound-care efforts in Haiti following the 2010 earthquake. The foundation has since supported such philanthropic efforts as helping people with albinism, offering scholarships, and raising awareness of the importance of sun protection among youth athletes.
“One day, 3 years ago or so, I was reading the New York Times and I came across this article – it was called ‘The Hunted,’ ” Dr. Colbert recalled. “It was something I knew nothing about. In Eastern Africa, people with albinism are often hunted down for body parts and their lives are at risk” from being hunted and murdered – but also because their body parts are used for witchcraft and magic, he noted.
“I was captivated by that, and I remember I called Stephan, and I said, ‘I have a project for you.’ ” Because of extensive previous work with international nongovernmental organizations and the United Nations, Mr. Bognar, who is now the executive director of the NYDG Foundation, “had the pedigree to make things happen instead of spinning our wheels,” Dr. Colbert said.
Albinism is more common by a factor of about 10 in certain sub-Saharan African populations in Tanzania and Malawi, compared with worldwide prevalence. The condition is stigmatized, but people with albinism are also believed to possess some magical powers. People with albinism are attacked, maimed, and even killed for their body parts, which are used by traditional “witch doctors” in ceremonies designed to generate wealth and good fortune. Raping a woman with albinism is thought by some to cure HIV/AIDS and infertility.
If African individuals with albinism escapes these horrors, they are still at high risk of developing a disfiguring, or even fatal, skin cancer. Even in higher-resource countries and in places farther from the equator, though, people with albinism still need stringent sun-exposure precautions and frequent dermatologic surveillance.
Philanthropic work in dermatology
Despite his busy practice, Dr. Colbert said he has found great satisfaction in pursuing philanthropic work. For physicians considering similar efforts, he said that genuine engagement with the issue is critical and global travel isn’t necessary to make a real difference.
“I think that, first, this should be something that you’re interested in and that you have the means to make some impact,” Dr. Colbert said. “Doing something doesn’t need to be a global campaign. You don’t need to have a home run – every little thing counts. Catching one squamous cell cancer on one patient with albinism makes a difference. But if you want to go bigger, you have to look at your community and see who has the resources and who might also be interested” in a cause you’re passionate about.
He added that a busy physician shouldn’t expect to do it all. “You have to find the right partner because we as physicians are taking care of our patients and paying the rent, so taking on a partner who is trained to do that can ... help you achieve what you envision.”
Though the NYDG Foundation has funded trips to Africa and participates in teledermatology there, Dr. Colbert said that the awareness campaign the NYDG is cosponsoring with the United Nations is of fundamental importance as well. “This is a really great example of the positive impact that social media can have on our society – in a good way, instead of a negative or self-serving way,” he said.
“I think that the ColorFull campaign will normalize the idea of people who are living without melanin in their skin. It keeps it out of the realm of ‘Don’t say anything.’ People don’t know what it means, so if we bring out the science, and show successful people who have normal lives, who have children, and we explain what it is, it demystifies it – and everybody wins. ... We’re all just people, no matter how many melanin granules we have.”
Dr. Colbert reported that he has no relevant conflicts of interest.
Standing Voice also provides resources in East Africa
The work of other nongovernmental organizations is also making a difference for people in East Africa with albinism.
Standing Voice is a United Kingdom–based nonprofit that provides education and resources that include sunscreen, as well as assessment and treatment of skin conditions for people with albinism in Tanzania and Malawi.

This and other work by Standing Voice were on display in an exhibit at the World Congress of Dermatology meeting in Milan in June 2019. In an interview at the meeting, Dr. Sharp, who spent his childhood in East Africa, contrasted access to dermatology care in the United States and United Kingdom with that in Africa, where an entire country may have hardly more than a few dermatologists.

“I go about three times a year, for about a week,” explained Dr. Sharp. “I’ll do a workshop to teach basic skin surgery techniques – excisions and biopsies. Very simple stuff. I’ll teach skin grafting as well because some of these patients have large lesions that won’t close directly,” he said. “On the whole, we like to use good grafts, rather than flaps, because often a local flap is just moving sun-damaged skin.”

Many patients have to travel great distances to reach a facility where general anesthesia and a full operating room suite are available, resources that are in high demand in resource-restricted African nations, according to Dr. Sharp. Teaching African practitioners regional anesthesia techniques that can be used for skin cancer surgery also helps ensure that more patients with albinism and squamous cell carcinoma can be treated – and treated closer to home.

Dr. Sharp reported that he has no relevant conflicts of interest.
Nonablative laser improved PIH in patients with darker skin
Yoon‐Soo Cindy Bae, MD, and colleagues reported.
Among patients treated with the nonablative fractional 1,927 nm laser, there was a mean improvement of about 43% in hyperpigmented areas, and no side effects were reported, wrote Dr. Bae, of the department of dermatology at New York University and the Laser & Skin Surgery Center of New York, and coauthors in Lasers in Surgery and Medicine.
Lasers have not been the first choice for hyperpigmentation in Fitzpatrick skin types IV, V, and VI, they pointed out. More commonly used treatments are hydroquinone and chemical peels that use glycolic acid or salicylic acid. But these are not always ideal options, Dr. Bae said in an interview.
“There are side effects to medical therapy. The drawbacks of medical therapy include compliance issues, risk of skin irritation from the product ... and a risk of hyperpigmentation specifically for hydroquinone. There are also risks to laser therapy, including dyspigmentation and scarring,” she added. “However, the laser we used is a low energy, nonablative type of laser, so the risk of scarring is extremely rare and the dyspigmentation is actually what we are aiming to treat.”
The retrospective study comprised 61 patients with PIH who had received more than one treatment with the low energy fractionated 1,927 nm diode laser between 2013 and 2016. Most were Fitzpatrick type IV (73.8%). The remainder were Type V (16.4%) and Type VI (9.8%). The most common treatment site was the face or cheeks (68.9%), followed by legs (13%), the rest of the cases were unspecified.
Patients had received treatment with the laser with fixed fluence at 5 mJ, fixed spot size of 140 micrometers, depth of 170 micrometers, and 5% coverage. They required several treatments: 15 had two, 14 had three, 16 had four, and the remainder had five or more. Topical treatment data were not collected. Photographs taken before treatment and before the last treatment were evaluated by dermatologists who had not treated the patients. Based on those evaluations, the mean improvement was a statistically significant 43.2%.
There did not, however, appear to be much difference between the treatment groups. The mean improvement among patients with two treatments was 44.5%; three treatments, 44.29%; four treatments, 40.63%; five or more treatments, 43.75%.
Although those with darker skin types tended to have better results, there were no statistically significant differences between the skin-type groups. Among those with Fitzpatrick skin type IV, the mean improvement was 40.39%; skin type V, 47.25%; and skin type VI, 57.92%.
“The fact that there was no correlation between Fitzpatrick skin type … and average percent improvement demonstrates that this laser is a viable treatment option for patients with very dark skin,” the authors wrote. “There were also no significant differences between the average percent improvements for people receiving different numbers of treatments. A trend was observed that favored treating patients with darker skin type; however, this lacked statistical significance. This may have been due to an underpowered study.”
Limitations of the study included the retrospective design and nonstandardization of photographs; “further studies with prospective controlled designs are needed to confirm our findings,” they added.
No funding or disclosure information was provided.
[email protected]
SOURCE: Bae YS et al. Lasers Surg Med. 2019 Oct 29. doi: 10.1002/lsm.23173.
Yoon‐Soo Cindy Bae, MD, and colleagues reported.
Among patients treated with the nonablative fractional 1,927 nm laser, there was a mean improvement of about 43% in hyperpigmented areas, and no side effects were reported, wrote Dr. Bae, of the department of dermatology at New York University and the Laser & Skin Surgery Center of New York, and coauthors in Lasers in Surgery and Medicine.
Lasers have not been the first choice for hyperpigmentation in Fitzpatrick skin types IV, V, and VI, they pointed out. More commonly used treatments are hydroquinone and chemical peels that use glycolic acid or salicylic acid. But these are not always ideal options, Dr. Bae said in an interview.
“There are side effects to medical therapy. The drawbacks of medical therapy include compliance issues, risk of skin irritation from the product ... and a risk of hyperpigmentation specifically for hydroquinone. There are also risks to laser therapy, including dyspigmentation and scarring,” she added. “However, the laser we used is a low energy, nonablative type of laser, so the risk of scarring is extremely rare and the dyspigmentation is actually what we are aiming to treat.”
The retrospective study comprised 61 patients with PIH who had received more than one treatment with the low energy fractionated 1,927 nm diode laser between 2013 and 2016. Most were Fitzpatrick type IV (73.8%). The remainder were Type V (16.4%) and Type VI (9.8%). The most common treatment site was the face or cheeks (68.9%), followed by legs (13%), the rest of the cases were unspecified.
Patients had received treatment with the laser with fixed fluence at 5 mJ, fixed spot size of 140 micrometers, depth of 170 micrometers, and 5% coverage. They required several treatments: 15 had two, 14 had three, 16 had four, and the remainder had five or more. Topical treatment data were not collected. Photographs taken before treatment and before the last treatment were evaluated by dermatologists who had not treated the patients. Based on those evaluations, the mean improvement was a statistically significant 43.2%.
There did not, however, appear to be much difference between the treatment groups. The mean improvement among patients with two treatments was 44.5%; three treatments, 44.29%; four treatments, 40.63%; five or more treatments, 43.75%.
Although those with darker skin types tended to have better results, there were no statistically significant differences between the skin-type groups. Among those with Fitzpatrick skin type IV, the mean improvement was 40.39%; skin type V, 47.25%; and skin type VI, 57.92%.
“The fact that there was no correlation between Fitzpatrick skin type … and average percent improvement demonstrates that this laser is a viable treatment option for patients with very dark skin,” the authors wrote. “There were also no significant differences between the average percent improvements for people receiving different numbers of treatments. A trend was observed that favored treating patients with darker skin type; however, this lacked statistical significance. This may have been due to an underpowered study.”
Limitations of the study included the retrospective design and nonstandardization of photographs; “further studies with prospective controlled designs are needed to confirm our findings,” they added.
No funding or disclosure information was provided.
[email protected]
SOURCE: Bae YS et al. Lasers Surg Med. 2019 Oct 29. doi: 10.1002/lsm.23173.
Yoon‐Soo Cindy Bae, MD, and colleagues reported.
Among patients treated with the nonablative fractional 1,927 nm laser, there was a mean improvement of about 43% in hyperpigmented areas, and no side effects were reported, wrote Dr. Bae, of the department of dermatology at New York University and the Laser & Skin Surgery Center of New York, and coauthors in Lasers in Surgery and Medicine.
Lasers have not been the first choice for hyperpigmentation in Fitzpatrick skin types IV, V, and VI, they pointed out. More commonly used treatments are hydroquinone and chemical peels that use glycolic acid or salicylic acid. But these are not always ideal options, Dr. Bae said in an interview.
“There are side effects to medical therapy. The drawbacks of medical therapy include compliance issues, risk of skin irritation from the product ... and a risk of hyperpigmentation specifically for hydroquinone. There are also risks to laser therapy, including dyspigmentation and scarring,” she added. “However, the laser we used is a low energy, nonablative type of laser, so the risk of scarring is extremely rare and the dyspigmentation is actually what we are aiming to treat.”
The retrospective study comprised 61 patients with PIH who had received more than one treatment with the low energy fractionated 1,927 nm diode laser between 2013 and 2016. Most were Fitzpatrick type IV (73.8%). The remainder were Type V (16.4%) and Type VI (9.8%). The most common treatment site was the face or cheeks (68.9%), followed by legs (13%), the rest of the cases were unspecified.
Patients had received treatment with the laser with fixed fluence at 5 mJ, fixed spot size of 140 micrometers, depth of 170 micrometers, and 5% coverage. They required several treatments: 15 had two, 14 had three, 16 had four, and the remainder had five or more. Topical treatment data were not collected. Photographs taken before treatment and before the last treatment were evaluated by dermatologists who had not treated the patients. Based on those evaluations, the mean improvement was a statistically significant 43.2%.
There did not, however, appear to be much difference between the treatment groups. The mean improvement among patients with two treatments was 44.5%; three treatments, 44.29%; four treatments, 40.63%; five or more treatments, 43.75%.
Although those with darker skin types tended to have better results, there were no statistically significant differences between the skin-type groups. Among those with Fitzpatrick skin type IV, the mean improvement was 40.39%; skin type V, 47.25%; and skin type VI, 57.92%.
“The fact that there was no correlation between Fitzpatrick skin type … and average percent improvement demonstrates that this laser is a viable treatment option for patients with very dark skin,” the authors wrote. “There were also no significant differences between the average percent improvements for people receiving different numbers of treatments. A trend was observed that favored treating patients with darker skin type; however, this lacked statistical significance. This may have been due to an underpowered study.”
Limitations of the study included the retrospective design and nonstandardization of photographs; “further studies with prospective controlled designs are needed to confirm our findings,” they added.
No funding or disclosure information was provided.
[email protected]
SOURCE: Bae YS et al. Lasers Surg Med. 2019 Oct 29. doi: 10.1002/lsm.23173.
FROM LASERS IN SURGERY AND MEDICINE
Vitiligo: First-ever RCT is smashing success
MADRID – cream for the treatment of vitiligo, Amit G. Pandya, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“I have been waiting 30 years for the first clinical trial for vitiligo. I know many of you dermatologists have been waiting for something for vitiligo, so I’m happy to present the results of the first randomized, placebo-controlled, double-blind, prospective trial of a topical agent in history for vitiligo,” said Dr. Pandya, who was clearly overjoyed to present the final results of the 52-week trial.
Ruxolitinib is a Janus kinase (JAK) 1 and 2 inhibitor. Topical ruxolitinib is under study for vitiligo because this chronic autoimmune disease targeting melanocytes is now recognized as being driven by signaling through the JAK 1/2 pathways.
The interim 24-week results of the phase 2 trial, presented earlier in the year at the World Congress of Dermatology in Milan, showed significant repigmentation with ruxolitinib cream. Dr. Pandya’s key message at EADV 2019 was that continued treatment out to a year brought substantial further improvement, and with a benign safety profile indistinguishable from vehicle control.
“We see a tremendous difference between 6 months and 1 year,” said Dr. Pandya, professor of dermatology at the University of Texas, Dallas. “For the first time, we dare talk about F-VASI75 [Facial Vitiligo Area Scoring Index] and F-VASI90 responses. We don’t usually tell patients that they can get 75% or 90% of their color back, and yet the week-52 F-VASI75 rate was 51.5%, up from 30.3% at week 24. And the F-VASI90 response was 33.3%, versus 12.1% at week 24.”
F-VASI is measured using the patient’s hand, which is typically equivalent to about 1% of body surface area. The mean baseline F-VASI was 1.26% in this study of 157 mostly middle-aged adults with longstanding vitiligo of a mean 14-year duration. That’s fairly severe vitiligo, since the total face occupies only about 4% of total body surface area.
The primary study endpoint was achievement of greater than 50% repigmentation in the F-VASI, or an F-VASI50 response. Under double-blind conditions at 52 weeks in the group randomized to 1.5% ruxolitinib cream twice a day, the highest dose used in the trial, the F-VASI50 rate was 57.6%. That’s up from a week-24 F-VASI50 of 45.5%, and a week-34 response rate of 51.5%.
A key secondary endpoint was T-VASI50, reflecting the total body response.
“Patients don’t just want their face to be better, they want their chest, arms, elbows, knees, hands, and feet to be better,” the dermatologist commented.
The week-52 T-VASI50 rate was 36.4%, up substantially from 12.1% at week 24. And that week-52 T-VASI50 rate probably underestimates the full potential benefit. That’s because a safety-based study rule prohibited patients from applying the cream to more than 20% of their body surface area. Adverse effects reported for oral ruxolitinib, approved for treatment of myelofibrosis, polycythemia vera, and acute graft-versus-host disease, include thrombocytopenia and anemia.
“In this early study we didn’t want to take a chance of systemic absorption with serum levels that would potentially affect the bone marrow,” Dr. Pandya explained.
He noted that 57 study participants had a baseline T-VASI greater than 20% of their body surface area and thus weren’t able to treat all of their disease. In the 100 patients with a vitiligo-involved total body surface area of 20% or less, however, the week-52 T-VASI50 reached 45%, compared with 20% at week 24.
Another prespecified secondary endpoint was the proportion of patients who received a facial physician’s global assessment of clear or almost clear. About 21% of patients in the highest-dose group achieved this milestone at 52 weeks.
A phase 3, randomized, controlled trial of ruxolitinib cream is ongoing and should be completed next year. Dr. Pandya reported receiving research funding from and serving as a consultant to Incyte, the study sponsor. He has similar financial relationships with Pfizer, Aclaris Therapeutics, and the Immune Tolerance Network.
MADRID – cream for the treatment of vitiligo, Amit G. Pandya, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“I have been waiting 30 years for the first clinical trial for vitiligo. I know many of you dermatologists have been waiting for something for vitiligo, so I’m happy to present the results of the first randomized, placebo-controlled, double-blind, prospective trial of a topical agent in history for vitiligo,” said Dr. Pandya, who was clearly overjoyed to present the final results of the 52-week trial.
Ruxolitinib is a Janus kinase (JAK) 1 and 2 inhibitor. Topical ruxolitinib is under study for vitiligo because this chronic autoimmune disease targeting melanocytes is now recognized as being driven by signaling through the JAK 1/2 pathways.
The interim 24-week results of the phase 2 trial, presented earlier in the year at the World Congress of Dermatology in Milan, showed significant repigmentation with ruxolitinib cream. Dr. Pandya’s key message at EADV 2019 was that continued treatment out to a year brought substantial further improvement, and with a benign safety profile indistinguishable from vehicle control.
“We see a tremendous difference between 6 months and 1 year,” said Dr. Pandya, professor of dermatology at the University of Texas, Dallas. “For the first time, we dare talk about F-VASI75 [Facial Vitiligo Area Scoring Index] and F-VASI90 responses. We don’t usually tell patients that they can get 75% or 90% of their color back, and yet the week-52 F-VASI75 rate was 51.5%, up from 30.3% at week 24. And the F-VASI90 response was 33.3%, versus 12.1% at week 24.”
F-VASI is measured using the patient’s hand, which is typically equivalent to about 1% of body surface area. The mean baseline F-VASI was 1.26% in this study of 157 mostly middle-aged adults with longstanding vitiligo of a mean 14-year duration. That’s fairly severe vitiligo, since the total face occupies only about 4% of total body surface area.
The primary study endpoint was achievement of greater than 50% repigmentation in the F-VASI, or an F-VASI50 response. Under double-blind conditions at 52 weeks in the group randomized to 1.5% ruxolitinib cream twice a day, the highest dose used in the trial, the F-VASI50 rate was 57.6%. That’s up from a week-24 F-VASI50 of 45.5%, and a week-34 response rate of 51.5%.
A key secondary endpoint was T-VASI50, reflecting the total body response.
“Patients don’t just want their face to be better, they want their chest, arms, elbows, knees, hands, and feet to be better,” the dermatologist commented.
The week-52 T-VASI50 rate was 36.4%, up substantially from 12.1% at week 24. And that week-52 T-VASI50 rate probably underestimates the full potential benefit. That’s because a safety-based study rule prohibited patients from applying the cream to more than 20% of their body surface area. Adverse effects reported for oral ruxolitinib, approved for treatment of myelofibrosis, polycythemia vera, and acute graft-versus-host disease, include thrombocytopenia and anemia.
“In this early study we didn’t want to take a chance of systemic absorption with serum levels that would potentially affect the bone marrow,” Dr. Pandya explained.
He noted that 57 study participants had a baseline T-VASI greater than 20% of their body surface area and thus weren’t able to treat all of their disease. In the 100 patients with a vitiligo-involved total body surface area of 20% or less, however, the week-52 T-VASI50 reached 45%, compared with 20% at week 24.
Another prespecified secondary endpoint was the proportion of patients who received a facial physician’s global assessment of clear or almost clear. About 21% of patients in the highest-dose group achieved this milestone at 52 weeks.
A phase 3, randomized, controlled trial of ruxolitinib cream is ongoing and should be completed next year. Dr. Pandya reported receiving research funding from and serving as a consultant to Incyte, the study sponsor. He has similar financial relationships with Pfizer, Aclaris Therapeutics, and the Immune Tolerance Network.
MADRID – cream for the treatment of vitiligo, Amit G. Pandya, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“I have been waiting 30 years for the first clinical trial for vitiligo. I know many of you dermatologists have been waiting for something for vitiligo, so I’m happy to present the results of the first randomized, placebo-controlled, double-blind, prospective trial of a topical agent in history for vitiligo,” said Dr. Pandya, who was clearly overjoyed to present the final results of the 52-week trial.
Ruxolitinib is a Janus kinase (JAK) 1 and 2 inhibitor. Topical ruxolitinib is under study for vitiligo because this chronic autoimmune disease targeting melanocytes is now recognized as being driven by signaling through the JAK 1/2 pathways.
The interim 24-week results of the phase 2 trial, presented earlier in the year at the World Congress of Dermatology in Milan, showed significant repigmentation with ruxolitinib cream. Dr. Pandya’s key message at EADV 2019 was that continued treatment out to a year brought substantial further improvement, and with a benign safety profile indistinguishable from vehicle control.
“We see a tremendous difference between 6 months and 1 year,” said Dr. Pandya, professor of dermatology at the University of Texas, Dallas. “For the first time, we dare talk about F-VASI75 [Facial Vitiligo Area Scoring Index] and F-VASI90 responses. We don’t usually tell patients that they can get 75% or 90% of their color back, and yet the week-52 F-VASI75 rate was 51.5%, up from 30.3% at week 24. And the F-VASI90 response was 33.3%, versus 12.1% at week 24.”
F-VASI is measured using the patient’s hand, which is typically equivalent to about 1% of body surface area. The mean baseline F-VASI was 1.26% in this study of 157 mostly middle-aged adults with longstanding vitiligo of a mean 14-year duration. That’s fairly severe vitiligo, since the total face occupies only about 4% of total body surface area.
The primary study endpoint was achievement of greater than 50% repigmentation in the F-VASI, or an F-VASI50 response. Under double-blind conditions at 52 weeks in the group randomized to 1.5% ruxolitinib cream twice a day, the highest dose used in the trial, the F-VASI50 rate was 57.6%. That’s up from a week-24 F-VASI50 of 45.5%, and a week-34 response rate of 51.5%.
A key secondary endpoint was T-VASI50, reflecting the total body response.
“Patients don’t just want their face to be better, they want their chest, arms, elbows, knees, hands, and feet to be better,” the dermatologist commented.
The week-52 T-VASI50 rate was 36.4%, up substantially from 12.1% at week 24. And that week-52 T-VASI50 rate probably underestimates the full potential benefit. That’s because a safety-based study rule prohibited patients from applying the cream to more than 20% of their body surface area. Adverse effects reported for oral ruxolitinib, approved for treatment of myelofibrosis, polycythemia vera, and acute graft-versus-host disease, include thrombocytopenia and anemia.
“In this early study we didn’t want to take a chance of systemic absorption with serum levels that would potentially affect the bone marrow,” Dr. Pandya explained.
He noted that 57 study participants had a baseline T-VASI greater than 20% of their body surface area and thus weren’t able to treat all of their disease. In the 100 patients with a vitiligo-involved total body surface area of 20% or less, however, the week-52 T-VASI50 reached 45%, compared with 20% at week 24.
Another prespecified secondary endpoint was the proportion of patients who received a facial physician’s global assessment of clear or almost clear. About 21% of patients in the highest-dose group achieved this milestone at 52 weeks.
A phase 3, randomized, controlled trial of ruxolitinib cream is ongoing and should be completed next year. Dr. Pandya reported receiving research funding from and serving as a consultant to Incyte, the study sponsor. He has similar financial relationships with Pfizer, Aclaris Therapeutics, and the Immune Tolerance Network.
REPORTING FROM THE EADV CONGRESS
Streaked Discoloration on the Upper Body
The Diagnosis: Bleomycin-Induced Flagellate Hyperpigmentation
Histopathology of the affected skin demonstrated a slight increase in collagen bundle thickness, a chronic dermal perivascular inflammation, and associated pigment incontinence with dermal melanophages compared to unaffected skin (Figure). CD34 was faintly decreased, and dermal mucin increased in affected skin. This postinflammatory pigmentary alteration with subtle dermal sclerosis had persisted unchanged for more than 5 years after cessation of bleomycin therapy. Topical hydroquinone, physical blocker photoprotection, and laser modalities such as the Q-switched alexandrite (755-nm)/Nd:YAG (1064-nm) and ablative CO2 resurfacing lasers were attempted with minimal overall impact on cosmesis.

Bleomycin is a chemotherapeutic antibiotic that has been commonly used to treat Hodgkin lymphoma, germ cell tumors, and recurrent malignant pleural effusions.1 The drug is inactivated in most tissues by the enzyme bleomycin hydrolase. This enzyme is not present in skin and lung tissue; as a result, these organs are the most common sites of bleomycin toxicity.1 There are a variety of cutaneous effects associated with bleomycin including alopecia, hyperpigmentation, acral erythema, Raynaud phenomenon, and nail dystrophy.2 Flagellate hyperpigmentation is a less common cutaneous toxicity. It is an unusual eruption that appears as whiplike linear streaks on the upper chest and back, limbs, and flanks.3 This cutaneous manifestation was once thought to be specific to bleomycin use; however, it also has been described in dermatomyositis, adult-onset Still disease, and after the ingestion of uncooked or undercooked shiitake mushrooms.4 Flagellate hyperpigmentation also was once thought to be dose dependent; however, it has been described in even very small doses.5 The eruption has been described as independent of the route of drug administration, appearing with intravenous, subcutaneous, and intramuscular bleomycin.2 The association of bleomycin and flagellate hyperpigmentation has been reported since 1970; however, it is less commonly seen in clinical practice with the declining use of bleomycin.1
The exact mechanism for the hyperpigmentation is unknown. It has been proposed that the linear lesions are related to areas of pruritus and subsequent excoriations.1 Dermatographism may be present to a limited extent, but it is unlikely to be a chief cause of flagellate hyperpigmentation, as linear streaks have been reported in the absence of trauma. It also has been proposed that bleomycin has a direct toxic effect on the melanocytes, which stimulates increased melanin secretion.2 The hyperpigmentation also may be due to pigmentary incontinence secondary to inflammation.5 Histopathologic findings usually are varied and nonspecific.2 There may be a deep perivascular lymphocytic infiltrate, which is nonspecific but can be associated with drug-induced pathology.4 Bleomycin also is used to induce localized scleroderma in mouse-model research6 and has been reported to cause localized scleroderma at an infusion site or after an intralesional injection,7,8 which is not typically reported in flagellate erythema, but bleomycin's sclerosing effects may have played a role in the visible and sclerosing atrophy noted in our patient. Yamamoto et al9 reported a similar case of dermal sclerosis induced by bleomycin.
Flagellate hyperpigmentation typically lasts for up to 6 months.3 Patients with cutaneous manifestations from bleomycin therapy usually respond to steroid therapy and discontinuation of the drug. Bleomycin re-exposure should be avoided, as it may cause extension or widespread recurrence of flagellate hyperpigmentation.3 Postinflammatory pigment alteration may persist in patients with darker skin types and in patients with dramatic inciting inflammation.
Atrophoderma of Pasini and Pierini is a form of dermal atrophy that presents with 1 or more sharply demarcated depressed patches. There is some debate whether it is a distinct entity or a primary atrophic morphea.10 Linear atrophoderma of Moulin has a similar morphology with hyperpigmented depressions and "cliff-drop" borders, but these lesions follow the lines of Blaschko.11 Linear morphea initially can present as a linear erythematous streak but more commonly appears as a plaque-type morphea lesion that forms a scarlike band.12 Erythema dyschromicum perstans is an ashy dermatosis characterized by gray or blue-brown macules seen in Fitzpatrick skin types III through V and typically is chronic and progressive.13
- Lee HY, Lim KH, Ryu Y, et al. Bleomycininduced flagellate erythema: a case report and review of the literature. Oncol Lett. 2014;8:933-935.
- Simpson RC, Da Forno P, Nagarajan C, et al. A pruritic rash in a patient with Hodgkin lymphoma. Clin Exp Dermatol. 2011;36:680-682.
- Fyfe AJ, McKay P. Toxicities associated with bleomycin. J R Coll Physicians Edinb. 2010;40:213-215.
- Lu CC, Lu YY, Wang QR, et al. Bleomycin-induced flagellate erythema. Balkan Med J. 2014;31:189-190.
- Abess A, Keel DM, Graham BS. Flagellate hyperpigmentation following intralesional bleomycin treatment of verruca plantaris. Arch Dermatol. 2003;139:337-339.
- Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297:333-344.
- Kim KH, Yoon TJ, Oh CW, et al. A case of bleomycin-induced scleroderma. J Korean Med Sci. 1996;11:454-456.
- Kerr LD, Spiera H. Scleroderma in association with the use of bleomycin: a report of 3 cases. J Rheumatol. 1992;19:294-296.
- Yamamoto T, Yokozeki H, Nishioka K. Dermal sclerosis in the lesional skin of 'flagellate' erythema (scratch dermatitis) induced by bleomycin. Dermatology. 1998;197:399-400.
- Kencka D, Blaszczyk M, Jablońska S. Atrophoderma Pasini-Pierini is a primary atrophic abortive morphea. Dermatology. 1995;190:203-206.
- Moulin G, Hill MP, Guillaud V, et al. Acquired atrophic pigmented band-like lesions following Blaschko's lines. Ann Dermatol Venereol. 1992;119:729-736.
- Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228.
- Zaynoun S, Rubeiz N, Kibbi AG. Ashy dermatosis--a critical review of literature and a proposed simplified clinical classification. Int J Dermatol. 2008;47:542-544.
The Diagnosis: Bleomycin-Induced Flagellate Hyperpigmentation
Histopathology of the affected skin demonstrated a slight increase in collagen bundle thickness, a chronic dermal perivascular inflammation, and associated pigment incontinence with dermal melanophages compared to unaffected skin (Figure). CD34 was faintly decreased, and dermal mucin increased in affected skin. This postinflammatory pigmentary alteration with subtle dermal sclerosis had persisted unchanged for more than 5 years after cessation of bleomycin therapy. Topical hydroquinone, physical blocker photoprotection, and laser modalities such as the Q-switched alexandrite (755-nm)/Nd:YAG (1064-nm) and ablative CO2 resurfacing lasers were attempted with minimal overall impact on cosmesis.

Bleomycin is a chemotherapeutic antibiotic that has been commonly used to treat Hodgkin lymphoma, germ cell tumors, and recurrent malignant pleural effusions.1 The drug is inactivated in most tissues by the enzyme bleomycin hydrolase. This enzyme is not present in skin and lung tissue; as a result, these organs are the most common sites of bleomycin toxicity.1 There are a variety of cutaneous effects associated with bleomycin including alopecia, hyperpigmentation, acral erythema, Raynaud phenomenon, and nail dystrophy.2 Flagellate hyperpigmentation is a less common cutaneous toxicity. It is an unusual eruption that appears as whiplike linear streaks on the upper chest and back, limbs, and flanks.3 This cutaneous manifestation was once thought to be specific to bleomycin use; however, it also has been described in dermatomyositis, adult-onset Still disease, and after the ingestion of uncooked or undercooked shiitake mushrooms.4 Flagellate hyperpigmentation also was once thought to be dose dependent; however, it has been described in even very small doses.5 The eruption has been described as independent of the route of drug administration, appearing with intravenous, subcutaneous, and intramuscular bleomycin.2 The association of bleomycin and flagellate hyperpigmentation has been reported since 1970; however, it is less commonly seen in clinical practice with the declining use of bleomycin.1
The exact mechanism for the hyperpigmentation is unknown. It has been proposed that the linear lesions are related to areas of pruritus and subsequent excoriations.1 Dermatographism may be present to a limited extent, but it is unlikely to be a chief cause of flagellate hyperpigmentation, as linear streaks have been reported in the absence of trauma. It also has been proposed that bleomycin has a direct toxic effect on the melanocytes, which stimulates increased melanin secretion.2 The hyperpigmentation also may be due to pigmentary incontinence secondary to inflammation.5 Histopathologic findings usually are varied and nonspecific.2 There may be a deep perivascular lymphocytic infiltrate, which is nonspecific but can be associated with drug-induced pathology.4 Bleomycin also is used to induce localized scleroderma in mouse-model research6 and has been reported to cause localized scleroderma at an infusion site or after an intralesional injection,7,8 which is not typically reported in flagellate erythema, but bleomycin's sclerosing effects may have played a role in the visible and sclerosing atrophy noted in our patient. Yamamoto et al9 reported a similar case of dermal sclerosis induced by bleomycin.
Flagellate hyperpigmentation typically lasts for up to 6 months.3 Patients with cutaneous manifestations from bleomycin therapy usually respond to steroid therapy and discontinuation of the drug. Bleomycin re-exposure should be avoided, as it may cause extension or widespread recurrence of flagellate hyperpigmentation.3 Postinflammatory pigment alteration may persist in patients with darker skin types and in patients with dramatic inciting inflammation.
Atrophoderma of Pasini and Pierini is a form of dermal atrophy that presents with 1 or more sharply demarcated depressed patches. There is some debate whether it is a distinct entity or a primary atrophic morphea.10 Linear atrophoderma of Moulin has a similar morphology with hyperpigmented depressions and "cliff-drop" borders, but these lesions follow the lines of Blaschko.11 Linear morphea initially can present as a linear erythematous streak but more commonly appears as a plaque-type morphea lesion that forms a scarlike band.12 Erythema dyschromicum perstans is an ashy dermatosis characterized by gray or blue-brown macules seen in Fitzpatrick skin types III through V and typically is chronic and progressive.13
The Diagnosis: Bleomycin-Induced Flagellate Hyperpigmentation
Histopathology of the affected skin demonstrated a slight increase in collagen bundle thickness, a chronic dermal perivascular inflammation, and associated pigment incontinence with dermal melanophages compared to unaffected skin (Figure). CD34 was faintly decreased, and dermal mucin increased in affected skin. This postinflammatory pigmentary alteration with subtle dermal sclerosis had persisted unchanged for more than 5 years after cessation of bleomycin therapy. Topical hydroquinone, physical blocker photoprotection, and laser modalities such as the Q-switched alexandrite (755-nm)/Nd:YAG (1064-nm) and ablative CO2 resurfacing lasers were attempted with minimal overall impact on cosmesis.

Bleomycin is a chemotherapeutic antibiotic that has been commonly used to treat Hodgkin lymphoma, germ cell tumors, and recurrent malignant pleural effusions.1 The drug is inactivated in most tissues by the enzyme bleomycin hydrolase. This enzyme is not present in skin and lung tissue; as a result, these organs are the most common sites of bleomycin toxicity.1 There are a variety of cutaneous effects associated with bleomycin including alopecia, hyperpigmentation, acral erythema, Raynaud phenomenon, and nail dystrophy.2 Flagellate hyperpigmentation is a less common cutaneous toxicity. It is an unusual eruption that appears as whiplike linear streaks on the upper chest and back, limbs, and flanks.3 This cutaneous manifestation was once thought to be specific to bleomycin use; however, it also has been described in dermatomyositis, adult-onset Still disease, and after the ingestion of uncooked or undercooked shiitake mushrooms.4 Flagellate hyperpigmentation also was once thought to be dose dependent; however, it has been described in even very small doses.5 The eruption has been described as independent of the route of drug administration, appearing with intravenous, subcutaneous, and intramuscular bleomycin.2 The association of bleomycin and flagellate hyperpigmentation has been reported since 1970; however, it is less commonly seen in clinical practice with the declining use of bleomycin.1
The exact mechanism for the hyperpigmentation is unknown. It has been proposed that the linear lesions are related to areas of pruritus and subsequent excoriations.1 Dermatographism may be present to a limited extent, but it is unlikely to be a chief cause of flagellate hyperpigmentation, as linear streaks have been reported in the absence of trauma. It also has been proposed that bleomycin has a direct toxic effect on the melanocytes, which stimulates increased melanin secretion.2 The hyperpigmentation also may be due to pigmentary incontinence secondary to inflammation.5 Histopathologic findings usually are varied and nonspecific.2 There may be a deep perivascular lymphocytic infiltrate, which is nonspecific but can be associated with drug-induced pathology.4 Bleomycin also is used to induce localized scleroderma in mouse-model research6 and has been reported to cause localized scleroderma at an infusion site or after an intralesional injection,7,8 which is not typically reported in flagellate erythema, but bleomycin's sclerosing effects may have played a role in the visible and sclerosing atrophy noted in our patient. Yamamoto et al9 reported a similar case of dermal sclerosis induced by bleomycin.
Flagellate hyperpigmentation typically lasts for up to 6 months.3 Patients with cutaneous manifestations from bleomycin therapy usually respond to steroid therapy and discontinuation of the drug. Bleomycin re-exposure should be avoided, as it may cause extension or widespread recurrence of flagellate hyperpigmentation.3 Postinflammatory pigment alteration may persist in patients with darker skin types and in patients with dramatic inciting inflammation.
Atrophoderma of Pasini and Pierini is a form of dermal atrophy that presents with 1 or more sharply demarcated depressed patches. There is some debate whether it is a distinct entity or a primary atrophic morphea.10 Linear atrophoderma of Moulin has a similar morphology with hyperpigmented depressions and "cliff-drop" borders, but these lesions follow the lines of Blaschko.11 Linear morphea initially can present as a linear erythematous streak but more commonly appears as a plaque-type morphea lesion that forms a scarlike band.12 Erythema dyschromicum perstans is an ashy dermatosis characterized by gray or blue-brown macules seen in Fitzpatrick skin types III through V and typically is chronic and progressive.13
- Lee HY, Lim KH, Ryu Y, et al. Bleomycininduced flagellate erythema: a case report and review of the literature. Oncol Lett. 2014;8:933-935.
- Simpson RC, Da Forno P, Nagarajan C, et al. A pruritic rash in a patient with Hodgkin lymphoma. Clin Exp Dermatol. 2011;36:680-682.
- Fyfe AJ, McKay P. Toxicities associated with bleomycin. J R Coll Physicians Edinb. 2010;40:213-215.
- Lu CC, Lu YY, Wang QR, et al. Bleomycin-induced flagellate erythema. Balkan Med J. 2014;31:189-190.
- Abess A, Keel DM, Graham BS. Flagellate hyperpigmentation following intralesional bleomycin treatment of verruca plantaris. Arch Dermatol. 2003;139:337-339.
- Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297:333-344.
- Kim KH, Yoon TJ, Oh CW, et al. A case of bleomycin-induced scleroderma. J Korean Med Sci. 1996;11:454-456.
- Kerr LD, Spiera H. Scleroderma in association with the use of bleomycin: a report of 3 cases. J Rheumatol. 1992;19:294-296.
- Yamamoto T, Yokozeki H, Nishioka K. Dermal sclerosis in the lesional skin of 'flagellate' erythema (scratch dermatitis) induced by bleomycin. Dermatology. 1998;197:399-400.
- Kencka D, Blaszczyk M, Jablońska S. Atrophoderma Pasini-Pierini is a primary atrophic abortive morphea. Dermatology. 1995;190:203-206.
- Moulin G, Hill MP, Guillaud V, et al. Acquired atrophic pigmented band-like lesions following Blaschko's lines. Ann Dermatol Venereol. 1992;119:729-736.
- Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228.
- Zaynoun S, Rubeiz N, Kibbi AG. Ashy dermatosis--a critical review of literature and a proposed simplified clinical classification. Int J Dermatol. 2008;47:542-544.
- Lee HY, Lim KH, Ryu Y, et al. Bleomycininduced flagellate erythema: a case report and review of the literature. Oncol Lett. 2014;8:933-935.
- Simpson RC, Da Forno P, Nagarajan C, et al. A pruritic rash in a patient with Hodgkin lymphoma. Clin Exp Dermatol. 2011;36:680-682.
- Fyfe AJ, McKay P. Toxicities associated with bleomycin. J R Coll Physicians Edinb. 2010;40:213-215.
- Lu CC, Lu YY, Wang QR, et al. Bleomycin-induced flagellate erythema. Balkan Med J. 2014;31:189-190.
- Abess A, Keel DM, Graham BS. Flagellate hyperpigmentation following intralesional bleomycin treatment of verruca plantaris. Arch Dermatol. 2003;139:337-339.
- Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297:333-344.
- Kim KH, Yoon TJ, Oh CW, et al. A case of bleomycin-induced scleroderma. J Korean Med Sci. 1996;11:454-456.
- Kerr LD, Spiera H. Scleroderma in association with the use of bleomycin: a report of 3 cases. J Rheumatol. 1992;19:294-296.
- Yamamoto T, Yokozeki H, Nishioka K. Dermal sclerosis in the lesional skin of 'flagellate' erythema (scratch dermatitis) induced by bleomycin. Dermatology. 1998;197:399-400.
- Kencka D, Blaszczyk M, Jablońska S. Atrophoderma Pasini-Pierini is a primary atrophic abortive morphea. Dermatology. 1995;190:203-206.
- Moulin G, Hill MP, Guillaud V, et al. Acquired atrophic pigmented band-like lesions following Blaschko's lines. Ann Dermatol Venereol. 1992;119:729-736.
- Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228.
- Zaynoun S, Rubeiz N, Kibbi AG. Ashy dermatosis--a critical review of literature and a proposed simplified clinical classification. Int J Dermatol. 2008;47:542-544.

An 18-year-old woman presented to our dermatology clinic with persistent diffuse discoloration on the upper body of more than 5 years’ duration. Her medical history was notable for primary mediastinal classical Hodgkin lymphoma treated with ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide) chemotherapy and 22 Gy radiation therapy to the chest 5 years prior. She reported the initial onset of diffuse pruritus with associated scratching and persistent skin discoloration while receiving a course of chemotherapy. Physical examination revealed numerous thin, flagellate, faintly hyperpigmented streaks with subtle atrophy in a parallel configuration on the bilateral shoulders (top), upper back (bottom), and abdomen. Punch biopsies (5 mm) of both affected and unaffected skin on the left side of the lateral upper back were performed.
Postinflammatory Hyperpigmentation Following Treatment of Hyperkeratosis Lenticularis Perstans With Tazarotene Cream 0.1%
To the Editor:
Hyperkeratosis lenticularis perstans (HLP), or Flegel disease, is a rare keratinization disorder characterized by asymptomatic, red-brown, 1- to 5-mm papules with irregular horny scales commonly seen on the dorsal feet and lower legs.1 Hyperkeratosis lenticularis perstans is notorious for being difficult to treat. Various treatment options, including 5-fluorouracil, topical and oral retinoids, vitamin D3 derivatives, psoralen plus UVA therapy, and dermabrasion, have been explored but none have proven to be consistently effective.
A woman in her 50s presented with an asymptomatic eruption on the legs and thighs that had been present for the last 20 years. She had been misdiagnosed by multiple outside providers with atopic dermatitis and was treated with topical steroids without considerable improvement. Upon initial presentation to our clinic , physical examination revealed a woman with Fitzpatrick skin type II with multiple hyperpigmented, red-brown, 2- to 6-mm papules on the extensor surfaces of the lower legs and upper thighs (Figure, A). A 3-mm punch biopsy of a lesion on the right upper thigh revealed hyperkeratosis and parakeratosis with basal layer degeneration and a perivascular lymphocytic infiltrate. The clinical and histopathologic findings were consistent with HLP.
The patient was started on treatment with 5-fluorouracil cream on the right leg and tazarotene cream 0.1% on the left leg to determine which agent would work best. After 9 weeks of treatment, slight improvement was observed on both legs, but the lesions were still erythematous (Figure, B). Treatment was continued, and after 14 weeks complete resolution of the lesions was noted on both legs; however, postinflammatory hyperpigmentation (PIH) was observed on the left leg, which had been treated with tazarotene (Figure, C). The patient was lost to follow-up prior to treatment of the PIH.
Postinflammatory hyperpigmentation is an acquired excess of pigment due to a prior disease process such as an infection, allergic reaction, trauma, inflammatory disease, or drug reaction. In our patient, this finding was unusual because tazarotene has been shown to be an effective treatment of PIH.2,3
In PIH, there is either abnormal production or distribution of melanin pigment in the epidermis and/or dermis. Several mechanisms for PIH have been suggested. One potential mechanism is disruption of the basal cell layer due to dermal lymphocytic inflammation, causing melanin to be released and trapped by macrophages present in the dermal papillae. Another possible mechanism is epidermal hypermelanosis, in which the release and oxidation of arachidonic acid to prostaglandins and leukotrienes alters immune cells and melanocytes, causing an increase in melanin and increased transfer of melanin to keratinocytes in the surrounding epidermis.4
Treatment of PIH can be a difficult and prolonged process, especially when a dermal rather than epidermal melanosis is observed. Topical retinoids, topical hydroquinone, azelaic acid, corticosteroids, tretinoin cream, glycolic acid, and trichloroacetic acid have been shown to be effective in treating epidermal PIH. Tazarotene is a synthetic retinoid that has been proven to be an effective treatment of PIH3; however, in our patient the PIH progressed with treatment. One plausible explanation is that irritation caused by the medication led to further PIH.2,5
It is uncommon for tazarotene to cause PIH. Hyperpigmentation is listed as an adverse effect observed during the postmarketing experience according to one manufacturer6 and the US Food and Drug Administration; however, details about prior incidents of hyperpigmentation have not been reported in the literature. Our case is unique because both treatments showed considerable improvement in HLP, but more PIH was observed on the tazarotene-treated leg.
- Bean SF. Hyperkeratosis lenticularis perstans. a clinical, histopathologic, and genetic study. Arch Dermatol. 1969;99:705-709.
- Callender V, St. Surin-Lord S, Davis E, et al. Postinflammatory hyperpigmentation: etiologic and therapeutic considerations. Am J Clin Dermatol. 2011;12:87-99.
- McEvoy G. Tazarotene (topical). In: AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists, Inc; 2014:84-92.
- Lacz N, Vafaie J, Kihiczak N, et al. Postinflammatory hyperpigmentation: a common but troubling condition. Int J Dermatol. 2004;43:362-365.
- Tazorac (tazarotene) cream [package insert]. Irvine, CA: Allergan, Inc; 2013.
- Tazorac (tazarotene) gel [package insert]. Irvine, CA: Allergan, Inc; 2014.
To the Editor:
Hyperkeratosis lenticularis perstans (HLP), or Flegel disease, is a rare keratinization disorder characterized by asymptomatic, red-brown, 1- to 5-mm papules with irregular horny scales commonly seen on the dorsal feet and lower legs.1 Hyperkeratosis lenticularis perstans is notorious for being difficult to treat. Various treatment options, including 5-fluorouracil, topical and oral retinoids, vitamin D3 derivatives, psoralen plus UVA therapy, and dermabrasion, have been explored but none have proven to be consistently effective.
A woman in her 50s presented with an asymptomatic eruption on the legs and thighs that had been present for the last 20 years. She had been misdiagnosed by multiple outside providers with atopic dermatitis and was treated with topical steroids without considerable improvement. Upon initial presentation to our clinic , physical examination revealed a woman with Fitzpatrick skin type II with multiple hyperpigmented, red-brown, 2- to 6-mm papules on the extensor surfaces of the lower legs and upper thighs (Figure, A). A 3-mm punch biopsy of a lesion on the right upper thigh revealed hyperkeratosis and parakeratosis with basal layer degeneration and a perivascular lymphocytic infiltrate. The clinical and histopathologic findings were consistent with HLP.
The patient was started on treatment with 5-fluorouracil cream on the right leg and tazarotene cream 0.1% on the left leg to determine which agent would work best. After 9 weeks of treatment, slight improvement was observed on both legs, but the lesions were still erythematous (Figure, B). Treatment was continued, and after 14 weeks complete resolution of the lesions was noted on both legs; however, postinflammatory hyperpigmentation (PIH) was observed on the left leg, which had been treated with tazarotene (Figure, C). The patient was lost to follow-up prior to treatment of the PIH.

Postinflammatory hyperpigmentation is an acquired excess of pigment due to a prior disease process such as an infection, allergic reaction, trauma, inflammatory disease, or drug reaction. In our patient, this finding was unusual because tazarotene has been shown to be an effective treatment of PIH.2,3
In PIH, there is either abnormal production or distribution of melanin pigment in the epidermis and/or dermis. Several mechanisms for PIH have been suggested. One potential mechanism is disruption of the basal cell layer due to dermal lymphocytic inflammation, causing melanin to be released and trapped by macrophages present in the dermal papillae. Another possible mechanism is epidermal hypermelanosis, in which the release and oxidation of arachidonic acid to prostaglandins and leukotrienes alters immune cells and melanocytes, causing an increase in melanin and increased transfer of melanin to keratinocytes in the surrounding epidermis.4
Treatment of PIH can be a difficult and prolonged process, especially when a dermal rather than epidermal melanosis is observed. Topical retinoids, topical hydroquinone, azelaic acid, corticosteroids, tretinoin cream, glycolic acid, and trichloroacetic acid have been shown to be effective in treating epidermal PIH. Tazarotene is a synthetic retinoid that has been proven to be an effective treatment of PIH3; however, in our patient the PIH progressed with treatment. One plausible explanation is that irritation caused by the medication led to further PIH.2,5
It is uncommon for tazarotene to cause PIH. Hyperpigmentation is listed as an adverse effect observed during the postmarketing experience according to one manufacturer6 and the US Food and Drug Administration; however, details about prior incidents of hyperpigmentation have not been reported in the literature. Our case is unique because both treatments showed considerable improvement in HLP, but more PIH was observed on the tazarotene-treated leg.
To the Editor:
Hyperkeratosis lenticularis perstans (HLP), or Flegel disease, is a rare keratinization disorder characterized by asymptomatic, red-brown, 1- to 5-mm papules with irregular horny scales commonly seen on the dorsal feet and lower legs.1 Hyperkeratosis lenticularis perstans is notorious for being difficult to treat. Various treatment options, including 5-fluorouracil, topical and oral retinoids, vitamin D3 derivatives, psoralen plus UVA therapy, and dermabrasion, have been explored but none have proven to be consistently effective.
A woman in her 50s presented with an asymptomatic eruption on the legs and thighs that had been present for the last 20 years. She had been misdiagnosed by multiple outside providers with atopic dermatitis and was treated with topical steroids without considerable improvement. Upon initial presentation to our clinic , physical examination revealed a woman with Fitzpatrick skin type II with multiple hyperpigmented, red-brown, 2- to 6-mm papules on the extensor surfaces of the lower legs and upper thighs (Figure, A). A 3-mm punch biopsy of a lesion on the right upper thigh revealed hyperkeratosis and parakeratosis with basal layer degeneration and a perivascular lymphocytic infiltrate. The clinical and histopathologic findings were consistent with HLP.
The patient was started on treatment with 5-fluorouracil cream on the right leg and tazarotene cream 0.1% on the left leg to determine which agent would work best. After 9 weeks of treatment, slight improvement was observed on both legs, but the lesions were still erythematous (Figure, B). Treatment was continued, and after 14 weeks complete resolution of the lesions was noted on both legs; however, postinflammatory hyperpigmentation (PIH) was observed on the left leg, which had been treated with tazarotene (Figure, C). The patient was lost to follow-up prior to treatment of the PIH.

Postinflammatory hyperpigmentation is an acquired excess of pigment due to a prior disease process such as an infection, allergic reaction, trauma, inflammatory disease, or drug reaction. In our patient, this finding was unusual because tazarotene has been shown to be an effective treatment of PIH.2,3
In PIH, there is either abnormal production or distribution of melanin pigment in the epidermis and/or dermis. Several mechanisms for PIH have been suggested. One potential mechanism is disruption of the basal cell layer due to dermal lymphocytic inflammation, causing melanin to be released and trapped by macrophages present in the dermal papillae. Another possible mechanism is epidermal hypermelanosis, in which the release and oxidation of arachidonic acid to prostaglandins and leukotrienes alters immune cells and melanocytes, causing an increase in melanin and increased transfer of melanin to keratinocytes in the surrounding epidermis.4
Treatment of PIH can be a difficult and prolonged process, especially when a dermal rather than epidermal melanosis is observed. Topical retinoids, topical hydroquinone, azelaic acid, corticosteroids, tretinoin cream, glycolic acid, and trichloroacetic acid have been shown to be effective in treating epidermal PIH. Tazarotene is a synthetic retinoid that has been proven to be an effective treatment of PIH3; however, in our patient the PIH progressed with treatment. One plausible explanation is that irritation caused by the medication led to further PIH.2,5
It is uncommon for tazarotene to cause PIH. Hyperpigmentation is listed as an adverse effect observed during the postmarketing experience according to one manufacturer6 and the US Food and Drug Administration; however, details about prior incidents of hyperpigmentation have not been reported in the literature. Our case is unique because both treatments showed considerable improvement in HLP, but more PIH was observed on the tazarotene-treated leg.
- Bean SF. Hyperkeratosis lenticularis perstans. a clinical, histopathologic, and genetic study. Arch Dermatol. 1969;99:705-709.
- Callender V, St. Surin-Lord S, Davis E, et al. Postinflammatory hyperpigmentation: etiologic and therapeutic considerations. Am J Clin Dermatol. 2011;12:87-99.
- McEvoy G. Tazarotene (topical). In: AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists, Inc; 2014:84-92.
- Lacz N, Vafaie J, Kihiczak N, et al. Postinflammatory hyperpigmentation: a common but troubling condition. Int J Dermatol. 2004;43:362-365.
- Tazorac (tazarotene) cream [package insert]. Irvine, CA: Allergan, Inc; 2013.
- Tazorac (tazarotene) gel [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Bean SF. Hyperkeratosis lenticularis perstans. a clinical, histopathologic, and genetic study. Arch Dermatol. 1969;99:705-709.
- Callender V, St. Surin-Lord S, Davis E, et al. Postinflammatory hyperpigmentation: etiologic and therapeutic considerations. Am J Clin Dermatol. 2011;12:87-99.
- McEvoy G. Tazarotene (topical). In: AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists, Inc; 2014:84-92.
- Lacz N, Vafaie J, Kihiczak N, et al. Postinflammatory hyperpigmentation: a common but troubling condition. Int J Dermatol. 2004;43:362-365.
- Tazorac (tazarotene) cream [package insert]. Irvine, CA: Allergan, Inc; 2013.
- Tazorac (tazarotene) gel [package insert]. Irvine, CA: Allergan, Inc; 2014.
Practice Points
- Hyperkeratosis lenticularis perstans is a rare keratinization disorder that presents with asymptomatic red-brown papules with irregular horny scales on the lower extremities.
- Hyperkeratosis lenticularis perstans can be difficult to diagnose and treat. Hematoxylin and eosin staining generally will show hyperkeratosis and parakeratosis with basal layer degeneration and a perivascular lymphocytic infiltrate.
- Tazarotene cream 0.1% is a synthetic retinoid sometimes used for treatment of hyperpigmentation, but it also can cause postinflammatory hyperpigmentation.
Violaceous Patches on the Arm
The Diagnosis: Phacomatosis Cesioflammea
Phacomatosis pigmentovascularis (PPV) encompasses a group of diseases that have a vascular nevus coupled with a pigmented nevus.1 It is divided into 5 types: Type I is defined by the presence of a vascular malformation and epidermal nevus; type II by a vascular malformation and dermal melanosis with or without nevus anemicus; type III by a vascular malformation and nevus spilus with or without nevus anemicus; type IV by a vascular malformation, dermal melanosis, and nevus spilus with or without nevus anemicus; and type V as cutis marmorata telangiectatica congenita and dermal melanosis.1
Happle2 proposed a descriptive classification system in 2005 that eliminated type I PPV because neither linear epidermal nevus nor Becker nevus are derived from pigmentary cells. An appended "a" denotes a subtype with isolated cutaneous findings, while "b" is associated with extracutaneous manifestations. Phacomatosis cesioflammea (type IIa/b) refers to blue-hued dermal melanocytosis and nevus flammeus. Phacomatosis spilorosea (type IIIa/b) refers to nevus spilus and rose-colored nevus flammeus. Phacomatosis cesiomarmorata (type Va/b) refers to dermal melanocytosis and cutis marmorata telangiectasia congenita. The last group (type IVa/b) is unclassifiable phacomatosis pigmentovascularis.2,3
Phacomatosis pigmentovascularis can be isolated to the skin or have associated extracutaneous findings, including ocular melanocytosis, seizures, or cognitive delay due to intracerebral vascular malformations. Patients also can develop limb and soft-tissue overgrowth.4 Phacomatosis pigmentovascularis has been found to be associated with mutations in the GNA11 and GNAQ genes. The theory behind PPV is twin spotting, resulting from a somatic mutation that leads to mosaic proliferation of 2 different cell lines.5 Phacomatosis pigmentovascularis can occur in isolation or can demonstrate the phenotype of Sturge-Weber syndrome or Klippel-Trenaunay syndrome. In Sturge-Weber syndrome, capillary malformations involve the face and underlying leptomeninges and cerebral cortex. Glaucoma and epilepsy also may be present. In Klippel-Trenaunay syndrome, capillary malformations involve the extremities (usually the legs) in association with varicose veins, soft-tissue hypertrophy, and skeletal overgrowth.6-9 Tuberous sclerosis is an autosomal-dominant neurocutaneous disease in which patients develop hamartomas throughout the body, including the brain, skin, eyes, kidneys, heart, and lungs. Cutaneous manifestations include facial angiofibromas, ungual fibromas, hypomelanotic macules (ash leaf spots, confetti-like lesions), shagreen patches or connective tissue hamartomas, and fibrous plaques on the forehead. Tuberous sclerosis does not include vascular malformations.10
Our patient was diagnosed with PPV type IIb, or phacomatosis cesioflammea. He had a large port-wine stain involving the right upper arm, back (Figure, A), and chest (Figure, B) with involvement of the bilateral conjunctivae (Figure, C). Our case is unique because our patient did not have dermal melanocytosis, only ocular melanocytosis.
Once underlying neurologic and vascular anomalies have been ruled out, port-wine stains can be treated cosmetically. Pulsed dye laser is the gold standard therapy for capillary malformations, especially when instituted early. Follow-up with ophthalmology is advised to monitor ocular involvement. Shields et al11 suggested dilated fundoscopy for patients with port-wine stains because choroidal pigmentation often is the only ocular change seen. Ocular melanocytosis can progress to pigmented glaucoma or choroidal melanoma.
- Fernandez-Guarino M, Boixeda P, De las Heras E, et al. Phakomatosis pigmentovascularis: clinical findings in 15 patients and review of the literature. J Am Acad Dermatol. 2008;58:88-93.
- Happle R. Phacomatosis pigmentovascularis revisited and reclassified. Arch Dermatol. 2005;141:385-388.
- Villarreal DJ, Leal F. Phacomatosis pigmentovascularis of cesioflammea type. An Bras Dermatol. 2016;91(5 suppl 1):54-56.
- Thomas AC, Zeng Z, Riviere JB, et al. Mosaic activating mutations in GNA11 and GNAQ are associated with phakomatosis pigmentovascularis and extensive dermal melanocytosis. J Invest Dermatol. 2016;136:770-778.
- Krema H, Simpson R, McGowan H. Choroidal melanoma in phacomatosis pigmentovascularis cesioflammea. Can J Ophthalmol. 2013;48:E41-E42.
- Wu CY, Chen PH, Chen GS. Phacomatosis cesioflammea associated with pectus excavatum. Acta Derm Venereol. 2009;89:301-310.
- Pradhan S, Patnaik S, Padhi T, et al. Phakomatosis pigmentovascularis type IIb, Sturge-Weber syndrome and cone shaped tongue: an unusual association. Indian J Dermatol Venereol Leprol. 2015;81:614-616.
- Turk BG, Turkmen M, Tuna A, et al. Phakomatosis pigmentovascularis type IIb associated with Klippel-Trenaunay syndrome and congenital triangular alopecia. J Am Acad Dermatol. 2011;65:E46-E49.
- Sen S, Bala S, Halder C, et al. Phakomatosis pigmentovascularis presenting with Sturge-Weber syndrome and Klippel-Trenaunay syndrome. Indian J Dermatol. 2015;60:77-79.
- Schwartz RA, Fernandez G, Kotulska K, et al. Tuberous sclerosis complex: advances in diagnosis, genetics, and management. J Am Acad Dermatol. 2007;57:189-202.
- Shields CL, Kligman BE, Suriano M, et al. Phacomatosis pigmentovascularis of cesioflammea type in 7 patients: combination of ocular pigmentation (melanocytosis or melanosis) and nevus flammeus with risk for melanoma. Arch Ophthalmol. 2011;129:746-750.
The Diagnosis: Phacomatosis Cesioflammea
Phacomatosis pigmentovascularis (PPV) encompasses a group of diseases that have a vascular nevus coupled with a pigmented nevus.1 It is divided into 5 types: Type I is defined by the presence of a vascular malformation and epidermal nevus; type II by a vascular malformation and dermal melanosis with or without nevus anemicus; type III by a vascular malformation and nevus spilus with or without nevus anemicus; type IV by a vascular malformation, dermal melanosis, and nevus spilus with or without nevus anemicus; and type V as cutis marmorata telangiectatica congenita and dermal melanosis.1
Happle2 proposed a descriptive classification system in 2005 that eliminated type I PPV because neither linear epidermal nevus nor Becker nevus are derived from pigmentary cells. An appended "a" denotes a subtype with isolated cutaneous findings, while "b" is associated with extracutaneous manifestations. Phacomatosis cesioflammea (type IIa/b) refers to blue-hued dermal melanocytosis and nevus flammeus. Phacomatosis spilorosea (type IIIa/b) refers to nevus spilus and rose-colored nevus flammeus. Phacomatosis cesiomarmorata (type Va/b) refers to dermal melanocytosis and cutis marmorata telangiectasia congenita. The last group (type IVa/b) is unclassifiable phacomatosis pigmentovascularis.2,3
Phacomatosis pigmentovascularis can be isolated to the skin or have associated extracutaneous findings, including ocular melanocytosis, seizures, or cognitive delay due to intracerebral vascular malformations. Patients also can develop limb and soft-tissue overgrowth.4 Phacomatosis pigmentovascularis has been found to be associated with mutations in the GNA11 and GNAQ genes. The theory behind PPV is twin spotting, resulting from a somatic mutation that leads to mosaic proliferation of 2 different cell lines.5 Phacomatosis pigmentovascularis can occur in isolation or can demonstrate the phenotype of Sturge-Weber syndrome or Klippel-Trenaunay syndrome. In Sturge-Weber syndrome, capillary malformations involve the face and underlying leptomeninges and cerebral cortex. Glaucoma and epilepsy also may be present. In Klippel-Trenaunay syndrome, capillary malformations involve the extremities (usually the legs) in association with varicose veins, soft-tissue hypertrophy, and skeletal overgrowth.6-9 Tuberous sclerosis is an autosomal-dominant neurocutaneous disease in which patients develop hamartomas throughout the body, including the brain, skin, eyes, kidneys, heart, and lungs. Cutaneous manifestations include facial angiofibromas, ungual fibromas, hypomelanotic macules (ash leaf spots, confetti-like lesions), shagreen patches or connective tissue hamartomas, and fibrous plaques on the forehead. Tuberous sclerosis does not include vascular malformations.10
Our patient was diagnosed with PPV type IIb, or phacomatosis cesioflammea. He had a large port-wine stain involving the right upper arm, back (Figure, A), and chest (Figure, B) with involvement of the bilateral conjunctivae (Figure, C). Our case is unique because our patient did not have dermal melanocytosis, only ocular melanocytosis.

Once underlying neurologic and vascular anomalies have been ruled out, port-wine stains can be treated cosmetically. Pulsed dye laser is the gold standard therapy for capillary malformations, especially when instituted early. Follow-up with ophthalmology is advised to monitor ocular involvement. Shields et al11 suggested dilated fundoscopy for patients with port-wine stains because choroidal pigmentation often is the only ocular change seen. Ocular melanocytosis can progress to pigmented glaucoma or choroidal melanoma.
The Diagnosis: Phacomatosis Cesioflammea
Phacomatosis pigmentovascularis (PPV) encompasses a group of diseases that have a vascular nevus coupled with a pigmented nevus.1 It is divided into 5 types: Type I is defined by the presence of a vascular malformation and epidermal nevus; type II by a vascular malformation and dermal melanosis with or without nevus anemicus; type III by a vascular malformation and nevus spilus with or without nevus anemicus; type IV by a vascular malformation, dermal melanosis, and nevus spilus with or without nevus anemicus; and type V as cutis marmorata telangiectatica congenita and dermal melanosis.1
Happle2 proposed a descriptive classification system in 2005 that eliminated type I PPV because neither linear epidermal nevus nor Becker nevus are derived from pigmentary cells. An appended "a" denotes a subtype with isolated cutaneous findings, while "b" is associated with extracutaneous manifestations. Phacomatosis cesioflammea (type IIa/b) refers to blue-hued dermal melanocytosis and nevus flammeus. Phacomatosis spilorosea (type IIIa/b) refers to nevus spilus and rose-colored nevus flammeus. Phacomatosis cesiomarmorata (type Va/b) refers to dermal melanocytosis and cutis marmorata telangiectasia congenita. The last group (type IVa/b) is unclassifiable phacomatosis pigmentovascularis.2,3
Phacomatosis pigmentovascularis can be isolated to the skin or have associated extracutaneous findings, including ocular melanocytosis, seizures, or cognitive delay due to intracerebral vascular malformations. Patients also can develop limb and soft-tissue overgrowth.4 Phacomatosis pigmentovascularis has been found to be associated with mutations in the GNA11 and GNAQ genes. The theory behind PPV is twin spotting, resulting from a somatic mutation that leads to mosaic proliferation of 2 different cell lines.5 Phacomatosis pigmentovascularis can occur in isolation or can demonstrate the phenotype of Sturge-Weber syndrome or Klippel-Trenaunay syndrome. In Sturge-Weber syndrome, capillary malformations involve the face and underlying leptomeninges and cerebral cortex. Glaucoma and epilepsy also may be present. In Klippel-Trenaunay syndrome, capillary malformations involve the extremities (usually the legs) in association with varicose veins, soft-tissue hypertrophy, and skeletal overgrowth.6-9 Tuberous sclerosis is an autosomal-dominant neurocutaneous disease in which patients develop hamartomas throughout the body, including the brain, skin, eyes, kidneys, heart, and lungs. Cutaneous manifestations include facial angiofibromas, ungual fibromas, hypomelanotic macules (ash leaf spots, confetti-like lesions), shagreen patches or connective tissue hamartomas, and fibrous plaques on the forehead. Tuberous sclerosis does not include vascular malformations.10
Our patient was diagnosed with PPV type IIb, or phacomatosis cesioflammea. He had a large port-wine stain involving the right upper arm, back (Figure, A), and chest (Figure, B) with involvement of the bilateral conjunctivae (Figure, C). Our case is unique because our patient did not have dermal melanocytosis, only ocular melanocytosis.

Once underlying neurologic and vascular anomalies have been ruled out, port-wine stains can be treated cosmetically. Pulsed dye laser is the gold standard therapy for capillary malformations, especially when instituted early. Follow-up with ophthalmology is advised to monitor ocular involvement. Shields et al11 suggested dilated fundoscopy for patients with port-wine stains because choroidal pigmentation often is the only ocular change seen. Ocular melanocytosis can progress to pigmented glaucoma or choroidal melanoma.
- Fernandez-Guarino M, Boixeda P, De las Heras E, et al. Phakomatosis pigmentovascularis: clinical findings in 15 patients and review of the literature. J Am Acad Dermatol. 2008;58:88-93.
- Happle R. Phacomatosis pigmentovascularis revisited and reclassified. Arch Dermatol. 2005;141:385-388.
- Villarreal DJ, Leal F. Phacomatosis pigmentovascularis of cesioflammea type. An Bras Dermatol. 2016;91(5 suppl 1):54-56.
- Thomas AC, Zeng Z, Riviere JB, et al. Mosaic activating mutations in GNA11 and GNAQ are associated with phakomatosis pigmentovascularis and extensive dermal melanocytosis. J Invest Dermatol. 2016;136:770-778.
- Krema H, Simpson R, McGowan H. Choroidal melanoma in phacomatosis pigmentovascularis cesioflammea. Can J Ophthalmol. 2013;48:E41-E42.
- Wu CY, Chen PH, Chen GS. Phacomatosis cesioflammea associated with pectus excavatum. Acta Derm Venereol. 2009;89:301-310.
- Pradhan S, Patnaik S, Padhi T, et al. Phakomatosis pigmentovascularis type IIb, Sturge-Weber syndrome and cone shaped tongue: an unusual association. Indian J Dermatol Venereol Leprol. 2015;81:614-616.
- Turk BG, Turkmen M, Tuna A, et al. Phakomatosis pigmentovascularis type IIb associated with Klippel-Trenaunay syndrome and congenital triangular alopecia. J Am Acad Dermatol. 2011;65:E46-E49.
- Sen S, Bala S, Halder C, et al. Phakomatosis pigmentovascularis presenting with Sturge-Weber syndrome and Klippel-Trenaunay syndrome. Indian J Dermatol. 2015;60:77-79.
- Schwartz RA, Fernandez G, Kotulska K, et al. Tuberous sclerosis complex: advances in diagnosis, genetics, and management. J Am Acad Dermatol. 2007;57:189-202.
- Shields CL, Kligman BE, Suriano M, et al. Phacomatosis pigmentovascularis of cesioflammea type in 7 patients: combination of ocular pigmentation (melanocytosis or melanosis) and nevus flammeus with risk for melanoma. Arch Ophthalmol. 2011;129:746-750.
- Fernandez-Guarino M, Boixeda P, De las Heras E, et al. Phakomatosis pigmentovascularis: clinical findings in 15 patients and review of the literature. J Am Acad Dermatol. 2008;58:88-93.
- Happle R. Phacomatosis pigmentovascularis revisited and reclassified. Arch Dermatol. 2005;141:385-388.
- Villarreal DJ, Leal F. Phacomatosis pigmentovascularis of cesioflammea type. An Bras Dermatol. 2016;91(5 suppl 1):54-56.
- Thomas AC, Zeng Z, Riviere JB, et al. Mosaic activating mutations in GNA11 and GNAQ are associated with phakomatosis pigmentovascularis and extensive dermal melanocytosis. J Invest Dermatol. 2016;136:770-778.
- Krema H, Simpson R, McGowan H. Choroidal melanoma in phacomatosis pigmentovascularis cesioflammea. Can J Ophthalmol. 2013;48:E41-E42.
- Wu CY, Chen PH, Chen GS. Phacomatosis cesioflammea associated with pectus excavatum. Acta Derm Venereol. 2009;89:301-310.
- Pradhan S, Patnaik S, Padhi T, et al. Phakomatosis pigmentovascularis type IIb, Sturge-Weber syndrome and cone shaped tongue: an unusual association. Indian J Dermatol Venereol Leprol. 2015;81:614-616.
- Turk BG, Turkmen M, Tuna A, et al. Phakomatosis pigmentovascularis type IIb associated with Klippel-Trenaunay syndrome and congenital triangular alopecia. J Am Acad Dermatol. 2011;65:E46-E49.
- Sen S, Bala S, Halder C, et al. Phakomatosis pigmentovascularis presenting with Sturge-Weber syndrome and Klippel-Trenaunay syndrome. Indian J Dermatol. 2015;60:77-79.
- Schwartz RA, Fernandez G, Kotulska K, et al. Tuberous sclerosis complex: advances in diagnosis, genetics, and management. J Am Acad Dermatol. 2007;57:189-202.
- Shields CL, Kligman BE, Suriano M, et al. Phacomatosis pigmentovascularis of cesioflammea type in 7 patients: combination of ocular pigmentation (melanocytosis or melanosis) and nevus flammeus with risk for melanoma. Arch Ophthalmol. 2011;129:746-750.
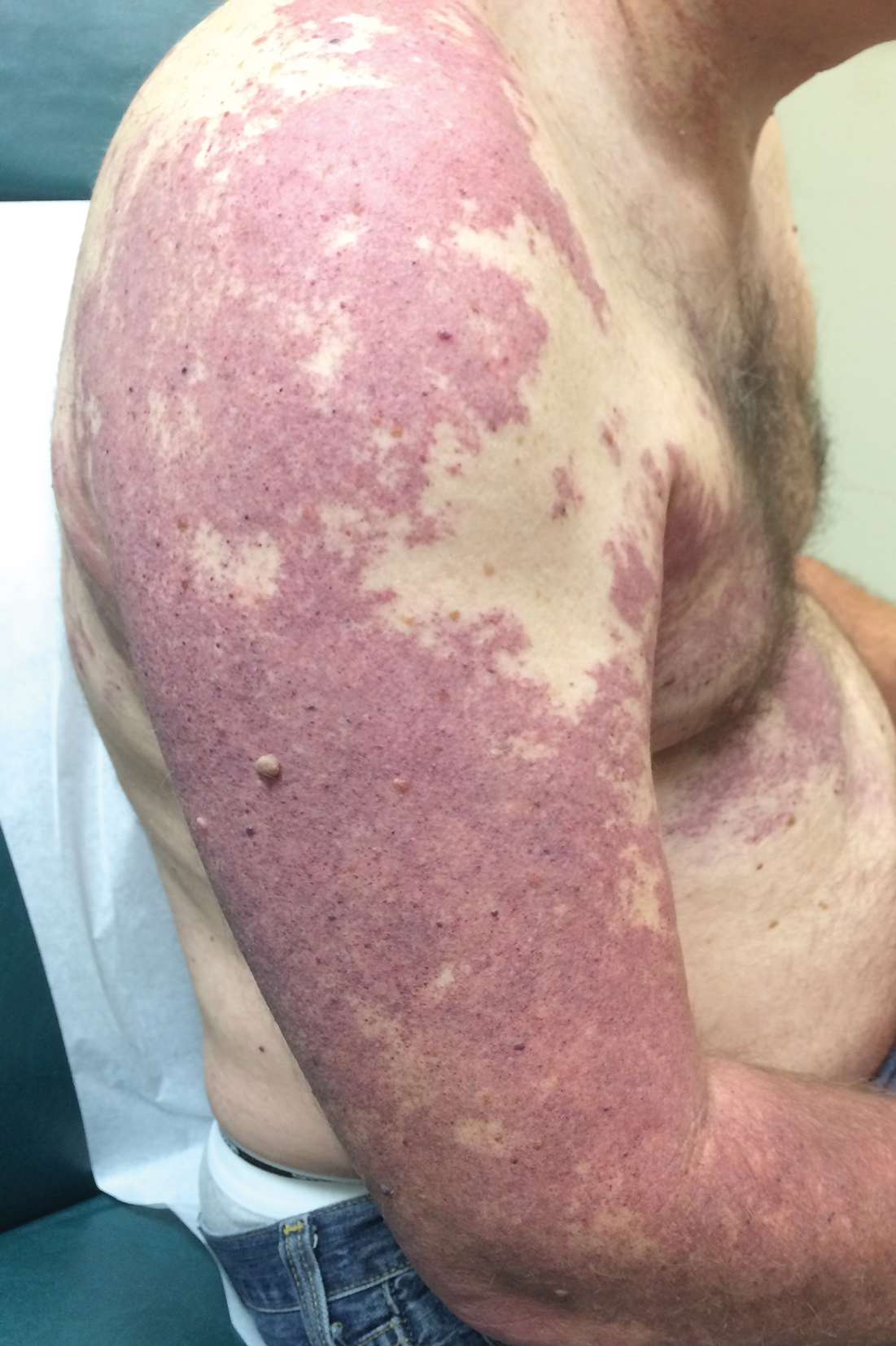
A 55-year-old man presented with red-violet patches on the right arm and chest that had been present since birth. The patches were asymptomatic and stable in size and shape. He denied any personal or family history of glaucoma or epilepsy. Physical examination demonstrated nonblanchable, violaceous to red patches on the right arm, back, and chest. No thrills or bruits were appreciable, and the right and left arms were of equal circumference and length. Further examination revealed hyperpigmented patches on the bilateral conjunctivae.
Recent progress in vitiligo treatment might be heading to vitiligo cure
NEW YORK – but also might be leading to a strategy that will prevent the inevitable relapse that occurs after treatment is stopped, according to an update at the American Academy of Dermatology summer meeting.
Recently, trial results with a Janus kinase (JAK) pathway inhibitor have shown promise for treatment of vitiligo, but the ultimate fix for this recurring autoimmune disease might be elimination of resident-memory T cells, according to John Harris, MD, PhD, of the department of dermatology at the University of Massachusetts, Worcester.
In a murine vitiligo model, targeting interleukin-15, a cytokine thought to be essential for maintaining memory T cells, produced rapid and durable repigmentation without apparent adverse effects in a series of studies sufficiently promising that clinical trials are now being actively planned, Dr. Harris said. The ongoing work to eliminate resident-memory T cells to prevent relapse of vitiligo comes at the end of other recent advances that have provided major insights into the pathophysiology of vitiligo.
As outlined by Dr. Harris, vitiligo involves an autoimmune sequence that includes up-regulation of interferon-gamma, activation of the JAK signaling pathway, and mobilization of the cytokine CXCl10, all of which are part of the sequence of events culminating in activation of T cells that attack the melanocyte. The process can be stopped when any of these events are targeted, according to the experimental studies. These findings have already been translated into new drug development.
“There are now three ongoing clinical trials with JAK inhibitors. This is a tremendous advance in a disease for which there have been no clinical trials for decades,” Dr. Harris said. He cited highly positive data with the JAK inhibitor ruxolitinib, which were reported just weeks earlier at the World Congress of Dermatology, to confirm that this principle of intervention is viable.
However, relapse after discontinuation of ruxolitinib, like other treatments for vitiligo, is high. The observation that relapses typically occur in the exact spot where skin lesions occurred previously created the framework of a new potential wave of advances, according to Dr. Harris, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester.
These advances involve progress in understanding the role of resident-memory T cells in driving autoimmune disease relapse.
In principle, memory-resident T cells are left behind in order to stimulate a rapid immune response in the event of a recurrence of a virus or another pathogen. According to work performed in animal models of vitiligo, they also appear to play a critical role in reactivation of this autoimmune disease, Dr. Harris said.
This role was not surprising, but the potential breakthrough in vitiligo surrounds evidence that the cytokine IL-15 is essential to the creation and maintenance of these memory cells. Evidence suggests vitiligo in animal models does not recur in the absence of IL-15, making it a potential target for treatment.
Initially, there was concern that inhibition of IL-15 would have off-target effects, but this concern has diminished with antibodies designed to inhibit IL-15 signaling in the animal model.
“It turns out that autoreactive cells are much more dependent on the cytokine than other T cells,” he said.
In the animal model, repigmentation has occurred more rapidly with anti-IL-15 therapy than with any other treatment tested to date, but more importantly, these mice then appear to be protected from vitiligo recurrence for extended periods, Dr. Harris noted.
Studies conducted with human tissue have provided strong evidence that the same mechanisms are in play. There are now several approaches to blocking IL-15 signaling, including a monoclonal antibody targeted at the IL-15 receptor, in development. This latter approach is now the focus of a company formed by Dr. Harris.
It is not yet clear if one approach to the inhibition of IL-15 will be superior to another, but Dr. Harris is highly optimistic that this will be a viable approach to control of vitiligo. Noting that good results have been achieved in experimental models by skin injections, thereby avoiding systemic exposure, he is also optimistic that this approach will be well tolerated.
“Based on these data, we are expecting clinical trials soon,” he said.
Dr. Harris reported serving as a consultant and/or investigator for multiple pharmaceutical companies including Aclaris Therapeutics, Celgene, EMD Serono, Genzyme, Incyte, and Janssen Biotech.
NEW YORK – but also might be leading to a strategy that will prevent the inevitable relapse that occurs after treatment is stopped, according to an update at the American Academy of Dermatology summer meeting.
Recently, trial results with a Janus kinase (JAK) pathway inhibitor have shown promise for treatment of vitiligo, but the ultimate fix for this recurring autoimmune disease might be elimination of resident-memory T cells, according to John Harris, MD, PhD, of the department of dermatology at the University of Massachusetts, Worcester.
In a murine vitiligo model, targeting interleukin-15, a cytokine thought to be essential for maintaining memory T cells, produced rapid and durable repigmentation without apparent adverse effects in a series of studies sufficiently promising that clinical trials are now being actively planned, Dr. Harris said. The ongoing work to eliminate resident-memory T cells to prevent relapse of vitiligo comes at the end of other recent advances that have provided major insights into the pathophysiology of vitiligo.
As outlined by Dr. Harris, vitiligo involves an autoimmune sequence that includes up-regulation of interferon-gamma, activation of the JAK signaling pathway, and mobilization of the cytokine CXCl10, all of which are part of the sequence of events culminating in activation of T cells that attack the melanocyte. The process can be stopped when any of these events are targeted, according to the experimental studies. These findings have already been translated into new drug development.
“There are now three ongoing clinical trials with JAK inhibitors. This is a tremendous advance in a disease for which there have been no clinical trials for decades,” Dr. Harris said. He cited highly positive data with the JAK inhibitor ruxolitinib, which were reported just weeks earlier at the World Congress of Dermatology, to confirm that this principle of intervention is viable.
However, relapse after discontinuation of ruxolitinib, like other treatments for vitiligo, is high. The observation that relapses typically occur in the exact spot where skin lesions occurred previously created the framework of a new potential wave of advances, according to Dr. Harris, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester.
These advances involve progress in understanding the role of resident-memory T cells in driving autoimmune disease relapse.
In principle, memory-resident T cells are left behind in order to stimulate a rapid immune response in the event of a recurrence of a virus or another pathogen. According to work performed in animal models of vitiligo, they also appear to play a critical role in reactivation of this autoimmune disease, Dr. Harris said.
This role was not surprising, but the potential breakthrough in vitiligo surrounds evidence that the cytokine IL-15 is essential to the creation and maintenance of these memory cells. Evidence suggests vitiligo in animal models does not recur in the absence of IL-15, making it a potential target for treatment.
Initially, there was concern that inhibition of IL-15 would have off-target effects, but this concern has diminished with antibodies designed to inhibit IL-15 signaling in the animal model.
“It turns out that autoreactive cells are much more dependent on the cytokine than other T cells,” he said.
In the animal model, repigmentation has occurred more rapidly with anti-IL-15 therapy than with any other treatment tested to date, but more importantly, these mice then appear to be protected from vitiligo recurrence for extended periods, Dr. Harris noted.
Studies conducted with human tissue have provided strong evidence that the same mechanisms are in play. There are now several approaches to blocking IL-15 signaling, including a monoclonal antibody targeted at the IL-15 receptor, in development. This latter approach is now the focus of a company formed by Dr. Harris.
It is not yet clear if one approach to the inhibition of IL-15 will be superior to another, but Dr. Harris is highly optimistic that this will be a viable approach to control of vitiligo. Noting that good results have been achieved in experimental models by skin injections, thereby avoiding systemic exposure, he is also optimistic that this approach will be well tolerated.
“Based on these data, we are expecting clinical trials soon,” he said.
Dr. Harris reported serving as a consultant and/or investigator for multiple pharmaceutical companies including Aclaris Therapeutics, Celgene, EMD Serono, Genzyme, Incyte, and Janssen Biotech.
NEW YORK – but also might be leading to a strategy that will prevent the inevitable relapse that occurs after treatment is stopped, according to an update at the American Academy of Dermatology summer meeting.
Recently, trial results with a Janus kinase (JAK) pathway inhibitor have shown promise for treatment of vitiligo, but the ultimate fix for this recurring autoimmune disease might be elimination of resident-memory T cells, according to John Harris, MD, PhD, of the department of dermatology at the University of Massachusetts, Worcester.
In a murine vitiligo model, targeting interleukin-15, a cytokine thought to be essential for maintaining memory T cells, produced rapid and durable repigmentation without apparent adverse effects in a series of studies sufficiently promising that clinical trials are now being actively planned, Dr. Harris said. The ongoing work to eliminate resident-memory T cells to prevent relapse of vitiligo comes at the end of other recent advances that have provided major insights into the pathophysiology of vitiligo.
As outlined by Dr. Harris, vitiligo involves an autoimmune sequence that includes up-regulation of interferon-gamma, activation of the JAK signaling pathway, and mobilization of the cytokine CXCl10, all of which are part of the sequence of events culminating in activation of T cells that attack the melanocyte. The process can be stopped when any of these events are targeted, according to the experimental studies. These findings have already been translated into new drug development.
“There are now three ongoing clinical trials with JAK inhibitors. This is a tremendous advance in a disease for which there have been no clinical trials for decades,” Dr. Harris said. He cited highly positive data with the JAK inhibitor ruxolitinib, which were reported just weeks earlier at the World Congress of Dermatology, to confirm that this principle of intervention is viable.
However, relapse after discontinuation of ruxolitinib, like other treatments for vitiligo, is high. The observation that relapses typically occur in the exact spot where skin lesions occurred previously created the framework of a new potential wave of advances, according to Dr. Harris, director of the Vitiligo Clinic and Research Center at the University of Massachusetts, Worcester.
These advances involve progress in understanding the role of resident-memory T cells in driving autoimmune disease relapse.
In principle, memory-resident T cells are left behind in order to stimulate a rapid immune response in the event of a recurrence of a virus or another pathogen. According to work performed in animal models of vitiligo, they also appear to play a critical role in reactivation of this autoimmune disease, Dr. Harris said.
This role was not surprising, but the potential breakthrough in vitiligo surrounds evidence that the cytokine IL-15 is essential to the creation and maintenance of these memory cells. Evidence suggests vitiligo in animal models does not recur in the absence of IL-15, making it a potential target for treatment.
Initially, there was concern that inhibition of IL-15 would have off-target effects, but this concern has diminished with antibodies designed to inhibit IL-15 signaling in the animal model.
“It turns out that autoreactive cells are much more dependent on the cytokine than other T cells,” he said.
In the animal model, repigmentation has occurred more rapidly with anti-IL-15 therapy than with any other treatment tested to date, but more importantly, these mice then appear to be protected from vitiligo recurrence for extended periods, Dr. Harris noted.
Studies conducted with human tissue have provided strong evidence that the same mechanisms are in play. There are now several approaches to blocking IL-15 signaling, including a monoclonal antibody targeted at the IL-15 receptor, in development. This latter approach is now the focus of a company formed by Dr. Harris.
It is not yet clear if one approach to the inhibition of IL-15 will be superior to another, but Dr. Harris is highly optimistic that this will be a viable approach to control of vitiligo. Noting that good results have been achieved in experimental models by skin injections, thereby avoiding systemic exposure, he is also optimistic that this approach will be well tolerated.
“Based on these data, we are expecting clinical trials soon,” he said.
Dr. Harris reported serving as a consultant and/or investigator for multiple pharmaceutical companies including Aclaris Therapeutics, Celgene, EMD Serono, Genzyme, Incyte, and Janssen Biotech.
EXPERT ANALYSIS FROM SUMMER AAD 2019
Bimatoprost-Induced Iris Hyperpigmentation: Beauty in the Darkened Eye of the Beholder
To the Editor:
Long, dark, and thick eyelashes have been a focal point of society’s perception of beauty for thousands of years,1 and the use of makeup products such as mascaras, eyeliners, and eye shadows has further increased the perception of attractiveness of the eyes.2 Many eyelash enhancement methods have been developed or in some instances have been serendipitously discovered. Bimatoprost ophthalmic solution 0.03% originally was developed as an eye drop that was approved by the US Food and Drug Association (FDA) in 2001 for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. An unexpected side effect of this product was eyelash hypertrichosis.3,4 As a result, the FDA approved
Because all follicular development occurs during embryogenesis, the number of eyelash follicles does not increase over time.6 Bitmatoprost eyelash solution works by prolonging the anagen (growth) phase of the eyelashes and stimulating the transition from the telogen (dormant) phase to the anagen phase. It also has been shown to increase the hair bulb diameter of follicles undergoing the anagen phase, resulting in thicker eyelashes.7 Although many patients have enjoyed this unexpected indication, prostaglandin (PG) analogues such as bimatoprost and latanoprost have a well-documented history of ocular side effects when applied directly to the eye. The most common adverse reactions include eye pruritus, conjunctival hyperemia, and eyelid pigmentation.3 The product safety information indicates that eyelid pigmentation typically is reversible.3,5 Iris pigmentation is perhaps the least desirable side effect of PG analogues and was first noted in latanoprost studies on primates.8 The underlying mechanism appears to be due to an increase in melanogenesis that results in an increase in melanin granules without concomitant proliferation of melanocytes, cellular atypia, or evidence of inflammatory reaction. Unfortunately, this pigmentation typically is permanent.3,5,9
Studies have shown that
An otherwise healthy 63-year-old woman presented to our clinic for an annual skin examination. She noted that she had worsening dark pigmentation of the bilateral irises. The patient did not have any personal or family history of melanoma or ocular nevi, and there were no associated symptoms of eye tearing, pruritus, burning, or discharge. No prior surgical procedures had been performed on or around the eyes, and the patient never used contact lenses. She had been intermittently using bimatoprost eyelash solution prescribed by an outside physician for approximately 3 years to enhance her eyelashes. Although she never applied the product directly into her eyes, she noted that she often was unmethodical in application of the product and that runoff from the product may have occasionally leaked into the eyes. Physical examination revealed bilateral blue irises with ink spot–like, grayish black patches encircling the bilateral pupils (Figure).
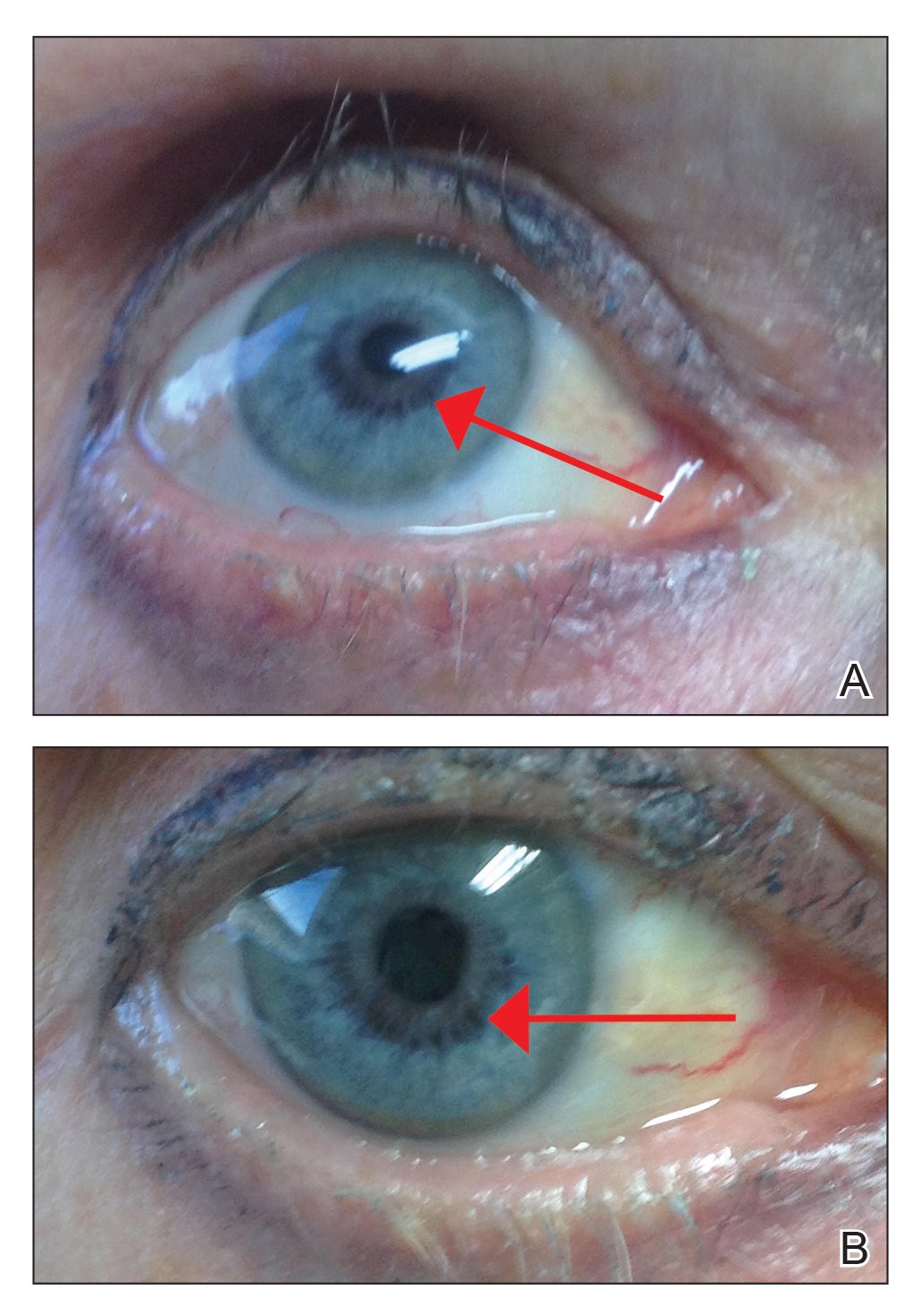
The patient was advised to stop using the product, but no improvement of the iris hyperpigmentation was appreciated at 6-month follow-up. The patient declined referral to ophthalmology for evaluation to confirm a diagnosis and discuss treatment because the hyperpigmentation did not bother her.
There have been several studies of iris hyperpigmentation with use of PG analogues in the treatment of glaucoma. In a phase 3 clinical trial of the safety and efficacy of latanoprost for treatment of ocular hypertension, it was noted that 24 (12%) of 198 patients experienced iris hyperpigmentation and that patients with heterogeneous pigmentation (ie, hazel irises and mixed coloring) were at an increased risk.11 Other studies also have shown an increased risk of iris hyperpigmentation due to heterogeneous phenotype12 as well as older age.13
Reports of bimatoprost eye drops used for treatment of glaucoma have shown a high incidence of iris hyperpigmentation with long-term use. A prospective study conducted in 2012 investigated the adverse events of bimatoprost eye drops in 52 Japanese patients with glaucoma or ocular hypertension. Clinical photographs of the irises, eyelids, and eyelashes were taken at baseline and after 6 months of treatment. It was noted that 50% (26/52) of participants experienced iris hyperpigmentation upon completion of treatment.10
In our patient, bimatoprost eyelash solution was applied to the top eyelid margins using an applicator; our patient did not use the eye drop formulation, which is directed for use in ocular hypertension or glaucoma. A PubMed search of articles indexed for MEDLINE using the terms bimatoprost and iris hyperpigmentation yielded no published peer-reviewed studies or case reports of iris hyperpigmentation caused by bimatoprost eyelash solution for treatment of eyelid hypotrichosis, which makes this case report novel. With that said, the package insert states iris hyperpigmentation as a side effect in the prescribing information for both a bimatoprost eye drop formulation used to treat ocular hypertension3 as well as a formulation for topical application on the eyelids/eyelashes.5 A 2014 retrospective review of long-term safety with bimatoprost eyelash solution for eyelash hypotrichosis reported 4 instances (0.7%) of documented adverse events after 12 months of use in 585 patients, including dry eye, eyelid erythema, ocular pruritus, and low ocular pressure. Iris hyperpigmentation was not reported.14
The method of bimatoprost application likely is a determining factor in the number of reported adverse events. Studies with similar treatment periods have demonstrated more adverse events associated with bimatoprost eye drops vs eyelash solution.15,16 When bimatoprost is used in the eye drop formulation for treatment of glaucoma, iris hyperpigmentation has been estimated to occur in 1.5%4 to 50%9 of cases. To our knowledge, there are no documented cases when bimatoprost eyelash solution is applied with a dermal applicator for treatment of eyelash hypotrichosis.15,17 These results may be explained using an ocular splash test. In one study using lissamine green dye, decreased delivery of bimatoprost eyelash solution with the dermal applicator was noted vs eye drop application. Additionally, it has been demonstrated that approximately 5% (based on weight) of a one-drop dose of bimatoprost eyelash solution applied to the dermal applicator is actually delivered to the patient.18 The rest of the solution remains on the applicator.
It is important that patients use bimatoprost eyelash solution as instructed in the prescribing information (eg, clean the face, remove makeup and contact lenses prior to applying the product). The eyelid should not be rinsed after application, which limits the possibility of the bimatoprost solution from contacting or pooling in the eye. One drop of bimatoprost eyelash solution should be applied to the applicator supplied by the manufacturer and distributed evenly along the skin of the upper eyelid margin at the base of the eyelashes. It is important to blot any excess solution runoff outside the upper eyelid margin.5 Of note, our patient admitted to not always doing this step, which may have contributed to her susceptibility to this rare side effect.
Prostaglandin analogues have been observed to cause iris hyperpigmentation when applied directly to the eye for use in the treatment of glaucoma.19 Theoretically, the same side-effect profile should apply in their use as a dermal application on the eyelids. For this reason, one manufacturer includes iris hyperpigmentation as an adverse side effect in the prescribing information.5 It is important for physicians who prescribe bimatoprost eyelash solution to inform patients of this rare yet possible side effect and to instruct patients on proper application to minimize hyperpigmentation.
Our literature review did not demonstrate previous cases of iris hyperpigmentation associated with bimatoprost eyelash solution. One study suggested that 2 patients experienced hypopigmentation; however, this was not clinically significant and was not consistent with the proposed iris pigmentation thought to be caused by bimatoprost eyelash solution.20
Potential future applications and off-label uses of bimatoprost include treatment of eyelash hypotrichosis on the lower eyelid margin and eyebrow hypertrichosis, as well as androgenic alopecia, alopecia areata, chemotherapy-induced alopecia, vitiligo, and hypopigmented scarring.21 Currently, investigational studies are looking at bimatoprost ophthalmic solution 0.03% for chemotherapy-induced eyelash hypotrichosis with positive results.22 In the future, bimatoprost may be used for other off-label and possibly FDA-approved uses.
- Draelos ZD. Special considerations in eye cosmetics. Clin Dermatol. 2001;19:424-430.
- Mulhern R, Fieldman G, Hussey T, et al. Do cosmetics enhance female Caucasian facial attractiveness? Int J Cosmet Sci. 2003;25:199-205.
- Lumigan [package insert]. Irvine, CA: Allergan, Inc; 2012.
- Higginbotham EJ, Schuman JS, Goldberg I, et al; Bimatoprost Study Groups 1 and 2. one-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch Ophthalmol. 2002;120:1286-1293.
- Latisse [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Hair diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Treatment. 4th ed. St. Louis, MO: C.V. Mosby Company; 2003. 7. Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Selen G, Stjernschantz J, Resul B. Prostaglandin-induced iridial pigmentation in primates. Surv Opthalmol. 1997;41(suppl 2):S125-128.
- Stjernschantz JW, Albert DM, Hu D-N, et al. Mechanism and clinical significance of prostaglandin-induced iris pigmentation. Surv Ophthalmol. 2002;47(suppl 1):162S-S175S.
- Inoue K, Shiokawa M, Sugahara M, et al. Iris and periocular adverse reactions to bimatoprost in Japanese patients with glaucoma or ocular hypertension. Clin Ophthalmol. 2012;6:111-116.
- Alm A, Camras C, Watson P. Phase III latanoprost studies in Scandinavia, the United Kingdom and the United States. Surv Ophthalmol. 1997;41(suppl 2):S105-S110.
- Wistrand PJ, Stjernschantz J, Olsson K. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv Ophthalmol. 1997;41(suppl 2):S129-S138.
- Arranz-Marquez E, Teus MA. Effect of age on the development of a latanoprost-induced increase in iris pigmentation. Ophthalmology. 2007;114:1255-1258.
- Yoelin S, Fagien S, Cox S, et al. A retrospective review and observational study of outcomes and safety of bimatoprost ophthalmic solution 0.03% for treating eyelash hypotrichosis. Dermatol Surg. 2014;40:1118-1124.
- Brandt JD, VanDenburgh AM, Chen K, et al; Bimatoprost Study Group. Comparison of once- or twice-daily bimatoprost with twice-daily timolol in patients with elevated IOP: a 3-month clinical trial. Ophthalmology. 2001;108:1023-1031; discussion 1032.
- Fagien S, Walt JG, Carruthers J, et al. Patient-reported outcomes of bimatoprost for eyelash growth: results from a randomized, double-masked, vehicle-controlled, parallel-group study. Aesthet Surg J. 2013;33:789-798.
- Yoelin S, Walt JG, Earl M. Safety, effectiveness, and subjective experience with topical bimatoprost 0.03% for eyelash growth. Dermatol Surg. 2010;36:638-649.
- Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Rodríguez-Agramonte F, Jiménez JC, Montes JR. Periorbital changes associated with topical prostaglandins analogues in a Hispanic population. P R Health Sci J. 2017;36:218-222.
- Wirta D, Baumann L, Bruce S, et al. Safety and efficacy of bimatoprost for eyelash growth in postchemotherapy subjects. J Clin Aesthet Dermatol. 2015;8:11-20.
- Choi YM, Diehl J, Levins PC. Promising alternative clinical uses of prostaglandin F2α analogs: beyond the eyelashes [published online January 16, 2015]. J Am Acad Dermatol. 2015;72:712-716.
- Ahluwalia GS. Safety and efficacy of bimatoprost solution 0.03% topical application in patients with chemotherapy-induced eyelash loss. J Investig Dermatol Symp Proc. 2013;16:S73-S76.
To the Editor:
Long, dark, and thick eyelashes have been a focal point of society’s perception of beauty for thousands of years,1 and the use of makeup products such as mascaras, eyeliners, and eye shadows has further increased the perception of attractiveness of the eyes.2 Many eyelash enhancement methods have been developed or in some instances have been serendipitously discovered. Bimatoprost ophthalmic solution 0.03% originally was developed as an eye drop that was approved by the US Food and Drug Association (FDA) in 2001 for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. An unexpected side effect of this product was eyelash hypertrichosis.3,4 As a result, the FDA approved
Because all follicular development occurs during embryogenesis, the number of eyelash follicles does not increase over time.6 Bitmatoprost eyelash solution works by prolonging the anagen (growth) phase of the eyelashes and stimulating the transition from the telogen (dormant) phase to the anagen phase. It also has been shown to increase the hair bulb diameter of follicles undergoing the anagen phase, resulting in thicker eyelashes.7 Although many patients have enjoyed this unexpected indication, prostaglandin (PG) analogues such as bimatoprost and latanoprost have a well-documented history of ocular side effects when applied directly to the eye. The most common adverse reactions include eye pruritus, conjunctival hyperemia, and eyelid pigmentation.3 The product safety information indicates that eyelid pigmentation typically is reversible.3,5 Iris pigmentation is perhaps the least desirable side effect of PG analogues and was first noted in latanoprost studies on primates.8 The underlying mechanism appears to be due to an increase in melanogenesis that results in an increase in melanin granules without concomitant proliferation of melanocytes, cellular atypia, or evidence of inflammatory reaction. Unfortunately, this pigmentation typically is permanent.3,5,9
Studies have shown that
An otherwise healthy 63-year-old woman presented to our clinic for an annual skin examination. She noted that she had worsening dark pigmentation of the bilateral irises. The patient did not have any personal or family history of melanoma or ocular nevi, and there were no associated symptoms of eye tearing, pruritus, burning, or discharge. No prior surgical procedures had been performed on or around the eyes, and the patient never used contact lenses. She had been intermittently using bimatoprost eyelash solution prescribed by an outside physician for approximately 3 years to enhance her eyelashes. Although she never applied the product directly into her eyes, she noted that she often was unmethodical in application of the product and that runoff from the product may have occasionally leaked into the eyes. Physical examination revealed bilateral blue irises with ink spot–like, grayish black patches encircling the bilateral pupils (Figure).

The patient was advised to stop using the product, but no improvement of the iris hyperpigmentation was appreciated at 6-month follow-up. The patient declined referral to ophthalmology for evaluation to confirm a diagnosis and discuss treatment because the hyperpigmentation did not bother her.
There have been several studies of iris hyperpigmentation with use of PG analogues in the treatment of glaucoma. In a phase 3 clinical trial of the safety and efficacy of latanoprost for treatment of ocular hypertension, it was noted that 24 (12%) of 198 patients experienced iris hyperpigmentation and that patients with heterogeneous pigmentation (ie, hazel irises and mixed coloring) were at an increased risk.11 Other studies also have shown an increased risk of iris hyperpigmentation due to heterogeneous phenotype12 as well as older age.13
Reports of bimatoprost eye drops used for treatment of glaucoma have shown a high incidence of iris hyperpigmentation with long-term use. A prospective study conducted in 2012 investigated the adverse events of bimatoprost eye drops in 52 Japanese patients with glaucoma or ocular hypertension. Clinical photographs of the irises, eyelids, and eyelashes were taken at baseline and after 6 months of treatment. It was noted that 50% (26/52) of participants experienced iris hyperpigmentation upon completion of treatment.10
In our patient, bimatoprost eyelash solution was applied to the top eyelid margins using an applicator; our patient did not use the eye drop formulation, which is directed for use in ocular hypertension or glaucoma. A PubMed search of articles indexed for MEDLINE using the terms bimatoprost and iris hyperpigmentation yielded no published peer-reviewed studies or case reports of iris hyperpigmentation caused by bimatoprost eyelash solution for treatment of eyelid hypotrichosis, which makes this case report novel. With that said, the package insert states iris hyperpigmentation as a side effect in the prescribing information for both a bimatoprost eye drop formulation used to treat ocular hypertension3 as well as a formulation for topical application on the eyelids/eyelashes.5 A 2014 retrospective review of long-term safety with bimatoprost eyelash solution for eyelash hypotrichosis reported 4 instances (0.7%) of documented adverse events after 12 months of use in 585 patients, including dry eye, eyelid erythema, ocular pruritus, and low ocular pressure. Iris hyperpigmentation was not reported.14
The method of bimatoprost application likely is a determining factor in the number of reported adverse events. Studies with similar treatment periods have demonstrated more adverse events associated with bimatoprost eye drops vs eyelash solution.15,16 When bimatoprost is used in the eye drop formulation for treatment of glaucoma, iris hyperpigmentation has been estimated to occur in 1.5%4 to 50%9 of cases. To our knowledge, there are no documented cases when bimatoprost eyelash solution is applied with a dermal applicator for treatment of eyelash hypotrichosis.15,17 These results may be explained using an ocular splash test. In one study using lissamine green dye, decreased delivery of bimatoprost eyelash solution with the dermal applicator was noted vs eye drop application. Additionally, it has been demonstrated that approximately 5% (based on weight) of a one-drop dose of bimatoprost eyelash solution applied to the dermal applicator is actually delivered to the patient.18 The rest of the solution remains on the applicator.
It is important that patients use bimatoprost eyelash solution as instructed in the prescribing information (eg, clean the face, remove makeup and contact lenses prior to applying the product). The eyelid should not be rinsed after application, which limits the possibility of the bimatoprost solution from contacting or pooling in the eye. One drop of bimatoprost eyelash solution should be applied to the applicator supplied by the manufacturer and distributed evenly along the skin of the upper eyelid margin at the base of the eyelashes. It is important to blot any excess solution runoff outside the upper eyelid margin.5 Of note, our patient admitted to not always doing this step, which may have contributed to her susceptibility to this rare side effect.
Prostaglandin analogues have been observed to cause iris hyperpigmentation when applied directly to the eye for use in the treatment of glaucoma.19 Theoretically, the same side-effect profile should apply in their use as a dermal application on the eyelids. For this reason, one manufacturer includes iris hyperpigmentation as an adverse side effect in the prescribing information.5 It is important for physicians who prescribe bimatoprost eyelash solution to inform patients of this rare yet possible side effect and to instruct patients on proper application to minimize hyperpigmentation.
Our literature review did not demonstrate previous cases of iris hyperpigmentation associated with bimatoprost eyelash solution. One study suggested that 2 patients experienced hypopigmentation; however, this was not clinically significant and was not consistent with the proposed iris pigmentation thought to be caused by bimatoprost eyelash solution.20
Potential future applications and off-label uses of bimatoprost include treatment of eyelash hypotrichosis on the lower eyelid margin and eyebrow hypertrichosis, as well as androgenic alopecia, alopecia areata, chemotherapy-induced alopecia, vitiligo, and hypopigmented scarring.21 Currently, investigational studies are looking at bimatoprost ophthalmic solution 0.03% for chemotherapy-induced eyelash hypotrichosis with positive results.22 In the future, bimatoprost may be used for other off-label and possibly FDA-approved uses.
To the Editor:
Long, dark, and thick eyelashes have been a focal point of society’s perception of beauty for thousands of years,1 and the use of makeup products such as mascaras, eyeliners, and eye shadows has further increased the perception of attractiveness of the eyes.2 Many eyelash enhancement methods have been developed or in some instances have been serendipitously discovered. Bimatoprost ophthalmic solution 0.03% originally was developed as an eye drop that was approved by the US Food and Drug Association (FDA) in 2001 for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. An unexpected side effect of this product was eyelash hypertrichosis.3,4 As a result, the FDA approved
Because all follicular development occurs during embryogenesis, the number of eyelash follicles does not increase over time.6 Bitmatoprost eyelash solution works by prolonging the anagen (growth) phase of the eyelashes and stimulating the transition from the telogen (dormant) phase to the anagen phase. It also has been shown to increase the hair bulb diameter of follicles undergoing the anagen phase, resulting in thicker eyelashes.7 Although many patients have enjoyed this unexpected indication, prostaglandin (PG) analogues such as bimatoprost and latanoprost have a well-documented history of ocular side effects when applied directly to the eye. The most common adverse reactions include eye pruritus, conjunctival hyperemia, and eyelid pigmentation.3 The product safety information indicates that eyelid pigmentation typically is reversible.3,5 Iris pigmentation is perhaps the least desirable side effect of PG analogues and was first noted in latanoprost studies on primates.8 The underlying mechanism appears to be due to an increase in melanogenesis that results in an increase in melanin granules without concomitant proliferation of melanocytes, cellular atypia, or evidence of inflammatory reaction. Unfortunately, this pigmentation typically is permanent.3,5,9
Studies have shown that
An otherwise healthy 63-year-old woman presented to our clinic for an annual skin examination. She noted that she had worsening dark pigmentation of the bilateral irises. The patient did not have any personal or family history of melanoma or ocular nevi, and there were no associated symptoms of eye tearing, pruritus, burning, or discharge. No prior surgical procedures had been performed on or around the eyes, and the patient never used contact lenses. She had been intermittently using bimatoprost eyelash solution prescribed by an outside physician for approximately 3 years to enhance her eyelashes. Although she never applied the product directly into her eyes, she noted that she often was unmethodical in application of the product and that runoff from the product may have occasionally leaked into the eyes. Physical examination revealed bilateral blue irises with ink spot–like, grayish black patches encircling the bilateral pupils (Figure).

The patient was advised to stop using the product, but no improvement of the iris hyperpigmentation was appreciated at 6-month follow-up. The patient declined referral to ophthalmology for evaluation to confirm a diagnosis and discuss treatment because the hyperpigmentation did not bother her.
There have been several studies of iris hyperpigmentation with use of PG analogues in the treatment of glaucoma. In a phase 3 clinical trial of the safety and efficacy of latanoprost for treatment of ocular hypertension, it was noted that 24 (12%) of 198 patients experienced iris hyperpigmentation and that patients with heterogeneous pigmentation (ie, hazel irises and mixed coloring) were at an increased risk.11 Other studies also have shown an increased risk of iris hyperpigmentation due to heterogeneous phenotype12 as well as older age.13
Reports of bimatoprost eye drops used for treatment of glaucoma have shown a high incidence of iris hyperpigmentation with long-term use. A prospective study conducted in 2012 investigated the adverse events of bimatoprost eye drops in 52 Japanese patients with glaucoma or ocular hypertension. Clinical photographs of the irises, eyelids, and eyelashes were taken at baseline and after 6 months of treatment. It was noted that 50% (26/52) of participants experienced iris hyperpigmentation upon completion of treatment.10
In our patient, bimatoprost eyelash solution was applied to the top eyelid margins using an applicator; our patient did not use the eye drop formulation, which is directed for use in ocular hypertension or glaucoma. A PubMed search of articles indexed for MEDLINE using the terms bimatoprost and iris hyperpigmentation yielded no published peer-reviewed studies or case reports of iris hyperpigmentation caused by bimatoprost eyelash solution for treatment of eyelid hypotrichosis, which makes this case report novel. With that said, the package insert states iris hyperpigmentation as a side effect in the prescribing information for both a bimatoprost eye drop formulation used to treat ocular hypertension3 as well as a formulation for topical application on the eyelids/eyelashes.5 A 2014 retrospective review of long-term safety with bimatoprost eyelash solution for eyelash hypotrichosis reported 4 instances (0.7%) of documented adverse events after 12 months of use in 585 patients, including dry eye, eyelid erythema, ocular pruritus, and low ocular pressure. Iris hyperpigmentation was not reported.14
The method of bimatoprost application likely is a determining factor in the number of reported adverse events. Studies with similar treatment periods have demonstrated more adverse events associated with bimatoprost eye drops vs eyelash solution.15,16 When bimatoprost is used in the eye drop formulation for treatment of glaucoma, iris hyperpigmentation has been estimated to occur in 1.5%4 to 50%9 of cases. To our knowledge, there are no documented cases when bimatoprost eyelash solution is applied with a dermal applicator for treatment of eyelash hypotrichosis.15,17 These results may be explained using an ocular splash test. In one study using lissamine green dye, decreased delivery of bimatoprost eyelash solution with the dermal applicator was noted vs eye drop application. Additionally, it has been demonstrated that approximately 5% (based on weight) of a one-drop dose of bimatoprost eyelash solution applied to the dermal applicator is actually delivered to the patient.18 The rest of the solution remains on the applicator.
It is important that patients use bimatoprost eyelash solution as instructed in the prescribing information (eg, clean the face, remove makeup and contact lenses prior to applying the product). The eyelid should not be rinsed after application, which limits the possibility of the bimatoprost solution from contacting or pooling in the eye. One drop of bimatoprost eyelash solution should be applied to the applicator supplied by the manufacturer and distributed evenly along the skin of the upper eyelid margin at the base of the eyelashes. It is important to blot any excess solution runoff outside the upper eyelid margin.5 Of note, our patient admitted to not always doing this step, which may have contributed to her susceptibility to this rare side effect.
Prostaglandin analogues have been observed to cause iris hyperpigmentation when applied directly to the eye for use in the treatment of glaucoma.19 Theoretically, the same side-effect profile should apply in their use as a dermal application on the eyelids. For this reason, one manufacturer includes iris hyperpigmentation as an adverse side effect in the prescribing information.5 It is important for physicians who prescribe bimatoprost eyelash solution to inform patients of this rare yet possible side effect and to instruct patients on proper application to minimize hyperpigmentation.
Our literature review did not demonstrate previous cases of iris hyperpigmentation associated with bimatoprost eyelash solution. One study suggested that 2 patients experienced hypopigmentation; however, this was not clinically significant and was not consistent with the proposed iris pigmentation thought to be caused by bimatoprost eyelash solution.20
Potential future applications and off-label uses of bimatoprost include treatment of eyelash hypotrichosis on the lower eyelid margin and eyebrow hypertrichosis, as well as androgenic alopecia, alopecia areata, chemotherapy-induced alopecia, vitiligo, and hypopigmented scarring.21 Currently, investigational studies are looking at bimatoprost ophthalmic solution 0.03% for chemotherapy-induced eyelash hypotrichosis with positive results.22 In the future, bimatoprost may be used for other off-label and possibly FDA-approved uses.
- Draelos ZD. Special considerations in eye cosmetics. Clin Dermatol. 2001;19:424-430.
- Mulhern R, Fieldman G, Hussey T, et al. Do cosmetics enhance female Caucasian facial attractiveness? Int J Cosmet Sci. 2003;25:199-205.
- Lumigan [package insert]. Irvine, CA: Allergan, Inc; 2012.
- Higginbotham EJ, Schuman JS, Goldberg I, et al; Bimatoprost Study Groups 1 and 2. one-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch Ophthalmol. 2002;120:1286-1293.
- Latisse [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Hair diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Treatment. 4th ed. St. Louis, MO: C.V. Mosby Company; 2003. 7. Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Selen G, Stjernschantz J, Resul B. Prostaglandin-induced iridial pigmentation in primates. Surv Opthalmol. 1997;41(suppl 2):S125-128.
- Stjernschantz JW, Albert DM, Hu D-N, et al. Mechanism and clinical significance of prostaglandin-induced iris pigmentation. Surv Ophthalmol. 2002;47(suppl 1):162S-S175S.
- Inoue K, Shiokawa M, Sugahara M, et al. Iris and periocular adverse reactions to bimatoprost in Japanese patients with glaucoma or ocular hypertension. Clin Ophthalmol. 2012;6:111-116.
- Alm A, Camras C, Watson P. Phase III latanoprost studies in Scandinavia, the United Kingdom and the United States. Surv Ophthalmol. 1997;41(suppl 2):S105-S110.
- Wistrand PJ, Stjernschantz J, Olsson K. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv Ophthalmol. 1997;41(suppl 2):S129-S138.
- Arranz-Marquez E, Teus MA. Effect of age on the development of a latanoprost-induced increase in iris pigmentation. Ophthalmology. 2007;114:1255-1258.
- Yoelin S, Fagien S, Cox S, et al. A retrospective review and observational study of outcomes and safety of bimatoprost ophthalmic solution 0.03% for treating eyelash hypotrichosis. Dermatol Surg. 2014;40:1118-1124.
- Brandt JD, VanDenburgh AM, Chen K, et al; Bimatoprost Study Group. Comparison of once- or twice-daily bimatoprost with twice-daily timolol in patients with elevated IOP: a 3-month clinical trial. Ophthalmology. 2001;108:1023-1031; discussion 1032.
- Fagien S, Walt JG, Carruthers J, et al. Patient-reported outcomes of bimatoprost for eyelash growth: results from a randomized, double-masked, vehicle-controlled, parallel-group study. Aesthet Surg J. 2013;33:789-798.
- Yoelin S, Walt JG, Earl M. Safety, effectiveness, and subjective experience with topical bimatoprost 0.03% for eyelash growth. Dermatol Surg. 2010;36:638-649.
- Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Rodríguez-Agramonte F, Jiménez JC, Montes JR. Periorbital changes associated with topical prostaglandins analogues in a Hispanic population. P R Health Sci J. 2017;36:218-222.
- Wirta D, Baumann L, Bruce S, et al. Safety and efficacy of bimatoprost for eyelash growth in postchemotherapy subjects. J Clin Aesthet Dermatol. 2015;8:11-20.
- Choi YM, Diehl J, Levins PC. Promising alternative clinical uses of prostaglandin F2α analogs: beyond the eyelashes [published online January 16, 2015]. J Am Acad Dermatol. 2015;72:712-716.
- Ahluwalia GS. Safety and efficacy of bimatoprost solution 0.03% topical application in patients with chemotherapy-induced eyelash loss. J Investig Dermatol Symp Proc. 2013;16:S73-S76.
- Draelos ZD. Special considerations in eye cosmetics. Clin Dermatol. 2001;19:424-430.
- Mulhern R, Fieldman G, Hussey T, et al. Do cosmetics enhance female Caucasian facial attractiveness? Int J Cosmet Sci. 2003;25:199-205.
- Lumigan [package insert]. Irvine, CA: Allergan, Inc; 2012.
- Higginbotham EJ, Schuman JS, Goldberg I, et al; Bimatoprost Study Groups 1 and 2. one-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch Ophthalmol. 2002;120:1286-1293.
- Latisse [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Hair diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Treatment. 4th ed. St. Louis, MO: C.V. Mosby Company; 2003. 7. Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Selen G, Stjernschantz J, Resul B. Prostaglandin-induced iridial pigmentation in primates. Surv Opthalmol. 1997;41(suppl 2):S125-128.
- Stjernschantz JW, Albert DM, Hu D-N, et al. Mechanism and clinical significance of prostaglandin-induced iris pigmentation. Surv Ophthalmol. 2002;47(suppl 1):162S-S175S.
- Inoue K, Shiokawa M, Sugahara M, et al. Iris and periocular adverse reactions to bimatoprost in Japanese patients with glaucoma or ocular hypertension. Clin Ophthalmol. 2012;6:111-116.
- Alm A, Camras C, Watson P. Phase III latanoprost studies in Scandinavia, the United Kingdom and the United States. Surv Ophthalmol. 1997;41(suppl 2):S105-S110.
- Wistrand PJ, Stjernschantz J, Olsson K. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv Ophthalmol. 1997;41(suppl 2):S129-S138.
- Arranz-Marquez E, Teus MA. Effect of age on the development of a latanoprost-induced increase in iris pigmentation. Ophthalmology. 2007;114:1255-1258.
- Yoelin S, Fagien S, Cox S, et al. A retrospective review and observational study of outcomes and safety of bimatoprost ophthalmic solution 0.03% for treating eyelash hypotrichosis. Dermatol Surg. 2014;40:1118-1124.
- Brandt JD, VanDenburgh AM, Chen K, et al; Bimatoprost Study Group. Comparison of once- or twice-daily bimatoprost with twice-daily timolol in patients with elevated IOP: a 3-month clinical trial. Ophthalmology. 2001;108:1023-1031; discussion 1032.
- Fagien S, Walt JG, Carruthers J, et al. Patient-reported outcomes of bimatoprost for eyelash growth: results from a randomized, double-masked, vehicle-controlled, parallel-group study. Aesthet Surg J. 2013;33:789-798.
- Yoelin S, Walt JG, Earl M. Safety, effectiveness, and subjective experience with topical bimatoprost 0.03% for eyelash growth. Dermatol Surg. 2010;36:638-649.
- Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Rodríguez-Agramonte F, Jiménez JC, Montes JR. Periorbital changes associated with topical prostaglandins analogues in a Hispanic population. P R Health Sci J. 2017;36:218-222.
- Wirta D, Baumann L, Bruce S, et al. Safety and efficacy of bimatoprost for eyelash growth in postchemotherapy subjects. J Clin Aesthet Dermatol. 2015;8:11-20.
- Choi YM, Diehl J, Levins PC. Promising alternative clinical uses of prostaglandin F2α analogs: beyond the eyelashes [published online January 16, 2015]. J Am Acad Dermatol. 2015;72:712-716.
- Ahluwalia GS. Safety and efficacy of bimatoprost solution 0.03% topical application in patients with chemotherapy-induced eyelash loss. J Investig Dermatol Symp Proc. 2013;16:S73-S76.
Practice Points
- Bimatoprost ophthalmic solution 0.03% was approved by the US Food and Drug Administration in 2008 as an eyelash solution with an eyelid applicator for treatment of eyelash hypotrichosis.
- Iris hyperpigmentation can occur when bimatoprost eye drops are applied to the eyes for treatment of ocular hypertension and glaucoma, but reports associated with bimatoprost eyelash solution are rare.
- It is important that patients use bimatoprost eyelash solution as instructed in the prescribing information to avoid potential adverse events. The eyelid should not be rinsed after application, which limits the possibility of the bimatoprost solution from contacting or pooling in the eye.