User login
FDA proposes withdrawing Makena’s approval
Makena should be withdrawn from the market because a postmarketing study did not show clinical benefit, according to a statement released today from the Center for Drug Evaluation and Research at the Food and Drug Administration.
The drug, hydroxyprogesterone caproate injection, was approved in 2011 to reduce the risk of preterm birth in women who with previous spontaneous preterm birth. The FDA approved the medication under an accelerated pathway that required another trial to confirm clinical benefit.
The required postmarketing study “not only failed to demonstrate Makena’s benefit to the neonate, but also failed to substantiate any effect of Makena on the surrogate endpoint of gestational age at delivery that was the basis of the initial approval,” Patrizia Cavazzoni, MD, acting director of the CDER, wrote in a letter to AMAG Pharma USA, which markets Makena. The letter also was sent to other companies developing products that use the drug.
Beyond the lack of efficacy, risks associated with the drug include thromboembolic disorders, allergic reactions, decreased glucose tolerance, and fluid retention. “The risk of exposing treated pregnant women to these harms, in addition to false hopes, costs, and additional healthcare utilization outweighs Makena’s unproven benefit,” Dr. Cavazzoni said.
The letter notifies companies about the opportunity for a hearing on the proposed withdrawal of marketing approval. Makena and its generic equivalents will remain on the market until the manufacturers remove the drugs or the FDA commissioner mandates their removal, the CDER said.
The FDA commissioner ultimately will decide whether to withdraw approval of the drug. An FDA panel previously voted to withdraw the drug from the market in October 2019, and the drug has remained in limbo since.
Health care professionals should discuss “Makena’s benefits, risks, and uncertainties with their patients to decide whether to use Makena while a final decision is being made about the drug’s marketing status,” the CDER announcement said.
A version of this article originally appeared on Medscape.com.
Makena should be withdrawn from the market because a postmarketing study did not show clinical benefit, according to a statement released today from the Center for Drug Evaluation and Research at the Food and Drug Administration.
The drug, hydroxyprogesterone caproate injection, was approved in 2011 to reduce the risk of preterm birth in women who with previous spontaneous preterm birth. The FDA approved the medication under an accelerated pathway that required another trial to confirm clinical benefit.
The required postmarketing study “not only failed to demonstrate Makena’s benefit to the neonate, but also failed to substantiate any effect of Makena on the surrogate endpoint of gestational age at delivery that was the basis of the initial approval,” Patrizia Cavazzoni, MD, acting director of the CDER, wrote in a letter to AMAG Pharma USA, which markets Makena. The letter also was sent to other companies developing products that use the drug.
Beyond the lack of efficacy, risks associated with the drug include thromboembolic disorders, allergic reactions, decreased glucose tolerance, and fluid retention. “The risk of exposing treated pregnant women to these harms, in addition to false hopes, costs, and additional healthcare utilization outweighs Makena’s unproven benefit,” Dr. Cavazzoni said.
The letter notifies companies about the opportunity for a hearing on the proposed withdrawal of marketing approval. Makena and its generic equivalents will remain on the market until the manufacturers remove the drugs or the FDA commissioner mandates their removal, the CDER said.
The FDA commissioner ultimately will decide whether to withdraw approval of the drug. An FDA panel previously voted to withdraw the drug from the market in October 2019, and the drug has remained in limbo since.
Health care professionals should discuss “Makena’s benefits, risks, and uncertainties with their patients to decide whether to use Makena while a final decision is being made about the drug’s marketing status,” the CDER announcement said.
A version of this article originally appeared on Medscape.com.
Makena should be withdrawn from the market because a postmarketing study did not show clinical benefit, according to a statement released today from the Center for Drug Evaluation and Research at the Food and Drug Administration.
The drug, hydroxyprogesterone caproate injection, was approved in 2011 to reduce the risk of preterm birth in women who with previous spontaneous preterm birth. The FDA approved the medication under an accelerated pathway that required another trial to confirm clinical benefit.
The required postmarketing study “not only failed to demonstrate Makena’s benefit to the neonate, but also failed to substantiate any effect of Makena on the surrogate endpoint of gestational age at delivery that was the basis of the initial approval,” Patrizia Cavazzoni, MD, acting director of the CDER, wrote in a letter to AMAG Pharma USA, which markets Makena. The letter also was sent to other companies developing products that use the drug.
Beyond the lack of efficacy, risks associated with the drug include thromboembolic disorders, allergic reactions, decreased glucose tolerance, and fluid retention. “The risk of exposing treated pregnant women to these harms, in addition to false hopes, costs, and additional healthcare utilization outweighs Makena’s unproven benefit,” Dr. Cavazzoni said.
The letter notifies companies about the opportunity for a hearing on the proposed withdrawal of marketing approval. Makena and its generic equivalents will remain on the market until the manufacturers remove the drugs or the FDA commissioner mandates their removal, the CDER said.
The FDA commissioner ultimately will decide whether to withdraw approval of the drug. An FDA panel previously voted to withdraw the drug from the market in October 2019, and the drug has remained in limbo since.
Health care professionals should discuss “Makena’s benefits, risks, and uncertainties with their patients to decide whether to use Makena while a final decision is being made about the drug’s marketing status,” the CDER announcement said.
A version of this article originally appeared on Medscape.com.
Rural areas with local obstetrical care have better perinatal outcomes
according to a retrospective study using county-level data from the Alabama Department of Public Health.
Although association does not establish causation, these data raise concern “for the current trend of diminishing L&D units that is occurring in many rural settings,” according to the authors of the study, led by John B. Waits, MD, of Cahaba Medical Care, Centreville, Ala., in Annals of Family Medicine.
When mortality per 1,000 live births was compared over a 15-year period (2003-2017) between 15 counties with and 21 counties without local L&D units, those with the units had lower overall infant mortality (9.23 vs. 7.89; P = .0011), perinatal mortality (8.89 vs. 10.82; P < .001), and neonatal mortality (4.74 vs. 5.67; P = .0034). The percentages of low-birth-weight babies born between 2003 and 2014 were 9.86% versus 10.61% (P < .001) for counties with and without L&D units, respectively.
The relative increased risks (RR) for these adverse outcomes in counties without L&D units were statistically significant and substantial, ranging from about 8% for a pregnancy resulting in a low-birth-weight infant to slightly more than 21% for perinatal mortality.
Over the study period, there were 165,525 live births in the 15 counties with L&D units and 72,177 births in the 21 counties with no such units. In counties without L&D units, the average proportion of White people was higher (73.47% vs. 60.86%), and that of African Americans was lower (22.76% vs. 36.23%). Median income ($40,759 vs. $35,604) and per capita income ($22,474 vs. $20,641) was slightly higher.
Of the 67 counties in Alabama, this study did not include those considered urbanized by the Alabama Office of Management and Budget even if classified rural by other statewide offices, such as the Alabama Rural Health Association. Any county with at least one L&D unit was considered to have a local unit. Three counties with L&D units that closed before the observation period was completed were excluded from the analysis.
The Alabama data appear to identify a major problem in need of an urgent solution, according to John S. Cullen, MD, a family physician in Valdez, Alaska, and chair of the American Academy of Family Physicians Board of Directors.
“Almost 20% of U.S. women of reproductive age live in rural communities,” he said in an interview. The data from this study provides compelling evidence “that the loss of rural maternity care in this country has contributed to the increase in newborn mortality in rural communities.”
There are many limitations for this study, according to the authors. They acknowledged that they could not control for many potentially important variables, such as travel time to hospitals for those in counties with L&D units when compared with those without. They also acknowledged the lack of data regarding availability of prenatal care in places with or without L&D units.
If lack of L&D services in rural areas is a source of adverse outcomes, data suggesting that the ongoing decline in L&D units are worrisome, according to the authors. Of studies they cited, one showed nearly a 10% loss in rural L&D services in a recent 10-year period.
The authors also noted that about half of the 3,143 counties in the United States do not have a practicing obstetrician, and that fewer than 7% of obstetricians-gynecologists practice in rural settings.
In many rural counties, including the county where the lead author practices, family practitioners provide 100% of local obstetric care, but access to these clinicians also appears to be declining, according to the paper. The ratio of primary care physicians to patients is already lower in non-metropolitan than metropolitan areas (39.8 vs. 53.3). The American Board of Family Medicine has reported that fewer than 10% of family physicians now provide maternity care, the authors wrote.
“If a causal relationship does exist [between lack of L&D units and adverse perinatal outcomes], then rural populations would definitively benefit from having local access to a L&D unit,” the authors stated.
The lead author, Dr. Waits, said in an interview that there are two obstacles to an increase in rural L&D units: malpractice premiums and reimbursement for indigent deliveries. The large malpractice premiums required to cover OB care are hurdles for caregivers, such as family physicians, as well as the hospitals where they practice.
Reforms from the legislative or regulatory perspective are needed to permit malpractice insurance to be issued at a reasonable cost, according to Dr. Waits. Such reforms are a “moral imperative” so that the malpractice issue is not allowed to “shipwreck infant and maternal mortality,” he said.
Of the many potential solutions, such as increased use of telemedicine, legislative initiatives to reduce the malpractice burden, or new support and incentives for family physicians to deliver OB care, each is burdened with obstacles to overcome, according to Dr. Waits. This does not mean these solutions should not be pursued alone or together, but he made it clear that the no solution is easy. In the meantime, Dr. Waits indicated a need to consider practical and immediate strategies to fix the problem.
“There should be incentives for rural emergency departments and ambulance systems to train in the [American Academy of Family Physicians’] Basic Life Support in Obstetrics (BLSO) certification courses each year. I am not aware of any specific evidence around this, but it is a known fact that, when L&Ds close, institutional memory of OB emergencies recede, and preparedness suffers,” he said.
Dr. Cullen agreed that if the closing of L&D units explains the higher rate of perinatal mortality in rural areas, both short-term and long-term solutions are needed.
“Every community must have a plan for obstetric and newborn emergencies. The decision to not offer maternity care means that rural providers will still provide maternity care but not be ready for emergencies,” he said, echoing a point made by Dr. Waits.
The study authors disclosed no conflicts. Dr. Cullen reported having no disclosures.
SOURCE: Waits JB et al. Ann Fam Med. 2020;18:446-51.
according to a retrospective study using county-level data from the Alabama Department of Public Health.
Although association does not establish causation, these data raise concern “for the current trend of diminishing L&D units that is occurring in many rural settings,” according to the authors of the study, led by John B. Waits, MD, of Cahaba Medical Care, Centreville, Ala., in Annals of Family Medicine.
When mortality per 1,000 live births was compared over a 15-year period (2003-2017) between 15 counties with and 21 counties without local L&D units, those with the units had lower overall infant mortality (9.23 vs. 7.89; P = .0011), perinatal mortality (8.89 vs. 10.82; P < .001), and neonatal mortality (4.74 vs. 5.67; P = .0034). The percentages of low-birth-weight babies born between 2003 and 2014 were 9.86% versus 10.61% (P < .001) for counties with and without L&D units, respectively.
The relative increased risks (RR) for these adverse outcomes in counties without L&D units were statistically significant and substantial, ranging from about 8% for a pregnancy resulting in a low-birth-weight infant to slightly more than 21% for perinatal mortality.
Over the study period, there were 165,525 live births in the 15 counties with L&D units and 72,177 births in the 21 counties with no such units. In counties without L&D units, the average proportion of White people was higher (73.47% vs. 60.86%), and that of African Americans was lower (22.76% vs. 36.23%). Median income ($40,759 vs. $35,604) and per capita income ($22,474 vs. $20,641) was slightly higher.
Of the 67 counties in Alabama, this study did not include those considered urbanized by the Alabama Office of Management and Budget even if classified rural by other statewide offices, such as the Alabama Rural Health Association. Any county with at least one L&D unit was considered to have a local unit. Three counties with L&D units that closed before the observation period was completed were excluded from the analysis.
The Alabama data appear to identify a major problem in need of an urgent solution, according to John S. Cullen, MD, a family physician in Valdez, Alaska, and chair of the American Academy of Family Physicians Board of Directors.
“Almost 20% of U.S. women of reproductive age live in rural communities,” he said in an interview. The data from this study provides compelling evidence “that the loss of rural maternity care in this country has contributed to the increase in newborn mortality in rural communities.”
There are many limitations for this study, according to the authors. They acknowledged that they could not control for many potentially important variables, such as travel time to hospitals for those in counties with L&D units when compared with those without. They also acknowledged the lack of data regarding availability of prenatal care in places with or without L&D units.
If lack of L&D services in rural areas is a source of adverse outcomes, data suggesting that the ongoing decline in L&D units are worrisome, according to the authors. Of studies they cited, one showed nearly a 10% loss in rural L&D services in a recent 10-year period.
The authors also noted that about half of the 3,143 counties in the United States do not have a practicing obstetrician, and that fewer than 7% of obstetricians-gynecologists practice in rural settings.
In many rural counties, including the county where the lead author practices, family practitioners provide 100% of local obstetric care, but access to these clinicians also appears to be declining, according to the paper. The ratio of primary care physicians to patients is already lower in non-metropolitan than metropolitan areas (39.8 vs. 53.3). The American Board of Family Medicine has reported that fewer than 10% of family physicians now provide maternity care, the authors wrote.
“If a causal relationship does exist [between lack of L&D units and adverse perinatal outcomes], then rural populations would definitively benefit from having local access to a L&D unit,” the authors stated.
The lead author, Dr. Waits, said in an interview that there are two obstacles to an increase in rural L&D units: malpractice premiums and reimbursement for indigent deliveries. The large malpractice premiums required to cover OB care are hurdles for caregivers, such as family physicians, as well as the hospitals where they practice.
Reforms from the legislative or regulatory perspective are needed to permit malpractice insurance to be issued at a reasonable cost, according to Dr. Waits. Such reforms are a “moral imperative” so that the malpractice issue is not allowed to “shipwreck infant and maternal mortality,” he said.
Of the many potential solutions, such as increased use of telemedicine, legislative initiatives to reduce the malpractice burden, or new support and incentives for family physicians to deliver OB care, each is burdened with obstacles to overcome, according to Dr. Waits. This does not mean these solutions should not be pursued alone or together, but he made it clear that the no solution is easy. In the meantime, Dr. Waits indicated a need to consider practical and immediate strategies to fix the problem.
“There should be incentives for rural emergency departments and ambulance systems to train in the [American Academy of Family Physicians’] Basic Life Support in Obstetrics (BLSO) certification courses each year. I am not aware of any specific evidence around this, but it is a known fact that, when L&Ds close, institutional memory of OB emergencies recede, and preparedness suffers,” he said.
Dr. Cullen agreed that if the closing of L&D units explains the higher rate of perinatal mortality in rural areas, both short-term and long-term solutions are needed.
“Every community must have a plan for obstetric and newborn emergencies. The decision to not offer maternity care means that rural providers will still provide maternity care but not be ready for emergencies,” he said, echoing a point made by Dr. Waits.
The study authors disclosed no conflicts. Dr. Cullen reported having no disclosures.
SOURCE: Waits JB et al. Ann Fam Med. 2020;18:446-51.
according to a retrospective study using county-level data from the Alabama Department of Public Health.
Although association does not establish causation, these data raise concern “for the current trend of diminishing L&D units that is occurring in many rural settings,” according to the authors of the study, led by John B. Waits, MD, of Cahaba Medical Care, Centreville, Ala., in Annals of Family Medicine.
When mortality per 1,000 live births was compared over a 15-year period (2003-2017) between 15 counties with and 21 counties without local L&D units, those with the units had lower overall infant mortality (9.23 vs. 7.89; P = .0011), perinatal mortality (8.89 vs. 10.82; P < .001), and neonatal mortality (4.74 vs. 5.67; P = .0034). The percentages of low-birth-weight babies born between 2003 and 2014 were 9.86% versus 10.61% (P < .001) for counties with and without L&D units, respectively.
The relative increased risks (RR) for these adverse outcomes in counties without L&D units were statistically significant and substantial, ranging from about 8% for a pregnancy resulting in a low-birth-weight infant to slightly more than 21% for perinatal mortality.
Over the study period, there were 165,525 live births in the 15 counties with L&D units and 72,177 births in the 21 counties with no such units. In counties without L&D units, the average proportion of White people was higher (73.47% vs. 60.86%), and that of African Americans was lower (22.76% vs. 36.23%). Median income ($40,759 vs. $35,604) and per capita income ($22,474 vs. $20,641) was slightly higher.
Of the 67 counties in Alabama, this study did not include those considered urbanized by the Alabama Office of Management and Budget even if classified rural by other statewide offices, such as the Alabama Rural Health Association. Any county with at least one L&D unit was considered to have a local unit. Three counties with L&D units that closed before the observation period was completed were excluded from the analysis.
The Alabama data appear to identify a major problem in need of an urgent solution, according to John S. Cullen, MD, a family physician in Valdez, Alaska, and chair of the American Academy of Family Physicians Board of Directors.
“Almost 20% of U.S. women of reproductive age live in rural communities,” he said in an interview. The data from this study provides compelling evidence “that the loss of rural maternity care in this country has contributed to the increase in newborn mortality in rural communities.”
There are many limitations for this study, according to the authors. They acknowledged that they could not control for many potentially important variables, such as travel time to hospitals for those in counties with L&D units when compared with those without. They also acknowledged the lack of data regarding availability of prenatal care in places with or without L&D units.
If lack of L&D services in rural areas is a source of adverse outcomes, data suggesting that the ongoing decline in L&D units are worrisome, according to the authors. Of studies they cited, one showed nearly a 10% loss in rural L&D services in a recent 10-year period.
The authors also noted that about half of the 3,143 counties in the United States do not have a practicing obstetrician, and that fewer than 7% of obstetricians-gynecologists practice in rural settings.
In many rural counties, including the county where the lead author practices, family practitioners provide 100% of local obstetric care, but access to these clinicians also appears to be declining, according to the paper. The ratio of primary care physicians to patients is already lower in non-metropolitan than metropolitan areas (39.8 vs. 53.3). The American Board of Family Medicine has reported that fewer than 10% of family physicians now provide maternity care, the authors wrote.
“If a causal relationship does exist [between lack of L&D units and adverse perinatal outcomes], then rural populations would definitively benefit from having local access to a L&D unit,” the authors stated.
The lead author, Dr. Waits, said in an interview that there are two obstacles to an increase in rural L&D units: malpractice premiums and reimbursement for indigent deliveries. The large malpractice premiums required to cover OB care are hurdles for caregivers, such as family physicians, as well as the hospitals where they practice.
Reforms from the legislative or regulatory perspective are needed to permit malpractice insurance to be issued at a reasonable cost, according to Dr. Waits. Such reforms are a “moral imperative” so that the malpractice issue is not allowed to “shipwreck infant and maternal mortality,” he said.
Of the many potential solutions, such as increased use of telemedicine, legislative initiatives to reduce the malpractice burden, or new support and incentives for family physicians to deliver OB care, each is burdened with obstacles to overcome, according to Dr. Waits. This does not mean these solutions should not be pursued alone or together, but he made it clear that the no solution is easy. In the meantime, Dr. Waits indicated a need to consider practical and immediate strategies to fix the problem.
“There should be incentives for rural emergency departments and ambulance systems to train in the [American Academy of Family Physicians’] Basic Life Support in Obstetrics (BLSO) certification courses each year. I am not aware of any specific evidence around this, but it is a known fact that, when L&Ds close, institutional memory of OB emergencies recede, and preparedness suffers,” he said.
Dr. Cullen agreed that if the closing of L&D units explains the higher rate of perinatal mortality in rural areas, both short-term and long-term solutions are needed.
“Every community must have a plan for obstetric and newborn emergencies. The decision to not offer maternity care means that rural providers will still provide maternity care but not be ready for emergencies,” he said, echoing a point made by Dr. Waits.
The study authors disclosed no conflicts. Dr. Cullen reported having no disclosures.
SOURCE: Waits JB et al. Ann Fam Med. 2020;18:446-51.
FROM ANNALS OF FAMILY MEDICINE
Key clinical point: The absence of labor and delivery (L&D) services in rural counties predicts adverse outcomes, including higher child mortality.
Major finding: In the absence of L&D units, the risk of perinatal mortality per 1,000 live births is 19% higher (5.67 vs. 4.74; P = .0034).
Data Source: Retrospective cohort study.
Disclosures: Potential conflicts of interest involving this topic were not reported.
Source: Waits JB et al. Ann Fam Med. 2020;18:446-51.
Health disparity: Race, mortality, and infants of teenage mothers
according to a new analysis from the National Center for Health Statistics.

In 2017-2018, overall mortality rates were 12.5 per 100,000 live births for infants born to Black mothers aged 15-19 years, 8.4 per 100,000 for infants born to White teenagers, and 6.5 per 100,000 for those born to Hispanic teens, Ashley M. Woodall, MPH, and Anne K. Driscoll, PhD, of the NCHS said in a data brief.
Looking at the five leading causes of those deaths shows that deaths of Black infants were the highest by significant margins in four, although, when it comes to “disorders related to short gestation and low birth weight,” significant may be an understatement.
The rate of preterm/low-birth-weight deaths for white infants in 2017-2018 was 119 per 100,000 live births; for Hispanic infants it was 94 per 100,000. Among infants born to Black teenagers, however, it was 284 deaths per 100,000, they reported based on data from the National Vital Statistics System’s linked birth/infant death file.
The numbers for congenital malformations and accidents were closer but still significantly different, and with each of the three most common causes, the rates for infants of Hispanic mothers also were significantly lower than those of White infants, the researchers said.
The situation changes for mortality-cause No. 4, sudden infant death syndrome, which was significantly more common among infants born to White teenagers, with a rate of 91 deaths per 100,000 live births, compared with either black (77) or Hispanic (44) infants, Ms. Woodall and Dr. Driscoll said.
Infants born to Black teens had the highest death rate again (68 per 100,000) for maternal complications of pregnancy, the fifth-leading cause of mortality, but for the first time Hispanic infants had a higher rate (36) than did those of White teenagers (29), they reported.
according to a new analysis from the National Center for Health Statistics.

In 2017-2018, overall mortality rates were 12.5 per 100,000 live births for infants born to Black mothers aged 15-19 years, 8.4 per 100,000 for infants born to White teenagers, and 6.5 per 100,000 for those born to Hispanic teens, Ashley M. Woodall, MPH, and Anne K. Driscoll, PhD, of the NCHS said in a data brief.
Looking at the five leading causes of those deaths shows that deaths of Black infants were the highest by significant margins in four, although, when it comes to “disorders related to short gestation and low birth weight,” significant may be an understatement.
The rate of preterm/low-birth-weight deaths for white infants in 2017-2018 was 119 per 100,000 live births; for Hispanic infants it was 94 per 100,000. Among infants born to Black teenagers, however, it was 284 deaths per 100,000, they reported based on data from the National Vital Statistics System’s linked birth/infant death file.
The numbers for congenital malformations and accidents were closer but still significantly different, and with each of the three most common causes, the rates for infants of Hispanic mothers also were significantly lower than those of White infants, the researchers said.
The situation changes for mortality-cause No. 4, sudden infant death syndrome, which was significantly more common among infants born to White teenagers, with a rate of 91 deaths per 100,000 live births, compared with either black (77) or Hispanic (44) infants, Ms. Woodall and Dr. Driscoll said.
Infants born to Black teens had the highest death rate again (68 per 100,000) for maternal complications of pregnancy, the fifth-leading cause of mortality, but for the first time Hispanic infants had a higher rate (36) than did those of White teenagers (29), they reported.
according to a new analysis from the National Center for Health Statistics.

In 2017-2018, overall mortality rates were 12.5 per 100,000 live births for infants born to Black mothers aged 15-19 years, 8.4 per 100,000 for infants born to White teenagers, and 6.5 per 100,000 for those born to Hispanic teens, Ashley M. Woodall, MPH, and Anne K. Driscoll, PhD, of the NCHS said in a data brief.
Looking at the five leading causes of those deaths shows that deaths of Black infants were the highest by significant margins in four, although, when it comes to “disorders related to short gestation and low birth weight,” significant may be an understatement.
The rate of preterm/low-birth-weight deaths for white infants in 2017-2018 was 119 per 100,000 live births; for Hispanic infants it was 94 per 100,000. Among infants born to Black teenagers, however, it was 284 deaths per 100,000, they reported based on data from the National Vital Statistics System’s linked birth/infant death file.
The numbers for congenital malformations and accidents were closer but still significantly different, and with each of the three most common causes, the rates for infants of Hispanic mothers also were significantly lower than those of White infants, the researchers said.
The situation changes for mortality-cause No. 4, sudden infant death syndrome, which was significantly more common among infants born to White teenagers, with a rate of 91 deaths per 100,000 live births, compared with either black (77) or Hispanic (44) infants, Ms. Woodall and Dr. Driscoll said.
Infants born to Black teens had the highest death rate again (68 per 100,000) for maternal complications of pregnancy, the fifth-leading cause of mortality, but for the first time Hispanic infants had a higher rate (36) than did those of White teenagers (29), they reported.
Declines in infant mortality tempered by disparities
Age-adjusted infant mortality dropped 11% from 2000 to 2017 in the United States, but the even larger decline for infants born to black women still left a death rate more than twice as high as those of white or Hispanic infants, according to a new analysis from the National Center for Health Statistics.

while the crude mortality rate fell 16% from 6.89 to 5.79, reported Anne K. Driscoll, PhD, and Danielle M. Ely, PhD, of the NCHS.
Over that same time period, age-adjusted infant mortality for births to black women went from 13.59 per 1,000 to 11.19, a drop of 18%. By comparison, age-adjusted mortality declined 7% from 5.59 per 1,000 for infants born to Hispanic women to 5.21 in 2017, they said in a National Vital Statistics Report.
Changes in maternal age distribution had an important effect on infant mortality. Women aged under 25 years, who have higher mortality rates, were less likely to give birth in 2017 than in 2000, and women aged 30-39 years, who have the lowest rates, made up a larger share of births in 2017, they pointed out.
It was, however, changes in age-specific mortality rates (ASMRs) that had the largest influence on the overall drop in the crude mortality rate, accounting for about two-thirds of the overall decline, the NCHS researchers said, noting that the effect varied by race and Hispanic origin.
Births to non-Hispanic white women mirrored the national situation: Approximately two-thirds (68.7%) of the decrease in infant mortality came from changes in ASMRs and one-third (31.3%) from changes in maternal age distribution. Among non-Hispanic black women, the distribution was 95.2% ASMRs and 4.8% age distribution, Dr. Driscoll and Dr. Ely reported based on data from the National Vital Statistics System.
The disparity between the two trends went even further for infants born to Hispanic women. Changes in ASMRs were responsible for 133.7% of the overall change in crude mortality versus –33.7% for changes in maternal age distribution. “If no changes occurred in the ASMRs, the changes in the maternal age distribution would have resulted in a higher mortality rate in 2017,” they explained.
The declines in the ASMRs may be related to incremental improved survival of preterm and low-birthweight infants in certain groups. “While little or no progress has been made to lower [these] two key risk factors for poor birth outcomes, progress has been made in lowering the mortality rates of at-risk infants across maternal age and race and Hispanic origin, resulting in lower ASMRs for all age groups,” the investigators suggested.
It also is possible that “changes in other factors, such as maternal education and cigarette smoking during pregnancy, may have indirectly resulted in declining ASMRs for all age groups over time,” they added.
SOURCE: Driscoll AK, Ely DM. National Vital Statistics Reports. 2020;69(5):1-18.
Age-adjusted infant mortality dropped 11% from 2000 to 2017 in the United States, but the even larger decline for infants born to black women still left a death rate more than twice as high as those of white or Hispanic infants, according to a new analysis from the National Center for Health Statistics.

while the crude mortality rate fell 16% from 6.89 to 5.79, reported Anne K. Driscoll, PhD, and Danielle M. Ely, PhD, of the NCHS.
Over that same time period, age-adjusted infant mortality for births to black women went from 13.59 per 1,000 to 11.19, a drop of 18%. By comparison, age-adjusted mortality declined 7% from 5.59 per 1,000 for infants born to Hispanic women to 5.21 in 2017, they said in a National Vital Statistics Report.
Changes in maternal age distribution had an important effect on infant mortality. Women aged under 25 years, who have higher mortality rates, were less likely to give birth in 2017 than in 2000, and women aged 30-39 years, who have the lowest rates, made up a larger share of births in 2017, they pointed out.
It was, however, changes in age-specific mortality rates (ASMRs) that had the largest influence on the overall drop in the crude mortality rate, accounting for about two-thirds of the overall decline, the NCHS researchers said, noting that the effect varied by race and Hispanic origin.
Births to non-Hispanic white women mirrored the national situation: Approximately two-thirds (68.7%) of the decrease in infant mortality came from changes in ASMRs and one-third (31.3%) from changes in maternal age distribution. Among non-Hispanic black women, the distribution was 95.2% ASMRs and 4.8% age distribution, Dr. Driscoll and Dr. Ely reported based on data from the National Vital Statistics System.
The disparity between the two trends went even further for infants born to Hispanic women. Changes in ASMRs were responsible for 133.7% of the overall change in crude mortality versus –33.7% for changes in maternal age distribution. “If no changes occurred in the ASMRs, the changes in the maternal age distribution would have resulted in a higher mortality rate in 2017,” they explained.
The declines in the ASMRs may be related to incremental improved survival of preterm and low-birthweight infants in certain groups. “While little or no progress has been made to lower [these] two key risk factors for poor birth outcomes, progress has been made in lowering the mortality rates of at-risk infants across maternal age and race and Hispanic origin, resulting in lower ASMRs for all age groups,” the investigators suggested.
It also is possible that “changes in other factors, such as maternal education and cigarette smoking during pregnancy, may have indirectly resulted in declining ASMRs for all age groups over time,” they added.
SOURCE: Driscoll AK, Ely DM. National Vital Statistics Reports. 2020;69(5):1-18.
Age-adjusted infant mortality dropped 11% from 2000 to 2017 in the United States, but the even larger decline for infants born to black women still left a death rate more than twice as high as those of white or Hispanic infants, according to a new analysis from the National Center for Health Statistics.

while the crude mortality rate fell 16% from 6.89 to 5.79, reported Anne K. Driscoll, PhD, and Danielle M. Ely, PhD, of the NCHS.
Over that same time period, age-adjusted infant mortality for births to black women went from 13.59 per 1,000 to 11.19, a drop of 18%. By comparison, age-adjusted mortality declined 7% from 5.59 per 1,000 for infants born to Hispanic women to 5.21 in 2017, they said in a National Vital Statistics Report.
Changes in maternal age distribution had an important effect on infant mortality. Women aged under 25 years, who have higher mortality rates, were less likely to give birth in 2017 than in 2000, and women aged 30-39 years, who have the lowest rates, made up a larger share of births in 2017, they pointed out.
It was, however, changes in age-specific mortality rates (ASMRs) that had the largest influence on the overall drop in the crude mortality rate, accounting for about two-thirds of the overall decline, the NCHS researchers said, noting that the effect varied by race and Hispanic origin.
Births to non-Hispanic white women mirrored the national situation: Approximately two-thirds (68.7%) of the decrease in infant mortality came from changes in ASMRs and one-third (31.3%) from changes in maternal age distribution. Among non-Hispanic black women, the distribution was 95.2% ASMRs and 4.8% age distribution, Dr. Driscoll and Dr. Ely reported based on data from the National Vital Statistics System.
The disparity between the two trends went even further for infants born to Hispanic women. Changes in ASMRs were responsible for 133.7% of the overall change in crude mortality versus –33.7% for changes in maternal age distribution. “If no changes occurred in the ASMRs, the changes in the maternal age distribution would have resulted in a higher mortality rate in 2017,” they explained.
The declines in the ASMRs may be related to incremental improved survival of preterm and low-birthweight infants in certain groups. “While little or no progress has been made to lower [these] two key risk factors for poor birth outcomes, progress has been made in lowering the mortality rates of at-risk infants across maternal age and race and Hispanic origin, resulting in lower ASMRs for all age groups,” the investigators suggested.
It also is possible that “changes in other factors, such as maternal education and cigarette smoking during pregnancy, may have indirectly resulted in declining ASMRs for all age groups over time,” they added.
SOURCE: Driscoll AK, Ely DM. National Vital Statistics Reports. 2020;69(5):1-18.
Antenatal corticosteroids may increase risk for mental and behavioral disorders
according to a Finnish population-based study published in JAMA. The findings may lead to changes in clinical practice, particularly for infants who may be born full term.
After adjustment for variables such as maternal age, smoking during pregnancy, any lifetime mental disorder diagnosis, and gestational age at birth, exposure to maternal antenatal corticosteroid treatment was significantly associated with mental and behavioral disorders in children, compared with nonexposure, with a hazard ratio of 1.33. Among children born at term, the adjusted hazard ratio was 1.47. Among preterm children, the hazard ratio was not significant.
“Although benefits of this therapy outweigh risks in the most vulnerable infants, this may not be true for all infants,” wrote Sara B. DeMauro, MD, an attending neonatologist and program director of the neonatal follow-up program at Children’s Hospital of Philadelphia, in an editorial also published in JAMA. “Recommendations to administer this therapy to broader populations of pregnant women may need to be reexamined until sufficient safety data, particularly among more mature infants, are available.”
Corticosteroid treatment to accelerate fetal maturation is standard care before 34 weeks’ gestation when there is a likelihood of delivery within 7 days, and studies have found that providing this therapy reduces the risk for respiratory problems when administered beyond 34 weeks. In 2016, updates to U.S. guidelines allowed for the use of corticosteroid treatment between 34 weeks and 36 weeks 6 days when women are at risk for preterm delivery within 7 days and have not received a previous course of antenatal corticosteroids.
The data from Finland indicate that “a significant number of very preterm children who might have benefited from this treatment did not receive it,” Dr. DeMauro wrote. At the same time, “45% of steroid-exposed infants were delivered at term. In these infants, minor short-term benefit may have been outweighed by significant longer-term risks. These data elucidate both the continuing struggle to accurately predict preterm birth and the incomplete uptake of an effective therapy that is beneficial when administered to the correct patients.”
Pause expanded use?
“Since the recommendations came out to expand the use of corticosteroids for preterm labor up until 37 weeks gestational age, my practice has incorporated these guidelines,” said Santina Wheat, MD, assistant professor of family and community medicine at Northwestern University in Chicago. “We have incorporated the guidelines though with the understanding that the benefits outweigh the risk. This article indicates that we may have been wrong in that understanding.” Although the association does not establish that the treatment causes mental and behavioral disorders, it “raises the question of whether we should halt this practice until additional information can be gathered,” noted Dr. Wheat, who also serves on the editorial advisory board of Family Practice News.
When administered before delivery of a very premature infant, corticosteroid therapy accelerates fetal lung maturation and helps prevent neonatal mortality, respiratory distress syndrome, and brain injury. Investigators demonstrated the benefits of antenatal corticosteroids in 1972, and the treatment – “one of the most important advances in perinatal care” – became widely used in the 1990s, Dr. DeMauro said.
To examine whether treatment exposure is associated with a risk of childhood mental and behavioral disorders and whether the risk is similar in infants born at term and preterm, Katri Räikkönen, PhD, a researcher at the University of Helsinki, and colleagues conducted a population-based retrospective study of more than 670,000 children.
The researchers identified all singleton pregnancies ending in a live birth in Finland during Jan. 1, 2006–Dec.31, 2017. In addition, they identified all consecutive maternal sibling pairs born at term, including sibling pairs discordant for maternal antenatal corticosteroid treatment exposure and sibling pairs concordant for treatment exposure or nonexposure. The investigators identified diagnoses of childhood mental and behavioral disorders using the Finnish Care Register for Health Care using ICD-10 codes on hospital inpatient and outpatient treatments by physicians in specialized medical care.
A range of disorders
In all, 670,097 infants with a median follow-up duration of 5.8 years were included in the analysis, and 14,868 (2.22%) were exposed to antenatal corticosteroids. Of the treatment-exposed children, about 45% were born at term. Of the nonexposed children, approximately 97% were born at term. Cumulative incidence rates for any mental and behavioral disorder were significantly higher for treatment-exposed children, compared with nonexposed children, in the entire cohort (12.01% vs. 6.45%; P less than .001) and in term-born children (8.89% vs. 6.31%; P less than .001).
In preterm children, the incidence rate of any mental and behavioral disorder was significantly higher among those with treatment exposure (14.59% vs. 10.71%; P less than .001). Associations persisted when the investigators focused on 241,621 sibling pairs, “suggesting that unmeasured familial confounding did not explain these associations,” the authors said.
“[In] the entire cohort and term-born children, treatment exposure ... was significantly associated with psychological development disorders; attention-deficit/hyperactivity or conduct disorders; mixed disorders of conduct and emotions, emotional disorders, disorders of social functioning or tic disorders; other behavioral or emotional disorders; and sleep disorders,” Dr. Räikkönen and colleagues reported. Among preterm-born, treatment-exposed children, the adjusted hazard ratio was significantly lower for intellectual disability and higher for sleep disorders.
Dr. DeMauro noted potential confounders in this observational study, including abnormal pregnancy events that lead clinicians to administer steroids. Such events “predispose the exposed children to adverse cognitive outcomes,” suggests some research. “Alternately, after a pregnancy at high risk for preterm delivery, families may perceive their children as vulnerable and therefore may be more likely to seek care and earlier diagnosis of mental or behavioral disorders,” Dr. DeMauro said.
The study was funded by the Academy of Finland, European Commission, Foundation for Pediatric Research, the Signe and Ane Gyllenberg Foundation, the Novo Nordisk Foundation, the Sigrid Juselius Foundation, and the Juho Vainio Foundation. The investigators and Dr. DeMauro had no conflict of interest disclosures.
SOURCE: Räikkönen K et al. JAMA. 2020;323(19):1924-33. doi: 10.1001/jama.2020.3937.
according to a Finnish population-based study published in JAMA. The findings may lead to changes in clinical practice, particularly for infants who may be born full term.
After adjustment for variables such as maternal age, smoking during pregnancy, any lifetime mental disorder diagnosis, and gestational age at birth, exposure to maternal antenatal corticosteroid treatment was significantly associated with mental and behavioral disorders in children, compared with nonexposure, with a hazard ratio of 1.33. Among children born at term, the adjusted hazard ratio was 1.47. Among preterm children, the hazard ratio was not significant.
“Although benefits of this therapy outweigh risks in the most vulnerable infants, this may not be true for all infants,” wrote Sara B. DeMauro, MD, an attending neonatologist and program director of the neonatal follow-up program at Children’s Hospital of Philadelphia, in an editorial also published in JAMA. “Recommendations to administer this therapy to broader populations of pregnant women may need to be reexamined until sufficient safety data, particularly among more mature infants, are available.”
Corticosteroid treatment to accelerate fetal maturation is standard care before 34 weeks’ gestation when there is a likelihood of delivery within 7 days, and studies have found that providing this therapy reduces the risk for respiratory problems when administered beyond 34 weeks. In 2016, updates to U.S. guidelines allowed for the use of corticosteroid treatment between 34 weeks and 36 weeks 6 days when women are at risk for preterm delivery within 7 days and have not received a previous course of antenatal corticosteroids.
The data from Finland indicate that “a significant number of very preterm children who might have benefited from this treatment did not receive it,” Dr. DeMauro wrote. At the same time, “45% of steroid-exposed infants were delivered at term. In these infants, minor short-term benefit may have been outweighed by significant longer-term risks. These data elucidate both the continuing struggle to accurately predict preterm birth and the incomplete uptake of an effective therapy that is beneficial when administered to the correct patients.”
Pause expanded use?
“Since the recommendations came out to expand the use of corticosteroids for preterm labor up until 37 weeks gestational age, my practice has incorporated these guidelines,” said Santina Wheat, MD, assistant professor of family and community medicine at Northwestern University in Chicago. “We have incorporated the guidelines though with the understanding that the benefits outweigh the risk. This article indicates that we may have been wrong in that understanding.” Although the association does not establish that the treatment causes mental and behavioral disorders, it “raises the question of whether we should halt this practice until additional information can be gathered,” noted Dr. Wheat, who also serves on the editorial advisory board of Family Practice News.
When administered before delivery of a very premature infant, corticosteroid therapy accelerates fetal lung maturation and helps prevent neonatal mortality, respiratory distress syndrome, and brain injury. Investigators demonstrated the benefits of antenatal corticosteroids in 1972, and the treatment – “one of the most important advances in perinatal care” – became widely used in the 1990s, Dr. DeMauro said.
To examine whether treatment exposure is associated with a risk of childhood mental and behavioral disorders and whether the risk is similar in infants born at term and preterm, Katri Räikkönen, PhD, a researcher at the University of Helsinki, and colleagues conducted a population-based retrospective study of more than 670,000 children.
The researchers identified all singleton pregnancies ending in a live birth in Finland during Jan. 1, 2006–Dec.31, 2017. In addition, they identified all consecutive maternal sibling pairs born at term, including sibling pairs discordant for maternal antenatal corticosteroid treatment exposure and sibling pairs concordant for treatment exposure or nonexposure. The investigators identified diagnoses of childhood mental and behavioral disorders using the Finnish Care Register for Health Care using ICD-10 codes on hospital inpatient and outpatient treatments by physicians in specialized medical care.
A range of disorders
In all, 670,097 infants with a median follow-up duration of 5.8 years were included in the analysis, and 14,868 (2.22%) were exposed to antenatal corticosteroids. Of the treatment-exposed children, about 45% were born at term. Of the nonexposed children, approximately 97% were born at term. Cumulative incidence rates for any mental and behavioral disorder were significantly higher for treatment-exposed children, compared with nonexposed children, in the entire cohort (12.01% vs. 6.45%; P less than .001) and in term-born children (8.89% vs. 6.31%; P less than .001).
In preterm children, the incidence rate of any mental and behavioral disorder was significantly higher among those with treatment exposure (14.59% vs. 10.71%; P less than .001). Associations persisted when the investigators focused on 241,621 sibling pairs, “suggesting that unmeasured familial confounding did not explain these associations,” the authors said.
“[In] the entire cohort and term-born children, treatment exposure ... was significantly associated with psychological development disorders; attention-deficit/hyperactivity or conduct disorders; mixed disorders of conduct and emotions, emotional disorders, disorders of social functioning or tic disorders; other behavioral or emotional disorders; and sleep disorders,” Dr. Räikkönen and colleagues reported. Among preterm-born, treatment-exposed children, the adjusted hazard ratio was significantly lower for intellectual disability and higher for sleep disorders.
Dr. DeMauro noted potential confounders in this observational study, including abnormal pregnancy events that lead clinicians to administer steroids. Such events “predispose the exposed children to adverse cognitive outcomes,” suggests some research. “Alternately, after a pregnancy at high risk for preterm delivery, families may perceive their children as vulnerable and therefore may be more likely to seek care and earlier diagnosis of mental or behavioral disorders,” Dr. DeMauro said.
The study was funded by the Academy of Finland, European Commission, Foundation for Pediatric Research, the Signe and Ane Gyllenberg Foundation, the Novo Nordisk Foundation, the Sigrid Juselius Foundation, and the Juho Vainio Foundation. The investigators and Dr. DeMauro had no conflict of interest disclosures.
SOURCE: Räikkönen K et al. JAMA. 2020;323(19):1924-33. doi: 10.1001/jama.2020.3937.
according to a Finnish population-based study published in JAMA. The findings may lead to changes in clinical practice, particularly for infants who may be born full term.
After adjustment for variables such as maternal age, smoking during pregnancy, any lifetime mental disorder diagnosis, and gestational age at birth, exposure to maternal antenatal corticosteroid treatment was significantly associated with mental and behavioral disorders in children, compared with nonexposure, with a hazard ratio of 1.33. Among children born at term, the adjusted hazard ratio was 1.47. Among preterm children, the hazard ratio was not significant.
“Although benefits of this therapy outweigh risks in the most vulnerable infants, this may not be true for all infants,” wrote Sara B. DeMauro, MD, an attending neonatologist and program director of the neonatal follow-up program at Children’s Hospital of Philadelphia, in an editorial also published in JAMA. “Recommendations to administer this therapy to broader populations of pregnant women may need to be reexamined until sufficient safety data, particularly among more mature infants, are available.”
Corticosteroid treatment to accelerate fetal maturation is standard care before 34 weeks’ gestation when there is a likelihood of delivery within 7 days, and studies have found that providing this therapy reduces the risk for respiratory problems when administered beyond 34 weeks. In 2016, updates to U.S. guidelines allowed for the use of corticosteroid treatment between 34 weeks and 36 weeks 6 days when women are at risk for preterm delivery within 7 days and have not received a previous course of antenatal corticosteroids.
The data from Finland indicate that “a significant number of very preterm children who might have benefited from this treatment did not receive it,” Dr. DeMauro wrote. At the same time, “45% of steroid-exposed infants were delivered at term. In these infants, minor short-term benefit may have been outweighed by significant longer-term risks. These data elucidate both the continuing struggle to accurately predict preterm birth and the incomplete uptake of an effective therapy that is beneficial when administered to the correct patients.”
Pause expanded use?
“Since the recommendations came out to expand the use of corticosteroids for preterm labor up until 37 weeks gestational age, my practice has incorporated these guidelines,” said Santina Wheat, MD, assistant professor of family and community medicine at Northwestern University in Chicago. “We have incorporated the guidelines though with the understanding that the benefits outweigh the risk. This article indicates that we may have been wrong in that understanding.” Although the association does not establish that the treatment causes mental and behavioral disorders, it “raises the question of whether we should halt this practice until additional information can be gathered,” noted Dr. Wheat, who also serves on the editorial advisory board of Family Practice News.
When administered before delivery of a very premature infant, corticosteroid therapy accelerates fetal lung maturation and helps prevent neonatal mortality, respiratory distress syndrome, and brain injury. Investigators demonstrated the benefits of antenatal corticosteroids in 1972, and the treatment – “one of the most important advances in perinatal care” – became widely used in the 1990s, Dr. DeMauro said.
To examine whether treatment exposure is associated with a risk of childhood mental and behavioral disorders and whether the risk is similar in infants born at term and preterm, Katri Räikkönen, PhD, a researcher at the University of Helsinki, and colleagues conducted a population-based retrospective study of more than 670,000 children.
The researchers identified all singleton pregnancies ending in a live birth in Finland during Jan. 1, 2006–Dec.31, 2017. In addition, they identified all consecutive maternal sibling pairs born at term, including sibling pairs discordant for maternal antenatal corticosteroid treatment exposure and sibling pairs concordant for treatment exposure or nonexposure. The investigators identified diagnoses of childhood mental and behavioral disorders using the Finnish Care Register for Health Care using ICD-10 codes on hospital inpatient and outpatient treatments by physicians in specialized medical care.
A range of disorders
In all, 670,097 infants with a median follow-up duration of 5.8 years were included in the analysis, and 14,868 (2.22%) were exposed to antenatal corticosteroids. Of the treatment-exposed children, about 45% were born at term. Of the nonexposed children, approximately 97% were born at term. Cumulative incidence rates for any mental and behavioral disorder were significantly higher for treatment-exposed children, compared with nonexposed children, in the entire cohort (12.01% vs. 6.45%; P less than .001) and in term-born children (8.89% vs. 6.31%; P less than .001).
In preterm children, the incidence rate of any mental and behavioral disorder was significantly higher among those with treatment exposure (14.59% vs. 10.71%; P less than .001). Associations persisted when the investigators focused on 241,621 sibling pairs, “suggesting that unmeasured familial confounding did not explain these associations,” the authors said.
“[In] the entire cohort and term-born children, treatment exposure ... was significantly associated with psychological development disorders; attention-deficit/hyperactivity or conduct disorders; mixed disorders of conduct and emotions, emotional disorders, disorders of social functioning or tic disorders; other behavioral or emotional disorders; and sleep disorders,” Dr. Räikkönen and colleagues reported. Among preterm-born, treatment-exposed children, the adjusted hazard ratio was significantly lower for intellectual disability and higher for sleep disorders.
Dr. DeMauro noted potential confounders in this observational study, including abnormal pregnancy events that lead clinicians to administer steroids. Such events “predispose the exposed children to adverse cognitive outcomes,” suggests some research. “Alternately, after a pregnancy at high risk for preterm delivery, families may perceive their children as vulnerable and therefore may be more likely to seek care and earlier diagnosis of mental or behavioral disorders,” Dr. DeMauro said.
The study was funded by the Academy of Finland, European Commission, Foundation for Pediatric Research, the Signe and Ane Gyllenberg Foundation, the Novo Nordisk Foundation, the Sigrid Juselius Foundation, and the Juho Vainio Foundation. The investigators and Dr. DeMauro had no conflict of interest disclosures.
SOURCE: Räikkönen K et al. JAMA. 2020;323(19):1924-33. doi: 10.1001/jama.2020.3937.
FROM JAMA
Key clinical point: Exposure to maternal antenatal corticosteroid treatment is significantly associated with mental and behavioral disorders in children, compared with nonexposure.
Major finding: After adjustment for such variables as maternal age, smoking during pregnancy, any lifetime mental disorder diagnosis, and gestational age at birth, exposure to maternal antenatal corticosteroid treatment was significantly associated with mental and behavioral disorders in children, compared with nonexposure (HR, 1.33). Among children born at term, the adjusted HR was 1.47.
Study details: A population-based retrospective cohort study that included 670,097 children in Finland.
Disclosures: The study was funded by the Academy of Finland, European Commission, Foundation for Pediatric Research, the Signe and Ane Gyllenberg Foundation, the Novo Nordisk Foundation, the Sigrid Juselius Foundation, and the Juho Vainio Foundation. The authors had no conflict of interest disclosures.
Source: Räikkönen K et al. JAMA. 2020;323(19):1924-33. doi: 10.1001/jama.2020.3937.
A multicenter RCT makes a case for transabdominal cerclage
Since the 1950s, when Shirodkar (1955) and McDonald (1957) published their seminal works detailing a transvaginal method to suture a “weak” cervix, clinicians and researchers have debated the indications for and utility of cerclage for preventing pregnancy loss and preterm birth.1,2
Originally based on a history of recurrent mid-trimester loss (that is, a clinical diagnosis of cervical insufficiency), cerclage has been expanded to capture both ultrasonography and physical-exam indications. While cerclage has proven useful in select patient populations, an infrequent but vexing problem is what to do when a woman has experienced 1 or more (transvaginal) cerclage “failures.”
With a dearth of well-controlled, randomized data to support the use of cerclage for either history- or physical-exam indications, it is not surprising that we still debate whether the Shirodkar method is superior to the McDonald technique as well as how to best manage a patient when either or both methods previously resulted in an unsatisfactory outcome.
First randomized study to directly compare cerclage techniques
Fortunately, Shennan and colleagues in the United Kingdom have greatly enlarged our knowledge in this area by performing the first well-powered, 3-arm, randomized trial of transabdominal cerclage (TAC) compared with both high and low vaginal cerclage (HVC, LVC).3 They analyzed data for 111 women who were randomly assigned to TAC
(n = 39), HVC (n = 39), or LVC (n = 33).
Interestingly, the investigators chose to not attach conventional eponymous labels to their transvaginal methods, and they do not even provide a reference or detailed description of the surgical methods, telling us instead that, “Techniques used were left to the local clinician’s discretion.” Writing also that HVC cases, like the transabdominal surgeries, were carried out in specialty centers, they implied that additional training was required for the HVC. I inferred that indeed they actually were performing the McDonald and Shirodkar transvaginal methods and with possible by-physician, local modifications.
I am certain that the authors’ results did not surprise proponents of transabdominal cerclage for transvaginal cerclage failures, defined in this trial as prior birth from 14 to 28 weeks’ gestation. Since some clinicians use a more generous definition of cerclage failure (such as birth at less than 34 weeks), this study population was clearly at high risk for poor outcomes; in fact, more than 90% of each group had experienced at least 2 prior mid-trimester losses. As anticipated with randomization, other characteristics were well distributed across the 3 groups.
Continue to: Transabdominal cerclage significantly reduced preterm birth rates...
Transabdominal cerclage significantly reduced preterm birth rates
Using a primary outcome of preterm birth less than 32 weeks, which concentrates neonatal morbidities, the investigators observed an overall 4.5-fold higher rate of preterm birth in the transvaginal cohorts compared with the transabdominal patients (33% and 38% versus 8%, respectively). Comparing the TAC group individually with both LVC and HVC groups, the relative risk of preterm birth was 0.20 compared with the HVC group and 0.23 compared with the LVC group, reflecting an approximate 80% reduction.
Not surprising to me, the investigators observed nearly identical outcomes between the HVC and LVC cohorts, substantiating my bias that the 2 transvaginal methods are similarly effective. Opponents will quickly remind me that the study was not well-powered to detect a clinically significant difference between these 2 groups; touché!
Risks of TAC. We all know that, despite its now-proven benefits, the transabdominal approach is associated with a risk of special complications, including the surgical risks of placement (and removal) of the cerclage, the management of fetal death beyond approximately 14 weeks, and the absolute requisite for hysterotomy/cesarean birth. While serious complications are rare, in the trial by Shennan and colleagues none were recorded in the 39 TAC cases. Nevertheless, for women with no children or only prior early births, the risks seem to be justified; the number needed to treat was less than 4 to prevent 1 birth at less than 32 weeks and was 5.3 to prevent a fetal loss.
TAC is an option for select patients
Given that TAC now can be successfully placed using minimally invasive surgery, either prior to or following conception, this study provides unique level I evidence that should not be discounted and should further be considered in the context of confirming prior cohort studies that suggested a significant benefit. Although specialized training is required and the procedure may involve travel to a specialty center, the weight of clinical data clearly supports the use of TAC.
In summary, based largely on the trial by Shennan and colleagues, women with prior failed vaginal cerclage can and should be counseled regarding the availability of TAC and given the opportunity to weigh the reported risks and benefits. ●
1. Shirodkar VN. A new method of operative treatment for habitual abortion in the second trimester of pregnancy. Antiseptic. 1955;52:299-303.
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynecol Br Emp. 1957;64:346-350.
3. Shennan A, Chandiramani M, Bennett P, et al. MAVRIC: a multicenter randomized trial of transabdominal vs transvaginal cervical cerclage. Am J Obstet Gynecol. 2020;222:261.e1-261.e9.
Since the 1950s, when Shirodkar (1955) and McDonald (1957) published their seminal works detailing a transvaginal method to suture a “weak” cervix, clinicians and researchers have debated the indications for and utility of cerclage for preventing pregnancy loss and preterm birth.1,2
Originally based on a history of recurrent mid-trimester loss (that is, a clinical diagnosis of cervical insufficiency), cerclage has been expanded to capture both ultrasonography and physical-exam indications. While cerclage has proven useful in select patient populations, an infrequent but vexing problem is what to do when a woman has experienced 1 or more (transvaginal) cerclage “failures.”
With a dearth of well-controlled, randomized data to support the use of cerclage for either history- or physical-exam indications, it is not surprising that we still debate whether the Shirodkar method is superior to the McDonald technique as well as how to best manage a patient when either or both methods previously resulted in an unsatisfactory outcome.
First randomized study to directly compare cerclage techniques
Fortunately, Shennan and colleagues in the United Kingdom have greatly enlarged our knowledge in this area by performing the first well-powered, 3-arm, randomized trial of transabdominal cerclage (TAC) compared with both high and low vaginal cerclage (HVC, LVC).3 They analyzed data for 111 women who were randomly assigned to TAC
(n = 39), HVC (n = 39), or LVC (n = 33).
Interestingly, the investigators chose to not attach conventional eponymous labels to their transvaginal methods, and they do not even provide a reference or detailed description of the surgical methods, telling us instead that, “Techniques used were left to the local clinician’s discretion.” Writing also that HVC cases, like the transabdominal surgeries, were carried out in specialty centers, they implied that additional training was required for the HVC. I inferred that indeed they actually were performing the McDonald and Shirodkar transvaginal methods and with possible by-physician, local modifications.
I am certain that the authors’ results did not surprise proponents of transabdominal cerclage for transvaginal cerclage failures, defined in this trial as prior birth from 14 to 28 weeks’ gestation. Since some clinicians use a more generous definition of cerclage failure (such as birth at less than 34 weeks), this study population was clearly at high risk for poor outcomes; in fact, more than 90% of each group had experienced at least 2 prior mid-trimester losses. As anticipated with randomization, other characteristics were well distributed across the 3 groups.
Continue to: Transabdominal cerclage significantly reduced preterm birth rates...
Transabdominal cerclage significantly reduced preterm birth rates
Using a primary outcome of preterm birth less than 32 weeks, which concentrates neonatal morbidities, the investigators observed an overall 4.5-fold higher rate of preterm birth in the transvaginal cohorts compared with the transabdominal patients (33% and 38% versus 8%, respectively). Comparing the TAC group individually with both LVC and HVC groups, the relative risk of preterm birth was 0.20 compared with the HVC group and 0.23 compared with the LVC group, reflecting an approximate 80% reduction.
Not surprising to me, the investigators observed nearly identical outcomes between the HVC and LVC cohorts, substantiating my bias that the 2 transvaginal methods are similarly effective. Opponents will quickly remind me that the study was not well-powered to detect a clinically significant difference between these 2 groups; touché!
Risks of TAC. We all know that, despite its now-proven benefits, the transabdominal approach is associated with a risk of special complications, including the surgical risks of placement (and removal) of the cerclage, the management of fetal death beyond approximately 14 weeks, and the absolute requisite for hysterotomy/cesarean birth. While serious complications are rare, in the trial by Shennan and colleagues none were recorded in the 39 TAC cases. Nevertheless, for women with no children or only prior early births, the risks seem to be justified; the number needed to treat was less than 4 to prevent 1 birth at less than 32 weeks and was 5.3 to prevent a fetal loss.
TAC is an option for select patients
Given that TAC now can be successfully placed using minimally invasive surgery, either prior to or following conception, this study provides unique level I evidence that should not be discounted and should further be considered in the context of confirming prior cohort studies that suggested a significant benefit. Although specialized training is required and the procedure may involve travel to a specialty center, the weight of clinical data clearly supports the use of TAC.
In summary, based largely on the trial by Shennan and colleagues, women with prior failed vaginal cerclage can and should be counseled regarding the availability of TAC and given the opportunity to weigh the reported risks and benefits. ●
Since the 1950s, when Shirodkar (1955) and McDonald (1957) published their seminal works detailing a transvaginal method to suture a “weak” cervix, clinicians and researchers have debated the indications for and utility of cerclage for preventing pregnancy loss and preterm birth.1,2
Originally based on a history of recurrent mid-trimester loss (that is, a clinical diagnosis of cervical insufficiency), cerclage has been expanded to capture both ultrasonography and physical-exam indications. While cerclage has proven useful in select patient populations, an infrequent but vexing problem is what to do when a woman has experienced 1 or more (transvaginal) cerclage “failures.”
With a dearth of well-controlled, randomized data to support the use of cerclage for either history- or physical-exam indications, it is not surprising that we still debate whether the Shirodkar method is superior to the McDonald technique as well as how to best manage a patient when either or both methods previously resulted in an unsatisfactory outcome.
First randomized study to directly compare cerclage techniques
Fortunately, Shennan and colleagues in the United Kingdom have greatly enlarged our knowledge in this area by performing the first well-powered, 3-arm, randomized trial of transabdominal cerclage (TAC) compared with both high and low vaginal cerclage (HVC, LVC).3 They analyzed data for 111 women who were randomly assigned to TAC
(n = 39), HVC (n = 39), or LVC (n = 33).
Interestingly, the investigators chose to not attach conventional eponymous labels to their transvaginal methods, and they do not even provide a reference or detailed description of the surgical methods, telling us instead that, “Techniques used were left to the local clinician’s discretion.” Writing also that HVC cases, like the transabdominal surgeries, were carried out in specialty centers, they implied that additional training was required for the HVC. I inferred that indeed they actually were performing the McDonald and Shirodkar transvaginal methods and with possible by-physician, local modifications.
I am certain that the authors’ results did not surprise proponents of transabdominal cerclage for transvaginal cerclage failures, defined in this trial as prior birth from 14 to 28 weeks’ gestation. Since some clinicians use a more generous definition of cerclage failure (such as birth at less than 34 weeks), this study population was clearly at high risk for poor outcomes; in fact, more than 90% of each group had experienced at least 2 prior mid-trimester losses. As anticipated with randomization, other characteristics were well distributed across the 3 groups.
Continue to: Transabdominal cerclage significantly reduced preterm birth rates...
Transabdominal cerclage significantly reduced preterm birth rates
Using a primary outcome of preterm birth less than 32 weeks, which concentrates neonatal morbidities, the investigators observed an overall 4.5-fold higher rate of preterm birth in the transvaginal cohorts compared with the transabdominal patients (33% and 38% versus 8%, respectively). Comparing the TAC group individually with both LVC and HVC groups, the relative risk of preterm birth was 0.20 compared with the HVC group and 0.23 compared with the LVC group, reflecting an approximate 80% reduction.
Not surprising to me, the investigators observed nearly identical outcomes between the HVC and LVC cohorts, substantiating my bias that the 2 transvaginal methods are similarly effective. Opponents will quickly remind me that the study was not well-powered to detect a clinically significant difference between these 2 groups; touché!
Risks of TAC. We all know that, despite its now-proven benefits, the transabdominal approach is associated with a risk of special complications, including the surgical risks of placement (and removal) of the cerclage, the management of fetal death beyond approximately 14 weeks, and the absolute requisite for hysterotomy/cesarean birth. While serious complications are rare, in the trial by Shennan and colleagues none were recorded in the 39 TAC cases. Nevertheless, for women with no children or only prior early births, the risks seem to be justified; the number needed to treat was less than 4 to prevent 1 birth at less than 32 weeks and was 5.3 to prevent a fetal loss.
TAC is an option for select patients
Given that TAC now can be successfully placed using minimally invasive surgery, either prior to or following conception, this study provides unique level I evidence that should not be discounted and should further be considered in the context of confirming prior cohort studies that suggested a significant benefit. Although specialized training is required and the procedure may involve travel to a specialty center, the weight of clinical data clearly supports the use of TAC.
In summary, based largely on the trial by Shennan and colleagues, women with prior failed vaginal cerclage can and should be counseled regarding the availability of TAC and given the opportunity to weigh the reported risks and benefits. ●
1. Shirodkar VN. A new method of operative treatment for habitual abortion in the second trimester of pregnancy. Antiseptic. 1955;52:299-303.
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynecol Br Emp. 1957;64:346-350.
3. Shennan A, Chandiramani M, Bennett P, et al. MAVRIC: a multicenter randomized trial of transabdominal vs transvaginal cervical cerclage. Am J Obstet Gynecol. 2020;222:261.e1-261.e9.
1. Shirodkar VN. A new method of operative treatment for habitual abortion in the second trimester of pregnancy. Antiseptic. 1955;52:299-303.
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynecol Br Emp. 1957;64:346-350.
3. Shennan A, Chandiramani M, Bennett P, et al. MAVRIC: a multicenter randomized trial of transabdominal vs transvaginal cervical cerclage. Am J Obstet Gynecol. 2020;222:261.e1-261.e9.
Transabdominal cerclage for managing recurrent pregnancy loss
CASE A woman with recurrent pregnancy loss
A 38-year-old woman (G4P0221) presents to your office for preconception counseling. Her history is significant for the following: a spontaneous pregnancy loss at 15 weeks’ gestation; a pregnancy loss at 17 weeks secondary to preterm premature rupture of membranes (PPROM); a cesarean delivery at 30 weeks and 6 days’ gestation after placement of a transvaginal cerclage at 20 weeks for cervical dilation noted on physical exam (the child now has developmental delays); and most recently a delivery at 24 weeks and 4 days due to preterm labor with subsequent neonatal demise (this followed a transvaginal cerclage placed at 13 weeks and 6 days).
How would you counsel this patient?
Cervical insufficiency describes the inability of the cervix to retain a pregnancy in the absence of the signs and symptoms of clinical contractions, labor, or both in the second trimester.1 This condition affects an estimated 1% of obstetric patients and 8% of women with recurrent losses who have experienced a second-trimester loss.2
Diagnosis of cervical insufficiency is based on a history of painless cervical dilation after the first trimester with expulsion of the pregnancy in the second trimester before 24 weeks of gestation without contractions and in the absence of other pathology, such as bleeding, infection, or ruptured membranes.1 Diagnosis also can be made by noting cervical dilation on physical exam during the second trimester; more recently, short cervical length on transvaginal ultrasonography in the second trimester has been used to try to predict when a cervical cerclage may be indicated, although sonographic cervical length is more a marker for risk of preterm birth than for cervical insufficiency specifically.1,3
Given the considerable emotional and physical distress that patients experience with recurrent second-trimester losses and the significant neonatal morbidity and mortality that can occur with preterm delivery, substantial efforts are made to prevent these outcomes by treating patients with cervical insufficiency and those at risk for preterm delivery.
Transvaginal cerclage: A treatment mainstay
Standard treatment options for cervical insufficiency depend on the patient’s history. One of the treatment mainstays for women with prior second-trimester losses or preterm deliveries is transvaginal cervical cerclage. A transvaginal cerclage can be placed using either a Shirodkar technique, in which the vesicocervical mucosa is dissected and a suture is placed as close to the internal cervical os as possible, or a McDonald technique, in which a purse-string suture is placed around the cervicovaginal junction. No randomized trials have compared the effectiveness of these 2 methods, but most observational studies show no difference, and one suggests that the Shirodkar technique may be more effective in obese women specifically.4-6
Indications for transvaginal cerclage. The indication for transvaginal cerclage is based on history, physical exam, or ultrasonography.
A physical-exam indication is the most straightforward of the 3. Transvaginal cerclage placement is indicated if on physical exam in the second trimester a patient has cervical dilation without contractions or infection.1,7
A history-indicated cerclage (typically placed between 12 and 14 weeks’ gestation) is based on a cerclage having been placed in a prior pregnancy due to painless cervical dilation in the second trimester (either ultrasonography- or physical-exam indicated), and it also can be considered in the case of a history of 1 or more second-trimester pregnancy losses related to painless cervical dilation.1
More recent evidence suggests that in patients with 1 prior second-trimester loss or preterm delivery, serial sonographic cervical length can be measured safely from 16 to 24 weeks, with a cerclage being placed only if cervical length decreases to less than 25 mm. By using the ultrasonography-based indication, unnecessary history-indicated cerclages for 1 prior second-trimester or preterm birth can be avoided in more than one-half of patients (FIGURE 1).1,7
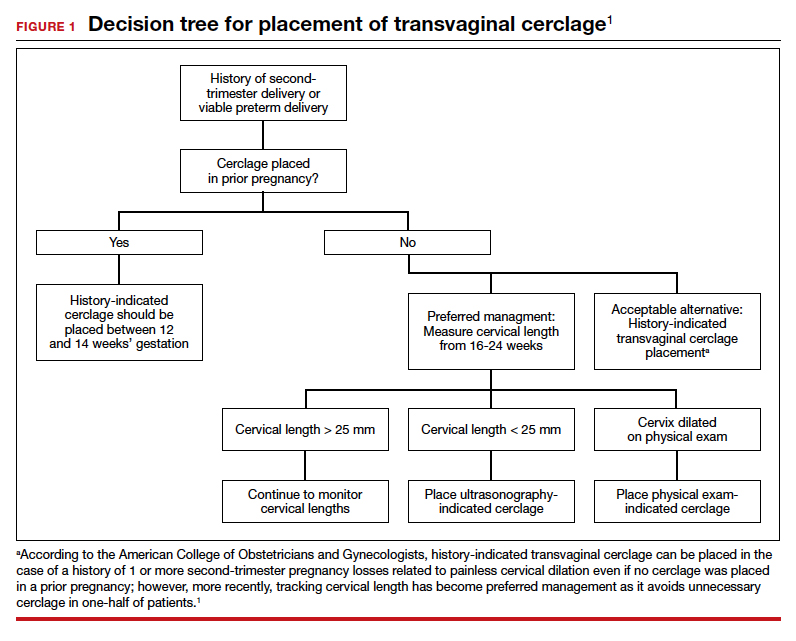
Efficacy. The effectiveness of transvaginal cerclage varies by the indication. Authors of a 2017 Cochrane review found an overall reduced risk of giving birth before 34 weeks’ gestation for any indication, with an average relative risk of 0.77.2 Other recent studies showed the following8-10:
- a 63% delivery rate after 28 weeks’ gestation for physical-exam indicated cerclages in the presence of bulging amniotic membranes
- an 86.2% delivery rate after 32 weeks’ gestation for ultrasonography-indicated cerclages
- an 86% delivery rate after 32 weeks’ gestation for a history-indicated cerclage in patients with 2 or more prior second-trimester losses.
Success rates, especially for ultrasonography- and history-indicated cerclage, are thus high. For the 14% who still fail these methods, however, a different management strategy is needed, which is where transabdominal cerclage comes into play.
Continue to: Transabdominal cerclage is an option for certain patients...
Transabdominal cerclage is an option for certain patients
In transabdominal cerclage, an abdominal approach is used to place a stitch at the cervicouterine junction. With this approach, the cerclage can reach a closer proximity to the internal os compared with the vaginal approach, providing better support of the cervical tissue (FIGURE 2).11 Whether performed via laparotomy or laparoscopy, the transabdominal cerclage procedure likely carries higher morbidity than a transvaginal approach, and cesarean delivery is required after placement.

Since transvaginal cerclage often is successful, in most cases the transabdominal approach should not be viewed as the first-line treatment for cervical insufficiency if a history-indicated transvaginal cerclage has not been attempted. For women who fail a history-indicated transvaginal cerclage, however, a transabdominal cerclage has been proven to decrease the rate of preterm delivery and PPROM compared with attempting another history-indicated transvaginal cerclage.11,12
A recent systematic review of pregnancy outcomes after transabdominal cerclage placement reported neonatal survival of 96.5% and an 83% delivery rate after 34 weeks’ gestation.13 Thus, even among a population that failed transvaginal cerclage, a transabdominal cerclage has a high success rate in providing a good pregnancy outcome (TABLE). Transabdominal cerclage also can be considered as first-line treatment in patients who had prior cervical surgery or cervical deformities that might preclude the ability to place a cerclage transvaginally.

CASE Continued: A candidate for transabdominal cerclage
Given the patient’s poor obstetric history, which includes a preterm delivery and neonatal loss despite a history-indicated cerclage, you recommend that the patient have a transabdominal cerclage placed as the procedure has been proven to increase the chances of neonatal survival and delivery after 34 weeks in women with a similar obstetric history. The patient is interested in this option and asks about how this cerclage is placed and when it would need to be placed during her next pregnancy.
Surgical technique for transabdominal cerclage placement
A transabdominal cerclage can be placed via laparotomy, laparoscopy, or robot-assisted laparoscopy. No differences in obstetric outcomes have been shown between the laparotomy and laparoscopic approaches.14,15 Given the benefits of minimally invasive surgery, a laparoscopic or robot-assisted approach is preferred when feasible.
Additionally, for ease of placement, transabdominal cerclage can be placed prior to conception—known as interval placement—or during pregnancy between 10 and 14 weeks (preferably closer to 10 weeks). Because of the increased difficulty in placing a cerclage in the gravid uterus, interval transabdominal cerclage placement is recommended when possible.13,16 Authors of one observational study noted that improved obstetric outcomes occurred with interval placement compared with cerclage placement between 9 and 10 weeks’ gestation, with a delivery rate at more than 34 weeks’ gestation in 90% versus 74% of patients, respectively.16
Continue to: Steps for interval cerclage and during pregnancy...
Steps for interval cerclage and during pregnancy
Our practice is to place transabdominal cerclage via conventional laparoscopy as an interval procedure when possible. We find no benefit in using robotic assistance.
For an interval procedure, the patient is placed in a dorsal lithotomy position, and we place a 10-mm umbilical port, 2 lateral 5-mm ports, 1 suprapubic 5-mm port, and a uterine manipulator. We use a flexible laparoscope to provide optimal visualization of the pelvis from any angle.
The first step of the surgery involves dissecting the vesicouterine peritoneum in order to move the bladder inferiorly (FIGURE 3A). Uterine arteries are then identified lateral to the cervix as part of this dissection, and a window is created in the inferior aspect of the broad ligament just anterior and lateral to the insertion of the uterosacral ligaments onto the uterus, with care taken to avoid the uterine vessels superiorly (FIGURE 3B). Two 5-mm Mersilene tape sutures are then tied together to create 1 suture with a needle at each end. This is then passed into the abdomen, and 1 needle is passed through the parametrial space at the level of the internal os inferior to the uterine vessels on 1 side of the uterus while the other needle is passed through the parametrial space on the opposite side.
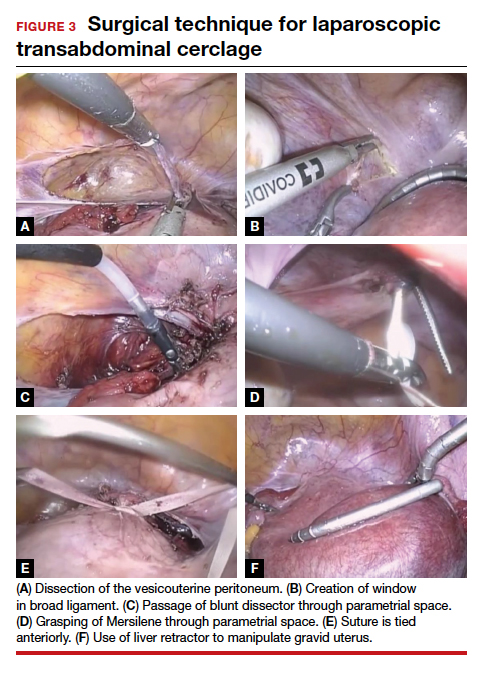
Alternatively, rather than using the suture needles, a blunt dissector can be passed through this same space bilaterally (FIGURE 3C) via the suprapubic port and can pull the Mersilene tape through the parametrial space (FIGURE 3D). The suture is then tied anterior at the level of the internal os intracorporally (FIGURE 3E), and the needles are cut off the suture and removed from the abdomen.
To perform transabdominal cerclage when the patient is pregnant, a few modifications are needed to help with placement. First, the patient may be placed in supine position since a uterine manipulator cannot be used. Second, use of a flexible laparoscope becomes even more imperative in order to properly see around the gravid uterus. Lastly, a 5-mm laparoscopic liver retractor can be used to aid in blunt manipulation of the gravid uterus (FIGURE 3F). (The surgical video below highlights the steps to transabdominal cerclage placement in a pregnant patient.) All other port placements and steps to dissection and suture placement are the same as in interval placement.

CASE Continued: Patient pursues transabdominal cerclage
You explain to your patient that ideally the cerclage should be placed now in a laparoscopic fashion before she becomes pregnant. You then refer her to a local gynecologic surgeon who places many laparoscopic transabdominal cerclages. She undergoes the procedure, becomes pregnant, and after presenting in labor at 35 weeks’ gestation has a cesarean delivery. Her baby is born without any neonatal complications, and the patient is overjoyed with the outcome.
Management during and after pregnancy
Pregnant patients with a transabdominal cerclage are precluded from having a vaginal delivery and must deliver via cesarean. During the antepartum period, patients are managed in the same manner as those who have a transvaginal cerclage. Delivery via cesarean at the onset of regular contractions is recommended to reduce the risk of uterine rupture. In the absence of labor, scheduled cesarean is performed at term.
Our practice is to schedule cesarean delivery at 38 weeks’ gestation, although there are no data or consensus to support a specific gestational age between 37 and 39 weeks. Unlike a transvaginal cerclage, a transabdominal cerclage can be left in place for use in subsequent pregnancies. Data are limited on whether the transabdominal cerclage should be removed in women who no longer desire childbearing and whether there are long-term sequelae if the suture is left in situ.17
Continue to: Complications and risks of abdominal cerclage...
Complications and risks of abdominal cerclage
As the data suggest and our experience confirms, transabdominal cerclage is highly successful in patients who have failed a history-indicated transvaginal cerclage; however, the transabdominal approach carries a higher surgical risk. Risks include intraoperative hemorrhage, conversion to laparotomy, and a range of rare surgical and obstetric complications, such as bladder injury and PPROM.13,18
If a patient experiences a fetal loss in the first trimester, a dilation and curettage (D&C) can be performed, with good obstetric outcomes in subsequent pregnancies.19 If the patient experiences an early-to-mid second-trimester loss, some studies suggest that a dilation and evacuation (D&E) of the uterus can be done with sufficient dilation of the cervix to accommodate up to a 15-mm cannula and Sopher forceps.19 Laminaria also may be used in this process. However, no data exist regarding success of future pregnancies and transabdominal cerclage integrity after a D&E.20 If the cerclage prevents successful dilation of the cervix, the cerclage must be removed laparoscopically prior to performing the D&E.
In late second-trimester and third-trimester loss, the cerclage must be removed to allow passage of the fetus and placenta prior to a D&E or an induction of labor.20
For patients with PPROM or preterm labor, data are limited regarding management recommendations. However, in these complex cases, we strongly recommend an individualized approach and co-management with maternal-fetal medicine specialists.
CASE Resolved
The cerclage is left in place during the patient’s cesarean delivery, and her postpartum course is uneventful. She continued without complications for the next year, at which time she sees you in the office with plans to have another pregnancy later in the year. You counsel her that her abdominal cerclage will still be effective and that she can get pregnant with expectations of similar outcomes as her previous pregnancy. She thanks you for everything and reports that she hopes to return later in the year for her first prenatal visit. ●
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 142: Cerclage for the management of cervical insufficiency. Obstet Gynecol. 2014;123(2 pt 1): 372-379.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6(6):CD008991.
- Brown R, Gagnon R, Delisle M-F. No. 373—cervical insufficiency and cervical cerclage. J Obstet Gynaecol Can. 2019;41:233-247.
- Odibo AO, Berghella V, To MS, et al. Shirodkar versus McDonald cerclage for the prevention of preterm birth in women with short cervical length. Am J Perinatol. 2007;24: 55-60.
- Basbug A, Bayrak M, Dogan O, et al. McDonald versus modified Shirodkar rescue cerclage in women with prolapsed fetal membranes. J Matern Fetal Neonatal Med. 2020;33: 1075-1097.
- Figueroa R, Crowell R, Martinez A, et al. McDonald versus Shirodkar cervical cerclage for the prevention of preterm birth: impact of body mass index. J Matern Fetal Neonatal Med. 2019;32:3408-3414.
- Suhag A, Berghella V. Cervical cerclage. Clin Obstet Gynecol. 2014;57:557-567.
- Bayrak M, Gul A, Goynumer G. Rescue cerclage when foetal membranes prolapse into the vagina. J Obstet Gynaecol. 2017;37:471-475.
- Drassinower D, Coviello E, Landy HJ, et al. Outcomes after periviable ultrasound-indicated cerclage. J Matern Fetal Neonatal Med. 2019;32:932-938.
- Lee KN, Whang EJ, Chang KH, et al. History-indicated cerclage: the association between previous preterm history and cerclage outcome. Obstet Gynecol Sci. 2018;61:23-29. doi:10.5468/ogs.2018.61.1.23.
- Sneider K, Christiansen OB, Sundtoft IB, et al. Recurrence rates after abdominal and vaginal cerclages in women with cervical insufficiency: a validated cohort study. Arch Gynecol Obstet. 2017;295:859-866.
- Davis G, Berghella V, Talucci M, et al. Patients with a prior failed transvaginal cerclage: a comparison of obstetric outcomes with either transabdominal or transvaginal cerclage. Am J Obstet Gynecol. 2000;183:836-839.
- Moawad GN, Tyan P, Bracke T, et al. Systematic review of transabdominal cerclage placed via laparoscopy for the prevention of preterm birth. J Mimim Invasive Gynecol. 2018;25:277-286.
- Burger NB, Brölmann HAM, Einarsson JI, et al. Effectiveness of abdominal cerclage placed via laparotomy or laparoscopy: systematic review. J Minim Invasive Gynecol. 2011;18:696-704.
- Kim S, Hill A, Menderes G, et al. Minimally invasive abdominal cerclage compared to laparotomy: a comparison of surgical and obstetric outcomes. J Robot Surg. 2018;12:295-301.
- Dawood F, Farquharson RG. Transabdominal cerclage: preconceptual versus first trimester insertion. Eur J Obstet Gynecol Reprod Biol. 2016;199:27-31.
- Hawkins E, Nimaroff M. Vaginal erosion of an abdominal cerclage 7 years after laparoscopic placement. Obstet Gynecol. 2014;123(2 pt 2 suppl 2):420-423.
- Foster TL, Moore ES, Sumners JE. Operative complications and fetal morbidity encountered in 300 prophylactic transabdominal cervical cerclage procedures by one obstetric surgeon. J Obstet Gynaecol. 2011;31:713-717.
- Dethier D, Lassey SC, Pilliod R, et al. Uterine evacuation in the setting of transabdominal cerclage. Contraception. 2020;101:174-177.
- Martin A, Lathrop E. Controversies in family planning: management of second-trimester losses in the setting of an abdominal cerclage. Contraception. 2013;87:728-731.
CASE A woman with recurrent pregnancy loss
A 38-year-old woman (G4P0221) presents to your office for preconception counseling. Her history is significant for the following: a spontaneous pregnancy loss at 15 weeks’ gestation; a pregnancy loss at 17 weeks secondary to preterm premature rupture of membranes (PPROM); a cesarean delivery at 30 weeks and 6 days’ gestation after placement of a transvaginal cerclage at 20 weeks for cervical dilation noted on physical exam (the child now has developmental delays); and most recently a delivery at 24 weeks and 4 days due to preterm labor with subsequent neonatal demise (this followed a transvaginal cerclage placed at 13 weeks and 6 days).
How would you counsel this patient?
Cervical insufficiency describes the inability of the cervix to retain a pregnancy in the absence of the signs and symptoms of clinical contractions, labor, or both in the second trimester.1 This condition affects an estimated 1% of obstetric patients and 8% of women with recurrent losses who have experienced a second-trimester loss.2
Diagnosis of cervical insufficiency is based on a history of painless cervical dilation after the first trimester with expulsion of the pregnancy in the second trimester before 24 weeks of gestation without contractions and in the absence of other pathology, such as bleeding, infection, or ruptured membranes.1 Diagnosis also can be made by noting cervical dilation on physical exam during the second trimester; more recently, short cervical length on transvaginal ultrasonography in the second trimester has been used to try to predict when a cervical cerclage may be indicated, although sonographic cervical length is more a marker for risk of preterm birth than for cervical insufficiency specifically.1,3
Given the considerable emotional and physical distress that patients experience with recurrent second-trimester losses and the significant neonatal morbidity and mortality that can occur with preterm delivery, substantial efforts are made to prevent these outcomes by treating patients with cervical insufficiency and those at risk for preterm delivery.
Transvaginal cerclage: A treatment mainstay
Standard treatment options for cervical insufficiency depend on the patient’s history. One of the treatment mainstays for women with prior second-trimester losses or preterm deliveries is transvaginal cervical cerclage. A transvaginal cerclage can be placed using either a Shirodkar technique, in which the vesicocervical mucosa is dissected and a suture is placed as close to the internal cervical os as possible, or a McDonald technique, in which a purse-string suture is placed around the cervicovaginal junction. No randomized trials have compared the effectiveness of these 2 methods, but most observational studies show no difference, and one suggests that the Shirodkar technique may be more effective in obese women specifically.4-6
Indications for transvaginal cerclage. The indication for transvaginal cerclage is based on history, physical exam, or ultrasonography.
A physical-exam indication is the most straightforward of the 3. Transvaginal cerclage placement is indicated if on physical exam in the second trimester a patient has cervical dilation without contractions or infection.1,7
A history-indicated cerclage (typically placed between 12 and 14 weeks’ gestation) is based on a cerclage having been placed in a prior pregnancy due to painless cervical dilation in the second trimester (either ultrasonography- or physical-exam indicated), and it also can be considered in the case of a history of 1 or more second-trimester pregnancy losses related to painless cervical dilation.1
More recent evidence suggests that in patients with 1 prior second-trimester loss or preterm delivery, serial sonographic cervical length can be measured safely from 16 to 24 weeks, with a cerclage being placed only if cervical length decreases to less than 25 mm. By using the ultrasonography-based indication, unnecessary history-indicated cerclages for 1 prior second-trimester or preterm birth can be avoided in more than one-half of patients (FIGURE 1).1,7

Efficacy. The effectiveness of transvaginal cerclage varies by the indication. Authors of a 2017 Cochrane review found an overall reduced risk of giving birth before 34 weeks’ gestation for any indication, with an average relative risk of 0.77.2 Other recent studies showed the following8-10:
- a 63% delivery rate after 28 weeks’ gestation for physical-exam indicated cerclages in the presence of bulging amniotic membranes
- an 86.2% delivery rate after 32 weeks’ gestation for ultrasonography-indicated cerclages
- an 86% delivery rate after 32 weeks’ gestation for a history-indicated cerclage in patients with 2 or more prior second-trimester losses.
Success rates, especially for ultrasonography- and history-indicated cerclage, are thus high. For the 14% who still fail these methods, however, a different management strategy is needed, which is where transabdominal cerclage comes into play.
Continue to: Transabdominal cerclage is an option for certain patients...
Transabdominal cerclage is an option for certain patients
In transabdominal cerclage, an abdominal approach is used to place a stitch at the cervicouterine junction. With this approach, the cerclage can reach a closer proximity to the internal os compared with the vaginal approach, providing better support of the cervical tissue (FIGURE 2).11 Whether performed via laparotomy or laparoscopy, the transabdominal cerclage procedure likely carries higher morbidity than a transvaginal approach, and cesarean delivery is required after placement.

Since transvaginal cerclage often is successful, in most cases the transabdominal approach should not be viewed as the first-line treatment for cervical insufficiency if a history-indicated transvaginal cerclage has not been attempted. For women who fail a history-indicated transvaginal cerclage, however, a transabdominal cerclage has been proven to decrease the rate of preterm delivery and PPROM compared with attempting another history-indicated transvaginal cerclage.11,12
A recent systematic review of pregnancy outcomes after transabdominal cerclage placement reported neonatal survival of 96.5% and an 83% delivery rate after 34 weeks’ gestation.13 Thus, even among a population that failed transvaginal cerclage, a transabdominal cerclage has a high success rate in providing a good pregnancy outcome (TABLE). Transabdominal cerclage also can be considered as first-line treatment in patients who had prior cervical surgery or cervical deformities that might preclude the ability to place a cerclage transvaginally.

CASE Continued: A candidate for transabdominal cerclage
Given the patient’s poor obstetric history, which includes a preterm delivery and neonatal loss despite a history-indicated cerclage, you recommend that the patient have a transabdominal cerclage placed as the procedure has been proven to increase the chances of neonatal survival and delivery after 34 weeks in women with a similar obstetric history. The patient is interested in this option and asks about how this cerclage is placed and when it would need to be placed during her next pregnancy.
Surgical technique for transabdominal cerclage placement
A transabdominal cerclage can be placed via laparotomy, laparoscopy, or robot-assisted laparoscopy. No differences in obstetric outcomes have been shown between the laparotomy and laparoscopic approaches.14,15 Given the benefits of minimally invasive surgery, a laparoscopic or robot-assisted approach is preferred when feasible.
Additionally, for ease of placement, transabdominal cerclage can be placed prior to conception—known as interval placement—or during pregnancy between 10 and 14 weeks (preferably closer to 10 weeks). Because of the increased difficulty in placing a cerclage in the gravid uterus, interval transabdominal cerclage placement is recommended when possible.13,16 Authors of one observational study noted that improved obstetric outcomes occurred with interval placement compared with cerclage placement between 9 and 10 weeks’ gestation, with a delivery rate at more than 34 weeks’ gestation in 90% versus 74% of patients, respectively.16
Continue to: Steps for interval cerclage and during pregnancy...
Steps for interval cerclage and during pregnancy
Our practice is to place transabdominal cerclage via conventional laparoscopy as an interval procedure when possible. We find no benefit in using robotic assistance.
For an interval procedure, the patient is placed in a dorsal lithotomy position, and we place a 10-mm umbilical port, 2 lateral 5-mm ports, 1 suprapubic 5-mm port, and a uterine manipulator. We use a flexible laparoscope to provide optimal visualization of the pelvis from any angle.
The first step of the surgery involves dissecting the vesicouterine peritoneum in order to move the bladder inferiorly (FIGURE 3A). Uterine arteries are then identified lateral to the cervix as part of this dissection, and a window is created in the inferior aspect of the broad ligament just anterior and lateral to the insertion of the uterosacral ligaments onto the uterus, with care taken to avoid the uterine vessels superiorly (FIGURE 3B). Two 5-mm Mersilene tape sutures are then tied together to create 1 suture with a needle at each end. This is then passed into the abdomen, and 1 needle is passed through the parametrial space at the level of the internal os inferior to the uterine vessels on 1 side of the uterus while the other needle is passed through the parametrial space on the opposite side.

Alternatively, rather than using the suture needles, a blunt dissector can be passed through this same space bilaterally (FIGURE 3C) via the suprapubic port and can pull the Mersilene tape through the parametrial space (FIGURE 3D). The suture is then tied anterior at the level of the internal os intracorporally (FIGURE 3E), and the needles are cut off the suture and removed from the abdomen.
To perform transabdominal cerclage when the patient is pregnant, a few modifications are needed to help with placement. First, the patient may be placed in supine position since a uterine manipulator cannot be used. Second, use of a flexible laparoscope becomes even more imperative in order to properly see around the gravid uterus. Lastly, a 5-mm laparoscopic liver retractor can be used to aid in blunt manipulation of the gravid uterus (FIGURE 3F). (The surgical video below highlights the steps to transabdominal cerclage placement in a pregnant patient.) All other port placements and steps to dissection and suture placement are the same as in interval placement.

CASE Continued: Patient pursues transabdominal cerclage
You explain to your patient that ideally the cerclage should be placed now in a laparoscopic fashion before she becomes pregnant. You then refer her to a local gynecologic surgeon who places many laparoscopic transabdominal cerclages. She undergoes the procedure, becomes pregnant, and after presenting in labor at 35 weeks’ gestation has a cesarean delivery. Her baby is born without any neonatal complications, and the patient is overjoyed with the outcome.
Management during and after pregnancy
Pregnant patients with a transabdominal cerclage are precluded from having a vaginal delivery and must deliver via cesarean. During the antepartum period, patients are managed in the same manner as those who have a transvaginal cerclage. Delivery via cesarean at the onset of regular contractions is recommended to reduce the risk of uterine rupture. In the absence of labor, scheduled cesarean is performed at term.
Our practice is to schedule cesarean delivery at 38 weeks’ gestation, although there are no data or consensus to support a specific gestational age between 37 and 39 weeks. Unlike a transvaginal cerclage, a transabdominal cerclage can be left in place for use in subsequent pregnancies. Data are limited on whether the transabdominal cerclage should be removed in women who no longer desire childbearing and whether there are long-term sequelae if the suture is left in situ.17
Continue to: Complications and risks of abdominal cerclage...
Complications and risks of abdominal cerclage
As the data suggest and our experience confirms, transabdominal cerclage is highly successful in patients who have failed a history-indicated transvaginal cerclage; however, the transabdominal approach carries a higher surgical risk. Risks include intraoperative hemorrhage, conversion to laparotomy, and a range of rare surgical and obstetric complications, such as bladder injury and PPROM.13,18
If a patient experiences a fetal loss in the first trimester, a dilation and curettage (D&C) can be performed, with good obstetric outcomes in subsequent pregnancies.19 If the patient experiences an early-to-mid second-trimester loss, some studies suggest that a dilation and evacuation (D&E) of the uterus can be done with sufficient dilation of the cervix to accommodate up to a 15-mm cannula and Sopher forceps.19 Laminaria also may be used in this process. However, no data exist regarding success of future pregnancies and transabdominal cerclage integrity after a D&E.20 If the cerclage prevents successful dilation of the cervix, the cerclage must be removed laparoscopically prior to performing the D&E.
In late second-trimester and third-trimester loss, the cerclage must be removed to allow passage of the fetus and placenta prior to a D&E or an induction of labor.20
For patients with PPROM or preterm labor, data are limited regarding management recommendations. However, in these complex cases, we strongly recommend an individualized approach and co-management with maternal-fetal medicine specialists.
CASE Resolved
The cerclage is left in place during the patient’s cesarean delivery, and her postpartum course is uneventful. She continued without complications for the next year, at which time she sees you in the office with plans to have another pregnancy later in the year. You counsel her that her abdominal cerclage will still be effective and that she can get pregnant with expectations of similar outcomes as her previous pregnancy. She thanks you for everything and reports that she hopes to return later in the year for her first prenatal visit. ●
CASE A woman with recurrent pregnancy loss
A 38-year-old woman (G4P0221) presents to your office for preconception counseling. Her history is significant for the following: a spontaneous pregnancy loss at 15 weeks’ gestation; a pregnancy loss at 17 weeks secondary to preterm premature rupture of membranes (PPROM); a cesarean delivery at 30 weeks and 6 days’ gestation after placement of a transvaginal cerclage at 20 weeks for cervical dilation noted on physical exam (the child now has developmental delays); and most recently a delivery at 24 weeks and 4 days due to preterm labor with subsequent neonatal demise (this followed a transvaginal cerclage placed at 13 weeks and 6 days).
How would you counsel this patient?
Cervical insufficiency describes the inability of the cervix to retain a pregnancy in the absence of the signs and symptoms of clinical contractions, labor, or both in the second trimester.1 This condition affects an estimated 1% of obstetric patients and 8% of women with recurrent losses who have experienced a second-trimester loss.2
Diagnosis of cervical insufficiency is based on a history of painless cervical dilation after the first trimester with expulsion of the pregnancy in the second trimester before 24 weeks of gestation without contractions and in the absence of other pathology, such as bleeding, infection, or ruptured membranes.1 Diagnosis also can be made by noting cervical dilation on physical exam during the second trimester; more recently, short cervical length on transvaginal ultrasonography in the second trimester has been used to try to predict when a cervical cerclage may be indicated, although sonographic cervical length is more a marker for risk of preterm birth than for cervical insufficiency specifically.1,3
Given the considerable emotional and physical distress that patients experience with recurrent second-trimester losses and the significant neonatal morbidity and mortality that can occur with preterm delivery, substantial efforts are made to prevent these outcomes by treating patients with cervical insufficiency and those at risk for preterm delivery.
Transvaginal cerclage: A treatment mainstay
Standard treatment options for cervical insufficiency depend on the patient’s history. One of the treatment mainstays for women with prior second-trimester losses or preterm deliveries is transvaginal cervical cerclage. A transvaginal cerclage can be placed using either a Shirodkar technique, in which the vesicocervical mucosa is dissected and a suture is placed as close to the internal cervical os as possible, or a McDonald technique, in which a purse-string suture is placed around the cervicovaginal junction. No randomized trials have compared the effectiveness of these 2 methods, but most observational studies show no difference, and one suggests that the Shirodkar technique may be more effective in obese women specifically.4-6
Indications for transvaginal cerclage. The indication for transvaginal cerclage is based on history, physical exam, or ultrasonography.
A physical-exam indication is the most straightforward of the 3. Transvaginal cerclage placement is indicated if on physical exam in the second trimester a patient has cervical dilation without contractions or infection.1,7
A history-indicated cerclage (typically placed between 12 and 14 weeks’ gestation) is based on a cerclage having been placed in a prior pregnancy due to painless cervical dilation in the second trimester (either ultrasonography- or physical-exam indicated), and it also can be considered in the case of a history of 1 or more second-trimester pregnancy losses related to painless cervical dilation.1
More recent evidence suggests that in patients with 1 prior second-trimester loss or preterm delivery, serial sonographic cervical length can be measured safely from 16 to 24 weeks, with a cerclage being placed only if cervical length decreases to less than 25 mm. By using the ultrasonography-based indication, unnecessary history-indicated cerclages for 1 prior second-trimester or preterm birth can be avoided in more than one-half of patients (FIGURE 1).1,7

Efficacy. The effectiveness of transvaginal cerclage varies by the indication. Authors of a 2017 Cochrane review found an overall reduced risk of giving birth before 34 weeks’ gestation for any indication, with an average relative risk of 0.77.2 Other recent studies showed the following8-10:
- a 63% delivery rate after 28 weeks’ gestation for physical-exam indicated cerclages in the presence of bulging amniotic membranes
- an 86.2% delivery rate after 32 weeks’ gestation for ultrasonography-indicated cerclages
- an 86% delivery rate after 32 weeks’ gestation for a history-indicated cerclage in patients with 2 or more prior second-trimester losses.
Success rates, especially for ultrasonography- and history-indicated cerclage, are thus high. For the 14% who still fail these methods, however, a different management strategy is needed, which is where transabdominal cerclage comes into play.
Continue to: Transabdominal cerclage is an option for certain patients...
Transabdominal cerclage is an option for certain patients
In transabdominal cerclage, an abdominal approach is used to place a stitch at the cervicouterine junction. With this approach, the cerclage can reach a closer proximity to the internal os compared with the vaginal approach, providing better support of the cervical tissue (FIGURE 2).11 Whether performed via laparotomy or laparoscopy, the transabdominal cerclage procedure likely carries higher morbidity than a transvaginal approach, and cesarean delivery is required after placement.

Since transvaginal cerclage often is successful, in most cases the transabdominal approach should not be viewed as the first-line treatment for cervical insufficiency if a history-indicated transvaginal cerclage has not been attempted. For women who fail a history-indicated transvaginal cerclage, however, a transabdominal cerclage has been proven to decrease the rate of preterm delivery and PPROM compared with attempting another history-indicated transvaginal cerclage.11,12
A recent systematic review of pregnancy outcomes after transabdominal cerclage placement reported neonatal survival of 96.5% and an 83% delivery rate after 34 weeks’ gestation.13 Thus, even among a population that failed transvaginal cerclage, a transabdominal cerclage has a high success rate in providing a good pregnancy outcome (TABLE). Transabdominal cerclage also can be considered as first-line treatment in patients who had prior cervical surgery or cervical deformities that might preclude the ability to place a cerclage transvaginally.

CASE Continued: A candidate for transabdominal cerclage
Given the patient’s poor obstetric history, which includes a preterm delivery and neonatal loss despite a history-indicated cerclage, you recommend that the patient have a transabdominal cerclage placed as the procedure has been proven to increase the chances of neonatal survival and delivery after 34 weeks in women with a similar obstetric history. The patient is interested in this option and asks about how this cerclage is placed and when it would need to be placed during her next pregnancy.
Surgical technique for transabdominal cerclage placement
A transabdominal cerclage can be placed via laparotomy, laparoscopy, or robot-assisted laparoscopy. No differences in obstetric outcomes have been shown between the laparotomy and laparoscopic approaches.14,15 Given the benefits of minimally invasive surgery, a laparoscopic or robot-assisted approach is preferred when feasible.
Additionally, for ease of placement, transabdominal cerclage can be placed prior to conception—known as interval placement—or during pregnancy between 10 and 14 weeks (preferably closer to 10 weeks). Because of the increased difficulty in placing a cerclage in the gravid uterus, interval transabdominal cerclage placement is recommended when possible.13,16 Authors of one observational study noted that improved obstetric outcomes occurred with interval placement compared with cerclage placement between 9 and 10 weeks’ gestation, with a delivery rate at more than 34 weeks’ gestation in 90% versus 74% of patients, respectively.16
Continue to: Steps for interval cerclage and during pregnancy...
Steps for interval cerclage and during pregnancy
Our practice is to place transabdominal cerclage via conventional laparoscopy as an interval procedure when possible. We find no benefit in using robotic assistance.
For an interval procedure, the patient is placed in a dorsal lithotomy position, and we place a 10-mm umbilical port, 2 lateral 5-mm ports, 1 suprapubic 5-mm port, and a uterine manipulator. We use a flexible laparoscope to provide optimal visualization of the pelvis from any angle.
The first step of the surgery involves dissecting the vesicouterine peritoneum in order to move the bladder inferiorly (FIGURE 3A). Uterine arteries are then identified lateral to the cervix as part of this dissection, and a window is created in the inferior aspect of the broad ligament just anterior and lateral to the insertion of the uterosacral ligaments onto the uterus, with care taken to avoid the uterine vessels superiorly (FIGURE 3B). Two 5-mm Mersilene tape sutures are then tied together to create 1 suture with a needle at each end. This is then passed into the abdomen, and 1 needle is passed through the parametrial space at the level of the internal os inferior to the uterine vessels on 1 side of the uterus while the other needle is passed through the parametrial space on the opposite side.

Alternatively, rather than using the suture needles, a blunt dissector can be passed through this same space bilaterally (FIGURE 3C) via the suprapubic port and can pull the Mersilene tape through the parametrial space (FIGURE 3D). The suture is then tied anterior at the level of the internal os intracorporally (FIGURE 3E), and the needles are cut off the suture and removed from the abdomen.
To perform transabdominal cerclage when the patient is pregnant, a few modifications are needed to help with placement. First, the patient may be placed in supine position since a uterine manipulator cannot be used. Second, use of a flexible laparoscope becomes even more imperative in order to properly see around the gravid uterus. Lastly, a 5-mm laparoscopic liver retractor can be used to aid in blunt manipulation of the gravid uterus (FIGURE 3F). (The surgical video below highlights the steps to transabdominal cerclage placement in a pregnant patient.) All other port placements and steps to dissection and suture placement are the same as in interval placement.

CASE Continued: Patient pursues transabdominal cerclage
You explain to your patient that ideally the cerclage should be placed now in a laparoscopic fashion before she becomes pregnant. You then refer her to a local gynecologic surgeon who places many laparoscopic transabdominal cerclages. She undergoes the procedure, becomes pregnant, and after presenting in labor at 35 weeks’ gestation has a cesarean delivery. Her baby is born without any neonatal complications, and the patient is overjoyed with the outcome.
Management during and after pregnancy
Pregnant patients with a transabdominal cerclage are precluded from having a vaginal delivery and must deliver via cesarean. During the antepartum period, patients are managed in the same manner as those who have a transvaginal cerclage. Delivery via cesarean at the onset of regular contractions is recommended to reduce the risk of uterine rupture. In the absence of labor, scheduled cesarean is performed at term.
Our practice is to schedule cesarean delivery at 38 weeks’ gestation, although there are no data or consensus to support a specific gestational age between 37 and 39 weeks. Unlike a transvaginal cerclage, a transabdominal cerclage can be left in place for use in subsequent pregnancies. Data are limited on whether the transabdominal cerclage should be removed in women who no longer desire childbearing and whether there are long-term sequelae if the suture is left in situ.17
Continue to: Complications and risks of abdominal cerclage...
Complications and risks of abdominal cerclage
As the data suggest and our experience confirms, transabdominal cerclage is highly successful in patients who have failed a history-indicated transvaginal cerclage; however, the transabdominal approach carries a higher surgical risk. Risks include intraoperative hemorrhage, conversion to laparotomy, and a range of rare surgical and obstetric complications, such as bladder injury and PPROM.13,18
If a patient experiences a fetal loss in the first trimester, a dilation and curettage (D&C) can be performed, with good obstetric outcomes in subsequent pregnancies.19 If the patient experiences an early-to-mid second-trimester loss, some studies suggest that a dilation and evacuation (D&E) of the uterus can be done with sufficient dilation of the cervix to accommodate up to a 15-mm cannula and Sopher forceps.19 Laminaria also may be used in this process. However, no data exist regarding success of future pregnancies and transabdominal cerclage integrity after a D&E.20 If the cerclage prevents successful dilation of the cervix, the cerclage must be removed laparoscopically prior to performing the D&E.
In late second-trimester and third-trimester loss, the cerclage must be removed to allow passage of the fetus and placenta prior to a D&E or an induction of labor.20
For patients with PPROM or preterm labor, data are limited regarding management recommendations. However, in these complex cases, we strongly recommend an individualized approach and co-management with maternal-fetal medicine specialists.
CASE Resolved
The cerclage is left in place during the patient’s cesarean delivery, and her postpartum course is uneventful. She continued without complications for the next year, at which time she sees you in the office with plans to have another pregnancy later in the year. You counsel her that her abdominal cerclage will still be effective and that she can get pregnant with expectations of similar outcomes as her previous pregnancy. She thanks you for everything and reports that she hopes to return later in the year for her first prenatal visit. ●
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 142: Cerclage for the management of cervical insufficiency. Obstet Gynecol. 2014;123(2 pt 1): 372-379.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6(6):CD008991.
- Brown R, Gagnon R, Delisle M-F. No. 373—cervical insufficiency and cervical cerclage. J Obstet Gynaecol Can. 2019;41:233-247.
- Odibo AO, Berghella V, To MS, et al. Shirodkar versus McDonald cerclage for the prevention of preterm birth in women with short cervical length. Am J Perinatol. 2007;24: 55-60.
- Basbug A, Bayrak M, Dogan O, et al. McDonald versus modified Shirodkar rescue cerclage in women with prolapsed fetal membranes. J Matern Fetal Neonatal Med. 2020;33: 1075-1097.
- Figueroa R, Crowell R, Martinez A, et al. McDonald versus Shirodkar cervical cerclage for the prevention of preterm birth: impact of body mass index. J Matern Fetal Neonatal Med. 2019;32:3408-3414.
- Suhag A, Berghella V. Cervical cerclage. Clin Obstet Gynecol. 2014;57:557-567.
- Bayrak M, Gul A, Goynumer G. Rescue cerclage when foetal membranes prolapse into the vagina. J Obstet Gynaecol. 2017;37:471-475.
- Drassinower D, Coviello E, Landy HJ, et al. Outcomes after periviable ultrasound-indicated cerclage. J Matern Fetal Neonatal Med. 2019;32:932-938.
- Lee KN, Whang EJ, Chang KH, et al. History-indicated cerclage: the association between previous preterm history and cerclage outcome. Obstet Gynecol Sci. 2018;61:23-29. doi:10.5468/ogs.2018.61.1.23.
- Sneider K, Christiansen OB, Sundtoft IB, et al. Recurrence rates after abdominal and vaginal cerclages in women with cervical insufficiency: a validated cohort study. Arch Gynecol Obstet. 2017;295:859-866.
- Davis G, Berghella V, Talucci M, et al. Patients with a prior failed transvaginal cerclage: a comparison of obstetric outcomes with either transabdominal or transvaginal cerclage. Am J Obstet Gynecol. 2000;183:836-839.
- Moawad GN, Tyan P, Bracke T, et al. Systematic review of transabdominal cerclage placed via laparoscopy for the prevention of preterm birth. J Mimim Invasive Gynecol. 2018;25:277-286.
- Burger NB, Brölmann HAM, Einarsson JI, et al. Effectiveness of abdominal cerclage placed via laparotomy or laparoscopy: systematic review. J Minim Invasive Gynecol. 2011;18:696-704.
- Kim S, Hill A, Menderes G, et al. Minimally invasive abdominal cerclage compared to laparotomy: a comparison of surgical and obstetric outcomes. J Robot Surg. 2018;12:295-301.
- Dawood F, Farquharson RG. Transabdominal cerclage: preconceptual versus first trimester insertion. Eur J Obstet Gynecol Reprod Biol. 2016;199:27-31.
- Hawkins E, Nimaroff M. Vaginal erosion of an abdominal cerclage 7 years after laparoscopic placement. Obstet Gynecol. 2014;123(2 pt 2 suppl 2):420-423.
- Foster TL, Moore ES, Sumners JE. Operative complications and fetal morbidity encountered in 300 prophylactic transabdominal cervical cerclage procedures by one obstetric surgeon. J Obstet Gynaecol. 2011;31:713-717.
- Dethier D, Lassey SC, Pilliod R, et al. Uterine evacuation in the setting of transabdominal cerclage. Contraception. 2020;101:174-177.
- Martin A, Lathrop E. Controversies in family planning: management of second-trimester losses in the setting of an abdominal cerclage. Contraception. 2013;87:728-731.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 142: Cerclage for the management of cervical insufficiency. Obstet Gynecol. 2014;123(2 pt 1): 372-379.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6(6):CD008991.
- Brown R, Gagnon R, Delisle M-F. No. 373—cervical insufficiency and cervical cerclage. J Obstet Gynaecol Can. 2019;41:233-247.
- Odibo AO, Berghella V, To MS, et al. Shirodkar versus McDonald cerclage for the prevention of preterm birth in women with short cervical length. Am J Perinatol. 2007;24: 55-60.
- Basbug A, Bayrak M, Dogan O, et al. McDonald versus modified Shirodkar rescue cerclage in women with prolapsed fetal membranes. J Matern Fetal Neonatal Med. 2020;33: 1075-1097.
- Figueroa R, Crowell R, Martinez A, et al. McDonald versus Shirodkar cervical cerclage for the prevention of preterm birth: impact of body mass index. J Matern Fetal Neonatal Med. 2019;32:3408-3414.
- Suhag A, Berghella V. Cervical cerclage. Clin Obstet Gynecol. 2014;57:557-567.
- Bayrak M, Gul A, Goynumer G. Rescue cerclage when foetal membranes prolapse into the vagina. J Obstet Gynaecol. 2017;37:471-475.
- Drassinower D, Coviello E, Landy HJ, et al. Outcomes after periviable ultrasound-indicated cerclage. J Matern Fetal Neonatal Med. 2019;32:932-938.
- Lee KN, Whang EJ, Chang KH, et al. History-indicated cerclage: the association between previous preterm history and cerclage outcome. Obstet Gynecol Sci. 2018;61:23-29. doi:10.5468/ogs.2018.61.1.23.
- Sneider K, Christiansen OB, Sundtoft IB, et al. Recurrence rates after abdominal and vaginal cerclages in women with cervical insufficiency: a validated cohort study. Arch Gynecol Obstet. 2017;295:859-866.
- Davis G, Berghella V, Talucci M, et al. Patients with a prior failed transvaginal cerclage: a comparison of obstetric outcomes with either transabdominal or transvaginal cerclage. Am J Obstet Gynecol. 2000;183:836-839.
- Moawad GN, Tyan P, Bracke T, et al. Systematic review of transabdominal cerclage placed via laparoscopy for the prevention of preterm birth. J Mimim Invasive Gynecol. 2018;25:277-286.
- Burger NB, Brölmann HAM, Einarsson JI, et al. Effectiveness of abdominal cerclage placed via laparotomy or laparoscopy: systematic review. J Minim Invasive Gynecol. 2011;18:696-704.
- Kim S, Hill A, Menderes G, et al. Minimally invasive abdominal cerclage compared to laparotomy: a comparison of surgical and obstetric outcomes. J Robot Surg. 2018;12:295-301.
- Dawood F, Farquharson RG. Transabdominal cerclage: preconceptual versus first trimester insertion. Eur J Obstet Gynecol Reprod Biol. 2016;199:27-31.
- Hawkins E, Nimaroff M. Vaginal erosion of an abdominal cerclage 7 years after laparoscopic placement. Obstet Gynecol. 2014;123(2 pt 2 suppl 2):420-423.
- Foster TL, Moore ES, Sumners JE. Operative complications and fetal morbidity encountered in 300 prophylactic transabdominal cervical cerclage procedures by one obstetric surgeon. J Obstet Gynaecol. 2011;31:713-717.
- Dethier D, Lassey SC, Pilliod R, et al. Uterine evacuation in the setting of transabdominal cerclage. Contraception. 2020;101:174-177.
- Martin A, Lathrop E. Controversies in family planning: management of second-trimester losses in the setting of an abdominal cerclage. Contraception. 2013;87:728-731.
Progesterone for preterm delivery prevention
Researchers have been studying the use of exogenous progestins for prevention of preterm delivery (PTD) for almost 60 years, but conflicting results contribute to an ongoing debate. Interpretation of the available data is particularly difficult because different forms and doses of progestins have been used in disparate study populations.
Based on available data, progesterone supplementation is not effective as a primary prevention strategy for PTD in the general low-risk obstetric population. PTD is a complex problem with varied and incompletely elucidated pathogenic pathways, making it unlikely that one interventional approach would be effective for all pregnant women. As a result, emerging indications for the use of progesterone are based on risk factors for PTD (ie, prior PTD and/or short cervix). However, this secondary prevention approach is a limiting factor in itself because 50% of women destined to have a PTD have no identifiable risk factors.1 In addition, researchers have found that progestins are ineffective at delaying delivery for women with multiple gestation, suggesting that a distinct underlying mechanism of early parturition is present in these women, and that this mechanism is unresponsive to progestins.2
The formulations used in the study of progestin supplementation for PTD prevention have been almost exclusively either the synthetic 17 alpha-hydroxyprogesterone caproate (17-OHPC) or natural progesterone administered orally or vaginally. In 2003, the American College of Obstetricians and Gynecologists (ACOG) supported the use of progesterone to reduce the rate of PTD,3 and in 2011, the US Food and Drug Administration (FDA) approved 17-OHPC for use as prophylaxis against recurrent PTD. As a result, in recent years, the perceived standard of care for a majority of practitioners in the United States had been that all women with a previous preterm birth should be offered 17-OHPC. It may be interesting to note that in other parts of the world, the same enthusiastic adoption did not occur. For example, in Australia and New Zealand in 2007, only 5% of practitioners were using progesterone for this indication.4 Further, 17-OHPC is not recommended by professional guidelines in the United Kingdom and has remained unavailable in Germany.
The publication in 2019 of the PROLONG trial called into question the use of 17-OHPC for the prevention of PTD.5 In the December 2019 issue of OBG Management (“Managing preterm birth in those at risk: Expert strategies”), I expressed the opinion that with only rare exceptions, 17-OHPC is no longer a viable option for recurrent PTD prevention.6 In light of these developments, what scientific evidence is relevant and applicable to the care of women at risk for PTD?
Continue to: Case 1 Previous spontaneous PTD at 31 weeks...
Case 1 Previous spontaneous PTD at 31 weeks
MC is an asymptomatic 32-year-old woman with a singleton pregnancy at 13 weeks’ gestation. You see her for a maternal-fetal medicine consultation because 2 years ago she had a spontaneous PTD at 31 weeks’ gestation. What management recommendations can you make to decrease her risk of recurrent PTD?
Cervical length measurement narrows in on risk
The indication “previous preterm birth” is largely meaningless because of the heterogeneity in preterm birth pathways (preterm birth as a syndrome7) and inadequate risk characterization. Among women who experience a spontaneous PTD, 70% to 80% do not deliver prematurely in subsequent pregnancies.8 To better characterize the risk of PTD recurrence, ultrasound assessment of cervical length should be used. Research has shown that among women with a prior spontaneous PTD who maintain a normal cervical length until 24 weeks’ gestation, more than 90% will deliver at 35 weeks or after without intervention.9 Such an approach not only identifies the subgroup of women at significantly increased risk of recurrence but also eliminates unnecessary interventions.
Cervical ultrasound surveillance should be initiated at 16 weeks’ gestation. A short cervix before 16 weeks is not associated with a statistically significant increase in risk for PTD.10 Shortening of the cervix begins approximately 10 weeks before delivery in any gestational age group.11 Therefore, ultrasound assessment of the cervix at 28 weeks and after is irrelevant. In addition, after 28 weeks, cervical length varies greatly leading to loss in the predictive power of the cervical measurement.12 Based on these considerations, cervical surveillance may be extended up to 26 weeks. Although cervical cerclage is not an option in the United States in cases in which a short cervix is detected between 24 and 26 weeks, vaginal progesterone supplementation may still be considered.
Case 1 Continued
MC was started on ultrasound cervical surveillance at 16 weeks’ gestation. Her cervical length was initially normal (> 2.5 cm), but at 18 weeks the measurement was 2.2 cm. What is your recommendation?
The value of vaginal progesterone
There appears to be increasing consensus on the value of vaginal progesterone for women with a midtrimester short cervix on sonography, with or without a history of PTD. An individual patient data meta-analysis demonstrated the benefits of vaginal progesterone.13 Although there was no evidence of an effect on PTD at less than 37 weeks, the rates of PTD at less than 36 weeks and spontaneous PTD at less than 34 weeks were significantly reduced (by 20% and 28%, respectively). Also, there was a significant reduction in the risk of respiratory distress syndrome (53%) and composite neonatal morbidity and mortality (41%), with no significant impact on infant development up to the second year of life.13
The lack of generalizable evidence of benefit on childhood outcomes, combined with considerable uncertainty about the exact role and mechanism of action of exogenous progestins, contribute to the ongoing debate. Vaginal progesterone dosage regimens have been based on extrapolations from experience with progesterone in nonpregnant women, and recent pharmacokinetic studies have revealed how precarious such extrapolations may be. As an example, in nonpregnant women, the bioavailability of oral and vaginal progesterone is similar.14 In pregnancy, however, while daily oral progesterone doubles a pregnant woman’s serum progesterone level,15 daily vaginal administration of progesterone results in only a modest rise in serum progesterone, with a coefficient of variation among individuals that is double that outside of pregnancy.16 It is, therefore, considered that vaginal progesterone in pregnancy may have a local action secondary to the uterine first-pass effect. The uterine first-pass effect for vaginal progesterone was described in nonpregnant women and is only assumed to occur in pregnancy as well. 17
After evaluating the data from the largest available study of vaginal progesterone,18 the FDA concluded in 2012 that the study did not meet the statistical significance generally expected to support the approval of a new product. However, according to a more comprehensive evidence review developed in 2019 by the National Guideline Alliance in the United Kingdom, women with a history of PTD and women with a short cervix derive an important benefit from the use of vaginal progesterone; thus, this intervention should be offered to them.19 At this time, a short cervix and PTD prevention are not considered FDA-approved indications for progesterone supplementation in pregnancy. However, vaginal progesterone is FDA approved for use in pregnant women with a history of infertility.
Continue to: Case 1 Continued...
Case 1 Continued
MC initiated treatment with daily vaginal progesterone at 18 weeks’ gestation and returned for ultrasound cervical length examination weekly instead of every other week. At 20 weeks’ gestation, cervical length was 2.0 cm; the following week it was 1.4 cm. What would you recommend at this point?
When to consider cerclage
If cervical shortening progresses to about 1.5 cm while a woman is being treated with vaginal progesterone, cerclage may be considered. The benefit of cerclage in patients with prior PTD and a short cervix was highlighted in a 2018 Cochrane Review.20 In this stepwise management approach to a short cervix, waiting for a cervix to be less than 1.5 cm may be unadvisable. Under conditions of a very short cervix that is frequently dilated with exposure of fetal membranes, ascending subclinical intra-amniotic infection may already be present, reducing the efficacy of any preventive measures. Preferential consideration for cerclage from the start over initial vaginal progesterone also may be appropriate when there is a history of 2 spontaneous PTDs or mid-trimester losses, a history of a successful cerclage, or with a very short cervix (< 1.0 cm) at the initial evaluation. As for the latter, a 2018 individual patient data meta-analysis of vaginal progesterone found no benefit when the cervix was less than 1.0 cm.13
Progesterone plus cerclage likely to add benefit
The results of an adjusted indirect comparison meta-analysis suggest that both interventions—vaginal progesterone and cerclage—are equally effective.21 Assuming that there is no clinically meaningful difference in benefit associated with these 2 treatments, the next logical question is whether combining the 2 therapies provides any added benefit; limited observational data seem to suggest that it does. In a retrospective cohort of 86 consecutive singleton pregnancies among women who underwent ultrasound-indicated cerclage, those who used vaginal progesterone after cerclage (n = 45) had a lower rate of PTD.22 Also, a small (66 cases) case-control study demonstrated the benefit of administration of vaginal progesterone as a rescue intervention in women with cerclage and progressive cervical shortening despite cerclage.23
Case 2 Woman experiences adverse effects from vaginal progesterone
MS is a 25-year-old G2P0101 who was started on vaginal progesterone as prophylaxis for recurrent PTD. She is now at 20 weeks’ gestation, with a stable remnant cervical length of 2.0 cm. She is reporting an increasing vaginal burning sensation and vaginal discharge caused by the nightly vaginal progesterone applications, to the point that she is unwilling to continue the treatment. She asks if any alternatives to vaginal progesterone are available to decrease her risk of PTD.
Continue to: Is oral progesterone an option?...
Is oral progesterone an option?
In the 1980s and 1990s, oral micronized progesterone was widely used in France at doses of 900 to 1,200 mg/d for women at risk for PTD. The practice was stopped when secondary hepatic effects, including cholestasis of pregnancy, were reported at a higher rate in treated women.24 A rise in the serum concentration of progesterone metabolites has been associated with impaired biliary excretion and subsequent accumulation of bile acids.25 In other reports, elevated serum transaminase activity was found in pregnant women treated with oral micronized progesterone, and withdrawal of treatment frequently has led to improvement in transaminase levels.26 The synthesis of endogenous progesterone during normal pregnancy is between 250 and 500 mg/d,26 and experts have expressed concern that exogenous progesterone supplementation may impose an additional load on the hepatic transport of sulfated metabolites. Unlike orally administered progesterone, progestins given by the vaginal route avoid the hepatic first-pass effect. For this reason, they may be associated with less hepatic dysfunction.
Although not recommended by professional guidelines, oral progesterone administration for the prevention of PTD has been used in the United States. A 2015 survey of Wisconsin prenatal care providers found that of those who prescribed any progesterone for PTD prevention, oral progesterone was prescribed by 13.1% of obstetricians, 24.4% of midwives, and 40.7% of family medicine practitioners.27
Some limited recent evidence from a meta-analysis of 3 trials investigating oral progesterone versus placebo suggests effectiveness in the prevention of recurrent PTD and reduction in perinatal morbidity and mortality.15 However, the number of cases included in the meta-analysis (386) was too small to support definitive clinical recommendations. Furthermore, questions have been raised in the literature about the reliability of the largest trial included in that meta-analysis.28
Case 3 Two previous spontaneous PTDs
A 29-year-old G3P0201 presents for her first prenatal appointment at 10 weeks’ gestation. With her first pregnancy she had a spontaneous PTD at 23 weeks, and the neonate did not survive. In her second pregnancy, she was treated with 17-OHPC from 16 weeks’ gestation. She had a spontaneous PTD at 29 weeks, and that child is developing normally by her report. She believes that 17-OHPC helped her in her last pregnancy and is anxious about the risk for still another PTD. Consistent with the concept of shared decision-making, you inform her of the results of the recent PROLONG trial and statements on the subject released by professional organizations such as ACOG and the Society for Maternal-Fetal Medicine (SMFM). What options does she have?
17-OHPC may be a possibility in very high-risk women
According to a SMFM statement released in the wake of the PROLONG trial publication, “. . . SMFM believes that it is reasonable for providers to use 17-OHPC in women with a profile more representative of the very high-risk population reported in the Meis trial”.29 Only a few women will have a recurrence risk of PTD over 50%, as was the background event rate in the Meis trial.30 Such a risk level may be suspected, as an example, in women with 2 or more prior early (before 34 weeks) PTDs without intervening term deliveries. Even in those cases, if treatment with 17-OHPC is decided upon, ultrasound cervical surveillance should be added as an additional safety measure. ●
- Iams JD, Goldenberg RL, Mercer BM, et al. The preterm prediction study: can low-risk women destined for spontaneous preterm birth be identified? Am J Obstet Gynecol. 2001;184:652-655.
- Murray SR, Stock SJ, Cowan S, et al. Spontaneous preterm birth prevention in multiple pregnancy. Obstet Gynecol. 2018;20:57-63.
- American College of Obstetricians and Gynecologists. ACOG committee opinion. Use of progesterone to reduce preterm birth. Obstet Gynecol. 2003;102:1115-1116.
- Dodd JM, Ashwood P, Flenady V, et al. A survey of clinician and patient attitudes towards the use of progesterone for women at risk of preterm birth. Aust N Z J Obstet Gynaecol. 2007;47:106-109.
- Blackwell SC, Gyamfi -Bannerman C, Biggio JR, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- Duff P, Vidaeff AC, Ross MG, Norwitz ER. Managing preterm birth in those at risk: expert strategies. OBG Manag. 2019;31:39-42.
- Romero R, Mazor M, Munoz H, et al. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414-429.
- Phillips C, Velji Z, Hanly C, et al. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402.
- Berghella V, Seibel-Seamon J. Contemporary use of cervical cerclage. Clin Obstet Gynecol. 2007;50:468-477.
- Naim A, Haberman S, Burgess T, et al. Changes in cervical length and the risk of preterm labor. Am J Obstet Gynecol. 2002;186:887-889.
- Zilianti M, Azuaga A, Calderon F, et al. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14:719-724.
- Goldenberg RL, Iams JD, Miodovnik M, et al. The preterm prediction study: risk factors in twin gestation. Am J Obstet Gynecol. 1996;175:1047-1053.
- Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161-180.
- Norman T, Morse C, Dennerstein L. Comparative bioavailability of orally and vaginally administered progesterone. Fertil Steril. 1991;56:1034-1039.
- Boelig RC, Della Corte L, Ashoush S, et al. Oral progesterone for the prevention of recurrent preterm birth: systematic review and metaanalysis. Am J Obstet Gynecol MFM. 2019;1:50-62.
- Boelig RC, Zuppa AF, Kraft WK, et al. Pharmacokinetics of vaginal progesterone in pregnancy. Am J Obstet Gynecol. 2019;221:263.e1-7.
- Bulletti C, de Ziegler D, Flamigni C, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997;12:1073-1079.
- Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebocontrolled trial. Ultrasound Obstet Gynecol. 2011;38:18-31.
- Preterm labour and birth. Evidence review for clinical effectiveness of prophylactic progesterone in preventing preterm labour. London: National Institute for Health and Care Excellence (UK); August 2019.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6:CD008991.
- Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. 2018;219:10-25.
- Park JY, Jung YM, Kook S-Y, et al. The effect of postoperative vaginal progesterone in ultrasound-indicated cerclage to prevent preterm birth. J Matern Fetal Neonatal Med. 2019:1-8.
- Roman AR, Da Silva Costa F, et al. Rescue adjuvant vaginal progesterone may improve outcomes in cervical cerclage failure. Geburt Frauen. 2018;78:785-790.
- Benifle JL, Dumont M, Levardon M, et al. Effects of natural micronized progesterone on the liver in the third trimester of pregnancy. Contracept Fertil Sex. 1997;25:165-169.
- Vallejo M, Briz O, Serrano MA, et al. Potential role of transinhibition of the bile salt export pump by progesterone metabolites in the etiopathogenesis of intrahepatic cholestasis of pregnancy. J Hepatol. 2006;44:1150-1157.
- Bacq Y, Sapey T, Bréchot MC, et al. Intrahepatic cholestasis of pregnancy: a French prospective study. Hepatology. 1997;26:358-364.
- Hoppe K, Kramer RD, Ha B, et al. Progesterone supplementation for the prevention of preterm birth: provider practice in Wisconsin. WMJ. 2019;118:126-131.
- Katsanevakis E, Mol BW, Thornton J. A question about the reliability of a recent trial of progesterone for preterm birth prevention, published in Acta. Acta Obstet Gynecol Scand. 2020;99:426.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280smfm-statement-use-of-17-alpha-hydroxyprogesteronecaproate-for-prevention-of-recurrent-preterm-birth. Accessed March 23, 2020.
- Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;384:2379-2385.
Researchers have been studying the use of exogenous progestins for prevention of preterm delivery (PTD) for almost 60 years, but conflicting results contribute to an ongoing debate. Interpretation of the available data is particularly difficult because different forms and doses of progestins have been used in disparate study populations.
Based on available data, progesterone supplementation is not effective as a primary prevention strategy for PTD in the general low-risk obstetric population. PTD is a complex problem with varied and incompletely elucidated pathogenic pathways, making it unlikely that one interventional approach would be effective for all pregnant women. As a result, emerging indications for the use of progesterone are based on risk factors for PTD (ie, prior PTD and/or short cervix). However, this secondary prevention approach is a limiting factor in itself because 50% of women destined to have a PTD have no identifiable risk factors.1 In addition, researchers have found that progestins are ineffective at delaying delivery for women with multiple gestation, suggesting that a distinct underlying mechanism of early parturition is present in these women, and that this mechanism is unresponsive to progestins.2
The formulations used in the study of progestin supplementation for PTD prevention have been almost exclusively either the synthetic 17 alpha-hydroxyprogesterone caproate (17-OHPC) or natural progesterone administered orally or vaginally. In 2003, the American College of Obstetricians and Gynecologists (ACOG) supported the use of progesterone to reduce the rate of PTD,3 and in 2011, the US Food and Drug Administration (FDA) approved 17-OHPC for use as prophylaxis against recurrent PTD. As a result, in recent years, the perceived standard of care for a majority of practitioners in the United States had been that all women with a previous preterm birth should be offered 17-OHPC. It may be interesting to note that in other parts of the world, the same enthusiastic adoption did not occur. For example, in Australia and New Zealand in 2007, only 5% of practitioners were using progesterone for this indication.4 Further, 17-OHPC is not recommended by professional guidelines in the United Kingdom and has remained unavailable in Germany.
The publication in 2019 of the PROLONG trial called into question the use of 17-OHPC for the prevention of PTD.5 In the December 2019 issue of OBG Management (“Managing preterm birth in those at risk: Expert strategies”), I expressed the opinion that with only rare exceptions, 17-OHPC is no longer a viable option for recurrent PTD prevention.6 In light of these developments, what scientific evidence is relevant and applicable to the care of women at risk for PTD?
Continue to: Case 1 Previous spontaneous PTD at 31 weeks...
Case 1 Previous spontaneous PTD at 31 weeks
MC is an asymptomatic 32-year-old woman with a singleton pregnancy at 13 weeks’ gestation. You see her for a maternal-fetal medicine consultation because 2 years ago she had a spontaneous PTD at 31 weeks’ gestation. What management recommendations can you make to decrease her risk of recurrent PTD?
Cervical length measurement narrows in on risk
The indication “previous preterm birth” is largely meaningless because of the heterogeneity in preterm birth pathways (preterm birth as a syndrome7) and inadequate risk characterization. Among women who experience a spontaneous PTD, 70% to 80% do not deliver prematurely in subsequent pregnancies.8 To better characterize the risk of PTD recurrence, ultrasound assessment of cervical length should be used. Research has shown that among women with a prior spontaneous PTD who maintain a normal cervical length until 24 weeks’ gestation, more than 90% will deliver at 35 weeks or after without intervention.9 Such an approach not only identifies the subgroup of women at significantly increased risk of recurrence but also eliminates unnecessary interventions.
Cervical ultrasound surveillance should be initiated at 16 weeks’ gestation. A short cervix before 16 weeks is not associated with a statistically significant increase in risk for PTD.10 Shortening of the cervix begins approximately 10 weeks before delivery in any gestational age group.11 Therefore, ultrasound assessment of the cervix at 28 weeks and after is irrelevant. In addition, after 28 weeks, cervical length varies greatly leading to loss in the predictive power of the cervical measurement.12 Based on these considerations, cervical surveillance may be extended up to 26 weeks. Although cervical cerclage is not an option in the United States in cases in which a short cervix is detected between 24 and 26 weeks, vaginal progesterone supplementation may still be considered.
Case 1 Continued
MC was started on ultrasound cervical surveillance at 16 weeks’ gestation. Her cervical length was initially normal (> 2.5 cm), but at 18 weeks the measurement was 2.2 cm. What is your recommendation?
The value of vaginal progesterone
There appears to be increasing consensus on the value of vaginal progesterone for women with a midtrimester short cervix on sonography, with or without a history of PTD. An individual patient data meta-analysis demonstrated the benefits of vaginal progesterone.13 Although there was no evidence of an effect on PTD at less than 37 weeks, the rates of PTD at less than 36 weeks and spontaneous PTD at less than 34 weeks were significantly reduced (by 20% and 28%, respectively). Also, there was a significant reduction in the risk of respiratory distress syndrome (53%) and composite neonatal morbidity and mortality (41%), with no significant impact on infant development up to the second year of life.13
The lack of generalizable evidence of benefit on childhood outcomes, combined with considerable uncertainty about the exact role and mechanism of action of exogenous progestins, contribute to the ongoing debate. Vaginal progesterone dosage regimens have been based on extrapolations from experience with progesterone in nonpregnant women, and recent pharmacokinetic studies have revealed how precarious such extrapolations may be. As an example, in nonpregnant women, the bioavailability of oral and vaginal progesterone is similar.14 In pregnancy, however, while daily oral progesterone doubles a pregnant woman’s serum progesterone level,15 daily vaginal administration of progesterone results in only a modest rise in serum progesterone, with a coefficient of variation among individuals that is double that outside of pregnancy.16 It is, therefore, considered that vaginal progesterone in pregnancy may have a local action secondary to the uterine first-pass effect. The uterine first-pass effect for vaginal progesterone was described in nonpregnant women and is only assumed to occur in pregnancy as well. 17
After evaluating the data from the largest available study of vaginal progesterone,18 the FDA concluded in 2012 that the study did not meet the statistical significance generally expected to support the approval of a new product. However, according to a more comprehensive evidence review developed in 2019 by the National Guideline Alliance in the United Kingdom, women with a history of PTD and women with a short cervix derive an important benefit from the use of vaginal progesterone; thus, this intervention should be offered to them.19 At this time, a short cervix and PTD prevention are not considered FDA-approved indications for progesterone supplementation in pregnancy. However, vaginal progesterone is FDA approved for use in pregnant women with a history of infertility.
Continue to: Case 1 Continued...
Case 1 Continued
MC initiated treatment with daily vaginal progesterone at 18 weeks’ gestation and returned for ultrasound cervical length examination weekly instead of every other week. At 20 weeks’ gestation, cervical length was 2.0 cm; the following week it was 1.4 cm. What would you recommend at this point?
When to consider cerclage
If cervical shortening progresses to about 1.5 cm while a woman is being treated with vaginal progesterone, cerclage may be considered. The benefit of cerclage in patients with prior PTD and a short cervix was highlighted in a 2018 Cochrane Review.20 In this stepwise management approach to a short cervix, waiting for a cervix to be less than 1.5 cm may be unadvisable. Under conditions of a very short cervix that is frequently dilated with exposure of fetal membranes, ascending subclinical intra-amniotic infection may already be present, reducing the efficacy of any preventive measures. Preferential consideration for cerclage from the start over initial vaginal progesterone also may be appropriate when there is a history of 2 spontaneous PTDs or mid-trimester losses, a history of a successful cerclage, or with a very short cervix (< 1.0 cm) at the initial evaluation. As for the latter, a 2018 individual patient data meta-analysis of vaginal progesterone found no benefit when the cervix was less than 1.0 cm.13
Progesterone plus cerclage likely to add benefit
The results of an adjusted indirect comparison meta-analysis suggest that both interventions—vaginal progesterone and cerclage—are equally effective.21 Assuming that there is no clinically meaningful difference in benefit associated with these 2 treatments, the next logical question is whether combining the 2 therapies provides any added benefit; limited observational data seem to suggest that it does. In a retrospective cohort of 86 consecutive singleton pregnancies among women who underwent ultrasound-indicated cerclage, those who used vaginal progesterone after cerclage (n = 45) had a lower rate of PTD.22 Also, a small (66 cases) case-control study demonstrated the benefit of administration of vaginal progesterone as a rescue intervention in women with cerclage and progressive cervical shortening despite cerclage.23
Case 2 Woman experiences adverse effects from vaginal progesterone
MS is a 25-year-old G2P0101 who was started on vaginal progesterone as prophylaxis for recurrent PTD. She is now at 20 weeks’ gestation, with a stable remnant cervical length of 2.0 cm. She is reporting an increasing vaginal burning sensation and vaginal discharge caused by the nightly vaginal progesterone applications, to the point that she is unwilling to continue the treatment. She asks if any alternatives to vaginal progesterone are available to decrease her risk of PTD.
Continue to: Is oral progesterone an option?...
Is oral progesterone an option?
In the 1980s and 1990s, oral micronized progesterone was widely used in France at doses of 900 to 1,200 mg/d for women at risk for PTD. The practice was stopped when secondary hepatic effects, including cholestasis of pregnancy, were reported at a higher rate in treated women.24 A rise in the serum concentration of progesterone metabolites has been associated with impaired biliary excretion and subsequent accumulation of bile acids.25 In other reports, elevated serum transaminase activity was found in pregnant women treated with oral micronized progesterone, and withdrawal of treatment frequently has led to improvement in transaminase levels.26 The synthesis of endogenous progesterone during normal pregnancy is between 250 and 500 mg/d,26 and experts have expressed concern that exogenous progesterone supplementation may impose an additional load on the hepatic transport of sulfated metabolites. Unlike orally administered progesterone, progestins given by the vaginal route avoid the hepatic first-pass effect. For this reason, they may be associated with less hepatic dysfunction.
Although not recommended by professional guidelines, oral progesterone administration for the prevention of PTD has been used in the United States. A 2015 survey of Wisconsin prenatal care providers found that of those who prescribed any progesterone for PTD prevention, oral progesterone was prescribed by 13.1% of obstetricians, 24.4% of midwives, and 40.7% of family medicine practitioners.27
Some limited recent evidence from a meta-analysis of 3 trials investigating oral progesterone versus placebo suggests effectiveness in the prevention of recurrent PTD and reduction in perinatal morbidity and mortality.15 However, the number of cases included in the meta-analysis (386) was too small to support definitive clinical recommendations. Furthermore, questions have been raised in the literature about the reliability of the largest trial included in that meta-analysis.28
Case 3 Two previous spontaneous PTDs
A 29-year-old G3P0201 presents for her first prenatal appointment at 10 weeks’ gestation. With her first pregnancy she had a spontaneous PTD at 23 weeks, and the neonate did not survive. In her second pregnancy, she was treated with 17-OHPC from 16 weeks’ gestation. She had a spontaneous PTD at 29 weeks, and that child is developing normally by her report. She believes that 17-OHPC helped her in her last pregnancy and is anxious about the risk for still another PTD. Consistent with the concept of shared decision-making, you inform her of the results of the recent PROLONG trial and statements on the subject released by professional organizations such as ACOG and the Society for Maternal-Fetal Medicine (SMFM). What options does she have?
17-OHPC may be a possibility in very high-risk women
According to a SMFM statement released in the wake of the PROLONG trial publication, “. . . SMFM believes that it is reasonable for providers to use 17-OHPC in women with a profile more representative of the very high-risk population reported in the Meis trial”.29 Only a few women will have a recurrence risk of PTD over 50%, as was the background event rate in the Meis trial.30 Such a risk level may be suspected, as an example, in women with 2 or more prior early (before 34 weeks) PTDs without intervening term deliveries. Even in those cases, if treatment with 17-OHPC is decided upon, ultrasound cervical surveillance should be added as an additional safety measure. ●
Researchers have been studying the use of exogenous progestins for prevention of preterm delivery (PTD) for almost 60 years, but conflicting results contribute to an ongoing debate. Interpretation of the available data is particularly difficult because different forms and doses of progestins have been used in disparate study populations.
Based on available data, progesterone supplementation is not effective as a primary prevention strategy for PTD in the general low-risk obstetric population. PTD is a complex problem with varied and incompletely elucidated pathogenic pathways, making it unlikely that one interventional approach would be effective for all pregnant women. As a result, emerging indications for the use of progesterone are based on risk factors for PTD (ie, prior PTD and/or short cervix). However, this secondary prevention approach is a limiting factor in itself because 50% of women destined to have a PTD have no identifiable risk factors.1 In addition, researchers have found that progestins are ineffective at delaying delivery for women with multiple gestation, suggesting that a distinct underlying mechanism of early parturition is present in these women, and that this mechanism is unresponsive to progestins.2
The formulations used in the study of progestin supplementation for PTD prevention have been almost exclusively either the synthetic 17 alpha-hydroxyprogesterone caproate (17-OHPC) or natural progesterone administered orally or vaginally. In 2003, the American College of Obstetricians and Gynecologists (ACOG) supported the use of progesterone to reduce the rate of PTD,3 and in 2011, the US Food and Drug Administration (FDA) approved 17-OHPC for use as prophylaxis against recurrent PTD. As a result, in recent years, the perceived standard of care for a majority of practitioners in the United States had been that all women with a previous preterm birth should be offered 17-OHPC. It may be interesting to note that in other parts of the world, the same enthusiastic adoption did not occur. For example, in Australia and New Zealand in 2007, only 5% of practitioners were using progesterone for this indication.4 Further, 17-OHPC is not recommended by professional guidelines in the United Kingdom and has remained unavailable in Germany.
The publication in 2019 of the PROLONG trial called into question the use of 17-OHPC for the prevention of PTD.5 In the December 2019 issue of OBG Management (“Managing preterm birth in those at risk: Expert strategies”), I expressed the opinion that with only rare exceptions, 17-OHPC is no longer a viable option for recurrent PTD prevention.6 In light of these developments, what scientific evidence is relevant and applicable to the care of women at risk for PTD?
Continue to: Case 1 Previous spontaneous PTD at 31 weeks...
Case 1 Previous spontaneous PTD at 31 weeks
MC is an asymptomatic 32-year-old woman with a singleton pregnancy at 13 weeks’ gestation. You see her for a maternal-fetal medicine consultation because 2 years ago she had a spontaneous PTD at 31 weeks’ gestation. What management recommendations can you make to decrease her risk of recurrent PTD?
Cervical length measurement narrows in on risk
The indication “previous preterm birth” is largely meaningless because of the heterogeneity in preterm birth pathways (preterm birth as a syndrome7) and inadequate risk characterization. Among women who experience a spontaneous PTD, 70% to 80% do not deliver prematurely in subsequent pregnancies.8 To better characterize the risk of PTD recurrence, ultrasound assessment of cervical length should be used. Research has shown that among women with a prior spontaneous PTD who maintain a normal cervical length until 24 weeks’ gestation, more than 90% will deliver at 35 weeks or after without intervention.9 Such an approach not only identifies the subgroup of women at significantly increased risk of recurrence but also eliminates unnecessary interventions.
Cervical ultrasound surveillance should be initiated at 16 weeks’ gestation. A short cervix before 16 weeks is not associated with a statistically significant increase in risk for PTD.10 Shortening of the cervix begins approximately 10 weeks before delivery in any gestational age group.11 Therefore, ultrasound assessment of the cervix at 28 weeks and after is irrelevant. In addition, after 28 weeks, cervical length varies greatly leading to loss in the predictive power of the cervical measurement.12 Based on these considerations, cervical surveillance may be extended up to 26 weeks. Although cervical cerclage is not an option in the United States in cases in which a short cervix is detected between 24 and 26 weeks, vaginal progesterone supplementation may still be considered.
Case 1 Continued
MC was started on ultrasound cervical surveillance at 16 weeks’ gestation. Her cervical length was initially normal (> 2.5 cm), but at 18 weeks the measurement was 2.2 cm. What is your recommendation?
The value of vaginal progesterone
There appears to be increasing consensus on the value of vaginal progesterone for women with a midtrimester short cervix on sonography, with or without a history of PTD. An individual patient data meta-analysis demonstrated the benefits of vaginal progesterone.13 Although there was no evidence of an effect on PTD at less than 37 weeks, the rates of PTD at less than 36 weeks and spontaneous PTD at less than 34 weeks were significantly reduced (by 20% and 28%, respectively). Also, there was a significant reduction in the risk of respiratory distress syndrome (53%) and composite neonatal morbidity and mortality (41%), with no significant impact on infant development up to the second year of life.13
The lack of generalizable evidence of benefit on childhood outcomes, combined with considerable uncertainty about the exact role and mechanism of action of exogenous progestins, contribute to the ongoing debate. Vaginal progesterone dosage regimens have been based on extrapolations from experience with progesterone in nonpregnant women, and recent pharmacokinetic studies have revealed how precarious such extrapolations may be. As an example, in nonpregnant women, the bioavailability of oral and vaginal progesterone is similar.14 In pregnancy, however, while daily oral progesterone doubles a pregnant woman’s serum progesterone level,15 daily vaginal administration of progesterone results in only a modest rise in serum progesterone, with a coefficient of variation among individuals that is double that outside of pregnancy.16 It is, therefore, considered that vaginal progesterone in pregnancy may have a local action secondary to the uterine first-pass effect. The uterine first-pass effect for vaginal progesterone was described in nonpregnant women and is only assumed to occur in pregnancy as well. 17
After evaluating the data from the largest available study of vaginal progesterone,18 the FDA concluded in 2012 that the study did not meet the statistical significance generally expected to support the approval of a new product. However, according to a more comprehensive evidence review developed in 2019 by the National Guideline Alliance in the United Kingdom, women with a history of PTD and women with a short cervix derive an important benefit from the use of vaginal progesterone; thus, this intervention should be offered to them.19 At this time, a short cervix and PTD prevention are not considered FDA-approved indications for progesterone supplementation in pregnancy. However, vaginal progesterone is FDA approved for use in pregnant women with a history of infertility.
Continue to: Case 1 Continued...
Case 1 Continued
MC initiated treatment with daily vaginal progesterone at 18 weeks’ gestation and returned for ultrasound cervical length examination weekly instead of every other week. At 20 weeks’ gestation, cervical length was 2.0 cm; the following week it was 1.4 cm. What would you recommend at this point?
When to consider cerclage
If cervical shortening progresses to about 1.5 cm while a woman is being treated with vaginal progesterone, cerclage may be considered. The benefit of cerclage in patients with prior PTD and a short cervix was highlighted in a 2018 Cochrane Review.20 In this stepwise management approach to a short cervix, waiting for a cervix to be less than 1.5 cm may be unadvisable. Under conditions of a very short cervix that is frequently dilated with exposure of fetal membranes, ascending subclinical intra-amniotic infection may already be present, reducing the efficacy of any preventive measures. Preferential consideration for cerclage from the start over initial vaginal progesterone also may be appropriate when there is a history of 2 spontaneous PTDs or mid-trimester losses, a history of a successful cerclage, or with a very short cervix (< 1.0 cm) at the initial evaluation. As for the latter, a 2018 individual patient data meta-analysis of vaginal progesterone found no benefit when the cervix was less than 1.0 cm.13
Progesterone plus cerclage likely to add benefit
The results of an adjusted indirect comparison meta-analysis suggest that both interventions—vaginal progesterone and cerclage—are equally effective.21 Assuming that there is no clinically meaningful difference in benefit associated with these 2 treatments, the next logical question is whether combining the 2 therapies provides any added benefit; limited observational data seem to suggest that it does. In a retrospective cohort of 86 consecutive singleton pregnancies among women who underwent ultrasound-indicated cerclage, those who used vaginal progesterone after cerclage (n = 45) had a lower rate of PTD.22 Also, a small (66 cases) case-control study demonstrated the benefit of administration of vaginal progesterone as a rescue intervention in women with cerclage and progressive cervical shortening despite cerclage.23
Case 2 Woman experiences adverse effects from vaginal progesterone
MS is a 25-year-old G2P0101 who was started on vaginal progesterone as prophylaxis for recurrent PTD. She is now at 20 weeks’ gestation, with a stable remnant cervical length of 2.0 cm. She is reporting an increasing vaginal burning sensation and vaginal discharge caused by the nightly vaginal progesterone applications, to the point that she is unwilling to continue the treatment. She asks if any alternatives to vaginal progesterone are available to decrease her risk of PTD.
Continue to: Is oral progesterone an option?...
Is oral progesterone an option?
In the 1980s and 1990s, oral micronized progesterone was widely used in France at doses of 900 to 1,200 mg/d for women at risk for PTD. The practice was stopped when secondary hepatic effects, including cholestasis of pregnancy, were reported at a higher rate in treated women.24 A rise in the serum concentration of progesterone metabolites has been associated with impaired biliary excretion and subsequent accumulation of bile acids.25 In other reports, elevated serum transaminase activity was found in pregnant women treated with oral micronized progesterone, and withdrawal of treatment frequently has led to improvement in transaminase levels.26 The synthesis of endogenous progesterone during normal pregnancy is between 250 and 500 mg/d,26 and experts have expressed concern that exogenous progesterone supplementation may impose an additional load on the hepatic transport of sulfated metabolites. Unlike orally administered progesterone, progestins given by the vaginal route avoid the hepatic first-pass effect. For this reason, they may be associated with less hepatic dysfunction.
Although not recommended by professional guidelines, oral progesterone administration for the prevention of PTD has been used in the United States. A 2015 survey of Wisconsin prenatal care providers found that of those who prescribed any progesterone for PTD prevention, oral progesterone was prescribed by 13.1% of obstetricians, 24.4% of midwives, and 40.7% of family medicine practitioners.27
Some limited recent evidence from a meta-analysis of 3 trials investigating oral progesterone versus placebo suggests effectiveness in the prevention of recurrent PTD and reduction in perinatal morbidity and mortality.15 However, the number of cases included in the meta-analysis (386) was too small to support definitive clinical recommendations. Furthermore, questions have been raised in the literature about the reliability of the largest trial included in that meta-analysis.28
Case 3 Two previous spontaneous PTDs
A 29-year-old G3P0201 presents for her first prenatal appointment at 10 weeks’ gestation. With her first pregnancy she had a spontaneous PTD at 23 weeks, and the neonate did not survive. In her second pregnancy, she was treated with 17-OHPC from 16 weeks’ gestation. She had a spontaneous PTD at 29 weeks, and that child is developing normally by her report. She believes that 17-OHPC helped her in her last pregnancy and is anxious about the risk for still another PTD. Consistent with the concept of shared decision-making, you inform her of the results of the recent PROLONG trial and statements on the subject released by professional organizations such as ACOG and the Society for Maternal-Fetal Medicine (SMFM). What options does she have?
17-OHPC may be a possibility in very high-risk women
According to a SMFM statement released in the wake of the PROLONG trial publication, “. . . SMFM believes that it is reasonable for providers to use 17-OHPC in women with a profile more representative of the very high-risk population reported in the Meis trial”.29 Only a few women will have a recurrence risk of PTD over 50%, as was the background event rate in the Meis trial.30 Such a risk level may be suspected, as an example, in women with 2 or more prior early (before 34 weeks) PTDs without intervening term deliveries. Even in those cases, if treatment with 17-OHPC is decided upon, ultrasound cervical surveillance should be added as an additional safety measure. ●
- Iams JD, Goldenberg RL, Mercer BM, et al. The preterm prediction study: can low-risk women destined for spontaneous preterm birth be identified? Am J Obstet Gynecol. 2001;184:652-655.
- Murray SR, Stock SJ, Cowan S, et al. Spontaneous preterm birth prevention in multiple pregnancy. Obstet Gynecol. 2018;20:57-63.
- American College of Obstetricians and Gynecologists. ACOG committee opinion. Use of progesterone to reduce preterm birth. Obstet Gynecol. 2003;102:1115-1116.
- Dodd JM, Ashwood P, Flenady V, et al. A survey of clinician and patient attitudes towards the use of progesterone for women at risk of preterm birth. Aust N Z J Obstet Gynaecol. 2007;47:106-109.
- Blackwell SC, Gyamfi -Bannerman C, Biggio JR, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- Duff P, Vidaeff AC, Ross MG, Norwitz ER. Managing preterm birth in those at risk: expert strategies. OBG Manag. 2019;31:39-42.
- Romero R, Mazor M, Munoz H, et al. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414-429.
- Phillips C, Velji Z, Hanly C, et al. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402.
- Berghella V, Seibel-Seamon J. Contemporary use of cervical cerclage. Clin Obstet Gynecol. 2007;50:468-477.
- Naim A, Haberman S, Burgess T, et al. Changes in cervical length and the risk of preterm labor. Am J Obstet Gynecol. 2002;186:887-889.
- Zilianti M, Azuaga A, Calderon F, et al. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14:719-724.
- Goldenberg RL, Iams JD, Miodovnik M, et al. The preterm prediction study: risk factors in twin gestation. Am J Obstet Gynecol. 1996;175:1047-1053.
- Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161-180.
- Norman T, Morse C, Dennerstein L. Comparative bioavailability of orally and vaginally administered progesterone. Fertil Steril. 1991;56:1034-1039.
- Boelig RC, Della Corte L, Ashoush S, et al. Oral progesterone for the prevention of recurrent preterm birth: systematic review and metaanalysis. Am J Obstet Gynecol MFM. 2019;1:50-62.
- Boelig RC, Zuppa AF, Kraft WK, et al. Pharmacokinetics of vaginal progesterone in pregnancy. Am J Obstet Gynecol. 2019;221:263.e1-7.
- Bulletti C, de Ziegler D, Flamigni C, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997;12:1073-1079.
- Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebocontrolled trial. Ultrasound Obstet Gynecol. 2011;38:18-31.
- Preterm labour and birth. Evidence review for clinical effectiveness of prophylactic progesterone in preventing preterm labour. London: National Institute for Health and Care Excellence (UK); August 2019.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6:CD008991.
- Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. 2018;219:10-25.
- Park JY, Jung YM, Kook S-Y, et al. The effect of postoperative vaginal progesterone in ultrasound-indicated cerclage to prevent preterm birth. J Matern Fetal Neonatal Med. 2019:1-8.
- Roman AR, Da Silva Costa F, et al. Rescue adjuvant vaginal progesterone may improve outcomes in cervical cerclage failure. Geburt Frauen. 2018;78:785-790.
- Benifle JL, Dumont M, Levardon M, et al. Effects of natural micronized progesterone on the liver in the third trimester of pregnancy. Contracept Fertil Sex. 1997;25:165-169.
- Vallejo M, Briz O, Serrano MA, et al. Potential role of transinhibition of the bile salt export pump by progesterone metabolites in the etiopathogenesis of intrahepatic cholestasis of pregnancy. J Hepatol. 2006;44:1150-1157.
- Bacq Y, Sapey T, Bréchot MC, et al. Intrahepatic cholestasis of pregnancy: a French prospective study. Hepatology. 1997;26:358-364.
- Hoppe K, Kramer RD, Ha B, et al. Progesterone supplementation for the prevention of preterm birth: provider practice in Wisconsin. WMJ. 2019;118:126-131.
- Katsanevakis E, Mol BW, Thornton J. A question about the reliability of a recent trial of progesterone for preterm birth prevention, published in Acta. Acta Obstet Gynecol Scand. 2020;99:426.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280smfm-statement-use-of-17-alpha-hydroxyprogesteronecaproate-for-prevention-of-recurrent-preterm-birth. Accessed March 23, 2020.
- Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;384:2379-2385.
- Iams JD, Goldenberg RL, Mercer BM, et al. The preterm prediction study: can low-risk women destined for spontaneous preterm birth be identified? Am J Obstet Gynecol. 2001;184:652-655.
- Murray SR, Stock SJ, Cowan S, et al. Spontaneous preterm birth prevention in multiple pregnancy. Obstet Gynecol. 2018;20:57-63.
- American College of Obstetricians and Gynecologists. ACOG committee opinion. Use of progesterone to reduce preterm birth. Obstet Gynecol. 2003;102:1115-1116.
- Dodd JM, Ashwood P, Flenady V, et al. A survey of clinician and patient attitudes towards the use of progesterone for women at risk of preterm birth. Aust N Z J Obstet Gynaecol. 2007;47:106-109.
- Blackwell SC, Gyamfi -Bannerman C, Biggio JR, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- Duff P, Vidaeff AC, Ross MG, Norwitz ER. Managing preterm birth in those at risk: expert strategies. OBG Manag. 2019;31:39-42.
- Romero R, Mazor M, Munoz H, et al. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414-429.
- Phillips C, Velji Z, Hanly C, et al. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402.
- Berghella V, Seibel-Seamon J. Contemporary use of cervical cerclage. Clin Obstet Gynecol. 2007;50:468-477.
- Naim A, Haberman S, Burgess T, et al. Changes in cervical length and the risk of preterm labor. Am J Obstet Gynecol. 2002;186:887-889.
- Zilianti M, Azuaga A, Calderon F, et al. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14:719-724.
- Goldenberg RL, Iams JD, Miodovnik M, et al. The preterm prediction study: risk factors in twin gestation. Am J Obstet Gynecol. 1996;175:1047-1053.
- Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161-180.
- Norman T, Morse C, Dennerstein L. Comparative bioavailability of orally and vaginally administered progesterone. Fertil Steril. 1991;56:1034-1039.
- Boelig RC, Della Corte L, Ashoush S, et al. Oral progesterone for the prevention of recurrent preterm birth: systematic review and metaanalysis. Am J Obstet Gynecol MFM. 2019;1:50-62.
- Boelig RC, Zuppa AF, Kraft WK, et al. Pharmacokinetics of vaginal progesterone in pregnancy. Am J Obstet Gynecol. 2019;221:263.e1-7.
- Bulletti C, de Ziegler D, Flamigni C, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997;12:1073-1079.
- Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebocontrolled trial. Ultrasound Obstet Gynecol. 2011;38:18-31.
- Preterm labour and birth. Evidence review for clinical effectiveness of prophylactic progesterone in preventing preterm labour. London: National Institute for Health and Care Excellence (UK); August 2019.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6:CD008991.
- Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. 2018;219:10-25.
- Park JY, Jung YM, Kook S-Y, et al. The effect of postoperative vaginal progesterone in ultrasound-indicated cerclage to prevent preterm birth. J Matern Fetal Neonatal Med. 2019:1-8.
- Roman AR, Da Silva Costa F, et al. Rescue adjuvant vaginal progesterone may improve outcomes in cervical cerclage failure. Geburt Frauen. 2018;78:785-790.
- Benifle JL, Dumont M, Levardon M, et al. Effects of natural micronized progesterone on the liver in the third trimester of pregnancy. Contracept Fertil Sex. 1997;25:165-169.
- Vallejo M, Briz O, Serrano MA, et al. Potential role of transinhibition of the bile salt export pump by progesterone metabolites in the etiopathogenesis of intrahepatic cholestasis of pregnancy. J Hepatol. 2006;44:1150-1157.
- Bacq Y, Sapey T, Bréchot MC, et al. Intrahepatic cholestasis of pregnancy: a French prospective study. Hepatology. 1997;26:358-364.
- Hoppe K, Kramer RD, Ha B, et al. Progesterone supplementation for the prevention of preterm birth: provider practice in Wisconsin. WMJ. 2019;118:126-131.
- Katsanevakis E, Mol BW, Thornton J. A question about the reliability of a recent trial of progesterone for preterm birth prevention, published in Acta. Acta Obstet Gynecol Scand. 2020;99:426.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280smfm-statement-use-of-17-alpha-hydroxyprogesteronecaproate-for-prevention-of-recurrent-preterm-birth. Accessed March 23, 2020.
- Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;384:2379-2385.
COVID-19 during pregnancy: How would you proceed in this case of a novel and ominous emerging pathogen?
CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).
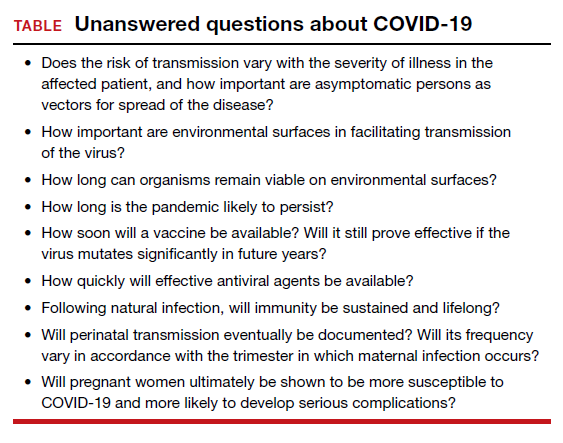
Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).

Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).

Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
The Clinical Conundrum in Managing Preterm Birth: Balancing Historical Trial Results, Society Guidelines, and Clinical Experience with a Contradictory Trial Outcome
This CME supplement details results of the PROLONG (Progestin’s Role in Optimizing Neonatal Gestation) trial evaluating 17-OHPC in patients with a history of a prior spontaneous singleton preterm delivery.
To receive CME credit, please
read the article and go to www.omniaeducation.com/ptb
to access the posttest and evaluation.
This CME supplement details results of the PROLONG (Progestin’s Role in Optimizing Neonatal Gestation) trial evaluating 17-OHPC in patients with a history of a prior spontaneous singleton preterm delivery.
To receive CME credit, please
read the article and go to www.omniaeducation.com/ptb
to access the posttest and evaluation.
This CME supplement details results of the PROLONG (Progestin’s Role in Optimizing Neonatal Gestation) trial evaluating 17-OHPC in patients with a history of a prior spontaneous singleton preterm delivery.
To receive CME credit, please
read the article and go to www.omniaeducation.com/ptb
to access the posttest and evaluation.