User login
VIDEO: Secukinumab ‘exciting’ new agent for psoriasis
KAUAI, HAWAII – The Food and Drug administration recently approved a new class of drug for psoriasis. Secukinumab, indicated for the treatment of mild to severe psoriasis, targets IL-17, a cytokine highly implicated in the disease.
“I’m very excited to try this drug in my patients,” Dr. Kristina Callis Duffin said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
In this report, Dr. Duffin of the University of Utah explains the drug’s mechanism of action, describes how it differs from other biologic agents used to treat psoriasis, and shares promising data showing improved scores on the Psoriasis Area and Severity Index (PASI).
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
KAUAI, HAWAII – The Food and Drug administration recently approved a new class of drug for psoriasis. Secukinumab, indicated for the treatment of mild to severe psoriasis, targets IL-17, a cytokine highly implicated in the disease.
“I’m very excited to try this drug in my patients,” Dr. Kristina Callis Duffin said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
In this report, Dr. Duffin of the University of Utah explains the drug’s mechanism of action, describes how it differs from other biologic agents used to treat psoriasis, and shares promising data showing improved scores on the Psoriasis Area and Severity Index (PASI).
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
KAUAI, HAWAII – The Food and Drug administration recently approved a new class of drug for psoriasis. Secukinumab, indicated for the treatment of mild to severe psoriasis, targets IL-17, a cytokine highly implicated in the disease.
“I’m very excited to try this drug in my patients,” Dr. Kristina Callis Duffin said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
In this report, Dr. Duffin of the University of Utah explains the drug’s mechanism of action, describes how it differs from other biologic agents used to treat psoriasis, and shares promising data showing improved scores on the Psoriasis Area and Severity Index (PASI).
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
AT SDEF HAWAII DERMATOLOGY SEMINAR
Infliximab most common cause of drug-induced liver injury
Infliximab caused liver injury in 8.3% of treated patients in a prospective study, exceeding rates for other tumor necrosis factor-alpha antagonists, investigators reported online in Clinical Gastroenterology and Hepatology.
The findings show that “liver injury associated with the use of TNF-alpha antagonists is more common than previously reported, occurring in 1 in 120 of those exposed to infliximab,” said Dr. Einar S. Björnsson at the University of Iceland in Reykjavik and his associates. Furthermore, neither anti-TNF treatment dose nor baseline antinuclear acid antibody (ANA) status predicted which patients would develop drug-induced liver injury (DILI), the researchers said.
Since emerging in the 1990s, anti-TNF agents have dramatically altered the treatment landscape for autoimmune diseases such as rheumatoid arthritis, psoriasis, and inflammatory bowel disease. Although they are known to cause liver damage in some patients, data on the topic mainly come from single case reports, the researchers said (Clin. Gastroenterol. Hepatol. 2014 [doi: http://dx.doi.org/10.1016/j.cgh.2014.07.062]) To better understand the association, the researchers prospectively studied patients who received anti-TNF agents between 2009 and 2013 at the University Hospital in Iceland. They defined liver injury as aspartate aminotransferase or alanine aminotransferase (ALT) levels that were at least triple the normal upper limit, or alkaline phosphatase levels of at least double the upper limit.
A total of 1,776 patients were treated with anti-TNF agents during the 5-year study period, the researchers reported. In all, 11 developed drug-induced liver injury (DILI), of which nine cases were caused by infliximab, they said. Liver injury developed in 8.3% of patients treated with infliximab, compared with only 3.7% of those who received adalimumab and 2.3% of those given etanercept, they added. In a past analysis, the researchers calculated that one in every 148 patients would develop DILI during 2 years of treatment with infliximab (Gastroenterology 2014;144:1419-25). Patients who developed DILI on one anti-TNF agent were able to switch therapies without DILI recurring, the investigators said. Seven patients were switched from infliximab to adalimumab, etanercept, or both, and one was switched to infliximab after developing DILI on adalimumab, they added.
The researchers also compared the 11 cases to 22 randomized controls matched by age, sex, underlying condition, and treatment. Notably, among the 11 patients diagnosed with DILI, just 1 (9%) was receiving methotrexate at the time of diagnosis, compared with 59% of the controls (P = .009), they reported. “The reason for this is not clear,” they added. “Methotrexate has been shown to lead to a decrease in circulating autoantibodies in cutaneous lupus erythematosus, but the influence of methotrexate could not be confirmed during infliximab treatment.”
Five of the 11 patients with DILI had liver biopsies, of which three showed severe acute hepatitis, two indicated mild unspecified chronic hepatitis, and one showed pure canalicular cholestasis, the researchers reported. About half the patients needed steroids acutely, but “the vast majority” did not need long-term steroid treatment, they said. Exactly how anti-TNF agents cause liver injury remains unclear, they added. Future studies might evaluate whether these drugs trigger CD4 T cells to react against liver cells, as is the case in classic autoimmune hepatitis, they said.
The researchers reported no funding sources and declared having no conflicts of interest.
Infliximab caused liver injury in 8.3% of treated patients in a prospective study, exceeding rates for other tumor necrosis factor-alpha antagonists, investigators reported online in Clinical Gastroenterology and Hepatology.
The findings show that “liver injury associated with the use of TNF-alpha antagonists is more common than previously reported, occurring in 1 in 120 of those exposed to infliximab,” said Dr. Einar S. Björnsson at the University of Iceland in Reykjavik and his associates. Furthermore, neither anti-TNF treatment dose nor baseline antinuclear acid antibody (ANA) status predicted which patients would develop drug-induced liver injury (DILI), the researchers said.
Since emerging in the 1990s, anti-TNF agents have dramatically altered the treatment landscape for autoimmune diseases such as rheumatoid arthritis, psoriasis, and inflammatory bowel disease. Although they are known to cause liver damage in some patients, data on the topic mainly come from single case reports, the researchers said (Clin. Gastroenterol. Hepatol. 2014 [doi: http://dx.doi.org/10.1016/j.cgh.2014.07.062]) To better understand the association, the researchers prospectively studied patients who received anti-TNF agents between 2009 and 2013 at the University Hospital in Iceland. They defined liver injury as aspartate aminotransferase or alanine aminotransferase (ALT) levels that were at least triple the normal upper limit, or alkaline phosphatase levels of at least double the upper limit.
A total of 1,776 patients were treated with anti-TNF agents during the 5-year study period, the researchers reported. In all, 11 developed drug-induced liver injury (DILI), of which nine cases were caused by infliximab, they said. Liver injury developed in 8.3% of patients treated with infliximab, compared with only 3.7% of those who received adalimumab and 2.3% of those given etanercept, they added. In a past analysis, the researchers calculated that one in every 148 patients would develop DILI during 2 years of treatment with infliximab (Gastroenterology 2014;144:1419-25). Patients who developed DILI on one anti-TNF agent were able to switch therapies without DILI recurring, the investigators said. Seven patients were switched from infliximab to adalimumab, etanercept, or both, and one was switched to infliximab after developing DILI on adalimumab, they added.
The researchers also compared the 11 cases to 22 randomized controls matched by age, sex, underlying condition, and treatment. Notably, among the 11 patients diagnosed with DILI, just 1 (9%) was receiving methotrexate at the time of diagnosis, compared with 59% of the controls (P = .009), they reported. “The reason for this is not clear,” they added. “Methotrexate has been shown to lead to a decrease in circulating autoantibodies in cutaneous lupus erythematosus, but the influence of methotrexate could not be confirmed during infliximab treatment.”
Five of the 11 patients with DILI had liver biopsies, of which three showed severe acute hepatitis, two indicated mild unspecified chronic hepatitis, and one showed pure canalicular cholestasis, the researchers reported. About half the patients needed steroids acutely, but “the vast majority” did not need long-term steroid treatment, they said. Exactly how anti-TNF agents cause liver injury remains unclear, they added. Future studies might evaluate whether these drugs trigger CD4 T cells to react against liver cells, as is the case in classic autoimmune hepatitis, they said.
The researchers reported no funding sources and declared having no conflicts of interest.
Infliximab caused liver injury in 8.3% of treated patients in a prospective study, exceeding rates for other tumor necrosis factor-alpha antagonists, investigators reported online in Clinical Gastroenterology and Hepatology.
The findings show that “liver injury associated with the use of TNF-alpha antagonists is more common than previously reported, occurring in 1 in 120 of those exposed to infliximab,” said Dr. Einar S. Björnsson at the University of Iceland in Reykjavik and his associates. Furthermore, neither anti-TNF treatment dose nor baseline antinuclear acid antibody (ANA) status predicted which patients would develop drug-induced liver injury (DILI), the researchers said.
Since emerging in the 1990s, anti-TNF agents have dramatically altered the treatment landscape for autoimmune diseases such as rheumatoid arthritis, psoriasis, and inflammatory bowel disease. Although they are known to cause liver damage in some patients, data on the topic mainly come from single case reports, the researchers said (Clin. Gastroenterol. Hepatol. 2014 [doi: http://dx.doi.org/10.1016/j.cgh.2014.07.062]) To better understand the association, the researchers prospectively studied patients who received anti-TNF agents between 2009 and 2013 at the University Hospital in Iceland. They defined liver injury as aspartate aminotransferase or alanine aminotransferase (ALT) levels that were at least triple the normal upper limit, or alkaline phosphatase levels of at least double the upper limit.
A total of 1,776 patients were treated with anti-TNF agents during the 5-year study period, the researchers reported. In all, 11 developed drug-induced liver injury (DILI), of which nine cases were caused by infliximab, they said. Liver injury developed in 8.3% of patients treated with infliximab, compared with only 3.7% of those who received adalimumab and 2.3% of those given etanercept, they added. In a past analysis, the researchers calculated that one in every 148 patients would develop DILI during 2 years of treatment with infliximab (Gastroenterology 2014;144:1419-25). Patients who developed DILI on one anti-TNF agent were able to switch therapies without DILI recurring, the investigators said. Seven patients were switched from infliximab to adalimumab, etanercept, or both, and one was switched to infliximab after developing DILI on adalimumab, they added.
The researchers also compared the 11 cases to 22 randomized controls matched by age, sex, underlying condition, and treatment. Notably, among the 11 patients diagnosed with DILI, just 1 (9%) was receiving methotrexate at the time of diagnosis, compared with 59% of the controls (P = .009), they reported. “The reason for this is not clear,” they added. “Methotrexate has been shown to lead to a decrease in circulating autoantibodies in cutaneous lupus erythematosus, but the influence of methotrexate could not be confirmed during infliximab treatment.”
Five of the 11 patients with DILI had liver biopsies, of which three showed severe acute hepatitis, two indicated mild unspecified chronic hepatitis, and one showed pure canalicular cholestasis, the researchers reported. About half the patients needed steroids acutely, but “the vast majority” did not need long-term steroid treatment, they said. Exactly how anti-TNF agents cause liver injury remains unclear, they added. Future studies might evaluate whether these drugs trigger CD4 T cells to react against liver cells, as is the case in classic autoimmune hepatitis, they said.
The researchers reported no funding sources and declared having no conflicts of interest.
Key clinical point: Infliximab was the most common anti–tumor necrosis factor-alpha agent linked to liver injury.
Major finding: Rates of drug-induced liver injury were highest among patients treated with infliximab (8.3%), compared with 3.7% for adalimumab and 2.3% for etanercept.
Data source: Prospective study of 11 cases of drug-induced liver injury and 22 controls.
Disclosures: The researchers declared no funding sources or conflicts of interest.
New psoriasis drugs offer treatment advantages
MIAMI BEACH – Two of the newest treatments available for psoriasis – apremilast and secukinumab – are true “game-changers,” according to Dr. David M. Pariser.
Apremilast (Otezla), a recently approved oral phosphodiesterase 4 inhibitor, will be a particularly attractive treatment option for many dermatologists and patients, he said at the South Beach Symposium.
Apremilast has a very limited effect on the immune system, and it’s an oral therapy and thus requires no needles. It is very safe – with “strikingly few” serious adverse events – and no laboratory monitoring is required, he explained.
Efficacy results with apremilast are modest. In the phase III ESTEEM trial, for example, 33% of patients achieved at least 75% improvement (PASI-75), compared with 5% of patients who received placebo, said Dr. Pariser of Eastern Virginia Medical School, Norfolk, and an investigator for the trial.
Further, the drug can be used for almost any patient and type of psoriasis; it is an option for those who want systemic therapy, but who don’t want to go on a biologic or methotrexate, and its use is not precluded by a history of cancer or infections, as is the case with biologics, he added.
Apremilast also will be attractive for dermatologists who do not currently prescribe systemic therapy for psoriasis, or who don’t use aggressive systemic therapy for psoriasis, he said.
“If a prescriber feels safety is more important than efficacy, this might be a good choice,” he said.
Secukinumab (Cosentyx), on the other hand, is a “big gun,” Dr. Pariser said of the biologic, which was approved in January 2015 for the treatment of adults with moderate to severe plaque psoriasis.
“It’s the biggest gun we’ve got now … and it really has a safety profile similar to existing biologics so far,” he said.
The fully human monoclonal antibody inhibits interleukin-17A and is administered by subcutaneous injection. Its safety and efficacy were demonstrated in numerous of studies involving about 4,500 patients. Treatment was associated with significant improvement, compared with placebo, said Dr. Pariser, who also was an investigator on secukinumab trials.
Of note, while the PASI-75 findings for secukinumab are “a nice number but not dramatically higher than things we have had in the past,” the PASI-90 and PASI-100 scores are remarkable, he said.
At the highest dose studied (300 mg given at weeks 1, 2, 3, 4, and 8, and monthly thereafter), PASI-90 was achieved by 59% of patients in one study, and PASI-100 was achieved in 28%.
“That’s significant. We haven’t had that before, and that is really, really, really nice,” he said.
Further, the primary efficacy endpoint of the study was outcome at 12 weeks, but patients continued to improve at least until 16 weeks.
PASI-100 – no psoriasis whatsoever – was 40% at 16 weeks, he said.
In another phase III trial, secukinumab was again shown to be superior to placebo, but it also compared favorably with etanercept, he noted.
Safety was reasonable in the secukinumab trials. No deaths occurred, but there were more serious adverse events and discontinuations in the active treatment group. Nasopharyngitis was the most common serious adverse events, and it occurred in all groups. Upper respiratory tract infections appeared to be more common in the secukinumab patients, he noted.
An important consideration with secukinumab, however, is the need for continuous treatment, he said.
“The bottom line, really, is that patients should stay on it. They will lose effectiveness if they go off of it or take it on an as-needed basis,” he said.
Dr. Pariser is a consultant and/or researcher for Amgen, AbbVie, Celgene, Eli Lilly, Janssen Pharmaceuticals, Merck, and Pfizer.
MIAMI BEACH – Two of the newest treatments available for psoriasis – apremilast and secukinumab – are true “game-changers,” according to Dr. David M. Pariser.
Apremilast (Otezla), a recently approved oral phosphodiesterase 4 inhibitor, will be a particularly attractive treatment option for many dermatologists and patients, he said at the South Beach Symposium.
Apremilast has a very limited effect on the immune system, and it’s an oral therapy and thus requires no needles. It is very safe – with “strikingly few” serious adverse events – and no laboratory monitoring is required, he explained.
Efficacy results with apremilast are modest. In the phase III ESTEEM trial, for example, 33% of patients achieved at least 75% improvement (PASI-75), compared with 5% of patients who received placebo, said Dr. Pariser of Eastern Virginia Medical School, Norfolk, and an investigator for the trial.
Further, the drug can be used for almost any patient and type of psoriasis; it is an option for those who want systemic therapy, but who don’t want to go on a biologic or methotrexate, and its use is not precluded by a history of cancer or infections, as is the case with biologics, he added.
Apremilast also will be attractive for dermatologists who do not currently prescribe systemic therapy for psoriasis, or who don’t use aggressive systemic therapy for psoriasis, he said.
“If a prescriber feels safety is more important than efficacy, this might be a good choice,” he said.
Secukinumab (Cosentyx), on the other hand, is a “big gun,” Dr. Pariser said of the biologic, which was approved in January 2015 for the treatment of adults with moderate to severe plaque psoriasis.
“It’s the biggest gun we’ve got now … and it really has a safety profile similar to existing biologics so far,” he said.
The fully human monoclonal antibody inhibits interleukin-17A and is administered by subcutaneous injection. Its safety and efficacy were demonstrated in numerous of studies involving about 4,500 patients. Treatment was associated with significant improvement, compared with placebo, said Dr. Pariser, who also was an investigator on secukinumab trials.
Of note, while the PASI-75 findings for secukinumab are “a nice number but not dramatically higher than things we have had in the past,” the PASI-90 and PASI-100 scores are remarkable, he said.
At the highest dose studied (300 mg given at weeks 1, 2, 3, 4, and 8, and monthly thereafter), PASI-90 was achieved by 59% of patients in one study, and PASI-100 was achieved in 28%.
“That’s significant. We haven’t had that before, and that is really, really, really nice,” he said.
Further, the primary efficacy endpoint of the study was outcome at 12 weeks, but patients continued to improve at least until 16 weeks.
PASI-100 – no psoriasis whatsoever – was 40% at 16 weeks, he said.
In another phase III trial, secukinumab was again shown to be superior to placebo, but it also compared favorably with etanercept, he noted.
Safety was reasonable in the secukinumab trials. No deaths occurred, but there were more serious adverse events and discontinuations in the active treatment group. Nasopharyngitis was the most common serious adverse events, and it occurred in all groups. Upper respiratory tract infections appeared to be more common in the secukinumab patients, he noted.
An important consideration with secukinumab, however, is the need for continuous treatment, he said.
“The bottom line, really, is that patients should stay on it. They will lose effectiveness if they go off of it or take it on an as-needed basis,” he said.
Dr. Pariser is a consultant and/or researcher for Amgen, AbbVie, Celgene, Eli Lilly, Janssen Pharmaceuticals, Merck, and Pfizer.
MIAMI BEACH – Two of the newest treatments available for psoriasis – apremilast and secukinumab – are true “game-changers,” according to Dr. David M. Pariser.
Apremilast (Otezla), a recently approved oral phosphodiesterase 4 inhibitor, will be a particularly attractive treatment option for many dermatologists and patients, he said at the South Beach Symposium.
Apremilast has a very limited effect on the immune system, and it’s an oral therapy and thus requires no needles. It is very safe – with “strikingly few” serious adverse events – and no laboratory monitoring is required, he explained.
Efficacy results with apremilast are modest. In the phase III ESTEEM trial, for example, 33% of patients achieved at least 75% improvement (PASI-75), compared with 5% of patients who received placebo, said Dr. Pariser of Eastern Virginia Medical School, Norfolk, and an investigator for the trial.
Further, the drug can be used for almost any patient and type of psoriasis; it is an option for those who want systemic therapy, but who don’t want to go on a biologic or methotrexate, and its use is not precluded by a history of cancer or infections, as is the case with biologics, he added.
Apremilast also will be attractive for dermatologists who do not currently prescribe systemic therapy for psoriasis, or who don’t use aggressive systemic therapy for psoriasis, he said.
“If a prescriber feels safety is more important than efficacy, this might be a good choice,” he said.
Secukinumab (Cosentyx), on the other hand, is a “big gun,” Dr. Pariser said of the biologic, which was approved in January 2015 for the treatment of adults with moderate to severe plaque psoriasis.
“It’s the biggest gun we’ve got now … and it really has a safety profile similar to existing biologics so far,” he said.
The fully human monoclonal antibody inhibits interleukin-17A and is administered by subcutaneous injection. Its safety and efficacy were demonstrated in numerous of studies involving about 4,500 patients. Treatment was associated with significant improvement, compared with placebo, said Dr. Pariser, who also was an investigator on secukinumab trials.
Of note, while the PASI-75 findings for secukinumab are “a nice number but not dramatically higher than things we have had in the past,” the PASI-90 and PASI-100 scores are remarkable, he said.
At the highest dose studied (300 mg given at weeks 1, 2, 3, 4, and 8, and monthly thereafter), PASI-90 was achieved by 59% of patients in one study, and PASI-100 was achieved in 28%.
“That’s significant. We haven’t had that before, and that is really, really, really nice,” he said.
Further, the primary efficacy endpoint of the study was outcome at 12 weeks, but patients continued to improve at least until 16 weeks.
PASI-100 – no psoriasis whatsoever – was 40% at 16 weeks, he said.
In another phase III trial, secukinumab was again shown to be superior to placebo, but it also compared favorably with etanercept, he noted.
Safety was reasonable in the secukinumab trials. No deaths occurred, but there were more serious adverse events and discontinuations in the active treatment group. Nasopharyngitis was the most common serious adverse events, and it occurred in all groups. Upper respiratory tract infections appeared to be more common in the secukinumab patients, he noted.
An important consideration with secukinumab, however, is the need for continuous treatment, he said.
“The bottom line, really, is that patients should stay on it. They will lose effectiveness if they go off of it or take it on an as-needed basis,” he said.
Dr. Pariser is a consultant and/or researcher for Amgen, AbbVie, Celgene, Eli Lilly, Janssen Pharmaceuticals, Merck, and Pfizer.
EXPERT ANALYSIS FROM THE ANNUAL SOUTH BEACH SYMPOSIUM
Data suggest link between tonsillectomy, psoriasis improvement
MIAMI BEACH – If all else fails in treating psoriasis, a tonsillectomy may do the trick – at least in patients whose psoriasis is associated with recurrent tonsillitis, according to findings from a systematic review of available data.
“Maybe taking the tonsils out removes resident bacteria like strep whose antigens tend to promote psoriasis,” Dr. Theodore Rosen, professor of dermatology at Baylor College of Medicine, Houston, suggested during a presentation at the South Beach Symposium.
The relationship between streptococcal pharyngitis and guttate psoriasis is well known, he explained during the talk on the latest findings in clinical dermatology.
Indeed, the authors of the systematic review noted that streptococcal infection is associated with psoriasis onset in some patients, but said it was unknown whether tonsillectomy decreases psoriasis symptoms in those patients.
Dr. Tara D. Rachakonda of Salt Lake City and her colleagues included studies dating back to 1960, and identified 20 relevant studies – including 5 controlled studies – involving 545 patients with psoriasis who were evaluated for or underwent tonsillectomy. The overall improvement rate across the studies was about 70% in 410 patients who underwent tonsillectomy, and in one of the studies the response rate was 86%.
In some cases, the response was sustained, but some patients experienced relapse, they reported (JAAD 2015;72:261-75).
The authors concluded that tonsillectomy may be a potential treatment option in patients with recalcitrant psoriasis associated with episodes of tonsillitis, but noted that additional study with long-term follow-up is needed to examine both the extent and persistence of benefit of tonsillectomy in psoriasis patients.
“The evidence is not sufficient to recommend tonsillectomy in all of your psoriasis patients,” Dr. Rosen said, but he noted that it may be something worth considering in patients who still have their tonsils and who are not responding to psoriasis treatments.
Dr. Rosen reported having no relevant financial disclosures.
MIAMI BEACH – If all else fails in treating psoriasis, a tonsillectomy may do the trick – at least in patients whose psoriasis is associated with recurrent tonsillitis, according to findings from a systematic review of available data.
“Maybe taking the tonsils out removes resident bacteria like strep whose antigens tend to promote psoriasis,” Dr. Theodore Rosen, professor of dermatology at Baylor College of Medicine, Houston, suggested during a presentation at the South Beach Symposium.
The relationship between streptococcal pharyngitis and guttate psoriasis is well known, he explained during the talk on the latest findings in clinical dermatology.
Indeed, the authors of the systematic review noted that streptococcal infection is associated with psoriasis onset in some patients, but said it was unknown whether tonsillectomy decreases psoriasis symptoms in those patients.
Dr. Tara D. Rachakonda of Salt Lake City and her colleagues included studies dating back to 1960, and identified 20 relevant studies – including 5 controlled studies – involving 545 patients with psoriasis who were evaluated for or underwent tonsillectomy. The overall improvement rate across the studies was about 70% in 410 patients who underwent tonsillectomy, and in one of the studies the response rate was 86%.
In some cases, the response was sustained, but some patients experienced relapse, they reported (JAAD 2015;72:261-75).
The authors concluded that tonsillectomy may be a potential treatment option in patients with recalcitrant psoriasis associated with episodes of tonsillitis, but noted that additional study with long-term follow-up is needed to examine both the extent and persistence of benefit of tonsillectomy in psoriasis patients.
“The evidence is not sufficient to recommend tonsillectomy in all of your psoriasis patients,” Dr. Rosen said, but he noted that it may be something worth considering in patients who still have their tonsils and who are not responding to psoriasis treatments.
Dr. Rosen reported having no relevant financial disclosures.
MIAMI BEACH – If all else fails in treating psoriasis, a tonsillectomy may do the trick – at least in patients whose psoriasis is associated with recurrent tonsillitis, according to findings from a systematic review of available data.
“Maybe taking the tonsils out removes resident bacteria like strep whose antigens tend to promote psoriasis,” Dr. Theodore Rosen, professor of dermatology at Baylor College of Medicine, Houston, suggested during a presentation at the South Beach Symposium.
The relationship between streptococcal pharyngitis and guttate psoriasis is well known, he explained during the talk on the latest findings in clinical dermatology.
Indeed, the authors of the systematic review noted that streptococcal infection is associated with psoriasis onset in some patients, but said it was unknown whether tonsillectomy decreases psoriasis symptoms in those patients.
Dr. Tara D. Rachakonda of Salt Lake City and her colleagues included studies dating back to 1960, and identified 20 relevant studies – including 5 controlled studies – involving 545 patients with psoriasis who were evaluated for or underwent tonsillectomy. The overall improvement rate across the studies was about 70% in 410 patients who underwent tonsillectomy, and in one of the studies the response rate was 86%.
In some cases, the response was sustained, but some patients experienced relapse, they reported (JAAD 2015;72:261-75).
The authors concluded that tonsillectomy may be a potential treatment option in patients with recalcitrant psoriasis associated with episodes of tonsillitis, but noted that additional study with long-term follow-up is needed to examine both the extent and persistence of benefit of tonsillectomy in psoriasis patients.
“The evidence is not sufficient to recommend tonsillectomy in all of your psoriasis patients,” Dr. Rosen said, but he noted that it may be something worth considering in patients who still have their tonsils and who are not responding to psoriasis treatments.
Dr. Rosen reported having no relevant financial disclosures.
Ixekizumab improves lesions in patients with chronic plaque psoriasis after 20 weeks
Patients with chronic plaque psoriasis who were given ixekizumab improved the severity of scalp and nail lesions in a phase 2 trial, according to Dr. Richard G. Langley of Dalhousie University in Halifax, N.S., and his associates.
In a 20-week randomized, placebo-controlled trial, 142 patients with moderate to severe plaque psoriasis at baseline were injected subcutaneously with ixekizumab at weeks 0, 2, 4, 8, 12, and 16. Ixekizumab, a monoclonal antibody, specifically targets IL-17A, a cytokine involved in the development of psoriasis.
Patients with scalp psoriasis were split into groups and given 10-, 25-, 75- and 150-mg doses of ixekizumab, or placebo; patients with nail psoriasis received 75- and 150-mg doses of ixekizumab. After 20 weeks, patients scalp psoriasis patients in the 25-, 75-, and 150-mg groups and nail psoriasis patients in the 75- and 150-mg groups showed significant improvement from baseline. By week 48, 78% of patients with scalp psoriasis and 51% of patients with nail psoriasis experienced complete resolution of lesions, the investigators reported.
Read the full article in the Journal of the European Academy of Dermatology and Venereology.
Patients with chronic plaque psoriasis who were given ixekizumab improved the severity of scalp and nail lesions in a phase 2 trial, according to Dr. Richard G. Langley of Dalhousie University in Halifax, N.S., and his associates.
In a 20-week randomized, placebo-controlled trial, 142 patients with moderate to severe plaque psoriasis at baseline were injected subcutaneously with ixekizumab at weeks 0, 2, 4, 8, 12, and 16. Ixekizumab, a monoclonal antibody, specifically targets IL-17A, a cytokine involved in the development of psoriasis.
Patients with scalp psoriasis were split into groups and given 10-, 25-, 75- and 150-mg doses of ixekizumab, or placebo; patients with nail psoriasis received 75- and 150-mg doses of ixekizumab. After 20 weeks, patients scalp psoriasis patients in the 25-, 75-, and 150-mg groups and nail psoriasis patients in the 75- and 150-mg groups showed significant improvement from baseline. By week 48, 78% of patients with scalp psoriasis and 51% of patients with nail psoriasis experienced complete resolution of lesions, the investigators reported.
Read the full article in the Journal of the European Academy of Dermatology and Venereology.
Patients with chronic plaque psoriasis who were given ixekizumab improved the severity of scalp and nail lesions in a phase 2 trial, according to Dr. Richard G. Langley of Dalhousie University in Halifax, N.S., and his associates.
In a 20-week randomized, placebo-controlled trial, 142 patients with moderate to severe plaque psoriasis at baseline were injected subcutaneously with ixekizumab at weeks 0, 2, 4, 8, 12, and 16. Ixekizumab, a monoclonal antibody, specifically targets IL-17A, a cytokine involved in the development of psoriasis.
Patients with scalp psoriasis were split into groups and given 10-, 25-, 75- and 150-mg doses of ixekizumab, or placebo; patients with nail psoriasis received 75- and 150-mg doses of ixekizumab. After 20 weeks, patients scalp psoriasis patients in the 25-, 75-, and 150-mg groups and nail psoriasis patients in the 75- and 150-mg groups showed significant improvement from baseline. By week 48, 78% of patients with scalp psoriasis and 51% of patients with nail psoriasis experienced complete resolution of lesions, the investigators reported.
Read the full article in the Journal of the European Academy of Dermatology and Venereology.
Counting Costs
We are all aware of the rising costs of medical care, especially for complex diseases such as psoriasis. The total cost of psoriasis in the United States is unknown. Brezinski et al (JAMA Dermatol. doi:10.1001/jamadermatol.2014.3593) sought to define the economic burden of psoriasis in the United States. They argued that this information is needed to provide the foundation for research, advocacy, and educational efforts within the disease.
The authors searched PubMed and MEDLINE databases for economic investigations on the costs of adult psoriasis in the United States. The primary objective of the analysis was to provide a comprehensive analysis of the literature on the economic burden of psoriasis in the United States. The direct, indirect, intangible, and comorbidity costs of psoriasis were reported based on this systematic literature review and adjusted to 2013 US dollars.
The direct costs included medical costs associated with (1) specialist medical evaluations, (2) hospitalization, (3) prescription medications, (4) phototherapy, (5) medication administration costs, (6) laboratory tests and monitoring studies, and (7) over-the-counter medications and self-care products. The indirect costs were determined by absenteeism and impaired work productivity. Intangible costs were calculated as a measure of the negative effect of psoriasis on quality of life. Finally, comorbidity costs measured the medical evaluations, treatment, and lab monitoring that were directly attributed to comorbid conditions associated with psoriasis.
An initial review of the literature generated 100 articles; 22 studies were included in the systematic review. The direct psoriasis costs ranged from $51.7 billion to $63.2 billion, the indirect costs ranged from $23.9 billion to $35.4 billion, and medical comorbidities were estimated to contribute $36.4 billion annually in 2013 US dollars. The annual cost of psoriasis in the United States amounted to approximately $112 billion in 2013.
The authors concluded that the economic burden of psoriasis was substantial and significant in the United States.
What’s the issue?
In the United States, the economic burden of psoriasis is substantial because this disease is associated with negative physical, psychiatric, and social consequences. In addition, treatment costs continue to rise. How will this analysis of cost influence your future management of psoriasis?
We are all aware of the rising costs of medical care, especially for complex diseases such as psoriasis. The total cost of psoriasis in the United States is unknown. Brezinski et al (JAMA Dermatol. doi:10.1001/jamadermatol.2014.3593) sought to define the economic burden of psoriasis in the United States. They argued that this information is needed to provide the foundation for research, advocacy, and educational efforts within the disease.
The authors searched PubMed and MEDLINE databases for economic investigations on the costs of adult psoriasis in the United States. The primary objective of the analysis was to provide a comprehensive analysis of the literature on the economic burden of psoriasis in the United States. The direct, indirect, intangible, and comorbidity costs of psoriasis were reported based on this systematic literature review and adjusted to 2013 US dollars.
The direct costs included medical costs associated with (1) specialist medical evaluations, (2) hospitalization, (3) prescription medications, (4) phototherapy, (5) medication administration costs, (6) laboratory tests and monitoring studies, and (7) over-the-counter medications and self-care products. The indirect costs were determined by absenteeism and impaired work productivity. Intangible costs were calculated as a measure of the negative effect of psoriasis on quality of life. Finally, comorbidity costs measured the medical evaluations, treatment, and lab monitoring that were directly attributed to comorbid conditions associated with psoriasis.
An initial review of the literature generated 100 articles; 22 studies were included in the systematic review. The direct psoriasis costs ranged from $51.7 billion to $63.2 billion, the indirect costs ranged from $23.9 billion to $35.4 billion, and medical comorbidities were estimated to contribute $36.4 billion annually in 2013 US dollars. The annual cost of psoriasis in the United States amounted to approximately $112 billion in 2013.
The authors concluded that the economic burden of psoriasis was substantial and significant in the United States.
What’s the issue?
In the United States, the economic burden of psoriasis is substantial because this disease is associated with negative physical, psychiatric, and social consequences. In addition, treatment costs continue to rise. How will this analysis of cost influence your future management of psoriasis?
We are all aware of the rising costs of medical care, especially for complex diseases such as psoriasis. The total cost of psoriasis in the United States is unknown. Brezinski et al (JAMA Dermatol. doi:10.1001/jamadermatol.2014.3593) sought to define the economic burden of psoriasis in the United States. They argued that this information is needed to provide the foundation for research, advocacy, and educational efforts within the disease.
The authors searched PubMed and MEDLINE databases for economic investigations on the costs of adult psoriasis in the United States. The primary objective of the analysis was to provide a comprehensive analysis of the literature on the economic burden of psoriasis in the United States. The direct, indirect, intangible, and comorbidity costs of psoriasis were reported based on this systematic literature review and adjusted to 2013 US dollars.
The direct costs included medical costs associated with (1) specialist medical evaluations, (2) hospitalization, (3) prescription medications, (4) phototherapy, (5) medication administration costs, (6) laboratory tests and monitoring studies, and (7) over-the-counter medications and self-care products. The indirect costs were determined by absenteeism and impaired work productivity. Intangible costs were calculated as a measure of the negative effect of psoriasis on quality of life. Finally, comorbidity costs measured the medical evaluations, treatment, and lab monitoring that were directly attributed to comorbid conditions associated with psoriasis.
An initial review of the literature generated 100 articles; 22 studies were included in the systematic review. The direct psoriasis costs ranged from $51.7 billion to $63.2 billion, the indirect costs ranged from $23.9 billion to $35.4 billion, and medical comorbidities were estimated to contribute $36.4 billion annually in 2013 US dollars. The annual cost of psoriasis in the United States amounted to approximately $112 billion in 2013.
The authors concluded that the economic burden of psoriasis was substantial and significant in the United States.
What’s the issue?
In the United States, the economic burden of psoriasis is substantial because this disease is associated with negative physical, psychiatric, and social consequences. In addition, treatment costs continue to rise. How will this analysis of cost influence your future management of psoriasis?
VIDEO: As biosimilars arrive in U.S., treatment questions arise
MAUI, HAWAII – There’s been fairly brisk uptake of biosimilar infliximab in Europe, both for new patients and as a possible switch from Remicade.
That biosimilar infliximab, Remsima, was submitted for U.S. approval in 2014, and a Food and Drug Administration advisory panel recently recommended approval of a biosimilar for filgrastim (Neupogen).
The agents are in the vanguard of what is sure to be an expanding market in the United States as biologics come off patent. Additional infliximab replacements are in the works, as well as biosimilars for etanercept, adalimumab, rituximab, and others.
For rheumatologists, that could mean less expensive treatments for patients, but it’s also likely to make treatment more complicated. Given the complexity of the molecules, the differences between biosimilars and familiar brands could be a bit more marked than those between small-molecule generics and their branded counterparts.
In a video interview at the 2015 Rheumatology Winter Clinical Symposium, Dr. Arthur F. Kavanaugh, a rheumatology professor at the University of California, San Diego, outlined the latest developments and shared his thoughts on the rapidly evolving field.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MAUI, HAWAII – There’s been fairly brisk uptake of biosimilar infliximab in Europe, both for new patients and as a possible switch from Remicade.
That biosimilar infliximab, Remsima, was submitted for U.S. approval in 2014, and a Food and Drug Administration advisory panel recently recommended approval of a biosimilar for filgrastim (Neupogen).
The agents are in the vanguard of what is sure to be an expanding market in the United States as biologics come off patent. Additional infliximab replacements are in the works, as well as biosimilars for etanercept, adalimumab, rituximab, and others.
For rheumatologists, that could mean less expensive treatments for patients, but it’s also likely to make treatment more complicated. Given the complexity of the molecules, the differences between biosimilars and familiar brands could be a bit more marked than those between small-molecule generics and their branded counterparts.
In a video interview at the 2015 Rheumatology Winter Clinical Symposium, Dr. Arthur F. Kavanaugh, a rheumatology professor at the University of California, San Diego, outlined the latest developments and shared his thoughts on the rapidly evolving field.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MAUI, HAWAII – There’s been fairly brisk uptake of biosimilar infliximab in Europe, both for new patients and as a possible switch from Remicade.
That biosimilar infliximab, Remsima, was submitted for U.S. approval in 2014, and a Food and Drug Administration advisory panel recently recommended approval of a biosimilar for filgrastim (Neupogen).
The agents are in the vanguard of what is sure to be an expanding market in the United States as biologics come off patent. Additional infliximab replacements are in the works, as well as biosimilars for etanercept, adalimumab, rituximab, and others.
For rheumatologists, that could mean less expensive treatments for patients, but it’s also likely to make treatment more complicated. Given the complexity of the molecules, the differences between biosimilars and familiar brands could be a bit more marked than those between small-molecule generics and their branded counterparts.
In a video interview at the 2015 Rheumatology Winter Clinical Symposium, Dr. Arthur F. Kavanaugh, a rheumatology professor at the University of California, San Diego, outlined the latest developments and shared his thoughts on the rapidly evolving field.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT RWCS 2015
Unique psoriatic arthritis genetic risk loci discovered
Several genetic risk loci unique to psoriatic arthritis have been identified in a study of samples from 1,962 patients with the disease and 8,923 healthy population controls of white ancestry, according to Dr. John Bowes and his associates.
The investigators used Immunochip genotyping array to fine map previously reported immune-related susceptibility loci, including known psoriasis susceptibility loci, to identify novel psoriatic arthritis (PsA) susceptibility loci.
They found a risk locus specific to PsA at chromosome 5q31, and they detected PsA risk variants at chromosome 1q31, a known psoriasis susceptibility locus. They found independent associations between PsA and three human leukocyte antigen (HLA) genes as well as three non-HLA loci. Finally, they determined that the PsA genetic risk variants preferentially associated with epigenetic markers of open chromatin in CD8+ memory primary T cells, supporting “their importance in the underlying disease mechanism.”
Until this point, the majority of risk loci associated with PsA were shared with psoriasis, but the discovery of unique risk loci could begin to explain fundamental differences between psoriasis and PsA, the investigators said.
Read the full study published Feb. 5 in Nature Communications (doi:10.1038/ncomms7046).
Several genetic risk loci unique to psoriatic arthritis have been identified in a study of samples from 1,962 patients with the disease and 8,923 healthy population controls of white ancestry, according to Dr. John Bowes and his associates.
The investigators used Immunochip genotyping array to fine map previously reported immune-related susceptibility loci, including known psoriasis susceptibility loci, to identify novel psoriatic arthritis (PsA) susceptibility loci.
They found a risk locus specific to PsA at chromosome 5q31, and they detected PsA risk variants at chromosome 1q31, a known psoriasis susceptibility locus. They found independent associations between PsA and three human leukocyte antigen (HLA) genes as well as three non-HLA loci. Finally, they determined that the PsA genetic risk variants preferentially associated with epigenetic markers of open chromatin in CD8+ memory primary T cells, supporting “their importance in the underlying disease mechanism.”
Until this point, the majority of risk loci associated with PsA were shared with psoriasis, but the discovery of unique risk loci could begin to explain fundamental differences between psoriasis and PsA, the investigators said.
Read the full study published Feb. 5 in Nature Communications (doi:10.1038/ncomms7046).
Several genetic risk loci unique to psoriatic arthritis have been identified in a study of samples from 1,962 patients with the disease and 8,923 healthy population controls of white ancestry, according to Dr. John Bowes and his associates.
The investigators used Immunochip genotyping array to fine map previously reported immune-related susceptibility loci, including known psoriasis susceptibility loci, to identify novel psoriatic arthritis (PsA) susceptibility loci.
They found a risk locus specific to PsA at chromosome 5q31, and they detected PsA risk variants at chromosome 1q31, a known psoriasis susceptibility locus. They found independent associations between PsA and three human leukocyte antigen (HLA) genes as well as three non-HLA loci. Finally, they determined that the PsA genetic risk variants preferentially associated with epigenetic markers of open chromatin in CD8+ memory primary T cells, supporting “their importance in the underlying disease mechanism.”
Until this point, the majority of risk loci associated with PsA were shared with psoriasis, but the discovery of unique risk loci could begin to explain fundamental differences between psoriasis and PsA, the investigators said.
Read the full study published Feb. 5 in Nature Communications (doi:10.1038/ncomms7046).
Manage Your Dermatology Practice: Managing Difficult Patient Encounters
Difficult patient encounters in the dermatology office can be navigated through honest physician-patient communication regarding problems within the office and insurance coverage. Dr. Gary Goldenberg provides tips on communicating with patients about cosmetic procedures that may be noncovered services as well as diagnoses such as melanoma and psoriasis. He also advises how to work through a long list of questions patients may bring to their visit.
Difficult patient encounters in the dermatology office can be navigated through honest physician-patient communication regarding problems within the office and insurance coverage. Dr. Gary Goldenberg provides tips on communicating with patients about cosmetic procedures that may be noncovered services as well as diagnoses such as melanoma and psoriasis. He also advises how to work through a long list of questions patients may bring to their visit.
Difficult patient encounters in the dermatology office can be navigated through honest physician-patient communication regarding problems within the office and insurance coverage. Dr. Gary Goldenberg provides tips on communicating with patients about cosmetic procedures that may be noncovered services as well as diagnoses such as melanoma and psoriasis. He also advises how to work through a long list of questions patients may bring to their visit.
Most cost-effective psoriasis treatment: methotrexate
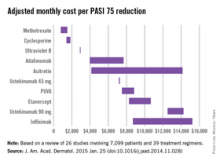
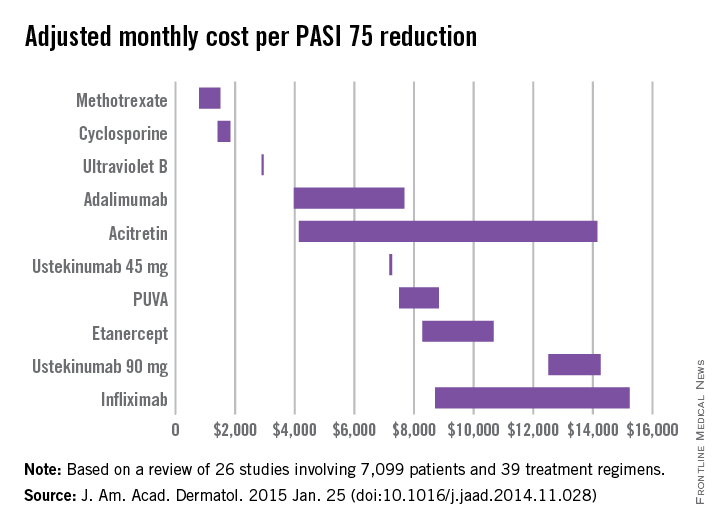
Methotrexate is the most cost-effective systemic treatment for psoriasis in terms of the number of patients needed to treat to achieve a PASI 75, according to a literature review in the Journal of the American Academy of Dermatology.
Methotrexate (2.5-mg tablet) cost an adjusted $794-$1,503 a month per number needed to treat (NNT) for a 75% reduction in Psoriasis Area and Severity Index (PASI 75) score, Dr. Logan S. D’Souza and Dr. Michael J. Payette of the department of dermatology at the University of Connecticut in Farmington reported (J. Am. Acad. Dermatol. 2015 Jan. 25 [doi:10.1016/j.jaad.2014.11.028]).
Coming in second was cyclosporine (25- and 100-mg tablets) at $1,410-$1,844 in monthly cost per PASI 75, followed by narrow-band ultraviolet B light phototherapy at $2,925 and adalimumab (40-mg subcutaneous injection) at $3,975-$7,679, the investigators said.
The most expensive of the 10 Food and Drug Administration–approved treatments included in the study was infliximab (100-mg vial) at $8,705-$15,236 per month. The next most expensive treatment was ustekinumab (90-mg subcutaneous injection), which cost $12,505-$14,257 per month.
“While our data indicate that methotrexate and cyclosporine are more cost efficacious in reaching PASI 75 than other medications approved by the FDA, their potential toxicities may limit their long-term use,” Dr. D’Souza and Dr. Payette noted. “Our study is not meant to dictate treatments based solely on costs; rather, our analysis should help both clinicians and patients in choosing between potential treatments,” they added.
For this analysis, 26 studies involving 7,099 patients and 39 treatment regimens met the investigators’ search criteria.
Dr. D’Souza declared no conflicts of interest. Dr. Payette has been an adviser to Amgen.
Methotrexate is the most cost-effective systemic treatment for psoriasis in terms of the number of patients needed to treat to achieve a PASI 75, according to a literature review in the Journal of the American Academy of Dermatology.
Methotrexate (2.5-mg tablet) cost an adjusted $794-$1,503 a month per number needed to treat (NNT) for a 75% reduction in Psoriasis Area and Severity Index (PASI 75) score, Dr. Logan S. D’Souza and Dr. Michael J. Payette of the department of dermatology at the University of Connecticut in Farmington reported (J. Am. Acad. Dermatol. 2015 Jan. 25 [doi:10.1016/j.jaad.2014.11.028]).
Coming in second was cyclosporine (25- and 100-mg tablets) at $1,410-$1,844 in monthly cost per PASI 75, followed by narrow-band ultraviolet B light phototherapy at $2,925 and adalimumab (40-mg subcutaneous injection) at $3,975-$7,679, the investigators said.
The most expensive of the 10 Food and Drug Administration–approved treatments included in the study was infliximab (100-mg vial) at $8,705-$15,236 per month. The next most expensive treatment was ustekinumab (90-mg subcutaneous injection), which cost $12,505-$14,257 per month.
“While our data indicate that methotrexate and cyclosporine are more cost efficacious in reaching PASI 75 than other medications approved by the FDA, their potential toxicities may limit their long-term use,” Dr. D’Souza and Dr. Payette noted. “Our study is not meant to dictate treatments based solely on costs; rather, our analysis should help both clinicians and patients in choosing between potential treatments,” they added.
For this analysis, 26 studies involving 7,099 patients and 39 treatment regimens met the investigators’ search criteria.
Dr. D’Souza declared no conflicts of interest. Dr. Payette has been an adviser to Amgen.
Methotrexate is the most cost-effective systemic treatment for psoriasis in terms of the number of patients needed to treat to achieve a PASI 75, according to a literature review in the Journal of the American Academy of Dermatology.
Methotrexate (2.5-mg tablet) cost an adjusted $794-$1,503 a month per number needed to treat (NNT) for a 75% reduction in Psoriasis Area and Severity Index (PASI 75) score, Dr. Logan S. D’Souza and Dr. Michael J. Payette of the department of dermatology at the University of Connecticut in Farmington reported (J. Am. Acad. Dermatol. 2015 Jan. 25 [doi:10.1016/j.jaad.2014.11.028]).
Coming in second was cyclosporine (25- and 100-mg tablets) at $1,410-$1,844 in monthly cost per PASI 75, followed by narrow-band ultraviolet B light phototherapy at $2,925 and adalimumab (40-mg subcutaneous injection) at $3,975-$7,679, the investigators said.
The most expensive of the 10 Food and Drug Administration–approved treatments included in the study was infliximab (100-mg vial) at $8,705-$15,236 per month. The next most expensive treatment was ustekinumab (90-mg subcutaneous injection), which cost $12,505-$14,257 per month.
“While our data indicate that methotrexate and cyclosporine are more cost efficacious in reaching PASI 75 than other medications approved by the FDA, their potential toxicities may limit their long-term use,” Dr. D’Souza and Dr. Payette noted. “Our study is not meant to dictate treatments based solely on costs; rather, our analysis should help both clinicians and patients in choosing between potential treatments,” they added.
For this analysis, 26 studies involving 7,099 patients and 39 treatment regimens met the investigators’ search criteria.
Dr. D’Souza declared no conflicts of interest. Dr. Payette has been an adviser to Amgen.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY