User login
A New Appraisal of Dermatologic Manifestations of Diabetes Mellitus
Diabetes mellitus is a morbid and costly condition that carries a high burden of disease for patients (both with and without a diagnosis) and for society as a whole. The economic burden of diabetes in the United States recently was estimated at nearly $250 billion annually,1 and this number continues to rise. The cutaneous manifestations of diabetes are diverse and far-reaching, ranging from benign cosmetic concerns to severe dermatologic conditions. Given the wide range of etiology for diabetes mellitus and its existence on a spectrum of severity, it is perhaps not surprising that some of these entities are the subject of debate (vis-à-vis the strength of association between these skin conditions and diabetes) and can manifest in different forms. However, it is clear that the cutaneous manifestations of diabetes are equally as important to consider and manage as the systemic complications of the disease. In analyzing associations with diabetes, it is important to note that given such a high incidence of diabetes among the general population and its close association with other disease states, such as the metabolic syndrome, studies aimed at determining direct relationships to this entity must be well controlled for confounding factors, which may not even always be possible. Regardless, it is important for dermatologists and dermatology residents to recognize and understand the protean cutaneous manifestations of diabetes mellitus, and this column will explore skin findings that are characteristic of diabetes (Table 1) as well as other dermatoses with a reported but less clear association with diabetes (Table 2).
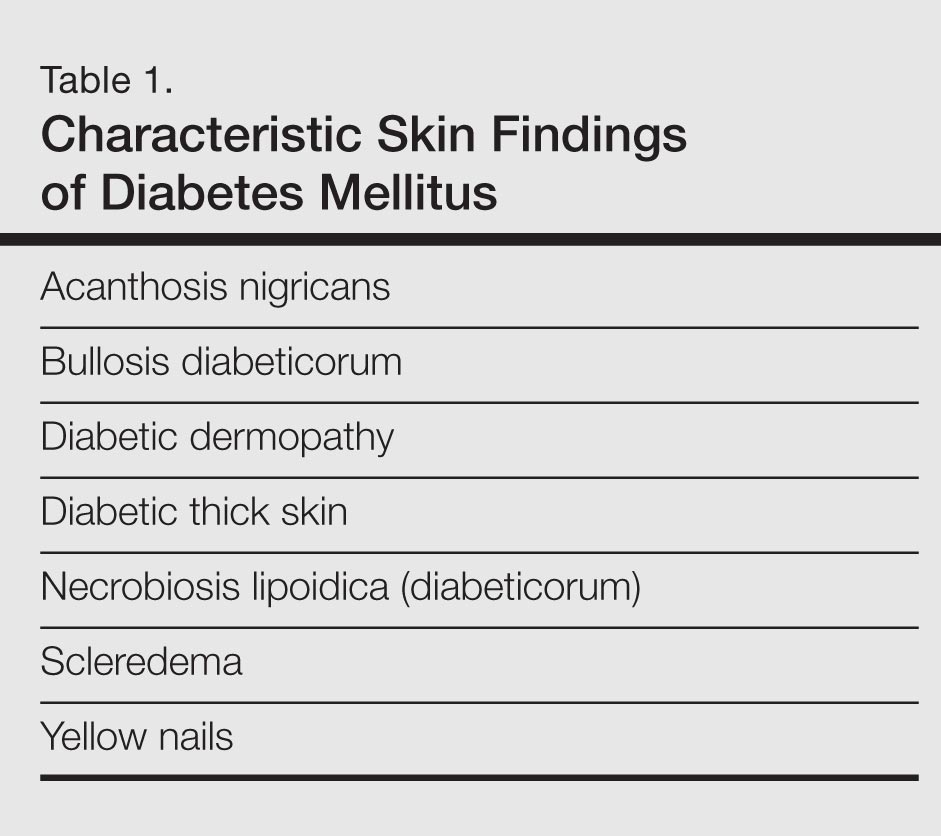

Skin Findings Characteristic of Diabetes
Diabetic Thick Skin
The association between diabetes and thick skin is well described as either a mobility-limiting affliction of the joints of the hands (cheiroarthropathy) or as an asymptomatic thickening of the skin. It has been estimated that 8% to 36% of patients with insulin-dependent diabetes develop some form of skin thickening2; one series also found this association to be true for patients with non–insulin-dependent diabetes mellitus (NIDDM).3 Skin thickening is readily observable on clinical presentation or ultrasonography, with increasing thickness in many cases associated with long-term disease progression. This increasing thickness was shown histopathologically to be a direct result of activated fibroblasts and increased collagen polymerization, with some similar features to progressive systemic sclerosis.4 Interestingly, even clinically normal skin showed some degree of fibroblast activation in diabetic patients, but collagen fibers in each case were smaller in diameter than those found in progressive systemic sclerosis. This finding clearly has implications on quality of life, as a lack of hand mobility due to the cheiroarthropathy can be severely disabling. Underlining the need for strict glycemic control, it has been suggested that tight control of blood sugar levels can lead to improvement in diabetic thick skin; however, reports of improvement are based on a small sample population.5 Huntley papules are localized to areas on the dorsum of the hands overlying the joints, demonstrating hyperkeratosis and enlarged dermal papillae.6 They also can be found in a minority of patients without diabetes. Interestingly, diabetic thick skin also has been associated with neurologic disorders in diabetes.7 Diabetic thick skin was found to be significantly (P<.05) correlated with diabetic neuropathy, independent of duration of diabetes, age, or glycosylated hemoglobin levels, though no causal or etiologic link between these entities has been proven.
Yellow Nails
Nail changes are well described in diabetes, ranging from periungual telangiectases to complications from infections, such as paronychia; however, a well-recognized finding, especially in elderly diabetic patients, is a characteristic yellowing of the nails, reported to affect up to 40% of patients with diabetes.8 The mechanism behind it likely includes accumulation of glycation end products, which also has been thought to lead to yellowing of the skin, and vascular impairment.9 These nails tend to exhibit slow growth, likely resulting from a nail matrix that is poorly supplied with blood, and also can be more curved than normal with longitudinal ridges (onychorrhexis).10 It is important, however, not to attribute yellow nails to diabetes without considering other causes of yellow nails, such as onychomycosis, yellow nail syndrome, and yellow nails associated with lymphedema or respiratory tract disease (eg, pleural effusion, bronchiectasis).11
Diabetic Dermopathy
Colloquially known as shin spots, diabetic dermopathy is perhaps the most common skin finding in this patient population, though it also can occur in up to 1 in 5 individuals without diabetes.12 Although it is very common, it is not a condition that should be overlooked, as numerous studies have shown an increase in microangiopathic complications such as retinopathy in patients with diabetic dermopathy.13,14 Although follow-up studies may be necessary to fully characterize the relationship between shin spots and diabetes, it certainly is reasonable to be more wary of diabetic patients presenting with many shin spots, as the general consensus is that these areas represent postinflammatory hyperpigmentation and cutaneous atrophy in the setting of poor vascular supply, which should prompt analysis of other areas that might be affected by poor vasculature, such as an ophthalmologic examination. Antecedent and perhaps unnoticed trauma has been implicated given a possible underlying neuropathy, but this theory has not been supported by studies.
Bullosis Diabeticorum
Bullosis diabeticorum is a rare but well-described occurrence of self-resolving, nonscarring blisters that arise on the extremities of diabetic patients. This entity should be distinguished from other primary autoimmune blistering disorders and from simple mechanobullous lesions. Several types of bullosis diabeticorum have been described, with the most classic form showing an intraepidermal cleavage plane.15 These lesions tend to resolve in weeks but can be recurrent. The location of the pathology underlines its nonscarring nature, though similar lesions have been reported showing a cleavage plane in the lamina lucida of the dermoepidermal junction, which underlines the confusion in the literature surrounding diabetic bullae.16 Some may even use this term interchangeably with trauma or friction-induced blisters, which diabetics may be prone to develop due to peripheral neuropathy. Confounding reports have stated there is a correlation between bullosis diabeticorum and neuropathy as well as the acral location of these blisters. Although many authors cite the incidence of bullosis diabeticorum being 0.5%,17 no population-based studies have confirmed this figure and some have speculated that the actual incidence is higher.18 In the end, the term bullosis diabeticorum is probably best reserved for a rapidly appearing blister on the extremities of diabetic patients with at most minimal trauma, with a lesion containing sterile fluid and negative immunofluorescence. The mechanism for these blisters is thought to be microangiopathy, with scant blood supply to the skin causing it to be more prone to acantholysis and blister formation.19 This theory was reinforced in a study showing a reduced threshold for suction blister formation in diabetic patients.20 Care should be taken to prevent secondary infections at these sites.
Acanthosis Nigricans
Acanthosis nigricans, which consists of dark brown plaques in the flexural areas, especially the posterior neck and axillae, is a common finding in diabetic patients and is no doubt familiar to clinicians. The pathophysiology of these lesions has been well studied and is a prototype for the effects of insulin resistance in the skin. In this model, high concentrations of insulin binding to insulinlike growth factor receptor in the skin stimulate keratinocyte proliferation,21 leading to the clinical appearance and the histologic finding of hyperkeratosis and papillomatosis, which in turn is responsible for the observed hyperpigmentation. It is an important finding, especially in those without a known history of diabetes, as it can also signal an underlying endocrinopathy (eg, Cushing syndrome, acromegaly, polycystic ovary syndrome) or malignancy (ie, adenocarcinoma of the gastrointestinal tract). Several distinct mechanisms of insulin resistance have been described, including insulin resistance due to receptor defects, such as those seen with insulin resistance in NIDDM; autoimmune processes; and postreceptor defects in insulin action.22 Keratolytics and topical retinoids have been used to ameliorate the appearance of these lesions.
Necrobiosis Lipoidica (Diabeticorum)
Necrobiosis lipoidica diabeticorum was first described by Urback23 in 1932, but reports of similar lesions were described in nondiabetic patients soon after. The dermatologic community has since come to realize that perhaps a more accurate nomenclature is necrobiosis lipoidica to fully encompass this entity. Clinically, lesions appear as erythematous papules and plaques that expand into a larger well-circumscribed plaque with a waxy yellowish atrophic center, often with telangiectases, and usually presenting in the pretibial area. Lesions can become ulcerated in up to one-third of cases. Necrobiosis lipoidica also is defined by characteristic histologic findings, including important features such as palisaded granulomas arranged in a tierlike fashion, necrotizing vasculitis, collagen degradation, and panniculitis. Necrobiosis lipoidica is still relatively rare, developing in approximately 0.3% of patients with diabetes,24 though its relationship with insulin resistance and diabetes is strong. Approximately two-thirds of patients with necrobiosis lipoidica have diabetes and an even higher number go on to develop diabetes or have a positive family history of diabetes.
Although these figures are interesting, the data are nearly a half-century-old, and it is unclear if these findings still hold true today. The etiology of necrobiosis lipoidica also remains elusive, with theories focusing on the role of microangiopathy, immunoglobulin deposition leading to vasculitis, structural abnormalities in collagen or fibroblasts, and trauma; however, the true nature of this condition is likely some combination of these factors.25 These lesions are difficult to treat, especially at an advanced stage. Management with topical steroids to limit the inflammatory progression of the lesions is the mainstay of therapy.
Scleredema
Scleredema adultorum (Buschke disease) refers to indurated plaques over the posterior neck and upper back. It is usually thought of as 3 distinct forms. The form that is known to occur in diabetic patients is sometimes referred to as scleredema diabeticorum; the other 2 occur as postinfectious, usually Streptococcus, or malignancy-related forms. The prevalence of scleredema diabeticorum among diabetic individuals most frequently is reported as 2.5%26; however, it is worth noting that other estimates have been as high as 14%.27 Although there has been some correlation between poorly controlled NIDDM, treatment and tight glucose control does not seem to readily resolve these lesions with only few conflicting case studies serving as evidence for and against this premise.28-30 The lesions often are recalcitrant toward a wide variety of treatment approaches. Histopathologic analysis generally reveals a thickened dermis with large collagen bundles, with clear spaces between the collagen representing mucin and increased numbers of mast cells. Proposed mechanisms include stimulation of collagen synthesis by fibroblasts and retarded collagen degradation, likely due to excess glucose.31
Dermatoses Demonstrating an Association With Diabetes
Granuloma Annulare
Granuloma annulare (GA) is a dermatologic condition existing in numerous forms. The generalized form has been suggested to have some association with diabetes. The lesions of GA are classically round, flesh-colored to erythematous papules arising in the dermis that may start on the dorsal extremities where the localized form typically presents, though larger annular plaques or patches may exist in the generalized form. Histologically, GA has a characteristic granulomatous infiltrate and palisaded granulomatous dermatitis, depending on the stage of the evolution. Many studies dating back to the mid-20th century have attempted to elucidate a link between GA and diabetes, with numerous reports showing conflicting results across their study populations.32-36 This issue is further muddled by links between generalized GA and a host of other diseases, such as malignancy, thyroid disease, hepatitis, and human immunodeficiency virus infection. The usual course of GA is spontaneous resolution, including a peculiar phenomenon noted in the literature whereby biopsy of one of the lesions led to clearance of other lesions on the body.37 However, the generalized form may be more difficult to treat, with therapeutic approaches including topical steroids, light therapy, and systemic immunomodulators.
Lichen Planus
A recent small population study in Turkey demonstrated a strong relationship between lichen planus and abnormal glucose tolerance. In this study of 30 patients with lichen planus, approximately half (14/30) had abnormal glucose metabolism and a quarter (8/30) had known diabetes, but larger studies are needed to clarify this relationship.38 Prior to this report, a link between oral lichen planus and diabetes had been shown in larger case series.39,40 Clinically, one may see white plaques with a characteristic lacy reticular pattern in the mouth. At other cutaneous sites, lichen planus generally appears as pruritic, purple, flat-topped polygonal papules. The clinical finding of lichen planus also is linked with many other disease states, most notably hepatitis C virus, but also thymoma, liver disease, and inflammatory bowel disease, among other associations.41
Vitiligo
As an autoimmune entity, it stands to reason that vitiligo may be seen more commonly associated with insulin-dependent diabetes, which has been shown to hold true in one study, while no association was found between later-onset NIDDM and vitiligo.42 Given the nonspecific nature of this association and the relatively common presentation of vitiligo, no special consideration is likely needed when examining a patient with vitiligo, but its presence should remind the clinician that these autoimmune entities tend to travel together.
Acquired Perforating Dermatosis
Although the classic presentation of acquired perforating dermatosis (Kyrle disease) is linked to renal failure, diabetes also has been connected to its presentation. Extremely rare outside of the setting of chronic renal failure, acquired perforating dermatosis occurs in up to 10% of dialysis patients.43,44 It is characterized by papules with a central keratin plug, representing transepidermal elimination of keratin, collagen, and other cellular material; its etiology has not been elucidated. The connection between acquired perforating dermatosis and diabetes is not completely clear; it would seem that renal failure is a prerequisite for itspresentation. A large proportion of renal failure necessitating hemodialysis occurs in patients with diabetic nephropathy, which may explain the coincidence of diabetes, renal failure, and acquired perforating dermatosis.45 The presentation of this cutaneous finding should not, however, affect treatment of the underlying conditions. Symptom relief in the form of topical steroids can be used as a first-line treatment of these often pruritic lesions.
Eruptive Xanthomas
The link between diabetes and eruptive xanthomas seems to be a rather tenuous one, hinging on the fact that many diabetic patients have abnormalities in carbohydrate and lipid metabolism. A central feature of eruptive xanthomas is an elevation in triglycerides, which can occur in diabetes. Indeed, it has been estimated that only 0.1% of diabetics will develop eruptive xanthomas,46 and its main importance may be to prompt the physician to treat the hypertriglyceridemia and consider other concerning possibilities such as acute pancreatitis.
Psoriasis
Psoriasis is a common dermatologic condition that has been shown to have a far-reaching impact both on patients’ quality of life and cardiovascular risk profiles. Data have emerged linking psoriasis with diabetes as an independent risk factor47; although this retrospective study had its limitations, it certainly is interesting to note that patients with psoriasis may have an increased risk for developing diabetes. Perhaps more importantly, though, this study also implied that patients with severe psoriasis may present with diabetes that is more difficult to control, evidenced by increased treatment with systemic therapies as opposed to milder forms of intervention such as diet control.47 There almost certainly are other confounding factors and further studies would serve to reveal the strength of this association, but it is certainly an intriguing concept. Echoing these findings, a more recent nationwide study from Denmark demonstrated that psoriasis is associated with increased incidence of new-onset diabetes, adjusting for numerous confounding factors.48 The relationship between psoriasis and diabetes is worth noting as evidence continues to emerge.
Conclusion
Given the diverse cutaneous manifestations of diabetes, it is important to distinguish those that are directly related to diabetes from those that suggest there may be another underlying process. For example, a new patient presenting to a primary care physician with acanthosis nigricans and yellow nails should immediately trigger a test for a hemoglobin A1c (glycated hemoglobin) level to investigate for diabetes; however, clinicians also should be wary of patients with acanthosis nigricans who report early satiety, as this asso-ciation may be a sign of underlying malignancy. Conversely, the presence of yellow nails in a patient with chronic diabetes should not be ignored. The physician should consider onychomycosis and query the patient about possible respiratory symptoms. In the case of a multisystem disease such as diabetes, it may be challenging to reconcile seemingly disparate skin findings, but having a framework to approach the cutaneous manifestations of diabetes can help to properly identify and treat an individual patient’s afflictions.
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012 [published online instead of print March 16, 2013]. Diabetes Care. 2013;36:1033-1046.
- Collier A, Matthews DM, Kellett HA, et al. Change in skin thickness associated with cheiroarthropathy in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed). 1986;292:936.
- Fitzcharles MA, Duby S, Waddell RW, et al. Limitation of joint mobility (cheiroarthropathy) in adult noninsulin-dependent diabetic patients. Ann Rheum Dis. 1984;43:251-254.
- Hanna W, Friesen D, Bombardier C, et al. Pathologic features of diabetic thick skin. J Am Acad Dermatol. 1987;16:546-553.
- Lieberman LS, Rosenbloom AL, Riley WJ, et al. Reduced skin thickness with pump administration of insulin. N Engl J Med. 1980;303:940-941.
- Guarneri C, Guarneri F, Borgia F, et al. Finger pebbles in a diabetic patient: Huntley's papules. Int J Dermatol. 2005;44:755-756.
- Forst T, Kann P, Pfützner A, et al. Association between "diabetic thick skin syndrome" and neurological disorders in diabetes mellitus. Acta Diabetol. 1994;31:73-77.
- Nikoleishvili LR, Kurashvili RB, Virsaladze DK, et al. Characteristic changes of skin and its accessories in type 2 diabetes mellitus [in Russian]. Georgian Med News. 2006:43-46.
- Lithner F. Purpura, pigmentation and yellow nails of the lower extremities in diabetics. Acta Med Scand. 1976;199:203-208.
- Greene RA, Scher RK. Nail changes associated with diabetes mellitus. J Am Acad Dermatol. 1987;16:1015-1021.
- Hiller E, Rosenow EC 3rd, Olsen AM. Pulmonary manifestations of the yellow nail syndrome. Chest. 1972;61:452-458.
- Feingold KR, Elias PM. Endocrine-skin interactions. cutaneous manifestations of pituitary disease, thyroid disease, calcium disorders, and diabetes. J Am Acad Dermatol. 1987;17:921-940.
- Abdollahi A, Daneshpazhooh M, Amirchaghmaghi E, et al. Dermopathy and retinopathy in diabetes: is there an association? Dermatology, 2007;214:133-136.
- Morgan AJ, Schwartz RA. Diabetic dermopathy: A subtle sign with grave implications. J Am Acad Dermatol. 2008;58:447-451.
- Perez MI, Kohn SR. Cutaneous manifestations of diabetes mellitus. J Am Acad Dermatol. 1994;30:519-531.
- Cantwell AR, Martz W. Idiopathic bullae in diabetics. Bullosis diabeticorum. Arch Dermatol. 1967;96:42-44.
- Larsen K, Jensen T, Karlsmark T, Holstein PE. Incidence of bullosis diabeticorum – a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596.
- Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200.
- Basarab T, Munn SE, McGrath J, et al. Bullosis diabeticorum. a case report and literature review. Clin Exp Dermatol. 1995;20:218-220.
- Bernstein JE, Levine LE, Medenica MM, et al. Reduced threshold to suction-induced blister formation in insulin-dependent diabetics. J Am Acad Dermatol. 1983;8:790-791.
- Cruz PD Jr, Hud JA Jr. Excess insulin binding to insulin-like growth factor receptors: proposed mechanism for acanthosis nigricans. J Invest Dermatol. 1992;98(suppl 6):S82-S85.
- Romano G, Moretti G, Di Benedetto A, et al. Skin lesions in diabetes mellitus: prevalence and clinical correlations. Diabetes Res Clin Pract. 1998;39:101-106.
- Urback E. Eine neue diabetische Stoffwechseldermatose: Nekrobiosis lipoidica diabeticorum. Arch. f. Dermat. u Syph. 1932;166:273.
- Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum. a clinical and pathological investigation of 171 cases. Arch Dermatol. 1966;93:272-281.
- Engel MF, Smith JG Jr. The pathogenesis of necrobiosis lipoidica. necrobiosis lipoidica, a form fruste of diabetes mellitus. Arch Dermatol. 1960;82:791-797.
- Cole GW, Headley J, Skowsky R. Scleredema diabeticorum: a common and distinct cutaneous manifestation of diabetes mellitus. Diabetes Care. 1983;6:189-192.
- Sattar MA, Diab S, Sugathan TN, et al. Scleroedema diabeticorum: a minor but often unrecognized complication of diabetes mellitus. Diabet Med. 1988;5:465-468.
- Rho YW, Suhr KB, Lee JH, et al. A clinical observation of scleredema adultorum and its relationship to diabetes. J Dermatol. 1998;25:103-107.
- Baillot-Rudoni S, Apostol D, Vaillant G, et al. Implantable pump therapy restores metabolic control and quality of life in type 1 diabetic patients with Buschke's nonsystemic scleroderma. Diabetes Care. 2006;29:1710.
- Meguerditchian C, Jacquet P, Béliard S, et al. Scleredema adultorum of Buschke: an under recognized skin complication of diabetes. Diabetes Metab. 2006;32:481-484.
- Behm B, Schreml S, Landthaler M, et al. Skin signs in diabetes mellitus. J Eur Acad Dermatol Venereol. 2012;26:1203-1211.
- Nebesio CL, Lewis C, Chuang TY. Lack of an association between granuloma annulare and type 2 diabetes mellitus. Br J Dermatol. 2002;146:122-124.
- Stankler L, Leslie G. Generalized granuloma annulare. a report of a case and review of the literature. Arch Dermatol. 1976;95:509-513.
- Williamson DM, Dykes JR. Carbohydrate metabolism in granuloma annulare. J Invest Dermatol. 1972;58:400-404.
- Dabski K, Winkelmann RK. Generalized granuloma annulare: clinical and laboratory findings in 100 patients. J Am Acad Dermatol. 1989;20:39-47.
- Veraldi S, Bencini PL, Drudi E, et al. Laboratory abnormalities in granuloma annulare: a case-control study. Br J Dermatol. 1997;136:652-653.
- Levin NA, Patterson JW, Yao LL, et al. Resolution of patch-type granuloma annulare lesions after biopsy. J Am Acad Dermatol. 2002;46:426-429.
- Seyhan M, Ozcan H, Sahin I, et al. High prevalence of glucose metabolism disturbance in patients with lichen planus. Diabetes Res Clin Pract. 2007;77:198-202.
- Romero MA, Seoane J, Varela-Centelles P, et al. Prevalence of diabetes mellitus amongst oral lichen planus patients. clinical and pathological characteristics. Med Oral. 2002;7:121-129.
- Albrecht M, Banoczy J, Dinya E, et al. Occurrence of oral leukoplakia and lichen planus in diabetes mellitus. J Oral Pathol Med. 1992;21:364-366.
- Lehman JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48:682-694.
- Gould IM, Gray RS, Urbaniak SJ, et al. Vitiligo in diabetes mellitus. Br J Dermatol. 1985;113:153-155.
- White CR Jr, Heskel NS, Pokorny DJ. Perforating folliculitis of hemodialysis. Am J Dermatopathol. 1982;4:109-116.
- Hurwitz RM, Melton ME, Creech FT 3rd, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
- Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989;125:1074-1078.
- Muller SA. Dermatologic disorders associated with diabetes mellitus. Mayo Clin Proc. 1966;41:689-703.
- Azfar RS, Seminara NM, Shin DB, et al. Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol. 2012;148:995-1000.
- Khalid U, Hansen PR, Gislason GH, et al. Psoriasis and new-onset diabetes: a Danish nationwide cohort study. Diabetes Care. 2013;36:2402-2407.
Diabetes mellitus is a morbid and costly condition that carries a high burden of disease for patients (both with and without a diagnosis) and for society as a whole. The economic burden of diabetes in the United States recently was estimated at nearly $250 billion annually,1 and this number continues to rise. The cutaneous manifestations of diabetes are diverse and far-reaching, ranging from benign cosmetic concerns to severe dermatologic conditions. Given the wide range of etiology for diabetes mellitus and its existence on a spectrum of severity, it is perhaps not surprising that some of these entities are the subject of debate (vis-à-vis the strength of association between these skin conditions and diabetes) and can manifest in different forms. However, it is clear that the cutaneous manifestations of diabetes are equally as important to consider and manage as the systemic complications of the disease. In analyzing associations with diabetes, it is important to note that given such a high incidence of diabetes among the general population and its close association with other disease states, such as the metabolic syndrome, studies aimed at determining direct relationships to this entity must be well controlled for confounding factors, which may not even always be possible. Regardless, it is important for dermatologists and dermatology residents to recognize and understand the protean cutaneous manifestations of diabetes mellitus, and this column will explore skin findings that are characteristic of diabetes (Table 1) as well as other dermatoses with a reported but less clear association with diabetes (Table 2).


Skin Findings Characteristic of Diabetes
Diabetic Thick Skin
The association between diabetes and thick skin is well described as either a mobility-limiting affliction of the joints of the hands (cheiroarthropathy) or as an asymptomatic thickening of the skin. It has been estimated that 8% to 36% of patients with insulin-dependent diabetes develop some form of skin thickening2; one series also found this association to be true for patients with non–insulin-dependent diabetes mellitus (NIDDM).3 Skin thickening is readily observable on clinical presentation or ultrasonography, with increasing thickness in many cases associated with long-term disease progression. This increasing thickness was shown histopathologically to be a direct result of activated fibroblasts and increased collagen polymerization, with some similar features to progressive systemic sclerosis.4 Interestingly, even clinically normal skin showed some degree of fibroblast activation in diabetic patients, but collagen fibers in each case were smaller in diameter than those found in progressive systemic sclerosis. This finding clearly has implications on quality of life, as a lack of hand mobility due to the cheiroarthropathy can be severely disabling. Underlining the need for strict glycemic control, it has been suggested that tight control of blood sugar levels can lead to improvement in diabetic thick skin; however, reports of improvement are based on a small sample population.5 Huntley papules are localized to areas on the dorsum of the hands overlying the joints, demonstrating hyperkeratosis and enlarged dermal papillae.6 They also can be found in a minority of patients without diabetes. Interestingly, diabetic thick skin also has been associated with neurologic disorders in diabetes.7 Diabetic thick skin was found to be significantly (P<.05) correlated with diabetic neuropathy, independent of duration of diabetes, age, or glycosylated hemoglobin levels, though no causal or etiologic link between these entities has been proven.
Yellow Nails
Nail changes are well described in diabetes, ranging from periungual telangiectases to complications from infections, such as paronychia; however, a well-recognized finding, especially in elderly diabetic patients, is a characteristic yellowing of the nails, reported to affect up to 40% of patients with diabetes.8 The mechanism behind it likely includes accumulation of glycation end products, which also has been thought to lead to yellowing of the skin, and vascular impairment.9 These nails tend to exhibit slow growth, likely resulting from a nail matrix that is poorly supplied with blood, and also can be more curved than normal with longitudinal ridges (onychorrhexis).10 It is important, however, not to attribute yellow nails to diabetes without considering other causes of yellow nails, such as onychomycosis, yellow nail syndrome, and yellow nails associated with lymphedema or respiratory tract disease (eg, pleural effusion, bronchiectasis).11
Diabetic Dermopathy
Colloquially known as shin spots, diabetic dermopathy is perhaps the most common skin finding in this patient population, though it also can occur in up to 1 in 5 individuals without diabetes.12 Although it is very common, it is not a condition that should be overlooked, as numerous studies have shown an increase in microangiopathic complications such as retinopathy in patients with diabetic dermopathy.13,14 Although follow-up studies may be necessary to fully characterize the relationship between shin spots and diabetes, it certainly is reasonable to be more wary of diabetic patients presenting with many shin spots, as the general consensus is that these areas represent postinflammatory hyperpigmentation and cutaneous atrophy in the setting of poor vascular supply, which should prompt analysis of other areas that might be affected by poor vasculature, such as an ophthalmologic examination. Antecedent and perhaps unnoticed trauma has been implicated given a possible underlying neuropathy, but this theory has not been supported by studies.
Bullosis Diabeticorum
Bullosis diabeticorum is a rare but well-described occurrence of self-resolving, nonscarring blisters that arise on the extremities of diabetic patients. This entity should be distinguished from other primary autoimmune blistering disorders and from simple mechanobullous lesions. Several types of bullosis diabeticorum have been described, with the most classic form showing an intraepidermal cleavage plane.15 These lesions tend to resolve in weeks but can be recurrent. The location of the pathology underlines its nonscarring nature, though similar lesions have been reported showing a cleavage plane in the lamina lucida of the dermoepidermal junction, which underlines the confusion in the literature surrounding diabetic bullae.16 Some may even use this term interchangeably with trauma or friction-induced blisters, which diabetics may be prone to develop due to peripheral neuropathy. Confounding reports have stated there is a correlation between bullosis diabeticorum and neuropathy as well as the acral location of these blisters. Although many authors cite the incidence of bullosis diabeticorum being 0.5%,17 no population-based studies have confirmed this figure and some have speculated that the actual incidence is higher.18 In the end, the term bullosis diabeticorum is probably best reserved for a rapidly appearing blister on the extremities of diabetic patients with at most minimal trauma, with a lesion containing sterile fluid and negative immunofluorescence. The mechanism for these blisters is thought to be microangiopathy, with scant blood supply to the skin causing it to be more prone to acantholysis and blister formation.19 This theory was reinforced in a study showing a reduced threshold for suction blister formation in diabetic patients.20 Care should be taken to prevent secondary infections at these sites.
Acanthosis Nigricans
Acanthosis nigricans, which consists of dark brown plaques in the flexural areas, especially the posterior neck and axillae, is a common finding in diabetic patients and is no doubt familiar to clinicians. The pathophysiology of these lesions has been well studied and is a prototype for the effects of insulin resistance in the skin. In this model, high concentrations of insulin binding to insulinlike growth factor receptor in the skin stimulate keratinocyte proliferation,21 leading to the clinical appearance and the histologic finding of hyperkeratosis and papillomatosis, which in turn is responsible for the observed hyperpigmentation. It is an important finding, especially in those without a known history of diabetes, as it can also signal an underlying endocrinopathy (eg, Cushing syndrome, acromegaly, polycystic ovary syndrome) or malignancy (ie, adenocarcinoma of the gastrointestinal tract). Several distinct mechanisms of insulin resistance have been described, including insulin resistance due to receptor defects, such as those seen with insulin resistance in NIDDM; autoimmune processes; and postreceptor defects in insulin action.22 Keratolytics and topical retinoids have been used to ameliorate the appearance of these lesions.
Necrobiosis Lipoidica (Diabeticorum)
Necrobiosis lipoidica diabeticorum was first described by Urback23 in 1932, but reports of similar lesions were described in nondiabetic patients soon after. The dermatologic community has since come to realize that perhaps a more accurate nomenclature is necrobiosis lipoidica to fully encompass this entity. Clinically, lesions appear as erythematous papules and plaques that expand into a larger well-circumscribed plaque with a waxy yellowish atrophic center, often with telangiectases, and usually presenting in the pretibial area. Lesions can become ulcerated in up to one-third of cases. Necrobiosis lipoidica also is defined by characteristic histologic findings, including important features such as palisaded granulomas arranged in a tierlike fashion, necrotizing vasculitis, collagen degradation, and panniculitis. Necrobiosis lipoidica is still relatively rare, developing in approximately 0.3% of patients with diabetes,24 though its relationship with insulin resistance and diabetes is strong. Approximately two-thirds of patients with necrobiosis lipoidica have diabetes and an even higher number go on to develop diabetes or have a positive family history of diabetes.
Although these figures are interesting, the data are nearly a half-century-old, and it is unclear if these findings still hold true today. The etiology of necrobiosis lipoidica also remains elusive, with theories focusing on the role of microangiopathy, immunoglobulin deposition leading to vasculitis, structural abnormalities in collagen or fibroblasts, and trauma; however, the true nature of this condition is likely some combination of these factors.25 These lesions are difficult to treat, especially at an advanced stage. Management with topical steroids to limit the inflammatory progression of the lesions is the mainstay of therapy.
Scleredema
Scleredema adultorum (Buschke disease) refers to indurated plaques over the posterior neck and upper back. It is usually thought of as 3 distinct forms. The form that is known to occur in diabetic patients is sometimes referred to as scleredema diabeticorum; the other 2 occur as postinfectious, usually Streptococcus, or malignancy-related forms. The prevalence of scleredema diabeticorum among diabetic individuals most frequently is reported as 2.5%26; however, it is worth noting that other estimates have been as high as 14%.27 Although there has been some correlation between poorly controlled NIDDM, treatment and tight glucose control does not seem to readily resolve these lesions with only few conflicting case studies serving as evidence for and against this premise.28-30 The lesions often are recalcitrant toward a wide variety of treatment approaches. Histopathologic analysis generally reveals a thickened dermis with large collagen bundles, with clear spaces between the collagen representing mucin and increased numbers of mast cells. Proposed mechanisms include stimulation of collagen synthesis by fibroblasts and retarded collagen degradation, likely due to excess glucose.31
Dermatoses Demonstrating an Association With Diabetes
Granuloma Annulare
Granuloma annulare (GA) is a dermatologic condition existing in numerous forms. The generalized form has been suggested to have some association with diabetes. The lesions of GA are classically round, flesh-colored to erythematous papules arising in the dermis that may start on the dorsal extremities where the localized form typically presents, though larger annular plaques or patches may exist in the generalized form. Histologically, GA has a characteristic granulomatous infiltrate and palisaded granulomatous dermatitis, depending on the stage of the evolution. Many studies dating back to the mid-20th century have attempted to elucidate a link between GA and diabetes, with numerous reports showing conflicting results across their study populations.32-36 This issue is further muddled by links between generalized GA and a host of other diseases, such as malignancy, thyroid disease, hepatitis, and human immunodeficiency virus infection. The usual course of GA is spontaneous resolution, including a peculiar phenomenon noted in the literature whereby biopsy of one of the lesions led to clearance of other lesions on the body.37 However, the generalized form may be more difficult to treat, with therapeutic approaches including topical steroids, light therapy, and systemic immunomodulators.
Lichen Planus
A recent small population study in Turkey demonstrated a strong relationship between lichen planus and abnormal glucose tolerance. In this study of 30 patients with lichen planus, approximately half (14/30) had abnormal glucose metabolism and a quarter (8/30) had known diabetes, but larger studies are needed to clarify this relationship.38 Prior to this report, a link between oral lichen planus and diabetes had been shown in larger case series.39,40 Clinically, one may see white plaques with a characteristic lacy reticular pattern in the mouth. At other cutaneous sites, lichen planus generally appears as pruritic, purple, flat-topped polygonal papules. The clinical finding of lichen planus also is linked with many other disease states, most notably hepatitis C virus, but also thymoma, liver disease, and inflammatory bowel disease, among other associations.41
Vitiligo
As an autoimmune entity, it stands to reason that vitiligo may be seen more commonly associated with insulin-dependent diabetes, which has been shown to hold true in one study, while no association was found between later-onset NIDDM and vitiligo.42 Given the nonspecific nature of this association and the relatively common presentation of vitiligo, no special consideration is likely needed when examining a patient with vitiligo, but its presence should remind the clinician that these autoimmune entities tend to travel together.
Acquired Perforating Dermatosis
Although the classic presentation of acquired perforating dermatosis (Kyrle disease) is linked to renal failure, diabetes also has been connected to its presentation. Extremely rare outside of the setting of chronic renal failure, acquired perforating dermatosis occurs in up to 10% of dialysis patients.43,44 It is characterized by papules with a central keratin plug, representing transepidermal elimination of keratin, collagen, and other cellular material; its etiology has not been elucidated. The connection between acquired perforating dermatosis and diabetes is not completely clear; it would seem that renal failure is a prerequisite for itspresentation. A large proportion of renal failure necessitating hemodialysis occurs in patients with diabetic nephropathy, which may explain the coincidence of diabetes, renal failure, and acquired perforating dermatosis.45 The presentation of this cutaneous finding should not, however, affect treatment of the underlying conditions. Symptom relief in the form of topical steroids can be used as a first-line treatment of these often pruritic lesions.
Eruptive Xanthomas
The link between diabetes and eruptive xanthomas seems to be a rather tenuous one, hinging on the fact that many diabetic patients have abnormalities in carbohydrate and lipid metabolism. A central feature of eruptive xanthomas is an elevation in triglycerides, which can occur in diabetes. Indeed, it has been estimated that only 0.1% of diabetics will develop eruptive xanthomas,46 and its main importance may be to prompt the physician to treat the hypertriglyceridemia and consider other concerning possibilities such as acute pancreatitis.
Psoriasis
Psoriasis is a common dermatologic condition that has been shown to have a far-reaching impact both on patients’ quality of life and cardiovascular risk profiles. Data have emerged linking psoriasis with diabetes as an independent risk factor47; although this retrospective study had its limitations, it certainly is interesting to note that patients with psoriasis may have an increased risk for developing diabetes. Perhaps more importantly, though, this study also implied that patients with severe psoriasis may present with diabetes that is more difficult to control, evidenced by increased treatment with systemic therapies as opposed to milder forms of intervention such as diet control.47 There almost certainly are other confounding factors and further studies would serve to reveal the strength of this association, but it is certainly an intriguing concept. Echoing these findings, a more recent nationwide study from Denmark demonstrated that psoriasis is associated with increased incidence of new-onset diabetes, adjusting for numerous confounding factors.48 The relationship between psoriasis and diabetes is worth noting as evidence continues to emerge.
Conclusion
Given the diverse cutaneous manifestations of diabetes, it is important to distinguish those that are directly related to diabetes from those that suggest there may be another underlying process. For example, a new patient presenting to a primary care physician with acanthosis nigricans and yellow nails should immediately trigger a test for a hemoglobin A1c (glycated hemoglobin) level to investigate for diabetes; however, clinicians also should be wary of patients with acanthosis nigricans who report early satiety, as this asso-ciation may be a sign of underlying malignancy. Conversely, the presence of yellow nails in a patient with chronic diabetes should not be ignored. The physician should consider onychomycosis and query the patient about possible respiratory symptoms. In the case of a multisystem disease such as diabetes, it may be challenging to reconcile seemingly disparate skin findings, but having a framework to approach the cutaneous manifestations of diabetes can help to properly identify and treat an individual patient’s afflictions.
Diabetes mellitus is a morbid and costly condition that carries a high burden of disease for patients (both with and without a diagnosis) and for society as a whole. The economic burden of diabetes in the United States recently was estimated at nearly $250 billion annually,1 and this number continues to rise. The cutaneous manifestations of diabetes are diverse and far-reaching, ranging from benign cosmetic concerns to severe dermatologic conditions. Given the wide range of etiology for diabetes mellitus and its existence on a spectrum of severity, it is perhaps not surprising that some of these entities are the subject of debate (vis-à-vis the strength of association between these skin conditions and diabetes) and can manifest in different forms. However, it is clear that the cutaneous manifestations of diabetes are equally as important to consider and manage as the systemic complications of the disease. In analyzing associations with diabetes, it is important to note that given such a high incidence of diabetes among the general population and its close association with other disease states, such as the metabolic syndrome, studies aimed at determining direct relationships to this entity must be well controlled for confounding factors, which may not even always be possible. Regardless, it is important for dermatologists and dermatology residents to recognize and understand the protean cutaneous manifestations of diabetes mellitus, and this column will explore skin findings that are characteristic of diabetes (Table 1) as well as other dermatoses with a reported but less clear association with diabetes (Table 2).


Skin Findings Characteristic of Diabetes
Diabetic Thick Skin
The association between diabetes and thick skin is well described as either a mobility-limiting affliction of the joints of the hands (cheiroarthropathy) or as an asymptomatic thickening of the skin. It has been estimated that 8% to 36% of patients with insulin-dependent diabetes develop some form of skin thickening2; one series also found this association to be true for patients with non–insulin-dependent diabetes mellitus (NIDDM).3 Skin thickening is readily observable on clinical presentation or ultrasonography, with increasing thickness in many cases associated with long-term disease progression. This increasing thickness was shown histopathologically to be a direct result of activated fibroblasts and increased collagen polymerization, with some similar features to progressive systemic sclerosis.4 Interestingly, even clinically normal skin showed some degree of fibroblast activation in diabetic patients, but collagen fibers in each case were smaller in diameter than those found in progressive systemic sclerosis. This finding clearly has implications on quality of life, as a lack of hand mobility due to the cheiroarthropathy can be severely disabling. Underlining the need for strict glycemic control, it has been suggested that tight control of blood sugar levels can lead to improvement in diabetic thick skin; however, reports of improvement are based on a small sample population.5 Huntley papules are localized to areas on the dorsum of the hands overlying the joints, demonstrating hyperkeratosis and enlarged dermal papillae.6 They also can be found in a minority of patients without diabetes. Interestingly, diabetic thick skin also has been associated with neurologic disorders in diabetes.7 Diabetic thick skin was found to be significantly (P<.05) correlated with diabetic neuropathy, independent of duration of diabetes, age, or glycosylated hemoglobin levels, though no causal or etiologic link between these entities has been proven.
Yellow Nails
Nail changes are well described in diabetes, ranging from periungual telangiectases to complications from infections, such as paronychia; however, a well-recognized finding, especially in elderly diabetic patients, is a characteristic yellowing of the nails, reported to affect up to 40% of patients with diabetes.8 The mechanism behind it likely includes accumulation of glycation end products, which also has been thought to lead to yellowing of the skin, and vascular impairment.9 These nails tend to exhibit slow growth, likely resulting from a nail matrix that is poorly supplied with blood, and also can be more curved than normal with longitudinal ridges (onychorrhexis).10 It is important, however, not to attribute yellow nails to diabetes without considering other causes of yellow nails, such as onychomycosis, yellow nail syndrome, and yellow nails associated with lymphedema or respiratory tract disease (eg, pleural effusion, bronchiectasis).11
Diabetic Dermopathy
Colloquially known as shin spots, diabetic dermopathy is perhaps the most common skin finding in this patient population, though it also can occur in up to 1 in 5 individuals without diabetes.12 Although it is very common, it is not a condition that should be overlooked, as numerous studies have shown an increase in microangiopathic complications such as retinopathy in patients with diabetic dermopathy.13,14 Although follow-up studies may be necessary to fully characterize the relationship between shin spots and diabetes, it certainly is reasonable to be more wary of diabetic patients presenting with many shin spots, as the general consensus is that these areas represent postinflammatory hyperpigmentation and cutaneous atrophy in the setting of poor vascular supply, which should prompt analysis of other areas that might be affected by poor vasculature, such as an ophthalmologic examination. Antecedent and perhaps unnoticed trauma has been implicated given a possible underlying neuropathy, but this theory has not been supported by studies.
Bullosis Diabeticorum
Bullosis diabeticorum is a rare but well-described occurrence of self-resolving, nonscarring blisters that arise on the extremities of diabetic patients. This entity should be distinguished from other primary autoimmune blistering disorders and from simple mechanobullous lesions. Several types of bullosis diabeticorum have been described, with the most classic form showing an intraepidermal cleavage plane.15 These lesions tend to resolve in weeks but can be recurrent. The location of the pathology underlines its nonscarring nature, though similar lesions have been reported showing a cleavage plane in the lamina lucida of the dermoepidermal junction, which underlines the confusion in the literature surrounding diabetic bullae.16 Some may even use this term interchangeably with trauma or friction-induced blisters, which diabetics may be prone to develop due to peripheral neuropathy. Confounding reports have stated there is a correlation between bullosis diabeticorum and neuropathy as well as the acral location of these blisters. Although many authors cite the incidence of bullosis diabeticorum being 0.5%,17 no population-based studies have confirmed this figure and some have speculated that the actual incidence is higher.18 In the end, the term bullosis diabeticorum is probably best reserved for a rapidly appearing blister on the extremities of diabetic patients with at most minimal trauma, with a lesion containing sterile fluid and negative immunofluorescence. The mechanism for these blisters is thought to be microangiopathy, with scant blood supply to the skin causing it to be more prone to acantholysis and blister formation.19 This theory was reinforced in a study showing a reduced threshold for suction blister formation in diabetic patients.20 Care should be taken to prevent secondary infections at these sites.
Acanthosis Nigricans
Acanthosis nigricans, which consists of dark brown plaques in the flexural areas, especially the posterior neck and axillae, is a common finding in diabetic patients and is no doubt familiar to clinicians. The pathophysiology of these lesions has been well studied and is a prototype for the effects of insulin resistance in the skin. In this model, high concentrations of insulin binding to insulinlike growth factor receptor in the skin stimulate keratinocyte proliferation,21 leading to the clinical appearance and the histologic finding of hyperkeratosis and papillomatosis, which in turn is responsible for the observed hyperpigmentation. It is an important finding, especially in those without a known history of diabetes, as it can also signal an underlying endocrinopathy (eg, Cushing syndrome, acromegaly, polycystic ovary syndrome) or malignancy (ie, adenocarcinoma of the gastrointestinal tract). Several distinct mechanisms of insulin resistance have been described, including insulin resistance due to receptor defects, such as those seen with insulin resistance in NIDDM; autoimmune processes; and postreceptor defects in insulin action.22 Keratolytics and topical retinoids have been used to ameliorate the appearance of these lesions.
Necrobiosis Lipoidica (Diabeticorum)
Necrobiosis lipoidica diabeticorum was first described by Urback23 in 1932, but reports of similar lesions were described in nondiabetic patients soon after. The dermatologic community has since come to realize that perhaps a more accurate nomenclature is necrobiosis lipoidica to fully encompass this entity. Clinically, lesions appear as erythematous papules and plaques that expand into a larger well-circumscribed plaque with a waxy yellowish atrophic center, often with telangiectases, and usually presenting in the pretibial area. Lesions can become ulcerated in up to one-third of cases. Necrobiosis lipoidica also is defined by characteristic histologic findings, including important features such as palisaded granulomas arranged in a tierlike fashion, necrotizing vasculitis, collagen degradation, and panniculitis. Necrobiosis lipoidica is still relatively rare, developing in approximately 0.3% of patients with diabetes,24 though its relationship with insulin resistance and diabetes is strong. Approximately two-thirds of patients with necrobiosis lipoidica have diabetes and an even higher number go on to develop diabetes or have a positive family history of diabetes.
Although these figures are interesting, the data are nearly a half-century-old, and it is unclear if these findings still hold true today. The etiology of necrobiosis lipoidica also remains elusive, with theories focusing on the role of microangiopathy, immunoglobulin deposition leading to vasculitis, structural abnormalities in collagen or fibroblasts, and trauma; however, the true nature of this condition is likely some combination of these factors.25 These lesions are difficult to treat, especially at an advanced stage. Management with topical steroids to limit the inflammatory progression of the lesions is the mainstay of therapy.
Scleredema
Scleredema adultorum (Buschke disease) refers to indurated plaques over the posterior neck and upper back. It is usually thought of as 3 distinct forms. The form that is known to occur in diabetic patients is sometimes referred to as scleredema diabeticorum; the other 2 occur as postinfectious, usually Streptococcus, or malignancy-related forms. The prevalence of scleredema diabeticorum among diabetic individuals most frequently is reported as 2.5%26; however, it is worth noting that other estimates have been as high as 14%.27 Although there has been some correlation between poorly controlled NIDDM, treatment and tight glucose control does not seem to readily resolve these lesions with only few conflicting case studies serving as evidence for and against this premise.28-30 The lesions often are recalcitrant toward a wide variety of treatment approaches. Histopathologic analysis generally reveals a thickened dermis with large collagen bundles, with clear spaces between the collagen representing mucin and increased numbers of mast cells. Proposed mechanisms include stimulation of collagen synthesis by fibroblasts and retarded collagen degradation, likely due to excess glucose.31
Dermatoses Demonstrating an Association With Diabetes
Granuloma Annulare
Granuloma annulare (GA) is a dermatologic condition existing in numerous forms. The generalized form has been suggested to have some association with diabetes. The lesions of GA are classically round, flesh-colored to erythematous papules arising in the dermis that may start on the dorsal extremities where the localized form typically presents, though larger annular plaques or patches may exist in the generalized form. Histologically, GA has a characteristic granulomatous infiltrate and palisaded granulomatous dermatitis, depending on the stage of the evolution. Many studies dating back to the mid-20th century have attempted to elucidate a link between GA and diabetes, with numerous reports showing conflicting results across their study populations.32-36 This issue is further muddled by links between generalized GA and a host of other diseases, such as malignancy, thyroid disease, hepatitis, and human immunodeficiency virus infection. The usual course of GA is spontaneous resolution, including a peculiar phenomenon noted in the literature whereby biopsy of one of the lesions led to clearance of other lesions on the body.37 However, the generalized form may be more difficult to treat, with therapeutic approaches including topical steroids, light therapy, and systemic immunomodulators.
Lichen Planus
A recent small population study in Turkey demonstrated a strong relationship between lichen planus and abnormal glucose tolerance. In this study of 30 patients with lichen planus, approximately half (14/30) had abnormal glucose metabolism and a quarter (8/30) had known diabetes, but larger studies are needed to clarify this relationship.38 Prior to this report, a link between oral lichen planus and diabetes had been shown in larger case series.39,40 Clinically, one may see white plaques with a characteristic lacy reticular pattern in the mouth. At other cutaneous sites, lichen planus generally appears as pruritic, purple, flat-topped polygonal papules. The clinical finding of lichen planus also is linked with many other disease states, most notably hepatitis C virus, but also thymoma, liver disease, and inflammatory bowel disease, among other associations.41
Vitiligo
As an autoimmune entity, it stands to reason that vitiligo may be seen more commonly associated with insulin-dependent diabetes, which has been shown to hold true in one study, while no association was found between later-onset NIDDM and vitiligo.42 Given the nonspecific nature of this association and the relatively common presentation of vitiligo, no special consideration is likely needed when examining a patient with vitiligo, but its presence should remind the clinician that these autoimmune entities tend to travel together.
Acquired Perforating Dermatosis
Although the classic presentation of acquired perforating dermatosis (Kyrle disease) is linked to renal failure, diabetes also has been connected to its presentation. Extremely rare outside of the setting of chronic renal failure, acquired perforating dermatosis occurs in up to 10% of dialysis patients.43,44 It is characterized by papules with a central keratin plug, representing transepidermal elimination of keratin, collagen, and other cellular material; its etiology has not been elucidated. The connection between acquired perforating dermatosis and diabetes is not completely clear; it would seem that renal failure is a prerequisite for itspresentation. A large proportion of renal failure necessitating hemodialysis occurs in patients with diabetic nephropathy, which may explain the coincidence of diabetes, renal failure, and acquired perforating dermatosis.45 The presentation of this cutaneous finding should not, however, affect treatment of the underlying conditions. Symptom relief in the form of topical steroids can be used as a first-line treatment of these often pruritic lesions.
Eruptive Xanthomas
The link between diabetes and eruptive xanthomas seems to be a rather tenuous one, hinging on the fact that many diabetic patients have abnormalities in carbohydrate and lipid metabolism. A central feature of eruptive xanthomas is an elevation in triglycerides, which can occur in diabetes. Indeed, it has been estimated that only 0.1% of diabetics will develop eruptive xanthomas,46 and its main importance may be to prompt the physician to treat the hypertriglyceridemia and consider other concerning possibilities such as acute pancreatitis.
Psoriasis
Psoriasis is a common dermatologic condition that has been shown to have a far-reaching impact both on patients’ quality of life and cardiovascular risk profiles. Data have emerged linking psoriasis with diabetes as an independent risk factor47; although this retrospective study had its limitations, it certainly is interesting to note that patients with psoriasis may have an increased risk for developing diabetes. Perhaps more importantly, though, this study also implied that patients with severe psoriasis may present with diabetes that is more difficult to control, evidenced by increased treatment with systemic therapies as opposed to milder forms of intervention such as diet control.47 There almost certainly are other confounding factors and further studies would serve to reveal the strength of this association, but it is certainly an intriguing concept. Echoing these findings, a more recent nationwide study from Denmark demonstrated that psoriasis is associated with increased incidence of new-onset diabetes, adjusting for numerous confounding factors.48 The relationship between psoriasis and diabetes is worth noting as evidence continues to emerge.
Conclusion
Given the diverse cutaneous manifestations of diabetes, it is important to distinguish those that are directly related to diabetes from those that suggest there may be another underlying process. For example, a new patient presenting to a primary care physician with acanthosis nigricans and yellow nails should immediately trigger a test for a hemoglobin A1c (glycated hemoglobin) level to investigate for diabetes; however, clinicians also should be wary of patients with acanthosis nigricans who report early satiety, as this asso-ciation may be a sign of underlying malignancy. Conversely, the presence of yellow nails in a patient with chronic diabetes should not be ignored. The physician should consider onychomycosis and query the patient about possible respiratory symptoms. In the case of a multisystem disease such as diabetes, it may be challenging to reconcile seemingly disparate skin findings, but having a framework to approach the cutaneous manifestations of diabetes can help to properly identify and treat an individual patient’s afflictions.
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012 [published online instead of print March 16, 2013]. Diabetes Care. 2013;36:1033-1046.
- Collier A, Matthews DM, Kellett HA, et al. Change in skin thickness associated with cheiroarthropathy in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed). 1986;292:936.
- Fitzcharles MA, Duby S, Waddell RW, et al. Limitation of joint mobility (cheiroarthropathy) in adult noninsulin-dependent diabetic patients. Ann Rheum Dis. 1984;43:251-254.
- Hanna W, Friesen D, Bombardier C, et al. Pathologic features of diabetic thick skin. J Am Acad Dermatol. 1987;16:546-553.
- Lieberman LS, Rosenbloom AL, Riley WJ, et al. Reduced skin thickness with pump administration of insulin. N Engl J Med. 1980;303:940-941.
- Guarneri C, Guarneri F, Borgia F, et al. Finger pebbles in a diabetic patient: Huntley's papules. Int J Dermatol. 2005;44:755-756.
- Forst T, Kann P, Pfützner A, et al. Association between "diabetic thick skin syndrome" and neurological disorders in diabetes mellitus. Acta Diabetol. 1994;31:73-77.
- Nikoleishvili LR, Kurashvili RB, Virsaladze DK, et al. Characteristic changes of skin and its accessories in type 2 diabetes mellitus [in Russian]. Georgian Med News. 2006:43-46.
- Lithner F. Purpura, pigmentation and yellow nails of the lower extremities in diabetics. Acta Med Scand. 1976;199:203-208.
- Greene RA, Scher RK. Nail changes associated with diabetes mellitus. J Am Acad Dermatol. 1987;16:1015-1021.
- Hiller E, Rosenow EC 3rd, Olsen AM. Pulmonary manifestations of the yellow nail syndrome. Chest. 1972;61:452-458.
- Feingold KR, Elias PM. Endocrine-skin interactions. cutaneous manifestations of pituitary disease, thyroid disease, calcium disorders, and diabetes. J Am Acad Dermatol. 1987;17:921-940.
- Abdollahi A, Daneshpazhooh M, Amirchaghmaghi E, et al. Dermopathy and retinopathy in diabetes: is there an association? Dermatology, 2007;214:133-136.
- Morgan AJ, Schwartz RA. Diabetic dermopathy: A subtle sign with grave implications. J Am Acad Dermatol. 2008;58:447-451.
- Perez MI, Kohn SR. Cutaneous manifestations of diabetes mellitus. J Am Acad Dermatol. 1994;30:519-531.
- Cantwell AR, Martz W. Idiopathic bullae in diabetics. Bullosis diabeticorum. Arch Dermatol. 1967;96:42-44.
- Larsen K, Jensen T, Karlsmark T, Holstein PE. Incidence of bullosis diabeticorum – a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596.
- Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200.
- Basarab T, Munn SE, McGrath J, et al. Bullosis diabeticorum. a case report and literature review. Clin Exp Dermatol. 1995;20:218-220.
- Bernstein JE, Levine LE, Medenica MM, et al. Reduced threshold to suction-induced blister formation in insulin-dependent diabetics. J Am Acad Dermatol. 1983;8:790-791.
- Cruz PD Jr, Hud JA Jr. Excess insulin binding to insulin-like growth factor receptors: proposed mechanism for acanthosis nigricans. J Invest Dermatol. 1992;98(suppl 6):S82-S85.
- Romano G, Moretti G, Di Benedetto A, et al. Skin lesions in diabetes mellitus: prevalence and clinical correlations. Diabetes Res Clin Pract. 1998;39:101-106.
- Urback E. Eine neue diabetische Stoffwechseldermatose: Nekrobiosis lipoidica diabeticorum. Arch. f. Dermat. u Syph. 1932;166:273.
- Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum. a clinical and pathological investigation of 171 cases. Arch Dermatol. 1966;93:272-281.
- Engel MF, Smith JG Jr. The pathogenesis of necrobiosis lipoidica. necrobiosis lipoidica, a form fruste of diabetes mellitus. Arch Dermatol. 1960;82:791-797.
- Cole GW, Headley J, Skowsky R. Scleredema diabeticorum: a common and distinct cutaneous manifestation of diabetes mellitus. Diabetes Care. 1983;6:189-192.
- Sattar MA, Diab S, Sugathan TN, et al. Scleroedema diabeticorum: a minor but often unrecognized complication of diabetes mellitus. Diabet Med. 1988;5:465-468.
- Rho YW, Suhr KB, Lee JH, et al. A clinical observation of scleredema adultorum and its relationship to diabetes. J Dermatol. 1998;25:103-107.
- Baillot-Rudoni S, Apostol D, Vaillant G, et al. Implantable pump therapy restores metabolic control and quality of life in type 1 diabetic patients with Buschke's nonsystemic scleroderma. Diabetes Care. 2006;29:1710.
- Meguerditchian C, Jacquet P, Béliard S, et al. Scleredema adultorum of Buschke: an under recognized skin complication of diabetes. Diabetes Metab. 2006;32:481-484.
- Behm B, Schreml S, Landthaler M, et al. Skin signs in diabetes mellitus. J Eur Acad Dermatol Venereol. 2012;26:1203-1211.
- Nebesio CL, Lewis C, Chuang TY. Lack of an association between granuloma annulare and type 2 diabetes mellitus. Br J Dermatol. 2002;146:122-124.
- Stankler L, Leslie G. Generalized granuloma annulare. a report of a case and review of the literature. Arch Dermatol. 1976;95:509-513.
- Williamson DM, Dykes JR. Carbohydrate metabolism in granuloma annulare. J Invest Dermatol. 1972;58:400-404.
- Dabski K, Winkelmann RK. Generalized granuloma annulare: clinical and laboratory findings in 100 patients. J Am Acad Dermatol. 1989;20:39-47.
- Veraldi S, Bencini PL, Drudi E, et al. Laboratory abnormalities in granuloma annulare: a case-control study. Br J Dermatol. 1997;136:652-653.
- Levin NA, Patterson JW, Yao LL, et al. Resolution of patch-type granuloma annulare lesions after biopsy. J Am Acad Dermatol. 2002;46:426-429.
- Seyhan M, Ozcan H, Sahin I, et al. High prevalence of glucose metabolism disturbance in patients with lichen planus. Diabetes Res Clin Pract. 2007;77:198-202.
- Romero MA, Seoane J, Varela-Centelles P, et al. Prevalence of diabetes mellitus amongst oral lichen planus patients. clinical and pathological characteristics. Med Oral. 2002;7:121-129.
- Albrecht M, Banoczy J, Dinya E, et al. Occurrence of oral leukoplakia and lichen planus in diabetes mellitus. J Oral Pathol Med. 1992;21:364-366.
- Lehman JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48:682-694.
- Gould IM, Gray RS, Urbaniak SJ, et al. Vitiligo in diabetes mellitus. Br J Dermatol. 1985;113:153-155.
- White CR Jr, Heskel NS, Pokorny DJ. Perforating folliculitis of hemodialysis. Am J Dermatopathol. 1982;4:109-116.
- Hurwitz RM, Melton ME, Creech FT 3rd, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
- Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989;125:1074-1078.
- Muller SA. Dermatologic disorders associated with diabetes mellitus. Mayo Clin Proc. 1966;41:689-703.
- Azfar RS, Seminara NM, Shin DB, et al. Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol. 2012;148:995-1000.
- Khalid U, Hansen PR, Gislason GH, et al. Psoriasis and new-onset diabetes: a Danish nationwide cohort study. Diabetes Care. 2013;36:2402-2407.
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012 [published online instead of print March 16, 2013]. Diabetes Care. 2013;36:1033-1046.
- Collier A, Matthews DM, Kellett HA, et al. Change in skin thickness associated with cheiroarthropathy in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed). 1986;292:936.
- Fitzcharles MA, Duby S, Waddell RW, et al. Limitation of joint mobility (cheiroarthropathy) in adult noninsulin-dependent diabetic patients. Ann Rheum Dis. 1984;43:251-254.
- Hanna W, Friesen D, Bombardier C, et al. Pathologic features of diabetic thick skin. J Am Acad Dermatol. 1987;16:546-553.
- Lieberman LS, Rosenbloom AL, Riley WJ, et al. Reduced skin thickness with pump administration of insulin. N Engl J Med. 1980;303:940-941.
- Guarneri C, Guarneri F, Borgia F, et al. Finger pebbles in a diabetic patient: Huntley's papules. Int J Dermatol. 2005;44:755-756.
- Forst T, Kann P, Pfützner A, et al. Association between "diabetic thick skin syndrome" and neurological disorders in diabetes mellitus. Acta Diabetol. 1994;31:73-77.
- Nikoleishvili LR, Kurashvili RB, Virsaladze DK, et al. Characteristic changes of skin and its accessories in type 2 diabetes mellitus [in Russian]. Georgian Med News. 2006:43-46.
- Lithner F. Purpura, pigmentation and yellow nails of the lower extremities in diabetics. Acta Med Scand. 1976;199:203-208.
- Greene RA, Scher RK. Nail changes associated with diabetes mellitus. J Am Acad Dermatol. 1987;16:1015-1021.
- Hiller E, Rosenow EC 3rd, Olsen AM. Pulmonary manifestations of the yellow nail syndrome. Chest. 1972;61:452-458.
- Feingold KR, Elias PM. Endocrine-skin interactions. cutaneous manifestations of pituitary disease, thyroid disease, calcium disorders, and diabetes. J Am Acad Dermatol. 1987;17:921-940.
- Abdollahi A, Daneshpazhooh M, Amirchaghmaghi E, et al. Dermopathy and retinopathy in diabetes: is there an association? Dermatology, 2007;214:133-136.
- Morgan AJ, Schwartz RA. Diabetic dermopathy: A subtle sign with grave implications. J Am Acad Dermatol. 2008;58:447-451.
- Perez MI, Kohn SR. Cutaneous manifestations of diabetes mellitus. J Am Acad Dermatol. 1994;30:519-531.
- Cantwell AR, Martz W. Idiopathic bullae in diabetics. Bullosis diabeticorum. Arch Dermatol. 1967;96:42-44.
- Larsen K, Jensen T, Karlsmark T, Holstein PE. Incidence of bullosis diabeticorum – a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596.
- Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200.
- Basarab T, Munn SE, McGrath J, et al. Bullosis diabeticorum. a case report and literature review. Clin Exp Dermatol. 1995;20:218-220.
- Bernstein JE, Levine LE, Medenica MM, et al. Reduced threshold to suction-induced blister formation in insulin-dependent diabetics. J Am Acad Dermatol. 1983;8:790-791.
- Cruz PD Jr, Hud JA Jr. Excess insulin binding to insulin-like growth factor receptors: proposed mechanism for acanthosis nigricans. J Invest Dermatol. 1992;98(suppl 6):S82-S85.
- Romano G, Moretti G, Di Benedetto A, et al. Skin lesions in diabetes mellitus: prevalence and clinical correlations. Diabetes Res Clin Pract. 1998;39:101-106.
- Urback E. Eine neue diabetische Stoffwechseldermatose: Nekrobiosis lipoidica diabeticorum. Arch. f. Dermat. u Syph. 1932;166:273.
- Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum. a clinical and pathological investigation of 171 cases. Arch Dermatol. 1966;93:272-281.
- Engel MF, Smith JG Jr. The pathogenesis of necrobiosis lipoidica. necrobiosis lipoidica, a form fruste of diabetes mellitus. Arch Dermatol. 1960;82:791-797.
- Cole GW, Headley J, Skowsky R. Scleredema diabeticorum: a common and distinct cutaneous manifestation of diabetes mellitus. Diabetes Care. 1983;6:189-192.
- Sattar MA, Diab S, Sugathan TN, et al. Scleroedema diabeticorum: a minor but often unrecognized complication of diabetes mellitus. Diabet Med. 1988;5:465-468.
- Rho YW, Suhr KB, Lee JH, et al. A clinical observation of scleredema adultorum and its relationship to diabetes. J Dermatol. 1998;25:103-107.
- Baillot-Rudoni S, Apostol D, Vaillant G, et al. Implantable pump therapy restores metabolic control and quality of life in type 1 diabetic patients with Buschke's nonsystemic scleroderma. Diabetes Care. 2006;29:1710.
- Meguerditchian C, Jacquet P, Béliard S, et al. Scleredema adultorum of Buschke: an under recognized skin complication of diabetes. Diabetes Metab. 2006;32:481-484.
- Behm B, Schreml S, Landthaler M, et al. Skin signs in diabetes mellitus. J Eur Acad Dermatol Venereol. 2012;26:1203-1211.
- Nebesio CL, Lewis C, Chuang TY. Lack of an association between granuloma annulare and type 2 diabetes mellitus. Br J Dermatol. 2002;146:122-124.
- Stankler L, Leslie G. Generalized granuloma annulare. a report of a case and review of the literature. Arch Dermatol. 1976;95:509-513.
- Williamson DM, Dykes JR. Carbohydrate metabolism in granuloma annulare. J Invest Dermatol. 1972;58:400-404.
- Dabski K, Winkelmann RK. Generalized granuloma annulare: clinical and laboratory findings in 100 patients. J Am Acad Dermatol. 1989;20:39-47.
- Veraldi S, Bencini PL, Drudi E, et al. Laboratory abnormalities in granuloma annulare: a case-control study. Br J Dermatol. 1997;136:652-653.
- Levin NA, Patterson JW, Yao LL, et al. Resolution of patch-type granuloma annulare lesions after biopsy. J Am Acad Dermatol. 2002;46:426-429.
- Seyhan M, Ozcan H, Sahin I, et al. High prevalence of glucose metabolism disturbance in patients with lichen planus. Diabetes Res Clin Pract. 2007;77:198-202.
- Romero MA, Seoane J, Varela-Centelles P, et al. Prevalence of diabetes mellitus amongst oral lichen planus patients. clinical and pathological characteristics. Med Oral. 2002;7:121-129.
- Albrecht M, Banoczy J, Dinya E, et al. Occurrence of oral leukoplakia and lichen planus in diabetes mellitus. J Oral Pathol Med. 1992;21:364-366.
- Lehman JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48:682-694.
- Gould IM, Gray RS, Urbaniak SJ, et al. Vitiligo in diabetes mellitus. Br J Dermatol. 1985;113:153-155.
- White CR Jr, Heskel NS, Pokorny DJ. Perforating folliculitis of hemodialysis. Am J Dermatopathol. 1982;4:109-116.
- Hurwitz RM, Melton ME, Creech FT 3rd, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
- Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989;125:1074-1078.
- Muller SA. Dermatologic disorders associated with diabetes mellitus. Mayo Clin Proc. 1966;41:689-703.
- Azfar RS, Seminara NM, Shin DB, et al. Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol. 2012;148:995-1000.
- Khalid U, Hansen PR, Gislason GH, et al. Psoriasis and new-onset diabetes: a Danish nationwide cohort study. Diabetes Care. 2013;36:2402-2407.
Experts disagree on skin endpoint in psoriatic arthritis
NEW YORK – For the skin component of psoriatic arthritis, consensus about how high to set the bar for disease control continues to elude experts, judging from a debate about the value of "clear" or "almost clear" as the most acceptable treatment endpoints.
Current therapies in psoriatic arthritis (PsA) are sufficiently effective to support a treat-to-target approach for both skin lesions and joint inflammation, but there is lack of consensus about which metrics should be employed to define any specific target for skin lesions, according to Dr. Joel Gelfand, medical director of the clinical studies unit in the department of dermatology at the University of Pennsylvania, Philadelphia. He noted that even if the goal is the absence of skin involvement, no one agrees on what method should be used to document this endpoint.
"No one in clinical practice uses PASI," maintained Dr. Gelfand, referring to the Psoriasis and Area and Severity Index, which is one of the most widely accepted standards for judging efficacy in treatment trials. Speaking at the joint meetings of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the Spondyloarthritis Research & Treatment Network, Dr. Gelfand said most objective scoring systems, such as PASI, are too labor intensive. Clinicians tend to use their own system for documenting relative control of skin lesions in the medical record.
Evaluating disease control without applying a reproducible, broadly used metric has numerous potential problems, according to Dr. Gelfand. Most importantly, it prevents objective evaluation of whether treatment targets are being met. This obscures efforts to document benefit relative to either clinical goals or optimal care. Dr. Gelfand made the analogy to the treatment goals created for blood pressure, blood glucose, or cholesterol, where the targets are clear and a change in treatment is warranted if the target is not reached.
This point of view resonated with the audience of dermatologists, rheumatologists, and PsA patients attending the meeting. In an electronic audience poll, there was nearly 100% agreement that a treat-to-target approach is appropriate for skin lesions but no strong consensus on what the target should be. In the poll attempting to define consensus, 32% agreed with a target of less than 3% body surface area (BSA) while 48% agreed the BSA target should be less than 1%. It is notable that dermatologists were more likely to accept a less rigorous BSA than rheumatologists. The majority of patients agreed the target should be less than 1% BSA, although this group represented less than 10% of the audience.
Not surprisingly, health-related quality of life studies show measurable differences between "clear" and "almost clear" skin lesions in PsA patients whether measured by PASI, BSA, or other measures, according to Dr. Gelfand, but he cautioned that it is important to be realistic about goals. He suggested that a target that exposes patients to unacceptable side effects is the wrong target, and this may be the source of dissension among experts.
Asked for a comment, Dr. Wolf-Henning Boehncke, professor and chair of the department of dermatology at the University of Geneva agreed in principle with the treat-to-target approach. However, Dr. Boehncke, who is the current president of GRAPPA, also emphasized simple and practical assessment tools and achievable endpoints.
"We must define targets that are reasonable," Dr. Boehncke said. While complex patient assessment tools are not likely to be widely used by clinicians, unrealistic targets can be counterproductive if they are not considered in the overall context of tolerability and patient satisfaction.
Dr. Gelfand has or has had financial relationships with AbbVie, Amgen, Celgene, Eli Lilly, Genentech, Janssen, Merck, Novartis, and Pfizer.
NEW YORK – For the skin component of psoriatic arthritis, consensus about how high to set the bar for disease control continues to elude experts, judging from a debate about the value of "clear" or "almost clear" as the most acceptable treatment endpoints.
Current therapies in psoriatic arthritis (PsA) are sufficiently effective to support a treat-to-target approach for both skin lesions and joint inflammation, but there is lack of consensus about which metrics should be employed to define any specific target for skin lesions, according to Dr. Joel Gelfand, medical director of the clinical studies unit in the department of dermatology at the University of Pennsylvania, Philadelphia. He noted that even if the goal is the absence of skin involvement, no one agrees on what method should be used to document this endpoint.
"No one in clinical practice uses PASI," maintained Dr. Gelfand, referring to the Psoriasis and Area and Severity Index, which is one of the most widely accepted standards for judging efficacy in treatment trials. Speaking at the joint meetings of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the Spondyloarthritis Research & Treatment Network, Dr. Gelfand said most objective scoring systems, such as PASI, are too labor intensive. Clinicians tend to use their own system for documenting relative control of skin lesions in the medical record.
Evaluating disease control without applying a reproducible, broadly used metric has numerous potential problems, according to Dr. Gelfand. Most importantly, it prevents objective evaluation of whether treatment targets are being met. This obscures efforts to document benefit relative to either clinical goals or optimal care. Dr. Gelfand made the analogy to the treatment goals created for blood pressure, blood glucose, or cholesterol, where the targets are clear and a change in treatment is warranted if the target is not reached.
This point of view resonated with the audience of dermatologists, rheumatologists, and PsA patients attending the meeting. In an electronic audience poll, there was nearly 100% agreement that a treat-to-target approach is appropriate for skin lesions but no strong consensus on what the target should be. In the poll attempting to define consensus, 32% agreed with a target of less than 3% body surface area (BSA) while 48% agreed the BSA target should be less than 1%. It is notable that dermatologists were more likely to accept a less rigorous BSA than rheumatologists. The majority of patients agreed the target should be less than 1% BSA, although this group represented less than 10% of the audience.
Not surprisingly, health-related quality of life studies show measurable differences between "clear" and "almost clear" skin lesions in PsA patients whether measured by PASI, BSA, or other measures, according to Dr. Gelfand, but he cautioned that it is important to be realistic about goals. He suggested that a target that exposes patients to unacceptable side effects is the wrong target, and this may be the source of dissension among experts.
Asked for a comment, Dr. Wolf-Henning Boehncke, professor and chair of the department of dermatology at the University of Geneva agreed in principle with the treat-to-target approach. However, Dr. Boehncke, who is the current president of GRAPPA, also emphasized simple and practical assessment tools and achievable endpoints.
"We must define targets that are reasonable," Dr. Boehncke said. While complex patient assessment tools are not likely to be widely used by clinicians, unrealistic targets can be counterproductive if they are not considered in the overall context of tolerability and patient satisfaction.
Dr. Gelfand has or has had financial relationships with AbbVie, Amgen, Celgene, Eli Lilly, Genentech, Janssen, Merck, Novartis, and Pfizer.
NEW YORK – For the skin component of psoriatic arthritis, consensus about how high to set the bar for disease control continues to elude experts, judging from a debate about the value of "clear" or "almost clear" as the most acceptable treatment endpoints.
Current therapies in psoriatic arthritis (PsA) are sufficiently effective to support a treat-to-target approach for both skin lesions and joint inflammation, but there is lack of consensus about which metrics should be employed to define any specific target for skin lesions, according to Dr. Joel Gelfand, medical director of the clinical studies unit in the department of dermatology at the University of Pennsylvania, Philadelphia. He noted that even if the goal is the absence of skin involvement, no one agrees on what method should be used to document this endpoint.
"No one in clinical practice uses PASI," maintained Dr. Gelfand, referring to the Psoriasis and Area and Severity Index, which is one of the most widely accepted standards for judging efficacy in treatment trials. Speaking at the joint meetings of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the Spondyloarthritis Research & Treatment Network, Dr. Gelfand said most objective scoring systems, such as PASI, are too labor intensive. Clinicians tend to use their own system for documenting relative control of skin lesions in the medical record.
Evaluating disease control without applying a reproducible, broadly used metric has numerous potential problems, according to Dr. Gelfand. Most importantly, it prevents objective evaluation of whether treatment targets are being met. This obscures efforts to document benefit relative to either clinical goals or optimal care. Dr. Gelfand made the analogy to the treatment goals created for blood pressure, blood glucose, or cholesterol, where the targets are clear and a change in treatment is warranted if the target is not reached.
This point of view resonated with the audience of dermatologists, rheumatologists, and PsA patients attending the meeting. In an electronic audience poll, there was nearly 100% agreement that a treat-to-target approach is appropriate for skin lesions but no strong consensus on what the target should be. In the poll attempting to define consensus, 32% agreed with a target of less than 3% body surface area (BSA) while 48% agreed the BSA target should be less than 1%. It is notable that dermatologists were more likely to accept a less rigorous BSA than rheumatologists. The majority of patients agreed the target should be less than 1% BSA, although this group represented less than 10% of the audience.
Not surprisingly, health-related quality of life studies show measurable differences between "clear" and "almost clear" skin lesions in PsA patients whether measured by PASI, BSA, or other measures, according to Dr. Gelfand, but he cautioned that it is important to be realistic about goals. He suggested that a target that exposes patients to unacceptable side effects is the wrong target, and this may be the source of dissension among experts.
Asked for a comment, Dr. Wolf-Henning Boehncke, professor and chair of the department of dermatology at the University of Geneva agreed in principle with the treat-to-target approach. However, Dr. Boehncke, who is the current president of GRAPPA, also emphasized simple and practical assessment tools and achievable endpoints.
"We must define targets that are reasonable," Dr. Boehncke said. While complex patient assessment tools are not likely to be widely used by clinicians, unrealistic targets can be counterproductive if they are not considered in the overall context of tolerability and patient satisfaction.
Dr. Gelfand has or has had financial relationships with AbbVie, Amgen, Celgene, Eli Lilly, Genentech, Janssen, Merck, Novartis, and Pfizer.
AT THE 2014 GRAPPA AND SPARTAN ANNUAL MEETINGS
Key clinical point: Experts in psoriatic arthritis agree that a treat-to-target approach should be applied to skin involvement but remain divided about how the targets should be defined and measured.
Major finding: In the poll attempting to define consensus, 32% agreed with a target of less than 3% body surface area (BSA) while 48% agreed the BSA target should be less than 1%.
Data source: Consensus review of outcome tools.
Disclosures: Dr. Joel Gelfand has or has had financial relationships with AbbVie, Amgen, Celgene, Eli Lilly, Genentech, Janssen, Merck, Novartis, and Pfizer.
Secukinumab bests etanercept, placebo for sustained improvement of psoriasis
At least two-thirds of adults with plaque psoriasis treated with the fully human anti-interleukin-17A monoclonal antibody secukinumab achieved PASI 75 at 12 weeks in two related randomized phase III trials. The findings were reported online July 9 in the New England Journal of Medicine.
In the ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) trial, a multicenter, double-blind study involving 738 patients, the proportions of patients who achieved a 75% or greater reduction in Psoriasis Area and Severity Index (PASI 75) score at 12 weeks were 81.6%, 71.6%, and 4.5% with 300 mg of secukinumab, 150 mg of secukinumab, and placebo, respectively.
In the FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using Two Dosing Regimens) study, a multicenter, double-blind, active-controlled study involving 1,306 patients, the proportions meeting PASI 75 were 77.1% and 67% for 300 and 150 mg, respectively, compared with 44% for etanercept and 4.9% for placebo.
The difference between each secukinumab dose and its comparators was statistically significant in both studies, Dr. Richard G. Langley of Dalhousie University, Halifax, N.S., Dr. Boni Elewski of the University of Alabama, Birmingham, and their colleagues reported on behalf of the ERASURE and FIXTURE Study Groups.
The investigators also found that the proportions of patients with a response of 0 or 1 on the modified investigator’s global assessment at 12 weeks were 65.3%, 51.2%, and 2.4% for the 300- and 150-mg secukinumab doses and placebo, respectively, in ERASURE; and 62.5% and 51.1% for the 300- and 150-mg doses, 27.2% for etanercept, and 2.8% for placebo in FIXTURE (N. Engl. J. Med. 2014 July 9 [doi: 10.1056/NE/NEJMoa314258]).
The differences between each secukinumab dose and its comparators were significant for this measure as well, the investigators reported.
Secukinumab at the 300- and 150-mg doses in both ERASURE and FIXTURE was also superior to placebo (and in FIXTURE, superior to etanercept) for all secondary efficacy endpoints, including PASI 90 and PASI 100 response at week 12; patient reports of itching, pain, and scaling on the Psoriasis Symptom Diary at week 12; and Dermatology Life Quality Index scores.
Both ERASURE and FIXTURE were designed to evaluate the safety of secukinumab as induction therapy with assessment at week 12, and as maintenance therapy with assessment at week 52. Subjects were aged 18 years or older with poorly controlled, moderate to severe plaque psoriasis that had been diagnosed at least 6 months prior, and with a PASI score of 12 or higher, a modified investigator’s global assessment score of 3 or 4, and involvement of 10% or more of their body surface area.
ERASURE was conducted from June 2011 to April 2013 at 88 sites worldwide, and FIXTURE was conducted from June 2011 to June 2013 at 231 sites. Patients in the 300-mg groups received two 150-mg subcutaneous injections of secukinumab, and those in the 150-mg group received one 150-mg injection of secukinumab, and one placebo injection. Etanercept was given at a dose of 50 mg administered subcutaneously twice weekly from baseline until week 12, and then once weekly through week 51.
Adverse events were more common in patients treated with secukinumab in ERASURE; 55% of patients in the 300-mg group, 60.4% in the 150-mg group, and 47% in the placebo group experienced at least one adverse event during the induction period, and 29.4%, 26.9%, and 16.2% in those groups, respectively, experienced infections and/or infestations. The most common adverse events were nasopharyngitis, headache, and upper respiratory tract infection.
The incidence of adverse events in FIXTURE was similar in those receiving secukinumab and etanercept, and was greater in both groups than in the placebo group. Infections and infestations occurred in 26.7%, 30.9%, 24.5%, and 19.3% of those receiving 300 mg of secukinumab, 150 mg of secukinumab, etanercept, and placebo, respectively. However, candida infections were more common with secukinumab than with etanercept (4.7%, 2.3%, and 1.2% of patients in the 300- and 150-mg secukinumab groups and the etanercept group, respectively, had candida infections). All candida infections in the secukinumab group were mild or moderate, and two of four infections in the etanercept group were severe.
Grade 3 neutropenia occurred in nine patients receiving secukinumab and in no patients receiving etanercept.
"The results of these phase 3 studies validate interleukin-17A as an important therapeutic target in moderate to severe plaque psoriasis, confirming earlier findings from basic research and phase 2 trials of secukinumab that suggested that interleukin-17A plays a role in the pathogenesis of psoriasis," the investigators said.
Interleukin-17A plays a key role in host defense, specifically in mucocutaneous microbial surveillance, they added.
"Continued vigilance with respect to the potential for candida infection will be necessary for interleukin-17A inhibitors. Neutropenia may also be of potential concern because of the reported role of interleukin-17A in the stimulation of granulopoiesis and neutrophil trafficking," they noted.
A limitation of both ERASURE and FIXTURE was that few patients continued to receive placebo after week 12, thus limiting the comparisons with the placebo group during the maintenance periods. Also, the study populations may have been too small to detect rare adverse events, the authors wrote, noting that extension studies to evaluate long-term efficacy are ongoing.
ERASURE and FIXTURE were supported by Novartis Pharmaceuticals. Dr. Langley reported no conflicts related to these studies. Dr. Elewski disclosed funding from Novartis during the study period.
At least two-thirds of adults with plaque psoriasis treated with the fully human anti-interleukin-17A monoclonal antibody secukinumab achieved PASI 75 at 12 weeks in two related randomized phase III trials. The findings were reported online July 9 in the New England Journal of Medicine.
In the ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) trial, a multicenter, double-blind study involving 738 patients, the proportions of patients who achieved a 75% or greater reduction in Psoriasis Area and Severity Index (PASI 75) score at 12 weeks were 81.6%, 71.6%, and 4.5% with 300 mg of secukinumab, 150 mg of secukinumab, and placebo, respectively.
In the FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using Two Dosing Regimens) study, a multicenter, double-blind, active-controlled study involving 1,306 patients, the proportions meeting PASI 75 were 77.1% and 67% for 300 and 150 mg, respectively, compared with 44% for etanercept and 4.9% for placebo.
The difference between each secukinumab dose and its comparators was statistically significant in both studies, Dr. Richard G. Langley of Dalhousie University, Halifax, N.S., Dr. Boni Elewski of the University of Alabama, Birmingham, and their colleagues reported on behalf of the ERASURE and FIXTURE Study Groups.
The investigators also found that the proportions of patients with a response of 0 or 1 on the modified investigator’s global assessment at 12 weeks were 65.3%, 51.2%, and 2.4% for the 300- and 150-mg secukinumab doses and placebo, respectively, in ERASURE; and 62.5% and 51.1% for the 300- and 150-mg doses, 27.2% for etanercept, and 2.8% for placebo in FIXTURE (N. Engl. J. Med. 2014 July 9 [doi: 10.1056/NE/NEJMoa314258]).
The differences between each secukinumab dose and its comparators were significant for this measure as well, the investigators reported.
Secukinumab at the 300- and 150-mg doses in both ERASURE and FIXTURE was also superior to placebo (and in FIXTURE, superior to etanercept) for all secondary efficacy endpoints, including PASI 90 and PASI 100 response at week 12; patient reports of itching, pain, and scaling on the Psoriasis Symptom Diary at week 12; and Dermatology Life Quality Index scores.
Both ERASURE and FIXTURE were designed to evaluate the safety of secukinumab as induction therapy with assessment at week 12, and as maintenance therapy with assessment at week 52. Subjects were aged 18 years or older with poorly controlled, moderate to severe plaque psoriasis that had been diagnosed at least 6 months prior, and with a PASI score of 12 or higher, a modified investigator’s global assessment score of 3 or 4, and involvement of 10% or more of their body surface area.
ERASURE was conducted from June 2011 to April 2013 at 88 sites worldwide, and FIXTURE was conducted from June 2011 to June 2013 at 231 sites. Patients in the 300-mg groups received two 150-mg subcutaneous injections of secukinumab, and those in the 150-mg group received one 150-mg injection of secukinumab, and one placebo injection. Etanercept was given at a dose of 50 mg administered subcutaneously twice weekly from baseline until week 12, and then once weekly through week 51.
Adverse events were more common in patients treated with secukinumab in ERASURE; 55% of patients in the 300-mg group, 60.4% in the 150-mg group, and 47% in the placebo group experienced at least one adverse event during the induction period, and 29.4%, 26.9%, and 16.2% in those groups, respectively, experienced infections and/or infestations. The most common adverse events were nasopharyngitis, headache, and upper respiratory tract infection.
The incidence of adverse events in FIXTURE was similar in those receiving secukinumab and etanercept, and was greater in both groups than in the placebo group. Infections and infestations occurred in 26.7%, 30.9%, 24.5%, and 19.3% of those receiving 300 mg of secukinumab, 150 mg of secukinumab, etanercept, and placebo, respectively. However, candida infections were more common with secukinumab than with etanercept (4.7%, 2.3%, and 1.2% of patients in the 300- and 150-mg secukinumab groups and the etanercept group, respectively, had candida infections). All candida infections in the secukinumab group were mild or moderate, and two of four infections in the etanercept group were severe.
Grade 3 neutropenia occurred in nine patients receiving secukinumab and in no patients receiving etanercept.
"The results of these phase 3 studies validate interleukin-17A as an important therapeutic target in moderate to severe plaque psoriasis, confirming earlier findings from basic research and phase 2 trials of secukinumab that suggested that interleukin-17A plays a role in the pathogenesis of psoriasis," the investigators said.
Interleukin-17A plays a key role in host defense, specifically in mucocutaneous microbial surveillance, they added.
"Continued vigilance with respect to the potential for candida infection will be necessary for interleukin-17A inhibitors. Neutropenia may also be of potential concern because of the reported role of interleukin-17A in the stimulation of granulopoiesis and neutrophil trafficking," they noted.
A limitation of both ERASURE and FIXTURE was that few patients continued to receive placebo after week 12, thus limiting the comparisons with the placebo group during the maintenance periods. Also, the study populations may have been too small to detect rare adverse events, the authors wrote, noting that extension studies to evaluate long-term efficacy are ongoing.
ERASURE and FIXTURE were supported by Novartis Pharmaceuticals. Dr. Langley reported no conflicts related to these studies. Dr. Elewski disclosed funding from Novartis during the study period.
At least two-thirds of adults with plaque psoriasis treated with the fully human anti-interleukin-17A monoclonal antibody secukinumab achieved PASI 75 at 12 weeks in two related randomized phase III trials. The findings were reported online July 9 in the New England Journal of Medicine.
In the ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) trial, a multicenter, double-blind study involving 738 patients, the proportions of patients who achieved a 75% or greater reduction in Psoriasis Area and Severity Index (PASI 75) score at 12 weeks were 81.6%, 71.6%, and 4.5% with 300 mg of secukinumab, 150 mg of secukinumab, and placebo, respectively.
In the FIXTURE (Full Year Investigative Examination of Secukinumab vs. Etanercept Using Two Dosing Regimens) study, a multicenter, double-blind, active-controlled study involving 1,306 patients, the proportions meeting PASI 75 were 77.1% and 67% for 300 and 150 mg, respectively, compared with 44% for etanercept and 4.9% for placebo.
The difference between each secukinumab dose and its comparators was statistically significant in both studies, Dr. Richard G. Langley of Dalhousie University, Halifax, N.S., Dr. Boni Elewski of the University of Alabama, Birmingham, and their colleagues reported on behalf of the ERASURE and FIXTURE Study Groups.
The investigators also found that the proportions of patients with a response of 0 or 1 on the modified investigator’s global assessment at 12 weeks were 65.3%, 51.2%, and 2.4% for the 300- and 150-mg secukinumab doses and placebo, respectively, in ERASURE; and 62.5% and 51.1% for the 300- and 150-mg doses, 27.2% for etanercept, and 2.8% for placebo in FIXTURE (N. Engl. J. Med. 2014 July 9 [doi: 10.1056/NE/NEJMoa314258]).
The differences between each secukinumab dose and its comparators were significant for this measure as well, the investigators reported.
Secukinumab at the 300- and 150-mg doses in both ERASURE and FIXTURE was also superior to placebo (and in FIXTURE, superior to etanercept) for all secondary efficacy endpoints, including PASI 90 and PASI 100 response at week 12; patient reports of itching, pain, and scaling on the Psoriasis Symptom Diary at week 12; and Dermatology Life Quality Index scores.
Both ERASURE and FIXTURE were designed to evaluate the safety of secukinumab as induction therapy with assessment at week 12, and as maintenance therapy with assessment at week 52. Subjects were aged 18 years or older with poorly controlled, moderate to severe plaque psoriasis that had been diagnosed at least 6 months prior, and with a PASI score of 12 or higher, a modified investigator’s global assessment score of 3 or 4, and involvement of 10% or more of their body surface area.
ERASURE was conducted from June 2011 to April 2013 at 88 sites worldwide, and FIXTURE was conducted from June 2011 to June 2013 at 231 sites. Patients in the 300-mg groups received two 150-mg subcutaneous injections of secukinumab, and those in the 150-mg group received one 150-mg injection of secukinumab, and one placebo injection. Etanercept was given at a dose of 50 mg administered subcutaneously twice weekly from baseline until week 12, and then once weekly through week 51.
Adverse events were more common in patients treated with secukinumab in ERASURE; 55% of patients in the 300-mg group, 60.4% in the 150-mg group, and 47% in the placebo group experienced at least one adverse event during the induction period, and 29.4%, 26.9%, and 16.2% in those groups, respectively, experienced infections and/or infestations. The most common adverse events were nasopharyngitis, headache, and upper respiratory tract infection.
The incidence of adverse events in FIXTURE was similar in those receiving secukinumab and etanercept, and was greater in both groups than in the placebo group. Infections and infestations occurred in 26.7%, 30.9%, 24.5%, and 19.3% of those receiving 300 mg of secukinumab, 150 mg of secukinumab, etanercept, and placebo, respectively. However, candida infections were more common with secukinumab than with etanercept (4.7%, 2.3%, and 1.2% of patients in the 300- and 150-mg secukinumab groups and the etanercept group, respectively, had candida infections). All candida infections in the secukinumab group were mild or moderate, and two of four infections in the etanercept group were severe.
Grade 3 neutropenia occurred in nine patients receiving secukinumab and in no patients receiving etanercept.
"The results of these phase 3 studies validate interleukin-17A as an important therapeutic target in moderate to severe plaque psoriasis, confirming earlier findings from basic research and phase 2 trials of secukinumab that suggested that interleukin-17A plays a role in the pathogenesis of psoriasis," the investigators said.
Interleukin-17A plays a key role in host defense, specifically in mucocutaneous microbial surveillance, they added.
"Continued vigilance with respect to the potential for candida infection will be necessary for interleukin-17A inhibitors. Neutropenia may also be of potential concern because of the reported role of interleukin-17A in the stimulation of granulopoiesis and neutrophil trafficking," they noted.
A limitation of both ERASURE and FIXTURE was that few patients continued to receive placebo after week 12, thus limiting the comparisons with the placebo group during the maintenance periods. Also, the study populations may have been too small to detect rare adverse events, the authors wrote, noting that extension studies to evaluate long-term efficacy are ongoing.
ERASURE and FIXTURE were supported by Novartis Pharmaceuticals. Dr. Langley reported no conflicts related to these studies. Dr. Elewski disclosed funding from Novartis during the study period.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: The study findings validate interleukin-17A as a therapeutic target for moderate to severe plaque psoriasis, and a majority of patients maintained a clinical response for up to a year with treatments every 4 weeks.
Major finding: In ERASURE, the proportions of patients achieving PASI 75 at 12 weeks were 81.6%, 71.6%, and 4.5% with 300 mg of secukinumab, 150 mg of secukinumab, and placebo, respectively. In FIXTURE, they were 77.1% and 67% for 300 and 150 mg of secukinumab, respectively, compared with 44% for etanercept and 4.9% for placebo.
Data source: Two randomized, controlled trials: ERASURE, which included 738 patients, and FIXTURE, which included 1,306 patients.
Disclosures: ERASURE and FIXTURE were supported by Novartis Pharmaceuticals. Dr. Langley reported no conflicts related to these studies. Dr. Elewski disclosed funding from Novartis during the study period.
Assessing Attributes of Topical Vehicles for the Treatment of Acne, Atopic Dermatitis, and Plaque Psoriasis
The skin care market includes topical product formulations (eg, foams, lotions, ointments, creams) for a number of dermatologic therapeutic targets; however, there is limited information available regarding patient preference for product vehicles by specific dermatologic disease. There are few studies in the literature examining patient adherence to topical medications.1 In the current study, 6 focus groups comprised of patients with 3 dermatologic conditions of interest—acne, atopic dermatitis (AD), or plaque psoriasis (PP)—were surveyed to gain a better understanding of treatment preferences among patients and attributes of various formulations that are most desirable and important to this patient population.
Patient preference for a vehicle is relevant to treatment adherence in patients with conditions such as acne, AD, and PP because noncompliance is a major factor in the high rates of failure that have been associated with topical dermatologic treatments.2 The aesthetic attributes of a given vehicle formulation depend on the disease state being treated, the site of application, and the length of treatment.3 A limited number of studies have linked patient preferences to the attributes of topical medications. In one study, participants preferred an aqueous gel formulation compared with previously used topical treatments for AD.3 In another study, participants ranked the following properties of a hypothetical topical medication as most important: gel formulation, room temperature storage, product life of up to 18 months once opened, application with fingers, and a once-daily regimen.2 A third study found that foams were preferred among other formulations (ie, creams, gels, and ointments) for a variety of disease states, including AD, PP, and seborrheic dermatitis.4 The current study uses a qualitative analysis to further explore patient preferences of vehicle attributes for the topical treatment of acne, AD, and PP.
Methods
Study Participants
Six focus groups were conducted with patients who were using topical prescription medications for the treatment of acne, AD, or PP from March to April 2012. Participants were recruited from Raleigh, North Carolina, and New York, New York, with 3 focus groups (1 for each condition) from each city.
Following institutional review board approval of the study processes and recruitment materials, participants meeting the following criteria were included in the study: 18 years or older; diagnosed with acne, AD, or PP by a physician (participants who currently reported more than 1 of these conditions were not eligible); diagnosed with the respective skin condition at least 6 months prior to screening; current or prior use of topical prescription medications in at least 2 different vehicle formulations (eg, cream and foam, ointment and gel); use of at least 1 topical prescription treatment 5 or more times per month; and English speaking and able to provide written consent.
Study Design
A semistructured discussion guide was developed to ensure consistency in the topics surveyed among all 6 focus groups, and the same 2 moderators conducted the discussion for all 6 groups. At the beginning of the study, after providing written informed consent, participants were given an overview of the study and were asked general questions intended to get the participants talking about their experiences with their respective conditions. To avoid or minimize bias, participants were only asked open-ended questions designed to ascertain what symptoms they experienced in relation to their respective conditions. Finally, the discussions were focused on the topical prescription treatments that participants had tried and the properties of each treatment they liked and disliked.
The focus groups were recorded (audio) and transcribed; transcriptions were then verified through an iterative process of technical and editorial review. Analysis of the results was conducted by evaluation and review of the field notes as well as the transcripts from the focus groups.
Results
A total of 54 participants were surveyed (average age, 40.9 years). Although the average age of participants in the acne and AD groups was generally the same (35.2 and 35.4 years, respectively), the average age of participants in the PP group was higher (52.2 years). The majority of participants were white females who had at least a college degree. On average, participants had been diagnosed with their respective condition approximately 15.5 years prior to screening. Participant demographics and clinical characteristics are presented in more detail in Table 1.
At the time of screening, participants reported prior or present use of topical prescription medications in various formulations for treatment of their respective conditions. The most commonly reported vehicles across all 3 conditions were creams and ointments, followed by lotions, gels, and foams.
Symptoms Across Conditions
At the beginning of the study, participants reported symptoms they experienced in association with their respective skin conditions. Itching and redness were the only symptoms reported across all 3 conditions in all 6 focus groups. Dry skin/dryness, flaking/scaling/peeling, and pain were reported by at least 1 participant in 5 of 6 focus groups. Other common symptoms reported in at least 3 focus groups included pimples/bumps/boils, sensitivity, bleeding, discoloration of skin, cracking/cuts/skin breaking, and buildup of dead or thickened skin/chunks of skin/plaque. As shown in Table 2, there was more variability in the symptoms reported across the 2 acne groups compared with those reported by the AD and PP groups. Table 2 displays all symptoms spontaneously reported across each of the 6 focus groups. Symptoms are listed according to the terms/descriptions provided by focus group participants.
Results by Condition
Unless otherwise noted, participant responses generally were consistent across the North Carolina and New York focus groups. Results from the study, which included a total of 54 participants, suggested a high degree of similarity among preferences for topical treatment attributes across the 3 conditions. Moisturizing was the single attribute that was mentioned across all 3 conditions in all 6 focus groups as an important characteristic in a topical dermatologic treatment. Other attributes mentioned by at least 1 participant in 5 of 6 focus groups included the following: absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy/oily, is not sticky/tacky, is long lasting/long acting/stays on/lasts through sweating or hand washing, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
A few condition-specific attributes also were noted. An attribute was considered condition specific if it was mentioned by at least 1 participant in both groups for any condition. Preferred properties for topical medications that were specific to acne patients included the following: easy to dispense/dispenses right amount, nondrying, product goes on/spreads smoothly, container (eg, tube, pump) is not easily broken/does not leak, and creamy. Preferred properties that were specific to AD patients included the following: is not noticeable to others/conceals area, good consistency, and cooling. Preferred properties that were specific to 2 conditions included no residue among acne and AD patients and soothing among AD and PP patients. Preferred properties for topical medications across all focus groups are described in further detail in Table 3.
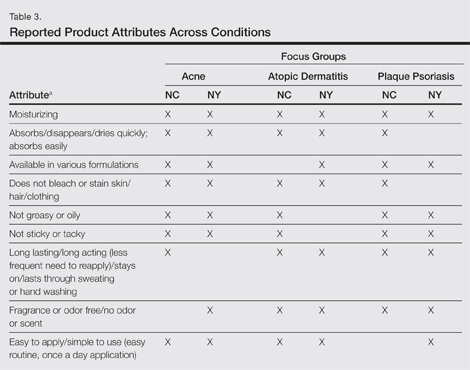
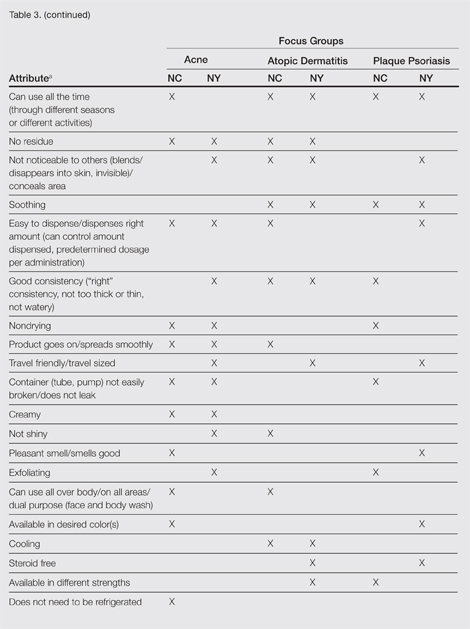
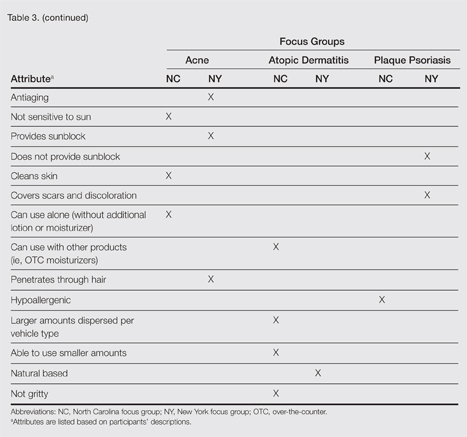
Regarding vehicle-type preferences, acne patients tended to prefer washes, creams, and lotions; AD patients preferred creams; and psoriasis patients preferred creams, ointments, and foams (particularly for the scalp).
Participants across all 3 conditions reported that during daytime hours (versus at night), they would be less likely to use products that are oily, shiny, thick (eg, ointments, oils); bleach or stain the skin/clothing; interfere with makeup, work, or other activities; or are visible to others. Rather, a majority of participants noted that they were more likely to use thinner, less oily products (eg, creams, lotions) during daytime hours, in social situations, or during certain activities (eg, exercise). Participants across all 3 conditions noted that they only used prescription shampoos when they had enough time to repeatedly rinse their hair to eliminate the smell. Participants across all 6 groups noted that they tended to choose creams or lotions over ointments (when available) during the summer because they considered these products to be lighter and less greasy.
Overall, participants indicated that condition-specific symptoms did not influence their preference for topical formulations; rather, they used whatever prescriptions they currently had. However, in some instances, participants noted that the location of the affected area might influence the type of product selected. For example, some participants tended not to use ointments on the scalp or in locations where their clothes might come into contact with the medicated area to avoid clothes sticking to medicated areas. Other participants used lighter, less stringent products on the face versus other locations. Participants noted that foams were preferred for more discrete localized areas.
Comment
Despite the variability in symptoms and conditions across the study population as well as participants’ varying experience with and access to topical treatments, the attributes participants valued most in topical prescription treatments were relatively consistent across the 3 conditions: moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time. Preferred vehicle attributes were generally consistent across the 3 conditions, but preferences for vehicle types tended to vary by condition. Although some vehicles were more closely associated with specific attributes (eg, the majority considered lotions to be moisturizing), no one vehicle was associated with the complete list of attributes for the ideal topical medication. Regardless of the symptoms experienced or particular situations/activities, participants noted that they tended to use the topical medications that were available to them, even if intended for different locations of the body, and nearly all reported using more than 1 type of topical treatment.
Caution should be used in interpreting these findings, as participant preferences are largely dependent on prior experience with different vehicle types. The small number of participants across the 3 conditions in this analysis also limits conclusions that can be drawn from the data sets, as each condition has different though somewhat overlapping treatment algorithms. A focused inquiry into each of these conditions through separate evaluations may provide for a more robust analysis. Future research might employ questionnaire methodology to develop items assessing the attributes of interest (eg, moisturizing ability, rate of absorption, greasiness/oiliness, stickiness/tackiness, fragrance/odor). The final set of desirable attributes may vary depending on the condition (eg, a moisturizing product may be more important to PP and AD patients than acne patients) as well as comparator products.
Acknowledgment—The authors would like to thank Jennifer Gwazdauskas, MBA, Research Triangle Park, North Carolina, of Stiefel, a GSK company, for her assistance with manuscript review.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient p for topical antibiotic treatment for acne. a randomised controlled trial. Br J Dermatol. 2006;154:524-532.
- Trookman NS, Rizer RL, Ho ET, et al. The importance of vehicle properties to patients with atopic dermatitis. Cutis. 2011;88:13-17.
- Weiss S, Wyres M, Brundage T. A novel foam vehicle is consistently preferred by patients for dermatologic conditions. J Am Acad Dermatol. 2011;64(suppl 1):AB50.
The skin care market includes topical product formulations (eg, foams, lotions, ointments, creams) for a number of dermatologic therapeutic targets; however, there is limited information available regarding patient preference for product vehicles by specific dermatologic disease. There are few studies in the literature examining patient adherence to topical medications.1 In the current study, 6 focus groups comprised of patients with 3 dermatologic conditions of interest—acne, atopic dermatitis (AD), or plaque psoriasis (PP)—were surveyed to gain a better understanding of treatment preferences among patients and attributes of various formulations that are most desirable and important to this patient population.
Patient preference for a vehicle is relevant to treatment adherence in patients with conditions such as acne, AD, and PP because noncompliance is a major factor in the high rates of failure that have been associated with topical dermatologic treatments.2 The aesthetic attributes of a given vehicle formulation depend on the disease state being treated, the site of application, and the length of treatment.3 A limited number of studies have linked patient preferences to the attributes of topical medications. In one study, participants preferred an aqueous gel formulation compared with previously used topical treatments for AD.3 In another study, participants ranked the following properties of a hypothetical topical medication as most important: gel formulation, room temperature storage, product life of up to 18 months once opened, application with fingers, and a once-daily regimen.2 A third study found that foams were preferred among other formulations (ie, creams, gels, and ointments) for a variety of disease states, including AD, PP, and seborrheic dermatitis.4 The current study uses a qualitative analysis to further explore patient preferences of vehicle attributes for the topical treatment of acne, AD, and PP.
Methods
Study Participants
Six focus groups were conducted with patients who were using topical prescription medications for the treatment of acne, AD, or PP from March to April 2012. Participants were recruited from Raleigh, North Carolina, and New York, New York, with 3 focus groups (1 for each condition) from each city.
Following institutional review board approval of the study processes and recruitment materials, participants meeting the following criteria were included in the study: 18 years or older; diagnosed with acne, AD, or PP by a physician (participants who currently reported more than 1 of these conditions were not eligible); diagnosed with the respective skin condition at least 6 months prior to screening; current or prior use of topical prescription medications in at least 2 different vehicle formulations (eg, cream and foam, ointment and gel); use of at least 1 topical prescription treatment 5 or more times per month; and English speaking and able to provide written consent.
Study Design
A semistructured discussion guide was developed to ensure consistency in the topics surveyed among all 6 focus groups, and the same 2 moderators conducted the discussion for all 6 groups. At the beginning of the study, after providing written informed consent, participants were given an overview of the study and were asked general questions intended to get the participants talking about their experiences with their respective conditions. To avoid or minimize bias, participants were only asked open-ended questions designed to ascertain what symptoms they experienced in relation to their respective conditions. Finally, the discussions were focused on the topical prescription treatments that participants had tried and the properties of each treatment they liked and disliked.
The focus groups were recorded (audio) and transcribed; transcriptions were then verified through an iterative process of technical and editorial review. Analysis of the results was conducted by evaluation and review of the field notes as well as the transcripts from the focus groups.
Results
A total of 54 participants were surveyed (average age, 40.9 years). Although the average age of participants in the acne and AD groups was generally the same (35.2 and 35.4 years, respectively), the average age of participants in the PP group was higher (52.2 years). The majority of participants were white females who had at least a college degree. On average, participants had been diagnosed with their respective condition approximately 15.5 years prior to screening. Participant demographics and clinical characteristics are presented in more detail in Table 1.

At the time of screening, participants reported prior or present use of topical prescription medications in various formulations for treatment of their respective conditions. The most commonly reported vehicles across all 3 conditions were creams and ointments, followed by lotions, gels, and foams.
Symptoms Across Conditions
At the beginning of the study, participants reported symptoms they experienced in association with their respective skin conditions. Itching and redness were the only symptoms reported across all 3 conditions in all 6 focus groups. Dry skin/dryness, flaking/scaling/peeling, and pain were reported by at least 1 participant in 5 of 6 focus groups. Other common symptoms reported in at least 3 focus groups included pimples/bumps/boils, sensitivity, bleeding, discoloration of skin, cracking/cuts/skin breaking, and buildup of dead or thickened skin/chunks of skin/plaque. As shown in Table 2, there was more variability in the symptoms reported across the 2 acne groups compared with those reported by the AD and PP groups. Table 2 displays all symptoms spontaneously reported across each of the 6 focus groups. Symptoms are listed according to the terms/descriptions provided by focus group participants.


Results by Condition
Unless otherwise noted, participant responses generally were consistent across the North Carolina and New York focus groups. Results from the study, which included a total of 54 participants, suggested a high degree of similarity among preferences for topical treatment attributes across the 3 conditions. Moisturizing was the single attribute that was mentioned across all 3 conditions in all 6 focus groups as an important characteristic in a topical dermatologic treatment. Other attributes mentioned by at least 1 participant in 5 of 6 focus groups included the following: absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy/oily, is not sticky/tacky, is long lasting/long acting/stays on/lasts through sweating or hand washing, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
A few condition-specific attributes also were noted. An attribute was considered condition specific if it was mentioned by at least 1 participant in both groups for any condition. Preferred properties for topical medications that were specific to acne patients included the following: easy to dispense/dispenses right amount, nondrying, product goes on/spreads smoothly, container (eg, tube, pump) is not easily broken/does not leak, and creamy. Preferred properties that were specific to AD patients included the following: is not noticeable to others/conceals area, good consistency, and cooling. Preferred properties that were specific to 2 conditions included no residue among acne and AD patients and soothing among AD and PP patients. Preferred properties for topical medications across all focus groups are described in further detail in Table 3.



Regarding vehicle-type preferences, acne patients tended to prefer washes, creams, and lotions; AD patients preferred creams; and psoriasis patients preferred creams, ointments, and foams (particularly for the scalp).
Participants across all 3 conditions reported that during daytime hours (versus at night), they would be less likely to use products that are oily, shiny, thick (eg, ointments, oils); bleach or stain the skin/clothing; interfere with makeup, work, or other activities; or are visible to others. Rather, a majority of participants noted that they were more likely to use thinner, less oily products (eg, creams, lotions) during daytime hours, in social situations, or during certain activities (eg, exercise). Participants across all 3 conditions noted that they only used prescription shampoos when they had enough time to repeatedly rinse their hair to eliminate the smell. Participants across all 6 groups noted that they tended to choose creams or lotions over ointments (when available) during the summer because they considered these products to be lighter and less greasy.
Overall, participants indicated that condition-specific symptoms did not influence their preference for topical formulations; rather, they used whatever prescriptions they currently had. However, in some instances, participants noted that the location of the affected area might influence the type of product selected. For example, some participants tended not to use ointments on the scalp or in locations where their clothes might come into contact with the medicated area to avoid clothes sticking to medicated areas. Other participants used lighter, less stringent products on the face versus other locations. Participants noted that foams were preferred for more discrete localized areas.
Comment
Despite the variability in symptoms and conditions across the study population as well as participants’ varying experience with and access to topical treatments, the attributes participants valued most in topical prescription treatments were relatively consistent across the 3 conditions: moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time. Preferred vehicle attributes were generally consistent across the 3 conditions, but preferences for vehicle types tended to vary by condition. Although some vehicles were more closely associated with specific attributes (eg, the majority considered lotions to be moisturizing), no one vehicle was associated with the complete list of attributes for the ideal topical medication. Regardless of the symptoms experienced or particular situations/activities, participants noted that they tended to use the topical medications that were available to them, even if intended for different locations of the body, and nearly all reported using more than 1 type of topical treatment.
Caution should be used in interpreting these findings, as participant preferences are largely dependent on prior experience with different vehicle types. The small number of participants across the 3 conditions in this analysis also limits conclusions that can be drawn from the data sets, as each condition has different though somewhat overlapping treatment algorithms. A focused inquiry into each of these conditions through separate evaluations may provide for a more robust analysis. Future research might employ questionnaire methodology to develop items assessing the attributes of interest (eg, moisturizing ability, rate of absorption, greasiness/oiliness, stickiness/tackiness, fragrance/odor). The final set of desirable attributes may vary depending on the condition (eg, a moisturizing product may be more important to PP and AD patients than acne patients) as well as comparator products.
Acknowledgment—The authors would like to thank Jennifer Gwazdauskas, MBA, Research Triangle Park, North Carolina, of Stiefel, a GSK company, for her assistance with manuscript review.
The skin care market includes topical product formulations (eg, foams, lotions, ointments, creams) for a number of dermatologic therapeutic targets; however, there is limited information available regarding patient preference for product vehicles by specific dermatologic disease. There are few studies in the literature examining patient adherence to topical medications.1 In the current study, 6 focus groups comprised of patients with 3 dermatologic conditions of interest—acne, atopic dermatitis (AD), or plaque psoriasis (PP)—were surveyed to gain a better understanding of treatment preferences among patients and attributes of various formulations that are most desirable and important to this patient population.
Patient preference for a vehicle is relevant to treatment adherence in patients with conditions such as acne, AD, and PP because noncompliance is a major factor in the high rates of failure that have been associated with topical dermatologic treatments.2 The aesthetic attributes of a given vehicle formulation depend on the disease state being treated, the site of application, and the length of treatment.3 A limited number of studies have linked patient preferences to the attributes of topical medications. In one study, participants preferred an aqueous gel formulation compared with previously used topical treatments for AD.3 In another study, participants ranked the following properties of a hypothetical topical medication as most important: gel formulation, room temperature storage, product life of up to 18 months once opened, application with fingers, and a once-daily regimen.2 A third study found that foams were preferred among other formulations (ie, creams, gels, and ointments) for a variety of disease states, including AD, PP, and seborrheic dermatitis.4 The current study uses a qualitative analysis to further explore patient preferences of vehicle attributes for the topical treatment of acne, AD, and PP.
Methods
Study Participants
Six focus groups were conducted with patients who were using topical prescription medications for the treatment of acne, AD, or PP from March to April 2012. Participants were recruited from Raleigh, North Carolina, and New York, New York, with 3 focus groups (1 for each condition) from each city.
Following institutional review board approval of the study processes and recruitment materials, participants meeting the following criteria were included in the study: 18 years or older; diagnosed with acne, AD, or PP by a physician (participants who currently reported more than 1 of these conditions were not eligible); diagnosed with the respective skin condition at least 6 months prior to screening; current or prior use of topical prescription medications in at least 2 different vehicle formulations (eg, cream and foam, ointment and gel); use of at least 1 topical prescription treatment 5 or more times per month; and English speaking and able to provide written consent.
Study Design
A semistructured discussion guide was developed to ensure consistency in the topics surveyed among all 6 focus groups, and the same 2 moderators conducted the discussion for all 6 groups. At the beginning of the study, after providing written informed consent, participants were given an overview of the study and were asked general questions intended to get the participants talking about their experiences with their respective conditions. To avoid or minimize bias, participants were only asked open-ended questions designed to ascertain what symptoms they experienced in relation to their respective conditions. Finally, the discussions were focused on the topical prescription treatments that participants had tried and the properties of each treatment they liked and disliked.
The focus groups were recorded (audio) and transcribed; transcriptions were then verified through an iterative process of technical and editorial review. Analysis of the results was conducted by evaluation and review of the field notes as well as the transcripts from the focus groups.
Results
A total of 54 participants were surveyed (average age, 40.9 years). Although the average age of participants in the acne and AD groups was generally the same (35.2 and 35.4 years, respectively), the average age of participants in the PP group was higher (52.2 years). The majority of participants were white females who had at least a college degree. On average, participants had been diagnosed with their respective condition approximately 15.5 years prior to screening. Participant demographics and clinical characteristics are presented in more detail in Table 1.

At the time of screening, participants reported prior or present use of topical prescription medications in various formulations for treatment of their respective conditions. The most commonly reported vehicles across all 3 conditions were creams and ointments, followed by lotions, gels, and foams.
Symptoms Across Conditions
At the beginning of the study, participants reported symptoms they experienced in association with their respective skin conditions. Itching and redness were the only symptoms reported across all 3 conditions in all 6 focus groups. Dry skin/dryness, flaking/scaling/peeling, and pain were reported by at least 1 participant in 5 of 6 focus groups. Other common symptoms reported in at least 3 focus groups included pimples/bumps/boils, sensitivity, bleeding, discoloration of skin, cracking/cuts/skin breaking, and buildup of dead or thickened skin/chunks of skin/plaque. As shown in Table 2, there was more variability in the symptoms reported across the 2 acne groups compared with those reported by the AD and PP groups. Table 2 displays all symptoms spontaneously reported across each of the 6 focus groups. Symptoms are listed according to the terms/descriptions provided by focus group participants.


Results by Condition
Unless otherwise noted, participant responses generally were consistent across the North Carolina and New York focus groups. Results from the study, which included a total of 54 participants, suggested a high degree of similarity among preferences for topical treatment attributes across the 3 conditions. Moisturizing was the single attribute that was mentioned across all 3 conditions in all 6 focus groups as an important characteristic in a topical dermatologic treatment. Other attributes mentioned by at least 1 participant in 5 of 6 focus groups included the following: absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy/oily, is not sticky/tacky, is long lasting/long acting/stays on/lasts through sweating or hand washing, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
A few condition-specific attributes also were noted. An attribute was considered condition specific if it was mentioned by at least 1 participant in both groups for any condition. Preferred properties for topical medications that were specific to acne patients included the following: easy to dispense/dispenses right amount, nondrying, product goes on/spreads smoothly, container (eg, tube, pump) is not easily broken/does not leak, and creamy. Preferred properties that were specific to AD patients included the following: is not noticeable to others/conceals area, good consistency, and cooling. Preferred properties that were specific to 2 conditions included no residue among acne and AD patients and soothing among AD and PP patients. Preferred properties for topical medications across all focus groups are described in further detail in Table 3.



Regarding vehicle-type preferences, acne patients tended to prefer washes, creams, and lotions; AD patients preferred creams; and psoriasis patients preferred creams, ointments, and foams (particularly for the scalp).
Participants across all 3 conditions reported that during daytime hours (versus at night), they would be less likely to use products that are oily, shiny, thick (eg, ointments, oils); bleach or stain the skin/clothing; interfere with makeup, work, or other activities; or are visible to others. Rather, a majority of participants noted that they were more likely to use thinner, less oily products (eg, creams, lotions) during daytime hours, in social situations, or during certain activities (eg, exercise). Participants across all 3 conditions noted that they only used prescription shampoos when they had enough time to repeatedly rinse their hair to eliminate the smell. Participants across all 6 groups noted that they tended to choose creams or lotions over ointments (when available) during the summer because they considered these products to be lighter and less greasy.
Overall, participants indicated that condition-specific symptoms did not influence their preference for topical formulations; rather, they used whatever prescriptions they currently had. However, in some instances, participants noted that the location of the affected area might influence the type of product selected. For example, some participants tended not to use ointments on the scalp or in locations where their clothes might come into contact with the medicated area to avoid clothes sticking to medicated areas. Other participants used lighter, less stringent products on the face versus other locations. Participants noted that foams were preferred for more discrete localized areas.
Comment
Despite the variability in symptoms and conditions across the study population as well as participants’ varying experience with and access to topical treatments, the attributes participants valued most in topical prescription treatments were relatively consistent across the 3 conditions: moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time. Preferred vehicle attributes were generally consistent across the 3 conditions, but preferences for vehicle types tended to vary by condition. Although some vehicles were more closely associated with specific attributes (eg, the majority considered lotions to be moisturizing), no one vehicle was associated with the complete list of attributes for the ideal topical medication. Regardless of the symptoms experienced or particular situations/activities, participants noted that they tended to use the topical medications that were available to them, even if intended for different locations of the body, and nearly all reported using more than 1 type of topical treatment.
Caution should be used in interpreting these findings, as participant preferences are largely dependent on prior experience with different vehicle types. The small number of participants across the 3 conditions in this analysis also limits conclusions that can be drawn from the data sets, as each condition has different though somewhat overlapping treatment algorithms. A focused inquiry into each of these conditions through separate evaluations may provide for a more robust analysis. Future research might employ questionnaire methodology to develop items assessing the attributes of interest (eg, moisturizing ability, rate of absorption, greasiness/oiliness, stickiness/tackiness, fragrance/odor). The final set of desirable attributes may vary depending on the condition (eg, a moisturizing product may be more important to PP and AD patients than acne patients) as well as comparator products.
Acknowledgment—The authors would like to thank Jennifer Gwazdauskas, MBA, Research Triangle Park, North Carolina, of Stiefel, a GSK company, for her assistance with manuscript review.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient p for topical antibiotic treatment for acne. a randomised controlled trial. Br J Dermatol. 2006;154:524-532.
- Trookman NS, Rizer RL, Ho ET, et al. The importance of vehicle properties to patients with atopic dermatitis. Cutis. 2011;88:13-17.
- Weiss S, Wyres M, Brundage T. A novel foam vehicle is consistently preferred by patients for dermatologic conditions. J Am Acad Dermatol. 2011;64(suppl 1):AB50.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient p for topical antibiotic treatment for acne. a randomised controlled trial. Br J Dermatol. 2006;154:524-532.
- Trookman NS, Rizer RL, Ho ET, et al. The importance of vehicle properties to patients with atopic dermatitis. Cutis. 2011;88:13-17.
- Weiss S, Wyres M, Brundage T. A novel foam vehicle is consistently preferred by patients for dermatologic conditions. J Am Acad Dermatol. 2011;64(suppl 1):AB50.
- Patient preference for topical product formulation varies by dermatologic condition being treated.
- Most important vehicle attributes cited across study conditions include moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
- Patient preference for product vehicle is relevant to treatment adherence and may vary depending on the condition being treated.
Arthritis drug restores hair in man with alopecia universalis and psoriasis
A 25-year-old man with plaque psoriasis and virtually no hair of any sort now sports a full head of hair plus body hair after treatment with the arthritis drug tofacitinib, according to Dr. Brittany G. Craiglow and Dr. Brett A. King of Yale University, New Haven, Conn.
The treatment has been so successful that Dr. King has submitted a proposal for a clinical trial involving a cream form of tofacitinib as a treatment for alopecia areata, according to a statement from the university.
Tofacitinib is approved only for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to methotrexate, but it is in clinical development for the treatment of psoriasis.
Dr. Craiglow and Dr. King began treating the patient with 10 mg oral tofacitinib (Xeljanz) daily. At baseline, the patient had been diagnosed with plaque psoriasis and alopecia universalis. His only body hair was a small amount of hair within the psoriasis plaques on his head, the researchers said (J. Invest. Dermatol. 2014 June 18 [doi:10.1038/jid.2014.260]).
After 2 months of tofacitinib dosed at 5 mg twice daily, the psoriasis on the patient’s scalp, torso, and elbows showed some improvement, and there was some hair growth on his face and scalp. The researchers increased the dose to 10 mg in the morning and 5 mg at night. After 3 more months, the patient had complete regrowth of scalp hair, as well as some growth of eyebrows, eyelashes, armpit hair, and pubic hair. After 8 months, the patient had full regrowth of all body hair, with the exception of hair on the arms and legs (which had been sparse prior to his alopecia diagnosis, the researchers said).
Although the hair growth has been dramatic, improvements in the patient’s psoriasis have been slower, likely because of the dosage.
“While we considered increasing the dose of tofacitinib, the patient is so pleased with the regrowth of his hair (and is not particularly bothered by the remaining psoriasis) that he has chosen to continue at the present dose,” the researchers noted.
The researchers considered using tofacitinib to treat the patient’s alopecia universalis based on the research of Angela M. Christiano, Ph.D., of Columbia University, New York, in which the drug reversed hair loss in a mouse model of alopecia areata.
The patient has reported no side effects, and lab testing has shown no abnormalities in complete blood count, serum creatinine, electrolytes, liver function, glucose, or lipids, the researchers noted.
The researchers had no financial conflicts to disclose.
[email protected]
A 25-year-old man with plaque psoriasis and virtually no hair of any sort now sports a full head of hair plus body hair after treatment with the arthritis drug tofacitinib, according to Dr. Brittany G. Craiglow and Dr. Brett A. King of Yale University, New Haven, Conn.
The treatment has been so successful that Dr. King has submitted a proposal for a clinical trial involving a cream form of tofacitinib as a treatment for alopecia areata, according to a statement from the university.
Tofacitinib is approved only for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to methotrexate, but it is in clinical development for the treatment of psoriasis.
Dr. Craiglow and Dr. King began treating the patient with 10 mg oral tofacitinib (Xeljanz) daily. At baseline, the patient had been diagnosed with plaque psoriasis and alopecia universalis. His only body hair was a small amount of hair within the psoriasis plaques on his head, the researchers said (J. Invest. Dermatol. 2014 June 18 [doi:10.1038/jid.2014.260]).
After 2 months of tofacitinib dosed at 5 mg twice daily, the psoriasis on the patient’s scalp, torso, and elbows showed some improvement, and there was some hair growth on his face and scalp. The researchers increased the dose to 10 mg in the morning and 5 mg at night. After 3 more months, the patient had complete regrowth of scalp hair, as well as some growth of eyebrows, eyelashes, armpit hair, and pubic hair. After 8 months, the patient had full regrowth of all body hair, with the exception of hair on the arms and legs (which had been sparse prior to his alopecia diagnosis, the researchers said).
Although the hair growth has been dramatic, improvements in the patient’s psoriasis have been slower, likely because of the dosage.
“While we considered increasing the dose of tofacitinib, the patient is so pleased with the regrowth of his hair (and is not particularly bothered by the remaining psoriasis) that he has chosen to continue at the present dose,” the researchers noted.
The researchers considered using tofacitinib to treat the patient’s alopecia universalis based on the research of Angela M. Christiano, Ph.D., of Columbia University, New York, in which the drug reversed hair loss in a mouse model of alopecia areata.
The patient has reported no side effects, and lab testing has shown no abnormalities in complete blood count, serum creatinine, electrolytes, liver function, glucose, or lipids, the researchers noted.
The researchers had no financial conflicts to disclose.
[email protected]
A 25-year-old man with plaque psoriasis and virtually no hair of any sort now sports a full head of hair plus body hair after treatment with the arthritis drug tofacitinib, according to Dr. Brittany G. Craiglow and Dr. Brett A. King of Yale University, New Haven, Conn.
The treatment has been so successful that Dr. King has submitted a proposal for a clinical trial involving a cream form of tofacitinib as a treatment for alopecia areata, according to a statement from the university.
Tofacitinib is approved only for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to methotrexate, but it is in clinical development for the treatment of psoriasis.
Dr. Craiglow and Dr. King began treating the patient with 10 mg oral tofacitinib (Xeljanz) daily. At baseline, the patient had been diagnosed with plaque psoriasis and alopecia universalis. His only body hair was a small amount of hair within the psoriasis plaques on his head, the researchers said (J. Invest. Dermatol. 2014 June 18 [doi:10.1038/jid.2014.260]).
After 2 months of tofacitinib dosed at 5 mg twice daily, the psoriasis on the patient’s scalp, torso, and elbows showed some improvement, and there was some hair growth on his face and scalp. The researchers increased the dose to 10 mg in the morning and 5 mg at night. After 3 more months, the patient had complete regrowth of scalp hair, as well as some growth of eyebrows, eyelashes, armpit hair, and pubic hair. After 8 months, the patient had full regrowth of all body hair, with the exception of hair on the arms and legs (which had been sparse prior to his alopecia diagnosis, the researchers said).
Although the hair growth has been dramatic, improvements in the patient’s psoriasis have been slower, likely because of the dosage.
“While we considered increasing the dose of tofacitinib, the patient is so pleased with the regrowth of his hair (and is not particularly bothered by the remaining psoriasis) that he has chosen to continue at the present dose,” the researchers noted.
The researchers considered using tofacitinib to treat the patient’s alopecia universalis based on the research of Angela M. Christiano, Ph.D., of Columbia University, New York, in which the drug reversed hair loss in a mouse model of alopecia areata.
The patient has reported no side effects, and lab testing has shown no abnormalities in complete blood count, serum creatinine, electrolytes, liver function, glucose, or lipids, the researchers noted.
The researchers had no financial conflicts to disclose.
[email protected]
Arthritis drug restores hair in man with alopecia universalis and psoriasis
A 25-year-old man with plaque psoriasis and virtually no hair of any sort now sports a full head of hair plus body hair after treatment with the arthritis drug tofacitinib, according to Dr. Brittany G. Craiglow and Dr. Brett A. King of Yale University, New Haven, Conn.
The treatment has been so successful that Dr. King has submitted a proposal for a clinical trial involving a cream form of tofacitinib as a treatment for alopecia areata, according to a statement from the university.
Tofacitinib is approved only for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to methotrexate, but it is in clinical development for the treatment of psoriasis.
Dr. Craiglow and Dr. King began treating the patient with 10 mg oral tofacitinib (Xeljanz) daily. At baseline, the patient had been diagnosed with plaque psoriasis and alopecia universalis. His only body hair was a small amount of hair within the psoriasis plaques on his head, the researchers said (J. Invest. Dermatol. 2014 June 18 [doi:10.1038/jid.2014.260]).
After 2 months of tofacitinib dosed at 5 mg twice daily, the psoriasis on the patient’s scalp, torso, and elbows showed some improvement, and there was some hair growth on his face and scalp. The researchers increased the dose to 10 mg in the morning and 5 mg at night. After 3 more months, the patient had complete regrowth of scalp hair, as well as some growth of eyebrows, eyelashes, armpit hair, and pubic hair. After 8 months, the patient had full regrowth of all body hair, with the exception of hair on the arms and legs (which had been sparse prior to his alopecia diagnosis, the researchers said).
Although the hair growth has been dramatic, improvements in the patient’s psoriasis have been slower, likely because of the dosage.
“While we considered increasing the dose of tofacitinib, the patient is so pleased with the regrowth of his hair (and is not particularly bothered by the remaining psoriasis) that he has chosen to continue at the present dose,” the researchers noted.
The researchers considered using tofacitinib to treat the patient’s alopecia universalis based on the research of Angela M. Christiano, Ph.D., of Columbia University, New York, in which the drug reversed hair loss in a mouse model of alopecia areata.
The patient has reported no side effects, and lab testing has shown no abnormalities in complete blood count, serum creatinine, electrolytes, liver function, glucose, or lipids, the researchers noted.
The researchers had no financial conflicts to disclose.
[email protected]
A 25-year-old man with plaque psoriasis and virtually no hair of any sort now sports a full head of hair plus body hair after treatment with the arthritis drug tofacitinib, according to Dr. Brittany G. Craiglow and Dr. Brett A. King of Yale University, New Haven, Conn.
The treatment has been so successful that Dr. King has submitted a proposal for a clinical trial involving a cream form of tofacitinib as a treatment for alopecia areata, according to a statement from the university.
Tofacitinib is approved only for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to methotrexate, but it is in clinical development for the treatment of psoriasis.
Dr. Craiglow and Dr. King began treating the patient with 10 mg oral tofacitinib (Xeljanz) daily. At baseline, the patient had been diagnosed with plaque psoriasis and alopecia universalis. His only body hair was a small amount of hair within the psoriasis plaques on his head, the researchers said (J. Invest. Dermatol. 2014 June 18 [doi:10.1038/jid.2014.260]).
After 2 months of tofacitinib dosed at 5 mg twice daily, the psoriasis on the patient’s scalp, torso, and elbows showed some improvement, and there was some hair growth on his face and scalp. The researchers increased the dose to 10 mg in the morning and 5 mg at night. After 3 more months, the patient had complete regrowth of scalp hair, as well as some growth of eyebrows, eyelashes, armpit hair, and pubic hair. After 8 months, the patient had full regrowth of all body hair, with the exception of hair on the arms and legs (which had been sparse prior to his alopecia diagnosis, the researchers said).
Although the hair growth has been dramatic, improvements in the patient’s psoriasis have been slower, likely because of the dosage.
“While we considered increasing the dose of tofacitinib, the patient is so pleased with the regrowth of his hair (and is not particularly bothered by the remaining psoriasis) that he has chosen to continue at the present dose,” the researchers noted.
The researchers considered using tofacitinib to treat the patient’s alopecia universalis based on the research of Angela M. Christiano, Ph.D., of Columbia University, New York, in which the drug reversed hair loss in a mouse model of alopecia areata.
The patient has reported no side effects, and lab testing has shown no abnormalities in complete blood count, serum creatinine, electrolytes, liver function, glucose, or lipids, the researchers noted.
The researchers had no financial conflicts to disclose.
[email protected]
A 25-year-old man with plaque psoriasis and virtually no hair of any sort now sports a full head of hair plus body hair after treatment with the arthritis drug tofacitinib, according to Dr. Brittany G. Craiglow and Dr. Brett A. King of Yale University, New Haven, Conn.
The treatment has been so successful that Dr. King has submitted a proposal for a clinical trial involving a cream form of tofacitinib as a treatment for alopecia areata, according to a statement from the university.
Tofacitinib is approved only for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to methotrexate, but it is in clinical development for the treatment of psoriasis.
Dr. Craiglow and Dr. King began treating the patient with 10 mg oral tofacitinib (Xeljanz) daily. At baseline, the patient had been diagnosed with plaque psoriasis and alopecia universalis. His only body hair was a small amount of hair within the psoriasis plaques on his head, the researchers said (J. Invest. Dermatol. 2014 June 18 [doi:10.1038/jid.2014.260]).
After 2 months of tofacitinib dosed at 5 mg twice daily, the psoriasis on the patient’s scalp, torso, and elbows showed some improvement, and there was some hair growth on his face and scalp. The researchers increased the dose to 10 mg in the morning and 5 mg at night. After 3 more months, the patient had complete regrowth of scalp hair, as well as some growth of eyebrows, eyelashes, armpit hair, and pubic hair. After 8 months, the patient had full regrowth of all body hair, with the exception of hair on the arms and legs (which had been sparse prior to his alopecia diagnosis, the researchers said).
Although the hair growth has been dramatic, improvements in the patient’s psoriasis have been slower, likely because of the dosage.
“While we considered increasing the dose of tofacitinib, the patient is so pleased with the regrowth of his hair (and is not particularly bothered by the remaining psoriasis) that he has chosen to continue at the present dose,” the researchers noted.
The researchers considered using tofacitinib to treat the patient’s alopecia universalis based on the research of Angela M. Christiano, Ph.D., of Columbia University, New York, in which the drug reversed hair loss in a mouse model of alopecia areata.
The patient has reported no side effects, and lab testing has shown no abnormalities in complete blood count, serum creatinine, electrolytes, liver function, glucose, or lipids, the researchers noted.
The researchers had no financial conflicts to disclose.
[email protected]
Metabolic syndrome, insulin resistance occur frequently in psoriatic arthritis patients
The metabolic syndrome and insulin resistance are not just common among patients with psoriatic arthritis, but both also correlate with the severity of the inflammatory musculoskeletal disease, according to a single-center, cross-sectional cohort study.
In the study of 283 consecutive white patients with longstanding psoriatic arthritis who attended a rheumatology clinic during a 1-year period, 44% were found to have the metabolic syndrome and 16% to have insulin resistance. "Our findings are novel and support our pretest hypothesis that the risk of metabolic syndrome and insulin resistance increases with the severity of underlying psoriatic arthritis, probably reflecting the increasing burden of inflammation," said Dr. Muhammad Haroon of the department of rheumatology and his associates at St. Vincent’s University Hospital, Dublin.
Psoriatic arthritis is known to be associated with heightened cardiovascular risk, and CV diseases are the leading causes of death in patients with psoriatic arthritis. Until now, however, the prevalences of these two major CV risk factors have not been well studied in patients with psoriatic arthritis. "We hypothesized, therefore, that there might be a greater burden of metabolic syndrome and insulin resistance in psoriatic arthritis, and consequently of cardiovascular diseases because of a greater inflammatory load," Dr. Haroon and his colleagues wrote (J. Rheumatol. 2014;41:1357-65).
The patients had psoriatic arthritis for a duration of at least 10 years (mean of 19 years), and just over half of the patients were women. Their mean age was 54.6 years.
A total of 124 patients (44%) were found to have the metabolic syndrome. "Even more alarming was the finding that about 50% of these newly diagnosed patients with metabolic syndrome had a combination of 4 or 5 of these risk features," the investigators said. In particular, elevated blood pressure (74%), greater waist circumference (56%), and elevated triglycerides (44%) were common among these psoriatic arthritis patients.
On multivariate analysis, metabolic syndrome was significantly associated with more severe disease, higher smoking pack-years, and worse EuroQol-5 dimension (EQ-5D). Metabolic syndrome was significantly associated with severe disease, even after adjustment for the presence of insulin resistance.
A total of 41 patients (16%) had insulin resistance of the 263 for whom insulin resistance data were available, which on multivariate analysis was significantly associated more severe disease, older age at the onset of psoriasis, and higher body-mass index, even after adjusting for the presence of metabolic syndrome.
Both the metabolic syndrome and insulin resistance were more frequent among patients with the most severe psoriatic arthritis, as measured by current and previous skin assessments; inflammatory markers; measures of disease activity; the number of deformed joints; and the presence of dactylitis, enthesitis, peripheral joint erosions, osteolysis, and sacroiliitis, the investigators said.
The study findings suggest, but do not establish, that "the higher burden of inflammatory arthritis or the combination of severe psoriatic disease features play major roles in the development of the metabolic syndrome and/or insulin resistance," Dr. Haroon and his associates said.
Their observations "can also help inform risk stratification" of patients with psoriatic arthritis, they added.
No disclosures were provided.
The metabolic syndrome and insulin resistance are not just common among patients with psoriatic arthritis, but both also correlate with the severity of the inflammatory musculoskeletal disease, according to a single-center, cross-sectional cohort study.
In the study of 283 consecutive white patients with longstanding psoriatic arthritis who attended a rheumatology clinic during a 1-year period, 44% were found to have the metabolic syndrome and 16% to have insulin resistance. "Our findings are novel and support our pretest hypothesis that the risk of metabolic syndrome and insulin resistance increases with the severity of underlying psoriatic arthritis, probably reflecting the increasing burden of inflammation," said Dr. Muhammad Haroon of the department of rheumatology and his associates at St. Vincent’s University Hospital, Dublin.
Psoriatic arthritis is known to be associated with heightened cardiovascular risk, and CV diseases are the leading causes of death in patients with psoriatic arthritis. Until now, however, the prevalences of these two major CV risk factors have not been well studied in patients with psoriatic arthritis. "We hypothesized, therefore, that there might be a greater burden of metabolic syndrome and insulin resistance in psoriatic arthritis, and consequently of cardiovascular diseases because of a greater inflammatory load," Dr. Haroon and his colleagues wrote (J. Rheumatol. 2014;41:1357-65).
The patients had psoriatic arthritis for a duration of at least 10 years (mean of 19 years), and just over half of the patients were women. Their mean age was 54.6 years.
A total of 124 patients (44%) were found to have the metabolic syndrome. "Even more alarming was the finding that about 50% of these newly diagnosed patients with metabolic syndrome had a combination of 4 or 5 of these risk features," the investigators said. In particular, elevated blood pressure (74%), greater waist circumference (56%), and elevated triglycerides (44%) were common among these psoriatic arthritis patients.
On multivariate analysis, metabolic syndrome was significantly associated with more severe disease, higher smoking pack-years, and worse EuroQol-5 dimension (EQ-5D). Metabolic syndrome was significantly associated with severe disease, even after adjustment for the presence of insulin resistance.
A total of 41 patients (16%) had insulin resistance of the 263 for whom insulin resistance data were available, which on multivariate analysis was significantly associated more severe disease, older age at the onset of psoriasis, and higher body-mass index, even after adjusting for the presence of metabolic syndrome.
Both the metabolic syndrome and insulin resistance were more frequent among patients with the most severe psoriatic arthritis, as measured by current and previous skin assessments; inflammatory markers; measures of disease activity; the number of deformed joints; and the presence of dactylitis, enthesitis, peripheral joint erosions, osteolysis, and sacroiliitis, the investigators said.
The study findings suggest, but do not establish, that "the higher burden of inflammatory arthritis or the combination of severe psoriatic disease features play major roles in the development of the metabolic syndrome and/or insulin resistance," Dr. Haroon and his associates said.
Their observations "can also help inform risk stratification" of patients with psoriatic arthritis, they added.
No disclosures were provided.
The metabolic syndrome and insulin resistance are not just common among patients with psoriatic arthritis, but both also correlate with the severity of the inflammatory musculoskeletal disease, according to a single-center, cross-sectional cohort study.
In the study of 283 consecutive white patients with longstanding psoriatic arthritis who attended a rheumatology clinic during a 1-year period, 44% were found to have the metabolic syndrome and 16% to have insulin resistance. "Our findings are novel and support our pretest hypothesis that the risk of metabolic syndrome and insulin resistance increases with the severity of underlying psoriatic arthritis, probably reflecting the increasing burden of inflammation," said Dr. Muhammad Haroon of the department of rheumatology and his associates at St. Vincent’s University Hospital, Dublin.
Psoriatic arthritis is known to be associated with heightened cardiovascular risk, and CV diseases are the leading causes of death in patients with psoriatic arthritis. Until now, however, the prevalences of these two major CV risk factors have not been well studied in patients with psoriatic arthritis. "We hypothesized, therefore, that there might be a greater burden of metabolic syndrome and insulin resistance in psoriatic arthritis, and consequently of cardiovascular diseases because of a greater inflammatory load," Dr. Haroon and his colleagues wrote (J. Rheumatol. 2014;41:1357-65).
The patients had psoriatic arthritis for a duration of at least 10 years (mean of 19 years), and just over half of the patients were women. Their mean age was 54.6 years.
A total of 124 patients (44%) were found to have the metabolic syndrome. "Even more alarming was the finding that about 50% of these newly diagnosed patients with metabolic syndrome had a combination of 4 or 5 of these risk features," the investigators said. In particular, elevated blood pressure (74%), greater waist circumference (56%), and elevated triglycerides (44%) were common among these psoriatic arthritis patients.
On multivariate analysis, metabolic syndrome was significantly associated with more severe disease, higher smoking pack-years, and worse EuroQol-5 dimension (EQ-5D). Metabolic syndrome was significantly associated with severe disease, even after adjustment for the presence of insulin resistance.
A total of 41 patients (16%) had insulin resistance of the 263 for whom insulin resistance data were available, which on multivariate analysis was significantly associated more severe disease, older age at the onset of psoriasis, and higher body-mass index, even after adjusting for the presence of metabolic syndrome.
Both the metabolic syndrome and insulin resistance were more frequent among patients with the most severe psoriatic arthritis, as measured by current and previous skin assessments; inflammatory markers; measures of disease activity; the number of deformed joints; and the presence of dactylitis, enthesitis, peripheral joint erosions, osteolysis, and sacroiliitis, the investigators said.
The study findings suggest, but do not establish, that "the higher burden of inflammatory arthritis or the combination of severe psoriatic disease features play major roles in the development of the metabolic syndrome and/or insulin resistance," Dr. Haroon and his associates said.
Their observations "can also help inform risk stratification" of patients with psoriatic arthritis, they added.
No disclosures were provided.
FROM THE JOURNAL OF RHEUMATOLOGY
Key clinical point: The presence of metabolic syndrome or insulin resistance may be a marker for more severe psoriatic arthritis.
Major finding: 124 patients (44%) with psoriatic arthritis were found to have the metabolic syndrome and 41 (16%) were found to have insulin resistance.
Data source: A cross-sectional cohort study involving 283 consecutive white patients with psoriatic arthritis of at least 10 years’ duration who were treated at a single rheumatology clinic in a 1-year period.
Disclosures: No disclosures were provided.
Psoriasis risk rises in women with history of hypertension and beta-blocker use
Women with a 6-year or longer history of hypertension, and women with a 6-year or longer use of beta-blocker medications to treat hypertension, may be at increased risk of developing psoriasis, compared with women who have normal blood pressure, according to data from more than 77,000 women.
"Women with hypertension tended to be older; had higher [body mass indexes]; had proportionately higher prevalence rates of cardiovascular disease, type 2 diabetes, and hypercholesterolemia; and were less physically active than those without hypertension," the researchers wrote.
The report was published online July 2 in JAMA Dermatology [doi:10.1001/jamadermatol.2013.9957].
Dr. Shaowei Wu of Brown University, Providence, R.I., and colleagues performed a prospective cohort study of 77,728 women participating in the Nurses’ Health Study between June 1996 and June 2008. The women provided biennially updated data on hypertension and antihypertensive medications. The researchers identified 843 psoriasis cases during more than 1 million person-years of follow-up.
Women with hypertension lasting 6 years or more were at higher risk of developing psoriasis than were normotensive women [HR 1.27]. In further analysis, researchers found "a higher risk of psoriasis among hypertensive women without medication use [HR 1.49] and among hypertensive women with current medication use [HR 1.31] when compared with normotensive women without medication use." In an analysis of individual antihypertensive medications, beta-blockers were the only drugs associated with psoriasis development. Although this association disappeared in a fully-adjusted model, it "persisted in a duration-dependent manner" [HR 1.39] among women taking the medications for 6 years or more, and this trend was statistically significant.
"Special attention on psoriasis screening may be needed for patients with long-term duration of hypertension and related antihypertensive medication use in clinical practices," the authors wrote. The findings "provide novel insights into the association among hypertension, antihypertensive medications, and psoriasis," they said. "However, further work is necessary to confirm our findings and clarify the biological mechanisms that underlie these associations."
The study was supported in part by the National Institutes of Health. Senior author Dr. Abrar Qureshi has served as a consultant for Abbott, Centocor, Novartis, and the Centers for Disease Control and Prevention.
"A critical practice gap exists in identifying the causes of psoriasis flares, especially medication-related causes," said Dr. April Armstrong. "Some physicians may not consistently examine medications for their contribution to psoriasis flares. However, a careful consideration of the role of medications in psoriasis exacerbation may improve long-term psoriasis control."
Solutions to narrow these gaps "include a careful review of a patient’s medication list with special attention to medications with strong evidence of contributing to psoriasis exacerbation," including beta-blockers, lithium, antimalarials, and interferons, she said. It also is important for dermatologists "to recognize medications with latency periods beyond the typical 2-4 weeks and to inquire about historical use of these medications with known long latency periods." If dermatologists recommend discontinued use of a medication, "they need to coordinate care with other health care professionals to ensure that the patient is offered appropriate alternative treatments."
Dr. Armstrong is in the department of dermatology at the University of Colorado, Denver. She had no related financial disclosures. These remarks were taken from her editorial accompanying the report by Dr. Wu (JAMA Dermatology 2014 [doi: 10.1001/jamadermatol.2014.1019]).
"A critical practice gap exists in identifying the causes of psoriasis flares, especially medication-related causes," said Dr. April Armstrong. "Some physicians may not consistently examine medications for their contribution to psoriasis flares. However, a careful consideration of the role of medications in psoriasis exacerbation may improve long-term psoriasis control."
Solutions to narrow these gaps "include a careful review of a patient’s medication list with special attention to medications with strong evidence of contributing to psoriasis exacerbation," including beta-blockers, lithium, antimalarials, and interferons, she said. It also is important for dermatologists "to recognize medications with latency periods beyond the typical 2-4 weeks and to inquire about historical use of these medications with known long latency periods." If dermatologists recommend discontinued use of a medication, "they need to coordinate care with other health care professionals to ensure that the patient is offered appropriate alternative treatments."
Dr. Armstrong is in the department of dermatology at the University of Colorado, Denver. She had no related financial disclosures. These remarks were taken from her editorial accompanying the report by Dr. Wu (JAMA Dermatology 2014 [doi: 10.1001/jamadermatol.2014.1019]).
"A critical practice gap exists in identifying the causes of psoriasis flares, especially medication-related causes," said Dr. April Armstrong. "Some physicians may not consistently examine medications for their contribution to psoriasis flares. However, a careful consideration of the role of medications in psoriasis exacerbation may improve long-term psoriasis control."
Solutions to narrow these gaps "include a careful review of a patient’s medication list with special attention to medications with strong evidence of contributing to psoriasis exacerbation," including beta-blockers, lithium, antimalarials, and interferons, she said. It also is important for dermatologists "to recognize medications with latency periods beyond the typical 2-4 weeks and to inquire about historical use of these medications with known long latency periods." If dermatologists recommend discontinued use of a medication, "they need to coordinate care with other health care professionals to ensure that the patient is offered appropriate alternative treatments."
Dr. Armstrong is in the department of dermatology at the University of Colorado, Denver. She had no related financial disclosures. These remarks were taken from her editorial accompanying the report by Dr. Wu (JAMA Dermatology 2014 [doi: 10.1001/jamadermatol.2014.1019]).
Women with a 6-year or longer history of hypertension, and women with a 6-year or longer use of beta-blocker medications to treat hypertension, may be at increased risk of developing psoriasis, compared with women who have normal blood pressure, according to data from more than 77,000 women.
"Women with hypertension tended to be older; had higher [body mass indexes]; had proportionately higher prevalence rates of cardiovascular disease, type 2 diabetes, and hypercholesterolemia; and were less physically active than those without hypertension," the researchers wrote.
The report was published online July 2 in JAMA Dermatology [doi:10.1001/jamadermatol.2013.9957].
Dr. Shaowei Wu of Brown University, Providence, R.I., and colleagues performed a prospective cohort study of 77,728 women participating in the Nurses’ Health Study between June 1996 and June 2008. The women provided biennially updated data on hypertension and antihypertensive medications. The researchers identified 843 psoriasis cases during more than 1 million person-years of follow-up.
Women with hypertension lasting 6 years or more were at higher risk of developing psoriasis than were normotensive women [HR 1.27]. In further analysis, researchers found "a higher risk of psoriasis among hypertensive women without medication use [HR 1.49] and among hypertensive women with current medication use [HR 1.31] when compared with normotensive women without medication use." In an analysis of individual antihypertensive medications, beta-blockers were the only drugs associated with psoriasis development. Although this association disappeared in a fully-adjusted model, it "persisted in a duration-dependent manner" [HR 1.39] among women taking the medications for 6 years or more, and this trend was statistically significant.
"Special attention on psoriasis screening may be needed for patients with long-term duration of hypertension and related antihypertensive medication use in clinical practices," the authors wrote. The findings "provide novel insights into the association among hypertension, antihypertensive medications, and psoriasis," they said. "However, further work is necessary to confirm our findings and clarify the biological mechanisms that underlie these associations."
The study was supported in part by the National Institutes of Health. Senior author Dr. Abrar Qureshi has served as a consultant for Abbott, Centocor, Novartis, and the Centers for Disease Control and Prevention.
Women with a 6-year or longer history of hypertension, and women with a 6-year or longer use of beta-blocker medications to treat hypertension, may be at increased risk of developing psoriasis, compared with women who have normal blood pressure, according to data from more than 77,000 women.
"Women with hypertension tended to be older; had higher [body mass indexes]; had proportionately higher prevalence rates of cardiovascular disease, type 2 diabetes, and hypercholesterolemia; and were less physically active than those without hypertension," the researchers wrote.
The report was published online July 2 in JAMA Dermatology [doi:10.1001/jamadermatol.2013.9957].
Dr. Shaowei Wu of Brown University, Providence, R.I., and colleagues performed a prospective cohort study of 77,728 women participating in the Nurses’ Health Study between June 1996 and June 2008. The women provided biennially updated data on hypertension and antihypertensive medications. The researchers identified 843 psoriasis cases during more than 1 million person-years of follow-up.
Women with hypertension lasting 6 years or more were at higher risk of developing psoriasis than were normotensive women [HR 1.27]. In further analysis, researchers found "a higher risk of psoriasis among hypertensive women without medication use [HR 1.49] and among hypertensive women with current medication use [HR 1.31] when compared with normotensive women without medication use." In an analysis of individual antihypertensive medications, beta-blockers were the only drugs associated with psoriasis development. Although this association disappeared in a fully-adjusted model, it "persisted in a duration-dependent manner" [HR 1.39] among women taking the medications for 6 years or more, and this trend was statistically significant.
"Special attention on psoriasis screening may be needed for patients with long-term duration of hypertension and related antihypertensive medication use in clinical practices," the authors wrote. The findings "provide novel insights into the association among hypertension, antihypertensive medications, and psoriasis," they said. "However, further work is necessary to confirm our findings and clarify the biological mechanisms that underlie these associations."
The study was supported in part by the National Institutes of Health. Senior author Dr. Abrar Qureshi has served as a consultant for Abbott, Centocor, Novartis, and the Centers for Disease Control and Prevention.
FROM JAMA DERMATOLOGY
Key clinical point: Women with a long history of hypertension, or a long history of beta-blocker use to treat hypertension, may be at increased risk of developing psoriasis.
Major finding: Women with hypertension for 6 years or more were at higher risk of developing psoriasis (HR 1.27) than were normotensive women. The risk of psoriasis was higher among hypertensive women not taking medication (HR 1.49) and among hypertensive women taking medication (HR 1.31) compared with that of normotensive women not taking medication.
Data source: A group of 77,728 women who participated in the Nurses’ Health Study from 1996 to 2008.
Disclosures: The study was supported in part by the National Institutes of Health. The senior study author has served as a consultant for Abbott, Centocor, Novartis, and the Centers for Disease Control and Prevention.
Etanercept-Induced Cystic Acne
Case Report
A 35-year-old man with chronic plaque-type psoriasis presented for treatment of a flare-up caused by a combination of hot tub use and 3 missed etanercept injections. The patient had been prescribed subcutaneous etanercept (50 mg once weekly) approximately 2 years prior to presentation for treatment of psoriasis and had used it consistently with 2 brief periods of discontinuation due to upper respiratory infections; treatment was discontinued for 2 weeks until the infections resolved and was restarted at the same dose. Recent hot tub use induced localized folliculitis of the leg and caused koebnerization of his psoriasis. Treatment with etanercept (50 mg twice weekly) was reinitiated, but after 1 month of therapy, the patient developed a nodulocystic eruption on the face. The patient discontinued use of etanercept and subsequently was started on oral minocycline (50 mgonce daily) by his primary care physician. After 6 weeks of minocycline therapy, the patient reported gradual improvement of his cystic acne. At follow-up, physical examination revealed approximately 15 erythematous cystic papules and nodules on the bilateral cheeks. The dose of oral minocycline was increased to 100 mg twice daily and his face subsequently cleared after 12 weeks of treatment.
On rechallenge with etanercept (50 mg weekly) after a separate flare-up of his psoriasis and psoriatic arthritis, the patient again developed nodulocystic acne within 3 weeks of restarting the medication, which confirmed the association between etanercept and the eruption. Etanercept was subsequently discontinued and the patient was started on ustekinumab for treatment of psoriasis and psoriatic arthritis as well as minocycline (100 mg twice daily) for treatment of nodulocystic acne. His acne subsequently cleared after 12 weeks of therapy. The patient’s psoriasis currently is well controlled on a regimen of methotrexate 20 mg weekly, as continued use of ustekinumab was not covered by his health insurance policy.
Comment
Etanercept, along with infliximab and adalimumab, is a tumor necrosis factor α (TNF-α) antagonist. Tumor necrosis factor α is a major cytokine of the immune system that is deregulated in autoimmune disorders, causing inflammation and a variety of systemic effects. Inhibition of TNF-α results in a decrease in inflammatory markers such as IL-1 and IL-6, leading to a reduction in endothelial permeability and leukocyte migration.1 Etanercept is unique in that it is a fully humanized soluble fusion protein consisting of a TNF-α receptor linked to the Fc region of IgG1, whereas infliximab and adalimumab are monoclonal antibodies that target TNF-α. Etanercept works by acting as a decoy receptor for TNF-α, thereby preventing it from binding cell surface receptors and subsequently decreasing inflammation. It is an approved treatment of rheumatoid arthritis, polyarticular juvenile rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis.2
Many drugs have been implicated in acneform eruptions mimicking acne vulgaris, such as corticosteroids, cyclosporine, antipsychotics, anticonvulsants, epidermal growth factor receptor inhibitors, antidepressants, danazol, antituberculosis drugs, quinidine, azathioprine, testosterone, and TNF-α antagonists.1 The TNF-α antagonists that have been associated with acne are infliximab and adalimumab, especially when used to treat Crohn disease or rheumatoid arthritis, though there also are reports of acneform eruptions when using these drugs to treat psoriasis.1,3 There have been reports of the use of etanercept in treating severe acne and acne conglobata4,5; our case documents etanercept-induced acne associated with psoriasis treatment. Theoretically, anti–TNF-α agents should suppress acne rather than induce it due to their inhibition of the inflammatory markers TNF-α, IL-1a, and IFN-γ, which are thought to play a role in hypercornification of the infundibulum.6 Thus the mechanism of TNF-α antagonists and associated acneform eruptions remains unknown. This observation should prompt further research to investigate the correlation between the use of TNF-α inhibitors, specifically etanercept, and cystic acne.
- Momin SB, Peterson A, Del Rosso JQ. A status report on drug-associated acne and acneiform eruptions. J Drugs Dermatol. 2010;9:627-636.
- Zhou H. Clinical pharmacokinetics of etanercept: a fully humanized soluble recombinant tumor necrosis factor receptor fusion protein. J Clin Pharmacol. 2005;45:490-497.
- Sun G, Wasko CA, Hsu S. Acneiform eruption following anti-TNF-alpha treatment: a report of three cases. J Drugs Dermatol. 2008;7:69-71.
- Campione E, Mazzotta AM, Bianchi L, et al. Severe acne successfully treated with etanercept. Acta Derm Venereol. 2006;86:256-257.
- Vega J, Sánchez-Velicia L, Pozo T. Efficacy of etanercept in the treatment of acne conglobata [in Spanish]. Actas Dermosifiliogr. 2010;101:553-554.
- Bassi E, Poli F, Charachon A, et al. Infliximab-induced acne: report of two cases. Br J Dermatol. 2007;156:402-403.
Case Report
A 35-year-old man with chronic plaque-type psoriasis presented for treatment of a flare-up caused by a combination of hot tub use and 3 missed etanercept injections. The patient had been prescribed subcutaneous etanercept (50 mg once weekly) approximately 2 years prior to presentation for treatment of psoriasis and had used it consistently with 2 brief periods of discontinuation due to upper respiratory infections; treatment was discontinued for 2 weeks until the infections resolved and was restarted at the same dose. Recent hot tub use induced localized folliculitis of the leg and caused koebnerization of his psoriasis. Treatment with etanercept (50 mg twice weekly) was reinitiated, but after 1 month of therapy, the patient developed a nodulocystic eruption on the face. The patient discontinued use of etanercept and subsequently was started on oral minocycline (50 mgonce daily) by his primary care physician. After 6 weeks of minocycline therapy, the patient reported gradual improvement of his cystic acne. At follow-up, physical examination revealed approximately 15 erythematous cystic papules and nodules on the bilateral cheeks. The dose of oral minocycline was increased to 100 mg twice daily and his face subsequently cleared after 12 weeks of treatment.
On rechallenge with etanercept (50 mg weekly) after a separate flare-up of his psoriasis and psoriatic arthritis, the patient again developed nodulocystic acne within 3 weeks of restarting the medication, which confirmed the association between etanercept and the eruption. Etanercept was subsequently discontinued and the patient was started on ustekinumab for treatment of psoriasis and psoriatic arthritis as well as minocycline (100 mg twice daily) for treatment of nodulocystic acne. His acne subsequently cleared after 12 weeks of therapy. The patient’s psoriasis currently is well controlled on a regimen of methotrexate 20 mg weekly, as continued use of ustekinumab was not covered by his health insurance policy.
Comment
Etanercept, along with infliximab and adalimumab, is a tumor necrosis factor α (TNF-α) antagonist. Tumor necrosis factor α is a major cytokine of the immune system that is deregulated in autoimmune disorders, causing inflammation and a variety of systemic effects. Inhibition of TNF-α results in a decrease in inflammatory markers such as IL-1 and IL-6, leading to a reduction in endothelial permeability and leukocyte migration.1 Etanercept is unique in that it is a fully humanized soluble fusion protein consisting of a TNF-α receptor linked to the Fc region of IgG1, whereas infliximab and adalimumab are monoclonal antibodies that target TNF-α. Etanercept works by acting as a decoy receptor for TNF-α, thereby preventing it from binding cell surface receptors and subsequently decreasing inflammation. It is an approved treatment of rheumatoid arthritis, polyarticular juvenile rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis.2
Many drugs have been implicated in acneform eruptions mimicking acne vulgaris, such as corticosteroids, cyclosporine, antipsychotics, anticonvulsants, epidermal growth factor receptor inhibitors, antidepressants, danazol, antituberculosis drugs, quinidine, azathioprine, testosterone, and TNF-α antagonists.1 The TNF-α antagonists that have been associated with acne are infliximab and adalimumab, especially when used to treat Crohn disease or rheumatoid arthritis, though there also are reports of acneform eruptions when using these drugs to treat psoriasis.1,3 There have been reports of the use of etanercept in treating severe acne and acne conglobata4,5; our case documents etanercept-induced acne associated with psoriasis treatment. Theoretically, anti–TNF-α agents should suppress acne rather than induce it due to their inhibition of the inflammatory markers TNF-α, IL-1a, and IFN-γ, which are thought to play a role in hypercornification of the infundibulum.6 Thus the mechanism of TNF-α antagonists and associated acneform eruptions remains unknown. This observation should prompt further research to investigate the correlation between the use of TNF-α inhibitors, specifically etanercept, and cystic acne.
Case Report
A 35-year-old man with chronic plaque-type psoriasis presented for treatment of a flare-up caused by a combination of hot tub use and 3 missed etanercept injections. The patient had been prescribed subcutaneous etanercept (50 mg once weekly) approximately 2 years prior to presentation for treatment of psoriasis and had used it consistently with 2 brief periods of discontinuation due to upper respiratory infections; treatment was discontinued for 2 weeks until the infections resolved and was restarted at the same dose. Recent hot tub use induced localized folliculitis of the leg and caused koebnerization of his psoriasis. Treatment with etanercept (50 mg twice weekly) was reinitiated, but after 1 month of therapy, the patient developed a nodulocystic eruption on the face. The patient discontinued use of etanercept and subsequently was started on oral minocycline (50 mgonce daily) by his primary care physician. After 6 weeks of minocycline therapy, the patient reported gradual improvement of his cystic acne. At follow-up, physical examination revealed approximately 15 erythematous cystic papules and nodules on the bilateral cheeks. The dose of oral minocycline was increased to 100 mg twice daily and his face subsequently cleared after 12 weeks of treatment.
On rechallenge with etanercept (50 mg weekly) after a separate flare-up of his psoriasis and psoriatic arthritis, the patient again developed nodulocystic acne within 3 weeks of restarting the medication, which confirmed the association between etanercept and the eruption. Etanercept was subsequently discontinued and the patient was started on ustekinumab for treatment of psoriasis and psoriatic arthritis as well as minocycline (100 mg twice daily) for treatment of nodulocystic acne. His acne subsequently cleared after 12 weeks of therapy. The patient’s psoriasis currently is well controlled on a regimen of methotrexate 20 mg weekly, as continued use of ustekinumab was not covered by his health insurance policy.
Comment
Etanercept, along with infliximab and adalimumab, is a tumor necrosis factor α (TNF-α) antagonist. Tumor necrosis factor α is a major cytokine of the immune system that is deregulated in autoimmune disorders, causing inflammation and a variety of systemic effects. Inhibition of TNF-α results in a decrease in inflammatory markers such as IL-1 and IL-6, leading to a reduction in endothelial permeability and leukocyte migration.1 Etanercept is unique in that it is a fully humanized soluble fusion protein consisting of a TNF-α receptor linked to the Fc region of IgG1, whereas infliximab and adalimumab are monoclonal antibodies that target TNF-α. Etanercept works by acting as a decoy receptor for TNF-α, thereby preventing it from binding cell surface receptors and subsequently decreasing inflammation. It is an approved treatment of rheumatoid arthritis, polyarticular juvenile rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis.2
Many drugs have been implicated in acneform eruptions mimicking acne vulgaris, such as corticosteroids, cyclosporine, antipsychotics, anticonvulsants, epidermal growth factor receptor inhibitors, antidepressants, danazol, antituberculosis drugs, quinidine, azathioprine, testosterone, and TNF-α antagonists.1 The TNF-α antagonists that have been associated with acne are infliximab and adalimumab, especially when used to treat Crohn disease or rheumatoid arthritis, though there also are reports of acneform eruptions when using these drugs to treat psoriasis.1,3 There have been reports of the use of etanercept in treating severe acne and acne conglobata4,5; our case documents etanercept-induced acne associated with psoriasis treatment. Theoretically, anti–TNF-α agents should suppress acne rather than induce it due to their inhibition of the inflammatory markers TNF-α, IL-1a, and IFN-γ, which are thought to play a role in hypercornification of the infundibulum.6 Thus the mechanism of TNF-α antagonists and associated acneform eruptions remains unknown. This observation should prompt further research to investigate the correlation between the use of TNF-α inhibitors, specifically etanercept, and cystic acne.
- Momin SB, Peterson A, Del Rosso JQ. A status report on drug-associated acne and acneiform eruptions. J Drugs Dermatol. 2010;9:627-636.
- Zhou H. Clinical pharmacokinetics of etanercept: a fully humanized soluble recombinant tumor necrosis factor receptor fusion protein. J Clin Pharmacol. 2005;45:490-497.
- Sun G, Wasko CA, Hsu S. Acneiform eruption following anti-TNF-alpha treatment: a report of three cases. J Drugs Dermatol. 2008;7:69-71.
- Campione E, Mazzotta AM, Bianchi L, et al. Severe acne successfully treated with etanercept. Acta Derm Venereol. 2006;86:256-257.
- Vega J, Sánchez-Velicia L, Pozo T. Efficacy of etanercept in the treatment of acne conglobata [in Spanish]. Actas Dermosifiliogr. 2010;101:553-554.
- Bassi E, Poli F, Charachon A, et al. Infliximab-induced acne: report of two cases. Br J Dermatol. 2007;156:402-403.
- Momin SB, Peterson A, Del Rosso JQ. A status report on drug-associated acne and acneiform eruptions. J Drugs Dermatol. 2010;9:627-636.
- Zhou H. Clinical pharmacokinetics of etanercept: a fully humanized soluble recombinant tumor necrosis factor receptor fusion protein. J Clin Pharmacol. 2005;45:490-497.
- Sun G, Wasko CA, Hsu S. Acneiform eruption following anti-TNF-alpha treatment: a report of three cases. J Drugs Dermatol. 2008;7:69-71.
- Campione E, Mazzotta AM, Bianchi L, et al. Severe acne successfully treated with etanercept. Acta Derm Venereol. 2006;86:256-257.
- Vega J, Sánchez-Velicia L, Pozo T. Efficacy of etanercept in the treatment of acne conglobata [in Spanish]. Actas Dermosifiliogr. 2010;101:553-554.
- Bassi E, Poli F, Charachon A, et al. Infliximab-induced acne: report of two cases. Br J Dermatol. 2007;156:402-403.
- Tumor necrosis factor α antagonists have been implicated in causing acneform eruptions.
- Discontinuation of the agent along with initiation of oral antibiotics or isotretinoin can treat medication-induced nodulocystic acne.
- Ustekinumab is an alternative non–tumor necrosis factor α antagonist that can be used to treat psoriasis and psoriatic arthritis that has not been associated with acneform eruptions.
Foundation turns spotlight on psoriatic arthritis
The National Psoriasis Foundation has relaunched its psoriatic arthritis program to aggressively address the disparities in diagnosis and treatment for psoriatic arthritis patients in the United States.
"There are significantly fewer resources for people with psoriatic arthritis than for those with rheumatoid arthritis," despite similar symptoms and prevalence, according to a press release issued by the foundation June 26.
The National Psoriasis Foundation PsA Project states four goals: Reduce the time to diagnosis of PsA; improve clinician understanding of PsA symptoms, treatment options, and the effect of the disease on quality of life; reduce barriers to treatment; and help patients better manage their disease.
Specifically, the foundation aims to reduce from 4 years to 1 year the average time to diagnosis for PsA patients, reduce from 50% to 30% the percentage of patients who report PsA is a problem in their everyday lives; and double the number of PsA patients receiving proper treatment, the number of National Institutes of Health scientists studying psoriatic disease, and the health resources available for PsA patients.
The National Psoriasis Foundation has relaunched its psoriatic arthritis program to aggressively address the disparities in diagnosis and treatment for psoriatic arthritis patients in the United States.
"There are significantly fewer resources for people with psoriatic arthritis than for those with rheumatoid arthritis," despite similar symptoms and prevalence, according to a press release issued by the foundation June 26.
The National Psoriasis Foundation PsA Project states four goals: Reduce the time to diagnosis of PsA; improve clinician understanding of PsA symptoms, treatment options, and the effect of the disease on quality of life; reduce barriers to treatment; and help patients better manage their disease.
Specifically, the foundation aims to reduce from 4 years to 1 year the average time to diagnosis for PsA patients, reduce from 50% to 30% the percentage of patients who report PsA is a problem in their everyday lives; and double the number of PsA patients receiving proper treatment, the number of National Institutes of Health scientists studying psoriatic disease, and the health resources available for PsA patients.
The National Psoriasis Foundation has relaunched its psoriatic arthritis program to aggressively address the disparities in diagnosis and treatment for psoriatic arthritis patients in the United States.
"There are significantly fewer resources for people with psoriatic arthritis than for those with rheumatoid arthritis," despite similar symptoms and prevalence, according to a press release issued by the foundation June 26.
The National Psoriasis Foundation PsA Project states four goals: Reduce the time to diagnosis of PsA; improve clinician understanding of PsA symptoms, treatment options, and the effect of the disease on quality of life; reduce barriers to treatment; and help patients better manage their disease.
Specifically, the foundation aims to reduce from 4 years to 1 year the average time to diagnosis for PsA patients, reduce from 50% to 30% the percentage of patients who report PsA is a problem in their everyday lives; and double the number of PsA patients receiving proper treatment, the number of National Institutes of Health scientists studying psoriatic disease, and the health resources available for PsA patients.




