User login
Genital psoriasis is the worst: Patients sound off
GENEVA – The great majority of patients with genital psoriasis say their symptoms in the genital area are worse than elsewhere on the body, Kim A. Meeuwis, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
She presented a qualitative study in which 20 patients with longstanding genital psoriasis sounded off, sharing their perspectives on the disease in one-on-one, semistructured, face-to-face interviews.
Genital psoriasis is common. Epidemiologic studies show 30%-60% of psoriasis patients experience genital involvement at some point in the course of their disease. Yet patients seldom discuss their genital psoriasis with their physicians, and the patient perspective on how the experience of genital psoriasis differs from that of having psoriasis at other locations has been addressed only sparsely in the literature. This lack of attention was the impetus for the current study, she explained.
The 20 participants in the study had an average 18-year history of plaque psoriasis, with an average 7.5-year history of genital involvement. The genital psoriasis was rated moderate or severe in 70% of subjects at the time of the study.
The most commonly reported symptoms of genital psoriasis were itch and discomfort, each of which was cited by all study participants. This was followed by erythema, cited by 95%; stinging and burning, also cited by 95%; pain, cited by 85%; scaling, by 75%; and cracking, by 30%.
Of the patients in the study, 85% reported that their pain and/or discomfort were worse in the genital area than at other sites, and 10% said they were highly self-conscious about their genital psoriasis because others had misidentified them as having a sexually transmitted infection.
Since this was a qualitative study, Dr. Meeuwis provided representative quotes from several patients, including one who asserted, “I really only have discomfort on my psoriasis on the rest of my body ... in my genitals is the only place that actually has pain, or the itching is ... really, really bad.”
Dr. Meeuwis said the study results hold an important lesson for physicians who treat psoriasis: “Due to differences in patient experiences between genital and nongenital skin, it’s really important to make time for the specific evaluation of genital involvement in taking care of patients with psoriasis – and to be sure to ask about it.”
Dr. Meeuwis reported serving as a consultant to Eli Lilly, which sponsored the study, as well as being on an advisory board to Beiersdorf.
GENEVA – The great majority of patients with genital psoriasis say their symptoms in the genital area are worse than elsewhere on the body, Kim A. Meeuwis, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
She presented a qualitative study in which 20 patients with longstanding genital psoriasis sounded off, sharing their perspectives on the disease in one-on-one, semistructured, face-to-face interviews.
Genital psoriasis is common. Epidemiologic studies show 30%-60% of psoriasis patients experience genital involvement at some point in the course of their disease. Yet patients seldom discuss their genital psoriasis with their physicians, and the patient perspective on how the experience of genital psoriasis differs from that of having psoriasis at other locations has been addressed only sparsely in the literature. This lack of attention was the impetus for the current study, she explained.
The 20 participants in the study had an average 18-year history of plaque psoriasis, with an average 7.5-year history of genital involvement. The genital psoriasis was rated moderate or severe in 70% of subjects at the time of the study.
The most commonly reported symptoms of genital psoriasis were itch and discomfort, each of which was cited by all study participants. This was followed by erythema, cited by 95%; stinging and burning, also cited by 95%; pain, cited by 85%; scaling, by 75%; and cracking, by 30%.
Of the patients in the study, 85% reported that their pain and/or discomfort were worse in the genital area than at other sites, and 10% said they were highly self-conscious about their genital psoriasis because others had misidentified them as having a sexually transmitted infection.
Since this was a qualitative study, Dr. Meeuwis provided representative quotes from several patients, including one who asserted, “I really only have discomfort on my psoriasis on the rest of my body ... in my genitals is the only place that actually has pain, or the itching is ... really, really bad.”
Dr. Meeuwis said the study results hold an important lesson for physicians who treat psoriasis: “Due to differences in patient experiences between genital and nongenital skin, it’s really important to make time for the specific evaluation of genital involvement in taking care of patients with psoriasis – and to be sure to ask about it.”
Dr. Meeuwis reported serving as a consultant to Eli Lilly, which sponsored the study, as well as being on an advisory board to Beiersdorf.
GENEVA – The great majority of patients with genital psoriasis say their symptoms in the genital area are worse than elsewhere on the body, Kim A. Meeuwis, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
She presented a qualitative study in which 20 patients with longstanding genital psoriasis sounded off, sharing their perspectives on the disease in one-on-one, semistructured, face-to-face interviews.
Genital psoriasis is common. Epidemiologic studies show 30%-60% of psoriasis patients experience genital involvement at some point in the course of their disease. Yet patients seldom discuss their genital psoriasis with their physicians, and the patient perspective on how the experience of genital psoriasis differs from that of having psoriasis at other locations has been addressed only sparsely in the literature. This lack of attention was the impetus for the current study, she explained.
The 20 participants in the study had an average 18-year history of plaque psoriasis, with an average 7.5-year history of genital involvement. The genital psoriasis was rated moderate or severe in 70% of subjects at the time of the study.
The most commonly reported symptoms of genital psoriasis were itch and discomfort, each of which was cited by all study participants. This was followed by erythema, cited by 95%; stinging and burning, also cited by 95%; pain, cited by 85%; scaling, by 75%; and cracking, by 30%.
Of the patients in the study, 85% reported that their pain and/or discomfort were worse in the genital area than at other sites, and 10% said they were highly self-conscious about their genital psoriasis because others had misidentified them as having a sexually transmitted infection.
Since this was a qualitative study, Dr. Meeuwis provided representative quotes from several patients, including one who asserted, “I really only have discomfort on my psoriasis on the rest of my body ... in my genitals is the only place that actually has pain, or the itching is ... really, really bad.”
Dr. Meeuwis said the study results hold an important lesson for physicians who treat psoriasis: “Due to differences in patient experiences between genital and nongenital skin, it’s really important to make time for the specific evaluation of genital involvement in taking care of patients with psoriasis – and to be sure to ask about it.”
Dr. Meeuwis reported serving as a consultant to Eli Lilly, which sponsored the study, as well as being on an advisory board to Beiersdorf.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Of the participants in a study of genital psoriasis, 100% reported that a hallmark of their genital disease was itching and discomfort.
Data source: A qualitative study that involved one-on-one interviews with 20 patients with genital psoriasis, who shared their experiences as to how genital involvement differs from their psoriasis elsewhere.
Disclosures: The study was sponsored by Eli Lilly. The presenter reported serving as a consultant to the company.
Ixekizumab has profound impact on genital psoriasis
GENEVA – The interleukin-17A inhibitor ixekizumab provided rapid clearance of genital psoriasis in a phase 3b clinical trial, with significant improvement seen as early as week 1, Caitriona Ryan, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The highly targeted monoclonal antibody also improved the intense itching that’s a particularly prominent feature of genital psoriasis.

“Genital psoriasis is a hidden part of psoriasis. Unfortunately, as dermatologists we do a bad job of evaluating our patients for it. They are ashamed and embarrassed to bring up the topic with their dermatologists. Hopefully, this study will create some awareness around the topic,” she said.
This was the first-ever randomized trial to evaluate the effect of a biologic agent specifically on genital psoriasis. It was also the first study of a biologic in psoriasis patients with less than 10% body surface area involved.
“That’s a very important thing,” according to the dermatologist. “There are lots of patients with genital psoriasis who have less than 10% body surface area involved and therefore don’t qualify for biologic therapy, even though their genital psoriasis can be incredibly debilitating.”
The 12-week, multicenter, double-blind trial included 149 patients with a baseline static Physician’s Global Assessment of Genitalia (sPGA-G) score of at least 3 on a 0-5 scale. All participants had failed to respond to at least one topical therapy for their genital psoriasis, such as a corticosteroid, a calcineurin inhibitor, or a vitamin D analog. The subjects averaged a 16-year history of psoriasis and a 9-year history of genital psoriasis. Thirty-eight percent of participants had an involved body surface area of at least 1% but less than 10%.
Patients were randomized to ixekizumab (Taltz) given in the usual way – a subcutaneous loading dose of 160 mg, followed by repeat 80-mg injections every 2 weeks – or placebo.
The primary study endpoint was achievement of an sPGA-G score of 0 or 1, meaning clear or almost clear, as assessed by blinded investigators. At the 12-week mark, the rate was 73% in the ixekizumab group and 8% in controls. The sPGA-G score already differed significantly between the two study arms at the first assessment, after 1 week. The treatment success rate was closely similar in patients with or without at least 10% total body surface area involved.
A key secondary endpoint concerned sexual health. Among patients who at baseline indicated that in the past week, their genital psoriasis “sometimes,” “often,” or “always” limited the frequency of their sexual activity, at week 12, 78% of those in the ixekizumab group answered the same question on the Sexual Frequency Questionnaire “never” or “rarely,” compared with 21% of controls.
“This is huge. It’s such an important part of our patients’ lives, and there was a big difference by week 1,” Dr. Ryan noted.
On another secondary endpoint, 60% of the ixekizumab group reported at least a 3-point improvement in the 0-10 Genital Itch Numeric Rating Scale at week 12, compared with 8% of controls, with a statistically significant difference apparent at week 2.
“Itch is the most frequently reported symptom in our patients with genital psoriasis, and it seems to be much more impactful than itch from psoriasis elsewhere,” Dr. Ryan commented.
The side effect profile of ixekizumab was the same as has been seen in larger, longer-term studies. There were no serious ixekizumab-related adverse events, and no cases of candidiasis.
The study was sponsored by Eli Lilly. Dr. Ryan reported serving as an advisory board member to and/or receiving honoraria from that company and more than half a dozen other pharmaceutical companies.
GENEVA – The interleukin-17A inhibitor ixekizumab provided rapid clearance of genital psoriasis in a phase 3b clinical trial, with significant improvement seen as early as week 1, Caitriona Ryan, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The highly targeted monoclonal antibody also improved the intense itching that’s a particularly prominent feature of genital psoriasis.

“Genital psoriasis is a hidden part of psoriasis. Unfortunately, as dermatologists we do a bad job of evaluating our patients for it. They are ashamed and embarrassed to bring up the topic with their dermatologists. Hopefully, this study will create some awareness around the topic,” she said.
This was the first-ever randomized trial to evaluate the effect of a biologic agent specifically on genital psoriasis. It was also the first study of a biologic in psoriasis patients with less than 10% body surface area involved.
“That’s a very important thing,” according to the dermatologist. “There are lots of patients with genital psoriasis who have less than 10% body surface area involved and therefore don’t qualify for biologic therapy, even though their genital psoriasis can be incredibly debilitating.”
The 12-week, multicenter, double-blind trial included 149 patients with a baseline static Physician’s Global Assessment of Genitalia (sPGA-G) score of at least 3 on a 0-5 scale. All participants had failed to respond to at least one topical therapy for their genital psoriasis, such as a corticosteroid, a calcineurin inhibitor, or a vitamin D analog. The subjects averaged a 16-year history of psoriasis and a 9-year history of genital psoriasis. Thirty-eight percent of participants had an involved body surface area of at least 1% but less than 10%.
Patients were randomized to ixekizumab (Taltz) given in the usual way – a subcutaneous loading dose of 160 mg, followed by repeat 80-mg injections every 2 weeks – or placebo.
The primary study endpoint was achievement of an sPGA-G score of 0 or 1, meaning clear or almost clear, as assessed by blinded investigators. At the 12-week mark, the rate was 73% in the ixekizumab group and 8% in controls. The sPGA-G score already differed significantly between the two study arms at the first assessment, after 1 week. The treatment success rate was closely similar in patients with or without at least 10% total body surface area involved.
A key secondary endpoint concerned sexual health. Among patients who at baseline indicated that in the past week, their genital psoriasis “sometimes,” “often,” or “always” limited the frequency of their sexual activity, at week 12, 78% of those in the ixekizumab group answered the same question on the Sexual Frequency Questionnaire “never” or “rarely,” compared with 21% of controls.
“This is huge. It’s such an important part of our patients’ lives, and there was a big difference by week 1,” Dr. Ryan noted.
On another secondary endpoint, 60% of the ixekizumab group reported at least a 3-point improvement in the 0-10 Genital Itch Numeric Rating Scale at week 12, compared with 8% of controls, with a statistically significant difference apparent at week 2.
“Itch is the most frequently reported symptom in our patients with genital psoriasis, and it seems to be much more impactful than itch from psoriasis elsewhere,” Dr. Ryan commented.
The side effect profile of ixekizumab was the same as has been seen in larger, longer-term studies. There were no serious ixekizumab-related adverse events, and no cases of candidiasis.
The study was sponsored by Eli Lilly. Dr. Ryan reported serving as an advisory board member to and/or receiving honoraria from that company and more than half a dozen other pharmaceutical companies.
GENEVA – The interleukin-17A inhibitor ixekizumab provided rapid clearance of genital psoriasis in a phase 3b clinical trial, with significant improvement seen as early as week 1, Caitriona Ryan, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The highly targeted monoclonal antibody also improved the intense itching that’s a particularly prominent feature of genital psoriasis.

“Genital psoriasis is a hidden part of psoriasis. Unfortunately, as dermatologists we do a bad job of evaluating our patients for it. They are ashamed and embarrassed to bring up the topic with their dermatologists. Hopefully, this study will create some awareness around the topic,” she said.
This was the first-ever randomized trial to evaluate the effect of a biologic agent specifically on genital psoriasis. It was also the first study of a biologic in psoriasis patients with less than 10% body surface area involved.
“That’s a very important thing,” according to the dermatologist. “There are lots of patients with genital psoriasis who have less than 10% body surface area involved and therefore don’t qualify for biologic therapy, even though their genital psoriasis can be incredibly debilitating.”
The 12-week, multicenter, double-blind trial included 149 patients with a baseline static Physician’s Global Assessment of Genitalia (sPGA-G) score of at least 3 on a 0-5 scale. All participants had failed to respond to at least one topical therapy for their genital psoriasis, such as a corticosteroid, a calcineurin inhibitor, or a vitamin D analog. The subjects averaged a 16-year history of psoriasis and a 9-year history of genital psoriasis. Thirty-eight percent of participants had an involved body surface area of at least 1% but less than 10%.
Patients were randomized to ixekizumab (Taltz) given in the usual way – a subcutaneous loading dose of 160 mg, followed by repeat 80-mg injections every 2 weeks – or placebo.
The primary study endpoint was achievement of an sPGA-G score of 0 or 1, meaning clear or almost clear, as assessed by blinded investigators. At the 12-week mark, the rate was 73% in the ixekizumab group and 8% in controls. The sPGA-G score already differed significantly between the two study arms at the first assessment, after 1 week. The treatment success rate was closely similar in patients with or without at least 10% total body surface area involved.
A key secondary endpoint concerned sexual health. Among patients who at baseline indicated that in the past week, their genital psoriasis “sometimes,” “often,” or “always” limited the frequency of their sexual activity, at week 12, 78% of those in the ixekizumab group answered the same question on the Sexual Frequency Questionnaire “never” or “rarely,” compared with 21% of controls.
“This is huge. It’s such an important part of our patients’ lives, and there was a big difference by week 1,” Dr. Ryan noted.
On another secondary endpoint, 60% of the ixekizumab group reported at least a 3-point improvement in the 0-10 Genital Itch Numeric Rating Scale at week 12, compared with 8% of controls, with a statistically significant difference apparent at week 2.
“Itch is the most frequently reported symptom in our patients with genital psoriasis, and it seems to be much more impactful than itch from psoriasis elsewhere,” Dr. Ryan commented.
The side effect profile of ixekizumab was the same as has been seen in larger, longer-term studies. There were no serious ixekizumab-related adverse events, and no cases of candidiasis.
The study was sponsored by Eli Lilly. Dr. Ryan reported serving as an advisory board member to and/or receiving honoraria from that company and more than half a dozen other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point: First-ever trial of a biologic agent in genital psoriasis shows heartening results.
Major finding: 73% of patients with moderate to severe genital psoriasis were clear or almost clear of their genital disease after 12 weeks of ixekizumab, vs. 8% of controls.
Data source: This was a randomized, double-blind, placebo-controlled, multicenter, 12-week clinical trial in 149 patients with moderate to severe genital psoriasis.
Disclosures: The study was sponsored by Eli Lilly. The presenter reported serving as an advisory board member to and/or receiving honoraria from that company and more than half a dozen other pharmaceutical companies.
Innovations in Dermatology: Brodalumab for Plaque Psoriasis
Baseline lab data is class specific for biologics to treat psoriasis patients
said April Armstrong, MD, of the University of Southern California, Los Angeles.
Keep class-specific considerations in mind when collecting baseline lab data to help support the success of biologics in treating psoriasis, Dr. Armstrong said at the annual Coastal Dermatology Symposium.
When clinicians consider biologics, they must balance efficacy, safety, convenience, and costs of treatment, Dr. Armstrong said.
She addressed general considerations when selecting biologics for psoriasis and stressed the importance of assessing patients for tuberculosis and reviewing underlying cancer risk. Confirm that a patient has no active infections and consider whether a patient has completed all age-appropriate immunizations. Consider a complete blood count and metabolic panel for the following biologics:
- Ustekinumab: Baseline HIV or pregnancy test, and a TB evaluation at baseline as well as annual monitoring.
- Etanercept, adalimumab, infliximab: Baseline TB evaluation and screening hepatitis panel, liver function tests, and blood count, with option to add pregnancy test or HIV test. A liver function test/hepatitis panel is indicated annually, and TB should be monitored annually. Be cautious about using this class of drugs in patients with heart failure, and verify the absence of demyelinating disease in patients prior to prescribing this class of drugs.
- Guselkumab: Baseline TB evaluation, possible pregnancy or HIV tests, followed by annual TB evaluation.
- Secukinumab, ixekizumab, and brodalumab: Baseline TB evaluation, consider HIV or pregnancy tests, followed by annual TB evaluation. Be cautious about using this class of drugs in patients with ulcerative colitis or Crohn’s disease; assess and counsel for increased risk of suicidality when considering brodalumab.
Beyond the general considerations, several other factors can help maximize success with particular biologics, Dr. Armstrong said at the meeting, which is jointly presented by the University of Louisville and Global Academy for Medical Education.
The number of injections given in the first year, which range from 5 (ustekinumab) to 64 (etanercept) is an important consideration for some patients, Dr. Armstrong noted; the number of injections for the remaining biologics are guselkumab, 8; ixekizumab, 17; brodalumab and adalimumab, both 27, and secukinumab, 32. In addition, the IL-17 inhibitors carry some risk of oral candidiasis and inflammatory bowel disease.
This publication and the Global Academy for Medical Education are owned by Frontline Medical News.
Dr. Armstrong disclosed relationships with multiple companies including AbbVie, Janssen, Novartis, Lilly, Regeneron, Sanofi, Modernizing Medicine, and Valeant.
said April Armstrong, MD, of the University of Southern California, Los Angeles.
Keep class-specific considerations in mind when collecting baseline lab data to help support the success of biologics in treating psoriasis, Dr. Armstrong said at the annual Coastal Dermatology Symposium.
When clinicians consider biologics, they must balance efficacy, safety, convenience, and costs of treatment, Dr. Armstrong said.
She addressed general considerations when selecting biologics for psoriasis and stressed the importance of assessing patients for tuberculosis and reviewing underlying cancer risk. Confirm that a patient has no active infections and consider whether a patient has completed all age-appropriate immunizations. Consider a complete blood count and metabolic panel for the following biologics:
- Ustekinumab: Baseline HIV or pregnancy test, and a TB evaluation at baseline as well as annual monitoring.
- Etanercept, adalimumab, infliximab: Baseline TB evaluation and screening hepatitis panel, liver function tests, and blood count, with option to add pregnancy test or HIV test. A liver function test/hepatitis panel is indicated annually, and TB should be monitored annually. Be cautious about using this class of drugs in patients with heart failure, and verify the absence of demyelinating disease in patients prior to prescribing this class of drugs.
- Guselkumab: Baseline TB evaluation, possible pregnancy or HIV tests, followed by annual TB evaluation.
- Secukinumab, ixekizumab, and brodalumab: Baseline TB evaluation, consider HIV or pregnancy tests, followed by annual TB evaluation. Be cautious about using this class of drugs in patients with ulcerative colitis or Crohn’s disease; assess and counsel for increased risk of suicidality when considering brodalumab.
Beyond the general considerations, several other factors can help maximize success with particular biologics, Dr. Armstrong said at the meeting, which is jointly presented by the University of Louisville and Global Academy for Medical Education.
The number of injections given in the first year, which range from 5 (ustekinumab) to 64 (etanercept) is an important consideration for some patients, Dr. Armstrong noted; the number of injections for the remaining biologics are guselkumab, 8; ixekizumab, 17; brodalumab and adalimumab, both 27, and secukinumab, 32. In addition, the IL-17 inhibitors carry some risk of oral candidiasis and inflammatory bowel disease.
This publication and the Global Academy for Medical Education are owned by Frontline Medical News.
Dr. Armstrong disclosed relationships with multiple companies including AbbVie, Janssen, Novartis, Lilly, Regeneron, Sanofi, Modernizing Medicine, and Valeant.
said April Armstrong, MD, of the University of Southern California, Los Angeles.
Keep class-specific considerations in mind when collecting baseline lab data to help support the success of biologics in treating psoriasis, Dr. Armstrong said at the annual Coastal Dermatology Symposium.
When clinicians consider biologics, they must balance efficacy, safety, convenience, and costs of treatment, Dr. Armstrong said.
She addressed general considerations when selecting biologics for psoriasis and stressed the importance of assessing patients for tuberculosis and reviewing underlying cancer risk. Confirm that a patient has no active infections and consider whether a patient has completed all age-appropriate immunizations. Consider a complete blood count and metabolic panel for the following biologics:
- Ustekinumab: Baseline HIV or pregnancy test, and a TB evaluation at baseline as well as annual monitoring.
- Etanercept, adalimumab, infliximab: Baseline TB evaluation and screening hepatitis panel, liver function tests, and blood count, with option to add pregnancy test or HIV test. A liver function test/hepatitis panel is indicated annually, and TB should be monitored annually. Be cautious about using this class of drugs in patients with heart failure, and verify the absence of demyelinating disease in patients prior to prescribing this class of drugs.
- Guselkumab: Baseline TB evaluation, possible pregnancy or HIV tests, followed by annual TB evaluation.
- Secukinumab, ixekizumab, and brodalumab: Baseline TB evaluation, consider HIV or pregnancy tests, followed by annual TB evaluation. Be cautious about using this class of drugs in patients with ulcerative colitis or Crohn’s disease; assess and counsel for increased risk of suicidality when considering brodalumab.
Beyond the general considerations, several other factors can help maximize success with particular biologics, Dr. Armstrong said at the meeting, which is jointly presented by the University of Louisville and Global Academy for Medical Education.
The number of injections given in the first year, which range from 5 (ustekinumab) to 64 (etanercept) is an important consideration for some patients, Dr. Armstrong noted; the number of injections for the remaining biologics are guselkumab, 8; ixekizumab, 17; brodalumab and adalimumab, both 27, and secukinumab, 32. In addition, the IL-17 inhibitors carry some risk of oral candidiasis and inflammatory bowel disease.
This publication and the Global Academy for Medical Education are owned by Frontline Medical News.
Dr. Armstrong disclosed relationships with multiple companies including AbbVie, Janssen, Novartis, Lilly, Regeneron, Sanofi, Modernizing Medicine, and Valeant.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
New psoriasis therapies coming of age
The pathogenesis theories and treatment approaches to psoriasis have evolved over the past 3 decades, and the latest treatments continue to show safety and effectiveness, according to Alan Menter, MD, chairman of dermatology at Baylor University Medical Center, Dallas.
Before the 1980s, psoriasis was seen as a disease of keratinocyte dysfunction, with treatments that included methotrexate, UVB, and retinoids, Dr. Menter said in a presentation at the annual Coastal Dermatology Symposium. In the 1980s, it was considered an immunologic disease, and then an interleukin (IL)–12/Th1–mediated disease, with anti-CD2, anti-CD11a, and tumor necrosis factor–alpha blocker treatments from 1990 to 2004.
These include risankizumab, which targets the p19 subunit of IL-23 and is being studied for treatment of moderate to severe psoriasis. After one intravenous or subcutaneous dose of risankizumab in a phase 1 study, 16% of patients achieved a Psoriasis Area and Severity Index (PASI) 100, 58% achieved a PASI 90, and 87% achieved a PASI 75, and the publication of phase 2 results are pending, Dr. Menter said. The most common side effects included mild to moderate upper respiratory infections, mild nasopharyngitis, and mild to moderate headaches.
Psoriasis patients treated with guselkumab, which also targets the p19 subunit of IL-23 and was approved in July 2017 for patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy, were significantly more likely to be clear or almost clear at 16 weeks, compared with those on placebo in a phase 2 randomized, controlled trial.
“Both IL-23 and IL-17 are promising targets in the treatment of moderate to severe plaque psoriasis,” said Dr. Menter. “It is important to be vigilant in following the safety profile of these drugs both in clinical trials and in postmarketing registries to ensure their long-term safety,” he added.
Additional research on how to curb side effects associated with these new and emerging therapies should target receptors downstream along the IL-23/Th17 pathway, Dr. Menter explained. Findings from a 2015 study suggest that deficiencies in cytokines and receptors further downstream in the IL-23/Th17 pathway “are associated with fewer disorders than deficiencies in upstream components of the pathway,” he said (J Invest Dermatol. 2015 Aug;135[8]:1946-53).
Although concerns about safety remain, avoiding biologics may have a negative impact as well, as moderate to severe psoriasis patients may experience deformed joints, decreased quality of life, heart attacks, strokes, and early death, Dr. Menter said.
Dr. Menter disclosed having received research support and/or serving as a consultant and/or lecturer for AbbVie, Allergan, Amgen, Anacor, Celgene, Dermira, Eli Lilly, Galderma, Janssen Biotech, LEO Pharma, Merck, Neothetics, Novartis, Pfizer, Regeneron, Stiefel, Symbio/Maruho, Vitae, and Xenoport.
The symposium was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical News.
The pathogenesis theories and treatment approaches to psoriasis have evolved over the past 3 decades, and the latest treatments continue to show safety and effectiveness, according to Alan Menter, MD, chairman of dermatology at Baylor University Medical Center, Dallas.
Before the 1980s, psoriasis was seen as a disease of keratinocyte dysfunction, with treatments that included methotrexate, UVB, and retinoids, Dr. Menter said in a presentation at the annual Coastal Dermatology Symposium. In the 1980s, it was considered an immunologic disease, and then an interleukin (IL)–12/Th1–mediated disease, with anti-CD2, anti-CD11a, and tumor necrosis factor–alpha blocker treatments from 1990 to 2004.
These include risankizumab, which targets the p19 subunit of IL-23 and is being studied for treatment of moderate to severe psoriasis. After one intravenous or subcutaneous dose of risankizumab in a phase 1 study, 16% of patients achieved a Psoriasis Area and Severity Index (PASI) 100, 58% achieved a PASI 90, and 87% achieved a PASI 75, and the publication of phase 2 results are pending, Dr. Menter said. The most common side effects included mild to moderate upper respiratory infections, mild nasopharyngitis, and mild to moderate headaches.
Psoriasis patients treated with guselkumab, which also targets the p19 subunit of IL-23 and was approved in July 2017 for patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy, were significantly more likely to be clear or almost clear at 16 weeks, compared with those on placebo in a phase 2 randomized, controlled trial.
“Both IL-23 and IL-17 are promising targets in the treatment of moderate to severe plaque psoriasis,” said Dr. Menter. “It is important to be vigilant in following the safety profile of these drugs both in clinical trials and in postmarketing registries to ensure their long-term safety,” he added.
Additional research on how to curb side effects associated with these new and emerging therapies should target receptors downstream along the IL-23/Th17 pathway, Dr. Menter explained. Findings from a 2015 study suggest that deficiencies in cytokines and receptors further downstream in the IL-23/Th17 pathway “are associated with fewer disorders than deficiencies in upstream components of the pathway,” he said (J Invest Dermatol. 2015 Aug;135[8]:1946-53).
Although concerns about safety remain, avoiding biologics may have a negative impact as well, as moderate to severe psoriasis patients may experience deformed joints, decreased quality of life, heart attacks, strokes, and early death, Dr. Menter said.
Dr. Menter disclosed having received research support and/or serving as a consultant and/or lecturer for AbbVie, Allergan, Amgen, Anacor, Celgene, Dermira, Eli Lilly, Galderma, Janssen Biotech, LEO Pharma, Merck, Neothetics, Novartis, Pfizer, Regeneron, Stiefel, Symbio/Maruho, Vitae, and Xenoport.
The symposium was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical News.
The pathogenesis theories and treatment approaches to psoriasis have evolved over the past 3 decades, and the latest treatments continue to show safety and effectiveness, according to Alan Menter, MD, chairman of dermatology at Baylor University Medical Center, Dallas.
Before the 1980s, psoriasis was seen as a disease of keratinocyte dysfunction, with treatments that included methotrexate, UVB, and retinoids, Dr. Menter said in a presentation at the annual Coastal Dermatology Symposium. In the 1980s, it was considered an immunologic disease, and then an interleukin (IL)–12/Th1–mediated disease, with anti-CD2, anti-CD11a, and tumor necrosis factor–alpha blocker treatments from 1990 to 2004.
These include risankizumab, which targets the p19 subunit of IL-23 and is being studied for treatment of moderate to severe psoriasis. After one intravenous or subcutaneous dose of risankizumab in a phase 1 study, 16% of patients achieved a Psoriasis Area and Severity Index (PASI) 100, 58% achieved a PASI 90, and 87% achieved a PASI 75, and the publication of phase 2 results are pending, Dr. Menter said. The most common side effects included mild to moderate upper respiratory infections, mild nasopharyngitis, and mild to moderate headaches.
Psoriasis patients treated with guselkumab, which also targets the p19 subunit of IL-23 and was approved in July 2017 for patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy, were significantly more likely to be clear or almost clear at 16 weeks, compared with those on placebo in a phase 2 randomized, controlled trial.
“Both IL-23 and IL-17 are promising targets in the treatment of moderate to severe plaque psoriasis,” said Dr. Menter. “It is important to be vigilant in following the safety profile of these drugs both in clinical trials and in postmarketing registries to ensure their long-term safety,” he added.
Additional research on how to curb side effects associated with these new and emerging therapies should target receptors downstream along the IL-23/Th17 pathway, Dr. Menter explained. Findings from a 2015 study suggest that deficiencies in cytokines and receptors further downstream in the IL-23/Th17 pathway “are associated with fewer disorders than deficiencies in upstream components of the pathway,” he said (J Invest Dermatol. 2015 Aug;135[8]:1946-53).
Although concerns about safety remain, avoiding biologics may have a negative impact as well, as moderate to severe psoriasis patients may experience deformed joints, decreased quality of life, heart attacks, strokes, and early death, Dr. Menter said.
Dr. Menter disclosed having received research support and/or serving as a consultant and/or lecturer for AbbVie, Allergan, Amgen, Anacor, Celgene, Dermira, Eli Lilly, Galderma, Janssen Biotech, LEO Pharma, Merck, Neothetics, Novartis, Pfizer, Regeneron, Stiefel, Symbio/Maruho, Vitae, and Xenoport.
The symposium was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical News.
FROM THE COASTAL DERMATOLOGY SYMPOSIUM
TNF inhibitors associated with fewer side effects than methotrexate in children with psoriasis
Treatment with tumor necrosis factor (TNF) inhibitors was associated with fewer adverse events (AEs) than with methotrexate, in an international, retrospective study of children with psoriasis.
“Patients with pediatric psoriasis treated with methotrexate had a greater risk of having one or more AEs than those treated with TNF-I [tumor necrosis factor inhibitors], although fewer AEs occurred with methotrexate or TNF-I than with other drug classes,” Inge M.G.J. Bronckers, MD, of the department of dermatology at Radboud University, Nijmegen, the Netherlands, and his coauthors reported.
Among those treated with methotrexate, administration of folic acid six to seven times a week was more protective against methotrexate-associated gastrointestinal AEs, than when administered only once a week. The study was published on Sept. 13 in JAMA Dermatology (2017. doi: 10.1001/jamadermatol.2017.3029).
The study evaluated 390 children with moderate to severe psoriasis, treated with at least one systemic medication at 20 centers in Canada, Europe, and the United States, during December 1990-September 2014. They were diagnosed at a mean age of about 8 years, and started systemic therapy a mean of 3 years later. Of the 390 children treated for psoriasis, 270 were treated with methotrexate and 106 were treated with biologics, most often the TNF inhibitor etanercept. The remaining treatments were acitretin, cyclosporine, and fumaric acid esters; almost 19% were treated with more than one medication.
Of those treated with methotrexate, 130 (48.1%) experienced one or more treatment-related AEs, compared with 41 (38.7%) of those treated with a biologic agent (odds ratio, 1.76; P = .03). Almost 25% of those on methotrexate had GI-related AEs, the most common AE; other AEs included elevated transaminase levels and fatigue. Among those on biologics, injection site reactions were the most common (in 18.9%); 12 patients (11.3%) of those on biologics had infections, primarily airway infections.
Compared with those on a TNF inhibitor, patients on methotrexate were more likely to experience GI-related AEs (OR, 11.49; P less than .001) or to discontinue treatment (OR, 5.69; P = .02), the investigators said. But associated infections were more common with TNF inhibitors (OR, 0.36; P = .03), compared with methotrexate. There were no cases of malignancies or tuberculosis.
Folic acid was prescribed to 239 patients receiving methotrexate in one of three regimens: once weekly; six times weekly, avoiding the methotrexate day; and seven times weekly, according to the investigators. Compared with once-weekly treatment, administration six or seven times weekly was associated with a lower probability of developing a GI-related AE (OR, 0.16; P less than .001; OR, 0.21; P = .003, respectively).
“Data are sparse on the relative use of systemic agents and their toxic effects in the pediatric population,” Dr. Bronckers and his coauthors wrote, adding that standardized guidelines and more data concerning children are needed. “Our data suggest that a weekly administration of folic acid could be replaced with a daily or six times weekly administration to reduce GI AEs, although the potential efficacy of six vs. seven times weekly dosing deserves further investigation,” they concluded.
The study was supported by a grant from the International Psoriasis Council. Of the 23 authors, 11 had financial disclosures with various pharmaceutical manufacturers.
Treatment with tumor necrosis factor (TNF) inhibitors was associated with fewer adverse events (AEs) than with methotrexate, in an international, retrospective study of children with psoriasis.
“Patients with pediatric psoriasis treated with methotrexate had a greater risk of having one or more AEs than those treated with TNF-I [tumor necrosis factor inhibitors], although fewer AEs occurred with methotrexate or TNF-I than with other drug classes,” Inge M.G.J. Bronckers, MD, of the department of dermatology at Radboud University, Nijmegen, the Netherlands, and his coauthors reported.
Among those treated with methotrexate, administration of folic acid six to seven times a week was more protective against methotrexate-associated gastrointestinal AEs, than when administered only once a week. The study was published on Sept. 13 in JAMA Dermatology (2017. doi: 10.1001/jamadermatol.2017.3029).
The study evaluated 390 children with moderate to severe psoriasis, treated with at least one systemic medication at 20 centers in Canada, Europe, and the United States, during December 1990-September 2014. They were diagnosed at a mean age of about 8 years, and started systemic therapy a mean of 3 years later. Of the 390 children treated for psoriasis, 270 were treated with methotrexate and 106 were treated with biologics, most often the TNF inhibitor etanercept. The remaining treatments were acitretin, cyclosporine, and fumaric acid esters; almost 19% were treated with more than one medication.
Of those treated with methotrexate, 130 (48.1%) experienced one or more treatment-related AEs, compared with 41 (38.7%) of those treated with a biologic agent (odds ratio, 1.76; P = .03). Almost 25% of those on methotrexate had GI-related AEs, the most common AE; other AEs included elevated transaminase levels and fatigue. Among those on biologics, injection site reactions were the most common (in 18.9%); 12 patients (11.3%) of those on biologics had infections, primarily airway infections.
Compared with those on a TNF inhibitor, patients on methotrexate were more likely to experience GI-related AEs (OR, 11.49; P less than .001) or to discontinue treatment (OR, 5.69; P = .02), the investigators said. But associated infections were more common with TNF inhibitors (OR, 0.36; P = .03), compared with methotrexate. There were no cases of malignancies or tuberculosis.
Folic acid was prescribed to 239 patients receiving methotrexate in one of three regimens: once weekly; six times weekly, avoiding the methotrexate day; and seven times weekly, according to the investigators. Compared with once-weekly treatment, administration six or seven times weekly was associated with a lower probability of developing a GI-related AE (OR, 0.16; P less than .001; OR, 0.21; P = .003, respectively).
“Data are sparse on the relative use of systemic agents and their toxic effects in the pediatric population,” Dr. Bronckers and his coauthors wrote, adding that standardized guidelines and more data concerning children are needed. “Our data suggest that a weekly administration of folic acid could be replaced with a daily or six times weekly administration to reduce GI AEs, although the potential efficacy of six vs. seven times weekly dosing deserves further investigation,” they concluded.
The study was supported by a grant from the International Psoriasis Council. Of the 23 authors, 11 had financial disclosures with various pharmaceutical manufacturers.
Treatment with tumor necrosis factor (TNF) inhibitors was associated with fewer adverse events (AEs) than with methotrexate, in an international, retrospective study of children with psoriasis.
“Patients with pediatric psoriasis treated with methotrexate had a greater risk of having one or more AEs than those treated with TNF-I [tumor necrosis factor inhibitors], although fewer AEs occurred with methotrexate or TNF-I than with other drug classes,” Inge M.G.J. Bronckers, MD, of the department of dermatology at Radboud University, Nijmegen, the Netherlands, and his coauthors reported.
Among those treated with methotrexate, administration of folic acid six to seven times a week was more protective against methotrexate-associated gastrointestinal AEs, than when administered only once a week. The study was published on Sept. 13 in JAMA Dermatology (2017. doi: 10.1001/jamadermatol.2017.3029).
The study evaluated 390 children with moderate to severe psoriasis, treated with at least one systemic medication at 20 centers in Canada, Europe, and the United States, during December 1990-September 2014. They were diagnosed at a mean age of about 8 years, and started systemic therapy a mean of 3 years later. Of the 390 children treated for psoriasis, 270 were treated with methotrexate and 106 were treated with biologics, most often the TNF inhibitor etanercept. The remaining treatments were acitretin, cyclosporine, and fumaric acid esters; almost 19% were treated with more than one medication.
Of those treated with methotrexate, 130 (48.1%) experienced one or more treatment-related AEs, compared with 41 (38.7%) of those treated with a biologic agent (odds ratio, 1.76; P = .03). Almost 25% of those on methotrexate had GI-related AEs, the most common AE; other AEs included elevated transaminase levels and fatigue. Among those on biologics, injection site reactions were the most common (in 18.9%); 12 patients (11.3%) of those on biologics had infections, primarily airway infections.
Compared with those on a TNF inhibitor, patients on methotrexate were more likely to experience GI-related AEs (OR, 11.49; P less than .001) or to discontinue treatment (OR, 5.69; P = .02), the investigators said. But associated infections were more common with TNF inhibitors (OR, 0.36; P = .03), compared with methotrexate. There were no cases of malignancies or tuberculosis.
Folic acid was prescribed to 239 patients receiving methotrexate in one of three regimens: once weekly; six times weekly, avoiding the methotrexate day; and seven times weekly, according to the investigators. Compared with once-weekly treatment, administration six or seven times weekly was associated with a lower probability of developing a GI-related AE (OR, 0.16; P less than .001; OR, 0.21; P = .003, respectively).
“Data are sparse on the relative use of systemic agents and their toxic effects in the pediatric population,” Dr. Bronckers and his coauthors wrote, adding that standardized guidelines and more data concerning children are needed. “Our data suggest that a weekly administration of folic acid could be replaced with a daily or six times weekly administration to reduce GI AEs, although the potential efficacy of six vs. seven times weekly dosing deserves further investigation,” they concluded.
The study was supported by a grant from the International Psoriasis Council. Of the 23 authors, 11 had financial disclosures with various pharmaceutical manufacturers.
FROM JAMA DERMATOLOGY
Key clinical point:
Major finding: Almost half (48.1%) of the children treated with methotrexate experienced one or more treatment-related adverse events, compared with 41 (38.7%) of those treated with a biologic agent (OR, 1.76; P = .03).
Data source: An international, retrospective study of 390 children with moderate to severe psoriasis compared the adverse events associated with different systemic therapies.
Disclosures: The study was supported by a grant from the International Psoriasis Council.
Launch of adalimumab biosimilar Amjevita postponed
Amgen, maker of the adalimumab biosimilar Amjevita (adalimumab-atto) has reached an agreement with AbbVie, manufacturer of the originator adalimumab Humira, that halts marketing of Amjevita in the United States until 2023 and in Europe until 2018, according to a company statement.
The deal between the two manufacturers settles a patent infringement lawsuit that AbbVie brought against Amgen after it received Food and Drug Administration approval in September 2016 for seven of Humira’s nine indications: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, plaque psoriasis, and polyarticular juvenile idiopathic arthritis. Amjevita is not approved for two of Humira’s indications, hidradenitis suppurativa and uveitis.
Amgen said in its statement that AbbVie will grant patent licenses for the use and sale of Amjevita worldwide, on a country-by-country basis, with current expectations that marketing will begin in Europe on Oct. 16, 2018, and in the United States on Jan. 31, 2023. Amjevita is named Amgevita in Europe.
Amgen, maker of the adalimumab biosimilar Amjevita (adalimumab-atto) has reached an agreement with AbbVie, manufacturer of the originator adalimumab Humira, that halts marketing of Amjevita in the United States until 2023 and in Europe until 2018, according to a company statement.
The deal between the two manufacturers settles a patent infringement lawsuit that AbbVie brought against Amgen after it received Food and Drug Administration approval in September 2016 for seven of Humira’s nine indications: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, plaque psoriasis, and polyarticular juvenile idiopathic arthritis. Amjevita is not approved for two of Humira’s indications, hidradenitis suppurativa and uveitis.
Amgen said in its statement that AbbVie will grant patent licenses for the use and sale of Amjevita worldwide, on a country-by-country basis, with current expectations that marketing will begin in Europe on Oct. 16, 2018, and in the United States on Jan. 31, 2023. Amjevita is named Amgevita in Europe.
Amgen, maker of the adalimumab biosimilar Amjevita (adalimumab-atto) has reached an agreement with AbbVie, manufacturer of the originator adalimumab Humira, that halts marketing of Amjevita in the United States until 2023 and in Europe until 2018, according to a company statement.
The deal between the two manufacturers settles a patent infringement lawsuit that AbbVie brought against Amgen after it received Food and Drug Administration approval in September 2016 for seven of Humira’s nine indications: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, plaque psoriasis, and polyarticular juvenile idiopathic arthritis. Amjevita is not approved for two of Humira’s indications, hidradenitis suppurativa and uveitis.
Amgen said in its statement that AbbVie will grant patent licenses for the use and sale of Amjevita worldwide, on a country-by-country basis, with current expectations that marketing will begin in Europe on Oct. 16, 2018, and in the United States on Jan. 31, 2023. Amjevita is named Amgevita in Europe.
Swedish study finds low risk of developing psoriasis in bariatric surgery patients
Obese patients who undergo bariatric surgery have a lower risk of later developing psoriasis, according to results of nonrandomized, longitudinal intervention trial.
Cristina Maglio, MD, of the University of Gothenburg, Sweden, and her associates found that over a 26-year follow-up period, the adjusted hazard ratio (HR) of developing psoriasis was 0.65 (95% confidence interval [CI], 0.47-0.89; P = .008) for patients who underwent bariatric surgery, compared with those who received conventional, nonsurgical obesity treatments. Psoriasis developed in 3.6% of 1,991 patients in the surgery group during follow-up and in 5.1% of 2,018 control patients during follow-up.
Conversely, the difference in the risk of developing psoriatic arthritis (PsA), experienced by up to one-third of patients with psoriasis, was not statistically significant (HR, 0.77; 95% CI, 0.43-1.37; P = .287). PsA developed in 1% of subjects from the surgery group and 1.3% from the control group.
To understand how surgery affected the development of psoriasis or psoriatic arthritis, the researchers conducted a trial with a control group and surgery group. In the control group, 2,018 patients received standard obesity treatments that included recommendations on eating behavior, food selection, and physical activity. The 1,991 patients in the surgery group underwent gastric banding (375), vertical banded gastroplasty (1,354), or gastric bypass (262). At the start of the study, patients were evaluated for baseline measurements, then again at 6 months. After the 6-month mark, patients were reevaluated at 1, 2, 3, 4, 6, 8, 10, 15, and 20 years, respectively. All study participants, regardless of trial group, were examined and presented patient health questionnaires at each follow-up. The endpoint for this study was the first diagnosis of either psoriasis or PsA. Body mass index decreased significantly in the surgery group, compared with virtually no change in the control group.
Vertical banded gastroplasty was found to significantly lower the incidence of psoriasis, compared with usual treatment. But using gastric banding as a reference, vertical banded gastroplasty (HR, 0.80; 95% CI, 0.46-1.39; P = .418) and gastric bypass (HR, 0.71; 95% CI, 0.29-1.71; P = 0.439) were found to have similar effects on the prevention of psoriasis.
The researchers also identified several risk factors that significantly increased the risk of developing psoriasis. Smoking (HR, 1.75; 95% CI, 1.26-2.42; P = .001), a known risk factor in the development of psoriasis, and the length of time a patient had been obese (HR, 1.28; 95% CI, 1.05-1.55; P = .014) were found to be independently associated with an increased risk of psoriasis.
As part of their risk analysis, Dr. Maglio and her colleagues analyzed the interactions of baseline risk factors such as BMI and obesity duration with the bariatric surgery. This analysis found no significant interactions between baseline risk factors and bariatric surgery. It did reveal that patients who were older at baseline evaluation had slightly better responses to bariatric surgery with lower incidences of psoriasis, compared with younger patients, but the differences were not statistically significant.
“The preventive role of bariatric surgery on the risk of psoriasis has been recently highlighted by a retrospective Danish study (JAMA Surg. 2017 Apr 1;152[4]:344-9),” noted Dr. Maglio and her colleagues. “However, we lent strength to the previous results by confirming this association in a large prospective intervention trial designed to examine the effect of bariatric surgery on obesity-related comorbidities in comparison with usual obesity care.
This study was funded in part by the National Institutes of Diabetes and Digestive and Kidney Diseases, the Swedish Rheumatism association, the Swedish Research Council, the University of Gothenburg, and the Swedish federal government. Dr. Anna Rudin reported that part of her salary at Sahlgrenska University is supported by a grant from AstraZeneca. Dr. Lena M.S. Carlsson has received lecture fees from AstraZeneca, Johnson & Johnson, and Merck Sharp and Dohme. Dr. Maglio and Dr. Markku Peltonen had no relevant financial disclosures.
SOURCE: Maglio et al. Obesity. 2017. Dec; 25[12]:2068-73.
Obese patients who undergo bariatric surgery have a lower risk of later developing psoriasis, according to results of nonrandomized, longitudinal intervention trial.
Cristina Maglio, MD, of the University of Gothenburg, Sweden, and her associates found that over a 26-year follow-up period, the adjusted hazard ratio (HR) of developing psoriasis was 0.65 (95% confidence interval [CI], 0.47-0.89; P = .008) for patients who underwent bariatric surgery, compared with those who received conventional, nonsurgical obesity treatments. Psoriasis developed in 3.6% of 1,991 patients in the surgery group during follow-up and in 5.1% of 2,018 control patients during follow-up.
Conversely, the difference in the risk of developing psoriatic arthritis (PsA), experienced by up to one-third of patients with psoriasis, was not statistically significant (HR, 0.77; 95% CI, 0.43-1.37; P = .287). PsA developed in 1% of subjects from the surgery group and 1.3% from the control group.
To understand how surgery affected the development of psoriasis or psoriatic arthritis, the researchers conducted a trial with a control group and surgery group. In the control group, 2,018 patients received standard obesity treatments that included recommendations on eating behavior, food selection, and physical activity. The 1,991 patients in the surgery group underwent gastric banding (375), vertical banded gastroplasty (1,354), or gastric bypass (262). At the start of the study, patients were evaluated for baseline measurements, then again at 6 months. After the 6-month mark, patients were reevaluated at 1, 2, 3, 4, 6, 8, 10, 15, and 20 years, respectively. All study participants, regardless of trial group, were examined and presented patient health questionnaires at each follow-up. The endpoint for this study was the first diagnosis of either psoriasis or PsA. Body mass index decreased significantly in the surgery group, compared with virtually no change in the control group.
Vertical banded gastroplasty was found to significantly lower the incidence of psoriasis, compared with usual treatment. But using gastric banding as a reference, vertical banded gastroplasty (HR, 0.80; 95% CI, 0.46-1.39; P = .418) and gastric bypass (HR, 0.71; 95% CI, 0.29-1.71; P = 0.439) were found to have similar effects on the prevention of psoriasis.
The researchers also identified several risk factors that significantly increased the risk of developing psoriasis. Smoking (HR, 1.75; 95% CI, 1.26-2.42; P = .001), a known risk factor in the development of psoriasis, and the length of time a patient had been obese (HR, 1.28; 95% CI, 1.05-1.55; P = .014) were found to be independently associated with an increased risk of psoriasis.
As part of their risk analysis, Dr. Maglio and her colleagues analyzed the interactions of baseline risk factors such as BMI and obesity duration with the bariatric surgery. This analysis found no significant interactions between baseline risk factors and bariatric surgery. It did reveal that patients who were older at baseline evaluation had slightly better responses to bariatric surgery with lower incidences of psoriasis, compared with younger patients, but the differences were not statistically significant.
“The preventive role of bariatric surgery on the risk of psoriasis has been recently highlighted by a retrospective Danish study (JAMA Surg. 2017 Apr 1;152[4]:344-9),” noted Dr. Maglio and her colleagues. “However, we lent strength to the previous results by confirming this association in a large prospective intervention trial designed to examine the effect of bariatric surgery on obesity-related comorbidities in comparison with usual obesity care.
This study was funded in part by the National Institutes of Diabetes and Digestive and Kidney Diseases, the Swedish Rheumatism association, the Swedish Research Council, the University of Gothenburg, and the Swedish federal government. Dr. Anna Rudin reported that part of her salary at Sahlgrenska University is supported by a grant from AstraZeneca. Dr. Lena M.S. Carlsson has received lecture fees from AstraZeneca, Johnson & Johnson, and Merck Sharp and Dohme. Dr. Maglio and Dr. Markku Peltonen had no relevant financial disclosures.
SOURCE: Maglio et al. Obesity. 2017. Dec; 25[12]:2068-73.
Obese patients who undergo bariatric surgery have a lower risk of later developing psoriasis, according to results of nonrandomized, longitudinal intervention trial.
Cristina Maglio, MD, of the University of Gothenburg, Sweden, and her associates found that over a 26-year follow-up period, the adjusted hazard ratio (HR) of developing psoriasis was 0.65 (95% confidence interval [CI], 0.47-0.89; P = .008) for patients who underwent bariatric surgery, compared with those who received conventional, nonsurgical obesity treatments. Psoriasis developed in 3.6% of 1,991 patients in the surgery group during follow-up and in 5.1% of 2,018 control patients during follow-up.
Conversely, the difference in the risk of developing psoriatic arthritis (PsA), experienced by up to one-third of patients with psoriasis, was not statistically significant (HR, 0.77; 95% CI, 0.43-1.37; P = .287). PsA developed in 1% of subjects from the surgery group and 1.3% from the control group.
To understand how surgery affected the development of psoriasis or psoriatic arthritis, the researchers conducted a trial with a control group and surgery group. In the control group, 2,018 patients received standard obesity treatments that included recommendations on eating behavior, food selection, and physical activity. The 1,991 patients in the surgery group underwent gastric banding (375), vertical banded gastroplasty (1,354), or gastric bypass (262). At the start of the study, patients were evaluated for baseline measurements, then again at 6 months. After the 6-month mark, patients were reevaluated at 1, 2, 3, 4, 6, 8, 10, 15, and 20 years, respectively. All study participants, regardless of trial group, were examined and presented patient health questionnaires at each follow-up. The endpoint for this study was the first diagnosis of either psoriasis or PsA. Body mass index decreased significantly in the surgery group, compared with virtually no change in the control group.
Vertical banded gastroplasty was found to significantly lower the incidence of psoriasis, compared with usual treatment. But using gastric banding as a reference, vertical banded gastroplasty (HR, 0.80; 95% CI, 0.46-1.39; P = .418) and gastric bypass (HR, 0.71; 95% CI, 0.29-1.71; P = 0.439) were found to have similar effects on the prevention of psoriasis.
The researchers also identified several risk factors that significantly increased the risk of developing psoriasis. Smoking (HR, 1.75; 95% CI, 1.26-2.42; P = .001), a known risk factor in the development of psoriasis, and the length of time a patient had been obese (HR, 1.28; 95% CI, 1.05-1.55; P = .014) were found to be independently associated with an increased risk of psoriasis.
As part of their risk analysis, Dr. Maglio and her colleagues analyzed the interactions of baseline risk factors such as BMI and obesity duration with the bariatric surgery. This analysis found no significant interactions between baseline risk factors and bariatric surgery. It did reveal that patients who were older at baseline evaluation had slightly better responses to bariatric surgery with lower incidences of psoriasis, compared with younger patients, but the differences were not statistically significant.
“The preventive role of bariatric surgery on the risk of psoriasis has been recently highlighted by a retrospective Danish study (JAMA Surg. 2017 Apr 1;152[4]:344-9),” noted Dr. Maglio and her colleagues. “However, we lent strength to the previous results by confirming this association in a large prospective intervention trial designed to examine the effect of bariatric surgery on obesity-related comorbidities in comparison with usual obesity care.
This study was funded in part by the National Institutes of Diabetes and Digestive and Kidney Diseases, the Swedish Rheumatism association, the Swedish Research Council, the University of Gothenburg, and the Swedish federal government. Dr. Anna Rudin reported that part of her salary at Sahlgrenska University is supported by a grant from AstraZeneca. Dr. Lena M.S. Carlsson has received lecture fees from AstraZeneca, Johnson & Johnson, and Merck Sharp and Dohme. Dr. Maglio and Dr. Markku Peltonen had no relevant financial disclosures.
SOURCE: Maglio et al. Obesity. 2017. Dec; 25[12]:2068-73.
FROM OBESITY
Key clinical point:
Major finding: Obese patients who underwent bariatric surgery had a lower incidence of psoriasis over a 26-year period (HR, 0.65; 95% CI: 0.47-0.89; P = .008), compared with usual care.
Study details: Swedish Obese Subjects study, a longitudinal, nonrandomized intervention trial comprising 1,991 surgery group patients and 2,018 control patients.
Disclosures: This study was funded in part by the National Institutes of Diabetes and Digestive and Kidney Diseases, the Swedish Rheumatism association, the Swedish Research Council, the University of Gothenburg, and the Swedish federal government. Dr. Anna Rudin reported that part of her salary at Sahlgrenska University is supported by a grant from AstraZeneca. Dr. Lena M.S. Carlsson has received lecture fees from AstraZeneca, Johnson & Johnson, and Merck Sharp and Dohme. Dr. Maglio and Dr. Markku Peltonen had no relevant financial disclosures.
Source: Maglio et al. Obesity. 2017. Dec; 25[12]:2068-2073.
Smoking linked to increased psoriasis risk
Current and former smokers were significantly more likely to have psoriasis than were nonsmokers in an analysis of the Korean National Health Insurance database.
Multivariate analyses produced adjusted incidence rates of 1.14 for current smokers (n = 132,566) and 1.11 for former smokers (n = 47,477), compared with nonsmokers (n = 320,435), indicating “that smoking status is an independent potential risk factor for psoriasis,” reported Eun Joo Lee, PhD, of the National Health Insurance Service in Wonjusi, South Korea, and associates (J Am Acad Dermatol. 2017;77[3]:573-5).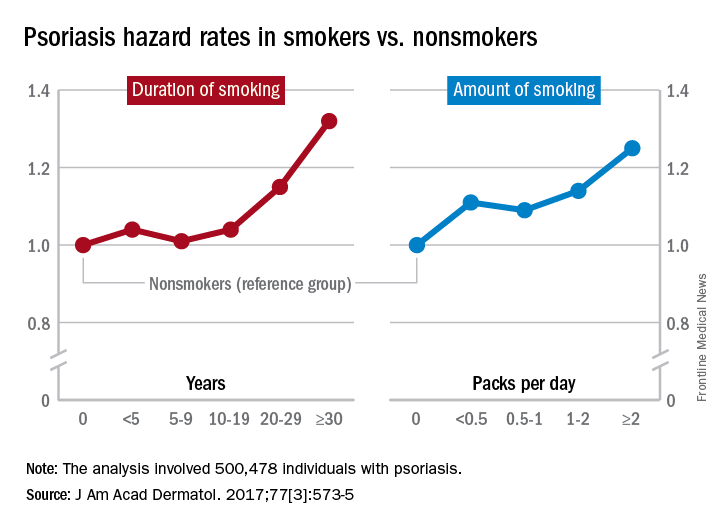
The study was supported by a grant from the National Research Foundation of Korea that was funded by the Korean government. The investigators did not declare any conflicts of interest.
Current and former smokers were significantly more likely to have psoriasis than were nonsmokers in an analysis of the Korean National Health Insurance database.
Multivariate analyses produced adjusted incidence rates of 1.14 for current smokers (n = 132,566) and 1.11 for former smokers (n = 47,477), compared with nonsmokers (n = 320,435), indicating “that smoking status is an independent potential risk factor for psoriasis,” reported Eun Joo Lee, PhD, of the National Health Insurance Service in Wonjusi, South Korea, and associates (J Am Acad Dermatol. 2017;77[3]:573-5).
The study was supported by a grant from the National Research Foundation of Korea that was funded by the Korean government. The investigators did not declare any conflicts of interest.
Current and former smokers were significantly more likely to have psoriasis than were nonsmokers in an analysis of the Korean National Health Insurance database.
Multivariate analyses produced adjusted incidence rates of 1.14 for current smokers (n = 132,566) and 1.11 for former smokers (n = 47,477), compared with nonsmokers (n = 320,435), indicating “that smoking status is an independent potential risk factor for psoriasis,” reported Eun Joo Lee, PhD, of the National Health Insurance Service in Wonjusi, South Korea, and associates (J Am Acad Dermatol. 2017;77[3]:573-5).
The study was supported by a grant from the National Research Foundation of Korea that was funded by the Korean government. The investigators did not declare any conflicts of interest.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
50 years of pediatric dermatology
The world in pediatric dermatology has changed in incredible ways since 1967. In fact, pediatric dermatology was not an organized specialty until years later! This article will look back at some of the history of pediatric dermatology, exploring how different the field was 50 years ago, and how it has evolved into the vibrant field that it is. By looking at some disease states, and differences in practice in relation to the care of dermatologic conditions in children both by pediatricians and dermatologists, we can see the tremendous evolution in our understanding and management of pediatric skin conditions, and perhaps gain insight into the future.
Pediatric dermatology was fairly “neonatal” 50 years ago, with only a few practitioners in the field. Recognizing that up to 30% of pediatric primary care visits include a skin-related problem, and that there was limited training about skin diseases among primary care practitioners and inconsistent training amongst dermatologists, there was a clinical need for establishing the subspecialty of pediatric dermatology. The first international symposium was held in Mexico City in October 1972, and with this meeting the International Society of Pediatric Dermatology was founded. The Society for Pediatric Dermatology (SPD) began in 1973, with Alvin Jacobs, MD, Samuel Weinberg, MD, Nancy Esterly, MD, Sidney Hurwitz, MD, William Weston, MD, and Coleman Jacobson, MD, as some of the initial “founding mothers and fathers.” The journal Pediatric Dermatology released its first issue in 1982 (35 years ago), and the American Academy of Pediatrics did not have a section of dermatology until 1986.
Pediatrics and dermatology: The interface
Many of the first generation of pediatric dermatologists trained as pediatricians prior to pursuing their dermatology work, with some being “assigned” dermatology as pediatric experts, while others did formal residencies in dermatology. This history is important, as pediatric dermatology was, and remains, integrated with pediatrics, even while training in dermatology residencies became standard practice. An important part of the development of the field has been the education of pediatricians and dermatologists by pediatric dermatologists, with a strong sensibility that improved training for both generalists and specialists about pediatric skin disease would yield better care for patients and families.
Initially, there were very few pediatric or dermatology programs in the United States that had pediatric dermatologists. Over the succeeding decades, this is now less common, although even now there are still dermatology and pediatric residency programs that do not have a pediatric dermatologist for either training or to serve their patients. The founding leaders of the SPD set a tone of collaboration nationally and internationally, reaching out to pediatric colleagues and dermatology associates from around the world, and establishing superb educational programs for the exchange of ideas, presentation of challenging cases, and promoting state of the art knowledge of the field. Through annual meetings of the SPD, conferences immediately preceding the American Academy of Dermatology annual meetings, the World Congress of Pediatric Dermatology, and other regional and international meetings, the field developed as the number of practitioners grew, and as the specialized published literature reflected new knowledge in diagnosis and therapy.
Building upon the history of collaboration and reflecting the maturation of the field with a desire to influence the breadth and quantity of research in pediatric dermatology, the Pediatric Dermatology Research Alliance (PeDRA) was formed in 2012. This organization was formed to promote and facilitate high quality collaborative clinical, translational, educational, and basic science research in pediatric dermatology with a vision to create sustainable, collaborative networks to better understand, prevent, treat, and cure dermatologic diseases in children. This network is now composed of over 230 members representing over 68 institutions from the United States and Canada, but including involvement globally from Mexico, Europe, and the Middle East.
Examples of changing perspectives: hemangiomas
A good way to look at evolution of the field is take a look at some of the similarities and differences in clinical practice in relation to common and uncommon disease states.
A great example is hemangiomas. Some of the first natural history studies on hemangiomas were done in the early 1960s, establishing that many lesions had a typical clinical course of fairly rapid growth, plateau, and involution over time. Of course, the identification of hemangiomas of infancy (or “HOI” in the trade), was confused with vascular malformations, and no one had recognized variant tumors that were distinct, such as rapidly involuting and noninvoluting congenital hemangiomas (RICHs or NICHs), tufted angiomas, and hemangioendotheliomas. PHACE syndrome (posterior fossa brain malformations) had yet to be described (that was done in 1996 by Ilona Frieden and her colleagues). For a time period, hemangiomas were treated with X-rays, before the negative impact of such radiation was acknowledged. For many years after that, even deforming and functionally significant lesions were “followed clinically” for natural involution, presumably a backlash from the radiation therapy interventions.
This story also reflects how organized research efforts helped with the evolution of knowledge and clinical care. The Hemangioma of Infancy Group was formed to take a collaborative approach to characterize and study hemangiomas and related tumors. Beginning with energetic, insightful pediatric dermatologists, and little funding, they changed our knowledge base of how hemangiomas present, the risk factors for their development and the characteristics and multiple organ findings associated with PHACE and other syndromic hemangiomas.
Procedural pediatric dermatology: Tremendous revolution in surgery and laser
The first generation of pediatric dermatologists were considered medical dermatologist specialists. And how important this specialty work was! Acne, atopic dermatitis, psoriasis, diaper and seborrheic dermatitis, and rare genetic syndromes, these conditions were a major part of the work of early pediatric dermatologists (and remain so now). What was not common was for pediatric dermatologists to have procedural or surgical practices, while this now is routinely part of the work of specialists in the field. How did this shift occur?
The fundamental shift began to occur with the introduction of the pulsed dye laser in 1989 and the publication of a seminal article in the New England Journal of Medicine (1989 Feb 16;320[7]:416-21) on its utility in treating port-wine stains in children with minimal scarring. Vascular lesions including port-wine stains were common, and pediatric dermatologists managed these patients for both diagnosis and medical management. Also, dermatology residencies at this time offered training in cutaneous surgery, excisions (including Mohs surgery) and repairs, and trainees in pediatric dermatology were “trained up” to high levels of expertise. As lasers were incorporated into dermatology residency work and practices, pediatric dermatologists had the exposure and skill to do this work. An added advantage was having the pediatric knowledge of how to handle children and adolescents in an age appropriate manner, and consideration of methods to minimize the pain and anxiety of procedures. Within a few years, pediatric dermatologists were at the forefront of the use of topical anesthetics (EMLA and liposomal lidocaine) and had general anesthesia privileges for laser and excisional surgery.
So while pediatric dermatologists still do “small procedures” every hour in most practices (cryotherapy for warts, cantharidin for molluscum, shave and punch biopsies), a subset now have extensive procedural practices, which in recent years has extended to pigment lesion lasers (to treat nevus of Ota), hair lasers (to treat perineal areas to prevent pilonidal cyst recurrence or to treat hirsutism), and combinations of lasers to treat hypertrophic, constrictive, and/or deforming scars).
Inflammatory skin disorders: Bread and butter ... and peanut butter?
The care of pediatric inflammatory skin disorders has evolved, but more slowly for some diseases than others. Acne vulgaris now is recognized as much more common under age 12 years than previously, presumably reflecting earlier pubertal changes in our preteens. Over the past 30 years, therapy has evolved with the use of topical retinoids (still underused by pediatricians, considered a “practice gap”), hormonal therapy with combined oral contraceptives, and oral isotretinoin, a powerful but highly effective systemic agent for severe and refractory acne. Specific pediatric guidelines came much later. Pediatric acne expert recommendations were formulated by the American Acne and Rosacea Society and endorsed by the American Academy of Pediatrics in 2013 (Pediatrics. 2013;131:S163-86). Over the past few years, there is a push by experts for more judicious use of antibiotics for acne (oral and topical) to minimize the emergence of bacterial resistance.
Psoriasis has been a condition that has been “behind the revolution,” in that no biologic agent was approved for pediatric psoriasis in the United States until several months ago, lagging behind Europe and elsewhere in the world by almost a decade. Adult psoriasis has been recognized to be associated with a broad set of comorbidities, including obesity and early heart disease, and there is now research on how children are at risk as well, and new recommendations on how to screen children with psoriasis. Moderate to severe psoriasis in adults is now tremendously controllable with biologic agents targeting TNF-alpha, IL 12/23, and IL-17. Etanercept has been approved for children with psoriasis aged 4 years and older, and other biologic agents are under study.
Atopic dermatitis now is ready for its revolution! AD has increased in prevalence from around 5% of the pediatric population 30-plus years ago to 10%-15%. Treatment of most individuals has remained the same over the decades: Good skin care, frequent moisturizers, topical corticosteroids for flares, management of infection if noted. The topical calcineurin inhibitors (TCIs) broadened the therapeutic approach when introduced in 2000 and 2001, but the boxed warning resulted in some practitioners minimizing their utilization of these useful agents.

It has been recognized for years that children with AD have higher risk of developing food allergies than children without AD. A changing understanding of how early food exposure may induce tolerance is changing the world of allergy and influencing the care of children with AD. This is where the peanut butter (or other processed peanut, such as “Bamba”) may be life saving. New guidelines have come from the National Institute of Allergy and Infectious Diseases recommending that infants with severe eczema (or egg allergy, or both) have introduction of age-appropriate peanut-containing food as early as 4-6 months of age to reduce the risk of development of peanut allergy. It is recommended that these infants undergo early evaluation for possible sensitization to peanut protein, with referral to allergists for skin prick tests or serum IgE screens (though if positive, referral to allergists is appropriate), and assess the safety of going ahead with early feeding. It is hoped that following these new guidelines can minimize the development of peanut allergy.
The future
Where will pediatric skin disease, or more importantly, skin health over a lifetime be in 50 years? Can we cure or prevent the consequences of our lethal and life altering genetic diseases such as epidermolysis bullosa or our neurocutaneous disorders? Will our new insights into birthmarks (they are mostly somatic mutations) allow us to form specific, personalized therapies to minimize their impact? Will we be using computers equipped with imaging devices and algorithms to assess our patients’ moles, papules, and nodules? Will our vaccines have wiped out warts, molluscum, and perhaps, acne? Will we have cured our inflammatory skin disorders, or perhaps prevented them by interventions in the neonatal period? No predictions will be offered here, other than that we can look forward to incredible changes for our future generations of health care practitioners, patients, and families.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Eichenfield has served as a consultant for Anacor/Pfizer and Regeneron/Sanofi. Email him at [email protected].
The world in pediatric dermatology has changed in incredible ways since 1967. In fact, pediatric dermatology was not an organized specialty until years later! This article will look back at some of the history of pediatric dermatology, exploring how different the field was 50 years ago, and how it has evolved into the vibrant field that it is. By looking at some disease states, and differences in practice in relation to the care of dermatologic conditions in children both by pediatricians and dermatologists, we can see the tremendous evolution in our understanding and management of pediatric skin conditions, and perhaps gain insight into the future.
Pediatric dermatology was fairly “neonatal” 50 years ago, with only a few practitioners in the field. Recognizing that up to 30% of pediatric primary care visits include a skin-related problem, and that there was limited training about skin diseases among primary care practitioners and inconsistent training amongst dermatologists, there was a clinical need for establishing the subspecialty of pediatric dermatology. The first international symposium was held in Mexico City in October 1972, and with this meeting the International Society of Pediatric Dermatology was founded. The Society for Pediatric Dermatology (SPD) began in 1973, with Alvin Jacobs, MD, Samuel Weinberg, MD, Nancy Esterly, MD, Sidney Hurwitz, MD, William Weston, MD, and Coleman Jacobson, MD, as some of the initial “founding mothers and fathers.” The journal Pediatric Dermatology released its first issue in 1982 (35 years ago), and the American Academy of Pediatrics did not have a section of dermatology until 1986.
Pediatrics and dermatology: The interface
Many of the first generation of pediatric dermatologists trained as pediatricians prior to pursuing their dermatology work, with some being “assigned” dermatology as pediatric experts, while others did formal residencies in dermatology. This history is important, as pediatric dermatology was, and remains, integrated with pediatrics, even while training in dermatology residencies became standard practice. An important part of the development of the field has been the education of pediatricians and dermatologists by pediatric dermatologists, with a strong sensibility that improved training for both generalists and specialists about pediatric skin disease would yield better care for patients and families.
Initially, there were very few pediatric or dermatology programs in the United States that had pediatric dermatologists. Over the succeeding decades, this is now less common, although even now there are still dermatology and pediatric residency programs that do not have a pediatric dermatologist for either training or to serve their patients. The founding leaders of the SPD set a tone of collaboration nationally and internationally, reaching out to pediatric colleagues and dermatology associates from around the world, and establishing superb educational programs for the exchange of ideas, presentation of challenging cases, and promoting state of the art knowledge of the field. Through annual meetings of the SPD, conferences immediately preceding the American Academy of Dermatology annual meetings, the World Congress of Pediatric Dermatology, and other regional and international meetings, the field developed as the number of practitioners grew, and as the specialized published literature reflected new knowledge in diagnosis and therapy.
Building upon the history of collaboration and reflecting the maturation of the field with a desire to influence the breadth and quantity of research in pediatric dermatology, the Pediatric Dermatology Research Alliance (PeDRA) was formed in 2012. This organization was formed to promote and facilitate high quality collaborative clinical, translational, educational, and basic science research in pediatric dermatology with a vision to create sustainable, collaborative networks to better understand, prevent, treat, and cure dermatologic diseases in children. This network is now composed of over 230 members representing over 68 institutions from the United States and Canada, but including involvement globally from Mexico, Europe, and the Middle East.
Examples of changing perspectives: hemangiomas
A good way to look at evolution of the field is take a look at some of the similarities and differences in clinical practice in relation to common and uncommon disease states.
A great example is hemangiomas. Some of the first natural history studies on hemangiomas were done in the early 1960s, establishing that many lesions had a typical clinical course of fairly rapid growth, plateau, and involution over time. Of course, the identification of hemangiomas of infancy (or “HOI” in the trade), was confused with vascular malformations, and no one had recognized variant tumors that were distinct, such as rapidly involuting and noninvoluting congenital hemangiomas (RICHs or NICHs), tufted angiomas, and hemangioendotheliomas. PHACE syndrome (posterior fossa brain malformations) had yet to be described (that was done in 1996 by Ilona Frieden and her colleagues). For a time period, hemangiomas were treated with X-rays, before the negative impact of such radiation was acknowledged. For many years after that, even deforming and functionally significant lesions were “followed clinically” for natural involution, presumably a backlash from the radiation therapy interventions.
This story also reflects how organized research efforts helped with the evolution of knowledge and clinical care. The Hemangioma of Infancy Group was formed to take a collaborative approach to characterize and study hemangiomas and related tumors. Beginning with energetic, insightful pediatric dermatologists, and little funding, they changed our knowledge base of how hemangiomas present, the risk factors for their development and the characteristics and multiple organ findings associated with PHACE and other syndromic hemangiomas.
Procedural pediatric dermatology: Tremendous revolution in surgery and laser
The first generation of pediatric dermatologists were considered medical dermatologist specialists. And how important this specialty work was! Acne, atopic dermatitis, psoriasis, diaper and seborrheic dermatitis, and rare genetic syndromes, these conditions were a major part of the work of early pediatric dermatologists (and remain so now). What was not common was for pediatric dermatologists to have procedural or surgical practices, while this now is routinely part of the work of specialists in the field. How did this shift occur?
The fundamental shift began to occur with the introduction of the pulsed dye laser in 1989 and the publication of a seminal article in the New England Journal of Medicine (1989 Feb 16;320[7]:416-21) on its utility in treating port-wine stains in children with minimal scarring. Vascular lesions including port-wine stains were common, and pediatric dermatologists managed these patients for both diagnosis and medical management. Also, dermatology residencies at this time offered training in cutaneous surgery, excisions (including Mohs surgery) and repairs, and trainees in pediatric dermatology were “trained up” to high levels of expertise. As lasers were incorporated into dermatology residency work and practices, pediatric dermatologists had the exposure and skill to do this work. An added advantage was having the pediatric knowledge of how to handle children and adolescents in an age appropriate manner, and consideration of methods to minimize the pain and anxiety of procedures. Within a few years, pediatric dermatologists were at the forefront of the use of topical anesthetics (EMLA and liposomal lidocaine) and had general anesthesia privileges for laser and excisional surgery.
So while pediatric dermatologists still do “small procedures” every hour in most practices (cryotherapy for warts, cantharidin for molluscum, shave and punch biopsies), a subset now have extensive procedural practices, which in recent years has extended to pigment lesion lasers (to treat nevus of Ota), hair lasers (to treat perineal areas to prevent pilonidal cyst recurrence or to treat hirsutism), and combinations of lasers to treat hypertrophic, constrictive, and/or deforming scars).
Inflammatory skin disorders: Bread and butter ... and peanut butter?
The care of pediatric inflammatory skin disorders has evolved, but more slowly for some diseases than others. Acne vulgaris now is recognized as much more common under age 12 years than previously, presumably reflecting earlier pubertal changes in our preteens. Over the past 30 years, therapy has evolved with the use of topical retinoids (still underused by pediatricians, considered a “practice gap”), hormonal therapy with combined oral contraceptives, and oral isotretinoin, a powerful but highly effective systemic agent for severe and refractory acne. Specific pediatric guidelines came much later. Pediatric acne expert recommendations were formulated by the American Acne and Rosacea Society and endorsed by the American Academy of Pediatrics in 2013 (Pediatrics. 2013;131:S163-86). Over the past few years, there is a push by experts for more judicious use of antibiotics for acne (oral and topical) to minimize the emergence of bacterial resistance.
Psoriasis has been a condition that has been “behind the revolution,” in that no biologic agent was approved for pediatric psoriasis in the United States until several months ago, lagging behind Europe and elsewhere in the world by almost a decade. Adult psoriasis has been recognized to be associated with a broad set of comorbidities, including obesity and early heart disease, and there is now research on how children are at risk as well, and new recommendations on how to screen children with psoriasis. Moderate to severe psoriasis in adults is now tremendously controllable with biologic agents targeting TNF-alpha, IL 12/23, and IL-17. Etanercept has been approved for children with psoriasis aged 4 years and older, and other biologic agents are under study.
Atopic dermatitis now is ready for its revolution! AD has increased in prevalence from around 5% of the pediatric population 30-plus years ago to 10%-15%. Treatment of most individuals has remained the same over the decades: Good skin care, frequent moisturizers, topical corticosteroids for flares, management of infection if noted. The topical calcineurin inhibitors (TCIs) broadened the therapeutic approach when introduced in 2000 and 2001, but the boxed warning resulted in some practitioners minimizing their utilization of these useful agents.

It has been recognized for years that children with AD have higher risk of developing food allergies than children without AD. A changing understanding of how early food exposure may induce tolerance is changing the world of allergy and influencing the care of children with AD. This is where the peanut butter (or other processed peanut, such as “Bamba”) may be life saving. New guidelines have come from the National Institute of Allergy and Infectious Diseases recommending that infants with severe eczema (or egg allergy, or both) have introduction of age-appropriate peanut-containing food as early as 4-6 months of age to reduce the risk of development of peanut allergy. It is recommended that these infants undergo early evaluation for possible sensitization to peanut protein, with referral to allergists for skin prick tests or serum IgE screens (though if positive, referral to allergists is appropriate), and assess the safety of going ahead with early feeding. It is hoped that following these new guidelines can minimize the development of peanut allergy.
The future
Where will pediatric skin disease, or more importantly, skin health over a lifetime be in 50 years? Can we cure or prevent the consequences of our lethal and life altering genetic diseases such as epidermolysis bullosa or our neurocutaneous disorders? Will our new insights into birthmarks (they are mostly somatic mutations) allow us to form specific, personalized therapies to minimize their impact? Will we be using computers equipped with imaging devices and algorithms to assess our patients’ moles, papules, and nodules? Will our vaccines have wiped out warts, molluscum, and perhaps, acne? Will we have cured our inflammatory skin disorders, or perhaps prevented them by interventions in the neonatal period? No predictions will be offered here, other than that we can look forward to incredible changes for our future generations of health care practitioners, patients, and families.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Eichenfield has served as a consultant for Anacor/Pfizer and Regeneron/Sanofi. Email him at [email protected].
The world in pediatric dermatology has changed in incredible ways since 1967. In fact, pediatric dermatology was not an organized specialty until years later! This article will look back at some of the history of pediatric dermatology, exploring how different the field was 50 years ago, and how it has evolved into the vibrant field that it is. By looking at some disease states, and differences in practice in relation to the care of dermatologic conditions in children both by pediatricians and dermatologists, we can see the tremendous evolution in our understanding and management of pediatric skin conditions, and perhaps gain insight into the future.
Pediatric dermatology was fairly “neonatal” 50 years ago, with only a few practitioners in the field. Recognizing that up to 30% of pediatric primary care visits include a skin-related problem, and that there was limited training about skin diseases among primary care practitioners and inconsistent training amongst dermatologists, there was a clinical need for establishing the subspecialty of pediatric dermatology. The first international symposium was held in Mexico City in October 1972, and with this meeting the International Society of Pediatric Dermatology was founded. The Society for Pediatric Dermatology (SPD) began in 1973, with Alvin Jacobs, MD, Samuel Weinberg, MD, Nancy Esterly, MD, Sidney Hurwitz, MD, William Weston, MD, and Coleman Jacobson, MD, as some of the initial “founding mothers and fathers.” The journal Pediatric Dermatology released its first issue in 1982 (35 years ago), and the American Academy of Pediatrics did not have a section of dermatology until 1986.
Pediatrics and dermatology: The interface
Many of the first generation of pediatric dermatologists trained as pediatricians prior to pursuing their dermatology work, with some being “assigned” dermatology as pediatric experts, while others did formal residencies in dermatology. This history is important, as pediatric dermatology was, and remains, integrated with pediatrics, even while training in dermatology residencies became standard practice. An important part of the development of the field has been the education of pediatricians and dermatologists by pediatric dermatologists, with a strong sensibility that improved training for both generalists and specialists about pediatric skin disease would yield better care for patients and families.
Initially, there were very few pediatric or dermatology programs in the United States that had pediatric dermatologists. Over the succeeding decades, this is now less common, although even now there are still dermatology and pediatric residency programs that do not have a pediatric dermatologist for either training or to serve their patients. The founding leaders of the SPD set a tone of collaboration nationally and internationally, reaching out to pediatric colleagues and dermatology associates from around the world, and establishing superb educational programs for the exchange of ideas, presentation of challenging cases, and promoting state of the art knowledge of the field. Through annual meetings of the SPD, conferences immediately preceding the American Academy of Dermatology annual meetings, the World Congress of Pediatric Dermatology, and other regional and international meetings, the field developed as the number of practitioners grew, and as the specialized published literature reflected new knowledge in diagnosis and therapy.
Building upon the history of collaboration and reflecting the maturation of the field with a desire to influence the breadth and quantity of research in pediatric dermatology, the Pediatric Dermatology Research Alliance (PeDRA) was formed in 2012. This organization was formed to promote and facilitate high quality collaborative clinical, translational, educational, and basic science research in pediatric dermatology with a vision to create sustainable, collaborative networks to better understand, prevent, treat, and cure dermatologic diseases in children. This network is now composed of over 230 members representing over 68 institutions from the United States and Canada, but including involvement globally from Mexico, Europe, and the Middle East.
Examples of changing perspectives: hemangiomas
A good way to look at evolution of the field is take a look at some of the similarities and differences in clinical practice in relation to common and uncommon disease states.
A great example is hemangiomas. Some of the first natural history studies on hemangiomas were done in the early 1960s, establishing that many lesions had a typical clinical course of fairly rapid growth, plateau, and involution over time. Of course, the identification of hemangiomas of infancy (or “HOI” in the trade), was confused with vascular malformations, and no one had recognized variant tumors that were distinct, such as rapidly involuting and noninvoluting congenital hemangiomas (RICHs or NICHs), tufted angiomas, and hemangioendotheliomas. PHACE syndrome (posterior fossa brain malformations) had yet to be described (that was done in 1996 by Ilona Frieden and her colleagues). For a time period, hemangiomas were treated with X-rays, before the negative impact of such radiation was acknowledged. For many years after that, even deforming and functionally significant lesions were “followed clinically” for natural involution, presumably a backlash from the radiation therapy interventions.
This story also reflects how organized research efforts helped with the evolution of knowledge and clinical care. The Hemangioma of Infancy Group was formed to take a collaborative approach to characterize and study hemangiomas and related tumors. Beginning with energetic, insightful pediatric dermatologists, and little funding, they changed our knowledge base of how hemangiomas present, the risk factors for their development and the characteristics and multiple organ findings associated with PHACE and other syndromic hemangiomas.
Procedural pediatric dermatology: Tremendous revolution in surgery and laser
The first generation of pediatric dermatologists were considered medical dermatologist specialists. And how important this specialty work was! Acne, atopic dermatitis, psoriasis, diaper and seborrheic dermatitis, and rare genetic syndromes, these conditions were a major part of the work of early pediatric dermatologists (and remain so now). What was not common was for pediatric dermatologists to have procedural or surgical practices, while this now is routinely part of the work of specialists in the field. How did this shift occur?
The fundamental shift began to occur with the introduction of the pulsed dye laser in 1989 and the publication of a seminal article in the New England Journal of Medicine (1989 Feb 16;320[7]:416-21) on its utility in treating port-wine stains in children with minimal scarring. Vascular lesions including port-wine stains were common, and pediatric dermatologists managed these patients for both diagnosis and medical management. Also, dermatology residencies at this time offered training in cutaneous surgery, excisions (including Mohs surgery) and repairs, and trainees in pediatric dermatology were “trained up” to high levels of expertise. As lasers were incorporated into dermatology residency work and practices, pediatric dermatologists had the exposure and skill to do this work. An added advantage was having the pediatric knowledge of how to handle children and adolescents in an age appropriate manner, and consideration of methods to minimize the pain and anxiety of procedures. Within a few years, pediatric dermatologists were at the forefront of the use of topical anesthetics (EMLA and liposomal lidocaine) and had general anesthesia privileges for laser and excisional surgery.
So while pediatric dermatologists still do “small procedures” every hour in most practices (cryotherapy for warts, cantharidin for molluscum, shave and punch biopsies), a subset now have extensive procedural practices, which in recent years has extended to pigment lesion lasers (to treat nevus of Ota), hair lasers (to treat perineal areas to prevent pilonidal cyst recurrence or to treat hirsutism), and combinations of lasers to treat hypertrophic, constrictive, and/or deforming scars).
Inflammatory skin disorders: Bread and butter ... and peanut butter?
The care of pediatric inflammatory skin disorders has evolved, but more slowly for some diseases than others. Acne vulgaris now is recognized as much more common under age 12 years than previously, presumably reflecting earlier pubertal changes in our preteens. Over the past 30 years, therapy has evolved with the use of topical retinoids (still underused by pediatricians, considered a “practice gap”), hormonal therapy with combined oral contraceptives, and oral isotretinoin, a powerful but highly effective systemic agent for severe and refractory acne. Specific pediatric guidelines came much later. Pediatric acne expert recommendations were formulated by the American Acne and Rosacea Society and endorsed by the American Academy of Pediatrics in 2013 (Pediatrics. 2013;131:S163-86). Over the past few years, there is a push by experts for more judicious use of antibiotics for acne (oral and topical) to minimize the emergence of bacterial resistance.
Psoriasis has been a condition that has been “behind the revolution,” in that no biologic agent was approved for pediatric psoriasis in the United States until several months ago, lagging behind Europe and elsewhere in the world by almost a decade. Adult psoriasis has been recognized to be associated with a broad set of comorbidities, including obesity and early heart disease, and there is now research on how children are at risk as well, and new recommendations on how to screen children with psoriasis. Moderate to severe psoriasis in adults is now tremendously controllable with biologic agents targeting TNF-alpha, IL 12/23, and IL-17. Etanercept has been approved for children with psoriasis aged 4 years and older, and other biologic agents are under study.
Atopic dermatitis now is ready for its revolution! AD has increased in prevalence from around 5% of the pediatric population 30-plus years ago to 10%-15%. Treatment of most individuals has remained the same over the decades: Good skin care, frequent moisturizers, topical corticosteroids for flares, management of infection if noted. The topical calcineurin inhibitors (TCIs) broadened the therapeutic approach when introduced in 2000 and 2001, but the boxed warning resulted in some practitioners minimizing their utilization of these useful agents.

It has been recognized for years that children with AD have higher risk of developing food allergies than children without AD. A changing understanding of how early food exposure may induce tolerance is changing the world of allergy and influencing the care of children with AD. This is where the peanut butter (or other processed peanut, such as “Bamba”) may be life saving. New guidelines have come from the National Institute of Allergy and Infectious Diseases recommending that infants with severe eczema (or egg allergy, or both) have introduction of age-appropriate peanut-containing food as early as 4-6 months of age to reduce the risk of development of peanut allergy. It is recommended that these infants undergo early evaluation for possible sensitization to peanut protein, with referral to allergists for skin prick tests or serum IgE screens (though if positive, referral to allergists is appropriate), and assess the safety of going ahead with early feeding. It is hoped that following these new guidelines can minimize the development of peanut allergy.
The future
Where will pediatric skin disease, or more importantly, skin health over a lifetime be in 50 years? Can we cure or prevent the consequences of our lethal and life altering genetic diseases such as epidermolysis bullosa or our neurocutaneous disorders? Will our new insights into birthmarks (they are mostly somatic mutations) allow us to form specific, personalized therapies to minimize their impact? Will we be using computers equipped with imaging devices and algorithms to assess our patients’ moles, papules, and nodules? Will our vaccines have wiped out warts, molluscum, and perhaps, acne? Will we have cured our inflammatory skin disorders, or perhaps prevented them by interventions in the neonatal period? No predictions will be offered here, other than that we can look forward to incredible changes for our future generations of health care practitioners, patients, and families.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Eichenfield has served as a consultant for Anacor/Pfizer and Regeneron/Sanofi. Email him at [email protected].