User login
VIDEO: Consider depression in patients with psoriasis
LAS VEGAS – When treating patients with psoriasis, “it is very important for us to treat the entire patient,” and consider the comorbidities, including depression, associated with psoriasis, Jeffrey M. Sobell, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
Depression can be a particular concern for younger patients with more severe psoriasis, said Dr. Sobell of Tufts University, Boston.
When he sees patients aged 18-35 years with significant psoriasis in his practice, he has made it a habit to ask them about depression “and if they’ve ever had thoughts of hurting themselves,” and arranges for mental health follow-up visits for patients about whom he is concerned. “It’s something that’s hard to talk about, but so important,” he said.
Dr. Sobell disclosed relationships with multiple companies including AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Merck, Novartis, Regeneron, Sanofi, and Sun Pharma.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – When treating patients with psoriasis, “it is very important for us to treat the entire patient,” and consider the comorbidities, including depression, associated with psoriasis, Jeffrey M. Sobell, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
Depression can be a particular concern for younger patients with more severe psoriasis, said Dr. Sobell of Tufts University, Boston.
When he sees patients aged 18-35 years with significant psoriasis in his practice, he has made it a habit to ask them about depression “and if they’ve ever had thoughts of hurting themselves,” and arranges for mental health follow-up visits for patients about whom he is concerned. “It’s something that’s hard to talk about, but so important,” he said.
Dr. Sobell disclosed relationships with multiple companies including AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Merck, Novartis, Regeneron, Sanofi, and Sun Pharma.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – When treating patients with psoriasis, “it is very important for us to treat the entire patient,” and consider the comorbidities, including depression, associated with psoriasis, Jeffrey M. Sobell, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
Depression can be a particular concern for younger patients with more severe psoriasis, said Dr. Sobell of Tufts University, Boston.
When he sees patients aged 18-35 years with significant psoriasis in his practice, he has made it a habit to ask them about depression “and if they’ve ever had thoughts of hurting themselves,” and arranges for mental health follow-up visits for patients about whom he is concerned. “It’s something that’s hard to talk about, but so important,” he said.
Dr. Sobell disclosed relationships with multiple companies including AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Merck, Novartis, Regeneron, Sanofi, and Sun Pharma.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Ustekinumab may reduce risk of nonmelanoma skin cancer
GENEVA – Ustekinumab therapy appears to protect psoriasis patients against nonmelanoma skin cancer (NMSC), according to a new analysis from the PSOLAR registry.
Compared with psoriasis patients on methotrexate, the risk of developing on-treatment NMSC was lower among patients on the interleukin-12-/23 inhibitor ustekinumab (Stelara) and those on the three tumor necrosis factor (TNF) inhibitors included in the PSOLAR registry – infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira). The lower risk was statistically significant only for ustekinumab, although there was a favorable trend with the TNF inhibitors showing a 19% relative risk reduction, Bhaskar Srivastava, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
PSOLAR (Psoriasis Longitudinal Assessment and Registry) is an ongoing international prospective observational study evaluating long-term safety and clinical outcomes in psoriasis patients eligible for systemic therapies. The study is now fully enrolled, with 12,090 psoriasis patients and 48,870 patient-years of follow-up and climbing, noted Dr. Srivastava, an employee of Janssen Scientific Affairs, Spring House, Pa.
This analysis focused on 6,782 PSOLAR participants with a mean 18-year history of psoriasis and no history of NMSC at enrollment: 2,623 patients on ustekinumab with 7,900 patient-years of prospective follow-up, 3,727 on a TNF inhibitor with 10,580 patient-years of follow-up, and 432 controls on methotrexate with 781 patient-years of follow-up.
Patients on a biologic were significantly younger, with a mean age of 46.7 years, versus 53.6 years for those on methotrexate. Rates of past or current smoking were similar, in the 55%-60% range, regardless of which systemic agent patients were using.
The crude unadjusted incidence rate for NMSC among all patients on a biologic was 0.33 cancers/100 patient-years, compared with 1.41/100 patient-years for psoriasis patients on methotrexate.
Patients on ustekinumab had an NMSC incidence rate of 0.19/100 patient-years, with a basal cell carcinoma rate of 0.13/100 patient-years and a squamous cell carcinoma rate of 0.06/100 patient-years. Psoriasis patients on a TNF inhibitor had an NMSC incidence rate of 0.43/100 patient-years, with a basal cell carcinoma rate of 0.26/100 patient-years and a squamous cell carcinoma rate of 0.17/100 patient-years.
In a multivariate analysis adjusted for age, sex, race, location, duration of psoriasis, smoking, prior malignancy, skin type, and history of treatment with cyclosporine, methotrexate, other systemic agents, or phototherapy, patients taking ustekinumab had a statistically significant 65% reduction in the risk of NMSC compared with patients on methotrexate and a 74% relative risk reduction for basal cell carcinoma; however, the squamous cell carcinoma risk in the two patient groups was similar.
Dr. Srivastava said the PSOLAR data shouldn’t be taken as the final word regarding NMSC risk and the use of biologics. He noted that psoriasis itself is associated with an increased risk of NMSC. And methotrexate, which was used as the reference standard in this analysis, may alter the risk of NMSC.
“Overall, these results require further validation in psoriasis populations with larger numbers of exposed patients,” he said.
The PSOLAR registry is funded by Janssen, where Dr. Srivastava is employed.
GENEVA – Ustekinumab therapy appears to protect psoriasis patients against nonmelanoma skin cancer (NMSC), according to a new analysis from the PSOLAR registry.
Compared with psoriasis patients on methotrexate, the risk of developing on-treatment NMSC was lower among patients on the interleukin-12-/23 inhibitor ustekinumab (Stelara) and those on the three tumor necrosis factor (TNF) inhibitors included in the PSOLAR registry – infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira). The lower risk was statistically significant only for ustekinumab, although there was a favorable trend with the TNF inhibitors showing a 19% relative risk reduction, Bhaskar Srivastava, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
PSOLAR (Psoriasis Longitudinal Assessment and Registry) is an ongoing international prospective observational study evaluating long-term safety and clinical outcomes in psoriasis patients eligible for systemic therapies. The study is now fully enrolled, with 12,090 psoriasis patients and 48,870 patient-years of follow-up and climbing, noted Dr. Srivastava, an employee of Janssen Scientific Affairs, Spring House, Pa.
This analysis focused on 6,782 PSOLAR participants with a mean 18-year history of psoriasis and no history of NMSC at enrollment: 2,623 patients on ustekinumab with 7,900 patient-years of prospective follow-up, 3,727 on a TNF inhibitor with 10,580 patient-years of follow-up, and 432 controls on methotrexate with 781 patient-years of follow-up.
Patients on a biologic were significantly younger, with a mean age of 46.7 years, versus 53.6 years for those on methotrexate. Rates of past or current smoking were similar, in the 55%-60% range, regardless of which systemic agent patients were using.
The crude unadjusted incidence rate for NMSC among all patients on a biologic was 0.33 cancers/100 patient-years, compared with 1.41/100 patient-years for psoriasis patients on methotrexate.
Patients on ustekinumab had an NMSC incidence rate of 0.19/100 patient-years, with a basal cell carcinoma rate of 0.13/100 patient-years and a squamous cell carcinoma rate of 0.06/100 patient-years. Psoriasis patients on a TNF inhibitor had an NMSC incidence rate of 0.43/100 patient-years, with a basal cell carcinoma rate of 0.26/100 patient-years and a squamous cell carcinoma rate of 0.17/100 patient-years.
In a multivariate analysis adjusted for age, sex, race, location, duration of psoriasis, smoking, prior malignancy, skin type, and history of treatment with cyclosporine, methotrexate, other systemic agents, or phototherapy, patients taking ustekinumab had a statistically significant 65% reduction in the risk of NMSC compared with patients on methotrexate and a 74% relative risk reduction for basal cell carcinoma; however, the squamous cell carcinoma risk in the two patient groups was similar.
Dr. Srivastava said the PSOLAR data shouldn’t be taken as the final word regarding NMSC risk and the use of biologics. He noted that psoriasis itself is associated with an increased risk of NMSC. And methotrexate, which was used as the reference standard in this analysis, may alter the risk of NMSC.
“Overall, these results require further validation in psoriasis populations with larger numbers of exposed patients,” he said.
The PSOLAR registry is funded by Janssen, where Dr. Srivastava is employed.
GENEVA – Ustekinumab therapy appears to protect psoriasis patients against nonmelanoma skin cancer (NMSC), according to a new analysis from the PSOLAR registry.
Compared with psoriasis patients on methotrexate, the risk of developing on-treatment NMSC was lower among patients on the interleukin-12-/23 inhibitor ustekinumab (Stelara) and those on the three tumor necrosis factor (TNF) inhibitors included in the PSOLAR registry – infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira). The lower risk was statistically significant only for ustekinumab, although there was a favorable trend with the TNF inhibitors showing a 19% relative risk reduction, Bhaskar Srivastava, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
PSOLAR (Psoriasis Longitudinal Assessment and Registry) is an ongoing international prospective observational study evaluating long-term safety and clinical outcomes in psoriasis patients eligible for systemic therapies. The study is now fully enrolled, with 12,090 psoriasis patients and 48,870 patient-years of follow-up and climbing, noted Dr. Srivastava, an employee of Janssen Scientific Affairs, Spring House, Pa.
This analysis focused on 6,782 PSOLAR participants with a mean 18-year history of psoriasis and no history of NMSC at enrollment: 2,623 patients on ustekinumab with 7,900 patient-years of prospective follow-up, 3,727 on a TNF inhibitor with 10,580 patient-years of follow-up, and 432 controls on methotrexate with 781 patient-years of follow-up.
Patients on a biologic were significantly younger, with a mean age of 46.7 years, versus 53.6 years for those on methotrexate. Rates of past or current smoking were similar, in the 55%-60% range, regardless of which systemic agent patients were using.
The crude unadjusted incidence rate for NMSC among all patients on a biologic was 0.33 cancers/100 patient-years, compared with 1.41/100 patient-years for psoriasis patients on methotrexate.
Patients on ustekinumab had an NMSC incidence rate of 0.19/100 patient-years, with a basal cell carcinoma rate of 0.13/100 patient-years and a squamous cell carcinoma rate of 0.06/100 patient-years. Psoriasis patients on a TNF inhibitor had an NMSC incidence rate of 0.43/100 patient-years, with a basal cell carcinoma rate of 0.26/100 patient-years and a squamous cell carcinoma rate of 0.17/100 patient-years.
In a multivariate analysis adjusted for age, sex, race, location, duration of psoriasis, smoking, prior malignancy, skin type, and history of treatment with cyclosporine, methotrexate, other systemic agents, or phototherapy, patients taking ustekinumab had a statistically significant 65% reduction in the risk of NMSC compared with patients on methotrexate and a 74% relative risk reduction for basal cell carcinoma; however, the squamous cell carcinoma risk in the two patient groups was similar.
Dr. Srivastava said the PSOLAR data shouldn’t be taken as the final word regarding NMSC risk and the use of biologics. He noted that psoriasis itself is associated with an increased risk of NMSC. And methotrexate, which was used as the reference standard in this analysis, may alter the risk of NMSC.
“Overall, these results require further validation in psoriasis populations with larger numbers of exposed patients,” he said.
The PSOLAR registry is funded by Janssen, where Dr. Srivastava is employed.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Psoriasis patients on ustekinumab had an adjusted 65% reduction in the risk of developing nonmelanoma skin cancer compared with patients on methotrexate.
Data source: An analysis of 6,782 psoriasis patients participating in an international prospective observational registry evaluating the long-term safety and clinical outcomes of systemic therapies.
Disclosures: The PSOLAR registry is funded by ustekinumab manufacturer Janssen; the study presenter is a company employee.
Moderate psoriasis: the new frontier for systemic therapies
GENEVA – Apremilast showed substantial efficacy for patients with truly moderate psoriasis as defined by an involved body surface area of 5%-10% in the first-of-its-kind UNVEIL trial, Bruce Strober, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Patients with moderate psoriasis constitute a very large and underserved population, said Dr. Strober, professor and chair of the department of dermatology at the University of Connecticut, Farmington. “I would say the moderate psoriasis group defined by 5%-10% psoriasis-involved body surface area represents a gray area in our treatment. Often, people with this degree of psoriasis receive no treatment or are relegated to topical monotherapy, yet unfortunately do not respond to those treatments. Nevertheless, clinical trials for systemic therapies, including biologics, exclude this population solely because they’re under 10% involved body surface area,” he noted.
“A large percentage of my patients who are on biologic therapy have less than 10% involved body surface area and would have a PASI [Psoriasis Area Severity Index] score, if I were to measure it, of under 12. Therefore they couldn’t get into a typical registration study for moderate to severe psoriasis,” he continued.
The UNVEIL trial included a 16-week, double-blind, placebo-controlled phase in which 221 systemic therapy–naive patients with plaque psoriasis on 5%-10% of their body surface area (BSA) were randomized 2:1 to apremilast at 30 mg twice a day or placebo. Thereafter, the placebo group was switched over to apremilast and the trial continued in open-label fashion out to 52 weeks. Apremilast (Otezla) is approved only for use in moderate to severe psoriasis.
At baseline, participants had a mean 15-year duration of psoriasis, an involved BSA of 7.1%, a PASI score of 8.1, a static Physician’s Global Assessment (PGA) score of 3 on a 0-5 scale, and a Dermatology Life Quality Index (DLQI) score of 11.
UNVEIL not only targeted a new population for a modern systemic therapy, it also debuted as its primary endpoint a novel metric for disease severity. Because PASI score is a relatively crude measure of change in a population with moderate psoriasis, Dr. Strober and his coinvestigators developed and employed as the primary outcome measure PGA+BSA, which can range from 15 to 30.
The mean baseline PGA+BSA was 21.8. At week 16, the placebo group averaged a 10% decrease in this metric, while the apremilast group showed a 48% reduction. At week 52, the group switched from placebo to apremilast at 16 weeks had a 42% improvement in PGA+BSA and patients on apremilast for the full study had a 49% improvement.
“So you can assume about half of patients with moderate psoriasis will experience a 50% reduction in the product of PGA+BSA on apremilast,” Dr. Strober observed.
At week 52, a PGA score of 0 or 1, meaning clear or almost clear, was present in 36% of the switchover group and 29% of patients on apremilast for 52 weeks.
A 75% improvement in PGA+BSA, or a PGA+BSA–75 response, occurred at week 52 in 45% of the switchover group and 37% of those on apremilast for the entire study.
The mean improvement in the DLQI at week 16 was 2.4 points in the placebo arm and twice that amount with apremilast. At 52 weeks, the switchover group averaged a 5.1-point reduction in DLQI from baseline and the full-time apremilast group had a 4.3-point reduction.
The incidence of apremilast-related adverse events didn’t increase over time. The main issues were diarrhea, nausea, and headache, as is the case when the oral drug is prescribed for its approved indication in patients with moderate to severe psoriasis.
In an interview, Dr. Strober said that despite the encouraging results of UNVEIL, the study is not by itself sufficient evidence to win an expanded indication for apremilast from regulatory agencies and the drug’s manufacturer, Celgene, is not interested in pursuing that course.
UNVEIL, a phase 4 study, was funded by Celgene. Dr. Strober reported serving as a consultant to and/or receiving research funding from that company and more than a dozen others.
GENEVA – Apremilast showed substantial efficacy for patients with truly moderate psoriasis as defined by an involved body surface area of 5%-10% in the first-of-its-kind UNVEIL trial, Bruce Strober, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Patients with moderate psoriasis constitute a very large and underserved population, said Dr. Strober, professor and chair of the department of dermatology at the University of Connecticut, Farmington. “I would say the moderate psoriasis group defined by 5%-10% psoriasis-involved body surface area represents a gray area in our treatment. Often, people with this degree of psoriasis receive no treatment or are relegated to topical monotherapy, yet unfortunately do not respond to those treatments. Nevertheless, clinical trials for systemic therapies, including biologics, exclude this population solely because they’re under 10% involved body surface area,” he noted.
“A large percentage of my patients who are on biologic therapy have less than 10% involved body surface area and would have a PASI [Psoriasis Area Severity Index] score, if I were to measure it, of under 12. Therefore they couldn’t get into a typical registration study for moderate to severe psoriasis,” he continued.
The UNVEIL trial included a 16-week, double-blind, placebo-controlled phase in which 221 systemic therapy–naive patients with plaque psoriasis on 5%-10% of their body surface area (BSA) were randomized 2:1 to apremilast at 30 mg twice a day or placebo. Thereafter, the placebo group was switched over to apremilast and the trial continued in open-label fashion out to 52 weeks. Apremilast (Otezla) is approved only for use in moderate to severe psoriasis.
At baseline, participants had a mean 15-year duration of psoriasis, an involved BSA of 7.1%, a PASI score of 8.1, a static Physician’s Global Assessment (PGA) score of 3 on a 0-5 scale, and a Dermatology Life Quality Index (DLQI) score of 11.
UNVEIL not only targeted a new population for a modern systemic therapy, it also debuted as its primary endpoint a novel metric for disease severity. Because PASI score is a relatively crude measure of change in a population with moderate psoriasis, Dr. Strober and his coinvestigators developed and employed as the primary outcome measure PGA+BSA, which can range from 15 to 30.
The mean baseline PGA+BSA was 21.8. At week 16, the placebo group averaged a 10% decrease in this metric, while the apremilast group showed a 48% reduction. At week 52, the group switched from placebo to apremilast at 16 weeks had a 42% improvement in PGA+BSA and patients on apremilast for the full study had a 49% improvement.
“So you can assume about half of patients with moderate psoriasis will experience a 50% reduction in the product of PGA+BSA on apremilast,” Dr. Strober observed.
At week 52, a PGA score of 0 or 1, meaning clear or almost clear, was present in 36% of the switchover group and 29% of patients on apremilast for 52 weeks.
A 75% improvement in PGA+BSA, or a PGA+BSA–75 response, occurred at week 52 in 45% of the switchover group and 37% of those on apremilast for the entire study.
The mean improvement in the DLQI at week 16 was 2.4 points in the placebo arm and twice that amount with apremilast. At 52 weeks, the switchover group averaged a 5.1-point reduction in DLQI from baseline and the full-time apremilast group had a 4.3-point reduction.
The incidence of apremilast-related adverse events didn’t increase over time. The main issues were diarrhea, nausea, and headache, as is the case when the oral drug is prescribed for its approved indication in patients with moderate to severe psoriasis.
In an interview, Dr. Strober said that despite the encouraging results of UNVEIL, the study is not by itself sufficient evidence to win an expanded indication for apremilast from regulatory agencies and the drug’s manufacturer, Celgene, is not interested in pursuing that course.
UNVEIL, a phase 4 study, was funded by Celgene. Dr. Strober reported serving as a consultant to and/or receiving research funding from that company and more than a dozen others.
GENEVA – Apremilast showed substantial efficacy for patients with truly moderate psoriasis as defined by an involved body surface area of 5%-10% in the first-of-its-kind UNVEIL trial, Bruce Strober, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Patients with moderate psoriasis constitute a very large and underserved population, said Dr. Strober, professor and chair of the department of dermatology at the University of Connecticut, Farmington. “I would say the moderate psoriasis group defined by 5%-10% psoriasis-involved body surface area represents a gray area in our treatment. Often, people with this degree of psoriasis receive no treatment or are relegated to topical monotherapy, yet unfortunately do not respond to those treatments. Nevertheless, clinical trials for systemic therapies, including biologics, exclude this population solely because they’re under 10% involved body surface area,” he noted.
“A large percentage of my patients who are on biologic therapy have less than 10% involved body surface area and would have a PASI [Psoriasis Area Severity Index] score, if I were to measure it, of under 12. Therefore they couldn’t get into a typical registration study for moderate to severe psoriasis,” he continued.
The UNVEIL trial included a 16-week, double-blind, placebo-controlled phase in which 221 systemic therapy–naive patients with plaque psoriasis on 5%-10% of their body surface area (BSA) were randomized 2:1 to apremilast at 30 mg twice a day or placebo. Thereafter, the placebo group was switched over to apremilast and the trial continued in open-label fashion out to 52 weeks. Apremilast (Otezla) is approved only for use in moderate to severe psoriasis.
At baseline, participants had a mean 15-year duration of psoriasis, an involved BSA of 7.1%, a PASI score of 8.1, a static Physician’s Global Assessment (PGA) score of 3 on a 0-5 scale, and a Dermatology Life Quality Index (DLQI) score of 11.
UNVEIL not only targeted a new population for a modern systemic therapy, it also debuted as its primary endpoint a novel metric for disease severity. Because PASI score is a relatively crude measure of change in a population with moderate psoriasis, Dr. Strober and his coinvestigators developed and employed as the primary outcome measure PGA+BSA, which can range from 15 to 30.
The mean baseline PGA+BSA was 21.8. At week 16, the placebo group averaged a 10% decrease in this metric, while the apremilast group showed a 48% reduction. At week 52, the group switched from placebo to apremilast at 16 weeks had a 42% improvement in PGA+BSA and patients on apremilast for the full study had a 49% improvement.
“So you can assume about half of patients with moderate psoriasis will experience a 50% reduction in the product of PGA+BSA on apremilast,” Dr. Strober observed.
At week 52, a PGA score of 0 or 1, meaning clear or almost clear, was present in 36% of the switchover group and 29% of patients on apremilast for 52 weeks.
A 75% improvement in PGA+BSA, or a PGA+BSA–75 response, occurred at week 52 in 45% of the switchover group and 37% of those on apremilast for the entire study.
The mean improvement in the DLQI at week 16 was 2.4 points in the placebo arm and twice that amount with apremilast. At 52 weeks, the switchover group averaged a 5.1-point reduction in DLQI from baseline and the full-time apremilast group had a 4.3-point reduction.
The incidence of apremilast-related adverse events didn’t increase over time. The main issues were diarrhea, nausea, and headache, as is the case when the oral drug is prescribed for its approved indication in patients with moderate to severe psoriasis.
In an interview, Dr. Strober said that despite the encouraging results of UNVEIL, the study is not by itself sufficient evidence to win an expanded indication for apremilast from regulatory agencies and the drug’s manufacturer, Celgene, is not interested in pursuing that course.
UNVEIL, a phase 4 study, was funded by Celgene. Dr. Strober reported serving as a consultant to and/or receiving research funding from that company and more than a dozen others.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Roughly half of apremilast-treated patients with moderate psoriasis as defined by an involved body surface area of 5%-10% experienced a 50% reduction in a novel outcome metric: the product of the Physician’s Global Assessment plus the involved body surface area.
Data source: This 52-week study of 221 patients with truly moderate psoriasis featured a 16-week, double-blind, placebo-controlled phase, after which everyone continued on open-label apremilast out to 51 weeks.
Disclosures: The UNVEIL study was sponsored by Celgene. The presenter reported receiving research funding from and serving as a consultant to that company and numerous others.
Red Scaly Rash Following Tattoo Application
The Diagnosis: Isomorphic Psoriasis
Tattooing has become an increasingly popular trend among young people. Currently, there are no guidelines in the United States regulating the production of tattoo ink and pigments.1 Henna tattooing, a form of temporary skin painting, also has risks of allergic contact dermatitis from paraphenylenediamine dye.2 Complications following tattoo application include an allergic contact dermatitis to tattoo pigments, infection, granulomatous and lichenoid reactions, and skin disease localized to the tattooed area.3
Localized dermatosis arising in a traumatized area, or the Koebner phenomenon, was first described by Heinrich Koebner in 1877.4 He described the formation of psoriasiform lesions at the site of cutaneous trauma.5 These isomorphic lesions can occur in 25% of patients with psoriasis after trauma to the skin such as tattooing.6 Other dermatologic diseases that can present as an isomorphic response to tattooing include lichen planus, Darier disease, vitiligo, and autoimmune bullous disease.3,5,6
Various causes of trauma such as burns, insect bites, physical trauma, and needle trauma have been shown to produce new psoriatic lesions.6 The time period from trauma to formation of psoriasiform lesions usually ranges from 10 to 20 days; however, an initial reaction can occur as early as 3 days or as long as 2 years after trauma.4 Although the pathophysiology of the isomorphic response is not well known, it has been shown that nerve growth factor has a role. Raychaudhuri et al7 demonstrated the upregulation of nerve growth factor in the development of a psoriatic lesion, influencing keratinocyte proliferation, angiogenesis, and T-cell activation.
Physical trauma such as tattooing has been shown to cause an isomorphic response in psoriasis. We describe a case of isomorphic psoriasis in a patient after tattoo application. Our patient had a several-month history of well-controlled psoriasis prior to obtaining the new tattoo. Several days after receiving the tattoo, the patient reported an increase in psoriatic lesions, including at the site of the tattoo. The trauma causing the isomorphic response could have been either a response to the tattoo pigment or needle injury to the skin.6
Psoriasis and isomorphic lesions can be treated with topical corticosteroids as well as systemic and biologic agents. Our patient was treated with triamcinolone cream with good response.8
- Haugh IM, Laumann SL, Laumann AE. Regulation of tattoo ink production and the tattoo business in the US. Curr Probl Dermatol. 2015;48:248-252.
- Marcoux D, Couture-Trudel PM, Rboulet-Delmas G, et al. Sensitization to paraphenylenediame from a streetside temporary tattoo. Pediatr Dermatol. 2002;19:498-502.
- Bassi A, Campolmi P, Cannarozzo G, et al. Tattoo-associated skin reaction: the importance of an early diagnosis and proper treatment [published online July 23, 2014]. Biomed Res Int. 2014;2014:354608.
- Weiss G, Shemer A, Trau H. The Koebner phenomenon: review of the literature. J Eur Acad Dermatol Venereol. 2002;16:241-248.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Orzan OA, Popa LG, Vexler ES, et al. Tattoo-induced psoriasis. J Med Life. 2014;7:65-68.
- Raychaudhuri SP, Jiang WY, Raychaudhuri SK. Revisiting the Koebner phenomenon: role of NGF and its receptor system in the pathogenesis of psoriasis. Am J Pathol. 2008;172:961-971.
- Gottlieb AB. Therapeutic options in the treatment of psoriasis and atopic dermatitis. J Am Acad Dermatol. 2005;53(1 suppl 1):S3-S16.
The Diagnosis: Isomorphic Psoriasis
Tattooing has become an increasingly popular trend among young people. Currently, there are no guidelines in the United States regulating the production of tattoo ink and pigments.1 Henna tattooing, a form of temporary skin painting, also has risks of allergic contact dermatitis from paraphenylenediamine dye.2 Complications following tattoo application include an allergic contact dermatitis to tattoo pigments, infection, granulomatous and lichenoid reactions, and skin disease localized to the tattooed area.3
Localized dermatosis arising in a traumatized area, or the Koebner phenomenon, was first described by Heinrich Koebner in 1877.4 He described the formation of psoriasiform lesions at the site of cutaneous trauma.5 These isomorphic lesions can occur in 25% of patients with psoriasis after trauma to the skin such as tattooing.6 Other dermatologic diseases that can present as an isomorphic response to tattooing include lichen planus, Darier disease, vitiligo, and autoimmune bullous disease.3,5,6
Various causes of trauma such as burns, insect bites, physical trauma, and needle trauma have been shown to produce new psoriatic lesions.6 The time period from trauma to formation of psoriasiform lesions usually ranges from 10 to 20 days; however, an initial reaction can occur as early as 3 days or as long as 2 years after trauma.4 Although the pathophysiology of the isomorphic response is not well known, it has been shown that nerve growth factor has a role. Raychaudhuri et al7 demonstrated the upregulation of nerve growth factor in the development of a psoriatic lesion, influencing keratinocyte proliferation, angiogenesis, and T-cell activation.
Physical trauma such as tattooing has been shown to cause an isomorphic response in psoriasis. We describe a case of isomorphic psoriasis in a patient after tattoo application. Our patient had a several-month history of well-controlled psoriasis prior to obtaining the new tattoo. Several days after receiving the tattoo, the patient reported an increase in psoriatic lesions, including at the site of the tattoo. The trauma causing the isomorphic response could have been either a response to the tattoo pigment or needle injury to the skin.6
Psoriasis and isomorphic lesions can be treated with topical corticosteroids as well as systemic and biologic agents. Our patient was treated with triamcinolone cream with good response.8
The Diagnosis: Isomorphic Psoriasis
Tattooing has become an increasingly popular trend among young people. Currently, there are no guidelines in the United States regulating the production of tattoo ink and pigments.1 Henna tattooing, a form of temporary skin painting, also has risks of allergic contact dermatitis from paraphenylenediamine dye.2 Complications following tattoo application include an allergic contact dermatitis to tattoo pigments, infection, granulomatous and lichenoid reactions, and skin disease localized to the tattooed area.3
Localized dermatosis arising in a traumatized area, or the Koebner phenomenon, was first described by Heinrich Koebner in 1877.4 He described the formation of psoriasiform lesions at the site of cutaneous trauma.5 These isomorphic lesions can occur in 25% of patients with psoriasis after trauma to the skin such as tattooing.6 Other dermatologic diseases that can present as an isomorphic response to tattooing include lichen planus, Darier disease, vitiligo, and autoimmune bullous disease.3,5,6
Various causes of trauma such as burns, insect bites, physical trauma, and needle trauma have been shown to produce new psoriatic lesions.6 The time period from trauma to formation of psoriasiform lesions usually ranges from 10 to 20 days; however, an initial reaction can occur as early as 3 days or as long as 2 years after trauma.4 Although the pathophysiology of the isomorphic response is not well known, it has been shown that nerve growth factor has a role. Raychaudhuri et al7 demonstrated the upregulation of nerve growth factor in the development of a psoriatic lesion, influencing keratinocyte proliferation, angiogenesis, and T-cell activation.
Physical trauma such as tattooing has been shown to cause an isomorphic response in psoriasis. We describe a case of isomorphic psoriasis in a patient after tattoo application. Our patient had a several-month history of well-controlled psoriasis prior to obtaining the new tattoo. Several days after receiving the tattoo, the patient reported an increase in psoriatic lesions, including at the site of the tattoo. The trauma causing the isomorphic response could have been either a response to the tattoo pigment or needle injury to the skin.6
Psoriasis and isomorphic lesions can be treated with topical corticosteroids as well as systemic and biologic agents. Our patient was treated with triamcinolone cream with good response.8
- Haugh IM, Laumann SL, Laumann AE. Regulation of tattoo ink production and the tattoo business in the US. Curr Probl Dermatol. 2015;48:248-252.
- Marcoux D, Couture-Trudel PM, Rboulet-Delmas G, et al. Sensitization to paraphenylenediame from a streetside temporary tattoo. Pediatr Dermatol. 2002;19:498-502.
- Bassi A, Campolmi P, Cannarozzo G, et al. Tattoo-associated skin reaction: the importance of an early diagnosis and proper treatment [published online July 23, 2014]. Biomed Res Int. 2014;2014:354608.
- Weiss G, Shemer A, Trau H. The Koebner phenomenon: review of the literature. J Eur Acad Dermatol Venereol. 2002;16:241-248.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Orzan OA, Popa LG, Vexler ES, et al. Tattoo-induced psoriasis. J Med Life. 2014;7:65-68.
- Raychaudhuri SP, Jiang WY, Raychaudhuri SK. Revisiting the Koebner phenomenon: role of NGF and its receptor system in the pathogenesis of psoriasis. Am J Pathol. 2008;172:961-971.
- Gottlieb AB. Therapeutic options in the treatment of psoriasis and atopic dermatitis. J Am Acad Dermatol. 2005;53(1 suppl 1):S3-S16.
- Haugh IM, Laumann SL, Laumann AE. Regulation of tattoo ink production and the tattoo business in the US. Curr Probl Dermatol. 2015;48:248-252.
- Marcoux D, Couture-Trudel PM, Rboulet-Delmas G, et al. Sensitization to paraphenylenediame from a streetside temporary tattoo. Pediatr Dermatol. 2002;19:498-502.
- Bassi A, Campolmi P, Cannarozzo G, et al. Tattoo-associated skin reaction: the importance of an early diagnosis and proper treatment [published online July 23, 2014]. Biomed Res Int. 2014;2014:354608.
- Weiss G, Shemer A, Trau H. The Koebner phenomenon: review of the literature. J Eur Acad Dermatol Venereol. 2002;16:241-248.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Orzan OA, Popa LG, Vexler ES, et al. Tattoo-induced psoriasis. J Med Life. 2014;7:65-68.
- Raychaudhuri SP, Jiang WY, Raychaudhuri SK. Revisiting the Koebner phenomenon: role of NGF and its receptor system in the pathogenesis of psoriasis. Am J Pathol. 2008;172:961-971.
- Gottlieb AB. Therapeutic options in the treatment of psoriasis and atopic dermatitis. J Am Acad Dermatol. 2005;53(1 suppl 1):S3-S16.

A 26-year-old man presented with a mildly pruritic red scaly rash on the right arm of 3 weeks' duration. He reported having a tattoo placed on previously normal skin on the right lateral arm prior to the development of the rash. Two weeks after receiving the tattoo, he developed scaling and redness of the skin involved in the tattoo. He also had similar papules and plaques over the rest of his body. Physical examination showed well-demarcated, erythematous, scaly papules and plaques following the design of a black-pigmented tattoo on the lateral aspect of the right arm. There also were similar erythematous scaly plaques scattered over both arms and the trunk. He denied any pain or blister formation of the involved areas.
Guselkumab tops adalimumab for psychiatric comorbidities in psoriasis
GENEVA – Guselkumab for treatment of moderate to severe plaque psoriasis generated significantly greater improvement in comorbid anxiety and depression than adalimumab in the head-to-head VOYAGE 2 randomized trial, Kenneth B. Gordon, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The population enrolled in VOYAGE 2, which did not preselect to enrich the study population for anxiety and depression, reflected how common these mental health problems are among psoriasis patients. Fully 38.6% of the 992 randomized patients had a baseline Hospital Anxiety and Depression Score-Anxiety (HADS-A) of 8 or more (considered clinically important anxiety) on the 0-21 scale, and 27.7% had a HADS-D score of at least 8 (indicative of clinically meaningful depression), noted Dr. Gordon, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee.
The on-treatment reductions in psychiatric symptoms in VOYAGE 2 correlated closely with greater improvement in psoriasis, he added. For example, regardless of which drug they were on, patients with a baseline HADS-D of 8 or more averaged a 3.6-point reduction in HADS-D at week 24 if, at that point, they had a Psoriasis Area Severity Index (PASI) response of 75-89, a 4.0-point improvement if they had a PASI 90-99 response, and a 5.2-point reduction in HADS-D if they had a PASI 100 response.
VOYAGE 2 was a phase 3, double-blind, multicenter, placebo-controlled comparison of guselkumab (Tremfya), a human monoclonal antibody targeting the interleukin-23 p 19 subunit, and the tumor necrosis factor-alpha inhibitor adalimumab (Humira) in their standard dosing. Participants were randomized 2:1:1 to guselkumab, adalimumab, or placebo. The study’s primary outcomes have previously been reported (J Am Acad Dermatol. 2017 Mar;76[3]:418-31). For example, at week 24 the guselkumab group demonstrated PASI 75, 90, and 100 responses of 89.1%, 75.2%, and 44.2%, respectively, compared with adalimumab, with PASI 75, 90, and 100 responses of 71%, 54.8%, and 26.6%.
Serial measurement of anxiety and depression symptoms using the HADS-A and HADS-D were part of the study protocol. The mean baseline HADS-A and HADS-D scores were 6.8 and 5.3. At week 24, the mean reduction in the HADS-A score was 2.0 points in the guselkumab group, compared with 1.0 point in the adalimumab arm. HADS-D scores improved by 1.7 and 1.1 points, respectively, in the overall guselkumab and adalimumab groups.
More importantly, among patients with a baseline HADS-A of 8 or higher, by week 16 of the trial, the HADS-A score had dropped below 8 in 51.4% of those on guselkumab, compared with 44.9% of patients on adalimumab and 25.9% on placebo, Dr. Gordon reported. Similarly, 59.2% of guselkumab-treated patients with a high baseline HADS-D improved to a score below 8 at week 16, compared with 54.3% on adalimumab and 27% on placebo.
At week 24, 58.4% of guselkumab-treated patients with a high baseline HADS-A and 59.4% of those with a high HADS-D had scores below 8. Again, these responses were significantly better than the 42.9% and 46.4% rates, respectively, in the adalimumab arm.
VOYAGE 2 was sponsored by Janssen, which markets guselkumab, approved by the Food and Drug Administration in July 2017 for treating adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Dr. Gordon reported receiving research support from and serving as a consultant to Janssen and numerous other pharmaceutical companies.
GENEVA – Guselkumab for treatment of moderate to severe plaque psoriasis generated significantly greater improvement in comorbid anxiety and depression than adalimumab in the head-to-head VOYAGE 2 randomized trial, Kenneth B. Gordon, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The population enrolled in VOYAGE 2, which did not preselect to enrich the study population for anxiety and depression, reflected how common these mental health problems are among psoriasis patients. Fully 38.6% of the 992 randomized patients had a baseline Hospital Anxiety and Depression Score-Anxiety (HADS-A) of 8 or more (considered clinically important anxiety) on the 0-21 scale, and 27.7% had a HADS-D score of at least 8 (indicative of clinically meaningful depression), noted Dr. Gordon, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee.
The on-treatment reductions in psychiatric symptoms in VOYAGE 2 correlated closely with greater improvement in psoriasis, he added. For example, regardless of which drug they were on, patients with a baseline HADS-D of 8 or more averaged a 3.6-point reduction in HADS-D at week 24 if, at that point, they had a Psoriasis Area Severity Index (PASI) response of 75-89, a 4.0-point improvement if they had a PASI 90-99 response, and a 5.2-point reduction in HADS-D if they had a PASI 100 response.
VOYAGE 2 was a phase 3, double-blind, multicenter, placebo-controlled comparison of guselkumab (Tremfya), a human monoclonal antibody targeting the interleukin-23 p 19 subunit, and the tumor necrosis factor-alpha inhibitor adalimumab (Humira) in their standard dosing. Participants were randomized 2:1:1 to guselkumab, adalimumab, or placebo. The study’s primary outcomes have previously been reported (J Am Acad Dermatol. 2017 Mar;76[3]:418-31). For example, at week 24 the guselkumab group demonstrated PASI 75, 90, and 100 responses of 89.1%, 75.2%, and 44.2%, respectively, compared with adalimumab, with PASI 75, 90, and 100 responses of 71%, 54.8%, and 26.6%.
Serial measurement of anxiety and depression symptoms using the HADS-A and HADS-D were part of the study protocol. The mean baseline HADS-A and HADS-D scores were 6.8 and 5.3. At week 24, the mean reduction in the HADS-A score was 2.0 points in the guselkumab group, compared with 1.0 point in the adalimumab arm. HADS-D scores improved by 1.7 and 1.1 points, respectively, in the overall guselkumab and adalimumab groups.
More importantly, among patients with a baseline HADS-A of 8 or higher, by week 16 of the trial, the HADS-A score had dropped below 8 in 51.4% of those on guselkumab, compared with 44.9% of patients on adalimumab and 25.9% on placebo, Dr. Gordon reported. Similarly, 59.2% of guselkumab-treated patients with a high baseline HADS-D improved to a score below 8 at week 16, compared with 54.3% on adalimumab and 27% on placebo.
At week 24, 58.4% of guselkumab-treated patients with a high baseline HADS-A and 59.4% of those with a high HADS-D had scores below 8. Again, these responses were significantly better than the 42.9% and 46.4% rates, respectively, in the adalimumab arm.
VOYAGE 2 was sponsored by Janssen, which markets guselkumab, approved by the Food and Drug Administration in July 2017 for treating adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Dr. Gordon reported receiving research support from and serving as a consultant to Janssen and numerous other pharmaceutical companies.
GENEVA – Guselkumab for treatment of moderate to severe plaque psoriasis generated significantly greater improvement in comorbid anxiety and depression than adalimumab in the head-to-head VOYAGE 2 randomized trial, Kenneth B. Gordon, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The population enrolled in VOYAGE 2, which did not preselect to enrich the study population for anxiety and depression, reflected how common these mental health problems are among psoriasis patients. Fully 38.6% of the 992 randomized patients had a baseline Hospital Anxiety and Depression Score-Anxiety (HADS-A) of 8 or more (considered clinically important anxiety) on the 0-21 scale, and 27.7% had a HADS-D score of at least 8 (indicative of clinically meaningful depression), noted Dr. Gordon, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee.
The on-treatment reductions in psychiatric symptoms in VOYAGE 2 correlated closely with greater improvement in psoriasis, he added. For example, regardless of which drug they were on, patients with a baseline HADS-D of 8 or more averaged a 3.6-point reduction in HADS-D at week 24 if, at that point, they had a Psoriasis Area Severity Index (PASI) response of 75-89, a 4.0-point improvement if they had a PASI 90-99 response, and a 5.2-point reduction in HADS-D if they had a PASI 100 response.
VOYAGE 2 was a phase 3, double-blind, multicenter, placebo-controlled comparison of guselkumab (Tremfya), a human monoclonal antibody targeting the interleukin-23 p 19 subunit, and the tumor necrosis factor-alpha inhibitor adalimumab (Humira) in their standard dosing. Participants were randomized 2:1:1 to guselkumab, adalimumab, or placebo. The study’s primary outcomes have previously been reported (J Am Acad Dermatol. 2017 Mar;76[3]:418-31). For example, at week 24 the guselkumab group demonstrated PASI 75, 90, and 100 responses of 89.1%, 75.2%, and 44.2%, respectively, compared with adalimumab, with PASI 75, 90, and 100 responses of 71%, 54.8%, and 26.6%.
Serial measurement of anxiety and depression symptoms using the HADS-A and HADS-D were part of the study protocol. The mean baseline HADS-A and HADS-D scores were 6.8 and 5.3. At week 24, the mean reduction in the HADS-A score was 2.0 points in the guselkumab group, compared with 1.0 point in the adalimumab arm. HADS-D scores improved by 1.7 and 1.1 points, respectively, in the overall guselkumab and adalimumab groups.
More importantly, among patients with a baseline HADS-A of 8 or higher, by week 16 of the trial, the HADS-A score had dropped below 8 in 51.4% of those on guselkumab, compared with 44.9% of patients on adalimumab and 25.9% on placebo, Dr. Gordon reported. Similarly, 59.2% of guselkumab-treated patients with a high baseline HADS-D improved to a score below 8 at week 16, compared with 54.3% on adalimumab and 27% on placebo.
At week 24, 58.4% of guselkumab-treated patients with a high baseline HADS-A and 59.4% of those with a high HADS-D had scores below 8. Again, these responses were significantly better than the 42.9% and 46.4% rates, respectively, in the adalimumab arm.
VOYAGE 2 was sponsored by Janssen, which markets guselkumab, approved by the Food and Drug Administration in July 2017 for treating adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Dr. Gordon reported receiving research support from and serving as a consultant to Janssen and numerous other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Fifty-nine percent of gesulkumab-treated patients with moderate to severe psoriasis and clinically significant depression at baseline were below the depression threshold at week 24, compared with 46% of adalimumab-treated patients.
Data source: A phase 3, double-blind, multicenter, placebo-controlled comparison of guselkumab and adalimumab in 992 randomized patients with moderate to severe psoriasis.
Disclosures: VOYAGE 2 was sponsored by Janssen, which markets guselkumab. The presenter reported receiving research support from and serving as a consultant to Janssen and numerous other pharmaceutical companies.
Psoriasis patients face higher risk of alcohol-related death – but lower suicide risk
GENEVA – Psoriasis patients are roughly two-thirds more likely to die of alcohol-related causes than their peers in the general population, Rosa Parisi, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Indeed, alcohol-related mortality appears to be an important contributor to the long-recognized elevated risk of premature mortality in patients who’ve been diagnosed with psoriasis, according to Dr. Parisi of the University of Manchester (England), who presented the results of a retrospective cohort study of alcohol-related deaths. The reasons for psoriasis patients’ increased risk of early mortality have been unclear, although it’s well established that psoriasis is associated with increased rates of unhealthy behaviors, including smoking and high alcohol consumption.
The study of alcohol-related deaths utilized a database of 398 primary care practices in England. The study population consisted of 55,537 patients diagnosed with psoriasis in 1998-2014 and 854,314 controls without psoriasis who were matched based on age, sex, and membership in the same primary care practice.
During a median 4.4 years of follow-up, the rate of deaths directly attributable to alcohol consumption was 4.8 per 10,000 person-years in the psoriasis group and 2.5 per 10,000 person-years in controls. Nearly two-thirds of the alcohol-related deaths were recorded as due to alcoholic liver disease and 24% to hepatic fibrosis and cirrhosis; 8% were attributed to mental and behavioral disorders due to alcohol.
In an analysis adjusted for socioeconomic status, the risk for alcohol-related death was increased 1.58-fold in the psoriasis group, compared with controls. Upon exclusion of all subjects who’d been on methotrexate on the grounds that the drug interacts with alcohol to accelerate liver damage, having psoriasis was associated with a 1.66-fold increased risk of alcohol-related death.
Such deaths occurred in women with psoriasis at a median age of 55 – a full 5 years younger than alcohol-related deaths in nonpsoriatic women. The age gap was smaller in men.
Medical records indicated that 82.2% of psoriasis patients who died of alcohol-related causes had previously been coded by their primary care practitioner as a heavy drinker, as were 75.5% of those without psoriasis who died of similar causes. Yet fewer than 11% of the psoriasis patients and controls who experienced alcohol-related mortality had ever received a prescription for a medication for alcohol dependence, such as disulfiram. Psychologic support for alcohol dependence had been offered within the primary care practices for only 19.7% of psoriasis patients with an alcohol-related death and 14.4% of controls.
These statistics contain an important message for clinicians, Dr. Parisi said.
“Alcohol misuse often remains unidentified and undertreated in primary care. Health care practitioners should be more aware of the psychologic difficulties of people with psoriasis. The Alcohol Use Disorders Identification Test screening tool, which has been developed by the World Health Organization, should be implemented in both primary and secondary care to detect alcohol misuse in psoriasis patients,” she asserted.
Elsewhere at the meeting, she presented a study on the risks of suicide and nonfatal deliberate self-harm among psoriasis patients. She utilized the same database of 398 English primary care medical practices. This analysis included 56,961 psoriasis patients and 876,919 matched controls.
The suicide rate was 1.1 per 10,000 person-years in the psoriasis group and 1.5 per 10,000 person-years in controls. In a proportional hazard analysis adjusted for socioeconomic status, psoriasis patients were at a significant 41% lower risk of suicide than their nonpsoriatic peers. Breaking down the data further based upon age at diagnosis of psoriasis, patients diagnosed before age 40 were at a nonsignificant 8% lower risk of suicide, while those diagnosed at age 40 years or older experienced a highly significant 62% reduction in risk of suicide, compared with controls.
This was the case even though the psoriasis group had a significantly greater prevalence of psychiatric comorbidities overall, by a margin of 29.2% versus 26.4%.
The analysis of risk of nonfatal deliberate self-harm was done after excluding all individuals in the suicide study who had a baseline history of self-harm. This resulted in a study population of 54,709 psoriasis patients and 813,699 controls matched by age, sex, and primary care practice. Bottom line: There was no evidence of an association between having a diagnosis of psoriasis and nonfatal deliberate self-harm. The rates – 18.9 events per 10,000 person-years in the psoriasis group and 16.3 per 10,000 person-years in controls – were closely similar after adjusting for socioeconomic status.
Simultaneous with her EADV presentation on alcohol-related deaths in psoriasis patients, Dr. Parisi’s study was published online (JAMA Dermatol. 2017 Sep 15. doi: 10.1001/jamadermatol.2017.3225).
She reported having no financial conflicts of interest regarding her presentations.
GENEVA – Psoriasis patients are roughly two-thirds more likely to die of alcohol-related causes than their peers in the general population, Rosa Parisi, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Indeed, alcohol-related mortality appears to be an important contributor to the long-recognized elevated risk of premature mortality in patients who’ve been diagnosed with psoriasis, according to Dr. Parisi of the University of Manchester (England), who presented the results of a retrospective cohort study of alcohol-related deaths. The reasons for psoriasis patients’ increased risk of early mortality have been unclear, although it’s well established that psoriasis is associated with increased rates of unhealthy behaviors, including smoking and high alcohol consumption.
The study of alcohol-related deaths utilized a database of 398 primary care practices in England. The study population consisted of 55,537 patients diagnosed with psoriasis in 1998-2014 and 854,314 controls without psoriasis who were matched based on age, sex, and membership in the same primary care practice.
During a median 4.4 years of follow-up, the rate of deaths directly attributable to alcohol consumption was 4.8 per 10,000 person-years in the psoriasis group and 2.5 per 10,000 person-years in controls. Nearly two-thirds of the alcohol-related deaths were recorded as due to alcoholic liver disease and 24% to hepatic fibrosis and cirrhosis; 8% were attributed to mental and behavioral disorders due to alcohol.
In an analysis adjusted for socioeconomic status, the risk for alcohol-related death was increased 1.58-fold in the psoriasis group, compared with controls. Upon exclusion of all subjects who’d been on methotrexate on the grounds that the drug interacts with alcohol to accelerate liver damage, having psoriasis was associated with a 1.66-fold increased risk of alcohol-related death.
Such deaths occurred in women with psoriasis at a median age of 55 – a full 5 years younger than alcohol-related deaths in nonpsoriatic women. The age gap was smaller in men.
Medical records indicated that 82.2% of psoriasis patients who died of alcohol-related causes had previously been coded by their primary care practitioner as a heavy drinker, as were 75.5% of those without psoriasis who died of similar causes. Yet fewer than 11% of the psoriasis patients and controls who experienced alcohol-related mortality had ever received a prescription for a medication for alcohol dependence, such as disulfiram. Psychologic support for alcohol dependence had been offered within the primary care practices for only 19.7% of psoriasis patients with an alcohol-related death and 14.4% of controls.
These statistics contain an important message for clinicians, Dr. Parisi said.
“Alcohol misuse often remains unidentified and undertreated in primary care. Health care practitioners should be more aware of the psychologic difficulties of people with psoriasis. The Alcohol Use Disorders Identification Test screening tool, which has been developed by the World Health Organization, should be implemented in both primary and secondary care to detect alcohol misuse in psoriasis patients,” she asserted.
Elsewhere at the meeting, she presented a study on the risks of suicide and nonfatal deliberate self-harm among psoriasis patients. She utilized the same database of 398 English primary care medical practices. This analysis included 56,961 psoriasis patients and 876,919 matched controls.
The suicide rate was 1.1 per 10,000 person-years in the psoriasis group and 1.5 per 10,000 person-years in controls. In a proportional hazard analysis adjusted for socioeconomic status, psoriasis patients were at a significant 41% lower risk of suicide than their nonpsoriatic peers. Breaking down the data further based upon age at diagnosis of psoriasis, patients diagnosed before age 40 were at a nonsignificant 8% lower risk of suicide, while those diagnosed at age 40 years or older experienced a highly significant 62% reduction in risk of suicide, compared with controls.
This was the case even though the psoriasis group had a significantly greater prevalence of psychiatric comorbidities overall, by a margin of 29.2% versus 26.4%.
The analysis of risk of nonfatal deliberate self-harm was done after excluding all individuals in the suicide study who had a baseline history of self-harm. This resulted in a study population of 54,709 psoriasis patients and 813,699 controls matched by age, sex, and primary care practice. Bottom line: There was no evidence of an association between having a diagnosis of psoriasis and nonfatal deliberate self-harm. The rates – 18.9 events per 10,000 person-years in the psoriasis group and 16.3 per 10,000 person-years in controls – were closely similar after adjusting for socioeconomic status.
Simultaneous with her EADV presentation on alcohol-related deaths in psoriasis patients, Dr. Parisi’s study was published online (JAMA Dermatol. 2017 Sep 15. doi: 10.1001/jamadermatol.2017.3225).
She reported having no financial conflicts of interest regarding her presentations.
GENEVA – Psoriasis patients are roughly two-thirds more likely to die of alcohol-related causes than their peers in the general population, Rosa Parisi, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Indeed, alcohol-related mortality appears to be an important contributor to the long-recognized elevated risk of premature mortality in patients who’ve been diagnosed with psoriasis, according to Dr. Parisi of the University of Manchester (England), who presented the results of a retrospective cohort study of alcohol-related deaths. The reasons for psoriasis patients’ increased risk of early mortality have been unclear, although it’s well established that psoriasis is associated with increased rates of unhealthy behaviors, including smoking and high alcohol consumption.
The study of alcohol-related deaths utilized a database of 398 primary care practices in England. The study population consisted of 55,537 patients diagnosed with psoriasis in 1998-2014 and 854,314 controls without psoriasis who were matched based on age, sex, and membership in the same primary care practice.
During a median 4.4 years of follow-up, the rate of deaths directly attributable to alcohol consumption was 4.8 per 10,000 person-years in the psoriasis group and 2.5 per 10,000 person-years in controls. Nearly two-thirds of the alcohol-related deaths were recorded as due to alcoholic liver disease and 24% to hepatic fibrosis and cirrhosis; 8% were attributed to mental and behavioral disorders due to alcohol.
In an analysis adjusted for socioeconomic status, the risk for alcohol-related death was increased 1.58-fold in the psoriasis group, compared with controls. Upon exclusion of all subjects who’d been on methotrexate on the grounds that the drug interacts with alcohol to accelerate liver damage, having psoriasis was associated with a 1.66-fold increased risk of alcohol-related death.
Such deaths occurred in women with psoriasis at a median age of 55 – a full 5 years younger than alcohol-related deaths in nonpsoriatic women. The age gap was smaller in men.
Medical records indicated that 82.2% of psoriasis patients who died of alcohol-related causes had previously been coded by their primary care practitioner as a heavy drinker, as were 75.5% of those without psoriasis who died of similar causes. Yet fewer than 11% of the psoriasis patients and controls who experienced alcohol-related mortality had ever received a prescription for a medication for alcohol dependence, such as disulfiram. Psychologic support for alcohol dependence had been offered within the primary care practices for only 19.7% of psoriasis patients with an alcohol-related death and 14.4% of controls.
These statistics contain an important message for clinicians, Dr. Parisi said.
“Alcohol misuse often remains unidentified and undertreated in primary care. Health care practitioners should be more aware of the psychologic difficulties of people with psoriasis. The Alcohol Use Disorders Identification Test screening tool, which has been developed by the World Health Organization, should be implemented in both primary and secondary care to detect alcohol misuse in psoriasis patients,” she asserted.
Elsewhere at the meeting, she presented a study on the risks of suicide and nonfatal deliberate self-harm among psoriasis patients. She utilized the same database of 398 English primary care medical practices. This analysis included 56,961 psoriasis patients and 876,919 matched controls.
The suicide rate was 1.1 per 10,000 person-years in the psoriasis group and 1.5 per 10,000 person-years in controls. In a proportional hazard analysis adjusted for socioeconomic status, psoriasis patients were at a significant 41% lower risk of suicide than their nonpsoriatic peers. Breaking down the data further based upon age at diagnosis of psoriasis, patients diagnosed before age 40 were at a nonsignificant 8% lower risk of suicide, while those diagnosed at age 40 years or older experienced a highly significant 62% reduction in risk of suicide, compared with controls.
This was the case even though the psoriasis group had a significantly greater prevalence of psychiatric comorbidities overall, by a margin of 29.2% versus 26.4%.
The analysis of risk of nonfatal deliberate self-harm was done after excluding all individuals in the suicide study who had a baseline history of self-harm. This resulted in a study population of 54,709 psoriasis patients and 813,699 controls matched by age, sex, and primary care practice. Bottom line: There was no evidence of an association between having a diagnosis of psoriasis and nonfatal deliberate self-harm. The rates – 18.9 events per 10,000 person-years in the psoriasis group and 16.3 per 10,000 person-years in controls – were closely similar after adjusting for socioeconomic status.
Simultaneous with her EADV presentation on alcohol-related deaths in psoriasis patients, Dr. Parisi’s study was published online (JAMA Dermatol. 2017 Sep 15. doi: 10.1001/jamadermatol.2017.3225).
She reported having no financial conflicts of interest regarding her presentations.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Psoriasis patients who had never been on methotrexate had a 66% higher rate of alcohol-related death than matched controls without psoriasis.
Data source: This retrospective cohort study of alcohol-related deaths included 55,537 psoriasis patients and 854,314 controls without psoriasis matched based on age, sex, and membership in the same primary care practice.
Disclosures: The study presenter reported having no financial conflicts of interest.
Biosimilars poised to save $54 billion over the next decade
Biosimilars could reduce overall spending on biologic products by $54 billion from 2017 to 2026, according to new research from the Rand Corp.
Given the level of uncertainty surrounding the biosimilars market, however, the range of savings could be as low at $24 billion or as high as $150 billion.
“Because of limited U.S. experience with biosimilars, the key assumptions on market share and biosimilar prices are ‘best guesses’ based on anecdotes or professional opinion,” Andrew Mulcahy, PhD, a health policy researcher at Rand, and his colleagues, wrote in a perspective report.
“Whether actual cost savings end up above or below our baseline estimate hinges in large part on whether manufacturers continue to have a business case to invest in developing and marketing biosimilars,” the authors noted, citing a number of areas, including intellectual property litigation, payment, price competition, nonprice competition from reference biologic manufacturers, naming convention, and interchangeability.
Getting over these hurdles could require legislative or regulatory solutions.
“The pervasive uncertainty in the U.S. biosimilar market – including questions as to whether the market will be sustainable and lead to cost savings, as intended – presents two choices for policymakers,” Dr. Mulcahy and his colleagues wrote. “One strategy is to let the market continue to develop under current policies,” with stability coming from experience.
The alternative could be policy levers to “help steer the U.S. biosimilar market more quickly to a sustainable, competitive state,” they continued. “For example, regulators at the FDA could experiment with new approaches to provide stronger, earlier signals through guidance documents or other mechanisms on expectations surrounding interchangeability and other topics.”
The FDA appears to be moving on the latter. In an Oct. 23 blog post, FDA Commissioner Scott Gottlieb, MD, and Leah Christl, PhD, associate director for therapeutic biologics in the office of new drugs at the FDA’s Center for Drug Evaluation and Research, outlined a number of recent tools to help biosimilar adoption. The resources provide basics such as the basic definition associated with biosimilars (i.e., what is a biosimilar and a reference product, and what it means to be interchangeable), the standards of approval that biosimilars must go through, and easily accessible information on what the FDA is using to review biosimilarity.
“Next, FDA plans to embark on additional research with health care professionals to learn more about the types of information prescribers need to properly communicate with their patients about biosimilars,” Commissioner Gottlieb and Dr. Christl wrote. “An increase in market competition, offered by a growing complement of biosimilars, may lead to meaningfully reduced costs for both patients and our health care system.”
The Centers for Medicare & Medicaid Services also plays a role in developing policy to spur biosimilar adoption, Dr. Mulcahy and his colleagues wrote. They note work being done by the Medicare Payment Advisory Commission on recommendations that could address payment for physician-administered biosimilars under Part B, as well as incentives in the Part D prescription drug program to steer patients and providers toward lower cost biosimilars when appropriate. CMS changed current payment policy for biosimilars for 2018, which may have an effect.
“Beyond FDA regulation, payment, and coverage, both government and industry could play a role in educating patients and providers about the potential cost savings from biosimilars, much like both groups have done for generic drugs,” they stated. “While our study does not address whether policy action is needed now, it is likely that the answer will become clearer over the next 1 to 3 years as the market continues to develop.”
Biosimilars could reduce overall spending on biologic products by $54 billion from 2017 to 2026, according to new research from the Rand Corp.
Given the level of uncertainty surrounding the biosimilars market, however, the range of savings could be as low at $24 billion or as high as $150 billion.
“Because of limited U.S. experience with biosimilars, the key assumptions on market share and biosimilar prices are ‘best guesses’ based on anecdotes or professional opinion,” Andrew Mulcahy, PhD, a health policy researcher at Rand, and his colleagues, wrote in a perspective report.
“Whether actual cost savings end up above or below our baseline estimate hinges in large part on whether manufacturers continue to have a business case to invest in developing and marketing biosimilars,” the authors noted, citing a number of areas, including intellectual property litigation, payment, price competition, nonprice competition from reference biologic manufacturers, naming convention, and interchangeability.
Getting over these hurdles could require legislative or regulatory solutions.
“The pervasive uncertainty in the U.S. biosimilar market – including questions as to whether the market will be sustainable and lead to cost savings, as intended – presents two choices for policymakers,” Dr. Mulcahy and his colleagues wrote. “One strategy is to let the market continue to develop under current policies,” with stability coming from experience.
The alternative could be policy levers to “help steer the U.S. biosimilar market more quickly to a sustainable, competitive state,” they continued. “For example, regulators at the FDA could experiment with new approaches to provide stronger, earlier signals through guidance documents or other mechanisms on expectations surrounding interchangeability and other topics.”
The FDA appears to be moving on the latter. In an Oct. 23 blog post, FDA Commissioner Scott Gottlieb, MD, and Leah Christl, PhD, associate director for therapeutic biologics in the office of new drugs at the FDA’s Center for Drug Evaluation and Research, outlined a number of recent tools to help biosimilar adoption. The resources provide basics such as the basic definition associated with biosimilars (i.e., what is a biosimilar and a reference product, and what it means to be interchangeable), the standards of approval that biosimilars must go through, and easily accessible information on what the FDA is using to review biosimilarity.
“Next, FDA plans to embark on additional research with health care professionals to learn more about the types of information prescribers need to properly communicate with their patients about biosimilars,” Commissioner Gottlieb and Dr. Christl wrote. “An increase in market competition, offered by a growing complement of biosimilars, may lead to meaningfully reduced costs for both patients and our health care system.”
The Centers for Medicare & Medicaid Services also plays a role in developing policy to spur biosimilar adoption, Dr. Mulcahy and his colleagues wrote. They note work being done by the Medicare Payment Advisory Commission on recommendations that could address payment for physician-administered biosimilars under Part B, as well as incentives in the Part D prescription drug program to steer patients and providers toward lower cost biosimilars when appropriate. CMS changed current payment policy for biosimilars for 2018, which may have an effect.
“Beyond FDA regulation, payment, and coverage, both government and industry could play a role in educating patients and providers about the potential cost savings from biosimilars, much like both groups have done for generic drugs,” they stated. “While our study does not address whether policy action is needed now, it is likely that the answer will become clearer over the next 1 to 3 years as the market continues to develop.”
Biosimilars could reduce overall spending on biologic products by $54 billion from 2017 to 2026, according to new research from the Rand Corp.
Given the level of uncertainty surrounding the biosimilars market, however, the range of savings could be as low at $24 billion or as high as $150 billion.
“Because of limited U.S. experience with biosimilars, the key assumptions on market share and biosimilar prices are ‘best guesses’ based on anecdotes or professional opinion,” Andrew Mulcahy, PhD, a health policy researcher at Rand, and his colleagues, wrote in a perspective report.
“Whether actual cost savings end up above or below our baseline estimate hinges in large part on whether manufacturers continue to have a business case to invest in developing and marketing biosimilars,” the authors noted, citing a number of areas, including intellectual property litigation, payment, price competition, nonprice competition from reference biologic manufacturers, naming convention, and interchangeability.
Getting over these hurdles could require legislative or regulatory solutions.
“The pervasive uncertainty in the U.S. biosimilar market – including questions as to whether the market will be sustainable and lead to cost savings, as intended – presents two choices for policymakers,” Dr. Mulcahy and his colleagues wrote. “One strategy is to let the market continue to develop under current policies,” with stability coming from experience.
The alternative could be policy levers to “help steer the U.S. biosimilar market more quickly to a sustainable, competitive state,” they continued. “For example, regulators at the FDA could experiment with new approaches to provide stronger, earlier signals through guidance documents or other mechanisms on expectations surrounding interchangeability and other topics.”
The FDA appears to be moving on the latter. In an Oct. 23 blog post, FDA Commissioner Scott Gottlieb, MD, and Leah Christl, PhD, associate director for therapeutic biologics in the office of new drugs at the FDA’s Center for Drug Evaluation and Research, outlined a number of recent tools to help biosimilar adoption. The resources provide basics such as the basic definition associated with biosimilars (i.e., what is a biosimilar and a reference product, and what it means to be interchangeable), the standards of approval that biosimilars must go through, and easily accessible information on what the FDA is using to review biosimilarity.
“Next, FDA plans to embark on additional research with health care professionals to learn more about the types of information prescribers need to properly communicate with their patients about biosimilars,” Commissioner Gottlieb and Dr. Christl wrote. “An increase in market competition, offered by a growing complement of biosimilars, may lead to meaningfully reduced costs for both patients and our health care system.”
The Centers for Medicare & Medicaid Services also plays a role in developing policy to spur biosimilar adoption, Dr. Mulcahy and his colleagues wrote. They note work being done by the Medicare Payment Advisory Commission on recommendations that could address payment for physician-administered biosimilars under Part B, as well as incentives in the Part D prescription drug program to steer patients and providers toward lower cost biosimilars when appropriate. CMS changed current payment policy for biosimilars for 2018, which may have an effect.
“Beyond FDA regulation, payment, and coverage, both government and industry could play a role in educating patients and providers about the potential cost savings from biosimilars, much like both groups have done for generic drugs,” they stated. “While our study does not address whether policy action is needed now, it is likely that the answer will become clearer over the next 1 to 3 years as the market continues to develop.”
Psoriasis: Biologics bring potential for long-term remission off treatment
GENEVA – Can potent, highly efficacious biologic therapy induce longstanding remission of moderate to severe plaque psoriasis following discontinuation of all treatment?
The answer appears to be yes, in a minority of patients, based on results of a long-term extension study of two pivotal phase 3 clinical trials of secukinumab (Cosentyx), Mark G. Lebwohl, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.

This 2-year extension study provides the first double-blind, long-term evidence regarding the natural history of psoriasis following discontinuation of a contemporary potent targeted therapy.
The results are exciting because spontaneous remission of moderate to severe psoriasis is rare; psoriasis typically reverts to baseline severity following discontinuation of treatment. In the extension study, though, 21% of patients who achieved a 75% reduction in the Psoriasis Area Severity Index (PASI 75) response on secukinumab at the 300-mg dose for 52 weeks and were then crossed over double-blind to placebo remained continuously relapse-free after 12 months on placebo, and 10% remained relapse-free at 2 years, at which point they were unblinded and put back on secukinumab. No psoriasis therapies were permitted during the placebo phase.
Those findings are particularly impressive because participants in the two pivotal trials of secukinumab combined for the treatment withdrawal extension study – ERASURE and FIXTURE – had a mean 14- and 16-year history of psoriasis at baseline.
“Secukinumab appears to have modified the course of chronic moderate to severe psoriasis, despite intervening late in its course. Treating patients early is expected to have an even greater potential for disease modification,” commented Dr. Lebwohl, the Sol and Clara Kest Professor and chair of the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
That hypothesis is under investigation in the ongoing multinational STEPin trial (Study of the Efficacy of Early Intervention With Secukinumab 300 mg s.c. [subcutaneous] Compared to Narrow-band UVB in Patients With New-onset Moderate to Severe Plaque Psoriasis). STEPin involves about 200 patients with newly diagnosed disease not previously treated with systemic agents or phototherapy.
Dr. Lebwohl focused on the 120 patients in the extension study who had a PASI 75 response to secukinumab at the 300-mg dose and the 100 patients with a PASI 75 response to the 150-mg dose who, after 52 weeks on the fully human anti-interleukin-17A monoclonal antibody, were crossed over double-blind to placebo for 2 years or until relapse occurred. Patients were assessed monthly during the extension study.
Relapse was defined as loss of 50% of the maximum PASI improvement achieved while on secukinumab. The relapse-free rate was better in patients who had been on secukinumab 300 mg than in those on 150 mg. At 1 year on placebo, 21% of patients formerly on secukinumab at the higher dose remained free of relapse, as did 10% at 2 years. In contrast, the 1- and 2-year relapse-free rates in the group formerly on secukinumab 150 mg were lower at 14% and 6%, respectively, even though everyone in the extension study had a PASI 75 response when they went off treatment.
The mean baseline PASI score in the ERASURE and FIXTURE trials was 23. Among patients who remained relapse-free for 1 year, the mean PASI score was dramatically lower, at 0.7; among those in remission for 2 years, it was 0.4.
Importantly, the longer a patient’s history of psoriasis, the less likely a long-term relapse-free experience, the dermatologist observed.
A separate mechanistic study in a different group of psoriasis patients provided a likely explanation for the long relapse-free period seen off therapy in some patients in the extension study. The mechanistic study entailed transcriptional analysis of psoriasis-related genes in lesional and nonlesional skin before and after 1 year of treatment with secukinumab 300 mg. The major finding: 85% of the upregulated genes and 75% of downregulated genes in lesional skin pretreatment reverted to normal levels in healed lesional skin post treatment.
Moreover, global mRNA expression for key genes involved in the interleukin-23/interleukin-17 inflammatory cytokine pathway closely resembled healthy skin following treatment with secukinumab. This is evidence of profound molecular normalization, Dr. Lebwohl said.
These secukinumab studies were sponsored by Novartis. Dr. Lebwohl reported having no personal financial conflicts of interest, although his department receives research funding from Novartis and numerous other pharmaceutical companies.
GENEVA – Can potent, highly efficacious biologic therapy induce longstanding remission of moderate to severe plaque psoriasis following discontinuation of all treatment?
The answer appears to be yes, in a minority of patients, based on results of a long-term extension study of two pivotal phase 3 clinical trials of secukinumab (Cosentyx), Mark G. Lebwohl, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.

This 2-year extension study provides the first double-blind, long-term evidence regarding the natural history of psoriasis following discontinuation of a contemporary potent targeted therapy.
The results are exciting because spontaneous remission of moderate to severe psoriasis is rare; psoriasis typically reverts to baseline severity following discontinuation of treatment. In the extension study, though, 21% of patients who achieved a 75% reduction in the Psoriasis Area Severity Index (PASI 75) response on secukinumab at the 300-mg dose for 52 weeks and were then crossed over double-blind to placebo remained continuously relapse-free after 12 months on placebo, and 10% remained relapse-free at 2 years, at which point they were unblinded and put back on secukinumab. No psoriasis therapies were permitted during the placebo phase.
Those findings are particularly impressive because participants in the two pivotal trials of secukinumab combined for the treatment withdrawal extension study – ERASURE and FIXTURE – had a mean 14- and 16-year history of psoriasis at baseline.
“Secukinumab appears to have modified the course of chronic moderate to severe psoriasis, despite intervening late in its course. Treating patients early is expected to have an even greater potential for disease modification,” commented Dr. Lebwohl, the Sol and Clara Kest Professor and chair of the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
That hypothesis is under investigation in the ongoing multinational STEPin trial (Study of the Efficacy of Early Intervention With Secukinumab 300 mg s.c. [subcutaneous] Compared to Narrow-band UVB in Patients With New-onset Moderate to Severe Plaque Psoriasis). STEPin involves about 200 patients with newly diagnosed disease not previously treated with systemic agents or phototherapy.
Dr. Lebwohl focused on the 120 patients in the extension study who had a PASI 75 response to secukinumab at the 300-mg dose and the 100 patients with a PASI 75 response to the 150-mg dose who, after 52 weeks on the fully human anti-interleukin-17A monoclonal antibody, were crossed over double-blind to placebo for 2 years or until relapse occurred. Patients were assessed monthly during the extension study.
Relapse was defined as loss of 50% of the maximum PASI improvement achieved while on secukinumab. The relapse-free rate was better in patients who had been on secukinumab 300 mg than in those on 150 mg. At 1 year on placebo, 21% of patients formerly on secukinumab at the higher dose remained free of relapse, as did 10% at 2 years. In contrast, the 1- and 2-year relapse-free rates in the group formerly on secukinumab 150 mg were lower at 14% and 6%, respectively, even though everyone in the extension study had a PASI 75 response when they went off treatment.
The mean baseline PASI score in the ERASURE and FIXTURE trials was 23. Among patients who remained relapse-free for 1 year, the mean PASI score was dramatically lower, at 0.7; among those in remission for 2 years, it was 0.4.
Importantly, the longer a patient’s history of psoriasis, the less likely a long-term relapse-free experience, the dermatologist observed.
A separate mechanistic study in a different group of psoriasis patients provided a likely explanation for the long relapse-free period seen off therapy in some patients in the extension study. The mechanistic study entailed transcriptional analysis of psoriasis-related genes in lesional and nonlesional skin before and after 1 year of treatment with secukinumab 300 mg. The major finding: 85% of the upregulated genes and 75% of downregulated genes in lesional skin pretreatment reverted to normal levels in healed lesional skin post treatment.
Moreover, global mRNA expression for key genes involved in the interleukin-23/interleukin-17 inflammatory cytokine pathway closely resembled healthy skin following treatment with secukinumab. This is evidence of profound molecular normalization, Dr. Lebwohl said.
These secukinumab studies were sponsored by Novartis. Dr. Lebwohl reported having no personal financial conflicts of interest, although his department receives research funding from Novartis and numerous other pharmaceutical companies.
GENEVA – Can potent, highly efficacious biologic therapy induce longstanding remission of moderate to severe plaque psoriasis following discontinuation of all treatment?
The answer appears to be yes, in a minority of patients, based on results of a long-term extension study of two pivotal phase 3 clinical trials of secukinumab (Cosentyx), Mark G. Lebwohl, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.

This 2-year extension study provides the first double-blind, long-term evidence regarding the natural history of psoriasis following discontinuation of a contemporary potent targeted therapy.
The results are exciting because spontaneous remission of moderate to severe psoriasis is rare; psoriasis typically reverts to baseline severity following discontinuation of treatment. In the extension study, though, 21% of patients who achieved a 75% reduction in the Psoriasis Area Severity Index (PASI 75) response on secukinumab at the 300-mg dose for 52 weeks and were then crossed over double-blind to placebo remained continuously relapse-free after 12 months on placebo, and 10% remained relapse-free at 2 years, at which point they were unblinded and put back on secukinumab. No psoriasis therapies were permitted during the placebo phase.
Those findings are particularly impressive because participants in the two pivotal trials of secukinumab combined for the treatment withdrawal extension study – ERASURE and FIXTURE – had a mean 14- and 16-year history of psoriasis at baseline.
“Secukinumab appears to have modified the course of chronic moderate to severe psoriasis, despite intervening late in its course. Treating patients early is expected to have an even greater potential for disease modification,” commented Dr. Lebwohl, the Sol and Clara Kest Professor and chair of the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
That hypothesis is under investigation in the ongoing multinational STEPin trial (Study of the Efficacy of Early Intervention With Secukinumab 300 mg s.c. [subcutaneous] Compared to Narrow-band UVB in Patients With New-onset Moderate to Severe Plaque Psoriasis). STEPin involves about 200 patients with newly diagnosed disease not previously treated with systemic agents or phototherapy.
Dr. Lebwohl focused on the 120 patients in the extension study who had a PASI 75 response to secukinumab at the 300-mg dose and the 100 patients with a PASI 75 response to the 150-mg dose who, after 52 weeks on the fully human anti-interleukin-17A monoclonal antibody, were crossed over double-blind to placebo for 2 years or until relapse occurred. Patients were assessed monthly during the extension study.
Relapse was defined as loss of 50% of the maximum PASI improvement achieved while on secukinumab. The relapse-free rate was better in patients who had been on secukinumab 300 mg than in those on 150 mg. At 1 year on placebo, 21% of patients formerly on secukinumab at the higher dose remained free of relapse, as did 10% at 2 years. In contrast, the 1- and 2-year relapse-free rates in the group formerly on secukinumab 150 mg were lower at 14% and 6%, respectively, even though everyone in the extension study had a PASI 75 response when they went off treatment.
The mean baseline PASI score in the ERASURE and FIXTURE trials was 23. Among patients who remained relapse-free for 1 year, the mean PASI score was dramatically lower, at 0.7; among those in remission for 2 years, it was 0.4.
Importantly, the longer a patient’s history of psoriasis, the less likely a long-term relapse-free experience, the dermatologist observed.
A separate mechanistic study in a different group of psoriasis patients provided a likely explanation for the long relapse-free period seen off therapy in some patients in the extension study. The mechanistic study entailed transcriptional analysis of psoriasis-related genes in lesional and nonlesional skin before and after 1 year of treatment with secukinumab 300 mg. The major finding: 85% of the upregulated genes and 75% of downregulated genes in lesional skin pretreatment reverted to normal levels in healed lesional skin post treatment.
Moreover, global mRNA expression for key genes involved in the interleukin-23/interleukin-17 inflammatory cytokine pathway closely resembled healthy skin following treatment with secukinumab. This is evidence of profound molecular normalization, Dr. Lebwohl said.
These secukinumab studies were sponsored by Novartis. Dr. Lebwohl reported having no personal financial conflicts of interest, although his department receives research funding from Novartis and numerous other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point: , enabling some patients to experience long-term remission off therapy.
Major finding: After 1 and 2 years without any psoriasis therapy following successful treatment with secukinumab for 52 weeks, 21% and 10% of patients, respectively, remained relapse-free.
Data source: This prospective extension of two phase 3 pivotal trials of secukinumab included 220 psoriasis patients who were taken off the biologic after 52 weeks and crossed over double blind to placebo for up to 2 years.
Disclosures: The study was sponsored by Novartis. Dr. Lebwohl reported having no financial conflicts.
Pediatric psoriasis carries sharply increased risk of selected autoimmune comorbidities
GENEVA – Pediatric psoriasis is associated with sharply increased risks of selected autoimmune diseases, according to a cross-sectional study encompassing every child and adolescent in Denmark.
"Even though the absolute risk of many of these conditions remains rare in childhood, clinicians should keep these associations in mind because they can greatly add to the total disease burden. In particular, we advise focusing on extracutaneous symptoms when treating psoriasis in children,” Christoffer Blegvad, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional study in which Denmark’s vaunted system of comprehensive national registries was harnessed to obtain health information on all individuals under age 18 years living in Denmark as of the end of 2012. The study population comprised 1,925 children and adolescents with dermatologist-diagnosed psoriasis, including those with psoriasis mild enough to be managed with topical therapies, and 1,194,712 youths without psoriasis.
In a first-pass unadjusted analysis, the psoriasis patients were at significantly increased risk for all nine of the autoimmune diseases examined. When the investigators adjusted for age, sex, and an individual’s health care utilization as reflected in his or her number of dermatology visits, the children and adolescents with psoriasis remained at tenfold increased risk for comorbid psoriatic arthritis, 6.6-fold risk for rheumatoid arthritis, 4.8-fold risk for vitiligo, and smaller yet significantly increased risks for several other autoimmune diseases, compared with individuals without psoriasis.
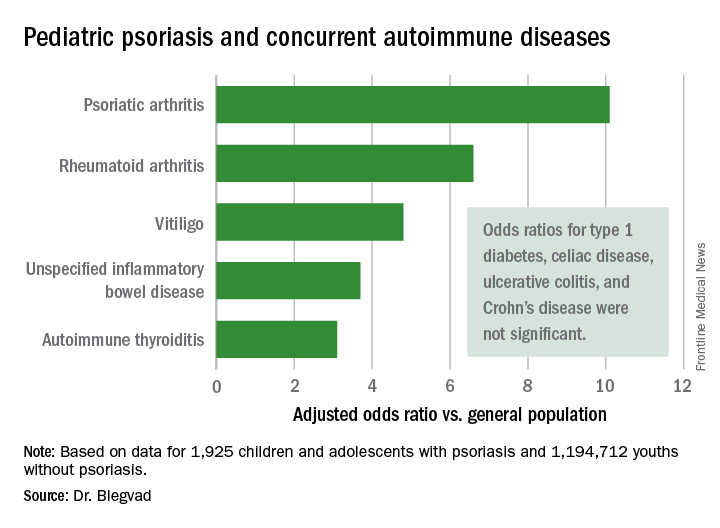
Indeed, while the presence of pediatric psoriasis was associated with an adjusted 4.4-fold increased risk of having at least one autoimmune disease, psoriasis patients with one of the selected autoimmune diseases were at 7.3-fold greater risk of having two or more autoimmune diseases, compared with individuals with one autoimmune disease who didn’t have psoriasis.
This is the first study to show such a clustering effect in either pediatric or adult psoriasis patients. The finding highlights the complex genetic underpinnings of psoriasis, which has previously been shown to share genetic susceptibility loci with inflammatory bowel disease and various other autoimmune diseases, Dr. Blegvad noted.
A small caveat: The psoriatic arthritis category also included individuals with juvenile idiopathic arthritis and juvenile psoriatic arthritis, because the clinical signs and symptoms of the three disorders often are difficult to distinguish in young patients.
Dr. Blegvad reported having no financial conflicts regarding the study, funded by Herlev and Gentofte Hospital and the LEO Foundation.
GENEVA – Pediatric psoriasis is associated with sharply increased risks of selected autoimmune diseases, according to a cross-sectional study encompassing every child and adolescent in Denmark.
"Even though the absolute risk of many of these conditions remains rare in childhood, clinicians should keep these associations in mind because they can greatly add to the total disease burden. In particular, we advise focusing on extracutaneous symptoms when treating psoriasis in children,” Christoffer Blegvad, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional study in which Denmark’s vaunted system of comprehensive national registries was harnessed to obtain health information on all individuals under age 18 years living in Denmark as of the end of 2012. The study population comprised 1,925 children and adolescents with dermatologist-diagnosed psoriasis, including those with psoriasis mild enough to be managed with topical therapies, and 1,194,712 youths without psoriasis.
In a first-pass unadjusted analysis, the psoriasis patients were at significantly increased risk for all nine of the autoimmune diseases examined. When the investigators adjusted for age, sex, and an individual’s health care utilization as reflected in his or her number of dermatology visits, the children and adolescents with psoriasis remained at tenfold increased risk for comorbid psoriatic arthritis, 6.6-fold risk for rheumatoid arthritis, 4.8-fold risk for vitiligo, and smaller yet significantly increased risks for several other autoimmune diseases, compared with individuals without psoriasis.

Indeed, while the presence of pediatric psoriasis was associated with an adjusted 4.4-fold increased risk of having at least one autoimmune disease, psoriasis patients with one of the selected autoimmune diseases were at 7.3-fold greater risk of having two or more autoimmune diseases, compared with individuals with one autoimmune disease who didn’t have psoriasis.
This is the first study to show such a clustering effect in either pediatric or adult psoriasis patients. The finding highlights the complex genetic underpinnings of psoriasis, which has previously been shown to share genetic susceptibility loci with inflammatory bowel disease and various other autoimmune diseases, Dr. Blegvad noted.
A small caveat: The psoriatic arthritis category also included individuals with juvenile idiopathic arthritis and juvenile psoriatic arthritis, because the clinical signs and symptoms of the three disorders often are difficult to distinguish in young patients.
Dr. Blegvad reported having no financial conflicts regarding the study, funded by Herlev and Gentofte Hospital and the LEO Foundation.
GENEVA – Pediatric psoriasis is associated with sharply increased risks of selected autoimmune diseases, according to a cross-sectional study encompassing every child and adolescent in Denmark.
"Even though the absolute risk of many of these conditions remains rare in childhood, clinicians should keep these associations in mind because they can greatly add to the total disease burden. In particular, we advise focusing on extracutaneous symptoms when treating psoriasis in children,” Christoffer Blegvad, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
He presented a cross-sectional study in which Denmark’s vaunted system of comprehensive national registries was harnessed to obtain health information on all individuals under age 18 years living in Denmark as of the end of 2012. The study population comprised 1,925 children and adolescents with dermatologist-diagnosed psoriasis, including those with psoriasis mild enough to be managed with topical therapies, and 1,194,712 youths without psoriasis.
In a first-pass unadjusted analysis, the psoriasis patients were at significantly increased risk for all nine of the autoimmune diseases examined. When the investigators adjusted for age, sex, and an individual’s health care utilization as reflected in his or her number of dermatology visits, the children and adolescents with psoriasis remained at tenfold increased risk for comorbid psoriatic arthritis, 6.6-fold risk for rheumatoid arthritis, 4.8-fold risk for vitiligo, and smaller yet significantly increased risks for several other autoimmune diseases, compared with individuals without psoriasis.

Indeed, while the presence of pediatric psoriasis was associated with an adjusted 4.4-fold increased risk of having at least one autoimmune disease, psoriasis patients with one of the selected autoimmune diseases were at 7.3-fold greater risk of having two or more autoimmune diseases, compared with individuals with one autoimmune disease who didn’t have psoriasis.
This is the first study to show such a clustering effect in either pediatric or adult psoriasis patients. The finding highlights the complex genetic underpinnings of psoriasis, which has previously been shown to share genetic susceptibility loci with inflammatory bowel disease and various other autoimmune diseases, Dr. Blegvad noted.
A small caveat: The psoriatic arthritis category also included individuals with juvenile idiopathic arthritis and juvenile psoriatic arthritis, because the clinical signs and symptoms of the three disorders often are difficult to distinguish in young patients.
Dr. Blegvad reported having no financial conflicts regarding the study, funded by Herlev and Gentofte Hospital and the LEO Foundation.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Pediatric psoriasis patients were at an adjusted 6.6-fold increased risk of comorbid rheumatoid arthritis, 4.8-fold risk of vitiligo, and significantly increased risks of several other autoimmune diseases, compared with matched youths without psoriasis.
Data source: A cross-sectional study of all children and adolescents living in Denmark at the end of 2012.
Disclosures: The presenter reported having no financial conflicts regarding the study, funded by Herlev and Gentofte Hospital and the LEO Foundation.
Biologic approved for moderate to severe psoriasis in adolescents
The Food and Drug Administration approval of ustekinumab has been expanded to include adolescents aged 12 and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, based on the results of a phase 3 study.
The manufacturer, Janssen Biotech, announced the expanded indication in a press release on Oct. 13.
Ustekinumab, an interleukin-12 and -23 antagonist administered subcutaneously, was first approved by the FDA in 2009 for the same indication in adults; it is also approved for adults with active psoriatic arthritis, and for adults with moderately to severely active Crohn’s disease.
Ustekinumab is marketed as Stelara.
The Food and Drug Administration approval of ustekinumab has been expanded to include adolescents aged 12 and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, based on the results of a phase 3 study.
The manufacturer, Janssen Biotech, announced the expanded indication in a press release on Oct. 13.
Ustekinumab, an interleukin-12 and -23 antagonist administered subcutaneously, was first approved by the FDA in 2009 for the same indication in adults; it is also approved for adults with active psoriatic arthritis, and for adults with moderately to severely active Crohn’s disease.
Ustekinumab is marketed as Stelara.
The Food and Drug Administration approval of ustekinumab has been expanded to include adolescents aged 12 and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, based on the results of a phase 3 study.
The manufacturer, Janssen Biotech, announced the expanded indication in a press release on Oct. 13.
Ustekinumab, an interleukin-12 and -23 antagonist administered subcutaneously, was first approved by the FDA in 2009 for the same indication in adults; it is also approved for adults with active psoriatic arthritis, and for adults with moderately to severely active Crohn’s disease.
Ustekinumab is marketed as Stelara.











